User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Capivasertib/fulvestrant improves progression free survival in breast cancer
SAN ANTONIO – For patients with hormone receptor-positive/HER2-negative (HR+/HER2–) breast cancers resistant to aromatase inhibitors, the combination of the investigational AKT inhibitor capivasertib with the selective estrogen receptor degrader fulvestrant (Faslodex) was associated with significant improvement in progression-free survival compared with fulvestrant alone in the CAPItelllo-291 study recently presented at the San Antonio Breast Cancer Symposium.
, reported Nicholas Turner, MD, PhD, of the Institute of Cancer Research and Royal Marsden NHS Foundation Trust in London.
“Capivasertib plus fulvestrant has the potential to be a future treatment option for patients with hormone receptor–positive advanced breast cancer who have progressed on an endocrine-based regimen,” he said.
AKT alterations
Many HR+/HER2– advanced breast cancers have activation of the AKT pathway through alteration in PIK3CA, AKT1, and PTEN, but this activation can also occur in the absence of genetic alterations. AKT signaling is also a mechanism of resistance to endocrine therapy, Dr. Turner said.
Capivasertib, a select inhibitor of the AKT isoforms 1, 2, and 3, was combined with fulvestrant in the phase 2 FAKTION trial. The combination was associated with significant improvements in both progression-free survival (PFS) and overall survival (OS) compared with fulvestrant plus placebo in CDK4/6-naive postmenopausal women with aromatase inhibitor–resistant HR+/HER2– advanced breast cancer. The clinical benefit in this trial was more pronounced among patients with tumors bearing AKT pathway alterations, he said.
In the phase 3 CAPItello study, Dr. Turner and colleagues enrolled men and both pre- and postmenopausal women with HR+/HER2– advanced breast cancer who experienced recurrence either during therapy with adjuvant aromatase inhibitor or within 12 months of the end of therapy, or who had disease progression while on prior aromatase inhibitor therapy for advanced breast cancer.
The patients could have no more than two prior lines of endocrine therapy and no more than one prior line of chemotherapy for advanced breast cancer, and no prior selective estrogen receptor degrader (SERD), mTOR inhibitor, PI3K inhibitor, or AKT inhibitor. Patients with hemoglobin A1c below 8% and with diabetes not requiring insulin were eligible for the study. After stratification for liver metastases, prior CDK4/6 inhibitor therapy, and geographic region, 708 patients were randomized to either capivasertib 400 mg twice daily 4 days on and 3 days off plus fulvestrant 500 mg on days 1 and 15 of cycle 1 and then every 4 weeks, or to fulvestrant in the same dose and schedule plus placebo.
Results
The dual primary endpoint was investigator assessed PFS in both the overall population and in those with AKT pathway alterations. The median PFS in the overall population was 7.2 months with the combination, compared with 3.6 months for fulvestrant alone, translating into an adjusted hazard ratio for progression of 0.60 (P < .001).
In the pathway-altered population, the median PFS was 7.3 months with capivasertib/fulvestrant vs. 3.1 months with fulvestrant placebo, which translated into an adjusted hazard ratio for progression on the combination of 0.50 (P < .001).
An exploratory analysis of PFS among patients either without pathway alterations or unknown AKT status showed median PFS of 7.2 months and 3.7 months, respectively, with a hazard ratio of 0.70.
An analysis of benefit by subgroups in the overall population showed that the balance tipped in favor of the combination in nearly all categories, including among patients with or without liver metastases and with or without prior CDK4/6 inhibitor use.
Among patients with measurable disease at baseline the combination was associated with objective response rates (ORR) of 22.9% in the overall population and 28.8% in the pathway-altered population. The respective ORR for fulvestrant/placebo were 12.2% and 9.7%.
Overall survival data were not mature at the time of data cutoff, but showed trends favoring capivasertib plus fulvestrant in both the overall and AKT-pathway-altered population.
There were four fatal adverse events in the combination arm (acute myocardial infarction, cerebral hemorrhage, pneumonia aspiration, and sepsis), and one in the fulvestrant alone arm (COVID-19).
The most common grade 3 or greater adverse events among patients treated with the combination were rash (12.1%), diarrhea (9.3 %), and hyperglycemia (2.3%). In all, 13% of patients randomized to capivasertib/fulvestrant discontinued therapy due to adverse events, compared with 2.3% of patients assigned to fulvestrant/placebo.
Dr. Turner said that the overall adverse event profile with the combination was manageable and consistent with data from previous studies.
‘Clinically relevant benefit’
Invited discussant Fabrice André, MD, PhD, of Gustave Roussy Cancer Center in Villejuif, France, noted that the CAPItello-291 study is one of the first randomized trials enriched with patients whose tumors are resistant to CDK4/6 inhibitors.
“What are the take-home messages? First, there is a clinically relevant benefit in the overall population and in the PIK3CA mutant/AKT/PTEN altered population,” he said.
He noted that the exploratory analysis showed a small clinical benefit with an impressive hazard ratio but broad confidence interval in patients with biomarker-negative tumors, and noted that the study lacked either circulating tumor DNA analysis or exploration of other mechanisms of AKT pathway alteration.
The study was funded by AstraZeneca. Dr. Turner has served on the advisory board for AstraZeneca, and his institution has received research funding from the company. Dr. Andre disclosed fees to his hospital on his behalf from AstraZeneca, Daiichi Sankyo, Sanofi, Pfizer, Lilly, and Roche.
SAN ANTONIO – For patients with hormone receptor-positive/HER2-negative (HR+/HER2–) breast cancers resistant to aromatase inhibitors, the combination of the investigational AKT inhibitor capivasertib with the selective estrogen receptor degrader fulvestrant (Faslodex) was associated with significant improvement in progression-free survival compared with fulvestrant alone in the CAPItelllo-291 study recently presented at the San Antonio Breast Cancer Symposium.
, reported Nicholas Turner, MD, PhD, of the Institute of Cancer Research and Royal Marsden NHS Foundation Trust in London.
“Capivasertib plus fulvestrant has the potential to be a future treatment option for patients with hormone receptor–positive advanced breast cancer who have progressed on an endocrine-based regimen,” he said.
AKT alterations
Many HR+/HER2– advanced breast cancers have activation of the AKT pathway through alteration in PIK3CA, AKT1, and PTEN, but this activation can also occur in the absence of genetic alterations. AKT signaling is also a mechanism of resistance to endocrine therapy, Dr. Turner said.
Capivasertib, a select inhibitor of the AKT isoforms 1, 2, and 3, was combined with fulvestrant in the phase 2 FAKTION trial. The combination was associated with significant improvements in both progression-free survival (PFS) and overall survival (OS) compared with fulvestrant plus placebo in CDK4/6-naive postmenopausal women with aromatase inhibitor–resistant HR+/HER2– advanced breast cancer. The clinical benefit in this trial was more pronounced among patients with tumors bearing AKT pathway alterations, he said.
In the phase 3 CAPItello study, Dr. Turner and colleagues enrolled men and both pre- and postmenopausal women with HR+/HER2– advanced breast cancer who experienced recurrence either during therapy with adjuvant aromatase inhibitor or within 12 months of the end of therapy, or who had disease progression while on prior aromatase inhibitor therapy for advanced breast cancer.
The patients could have no more than two prior lines of endocrine therapy and no more than one prior line of chemotherapy for advanced breast cancer, and no prior selective estrogen receptor degrader (SERD), mTOR inhibitor, PI3K inhibitor, or AKT inhibitor. Patients with hemoglobin A1c below 8% and with diabetes not requiring insulin were eligible for the study. After stratification for liver metastases, prior CDK4/6 inhibitor therapy, and geographic region, 708 patients were randomized to either capivasertib 400 mg twice daily 4 days on and 3 days off plus fulvestrant 500 mg on days 1 and 15 of cycle 1 and then every 4 weeks, or to fulvestrant in the same dose and schedule plus placebo.
Results
The dual primary endpoint was investigator assessed PFS in both the overall population and in those with AKT pathway alterations. The median PFS in the overall population was 7.2 months with the combination, compared with 3.6 months for fulvestrant alone, translating into an adjusted hazard ratio for progression of 0.60 (P < .001).
In the pathway-altered population, the median PFS was 7.3 months with capivasertib/fulvestrant vs. 3.1 months with fulvestrant placebo, which translated into an adjusted hazard ratio for progression on the combination of 0.50 (P < .001).
An exploratory analysis of PFS among patients either without pathway alterations or unknown AKT status showed median PFS of 7.2 months and 3.7 months, respectively, with a hazard ratio of 0.70.
An analysis of benefit by subgroups in the overall population showed that the balance tipped in favor of the combination in nearly all categories, including among patients with or without liver metastases and with or without prior CDK4/6 inhibitor use.
Among patients with measurable disease at baseline the combination was associated with objective response rates (ORR) of 22.9% in the overall population and 28.8% in the pathway-altered population. The respective ORR for fulvestrant/placebo were 12.2% and 9.7%.
Overall survival data were not mature at the time of data cutoff, but showed trends favoring capivasertib plus fulvestrant in both the overall and AKT-pathway-altered population.
There were four fatal adverse events in the combination arm (acute myocardial infarction, cerebral hemorrhage, pneumonia aspiration, and sepsis), and one in the fulvestrant alone arm (COVID-19).
The most common grade 3 or greater adverse events among patients treated with the combination were rash (12.1%), diarrhea (9.3 %), and hyperglycemia (2.3%). In all, 13% of patients randomized to capivasertib/fulvestrant discontinued therapy due to adverse events, compared with 2.3% of patients assigned to fulvestrant/placebo.
Dr. Turner said that the overall adverse event profile with the combination was manageable and consistent with data from previous studies.
‘Clinically relevant benefit’
Invited discussant Fabrice André, MD, PhD, of Gustave Roussy Cancer Center in Villejuif, France, noted that the CAPItello-291 study is one of the first randomized trials enriched with patients whose tumors are resistant to CDK4/6 inhibitors.
“What are the take-home messages? First, there is a clinically relevant benefit in the overall population and in the PIK3CA mutant/AKT/PTEN altered population,” he said.
He noted that the exploratory analysis showed a small clinical benefit with an impressive hazard ratio but broad confidence interval in patients with biomarker-negative tumors, and noted that the study lacked either circulating tumor DNA analysis or exploration of other mechanisms of AKT pathway alteration.
The study was funded by AstraZeneca. Dr. Turner has served on the advisory board for AstraZeneca, and his institution has received research funding from the company. Dr. Andre disclosed fees to his hospital on his behalf from AstraZeneca, Daiichi Sankyo, Sanofi, Pfizer, Lilly, and Roche.
SAN ANTONIO – For patients with hormone receptor-positive/HER2-negative (HR+/HER2–) breast cancers resistant to aromatase inhibitors, the combination of the investigational AKT inhibitor capivasertib with the selective estrogen receptor degrader fulvestrant (Faslodex) was associated with significant improvement in progression-free survival compared with fulvestrant alone in the CAPItelllo-291 study recently presented at the San Antonio Breast Cancer Symposium.
, reported Nicholas Turner, MD, PhD, of the Institute of Cancer Research and Royal Marsden NHS Foundation Trust in London.
“Capivasertib plus fulvestrant has the potential to be a future treatment option for patients with hormone receptor–positive advanced breast cancer who have progressed on an endocrine-based regimen,” he said.
AKT alterations
Many HR+/HER2– advanced breast cancers have activation of the AKT pathway through alteration in PIK3CA, AKT1, and PTEN, but this activation can also occur in the absence of genetic alterations. AKT signaling is also a mechanism of resistance to endocrine therapy, Dr. Turner said.
Capivasertib, a select inhibitor of the AKT isoforms 1, 2, and 3, was combined with fulvestrant in the phase 2 FAKTION trial. The combination was associated with significant improvements in both progression-free survival (PFS) and overall survival (OS) compared with fulvestrant plus placebo in CDK4/6-naive postmenopausal women with aromatase inhibitor–resistant HR+/HER2– advanced breast cancer. The clinical benefit in this trial was more pronounced among patients with tumors bearing AKT pathway alterations, he said.
In the phase 3 CAPItello study, Dr. Turner and colleagues enrolled men and both pre- and postmenopausal women with HR+/HER2– advanced breast cancer who experienced recurrence either during therapy with adjuvant aromatase inhibitor or within 12 months of the end of therapy, or who had disease progression while on prior aromatase inhibitor therapy for advanced breast cancer.
The patients could have no more than two prior lines of endocrine therapy and no more than one prior line of chemotherapy for advanced breast cancer, and no prior selective estrogen receptor degrader (SERD), mTOR inhibitor, PI3K inhibitor, or AKT inhibitor. Patients with hemoglobin A1c below 8% and with diabetes not requiring insulin were eligible for the study. After stratification for liver metastases, prior CDK4/6 inhibitor therapy, and geographic region, 708 patients were randomized to either capivasertib 400 mg twice daily 4 days on and 3 days off plus fulvestrant 500 mg on days 1 and 15 of cycle 1 and then every 4 weeks, or to fulvestrant in the same dose and schedule plus placebo.
Results
The dual primary endpoint was investigator assessed PFS in both the overall population and in those with AKT pathway alterations. The median PFS in the overall population was 7.2 months with the combination, compared with 3.6 months for fulvestrant alone, translating into an adjusted hazard ratio for progression of 0.60 (P < .001).
In the pathway-altered population, the median PFS was 7.3 months with capivasertib/fulvestrant vs. 3.1 months with fulvestrant placebo, which translated into an adjusted hazard ratio for progression on the combination of 0.50 (P < .001).
An exploratory analysis of PFS among patients either without pathway alterations or unknown AKT status showed median PFS of 7.2 months and 3.7 months, respectively, with a hazard ratio of 0.70.
An analysis of benefit by subgroups in the overall population showed that the balance tipped in favor of the combination in nearly all categories, including among patients with or without liver metastases and with or without prior CDK4/6 inhibitor use.
Among patients with measurable disease at baseline the combination was associated with objective response rates (ORR) of 22.9% in the overall population and 28.8% in the pathway-altered population. The respective ORR for fulvestrant/placebo were 12.2% and 9.7%.
Overall survival data were not mature at the time of data cutoff, but showed trends favoring capivasertib plus fulvestrant in both the overall and AKT-pathway-altered population.
There were four fatal adverse events in the combination arm (acute myocardial infarction, cerebral hemorrhage, pneumonia aspiration, and sepsis), and one in the fulvestrant alone arm (COVID-19).
The most common grade 3 or greater adverse events among patients treated with the combination were rash (12.1%), diarrhea (9.3 %), and hyperglycemia (2.3%). In all, 13% of patients randomized to capivasertib/fulvestrant discontinued therapy due to adverse events, compared with 2.3% of patients assigned to fulvestrant/placebo.
Dr. Turner said that the overall adverse event profile with the combination was manageable and consistent with data from previous studies.
‘Clinically relevant benefit’
Invited discussant Fabrice André, MD, PhD, of Gustave Roussy Cancer Center in Villejuif, France, noted that the CAPItello-291 study is one of the first randomized trials enriched with patients whose tumors are resistant to CDK4/6 inhibitors.
“What are the take-home messages? First, there is a clinically relevant benefit in the overall population and in the PIK3CA mutant/AKT/PTEN altered population,” he said.
He noted that the exploratory analysis showed a small clinical benefit with an impressive hazard ratio but broad confidence interval in patients with biomarker-negative tumors, and noted that the study lacked either circulating tumor DNA analysis or exploration of other mechanisms of AKT pathway alteration.
The study was funded by AstraZeneca. Dr. Turner has served on the advisory board for AstraZeneca, and his institution has received research funding from the company. Dr. Andre disclosed fees to his hospital on his behalf from AstraZeneca, Daiichi Sankyo, Sanofi, Pfizer, Lilly, and Roche.
AT SABCS 2022
Gene signature may spare some breast cancer patients from radiation
San Antonio – as well as those who can be safely spared from breast radiation following breast-conserving surgery, an international team of investigators said.
In combined data from three independent randomized trials grouped into a meta-analysis, patients who had low scores on the messenger RNA–based signature, dubbed “Profile for the Omission of Local Adjuvant Radiotherapy” (POLAR), derived only minimal benefit from radiotherapy following breast-conserving surgery. In contrast, patients with high POLAR scores had significant clinical benefit from adjuvant radiotherapy, reported Per Karlsson, MD, chief physician with the Sahlgrenska Comprehensive Cancer Center and the University of Gothenburg (Sweden). Dr. Karlsson reported his findings at the San Antonio Breast Cancer Symposium.
“To our knowledge, POLAR is the first genomic classifier that is not only prognostic but also predictive of radiotherapy benefit, showing a significant interaction between radiotherapy and the classifier,” he said. “These important retrospective findings warrant further investigation, including in contemporary clinical studies.”
Investigators with the Swedish SweBCG91RT trial (Swedish Breast Cancer Group 91 Radiotherapy), the Scottish Conservation (radiotherapy) Trial (SCT), and a trial from the Princess Margaret Cancer Hospital in Toronto, collaborated on improving and validating the POLAR signature, which was originally developed for use in the SweBCG91RT trial in patients with lymph node–negative breast cancer who underwent breast-conserving surgery. The patients were randomized to whole breast irradiation or no radiotherapy.
To develop the signature, researchers collected tumor blocks from 1,004 patients, and extracted RNA from the samples. Gene expression data were obtained from primary tumors of 764 patients. The subset of 597 patients with estrogen receptor–positive, HER2-negative tumors (ER+/HER2–) who did not receive systemic therapy were divided into a training set with 243 patients, and a validation cohort with 354 patients.
They identified a total of 16 genes involved in cellular proliferation and immune response, and then validated the signature using retrospective data from three clinical trials of patients randomized to radiotherapy or no radiation following breast-conserving surgery.
Of 623 patients with node-negative ER+/HER2– tumors who were included in the meta-analysis, 429 patients were found to have high POLAR scores. These patients benefited from adjuvant radiation therapy after breast-conserving surgery with a 10-year cumulative incidence of low risk of locoregional recurrence ranging from 15% to 26% for those who were not treated with radiation therapy, compared with only 4%-11% percent for those who received radiation therapy (hazard ratio, 0.37; P < .001).
In contrast, among the 194 patients whose tumors had POLAR low scores, there was no apparent benefit from radiation therapy with a nonsignificant HR of 0.92 (P = .832).
In Cox proportional hazard models for time to locoregional recurrences for 309 patients who did not undergo radiation, POLAR scores were significantly prognostic for recurrence, with a HR of 1.53 (P < .001) in univariable analysis, and 1.43 (P = .005) in multivariable analysis controlling for age, tumor size, tumor grade and molecular groupings.
New modalities may make findings less relevant
Alphonse Taghian, MD, PhD, a breast radiation oncologist with Mass General Cancer Center, Boston, who was not involved in the study, said there have been major changes in radiation therapy since the studies used for development of the POLAR signature were performed. For example, the Scottish Conservation Trial ran from 1985 to 1991, while the SweBCGR91RT trial and Princess Margaret trial were both conducted in the 1990s.
He noted that patients in those studies would likely experience more morbidities from radiation than patients treated with more recent modalities such as intensity modulated radiation therapy, and that patients treated 30 years ago would have to put up with lengthy fractionation schedules that required daily trips to the hospital over as long as 6 weeks, whereas a majority of patients can now be treated with hypofractionated radiation that can be performed in a much shorter time and with minimal comorbidities.
He acknowledged, however, that “it will help to have a signature proved, confirmed, or validated retrospectively with a different set of data.”
Dr. Taghian also said that it would be helpful to have more data about the age of patients, because omitting radiation is more common for elderly patients than it is for younger patients.
“It will maybe be beneficial to look at this signature in patients that we think might not need radiation,” he said.
The study was supported by the Swedish Cancer Society, Swedish Research Council, King Gustav 5 Jubilee Clinic Foundation, the ALF Agreement of the Swedish government, PFS Genomics, and Exact Sciences. Dr. Karlsson has pending patents with and receives royalties from Exact Sciences and PreludeDX. Dr. Taghian reported having no relevant disclosures.
