User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Canadian guidance recommends reducing alcohol consumption
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
Transgender people in rural America struggle to find doctors willing or able to provide care
For Tammy Rainey, finding a health care provider who knows about gender-affirming care has been a challenge in the rural northern Mississippi town where she lives.
As a transgender woman, Ms. Rainey needs the hormone estrogen, which allows her to physically transition by developing more feminine features. But when she asked her doctor for an estrogen prescription, he said he couldn’t provide that type of care.
“He’s generally a good guy and doesn’t act prejudiced. He gets my name and pronouns right,” said Ms. Rainey. “But when I asked him about hormones, he said, ‘I just don’t feel like I know enough about that. I don’t want to get involved in that.’ ”
So Ms. Rainey drives around 170 miles round trip every 6 months to get a supply of estrogen from a clinic in Memphis, Tenn., to take home with her.
The obstacles Ms. Rainey overcomes to access care illustrate a type of medical inequity that transgender people who live in the rural United States often face: A general lack of education about trans-related care among small-town health professionals who might also be reluctant to learn.
“Medical communities across the country are seeing clearly that there is a knowledge gap in the provision of gender-affirming care,” said Morissa Ladinsky, MD, a pediatrician who co-leads the Youth Multidisciplinary Gender Team at the University of Alabama–Birmingham (UAB).
Accurately counting the number of transgender people in rural America is hindered by a lack of U.S. census data and uniform state data. However, the Movement Advancement Project, a nonprofit organization that advocates for LGBTQ+ issues, used 2014-17 Centers for Disease Control and Prevention data from selected ZIP codes in 35 states to estimate that roughly one in six transgender adults in the United States live in a rural area. When that report was released in 2019, there were an estimated 1.4 million transgender people 13 and older nationwide. That number is now at least 1.6 million, according to the Williams Institute, a nonprofit think tank at the UCLA School of Law.
One in three trans people in rural areas experienced discrimination by a health care provider in the year leading up to the 2015 U.S. Transgender Survey Report, according to an analysis by MAP. A third of all trans individuals report having to teach their doctors about their health care needs to receive appropriate care, and 62% worry about being negatively judged by a health care provider because of their sexual orientation or gender identity, according to data collected by the Williams Institute and other organizations.
A lack of local rural providers knowledgeable in trans care can mean long drives to gender-affirming clinics in metropolitan areas. Rural trans people are three times as likely as are all transgender adults to travel 25-49 miles for routine care.
In Colorado, for example, many trans people outside Denver struggle to find proper care. Those who do have a trans-inclusive provider are more likely to receive wellness exams, less likely to delay care due to discrimination, and less likely to attempt suicide, according to results from the Colorado Transgender Health Survey published in 2018.
Much of the lack of care experienced by trans people is linked to insufficient education on LGBTQ+ health in medical schools across the country. In 2014, the Association of American Medical Colleges, which represents 170 accredited medical schools in the United States and Canada, released its first curriculum guidelines on caring for LGBTQ+ patients. As of 2018, 76% of medical schools included LGBTQ health themes in their curriculum, with half providing three or fewer classes on this topic.
Perhaps because of this, almost 77% of students from 10 medical schools in New England felt “not competent” or “somewhat not competent” in treating gender minority patients, according to a 2018 pilot study. Another paper, published last year, found that even clinicians who work in trans-friendly clinics lack knowledge about hormones, gender-affirming surgical options, and how to use appropriate pronouns and trans-inclusive language.
Throughout medical school, trans care was only briefly mentioned in endocrinology class, said Justin Bailey, MD, who received his medical degree from UAB in 2021 and is now a resident there. “I don’t want to say the wrong thing or use the wrong pronouns, so I was hesitant and a little bit tepid in my approach to interviewing and treating this population of patients,” he said.
On top of insufficient medical school education, some practicing doctors don’t take the time to teach themselves about trans people, said Kathie Moehlig, founder of TransFamily Support Services, a nonprofit organization that offers a range of services to transgender people and their families. They are very well intentioned yet uneducated when it comes to transgender care, she said.
Some medical schools, like the one at UAB, have pushed for change. Since 2017, Dr. Ladinsky and her colleagues have worked to include trans people in their standardized patient program, which gives medical students hands-on experience and feedback by interacting with “patients” in simulated clinical environments.
For example, a trans individual acting as a patient will simulate acid reflux by pretending to have pain in their stomach and chest. Then, over the course of the examination, they will reveal that they are transgender.
In the early years of this program, some students’ bedside manner would change once the patient’s gender identity was revealed, said Elaine Stephens, a trans woman who participates in UAB’s standardized patient program. “Sometimes they would immediately start asking about sexual activity,” Stephens said.
Since UAB launched its program, students’ reactions have improved significantly, she said.
This progress is being replicated by other medical schools, said Ms. Moehlig. “But it’s a slow start, and these are large institutions that take a long time to move forward.”
Advocates also are working outside medical schools to improve care in rural areas. In Colorado, the nonprofit Extension for Community Health Outcomes, or ECHO Colorado, has been offering monthly virtual classes on gender-affirming care to rural providers since 2020. The classes became so popular that the organization created a 4-week boot camp in 2021 for providers to learn about hormone therapy management, proper terminologies, surgical options, and supporting patients’ mental health.
For many years, doctors failed to recognize the need to learn about gender-affirming care, said Caroline Kirsch, DO, director of osteopathic education at the University of Wyoming Family Medicine Residency Program–Casper. In Casper, this led to “a number of patients traveling to Colorado to access care, which is a large burden for them financially,” said Dr. Kirsch, who has participated in the ECHO Colorado program.
“Things that haven’t been as well taught historically in medical school are things that I think many physicians feel anxious about initially,” she said. “The earlier you learn about this type of care in your career, the more likely you are to see its potential and be less anxious about it.”
