User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
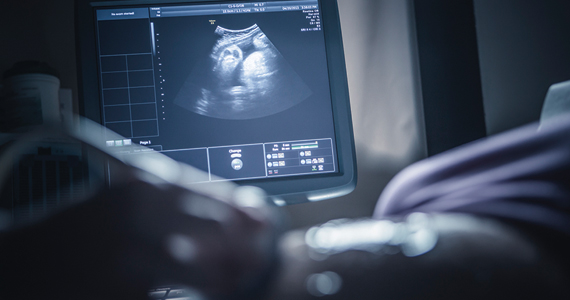
Trauma rates with operative vaginal delivery unexpectedly high, study finds
A new investigation has found that rates of physical trauma following operative vaginal delivery (OVD) in Canada are higher than previously reported.
The cohort study of more than 1.3 million deliveries in the country found trauma rates were highest with forceps delivery, with more than 1 in 4 pregnancies resulting in maternal trauma and 1 in 105 infants experiencing neonatal trauma. Maternal and neonatal trauma following vacuum deliveries was less common, occurring in 1 in 8 pregnancies and 1 in 104 infants, according to the researchers, who reported their findings in the Canadian Medical Association Journal .
“The rates of trauma following OVD in Canada are higher than previously reported, irrespective of region, level of obstetric care, and volume of instrument use among hospitals,” lead author Giulia Muraca, PhD, MPH, assistant professor of obstetrics and gynecology at McMaster University, Hamilton, Ont., said in an interview. “While OVDs may be associated with low rates of morbidity in carefully selected circumstances, the uniformly high rates of trauma among forceps and vacuum deliveries documented across regions, levels of obstetric care, and hospitals show that such conditions do not apply to routine obstetric practice in Canada.”
The American College of Obstetricians and Gynecologists considers OVD a way to reduce the rate of cesarean deliveries. However, the group has also pointed to a decline in familiarity with the procedures among clinicians new to the field.
Current reports also show that while OVD accounts for up to 15% of deliveries in Canada, Australia, and the United Kingdom, the risks associated with the approach are heavily dependent on the expertise of the provider. Declining use of OVD in favor of cesarean delivery has reduced opportunities for clinicians to acquire proficiency in performing these deliveries, according to the researchers.
Given these various factors, the investigators said the consensus on the safety of OVD is under scrutiny.
“An examination of maternal and neonatal trauma among OVD in contemporary practice is necessary to ensure that health care providers, policy makers, and pregnant individuals are informed regarding the risks of OVD typically experienced in routine obstetric practice, rather than those encountered under ideal conditions,” Dr. Muraca said.
Over 1 million deliveries studied
Dr. Muraca and colleagues looked at 1,326,191 deliveries occurring across Canada (except Quebec) between April 2013 and March 2019. The researchers included all singleton, term (≥37 weeks), in-hospital deliveries to women who had not undergone a previous cesarean delivery.
The study’s primary outcome measures were composite maternal trauma and composite neonatal trauma. Maternal trauma included obstetric anal sphincter injury (OASI); cervical or high vaginal laceration; pelvic hematoma; obstetric injury to the pelvic organs, pelvic joints, or ligaments; injury to the bladder or urethra; and other pelvic trauma. Neonatal trauma comprised intracranial hemorrhage and laceration, skull fracture, severe injury to the central or peripheral nervous system, fracture of the long bones, injury to the liver or spleen, seizures, and neonatal death.
The analysis found that 38,500 (2.9%) of the cases involved attempted forceps deliveries while 110,987 (8.4%) were attempted vacuum deliveries. Of the attempted forceps deliveries, 1,606 (4.2%) failed, while 8,791 (7.9%) of attempted vacuum deliveries failed.
Maternal trauma was observed in 25.3% of all forceps deliveries (n = 9,728) and 13.2% of all vacuum deliveries (n = 14,614), the researchers reported. The most common form of maternal trauma was OASI, which was observed in 21.52% of women undergoing forceps delivery and 11.67% of those undergoing vacuum delivery. The rates of all other forms of maternal trauma were higher among patients undergoing attempted forceps delivery than among their counterparts undergoing attempted vacuum delivery.
After adjusting for possible confounders, rates of maternal trauma remained higher with forceps than with vacuum deliveries (adjusted rate ratio, 1.70).
The rate of neonatal trauma was comparable for forceps (9.56/1,000 live births) and vacuum deliveries (9.58/1,000 live births). In these cases, damage to the peripheral nervous system was the most common form of neonatal trauma, occurring in 4.85/1,000 live births with forceps delivery and 3.41/1,000 live births for vacuum delivery, the researchers found.
Consider morbidity following OVD against potential alternatives, authors say
According to Dr. Muraca, the rates of maternal trauma in her study – along with accumulating evidence of the severe long-term consequences of these injuries – demonstrates the importance of reporting timely, empirically derived risk measures that accurately reflect those that pregnant individuals may encounter in typical obstetric practice.
“Although there is merit in understanding the estimates of risk that can be achieved when conditions are optimal, the interpretation of these estimates can be misleading, especially given secular shifts in patterns of practice,” she said. “The failure to do so compromises women’s autonomy in making evidence-informed decisions regarding childbirth interventions, such as evaluating the short- and long-term risks of OVD and cesarean delivery.
Her group recommended that morbidity following OVD be weighed against potential alternatives to such procedures, which carry their own risks. “This includes an extended second stage of labor and a spontaneous vaginal delivery, or a second-stage cesarean delivery, both of which are associated with significant morbidity,” Dr. Muraca said. “However, a comprehensive consideration of high population rates of OVD morbidity also prompts questions about choice of instrument, obstetrician training in OVD use, and for recognizing cases that would benefit from a cesarean delivery earlier in labor.”
Alan Peaceman, MD, professor of obstetrics and gynecology at Northwestern University, Chicago, said he was not surprised by the rates of sphincter injury, but that the rate of severe neonatal injury rate was higher than he expected. However, he added, “I don’t think clinicians should change their approach based on a single study. They should continue with the approach that they are most skilled at and is appropriate for the clinical circumstances.”
The study was funded by a grant from the Canadian Institutes of Health Research. Dr. Muraca and Dr. Peaceman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new investigation has found that rates of physical trauma following operative vaginal delivery (OVD) in Canada are higher than previously reported.
The cohort study of more than 1.3 million deliveries in the country found trauma rates were highest with forceps delivery, with more than 1 in 4 pregnancies resulting in maternal trauma and 1 in 105 infants experiencing neonatal trauma. Maternal and neonatal trauma following vacuum deliveries was less common, occurring in 1 in 8 pregnancies and 1 in 104 infants, according to the researchers, who reported their findings in the Canadian Medical Association Journal .
“The rates of trauma following OVD in Canada are higher than previously reported, irrespective of region, level of obstetric care, and volume of instrument use among hospitals,” lead author Giulia Muraca, PhD, MPH, assistant professor of obstetrics and gynecology at McMaster University, Hamilton, Ont., said in an interview. “While OVDs may be associated with low rates of morbidity in carefully selected circumstances, the uniformly high rates of trauma among forceps and vacuum deliveries documented across regions, levels of obstetric care, and hospitals show that such conditions do not apply to routine obstetric practice in Canada.”
The American College of Obstetricians and Gynecologists considers OVD a way to reduce the rate of cesarean deliveries. However, the group has also pointed to a decline in familiarity with the procedures among clinicians new to the field.
Current reports also show that while OVD accounts for up to 15% of deliveries in Canada, Australia, and the United Kingdom, the risks associated with the approach are heavily dependent on the expertise of the provider. Declining use of OVD in favor of cesarean delivery has reduced opportunities for clinicians to acquire proficiency in performing these deliveries, according to the researchers.
Given these various factors, the investigators said the consensus on the safety of OVD is under scrutiny.
“An examination of maternal and neonatal trauma among OVD in contemporary practice is necessary to ensure that health care providers, policy makers, and pregnant individuals are informed regarding the risks of OVD typically experienced in routine obstetric practice, rather than those encountered under ideal conditions,” Dr. Muraca said.
Over 1 million deliveries studied
Dr. Muraca and colleagues looked at 1,326,191 deliveries occurring across Canada (except Quebec) between April 2013 and March 2019. The researchers included all singleton, term (≥37 weeks), in-hospital deliveries to women who had not undergone a previous cesarean delivery.
The study’s primary outcome measures were composite maternal trauma and composite neonatal trauma. Maternal trauma included obstetric anal sphincter injury (OASI); cervical or high vaginal laceration; pelvic hematoma; obstetric injury to the pelvic organs, pelvic joints, or ligaments; injury to the bladder or urethra; and other pelvic trauma. Neonatal trauma comprised intracranial hemorrhage and laceration, skull fracture, severe injury to the central or peripheral nervous system, fracture of the long bones, injury to the liver or spleen, seizures, and neonatal death.
The analysis found that 38,500 (2.9%) of the cases involved attempted forceps deliveries while 110,987 (8.4%) were attempted vacuum deliveries. Of the attempted forceps deliveries, 1,606 (4.2%) failed, while 8,791 (7.9%) of attempted vacuum deliveries failed.
Maternal trauma was observed in 25.3% of all forceps deliveries (n = 9,728) and 13.2% of all vacuum deliveries (n = 14,614), the researchers reported. The most common form of maternal trauma was OASI, which was observed in 21.52% of women undergoing forceps delivery and 11.67% of those undergoing vacuum delivery. The rates of all other forms of maternal trauma were higher among patients undergoing attempted forceps delivery than among their counterparts undergoing attempted vacuum delivery.
After adjusting for possible confounders, rates of maternal trauma remained higher with forceps than with vacuum deliveries (adjusted rate ratio, 1.70).
The rate of neonatal trauma was comparable for forceps (9.56/1,000 live births) and vacuum deliveries (9.58/1,000 live births). In these cases, damage to the peripheral nervous system was the most common form of neonatal trauma, occurring in 4.85/1,000 live births with forceps delivery and 3.41/1,000 live births for vacuum delivery, the researchers found.
Consider morbidity following OVD against potential alternatives, authors say
According to Dr. Muraca, the rates of maternal trauma in her study – along with accumulating evidence of the severe long-term consequences of these injuries – demonstrates the importance of reporting timely, empirically derived risk measures that accurately reflect those that pregnant individuals may encounter in typical obstetric practice.
“Although there is merit in understanding the estimates of risk that can be achieved when conditions are optimal, the interpretation of these estimates can be misleading, especially given secular shifts in patterns of practice,” she said. “The failure to do so compromises women’s autonomy in making evidence-informed decisions regarding childbirth interventions, such as evaluating the short- and long-term risks of OVD and cesarean delivery.
Her group recommended that morbidity following OVD be weighed against potential alternatives to such procedures, which carry their own risks. “This includes an extended second stage of labor and a spontaneous vaginal delivery, or a second-stage cesarean delivery, both of which are associated with significant morbidity,” Dr. Muraca said. “However, a comprehensive consideration of high population rates of OVD morbidity also prompts questions about choice of instrument, obstetrician training in OVD use, and for recognizing cases that would benefit from a cesarean delivery earlier in labor.”
Alan Peaceman, MD, professor of obstetrics and gynecology at Northwestern University, Chicago, said he was not surprised by the rates of sphincter injury, but that the rate of severe neonatal injury rate was higher than he expected. However, he added, “I don’t think clinicians should change their approach based on a single study. They should continue with the approach that they are most skilled at and is appropriate for the clinical circumstances.”
The study was funded by a grant from the Canadian Institutes of Health Research. Dr. Muraca and Dr. Peaceman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new investigation has found that rates of physical trauma following operative vaginal delivery (OVD) in Canada are higher than previously reported.
The cohort study of more than 1.3 million deliveries in the country found trauma rates were highest with forceps delivery, with more than 1 in 4 pregnancies resulting in maternal trauma and 1 in 105 infants experiencing neonatal trauma. Maternal and neonatal trauma following vacuum deliveries was less common, occurring in 1 in 8 pregnancies and 1 in 104 infants, according to the researchers, who reported their findings in the Canadian Medical Association Journal .
“The rates of trauma following OVD in Canada are higher than previously reported, irrespective of region, level of obstetric care, and volume of instrument use among hospitals,” lead author Giulia Muraca, PhD, MPH, assistant professor of obstetrics and gynecology at McMaster University, Hamilton, Ont., said in an interview. “While OVDs may be associated with low rates of morbidity in carefully selected circumstances, the uniformly high rates of trauma among forceps and vacuum deliveries documented across regions, levels of obstetric care, and hospitals show that such conditions do not apply to routine obstetric practice in Canada.”
The American College of Obstetricians and Gynecologists considers OVD a way to reduce the rate of cesarean deliveries. However, the group has also pointed to a decline in familiarity with the procedures among clinicians new to the field.
Current reports also show that while OVD accounts for up to 15% of deliveries in Canada, Australia, and the United Kingdom, the risks associated with the approach are heavily dependent on the expertise of the provider. Declining use of OVD in favor of cesarean delivery has reduced opportunities for clinicians to acquire proficiency in performing these deliveries, according to the researchers.
Given these various factors, the investigators said the consensus on the safety of OVD is under scrutiny.
“An examination of maternal and neonatal trauma among OVD in contemporary practice is necessary to ensure that health care providers, policy makers, and pregnant individuals are informed regarding the risks of OVD typically experienced in routine obstetric practice, rather than those encountered under ideal conditions,” Dr. Muraca said.
Over 1 million deliveries studied
Dr. Muraca and colleagues looked at 1,326,191 deliveries occurring across Canada (except Quebec) between April 2013 and March 2019. The researchers included all singleton, term (≥37 weeks), in-hospital deliveries to women who had not undergone a previous cesarean delivery.
The study’s primary outcome measures were composite maternal trauma and composite neonatal trauma. Maternal trauma included obstetric anal sphincter injury (OASI); cervical or high vaginal laceration; pelvic hematoma; obstetric injury to the pelvic organs, pelvic joints, or ligaments; injury to the bladder or urethra; and other pelvic trauma. Neonatal trauma comprised intracranial hemorrhage and laceration, skull fracture, severe injury to the central or peripheral nervous system, fracture of the long bones, injury to the liver or spleen, seizures, and neonatal death.
The analysis found that 38,500 (2.9%) of the cases involved attempted forceps deliveries while 110,987 (8.4%) were attempted vacuum deliveries. Of the attempted forceps deliveries, 1,606 (4.2%) failed, while 8,791 (7.9%) of attempted vacuum deliveries failed.
Maternal trauma was observed in 25.3% of all forceps deliveries (n = 9,728) and 13.2% of all vacuum deliveries (n = 14,614), the researchers reported. The most common form of maternal trauma was OASI, which was observed in 21.52% of women undergoing forceps delivery and 11.67% of those undergoing vacuum delivery. The rates of all other forms of maternal trauma were higher among patients undergoing attempted forceps delivery than among their counterparts undergoing attempted vacuum delivery.
After adjusting for possible confounders, rates of maternal trauma remained higher with forceps than with vacuum deliveries (adjusted rate ratio, 1.70).
The rate of neonatal trauma was comparable for forceps (9.56/1,000 live births) and vacuum deliveries (9.58/1,000 live births). In these cases, damage to the peripheral nervous system was the most common form of neonatal trauma, occurring in 4.85/1,000 live births with forceps delivery and 3.41/1,000 live births for vacuum delivery, the researchers found.
Consider morbidity following OVD against potential alternatives, authors say
According to Dr. Muraca, the rates of maternal trauma in her study – along with accumulating evidence of the severe long-term consequences of these injuries – demonstrates the importance of reporting timely, empirically derived risk measures that accurately reflect those that pregnant individuals may encounter in typical obstetric practice.
“Although there is merit in understanding the estimates of risk that can be achieved when conditions are optimal, the interpretation of these estimates can be misleading, especially given secular shifts in patterns of practice,” she said. “The failure to do so compromises women’s autonomy in making evidence-informed decisions regarding childbirth interventions, such as evaluating the short- and long-term risks of OVD and cesarean delivery.
Her group recommended that morbidity following OVD be weighed against potential alternatives to such procedures, which carry their own risks. “This includes an extended second stage of labor and a spontaneous vaginal delivery, or a second-stage cesarean delivery, both of which are associated with significant morbidity,” Dr. Muraca said. “However, a comprehensive consideration of high population rates of OVD morbidity also prompts questions about choice of instrument, obstetrician training in OVD use, and for recognizing cases that would benefit from a cesarean delivery earlier in labor.”