San Antonio – as well as those who can be safely spared from breast radiation following breast-conserving surgery, an international team of investigators said.
In combined data from three independent randomized trials grouped into a meta-analysis, patients who had low scores on the messenger RNA–based signature, dubbed “Profile for the Omission of Local Adjuvant Radiotherapy” (POLAR), derived only minimal benefit from radiotherapy following breast-conserving surgery. In contrast, patients with high POLAR scores had significant clinical benefit from adjuvant radiotherapy, reported Per Karlsson, MD, chief physician with the Sahlgrenska Comprehensive Cancer Center and the University of Gothenburg (Sweden). Dr. Karlsson reported his findings at the San Antonio Breast Cancer Symposium.
“To our knowledge, POLAR is the first genomic classifier that is not only prognostic but also predictive of radiotherapy benefit, showing a significant interaction between radiotherapy and the classifier,” he said. “These important retrospective findings warrant further investigation, including in contemporary clinical studies.”
Investigators with the Swedish SweBCG91RT trial (Swedish Breast Cancer Group 91 Radiotherapy), the Scottish Conservation (radiotherapy) Trial (SCT), and a trial from the Princess Margaret Cancer Hospital in Toronto, collaborated on improving and validating the POLAR signature, which was originally developed for use in the SweBCG91RT trial in patients with lymph node–negative breast cancer who underwent breast-conserving surgery. The patients were randomized to whole breast irradiation or no radiotherapy.
To develop the signature, researchers collected tumor blocks from 1,004 patients, and extracted RNA from the samples. Gene expression data were obtained from primary tumors of 764 patients. The subset of 597 patients with estrogen receptor–positive, HER2-negative tumors (ER+/HER2–) who did not receive systemic therapy were divided into a training set with 243 patients, and a validation cohort with 354 patients.
They identified a total of 16 genes involved in cellular proliferation and immune response, and then validated the signature using retrospective data from three clinical trials of patients randomized to radiotherapy or no radiation following breast-conserving surgery.
Of 623 patients with node-negative ER+/HER2– tumors who were included in the meta-analysis, 429 patients were found to have high POLAR scores. These patients benefited from adjuvant radiation therapy after breast-conserving surgery with a 10-year cumulative incidence of low risk of locoregional recurrence ranging from 15% to 26% for those who were not treated with radiation therapy, compared with only 4%-11% percent for those who received radiation therapy (hazard ratio, 0.37; P < .001).
In contrast, among the 194 patients whose tumors had POLAR low scores, there was no apparent benefit from radiation therapy with a nonsignificant HR of 0.92 (P = .832).
In Cox proportional hazard models for time to locoregional recurrences for 309 patients who did not undergo radiation, POLAR scores were significantly prognostic for recurrence, with a HR of 1.53 (P < .001) in univariable analysis, and 1.43 (P = .005) in multivariable analysis controlling for age, tumor size, tumor grade and molecular groupings.
New modalities may make findings less relevant
Alphonse Taghian, MD, PhD, a breast radiation oncologist with Mass General Cancer Center, Boston, who was not involved in the study, said there have been major changes in radiation therapy since the studies used for development of the POLAR signature were performed. For example, the Scottish Conservation Trial ran from 1985 to 1991, while the SweBCGR91RT trial and Princess Margaret trial were both conducted in the 1990s.
He noted that patients in those studies would likely experience more morbidities from radiation than patients treated with more recent modalities such as intensity modulated radiation therapy, and that patients treated 30 years ago would have to put up with lengthy fractionation schedules that required daily trips to the hospital over as long as 6 weeks, whereas a majority of patients can now be treated with hypofractionated radiation that can be performed in a much shorter time and with minimal comorbidities.
He acknowledged, however, that “it will help to have a signature proved, confirmed, or validated retrospectively with a different set of data.”
Dr. Taghian also said that it would be helpful to have more data about the age of patients, because omitting radiation is more common for elderly patients than it is for younger patients.
“It will maybe be beneficial to look at this signature in patients that we think might not need radiation,” he said.
The study was supported by the Swedish Cancer Society, Swedish Research Council, King Gustav 5 Jubilee Clinic Foundation, the ALF Agreement of the Swedish government, PFS Genomics, and Exact Sciences. Dr. Karlsson has pending patents with and receives royalties from Exact Sciences and PreludeDX. Dr. Taghian reported having no relevant disclosures.
San Antonio – as well as those who can be safely spared from breast radiation following breast-conserving surgery, an international team of investigators said.
In combined data from three independent randomized trials grouped into a meta-analysis, patients who had low scores on the messenger RNA–based signature, dubbed “Profile for the Omission of Local Adjuvant Radiotherapy” (POLAR), derived only minimal benefit from radiotherapy following breast-conserving surgery. In contrast, patients with high POLAR scores had significant clinical benefit from adjuvant radiotherapy, reported Per Karlsson, MD, chief physician with the Sahlgrenska Comprehensive Cancer Center and the University of Gothenburg (Sweden). Dr. Karlsson reported his findings at the San Antonio Breast Cancer Symposium.
“To our knowledge, POLAR is the first genomic classifier that is not only prognostic but also predictive of radiotherapy benefit, showing a significant interaction between radiotherapy and the classifier,” he said. “These important retrospective findings warrant further investigation, including in contemporary clinical studies.”
Investigators with the Swedish SweBCG91RT trial (Swedish Breast Cancer Group 91 Radiotherapy), the Scottish Conservation (radiotherapy) Trial (SCT), and a trial from the Princess Margaret Cancer Hospital in Toronto, collaborated on improving and validating the POLAR signature, which was originally developed for use in the SweBCG91RT trial in patients with lymph node–negative breast cancer who underwent breast-conserving surgery. The patients were randomized to whole breast irradiation or no radiotherapy.
To develop the signature, researchers collected tumor blocks from 1,004 patients, and extracted RNA from the samples. Gene expression data were obtained from primary tumors of 764 patients. The subset of 597 patients with estrogen receptor–positive, HER2-negative tumors (ER+/HER2–) who did not receive systemic therapy were divided into a training set with 243 patients, and a validation cohort with 354 patients.
They identified a total of 16 genes involved in cellular proliferation and immune response, and then validated the signature using retrospective data from three clinical trials of patients randomized to radiotherapy or no radiation following breast-conserving surgery.
Of 623 patients with node-negative ER+/HER2– tumors who were included in the meta-analysis, 429 patients were found to have high POLAR scores. These patients benefited from adjuvant radiation therapy after breast-conserving surgery with a 10-year cumulative incidence of low risk of locoregional recurrence ranging from 15% to 26% for those who were not treated with radiation therapy, compared with only 4%-11% percent for those who received radiation therapy (hazard ratio, 0.37; P < .001).
In contrast, among the 194 patients whose tumors had POLAR low scores, there was no apparent benefit from radiation therapy with a nonsignificant HR of 0.92 (P = .832).
In Cox proportional hazard models for time to locoregional recurrences for 309 patients who did not undergo radiation, POLAR scores were significantly prognostic for recurrence, with a HR of 1.53 (P < .001) in univariable analysis, and 1.43 (P = .005) in multivariable analysis controlling for age, tumor size, tumor grade and molecular groupings.
New modalities may make findings less relevant
Alphonse Taghian, MD, PhD, a breast radiation oncologist with Mass General Cancer Center, Boston, who was not involved in the study, said there have been major changes in radiation therapy since the studies used for development of the POLAR signature were performed. For example, the Scottish Conservation Trial ran from 1985 to 1991, while the SweBCGR91RT trial and Princess Margaret trial were both conducted in the 1990s.
He noted that patients in those studies would likely experience more morbidities from radiation than patients treated with more recent modalities such as intensity modulated radiation therapy, and that patients treated 30 years ago would have to put up with lengthy fractionation schedules that required daily trips to the hospital over as long as 6 weeks, whereas a majority of patients can now be treated with hypofractionated radiation that can be performed in a much shorter time and with minimal comorbidities.
He acknowledged, however, that “it will help to have a signature proved, confirmed, or validated retrospectively with a different set of data.”
Dr. Taghian also said that it would be helpful to have more data about the age of patients, because omitting radiation is more common for elderly patients than it is for younger patients.
“It will maybe be beneficial to look at this signature in patients that we think might not need radiation,” he said.
The study was supported by the Swedish Cancer Society, Swedish Research Council, King Gustav 5 Jubilee Clinic Foundation, the ALF Agreement of the Swedish government, PFS Genomics, and Exact Sciences. Dr. Karlsson has pending patents with and receives royalties from Exact Sciences and PreludeDX. Dr. Taghian reported having no relevant disclosures.
AT SABCS 2022
Endometrial receptivity testing before IVF seen as unnecessary
Endometrial receptivity testing (ERT) did not increase the chances of achieving live birth in patients undergoing in vitro fertilization.
The study was promoted by widespread use of the tests in reproductive medicine and earlier conflicting studies regarding its effectiveness. The procedure, in which doctors extract cells from a woman’s endometrial lining in an effort to determine the best day to perform in vitro fertilization, requires a biopsy, can take a month to generate results, and costs up to $1,000.
But the new study, published online December 6 in the Journal of the American Medical Association, found that embryo transfer based on the timing of ERT was no better than that based on the standard protocol.
“Endometrial receptivity testing ended up not being beneficial in the population of interest, a good-prognosis IVF patient population,” said Nicole Doyle, MD, PhD, of Shady Grove Fertility, in Arlington, Va., who led the study. “For this particular patient population I would not recommend ERT based on the results of the trial.”
“Unfortunately, as is our history in reproductive medicine, we may embrace technology prematurely given patient desperation and physician eagerness to improve pregnancy outcomes,” said Mark P. Trolice, MD, director of the IVF Center in Orlando, who was not involved in the new research.
The double-blind, randomized clinical trial enrolled 726 women treated at Dr. Doyle’s clinic between May 2018 and September 2020.
All the women underwent ERT. Of those who received adjusted progesterone exposure after the test, live birth occurred in 58.5% of transfers (223 of 381). Among those in a control group who did not have their progesterone adjusted after ERT and underwent IVF on a standardized schedule, 61.9% of transfers (239 of 386) resulted in live birth, according to the researchers.
The differences in rates of clinical (77.2% vs. 79.5% [95% confidence interval, −10.4% to 2.4%]) and biochemical pregnancy (68.8% vs. 72.8% [95% CI, −8.2% to 3.5%]) were not statistically significant between the two groups, Dr. Doyle and her colleagues reported.
Women who experienced recurrent implantation failure (RIF), defined as more than two failed embryo transfers, were excluded from the study. “We can’t assess the benefit of an endometrial receptivity testing in this particular patient population,” Dr. Doyle said.
However, she noted that the number of women who undergo RIF is “a very small fraction of all IVF patients, less than 5%.” Of those, half are expected to have embryos that are not suitable for implantation, Dr. Doyle said.
As a result, she said, “it’s really only about 2.5% of IVF patients for which we don’t yet have an answer regarding the utility of ERT.”
Dr. Trolice, also a professor at the University of Central Florida, Orlando, expressed certainty that the “one-size-fits-all approach” for ERT has been disproven by the study’s failure to find a benefit from the procedure in women with a “good prognosis.” But, he added, whether ERT is of value in a subset of patients, such as those with recurrent implantation failure, remains “a question of vital importance.”
Dr. Doyle and Dr. Trolice reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Endometrial receptivity testing (ERT) did not increase the chances of achieving live birth in patients undergoing in vitro fertilization.
The study was promoted by widespread use of the tests in reproductive medicine and earlier conflicting studies regarding its effectiveness. The procedure, in which doctors extract cells from a woman’s endometrial lining in an effort to determine the best day to perform in vitro fertilization, requires a biopsy, can take a month to generate results, and costs up to $1,000.
But the new study, published online December 6 in the Journal of the American Medical Association, found that embryo transfer based on the timing of ERT was no better than that based on the standard protocol.
“Endometrial receptivity testing ended up not being beneficial in the population of interest, a good-prognosis IVF patient population,” said Nicole Doyle, MD, PhD, of Shady Grove Fertility, in Arlington, Va., who led the study. “For this particular patient population I would not recommend ERT based on the results of the trial.”
“Unfortunately, as is our history in reproductive medicine, we may embrace technology prematurely given patient desperation and physician eagerness to improve pregnancy outcomes,” said Mark P. Trolice, MD, director of the IVF Center in Orlando, who was not involved in the new research.
The double-blind, randomized clinical trial enrolled 726 women treated at Dr. Doyle’s clinic between May 2018 and September 2020.
All the women underwent ERT. Of those who received adjusted progesterone exposure after the test, live birth occurred in 58.5% of transfers (223 of 381). Among those in a control group who did not have their progesterone adjusted after ERT and underwent IVF on a standardized schedule, 61.9% of transfers (239 of 386) resulted in live birth, according to the researchers.
The differences in rates of clinical (77.2% vs. 79.5% [95% confidence interval, −10.4% to 2.4%]) and biochemical pregnancy (68.8% vs. 72.8% [95% CI, −8.2% to 3.5%]) were not statistically significant between the two groups, Dr. Doyle and her colleagues reported.
Women who experienced recurrent implantation failure (RIF), defined as more than two failed embryo transfers, were excluded from the study. “We can’t assess the benefit of an endometrial receptivity testing in this particular patient population,” Dr. Doyle said.
However, she noted that the number of women who undergo RIF is “a very small fraction of all IVF patients, less than 5%.” Of those, half are expected to have embryos that are not suitable for implantation, Dr. Doyle said.
As a result, she said, “it’s really only about 2.5% of IVF patients for which we don’t yet have an answer regarding the utility of ERT.”
Dr. Trolice, also a professor at the University of Central Florida, Orlando, expressed certainty that the “one-size-fits-all approach” for ERT has been disproven by the study’s failure to find a benefit from the procedure in women with a “good prognosis.” But, he added, whether ERT is of value in a subset of patients, such as those with recurrent implantation failure, remains “a question of vital importance.”
Dr. Doyle and Dr. Trolice reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Endometrial receptivity testing (ERT) did not increase the chances of achieving live birth in patients undergoing in vitro fertilization.
The study was promoted by widespread use of the tests in reproductive medicine and earlier conflicting studies regarding its effectiveness. The procedure, in which doctors extract cells from a woman’s endometrial lining in an effort to determine the best day to perform in vitro fertilization, requires a biopsy, can take a month to generate results, and costs up to $1,000.
But the new study, published online December 6 in the Journal of the American Medical Association, found that embryo transfer based on the timing of ERT was no better than that based on the standard protocol.
“Endometrial receptivity testing ended up not being beneficial in the population of interest, a good-prognosis IVF patient population,” said Nicole Doyle, MD, PhD, of Shady Grove Fertility, in Arlington, Va., who led the study. “For this particular patient population I would not recommend ERT based on the results of the trial.”
“Unfortunately, as is our history in reproductive medicine, we may embrace technology prematurely given patient desperation and physician eagerness to improve pregnancy outcomes,” said Mark P. Trolice, MD, director of the IVF Center in Orlando, who was not involved in the new research.
The double-blind, randomized clinical trial enrolled 726 women treated at Dr. Doyle’s clinic between May 2018 and September 2020.
All the women underwent ERT. Of those who received adjusted progesterone exposure after the test, live birth occurred in 58.5% of transfers (223 of 381). Among those in a control group who did not have their progesterone adjusted after ERT and underwent IVF on a standardized schedule, 61.9% of transfers (239 of 386) resulted in live birth, according to the researchers.
The differences in rates of clinical (77.2% vs. 79.5% [95% confidence interval, −10.4% to 2.4%]) and biochemical pregnancy (68.8% vs. 72.8% [95% CI, −8.2% to 3.5%]) were not statistically significant between the two groups, Dr. Doyle and her colleagues reported.
Women who experienced recurrent implantation failure (RIF), defined as more than two failed embryo transfers, were excluded from the study. “We can’t assess the benefit of an endometrial receptivity testing in this particular patient population,” Dr. Doyle said.
However, she noted that the number of women who undergo RIF is “a very small fraction of all IVF patients, less than 5%.” Of those, half are expected to have embryos that are not suitable for implantation, Dr. Doyle said.
As a result, she said, “it’s really only about 2.5% of IVF patients for which we don’t yet have an answer regarding the utility of ERT.”
Dr. Trolice, also a professor at the University of Central Florida, Orlando, expressed certainty that the “one-size-fits-all approach” for ERT has been disproven by the study’s failure to find a benefit from the procedure in women with a “good prognosis.” But, he added, whether ERT is of value in a subset of patients, such as those with recurrent implantation failure, remains “a question of vital importance.”
Dr. Doyle and Dr. Trolice reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
How to advocate in a post-Roe world, no matter your zip code
For many, the recent Supreme Court decision in the Dobbs v Jackson case that removed the constitutional right to an abortion has introduced outrage, fear, and confusion throughout the country. While the American College of Obstetricians and Gynecologists (ACOG) clearly has established that abortion is essential health care and has published resources regarding the issue (www.acog.org/advocacy/abortion-is-essential), and many providers know what to do medically, they do not know what they can do legally. In a country where 45% of pregnancies are unplanned and 25% of women will access abortion services in their lifetime, this decision will completely change the landscape of providing and receiving abortion care. This decision will affect every provider and their patients and will affect them differently in each state. The country likely will be divided into 24 destination states that will protect the right to abortion and another 26 states that have or will soon ban abortion or severely restrict access to it.
Regardless of the state you practice in, it is clear that our voices, actions, and advocacy are essential during these challenging times. It can feel difficult to find ways to advocate, especially if you are in a state or have an employer that supports anti-abortion legislation or has been silent after the Dobbs decision was released. We have created a guide to help and encourage all ObGyn providers to find ways to advocate, no matter their zip code.
1. Donate
Many of our patients will need to travel out of state to seek abortion care. The cost of abortion care can be expensive, and travel, child care, and time off of work add to the costs of the procedure itself, making access to abortion care financially out of reach for some. There are many well-established abortion funds throughout the country; consider donating to one of them or organizing a fundraiser in your community. Go to abortionfunds.org/funds to find an abortion fund that will support patients in your community, or donate generally to support them all.
2. Save your stories
We already are hearing the devastating impact abortion bans have on patient care around the country. If you had to deny or delay care because of the new legal landscape surrounding abortion, write down or record the experience. Your stories can be critical in discussing the impact of legislation. If you choose to share on social media, ask the involved patients if they are comfortable with their story being shared online (as long as their identity is protected).
3. Talk about it
Talking about abortion is a critical step in destigmatizing it and supporting our patients as well as our field. These conversations can be challenging, but ACOG has provided an important guide that includes key phrases and statements to help shape the conversation and avoid polarizing language (https://www.acog.org/advocacy/abortion-is-essential/come-prepared). This guide also can be helpful to keep in mind when talking to members of the media.
Continue to: 4. Write about it...
4. Write about it
There are many opportunities to write about the impact of the Dobbs decision, especially locally. As a clinician and trusted member of the community, you can uniquely share your and your patients’ experiences. Your article does not have to appear in a major publication; you can still have an important impact in your local paper. See resources on how to write an op-ed and letter to the editor (https://www.acog.org/advocacy/abortion-is-essential/connect-in-your-community/legislative-rx-op-eds-and-letters-to-the-editor).
5. Teach about it
These legislative changes uniquely impact our ObGyn residents; 44% of residents likely will be in a training program in a state that will ban or severely restrict abortion access. Abortion is health care, and a vast majority of our residents could graduate without important skills to save lives. As we strategize to ensure all ObGyn residents are able to receive this important training, work on incorporating an advocacy curriculum into your residents’ educational experience. Teaching about how to advocate is an important skill for supporting our patients and ensuring critical health policy. ACOG has published guides focused on education and training (www.acog.org/advocacy/abortion-is-essential/education-and-training). We also have included our own medical center’s advocacy curriculum (https://docs.google.com/document/d/1STxLzE0j55mlDEbF0_wZbo9O QryAcs6RpfZ47Mwfs4I/edit).
6. Get involved and seek out allies
It’s important that ObGyns be at the table for all discussions surrounding abortion care and reproductive health. Join hospital committees and help influence policy within your own institution. Refer back to those abortion talking points—this will help in some of these challenging conversations.
