User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
A focus on women with diabetes and their offspring
In 2021, diabetes and related complications was the 8th leading cause of death in the United States.1 As of 2022, more than 11% of the U.S. population had diabetes and 38% of the adult U.S. population had prediabetes.2 Diabetes is the most expensive chronic condition in the United States, where $1 of every $4 in health care costs is spent on care.3
Where this is most concerning is diabetes in pregnancy. While childbirth rates in the United States have decreased since the 2007 high of 4.32 million births4 to 3.66 million in 2021,5 the incidence of diabetes in pregnancy – both pregestational and gestational – has increased. The rate of pregestational diabetes in 2021 was 10.9 per 1,000 births, a 27% increase from 2016 (8.6 per 1,000).6 The percentage of those giving birth who also were diagnosed with gestational diabetes mellitus (GDM) was 8.3% in 2021, up from 6.0% in 2016.7
Adverse outcomes for an infant born to a mother with diabetes include a higher risk of obesity and diabetes as adults, potentially leading to a forward-feeding cycle.
We and our colleagues established the Diabetes in Pregnancy Study Group of North America in 1997 because we had witnessed too frequently the devastating diabetes-induced pregnancy complications in our patients. The mission we set forth was to provide a forum for dialogue among maternal-fetal medicine subspecialists. The three main goals we set forth to support this mission were to provide a catalyst for research, contribute to the creation and refinement of medical policies, and influence professional practices in diabetes in pregnancy.8
In the last quarter century, DPSG-NA, through its annual and biennial meetings, has brought together several hundred practitioners that include physicians, nurses, statisticians, researchers, nutritionists, and allied health professionals, among others. As a group, it has improved the detection and management of diabetes in pregnant women and their offspring through knowledge sharing and influencing policies on GDM screening, diagnosis, management, and treatment. Our members have shown that preconceptional counseling for women with diabetes can significantly reduce congenital malformation and perinatal mortality compared with those women with pregestational diabetes who receive no counseling.9,10
We have addressed a wide variety of topics including the paucity of data in determining the timing of delivery for women with diabetes and the Institute of Medicine/National Academy of Medicine recommendations of gestational weight gain and risks of not adhering to them. We have learned about new scientific discoveries that reveal underlying mechanisms to diabetes-related birth defects and potential therapeutic targets; and we have discussed the health literacy requirements, ethics, and opportunities for lifestyle intervention.11-16
But we need to do more.
Two risk factors are at play: Women continue to choose to have babies at later ages and their pregnancies continue to be complicated by the rising incidence of obesity (see Figure 1 and Figure 2). 
The global obesity epidemic has become a significant concern for all aspects of health and particularly for diabetes in pregnancy. 
In 1990, 24.9% of women in the United States were obese; in 2010, 35.8%; and now more than 41%. Some experts project that by 2030 more than 80% of women in the United States will be overweight or obese.21
If we are to stop this cycle of diabetes begets more diabetes, now more than ever we need to come together and accelerate the research and education around the diabetes in pregnancy. Join us at this year’s DPSG-NA meeting Oct. 26-28 to take part in the knowledge sharing, discussions, and planning. More information can be found online at https://events.dpsg-na.com/home.
Dr. Miodovnik is adjunct professor of obstetrics, gynecology, and reproductive sciences at University of Maryland School of Medicine. Dr. Reece is professor of obstetrics, gynecology, and reproductive sciences and senior scientist at the Center for Birth Defects Research at University of Maryland School of Medicine.
References
1. Xu J et al. Mortality in the United States, 2021. NCHS Data Brief. 2022 Dec;(456):1-8. PMID: 36598387.
2. Centers for Disease Control and Prevention, diabetes data and statistics.
3. American Diabetes Association. The Cost of Diabetes.
4. Martin JA et al. Births: Final data for 2007. Natl Vital Stat Rep. 2010 Aug 9;58(24):1-85. PMID: 21254725.
5. Osterman MJK et al. Births: Final data for 2021. Natl Vital Stat Rep. 2023 Jan;72(1):1-53. PMID: 36723449.
6. Gregory ECW and Ely DM. Trends and characteristics in prepregnancy diabetes: United States, 2016-2021. Natl Vital Stat Rep. 2023 May;72(6):1-13. PMID: 37256333.
7. QuickStats: Percentage of mothers with gestational diabetes, by maternal age – National Vital Statistics System, United States, 2016 and 2021. MMWR Morb Mortal Wkly Rep. 2023 Jan 6;72(1):16. doi: 10.15585/mmwr.mm7201a4.
8. Langer O et al. The Diabetes in Pregnancy Study Group of North America – Introduction and summary statement. Prenat Neonat Med. 1998;3(6):514-6.
9. Willhoite MB et al. The impact of preconception counseling on pregnancy outcomes. The experience of the Maine Diabetes in Pregnancy Program. Diabetes Care. 1993 Feb;16(2):450-5. doi: 10.2337/diacare.16.2.450.
10. McElvy SS et al. A focused preconceptional and early pregnancy program in women with type 1 diabetes reduces perinatal mortality and malformation rates to general population levels. J Matern Fetal Med. 2000 Jan-Feb;9(1):14-20. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<14::AID-MFM5>3.0.CO;2-K.
11. Rosen JA et al. The history and contributions of the Diabetes in Pregnancy Study Group of North America (1997-2015). Am J Perinatol. 2016 Nov;33(13):1223-6. doi: 10.1055/s-0036-1585082.
12. Driggers RW and Baschat A. The 12th meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA): Introduction and overview. J Matern Fetal Neonatal Med. 2012 Jan;25(1):3-4. doi: 10.3109/14767058.2012.626917.
13. Langer O et al. The proceedings of the Diabetes in Pregnancy Study Group of North America 2009 conference. J Matern Fetal Neonatal Med. 2010 Mar;23(3):196-8. doi: 10.3109/14767050903550634.
14. Reece EA et al. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Ark., May 2002. J Matern Fetal Neonatal Med. 2002 Dec;12(6):362-4. doi: 10.1080/jmf.12.6.362.364.
15. Reece EA and Maulik D. A consensus conference of the Diabetes in Pregnancy Study Group of North America. J Matern Fetal Neonatal Med. 2002 Dec;12(6):361. doi: 10.1080/jmf.12.6.361.361.
16. Gabbe SG. Summation of the second meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA). J Matern Fetal Med. 2000 Jan-Feb;9(1):3-9.
17. Vital Statistics of the United States 1990: Volume I – Natality.
18. Martin JA et al. Births: final data for 2000. Natl Vital Stat Rep. 2002 Feb 12;50(5):1-101. PMID: 11876093.
19. Martin JA et al. Births: final data for 2010. Natl Vital Stat Rep. 2012 Aug 28;61(1):1-72. PMID: 24974589.
20. CDC Website. Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States.
21. Wang Y et al. Has the prevalence of overweight, obesity, and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020 Jun 1;49(3):810-23. doi: 10.1093/ije/dyz273.
In 2021, diabetes and related complications was the 8th leading cause of death in the United States.1 As of 2022, more than 11% of the U.S. population had diabetes and 38% of the adult U.S. population had prediabetes.2 Diabetes is the most expensive chronic condition in the United States, where $1 of every $4 in health care costs is spent on care.3
Where this is most concerning is diabetes in pregnancy. While childbirth rates in the United States have decreased since the 2007 high of 4.32 million births4 to 3.66 million in 2021,5 the incidence of diabetes in pregnancy – both pregestational and gestational – has increased. The rate of pregestational diabetes in 2021 was 10.9 per 1,000 births, a 27% increase from 2016 (8.6 per 1,000).6 The percentage of those giving birth who also were diagnosed with gestational diabetes mellitus (GDM) was 8.3% in 2021, up from 6.0% in 2016.7
Adverse outcomes for an infant born to a mother with diabetes include a higher risk of obesity and diabetes as adults, potentially leading to a forward-feeding cycle.
We and our colleagues established the Diabetes in Pregnancy Study Group of North America in 1997 because we had witnessed too frequently the devastating diabetes-induced pregnancy complications in our patients. The mission we set forth was to provide a forum for dialogue among maternal-fetal medicine subspecialists. The three main goals we set forth to support this mission were to provide a catalyst for research, contribute to the creation and refinement of medical policies, and influence professional practices in diabetes in pregnancy.8
In the last quarter century, DPSG-NA, through its annual and biennial meetings, has brought together several hundred practitioners that include physicians, nurses, statisticians, researchers, nutritionists, and allied health professionals, among others. As a group, it has improved the detection and management of diabetes in pregnant women and their offspring through knowledge sharing and influencing policies on GDM screening, diagnosis, management, and treatment. Our members have shown that preconceptional counseling for women with diabetes can significantly reduce congenital malformation and perinatal mortality compared with those women with pregestational diabetes who receive no counseling.9,10
We have addressed a wide variety of topics including the paucity of data in determining the timing of delivery for women with diabetes and the Institute of Medicine/National Academy of Medicine recommendations of gestational weight gain and risks of not adhering to them. We have learned about new scientific discoveries that reveal underlying mechanisms to diabetes-related birth defects and potential therapeutic targets; and we have discussed the health literacy requirements, ethics, and opportunities for lifestyle intervention.11-16
But we need to do more.
Two risk factors are at play: Women continue to choose to have babies at later ages and their pregnancies continue to be complicated by the rising incidence of obesity (see Figure 1 and Figure 2). 
The global obesity epidemic has become a significant concern for all aspects of health and particularly for diabetes in pregnancy. 
In 1990, 24.9% of women in the United States were obese; in 2010, 35.8%; and now more than 41%. Some experts project that by 2030 more than 80% of women in the United States will be overweight or obese.21
If we are to stop this cycle of diabetes begets more diabetes, now more than ever we need to come together and accelerate the research and education around the diabetes in pregnancy. Join us at this year’s DPSG-NA meeting Oct. 26-28 to take part in the knowledge sharing, discussions, and planning. More information can be found online at https://events.dpsg-na.com/home.
Dr. Miodovnik is adjunct professor of obstetrics, gynecology, and reproductive sciences at University of Maryland School of Medicine. Dr. Reece is professor of obstetrics, gynecology, and reproductive sciences and senior scientist at the Center for Birth Defects Research at University of Maryland School of Medicine.
References
1. Xu J et al. Mortality in the United States, 2021. NCHS Data Brief. 2022 Dec;(456):1-8. PMID: 36598387.
2. Centers for Disease Control and Prevention, diabetes data and statistics.
3. American Diabetes Association. The Cost of Diabetes.
4. Martin JA et al. Births: Final data for 2007. Natl Vital Stat Rep. 2010 Aug 9;58(24):1-85. PMID: 21254725.
5. Osterman MJK et al. Births: Final data for 2021. Natl Vital Stat Rep. 2023 Jan;72(1):1-53. PMID: 36723449.
6. Gregory ECW and Ely DM. Trends and characteristics in prepregnancy diabetes: United States, 2016-2021. Natl Vital Stat Rep. 2023 May;72(6):1-13. PMID: 37256333.
7. QuickStats: Percentage of mothers with gestational diabetes, by maternal age – National Vital Statistics System, United States, 2016 and 2021. MMWR Morb Mortal Wkly Rep. 2023 Jan 6;72(1):16. doi: 10.15585/mmwr.mm7201a4.
8. Langer O et al. The Diabetes in Pregnancy Study Group of North America – Introduction and summary statement. Prenat Neonat Med. 1998;3(6):514-6.
9. Willhoite MB et al. The impact of preconception counseling on pregnancy outcomes. The experience of the Maine Diabetes in Pregnancy Program. Diabetes Care. 1993 Feb;16(2):450-5. doi: 10.2337/diacare.16.2.450.
10. McElvy SS et al. A focused preconceptional and early pregnancy program in women with type 1 diabetes reduces perinatal mortality and malformation rates to general population levels. J Matern Fetal Med. 2000 Jan-Feb;9(1):14-20. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<14::AID-MFM5>3.0.CO;2-K.
11. Rosen JA et al. The history and contributions of the Diabetes in Pregnancy Study Group of North America (1997-2015). Am J Perinatol. 2016 Nov;33(13):1223-6. doi: 10.1055/s-0036-1585082.
12. Driggers RW and Baschat A. The 12th meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA): Introduction and overview. J Matern Fetal Neonatal Med. 2012 Jan;25(1):3-4. doi: 10.3109/14767058.2012.626917.
13. Langer O et al. The proceedings of the Diabetes in Pregnancy Study Group of North America 2009 conference. J Matern Fetal Neonatal Med. 2010 Mar;23(3):196-8. doi: 10.3109/14767050903550634.
14. Reece EA et al. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Ark., May 2002. J Matern Fetal Neonatal Med. 2002 Dec;12(6):362-4. doi: 10.1080/jmf.12.6.362.364.
15. Reece EA and Maulik D. A consensus conference of the Diabetes in Pregnancy Study Group of North America. J Matern Fetal Neonatal Med. 2002 Dec;12(6):361. doi: 10.1080/jmf.12.6.361.361.
16. Gabbe SG. Summation of the second meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA). J Matern Fetal Med. 2000 Jan-Feb;9(1):3-9.
17. Vital Statistics of the United States 1990: Volume I – Natality.
18. Martin JA et al. Births: final data for 2000. Natl Vital Stat Rep. 2002 Feb 12;50(5):1-101. PMID: 11876093.
19. Martin JA et al. Births: final data for 2010. Natl Vital Stat Rep. 2012 Aug 28;61(1):1-72. PMID: 24974589.
20. CDC Website. Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States.
21. Wang Y et al. Has the prevalence of overweight, obesity, and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020 Jun 1;49(3):810-23. doi: 10.1093/ije/dyz273.
In 2021, diabetes and related complications was the 8th leading cause of death in the United States.1 As of 2022, more than 11% of the U.S. population had diabetes and 38% of the adult U.S. population had prediabetes.2 Diabetes is the most expensive chronic condition in the United States, where $1 of every $4 in health care costs is spent on care.3
Where this is most concerning is diabetes in pregnancy. While childbirth rates in the United States have decreased since the 2007 high of 4.32 million births4 to 3.66 million in 2021,5 the incidence of diabetes in pregnancy – both pregestational and gestational – has increased. The rate of pregestational diabetes in 2021 was 10.9 per 1,000 births, a 27% increase from 2016 (8.6 per 1,000).6 The percentage of those giving birth who also were diagnosed with gestational diabetes mellitus (GDM) was 8.3% in 2021, up from 6.0% in 2016.7
Adverse outcomes for an infant born to a mother with diabetes include a higher risk of obesity and diabetes as adults, potentially leading to a forward-feeding cycle.
We and our colleagues established the Diabetes in Pregnancy Study Group of North America in 1997 because we had witnessed too frequently the devastating diabetes-induced pregnancy complications in our patients. The mission we set forth was to provide a forum for dialogue among maternal-fetal medicine subspecialists. The three main goals we set forth to support this mission were to provide a catalyst for research, contribute to the creation and refinement of medical policies, and influence professional practices in diabetes in pregnancy.8
In the last quarter century, DPSG-NA, through its annual and biennial meetings, has brought together several hundred practitioners that include physicians, nurses, statisticians, researchers, nutritionists, and allied health professionals, among others. As a group, it has improved the detection and management of diabetes in pregnant women and their offspring through knowledge sharing and influencing policies on GDM screening, diagnosis, management, and treatment. Our members have shown that preconceptional counseling for women with diabetes can significantly reduce congenital malformation and perinatal mortality compared with those women with pregestational diabetes who receive no counseling.9,10
We have addressed a wide variety of topics including the paucity of data in determining the timing of delivery for women with diabetes and the Institute of Medicine/National Academy of Medicine recommendations of gestational weight gain and risks of not adhering to them. We have learned about new scientific discoveries that reveal underlying mechanisms to diabetes-related birth defects and potential therapeutic targets; and we have discussed the health literacy requirements, ethics, and opportunities for lifestyle intervention.11-16
But we need to do more.
Two risk factors are at play: Women continue to choose to have babies at later ages and their pregnancies continue to be complicated by the rising incidence of obesity (see Figure 1 and Figure 2). 
The global obesity epidemic has become a significant concern for all aspects of health and particularly for diabetes in pregnancy. 
In 1990, 24.9% of women in the United States were obese; in 2010, 35.8%; and now more than 41%. Some experts project that by 2030 more than 80% of women in the United States will be overweight or obese.21
If we are to stop this cycle of diabetes begets more diabetes, now more than ever we need to come together and accelerate the research and education around the diabetes in pregnancy. Join us at this year’s DPSG-NA meeting Oct. 26-28 to take part in the knowledge sharing, discussions, and planning. More information can be found online at https://events.dpsg-na.com/home.
Dr. Miodovnik is adjunct professor of obstetrics, gynecology, and reproductive sciences at University of Maryland School of Medicine. Dr. Reece is professor of obstetrics, gynecology, and reproductive sciences and senior scientist at the Center for Birth Defects Research at University of Maryland School of Medicine.
References
1. Xu J et al. Mortality in the United States, 2021. NCHS Data Brief. 2022 Dec;(456):1-8. PMID: 36598387.
2. Centers for Disease Control and Prevention, diabetes data and statistics.
3. American Diabetes Association. The Cost of Diabetes.
4. Martin JA et al. Births: Final data for 2007. Natl Vital Stat Rep. 2010 Aug 9;58(24):1-85. PMID: 21254725.
5. Osterman MJK et al. Births: Final data for 2021. Natl Vital Stat Rep. 2023 Jan;72(1):1-53. PMID: 36723449.
6. Gregory ECW and Ely DM. Trends and characteristics in prepregnancy diabetes: United States, 2016-2021. Natl Vital Stat Rep. 2023 May;72(6):1-13. PMID: 37256333.
7. QuickStats: Percentage of mothers with gestational diabetes, by maternal age – National Vital Statistics System, United States, 2016 and 2021. MMWR Morb Mortal Wkly Rep. 2023 Jan 6;72(1):16. doi: 10.15585/mmwr.mm7201a4.
8. Langer O et al. The Diabetes in Pregnancy Study Group of North America – Introduction and summary statement. Prenat Neonat Med. 1998;3(6):514-6.
9. Willhoite MB et al. The impact of preconception counseling on pregnancy outcomes. The experience of the Maine Diabetes in Pregnancy Program. Diabetes Care. 1993 Feb;16(2):450-5. doi: 10.2337/diacare.16.2.450.
10. McElvy SS et al. A focused preconceptional and early pregnancy program in women with type 1 diabetes reduces perinatal mortality and malformation rates to general population levels. J Matern Fetal Med. 2000 Jan-Feb;9(1):14-20. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<14::AID-MFM5>3.0.CO;2-K.
11. Rosen JA et al. The history and contributions of the Diabetes in Pregnancy Study Group of North America (1997-2015). Am J Perinatol. 2016 Nov;33(13):1223-6. doi: 10.1055/s-0036-1585082.
12. Driggers RW and Baschat A. The 12th meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA): Introduction and overview. J Matern Fetal Neonatal Med. 2012 Jan;25(1):3-4. doi: 10.3109/14767058.2012.626917.
13. Langer O et al. The proceedings of the Diabetes in Pregnancy Study Group of North America 2009 conference. J Matern Fetal Neonatal Med. 2010 Mar;23(3):196-8. doi: 10.3109/14767050903550634.
14. Reece EA et al. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Ark., May 2002. J Matern Fetal Neonatal Med. 2002 Dec;12(6):362-4. doi: 10.1080/jmf.12.6.362.364.
15. Reece EA and Maulik D. A consensus conference of the Diabetes in Pregnancy Study Group of North America. J Matern Fetal Neonatal Med. 2002 Dec;12(6):361. doi: 10.1080/jmf.12.6.361.361.
16. Gabbe SG. Summation of the second meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA). J Matern Fetal Med. 2000 Jan-Feb;9(1):3-9.
17. Vital Statistics of the United States 1990: Volume I – Natality.
18. Martin JA et al. Births: final data for 2000. Natl Vital Stat Rep. 2002 Feb 12;50(5):1-101. PMID: 11876093.
19. Martin JA et al. Births: final data for 2010. Natl Vital Stat Rep. 2012 Aug 28;61(1):1-72. PMID: 24974589.
20. CDC Website. Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States.
21. Wang Y et al. Has the prevalence of overweight, obesity, and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020 Jun 1;49(3):810-23. doi: 10.1093/ije/dyz273.
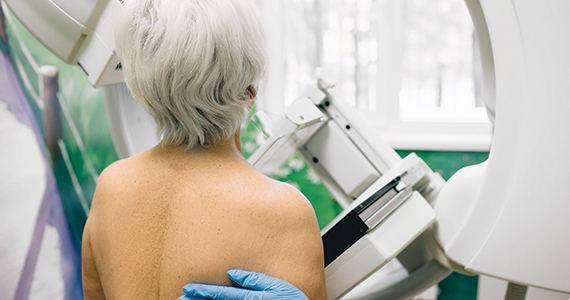
Taking a new obesity drug and birth control pills? Be careful
For women who are obese, daily life is wrought with landmines. Whether it’s the challenges of air travel because plane seats are too small, the need to shield themselves from the world’s discriminating eyes, or the great lengths many will go to achieve better health and the promise of longevity, navigating life as an obese person requires a thick skin.
So, it’s no wonder so many are willing to pay more than $1,000 a month out of pocket to get their hands on drugs like semaglutide (Ozempic and Wegovy) or tirzepatide (Mounjaro). The benefits of these drugs, which are part of a new class called glucagonlike peptide–1 (GLP-1) receptor agonists, include significant and rapid weight loss, blood sugar control, and improved life quality; they are unprecedented in a setting where surgery has long been considered the most effective long-term option.
On the flip side, the desire for rapid weight loss and better blood sugar control also comes with an unexpected cost. , making an unintended pregnancy more likely.
