User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Avoid anti-HER2 cancer therapies during pregnancy
TOPLINE:
, according to a recent analysis.
METHODOLOGY:
- Current guidelines do not recommend treating pregnant women with trastuzumab, given documented safety concerns. Other anti-HER2 agents are also discouraged in this setting because of a lack of safety data. However, when considering the efficacy of these drugs in HER2-positive breast cancer, having a better understanding of the potential toxicities in pregnant patients is important.
- In the current case-control analysis, the team explored the risk for adverse effects among pregnant women exposed to anti-HER2 agents vs other anticancer drugs.
- The researchers leveraged the World Health Organization’s pharmacovigilance database, VigiBase, to identify reports with at least one pregnancy-related complication and one suspected anticancer drug.
- The researchers classified exposure to the drugs as occurring before pregnancy, during pregnancy, or via breast milk, semen, or skin. The team then examined 30 maternal and fetal or neonatal adverse outcomes and grouped them into seven categories: abortions, stillbirths, congenital malformations, pregnancy complications, preterm birth, neonatal complications, and delivery complications.
- The most used anti-HER2 agent was trastuzumab (n = 302), followed by pertuzumab (n = 55), trastuzumab-emtansine (n = 20), and lapatinib (n = 18).
TAKEAWAY:
- Among 3,558 reports included in the analysis, 328 patients were exposed to anti-HER2 drugs compared with 3,230 patients who received other anticancer agents.
- Pregnancy, fetal, or newborn adverse outcomes were reported in 61.3% of women treated with anti-HER2 agents and 56.3% of those receiving other anticancer drugs.
- The five most frequently reported complications in the anti-HER2 group were oligohydramnios (23.8%), preterm birth (17.4%), intrauterine growth restriction (9.8%), neonatal respiratory disorder (7.3%), and spontaneous abortion (7.3%).
- Adverse outcomes overreported in women who received anti-HER2 agents included oligohydramnios (reporting odds ratio [ROR], 17.68), congenital tract disorders (ROR, 9.98), and neonatal kidney failure (ROR, 9.15). Cardiovascular malformations were also overreported among women receiving trastuzumab-emtansine (ROR, 4.46), as were intrauterine growth restrictions for those treated with lapatinib (ROR, 7.68).
IN PRACTICE:
Exposure to anti-HER2 agents was associated with “severe specific adverse pregnancy and fetal or newborn outcomes compared with exposure to other anticancer treatments,” with a “strong, highly significant overreporting of congenital respiratory tract disorders and neonatal kidney failure,” which can lead to oligohydramnios, the authors wrote. The authors also noted that when delaying anti-HER2 therapy is not possible, it’s imperative to monitor patients closely for oligohydramnios.
SOURCE:
The study, led by Paul Gougis, MD, Institut Curie Centre de Recherche, Paris, , was published online in JAMA Network Open.
LIMITATIONS:
Potential inconsistencies in the collection of pharmacovigilance data could limit the generalizability of the results in the general population. The group of women exposed to other anticancer therapies may also constitute a different patient population from that given anti-HER2 therapies.
DISCLOSURES:
Coauthor Jean-Philippe Spano, MD, PhD, declared relationships Gilead, AstraZeneca, Lilly, Pfizer, Novartis, Daiichi Sankyo, and GSK.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a recent analysis.
METHODOLOGY:
- Current guidelines do not recommend treating pregnant women with trastuzumab, given documented safety concerns. Other anti-HER2 agents are also discouraged in this setting because of a lack of safety data. However, when considering the efficacy of these drugs in HER2-positive breast cancer, having a better understanding of the potential toxicities in pregnant patients is important.
- In the current case-control analysis, the team explored the risk for adverse effects among pregnant women exposed to anti-HER2 agents vs other anticancer drugs.
- The researchers leveraged the World Health Organization’s pharmacovigilance database, VigiBase, to identify reports with at least one pregnancy-related complication and one suspected anticancer drug.
- The researchers classified exposure to the drugs as occurring before pregnancy, during pregnancy, or via breast milk, semen, or skin. The team then examined 30 maternal and fetal or neonatal adverse outcomes and grouped them into seven categories: abortions, stillbirths, congenital malformations, pregnancy complications, preterm birth, neonatal complications, and delivery complications.
- The most used anti-HER2 agent was trastuzumab (n = 302), followed by pertuzumab (n = 55), trastuzumab-emtansine (n = 20), and lapatinib (n = 18).
TAKEAWAY:
- Among 3,558 reports included in the analysis, 328 patients were exposed to anti-HER2 drugs compared with 3,230 patients who received other anticancer agents.
- Pregnancy, fetal, or newborn adverse outcomes were reported in 61.3% of women treated with anti-HER2 agents and 56.3% of those receiving other anticancer drugs.
- The five most frequently reported complications in the anti-HER2 group were oligohydramnios (23.8%), preterm birth (17.4%), intrauterine growth restriction (9.8%), neonatal respiratory disorder (7.3%), and spontaneous abortion (7.3%).
- Adverse outcomes overreported in women who received anti-HER2 agents included oligohydramnios (reporting odds ratio [ROR], 17.68), congenital tract disorders (ROR, 9.98), and neonatal kidney failure (ROR, 9.15). Cardiovascular malformations were also overreported among women receiving trastuzumab-emtansine (ROR, 4.46), as were intrauterine growth restrictions for those treated with lapatinib (ROR, 7.68).
IN PRACTICE:
Exposure to anti-HER2 agents was associated with “severe specific adverse pregnancy and fetal or newborn outcomes compared with exposure to other anticancer treatments,” with a “strong, highly significant overreporting of congenital respiratory tract disorders and neonatal kidney failure,” which can lead to oligohydramnios, the authors wrote. The authors also noted that when delaying anti-HER2 therapy is not possible, it’s imperative to monitor patients closely for oligohydramnios.
SOURCE:
The study, led by Paul Gougis, MD, Institut Curie Centre de Recherche, Paris, , was published online in JAMA Network Open.
LIMITATIONS:
Potential inconsistencies in the collection of pharmacovigilance data could limit the generalizability of the results in the general population. The group of women exposed to other anticancer therapies may also constitute a different patient population from that given anti-HER2 therapies.
DISCLOSURES:
Coauthor Jean-Philippe Spano, MD, PhD, declared relationships Gilead, AstraZeneca, Lilly, Pfizer, Novartis, Daiichi Sankyo, and GSK.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a recent analysis.
METHODOLOGY:
- Current guidelines do not recommend treating pregnant women with trastuzumab, given documented safety concerns. Other anti-HER2 agents are also discouraged in this setting because of a lack of safety data. However, when considering the efficacy of these drugs in HER2-positive breast cancer, having a better understanding of the potential toxicities in pregnant patients is important.
- In the current case-control analysis, the team explored the risk for adverse effects among pregnant women exposed to anti-HER2 agents vs other anticancer drugs.
- The researchers leveraged the World Health Organization’s pharmacovigilance database, VigiBase, to identify reports with at least one pregnancy-related complication and one suspected anticancer drug.
- The researchers classified exposure to the drugs as occurring before pregnancy, during pregnancy, or via breast milk, semen, or skin. The team then examined 30 maternal and fetal or neonatal adverse outcomes and grouped them into seven categories: abortions, stillbirths, congenital malformations, pregnancy complications, preterm birth, neonatal complications, and delivery complications.
- The most used anti-HER2 agent was trastuzumab (n = 302), followed by pertuzumab (n = 55), trastuzumab-emtansine (n = 20), and lapatinib (n = 18).
TAKEAWAY:
- Among 3,558 reports included in the analysis, 328 patients were exposed to anti-HER2 drugs compared with 3,230 patients who received other anticancer agents.
- Pregnancy, fetal, or newborn adverse outcomes were reported in 61.3% of women treated with anti-HER2 agents and 56.3% of those receiving other anticancer drugs.
- The five most frequently reported complications in the anti-HER2 group were oligohydramnios (23.8%), preterm birth (17.4%), intrauterine growth restriction (9.8%), neonatal respiratory disorder (7.3%), and spontaneous abortion (7.3%).
- Adverse outcomes overreported in women who received anti-HER2 agents included oligohydramnios (reporting odds ratio [ROR], 17.68), congenital tract disorders (ROR, 9.98), and neonatal kidney failure (ROR, 9.15). Cardiovascular malformations were also overreported among women receiving trastuzumab-emtansine (ROR, 4.46), as were intrauterine growth restrictions for those treated with lapatinib (ROR, 7.68).
IN PRACTICE:
Exposure to anti-HER2 agents was associated with “severe specific adverse pregnancy and fetal or newborn outcomes compared with exposure to other anticancer treatments,” with a “strong, highly significant overreporting of congenital respiratory tract disorders and neonatal kidney failure,” which can lead to oligohydramnios, the authors wrote. The authors also noted that when delaying anti-HER2 therapy is not possible, it’s imperative to monitor patients closely for oligohydramnios.
SOURCE:
The study, led by Paul Gougis, MD, Institut Curie Centre de Recherche, Paris, , was published online in JAMA Network Open.
LIMITATIONS:
Potential inconsistencies in the collection of pharmacovigilance data could limit the generalizability of the results in the general population. The group of women exposed to other anticancer therapies may also constitute a different patient population from that given anti-HER2 therapies.
DISCLOSURES:
Coauthor Jean-Philippe Spano, MD, PhD, declared relationships Gilead, AstraZeneca, Lilly, Pfizer, Novartis, Daiichi Sankyo, and GSK.
A version of this article appeared on Medscape.com.
Pregnant women with eosinophilic esophagitis show no ill effects from inhaled steroids
, according to new research presented at the annual meeting of the American College of Gastroenterology.
“Currently, there are no specific recommendations about the safe use of steroids in pregnant women with eosinophilic esophagitis (EoE), Julton Tomanguillo Chumbe, MD, said in an interview. “Our recommendations about the use of steroids among this population are based on the safety data extrapolated mainly from pregnant women with asthma.”
In the study, Dr. Chumbe, an internal medicine resident at Charleston Area Medical Center, West Virginia University, Charleston, and colleagues identified pregnant patients aged 18 years and older with a diagnosis of EoE between January 2011 and December 2022 through the TriNetx Global Collaborative Network, which includes 101 health care organizations in 14 countries. The study population consisted of 1,263 individuals.
The researchers used propensity score matching (PSM) to compare the rates of spontaneous abortion, placenta previa, preeclampsia, premature delivery, HELLP syndrome, eclampsia, hyperemesis gravidarum, and major congenital abnormalities between women with EoE who did and did not use steroids during pregnancy. The PSM cohorts included 268 women in each group.
Overall, pregnant women who used steroids were not significantly more likely than were those who did not use steroids to experience spontaneous abortion (3.73% vs. 4.85%, P = .52). Rates of placenta previa, preeclampsia, premature delivery, HELLP syndrome, and hyperemesis gravidarum were equal between the groups (3.73% vs. 3.73%, P = 1.00 for all). No cases of eclampsia occurred in the steroid group, compared with a 3.73% rate in women who did not use steroids.
Incidence of major congenital abnormalities including but not limited to malformations of the eye, ear, face, neck, skull and face bones, and of the circulatory, respiratory, and digestive systems, were similar between the steroid and no steroid groups (7.09% vs. 8.20%, P = .62)
Dr. Chumbe said he was not surprised by the findings, given the robust data about the safe use of steroids in pregnant women with asthma, in terms of pregnancy outcomes and fetal outcomes.
“The findings of this study provide reassurance that the use of steroids in pregnant patients with eosinophilic esophagitis is not significantly associated with an increased risk of worse maternal or fetal outcomes,” he said. “During pregnancy, some patients may discontinue treatment due to safety concerns. However, this study suggests that this may not be necessary.” Consequently, patients can maintain EoE management while reducing the risk of complications.
Looking ahead, “it will be important to have some data about the safe use of dupilumab during pregnancy in patients with eosinophilic esophagitis,” he said.
Pregnant patients can maintain EoE management
“This study is able to address an important concern that many patients have regarding the safety of steroid therapy for EoE, particularly during pregnancy,” said Anita Afzali, MD, MPH, AGAF, a gastroenterologist specializing in inflammatory bowel disease and executive vice chair of internal medicine at the University of Cincinnati. “As EoE impacts over 40% of women, most who are in childbearing age, it is important to review the safety of treatment and management of EoE so a mother does not have to choose between EoE management and pregnancy.”
The results from this study were certainly reassuring, though not surprising, Dr. Afzali said. “Previously, the safety profile of steroids during pregnancy was mostly extrapolated from asthma, and other diseases such as inflammatory bowel disease. The results from this study confirm that there are no significant associations with adverse maternal or birth outcomes among women with EoE treated with steroids during pregnancy,” she said.
The study has some limitations, including the retrospective design and potential for selection bias, Dr. Afzali noted. “Further research is needed for the evaluation of newer therapies in the pipeline for treatment of EoE and its safety profile with pregnancy,” she said.
However, “sharing this information in clinical practice “will allow our patients to feel comfortable with continuation of appropriate steroid therapy for treatment and management of their EoE, without having to choose between family planning or pregnancy and EoE care management,” Dr. Afzali said.
The study received no outside funding. Dr. Chumbe an Dr. Afzali indicated having no relevant financial conflicts to disclose.
, according to new research presented at the annual meeting of the American College of Gastroenterology.
“Currently, there are no specific recommendations about the safe use of steroids in pregnant women with eosinophilic esophagitis (EoE), Julton Tomanguillo Chumbe, MD, said in an interview. “Our recommendations about the use of steroids among this population are based on the safety data extrapolated mainly from pregnant women with asthma.”
In the study, Dr. Chumbe, an internal medicine resident at Charleston Area Medical Center, West Virginia University, Charleston, and colleagues identified pregnant patients aged 18 years and older with a diagnosis of EoE between January 2011 and December 2022 through the TriNetx Global Collaborative Network, which includes 101 health care organizations in 14 countries. The study population consisted of 1,263 individuals.
The researchers used propensity score matching (PSM) to compare the rates of spontaneous abortion, placenta previa, preeclampsia, premature delivery, HELLP syndrome, eclampsia, hyperemesis gravidarum, and major congenital abnormalities between women with EoE who did and did not use steroids during pregnancy. The PSM cohorts included 268 women in each group.
Overall, pregnant women who used steroids were not significantly more likely than were those who did not use steroids to experience spontaneous abortion (3.73% vs. 4.85%, P = .52). Rates of placenta previa, preeclampsia, premature delivery, HELLP syndrome, and hyperemesis gravidarum were equal between the groups (3.73% vs. 3.73%, P = 1.00 for all). No cases of eclampsia occurred in the steroid group, compared with a 3.73% rate in women who did not use steroids.
Incidence of major congenital abnormalities including but not limited to malformations of the eye, ear, face, neck, skull and face bones, and of the circulatory, respiratory, and digestive systems, were similar between the steroid and no steroid groups (7.09% vs. 8.20%, P = .62)
Dr. Chumbe said he was not surprised by the findings, given the robust data about the safe use of steroids in pregnant women with asthma, in terms of pregnancy outcomes and fetal outcomes.
“The findings of this study provide reassurance that the use of steroids in pregnant patients with eosinophilic esophagitis is not significantly associated with an increased risk of worse maternal or fetal outcomes,” he said. “During pregnancy, some patients may discontinue treatment due to safety concerns. However, this study suggests that this may not be necessary.” Consequently, patients can maintain EoE management while reducing the risk of complications.
Looking ahead, “it will be important to have some data about the safe use of dupilumab during pregnancy in patients with eosinophilic esophagitis,” he said.
Pregnant patients can maintain EoE management
“This study is able to address an important concern that many patients have regarding the safety of steroid therapy for EoE, particularly during pregnancy,” said Anita Afzali, MD, MPH, AGAF, a gastroenterologist specializing in inflammatory bowel disease and executive vice chair of internal medicine at the University of Cincinnati. “As EoE impacts over 40% of women, most who are in childbearing age, it is important to review the safety of treatment and management of EoE so a mother does not have to choose between EoE management and pregnancy.”
The results from this study were certainly reassuring, though not surprising, Dr. Afzali said. “Previously, the safety profile of steroids during pregnancy was mostly extrapolated from asthma, and other diseases such as inflammatory bowel disease. The results from this study confirm that there are no significant associations with adverse maternal or birth outcomes among women with EoE treated with steroids during pregnancy,” she said.
The study has some limitations, including the retrospective design and potential for selection bias, Dr. Afzali noted. “Further research is needed for the evaluation of newer therapies in the pipeline for treatment of EoE and its safety profile with pregnancy,” she said.
However, “sharing this information in clinical practice “will allow our patients to feel comfortable with continuation of appropriate steroid therapy for treatment and management of their EoE, without having to choose between family planning or pregnancy and EoE care management,” Dr. Afzali said.
The study received no outside funding. Dr. Chumbe an Dr. Afzali indicated having no relevant financial conflicts to disclose.
, according to new research presented at the annual meeting of the American College of Gastroenterology.
“Currently, there are no specific recommendations about the safe use of steroids in pregnant women with eosinophilic esophagitis (EoE), Julton Tomanguillo Chumbe, MD, said in an interview. “Our recommendations about the use of steroids among this population are based on the safety data extrapolated mainly from pregnant women with asthma.”
In the study, Dr. Chumbe, an internal medicine resident at Charleston Area Medical Center, West Virginia University, Charleston, and colleagues identified pregnant patients aged 18 years and older with a diagnosis of EoE between January 2011 and December 2022 through the TriNetx Global Collaborative Network, which includes 101 health care organizations in 14 countries. The study population consisted of 1,263 individuals.
The researchers used propensity score matching (PSM) to compare the rates of spontaneous abortion, placenta previa, preeclampsia, premature delivery, HELLP syndrome, eclampsia, hyperemesis gravidarum, and major congenital abnormalities between women with EoE who did and did not use steroids during pregnancy. The PSM cohorts included 268 women in each group.
Overall, pregnant women who used steroids were not significantly more likely than were those who did not use steroids to experience spontaneous abortion (3.73% vs. 4.85%, P = .52). Rates of placenta previa, preeclampsia, premature delivery, HELLP syndrome, and hyperemesis gravidarum were equal between the groups (3.73% vs. 3.73%, P = 1.00 for all). No cases of eclampsia occurred in the steroid group, compared with a 3.73% rate in women who did not use steroids.
Incidence of major congenital abnormalities including but not limited to malformations of the eye, ear, face, neck, skull and face bones, and of the circulatory, respiratory, and digestive systems, were similar between the steroid and no steroid groups (7.09% vs. 8.20%, P = .62)
Dr. Chumbe said he was not surprised by the findings, given the robust data about the safe use of steroids in pregnant women with asthma, in terms of pregnancy outcomes and fetal outcomes.
“The findings of this study provide reassurance that the use of steroids in pregnant patients with eosinophilic esophagitis is not significantly associated with an increased risk of worse maternal or fetal outcomes,” he said. “During pregnancy, some patients may discontinue treatment due to safety concerns. However, this study suggests that this may not be necessary.” Consequently, patients can maintain EoE management while reducing the risk of complications.
Looking ahead, “it will be important to have some data about the safe use of dupilumab during pregnancy in patients with eosinophilic esophagitis,” he said.
Pregnant patients can maintain EoE management
“This study is able to address an important concern that many patients have regarding the safety of steroid therapy for EoE, particularly during pregnancy,” said Anita Afzali, MD, MPH, AGAF, a gastroenterologist specializing in inflammatory bowel disease and executive vice chair of internal medicine at the University of Cincinnati. “As EoE impacts over 40% of women, most who are in childbearing age, it is important to review the safety of treatment and management of EoE so a mother does not have to choose between EoE management and pregnancy.”
The results from this study were certainly reassuring, though not surprising, Dr. Afzali said. “Previously, the safety profile of steroids during pregnancy was mostly extrapolated from asthma, and other diseases such as inflammatory bowel disease. The results from this study confirm that there are no significant associations with adverse maternal or birth outcomes among women with EoE treated with steroids during pregnancy,” she said.
The study has some limitations, including the retrospective design and potential for selection bias, Dr. Afzali noted. “Further research is needed for the evaluation of newer therapies in the pipeline for treatment of EoE and its safety profile with pregnancy,” she said.
However, “sharing this information in clinical practice “will allow our patients to feel comfortable with continuation of appropriate steroid therapy for treatment and management of their EoE, without having to choose between family planning or pregnancy and EoE care management,” Dr. Afzali said.
The study received no outside funding. Dr. Chumbe an Dr. Afzali indicated having no relevant financial conflicts to disclose.
FROM ACG 2023
Rx for resilience: Five prescriptions for physician burnout
Physician burnout persists even as the height of the COVID-19 crisis fades farther into the rear-view mirror. The causes for the sadness, stress, and frustration among doctors vary, but the effects are universal and often debilitating: exhaustion, emotional detachment, lethargy, feeling useless, and lacking purpose.