7. Get on social media
Using social media can be a powerful tool for advocacy. You can help elevate issues and encourage others to get active as well. Using a common hashtag, such as #AbortionisHealthcare, on different platforms can help connect you to other advocates. Share simple and important graphics provided by ACOG on important topics in our field (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state/social-media) and review ACOG’s recommendation for professionalism in social media (https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2019/10/professional-use-of-digital-and-social-media).
8. Get active locally
We have seen the introduction of hundreds of bills in states around the country not only on abortion but also on other legislation that directly impacts the care we provide. It is critical that we get involved in advocating for important reproductive health legislation and against bills that cause harm and interfere with the doctor-patient relationship. Stay up to date on legislative issues with your local ACOG and medical chapters (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state). Consider testifying at your State house, providing written or oral testimony. Connect with ACOG or your state medical chapter to help with talking points!
9. Read up
There have been many new policies at the federal level that could impact the care you provide. Take some time to read up on these new changes. Patients also may ask you about self-managed abortion. There are guides and resources (https://www.acog.org/advocacy/abortion-is-essential/practice-management) for patients that may seek medication online, and we want to ensure that patients have the resources to make informed decisions.
10. Hit the Capitol
Consider making time to come to the annual Congressional Leadership Conference in Washington, DC (https://www.acog.org/education-and-events/meetings/acog-congressional-leadership-conference), or other advocacy events offered through the American Medical Association or other subspecialty organizations. When we all come together as an organization, a field, and a community, it sends a powerful message that we are standing up together for our patients and our colleagues.
Make a difference
There is no advocacy too big or too small. It is critical that we continue to use our voices and our platforms to stand up for health care and access to critical services, including abortion care. ●
For many, the recent Supreme Court decision in the Dobbs v Jackson case that removed the constitutional right to an abortion has introduced outrage, fear, and confusion throughout the country. While the American College of Obstetricians and Gynecologists (ACOG) clearly has established that abortion is essential health care and has published resources regarding the issue (www.acog.org/advocacy/abortion-is-essential), and many providers know what to do medically, they do not know what they can do legally. In a country where 45% of pregnancies are unplanned and 25% of women will access abortion services in their lifetime, this decision will completely change the landscape of providing and receiving abortion care. This decision will affect every provider and their patients and will affect them differently in each state. The country likely will be divided into 24 destination states that will protect the right to abortion and another 26 states that have or will soon ban abortion or severely restrict access to it.
Regardless of the state you practice in, it is clear that our voices, actions, and advocacy are essential during these challenging times. It can feel difficult to find ways to advocate, especially if you are in a state or have an employer that supports anti-abortion legislation or has been silent after the Dobbs decision was released. We have created a guide to help and encourage all ObGyn providers to find ways to advocate, no matter their zip code.
1. Donate
Many of our patients will need to travel out of state to seek abortion care. The cost of abortion care can be expensive, and travel, child care, and time off of work add to the costs of the procedure itself, making access to abortion care financially out of reach for some. There are many well-established abortion funds throughout the country; consider donating to one of them or organizing a fundraiser in your community. Go to abortionfunds.org/funds to find an abortion fund that will support patients in your community, or donate generally to support them all.
2. Save your stories
We already are hearing the devastating impact abortion bans have on patient care around the country. If you had to deny or delay care because of the new legal landscape surrounding abortion, write down or record the experience. Your stories can be critical in discussing the impact of legislation. If you choose to share on social media, ask the involved patients if they are comfortable with their story being shared online (as long as their identity is protected).
3. Talk about it
Talking about abortion is a critical step in destigmatizing it and supporting our patients as well as our field. These conversations can be challenging, but ACOG has provided an important guide that includes key phrases and statements to help shape the conversation and avoid polarizing language (https://www.acog.org/advocacy/abortion-is-essential/come-prepared). This guide also can be helpful to keep in mind when talking to members of the media.
Continue to: 4. Write about it...
4. Write about it
There are many opportunities to write about the impact of the Dobbs decision, especially locally. As a clinician and trusted member of the community, you can uniquely share your and your patients’ experiences. Your article does not have to appear in a major publication; you can still have an important impact in your local paper. See resources on how to write an op-ed and letter to the editor (https://www.acog.org/advocacy/abortion-is-essential/connect-in-your-community/legislative-rx-op-eds-and-letters-to-the-editor).
5. Teach about it
These legislative changes uniquely impact our ObGyn residents; 44% of residents likely will be in a training program in a state that will ban or severely restrict abortion access. Abortion is health care, and a vast majority of our residents could graduate without important skills to save lives. As we strategize to ensure all ObGyn residents are able to receive this important training, work on incorporating an advocacy curriculum into your residents’ educational experience. Teaching about how to advocate is an important skill for supporting our patients and ensuring critical health policy. ACOG has published guides focused on education and training (www.acog.org/advocacy/abortion-is-essential/education-and-training). We also have included our own medical center’s advocacy curriculum (https://docs.google.com/document/d/1STxLzE0j55mlDEbF0_wZbo9O QryAcs6RpfZ47Mwfs4I/edit).
6. Get involved and seek out allies
It’s important that ObGyns be at the table for all discussions surrounding abortion care and reproductive health. Join hospital committees and help influence policy within your own institution. Refer back to those abortion talking points—this will help in some of these challenging conversations.
7. Get on social media
Using social media can be a powerful tool for advocacy. You can help elevate issues and encourage others to get active as well. Using a common hashtag, such as #AbortionisHealthcare, on different platforms can help connect you to other advocates. Share simple and important graphics provided by ACOG on important topics in our field (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state/social-media) and review ACOG’s recommendation for professionalism in social media (https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2019/10/professional-use-of-digital-and-social-media).
8. Get active locally
We have seen the introduction of hundreds of bills in states around the country not only on abortion but also on other legislation that directly impacts the care we provide. It is critical that we get involved in advocating for important reproductive health legislation and against bills that cause harm and interfere with the doctor-patient relationship. Stay up to date on legislative issues with your local ACOG and medical chapters (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state). Consider testifying at your State house, providing written or oral testimony. Connect with ACOG or your state medical chapter to help with talking points!
9. Read up
There have been many new policies at the federal level that could impact the care you provide. Take some time to read up on these new changes. Patients also may ask you about self-managed abortion. There are guides and resources (https://www.acog.org/advocacy/abortion-is-essential/practice-management) for patients that may seek medication online, and we want to ensure that patients have the resources to make informed decisions.
10. Hit the Capitol
Consider making time to come to the annual Congressional Leadership Conference in Washington, DC (https://www.acog.org/education-and-events/meetings/acog-congressional-leadership-conference), or other advocacy events offered through the American Medical Association or other subspecialty organizations. When we all come together as an organization, a field, and a community, it sends a powerful message that we are standing up together for our patients and our colleagues.
Make a difference
There is no advocacy too big or too small. It is critical that we continue to use our voices and our platforms to stand up for health care and access to critical services, including abortion care. ●
For many, the recent Supreme Court decision in the Dobbs v Jackson case that removed the constitutional right to an abortion has introduced outrage, fear, and confusion throughout the country. While the American College of Obstetricians and Gynecologists (ACOG) clearly has established that abortion is essential health care and has published resources regarding the issue (www.acog.org/advocacy/abortion-is-essential), and many providers know what to do medically, they do not know what they can do legally. In a country where 45% of pregnancies are unplanned and 25% of women will access abortion services in their lifetime, this decision will completely change the landscape of providing and receiving abortion care. This decision will affect every provider and their patients and will affect them differently in each state. The country likely will be divided into 24 destination states that will protect the right to abortion and another 26 states that have or will soon ban abortion or severely restrict access to it.
Regardless of the state you practice in, it is clear that our voices, actions, and advocacy are essential during these challenging times. It can feel difficult to find ways to advocate, especially if you are in a state or have an employer that supports anti-abortion legislation or has been silent after the Dobbs decision was released. We have created a guide to help and encourage all ObGyn providers to find ways to advocate, no matter their zip code.
1. Donate
Many of our patients will need to travel out of state to seek abortion care. The cost of abortion care can be expensive, and travel, child care, and time off of work add to the costs of the procedure itself, making access to abortion care financially out of reach for some. There are many well-established abortion funds throughout the country; consider donating to one of them or organizing a fundraiser in your community. Go to abortionfunds.org/funds to find an abortion fund that will support patients in your community, or donate generally to support them all.
2. Save your stories
We already are hearing the devastating impact abortion bans have on patient care around the country. If you had to deny or delay care because of the new legal landscape surrounding abortion, write down or record the experience. Your stories can be critical in discussing the impact of legislation. If you choose to share on social media, ask the involved patients if they are comfortable with their story being shared online (as long as their identity is protected).
3. Talk about it
Talking about abortion is a critical step in destigmatizing it and supporting our patients as well as our field. These conversations can be challenging, but ACOG has provided an important guide that includes key phrases and statements to help shape the conversation and avoid polarizing language (https://www.acog.org/advocacy/abortion-is-essential/come-prepared). This guide also can be helpful to keep in mind when talking to members of the media.
Continue to: 4. Write about it...
4. Write about it
There are many opportunities to write about the impact of the Dobbs decision, especially locally. As a clinician and trusted member of the community, you can uniquely share your and your patients’ experiences. Your article does not have to appear in a major publication; you can still have an important impact in your local paper. See resources on how to write an op-ed and letter to the editor (https://www.acog.org/advocacy/abortion-is-essential/connect-in-your-community/legislative-rx-op-eds-and-letters-to-the-editor).
5. Teach about it
These legislative changes uniquely impact our ObGyn residents; 44% of residents likely will be in a training program in a state that will ban or severely restrict abortion access. Abortion is health care, and a vast majority of our residents could graduate without important skills to save lives. As we strategize to ensure all ObGyn residents are able to receive this important training, work on incorporating an advocacy curriculum into your residents’ educational experience. Teaching about how to advocate is an important skill for supporting our patients and ensuring critical health policy. ACOG has published guides focused on education and training (www.acog.org/advocacy/abortion-is-essential/education-and-training). We also have included our own medical center’s advocacy curriculum (https://docs.google.com/document/d/1STxLzE0j55mlDEbF0_wZbo9O QryAcs6RpfZ47Mwfs4I/edit).
6. Get involved and seek out allies
It’s important that ObGyns be at the table for all discussions surrounding abortion care and reproductive health. Join hospital committees and help influence policy within your own institution. Refer back to those abortion talking points—this will help in some of these challenging conversations.
7. Get on social media
Using social media can be a powerful tool for advocacy. You can help elevate issues and encourage others to get active as well. Using a common hashtag, such as #AbortionisHealthcare, on different platforms can help connect you to other advocates. Share simple and important graphics provided by ACOG on important topics in our field (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state/social-media) and review ACOG’s recommendation for professionalism in social media (https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2019/10/professional-use-of-digital-and-social-media).
8. Get active locally
We have seen the introduction of hundreds of bills in states around the country not only on abortion but also on other legislation that directly impacts the care we provide. It is critical that we get involved in advocating for important reproductive health legislation and against bills that cause harm and interfere with the doctor-patient relationship. Stay up to date on legislative issues with your local ACOG and medical chapters (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state). Consider testifying at your State house, providing written or oral testimony. Connect with ACOG or your state medical chapter to help with talking points!
9. Read up
There have been many new policies at the federal level that could impact the care you provide. Take some time to read up on these new changes. Patients also may ask you about self-managed abortion. There are guides and resources (https://www.acog.org/advocacy/abortion-is-essential/practice-management) for patients that may seek medication online, and we want to ensure that patients have the resources to make informed decisions.
10. Hit the Capitol
Consider making time to come to the annual Congressional Leadership Conference in Washington, DC (https://www.acog.org/education-and-events/meetings/acog-congressional-leadership-conference), or other advocacy events offered through the American Medical Association or other subspecialty organizations. When we all come together as an organization, a field, and a community, it sends a powerful message that we are standing up together for our patients and our colleagues.
Make a difference
There is no advocacy too big or too small. It is critical that we continue to use our voices and our platforms to stand up for health care and access to critical services, including abortion care. ●
Simplify your approach to the diagnosis and treatment of PCOS
PCOS is a common problem, with a prevalence of 6% to 10% among women of reproductive age.1 Patients with PCOS often present with hirsutism, acne, female androgenetic alopecia, oligomenorrhea (also known as infrequent menstrual bleeding), amenorrhea, infertility, overweight, or obesity. In addition, many patients with PCOS have insulin resistance, dyslipidemia, metabolic syndrome, and an increased risk for developing type 2 diabetes mellitus (DM).2 A simplified approach to the diagnosis of PCOS will save health care resources by reducing the use of low-value diagnostic tests. A simplified approach to the treatment of PCOS will support patient medication adherence and improve health outcomes.
Simplify the diagnosis of PCOS
Simplify PCOS diagnosis by focusing on the core criteria of hyperandrogenism and oligo-ovulation. There are 3 major approaches to diagnosis:
- the 1990 National Institutes of Health (NIH) criteria3
- the 2003 Rotterdam criteria4,5
- the 2008 Androgen Excess and PCOS Society (AES) criteria.6
Using the 1990 NIH approach, the diagnosis of PCOS is made by the presence of 2 core criteria: hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea. In addition, other causes of hyperandrogenism should be excluded, including nonclassical adrenal hyperplasia (NCAH) due to 21-hydroxylase deficiency.3 Using the 1990 NIH criteria, PCOS can be diagnosed based on history (oligomenorrhea) and physical examination (assessment of the severity of hirsutism), but laboratory tests including total testosterone are often ordered.7
The Rotterdam approach to the diagnosis added a third criteria, the detection by ultrasonography of a multifollicular ovary and/or increased ovarian volume.4,5 Using the Rotterdam approach, PCOS is diagnosed in the presence of any 2 of the following 3 criteria: hyperandrogenism, oligo-ovulation, or ultrasound imaging showing the presence of a multifollicular ovary, identified by ≥ 12 antral follicles (2 to 9 mm in diameter) in each ovary or increased ovarian volume (> 10 mL).4,5
The Rotterdam approach using ovarian ultrasound as a criterion to diagnose PCOS is rife with serious problems, including:
- The number of small antral follicles in the normal ovary is age dependent, and many ovulatory and nonhirsute patients have ≥ 12 small antral follicles in each ovary.8,9
- There is no consensus on the number of small antral follicles needed to diagnose a multifollicular ovary, with recommendations to use thresholds of 124,5 or 20 follicles10 as the diagnostic cut-off.
- Accurate counting of the number of small ovarian follicles requires transvaginal ultrasound, which is not appropriate for many young adolescent patients.
- The process of counting ovarian follicles is operator-dependent.
- The high cost of ultrasound assessment of ovarian follicles (≥ $500 per examination).
The Rotterdam approach supports the diagnosis of PCOS in a patient with oligo-ovulation plus an ultrasound showing a multifollicular ovary in the absence of any clinical or laboratory evidence of hyperandrogenism.3,4,5 This approach to the diagnosis of PCOS is rejected by both the 1990 NIH3 and AES6 recommendations, which require the presence of hyperandrogenism as the sine qua non in the diagnosis of PCOS. I recommend against diagnosing PCOS in a non-hyperandrogenic patient with oligo-ovulation and a multifollicular ovary because other diagnoses are also possible, such as functional hypothalamic oligo-ovulation, especially in young patients. The Rotterdam approach also supports the diagnosis of PCOS in a patient with hyperandrogenism, an ultrasound showing a multifollicular ovary, and normal ovulation and menses.3,4 For most patients with normal, regular ovulation and menses, the testosterone concentration is normal and the only evidence of hyperandrogenism is hirsutism. Patients with normal, regular ovulation and menses plus hirsutism usually have idiopathic hirsutism. Idiopathic hirsutism is a problem caused by excessive 5-alpha-reductase activity in the hair pilosebaceous unit, which catalyzes the conversion of weak androgens into dihydrotestosterone, a potent intracellular androgen that stimulates terminal hair growth.11 In my opinion, the Rotterdam approach to diagnosing PCOS has created unnecessary confusion and complexity for both clinicians and patients. I believe we should simplify the diagnosis of PCOS and return to the 1990 NIH criteria.3
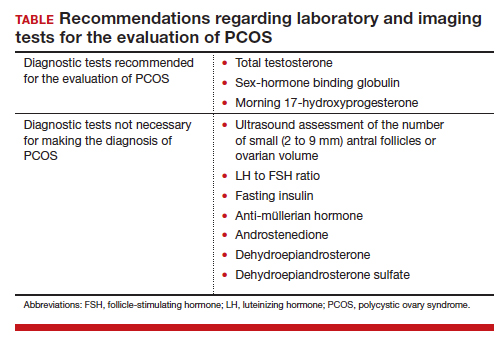
On occasion, a patient presents for a consultation and has already had an ovarian ultrasound to assess for a multifollicular ovary. I carefully read the report and, if a multifollicular ovary has been identified, I consider it as a secondary supporting finding of PCOS in my clinical assessment. But I do not base my diagnosis on the ultrasound finding. Patients often present with other laboratory tests that are secondary supporting findings of PCOS, which I carefully consider but do not use to make a diagnosis of PCOS. Secondary supporting laboratory findings consistent with PCOS include: 1) a markedly elevated anti-müllerian hormone (AMH) level,12 2) an elevated fasting insulin level,2,13 and 3) an elevated luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio.13,14 But it is not necessary to measure AMH, fasting insulin, LH, and FSH levels. To conserve health care resources, I recommend against measuring those analytes to diagnose PCOS.
Continue to: Simplify the core laboratory tests...
Simplify the core laboratory tests
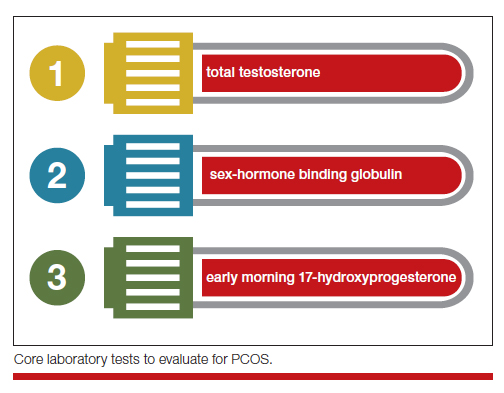
Simplify the testing used to support the diagnosis of PCOS by measuring total testosterone, sex-hormone binding globulin (SHBG) and early morning 17-hydroxyprogesterone (17-OH Prog).
The core criteria for diagnosis of PCOS are hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea or amenorrhea. Hyperandrogenism can be clinically diagnosed by assessing for the presence of hirsutism.7 Elevated levels of total testosterone, free testosterone, androstenedione, and/or dehydroepiandrosterone sulfate (DHEAS) suggest the presence of hyperandrogenism. In clinical practice, the laboratory approach to the diagnosis of hyperandrogenism can be simplified to the measurement of total testosterone, SHBG, and 17-OH Prog. By measuring total testosterone and SHBG, an estimate of free testosterone can be made. If the total testosterone is elevated, it is highly likely that the free testosterone is elevated. If the SHBG is abnormally low and the total testosterone level is in the upper limit of the normal range, the free testosterone is likely to be elevated.15 Using this approach, either an elevated total testosterone or an abnormally low SHBG indicate elevated free testosterone. For patients with hyperandrogenism and oligo-ovulation, an early morning (8 to 9 AM) 17-OH Prog level ≤ 2 ng/mL rules out the presence of NCAH due to a 21-hydroxylase deficiency.16 In my practice, the core laboratory tests I order when considering the diagnosis of PCOS are a total testosterone, SHBG, and 17-OH Prog.
Additional laboratory tests may be warranted to assess the patient diagnosed with PCOS. For example, if the patient has amenorrhea due to anovulation, tests for prolactin, FSH, and thyroid-stimulating hormone levels are warranted to assess for the presence of a prolactinoma, primary ovarian insufficiency, or thyroid disease, respectively. If the patient has a body mass index (BMI) ≥ 25 kg/m2, a hemoglobin A1c concentration is warranted to assess for the presence of prediabetes or DM.2 Many patients with PCOS have dyslipidemia, manifested through low high-density lipoprotein cholesterol levels and elevated low-density lipoprotein cholesterol levels, and a lipid panel assessment may be indicated. Among patients with PCOS, the most common lipid abnormality is a low high-density lipoprotein cholesterol level.17
Simplify the treatment of PCOS
Simplify treatment by counseling about lifestyle changes and prescribing an estrogen-progestin contraceptive, spironolactone, and metformin.
