User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Medical coding creates barriers to care for transgender patients
In 2021, Tim Chevalier received the first of many coverage denials from his insurance company for the hair-removal procedure he needed as part of a phalloplasty, the creation of a penis.
Electrolysis is a common procedure among transgender people like Mr. Chevalier, a software developer in Oakland, Calif.. In some cases, it’s used to remove unwanted hair from the face or body. But it’s also required for a phalloplasty or a vaginoplasty, the creation of a vagina, because all hair must be removed from the tissue that will be relocated during surgery.
Mr. Chevalier’s insurer, Anthem Blue Cross, told him he needed what’s known as a prior authorization for the procedure. Even after Mr. Chevalier received the authorization, he said, his reimbursement claims kept getting denied. According to Mr. Chevalier, Anthem said the procedure was considered cosmetic.
Many trans patients have trouble getting their insurers to cover gender-affirming care. One reason is transphobia within the U.S. health care system, but another involves how medical diagnoses and procedures are coded for insurance companies. Nationwide, health care providers use a list of diagnostic codes provided by the ICD-10. And many of those, advocates for transgender people say, haven’t caught up to the needs of patients. Such diagnostic codes provide the basis for determining which procedures, such as electrolysis or surgery, insurance will cover.
“It’s widely regarded that the codes are very limited in ICD-10,” said Johanna Olson-Kennedy, MD, medical director of the Center for Transyouth Health and Development at Children’s Hospital Los Angeles.
She advocates for a move to the 11th edition of the coding system, which was endorsed by the World Health Organization in 2019 and began to be adopted around the globe in February. Today, more than 34 countries use ICD-11.
The new edition has replaced outdated terms like “transsexualism” and “gender identity disorder” with “gender incongruence,” which is no longer classified as a mental health condition, but as a sexual health one. This is crucial in reducing the stigmatization of trans people in health care, said Dr. Olson-Kennedy.
A move away from the mental health classification may also mean more coverage of gender-affirming care by insurance companies, which sometimes question mental health claims more rigorously than those for physical illnesses. WHO officials have said they hope that adding gender incongruence to a sexual health chapter will “help increase access to care for health interventions” and “destigmatize the condition,” according to the WHO website.
However, history suggests that ICD-11 likely won’t be implemented in the United States for years. The WHO first endorsed ICD-10 in 1990, but the United States didn’t implement it for 25 years.
Meanwhile, patients who identify as transgender and their doctors are spending hours trying to get coverage – or using crowdfunding to cover big out-of-pocket bills. Mr. Chevalier estimated he has received 78 hours of electrolysis at $140 per hour, costing $10,920.
Anthem spokesperson Michael Bowman wrote in an email that “there has been no medical denials or denial of coverage” because Anthem “preapproved coverage for these services.”
However, even after the preapproval was given, Anthem responded to Mr. Chevalier’s claims by stating the electrolysis would not be reimbursed because the procedure is considered cosmetic, rather than medically necessary. This is regardless of Mr. Chevalier’s diagnosis of gender dysphoria – the psychological distress felt when someone’s biological sex and gender identity don’t match – which many doctors consider a medically legitimate reason for hair removal.
Bowman wrote that “once this issue was identified, Anthem implemented an internal process which included a manual override in the billing system.”
Still, Mr. Chevalier filed a complaint with the California Department of Managed Health Care, and the state declared Anthem Blue Cross out of compliance. Additionally, after KHN started asking Anthem questions about Chevalier’s bills, two claims that had not been addressed since April were resolved in July. So far, Anthem has reimbursed Chevalier around $8,000.
Some procedures that trans patients receive can also be excluded from coverage because insurance companies consider them “sex specific.” For example, a transgender man’s gynecological visit may not be covered because his insurance plan covers those visits only for people enrolled as women.
“There is always this question of: What gender should you tell the insurance company?” said Nick Gorton, MD, an emergency medicine physician in Davis, Calif. Dr. Gorton, who is trans, recommends his patients with insurance plans that exclude trans care calculate the out-of-pocket costs that would be required for certain procedures based on whether the patient lists themselves as male or female on their insurance paperwork. For example, Dr. Gorton said, the question for a trans man becomes “what’s more expensive – paying for testosterone or paying for a Pap smear?” – since insurance likely won’t cover both.
For years, some physicians helped trans patients get coverage by finding other medical reasons for their trans-related care. Dr. Gorton said that if, for instance, a transgender man wanted a hysterectomy but his insurance didn’t cover gender-affirming care, Dr. Gorton would enter the ICD-10 code for pelvic pain, as opposed to gender dysphoria, into the patient’s billing record. Pelvic pain is a legitimate reason for the surgery and is commonly accepted by insurance providers, Dr. Gorton said. But some insurance companies pushed back, and he had to find other ways to help his patients.
In 2005, California passed a first-of-its-kind law that prohibits discrimination by health insurance on the basis of gender or gender identity. Now, 24 states and Washington, D.C., forbid private insurance from excluding transgender-related health care benefits.
Consequently, Dr. Gorton no longer needs to use different codes for patients seeking gender-affirming care at his practice in California. But physicians in other states are still struggling.
When Eric Meininger, MD, MPH, an internist and pediatrician at Indiana University Health’s gender health program in Indianapolis, treats a trans kid seeking hormone therapy, he commonly uses the ICD-10 code for “medication management” as the primary reason for the patient’s visit. That’s because Indiana has no law providing insurance protections for LGBTQ+ people, and when gender dysphoria is listed as the primary reason, insurance companies have denied coverage.
“It’s frustrating,” Dr. Meininger said. In a patient’s billing record, he sometimes provides multiple diagnoses, including gender dysphoria, to increase the likelihood that a procedure will be covered. “It’s not hard usually to come up with five or seven or eight diagnoses for someone because there’s lots of vague ones out there.”
Implementing ICD-11 won’t fix all the coding problems, as insurance companies may still refuse to cover procedures related to gender incongruence even though it is listed as a sexual health condition. It also won’t change the fact that many states still allow insurance to exclude gender-affirming care. But in terms of reducing stigma, it’s a step forward, Dr. Olson-Kennedy said.
One reason the United States took so long to switch to ICD-10 is that the American Medical Association strongly opposed the move. It argued the new system would put an incredible burden on doctors. Physicians would have to “contend with 68,000 diagnosis codes – a fivefold increase from the approximately 13,000 diagnosis codes in use today,” the AMA wrote in a 2014 letter. Implementing software to update providers’ coding systems would also be costly, dealing a financial blow to small medical practices, the association argued.
Unlike past coding systems, ICD-11 is fully electronic, with no physical manual of codes, and can be incorporated into a medical facility’s current coding system without requiring a new rollout, said Christian Lindmeier, a WHO spokesperson.
Whether these changes will make the adoption of the new edition easier in the United States is yet to be seen. For now, many trans patients in need of gender-affirming care must pay their bills out of pocket, fight their insurance company for coverage, or rely on the generosity of others.
“Even though I did get reimbursed eventually, the reimbursements were delayed, and it burned up a lot of my time,” Mr. Chevalier said. “Most people would have just given up.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
In 2021, Tim Chevalier received the first of many coverage denials from his insurance company for the hair-removal procedure he needed as part of a phalloplasty, the creation of a penis.
Electrolysis is a common procedure among transgender people like Mr. Chevalier, a software developer in Oakland, Calif.. In some cases, it’s used to remove unwanted hair from the face or body. But it’s also required for a phalloplasty or a vaginoplasty, the creation of a vagina, because all hair must be removed from the tissue that will be relocated during surgery.
Mr. Chevalier’s insurer, Anthem Blue Cross, told him he needed what’s known as a prior authorization for the procedure. Even after Mr. Chevalier received the authorization, he said, his reimbursement claims kept getting denied. According to Mr. Chevalier, Anthem said the procedure was considered cosmetic.
Many trans patients have trouble getting their insurers to cover gender-affirming care. One reason is transphobia within the U.S. health care system, but another involves how medical diagnoses and procedures are coded for insurance companies. Nationwide, health care providers use a list of diagnostic codes provided by the ICD-10. And many of those, advocates for transgender people say, haven’t caught up to the needs of patients. Such diagnostic codes provide the basis for determining which procedures, such as electrolysis or surgery, insurance will cover.
“It’s widely regarded that the codes are very limited in ICD-10,” said Johanna Olson-Kennedy, MD, medical director of the Center for Transyouth Health and Development at Children’s Hospital Los Angeles.
She advocates for a move to the 11th edition of the coding system, which was endorsed by the World Health Organization in 2019 and began to be adopted around the globe in February. Today, more than 34 countries use ICD-11.
The new edition has replaced outdated terms like “transsexualism” and “gender identity disorder” with “gender incongruence,” which is no longer classified as a mental health condition, but as a sexual health one. This is crucial in reducing the stigmatization of trans people in health care, said Dr. Olson-Kennedy.
A move away from the mental health classification may also mean more coverage of gender-affirming care by insurance companies, which sometimes question mental health claims more rigorously than those for physical illnesses. WHO officials have said they hope that adding gender incongruence to a sexual health chapter will “help increase access to care for health interventions” and “destigmatize the condition,” according to the WHO website.
However, history suggests that ICD-11 likely won’t be implemented in the United States for years. The WHO first endorsed ICD-10 in 1990, but the United States didn’t implement it for 25 years.
Meanwhile, patients who identify as transgender and their doctors are spending hours trying to get coverage – or using crowdfunding to cover big out-of-pocket bills. Mr. Chevalier estimated he has received 78 hours of electrolysis at $140 per hour, costing $10,920.
Anthem spokesperson Michael Bowman wrote in an email that “there has been no medical denials or denial of coverage” because Anthem “preapproved coverage for these services.”
However, even after the preapproval was given, Anthem responded to Mr. Chevalier’s claims by stating the electrolysis would not be reimbursed because the procedure is considered cosmetic, rather than medically necessary. This is regardless of Mr. Chevalier’s diagnosis of gender dysphoria – the psychological distress felt when someone’s biological sex and gender identity don’t match – which many doctors consider a medically legitimate reason for hair removal.
Bowman wrote that “once this issue was identified, Anthem implemented an internal process which included a manual override in the billing system.”
Still, Mr. Chevalier filed a complaint with the California Department of Managed Health Care, and the state declared Anthem Blue Cross out of compliance. Additionally, after KHN started asking Anthem questions about Chevalier’s bills, two claims that had not been addressed since April were resolved in July. So far, Anthem has reimbursed Chevalier around $8,000.
Some procedures that trans patients receive can also be excluded from coverage because insurance companies consider them “sex specific.” For example, a transgender man’s gynecological visit may not be covered because his insurance plan covers those visits only for people enrolled as women.
“There is always this question of: What gender should you tell the insurance company?” said Nick Gorton, MD, an emergency medicine physician in Davis, Calif. Dr. Gorton, who is trans, recommends his patients with insurance plans that exclude trans care calculate the out-of-pocket costs that would be required for certain procedures based on whether the patient lists themselves as male or female on their insurance paperwork. For example, Dr. Gorton said, the question for a trans man becomes “what’s more expensive – paying for testosterone or paying for a Pap smear?” – since insurance likely won’t cover both.
For years, some physicians helped trans patients get coverage by finding other medical reasons for their trans-related care. Dr. Gorton said that if, for instance, a transgender man wanted a hysterectomy but his insurance didn’t cover gender-affirming care, Dr. Gorton would enter the ICD-10 code for pelvic pain, as opposed to gender dysphoria, into the patient’s billing record. Pelvic pain is a legitimate reason for the surgery and is commonly accepted by insurance providers, Dr. Gorton said. But some insurance companies pushed back, and he had to find other ways to help his patients.
In 2005, California passed a first-of-its-kind law that prohibits discrimination by health insurance on the basis of gender or gender identity. Now, 24 states and Washington, D.C., forbid private insurance from excluding transgender-related health care benefits.
Consequently, Dr. Gorton no longer needs to use different codes for patients seeking gender-affirming care at his practice in California. But physicians in other states are still struggling.
When Eric Meininger, MD, MPH, an internist and pediatrician at Indiana University Health’s gender health program in Indianapolis, treats a trans kid seeking hormone therapy, he commonly uses the ICD-10 code for “medication management” as the primary reason for the patient’s visit. That’s because Indiana has no law providing insurance protections for LGBTQ+ people, and when gender dysphoria is listed as the primary reason, insurance companies have denied coverage.