Neel Shah, MD, an endocrinologist and associate professor at the University of Texas Health Science Center at Houston, said he has had several patients become pregnant without intending to.
“It was when Mounjaro came out on the market when we started using it,” he said of the drug the Food and Drug Administration approved for type 2 diabetes in 2022. “It [the warning] was in the product insert, but clinically speaking, I don’t know if it was at the top of providers’ minds when they were prescribing Mounjaro.”
When asked if he believed that we were going to be seeing a significant increase in so-called Mounjaro babies, Dr. Shah was sure in his response.
“Absolutely. We will because the sheer volume [of patients] will increase,” he said.
It’s all in the gut
One of the ways that drugs like Mounjaro work is by delaying the time that it takes for food to move from the stomach to the small intestine. Although data are still evolving, it is believed that this process – delayed gastric emptying – may affect the absorption of birth control pills.
Dr. Shah said another theory is that vomiting, which is a common side effect of these types of drugs, also affects the pills’ ability to prevent pregnancy.
And “there’s a prolonged period of ramping up the dose because of the GI side effects,” said Pinar Kodaman, MD, PhD, a reproductive endocrinologist and assistant professor of gynecology at Yale University in New Haven, Conn.
“Initially, at the lowest dose, there may not be a lot of potential effect on absorption and gastric emptying. But as the dose goes up, it becomes more common, and it can cause diarrhea, which is another condition that can affect the absorption of any medication,” she said.
Unanticipated outcomes, extra prevention
Roughly 42% of women in the United States are obese, 40% of whom are between the ages of 20 and 39. Although these new drugs can improve fertility outcomes for women who are obese (especially those with polycystic ovary syndrome, or PCOS), only one – Mounjaro – currently carries a warning about birth control pill effectiveness on its label. Unfortunately, it appears that some doctors are unaware or not counseling patients about this risk, and the data are unclear about whether other drugs in this class, like Ozempic and Wegovy, have the same risks.
“To date, it hasn’t been a typical thing that we counsel about,” said Dr. Kodaman. “It’s all fairly new, but when we have patients on birth control pills, we do review other medications that they are on because some can affect efficacy, and it’s something to keep in mind.”
It’s also unclear if other forms of birth control – for example, birth control patches that deliver through the skin – might carry similar pregnancy risks. Dr. Shah said some of his patients who became pregnant without intending to were using these patches. This raises even more questions, since they deliver drugs through the skin directly into the bloodstream and not through the GI system.
What can women do to help ensure that they don’t become pregnant while using these drugs?
“I really think that if patients want to protect themselves from an unplanned pregnancy, that as soon as they start the GLP receptor agonists, it wouldn’t be a bad idea to use condoms, because the onset of action is pretty quick,” said Dr. Kodaman, noting also that “at the lowest dose there may not be a lot of potential effect on gastric emptying. But as the dose goes up, it becomes much more common or can cause diarrhea.”
Dr. Shah said that in his practice he’s “been telling patients to add barrier contraception” 4 weeks before they start their first dose “and at any dose adjustment.”
Zoobia Chaudhry, an obesity medicine doctor and assistant professor of medicine at Johns Hopkins University in Baltimore, recommends that “patients just make sure that the injection and medication that they take are at least 1 hour apart.”
“Most of the time, patients do take birth control before bedtime, so if the two are spaced, it should be OK,” she said.
Another option is for women to speak to their doctors about other contraceptive options like IUDs or implantable rods, where gastric absorption is not going to be an issue.
“There’s very little research on this class of drugs,” said Emily Goodstein, a 40-year-old small-business owner in Washington, who recently switched from Ozempic to Mounjaro. “Being a person who lives in a larger body is such a horrifying experience because of the way that the world discriminates against you.”
She appreciates the feeling of being proactive that these new drugs grant. It has “opened up a bunch of opportunities for me to be seen as a full individual by the medical establishment,” she said. “I was willing to take the risk, knowing that I would be on these drugs for the rest of my life.”
In addition to being what Dr. Goodstein refers to as a guinea pig, she said she made sure that her primary care doctor was aware that she was not trying or planning to become pregnant again. (She has a 3-year-old child.) Still, her doctor mentioned only the most common side effects linked to these drugs, like nausea, vomiting, and diarrhea, and did not mention the risk of pregnancy.
“Folks are really not talking about the reproductive implications,” she said, referring to members of a Facebook group on these drugs that she belongs to.
Like patients themselves, many doctors are just beginning to get their arms around these agents. “Awareness, education, provider involvement, and having a multidisciplinary team could help patients achieve the goals that they set out for themselves,” said Dr. Shah.
Clear conversations are key.
A version of this article first appeared on WebMD.com.
For women who are obese, daily life is wrought with landmines. Whether it’s the challenges of air travel because plane seats are too small, the need to shield themselves from the world’s discriminating eyes, or the great lengths many will go to achieve better health and the promise of longevity, navigating life as an obese person requires a thick skin.
So, it’s no wonder so many are willing to pay more than $1,000 a month out of pocket to get their hands on drugs like semaglutide (Ozempic and Wegovy) or tirzepatide (Mounjaro). The benefits of these drugs, which are part of a new class called glucagonlike peptide–1 (GLP-1) receptor agonists, include significant and rapid weight loss, blood sugar control, and improved life quality; they are unprecedented in a setting where surgery has long been considered the most effective long-term option.
On the flip side, the desire for rapid weight loss and better blood sugar control also comes with an unexpected cost. , making an unintended pregnancy more likely.
Neel Shah, MD, an endocrinologist and associate professor at the University of Texas Health Science Center at Houston, said he has had several patients become pregnant without intending to.
“It was when Mounjaro came out on the market when we started using it,” he said of the drug the Food and Drug Administration approved for type 2 diabetes in 2022. “It [the warning] was in the product insert, but clinically speaking, I don’t know if it was at the top of providers’ minds when they were prescribing Mounjaro.”
When asked if he believed that we were going to be seeing a significant increase in so-called Mounjaro babies, Dr. Shah was sure in his response.
“Absolutely. We will because the sheer volume [of patients] will increase,” he said.
It’s all in the gut
One of the ways that drugs like Mounjaro work is by delaying the time that it takes for food to move from the stomach to the small intestine. Although data are still evolving, it is believed that this process – delayed gastric emptying – may affect the absorption of birth control pills.
Dr. Shah said another theory is that vomiting, which is a common side effect of these types of drugs, also affects the pills’ ability to prevent pregnancy.
And “there’s a prolonged period of ramping up the dose because of the GI side effects,” said Pinar Kodaman, MD, PhD, a reproductive endocrinologist and assistant professor of gynecology at Yale University in New Haven, Conn.
“Initially, at the lowest dose, there may not be a lot of potential effect on absorption and gastric emptying. But as the dose goes up, it becomes more common, and it can cause diarrhea, which is another condition that can affect the absorption of any medication,” she said.
Unanticipated outcomes, extra prevention
Roughly 42% of women in the United States are obese, 40% of whom are between the ages of 20 and 39. Although these new drugs can improve fertility outcomes for women who are obese (especially those with polycystic ovary syndrome, or PCOS), only one – Mounjaro – currently carries a warning about birth control pill effectiveness on its label. Unfortunately, it appears that some doctors are unaware or not counseling patients about this risk, and the data are unclear about whether other drugs in this class, like Ozempic and Wegovy, have the same risks.
“To date, it hasn’t been a typical thing that we counsel about,” said Dr. Kodaman. “It’s all fairly new, but when we have patients on birth control pills, we do review other medications that they are on because some can affect efficacy, and it’s something to keep in mind.”
It’s also unclear if other forms of birth control – for example, birth control patches that deliver through the skin – might carry similar pregnancy risks. Dr. Shah said some of his patients who became pregnant without intending to were using these patches. This raises even more questions, since they deliver drugs through the skin directly into the bloodstream and not through the GI system.
What can women do to help ensure that they don’t become pregnant while using these drugs?
“I really think that if patients want to protect themselves from an unplanned pregnancy, that as soon as they start the GLP receptor agonists, it wouldn’t be a bad idea to use condoms, because the onset of action is pretty quick,” said Dr. Kodaman, noting also that “at the lowest dose there may not be a lot of potential effect on gastric emptying. But as the dose goes up, it becomes much more common or can cause diarrhea.”
Dr. Shah said that in his practice he’s “been telling patients to add barrier contraception” 4 weeks before they start their first dose “and at any dose adjustment.”
Zoobia Chaudhry, an obesity medicine doctor and assistant professor of medicine at Johns Hopkins University in Baltimore, recommends that “patients just make sure that the injection and medication that they take are at least 1 hour apart.”
“Most of the time, patients do take birth control before bedtime, so if the two are spaced, it should be OK,” she said.
Another option is for women to speak to their doctors about other contraceptive options like IUDs or implantable rods, where gastric absorption is not going to be an issue.
“There’s very little research on this class of drugs,” said Emily Goodstein, a 40-year-old small-business owner in Washington, who recently switched from Ozempic to Mounjaro. “Being a person who lives in a larger body is such a horrifying experience because of the way that the world discriminates against you.”
She appreciates the feeling of being proactive that these new drugs grant. It has “opened up a bunch of opportunities for me to be seen as a full individual by the medical establishment,” she said. “I was willing to take the risk, knowing that I would be on these drugs for the rest of my life.”
In addition to being what Dr. Goodstein refers to as a guinea pig, she said she made sure that her primary care doctor was aware that she was not trying or planning to become pregnant again. (She has a 3-year-old child.) Still, her doctor mentioned only the most common side effects linked to these drugs, like nausea, vomiting, and diarrhea, and did not mention the risk of pregnancy.
“Folks are really not talking about the reproductive implications,” she said, referring to members of a Facebook group on these drugs that she belongs to.
Like patients themselves, many doctors are just beginning to get their arms around these agents. “Awareness, education, provider involvement, and having a multidisciplinary team could help patients achieve the goals that they set out for themselves,” said Dr. Shah.
Clear conversations are key.
A version of this article first appeared on WebMD.com.
For women who are obese, daily life is wrought with landmines. Whether it’s the challenges of air travel because plane seats are too small, the need to shield themselves from the world’s discriminating eyes, or the great lengths many will go to achieve better health and the promise of longevity, navigating life as an obese person requires a thick skin.
So, it’s no wonder so many are willing to pay more than $1,000 a month out of pocket to get their hands on drugs like semaglutide (Ozempic and Wegovy) or tirzepatide (Mounjaro). The benefits of these drugs, which are part of a new class called glucagonlike peptide–1 (GLP-1) receptor agonists, include significant and rapid weight loss, blood sugar control, and improved life quality; they are unprecedented in a setting where surgery has long been considered the most effective long-term option.
On the flip side, the desire for rapid weight loss and better blood sugar control also comes with an unexpected cost. , making an unintended pregnancy more likely.
Neel Shah, MD, an endocrinologist and associate professor at the University of Texas Health Science Center at Houston, said he has had several patients become pregnant without intending to.
“It was when Mounjaro came out on the market when we started using it,” he said of the drug the Food and Drug Administration approved for type 2 diabetes in 2022. “It [the warning] was in the product insert, but clinically speaking, I don’t know if it was at the top of providers’ minds when they were prescribing Mounjaro.”
When asked if he believed that we were going to be seeing a significant increase in so-called Mounjaro babies, Dr. Shah was sure in his response.
“Absolutely. We will because the sheer volume [of patients] will increase,” he said.
It’s all in the gut
One of the ways that drugs like Mounjaro work is by delaying the time that it takes for food to move from the stomach to the small intestine. Although data are still evolving, it is believed that this process – delayed gastric emptying – may affect the absorption of birth control pills.
Dr. Shah said another theory is that vomiting, which is a common side effect of these types of drugs, also affects the pills’ ability to prevent pregnancy.
And “there’s a prolonged period of ramping up the dose because of the GI side effects,” said Pinar Kodaman, MD, PhD, a reproductive endocrinologist and assistant professor of gynecology at Yale University in New Haven, Conn.
“Initially, at the lowest dose, there may not be a lot of potential effect on absorption and gastric emptying. But as the dose goes up, it becomes more common, and it can cause diarrhea, which is another condition that can affect the absorption of any medication,” she said.
Unanticipated outcomes, extra prevention
Roughly 42% of women in the United States are obese, 40% of whom are between the ages of 20 and 39. Although these new drugs can improve fertility outcomes for women who are obese (especially those with polycystic ovary syndrome, or PCOS), only one – Mounjaro – currently carries a warning about birth control pill effectiveness on its label. Unfortunately, it appears that some doctors are unaware or not counseling patients about this risk, and the data are unclear about whether other drugs in this class, like Ozempic and Wegovy, have the same risks.
“To date, it hasn’t been a typical thing that we counsel about,” said Dr. Kodaman. “It’s all fairly new, but when we have patients on birth control pills, we do review other medications that they are on because some can affect efficacy, and it’s something to keep in mind.”
It’s also unclear if other forms of birth control – for example, birth control patches that deliver through the skin – might carry similar pregnancy risks. Dr. Shah said some of his patients who became pregnant without intending to were using these patches. This raises even more questions, since they deliver drugs through the skin directly into the bloodstream and not through the GI system.
What can women do to help ensure that they don’t become pregnant while using these drugs?
“I really think that if patients want to protect themselves from an unplanned pregnancy, that as soon as they start the GLP receptor agonists, it wouldn’t be a bad idea to use condoms, because the onset of action is pretty quick,” said Dr. Kodaman, noting also that “at the lowest dose there may not be a lot of potential effect on gastric emptying. But as the dose goes up, it becomes much more common or can cause diarrhea.”
Dr. Shah said that in his practice he’s “been telling patients to add barrier contraception” 4 weeks before they start their first dose “and at any dose adjustment.”
Zoobia Chaudhry, an obesity medicine doctor and assistant professor of medicine at Johns Hopkins University in Baltimore, recommends that “patients just make sure that the injection and medication that they take are at least 1 hour apart.”
“Most of the time, patients do take birth control before bedtime, so if the two are spaced, it should be OK,” she said.
Another option is for women to speak to their doctors about other contraceptive options like IUDs or implantable rods, where gastric absorption is not going to be an issue.
“There’s very little research on this class of drugs,” said Emily Goodstein, a 40-year-old small-business owner in Washington, who recently switched from Ozempic to Mounjaro. “Being a person who lives in a larger body is such a horrifying experience because of the way that the world discriminates against you.”
She appreciates the feeling of being proactive that these new drugs grant. It has “opened up a bunch of opportunities for me to be seen as a full individual by the medical establishment,” she said. “I was willing to take the risk, knowing that I would be on these drugs for the rest of my life.”
In addition to being what Dr. Goodstein refers to as a guinea pig, she said she made sure that her primary care doctor was aware that she was not trying or planning to become pregnant again. (She has a 3-year-old child.) Still, her doctor mentioned only the most common side effects linked to these drugs, like nausea, vomiting, and diarrhea, and did not mention the risk of pregnancy.
“Folks are really not talking about the reproductive implications,” she said, referring to members of a Facebook group on these drugs that she belongs to.
Like patients themselves, many doctors are just beginning to get their arms around these agents. “Awareness, education, provider involvement, and having a multidisciplinary team could help patients achieve the goals that they set out for themselves,” said Dr. Shah.
Clear conversations are key.
A version of this article first appeared on WebMD.com.
Employed physicians: A survival guide
The strike by health care workers at Kaiser Permanente may not involve physicians (yet). But as more doctors in the United States are finding themselves working as salaried employees, physicians can – and probably will – become a powerful force for change in a health care system that has shown itself to be increasingly hostile to employee concerns over issues involving patient care, wages and benefits, safety, and well-being.
Salaried employment has its challenges. Physician-employees may have less autonomy and voice in decision-making that affects patients. They may splinter into fragmented work groups; feel isolated; and have different imperatives based on who they are, what they want, and where they work. They may feel more removed from their patients and struggle to build strong relationships, with their employers in the way.
Yet important opportunities exist for doctors when embracing their employee side. Examples of these interests include adequate compensation, wellness, job security, patient and worker safety, health care quality, reasonable workloads and schedules, and fair treatment by employers, including the need to exhibit a strong collective voice in organizational decision-making.
Some believe that physician-employees must be unionized to maximize their rights and power as employees. Many expect physician unionization to take hold more fully over time. Medical residents, the doctors of tomorrow, are already considering unionization in greater numbers. Some are also doing it in the same employment setting alongside other health professionals, such as nurses.
Having studied doctors and their employment situations for years, I am convinced that whether through unionization or another approach, physicians must also change how they think about control; train and learn alongside other health care workers who share similar interests; and elevate at an early career stage their knowledge of the business side of health care.
Adopt a more pragmatic definition of autonomy
Doctors must embrace an updated definition of autonomy – one that matches their status as highly paid labor.
When I have spoken to physicians in my research about what autonomy means to them, many seem unable to reconceptualize it from a vague and absolute form of their profession’s strategic control over their economic fates and technical skills toward an individualized control that is situation-specific, one centered on winning the daily fights about workplace bread-and-butter issues such as those mentioned above.
But a more pragmatic definition of autonomy could get doctors focused on influencing important issues of the patient-care day and enhance their negotiating power with employers. It would allow physicians to break out of what often seems a paralysis of inaction – waiting for employers, insurers, or the government to reinstate the profession’s idealized version of control by handing it back the keys to the health care system through major regulatory, structural, and reimbursement-related changes. This fantasy is unlikely to become reality.
Physician-employees I’ve talked to over the years understand their everyday challenges. But when it comes to engaging in localized and sustained action to overcome them, they often perform less well, leading to feelings of helplessness and burnout. Valuing tactical control over their jobs and work setting will yield smaller but more impactful wins as employees intent on making their everyday work lives better.
Train alongside other health care professionals
Physicians must accept that how they are trained no longer prepares them for the employee world into which most are dropped. For instance, unless doctors are trained collaboratively alongside other health care professionals – such as nurses – they are less likely to identify closely with these colleagues once in practice. There is strength in numbers, so this mutual identification empowers both groups of employees. Yet, medical education remains largely the same: training young medical students in isolation for the first couple of years, then placing them into clerkships and residencies where true interprofessional care opportunities remain stunted and secondary to the “physician as captain of the team” mantra.
Unfortunately, the “hidden curriculum” of medicine helps convince medical students and residents early in their careers that they are the unquestioned leaders in patient care settings. This hierarchy encourages some doctors to keep their psychological distance from other members of the health care team and to resist sharing power, concerns, or insights with less skilled health care workers. This socialization harms the ability of physicians to act in a unified fashion alongside these other workers. Having physicians learn and train alongside other health professionals yields positive benefits for collective advocacy, including a shared sense of purpose, positive views on collaboration with others in the health setting, and greater development of bonds with nonphysician coworkers.
Integrate business with medical training in real time
Medical students and residents generally lack exposure to the everyday business realities of the U.S. health care system. This gap hinders their ability to understand the employee world and push for the types of changes and work conditions that benefit all health care workers. Formal business and management training should be a required part of every U.S. medical school and residency curriculum from day one. If you see it at all in medical schools now, it is mostly by accident, or given separate treatment in the form of standalone MBA or MPH degrees that rarely integrate organically and in real time with actual medical training. Not every doctor needs an MBA or MPH degree. However, all of them require a stronger contextual understanding of how the medicine they wish to practice is shaped by the economic and fiscal circumstances surrounding it – circumstances they do not control.
This is another reason why young doctors are unhappy and burned out. They cannot push for specific changes or properly critique the pros and cons of how their work is structured because they have not been made aware, in real time as they learn clinical practice, how their jobs are shaped by realities such as insurance coverage and reimbursement, the fragmentation of the care delivery system, their employer’s financial health , and the socioeconomic circumstances of their patients. They aren’t given the methods and tools related to process and quality improvement, budgeting, negotiation, risk management, leadership, and talent management that might help them navigate these undermining forces. They also get little advance exposure in their training to important workplace “soft” skills in such areas as how to work in teams, networking, communication and listening, empathy, and problem-solving – all necessary foci for bringing them closer to other health care workers and advocating alongside them effectively with health care employers.
Now is the time for physicians to embrace their identity as employees. Doing so is in their own best interest as professionals. It will help others in the health care workforce as well as patients. Moreover, it provides a needed counterbalance to the powerful corporate ethos now ascendant in U.S. health care.
Timothy Hoff, PhD, is a professor of management and healthcare systems at Northeastern University, Boston, and an associate fellow at the University of Oxford, England. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The strike by health care workers at Kaiser Permanente may not involve physicians (yet). But as more doctors in the United States are finding themselves working as salaried employees, physicians can – and probably will – become a powerful force for change in a health care system that has shown itself to be increasingly hostile to employee concerns over issues involving patient care, wages and benefits, safety, and well-being.
Salaried employment has its challenges. Physician-employees may have less autonomy and voice in decision-making that affects patients. They may splinter into fragmented work groups; feel isolated; and have different imperatives based on who they are, what they want, and where they work. They may feel more removed from their patients and struggle to build strong relationships, with their employers in the way.
Yet important opportunities exist for doctors when embracing their employee side. Examples of these interests include adequate compensation, wellness, job security, patient and worker safety, health care quality, reasonable workloads and schedules, and fair treatment by employers, including the need to exhibit a strong collective voice in organizational decision-making.
Some believe that physician-employees must be unionized to maximize their rights and power as employees. Many expect physician unionization to take hold more fully over time. Medical residents, the doctors of tomorrow, are already considering unionization in greater numbers. Some are also doing it in the same employment setting alongside other health professionals, such as nurses.
Having studied doctors and their employment situations for years, I am convinced that whether through unionization or another approach, physicians must also change how they think about control; train and learn alongside other health care workers who share similar interests; and elevate at an early career stage their knowledge of the business side of health care.
