User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Vegan diet may curb hot flashes by altering the gut microbiome
TOPLINE:
METHODOLOGY:
- For this exploratory analysis, postmenopausal women with two or more moderate to severe hot flashes daily were randomly assigned in two successive cohorts to consume a low-fat vegan diet with cooked soybeans or their usual diet.
- Over a 12-week period, frequency and severity of hot flashes were recorded on a mobile application.
- Researchers used deep shotgun metagenomic sequencing to analyze the gut microbiome at baseline and 12 weeks in a subset of 11 women in the dietary intervention group.
TAKEAWAY:
- In the subset receiving microbiome analysis, total hot flashes decreased by 95%, moderate to severe hot flashes decreased by 96%, and severe hot flashes disappeared during the dietary intervention.
- The relative abundance of Porphyromonas and Prevotella corporis decreased in participants on the diet intervention, and this correlated with a reduction in severe daytime hot flashes.
- The relative abundance of Clostridium asparagiforme also decreased in participants on the low-fat vegan diet, and this change correlated with a reduction in total severe and severe nighttime hot flashes.
- However, after correction for multiple comparisons, these associations were no longer significant.
IN PRACTICE:
“The targeted and untargeted gut microbiome analysis was robust and revealed important changes in the gut microbiome composition in response to a low-fat vegan diet and large correlations with symptomatic changes,” the authors write. “Larger randomized clinical trials are needed to further investigate these findings.”
SOURCE:
The study, with first author Hana Kahleova, MD, PhD, with the Physicians Committee for Responsible Medicine, in Washington, DC, was published online November 8 in Complementary Therapies in Medicine.
LIMITATIONS:
The gut microbiome analysis was only performed in a small subset of women on the diet intervention, with no control group. Although strong associations were noted between several gut bacteria and changes in hot flash frequency, and nominally statistically significant relative abundance changes were observed, robust statistical significance cannot be concluded for any of the reported gut microbiome assessments when the modestly large number of total comparisons is taken into account.
DISCLOSURES:
The study was funded by the Physicians Committee for Responsible Medicine. The authors report no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- For this exploratory analysis, postmenopausal women with two or more moderate to severe hot flashes daily were randomly assigned in two successive cohorts to consume a low-fat vegan diet with cooked soybeans or their usual diet.
- Over a 12-week period, frequency and severity of hot flashes were recorded on a mobile application.
- Researchers used deep shotgun metagenomic sequencing to analyze the gut microbiome at baseline and 12 weeks in a subset of 11 women in the dietary intervention group.
TAKEAWAY:
- In the subset receiving microbiome analysis, total hot flashes decreased by 95%, moderate to severe hot flashes decreased by 96%, and severe hot flashes disappeared during the dietary intervention.
- The relative abundance of Porphyromonas and Prevotella corporis decreased in participants on the diet intervention, and this correlated with a reduction in severe daytime hot flashes.
- The relative abundance of Clostridium asparagiforme also decreased in participants on the low-fat vegan diet, and this change correlated with a reduction in total severe and severe nighttime hot flashes.
- However, after correction for multiple comparisons, these associations were no longer significant.
IN PRACTICE:
“The targeted and untargeted gut microbiome analysis was robust and revealed important changes in the gut microbiome composition in response to a low-fat vegan diet and large correlations with symptomatic changes,” the authors write. “Larger randomized clinical trials are needed to further investigate these findings.”
SOURCE:
The study, with first author Hana Kahleova, MD, PhD, with the Physicians Committee for Responsible Medicine, in Washington, DC, was published online November 8 in Complementary Therapies in Medicine.
LIMITATIONS:
The gut microbiome analysis was only performed in a small subset of women on the diet intervention, with no control group. Although strong associations were noted between several gut bacteria and changes in hot flash frequency, and nominally statistically significant relative abundance changes were observed, robust statistical significance cannot be concluded for any of the reported gut microbiome assessments when the modestly large number of total comparisons is taken into account.
DISCLOSURES:
The study was funded by the Physicians Committee for Responsible Medicine. The authors report no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- For this exploratory analysis, postmenopausal women with two or more moderate to severe hot flashes daily were randomly assigned in two successive cohorts to consume a low-fat vegan diet with cooked soybeans or their usual diet.
- Over a 12-week period, frequency and severity of hot flashes were recorded on a mobile application.
- Researchers used deep shotgun metagenomic sequencing to analyze the gut microbiome at baseline and 12 weeks in a subset of 11 women in the dietary intervention group.
TAKEAWAY:
- In the subset receiving microbiome analysis, total hot flashes decreased by 95%, moderate to severe hot flashes decreased by 96%, and severe hot flashes disappeared during the dietary intervention.
- The relative abundance of Porphyromonas and Prevotella corporis decreased in participants on the diet intervention, and this correlated with a reduction in severe daytime hot flashes.
- The relative abundance of Clostridium asparagiforme also decreased in participants on the low-fat vegan diet, and this change correlated with a reduction in total severe and severe nighttime hot flashes.
- However, after correction for multiple comparisons, these associations were no longer significant.
IN PRACTICE:
“The targeted and untargeted gut microbiome analysis was robust and revealed important changes in the gut microbiome composition in response to a low-fat vegan diet and large correlations with symptomatic changes,” the authors write. “Larger randomized clinical trials are needed to further investigate these findings.”
SOURCE:
The study, with first author Hana Kahleova, MD, PhD, with the Physicians Committee for Responsible Medicine, in Washington, DC, was published online November 8 in Complementary Therapies in Medicine.
LIMITATIONS:
The gut microbiome analysis was only performed in a small subset of women on the diet intervention, with no control group. Although strong associations were noted between several gut bacteria and changes in hot flash frequency, and nominally statistically significant relative abundance changes were observed, robust statistical significance cannot be concluded for any of the reported gut microbiome assessments when the modestly large number of total comparisons is taken into account.
DISCLOSURES:
The study was funded by the Physicians Committee for Responsible Medicine. The authors report no conflicts of interest.
A version of this article appeared on Medscape.com.
FDA mandates five changes to iPLEDGE program for isotretinoin
In a letter dated Nov. 30, 2023, the .
The development follows a March 2023 joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee about iPLEDGE REMS requirements, which included feedback from patients and dermatologists and recommendations for changes to the REMS program, aimed at minimizing the burden of the program on patients, pharmacies, and prescribers while continuing to maintain safe use of the highly teratogenic drug for patients.
The five changes include the following:
- Remove the requirement that pregnancy tests must be performed in a specially certified (i.e., Clinical Laboratory Improvement Amendments [CLIA]) laboratory. In the opinion of John S. Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, this change “may make it easier to perform pregnancy tests in a clinic setting without needing to send the patient to a separate lab,” he said in an interview.
- Allow prescribers the option of using home pregnancy testing for their patients during and after isotretinoin treatment. Prescribers who rely on the patient to perform a home pregnancy test need to take steps to minimize patients falsifying the results of these tests. According to Dr. Barbieri, this means that two pregnancy tests prior to starting isotretinoin must be done in a lab or office setting. “However, all the pregnancy tests on therapy can be either in a medical setting or using a home pregnancy test,” he told this news organization. “This option facilitates the use of telemedicine so that patients would not need to come in; they can just share a pregnancy test with their name and date with their dermatologist.”
- Remove the waiting period requirement — also known as the “19-day lockout” — for patients if they do not obtain isotretinoin within the first 7-day prescription window. According to Dr. Barbieri, this change helps to ensure that patients can begin isotretinoin in a timely manner. “Insurance and pharmacy delays that are no fault of the patient can commonly cause missed initial window periods,” he said. “Allowing for immediate repeat of a pregnancy test to start a new window period, rather than requiring the patient to wait 19 more days, can ensure patient safety and pregnancy prevention without negatively impacting access.”
- Revise the pregnancy registry requirement to remove the objective to document the pregnancy and fetal outcomes for each pregnancy.
- Revise the requirement for prescribers to document patient counseling in patients who cannot become pregnant from monthly to only at enrollment. Dr. Barbieri characterized this change as “major” and said that it could eliminate the need for monthly visits for persons of non–childbearing potential. “This could substantially reduce logistical burdens for patients and reduce wait times to see a dermatologist,” he said.
Future changes to iPLEDGE that Dr. Barbieri would like to see include allowing for home pregnancy tests prior to starting therapy — particularly the test after the 30-day window period. “In addition, it would be good to be able to reduce the 30-day waiting period prior to therapy to something shorter,” such as 14 days, which would still “reliably exclude pregnancy, particularly for those on stable long-acting reversible contraception,” he said. There are also opportunities to improve the iPLEDGE website functionality and to ensure that the website is accessible to patients with limited English proficiency, he added.
He also recommended greater transparency by the Isotretinoin Products Manufacturers Group and inclusion of input from diverse stakeholders such as dermatologists, patients, and pharmacists.
Dr. Barbieri reported personal fees from Dexcel Pharma.
In a letter dated Nov. 30, 2023, the .
The development follows a March 2023 joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee about iPLEDGE REMS requirements, which included feedback from patients and dermatologists and recommendations for changes to the REMS program, aimed at minimizing the burden of the program on patients, pharmacies, and prescribers while continuing to maintain safe use of the highly teratogenic drug for patients.
The five changes include the following:
- Remove the requirement that pregnancy tests must be performed in a specially certified (i.e., Clinical Laboratory Improvement Amendments [CLIA]) laboratory. In the opinion of John S. Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, this change “may make it easier to perform pregnancy tests in a clinic setting without needing to send the patient to a separate lab,” he said in an interview.
- Allow prescribers the option of using home pregnancy testing for their patients during and after isotretinoin treatment. Prescribers who rely on the patient to perform a home pregnancy test need to take steps to minimize patients falsifying the results of these tests. According to Dr. Barbieri, this means that two pregnancy tests prior to starting isotretinoin must be done in a lab or office setting. “However, all the pregnancy tests on therapy can be either in a medical setting or using a home pregnancy test,” he told this news organization. “This option facilitates the use of telemedicine so that patients would not need to come in; they can just share a pregnancy test with their name and date with their dermatologist.”
- Remove the waiting period requirement — also known as the “19-day lockout” — for patients if they do not obtain isotretinoin within the first 7-day prescription window. According to Dr. Barbieri, this change helps to ensure that patients can begin isotretinoin in a timely manner. “Insurance and pharmacy delays that are no fault of the patient can commonly cause missed initial window periods,” he said. “Allowing for immediate repeat of a pregnancy test to start a new window period, rather than requiring the patient to wait 19 more days, can ensure patient safety and pregnancy prevention without negatively impacting access.”
- Revise the pregnancy registry requirement to remove the objective to document the pregnancy and fetal outcomes for each pregnancy.
- Revise the requirement for prescribers to document patient counseling in patients who cannot become pregnant from monthly to only at enrollment. Dr. Barbieri characterized this change as “major” and said that it could eliminate the need for monthly visits for persons of non–childbearing potential. “This could substantially reduce logistical burdens for patients and reduce wait times to see a dermatologist,” he said.
Future changes to iPLEDGE that Dr. Barbieri would like to see include allowing for home pregnancy tests prior to starting therapy — particularly the test after the 30-day window period. “In addition, it would be good to be able to reduce the 30-day waiting period prior to therapy to something shorter,” such as 14 days, which would still “reliably exclude pregnancy, particularly for those on stable long-acting reversible contraception,” he said. There are also opportunities to improve the iPLEDGE website functionality and to ensure that the website is accessible to patients with limited English proficiency, he added.
He also recommended greater transparency by the Isotretinoin Products Manufacturers Group and inclusion of input from diverse stakeholders such as dermatologists, patients, and pharmacists.
Dr. Barbieri reported personal fees from Dexcel Pharma.
In a letter dated Nov. 30, 2023, the .
The development follows a March 2023 joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee about iPLEDGE REMS requirements, which included feedback from patients and dermatologists and recommendations for changes to the REMS program, aimed at minimizing the burden of the program on patients, pharmacies, and prescribers while continuing to maintain safe use of the highly teratogenic drug for patients.
The five changes include the following:
- Remove the requirement that pregnancy tests must be performed in a specially certified (i.e., Clinical Laboratory Improvement Amendments [CLIA]) laboratory. In the opinion of John S. Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, this change “may make it easier to perform pregnancy tests in a clinic setting without needing to send the patient to a separate lab,” he said in an interview.
- Allow prescribers the option of using home pregnancy testing for their patients during and after isotretinoin treatment. Prescribers who rely on the patient to perform a home pregnancy test need to take steps to minimize patients falsifying the results of these tests. According to Dr. Barbieri, this means that two pregnancy tests prior to starting isotretinoin must be done in a lab or office setting. “However, all the pregnancy tests on therapy can be either in a medical setting or using a home pregnancy test,” he told this news organization. “This option facilitates the use of telemedicine so that patients would not need to come in; they can just share a pregnancy test with their name and date with their dermatologist.”
- Remove the waiting period requirement — also known as the “19-day lockout” — for patients if they do not obtain isotretinoin within the first 7-day prescription window. According to Dr. Barbieri, this change helps to ensure that patients can begin isotretinoin in a timely manner. “Insurance and pharmacy delays that are no fault of the patient can commonly cause missed initial window periods,” he said. “Allowing for immediate repeat of a pregnancy test to start a new window period, rather than requiring the patient to wait 19 more days, can ensure patient safety and pregnancy prevention without negatively impacting access.”
- Revise the pregnancy registry requirement to remove the objective to document the pregnancy and fetal outcomes for each pregnancy.
- Revise the requirement for prescribers to document patient counseling in patients who cannot become pregnant from monthly to only at enrollment. Dr. Barbieri characterized this change as “major” and said that it could eliminate the need for monthly visits for persons of non–childbearing potential. “This could substantially reduce logistical burdens for patients and reduce wait times to see a dermatologist,” he said.
Future changes to iPLEDGE that Dr. Barbieri would like to see include allowing for home pregnancy tests prior to starting therapy — particularly the test after the 30-day window period. “In addition, it would be good to be able to reduce the 30-day waiting period prior to therapy to something shorter,” such as 14 days, which would still “reliably exclude pregnancy, particularly for those on stable long-acting reversible contraception,” he said. There are also opportunities to improve the iPLEDGE website functionality and to ensure that the website is accessible to patients with limited English proficiency, he added.
He also recommended greater transparency by the Isotretinoin Products Manufacturers Group and inclusion of input from diverse stakeholders such as dermatologists, patients, and pharmacists.
Dr. Barbieri reported personal fees from Dexcel Pharma.
Are you sure your patient is alive?
This transcript has been edited for clarity.
Much of my research focuses on what is known as clinical decision support — prompts and messages to providers to help them make good decisions for their patients. I know that these things can be annoying, which is exactly why I study them — to figure out which ones actually help.
When I got started on this about 10 years ago, we were learning a lot about how best to message providers about their patients. My team had developed a simple alert for acute kidney injury (AKI). We knew that providers often missed the diagnosis, so maybe letting them know would improve patient outcomes.