Alan Peaceman, MD, professor of obstetrics and gynecology at Northwestern University, Chicago, said he was not surprised by the rates of sphincter injury, but that the rate of severe neonatal injury rate was higher than he expected. However, he added, “I don’t think clinicians should change their approach based on a single study. They should continue with the approach that they are most skilled at and is appropriate for the clinical circumstances.”
The study was funded by a grant from the Canadian Institutes of Health Research. Dr. Muraca and Dr. Peaceman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
When the patient wants to speak to a manager
A patient swore at me the other day. Not as in “she used a curse word.” As in she spewed fury, spitting out a vulgar, adverbial word before “... terrible doctor” while jabbing her finger toward me. In my 15 years of practice, I’d never had that happen before. Equally surprising, I was not surprised by her outburst. The level of incivility from patients is at an all-time high.
Her anger was misdirected. She wanted me to write a letter to her employer excusing her from getting a vaccine. It was neither indicated nor ethical for me to do so. I did my best to redirect her, but without success. As our chief of service, I often help with service concerns and am happy to see patients who want another opinion or want to speak with the department head (aka, “the manager”). Usually I can help. Lately, it’s become harder.
Not only are such rude incidents more frequent, but they are also more dramatic and inappropriate. For example, I cannot imagine writing a complaint against a doctor stating that she must be a foreign medical grad (as it happens, she’s Ivy League-trained) or demanding money back when a biopsy result turned out to be benign, or threatening to report a doctor to the medical board because he failed to schedule a follow-up appointment (that doctor had been retired for months). Patients have hung up on our staff mid-sentence and slammed a clinic door when they left in a huff. Why are so many previously sensible people throwing childlike tantrums?
It’s the same phenomenon happening to our fellow service agents across all industries. The Federal Aviation Administration’s graph of unruly passenger incidents is a flat line from 1995 to 2019, then it goes straight vertical. A recent survey showed that Americans’ sense of civility is low and worse, that people’s expectations that civility will improve is going down. It’s palpable. Last month, I witnessed a man and woman screaming at each other over Christmas lights in a busy store. An army of aproned walkie-talkie staff surrounded them and escorted them out – their coordination and efficiency clearly indicated they’d done this before. Customers everywhere are mad, frustrated, disenfranchised. Lately, a lot of things just are not working out for them. Supplies are out. Kids are sent home from school. No elective surgery appointments are available. The insta-gratification they’ve grown accustomed to from Amazon and DoorDash is colliding with the reality that not everything works that way.
The word “patient’’ you’ll recall comes from the Latin “patior,” meaning to suffer or bear. With virus variants raging, inflation growing, and call center wait times approaching infinity, many of our patients, it seems, cannot bear any more. I’m confident this situation will improve and our patients will be more reasonable in their expectations, but I am afraid that, in the end, we’ll have lost some decorum and dignity that we may never find again in medicine.
For my potty-mouthed patient, I made an excuse to leave the room to get my dermatoscope and walked out. It gave her time to calm down. I returned in a few minutes to do a skin exam. As I was wrapping up, I advised her that she cannot raise her voice or use offensive language and that she should know that I and everyone in our office cares about her and wants to help. She did apologize for her behavior, but then had to add that, if I really cared, I’d write the letter for her.
I guess the customer is not always right.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
A patient swore at me the other day. Not as in “she used a curse word.” As in she spewed fury, spitting out a vulgar, adverbial word before “... terrible doctor” while jabbing her finger toward me. In my 15 years of practice, I’d never had that happen before. Equally surprising, I was not surprised by her outburst. The level of incivility from patients is at an all-time high.
Her anger was misdirected. She wanted me to write a letter to her employer excusing her from getting a vaccine. It was neither indicated nor ethical for me to do so. I did my best to redirect her, but without success. As our chief of service, I often help with service concerns and am happy to see patients who want another opinion or want to speak with the department head (aka, “the manager”). Usually I can help. Lately, it’s become harder.
Not only are such rude incidents more frequent, but they are also more dramatic and inappropriate. For example, I cannot imagine writing a complaint against a doctor stating that she must be a foreign medical grad (as it happens, she’s Ivy League-trained) or demanding money back when a biopsy result turned out to be benign, or threatening to report a doctor to the medical board because he failed to schedule a follow-up appointment (that doctor had been retired for months). Patients have hung up on our staff mid-sentence and slammed a clinic door when they left in a huff. Why are so many previously sensible people throwing childlike tantrums?
It’s the same phenomenon happening to our fellow service agents across all industries. The Federal Aviation Administration’s graph of unruly passenger incidents is a flat line from 1995 to 2019, then it goes straight vertical. A recent survey showed that Americans’ sense of civility is low and worse, that people’s expectations that civility will improve is going down. It’s palpable. Last month, I witnessed a man and woman screaming at each other over Christmas lights in a busy store. An army of aproned walkie-talkie staff surrounded them and escorted them out – their coordination and efficiency clearly indicated they’d done this before. Customers everywhere are mad, frustrated, disenfranchised. Lately, a lot of things just are not working out for them. Supplies are out. Kids are sent home from school. No elective surgery appointments are available. The insta-gratification they’ve grown accustomed to from Amazon and DoorDash is colliding with the reality that not everything works that way.
The word “patient’’ you’ll recall comes from the Latin “patior,” meaning to suffer or bear. With virus variants raging, inflation growing, and call center wait times approaching infinity, many of our patients, it seems, cannot bear any more. I’m confident this situation will improve and our patients will be more reasonable in their expectations, but I am afraid that, in the end, we’ll have lost some decorum and dignity that we may never find again in medicine.
For my potty-mouthed patient, I made an excuse to leave the room to get my dermatoscope and walked out. It gave her time to calm down. I returned in a few minutes to do a skin exam. As I was wrapping up, I advised her that she cannot raise her voice or use offensive language and that she should know that I and everyone in our office cares about her and wants to help. She did apologize for her behavior, but then had to add that, if I really cared, I’d write the letter for her.
I guess the customer is not always right.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
A patient swore at me the other day. Not as in “she used a curse word.” As in she spewed fury, spitting out a vulgar, adverbial word before “... terrible doctor” while jabbing her finger toward me. In my 15 years of practice, I’d never had that happen before. Equally surprising, I was not surprised by her outburst. The level of incivility from patients is at an all-time high.
Her anger was misdirected. She wanted me to write a letter to her employer excusing her from getting a vaccine. It was neither indicated nor ethical for me to do so. I did my best to redirect her, but without success. As our chief of service, I often help with service concerns and am happy to see patients who want another opinion or want to speak with the department head (aka, “the manager”). Usually I can help. Lately, it’s become harder.
Not only are such rude incidents more frequent, but they are also more dramatic and inappropriate. For example, I cannot imagine writing a complaint against a doctor stating that she must be a foreign medical grad (as it happens, she’s Ivy League-trained) or demanding money back when a biopsy result turned out to be benign, or threatening to report a doctor to the medical board because he failed to schedule a follow-up appointment (that doctor had been retired for months). Patients have hung up on our staff mid-sentence and slammed a clinic door when they left in a huff. Why are so many previously sensible people throwing childlike tantrums?
It’s the same phenomenon happening to our fellow service agents across all industries. The Federal Aviation Administration’s graph of unruly passenger incidents is a flat line from 1995 to 2019, then it goes straight vertical. A recent survey showed that Americans’ sense of civility is low and worse, that people’s expectations that civility will improve is going down. It’s palpable. Last month, I witnessed a man and woman screaming at each other over Christmas lights in a busy store. An army of aproned walkie-talkie staff surrounded them and escorted them out – their coordination and efficiency clearly indicated they’d done this before. Customers everywhere are mad, frustrated, disenfranchised. Lately, a lot of things just are not working out for them. Supplies are out. Kids are sent home from school. No elective surgery appointments are available. The insta-gratification they’ve grown accustomed to from Amazon and DoorDash is colliding with the reality that not everything works that way.
The word “patient’’ you’ll recall comes from the Latin “patior,” meaning to suffer or bear. With virus variants raging, inflation growing, and call center wait times approaching infinity, many of our patients, it seems, cannot bear any more. I’m confident this situation will improve and our patients will be more reasonable in their expectations, but I am afraid that, in the end, we’ll have lost some decorum and dignity that we may never find again in medicine.
For my potty-mouthed patient, I made an excuse to leave the room to get my dermatoscope and walked out. It gave her time to calm down. I returned in a few minutes to do a skin exam. As I was wrapping up, I advised her that she cannot raise her voice or use offensive language and that she should know that I and everyone in our office cares about her and wants to help. She did apologize for her behavior, but then had to add that, if I really cared, I’d write the letter for her.
I guess the customer is not always right.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Study finds genetic factor for COVID smell and taste loss
, according to a new study published in the journal Nature Genetics
The finding could eventually help the 1.6 million people in the United States who still can’t smell or have had a change in their ability to smell more than 6 months after getting the coronavirus. The exact cause related to COVID-19 is still unknown, but researchers believe it could be because of damage in a part of the nose called the olfactory epithelium.
“How we get from infection to smell loss remains unclear,” Justin Turner, MD, an associate professor of otolaryngology at Vanderbilt University, Nashville, Tenn., told NBC News. Dr. Turner was not part of the research team.
“Early data suggest that supporting cells of the olfactory epithelium are the ones mostly being infected by the virus, and presumably this leads to the death of the neurons themselves,” he said. “But we don’t really, really know why and when that happens, and why it seems to preferentially happen in certain individuals.”
Researchers at 23andMe, a genomics and biotechnology company, did the study as part of a larger COVID-19 project, which includes people in the United States and the United Kingdom. They analyzed data from nearly 70,000 people who took online surveys after receiving a positive coronavirus test. Among those, 68% reported a loss of smell or taste as a symptom.
The study team compared the genetic differences between those who lost their sense of smell and taste and those who didn’t. They found that a location near two olfactory genes – UGT2A1 and UGT2A2 – is associated with COVID-19 loss of smell and taste. The genetic risk factor makes it 11% more likely for a person with COVID-19 to lose their sense of smell or taste.
The research team also found that women were 11% more likely than men to report a loss of smell and taste. About 73% of those who reported a loss of smell and taste were ages 26-35.
The researchers aren’t sure how the genes are involved, though they suspect that infected cells could lead to smell loss. Typically, the genes are expressed in tissue inside the nose involved with smell and play a role in processing things that have an odor. To use the findings, researchers need to learn more about the genes, how they are expressed, and what their functions are, NBC News reported.
The findings could help lead to treatments. Other research has shown that the loss of taste and smell is related to a “failure to protect the sensory cells of the nose and tongue from viral infection,” Danielle Reed, PhD, associate director of the Monell Chemical Senses Center in Philadelphia, told NBC News. She was not part of the research team but studies person-to-person differences in the loss of these senses because of COVID-19.
“This study suggests a different direction,” she said. “The pathways that break down the chemicals that cause taste and smell in the first place might be over or underactive, reducing or distorting the ability to taste and smell.”
A version of this article first appeared on WebMD.com.
, according to a new study published in the journal Nature Genetics
The finding could eventually help the 1.6 million people in the United States who still can’t smell or have had a change in their ability to smell more than 6 months after getting the coronavirus. The exact cause related to COVID-19 is still unknown, but researchers believe it could be because of damage in a part of the nose called the olfactory epithelium.
“How we get from infection to smell loss remains unclear,” Justin Turner, MD, an associate professor of otolaryngology at Vanderbilt University, Nashville, Tenn., told NBC News. Dr. Turner was not part of the research team.
“Early data suggest that supporting cells of the olfactory epithelium are the ones mostly being infected by the virus, and presumably this leads to the death of the neurons themselves,” he said. “But we don’t really, really know why and when that happens, and why it seems to preferentially happen in certain individuals.”
Researchers at 23andMe, a genomics and biotechnology company, did the study as part of a larger COVID-19 project, which includes people in the United States and the United Kingdom. They analyzed data from nearly 70,000 people who took online surveys after receiving a positive coronavirus test. Among those, 68% reported a loss of smell or taste as a symptom.
The study team compared the genetic differences between those who lost their sense of smell and taste and those who didn’t. They found that a location near two olfactory genes – UGT2A1 and UGT2A2 – is associated with COVID-19 loss of smell and taste. The genetic risk factor makes it 11% more likely for a person with COVID-19 to lose their sense of smell or taste.
The research team also found that women were 11% more likely than men to report a loss of smell and taste. About 73% of those who reported a loss of smell and taste were ages 26-35.
The researchers aren’t sure how the genes are involved, though they suspect that infected cells could lead to smell loss. Typically, the genes are expressed in tissue inside the nose involved with smell and play a role in processing things that have an odor. To use the findings, researchers need to learn more about the genes, how they are expressed, and what their functions are, NBC News reported.
The findings could help lead to treatments. Other research has shown that the loss of taste and smell is related to a “failure to protect the sensory cells of the nose and tongue from viral infection,” Danielle Reed, PhD, associate director of the Monell Chemical Senses Center in Philadelphia, told NBC News. She was not part of the research team but studies person-to-person differences in the loss of these senses because of COVID-19.
“This study suggests a different direction,” she said. “The pathways that break down the chemicals that cause taste and smell in the first place might be over or underactive, reducing or distorting the ability to taste and smell.”
A version of this article first appeared on WebMD.com.
, according to a new study published in the journal Nature Genetics
The finding could eventually help the 1.6 million people in the United States who still can’t smell or have had a change in their ability to smell more than 6 months after getting the coronavirus. The exact cause related to COVID-19 is still unknown, but researchers believe it could be because of damage in a part of the nose called the olfactory epithelium.
“How we get from infection to smell loss remains unclear,” Justin Turner, MD, an associate professor of otolaryngology at Vanderbilt University, Nashville, Tenn., told NBC News. Dr. Turner was not part of the research team.
“Early data suggest that supporting cells of the olfactory epithelium are the ones mostly being infected by the virus, and presumably this leads to the death of the neurons themselves,” he said. “But we don’t really, really know why and when that happens, and why it seems to preferentially happen in certain individuals.”
Researchers at 23andMe, a genomics and biotechnology company, did the study as part of a larger COVID-19 project, which includes people in the United States and the United Kingdom. They analyzed data from nearly 70,000 people who took online surveys after receiving a positive coronavirus test. Among those, 68% reported a loss of smell or taste as a symptom.
The study team compared the genetic differences between those who lost their sense of smell and taste and those who didn’t. They found that a location near two olfactory genes – UGT2A1 and UGT2A2 – is associated with COVID-19 loss of smell and taste. The genetic risk factor makes it 11% more likely for a person with COVID-19 to lose their sense of smell or taste.
The research team also found that women were 11% more likely than men to report a loss of smell and taste. About 73% of those who reported a loss of smell and taste were ages 26-35.
The researchers aren’t sure how the genes are involved, though they suspect that infected cells could lead to smell loss. Typically, the genes are expressed in tissue inside the nose involved with smell and play a role in processing things that have an odor. To use the findings, researchers need to learn more about the genes, how they are expressed, and what their functions are, NBC News reported.
The findings could help lead to treatments. Other research has shown that the loss of taste and smell is related to a “failure to protect the sensory cells of the nose and tongue from viral infection,” Danielle Reed, PhD, associate director of the Monell Chemical Senses Center in Philadelphia, told NBC News. She was not part of the research team but studies person-to-person differences in the loss of these senses because of COVID-19.
“This study suggests a different direction,” she said. “The pathways that break down the chemicals that cause taste and smell in the first place might be over or underactive, reducing or distorting the ability to taste and smell.”
A version of this article first appeared on WebMD.com.
FROM NATURE GENETICS
Fourth vaccine shot less effective against Omicron, Israeli study says
, according to new research at an Israeli hospital.
The preliminary results, released on Jan. 17, challenge the idea of giving a second booster dose to slow the spread of the coronavirus, according to USA Today.
“Despite increased antibody levels, the fourth vaccine only offers a partial defense against the virus,” Gili Regev-Yochay, MD, director of the hospital’s infection prevention and control units, told reporters.
“The vaccines, which were more effective against previous variants, offer less protection versus Omicron,” she said.
In a clinical trial, 274 medical workers at Sheba Medical Center near Tel Aviv received a fourth vaccine dose in December – 154 got the Pfizer vaccine and 120 got the Moderna vaccine – after previously getting three Pfizer shots.
Both groups received a boost in antibodies that was “slightly higher” than after the third shot, Dr. Regev-Yochay said. But when compared with a control group that didn’t receive the fourth dose, the extra boost didn’t prevent the spread of Omicron.
“We see many infected with Omicron who received the fourth dose,” Dr. Regev-Yochay said. “Granted, a bit less than in the control group, but still a lot of infections.”