Most patients with PCOS have dysfunction in reproductive, metabolic, and dermatologic systems. For patients who are overweight or obese, lifestyle changes, including diet and exercise, that result in a 5% to 10% decrease in weight can improve metabolic balance, reduce circulating androgens, and increase menstrual frequency.18 For patients with PCOS and weight issues, referral to nutrition counseling or a full-service weight loss program can be very beneficial. In addition to lifestyle changes, patients with PCOS benefit from treatment with estrogen-progestin medications, spironolactone, and metformin.
Combination estrogen-progestin medications will lower LH secretion, decrease ovarian androgen production, increase SHBG production, decrease free testosterone levels and, if given cyclically, cause regular withdrawal bleeding.19 Spironolactone is an antiandrogen, which blocks the intracellular action of dihydrotestosterone and improves hirsutism and acne. Spironolactone also modestly decreases circulating levels of testosterone and DHEAS.20 For patients with metabolic problems, including insulin resistance and obesity, weight loss and/or treatment with metformin can help improve metabolic balance, which may result in restoration of ovulatory menses.21,22 Metformin can be effective in restoring ovulatory menses in both obese and lean patients with PCOS.22 The most common dermatologic problem caused by PCOS are hirsutism and acne. Both combination estrogen-progestin medications and spironolactone are effective treatments for hirsutism and acne.23
Estrogen-progestin hormones, spironolactone, and metformin are low-cost medications for the treatment of PCOS. Additional high-cost options for treatment of PCOS in obese patients include bariatric surgery and glucagon-like peptide (GLP-1) agonist medications (liraglutide and exenatide). For patients with PCOS and a body mass index (BMI) ≥ 35 kg/m2, bariatric surgery often results in sufficient weight loss to resolve the patient’s hyperandrogenism and oligo-ovulation, restoring spontaneous ovulatory cycles.24 In a study of more than 1,000 patients with: PCOS; mean BMI, 44 kg/m2; mean age, 31 years who were followed post-bariatric surgery for 5 years, > 90% of patients reported reductions in hirsutism and resumption of regular menses.25 For patients with PCOS seeking fertility, bariatric surgery often results in spontaneous pregnancy and live birth.26 GLP-1 agonists, including liraglutide or exenatide with or without metformin are effective in reducing weight, decreasing androgen levels, and restoring ovulatory menses.27,28
In my practice, I often prescribe 2 or 3 core medications for a patient with PCOS: 1) combination estrogen-progestin used cyclically or continuously, 2) spironolactone, and 3) metformin.19 Any estrogen-progestin contraceptive will suppress LH and ovarian androgen production; however, in the treatment of patients with PCOS, I prefer to use an estrogen-progestin combination that does not contain the androgenic progestin levonorgestrel.29 For the treatment of PCOS, I prefer to use an estrogen-progestin contraceptive with a non-androgenic progestin such as drospirenone, desogestrel, or gestodene. I routinely prescribe spironolactone at a dose of 100 mg, once daily, a dose near the top of the dose-response curve. A daily dose ≤ 50 mg of spironolactone is subtherapeutic for the treatment of hirsutism. A daily dose of 200 mg of spironolactone may cause bothersome breakthrough bleeding. When prescribing metformin, I usually recommend the extended-release formulation, at a dose of 750 mg with dinner. If well tolerated, I will increase the dose to 1,500 mg with dinner. Most of my patients with PCOS are taking a combination of 2 medications, either an estrogen-progestin contraceptive plus spironolactone or an estrogen-progestin contraceptive plus metformin.19 Some of my patients are taking all 3 medications. All 3 medications are very low cost.
For patients with PCOS and anovulatory infertility, letrozole treatment often results in ovulatory cycles and pregnancy with live birth. In obese PCOS patients, compared with clomiphene, letrozole results in superior live birth rates.30 Unlike clomiphene, letrozole is not approved by the US Food and Drug Administration for the treatment of anovulatory infertility.
The diagnosis of PCOS is often delayed due to confusion about how to make the diagnosis.31 To simplify the diagnosis of PCOS and improve patient encounters for PCOS, I focus on 2 core criteria: hyperandrogenism and oligo-ovulation. I recommend against ordering ultrasound imaging to assess for the presence of a multifollicular ovary. To simplify the treatment of PCOS I frequently prescribe an estrogen-progestin contraceptive, spironolactone, and metformin. By simplifying the diagnosis and treatment of PCOS, ObGyns will reduce patient confusion, improve outcomes, and save health care resources. ●
PCOS and adolescent patients
It is difficult to diagnose polycystic ovary syndrome (PCOS) in adolescents because oligo-ovulation is a common physiological feature of adolescence. Based on consensus among experts, PCOS should not be diagnosed within the first 2 years following menarche because the prevalence of oligo-ovulation is common at this stage of pubertal development. Two years after menarche, if an adolescent has a cycle length that is routinely > 45 days, it is likely that the pattern will persist, suggesting the presence of oligo-ovulation. Hyperandrogenism can be diagnosed based on the presence of moderate to severe hirsutism and/or an elevated testosterone or abnormally low sex-hormone binding globulin (SHBG) concentration. Two years after menarche the presence of oligo-ovulation and hyperandrogenism establishes the diagnosis of PCOS.1
PCOS and thrombophilia or migraine with aura
For patients with PCOS and a Factor V Leiden allele, where an estrogen-progestin contraceptive is contraindicated because of an increased risk of a venous thrombus, I prescribe spironolactone plus a levonorgestrel-intrauterine device. A low-dose oral progestin also may be considered because it will modestly suppress LH and ovarian androgen production. Similarly for patients with migraine with aura, where an estrogen-progestin contraceptive is contraindicated because of an increased of stroke, spironolactone plus a levonorgesterel intrauterine device may be effective in the treatment of hirsutism.
Androgen secreting tumors
Occasionally during the evaluation of a patient with hyperandrogenism and oligo-ovulation, measurement of total testosterone levels will reveal a value > 1.5 ng/mL. Most patients with PCOS have a total testosterone level ≤ 1.5 ng/mL. A total testosterone concentration > 1.5 ng/mL may be caused by ovarian stromal hyperthecosis or an androgen-producing tumor.2
Strongly-held patient perspectives on PCOS
At the first consultation visit, some patients are fearful and not receptive to a diagnosis of PCOS. If a clinician senses that the patient is not prepared to hear that they have PCOS, the clinician can be supportive of the patient’s perspective and focus on the patient’s chief health concerns, which may include abnormal cycle length, hirsutism, and/or overweight or obesity. During follow-up visits, as the patient builds trust with the clinician, the patient will be better prepared to discuss the diagnosis of PCOS. At the first consultation visit, some patients present with a strong belief that they have PCOS but have seen clinicians who conclude that they do not have PCOS. The diagnosis of PCOS is confusing because of competing diagnostic frameworks (NIH, Rotterdam, and AES). I avoid engaging in an argument with a patient who strongly believes that they have PCOS. In these situations, I focus on identifying the patient’s chief health concerns and discussing interventions to support their health goals.
References
1. Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol. 2020;33:445-447.
2. Meczekalski B, Szeliga A, Maciejewska-Jeske M, et al. Hyperthecosis: an underestimated nontumorous cause of hyperandrogenism. Gynecol Endocrinol. 2021;37:677-682.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome. Current Issues in Endocrinology and Metabolism. Dunaif A, Givens JR, Haseltine FP, Merriam GE (eds.). Blackwell Scientific Inc. Boston, Massachusetts; 1992:377.
- Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reprod. 2004;19:41-47.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Hatch R, Rosenfield RS, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-830.
- Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-Rotterdam: a common age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95:4965-4972.
- Alsamarai S, Adams JM, Murphy MK, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961-4970.
- Teede HJ, Misso ML, Costello MF, et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364-379.
- Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic hirsutism. Fertil Steril. 1985;43:74-78.
- Pigny P, Jonard S, Robert Y, et al. Serum anti-Müllerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941-945.
- Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812-841.
- Kumar N, Agarwal H. Early clinical, biochemical and radiologic features in obese and non-obese young women with polycystic ovarian syndrome: a comparative study. Horm Metab Res. 2022;54:620-624.
- Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95-109.
- Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180:R127-145.
- Guo F, Gong Z, Fernando T, et al. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: a retrospective study in Chinese population. Front Endocrinol. 2022;13:892125.
- Dietz de Loos ALP, Jiskoot G, Timman R, et al. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod Biomed Online. 2021;43:298-309.
- Ezeh U, Huang A, Landay M, et al. Long-term response of hirsutism and other hyperandrogenic symptoms to combination therapy in polycystic ovary syndrome. J Women’s Health. 2018;27:892-902.
- Ashraf Ganie M, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Pasquali R, Gambineri A, Cavazza C, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:53-60.
- Yang PK, Hsu CY, Chen MJ, et al. The efficacy of 24-month metformin for improving menses, hormones and metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:890-899.
- Garg V, Choi J, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355.
- Hu L, Ma L, Ying T, et al. Efficacy of bariatric surgery in the treatment of women with obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107:e3217-3229.
- Bhandari M, Kosta S, Bhandari M, et al. Effects of bariatric surgery on people with obesity and polycystic ovary syndrome: a large single center study from India. Obes Surg. 2022;32:3305-3312.
- Benito E, Gomez-Martin JM, Vega-Pinero B, et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J Clin Endocrinol Metab. 2020;105:e3384-3391.
- Xing C, Li C, He B. Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J Clin Endocrinol Metab. 2020;105:2950-2963.
- Elkind-Hirsch KE, Chappell N, Shaler D, et al. Liraglutide 3 mg on weight, body composition and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil Steril. 2022;118:371-381.
- Amiri M, Nahidi F, Bidhendi-Yarandi R, et al. A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: a crossover randomized controlled trial. Hum Reprod. 2020;35:175-186.
- Legro RS, Brzyski RG, Diamond NP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119-129.
- Gibson-Helm M, Teede H, Dunaif A, et al. Delayed diagnosis and lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:604-612.
PCOS is a common problem, with a prevalence of 6% to 10% among women of reproductive age.1 Patients with PCOS often present with hirsutism, acne, female androgenetic alopecia, oligomenorrhea (also known as infrequent menstrual bleeding), amenorrhea, infertility, overweight, or obesity. In addition, many patients with PCOS have insulin resistance, dyslipidemia, metabolic syndrome, and an increased risk for developing type 2 diabetes mellitus (DM).2 A simplified approach to the diagnosis of PCOS will save health care resources by reducing the use of low-value diagnostic tests. A simplified approach to the treatment of PCOS will support patient medication adherence and improve health outcomes.
Simplify the diagnosis of PCOS
Simplify PCOS diagnosis by focusing on the core criteria of hyperandrogenism and oligo-ovulation. There are 3 major approaches to diagnosis:
- the 1990 National Institutes of Health (NIH) criteria3
- the 2003 Rotterdam criteria4,5
- the 2008 Androgen Excess and PCOS Society (AES) criteria.6
Using the 1990 NIH approach, the diagnosis of PCOS is made by the presence of 2 core criteria: hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea. In addition, other causes of hyperandrogenism should be excluded, including nonclassical adrenal hyperplasia (NCAH) due to 21-hydroxylase deficiency.3 Using the 1990 NIH criteria, PCOS can be diagnosed based on history (oligomenorrhea) and physical examination (assessment of the severity of hirsutism), but laboratory tests including total testosterone are often ordered.7
The Rotterdam approach to the diagnosis added a third criteria, the detection by ultrasonography of a multifollicular ovary and/or increased ovarian volume.4,5 Using the Rotterdam approach, PCOS is diagnosed in the presence of any 2 of the following 3 criteria: hyperandrogenism, oligo-ovulation, or ultrasound imaging showing the presence of a multifollicular ovary, identified by ≥ 12 antral follicles (2 to 9 mm in diameter) in each ovary or increased ovarian volume (> 10 mL).4,5
The Rotterdam approach using ovarian ultrasound as a criterion to diagnose PCOS is rife with serious problems, including:
- The number of small antral follicles in the normal ovary is age dependent, and many ovulatory and nonhirsute patients have ≥ 12 small antral follicles in each ovary.8,9
- There is no consensus on the number of small antral follicles needed to diagnose a multifollicular ovary, with recommendations to use thresholds of 124,5 or 20 follicles10 as the diagnostic cut-off.
- Accurate counting of the number of small ovarian follicles requires transvaginal ultrasound, which is not appropriate for many young adolescent patients.
- The process of counting ovarian follicles is operator-dependent.
- The high cost of ultrasound assessment of ovarian follicles (≥ $500 per examination).

The Rotterdam approach supports the diagnosis of PCOS in a patient with oligo-ovulation plus an ultrasound showing a multifollicular ovary in the absence of any clinical or laboratory evidence of hyperandrogenism.3,4,5 This approach to the diagnosis of PCOS is rejected by both the 1990 NIH3 and AES6 recommendations, which require the presence of hyperandrogenism as the sine qua non in the diagnosis of PCOS. I recommend against diagnosing PCOS in a non-hyperandrogenic patient with oligo-ovulation and a multifollicular ovary because other diagnoses are also possible, such as functional hypothalamic oligo-ovulation, especially in young patients. The Rotterdam approach also supports the diagnosis of PCOS in a patient with hyperandrogenism, an ultrasound showing a multifollicular ovary, and normal ovulation and menses.3,4 For most patients with normal, regular ovulation and menses, the testosterone concentration is normal and the only evidence of hyperandrogenism is hirsutism. Patients with normal, regular ovulation and menses plus hirsutism usually have idiopathic hirsutism. Idiopathic hirsutism is a problem caused by excessive 5-alpha-reductase activity in the hair pilosebaceous unit, which catalyzes the conversion of weak androgens into dihydrotestosterone, a potent intracellular androgen that stimulates terminal hair growth.11 In my opinion, the Rotterdam approach to diagnosing PCOS has created unnecessary confusion and complexity for both clinicians and patients. I believe we should simplify the diagnosis of PCOS and return to the 1990 NIH criteria.3
On occasion, a patient presents for a consultation and has already had an ovarian ultrasound to assess for a multifollicular ovary. I carefully read the report and, if a multifollicular ovary has been identified, I consider it as a secondary supporting finding of PCOS in my clinical assessment. But I do not base my diagnosis on the ultrasound finding. Patients often present with other laboratory tests that are secondary supporting findings of PCOS, which I carefully consider but do not use to make a diagnosis of PCOS. Secondary supporting laboratory findings consistent with PCOS include: 1) a markedly elevated anti-müllerian hormone (AMH) level,12 2) an elevated fasting insulin level,2,13 and 3) an elevated luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio.13,14 But it is not necessary to measure AMH, fasting insulin, LH, and FSH levels. To conserve health care resources, I recommend against measuring those analytes to diagnose PCOS.

Continue to: Simplify the core laboratory tests...
Simplify the core laboratory tests
Simplify the testing used to support the diagnosis of PCOS by measuring total testosterone, sex-hormone binding globulin (SHBG) and early morning 17-hydroxyprogesterone (17-OH Prog).
The core criteria for diagnosis of PCOS are hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea or amenorrhea. Hyperandrogenism can be clinically diagnosed by assessing for the presence of hirsutism.7 Elevated levels of total testosterone, free testosterone, androstenedione, and/or dehydroepiandrosterone sulfate (DHEAS) suggest the presence of hyperandrogenism. In clinical practice, the laboratory approach to the diagnosis of hyperandrogenism can be simplified to the measurement of total testosterone, SHBG, and 17-OH Prog. By measuring total testosterone and SHBG, an estimate of free testosterone can be made. If the total testosterone is elevated, it is highly likely that the free testosterone is elevated. If the SHBG is abnormally low and the total testosterone level is in the upper limit of the normal range, the free testosterone is likely to be elevated.15 Using this approach, either an elevated total testosterone or an abnormally low SHBG indicate elevated free testosterone. For patients with hyperandrogenism and oligo-ovulation, an early morning (8 to 9 AM) 17-OH Prog level ≤ 2 ng/mL rules out the presence of NCAH due to a 21-hydroxylase deficiency.16 In my practice, the core laboratory tests I order when considering the diagnosis of PCOS are a total testosterone, SHBG, and 17-OH Prog.
Additional laboratory tests may be warranted to assess the patient diagnosed with PCOS. For example, if the patient has amenorrhea due to anovulation, tests for prolactin, FSH, and thyroid-stimulating hormone levels are warranted to assess for the presence of a prolactinoma, primary ovarian insufficiency, or thyroid disease, respectively. If the patient has a body mass index (BMI) ≥ 25 kg/m2, a hemoglobin A1c concentration is warranted to assess for the presence of prediabetes or DM.2 Many patients with PCOS have dyslipidemia, manifested through low high-density lipoprotein cholesterol levels and elevated low-density lipoprotein cholesterol levels, and a lipid panel assessment may be indicated. Among patients with PCOS, the most common lipid abnormality is a low high-density lipoprotein cholesterol level.17
Simplify the treatment of PCOS
Simplify treatment by counseling about lifestyle changes and prescribing an estrogen-progestin contraceptive, spironolactone, and metformin.
Most patients with PCOS have dysfunction in reproductive, metabolic, and dermatologic systems. For patients who are overweight or obese, lifestyle changes, including diet and exercise, that result in a 5% to 10% decrease in weight can improve metabolic balance, reduce circulating androgens, and increase menstrual frequency.18 For patients with PCOS and weight issues, referral to nutrition counseling or a full-service weight loss program can be very beneficial. In addition to lifestyle changes, patients with PCOS benefit from treatment with estrogen-progestin medications, spironolactone, and metformin.
Combination estrogen-progestin medications will lower LH secretion, decrease ovarian androgen production, increase SHBG production, decrease free testosterone levels and, if given cyclically, cause regular withdrawal bleeding.19 Spironolactone is an antiandrogen, which blocks the intracellular action of dihydrotestosterone and improves hirsutism and acne. Spironolactone also modestly decreases circulating levels of testosterone and DHEAS.20 For patients with metabolic problems, including insulin resistance and obesity, weight loss and/or treatment with metformin can help improve metabolic balance, which may result in restoration of ovulatory menses.21,22 Metformin can be effective in restoring ovulatory menses in both obese and lean patients with PCOS.22 The most common dermatologic problem caused by PCOS are hirsutism and acne. Both combination estrogen-progestin medications and spironolactone are effective treatments for hirsutism and acne.23
Estrogen-progestin hormones, spironolactone, and metformin are low-cost medications for the treatment of PCOS. Additional high-cost options for treatment of PCOS in obese patients include bariatric surgery and glucagon-like peptide (GLP-1) agonist medications (liraglutide and exenatide). For patients with PCOS and a body mass index (BMI) ≥ 35 kg/m2, bariatric surgery often results in sufficient weight loss to resolve the patient’s hyperandrogenism and oligo-ovulation, restoring spontaneous ovulatory cycles.24 In a study of more than 1,000 patients with: PCOS; mean BMI, 44 kg/m2; mean age, 31 years who were followed post-bariatric surgery for 5 years, > 90% of patients reported reductions in hirsutism and resumption of regular menses.25 For patients with PCOS seeking fertility, bariatric surgery often results in spontaneous pregnancy and live birth.26 GLP-1 agonists, including liraglutide or exenatide with or without metformin are effective in reducing weight, decreasing androgen levels, and restoring ovulatory menses.27,28
In my practice, I often prescribe 2 or 3 core medications for a patient with PCOS: 1) combination estrogen-progestin used cyclically or continuously, 2) spironolactone, and 3) metformin.19 Any estrogen-progestin contraceptive will suppress LH and ovarian androgen production; however, in the treatment of patients with PCOS, I prefer to use an estrogen-progestin combination that does not contain the androgenic progestin levonorgestrel.29 For the treatment of PCOS, I prefer to use an estrogen-progestin contraceptive with a non-androgenic progestin such as drospirenone, desogestrel, or gestodene. I routinely prescribe spironolactone at a dose of 100 mg, once daily, a dose near the top of the dose-response curve. A daily dose ≤ 50 mg of spironolactone is subtherapeutic for the treatment of hirsutism. A daily dose of 200 mg of spironolactone may cause bothersome breakthrough bleeding. When prescribing metformin, I usually recommend the extended-release formulation, at a dose of 750 mg with dinner. If well tolerated, I will increase the dose to 1,500 mg with dinner. Most of my patients with PCOS are taking a combination of 2 medications, either an estrogen-progestin contraceptive plus spironolactone or an estrogen-progestin contraceptive plus metformin.19 Some of my patients are taking all 3 medications. All 3 medications are very low cost.