Educating more providers about trans-related care has become increasingly vital in recent years as gender-affirming clinics nationwide experience a rise in harassment and threats. For instance, Vanderbilt University Medical Center’s Clinic for Transgender Health became the target of far-right hate on social media last year. After growing pressure from Tennessee’s Republican lawmakers, the clinic paused gender-affirmation surgeries on patients younger than 18, potentially leaving many trans individuals without necessary care.
Stephens hopes to see more medical schools include coursework on trans health care. She also wishes for doctors to treat trans people as they would any other patient.
“Just provide quality health care,” she tells the medical students at UAB. “We need health care like everyone else does.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
For Tammy Rainey, finding a health care provider who knows about gender-affirming care has been a challenge in the rural northern Mississippi town where she lives.
As a transgender woman, Ms. Rainey needs the hormone estrogen, which allows her to physically transition by developing more feminine features. But when she asked her doctor for an estrogen prescription, he said he couldn’t provide that type of care.
“He’s generally a good guy and doesn’t act prejudiced. He gets my name and pronouns right,” said Ms. Rainey. “But when I asked him about hormones, he said, ‘I just don’t feel like I know enough about that. I don’t want to get involved in that.’ ”
So Ms. Rainey drives around 170 miles round trip every 6 months to get a supply of estrogen from a clinic in Memphis, Tenn., to take home with her.
The obstacles Ms. Rainey overcomes to access care illustrate a type of medical inequity that transgender people who live in the rural United States often face: A general lack of education about trans-related care among small-town health professionals who might also be reluctant to learn.
“Medical communities across the country are seeing clearly that there is a knowledge gap in the provision of gender-affirming care,” said Morissa Ladinsky, MD, a pediatrician who co-leads the Youth Multidisciplinary Gender Team at the University of Alabama–Birmingham (UAB).
Accurately counting the number of transgender people in rural America is hindered by a lack of U.S. census data and uniform state data. However, the Movement Advancement Project, a nonprofit organization that advocates for LGBTQ+ issues, used 2014-17 Centers for Disease Control and Prevention data from selected ZIP codes in 35 states to estimate that roughly one in six transgender adults in the United States live in a rural area. When that report was released in 2019, there were an estimated 1.4 million transgender people 13 and older nationwide. That number is now at least 1.6 million, according to the Williams Institute, a nonprofit think tank at the UCLA School of Law.
One in three trans people in rural areas experienced discrimination by a health care provider in the year leading up to the 2015 U.S. Transgender Survey Report, according to an analysis by MAP. A third of all trans individuals report having to teach their doctors about their health care needs to receive appropriate care, and 62% worry about being negatively judged by a health care provider because of their sexual orientation or gender identity, according to data collected by the Williams Institute and other organizations.
A lack of local rural providers knowledgeable in trans care can mean long drives to gender-affirming clinics in metropolitan areas. Rural trans people are three times as likely as are all transgender adults to travel 25-49 miles for routine care.
In Colorado, for example, many trans people outside Denver struggle to find proper care. Those who do have a trans-inclusive provider are more likely to receive wellness exams, less likely to delay care due to discrimination, and less likely to attempt suicide, according to results from the Colorado Transgender Health Survey published in 2018.
Much of the lack of care experienced by trans people is linked to insufficient education on LGBTQ+ health in medical schools across the country. In 2014, the Association of American Medical Colleges, which represents 170 accredited medical schools in the United States and Canada, released its first curriculum guidelines on caring for LGBTQ+ patients. As of 2018, 76% of medical schools included LGBTQ health themes in their curriculum, with half providing three or fewer classes on this topic.
Perhaps because of this, almost 77% of students from 10 medical schools in New England felt “not competent” or “somewhat not competent” in treating gender minority patients, according to a 2018 pilot study. Another paper, published last year, found that even clinicians who work in trans-friendly clinics lack knowledge about hormones, gender-affirming surgical options, and how to use appropriate pronouns and trans-inclusive language.
Throughout medical school, trans care was only briefly mentioned in endocrinology class, said Justin Bailey, MD, who received his medical degree from UAB in 2021 and is now a resident there. “I don’t want to say the wrong thing or use the wrong pronouns, so I was hesitant and a little bit tepid in my approach to interviewing and treating this population of patients,” he said.
On top of insufficient medical school education, some practicing doctors don’t take the time to teach themselves about trans people, said Kathie Moehlig, founder of TransFamily Support Services, a nonprofit organization that offers a range of services to transgender people and their families. They are very well intentioned yet uneducated when it comes to transgender care, she said.
Some medical schools, like the one at UAB, have pushed for change. Since 2017, Dr. Ladinsky and her colleagues have worked to include trans people in their standardized patient program, which gives medical students hands-on experience and feedback by interacting with “patients” in simulated clinical environments.
For example, a trans individual acting as a patient will simulate acid reflux by pretending to have pain in their stomach and chest. Then, over the course of the examination, they will reveal that they are transgender.
In the early years of this program, some students’ bedside manner would change once the patient’s gender identity was revealed, said Elaine Stephens, a trans woman who participates in UAB’s standardized patient program. “Sometimes they would immediately start asking about sexual activity,” Stephens said.
Since UAB launched its program, students’ reactions have improved significantly, she said.
This progress is being replicated by other medical schools, said Ms. Moehlig. “But it’s a slow start, and these are large institutions that take a long time to move forward.”
Advocates also are working outside medical schools to improve care in rural areas. In Colorado, the nonprofit Extension for Community Health Outcomes, or ECHO Colorado, has been offering monthly virtual classes on gender-affirming care to rural providers since 2020. The classes became so popular that the organization created a 4-week boot camp in 2021 for providers to learn about hormone therapy management, proper terminologies, surgical options, and supporting patients’ mental health.