“It’s frustrating,” Dr. Meininger said. In a patient’s billing record, he sometimes provides multiple diagnoses, including gender dysphoria, to increase the likelihood that a procedure will be covered. “It’s not hard usually to come up with five or seven or eight diagnoses for someone because there’s lots of vague ones out there.”
Implementing ICD-11 won’t fix all the coding problems, as insurance companies may still refuse to cover procedures related to gender incongruence even though it is listed as a sexual health condition. It also won’t change the fact that many states still allow insurance to exclude gender-affirming care. But in terms of reducing stigma, it’s a step forward, Dr. Olson-Kennedy said.
One reason the United States took so long to switch to ICD-10 is that the American Medical Association strongly opposed the move. It argued the new system would put an incredible burden on doctors. Physicians would have to “contend with 68,000 diagnosis codes – a fivefold increase from the approximately 13,000 diagnosis codes in use today,” the AMA wrote in a 2014 letter. Implementing software to update providers’ coding systems would also be costly, dealing a financial blow to small medical practices, the association argued.
Unlike past coding systems, ICD-11 is fully electronic, with no physical manual of codes, and can be incorporated into a medical facility’s current coding system without requiring a new rollout, said Christian Lindmeier, a WHO spokesperson.
Whether these changes will make the adoption of the new edition easier in the United States is yet to be seen. For now, many trans patients in need of gender-affirming care must pay their bills out of pocket, fight their insurance company for coverage, or rely on the generosity of others.
“Even though I did get reimbursed eventually, the reimbursements were delayed, and it burned up a lot of my time,” Mr. Chevalier said. “Most people would have just given up.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
In 2021, Tim Chevalier received the first of many coverage denials from his insurance company for the hair-removal procedure he needed as part of a phalloplasty, the creation of a penis.
Electrolysis is a common procedure among transgender people like Mr. Chevalier, a software developer in Oakland, Calif.. In some cases, it’s used to remove unwanted hair from the face or body. But it’s also required for a phalloplasty or a vaginoplasty, the creation of a vagina, because all hair must be removed from the tissue that will be relocated during surgery.
Mr. Chevalier’s insurer, Anthem Blue Cross, told him he needed what’s known as a prior authorization for the procedure. Even after Mr. Chevalier received the authorization, he said, his reimbursement claims kept getting denied. According to Mr. Chevalier, Anthem said the procedure was considered cosmetic.
Many trans patients have trouble getting their insurers to cover gender-affirming care. One reason is transphobia within the U.S. health care system, but another involves how medical diagnoses and procedures are coded for insurance companies. Nationwide, health care providers use a list of diagnostic codes provided by the ICD-10. And many of those, advocates for transgender people say, haven’t caught up to the needs of patients. Such diagnostic codes provide the basis for determining which procedures, such as electrolysis or surgery, insurance will cover.
“It’s widely regarded that the codes are very limited in ICD-10,” said Johanna Olson-Kennedy, MD, medical director of the Center for Transyouth Health and Development at Children’s Hospital Los Angeles.
She advocates for a move to the 11th edition of the coding system, which was endorsed by the World Health Organization in 2019 and began to be adopted around the globe in February. Today, more than 34 countries use ICD-11.
The new edition has replaced outdated terms like “transsexualism” and “gender identity disorder” with “gender incongruence,” which is no longer classified as a mental health condition, but as a sexual health one. This is crucial in reducing the stigmatization of trans people in health care, said Dr. Olson-Kennedy.
A move away from the mental health classification may also mean more coverage of gender-affirming care by insurance companies, which sometimes question mental health claims more rigorously than those for physical illnesses. WHO officials have said they hope that adding gender incongruence to a sexual health chapter will “help increase access to care for health interventions” and “destigmatize the condition,” according to the WHO website.
However, history suggests that ICD-11 likely won’t be implemented in the United States for years. The WHO first endorsed ICD-10 in 1990, but the United States didn’t implement it for 25 years.
Meanwhile, patients who identify as transgender and their doctors are spending hours trying to get coverage – or using crowdfunding to cover big out-of-pocket bills. Mr. Chevalier estimated he has received 78 hours of electrolysis at $140 per hour, costing $10,920.
Anthem spokesperson Michael Bowman wrote in an email that “there has been no medical denials or denial of coverage” because Anthem “preapproved coverage for these services.”
However, even after the preapproval was given, Anthem responded to Mr. Chevalier’s claims by stating the electrolysis would not be reimbursed because the procedure is considered cosmetic, rather than medically necessary. This is regardless of Mr. Chevalier’s diagnosis of gender dysphoria – the psychological distress felt when someone’s biological sex and gender identity don’t match – which many doctors consider a medically legitimate reason for hair removal.
Bowman wrote that “once this issue was identified, Anthem implemented an internal process which included a manual override in the billing system.”
Still, Mr. Chevalier filed a complaint with the California Department of Managed Health Care, and the state declared Anthem Blue Cross out of compliance. Additionally, after KHN started asking Anthem questions about Chevalier’s bills, two claims that had not been addressed since April were resolved in July. So far, Anthem has reimbursed Chevalier around $8,000.
Some procedures that trans patients receive can also be excluded from coverage because insurance companies consider them “sex specific.” For example, a transgender man’s gynecological visit may not be covered because his insurance plan covers those visits only for people enrolled as women.
“There is always this question of: What gender should you tell the insurance company?” said Nick Gorton, MD, an emergency medicine physician in Davis, Calif. Dr. Gorton, who is trans, recommends his patients with insurance plans that exclude trans care calculate the out-of-pocket costs that would be required for certain procedures based on whether the patient lists themselves as male or female on their insurance paperwork. For example, Dr. Gorton said, the question for a trans man becomes “what’s more expensive – paying for testosterone or paying for a Pap smear?” – since insurance likely won’t cover both.
For years, some physicians helped trans patients get coverage by finding other medical reasons for their trans-related care. Dr. Gorton said that if, for instance, a transgender man wanted a hysterectomy but his insurance didn’t cover gender-affirming care, Dr. Gorton would enter the ICD-10 code for pelvic pain, as opposed to gender dysphoria, into the patient’s billing record. Pelvic pain is a legitimate reason for the surgery and is commonly accepted by insurance providers, Dr. Gorton said. But some insurance companies pushed back, and he had to find other ways to help his patients.
In 2005, California passed a first-of-its-kind law that prohibits discrimination by health insurance on the basis of gender or gender identity. Now, 24 states and Washington, D.C., forbid private insurance from excluding transgender-related health care benefits.
Consequently, Dr. Gorton no longer needs to use different codes for patients seeking gender-affirming care at his practice in California. But physicians in other states are still struggling.
When Eric Meininger, MD, MPH, an internist and pediatrician at Indiana University Health’s gender health program in Indianapolis, treats a trans kid seeking hormone therapy, he commonly uses the ICD-10 code for “medication management” as the primary reason for the patient’s visit. That’s because Indiana has no law providing insurance protections for LGBTQ+ people, and when gender dysphoria is listed as the primary reason, insurance companies have denied coverage.
“It’s frustrating,” Dr. Meininger said. In a patient’s billing record, he sometimes provides multiple diagnoses, including gender dysphoria, to increase the likelihood that a procedure will be covered. “It’s not hard usually to come up with five or seven or eight diagnoses for someone because there’s lots of vague ones out there.”
Implementing ICD-11 won’t fix all the coding problems, as insurance companies may still refuse to cover procedures related to gender incongruence even though it is listed as a sexual health condition. It also won’t change the fact that many states still allow insurance to exclude gender-affirming care. But in terms of reducing stigma, it’s a step forward, Dr. Olson-Kennedy said.
One reason the United States took so long to switch to ICD-10 is that the American Medical Association strongly opposed the move. It argued the new system would put an incredible burden on doctors. Physicians would have to “contend with 68,000 diagnosis codes – a fivefold increase from the approximately 13,000 diagnosis codes in use today,” the AMA wrote in a 2014 letter. Implementing software to update providers’ coding systems would also be costly, dealing a financial blow to small medical practices, the association argued.
Unlike past coding systems, ICD-11 is fully electronic, with no physical manual of codes, and can be incorporated into a medical facility’s current coding system without requiring a new rollout, said Christian Lindmeier, a WHO spokesperson.
Whether these changes will make the adoption of the new edition easier in the United States is yet to be seen. For now, many trans patients in need of gender-affirming care must pay their bills out of pocket, fight their insurance company for coverage, or rely on the generosity of others.
“Even though I did get reimbursed eventually, the reimbursements were delayed, and it burned up a lot of my time,” Mr. Chevalier said. “Most people would have just given up.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Hidradenitis Suppurativa Overview
Hidradenitis Suppurativa Pathophysiology
‘Dr. Caveman’ had a leg up on amputation
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
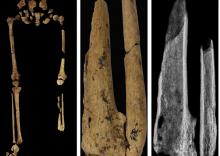
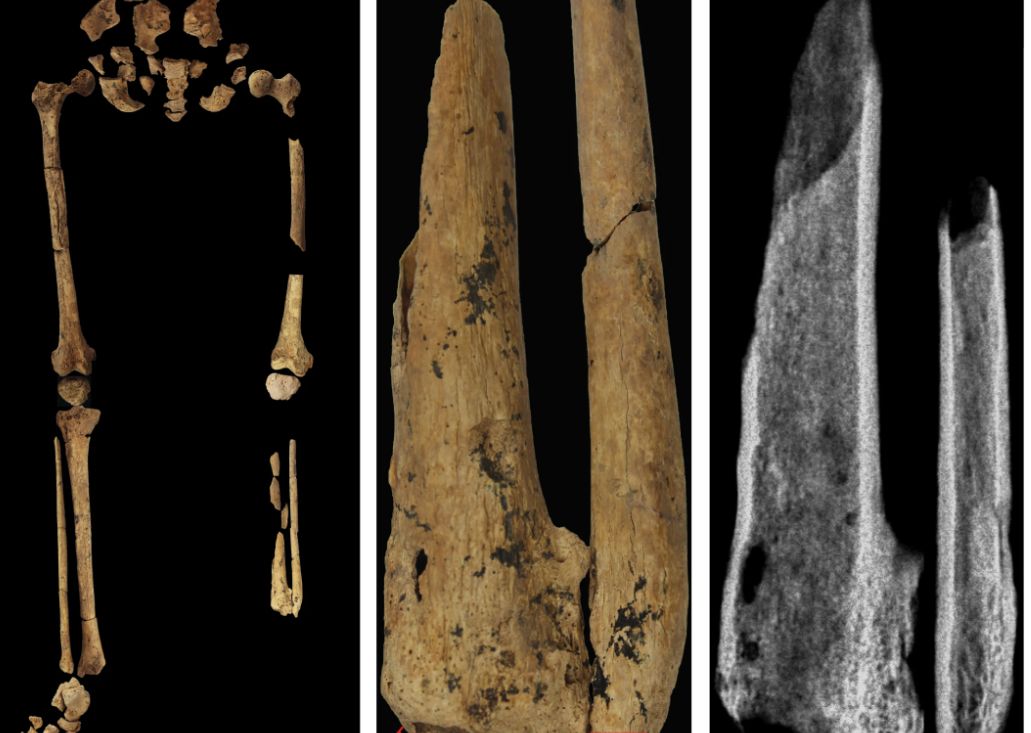
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
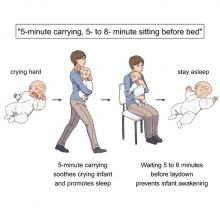
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
FDA warns of cancer risk in scar tissue around breast implants
.
The FDA safety communication is based on several dozen reports of these cancers occurring in the capsule or scar tissue around breast implants. This issue differs from breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) – a known risk among implant recipients.
“After preliminary review of published literature as part of our ongoing monitoring of the safety of breast implants, the FDA is aware of less than 20 cases of SCC and less than 30 cases of various lymphomas in the capsule around the breast implant,” the agency’s alert explains.
One avenue through which the FDA has identified cases is via medical device reports. As of Sept. 1, the FDA has received 10 medical device reports about SCC related to breast implants and 12 about various lymphomas.