Adopt a more pragmatic definition of autonomy
Doctors must embrace an updated definition of autonomy – one that matches their status as highly paid labor.
When I have spoken to physicians in my research about what autonomy means to them, many seem unable to reconceptualize it from a vague and absolute form of their profession’s strategic control over their economic fates and technical skills toward an individualized control that is situation-specific, one centered on winning the daily fights about workplace bread-and-butter issues such as those mentioned above.
But a more pragmatic definition of autonomy could get doctors focused on influencing important issues of the patient-care day and enhance their negotiating power with employers. It would allow physicians to break out of what often seems a paralysis of inaction – waiting for employers, insurers, or the government to reinstate the profession’s idealized version of control by handing it back the keys to the health care system through major regulatory, structural, and reimbursement-related changes. This fantasy is unlikely to become reality.
Physician-employees I’ve talked to over the years understand their everyday challenges. But when it comes to engaging in localized and sustained action to overcome them, they often perform less well, leading to feelings of helplessness and burnout. Valuing tactical control over their jobs and work setting will yield smaller but more impactful wins as employees intent on making their everyday work lives better.
Train alongside other health care professionals
Physicians must accept that how they are trained no longer prepares them for the employee world into which most are dropped. For instance, unless doctors are trained collaboratively alongside other health care professionals – such as nurses – they are less likely to identify closely with these colleagues once in practice. There is strength in numbers, so this mutual identification empowers both groups of employees. Yet, medical education remains largely the same: training young medical students in isolation for the first couple of years, then placing them into clerkships and residencies where true interprofessional care opportunities remain stunted and secondary to the “physician as captain of the team” mantra.
Unfortunately, the “hidden curriculum” of medicine helps convince medical students and residents early in their careers that they are the unquestioned leaders in patient care settings. This hierarchy encourages some doctors to keep their psychological distance from other members of the health care team and to resist sharing power, concerns, or insights with less skilled health care workers. This socialization harms the ability of physicians to act in a unified fashion alongside these other workers. Having physicians learn and train alongside other health professionals yields positive benefits for collective advocacy, including a shared sense of purpose, positive views on collaboration with others in the health setting, and greater development of bonds with nonphysician coworkers.
Integrate business with medical training in real time
Medical students and residents generally lack exposure to the everyday business realities of the U.S. health care system. This gap hinders their ability to understand the employee world and push for the types of changes and work conditions that benefit all health care workers. Formal business and management training should be a required part of every U.S. medical school and residency curriculum from day one. If you see it at all in medical schools now, it is mostly by accident, or given separate treatment in the form of standalone MBA or MPH degrees that rarely integrate organically and in real time with actual medical training. Not every doctor needs an MBA or MPH degree. However, all of them require a stronger contextual understanding of how the medicine they wish to practice is shaped by the economic and fiscal circumstances surrounding it – circumstances they do not control.
This is another reason why young doctors are unhappy and burned out. They cannot push for specific changes or properly critique the pros and cons of how their work is structured because they have not been made aware, in real time as they learn clinical practice, how their jobs are shaped by realities such as insurance coverage and reimbursement, the fragmentation of the care delivery system, their employer’s financial health , and the socioeconomic circumstances of their patients. They aren’t given the methods and tools related to process and quality improvement, budgeting, negotiation, risk management, leadership, and talent management that might help them navigate these undermining forces. They also get little advance exposure in their training to important workplace “soft” skills in such areas as how to work in teams, networking, communication and listening, empathy, and problem-solving – all necessary foci for bringing them closer to other health care workers and advocating alongside them effectively with health care employers.
Now is the time for physicians to embrace their identity as employees. Doing so is in their own best interest as professionals. It will help others in the health care workforce as well as patients. Moreover, it provides a needed counterbalance to the powerful corporate ethos now ascendant in U.S. health care.
Timothy Hoff, PhD, is a professor of management and healthcare systems at Northeastern University, Boston, and an associate fellow at the University of Oxford, England. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The strike by health care workers at Kaiser Permanente may not involve physicians (yet). But as more doctors in the United States are finding themselves working as salaried employees, physicians can – and probably will – become a powerful force for change in a health care system that has shown itself to be increasingly hostile to employee concerns over issues involving patient care, wages and benefits, safety, and well-being.
Salaried employment has its challenges. Physician-employees may have less autonomy and voice in decision-making that affects patients. They may splinter into fragmented work groups; feel isolated; and have different imperatives based on who they are, what they want, and where they work. They may feel more removed from their patients and struggle to build strong relationships, with their employers in the way.
Yet important opportunities exist for doctors when embracing their employee side. Examples of these interests include adequate compensation, wellness, job security, patient and worker safety, health care quality, reasonable workloads and schedules, and fair treatment by employers, including the need to exhibit a strong collective voice in organizational decision-making.
Some believe that physician-employees must be unionized to maximize their rights and power as employees. Many expect physician unionization to take hold more fully over time. Medical residents, the doctors of tomorrow, are already considering unionization in greater numbers. Some are also doing it in the same employment setting alongside other health professionals, such as nurses.
Having studied doctors and their employment situations for years, I am convinced that whether through unionization or another approach, physicians must also change how they think about control; train and learn alongside other health care workers who share similar interests; and elevate at an early career stage their knowledge of the business side of health care.
Adopt a more pragmatic definition of autonomy
Doctors must embrace an updated definition of autonomy – one that matches their status as highly paid labor.
When I have spoken to physicians in my research about what autonomy means to them, many seem unable to reconceptualize it from a vague and absolute form of their profession’s strategic control over their economic fates and technical skills toward an individualized control that is situation-specific, one centered on winning the daily fights about workplace bread-and-butter issues such as those mentioned above.
But a more pragmatic definition of autonomy could get doctors focused on influencing important issues of the patient-care day and enhance their negotiating power with employers. It would allow physicians to break out of what often seems a paralysis of inaction – waiting for employers, insurers, or the government to reinstate the profession’s idealized version of control by handing it back the keys to the health care system through major regulatory, structural, and reimbursement-related changes. This fantasy is unlikely to become reality.
Physician-employees I’ve talked to over the years understand their everyday challenges. But when it comes to engaging in localized and sustained action to overcome them, they often perform less well, leading to feelings of helplessness and burnout. Valuing tactical control over their jobs and work setting will yield smaller but more impactful wins as employees intent on making their everyday work lives better.
Train alongside other health care professionals
Physicians must accept that how they are trained no longer prepares them for the employee world into which most are dropped. For instance, unless doctors are trained collaboratively alongside other health care professionals – such as nurses – they are less likely to identify closely with these colleagues once in practice. There is strength in numbers, so this mutual identification empowers both groups of employees. Yet, medical education remains largely the same: training young medical students in isolation for the first couple of years, then placing them into clerkships and residencies where true interprofessional care opportunities remain stunted and secondary to the “physician as captain of the team” mantra.
Unfortunately, the “hidden curriculum” of medicine helps convince medical students and residents early in their careers that they are the unquestioned leaders in patient care settings. This hierarchy encourages some doctors to keep their psychological distance from other members of the health care team and to resist sharing power, concerns, or insights with less skilled health care workers. This socialization harms the ability of physicians to act in a unified fashion alongside these other workers. Having physicians learn and train alongside other health professionals yields positive benefits for collective advocacy, including a shared sense of purpose, positive views on collaboration with others in the health setting, and greater development of bonds with nonphysician coworkers.
Integrate business with medical training in real time
Medical students and residents generally lack exposure to the everyday business realities of the U.S. health care system. This gap hinders their ability to understand the employee world and push for the types of changes and work conditions that benefit all health care workers. Formal business and management training should be a required part of every U.S. medical school and residency curriculum from day one. If you see it at all in medical schools now, it is mostly by accident, or given separate treatment in the form of standalone MBA or MPH degrees that rarely integrate organically and in real time with actual medical training. Not every doctor needs an MBA or MPH degree. However, all of them require a stronger contextual understanding of how the medicine they wish to practice is shaped by the economic and fiscal circumstances surrounding it – circumstances they do not control.
This is another reason why young doctors are unhappy and burned out. They cannot push for specific changes or properly critique the pros and cons of how their work is structured because they have not been made aware, in real time as they learn clinical practice, how their jobs are shaped by realities such as insurance coverage and reimbursement, the fragmentation of the care delivery system, their employer’s financial health , and the socioeconomic circumstances of their patients. They aren’t given the methods and tools related to process and quality improvement, budgeting, negotiation, risk management, leadership, and talent management that might help them navigate these undermining forces. They also get little advance exposure in their training to important workplace “soft” skills in such areas as how to work in teams, networking, communication and listening, empathy, and problem-solving – all necessary foci for bringing them closer to other health care workers and advocating alongside them effectively with health care employers.
Now is the time for physicians to embrace their identity as employees. Doing so is in their own best interest as professionals. It will help others in the health care workforce as well as patients. Moreover, it provides a needed counterbalance to the powerful corporate ethos now ascendant in U.S. health care.
Timothy Hoff, PhD, is a professor of management and healthcare systems at Northeastern University, Boston, and an associate fellow at the University of Oxford, England. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Why legal pot makes this physician sick
Last year, my husband and I took a 16-day road trip from Kentucky through Massachusetts to Maine. On our first morning in Boston, we exited the Park Street Station en route to Boston Common, but instead of being greeted by the aroma of molasses, we were hit full-on with a pungent, repulsive odor. “That’s skunk weed,” my husband chuckled as we stepped right into the middle of the Boston Freedom Rally, a celebration of all things cannabis.
As we boarded a hop-on-hop-off bus, we learned that this was the one week of the year that the city skips testing tour bus drivers for tetrahydrocannabinol (THC), “because we all test positive,” the driver quipped. As our open-air bus circled the Common, a crowd of pot enthusiasts displayed signs in support of relaxed regulation for public consumption.
The 34-year-old Boston Freedom Rally is a sign that U.S. culture has transformed forever. Mary Jane is no friend of emergency physicians nor of staff on hospital wards and offices.
Toking boomers and millennials
Researchers at the University of California, San Diego, looked at cannabis-related emergency department visits from all acute-care hospitals in the state from 2005 to 2019 and found an 1,808% increase in patients aged 65 or older (that is not a typo) who were there for complications from cannabis use.
The lead author said in an interview that, “older patients taking marijuana or related products may have dizziness and falls, heart palpitations, panic attacks, confusion, anxiety or worsening of underlying lung diseases, such as asthma or [chronic obstructive pulmonary disease].”
A recent study from Canada suggests that commercialization has been associated with an increase in related hospitalizations, including cannabis-induced psychosis.
According to a National Study of Drug Use and Health, marijuana use in young adults reached an all-time high (pun intended) in 2021. Nearly 10% of eighth graders and 20% of 10th graders reported using marijuana this past year.
The full downside of any drug, legal or illegal, is largely unknown until it infiltrates the mainstream market, but these are the typical cases we see:
Let’s start with the demotivated high school honors student who dropped out of college to work at the local cinema. He stumbled and broke his clavicle outside a bar at 2 AM, but he wasn’t sure if he passed out, so a cardiology consult was requested to “rule out” arrhythmia associated with syncope. He related that his plan to become a railway conductor had been upended because he knew he would be drug tested and just couldn’t give up pot. After a normal cardiac exam, ECG, labs, a Holter, and an echocardiogram were also requested and normal at a significant cost.
Cannabinoid hyperemesis syndrome
One of my Midwest colleagues related her encounter with two middle-aged pot users with ventricular tachycardia (VT). These episodes coincided with potassium levels less than 3.0 mEq/L in the setting of repetitive vomiting. The QTc interval didn’t normalize despite a corrected potassium level in one patient. They were both informed that they should never smoke pot because vomiting would predictably drop their K+ levels again and prolong their QTc intervals. Then began “the circular argument,” as my friend described it. The patient claims, “I smoke pot to relieve my nausea,” to which she explains that “in many folks, pot use induces nausea.” Of course, the classic reply is, “Not me.” Predictably one of these stoners soon returned with more VT, more puking, and more hypokalemia. “Consider yourself ‘allergic’ to pot smoke,” my friend advised, but “was met with no meaningful hint of understanding or hope for transformative change,” she told me.
I’ve seen cannabinoid hyperemesis syndrome several times in the past few years. It occurs in daily to weekly pot users. Very rarely, it can cause cerebral edema, but it is also associated with seizures and dehydration that can lead to hypovolemic shock and kidney failure.
Heart and brain harm
Then there are the young patients who for various reasons have developed heart failure. Unfortunately, some are repetitively tox screen positive with varying trifectas of methamphetamine (meth), cocaine, and THC; opiates, meth, and THC; alcohol, meth, and THC; or heroin, meth, and THC. THC, the ever present and essential third leg of the stool of stupor. These unfortunate patients often need heart failure medications that they can’t afford or won’t take because illicit drug use is expensive and dulls their ability to prioritize their health. Some desperately need a heart transplant, but the necessary negative drug screen is a pipe dream.
And it’s not just the heart that is affected. There are data linking cannabis use to a higher risk for both ischemic and hemorrhagic stroke. A retrospective study published in Stroke, of more than 1,000 people diagnosed with an aneurysmal subarachnoid hemorrhage, found that more than half of the 46 who tested positive for THC at admission developed delayed cerebral ischemia (DCI), which increases the risk for disability or early death. This was after adjusting for several patient characteristics as well as recent exposure to other illicit substances; cocaine, meth, and tobacco use were not associated with DCI.
Natural my ...
I’m certain my anti-cannabis stance will strike a nerve with those who love their recreational THC and push for its legal sale; after all, “It’s perfectly natural.” But I counter with the fact that tornadoes, earthquakes, cyanide, and appendicitis are all natural but certainly not optimal. And what we are seeing in the vascular specialties is completely unnatural. We are treating a different mix of complications than before pot was readily accessible across several states.
Our most effective action is to educate our patients. We should encourage those who don’t currently smoke cannabis to never start and those who do to quit. People who require marijuana for improved quality of life for terminal care or true (not supposed) disorders that mainstream medicine fails should be approached with empathy and caution.
A good rule of thumb is to never breathe anything you can see. Never put anything in your body that comes off the street: Drug dealers who sell cannabis cut with fentanyl will be ecstatic to take someone’s money then merely keep scrolling when their obituary comes up.
Let’s try to reverse the rise of vascular complications, orthopedic injuries, and vomiting across America. We can start by encouraging our patients to avoid “skunk weed” and get back to the sweet smells of nature in our cities and parks.
Some details have been changed to protect the patients’ identities, but the essence of their diagnoses has been preserved.
Dr. Walton-Shirley is a retired clinical cardiologist from Nashville, Tenn. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Last year, my husband and I took a 16-day road trip from Kentucky through Massachusetts to Maine. On our first morning in Boston, we exited the Park Street Station en route to Boston Common, but instead of being greeted by the aroma of molasses, we were hit full-on with a pungent, repulsive odor. “That’s skunk weed,” my husband chuckled as we stepped right into the middle of the Boston Freedom Rally, a celebration of all things cannabis.
As we boarded a hop-on-hop-off bus, we learned that this was the one week of the year that the city skips testing tour bus drivers for tetrahydrocannabinol (THC), “because we all test positive,” the driver quipped. As our open-air bus circled the Common, a crowd of pot enthusiasts displayed signs in support of relaxed regulation for public consumption.
The 34-year-old Boston Freedom Rally is a sign that U.S. culture has transformed forever. Mary Jane is no friend of emergency physicians nor of staff on hospital wards and offices.
Toking boomers and millennials
Researchers at the University of California, San Diego, looked at cannabis-related emergency department visits from all acute-care hospitals in the state from 2005 to 2019 and found an 1,808% increase in patients aged 65 or older (that is not a typo) who were there for complications from cannabis use.
The lead author said in an interview that, “older patients taking marijuana or related products may have dizziness and falls, heart palpitations, panic attacks, confusion, anxiety or worsening of underlying lung diseases, such as asthma or [chronic obstructive pulmonary disease].”
A recent study from Canada suggests that commercialization has been associated with an increase in related hospitalizations, including cannabis-induced psychosis.
According to a National Study of Drug Use and Health, marijuana use in young adults reached an all-time high (pun intended) in 2021. Nearly 10% of eighth graders and 20% of 10th graders reported using marijuana this past year.
The full downside of any drug, legal or illegal, is largely unknown until it infiltrates the mainstream market, but these are the typical cases we see:
Let’s start with the demotivated high school honors student who dropped out of college to work at the local cinema. He stumbled and broke his clavicle outside a bar at 2 AM, but he wasn’t sure if he passed out, so a cardiology consult was requested to “rule out” arrhythmia associated with syncope. He related that his plan to become a railway conductor had been upended because he knew he would be drug tested and just couldn’t give up pot. After a normal cardiac exam, ECG, labs, a Holter, and an echocardiogram were also requested and normal at a significant cost.
Cannabinoid hyperemesis syndrome
One of my Midwest colleagues related her encounter with two middle-aged pot users with ventricular tachycardia (VT). These episodes coincided with potassium levels less than 3.0 mEq/L in the setting of repetitive vomiting. The QTc interval didn’t normalize despite a corrected potassium level in one patient. They were both informed that they should never smoke pot because vomiting would predictably drop their K+ levels again and prolong their QTc intervals. Then began “the circular argument,” as my friend described it. The patient claims, “I smoke pot to relieve my nausea,” to which she explains that “in many folks, pot use induces nausea.” Of course, the classic reply is, “Not me.” Predictably one of these stoners soon returned with more VT, more puking, and more hypokalemia. “Consider yourself ‘allergic’ to pot smoke,” my friend advised, but “was met with no meaningful hint of understanding or hope for transformative change,” she told me.
I’ve seen cannabinoid hyperemesis syndrome several times in the past few years. It occurs in daily to weekly pot users. Very rarely, it can cause cerebral edema, but it is also associated with seizures and dehydration that can lead to hypovolemic shock and kidney failure.
Heart and brain harm
Then there are the young patients who for various reasons have developed heart failure. Unfortunately, some are repetitively tox screen positive with varying trifectas of methamphetamine (meth), cocaine, and THC; opiates, meth, and THC; alcohol, meth, and THC; or heroin, meth, and THC. THC, the ever present and essential third leg of the stool of stupor. These unfortunate patients often need heart failure medications that they can’t afford or won’t take because illicit drug use is expensive and dulls their ability to prioritize their health. Some desperately need a heart transplant, but the necessary negative drug screen is a pipe dream.
And it’s not just the heart that is affected. There are data linking cannabis use to a higher risk for both ischemic and hemorrhagic stroke. A retrospective study published in Stroke, of more than 1,000 people diagnosed with an aneurysmal subarachnoid hemorrhage, found that more than half of the 46 who tested positive for THC at admission developed delayed cerebral ischemia (DCI), which increases the risk for disability or early death. This was after adjusting for several patient characteristics as well as recent exposure to other illicit substances; cocaine, meth, and tobacco use were not associated with DCI.
Natural my ...
I’m certain my anti-cannabis stance will strike a nerve with those who love their recreational THC and push for its legal sale; after all, “It’s perfectly natural.” But I counter with the fact that tornadoes, earthquakes, cyanide, and appendicitis are all natural but certainly not optimal. And what we are seeing in the vascular specialties is completely unnatural. We are treating a different mix of complications than before pot was readily accessible across several states.
Our most effective action is to educate our patients. We should encourage those who don’t currently smoke cannabis to never start and those who do to quit. People who require marijuana for improved quality of life for terminal care or true (not supposed) disorders that mainstream medicine fails should be approached with empathy and caution.
A good rule of thumb is to never breathe anything you can see. Never put anything in your body that comes off the street: Drug dealers who sell cannabis cut with fentanyl will be ecstatic to take someone’s money then merely keep scrolling when their obituary comes up.
Let’s try to reverse the rise of vascular complications, orthopedic injuries, and vomiting across America. We can start by encouraging our patients to avoid “skunk weed” and get back to the sweet smells of nature in our cities and parks.
Some details have been changed to protect the patients’ identities, but the essence of their diagnoses has been preserved.
Dr. Walton-Shirley is a retired clinical cardiologist from Nashville, Tenn. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Last year, my husband and I took a 16-day road trip from Kentucky through Massachusetts to Maine. On our first morning in Boston, we exited the Park Street Station en route to Boston Common, but instead of being greeted by the aroma of molasses, we were hit full-on with a pungent, repulsive odor. “That’s skunk weed,” my husband chuckled as we stepped right into the middle of the Boston Freedom Rally, a celebration of all things cannabis.
As we boarded a hop-on-hop-off bus, we learned that this was the one week of the year that the city skips testing tour bus drivers for tetrahydrocannabinol (THC), “because we all test positive,” the driver quipped. As our open-air bus circled the Common, a crowd of pot enthusiasts displayed signs in support of relaxed regulation for public consumption.
The 34-year-old Boston Freedom Rally is a sign that U.S. culture has transformed forever. Mary Jane is no friend of emergency physicians nor of staff on hospital wards and offices.
Toking boomers and millennials
Researchers at the University of California, San Diego, looked at cannabis-related emergency department visits from all acute-care hospitals in the state from 2005 to 2019 and found an 1,808% increase in patients aged 65 or older (that is not a typo) who were there for complications from cannabis use.
The lead author said in an interview that, “older patients taking marijuana or related products may have dizziness and falls, heart palpitations, panic attacks, confusion, anxiety or worsening of underlying lung diseases, such as asthma or [chronic obstructive pulmonary disease].”
A recent study from Canada suggests that commercialization has been associated with an increase in related hospitalizations, including cannabis-induced psychosis.
According to a National Study of Drug Use and Health, marijuana use in young adults reached an all-time high (pun intended) in 2021. Nearly 10% of eighth graders and 20% of 10th graders reported using marijuana this past year.