For many years, doctors failed to recognize the need to learn about gender-affirming care, said Caroline Kirsch, DO, director of osteopathic education at the University of Wyoming Family Medicine Residency Program–Casper. In Casper, this led to “a number of patients traveling to Colorado to access care, which is a large burden for them financially,” said Dr. Kirsch, who has participated in the ECHO Colorado program.
“Things that haven’t been as well taught historically in medical school are things that I think many physicians feel anxious about initially,” she said. “The earlier you learn about this type of care in your career, the more likely you are to see its potential and be less anxious about it.”
Educating more providers about trans-related care has become increasingly vital in recent years as gender-affirming clinics nationwide experience a rise in harassment and threats. For instance, Vanderbilt University Medical Center’s Clinic for Transgender Health became the target of far-right hate on social media last year. After growing pressure from Tennessee’s Republican lawmakers, the clinic paused gender-affirmation surgeries on patients younger than 18, potentially leaving many trans individuals without necessary care.
Stephens hopes to see more medical schools include coursework on trans health care. She also wishes for doctors to treat trans people as they would any other patient.
“Just provide quality health care,” she tells the medical students at UAB. “We need health care like everyone else does.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
For Tammy Rainey, finding a health care provider who knows about gender-affirming care has been a challenge in the rural northern Mississippi town where she lives.
As a transgender woman, Ms. Rainey needs the hormone estrogen, which allows her to physically transition by developing more feminine features. But when she asked her doctor for an estrogen prescription, he said he couldn’t provide that type of care.
“He’s generally a good guy and doesn’t act prejudiced. He gets my name and pronouns right,” said Ms. Rainey. “But when I asked him about hormones, he said, ‘I just don’t feel like I know enough about that. I don’t want to get involved in that.’ ”
So Ms. Rainey drives around 170 miles round trip every 6 months to get a supply of estrogen from a clinic in Memphis, Tenn., to take home with her.
The obstacles Ms. Rainey overcomes to access care illustrate a type of medical inequity that transgender people who live in the rural United States often face: A general lack of education about trans-related care among small-town health professionals who might also be reluctant to learn.
“Medical communities across the country are seeing clearly that there is a knowledge gap in the provision of gender-affirming care,” said Morissa Ladinsky, MD, a pediatrician who co-leads the Youth Multidisciplinary Gender Team at the University of Alabama–Birmingham (UAB).
Accurately counting the number of transgender people in rural America is hindered by a lack of U.S. census data and uniform state data. However, the Movement Advancement Project, a nonprofit organization that advocates for LGBTQ+ issues, used 2014-17 Centers for Disease Control and Prevention data from selected ZIP codes in 35 states to estimate that roughly one in six transgender adults in the United States live in a rural area. When that report was released in 2019, there were an estimated 1.4 million transgender people 13 and older nationwide. That number is now at least 1.6 million, according to the Williams Institute, a nonprofit think tank at the UCLA School of Law.
One in three trans people in rural areas experienced discrimination by a health care provider in the year leading up to the 2015 U.S. Transgender Survey Report, according to an analysis by MAP. A third of all trans individuals report having to teach their doctors about their health care needs to receive appropriate care, and 62% worry about being negatively judged by a health care provider because of their sexual orientation or gender identity, according to data collected by the Williams Institute and other organizations.
A lack of local rural providers knowledgeable in trans care can mean long drives to gender-affirming clinics in metropolitan areas. Rural trans people are three times as likely as are all transgender adults to travel 25-49 miles for routine care.
In Colorado, for example, many trans people outside Denver struggle to find proper care. Those who do have a trans-inclusive provider are more likely to receive wellness exams, less likely to delay care due to discrimination, and less likely to attempt suicide, according to results from the Colorado Transgender Health Survey published in 2018.
Much of the lack of care experienced by trans people is linked to insufficient education on LGBTQ+ health in medical schools across the country. In 2014, the Association of American Medical Colleges, which represents 170 accredited medical schools in the United States and Canada, released its first curriculum guidelines on caring for LGBTQ+ patients. As of 2018, 76% of medical schools included LGBTQ health themes in their curriculum, with half providing three or fewer classes on this topic.
Perhaps because of this, almost 77% of students from 10 medical schools in New England felt “not competent” or “somewhat not competent” in treating gender minority patients, according to a 2018 pilot study. Another paper, published last year, found that even clinicians who work in trans-friendly clinics lack knowledge about hormones, gender-affirming surgical options, and how to use appropriate pronouns and trans-inclusive language.
Throughout medical school, trans care was only briefly mentioned in endocrinology class, said Justin Bailey, MD, who received his medical degree from UAB in 2021 and is now a resident there. “I don’t want to say the wrong thing or use the wrong pronouns, so I was hesitant and a little bit tepid in my approach to interviewing and treating this population of patients,” he said.
On top of insufficient medical school education, some practicing doctors don’t take the time to teach themselves about trans people, said Kathie Moehlig, founder of TransFamily Support Services, a nonprofit organization that offers a range of services to transgender people and their families. They are very well intentioned yet uneducated when it comes to transgender care, she said.
Some medical schools, like the one at UAB, have pushed for change. Since 2017, Dr. Ladinsky and her colleagues have worked to include trans people in their standardized patient program, which gives medical students hands-on experience and feedback by interacting with “patients” in simulated clinical environments.
For example, a trans individual acting as a patient will simulate acid reflux by pretending to have pain in their stomach and chest. Then, over the course of the examination, they will reveal that they are transgender.
In the early years of this program, some students’ bedside manner would change once the patient’s gender identity was revealed, said Elaine Stephens, a trans woman who participates in UAB’s standardized patient program. “Sometimes they would immediately start asking about sexual activity,” Stephens said.
Since UAB launched its program, students’ reactions have improved significantly, she said.
This progress is being replicated by other medical schools, said Ms. Moehlig. “But it’s a slow start, and these are large institutions that take a long time to move forward.”
