User login
News and Views that Matter to Pediatricians
The leading independent newspaper covering news and commentary in pediatrics.
Children and COVID: Vaccinations lower than ever as cases continue to drop
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.
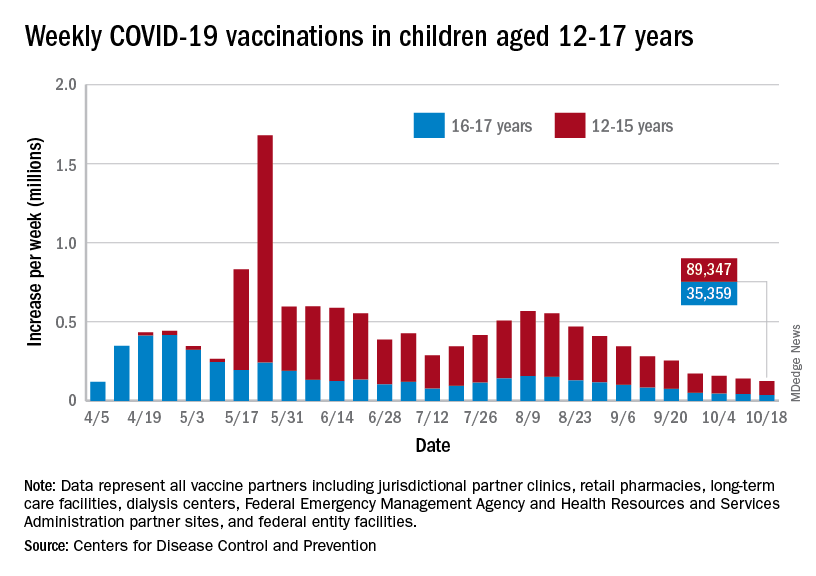
Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.

Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.

Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.
FDA expands use of HIV drug to young children
The new lower dose is approved for children weighing from at least 14 kg (30 pounds) to 25 kg (55 pounds) who are virologically suppressed or new to antiretroviral therapy.
“Children living with HIV are in need of effective and accessible formulations of antiretroviral therapy,” said Merdad Parsey, MD, PhD, chief medical officer of Gilead Sciences, the company that produces Biktarvy, in a press release. “The New Drug Application approval is an important step in fulfilling Gilead’s commitment to a goal of bringing pediatric formulations of Biktarvy to children living with HIV around the world,” he said.
Although advances in treatment for pregnant women with HIV have lowered the likelihood of perinatal HIV transmission, pediatric HIV remains a global public health challenge. In 2020, about 1.7 million children younger than 15 years were living with HIV worldwide; 850 children become infected every day.
The approval, announced October 18, expands the use of Biktarvy to younger children. The medication was originally approved in February 2018 for treatment-naive or virologically suppressed adults. In June 2019, the FDA approved updating of the label to include pediatric patients weighing at least 25 kg. This new lower dose of Biktarvy is for a three-drug combo containing bictegravir 30 mg, emtricitabine 120 mg, and tenofovir alafenamide 15 mg. It is given once a day in tablet form.
The most recent expanded indication was based on data from an open-label, single-arm study that included 22 virologically suppressed children living with HIV. After switching to Biktarvy, 91% of participants (20 of 22) remained virologically suppressed at 24 weeks. HIV-1 RNA was not collected for two patients because of «pandemic-related study disruption,» the press release said.
“As children living with HIV will be on therapy for the foreseeable future and from such a young age, there are a number of factors I weigh as a clinician when prescribing the right HIV treatment option to my pediatric patients,” said Carina Rodriguez, MD, the division chief of pediatric infectious diseases at the University of South Florida, who was one of the study investigators. “Finding an efficacious treatment option is paramount, but tolerability and safety are keys to ensuring treatment success. With this expanded approval, clinicians can add Biktarvy to their arsenal of options to help ensure these children maintain virologic suppression with a treatment option that makes sense for them.”
A version of this article first appeared on Medscape.com.
The new lower dose is approved for children weighing from at least 14 kg (30 pounds) to 25 kg (55 pounds) who are virologically suppressed or new to antiretroviral therapy.
“Children living with HIV are in need of effective and accessible formulations of antiretroviral therapy,” said Merdad Parsey, MD, PhD, chief medical officer of Gilead Sciences, the company that produces Biktarvy, in a press release. “The New Drug Application approval is an important step in fulfilling Gilead’s commitment to a goal of bringing pediatric formulations of Biktarvy to children living with HIV around the world,” he said.
Although advances in treatment for pregnant women with HIV have lowered the likelihood of perinatal HIV transmission, pediatric HIV remains a global public health challenge. In 2020, about 1.7 million children younger than 15 years were living with HIV worldwide; 850 children become infected every day.
The approval, announced October 18, expands the use of Biktarvy to younger children. The medication was originally approved in February 2018 for treatment-naive or virologically suppressed adults. In June 2019, the FDA approved updating of the label to include pediatric patients weighing at least 25 kg. This new lower dose of Biktarvy is for a three-drug combo containing bictegravir 30 mg, emtricitabine 120 mg, and tenofovir alafenamide 15 mg. It is given once a day in tablet form.
The most recent expanded indication was based on data from an open-label, single-arm study that included 22 virologically suppressed children living with HIV. After switching to Biktarvy, 91% of participants (20 of 22) remained virologically suppressed at 24 weeks. HIV-1 RNA was not collected for two patients because of «pandemic-related study disruption,» the press release said.
“As children living with HIV will be on therapy for the foreseeable future and from such a young age, there are a number of factors I weigh as a clinician when prescribing the right HIV treatment option to my pediatric patients,” said Carina Rodriguez, MD, the division chief of pediatric infectious diseases at the University of South Florida, who was one of the study investigators. “Finding an efficacious treatment option is paramount, but tolerability and safety are keys to ensuring treatment success. With this expanded approval, clinicians can add Biktarvy to their arsenal of options to help ensure these children maintain virologic suppression with a treatment option that makes sense for them.”
A version of this article first appeared on Medscape.com.
The new lower dose is approved for children weighing from at least 14 kg (30 pounds) to 25 kg (55 pounds) who are virologically suppressed or new to antiretroviral therapy.
“Children living with HIV are in need of effective and accessible formulations of antiretroviral therapy,” said Merdad Parsey, MD, PhD, chief medical officer of Gilead Sciences, the company that produces Biktarvy, in a press release. “The New Drug Application approval is an important step in fulfilling Gilead’s commitment to a goal of bringing pediatric formulations of Biktarvy to children living with HIV around the world,” he said.
Although advances in treatment for pregnant women with HIV have lowered the likelihood of perinatal HIV transmission, pediatric HIV remains a global public health challenge. In 2020, about 1.7 million children younger than 15 years were living with HIV worldwide; 850 children become infected every day.
The approval, announced October 18, expands the use of Biktarvy to younger children. The medication was originally approved in February 2018 for treatment-naive or virologically suppressed adults. In June 2019, the FDA approved updating of the label to include pediatric patients weighing at least 25 kg. This new lower dose of Biktarvy is for a three-drug combo containing bictegravir 30 mg, emtricitabine 120 mg, and tenofovir alafenamide 15 mg. It is given once a day in tablet form.
The most recent expanded indication was based on data from an open-label, single-arm study that included 22 virologically suppressed children living with HIV. After switching to Biktarvy, 91% of participants (20 of 22) remained virologically suppressed at 24 weeks. HIV-1 RNA was not collected for two patients because of «pandemic-related study disruption,» the press release said.
“As children living with HIV will be on therapy for the foreseeable future and from such a young age, there are a number of factors I weigh as a clinician when prescribing the right HIV treatment option to my pediatric patients,” said Carina Rodriguez, MD, the division chief of pediatric infectious diseases at the University of South Florida, who was one of the study investigators. “Finding an efficacious treatment option is paramount, but tolerability and safety are keys to ensuring treatment success. With this expanded approval, clinicians can add Biktarvy to their arsenal of options to help ensure these children maintain virologic suppression with a treatment option that makes sense for them.”
A version of this article first appeared on Medscape.com.
National Academies issue guidance for childhood COVID-19 vaccines
While the U.S. Food and Drug Administration has yet to give the green light to COVID-19 vaccination for children who are under age 12, it is expected that approval will be granted. In anticipation of the FDA’s go-ahead, which is expected in the coming weeks, a new “rapid expert consultation” has identified “actionable guidance” that state and local decision-makers can use to communicate with the public. The goal is to build confidence in and promote the uptake of COVID-19 vaccines, especially for parents who are contemplating vaccinating their children.
They note that key factors in decision-making concern vaccine side effects, the efficacy of the vaccine in children, availability of research in their child’s age group, research conducted by the parents themselves, and recommendations by the child’s health care provider.
“One of the reasons that the COVID vaccine only became available for children 12 and over months after it was approved for adults is that it takes time and many, many trial participants who are closely monitored before the vaccine ever reaches the general public,” said Nusheen Ameenuddin, MD, MPH, MPA, an assistant professor of pediatrics at the Mayo Clinic, Rochester, Minn. “We continue to talk to parents about the fact that the vaccines have been very safe and effective in this group, and even though people are concerned about side effects, they are much milder and less frequent than the effects of the disease itself.”
Dr. Ameenuddin noted that the lack of data in this age group can be concerning for parents. “It’s not like other vaccines which have been available for a long time, and the clinical trial data are still limited for this age group,” she said. “But I think the main point that practitioners need to emphasize is that, even though the vaccine is new, the science for this vaccine has been around for about a decade.”
The unique circumstances of a pandemic, she pointed out, allowed for important information about effectiveness, safety, and side effects to be obtained more quickly from clinical trial data.
“We have really good evidence for kids 12 and over, about safety and effectiveness, and even though children are not small adults and have their own unique physiology, this has provided a good starting point to suggest that kids slightly younger will also respond well to the vaccines,” said Dr. Ameenuddin, who is also chair of the American Academy of Pediatrics Council on Communications and Media. “As we learn more, we can start gathering more information about even younger kids to ensure that the right dosage and spacing of vaccines can provide maximum vaccine effectiveness and protection from disease.”
The guidance was published Oct. 13 by the National Academies of Sciences, Engineering, and Medicine.
The rapid expert consultation was produced through the Societal Experts Action Network, an activity of the National Academies that is sponsored by the NASEM and the Alfred P. Sloan Foundation. The goal of SEAN is to connect researchers in the social, behavioral, and economic sciences with decision-makers to respond to policy questions related to the COVID-19 pandemic.
In their expert consultation, the authors emphasize that vaccination is critical for decreasing transmission and controlling infection, as well as limiting the emergence of future serious variants. As of Oct. 3, 2021, about 65% of the U.S. population had received at least one dose of the vaccine, and the rate has begun to lag in many areas of the country. There are a variety of reasons for vaccine hesitancy, they note, including perception of low risks from COVID-19 or of high risks from COVID-19 vaccines, exposure to media, political agendas, lack of confidence in science, and distrust of the medical establishment. The Pfizer/BioNTech vaccine is currently authorized for emergency use for individuals 12 years of age and older and fully approved for those aged 16 and older, while the Moderna and the Johnson & Johnson vaccines are authorized for emergency use for those 18 years of age and older.
Many children between the ages of 12 and 17 have not been vaccinated, and the major concerns reported by parents include not knowing enough about the long-term effects of the COVID-19 vaccine in children (88%), concerns about children experiencing serious side effects (79%), and concerns that the COVID-19 vaccine might negatively affect future fertility (73%).
The National Academies have previously released two other “rapid expert consultations” which have addressed building vaccine confidence, and both reports provide key strategies for communicating information about COVID-19 vaccines. In this paper, the focus was on communicating with parents to gain confidence in the vaccine and address concerns.
Key points
The key strategies highlighted for communicating with parents include the following:
- Emphasizing safety and efficacy: Parents should be informed about the ongoing research and clinical trials that will answer more questions about the vaccine and that there is continued monitoring for any safety risks. Pointing to the safety data from the clinical trials for 12- to 17-year-olds, and the lack of serious adverse events from the vaccine in this age group may help alleviate concerns.
- CalibriEncouraging parents to talk with a primary care provider: Research shows that parents trust family physicians and other health care practitioners to provide them with accurate information about vaccines. Local, state, and national leaders can provide messaging templates and other resources to health care professionals who are engaged in these conversations.
- Leveraging social networks to influence parents’ vaccination decisions: Parents are influenced by their social network connections. It is important to engage these networks, especially with members of their community who are considered trustworthy and influential. Social networks may also be very diverse, and include family members, friends, coworkers, social media, and members of their religious community.
While the guidance states that different groups of parents will require different messaging, they suggest that communication can begin with a focus on the things that vaccination can accomplish. In addition to preventing infection with COVID-19, it will allow children to attend school in person and participate in extracurricular activities such as sports, without risking their health. “One thing I’ve learned over several years of working with vaccine-hesitant parents is that you have to tailor each approach to the individual,” said Dr. Ameenuddin. “Different people have different concerns, and first and foremost, it’s important to listen.”
For some parents, emphasizing that the more people that can be vaccinated and the sooner it can be done, the sooner everyone can return to a normal life is a good approach, she added. “I think it’s important to emphasize both the individual and communal benefits of vaccines, but that won’t necessarily reach every person with concerns. I think it’s important to find out what is most important to individuals and work from there to find a way to connect with that family to encourage vaccination.”
Dr. Ameenuddin has no disclosures.
While the U.S. Food and Drug Administration has yet to give the green light to COVID-19 vaccination for children who are under age 12, it is expected that approval will be granted. In anticipation of the FDA’s go-ahead, which is expected in the coming weeks, a new “rapid expert consultation” has identified “actionable guidance” that state and local decision-makers can use to communicate with the public. The goal is to build confidence in and promote the uptake of COVID-19 vaccines, especially for parents who are contemplating vaccinating their children.