As we tested the alert, we got feedback, and I have kept an email from an ICU doctor from those early days. It read:
Dear Dr. Wilson: Thank you for the automated alert informing me that my patient had AKI. Regrettably, the alert fired about an hour after the patient had died. I feel that the information is less than actionable at this time.
Our early system had neglected to add a conditional flag ensuring that the patient was still alive at the time it sent the alert message. A small oversight, but one that had very large implications. Future studies would show that “false positive” alerts like this seriously degrade physician confidence in the system. And why wouldn’t they?
Not knowing the vital status of a patient can have major consequences.
Health systems send messages to their patients all the time: reminders of appointments, reminders for preventive care, reminders for vaccinations, and so on.
But what if the patient being reminded has died? It’s a waste of resources, of course, but more than that, it can be painful for their families and reflects poorly on the health care system. Of all the people who should know whether someone is alive or dead, shouldn’t their doctor be at the top of the list?
A new study in JAMA Internal Medicine quantifies this very phenomenon.
Researchers examined 11,658 primary care patients in their health system who met the criteria of being “seriously ill” and followed them for 2 years. During that period of time, 25% were recorded as deceased in the electronic health record. But 30.8% had died. That left 676 patients who had died, but were not known to have died, left in the system.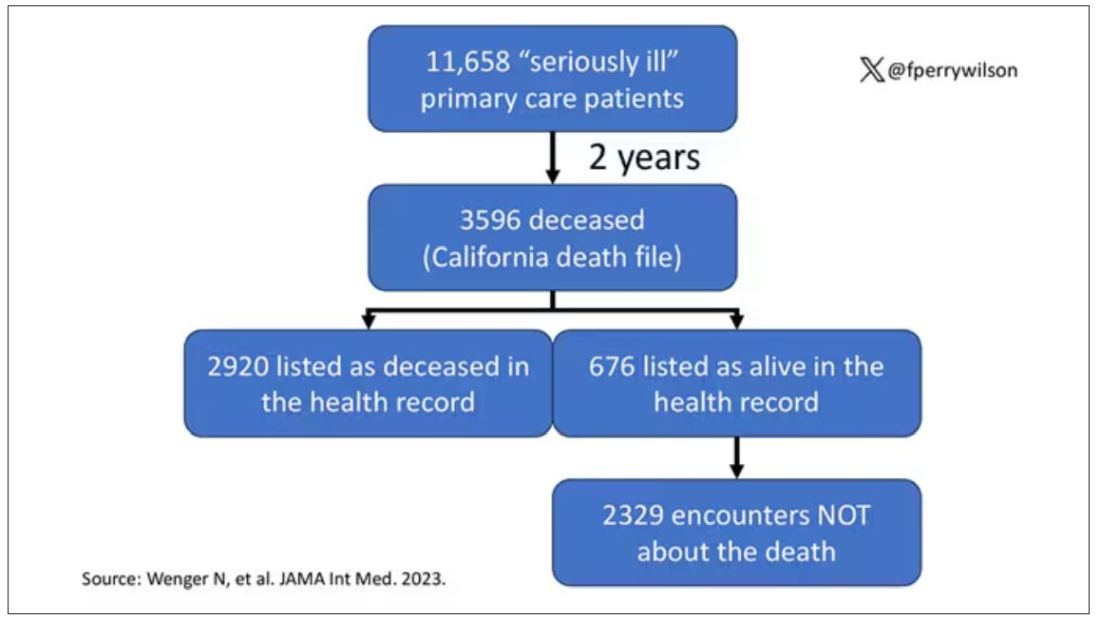
And those 676 were not left to rest in peace. They received 221 telephone and 338 health portal messages not related to death, and 920 letters reminding them about unmet primary care metrics like flu shots and cancer screening. Orders were entered into the health record for things like vaccines and routine screenings for 158 patients, and 310 future appointments — destined to be no-shows — were still on the books. One can only imagine the frustration of families checking their mail and finding yet another letter reminding their deceased loved one to get a mammogram.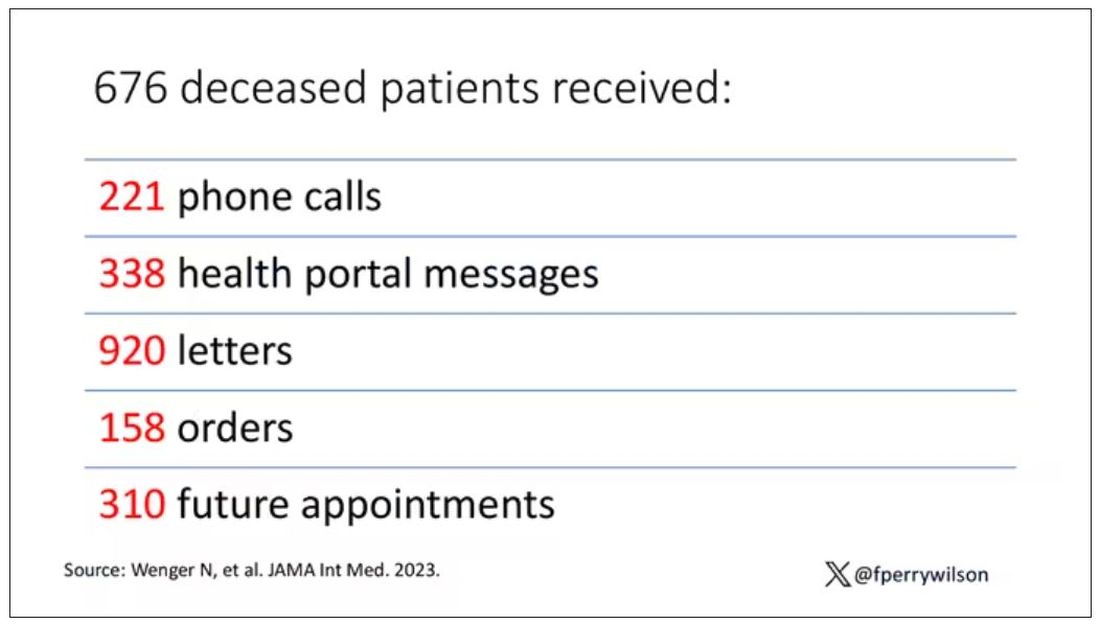
How did the researchers figure out who had died? It turns out it’s not that hard. California keeps a record of all deaths in the state; they simply had to search it. Like all state death records, they tend to lag a bit so it’s not clinically terribly useful, but it works. California and most other states also have a very accurate and up-to-date death file which can only be used by law enforcement to investigate criminal activity and fraud; health care is left in the lurch.
Nationwide, there is the real-time fact of death service, supported by the National Association for Public Health Statistics and Information Systems. This allows employers to verify, in real time, whether the person applying for a job is alive. Healthcare systems are not allowed to use it.
Let’s also remember that very few people die in this country without some health care agency knowing about it and recording it. But sharing of medical information is so poor in the United States that your patient could die in a hospital one city away from you and you might not find out until you’re calling them to see why they missed a scheduled follow-up appointment.
These events — the embarrassing lack of knowledge about the very vital status of our patients — highlight a huge problem with health care in our country. The fragmented health care system is terrible at data sharing, in part because of poor protocols, in part because of unfounded concerns about patient privacy, and in part because of a tendency to hoard data that might be valuable in the future. It has to stop. We need to know how our patients are doing even when they are not sitting in front of us. When it comes to life and death, the knowledge is out there; we just can’t access it. Seems like a pretty easy fix.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com .
This transcript has been edited for clarity.
Much of my research focuses on what is known as clinical decision support — prompts and messages to providers to help them make good decisions for their patients. I know that these things can be annoying, which is exactly why I study them — to figure out which ones actually help.
When I got started on this about 10 years ago, we were learning a lot about how best to message providers about their patients. My team had developed a simple alert for acute kidney injury (AKI). We knew that providers often missed the diagnosis, so maybe letting them know would improve patient outcomes.
As we tested the alert, we got feedback, and I have kept an email from an ICU doctor from those early days. It read:
Dear Dr. Wilson: Thank you for the automated alert informing me that my patient had AKI. Regrettably, the alert fired about an hour after the patient had died. I feel that the information is less than actionable at this time.
Our early system had neglected to add a conditional flag ensuring that the patient was still alive at the time it sent the alert message. A small oversight, but one that had very large implications. Future studies would show that “false positive” alerts like this seriously degrade physician confidence in the system. And why wouldn’t they?
Not knowing the vital status of a patient can have major consequences.
Health systems send messages to their patients all the time: reminders of appointments, reminders for preventive care, reminders for vaccinations, and so on.
But what if the patient being reminded has died? It’s a waste of resources, of course, but more than that, it can be painful for their families and reflects poorly on the health care system. Of all the people who should know whether someone is alive or dead, shouldn’t their doctor be at the top of the list?
A new study in JAMA Internal Medicine quantifies this very phenomenon.
Researchers examined 11,658 primary care patients in their health system who met the criteria of being “seriously ill” and followed them for 2 years. During that period of time, 25% were recorded as deceased in the electronic health record. But 30.8% had died. That left 676 patients who had died, but were not known to have died, left in the system.
And those 676 were not left to rest in peace. They received 221 telephone and 338 health portal messages not related to death, and 920 letters reminding them about unmet primary care metrics like flu shots and cancer screening. Orders were entered into the health record for things like vaccines and routine screenings for 158 patients, and 310 future appointments — destined to be no-shows — were still on the books. One can only imagine the frustration of families checking their mail and finding yet another letter reminding their deceased loved one to get a mammogram.
How did the researchers figure out who had died? It turns out it’s not that hard. California keeps a record of all deaths in the state; they simply had to search it. Like all state death records, they tend to lag a bit so it’s not clinically terribly useful, but it works. California and most other states also have a very accurate and up-to-date death file which can only be used by law enforcement to investigate criminal activity and fraud; health care is left in the lurch.
Nationwide, there is the real-time fact of death service, supported by the National Association for Public Health Statistics and Information Systems. This allows employers to verify, in real time, whether the person applying for a job is alive. Healthcare systems are not allowed to use it.
Let’s also remember that very few people die in this country without some health care agency knowing about it and recording it. But sharing of medical information is so poor in the United States that your patient could die in a hospital one city away from you and you might not find out until you’re calling them to see why they missed a scheduled follow-up appointment.
These events — the embarrassing lack of knowledge about the very vital status of our patients — highlight a huge problem with health care in our country. The fragmented health care system is terrible at data sharing, in part because of poor protocols, in part because of unfounded concerns about patient privacy, and in part because of a tendency to hoard data that might be valuable in the future. It has to stop. We need to know how our patients are doing even when they are not sitting in front of us. When it comes to life and death, the knowledge is out there; we just can’t access it. Seems like a pretty easy fix.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com .
This transcript has been edited for clarity.
Much of my research focuses on what is known as clinical decision support — prompts and messages to providers to help them make good decisions for their patients. I know that these things can be annoying, which is exactly why I study them — to figure out which ones actually help.
When I got started on this about 10 years ago, we were learning a lot about how best to message providers about their patients. My team had developed a simple alert for acute kidney injury (AKI). We knew that providers often missed the diagnosis, so maybe letting them know would improve patient outcomes.
As we tested the alert, we got feedback, and I have kept an email from an ICU doctor from those early days. It read:
Dear Dr. Wilson: Thank you for the automated alert informing me that my patient had AKI. Regrettably, the alert fired about an hour after the patient had died. I feel that the information is less than actionable at this time.
Our early system had neglected to add a conditional flag ensuring that the patient was still alive at the time it sent the alert message. A small oversight, but one that had very large implications. Future studies would show that “false positive” alerts like this seriously degrade physician confidence in the system. And why wouldn’t they?
Not knowing the vital status of a patient can have major consequences.
Health systems send messages to their patients all the time: reminders of appointments, reminders for preventive care, reminders for vaccinations, and so on.
But what if the patient being reminded has died? It’s a waste of resources, of course, but more than that, it can be painful for their families and reflects poorly on the health care system. Of all the people who should know whether someone is alive or dead, shouldn’t their doctor be at the top of the list?
A new study in JAMA Internal Medicine quantifies this very phenomenon.
Researchers examined 11,658 primary care patients in their health system who met the criteria of being “seriously ill” and followed them for 2 years. During that period of time, 25% were recorded as deceased in the electronic health record. But 30.8% had died. That left 676 patients who had died, but were not known to have died, left in the system.
And those 676 were not left to rest in peace. They received 221 telephone and 338 health portal messages not related to death, and 920 letters reminding them about unmet primary care metrics like flu shots and cancer screening. Orders were entered into the health record for things like vaccines and routine screenings for 158 patients, and 310 future appointments — destined to be no-shows — were still on the books. One can only imagine the frustration of families checking their mail and finding yet another letter reminding their deceased loved one to get a mammogram.
How did the researchers figure out who had died? It turns out it’s not that hard. California keeps a record of all deaths in the state; they simply had to search it. Like all state death records, they tend to lag a bit so it’s not clinically terribly useful, but it works. California and most other states also have a very accurate and up-to-date death file which can only be used by law enforcement to investigate criminal activity and fraud; health care is left in the lurch.
Nationwide, there is the real-time fact of death service, supported by the National Association for Public Health Statistics and Information Systems. This allows employers to verify, in real time, whether the person applying for a job is alive. Healthcare systems are not allowed to use it.
Let’s also remember that very few people die in this country without some health care agency knowing about it and recording it. But sharing of medical information is so poor in the United States that your patient could die in a hospital one city away from you and you might not find out until you’re calling them to see why they missed a scheduled follow-up appointment.
These events — the embarrassing lack of knowledge about the very vital status of our patients — highlight a huge problem with health care in our country. The fragmented health care system is terrible at data sharing, in part because of poor protocols, in part because of unfounded concerns about patient privacy, and in part because of a tendency to hoard data that might be valuable in the future. It has to stop. We need to know how our patients are doing even when they are not sitting in front of us. When it comes to life and death, the knowledge is out there; we just can’t access it. Seems like a pretty easy fix.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com .
Early age at first period raises type 2 diabetes risk
TOPLINE:
, a retrospective study of US women under age 65 found.
METHODOLOGY:
- Researchers analyzed data from 17,377 women who were aged 20-65 years when they participated in a National Health and Nutrition Examination Survey (NHANES) from 1999 to 2018 and reported their age at first menstruation, which was classified as ≤ 10, 11, 12, 13, 14, or ≥ 15 years of age.
- In total, 0.2% of the women (1773) had type 2 diabetes; of these, 11.5% (205) had cardiovascular disease (CVD), defined as coronary heart disease (CHD), myocardial infarction, or stroke.
- Compared with women who had their first menstrual period at age 13 (the mean age in this population), those who had their period at age ≤ 10 had a significantly greater risk of having type 2 diabetes, after adjustment for age, race/ethnicity, education, parity, menopause status, family history of diabetes, smoking status, physical activity, alcohol consumption, and body mass index (odds ratio, 1.32; 95% CI, 1.03-1.69; P trend = .03).