Advocates also are working outside medical schools to improve care in rural areas. In Colorado, the nonprofit Extension for Community Health Outcomes, or ECHO Colorado, has been offering monthly virtual classes on gender-affirming care to rural providers since 2020. The classes became so popular that the organization created a 4-week boot camp in 2021 for providers to learn about hormone therapy management, proper terminologies, surgical options, and supporting patients’ mental health.
For many years, doctors failed to recognize the need to learn about gender-affirming care, said Caroline Kirsch, DO, director of osteopathic education at the University of Wyoming Family Medicine Residency Program–Casper. In Casper, this led to “a number of patients traveling to Colorado to access care, which is a large burden for them financially,” said Dr. Kirsch, who has participated in the ECHO Colorado program.
“Things that haven’t been as well taught historically in medical school are things that I think many physicians feel anxious about initially,” she said. “The earlier you learn about this type of care in your career, the more likely you are to see its potential and be less anxious about it.”
Educating more providers about trans-related care has become increasingly vital in recent years as gender-affirming clinics nationwide experience a rise in harassment and threats. For instance, Vanderbilt University Medical Center’s Clinic for Transgender Health became the target of far-right hate on social media last year. After growing pressure from Tennessee’s Republican lawmakers, the clinic paused gender-affirmation surgeries on patients younger than 18, potentially leaving many trans individuals without necessary care.
Stephens hopes to see more medical schools include coursework on trans health care. She also wishes for doctors to treat trans people as they would any other patient.
“Just provide quality health care,” she tells the medical students at UAB. “We need health care like everyone else does.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Ospemifene and HT boost vaginal microbiome in vulvovaginal atrophy
The selective estrogen receptor modulator ospemifene appears to improve the vaginal microbiome of postmenopausal women with vulvovaginal atrophy (VVA), according to results from a small Italian case-control study in the journal Menopause.
The study sheds microbiological light on the mechanisms of ospemifene and low-dose systemic hormone therapy, which are widely used to treat genitourinary symptoms. Both had a positive effect on vaginal well-being, likely by reducing potentially harmful bacteria and increasing health-promoting acid-friendly microorganisms, writes a group led by M. Cristina Meriggiola, MD, PhD, of the gynecology and physiopathology of human reproduction unit at the University of Bologna, Italy.
VVA occurs in about 50% of postmenopausal women and produces a less favorable, less acidic vaginal microbiome profile than that of unaffected women. “The loss of estrogen leads to lower concentrations of Lactobacilli, bacteria that lower the pH. As a result, other bacterial species fill in the void,” explained Stephanie S. Faubion, MD, MBA, director of the Mayo Clinic Center for Women’s Health in Jacksonville, Fla., and medical director of the North American Menopause Society.
Added Tina Murphy, APN, a NAMS-certified menopause practitioner at Northwestern Medicine Orland Park in Illinois, “When this protective flora declines, then pathogenic bacteria can predominate the microbiome, which can contribute to vaginal irritation, infection, UTI’s, dyspareunia, and discomfort. Balancing and restoring the microbiome can mitigate the effects of estrogen depletion on the vaginal tissue and prevent the untoward effects of the hypoestrogenic state.” While ospemifene and hormone therapy are common therapies for the genitourinary symptoms of menopause, the focus has been on their treatment efficacy, not their effect on the microbiome profile, added Dr. Faubion. Only about 9% of women with menopause-related genitourinary symptoms receive prescription treatment, she added.
The study
Of 67 eligible postmenopausal participants in their mid-50s enrolled at a gynecology clinic from April 2019 to February 2020, 39 were diagnosed with VVA and 28 were considered healthy controls. In the atrophic group, 20 were prescribed ospemifene and 19 received hormone treatment.
Only those women with VVA but no menopausal vasomotor symptoms received ospemifene (60 mg/day); symptomatic women received hormone therapy according to guidelines.
The researchers calculated the women’s vaginal health index (VHI) based on elasticity, secretions, pH level, epithelial mucosa, and hydration. They used swabs to assess vaginal maturation index (VMI) by percentages of superficial, intermediate, and parabasal cells. Evaluation of the vaginal microbiome was done with 16S rRNA gene sequencing, and clinical and microbiological analyses were repeated after 3 months.
The vaginal microbiome of atrophic women was characterized by a significant reduction of benign Lactobacillus bacteria (P = .002) and an increase of potentially pathogenic Streptococcus (P = .008) and Sneathia (P = .02) bacteria.
The vaginal microbiome of women with VVA was depleted, within the Lactobacillus genus, in the L. crispatus species, a hallmark of vaginal health that has significant antimicrobial activity against endogenous and exogenous pathogens.
Furthermore, there was a positive correlation between the VHI/VMI and Lactobacillus abundance (P = .002 and P = 0.035, respectively).
While the lactic acid–producing Lactobacillus and Bifidobacterium genera were strongly associated with healthy controls, the characteristics of VVA patients were strongly associated with Streptococcus, Prevotella, Alloscardovia, and Staphylococcus.
Both therapeutic approaches effectively improved vaginal indices but by different routes. Systemic hormone treatment induced changes in minority bacterial groups in the vaginal microbiome, whereas ospemifene eliminated specific harmful bacterial taxa, such as Staphylococcus (P = .04) and Clostridium (P = .01). Both treatments induced a trend in the increase of beneficial Bifidobacteria.
A 2022 study reported that vaginal estradiol tablets significantly changed the vaginal microbiota in postmenopausal women compared with vaginal moisturizer or placebo, but the reductions in bothersome symptoms were similar.
The future
“Areas for future study include the assessment of changes in the vaginal microbiome, proteomic profiles, and immunologic markers with various treatments and the associations between these changes and genitourinary symptoms,” Dr. Faubion said. She added that, while there may be a role at some point for oral or topical probiotics, “Thus far, probiotics have not demonstrated significant benefits.”