Some public health officials in Israel say the campaign for fourth doses is still worthwhile, according to The Times of Israel. The vaccine still works well against the Alpha and Delta variants, Dr. Regev-Yochay said, and a fourth shot should go to older adults and those who face higher risks for severe COVID-19.
Hours after releasing the preliminary results, Sheba Medical Center published a statement calling for “continuing the vaccination drive for risk groups at this time, even though the vaccine doesn’t provide optimal protection against getting infected with the variant.” News outlets reported that the hospital was pressured into issuing the statement after Israel’s Health Ministry didn’t like the release of the early study results, The Times of Israel reported.
The second booster “returns the level of antibodies to what it was at the beginning of the third booster,” Nachman Ash, MD, director of Israel’s Health Ministry, told Channel 13 TV in Israel, according to The Associated Press.
“That has great importance, especially among the older population,” he said.
As of Sunday, more than 500,000 people in Israel had received fourth doses since the country began offering them last month to medical workers, immunocompromised patients, and people ages 60 years and older, the AP reported. At the same time, the country has faced a recent coronavirus surge that has led to record-breaking numbers of cases and rising hospitalizations.
On Tuesday, the Israeli government said it would shorten the mandatory quarantine period from 7 days to 5 days, the AP reported.
“This decision will enable us to continue safeguarding public health on the one hand and to keep the economy going at this time on the other, even though it is difficult, so that we can get through this wave safely,” Prime Minister Naftali Bennett said.
A version of this article first appeared on WebMD.com.
, according to new research at an Israeli hospital.
The preliminary results, released on Jan. 17, challenge the idea of giving a second booster dose to slow the spread of the coronavirus, according to USA Today.
“Despite increased antibody levels, the fourth vaccine only offers a partial defense against the virus,” Gili Regev-Yochay, MD, director of the hospital’s infection prevention and control units, told reporters.
“The vaccines, which were more effective against previous variants, offer less protection versus Omicron,” she said.
In a clinical trial, 274 medical workers at Sheba Medical Center near Tel Aviv received a fourth vaccine dose in December – 154 got the Pfizer vaccine and 120 got the Moderna vaccine – after previously getting three Pfizer shots.
Both groups received a boost in antibodies that was “slightly higher” than after the third shot, Dr. Regev-Yochay said. But when compared with a control group that didn’t receive the fourth dose, the extra boost didn’t prevent the spread of Omicron.
“We see many infected with Omicron who received the fourth dose,” Dr. Regev-Yochay said. “Granted, a bit less than in the control group, but still a lot of infections.”
Some public health officials in Israel say the campaign for fourth doses is still worthwhile, according to The Times of Israel. The vaccine still works well against the Alpha and Delta variants, Dr. Regev-Yochay said, and a fourth shot should go to older adults and those who face higher risks for severe COVID-19.
Hours after releasing the preliminary results, Sheba Medical Center published a statement calling for “continuing the vaccination drive for risk groups at this time, even though the vaccine doesn’t provide optimal protection against getting infected with the variant.” News outlets reported that the hospital was pressured into issuing the statement after Israel’s Health Ministry didn’t like the release of the early study results, The Times of Israel reported.
The second booster “returns the level of antibodies to what it was at the beginning of the third booster,” Nachman Ash, MD, director of Israel’s Health Ministry, told Channel 13 TV in Israel, according to The Associated Press.
“That has great importance, especially among the older population,” he said.
As of Sunday, more than 500,000 people in Israel had received fourth doses since the country began offering them last month to medical workers, immunocompromised patients, and people ages 60 years and older, the AP reported. At the same time, the country has faced a recent coronavirus surge that has led to record-breaking numbers of cases and rising hospitalizations.
On Tuesday, the Israeli government said it would shorten the mandatory quarantine period from 7 days to 5 days, the AP reported.
“This decision will enable us to continue safeguarding public health on the one hand and to keep the economy going at this time on the other, even though it is difficult, so that we can get through this wave safely,” Prime Minister Naftali Bennett said.
A version of this article first appeared on WebMD.com.
, according to new research at an Israeli hospital.
The preliminary results, released on Jan. 17, challenge the idea of giving a second booster dose to slow the spread of the coronavirus, according to USA Today.
“Despite increased antibody levels, the fourth vaccine only offers a partial defense against the virus,” Gili Regev-Yochay, MD, director of the hospital’s infection prevention and control units, told reporters.
“The vaccines, which were more effective against previous variants, offer less protection versus Omicron,” she said.
In a clinical trial, 274 medical workers at Sheba Medical Center near Tel Aviv received a fourth vaccine dose in December – 154 got the Pfizer vaccine and 120 got the Moderna vaccine – after previously getting three Pfizer shots.
Both groups received a boost in antibodies that was “slightly higher” than after the third shot, Dr. Regev-Yochay said. But when compared with a control group that didn’t receive the fourth dose, the extra boost didn’t prevent the spread of Omicron.
“We see many infected with Omicron who received the fourth dose,” Dr. Regev-Yochay said. “Granted, a bit less than in the control group, but still a lot of infections.”
Some public health officials in Israel say the campaign for fourth doses is still worthwhile, according to The Times of Israel. The vaccine still works well against the Alpha and Delta variants, Dr. Regev-Yochay said, and a fourth shot should go to older adults and those who face higher risks for severe COVID-19.
Hours after releasing the preliminary results, Sheba Medical Center published a statement calling for “continuing the vaccination drive for risk groups at this time, even though the vaccine doesn’t provide optimal protection against getting infected with the variant.” News outlets reported that the hospital was pressured into issuing the statement after Israel’s Health Ministry didn’t like the release of the early study results, The Times of Israel reported.
The second booster “returns the level of antibodies to what it was at the beginning of the third booster,” Nachman Ash, MD, director of Israel’s Health Ministry, told Channel 13 TV in Israel, according to The Associated Press.
“That has great importance, especially among the older population,” he said.
As of Sunday, more than 500,000 people in Israel had received fourth doses since the country began offering them last month to medical workers, immunocompromised patients, and people ages 60 years and older, the AP reported. At the same time, the country has faced a recent coronavirus surge that has led to record-breaking numbers of cases and rising hospitalizations.
On Tuesday, the Israeli government said it would shorten the mandatory quarantine period from 7 days to 5 days, the AP reported.
“This decision will enable us to continue safeguarding public health on the one hand and to keep the economy going at this time on the other, even though it is difficult, so that we can get through this wave safely,” Prime Minister Naftali Bennett said.
A version of this article first appeared on WebMD.com.
2022 Update on obstetrics
Obstetrical practice saw updates in 2021 to 3 major areas of pregnancy management: preterm birth prevention, antepartum fetal surveillance, and the use of antenatal corticosteroids.
Updated guidance on predicting and preventing spontaneous PTB
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
Preterm birth (PTB) continues to pose a challenge in clinical obstetrics, with the most recently reported rate of 10.2% in the United States.1 This accounts for almost 75% of perinatal mortality and more than half of neonatal morbidity, in which effects last well past the neonatal period. PTB is classified as spontaneous (following preterm labor, preterm prelabor rupture of membranes, or cervical insufficiency) or iatrogenic (indicated due to maternal and/or fetal complications).
Assessing risk for PTB
The single strongest predictor of subsequent PTB is a history of spontaneous PTB. Recurrence risk is further increased by the number of prior PTBs and the gestational age at prior PTB. Identification of and intervention for a short cervix has been shown to prolong gestation. Transvaginal ultrasonography of the cervix is the most accurate method for evaluating cervical length (CL). Specific examination criteria exist to ensure that CL measurements are reproducible and reliable.2 A short CL is generally defined as a measurement of less than 25 mm between 16 and 24 weeks’ gestation.
Screening strategies
The American College of Obstetricians and Gynecologists (ACOG), with an endorsement from the Society for Maternal-Fetal Medicine (SMFM), recommends cervical evaluation during the anatomy ultrasound exam between 18 0/7 and 22 6/7 weeks’ gestation in all pregnant patients regardless of prior PTB.3 If transabdominal imaging is concerning for a shortened cervix, transvaginal ultrasonography should be performed to assess the CL.
Serial transvaginal CL measurements are recommended between 16 0/7 and 24 0/7 weeks’ gestation for patients with a current singleton pregnancy and history of a spontaneous PTB, but not for patients with a history of iatrogenic or indicated PTB.
Interventions: Mind your p’s and c’s
Interventions to reduce the risk of spontaneous PTB depend on whether the current pregnancy is a singleton, twins, or higher-order multiples; CL measurement; and history of spontaneous PTB. Preconception optimization of underlying medical conditions also is important to reduce the risk of recurrent indicated PTB.
Continue to: Progesterone...
Progesterone
Vaginal administration. Several trials have shown that vaginal progesterone can be used to reduce the risk of spontaneous PTB in asymptomatic patients with a singleton pregnancy, incidental finding of a short cervix (<25 mm), and no history of spontaneous PTB. This is a change from the prior recommendation of CL of less than 20 mm. In the setting of a twin pregnancy, regardless of CL, data do not definitively support the use of vaginal progesterone.
Intramuscular administration.4,5 The popularity of intramuscular progesterone has waxed and waned. At present, ACOG recommends that all patients with a singleton pregnancy and history of spontaneous PTB be offered progesterone beginning at 16 0/7 weeks’ gestation following a shared decision-making process that includes the limited data of efficacy noted in existing studies.
In a twin pregnancy with no history of spontaneous PTB, the use of intramuscular progesterone has been shown to potentially increase the risk of PTB and admission to the neonatal intensive care unit. As such, intramuscular progesterone in the setting of a twin gestation without a history of spontaneous PTB is not recommended. When a prior spontaneous PTB has occurred, there may be some benefit to intramuscular progesterone in twin gestations.
Cerclage
Ultrasound indicated. In a singleton pregnancy with an incidental finding of short cervix (<25 mm) and no history of PTB, the use of cerclage is of uncertain benefit. Effectiveness may be seen if the cervix is less than 10 mm. Ultrasound-indicated cerclage should be considered in a singleton pregnancy with a CL less than 25 mm and a history of spontaneous PTB.
Possibly one of the most controversial topics is ultrasound-indicated cerclage placement in twin gestation. As with many situations in obstetrics, data regarding ultrasound-indicated cerclage in twin gestation is based on small retrospective studies fraught with bias. Results from these studies range from no benefit, to potential benefit, to even possible increased risk of PTB. Since data are limited, as we await more evidence, it is recommended that the clinician and patient use shared decision making to decide on cerclage placement in a twin gestation.
Exam indicated. In a singleton pregnancy with a dilated cervix on digital or speculum exam between 16 0/7 to 23 6/7 weeks’ gestation, a physical exam–indicated cerclage should be offered. Exam-indicated cerclage also may reduce the incidence of PTB in twin gestations with cervical dilation between 16 0/7 and 23 6/7 weeks’ gestation. Indomethacin tocolysis and perioperative antibiotics should be considered when an exam-indicated cerclage is placed.
As the limits of viability are continually pushed earlier, more in-depth conversation is needed with patients who are considering an exam-indicated cerclage. The nuances of periviability and the likelihood that an exam-indicated cerclage will commit a pregnancy to a periviable or extremely preterm birth should be discussed in detail using a shared decision making model.
Regardless of whether the cerclage is ultrasound or exam indicated, once it is placed there is no utility in additional CL ultrasound monitoring.
Pessary
Vaginal pessaries for prevention of PTB have not gained popularity in the United States as they have in other countries. Trials are being conducted to determine the utility of vaginal pessary, but current data have not proven its effectiveness in preventing PTB in the setting of singleton pregnancy, short cervix, and no history of spontaneous PTB. So for now, pessary is not recommended. The same can be said for use in the twin gestation.
- All patients should have cervical evaluation during pregnancy. Serial imaging is reserved for those with a history of spontaneous PTB.
- Progesterone supplementation should be offered to patients with a singleton pregnancy and a history of spontaneous PTB or to patients with a singleton pregnancy and no history of spontaneous PTB who have cervical shortening at less than 24 weeks.
- Cerclage may be offered between 16 and 24 weeks for a cervical length less than 25 mm in a patient with a singleton gestation who has a history of spontaneous PTB (<10 mm if no history of spontaneous PTB) or for a dilated cervix on exam regardless of history.
- Women who have a twin gestation with cervical dilation may be offered physical exam–indicated cerclage.
Which patients may benefit from antepartum fetal surveillance and when to initiate it
American College of Obstetricians and Gynecologists’ Committee on Obstetrics Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Antepartum fetal surveillance: ACOG practice bulletin, number 229. Obstet Gynecol. 2021;137:e116-e127.
The ultimate purpose of antenatal fetal surveillance is to prevent stillbirth. However, stillbirth has multiple etiologies, not all of which are preventable with testing. In June 2021, ACOG released a new Committee Opinion containing guidelines for fetal surveillance, including suggested gestational age at initiation and frequency of testing, for the most common high-risk conditions. ACOG also released an update to the Practice Bulletin on antepartum fetal surveillance; additions include randomized controlled trial level data on the utility of fetal kick counts (FKCs) and recommendations that align with the new Committee Opinion.
Data for the efficacy of antepartum fetal surveillance are lacking, mainly due to the difficulty of performing prospective studies in stillbirth. The existing evidence is subject to intervention bias, as deliveries increase in tested patients, and recommendations rely heavily on expert consensus and nonrandomized studies. Antenatal testing is also time, cost, and labor intensive, with the risk of intervention for a false-positive result. Despite these limitations, obstetrical practices routinely perform antenatal fetal surveillance.
The new guidelines: The why, when, and how often
Why. Antepartum fetal surveillance is suggested for conditions that have a risk of stillbirth greater than 0.8 per 1,000 (that is, the false-negative rate of a biophysical profile or a modified biophysical profile) and the relative risk or odds ratio is greater than 2.0 for stillbirth compared with unaffected pregnancies.
When. For most conditions, ACOG recommends initiation of testing at 32 weeks or later, with notable earlier exceptions for some of the highest-risk patients. For certain conditions, such as fetal growth restriction and hypertensive disorders of pregnancy, the recommendation is to start “at diagnosis,” with the corollary “or at a gestational age when delivery would be considered because of abnormal results.” Shared decision making with the patient about pregnancy goals therefore is required, particularly in cases of fetal anomalies, genetic conditions, or at very early gestational ages.
How often. The recommended frequency of testing is at least weekly. Testing frequency should be increased to twice-weekly outpatient or daily inpatient for the most complicated pregnancies (for example, fetal growth restriction with abnormal umbilical artery Doppler studies, preeclampsia with severe features).
Once or twice weekly is an option for many conditions, which gives the clinician the opportunity to assess clinical stability as well as the patient’s input in terms of logistics and anxiety.
Patients with multiple conditions may fall into the “individualized” category, as do patients with suboptimal control of conditions (for example, diabetes, hypertension) that may affect the fetus as the pregnancy progresses.
New diagnoses included for surveillance
Several diagnoses not previously included now qualify for antepartum fetal surveillance under the new guidelines, most notably:
- history of obstetrical complications in the immediate preceding pregnancy
—history of prior fetal growth restriction requiring preterm delivery
—history of prior preeclampsia requiring delivery
- alcohol use of 5 or more drinks per day
- in vitro fertilization
- abnormal serum markers
—pregnancy-associated plasma protein A (PAPP-A) in the fifth or lower percentile or 0.4 multiples of the median (MoM)
—second trimester inhibin A of 2 or greater MoM
- prepregnancy body mass index (BMI)
—this is divided into 2 categories for timing of initiation of testing:
- 37 weeks for BMI of 35 to 39.9 kg/m2
- 34 weeks for BMI of or greater than 40 kg/m2.
Fetal kick counts
The major change to the updated Practice Bulletin on antenatal surveillance is the inclusion of data on FKCs, a simple modality of fetal surveillance that does not require a clinical visit. For FKCs, a meta-analysis of more than 450,000 patients did not demonstrate a difference in perinatal death between the FKC intervention group (0.54%) and the control group (0.59%). Of note, there were small but statistically significant increases in the rates of induction of labor, cesarean delivery, and preterm delivery in the FKC intervention group. Therefore, this update does not recommend a formal program of FKCs for all patients.
- The antenatal fetal surveillance guidelines are just that—guidelines, not mandates. Their use will need to be adapted for specific patient populations and practice management patterns.
- Many conditions qualify for “individualized” surveillance, which offers the opportunity for detailed discussions on the patient’s care. This includes shared decision making with patients to meet their goals for the pregnancy.