When surveyed, physicians pointed to many systemic solutions for burnout in Medscape’s Physician Burnout & Depression Report 2023, such as a need for greater compensation, more manageable workloads and schedules, and more support staff. But for many doctors, these fixes may be years if not decades away. Equally important are strategies for relieving burnout symptoms now, especially as we head into a busy holiday season.
Because not every stress-relief practice works for everyone, it’s crucial to try various methods until you find something that makes a difference for you, said Christine Gibson, MD, a family physician and trauma therapist in Calgary, Alta., and author of The Modern Trauma Toolkit.
“Every person should have a toolkit of the things that bring them out of the psychological and physical distress that dysregulates their nervous system,” said Dr. Gibson.
Once you learn the personal ways to alleviate your specific brand of burnout, you can start working on systemic changes that might help the culture of medicine overall.
Symptoms speak louder than words
It seems obvious, but if you aren’t aware that what you’re feeling is burnout, you probably aren’t going to find effective steps to relieve it. Jessi Gold, MD, assistant professor and director of wellness, engagement, and outreach in the department of psychiatry, Washington University in St. Louis, is a psychiatrist who treats health care professionals, including frontline workers during the height of the pandemic. But even as a burnout expert, she admits that she misses the signs in herself.
“I was fighting constant fatigue, falling asleep the minute I got home from work every day, but I thought a B12 shot would solve all my problems. I didn’t realize I was having symptoms of burnout until my own therapist told me,” said Dr. Gold. “As doctors, we spend so much time focusing on other people that we don’t necessarily notice very much in ourselves – usually once it starts to impact our job.”
Practices like meditation and mindfulness can help you delve into your feelings and emotions and notice how you’re doing. But you may also need to ask spouses, partners, and friends and family – or better yet, a mental health professional – if they notice that you seem burnt out.
Practice ‘in the moment’ relief
Sometimes, walking away at the moment of stress helps like when stepping away from a heated argument. “Step out of a frustrating staff meeting to go to the bathroom and splash your face,” said Eran Magan, PhD, a psychologist at the University of Pennsylvania, Philadelphia, and founder and CEO of the suicide prevention system EarlyAlert.me. “Tell a patient you need to check something in the next room, so you have time to take a breath.”
Dr. Magan recommended finding techniques that help lower acute stress while it’s actually happening. First, find a way to escape or excuse yourself from the event, and when possible, stop situations that are actively upsetting or triggering in their tracks.
Next, recharge by doing something that helps you feel better, like looking at a cute video of your child or grandchild or closing your eyes and taking a deep breath. You can also try to “catch” good feelings from someone else, said Dr. Magan. Ask someone about a trip, vacation, holiday, or pleasant event. “Ask a colleague about something that makes [them] happy,” he said. “Happiness can be infectious too.”
Burnout is also in the body
“Body psychotherapy” or somatic therapy is a treatment that focuses on how emotions appear within your body. Dr. Gibson said it’s a valuable tool for addressing trauma and a mainstay in many a medical career; it’s useful to help physicians learn to “befriend” their nervous system.
Somatic therapy exercises involve things like body scanning, scanning for physical sensations; conscious breathing, connecting to each inhale and exhale; grounding your weight by releasing tension through your feet, doing a total body stretch; or releasing shoulder and neck tension by consciously relaxing each of these muscle groups.
“We spend our whole day in sympathetic tone; our amygdala’s are firing, telling us that we’re in danger,” said Dr. Gibson. “We actually have to practice getting into and spending time in our parasympathetic nervous system to restore the balance in our autonomic nervous system.”
Somatic therapy includes a wide array of exercises that help reconnect you to your body through calming or activation. The movements release tension, ground you, and restore balance.
Bite-sized tools for well-being
Because of the prevalence of physician burnout, there’s been a groundswell of researchers and organizations who have turned their focus toward improving the well-being in the health care workforce.
One such effort comes from the Duke Center for the Advancement of Well-being Science, which “camouflages” well-being tools as continuing education credits to make them accessible for busy, stressed, and overworked physicians.
“They’re called bite-sized tools for well-being, and they have actual evidence behind them,” said Dr. Gold. For example, she said, one tools is a text program called Three Good Things that encourages physicians to send a text listing three positive things that happened during the day. The exercise lasts 15 days, and texters have access to others’ answers as well. After 3 months, participants’ baseline depression, gratitude, and life satisfaction had all “significantly improved.”
“It feels almost ridiculous that that could work, but it does,” said Dr. Gold. “I’ve had patients push back and say: ‘Well, isn’t that toxic positivity?’ But really what it is is dialectics. It’s not saying there’s only positive; it’s just making you realize there is more than just the negative.”
These and other short interventions focus on concepts such as joy, humor, awe, engagement, and self-kindness to build resilience and help physicians recover from burnout symptoms.
Cognitive restructuring could work
Cognitive restructuring is a therapeutic process of learning new ways of interpreting and responding to people and situations. It helps you change the “filter” through which you interact with your environment. Dr. Gibson said it’s a tool to use with care after other modes of therapy that help you understand your patterns and how they developed because of how you view and understand the world.
“The message of [cognitive-behavioral therapy] or cognitive restructuring is there’s something wrong with the way you’re thinking, and we need to change it or fix it, but in a traumatic system [like health care], you’re thinking has been an adaptive process related to the harm in the environment you’re in,” said Dr. Gibson.
“So, if you [jump straight to cognitive restructuring before other types of therapy], then we just gaslight ourselves into believing that there’s something wrong with us, that we haven’t adapted sufficiently to an environment that’s actually harmful.”
Strive for a few systemic changes
Systemic changes can be small ones within your own sphere. For example, Dr. Magan said, work toward making little tweaks to the flow of your day that will increase calm and reduce frustration.
“Make a ‘bug list,’ little, regular demands that drain your energy, and discuss them with your colleagues and supervisors to see if they can be improved,” he said. Examples include everyday frustrations like having unsolicited visitors popping into your office, scheduling complex patients too late in the day, or having a computer freeze whenever you access patient charts.
Though not always financially feasible, affecting real change and finding relief from all these insidious bugs can improve your mental health and burnout symptoms.
“Physicians tend to work extremely hard in order to keep holding together a system that is often not inherently sustainable, like the fascia of a body under tremendous strain,” said Dr. Magan. “Sometimes the brave thing to do is to refuse to continue being the lynchpin and let things break, so the system will have to start improving itself, rather than demanding more and more of the people in it.”
A version of this article first appeared on Medscape.com.
Physician burnout persists even as the height of the COVID-19 crisis fades farther into the rear-view mirror. The causes for the sadness, stress, and frustration among doctors vary, but the effects are universal and often debilitating: exhaustion, emotional detachment, lethargy, feeling useless, and lacking purpose.
When surveyed, physicians pointed to many systemic solutions for burnout in Medscape’s Physician Burnout & Depression Report 2023, such as a need for greater compensation, more manageable workloads and schedules, and more support staff. But for many doctors, these fixes may be years if not decades away. Equally important are strategies for relieving burnout symptoms now, especially as we head into a busy holiday season.
Because not every stress-relief practice works for everyone, it’s crucial to try various methods until you find something that makes a difference for you, said Christine Gibson, MD, a family physician and trauma therapist in Calgary, Alta., and author of The Modern Trauma Toolkit.
“Every person should have a toolkit of the things that bring them out of the psychological and physical distress that dysregulates their nervous system,” said Dr. Gibson.
Once you learn the personal ways to alleviate your specific brand of burnout, you can start working on systemic changes that might help the culture of medicine overall.
Symptoms speak louder than words
It seems obvious, but if you aren’t aware that what you’re feeling is burnout, you probably aren’t going to find effective steps to relieve it. Jessi Gold, MD, assistant professor and director of wellness, engagement, and outreach in the department of psychiatry, Washington University in St. Louis, is a psychiatrist who treats health care professionals, including frontline workers during the height of the pandemic. But even as a burnout expert, she admits that she misses the signs in herself.
“I was fighting constant fatigue, falling asleep the minute I got home from work every day, but I thought a B12 shot would solve all my problems. I didn’t realize I was having symptoms of burnout until my own therapist told me,” said Dr. Gold. “As doctors, we spend so much time focusing on other people that we don’t necessarily notice very much in ourselves – usually once it starts to impact our job.”
Practices like meditation and mindfulness can help you delve into your feelings and emotions and notice how you’re doing. But you may also need to ask spouses, partners, and friends and family – or better yet, a mental health professional – if they notice that you seem burnt out.
Practice ‘in the moment’ relief
Sometimes, walking away at the moment of stress helps like when stepping away from a heated argument. “Step out of a frustrating staff meeting to go to the bathroom and splash your face,” said Eran Magan, PhD, a psychologist at the University of Pennsylvania, Philadelphia, and founder and CEO of the suicide prevention system EarlyAlert.me. “Tell a patient you need to check something in the next room, so you have time to take a breath.”
Dr. Magan recommended finding techniques that help lower acute stress while it’s actually happening. First, find a way to escape or excuse yourself from the event, and when possible, stop situations that are actively upsetting or triggering in their tracks.
Next, recharge by doing something that helps you feel better, like looking at a cute video of your child or grandchild or closing your eyes and taking a deep breath. You can also try to “catch” good feelings from someone else, said Dr. Magan. Ask someone about a trip, vacation, holiday, or pleasant event. “Ask a colleague about something that makes [them] happy,” he said. “Happiness can be infectious too.”
Burnout is also in the body
“Body psychotherapy” or somatic therapy is a treatment that focuses on how emotions appear within your body. Dr. Gibson said it’s a valuable tool for addressing trauma and a mainstay in many a medical career; it’s useful to help physicians learn to “befriend” their nervous system.
Somatic therapy exercises involve things like body scanning, scanning for physical sensations; conscious breathing, connecting to each inhale and exhale; grounding your weight by releasing tension through your feet, doing a total body stretch; or releasing shoulder and neck tension by consciously relaxing each of these muscle groups.
“We spend our whole day in sympathetic tone; our amygdala’s are firing, telling us that we’re in danger,” said Dr. Gibson. “We actually have to practice getting into and spending time in our parasympathetic nervous system to restore the balance in our autonomic nervous system.”
Somatic therapy includes a wide array of exercises that help reconnect you to your body through calming or activation. The movements release tension, ground you, and restore balance.
Bite-sized tools for well-being
Because of the prevalence of physician burnout, there’s been a groundswell of researchers and organizations who have turned their focus toward improving the well-being in the health care workforce.
One such effort comes from the Duke Center for the Advancement of Well-being Science, which “camouflages” well-being tools as continuing education credits to make them accessible for busy, stressed, and overworked physicians.
“They’re called bite-sized tools for well-being, and they have actual evidence behind them,” said Dr. Gold. For example, she said, one tools is a text program called Three Good Things that encourages physicians to send a text listing three positive things that happened during the day. The exercise lasts 15 days, and texters have access to others’ answers as well. After 3 months, participants’ baseline depression, gratitude, and life satisfaction had all “significantly improved.”
“It feels almost ridiculous that that could work, but it does,” said Dr. Gold. “I’ve had patients push back and say: ‘Well, isn’t that toxic positivity?’ But really what it is is dialectics. It’s not saying there’s only positive; it’s just making you realize there is more than just the negative.”
These and other short interventions focus on concepts such as joy, humor, awe, engagement, and self-kindness to build resilience and help physicians recover from burnout symptoms.
Cognitive restructuring could work
Cognitive restructuring is a therapeutic process of learning new ways of interpreting and responding to people and situations. It helps you change the “filter” through which you interact with your environment. Dr. Gibson said it’s a tool to use with care after other modes of therapy that help you understand your patterns and how they developed because of how you view and understand the world.
“The message of [cognitive-behavioral therapy] or cognitive restructuring is there’s something wrong with the way you’re thinking, and we need to change it or fix it, but in a traumatic system [like health care], you’re thinking has been an adaptive process related to the harm in the environment you’re in,” said Dr. Gibson.
“So, if you [jump straight to cognitive restructuring before other types of therapy], then we just gaslight ourselves into believing that there’s something wrong with us, that we haven’t adapted sufficiently to an environment that’s actually harmful.”
Strive for a few systemic changes
Systemic changes can be small ones within your own sphere. For example, Dr. Magan said, work toward making little tweaks to the flow of your day that will increase calm and reduce frustration.
“Make a ‘bug list,’ little, regular demands that drain your energy, and discuss them with your colleagues and supervisors to see if they can be improved,” he said. Examples include everyday frustrations like having unsolicited visitors popping into your office, scheduling complex patients too late in the day, or having a computer freeze whenever you access patient charts.
Though not always financially feasible, affecting real change and finding relief from all these insidious bugs can improve your mental health and burnout symptoms.
“Physicians tend to work extremely hard in order to keep holding together a system that is often not inherently sustainable, like the fascia of a body under tremendous strain,” said Dr. Magan. “Sometimes the brave thing to do is to refuse to continue being the lynchpin and let things break, so the system will have to start improving itself, rather than demanding more and more of the people in it.”
A version of this article first appeared on Medscape.com.
Physician burnout persists even as the height of the COVID-19 crisis fades farther into the rear-view mirror. The causes for the sadness, stress, and frustration among doctors vary, but the effects are universal and often debilitating: exhaustion, emotional detachment, lethargy, feeling useless, and lacking purpose.
When surveyed, physicians pointed to many systemic solutions for burnout in Medscape’s Physician Burnout & Depression Report 2023, such as a need for greater compensation, more manageable workloads and schedules, and more support staff. But for many doctors, these fixes may be years if not decades away. Equally important are strategies for relieving burnout symptoms now, especially as we head into a busy holiday season.
Because not every stress-relief practice works for everyone, it’s crucial to try various methods until you find something that makes a difference for you, said Christine Gibson, MD, a family physician and trauma therapist in Calgary, Alta., and author of The Modern Trauma Toolkit.
“Every person should have a toolkit of the things that bring them out of the psychological and physical distress that dysregulates their nervous system,” said Dr. Gibson.
Once you learn the personal ways to alleviate your specific brand of burnout, you can start working on systemic changes that might help the culture of medicine overall.
Symptoms speak louder than words
It seems obvious, but if you aren’t aware that what you’re feeling is burnout, you probably aren’t going to find effective steps to relieve it. Jessi Gold, MD, assistant professor and director of wellness, engagement, and outreach in the department of psychiatry, Washington University in St. Louis, is a psychiatrist who treats health care professionals, including frontline workers during the height of the pandemic. But even as a burnout expert, she admits that she misses the signs in herself.
“I was fighting constant fatigue, falling asleep the minute I got home from work every day, but I thought a B12 shot would solve all my problems. I didn’t realize I was having symptoms of burnout until my own therapist told me,” said Dr. Gold. “As doctors, we spend so much time focusing on other people that we don’t necessarily notice very much in ourselves – usually once it starts to impact our job.”
Practices like meditation and mindfulness can help you delve into your feelings and emotions and notice how you’re doing. But you may also need to ask spouses, partners, and friends and family – or better yet, a mental health professional – if they notice that you seem burnt out.
Practice ‘in the moment’ relief
Sometimes, walking away at the moment of stress helps like when stepping away from a heated argument. “Step out of a frustrating staff meeting to go to the bathroom and splash your face,” said Eran Magan, PhD, a psychologist at the University of Pennsylvania, Philadelphia, and founder and CEO of the suicide prevention system EarlyAlert.me. “Tell a patient you need to check something in the next room, so you have time to take a breath.”
Dr. Magan recommended finding techniques that help lower acute stress while it’s actually happening. First, find a way to escape or excuse yourself from the event, and when possible, stop situations that are actively upsetting or triggering in their tracks.
Next, recharge by doing something that helps you feel better, like looking at a cute video of your child or grandchild or closing your eyes and taking a deep breath. You can also try to “catch” good feelings from someone else, said Dr. Magan. Ask someone about a trip, vacation, holiday, or pleasant event. “Ask a colleague about something that makes [them] happy,” he said. “Happiness can be infectious too.”
Burnout is also in the body
“Body psychotherapy” or somatic therapy is a treatment that focuses on how emotions appear within your body. Dr. Gibson said it’s a valuable tool for addressing trauma and a mainstay in many a medical career; it’s useful to help physicians learn to “befriend” their nervous system.
Somatic therapy exercises involve things like body scanning, scanning for physical sensations; conscious breathing, connecting to each inhale and exhale; grounding your weight by releasing tension through your feet, doing a total body stretch; or releasing shoulder and neck tension by consciously relaxing each of these muscle groups.
“We spend our whole day in sympathetic tone; our amygdala’s are firing, telling us that we’re in danger,” said Dr. Gibson. “We actually have to practice getting into and spending time in our parasympathetic nervous system to restore the balance in our autonomic nervous system.”
Somatic therapy includes a wide array of exercises that help reconnect you to your body through calming or activation. The movements release tension, ground you, and restore balance.
Bite-sized tools for well-being
Because of the prevalence of physician burnout, there’s been a groundswell of researchers and organizations who have turned their focus toward improving the well-being in the health care workforce.
One such effort comes from the Duke Center for the Advancement of Well-being Science, which “camouflages” well-being tools as continuing education credits to make them accessible for busy, stressed, and overworked physicians.
“They’re called bite-sized tools for well-being, and they have actual evidence behind them,” said Dr. Gold. For example, she said, one tools is a text program called Three Good Things that encourages physicians to send a text listing three positive things that happened during the day. The exercise lasts 15 days, and texters have access to others’ answers as well. After 3 months, participants’ baseline depression, gratitude, and life satisfaction had all “significantly improved.”
“It feels almost ridiculous that that could work, but it does,” said Dr. Gold. “I’ve had patients push back and say: ‘Well, isn’t that toxic positivity?’ But really what it is is dialectics. It’s not saying there’s only positive; it’s just making you realize there is more than just the negative.”
These and other short interventions focus on concepts such as joy, humor, awe, engagement, and self-kindness to build resilience and help physicians recover from burnout symptoms.
Cognitive restructuring could work
Cognitive restructuring is a therapeutic process of learning new ways of interpreting and responding to people and situations. It helps you change the “filter” through which you interact with your environment. Dr. Gibson said it’s a tool to use with care after other modes of therapy that help you understand your patterns and how they developed because of how you view and understand the world.
“The message of [cognitive-behavioral therapy] or cognitive restructuring is there’s something wrong with the way you’re thinking, and we need to change it or fix it, but in a traumatic system [like health care], you’re thinking has been an adaptive process related to the harm in the environment you’re in,” said Dr. Gibson.
“So, if you [jump straight to cognitive restructuring before other types of therapy], then we just gaslight ourselves into believing that there’s something wrong with us, that we haven’t adapted sufficiently to an environment that’s actually harmful.”
Strive for a few systemic changes
Systemic changes can be small ones within your own sphere. For example, Dr. Magan said, work toward making little tweaks to the flow of your day that will increase calm and reduce frustration.
“Make a ‘bug list,’ little, regular demands that drain your energy, and discuss them with your colleagues and supervisors to see if they can be improved,” he said. Examples include everyday frustrations like having unsolicited visitors popping into your office, scheduling complex patients too late in the day, or having a computer freeze whenever you access patient charts.
Though not always financially feasible, affecting real change and finding relief from all these insidious bugs can improve your mental health and burnout symptoms.
“Physicians tend to work extremely hard in order to keep holding together a system that is often not inherently sustainable, like the fascia of a body under tremendous strain,” said Dr. Magan. “Sometimes the brave thing to do is to refuse to continue being the lynchpin and let things break, so the system will have to start improving itself, rather than demanding more and more of the people in it.”
A version of this article first appeared on Medscape.com.
Hemorrhage-control device holds up in real-world review
Morbidity and mortality related to postpartum hemorrhage (PPH) are often preventable if caught early, but the persistent rise in PPH-associated morbidity illustrates the need for new and innovative treatments, wrote Dena Goffman, MD, of New York-Presbyterian/Columbia University Irving Medical Center, New York, and colleagues.
The device, known as the Jada System, was cleared by the Food and Drug Administration for management of abnormal postpartum uterine bleeding or postpartum hemorrhage (PPH) in August 2020 and showed safety and effectiveness in a registrational study of 106 patients, the researchers said.
In a postmarket registry medical record review known as RUBY (Treating Abnormal Postpartum Uterine Bleeding or Postpartum Hemorrhage with the Jada System), the researchers examined data collected from Oct. 8, 2020, to March 31, 2022, at 16 centers in the United States. The findings were published in Obstetrics & Gynecology.
The study population included all individuals treated with an intrauterine vacuum-induced hemorrhage control device; of these, 530 were vaginal births and 270 were cesarean births. A total of 94.3% had uterine atony, alone or in conjunction with other causes of bleeding. The median maternal age was 30.3 years; approximately 60% and 53% of patients in the vaginal and cesarean groups were White, and approximately 43% and 49% of patients in the two groups, respectively, were nulliparous.