Meanwhile, said Ms. Murphy, “There are many options available that may benefit our patients. As a provider, meeting with your patient, discussing her concerns and individual risk factors is the most important part of choosing the correct treatment plan.”
The authors call for further studies to confirm the observed modifications of the vaginal ecosystem. In the meantime, Dr. Meriggiola said in an interview, “My best advice to physicians is to ask women if they have this problem. Do not ignore it; be proactive and treat. There are many options on the market for genitourinary symptoms – not just for postmenopausal women but breast cancer survivors as well.”
Dr. Meriggiola’s group is planning to study ospemifene in cancer patients, whose quality of life is severely affected by VVA.
This study received no financial support. Dr. Meriggiola reported past financial relationships with Shionogi Limited, Teramex, Organon, Italfarmaco, MDS Italia, and Bayer. Coauthor Dr. Baldassarre disclosed past financial relationships with Shionogi. Ms. Murphy disclosed no relevant conflicts of interest with respect to her comments. Dr. Faubion is medical director of the North American Menopause Society and editor of the journal Menopause.
The selective estrogen receptor modulator ospemifene appears to improve the vaginal microbiome of postmenopausal women with vulvovaginal atrophy (VVA), according to results from a small Italian case-control study in the journal Menopause.
The study sheds microbiological light on the mechanisms of ospemifene and low-dose systemic hormone therapy, which are widely used to treat genitourinary symptoms. Both had a positive effect on vaginal well-being, likely by reducing potentially harmful bacteria and increasing health-promoting acid-friendly microorganisms, writes a group led by M. Cristina Meriggiola, MD, PhD, of the gynecology and physiopathology of human reproduction unit at the University of Bologna, Italy.
VVA occurs in about 50% of postmenopausal women and produces a less favorable, less acidic vaginal microbiome profile than that of unaffected women. “The loss of estrogen leads to lower concentrations of Lactobacilli, bacteria that lower the pH. As a result, other bacterial species fill in the void,” explained Stephanie S. Faubion, MD, MBA, director of the Mayo Clinic Center for Women’s Health in Jacksonville, Fla., and medical director of the North American Menopause Society.
Added Tina Murphy, APN, a NAMS-certified menopause practitioner at Northwestern Medicine Orland Park in Illinois, “When this protective flora declines, then pathogenic bacteria can predominate the microbiome, which can contribute to vaginal irritation, infection, UTI’s, dyspareunia, and discomfort. Balancing and restoring the microbiome can mitigate the effects of estrogen depletion on the vaginal tissue and prevent the untoward effects of the hypoestrogenic state.” While ospemifene and hormone therapy are common therapies for the genitourinary symptoms of menopause, the focus has been on their treatment efficacy, not their effect on the microbiome profile, added Dr. Faubion. Only about 9% of women with menopause-related genitourinary symptoms receive prescription treatment, she added.
The study
Of 67 eligible postmenopausal participants in their mid-50s enrolled at a gynecology clinic from April 2019 to February 2020, 39 were diagnosed with VVA and 28 were considered healthy controls. In the atrophic group, 20 were prescribed ospemifene and 19 received hormone treatment.
Only those women with VVA but no menopausal vasomotor symptoms received ospemifene (60 mg/day); symptomatic women received hormone therapy according to guidelines.
The researchers calculated the women’s vaginal health index (VHI) based on elasticity, secretions, pH level, epithelial mucosa, and hydration. They used swabs to assess vaginal maturation index (VMI) by percentages of superficial, intermediate, and parabasal cells. Evaluation of the vaginal microbiome was done with 16S rRNA gene sequencing, and clinical and microbiological analyses were repeated after 3 months.
The vaginal microbiome of atrophic women was characterized by a significant reduction of benign Lactobacillus bacteria (P = .002) and an increase of potentially pathogenic Streptococcus (P = .008) and Sneathia (P = .02) bacteria.
The vaginal microbiome of women with VVA was depleted, within the Lactobacillus genus, in the L. crispatus species, a hallmark of vaginal health that has significant antimicrobial activity against endogenous and exogenous pathogens.
Furthermore, there was a positive correlation between the VHI/VMI and Lactobacillus abundance (P = .002 and P = 0.035, respectively).
While the lactic acid–producing Lactobacillus and Bifidobacterium genera were strongly associated with healthy controls, the characteristics of VVA patients were strongly associated with Streptococcus, Prevotella, Alloscardovia, and Staphylococcus.
Both therapeutic approaches effectively improved vaginal indices but by different routes. Systemic hormone treatment induced changes in minority bacterial groups in the vaginal microbiome, whereas ospemifene eliminated specific harmful bacterial taxa, such as Staphylococcus (P = .04) and Clostridium (P = .01). Both treatments induced a trend in the increase of beneficial Bifidobacteria.
A 2022 study reported that vaginal estradiol tablets significantly changed the vaginal microbiota in postmenopausal women compared with vaginal moisturizer or placebo, but the reductions in bothersome symptoms were similar.
The future
“Areas for future study include the assessment of changes in the vaginal microbiome, proteomic profiles, and immunologic markers with various treatments and the associations between these changes and genitourinary symptoms,” Dr. Faubion said. She added that, while there may be a role at some point for oral or topical probiotics, “Thus far, probiotics have not demonstrated significant benefits.”
Meanwhile, said Ms. Murphy, “There are many options available that may benefit our patients. As a provider, meeting with your patient, discussing her concerns and individual risk factors is the most important part of choosing the correct treatment plan.”
The authors call for further studies to confirm the observed modifications of the vaginal ecosystem. In the meantime, Dr. Meriggiola said in an interview, “My best advice to physicians is to ask women if they have this problem. Do not ignore it; be proactive and treat. There are many options on the market for genitourinary symptoms – not just for postmenopausal women but breast cancer survivors as well.”
