User login
News and Views that Matter to Pediatricians
The leading independent newspaper covering news and commentary in pediatrics.
New study shows natural immunity to COVID has enduring strength
It’s a matter of quality, not quantity. That’s the gist of a new Israeli study that shows that unvaccinated people with a prior SARS-CoV-2 infection create antibodies that are more effective in the long run compared with others who were vaccinated but never infected.
“While the quantity of antibodies decreases with time in both COVID-19 recovered patients and vaccinated individuals, ” lead author Carmit Cohen, PhD, said in an interview.
This difference could explain why previously infected patients appear to be better protected against a new infection than those who have only been vaccinated, according to a news release attached to the research.
One key caveat: This research does not include people from the later part of the pandemic.
This means there is a catch in terms of timing, William Schaffner, MD, Vanderbilt University School of Medicine, Nashville, Tenn., said when asked to comment on the study: “The study involved only the early COVID strains – it has no information on either the Delta or Omicron variants. Thus, the results primarily are of scientific or historical interest but are not immediately relevant to the current situation.”
The findings come from an early release of a study to be presented at the European Congress of Clinical Microbiology & Infectious Diseases in April.
An unexpected finding of the study showed that obese people had better protection – a higher and more sustained immune response – compared with overweight and normal-weight individuals.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” Dr. Schaffner said. “Obesity does predispose to more severe disease.”
A focus on earlier strains
Dr. Cohen – a senior research assistant in infectious disease prevention at the Sheba Medical Center in Ramat Gan, Israel – and her colleagues recruited participants between March 25, 2020 and Nov. 25, 2020 and completed analysis in April 2021. This means they assessed people with a history of infection from the original, the Alpha, and some Beta strains of SARS-CoV-2.
Dr. Cohen indicated that the next phase of their research will examine innate and acquired immune responses to the more recent Delta and Omicron variants.
The investigators analyzed the antibody-induced immune response up to 1 year in 130 COVID-19 recovered but unvaccinated individuals versus up to 8 months among 402 others matched by age and body mass index (BMI) and without previous infection who received two doses of the Pfizer vaccine.
The numbers of antibodies a month after vaccination were higher than those in the COVID-19 recovered patients. However, these numbers also declined more steeply in the vaccinated group, they note.
To assess the antibody performance, the investigators used the avidity index. This assay measures antibody function based on the strength of the interactions between the antibody and the viral antigen.
They found that the avidity index was higher in vaccinated individuals than in recovered patients initially but changes over time. At up to 6 months, the index did not significantly change in vaccinated individuals, whereas it gradually increased in recovered patients. This increase would potentially protect them from reinfection, the authors note.
These findings stand in stark contrast to an Oct. 29, 2021, Centers for Disease Control and Prevention study that found that COVID-19 vaccines provided five times the protection of natural immunity.
Those results, published in the organization’s Morbidity and Mortality Weekly Report, suggest that vaccination helps people mount a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least 6 months.
Protection linked to obesity
Another finding that ran against the scientific grain was the data about obesity.
There was a higher and more persistent antibody performance among people with a BMI of 30 kg/m2.
This could relate to greater disease severity and/or a more pronounced initial response to infection among the obese group.
“Our hypothesis is that patients with obesity begin with a more pronounced response – reflected also by the disease manifestation – and the trend of decline is similar, therefore the kinetics of immune response remain higher throughout the study,” Dr. Cohen said.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” said Dr. Schaffner, who is also the current medical director of the National Foundation for Infectious Diseases. “Obesity does predispose to more severe disease.”
Before the boosters
Along with using participants from only the earlier part of the pandemic, another limitation of the study was that the vaccinated group had only two doses of vaccine; boosters were not given during the time of the study, Dr. Schaffner said.
“Again, not the current situation.”
“That said, the strength and duration of natural immunity provided by the early variants was solid for up to a year, confirming previous reports,” he said.
A version of this article first appeared on Medscape.com.
It’s a matter of quality, not quantity. That’s the gist of a new Israeli study that shows that unvaccinated people with a prior SARS-CoV-2 infection create antibodies that are more effective in the long run compared with others who were vaccinated but never infected.
“While the quantity of antibodies decreases with time in both COVID-19 recovered patients and vaccinated individuals, ” lead author Carmit Cohen, PhD, said in an interview.
This difference could explain why previously infected patients appear to be better protected against a new infection than those who have only been vaccinated, according to a news release attached to the research.
One key caveat: This research does not include people from the later part of the pandemic.
This means there is a catch in terms of timing, William Schaffner, MD, Vanderbilt University School of Medicine, Nashville, Tenn., said when asked to comment on the study: “The study involved only the early COVID strains – it has no information on either the Delta or Omicron variants. Thus, the results primarily are of scientific or historical interest but are not immediately relevant to the current situation.”
The findings come from an early release of a study to be presented at the European Congress of Clinical Microbiology & Infectious Diseases in April.
An unexpected finding of the study showed that obese people had better protection – a higher and more sustained immune response – compared with overweight and normal-weight individuals.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” Dr. Schaffner said. “Obesity does predispose to more severe disease.”
A focus on earlier strains
Dr. Cohen – a senior research assistant in infectious disease prevention at the Sheba Medical Center in Ramat Gan, Israel – and her colleagues recruited participants between March 25, 2020 and Nov. 25, 2020 and completed analysis in April 2021. This means they assessed people with a history of infection from the original, the Alpha, and some Beta strains of SARS-CoV-2.
Dr. Cohen indicated that the next phase of their research will examine innate and acquired immune responses to the more recent Delta and Omicron variants.
The investigators analyzed the antibody-induced immune response up to 1 year in 130 COVID-19 recovered but unvaccinated individuals versus up to 8 months among 402 others matched by age and body mass index (BMI) and without previous infection who received two doses of the Pfizer vaccine.
The numbers of antibodies a month after vaccination were higher than those in the COVID-19 recovered patients. However, these numbers also declined more steeply in the vaccinated group, they note.
To assess the antibody performance, the investigators used the avidity index. This assay measures antibody function based on the strength of the interactions between the antibody and the viral antigen.
They found that the avidity index was higher in vaccinated individuals than in recovered patients initially but changes over time. At up to 6 months, the index did not significantly change in vaccinated individuals, whereas it gradually increased in recovered patients. This increase would potentially protect them from reinfection, the authors note.
These findings stand in stark contrast to an Oct. 29, 2021, Centers for Disease Control and Prevention study that found that COVID-19 vaccines provided five times the protection of natural immunity.
Those results, published in the organization’s Morbidity and Mortality Weekly Report, suggest that vaccination helps people mount a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least 6 months.
Protection linked to obesity
Another finding that ran against the scientific grain was the data about obesity.
There was a higher and more persistent antibody performance among people with a BMI of 30 kg/m2.
This could relate to greater disease severity and/or a more pronounced initial response to infection among the obese group.
“Our hypothesis is that patients with obesity begin with a more pronounced response – reflected also by the disease manifestation – and the trend of decline is similar, therefore the kinetics of immune response remain higher throughout the study,” Dr. Cohen said.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” said Dr. Schaffner, who is also the current medical director of the National Foundation for Infectious Diseases. “Obesity does predispose to more severe disease.”
Before the boosters
Along with using participants from only the earlier part of the pandemic, another limitation of the study was that the vaccinated group had only two doses of vaccine; boosters were not given during the time of the study, Dr. Schaffner said.
“Again, not the current situation.”
“That said, the strength and duration of natural immunity provided by the early variants was solid for up to a year, confirming previous reports,” he said.
A version of this article first appeared on Medscape.com.
It’s a matter of quality, not quantity. That’s the gist of a new Israeli study that shows that unvaccinated people with a prior SARS-CoV-2 infection create antibodies that are more effective in the long run compared with others who were vaccinated but never infected.
“While the quantity of antibodies decreases with time in both COVID-19 recovered patients and vaccinated individuals, ” lead author Carmit Cohen, PhD, said in an interview.
This difference could explain why previously infected patients appear to be better protected against a new infection than those who have only been vaccinated, according to a news release attached to the research.
One key caveat: This research does not include people from the later part of the pandemic.
This means there is a catch in terms of timing, William Schaffner, MD, Vanderbilt University School of Medicine, Nashville, Tenn., said when asked to comment on the study: “The study involved only the early COVID strains – it has no information on either the Delta or Omicron variants. Thus, the results primarily are of scientific or historical interest but are not immediately relevant to the current situation.”
The findings come from an early release of a study to be presented at the European Congress of Clinical Microbiology & Infectious Diseases in April.
An unexpected finding of the study showed that obese people had better protection – a higher and more sustained immune response – compared with overweight and normal-weight individuals.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” Dr. Schaffner said. “Obesity does predispose to more severe disease.”
A focus on earlier strains
Dr. Cohen – a senior research assistant in infectious disease prevention at the Sheba Medical Center in Ramat Gan, Israel – and her colleagues recruited participants between March 25, 2020 and Nov. 25, 2020 and completed analysis in April 2021. This means they assessed people with a history of infection from the original, the Alpha, and some Beta strains of SARS-CoV-2.
Dr. Cohen indicated that the next phase of their research will examine innate and acquired immune responses to the more recent Delta and Omicron variants.
The investigators analyzed the antibody-induced immune response up to 1 year in 130 COVID-19 recovered but unvaccinated individuals versus up to 8 months among 402 others matched by age and body mass index (BMI) and without previous infection who received two doses of the Pfizer vaccine.
The numbers of antibodies a month after vaccination were higher than those in the COVID-19 recovered patients. However, these numbers also declined more steeply in the vaccinated group, they note.
To assess the antibody performance, the investigators used the avidity index. This assay measures antibody function based on the strength of the interactions between the antibody and the viral antigen.
They found that the avidity index was higher in vaccinated individuals than in recovered patients initially but changes over time. At up to 6 months, the index did not significantly change in vaccinated individuals, whereas it gradually increased in recovered patients. This increase would potentially protect them from reinfection, the authors note.
These findings stand in stark contrast to an Oct. 29, 2021, Centers for Disease Control and Prevention study that found that COVID-19 vaccines provided five times the protection of natural immunity.
Those results, published in the organization’s Morbidity and Mortality Weekly Report, suggest that vaccination helps people mount a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least 6 months.
Protection linked to obesity
Another finding that ran against the scientific grain was the data about obesity.
There was a higher and more persistent antibody performance among people with a BMI of 30 kg/m2.
This could relate to greater disease severity and/or a more pronounced initial response to infection among the obese group.
“Our hypothesis is that patients with obesity begin with a more pronounced response – reflected also by the disease manifestation – and the trend of decline is similar, therefore the kinetics of immune response remain higher throughout the study,” Dr. Cohen said.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” said Dr. Schaffner, who is also the current medical director of the National Foundation for Infectious Diseases. “Obesity does predispose to more severe disease.”
Before the boosters
Along with using participants from only the earlier part of the pandemic, another limitation of the study was that the vaccinated group had only two doses of vaccine; boosters were not given during the time of the study, Dr. Schaffner said.
“Again, not the current situation.”
“That said, the strength and duration of natural immunity provided by the early variants was solid for up to a year, confirming previous reports,” he said.
A version of this article first appeared on Medscape.com.
To a perfect day
Motionless, every Olympic skater starts off perfectly. Once the music starts, it’s up to them whether they will continue on perfectly or not. In this way, you’re just like an Olympic skater. Each day, a skating program. The music starts the moment your foot touches the floor in the morning. It’s up to you if the rest of the day will continue on flawlessly or not. To this point, I’ve yet to have a perfect day.
If I’m honest, my “perfect day” streak typically ends once I’ve made coffee. By then, I’ll have spilled a few grains of grounds or clinked mugs together when taking one from the cupboard. (D’oh!) Hardly ever can I make it to backing out of the driveway, let alone through a patient encounter. I’ve had a few procedures that when complete I’ve thought, “well, that looks great.” I can remember encounters that went brilliantly despite a high technical difficulty. I’ve also tagged a 7-iron shot 160 downwind yards to within inches of the cup. But I’ve hardly ever done anything in my life perfectly.
What does it mean to be perfect? Well, there have been 23 perfect baseball games. In 1972, the Miami Dolphins had the only perfect NFL season, 14-0 (although my 2007 Patriots went 18-0 before losing to the – ugh – Giants). Every year, several hundred students score a perfect 1600 on the SAT. In an underground vault somewhere in France is a perfect sphere, a perfectly spherical 1-kg mass of pure silicon. There are at least 51 perfect numbers. And model Bella Hadid’s exactly 1.62-ratioed face is said to be perfectly beautiful. But yet, U.S. skater Nathan Chen’s seemingly flawless 113.97-point short program in Beijing, still imperfect.
Attempting a perfect day or perfect surgery or a perfect pour over coffee is a fun game, but perfectionism has an insidious side. Some of us feel this way every day: We must do it exactly right, every time. Even an insignificant imperfection or error feels like failure. A 3.90 GPA is a fail. 515 on the MCAT, not nearly good enough. For them, the burden of perfection is crushing. It is hard for some to recognize that even if your performance could not be improved, the outcome can still be flawed. A chip in the ice, a patient showing up late, an interviewer with an agenda, a missed referee call can all flub up an otherwise flawless day. It isn’t necessary to abandon hope, all ye who live in the real world. Although achieving perfection is usually impossible, reward comes from the pursuit of perfection, not from holding it. It is called perfectionistic striving and in contrast to perfectionistic concerns, it is associated with resilience and positive mood. To do so you must combine giving your all with acceptance of whatever the outcome.
Keith Jarrett is one of the greatest jazz pianists of all time. He is a true perfectionist, precise in his standards and exacting in expectations. In 1975 in Cologne, Germany, he agreed to play at the behest of a teenage girl who arranged to have him perform at the opera house. Except, there was a miscommunication and only a small, broken rehearsal piano was available. As the story goes, she approached him as he waited to be taken back to his hotel, the concert was canceled and she somehow convinced him to play on the nearly unplayable instrument. The result is the Köln Concert, one of the greatest jazz performances in history. It was perfectly imperfect.
Yes, even the 1-kg sphere has femtogram quantities of other elements mixed in – the universal standard for perfect is itself, imperfect. It doesn’t matter. It’s the pursuit of such that makes life worthwhile. There’s always tomorrow. Have your coffee grinders ready.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Motionless, every Olympic skater starts off perfectly. Once the music starts, it’s up to them whether they will continue on perfectly or not. In this way, you’re just like an Olympic skater. Each day, a skating program. The music starts the moment your foot touches the floor in the morning. It’s up to you if the rest of the day will continue on flawlessly or not. To this point, I’ve yet to have a perfect day.
If I’m honest, my “perfect day” streak typically ends once I’ve made coffee. By then, I’ll have spilled a few grains of grounds or clinked mugs together when taking one from the cupboard. (D’oh!) Hardly ever can I make it to backing out of the driveway, let alone through a patient encounter. I’ve had a few procedures that when complete I’ve thought, “well, that looks great.” I can remember encounters that went brilliantly despite a high technical difficulty. I’ve also tagged a 7-iron shot 160 downwind yards to within inches of the cup. But I’ve hardly ever done anything in my life perfectly.