The incidence rate and risk factors for these events are currently unknown, but reports of SCC and various lymphomas in the capsule around the breast implants have been reported for both textured and smooth breast implants, as well as for both saline and silicone breast implants. In some cases, the cancers were diagnosed years after breast implant surgery.
Reported signs and symptoms included swelling, pain, lumps, or skin changes.
Although the risks of SCC and lymphomas in the tissue around breast implants appears rare, “when safety risks with medical devices are identified, we wanted to provide clear and understandable information to the public as quickly as possible,” Binita Ashar, MD, director of the Office of Surgical and Infection Control Devices, FDA Center for Devices and Radiological Health, explained in a press release.
Patients and providers are strongly encouraged to report breast implant–related problems and cases of SCC or lymphoma of the breast implant capsule to MedWatch, the FDA’s adverse event reporting program.
The FDA plans to complete “a thorough literature review” as well as “identify ways to collect more detailed information regarding patient cases.”
A version of this article first appeared on Medscape.com.
.
The FDA safety communication is based on several dozen reports of these cancers occurring in the capsule or scar tissue around breast implants. This issue differs from breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) – a known risk among implant recipients.
“After preliminary review of published literature as part of our ongoing monitoring of the safety of breast implants, the FDA is aware of less than 20 cases of SCC and less than 30 cases of various lymphomas in the capsule around the breast implant,” the agency’s alert explains.
One avenue through which the FDA has identified cases is via medical device reports. As of Sept. 1, the FDA has received 10 medical device reports about SCC related to breast implants and 12 about various lymphomas.
The incidence rate and risk factors for these events are currently unknown, but reports of SCC and various lymphomas in the capsule around the breast implants have been reported for both textured and smooth breast implants, as well as for both saline and silicone breast implants. In some cases, the cancers were diagnosed years after breast implant surgery.
Reported signs and symptoms included swelling, pain, lumps, or skin changes.
Although the risks of SCC and lymphomas in the tissue around breast implants appears rare, “when safety risks with medical devices are identified, we wanted to provide clear and understandable information to the public as quickly as possible,” Binita Ashar, MD, director of the Office of Surgical and Infection Control Devices, FDA Center for Devices and Radiological Health, explained in a press release.
Patients and providers are strongly encouraged to report breast implant–related problems and cases of SCC or lymphoma of the breast implant capsule to MedWatch, the FDA’s adverse event reporting program.
The FDA plans to complete “a thorough literature review” as well as “identify ways to collect more detailed information regarding patient cases.”
A version of this article first appeared on Medscape.com.
.
The FDA safety communication is based on several dozen reports of these cancers occurring in the capsule or scar tissue around breast implants. This issue differs from breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) – a known risk among implant recipients.
“After preliminary review of published literature as part of our ongoing monitoring of the safety of breast implants, the FDA is aware of less than 20 cases of SCC and less than 30 cases of various lymphomas in the capsule around the breast implant,” the agency’s alert explains.
One avenue through which the FDA has identified cases is via medical device reports. As of Sept. 1, the FDA has received 10 medical device reports about SCC related to breast implants and 12 about various lymphomas.
The incidence rate and risk factors for these events are currently unknown, but reports of SCC and various lymphomas in the capsule around the breast implants have been reported for both textured and smooth breast implants, as well as for both saline and silicone breast implants. In some cases, the cancers were diagnosed years after breast implant surgery.
Reported signs and symptoms included swelling, pain, lumps, or skin changes.
Although the risks of SCC and lymphomas in the tissue around breast implants appears rare, “when safety risks with medical devices are identified, we wanted to provide clear and understandable information to the public as quickly as possible,” Binita Ashar, MD, director of the Office of Surgical and Infection Control Devices, FDA Center for Devices and Radiological Health, explained in a press release.
Patients and providers are strongly encouraged to report breast implant–related problems and cases of SCC or lymphoma of the breast implant capsule to MedWatch, the FDA’s adverse event reporting program.
The FDA plans to complete “a thorough literature review” as well as “identify ways to collect more detailed information regarding patient cases.”
A version of this article first appeared on Medscape.com.
Fish oil pills do not reduce fractures in healthy seniors: VITAL
Omega-3 supplements did not reduce fractures during a median 5.3-year follow-up in the more than 25,000 generally healthy men and women (≥ age 50 and ≥ age 55, respectively) in the Vitamin D and Omega-3 Trial (VITAL).
The large randomized controlled trial tested whether omega-3 fatty acid or vitamin D supplements prevented cardiovascular disease or cancer in a representative sample of midlife and older adults from 50 U.S. states – which they did not. In a further analysis of VITAL, vitamin D supplements (cholecalciferol, 2,000 IU/day) did not lower the risk of incident total, nonvertebral, and hip fractures, compared with placebo.
Now this new analysis shows that omega-3 fatty acid supplements (1 g/day of fish oil) did not reduce the risk of such fractures in the VITAL population either. Meryl S. LeBoff, MD, presented the latest findings during an oral session at the annual meeting of the American Society for Bone and Mineral Research.
“In this, the largest randomized controlled trial in the world, we did not find an effect of omega-3 fatty acid supplements on fractures,” Dr. LeBoff, from Brigham and Women’s Hospital and Harvard Medical School, both in Boston, told this news organization.
The current analysis did “unexpectedly” show that among participants who received the omega-3 fatty acid supplements, there was an increase in fractures in men, and fracture risk was higher in people with a normal or low body mass index and lower in people with higher BMI.
However, these subgroup findings need to be interpreted with caution and may be caused by chance, Dr. LeBoff warned. The researchers will be investigating these findings in further analyses.
Should patients take omega-3 supplements or not?
Asked whether, in the meantime, patients should start or keep taking fish oil supplements for possible health benefits, she noted that certain individuals might benefit.
For example, in VITAL, participants who ate less than 1.5 servings of fish per week and received omega-3 fatty acid supplements had a decrease in the combined cardiovascular endpoint, and Black participants who took fish oil supplements had a substantially reduced risk of the outcome, regardless of fish intake.
“I think everybody needs to review [the study findings] with clinicians and make a decision in terms of what would be best for them,” she said.
Session comoderator Bente Langdahl, MD, PhD, commented that “many people take omega-3 because they think it will help” knee, hip, or other joint pain.
Perhaps men are more prone to joint pain because of osteoarthritis and the supplements lessen the pain, so these men became more physically active and more prone to fractures, she speculated.
The current study shows that, “so far, we haven’t been able to demonstrate a reduced rate of fractures with fish oil supplements in clinical randomized trials” conducted in relatively healthy and not the oldest patients, she summarized. “We’re not talking about 80-year-olds.”
In this “well-conducted study, they were not able to see any difference” with omega-3 fatty acid supplements versus placebo, but apparently, there are no harms associated with taking these supplements, she said.
To patients who ask her about such supplements, Dr. Langdahl advised: “Try it out for 3 months. If it really helps you, if it takes away your joint pain or whatever, then that might work for you. But then remember to stop again because it might just be a temporary effect.”
Could fish oil supplements protect against fractures?
An estimated 22% of U.S. adults aged 60 and older take omega-3 fatty acid supplements, Dr. LeBoff noted.
Preclinical studies have shown that omega-3 fatty acids reduce bone resorption and have anti-inflammatory effects, but observational studies have reported conflicting findings.
The researchers conducted this ancillary study of VITAL to fill these knowledge gaps.
VITAL enrolled a national sample of 25,871 U.S. men and women, including 5,106 Black participants, with a mean age of 67 and a mean BMI of 28 kg/m2.
Importantly, participants were not recruited by low bone density, fractures, or vitamin D deficiency. Prior to entry, participants were required to stop taking omega-3 supplements and limit nonstudy vitamin D and calcium supplements.
The omega-3 fatty acid supplements used in the study contained eicosapentaenoic acid and docosahexaenoic acid in a 1.2:1 ratio.
VITAL had a 2x2 factorial design whereby 6,463 participants were randomized to receive the omega-3 fatty acid supplement and 6,474 were randomized to placebo. (Remaining participants were randomized to receive vitamin D or placebo.)
Participants in the omega-3 fatty acid and placebo groups had similar baseline characteristics. For example, about half (50.5%) were women, and on average, they ate 1.1 servings of dark-meat fish (such as salmon) per week.
Participants completed detailed questionnaires at baseline and each year.
Plasma omega-3 levels were measured at baseline and, in 1,583 participants, at 1 year of follow-up. The mean omega-3 index rose 54.7% in the omega-3 fatty acid group and changed less than 2% in the placebo group at 1 year.
Study pill adherence was 87.0% at 2 years and 85.7% at 5 years.
Fractures were self-reported on annual questionnaires and centrally adjudicated in medical record review.
No clinically meaningful effect of omega-3 fatty acids on fractures
During a median 5.3-year follow-up, researchers adjudicated 2,133 total fractures and confirmed 1,991 fractures (93%) in 1551 participants.
Incidences of total, nonvertebral, and hip fractures were similar in both groups.
Compared with placebo, omega-3 fatty acid supplements had no significant effect on risk of total fractures (hazard ratio, 1.02; 95% confidence interval, 0.92-1.13), nonvertebral fractures (HR, 1.01; 95% CI, 0.91-1.12), or hip fractures (HR, 0.89; 95% CI, 0.61-1.30), all adjusted for age, sex, and race.
The “confidence intervals were narrow, likely excluding a clinically meaningful effect,” Dr. LeBoff noted.
Among men, those who received fish oil supplements had a greater risk of fracture than those who received placebo (HR, 1.27; 95% CI, 1.07-1.51), but this result “was not corrected for multiple hypothesis testing,” Dr. LeBoff cautioned.
In the overall population, participants with a BMI less than 25 who received fish oil versus placebo had an increased risk of fracture, and those with a BMI of at least 30 who received fish oil versus placebo had a decreased risk of fracture, but the limits of the confidence intervals crossed 1.00.
After excluding digit, skull, and pathologic fractures, there was no significant reduction in total fractures (HR, 1.02; 95% CI, 0.92-1.14), nonvertebral fractures (HR, 1.02; 95% CI, 0.92-1.14), or hip fractures (HR, 0.90; 95% CI, 0.61-1.33), with omega-3 supplements versus placebo.
Similarly, there was no significant reduction in risk of major osteoporotic fractures (hip, wrist, humerus, and clinical spine fractures) or wrist fractures with omega-3 supplements versus placebo.
VITAL only studied one dose of omega-3 fatty acid supplements, and results may not be generalizable to younger adults, or older adults living in residential communities, Dr. LeBoff noted.
The study was supported by grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. VITAL was funded by the National Cancer Institute and the National Heart, Lung, and Blood Institute. Dr. LeBoff and Dr. Langdahl have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Omega-3 supplements did not reduce fractures during a median 5.3-year follow-up in the more than 25,000 generally healthy men and women (≥ age 50 and ≥ age 55, respectively) in the Vitamin D and Omega-3 Trial (VITAL).
The large randomized controlled trial tested whether omega-3 fatty acid or vitamin D supplements prevented cardiovascular disease or cancer in a representative sample of midlife and older adults from 50 U.S. states – which they did not. In a further analysis of VITAL, vitamin D supplements (cholecalciferol, 2,000 IU/day) did not lower the risk of incident total, nonvertebral, and hip fractures, compared with placebo.
Now this new analysis shows that omega-3 fatty acid supplements (1 g/day of fish oil) did not reduce the risk of such fractures in the VITAL population either. Meryl S. LeBoff, MD, presented the latest findings during an oral session at the annual meeting of the American Society for Bone and Mineral Research.
“In this, the largest randomized controlled trial in the world, we did not find an effect of omega-3 fatty acid supplements on fractures,” Dr. LeBoff, from Brigham and Women’s Hospital and Harvard Medical School, both in Boston, told this news organization.
The current analysis did “unexpectedly” show that among participants who received the omega-3 fatty acid supplements, there was an increase in fractures in men, and fracture risk was higher in people with a normal or low body mass index and lower in people with higher BMI.
However, these subgroup findings need to be interpreted with caution and may be caused by chance, Dr. LeBoff warned. The researchers will be investigating these findings in further analyses.