Dr. Meriggiola’s group is planning to study ospemifene in cancer patients, whose quality of life is severely affected by VVA.
This study received no financial support. Dr. Meriggiola reported past financial relationships with Shionogi Limited, Teramex, Organon, Italfarmaco, MDS Italia, and Bayer. Coauthor Dr. Baldassarre disclosed past financial relationships with Shionogi. Ms. Murphy disclosed no relevant conflicts of interest with respect to her comments. Dr. Faubion is medical director of the North American Menopause Society and editor of the journal Menopause.
The selective estrogen receptor modulator ospemifene appears to improve the vaginal microbiome of postmenopausal women with vulvovaginal atrophy (VVA), according to results from a small Italian case-control study in the journal Menopause.
The study sheds microbiological light on the mechanisms of ospemifene and low-dose systemic hormone therapy, which are widely used to treat genitourinary symptoms. Both had a positive effect on vaginal well-being, likely by reducing potentially harmful bacteria and increasing health-promoting acid-friendly microorganisms, writes a group led by M. Cristina Meriggiola, MD, PhD, of the gynecology and physiopathology of human reproduction unit at the University of Bologna, Italy.
VVA occurs in about 50% of postmenopausal women and produces a less favorable, less acidic vaginal microbiome profile than that of unaffected women. “The loss of estrogen leads to lower concentrations of Lactobacilli, bacteria that lower the pH. As a result, other bacterial species fill in the void,” explained Stephanie S. Faubion, MD, MBA, director of the Mayo Clinic Center for Women’s Health in Jacksonville, Fla., and medical director of the North American Menopause Society.
Added Tina Murphy, APN, a NAMS-certified menopause practitioner at Northwestern Medicine Orland Park in Illinois, “When this protective flora declines, then pathogenic bacteria can predominate the microbiome, which can contribute to vaginal irritation, infection, UTI’s, dyspareunia, and discomfort. Balancing and restoring the microbiome can mitigate the effects of estrogen depletion on the vaginal tissue and prevent the untoward effects of the hypoestrogenic state.” While ospemifene and hormone therapy are common therapies for the genitourinary symptoms of menopause, the focus has been on their treatment efficacy, not their effect on the microbiome profile, added Dr. Faubion. Only about 9% of women with menopause-related genitourinary symptoms receive prescription treatment, she added.
The study
Of 67 eligible postmenopausal participants in their mid-50s enrolled at a gynecology clinic from April 2019 to February 2020, 39 were diagnosed with VVA and 28 were considered healthy controls. In the atrophic group, 20 were prescribed ospemifene and 19 received hormone treatment.
Only those women with VVA but no menopausal vasomotor symptoms received ospemifene (60 mg/day); symptomatic women received hormone therapy according to guidelines.
The researchers calculated the women’s vaginal health index (VHI) based on elasticity, secretions, pH level, epithelial mucosa, and hydration. They used swabs to assess vaginal maturation index (VMI) by percentages of superficial, intermediate, and parabasal cells. Evaluation of the vaginal microbiome was done with 16S rRNA gene sequencing, and clinical and microbiological analyses were repeated after 3 months.
The vaginal microbiome of atrophic women was characterized by a significant reduction of benign Lactobacillus bacteria (P = .002) and an increase of potentially pathogenic Streptococcus (P = .008) and Sneathia (P = .02) bacteria.
The vaginal microbiome of women with VVA was depleted, within the Lactobacillus genus, in the L. crispatus species, a hallmark of vaginal health that has significant antimicrobial activity against endogenous and exogenous pathogens.
Furthermore, there was a positive correlation between the VHI/VMI and Lactobacillus abundance (P = .002 and P = 0.035, respectively).
While the lactic acid–producing Lactobacillus and Bifidobacterium genera were strongly associated with healthy controls, the characteristics of VVA patients were strongly associated with Streptococcus, Prevotella, Alloscardovia, and Staphylococcus.
Both therapeutic approaches effectively improved vaginal indices but by different routes. Systemic hormone treatment induced changes in minority bacterial groups in the vaginal microbiome, whereas ospemifene eliminated specific harmful bacterial taxa, such as Staphylococcus (P = .04) and Clostridium (P = .01). Both treatments induced a trend in the increase of beneficial Bifidobacteria.
A 2022 study reported that vaginal estradiol tablets significantly changed the vaginal microbiota in postmenopausal women compared with vaginal moisturizer or placebo, but the reductions in bothersome symptoms were similar.
The future
“Areas for future study include the assessment of changes in the vaginal microbiome, proteomic profiles, and immunologic markers with various treatments and the associations between these changes and genitourinary symptoms,” Dr. Faubion said. She added that, while there may be a role at some point for oral or topical probiotics, “Thus far, probiotics have not demonstrated significant benefits.”
Meanwhile, said Ms. Murphy, “There are many options available that may benefit our patients. As a provider, meeting with your patient, discussing her concerns and individual risk factors is the most important part of choosing the correct treatment plan.”
The authors call for further studies to confirm the observed modifications of the vaginal ecosystem. In the meantime, Dr. Meriggiola said in an interview, “My best advice to physicians is to ask women if they have this problem. Do not ignore it; be proactive and treat. There are many options on the market for genitourinary symptoms – not just for postmenopausal women but breast cancer survivors as well.”
Dr. Meriggiola’s group is planning to study ospemifene in cancer patients, whose quality of life is severely affected by VVA.
This study received no financial support. Dr. Meriggiola reported past financial relationships with Shionogi Limited, Teramex, Organon, Italfarmaco, MDS Italia, and Bayer. Coauthor Dr. Baldassarre disclosed past financial relationships with Shionogi. Ms. Murphy disclosed no relevant conflicts of interest with respect to her comments. Dr. Faubion is medical director of the North American Menopause Society and editor of the journal Menopause.