For patients with PCOS and anovulatory infertility, letrozole treatment often results in ovulatory cycles and pregnancy with live birth. In obese PCOS patients, compared with clomiphene, letrozole results in superior live birth rates.30 Unlike clomiphene, letrozole is not approved by the US Food and Drug Administration for the treatment of anovulatory infertility.
The diagnosis of PCOS is often delayed due to confusion about how to make the diagnosis.31 To simplify the diagnosis of PCOS and improve patient encounters for PCOS, I focus on 2 core criteria: hyperandrogenism and oligo-ovulation. I recommend against ordering ultrasound imaging to assess for the presence of a multifollicular ovary. To simplify the treatment of PCOS I frequently prescribe an estrogen-progestin contraceptive, spironolactone, and metformin. By simplifying the diagnosis and treatment of PCOS, ObGyns will reduce patient confusion, improve outcomes, and save health care resources. ●
PCOS and adolescent patients
It is difficult to diagnose polycystic ovary syndrome (PCOS) in adolescents because oligo-ovulation is a common physiological feature of adolescence. Based on consensus among experts, PCOS should not be diagnosed within the first 2 years following menarche because the prevalence of oligo-ovulation is common at this stage of pubertal development. Two years after menarche, if an adolescent has a cycle length that is routinely > 45 days, it is likely that the pattern will persist, suggesting the presence of oligo-ovulation. Hyperandrogenism can be diagnosed based on the presence of moderate to severe hirsutism and/or an elevated testosterone or abnormally low sex-hormone binding globulin (SHBG) concentration. Two years after menarche the presence of oligo-ovulation and hyperandrogenism establishes the diagnosis of PCOS.1
PCOS and thrombophilia or migraine with aura
For patients with PCOS and a Factor V Leiden allele, where an estrogen-progestin contraceptive is contraindicated because of an increased risk of a venous thrombus, I prescribe spironolactone plus a levonorgestrel-intrauterine device. A low-dose oral progestin also may be considered because it will modestly suppress LH and ovarian androgen production. Similarly for patients with migraine with aura, where an estrogen-progestin contraceptive is contraindicated because of an increased of stroke, spironolactone plus a levonorgesterel intrauterine device may be effective in the treatment of hirsutism.
Androgen secreting tumors
Occasionally during the evaluation of a patient with hyperandrogenism and oligo-ovulation, measurement of total testosterone levels will reveal a value > 1.5 ng/mL. Most patients with PCOS have a total testosterone level ≤ 1.5 ng/mL. A total testosterone concentration > 1.5 ng/mL may be caused by ovarian stromal hyperthecosis or an androgen-producing tumor.2
Strongly-held patient perspectives on PCOS
At the first consultation visit, some patients are fearful and not receptive to a diagnosis of PCOS. If a clinician senses that the patient is not prepared to hear that they have PCOS, the clinician can be supportive of the patient’s perspective and focus on the patient’s chief health concerns, which may include abnormal cycle length, hirsutism, and/or overweight or obesity. During follow-up visits, as the patient builds trust with the clinician, the patient will be better prepared to discuss the diagnosis of PCOS. At the first consultation visit, some patients present with a strong belief that they have PCOS but have seen clinicians who conclude that they do not have PCOS. The diagnosis of PCOS is confusing because of competing diagnostic frameworks (NIH, Rotterdam, and AES). I avoid engaging in an argument with a patient who strongly believes that they have PCOS. In these situations, I focus on identifying the patient’s chief health concerns and discussing interventions to support their health goals.
References
1. Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol. 2020;33:445-447.
2. Meczekalski B, Szeliga A, Maciejewska-Jeske M, et al. Hyperthecosis: an underestimated nontumorous cause of hyperandrogenism. Gynecol Endocrinol. 2021;37:677-682.
PCOS is a common problem, with a prevalence of 6% to 10% among women of reproductive age.1 Patients with PCOS often present with hirsutism, acne, female androgenetic alopecia, oligomenorrhea (also known as infrequent menstrual bleeding), amenorrhea, infertility, overweight, or obesity. In addition, many patients with PCOS have insulin resistance, dyslipidemia, metabolic syndrome, and an increased risk for developing type 2 diabetes mellitus (DM).2 A simplified approach to the diagnosis of PCOS will save health care resources by reducing the use of low-value diagnostic tests. A simplified approach to the treatment of PCOS will support patient medication adherence and improve health outcomes.
Simplify the diagnosis of PCOS
Simplify PCOS diagnosis by focusing on the core criteria of hyperandrogenism and oligo-ovulation. There are 3 major approaches to diagnosis:
- the 1990 National Institutes of Health (NIH) criteria3
- the 2003 Rotterdam criteria4,5
- the 2008 Androgen Excess and PCOS Society (AES) criteria.6
Using the 1990 NIH approach, the diagnosis of PCOS is made by the presence of 2 core criteria: hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea. In addition, other causes of hyperandrogenism should be excluded, including nonclassical adrenal hyperplasia (NCAH) due to 21-hydroxylase deficiency.3 Using the 1990 NIH criteria, PCOS can be diagnosed based on history (oligomenorrhea) and physical examination (assessment of the severity of hirsutism), but laboratory tests including total testosterone are often ordered.7
The Rotterdam approach to the diagnosis added a third criteria, the detection by ultrasonography of a multifollicular ovary and/or increased ovarian volume.4,5 Using the Rotterdam approach, PCOS is diagnosed in the presence of any 2 of the following 3 criteria: hyperandrogenism, oligo-ovulation, or ultrasound imaging showing the presence of a multifollicular ovary, identified by ≥ 12 antral follicles (2 to 9 mm in diameter) in each ovary or increased ovarian volume (> 10 mL).4,5
The Rotterdam approach using ovarian ultrasound as a criterion to diagnose PCOS is rife with serious problems, including:
- The number of small antral follicles in the normal ovary is age dependent, and many ovulatory and nonhirsute patients have ≥ 12 small antral follicles in each ovary.8,9
- There is no consensus on the number of small antral follicles needed to diagnose a multifollicular ovary, with recommendations to use thresholds of 124,5 or 20 follicles10 as the diagnostic cut-off.
- Accurate counting of the number of small ovarian follicles requires transvaginal ultrasound, which is not appropriate for many young adolescent patients.
- The process of counting ovarian follicles is operator-dependent.
- The high cost of ultrasound assessment of ovarian follicles (≥ $500 per examination).

The Rotterdam approach supports the diagnosis of PCOS in a patient with oligo-ovulation plus an ultrasound showing a multifollicular ovary in the absence of any clinical or laboratory evidence of hyperandrogenism.3,4,5 This approach to the diagnosis of PCOS is rejected by both the 1990 NIH3 and AES6 recommendations, which require the presence of hyperandrogenism as the sine qua non in the diagnosis of PCOS. I recommend against diagnosing PCOS in a non-hyperandrogenic patient with oligo-ovulation and a multifollicular ovary because other diagnoses are also possible, such as functional hypothalamic oligo-ovulation, especially in young patients. The Rotterdam approach also supports the diagnosis of PCOS in a patient with hyperandrogenism, an ultrasound showing a multifollicular ovary, and normal ovulation and menses.3,4 For most patients with normal, regular ovulation and menses, the testosterone concentration is normal and the only evidence of hyperandrogenism is hirsutism. Patients with normal, regular ovulation and menses plus hirsutism usually have idiopathic hirsutism. Idiopathic hirsutism is a problem caused by excessive 5-alpha-reductase activity in the hair pilosebaceous unit, which catalyzes the conversion of weak androgens into dihydrotestosterone, a potent intracellular androgen that stimulates terminal hair growth.11 In my opinion, the Rotterdam approach to diagnosing PCOS has created unnecessary confusion and complexity for both clinicians and patients. I believe we should simplify the diagnosis of PCOS and return to the 1990 NIH criteria.3
On occasion, a patient presents for a consultation and has already had an ovarian ultrasound to assess for a multifollicular ovary. I carefully read the report and, if a multifollicular ovary has been identified, I consider it as a secondary supporting finding of PCOS in my clinical assessment. But I do not base my diagnosis on the ultrasound finding. Patients often present with other laboratory tests that are secondary supporting findings of PCOS, which I carefully consider but do not use to make a diagnosis of PCOS. Secondary supporting laboratory findings consistent with PCOS include: 1) a markedly elevated anti-müllerian hormone (AMH) level,12 2) an elevated fasting insulin level,2,13 and 3) an elevated luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio.13,14 But it is not necessary to measure AMH, fasting insulin, LH, and FSH levels. To conserve health care resources, I recommend against measuring those analytes to diagnose PCOS.

Continue to: Simplify the core laboratory tests...
Simplify the core laboratory tests
Simplify the testing used to support the diagnosis of PCOS by measuring total testosterone, sex-hormone binding globulin (SHBG) and early morning 17-hydroxyprogesterone (17-OH Prog).
The core criteria for diagnosis of PCOS are hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea or amenorrhea. Hyperandrogenism can be clinically diagnosed by assessing for the presence of hirsutism.7 Elevated levels of total testosterone, free testosterone, androstenedione, and/or dehydroepiandrosterone sulfate (DHEAS) suggest the presence of hyperandrogenism. In clinical practice, the laboratory approach to the diagnosis of hyperandrogenism can be simplified to the measurement of total testosterone, SHBG, and 17-OH Prog. By measuring total testosterone and SHBG, an estimate of free testosterone can be made. If the total testosterone is elevated, it is highly likely that the free testosterone is elevated. If the SHBG is abnormally low and the total testosterone level is in the upper limit of the normal range, the free testosterone is likely to be elevated.15 Using this approach, either an elevated total testosterone or an abnormally low SHBG indicate elevated free testosterone. For patients with hyperandrogenism and oligo-ovulation, an early morning (8 to 9 AM) 17-OH Prog level ≤ 2 ng/mL rules out the presence of NCAH due to a 21-hydroxylase deficiency.16 In my practice, the core laboratory tests I order when considering the diagnosis of PCOS are a total testosterone, SHBG, and 17-OH Prog.
Additional laboratory tests may be warranted to assess the patient diagnosed with PCOS. For example, if the patient has amenorrhea due to anovulation, tests for prolactin, FSH, and thyroid-stimulating hormone levels are warranted to assess for the presence of a prolactinoma, primary ovarian insufficiency, or thyroid disease, respectively. If the patient has a body mass index (BMI) ≥ 25 kg/m2, a hemoglobin A1c concentration is warranted to assess for the presence of prediabetes or DM.2 Many patients with PCOS have dyslipidemia, manifested through low high-density lipoprotein cholesterol levels and elevated low-density lipoprotein cholesterol levels, and a lipid panel assessment may be indicated. Among patients with PCOS, the most common lipid abnormality is a low high-density lipoprotein cholesterol level.17
Simplify the treatment of PCOS
Simplify treatment by counseling about lifestyle changes and prescribing an estrogen-progestin contraceptive, spironolactone, and metformin.
Most patients with PCOS have dysfunction in reproductive, metabolic, and dermatologic systems. For patients who are overweight or obese, lifestyle changes, including diet and exercise, that result in a 5% to 10% decrease in weight can improve metabolic balance, reduce circulating androgens, and increase menstrual frequency.18 For patients with PCOS and weight issues, referral to nutrition counseling or a full-service weight loss program can be very beneficial. In addition to lifestyle changes, patients with PCOS benefit from treatment with estrogen-progestin medications, spironolactone, and metformin.
Combination estrogen-progestin medications will lower LH secretion, decrease ovarian androgen production, increase SHBG production, decrease free testosterone levels and, if given cyclically, cause regular withdrawal bleeding.19 Spironolactone is an antiandrogen, which blocks the intracellular action of dihydrotestosterone and improves hirsutism and acne. Spironolactone also modestly decreases circulating levels of testosterone and DHEAS.20 For patients with metabolic problems, including insulin resistance and obesity, weight loss and/or treatment with metformin can help improve metabolic balance, which may result in restoration of ovulatory menses.21,22 Metformin can be effective in restoring ovulatory menses in both obese and lean patients with PCOS.22 The most common dermatologic problem caused by PCOS are hirsutism and acne. Both combination estrogen-progestin medications and spironolactone are effective treatments for hirsutism and acne.23
Estrogen-progestin hormones, spironolactone, and metformin are low-cost medications for the treatment of PCOS. Additional high-cost options for treatment of PCOS in obese patients include bariatric surgery and glucagon-like peptide (GLP-1) agonist medications (liraglutide and exenatide). For patients with PCOS and a body mass index (BMI) ≥ 35 kg/m2, bariatric surgery often results in sufficient weight loss to resolve the patient’s hyperandrogenism and oligo-ovulation, restoring spontaneous ovulatory cycles.24 In a study of more than 1,000 patients with: PCOS; mean BMI, 44 kg/m2; mean age, 31 years who were followed post-bariatric surgery for 5 years, > 90% of patients reported reductions in hirsutism and resumption of regular menses.25 For patients with PCOS seeking fertility, bariatric surgery often results in spontaneous pregnancy and live birth.26 GLP-1 agonists, including liraglutide or exenatide with or without metformin are effective in reducing weight, decreasing androgen levels, and restoring ovulatory menses.27,28
In my practice, I often prescribe 2 or 3 core medications for a patient with PCOS: 1) combination estrogen-progestin used cyclically or continuously, 2) spironolactone, and 3) metformin.19 Any estrogen-progestin contraceptive will suppress LH and ovarian androgen production; however, in the treatment of patients with PCOS, I prefer to use an estrogen-progestin combination that does not contain the androgenic progestin levonorgestrel.29 For the treatment of PCOS, I prefer to use an estrogen-progestin contraceptive with a non-androgenic progestin such as drospirenone, desogestrel, or gestodene. I routinely prescribe spironolactone at a dose of 100 mg, once daily, a dose near the top of the dose-response curve. A daily dose ≤ 50 mg of spironolactone is subtherapeutic for the treatment of hirsutism. A daily dose of 200 mg of spironolactone may cause bothersome breakthrough bleeding. When prescribing metformin, I usually recommend the extended-release formulation, at a dose of 750 mg with dinner. If well tolerated, I will increase the dose to 1,500 mg with dinner. Most of my patients with PCOS are taking a combination of 2 medications, either an estrogen-progestin contraceptive plus spironolactone or an estrogen-progestin contraceptive plus metformin.19 Some of my patients are taking all 3 medications. All 3 medications are very low cost.
For patients with PCOS and anovulatory infertility, letrozole treatment often results in ovulatory cycles and pregnancy with live birth. In obese PCOS patients, compared with clomiphene, letrozole results in superior live birth rates.30 Unlike clomiphene, letrozole is not approved by the US Food and Drug Administration for the treatment of anovulatory infertility.
The diagnosis of PCOS is often delayed due to confusion about how to make the diagnosis.31 To simplify the diagnosis of PCOS and improve patient encounters for PCOS, I focus on 2 core criteria: hyperandrogenism and oligo-ovulation. I recommend against ordering ultrasound imaging to assess for the presence of a multifollicular ovary. To simplify the treatment of PCOS I frequently prescribe an estrogen-progestin contraceptive, spironolactone, and metformin. By simplifying the diagnosis and treatment of PCOS, ObGyns will reduce patient confusion, improve outcomes, and save health care resources. ●
PCOS and adolescent patients
It is difficult to diagnose polycystic ovary syndrome (PCOS) in adolescents because oligo-ovulation is a common physiological feature of adolescence. Based on consensus among experts, PCOS should not be diagnosed within the first 2 years following menarche because the prevalence of oligo-ovulation is common at this stage of pubertal development. Two years after menarche, if an adolescent has a cycle length that is routinely > 45 days, it is likely that the pattern will persist, suggesting the presence of oligo-ovulation. Hyperandrogenism can be diagnosed based on the presence of moderate to severe hirsutism and/or an elevated testosterone or abnormally low sex-hormone binding globulin (SHBG) concentration. Two years after menarche the presence of oligo-ovulation and hyperandrogenism establishes the diagnosis of PCOS.1
PCOS and thrombophilia or migraine with aura
For patients with PCOS and a Factor V Leiden allele, where an estrogen-progestin contraceptive is contraindicated because of an increased risk of a venous thrombus, I prescribe spironolactone plus a levonorgestrel-intrauterine device. A low-dose oral progestin also may be considered because it will modestly suppress LH and ovarian androgen production. Similarly for patients with migraine with aura, where an estrogen-progestin contraceptive is contraindicated because of an increased of stroke, spironolactone plus a levonorgesterel intrauterine device may be effective in the treatment of hirsutism.
Androgen secreting tumors
Occasionally during the evaluation of a patient with hyperandrogenism and oligo-ovulation, measurement of total testosterone levels will reveal a value > 1.5 ng/mL. Most patients with PCOS have a total testosterone level ≤ 1.5 ng/mL. A total testosterone concentration > 1.5 ng/mL may be caused by ovarian stromal hyperthecosis or an androgen-producing tumor.2
Strongly-held patient perspectives on PCOS
At the first consultation visit, some patients are fearful and not receptive to a diagnosis of PCOS. If a clinician senses that the patient is not prepared to hear that they have PCOS, the clinician can be supportive of the patient’s perspective and focus on the patient’s chief health concerns, which may include abnormal cycle length, hirsutism, and/or overweight or obesity. During follow-up visits, as the patient builds trust with the clinician, the patient will be better prepared to discuss the diagnosis of PCOS. At the first consultation visit, some patients present with a strong belief that they have PCOS but have seen clinicians who conclude that they do not have PCOS. The diagnosis of PCOS is confusing because of competing diagnostic frameworks (NIH, Rotterdam, and AES). I avoid engaging in an argument with a patient who strongly believes that they have PCOS. In these situations, I focus on identifying the patient’s chief health concerns and discussing interventions to support their health goals.
References
1. Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol. 2020;33:445-447.
2. Meczekalski B, Szeliga A, Maciejewska-Jeske M, et al. Hyperthecosis: an underestimated nontumorous cause of hyperandrogenism. Gynecol Endocrinol. 2021;37:677-682.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome. Current Issues in Endocrinology and Metabolism. Dunaif A, Givens JR, Haseltine FP, Merriam GE (eds.). Blackwell Scientific Inc. Boston, Massachusetts; 1992:377.
- Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reprod. 2004;19:41-47.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Hatch R, Rosenfield RS, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-830.
- Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-Rotterdam: a common age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95:4965-4972.
- Alsamarai S, Adams JM, Murphy MK, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961-4970.
- Teede HJ, Misso ML, Costello MF, et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364-379.
- Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic hirsutism. Fertil Steril. 1985;43:74-78.
- Pigny P, Jonard S, Robert Y, et al. Serum anti-Müllerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941-945.
- Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812-841.
- Kumar N, Agarwal H. Early clinical, biochemical and radiologic features in obese and non-obese young women with polycystic ovarian syndrome: a comparative study. Horm Metab Res. 2022;54:620-624.
- Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95-109.
- Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180:R127-145.
- Guo F, Gong Z, Fernando T, et al. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: a retrospective study in Chinese population. Front Endocrinol. 2022;13:892125.
- Dietz de Loos ALP, Jiskoot G, Timman R, et al. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod Biomed Online. 2021;43:298-309.