The median blood loss at the time of device insertion was 1,250 mL in vaginal births and 1,980 mL in cesarean births, and the median time from delivery of the placenta to device insertion was 31 minutes and 108 minutes in the two groups, respectively.
The primary endpoint was treatment success, defined as control of bleeding after device insertion, with no escalation of treatment or recurrence of bleeding after the initial bleeding control and device removal.
Treatment success was achieved in 92.5% of vaginal births and 83.7% of cesarean births, and in 95.8% and 88.2%, respectively, among patients with isolated uterine atony. The median insertion time was 3.1 hours for vaginal births and 4.6 hours for cesarean births.
The safety profile was similar to that in the registrational trial and adverse effects were those expected in patients with PPH, the researchers noted.
A total of 14 SAEs were reported in 13 patients with vaginal births, and 22 SAEs were reported in 21 patients with cesarean births. Of these, three were identified as possibly related to the device or procedure (two cases of endometritis in the vaginal birth group and one case of hemorrhagic shock in the cesarean group); no uterine perforations of deaths were reported during the study.
The study was limited by several factors including the use of data mainly from academic centers, which could limit generalizability, and by the use of a mix of estimated and quantitative reporting of blood loss, the researchers noted. Other limitations include the inability to make direct comparisons to other treatments for PPH.
However, the results confirm the safety and efficacy of the device in a real-world setting and support its use as an important new tool in the management of PPH and reducing maternal morbidity and mortality, they concluded.
Two companies were involved in the study; Alydia Health contributed to the concept, design, and analysis, and Organon contributed to data analysis and reviewed the manuscript.
Dr. Goffman disclosed research support from Organon and Alydia Health, as well as serving as a speaker for Haymarket and PRIME PPH education and for Laborie, participation in the Cooper Surgical Obstetrical Safety Council, and serving as an editor for UpToDate. Several coauthors disclosed relationships with multiple companies including Organon and Alydia Health.
Morbidity and mortality related to postpartum hemorrhage (PPH) are often preventable if caught early, but the persistent rise in PPH-associated morbidity illustrates the need for new and innovative treatments, wrote Dena Goffman, MD, of New York-Presbyterian/Columbia University Irving Medical Center, New York, and colleagues.
The device, known as the Jada System, was cleared by the Food and Drug Administration for management of abnormal postpartum uterine bleeding or postpartum hemorrhage (PPH) in August 2020 and showed safety and effectiveness in a registrational study of 106 patients, the researchers said.
In a postmarket registry medical record review known as RUBY (Treating Abnormal Postpartum Uterine Bleeding or Postpartum Hemorrhage with the Jada System), the researchers examined data collected from Oct. 8, 2020, to March 31, 2022, at 16 centers in the United States. The findings were published in Obstetrics & Gynecology.
The study population included all individuals treated with an intrauterine vacuum-induced hemorrhage control device; of these, 530 were vaginal births and 270 were cesarean births. A total of 94.3% had uterine atony, alone or in conjunction with other causes of bleeding. The median maternal age was 30.3 years; approximately 60% and 53% of patients in the vaginal and cesarean groups were White, and approximately 43% and 49% of patients in the two groups, respectively, were nulliparous.
The median blood loss at the time of device insertion was 1,250 mL in vaginal births and 1,980 mL in cesarean births, and the median time from delivery of the placenta to device insertion was 31 minutes and 108 minutes in the two groups, respectively.
The primary endpoint was treatment success, defined as control of bleeding after device insertion, with no escalation of treatment or recurrence of bleeding after the initial bleeding control and device removal.
Treatment success was achieved in 92.5% of vaginal births and 83.7% of cesarean births, and in 95.8% and 88.2%, respectively, among patients with isolated uterine atony. The median insertion time was 3.1 hours for vaginal births and 4.6 hours for cesarean births.
The safety profile was similar to that in the registrational trial and adverse effects were those expected in patients with PPH, the researchers noted.
A total of 14 SAEs were reported in 13 patients with vaginal births, and 22 SAEs were reported in 21 patients with cesarean births. Of these, three were identified as possibly related to the device or procedure (two cases of endometritis in the vaginal birth group and one case of hemorrhagic shock in the cesarean group); no uterine perforations of deaths were reported during the study.
The study was limited by several factors including the use of data mainly from academic centers, which could limit generalizability, and by the use of a mix of estimated and quantitative reporting of blood loss, the researchers noted. Other limitations include the inability to make direct comparisons to other treatments for PPH.
However, the results confirm the safety and efficacy of the device in a real-world setting and support its use as an important new tool in the management of PPH and reducing maternal morbidity and mortality, they concluded.
Two companies were involved in the study; Alydia Health contributed to the concept, design, and analysis, and Organon contributed to data analysis and reviewed the manuscript.
Dr. Goffman disclosed research support from Organon and Alydia Health, as well as serving as a speaker for Haymarket and PRIME PPH education and for Laborie, participation in the Cooper Surgical Obstetrical Safety Council, and serving as an editor for UpToDate. Several coauthors disclosed relationships with multiple companies including Organon and Alydia Health.
Morbidity and mortality related to postpartum hemorrhage (PPH) are often preventable if caught early, but the persistent rise in PPH-associated morbidity illustrates the need for new and innovative treatments, wrote Dena Goffman, MD, of New York-Presbyterian/Columbia University Irving Medical Center, New York, and colleagues.
The device, known as the Jada System, was cleared by the Food and Drug Administration for management of abnormal postpartum uterine bleeding or postpartum hemorrhage (PPH) in August 2020 and showed safety and effectiveness in a registrational study of 106 patients, the researchers said.
In a postmarket registry medical record review known as RUBY (Treating Abnormal Postpartum Uterine Bleeding or Postpartum Hemorrhage with the Jada System), the researchers examined data collected from Oct. 8, 2020, to March 31, 2022, at 16 centers in the United States. The findings were published in Obstetrics & Gynecology.
The study population included all individuals treated with an intrauterine vacuum-induced hemorrhage control device; of these, 530 were vaginal births and 270 were cesarean births. A total of 94.3% had uterine atony, alone or in conjunction with other causes of bleeding. The median maternal age was 30.3 years; approximately 60% and 53% of patients in the vaginal and cesarean groups were White, and approximately 43% and 49% of patients in the two groups, respectively, were nulliparous.
The median blood loss at the time of device insertion was 1,250 mL in vaginal births and 1,980 mL in cesarean births, and the median time from delivery of the placenta to device insertion was 31 minutes and 108 minutes in the two groups, respectively.
The primary endpoint was treatment success, defined as control of bleeding after device insertion, with no escalation of treatment or recurrence of bleeding after the initial bleeding control and device removal.
Treatment success was achieved in 92.5% of vaginal births and 83.7% of cesarean births, and in 95.8% and 88.2%, respectively, among patients with isolated uterine atony. The median insertion time was 3.1 hours for vaginal births and 4.6 hours for cesarean births.
The safety profile was similar to that in the registrational trial and adverse effects were those expected in patients with PPH, the researchers noted.
A total of 14 SAEs were reported in 13 patients with vaginal births, and 22 SAEs were reported in 21 patients with cesarean births. Of these, three were identified as possibly related to the device or procedure (two cases of endometritis in the vaginal birth group and one case of hemorrhagic shock in the cesarean group); no uterine perforations of deaths were reported during the study.
The study was limited by several factors including the use of data mainly from academic centers, which could limit generalizability, and by the use of a mix of estimated and quantitative reporting of blood loss, the researchers noted. Other limitations include the inability to make direct comparisons to other treatments for PPH.
However, the results confirm the safety and efficacy of the device in a real-world setting and support its use as an important new tool in the management of PPH and reducing maternal morbidity and mortality, they concluded.
Two companies were involved in the study; Alydia Health contributed to the concept, design, and analysis, and Organon contributed to data analysis and reviewed the manuscript.
Dr. Goffman disclosed research support from Organon and Alydia Health, as well as serving as a speaker for Haymarket and PRIME PPH education and for Laborie, participation in the Cooper Surgical Obstetrical Safety Council, and serving as an editor for UpToDate. Several coauthors disclosed relationships with multiple companies including Organon and Alydia Health.
FROM OBSTETRICS & GYNECOLOGY
Despite effective therapies, fibroid care still lacking
In 2022, two colleagues from Johns Hopkins University, Baltimore, Bhuchitra Singh, MD, MPH, MS, MBA, and James Segars Jr., MD, reviewed the available literature to evaluate the effectiveness of newer minimally invasive therapies in reducing bleeding and improving the quality of life and control of symptoms linked to uterine fibroids.
Their goal, according to Dr. Segars, a professor of obstetrics and gynecology and director of the division of women’s health research at Johns Hopkins, was to help guide clinicians and patients in making decisions about the use of the newer therapies, including radiofrequency ablation and ultrasound-guided removal of lesions.
But he and Dr. Singh, the director of clinical research at the Howard W. and Georgeanna Seegar Jones Laboratory of Reproductive Sciences and Women’s Health Research, were surprised by their findings. “The outcomes were relatively the same,” Dr. Segars said. “All of the modalities lead to significant reduction in bleeding and other fibroid-related symptoms.”
The data on long-term complications and risk for recurrence are sparse for some of the newer approaches, and not enough high-quality long-term studies have been conducted for the Food and Drug Administration to approve them as fertility-sparing treatments.
But perhaps, the biggest challenge now is to ensure that women can take advantage of these newer therapies, with large gaps in both the diagnosis of fibroids and geographic access to minimally invasive treatments.
A widespread condition widely underdiagnosed
Uterine fibroids occur in most women (the incidence rises with age) and can be found in up to 70% of women by the time they reach menopause. Risk factors include family history, increasing interval since last birth, hypertension, and obesity. Increasing parity and use of oral contraceptives are protective.
But as many as 50% of cases go undiagnosed, and one reason for this is the failure of clinicians to dig deeply enough into women’s menstrual histories to diagnose fibroids.
“The most common cause of anemia is heavy menstrual bleeding,” said Shannon Laughlin-Tommaso, MD, MPH, a professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minn. She frequently sees patients who have already undergone colonoscopy to work-up the source of their anemia before anyone suspects that fibroids are the culprit.
“When women tell us about their periods, what they’ve been told is normal [bleeding] – or what they’ve always had and considered normal – is actually kind of on the heavier spectrum,” she said.
Ideally, treatment for uterine fibroids would fix abnormally prolonged or heavy menstrual bleeding, relieve pain, and ameliorate symptoms associated with an enlarged uterus, such as pelvic pressure, urinary frequency, and constipation. And the fibroids would never recur.
By those measures, hysterectomy fits the bill: Success rates in relieving symptoms are high, and the risk for recurrence is zero. But the procedure carries significant drawbacks: short-term complications of surgery, including infection, bleeding, and injury to the bowels and bladder along with potential long-term risks for cardiovascular disease, cancer, ovarian failure and premature menopause, depression, and decline in cognitive function. Those factors loom even larger for women who still hope to have children.
For that reason, the American College of Obstetricians and Gynecologists recommends myomectomy, or surgical removal of individual fibroids, for women who desire uterine preservation or future pregnancy. And the literature here is solid, according to Dr. Singh, who found that 95% of myomectomy patients achieved control of their bleeding symptoms, whether it was via laparoscopy, hysteroscopy, or laparotomy. Up to 40% of women may develop new fibroids, or leiomyomas, within 3 years, although only 12.2% required a second surgery up to after 5 years.
But myomectomy is invasive, requiring general anesthesia, incisions in the uterus, and stitches to close the organ.
Newer techniques have emerged that can effectively treat symptoms of fibroids without requiring surgery. Uterine artery embolization (UAE), which involves passing a catheter into the femoral artery, or laparoscopic uterine artery occlusion can be used to cut off the blood supply of the fibroid. Other techniques, including focused ultrasound surgery and radiofrequency ablation (RFA), use various forms of energy to heat and ablate fibroids. The latter two can be performed in outpatient settings and often without general anesthesia.
Approved for use in 1994, UAE has the most data available, with reduction in the volume of fibroids and uterine tissue lasting up to 5 years, and rates of reintervention of 19%-38% between 2 and 5 years after the procedure. Dr. Singh’s review found that 79%-98.5% of recipients of the procedure reported declines in bleeding that persisted for several years, which is comparable to myomectomy. Quality of life and pain scores also showed good improvement, with follow-up in the different studies ranging from 12 months to over 5 years, the analysis showed.
UAE does have its drawbacks. In rare cases, embolization can deprive the entire uterus and ovaries of blood, which can cause ovarian dysfunction and potentially result in premature menopause, although this outcome is most common in women who are older than 45 years. The procedure can often also be painful enough that overnight hospitalization is required.
Focused ultrasound surgeries, which include magnetic resonance–guided focused ultrasound surgery (MRgFUS) and high-intensity focused ultrasound (HIFU), were approved by the FDA in 2004. Focused ultrasound waves pass through the abdominal wall and produce significant heating, causing a burn that destroys the targeted tissue without damaging surrounding tissue. As with UAE, improvements in fibroid-associated bleeding and measures of quality of life were similar to those after myomectomy up to 3 years later.
But Dr. Singh noted that both focused ultrasound and RFA can damage the skin or internal organs. “[As] always with the thermal interventions, there is the probability of skin as well as internal organs that might get the thermal energy if it’s not focused correctly on to the fibroid itself,” he said. In addition, MRgFUS is not an option for women who are not good candidates to undergo an MRI, such as those with claustrophobia or pacemakers.
Also, with focus ultrasound and RFA, “we do worry about that fibroid getting blood flow back,” which can lead to recurrence of heavy menstrual bleeding, Dr. Laughlin-Tommaso noted.
Although data on RFA are limited to 12 months of follow-up, most women reported meaningful reductions in bleeding symptoms. Longer follow-up has been reported for bleeding symptoms after MRgFUS, with similar results up to 3 years later.
For Leslie Hansen-Lindner, MD, chief of obstetrics and gynecology at Atrium Health in Charlotte, N.C., choosing the right procedure starts with a patient-centered conversation weighing the pros and cons of the options and the woman’s goals.
“Is their goal to reduce the size and impact of their fibroid, bleed less, and have a better quality of life on their period?” Dr. Hansen-Lindner said. “Or is their goal to have the entire fibroid removed?”
If the former, an RFA is appealing to many women. If the latter, laparoscopic or mini-laparotomy myomectomy might be a better choice. Although fewer than 10% of patients require surgical reintervention at 3 years of follow-up for RFA, myomectomy has more consistent long-term evidence showing that fewer women require re-intervention and preserve their fertility, she added.
Age also plays a role in the decision: The closer a woman is to menopause, the less likely she is to experience a recurrence, so a less-invasive procedure is preferable. But for younger women hoping to become pregnant, the lower risk for recurrence and good prognosis for future fertility might sway the choice toward myomectomy.
The first laparoscopic RFA procedures were approved for uterine fibroids in 2012. Dr. Hansen-Lindner is a proponent of transcervical fibroid ablation (TFA), a newer RFA procedure that the FDA approved in 2018. Performed through the cervix, TFA requires no incisions and can generally be done without general anesthesia. Eligible candidates would be any woman with symptomatic fibroids, such as heavy menstrual bleeding, pain, or bulk symptoms. The contraindications are few.
“It’s going to come down to size and location of fibroids, and whether or not they would be accessible by the TFA,” Dr. Hansen-Lindner said. “I have to make sure that there isn’t a fibroid blocking their cervix and that the fibroids are accessible with this device.”
TFA also is not suitable for removing most submucosal lesions, which typically must be removed by hysteroscopic myomectomy. Dr. Hansen-Lindner said that she often uses TFA in conjunction with hysteroscopic myomectomy for this scenario. Although data on pregnancy after RFA (including TFA), MRgFUS, and HIFU are lacking, Gynesonics, the manufacturer of the Sonata System (the device that delivers radiofrequency energy to shrink the fibroid) has documented 79 pregnancies among the 2,200 women who have undergone TFA in the United States since 2018.
Disparities hampering care
Uterine fibroids are a particular problem for Black women, whose symptoms are more likely to be ignored by clinicians, according to Jodie Katon, PhD, a core investigator at the Veterans Affairs Greater Los Angeles Center for the Study of Healthcare Innovation, Implementation and Policy. Dr. Katon cited studies in which Black women interviewed about their experiences reported a consistent theme: Clinicians dismissed their symptoms, told them these were nothing to worry about, and advised them to lose weight. Those interactions not only delayed diagnosis among Black women but also led many of them to mistrust clinicians and avoid the health care system altogether.
The failure of clinicians to take their complaints seriously is just one of the disparities affecting Black women. In reviewing the literature, Dr. Laughlin-Tommaso, who also serves as the associate dean for Education Diversity, Equity, and Inclusion at the Mayo Clinic, found that African American women experience two to three times the risk for fibroids, compared with White women, as well as earlier onset and more severe disease, as measured by number and size of the lesions.
According to Dr. Katon, the etiology of fibroids is still poorly understood. “What we do know is that Black women are disproportionately exposed to a variety of factors that we have shown through observational studies are associated with increased risk of development of uterine fibroids.”
The list includes factors like stress; interpersonal racism; early age at menarche; various indicators of poor diets, such as vitamin D deficiency; the use of certain beauty products, specifically hair straighteners; as well as exposure to air pollution and other environmental toxins.
Laughlin-Tommaso also pointed to historical disparities in management, citing a doubled risk for hysterectomy for Black women in a study published in 2007 despite survey data suggesting that Black women report being more interested in uterine-preserving therapies rather than a hysterectomy.
Breaking down barriers of access to new treatments
Dr. Laughlin-Tommaso looked at more recent trends in the management of fibroids using data from the multicenter COMPARE-UF study, which enrolled women between 2015 and 2020 undergoing fibroid treatment into a longitudinal registry to track their outcomes. She found that Black women underwent hysterectomies at a lower rate than did White women and were instead more likely to undergo myomectomy or UAE.
Some of the change may reflect lack of approved minimally invasive procedures before 2000. “But now that we have expanded options, I think most women are opting not to have a hysterectomy,” Dr. Laughlin-Tommaso said.
Dr. Katon has research funding from the VA to look more closely at racial disparities in the treatment of fibroids. In a study published in April 2023, she reported some surprising trends.
During the period from 2010 to 2018, she found that Black veterans diagnosed with fibroids were less likely than White veterans were to receive treatment, regardless of their age or the severity of their symptoms. This finding held even among women with anemia, which should have been a clear indication for treatment.
But, as in the COMPARE-UF study, the subset of Black veterans who received an interventional treatment were less likely than their White peers were to undergo hysterectomy in favor of a fertility-sparing treatment as their initial procedure. Dr. Katon called it a “welcome but unexpected finding.”
But another significant barrier remains: The two newest types of procedures, RFA and guided focused ultrasound, are not commonly performed outside of tertiary care facilities. However, studies have found that all these procedures are cost effective (studies for myomectomy, UAE, MRgFUS, and TFA). The implementation of a category 1 billing code for laparoscopic RFA in 2017 has led more insurance companies to cover the service, and a category 1 code will be available for TFA effective January 2024.
Although RFA does require investment in specialized equipment, which limits facilities from offering the procedure, any gynecologist who routinely performs hysteroscopy can easily learn to do TFA. And the VA, which is committed to eliminating disparities in women’s health, established a 2-year advanced fellowship in minimally invasive gynecologic surgery in 2022 to help expand their capacity to offer these procedures.
The VA has been rapidly expanding their gynecology services, and Katon said that she is confident that ultrasound-guided procedures and RFA will become more available within the system. “I would say we’re keeping pace. And in some ways, you know, as a national system we may be positioned to actually outpace the rest of the U.S.”
Dr. Segars reported prior research funding for clinical trials from BioSpecifics Technologies, Bayer, Allergan, AbbVie, and ObsEva and currently receives funding from Myovant Sciences. Dr. Hansen-Lindner reported personal fees from Gynesonics. Dr. Singh, Dr. Laughlin-Tommaso, and Dr. Katon reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
In 2022, two colleagues from Johns Hopkins University, Baltimore, Bhuchitra Singh, MD, MPH, MS, MBA, and James Segars Jr., MD, reviewed the available literature to evaluate the effectiveness of newer minimally invasive therapies in reducing bleeding and improving the quality of life and control of symptoms linked to uterine fibroids.
Their goal, according to Dr. Segars, a professor of obstetrics and gynecology and director of the division of women’s health research at Johns Hopkins, was to help guide clinicians and patients in making decisions about the use of the newer therapies, including radiofrequency ablation and ultrasound-guided removal of lesions.