FROM MENOPAUSE
Update on secondary cytoreduction in recurrent ovarian cancer
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.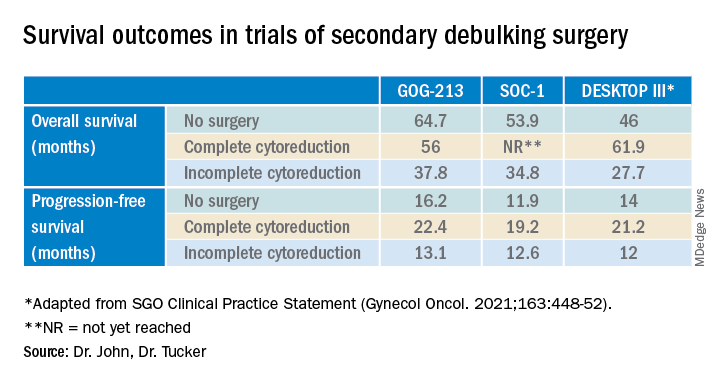
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Advances in fertility preservation: Q & A
From the first obscure reference until the 19th century, the maternal mortality rate from an ectopic pregnancy was nearly 100%. In the past 140 years, because of early detection and prompt surgical management, the mortality rate from an ectopic pregnancy declined from 72%-90% in 1880 to 0.48% from 2004 to 2008.1 Given this remarkable reduction in mortality, the 20th-century approach to ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing conservative treatment with methotrexate and/or tubal surgery.
Why the reference to ectopic pregnancy? Advances in oncology have comparably affected our approach to cancer patients. The increase in survival rates following a cancer diagnosis has fostered revolutionary developments in fertility preservation to obviate the effect of gonadotoxic therapy. We have evolved from shielding and transposing ovaries to ovarian tissue cryopreservation2,3 with rapid implementation.
One of the leaders in the field of female fertility preservation is Kutluk Oktay, MD, of Yale University, New Haven, Conn. I posed the following salient questions to him on the state of fertility preservation as well as expectations for the future.
Q1. What medication/treatment is gonadotoxic that warrants a consultation for fertility preservation?
A: While new drugs for cancer treatment continue to be approved and require testing for gonadotoxicity, evidence is clear on the damaging effects of alkylating agents such as cyclophosphamide, ifosfamide, chlorambucil, and melphalan on primordial follicle reserve.4 A useful tool to determine the risk of alkylating agents affecting fertility is the Cyclophosphamide Equivalent Dose (CED) Calculator. Likewise, topoisomerase inhibitors, such as doxorubicin4 induce ovarian reserve damage by causing double-strand DNA breaks (DSBs) in oocytes.5-7 Contrary to common belief, chemotherapy exposure suppresses the mechanisms that can initiate follicle growth.6 When DSBs occur, some oocytes may be able to repair such damage, otherwise apoptosis is triggered, which results in irreversible ovarian reserve loss.7 Younger individuals have much higher repair capacity, the magnitude of damage can be hard to predict, and it is variable.8,9 So, prior exposure to gonadotoxic drugs does not preclude consideration of fertility preservation.10
In addition, pelvic radiation, in a dose-dependent manner, causes severe DSBs and triggers the same cell suicide mechanisms while also potentially damaging uterine function. Additional information can be found in the American Society of Clinical Oncology Fertility Preservation Guidelines.4
Q2. What are the current options for fertility preservation in patients who will be exposed to gonadotoxic medication/treatment?
A: The current fertility preservation options for female patients faced with gonadotoxic treatments are embryo, oocyte, and ovarian tissue cryopreservation (OTC). Selection of fertility preservation is typically contingent upon the timetable of treatment. Oocyte and embryo cryopreservation have been the standard of care. Recently, OTC had its experimental designation removed by American Society for Reproductive Medicine11 with the advantage of not requiring ovarian stimulation or sexual maturity; and it may to be performed while patients are receiving chemotherapy. If successful, OTC followed by orthotopic transplantation has the potential to restore natural ovarian function, thereby allowing spontaneous conception.10 Especially in young adults, ovarian reserve loss is fractional and can remain at reasonable levels after a few courses of chemotherapy. Ovarian stimulation is risky after the initiation of chemotherapy because of the severe DNA damage to oocytes of developing follicles and the associated poor response.7 Hence, ovarian stimulation should be initiated and completed before the initiation of chemotherapy.
Q3. How successful are the approved fertility preservation options in obtaining oocytes for future utilization by ART?
A: We have decades of experience with embryo cryopreservation and proven success rates that patients can check on the SART.org website for individual clinics. For oocyte cryopreservation, models are used to provide calculation estimates because the technique is less established.12 Although success rates are approaching those with fresh oocytes, they are still not equal.13 OTC followed by orthotopic tissue transplantation has the least outcomes data (approximately 200 reported livebirths to date with a 25% live birth rate per recipient worldwide10 since the first success was reported in 2000.2,14
With our robotic surgical approach to orthotopic and heterotopic ovarian tissue transplantation and the utility of neovascularizing agents, we have found that ovarian graft longevity is extended. Oocytes/embryos can be obtained and has resulted in one to two livebirths in all our recipients to date.10 Unfortunately, if any of the critical steps are not up to standards (freezing, thawing, or transplantation), success rates can dramatically decline. Therefore, providers and patients should seek centers with experience in all three stages of this procedure to maximize outcomes.
Q4. Are there concerns of increasing recurrence/mortality with fertility preservation given hormonal exposure?
A: Yes, this concern exists, at least in theory for estrogen-sensitive cancers, most commonly breast cancer. We developed ovarian stimulation protocols supplemented with anti-estrogen treatments (tamoxifen, an estrogen-receptor antagonist, and letrozole, an aromatase inhibitor) that appear equally effective and reduce estrogen exposure in any susceptible cancer.15,16 Even in estrogen receptor–negative tumors, high estrogen exposure may activate non–estrogen receptor–dependent pathways. In addition, even those tumors that are practically deemed estrogen receptor negative may still contain a small percentage of estrogen receptors, which may become active at high estrogen levels.