The full downside of any drug, legal or illegal, is largely unknown until it infiltrates the mainstream market, but these are the typical cases we see:
Let’s start with the demotivated high school honors student who dropped out of college to work at the local cinema. He stumbled and broke his clavicle outside a bar at 2 AM, but he wasn’t sure if he passed out, so a cardiology consult was requested to “rule out” arrhythmia associated with syncope. He related that his plan to become a railway conductor had been upended because he knew he would be drug tested and just couldn’t give up pot. After a normal cardiac exam, ECG, labs, a Holter, and an echocardiogram were also requested and normal at a significant cost.
Cannabinoid hyperemesis syndrome
One of my Midwest colleagues related her encounter with two middle-aged pot users with ventricular tachycardia (VT). These episodes coincided with potassium levels less than 3.0 mEq/L in the setting of repetitive vomiting. The QTc interval didn’t normalize despite a corrected potassium level in one patient. They were both informed that they should never smoke pot because vomiting would predictably drop their K+ levels again and prolong their QTc intervals. Then began “the circular argument,” as my friend described it. The patient claims, “I smoke pot to relieve my nausea,” to which she explains that “in many folks, pot use induces nausea.” Of course, the classic reply is, “Not me.” Predictably one of these stoners soon returned with more VT, more puking, and more hypokalemia. “Consider yourself ‘allergic’ to pot smoke,” my friend advised, but “was met with no meaningful hint of understanding or hope for transformative change,” she told me.
I’ve seen cannabinoid hyperemesis syndrome several times in the past few years. It occurs in daily to weekly pot users. Very rarely, it can cause cerebral edema, but it is also associated with seizures and dehydration that can lead to hypovolemic shock and kidney failure.
Heart and brain harm
Then there are the young patients who for various reasons have developed heart failure. Unfortunately, some are repetitively tox screen positive with varying trifectas of methamphetamine (meth), cocaine, and THC; opiates, meth, and THC; alcohol, meth, and THC; or heroin, meth, and THC. THC, the ever present and essential third leg of the stool of stupor. These unfortunate patients often need heart failure medications that they can’t afford or won’t take because illicit drug use is expensive and dulls their ability to prioritize their health. Some desperately need a heart transplant, but the necessary negative drug screen is a pipe dream.
And it’s not just the heart that is affected. There are data linking cannabis use to a higher risk for both ischemic and hemorrhagic stroke. A retrospective study published in Stroke, of more than 1,000 people diagnosed with an aneurysmal subarachnoid hemorrhage, found that more than half of the 46 who tested positive for THC at admission developed delayed cerebral ischemia (DCI), which increases the risk for disability or early death. This was after adjusting for several patient characteristics as well as recent exposure to other illicit substances; cocaine, meth, and tobacco use were not associated with DCI.
Natural my ...
I’m certain my anti-cannabis stance will strike a nerve with those who love their recreational THC and push for its legal sale; after all, “It’s perfectly natural.” But I counter with the fact that tornadoes, earthquakes, cyanide, and appendicitis are all natural but certainly not optimal. And what we are seeing in the vascular specialties is completely unnatural. We are treating a different mix of complications than before pot was readily accessible across several states.
Our most effective action is to educate our patients. We should encourage those who don’t currently smoke cannabis to never start and those who do to quit. People who require marijuana for improved quality of life for terminal care or true (not supposed) disorders that mainstream medicine fails should be approached with empathy and caution.
A good rule of thumb is to never breathe anything you can see. Never put anything in your body that comes off the street: Drug dealers who sell cannabis cut with fentanyl will be ecstatic to take someone’s money then merely keep scrolling when their obituary comes up.
Let’s try to reverse the rise of vascular complications, orthopedic injuries, and vomiting across America. We can start by encouraging our patients to avoid “skunk weed” and get back to the sweet smells of nature in our cities and parks.
Some details have been changed to protect the patients’ identities, but the essence of their diagnoses has been preserved.
Dr. Walton-Shirley is a retired clinical cardiologist from Nashville, Tenn. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Prior authorization software: Saves time but hurdles remain
New England Baptist Hospital has been grappling with a serious problem facing health care today: insurers demanding prior authorizations for services ordered by physicians. Meeting payers’ requirements eats up time, delays treatment, and can be a costly drain on doctors’ practices.
To deal with this problem, the Boston orthopedic hospital has opted to automate submission of prior authorization requests on behalf of more than 100 mostly orthopedic surgeons on staff.
After 5 years using this system, “we can say that automation definitely works,” said Lidiya Hadzhieva, director of patient access at the hospital. The software has reduced write-offs by 30% and staff costs by 25%. Prior authorization gets approved 3 days after scheduling, compared with 11 days previously, she said.
“This software not only saves staff time, but it can also more accurately predict when prior authorization is needed,” she added.
For practices deluged with required prior authorizations by insurers, automation is emerging as a way for practices to make the process less time-consuming and save money. However, the software can be costly and may not be adoptable to many practices, and many physicians are not even aware it exists.
So far, the software is mainly used at large organizations like hospital systems. But as word gets out and the software becomes easier to use, private practices and other smaller entities may join the automation trend.
There is definitely a need to automate prior authorization. The American Medical Association reports that physicians spend 16 hours per week on prior authorizations. In a recent AMA survey, more than 60% of physicians indicated that it’s difficult to know when prior authorization is needed. And 93% of physicians reported care delays while waiting for authorization, the AMA said.
Experts estimate that 80% of prior authorization work could be automated, but most practices still use the phone or fax, even as numbers of prior authorizations continue to increase.
How it works
Automation software connects directly to the practice’s electronic health record (EHR). “When the doctor places an order in the EHR, the process starts automatically,” Ms. Hadzhieva said. “The doctor may not even notice it.”
In addition to using an EHR connection, many software products can communicate with the payer through its portal or by fax or phone, while still automating other parts of the process.
The software’s first step is to decide whether prior authorization is needed. This requires having an updated list of the rules that each payer uses for prior authorization. Manually keeping track of payer rules is very time-consuming, but automation uses bots to visit each payer site to look for rules changes. One vendor, Infinitus, uses a voice-based bot called Eva that calls up each payer and speaks with a representative.
“Automatically updating payer rules is not a new technology,” said YiDing Yu, MD, chief product officer at Olive, the automation vendor for New England Baptist. “What is new in the last 5 years is extracting the information needed for the prior authorization out of the clinical notes.”
This is challenging because each doctor has different ways to describe each step of clinical work. To identify this shorthand, Dr. Yu said Olive uses natural language processing, which is a form of artificial intelligence that learns how each doctor describes things.
Dr. Yu asserts that Olive is actually better than a practice’s staff at digging out clinical information. She said staff without much clinical training may miss terms that the software can catch, and they don’t have the time to go back many months into the record to find valuable information. But automation can do that.
In some instances, however, the software may not be able to find the information, in which case it alerts staff through a prompt in the EHR and the information is retrieved manually, Dr. Yu said.
Next, the Olive software puts the information it found into the request form and sends it to the payer. After submission, the software constantly checks on the status of each request, again visiting payer sites with a bot.
At New England Baptist, the software is used mainly by physicians in fairly small private practices who are on staff. They are using the software on the hospital’s dime, but it only works inside the hospital, Ms. Hadzhieva said. For their work outside of the hospital, they would have to purchase the Olive software on their own, she said.
Automation hasn’t spread to practices yet
Despite the promising outcomes for products like Olive, automation software is still primarily used by large organizations. Vendors say very few private practices have bought it yet. “The technology works, but it is still in the early-adopter phase,” Dr. Yu said.
For one thing, the software can be expensive. Very few vendors reveal their prices, but Dr. Yu did so. She said Olive normally costs about $50,000 a year for even a small organization. She insisted, however, that the savings from avoiding just one denial each month for a hip surgery would justify the expense.
On the other hand, some automation software is free, such as the Surescripts product for prior authorization of prescriptions. But it is unclear whether Surescripts does as much as Olive. Vendors’ descriptions of their products tend to be vague.
Also, Surescripts and Olive have entirely separate functions. Dr. Yu said Olive is limited to procedures, so it benefits specialties like oncology, neurosurgery, colorectal surgery, vascular surgery, and cardiology. Olive does not cover prescriptions, because they operate on a different technology.
Dr. Yu said another hurdle for adopting the software is the kind of EHR systems that doctors use. At this point, only a few EHR systems – such as Epic, Cerner, and Athena – are compatible with Olive. Large organizations tend to use Epic and Cerner, while many practices often use Athena or a variety of other systems, she said.
Despite stunted demand, there is no shortage of companies offering automation software for medical (that is, non-prescription) prior authorization. One compilation lists 25 such vendors, including companies like Myndshft, Rhyme, Infinitus, Infinx, and Waystar. As with any start-up technology, companies occasionally buy each other out.
In addition to issues like cost, specialty, and EHR compatibility, another hurdle is that few doctors even know the technology exists. Vendors say marketing focuses on larger provider organizations, not smaller practices.
Even many tech-savvy doctors, like Adam Bruggeman, MD, an orthopedist and CEO of Texas Spine Care Center in San Antonio, say they know little about the technology. “There is definitely a need to automate prior authorization,” he said. “But I don’t know of any colleagues who use it.” He has only just begun to explore vendors, he said.
Many medical practice consultants also have not yet explored the technology. “Automation makes a lot of sense, because there are a lot of repetitive tasks in prior authorization,” said Jill Arena, CEO of Portland, Ore.–based Health e Practices. “But I haven’t looked into it yet, and none of my clients has even asked about it.”
“I could see how it could be an easier sell for large organizations,” she added. “They have an IT person and a CFO who can explore the issue. Smaller practices usually don’t have that kind of expertise.”
Where does automation go from here?
Until now, clinicians who want to fully automate prior authorizations would have to buy two products – one for medical procedures and one for prescriptions. This has to do with incompatible electronic transmission standards, which are used to digitize information, said Susan Lawson-Dawson, content marketing strategist for the vendor Myndshft Health.
Myndshft has long been selling automation software for medical prior authorizations, but now it is introducing a product for prescriptions, Ms. Lawson-Dawson said. She said Myndshft will then be the only vendor to automate both kinds of prior authorizations.
Ms. Lawson-Dawson said Myndshft has 685 customers to date and is looking for more business. Recently the company entered the Google Cloud Marketplace. Google Cloud customers can now direct their committed spend with Google to purchasing Myndshft, meaning they could get it at a discount.
Software like Olive and Myndshft can operate independently of payers, but a vendor called Rhyme depends on payers for its software to function, said Rhyme CEO Joe Anstine. He said more than 300 payers have agreed to install the Rhyme system, and Rhyme has signed up a number of large health systems to use the product. Initially, he said, clinicians paid for the service, but now Rhyme is beginning to find payers to foot the costs and to let clinicians use it for free, which would open Rhyme up to smaller practices.
EHR companies themselves are beginning to offer automation, too. Epic, for example, has created a tool for prior authorization as part of its Epic Payer Platform. Like Rhyme, it requires payer cooperation, because information goes back and forth between clinician and payer in what is called bi-directional exchange.
The Epic product is still in its pilot phase. Epic reported that several large health systems were using its product in conjunction with a specific payer – for instance, Mayo Clinic with Blue Cross and Blue Shield of Minnesota and Ochsner Health with Humana. According to Epic, the arrangement reduced Mayo’s denials due to additional documentation requests by 63% for professional billing.
Automating with just one payer still means the clinician has to deal with manual processes at other payers, but a large clinician could have sufficient volume with that one payer to make the arrangement useful.
Will payers automate prior authorization?
Ultimately, payers may take the automation business away from vendors, offering a free product to all clinicians. But don’t hold your breath. Payers first have to rebuild their electronic systems to accommodate an electronic connection with providers. Even then, some payers might hold back from automating, forcing practices to continue manually processing some prior authorizations.
Efforts are underway, however, to mandate payers to support prior authorization automation. For this to happen, payers would have to revamp their data so that it could be easily read by practices’ EHRs. This would mean adopting a specific interoperability standard called Health Level 7 Fast Healthcare Interoperability Resources (FHIR).
Toward this goal, the Centers for Medicare & Medicaid Services proposes to require payers to adopt FHIR by January 2026. (CMS still has to finalize the rule.) Experts say the two-year ramp-up time is needed because it takes extensive work for payers to translate their data into FHIR.
The only payer so far to switch to FHIR for prior authorization is Regence in Washington state. In a pilot project, it has automated prior authorization with just one provider, MultiCare Connected Care, an accountable care organization (ACO), also in Washington state.
Anna Taylor, associate vice president of population health and value-based care at MultiCare, explained how the arrangement works. “Two separate entities are sharing one operational process,” she told this news organization. “That means they can have a digital conversation back and forth, so it is much easier to resolve prior authorization issues.”
Unlike many vendor products, the Regence service is free. And while the vendors market only to large organizations, most doctors in the MultiCare arrangement are in independent practices. Ms. Taylor said these doctors have been “enthusiastic” about the arrangement.
The results of the pilot are impressive. Ms. Taylor said automation has resulted in a 233% productivity gain for MultiCare clinicians, and 89% of submissions to Regence get an immediate response.
There is a potential downside, however, to working directly with payers. A direct connection to clinicians allows payers to access the doctor’s clinical notes, which could make many doctors uneasy. But Ms. Taylor said Regence only has access to the “discrete data fields” on MultiCare’s EHR dashboard, not to the notes themselves.
The ultimate goal of the Regence-Multicare project is to include more payers and clinicians. Ms. Taylor said two of the 27 other payers that MultiCare works with are “highly interested,” but it would take a lot of work for them to get connected with practices and other clinicians.
Ultimately, payers could offer automation and third-party vendors might then fade away. However, physicians may resist working directly with payers if the arrangement requires full access to their medical records.
A version of this article first appeared on Medscape.com.
New England Baptist Hospital has been grappling with a serious problem facing health care today: insurers demanding prior authorizations for services ordered by physicians. Meeting payers’ requirements eats up time, delays treatment, and can be a costly drain on doctors’ practices.
To deal with this problem, the Boston orthopedic hospital has opted to automate submission of prior authorization requests on behalf of more than 100 mostly orthopedic surgeons on staff.
After 5 years using this system, “we can say that automation definitely works,” said Lidiya Hadzhieva, director of patient access at the hospital. The software has reduced write-offs by 30% and staff costs by 25%. Prior authorization gets approved 3 days after scheduling, compared with 11 days previously, she said.
“This software not only saves staff time, but it can also more accurately predict when prior authorization is needed,” she added.
For practices deluged with required prior authorizations by insurers, automation is emerging as a way for practices to make the process less time-consuming and save money. However, the software can be costly and may not be adoptable to many practices, and many physicians are not even aware it exists.
So far, the software is mainly used at large organizations like hospital systems. But as word gets out and the software becomes easier to use, private practices and other smaller entities may join the automation trend.
There is definitely a need to automate prior authorization. The American Medical Association reports that physicians spend 16 hours per week on prior authorizations. In a recent AMA survey, more than 60% of physicians indicated that it’s difficult to know when prior authorization is needed. And 93% of physicians reported care delays while waiting for authorization, the AMA said.
Experts estimate that 80% of prior authorization work could be automated, but most practices still use the phone or fax, even as numbers of prior authorizations continue to increase.
How it works
Automation software connects directly to the practice’s electronic health record (EHR). “When the doctor places an order in the EHR, the process starts automatically,” Ms. Hadzhieva said. “The doctor may not even notice it.”
In addition to using an EHR connection, many software products can communicate with the payer through its portal or by fax or phone, while still automating other parts of the process.
The software’s first step is to decide whether prior authorization is needed. This requires having an updated list of the rules that each payer uses for prior authorization. Manually keeping track of payer rules is very time-consuming, but automation uses bots to visit each payer site to look for rules changes. One vendor, Infinitus, uses a voice-based bot called Eva that calls up each payer and speaks with a representative.
“Automatically updating payer rules is not a new technology,” said YiDing Yu, MD, chief product officer at Olive, the automation vendor for New England Baptist. “What is new in the last 5 years is extracting the information needed for the prior authorization out of the clinical notes.”
This is challenging because each doctor has different ways to describe each step of clinical work. To identify this shorthand, Dr. Yu said Olive uses natural language processing, which is a form of artificial intelligence that learns how each doctor describes things.
Dr. Yu asserts that Olive is actually better than a practice’s staff at digging out clinical information. She said staff without much clinical training may miss terms that the software can catch, and they don’t have the time to go back many months into the record to find valuable information. But automation can do that.
In some instances, however, the software may not be able to find the information, in which case it alerts staff through a prompt in the EHR and the information is retrieved manually, Dr. Yu said.
Next, the Olive software puts the information it found into the request form and sends it to the payer. After submission, the software constantly checks on the status of each request, again visiting payer sites with a bot.
At New England Baptist, the software is used mainly by physicians in fairly small private practices who are on staff. They are using the software on the hospital’s dime, but it only works inside the hospital, Ms. Hadzhieva said. For their work outside of the hospital, they would have to purchase the Olive software on their own, she said.
Automation hasn’t spread to practices yet
Despite the promising outcomes for products like Olive, automation software is still primarily used by large organizations. Vendors say very few private practices have bought it yet. “The technology works, but it is still in the early-adopter phase,” Dr. Yu said.
For one thing, the software can be expensive. Very few vendors reveal their prices, but Dr. Yu did so. She said Olive normally costs about $50,000 a year for even a small organization. She insisted, however, that the savings from avoiding just one denial each month for a hip surgery would justify the expense.
On the other hand, some automation software is free, such as the Surescripts product for prior authorization of prescriptions. But it is unclear whether Surescripts does as much as Olive. Vendors’ descriptions of their products tend to be vague.
Also, Surescripts and Olive have entirely separate functions. Dr. Yu said Olive is limited to procedures, so it benefits specialties like oncology, neurosurgery, colorectal surgery, vascular surgery, and cardiology. Olive does not cover prescriptions, because they operate on a different technology.
Dr. Yu said another hurdle for adopting the software is the kind of EHR systems that doctors use. At this point, only a few EHR systems – such as Epic, Cerner, and Athena – are compatible with Olive. Large organizations tend to use Epic and Cerner, while many practices often use Athena or a variety of other systems, she said.
Despite stunted demand, there is no shortage of companies offering automation software for medical (that is, non-prescription) prior authorization. One compilation lists 25 such vendors, including companies like Myndshft, Rhyme, Infinitus, Infinx, and Waystar. As with any start-up technology, companies occasionally buy each other out.
In addition to issues like cost, specialty, and EHR compatibility, another hurdle is that few doctors even know the technology exists. Vendors say marketing focuses on larger provider organizations, not smaller practices.
Even many tech-savvy doctors, like Adam Bruggeman, MD, an orthopedist and CEO of Texas Spine Care Center in San Antonio, say they know little about the technology. “There is definitely a need to automate prior authorization,” he said. “But I don’t know of any colleagues who use it.” He has only just begun to explore vendors, he said.
Many medical practice consultants also have not yet explored the technology. “Automation makes a lot of sense, because there are a lot of repetitive tasks in prior authorization,” said Jill Arena, CEO of Portland, Ore.–based Health e Practices. “But I haven’t looked into it yet, and none of my clients has even asked about it.”
“I could see how it could be an easier sell for large organizations,” she added. “They have an IT person and a CFO who can explore the issue. Smaller practices usually don’t have that kind of expertise.”
Where does automation go from here?
Until now, clinicians who want to fully automate prior authorizations would have to buy two products – one for medical procedures and one for prescriptions. This has to do with incompatible electronic transmission standards, which are used to digitize information, said Susan Lawson-Dawson, content marketing strategist for the vendor Myndshft Health.
Myndshft has long been selling automation software for medical prior authorizations, but now it is introducing a product for prescriptions, Ms. Lawson-Dawson said. She said Myndshft will then be the only vendor to automate both kinds of prior authorizations.
Ms. Lawson-Dawson said Myndshft has 685 customers to date and is looking for more business. Recently the company entered the Google Cloud Marketplace. Google Cloud customers can now direct their committed spend with Google to purchasing Myndshft, meaning they could get it at a discount.
Software like Olive and Myndshft can operate independently of payers, but a vendor called Rhyme depends on payers for its software to function, said Rhyme CEO Joe Anstine. He said more than 300 payers have agreed to install the Rhyme system, and Rhyme has signed up a number of large health systems to use the product. Initially, he said, clinicians paid for the service, but now Rhyme is beginning to find payers to foot the costs and to let clinicians use it for free, which would open Rhyme up to smaller practices.
EHR companies themselves are beginning to offer automation, too. Epic, for example, has created a tool for prior authorization as part of its Epic Payer Platform. Like Rhyme, it requires payer cooperation, because information goes back and forth between clinician and payer in what is called bi-directional exchange.
The Epic product is still in its pilot phase. Epic reported that several large health systems were using its product in conjunction with a specific payer – for instance, Mayo Clinic with Blue Cross and Blue Shield of Minnesota and Ochsner Health with Humana. According to Epic, the arrangement reduced Mayo’s denials due to additional documentation requests by 63% for professional billing.
Automating with just one payer still means the clinician has to deal with manual processes at other payers, but a large clinician could have sufficient volume with that one payer to make the arrangement useful.
Will payers automate prior authorization?
Ultimately, payers may take the automation business away from vendors, offering a free product to all clinicians. But don’t hold your breath. Payers first have to rebuild their electronic systems to accommodate an electronic connection with providers. Even then, some payers might hold back from automating, forcing practices to continue manually processing some prior authorizations.
Efforts are underway, however, to mandate payers to support prior authorization automation. For this to happen, payers would have to revamp their data so that it could be easily read by practices’ EHRs. This would mean adopting a specific interoperability standard called Health Level 7 Fast Healthcare Interoperability Resources (FHIR).