They note that key factors in decision-making concern vaccine side effects, the efficacy of the vaccine in children, availability of research in their child’s age group, research conducted by the parents themselves, and recommendations by the child’s health care provider.
“One of the reasons that the COVID vaccine only became available for children 12 and over months after it was approved for adults is that it takes time and many, many trial participants who are closely monitored before the vaccine ever reaches the general public,” said Nusheen Ameenuddin, MD, MPH, MPA, an assistant professor of pediatrics at the Mayo Clinic, Rochester, Minn. “We continue to talk to parents about the fact that the vaccines have been very safe and effective in this group, and even though people are concerned about side effects, they are much milder and less frequent than the effects of the disease itself.”
Dr. Ameenuddin noted that the lack of data in this age group can be concerning for parents. “It’s not like other vaccines which have been available for a long time, and the clinical trial data are still limited for this age group,” she said. “But I think the main point that practitioners need to emphasize is that, even though the vaccine is new, the science for this vaccine has been around for about a decade.”
The unique circumstances of a pandemic, she pointed out, allowed for important information about effectiveness, safety, and side effects to be obtained more quickly from clinical trial data.
“We have really good evidence for kids 12 and over, about safety and effectiveness, and even though children are not small adults and have their own unique physiology, this has provided a good starting point to suggest that kids slightly younger will also respond well to the vaccines,” said Dr. Ameenuddin, who is also chair of the American Academy of Pediatrics Council on Communications and Media. “As we learn more, we can start gathering more information about even younger kids to ensure that the right dosage and spacing of vaccines can provide maximum vaccine effectiveness and protection from disease.”
The guidance was published Oct. 13 by the National Academies of Sciences, Engineering, and Medicine.
The rapid expert consultation was produced through the Societal Experts Action Network, an activity of the National Academies that is sponsored by the NASEM and the Alfred P. Sloan Foundation. The goal of SEAN is to connect researchers in the social, behavioral, and economic sciences with decision-makers to respond to policy questions related to the COVID-19 pandemic.
In their expert consultation, the authors emphasize that vaccination is critical for decreasing transmission and controlling infection, as well as limiting the emergence of future serious variants. As of Oct. 3, 2021, about 65% of the U.S. population had received at least one dose of the vaccine, and the rate has begun to lag in many areas of the country. There are a variety of reasons for vaccine hesitancy, they note, including perception of low risks from COVID-19 or of high risks from COVID-19 vaccines, exposure to media, political agendas, lack of confidence in science, and distrust of the medical establishment. The Pfizer/BioNTech vaccine is currently authorized for emergency use for individuals 12 years of age and older and fully approved for those aged 16 and older, while the Moderna and the Johnson & Johnson vaccines are authorized for emergency use for those 18 years of age and older.
Many children between the ages of 12 and 17 have not been vaccinated, and the major concerns reported by parents include not knowing enough about the long-term effects of the COVID-19 vaccine in children (88%), concerns about children experiencing serious side effects (79%), and concerns that the COVID-19 vaccine might negatively affect future fertility (73%).
The National Academies have previously released two other “rapid expert consultations” which have addressed building vaccine confidence, and both reports provide key strategies for communicating information about COVID-19 vaccines. In this paper, the focus was on communicating with parents to gain confidence in the vaccine and address concerns.
Key points
The key strategies highlighted for communicating with parents include the following:
- Emphasizing safety and efficacy: Parents should be informed about the ongoing research and clinical trials that will answer more questions about the vaccine and that there is continued monitoring for any safety risks. Pointing to the safety data from the clinical trials for 12- to 17-year-olds, and the lack of serious adverse events from the vaccine in this age group may help alleviate concerns.
- CalibriEncouraging parents to talk with a primary care provider: Research shows that parents trust family physicians and other health care practitioners to provide them with accurate information about vaccines. Local, state, and national leaders can provide messaging templates and other resources to health care professionals who are engaged in these conversations.
- Leveraging social networks to influence parents’ vaccination decisions: Parents are influenced by their social network connections. It is important to engage these networks, especially with members of their community who are considered trustworthy and influential. Social networks may also be very diverse, and include family members, friends, coworkers, social media, and members of their religious community.
While the guidance states that different groups of parents will require different messaging, they suggest that communication can begin with a focus on the things that vaccination can accomplish. In addition to preventing infection with COVID-19, it will allow children to attend school in person and participate in extracurricular activities such as sports, without risking their health. “One thing I’ve learned over several years of working with vaccine-hesitant parents is that you have to tailor each approach to the individual,” said Dr. Ameenuddin. “Different people have different concerns, and first and foremost, it’s important to listen.”
For some parents, emphasizing that the more people that can be vaccinated and the sooner it can be done, the sooner everyone can return to a normal life is a good approach, she added. “I think it’s important to emphasize both the individual and communal benefits of vaccines, but that won’t necessarily reach every person with concerns. I think it’s important to find out what is most important to individuals and work from there to find a way to connect with that family to encourage vaccination.”
Dr. Ameenuddin has no disclosures.
While the U.S. Food and Drug Administration has yet to give the green light to COVID-19 vaccination for children who are under age 12, it is expected that approval will be granted. In anticipation of the FDA’s go-ahead, which is expected in the coming weeks, a new “rapid expert consultation” has identified “actionable guidance” that state and local decision-makers can use to communicate with the public. The goal is to build confidence in and promote the uptake of COVID-19 vaccines, especially for parents who are contemplating vaccinating their children.
They note that key factors in decision-making concern vaccine side effects, the efficacy of the vaccine in children, availability of research in their child’s age group, research conducted by the parents themselves, and recommendations by the child’s health care provider.
“One of the reasons that the COVID vaccine only became available for children 12 and over months after it was approved for adults is that it takes time and many, many trial participants who are closely monitored before the vaccine ever reaches the general public,” said Nusheen Ameenuddin, MD, MPH, MPA, an assistant professor of pediatrics at the Mayo Clinic, Rochester, Minn. “We continue to talk to parents about the fact that the vaccines have been very safe and effective in this group, and even though people are concerned about side effects, they are much milder and less frequent than the effects of the disease itself.”
Dr. Ameenuddin noted that the lack of data in this age group can be concerning for parents. “It’s not like other vaccines which have been available for a long time, and the clinical trial data are still limited for this age group,” she said. “But I think the main point that practitioners need to emphasize is that, even though the vaccine is new, the science for this vaccine has been around for about a decade.”
The unique circumstances of a pandemic, she pointed out, allowed for important information about effectiveness, safety, and side effects to be obtained more quickly from clinical trial data.
“We have really good evidence for kids 12 and over, about safety and effectiveness, and even though children are not small adults and have their own unique physiology, this has provided a good starting point to suggest that kids slightly younger will also respond well to the vaccines,” said Dr. Ameenuddin, who is also chair of the American Academy of Pediatrics Council on Communications and Media. “As we learn more, we can start gathering more information about even younger kids to ensure that the right dosage and spacing of vaccines can provide maximum vaccine effectiveness and protection from disease.”
The guidance was published Oct. 13 by the National Academies of Sciences, Engineering, and Medicine.
The rapid expert consultation was produced through the Societal Experts Action Network, an activity of the National Academies that is sponsored by the NASEM and the Alfred P. Sloan Foundation. The goal of SEAN is to connect researchers in the social, behavioral, and economic sciences with decision-makers to respond to policy questions related to the COVID-19 pandemic.
In their expert consultation, the authors emphasize that vaccination is critical for decreasing transmission and controlling infection, as well as limiting the emergence of future serious variants. As of Oct. 3, 2021, about 65% of the U.S. population had received at least one dose of the vaccine, and the rate has begun to lag in many areas of the country. There are a variety of reasons for vaccine hesitancy, they note, including perception of low risks from COVID-19 or of high risks from COVID-19 vaccines, exposure to media, political agendas, lack of confidence in science, and distrust of the medical establishment. The Pfizer/BioNTech vaccine is currently authorized for emergency use for individuals 12 years of age and older and fully approved for those aged 16 and older, while the Moderna and the Johnson & Johnson vaccines are authorized for emergency use for those 18 years of age and older.
Many children between the ages of 12 and 17 have not been vaccinated, and the major concerns reported by parents include not knowing enough about the long-term effects of the COVID-19 vaccine in children (88%), concerns about children experiencing serious side effects (79%), and concerns that the COVID-19 vaccine might negatively affect future fertility (73%).
The National Academies have previously released two other “rapid expert consultations” which have addressed building vaccine confidence, and both reports provide key strategies for communicating information about COVID-19 vaccines. In this paper, the focus was on communicating with parents to gain confidence in the vaccine and address concerns.
Key points
The key strategies highlighted for communicating with parents include the following:
- Emphasizing safety and efficacy: Parents should be informed about the ongoing research and clinical trials that will answer more questions about the vaccine and that there is continued monitoring for any safety risks. Pointing to the safety data from the clinical trials for 12- to 17-year-olds, and the lack of serious adverse events from the vaccine in this age group may help alleviate concerns.
- CalibriEncouraging parents to talk with a primary care provider: Research shows that parents trust family physicians and other health care practitioners to provide them with accurate information about vaccines. Local, state, and national leaders can provide messaging templates and other resources to health care professionals who are engaged in these conversations.
- Leveraging social networks to influence parents’ vaccination decisions: Parents are influenced by their social network connections. It is important to engage these networks, especially with members of their community who are considered trustworthy and influential. Social networks may also be very diverse, and include family members, friends, coworkers, social media, and members of their religious community.
While the guidance states that different groups of parents will require different messaging, they suggest that communication can begin with a focus on the things that vaccination can accomplish. In addition to preventing infection with COVID-19, it will allow children to attend school in person and participate in extracurricular activities such as sports, without risking their health. “One thing I’ve learned over several years of working with vaccine-hesitant parents is that you have to tailor each approach to the individual,” said Dr. Ameenuddin. “Different people have different concerns, and first and foremost, it’s important to listen.”
For some parents, emphasizing that the more people that can be vaccinated and the sooner it can be done, the sooner everyone can return to a normal life is a good approach, she added. “I think it’s important to emphasize both the individual and communal benefits of vaccines, but that won’t necessarily reach every person with concerns. I think it’s important to find out what is most important to individuals and work from there to find a way to connect with that family to encourage vaccination.”
Dr. Ameenuddin has no disclosures.
PA defends against license suspension for COVID treatment
The suspension stemmed from allegations against Scott C. Miller, PA-C, by at least six COVID patients, including some who weren’t his patients or whom he never examined and a few who later died from the virus, according to the Washington Medical Commission.
“Miller’s treatment of COVID-19 patients fell below the standard of care,” the suspension report states. “Miller began a public campaign promoting ivermectin as a curative for COVID-19, and prescribing it without adequate examination to at least one person, with no reliable clinical studies that establish its efficacy in preventing or treating COVID-19.”
Mr. Miller has until early November to respond to the allegations. On his clinic’s website, Mr. Miller stated, “In response to the charges, I want to reassure all of you that the initial attacks against me have been brought on by a small handful of people that have no ties to our medical practice, and by pharmacies and hospitals that have a zero tolerance policy on family members asking that I help them advocate for loved ones that have been admitted and written off in our current system of dismissiveness and neglect.”
Mr. Miller also expressed gratitude for the support he has received recently. A GoFundMe campaign to raise money for Mr. Miller’s legal fund had raised more than $59,000 at press time. His GoFundMe page had been shared 2,400 times. He has more than 550 followers and more than 400 donors.
“I don’t know that I have the words to adequately describe the deep sense of love and connection I have received from you, the families I serve, and those that have reached out to me in this deeply challenging time,” he wrote on the clinic website.
Mr. Miller has spoken publicly about his anti-mask views and his support for ivermectin, according to the commission report. As part of the suspension, he was charged with making “misleading representations regarding the efficacy of non-FDA approved treatment and mask use.”
In one case that was cited in the report, a 39-year-old patient contacted the pediatric clinic, and Mr. Miller spoke with the patient by phone. The patient reported that he had tested positive for COVID. Mr. Miller advised the patient to take supplements, including vitamin D and C, zinc, and melatonin, and he prescribed ivermectin, dexamethasone, and azithromycin. He did not perform an exam, verify the information that the patient had provided, advise the patient regarding interactions, or order follow-up testing, the report states.
Other charges against Mr. Miller include harassing hospital staff by making threatening statements about hospitals and doctors who treat COVID-19 patients and misrepresenting his original 2013 license application. He denied on the application that he was being investigated by another licensing board. At the time, the California Physician Assistant Board was investigating him for providing medical care and prescribing without a supervising doctor’s authorization and without conducting physical exams, among other charges.
A version of this article first appeared on Medscape.com.
The suspension stemmed from allegations against Scott C. Miller, PA-C, by at least six COVID patients, including some who weren’t his patients or whom he never examined and a few who later died from the virus, according to the Washington Medical Commission.
“Miller’s treatment of COVID-19 patients fell below the standard of care,” the suspension report states. “Miller began a public campaign promoting ivermectin as a curative for COVID-19, and prescribing it without adequate examination to at least one person, with no reliable clinical studies that establish its efficacy in preventing or treating COVID-19.”
Mr. Miller has until early November to respond to the allegations. On his clinic’s website, Mr. Miller stated, “In response to the charges, I want to reassure all of you that the initial attacks against me have been brought on by a small handful of people that have no ties to our medical practice, and by pharmacies and hospitals that have a zero tolerance policy on family members asking that I help them advocate for loved ones that have been admitted and written off in our current system of dismissiveness and neglect.”
Mr. Miller also expressed gratitude for the support he has received recently. A GoFundMe campaign to raise money for Mr. Miller’s legal fund had raised more than $59,000 at press time. His GoFundMe page had been shared 2,400 times. He has more than 550 followers and more than 400 donors.