What does it mean to be perfect? Well, there have been 23 perfect baseball games. In 1972, the Miami Dolphins had the only perfect NFL season, 14-0 (although my 2007 Patriots went 18-0 before losing to the – ugh – Giants). Every year, several hundred students score a perfect 1600 on the SAT. In an underground vault somewhere in France is a perfect sphere, a perfectly spherical 1-kg mass of pure silicon. There are at least 51 perfect numbers. And model Bella Hadid’s exactly 1.62-ratioed face is said to be perfectly beautiful. But yet, U.S. skater Nathan Chen’s seemingly flawless 113.97-point short program in Beijing, still imperfect.
Attempting a perfect day or perfect surgery or a perfect pour over coffee is a fun game, but perfectionism has an insidious side. Some of us feel this way every day: We must do it exactly right, every time. Even an insignificant imperfection or error feels like failure. A 3.90 GPA is a fail. 515 on the MCAT, not nearly good enough. For them, the burden of perfection is crushing. It is hard for some to recognize that even if your performance could not be improved, the outcome can still be flawed. A chip in the ice, a patient showing up late, an interviewer with an agenda, a missed referee call can all flub up an otherwise flawless day. It isn’t necessary to abandon hope, all ye who live in the real world. Although achieving perfection is usually impossible, reward comes from the pursuit of perfection, not from holding it. It is called perfectionistic striving and in contrast to perfectionistic concerns, it is associated with resilience and positive mood. To do so you must combine giving your all with acceptance of whatever the outcome.
Keith Jarrett is one of the greatest jazz pianists of all time. He is a true perfectionist, precise in his standards and exacting in expectations. In 1975 in Cologne, Germany, he agreed to play at the behest of a teenage girl who arranged to have him perform at the opera house. Except, there was a miscommunication and only a small, broken rehearsal piano was available. As the story goes, she approached him as he waited to be taken back to his hotel, the concert was canceled and she somehow convinced him to play on the nearly unplayable instrument. The result is the Köln Concert, one of the greatest jazz performances in history. It was perfectly imperfect.
Yes, even the 1-kg sphere has femtogram quantities of other elements mixed in – the universal standard for perfect is itself, imperfect. It doesn’t matter. It’s the pursuit of such that makes life worthwhile. There’s always tomorrow. Have your coffee grinders ready.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Motionless, every Olympic skater starts off perfectly. Once the music starts, it’s up to them whether they will continue on perfectly or not. In this way, you’re just like an Olympic skater. Each day, a skating program. The music starts the moment your foot touches the floor in the morning. It’s up to you if the rest of the day will continue on flawlessly or not. To this point, I’ve yet to have a perfect day.
If I’m honest, my “perfect day” streak typically ends once I’ve made coffee. By then, I’ll have spilled a few grains of grounds or clinked mugs together when taking one from the cupboard. (D’oh!) Hardly ever can I make it to backing out of the driveway, let alone through a patient encounter. I’ve had a few procedures that when complete I’ve thought, “well, that looks great.” I can remember encounters that went brilliantly despite a high technical difficulty. I’ve also tagged a 7-iron shot 160 downwind yards to within inches of the cup. But I’ve hardly ever done anything in my life perfectly.
What does it mean to be perfect? Well, there have been 23 perfect baseball games. In 1972, the Miami Dolphins had the only perfect NFL season, 14-0 (although my 2007 Patriots went 18-0 before losing to the – ugh – Giants). Every year, several hundred students score a perfect 1600 on the SAT. In an underground vault somewhere in France is a perfect sphere, a perfectly spherical 1-kg mass of pure silicon. There are at least 51 perfect numbers. And model Bella Hadid’s exactly 1.62-ratioed face is said to be perfectly beautiful. But yet, U.S. skater Nathan Chen’s seemingly flawless 113.97-point short program in Beijing, still imperfect.
Attempting a perfect day or perfect surgery or a perfect pour over coffee is a fun game, but perfectionism has an insidious side. Some of us feel this way every day: We must do it exactly right, every time. Even an insignificant imperfection or error feels like failure. A 3.90 GPA is a fail. 515 on the MCAT, not nearly good enough. For them, the burden of perfection is crushing. It is hard for some to recognize that even if your performance could not be improved, the outcome can still be flawed. A chip in the ice, a patient showing up late, an interviewer with an agenda, a missed referee call can all flub up an otherwise flawless day. It isn’t necessary to abandon hope, all ye who live in the real world. Although achieving perfection is usually impossible, reward comes from the pursuit of perfection, not from holding it. It is called perfectionistic striving and in contrast to perfectionistic concerns, it is associated with resilience and positive mood. To do so you must combine giving your all with acceptance of whatever the outcome.
Keith Jarrett is one of the greatest jazz pianists of all time. He is a true perfectionist, precise in his standards and exacting in expectations. In 1975 in Cologne, Germany, he agreed to play at the behest of a teenage girl who arranged to have him perform at the opera house. Except, there was a miscommunication and only a small, broken rehearsal piano was available. As the story goes, she approached him as he waited to be taken back to his hotel, the concert was canceled and she somehow convinced him to play on the nearly unplayable instrument. The result is the Köln Concert, one of the greatest jazz performances in history. It was perfectly imperfect.
Yes, even the 1-kg sphere has femtogram quantities of other elements mixed in – the universal standard for perfect is itself, imperfect. It doesn’t matter. It’s the pursuit of such that makes life worthwhile. There’s always tomorrow. Have your coffee grinders ready.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Eighteen-year study shows inconsistencies in treating, classifying JIA
“Children are not little adults” is a common refrain in pediatric medicine, but when it comes to a condition like juvenile idiopathic arthritis (JIA), rheumatologists might be better off treating pediatric and adult rheumatic disease more similarly.
A recent study published in Arthritis Care & Research followed children diagnosed with JIA for 18 years. Although not the first long-term study to examine children with JIA, it is unique in that it took place “during a time where biologic DMARDs [disease-modifying antirheumatic drugs] were emerging as a fundamental therapy in the management of children with JIA,” said Dawn M. Wahezi, MD, chief of the division of pediatric rheumatology at the Children’s Hospital at Montefiore in New York, who was not involved with the study.
Additionally, the study highlights the International League of Associations for Rheumatology (ILAR) consensus-based classification criteria as an imperfect method to categorize patients with JIA.
Mia Glerup, MD, PhD, of the department of pediatrics at Aarhus (Denmark) University Hospital and colleagues prospectively analyzed 373 patients from Denmark, Norway, Sweden, and Finland with new-onset JIA between 1997 and 2000 and evaluated them at baseline, 8 years, and 18 years. At each visit, the researchers collected data on demographics, disease activity, ILAR category, treatment, and blood samples.
Patients in the cohort were mostly girls (66.7%) with a median age of 5.9 years at onset. Approximately one-third (34.8%) of patients were antinuclear antibody (ANA) positive and 21.6% were HLA-B27 positive. The most common JIA categories at baseline were persistent oligoarthritis (53.9%), polyarticular rheumatoid factor (RF) negative (21.1%), and undifferentiated arthritis (10.2%).
Dr. Glerup and colleagues found that the proportion of patients not receiving DMARDs declined from 73.2% at baseline to 59.7% at 8 years, and then rose again to 70% at 18 years (risk ratio, 1.3; P = .003). The group of 103 patients who used conventional DMARDs (cDMARDs) either as monotherapy or in combination with a biologic DMARD (bDMARD) at 8 years dwindled to 44 (42.7%) at 18 years (RR, 0.4; P < .001), whereas 32 of 52 patients (61.5%) using bDMARDs at 8 years were still taking them at 18 years (RR, 0.6; P = .02). Across the whole study, 14.7% of patients never received any JIA treatment, and 33 of 85 patients (38.8%) on continuous DMARDs developed uveitis during the study period.
Overall, 62.7% of patients received DMARDs at least once, including 89.7% with polyarticular RF negative, 77.3% with oligoarticular extended, 76.9% with systemic, 75.7% with juvenile enthesitis-related arthritis (ERA), 66.7% with polyarticular RF-positive, 65.2% with juvenile psoriatic arthritis (JPsA), 58.9% with undifferentiated JIA, and 27.6% of patients with persistent oligoarticular disease.
The median number of active joints dropped from 3 (range, 1-30) at baseline to 0 at 8 years (range, 0-13), whereas the median cumulative number of affected joints rose from 3 at baseline (range, 1-30) to 6 at 8 years (range, 1-41). At last follow-up, the median number of active joints was 0 (range, 0-5) and median cumulative number of affected joints was 7 (range, 1-47). The percentage of patients in remission barely changed from 52% at 8 years to 51% at 18.
Some patients also changed ILAR categories during the study period, with 7% shifting between baseline and 8 years, and 11% shifting between 8-year and 18-year follow-up. Compared with baseline, by the 18-year follow-up time point there was a significant decrease in the number of patients categorized as oligoarticular (230 vs. 197 patients; P = .02), a significant increase in patients in the psoriatic ILAR category (8 vs. 28 patients; P < .001), and a nonsignificant increase in the number of patients in the undifferentiated category (45 vs. 63 patients; P = .06).
“Almost half of the changes in the distribution between the ILAR categories were caused by updated information on heredity in a first-degree relative obtained at the follow-up visits,” Dr. Glerup and colleagues write.
The results of the long-term study show that patients are “likely to remain in remission – with the converse also evident, as patients still with evidence of disease activity at 8 years after disease onset were more likely to have refractory disease,” Dr. Wahezi said.
Commenting on the study’s findings, Lisa F. Imundo, MD, director of adolescent rheumatology at Columbia University Medical Center in New York, said they are “great news to be able to give parents of young kids with arthritis.” However, she questioned whether the results are generalizable to populations of patients “who are in the worst prognostic group.”
For example, a substantial proportion of patients were classified under the oligoarticular category. “That’s already a group that we know from experience tends to have a better outcome than some of the other groups of JIA,” she said.
“That kind of weaves its way through the whole study, because then they show a lot of patients have come off their medication. Patients who had more severe disease in more joints would be less likely, I think, to just stop their medication and stop going to doctors,” Dr. Imundo explained.
Although the study is valuable for its long-term follow-up, there is also a question of generalizability across a more diverse ethnic and racial group. The authors do not elaborate on the racial breakdown of their patients, Dr. Imundo said, “so we’re going to have to assume that the vast majority are going to [have] Caucasian Nordic ethnic background, and that goes along with them having this high percentage of HLA-B27 positivity, which is a gene that’s more prevalent in northern European populations.”
Jonathan Hausmann, MD, a pediatric and adult rheumatologist at Boston Children’s Hospital, Boston,, told this news organization that he believes the overall conclusions from the study – that JIA persists over time and that ILAR classification is a somewhat imprecise measure of assessing JIA types in children – would be generalizable to other groups.
However, long-term registries evaluating JIA in more diverse populations, such as the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry, could confirm these results, said Dr. Hausmann, who is a registry informatics associate with CARRA and was not associated with the research.
Long-term management of JIA
In an accompanying editorial, Jaime Guzman, MD, MSc, and Ross E. Petty, MD, PhD, of British Columbia Children’s Hospital and the University of British Columbia, Vancouver, said a rheumatologist’s interpretation of the study would be tied to what they learned about children with arthritis in medical school. They would see the glass as “half full” if children who achieved remission stayed in remission if they learned that a child might end up outgrowing JIA but potentially develop lifelong disability, whereas others may focus on the outcome of approximately half of patients not achieving remission.
“When I was going through medical school, I remember learning that JIA is a disease of children, and typically, they outgrow it as they become adults,” Dr. Hausmann said. “I think this study and many other studies have shown that that’s actually not the case – that, in fact, it may be a majority of kids continue having active disease even through adulthood.”
If a rheumatologist knows JIA is likely to continue into adulthood, “that’s huge,” Dr. Hausmann said. “That means when we first diagnose patients with JIA as kids, we need to set expectations with the families that this may not just go away; this may be something that could be more lifelong.”
Education on the part of the patient, their parents, and their clinician on the expected trajectory of the disease is critical so that children can continue their own care as they transition to adulthood, Dr. Hausmann explained. “The earlier the kids develop the skills to discuss their medicines, their side effects, the better they’ll be able to transition to adult medicine,” he said.
For the patients who go into remission and stay in remission, the message is also important. “To have the reassurance that a lot of those kids won’t be having active joint symptoms or need to be on medication, that’s a huge positive message that can get out there, so I think that’s great,” Dr. Imundo said.
Time to move on from ILAR classification?
Another big takeaway from the study was how patients’ ILAR classification changed across the 18-year follow-up. First proposed in 1995, the JIA ILAR classification has been revised several times for clarification purposes. In its current form, the ILAR classification considers a patient’s history when categorizing JIA types but also includes factors such as immediate family history. This system of assessing JIA has been criticized and there are initiatives to create a new JIA classification system to replace it.
“The ILAR criteria were designed to classify patients 6 months after disease onset in an attempt to find some commonality in clinical phenotypes, prognosis, and suggested management,” Dr. Wahezi said. “While there continues to be debate as to whether we can improve our classification of JIA patients, it is not surprising that phenotypes may evolve over time as new clinical features develop. As pediatric rheumatologists, we are well accustomed to having to modify management plans as children manifest with new clinical features over time.”
Although the percentage of patients who switched ILAR classifications over the study period was “much higher” than she would have thought, Dr. Imundo said it was the reasons provided in the study that seemed odd to her. “The classification scheme relies on your family history, like someone else in your family now has psoriasis, so your arthritis classification changes,” she explained.
“We want to head toward a much more unified classification scheme, a simpler one. We now understand that some of the diseases that we see in pediatrics are really the equivalent or same disease in adults,” she said.
“Most of the pediatric categories of JIA have distinct adult correlates,” Dr. Hausmann agreed. RF-positive polyarthritis in children and rheumatoid arthritis in adults are correlated, as are systemic JIA and adult-onset Still’s disease, he explained. “That has been borne out also by genetic susceptibility studies that the genetic predispositions to systemic arthritis in children is the same as the genetic predisposition to adult-onset Still’s disease in adults. By and large, there are a lot of similarities between the two.
“I think we need to incorporate some of that knowledge in better classifying kids with JIA so that we can find the best treatments and the best outcomes, and we can provide information to families about the expected course of the disease over time so that can inform our discussions.”
Some pediatric rheumatologists accept the classification system is flawed, but not all concur with the degree to which these problems impact patient care. “While the ILAR classification criteria may be subject to criticism, it does provide general context and prognostic implications for patients and families,” Dr. Wahezi said.
“The medicines certainly are very similar across the JIA categories, so the implications are not as broad” when classification changes,” Dr. Hausmann said. “But it certainly shows that there are things that we still don’t know. I think classification is actually pretty important because it might give you a sense of how persistent the disease will be.”
Dr. Imundo said the ILAR classification’s “time is limited,” and rheumatologists may soon need to adopt a new way of classifying children with rheumatic disease – “a more data-driven, genetics-driven scheme.”