Should patients take omega-3 supplements or not?
Asked whether, in the meantime, patients should start or keep taking fish oil supplements for possible health benefits, she noted that certain individuals might benefit.
For example, in VITAL, participants who ate less than 1.5 servings of fish per week and received omega-3 fatty acid supplements had a decrease in the combined cardiovascular endpoint, and Black participants who took fish oil supplements had a substantially reduced risk of the outcome, regardless of fish intake.
“I think everybody needs to review [the study findings] with clinicians and make a decision in terms of what would be best for them,” she said.
Session comoderator Bente Langdahl, MD, PhD, commented that “many people take omega-3 because they think it will help” knee, hip, or other joint pain.
Perhaps men are more prone to joint pain because of osteoarthritis and the supplements lessen the pain, so these men became more physically active and more prone to fractures, she speculated.
The current study shows that, “so far, we haven’t been able to demonstrate a reduced rate of fractures with fish oil supplements in clinical randomized trials” conducted in relatively healthy and not the oldest patients, she summarized. “We’re not talking about 80-year-olds.”
In this “well-conducted study, they were not able to see any difference” with omega-3 fatty acid supplements versus placebo, but apparently, there are no harms associated with taking these supplements, she said.
To patients who ask her about such supplements, Dr. Langdahl advised: “Try it out for 3 months. If it really helps you, if it takes away your joint pain or whatever, then that might work for you. But then remember to stop again because it might just be a temporary effect.”
Could fish oil supplements protect against fractures?
An estimated 22% of U.S. adults aged 60 and older take omega-3 fatty acid supplements, Dr. LeBoff noted.
Preclinical studies have shown that omega-3 fatty acids reduce bone resorption and have anti-inflammatory effects, but observational studies have reported conflicting findings.
The researchers conducted this ancillary study of VITAL to fill these knowledge gaps.
VITAL enrolled a national sample of 25,871 U.S. men and women, including 5,106 Black participants, with a mean age of 67 and a mean BMI of 28 kg/m2.
Importantly, participants were not recruited by low bone density, fractures, or vitamin D deficiency. Prior to entry, participants were required to stop taking omega-3 supplements and limit nonstudy vitamin D and calcium supplements.
The omega-3 fatty acid supplements used in the study contained eicosapentaenoic acid and docosahexaenoic acid in a 1.2:1 ratio.
VITAL had a 2x2 factorial design whereby 6,463 participants were randomized to receive the omega-3 fatty acid supplement and 6,474 were randomized to placebo. (Remaining participants were randomized to receive vitamin D or placebo.)
Participants in the omega-3 fatty acid and placebo groups had similar baseline characteristics. For example, about half (50.5%) were women, and on average, they ate 1.1 servings of dark-meat fish (such as salmon) per week.
Participants completed detailed questionnaires at baseline and each year.
Plasma omega-3 levels were measured at baseline and, in 1,583 participants, at 1 year of follow-up. The mean omega-3 index rose 54.7% in the omega-3 fatty acid group and changed less than 2% in the placebo group at 1 year.
Study pill adherence was 87.0% at 2 years and 85.7% at 5 years.
Fractures were self-reported on annual questionnaires and centrally adjudicated in medical record review.
No clinically meaningful effect of omega-3 fatty acids on fractures
During a median 5.3-year follow-up, researchers adjudicated 2,133 total fractures and confirmed 1,991 fractures (93%) in 1551 participants.
Incidences of total, nonvertebral, and hip fractures were similar in both groups.
Compared with placebo, omega-3 fatty acid supplements had no significant effect on risk of total fractures (hazard ratio, 1.02; 95% confidence interval, 0.92-1.13), nonvertebral fractures (HR, 1.01; 95% CI, 0.91-1.12), or hip fractures (HR, 0.89; 95% CI, 0.61-1.30), all adjusted for age, sex, and race.
The “confidence intervals were narrow, likely excluding a clinically meaningful effect,” Dr. LeBoff noted.
Among men, those who received fish oil supplements had a greater risk of fracture than those who received placebo (HR, 1.27; 95% CI, 1.07-1.51), but this result “was not corrected for multiple hypothesis testing,” Dr. LeBoff cautioned.
In the overall population, participants with a BMI less than 25 who received fish oil versus placebo had an increased risk of fracture, and those with a BMI of at least 30 who received fish oil versus placebo had a decreased risk of fracture, but the limits of the confidence intervals crossed 1.00.
After excluding digit, skull, and pathologic fractures, there was no significant reduction in total fractures (HR, 1.02; 95% CI, 0.92-1.14), nonvertebral fractures (HR, 1.02; 95% CI, 0.92-1.14), or hip fractures (HR, 0.90; 95% CI, 0.61-1.33), with omega-3 supplements versus placebo.
Similarly, there was no significant reduction in risk of major osteoporotic fractures (hip, wrist, humerus, and clinical spine fractures) or wrist fractures with omega-3 supplements versus placebo.
VITAL only studied one dose of omega-3 fatty acid supplements, and results may not be generalizable to younger adults, or older adults living in residential communities, Dr. LeBoff noted.
The study was supported by grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. VITAL was funded by the National Cancer Institute and the National Heart, Lung, and Blood Institute. Dr. LeBoff and Dr. Langdahl have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Omega-3 supplements did not reduce fractures during a median 5.3-year follow-up in the more than 25,000 generally healthy men and women (≥ age 50 and ≥ age 55, respectively) in the Vitamin D and Omega-3 Trial (VITAL).
The large randomized controlled trial tested whether omega-3 fatty acid or vitamin D supplements prevented cardiovascular disease or cancer in a representative sample of midlife and older adults from 50 U.S. states – which they did not. In a further analysis of VITAL, vitamin D supplements (cholecalciferol, 2,000 IU/day) did not lower the risk of incident total, nonvertebral, and hip fractures, compared with placebo.
Now this new analysis shows that omega-3 fatty acid supplements (1 g/day of fish oil) did not reduce the risk of such fractures in the VITAL population either. Meryl S. LeBoff, MD, presented the latest findings during an oral session at the annual meeting of the American Society for Bone and Mineral Research.
“In this, the largest randomized controlled trial in the world, we did not find an effect of omega-3 fatty acid supplements on fractures,” Dr. LeBoff, from Brigham and Women’s Hospital and Harvard Medical School, both in Boston, told this news organization.
The current analysis did “unexpectedly” show that among participants who received the omega-3 fatty acid supplements, there was an increase in fractures in men, and fracture risk was higher in people with a normal or low body mass index and lower in people with higher BMI.
However, these subgroup findings need to be interpreted with caution and may be caused by chance, Dr. LeBoff warned. The researchers will be investigating these findings in further analyses.
Should patients take omega-3 supplements or not?
Asked whether, in the meantime, patients should start or keep taking fish oil supplements for possible health benefits, she noted that certain individuals might benefit.
For example, in VITAL, participants who ate less than 1.5 servings of fish per week and received omega-3 fatty acid supplements had a decrease in the combined cardiovascular endpoint, and Black participants who took fish oil supplements had a substantially reduced risk of the outcome, regardless of fish intake.
“I think everybody needs to review [the study findings] with clinicians and make a decision in terms of what would be best for them,” she said.
Session comoderator Bente Langdahl, MD, PhD, commented that “many people take omega-3 because they think it will help” knee, hip, or other joint pain.
Perhaps men are more prone to joint pain because of osteoarthritis and the supplements lessen the pain, so these men became more physically active and more prone to fractures, she speculated.
The current study shows that, “so far, we haven’t been able to demonstrate a reduced rate of fractures with fish oil supplements in clinical randomized trials” conducted in relatively healthy and not the oldest patients, she summarized. “We’re not talking about 80-year-olds.”
In this “well-conducted study, they were not able to see any difference” with omega-3 fatty acid supplements versus placebo, but apparently, there are no harms associated with taking these supplements, she said.
To patients who ask her about such supplements, Dr. Langdahl advised: “Try it out for 3 months. If it really helps you, if it takes away your joint pain or whatever, then that might work for you. But then remember to stop again because it might just be a temporary effect.”
Could fish oil supplements protect against fractures?
An estimated 22% of U.S. adults aged 60 and older take omega-3 fatty acid supplements, Dr. LeBoff noted.
Preclinical studies have shown that omega-3 fatty acids reduce bone resorption and have anti-inflammatory effects, but observational studies have reported conflicting findings.
The researchers conducted this ancillary study of VITAL to fill these knowledge gaps.
VITAL enrolled a national sample of 25,871 U.S. men and women, including 5,106 Black participants, with a mean age of 67 and a mean BMI of 28 kg/m2.
Importantly, participants were not recruited by low bone density, fractures, or vitamin D deficiency. Prior to entry, participants were required to stop taking omega-3 supplements and limit nonstudy vitamin D and calcium supplements.
The omega-3 fatty acid supplements used in the study contained eicosapentaenoic acid and docosahexaenoic acid in a 1.2:1 ratio.
VITAL had a 2x2 factorial design whereby 6,463 participants were randomized to receive the omega-3 fatty acid supplement and 6,474 were randomized to placebo. (Remaining participants were randomized to receive vitamin D or placebo.)
Participants in the omega-3 fatty acid and placebo groups had similar baseline characteristics. For example, about half (50.5%) were women, and on average, they ate 1.1 servings of dark-meat fish (such as salmon) per week.
Participants completed detailed questionnaires at baseline and each year.
Plasma omega-3 levels were measured at baseline and, in 1,583 participants, at 1 year of follow-up. The mean omega-3 index rose 54.7% in the omega-3 fatty acid group and changed less than 2% in the placebo group at 1 year.
Study pill adherence was 87.0% at 2 years and 85.7% at 5 years.
Fractures were self-reported on annual questionnaires and centrally adjudicated in medical record review.
No clinically meaningful effect of omega-3 fatty acids on fractures
During a median 5.3-year follow-up, researchers adjudicated 2,133 total fractures and confirmed 1,991 fractures (93%) in 1551 participants.
Incidences of total, nonvertebral, and hip fractures were similar in both groups.
Compared with placebo, omega-3 fatty acid supplements had no significant effect on risk of total fractures (hazard ratio, 1.02; 95% confidence interval, 0.92-1.13), nonvertebral fractures (HR, 1.01; 95% CI, 0.91-1.12), or hip fractures (HR, 0.89; 95% CI, 0.61-1.30), all adjusted for age, sex, and race.
The “confidence intervals were narrow, likely excluding a clinically meaningful effect,” Dr. LeBoff noted.
Among men, those who received fish oil supplements had a greater risk of fracture than those who received placebo (HR, 1.27; 95% CI, 1.07-1.51), but this result “was not corrected for multiple hypothesis testing,” Dr. LeBoff cautioned.
In the overall population, participants with a BMI less than 25 who received fish oil versus placebo had an increased risk of fracture, and those with a BMI of at least 30 who received fish oil versus placebo had a decreased risk of fracture, but the limits of the confidence intervals crossed 1.00.
After excluding digit, skull, and pathologic fractures, there was no significant reduction in total fractures (HR, 1.02; 95% CI, 0.92-1.14), nonvertebral fractures (HR, 1.02; 95% CI, 0.92-1.14), or hip fractures (HR, 0.90; 95% CI, 0.61-1.33), with omega-3 supplements versus placebo.
Similarly, there was no significant reduction in risk of major osteoporotic fractures (hip, wrist, humerus, and clinical spine fractures) or wrist fractures with omega-3 supplements versus placebo.
VITAL only studied one dose of omega-3 fatty acid supplements, and results may not be generalizable to younger adults, or older adults living in residential communities, Dr. LeBoff noted.
The study was supported by grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. VITAL was funded by the National Cancer Institute and the National Heart, Lung, and Blood Institute. Dr. LeBoff and Dr. Langdahl have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASMBR 2022
Integrase inhibitors and gestational weight gain: Should women worry?
In recent years, increased use of integrase strand transferase inhibitor (INSTI) antiviral treatment (ART) has raised concerns about weight gain and adverse outcomes in patients with HIV. This is especially true regarding possible excessive gestational weight gain, which in women without HIV has been associated with maternal gestational diabetes, hypertensive and liver conditions, as well as related risks for preterm birth, fetal macrosomia, and higher weight after birth.