- Among the women with diabetes, compared with those who had their first menstrual period at age 13, those who had it at age ≤ 10 had a significantly greater risk of having stroke (OR, 2.66; 95% CI, 1.07-6.64; P trend = .02), but not CVD or CHD, after adjustment for these multiple variables.
TAKEAWAY:
- In a racially and ethnically diverse national sample of US women younger than 65, “extremely early” age at first menstrual period was associated with significantly increased risk for type 2 diabetes; among the women with type 2 diabetes, it was associated with significantly increased risk for stroke but not CVD or CHD, after adjustment for multiple variables.
- Early age at menarche may be an early indicator of the cardiometabolic disease trajectory in women.
IN PRACTICE:
“Women with early-life exposures such as early age at menarche need to be further examined for diabetes and prevention research and strategies for progression of diabetes complications,” the study authors write.
SOURCE:
The authors, mainly from Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana, and also from Harvard Medical School, Boston, Massachusetts, published their findings in BMJ Nutrition, Prevention & Health.
LIMITATIONS:
- The women who participated in NHANES may not be representative of all women in the United States (selection bias).
- The study only included women who reported the age when they had their first menstrual period (selection bias).
- This was a cross-sectional, observational study, so it cannot show causality.
- The women may have reported the wrong age at which they had their first period (recall bias and social desirability bias).
- The women may have inaccurately reported CVD and type 2 diabetes (recall bias and social desirability bias).
DISCLOSURES:
The researchers were supported by grants from the National Heart, Lung, and Blood Institute and from the National Institute of General Medical Sciences of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
TOPLINE:
, a retrospective study of US women under age 65 found.
METHODOLOGY:
- Researchers analyzed data from 17,377 women who were aged 20-65 years when they participated in a National Health and Nutrition Examination Survey (NHANES) from 1999 to 2018 and reported their age at first menstruation, which was classified as ≤ 10, 11, 12, 13, 14, or ≥ 15 years of age.
- In total, 0.2% of the women (1773) had type 2 diabetes; of these, 11.5% (205) had cardiovascular disease (CVD), defined as coronary heart disease (CHD), myocardial infarction, or stroke.
- Compared with women who had their first menstrual period at age 13 (the mean age in this population), those who had their period at age ≤ 10 had a significantly greater risk of having type 2 diabetes, after adjustment for age, race/ethnicity, education, parity, menopause status, family history of diabetes, smoking status, physical activity, alcohol consumption, and body mass index (odds ratio, 1.32; 95% CI, 1.03-1.69; P trend = .03).
- Among the women with diabetes, compared with those who had their first menstrual period at age 13, those who had it at age ≤ 10 had a significantly greater risk of having stroke (OR, 2.66; 95% CI, 1.07-6.64; P trend = .02), but not CVD or CHD, after adjustment for these multiple variables.
TAKEAWAY:
- In a racially and ethnically diverse national sample of US women younger than 65, “extremely early” age at first menstrual period was associated with significantly increased risk for type 2 diabetes; among the women with type 2 diabetes, it was associated with significantly increased risk for stroke but not CVD or CHD, after adjustment for multiple variables.
- Early age at menarche may be an early indicator of the cardiometabolic disease trajectory in women.
IN PRACTICE:
“Women with early-life exposures such as early age at menarche need to be further examined for diabetes and prevention research and strategies for progression of diabetes complications,” the study authors write.
SOURCE:
The authors, mainly from Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana, and also from Harvard Medical School, Boston, Massachusetts, published their findings in BMJ Nutrition, Prevention & Health.
LIMITATIONS:
- The women who participated in NHANES may not be representative of all women in the United States (selection bias).
- The study only included women who reported the age when they had their first menstrual period (selection bias).
- This was a cross-sectional, observational study, so it cannot show causality.
- The women may have reported the wrong age at which they had their first period (recall bias and social desirability bias).
- The women may have inaccurately reported CVD and type 2 diabetes (recall bias and social desirability bias).
DISCLOSURES:
The researchers were supported by grants from the National Heart, Lung, and Blood Institute and from the National Institute of General Medical Sciences of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
TOPLINE:
, a retrospective study of US women under age 65 found.
METHODOLOGY:
- Researchers analyzed data from 17,377 women who were aged 20-65 years when they participated in a National Health and Nutrition Examination Survey (NHANES) from 1999 to 2018 and reported their age at first menstruation, which was classified as ≤ 10, 11, 12, 13, 14, or ≥ 15 years of age.
- In total, 0.2% of the women (1773) had type 2 diabetes; of these, 11.5% (205) had cardiovascular disease (CVD), defined as coronary heart disease (CHD), myocardial infarction, or stroke.
- Compared with women who had their first menstrual period at age 13 (the mean age in this population), those who had their period at age ≤ 10 had a significantly greater risk of having type 2 diabetes, after adjustment for age, race/ethnicity, education, parity, menopause status, family history of diabetes, smoking status, physical activity, alcohol consumption, and body mass index (odds ratio, 1.32; 95% CI, 1.03-1.69; P trend = .03).
- Among the women with diabetes, compared with those who had their first menstrual period at age 13, those who had it at age ≤ 10 had a significantly greater risk of having stroke (OR, 2.66; 95% CI, 1.07-6.64; P trend = .02), but not CVD or CHD, after adjustment for these multiple variables.
TAKEAWAY:
- In a racially and ethnically diverse national sample of US women younger than 65, “extremely early” age at first menstrual period was associated with significantly increased risk for type 2 diabetes; among the women with type 2 diabetes, it was associated with significantly increased risk for stroke but not CVD or CHD, after adjustment for multiple variables.
- Early age at menarche may be an early indicator of the cardiometabolic disease trajectory in women.
IN PRACTICE:
“Women with early-life exposures such as early age at menarche need to be further examined for diabetes and prevention research and strategies for progression of diabetes complications,” the study authors write.
SOURCE:
The authors, mainly from Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana, and also from Harvard Medical School, Boston, Massachusetts, published their findings in BMJ Nutrition, Prevention & Health.
LIMITATIONS:
- The women who participated in NHANES may not be representative of all women in the United States (selection bias).
- The study only included women who reported the age when they had their first menstrual period (selection bias).
- This was a cross-sectional, observational study, so it cannot show causality.
- The women may have reported the wrong age at which they had their first period (recall bias and social desirability bias).
- The women may have inaccurately reported CVD and type 2 diabetes (recall bias and social desirability bias).
DISCLOSURES:
The researchers were supported by grants from the National Heart, Lung, and Blood Institute and from the National Institute of General Medical Sciences of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Procedures may ease postmenopausal pain better than drugs
a new study shows.
“This study provides us a better understanding of pain management strategies for pre versus postmenopausal women,” said Tian Yu, MD, who presented the research at the annual pain medicine meeting of the American Society of Regional Anesthesia and Pain Medicine. “With our postmenopausal patients, we may no longer jump the gun and give them a lot of medications; we may first turn to physical therapy or procedural intervention, which they seem to benefit much more from than pharmacological therapy.”Pain perception is a multifaceted phenomenon influenced by age, gender, individual variations, and hormonal changes. Pain management in women, particularly in the context of menopausal status, still lacks consensus.
Menopause primarily results from diminished production of estrogen by the ovaries, leading to spinal and joint pain, hot flashes, night sweats, chronic fatigue, increased osteoclastic activity with a heightened risk for osteoporosis, psychological symptoms, and elevated risk for cardiovascular disease.
For their retrospective cohort study, Dr. Yu, department of anesthesiology, Advocate Illinois Masonic Medical Center, Chicago, Illinois, and his colleagues looked at 1215 women who had been treated for different chronic pain conditions for at least 3 months. The researchers used a predefined age cutoff of 51 years (considered the national average) to categorize participants as either premenopausal (n = 248) or postmenopausal (n = 967). Pain scores and subjective improvement were assessed after pharmacological and procedural interventions.
According to Dr. Yu, the results revealed distinct patterns in pain scores and response to interventions between the two groups.
Although postmenopausal women initially reported higher mean pain scores upon presentation (8.037 vs 7.613 in premenopausal women), they reported more improvement following intervention (63% vs 59%; P = .029). They responded more favorably to both procedural and pharmacological interventions, but were prescribed muscle relaxants, tricyclic antidepressants, and benzodiazepines less frequently than premenopausal women, Dr. Yu’s group found.
“So even though postmenopausal women had a higher initial pain score, they had better pain improvement after procedural intervention, although they were prescribed fewer pharmacological interventions,” Dr. Yu said.
The fact that postmenopausal women typically are older than women who have not reached menopause could act as a confounding factor in this study in terms of disease prevalence and intervention, Dr. Yu said. Additionally, the study’s reliance on a broad menopausal age cutoff of 51 years may limit the true characterization of menopausal status.
While acknowledging study limitations, the findings suggest a potential shift toward prioritizing nonpharmacological interventions in postmenopausal women. Further investigation into physical therapy and other approaches could provide a more comprehensive understanding of pain management strategies in this population.
“We hope to take these findings into consideration during our practice to better individualize care,” Yu said.
Robert Wenham, MD, MS, chair of gynecologic oncology, Moffitt Cancer Center, Tampa, Florida, who was not involved in the study, said: “Despite the many methodological challenges it has, including using age as a surrogate for menopause, I applaud the authors for investigating how pain and pain management may be individualized for women.”
Dr. Wenham added that he hoped the findings would prompt additional studies “that specifically address populations based on hormonal status and other confounding factors, so that interventional avenues may be identified for clinical trials.”
Dr. Yu and Dr. Wenham report no relevant financial relationships.
A version of this article appeared on Medscape.com.
a new study shows.
“This study provides us a better understanding of pain management strategies for pre versus postmenopausal women,” said Tian Yu, MD, who presented the research at the annual pain medicine meeting of the American Society of Regional Anesthesia and Pain Medicine. “With our postmenopausal patients, we may no longer jump the gun and give them a lot of medications; we may first turn to physical therapy or procedural intervention, which they seem to benefit much more from than pharmacological therapy.”Pain perception is a multifaceted phenomenon influenced by age, gender, individual variations, and hormonal changes. Pain management in women, particularly in the context of menopausal status, still lacks consensus.
Menopause primarily results from diminished production of estrogen by the ovaries, leading to spinal and joint pain, hot flashes, night sweats, chronic fatigue, increased osteoclastic activity with a heightened risk for osteoporosis, psychological symptoms, and elevated risk for cardiovascular disease.
For their retrospective cohort study, Dr. Yu, department of anesthesiology, Advocate Illinois Masonic Medical Center, Chicago, Illinois, and his colleagues looked at 1215 women who had been treated for different chronic pain conditions for at least 3 months. The researchers used a predefined age cutoff of 51 years (considered the national average) to categorize participants as either premenopausal (n = 248) or postmenopausal (n = 967). Pain scores and subjective improvement were assessed after pharmacological and procedural interventions.
According to Dr. Yu, the results revealed distinct patterns in pain scores and response to interventions between the two groups.
Although postmenopausal women initially reported higher mean pain scores upon presentation (8.037 vs 7.613 in premenopausal women), they reported more improvement following intervention (63% vs 59%; P = .029). They responded more favorably to both procedural and pharmacological interventions, but were prescribed muscle relaxants, tricyclic antidepressants, and benzodiazepines less frequently than premenopausal women, Dr. Yu’s group found.
“So even though postmenopausal women had a higher initial pain score, they had better pain improvement after procedural intervention, although they were prescribed fewer pharmacological interventions,” Dr. Yu said.
The fact that postmenopausal women typically are older than women who have not reached menopause could act as a confounding factor in this study in terms of disease prevalence and intervention, Dr. Yu said. Additionally, the study’s reliance on a broad menopausal age cutoff of 51 years may limit the true characterization of menopausal status.
While acknowledging study limitations, the findings suggest a potential shift toward prioritizing nonpharmacological interventions in postmenopausal women. Further investigation into physical therapy and other approaches could provide a more comprehensive understanding of pain management strategies in this population.
“We hope to take these findings into consideration during our practice to better individualize care,” Yu said.
Robert Wenham, MD, MS, chair of gynecologic oncology, Moffitt Cancer Center, Tampa, Florida, who was not involved in the study, said: “Despite the many methodological challenges it has, including using age as a surrogate for menopause, I applaud the authors for investigating how pain and pain management may be individualized for women.”
Dr. Wenham added that he hoped the findings would prompt additional studies “that specifically address populations based on hormonal status and other confounding factors, so that interventional avenues may be identified for clinical trials.”
Dr. Yu and Dr. Wenham report no relevant financial relationships.
A version of this article appeared on Medscape.com.
a new study shows.
“This study provides us a better understanding of pain management strategies for pre versus postmenopausal women,” said Tian Yu, MD, who presented the research at the annual pain medicine meeting of the American Society of Regional Anesthesia and Pain Medicine. “With our postmenopausal patients, we may no longer jump the gun and give them a lot of medications; we may first turn to physical therapy or procedural intervention, which they seem to benefit much more from than pharmacological therapy.”Pain perception is a multifaceted phenomenon influenced by age, gender, individual variations, and hormonal changes. Pain management in women, particularly in the context of menopausal status, still lacks consensus.
Menopause primarily results from diminished production of estrogen by the ovaries, leading to spinal and joint pain, hot flashes, night sweats, chronic fatigue, increased osteoclastic activity with a heightened risk for osteoporosis, psychological symptoms, and elevated risk for cardiovascular disease.
For their retrospective cohort study, Dr. Yu, department of anesthesiology, Advocate Illinois Masonic Medical Center, Chicago, Illinois, and his colleagues looked at 1215 women who had been treated for different chronic pain conditions for at least 3 months. The researchers used a predefined age cutoff of 51 years (considered the national average) to categorize participants as either premenopausal (n = 248) or postmenopausal (n = 967). Pain scores and subjective improvement were assessed after pharmacological and procedural interventions.
According to Dr. Yu, the results revealed distinct patterns in pain scores and response to interventions between the two groups.
Although postmenopausal women initially reported higher mean pain scores upon presentation (8.037 vs 7.613 in premenopausal women), they reported more improvement following intervention (63% vs 59%; P = .029). They responded more favorably to both procedural and pharmacological interventions, but were prescribed muscle relaxants, tricyclic antidepressants, and benzodiazepines less frequently than premenopausal women, Dr. Yu’s group found.
“So even though postmenopausal women had a higher initial pain score, they had better pain improvement after procedural intervention, although they were prescribed fewer pharmacological interventions,” Dr. Yu said.
The fact that postmenopausal women typically are older than women who have not reached menopause could act as a confounding factor in this study in terms of disease prevalence and intervention, Dr. Yu said. Additionally, the study’s reliance on a broad menopausal age cutoff of 51 years may limit the true characterization of menopausal status.
While acknowledging study limitations, the findings suggest a potential shift toward prioritizing nonpharmacological interventions in postmenopausal women. Further investigation into physical therapy and other approaches could provide a more comprehensive understanding of pain management strategies in this population.
“We hope to take these findings into consideration during our practice to better individualize care,” Yu said.