“I don’t know that I have the words to adequately describe the deep sense of love and connection I have received from you, the families I serve, and those that have reached out to me in this deeply challenging time,” he wrote on the clinic website.
Mr. Miller has spoken publicly about his anti-mask views and his support for ivermectin, according to the commission report. As part of the suspension, he was charged with making “misleading representations regarding the efficacy of non-FDA approved treatment and mask use.”
In one case that was cited in the report, a 39-year-old patient contacted the pediatric clinic, and Mr. Miller spoke with the patient by phone. The patient reported that he had tested positive for COVID. Mr. Miller advised the patient to take supplements, including vitamin D and C, zinc, and melatonin, and he prescribed ivermectin, dexamethasone, and azithromycin. He did not perform an exam, verify the information that the patient had provided, advise the patient regarding interactions, or order follow-up testing, the report states.
Other charges against Mr. Miller include harassing hospital staff by making threatening statements about hospitals and doctors who treat COVID-19 patients and misrepresenting his original 2013 license application. He denied on the application that he was being investigated by another licensing board. At the time, the California Physician Assistant Board was investigating him for providing medical care and prescribing without a supervising doctor’s authorization and without conducting physical exams, among other charges.
A version of this article first appeared on Medscape.com.
The suspension stemmed from allegations against Scott C. Miller, PA-C, by at least six COVID patients, including some who weren’t his patients or whom he never examined and a few who later died from the virus, according to the Washington Medical Commission.
“Miller’s treatment of COVID-19 patients fell below the standard of care,” the suspension report states. “Miller began a public campaign promoting ivermectin as a curative for COVID-19, and prescribing it without adequate examination to at least one person, with no reliable clinical studies that establish its efficacy in preventing or treating COVID-19.”
Mr. Miller has until early November to respond to the allegations. On his clinic’s website, Mr. Miller stated, “In response to the charges, I want to reassure all of you that the initial attacks against me have been brought on by a small handful of people that have no ties to our medical practice, and by pharmacies and hospitals that have a zero tolerance policy on family members asking that I help them advocate for loved ones that have been admitted and written off in our current system of dismissiveness and neglect.”
Mr. Miller also expressed gratitude for the support he has received recently. A GoFundMe campaign to raise money for Mr. Miller’s legal fund had raised more than $59,000 at press time. His GoFundMe page had been shared 2,400 times. He has more than 550 followers and more than 400 donors.
“I don’t know that I have the words to adequately describe the deep sense of love and connection I have received from you, the families I serve, and those that have reached out to me in this deeply challenging time,” he wrote on the clinic website.
Mr. Miller has spoken publicly about his anti-mask views and his support for ivermectin, according to the commission report. As part of the suspension, he was charged with making “misleading representations regarding the efficacy of non-FDA approved treatment and mask use.”
In one case that was cited in the report, a 39-year-old patient contacted the pediatric clinic, and Mr. Miller spoke with the patient by phone. The patient reported that he had tested positive for COVID. Mr. Miller advised the patient to take supplements, including vitamin D and C, zinc, and melatonin, and he prescribed ivermectin, dexamethasone, and azithromycin. He did not perform an exam, verify the information that the patient had provided, advise the patient regarding interactions, or order follow-up testing, the report states.
Other charges against Mr. Miller include harassing hospital staff by making threatening statements about hospitals and doctors who treat COVID-19 patients and misrepresenting his original 2013 license application. He denied on the application that he was being investigated by another licensing board. At the time, the California Physician Assistant Board was investigating him for providing medical care and prescribing without a supervising doctor’s authorization and without conducting physical exams, among other charges.
A version of this article first appeared on Medscape.com.
States can reserve COVID shots for kids 5-11 this week
States can preorder COVID-19 vaccine doses for younger children this week as they begin to set up vaccination campaigns for ages 5-11.
Vaccine advisory groups for the FDA and CDC are scheduled to discuss and approve the Pfizer shot for kids in the next three weeks. To help states and cities prepare for the rollout, the CDC issued guidance on how to set up expanded vaccination programs.
Immunization program managers can begin ordering doses on Wednesday, according to the guidance. The vials won’t be delivered until the FDA and CDC authorize the shot, but registering now will help federal officials ship doses quickly once they’re available.
Pharmacies in every state will be able to give COVID-19 shots to children, but they can only use doses that are prepared specifically for children. Ages 5-11 will need a 10-microgram dose, which is one-third of the dose administered to ages 12 and older. The guidance warns that doctors should not try to split up or fraction the adult doses.
The CDC guidance also recommends that pediatricians and family practice doctors should serve as primary places to give shots to kids. The document mentions other options, such as vaccination clinics at schools, but doesn’t endorse them as the first choice for vaccinating kids.
The CDC hasn’t yet addressed questions around whether kids should be required to get vaccinated to attend school. The decision will likely be left to state and city officials.
Federal health officials aren’t yet sure how many parents and guardians will seek shots for their younger kids right away, the AP reported. Demand may be high at first for some families, but it may not be as high as when shots first became available for adults, Marcus Plescia, MD, chief medical officer of the Association of State and Territorial Health Officials, told The Associated Press.
“We’re going to have potentially a very busy, and perhaps modestly chaotic time,” he said.
When vaccines were first authorized for adults, hospitals and pharmacies received priority for ordering shots. Some doctors felt left out. This time, however, the CDC has said that pediatricians will receive higher priority and be able to receive shipments quickly.
As the vaccine rollout begins, health officials should consider logistical concerns to address racial and economic disparities for younger kids, Richard Besser, MD, president and CEO of the Robert Wood Johnson Foundation and a former acting director of the CDC, told the AP.
If parents or guardians can’t leave work to take their kids to a pharmacy or doctor’s office, for instance, their kids may not receive a shot quickly – or at all.
“It’s really important that we recognize the barriers to vaccinations,” he said.
A version of this article first appeared on WebMD.com.
States can preorder COVID-19 vaccine doses for younger children this week as they begin to set up vaccination campaigns for ages 5-11.
Vaccine advisory groups for the FDA and CDC are scheduled to discuss and approve the Pfizer shot for kids in the next three weeks. To help states and cities prepare for the rollout, the CDC issued guidance on how to set up expanded vaccination programs.
Immunization program managers can begin ordering doses on Wednesday, according to the guidance. The vials won’t be delivered until the FDA and CDC authorize the shot, but registering now will help federal officials ship doses quickly once they’re available.
Pharmacies in every state will be able to give COVID-19 shots to children, but they can only use doses that are prepared specifically for children. Ages 5-11 will need a 10-microgram dose, which is one-third of the dose administered to ages 12 and older. The guidance warns that doctors should not try to split up or fraction the adult doses.
The CDC guidance also recommends that pediatricians and family practice doctors should serve as primary places to give shots to kids. The document mentions other options, such as vaccination clinics at schools, but doesn’t endorse them as the first choice for vaccinating kids.
The CDC hasn’t yet addressed questions around whether kids should be required to get vaccinated to attend school. The decision will likely be left to state and city officials.
Federal health officials aren’t yet sure how many parents and guardians will seek shots for their younger kids right away, the AP reported. Demand may be high at first for some families, but it may not be as high as when shots first became available for adults, Marcus Plescia, MD, chief medical officer of the Association of State and Territorial Health Officials, told The Associated Press.
“We’re going to have potentially a very busy, and perhaps modestly chaotic time,” he said.
When vaccines were first authorized for adults, hospitals and pharmacies received priority for ordering shots. Some doctors felt left out. This time, however, the CDC has said that pediatricians will receive higher priority and be able to receive shipments quickly.
As the vaccine rollout begins, health officials should consider logistical concerns to address racial and economic disparities for younger kids, Richard Besser, MD, president and CEO of the Robert Wood Johnson Foundation and a former acting director of the CDC, told the AP.
If parents or guardians can’t leave work to take their kids to a pharmacy or doctor’s office, for instance, their kids may not receive a shot quickly – or at all.
“It’s really important that we recognize the barriers to vaccinations,” he said.
A version of this article first appeared on WebMD.com.
States can preorder COVID-19 vaccine doses for younger children this week as they begin to set up vaccination campaigns for ages 5-11.
Vaccine advisory groups for the FDA and CDC are scheduled to discuss and approve the Pfizer shot for kids in the next three weeks. To help states and cities prepare for the rollout, the CDC issued guidance on how to set up expanded vaccination programs.
Immunization program managers can begin ordering doses on Wednesday, according to the guidance. The vials won’t be delivered until the FDA and CDC authorize the shot, but registering now will help federal officials ship doses quickly once they’re available.
Pharmacies in every state will be able to give COVID-19 shots to children, but they can only use doses that are prepared specifically for children. Ages 5-11 will need a 10-microgram dose, which is one-third of the dose administered to ages 12 and older. The guidance warns that doctors should not try to split up or fraction the adult doses.
The CDC guidance also recommends that pediatricians and family practice doctors should serve as primary places to give shots to kids. The document mentions other options, such as vaccination clinics at schools, but doesn’t endorse them as the first choice for vaccinating kids.
The CDC hasn’t yet addressed questions around whether kids should be required to get vaccinated to attend school. The decision will likely be left to state and city officials.
Federal health officials aren’t yet sure how many parents and guardians will seek shots for their younger kids right away, the AP reported. Demand may be high at first for some families, but it may not be as high as when shots first became available for adults, Marcus Plescia, MD, chief medical officer of the Association of State and Territorial Health Officials, told The Associated Press.
“We’re going to have potentially a very busy, and perhaps modestly chaotic time,” he said.
When vaccines were first authorized for adults, hospitals and pharmacies received priority for ordering shots. Some doctors felt left out. This time, however, the CDC has said that pediatricians will receive higher priority and be able to receive shipments quickly.
As the vaccine rollout begins, health officials should consider logistical concerns to address racial and economic disparities for younger kids, Richard Besser, MD, president and CEO of the Robert Wood Johnson Foundation and a former acting director of the CDC, told the AP.
If parents or guardians can’t leave work to take their kids to a pharmacy or doctor’s office, for instance, their kids may not receive a shot quickly – or at all.
“It’s really important that we recognize the barriers to vaccinations,” he said.
A version of this article first appeared on WebMD.com.
FDA approves cell-based flu shot for ages 6 months and older
The Food and Drug Administration has approved the Flucelvax quadrivalent vaccine for use in children aged 6 months and older, according to a statement from manufacturer Seqirus.
“This approval officially allows all eligible Americans to receive a cell-based influenza vaccine, increasing the potential for greater vaccine effectiveness,” according to the company.
The Centers for Disease Control and Prevention currently recommends annual influenza vaccination for all individuals aged 6 months and older without contraindications.
Flucelvax is manufactured using a cell-based process that yields a more precise match to the WHO-selected influenza strains for a given year. This process avoids the variation associated with traditional egg-based vaccines, and offers the potential for greater vaccine effectiveness, according to the company.
The approval was based in part on data from a phase 3 randomized, controlled noninferiority study of children aged 6-47 months. The data are the first for a cell-based flu vaccine in this age group, and were presented at the Pediatric Academic Societies meeting in 2021.
In the immunogenicity study of children aged 6 months through 3 years, described in the package insert, 1,597 children received Flucelvax quadrivalent and 805 received a control quadrivalent vaccine. After 28 days, Flucelvax showed noninferiority to the control quadrivalent against four influenza strains.
The most common side effects with Flucelvax quadrivalent vaccine overall are pain, redness, swelling, or a hardened area at the injection site, headache, low energy, muscle aches, and malaise. Additional side effects reported in children include tenderness or bruising at the injection site, sleepiness, diarrhea, changes in eating habits, and irritability. The vaccine is contraindicated for individuals with allergies to any of its ingredients.
Additional efficacy data on Flucelvax for children and adolescents aged 2-18 years were recently published in The New England Journal of Medicine.
Full prescribing information for Flucelvax is available here.
The FDA approval letter is available here.[email protected]
The Food and Drug Administration has approved the Flucelvax quadrivalent vaccine for use in children aged 6 months and older, according to a statement from manufacturer Seqirus.
“This approval officially allows all eligible Americans to receive a cell-based influenza vaccine, increasing the potential for greater vaccine effectiveness,” according to the company.
The Centers for Disease Control and Prevention currently recommends annual influenza vaccination for all individuals aged 6 months and older without contraindications.
Flucelvax is manufactured using a cell-based process that yields a more precise match to the WHO-selected influenza strains for a given year. This process avoids the variation associated with traditional egg-based vaccines, and offers the potential for greater vaccine effectiveness, according to the company.
The approval was based in part on data from a phase 3 randomized, controlled noninferiority study of children aged 6-47 months. The data are the first for a cell-based flu vaccine in this age group, and were presented at the Pediatric Academic Societies meeting in 2021.
In the immunogenicity study of children aged 6 months through 3 years, described in the package insert, 1,597 children received Flucelvax quadrivalent and 805 received a control quadrivalent vaccine. After 28 days, Flucelvax showed noninferiority to the control quadrivalent against four influenza strains.
The most common side effects with Flucelvax quadrivalent vaccine overall are pain, redness, swelling, or a hardened area at the injection site, headache, low energy, muscle aches, and malaise. Additional side effects reported in children include tenderness or bruising at the injection site, sleepiness, diarrhea, changes in eating habits, and irritability. The vaccine is contraindicated for individuals with allergies to any of its ingredients.
Additional efficacy data on Flucelvax for children and adolescents aged 2-18 years were recently published in The New England Journal of Medicine.
Full prescribing information for Flucelvax is available here.
The FDA approval letter is available here.[email protected]
The Food and Drug Administration has approved the Flucelvax quadrivalent vaccine for use in children aged 6 months and older, according to a statement from manufacturer Seqirus.