Unfortunately, few studies in pregnant women with HIV have moved out of the controlled environment into real-world settings, potentially limiting current knowledge about the impact of gestational weight gain – as well as strategies to both prevent it and the associated adverse outcomes.
That is what a team of infectious disease specialists at the Hospital Federal dos Servidores do Estado in Rio de Janeiro recently sought to answer among a cohort of INSTI-experienced and INSTI-naive women with BMIs less than 25 kg/m2 (underweight/normal weight) and higher than 25 kg/m2.
Surprising findings
The investigators determined that rates of excessive weight gain were significantly higher in INSTI-naive women with BMI less than 25 who experienced rates as high as 31.6%, compared with approximately 12% of women who conceived while on INSTIs, regardless of BMI values at baseline (P = .004).
However, rates of unfavorable pregnancy outcomes (for example, small for gestational age, preterm birth, stillbirth, death) appeared to be low overall and similar among all the study groups.
“We had some discussions when we were working on this and thought that the weight gain might have adverse effects,” Trevon Fuller, PhD, lead author and a postdoctoral student at the Hospital Federal dos Servidores do Estado, told this news organization.
“But it looked like the weight gain might actually be good, to the extent that we didn’t see any harm to the mom or the baby of those underweight or normal weight women who were naive to INSTIs,” he explained.
Dr. Fuller and his team enrolled 198 pregnant women living with HIV who sought care at the Hospital Federal dos Servidores do Estado – a national reference center for USAID’s Prevention of Mother to Child Transmission strategic program – between October 2014 and October 2021.
Participants were divided into two primary cohorts: BMI less than 25 at enrollment (n = 74) or BMI of 25 or higher (n = 124), then further divided by timing of INSTI-based combined ART:
- INSTI-naive: women using INSTI-based ART (raltegravir [Isentress] 400 mg twice per day or dolutegravir [Tivicay] 50 mg/day plus 2 non-nucleoside reverse transcriptase inhibitors – lamivudine plus tenofovir disoproxil fumarate or lamivudine plus zidovudine) for 4 weeks between baseline and near delivery.
- INSTI-experienced: women who became pregnant while using INSTIs for at least 6 months before conception.
Among underweight/normal weight participants, 77% (n = 57) were INSTI-naive and 23% (n = 17) INSTI-experienced, and among overweight/obese participants, 81.5% (n = 101) were INSTI-naive, and 18.5% (n = 23) were experienced.
Maternal age, which did not differ significantly by BMI or treatment experience, was a median of 28 years, and most participants were non-White. All participants were virally suppressed near delivery.
Study findings, which were published online in HIV Medicine, highlighted that median weight near delivery in participants who were overweight/obese at baseline was similar regardless of whether they were treatment-experienced (90 kg [198 lb]) or treatment-naive (82.3 kg [181 lb]), P = .026.
However, participants who were underweight/normal weight who were INSTI-naive had significantly higher rates of gestational weight gain (31.5%, 18/57), compared with those of underweight/normal weight who were INSTI-experienced (11.8%, 2/17), P = .004. Notably, this gain was significant in all categories of change (that is, low < 0.18 kg/week, normal 0.18-0.59 kg/week), and high > 0.59 kg/week).
“One of the things that we took away was that this weight gain is primarily happening with women who are starting INSTIs,” said Dr. Fuller.
“The data suggest that [it] might be temporary in the sense that there’s not going to be continuous weight gain but that it will probably approach some type of horizontal asymptote,” he added.
Although obstetric and neonatal outcomes were secondary measures, the investigators did not observe any significantly different outcomes when comparing the groups, and there were no stillbirths, neonatal deaths, or macrosomia.
Preterm delivery rates in underweight/normal weight participants who were INSTI-experienced (11.8%, 2/17) and INSTI-naive (5.3%, 3/57) were similar to overweight/obese participants who were INSTI-experienced (13%, 3/23) and INSTI-naive (6.9%, 7/101).
The same was true for low birthweight.
Still, the study appears to raise more questions than it answers, Sigal Yawetz, MD, an infectious disease specialist at Brigham and Women’s Hospital, Boston, said in an interview – a factor that she said is common also in some of the more recent randomized controlled studies, such as IMPAACT PROMISE.
Dr. Yawetz, who was not involved in the study, also noted, “The groups were small, so comparisons within the groups are difficult, and so many people were excluded that it’s hard to know if there were adverse outcomes related to this ... It’s very confounded.”
The World Health Organization estimates that there are roughly 1.3 million pregnant women with HIV, 81% of whom are on antiretroviral therapy. Although the literature continues to evolve, data suggest that in general, Black women are at greater risk for gestational weight gain.
“We have to remember that women who gain excess weight in pregnancy are still going to be with this weight following pregnancy as well,” Dr. Yawetz said. “So, it might impact their pregnancy but also their health after delivery and for subsequent pregnancies, which we don’t have data for yet.”
Dr. Fuller agrees that more data are needed and mentioned that the team plans to study this further, ideally with larger sample sizes.
Yet, despite the lingering questions, there is a silver lining, one that Dr. Yawetz was emphatic about.
“I really welcome people doing studies on this because we really need the data. By far, integrase inhibitors are the first-line regimen all over the world for pregnant women, and if you look at the gestalt or full picture, this is the best regimen to give pregnant women,” she said.
Dr. Fuller and Dr. Yawetz report no relevant financial relationships. The study was independently supported.
A version of this article first appeared on Medscape.com.
In recent years, increased use of integrase strand transferase inhibitor (INSTI) antiviral treatment (ART) has raised concerns about weight gain and adverse outcomes in patients with HIV. This is especially true regarding possible excessive gestational weight gain, which in women without HIV has been associated with maternal gestational diabetes, hypertensive and liver conditions, as well as related risks for preterm birth, fetal macrosomia, and higher weight after birth.
Unfortunately, few studies in pregnant women with HIV have moved out of the controlled environment into real-world settings, potentially limiting current knowledge about the impact of gestational weight gain – as well as strategies to both prevent it and the associated adverse outcomes.
That is what a team of infectious disease specialists at the Hospital Federal dos Servidores do Estado in Rio de Janeiro recently sought to answer among a cohort of INSTI-experienced and INSTI-naive women with BMIs less than 25 kg/m2 (underweight/normal weight) and higher than 25 kg/m2.
Surprising findings
The investigators determined that rates of excessive weight gain were significantly higher in INSTI-naive women with BMI less than 25 who experienced rates as high as 31.6%, compared with approximately 12% of women who conceived while on INSTIs, regardless of BMI values at baseline (P = .004).
However, rates of unfavorable pregnancy outcomes (for example, small for gestational age, preterm birth, stillbirth, death) appeared to be low overall and similar among all the study groups.
“We had some discussions when we were working on this and thought that the weight gain might have adverse effects,” Trevon Fuller, PhD, lead author and a postdoctoral student at the Hospital Federal dos Servidores do Estado, told this news organization.
“But it looked like the weight gain might actually be good, to the extent that we didn’t see any harm to the mom or the baby of those underweight or normal weight women who were naive to INSTIs,” he explained.
Dr. Fuller and his team enrolled 198 pregnant women living with HIV who sought care at the Hospital Federal dos Servidores do Estado – a national reference center for USAID’s Prevention of Mother to Child Transmission strategic program – between October 2014 and October 2021.
Participants were divided into two primary cohorts: BMI less than 25 at enrollment (n = 74) or BMI of 25 or higher (n = 124), then further divided by timing of INSTI-based combined ART:
- INSTI-naive: women using INSTI-based ART (raltegravir [Isentress] 400 mg twice per day or dolutegravir [Tivicay] 50 mg/day plus 2 non-nucleoside reverse transcriptase inhibitors – lamivudine plus tenofovir disoproxil fumarate or lamivudine plus zidovudine) for 4 weeks between baseline and near delivery.
- INSTI-experienced: women who became pregnant while using INSTIs for at least 6 months before conception.
Among underweight/normal weight participants, 77% (n = 57) were INSTI-naive and 23% (n = 17) INSTI-experienced, and among overweight/obese participants, 81.5% (n = 101) were INSTI-naive, and 18.5% (n = 23) were experienced.
Maternal age, which did not differ significantly by BMI or treatment experience, was a median of 28 years, and most participants were non-White. All participants were virally suppressed near delivery.
Study findings, which were published online in HIV Medicine, highlighted that median weight near delivery in participants who were overweight/obese at baseline was similar regardless of whether they were treatment-experienced (90 kg [198 lb]) or treatment-naive (82.3 kg [181 lb]), P = .026.
However, participants who were underweight/normal weight who were INSTI-naive had significantly higher rates of gestational weight gain (31.5%, 18/57), compared with those of underweight/normal weight who were INSTI-experienced (11.8%, 2/17), P = .004. Notably, this gain was significant in all categories of change (that is, low < 0.18 kg/week, normal 0.18-0.59 kg/week), and high > 0.59 kg/week).
“One of the things that we took away was that this weight gain is primarily happening with women who are starting INSTIs,” said Dr. Fuller.
“The data suggest that [it] might be temporary in the sense that there’s not going to be continuous weight gain but that it will probably approach some type of horizontal asymptote,” he added.
Although obstetric and neonatal outcomes were secondary measures, the investigators did not observe any significantly different outcomes when comparing the groups, and there were no stillbirths, neonatal deaths, or macrosomia.
Preterm delivery rates in underweight/normal weight participants who were INSTI-experienced (11.8%, 2/17) and INSTI-naive (5.3%, 3/57) were similar to overweight/obese participants who were INSTI-experienced (13%, 3/23) and INSTI-naive (6.9%, 7/101).
The same was true for low birthweight.
Still, the study appears to raise more questions than it answers, Sigal Yawetz, MD, an infectious disease specialist at Brigham and Women’s Hospital, Boston, said in an interview – a factor that she said is common also in some of the more recent randomized controlled studies, such as IMPAACT PROMISE.
Dr. Yawetz, who was not involved in the study, also noted, “The groups were small, so comparisons within the groups are difficult, and so many people were excluded that it’s hard to know if there were adverse outcomes related to this ... It’s very confounded.”
The World Health Organization estimates that there are roughly 1.3 million pregnant women with HIV, 81% of whom are on antiretroviral therapy. Although the literature continues to evolve, data suggest that in general, Black women are at greater risk for gestational weight gain.
“We have to remember that women who gain excess weight in pregnancy are still going to be with this weight following pregnancy as well,” Dr. Yawetz said. “So, it might impact their pregnancy but also their health after delivery and for subsequent pregnancies, which we don’t have data for yet.”
Dr. Fuller agrees that more data are needed and mentioned that the team plans to study this further, ideally with larger sample sizes.
Yet, despite the lingering questions, there is a silver lining, one that Dr. Yawetz was emphatic about.
“I really welcome people doing studies on this because we really need the data. By far, integrase inhibitors are the first-line regimen all over the world for pregnant women, and if you look at the gestalt or full picture, this is the best regimen to give pregnant women,” she said.
Dr. Fuller and Dr. Yawetz report no relevant financial relationships. The study was independently supported.
A version of this article first appeared on Medscape.com.
In recent years, increased use of integrase strand transferase inhibitor (INSTI) antiviral treatment (ART) has raised concerns about weight gain and adverse outcomes in patients with HIV. This is especially true regarding possible excessive gestational weight gain, which in women without HIV has been associated with maternal gestational diabetes, hypertensive and liver conditions, as well as related risks for preterm birth, fetal macrosomia, and higher weight after birth.
Unfortunately, few studies in pregnant women with HIV have moved out of the controlled environment into real-world settings, potentially limiting current knowledge about the impact of gestational weight gain – as well as strategies to both prevent it and the associated adverse outcomes.
That is what a team of infectious disease specialists at the Hospital Federal dos Servidores do Estado in Rio de Janeiro recently sought to answer among a cohort of INSTI-experienced and INSTI-naive women with BMIs less than 25 kg/m2 (underweight/normal weight) and higher than 25 kg/m2.
Surprising findings
The investigators determined that rates of excessive weight gain were significantly higher in INSTI-naive women with BMI less than 25 who experienced rates as high as 31.6%, compared with approximately 12% of women who conceived while on INSTIs, regardless of BMI values at baseline (P = .004).