Robert Wenham, MD, MS, chair of gynecologic oncology, Moffitt Cancer Center, Tampa, Florida, who was not involved in the study, said: “Despite the many methodological challenges it has, including using age as a surrogate for menopause, I applaud the authors for investigating how pain and pain management may be individualized for women.”
Dr. Wenham added that he hoped the findings would prompt additional studies “that specifically address populations based on hormonal status and other confounding factors, so that interventional avenues may be identified for clinical trials.”
Dr. Yu and Dr. Wenham report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Eight wealth tips just for doctors
The average physician makes $352,000, and some earn well into the $500,000s. So, doctors don’t have to worry about money, right?
You know the answer to that.
One thing all physicians have in common about money, says James M. Dahle, MD, FACEP, founder of The White Coat Investor, is that they don’t receive any training in business, personal finance, or investing throughout their schooling or careers unless they seek it out. This leaves many unprepared to make the best investing and money-saving decisions, while others get too frustrated about their lack of knowledge to even dip their toe into the investing pool.
Exhibit A: Four out of 10 physicians have a net worth below $1 million, according to the Medscape Physician Wealth & Debt Report 2023. Elizabeth Chiang, MD, PhD, an oculoplastic surgeon and a physician money coach at Grow Your Wealthy Mindset, notes that many of those doctors are over age 65, “which means they essentially can’t retire.”
And that’s just one pain point.
Physicians have money concerns specific to their profession and background. Luckily, some fellow doctors also serve as financial and wealth advisors just for other doctors.
Blind Spot #1
The early lean years skew doctors’ money outlook. “We have an extended training period, which commonly consists of taking on a large amount of debt, followed by 3 to 8 years of being paid a modest salary, and then finally a large boost in income,” explains Dr. Chiang. This can lay a shaky foundation for the earning years to come, and as a result, a lot of doctors just don’t think about money in healthy ways. Once their incomes increase, physicians may be surprised, for example, that making a multiple six-figure salary means paying six figures in taxes.
The Fix
Treat financial health like physical health. That means money cannot be a taboo subject. “The misguided mindset is that we didn’t become physicians to make money, we did it to help people,” explains Jordan Frey, MD, creator of the blog, The Prudent Plastic Surgeon.
Dr. Frey acknowledges that the desire to help is certainly true. But the result is a false idea that “to think about our personal finances makes us a worse doctor.”
Blind Spot #2
Because doctors know a lot about one thing (medicine), they might assume they know a lot about everything (such as investing). “Totally different fields with a different language and different way to think about it,” Dahle explains. This overconfidence could lead to some negligent or risky financial decisions.
The Fix
Educate yourself. There are several books on personal finance and investing written by physicians for physicians. Dr. Chiang recommends The Physician Philosopher’s Guide to Personal Finance, by James Turner, MD; Financial Freedom Rx, by Chirag Shah, MD, and Jayanth Sridhar, MD; and The Physician’s Guide to Finance, by Nicholas Christian and Amanda Christian, MD. There are also podcasts, blogs, and courses to help educate doctors on finance, such as the Fire Your Financial Advisor course by The White Coat Investor.
Blind Spot #3
Undersaving. Retirement saving is one thing, but 24% of doctors say they don’t even put money away in a taxable savings account, according to the Wealth & Debt Report.
Cobin Soelberg, MD, JD, a board-certified anesthesiologist and founder and principal advisor with Greeley Wealth Management, is the treasurer of his anesthesiology group. “I get to see every month how much people are saving, and even on an anesthesiologist salary, where everyone’s making about $400,000 a year, a lot of people are not saving anything, which is crazy.”
Undersaving can be both a time issue and a mindset one.
Time: Doctors often start investing in their retirement accounts later than the average professional, says Dr. Chiang. “A lot of physicians will max out their 401k or 403b,” she explains. “But if you’re putting in $20,000 a year and only starting when you’re in your early 30s, that’s not enough to get you to retirement.”
Mindset: Doctors also see people of all ages who are sick, dying, and injured. “They all know someone who worked hard and saved and then dropped dead at 55,” explains Dr. Dahle. This, he says, can lead to a bit of a “you only live once” attitude that prioritizes spending over saving.
The Fix
Shoot for 20%. If you can’t save 20% of your gross now, strive to get to that point. Think of it as telling a patient they have to change their behavior or trouble will come - not if, but when. “Develop a written investing plan and then stick with it through thick and thin,” says Dr. Dahle. “Once you have a reasonable plan, all you have to do is fund it adequately by saving 20% of your gross income, and a doctor will easily retire as a multimillionaire.”
Blind Spot #4
Bad investment strategies. Thirty-six percent of doctors experience their largest financial losses from lousy investments, according to the Wealth & Debt Report. Meanwhile, 17% of PCPs and 12% of specialists say they haven’t made any investments at all. That’s a terrible mix of doing the wrong thing and doing a worse thing.
The Fix
Don’t overthink investing, but don’t underthink it either. “As high-income earners, doctors just don’t need to take this high level of risk to reach their financial goals,” Dr. Frey says. A good investment plan doesn’t require you to time the stock market or predict individual stock winners. Consider what Vanguard founder Jack Bogle once said about investing: “Be bored by the process but elated by the outcome.”
Dr. Frey suggests going super-simple: index funds. Ignore investing strategies with actively managed mutual funds or individual stocks, as well as risky alternative investments such as cryptocurrency and angel investments. Everyone assumes doctors have money to burn, and they will push sketchy investment ideas at them. Avoid.
Blind Spot #5
Not taking debt seriously enough. The average medical student debt is $250,000 and can exceed $500,000, says Dr. Soelberg. Many doctors spend the first 10 to 20 years of their careers paying this off. Today’s graduates are paying more than 7% on their loans.
And it’s not just student debt: 39% of physicians carry five or more credit cards, and 34% have mortgages larger than $300,000 (with half of those are more than than $500K), per the Wealth & Debt Report.
The Fix
Treat debt like cancer. It’s a lethal enemy you can’t get rid of right away, but a steady, aggressive, long-term attack will have the best results. Dr. Soelberg suggests allocating the most you can afford per month, whether that’s $1000 or $5000, toward debt. Raise the amount as your income grows. Do the same with your 401k or retirement plan. Whatever is left, you can spend. Five to 10 years later, you will realize, “Wow. I’m debt free.”
Blind Spot #6
Not putting in the work to improve your situation. Seventy-one percent of doctors admit they haven’t done anything to reduce major expenses, according to the Wealth & Debt Report. Are you leaving major money on the table?
The Fix
Audit yourself in major areas like housing and taxes. While the average professional may need to put 10% to 20% down on a home, physicians can qualify for physician mortgage loans and can often put down 3% or less, says Dr. Chiang. If you can afford the higher mortgage payment, excess savings earmarked for a larger down payment can be put toward debt or invested.
Another trick, if you’re able, is to seek an area that is less in demand at a higher salary. “Physicians in places like New York City or San Francisco tend to make less than physicians in the Midwest or the South,” Dr. Chiang explains. A colleague of hers moved to rural Pennsylvania, where he made a high salary and had a low cost of living for 3½ years, paid off his student debt, and then relocated to an area where he wanted to live long term.
As for taxes, become familiar with tax law. Research things like, “What is considered a business expense for doctors?” says Brett Mollard, MD, a diagnostic radiologist who provides financial advice to younger physicians. “What will your estimated total tax burden be at the end of the year? Will you need to make extra payments to prevent owing a large sum of money from underpaying or to avoid tax penalties?”
Blind Spot #7
Living like a rock star on a doctor’s income. Getting caught up in trying to live the same lifestyle as your colleagues is a classic bear trap. “Sitting in the doctor’s lounge, it’s so crazy,” Dr. Soelberg says. He describes conversations like, “‘Where did you go on your trip?’ ‘What new toys are you buying?’” There’s pressure to live up to an image of what a doctor’s life is supposed to look like before you’ve sorted the basic things like paying off debt.
The Fix
Live like a resident even if you haven’t been one for years, at least until you’re in a better financial position. “You’re already used to living a life of lower means, and you’re an expert when it comes to delaying gratification,” says Dr. Mollard. “Do it a little longer.” Live frugally and spend only on things that bring you joy. “A lot of physicians are trying to be really rich in all areas of their life instead of the ones that actually matter to them,” Dr. Soelberg says. Identify what’s important to you and only splurge on that.
Blind Spot #8
Never asking for help. The right financial planner can provide expert help. Emphasis on right. “Doctors can be very trusting of other professionals, even when they should not be,” says Dr. Dahle. He notes that in financial services, many people masquerade as knowledgeable advisors who are really just salespeople. While legitimate financial advisors strive to make their clients money, they are also ultimately out to line their pockets and love to work with physician salaries. Thus, doctors can end up working with financial planners that don’t specifically understand their situations or end up taking too much from their clients.
The Fix
Find a planner who specializes in, or at least understands, physicians. Ask them how they make money, says Dr. Chiang. If someone hesitates to tell you about their fee structure or if it sounds like a lot, shop around and ask colleagues for recommendations.
“Ultimately, the path to wealth is to create and grow the margin between what you make and what you spend,” says Dr. Frey. Throw some investing into the mix and physicians can set themselves up on a path for a stress-free financial life.
A version of this article appeared on Medscape.com.
The average physician makes $352,000, and some earn well into the $500,000s. So, doctors don’t have to worry about money, right?
You know the answer to that.
One thing all physicians have in common about money, says James M. Dahle, MD, FACEP, founder of The White Coat Investor, is that they don’t receive any training in business, personal finance, or investing throughout their schooling or careers unless they seek it out. This leaves many unprepared to make the best investing and money-saving decisions, while others get too frustrated about their lack of knowledge to even dip their toe into the investing pool.
Exhibit A: Four out of 10 physicians have a net worth below $1 million, according to the Medscape Physician Wealth & Debt Report 2023. Elizabeth Chiang, MD, PhD, an oculoplastic surgeon and a physician money coach at Grow Your Wealthy Mindset, notes that many of those doctors are over age 65, “which means they essentially can’t retire.”
And that’s just one pain point.
Physicians have money concerns specific to their profession and background. Luckily, some fellow doctors also serve as financial and wealth advisors just for other doctors.
Blind Spot #1
The early lean years skew doctors’ money outlook. “We have an extended training period, which commonly consists of taking on a large amount of debt, followed by 3 to 8 years of being paid a modest salary, and then finally a large boost in income,” explains Dr. Chiang. This can lay a shaky foundation for the earning years to come, and as a result, a lot of doctors just don’t think about money in healthy ways. Once their incomes increase, physicians may be surprised, for example, that making a multiple six-figure salary means paying six figures in taxes.
The Fix
Treat financial health like physical health. That means money cannot be a taboo subject. “The misguided mindset is that we didn’t become physicians to make money, we did it to help people,” explains Jordan Frey, MD, creator of the blog, The Prudent Plastic Surgeon.
Dr. Frey acknowledges that the desire to help is certainly true. But the result is a false idea that “to think about our personal finances makes us a worse doctor.”
Blind Spot #2
Because doctors know a lot about one thing (medicine), they might assume they know a lot about everything (such as investing). “Totally different fields with a different language and different way to think about it,” Dahle explains. This overconfidence could lead to some negligent or risky financial decisions.
The Fix
Educate yourself. There are several books on personal finance and investing written by physicians for physicians. Dr. Chiang recommends The Physician Philosopher’s Guide to Personal Finance, by James Turner, MD; Financial Freedom Rx, by Chirag Shah, MD, and Jayanth Sridhar, MD; and The Physician’s Guide to Finance, by Nicholas Christian and Amanda Christian, MD. There are also podcasts, blogs, and courses to help educate doctors on finance, such as the Fire Your Financial Advisor course by The White Coat Investor.
Blind Spot #3
Undersaving. Retirement saving is one thing, but 24% of doctors say they don’t even put money away in a taxable savings account, according to the Wealth & Debt Report.
Cobin Soelberg, MD, JD, a board-certified anesthesiologist and founder and principal advisor with Greeley Wealth Management, is the treasurer of his anesthesiology group. “I get to see every month how much people are saving, and even on an anesthesiologist salary, where everyone’s making about $400,000 a year, a lot of people are not saving anything, which is crazy.”
Undersaving can be both a time issue and a mindset one.
Time: Doctors often start investing in their retirement accounts later than the average professional, says Dr. Chiang. “A lot of physicians will max out their 401k or 403b,” she explains. “But if you’re putting in $20,000 a year and only starting when you’re in your early 30s, that’s not enough to get you to retirement.”
Mindset: Doctors also see people of all ages who are sick, dying, and injured. “They all know someone who worked hard and saved and then dropped dead at 55,” explains Dr. Dahle. This, he says, can lead to a bit of a “you only live once” attitude that prioritizes spending over saving.
The Fix
Shoot for 20%. If you can’t save 20% of your gross now, strive to get to that point. Think of it as telling a patient they have to change their behavior or trouble will come - not if, but when. “Develop a written investing plan and then stick with it through thick and thin,” says Dr. Dahle. “Once you have a reasonable plan, all you have to do is fund it adequately by saving 20% of your gross income, and a doctor will easily retire as a multimillionaire.”
Blind Spot #4
Bad investment strategies. Thirty-six percent of doctors experience their largest financial losses from lousy investments, according to the Wealth & Debt Report. Meanwhile, 17% of PCPs and 12% of specialists say they haven’t made any investments at all. That’s a terrible mix of doing the wrong thing and doing a worse thing.
The Fix
Don’t overthink investing, but don’t underthink it either. “As high-income earners, doctors just don’t need to take this high level of risk to reach their financial goals,” Dr. Frey says. A good investment plan doesn’t require you to time the stock market or predict individual stock winners. Consider what Vanguard founder Jack Bogle once said about investing: “Be bored by the process but elated by the outcome.”
Dr. Frey suggests going super-simple: index funds. Ignore investing strategies with actively managed mutual funds or individual stocks, as well as risky alternative investments such as cryptocurrency and angel investments. Everyone assumes doctors have money to burn, and they will push sketchy investment ideas at them. Avoid.
Blind Spot #5
Not taking debt seriously enough. The average medical student debt is $250,000 and can exceed $500,000, says Dr. Soelberg. Many doctors spend the first 10 to 20 years of their careers paying this off. Today’s graduates are paying more than 7% on their loans.
And it’s not just student debt: 39% of physicians carry five or more credit cards, and 34% have mortgages larger than $300,000 (with half of those are more than than $500K), per the Wealth & Debt Report.
The Fix
Treat debt like cancer. It’s a lethal enemy you can’t get rid of right away, but a steady, aggressive, long-term attack will have the best results. Dr. Soelberg suggests allocating the most you can afford per month, whether that’s $1000 or $5000, toward debt. Raise the amount as your income grows. Do the same with your 401k or retirement plan. Whatever is left, you can spend. Five to 10 years later, you will realize, “Wow. I’m debt free.”
Blind Spot #6
Not putting in the work to improve your situation. Seventy-one percent of doctors admit they haven’t done anything to reduce major expenses, according to the Wealth & Debt Report. Are you leaving major money on the table?