“These categories are so imperfect, and the patients are changing. I feel like that says to me, let’s find something that’s more predictive that really helps us a little better than what we have now,” she said.
The study had no specific funding. The authors of the study and the editorial have disclosed no relevant financial relationships. Dr. Hausmann reports receiving salary support from CARRA. Dr. Imundo and Dr. Wahezi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Children are not little adults” is a common refrain in pediatric medicine, but when it comes to a condition like juvenile idiopathic arthritis (JIA), rheumatologists might be better off treating pediatric and adult rheumatic disease more similarly.
A recent study published in Arthritis Care & Research followed children diagnosed with JIA for 18 years. Although not the first long-term study to examine children with JIA, it is unique in that it took place “during a time where biologic DMARDs [disease-modifying antirheumatic drugs] were emerging as a fundamental therapy in the management of children with JIA,” said Dawn M. Wahezi, MD, chief of the division of pediatric rheumatology at the Children’s Hospital at Montefiore in New York, who was not involved with the study.
Additionally, the study highlights the International League of Associations for Rheumatology (ILAR) consensus-based classification criteria as an imperfect method to categorize patients with JIA.
Mia Glerup, MD, PhD, of the department of pediatrics at Aarhus (Denmark) University Hospital and colleagues prospectively analyzed 373 patients from Denmark, Norway, Sweden, and Finland with new-onset JIA between 1997 and 2000 and evaluated them at baseline, 8 years, and 18 years. At each visit, the researchers collected data on demographics, disease activity, ILAR category, treatment, and blood samples.
Patients in the cohort were mostly girls (66.7%) with a median age of 5.9 years at onset. Approximately one-third (34.8%) of patients were antinuclear antibody (ANA) positive and 21.6% were HLA-B27 positive. The most common JIA categories at baseline were persistent oligoarthritis (53.9%), polyarticular rheumatoid factor (RF) negative (21.1%), and undifferentiated arthritis (10.2%).
Dr. Glerup and colleagues found that the proportion of patients not receiving DMARDs declined from 73.2% at baseline to 59.7% at 8 years, and then rose again to 70% at 18 years (risk ratio, 1.3; P = .003). The group of 103 patients who used conventional DMARDs (cDMARDs) either as monotherapy or in combination with a biologic DMARD (bDMARD) at 8 years dwindled to 44 (42.7%) at 18 years (RR, 0.4; P < .001), whereas 32 of 52 patients (61.5%) using bDMARDs at 8 years were still taking them at 18 years (RR, 0.6; P = .02). Across the whole study, 14.7% of patients never received any JIA treatment, and 33 of 85 patients (38.8%) on continuous DMARDs developed uveitis during the study period.
Overall, 62.7% of patients received DMARDs at least once, including 89.7% with polyarticular RF negative, 77.3% with oligoarticular extended, 76.9% with systemic, 75.7% with juvenile enthesitis-related arthritis (ERA), 66.7% with polyarticular RF-positive, 65.2% with juvenile psoriatic arthritis (JPsA), 58.9% with undifferentiated JIA, and 27.6% of patients with persistent oligoarticular disease.
The median number of active joints dropped from 3 (range, 1-30) at baseline to 0 at 8 years (range, 0-13), whereas the median cumulative number of affected joints rose from 3 at baseline (range, 1-30) to 6 at 8 years (range, 1-41). At last follow-up, the median number of active joints was 0 (range, 0-5) and median cumulative number of affected joints was 7 (range, 1-47). The percentage of patients in remission barely changed from 52% at 8 years to 51% at 18.
Some patients also changed ILAR categories during the study period, with 7% shifting between baseline and 8 years, and 11% shifting between 8-year and 18-year follow-up. Compared with baseline, by the 18-year follow-up time point there was a significant decrease in the number of patients categorized as oligoarticular (230 vs. 197 patients; P = .02), a significant increase in patients in the psoriatic ILAR category (8 vs. 28 patients; P < .001), and a nonsignificant increase in the number of patients in the undifferentiated category (45 vs. 63 patients; P = .06).
“Almost half of the changes in the distribution between the ILAR categories were caused by updated information on heredity in a first-degree relative obtained at the follow-up visits,” Dr. Glerup and colleagues write.
The results of the long-term study show that patients are “likely to remain in remission – with the converse also evident, as patients still with evidence of disease activity at 8 years after disease onset were more likely to have refractory disease,” Dr. Wahezi said.
Commenting on the study’s findings, Lisa F. Imundo, MD, director of adolescent rheumatology at Columbia University Medical Center in New York, said they are “great news to be able to give parents of young kids with arthritis.” However, she questioned whether the results are generalizable to populations of patients “who are in the worst prognostic group.”
For example, a substantial proportion of patients were classified under the oligoarticular category. “That’s already a group that we know from experience tends to have a better outcome than some of the other groups of JIA,” she said.
“That kind of weaves its way through the whole study, because then they show a lot of patients have come off their medication. Patients who had more severe disease in more joints would be less likely, I think, to just stop their medication and stop going to doctors,” Dr. Imundo explained.
Although the study is valuable for its long-term follow-up, there is also a question of generalizability across a more diverse ethnic and racial group. The authors do not elaborate on the racial breakdown of their patients, Dr. Imundo said, “so we’re going to have to assume that the vast majority are going to [have] Caucasian Nordic ethnic background, and that goes along with them having this high percentage of HLA-B27 positivity, which is a gene that’s more prevalent in northern European populations.”
Jonathan Hausmann, MD, a pediatric and adult rheumatologist at Boston Children’s Hospital, Boston,, told this news organization that he believes the overall conclusions from the study – that JIA persists over time and that ILAR classification is a somewhat imprecise measure of assessing JIA types in children – would be generalizable to other groups.
However, long-term registries evaluating JIA in more diverse populations, such as the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry, could confirm these results, said Dr. Hausmann, who is a registry informatics associate with CARRA and was not associated with the research.
Long-term management of JIA
In an accompanying editorial, Jaime Guzman, MD, MSc, and Ross E. Petty, MD, PhD, of British Columbia Children’s Hospital and the University of British Columbia, Vancouver, said a rheumatologist’s interpretation of the study would be tied to what they learned about children with arthritis in medical school. They would see the glass as “half full” if children who achieved remission stayed in remission if they learned that a child might end up outgrowing JIA but potentially develop lifelong disability, whereas others may focus on the outcome of approximately half of patients not achieving remission.
“When I was going through medical school, I remember learning that JIA is a disease of children, and typically, they outgrow it as they become adults,” Dr. Hausmann said. “I think this study and many other studies have shown that that’s actually not the case – that, in fact, it may be a majority of kids continue having active disease even through adulthood.”
If a rheumatologist knows JIA is likely to continue into adulthood, “that’s huge,” Dr. Hausmann said. “That means when we first diagnose patients with JIA as kids, we need to set expectations with the families that this may not just go away; this may be something that could be more lifelong.”
Education on the part of the patient, their parents, and their clinician on the expected trajectory of the disease is critical so that children can continue their own care as they transition to adulthood, Dr. Hausmann explained. “The earlier the kids develop the skills to discuss their medicines, their side effects, the better they’ll be able to transition to adult medicine,” he said.
For the patients who go into remission and stay in remission, the message is also important. “To have the reassurance that a lot of those kids won’t be having active joint symptoms or need to be on medication, that’s a huge positive message that can get out there, so I think that’s great,” Dr. Imundo said.
Time to move on from ILAR classification?
Another big takeaway from the study was how patients’ ILAR classification changed across the 18-year follow-up. First proposed in 1995, the JIA ILAR classification has been revised several times for clarification purposes. In its current form, the ILAR classification considers a patient’s history when categorizing JIA types but also includes factors such as immediate family history. This system of assessing JIA has been criticized and there are initiatives to create a new JIA classification system to replace it.
“The ILAR criteria were designed to classify patients 6 months after disease onset in an attempt to find some commonality in clinical phenotypes, prognosis, and suggested management,” Dr. Wahezi said. “While there continues to be debate as to whether we can improve our classification of JIA patients, it is not surprising that phenotypes may evolve over time as new clinical features develop. As pediatric rheumatologists, we are well accustomed to having to modify management plans as children manifest with new clinical features over time.”
Although the percentage of patients who switched ILAR classifications over the study period was “much higher” than she would have thought, Dr. Imundo said it was the reasons provided in the study that seemed odd to her. “The classification scheme relies on your family history, like someone else in your family now has psoriasis, so your arthritis classification changes,” she explained.
“We want to head toward a much more unified classification scheme, a simpler one. We now understand that some of the diseases that we see in pediatrics are really the equivalent or same disease in adults,” she said.
“Most of the pediatric categories of JIA have distinct adult correlates,” Dr. Hausmann agreed. RF-positive polyarthritis in children and rheumatoid arthritis in adults are correlated, as are systemic JIA and adult-onset Still’s disease, he explained. “That has been borne out also by genetic susceptibility studies that the genetic predispositions to systemic arthritis in children is the same as the genetic predisposition to adult-onset Still’s disease in adults. By and large, there are a lot of similarities between the two.
“I think we need to incorporate some of that knowledge in better classifying kids with JIA so that we can find the best treatments and the best outcomes, and we can provide information to families about the expected course of the disease over time so that can inform our discussions.”
Some pediatric rheumatologists accept the classification system is flawed, but not all concur with the degree to which these problems impact patient care. “While the ILAR classification criteria may be subject to criticism, it does provide general context and prognostic implications for patients and families,” Dr. Wahezi said.
“The medicines certainly are very similar across the JIA categories, so the implications are not as broad” when classification changes,” Dr. Hausmann said. “But it certainly shows that there are things that we still don’t know. I think classification is actually pretty important because it might give you a sense of how persistent the disease will be.”
Dr. Imundo said the ILAR classification’s “time is limited,” and rheumatologists may soon need to adopt a new way of classifying children with rheumatic disease – “a more data-driven, genetics-driven scheme.”
“These categories are so imperfect, and the patients are changing. I feel like that says to me, let’s find something that’s more predictive that really helps us a little better than what we have now,” she said.
The study had no specific funding. The authors of the study and the editorial have disclosed no relevant financial relationships. Dr. Hausmann reports receiving salary support from CARRA. Dr. Imundo and Dr. Wahezi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Children are not little adults” is a common refrain in pediatric medicine, but when it comes to a condition like juvenile idiopathic arthritis (JIA), rheumatologists might be better off treating pediatric and adult rheumatic disease more similarly.
A recent study published in Arthritis Care & Research followed children diagnosed with JIA for 18 years. Although not the first long-term study to examine children with JIA, it is unique in that it took place “during a time where biologic DMARDs [disease-modifying antirheumatic drugs] were emerging as a fundamental therapy in the management of children with JIA,” said Dawn M. Wahezi, MD, chief of the division of pediatric rheumatology at the Children’s Hospital at Montefiore in New York, who was not involved with the study.
Additionally, the study highlights the International League of Associations for Rheumatology (ILAR) consensus-based classification criteria as an imperfect method to categorize patients with JIA.
Mia Glerup, MD, PhD, of the department of pediatrics at Aarhus (Denmark) University Hospital and colleagues prospectively analyzed 373 patients from Denmark, Norway, Sweden, and Finland with new-onset JIA between 1997 and 2000 and evaluated them at baseline, 8 years, and 18 years. At each visit, the researchers collected data on demographics, disease activity, ILAR category, treatment, and blood samples.
Patients in the cohort were mostly girls (66.7%) with a median age of 5.9 years at onset. Approximately one-third (34.8%) of patients were antinuclear antibody (ANA) positive and 21.6% were HLA-B27 positive. The most common JIA categories at baseline were persistent oligoarthritis (53.9%), polyarticular rheumatoid factor (RF) negative (21.1%), and undifferentiated arthritis (10.2%).
Dr. Glerup and colleagues found that the proportion of patients not receiving DMARDs declined from 73.2% at baseline to 59.7% at 8 years, and then rose again to 70% at 18 years (risk ratio, 1.3; P = .003). The group of 103 patients who used conventional DMARDs (cDMARDs) either as monotherapy or in combination with a biologic DMARD (bDMARD) at 8 years dwindled to 44 (42.7%) at 18 years (RR, 0.4; P < .001), whereas 32 of 52 patients (61.5%) using bDMARDs at 8 years were still taking them at 18 years (RR, 0.6; P = .02). Across the whole study, 14.7% of patients never received any JIA treatment, and 33 of 85 patients (38.8%) on continuous DMARDs developed uveitis during the study period.
Overall, 62.7% of patients received DMARDs at least once, including 89.7% with polyarticular RF negative, 77.3% with oligoarticular extended, 76.9% with systemic, 75.7% with juvenile enthesitis-related arthritis (ERA), 66.7% with polyarticular RF-positive, 65.2% with juvenile psoriatic arthritis (JPsA), 58.9% with undifferentiated JIA, and 27.6% of patients with persistent oligoarticular disease.
The median number of active joints dropped from 3 (range, 1-30) at baseline to 0 at 8 years (range, 0-13), whereas the median cumulative number of affected joints rose from 3 at baseline (range, 1-30) to 6 at 8 years (range, 1-41). At last follow-up, the median number of active joints was 0 (range, 0-5) and median cumulative number of affected joints was 7 (range, 1-47). The percentage of patients in remission barely changed from 52% at 8 years to 51% at 18.
Some patients also changed ILAR categories during the study period, with 7% shifting between baseline and 8 years, and 11% shifting between 8-year and 18-year follow-up. Compared with baseline, by the 18-year follow-up time point there was a significant decrease in the number of patients categorized as oligoarticular (230 vs. 197 patients; P = .02), a significant increase in patients in the psoriatic ILAR category (8 vs. 28 patients; P < .001), and a nonsignificant increase in the number of patients in the undifferentiated category (45 vs. 63 patients; P = .06).
“Almost half of the changes in the distribution between the ILAR categories were caused by updated information on heredity in a first-degree relative obtained at the follow-up visits,” Dr. Glerup and colleagues write.
The results of the long-term study show that patients are “likely to remain in remission – with the converse also evident, as patients still with evidence of disease activity at 8 years after disease onset were more likely to have refractory disease,” Dr. Wahezi said.
Commenting on the study’s findings, Lisa F. Imundo, MD, director of adolescent rheumatology at Columbia University Medical Center in New York, said they are “great news to be able to give parents of young kids with arthritis.” However, she questioned whether the results are generalizable to populations of patients “who are in the worst prognostic group.”
For example, a substantial proportion of patients were classified under the oligoarticular category. “That’s already a group that we know from experience tends to have a better outcome than some of the other groups of JIA,” she said.
“That kind of weaves its way through the whole study, because then they show a lot of patients have come off their medication. Patients who had more severe disease in more joints would be less likely, I think, to just stop their medication and stop going to doctors,” Dr. Imundo explained.