- Although patient-perceived decreased fetal movement always warrants clinical evaluation, a regular program of fetal kick count monitoring is not recommended for all patients due to lack of data supporting its benefit in reducing perinatal death.
- As with any change, new guidelines potentially are a source of frustration, so a concerted effort by obstetrical clinicians to agree on adoption of the guidelines is needed. Additional clinical resources and both clinician and patient education may be required depending on current practice style, as the new strategy may increase the number of appointments and ultrasound exams required.
Continue to: Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation...
Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation
American College of Obstetricians and Gynecologists. Use of antenatal corticosteroids at 22 weeks of gestation: ACOG practice advisory. September 2021. https://www .acog.org/clinical/clinical-guidance/practice-advisory /articles/2021/09/use-of-antenatal-corticosteroids-at -22-weeks-of-gestation. Accessed December 11, 2021.
In September 2021, ACOG and SMFM released a Practice Advisory updating the current recommendations for the administration of antenatal corticosteroids in the periviable period (22 to 25 6/7 weeks’ gestation). Whereas the prior lower limit of gestational age for consideration of steroids was 23 weeks, the new recommendation now extends this consideration down to 22 weeks.
The cited data include a meta-analysis of more than 2,200 patients in which the survival rate of infants born between 22 and 22 6/7 weeks who were exposed to antenatal steroids was 39% compared with 19.5% in the unexposed group. Another study of more than 1,000 patients demonstrated a statistically significant increase in overall survival in patients treated with antenatal steroids plus life support compared with life support only (38.5% vs 17.7%). Survival without major morbidity in this study, although increased from 1% to 4.4%, was still low.
Recommendation carries caveats
Given this information, the Practice Advisory offers a 2C level recommendation (weak recommendation, low quality of evidence) for antenatal steroids at 22 to 22 6/7 weeks’ gestation if neonatal resuscitation is planned, acknowledging the limitations and potential bias of the available data.
The Practice Advisory emphasizes the importance of counseling and patient involvement in the decision making. This requires a multidisciplinary collaboration among the neonatology and obstetrical teams, flexibility in the plan after birth depending on the infant’s condition, and redirection of care if appropriate. Estimated fetal weight, the presence of multiple gestations, fetal biologic sex, and any anomalies are also important in helping families make an informed decision for their particular pregnancy. As described in the Obstetric Care Consensus on periviable birth,6 it is important to remember that considerations and recommendations are not the same as requirements, and redirection of care to comfort and family memory making is not the same as withholding care.
The rest of the recommendations for the administration of antenatal steroids remain the same: Antenatal steroids are not recommended at less than 22 weeks due to lack of evidence of benefit, and they continue to be recommended at 24 weeks and beyond. ●
- Antenatal corticosteroids may be considered at 22 to 22 6/7 weeks’ gestation if, after thorough patient counseling, neonatal resuscitation is desired and planned by the family.
- The overall likelihood of survival and survival without major morbidities continues to be very low in the periviable period, especially at 22 weeks. Gestational age is only one of the many factors that must be considered in the shared decision making for this very difficult decision.
- Palliative care is a valid and appropriate option for patients facing a periviable delivery after appropriate counseling or after evaluation of the infant has occurred after birth.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2019. NCHS Data Brief, no 387. Hyattsville, MD: National Center for Health Statistics. October 2020. www.cdc.gov/nchs /data/databriefs/db387-H.pdf. Accessed December 20, 2021.
- To MS, Skentou C, Chan C, et al. Cervical assessment at the routine 23-week scan: standardizing techniques. Ultrasound Obstet Gynecol. 2001;17:217-219.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. Am J Obstet Gynecol. 2020;223:B16-B18.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 6: Periviable birth. Obstet Gynecol. 2017;130:e187-e199.
Obstetrical practice saw updates in 2021 to 3 major areas of pregnancy management: preterm birth prevention, antepartum fetal surveillance, and the use of antenatal corticosteroids.
Updated guidance on predicting and preventing spontaneous PTB
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
Preterm birth (PTB) continues to pose a challenge in clinical obstetrics, with the most recently reported rate of 10.2% in the United States.1 This accounts for almost 75% of perinatal mortality and more than half of neonatal morbidity, in which effects last well past the neonatal period. PTB is classified as spontaneous (following preterm labor, preterm prelabor rupture of membranes, or cervical insufficiency) or iatrogenic (indicated due to maternal and/or fetal complications).
Assessing risk for PTB
The single strongest predictor of subsequent PTB is a history of spontaneous PTB. Recurrence risk is further increased by the number of prior PTBs and the gestational age at prior PTB. Identification of and intervention for a short cervix has been shown to prolong gestation. Transvaginal ultrasonography of the cervix is the most accurate method for evaluating cervical length (CL). Specific examination criteria exist to ensure that CL measurements are reproducible and reliable.2 A short CL is generally defined as a measurement of less than 25 mm between 16 and 24 weeks’ gestation.
Screening strategies
The American College of Obstetricians and Gynecologists (ACOG), with an endorsement from the Society for Maternal-Fetal Medicine (SMFM), recommends cervical evaluation during the anatomy ultrasound exam between 18 0/7 and 22 6/7 weeks’ gestation in all pregnant patients regardless of prior PTB.3 If transabdominal imaging is concerning for a shortened cervix, transvaginal ultrasonography should be performed to assess the CL.
Serial transvaginal CL measurements are recommended between 16 0/7 and 24 0/7 weeks’ gestation for patients with a current singleton pregnancy and history of a spontaneous PTB, but not for patients with a history of iatrogenic or indicated PTB.
Interventions: Mind your p’s and c’s
Interventions to reduce the risk of spontaneous PTB depend on whether the current pregnancy is a singleton, twins, or higher-order multiples; CL measurement; and history of spontaneous PTB. Preconception optimization of underlying medical conditions also is important to reduce the risk of recurrent indicated PTB.
Continue to: Progesterone...
Progesterone
Vaginal administration. Several trials have shown that vaginal progesterone can be used to reduce the risk of spontaneous PTB in asymptomatic patients with a singleton pregnancy, incidental finding of a short cervix (<25 mm), and no history of spontaneous PTB. This is a change from the prior recommendation of CL of less than 20 mm. In the setting of a twin pregnancy, regardless of CL, data do not definitively support the use of vaginal progesterone.
Intramuscular administration.4,5 The popularity of intramuscular progesterone has waxed and waned. At present, ACOG recommends that all patients with a singleton pregnancy and history of spontaneous PTB be offered progesterone beginning at 16 0/7 weeks’ gestation following a shared decision-making process that includes the limited data of efficacy noted in existing studies.
In a twin pregnancy with no history of spontaneous PTB, the use of intramuscular progesterone has been shown to potentially increase the risk of PTB and admission to the neonatal intensive care unit. As such, intramuscular progesterone in the setting of a twin gestation without a history of spontaneous PTB is not recommended. When a prior spontaneous PTB has occurred, there may be some benefit to intramuscular progesterone in twin gestations.
Cerclage
Ultrasound indicated. In a singleton pregnancy with an incidental finding of short cervix (<25 mm) and no history of PTB, the use of cerclage is of uncertain benefit. Effectiveness may be seen if the cervix is less than 10 mm. Ultrasound-indicated cerclage should be considered in a singleton pregnancy with a CL less than 25 mm and a history of spontaneous PTB.
Possibly one of the most controversial topics is ultrasound-indicated cerclage placement in twin gestation. As with many situations in obstetrics, data regarding ultrasound-indicated cerclage in twin gestation is based on small retrospective studies fraught with bias. Results from these studies range from no benefit, to potential benefit, to even possible increased risk of PTB. Since data are limited, as we await more evidence, it is recommended that the clinician and patient use shared decision making to decide on cerclage placement in a twin gestation.
Exam indicated. In a singleton pregnancy with a dilated cervix on digital or speculum exam between 16 0/7 to 23 6/7 weeks’ gestation, a physical exam–indicated cerclage should be offered. Exam-indicated cerclage also may reduce the incidence of PTB in twin gestations with cervical dilation between 16 0/7 and 23 6/7 weeks’ gestation. Indomethacin tocolysis and perioperative antibiotics should be considered when an exam-indicated cerclage is placed.
As the limits of viability are continually pushed earlier, more in-depth conversation is needed with patients who are considering an exam-indicated cerclage. The nuances of periviability and the likelihood that an exam-indicated cerclage will commit a pregnancy to a periviable or extremely preterm birth should be discussed in detail using a shared decision making model.
Regardless of whether the cerclage is ultrasound or exam indicated, once it is placed there is no utility in additional CL ultrasound monitoring.
Pessary
Vaginal pessaries for prevention of PTB have not gained popularity in the United States as they have in other countries. Trials are being conducted to determine the utility of vaginal pessary, but current data have not proven its effectiveness in preventing PTB in the setting of singleton pregnancy, short cervix, and no history of spontaneous PTB. So for now, pessary is not recommended. The same can be said for use in the twin gestation.
- All patients should have cervical evaluation during pregnancy. Serial imaging is reserved for those with a history of spontaneous PTB.
- Progesterone supplementation should be offered to patients with a singleton pregnancy and a history of spontaneous PTB or to patients with a singleton pregnancy and no history of spontaneous PTB who have cervical shortening at less than 24 weeks.
- Cerclage may be offered between 16 and 24 weeks for a cervical length less than 25 mm in a patient with a singleton gestation who has a history of spontaneous PTB (<10 mm if no history of spontaneous PTB) or for a dilated cervix on exam regardless of history.
- Women who have a twin gestation with cervical dilation may be offered physical exam–indicated cerclage.
Which patients may benefit from antepartum fetal surveillance and when to initiate it
American College of Obstetricians and Gynecologists’ Committee on Obstetrics Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Antepartum fetal surveillance: ACOG practice bulletin, number 229. Obstet Gynecol. 2021;137:e116-e127.
The ultimate purpose of antenatal fetal surveillance is to prevent stillbirth. However, stillbirth has multiple etiologies, not all of which are preventable with testing. In June 2021, ACOG released a new Committee Opinion containing guidelines for fetal surveillance, including suggested gestational age at initiation and frequency of testing, for the most common high-risk conditions. ACOG also released an update to the Practice Bulletin on antepartum fetal surveillance; additions include randomized controlled trial level data on the utility of fetal kick counts (FKCs) and recommendations that align with the new Committee Opinion.
Data for the efficacy of antepartum fetal surveillance are lacking, mainly due to the difficulty of performing prospective studies in stillbirth. The existing evidence is subject to intervention bias, as deliveries increase in tested patients, and recommendations rely heavily on expert consensus and nonrandomized studies. Antenatal testing is also time, cost, and labor intensive, with the risk of intervention for a false-positive result. Despite these limitations, obstetrical practices routinely perform antenatal fetal surveillance.
The new guidelines: The why, when, and how often
Why. Antepartum fetal surveillance is suggested for conditions that have a risk of stillbirth greater than 0.8 per 1,000 (that is, the false-negative rate of a biophysical profile or a modified biophysical profile) and the relative risk or odds ratio is greater than 2.0 for stillbirth compared with unaffected pregnancies.
When. For most conditions, ACOG recommends initiation of testing at 32 weeks or later, with notable earlier exceptions for some of the highest-risk patients. For certain conditions, such as fetal growth restriction and hypertensive disorders of pregnancy, the recommendation is to start “at diagnosis,” with the corollary “or at a gestational age when delivery would be considered because of abnormal results.” Shared decision making with the patient about pregnancy goals therefore is required, particularly in cases of fetal anomalies, genetic conditions, or at very early gestational ages.
How often. The recommended frequency of testing is at least weekly. Testing frequency should be increased to twice-weekly outpatient or daily inpatient for the most complicated pregnancies (for example, fetal growth restriction with abnormal umbilical artery Doppler studies, preeclampsia with severe features).
Once or twice weekly is an option for many conditions, which gives the clinician the opportunity to assess clinical stability as well as the patient’s input in terms of logistics and anxiety.
Patients with multiple conditions may fall into the “individualized” category, as do patients with suboptimal control of conditions (for example, diabetes, hypertension) that may affect the fetus as the pregnancy progresses.
New diagnoses included for surveillance
Several diagnoses not previously included now qualify for antepartum fetal surveillance under the new guidelines, most notably:
- history of obstetrical complications in the immediate preceding pregnancy
—history of prior fetal growth restriction requiring preterm delivery
—history of prior preeclampsia requiring delivery
- alcohol use of 5 or more drinks per day
- in vitro fertilization
- abnormal serum markers
—pregnancy-associated plasma protein A (PAPP-A) in the fifth or lower percentile or 0.4 multiples of the median (MoM)
—second trimester inhibin A of 2 or greater MoM
- prepregnancy body mass index (BMI)
—this is divided into 2 categories for timing of initiation of testing:
- 37 weeks for BMI of 35 to 39.9 kg/m2
- 34 weeks for BMI of or greater than 40 kg/m2.
Fetal kick counts
The major change to the updated Practice Bulletin on antenatal surveillance is the inclusion of data on FKCs, a simple modality of fetal surveillance that does not require a clinical visit. For FKCs, a meta-analysis of more than 450,000 patients did not demonstrate a difference in perinatal death between the FKC intervention group (0.54%) and the control group (0.59%). Of note, there were small but statistically significant increases in the rates of induction of labor, cesarean delivery, and preterm delivery in the FKC intervention group. Therefore, this update does not recommend a formal program of FKCs for all patients.
- The antenatal fetal surveillance guidelines are just that—guidelines, not mandates. Their use will need to be adapted for specific patient populations and practice management patterns.
- Many conditions qualify for “individualized” surveillance, which offers the opportunity for detailed discussions on the patient’s care. This includes shared decision making with patients to meet their goals for the pregnancy.
- Although patient-perceived decreased fetal movement always warrants clinical evaluation, a regular program of fetal kick count monitoring is not recommended for all patients due to lack of data supporting its benefit in reducing perinatal death.
- As with any change, new guidelines potentially are a source of frustration, so a concerted effort by obstetrical clinicians to agree on adoption of the guidelines is needed. Additional clinical resources and both clinician and patient education may be required depending on current practice style, as the new strategy may increase the number of appointments and ultrasound exams required.
Continue to: Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation...
Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation
American College of Obstetricians and Gynecologists. Use of antenatal corticosteroids at 22 weeks of gestation: ACOG practice advisory. September 2021. https://www .acog.org/clinical/clinical-guidance/practice-advisory /articles/2021/09/use-of-antenatal-corticosteroids-at -22-weeks-of-gestation. Accessed December 11, 2021.
In September 2021, ACOG and SMFM released a Practice Advisory updating the current recommendations for the administration of antenatal corticosteroids in the periviable period (22 to 25 6/7 weeks’ gestation). Whereas the prior lower limit of gestational age for consideration of steroids was 23 weeks, the new recommendation now extends this consideration down to 22 weeks.
The cited data include a meta-analysis of more than 2,200 patients in which the survival rate of infants born between 22 and 22 6/7 weeks who were exposed to antenatal steroids was 39% compared with 19.5% in the unexposed group. Another study of more than 1,000 patients demonstrated a statistically significant increase in overall survival in patients treated with antenatal steroids plus life support compared with life support only (38.5% vs 17.7%). Survival without major morbidity in this study, although increased from 1% to 4.4%, was still low.
Recommendation carries caveats
Given this information, the Practice Advisory offers a 2C level recommendation (weak recommendation, low quality of evidence) for antenatal steroids at 22 to 22 6/7 weeks’ gestation if neonatal resuscitation is planned, acknowledging the limitations and potential bias of the available data.
The Practice Advisory emphasizes the importance of counseling and patient involvement in the decision making. This requires a multidisciplinary collaboration among the neonatology and obstetrical teams, flexibility in the plan after birth depending on the infant’s condition, and redirection of care if appropriate. Estimated fetal weight, the presence of multiple gestations, fetal biologic sex, and any anomalies are also important in helping families make an informed decision for their particular pregnancy. As described in the Obstetric Care Consensus on periviable birth,6 it is important to remember that considerations and recommendations are not the same as requirements, and redirection of care to comfort and family memory making is not the same as withholding care.
The rest of the recommendations for the administration of antenatal steroids remain the same: Antenatal steroids are not recommended at less than 22 weeks due to lack of evidence of benefit, and they continue to be recommended at 24 weeks and beyond. ●
- Antenatal corticosteroids may be considered at 22 to 22 6/7 weeks’ gestation if, after thorough patient counseling, neonatal resuscitation is desired and planned by the family.