The Fix
Audit yourself in major areas like housing and taxes. While the average professional may need to put 10% to 20% down on a home, physicians can qualify for physician mortgage loans and can often put down 3% or less, says Dr. Chiang. If you can afford the higher mortgage payment, excess savings earmarked for a larger down payment can be put toward debt or invested.
Another trick, if you’re able, is to seek an area that is less in demand at a higher salary. “Physicians in places like New York City or San Francisco tend to make less than physicians in the Midwest or the South,” Dr. Chiang explains. A colleague of hers moved to rural Pennsylvania, where he made a high salary and had a low cost of living for 3½ years, paid off his student debt, and then relocated to an area where he wanted to live long term.
As for taxes, become familiar with tax law. Research things like, “What is considered a business expense for doctors?” says Brett Mollard, MD, a diagnostic radiologist who provides financial advice to younger physicians. “What will your estimated total tax burden be at the end of the year? Will you need to make extra payments to prevent owing a large sum of money from underpaying or to avoid tax penalties?”
Blind Spot #7
Living like a rock star on a doctor’s income. Getting caught up in trying to live the same lifestyle as your colleagues is a classic bear trap. “Sitting in the doctor’s lounge, it’s so crazy,” Dr. Soelberg says. He describes conversations like, “‘Where did you go on your trip?’ ‘What new toys are you buying?’” There’s pressure to live up to an image of what a doctor’s life is supposed to look like before you’ve sorted the basic things like paying off debt.
The Fix
Live like a resident even if you haven’t been one for years, at least until you’re in a better financial position. “You’re already used to living a life of lower means, and you’re an expert when it comes to delaying gratification,” says Dr. Mollard. “Do it a little longer.” Live frugally and spend only on things that bring you joy. “A lot of physicians are trying to be really rich in all areas of their life instead of the ones that actually matter to them,” Dr. Soelberg says. Identify what’s important to you and only splurge on that.
Blind Spot #8
Never asking for help. The right financial planner can provide expert help. Emphasis on right. “Doctors can be very trusting of other professionals, even when they should not be,” says Dr. Dahle. He notes that in financial services, many people masquerade as knowledgeable advisors who are really just salespeople. While legitimate financial advisors strive to make their clients money, they are also ultimately out to line their pockets and love to work with physician salaries. Thus, doctors can end up working with financial planners that don’t specifically understand their situations or end up taking too much from their clients.
The Fix
Find a planner who specializes in, or at least understands, physicians. Ask them how they make money, says Dr. Chiang. If someone hesitates to tell you about their fee structure or if it sounds like a lot, shop around and ask colleagues for recommendations.
“Ultimately, the path to wealth is to create and grow the margin between what you make and what you spend,” says Dr. Frey. Throw some investing into the mix and physicians can set themselves up on a path for a stress-free financial life.
A version of this article appeared on Medscape.com.
The average physician makes $352,000, and some earn well into the $500,000s. So, doctors don’t have to worry about money, right?
You know the answer to that.
One thing all physicians have in common about money, says James M. Dahle, MD, FACEP, founder of The White Coat Investor, is that they don’t receive any training in business, personal finance, or investing throughout their schooling or careers unless they seek it out. This leaves many unprepared to make the best investing and money-saving decisions, while others get too frustrated about their lack of knowledge to even dip their toe into the investing pool.
Exhibit A: Four out of 10 physicians have a net worth below $1 million, according to the Medscape Physician Wealth & Debt Report 2023. Elizabeth Chiang, MD, PhD, an oculoplastic surgeon and a physician money coach at Grow Your Wealthy Mindset, notes that many of those doctors are over age 65, “which means they essentially can’t retire.”
And that’s just one pain point.
Physicians have money concerns specific to their profession and background. Luckily, some fellow doctors also serve as financial and wealth advisors just for other doctors.
Blind Spot #1
The early lean years skew doctors’ money outlook. “We have an extended training period, which commonly consists of taking on a large amount of debt, followed by 3 to 8 years of being paid a modest salary, and then finally a large boost in income,” explains Dr. Chiang. This can lay a shaky foundation for the earning years to come, and as a result, a lot of doctors just don’t think about money in healthy ways. Once their incomes increase, physicians may be surprised, for example, that making a multiple six-figure salary means paying six figures in taxes.
The Fix
Treat financial health like physical health. That means money cannot be a taboo subject. “The misguided mindset is that we didn’t become physicians to make money, we did it to help people,” explains Jordan Frey, MD, creator of the blog, The Prudent Plastic Surgeon.
Dr. Frey acknowledges that the desire to help is certainly true. But the result is a false idea that “to think about our personal finances makes us a worse doctor.”
Blind Spot #2
Because doctors know a lot about one thing (medicine), they might assume they know a lot about everything (such as investing). “Totally different fields with a different language and different way to think about it,” Dahle explains. This overconfidence could lead to some negligent or risky financial decisions.
The Fix
Educate yourself. There are several books on personal finance and investing written by physicians for physicians. Dr. Chiang recommends The Physician Philosopher’s Guide to Personal Finance, by James Turner, MD; Financial Freedom Rx, by Chirag Shah, MD, and Jayanth Sridhar, MD; and The Physician’s Guide to Finance, by Nicholas Christian and Amanda Christian, MD. There are also podcasts, blogs, and courses to help educate doctors on finance, such as the Fire Your Financial Advisor course by The White Coat Investor.
Blind Spot #3
Undersaving. Retirement saving is one thing, but 24% of doctors say they don’t even put money away in a taxable savings account, according to the Wealth & Debt Report.
Cobin Soelberg, MD, JD, a board-certified anesthesiologist and founder and principal advisor with Greeley Wealth Management, is the treasurer of his anesthesiology group. “I get to see every month how much people are saving, and even on an anesthesiologist salary, where everyone’s making about $400,000 a year, a lot of people are not saving anything, which is crazy.”
Undersaving can be both a time issue and a mindset one.
Time: Doctors often start investing in their retirement accounts later than the average professional, says Dr. Chiang. “A lot of physicians will max out their 401k or 403b,” she explains. “But if you’re putting in $20,000 a year and only starting when you’re in your early 30s, that’s not enough to get you to retirement.”
Mindset: Doctors also see people of all ages who are sick, dying, and injured. “They all know someone who worked hard and saved and then dropped dead at 55,” explains Dr. Dahle. This, he says, can lead to a bit of a “you only live once” attitude that prioritizes spending over saving.
The Fix
Shoot for 20%. If you can’t save 20% of your gross now, strive to get to that point. Think of it as telling a patient they have to change their behavior or trouble will come - not if, but when. “Develop a written investing plan and then stick with it through thick and thin,” says Dr. Dahle. “Once you have a reasonable plan, all you have to do is fund it adequately by saving 20% of your gross income, and a doctor will easily retire as a multimillionaire.”
Blind Spot #4
Bad investment strategies. Thirty-six percent of doctors experience their largest financial losses from lousy investments, according to the Wealth & Debt Report. Meanwhile, 17% of PCPs and 12% of specialists say they haven’t made any investments at all. That’s a terrible mix of doing the wrong thing and doing a worse thing.
The Fix
Don’t overthink investing, but don’t underthink it either. “As high-income earners, doctors just don’t need to take this high level of risk to reach their financial goals,” Dr. Frey says. A good investment plan doesn’t require you to time the stock market or predict individual stock winners. Consider what Vanguard founder Jack Bogle once said about investing: “Be bored by the process but elated by the outcome.”
Dr. Frey suggests going super-simple: index funds. Ignore investing strategies with actively managed mutual funds or individual stocks, as well as risky alternative investments such as cryptocurrency and angel investments. Everyone assumes doctors have money to burn, and they will push sketchy investment ideas at them. Avoid.
Blind Spot #5
Not taking debt seriously enough. The average medical student debt is $250,000 and can exceed $500,000, says Dr. Soelberg. Many doctors spend the first 10 to 20 years of their careers paying this off. Today’s graduates are paying more than 7% on their loans.
And it’s not just student debt: 39% of physicians carry five or more credit cards, and 34% have mortgages larger than $300,000 (with half of those are more than than $500K), per the Wealth & Debt Report.
The Fix
Treat debt like cancer. It’s a lethal enemy you can’t get rid of right away, but a steady, aggressive, long-term attack will have the best results. Dr. Soelberg suggests allocating the most you can afford per month, whether that’s $1000 or $5000, toward debt. Raise the amount as your income grows. Do the same with your 401k or retirement plan. Whatever is left, you can spend. Five to 10 years later, you will realize, “Wow. I’m debt free.”
Blind Spot #6
Not putting in the work to improve your situation. Seventy-one percent of doctors admit they haven’t done anything to reduce major expenses, according to the Wealth & Debt Report. Are you leaving major money on the table?
The Fix
Audit yourself in major areas like housing and taxes. While the average professional may need to put 10% to 20% down on a home, physicians can qualify for physician mortgage loans and can often put down 3% or less, says Dr. Chiang. If you can afford the higher mortgage payment, excess savings earmarked for a larger down payment can be put toward debt or invested.
Another trick, if you’re able, is to seek an area that is less in demand at a higher salary. “Physicians in places like New York City or San Francisco tend to make less than physicians in the Midwest or the South,” Dr. Chiang explains. A colleague of hers moved to rural Pennsylvania, where he made a high salary and had a low cost of living for 3½ years, paid off his student debt, and then relocated to an area where he wanted to live long term.
As for taxes, become familiar with tax law. Research things like, “What is considered a business expense for doctors?” says Brett Mollard, MD, a diagnostic radiologist who provides financial advice to younger physicians. “What will your estimated total tax burden be at the end of the year? Will you need to make extra payments to prevent owing a large sum of money from underpaying or to avoid tax penalties?”
Blind Spot #7
Living like a rock star on a doctor’s income. Getting caught up in trying to live the same lifestyle as your colleagues is a classic bear trap. “Sitting in the doctor’s lounge, it’s so crazy,” Dr. Soelberg says. He describes conversations like, “‘Where did you go on your trip?’ ‘What new toys are you buying?’” There’s pressure to live up to an image of what a doctor’s life is supposed to look like before you’ve sorted the basic things like paying off debt.
The Fix
Live like a resident even if you haven’t been one for years, at least until you’re in a better financial position. “You’re already used to living a life of lower means, and you’re an expert when it comes to delaying gratification,” says Dr. Mollard. “Do it a little longer.” Live frugally and spend only on things that bring you joy. “A lot of physicians are trying to be really rich in all areas of their life instead of the ones that actually matter to them,” Dr. Soelberg says. Identify what’s important to you and only splurge on that.
Blind Spot #8
Never asking for help. The right financial planner can provide expert help. Emphasis on right. “Doctors can be very trusting of other professionals, even when they should not be,” says Dr. Dahle. He notes that in financial services, many people masquerade as knowledgeable advisors who are really just salespeople. While legitimate financial advisors strive to make their clients money, they are also ultimately out to line their pockets and love to work with physician salaries. Thus, doctors can end up working with financial planners that don’t specifically understand their situations or end up taking too much from their clients.
The Fix
Find a planner who specializes in, or at least understands, physicians. Ask them how they make money, says Dr. Chiang. If someone hesitates to tell you about their fee structure or if it sounds like a lot, shop around and ask colleagues for recommendations.
“Ultimately, the path to wealth is to create and grow the margin between what you make and what you spend,” says Dr. Frey. Throw some investing into the mix and physicians can set themselves up on a path for a stress-free financial life.
A version of this article appeared on Medscape.com.
Autoimmune Skin Diseases Linked To Risk Of Adverse Pregnancy Outcomes
SAN DIEGO — , results from a large case-control study suggest.
Patients with systemic autoimmune conditions are known to have an increased risk for adverse pregnancy outcomes, “but we weren’t sure if that was the case for patients with autoimmune skin conditions,” presenting study author Heejo Keum, a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas, said in an interview during a poster session at the American College of Rheumatology (ACR) 2023 annual meeting. “There are case reports or nationwide population-based studies on patients with alopecia areata and vitiligo, but those were outside of the US, so we wanted to see if these outcomes could be studied in a larger population-based study in the US.”
Drawing from the TriNetX US Collaborative Network, a database of electronic medical records of 94 million patients in the United States, the researchers identified pregnant patients aged 15-44 years between January 1, 2016, and December 31, 2021. Cases were defined as patients diagnosed with at least one autoimmune skin disease (ASD) prior to the end of pregnancy, including alopecia areata, bullous pemphigoid, cicatricial pemphigoid, dermatitis herpetiformis, cutaneous lupus erythematosus, epidermolysis bullosa acquisita, morphea, pemphigus foliaceus, pemphigus vulgaris, vitiligo, and amyopathic DM. There were two control groups: healthy controls (those without ASDs, systemic lupus erythematosus or rheumatoid arthritis) and disease controls (those with SLE or RA). The researchers used ICD-10 codes to identify pregnancy endpoints, including live births, spontaneous abortion, and stillbirth. Patients with a history of hidradenitis suppurative were excluded from the analysis, as were those with common autoimmune disease such as Hashimoto’s thyroiditis, Grave’s disease, and type 1 diabetes.
The primary outcomes were adverse pregnancy outcomes defined as spontaneous abortion, gestational hypertension, preeclampsia/eclampsia, gestational diabetes, intrauterine growth restriction (IUGR), preterm premature rupture of membranes (PPROM), and preterm birth. The researchers used 1:1 propensity scoring to match patients with ASDs to controls by age, race, ethnicity, comorbidities, obesity, and substance use, and used odds ratio (OR) analysis with a 95% confidence interval (CI) to calculate each outcome.
Ms. Keum reported results from 3,654 women with ASDs, 3,654 healthy controls, 2,147 women with SLE, and 889 women with RA.
The three most common ASDs were vitiligo (30%), alopecia areata (30%), and cutaneous lupus erythematosus (27%). Compared with healthy controls, patients with ASDs were more likely to have spontaneous abortions (OR=1.5 [1.4-1.7], P<.001), and preeclampsia/eclampsia (OR=1.2 [1.0-1.3], P=.04). Compared with women with SLE, women with ASDs were less likely to have preeclampsia/eclampsia (OR=0.7 [0.6-0.9, P=.001); preterm birth (OR= 0.5 [0.4-0.7], P<.001); PPROM (OR=0.6 [0.4-0.9], P=.004), or an infant with IUGR (OR=0.6 [0.5-0.8], P<.001), but they were more likely to have a spontaneous abortion (OR=1.2 [1.1-1.3], P=.003). Overall, patients with ASDs had similar risks for adverse pregnancy outcomes as patients with RA.
“We found that patients with cutaneous lupus and vitiligo had higher rates of spontaneous abortion, which is interesting because we didn’t expect that,” Ms. Keum told this news organization. “Studies have shown that vitiligo patients might have an increased risk of pregnancy loss, so I think it’s important to have that discussion with those patients. It might benefit them to talk to a maternal-fetal medicine specialist. As for next steps, we want to look at how medication use and disease flare or disease severity play a role in APOs.”