Although the study is valuable for its long-term follow-up, there is also a question of generalizability across a more diverse ethnic and racial group. The authors do not elaborate on the racial breakdown of their patients, Dr. Imundo said, “so we’re going to have to assume that the vast majority are going to [have] Caucasian Nordic ethnic background, and that goes along with them having this high percentage of HLA-B27 positivity, which is a gene that’s more prevalent in northern European populations.”
Jonathan Hausmann, MD, a pediatric and adult rheumatologist at Boston Children’s Hospital, Boston,, told this news organization that he believes the overall conclusions from the study – that JIA persists over time and that ILAR classification is a somewhat imprecise measure of assessing JIA types in children – would be generalizable to other groups.
However, long-term registries evaluating JIA in more diverse populations, such as the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry, could confirm these results, said Dr. Hausmann, who is a registry informatics associate with CARRA and was not associated with the research.
Long-term management of JIA
In an accompanying editorial, Jaime Guzman, MD, MSc, and Ross E. Petty, MD, PhD, of British Columbia Children’s Hospital and the University of British Columbia, Vancouver, said a rheumatologist’s interpretation of the study would be tied to what they learned about children with arthritis in medical school. They would see the glass as “half full” if children who achieved remission stayed in remission if they learned that a child might end up outgrowing JIA but potentially develop lifelong disability, whereas others may focus on the outcome of approximately half of patients not achieving remission.
“When I was going through medical school, I remember learning that JIA is a disease of children, and typically, they outgrow it as they become adults,” Dr. Hausmann said. “I think this study and many other studies have shown that that’s actually not the case – that, in fact, it may be a majority of kids continue having active disease even through adulthood.”
If a rheumatologist knows JIA is likely to continue into adulthood, “that’s huge,” Dr. Hausmann said. “That means when we first diagnose patients with JIA as kids, we need to set expectations with the families that this may not just go away; this may be something that could be more lifelong.”
Education on the part of the patient, their parents, and their clinician on the expected trajectory of the disease is critical so that children can continue their own care as they transition to adulthood, Dr. Hausmann explained. “The earlier the kids develop the skills to discuss their medicines, their side effects, the better they’ll be able to transition to adult medicine,” he said.
For the patients who go into remission and stay in remission, the message is also important. “To have the reassurance that a lot of those kids won’t be having active joint symptoms or need to be on medication, that’s a huge positive message that can get out there, so I think that’s great,” Dr. Imundo said.
Time to move on from ILAR classification?
Another big takeaway from the study was how patients’ ILAR classification changed across the 18-year follow-up. First proposed in 1995, the JIA ILAR classification has been revised several times for clarification purposes. In its current form, the ILAR classification considers a patient’s history when categorizing JIA types but also includes factors such as immediate family history. This system of assessing JIA has been criticized and there are initiatives to create a new JIA classification system to replace it.
“The ILAR criteria were designed to classify patients 6 months after disease onset in an attempt to find some commonality in clinical phenotypes, prognosis, and suggested management,” Dr. Wahezi said. “While there continues to be debate as to whether we can improve our classification of JIA patients, it is not surprising that phenotypes may evolve over time as new clinical features develop. As pediatric rheumatologists, we are well accustomed to having to modify management plans as children manifest with new clinical features over time.”
Although the percentage of patients who switched ILAR classifications over the study period was “much higher” than she would have thought, Dr. Imundo said it was the reasons provided in the study that seemed odd to her. “The classification scheme relies on your family history, like someone else in your family now has psoriasis, so your arthritis classification changes,” she explained.
“We want to head toward a much more unified classification scheme, a simpler one. We now understand that some of the diseases that we see in pediatrics are really the equivalent or same disease in adults,” she said.
“Most of the pediatric categories of JIA have distinct adult correlates,” Dr. Hausmann agreed. RF-positive polyarthritis in children and rheumatoid arthritis in adults are correlated, as are systemic JIA and adult-onset Still’s disease, he explained. “That has been borne out also by genetic susceptibility studies that the genetic predispositions to systemic arthritis in children is the same as the genetic predisposition to adult-onset Still’s disease in adults. By and large, there are a lot of similarities between the two.
“I think we need to incorporate some of that knowledge in better classifying kids with JIA so that we can find the best treatments and the best outcomes, and we can provide information to families about the expected course of the disease over time so that can inform our discussions.”
Some pediatric rheumatologists accept the classification system is flawed, but not all concur with the degree to which these problems impact patient care. “While the ILAR classification criteria may be subject to criticism, it does provide general context and prognostic implications for patients and families,” Dr. Wahezi said.
“The medicines certainly are very similar across the JIA categories, so the implications are not as broad” when classification changes,” Dr. Hausmann said. “But it certainly shows that there are things that we still don’t know. I think classification is actually pretty important because it might give you a sense of how persistent the disease will be.”
Dr. Imundo said the ILAR classification’s “time is limited,” and rheumatologists may soon need to adopt a new way of classifying children with rheumatic disease – “a more data-driven, genetics-driven scheme.”
“These categories are so imperfect, and the patients are changing. I feel like that says to me, let’s find something that’s more predictive that really helps us a little better than what we have now,” she said.
The study had no specific funding. The authors of the study and the editorial have disclosed no relevant financial relationships. Dr. Hausmann reports receiving salary support from CARRA. Dr. Imundo and Dr. Wahezi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS CARE & RESEARCH
Tips for connecting with your patients
It is a tough time to be a doctor. With the stresses of the pandemic, the continued unfettered rise of insurance company BS, and so many medical groups being bought up that we often don’t even know who makes the decisions, the patient can sometimes be hidden in the equation.
Be curious
When physicians are curious about why patients have symptoms, how those symptoms will affect their lives, and how worried the patient is about them, patients feel cared about.
Ascertaining how concerned patients are about their symptoms will help you make decisions on whether symptoms you are not concerned about actually need to be treated.
Limit use of EHRs when possible
Use of the electronic health record during visits is essential, but focusing on it too much can put a barrier between the physician and the patient.
Marmor and colleagues found there is an inverse relationship between time spent on the EHR by a patient’s physician and the patient’s satisfaction.1
Eye contact with the patient is important, especially when patients are sharing concerns they are scared about and upsetting experiences. There can be awkward pauses when looking things up on the EHR. Fill those pauses by explaining to the patient what you are doing, or chatting with the patient.
Consider teaching medical students
When a medical student works with you, it doubles the time the patient gets with a concerned listener. Students also can do a great job with timely follow-up and checking in with worried patients.
By having the student present in the clinic room, with the patient present, the patient can really feel heard. The student shares all the details the patient shared, and now their physician is hearing an organized, thoughtful report of the patients concerns.
In fact, I was involved in a study that showed that patients preferred in room presentations, and that they were more satisfied when students presented in the room.2
Use healing words
Some words carry loaded emotions. The word chronic, for example, has negative connotations, whereas the term persisting does not.
I will often ask patients how long they have been suffering from a symptom to imply my concern for what they are going through. The term “chief complaint” is outdated, and upsets patients when they see it in their medical record.
As a patient of mine once said to me: “I never complained about that problem, I just brought it to your attention.” No one wants to be seen as a complainer. Substituting the word concern for complaint works well.
Explain as you examine
People love to hear the term normal. When you are examining a patient, let them know when findings are normal.
I also find it helpful to explain to patients why I am doing certain physical exam maneuvers. This helps them assess how thorough we are in our thought process.
When patients feel their physicians are thorough, they have more confidence in them.
In summary
- Be curious.
- Do not overly focus on the EHR.
- Consider teaching a medical student.
- Be careful of word choice.
- “Overexplain” the physical exam.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Marmor RA et al. Appl Clin Inform. 2018 Jan;9(1):11-4.
2. Rogers HD et al. Acad Med. 2003 Sep;78(9):945-9.
It is a tough time to be a doctor. With the stresses of the pandemic, the continued unfettered rise of insurance company BS, and so many medical groups being bought up that we often don’t even know who makes the decisions, the patient can sometimes be hidden in the equation.
Be curious
When physicians are curious about why patients have symptoms, how those symptoms will affect their lives, and how worried the patient is about them, patients feel cared about.
Ascertaining how concerned patients are about their symptoms will help you make decisions on whether symptoms you are not concerned about actually need to be treated.
Limit use of EHRs when possible
Use of the electronic health record during visits is essential, but focusing on it too much can put a barrier between the physician and the patient.
Marmor and colleagues found there is an inverse relationship between time spent on the EHR by a patient’s physician and the patient’s satisfaction.1
Eye contact with the patient is important, especially when patients are sharing concerns they are scared about and upsetting experiences. There can be awkward pauses when looking things up on the EHR. Fill those pauses by explaining to the patient what you are doing, or chatting with the patient.
Consider teaching medical students
When a medical student works with you, it doubles the time the patient gets with a concerned listener. Students also can do a great job with timely follow-up and checking in with worried patients.
By having the student present in the clinic room, with the patient present, the patient can really feel heard. The student shares all the details the patient shared, and now their physician is hearing an organized, thoughtful report of the patients concerns.
In fact, I was involved in a study that showed that patients preferred in room presentations, and that they were more satisfied when students presented in the room.2
Use healing words
Some words carry loaded emotions. The word chronic, for example, has negative connotations, whereas the term persisting does not.
I will often ask patients how long they have been suffering from a symptom to imply my concern for what they are going through. The term “chief complaint” is outdated, and upsets patients when they see it in their medical record.
As a patient of mine once said to me: “I never complained about that problem, I just brought it to your attention.” No one wants to be seen as a complainer. Substituting the word concern for complaint works well.
Explain as you examine
People love to hear the term normal. When you are examining a patient, let them know when findings are normal.
I also find it helpful to explain to patients why I am doing certain physical exam maneuvers. This helps them assess how thorough we are in our thought process.
When patients feel their physicians are thorough, they have more confidence in them.
In summary
- Be curious.
- Do not overly focus on the EHR.
- Consider teaching a medical student.
- Be careful of word choice.
- “Overexplain” the physical exam.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Marmor RA et al. Appl Clin Inform. 2018 Jan;9(1):11-4.
2. Rogers HD et al. Acad Med. 2003 Sep;78(9):945-9.
It is a tough time to be a doctor. With the stresses of the pandemic, the continued unfettered rise of insurance company BS, and so many medical groups being bought up that we often don’t even know who makes the decisions, the patient can sometimes be hidden in the equation.
Be curious
When physicians are curious about why patients have symptoms, how those symptoms will affect their lives, and how worried the patient is about them, patients feel cared about.
Ascertaining how concerned patients are about their symptoms will help you make decisions on whether symptoms you are not concerned about actually need to be treated.
Limit use of EHRs when possible
Use of the electronic health record during visits is essential, but focusing on it too much can put a barrier between the physician and the patient.
Marmor and colleagues found there is an inverse relationship between time spent on the EHR by a patient’s physician and the patient’s satisfaction.1
Eye contact with the patient is important, especially when patients are sharing concerns they are scared about and upsetting experiences. There can be awkward pauses when looking things up on the EHR. Fill those pauses by explaining to the patient what you are doing, or chatting with the patient.
Consider teaching medical students
When a medical student works with you, it doubles the time the patient gets with a concerned listener. Students also can do a great job with timely follow-up and checking in with worried patients.
By having the student present in the clinic room, with the patient present, the patient can really feel heard. The student shares all the details the patient shared, and now their physician is hearing an organized, thoughtful report of the patients concerns.
In fact, I was involved in a study that showed that patients preferred in room presentations, and that they were more satisfied when students presented in the room.2
Use healing words
Some words carry loaded emotions. The word chronic, for example, has negative connotations, whereas the term persisting does not.
I will often ask patients how long they have been suffering from a symptom to imply my concern for what they are going through. The term “chief complaint” is outdated, and upsets patients when they see it in their medical record.
As a patient of mine once said to me: “I never complained about that problem, I just brought it to your attention.” No one wants to be seen as a complainer. Substituting the word concern for complaint works well.
Explain as you examine
People love to hear the term normal. When you are examining a patient, let them know when findings are normal.
I also find it helpful to explain to patients why I am doing certain physical exam maneuvers. This helps them assess how thorough we are in our thought process.
When patients feel their physicians are thorough, they have more confidence in them.
In summary
- Be curious.
- Do not overly focus on the EHR.
- Consider teaching a medical student.
- Be careful of word choice.
- “Overexplain” the physical exam.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Marmor RA et al. Appl Clin Inform. 2018 Jan;9(1):11-4.
2. Rogers HD et al. Acad Med. 2003 Sep;78(9):945-9.
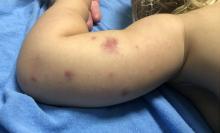
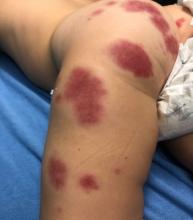
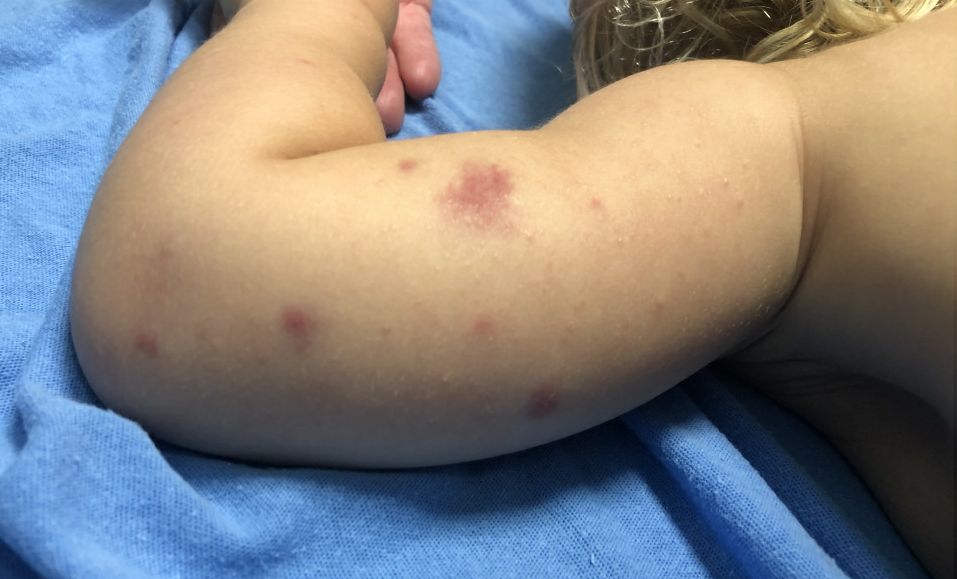
A 19-month-old vaccinated female with 2 days of rash
Acute hemorrhagic edema of infancy (AHEI) is a leukocytoclastic vasculitis that typically affects children between 4 months and 2 years of age.1 Etiology is unknown but the majority of cases are preceded by infections, vaccinations, or certain medications.2
AHEI is a self-limited disease that runs a benign course with spontaneous resolution within days to 3 weeks.3 Classic presentation involves acute onset of fever, purpura, ecchymosis, and inflammatory edema. Edema is often the first sign, and may involve the face, ears, scrotum, or extremities. Hemorrhagic lesions may vary in size but often coalesce and present in a distinctive “cockade” or rosette pattern with scalloped borders. Systemic manifestations are rare, but renal and joint involvement may occur.4 Despite the dramatic and sometimes extensive appearance of the dermatologic manifestations, patients with AHEI are usually not in significant distress.