But he and Dr. Singh, the director of clinical research at the Howard W. and Georgeanna Seegar Jones Laboratory of Reproductive Sciences and Women’s Health Research, were surprised by their findings. “The outcomes were relatively the same,” Dr. Segars said. “All of the modalities lead to significant reduction in bleeding and other fibroid-related symptoms.”
The data on long-term complications and risk for recurrence are sparse for some of the newer approaches, and not enough high-quality long-term studies have been conducted for the Food and Drug Administration to approve them as fertility-sparing treatments.
But perhaps, the biggest challenge now is to ensure that women can take advantage of these newer therapies, with large gaps in both the diagnosis of fibroids and geographic access to minimally invasive treatments.
A widespread condition widely underdiagnosed
Uterine fibroids occur in most women (the incidence rises with age) and can be found in up to 70% of women by the time they reach menopause. Risk factors include family history, increasing interval since last birth, hypertension, and obesity. Increasing parity and use of oral contraceptives are protective.
But as many as 50% of cases go undiagnosed, and one reason for this is the failure of clinicians to dig deeply enough into women’s menstrual histories to diagnose fibroids.
“The most common cause of anemia is heavy menstrual bleeding,” said Shannon Laughlin-Tommaso, MD, MPH, a professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minn. She frequently sees patients who have already undergone colonoscopy to work-up the source of their anemia before anyone suspects that fibroids are the culprit.
“When women tell us about their periods, what they’ve been told is normal [bleeding] – or what they’ve always had and considered normal – is actually kind of on the heavier spectrum,” she said.
Ideally, treatment for uterine fibroids would fix abnormally prolonged or heavy menstrual bleeding, relieve pain, and ameliorate symptoms associated with an enlarged uterus, such as pelvic pressure, urinary frequency, and constipation. And the fibroids would never recur.
By those measures, hysterectomy fits the bill: Success rates in relieving symptoms are high, and the risk for recurrence is zero. But the procedure carries significant drawbacks: short-term complications of surgery, including infection, bleeding, and injury to the bowels and bladder along with potential long-term risks for cardiovascular disease, cancer, ovarian failure and premature menopause, depression, and decline in cognitive function. Those factors loom even larger for women who still hope to have children.
For that reason, the American College of Obstetricians and Gynecologists recommends myomectomy, or surgical removal of individual fibroids, for women who desire uterine preservation or future pregnancy. And the literature here is solid, according to Dr. Singh, who found that 95% of myomectomy patients achieved control of their bleeding symptoms, whether it was via laparoscopy, hysteroscopy, or laparotomy. Up to 40% of women may develop new fibroids, or leiomyomas, within 3 years, although only 12.2% required a second surgery up to after 5 years.
But myomectomy is invasive, requiring general anesthesia, incisions in the uterus, and stitches to close the organ.
Newer techniques have emerged that can effectively treat symptoms of fibroids without requiring surgery. Uterine artery embolization (UAE), which involves passing a catheter into the femoral artery, or laparoscopic uterine artery occlusion can be used to cut off the blood supply of the fibroid. Other techniques, including focused ultrasound surgery and radiofrequency ablation (RFA), use various forms of energy to heat and ablate fibroids. The latter two can be performed in outpatient settings and often without general anesthesia.
Approved for use in 1994, UAE has the most data available, with reduction in the volume of fibroids and uterine tissue lasting up to 5 years, and rates of reintervention of 19%-38% between 2 and 5 years after the procedure. Dr. Singh’s review found that 79%-98.5% of recipients of the procedure reported declines in bleeding that persisted for several years, which is comparable to myomectomy. Quality of life and pain scores also showed good improvement, with follow-up in the different studies ranging from 12 months to over 5 years, the analysis showed.
UAE does have its drawbacks. In rare cases, embolization can deprive the entire uterus and ovaries of blood, which can cause ovarian dysfunction and potentially result in premature menopause, although this outcome is most common in women who are older than 45 years. The procedure can often also be painful enough that overnight hospitalization is required.
Focused ultrasound surgeries, which include magnetic resonance–guided focused ultrasound surgery (MRgFUS) and high-intensity focused ultrasound (HIFU), were approved by the FDA in 2004. Focused ultrasound waves pass through the abdominal wall and produce significant heating, causing a burn that destroys the targeted tissue without damaging surrounding tissue. As with UAE, improvements in fibroid-associated bleeding and measures of quality of life were similar to those after myomectomy up to 3 years later.
But Dr. Singh noted that both focused ultrasound and RFA can damage the skin or internal organs. “[As] always with the thermal interventions, there is the probability of skin as well as internal organs that might get the thermal energy if it’s not focused correctly on to the fibroid itself,” he said. In addition, MRgFUS is not an option for women who are not good candidates to undergo an MRI, such as those with claustrophobia or pacemakers.
Also, with focus ultrasound and RFA, “we do worry about that fibroid getting blood flow back,” which can lead to recurrence of heavy menstrual bleeding, Dr. Laughlin-Tommaso noted.
Although data on RFA are limited to 12 months of follow-up, most women reported meaningful reductions in bleeding symptoms. Longer follow-up has been reported for bleeding symptoms after MRgFUS, with similar results up to 3 years later.
For Leslie Hansen-Lindner, MD, chief of obstetrics and gynecology at Atrium Health in Charlotte, N.C., choosing the right procedure starts with a patient-centered conversation weighing the pros and cons of the options and the woman’s goals.
“Is their goal to reduce the size and impact of their fibroid, bleed less, and have a better quality of life on their period?” Dr. Hansen-Lindner said. “Or is their goal to have the entire fibroid removed?”
If the former, an RFA is appealing to many women. If the latter, laparoscopic or mini-laparotomy myomectomy might be a better choice. Although fewer than 10% of patients require surgical reintervention at 3 years of follow-up for RFA, myomectomy has more consistent long-term evidence showing that fewer women require re-intervention and preserve their fertility, she added.
Age also plays a role in the decision: The closer a woman is to menopause, the less likely she is to experience a recurrence, so a less-invasive procedure is preferable. But for younger women hoping to become pregnant, the lower risk for recurrence and good prognosis for future fertility might sway the choice toward myomectomy.
The first laparoscopic RFA procedures were approved for uterine fibroids in 2012. Dr. Hansen-Lindner is a proponent of transcervical fibroid ablation (TFA), a newer RFA procedure that the FDA approved in 2018. Performed through the cervix, TFA requires no incisions and can generally be done without general anesthesia. Eligible candidates would be any woman with symptomatic fibroids, such as heavy menstrual bleeding, pain, or bulk symptoms. The contraindications are few.
“It’s going to come down to size and location of fibroids, and whether or not they would be accessible by the TFA,” Dr. Hansen-Lindner said. “I have to make sure that there isn’t a fibroid blocking their cervix and that the fibroids are accessible with this device.”
TFA also is not suitable for removing most submucosal lesions, which typically must be removed by hysteroscopic myomectomy. Dr. Hansen-Lindner said that she often uses TFA in conjunction with hysteroscopic myomectomy for this scenario. Although data on pregnancy after RFA (including TFA), MRgFUS, and HIFU are lacking, Gynesonics, the manufacturer of the Sonata System (the device that delivers radiofrequency energy to shrink the fibroid) has documented 79 pregnancies among the 2,200 women who have undergone TFA in the United States since 2018.
Disparities hampering care
Uterine fibroids are a particular problem for Black women, whose symptoms are more likely to be ignored by clinicians, according to Jodie Katon, PhD, a core investigator at the Veterans Affairs Greater Los Angeles Center for the Study of Healthcare Innovation, Implementation and Policy. Dr. Katon cited studies in which Black women interviewed about their experiences reported a consistent theme: Clinicians dismissed their symptoms, told them these were nothing to worry about, and advised them to lose weight. Those interactions not only delayed diagnosis among Black women but also led many of them to mistrust clinicians and avoid the health care system altogether.
The failure of clinicians to take their complaints seriously is just one of the disparities affecting Black women. In reviewing the literature, Dr. Laughlin-Tommaso, who also serves as the associate dean for Education Diversity, Equity, and Inclusion at the Mayo Clinic, found that African American women experience two to three times the risk for fibroids, compared with White women, as well as earlier onset and more severe disease, as measured by number and size of the lesions.
According to Dr. Katon, the etiology of fibroids is still poorly understood. “What we do know is that Black women are disproportionately exposed to a variety of factors that we have shown through observational studies are associated with increased risk of development of uterine fibroids.”
The list includes factors like stress; interpersonal racism; early age at menarche; various indicators of poor diets, such as vitamin D deficiency; the use of certain beauty products, specifically hair straighteners; as well as exposure to air pollution and other environmental toxins.
Laughlin-Tommaso also pointed to historical disparities in management, citing a doubled risk for hysterectomy for Black women in a study published in 2007 despite survey data suggesting that Black women report being more interested in uterine-preserving therapies rather than a hysterectomy.
Breaking down barriers of access to new treatments
Dr. Laughlin-Tommaso looked at more recent trends in the management of fibroids using data from the multicenter COMPARE-UF study, which enrolled women between 2015 and 2020 undergoing fibroid treatment into a longitudinal registry to track their outcomes. She found that Black women underwent hysterectomies at a lower rate than did White women and were instead more likely to undergo myomectomy or UAE.
Some of the change may reflect lack of approved minimally invasive procedures before 2000. “But now that we have expanded options, I think most women are opting not to have a hysterectomy,” Dr. Laughlin-Tommaso said.
Dr. Katon has research funding from the VA to look more closely at racial disparities in the treatment of fibroids. In a study published in April 2023, she reported some surprising trends.
During the period from 2010 to 2018, she found that Black veterans diagnosed with fibroids were less likely than White veterans were to receive treatment, regardless of their age or the severity of their symptoms. This finding held even among women with anemia, which should have been a clear indication for treatment.
But, as in the COMPARE-UF study, the subset of Black veterans who received an interventional treatment were less likely than their White peers were to undergo hysterectomy in favor of a fertility-sparing treatment as their initial procedure. Dr. Katon called it a “welcome but unexpected finding.”
But another significant barrier remains: The two newest types of procedures, RFA and guided focused ultrasound, are not commonly performed outside of tertiary care facilities. However, studies have found that all these procedures are cost effective (studies for myomectomy, UAE, MRgFUS, and TFA). The implementation of a category 1 billing code for laparoscopic RFA in 2017 has led more insurance companies to cover the service, and a category 1 code will be available for TFA effective January 2024.
Although RFA does require investment in specialized equipment, which limits facilities from offering the procedure, any gynecologist who routinely performs hysteroscopy can easily learn to do TFA. And the VA, which is committed to eliminating disparities in women’s health, established a 2-year advanced fellowship in minimally invasive gynecologic surgery in 2022 to help expand their capacity to offer these procedures.
The VA has been rapidly expanding their gynecology services, and Katon said that she is confident that ultrasound-guided procedures and RFA will become more available within the system. “I would say we’re keeping pace. And in some ways, you know, as a national system we may be positioned to actually outpace the rest of the U.S.”
Dr. Segars reported prior research funding for clinical trials from BioSpecifics Technologies, Bayer, Allergan, AbbVie, and ObsEva and currently receives funding from Myovant Sciences. Dr. Hansen-Lindner reported personal fees from Gynesonics. Dr. Singh, Dr. Laughlin-Tommaso, and Dr. Katon reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
In 2022, two colleagues from Johns Hopkins University, Baltimore, Bhuchitra Singh, MD, MPH, MS, MBA, and James Segars Jr., MD, reviewed the available literature to evaluate the effectiveness of newer minimally invasive therapies in reducing bleeding and improving the quality of life and control of symptoms linked to uterine fibroids.
Their goal, according to Dr. Segars, a professor of obstetrics and gynecology and director of the division of women’s health research at Johns Hopkins, was to help guide clinicians and patients in making decisions about the use of the newer therapies, including radiofrequency ablation and ultrasound-guided removal of lesions.
But he and Dr. Singh, the director of clinical research at the Howard W. and Georgeanna Seegar Jones Laboratory of Reproductive Sciences and Women’s Health Research, were surprised by their findings. “The outcomes were relatively the same,” Dr. Segars said. “All of the modalities lead to significant reduction in bleeding and other fibroid-related symptoms.”
The data on long-term complications and risk for recurrence are sparse for some of the newer approaches, and not enough high-quality long-term studies have been conducted for the Food and Drug Administration to approve them as fertility-sparing treatments.
But perhaps, the biggest challenge now is to ensure that women can take advantage of these newer therapies, with large gaps in both the diagnosis of fibroids and geographic access to minimally invasive treatments.
A widespread condition widely underdiagnosed
Uterine fibroids occur in most women (the incidence rises with age) and can be found in up to 70% of women by the time they reach menopause. Risk factors include family history, increasing interval since last birth, hypertension, and obesity. Increasing parity and use of oral contraceptives are protective.
But as many as 50% of cases go undiagnosed, and one reason for this is the failure of clinicians to dig deeply enough into women’s menstrual histories to diagnose fibroids.
“The most common cause of anemia is heavy menstrual bleeding,” said Shannon Laughlin-Tommaso, MD, MPH, a professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minn. She frequently sees patients who have already undergone colonoscopy to work-up the source of their anemia before anyone suspects that fibroids are the culprit.
“When women tell us about their periods, what they’ve been told is normal [bleeding] – or what they’ve always had and considered normal – is actually kind of on the heavier spectrum,” she said.
Ideally, treatment for uterine fibroids would fix abnormally prolonged or heavy menstrual bleeding, relieve pain, and ameliorate symptoms associated with an enlarged uterus, such as pelvic pressure, urinary frequency, and constipation. And the fibroids would never recur.
By those measures, hysterectomy fits the bill: Success rates in relieving symptoms are high, and the risk for recurrence is zero. But the procedure carries significant drawbacks: short-term complications of surgery, including infection, bleeding, and injury to the bowels and bladder along with potential long-term risks for cardiovascular disease, cancer, ovarian failure and premature menopause, depression, and decline in cognitive function. Those factors loom even larger for women who still hope to have children.
For that reason, the American College of Obstetricians and Gynecologists recommends myomectomy, or surgical removal of individual fibroids, for women who desire uterine preservation or future pregnancy. And the literature here is solid, according to Dr. Singh, who found that 95% of myomectomy patients achieved control of their bleeding symptoms, whether it was via laparoscopy, hysteroscopy, or laparotomy. Up to 40% of women may develop new fibroids, or leiomyomas, within 3 years, although only 12.2% required a second surgery up to after 5 years.
But myomectomy is invasive, requiring general anesthesia, incisions in the uterus, and stitches to close the organ.
Newer techniques have emerged that can effectively treat symptoms of fibroids without requiring surgery. Uterine artery embolization (UAE), which involves passing a catheter into the femoral artery, or laparoscopic uterine artery occlusion can be used to cut off the blood supply of the fibroid. Other techniques, including focused ultrasound surgery and radiofrequency ablation (RFA), use various forms of energy to heat and ablate fibroids. The latter two can be performed in outpatient settings and often without general anesthesia.
Approved for use in 1994, UAE has the most data available, with reduction in the volume of fibroids and uterine tissue lasting up to 5 years, and rates of reintervention of 19%-38% between 2 and 5 years after the procedure. Dr. Singh’s review found that 79%-98.5% of recipients of the procedure reported declines in bleeding that persisted for several years, which is comparable to myomectomy. Quality of life and pain scores also showed good improvement, with follow-up in the different studies ranging from 12 months to over 5 years, the analysis showed.
UAE does have its drawbacks. In rare cases, embolization can deprive the entire uterus and ovaries of blood, which can cause ovarian dysfunction and potentially result in premature menopause, although this outcome is most common in women who are older than 45 years. The procedure can often also be painful enough that overnight hospitalization is required.
Focused ultrasound surgeries, which include magnetic resonance–guided focused ultrasound surgery (MRgFUS) and high-intensity focused ultrasound (HIFU), were approved by the FDA in 2004. Focused ultrasound waves pass through the abdominal wall and produce significant heating, causing a burn that destroys the targeted tissue without damaging surrounding tissue. As with UAE, improvements in fibroid-associated bleeding and measures of quality of life were similar to those after myomectomy up to 3 years later.
But Dr. Singh noted that both focused ultrasound and RFA can damage the skin or internal organs. “[As] always with the thermal interventions, there is the probability of skin as well as internal organs that might get the thermal energy if it’s not focused correctly on to the fibroid itself,” he said. In addition, MRgFUS is not an option for women who are not good candidates to undergo an MRI, such as those with claustrophobia or pacemakers.
Also, with focus ultrasound and RFA, “we do worry about that fibroid getting blood flow back,” which can lead to recurrence of heavy menstrual bleeding, Dr. Laughlin-Tommaso noted.
Although data on RFA are limited to 12 months of follow-up, most women reported meaningful reductions in bleeding symptoms. Longer follow-up has been reported for bleeding symptoms after MRgFUS, with similar results up to 3 years later.
For Leslie Hansen-Lindner, MD, chief of obstetrics and gynecology at Atrium Health in Charlotte, N.C., choosing the right procedure starts with a patient-centered conversation weighing the pros and cons of the options and the woman’s goals.
“Is their goal to reduce the size and impact of their fibroid, bleed less, and have a better quality of life on their period?” Dr. Hansen-Lindner said. “Or is their goal to have the entire fibroid removed?”
If the former, an RFA is appealing to many women. If the latter, laparoscopic or mini-laparotomy myomectomy might be a better choice. Although fewer than 10% of patients require surgical reintervention at 3 years of follow-up for RFA, myomectomy has more consistent long-term evidence showing that fewer women require re-intervention and preserve their fertility, she added.
Age also plays a role in the decision: The closer a woman is to menopause, the less likely she is to experience a recurrence, so a less-invasive procedure is preferable. But for younger women hoping to become pregnant, the lower risk for recurrence and good prognosis for future fertility might sway the choice toward myomectomy.
The first laparoscopic RFA procedures were approved for uterine fibroids in 2012. Dr. Hansen-Lindner is a proponent of transcervical fibroid ablation (TFA), a newer RFA procedure that the FDA approved in 2018. Performed through the cervix, TFA requires no incisions and can generally be done without general anesthesia. Eligible candidates would be any woman with symptomatic fibroids, such as heavy menstrual bleeding, pain, or bulk symptoms. The contraindications are few.
“It’s going to come down to size and location of fibroids, and whether or not they would be accessible by the TFA,” Dr. Hansen-Lindner said. “I have to make sure that there isn’t a fibroid blocking their cervix and that the fibroids are accessible with this device.”
TFA also is not suitable for removing most submucosal lesions, which typically must be removed by hysteroscopic myomectomy. Dr. Hansen-Lindner said that she often uses TFA in conjunction with hysteroscopic myomectomy for this scenario. Although data on pregnancy after RFA (including TFA), MRgFUS, and HIFU are lacking, Gynesonics, the manufacturer of the Sonata System (the device that delivers radiofrequency energy to shrink the fibroid) has documented 79 pregnancies among the 2,200 women who have undergone TFA in the United States since 2018.
Disparities hampering care
Uterine fibroids are a particular problem for Black women, whose symptoms are more likely to be ignored by clinicians, according to Jodie Katon, PhD, a core investigator at the Veterans Affairs Greater Los Angeles Center for the Study of Healthcare Innovation, Implementation and Policy. Dr. Katon cited studies in which Black women interviewed about their experiences reported a consistent theme: Clinicians dismissed their symptoms, told them these were nothing to worry about, and advised them to lose weight. Those interactions not only delayed diagnosis among Black women but also led many of them to mistrust clinicians and avoid the health care system altogether.
The failure of clinicians to take their complaints seriously is just one of the disparities affecting Black women. In reviewing the literature, Dr. Laughlin-Tommaso, who also serves as the associate dean for Education Diversity, Equity, and Inclusion at the Mayo Clinic, found that African American women experience two to three times the risk for fibroids, compared with White women, as well as earlier onset and more severe disease, as measured by number and size of the lesions.
According to Dr. Katon, the etiology of fibroids is still poorly understood. “What we do know is that Black women are disproportionately exposed to a variety of factors that we have shown through observational studies are associated with increased risk of development of uterine fibroids.”
The list includes factors like stress; interpersonal racism; early age at menarche; various indicators of poor diets, such as vitamin D deficiency; the use of certain beauty products, specifically hair straighteners; as well as exposure to air pollution and other environmental toxins.
Laughlin-Tommaso also pointed to historical disparities in management, citing a doubled risk for hysterectomy for Black women in a study published in 2007 despite survey data suggesting that Black women report being more interested in uterine-preserving therapies rather than a hysterectomy.
Breaking down barriers of access to new treatments
Dr. Laughlin-Tommaso looked at more recent trends in the management of fibroids using data from the multicenter COMPARE-UF study, which enrolled women between 2015 and 2020 undergoing fibroid treatment into a longitudinal registry to track their outcomes. She found that Black women underwent hysterectomies at a lower rate than did White women and were instead more likely to undergo myomectomy or UAE.