- The overall likelihood of survival and survival without major morbidities continues to be very low in the periviable period, especially at 22 weeks. Gestational age is only one of the many factors that must be considered in the shared decision making for this very difficult decision.
- Palliative care is a valid and appropriate option for patients facing a periviable delivery after appropriate counseling or after evaluation of the infant has occurred after birth.
Obstetrical practice saw updates in 2021 to 3 major areas of pregnancy management: preterm birth prevention, antepartum fetal surveillance, and the use of antenatal corticosteroids.
Updated guidance on predicting and preventing spontaneous PTB
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
Preterm birth (PTB) continues to pose a challenge in clinical obstetrics, with the most recently reported rate of 10.2% in the United States.1 This accounts for almost 75% of perinatal mortality and more than half of neonatal morbidity, in which effects last well past the neonatal period. PTB is classified as spontaneous (following preterm labor, preterm prelabor rupture of membranes, or cervical insufficiency) or iatrogenic (indicated due to maternal and/or fetal complications).
Assessing risk for PTB
The single strongest predictor of subsequent PTB is a history of spontaneous PTB. Recurrence risk is further increased by the number of prior PTBs and the gestational age at prior PTB. Identification of and intervention for a short cervix has been shown to prolong gestation. Transvaginal ultrasonography of the cervix is the most accurate method for evaluating cervical length (CL). Specific examination criteria exist to ensure that CL measurements are reproducible and reliable.2 A short CL is generally defined as a measurement of less than 25 mm between 16 and 24 weeks’ gestation.
Screening strategies
The American College of Obstetricians and Gynecologists (ACOG), with an endorsement from the Society for Maternal-Fetal Medicine (SMFM), recommends cervical evaluation during the anatomy ultrasound exam between 18 0/7 and 22 6/7 weeks’ gestation in all pregnant patients regardless of prior PTB.3 If transabdominal imaging is concerning for a shortened cervix, transvaginal ultrasonography should be performed to assess the CL.
Serial transvaginal CL measurements are recommended between 16 0/7 and 24 0/7 weeks’ gestation for patients with a current singleton pregnancy and history of a spontaneous PTB, but not for patients with a history of iatrogenic or indicated PTB.
Interventions: Mind your p’s and c’s
Interventions to reduce the risk of spontaneous PTB depend on whether the current pregnancy is a singleton, twins, or higher-order multiples; CL measurement; and history of spontaneous PTB. Preconception optimization of underlying medical conditions also is important to reduce the risk of recurrent indicated PTB.
Continue to: Progesterone...
Progesterone
Vaginal administration. Several trials have shown that vaginal progesterone can be used to reduce the risk of spontaneous PTB in asymptomatic patients with a singleton pregnancy, incidental finding of a short cervix (<25 mm), and no history of spontaneous PTB. This is a change from the prior recommendation of CL of less than 20 mm. In the setting of a twin pregnancy, regardless of CL, data do not definitively support the use of vaginal progesterone.
Intramuscular administration.4,5 The popularity of intramuscular progesterone has waxed and waned. At present, ACOG recommends that all patients with a singleton pregnancy and history of spontaneous PTB be offered progesterone beginning at 16 0/7 weeks’ gestation following a shared decision-making process that includes the limited data of efficacy noted in existing studies.
In a twin pregnancy with no history of spontaneous PTB, the use of intramuscular progesterone has been shown to potentially increase the risk of PTB and admission to the neonatal intensive care unit. As such, intramuscular progesterone in the setting of a twin gestation without a history of spontaneous PTB is not recommended. When a prior spontaneous PTB has occurred, there may be some benefit to intramuscular progesterone in twin gestations.
Cerclage
Ultrasound indicated. In a singleton pregnancy with an incidental finding of short cervix (<25 mm) and no history of PTB, the use of cerclage is of uncertain benefit. Effectiveness may be seen if the cervix is less than 10 mm. Ultrasound-indicated cerclage should be considered in a singleton pregnancy with a CL less than 25 mm and a history of spontaneous PTB.
Possibly one of the most controversial topics is ultrasound-indicated cerclage placement in twin gestation. As with many situations in obstetrics, data regarding ultrasound-indicated cerclage in twin gestation is based on small retrospective studies fraught with bias. Results from these studies range from no benefit, to potential benefit, to even possible increased risk of PTB. Since data are limited, as we await more evidence, it is recommended that the clinician and patient use shared decision making to decide on cerclage placement in a twin gestation.
Exam indicated. In a singleton pregnancy with a dilated cervix on digital or speculum exam between 16 0/7 to 23 6/7 weeks’ gestation, a physical exam–indicated cerclage should be offered. Exam-indicated cerclage also may reduce the incidence of PTB in twin gestations with cervical dilation between 16 0/7 and 23 6/7 weeks’ gestation. Indomethacin tocolysis and perioperative antibiotics should be considered when an exam-indicated cerclage is placed.
As the limits of viability are continually pushed earlier, more in-depth conversation is needed with patients who are considering an exam-indicated cerclage. The nuances of periviability and the likelihood that an exam-indicated cerclage will commit a pregnancy to a periviable or extremely preterm birth should be discussed in detail using a shared decision making model.
Regardless of whether the cerclage is ultrasound or exam indicated, once it is placed there is no utility in additional CL ultrasound monitoring.
Pessary
Vaginal pessaries for prevention of PTB have not gained popularity in the United States as they have in other countries. Trials are being conducted to determine the utility of vaginal pessary, but current data have not proven its effectiveness in preventing PTB in the setting of singleton pregnancy, short cervix, and no history of spontaneous PTB. So for now, pessary is not recommended. The same can be said for use in the twin gestation.
- All patients should have cervical evaluation during pregnancy. Serial imaging is reserved for those with a history of spontaneous PTB.
- Progesterone supplementation should be offered to patients with a singleton pregnancy and a history of spontaneous PTB or to patients with a singleton pregnancy and no history of spontaneous PTB who have cervical shortening at less than 24 weeks.
- Cerclage may be offered between 16 and 24 weeks for a cervical length less than 25 mm in a patient with a singleton gestation who has a history of spontaneous PTB (<10 mm if no history of spontaneous PTB) or for a dilated cervix on exam regardless of history.
- Women who have a twin gestation with cervical dilation may be offered physical exam–indicated cerclage.
Which patients may benefit from antepartum fetal surveillance and when to initiate it
American College of Obstetricians and Gynecologists’ Committee on Obstetrics Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Antepartum fetal surveillance: ACOG practice bulletin, number 229. Obstet Gynecol. 2021;137:e116-e127.
The ultimate purpose of antenatal fetal surveillance is to prevent stillbirth. However, stillbirth has multiple etiologies, not all of which are preventable with testing. In June 2021, ACOG released a new Committee Opinion containing guidelines for fetal surveillance, including suggested gestational age at initiation and frequency of testing, for the most common high-risk conditions. ACOG also released an update to the Practice Bulletin on antepartum fetal surveillance; additions include randomized controlled trial level data on the utility of fetal kick counts (FKCs) and recommendations that align with the new Committee Opinion.
Data for the efficacy of antepartum fetal surveillance are lacking, mainly due to the difficulty of performing prospective studies in stillbirth. The existing evidence is subject to intervention bias, as deliveries increase in tested patients, and recommendations rely heavily on expert consensus and nonrandomized studies. Antenatal testing is also time, cost, and labor intensive, with the risk of intervention for a false-positive result. Despite these limitations, obstetrical practices routinely perform antenatal fetal surveillance.
The new guidelines: The why, when, and how often
Why. Antepartum fetal surveillance is suggested for conditions that have a risk of stillbirth greater than 0.8 per 1,000 (that is, the false-negative rate of a biophysical profile or a modified biophysical profile) and the relative risk or odds ratio is greater than 2.0 for stillbirth compared with unaffected pregnancies.
When. For most conditions, ACOG recommends initiation of testing at 32 weeks or later, with notable earlier exceptions for some of the highest-risk patients. For certain conditions, such as fetal growth restriction and hypertensive disorders of pregnancy, the recommendation is to start “at diagnosis,” with the corollary “or at a gestational age when delivery would be considered because of abnormal results.” Shared decision making with the patient about pregnancy goals therefore is required, particularly in cases of fetal anomalies, genetic conditions, or at very early gestational ages.
How often. The recommended frequency of testing is at least weekly. Testing frequency should be increased to twice-weekly outpatient or daily inpatient for the most complicated pregnancies (for example, fetal growth restriction with abnormal umbilical artery Doppler studies, preeclampsia with severe features).
Once or twice weekly is an option for many conditions, which gives the clinician the opportunity to assess clinical stability as well as the patient’s input in terms of logistics and anxiety.
Patients with multiple conditions may fall into the “individualized” category, as do patients with suboptimal control of conditions (for example, diabetes, hypertension) that may affect the fetus as the pregnancy progresses.
New diagnoses included for surveillance
Several diagnoses not previously included now qualify for antepartum fetal surveillance under the new guidelines, most notably:
- history of obstetrical complications in the immediate preceding pregnancy
—history of prior fetal growth restriction requiring preterm delivery
—history of prior preeclampsia requiring delivery
- alcohol use of 5 or more drinks per day
- in vitro fertilization
- abnormal serum markers
—pregnancy-associated plasma protein A (PAPP-A) in the fifth or lower percentile or 0.4 multiples of the median (MoM)
—second trimester inhibin A of 2 or greater MoM
- prepregnancy body mass index (BMI)
—this is divided into 2 categories for timing of initiation of testing:
- 37 weeks for BMI of 35 to 39.9 kg/m2
- 34 weeks for BMI of or greater than 40 kg/m2.
Fetal kick counts
The major change to the updated Practice Bulletin on antenatal surveillance is the inclusion of data on FKCs, a simple modality of fetal surveillance that does not require a clinical visit. For FKCs, a meta-analysis of more than 450,000 patients did not demonstrate a difference in perinatal death between the FKC intervention group (0.54%) and the control group (0.59%). Of note, there were small but statistically significant increases in the rates of induction of labor, cesarean delivery, and preterm delivery in the FKC intervention group. Therefore, this update does not recommend a formal program of FKCs for all patients.
- The antenatal fetal surveillance guidelines are just that—guidelines, not mandates. Their use will need to be adapted for specific patient populations and practice management patterns.
- Many conditions qualify for “individualized” surveillance, which offers the opportunity for detailed discussions on the patient’s care. This includes shared decision making with patients to meet their goals for the pregnancy.
- Although patient-perceived decreased fetal movement always warrants clinical evaluation, a regular program of fetal kick count monitoring is not recommended for all patients due to lack of data supporting its benefit in reducing perinatal death.
- As with any change, new guidelines potentially are a source of frustration, so a concerted effort by obstetrical clinicians to agree on adoption of the guidelines is needed. Additional clinical resources and both clinician and patient education may be required depending on current practice style, as the new strategy may increase the number of appointments and ultrasound exams required.
Continue to: Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation...
Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation
American College of Obstetricians and Gynecologists. Use of antenatal corticosteroids at 22 weeks of gestation: ACOG practice advisory. September 2021. https://www .acog.org/clinical/clinical-guidance/practice-advisory /articles/2021/09/use-of-antenatal-corticosteroids-at -22-weeks-of-gestation. Accessed December 11, 2021.
In September 2021, ACOG and SMFM released a Practice Advisory updating the current recommendations for the administration of antenatal corticosteroids in the periviable period (22 to 25 6/7 weeks’ gestation). Whereas the prior lower limit of gestational age for consideration of steroids was 23 weeks, the new recommendation now extends this consideration down to 22 weeks.
The cited data include a meta-analysis of more than 2,200 patients in which the survival rate of infants born between 22 and 22 6/7 weeks who were exposed to antenatal steroids was 39% compared with 19.5% in the unexposed group. Another study of more than 1,000 patients demonstrated a statistically significant increase in overall survival in patients treated with antenatal steroids plus life support compared with life support only (38.5% vs 17.7%). Survival without major morbidity in this study, although increased from 1% to 4.4%, was still low.
Recommendation carries caveats
Given this information, the Practice Advisory offers a 2C level recommendation (weak recommendation, low quality of evidence) for antenatal steroids at 22 to 22 6/7 weeks’ gestation if neonatal resuscitation is planned, acknowledging the limitations and potential bias of the available data.
The Practice Advisory emphasizes the importance of counseling and patient involvement in the decision making. This requires a multidisciplinary collaboration among the neonatology and obstetrical teams, flexibility in the plan after birth depending on the infant’s condition, and redirection of care if appropriate. Estimated fetal weight, the presence of multiple gestations, fetal biologic sex, and any anomalies are also important in helping families make an informed decision for their particular pregnancy. As described in the Obstetric Care Consensus on periviable birth,6 it is important to remember that considerations and recommendations are not the same as requirements, and redirection of care to comfort and family memory making is not the same as withholding care.
The rest of the recommendations for the administration of antenatal steroids remain the same: Antenatal steroids are not recommended at less than 22 weeks due to lack of evidence of benefit, and they continue to be recommended at 24 weeks and beyond. ●
- Antenatal corticosteroids may be considered at 22 to 22 6/7 weeks’ gestation if, after thorough patient counseling, neonatal resuscitation is desired and planned by the family.
- The overall likelihood of survival and survival without major morbidities continues to be very low in the periviable period, especially at 22 weeks. Gestational age is only one of the many factors that must be considered in the shared decision making for this very difficult decision.
- Palliative care is a valid and appropriate option for patients facing a periviable delivery after appropriate counseling or after evaluation of the infant has occurred after birth.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2019. NCHS Data Brief, no 387. Hyattsville, MD: National Center for Health Statistics. October 2020. www.cdc.gov/nchs /data/databriefs/db387-H.pdf. Accessed December 20, 2021.
- To MS, Skentou C, Chan C, et al. Cervical assessment at the routine 23-week scan: standardizing techniques. Ultrasound Obstet Gynecol. 2001;17:217-219.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. Am J Obstet Gynecol. 2020;223:B16-B18.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 6: Periviable birth. Obstet Gynecol. 2017;130:e187-e199.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2019. NCHS Data Brief, no 387. Hyattsville, MD: National Center for Health Statistics. October 2020. www.cdc.gov/nchs /data/databriefs/db387-H.pdf. Accessed December 20, 2021.
- To MS, Skentou C, Chan C, et al. Cervical assessment at the routine 23-week scan: standardizing techniques. Ultrasound Obstet Gynecol. 2001;17:217-219.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. Am J Obstet Gynecol. 2020;223:B16-B18.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 6: Periviable birth. Obstet Gynecol. 2017;130:e187-e199.
Do recent data on use of menopausal HT and subsequent risk of dementia indicate an association?
Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
Much interest has surrounded whether the use of menopausal HT impacts future risk of cognitive decline. Recently, Vinogradova and colleagues conducted an observational study using data from 2 large primary care databases, QResearch and the Clinical Practice Research Datalink (CPRD), in the United Kingdom.1 The investigators conducted case-control studies that included women aged 55 and older diagnosed with dementia and up to 5 controls without dementia. Only cases and controls with at least 10 years of medical records prior to the index date (that is, the time of dementia diagnosis in cases) were included. Since early symptoms of dementia prior to diagnosis may cause sleep problems and dysphoria (which also may be symptoms of menopause), HT prescriptions during the 3 years prior to the index date were excluded.
Details of the study
Among 16,291 cases and 68,726 controls, the women’s mean age was approximately 83 years. Cases were identified by using codes for dementia from patients’ clinical records or records of prescriptions for drugs used to treat dementia.
More than half the women were being treated for hypertension, and 14% of women in both groups had used HT. Women were considered users of estrogen-only therapy if they had no prescriptions containing a progestogen after their first prescription for systemic estrogen as the start of exposure to HT. Those with any subsequent prescription containing a progestogen were classified as combined HT users.
Results. In an analysis adjusted for all available potential confounders—including lifestyle factors, ethnicity, family history of dementia, early menopause, oophorectomy/ hysterectomy, comorbidities, and use of other relevant drugs—the use of HT was not associated with risk of dementia.