Diagnosis is clinical, but skin biopsy may show leukocytoclastic vasculitis of the superficial small vessels with infiltrations of neutrophils, extravasation of red blood cells, and fibrinoid necrosis.5 In most cases, immunofluorescence is negative for perivascular IgA deposition. Treatment is symptomatic as the disease resolves spontaneously. Recurrence is uncommon but may occur, and usually occurs early.
What is on the differential?
Kawasaki disease. Similar to AHEI, patients with Kawasaki disease also may present with facial and extremity edema. However, patients with Kawasaki disease appear sicker, have associated lymphadenopathy, conjunctivitis, and fever longer than 5 days. The lack of elevated inflammatory markers, acute-onset, classic dermatologic lesions, and nontoxic appearance in our patient rule out Kawasaki disease and make AHEI more likely.
IgA vasculitis/Henoch-Schönlein purpura. The distinction between AHEI and Henoch-Schönlein purpura is among the most challenging. AHEI commonly afflicts younger children ranging from 4 months to 2 years, whereas Henoch-Schönlein purpura occurs in older children from 3 to 6 years of age. Visceral involvement is rare in AHEI, but frequently presents in Henoch-Schönlein purpura with gastrointestinal and renal complications. Although our patient had both mild renal involvement and a distribution primarily on the buttocks and lower limbs, similar to the classic distribution of Henoch-Schönlein purpura, the younger age and lack of gastrointestinal and arthritic manifestations make AHEI more likely.
Gianotti-Crosti syndrome. Gianotti-Crosti syndrome, also known as papulovesicular acrodermatitis of childhood, mainly affects children between the ages of 6 months and 12 years. Like AHEI, Gianotti-Crosti is a self-limiting condition likely triggered by viral infection or immunization. However, Gianotti-Crosti is characterized by a papular rash that may last for several weeks. Neither AHEI nor Gianotti-Crosti are pruritic, but patients with Gianotti-Crosti tend to have either inguinal or axillary lymphadenopathy. Our patient’s large, coalescing dusky red patches and edematous plaques without lymphadenopathy are more consistent with AHEI.
Erythema multiforme. Erythema multiforme is an acute, immune-mediated condition characterized by distinctive target-like lesions on the skin often accompanied by erosions or bullae. Unlike AHEI, erythema multiforme can involve the oral, genital, and/or ocular mucosae. Erythema multiforme is rare before the age of 4 years. Although the targetoid or annular purpuric configuration of erythema multiforme may present similarly to AHEI in some cases, the young age of our patient and the lack of mucosal involvement make AHEI more likely.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Ms. Kleinman is a pediatric dermatology research associate at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Dr. Matiz nor Ms. Kleinman has any relevant financial disclosures.
References
1. Savino F et al. Pediatr Dermatol. 2013;30(6):e149-e152.
2. Carboni E et al. F1000Res. 2019;8:1771. 2019 Oct 17.
3. Fiore E et al. J Am Acad Dermatol. 2008;59(4):684-95.
4. Watanabe T and Sato Y. Pediatr Nephrol. 2007;22(11):1979-81.
5. Cunha DF et al. Autops Case Rep. 2015;5(3):37-41.
Acute hemorrhagic edema of infancy (AHEI) is a leukocytoclastic vasculitis that typically affects children between 4 months and 2 years of age.1 Etiology is unknown but the majority of cases are preceded by infections, vaccinations, or certain medications.2
AHEI is a self-limited disease that runs a benign course with spontaneous resolution within days to 3 weeks.3 Classic presentation involves acute onset of fever, purpura, ecchymosis, and inflammatory edema. Edema is often the first sign, and may involve the face, ears, scrotum, or extremities. Hemorrhagic lesions may vary in size but often coalesce and present in a distinctive “cockade” or rosette pattern with scalloped borders. Systemic manifestations are rare, but renal and joint involvement may occur.4 Despite the dramatic and sometimes extensive appearance of the dermatologic manifestations, patients with AHEI are usually not in significant distress.
Diagnosis is clinical, but skin biopsy may show leukocytoclastic vasculitis of the superficial small vessels with infiltrations of neutrophils, extravasation of red blood cells, and fibrinoid necrosis.5 In most cases, immunofluorescence is negative for perivascular IgA deposition. Treatment is symptomatic as the disease resolves spontaneously. Recurrence is uncommon but may occur, and usually occurs early.
What is on the differential?
Kawasaki disease. Similar to AHEI, patients with Kawasaki disease also may present with facial and extremity edema. However, patients with Kawasaki disease appear sicker, have associated lymphadenopathy, conjunctivitis, and fever longer than 5 days. The lack of elevated inflammatory markers, acute-onset, classic dermatologic lesions, and nontoxic appearance in our patient rule out Kawasaki disease and make AHEI more likely.
IgA vasculitis/Henoch-Schönlein purpura. The distinction between AHEI and Henoch-Schönlein purpura is among the most challenging. AHEI commonly afflicts younger children ranging from 4 months to 2 years, whereas Henoch-Schönlein purpura occurs in older children from 3 to 6 years of age. Visceral involvement is rare in AHEI, but frequently presents in Henoch-Schönlein purpura with gastrointestinal and renal complications. Although our patient had both mild renal involvement and a distribution primarily on the buttocks and lower limbs, similar to the classic distribution of Henoch-Schönlein purpura, the younger age and lack of gastrointestinal and arthritic manifestations make AHEI more likely.
Gianotti-Crosti syndrome. Gianotti-Crosti syndrome, also known as papulovesicular acrodermatitis of childhood, mainly affects children between the ages of 6 months and 12 years. Like AHEI, Gianotti-Crosti is a self-limiting condition likely triggered by viral infection or immunization. However, Gianotti-Crosti is characterized by a papular rash that may last for several weeks. Neither AHEI nor Gianotti-Crosti are pruritic, but patients with Gianotti-Crosti tend to have either inguinal or axillary lymphadenopathy. Our patient’s large, coalescing dusky red patches and edematous plaques without lymphadenopathy are more consistent with AHEI.
Erythema multiforme. Erythema multiforme is an acute, immune-mediated condition characterized by distinctive target-like lesions on the skin often accompanied by erosions or bullae. Unlike AHEI, erythema multiforme can involve the oral, genital, and/or ocular mucosae. Erythema multiforme is rare before the age of 4 years. Although the targetoid or annular purpuric configuration of erythema multiforme may present similarly to AHEI in some cases, the young age of our patient and the lack of mucosal involvement make AHEI more likely.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Ms. Kleinman is a pediatric dermatology research associate at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Dr. Matiz nor Ms. Kleinman has any relevant financial disclosures.
References
1. Savino F et al. Pediatr Dermatol. 2013;30(6):e149-e152.
2. Carboni E et al. F1000Res. 2019;8:1771. 2019 Oct 17.
3. Fiore E et al. J Am Acad Dermatol. 2008;59(4):684-95.
4. Watanabe T and Sato Y. Pediatr Nephrol. 2007;22(11):1979-81.
5. Cunha DF et al. Autops Case Rep. 2015;5(3):37-41.
Acute hemorrhagic edema of infancy (AHEI) is a leukocytoclastic vasculitis that typically affects children between 4 months and 2 years of age.1 Etiology is unknown but the majority of cases are preceded by infections, vaccinations, or certain medications.2
AHEI is a self-limited disease that runs a benign course with spontaneous resolution within days to 3 weeks.3 Classic presentation involves acute onset of fever, purpura, ecchymosis, and inflammatory edema. Edema is often the first sign, and may involve the face, ears, scrotum, or extremities. Hemorrhagic lesions may vary in size but often coalesce and present in a distinctive “cockade” or rosette pattern with scalloped borders. Systemic manifestations are rare, but renal and joint involvement may occur.4 Despite the dramatic and sometimes extensive appearance of the dermatologic manifestations, patients with AHEI are usually not in significant distress.
Diagnosis is clinical, but skin biopsy may show leukocytoclastic vasculitis of the superficial small vessels with infiltrations of neutrophils, extravasation of red blood cells, and fibrinoid necrosis.5 In most cases, immunofluorescence is negative for perivascular IgA deposition. Treatment is symptomatic as the disease resolves spontaneously. Recurrence is uncommon but may occur, and usually occurs early.
What is on the differential?
Kawasaki disease. Similar to AHEI, patients with Kawasaki disease also may present with facial and extremity edema. However, patients with Kawasaki disease appear sicker, have associated lymphadenopathy, conjunctivitis, and fever longer than 5 days. The lack of elevated inflammatory markers, acute-onset, classic dermatologic lesions, and nontoxic appearance in our patient rule out Kawasaki disease and make AHEI more likely.
IgA vasculitis/Henoch-Schönlein purpura. The distinction between AHEI and Henoch-Schönlein purpura is among the most challenging. AHEI commonly afflicts younger children ranging from 4 months to 2 years, whereas Henoch-Schönlein purpura occurs in older children from 3 to 6 years of age. Visceral involvement is rare in AHEI, but frequently presents in Henoch-Schönlein purpura with gastrointestinal and renal complications. Although our patient had both mild renal involvement and a distribution primarily on the buttocks and lower limbs, similar to the classic distribution of Henoch-Schönlein purpura, the younger age and lack of gastrointestinal and arthritic manifestations make AHEI more likely.
Gianotti-Crosti syndrome. Gianotti-Crosti syndrome, also known as papulovesicular acrodermatitis of childhood, mainly affects children between the ages of 6 months and 12 years. Like AHEI, Gianotti-Crosti is a self-limiting condition likely triggered by viral infection or immunization. However, Gianotti-Crosti is characterized by a papular rash that may last for several weeks. Neither AHEI nor Gianotti-Crosti are pruritic, but patients with Gianotti-Crosti tend to have either inguinal or axillary lymphadenopathy. Our patient’s large, coalescing dusky red patches and edematous plaques without lymphadenopathy are more consistent with AHEI.
Erythema multiforme. Erythema multiforme is an acute, immune-mediated condition characterized by distinctive target-like lesions on the skin often accompanied by erosions or bullae. Unlike AHEI, erythema multiforme can involve the oral, genital, and/or ocular mucosae. Erythema multiforme is rare before the age of 4 years. Although the targetoid or annular purpuric configuration of erythema multiforme may present similarly to AHEI in some cases, the young age of our patient and the lack of mucosal involvement make AHEI more likely.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Ms. Kleinman is a pediatric dermatology research associate at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Dr. Matiz nor Ms. Kleinman has any relevant financial disclosures.
References
1. Savino F et al. Pediatr Dermatol. 2013;30(6):e149-e152.
2. Carboni E et al. F1000Res. 2019;8:1771. 2019 Oct 17.
3. Fiore E et al. J Am Acad Dermatol. 2008;59(4):684-95.
4. Watanabe T and Sato Y. Pediatr Nephrol. 2007;22(11):1979-81.
5. Cunha DF et al. Autops Case Rep. 2015;5(3):37-41.
Children and COVID: Weekly cases down by more than half
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
Growth hormone therapy for certain children may help them reach their potential
“Dr. Lilley, you’ll always be my favorite doctor; you helped me grow.”
These were the parting words from the last patient that I treated during my endocrinology fellowship. I had watched this young man grow from a prepubertal 17-year-old to a young man who had reached his predicted family height as I treated his delayed puberty caused by Kallmann syndrome, a problem that had been missed for years. It was the appropriate bookend for my chosen specialty.
Watching children grow and develop into who they were meant to be is one of my favorite things about endocrinology, as well as forming meaningful relationships with families. Treating detectable deficiencies in logical and measurable ways is also extremely satisfying.
Too little testosterone? That’s a problem I can solve. Too much thyroid hormone? There’s a blocker for that! Endocrinology can be a straightforward field, and when all goes well, everyone leaves happy.
Except when they don’t.
Gatekeepers for treatment for children’s growth
“Nice to meet you. We’re here to get growth hormone.”
“We’re here because his pediatrician made us come. We’ve already decided we’re not going to put hormones into his body.”
These are common statements I hear when I first meet new patients whose parents are concerned about their children’s growth. Pediatric endocrinologists, after all, are the usual gatekeepers for this treatment.
Growth hormone (GH) often makes the news for controversial reasons – most commonly for its abuse by elite athletes hoping to exploit its anabolic effects – causing parents to have varied opinions about its possible use in their children.
Some refuse endocrinology referrals at all owing to concerns that we will push daily injections on their children. Others demand referrals for their children of average height, hoping for every perceived advantage.
GH deficiency (GHD) – a condition where the pituitary gland fails to produce enough GH – can occur because of congenital pituitary malformations; anatomic, surgical, or traumatic interruptions to the gland; or enzyme deficiencies leading to faulty production.
GHD is just one reason for poor growth, however.
Growth is one of the most important indicators of health in children. A waning growth rate may be an early symptom of serious problems. In my clinic, I’ve diagnosed severe hypothyroidism in a marathon runner, a brain tumor, celiac disease in a teenager with no gastrointestinal complaints, autoimmune hepatitis, and several other diseases needing treatment in children who show no symptoms other than poor growth.
Barriers to normal growth
Sometimes, the die is cast for children to have barriers to normal growth. Several genetic conditions can lead to poor GH production or response, and GH treatment is often necessary to approximate normal height.
These may include:
- Turner syndrome (in females who are missing an X chromosome in whole or part) should be considered in every girl with abnormally short stature; mosaic forms of the condition may be subtle and lack classic features.
- Noonan syndrome is important to detect owing to the possibility of cardiac or renal malformations that may also occur in this condition, caused by a mutation in one of the genes in the RAS-MAPK pathway.
- Russell-Silver syndrome can cause intrauterine GH restriction and has been traced to uniparental disomy of chromosome 7 or duplications, mutations, or methylation defects in chromosome 11.
- Individuals with Prader-Willi syndrome, which is characterized by low muscle tone, hyperphagia, and hypogonadism, have demonstrated dramatic benefits from GH therapy, primarily in maintaining a normal body mass index.
Children who are small for their gestational age may be GH resistant, and those who do not catch up to their growth curve by the age of 2 years may require GH treatment to reach their height potential.
GH therapy isn’t entirely benign. Rare adverse effects of overtreatment can include slipped capital femoral epiphysis (a fracture to the growth plate) and pseudotumor cerebri (idiopathic intracranial hypertension).
Overtreatment can cause acromegaly, which results in coarsened features and large hands and feet.
When is GH therapy warranted?
“Growth hormone therapy has been denied by her insurer. They want you to fill out an appeal.”
Insurance approval in the United States can be a herculean effort because GH is expensive: Out-of-pocket costs are prohibitive for most people without insurance assistance, ranging from $7,000 to $30,000 annually.
Pediatric endocrinologists aren’t in the business of cosmetic endocrinology. Treatment of idiopathic short stature has been controversial since this became an indication for GH treatment.