However, rates of unfavorable pregnancy outcomes (for example, small for gestational age, preterm birth, stillbirth, death) appeared to be low overall and similar among all the study groups.
“We had some discussions when we were working on this and thought that the weight gain might have adverse effects,” Trevon Fuller, PhD, lead author and a postdoctoral student at the Hospital Federal dos Servidores do Estado, told this news organization.
“But it looked like the weight gain might actually be good, to the extent that we didn’t see any harm to the mom or the baby of those underweight or normal weight women who were naive to INSTIs,” he explained.
Dr. Fuller and his team enrolled 198 pregnant women living with HIV who sought care at the Hospital Federal dos Servidores do Estado – a national reference center for USAID’s Prevention of Mother to Child Transmission strategic program – between October 2014 and October 2021.
Participants were divided into two primary cohorts: BMI less than 25 at enrollment (n = 74) or BMI of 25 or higher (n = 124), then further divided by timing of INSTI-based combined ART:
- INSTI-naive: women using INSTI-based ART (raltegravir [Isentress] 400 mg twice per day or dolutegravir [Tivicay] 50 mg/day plus 2 non-nucleoside reverse transcriptase inhibitors – lamivudine plus tenofovir disoproxil fumarate or lamivudine plus zidovudine) for 4 weeks between baseline and near delivery.
- INSTI-experienced: women who became pregnant while using INSTIs for at least 6 months before conception.
Among underweight/normal weight participants, 77% (n = 57) were INSTI-naive and 23% (n = 17) INSTI-experienced, and among overweight/obese participants, 81.5% (n = 101) were INSTI-naive, and 18.5% (n = 23) were experienced.
Maternal age, which did not differ significantly by BMI or treatment experience, was a median of 28 years, and most participants were non-White. All participants were virally suppressed near delivery.
Study findings, which were published online in HIV Medicine, highlighted that median weight near delivery in participants who were overweight/obese at baseline was similar regardless of whether they were treatment-experienced (90 kg [198 lb]) or treatment-naive (82.3 kg [181 lb]), P = .026.
However, participants who were underweight/normal weight who were INSTI-naive had significantly higher rates of gestational weight gain (31.5%, 18/57), compared with those of underweight/normal weight who were INSTI-experienced (11.8%, 2/17), P = .004. Notably, this gain was significant in all categories of change (that is, low < 0.18 kg/week, normal 0.18-0.59 kg/week), and high > 0.59 kg/week).
“One of the things that we took away was that this weight gain is primarily happening with women who are starting INSTIs,” said Dr. Fuller.
“The data suggest that [it] might be temporary in the sense that there’s not going to be continuous weight gain but that it will probably approach some type of horizontal asymptote,” he added.
Although obstetric and neonatal outcomes were secondary measures, the investigators did not observe any significantly different outcomes when comparing the groups, and there were no stillbirths, neonatal deaths, or macrosomia.
Preterm delivery rates in underweight/normal weight participants who were INSTI-experienced (11.8%, 2/17) and INSTI-naive (5.3%, 3/57) were similar to overweight/obese participants who were INSTI-experienced (13%, 3/23) and INSTI-naive (6.9%, 7/101).
The same was true for low birthweight.
Still, the study appears to raise more questions than it answers, Sigal Yawetz, MD, an infectious disease specialist at Brigham and Women’s Hospital, Boston, said in an interview – a factor that she said is common also in some of the more recent randomized controlled studies, such as IMPAACT PROMISE.
Dr. Yawetz, who was not involved in the study, also noted, “The groups were small, so comparisons within the groups are difficult, and so many people were excluded that it’s hard to know if there were adverse outcomes related to this ... It’s very confounded.”
The World Health Organization estimates that there are roughly 1.3 million pregnant women with HIV, 81% of whom are on antiretroviral therapy. Although the literature continues to evolve, data suggest that in general, Black women are at greater risk for gestational weight gain.
“We have to remember that women who gain excess weight in pregnancy are still going to be with this weight following pregnancy as well,” Dr. Yawetz said. “So, it might impact their pregnancy but also their health after delivery and for subsequent pregnancies, which we don’t have data for yet.”
Dr. Fuller agrees that more data are needed and mentioned that the team plans to study this further, ideally with larger sample sizes.
Yet, despite the lingering questions, there is a silver lining, one that Dr. Yawetz was emphatic about.
“I really welcome people doing studies on this because we really need the data. By far, integrase inhibitors are the first-line regimen all over the world for pregnant women, and if you look at the gestalt or full picture, this is the best regimen to give pregnant women,” she said.
Dr. Fuller and Dr. Yawetz report no relevant financial relationships. The study was independently supported.
A version of this article first appeared on Medscape.com.
Weight gain during pregnancy may play role in child ADHD risk
Obesity in women of reproductive age has emerged as one of the main risk factors associated with neonatal complications and long-term neuropsychiatric consequences in offspring, including attention-deficit/hyperactivity disorder.
Research has also linked pregestational diabetes and gestational diabetes mellitus (GDM) to an increased risk for ADHD in offspring. Now, an observational study of 1,036 singleton births at one hospital between 1998 and 2008 suggests that in the presence of GDM, maternal obesity combined with excessive weight gain during pregnancy may be jointly associated with increased risk of offspring ADHD. The median follow-up was 17.7 years.
Maternal obesity was independently associated with ADHD (adjusted hazard ratio, 1.66; 95% confidence interval: 1.07-2.60), but excessive weight gain during pregnancy and maternal overweight were not, reported Verónica Perea, MD, PhD, of the Hospital Universitari Mútua de Terrassa, Barcelona, and colleagues in the Journal of Clinical Endocrinology & Metabolism.
However, in women with pregestation obesity who gained more weight than recommended by the National Academy of Medicine (NAM), the risk of offspring ADHD was higher, compared with women of normal weight whose pregnancy weight stayed within NAM guidelines (adjusted hazard ratio, 2.13; 95% confidence interval: 1.14-4.01).
“The results of this study suggest that the negative repercussions of excessive weight gain on children within the setting of a high-risk population with GDM and obesity were not only observed during the prenatal period but also years later with a development of ADHD,” the researchers wrote.
The study also showed that when maternal weight gain did not exceed NAM guidelines, maternal obesity was no longer independently associated with ADHD in offspring (aHR, 1.36; 95% CI: 0.78-2.36). This finding conflicts with earlier studies focusing primarily on the role of pregestational maternal weight, the researchers said. A 2018 nationwide Finnish cohort study in newborns showed an increased long-term risk of ADHD in those born to women with GDM, compared with the nondiabetic population. This long-term risk of ADHD increased in the presence of pregestational obesity (HR, 1.64).
Similarly, evidence from systematic reviews and meta-analyses has demonstrated that antenatal lifestyle interventions to prevent excessive weight gain during pregnancy were associated with a reduction in adverse pregnancy outcomes. However, evidence on offspring mental health was lacking, especially in high-risk pregnancies with gestational diabetes, the study authors said.
Although causal inferences can’t be drawn from the current observational study, “it seems that the higher risk [of ADHD] observed would be explained by the role of gestational weight gain during the antenatal period,” Dr. Perea said in an interview. Importantly, the study highlights a window of opportunity for promoting healthy weight gain during pregnancy, Dr. Perea said. ”This should be a priority in the current management of gestation.”
Fatima Cody Stanford, MD, MPH, an associate professor of medicine and pediatrics at Harvard Medical School, Boston, agreed. “I think one of the key issues is that there’s very little attention paid to how weight gain is regulated during pregnancy,” she said in an interview. On many other points, however, Dr. Stanford, who is a specialist in obesity medicine at Massachusetts General Hospital Weight Center, did not agree.
The association between ADHD and obesity has already been well established by a 2019 meta-analysis and systematic review of studies over the last 10 years, she emphasized. “These studies were able to show a much stronger association between maternal obesity and ADHD in offspring because they were powered to detect differences.”
The current study does not say “anything new or novel,” Dr. Stanford added. “Maternal obesity and the association with an increased risk of ADHD in offspring is the main issue. I don’t think there was any appreciable increase when weight gain during pregnancy was factored in. It’s mild at best.”
Eran Bornstein, MD, vice-chair of obstetrics and gynecology at Lenox Hill Hospital, New York, expressed a similar point of view. Although the study findings “add to the current literature,” they should be interpreted “cautiously,” Dr. Bornstein said in an interview.
The size of the effect on ADHD risk attributable to maternal weight gain during pregnancy “was not clear,” he said. “Cohort studies of this sort are excellent for finding associations which help us generate the hypothesis, but this doesn’t demonstrate a cause and effect or a magnitude for this effect.”
Physicians should follow cumulative data suggesting that maternal obesity is associated with a number of pregnancy complications and neonatal outcomes in women with and without diabetes, Dr. Bornstein suggested. “Optimizing maternal weight prior to pregnancy and adhering to recommendations regarding weight gain has the potential to improve some of these outcomes.”
Treating obesity prior to conception mitigates GDM risk, agreed Dr. Stanford. “The issue,” she explained, “is that all of the drugs approved for the treatment of obesity are contraindicated in pregnancy and lifestyle modification fails in 96% of cases, even when there is no pregnancy.” Drugs such as metformin are being used off-label to treat obesity and to safely manage gestational weight gain, she said. “Those of us who practice obesity medicine know that metformin can be safely used throughout pregnancy with no harm to the fetus.”
This study was partially funded by Fundació Docència i Recerca MútuaTerrassa. Dr. Perea and study coauthors reporting have no conflicts of interest. Dr. Stanford disclosed relationships with Novo Nordisk, Eli Lilly, Boehringer Ingelheim, Gelesis, Pfizer, Currax, and Rhythm. Dr. Bornstein reported having no conflicts of interest.
This story was updated on 11/7/2022.
Obesity in women of reproductive age has emerged as one of the main risk factors associated with neonatal complications and long-term neuropsychiatric consequences in offspring, including attention-deficit/hyperactivity disorder.
Research has also linked pregestational diabetes and gestational diabetes mellitus (GDM) to an increased risk for ADHD in offspring. Now, an observational study of 1,036 singleton births at one hospital between 1998 and 2008 suggests that in the presence of GDM, maternal obesity combined with excessive weight gain during pregnancy may be jointly associated with increased risk of offspring ADHD. The median follow-up was 17.7 years.
Maternal obesity was independently associated with ADHD (adjusted hazard ratio, 1.66; 95% confidence interval: 1.07-2.60), but excessive weight gain during pregnancy and maternal overweight were not, reported Verónica Perea, MD, PhD, of the Hospital Universitari Mútua de Terrassa, Barcelona, and colleagues in the Journal of Clinical Endocrinology & Metabolism.
However, in women with pregestation obesity who gained more weight than recommended by the National Academy of Medicine (NAM), the risk of offspring ADHD was higher, compared with women of normal weight whose pregnancy weight stayed within NAM guidelines (adjusted hazard ratio, 2.13; 95% confidence interval: 1.14-4.01).
“The results of this study suggest that the negative repercussions of excessive weight gain on children within the setting of a high-risk population with GDM and obesity were not only observed during the prenatal period but also years later with a development of ADHD,” the researchers wrote.
The study also showed that when maternal weight gain did not exceed NAM guidelines, maternal obesity was no longer independently associated with ADHD in offspring (aHR, 1.36; 95% CI: 0.78-2.36). This finding conflicts with earlier studies focusing primarily on the role of pregestational maternal weight, the researchers said. A 2018 nationwide Finnish cohort study in newborns showed an increased long-term risk of ADHD in those born to women with GDM, compared with the nondiabetic population. This long-term risk of ADHD increased in the presence of pregestational obesity (HR, 1.64).
Similarly, evidence from systematic reviews and meta-analyses has demonstrated that antenatal lifestyle interventions to prevent excessive weight gain during pregnancy were associated with a reduction in adverse pregnancy outcomes. However, evidence on offspring mental health was lacking, especially in high-risk pregnancies with gestational diabetes, the study authors said.
Although causal inferences can’t be drawn from the current observational study, “it seems that the higher risk [of ADHD] observed would be explained by the role of gestational weight gain during the antenatal period,” Dr. Perea said in an interview. Importantly, the study highlights a window of opportunity for promoting healthy weight gain during pregnancy, Dr. Perea said. ”This should be a priority in the current management of gestation.”