Some of the change may reflect lack of approved minimally invasive procedures before 2000. “But now that we have expanded options, I think most women are opting not to have a hysterectomy,” Dr. Laughlin-Tommaso said.
Dr. Katon has research funding from the VA to look more closely at racial disparities in the treatment of fibroids. In a study published in April 2023, she reported some surprising trends.
During the period from 2010 to 2018, she found that Black veterans diagnosed with fibroids were less likely than White veterans were to receive treatment, regardless of their age or the severity of their symptoms. This finding held even among women with anemia, which should have been a clear indication for treatment.
But, as in the COMPARE-UF study, the subset of Black veterans who received an interventional treatment were less likely than their White peers were to undergo hysterectomy in favor of a fertility-sparing treatment as their initial procedure. Dr. Katon called it a “welcome but unexpected finding.”
But another significant barrier remains: The two newest types of procedures, RFA and guided focused ultrasound, are not commonly performed outside of tertiary care facilities. However, studies have found that all these procedures are cost effective (studies for myomectomy, UAE, MRgFUS, and TFA). The implementation of a category 1 billing code for laparoscopic RFA in 2017 has led more insurance companies to cover the service, and a category 1 code will be available for TFA effective January 2024.
Although RFA does require investment in specialized equipment, which limits facilities from offering the procedure, any gynecologist who routinely performs hysteroscopy can easily learn to do TFA. And the VA, which is committed to eliminating disparities in women’s health, established a 2-year advanced fellowship in minimally invasive gynecologic surgery in 2022 to help expand their capacity to offer these procedures.
The VA has been rapidly expanding their gynecology services, and Katon said that she is confident that ultrasound-guided procedures and RFA will become more available within the system. “I would say we’re keeping pace. And in some ways, you know, as a national system we may be positioned to actually outpace the rest of the U.S.”
Dr. Segars reported prior research funding for clinical trials from BioSpecifics Technologies, Bayer, Allergan, AbbVie, and ObsEva and currently receives funding from Myovant Sciences. Dr. Hansen-Lindner reported personal fees from Gynesonics. Dr. Singh, Dr. Laughlin-Tommaso, and Dr. Katon reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Low-dose aspirin provokes no flares in patients with IBD during pregnancy
, shows new research presented in October at the American College of Gastroenterology (ACG) Annual Scientific Meeting.
Low-dose aspirin is recommended for pregnant women who are at risk of hypertensive disorders, such as eclampsia, preeclampsia, and gestational diabetes, said Uma Mahadevan, MD, AGAF, a gastroenterologist and director of the University of California, San Francisco Colitis and Crohn’s Disease Center, who presented the research at the meeting. Regular nonsteroidal anti-inflammatory drug use has been associated with increased disease activity in patients with inflammatory bowel disease (IBD), but the impact of low-dose aspirin on IBD during pregnancy has not been well studied, she said.
The study, which was conducted between January 2013 and December 2022 at a single clinic, included 325 women (mean age 34 years) with IBD who had at least one pregnancy. Of these, 53% had ulcerative colitis and 47% had Crohn’s disease. The primary outcome was IBD flare during pregnancy or within 6 months postpartum. Flares were defined as an IBD-related hospitalization and/or surgery, new initiation of IBD therapy, elevated level of fecal calprotectin greater than 150 micrograms per milligram, or new active endoscopic disease.
A total of 95 patients (29%) used low-dose aspirin during pregnancy; 59 took 81 mg and 36 took 162 mg. The cumulative flare rate was similar between patients who took low-dose aspirin and those who did not (24% vs. 26%, P = .83). However, patients who took low-dose aspirin were significantly more likely than were those who did not to experience preterm birth, younger gestational age at delivery, and cesarean delivery (22.1% vs. 6.1%, 38 weeks vs. 39 weeks, 51% vs. 27%, respectively, P < .01 for all).
Overall rates of hypertensive disorders of pregnancy were similar between the low-dose aspirin and non–low-dose aspirin groups (22% vs. 19%, respectively, P = .59), but individuals on low-dose aspirin were more likely to experience preeclampsia than were those not on low-dose aspirin (11.6% vs 4.3%, P = .03).
The study findings support the benefits of aspirin for pregnant women at increased risk for these conditions. “Pregnant patients with IBD should be offered low-dose aspirin without concern for increased risk of flares,” Dr. Mahadevan said.
“This is a very practical study with high relevance in our everyday management of IBD patients,” Shannon Chang, MD, a specialist in IBD with NYU Langone Health, said in an interview. “Having this study helps us understand the risk of increased IBD activity in the setting of aspirin use during pregnancy.”
Dr. Chang was not surprised by the findings. “Since the [ACOG] guidelines changed several years ago, there have been more and more patients with IBD who have taken aspirin during their pregnancies and the results of this study seem to match what we see in clinical practice,” she said. “This study will help us counsel our patients on the safety of aspirin use during pregnancy, and the findings will also be useful for discussions with our obstetrics colleagues who may seek guidance on the safety of aspirin [use] in our pregnant IBD patients.”
The study received no outside funding. Dr. Mahadevan disclosed relationships with AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Eli Lilly, Gilead, Janssen, Pfizer, Prometheus Biosciences, Protagonist Therapeutics, Rani Therapeutics, Roivant, and Takeda. Dr. Chang disclosed serving as a consultant for Pfizer, AbbVie, and BMS.
, shows new research presented in October at the American College of Gastroenterology (ACG) Annual Scientific Meeting.
Low-dose aspirin is recommended for pregnant women who are at risk of hypertensive disorders, such as eclampsia, preeclampsia, and gestational diabetes, said Uma Mahadevan, MD, AGAF, a gastroenterologist and director of the University of California, San Francisco Colitis and Crohn’s Disease Center, who presented the research at the meeting. Regular nonsteroidal anti-inflammatory drug use has been associated with increased disease activity in patients with inflammatory bowel disease (IBD), but the impact of low-dose aspirin on IBD during pregnancy has not been well studied, she said.
The study, which was conducted between January 2013 and December 2022 at a single clinic, included 325 women (mean age 34 years) with IBD who had at least one pregnancy. Of these, 53% had ulcerative colitis and 47% had Crohn’s disease. The primary outcome was IBD flare during pregnancy or within 6 months postpartum. Flares were defined as an IBD-related hospitalization and/or surgery, new initiation of IBD therapy, elevated level of fecal calprotectin greater than 150 micrograms per milligram, or new active endoscopic disease.
A total of 95 patients (29%) used low-dose aspirin during pregnancy; 59 took 81 mg and 36 took 162 mg. The cumulative flare rate was similar between patients who took low-dose aspirin and those who did not (24% vs. 26%, P = .83). However, patients who took low-dose aspirin were significantly more likely than were those who did not to experience preterm birth, younger gestational age at delivery, and cesarean delivery (22.1% vs. 6.1%, 38 weeks vs. 39 weeks, 51% vs. 27%, respectively, P < .01 for all).
Overall rates of hypertensive disorders of pregnancy were similar between the low-dose aspirin and non–low-dose aspirin groups (22% vs. 19%, respectively, P = .59), but individuals on low-dose aspirin were more likely to experience preeclampsia than were those not on low-dose aspirin (11.6% vs 4.3%, P = .03).
The study findings support the benefits of aspirin for pregnant women at increased risk for these conditions. “Pregnant patients with IBD should be offered low-dose aspirin without concern for increased risk of flares,” Dr. Mahadevan said.
“This is a very practical study with high relevance in our everyday management of IBD patients,” Shannon Chang, MD, a specialist in IBD with NYU Langone Health, said in an interview. “Having this study helps us understand the risk of increased IBD activity in the setting of aspirin use during pregnancy.”
Dr. Chang was not surprised by the findings. “Since the [ACOG] guidelines changed several years ago, there have been more and more patients with IBD who have taken aspirin during their pregnancies and the results of this study seem to match what we see in clinical practice,” she said. “This study will help us counsel our patients on the safety of aspirin use during pregnancy, and the findings will also be useful for discussions with our obstetrics colleagues who may seek guidance on the safety of aspirin [use] in our pregnant IBD patients.”
The study received no outside funding. Dr. Mahadevan disclosed relationships with AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Eli Lilly, Gilead, Janssen, Pfizer, Prometheus Biosciences, Protagonist Therapeutics, Rani Therapeutics, Roivant, and Takeda. Dr. Chang disclosed serving as a consultant for Pfizer, AbbVie, and BMS.
, shows new research presented in October at the American College of Gastroenterology (ACG) Annual Scientific Meeting.
Low-dose aspirin is recommended for pregnant women who are at risk of hypertensive disorders, such as eclampsia, preeclampsia, and gestational diabetes, said Uma Mahadevan, MD, AGAF, a gastroenterologist and director of the University of California, San Francisco Colitis and Crohn’s Disease Center, who presented the research at the meeting. Regular nonsteroidal anti-inflammatory drug use has been associated with increased disease activity in patients with inflammatory bowel disease (IBD), but the impact of low-dose aspirin on IBD during pregnancy has not been well studied, she said.
The study, which was conducted between January 2013 and December 2022 at a single clinic, included 325 women (mean age 34 years) with IBD who had at least one pregnancy. Of these, 53% had ulcerative colitis and 47% had Crohn’s disease. The primary outcome was IBD flare during pregnancy or within 6 months postpartum. Flares were defined as an IBD-related hospitalization and/or surgery, new initiation of IBD therapy, elevated level of fecal calprotectin greater than 150 micrograms per milligram, or new active endoscopic disease.
A total of 95 patients (29%) used low-dose aspirin during pregnancy; 59 took 81 mg and 36 took 162 mg. The cumulative flare rate was similar between patients who took low-dose aspirin and those who did not (24% vs. 26%, P = .83). However, patients who took low-dose aspirin were significantly more likely than were those who did not to experience preterm birth, younger gestational age at delivery, and cesarean delivery (22.1% vs. 6.1%, 38 weeks vs. 39 weeks, 51% vs. 27%, respectively, P < .01 for all).
Overall rates of hypertensive disorders of pregnancy were similar between the low-dose aspirin and non–low-dose aspirin groups (22% vs. 19%, respectively, P = .59), but individuals on low-dose aspirin were more likely to experience preeclampsia than were those not on low-dose aspirin (11.6% vs 4.3%, P = .03).
The study findings support the benefits of aspirin for pregnant women at increased risk for these conditions. “Pregnant patients with IBD should be offered low-dose aspirin without concern for increased risk of flares,” Dr. Mahadevan said.
“This is a very practical study with high relevance in our everyday management of IBD patients,” Shannon Chang, MD, a specialist in IBD with NYU Langone Health, said in an interview. “Having this study helps us understand the risk of increased IBD activity in the setting of aspirin use during pregnancy.”
Dr. Chang was not surprised by the findings. “Since the [ACOG] guidelines changed several years ago, there have been more and more patients with IBD who have taken aspirin during their pregnancies and the results of this study seem to match what we see in clinical practice,” she said. “This study will help us counsel our patients on the safety of aspirin use during pregnancy, and the findings will also be useful for discussions with our obstetrics colleagues who may seek guidance on the safety of aspirin [use] in our pregnant IBD patients.”
The study received no outside funding. Dr. Mahadevan disclosed relationships with AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Eli Lilly, Gilead, Janssen, Pfizer, Prometheus Biosciences, Protagonist Therapeutics, Rani Therapeutics, Roivant, and Takeda. Dr. Chang disclosed serving as a consultant for Pfizer, AbbVie, and BMS.
FROM ACG 2023
Physicians: Don’t ignore sexuality in your dying patients
I have a long history of being interested in conversations that others avoid. In medical school, I felt that we didn’t talk enough about death, so I organized a lecture series on end-of-life care for my fellow students. Now, as a sexual medicine specialist, I have other conversations from which many medical providers shy away. So, buckle up!
A key question in palliative care is: How do you want to live the life you have left? And where does the wide range of human pleasures fit in? In her book The Pleasure Zone, sex therapist Stella Resnick describes eight kinds of pleasure:
- pain relief
- play, humor, movement, and sound
- mental
- emotional
- sensual
- spiritual
- primal (just being)
- sexual
At the end of life, both medically and culturally, we pay attention to many of these pleasures. But sexuality is often ignored.
Sexuality – which can be defined as the experience of oneself as a sexual being – may include how sex is experienced in relationships or with oneself, sexual orientation, body image, gender expression and identity, as well as sexual satisfaction and pleasure. People may have different priorities at different times regarding their sexuality, but sexuality is a key aspect of feeling fully alive and human across the lifespan. At the end of life, sexuality, sexual expression, and physical connection may play even more important roles than previously.
‘I just want to be able to have sex with my husband again’
Z was a 75-year-old woman who came to me for help with vaginal stenosis. Her cancer treatments were not going well. I asked her one of my typical questions: “What does sex mean to you?”
Sexual pleasure was “glue” – a critical way for her to connect with her sense of self and with her husband, a man of few words. She described transcendent experiences with partnered sex during her life. Finally, she explained, she was saddened by the idea of not experiencing that again before she died.
As medical providers, we don’t all need to be sex experts, but our patients should be able to have open and shame-free conversations with us about these issues at all stages of life. Up to 86% of palliative care patients want the chance to discuss their sexual concerns with a skilled clinician, and many consider this issue important to their psychological well-being. And yet, 91% reported that sexuality had not been addressed in their care.
In a Canadian study of 10 palliative care patients (and their partners), all but one felt that their medical providers should initiate conversations about sexuality and the effect of illness on sexual experience. They felt that this communication should be an integral component of care. The one person who disagreed said it was appropriate for clinicians to ask patients whether they wanted to talk about sexuality.
Before this study, sexuality had been discussed with only one participant. Here’s the magic part: Several of the patients reported that the study itself was therapeutic. This is my clinical experience as well. More often than not, open and shame-free clinical discussions about sexuality led to patients reflecting: “I’ve never been able to say this to another person, and now I feel so much better.”
One study of palliative care nurses found that while the nurses acknowledged the importance of addressing sexuality, their way of addressing sexuality followed cultural myths and norms or relied on their own experience rather than knowledge-based guidelines. Why? One explanation could be that clinicians raised and educated in North America probably did not get adequate training on this topic. We need to do better.
Second, cultural concepts that equate sexuality with healthy and able bodies who are partnered, young, cisgender, and heterosexual make it hard to conceive of how to relate sexuality to other bodies. We’ve been steeped in the biases of our culture.
Some medical providers avoid the topic because they feel vulnerable, fearful that a conversation about sexuality with a patient will reveal something about themselves. Others may simply deny the possibility that sexual function changes in the face of serious illness or that this could be a priority for their patients. Of course, we have a million other things to talk about – I get it.
Views on sex and sexuality affect how clinicians approach these conversations as well. A study of palliative care professionals described themes among those who did and did not address the topic. The professionals who did not discuss sexuality endorsed a narrow definition of sex based on genital sexual acts between two partners, usually heterosexual. Among these clinicians, when the issue came up, patients had raised the topic. They talked about sex using jokes and euphemisms (“are you still enjoying ‘good moments’ with your partner?”), perhaps to ease their own discomfort.
On the other hand, professionals who more frequently discussed sexuality with their patients endorsed a more holistic concept of sexuality: including genital and nongenital contact as well as nonphysical components like verbal communication and emotions. These clinicians found sexuality applicable to all individuals across the lifespan. They were more likely to initiate discussions about the effect of medications or illness on sexual function and address the need for equipment, such as a larger hospital bed.
I’m hoping that you might one day find yourself in the second group. Our patients at the end of life need our help in accessing the full range of pleasure in their lives. We need better medical education on how to help with sexual concerns when they arise (an article for another day), but we can start right now by simply initiating open, shame-free sexual health conversations. This is often the most important therapeutic intervention.
Dr. Kranz, Clinical Assistant Professor of Obstetrics/Gynecology and Family Medicine, University of Rochester (N.Y.) Medical Center, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
I have a long history of being interested in conversations that others avoid. In medical school, I felt that we didn’t talk enough about death, so I organized a lecture series on end-of-life care for my fellow students. Now, as a sexual medicine specialist, I have other conversations from which many medical providers shy away. So, buckle up!
A key question in palliative care is: How do you want to live the life you have left? And where does the wide range of human pleasures fit in? In her book The Pleasure Zone, sex therapist Stella Resnick describes eight kinds of pleasure:
- pain relief
- play, humor, movement, and sound
- mental
- emotional
- sensual
- spiritual
- primal (just being)
- sexual
At the end of life, both medically and culturally, we pay attention to many of these pleasures. But sexuality is often ignored.
Sexuality – which can be defined as the experience of oneself as a sexual being – may include how sex is experienced in relationships or with oneself, sexual orientation, body image, gender expression and identity, as well as sexual satisfaction and pleasure. People may have different priorities at different times regarding their sexuality, but sexuality is a key aspect of feeling fully alive and human across the lifespan. At the end of life, sexuality, sexual expression, and physical connection may play even more important roles than previously.
‘I just want to be able to have sex with my husband again’
Z was a 75-year-old woman who came to me for help with vaginal stenosis. Her cancer treatments were not going well. I asked her one of my typical questions: “What does sex mean to you?”
Sexual pleasure was “glue” – a critical way for her to connect with her sense of self and with her husband, a man of few words. She described transcendent experiences with partnered sex during her life. Finally, she explained, she was saddened by the idea of not experiencing that again before she died.
As medical providers, we don’t all need to be sex experts, but our patients should be able to have open and shame-free conversations with us about these issues at all stages of life. Up to 86% of palliative care patients want the chance to discuss their sexual concerns with a skilled clinician, and many consider this issue important to their psychological well-being. And yet, 91% reported that sexuality had not been addressed in their care.
In a Canadian study of 10 palliative care patients (and their partners), all but one felt that their medical providers should initiate conversations about sexuality and the effect of illness on sexual experience. They felt that this communication should be an integral component of care. The one person who disagreed said it was appropriate for clinicians to ask patients whether they wanted to talk about sexuality.
Before this study, sexuality had been discussed with only one participant. Here’s the magic part: Several of the patients reported that the study itself was therapeutic. This is my clinical experience as well. More often than not, open and shame-free clinical discussions about sexuality led to patients reflecting: “I’ve never been able to say this to another person, and now I feel so much better.”
One study of palliative care nurses found that while the nurses acknowledged the importance of addressing sexuality, their way of addressing sexuality followed cultural myths and norms or relied on their own experience rather than knowledge-based guidelines. Why? One explanation could be that clinicians raised and educated in North America probably did not get adequate training on this topic. We need to do better.
Second, cultural concepts that equate sexuality with healthy and able bodies who are partnered, young, cisgender, and heterosexual make it hard to conceive of how to relate sexuality to other bodies. We’ve been steeped in the biases of our culture.
Some medical providers avoid the topic because they feel vulnerable, fearful that a conversation about sexuality with a patient will reveal something about themselves. Others may simply deny the possibility that sexual function changes in the face of serious illness or that this could be a priority for their patients. Of course, we have a million other things to talk about – I get it.
Views on sex and sexuality affect how clinicians approach these conversations as well. A study of palliative care professionals described themes among those who did and did not address the topic. The professionals who did not discuss sexuality endorsed a narrow definition of sex based on genital sexual acts between two partners, usually heterosexual. Among these clinicians, when the issue came up, patients had raised the topic. They talked about sex using jokes and euphemisms (“are you still enjoying ‘good moments’ with your partner?”), perhaps to ease their own discomfort.
On the other hand, professionals who more frequently discussed sexuality with their patients endorsed a more holistic concept of sexuality: including genital and nongenital contact as well as nonphysical components like verbal communication and emotions. These clinicians found sexuality applicable to all individuals across the lifespan. They were more likely to initiate discussions about the effect of medications or illness on sexual function and address the need for equipment, such as a larger hospital bed.
I’m hoping that you might one day find yourself in the second group. Our patients at the end of life need our help in accessing the full range of pleasure in their lives. We need better medical education on how to help with sexual concerns when they arise (an article for another day), but we can start right now by simply initiating open, shame-free sexual health conversations. This is often the most important therapeutic intervention.
Dr. Kranz, Clinical Assistant Professor of Obstetrics/Gynecology and Family Medicine, University of Rochester (N.Y.) Medical Center, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
I have a long history of being interested in conversations that others avoid. In medical school, I felt that we didn’t talk enough about death, so I organized a lecture series on end-of-life care for my fellow students. Now, as a sexual medicine specialist, I have other conversations from which many medical providers shy away. So, buckle up!