GH isn’t always necessary. Diagnosing the underlying cause for poor growth is the most important step.
Often, we find constitutional delay of growth and puberty, or “late bloomers.” This condition is characterized by a delayed bone age (growth plates more open than expected for age) and delayed pubertal onset. These children will often reach a normal height despite starting as some of the smallest of their peers.
However, GH plays other roles in the body than simply propelling height. Children with congenital GHD will require GH treatment to prevent hypoglycemia, especially in infancy.
GH is needed even in adults with fused growth plates for normal lipid metabolism, bone accrual, and maintaining normal muscle mass.
I have noticed marked improvements in muscle tone in many children with developmental delays who are treated with GH, and research supports cognitive benefits for certain populations.
The most common regimens for GH focus on treatment via subcutaneous injection nightly, when GH is naturally produced; sometimes, injections are given six nights out of seven to provide a break or for splitting time between households.
Newer once-weekly formulations have recently received approval, as reported by this news organization, and are coming into use.
Pediatric endocrinologists measure height and follow growth factors closely with visits every 3-6 months. GH levels are not useful outside of provocative diagnostic (stimulation) testing.
Insulinlike growth factor 1 or insulinlike growth factor binding protein levels are analyzed per Tanner stage of puberty to assess appropriate response and to make dose adjustments.
Annual standardized films of the left hand help predict progress and anticipated adult height. Treatment usually persists through puberty until growth plates are closed; if true GHD is noticed, much smaller doses are continued through adulthood.
Regardless, conversations about GH happen with your friendly local pediatric endocrinologist.
We are thrilled to help shepherd patients through their growing age to meet their potential. For more information about GH treatment for children, the MAGIC Foundation is the perfect place to start.
Dr. Lilley is director of the pediatric diabetes and lipid program, Mississippi Center for Advanced Medicine, Madison. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
“Dr. Lilley, you’ll always be my favorite doctor; you helped me grow.”
These were the parting words from the last patient that I treated during my endocrinology fellowship. I had watched this young man grow from a prepubertal 17-year-old to a young man who had reached his predicted family height as I treated his delayed puberty caused by Kallmann syndrome, a problem that had been missed for years. It was the appropriate bookend for my chosen specialty.
Watching children grow and develop into who they were meant to be is one of my favorite things about endocrinology, as well as forming meaningful relationships with families. Treating detectable deficiencies in logical and measurable ways is also extremely satisfying.
Too little testosterone? That’s a problem I can solve. Too much thyroid hormone? There’s a blocker for that! Endocrinology can be a straightforward field, and when all goes well, everyone leaves happy.
Except when they don’t.
Gatekeepers for treatment for children’s growth
“Nice to meet you. We’re here to get growth hormone.”
“We’re here because his pediatrician made us come. We’ve already decided we’re not going to put hormones into his body.”
These are common statements I hear when I first meet new patients whose parents are concerned about their children’s growth. Pediatric endocrinologists, after all, are the usual gatekeepers for this treatment.
Growth hormone (GH) often makes the news for controversial reasons – most commonly for its abuse by elite athletes hoping to exploit its anabolic effects – causing parents to have varied opinions about its possible use in their children.
Some refuse endocrinology referrals at all owing to concerns that we will push daily injections on their children. Others demand referrals for their children of average height, hoping for every perceived advantage.
GH deficiency (GHD) – a condition where the pituitary gland fails to produce enough GH – can occur because of congenital pituitary malformations; anatomic, surgical, or traumatic interruptions to the gland; or enzyme deficiencies leading to faulty production.
GHD is just one reason for poor growth, however.
Growth is one of the most important indicators of health in children. A waning growth rate may be an early symptom of serious problems. In my clinic, I’ve diagnosed severe hypothyroidism in a marathon runner, a brain tumor, celiac disease in a teenager with no gastrointestinal complaints, autoimmune hepatitis, and several other diseases needing treatment in children who show no symptoms other than poor growth.
Barriers to normal growth
Sometimes, the die is cast for children to have barriers to normal growth. Several genetic conditions can lead to poor GH production or response, and GH treatment is often necessary to approximate normal height.
These may include:
- Turner syndrome (in females who are missing an X chromosome in whole or part) should be considered in every girl with abnormally short stature; mosaic forms of the condition may be subtle and lack classic features.
- Noonan syndrome is important to detect owing to the possibility of cardiac or renal malformations that may also occur in this condition, caused by a mutation in one of the genes in the RAS-MAPK pathway.
- Russell-Silver syndrome can cause intrauterine GH restriction and has been traced to uniparental disomy of chromosome 7 or duplications, mutations, or methylation defects in chromosome 11.
- Individuals with Prader-Willi syndrome, which is characterized by low muscle tone, hyperphagia, and hypogonadism, have demonstrated dramatic benefits from GH therapy, primarily in maintaining a normal body mass index.
Children who are small for their gestational age may be GH resistant, and those who do not catch up to their growth curve by the age of 2 years may require GH treatment to reach their height potential.
GH therapy isn’t entirely benign. Rare adverse effects of overtreatment can include slipped capital femoral epiphysis (a fracture to the growth plate) and pseudotumor cerebri (idiopathic intracranial hypertension).
Overtreatment can cause acromegaly, which results in coarsened features and large hands and feet.
When is GH therapy warranted?
“Growth hormone therapy has been denied by her insurer. They want you to fill out an appeal.”
Insurance approval in the United States can be a herculean effort because GH is expensive: Out-of-pocket costs are prohibitive for most people without insurance assistance, ranging from $7,000 to $30,000 annually.
Pediatric endocrinologists aren’t in the business of cosmetic endocrinology. Treatment of idiopathic short stature has been controversial since this became an indication for GH treatment.
GH isn’t always necessary. Diagnosing the underlying cause for poor growth is the most important step.
Often, we find constitutional delay of growth and puberty, or “late bloomers.” This condition is characterized by a delayed bone age (growth plates more open than expected for age) and delayed pubertal onset. These children will often reach a normal height despite starting as some of the smallest of their peers.
However, GH plays other roles in the body than simply propelling height. Children with congenital GHD will require GH treatment to prevent hypoglycemia, especially in infancy.
GH is needed even in adults with fused growth plates for normal lipid metabolism, bone accrual, and maintaining normal muscle mass.
I have noticed marked improvements in muscle tone in many children with developmental delays who are treated with GH, and research supports cognitive benefits for certain populations.
The most common regimens for GH focus on treatment via subcutaneous injection nightly, when GH is naturally produced; sometimes, injections are given six nights out of seven to provide a break or for splitting time between households.
Newer once-weekly formulations have recently received approval, as reported by this news organization, and are coming into use.
Pediatric endocrinologists measure height and follow growth factors closely with visits every 3-6 months. GH levels are not useful outside of provocative diagnostic (stimulation) testing.
Insulinlike growth factor 1 or insulinlike growth factor binding protein levels are analyzed per Tanner stage of puberty to assess appropriate response and to make dose adjustments.
Annual standardized films of the left hand help predict progress and anticipated adult height. Treatment usually persists through puberty until growth plates are closed; if true GHD is noticed, much smaller doses are continued through adulthood.
Regardless, conversations about GH happen with your friendly local pediatric endocrinologist.
We are thrilled to help shepherd patients through their growing age to meet their potential. For more information about GH treatment for children, the MAGIC Foundation is the perfect place to start.
Dr. Lilley is director of the pediatric diabetes and lipid program, Mississippi Center for Advanced Medicine, Madison. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
“Dr. Lilley, you’ll always be my favorite doctor; you helped me grow.”
These were the parting words from the last patient that I treated during my endocrinology fellowship. I had watched this young man grow from a prepubertal 17-year-old to a young man who had reached his predicted family height as I treated his delayed puberty caused by Kallmann syndrome, a problem that had been missed for years. It was the appropriate bookend for my chosen specialty.
Watching children grow and develop into who they were meant to be is one of my favorite things about endocrinology, as well as forming meaningful relationships with families. Treating detectable deficiencies in logical and measurable ways is also extremely satisfying.
Too little testosterone? That’s a problem I can solve. Too much thyroid hormone? There’s a blocker for that! Endocrinology can be a straightforward field, and when all goes well, everyone leaves happy.
Except when they don’t.
Gatekeepers for treatment for children’s growth
“Nice to meet you. We’re here to get growth hormone.”
“We’re here because his pediatrician made us come. We’ve already decided we’re not going to put hormones into his body.”
These are common statements I hear when I first meet new patients whose parents are concerned about their children’s growth. Pediatric endocrinologists, after all, are the usual gatekeepers for this treatment.
Growth hormone (GH) often makes the news for controversial reasons – most commonly for its abuse by elite athletes hoping to exploit its anabolic effects – causing parents to have varied opinions about its possible use in their children.
Some refuse endocrinology referrals at all owing to concerns that we will push daily injections on their children. Others demand referrals for their children of average height, hoping for every perceived advantage.
GH deficiency (GHD) – a condition where the pituitary gland fails to produce enough GH – can occur because of congenital pituitary malformations; anatomic, surgical, or traumatic interruptions to the gland; or enzyme deficiencies leading to faulty production.
GHD is just one reason for poor growth, however.
Growth is one of the most important indicators of health in children. A waning growth rate may be an early symptom of serious problems. In my clinic, I’ve diagnosed severe hypothyroidism in a marathon runner, a brain tumor, celiac disease in a teenager with no gastrointestinal complaints, autoimmune hepatitis, and several other diseases needing treatment in children who show no symptoms other than poor growth.
Barriers to normal growth
Sometimes, the die is cast for children to have barriers to normal growth. Several genetic conditions can lead to poor GH production or response, and GH treatment is often necessary to approximate normal height.
These may include:
- Turner syndrome (in females who are missing an X chromosome in whole or part) should be considered in every girl with abnormally short stature; mosaic forms of the condition may be subtle and lack classic features.
- Noonan syndrome is important to detect owing to the possibility of cardiac or renal malformations that may also occur in this condition, caused by a mutation in one of the genes in the RAS-MAPK pathway.
- Russell-Silver syndrome can cause intrauterine GH restriction and has been traced to uniparental disomy of chromosome 7 or duplications, mutations, or methylation defects in chromosome 11.
- Individuals with Prader-Willi syndrome, which is characterized by low muscle tone, hyperphagia, and hypogonadism, have demonstrated dramatic benefits from GH therapy, primarily in maintaining a normal body mass index.
Children who are small for their gestational age may be GH resistant, and those who do not catch up to their growth curve by the age of 2 years may require GH treatment to reach their height potential.
GH therapy isn’t entirely benign. Rare adverse effects of overtreatment can include slipped capital femoral epiphysis (a fracture to the growth plate) and pseudotumor cerebri (idiopathic intracranial hypertension).
Overtreatment can cause acromegaly, which results in coarsened features and large hands and feet.
When is GH therapy warranted?
“Growth hormone therapy has been denied by her insurer. They want you to fill out an appeal.”
Insurance approval in the United States can be a herculean effort because GH is expensive: Out-of-pocket costs are prohibitive for most people without insurance assistance, ranging from $7,000 to $30,000 annually.
Pediatric endocrinologists aren’t in the business of cosmetic endocrinology. Treatment of idiopathic short stature has been controversial since this became an indication for GH treatment.
GH isn’t always necessary. Diagnosing the underlying cause for poor growth is the most important step.
Often, we find constitutional delay of growth and puberty, or “late bloomers.” This condition is characterized by a delayed bone age (growth plates more open than expected for age) and delayed pubertal onset. These children will often reach a normal height despite starting as some of the smallest of their peers.
However, GH plays other roles in the body than simply propelling height. Children with congenital GHD will require GH treatment to prevent hypoglycemia, especially in infancy.
GH is needed even in adults with fused growth plates for normal lipid metabolism, bone accrual, and maintaining normal muscle mass.
I have noticed marked improvements in muscle tone in many children with developmental delays who are treated with GH, and research supports cognitive benefits for certain populations.
The most common regimens for GH focus on treatment via subcutaneous injection nightly, when GH is naturally produced; sometimes, injections are given six nights out of seven to provide a break or for splitting time between households.
Newer once-weekly formulations have recently received approval, as reported by this news organization, and are coming into use.
Pediatric endocrinologists measure height and follow growth factors closely with visits every 3-6 months. GH levels are not useful outside of provocative diagnostic (stimulation) testing.
Insulinlike growth factor 1 or insulinlike growth factor binding protein levels are analyzed per Tanner stage of puberty to assess appropriate response and to make dose adjustments.
Annual standardized films of the left hand help predict progress and anticipated adult height. Treatment usually persists through puberty until growth plates are closed; if true GHD is noticed, much smaller doses are continued through adulthood.
Regardless, conversations about GH happen with your friendly local pediatric endocrinologist.
We are thrilled to help shepherd patients through their growing age to meet their potential. For more information about GH treatment for children, the MAGIC Foundation is the perfect place to start.
Dr. Lilley is director of the pediatric diabetes and lipid program, Mississippi Center for Advanced Medicine, Madison. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
Long COVID symptoms linked to effects on vagus nerve
Several long COVID symptoms could be linked to the effects of the coronavirus on a vital central nerve, according to new research being released in the spring.
The vagus nerve, which runs from the brain into the body, connects to the heart, lungs, intestines, and several muscles involved with swallowing. It plays a role in several body functions that control heart rate, speech, the gag reflex, sweating, and digestion.
Those with long COVID and vagus nerve problems could face long-term issues with their voice, a hard time swallowing, dizziness, a high heart rate, low blood pressure, and diarrhea, the study authors found.
Their findings will be presented at the 2022 European Congress of Clinical Microbiology and Infectious Diseases in late April.
“Most long COVID subjects with vagus nerve dysfunction symptoms had a range of significant, clinically relevant, structural and/or functional alterations in their vagus nerve, including nerve thickening, trouble swallowing, and symptoms of impaired breathing,” the study authors wrote. “Our findings so far thus point at vagus nerve dysfunction as a central pathophysiological feature of long COVID.”
Researchers from the University Hospital Germans Trias i Pujol in Barcelona performed a study to look at vagus nerve functioning in long COVID patients. Among 348 patients, about 66% had at least one symptom that suggested vagus nerve dysfunction. The researchers did a broad evaluation with imaging and functional tests for 22 patients in the university’s Long COVID Clinic from March to June 2021.
Of the 22 patients, 20 were women, and the median age was 44. The most frequent symptoms related to vagus nerve dysfunction were diarrhea (73%), high heart rates (59%), dizziness (45%), swallowing problems (45%), voice problems (45%), and low blood pressure (14%).
Almost all (19 of 22 patients) had three or more symptoms related to vagus nerve dysfunction. The average length of symptoms was 14 months.
Of 22 patients, 6 had a change in the vagus nerve in the neck, which the researchers observed by ultrasound. They had a thickening of the vagus nerve and increased “echogenicity,” which suggests inflammation.