Toward this goal, the Centers for Medicare & Medicaid Services proposes to require payers to adopt FHIR by January 2026. (CMS still has to finalize the rule.) Experts say the two-year ramp-up time is needed because it takes extensive work for payers to translate their data into FHIR.
The only payer so far to switch to FHIR for prior authorization is Regence in Washington state. In a pilot project, it has automated prior authorization with just one provider, MultiCare Connected Care, an accountable care organization (ACO), also in Washington state.
Anna Taylor, associate vice president of population health and value-based care at MultiCare, explained how the arrangement works. “Two separate entities are sharing one operational process,” she told this news organization. “That means they can have a digital conversation back and forth, so it is much easier to resolve prior authorization issues.”
Unlike many vendor products, the Regence service is free. And while the vendors market only to large organizations, most doctors in the MultiCare arrangement are in independent practices. Ms. Taylor said these doctors have been “enthusiastic” about the arrangement.
The results of the pilot are impressive. Ms. Taylor said automation has resulted in a 233% productivity gain for MultiCare clinicians, and 89% of submissions to Regence get an immediate response.
There is a potential downside, however, to working directly with payers. A direct connection to clinicians allows payers to access the doctor’s clinical notes, which could make many doctors uneasy. But Ms. Taylor said Regence only has access to the “discrete data fields” on MultiCare’s EHR dashboard, not to the notes themselves.
The ultimate goal of the Regence-Multicare project is to include more payers and clinicians. Ms. Taylor said two of the 27 other payers that MultiCare works with are “highly interested,” but it would take a lot of work for them to get connected with practices and other clinicians.
Ultimately, payers could offer automation and third-party vendors might then fade away. However, physicians may resist working directly with payers if the arrangement requires full access to their medical records.
A version of this article first appeared on Medscape.com.
New England Baptist Hospital has been grappling with a serious problem facing health care today: insurers demanding prior authorizations for services ordered by physicians. Meeting payers’ requirements eats up time, delays treatment, and can be a costly drain on doctors’ practices.
To deal with this problem, the Boston orthopedic hospital has opted to automate submission of prior authorization requests on behalf of more than 100 mostly orthopedic surgeons on staff.
After 5 years using this system, “we can say that automation definitely works,” said Lidiya Hadzhieva, director of patient access at the hospital. The software has reduced write-offs by 30% and staff costs by 25%. Prior authorization gets approved 3 days after scheduling, compared with 11 days previously, she said.
“This software not only saves staff time, but it can also more accurately predict when prior authorization is needed,” she added.
For practices deluged with required prior authorizations by insurers, automation is emerging as a way for practices to make the process less time-consuming and save money. However, the software can be costly and may not be adoptable to many practices, and many physicians are not even aware it exists.
So far, the software is mainly used at large organizations like hospital systems. But as word gets out and the software becomes easier to use, private practices and other smaller entities may join the automation trend.
There is definitely a need to automate prior authorization. The American Medical Association reports that physicians spend 16 hours per week on prior authorizations. In a recent AMA survey, more than 60% of physicians indicated that it’s difficult to know when prior authorization is needed. And 93% of physicians reported care delays while waiting for authorization, the AMA said.
Experts estimate that 80% of prior authorization work could be automated, but most practices still use the phone or fax, even as numbers of prior authorizations continue to increase.
How it works
Automation software connects directly to the practice’s electronic health record (EHR). “When the doctor places an order in the EHR, the process starts automatically,” Ms. Hadzhieva said. “The doctor may not even notice it.”
In addition to using an EHR connection, many software products can communicate with the payer through its portal or by fax or phone, while still automating other parts of the process.
The software’s first step is to decide whether prior authorization is needed. This requires having an updated list of the rules that each payer uses for prior authorization. Manually keeping track of payer rules is very time-consuming, but automation uses bots to visit each payer site to look for rules changes. One vendor, Infinitus, uses a voice-based bot called Eva that calls up each payer and speaks with a representative.
“Automatically updating payer rules is not a new technology,” said YiDing Yu, MD, chief product officer at Olive, the automation vendor for New England Baptist. “What is new in the last 5 years is extracting the information needed for the prior authorization out of the clinical notes.”
This is challenging because each doctor has different ways to describe each step of clinical work. To identify this shorthand, Dr. Yu said Olive uses natural language processing, which is a form of artificial intelligence that learns how each doctor describes things.
Dr. Yu asserts that Olive is actually better than a practice’s staff at digging out clinical information. She said staff without much clinical training may miss terms that the software can catch, and they don’t have the time to go back many months into the record to find valuable information. But automation can do that.
In some instances, however, the software may not be able to find the information, in which case it alerts staff through a prompt in the EHR and the information is retrieved manually, Dr. Yu said.
Next, the Olive software puts the information it found into the request form and sends it to the payer. After submission, the software constantly checks on the status of each request, again visiting payer sites with a bot.
At New England Baptist, the software is used mainly by physicians in fairly small private practices who are on staff. They are using the software on the hospital’s dime, but it only works inside the hospital, Ms. Hadzhieva said. For their work outside of the hospital, they would have to purchase the Olive software on their own, she said.
Automation hasn’t spread to practices yet
Despite the promising outcomes for products like Olive, automation software is still primarily used by large organizations. Vendors say very few private practices have bought it yet. “The technology works, but it is still in the early-adopter phase,” Dr. Yu said.
For one thing, the software can be expensive. Very few vendors reveal their prices, but Dr. Yu did so. She said Olive normally costs about $50,000 a year for even a small organization. She insisted, however, that the savings from avoiding just one denial each month for a hip surgery would justify the expense.
On the other hand, some automation software is free, such as the Surescripts product for prior authorization of prescriptions. But it is unclear whether Surescripts does as much as Olive. Vendors’ descriptions of their products tend to be vague.
Also, Surescripts and Olive have entirely separate functions. Dr. Yu said Olive is limited to procedures, so it benefits specialties like oncology, neurosurgery, colorectal surgery, vascular surgery, and cardiology. Olive does not cover prescriptions, because they operate on a different technology.
Dr. Yu said another hurdle for adopting the software is the kind of EHR systems that doctors use. At this point, only a few EHR systems – such as Epic, Cerner, and Athena – are compatible with Olive. Large organizations tend to use Epic and Cerner, while many practices often use Athena or a variety of other systems, she said.
Despite stunted demand, there is no shortage of companies offering automation software for medical (that is, non-prescription) prior authorization. One compilation lists 25 such vendors, including companies like Myndshft, Rhyme, Infinitus, Infinx, and Waystar. As with any start-up technology, companies occasionally buy each other out.
In addition to issues like cost, specialty, and EHR compatibility, another hurdle is that few doctors even know the technology exists. Vendors say marketing focuses on larger provider organizations, not smaller practices.
Even many tech-savvy doctors, like Adam Bruggeman, MD, an orthopedist and CEO of Texas Spine Care Center in San Antonio, say they know little about the technology. “There is definitely a need to automate prior authorization,” he said. “But I don’t know of any colleagues who use it.” He has only just begun to explore vendors, he said.
Many medical practice consultants also have not yet explored the technology. “Automation makes a lot of sense, because there are a lot of repetitive tasks in prior authorization,” said Jill Arena, CEO of Portland, Ore.–based Health e Practices. “But I haven’t looked into it yet, and none of my clients has even asked about it.”
“I could see how it could be an easier sell for large organizations,” she added. “They have an IT person and a CFO who can explore the issue. Smaller practices usually don’t have that kind of expertise.”
Where does automation go from here?
Until now, clinicians who want to fully automate prior authorizations would have to buy two products – one for medical procedures and one for prescriptions. This has to do with incompatible electronic transmission standards, which are used to digitize information, said Susan Lawson-Dawson, content marketing strategist for the vendor Myndshft Health.
Myndshft has long been selling automation software for medical prior authorizations, but now it is introducing a product for prescriptions, Ms. Lawson-Dawson said. She said Myndshft will then be the only vendor to automate both kinds of prior authorizations.
Ms. Lawson-Dawson said Myndshft has 685 customers to date and is looking for more business. Recently the company entered the Google Cloud Marketplace. Google Cloud customers can now direct their committed spend with Google to purchasing Myndshft, meaning they could get it at a discount.
Software like Olive and Myndshft can operate independently of payers, but a vendor called Rhyme depends on payers for its software to function, said Rhyme CEO Joe Anstine. He said more than 300 payers have agreed to install the Rhyme system, and Rhyme has signed up a number of large health systems to use the product. Initially, he said, clinicians paid for the service, but now Rhyme is beginning to find payers to foot the costs and to let clinicians use it for free, which would open Rhyme up to smaller practices.
EHR companies themselves are beginning to offer automation, too. Epic, for example, has created a tool for prior authorization as part of its Epic Payer Platform. Like Rhyme, it requires payer cooperation, because information goes back and forth between clinician and payer in what is called bi-directional exchange.
The Epic product is still in its pilot phase. Epic reported that several large health systems were using its product in conjunction with a specific payer – for instance, Mayo Clinic with Blue Cross and Blue Shield of Minnesota and Ochsner Health with Humana. According to Epic, the arrangement reduced Mayo’s denials due to additional documentation requests by 63% for professional billing.
Automating with just one payer still means the clinician has to deal with manual processes at other payers, but a large clinician could have sufficient volume with that one payer to make the arrangement useful.
Will payers automate prior authorization?
Ultimately, payers may take the automation business away from vendors, offering a free product to all clinicians. But don’t hold your breath. Payers first have to rebuild their electronic systems to accommodate an electronic connection with providers. Even then, some payers might hold back from automating, forcing practices to continue manually processing some prior authorizations.
Efforts are underway, however, to mandate payers to support prior authorization automation. For this to happen, payers would have to revamp their data so that it could be easily read by practices’ EHRs. This would mean adopting a specific interoperability standard called Health Level 7 Fast Healthcare Interoperability Resources (FHIR).
Toward this goal, the Centers for Medicare & Medicaid Services proposes to require payers to adopt FHIR by January 2026. (CMS still has to finalize the rule.) Experts say the two-year ramp-up time is needed because it takes extensive work for payers to translate their data into FHIR.
The only payer so far to switch to FHIR for prior authorization is Regence in Washington state. In a pilot project, it has automated prior authorization with just one provider, MultiCare Connected Care, an accountable care organization (ACO), also in Washington state.
Anna Taylor, associate vice president of population health and value-based care at MultiCare, explained how the arrangement works. “Two separate entities are sharing one operational process,” she told this news organization. “That means they can have a digital conversation back and forth, so it is much easier to resolve prior authorization issues.”
Unlike many vendor products, the Regence service is free. And while the vendors market only to large organizations, most doctors in the MultiCare arrangement are in independent practices. Ms. Taylor said these doctors have been “enthusiastic” about the arrangement.
The results of the pilot are impressive. Ms. Taylor said automation has resulted in a 233% productivity gain for MultiCare clinicians, and 89% of submissions to Regence get an immediate response.
There is a potential downside, however, to working directly with payers. A direct connection to clinicians allows payers to access the doctor’s clinical notes, which could make many doctors uneasy. But Ms. Taylor said Regence only has access to the “discrete data fields” on MultiCare’s EHR dashboard, not to the notes themselves.
The ultimate goal of the Regence-Multicare project is to include more payers and clinicians. Ms. Taylor said two of the 27 other payers that MultiCare works with are “highly interested,” but it would take a lot of work for them to get connected with practices and other clinicians.
Ultimately, payers could offer automation and third-party vendors might then fade away. However, physicians may resist working directly with payers if the arrangement requires full access to their medical records.
A version of this article first appeared on Medscape.com.
Maternal perinatal mortality: A pediatric issue
Checking on the well-being of mothers is one of the important acknowledged aspects of primary pediatric care. “How are you doing?” directed to the child’s mother has long been considered an appropriate question. The AAP recommends several checks in the Bright Futures Guidelines, including conducting several formal screens for depression and asking about “getting time alone with your partner” as well as other supports.
But I have recently become aware of new data that changes my ideas about what we pediatricians need to be doing as part of our care for children and their families, especially in the first year: Considering the risks to the mother of dying.
Maternal mortality increased by 26.6% from 2000 to 2014 across the United States such that it is higher now than it was for our own mothers. The U.S. now has the highest rates of maternal mortality among high-income nations, especially for Black, American Indian, or Alaska Native women, those of lower socioeconomic status, and those under 18 or over 35 years old.
You may be thinking, well, that is an issue for ob.gyns. Indeed, the most common reasons for maternal death are cardiovascular: hemorrhage, hypertensive disorders, deep vein thrombosis, and stroke, all usually occurring at or in the first week after birth. You may have heard about sudden unexpected heart failure from postpartum cardiomyopathy, although rare (1 in 1,000-4,000), presenting from 1 month pre birth to 5 months post delivery, which is when we may be the main clinicians seeing the mother, not the ob.gyns. This can be easily missed since it presents with shortness of breath and decreased exercise tolerance, fatigue, palpitations, and/or leg swelling. Serious eclampsia may have only symptoms of headache or abdominal pain. All of these may easily be mistaken for lingering pregnancy symptoms. But in higher income countries, such as the U.S., 38% of maternal deaths occur from 8 to 42 days after birth, the period for fatal infections as well as cardiac complications. Elevated risk for all of these causes of mortality include Black race, obesity, tobacco use, congenital heart disease, and being older than 40.
As pediatric providers, we may see mothers along with their infants as newborns in the hospital, at day 2, at 2 weeks, or even at 1-2 months after birth, potentially before their one recommended postnatal obstetric visit at 3-8 weeks. Asking the mother how she is feeling at those times should not just be a social nicety but rather an additional check for serious postnatal complications.
Additional concerns
But wait, it gets worse.
Did you know that the leading cause of maternal death from pregnancy up to 1 year after a birth is homicide?
Maternal perinatal mortality figures have not usually included “perinatal-associated” deaths, a maternal death attributable to a condition that is unaffected by the pregnancy and occurring within 1 year of delivery (that I will cite as perinatal henceforth). While half of maternal deaths occur during pregnancy, another half occur in the year following. There were 3.62 homicides per 100,000 live births among females who were pregnant or within 1 year postpartum, 16% more than for similarly aged nonpregnant and nonpostpartum women (3.12 deaths/100,000 population, P < .05). Homicides made up 8.4% of reported perinatal maternal deaths from all causes, with a rate of 1.7 per 100,000 live births, twice the rate of any one of the other leading causes noted above. Black women had seven times the risk of perinatal homicide as that of White women. Females under 20, many of them our own pediatric patients, had a greater than six times higher risk and those aged 20-24 had a 65% higher risk of pregnancy-associated homicide across race and ethnic groups. Homicide is most likely before 21 weeks of pregnancy, decreases in the third trimester, but increases again after birth. Two-thirds of pregnancy-associated homicide deaths occurred in the home, with the perpetrator a current or prior partner (> 59%, with 98% being male), 45%-50% were associated with reported intimate partner violence (IPV), and the most common method was a firearm (55%). Often the same women had histories of substance abuse, serious mental illness, and/or prior IPV, all risk factors for pregnancy-associated deaths, including from homicide.
Homicide? “Not the mothers in my practice,” you may say, but, if not homicide, drug-related deaths (3.68 per 100,000 person-years) and suicide (1.42 per 100,000 person-years) together comprise 18% of all maternal deaths. Non-Hispanic White women, Medicaid-insured women, and women residing in smaller cities were especially likely to die from drugs or suicide. More than half (54.3%) of perinatal suicides involve intimate partner conflict, which increases the risk ninefold. Perinatal mood disorders, affecting up to 15% of pregnant and postpartum U.S. women, is also a risk factor in substance abuse, opioid overdose death, and suicide.
And substance use has gotten more dangerous with the increase in fentanyl lacing. Pregnancy-associated deaths (4%-10% of deaths) involving opioids more than doubled between 2007 and 2016, and, although the rates are higher for Black women, the increase has been greater for non-Hispanic White women. Two-thirds of those deaths occur between 6 and 12 months postpartum, on our watch. Although many women decrease substance use during pregnancy, they may fall back into substance use (rates increase 4 times by 7-12 months after delivery) and not continue to receive treatment. Although pharmacotherapy (e.g., methadone, buprenorphine treatment) is the current standard of care for opioid use disorder (OUD) during pregnancy, nearly half receiving treatment in publicly funded centers are not receiving these medications and others may lose insurance or access to pregnancy-related treatment programs after delivery, increasing risk of relapse. Stigma, and punitive or discriminatory approaches to pregnant women with OUD (e.g., jail, removal of children) can dissuade them from participating in treatment, increasing overdose risk.
It is important to note that in more than half of the 41 deaths from violent trauma in one study (including 22 homicides), obstetrical providers knew of or suspected IPV. Also, the vast majority (74%) of those who died by drugs or suicide had made one or more emergency department or hospital visit between their delivery and death, and 39% had made three or more visits. Without knowing if anything was done in those cases, we also know that, in addition to thorough, compassionate providers, there is sometimes segmentation of responsibility, insensitivity, discrimination, racism, stigma, inequity, lack of resources, lack of access, lack of payment mechanisms, legal issues for immigrants, time constraints, and other systemic deficits that may hinder effective care for these and subsequent women.
Awareness and action
What should we, who are primary care pediatric providers, do about these threats to the mothers and pregnant young women we care for? Clearly, their children, our main patients, would be terribly and permanently hurt by harm coming to their mothers – the extreme adverse childhood experiences and social determinants of health to which we are already committed.
I hope this article will help alert pediatric providers to what is being published, mainly as women’s health and public health issues.
First, we need awareness of the physical symptoms that may come up in our interactions with pregnant and postpartum women so that we can educate them and expedite any indicated emergency care.
Next, we need to expand our routine screening of mothers and pregnant women from just the most impactful social determinants of health (including depression, substance use, and IPV) to include anxiety, past suicide attempts and current suicidal ideation, and the presence of firearms, early and repeatedly in the first year of the child’s life. Adults and teens are more likely to disclose risk for sensitive issues through questionnaires than through interviews, perhaps even more so when the identified patient is their child rather than themselves. Any screen can have false negatives, so asking directly when risk is suspected is important. The reason for screening could be framed as caring for the caregiver who is the most important person for the child. It could be accompanied by acknowledging that pregnancy and the first year of life can be difficult for mothers and their partners and that we want to support them and connect them to resources, if needed. When substance use disorder is acknowledged, we should prescribe and teach about Narcan for overdose. When there is IPV, we should discuss firearm removal/locking as well as counseling on a personal safety plan.
Working as part of an on-site or virtual team that includes professionals who know about community resources and can coordinate care is essential, in addition to educating about 211 for services and 988 for suicide risk.
Finally, we can advocate and vote for programs, people, and laws that support and safeguard women and families, address substance use, and reduce access to firearms.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Checking on the well-being of mothers is one of the important acknowledged aspects of primary pediatric care. “How are you doing?” directed to the child’s mother has long been considered an appropriate question. The AAP recommends several checks in the Bright Futures Guidelines, including conducting several formal screens for depression and asking about “getting time alone with your partner” as well as other supports.
But I have recently become aware of new data that changes my ideas about what we pediatricians need to be doing as part of our care for children and their families, especially in the first year: Considering the risks to the mother of dying.
Maternal mortality increased by 26.6% from 2000 to 2014 across the United States such that it is higher now than it was for our own mothers. The U.S. now has the highest rates of maternal mortality among high-income nations, especially for Black, American Indian, or Alaska Native women, those of lower socioeconomic status, and those under 18 or over 35 years old.
You may be thinking, well, that is an issue for ob.gyns. Indeed, the most common reasons for maternal death are cardiovascular: hemorrhage, hypertensive disorders, deep vein thrombosis, and stroke, all usually occurring at or in the first week after birth. You may have heard about sudden unexpected heart failure from postpartum cardiomyopathy, although rare (1 in 1,000-4,000), presenting from 1 month pre birth to 5 months post delivery, which is when we may be the main clinicians seeing the mother, not the ob.gyns. This can be easily missed since it presents with shortness of breath and decreased exercise tolerance, fatigue, palpitations, and/or leg swelling. Serious eclampsia may have only symptoms of headache or abdominal pain. All of these may easily be mistaken for lingering pregnancy symptoms. But in higher income countries, such as the U.S., 38% of maternal deaths occur from 8 to 42 days after birth, the period for fatal infections as well as cardiac complications. Elevated risk for all of these causes of mortality include Black race, obesity, tobacco use, congenital heart disease, and being older than 40.
As pediatric providers, we may see mothers along with their infants as newborns in the hospital, at day 2, at 2 weeks, or even at 1-2 months after birth, potentially before their one recommended postnatal obstetric visit at 3-8 weeks. Asking the mother how she is feeling at those times should not just be a social nicety but rather an additional check for serious postnatal complications.
Additional concerns
But wait, it gets worse.
Did you know that the leading cause of maternal death from pregnancy up to 1 year after a birth is homicide?
Maternal perinatal mortality figures have not usually included “perinatal-associated” deaths, a maternal death attributable to a condition that is unaffected by the pregnancy and occurring within 1 year of delivery (that I will cite as perinatal henceforth). While half of maternal deaths occur during pregnancy, another half occur in the year following. There were 3.62 homicides per 100,000 live births among females who were pregnant or within 1 year postpartum, 16% more than for similarly aged nonpregnant and nonpostpartum women (3.12 deaths/100,000 population, P < .05). Homicides made up 8.4% of reported perinatal maternal deaths from all causes, with a rate of 1.7 per 100,000 live births, twice the rate of any one of the other leading causes noted above. Black women had seven times the risk of perinatal homicide as that of White women. Females under 20, many of them our own pediatric patients, had a greater than six times higher risk and those aged 20-24 had a 65% higher risk of pregnancy-associated homicide across race and ethnic groups. Homicide is most likely before 21 weeks of pregnancy, decreases in the third trimester, but increases again after birth. Two-thirds of pregnancy-associated homicide deaths occurred in the home, with the perpetrator a current or prior partner (> 59%, with 98% being male), 45%-50% were associated with reported intimate partner violence (IPV), and the most common method was a firearm (55%). Often the same women had histories of substance abuse, serious mental illness, and/or prior IPV, all risk factors for pregnancy-associated deaths, including from homicide.