In their poster, the researchers acknowledged limitations of the study, including the inability to verify diagnoses or assess disease severity. Also, while medication use and concomitant antiphospholipid syndrome were evaluated as risk factors for advanced pregnancy outcomes, the number of patients per group was too small for analysis.
Karl Saardi, MD, director of the inpatient dermatology service at George Washington University Hospital, Washington, who was asked to comment on the study, said that in his view, the choice of disease states included in the analysis “is a bit arbitrary.” He added that “it would have been more helpful to compare controls versus discoid lupus versus systemic lupus or controls versus amyopathic dermatomyositis versus dermatomyositis with myopathy.”
The study received funding support from the Rheumatology Research Foundation and the UT Southwestern Dean’s Research Scholar program. Neither Ms. Keum nor Dr. Saardi reported having relevant disclosures.
SAN DIEGO — , results from a large case-control study suggest.
Patients with systemic autoimmune conditions are known to have an increased risk for adverse pregnancy outcomes, “but we weren’t sure if that was the case for patients with autoimmune skin conditions,” presenting study author Heejo Keum, a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas, said in an interview during a poster session at the American College of Rheumatology (ACR) 2023 annual meeting. “There are case reports or nationwide population-based studies on patients with alopecia areata and vitiligo, but those were outside of the US, so we wanted to see if these outcomes could be studied in a larger population-based study in the US.”
Drawing from the TriNetX US Collaborative Network, a database of electronic medical records of 94 million patients in the United States, the researchers identified pregnant patients aged 15-44 years between January 1, 2016, and December 31, 2021. Cases were defined as patients diagnosed with at least one autoimmune skin disease (ASD) prior to the end of pregnancy, including alopecia areata, bullous pemphigoid, cicatricial pemphigoid, dermatitis herpetiformis, cutaneous lupus erythematosus, epidermolysis bullosa acquisita, morphea, pemphigus foliaceus, pemphigus vulgaris, vitiligo, and amyopathic DM. There were two control groups: healthy controls (those without ASDs, systemic lupus erythematosus or rheumatoid arthritis) and disease controls (those with SLE or RA). The researchers used ICD-10 codes to identify pregnancy endpoints, including live births, spontaneous abortion, and stillbirth. Patients with a history of hidradenitis suppurative were excluded from the analysis, as were those with common autoimmune disease such as Hashimoto’s thyroiditis, Grave’s disease, and type 1 diabetes.
The primary outcomes were adverse pregnancy outcomes defined as spontaneous abortion, gestational hypertension, preeclampsia/eclampsia, gestational diabetes, intrauterine growth restriction (IUGR), preterm premature rupture of membranes (PPROM), and preterm birth. The researchers used 1:1 propensity scoring to match patients with ASDs to controls by age, race, ethnicity, comorbidities, obesity, and substance use, and used odds ratio (OR) analysis with a 95% confidence interval (CI) to calculate each outcome.
Ms. Keum reported results from 3,654 women with ASDs, 3,654 healthy controls, 2,147 women with SLE, and 889 women with RA.
The three most common ASDs were vitiligo (30%), alopecia areata (30%), and cutaneous lupus erythematosus (27%). Compared with healthy controls, patients with ASDs were more likely to have spontaneous abortions (OR=1.5 [1.4-1.7], P<.001), and preeclampsia/eclampsia (OR=1.2 [1.0-1.3], P=.04). Compared with women with SLE, women with ASDs were less likely to have preeclampsia/eclampsia (OR=0.7 [0.6-0.9, P=.001); preterm birth (OR= 0.5 [0.4-0.7], P<.001); PPROM (OR=0.6 [0.4-0.9], P=.004), or an infant with IUGR (OR=0.6 [0.5-0.8], P<.001), but they were more likely to have a spontaneous abortion (OR=1.2 [1.1-1.3], P=.003). Overall, patients with ASDs had similar risks for adverse pregnancy outcomes as patients with RA.
“We found that patients with cutaneous lupus and vitiligo had higher rates of spontaneous abortion, which is interesting because we didn’t expect that,” Ms. Keum told this news organization. “Studies have shown that vitiligo patients might have an increased risk of pregnancy loss, so I think it’s important to have that discussion with those patients. It might benefit them to talk to a maternal-fetal medicine specialist. As for next steps, we want to look at how medication use and disease flare or disease severity play a role in APOs.”
In their poster, the researchers acknowledged limitations of the study, including the inability to verify diagnoses or assess disease severity. Also, while medication use and concomitant antiphospholipid syndrome were evaluated as risk factors for advanced pregnancy outcomes, the number of patients per group was too small for analysis.
Karl Saardi, MD, director of the inpatient dermatology service at George Washington University Hospital, Washington, who was asked to comment on the study, said that in his view, the choice of disease states included in the analysis “is a bit arbitrary.” He added that “it would have been more helpful to compare controls versus discoid lupus versus systemic lupus or controls versus amyopathic dermatomyositis versus dermatomyositis with myopathy.”
The study received funding support from the Rheumatology Research Foundation and the UT Southwestern Dean’s Research Scholar program. Neither Ms. Keum nor Dr. Saardi reported having relevant disclosures.
SAN DIEGO — , results from a large case-control study suggest.
Patients with systemic autoimmune conditions are known to have an increased risk for adverse pregnancy outcomes, “but we weren’t sure if that was the case for patients with autoimmune skin conditions,” presenting study author Heejo Keum, a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas, said in an interview during a poster session at the American College of Rheumatology (ACR) 2023 annual meeting. “There are case reports or nationwide population-based studies on patients with alopecia areata and vitiligo, but those were outside of the US, so we wanted to see if these outcomes could be studied in a larger population-based study in the US.”
Drawing from the TriNetX US Collaborative Network, a database of electronic medical records of 94 million patients in the United States, the researchers identified pregnant patients aged 15-44 years between January 1, 2016, and December 31, 2021. Cases were defined as patients diagnosed with at least one autoimmune skin disease (ASD) prior to the end of pregnancy, including alopecia areata, bullous pemphigoid, cicatricial pemphigoid, dermatitis herpetiformis, cutaneous lupus erythematosus, epidermolysis bullosa acquisita, morphea, pemphigus foliaceus, pemphigus vulgaris, vitiligo, and amyopathic DM. There were two control groups: healthy controls (those without ASDs, systemic lupus erythematosus or rheumatoid arthritis) and disease controls (those with SLE or RA). The researchers used ICD-10 codes to identify pregnancy endpoints, including live births, spontaneous abortion, and stillbirth. Patients with a history of hidradenitis suppurative were excluded from the analysis, as were those with common autoimmune disease such as Hashimoto’s thyroiditis, Grave’s disease, and type 1 diabetes.
The primary outcomes were adverse pregnancy outcomes defined as spontaneous abortion, gestational hypertension, preeclampsia/eclampsia, gestational diabetes, intrauterine growth restriction (IUGR), preterm premature rupture of membranes (PPROM), and preterm birth. The researchers used 1:1 propensity scoring to match patients with ASDs to controls by age, race, ethnicity, comorbidities, obesity, and substance use, and used odds ratio (OR) analysis with a 95% confidence interval (CI) to calculate each outcome.
Ms. Keum reported results from 3,654 women with ASDs, 3,654 healthy controls, 2,147 women with SLE, and 889 women with RA.
The three most common ASDs were vitiligo (30%), alopecia areata (30%), and cutaneous lupus erythematosus (27%). Compared with healthy controls, patients with ASDs were more likely to have spontaneous abortions (OR=1.5 [1.4-1.7], P<.001), and preeclampsia/eclampsia (OR=1.2 [1.0-1.3], P=.04). Compared with women with SLE, women with ASDs were less likely to have preeclampsia/eclampsia (OR=0.7 [0.6-0.9, P=.001); preterm birth (OR= 0.5 [0.4-0.7], P<.001); PPROM (OR=0.6 [0.4-0.9], P=.004), or an infant with IUGR (OR=0.6 [0.5-0.8], P<.001), but they were more likely to have a spontaneous abortion (OR=1.2 [1.1-1.3], P=.003). Overall, patients with ASDs had similar risks for adverse pregnancy outcomes as patients with RA.
“We found that patients with cutaneous lupus and vitiligo had higher rates of spontaneous abortion, which is interesting because we didn’t expect that,” Ms. Keum told this news organization. “Studies have shown that vitiligo patients might have an increased risk of pregnancy loss, so I think it’s important to have that discussion with those patients. It might benefit them to talk to a maternal-fetal medicine specialist. As for next steps, we want to look at how medication use and disease flare or disease severity play a role in APOs.”
In their poster, the researchers acknowledged limitations of the study, including the inability to verify diagnoses or assess disease severity. Also, while medication use and concomitant antiphospholipid syndrome were evaluated as risk factors for advanced pregnancy outcomes, the number of patients per group was too small for analysis.
Karl Saardi, MD, director of the inpatient dermatology service at George Washington University Hospital, Washington, who was asked to comment on the study, said that in his view, the choice of disease states included in the analysis “is a bit arbitrary.” He added that “it would have been more helpful to compare controls versus discoid lupus versus systemic lupus or controls versus amyopathic dermatomyositis versus dermatomyositis with myopathy.”
The study received funding support from the Rheumatology Research Foundation and the UT Southwestern Dean’s Research Scholar program. Neither Ms. Keum nor Dr. Saardi reported having relevant disclosures.
FROM ACR 2023
AHA, AAP update neonatal resuscitation guidelines
The 2023 focused update was prompted by four systematic literature reviews by the International Liaison Committee on Resuscitation (ILCOR) Neonatal Life Support Task Force.
“Evidence evaluations by the ILCOR play a large role in the group’s process and timing of updates,” Henry Lee, MD, co-chair of the writing group, said in an interview.
He noted that updated recommendations do not change prior recommendations from the 2020 guidelines.
“However, they provide additional details to consider in neonatal resuscitation that could lead to changes in some practice in various settings,” said Dr. Lee, medical director of the University of California San Diego neonatal intensive care unit.
The focused update was simultaneously published online November 16 in Circulation and in Pediatrics.
Dr. Lee noted that effective positive-pressure ventilation (PPV) is the priority in newborn infants who need support after birth.
And while the 2020 update provided some details on devices to be used for PPV, the 2023 focused update gives guidance on use of T-piece resuscitators for providing PPV, which may be particularly helpful for preterm infants, and the use of supraglottic airways as a primary interface to deliver PPV, he explained.
Specifically, the updated guidelines state that use of a T-piece resuscitator to deliver PPV is preferred to the use of a self-inflating bag.
Because both T-piece resuscitators and flow-inflating bags require a compressed gas source to function, a self-inflating bag should be available as a backup in the event of compressed gas failure when using either of these devices.
Use of a supraglottic airway may be considered as the primary interface to administer PPV instead of a face mask for newborn infants delivered at 34 0/7 weeks’ gestation or later.
Continued Emphasis on Delayed Cord Clamping
The updated guidelines “continue to emphasize delayed cord clamping for both term and preterm newborn infants when clinically possible. There is also a new recommendation for nonvigorous infants born 35-42 weeks’ gestational age to consider umbilical cord milking,” Dr. Lee said in an interview.
Specifically, the guidelines state:
- For term and late preterm newborn infants ≥34 weeks’ gestation, and preterm newborn infants <34 weeks’ gestation, who do not require resuscitation, delayed cord clamping (≥30 seconds) can be beneficial compared with early cord clamping (<30 seconds).
- For term and late preterm newborn infants ≥34 weeks’ gestation who do not require resuscitation, intact cord milking is not known to be beneficial compared with delayed cord clamping (≥30 seconds).
- For preterm newborn infants between 28- and 34-weeks’ gestation who do not require resuscitation and in whom delayed cord clamping cannot be performed, intact cord milking may be reasonable.
- For preterm newborn infants <28 weeks’ gestation, intact cord milking is not recommended.
- For nonvigorous term and late preterm infants (35-42 weeks’ gestation), intact cord milking may be reasonable compared with early cord clamping (<30 seconds).
The guidelines also highlight the following knowledge gaps that require further research:
- Optimal management of the umbilical cord in term, late preterm, and preterm infants who require resuscitation at delivery
- Longer-term outcome data, such as anemia during infancy and neurodevelopmental outcomes, for all umbilical cord management strategies
- Cost-effectiveness of a T-piece resuscitator compared with a self-inflating bag
- The effect of a self-inflating bag with a positive end-expiratory pressure valve on outcomes in preterm newborn infants
- Comparison of either a T-piece resuscitator or a self-inflating bag with a flow-inflating bag for administering PPV
- Comparison of clinical outcomes by gestational age for any PPV device
- Comparison of supraglottic airway devices and face masks as the primary interface for PPV in high-resourced settings
- The amount and type of training required for successful supraglottic airway insertion and the potential for skill decay
- The utility of supraglottic airway devices for suctioning secretions from the airway
- The efficacy of a supraglottic airway during advanced neonatal resuscitation requiring chest compressions or the delivery of intratracheal medications
This research had no commercial funding. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
The 2023 focused update was prompted by four systematic literature reviews by the International Liaison Committee on Resuscitation (ILCOR) Neonatal Life Support Task Force.
“Evidence evaluations by the ILCOR play a large role in the group’s process and timing of updates,” Henry Lee, MD, co-chair of the writing group, said in an interview.
He noted that updated recommendations do not change prior recommendations from the 2020 guidelines.
“However, they provide additional details to consider in neonatal resuscitation that could lead to changes in some practice in various settings,” said Dr. Lee, medical director of the University of California San Diego neonatal intensive care unit.
The focused update was simultaneously published online November 16 in Circulation and in Pediatrics.
Dr. Lee noted that effective positive-pressure ventilation (PPV) is the priority in newborn infants who need support after birth.
And while the 2020 update provided some details on devices to be used for PPV, the 2023 focused update gives guidance on use of T-piece resuscitators for providing PPV, which may be particularly helpful for preterm infants, and the use of supraglottic airways as a primary interface to deliver PPV, he explained.
Specifically, the updated guidelines state that use of a T-piece resuscitator to deliver PPV is preferred to the use of a self-inflating bag.
Because both T-piece resuscitators and flow-inflating bags require a compressed gas source to function, a self-inflating bag should be available as a backup in the event of compressed gas failure when using either of these devices.
Use of a supraglottic airway may be considered as the primary interface to administer PPV instead of a face mask for newborn infants delivered at 34 0/7 weeks’ gestation or later.
Continued Emphasis on Delayed Cord Clamping
The updated guidelines “continue to emphasize delayed cord clamping for both term and preterm newborn infants when clinically possible. There is also a new recommendation for nonvigorous infants born 35-42 weeks’ gestational age to consider umbilical cord milking,” Dr. Lee said in an interview.
Specifically, the guidelines state:
- For term and late preterm newborn infants ≥34 weeks’ gestation, and preterm newborn infants <34 weeks’ gestation, who do not require resuscitation, delayed cord clamping (≥30 seconds) can be beneficial compared with early cord clamping (<30 seconds).