What’s more, 10 of 22 patients had flattened “diaphragmatic curves” during a thoracic ultrasound, which means the diaphragm doesn’t move as well as it should during breathing, and abnormal breathing. In another assessment, 10 of 16 patients had lower maximum inspiration pressures, suggesting a weakness in breathing muscles.
Eating and digestion were also impaired in some patients, with 13 reporting trouble with swallowing. During a gastric and bowel function assessment, eight patients couldn’t move food from the esophagus to the stomach as well as they should, while nine patients had acid reflux. Three patients had a hiatal hernia, which happens when the upper part of the stomach bulges through the diaphragm into the chest cavity.
The voices of some patients changed as well. Eight patients had an abnormal voice handicap index 30 test, which is a standard way to measure voice function. Among those, seven patients had dysphonia, or persistent voice problems.
The study is ongoing, and the research team is continuing to recruit patients to study the links between long COVID and the vagus nerve. The full paper isn’t yet available, and the research hasn’t yet been peer reviewed.
“The study appears to add to a growing collection of data suggesting at least some of the symptoms of long COVID is mediated through a direct impact on the nervous system,” David Strain, MD, a clinical senior lecturer at the University of Exeter (England), told the Science Media Centre.
“Establishing vagal nerve damage is useful information, as there are recognized, albeit not perfect, treatments for other causes of vagal nerve dysfunction that may be extrapolated to be beneficial for people with this type of long COVID,” he said.
A version of this article first appeared on WebMD.com.
Several long COVID symptoms could be linked to the effects of the coronavirus on a vital central nerve, according to new research being released in the spring.
The vagus nerve, which runs from the brain into the body, connects to the heart, lungs, intestines, and several muscles involved with swallowing. It plays a role in several body functions that control heart rate, speech, the gag reflex, sweating, and digestion.
Those with long COVID and vagus nerve problems could face long-term issues with their voice, a hard time swallowing, dizziness, a high heart rate, low blood pressure, and diarrhea, the study authors found.
Their findings will be presented at the 2022 European Congress of Clinical Microbiology and Infectious Diseases in late April.
“Most long COVID subjects with vagus nerve dysfunction symptoms had a range of significant, clinically relevant, structural and/or functional alterations in their vagus nerve, including nerve thickening, trouble swallowing, and symptoms of impaired breathing,” the study authors wrote. “Our findings so far thus point at vagus nerve dysfunction as a central pathophysiological feature of long COVID.”
Researchers from the University Hospital Germans Trias i Pujol in Barcelona performed a study to look at vagus nerve functioning in long COVID patients. Among 348 patients, about 66% had at least one symptom that suggested vagus nerve dysfunction. The researchers did a broad evaluation with imaging and functional tests for 22 patients in the university’s Long COVID Clinic from March to June 2021.
Of the 22 patients, 20 were women, and the median age was 44. The most frequent symptoms related to vagus nerve dysfunction were diarrhea (73%), high heart rates (59%), dizziness (45%), swallowing problems (45%), voice problems (45%), and low blood pressure (14%).
Almost all (19 of 22 patients) had three or more symptoms related to vagus nerve dysfunction. The average length of symptoms was 14 months.
Of 22 patients, 6 had a change in the vagus nerve in the neck, which the researchers observed by ultrasound. They had a thickening of the vagus nerve and increased “echogenicity,” which suggests inflammation.
What’s more, 10 of 22 patients had flattened “diaphragmatic curves” during a thoracic ultrasound, which means the diaphragm doesn’t move as well as it should during breathing, and abnormal breathing. In another assessment, 10 of 16 patients had lower maximum inspiration pressures, suggesting a weakness in breathing muscles.
Eating and digestion were also impaired in some patients, with 13 reporting trouble with swallowing. During a gastric and bowel function assessment, eight patients couldn’t move food from the esophagus to the stomach as well as they should, while nine patients had acid reflux. Three patients had a hiatal hernia, which happens when the upper part of the stomach bulges through the diaphragm into the chest cavity.
The voices of some patients changed as well. Eight patients had an abnormal voice handicap index 30 test, which is a standard way to measure voice function. Among those, seven patients had dysphonia, or persistent voice problems.
The study is ongoing, and the research team is continuing to recruit patients to study the links between long COVID and the vagus nerve. The full paper isn’t yet available, and the research hasn’t yet been peer reviewed.
“The study appears to add to a growing collection of data suggesting at least some of the symptoms of long COVID is mediated through a direct impact on the nervous system,” David Strain, MD, a clinical senior lecturer at the University of Exeter (England), told the Science Media Centre.
“Establishing vagal nerve damage is useful information, as there are recognized, albeit not perfect, treatments for other causes of vagal nerve dysfunction that may be extrapolated to be beneficial for people with this type of long COVID,” he said.
A version of this article first appeared on WebMD.com.
Several long COVID symptoms could be linked to the effects of the coronavirus on a vital central nerve, according to new research being released in the spring.
The vagus nerve, which runs from the brain into the body, connects to the heart, lungs, intestines, and several muscles involved with swallowing. It plays a role in several body functions that control heart rate, speech, the gag reflex, sweating, and digestion.
Those with long COVID and vagus nerve problems could face long-term issues with their voice, a hard time swallowing, dizziness, a high heart rate, low blood pressure, and diarrhea, the study authors found.
Their findings will be presented at the 2022 European Congress of Clinical Microbiology and Infectious Diseases in late April.
“Most long COVID subjects with vagus nerve dysfunction symptoms had a range of significant, clinically relevant, structural and/or functional alterations in their vagus nerve, including nerve thickening, trouble swallowing, and symptoms of impaired breathing,” the study authors wrote. “Our findings so far thus point at vagus nerve dysfunction as a central pathophysiological feature of long COVID.”
Researchers from the University Hospital Germans Trias i Pujol in Barcelona performed a study to look at vagus nerve functioning in long COVID patients. Among 348 patients, about 66% had at least one symptom that suggested vagus nerve dysfunction. The researchers did a broad evaluation with imaging and functional tests for 22 patients in the university’s Long COVID Clinic from March to June 2021.
Of the 22 patients, 20 were women, and the median age was 44. The most frequent symptoms related to vagus nerve dysfunction were diarrhea (73%), high heart rates (59%), dizziness (45%), swallowing problems (45%), voice problems (45%), and low blood pressure (14%).
Almost all (19 of 22 patients) had three or more symptoms related to vagus nerve dysfunction. The average length of symptoms was 14 months.
Of 22 patients, 6 had a change in the vagus nerve in the neck, which the researchers observed by ultrasound. They had a thickening of the vagus nerve and increased “echogenicity,” which suggests inflammation.
What’s more, 10 of 22 patients had flattened “diaphragmatic curves” during a thoracic ultrasound, which means the diaphragm doesn’t move as well as it should during breathing, and abnormal breathing. In another assessment, 10 of 16 patients had lower maximum inspiration pressures, suggesting a weakness in breathing muscles.
Eating and digestion were also impaired in some patients, with 13 reporting trouble with swallowing. During a gastric and bowel function assessment, eight patients couldn’t move food from the esophagus to the stomach as well as they should, while nine patients had acid reflux. Three patients had a hiatal hernia, which happens when the upper part of the stomach bulges through the diaphragm into the chest cavity.
The voices of some patients changed as well. Eight patients had an abnormal voice handicap index 30 test, which is a standard way to measure voice function. Among those, seven patients had dysphonia, or persistent voice problems.
The study is ongoing, and the research team is continuing to recruit patients to study the links between long COVID and the vagus nerve. The full paper isn’t yet available, and the research hasn’t yet been peer reviewed.
“The study appears to add to a growing collection of data suggesting at least some of the symptoms of long COVID is mediated through a direct impact on the nervous system,” David Strain, MD, a clinical senior lecturer at the University of Exeter (England), told the Science Media Centre.
“Establishing vagal nerve damage is useful information, as there are recognized, albeit not perfect, treatments for other causes of vagal nerve dysfunction that may be extrapolated to be beneficial for people with this type of long COVID,” he said.
A version of this article first appeared on WebMD.com.
Ear tubes not recommended for recurrent AOM without effusion, ENTs maintain
A practice guideline update from the ENT community on tympanostomy tubes in children reaffirms that tube insertion should not be considered in cases of otitis media with effusion (OME) lasting less than 3 months, or in children with recurrent acute otitis media (AOM) without middle ear effusion at the time of assessment for the procedure.
New in the update from the American Academy of Otolaryngology–Head and Neck Surgery Foundation (AAO-HNSF) is a strong recommendation for timely follow-up after surgery and recommendations against both routine use of prophylactic antibiotic ear drops after surgery and the initial use of long-term tubes except when there are specific reasons for doing so.
The update also expands the list of risk factors that place children with OME at increased risk of developmental difficulties – and often in need of timely ear tube placement – to include intellectual disability, learning disorder, and attention-deficit/hyperactivity disorder.
“Most of what we said in the 2013 [original] guideline was good and still valid ... and [important for] pediatricians, who are the key players” in managing otitis media, Jesse Hackell, MD, one of two general pediatricians who served on the Academy’s guideline update committee, said in an interview.
OME spontaneously clears up to 90% of the time within 3 months, said Dr. Hackell, of Pomona (New York) Pediatrics, and chair of the American Academy of Pediatrics (AAP) Committee on Practice and Ambulatory Medicine.
The updated guideline, for children 6 months to 12 years, reaffirms a recommendation that tube insertion be offered to children with “bilateral OME for 3 months or longer AND documented hearing difficulties.”
It also reaffirms “options” (a lesser quality of evidence) that in the absence of hearing difficulties, surgery may be performed for children with chronic OME (3 months or longer) in one or both ears if 1) they are at increased risk of developmental difficulties from OME or 2) effusion is likely contributing to balance problems, poor school performance, behavioral problems, ear discomfort, or reduced quality of life.
Children with chronic OME who do not undergo surgery should be reevaluated at 3- to 6-month intervals and monitored until effusion is no longer present, significant hearing loss is detected, or structural abnormalities of the tympanic membrane or middle ear are detected, the update again recommends.
Tympanostomy tube placement is the most common ambulatory surgery performed on children in the United States, the guideline authors say. In 2014, about 9% of children had undergone the surgery, they wrote, noting also that “tubes were placed in 25%-30% of children with frequent ear infections.”
Recurrent AOM
The AAO-HNSF guidance regarding tympanostomy tubes for OME is similar overall to management guidance issued by the AAP in its clinical practice guideline on OME.
The organizations differ, however, on their guidance for tube insertion for recurrent AOM. In its 2013 clinical practice guideline on AOM, the AAP recommends that clinicians may offer tube insertion for recurrent AOM, with no mention of the presence or absence of persistent fluid as a consideration.
According to the AAO-HNSF update, grade A evidence, including some research published since its original 2013 guideline, has shown little benefit to tube insertion in reducing the incidence of AOM in otherwise healthy children who don’t have middle ear effusion.
One study published in 2019 assessed outcomes after watchful waiting and found that only one-third of 123 children eventually went on to tympanostomy tube placement, noted Richard M. Rosenfeld, MD, distinguished professor and chairman of otolaryngology at SUNY Downstate Health Sciences University in Brooklyn, N.Y., and lead author of the original and updated guidelines.
In practice, “the real question [for the ENT] is the future. If the ears are perfectly clear, will tubes really reduce the frequency of infections going forward?” Dr. Rosenfeld said in an interview. “All the evidence seems to say no, it doesn’t make much of a difference.”
Dr. Hackell said he’s confident that the question “is settled enough.” While there “could be stronger research and higher quality studies, the evidence is still pretty good to suggest you gain little to no benefit with tubes when you’re dealing with recurrent AOM without effusion,” he said.
Asked to comment on the ENT update and its guidance on tympanostomy tubes for children with recurrent AOM, an AAP spokesperson said the “issue is under review” and that the AAP did not currently have a statement.
At-risk children
The AAO-HNSF update renews a recommendation to evaluate children with either recurrent AOM or OME of any duration for increased risk for speech, language, or learning problems from OME because of baseline factors (sensory, physical, cognitive, or behavioral).
When OME becomes chronic – or when a tympanogram gives a flat-line reading – OME is likely to persist, and families of at-risk children especially should be encouraged to pursue tube placement, Dr. Rosenfeld said.
Despite prior guidance to this effect, he said, ear tubes are being underutilized in at-risk children, with effusion being missed in primary care and with ENTs not expediting tube placement upon referral.
“These children have learning issues, cognitive issues, developmental issues,” he said in the interview. “It’s a population that does very poorly with ears full of fluid ... and despite guidance suggesting these children should be prioritized with tubes, it doesn’t seem to be happening enough.”
Formulating guidelines for at-risk children is challenging because they are often excluded from trials, Dr. Rosenfeld said, which limits evidence about the benefits of tubes and limits the strength of recommendations.
The addition of attention-deficit/hyperactivity disorder, intellectual disability, and learning disorder to the list of risk factors is notable, Dr. Hackell said. (The list includes autism spectrum disorder, developmental delay, and suspected or confirmed speech and language delay or disorder.)
“We know that kids with ADHD take in and process information a little differently ... it may be harder to get their attention with auditory stimulation,” he said. “So anything that would impact the taking in of information even for a short period of time increases their risk.”
Surgical practice
ENTs are advised in the new guidance to use long-term tubes and perioperative antibiotic ear drops more judiciously. “Long-term tubes have a role, but there are some doctors who routinely use them, even for a first-time surgery,” said Dr. Rosenfeld.
Overuse of long-term tubes results in a higher incidence of tympanic membrane perforation, chronic drainage, and other complications, as well as greater need for long-term follow-up. “There needs to be a reason – something to justify the need for prolonged ventilation,” he said.
Perioperative antibiotic ear drops are often administered during surgery and then prescribed routinely for all children afterward, but research has shown that saline irrigation during surgery and a single application of antibiotic/steroid drops is similarly efficacious in preventing otorrhea, the guideline says. Antibiotic ear drops are also “expensive,” noted Dr. Hackell. “There’s not enough benefit to justify it.”
The update also more explicitly advises selective use of adenoidectomy. A new option says that clinicians may perform the procedure as an adjunct to tube insertion for children 4 years or older to potentially reduce the future incidence of recurrent OME or the need for repeat surgery.
However, in younger children, it should not be offered unless there are symptoms directly related to adenoid infection or nasal obstruction. “Under 4 years, there’s no primary benefit for the ears,” said Dr. Rosenfeld.
Follow-up with the surgeon after tympanostomy tube insertion should occur within 3 months to assess outcomes and educate the family, the update strongly recommends.
And pediatricians should know, Dr. Hackell notes, that clinical evidence continues to show that earplugs and other water precautions are not routinely needed for children who have tubes in place. A good approach, the guideline says, is to “first avoid water precautions and instead reserve them for children with recurrent or persistent tympanostomy tube otorrhea.”
Asked to comment on the guideline update, Tim Joos, MD, MPH, who practices combined internal medicine/pediatrics in Seattle and is an editorial advisory board member of Pediatric News, noted the inclusion of patient information sheets with frequently asked questions – resources that can be useful for guiding parents through what’s often a shared decision-making process.