- Ezeh U, Huang A, Landay M, et al. Long-term response of hirsutism and other hyperandrogenic symptoms to combination therapy in polycystic ovary syndrome. J Women’s Health. 2018;27:892-902.
- Ashraf Ganie M, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Pasquali R, Gambineri A, Cavazza C, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:53-60.
- Yang PK, Hsu CY, Chen MJ, et al. The efficacy of 24-month metformin for improving menses, hormones and metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:890-899.
- Garg V, Choi J, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355.
- Hu L, Ma L, Ying T, et al. Efficacy of bariatric surgery in the treatment of women with obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107:e3217-3229.
- Bhandari M, Kosta S, Bhandari M, et al. Effects of bariatric surgery on people with obesity and polycystic ovary syndrome: a large single center study from India. Obes Surg. 2022;32:3305-3312.
- Benito E, Gomez-Martin JM, Vega-Pinero B, et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J Clin Endocrinol Metab. 2020;105:e3384-3391.
- Xing C, Li C, He B. Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J Clin Endocrinol Metab. 2020;105:2950-2963.
- Elkind-Hirsch KE, Chappell N, Shaler D, et al. Liraglutide 3 mg on weight, body composition and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil Steril. 2022;118:371-381.
- Amiri M, Nahidi F, Bidhendi-Yarandi R, et al. A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: a crossover randomized controlled trial. Hum Reprod. 2020;35:175-186.
- Legro RS, Brzyski RG, Diamond NP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119-129.
- Gibson-Helm M, Teede H, Dunaif A, et al. Delayed diagnosis and lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:604-612.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome. Current Issues in Endocrinology and Metabolism. Dunaif A, Givens JR, Haseltine FP, Merriam GE (eds.). Blackwell Scientific Inc. Boston, Massachusetts; 1992:377.
- Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reprod. 2004;19:41-47.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Hatch R, Rosenfield RS, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-830.
- Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-Rotterdam: a common age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95:4965-4972.
- Alsamarai S, Adams JM, Murphy MK, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961-4970.
- Teede HJ, Misso ML, Costello MF, et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364-379.
- Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic hirsutism. Fertil Steril. 1985;43:74-78.
- Pigny P, Jonard S, Robert Y, et al. Serum anti-Müllerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941-945.
- Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812-841.
- Kumar N, Agarwal H. Early clinical, biochemical and radiologic features in obese and non-obese young women with polycystic ovarian syndrome: a comparative study. Horm Metab Res. 2022;54:620-624.
- Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95-109.
- Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180:R127-145.
- Guo F, Gong Z, Fernando T, et al. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: a retrospective study in Chinese population. Front Endocrinol. 2022;13:892125.
- Dietz de Loos ALP, Jiskoot G, Timman R, et al. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod Biomed Online. 2021;43:298-309.
- Ezeh U, Huang A, Landay M, et al. Long-term response of hirsutism and other hyperandrogenic symptoms to combination therapy in polycystic ovary syndrome. J Women’s Health. 2018;27:892-902.
- Ashraf Ganie M, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Pasquali R, Gambineri A, Cavazza C, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:53-60.
- Yang PK, Hsu CY, Chen MJ, et al. The efficacy of 24-month metformin for improving menses, hormones and metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:890-899.
- Garg V, Choi J, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355.
- Hu L, Ma L, Ying T, et al. Efficacy of bariatric surgery in the treatment of women with obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107:e3217-3229.
- Bhandari M, Kosta S, Bhandari M, et al. Effects of bariatric surgery on people with obesity and polycystic ovary syndrome: a large single center study from India. Obes Surg. 2022;32:3305-3312.
- Benito E, Gomez-Martin JM, Vega-Pinero B, et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J Clin Endocrinol Metab. 2020;105:e3384-3391.
- Xing C, Li C, He B. Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J Clin Endocrinol Metab. 2020;105:2950-2963.
- Elkind-Hirsch KE, Chappell N, Shaler D, et al. Liraglutide 3 mg on weight, body composition and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil Steril. 2022;118:371-381.
- Amiri M, Nahidi F, Bidhendi-Yarandi R, et al. A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: a crossover randomized controlled trial. Hum Reprod. 2020;35:175-186.
- Legro RS, Brzyski RG, Diamond NP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119-129.
- Gibson-Helm M, Teede H, Dunaif A, et al. Delayed diagnosis and lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:604-612.
Cardiologist sues hospital, claims he was fired in retaliation
alleging that he was fired and maligned after raising concerns about poorly performed surgeries and poor ethical practices at the hospital.
Dr. Zelman, from Barnstable, Mass., has been affiliated with Cape Cod Hospital in Hyannis, Mass., for more than 30 years. He helped found the hospital’s Heart and Vascular Institute and has served as its medical director since 2018.
In his lawsuit filed Dec. 6, Dr. Zelman alleges that the defendants, under Mr. Lauf’s leadership, “placed profit above all else, including by prioritizing revenue generation over patient safety and public health.”
Dr. Zelman says the defendants supported him “to the extent his actions were profitable.”
Yet, when he raised patient safety concerns that harmed that bottom line, Dr. Zelman says the defendants retaliated against him, including by threatening his career and reputation and unlawfully terminating his employment with the hospital.
The complaint notes Dr. Zelman is bringing this action “to recover damages for violations of the Massachusetts Healthcare Provider Whistleblower Statute ... as well as for breach of contract and common law claims.”
Dr. Zelman’s complaint alleges the defendants refused to adequately address the “dangerous care and violations of the professional standards of practice” that he reported, “resulting in harmful and tragic consequences.”
It also alleges Mr. Lauf restricted the use of a cerebral protection device used in patients undergoing transcatheter aortic-valve replacement (TAVR) deemed to be at high risk for periprocedural stroke to only those patients whose insurance reimbursed at higher rates.
Dr. Zelman says he objected to this prohibition “in accordance with his contractual and ethical obligations to ensure treatment of patients without regard to their ability to pay.”
Dr. Zelman’s lawsuit further alleges that Mr. Lauf launched a “trumped-up” and “baseless, biased, and retaliatory sham” investigation against him.
In a statement sent to the Boston Globe, Cape Cod Hospital denied Dr. Zelman’s claims that the cardiologist was retaliated against for raising patient safety issues, or that the hospital didn’t take action to improve cardiac care at the facility.
Voiced concerns
In a statement sent to this news organization, Dr. Zelman, now in private practice, said, “Over the past 25 years, I have been instrumental in bringing advanced cardiac care to Cape Cod. My commitment has always been to delivering the same quality outcomes and safety as the academic centers in Boston.
“Unfortunately, over the past 5 years, there has been inadequate oversight by the hospital administration and problems have occurred that in my opinion have led to serious patient consequences,” Dr. Zelman stated.
He said he has “voiced concerns over several years and they have been ignored.”
He added that Cape Cod Hospital offered him a million-dollar contract as long as he agreed to immediately issue a written statement endorsing the quality and safety of the cardiac surgical program that no longer exists.
“No amount of money was going to buy my silence,” Dr. Zelman told this news organization.
In his lawsuit, Dr. Zelman is seeking an undisclosed amount in damages, including back and front pay, lost benefits, physical and emotional distress, and attorneys’ fees.
This news organization reached out to Cape Cod Hospital for comment but has not yet received a response.
A version of this article first appeared on Medscape.com.
alleging that he was fired and maligned after raising concerns about poorly performed surgeries and poor ethical practices at the hospital.
Dr. Zelman, from Barnstable, Mass., has been affiliated with Cape Cod Hospital in Hyannis, Mass., for more than 30 years. He helped found the hospital’s Heart and Vascular Institute and has served as its medical director since 2018.
In his lawsuit filed Dec. 6, Dr. Zelman alleges that the defendants, under Mr. Lauf’s leadership, “placed profit above all else, including by prioritizing revenue generation over patient safety and public health.”
Dr. Zelman says the defendants supported him “to the extent his actions were profitable.”
Yet, when he raised patient safety concerns that harmed that bottom line, Dr. Zelman says the defendants retaliated against him, including by threatening his career and reputation and unlawfully terminating his employment with the hospital.
The complaint notes Dr. Zelman is bringing this action “to recover damages for violations of the Massachusetts Healthcare Provider Whistleblower Statute ... as well as for breach of contract and common law claims.”
Dr. Zelman’s complaint alleges the defendants refused to adequately address the “dangerous care and violations of the professional standards of practice” that he reported, “resulting in harmful and tragic consequences.”
It also alleges Mr. Lauf restricted the use of a cerebral protection device used in patients undergoing transcatheter aortic-valve replacement (TAVR) deemed to be at high risk for periprocedural stroke to only those patients whose insurance reimbursed at higher rates.
Dr. Zelman says he objected to this prohibition “in accordance with his contractual and ethical obligations to ensure treatment of patients without regard to their ability to pay.”
Dr. Zelman’s lawsuit further alleges that Mr. Lauf launched a “trumped-up” and “baseless, biased, and retaliatory sham” investigation against him.
In a statement sent to the Boston Globe, Cape Cod Hospital denied Dr. Zelman’s claims that the cardiologist was retaliated against for raising patient safety issues, or that the hospital didn’t take action to improve cardiac care at the facility.
Voiced concerns
In a statement sent to this news organization, Dr. Zelman, now in private practice, said, “Over the past 25 years, I have been instrumental in bringing advanced cardiac care to Cape Cod. My commitment has always been to delivering the same quality outcomes and safety as the academic centers in Boston.
“Unfortunately, over the past 5 years, there has been inadequate oversight by the hospital administration and problems have occurred that in my opinion have led to serious patient consequences,” Dr. Zelman stated.
He said he has “voiced concerns over several years and they have been ignored.”
He added that Cape Cod Hospital offered him a million-dollar contract as long as he agreed to immediately issue a written statement endorsing the quality and safety of the cardiac surgical program that no longer exists.
“No amount of money was going to buy my silence,” Dr. Zelman told this news organization.
In his lawsuit, Dr. Zelman is seeking an undisclosed amount in damages, including back and front pay, lost benefits, physical and emotional distress, and attorneys’ fees.
This news organization reached out to Cape Cod Hospital for comment but has not yet received a response.
A version of this article first appeared on Medscape.com.
alleging that he was fired and maligned after raising concerns about poorly performed surgeries and poor ethical practices at the hospital.
Dr. Zelman, from Barnstable, Mass., has been affiliated with Cape Cod Hospital in Hyannis, Mass., for more than 30 years. He helped found the hospital’s Heart and Vascular Institute and has served as its medical director since 2018.
In his lawsuit filed Dec. 6, Dr. Zelman alleges that the defendants, under Mr. Lauf’s leadership, “placed profit above all else, including by prioritizing revenue generation over patient safety and public health.”
Dr. Zelman says the defendants supported him “to the extent his actions were profitable.”
Yet, when he raised patient safety concerns that harmed that bottom line, Dr. Zelman says the defendants retaliated against him, including by threatening his career and reputation and unlawfully terminating his employment with the hospital.
The complaint notes Dr. Zelman is bringing this action “to recover damages for violations of the Massachusetts Healthcare Provider Whistleblower Statute ... as well as for breach of contract and common law claims.”
Dr. Zelman’s complaint alleges the defendants refused to adequately address the “dangerous care and violations of the professional standards of practice” that he reported, “resulting in harmful and tragic consequences.”
It also alleges Mr. Lauf restricted the use of a cerebral protection device used in patients undergoing transcatheter aortic-valve replacement (TAVR) deemed to be at high risk for periprocedural stroke to only those patients whose insurance reimbursed at higher rates.
Dr. Zelman says he objected to this prohibition “in accordance with his contractual and ethical obligations to ensure treatment of patients without regard to their ability to pay.”
Dr. Zelman’s lawsuit further alleges that Mr. Lauf launched a “trumped-up” and “baseless, biased, and retaliatory sham” investigation against him.
In a statement sent to the Boston Globe, Cape Cod Hospital denied Dr. Zelman’s claims that the cardiologist was retaliated against for raising patient safety issues, or that the hospital didn’t take action to improve cardiac care at the facility.
Voiced concerns
In a statement sent to this news organization, Dr. Zelman, now in private practice, said, “Over the past 25 years, I have been instrumental in bringing advanced cardiac care to Cape Cod. My commitment has always been to delivering the same quality outcomes and safety as the academic centers in Boston.
“Unfortunately, over the past 5 years, there has been inadequate oversight by the hospital administration and problems have occurred that in my opinion have led to serious patient consequences,” Dr. Zelman stated.
He said he has “voiced concerns over several years and they have been ignored.”
He added that Cape Cod Hospital offered him a million-dollar contract as long as he agreed to immediately issue a written statement endorsing the quality and safety of the cardiac surgical program that no longer exists.
“No amount of money was going to buy my silence,” Dr. Zelman told this news organization.
In his lawsuit, Dr. Zelman is seeking an undisclosed amount in damages, including back and front pay, lost benefits, physical and emotional distress, and attorneys’ fees.
This news organization reached out to Cape Cod Hospital for comment but has not yet received a response.
A version of this article first appeared on Medscape.com.
Three antiseizure medications join list for newborn risks
NASHVILLE, TENN. – A study of more than 4 million births over 20 years in five Scandinavian countries has reported that three antiseizure medications should be used with caution in women of child-bearing age because they were associated with low birth weights.
In results presented at the annual meeting of the American Epilepsy Society, Jakob Christensen, MD, DSc, PhD, a professor at Aarhus University Hospital in Denmark, said that the study found that
“Because we have this large data set we were able to confirm the suspicion that’s been raised in the past that these drugs may be associated with low birth weight,” Dr. Christensen said in an interview.
The study analyzed records from population-based registers of 4.5 million births in Denmark, Finland, Iceland, Norway, and Sweden between 1996 and 2017, known as the SCAN-AED project. The researchers analyzed the association between prenatal use of antiseizure medications and birth weight, defining low birth weight as less than 5.5 pounds and small for gestational age as being in the lowest 10th percentile for sex, country, and gestational weight at birth.
The antiseizure medications and adjusted odds ratios for risk of low birth rate were:
- Carbamazepine, 1.44 (95% confidence interval [CI], 1.21-1.71).
- Oxcarbazepine, 1.32 (95% CI, 1.03-1.69).
- Topiramate, 1.60 (95% CI, 1.15-2.24).
- Pregabalin, 1.23 (95% CI, 1.02-1.48).
- Clobazam, 4.36 (95% CI, 1.66-11.45).
The odds ratios for being born small for gestational age were:
- Carbamazepine, 1.25 (95% CI, 1.11-1.41).
- Oxcarbazepine, 1.48 (95% CI, 1.27-1.73).
- Topiramate, 1.52 (95% CI, 1.20-1.91).
“Prenatal exposure to carbamazepine, oxcarbazepine, and topiramate were associated with all estimates of adverse birth weight outcomes, thus confirming results from preclinical studies in animals and previous smaller studies in humans,” Dr. Christensen said.
He noted a lack of evidence for newer medications because their use was relatively low over the 20 years of the study. “However, for drugs like lamotrigine where we have a high number of exposed children, the finding of no association with low birth weight is reassuring, indicating the drug is safe,” Dr. Christensen said.
Use with caution
This study adds supportive evidence for expanding the list of antiseizure medications associated with small for gestational age infants, Elizabeth Gerard, MD, director of the Women with Epilepsy Program and associate professor of neurology at Northwestern University in Chicago, said in an interview.
“Previous clinical trials demonstrated that topiramate and zonisamide as well as phenobarbital were associated with small for gestational age,” she said. “This study added to the list carbamazepine and oxcarbazepine. Previously it wasn’t clear from clinical data but there were some hints that carbamazepine and oxcarbazepine might be associated with small for gestational age, but this is the first study to present robust data that carbamazepine and oxcarbazepine are associated with small for gestational age infants as well.”
She noted that these drugs can be used cautiously in women of child-bearing age and pregnant women. “I think these lines of evidence suggest that women with epilepsy should be more carefully monitored, at least with these high-quality, standard-of-care drugs, for fetal growth monitoring and perhaps most of them, especially those on at-risk drugs, should have detailed growth gradings,” Dr. Gerard said. Pregnant women on these antiseizure medications should have ultrasound beginning at 24 weeks gestation to monitor fetal growth, she said.
The NordForsk Nordic Program and Health and Welfare and the Independent Research Fund Denmark provided funding for the study. Dr. Christensen disclosed financial relationships with Union Chimique Belge Nordic and Eisai. Dr. Gerard disclosed relationships with Xenon Pharmaceuticals and Eisai.
NASHVILLE, TENN. – A study of more than 4 million births over 20 years in five Scandinavian countries has reported that three antiseizure medications should be used with caution in women of child-bearing age because they were associated with low birth weights.
In results presented at the annual meeting of the American Epilepsy Society, Jakob Christensen, MD, DSc, PhD, a professor at Aarhus University Hospital in Denmark, said that the study found that
“Because we have this large data set we were able to confirm the suspicion that’s been raised in the past that these drugs may be associated with low birth weight,” Dr. Christensen said in an interview.
The study analyzed records from population-based registers of 4.5 million births in Denmark, Finland, Iceland, Norway, and Sweden between 1996 and 2017, known as the SCAN-AED project. The researchers analyzed the association between prenatal use of antiseizure medications and birth weight, defining low birth weight as less than 5.5 pounds and small for gestational age as being in the lowest 10th percentile for sex, country, and gestational weight at birth.
The antiseizure medications and adjusted odds ratios for risk of low birth rate were:
- Carbamazepine, 1.44 (95% confidence interval [CI], 1.21-1.71).
- Oxcarbazepine, 1.32 (95% CI, 1.03-1.69).
- Topiramate, 1.60 (95% CI, 1.15-2.24).
- Pregabalin, 1.23 (95% CI, 1.02-1.48).
- Clobazam, 4.36 (95% CI, 1.66-11.45).
The odds ratios for being born small for gestational age were:
- Carbamazepine, 1.25 (95% CI, 1.11-1.41).
- Oxcarbazepine, 1.48 (95% CI, 1.27-1.73).
- Topiramate, 1.52 (95% CI, 1.20-1.91).
“Prenatal exposure to carbamazepine, oxcarbazepine, and topiramate were associated with all estimates of adverse birth weight outcomes, thus confirming results from preclinical studies in animals and previous smaller studies in humans,” Dr. Christensen said.
He noted a lack of evidence for newer medications because their use was relatively low over the 20 years of the study. “However, for drugs like lamotrigine where we have a high number of exposed children, the finding of no association with low birth weight is reassuring, indicating the drug is safe,” Dr. Christensen said.
Use with caution
This study adds supportive evidence for expanding the list of antiseizure medications associated with small for gestational age infants, Elizabeth Gerard, MD, director of the Women with Epilepsy Program and associate professor of neurology at Northwestern University in Chicago, said in an interview.
“Previous clinical trials demonstrated that topiramate and zonisamide as well as phenobarbital were associated with small for gestational age,” she said. “This study added to the list carbamazepine and oxcarbazepine. Previously it wasn’t clear from clinical data but there were some hints that carbamazepine and oxcarbazepine might be associated with small for gestational age, but this is the first study to present robust data that carbamazepine and oxcarbazepine are associated with small for gestational age infants as well.”
She noted that these drugs can be used cautiously in women of child-bearing age and pregnant women. “I think these lines of evidence suggest that women with epilepsy should be more carefully monitored, at least with these high-quality, standard-of-care drugs, for fetal growth monitoring and perhaps most of them, especially those on at-risk drugs, should have detailed growth gradings,” Dr. Gerard said. Pregnant women on these antiseizure medications should have ultrasound beginning at 24 weeks gestation to monitor fetal growth, she said.
The NordForsk Nordic Program and Health and Welfare and the Independent Research Fund Denmark provided funding for the study. Dr. Christensen disclosed financial relationships with Union Chimique Belge Nordic and Eisai. Dr. Gerard disclosed relationships with Xenon Pharmaceuticals and Eisai.
NASHVILLE, TENN. – A study of more than 4 million births over 20 years in five Scandinavian countries has reported that three antiseizure medications should be used with caution in women of child-bearing age because they were associated with low birth weights.