A reduced risk of dementia was noted among women who had been taking estrogen-only HT for 10 years or more (odds ratio [OR], 0.85; 95% confidence interval [CI], 0.76–0.94). An elevated risk of Alzheimer disease was noted among women who had used estrogen-progestin HT for 5 to 9 years (OR, 1.19; CI, 1.06–1.33).1
Study strengths and limitations
The authors pointed out that this study’s main strengths were that it had a very large sample size representative of the general population and that its design permitted capture of all known cases as well as precision recording for prescribed drugs. On the other hand, the study is limited by the possible lack of data for some older women before the index date; that is, menopause in this latter group started before their registration or before these data were gathered electronically by their practice. ●
The authors of this British large observational study took pains to minimize potential bias. The finding that long-term use of estrogen-only HT may be neuroprotective is consistent with results of recent studies in the United States and Finland,2-4 as well as with the Women’s Health Initiative randomized trial, which found that with 18 years of follow-up, treatment with conjugated estrogen alone was associated with a 26% reduced risk of death from Alzheimer disease.5 Overall, however, the main message we should glean from this important study by Vinogradova and colleagues is that women with bothersome vasomotor symptoms considering use of menopausal HT can be reassured that such therapy has little if any impact on future risk of cognitive decline.
ANDREW M. KAUNITZ, MD, NCMP
- Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
- Matyi JM, Rattinger GB, Schwartz S, et al. Lifetime estrogen exposure and cognition in late life: the Cache County study. Menopause. 2019;26:1366-1374. doi: 10.1097 /GME.0000000000001405.
- Liu JH. Does estrogen provide “neuroprotection” for postmenopausal women? Menopause. 2019;26:1361-1362. doi: 10.1097/GME.0000000000001459.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88:1062-1068. doi: 10.1212 /WNL.0000000000003696.
- Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001 /jama.2017.11217.
Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
Much interest has surrounded whether the use of menopausal HT impacts future risk of cognitive decline. Recently, Vinogradova and colleagues conducted an observational study using data from 2 large primary care databases, QResearch and the Clinical Practice Research Datalink (CPRD), in the United Kingdom.1 The investigators conducted case-control studies that included women aged 55 and older diagnosed with dementia and up to 5 controls without dementia. Only cases and controls with at least 10 years of medical records prior to the index date (that is, the time of dementia diagnosis in cases) were included. Since early symptoms of dementia prior to diagnosis may cause sleep problems and dysphoria (which also may be symptoms of menopause), HT prescriptions during the 3 years prior to the index date were excluded.
Details of the study
Among 16,291 cases and 68,726 controls, the women’s mean age was approximately 83 years. Cases were identified by using codes for dementia from patients’ clinical records or records of prescriptions for drugs used to treat dementia.
More than half the women were being treated for hypertension, and 14% of women in both groups had used HT. Women were considered users of estrogen-only therapy if they had no prescriptions containing a progestogen after their first prescription for systemic estrogen as the start of exposure to HT. Those with any subsequent prescription containing a progestogen were classified as combined HT users.
Results. In an analysis adjusted for all available potential confounders—including lifestyle factors, ethnicity, family history of dementia, early menopause, oophorectomy/ hysterectomy, comorbidities, and use of other relevant drugs—the use of HT was not associated with risk of dementia.
A reduced risk of dementia was noted among women who had been taking estrogen-only HT for 10 years or more (odds ratio [OR], 0.85; 95% confidence interval [CI], 0.76–0.94). An elevated risk of Alzheimer disease was noted among women who had used estrogen-progestin HT for 5 to 9 years (OR, 1.19; CI, 1.06–1.33).1
Study strengths and limitations
The authors pointed out that this study’s main strengths were that it had a very large sample size representative of the general population and that its design permitted capture of all known cases as well as precision recording for prescribed drugs. On the other hand, the study is limited by the possible lack of data for some older women before the index date; that is, menopause in this latter group started before their registration or before these data were gathered electronically by their practice. ●
The authors of this British large observational study took pains to minimize potential bias. The finding that long-term use of estrogen-only HT may be neuroprotective is consistent with results of recent studies in the United States and Finland,2-4 as well as with the Women’s Health Initiative randomized trial, which found that with 18 years of follow-up, treatment with conjugated estrogen alone was associated with a 26% reduced risk of death from Alzheimer disease.5 Overall, however, the main message we should glean from this important study by Vinogradova and colleagues is that women with bothersome vasomotor symptoms considering use of menopausal HT can be reassured that such therapy has little if any impact on future risk of cognitive decline.
ANDREW M. KAUNITZ, MD, NCMP
Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
Much interest has surrounded whether the use of menopausal HT impacts future risk of cognitive decline. Recently, Vinogradova and colleagues conducted an observational study using data from 2 large primary care databases, QResearch and the Clinical Practice Research Datalink (CPRD), in the United Kingdom.1 The investigators conducted case-control studies that included women aged 55 and older diagnosed with dementia and up to 5 controls without dementia. Only cases and controls with at least 10 years of medical records prior to the index date (that is, the time of dementia diagnosis in cases) were included. Since early symptoms of dementia prior to diagnosis may cause sleep problems and dysphoria (which also may be symptoms of menopause), HT prescriptions during the 3 years prior to the index date were excluded.
Details of the study
Among 16,291 cases and 68,726 controls, the women’s mean age was approximately 83 years. Cases were identified by using codes for dementia from patients’ clinical records or records of prescriptions for drugs used to treat dementia.
More than half the women were being treated for hypertension, and 14% of women in both groups had used HT. Women were considered users of estrogen-only therapy if they had no prescriptions containing a progestogen after their first prescription for systemic estrogen as the start of exposure to HT. Those with any subsequent prescription containing a progestogen were classified as combined HT users.
Results. In an analysis adjusted for all available potential confounders—including lifestyle factors, ethnicity, family history of dementia, early menopause, oophorectomy/ hysterectomy, comorbidities, and use of other relevant drugs—the use of HT was not associated with risk of dementia.
A reduced risk of dementia was noted among women who had been taking estrogen-only HT for 10 years or more (odds ratio [OR], 0.85; 95% confidence interval [CI], 0.76–0.94). An elevated risk of Alzheimer disease was noted among women who had used estrogen-progestin HT for 5 to 9 years (OR, 1.19; CI, 1.06–1.33).1
Study strengths and limitations
The authors pointed out that this study’s main strengths were that it had a very large sample size representative of the general population and that its design permitted capture of all known cases as well as precision recording for prescribed drugs. On the other hand, the study is limited by the possible lack of data for some older women before the index date; that is, menopause in this latter group started before their registration or before these data were gathered electronically by their practice. ●
The authors of this British large observational study took pains to minimize potential bias. The finding that long-term use of estrogen-only HT may be neuroprotective is consistent with results of recent studies in the United States and Finland,2-4 as well as with the Women’s Health Initiative randomized trial, which found that with 18 years of follow-up, treatment with conjugated estrogen alone was associated with a 26% reduced risk of death from Alzheimer disease.5 Overall, however, the main message we should glean from this important study by Vinogradova and colleagues is that women with bothersome vasomotor symptoms considering use of menopausal HT can be reassured that such therapy has little if any impact on future risk of cognitive decline.
ANDREW M. KAUNITZ, MD, NCMP
- Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
- Matyi JM, Rattinger GB, Schwartz S, et al. Lifetime estrogen exposure and cognition in late life: the Cache County study. Menopause. 2019;26:1366-1374. doi: 10.1097 /GME.0000000000001405.
- Liu JH. Does estrogen provide “neuroprotection” for postmenopausal women? Menopause. 2019;26:1361-1362. doi: 10.1097/GME.0000000000001459.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88:1062-1068. doi: 10.1212 /WNL.0000000000003696.
- Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001 /jama.2017.11217.
- Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
- Matyi JM, Rattinger GB, Schwartz S, et al. Lifetime estrogen exposure and cognition in late life: the Cache County study. Menopause. 2019;26:1366-1374. doi: 10.1097 /GME.0000000000001405.
- Liu JH. Does estrogen provide “neuroprotection” for postmenopausal women? Menopause. 2019;26:1361-1362. doi: 10.1097/GME.0000000000001459.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88:1062-1068. doi: 10.1212 /WNL.0000000000003696.
- Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001 /jama.2017.11217.
Feds’ website for free at-home COVID tests launches day early
The Biden administration’s new no-cost, at-home testing program launched Jan. 18, a day ahead of schedule.
The administration said 500 million tests are available to be delivered to homes across the country. This accounts for half of the president’s recent pledge to purchase 1 billion free at-home COVID-19 tests to distribute to the American public.
On a Jan. 14 call with reporters, senior White House officials offered some details about the new program.
Here’s what we know so far.
How do I order my free tests?
Americans can visit COVIDtests.gov to order their rapid at-home tests. You can also order directly from the U.S. Postal Service website. After you order, you’ll receive a confirmation email that promises to send tracking information once your order ships.
What information do I need to order the tests?
You only need your name and home mailing address.
There is also an option to provide your email address to get updates on the status of your order.
What if someone needs help ordering the tests?
There will be a free call-in line for people needing more help, including those having trouble accessing the internet, according to White House officials.
What tests will be available?
There are nine at-home tests available through FDA emergency use authorization. According to the Frequently Asked Questions section of COVIDtests.gov, "You will not be able to choose the brand you order as part of this program.”
How long will it take to get the tests once I order them?
Tests are expected to ship 7 to 12 days after you order them.
But White House officials say that the time frame will likely shorten as the program gains steam.
How many can I order?
There’s a limit of four tests per residential mailing address.
For larger families, White House officials suggest trying other free testing options, like visiting COVID-19 testing sites or your local health center.
Is this a one-time opportunity?
The White House doesn’t say, but officials did mention that if you run out of your four free tests, there are many other ways to access free at-home tests, such as COVID-19 testing sites, pharmacies, and community health centers.
The free tests available through COVIDtests.gov are in addition to an estimated 375 million at-home rapid tests on the market in the U.S. this month.
When should people use a rapid at-home test?
The CDC and experts with other public health groups agree that Americans should consider using at-home rapid tests in the following situations:
- If they begin to have symptoms consistent with COVID-19;
- At least 5 days after close contact with someone who has COVID;
- If someone is indoors with a group of people who are at risk of severe disease or are unvaccinated.
Are at-home rapid tests accurate?
The U.S. Department of Health and Human Services and other federal officials confirmed through studies that all tests distributed through this program can detect the Omicron variant. These agencies also confirmed that their performance is consistent with the FDA’s emergency use authorization.
Is the website designed to handle high demand?
After the original website to sign up for health insurance under the Affordable Care Act crashed repeatedly at launch, the government says it has prepared for high demand for ordering at-home rapid tests.
The U.S. Digital Service (USDS), an organization founded after Healthcare.gov, has partnered with the Postal Service to plan for the launch.
The Postal Service has expanded its staffing, similar to what’s done during the holidays.
All orders in the continental United States will be shipped through first-class mail, with shipments to Alaska, Hawaii, U.S. territories, and military and overseas addresses sent through priority mail.
A version of this article first appeared on WebMD.com.
The Biden administration’s new no-cost, at-home testing program launched Jan. 18, a day ahead of schedule.
The administration said 500 million tests are available to be delivered to homes across the country. This accounts for half of the president’s recent pledge to purchase 1 billion free at-home COVID-19 tests to distribute to the American public.
On a Jan. 14 call with reporters, senior White House officials offered some details about the new program.
Here’s what we know so far.
How do I order my free tests?
Americans can visit COVIDtests.gov to order their rapid at-home tests. You can also order directly from the U.S. Postal Service website. After you order, you’ll receive a confirmation email that promises to send tracking information once your order ships.
What information do I need to order the tests?
You only need your name and home mailing address.
There is also an option to provide your email address to get updates on the status of your order.
What if someone needs help ordering the tests?
There will be a free call-in line for people needing more help, including those having trouble accessing the internet, according to White House officials.
What tests will be available?
There are nine at-home tests available through FDA emergency use authorization. According to the Frequently Asked Questions section of COVIDtests.gov, "You will not be able to choose the brand you order as part of this program.”
How long will it take to get the tests once I order them?
Tests are expected to ship 7 to 12 days after you order them.
But White House officials say that the time frame will likely shorten as the program gains steam.
How many can I order?
There’s a limit of four tests per residential mailing address.
For larger families, White House officials suggest trying other free testing options, like visiting COVID-19 testing sites or your local health center.
Is this a one-time opportunity?
The White House doesn’t say, but officials did mention that if you run out of your four free tests, there are many other ways to access free at-home tests, such as COVID-19 testing sites, pharmacies, and community health centers.
The free tests available through COVIDtests.gov are in addition to an estimated 375 million at-home rapid tests on the market in the U.S. this month.
When should people use a rapid at-home test?
The CDC and experts with other public health groups agree that Americans should consider using at-home rapid tests in the following situations:
- If they begin to have symptoms consistent with COVID-19;
- At least 5 days after close contact with someone who has COVID;
- If someone is indoors with a group of people who are at risk of severe disease or are unvaccinated.
Are at-home rapid tests accurate?
The U.S. Department of Health and Human Services and other federal officials confirmed through studies that all tests distributed through this program can detect the Omicron variant. These agencies also confirmed that their performance is consistent with the FDA’s emergency use authorization.
Is the website designed to handle high demand?
After the original website to sign up for health insurance under the Affordable Care Act crashed repeatedly at launch, the government says it has prepared for high demand for ordering at-home rapid tests.
The U.S. Digital Service (USDS), an organization founded after Healthcare.gov, has partnered with the Postal Service to plan for the launch.
The Postal Service has expanded its staffing, similar to what’s done during the holidays.
All orders in the continental United States will be shipped through first-class mail, with shipments to Alaska, Hawaii, U.S. territories, and military and overseas addresses sent through priority mail.
A version of this article first appeared on WebMD.com.
The Biden administration’s new no-cost, at-home testing program launched Jan. 18, a day ahead of schedule.
The administration said 500 million tests are available to be delivered to homes across the country. This accounts for half of the president’s recent pledge to purchase 1 billion free at-home COVID-19 tests to distribute to the American public.
On a Jan. 14 call with reporters, senior White House officials offered some details about the new program.
Here’s what we know so far.
How do I order my free tests?
Americans can visit COVIDtests.gov to order their rapid at-home tests. You can also order directly from the U.S. Postal Service website. After you order, you’ll receive a confirmation email that promises to send tracking information once your order ships.
What information do I need to order the tests?
You only need your name and home mailing address.
There is also an option to provide your email address to get updates on the status of your order.
What if someone needs help ordering the tests?
There will be a free call-in line for people needing more help, including those having trouble accessing the internet, according to White House officials.
What tests will be available?
There are nine at-home tests available through FDA emergency use authorization. According to the Frequently Asked Questions section of COVIDtests.gov, "You will not be able to choose the brand you order as part of this program.”
How long will it take to get the tests once I order them?
Tests are expected to ship 7 to 12 days after you order them.
But White House officials say that the time frame will likely shorten as the program gains steam.
How many can I order?
There’s a limit of four tests per residential mailing address.
For larger families, White House officials suggest trying other free testing options, like visiting COVID-19 testing sites or your local health center.
Is this a one-time opportunity?
The White House doesn’t say, but officials did mention that if you run out of your four free tests, there are many other ways to access free at-home tests, such as COVID-19 testing sites, pharmacies, and community health centers.
The free tests available through COVIDtests.gov are in addition to an estimated 375 million at-home rapid tests on the market in the U.S. this month.
When should people use a rapid at-home test?
The CDC and experts with other public health groups agree that Americans should consider using at-home rapid tests in the following situations:
- If they begin to have symptoms consistent with COVID-19;
- At least 5 days after close contact with someone who has COVID;
- If someone is indoors with a group of people who are at risk of severe disease or are unvaccinated.
Are at-home rapid tests accurate?
The U.S. Department of Health and Human Services and other federal officials confirmed through studies that all tests distributed through this program can detect the Omicron variant. These agencies also confirmed that their performance is consistent with the FDA’s emergency use authorization.
Is the website designed to handle high demand?
After the original website to sign up for health insurance under the Affordable Care Act crashed repeatedly at launch, the government says it has prepared for high demand for ordering at-home rapid tests.
The U.S. Digital Service (USDS), an organization founded after Healthcare.gov, has partnered with the Postal Service to plan for the launch.
The Postal Service has expanded its staffing, similar to what’s done during the holidays.
All orders in the continental United States will be shipped through first-class mail, with shipments to Alaska, Hawaii, U.S. territories, and military and overseas addresses sent through priority mail.
A version of this article first appeared on WebMD.com.
Preschool boys’ behaviors traced back to moms’ thyroid hormones
Pregnant women’s thyroid hormone trajectories (levels in the first, second, and third trimester) may predict whether their male offspring are aggressive or withdrawn at age 4.
Certain maternal thyroid hormone trajectories were associated with problem behaviors in preschool boys in a study of close to 2,000 mother-child pairs in China.
The researchers identified low, moderate, and high thyroid-stimulating hormone (TSH) and free thyroxine (FT4) trajectories.