A key question in palliative care is: How do you want to live the life you have left? And where does the wide range of human pleasures fit in? In her book The Pleasure Zone, sex therapist Stella Resnick describes eight kinds of pleasure:
- pain relief
- play, humor, movement, and sound
- mental
- emotional
- sensual
- spiritual
- primal (just being)
- sexual
At the end of life, both medically and culturally, we pay attention to many of these pleasures. But sexuality is often ignored.
Sexuality – which can be defined as the experience of oneself as a sexual being – may include how sex is experienced in relationships or with oneself, sexual orientation, body image, gender expression and identity, as well as sexual satisfaction and pleasure. People may have different priorities at different times regarding their sexuality, but sexuality is a key aspect of feeling fully alive and human across the lifespan. At the end of life, sexuality, sexual expression, and physical connection may play even more important roles than previously.
‘I just want to be able to have sex with my husband again’
Z was a 75-year-old woman who came to me for help with vaginal stenosis. Her cancer treatments were not going well. I asked her one of my typical questions: “What does sex mean to you?”
Sexual pleasure was “glue” – a critical way for her to connect with her sense of self and with her husband, a man of few words. She described transcendent experiences with partnered sex during her life. Finally, she explained, she was saddened by the idea of not experiencing that again before she died.
As medical providers, we don’t all need to be sex experts, but our patients should be able to have open and shame-free conversations with us about these issues at all stages of life. Up to 86% of palliative care patients want the chance to discuss their sexual concerns with a skilled clinician, and many consider this issue important to their psychological well-being. And yet, 91% reported that sexuality had not been addressed in their care.
In a Canadian study of 10 palliative care patients (and their partners), all but one felt that their medical providers should initiate conversations about sexuality and the effect of illness on sexual experience. They felt that this communication should be an integral component of care. The one person who disagreed said it was appropriate for clinicians to ask patients whether they wanted to talk about sexuality.
Before this study, sexuality had been discussed with only one participant. Here’s the magic part: Several of the patients reported that the study itself was therapeutic. This is my clinical experience as well. More often than not, open and shame-free clinical discussions about sexuality led to patients reflecting: “I’ve never been able to say this to another person, and now I feel so much better.”
One study of palliative care nurses found that while the nurses acknowledged the importance of addressing sexuality, their way of addressing sexuality followed cultural myths and norms or relied on their own experience rather than knowledge-based guidelines. Why? One explanation could be that clinicians raised and educated in North America probably did not get adequate training on this topic. We need to do better.
Second, cultural concepts that equate sexuality with healthy and able bodies who are partnered, young, cisgender, and heterosexual make it hard to conceive of how to relate sexuality to other bodies. We’ve been steeped in the biases of our culture.
Some medical providers avoid the topic because they feel vulnerable, fearful that a conversation about sexuality with a patient will reveal something about themselves. Others may simply deny the possibility that sexual function changes in the face of serious illness or that this could be a priority for their patients. Of course, we have a million other things to talk about – I get it.
Views on sex and sexuality affect how clinicians approach these conversations as well. A study of palliative care professionals described themes among those who did and did not address the topic. The professionals who did not discuss sexuality endorsed a narrow definition of sex based on genital sexual acts between two partners, usually heterosexual. Among these clinicians, when the issue came up, patients had raised the topic. They talked about sex using jokes and euphemisms (“are you still enjoying ‘good moments’ with your partner?”), perhaps to ease their own discomfort.
On the other hand, professionals who more frequently discussed sexuality with their patients endorsed a more holistic concept of sexuality: including genital and nongenital contact as well as nonphysical components like verbal communication and emotions. These clinicians found sexuality applicable to all individuals across the lifespan. They were more likely to initiate discussions about the effect of medications or illness on sexual function and address the need for equipment, such as a larger hospital bed.
I’m hoping that you might one day find yourself in the second group. Our patients at the end of life need our help in accessing the full range of pleasure in their lives. We need better medical education on how to help with sexual concerns when they arise (an article for another day), but we can start right now by simply initiating open, shame-free sexual health conversations. This is often the most important therapeutic intervention.
Dr. Kranz, Clinical Assistant Professor of Obstetrics/Gynecology and Family Medicine, University of Rochester (N.Y.) Medical Center, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
2023 USPSTF mammography age to start screening in average-risk patients: What’s new is old again
The US Preventive Services Task Force (USPSTF)1 is comprised of an independent panel of preventive services clinician experts who make evidence-based recommendations, with the letter grade assigned based on the strength of the evidence, from A through D (TABLE 1), on preventive services such as health screenings, shared decision making patient counseling, and preventive medications. Both A and B recommendations are generally accepted by both government and most private health insurance companies as a covered preventive benefit with no or minimal co-pays.

In 2002, the USPSTF released a Grade B recommendation that screening mammography for average-risk patients (with patients referring to persons assigned female at birth who have not undergone bilateral mastectomy) should take place starting at age 40 and be repeated every 1 to 2 years.2 This was consistent with or endorsed by most other national breast cancer screening guidelines, including the American College of Obstetricians and Gynecologists (ACOG), National Comprehensive Cancer Network (NCCN), the American Cancer Society (ACS), and the American College of Radiology.
In 2009, the USPSTF changed this Grade B recommendation, instead recommending biennial screening mammography for women aged 50 to 74.3 The most significant change in the revised guideline was for patients aged 40 to 49, where the recommendation was “against routine screening mammography.” They went on to say that the decision to start “biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms.” Other prominent national guideline groups (ACOG, NCCN, ACS) did not agree with this recommendation and maintained that patients aged 40 to 49 should continue to be offered routine screening mammography either annually (NCCN, ACS) or at 1-to-2-year intervals (ACOG).4-6 The American College of Physicians and the American Academy of Family Practice endorsed the 2016 USPSTF guidelines, creating a disparity in breast cancer mammography counseling for averagerisk patients in their 40s.7
In 2016, the USPSTF revisited their breast cancer screening recommendation and renewed their 2009 recommendation against routine screening in patients aged 40 to 49, with the American College of Physicians and the American Academy of Family Practice again endorsing these guidelines.8 ACOG, ACS, NCCN, and ACR continued to recommend age 40 as a starting age for routine mammography screening (TABLE 2). As a result, over the past 14 years, patients aged 40 to 49 were placed in an awkward position of potentially hearing different recommendations from their health care providers, those differences often depending on the specialty of the provider they were seeing.
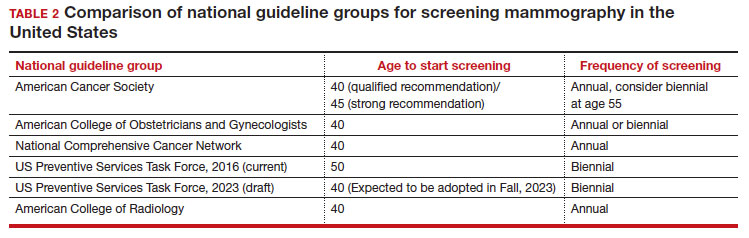
In 2023. On May 9, the USPSTF released a draft of their latest recommendation statement stating that all patients at average risk for breast cancer should get screened every other year beginning at age 40, bringing most of the national guideline groups into alignment with regard to age to start mammographic screening.9
- With an estimated more than 300,000 new cases in 2023, breast cancer has the highest incidence rate of any cancer in the United States
- The median age of patients with breast cancer in the United States is 58.0 years
- 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49
- Despite lower incidence rates among Black vs White patients, Black patients have higher death rates from breast cancer
Why the change?
To answer this question, we need to examine the relevant epidemiology of breast cancer.
Continue to: Incidence...
Incidence
It is estimated that, in the United States in 2023, there will be 300,590 new cases of breast cancer, resulting in 43,700 deaths.10 From 2015–2019, there were 128.1 new breast cancer cases/100,000 population, which is the highest rate of cancer in the United States, regardless of sex.11 Diagnoses among patients aged 40 to 49 are rising at a faster rate than previously, about 2% per year between 2015 and 2019.
Racial and ethnic differences
In addition to the racial and ethnic epidemiologic differences in breast cancer, there are also disparities in breast cancer care and outcomes that need to be considered when making national guidelines/policy recommendations.
Black women have high mortality rates from breast cancer. While non-Hispanic White patients have the highest rates of breast cancer (TABLE 3), non-Hispanic Black patients have the highest rates of death due to breast cancer.10 There appear to be several reasons for the estimated 40%-higher rate of mortality among Black women, including:
- systemic racism in primary research, guidelines, and policy
- inequities in diagnostic follow-up and access to evidence-based cancer treatments
- biologic differences in breast cancer (ie, the incidence of triple-negative breast cancer (TNBC) is 2-fold higher in Black women compared with the other racial and ethnic groups in the United States).12-14
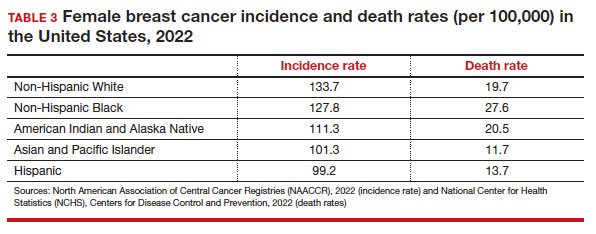
While prior studies have suggested that screening mammography might be less effective for patients with TNBC, a recent study demonstrated that patients who had mammography–screened-detected TNBC tumors were smaller and more likely to be node- negative compared with non-screened patients with TNBC.(14) Patients with screened-detected TNBCs were also more likely to undergo a lumpectomy instead of a mastectomy compared with non–screened detected TNBC (68.3% vs 46.1%; P = .002) (TABLE 4). These data strongly suggest that screening mammography is indeed effective in detecting TNBC at earlier stages, one of the best proxies for breast cancer mortality.
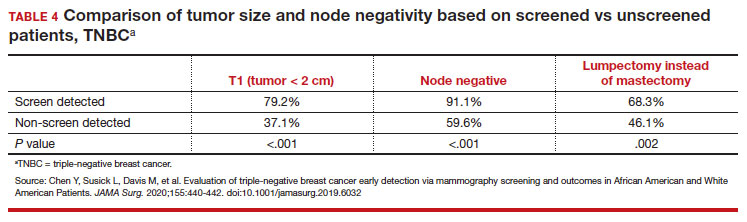
Non-White patients have higher incidence rates of breast cancer in their 40s. A second factor to consider in racial differences is the relatively higher incidence of breast cancer in Hispanic, Black, and Asian patients in their 40s compared with non-Hispanic White patients. In a recent analysis of data from 1973 to 2010 from the Surveillance, Epidemiology, and End Results (SEER) Program, the median age of patients with breast cancer in the United States was 58.0 years (interquartile range [IQR], 50.0–67.0 years).16 Across all US demographic populations by age at diagnosis, more than 20% of patients will have their initial diagnosis of breast cancer under the age of 50, and 1.55% (1 in 65) patients between ages 40 and 49 years will be diagnosed with breast cancer.4 However, among patients aged 50 and younger diagnosed with breast cancer, a significantly higher proportion are Black (31%), Hispanic (34.9%), or Asian (32.8%) versus White (23.1%) (P < .001 for all).16 So, for there to be similar racial and ethnic mammography capture rates with White patients, starting mammography screening ages would need to be lower for Black (age 47 years), Hispanic (and 46 years), and Asian (age 47 years) patients. Data from this study of the SEER database16 also demonstrated that more Black and Hispanic patients at age of diagnosis were diagnosed with advanced (regional or distant) breast cancer (46.6% and 42.9%, respectively) versus White or Asian patients (37.1% and 35.6%, respectively; P < .001 for all).
These findings led the authors of the study to conclude that the “Current [2016] USPSTF breast cancer screening recommendations do not reflect age-specific patterns based on race.” The USPSTF stated that this is one of the reasons why they reconsidered their stance on screening , and now recommend screening for all patients starting at age 40.
My current counseling approach
I encourage all racial and ethnic patients between the ages of 40 and 49 to undergo screening mammography because of the associated relative risk mortality reduction rates, which range from 15% to 50%. I also share that with my patients that, because of the younger average age of onset of breast cancer in Black, Hispanic, and Asian patients, they may derive additional benefit from screening starting at age 40.4
Impact of draft guidelines on breast cancer screening and mortality in younger patients
There is clear, unequivocal, and repeatable Level 1 evidence that screening mammography in the general population of patients aged 40 to 49 reduces breast cancer mortality. Breast cancer is the leading cause of cancer in the United States, the second leading cause of cancer mortality in patients, and 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49. While recent efforts have been made to come to consensus on a screening starting age of 40 for patients at average risk for breast cancer, the USPSTF appeared to be an outlier with their 2016 recommendation to routinely start mammography screening at age 50 instead of 40.17
The USPSTF is a very important national voice in cancer prevention, and their 2023 (draft) revised guidelines to age 40 as the recommended starting screening age now agrees with the leading US guideline groups listed in Table 2. These guideline groups have gone through varying processes, and now have finally arrived at the same conclusion for age to start screening mammography in women of average risk. This agreement should come as a significant comfort to health care providers and patients alike. Changing the starting age to 40 years will result in thousands of lives and hundreds of thousands of life-years saved for patients aged 40 to 49. ●
- US Preventive Services Task Force website. Task Force at a glance. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce.org /uspstf/about-uspstf/task-force-at-a-glance
- Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5_Part_1):347-360.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- American College of Obstetricans and Gynecologists. ACOG Practice Bulletin number 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1e16. doi: 10.1097/AOG. 0000000000002158.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Qaseem A, Lin JS, Mustafa RA, et al. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170: 547-560.
- Siu AL, US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- US Preventive Services Task Force. Draft Recommendation Statement Breast Cancer: Screening. May 9, 2023. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce .org/uspstf/draft-recommendation/breast -cancer-screening-adults#bcei-recommendation -title-area
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: Cancer J Clin. 2023;73:17-48.
- American Cancer Society. Cancer Statistics Center: Breast. 2023. Accessed October 25, 2023. https ://cancerstatisticscenter.cancer.org/#!/cancer-site /Breast
- Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol, Biomarkers Prev. 2021;30:53-60.
- Goel N, Westrick AC, Bailey ZD, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg. 2022;275:776-783.
- Chen Y, Susick L, Davis M, et al. Evaluation of triple-negative breast cancer early detection via mammography screening and outcomes in African American and White American patients. JAMA Surg. 2020;155:440-442.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Chelmow D, Pearlman MD, Young A, et al. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet Gynecol. 2020;135:1457-1478.
The US Preventive Services Task Force (USPSTF)1 is comprised of an independent panel of preventive services clinician experts who make evidence-based recommendations, with the letter grade assigned based on the strength of the evidence, from A through D (TABLE 1), on preventive services such as health screenings, shared decision making patient counseling, and preventive medications. Both A and B recommendations are generally accepted by both government and most private health insurance companies as a covered preventive benefit with no or minimal co-pays.

In 2002, the USPSTF released a Grade B recommendation that screening mammography for average-risk patients (with patients referring to persons assigned female at birth who have not undergone bilateral mastectomy) should take place starting at age 40 and be repeated every 1 to 2 years.2 This was consistent with or endorsed by most other national breast cancer screening guidelines, including the American College of Obstetricians and Gynecologists (ACOG), National Comprehensive Cancer Network (NCCN), the American Cancer Society (ACS), and the American College of Radiology.
In 2009, the USPSTF changed this Grade B recommendation, instead recommending biennial screening mammography for women aged 50 to 74.3 The most significant change in the revised guideline was for patients aged 40 to 49, where the recommendation was “against routine screening mammography.” They went on to say that the decision to start “biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms.” Other prominent national guideline groups (ACOG, NCCN, ACS) did not agree with this recommendation and maintained that patients aged 40 to 49 should continue to be offered routine screening mammography either annually (NCCN, ACS) or at 1-to-2-year intervals (ACOG).4-6 The American College of Physicians and the American Academy of Family Practice endorsed the 2016 USPSTF guidelines, creating a disparity in breast cancer mammography counseling for averagerisk patients in their 40s.7
In 2016, the USPSTF revisited their breast cancer screening recommendation and renewed their 2009 recommendation against routine screening in patients aged 40 to 49, with the American College of Physicians and the American Academy of Family Practice again endorsing these guidelines.8 ACOG, ACS, NCCN, and ACR continued to recommend age 40 as a starting age for routine mammography screening (TABLE 2). As a result, over the past 14 years, patients aged 40 to 49 were placed in an awkward position of potentially hearing different recommendations from their health care providers, those differences often depending on the specialty of the provider they were seeing.

In 2023. On May 9, the USPSTF released a draft of their latest recommendation statement stating that all patients at average risk for breast cancer should get screened every other year beginning at age 40, bringing most of the national guideline groups into alignment with regard to age to start mammographic screening.9
- With an estimated more than 300,000 new cases in 2023, breast cancer has the highest incidence rate of any cancer in the United States
- The median age of patients with breast cancer in the United States is 58.0 years
- 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49
- Despite lower incidence rates among Black vs White patients, Black patients have higher death rates from breast cancer
Why the change?
To answer this question, we need to examine the relevant epidemiology of breast cancer.
Continue to: Incidence...
Incidence
It is estimated that, in the United States in 2023, there will be 300,590 new cases of breast cancer, resulting in 43,700 deaths.10 From 2015–2019, there were 128.1 new breast cancer cases/100,000 population, which is the highest rate of cancer in the United States, regardless of sex.11 Diagnoses among patients aged 40 to 49 are rising at a faster rate than previously, about 2% per year between 2015 and 2019.
Racial and ethnic differences
In addition to the racial and ethnic epidemiologic differences in breast cancer, there are also disparities in breast cancer care and outcomes that need to be considered when making national guidelines/policy recommendations.
Black women have high mortality rates from breast cancer. While non-Hispanic White patients have the highest rates of breast cancer (TABLE 3), non-Hispanic Black patients have the highest rates of death due to breast cancer.10 There appear to be several reasons for the estimated 40%-higher rate of mortality among Black women, including:
- systemic racism in primary research, guidelines, and policy
- inequities in diagnostic follow-up and access to evidence-based cancer treatments
- biologic differences in breast cancer (ie, the incidence of triple-negative breast cancer (TNBC) is 2-fold higher in Black women compared with the other racial and ethnic groups in the United States).12-14

While prior studies have suggested that screening mammography might be less effective for patients with TNBC, a recent study demonstrated that patients who had mammography–screened-detected TNBC tumors were smaller and more likely to be node- negative compared with non-screened patients with TNBC.(14) Patients with screened-detected TNBCs were also more likely to undergo a lumpectomy instead of a mastectomy compared with non–screened detected TNBC (68.3% vs 46.1%; P = .002) (TABLE 4). These data strongly suggest that screening mammography is indeed effective in detecting TNBC at earlier stages, one of the best proxies for breast cancer mortality.

Non-White patients have higher incidence rates of breast cancer in their 40s. A second factor to consider in racial differences is the relatively higher incidence of breast cancer in Hispanic, Black, and Asian patients in their 40s compared with non-Hispanic White patients. In a recent analysis of data from 1973 to 2010 from the Surveillance, Epidemiology, and End Results (SEER) Program, the median age of patients with breast cancer in the United States was 58.0 years (interquartile range [IQR], 50.0–67.0 years).16 Across all US demographic populations by age at diagnosis, more than 20% of patients will have their initial diagnosis of breast cancer under the age of 50, and 1.55% (1 in 65) patients between ages 40 and 49 years will be diagnosed with breast cancer.4 However, among patients aged 50 and younger diagnosed with breast cancer, a significantly higher proportion are Black (31%), Hispanic (34.9%), or Asian (32.8%) versus White (23.1%) (P < .001 for all).16 So, for there to be similar racial and ethnic mammography capture rates with White patients, starting mammography screening ages would need to be lower for Black (age 47 years), Hispanic (and 46 years), and Asian (age 47 years) patients. Data from this study of the SEER database16 also demonstrated that more Black and Hispanic patients at age of diagnosis were diagnosed with advanced (regional or distant) breast cancer (46.6% and 42.9%, respectively) versus White or Asian patients (37.1% and 35.6%, respectively; P < .001 for all).
These findings led the authors of the study to conclude that the “Current [2016] USPSTF breast cancer screening recommendations do not reflect age-specific patterns based on race.” The USPSTF stated that this is one of the reasons why they reconsidered their stance on screening , and now recommend screening for all patients starting at age 40.