In results presented at the annual meeting of the American Epilepsy Society, Jakob Christensen, MD, DSc, PhD, a professor at Aarhus University Hospital in Denmark, said that the study found that
“Because we have this large data set we were able to confirm the suspicion that’s been raised in the past that these drugs may be associated with low birth weight,” Dr. Christensen said in an interview.
The study analyzed records from population-based registers of 4.5 million births in Denmark, Finland, Iceland, Norway, and Sweden between 1996 and 2017, known as the SCAN-AED project. The researchers analyzed the association between prenatal use of antiseizure medications and birth weight, defining low birth weight as less than 5.5 pounds and small for gestational age as being in the lowest 10th percentile for sex, country, and gestational weight at birth.
The antiseizure medications and adjusted odds ratios for risk of low birth rate were:
- Carbamazepine, 1.44 (95% confidence interval [CI], 1.21-1.71).
- Oxcarbazepine, 1.32 (95% CI, 1.03-1.69).
- Topiramate, 1.60 (95% CI, 1.15-2.24).
- Pregabalin, 1.23 (95% CI, 1.02-1.48).
- Clobazam, 4.36 (95% CI, 1.66-11.45).
The odds ratios for being born small for gestational age were:
- Carbamazepine, 1.25 (95% CI, 1.11-1.41).
- Oxcarbazepine, 1.48 (95% CI, 1.27-1.73).
- Topiramate, 1.52 (95% CI, 1.20-1.91).
“Prenatal exposure to carbamazepine, oxcarbazepine, and topiramate were associated with all estimates of adverse birth weight outcomes, thus confirming results from preclinical studies in animals and previous smaller studies in humans,” Dr. Christensen said.
He noted a lack of evidence for newer medications because their use was relatively low over the 20 years of the study. “However, for drugs like lamotrigine where we have a high number of exposed children, the finding of no association with low birth weight is reassuring, indicating the drug is safe,” Dr. Christensen said.
Use with caution
This study adds supportive evidence for expanding the list of antiseizure medications associated with small for gestational age infants, Elizabeth Gerard, MD, director of the Women with Epilepsy Program and associate professor of neurology at Northwestern University in Chicago, said in an interview.
“Previous clinical trials demonstrated that topiramate and zonisamide as well as phenobarbital were associated with small for gestational age,” she said. “This study added to the list carbamazepine and oxcarbazepine. Previously it wasn’t clear from clinical data but there were some hints that carbamazepine and oxcarbazepine might be associated with small for gestational age, but this is the first study to present robust data that carbamazepine and oxcarbazepine are associated with small for gestational age infants as well.”
She noted that these drugs can be used cautiously in women of child-bearing age and pregnant women. “I think these lines of evidence suggest that women with epilepsy should be more carefully monitored, at least with these high-quality, standard-of-care drugs, for fetal growth monitoring and perhaps most of them, especially those on at-risk drugs, should have detailed growth gradings,” Dr. Gerard said. Pregnant women on these antiseizure medications should have ultrasound beginning at 24 weeks gestation to monitor fetal growth, she said.
The NordForsk Nordic Program and Health and Welfare and the Independent Research Fund Denmark provided funding for the study. Dr. Christensen disclosed financial relationships with Union Chimique Belge Nordic and Eisai. Dr. Gerard disclosed relationships with Xenon Pharmaceuticals and Eisai.
AT AES 2022
Immune dysregulation may drive long-term postpartum depression
Postpartum depression, anxiety, and posttraumatic stress disorder that persist 2-3 years after birth are associated with a dysregulated immune system that is characterized by increased inflammatory signaling, according to investigators.
These findings suggest that mental health screening for women who have given birth should continue beyond the first year post partum, reported lead author Jennifer M. Nicoloro-SantaBarbara, PhD, of Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues.
“Delayed postpartum depression, also known as late-onset postpartum depression, can affect women up to 18 months after delivery,” the investigators wrote in the American Journal of Reproductive Immunology. “It can appear even later in some women, depending on the hormonal changes that occur after having a baby (for example, timing of weaning). However, the majority of research on maternal mental health focuses on the first year post birth, leaving a gap in research beyond 12 months post partum.”
To address this gap, the investigators enrolled 33 women who were 2-3 years post partum. Participants completed self-guided questionnaires on PTSD, depression, and anxiety, and provided blood samples for gene expression analysis.
Sixteen of the 33 women had clinically significant mood disturbances. and significantly reduced activation of genes associated with viral response.
“The results provide preliminary evidence of a mechanism (e.g., immune dysregulation) that might be contributing to mood disorders and bring us closer to the goal of identifying targetable biomarkers for mood disorders,” Dr. Nicoloro-SantaBarbara said in a written comment. “This work highlights the need for standardized and continual depression and anxiety screening in ob.gyn. and primary care settings that extends beyond the 6-week maternal visit and possibly beyond the first postpartum year.”
Findings draw skepticism
“The authors argue that mothers need to be screened for depression/anxiety longer than the first year post partum, and this is true, but it has nothing to do with their findings,” said Jennifer L. Payne, MD, an expert in reproductive psychiatry at the University of Virginia, Charlottesville.
In a written comment, she explained that the cross-sectional design makes it impossible to know whether the mood disturbances were linked with delivery at all.
“It is unclear if the depression/anxiety symptoms began after delivery or not,” Dr. Payne said. “In addition, it is unclear if the findings are causative or a result of depression/anxiety symptoms (the authors admit this in the limitations section). It is likely that the findings are not specific or even related to having delivered a child, but rather reflect a more general process related to depression/anxiety outside of the postpartum time period.”
Only prospective studies can answer these questions, she said.
Dr. Nicoloro-SantaBarbara agreed that further research is needed.
“Our findings are exciting, but still need to be replicated in larger samples with diverse women in order to make sure they generalize,” she said. “More work is needed to understand why inflammation plays a role in postpartum mental illness for some women and not others.”
The study was supported by a Cedars-Sinai Precision Health Grant, the Cousins Center for Psychoneuroimmunology, University of California, Los Angeles, and the National Institute of Mental Health. The investigators and Dr. Payne disclosed no relevant conflicts of interest.
Postpartum depression, anxiety, and posttraumatic stress disorder that persist 2-3 years after birth are associated with a dysregulated immune system that is characterized by increased inflammatory signaling, according to investigators.
These findings suggest that mental health screening for women who have given birth should continue beyond the first year post partum, reported lead author Jennifer M. Nicoloro-SantaBarbara, PhD, of Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues.
“Delayed postpartum depression, also known as late-onset postpartum depression, can affect women up to 18 months after delivery,” the investigators wrote in the American Journal of Reproductive Immunology. “It can appear even later in some women, depending on the hormonal changes that occur after having a baby (for example, timing of weaning). However, the majority of research on maternal mental health focuses on the first year post birth, leaving a gap in research beyond 12 months post partum.”
To address this gap, the investigators enrolled 33 women who were 2-3 years post partum. Participants completed self-guided questionnaires on PTSD, depression, and anxiety, and provided blood samples for gene expression analysis.
Sixteen of the 33 women had clinically significant mood disturbances. and significantly reduced activation of genes associated with viral response.
“The results provide preliminary evidence of a mechanism (e.g., immune dysregulation) that might be contributing to mood disorders and bring us closer to the goal of identifying targetable biomarkers for mood disorders,” Dr. Nicoloro-SantaBarbara said in a written comment. “This work highlights the need for standardized and continual depression and anxiety screening in ob.gyn. and primary care settings that extends beyond the 6-week maternal visit and possibly beyond the first postpartum year.”
Findings draw skepticism
“The authors argue that mothers need to be screened for depression/anxiety longer than the first year post partum, and this is true, but it has nothing to do with their findings,” said Jennifer L. Payne, MD, an expert in reproductive psychiatry at the University of Virginia, Charlottesville.
In a written comment, she explained that the cross-sectional design makes it impossible to know whether the mood disturbances were linked with delivery at all.
“It is unclear if the depression/anxiety symptoms began after delivery or not,” Dr. Payne said. “In addition, it is unclear if the findings are causative or a result of depression/anxiety symptoms (the authors admit this in the limitations section). It is likely that the findings are not specific or even related to having delivered a child, but rather reflect a more general process related to depression/anxiety outside of the postpartum time period.”
Only prospective studies can answer these questions, she said.
Dr. Nicoloro-SantaBarbara agreed that further research is needed.
“Our findings are exciting, but still need to be replicated in larger samples with diverse women in order to make sure they generalize,” she said. “More work is needed to understand why inflammation plays a role in postpartum mental illness for some women and not others.”
The study was supported by a Cedars-Sinai Precision Health Grant, the Cousins Center for Psychoneuroimmunology, University of California, Los Angeles, and the National Institute of Mental Health. The investigators and Dr. Payne disclosed no relevant conflicts of interest.
Postpartum depression, anxiety, and posttraumatic stress disorder that persist 2-3 years after birth are associated with a dysregulated immune system that is characterized by increased inflammatory signaling, according to investigators.
These findings suggest that mental health screening for women who have given birth should continue beyond the first year post partum, reported lead author Jennifer M. Nicoloro-SantaBarbara, PhD, of Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues.
“Delayed postpartum depression, also known as late-onset postpartum depression, can affect women up to 18 months after delivery,” the investigators wrote in the American Journal of Reproductive Immunology. “It can appear even later in some women, depending on the hormonal changes that occur after having a baby (for example, timing of weaning). However, the majority of research on maternal mental health focuses on the first year post birth, leaving a gap in research beyond 12 months post partum.”
To address this gap, the investigators enrolled 33 women who were 2-3 years post partum. Participants completed self-guided questionnaires on PTSD, depression, and anxiety, and provided blood samples for gene expression analysis.
Sixteen of the 33 women had clinically significant mood disturbances. and significantly reduced activation of genes associated with viral response.
“The results provide preliminary evidence of a mechanism (e.g., immune dysregulation) that might be contributing to mood disorders and bring us closer to the goal of identifying targetable biomarkers for mood disorders,” Dr. Nicoloro-SantaBarbara said in a written comment. “This work highlights the need for standardized and continual depression and anxiety screening in ob.gyn. and primary care settings that extends beyond the 6-week maternal visit and possibly beyond the first postpartum year.”
Findings draw skepticism
“The authors argue that mothers need to be screened for depression/anxiety longer than the first year post partum, and this is true, but it has nothing to do with their findings,” said Jennifer L. Payne, MD, an expert in reproductive psychiatry at the University of Virginia, Charlottesville.
In a written comment, she explained that the cross-sectional design makes it impossible to know whether the mood disturbances were linked with delivery at all.
“It is unclear if the depression/anxiety symptoms began after delivery or not,” Dr. Payne said. “In addition, it is unclear if the findings are causative or a result of depression/anxiety symptoms (the authors admit this in the limitations section). It is likely that the findings are not specific or even related to having delivered a child, but rather reflect a more general process related to depression/anxiety outside of the postpartum time period.”
Only prospective studies can answer these questions, she said.
Dr. Nicoloro-SantaBarbara agreed that further research is needed.
“Our findings are exciting, but still need to be replicated in larger samples with diverse women in order to make sure they generalize,” she said. “More work is needed to understand why inflammation plays a role in postpartum mental illness for some women and not others.”
The study was supported by a Cedars-Sinai Precision Health Grant, the Cousins Center for Psychoneuroimmunology, University of California, Los Angeles, and the National Institute of Mental Health. The investigators and Dr. Payne disclosed no relevant conflicts of interest.
FROM THE AMERICAN JOURNAL OF REPRODUCTIVE IMMUNOLOGY
Breaking bad news during IVF: How to soften the blow
Two years ago, Ashley Hingston had a miscarriage. The 35-year-old Floridian and her husband had been going through in vitro fertilization when they received the news no one wants to get, but which many in their position reflexively expect: “You are going to lose the pregnancy.”
On the other end of the line was Ms. Hingston’s physician, who offered her advice and comfort to ease the pain of the bad news.
“I was a complete wreck and could barely even talk,” Ms. Hingston recalled. “But I think my doctor knew what I was asking, and she was doing the best to answer the questions I had: Why did this happen? What does this mean? And she sat and listened to me.”
An estimated 2% of babies born in the United States each year are the result of IVF, according to 2019 data from the Centers for Disease Control and Prevention. The process is often emotionally, physically, and economically taxing for patients. According to the CDC, the chance a pregnancy will be successful through IVF is 21.3%. Consequently, doctors often find themselves the bearers of bad news.
But interaction with a care team or a string of nurses and providers, rather than a physician, is the norm for IVF patients, according to Aimee Eyvazzadeh, MD, a specialist in infertility and reproductive endocrinology in San Ramon, Calif.
“Patients see a doctor for all of 10 minutes and then they are handed off to a care team who don’t know their whole story,” she said.
For Dr. Eyvazzadeh and other health care professionals, physicians must improve the ways they share bad news, and how they divide tasks with care teams.
Personalized care works best
Providing personalized care will improve how IVF patients respond to bad news, according to Dr. Eyvazzadeh and others.
“When people have gone through so much trauma, anything you say to them, sometimes they can’t process it very well, so they have to see the information in different ways,” she told this news organization. “After each phone call, I’ll actually type up a summary for them, with links and articles for them to read, so they are directed in a way that I think is healthy so they aren’t pulled into a rabbit hole.”
Dr. Eyvazzadeh said she encourages her patients to seek counseling during IVF treatment, and even pays for their initial psychiatric consultation. Not many doctors do this, she noted.
“Taking the time to allow the patient to process the bad news is vital,” said Linda Kim, PhD, a psychiatrist at Moon Mental Health, to whom Dr. Eyvazzadeh refers couples. Sometimes, several calls are necessary.
“Rather than thinking of the conversation as a linear process, consider it a sphere of processing,” Dr. Kim said. “The patient may need space to grieve, may ask many questions, may need to clarify what happened, or may need to vent and release frustration. This is space that the patient needs to process the bad news.” (See below for more tips on how to share bad news with your patients.)
Many care teams are skilled in delivering bad news to patients, according to Liz Grill, PsyD, a psychologist at Weill Cornell Medicine, New York. The challenge for them is ensuring new nurses and clinicians continue to have empathy training, she said.
“You want to make sure clinicians are building relationships, and empathy. Whether there is a protocol to build that level of empathy, or if they have their own innate ability to build empathy, it’s about communicating in the right way,” Dr. Grill told this news organization.
But Mark Trolice, MD, a reproductive endocrinologist and infertility specialist at the University of Central Florida, Orlando, agreed that nurses should not deliver bad news, even if they have the expertise and the compassion to do so.
“It’s the doctor’s responsibility to make that call. It’s a very difficult call and it puts an unnecessary burden on your care team to be making these calls all the time,” Dr. Trolice said. “I feel the patient wants to hear from the physician who oversaw their cycle and did the procedure and embryo transfer. It shows a tremendous amount of responsibility and commitment on the part of the physician.”
Dr. Trolice also recommended clinicians refer to the HEART (Hearing, Empathy, Apology, Response, and Thanks) guidelines to ensure proper conversations with patients about bad news.
“You give the patient time to process the information and ask questions, and then we schedule another time to talk about plans going forward,” he said.
“Patients can feel powerless and not in control of what is happening, or even over their own bodies,” Dr. Kim added. “To counteract this, it can be helpful to outline projected steps as much as possible.”
For Dr. Eyvazzadeh, caring for an IVF patient is a matter of knowing your strengths.
Providing links to web resources, recommending an organic diet, and sending them to support groups (see below) are helpful, she said. “For some people, their strength isn’t engaging with patients on the same level that I do. But I still feel like there are ways that we can still make the patients feel cared for without being extreme.”
Tips on how to share bad news with patients
A guide often cited by clinicians when delivering bad news is the Buckman Six Step Protocol:
- Get the physical context right.
- Find out how much the patient knows.
- Find out how much the patient wants to know.
- Share the information.
- Respond to the patient’s feelings.
- Plan and follow through.
Linda Kim, PhD, notes that patient preference in receiving bad news is often culturally mediated. She recommends asking patients how they would want to receive bad news, especially in during IVF process, where there can be many challenges over the course of treatment. Dr. Kim also recommends these steps:
- Get as much information in advance as possible and ask your patient directly how they want bad news. When you are meeting a patient and their families for the first time, and they are filling out their intake paperwork or health forms, you may consider adding a section on “What is your preferred method of communication?” And after that, you might add, “What is the best way to tell you challenging or difficult news? Would you prefer to be by yourself or with a loved one? Please elaborate any additional preferences.” Everyone is different, and it can be helpful to hear from the patient directly how they would like to receive bad news. It will not only meet them where they are during a difficult time, it will also demonstrate to the patient that you are respecting their preferences and involving those preferences in the process.
- Try to leave enough time for a difficult conversation with a patient. Even better is if a clinician can prepare a patient that there is some disappointing or difficult news to share.
- Finally, offer discussion on next steps. It never hurts to ask the patient directly when they are ready to discuss next steps. This may take a few hours, a few days, or even a few months or longer.
Social media as support
Monica Wunderman, a patient of Dr. Eyvazzadeh’s since 2020, began her own social media campaign on Instagram to find support and give support to women experiencing IVF.
“I started scrolling and liking posts, and a girl reached out to me to ask if I needed help” in the form of emotional support, she recalls.
Instagram became a haven for Ms. Wunderman to share information, experiences, and support with others. It also allowed her to create a network of support and meet other women, like Ashley Hingston, going through similar struggles.
Ms. Wunderman has been through four rounds of IVF so far. Three were completely unsuccessful; the last ended in miscarriage. Although she and her husband are trying again with a surrogate, the uncertainty remains. And she feels the health care system should be offering her – and the other would-be parents she has met online – more support.
“We place such importance as a society on growing families,” she says. “But then we do very little to support those who want them and struggle.”
A version of this article first appeared on Medscape.com.
Two years ago, Ashley Hingston had a miscarriage. The 35-year-old Floridian and her husband had been going through in vitro fertilization when they received the news no one wants to get, but which many in their position reflexively expect: “You are going to lose the pregnancy.”
On the other end of the line was Ms. Hingston’s physician, who offered her advice and comfort to ease the pain of the bad news.
“I was a complete wreck and could barely even talk,” Ms. Hingston recalled. “But I think my doctor knew what I was asking, and she was doing the best to answer the questions I had: Why did this happen? What does this mean? And she sat and listened to me.”
An estimated 2% of babies born in the United States each year are the result of IVF, according to 2019 data from the Centers for Disease Control and Prevention. The process is often emotionally, physically, and economically taxing for patients. According to the CDC, the chance a pregnancy will be successful through IVF is 21.3%. Consequently, doctors often find themselves the bearers of bad news.
But interaction with a care team or a string of nurses and providers, rather than a physician, is the norm for IVF patients, according to Aimee Eyvazzadeh, MD, a specialist in infertility and reproductive endocrinology in San Ramon, Calif.
“Patients see a doctor for all of 10 minutes and then they are handed off to a care team who don’t know their whole story,” she said.
For Dr. Eyvazzadeh and other health care professionals, physicians must improve the ways they share bad news, and how they divide tasks with care teams.
Personalized care works best
Providing personalized care will improve how IVF patients respond to bad news, according to Dr. Eyvazzadeh and others.
“When people have gone through so much trauma, anything you say to them, sometimes they can’t process it very well, so they have to see the information in different ways,” she told this news organization. “After each phone call, I’ll actually type up a summary for them, with links and articles for them to read, so they are directed in a way that I think is healthy so they aren’t pulled into a rabbit hole.”
Dr. Eyvazzadeh said she encourages her patients to seek counseling during IVF treatment, and even pays for their initial psychiatric consultation. Not many doctors do this, she noted.
“Taking the time to allow the patient to process the bad news is vital,” said Linda Kim, PhD, a psychiatrist at Moon Mental Health, to whom Dr. Eyvazzadeh refers couples. Sometimes, several calls are necessary.
“Rather than thinking of the conversation as a linear process, consider it a sphere of processing,” Dr. Kim said. “The patient may need space to grieve, may ask many questions, may need to clarify what happened, or may need to vent and release frustration. This is space that the patient needs to process the bad news.” (See below for more tips on how to share bad news with your patients.)