Fatima Cody Stanford, MD, MPH, an associate professor of medicine and pediatrics at Harvard Medical School, Boston, agreed. “I think one of the key issues is that there’s very little attention paid to how weight gain is regulated during pregnancy,” she said in an interview. On many other points, however, Dr. Stanford, who is a specialist in obesity medicine at Massachusetts General Hospital Weight Center, did not agree.
The association between ADHD and obesity has already been well established by a 2019 meta-analysis and systematic review of studies over the last 10 years, she emphasized. “These studies were able to show a much stronger association between maternal obesity and ADHD in offspring because they were powered to detect differences.”
The current study does not say “anything new or novel,” Dr. Stanford added. “Maternal obesity and the association with an increased risk of ADHD in offspring is the main issue. I don’t think there was any appreciable increase when weight gain during pregnancy was factored in. It’s mild at best.”
Eran Bornstein, MD, vice-chair of obstetrics and gynecology at Lenox Hill Hospital, New York, expressed a similar point of view. Although the study findings “add to the current literature,” they should be interpreted “cautiously,” Dr. Bornstein said in an interview.
The size of the effect on ADHD risk attributable to maternal weight gain during pregnancy “was not clear,” he said. “Cohort studies of this sort are excellent for finding associations which help us generate the hypothesis, but this doesn’t demonstrate a cause and effect or a magnitude for this effect.”
Physicians should follow cumulative data suggesting that maternal obesity is associated with a number of pregnancy complications and neonatal outcomes in women with and without diabetes, Dr. Bornstein suggested. “Optimizing maternal weight prior to pregnancy and adhering to recommendations regarding weight gain has the potential to improve some of these outcomes.”
Treating obesity prior to conception mitigates GDM risk, agreed Dr. Stanford. “The issue,” she explained, “is that all of the drugs approved for the treatment of obesity are contraindicated in pregnancy and lifestyle modification fails in 96% of cases, even when there is no pregnancy.” Drugs such as metformin are being used off-label to treat obesity and to safely manage gestational weight gain, she said. “Those of us who practice obesity medicine know that metformin can be safely used throughout pregnancy with no harm to the fetus.”
This study was partially funded by Fundació Docència i Recerca MútuaTerrassa. Dr. Perea and study coauthors reporting have no conflicts of interest. Dr. Stanford disclosed relationships with Novo Nordisk, Eli Lilly, Boehringer Ingelheim, Gelesis, Pfizer, Currax, and Rhythm. Dr. Bornstein reported having no conflicts of interest.
This story was updated on 11/7/2022.
Obesity in women of reproductive age has emerged as one of the main risk factors associated with neonatal complications and long-term neuropsychiatric consequences in offspring, including attention-deficit/hyperactivity disorder.
Research has also linked pregestational diabetes and gestational diabetes mellitus (GDM) to an increased risk for ADHD in offspring. Now, an observational study of 1,036 singleton births at one hospital between 1998 and 2008 suggests that in the presence of GDM, maternal obesity combined with excessive weight gain during pregnancy may be jointly associated with increased risk of offspring ADHD. The median follow-up was 17.7 years.
Maternal obesity was independently associated with ADHD (adjusted hazard ratio, 1.66; 95% confidence interval: 1.07-2.60), but excessive weight gain during pregnancy and maternal overweight were not, reported Verónica Perea, MD, PhD, of the Hospital Universitari Mútua de Terrassa, Barcelona, and colleagues in the Journal of Clinical Endocrinology & Metabolism.
However, in women with pregestation obesity who gained more weight than recommended by the National Academy of Medicine (NAM), the risk of offspring ADHD was higher, compared with women of normal weight whose pregnancy weight stayed within NAM guidelines (adjusted hazard ratio, 2.13; 95% confidence interval: 1.14-4.01).
“The results of this study suggest that the negative repercussions of excessive weight gain on children within the setting of a high-risk population with GDM and obesity were not only observed during the prenatal period but also years later with a development of ADHD,” the researchers wrote.
The study also showed that when maternal weight gain did not exceed NAM guidelines, maternal obesity was no longer independently associated with ADHD in offspring (aHR, 1.36; 95% CI: 0.78-2.36). This finding conflicts with earlier studies focusing primarily on the role of pregestational maternal weight, the researchers said. A 2018 nationwide Finnish cohort study in newborns showed an increased long-term risk of ADHD in those born to women with GDM, compared with the nondiabetic population. This long-term risk of ADHD increased in the presence of pregestational obesity (HR, 1.64).
Similarly, evidence from systematic reviews and meta-analyses has demonstrated that antenatal lifestyle interventions to prevent excessive weight gain during pregnancy were associated with a reduction in adverse pregnancy outcomes. However, evidence on offspring mental health was lacking, especially in high-risk pregnancies with gestational diabetes, the study authors said.
Although causal inferences can’t be drawn from the current observational study, “it seems that the higher risk [of ADHD] observed would be explained by the role of gestational weight gain during the antenatal period,” Dr. Perea said in an interview. Importantly, the study highlights a window of opportunity for promoting healthy weight gain during pregnancy, Dr. Perea said. ”This should be a priority in the current management of gestation.”
Fatima Cody Stanford, MD, MPH, an associate professor of medicine and pediatrics at Harvard Medical School, Boston, agreed. “I think one of the key issues is that there’s very little attention paid to how weight gain is regulated during pregnancy,” she said in an interview. On many other points, however, Dr. Stanford, who is a specialist in obesity medicine at Massachusetts General Hospital Weight Center, did not agree.
The association between ADHD and obesity has already been well established by a 2019 meta-analysis and systematic review of studies over the last 10 years, she emphasized. “These studies were able to show a much stronger association between maternal obesity and ADHD in offspring because they were powered to detect differences.”
The current study does not say “anything new or novel,” Dr. Stanford added. “Maternal obesity and the association with an increased risk of ADHD in offspring is the main issue. I don’t think there was any appreciable increase when weight gain during pregnancy was factored in. It’s mild at best.”
Eran Bornstein, MD, vice-chair of obstetrics and gynecology at Lenox Hill Hospital, New York, expressed a similar point of view. Although the study findings “add to the current literature,” they should be interpreted “cautiously,” Dr. Bornstein said in an interview.
The size of the effect on ADHD risk attributable to maternal weight gain during pregnancy “was not clear,” he said. “Cohort studies of this sort are excellent for finding associations which help us generate the hypothesis, but this doesn’t demonstrate a cause and effect or a magnitude for this effect.”
Physicians should follow cumulative data suggesting that maternal obesity is associated with a number of pregnancy complications and neonatal outcomes in women with and without diabetes, Dr. Bornstein suggested. “Optimizing maternal weight prior to pregnancy and adhering to recommendations regarding weight gain has the potential to improve some of these outcomes.”
Treating obesity prior to conception mitigates GDM risk, agreed Dr. Stanford. “The issue,” she explained, “is that all of the drugs approved for the treatment of obesity are contraindicated in pregnancy and lifestyle modification fails in 96% of cases, even when there is no pregnancy.” Drugs such as metformin are being used off-label to treat obesity and to safely manage gestational weight gain, she said. “Those of us who practice obesity medicine know that metformin can be safely used throughout pregnancy with no harm to the fetus.”
This study was partially funded by Fundació Docència i Recerca MútuaTerrassa. Dr. Perea and study coauthors reporting have no conflicts of interest. Dr. Stanford disclosed relationships with Novo Nordisk, Eli Lilly, Boehringer Ingelheim, Gelesis, Pfizer, Currax, and Rhythm. Dr. Bornstein reported having no conflicts of interest.
This story was updated on 11/7/2022.
The Journal of Clinical Endocrinology & Metabolism
FAQ: New COVID Omicron boosters
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
When do we stop using BMI to diagnose obesity?
“BMI is trash. Full stop.” This controversial tweet received 26,500 likes and almost 3,000 retweets. The 400 comments from medical and non–health care personnel ranged from agreeable to contrary to offensive.
As a Black woman who is an obesity expert living with the impact of obesity in my own life, I know the emotion that a BMI conversation can evoke. Before emotions hijack the conversation, let’s discuss BMI’s past, present, and future.
BMI: From observational measurement to clinical use
Imagine walking into your favorite clothing store where an eager clerk greets you with a shirt to try on. The fit is off, but the clerk insists that the shirt must fit because everyone who’s your height should be able to wear it. This scenario seems ridiculous. But this is how we’ve come to use the BMI. Instead of thinking that people of the same height may be the same size, we declare that they must be the same size.
The idea behind the BMI was conceived in 1832 by Belgian anthropologist and mathematician Adolphe Quetelet, but he didn’t intend for it to be a health measure. Instead, it was simply an observation of how people’s weight changed in proportion to height over their lifetime.
Fast-forward to the 20th century, when insurance companies began using weight as an indicator of health status. Weights were recorded in a “Life Table.” Individual health status was determined on the basis of arbitrary cut-offs for weight on the Life Tables. Furthermore, White men set the “normal” weight standards because they were the primary insurance holders.
In 1972, Dr. Ancel Keys, a physician and leading expert in body composition at the time, cried foul on this practice and sought to standardize the use of weight as a health indicator. Dr. Keys used Quetelet’s calculation and termed it the Body Mass Index.
By 1985, the U.S. National Institutes of Health and the World Health Organization adopted the BMI. By the 21st century, BMI had become widely used in clinical settings. For example, the Centers for Medicare & Medicaid Services adopted BMI as a quality-of-care measure, placing even more pressure on clinicians to use BMI as a health screening tool.
BMI as a tool to diagnose obesity
We can’t discuss BMI without discussing the disease of obesity. BMI is the most widely used tool to diagnose obesity. In the United States, one-third of Americans meet the criteria for obesity. Another one-third are at risk for obesity.
Compared with BMI’s relatively quick acceptance into clinical practice, however, obesity was only recently recognized as a disease.
Historically, obesity has been viewed as a lifestyle choice, fueled by misinformation and multiple forms of bias. The historical bias associated with BMI and discrimination has led some public health officials and scholars to dismiss the use of BMI or fail to recognize obesity as disease.
This is a dangerous conclusion, because it comes to the detriment of the very people disproportionately impacted by obesity-related health disparities.
Furthermore, weight bias continues to prevent people living with obesity from receiving insurance coverage for life-enhancing obesity medications and interventions.
Is it time to phase out BMI?
The BMI is intertwined with many forms of bias: age, gender, racial, ethnic, and even weight. Therefore, it is time to phase out BMI. However, phasing out BMI is complex and will take time, given that:
- Obesity is still a relatively “young” disease. 2023 marks the 10th anniversary of obesity’s recognition as a disease by the American Medical Association. Currently, BMI is the most widely used tool to diagnose obesity. Tools such as waist circumference, body composition, and metabolic health assessment will need to replace the BMI. Shifting from BMI emphasizes that obesity is more than a number on the scale. Obesity, as defined by the Obesity Medicine Association, is indeed a “chronic, relapsing, multi-factorial, neurobehavioral disease, wherein an increase in body fat promotes adipose tissue dysfunction and abnormal fat mass physical forces, resulting in adverse metabolic, biomechanical, and psychosocial health consequences.”
- Much of our health research is tied to BMI. There have been some shifts in looking at non–weight-related health indicators. However, we need more robust studies evaluating other health indicators beyond weight and BMI. The availability of this data will help eliminate the need for BMI and promote individualized health assessment.
- Current treatment guidelines for obesity medications are based on BMI. (Note: Medications to treat obesity are called “anti-obesity” medications or AOMs. However, given the stigma associated with obesity, I prefer not to use the term “anti-obesity.”) Presently this interferes with long-term obesity treatment. Once BMI is “normal,” many patients lose insurance coverage for their obesity medication, despite needing long-term metabolic support to overcome the compensatory mechanism of weight regain. Obesity is a chronic disease that exists independent of weight status. Therefore, using non-BMI measures will help ensure appropriate lifetime support for obesity.
The preceding are barriers, not impossibilities. In the interim, if BMI is still used in any capacity, the BMI reference chart should be an adjusted BMI chart based on age, race, ethnicity, biological sex, and obesity-related conditions. Furthermore, BMI isn’t the sole determining factor of health status.