“This approval officially allows all eligible Americans to receive a cell-based influenza vaccine, increasing the potential for greater vaccine effectiveness,” according to the company.
The Centers for Disease Control and Prevention currently recommends annual influenza vaccination for all individuals aged 6 months and older without contraindications.
Flucelvax is manufactured using a cell-based process that yields a more precise match to the WHO-selected influenza strains for a given year. This process avoids the variation associated with traditional egg-based vaccines, and offers the potential for greater vaccine effectiveness, according to the company.
The approval was based in part on data from a phase 3 randomized, controlled noninferiority study of children aged 6-47 months. The data are the first for a cell-based flu vaccine in this age group, and were presented at the Pediatric Academic Societies meeting in 2021.
In the immunogenicity study of children aged 6 months through 3 years, described in the package insert, 1,597 children received Flucelvax quadrivalent and 805 received a control quadrivalent vaccine. After 28 days, Flucelvax showed noninferiority to the control quadrivalent against four influenza strains.
The most common side effects with Flucelvax quadrivalent vaccine overall are pain, redness, swelling, or a hardened area at the injection site, headache, low energy, muscle aches, and malaise. Additional side effects reported in children include tenderness or bruising at the injection site, sleepiness, diarrhea, changes in eating habits, and irritability. The vaccine is contraindicated for individuals with allergies to any of its ingredients.
Additional efficacy data on Flucelvax for children and adolescents aged 2-18 years were recently published in The New England Journal of Medicine.
Full prescribing information for Flucelvax is available here.
The FDA approval letter is available here.[email protected]
Paper linking COVID-19 vaccines to myocarditis is temporarily removed without explanation
The article, “A Report on Myocarditis Adverse Events in the U.S. Vaccine Adverse Events Reporting System (VAERS) in Association with COVID-19 Injectable Biological Products,” was published in Current Problems in Cardiology, an Elsevier journal, on October 1.
It was co-authored by Jessica Rose and Peter McCullough, whose affiliations are listed as the Public Health Policy Initiative at the Institute of Pure and Applied Knowledge — a group that has been critical of vaccines and of the response to COVID-19 and has funded one study that was retracted earlier this year — and Texas A&M’s Baylor Dallas campus. [See update at the end of the post.]
Last month, Baylor Scott & White obtained a restraining order against McCullough — whom Medscape says “has promoted the use of therapies seen as unproven for the treatment of COVID-19 and has questioned the effectiveness of COVID-19 vaccines” — for continuing to refer to an affiliation with the health care institution despite a separation agreement. “Since the Baylor suit, the Texas A&M College of Medicine, and the Texas Christian University (TCU) and University of North Texas Health Science Center (UNTHSC) School of Medicine have both removed McCullough from their faculties,” Medscape reported at the time.
Here are some highlights of the now temporarily retracted paper’s claims:
Within 8 weeks of the public offering of COVID-19 products to the 12-15-year-old age group, we found 19 times the expected number of myocarditis cases in the vaccination volunteers over background myocarditis rates for this age group. In addition, a 5-fold increase in myocarditis rate was observed subsequent to dose 2 as opposed to dose 1 in 15-year-old males.
While several studies have used the VAERS database and other similar datasets around the world to estimate rates of side effects from COVID-19 vaccines, the approach has been roundly criticized and has led to at least one retraction. VAERS itself includes caution against doing so. (Another paper about myocarditis cases linked to COVID-19 vaccines has been retracted for a serious math error.)
Here’s the notice:
The Publisher regrets that this article has been temporarily removed. A replacement will appear as soon as possible in which the reason for the removal of the article will be specified, or the article will be reinstated.
The full Elsevier Policy on Article Withdrawal can be found at http://www.elsevier.com/locate/withdrawalpolicy .
Rose, the corresponding author of the paper, told Retraction Watch that the publisher had “applied the ‘temporary withdrawal’ label to the paper without informing us.” The publisher, Rose said, “claimed that since ‘it wasn’t an invited paper’ that they were reconsidering publishing it and hence the ‘temporary withdrawal.’”
She said the move was “unheard of” and that Elsevier was “breaching the contract we signed – all fees have been paid for gorgeous color graphics.”
Elsevier has temporarily removed more than 100 papers since 2005, by our count. The papers are often reinstated without any mention of why the paper was removed.
Hector Ventura, the editor of the journal, did not immediately respond to a request for comment.
Update, 10/17/21, 1850 UTC: Rose tells us that the correct affiliations — now noted on the temporarily retracted version — are the Institute of Pure and Applied Knowledge’s Public Health Policy Initiative (PHPI) for her, and the Truth for Health Foundation in Tucson, Ariz. for McCullough. The foundation describes it mission as:
To provide truthful, balanced, medically sound, research-based information and cutting edge updates on prevention and treatment of common medical conditions, including COVID-19 and other infectious diseases, that affect health, quality of life and longevity.
To present faith-based integrated approaches to medical treatment, health and healing services that encompass all dimensions making us human: physical, psychological/emotional, spiritual, social and environmental.
The paper was submitted before McCullough’s departure from Baylor, Rose said.
A version of this article first appeared on Retraction Watch.
The article, “A Report on Myocarditis Adverse Events in the U.S. Vaccine Adverse Events Reporting System (VAERS) in Association with COVID-19 Injectable Biological Products,” was published in Current Problems in Cardiology, an Elsevier journal, on October 1.
It was co-authored by Jessica Rose and Peter McCullough, whose affiliations are listed as the Public Health Policy Initiative at the Institute of Pure and Applied Knowledge — a group that has been critical of vaccines and of the response to COVID-19 and has funded one study that was retracted earlier this year — and Texas A&M’s Baylor Dallas campus. [See update at the end of the post.]
Last month, Baylor Scott & White obtained a restraining order against McCullough — whom Medscape says “has promoted the use of therapies seen as unproven for the treatment of COVID-19 and has questioned the effectiveness of COVID-19 vaccines” — for continuing to refer to an affiliation with the health care institution despite a separation agreement. “Since the Baylor suit, the Texas A&M College of Medicine, and the Texas Christian University (TCU) and University of North Texas Health Science Center (UNTHSC) School of Medicine have both removed McCullough from their faculties,” Medscape reported at the time.
Here are some highlights of the now temporarily retracted paper’s claims:
Within 8 weeks of the public offering of COVID-19 products to the 12-15-year-old age group, we found 19 times the expected number of myocarditis cases in the vaccination volunteers over background myocarditis rates for this age group. In addition, a 5-fold increase in myocarditis rate was observed subsequent to dose 2 as opposed to dose 1 in 15-year-old males.
While several studies have used the VAERS database and other similar datasets around the world to estimate rates of side effects from COVID-19 vaccines, the approach has been roundly criticized and has led to at least one retraction. VAERS itself includes caution against doing so. (Another paper about myocarditis cases linked to COVID-19 vaccines has been retracted for a serious math error.)
Here’s the notice:
The Publisher regrets that this article has been temporarily removed. A replacement will appear as soon as possible in which the reason for the removal of the article will be specified, or the article will be reinstated.
The full Elsevier Policy on Article Withdrawal can be found at http://www.elsevier.com/locate/withdrawalpolicy .
Rose, the corresponding author of the paper, told Retraction Watch that the publisher had “applied the ‘temporary withdrawal’ label to the paper without informing us.” The publisher, Rose said, “claimed that since ‘it wasn’t an invited paper’ that they were reconsidering publishing it and hence the ‘temporary withdrawal.’”
She said the move was “unheard of” and that Elsevier was “breaching the contract we signed – all fees have been paid for gorgeous color graphics.”
Elsevier has temporarily removed more than 100 papers since 2005, by our count. The papers are often reinstated without any mention of why the paper was removed.
Hector Ventura, the editor of the journal, did not immediately respond to a request for comment.
Update, 10/17/21, 1850 UTC: Rose tells us that the correct affiliations — now noted on the temporarily retracted version — are the Institute of Pure and Applied Knowledge’s Public Health Policy Initiative (PHPI) for her, and the Truth for Health Foundation in Tucson, Ariz. for McCullough. The foundation describes it mission as:
To provide truthful, balanced, medically sound, research-based information and cutting edge updates on prevention and treatment of common medical conditions, including COVID-19 and other infectious diseases, that affect health, quality of life and longevity.
To present faith-based integrated approaches to medical treatment, health and healing services that encompass all dimensions making us human: physical, psychological/emotional, spiritual, social and environmental.
The paper was submitted before McCullough’s departure from Baylor, Rose said.
A version of this article first appeared on Retraction Watch.
The article, “A Report on Myocarditis Adverse Events in the U.S. Vaccine Adverse Events Reporting System (VAERS) in Association with COVID-19 Injectable Biological Products,” was published in Current Problems in Cardiology, an Elsevier journal, on October 1.
It was co-authored by Jessica Rose and Peter McCullough, whose affiliations are listed as the Public Health Policy Initiative at the Institute of Pure and Applied Knowledge — a group that has been critical of vaccines and of the response to COVID-19 and has funded one study that was retracted earlier this year — and Texas A&M’s Baylor Dallas campus. [See update at the end of the post.]
Last month, Baylor Scott & White obtained a restraining order against McCullough — whom Medscape says “has promoted the use of therapies seen as unproven for the treatment of COVID-19 and has questioned the effectiveness of COVID-19 vaccines” — for continuing to refer to an affiliation with the health care institution despite a separation agreement. “Since the Baylor suit, the Texas A&M College of Medicine, and the Texas Christian University (TCU) and University of North Texas Health Science Center (UNTHSC) School of Medicine have both removed McCullough from their faculties,” Medscape reported at the time.
Here are some highlights of the now temporarily retracted paper’s claims:
Within 8 weeks of the public offering of COVID-19 products to the 12-15-year-old age group, we found 19 times the expected number of myocarditis cases in the vaccination volunteers over background myocarditis rates for this age group. In addition, a 5-fold increase in myocarditis rate was observed subsequent to dose 2 as opposed to dose 1 in 15-year-old males.
While several studies have used the VAERS database and other similar datasets around the world to estimate rates of side effects from COVID-19 vaccines, the approach has been roundly criticized and has led to at least one retraction. VAERS itself includes caution against doing so. (Another paper about myocarditis cases linked to COVID-19 vaccines has been retracted for a serious math error.)
Here’s the notice:
The Publisher regrets that this article has been temporarily removed. A replacement will appear as soon as possible in which the reason for the removal of the article will be specified, or the article will be reinstated.
The full Elsevier Policy on Article Withdrawal can be found at http://www.elsevier.com/locate/withdrawalpolicy .
Rose, the corresponding author of the paper, told Retraction Watch that the publisher had “applied the ‘temporary withdrawal’ label to the paper without informing us.” The publisher, Rose said, “claimed that since ‘it wasn’t an invited paper’ that they were reconsidering publishing it and hence the ‘temporary withdrawal.’”
She said the move was “unheard of” and that Elsevier was “breaching the contract we signed – all fees have been paid for gorgeous color graphics.”
Elsevier has temporarily removed more than 100 papers since 2005, by our count. The papers are often reinstated without any mention of why the paper was removed.
Hector Ventura, the editor of the journal, did not immediately respond to a request for comment.
Update, 10/17/21, 1850 UTC: Rose tells us that the correct affiliations — now noted on the temporarily retracted version — are the Institute of Pure and Applied Knowledge’s Public Health Policy Initiative (PHPI) for her, and the Truth for Health Foundation in Tucson, Ariz. for McCullough. The foundation describes it mission as:
To provide truthful, balanced, medically sound, research-based information and cutting edge updates on prevention and treatment of common medical conditions, including COVID-19 and other infectious diseases, that affect health, quality of life and longevity.
To present faith-based integrated approaches to medical treatment, health and healing services that encompass all dimensions making us human: physical, psychological/emotional, spiritual, social and environmental.
The paper was submitted before McCullough’s departure from Baylor, Rose said.
A version of this article first appeared on Retraction Watch.
Sorting out the meaning of misbehavior: The invisible culprit
You have probably heard that determining the A(ntecedent)s, B(ehavior)s, and C(onsequence)s of a behavior is basic to counseling about oppositionality or aggression. But sorting out the As is especially important to going beyond disciplining a misbehavior to building insight for both parents and children.
Antecedents are of two types: triggers such as actions, words, or feelings that happen just before the behavior, and “setting events” that can occur intermittently hours or even days beforehand and lower the threshold for a trigger to cause a child to act out. Lack of sleep, hunger, or fatigue are common setting events that parents recognize and take into account as in “Oh, he missed his nap” to excuse a tantrum in younger children, but is less often considered or excused in older children in whom self-regulation is expected. Often, behavioral specialists in schools are asked to observe a child to identify the triggers and create a “functional behavioral assessment” based on what is observed.
While a functional behavioral assessment requires observations, invisible antecedents to consider include internal thoughts and feelings (meaning). A child feeling shame from a failed math test the day before may be on edge, then, when called on, may uncharacteristically talk back. The child may regard punishment for this “justified” response as unfair, accelerating anger. Feelings of shame or humiliation for failing one’s own standards (or perceived expectations of others the child cares about) are major setups for eliciting defiance.
Even more subtle are meanings the child creates for situations and people, whether real or imagined. A child’s behavior has meaning for the child and the family and can be initiated or maintained by that meaning. For example, a child may “live down” to what the family thinks of him/her; if you think I am bad, I will act badly.
Children may feel guilty about some real or imagined offense, such as divorce or death they think may be their fault, and act up with the family to elicit punishment as payment. When children feel conflicted in a relationship, such as a late adolescent feeling dependent on their mother when their age expectation is independence, they may act up expecting to be ejected from home when they are unable to gather the courage to voluntarily leave. This acting out may also occur with nonconflicted adults, who are actually safer targets. For example, school is often a safer place to express anger through aggression or bullying than home, the real source of the feelings, because family is the “lifeboat” of food and shelter they dare not upset.