Many care teams are skilled in delivering bad news to patients, according to Liz Grill, PsyD, a psychologist at Weill Cornell Medicine, New York. The challenge for them is ensuring new nurses and clinicians continue to have empathy training, she said.
“You want to make sure clinicians are building relationships, and empathy. Whether there is a protocol to build that level of empathy, or if they have their own innate ability to build empathy, it’s about communicating in the right way,” Dr. Grill told this news organization.
But Mark Trolice, MD, a reproductive endocrinologist and infertility specialist at the University of Central Florida, Orlando, agreed that nurses should not deliver bad news, even if they have the expertise and the compassion to do so.
“It’s the doctor’s responsibility to make that call. It’s a very difficult call and it puts an unnecessary burden on your care team to be making these calls all the time,” Dr. Trolice said. “I feel the patient wants to hear from the physician who oversaw their cycle and did the procedure and embryo transfer. It shows a tremendous amount of responsibility and commitment on the part of the physician.”
Dr. Trolice also recommended clinicians refer to the HEART (Hearing, Empathy, Apology, Response, and Thanks) guidelines to ensure proper conversations with patients about bad news.
“You give the patient time to process the information and ask questions, and then we schedule another time to talk about plans going forward,” he said.
“Patients can feel powerless and not in control of what is happening, or even over their own bodies,” Dr. Kim added. “To counteract this, it can be helpful to outline projected steps as much as possible.”
For Dr. Eyvazzadeh, caring for an IVF patient is a matter of knowing your strengths.
Providing links to web resources, recommending an organic diet, and sending them to support groups (see below) are helpful, she said. “For some people, their strength isn’t engaging with patients on the same level that I do. But I still feel like there are ways that we can still make the patients feel cared for without being extreme.”
Tips on how to share bad news with patients
A guide often cited by clinicians when delivering bad news is the Buckman Six Step Protocol:
- Get the physical context right.
- Find out how much the patient knows.
- Find out how much the patient wants to know.
- Share the information.
- Respond to the patient’s feelings.
- Plan and follow through.
Linda Kim, PhD, notes that patient preference in receiving bad news is often culturally mediated. She recommends asking patients how they would want to receive bad news, especially in during IVF process, where there can be many challenges over the course of treatment. Dr. Kim also recommends these steps:
- Get as much information in advance as possible and ask your patient directly how they want bad news. When you are meeting a patient and their families for the first time, and they are filling out their intake paperwork or health forms, you may consider adding a section on “What is your preferred method of communication?” And after that, you might add, “What is the best way to tell you challenging or difficult news? Would you prefer to be by yourself or with a loved one? Please elaborate any additional preferences.” Everyone is different, and it can be helpful to hear from the patient directly how they would like to receive bad news. It will not only meet them where they are during a difficult time, it will also demonstrate to the patient that you are respecting their preferences and involving those preferences in the process.
- Try to leave enough time for a difficult conversation with a patient. Even better is if a clinician can prepare a patient that there is some disappointing or difficult news to share.
- Finally, offer discussion on next steps. It never hurts to ask the patient directly when they are ready to discuss next steps. This may take a few hours, a few days, or even a few months or longer.
Social media as support
Monica Wunderman, a patient of Dr. Eyvazzadeh’s since 2020, began her own social media campaign on Instagram to find support and give support to women experiencing IVF.
“I started scrolling and liking posts, and a girl reached out to me to ask if I needed help” in the form of emotional support, she recalls.
Instagram became a haven for Ms. Wunderman to share information, experiences, and support with others. It also allowed her to create a network of support and meet other women, like Ashley Hingston, going through similar struggles.
Ms. Wunderman has been through four rounds of IVF so far. Three were completely unsuccessful; the last ended in miscarriage. Although she and her husband are trying again with a surrogate, the uncertainty remains. And she feels the health care system should be offering her – and the other would-be parents she has met online – more support.
“We place such importance as a society on growing families,” she says. “But then we do very little to support those who want them and struggle.”
A version of this article first appeared on Medscape.com.
Two years ago, Ashley Hingston had a miscarriage. The 35-year-old Floridian and her husband had been going through in vitro fertilization when they received the news no one wants to get, but which many in their position reflexively expect: “You are going to lose the pregnancy.”
On the other end of the line was Ms. Hingston’s physician, who offered her advice and comfort to ease the pain of the bad news.
“I was a complete wreck and could barely even talk,” Ms. Hingston recalled. “But I think my doctor knew what I was asking, and she was doing the best to answer the questions I had: Why did this happen? What does this mean? And she sat and listened to me.”
An estimated 2% of babies born in the United States each year are the result of IVF, according to 2019 data from the Centers for Disease Control and Prevention. The process is often emotionally, physically, and economically taxing for patients. According to the CDC, the chance a pregnancy will be successful through IVF is 21.3%. Consequently, doctors often find themselves the bearers of bad news.
But interaction with a care team or a string of nurses and providers, rather than a physician, is the norm for IVF patients, according to Aimee Eyvazzadeh, MD, a specialist in infertility and reproductive endocrinology in San Ramon, Calif.
“Patients see a doctor for all of 10 minutes and then they are handed off to a care team who don’t know their whole story,” she said.
For Dr. Eyvazzadeh and other health care professionals, physicians must improve the ways they share bad news, and how they divide tasks with care teams.
Personalized care works best
Providing personalized care will improve how IVF patients respond to bad news, according to Dr. Eyvazzadeh and others.
“When people have gone through so much trauma, anything you say to them, sometimes they can’t process it very well, so they have to see the information in different ways,” she told this news organization. “After each phone call, I’ll actually type up a summary for them, with links and articles for them to read, so they are directed in a way that I think is healthy so they aren’t pulled into a rabbit hole.”
Dr. Eyvazzadeh said she encourages her patients to seek counseling during IVF treatment, and even pays for their initial psychiatric consultation. Not many doctors do this, she noted.
“Taking the time to allow the patient to process the bad news is vital,” said Linda Kim, PhD, a psychiatrist at Moon Mental Health, to whom Dr. Eyvazzadeh refers couples. Sometimes, several calls are necessary.
“Rather than thinking of the conversation as a linear process, consider it a sphere of processing,” Dr. Kim said. “The patient may need space to grieve, may ask many questions, may need to clarify what happened, or may need to vent and release frustration. This is space that the patient needs to process the bad news.” (See below for more tips on how to share bad news with your patients.)
Many care teams are skilled in delivering bad news to patients, according to Liz Grill, PsyD, a psychologist at Weill Cornell Medicine, New York. The challenge for them is ensuring new nurses and clinicians continue to have empathy training, she said.
“You want to make sure clinicians are building relationships, and empathy. Whether there is a protocol to build that level of empathy, or if they have their own innate ability to build empathy, it’s about communicating in the right way,” Dr. Grill told this news organization.
But Mark Trolice, MD, a reproductive endocrinologist and infertility specialist at the University of Central Florida, Orlando, agreed that nurses should not deliver bad news, even if they have the expertise and the compassion to do so.
“It’s the doctor’s responsibility to make that call. It’s a very difficult call and it puts an unnecessary burden on your care team to be making these calls all the time,” Dr. Trolice said. “I feel the patient wants to hear from the physician who oversaw their cycle and did the procedure and embryo transfer. It shows a tremendous amount of responsibility and commitment on the part of the physician.”
Dr. Trolice also recommended clinicians refer to the HEART (Hearing, Empathy, Apology, Response, and Thanks) guidelines to ensure proper conversations with patients about bad news.
“You give the patient time to process the information and ask questions, and then we schedule another time to talk about plans going forward,” he said.
“Patients can feel powerless and not in control of what is happening, or even over their own bodies,” Dr. Kim added. “To counteract this, it can be helpful to outline projected steps as much as possible.”
For Dr. Eyvazzadeh, caring for an IVF patient is a matter of knowing your strengths.
Providing links to web resources, recommending an organic diet, and sending them to support groups (see below) are helpful, she said. “For some people, their strength isn’t engaging with patients on the same level that I do. But I still feel like there are ways that we can still make the patients feel cared for without being extreme.”
Tips on how to share bad news with patients
A guide often cited by clinicians when delivering bad news is the Buckman Six Step Protocol:
- Get the physical context right.
- Find out how much the patient knows.
- Find out how much the patient wants to know.
- Share the information.
- Respond to the patient’s feelings.
- Plan and follow through.
Linda Kim, PhD, notes that patient preference in receiving bad news is often culturally mediated. She recommends asking patients how they would want to receive bad news, especially in during IVF process, where there can be many challenges over the course of treatment. Dr. Kim also recommends these steps:
- Get as much information in advance as possible and ask your patient directly how they want bad news. When you are meeting a patient and their families for the first time, and they are filling out their intake paperwork or health forms, you may consider adding a section on “What is your preferred method of communication?” And after that, you might add, “What is the best way to tell you challenging or difficult news? Would you prefer to be by yourself or with a loved one? Please elaborate any additional preferences.” Everyone is different, and it can be helpful to hear from the patient directly how they would like to receive bad news. It will not only meet them where they are during a difficult time, it will also demonstrate to the patient that you are respecting their preferences and involving those preferences in the process.
- Try to leave enough time for a difficult conversation with a patient. Even better is if a clinician can prepare a patient that there is some disappointing or difficult news to share.
- Finally, offer discussion on next steps. It never hurts to ask the patient directly when they are ready to discuss next steps. This may take a few hours, a few days, or even a few months or longer.
Social media as support
Monica Wunderman, a patient of Dr. Eyvazzadeh’s since 2020, began her own social media campaign on Instagram to find support and give support to women experiencing IVF.
“I started scrolling and liking posts, and a girl reached out to me to ask if I needed help” in the form of emotional support, she recalls.
Instagram became a haven for Ms. Wunderman to share information, experiences, and support with others. It also allowed her to create a network of support and meet other women, like Ashley Hingston, going through similar struggles.
Ms. Wunderman has been through four rounds of IVF so far. Three were completely unsuccessful; the last ended in miscarriage. Although she and her husband are trying again with a surrogate, the uncertainty remains. And she feels the health care system should be offering her – and the other would-be parents she has met online – more support.
“We place such importance as a society on growing families,” she says. “But then we do very little to support those who want them and struggle.”
A version of this article first appeared on Medscape.com.
Why doctors are losing trust in patients; what should be done?
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University.
I want to talk about a paper that my colleagues in my division just published in Health Affairs.
As they pointed out, there’s a large amount of literature about what makes patients trust their doctor. There are many studies that show that, although patients sometimes have become more critical of the medical profession, in general they still try to trust their individual physician. Nurses remain in fairly high esteem among those who are getting hospital care.
What isn’t studied, as this paper properly points out, is, what can the doctor and the nurse do to trust the patient? How can that be assessed? Isn’t that just as important as saying that patients have to trust their doctors to do and comply with what they’re told?
What if doctors are afraid of violence? What if doctors are fearful that they can’t trust patients to listen, pay attention, or do what they’re being told? What if they think that patients are coming in with all kinds of disinformation, false information, or things they pick up on the Internet, so that even though you try your best to get across accurate and complete information about what to do about infectious diseases, taking care of a kid with strep throat, or whatever it might be, you’re thinking, Can I trust this patient to do what it is that I want them to do?
One particular problem that’s causing distrust is that more and more patients are showing stress and dependence on drugs and alcohol. That doesn’t make them less trustworthy per se, but it means they can’t regulate their own behavior as well.
That obviously has to be something that the physician or the nurse is thinking about. Is this person going to be able to contain anger? Is this person going to be able to handle bad news? Is this person going to deal with me when I tell them that some of the things they believe to be true about what’s good for their health care are false?
I think we have to really start to push administrators and people in positions of power to teach doctors and nurses how to defuse situations and how to make people more comfortable when they come in and the doctor suspects that they might be under the influence, impaired, or angry because of things they’ve seen on social media, whatever those might be – including concerns about racism, bigotry, and bias, which some patients are bringing into the clinic and the hospital setting.
We need more training. We’ve got to address this as a serious issue. What can we do to defuse situations where the doctor or the nurse rightly thinks that they can’t control or they can’t trust what the patient is thinking or how the patient might behave?
It’s also the case that I think we need more backup and quick access to security so that people feel safe and comfortable in providing care. We have to make sure that if you need someone to restrain a patient or to get somebody out of a situation, that they can get there quickly and respond rapidly, and that they know what to do to deescalate a situation.
It’s sad to say, but security in today’s health care world has to be something that we really test and check – not because we’re worried, as many places are, about a shooter entering the premises, which is its own bit of concern – but I’m just talking about when the doctor or the nurse says that this patient might be acting up, could get violent, or is someone I can’t trust.
My coauthors are basically saying that it’s not a one-way street. Yes, we have to figure out ways to make sure that our patients can trust what we say. Trust is absolutely the lubricant that makes health care flow. If patients don’t trust their doctors, they’re not going to do what they say. They’re not going to get their prescriptions filled. They’re not going to be compliant. They’re not going to try to lose weight or control their diabetes.
It also goes the other way. The doctor or the nurse has to trust the patient. They have to believe that they’re safe. They have to believe that the patient is capable of controlling themselves. They have to believe that the patient is capable of listening and hearing what they’re saying, and that they’re competent to follow up on instructions, including to come back if that’s what’s required.
Everybody has to feel secure in the environment in which they’re working. Security, sadly, has to be a priority if we’re going to have a health care workforce that really feels safe and comfortable dealing with a patient population that is increasingly aggressive and perhaps not as trustworthy.
That’s not news I like to read when my colleagues write it up, but it’s important and we have to take it seriously.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University.
I want to talk about a paper that my colleagues in my division just published in Health Affairs.
As they pointed out, there’s a large amount of literature about what makes patients trust their doctor. There are many studies that show that, although patients sometimes have become more critical of the medical profession, in general they still try to trust their individual physician. Nurses remain in fairly high esteem among those who are getting hospital care.
What isn’t studied, as this paper properly points out, is, what can the doctor and the nurse do to trust the patient? How can that be assessed? Isn’t that just as important as saying that patients have to trust their doctors to do and comply with what they’re told?
What if doctors are afraid of violence? What if doctors are fearful that they can’t trust patients to listen, pay attention, or do what they’re being told? What if they think that patients are coming in with all kinds of disinformation, false information, or things they pick up on the Internet, so that even though you try your best to get across accurate and complete information about what to do about infectious diseases, taking care of a kid with strep throat, or whatever it might be, you’re thinking, Can I trust this patient to do what it is that I want them to do?
One particular problem that’s causing distrust is that more and more patients are showing stress and dependence on drugs and alcohol. That doesn’t make them less trustworthy per se, but it means they can’t regulate their own behavior as well.
That obviously has to be something that the physician or the nurse is thinking about. Is this person going to be able to contain anger? Is this person going to be able to handle bad news? Is this person going to deal with me when I tell them that some of the things they believe to be true about what’s good for their health care are false?
I think we have to really start to push administrators and people in positions of power to teach doctors and nurses how to defuse situations and how to make people more comfortable when they come in and the doctor suspects that they might be under the influence, impaired, or angry because of things they’ve seen on social media, whatever those might be – including concerns about racism, bigotry, and bias, which some patients are bringing into the clinic and the hospital setting.
We need more training. We’ve got to address this as a serious issue. What can we do to defuse situations where the doctor or the nurse rightly thinks that they can’t control or they can’t trust what the patient is thinking or how the patient might behave?
It’s also the case that I think we need more backup and quick access to security so that people feel safe and comfortable in providing care. We have to make sure that if you need someone to restrain a patient or to get somebody out of a situation, that they can get there quickly and respond rapidly, and that they know what to do to deescalate a situation.
It’s sad to say, but security in today’s health care world has to be something that we really test and check – not because we’re worried, as many places are, about a shooter entering the premises, which is its own bit of concern – but I’m just talking about when the doctor or the nurse says that this patient might be acting up, could get violent, or is someone I can’t trust.
My coauthors are basically saying that it’s not a one-way street. Yes, we have to figure out ways to make sure that our patients can trust what we say. Trust is absolutely the lubricant that makes health care flow. If patients don’t trust their doctors, they’re not going to do what they say. They’re not going to get their prescriptions filled. They’re not going to be compliant. They’re not going to try to lose weight or control their diabetes.
It also goes the other way. The doctor or the nurse has to trust the patient. They have to believe that they’re safe. They have to believe that the patient is capable of controlling themselves. They have to believe that the patient is capable of listening and hearing what they’re saying, and that they’re competent to follow up on instructions, including to come back if that’s what’s required.
Everybody has to feel secure in the environment in which they’re working. Security, sadly, has to be a priority if we’re going to have a health care workforce that really feels safe and comfortable dealing with a patient population that is increasingly aggressive and perhaps not as trustworthy.
That’s not news I like to read when my colleagues write it up, but it’s important and we have to take it seriously.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University.
I want to talk about a paper that my colleagues in my division just published in Health Affairs.
As they pointed out, there’s a large amount of literature about what makes patients trust their doctor. There are many studies that show that, although patients sometimes have become more critical of the medical profession, in general they still try to trust their individual physician. Nurses remain in fairly high esteem among those who are getting hospital care.
What isn’t studied, as this paper properly points out, is, what can the doctor and the nurse do to trust the patient? How can that be assessed? Isn’t that just as important as saying that patients have to trust their doctors to do and comply with what they’re told?
What if doctors are afraid of violence? What if doctors are fearful that they can’t trust patients to listen, pay attention, or do what they’re being told? What if they think that patients are coming in with all kinds of disinformation, false information, or things they pick up on the Internet, so that even though you try your best to get across accurate and complete information about what to do about infectious diseases, taking care of a kid with strep throat, or whatever it might be, you’re thinking, Can I trust this patient to do what it is that I want them to do?
One particular problem that’s causing distrust is that more and more patients are showing stress and dependence on drugs and alcohol. That doesn’t make them less trustworthy per se, but it means they can’t regulate their own behavior as well.
That obviously has to be something that the physician or the nurse is thinking about. Is this person going to be able to contain anger? Is this person going to be able to handle bad news? Is this person going to deal with me when I tell them that some of the things they believe to be true about what’s good for their health care are false?
I think we have to really start to push administrators and people in positions of power to teach doctors and nurses how to defuse situations and how to make people more comfortable when they come in and the doctor suspects that they might be under the influence, impaired, or angry because of things they’ve seen on social media, whatever those might be – including concerns about racism, bigotry, and bias, which some patients are bringing into the clinic and the hospital setting.
We need more training. We’ve got to address this as a serious issue. What can we do to defuse situations where the doctor or the nurse rightly thinks that they can’t control or they can’t trust what the patient is thinking or how the patient might behave?
It’s also the case that I think we need more backup and quick access to security so that people feel safe and comfortable in providing care. We have to make sure that if you need someone to restrain a patient or to get somebody out of a situation, that they can get there quickly and respond rapidly, and that they know what to do to deescalate a situation.
It’s sad to say, but security in today’s health care world has to be something that we really test and check – not because we’re worried, as many places are, about a shooter entering the premises, which is its own bit of concern – but I’m just talking about when the doctor or the nurse says that this patient might be acting up, could get violent, or is someone I can’t trust.
My coauthors are basically saying that it’s not a one-way street. Yes, we have to figure out ways to make sure that our patients can trust what we say. Trust is absolutely the lubricant that makes health care flow. If patients don’t trust their doctors, they’re not going to do what they say. They’re not going to get their prescriptions filled. They’re not going to be compliant. They’re not going to try to lose weight or control their diabetes.
It also goes the other way. The doctor or the nurse has to trust the patient. They have to believe that they’re safe. They have to believe that the patient is capable of controlling themselves. They have to believe that the patient is capable of listening and hearing what they’re saying, and that they’re competent to follow up on instructions, including to come back if that’s what’s required.
Everybody has to feel secure in the environment in which they’re working. Security, sadly, has to be a priority if we’re going to have a health care workforce that really feels safe and comfortable dealing with a patient population that is increasingly aggressive and perhaps not as trustworthy.
That’s not news I like to read when my colleagues write it up, but it’s important and we have to take it seriously.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.