Most women had a low TSH trajectory and moderate FT4 trajectory, which the researchers deemed to be reference (normal) trajectories.
The children’s primary caregiver (parent or grandparent) completed an extensive questionnaire about their child’s behavior at age 4.
The 4-year-old boys whose mothers had a high TSH trajectory during pregnancy were more likely to be withdrawn and to externalize problems (odds ratio, 2.01 and 2.69, respectively).
Boys whose mothers had a high FT4 trajectory during pregnancy were more likely to be anxious/depressed (OR, 2.22).
And boys whose mothers had a moderate TSH trajectory or low FT4 trajectory were more likely to show aggressive behavior (OR, 3.76 and 4.17, respectively), compared with boys whose mothers had normal TSH and FT4 trajectories, after adjusting for potential confounders.
However, there was no association between abnormal maternal thyroid hormone trajectories and behavior problems in 4-year-old girls.
The study by Peixuan Li, BM, and colleagues was published online Jan. 6 in the Journal of Clinical Endocrinology & Metabolism.
‘Study supports monitoring thyroid function in pregnancy’
“Our findings highlight the significance of close monitoring and management of maternal thyroid function during pregnancy,” senior author Kun Huang, PhD, said in a press release from the Endocrine Society.
“This research presents a new perspective in early intervention of children’s emotional and behavioral problems,” added Dr. Huang, from Anhui Medical University, Hefei, China.
The results add to a growing body of literature about a controversial link between maternal thyroid hormones in pregnancy, when the fetal brain is developing, and subsequent behavior in preschool children, Caroline T. Nguyen, MD, who was not involved with this research, commented in an email.
“Some studies show an association between thyroid levels and behavioral outcomes, others not,” added Dr. Nguyen, assistant professor of clinical medicine, Keck School of Medicine, University of Southern California, Los Angeles. And “some studies have found sex-specific associations with maternal thyroid levels and neurocognitive/behavioral outcomes, others have not.”
Women considering pregnancy should be evaluated for possible thyroid disease, she continued. Currently, no universal screening mandates exist for thyroid disease in pregnancy, but the 2017 American Thyroid Association guidelines do recommend screening women at risk for thyroid dysfunction.
“I think screening for thyroid peroxidase antibody (TPOAb) positivity is helpful in women desiring pregnancy,” Dr. Nguyen continued, “because we know that patients with TPOAb positivity are at increased risk for miscarriage and have a blunted response to the increased demands of pregnancy for thyroid hormone production.”
TPOAb positivity is also associated with the increased risk of postpartum and long-term thyroid dysfunction.
This current study, Dr. Nguyen summarized, “adds to a growing body of research of the relationship of thyroid hormone levels and neurocognitive outcomes [in offspring] and supports the monitoring of thyroid disease in pregnancy.”
“However, we do not have sufficient data to demonstrate the benefits of intervention with levothyroxine treatment,” she noted.
Nevertheless, the lack of positive data does not suggest there is no theoretical benefit of intervention, she said, as such studies are very challenging to do.
“Physicians can help reduce stress and anxiety in patients desiring pregnancy by [recommending] preconception counseling, screening patients at risk for thyroid disease, and optimizing thyroid hormone levels before and during pregnancy,” according to Dr. Nguyen.
Maternal TSH and FT4 trajectories and preschoolers’ behaviors
Previous studies have reported that during pregnancy, maternal subclinical hypothyroidism (elevated TSH with normal FT4) as well as isolated hypothyroxinemia (decreased FT4 with normal TSH) are associated with adverse maternal and child outcomes, including preterm delivery and low birth weight.
However, most studies have not determined maternal thyroid hormone levels in different trimesters.
Researchers recruited pregnant women going for their first antenatal checkup at the Ma’anshan Maternal and Child Health Hospital in China from May 2013 to September 2014 and identified 1,860 mother-child pairs.
They determined maternal thyroid hormone levels from blood samples taken during the first, second, and third trimester: on average, gestational week 10, 25, and 34, respectively.
The researchers found that TSH levels increased somewhat from trimester 1 to trimester 2 and then decreased slightly in trimester 3. Most women (68%) had a low TSH trajectory, 28% had a moderate TSH trajectory, and 4% had a high TSH trajectory.
FT4 levels dropped sharply from trimester 1 to trimester 2 and then increased somewhat in trimester 3. About half of the women (52%) had a moderate FT4 trajectory, 33% had a low FT4 trajectory, and 15% had a high FT4 trajectory.
Most women (96.5%) had a low and stable TPOAb level, and the rest (3.5%) had high and decreasing TPOAb levels.
When the children in the study were 4 years old, their main caregiver (parent or grandparent) completed the 100-question Achenbach Child Behavior checklist to identify whether the child often, sometimes, or never displayed three internalizing problem behaviors (emotionally reactive, anxious/depressed, or withdrawn) and/or two externalizing problem behaviors (attention problems or aggressive behavior).
Study limitations, more research needed
It is not clear why the associations between maternal hormones and offspring behavior were only seen in boys. Perhaps male fetuses are more sensitive than female fetuses to changing maternal thyroid hormone levels in pregnancy, the researchers speculate.
They acknowledge that study limitations include there were few children with aggressive behavior, so the confidence interval for the association of the moderate TSH trajectory with aggressive behavior was very wide.
In addition, evaluation of children’s behavior by caregivers was subjective. Also, the researchers did not have information about iodine levels, and low iodine levels can impair child brain development.
And there may have been residual confounders that researchers did not account for, such as differences in family upbringing, parental marital status, and the mother’s exposure to endocrine disruptors.
Therefore, further research is needed.
The study was supported by grants from the National Natural Science Foundation of China, the University Synergy Innovation Program of Anhui Province, the Sci-Tech Basic Resources Research Program of China, the National Key Research and Development Program, the Chinese Academy of Medical Sciences, and the Research Fund of Anhui Institute of Translational Medicine. The researchers and Dr. Nguyen have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women’s thyroid hormone trajectories (levels in the first, second, and third trimester) may predict whether their male offspring are aggressive or withdrawn at age 4.
Certain maternal thyroid hormone trajectories were associated with problem behaviors in preschool boys in a study of close to 2,000 mother-child pairs in China.
The researchers identified low, moderate, and high thyroid-stimulating hormone (TSH) and free thyroxine (FT4) trajectories.
Most women had a low TSH trajectory and moderate FT4 trajectory, which the researchers deemed to be reference (normal) trajectories.
The children’s primary caregiver (parent or grandparent) completed an extensive questionnaire about their child’s behavior at age 4.
The 4-year-old boys whose mothers had a high TSH trajectory during pregnancy were more likely to be withdrawn and to externalize problems (odds ratio, 2.01 and 2.69, respectively).
Boys whose mothers had a high FT4 trajectory during pregnancy were more likely to be anxious/depressed (OR, 2.22).
And boys whose mothers had a moderate TSH trajectory or low FT4 trajectory were more likely to show aggressive behavior (OR, 3.76 and 4.17, respectively), compared with boys whose mothers had normal TSH and FT4 trajectories, after adjusting for potential confounders.
However, there was no association between abnormal maternal thyroid hormone trajectories and behavior problems in 4-year-old girls.
The study by Peixuan Li, BM, and colleagues was published online Jan. 6 in the Journal of Clinical Endocrinology & Metabolism.
‘Study supports monitoring thyroid function in pregnancy’
“Our findings highlight the significance of close monitoring and management of maternal thyroid function during pregnancy,” senior author Kun Huang, PhD, said in a press release from the Endocrine Society.
“This research presents a new perspective in early intervention of children’s emotional and behavioral problems,” added Dr. Huang, from Anhui Medical University, Hefei, China.
The results add to a growing body of literature about a controversial link between maternal thyroid hormones in pregnancy, when the fetal brain is developing, and subsequent behavior in preschool children, Caroline T. Nguyen, MD, who was not involved with this research, commented in an email.
“Some studies show an association between thyroid levels and behavioral outcomes, others not,” added Dr. Nguyen, assistant professor of clinical medicine, Keck School of Medicine, University of Southern California, Los Angeles. And “some studies have found sex-specific associations with maternal thyroid levels and neurocognitive/behavioral outcomes, others have not.”
Women considering pregnancy should be evaluated for possible thyroid disease, she continued. Currently, no universal screening mandates exist for thyroid disease in pregnancy, but the 2017 American Thyroid Association guidelines do recommend screening women at risk for thyroid dysfunction.
“I think screening for thyroid peroxidase antibody (TPOAb) positivity is helpful in women desiring pregnancy,” Dr. Nguyen continued, “because we know that patients with TPOAb positivity are at increased risk for miscarriage and have a blunted response to the increased demands of pregnancy for thyroid hormone production.”
TPOAb positivity is also associated with the increased risk of postpartum and long-term thyroid dysfunction.
This current study, Dr. Nguyen summarized, “adds to a growing body of research of the relationship of thyroid hormone levels and neurocognitive outcomes [in offspring] and supports the monitoring of thyroid disease in pregnancy.”
“However, we do not have sufficient data to demonstrate the benefits of intervention with levothyroxine treatment,” she noted.
Nevertheless, the lack of positive data does not suggest there is no theoretical benefit of intervention, she said, as such studies are very challenging to do.
“Physicians can help reduce stress and anxiety in patients desiring pregnancy by [recommending] preconception counseling, screening patients at risk for thyroid disease, and optimizing thyroid hormone levels before and during pregnancy,” according to Dr. Nguyen.
Maternal TSH and FT4 trajectories and preschoolers’ behaviors
Previous studies have reported that during pregnancy, maternal subclinical hypothyroidism (elevated TSH with normal FT4) as well as isolated hypothyroxinemia (decreased FT4 with normal TSH) are associated with adverse maternal and child outcomes, including preterm delivery and low birth weight.
However, most studies have not determined maternal thyroid hormone levels in different trimesters.
Researchers recruited pregnant women going for their first antenatal checkup at the Ma’anshan Maternal and Child Health Hospital in China from May 2013 to September 2014 and identified 1,860 mother-child pairs.
They determined maternal thyroid hormone levels from blood samples taken during the first, second, and third trimester: on average, gestational week 10, 25, and 34, respectively.
The researchers found that TSH levels increased somewhat from trimester 1 to trimester 2 and then decreased slightly in trimester 3. Most women (68%) had a low TSH trajectory, 28% had a moderate TSH trajectory, and 4% had a high TSH trajectory.
FT4 levels dropped sharply from trimester 1 to trimester 2 and then increased somewhat in trimester 3. About half of the women (52%) had a moderate FT4 trajectory, 33% had a low FT4 trajectory, and 15% had a high FT4 trajectory.
Most women (96.5%) had a low and stable TPOAb level, and the rest (3.5%) had high and decreasing TPOAb levels.
When the children in the study were 4 years old, their main caregiver (parent or grandparent) completed the 100-question Achenbach Child Behavior checklist to identify whether the child often, sometimes, or never displayed three internalizing problem behaviors (emotionally reactive, anxious/depressed, or withdrawn) and/or two externalizing problem behaviors (attention problems or aggressive behavior).
Study limitations, more research needed
It is not clear why the associations between maternal hormones and offspring behavior were only seen in boys. Perhaps male fetuses are more sensitive than female fetuses to changing maternal thyroid hormone levels in pregnancy, the researchers speculate.
They acknowledge that study limitations include there were few children with aggressive behavior, so the confidence interval for the association of the moderate TSH trajectory with aggressive behavior was very wide.
In addition, evaluation of children’s behavior by caregivers was subjective. Also, the researchers did not have information about iodine levels, and low iodine levels can impair child brain development.
And there may have been residual confounders that researchers did not account for, such as differences in family upbringing, parental marital status, and the mother’s exposure to endocrine disruptors.
Therefore, further research is needed.
The study was supported by grants from the National Natural Science Foundation of China, the University Synergy Innovation Program of Anhui Province, the Sci-Tech Basic Resources Research Program of China, the National Key Research and Development Program, the Chinese Academy of Medical Sciences, and the Research Fund of Anhui Institute of Translational Medicine. The researchers and Dr. Nguyen have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women’s thyroid hormone trajectories (levels in the first, second, and third trimester) may predict whether their male offspring are aggressive or withdrawn at age 4.
Certain maternal thyroid hormone trajectories were associated with problem behaviors in preschool boys in a study of close to 2,000 mother-child pairs in China.
The researchers identified low, moderate, and high thyroid-stimulating hormone (TSH) and free thyroxine (FT4) trajectories.
Most women had a low TSH trajectory and moderate FT4 trajectory, which the researchers deemed to be reference (normal) trajectories.
The children’s primary caregiver (parent or grandparent) completed an extensive questionnaire about their child’s behavior at age 4.
The 4-year-old boys whose mothers had a high TSH trajectory during pregnancy were more likely to be withdrawn and to externalize problems (odds ratio, 2.01 and 2.69, respectively).
Boys whose mothers had a high FT4 trajectory during pregnancy were more likely to be anxious/depressed (OR, 2.22).
And boys whose mothers had a moderate TSH trajectory or low FT4 trajectory were more likely to show aggressive behavior (OR, 3.76 and 4.17, respectively), compared with boys whose mothers had normal TSH and FT4 trajectories, after adjusting for potential confounders.
However, there was no association between abnormal maternal thyroid hormone trajectories and behavior problems in 4-year-old girls.
The study by Peixuan Li, BM, and colleagues was published online Jan. 6 in the Journal of Clinical Endocrinology & Metabolism.
‘Study supports monitoring thyroid function in pregnancy’
“Our findings highlight the significance of close monitoring and management of maternal thyroid function during pregnancy,” senior author Kun Huang, PhD, said in a press release from the Endocrine Society.
“This research presents a new perspective in early intervention of children’s emotional and behavioral problems,” added Dr. Huang, from Anhui Medical University, Hefei, China.
The results add to a growing body of literature about a controversial link between maternal thyroid hormones in pregnancy, when the fetal brain is developing, and subsequent behavior in preschool children, Caroline T. Nguyen, MD, who was not involved with this research, commented in an email.
“Some studies show an association between thyroid levels and behavioral outcomes, others not,” added Dr. Nguyen, assistant professor of clinical medicine, Keck School of Medicine, University of Southern California, Los Angeles. And “some studies have found sex-specific associations with maternal thyroid levels and neurocognitive/behavioral outcomes, others have not.”
Women considering pregnancy should be evaluated for possible thyroid disease, she continued. Currently, no universal screening mandates exist for thyroid disease in pregnancy, but the 2017 American Thyroid Association guidelines do recommend screening women at risk for thyroid dysfunction.
“I think screening for thyroid peroxidase antibody (TPOAb) positivity is helpful in women desiring pregnancy,” Dr. Nguyen continued, “because we know that patients with TPOAb positivity are at increased risk for miscarriage and have a blunted response to the increased demands of pregnancy for thyroid hormone production.”
TPOAb positivity is also associated with the increased risk of postpartum and long-term thyroid dysfunction.
This current study, Dr. Nguyen summarized, “adds to a growing body of research of the relationship of thyroid hormone levels and neurocognitive outcomes [in offspring] and supports the monitoring of thyroid disease in pregnancy.”
“However, we do not have sufficient data to demonstrate the benefits of intervention with levothyroxine treatment,” she noted.
Nevertheless, the lack of positive data does not suggest there is no theoretical benefit of intervention, she said, as such studies are very challenging to do.
“Physicians can help reduce stress and anxiety in patients desiring pregnancy by [recommending] preconception counseling, screening patients at risk for thyroid disease, and optimizing thyroid hormone levels before and during pregnancy,” according to Dr. Nguyen.
Maternal TSH and FT4 trajectories and preschoolers’ behaviors
Previous studies have reported that during pregnancy, maternal subclinical hypothyroidism (elevated TSH with normal FT4) as well as isolated hypothyroxinemia (decreased FT4 with normal TSH) are associated with adverse maternal and child outcomes, including preterm delivery and low birth weight.
However, most studies have not determined maternal thyroid hormone levels in different trimesters.
Researchers recruited pregnant women going for their first antenatal checkup at the Ma’anshan Maternal and Child Health Hospital in China from May 2013 to September 2014 and identified 1,860 mother-child pairs.
They determined maternal thyroid hormone levels from blood samples taken during the first, second, and third trimester: on average, gestational week 10, 25, and 34, respectively.
The researchers found that TSH levels increased somewhat from trimester 1 to trimester 2 and then decreased slightly in trimester 3. Most women (68%) had a low TSH trajectory, 28% had a moderate TSH trajectory, and 4% had a high TSH trajectory.