Conflicted relationships may be present in blended families, especially if the ex speaks negatively about the other parent. The child of divorce, feeling himself composed of parts of each parent, has diminished self-esteem and anger on behalf of that side being put down. Marital conflict may set children up to feel they have to take sides to angrily defend the parent of like-gender by being oppositional to the other.
Just as we ponder whether the color blue looks the same to someone else, neurologically based differences in perception may make a child misinterpret or act inflexibly or explode in situations that seem normal to adults. While people joke about “being a little OCD,” for some children the distress caused by a change in routine, a messy room, a delayed bus, or loud music is enough to disrupt their functioning and coping enough to explode. Such hypersensitivity can be part of autism or obsessive compulsive disorder or a subthreshold variant. Children vary by age and individually in their ability to understand language, especially sarcastic humor, and often misinterpret it as insulting, threatening, or scary and act accordingly. While most common in children with autism, those with a language learning disability, intellectual disability, or who have English as a second language, or are anxious or vigilant may also take sarcasm the wrong way. Anxious children also may react aggressively from a “hostile bias attribution” of expecting the worst from others.
Another possible meaning of a behavior is that it is being used by the child to manage their feelings. I have found it useful to remind depressed children and parents that it “feels better to be mad than sad” as a reason for irritability. Anger can also push away a person whose otherwise sympathetic approach might release a collapse into tears the child can’t tolerate or would find embarrassing.
The meaning of a child’s misbehavior also resides in the minds of the adults. In addition to all the categories of meaning just described, a parent may be reminded by the child of someone else for whom the adult has strong or conflicted feelings (“projection”) such as a now-hated ex, a sibling of whom the adult is jealous, or a bully from childhood, thus eliciting a reaction falsely triggered by that connection rather than the actual child. Asking parents whom the child “takes after” may elicit such parental projections based on appearance, behavior, or temperament. Helping them pick a feature of the child to focus on to differentiate him/her can serve as an anchor to remind them to control these reactions. Other useful questions to detect meanings of behavior might include asking the child “What’s up with that?” or “What did that make you think/feel?” We can ask parents “How is that for you?” or “What do you think things will be like in 10 years?” to determine despair, mood disorders, or family discord contributing to maladaptive responses possibly maintaining unwanted behaviors.
Throughout life, putting feelings into words is the main way meanings that are contributing to misbehaviors or parenting dysfunction can be uncovered and shifted. For this, the child or adult must feel emotionally safe to talk with a person who conveys curiosity rather than judgment. Helping families explain that divorce is not the child’s fault; admit they also make mistakes; rebuild conflicted relationships through play or talking; identify hypersensitivities or triggers to avoid; and express confidence that the child is a good person, still young, and sure to do better over time, are all things we pediatricians can do to help sort out the meanings of behaviors.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
You have probably heard that determining the A(ntecedent)s, B(ehavior)s, and C(onsequence)s of a behavior is basic to counseling about oppositionality or aggression. But sorting out the As is especially important to going beyond disciplining a misbehavior to building insight for both parents and children.
Antecedents are of two types: triggers such as actions, words, or feelings that happen just before the behavior, and “setting events” that can occur intermittently hours or even days beforehand and lower the threshold for a trigger to cause a child to act out. Lack of sleep, hunger, or fatigue are common setting events that parents recognize and take into account as in “Oh, he missed his nap” to excuse a tantrum in younger children, but is less often considered or excused in older children in whom self-regulation is expected. Often, behavioral specialists in schools are asked to observe a child to identify the triggers and create a “functional behavioral assessment” based on what is observed.
While a functional behavioral assessment requires observations, invisible antecedents to consider include internal thoughts and feelings (meaning). A child feeling shame from a failed math test the day before may be on edge, then, when called on, may uncharacteristically talk back. The child may regard punishment for this “justified” response as unfair, accelerating anger. Feelings of shame or humiliation for failing one’s own standards (or perceived expectations of others the child cares about) are major setups for eliciting defiance.
Even more subtle are meanings the child creates for situations and people, whether real or imagined. A child’s behavior has meaning for the child and the family and can be initiated or maintained by that meaning. For example, a child may “live down” to what the family thinks of him/her; if you think I am bad, I will act badly.
Children may feel guilty about some real or imagined offense, such as divorce or death they think may be their fault, and act up with the family to elicit punishment as payment. When children feel conflicted in a relationship, such as a late adolescent feeling dependent on their mother when their age expectation is independence, they may act up expecting to be ejected from home when they are unable to gather the courage to voluntarily leave. This acting out may also occur with nonconflicted adults, who are actually safer targets. For example, school is often a safer place to express anger through aggression or bullying than home, the real source of the feelings, because family is the “lifeboat” of food and shelter they dare not upset.
Conflicted relationships may be present in blended families, especially if the ex speaks negatively about the other parent. The child of divorce, feeling himself composed of parts of each parent, has diminished self-esteem and anger on behalf of that side being put down. Marital conflict may set children up to feel they have to take sides to angrily defend the parent of like-gender by being oppositional to the other.
Just as we ponder whether the color blue looks the same to someone else, neurologically based differences in perception may make a child misinterpret or act inflexibly or explode in situations that seem normal to adults. While people joke about “being a little OCD,” for some children the distress caused by a change in routine, a messy room, a delayed bus, or loud music is enough to disrupt their functioning and coping enough to explode. Such hypersensitivity can be part of autism or obsessive compulsive disorder or a subthreshold variant. Children vary by age and individually in their ability to understand language, especially sarcastic humor, and often misinterpret it as insulting, threatening, or scary and act accordingly. While most common in children with autism, those with a language learning disability, intellectual disability, or who have English as a second language, or are anxious or vigilant may also take sarcasm the wrong way. Anxious children also may react aggressively from a “hostile bias attribution” of expecting the worst from others.
Another possible meaning of a behavior is that it is being used by the child to manage their feelings. I have found it useful to remind depressed children and parents that it “feels better to be mad than sad” as a reason for irritability. Anger can also push away a person whose otherwise sympathetic approach might release a collapse into tears the child can’t tolerate or would find embarrassing.
The meaning of a child’s misbehavior also resides in the minds of the adults. In addition to all the categories of meaning just described, a parent may be reminded by the child of someone else for whom the adult has strong or conflicted feelings (“projection”) such as a now-hated ex, a sibling of whom the adult is jealous, or a bully from childhood, thus eliciting a reaction falsely triggered by that connection rather than the actual child. Asking parents whom the child “takes after” may elicit such parental projections based on appearance, behavior, or temperament. Helping them pick a feature of the child to focus on to differentiate him/her can serve as an anchor to remind them to control these reactions. Other useful questions to detect meanings of behavior might include asking the child “What’s up with that?” or “What did that make you think/feel?” We can ask parents “How is that for you?” or “What do you think things will be like in 10 years?” to determine despair, mood disorders, or family discord contributing to maladaptive responses possibly maintaining unwanted behaviors.
Throughout life, putting feelings into words is the main way meanings that are contributing to misbehaviors or parenting dysfunction can be uncovered and shifted. For this, the child or adult must feel emotionally safe to talk with a person who conveys curiosity rather than judgment. Helping families explain that divorce is not the child’s fault; admit they also make mistakes; rebuild conflicted relationships through play or talking; identify hypersensitivities or triggers to avoid; and express confidence that the child is a good person, still young, and sure to do better over time, are all things we pediatricians can do to help sort out the meanings of behaviors.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
You have probably heard that determining the A(ntecedent)s, B(ehavior)s, and C(onsequence)s of a behavior is basic to counseling about oppositionality or aggression. But sorting out the As is especially important to going beyond disciplining a misbehavior to building insight for both parents and children.
Antecedents are of two types: triggers such as actions, words, or feelings that happen just before the behavior, and “setting events” that can occur intermittently hours or even days beforehand and lower the threshold for a trigger to cause a child to act out. Lack of sleep, hunger, or fatigue are common setting events that parents recognize and take into account as in “Oh, he missed his nap” to excuse a tantrum in younger children, but is less often considered or excused in older children in whom self-regulation is expected. Often, behavioral specialists in schools are asked to observe a child to identify the triggers and create a “functional behavioral assessment” based on what is observed.
While a functional behavioral assessment requires observations, invisible antecedents to consider include internal thoughts and feelings (meaning). A child feeling shame from a failed math test the day before may be on edge, then, when called on, may uncharacteristically talk back. The child may regard punishment for this “justified” response as unfair, accelerating anger. Feelings of shame or humiliation for failing one’s own standards (or perceived expectations of others the child cares about) are major setups for eliciting defiance.
Even more subtle are meanings the child creates for situations and people, whether real or imagined. A child’s behavior has meaning for the child and the family and can be initiated or maintained by that meaning. For example, a child may “live down” to what the family thinks of him/her; if you think I am bad, I will act badly.
Children may feel guilty about some real or imagined offense, such as divorce or death they think may be their fault, and act up with the family to elicit punishment as payment. When children feel conflicted in a relationship, such as a late adolescent feeling dependent on their mother when their age expectation is independence, they may act up expecting to be ejected from home when they are unable to gather the courage to voluntarily leave. This acting out may also occur with nonconflicted adults, who are actually safer targets. For example, school is often a safer place to express anger through aggression or bullying than home, the real source of the feelings, because family is the “lifeboat” of food and shelter they dare not upset.
Conflicted relationships may be present in blended families, especially if the ex speaks negatively about the other parent. The child of divorce, feeling himself composed of parts of each parent, has diminished self-esteem and anger on behalf of that side being put down. Marital conflict may set children up to feel they have to take sides to angrily defend the parent of like-gender by being oppositional to the other.
Just as we ponder whether the color blue looks the same to someone else, neurologically based differences in perception may make a child misinterpret or act inflexibly or explode in situations that seem normal to adults. While people joke about “being a little OCD,” for some children the distress caused by a change in routine, a messy room, a delayed bus, or loud music is enough to disrupt their functioning and coping enough to explode. Such hypersensitivity can be part of autism or obsessive compulsive disorder or a subthreshold variant. Children vary by age and individually in their ability to understand language, especially sarcastic humor, and often misinterpret it as insulting, threatening, or scary and act accordingly. While most common in children with autism, those with a language learning disability, intellectual disability, or who have English as a second language, or are anxious or vigilant may also take sarcasm the wrong way. Anxious children also may react aggressively from a “hostile bias attribution” of expecting the worst from others.
Another possible meaning of a behavior is that it is being used by the child to manage their feelings. I have found it useful to remind depressed children and parents that it “feels better to be mad than sad” as a reason for irritability. Anger can also push away a person whose otherwise sympathetic approach might release a collapse into tears the child can’t tolerate or would find embarrassing.
The meaning of a child’s misbehavior also resides in the minds of the adults. In addition to all the categories of meaning just described, a parent may be reminded by the child of someone else for whom the adult has strong or conflicted feelings (“projection”) such as a now-hated ex, a sibling of whom the adult is jealous, or a bully from childhood, thus eliciting a reaction falsely triggered by that connection rather than the actual child. Asking parents whom the child “takes after” may elicit such parental projections based on appearance, behavior, or temperament. Helping them pick a feature of the child to focus on to differentiate him/her can serve as an anchor to remind them to control these reactions. Other useful questions to detect meanings of behavior might include asking the child “What’s up with that?” or “What did that make you think/feel?” We can ask parents “How is that for you?” or “What do you think things will be like in 10 years?” to determine despair, mood disorders, or family discord contributing to maladaptive responses possibly maintaining unwanted behaviors.
Throughout life, putting feelings into words is the main way meanings that are contributing to misbehaviors or parenting dysfunction can be uncovered and shifted. For this, the child or adult must feel emotionally safe to talk with a person who conveys curiosity rather than judgment. Helping families explain that divorce is not the child’s fault; admit they also make mistakes; rebuild conflicted relationships through play or talking; identify hypersensitivities or triggers to avoid; and express confidence that the child is a good person, still young, and sure to do better over time, are all things we pediatricians can do to help sort out the meanings of behaviors.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Dupilumab-improved lung function lasts in children with moderate to severe asthma
Add-on treatment with dupilumab may improve lung function in children aged 6-11 years with uncontrolled moderate to severe type 2 inflammatory asthma, results from a randomized, placebo-controlled, phase 3 study show.
Improvements in lung function parameters were observed as early as 2 weeks and persisted over the 52-week treatment period among children in the LIBERTY ASTHMA VOYAGE study, according to investigator Leonard B. Bacharier, MD, of Monroe Carell Jr. Children’s Hospital at Vanderbilt University Medical Center, Nashville, Tenn.
“Dupilumab led to clinically meaningful rapid and sustained improvements in lung function parameters,” Dr. Bacharier said in an online poster presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
The improvements in forced expiratory volume in 1 second (FEV1) and other measures reported for children with moderate to severe asthma who have the type 2 phenotype, which is the most common driver of pediatric asthma, according to Dr. Bacharier.
“Many children with moderate to severe asthma have abnormal lung function, and this can be a risk factor for future lung disease in adulthood,” Dr. Bacharier said in his presentation.
The VOYAGE continues
The findings presented at the meeting build on another report earlier this year from the LIBERTY ASTHMA VOYAGE study demonstrating that add-on dupilumab treatment led to a significant improvement versus placebo in FEV1 up to 12 weeks.
“We now have a long term data on this drug as well, showing its efficacy over a period of time,” said Muhammad Adrish, MD, MBA, FCCP, associate professor of pulmonary, critical care and sleep medicine at Baylor College of Medicine, Houston.
“I think that’s pretty exciting, and that’s another step towards precision medicine in treatment of asthma,” Dr. Adrish, who is Vice-Chair of CHEST’s Airways Disorders NetWork Steering Committee and was not involved in the study.