- For term and late preterm newborn infants ≥34 weeks’ gestation who do not require resuscitation, intact cord milking is not known to be beneficial compared with delayed cord clamping (≥30 seconds).
- For preterm newborn infants between 28- and 34-weeks’ gestation who do not require resuscitation and in whom delayed cord clamping cannot be performed, intact cord milking may be reasonable.
- For preterm newborn infants <28 weeks’ gestation, intact cord milking is not recommended.
- For nonvigorous term and late preterm infants (35-42 weeks’ gestation), intact cord milking may be reasonable compared with early cord clamping (<30 seconds).
The guidelines also highlight the following knowledge gaps that require further research:
- Optimal management of the umbilical cord in term, late preterm, and preterm infants who require resuscitation at delivery
- Longer-term outcome data, such as anemia during infancy and neurodevelopmental outcomes, for all umbilical cord management strategies
- Cost-effectiveness of a T-piece resuscitator compared with a self-inflating bag
- The effect of a self-inflating bag with a positive end-expiratory pressure valve on outcomes in preterm newborn infants
- Comparison of either a T-piece resuscitator or a self-inflating bag with a flow-inflating bag for administering PPV
- Comparison of clinical outcomes by gestational age for any PPV device
- Comparison of supraglottic airway devices and face masks as the primary interface for PPV in high-resourced settings
- The amount and type of training required for successful supraglottic airway insertion and the potential for skill decay
- The utility of supraglottic airway devices for suctioning secretions from the airway
- The efficacy of a supraglottic airway during advanced neonatal resuscitation requiring chest compressions or the delivery of intratracheal medications
This research had no commercial funding. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
The 2023 focused update was prompted by four systematic literature reviews by the International Liaison Committee on Resuscitation (ILCOR) Neonatal Life Support Task Force.
“Evidence evaluations by the ILCOR play a large role in the group’s process and timing of updates,” Henry Lee, MD, co-chair of the writing group, said in an interview.
He noted that updated recommendations do not change prior recommendations from the 2020 guidelines.
“However, they provide additional details to consider in neonatal resuscitation that could lead to changes in some practice in various settings,” said Dr. Lee, medical director of the University of California San Diego neonatal intensive care unit.
The focused update was simultaneously published online November 16 in Circulation and in Pediatrics.
Dr. Lee noted that effective positive-pressure ventilation (PPV) is the priority in newborn infants who need support after birth.
And while the 2020 update provided some details on devices to be used for PPV, the 2023 focused update gives guidance on use of T-piece resuscitators for providing PPV, which may be particularly helpful for preterm infants, and the use of supraglottic airways as a primary interface to deliver PPV, he explained.
Specifically, the updated guidelines state that use of a T-piece resuscitator to deliver PPV is preferred to the use of a self-inflating bag.
Because both T-piece resuscitators and flow-inflating bags require a compressed gas source to function, a self-inflating bag should be available as a backup in the event of compressed gas failure when using either of these devices.
Use of a supraglottic airway may be considered as the primary interface to administer PPV instead of a face mask for newborn infants delivered at 34 0/7 weeks’ gestation or later.
Continued Emphasis on Delayed Cord Clamping
The updated guidelines “continue to emphasize delayed cord clamping for both term and preterm newborn infants when clinically possible. There is also a new recommendation for nonvigorous infants born 35-42 weeks’ gestational age to consider umbilical cord milking,” Dr. Lee said in an interview.
Specifically, the guidelines state:
- For term and late preterm newborn infants ≥34 weeks’ gestation, and preterm newborn infants <34 weeks’ gestation, who do not require resuscitation, delayed cord clamping (≥30 seconds) can be beneficial compared with early cord clamping (<30 seconds).
- For term and late preterm newborn infants ≥34 weeks’ gestation who do not require resuscitation, intact cord milking is not known to be beneficial compared with delayed cord clamping (≥30 seconds).
- For preterm newborn infants between 28- and 34-weeks’ gestation who do not require resuscitation and in whom delayed cord clamping cannot be performed, intact cord milking may be reasonable.
- For preterm newborn infants <28 weeks’ gestation, intact cord milking is not recommended.
- For nonvigorous term and late preterm infants (35-42 weeks’ gestation), intact cord milking may be reasonable compared with early cord clamping (<30 seconds).
The guidelines also highlight the following knowledge gaps that require further research:
- Optimal management of the umbilical cord in term, late preterm, and preterm infants who require resuscitation at delivery
- Longer-term outcome data, such as anemia during infancy and neurodevelopmental outcomes, for all umbilical cord management strategies
- Cost-effectiveness of a T-piece resuscitator compared with a self-inflating bag
- The effect of a self-inflating bag with a positive end-expiratory pressure valve on outcomes in preterm newborn infants
- Comparison of either a T-piece resuscitator or a self-inflating bag with a flow-inflating bag for administering PPV
- Comparison of clinical outcomes by gestational age for any PPV device
- Comparison of supraglottic airway devices and face masks as the primary interface for PPV in high-resourced settings
- The amount and type of training required for successful supraglottic airway insertion and the potential for skill decay
- The utility of supraglottic airway devices for suctioning secretions from the airway
- The efficacy of a supraglottic airway during advanced neonatal resuscitation requiring chest compressions or the delivery of intratracheal medications
This research had no commercial funding. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
New tests may finally diagnose long COVID
One of the biggest challenges facing clinicians who treat long COVID is a lack of consensus when it comes to recognizing and diagnosing the condition. But
Effective diagnostic testing would be a game-changer in the long COVID fight, for it’s not just the fatigue, brain fog, heart palpitations, and other persistent symptoms that affect patients. Two out of three people with long COVID also suffer mental health challenges like depression and anxiety. Some patients say their symptoms are not taken seriously by their doctors. And as many as 12% of long COVID patients are unemployed because of the severity of their illness and their employers may be skeptical of their condition.
Quick, accurate diagnosis would eliminate all that. Now a new preprint study suggests that the elevation of certain immune system proteins are a commonality in long COVID patients and identifying them may be an accurate way to diagnose the condition.
Researchers at Cardiff (Wales) University, tracked 166 patients, 79 of whom had been diagnosed with long COVID and 87 who had not. All participants had recovered from a severe bout of acute COVID-19.
In an analysis of the blood plasma of the study participants, researchers found elevated levels of certain components. Four proteins in particular – Ba, iC3b, C5a, and TCC – predicted the presence of long COVID with 78.5% accuracy.
“I was gobsmacked by the results. We’re seeing a massive dysregulation in those four biomarkers,” says study author Wioleta Zelek, PhD, a research fellow at Cardiff University. “It’s a combination that we showed was predictive of long COVID.”
The study revealed that long COVID was associated with inflammation of the immune system causing these complement proteins to remain dysregulated. Proteins like C3, C4, and C5 are important parts of the immune system because they recruit phagocytes, cells that attack and engulf bacteria and viruses at the site of infection to destroy pathogens like SARS-coV-2.
In the case of long COVID, these proteins remain chronically elevated. While the symptoms of long COVID have seemed largely unrelated to one another, researchers point to elevated inflammation as a connecting factor that causes various systems in the body to go haywire.
“Anything that could help to better diagnose patients with long COVID is research we’re greatly appreciative of within the clinical community,” said Nisha Viswanathan, MD, director of the University of California, Los Angeles, Long COVID program at UCLA Health.
Testing for biomarkers highlighted in the study, as well as others like serotonin and cortisol, may help doctors separate patients who have long COVID from patients who have similar symptoms caused by other conditions, said Dr. Viswanathan. For example, a recent study published in the journal Cell found lower serotonin levels in long COVID patients, compared with patients who were diagnosed with acute COVID-19 but recovered from the condition.
Dr. Viswanathan cautions that the biomarker test does not answer all the questions about diagnosing long COVID. For example, Dr. Viswanathan said scientists don’t know whether complement dysregulation is caused by long COVID and not another underlying medical issue that patients had prior to infection, because “we don’t know where patients’ levels were prior to developing long COVID.” For example, those with autoimmune issues are more likely to develop long COVID, which means their levels could have been elevated prior to a COVID infection.
It is increasingly likely, said Dr. Viswanathan, that long COVID is an umbrella term for a host of conditions that could be caused by different impacts of the virus. Other research has pointed to the different phenotypes of long COVID. For example, some are focused on cardiopulmonary issues and others on fatigue and gastrointestinal problems.
“It looks like these different phenotypes have a different mechanism for disease,” she said. This means that it’s less likely to be a one-size-fits-all condition and the next step in the research should be identifying which biomarker is aligned with which phenotype of the disease.
Better diagnostics will open the door to better treatments, Dr. Zelek said. The more doctors understand about the mechanism causing immune dysregulation in long COVID patients, the more they can treat it with existing medications. Dr. Zelek’s lab has been studying certain medications like pegcetacoplan (C3 blocker), danicopan (anti-factor D), and iptacopan (anti-factor B) that can be used to break the body’s cycle of inflammation and reduce symptoms experienced in those with long COVID.
These drugs are approved by the U.S. Food and Drug Administration for the treatment of a rare blood disease called paroxysmal nocturnal hemoglobinuria. The C5 inhibitor zilucoplan has also been used in patients hospitalized with COVID-19 and researchers have found that the drug lowered serum C5 and interleukin-8 concentration in the blood, seeming to reduce certain aspects of the immune system’s inflammatory response to the virus.
The Cardiff University research is one of the most detailed studies to highlight long COVID biomarkers to date, said infectious disease specialist Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, Ohio. The research needs to be duplicated in a larger study population that might include the other biomarkers like serotonin and cortisol to see if they’re related, she said.
Researchers are learning more everyday about the various biomarkers that may be linked to long COVID, she added. This Cardiff study showed that a huge percentage of those patients had elevated levels of certain complements. The next step, said Dr. McComsey, “is to put all these puzzle pieces together” so that clinicians have a common diagnostic tool or tools that provide patients with some peace of mind in starting their road to recovery.
A version of this article first appeared on Medscape.com.
One of the biggest challenges facing clinicians who treat long COVID is a lack of consensus when it comes to recognizing and diagnosing the condition. But
Effective diagnostic testing would be a game-changer in the long COVID fight, for it’s not just the fatigue, brain fog, heart palpitations, and other persistent symptoms that affect patients. Two out of three people with long COVID also suffer mental health challenges like depression and anxiety. Some patients say their symptoms are not taken seriously by their doctors. And as many as 12% of long COVID patients are unemployed because of the severity of their illness and their employers may be skeptical of their condition.
Quick, accurate diagnosis would eliminate all that. Now a new preprint study suggests that the elevation of certain immune system proteins are a commonality in long COVID patients and identifying them may be an accurate way to diagnose the condition.
Researchers at Cardiff (Wales) University, tracked 166 patients, 79 of whom had been diagnosed with long COVID and 87 who had not. All participants had recovered from a severe bout of acute COVID-19.
In an analysis of the blood plasma of the study participants, researchers found elevated levels of certain components. Four proteins in particular – Ba, iC3b, C5a, and TCC – predicted the presence of long COVID with 78.5% accuracy.
“I was gobsmacked by the results. We’re seeing a massive dysregulation in those four biomarkers,” says study author Wioleta Zelek, PhD, a research fellow at Cardiff University. “It’s a combination that we showed was predictive of long COVID.”
The study revealed that long COVID was associated with inflammation of the immune system causing these complement proteins to remain dysregulated. Proteins like C3, C4, and C5 are important parts of the immune system because they recruit phagocytes, cells that attack and engulf bacteria and viruses at the site of infection to destroy pathogens like SARS-coV-2.
In the case of long COVID, these proteins remain chronically elevated. While the symptoms of long COVID have seemed largely unrelated to one another, researchers point to elevated inflammation as a connecting factor that causes various systems in the body to go haywire.
“Anything that could help to better diagnose patients with long COVID is research we’re greatly appreciative of within the clinical community,” said Nisha Viswanathan, MD, director of the University of California, Los Angeles, Long COVID program at UCLA Health.
Testing for biomarkers highlighted in the study, as well as others like serotonin and cortisol, may help doctors separate patients who have long COVID from patients who have similar symptoms caused by other conditions, said Dr. Viswanathan. For example, a recent study published in the journal Cell found lower serotonin levels in long COVID patients, compared with patients who were diagnosed with acute COVID-19 but recovered from the condition.
Dr. Viswanathan cautions that the biomarker test does not answer all the questions about diagnosing long COVID. For example, Dr. Viswanathan said scientists don’t know whether complement dysregulation is caused by long COVID and not another underlying medical issue that patients had prior to infection, because “we don’t know where patients’ levels were prior to developing long COVID.” For example, those with autoimmune issues are more likely to develop long COVID, which means their levels could have been elevated prior to a COVID infection.
It is increasingly likely, said Dr. Viswanathan, that long COVID is an umbrella term for a host of conditions that could be caused by different impacts of the virus. Other research has pointed to the different phenotypes of long COVID. For example, some are focused on cardiopulmonary issues and others on fatigue and gastrointestinal problems.
“It looks like these different phenotypes have a different mechanism for disease,” she said. This means that it’s less likely to be a one-size-fits-all condition and the next step in the research should be identifying which biomarker is aligned with which phenotype of the disease.
Better diagnostics will open the door to better treatments, Dr. Zelek said. The more doctors understand about the mechanism causing immune dysregulation in long COVID patients, the more they can treat it with existing medications. Dr. Zelek’s lab has been studying certain medications like pegcetacoplan (C3 blocker), danicopan (anti-factor D), and iptacopan (anti-factor B) that can be used to break the body’s cycle of inflammation and reduce symptoms experienced in those with long COVID.
These drugs are approved by the U.S. Food and Drug Administration for the treatment of a rare blood disease called paroxysmal nocturnal hemoglobinuria. The C5 inhibitor zilucoplan has also been used in patients hospitalized with COVID-19 and researchers have found that the drug lowered serum C5 and interleukin-8 concentration in the blood, seeming to reduce certain aspects of the immune system’s inflammatory response to the virus.
The Cardiff University research is one of the most detailed studies to highlight long COVID biomarkers to date, said infectious disease specialist Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, Ohio. The research needs to be duplicated in a larger study population that might include the other biomarkers like serotonin and cortisol to see if they’re related, she said.
Researchers are learning more everyday about the various biomarkers that may be linked to long COVID, she added. This Cardiff study showed that a huge percentage of those patients had elevated levels of certain complements. The next step, said Dr. McComsey, “is to put all these puzzle pieces together” so that clinicians have a common diagnostic tool or tools that provide patients with some peace of mind in starting their road to recovery.
A version of this article first appeared on Medscape.com.
One of the biggest challenges facing clinicians who treat long COVID is a lack of consensus when it comes to recognizing and diagnosing the condition. But
Effective diagnostic testing would be a game-changer in the long COVID fight, for it’s not just the fatigue, brain fog, heart palpitations, and other persistent symptoms that affect patients. Two out of three people with long COVID also suffer mental health challenges like depression and anxiety. Some patients say their symptoms are not taken seriously by their doctors. And as many as 12% of long COVID patients are unemployed because of the severity of their illness and their employers may be skeptical of their condition.