Instead, an “abnormal” BMI should initiate conversation and further testing, if needed, to determine an individual’s health. For example, compare two people of the same height with different BMIs and lifestyles. Current studies support that a person flagged as having a high adjusted BMI but practicing a healthy lifestyle and having no metabolic diseases is less at risk than a person with a “normal” BMI but high waist circumference and an unhealthy lifestyle.
Regardless of your personal feelings, the facts are clear. Technology empowers us with better tools than BMI to determine health status. Therefore, it’s not a matter of if we will stop using BMI but when.
Sylvia Gonsahn-Bollie, MD, DipABOM, is an integrative obesity specialist who specializes in individualized solutions for emotional and biological overeating. Connect with her at www.embraceyouweightloss.com or on Instagram @embraceyoumd. Her bestselling book, “Embrace You: Your Guide to Transforming Weight Loss Misconceptions Into Lifelong Wellness,” is Healthline.com’s Best Overall Weight Loss Book 2022 and one of Livestrong.com’s picks for the 8 Best Weight-Loss Books to Read in 2022.
A version of this article first appeared on Medscape.com.
“BMI is trash. Full stop.” This controversial tweet received 26,500 likes and almost 3,000 retweets. The 400 comments from medical and non–health care personnel ranged from agreeable to contrary to offensive.
As a Black woman who is an obesity expert living with the impact of obesity in my own life, I know the emotion that a BMI conversation can evoke. Before emotions hijack the conversation, let’s discuss BMI’s past, present, and future.
BMI: From observational measurement to clinical use
Imagine walking into your favorite clothing store where an eager clerk greets you with a shirt to try on. The fit is off, but the clerk insists that the shirt must fit because everyone who’s your height should be able to wear it. This scenario seems ridiculous. But this is how we’ve come to use the BMI. Instead of thinking that people of the same height may be the same size, we declare that they must be the same size.
The idea behind the BMI was conceived in 1832 by Belgian anthropologist and mathematician Adolphe Quetelet, but he didn’t intend for it to be a health measure. Instead, it was simply an observation of how people’s weight changed in proportion to height over their lifetime.
Fast-forward to the 20th century, when insurance companies began using weight as an indicator of health status. Weights were recorded in a “Life Table.” Individual health status was determined on the basis of arbitrary cut-offs for weight on the Life Tables. Furthermore, White men set the “normal” weight standards because they were the primary insurance holders.
In 1972, Dr. Ancel Keys, a physician and leading expert in body composition at the time, cried foul on this practice and sought to standardize the use of weight as a health indicator. Dr. Keys used Quetelet’s calculation and termed it the Body Mass Index.
By 1985, the U.S. National Institutes of Health and the World Health Organization adopted the BMI. By the 21st century, BMI had become widely used in clinical settings. For example, the Centers for Medicare & Medicaid Services adopted BMI as a quality-of-care measure, placing even more pressure on clinicians to use BMI as a health screening tool.
BMI as a tool to diagnose obesity
We can’t discuss BMI without discussing the disease of obesity. BMI is the most widely used tool to diagnose obesity. In the United States, one-third of Americans meet the criteria for obesity. Another one-third are at risk for obesity.
Compared with BMI’s relatively quick acceptance into clinical practice, however, obesity was only recently recognized as a disease.
Historically, obesity has been viewed as a lifestyle choice, fueled by misinformation and multiple forms of bias. The historical bias associated with BMI and discrimination has led some public health officials and scholars to dismiss the use of BMI or fail to recognize obesity as disease.
This is a dangerous conclusion, because it comes to the detriment of the very people disproportionately impacted by obesity-related health disparities.
Furthermore, weight bias continues to prevent people living with obesity from receiving insurance coverage for life-enhancing obesity medications and interventions.
Is it time to phase out BMI?
The BMI is intertwined with many forms of bias: age, gender, racial, ethnic, and even weight. Therefore, it is time to phase out BMI. However, phasing out BMI is complex and will take time, given that:
- Obesity is still a relatively “young” disease. 2023 marks the 10th anniversary of obesity’s recognition as a disease by the American Medical Association. Currently, BMI is the most widely used tool to diagnose obesity. Tools such as waist circumference, body composition, and metabolic health assessment will need to replace the BMI. Shifting from BMI emphasizes that obesity is more than a number on the scale. Obesity, as defined by the Obesity Medicine Association, is indeed a “chronic, relapsing, multi-factorial, neurobehavioral disease, wherein an increase in body fat promotes adipose tissue dysfunction and abnormal fat mass physical forces, resulting in adverse metabolic, biomechanical, and psychosocial health consequences.”
- Much of our health research is tied to BMI. There have been some shifts in looking at non–weight-related health indicators. However, we need more robust studies evaluating other health indicators beyond weight and BMI. The availability of this data will help eliminate the need for BMI and promote individualized health assessment.
- Current treatment guidelines for obesity medications are based on BMI. (Note: Medications to treat obesity are called “anti-obesity” medications or AOMs. However, given the stigma associated with obesity, I prefer not to use the term “anti-obesity.”) Presently this interferes with long-term obesity treatment. Once BMI is “normal,” many patients lose insurance coverage for their obesity medication, despite needing long-term metabolic support to overcome the compensatory mechanism of weight regain. Obesity is a chronic disease that exists independent of weight status. Therefore, using non-BMI measures will help ensure appropriate lifetime support for obesity.
The preceding are barriers, not impossibilities. In the interim, if BMI is still used in any capacity, the BMI reference chart should be an adjusted BMI chart based on age, race, ethnicity, biological sex, and obesity-related conditions. Furthermore, BMI isn’t the sole determining factor of health status.
Instead, an “abnormal” BMI should initiate conversation and further testing, if needed, to determine an individual’s health. For example, compare two people of the same height with different BMIs and lifestyles. Current studies support that a person flagged as having a high adjusted BMI but practicing a healthy lifestyle and having no metabolic diseases is less at risk than a person with a “normal” BMI but high waist circumference and an unhealthy lifestyle.
Regardless of your personal feelings, the facts are clear. Technology empowers us with better tools than BMI to determine health status. Therefore, it’s not a matter of if we will stop using BMI but when.
Sylvia Gonsahn-Bollie, MD, DipABOM, is an integrative obesity specialist who specializes in individualized solutions for emotional and biological overeating. Connect with her at www.embraceyouweightloss.com or on Instagram @embraceyoumd. Her bestselling book, “Embrace You: Your Guide to Transforming Weight Loss Misconceptions Into Lifelong Wellness,” is Healthline.com’s Best Overall Weight Loss Book 2022 and one of Livestrong.com’s picks for the 8 Best Weight-Loss Books to Read in 2022.
A version of this article first appeared on Medscape.com.
“BMI is trash. Full stop.” This controversial tweet received 26,500 likes and almost 3,000 retweets. The 400 comments from medical and non–health care personnel ranged from agreeable to contrary to offensive.
As a Black woman who is an obesity expert living with the impact of obesity in my own life, I know the emotion that a BMI conversation can evoke. Before emotions hijack the conversation, let’s discuss BMI’s past, present, and future.
BMI: From observational measurement to clinical use
Imagine walking into your favorite clothing store where an eager clerk greets you with a shirt to try on. The fit is off, but the clerk insists that the shirt must fit because everyone who’s your height should be able to wear it. This scenario seems ridiculous. But this is how we’ve come to use the BMI. Instead of thinking that people of the same height may be the same size, we declare that they must be the same size.
The idea behind the BMI was conceived in 1832 by Belgian anthropologist and mathematician Adolphe Quetelet, but he didn’t intend for it to be a health measure. Instead, it was simply an observation of how people’s weight changed in proportion to height over their lifetime.
Fast-forward to the 20th century, when insurance companies began using weight as an indicator of health status. Weights were recorded in a “Life Table.” Individual health status was determined on the basis of arbitrary cut-offs for weight on the Life Tables. Furthermore, White men set the “normal” weight standards because they were the primary insurance holders.
In 1972, Dr. Ancel Keys, a physician and leading expert in body composition at the time, cried foul on this practice and sought to standardize the use of weight as a health indicator. Dr. Keys used Quetelet’s calculation and termed it the Body Mass Index.
By 1985, the U.S. National Institutes of Health and the World Health Organization adopted the BMI. By the 21st century, BMI had become widely used in clinical settings. For example, the Centers for Medicare & Medicaid Services adopted BMI as a quality-of-care measure, placing even more pressure on clinicians to use BMI as a health screening tool.
BMI as a tool to diagnose obesity
We can’t discuss BMI without discussing the disease of obesity. BMI is the most widely used tool to diagnose obesity. In the United States, one-third of Americans meet the criteria for obesity. Another one-third are at risk for obesity.
Compared with BMI’s relatively quick acceptance into clinical practice, however, obesity was only recently recognized as a disease.
Historically, obesity has been viewed as a lifestyle choice, fueled by misinformation and multiple forms of bias. The historical bias associated with BMI and discrimination has led some public health officials and scholars to dismiss the use of BMI or fail to recognize obesity as disease.
This is a dangerous conclusion, because it comes to the detriment of the very people disproportionately impacted by obesity-related health disparities.
Furthermore, weight bias continues to prevent people living with obesity from receiving insurance coverage for life-enhancing obesity medications and interventions.
Is it time to phase out BMI?
The BMI is intertwined with many forms of bias: age, gender, racial, ethnic, and even weight. Therefore, it is time to phase out BMI. However, phasing out BMI is complex and will take time, given that:
- Obesity is still a relatively “young” disease. 2023 marks the 10th anniversary of obesity’s recognition as a disease by the American Medical Association. Currently, BMI is the most widely used tool to diagnose obesity. Tools such as waist circumference, body composition, and metabolic health assessment will need to replace the BMI. Shifting from BMI emphasizes that obesity is more than a number on the scale. Obesity, as defined by the Obesity Medicine Association, is indeed a “chronic, relapsing, multi-factorial, neurobehavioral disease, wherein an increase in body fat promotes adipose tissue dysfunction and abnormal fat mass physical forces, resulting in adverse metabolic, biomechanical, and psychosocial health consequences.”
- Much of our health research is tied to BMI. There have been some shifts in looking at non–weight-related health indicators. However, we need more robust studies evaluating other health indicators beyond weight and BMI. The availability of this data will help eliminate the need for BMI and promote individualized health assessment.
- Current treatment guidelines for obesity medications are based on BMI. (Note: Medications to treat obesity are called “anti-obesity” medications or AOMs. However, given the stigma associated with obesity, I prefer not to use the term “anti-obesity.”) Presently this interferes with long-term obesity treatment. Once BMI is “normal,” many patients lose insurance coverage for their obesity medication, despite needing long-term metabolic support to overcome the compensatory mechanism of weight regain. Obesity is a chronic disease that exists independent of weight status. Therefore, using non-BMI measures will help ensure appropriate lifetime support for obesity.
The preceding are barriers, not impossibilities. In the interim, if BMI is still used in any capacity, the BMI reference chart should be an adjusted BMI chart based on age, race, ethnicity, biological sex, and obesity-related conditions. Furthermore, BMI isn’t the sole determining factor of health status.
Instead, an “abnormal” BMI should initiate conversation and further testing, if needed, to determine an individual’s health. For example, compare two people of the same height with different BMIs and lifestyles. Current studies support that a person flagged as having a high adjusted BMI but practicing a healthy lifestyle and having no metabolic diseases is less at risk than a person with a “normal” BMI but high waist circumference and an unhealthy lifestyle.
Regardless of your personal feelings, the facts are clear. Technology empowers us with better tools than BMI to determine health status. Therefore, it’s not a matter of if we will stop using BMI but when.
Sylvia Gonsahn-Bollie, MD, DipABOM, is an integrative obesity specialist who specializes in individualized solutions for emotional and biological overeating. Connect with her at www.embraceyouweightloss.com or on Instagram @embraceyoumd. Her bestselling book, “Embrace You: Your Guide to Transforming Weight Loss Misconceptions Into Lifelong Wellness,” is Healthline.com’s Best Overall Weight Loss Book 2022 and one of Livestrong.com’s picks for the 8 Best Weight-Loss Books to Read in 2022.
A version of this article first appeared on Medscape.com.