Dupilumab received Food and Drug Administration approval in 2018 as add-on maintenance therapy for the treatment of patients aged 12 years or older with moderate to severe asthma that has an eosinophilic phenotype or that is dependent on oral corticosteroid treatment.
In March 2021, Sanofi and Regeneron announced that the FDA had accepted for review a supplemental Biologics License Application for dupilumab as an add-on treatment in children aged 6-11 years with uncontrolled moderate to severe asthma.
That sBLA is supported by data from the LIBERTY ASTHMA VOYAGE study, Sanofi and Regeneron said.
In results of the phase 3 study that Dr. Bacharier presented in May at the American Thoracic Society International Conference, add-on dupilumab dosed every 2 weeks significant improved percent predicted prebronchodilator FEV1 by an additional 5.21 percentage points versus placebo at week 12.
Dupilumab and the type 2 phenotype
The new data reported at the CHEST meeting come from a prespecified analysis evaluating the impact of dupilumab on lung function over a 52-week treatment period in patients with a T2 inflammatory asthma phenotype.
“Dupilumab, a fully human monoclonal antibody, blocks the shared receptor component for interleukin-4 and -13, key and central drivers of T2 inflammation in multiple diseases,” Dr. Bacharier and coinvestigators reported in their study abstract.
Of 408 patients in the study, 350 met the T2 phenotype criteria, including 236 in the dupilumab arm and 114 in the placebo arm.
Patients met T2 phenotype criteria if they had blood eosinophils of at least 150 cells/mcL or fractional exhaled nitric oxide FeNO of at least 20 parts per billion at baseline, investigators said.
Dr. Bacharier and coinvestigators reported on several different endpoints, including absolute and percent predicted prebronchodilator FEV1, percent predicted postbronchodilator FEV1, prebronchodilator forced expiratory flow at 25%-75% of pulmonary volume (FEF25%-75%), and forced vital capacity (FVC).
Dupilumab, when compared with placebo, significantly improved prebronchodilator FEV1 in pediatric patients with uncontrolled moderate to severe type 2 asthma, according to Dr. Bacharier.
“Patients receiving dupilumab experienced rapid improvements by week 2, and this was sustained for up to 52 weeks,” he said.
The prebronchodilator FEV1 improved from baseline for dupilumab versus placebo, with a least squares mean difference of 0.06 L at week 2, which reached 0.17 L by week 52, according to their data. Similarly, postbronchodilator FEV1 improved from baseline for dupilumab, with a least square mean difference versus placebo of 0.09 L at week 52.
Dupilumab compared to placebo also significantly improved percent predicted FEF25%-75%, and percent predicted FVC over the 52-week treatment period, according to Dr. Bacharier.
“Dupilumab led to significant, rapid, and sustained improvements in multiple aspects of lung function in children aged 6-11 years,” Dr. Bacharier added in a CHEST press release that described the findings.
The LIBERTY ASTHMA VOYAGE study was sponsored by Sanofi and Regeneron Pharmaceuticals. Dr. Bacharier provided disclosures related to AstraZeneca, GlaxoSmithKline, Regeneron Pharmaceuticals, Sanofi, CF Foundation, DBV Technologies, NIH, and Vectura.
Add-on treatment with dupilumab may improve lung function in children aged 6-11 years with uncontrolled moderate to severe type 2 inflammatory asthma, results from a randomized, placebo-controlled, phase 3 study show.
Improvements in lung function parameters were observed as early as 2 weeks and persisted over the 52-week treatment period among children in the LIBERTY ASTHMA VOYAGE study, according to investigator Leonard B. Bacharier, MD, of Monroe Carell Jr. Children’s Hospital at Vanderbilt University Medical Center, Nashville, Tenn.
“Dupilumab led to clinically meaningful rapid and sustained improvements in lung function parameters,” Dr. Bacharier said in an online poster presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
The improvements in forced expiratory volume in 1 second (FEV1) and other measures reported for children with moderate to severe asthma who have the type 2 phenotype, which is the most common driver of pediatric asthma, according to Dr. Bacharier.
“Many children with moderate to severe asthma have abnormal lung function, and this can be a risk factor for future lung disease in adulthood,” Dr. Bacharier said in his presentation.
The VOYAGE continues
The findings presented at the meeting build on another report earlier this year from the LIBERTY ASTHMA VOYAGE study demonstrating that add-on dupilumab treatment led to a significant improvement versus placebo in FEV1 up to 12 weeks.
“We now have a long term data on this drug as well, showing its efficacy over a period of time,” said Muhammad Adrish, MD, MBA, FCCP, associate professor of pulmonary, critical care and sleep medicine at Baylor College of Medicine, Houston.
“I think that’s pretty exciting, and that’s another step towards precision medicine in treatment of asthma,” Dr. Adrish, who is Vice-Chair of CHEST’s Airways Disorders NetWork Steering Committee and was not involved in the study.
Dupilumab received Food and Drug Administration approval in 2018 as add-on maintenance therapy for the treatment of patients aged 12 years or older with moderate to severe asthma that has an eosinophilic phenotype or that is dependent on oral corticosteroid treatment.
In March 2021, Sanofi and Regeneron announced that the FDA had accepted for review a supplemental Biologics License Application for dupilumab as an add-on treatment in children aged 6-11 years with uncontrolled moderate to severe asthma.
That sBLA is supported by data from the LIBERTY ASTHMA VOYAGE study, Sanofi and Regeneron said.
In results of the phase 3 study that Dr. Bacharier presented in May at the American Thoracic Society International Conference, add-on dupilumab dosed every 2 weeks significant improved percent predicted prebronchodilator FEV1 by an additional 5.21 percentage points versus placebo at week 12.
Dupilumab and the type 2 phenotype
The new data reported at the CHEST meeting come from a prespecified analysis evaluating the impact of dupilumab on lung function over a 52-week treatment period in patients with a T2 inflammatory asthma phenotype.
“Dupilumab, a fully human monoclonal antibody, blocks the shared receptor component for interleukin-4 and -13, key and central drivers of T2 inflammation in multiple diseases,” Dr. Bacharier and coinvestigators reported in their study abstract.
Of 408 patients in the study, 350 met the T2 phenotype criteria, including 236 in the dupilumab arm and 114 in the placebo arm.
Patients met T2 phenotype criteria if they had blood eosinophils of at least 150 cells/mcL or fractional exhaled nitric oxide FeNO of at least 20 parts per billion at baseline, investigators said.
Dr. Bacharier and coinvestigators reported on several different endpoints, including absolute and percent predicted prebronchodilator FEV1, percent predicted postbronchodilator FEV1, prebronchodilator forced expiratory flow at 25%-75% of pulmonary volume (FEF25%-75%), and forced vital capacity (FVC).
Dupilumab, when compared with placebo, significantly improved prebronchodilator FEV1 in pediatric patients with uncontrolled moderate to severe type 2 asthma, according to Dr. Bacharier.
“Patients receiving dupilumab experienced rapid improvements by week 2, and this was sustained for up to 52 weeks,” he said.
The prebronchodilator FEV1 improved from baseline for dupilumab versus placebo, with a least squares mean difference of 0.06 L at week 2, which reached 0.17 L by week 52, according to their data. Similarly, postbronchodilator FEV1 improved from baseline for dupilumab, with a least square mean difference versus placebo of 0.09 L at week 52.
Dupilumab compared to placebo also significantly improved percent predicted FEF25%-75%, and percent predicted FVC over the 52-week treatment period, according to Dr. Bacharier.
“Dupilumab led to significant, rapid, and sustained improvements in multiple aspects of lung function in children aged 6-11 years,” Dr. Bacharier added in a CHEST press release that described the findings.
The LIBERTY ASTHMA VOYAGE study was sponsored by Sanofi and Regeneron Pharmaceuticals. Dr. Bacharier provided disclosures related to AstraZeneca, GlaxoSmithKline, Regeneron Pharmaceuticals, Sanofi, CF Foundation, DBV Technologies, NIH, and Vectura.
Add-on treatment with dupilumab may improve lung function in children aged 6-11 years with uncontrolled moderate to severe type 2 inflammatory asthma, results from a randomized, placebo-controlled, phase 3 study show.
Improvements in lung function parameters were observed as early as 2 weeks and persisted over the 52-week treatment period among children in the LIBERTY ASTHMA VOYAGE study, according to investigator Leonard B. Bacharier, MD, of Monroe Carell Jr. Children’s Hospital at Vanderbilt University Medical Center, Nashville, Tenn.
“Dupilumab led to clinically meaningful rapid and sustained improvements in lung function parameters,” Dr. Bacharier said in an online poster presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
The improvements in forced expiratory volume in 1 second (FEV1) and other measures reported for children with moderate to severe asthma who have the type 2 phenotype, which is the most common driver of pediatric asthma, according to Dr. Bacharier.
“Many children with moderate to severe asthma have abnormal lung function, and this can be a risk factor for future lung disease in adulthood,” Dr. Bacharier said in his presentation.
The VOYAGE continues
The findings presented at the meeting build on another report earlier this year from the LIBERTY ASTHMA VOYAGE study demonstrating that add-on dupilumab treatment led to a significant improvement versus placebo in FEV1 up to 12 weeks.
“We now have a long term data on this drug as well, showing its efficacy over a period of time,” said Muhammad Adrish, MD, MBA, FCCP, associate professor of pulmonary, critical care and sleep medicine at Baylor College of Medicine, Houston.
“I think that’s pretty exciting, and that’s another step towards precision medicine in treatment of asthma,” Dr. Adrish, who is Vice-Chair of CHEST’s Airways Disorders NetWork Steering Committee and was not involved in the study.
Dupilumab received Food and Drug Administration approval in 2018 as add-on maintenance therapy for the treatment of patients aged 12 years or older with moderate to severe asthma that has an eosinophilic phenotype or that is dependent on oral corticosteroid treatment.
In March 2021, Sanofi and Regeneron announced that the FDA had accepted for review a supplemental Biologics License Application for dupilumab as an add-on treatment in children aged 6-11 years with uncontrolled moderate to severe asthma.
That sBLA is supported by data from the LIBERTY ASTHMA VOYAGE study, Sanofi and Regeneron said.
In results of the phase 3 study that Dr. Bacharier presented in May at the American Thoracic Society International Conference, add-on dupilumab dosed every 2 weeks significant improved percent predicted prebronchodilator FEV1 by an additional 5.21 percentage points versus placebo at week 12.
Dupilumab and the type 2 phenotype
The new data reported at the CHEST meeting come from a prespecified analysis evaluating the impact of dupilumab on lung function over a 52-week treatment period in patients with a T2 inflammatory asthma phenotype.
“Dupilumab, a fully human monoclonal antibody, blocks the shared receptor component for interleukin-4 and -13, key and central drivers of T2 inflammation in multiple diseases,” Dr. Bacharier and coinvestigators reported in their study abstract.
Of 408 patients in the study, 350 met the T2 phenotype criteria, including 236 in the dupilumab arm and 114 in the placebo arm.
Patients met T2 phenotype criteria if they had blood eosinophils of at least 150 cells/mcL or fractional exhaled nitric oxide FeNO of at least 20 parts per billion at baseline, investigators said.
Dr. Bacharier and coinvestigators reported on several different endpoints, including absolute and percent predicted prebronchodilator FEV1, percent predicted postbronchodilator FEV1, prebronchodilator forced expiratory flow at 25%-75% of pulmonary volume (FEF25%-75%), and forced vital capacity (FVC).
Dupilumab, when compared with placebo, significantly improved prebronchodilator FEV1 in pediatric patients with uncontrolled moderate to severe type 2 asthma, according to Dr. Bacharier.
“Patients receiving dupilumab experienced rapid improvements by week 2, and this was sustained for up to 52 weeks,” he said.
The prebronchodilator FEV1 improved from baseline for dupilumab versus placebo, with a least squares mean difference of 0.06 L at week 2, which reached 0.17 L by week 52, according to their data. Similarly, postbronchodilator FEV1 improved from baseline for dupilumab, with a least square mean difference versus placebo of 0.09 L at week 52.
Dupilumab compared to placebo also significantly improved percent predicted FEF25%-75%, and percent predicted FVC over the 52-week treatment period, according to Dr. Bacharier.
“Dupilumab led to significant, rapid, and sustained improvements in multiple aspects of lung function in children aged 6-11 years,” Dr. Bacharier added in a CHEST press release that described the findings.
The LIBERTY ASTHMA VOYAGE study was sponsored by Sanofi and Regeneron Pharmaceuticals. Dr. Bacharier provided disclosures related to AstraZeneca, GlaxoSmithKline, Regeneron Pharmaceuticals, Sanofi, CF Foundation, DBV Technologies, NIH, and Vectura.
FROM CHEST 2021
New land mines in your next (and even current) employment contract
Physician employment contracts include some new dangers. This includes physicians taking a new job, but it also includes already-employed doctors who are being asked to resign a new contract that contains new conditions. A number of these new clauses have arisen because of COVID-19. When the pandemic dramatically reduced patient flow, many employers didn’t have enough money to pay doctors and didn’t always have physicians in the right location or practice setting.
Vowing this would never happen again, some employers have rewritten their physician contracts to make it easier to reassign and terminate physicians.
Here are 12 potential land mines in a physician employment contract, some of which were added as a result of the pandemic.
You could be immediately terminated without notice
One outcome of the pandemic is the growing use of “force majeure” clauses, which give the employer the right to reduce your compensation or even terminate you due to a natural disaster, which could include COVID.
“COVID made employers aware of the potential impact of disasters on their operations,” said Dan Shay, a health law attorney at Alice Gosfield & Associates in Philadelphia. “Therefore, even as the threat of COVID abates in many places, employers are continuing to put this provision in the contract.”