My current counseling approach
I encourage all racial and ethnic patients between the ages of 40 and 49 to undergo screening mammography because of the associated relative risk mortality reduction rates, which range from 15% to 50%. I also share that with my patients that, because of the younger average age of onset of breast cancer in Black, Hispanic, and Asian patients, they may derive additional benefit from screening starting at age 40.4
Impact of draft guidelines on breast cancer screening and mortality in younger patients
There is clear, unequivocal, and repeatable Level 1 evidence that screening mammography in the general population of patients aged 40 to 49 reduces breast cancer mortality. Breast cancer is the leading cause of cancer in the United States, the second leading cause of cancer mortality in patients, and 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49. While recent efforts have been made to come to consensus on a screening starting age of 40 for patients at average risk for breast cancer, the USPSTF appeared to be an outlier with their 2016 recommendation to routinely start mammography screening at age 50 instead of 40.17
The USPSTF is a very important national voice in cancer prevention, and their 2023 (draft) revised guidelines to age 40 as the recommended starting screening age now agrees with the leading US guideline groups listed in Table 2. These guideline groups have gone through varying processes, and now have finally arrived at the same conclusion for age to start screening mammography in women of average risk. This agreement should come as a significant comfort to health care providers and patients alike. Changing the starting age to 40 years will result in thousands of lives and hundreds of thousands of life-years saved for patients aged 40 to 49. ●
The US Preventive Services Task Force (USPSTF)1 is comprised of an independent panel of preventive services clinician experts who make evidence-based recommendations, with the letter grade assigned based on the strength of the evidence, from A through D (TABLE 1), on preventive services such as health screenings, shared decision making patient counseling, and preventive medications. Both A and B recommendations are generally accepted by both government and most private health insurance companies as a covered preventive benefit with no or minimal co-pays.

In 2002, the USPSTF released a Grade B recommendation that screening mammography for average-risk patients (with patients referring to persons assigned female at birth who have not undergone bilateral mastectomy) should take place starting at age 40 and be repeated every 1 to 2 years.2 This was consistent with or endorsed by most other national breast cancer screening guidelines, including the American College of Obstetricians and Gynecologists (ACOG), National Comprehensive Cancer Network (NCCN), the American Cancer Society (ACS), and the American College of Radiology.
In 2009, the USPSTF changed this Grade B recommendation, instead recommending biennial screening mammography for women aged 50 to 74.3 The most significant change in the revised guideline was for patients aged 40 to 49, where the recommendation was “against routine screening mammography.” They went on to say that the decision to start “biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms.” Other prominent national guideline groups (ACOG, NCCN, ACS) did not agree with this recommendation and maintained that patients aged 40 to 49 should continue to be offered routine screening mammography either annually (NCCN, ACS) or at 1-to-2-year intervals (ACOG).4-6 The American College of Physicians and the American Academy of Family Practice endorsed the 2016 USPSTF guidelines, creating a disparity in breast cancer mammography counseling for averagerisk patients in their 40s.7
In 2016, the USPSTF revisited their breast cancer screening recommendation and renewed their 2009 recommendation against routine screening in patients aged 40 to 49, with the American College of Physicians and the American Academy of Family Practice again endorsing these guidelines.8 ACOG, ACS, NCCN, and ACR continued to recommend age 40 as a starting age for routine mammography screening (TABLE 2). As a result, over the past 14 years, patients aged 40 to 49 were placed in an awkward position of potentially hearing different recommendations from their health care providers, those differences often depending on the specialty of the provider they were seeing.

In 2023. On May 9, the USPSTF released a draft of their latest recommendation statement stating that all patients at average risk for breast cancer should get screened every other year beginning at age 40, bringing most of the national guideline groups into alignment with regard to age to start mammographic screening.9
- With an estimated more than 300,000 new cases in 2023, breast cancer has the highest incidence rate of any cancer in the United States
- The median age of patients with breast cancer in the United States is 58.0 years
- 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49
- Despite lower incidence rates among Black vs White patients, Black patients have higher death rates from breast cancer
Why the change?
To answer this question, we need to examine the relevant epidemiology of breast cancer.
Continue to: Incidence...
Incidence
It is estimated that, in the United States in 2023, there will be 300,590 new cases of breast cancer, resulting in 43,700 deaths.10 From 2015–2019, there were 128.1 new breast cancer cases/100,000 population, which is the highest rate of cancer in the United States, regardless of sex.11 Diagnoses among patients aged 40 to 49 are rising at a faster rate than previously, about 2% per year between 2015 and 2019.
Racial and ethnic differences
In addition to the racial and ethnic epidemiologic differences in breast cancer, there are also disparities in breast cancer care and outcomes that need to be considered when making national guidelines/policy recommendations.
Black women have high mortality rates from breast cancer. While non-Hispanic White patients have the highest rates of breast cancer (TABLE 3), non-Hispanic Black patients have the highest rates of death due to breast cancer.10 There appear to be several reasons for the estimated 40%-higher rate of mortality among Black women, including:
- systemic racism in primary research, guidelines, and policy
- inequities in diagnostic follow-up and access to evidence-based cancer treatments
- biologic differences in breast cancer (ie, the incidence of triple-negative breast cancer (TNBC) is 2-fold higher in Black women compared with the other racial and ethnic groups in the United States).12-14

While prior studies have suggested that screening mammography might be less effective for patients with TNBC, a recent study demonstrated that patients who had mammography–screened-detected TNBC tumors were smaller and more likely to be node- negative compared with non-screened patients with TNBC.(14) Patients with screened-detected TNBCs were also more likely to undergo a lumpectomy instead of a mastectomy compared with non–screened detected TNBC (68.3% vs 46.1%; P = .002) (TABLE 4). These data strongly suggest that screening mammography is indeed effective in detecting TNBC at earlier stages, one of the best proxies for breast cancer mortality.

Non-White patients have higher incidence rates of breast cancer in their 40s. A second factor to consider in racial differences is the relatively higher incidence of breast cancer in Hispanic, Black, and Asian patients in their 40s compared with non-Hispanic White patients. In a recent analysis of data from 1973 to 2010 from the Surveillance, Epidemiology, and End Results (SEER) Program, the median age of patients with breast cancer in the United States was 58.0 years (interquartile range [IQR], 50.0–67.0 years).16 Across all US demographic populations by age at diagnosis, more than 20% of patients will have their initial diagnosis of breast cancer under the age of 50, and 1.55% (1 in 65) patients between ages 40 and 49 years will be diagnosed with breast cancer.4 However, among patients aged 50 and younger diagnosed with breast cancer, a significantly higher proportion are Black (31%), Hispanic (34.9%), or Asian (32.8%) versus White (23.1%) (P < .001 for all).16 So, for there to be similar racial and ethnic mammography capture rates with White patients, starting mammography screening ages would need to be lower for Black (age 47 years), Hispanic (and 46 years), and Asian (age 47 years) patients. Data from this study of the SEER database16 also demonstrated that more Black and Hispanic patients at age of diagnosis were diagnosed with advanced (regional or distant) breast cancer (46.6% and 42.9%, respectively) versus White or Asian patients (37.1% and 35.6%, respectively; P < .001 for all).
These findings led the authors of the study to conclude that the “Current [2016] USPSTF breast cancer screening recommendations do not reflect age-specific patterns based on race.” The USPSTF stated that this is one of the reasons why they reconsidered their stance on screening , and now recommend screening for all patients starting at age 40.
My current counseling approach
I encourage all racial and ethnic patients between the ages of 40 and 49 to undergo screening mammography because of the associated relative risk mortality reduction rates, which range from 15% to 50%. I also share that with my patients that, because of the younger average age of onset of breast cancer in Black, Hispanic, and Asian patients, they may derive additional benefit from screening starting at age 40.4
Impact of draft guidelines on breast cancer screening and mortality in younger patients
There is clear, unequivocal, and repeatable Level 1 evidence that screening mammography in the general population of patients aged 40 to 49 reduces breast cancer mortality. Breast cancer is the leading cause of cancer in the United States, the second leading cause of cancer mortality in patients, and 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49. While recent efforts have been made to come to consensus on a screening starting age of 40 for patients at average risk for breast cancer, the USPSTF appeared to be an outlier with their 2016 recommendation to routinely start mammography screening at age 50 instead of 40.17
The USPSTF is a very important national voice in cancer prevention, and their 2023 (draft) revised guidelines to age 40 as the recommended starting screening age now agrees with the leading US guideline groups listed in Table 2. These guideline groups have gone through varying processes, and now have finally arrived at the same conclusion for age to start screening mammography in women of average risk. This agreement should come as a significant comfort to health care providers and patients alike. Changing the starting age to 40 years will result in thousands of lives and hundreds of thousands of life-years saved for patients aged 40 to 49. ●
- US Preventive Services Task Force website. Task Force at a glance. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce.org /uspstf/about-uspstf/task-force-at-a-glance
- Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5_Part_1):347-360.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- American College of Obstetricans and Gynecologists. ACOG Practice Bulletin number 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1e16. doi: 10.1097/AOG. 0000000000002158.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Qaseem A, Lin JS, Mustafa RA, et al. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170: 547-560.
- Siu AL, US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- US Preventive Services Task Force. Draft Recommendation Statement Breast Cancer: Screening. May 9, 2023. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce .org/uspstf/draft-recommendation/breast -cancer-screening-adults#bcei-recommendation -title-area
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: Cancer J Clin. 2023;73:17-48.
- American Cancer Society. Cancer Statistics Center: Breast. 2023. Accessed October 25, 2023. https ://cancerstatisticscenter.cancer.org/#!/cancer-site /Breast
- Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol, Biomarkers Prev. 2021;30:53-60.
- Goel N, Westrick AC, Bailey ZD, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg. 2022;275:776-783.
- Chen Y, Susick L, Davis M, et al. Evaluation of triple-negative breast cancer early detection via mammography screening and outcomes in African American and White American patients. JAMA Surg. 2020;155:440-442.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Chelmow D, Pearlman MD, Young A, et al. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet Gynecol. 2020;135:1457-1478.
- US Preventive Services Task Force website. Task Force at a glance. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce.org /uspstf/about-uspstf/task-force-at-a-glance
- Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5_Part_1):347-360.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- American College of Obstetricans and Gynecologists. ACOG Practice Bulletin number 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1e16. doi: 10.1097/AOG. 0000000000002158.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Qaseem A, Lin JS, Mustafa RA, et al. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170: 547-560.
- Siu AL, US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- US Preventive Services Task Force. Draft Recommendation Statement Breast Cancer: Screening. May 9, 2023. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce .org/uspstf/draft-recommendation/breast -cancer-screening-adults#bcei-recommendation -title-area
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: Cancer J Clin. 2023;73:17-48.
- American Cancer Society. Cancer Statistics Center: Breast. 2023. Accessed October 25, 2023. https ://cancerstatisticscenter.cancer.org/#!/cancer-site /Breast
- Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol, Biomarkers Prev. 2021;30:53-60.
- Goel N, Westrick AC, Bailey ZD, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg. 2022;275:776-783.
- Chen Y, Susick L, Davis M, et al. Evaluation of triple-negative breast cancer early detection via mammography screening and outcomes in African American and White American patients. JAMA Surg. 2020;155:440-442.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Chelmow D, Pearlman MD, Young A, et al. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet Gynecol. 2020;135:1457-1478.
Risks quantified in medically optimized pregnancy with lupus
SAN DIEGO – In pregnant women with systemic lupus erythematosus (SLE), those with ill-timed pregnancies had poorer pregnancy outcomes, including preeclampsia and preterm birth.
Women with autoimmune conditions are at an increased risk for pregnancy complications, including pregnancy loss, preterm delivery, and increased need for cesarean delivery, said Catherine Sims, MD, a rheumatologist at Duke Health in Durham, N.C., who is focused on reproductive rheumatology. “The natural question, both clinically and from a research perspective, is: ‘What can we do in order to mitigate or minimize these complications?’ ” she said during a presentation at the annual meeting of the American College of Rheumatology.
While research suggests that patients who plan their pregnancies during times of well-controlled disease have the “best chances of improved pregnancy outcomes,” Dr. Sims and colleagues wanted to quantify how planning for pregnancy affected reproductive outcomes.
Dr. Sims recruited pregnant women with SLE and assessed if the women were medically optimized for pregnancy, if the pregnancy was intended, or both. Intended pregnancy was assessed by using a validated self-reported survey called the London Measure of Unplanned Pregnancy. Pregnant women were considered “medically optimized” for pregnancy if they were not on teratogenic medication, had continued pregnancy-compatible SLE medications, and had a urine protein-creatinine ratio of less than 1 gram in the 6 months prior to or during the first trimester. Intended pregnancies that were medically optimized were classified as “well timed.”
Of the 115 women enrolled in the study, about half had well-timed pregnancies, 20% were neither intended nor medically optimized, 17% were not intended but medically optimized, and 13% were intended but not medically optimized.
Women with ill-timed pregnancy – either not medically optimized and/or unintended – were generally younger and more likely to be single, on Medicare or Medicaid, and on income of less than $50,000 per year.
Ill-timed pregnancies had higher rates of mycophenolate exposure and higher physician-reported SLE disease activity. While patient-reported SLE activity was higher in patients who were not personally ready for pregnancy, in patients who had an intended pregnancy, there was no difference in self-reported SLE activity between those that were medically optimized and those not medically optimized.
“About a third of our patients are actually underestimating their true disease activity level when they are preparing for pregnancy,” Dr. Sims said. For example, while persistent proteinuria in a patient would drive up physician assessment of disease activity, the patient may not be experiencing any symptoms and is unaware of her condition.
In terms of pregnancy outcomes, women with unintended pregnancies had a 2.5 times higher incidence of preeclampsia, compared with those with intended pregnancies. Patients with unplanned pregnancies were also significantly more likely to experience stillbirth.
Women who were not medically optimized for pregnancy were three times as likely to experience preterm birth and preeclampsia compared with those with optimized pregnancies.
These outcomes drive home the importance of optimizing patients for pregnancy, Dr. Sims said, and effectively communicating this importance to patients, especially when they might not be perceiving their disease activity.
The study’s findings show providers “what we thought we knew, which is that there are some patients that are not as aware of their risk,” commented Lisa R. Sammaritano, MD, Hospital for Special Surgery, New York, who moderated the session where the research was presented. “It brings home the importance of counselling our patients about contraception [as well as] the importance of planning.”
Dr. Sims added that it is “crucial” to make this information easily accessible and digestible to patients. One important resource she mentioned is the HOP-STEP program, which stands for Healthy Outcomes in Pregnancy with SLE Through Education of Providers. The program, directed by researchers at Duke University, is designed to improve pregnancy planning in people with lupus. Direct-to-patient resources are key, she said, as patients can often be nervous to ask about pregnancy planning during appointments.
“They won’t want to bring a pregnancy with me in clinic because they’re afraid I’m just going to say, ‘don’t do it,’ ” Dr. Sims said. “But we are making decisions with the patient. Our patients are not asking for permission, but telling us what they want, and we need to meet them where they are at.”
A version of this article appeared on Medscape.com.
SAN DIEGO – In pregnant women with systemic lupus erythematosus (SLE), those with ill-timed pregnancies had poorer pregnancy outcomes, including preeclampsia and preterm birth.
Women with autoimmune conditions are at an increased risk for pregnancy complications, including pregnancy loss, preterm delivery, and increased need for cesarean delivery, said Catherine Sims, MD, a rheumatologist at Duke Health in Durham, N.C., who is focused on reproductive rheumatology. “The natural question, both clinically and from a research perspective, is: ‘What can we do in order to mitigate or minimize these complications?’ ” she said during a presentation at the annual meeting of the American College of Rheumatology.
While research suggests that patients who plan their pregnancies during times of well-controlled disease have the “best chances of improved pregnancy outcomes,” Dr. Sims and colleagues wanted to quantify how planning for pregnancy affected reproductive outcomes.
Dr. Sims recruited pregnant women with SLE and assessed if the women were medically optimized for pregnancy, if the pregnancy was intended, or both. Intended pregnancy was assessed by using a validated self-reported survey called the London Measure of Unplanned Pregnancy. Pregnant women were considered “medically optimized” for pregnancy if they were not on teratogenic medication, had continued pregnancy-compatible SLE medications, and had a urine protein-creatinine ratio of less than 1 gram in the 6 months prior to or during the first trimester. Intended pregnancies that were medically optimized were classified as “well timed.”
Of the 115 women enrolled in the study, about half had well-timed pregnancies, 20% were neither intended nor medically optimized, 17% were not intended but medically optimized, and 13% were intended but not medically optimized.
Women with ill-timed pregnancy – either not medically optimized and/or unintended – were generally younger and more likely to be single, on Medicare or Medicaid, and on income of less than $50,000 per year.
Ill-timed pregnancies had higher rates of mycophenolate exposure and higher physician-reported SLE disease activity. While patient-reported SLE activity was higher in patients who were not personally ready for pregnancy, in patients who had an intended pregnancy, there was no difference in self-reported SLE activity between those that were medically optimized and those not medically optimized.
“About a third of our patients are actually underestimating their true disease activity level when they are preparing for pregnancy,” Dr. Sims said. For example, while persistent proteinuria in a patient would drive up physician assessment of disease activity, the patient may not be experiencing any symptoms and is unaware of her condition.
In terms of pregnancy outcomes, women with unintended pregnancies had a 2.5 times higher incidence of preeclampsia, compared with those with intended pregnancies. Patients with unplanned pregnancies were also significantly more likely to experience stillbirth.
Women who were not medically optimized for pregnancy were three times as likely to experience preterm birth and preeclampsia compared with those with optimized pregnancies.
These outcomes drive home the importance of optimizing patients for pregnancy, Dr. Sims said, and effectively communicating this importance to patients, especially when they might not be perceiving their disease activity.
The study’s findings show providers “what we thought we knew, which is that there are some patients that are not as aware of their risk,” commented Lisa R. Sammaritano, MD, Hospital for Special Surgery, New York, who moderated the session where the research was presented. “It brings home the importance of counselling our patients about contraception [as well as] the importance of planning.”
Dr. Sims added that it is “crucial” to make this information easily accessible and digestible to patients. One important resource she mentioned is the HOP-STEP program, which stands for Healthy Outcomes in Pregnancy with SLE Through Education of Providers. The program, directed by researchers at Duke University, is designed to improve pregnancy planning in people with lupus. Direct-to-patient resources are key, she said, as patients can often be nervous to ask about pregnancy planning during appointments.
“They won’t want to bring a pregnancy with me in clinic because they’re afraid I’m just going to say, ‘don’t do it,’ ” Dr. Sims said. “But we are making decisions with the patient. Our patients are not asking for permission, but telling us what they want, and we need to meet them where they are at.”
A version of this article appeared on Medscape.com.
SAN DIEGO – In pregnant women with systemic lupus erythematosus (SLE), those with ill-timed pregnancies had poorer pregnancy outcomes, including preeclampsia and preterm birth.
Women with autoimmune conditions are at an increased risk for pregnancy complications, including pregnancy loss, preterm delivery, and increased need for cesarean delivery, said Catherine Sims, MD, a rheumatologist at Duke Health in Durham, N.C., who is focused on reproductive rheumatology. “The natural question, both clinically and from a research perspective, is: ‘What can we do in order to mitigate or minimize these complications?’ ” she said during a presentation at the annual meeting of the American College of Rheumatology.
While research suggests that patients who plan their pregnancies during times of well-controlled disease have the “best chances of improved pregnancy outcomes,” Dr. Sims and colleagues wanted to quantify how planning for pregnancy affected reproductive outcomes.
Dr. Sims recruited pregnant women with SLE and assessed if the women were medically optimized for pregnancy, if the pregnancy was intended, or both. Intended pregnancy was assessed by using a validated self-reported survey called the London Measure of Unplanned Pregnancy. Pregnant women were considered “medically optimized” for pregnancy if they were not on teratogenic medication, had continued pregnancy-compatible SLE medications, and had a urine protein-creatinine ratio of less than 1 gram in the 6 months prior to or during the first trimester. Intended pregnancies that were medically optimized were classified as “well timed.”
Of the 115 women enrolled in the study, about half had well-timed pregnancies, 20% were neither intended nor medically optimized, 17% were not intended but medically optimized, and 13% were intended but not medically optimized.
Women with ill-timed pregnancy – either not medically optimized and/or unintended – were generally younger and more likely to be single, on Medicare or Medicaid, and on income of less than $50,000 per year.
Ill-timed pregnancies had higher rates of mycophenolate exposure and higher physician-reported SLE disease activity. While patient-reported SLE activity was higher in patients who were not personally ready for pregnancy, in patients who had an intended pregnancy, there was no difference in self-reported SLE activity between those that were medically optimized and those not medically optimized.
“About a third of our patients are actually underestimating their true disease activity level when they are preparing for pregnancy,” Dr. Sims said. For example, while persistent proteinuria in a patient would drive up physician assessment of disease activity, the patient may not be experiencing any symptoms and is unaware of her condition.
In terms of pregnancy outcomes, women with unintended pregnancies had a 2.5 times higher incidence of preeclampsia, compared with those with intended pregnancies. Patients with unplanned pregnancies were also significantly more likely to experience stillbirth.
Women who were not medically optimized for pregnancy were three times as likely to experience preterm birth and preeclampsia compared with those with optimized pregnancies.