Quick, accurate diagnosis would eliminate all that. Now a new preprint study suggests that the elevation of certain immune system proteins are a commonality in long COVID patients and identifying them may be an accurate way to diagnose the condition.
Researchers at Cardiff (Wales) University, tracked 166 patients, 79 of whom had been diagnosed with long COVID and 87 who had not. All participants had recovered from a severe bout of acute COVID-19.
In an analysis of the blood plasma of the study participants, researchers found elevated levels of certain components. Four proteins in particular – Ba, iC3b, C5a, and TCC – predicted the presence of long COVID with 78.5% accuracy.
“I was gobsmacked by the results. We’re seeing a massive dysregulation in those four biomarkers,” says study author Wioleta Zelek, PhD, a research fellow at Cardiff University. “It’s a combination that we showed was predictive of long COVID.”
The study revealed that long COVID was associated with inflammation of the immune system causing these complement proteins to remain dysregulated. Proteins like C3, C4, and C5 are important parts of the immune system because they recruit phagocytes, cells that attack and engulf bacteria and viruses at the site of infection to destroy pathogens like SARS-coV-2.
In the case of long COVID, these proteins remain chronically elevated. While the symptoms of long COVID have seemed largely unrelated to one another, researchers point to elevated inflammation as a connecting factor that causes various systems in the body to go haywire.
“Anything that could help to better diagnose patients with long COVID is research we’re greatly appreciative of within the clinical community,” said Nisha Viswanathan, MD, director of the University of California, Los Angeles, Long COVID program at UCLA Health.
Testing for biomarkers highlighted in the study, as well as others like serotonin and cortisol, may help doctors separate patients who have long COVID from patients who have similar symptoms caused by other conditions, said Dr. Viswanathan. For example, a recent study published in the journal Cell found lower serotonin levels in long COVID patients, compared with patients who were diagnosed with acute COVID-19 but recovered from the condition.
Dr. Viswanathan cautions that the biomarker test does not answer all the questions about diagnosing long COVID. For example, Dr. Viswanathan said scientists don’t know whether complement dysregulation is caused by long COVID and not another underlying medical issue that patients had prior to infection, because “we don’t know where patients’ levels were prior to developing long COVID.” For example, those with autoimmune issues are more likely to develop long COVID, which means their levels could have been elevated prior to a COVID infection.
It is increasingly likely, said Dr. Viswanathan, that long COVID is an umbrella term for a host of conditions that could be caused by different impacts of the virus. Other research has pointed to the different phenotypes of long COVID. For example, some are focused on cardiopulmonary issues and others on fatigue and gastrointestinal problems.
“It looks like these different phenotypes have a different mechanism for disease,” she said. This means that it’s less likely to be a one-size-fits-all condition and the next step in the research should be identifying which biomarker is aligned with which phenotype of the disease.
Better diagnostics will open the door to better treatments, Dr. Zelek said. The more doctors understand about the mechanism causing immune dysregulation in long COVID patients, the more they can treat it with existing medications. Dr. Zelek’s lab has been studying certain medications like pegcetacoplan (C3 blocker), danicopan (anti-factor D), and iptacopan (anti-factor B) that can be used to break the body’s cycle of inflammation and reduce symptoms experienced in those with long COVID.
These drugs are approved by the U.S. Food and Drug Administration for the treatment of a rare blood disease called paroxysmal nocturnal hemoglobinuria. The C5 inhibitor zilucoplan has also been used in patients hospitalized with COVID-19 and researchers have found that the drug lowered serum C5 and interleukin-8 concentration in the blood, seeming to reduce certain aspects of the immune system’s inflammatory response to the virus.
The Cardiff University research is one of the most detailed studies to highlight long COVID biomarkers to date, said infectious disease specialist Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, Ohio. The research needs to be duplicated in a larger study population that might include the other biomarkers like serotonin and cortisol to see if they’re related, she said.
Researchers are learning more everyday about the various biomarkers that may be linked to long COVID, she added. This Cardiff study showed that a huge percentage of those patients had elevated levels of certain complements. The next step, said Dr. McComsey, “is to put all these puzzle pieces together” so that clinicians have a common diagnostic tool or tools that provide patients with some peace of mind in starting their road to recovery.
A version of this article first appeared on Medscape.com.
FROM MEDRXIV
Underdiagnosed: Iron deficiency anemia during pregnancy
Jerome J. Federspiel, MD, often cares for patients who are about to deliver a baby but who have untreated iron deficiency anemia (IDA). Often, these patients require a blood transfusion after giving birth.
“I am sad to hear commonly from patients we treat that they have had iron-deficient anemia symptoms for many years. Correcting these conditions makes birth safer and, oftentimes, makes people feel much better – sometimes better than they have in years,” Dr. Federspiel, maternal-fetal medicine physician and assistant professor of obstetrics and gynecology and population health sciences at Duke University, Durham, N.C., said.
Even patients he is able to diagnose earlier “will have difficulties catching up during pregnancy.”
The condition is the most common type of anemia among people who are pregnant. IDA increases a patient’s risk of delivering preterm and developing postpartum depression and puts their infants at a risk for perinatal mortality. Without proper treatment of IDA throughout pregnancy, the condition can also lead to low birth weights in infants or failing to meet weight goals later on.
But of all women with a new diagnosis of IDA from 2021 to 2022, 10% were pregnant, according to an analysis by Komodo Health, a health care analytics company.
While estimates of the prevalence of IDA vary, research from 2021 found 6.5% of nearly 1,500 patients who were pregnant during the first trimester had the condition, a figure the researchers said might underrepresent the problem.
“In severe cases [fetal outcomes can include] abnormal fetal oxygenation, nonreassuring fetal heart rate patterns, reduced amniotic fluid volume, fetal cerebral vasodilation, and fetal death,” Alianne S. Tilley, NP, family nurse practitioner at Women’s Care of Lake Cumberland, Somerset, Ky., said.
Research has shown that adequate levels of iron are an integral component in the development of the fetal brain. Some studies have reported that IDA during pregnancy increases an infant’s risk for poor neurodevelopmental outcomes.
Lack of screening protocol
Discrepancies in guidance for testing patients who are pregnant for IDA may add to late diagnosis and low treatment, according to Katelin Zahn, MD, assistant professor of general obstetrics, gynecology, and midwifery at University of North Carolina at Chapel Hill.
“There’s no consistency, which leads to a lot of variation in individual practice, which creates variation in outcomes, too,” Dr. Zahn said. “You can only do so much as one independent physician, and you need to be able to create change in a system that functions and provides standard of care even when you aren’t there.”
The American College of Obstetricians and Gynecologists recommends screening all patients who are pregnant with a complete blood count in the first trimester and again between 24 and 27 weeks of gestation.
Patients who meet criteria for IDA based on hematocrit levels less than 33% in the first and third trimesters, and less than 32% in the second trimester, should be evaluated to determine the cause. Those with IDA should be treated with supplemental iron, in addition to prenatal vitamins, ACOG says.
But the U.S. Preventive Services Task Force in 2015 found insufficient evidence to recommend for or against screening patients without symptoms or signs of the condition. The organization is in the process of updating the recommendation.
Prevention as best practice
The most effective way to address IDA in patients who are pregnant is prevention, according to Dr. Federspiel.
“Having a systematic approach to screening and treatment is really important, and this starts before pregnancy,” Dr. Federspiel said. “On average, a typical pregnancy requires an additional 1 g of iron.”
Dr. Federspiel recommends clinicians discuss the causes and the effects of IDA with patients who are planning to or could become pregnant. Clinicians might recommend iron- and folate-rich foods and vitamins B12 and C and ask patients if they face any barriers to access.
“Prenatal vitamins with iron are the gold standard in preventing IDA in the pregnant population,” Ms. Tilley said. “Education on the significant risk factors associated with IDA in early pregnancy is key.”
A version of this article first appeared on Medscape.com.
Jerome J. Federspiel, MD, often cares for patients who are about to deliver a baby but who have untreated iron deficiency anemia (IDA). Often, these patients require a blood transfusion after giving birth.
“I am sad to hear commonly from patients we treat that they have had iron-deficient anemia symptoms for many years. Correcting these conditions makes birth safer and, oftentimes, makes people feel much better – sometimes better than they have in years,” Dr. Federspiel, maternal-fetal medicine physician and assistant professor of obstetrics and gynecology and population health sciences at Duke University, Durham, N.C., said.
Even patients he is able to diagnose earlier “will have difficulties catching up during pregnancy.”
The condition is the most common type of anemia among people who are pregnant. IDA increases a patient’s risk of delivering preterm and developing postpartum depression and puts their infants at a risk for perinatal mortality. Without proper treatment of IDA throughout pregnancy, the condition can also lead to low birth weights in infants or failing to meet weight goals later on.
But of all women with a new diagnosis of IDA from 2021 to 2022, 10% were pregnant, according to an analysis by Komodo Health, a health care analytics company.
While estimates of the prevalence of IDA vary, research from 2021 found 6.5% of nearly 1,500 patients who were pregnant during the first trimester had the condition, a figure the researchers said might underrepresent the problem.
“In severe cases [fetal outcomes can include] abnormal fetal oxygenation, nonreassuring fetal heart rate patterns, reduced amniotic fluid volume, fetal cerebral vasodilation, and fetal death,” Alianne S. Tilley, NP, family nurse practitioner at Women’s Care of Lake Cumberland, Somerset, Ky., said.
Research has shown that adequate levels of iron are an integral component in the development of the fetal brain. Some studies have reported that IDA during pregnancy increases an infant’s risk for poor neurodevelopmental outcomes.
Lack of screening protocol
Discrepancies in guidance for testing patients who are pregnant for IDA may add to late diagnosis and low treatment, according to Katelin Zahn, MD, assistant professor of general obstetrics, gynecology, and midwifery at University of North Carolina at Chapel Hill.
“There’s no consistency, which leads to a lot of variation in individual practice, which creates variation in outcomes, too,” Dr. Zahn said. “You can only do so much as one independent physician, and you need to be able to create change in a system that functions and provides standard of care even when you aren’t there.”
The American College of Obstetricians and Gynecologists recommends screening all patients who are pregnant with a complete blood count in the first trimester and again between 24 and 27 weeks of gestation.
Patients who meet criteria for IDA based on hematocrit levels less than 33% in the first and third trimesters, and less than 32% in the second trimester, should be evaluated to determine the cause. Those with IDA should be treated with supplemental iron, in addition to prenatal vitamins, ACOG says.
But the U.S. Preventive Services Task Force in 2015 found insufficient evidence to recommend for or against screening patients without symptoms or signs of the condition. The organization is in the process of updating the recommendation.
Prevention as best practice
The most effective way to address IDA in patients who are pregnant is prevention, according to Dr. Federspiel.
“Having a systematic approach to screening and treatment is really important, and this starts before pregnancy,” Dr. Federspiel said. “On average, a typical pregnancy requires an additional 1 g of iron.”
Dr. Federspiel recommends clinicians discuss the causes and the effects of IDA with patients who are planning to or could become pregnant. Clinicians might recommend iron- and folate-rich foods and vitamins B12 and C and ask patients if they face any barriers to access.
“Prenatal vitamins with iron are the gold standard in preventing IDA in the pregnant population,” Ms. Tilley said. “Education on the significant risk factors associated with IDA in early pregnancy is key.”
A version of this article first appeared on Medscape.com.
Jerome J. Federspiel, MD, often cares for patients who are about to deliver a baby but who have untreated iron deficiency anemia (IDA). Often, these patients require a blood transfusion after giving birth.
“I am sad to hear commonly from patients we treat that they have had iron-deficient anemia symptoms for many years. Correcting these conditions makes birth safer and, oftentimes, makes people feel much better – sometimes better than they have in years,” Dr. Federspiel, maternal-fetal medicine physician and assistant professor of obstetrics and gynecology and population health sciences at Duke University, Durham, N.C., said.
Even patients he is able to diagnose earlier “will have difficulties catching up during pregnancy.”
The condition is the most common type of anemia among people who are pregnant. IDA increases a patient’s risk of delivering preterm and developing postpartum depression and puts their infants at a risk for perinatal mortality. Without proper treatment of IDA throughout pregnancy, the condition can also lead to low birth weights in infants or failing to meet weight goals later on.
But of all women with a new diagnosis of IDA from 2021 to 2022, 10% were pregnant, according to an analysis by Komodo Health, a health care analytics company.
While estimates of the prevalence of IDA vary, research from 2021 found 6.5% of nearly 1,500 patients who were pregnant during the first trimester had the condition, a figure the researchers said might underrepresent the problem.
“In severe cases [fetal outcomes can include] abnormal fetal oxygenation, nonreassuring fetal heart rate patterns, reduced amniotic fluid volume, fetal cerebral vasodilation, and fetal death,” Alianne S. Tilley, NP, family nurse practitioner at Women’s Care of Lake Cumberland, Somerset, Ky., said.
Research has shown that adequate levels of iron are an integral component in the development of the fetal brain. Some studies have reported that IDA during pregnancy increases an infant’s risk for poor neurodevelopmental outcomes.
Lack of screening protocol
Discrepancies in guidance for testing patients who are pregnant for IDA may add to late diagnosis and low treatment, according to Katelin Zahn, MD, assistant professor of general obstetrics, gynecology, and midwifery at University of North Carolina at Chapel Hill.
“There’s no consistency, which leads to a lot of variation in individual practice, which creates variation in outcomes, too,” Dr. Zahn said. “You can only do so much as one independent physician, and you need to be able to create change in a system that functions and provides standard of care even when you aren’t there.”
The American College of Obstetricians and Gynecologists recommends screening all patients who are pregnant with a complete blood count in the first trimester and again between 24 and 27 weeks of gestation.
Patients who meet criteria for IDA based on hematocrit levels less than 33% in the first and third trimesters, and less than 32% in the second trimester, should be evaluated to determine the cause. Those with IDA should be treated with supplemental iron, in addition to prenatal vitamins, ACOG says.
But the U.S. Preventive Services Task Force in 2015 found insufficient evidence to recommend for or against screening patients without symptoms or signs of the condition. The organization is in the process of updating the recommendation.
Prevention as best practice
The most effective way to address IDA in patients who are pregnant is prevention, according to Dr. Federspiel.
“Having a systematic approach to screening and treatment is really important, and this starts before pregnancy,” Dr. Federspiel said. “On average, a typical pregnancy requires an additional 1 g of iron.”
Dr. Federspiel recommends clinicians discuss the causes and the effects of IDA with patients who are planning to or could become pregnant. Clinicians might recommend iron- and folate-rich foods and vitamins B12 and C and ask patients if they face any barriers to access.
“Prenatal vitamins with iron are the gold standard in preventing IDA in the pregnant population,” Ms. Tilley said. “Education on the significant risk factors associated with IDA in early pregnancy is key.”
A version of this article first appeared on Medscape.com.