What can you do? “One way to get some protection is to rule out a termination without cause in the first year,” said Michael A. Cassidy, a physician contract attorney at Tucker Arensberg in Pittsburgh.
The force majeure clause is less likely to affect salary, but could impact bonus and incentive tied to performance. It’s wise to try to specifically limit how much the force majeure could reduce pay tied to performance, and to be prepared to negotiate that aspect of your contract.
No protections if you’re let go through no fault of your own
You could lose your job if your employer could not generate enough business and has to let some doctors go. This happened quite often in the early days of the COVID pandemic.
In these situations, the doctor has not done anything wrong to prompt the termination, but the restrictive covenant may still apply, meaning that the doctor would have to leave the area to find work.
What can you do? You’re in a good position to get this changed, said Christopher L. Nuland, a solo physician contract attorney in Jacksonville, Fla. “Many employers recognize that it would be draconian to require a restrictive covenant in this case, and they will agree to modify this provision.”
Similarly, the employer may not cover your tail insurance even if you were let go from your work through no fault of your own. Most malpractice policies for employer physicians require buying an extra policy, called a tail, if you leave. In some cases, the employer won’t provide a tail and will make the departing doctor buy it.
In these cases, “try for a compromise, such as stipulating that the party that caused the termination should pay for the tail,” Mr. Nuland said. “The employer may not agree to anything more than that because they want to set up a disincentive against you leaving.”
Employer could unilaterally alter your compensation
Many recent contracts give the employer the option to unilaterally modify compensation, such as changing the base salary or raising the target required for meeting the productivity bonus, said Ericka L. Adler, a physician contract attorney at Roetzel & Andress in Chicago.
Ms. Adler thought this change could have been prompted by employers’ financial problems during the pandemic. In the early months of COVID, many physicians were not making much money for the employer but still had to be paid. So employers added a clause saying they could reduce compensation at any time, she said.
What can you do? Harsh provisions like this often come up in contracts with private equity firms, Mr. Cassidy said. “The contract might say the employer can adjust compensation or even terminate physicians based on productivity or their profitability. And it may say that if they reassign you to a new location and you refuse, they can terminate you.”
“If you can’t get these clauses removed, try to reduce the impact of a termination by providing longer notice periods or by inserting a severance agreement,” Mr. Cassidy said.
Accelerating notice for without-cause terminations
Physicians who are convicted of a felony or other moral issue can usually be terminated immediately. But if you are terminated for other reasons – that is, “without cause” – you are given notice at a certain number of days before you have to leave (typically 60-90 days), so that you have time to find a new job.
Some recent contracts, however, allow for very little notice in without-cause terminations, which allows the employer to fire you in as little as 0 days after providing notice, Ms. Adler said.
“This means that, even if 90 days’ notice is provided in the contract, the employer can decide that your last day will be an earlier date,” she said.
Why is this happening? Ms. Adler said employers want to begin reallocating resources and patients as soon as possible. The problem came to employers’ attention during the COVID pandemic, when they were contractually forced to pay doctors for doing little or nothing during the notice period.
What can you do? Possibly not much, other than attempt to negotiate. “Large employers typically don’t want to drop this provision, but at the least, the doctor needs to understand the risk it creates for them,” she said.
You could be assigned to far-off locations
As patient care needs changed dramatically during the pandemic, employers needed to reassign doctors to new locations.
Some new contracts allow employers to simply inform the doctor that they are changing the work location. However, “you don’t want to be assigned to a new work location that is 50 miles away,” Mr. Nuland said.
What can you do? Mr. Nuland recommended adding new language saying that, if the new assignment is more than 20 miles away, both parties would have to approve it.
You could end up working too many off-hours
“Most employers won’t issue a specific work schedule,” Mr. Nuland said. “They want the flexibility to assign evening or weekend work, and it would be difficult for a young doctor to change this.”
What can you do? Mr. Nuland recommended trying to set some limits. “You can try to limit off-hours work to two times a month or something like that,” she said. And if you need to have a special schedule, such as not working on Fridays, Adler advises that this should be put into the contract.
If you can’t get anything changed in the contract, Mr. Nuland said the next-best thing is to ask employers to tell you specifically what they plan to do with you. “Most employers will give you an informal idea of what’s expected – maybe not an exact schedule, but it’s quite likely they will honor it.”
You wouldn’t be able to work nearby if you left the job
Most contracts have a noncompete clause, also known as a “restrictive covenant,” which prevents employed physicians from working in the area if they left the job.
“Almost every doctor I represent has told me that they’re not concerned about the noncompete clause because, they believe, it is not enforceable anyway,” Ms. Adler said. “This is incorrect.”
Mr. Nuland said the faster pace of job-changing during the pandemic makes it all the more likely that doctors have to deal with a restrictive covenant. At the same time, some employers have been expanding the restriction – either by enlarging the radius where the restriction applies or by making the restriction apply to each of their sites, so that each one has a restricted radius around it.
For example, one contract Mr. Nuland is currently reviewing has a 20-mile radius that in effect becomes a 120-mile radius because the employer is counting four offices.
What can you do? Mr. Nuland advised trying to reduce the impact of the noncompete – for instance, making it apply only to the offices where you worked, or trading more time for less distance. “If you have a 2-year, 20-mile restriction, ask for a 3-year, 10-mile restriction, where the radius could be easier to deal with,” he said.
You might end up with too much call
Contracts rarely detail your call schedule because employers want flexibility to expand call as patient care needs change, but you can try adding some specificity, said Sanja Ord, a physician contract attorney at Greensfelder, Hemker & Gale in St. Louis.
Contracts often use wide-open language to describe call, such as simply making it “subject to the house call policies,” Mr. Cassidy said. Language that is more beneficial to the physician would say that call must be “equal” among “similarly situated” physicians.
But Ms. Ord said even provisions for equal call can turn out to be onerous if there are too few doctors in the call roster, so it’s a good idea to find out just how many doctors will be participating in call.
Still, Adler said even that strategy can’t remove all risk. What happens, she asked, if several physicians participating in call decide to leave? Then you might end up with call every other night.
What can you do? Mr. Cassidy recommends specifying a maximum amount of call – for example, no more frequent than one in four nights.
Physician must pay for reimbursement claw-backs by payers
When auditors for Medicare or other payers find overpayments after the fact, called a ‘claw-back,’ the provider must pay them back. But which provider has to do that – you or your employer?
In many cases, your employer’s billing office may have introduced the error, but there may be a clause in the contract stating that the physician is solely responsible for all claw-backs. That could be costly.
What can you do? Mr. Shay said the clause should state that you have to pay only when it is the result of your own error or omission, and also not when it was made at the direction of the employer.
Some work may be outside of your subspecialty
In some cases, the employer may assign subspecialized doctors to work outside their subspecialty, Mr. Nuland said.
For example, he said he represented an endocrinologist who expected to see only diabetes patients but was assigned to some general internal medicine work as well, and an otolaryngologist client of his who completed a fellowship on facial plastic surgery was expected to do liposuction in a cosmetic surgery group.
What can you do? To prevent this from happening, Mr. Nuland recommends a clause stating that your work will be restricted to your subspecialty.
What the employer promised isn’t in the contract
“Beware of promises that are not in the contract,” Mr. Shay said. “You might feel you can really trust your new boss and what he tells you, but what if that person resigns, or the organization gets a new owner who doesn’t honor unwritten agreements?”
Many contracts have an integration clause, which specifies that the contract constitutes the complete agreement between the two parties, and it nullifies any other oral or written promises made to the physician.
For example, the employer might have promised a relocation bonus and a sign-on bonus, but for some reason it didn’t get into the contract, Ms. Ord said. In those cases, the employer is under no obligation to honor the promise.
What can you do? Mr. Cassidy said it is possible to hold the employer to a commitment made outside the contract. The alternative document, such as an offer letter, has to specifically state that the commitment is protected from the integration clause in the contract, he said, adding: “It is still better to have the commitment put into the contract.”
Contract is simply accepted as is
“Generally, the bigger the employer, the less likely they will alter an agreement just to make you happy,” Mr. Shay said.
But even in these contracts, he said there is still opportunity to fix errors and ambiguities that could harm you later – or even alter a provision if you can’t remove it outright.
The back-and-forth is important, Ms. Adler said. “Negotiation means trying to have some control over your job and your life.”
Mr. Cassidy said a big part of contract review is facing up to the possibility that you may have to resign or be let go.
“Many physicians don’t like to think about leaving when they’re just starting a job, but they need to,” he said. “You need to begin with the end in mind. Think about what would happen if this job didn’t work out.”
A version of this article first appeared on Medscape.com.
Physician employment contracts include some new dangers. This includes physicians taking a new job, but it also includes already-employed doctors who are being asked to resign a new contract that contains new conditions. A number of these new clauses have arisen because of COVID-19. When the pandemic dramatically reduced patient flow, many employers didn’t have enough money to pay doctors and didn’t always have physicians in the right location or practice setting.
Vowing this would never happen again, some employers have rewritten their physician contracts to make it easier to reassign and terminate physicians.
Here are 12 potential land mines in a physician employment contract, some of which were added as a result of the pandemic.
You could be immediately terminated without notice
One outcome of the pandemic is the growing use of “force majeure” clauses, which give the employer the right to reduce your compensation or even terminate you due to a natural disaster, which could include COVID.
“COVID made employers aware of the potential impact of disasters on their operations,” said Dan Shay, a health law attorney at Alice Gosfield & Associates in Philadelphia. “Therefore, even as the threat of COVID abates in many places, employers are continuing to put this provision in the contract.”
What can you do? “One way to get some protection is to rule out a termination without cause in the first year,” said Michael A. Cassidy, a physician contract attorney at Tucker Arensberg in Pittsburgh.
The force majeure clause is less likely to affect salary, but could impact bonus and incentive tied to performance. It’s wise to try to specifically limit how much the force majeure could reduce pay tied to performance, and to be prepared to negotiate that aspect of your contract.
No protections if you’re let go through no fault of your own
You could lose your job if your employer could not generate enough business and has to let some doctors go. This happened quite often in the early days of the COVID pandemic.
In these situations, the doctor has not done anything wrong to prompt the termination, but the restrictive covenant may still apply, meaning that the doctor would have to leave the area to find work.
What can you do? You’re in a good position to get this changed, said Christopher L. Nuland, a solo physician contract attorney in Jacksonville, Fla. “Many employers recognize that it would be draconian to require a restrictive covenant in this case, and they will agree to modify this provision.”
Similarly, the employer may not cover your tail insurance even if you were let go from your work through no fault of your own. Most malpractice policies for employer physicians require buying an extra policy, called a tail, if you leave. In some cases, the employer won’t provide a tail and will make the departing doctor buy it.
In these cases, “try for a compromise, such as stipulating that the party that caused the termination should pay for the tail,” Mr. Nuland said. “The employer may not agree to anything more than that because they want to set up a disincentive against you leaving.”
Employer could unilaterally alter your compensation
Many recent contracts give the employer the option to unilaterally modify compensation, such as changing the base salary or raising the target required for meeting the productivity bonus, said Ericka L. Adler, a physician contract attorney at Roetzel & Andress in Chicago.
Ms. Adler thought this change could have been prompted by employers’ financial problems during the pandemic. In the early months of COVID, many physicians were not making much money for the employer but still had to be paid. So employers added a clause saying they could reduce compensation at any time, she said.
What can you do? Harsh provisions like this often come up in contracts with private equity firms, Mr. Cassidy said. “The contract might say the employer can adjust compensation or even terminate physicians based on productivity or their profitability. And it may say that if they reassign you to a new location and you refuse, they can terminate you.”
“If you can’t get these clauses removed, try to reduce the impact of a termination by providing longer notice periods or by inserting a severance agreement,” Mr. Cassidy said.
Accelerating notice for without-cause terminations
Physicians who are convicted of a felony or other moral issue can usually be terminated immediately. But if you are terminated for other reasons – that is, “without cause” – you are given notice at a certain number of days before you have to leave (typically 60-90 days), so that you have time to find a new job.
Some recent contracts, however, allow for very little notice in without-cause terminations, which allows the employer to fire you in as little as 0 days after providing notice, Ms. Adler said.
“This means that, even if 90 days’ notice is provided in the contract, the employer can decide that your last day will be an earlier date,” she said.
Why is this happening? Ms. Adler said employers want to begin reallocating resources and patients as soon as possible. The problem came to employers’ attention during the COVID pandemic, when they were contractually forced to pay doctors for doing little or nothing during the notice period.
What can you do? Possibly not much, other than attempt to negotiate. “Large employers typically don’t want to drop this provision, but at the least, the doctor needs to understand the risk it creates for them,” she said.
You could be assigned to far-off locations
As patient care needs changed dramatically during the pandemic, employers needed to reassign doctors to new locations.
Some new contracts allow employers to simply inform the doctor that they are changing the work location. However, “you don’t want to be assigned to a new work location that is 50 miles away,” Mr. Nuland said.
What can you do? Mr. Nuland recommended adding new language saying that, if the new assignment is more than 20 miles away, both parties would have to approve it.
You could end up working too many off-hours
“Most employers won’t issue a specific work schedule,” Mr. Nuland said. “They want the flexibility to assign evening or weekend work, and it would be difficult for a young doctor to change this.”
What can you do? Mr. Nuland recommended trying to set some limits. “You can try to limit off-hours work to two times a month or something like that,” she said. And if you need to have a special schedule, such as not working on Fridays, Adler advises that this should be put into the contract.
If you can’t get anything changed in the contract, Mr. Nuland said the next-best thing is to ask employers to tell you specifically what they plan to do with you. “Most employers will give you an informal idea of what’s expected – maybe not an exact schedule, but it’s quite likely they will honor it.”