Neither Dr. Rosenfeld nor Dr. Hackell reported any disclosures. Other members of the guideline update committee reported various book royalties, consulting fees, and other disclosures. Dr. Joos reported he has no connections to the guideline authors.
A practice guideline update from the ENT community on tympanostomy tubes in children reaffirms that tube insertion should not be considered in cases of otitis media with effusion (OME) lasting less than 3 months, or in children with recurrent acute otitis media (AOM) without middle ear effusion at the time of assessment for the procedure.
New in the update from the American Academy of Otolaryngology–Head and Neck Surgery Foundation (AAO-HNSF) is a strong recommendation for timely follow-up after surgery and recommendations against both routine use of prophylactic antibiotic ear drops after surgery and the initial use of long-term tubes except when there are specific reasons for doing so.
The update also expands the list of risk factors that place children with OME at increased risk of developmental difficulties – and often in need of timely ear tube placement – to include intellectual disability, learning disorder, and attention-deficit/hyperactivity disorder.
“Most of what we said in the 2013 [original] guideline was good and still valid ... and [important for] pediatricians, who are the key players” in managing otitis media, Jesse Hackell, MD, one of two general pediatricians who served on the Academy’s guideline update committee, said in an interview.
OME spontaneously clears up to 90% of the time within 3 months, said Dr. Hackell, of Pomona (New York) Pediatrics, and chair of the American Academy of Pediatrics (AAP) Committee on Practice and Ambulatory Medicine.
The updated guideline, for children 6 months to 12 years, reaffirms a recommendation that tube insertion be offered to children with “bilateral OME for 3 months or longer AND documented hearing difficulties.”
It also reaffirms “options” (a lesser quality of evidence) that in the absence of hearing difficulties, surgery may be performed for children with chronic OME (3 months or longer) in one or both ears if 1) they are at increased risk of developmental difficulties from OME or 2) effusion is likely contributing to balance problems, poor school performance, behavioral problems, ear discomfort, or reduced quality of life.
Children with chronic OME who do not undergo surgery should be reevaluated at 3- to 6-month intervals and monitored until effusion is no longer present, significant hearing loss is detected, or structural abnormalities of the tympanic membrane or middle ear are detected, the update again recommends.
Tympanostomy tube placement is the most common ambulatory surgery performed on children in the United States, the guideline authors say. In 2014, about 9% of children had undergone the surgery, they wrote, noting also that “tubes were placed in 25%-30% of children with frequent ear infections.”
Recurrent AOM
The AAO-HNSF guidance regarding tympanostomy tubes for OME is similar overall to management guidance issued by the AAP in its clinical practice guideline on OME.
The organizations differ, however, on their guidance for tube insertion for recurrent AOM. In its 2013 clinical practice guideline on AOM, the AAP recommends that clinicians may offer tube insertion for recurrent AOM, with no mention of the presence or absence of persistent fluid as a consideration.
According to the AAO-HNSF update, grade A evidence, including some research published since its original 2013 guideline, has shown little benefit to tube insertion in reducing the incidence of AOM in otherwise healthy children who don’t have middle ear effusion.
One study published in 2019 assessed outcomes after watchful waiting and found that only one-third of 123 children eventually went on to tympanostomy tube placement, noted Richard M. Rosenfeld, MD, distinguished professor and chairman of otolaryngology at SUNY Downstate Health Sciences University in Brooklyn, N.Y., and lead author of the original and updated guidelines.
In practice, “the real question [for the ENT] is the future. If the ears are perfectly clear, will tubes really reduce the frequency of infections going forward?” Dr. Rosenfeld said in an interview. “All the evidence seems to say no, it doesn’t make much of a difference.”
Dr. Hackell said he’s confident that the question “is settled enough.” While there “could be stronger research and higher quality studies, the evidence is still pretty good to suggest you gain little to no benefit with tubes when you’re dealing with recurrent AOM without effusion,” he said.
Asked to comment on the ENT update and its guidance on tympanostomy tubes for children with recurrent AOM, an AAP spokesperson said the “issue is under review” and that the AAP did not currently have a statement.
At-risk children
The AAO-HNSF update renews a recommendation to evaluate children with either recurrent AOM or OME of any duration for increased risk for speech, language, or learning problems from OME because of baseline factors (sensory, physical, cognitive, or behavioral).
When OME becomes chronic – or when a tympanogram gives a flat-line reading – OME is likely to persist, and families of at-risk children especially should be encouraged to pursue tube placement, Dr. Rosenfeld said.
Despite prior guidance to this effect, he said, ear tubes are being underutilized in at-risk children, with effusion being missed in primary care and with ENTs not expediting tube placement upon referral.
“These children have learning issues, cognitive issues, developmental issues,” he said in the interview. “It’s a population that does very poorly with ears full of fluid ... and despite guidance suggesting these children should be prioritized with tubes, it doesn’t seem to be happening enough.”
Formulating guidelines for at-risk children is challenging because they are often excluded from trials, Dr. Rosenfeld said, which limits evidence about the benefits of tubes and limits the strength of recommendations.
The addition of attention-deficit/hyperactivity disorder, intellectual disability, and learning disorder to the list of risk factors is notable, Dr. Hackell said. (The list includes autism spectrum disorder, developmental delay, and suspected or confirmed speech and language delay or disorder.)
“We know that kids with ADHD take in and process information a little differently ... it may be harder to get their attention with auditory stimulation,” he said. “So anything that would impact the taking in of information even for a short period of time increases their risk.”
Surgical practice
ENTs are advised in the new guidance to use long-term tubes and perioperative antibiotic ear drops more judiciously. “Long-term tubes have a role, but there are some doctors who routinely use them, even for a first-time surgery,” said Dr. Rosenfeld.
Overuse of long-term tubes results in a higher incidence of tympanic membrane perforation, chronic drainage, and other complications, as well as greater need for long-term follow-up. “There needs to be a reason – something to justify the need for prolonged ventilation,” he said.
Perioperative antibiotic ear drops are often administered during surgery and then prescribed routinely for all children afterward, but research has shown that saline irrigation during surgery and a single application of antibiotic/steroid drops is similarly efficacious in preventing otorrhea, the guideline says. Antibiotic ear drops are also “expensive,” noted Dr. Hackell. “There’s not enough benefit to justify it.”
The update also more explicitly advises selective use of adenoidectomy. A new option says that clinicians may perform the procedure as an adjunct to tube insertion for children 4 years or older to potentially reduce the future incidence of recurrent OME or the need for repeat surgery.
However, in younger children, it should not be offered unless there are symptoms directly related to adenoid infection or nasal obstruction. “Under 4 years, there’s no primary benefit for the ears,” said Dr. Rosenfeld.
Follow-up with the surgeon after tympanostomy tube insertion should occur within 3 months to assess outcomes and educate the family, the update strongly recommends.
And pediatricians should know, Dr. Hackell notes, that clinical evidence continues to show that earplugs and other water precautions are not routinely needed for children who have tubes in place. A good approach, the guideline says, is to “first avoid water precautions and instead reserve them for children with recurrent or persistent tympanostomy tube otorrhea.”
Asked to comment on the guideline update, Tim Joos, MD, MPH, who practices combined internal medicine/pediatrics in Seattle and is an editorial advisory board member of Pediatric News, noted the inclusion of patient information sheets with frequently asked questions – resources that can be useful for guiding parents through what’s often a shared decision-making process.
Neither Dr. Rosenfeld nor Dr. Hackell reported any disclosures. Other members of the guideline update committee reported various book royalties, consulting fees, and other disclosures. Dr. Joos reported he has no connections to the guideline authors.
A practice guideline update from the ENT community on tympanostomy tubes in children reaffirms that tube insertion should not be considered in cases of otitis media with effusion (OME) lasting less than 3 months, or in children with recurrent acute otitis media (AOM) without middle ear effusion at the time of assessment for the procedure.
New in the update from the American Academy of Otolaryngology–Head and Neck Surgery Foundation (AAO-HNSF) is a strong recommendation for timely follow-up after surgery and recommendations against both routine use of prophylactic antibiotic ear drops after surgery and the initial use of long-term tubes except when there are specific reasons for doing so.
The update also expands the list of risk factors that place children with OME at increased risk of developmental difficulties – and often in need of timely ear tube placement – to include intellectual disability, learning disorder, and attention-deficit/hyperactivity disorder.
“Most of what we said in the 2013 [original] guideline was good and still valid ... and [important for] pediatricians, who are the key players” in managing otitis media, Jesse Hackell, MD, one of two general pediatricians who served on the Academy’s guideline update committee, said in an interview.
OME spontaneously clears up to 90% of the time within 3 months, said Dr. Hackell, of Pomona (New York) Pediatrics, and chair of the American Academy of Pediatrics (AAP) Committee on Practice and Ambulatory Medicine.
The updated guideline, for children 6 months to 12 years, reaffirms a recommendation that tube insertion be offered to children with “bilateral OME for 3 months or longer AND documented hearing difficulties.”
It also reaffirms “options” (a lesser quality of evidence) that in the absence of hearing difficulties, surgery may be performed for children with chronic OME (3 months or longer) in one or both ears if 1) they are at increased risk of developmental difficulties from OME or 2) effusion is likely contributing to balance problems, poor school performance, behavioral problems, ear discomfort, or reduced quality of life.
Children with chronic OME who do not undergo surgery should be reevaluated at 3- to 6-month intervals and monitored until effusion is no longer present, significant hearing loss is detected, or structural abnormalities of the tympanic membrane or middle ear are detected, the update again recommends.
Tympanostomy tube placement is the most common ambulatory surgery performed on children in the United States, the guideline authors say. In 2014, about 9% of children had undergone the surgery, they wrote, noting also that “tubes were placed in 25%-30% of children with frequent ear infections.”
Recurrent AOM
The AAO-HNSF guidance regarding tympanostomy tubes for OME is similar overall to management guidance issued by the AAP in its clinical practice guideline on OME.
The organizations differ, however, on their guidance for tube insertion for recurrent AOM. In its 2013 clinical practice guideline on AOM, the AAP recommends that clinicians may offer tube insertion for recurrent AOM, with no mention of the presence or absence of persistent fluid as a consideration.
According to the AAO-HNSF update, grade A evidence, including some research published since its original 2013 guideline, has shown little benefit to tube insertion in reducing the incidence of AOM in otherwise healthy children who don’t have middle ear effusion.
One study published in 2019 assessed outcomes after watchful waiting and found that only one-third of 123 children eventually went on to tympanostomy tube placement, noted Richard M. Rosenfeld, MD, distinguished professor and chairman of otolaryngology at SUNY Downstate Health Sciences University in Brooklyn, N.Y., and lead author of the original and updated guidelines.
In practice, “the real question [for the ENT] is the future. If the ears are perfectly clear, will tubes really reduce the frequency of infections going forward?” Dr. Rosenfeld said in an interview. “All the evidence seems to say no, it doesn’t make much of a difference.”
Dr. Hackell said he’s confident that the question “is settled enough.” While there “could be stronger research and higher quality studies, the evidence is still pretty good to suggest you gain little to no benefit with tubes when you’re dealing with recurrent AOM without effusion,” he said.
Asked to comment on the ENT update and its guidance on tympanostomy tubes for children with recurrent AOM, an AAP spokesperson said the “issue is under review” and that the AAP did not currently have a statement.
At-risk children
The AAO-HNSF update renews a recommendation to evaluate children with either recurrent AOM or OME of any duration for increased risk for speech, language, or learning problems from OME because of baseline factors (sensory, physical, cognitive, or behavioral).
When OME becomes chronic – or when a tympanogram gives a flat-line reading – OME is likely to persist, and families of at-risk children especially should be encouraged to pursue tube placement, Dr. Rosenfeld said.
Despite prior guidance to this effect, he said, ear tubes are being underutilized in at-risk children, with effusion being missed in primary care and with ENTs not expediting tube placement upon referral.
“These children have learning issues, cognitive issues, developmental issues,” he said in the interview. “It’s a population that does very poorly with ears full of fluid ... and despite guidance suggesting these children should be prioritized with tubes, it doesn’t seem to be happening enough.”
Formulating guidelines for at-risk children is challenging because they are often excluded from trials, Dr. Rosenfeld said, which limits evidence about the benefits of tubes and limits the strength of recommendations.
The addition of attention-deficit/hyperactivity disorder, intellectual disability, and learning disorder to the list of risk factors is notable, Dr. Hackell said. (The list includes autism spectrum disorder, developmental delay, and suspected or confirmed speech and language delay or disorder.)
“We know that kids with ADHD take in and process information a little differently ... it may be harder to get their attention with auditory stimulation,” he said. “So anything that would impact the taking in of information even for a short period of time increases their risk.”
Surgical practice
ENTs are advised in the new guidance to use long-term tubes and perioperative antibiotic ear drops more judiciously. “Long-term tubes have a role, but there are some doctors who routinely use them, even for a first-time surgery,” said Dr. Rosenfeld.
Overuse of long-term tubes results in a higher incidence of tympanic membrane perforation, chronic drainage, and other complications, as well as greater need for long-term follow-up. “There needs to be a reason – something to justify the need for prolonged ventilation,” he said.
Perioperative antibiotic ear drops are often administered during surgery and then prescribed routinely for all children afterward, but research has shown that saline irrigation during surgery and a single application of antibiotic/steroid drops is similarly efficacious in preventing otorrhea, the guideline says. Antibiotic ear drops are also “expensive,” noted Dr. Hackell. “There’s not enough benefit to justify it.”
The update also more explicitly advises selective use of adenoidectomy. A new option says that clinicians may perform the procedure as an adjunct to tube insertion for children 4 years or older to potentially reduce the future incidence of recurrent OME or the need for repeat surgery.
However, in younger children, it should not be offered unless there are symptoms directly related to adenoid infection or nasal obstruction. “Under 4 years, there’s no primary benefit for the ears,” said Dr. Rosenfeld.
Follow-up with the surgeon after tympanostomy tube insertion should occur within 3 months to assess outcomes and educate the family, the update strongly recommends.
And pediatricians should know, Dr. Hackell notes, that clinical evidence continues to show that earplugs and other water precautions are not routinely needed for children who have tubes in place. A good approach, the guideline says, is to “first avoid water precautions and instead reserve them for children with recurrent or persistent tympanostomy tube otorrhea.”
Asked to comment on the guideline update, Tim Joos, MD, MPH, who practices combined internal medicine/pediatrics in Seattle and is an editorial advisory board member of Pediatric News, noted the inclusion of patient information sheets with frequently asked questions – resources that can be useful for guiding parents through what’s often a shared decision-making process.
Neither Dr. Rosenfeld nor Dr. Hackell reported any disclosures. Other members of the guideline update committee reported various book royalties, consulting fees, and other disclosures. Dr. Joos reported he has no connections to the guideline authors.
FROM OTOLARYNGOLOGY HEAD AND NECK SURGERY
PCOS common in adolescent girls with type 2 diabetes
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
FROM JAMA NETWORK OPEN