FT4 levels dropped sharply from trimester 1 to trimester 2 and then increased somewhat in trimester 3. About half of the women (52%) had a moderate FT4 trajectory, 33% had a low FT4 trajectory, and 15% had a high FT4 trajectory.
Most women (96.5%) had a low and stable TPOAb level, and the rest (3.5%) had high and decreasing TPOAb levels.
When the children in the study were 4 years old, their main caregiver (parent or grandparent) completed the 100-question Achenbach Child Behavior checklist to identify whether the child often, sometimes, or never displayed three internalizing problem behaviors (emotionally reactive, anxious/depressed, or withdrawn) and/or two externalizing problem behaviors (attention problems or aggressive behavior).
Study limitations, more research needed
It is not clear why the associations between maternal hormones and offspring behavior were only seen in boys. Perhaps male fetuses are more sensitive than female fetuses to changing maternal thyroid hormone levels in pregnancy, the researchers speculate.
They acknowledge that study limitations include there were few children with aggressive behavior, so the confidence interval for the association of the moderate TSH trajectory with aggressive behavior was very wide.
In addition, evaluation of children’s behavior by caregivers was subjective. Also, the researchers did not have information about iodine levels, and low iodine levels can impair child brain development.
And there may have been residual confounders that researchers did not account for, such as differences in family upbringing, parental marital status, and the mother’s exposure to endocrine disruptors.
Therefore, further research is needed.
The study was supported by grants from the National Natural Science Foundation of China, the University Synergy Innovation Program of Anhui Province, the Sci-Tech Basic Resources Research Program of China, the National Key Research and Development Program, the Chinese Academy of Medical Sciences, and the Research Fund of Anhui Institute of Translational Medicine. The researchers and Dr. Nguyen have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Federal website for free COVID-19 tests opens Jan. 19
The tests will ship within 7 to 12 days after being ordered, senior officials from President Joe Biden’s administration said Jan. 14. The U.S. Postal Service will handle the shipping and delivery through first-class mail.
People will input their name and mailing address on the website and can share an email address to receive updates on the order, according to NPR. People won’t need to pay shipping costs or enter a credit card number to order tests, according to the website’s homepage.
The website will be offered in both English and Spanish. The Biden administration will also set up a phone number so those without internet access can place orders.
Officials didn’t share a specific time that the website will open, according to he New York Times — simply that it will go live sometime on Jan. 19. Each household will be limited to ordering four tests.
Starting Jan. 15, people with private insurance were able to seek reimbursement for tests they purchase on their own. At the same time, some insurers have said it could take weeks to set up a system for smooth reimbursement, the newspaper reported.
Last week’s announcement is the latest step in the president’s pledge to get coronavirus tests to Americans. In December, Biden said his administration would purchase 500 million tests and distribute them to Americans for free. On Jan. 13, he announced that the administration would buy another 500 million tests, bringing the total to 1 billion.
So far, the administration has signed contracts to produce 420 million tests, the newspaper reported. With the website opening this week and the lag in shipping, the tests will likely arrive by the end of January at the earliest, which could be after the peak of the current coronavirus surge in some parts of the country.
At-home tests have been in high demand, with some pharmacies, retailers, and websites reporting no stock in recent weeks. People have lined up at community testing sites for hours to get tested as the national average of daily cases has climbed above 800,000 last week.
Some consumers have also been confused about how or when to use at-home tests. On Jan. 14, Biden administration officials said that people should use rapid tests for three reasons:
- If they begin to experience COVID-19 symptoms;
- When it has been five or more days after being exposed to someone who tests positive;
- If they are gathering indoors with a high-risk person and want to check if they are negative.
A version of this article first appeared on WebMD.com.
The tests will ship within 7 to 12 days after being ordered, senior officials from President Joe Biden’s administration said Jan. 14. The U.S. Postal Service will handle the shipping and delivery through first-class mail.
People will input their name and mailing address on the website and can share an email address to receive updates on the order, according to NPR. People won’t need to pay shipping costs or enter a credit card number to order tests, according to the website’s homepage.
The website will be offered in both English and Spanish. The Biden administration will also set up a phone number so those without internet access can place orders.
Officials didn’t share a specific time that the website will open, according to he New York Times — simply that it will go live sometime on Jan. 19. Each household will be limited to ordering four tests.
Starting Jan. 15, people with private insurance were able to seek reimbursement for tests they purchase on their own. At the same time, some insurers have said it could take weeks to set up a system for smooth reimbursement, the newspaper reported.
Last week’s announcement is the latest step in the president’s pledge to get coronavirus tests to Americans. In December, Biden said his administration would purchase 500 million tests and distribute them to Americans for free. On Jan. 13, he announced that the administration would buy another 500 million tests, bringing the total to 1 billion.
So far, the administration has signed contracts to produce 420 million tests, the newspaper reported. With the website opening this week and the lag in shipping, the tests will likely arrive by the end of January at the earliest, which could be after the peak of the current coronavirus surge in some parts of the country.
At-home tests have been in high demand, with some pharmacies, retailers, and websites reporting no stock in recent weeks. People have lined up at community testing sites for hours to get tested as the national average of daily cases has climbed above 800,000 last week.
Some consumers have also been confused about how or when to use at-home tests. On Jan. 14, Biden administration officials said that people should use rapid tests for three reasons:
- If they begin to experience COVID-19 symptoms;
- When it has been five or more days after being exposed to someone who tests positive;
- If they are gathering indoors with a high-risk person and want to check if they are negative.
A version of this article first appeared on WebMD.com.
The tests will ship within 7 to 12 days after being ordered, senior officials from President Joe Biden’s administration said Jan. 14. The U.S. Postal Service will handle the shipping and delivery through first-class mail.
People will input their name and mailing address on the website and can share an email address to receive updates on the order, according to NPR. People won’t need to pay shipping costs or enter a credit card number to order tests, according to the website’s homepage.
The website will be offered in both English and Spanish. The Biden administration will also set up a phone number so those without internet access can place orders.
Officials didn’t share a specific time that the website will open, according to he New York Times — simply that it will go live sometime on Jan. 19. Each household will be limited to ordering four tests.
Starting Jan. 15, people with private insurance were able to seek reimbursement for tests they purchase on their own. At the same time, some insurers have said it could take weeks to set up a system for smooth reimbursement, the newspaper reported.
Last week’s announcement is the latest step in the president’s pledge to get coronavirus tests to Americans. In December, Biden said his administration would purchase 500 million tests and distribute them to Americans for free. On Jan. 13, he announced that the administration would buy another 500 million tests, bringing the total to 1 billion.
So far, the administration has signed contracts to produce 420 million tests, the newspaper reported. With the website opening this week and the lag in shipping, the tests will likely arrive by the end of January at the earliest, which could be after the peak of the current coronavirus surge in some parts of the country.
At-home tests have been in high demand, with some pharmacies, retailers, and websites reporting no stock in recent weeks. People have lined up at community testing sites for hours to get tested as the national average of daily cases has climbed above 800,000 last week.
Some consumers have also been confused about how or when to use at-home tests. On Jan. 14, Biden administration officials said that people should use rapid tests for three reasons:
- If they begin to experience COVID-19 symptoms;
- When it has been five or more days after being exposed to someone who tests positive;
- If they are gathering indoors with a high-risk person and want to check if they are negative.
A version of this article first appeared on WebMD.com.
Should you dismiss that patient?
After a recent column about the dilemma of dealing with patients who refuse to be vaccinated against COVID-19, several readers raised the
Contrary to what seems to be the popular opinion, there are no statutory laws that I am aware of that directly apply to patient dismissal, beyond the obvious ones prohibiting discrimination that I’ve discussed many times. The more realistic concern is leaving yourself vulnerable to civil litigation – usually charges of abandonment.
Criteria will vary by region, jurisdiction, and practice. Since there are no hard and fast rules, your reasons for dismissal should be determined in advance, written out, and included in your practice manual. Once you have laid down your rules, follow them. Exceptions should be rare and made only under extraordinary circumstances.
Most patients are dismissed because of interpersonal conflicts between physician or staff members. Usually, that involves noncompliance with a reasonable treatment plan (including vaccinations), but there are other valid reasons. These include threats of violence, inappropriate sexual advances, providing false or misleading medical history, demands for inappropriate treatments or medications, and repeated failure to keep appointments or pay bills. And most ethics experts agree that you can dismiss someone who insists on treatment outside your area of expertise, or at a location other than your private office.
Even when circumstances warrant, dismissal should be a last resort. As with most interpersonal conflicts, your best option is usually reconciliation. Sit down with the patient, explain your concerns, and discuss what must be done if your doctor-patient relationship is to continue. Often, such patients are not aware (or willing to admit) that they are violating your office policies. Honest communication will often save such relationships. But be sure to make it clear that failure to address the problems you have outlined will result in dismissal from your practice. Document this conversation in detail in the patient’s chart, and follow up with a written communication reconfirming what you discussed.
If, despite your best (documented) efforts, the problems continue and dismissal becomes necessary, following a few generally accepted guidelines will help keep the process smooth and consequence free.
First, try to avoid dismissing a patient in the middle of a course of treatment. If that is unavoidable, you might want to contact your malpractice carrier and review the case with them prior to doing so.
Inform the patient, preferably by certified mail, of your decision. Spell out your reasons, with a reminder that these problems were discussed, and that a warning was issued and not heeded. If the patient belongs to a third-party health plan, be certain that you are acting within the stipulations of your contract with that plan, and inform the payer in writing of your action.
Once again, you must clearly document in the patient’s chart exactly how he or she violated your office policies. This will minimize grounds for charges of discrimination of any sort. Be especially diligent about this step if the patient has any known physical or mental disability.
Give the patient a reasonable amount of time (30 days is common) to find another physician, and mention that you will address any emergent problems within the scope of your specialty within that 30-day period. To minimize any potential allegations of abandonment, include a list of competent physicians in your area (without any guarantees) who might be willing to assume the patient’s care. Alternatively, you can list the phone number or website of a local medical society that they can contact to find a replacement. Offer to transfer medical records to the new physician upon receipt of written permission.
File a copy or scan of the letter, the certified delivery receipt, and the returned signature card in the patient’s chart. While the law states that a first-class letter, properly addressed and stamped, is presumed to have been delivered, you don’t want any question as to whether the patient received written notice of dismissal.
Forcibly ending a physician-patient relationship is a significant event that should not be undertaken lightly. Again, dismissal should be a rare occurrence, a last resort.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
After a recent column about the dilemma of dealing with patients who refuse to be vaccinated against COVID-19, several readers raised the
Contrary to what seems to be the popular opinion, there are no statutory laws that I am aware of that directly apply to patient dismissal, beyond the obvious ones prohibiting discrimination that I’ve discussed many times. The more realistic concern is leaving yourself vulnerable to civil litigation – usually charges of abandonment.
Criteria will vary by region, jurisdiction, and practice. Since there are no hard and fast rules, your reasons for dismissal should be determined in advance, written out, and included in your practice manual. Once you have laid down your rules, follow them. Exceptions should be rare and made only under extraordinary circumstances.
Most patients are dismissed because of interpersonal conflicts between physician or staff members. Usually, that involves noncompliance with a reasonable treatment plan (including vaccinations), but there are other valid reasons. These include threats of violence, inappropriate sexual advances, providing false or misleading medical history, demands for inappropriate treatments or medications, and repeated failure to keep appointments or pay bills. And most ethics experts agree that you can dismiss someone who insists on treatment outside your area of expertise, or at a location other than your private office.
Even when circumstances warrant, dismissal should be a last resort. As with most interpersonal conflicts, your best option is usually reconciliation. Sit down with the patient, explain your concerns, and discuss what must be done if your doctor-patient relationship is to continue. Often, such patients are not aware (or willing to admit) that they are violating your office policies. Honest communication will often save such relationships. But be sure to make it clear that failure to address the problems you have outlined will result in dismissal from your practice. Document this conversation in detail in the patient’s chart, and follow up with a written communication reconfirming what you discussed.
If, despite your best (documented) efforts, the problems continue and dismissal becomes necessary, following a few generally accepted guidelines will help keep the process smooth and consequence free.
First, try to avoid dismissing a patient in the middle of a course of treatment. If that is unavoidable, you might want to contact your malpractice carrier and review the case with them prior to doing so.
Inform the patient, preferably by certified mail, of your decision. Spell out your reasons, with a reminder that these problems were discussed, and that a warning was issued and not heeded. If the patient belongs to a third-party health plan, be certain that you are acting within the stipulations of your contract with that plan, and inform the payer in writing of your action.
Once again, you must clearly document in the patient’s chart exactly how he or she violated your office policies. This will minimize grounds for charges of discrimination of any sort. Be especially diligent about this step if the patient has any known physical or mental disability.
Give the patient a reasonable amount of time (30 days is common) to find another physician, and mention that you will address any emergent problems within the scope of your specialty within that 30-day period. To minimize any potential allegations of abandonment, include a list of competent physicians in your area (without any guarantees) who might be willing to assume the patient’s care. Alternatively, you can list the phone number or website of a local medical society that they can contact to find a replacement. Offer to transfer medical records to the new physician upon receipt of written permission.
File a copy or scan of the letter, the certified delivery receipt, and the returned signature card in the patient’s chart. While the law states that a first-class letter, properly addressed and stamped, is presumed to have been delivered, you don’t want any question as to whether the patient received written notice of dismissal.
Forcibly ending a physician-patient relationship is a significant event that should not be undertaken lightly. Again, dismissal should be a rare occurrence, a last resort.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
After a recent column about the dilemma of dealing with patients who refuse to be vaccinated against COVID-19, several readers raised the
Contrary to what seems to be the popular opinion, there are no statutory laws that I am aware of that directly apply to patient dismissal, beyond the obvious ones prohibiting discrimination that I’ve discussed many times. The more realistic concern is leaving yourself vulnerable to civil litigation – usually charges of abandonment.
Criteria will vary by region, jurisdiction, and practice. Since there are no hard and fast rules, your reasons for dismissal should be determined in advance, written out, and included in your practice manual. Once you have laid down your rules, follow them. Exceptions should be rare and made only under extraordinary circumstances.
Most patients are dismissed because of interpersonal conflicts between physician or staff members. Usually, that involves noncompliance with a reasonable treatment plan (including vaccinations), but there are other valid reasons. These include threats of violence, inappropriate sexual advances, providing false or misleading medical history, demands for inappropriate treatments or medications, and repeated failure to keep appointments or pay bills. And most ethics experts agree that you can dismiss someone who insists on treatment outside your area of expertise, or at a location other than your private office.
Even when circumstances warrant, dismissal should be a last resort. As with most interpersonal conflicts, your best option is usually reconciliation. Sit down with the patient, explain your concerns, and discuss what must be done if your doctor-patient relationship is to continue. Often, such patients are not aware (or willing to admit) that they are violating your office policies. Honest communication will often save such relationships. But be sure to make it clear that failure to address the problems you have outlined will result in dismissal from your practice. Document this conversation in detail in the patient’s chart, and follow up with a written communication reconfirming what you discussed.
If, despite your best (documented) efforts, the problems continue and dismissal becomes necessary, following a few generally accepted guidelines will help keep the process smooth and consequence free.
First, try to avoid dismissing a patient in the middle of a course of treatment. If that is unavoidable, you might want to contact your malpractice carrier and review the case with them prior to doing so.
Inform the patient, preferably by certified mail, of your decision. Spell out your reasons, with a reminder that these problems were discussed, and that a warning was issued and not heeded. If the patient belongs to a third-party health plan, be certain that you are acting within the stipulations of your contract with that plan, and inform the payer in writing of your action.
Once again, you must clearly document in the patient’s chart exactly how he or she violated your office policies. This will minimize grounds for charges of discrimination of any sort. Be especially diligent about this step if the patient has any known physical or mental disability.
Give the patient a reasonable amount of time (30 days is common) to find another physician, and mention that you will address any emergent problems within the scope of your specialty within that 30-day period. To minimize any potential allegations of abandonment, include a list of competent physicians in your area (without any guarantees) who might be willing to assume the patient’s care. Alternatively, you can list the phone number or website of a local medical society that they can contact to find a replacement. Offer to transfer medical records to the new physician upon receipt of written permission.
File a copy or scan of the letter, the certified delivery receipt, and the returned signature card in the patient’s chart. While the law states that a first-class letter, properly addressed and stamped, is presumed to have been delivered, you don’t want any question as to whether the patient received written notice of dismissal.
Forcibly ending a physician-patient relationship is a significant event that should not be undertaken lightly. Again, dismissal should be a rare occurrence, a last resort.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].