You wouldn’t be able to work nearby if you left the job
Most contracts have a noncompete clause, also known as a “restrictive covenant,” which prevents employed physicians from working in the area if they left the job.
“Almost every doctor I represent has told me that they’re not concerned about the noncompete clause because, they believe, it is not enforceable anyway,” Ms. Adler said. “This is incorrect.”
Mr. Nuland said the faster pace of job-changing during the pandemic makes it all the more likely that doctors have to deal with a restrictive covenant. At the same time, some employers have been expanding the restriction – either by enlarging the radius where the restriction applies or by making the restriction apply to each of their sites, so that each one has a restricted radius around it.
For example, one contract Mr. Nuland is currently reviewing has a 20-mile radius that in effect becomes a 120-mile radius because the employer is counting four offices.
What can you do? Mr. Nuland advised trying to reduce the impact of the noncompete – for instance, making it apply only to the offices where you worked, or trading more time for less distance. “If you have a 2-year, 20-mile restriction, ask for a 3-year, 10-mile restriction, where the radius could be easier to deal with,” he said.
You might end up with too much call
Contracts rarely detail your call schedule because employers want flexibility to expand call as patient care needs change, but you can try adding some specificity, said Sanja Ord, a physician contract attorney at Greensfelder, Hemker & Gale in St. Louis.
Contracts often use wide-open language to describe call, such as simply making it “subject to the house call policies,” Mr. Cassidy said. Language that is more beneficial to the physician would say that call must be “equal” among “similarly situated” physicians.
But Ms. Ord said even provisions for equal call can turn out to be onerous if there are too few doctors in the call roster, so it’s a good idea to find out just how many doctors will be participating in call.
Still, Adler said even that strategy can’t remove all risk. What happens, she asked, if several physicians participating in call decide to leave? Then you might end up with call every other night.
What can you do? Mr. Cassidy recommends specifying a maximum amount of call – for example, no more frequent than one in four nights.
Physician must pay for reimbursement claw-backs by payers
When auditors for Medicare or other payers find overpayments after the fact, called a ‘claw-back,’ the provider must pay them back. But which provider has to do that – you or your employer?
In many cases, your employer’s billing office may have introduced the error, but there may be a clause in the contract stating that the physician is solely responsible for all claw-backs. That could be costly.
What can you do? Mr. Shay said the clause should state that you have to pay only when it is the result of your own error or omission, and also not when it was made at the direction of the employer.
Some work may be outside of your subspecialty
In some cases, the employer may assign subspecialized doctors to work outside their subspecialty, Mr. Nuland said.
For example, he said he represented an endocrinologist who expected to see only diabetes patients but was assigned to some general internal medicine work as well, and an otolaryngologist client of his who completed a fellowship on facial plastic surgery was expected to do liposuction in a cosmetic surgery group.
What can you do? To prevent this from happening, Mr. Nuland recommends a clause stating that your work will be restricted to your subspecialty.
What the employer promised isn’t in the contract
“Beware of promises that are not in the contract,” Mr. Shay said. “You might feel you can really trust your new boss and what he tells you, but what if that person resigns, or the organization gets a new owner who doesn’t honor unwritten agreements?”
Many contracts have an integration clause, which specifies that the contract constitutes the complete agreement between the two parties, and it nullifies any other oral or written promises made to the physician.
For example, the employer might have promised a relocation bonus and a sign-on bonus, but for some reason it didn’t get into the contract, Ms. Ord said. In those cases, the employer is under no obligation to honor the promise.
What can you do? Mr. Cassidy said it is possible to hold the employer to a commitment made outside the contract. The alternative document, such as an offer letter, has to specifically state that the commitment is protected from the integration clause in the contract, he said, adding: “It is still better to have the commitment put into the contract.”
Contract is simply accepted as is
“Generally, the bigger the employer, the less likely they will alter an agreement just to make you happy,” Mr. Shay said.
But even in these contracts, he said there is still opportunity to fix errors and ambiguities that could harm you later – or even alter a provision if you can’t remove it outright.
The back-and-forth is important, Ms. Adler said. “Negotiation means trying to have some control over your job and your life.”
Mr. Cassidy said a big part of contract review is facing up to the possibility that you may have to resign or be let go.
“Many physicians don’t like to think about leaving when they’re just starting a job, but they need to,” he said. “You need to begin with the end in mind. Think about what would happen if this job didn’t work out.”
A version of this article first appeared on Medscape.com.
Physician employment contracts include some new dangers. This includes physicians taking a new job, but it also includes already-employed doctors who are being asked to resign a new contract that contains new conditions. A number of these new clauses have arisen because of COVID-19. When the pandemic dramatically reduced patient flow, many employers didn’t have enough money to pay doctors and didn’t always have physicians in the right location or practice setting.
Vowing this would never happen again, some employers have rewritten their physician contracts to make it easier to reassign and terminate physicians.
Here are 12 potential land mines in a physician employment contract, some of which were added as a result of the pandemic.
You could be immediately terminated without notice
One outcome of the pandemic is the growing use of “force majeure” clauses, which give the employer the right to reduce your compensation or even terminate you due to a natural disaster, which could include COVID.
“COVID made employers aware of the potential impact of disasters on their operations,” said Dan Shay, a health law attorney at Alice Gosfield & Associates in Philadelphia. “Therefore, even as the threat of COVID abates in many places, employers are continuing to put this provision in the contract.”
What can you do? “One way to get some protection is to rule out a termination without cause in the first year,” said Michael A. Cassidy, a physician contract attorney at Tucker Arensberg in Pittsburgh.
The force majeure clause is less likely to affect salary, but could impact bonus and incentive tied to performance. It’s wise to try to specifically limit how much the force majeure could reduce pay tied to performance, and to be prepared to negotiate that aspect of your contract.
No protections if you’re let go through no fault of your own
You could lose your job if your employer could not generate enough business and has to let some doctors go. This happened quite often in the early days of the COVID pandemic.
In these situations, the doctor has not done anything wrong to prompt the termination, but the restrictive covenant may still apply, meaning that the doctor would have to leave the area to find work.
What can you do? You’re in a good position to get this changed, said Christopher L. Nuland, a solo physician contract attorney in Jacksonville, Fla. “Many employers recognize that it would be draconian to require a restrictive covenant in this case, and they will agree to modify this provision.”
Similarly, the employer may not cover your tail insurance even if you were let go from your work through no fault of your own. Most malpractice policies for employer physicians require buying an extra policy, called a tail, if you leave. In some cases, the employer won’t provide a tail and will make the departing doctor buy it.
In these cases, “try for a compromise, such as stipulating that the party that caused the termination should pay for the tail,” Mr. Nuland said. “The employer may not agree to anything more than that because they want to set up a disincentive against you leaving.”
Employer could unilaterally alter your compensation
Many recent contracts give the employer the option to unilaterally modify compensation, such as changing the base salary or raising the target required for meeting the productivity bonus, said Ericka L. Adler, a physician contract attorney at Roetzel & Andress in Chicago.
Ms. Adler thought this change could have been prompted by employers’ financial problems during the pandemic. In the early months of COVID, many physicians were not making much money for the employer but still had to be paid. So employers added a clause saying they could reduce compensation at any time, she said.
What can you do? Harsh provisions like this often come up in contracts with private equity firms, Mr. Cassidy said. “The contract might say the employer can adjust compensation or even terminate physicians based on productivity or their profitability. And it may say that if they reassign you to a new location and you refuse, they can terminate you.”
“If you can’t get these clauses removed, try to reduce the impact of a termination by providing longer notice periods or by inserting a severance agreement,” Mr. Cassidy said.
Accelerating notice for without-cause terminations
Physicians who are convicted of a felony or other moral issue can usually be terminated immediately. But if you are terminated for other reasons – that is, “without cause” – you are given notice at a certain number of days before you have to leave (typically 60-90 days), so that you have time to find a new job.
Some recent contracts, however, allow for very little notice in without-cause terminations, which allows the employer to fire you in as little as 0 days after providing notice, Ms. Adler said.
“This means that, even if 90 days’ notice is provided in the contract, the employer can decide that your last day will be an earlier date,” she said.
Why is this happening? Ms. Adler said employers want to begin reallocating resources and patients as soon as possible. The problem came to employers’ attention during the COVID pandemic, when they were contractually forced to pay doctors for doing little or nothing during the notice period.
What can you do? Possibly not much, other than attempt to negotiate. “Large employers typically don’t want to drop this provision, but at the least, the doctor needs to understand the risk it creates for them,” she said.
You could be assigned to far-off locations
As patient care needs changed dramatically during the pandemic, employers needed to reassign doctors to new locations.
Some new contracts allow employers to simply inform the doctor that they are changing the work location. However, “you don’t want to be assigned to a new work location that is 50 miles away,” Mr. Nuland said.
What can you do? Mr. Nuland recommended adding new language saying that, if the new assignment is more than 20 miles away, both parties would have to approve it.
You could end up working too many off-hours
“Most employers won’t issue a specific work schedule,” Mr. Nuland said. “They want the flexibility to assign evening or weekend work, and it would be difficult for a young doctor to change this.”
What can you do? Mr. Nuland recommended trying to set some limits. “You can try to limit off-hours work to two times a month or something like that,” she said. And if you need to have a special schedule, such as not working on Fridays, Adler advises that this should be put into the contract.
If you can’t get anything changed in the contract, Mr. Nuland said the next-best thing is to ask employers to tell you specifically what they plan to do with you. “Most employers will give you an informal idea of what’s expected – maybe not an exact schedule, but it’s quite likely they will honor it.”
You wouldn’t be able to work nearby if you left the job
Most contracts have a noncompete clause, also known as a “restrictive covenant,” which prevents employed physicians from working in the area if they left the job.
“Almost every doctor I represent has told me that they’re not concerned about the noncompete clause because, they believe, it is not enforceable anyway,” Ms. Adler said. “This is incorrect.”
Mr. Nuland said the faster pace of job-changing during the pandemic makes it all the more likely that doctors have to deal with a restrictive covenant. At the same time, some employers have been expanding the restriction – either by enlarging the radius where the restriction applies or by making the restriction apply to each of their sites, so that each one has a restricted radius around it.
For example, one contract Mr. Nuland is currently reviewing has a 20-mile radius that in effect becomes a 120-mile radius because the employer is counting four offices.
What can you do? Mr. Nuland advised trying to reduce the impact of the noncompete – for instance, making it apply only to the offices where you worked, or trading more time for less distance. “If you have a 2-year, 20-mile restriction, ask for a 3-year, 10-mile restriction, where the radius could be easier to deal with,” he said.
You might end up with too much call
Contracts rarely detail your call schedule because employers want flexibility to expand call as patient care needs change, but you can try adding some specificity, said Sanja Ord, a physician contract attorney at Greensfelder, Hemker & Gale in St. Louis.
Contracts often use wide-open language to describe call, such as simply making it “subject to the house call policies,” Mr. Cassidy said. Language that is more beneficial to the physician would say that call must be “equal” among “similarly situated” physicians.
But Ms. Ord said even provisions for equal call can turn out to be onerous if there are too few doctors in the call roster, so it’s a good idea to find out just how many doctors will be participating in call.
Still, Adler said even that strategy can’t remove all risk. What happens, she asked, if several physicians participating in call decide to leave? Then you might end up with call every other night.
What can you do? Mr. Cassidy recommends specifying a maximum amount of call – for example, no more frequent than one in four nights.
Physician must pay for reimbursement claw-backs by payers
When auditors for Medicare or other payers find overpayments after the fact, called a ‘claw-back,’ the provider must pay them back. But which provider has to do that – you or your employer?
In many cases, your employer’s billing office may have introduced the error, but there may be a clause in the contract stating that the physician is solely responsible for all claw-backs. That could be costly.
What can you do? Mr. Shay said the clause should state that you have to pay only when it is the result of your own error or omission, and also not when it was made at the direction of the employer.
Some work may be outside of your subspecialty
In some cases, the employer may assign subspecialized doctors to work outside their subspecialty, Mr. Nuland said.
For example, he said he represented an endocrinologist who expected to see only diabetes patients but was assigned to some general internal medicine work as well, and an otolaryngologist client of his who completed a fellowship on facial plastic surgery was expected to do liposuction in a cosmetic surgery group.
What can you do? To prevent this from happening, Mr. Nuland recommends a clause stating that your work will be restricted to your subspecialty.
What the employer promised isn’t in the contract
“Beware of promises that are not in the contract,” Mr. Shay said. “You might feel you can really trust your new boss and what he tells you, but what if that person resigns, or the organization gets a new owner who doesn’t honor unwritten agreements?”
Many contracts have an integration clause, which specifies that the contract constitutes the complete agreement between the two parties, and it nullifies any other oral or written promises made to the physician.
For example, the employer might have promised a relocation bonus and a sign-on bonus, but for some reason it didn’t get into the contract, Ms. Ord said. In those cases, the employer is under no obligation to honor the promise.
What can you do? Mr. Cassidy said it is possible to hold the employer to a commitment made outside the contract. The alternative document, such as an offer letter, has to specifically state that the commitment is protected from the integration clause in the contract, he said, adding: “It is still better to have the commitment put into the contract.”
Contract is simply accepted as is
“Generally, the bigger the employer, the less likely they will alter an agreement just to make you happy,” Mr. Shay said.
But even in these contracts, he said there is still opportunity to fix errors and ambiguities that could harm you later – or even alter a provision if you can’t remove it outright.
The back-and-forth is important, Ms. Adler said. “Negotiation means trying to have some control over your job and your life.”
Mr. Cassidy said a big part of contract review is facing up to the possibility that you may have to resign or be let go.
“Many physicians don’t like to think about leaving when they’re just starting a job, but they need to,” he said. “You need to begin with the end in mind. Think about what would happen if this job didn’t work out.”
A version of this article first appeared on Medscape.com.