These outcomes drive home the importance of optimizing patients for pregnancy, Dr. Sims said, and effectively communicating this importance to patients, especially when they might not be perceiving their disease activity.
The study’s findings show providers “what we thought we knew, which is that there are some patients that are not as aware of their risk,” commented Lisa R. Sammaritano, MD, Hospital for Special Surgery, New York, who moderated the session where the research was presented. “It brings home the importance of counselling our patients about contraception [as well as] the importance of planning.”
Dr. Sims added that it is “crucial” to make this information easily accessible and digestible to patients. One important resource she mentioned is the HOP-STEP program, which stands for Healthy Outcomes in Pregnancy with SLE Through Education of Providers. The program, directed by researchers at Duke University, is designed to improve pregnancy planning in people with lupus. Direct-to-patient resources are key, she said, as patients can often be nervous to ask about pregnancy planning during appointments.
“They won’t want to bring a pregnancy with me in clinic because they’re afraid I’m just going to say, ‘don’t do it,’ ” Dr. Sims said. “But we are making decisions with the patient. Our patients are not asking for permission, but telling us what they want, and we need to meet them where they are at.”
A version of this article appeared on Medscape.com.
AT ACR 2023
‘Smart’ stethoscope spots peripartum cardiomyopathy
in a large study of obstetric patients in Nigeria.
Demilade A. Adedinsewo, MD, MPH, from Mayo Clinic, Jacksonville, Fla., reported these findings from the Screening for Pregnancy Related Heart Failure in Nigeria (SPEC-AI Nigeria) trial in a press briefing and in a late-breaking trial session at the annual scientific sessions of the American Heart Association.
“The key takeaway,” Dr. Adedinsewo said in an interview, “is recognizing that a simple, low-impact tool like a digital stethoscope can dramatically improve the diagnosis of a life-threatening condition, and we can treat it. A large proportion of the women will recover; if we identify them early and treat them appropriately, we can reduce the risk of dying.”
If the device predicted low ejection fraction, the patient went on to have an echocardiogram to confirm cardiomyopathy, defined as a left ventricular ejection fraction (LVEF) <50%.
Peripartum cardiomyopathy was detected in 4% of the women who were screened with this tool, compared with 1.8% of those who received usual care, which included a traditional ECG.
“I believe that the control arm also has about 4% of cardiomyopathy cases, but because they didn’t have the same screening and echo, we’re missing them,” Dr. Adedinsewo said.
Diagnosis of peripartum cardiomyopathy is challenging, she noted, owing to overlap of common symptoms in pregnancy, such as lower-extremity swelling, fatigue, and shortness of breath with mild activity, which are also cardinal symptoms of heart failure.
“We were really impressed by the effectiveness of the tool, looking at how accurate it was when it comes to the sensitivity,” she added. She noted that the digital stethoscope correctly identified 92% of women with LVEF < 50% and 100% of those with LVEF < 40%.
This was the first large, clinical trial to evaluate an AI intervention in pregnancy. The investigators used a portable, battery-operated device that yielded AI results in real time.
Nigeria has the highest rate of pericardium cardiomyopathy of any country. However, one study showed a 16-fold higher rate of cardiomyopathy among African American women, compared with White women in the United States, Dr. Adedinsewo noted. “It will be important to identify who we should be screening to identify more cases,” she said.
A digital stethoscope that provides an ECG is currently available, but the algorithm that powers detection of cardiomyopathy is not yet commercially available.
Findings ‘absolutely startling’
The study discussant in the press briefing, Alexander Tarlochan Singh Sandhu, MD, from Stanford (Calif.) University congratulated the authors on this “valuable study that uses AI tools to solve a real health problem.”
Finding that 4% of the women in the intervention arm had reduced ejection fraction is “absolutely startling,” he said, “and speaks to how important improving our diagnosis in this space is.
“Where the burden of disease is high, a tool like this can be so incredibly valuable,” he said. He noted that the investigators identified 2% more patients with peripartum cardiomyopathy.
“This is an example of the potential of AI tools that can actually improve access to care and improve quality of care in resource-limited settings,” he said. “We need to move to understanding how to implement this into subsequent care [and] figure out what the next steps are to improve their outcomes.”
“The main takeaway is that, in areas where there is a very high prevalence of a morbid condition, a prescreening tool like this may be helpful” for diagnosis, the assigned discussant in the session, Marco Perez, MD, also from Stanford University, told this news organization.
The number of women needed to screen to detect peripartum cardiomyopathy by echocardiography alone is 1 in 23 in Nigeria and 1 in 970 in the United States, he said.
With an AI tool such as this one (sensitivity, 92%; specificity, 80%), the number needed to screen would be 1 in 5.7 in Nigeria and 1 in 194 in the United States, he estimates on the basis of incidence data.
“Because it is so common in Nigeria, a screening method makes a lot of sense,” Dr. Perez said. “The big question that remains is, what is the best screening modality?
“Certainly, this tool helped in bringing down the number of echoes needed to find a case, from the mid 20s down to about 5 or 6, so it certainly does seem to be helpful.”
However, the investigators did not say whether this tool is better than a clinical review of ECG or an AI analysis of ECG alone. It’s not clear whether the phonocardiogram component is significant in conjunction with the ECG component.
Nevertheless, “In a place where there’s a very high prevalence of peripartum cardiomyopathy, like Haiti, like Nigeria, doing something like this makes a lot of sense.
“For the U.S. and the rest of the world, where the prevalence is much lower, even with a tool like this you still would need to do a lot of echoes to find one case, and that may end up not being cost-effective. You would need to screen 200 women with echo to find one case.”
AI-guided screening study
Nigeria has the highest reported incidence of peripartum cardiomyopathy mortality (1 in 100 live births) and the highest number of maternal deaths.
In the United States, where rates of peripartum cardiomyopathy are much lower, maternal deaths are nevertheless higher than in other developed countries and have trended up over the past 3 decades; cardiomyopathy is a key contributor.
The investigators enrolled 1,195 women who were pregnant or had given birth in the past 12 months. The patients were from six teaching hospitals in Nigeria (two in the north and four in the south). They were randomly assigned in a 1:1 ratio to the intervention group (587) or the control group (608).
In the intervention group, clinicians used a smart stethoscope to record a phonocardiogram and a single-lead ECG reading in the V2 position and in an angled position on the patient’s chest wall and to record an ECG from the patient’s fingers. The recordings were sent to a Bluetooth-enabled mobile device (tablet or smartphone), which displayed the phonocardiogram and ECG images and that indicated whether the ejection fraction was normal or low. All patients in the intervention group received an echocardiogram.
In the control group, patients received usual care plus a traditional ECG. They were not required to have an echocardiogram because undergoing an echocardiogram is not part of usual care; however, they could receive an echocardiogram if the ECG suggested that they might need further testing.
The mean age of all the patients was 31 years, and all were Black. At study entry, 73% were pregnant, and 26% were post partum. They had similar comorbidities.
The primary outcome, cardiomyopathy (LVEF <50%) was detected in 24 of 587 patients (4.1%) in the intervention group and in 11 of 608 patients (1.8%) in the control group (odds ratio, 2.3; 95% confidence interval, 1.1-4.8; P = .02).
For the detection of LVEF <50%, the sensitivity was 92% and the specificity was 80%. For the detection of LVEF <40% (a secondary outcome), the sensitivity was 100% and the specificity was 79%.
Dr. Adedinsewo is supported by the Mayo Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Program, which is funded by the National Institutes of Health. The trial was funded by Mayo Clinic (Centers for Digital Health and Community Health and Engagement Research) and in part by the Mayo Clinic BIRCWH Program. Portable ECG, phonocardiogram recordings, and AI predictions using the digital stethoscope were extracted by the Eko Health team and were sent to the coordinating center for analysis. Eko Health had no role in study design, data collection, data analysis, or data interpretation.
A version of this article appeared on Medscape.com.
in a large study of obstetric patients in Nigeria.
Demilade A. Adedinsewo, MD, MPH, from Mayo Clinic, Jacksonville, Fla., reported these findings from the Screening for Pregnancy Related Heart Failure in Nigeria (SPEC-AI Nigeria) trial in a press briefing and in a late-breaking trial session at the annual scientific sessions of the American Heart Association.
“The key takeaway,” Dr. Adedinsewo said in an interview, “is recognizing that a simple, low-impact tool like a digital stethoscope can dramatically improve the diagnosis of a life-threatening condition, and we can treat it. A large proportion of the women will recover; if we identify them early and treat them appropriately, we can reduce the risk of dying.”
If the device predicted low ejection fraction, the patient went on to have an echocardiogram to confirm cardiomyopathy, defined as a left ventricular ejection fraction (LVEF) <50%.
Peripartum cardiomyopathy was detected in 4% of the women who were screened with this tool, compared with 1.8% of those who received usual care, which included a traditional ECG.
“I believe that the control arm also has about 4% of cardiomyopathy cases, but because they didn’t have the same screening and echo, we’re missing them,” Dr. Adedinsewo said.
Diagnosis of peripartum cardiomyopathy is challenging, she noted, owing to overlap of common symptoms in pregnancy, such as lower-extremity swelling, fatigue, and shortness of breath with mild activity, which are also cardinal symptoms of heart failure.
“We were really impressed by the effectiveness of the tool, looking at how accurate it was when it comes to the sensitivity,” she added. She noted that the digital stethoscope correctly identified 92% of women with LVEF < 50% and 100% of those with LVEF < 40%.
This was the first large, clinical trial to evaluate an AI intervention in pregnancy. The investigators used a portable, battery-operated device that yielded AI results in real time.
Nigeria has the highest rate of pericardium cardiomyopathy of any country. However, one study showed a 16-fold higher rate of cardiomyopathy among African American women, compared with White women in the United States, Dr. Adedinsewo noted. “It will be important to identify who we should be screening to identify more cases,” she said.
A digital stethoscope that provides an ECG is currently available, but the algorithm that powers detection of cardiomyopathy is not yet commercially available.
Findings ‘absolutely startling’
The study discussant in the press briefing, Alexander Tarlochan Singh Sandhu, MD, from Stanford (Calif.) University congratulated the authors on this “valuable study that uses AI tools to solve a real health problem.”
Finding that 4% of the women in the intervention arm had reduced ejection fraction is “absolutely startling,” he said, “and speaks to how important improving our diagnosis in this space is.
“Where the burden of disease is high, a tool like this can be so incredibly valuable,” he said. He noted that the investigators identified 2% more patients with peripartum cardiomyopathy.
“This is an example of the potential of AI tools that can actually improve access to care and improve quality of care in resource-limited settings,” he said. “We need to move to understanding how to implement this into subsequent care [and] figure out what the next steps are to improve their outcomes.”
“The main takeaway is that, in areas where there is a very high prevalence of a morbid condition, a prescreening tool like this may be helpful” for diagnosis, the assigned discussant in the session, Marco Perez, MD, also from Stanford University, told this news organization.
The number of women needed to screen to detect peripartum cardiomyopathy by echocardiography alone is 1 in 23 in Nigeria and 1 in 970 in the United States, he said.
With an AI tool such as this one (sensitivity, 92%; specificity, 80%), the number needed to screen would be 1 in 5.7 in Nigeria and 1 in 194 in the United States, he estimates on the basis of incidence data.
“Because it is so common in Nigeria, a screening method makes a lot of sense,” Dr. Perez said. “The big question that remains is, what is the best screening modality?
“Certainly, this tool helped in bringing down the number of echoes needed to find a case, from the mid 20s down to about 5 or 6, so it certainly does seem to be helpful.”
However, the investigators did not say whether this tool is better than a clinical review of ECG or an AI analysis of ECG alone. It’s not clear whether the phonocardiogram component is significant in conjunction with the ECG component.
Nevertheless, “In a place where there’s a very high prevalence of peripartum cardiomyopathy, like Haiti, like Nigeria, doing something like this makes a lot of sense.
“For the U.S. and the rest of the world, where the prevalence is much lower, even with a tool like this you still would need to do a lot of echoes to find one case, and that may end up not being cost-effective. You would need to screen 200 women with echo to find one case.”
AI-guided screening study
Nigeria has the highest reported incidence of peripartum cardiomyopathy mortality (1 in 100 live births) and the highest number of maternal deaths.
In the United States, where rates of peripartum cardiomyopathy are much lower, maternal deaths are nevertheless higher than in other developed countries and have trended up over the past 3 decades; cardiomyopathy is a key contributor.
The investigators enrolled 1,195 women who were pregnant or had given birth in the past 12 months. The patients were from six teaching hospitals in Nigeria (two in the north and four in the south). They were randomly assigned in a 1:1 ratio to the intervention group (587) or the control group (608).
In the intervention group, clinicians used a smart stethoscope to record a phonocardiogram and a single-lead ECG reading in the V2 position and in an angled position on the patient’s chest wall and to record an ECG from the patient’s fingers. The recordings were sent to a Bluetooth-enabled mobile device (tablet or smartphone), which displayed the phonocardiogram and ECG images and that indicated whether the ejection fraction was normal or low. All patients in the intervention group received an echocardiogram.
In the control group, patients received usual care plus a traditional ECG. They were not required to have an echocardiogram because undergoing an echocardiogram is not part of usual care; however, they could receive an echocardiogram if the ECG suggested that they might need further testing.
The mean age of all the patients was 31 years, and all were Black. At study entry, 73% were pregnant, and 26% were post partum. They had similar comorbidities.
The primary outcome, cardiomyopathy (LVEF <50%) was detected in 24 of 587 patients (4.1%) in the intervention group and in 11 of 608 patients (1.8%) in the control group (odds ratio, 2.3; 95% confidence interval, 1.1-4.8; P = .02).
For the detection of LVEF <50%, the sensitivity was 92% and the specificity was 80%. For the detection of LVEF <40% (a secondary outcome), the sensitivity was 100% and the specificity was 79%.
Dr. Adedinsewo is supported by the Mayo Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Program, which is funded by the National Institutes of Health. The trial was funded by Mayo Clinic (Centers for Digital Health and Community Health and Engagement Research) and in part by the Mayo Clinic BIRCWH Program. Portable ECG, phonocardiogram recordings, and AI predictions using the digital stethoscope were extracted by the Eko Health team and were sent to the coordinating center for analysis. Eko Health had no role in study design, data collection, data analysis, or data interpretation.
A version of this article appeared on Medscape.com.
in a large study of obstetric patients in Nigeria.
Demilade A. Adedinsewo, MD, MPH, from Mayo Clinic, Jacksonville, Fla., reported these findings from the Screening for Pregnancy Related Heart Failure in Nigeria (SPEC-AI Nigeria) trial in a press briefing and in a late-breaking trial session at the annual scientific sessions of the American Heart Association.
“The key takeaway,” Dr. Adedinsewo said in an interview, “is recognizing that a simple, low-impact tool like a digital stethoscope can dramatically improve the diagnosis of a life-threatening condition, and we can treat it. A large proportion of the women will recover; if we identify them early and treat them appropriately, we can reduce the risk of dying.”
If the device predicted low ejection fraction, the patient went on to have an echocardiogram to confirm cardiomyopathy, defined as a left ventricular ejection fraction (LVEF) <50%.
Peripartum cardiomyopathy was detected in 4% of the women who were screened with this tool, compared with 1.8% of those who received usual care, which included a traditional ECG.
“I believe that the control arm also has about 4% of cardiomyopathy cases, but because they didn’t have the same screening and echo, we’re missing them,” Dr. Adedinsewo said.
Diagnosis of peripartum cardiomyopathy is challenging, she noted, owing to overlap of common symptoms in pregnancy, such as lower-extremity swelling, fatigue, and shortness of breath with mild activity, which are also cardinal symptoms of heart failure.
“We were really impressed by the effectiveness of the tool, looking at how accurate it was when it comes to the sensitivity,” she added. She noted that the digital stethoscope correctly identified 92% of women with LVEF < 50% and 100% of those with LVEF < 40%.
This was the first large, clinical trial to evaluate an AI intervention in pregnancy. The investigators used a portable, battery-operated device that yielded AI results in real time.
Nigeria has the highest rate of pericardium cardiomyopathy of any country. However, one study showed a 16-fold higher rate of cardiomyopathy among African American women, compared with White women in the United States, Dr. Adedinsewo noted. “It will be important to identify who we should be screening to identify more cases,” she said.
A digital stethoscope that provides an ECG is currently available, but the algorithm that powers detection of cardiomyopathy is not yet commercially available.
Findings ‘absolutely startling’
The study discussant in the press briefing, Alexander Tarlochan Singh Sandhu, MD, from Stanford (Calif.) University congratulated the authors on this “valuable study that uses AI tools to solve a real health problem.”
Finding that 4% of the women in the intervention arm had reduced ejection fraction is “absolutely startling,” he said, “and speaks to how important improving our diagnosis in this space is.
“Where the burden of disease is high, a tool like this can be so incredibly valuable,” he said. He noted that the investigators identified 2% more patients with peripartum cardiomyopathy.
“This is an example of the potential of AI tools that can actually improve access to care and improve quality of care in resource-limited settings,” he said. “We need to move to understanding how to implement this into subsequent care [and] figure out what the next steps are to improve their outcomes.”
“The main takeaway is that, in areas where there is a very high prevalence of a morbid condition, a prescreening tool like this may be helpful” for diagnosis, the assigned discussant in the session, Marco Perez, MD, also from Stanford University, told this news organization.
The number of women needed to screen to detect peripartum cardiomyopathy by echocardiography alone is 1 in 23 in Nigeria and 1 in 970 in the United States, he said.
With an AI tool such as this one (sensitivity, 92%; specificity, 80%), the number needed to screen would be 1 in 5.7 in Nigeria and 1 in 194 in the United States, he estimates on the basis of incidence data.
“Because it is so common in Nigeria, a screening method makes a lot of sense,” Dr. Perez said. “The big question that remains is, what is the best screening modality?
“Certainly, this tool helped in bringing down the number of echoes needed to find a case, from the mid 20s down to about 5 or 6, so it certainly does seem to be helpful.”
However, the investigators did not say whether this tool is better than a clinical review of ECG or an AI analysis of ECG alone. It’s not clear whether the phonocardiogram component is significant in conjunction with the ECG component.
Nevertheless, “In a place where there’s a very high prevalence of peripartum cardiomyopathy, like Haiti, like Nigeria, doing something like this makes a lot of sense.
“For the U.S. and the rest of the world, where the prevalence is much lower, even with a tool like this you still would need to do a lot of echoes to find one case, and that may end up not being cost-effective. You would need to screen 200 women with echo to find one case.”
AI-guided screening study
Nigeria has the highest reported incidence of peripartum cardiomyopathy mortality (1 in 100 live births) and the highest number of maternal deaths.
In the United States, where rates of peripartum cardiomyopathy are much lower, maternal deaths are nevertheless higher than in other developed countries and have trended up over the past 3 decades; cardiomyopathy is a key contributor.
The investigators enrolled 1,195 women who were pregnant or had given birth in the past 12 months. The patients were from six teaching hospitals in Nigeria (two in the north and four in the south). They were randomly assigned in a 1:1 ratio to the intervention group (587) or the control group (608).
In the intervention group, clinicians used a smart stethoscope to record a phonocardiogram and a single-lead ECG reading in the V2 position and in an angled position on the patient’s chest wall and to record an ECG from the patient’s fingers. The recordings were sent to a Bluetooth-enabled mobile device (tablet or smartphone), which displayed the phonocardiogram and ECG images and that indicated whether the ejection fraction was normal or low. All patients in the intervention group received an echocardiogram.
In the control group, patients received usual care plus a traditional ECG. They were not required to have an echocardiogram because undergoing an echocardiogram is not part of usual care; however, they could receive an echocardiogram if the ECG suggested that they might need further testing.
The mean age of all the patients was 31 years, and all were Black. At study entry, 73% were pregnant, and 26% were post partum. They had similar comorbidities.
The primary outcome, cardiomyopathy (LVEF <50%) was detected in 24 of 587 patients (4.1%) in the intervention group and in 11 of 608 patients (1.8%) in the control group (odds ratio, 2.3; 95% confidence interval, 1.1-4.8; P = .02).
For the detection of LVEF <50%, the sensitivity was 92% and the specificity was 80%. For the detection of LVEF <40% (a secondary outcome), the sensitivity was 100% and the specificity was 79%.
Dr. Adedinsewo is supported by the Mayo Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Program, which is funded by the National Institutes of Health. The trial was funded by Mayo Clinic (Centers for Digital Health and Community Health and Engagement Research) and in part by the Mayo Clinic BIRCWH Program. Portable ECG, phonocardiogram recordings, and AI predictions using the digital stethoscope were extracted by the Eko Health team and were sent to the coordinating center for analysis. Eko Health had no role in study design, data collection, data analysis, or data interpretation.
A version of this article appeared on Medscape.com.
FROM AHA 2023