Homicide? “Not the mothers in my practice,” you may say, but, if not homicide, drug-related deaths (3.68 per 100,000 person-years) and suicide (1.42 per 100,000 person-years) together comprise 18% of all maternal deaths. Non-Hispanic White women, Medicaid-insured women, and women residing in smaller cities were especially likely to die from drugs or suicide. More than half (54.3%) of perinatal suicides involve intimate partner conflict, which increases the risk ninefold. Perinatal mood disorders, affecting up to 15% of pregnant and postpartum U.S. women, is also a risk factor in substance abuse, opioid overdose death, and suicide.
And substance use has gotten more dangerous with the increase in fentanyl lacing. Pregnancy-associated deaths (4%-10% of deaths) involving opioids more than doubled between 2007 and 2016, and, although the rates are higher for Black women, the increase has been greater for non-Hispanic White women. Two-thirds of those deaths occur between 6 and 12 months postpartum, on our watch. Although many women decrease substance use during pregnancy, they may fall back into substance use (rates increase 4 times by 7-12 months after delivery) and not continue to receive treatment. Although pharmacotherapy (e.g., methadone, buprenorphine treatment) is the current standard of care for opioid use disorder (OUD) during pregnancy, nearly half receiving treatment in publicly funded centers are not receiving these medications and others may lose insurance or access to pregnancy-related treatment programs after delivery, increasing risk of relapse. Stigma, and punitive or discriminatory approaches to pregnant women with OUD (e.g., jail, removal of children) can dissuade them from participating in treatment, increasing overdose risk.
It is important to note that in more than half of the 41 deaths from violent trauma in one study (including 22 homicides), obstetrical providers knew of or suspected IPV. Also, the vast majority (74%) of those who died by drugs or suicide had made one or more emergency department or hospital visit between their delivery and death, and 39% had made three or more visits. Without knowing if anything was done in those cases, we also know that, in addition to thorough, compassionate providers, there is sometimes segmentation of responsibility, insensitivity, discrimination, racism, stigma, inequity, lack of resources, lack of access, lack of payment mechanisms, legal issues for immigrants, time constraints, and other systemic deficits that may hinder effective care for these and subsequent women.
Awareness and action
What should we, who are primary care pediatric providers, do about these threats to the mothers and pregnant young women we care for? Clearly, their children, our main patients, would be terribly and permanently hurt by harm coming to their mothers – the extreme adverse childhood experiences and social determinants of health to which we are already committed.
I hope this article will help alert pediatric providers to what is being published, mainly as women’s health and public health issues.
First, we need awareness of the physical symptoms that may come up in our interactions with pregnant and postpartum women so that we can educate them and expedite any indicated emergency care.
Next, we need to expand our routine screening of mothers and pregnant women from just the most impactful social determinants of health (including depression, substance use, and IPV) to include anxiety, past suicide attempts and current suicidal ideation, and the presence of firearms, early and repeatedly in the first year of the child’s life. Adults and teens are more likely to disclose risk for sensitive issues through questionnaires than through interviews, perhaps even more so when the identified patient is their child rather than themselves. Any screen can have false negatives, so asking directly when risk is suspected is important. The reason for screening could be framed as caring for the caregiver who is the most important person for the child. It could be accompanied by acknowledging that pregnancy and the first year of life can be difficult for mothers and their partners and that we want to support them and connect them to resources, if needed. When substance use disorder is acknowledged, we should prescribe and teach about Narcan for overdose. When there is IPV, we should discuss firearm removal/locking as well as counseling on a personal safety plan.
Working as part of an on-site or virtual team that includes professionals who know about community resources and can coordinate care is essential, in addition to educating about 211 for services and 988 for suicide risk.
Finally, we can advocate and vote for programs, people, and laws that support and safeguard women and families, address substance use, and reduce access to firearms.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Checking on the well-being of mothers is one of the important acknowledged aspects of primary pediatric care. “How are you doing?” directed to the child’s mother has long been considered an appropriate question. The AAP recommends several checks in the Bright Futures Guidelines, including conducting several formal screens for depression and asking about “getting time alone with your partner” as well as other supports.
But I have recently become aware of new data that changes my ideas about what we pediatricians need to be doing as part of our care for children and their families, especially in the first year: Considering the risks to the mother of dying.
Maternal mortality increased by 26.6% from 2000 to 2014 across the United States such that it is higher now than it was for our own mothers. The U.S. now has the highest rates of maternal mortality among high-income nations, especially for Black, American Indian, or Alaska Native women, those of lower socioeconomic status, and those under 18 or over 35 years old.
You may be thinking, well, that is an issue for ob.gyns. Indeed, the most common reasons for maternal death are cardiovascular: hemorrhage, hypertensive disorders, deep vein thrombosis, and stroke, all usually occurring at or in the first week after birth. You may have heard about sudden unexpected heart failure from postpartum cardiomyopathy, although rare (1 in 1,000-4,000), presenting from 1 month pre birth to 5 months post delivery, which is when we may be the main clinicians seeing the mother, not the ob.gyns. This can be easily missed since it presents with shortness of breath and decreased exercise tolerance, fatigue, palpitations, and/or leg swelling. Serious eclampsia may have only symptoms of headache or abdominal pain. All of these may easily be mistaken for lingering pregnancy symptoms. But in higher income countries, such as the U.S., 38% of maternal deaths occur from 8 to 42 days after birth, the period for fatal infections as well as cardiac complications. Elevated risk for all of these causes of mortality include Black race, obesity, tobacco use, congenital heart disease, and being older than 40.
As pediatric providers, we may see mothers along with their infants as newborns in the hospital, at day 2, at 2 weeks, or even at 1-2 months after birth, potentially before their one recommended postnatal obstetric visit at 3-8 weeks. Asking the mother how she is feeling at those times should not just be a social nicety but rather an additional check for serious postnatal complications.
Additional concerns
But wait, it gets worse.
Did you know that the leading cause of maternal death from pregnancy up to 1 year after a birth is homicide?
Maternal perinatal mortality figures have not usually included “perinatal-associated” deaths, a maternal death attributable to a condition that is unaffected by the pregnancy and occurring within 1 year of delivery (that I will cite as perinatal henceforth). While half of maternal deaths occur during pregnancy, another half occur in the year following. There were 3.62 homicides per 100,000 live births among females who were pregnant or within 1 year postpartum, 16% more than for similarly aged nonpregnant and nonpostpartum women (3.12 deaths/100,000 population, P < .05). Homicides made up 8.4% of reported perinatal maternal deaths from all causes, with a rate of 1.7 per 100,000 live births, twice the rate of any one of the other leading causes noted above. Black women had seven times the risk of perinatal homicide as that of White women. Females under 20, many of them our own pediatric patients, had a greater than six times higher risk and those aged 20-24 had a 65% higher risk of pregnancy-associated homicide across race and ethnic groups. Homicide is most likely before 21 weeks of pregnancy, decreases in the third trimester, but increases again after birth. Two-thirds of pregnancy-associated homicide deaths occurred in the home, with the perpetrator a current or prior partner (> 59%, with 98% being male), 45%-50% were associated with reported intimate partner violence (IPV), and the most common method was a firearm (55%). Often the same women had histories of substance abuse, serious mental illness, and/or prior IPV, all risk factors for pregnancy-associated deaths, including from homicide.
Homicide? “Not the mothers in my practice,” you may say, but, if not homicide, drug-related deaths (3.68 per 100,000 person-years) and suicide (1.42 per 100,000 person-years) together comprise 18% of all maternal deaths. Non-Hispanic White women, Medicaid-insured women, and women residing in smaller cities were especially likely to die from drugs or suicide. More than half (54.3%) of perinatal suicides involve intimate partner conflict, which increases the risk ninefold. Perinatal mood disorders, affecting up to 15% of pregnant and postpartum U.S. women, is also a risk factor in substance abuse, opioid overdose death, and suicide.
And substance use has gotten more dangerous with the increase in fentanyl lacing. Pregnancy-associated deaths (4%-10% of deaths) involving opioids more than doubled between 2007 and 2016, and, although the rates are higher for Black women, the increase has been greater for non-Hispanic White women. Two-thirds of those deaths occur between 6 and 12 months postpartum, on our watch. Although many women decrease substance use during pregnancy, they may fall back into substance use (rates increase 4 times by 7-12 months after delivery) and not continue to receive treatment. Although pharmacotherapy (e.g., methadone, buprenorphine treatment) is the current standard of care for opioid use disorder (OUD) during pregnancy, nearly half receiving treatment in publicly funded centers are not receiving these medications and others may lose insurance or access to pregnancy-related treatment programs after delivery, increasing risk of relapse. Stigma, and punitive or discriminatory approaches to pregnant women with OUD (e.g., jail, removal of children) can dissuade them from participating in treatment, increasing overdose risk.
It is important to note that in more than half of the 41 deaths from violent trauma in one study (including 22 homicides), obstetrical providers knew of or suspected IPV. Also, the vast majority (74%) of those who died by drugs or suicide had made one or more emergency department or hospital visit between their delivery and death, and 39% had made three or more visits. Without knowing if anything was done in those cases, we also know that, in addition to thorough, compassionate providers, there is sometimes segmentation of responsibility, insensitivity, discrimination, racism, stigma, inequity, lack of resources, lack of access, lack of payment mechanisms, legal issues for immigrants, time constraints, and other systemic deficits that may hinder effective care for these and subsequent women.
Awareness and action
What should we, who are primary care pediatric providers, do about these threats to the mothers and pregnant young women we care for? Clearly, their children, our main patients, would be terribly and permanently hurt by harm coming to their mothers – the extreme adverse childhood experiences and social determinants of health to which we are already committed.
I hope this article will help alert pediatric providers to what is being published, mainly as women’s health and public health issues.
First, we need awareness of the physical symptoms that may come up in our interactions with pregnant and postpartum women so that we can educate them and expedite any indicated emergency care.
Next, we need to expand our routine screening of mothers and pregnant women from just the most impactful social determinants of health (including depression, substance use, and IPV) to include anxiety, past suicide attempts and current suicidal ideation, and the presence of firearms, early and repeatedly in the first year of the child’s life. Adults and teens are more likely to disclose risk for sensitive issues through questionnaires than through interviews, perhaps even more so when the identified patient is their child rather than themselves. Any screen can have false negatives, so asking directly when risk is suspected is important. The reason for screening could be framed as caring for the caregiver who is the most important person for the child. It could be accompanied by acknowledging that pregnancy and the first year of life can be difficult for mothers and their partners and that we want to support them and connect them to resources, if needed. When substance use disorder is acknowledged, we should prescribe and teach about Narcan for overdose. When there is IPV, we should discuss firearm removal/locking as well as counseling on a personal safety plan.
Working as part of an on-site or virtual team that includes professionals who know about community resources and can coordinate care is essential, in addition to educating about 211 for services and 988 for suicide risk.
Finally, we can advocate and vote for programs, people, and laws that support and safeguard women and families, address substance use, and reduce access to firearms.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Fractures beget fractures at any age
VANCOUVER – The occurrence of a fracture predicts future fracture risk, but the increase in risk is the same no matter what the age of the patient, according to a new population-based study drawn from the Manitoba BMD Registry.
The work expands previous studies that focused mostly on fracture risk prediction after a first fracture among individuals aged 45-50 and older. Other limitations of prior studies include large age categories (such as “premenopausal”), reliance on self-reporting, and small sample sizes.
As a result, some guidelines recommend considering fracture history only for patients older than a certain age when assessing for future risk, such as with the Fracture Risk Assessment Tool (FRAX). The new study suggests a potential need to reconsider that stance.
“The [percentage] of increased risk from having had prevalent fractures in the past, no matter what your age, is about the same. I think that it’s really paradigm shifting because [when] most of us think [of] young people who fracture, we’re not thinking of osteoporosis or future fracture risk. We’re not saying, ‘Oh, I had a fracture when I was 25. When I’m 70, I should be thinking about osteoporosis.’ So, I think this study is quite eye-opening that way,” Carrie Ye, MD, who presented the study at the annual meeting of the American Society for Bone and Mineral Research, said in an interview.
Participants of younger age who are referred for dual-energy x-ray absorptiometry (DXA) likely represent a population at increased risk of osteoporosis, according to Dr. Ye. “Maybe they have Crohn’s disease or maybe they’re on a bunch of steroids, and so a clinician has flagged them,” said Dr. Ye, who is an assistant professor and rheumatologist at the University of Alberta, Edmonton.
The researchers limited the analysis to nontraumatic fractures, but session moderator Nicholas Harvey, MD, PhD, wondered if a similar finding would occur with traumatic fractures. In an interview, he noted that researchers led by William Leslie, MD, at the University of Manitoba, Winnipeg, found that prior traumatic fracture also predicted future low bone-mineral density (BMD) and osteoporotic fracture. “I think that would have been one interesting question,” said Dr. Harvey, director of the Medical Research Council Lifecourse Epidemiology Centre at the University of Southampton, England.
Dr. Ye’s study included 88,696 individuals who underwent a first DXA scan between 1996 and 2018, which researchers then linked to provincial administrative health data collected between 1979 and 2018. The mean age at first DXA was 64.6 years, and 90.3% were women. Their mean body mass index was 27.4 kg/m2. Current smokers made up 10.1% of the cohort, 5.5% had a history of prolonged glucocorticoid use, 3.1% had rheumatoid arthritis, and among 14.9% of patients, there was a secondary cause of osteoporosis. Over a median 25.1 years of observation prior to DXA, clinical fracture occurred in 23.8% of participants.
The mean age of the patients at the time of their first prior fracture was 57.7 years. Over a mean 9.0 years of follow-up, 14.6% of participants experienced a fracture of any kind, 14.0% had osteoporotic fractures, 10.6% had a major osteoporotic fracture (nonankle), and 3.5% had a hip fracture. Among persons aged 20-29 years to 80 years or older, the adjusted hazard ratios for future fractures were similar, ranging from 1.51 to 2.12 (P for trend = .120).
The results were similar when age groups were analyzed with regard to all fractures, osteoporotic fractures, major osteoporotic fractures, or hip fractures.
Going forward, Dr. Ye hopes to expand the research into childhood fractures. “They can break their bones pretty easily, especially as they’re going through growth spurts and things like that,” she said.
Asked what her advice to physicians would be, Dr. Ye responded: “Don’t ignore prior fractures, even if they occurred at an early age. I think if someone’s had a fracture, they bought themselves a fracture risk assessment, and that doesn’t mean necessarily a DXA scan. It means you go through their other risk factors: What medications are they on? Do they have a family history? Are they super low BMI? Look at other reasons why you should be worried about their bones, and if you should be worried about their bones, certainly [measure their] BMD and see what’s going on.”
Dr. Ye and Dr. Harvey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VANCOUVER – The occurrence of a fracture predicts future fracture risk, but the increase in risk is the same no matter what the age of the patient, according to a new population-based study drawn from the Manitoba BMD Registry.
The work expands previous studies that focused mostly on fracture risk prediction after a first fracture among individuals aged 45-50 and older. Other limitations of prior studies include large age categories (such as “premenopausal”), reliance on self-reporting, and small sample sizes.
As a result, some guidelines recommend considering fracture history only for patients older than a certain age when assessing for future risk, such as with the Fracture Risk Assessment Tool (FRAX). The new study suggests a potential need to reconsider that stance.
“The [percentage] of increased risk from having had prevalent fractures in the past, no matter what your age, is about the same. I think that it’s really paradigm shifting because [when] most of us think [of] young people who fracture, we’re not thinking of osteoporosis or future fracture risk. We’re not saying, ‘Oh, I had a fracture when I was 25. When I’m 70, I should be thinking about osteoporosis.’ So, I think this study is quite eye-opening that way,” Carrie Ye, MD, who presented the study at the annual meeting of the American Society for Bone and Mineral Research, said in an interview.
Participants of younger age who are referred for dual-energy x-ray absorptiometry (DXA) likely represent a population at increased risk of osteoporosis, according to Dr. Ye. “Maybe they have Crohn’s disease or maybe they’re on a bunch of steroids, and so a clinician has flagged them,” said Dr. Ye, who is an assistant professor and rheumatologist at the University of Alberta, Edmonton.
The researchers limited the analysis to nontraumatic fractures, but session moderator Nicholas Harvey, MD, PhD, wondered if a similar finding would occur with traumatic fractures. In an interview, he noted that researchers led by William Leslie, MD, at the University of Manitoba, Winnipeg, found that prior traumatic fracture also predicted future low bone-mineral density (BMD) and osteoporotic fracture. “I think that would have been one interesting question,” said Dr. Harvey, director of the Medical Research Council Lifecourse Epidemiology Centre at the University of Southampton, England.
Dr. Ye’s study included 88,696 individuals who underwent a first DXA scan between 1996 and 2018, which researchers then linked to provincial administrative health data collected between 1979 and 2018. The mean age at first DXA was 64.6 years, and 90.3% were women. Their mean body mass index was 27.4 kg/m2. Current smokers made up 10.1% of the cohort, 5.5% had a history of prolonged glucocorticoid use, 3.1% had rheumatoid arthritis, and among 14.9% of patients, there was a secondary cause of osteoporosis. Over a median 25.1 years of observation prior to DXA, clinical fracture occurred in 23.8% of participants.
The mean age of the patients at the time of their first prior fracture was 57.7 years. Over a mean 9.0 years of follow-up, 14.6% of participants experienced a fracture of any kind, 14.0% had osteoporotic fractures, 10.6% had a major osteoporotic fracture (nonankle), and 3.5% had a hip fracture. Among persons aged 20-29 years to 80 years or older, the adjusted hazard ratios for future fractures were similar, ranging from 1.51 to 2.12 (P for trend = .120).
The results were similar when age groups were analyzed with regard to all fractures, osteoporotic fractures, major osteoporotic fractures, or hip fractures.
Going forward, Dr. Ye hopes to expand the research into childhood fractures. “They can break their bones pretty easily, especially as they’re going through growth spurts and things like that,” she said.
Asked what her advice to physicians would be, Dr. Ye responded: “Don’t ignore prior fractures, even if they occurred at an early age. I think if someone’s had a fracture, they bought themselves a fracture risk assessment, and that doesn’t mean necessarily a DXA scan. It means you go through their other risk factors: What medications are they on? Do they have a family history? Are they super low BMI? Look at other reasons why you should be worried about their bones, and if you should be worried about their bones, certainly [measure their] BMD and see what’s going on.”
Dr. Ye and Dr. Harvey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VANCOUVER – The occurrence of a fracture predicts future fracture risk, but the increase in risk is the same no matter what the age of the patient, according to a new population-based study drawn from the Manitoba BMD Registry.
The work expands previous studies that focused mostly on fracture risk prediction after a first fracture among individuals aged 45-50 and older. Other limitations of prior studies include large age categories (such as “premenopausal”), reliance on self-reporting, and small sample sizes.
As a result, some guidelines recommend considering fracture history only for patients older than a certain age when assessing for future risk, such as with the Fracture Risk Assessment Tool (FRAX). The new study suggests a potential need to reconsider that stance.
“The [percentage] of increased risk from having had prevalent fractures in the past, no matter what your age, is about the same. I think that it’s really paradigm shifting because [when] most of us think [of] young people who fracture, we’re not thinking of osteoporosis or future fracture risk. We’re not saying, ‘Oh, I had a fracture when I was 25. When I’m 70, I should be thinking about osteoporosis.’ So, I think this study is quite eye-opening that way,” Carrie Ye, MD, who presented the study at the annual meeting of the American Society for Bone and Mineral Research, said in an interview.
Participants of younger age who are referred for dual-energy x-ray absorptiometry (DXA) likely represent a population at increased risk of osteoporosis, according to Dr. Ye. “Maybe they have Crohn’s disease or maybe they’re on a bunch of steroids, and so a clinician has flagged them,” said Dr. Ye, who is an assistant professor and rheumatologist at the University of Alberta, Edmonton.
The researchers limited the analysis to nontraumatic fractures, but session moderator Nicholas Harvey, MD, PhD, wondered if a similar finding would occur with traumatic fractures. In an interview, he noted that researchers led by William Leslie, MD, at the University of Manitoba, Winnipeg, found that prior traumatic fracture also predicted future low bone-mineral density (BMD) and osteoporotic fracture. “I think that would have been one interesting question,” said Dr. Harvey, director of the Medical Research Council Lifecourse Epidemiology Centre at the University of Southampton, England.
Dr. Ye’s study included 88,696 individuals who underwent a first DXA scan between 1996 and 2018, which researchers then linked to provincial administrative health data collected between 1979 and 2018. The mean age at first DXA was 64.6 years, and 90.3% were women. Their mean body mass index was 27.4 kg/m2. Current smokers made up 10.1% of the cohort, 5.5% had a history of prolonged glucocorticoid use, 3.1% had rheumatoid arthritis, and among 14.9% of patients, there was a secondary cause of osteoporosis. Over a median 25.1 years of observation prior to DXA, clinical fracture occurred in 23.8% of participants.
The mean age of the patients at the time of their first prior fracture was 57.7 years. Over a mean 9.0 years of follow-up, 14.6% of participants experienced a fracture of any kind, 14.0% had osteoporotic fractures, 10.6% had a major osteoporotic fracture (nonankle), and 3.5% had a hip fracture. Among persons aged 20-29 years to 80 years or older, the adjusted hazard ratios for future fractures were similar, ranging from 1.51 to 2.12 (P for trend = .120).
The results were similar when age groups were analyzed with regard to all fractures, osteoporotic fractures, major osteoporotic fractures, or hip fractures.
Going forward, Dr. Ye hopes to expand the research into childhood fractures. “They can break their bones pretty easily, especially as they’re going through growth spurts and things like that,” she said.
Asked what her advice to physicians would be, Dr. Ye responded: “Don’t ignore prior fractures, even if they occurred at an early age. I think if someone’s had a fracture, they bought themselves a fracture risk assessment, and that doesn’t mean necessarily a DXA scan. It means you go through their other risk factors: What medications are they on? Do they have a family history? Are they super low BMI? Look at other reasons why you should be worried about their bones, and if you should be worried about their bones, certainly [measure their] BMD and see what’s going on.”
Dr. Ye and Dr. Harvey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2023
Do screening mammograms in women aged 70 and older improve stage at diagnosis or breast cancer–specific mortality?
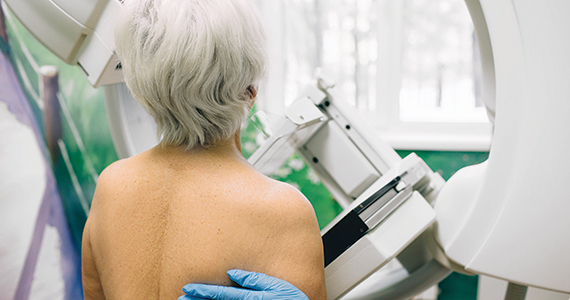
Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
EXPERT COMMENTARY
A screening test is performed to detect potential health disorders or diseases in people who do not have any symptoms of disease. The goal of screening is to detect the condition early enough to treat it most effectively, and ultimately to decrease morbidity and mortality related to the disease. Overdiagnosis refers to the finding of a cancer that would not have caused clinical problems during a person’s lifetime.
Current guidelines for the early detection of breast cancer vary considerably, including recommendations for what age to initiate screening, the cadence of screening (annual or biannual), the use of ancillary screening for people with dense breasts, and importantly the upper age limit for which screening is advised. The US Preventive Services Task Force recommends continuing screening to age 74. The American Cancer Society suggests ongoing screening if life expectancy is estimated at more than 10 years, and the American College of Physicians recommends stopping screening at age 75, or younger if life expectancy is less than 10 years. The American College of Obstetricians and Gynecologists states that women at average risk of breast cancer should continue screening mammography until at least age 75.
Overdiagnosis is a difficult concept for clinicians to understand let alone explain to our patients. Recently, Richman and colleagues published the results of their study aimed at estimating overdiagnosis associated with breast cancer screening among older women.1 As Dr. Otis Brawley, former Chief Medical and Scientific Officer of the American Cancer Society and current Distinguished Professor of Oncology and Epidemiology at Johns Hopkins University, states in the editorial that accompanies the study by Richman and colleagues, “Some tumors are not destined to grow, spread, and kill due to their genomics or their microenvironment. A second type of overdiagnosis involves small tumors that do have the potential to grow but will not grow fast enough to bother the patient within their natural lifetime.”2
Although screening mammography in older women results in frequent false positives that require additional imaging as well as biopsies, we have become more aware of the potential of overdiagnosis as an important downside of screening mammography in an elderly population.
Continue to: Details of the study...
Details of the study
Using the SEER registry to identify breast cancers linked to a 5% sample of Medicare beneficiaries, Richman and colleagues (funded by the National Cancer Institute and based at Yale University) conducted a retrospectivecohort study to estimate the likelihood of overdiagnosis associated with screening mammography among older women over 15 years of follow-up. Specifically, they assessed the difference in cumulative incidence of in situ and invasive breast cancer among women aged 70 years and older without a history of breast cancer when screened in 2002. During the subsequent 3 years, participants either continued screening (screened group) or did not (unscreened group). Women were followed through 2017.
Among almost 55,000 women followed, 88% were White, 6% were Black, and 3% were Hispanic. Mean follow-up was 13.7 years among women aged 70 to 74 years at baseline. For those aged 75 to 84 at baseline, mean follow-up was 10 years, and for those aged 85 years and older, mean follow-up was 5.7 years.
Estimated rates of overdiagnosis. Overall, among women aged 70 to 74 at baseline who were eventually diagnosed with breast cancer, the investigators estimated that 31% of these cancers were overdiagnosed. The corresponding percentage of breast cancers estimated to represent overdiagnosis climbed to 47% for those aged 75 to 84 years at baseline and to 54% for those aged 85 years and older at baseline.
The investigators assessed the impact of greater screening among women with a first-degree relative with a diagnosis of breast cancer and determined that this did not explain their results. With respect to cancer stage, the investigators noted that overdiagnosis was more prevalent among in situ and localized invasive cancers compared with those with regional or distant spread. Of note, the incidence of cancer with regional or distant spread was neither higher nor lower among those who were screened. Finally, the investigators did not observe significant differences in breast cancer–specific mortality by screening status.
The proportion of cancers that were overdiagnosed was particularly high among women with in situ as well as those with localized invasive disease. The investigators pointed out that as many as 90% of women aged 80 and older diagnosed with localized cancer undergo surgery, and almost two-thirds of those older than 70 years have radiation therapy for early-stage disease. In addition to the burdens associated with these treatments for overdiagnosed cancers in older women, simply being diagnosed with breast cancer profoundly affects the health and well-being of women, resulting in anxiety and substantial reductions in quality of life.
The authors also noted that some studies suggest that, among breast cancers diagnosed with screening, chemotherapy is less likely to be employed among older women, a screening benefit that must be weighed against the high likelihood of overdiagnosis. However, this benefit is unlikely to be meaningful for the majority of patients in this study who presented with in situ or early invasive lesions since chemotherapy often is not recommended for such women.
Study strengths and limitations
If screening mammography is effective, the incidence of advanced-stage tumors and breast cancer–specific mortality should be reduced in screened populations. Accordingly, in this large, long-term study using reliable sources of data, the findings that the incidence of advanced-stage disease as well as breast cancer–specific mortality were similar in the screened and unscreened cohorts provides powerful evidence that screening mammography is not effective in older women.3
As the authors pointed out, their findings regarding a high prevalence of overdiagnosis associated with screening mammography in older women are consistent with findings of other studies, some of which used different methodology.
The authors acknowledged that some women in their Medicare cohort who initially continued screening likely stopped screening subsequently, while some who initially did not continue screening might have been screened subsequently. They went on to indicate that if patients were completely adherent with subsequent screening (or not getting screened) the likelihood that cancers among screened women were overdiagnosed would be even higher.
Lead-time bias occurs when screening finds a cancer earlier than that cancer would have been diagnosed because of symptoms. This study followed the cohorts over a long timeframe to reduce the possibility that lead time was inappropriately identified as overdiagnosis. They also observed that, among women aged 85 and older, most cohort members had died by the end of study follow-up; accordingly, lead time is not likely to have explained their findings.
Limitations. The authors acknowledged that miscoding the mammogram type (screening vs diagnostic) could result in higher estimates of overdiagnosis. In their most conservative sensitivity analysis, the overdiagnosis rates could be as low as 15% for women aged 70 to 74, 36% for those aged 75 to 84, and 44% for people aged 85 and older.
Because this was an observational cohort study, unmeasured differences in breast cancer risk and underlying health factors may have been confounders. Specifically, people with severe life-threatening conditions that limited their expected life span may have chosen not to undergo regular screening. Although the authors did attempt to adjust for these factors, there may have been unrecognized confounders. This study was designed to estimate overdiagnosis, and therefore the specific benefits and harms of screening could not be addressed based on the data collected. ●
The high prevalence of overdiagnosis and lack of a breast cancer–specific mortality benefit among older women who undergo screening mammography is sobering. Clinician recommendations and shared decision making with our patients regarding screening mammography should take into consideration overdiagnosis and the considerable harms associated with overtreatment. Although we may recognize that overdiagnosed cancers are often indolent tumors with a long presymptomatic phase, in older women, even finding a biologically aggressive cancer may represent overdiagnosis if life expectancy is limited.
BARBARA LEVY, MD, MSCP; ANDREW M. KAUNITZ, MD, MSCP.
- Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
- Brawley OW, Ramalingam R. Understanding the varying biological behaviors of breast and other types of cancer to avoid overdiagnosis. Ann Intern Med. 2023;176:1273-1274. doi:10.7326/M23-18953
- Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer—lessons in cancer dynamics. N Engl J Med. 2015;373:1685-1687. doi:10.1056/NEJM p1510443

Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
EXPERT COMMENTARY
A screening test is performed to detect potential health disorders or diseases in people who do not have any symptoms of disease. The goal of screening is to detect the condition early enough to treat it most effectively, and ultimately to decrease morbidity and mortality related to the disease. Overdiagnosis refers to the finding of a cancer that would not have caused clinical problems during a person’s lifetime.
Current guidelines for the early detection of breast cancer vary considerably, including recommendations for what age to initiate screening, the cadence of screening (annual or biannual), the use of ancillary screening for people with dense breasts, and importantly the upper age limit for which screening is advised. The US Preventive Services Task Force recommends continuing screening to age 74. The American Cancer Society suggests ongoing screening if life expectancy is estimated at more than 10 years, and the American College of Physicians recommends stopping screening at age 75, or younger if life expectancy is less than 10 years. The American College of Obstetricians and Gynecologists states that women at average risk of breast cancer should continue screening mammography until at least age 75.
Overdiagnosis is a difficult concept for clinicians to understand let alone explain to our patients. Recently, Richman and colleagues published the results of their study aimed at estimating overdiagnosis associated with breast cancer screening among older women.1 As Dr. Otis Brawley, former Chief Medical and Scientific Officer of the American Cancer Society and current Distinguished Professor of Oncology and Epidemiology at Johns Hopkins University, states in the editorial that accompanies the study by Richman and colleagues, “Some tumors are not destined to grow, spread, and kill due to their genomics or their microenvironment. A second type of overdiagnosis involves small tumors that do have the potential to grow but will not grow fast enough to bother the patient within their natural lifetime.”2
Although screening mammography in older women results in frequent false positives that require additional imaging as well as biopsies, we have become more aware of the potential of overdiagnosis as an important downside of screening mammography in an elderly population.
Continue to: Details of the study...
Details of the study
Using the SEER registry to identify breast cancers linked to a 5% sample of Medicare beneficiaries, Richman and colleagues (funded by the National Cancer Institute and based at Yale University) conducted a retrospectivecohort study to estimate the likelihood of overdiagnosis associated with screening mammography among older women over 15 years of follow-up. Specifically, they assessed the difference in cumulative incidence of in situ and invasive breast cancer among women aged 70 years and older without a history of breast cancer when screened in 2002. During the subsequent 3 years, participants either continued screening (screened group) or did not (unscreened group). Women were followed through 2017.
Among almost 55,000 women followed, 88% were White, 6% were Black, and 3% were Hispanic. Mean follow-up was 13.7 years among women aged 70 to 74 years at baseline. For those aged 75 to 84 at baseline, mean follow-up was 10 years, and for those aged 85 years and older, mean follow-up was 5.7 years.
Estimated rates of overdiagnosis. Overall, among women aged 70 to 74 at baseline who were eventually diagnosed with breast cancer, the investigators estimated that 31% of these cancers were overdiagnosed. The corresponding percentage of breast cancers estimated to represent overdiagnosis climbed to 47% for those aged 75 to 84 years at baseline and to 54% for those aged 85 years and older at baseline.
The investigators assessed the impact of greater screening among women with a first-degree relative with a diagnosis of breast cancer and determined that this did not explain their results. With respect to cancer stage, the investigators noted that overdiagnosis was more prevalent among in situ and localized invasive cancers compared with those with regional or distant spread. Of note, the incidence of cancer with regional or distant spread was neither higher nor lower among those who were screened. Finally, the investigators did not observe significant differences in breast cancer–specific mortality by screening status.
The proportion of cancers that were overdiagnosed was particularly high among women with in situ as well as those with localized invasive disease. The investigators pointed out that as many as 90% of women aged 80 and older diagnosed with localized cancer undergo surgery, and almost two-thirds of those older than 70 years have radiation therapy for early-stage disease. In addition to the burdens associated with these treatments for overdiagnosed cancers in older women, simply being diagnosed with breast cancer profoundly affects the health and well-being of women, resulting in anxiety and substantial reductions in quality of life.
The authors also noted that some studies suggest that, among breast cancers diagnosed with screening, chemotherapy is less likely to be employed among older women, a screening benefit that must be weighed against the high likelihood of overdiagnosis. However, this benefit is unlikely to be meaningful for the majority of patients in this study who presented with in situ or early invasive lesions since chemotherapy often is not recommended for such women.
Study strengths and limitations
If screening mammography is effective, the incidence of advanced-stage tumors and breast cancer–specific mortality should be reduced in screened populations. Accordingly, in this large, long-term study using reliable sources of data, the findings that the incidence of advanced-stage disease as well as breast cancer–specific mortality were similar in the screened and unscreened cohorts provides powerful evidence that screening mammography is not effective in older women.3
As the authors pointed out, their findings regarding a high prevalence of overdiagnosis associated with screening mammography in older women are consistent with findings of other studies, some of which used different methodology.
The authors acknowledged that some women in their Medicare cohort who initially continued screening likely stopped screening subsequently, while some who initially did not continue screening might have been screened subsequently. They went on to indicate that if patients were completely adherent with subsequent screening (or not getting screened) the likelihood that cancers among screened women were overdiagnosed would be even higher.
Lead-time bias occurs when screening finds a cancer earlier than that cancer would have been diagnosed because of symptoms. This study followed the cohorts over a long timeframe to reduce the possibility that lead time was inappropriately identified as overdiagnosis. They also observed that, among women aged 85 and older, most cohort members had died by the end of study follow-up; accordingly, lead time is not likely to have explained their findings.
Limitations. The authors acknowledged that miscoding the mammogram type (screening vs diagnostic) could result in higher estimates of overdiagnosis. In their most conservative sensitivity analysis, the overdiagnosis rates could be as low as 15% for women aged 70 to 74, 36% for those aged 75 to 84, and 44% for people aged 85 and older.
Because this was an observational cohort study, unmeasured differences in breast cancer risk and underlying health factors may have been confounders. Specifically, people with severe life-threatening conditions that limited their expected life span may have chosen not to undergo regular screening. Although the authors did attempt to adjust for these factors, there may have been unrecognized confounders. This study was designed to estimate overdiagnosis, and therefore the specific benefits and harms of screening could not be addressed based on the data collected. ●
The high prevalence of overdiagnosis and lack of a breast cancer–specific mortality benefit among older women who undergo screening mammography is sobering. Clinician recommendations and shared decision making with our patients regarding screening mammography should take into consideration overdiagnosis and the considerable harms associated with overtreatment. Although we may recognize that overdiagnosed cancers are often indolent tumors with a long presymptomatic phase, in older women, even finding a biologically aggressive cancer may represent overdiagnosis if life expectancy is limited.
BARBARA LEVY, MD, MSCP; ANDREW M. KAUNITZ, MD, MSCP.

Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
EXPERT COMMENTARY
A screening test is performed to detect potential health disorders or diseases in people who do not have any symptoms of disease. The goal of screening is to detect the condition early enough to treat it most effectively, and ultimately to decrease morbidity and mortality related to the disease. Overdiagnosis refers to the finding of a cancer that would not have caused clinical problems during a person’s lifetime.
Current guidelines for the early detection of breast cancer vary considerably, including recommendations for what age to initiate screening, the cadence of screening (annual or biannual), the use of ancillary screening for people with dense breasts, and importantly the upper age limit for which screening is advised. The US Preventive Services Task Force recommends continuing screening to age 74. The American Cancer Society suggests ongoing screening if life expectancy is estimated at more than 10 years, and the American College of Physicians recommends stopping screening at age 75, or younger if life expectancy is less than 10 years. The American College of Obstetricians and Gynecologists states that women at average risk of breast cancer should continue screening mammography until at least age 75.
Overdiagnosis is a difficult concept for clinicians to understand let alone explain to our patients. Recently, Richman and colleagues published the results of their study aimed at estimating overdiagnosis associated with breast cancer screening among older women.1 As Dr. Otis Brawley, former Chief Medical and Scientific Officer of the American Cancer Society and current Distinguished Professor of Oncology and Epidemiology at Johns Hopkins University, states in the editorial that accompanies the study by Richman and colleagues, “Some tumors are not destined to grow, spread, and kill due to their genomics or their microenvironment. A second type of overdiagnosis involves small tumors that do have the potential to grow but will not grow fast enough to bother the patient within their natural lifetime.”2
Although screening mammography in older women results in frequent false positives that require additional imaging as well as biopsies, we have become more aware of the potential of overdiagnosis as an important downside of screening mammography in an elderly population.
Continue to: Details of the study...
Details of the study
Using the SEER registry to identify breast cancers linked to a 5% sample of Medicare beneficiaries, Richman and colleagues (funded by the National Cancer Institute and based at Yale University) conducted a retrospectivecohort study to estimate the likelihood of overdiagnosis associated with screening mammography among older women over 15 years of follow-up. Specifically, they assessed the difference in cumulative incidence of in situ and invasive breast cancer among women aged 70 years and older without a history of breast cancer when screened in 2002. During the subsequent 3 years, participants either continued screening (screened group) or did not (unscreened group). Women were followed through 2017.
Among almost 55,000 women followed, 88% were White, 6% were Black, and 3% were Hispanic. Mean follow-up was 13.7 years among women aged 70 to 74 years at baseline. For those aged 75 to 84 at baseline, mean follow-up was 10 years, and for those aged 85 years and older, mean follow-up was 5.7 years.
Estimated rates of overdiagnosis. Overall, among women aged 70 to 74 at baseline who were eventually diagnosed with breast cancer, the investigators estimated that 31% of these cancers were overdiagnosed. The corresponding percentage of breast cancers estimated to represent overdiagnosis climbed to 47% for those aged 75 to 84 years at baseline and to 54% for those aged 85 years and older at baseline.
The investigators assessed the impact of greater screening among women with a first-degree relative with a diagnosis of breast cancer and determined that this did not explain their results. With respect to cancer stage, the investigators noted that overdiagnosis was more prevalent among in situ and localized invasive cancers compared with those with regional or distant spread. Of note, the incidence of cancer with regional or distant spread was neither higher nor lower among those who were screened. Finally, the investigators did not observe significant differences in breast cancer–specific mortality by screening status.
The proportion of cancers that were overdiagnosed was particularly high among women with in situ as well as those with localized invasive disease. The investigators pointed out that as many as 90% of women aged 80 and older diagnosed with localized cancer undergo surgery, and almost two-thirds of those older than 70 years have radiation therapy for early-stage disease. In addition to the burdens associated with these treatments for overdiagnosed cancers in older women, simply being diagnosed with breast cancer profoundly affects the health and well-being of women, resulting in anxiety and substantial reductions in quality of life.
The authors also noted that some studies suggest that, among breast cancers diagnosed with screening, chemotherapy is less likely to be employed among older women, a screening benefit that must be weighed against the high likelihood of overdiagnosis. However, this benefit is unlikely to be meaningful for the majority of patients in this study who presented with in situ or early invasive lesions since chemotherapy often is not recommended for such women.
Study strengths and limitations
If screening mammography is effective, the incidence of advanced-stage tumors and breast cancer–specific mortality should be reduced in screened populations. Accordingly, in this large, long-term study using reliable sources of data, the findings that the incidence of advanced-stage disease as well as breast cancer–specific mortality were similar in the screened and unscreened cohorts provides powerful evidence that screening mammography is not effective in older women.3
As the authors pointed out, their findings regarding a high prevalence of overdiagnosis associated with screening mammography in older women are consistent with findings of other studies, some of which used different methodology.
The authors acknowledged that some women in their Medicare cohort who initially continued screening likely stopped screening subsequently, while some who initially did not continue screening might have been screened subsequently. They went on to indicate that if patients were completely adherent with subsequent screening (or not getting screened) the likelihood that cancers among screened women were overdiagnosed would be even higher.
Lead-time bias occurs when screening finds a cancer earlier than that cancer would have been diagnosed because of symptoms. This study followed the cohorts over a long timeframe to reduce the possibility that lead time was inappropriately identified as overdiagnosis. They also observed that, among women aged 85 and older, most cohort members had died by the end of study follow-up; accordingly, lead time is not likely to have explained their findings.
Limitations. The authors acknowledged that miscoding the mammogram type (screening vs diagnostic) could result in higher estimates of overdiagnosis. In their most conservative sensitivity analysis, the overdiagnosis rates could be as low as 15% for women aged 70 to 74, 36% for those aged 75 to 84, and 44% for people aged 85 and older.
Because this was an observational cohort study, unmeasured differences in breast cancer risk and underlying health factors may have been confounders. Specifically, people with severe life-threatening conditions that limited their expected life span may have chosen not to undergo regular screening. Although the authors did attempt to adjust for these factors, there may have been unrecognized confounders. This study was designed to estimate overdiagnosis, and therefore the specific benefits and harms of screening could not be addressed based on the data collected. ●
The high prevalence of overdiagnosis and lack of a breast cancer–specific mortality benefit among older women who undergo screening mammography is sobering. Clinician recommendations and shared decision making with our patients regarding screening mammography should take into consideration overdiagnosis and the considerable harms associated with overtreatment. Although we may recognize that overdiagnosed cancers are often indolent tumors with a long presymptomatic phase, in older women, even finding a biologically aggressive cancer may represent overdiagnosis if life expectancy is limited.
BARBARA LEVY, MD, MSCP; ANDREW M. KAUNITZ, MD, MSCP.
- Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
- Brawley OW, Ramalingam R. Understanding the varying biological behaviors of breast and other types of cancer to avoid overdiagnosis. Ann Intern Med. 2023;176:1273-1274. doi:10.7326/M23-18953
- Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer—lessons in cancer dynamics. N Engl J Med. 2015;373:1685-1687. doi:10.1056/NEJM p1510443
- Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
- Brawley OW, Ramalingam R. Understanding the varying biological behaviors of breast and other types of cancer to avoid overdiagnosis. Ann Intern Med. 2023;176:1273-1274. doi:10.7326/M23-18953
- Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer—lessons in cancer dynamics. N Engl J Med. 2015;373:1685-1687. doi:10.1056/NEJM p1510443
Greater fracture risk reduction seen with denosumab vs. zoledronic acid in postmenopausal women
VANCOUVER –
A previous head-to-head comparison showed that denosumab increased bone mineral density at key skeletal sites compared with zoledronic acid, but only a single, small observational study has examined fracture risk, and it found no difference.
The new study, presented at the annual meeting of the American Society for Bone and Mineral Research, used a relatively new method of real-world comparative effectiveness analysis called negative control outcome (NCO) to analyze Medicare fee-for-service data.
NCO analysis takes extra pains to remove bias through data that might be linked to potential confounders but could not reasonably be attributed to a drug. For example, people who have greater contact with the health care system may be more likely to get one drug or another. The researchers used the frequency of receiving a flu or pneumonia vaccine as a proxy for this. If the two comparison groups had a significant difference in a proxy, it suggested a hidden bias and forced the researchers to abandon those groupings. Another example used car accidents as a proxy for cognitive impairment.
“If you find meaningful differences between the two groups, and you can say there’s no way a bone drug could account for these differences, then we shouldn’t do this analysis because these groups just aren’t comparable. They probably differ by that confounding factor we couldn’t measure,” said Jeffrey Curtis, MD, who presented the study. He is a professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham.
The study strongly suggests superiority for denosumab. “There was a significant difference in multiple different groupings of fractures – beginning at year 2, extending to year 3 and even out to year 5 – that showed that there is a significant reduction in fracture risk if you get treated with denosumab [that was greater] than if you get treated with zoledronic acid,” Dr. Curtis said.
The researchers weighed 118 covariates and ultimately identified a population of 90,805 women taking denosumab and 37,328 taking zoledronic acid that was equally balanced in all patient characteristics. The mean age was about 75 years in the denosumab group and 74 in the zoledronic acid group.
The researchers found a 34% lower risk for hip fracture in the denosumab group by 5 years (relative risk, 0.66; 95% confidence interval, 0.43-0.90).
Similar patterns in fracture risk reduction were observed at 5 years for nonvertebral fracture (RR, 0.67; 95% CI, 0.52-0.82), nonhip nonvertebral fracture (RR, 0.69; 95% CI, 0.50-0.88), and major osteoporotic fracture (RR, 0.74; 95% CI, 0.59-0.89).
During the Q&A session after the talk, one audience member commented that the study was limited because the researchers only followed patients who received zoledronic acid for 60 days, which could have missed potential long-term benefits of the drug, especially since bisphosphonates have a lengthy skeletal retention time. Dr. Curtis acknowledged the point but said, “Usually, that’s not something we do, but these are different enough mechanisms of action that it may be warranted at least as a sensitivity analysis,” he said.
The study and its methodology were impressive, according to Yumie Rhee, MD, who comoderated the session where the study was presented. “I think they did a really good job by doing the negative control analysis. We’re not going to have a head-to-head clinical trial, so we don’t know the real fracture reduction differences [between denosumab and zoledronic acid]. [The NCO analysis] is more than the propensity matching score that we do usually,” said Dr. Rhee, who is a professor of endocrinology at Yonsei University College of Medicine in Seoul, South Korea.
In particular, the study showed a significantly greater reduction in hip fractures with denosumab. “Even in the RCTs, it was really hard to see the reduction in hip fracture, so I think this is showing much stronger data for denosumab. Especially in patients who have more [general fracture] risk and patients with higher hip fracture risk, I would go with denosumab,” Dr. Rhee said.
Her comoderator, Maria Zanchetta, MD, agreed. “It can have clinical implication, because we think denosumab is better than [zoledronic acid] for higher-risk patients, but we didn’t have the evidence. So at least we have a new [study] to look at, and I think it’s very important for our practice,” said Dr. Zanchetta, who is a professor of osteology at the Institute of Diagnostics and Metabolic Research, Universidad del Salvador, Buenos Aires.
The study was funded by Amgen, which markets denosumab. Dr. Curtis has consulted for Amgen. Dr. Rhee and Dr. Zanchetta report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VANCOUVER –
A previous head-to-head comparison showed that denosumab increased bone mineral density at key skeletal sites compared with zoledronic acid, but only a single, small observational study has examined fracture risk, and it found no difference.
The new study, presented at the annual meeting of the American Society for Bone and Mineral Research, used a relatively new method of real-world comparative effectiveness analysis called negative control outcome (NCO) to analyze Medicare fee-for-service data.
NCO analysis takes extra pains to remove bias through data that might be linked to potential confounders but could not reasonably be attributed to a drug. For example, people who have greater contact with the health care system may be more likely to get one drug or another. The researchers used the frequency of receiving a flu or pneumonia vaccine as a proxy for this. If the two comparison groups had a significant difference in a proxy, it suggested a hidden bias and forced the researchers to abandon those groupings. Another example used car accidents as a proxy for cognitive impairment.
“If you find meaningful differences between the two groups, and you can say there’s no way a bone drug could account for these differences, then we shouldn’t do this analysis because these groups just aren’t comparable. They probably differ by that confounding factor we couldn’t measure,” said Jeffrey Curtis, MD, who presented the study. He is a professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham.
The study strongly suggests superiority for denosumab. “There was a significant difference in multiple different groupings of fractures – beginning at year 2, extending to year 3 and even out to year 5 – that showed that there is a significant reduction in fracture risk if you get treated with denosumab [that was greater] than if you get treated with zoledronic acid,” Dr. Curtis said.
The researchers weighed 118 covariates and ultimately identified a population of 90,805 women taking denosumab and 37,328 taking zoledronic acid that was equally balanced in all patient characteristics. The mean age was about 75 years in the denosumab group and 74 in the zoledronic acid group.
The researchers found a 34% lower risk for hip fracture in the denosumab group by 5 years (relative risk, 0.66; 95% confidence interval, 0.43-0.90).
Similar patterns in fracture risk reduction were observed at 5 years for nonvertebral fracture (RR, 0.67; 95% CI, 0.52-0.82), nonhip nonvertebral fracture (RR, 0.69; 95% CI, 0.50-0.88), and major osteoporotic fracture (RR, 0.74; 95% CI, 0.59-0.89).
During the Q&A session after the talk, one audience member commented that the study was limited because the researchers only followed patients who received zoledronic acid for 60 days, which could have missed potential long-term benefits of the drug, especially since bisphosphonates have a lengthy skeletal retention time. Dr. Curtis acknowledged the point but said, “Usually, that’s not something we do, but these are different enough mechanisms of action that it may be warranted at least as a sensitivity analysis,” he said.
The study and its methodology were impressive, according to Yumie Rhee, MD, who comoderated the session where the study was presented. “I think they did a really good job by doing the negative control analysis. We’re not going to have a head-to-head clinical trial, so we don’t know the real fracture reduction differences [between denosumab and zoledronic acid]. [The NCO analysis] is more than the propensity matching score that we do usually,” said Dr. Rhee, who is a professor of endocrinology at Yonsei University College of Medicine in Seoul, South Korea.
In particular, the study showed a significantly greater reduction in hip fractures with denosumab. “Even in the RCTs, it was really hard to see the reduction in hip fracture, so I think this is showing much stronger data for denosumab. Especially in patients who have more [general fracture] risk and patients with higher hip fracture risk, I would go with denosumab,” Dr. Rhee said.
Her comoderator, Maria Zanchetta, MD, agreed. “It can have clinical implication, because we think denosumab is better than [zoledronic acid] for higher-risk patients, but we didn’t have the evidence. So at least we have a new [study] to look at, and I think it’s very important for our practice,” said Dr. Zanchetta, who is a professor of osteology at the Institute of Diagnostics and Metabolic Research, Universidad del Salvador, Buenos Aires.
The study was funded by Amgen, which markets denosumab. Dr. Curtis has consulted for Amgen. Dr. Rhee and Dr. Zanchetta report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VANCOUVER –
A previous head-to-head comparison showed that denosumab increased bone mineral density at key skeletal sites compared with zoledronic acid, but only a single, small observational study has examined fracture risk, and it found no difference.
The new study, presented at the annual meeting of the American Society for Bone and Mineral Research, used a relatively new method of real-world comparative effectiveness analysis called negative control outcome (NCO) to analyze Medicare fee-for-service data.
NCO analysis takes extra pains to remove bias through data that might be linked to potential confounders but could not reasonably be attributed to a drug. For example, people who have greater contact with the health care system may be more likely to get one drug or another. The researchers used the frequency of receiving a flu or pneumonia vaccine as a proxy for this. If the two comparison groups had a significant difference in a proxy, it suggested a hidden bias and forced the researchers to abandon those groupings. Another example used car accidents as a proxy for cognitive impairment.
“If you find meaningful differences between the two groups, and you can say there’s no way a bone drug could account for these differences, then we shouldn’t do this analysis because these groups just aren’t comparable. They probably differ by that confounding factor we couldn’t measure,” said Jeffrey Curtis, MD, who presented the study. He is a professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham.
The study strongly suggests superiority for denosumab. “There was a significant difference in multiple different groupings of fractures – beginning at year 2, extending to year 3 and even out to year 5 – that showed that there is a significant reduction in fracture risk if you get treated with denosumab [that was greater] than if you get treated with zoledronic acid,” Dr. Curtis said.
The researchers weighed 118 covariates and ultimately identified a population of 90,805 women taking denosumab and 37,328 taking zoledronic acid that was equally balanced in all patient characteristics. The mean age was about 75 years in the denosumab group and 74 in the zoledronic acid group.
The researchers found a 34% lower risk for hip fracture in the denosumab group by 5 years (relative risk, 0.66; 95% confidence interval, 0.43-0.90).
Similar patterns in fracture risk reduction were observed at 5 years for nonvertebral fracture (RR, 0.67; 95% CI, 0.52-0.82), nonhip nonvertebral fracture (RR, 0.69; 95% CI, 0.50-0.88), and major osteoporotic fracture (RR, 0.74; 95% CI, 0.59-0.89).
During the Q&A session after the talk, one audience member commented that the study was limited because the researchers only followed patients who received zoledronic acid for 60 days, which could have missed potential long-term benefits of the drug, especially since bisphosphonates have a lengthy skeletal retention time. Dr. Curtis acknowledged the point but said, “Usually, that’s not something we do, but these are different enough mechanisms of action that it may be warranted at least as a sensitivity analysis,” he said.
The study and its methodology were impressive, according to Yumie Rhee, MD, who comoderated the session where the study was presented. “I think they did a really good job by doing the negative control analysis. We’re not going to have a head-to-head clinical trial, so we don’t know the real fracture reduction differences [between denosumab and zoledronic acid]. [The NCO analysis] is more than the propensity matching score that we do usually,” said Dr. Rhee, who is a professor of endocrinology at Yonsei University College of Medicine in Seoul, South Korea.
In particular, the study showed a significantly greater reduction in hip fractures with denosumab. “Even in the RCTs, it was really hard to see the reduction in hip fracture, so I think this is showing much stronger data for denosumab. Especially in patients who have more [general fracture] risk and patients with higher hip fracture risk, I would go with denosumab,” Dr. Rhee said.
Her comoderator, Maria Zanchetta, MD, agreed. “It can have clinical implication, because we think denosumab is better than [zoledronic acid] for higher-risk patients, but we didn’t have the evidence. So at least we have a new [study] to look at, and I think it’s very important for our practice,” said Dr. Zanchetta, who is a professor of osteology at the Institute of Diagnostics and Metabolic Research, Universidad del Salvador, Buenos Aires.
The study was funded by Amgen, which markets denosumab. Dr. Curtis has consulted for Amgen. Dr. Rhee and Dr. Zanchetta report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ASBMR 2023
Suits or joggers? A doctor’s dress code
Look at this guy – NFL Chargers jersey and shorts with a RVCA hat on backward. And next to him, a woman wearing her spin-class-Lulu gear. There’s also a guy sporting a 2016 San Diego Rock ‘n Roll Marathon Tee. And that young woman is actually wearing slippers. A visitor from the 1950s would be thunderstruck to see such casual wear on people waiting to board a plane. Photos from that era show men buttoned up in white shirt and tie and women wearing Chanel with hats and white gloves. This dramatic transformation from formal to unfussy wear cuts through all social situations, including in my office. As a new doc out of residency, I used to wear a tie and shoes that could hold a shine. Now I wear jogger scrubs and sneakers. Rather than be offended by the lack of formality though, patients seem to appreciate it. Should they?
At first glance this seems to be a modern phenomenon. The reasons for casual wear today are manifold: about one-third of people work from home, Millennials are taking over with their TikTok values and general irreverence, COVID made us all fat and lazy. Heck, even the U.S. Senate briefly abolished the requirement to wear suits on the Senate floor. But getting dressed up was never to signal that you are elite or superior to others. It’s the opposite. To get dressed is a signal that you are serving others, a tradition that is as old as society.
Think of Downton Abbey as an example. The servants were always required to be smartly dressed when working, whereas members of the family could be dressed up or not. It’s clear who is serving whom. This tradition lives today in the hospitality industry. When you mosey into the lobby of a luxury hotel in your Rainbow sandals you can expect everyone who greets you will be in finery, signaling that they put in effort to serve you. You’ll find the same for all staff at the Mayo Clinic in Rochester, Minn., which is no coincidence.
Suits used to be standard in medicine. In the 19th century, physicians wore formal black-tie when seeing patients. Unlike hospitality however, we had good reason to eschew the tradition: germs. Once we figured out that our pus-stained ties and jackets were doing harm, we switched to wearing sanitized uniforms. Casual wear for doctors isn’t a modern phenomenon after all, then. For proof, compare Thomas Eakins painting “The Gross Clinic” (1875) with his later “The Agnew Clinic” (1889). In the former, Dr. Gross is portrayed in formal black wear, bloody hand and all. In the latter, Dr. Agnew is wearing white FIGS (or the 1890’s equivalent anyway). Similarly, nurses uniforms traditionally resembled kitchen servants, with criss-cross aprons and floor length skirts. It wasn’t until the 1980’s that nurses stopped wearing dresses and white caps.

In the operating theater it’s obviously critical that we wear sanitized scrubs to mitigate the risk of infection. Originally white to signal cleanliness, scrubs were changed to blue-green because surgeons were blinded by the lights bouncing off the uniforms. (Green is also opposite red on the color wheel, supposedly enhancing the ability to distinguish shades of red).
But Over time we’ve lost significant autonomy in our practice and lost a little respect from our patients. Payers tell us what to do. Patients question our expertise. Choosing what we wear is one of the few bits of medicine we still have agency. Pewter or pink, joggers or cargo pants, we get to choose.
The last time I flew British Airways everyone was in lounge wear, except the flight crew, of course. They were all smartly dressed. Recently British Airways rolled out updated, slightly more relaxed dress codes. Very modern, but I wonder if in a way we’re not all just a bit worse off.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Look at this guy – NFL Chargers jersey and shorts with a RVCA hat on backward. And next to him, a woman wearing her spin-class-Lulu gear. There’s also a guy sporting a 2016 San Diego Rock ‘n Roll Marathon Tee. And that young woman is actually wearing slippers. A visitor from the 1950s would be thunderstruck to see such casual wear on people waiting to board a plane. Photos from that era show men buttoned up in white shirt and tie and women wearing Chanel with hats and white gloves. This dramatic transformation from formal to unfussy wear cuts through all social situations, including in my office. As a new doc out of residency, I used to wear a tie and shoes that could hold a shine. Now I wear jogger scrubs and sneakers. Rather than be offended by the lack of formality though, patients seem to appreciate it. Should they?
At first glance this seems to be a modern phenomenon. The reasons for casual wear today are manifold: about one-third of people work from home, Millennials are taking over with their TikTok values and general irreverence, COVID made us all fat and lazy. Heck, even the U.S. Senate briefly abolished the requirement to wear suits on the Senate floor. But getting dressed up was never to signal that you are elite or superior to others. It’s the opposite. To get dressed is a signal that you are serving others, a tradition that is as old as society.
Think of Downton Abbey as an example. The servants were always required to be smartly dressed when working, whereas members of the family could be dressed up or not. It’s clear who is serving whom. This tradition lives today in the hospitality industry. When you mosey into the lobby of a luxury hotel in your Rainbow sandals you can expect everyone who greets you will be in finery, signaling that they put in effort to serve you. You’ll find the same for all staff at the Mayo Clinic in Rochester, Minn., which is no coincidence.
Suits used to be standard in medicine. In the 19th century, physicians wore formal black-tie when seeing patients. Unlike hospitality however, we had good reason to eschew the tradition: germs. Once we figured out that our pus-stained ties and jackets were doing harm, we switched to wearing sanitized uniforms. Casual wear for doctors isn’t a modern phenomenon after all, then. For proof, compare Thomas Eakins painting “The Gross Clinic” (1875) with his later “The Agnew Clinic” (1889). In the former, Dr. Gross is portrayed in formal black wear, bloody hand and all. In the latter, Dr. Agnew is wearing white FIGS (or the 1890’s equivalent anyway). Similarly, nurses uniforms traditionally resembled kitchen servants, with criss-cross aprons and floor length skirts. It wasn’t until the 1980’s that nurses stopped wearing dresses and white caps.

In the operating theater it’s obviously critical that we wear sanitized scrubs to mitigate the risk of infection. Originally white to signal cleanliness, scrubs were changed to blue-green because surgeons were blinded by the lights bouncing off the uniforms. (Green is also opposite red on the color wheel, supposedly enhancing the ability to distinguish shades of red).
But Over time we’ve lost significant autonomy in our practice and lost a little respect from our patients. Payers tell us what to do. Patients question our expertise. Choosing what we wear is one of the few bits of medicine we still have agency. Pewter or pink, joggers or cargo pants, we get to choose.
The last time I flew British Airways everyone was in lounge wear, except the flight crew, of course. They were all smartly dressed. Recently British Airways rolled out updated, slightly more relaxed dress codes. Very modern, but I wonder if in a way we’re not all just a bit worse off.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Look at this guy – NFL Chargers jersey and shorts with a RVCA hat on backward. And next to him, a woman wearing her spin-class-Lulu gear. There’s also a guy sporting a 2016 San Diego Rock ‘n Roll Marathon Tee. And that young woman is actually wearing slippers. A visitor from the 1950s would be thunderstruck to see such casual wear on people waiting to board a plane. Photos from that era show men buttoned up in white shirt and tie and women wearing Chanel with hats and white gloves. This dramatic transformation from formal to unfussy wear cuts through all social situations, including in my office. As a new doc out of residency, I used to wear a tie and shoes that could hold a shine. Now I wear jogger scrubs and sneakers. Rather than be offended by the lack of formality though, patients seem to appreciate it. Should they?
At first glance this seems to be a modern phenomenon. The reasons for casual wear today are manifold: about one-third of people work from home, Millennials are taking over with their TikTok values and general irreverence, COVID made us all fat and lazy. Heck, even the U.S. Senate briefly abolished the requirement to wear suits on the Senate floor. But getting dressed up was never to signal that you are elite or superior to others. It’s the opposite. To get dressed is a signal that you are serving others, a tradition that is as old as society.
Think of Downton Abbey as an example. The servants were always required to be smartly dressed when working, whereas members of the family could be dressed up or not. It’s clear who is serving whom. This tradition lives today in the hospitality industry. When you mosey into the lobby of a luxury hotel in your Rainbow sandals you can expect everyone who greets you will be in finery, signaling that they put in effort to serve you. You’ll find the same for all staff at the Mayo Clinic in Rochester, Minn., which is no coincidence.
Suits used to be standard in medicine. In the 19th century, physicians wore formal black-tie when seeing patients. Unlike hospitality however, we had good reason to eschew the tradition: germs. Once we figured out that our pus-stained ties and jackets were doing harm, we switched to wearing sanitized uniforms. Casual wear for doctors isn’t a modern phenomenon after all, then. For proof, compare Thomas Eakins painting “The Gross Clinic” (1875) with his later “The Agnew Clinic” (1889). In the former, Dr. Gross is portrayed in formal black wear, bloody hand and all. In the latter, Dr. Agnew is wearing white FIGS (or the 1890’s equivalent anyway). Similarly, nurses uniforms traditionally resembled kitchen servants, with criss-cross aprons and floor length skirts. It wasn’t until the 1980’s that nurses stopped wearing dresses and white caps.

In the operating theater it’s obviously critical that we wear sanitized scrubs to mitigate the risk of infection. Originally white to signal cleanliness, scrubs were changed to blue-green because surgeons were blinded by the lights bouncing off the uniforms. (Green is also opposite red on the color wheel, supposedly enhancing the ability to distinguish shades of red).
But Over time we’ve lost significant autonomy in our practice and lost a little respect from our patients. Payers tell us what to do. Patients question our expertise. Choosing what we wear is one of the few bits of medicine we still have agency. Pewter or pink, joggers or cargo pants, we get to choose.
The last time I flew British Airways everyone was in lounge wear, except the flight crew, of course. They were all smartly dressed. Recently British Airways rolled out updated, slightly more relaxed dress codes. Very modern, but I wonder if in a way we’re not all just a bit worse off.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]