Therefore, when we approach women with estrogen-sensitive cancers, e.g., breast and endometrial, we do not alter our approach based on receptor status. One exception occurs in women with BRCA mutations, especially the BRCA1, as they have 25% lower serum anti-müllerian hormone (AMH) levels,8,17 yield fewer oocytes in response to ovarian stimulation,18,19 and have lower fertilization rates and embryo numbers20 compared with those without the mutations.
Q5. Are all reproductive centers capable of offering fertility preservation? If not, how does a patient find a center?
A: All IVF clinics offer embryo and, presumably, oocyte cryopreservation. Pregnancy outcomes vary based on the center’s experience. Globally, major differences exist in the availability and competency of OTC along with the subsequent transplantation approach. A limited number of centers have competency in all aspects of OTC, i.e., cryopreservation, thawing, and transplantation. In general, fertility preservation patients have a multitude of medical issues that necessitate management expertise and the bandwidth to coordinate with cancer health professionals. The reproductive centers offering fertility preservation should be prepared to respond immediately and accommodate patients about to undergo gonadotoxic treatment.
Q6. How should a patient be counseled before proceeding with fertility preservation?
A: The candidate should be counseled on the likelihood of damage from gonadotoxic therapy and all fertility preservation options, on the basis of the urgency of treatment and the woman’s long-term goals. For example, the desire for a large family may compel a patient to undergo multiple cycles of ovarian stimulation or a combination of oocyte/embryo cryopreservation with OTC. In patients who are undergoing embryo cryopreservation, I recommend preimplantation genetic testing for aneuploidies, although there are limitations to its application. Other novel pieces of information we are using in counseling are baseline AMH levels and BRCA mutation status for women with breast cancer. In an 8-year-long NIH-funded prospective longitudinal study we found that women with both baseline AMH < 2 ng/mL and BRCA mutations are at significantly higher risk of losing their ovarian reserve and developing amenorrhea.21 Because the oocytes of women with BRCA mutations are deficient in DNA repair as we have previously shown,19 they are more liable to death upon exposure to DNA-damaging cancer drugs such as cyclophosphamide and doxorubicin.22
Q7. What is the time limit for use of cryopreserved oocytes/tissue?
A: Under optimal storage conditions, cryopreserved oocytes/tissue can be utilized indefinitely without a negative effect on pregnancy outcomes.
Q8. What does the future hold for fertility preservation?
A: The future holds promise for both the medical and nonmedical (planned) utility of fertility preservation. With the former, we will see that the utility of OTC and orthotopic and heterotopic tissue transplantation increase as success rates improve. Improved neovascularizing agents will make the transplants last longer and enhance pregnancy outcomes.23,24 I see planned fertility preservation increasing, based on the experience gained from cancer patients and some preliminary experience with planned OTC, especially for healthy women who wish to consider delaying menopause.25,26
Because of attrition from apoptosis, approximately 2,000 oocytes are wasted per ovulation. Through calculation models, we predict that if an equivalent of one-third of a woman’s ovarian cortex can be cryopreserved (which may not significantly affect the age at natural menopause) before age 40 years, transplantation at perimenopause may provide sufficient primordial follicles to delay menopause for 5 years or longer.26 Because ovarian tissue can also be transplanted subcutaneously under local anesthesia, as we have shown,27,28 repeated heterotopic transplants can be performed in an office setting at reduced cost, invasiveness, and with enhanced effectiveness. We can expect increasing reports and progress on this planned use of OTC and transplantation in the future.
Dr. Oktay is professor of obstetrics & gynecology and reproductive sciences and director of the Laboratory of Molecular Reproduction and Fertility Preservation at Yale University, New Haven, Conn. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Lurie S. Eur J Obstet Gynecol Reprod Biol. 1992 Jan 9;43(1):1-7.
2. Oktay K and Karlikaya G. N Engl J Med. 2000 Jun 22;342(25):1919.
3. Sonmezer and Oktay K. Hum Reprod Update. 2004;10(3):251-66.
4. Oktay K et al. J Clin Oncol. 2018 Jul 1;36(19):1994-2001.
5. Goldfarb SB et al. Breast Cancer Res Treat. 2021;185:165-73.
6. Titus S et al. Sci Rep. 2021 Jan 11;11(1):407.
7. Soleimani R et al. Aging (Albany NY). 2011 Aug;3(8):782-93.
8. Titus S et al. Sci Transl Med. 2013 Feb 13;5(172):172ra21.
9. Oktay KH et al. Fertil Steril. 2022 Jan 5:S0015-0282(21)02293-7.
10. Oktay K et al. Fertil Steril. 2022;117(1):181-92.
11. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2019;112(6):1022–33.
12. Cil A et al. Fertil Steril. 2013 Aug;100(2):492-9.e3.
13. Goldman KN et al. Fertil Steril. 2013 Sep;100(3):712-7.
14. Marin L and Oktay K. Scientific history of ovarian tissue cryopreservation and transplantation. In: Oktay K (ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:1-10.
15. Oktay K et al. J Clin Oncol. 2005 Jul 1;23(19):4347-53.
16. Kim JY et al. J Clin Endocrinol Metab. 2016 Apr;101(4):1364-71.
17. Turan V et al. J Clin Oncol. 2021;39:18.
18. Oktay K et al. J Clin Oncol. 2010 Jan 10;28(2):240-4.
19. Lin W et al. J Clin Endocrinol Metab. 2017;102(10):3839-47.
20. Turan V et al. Reprod Sci. 2018;(25):26-32.
21. Oktay K et al. Presence of BRCA mutations and a pre-chemotherapy AMH level of < 2ng/mL strongly predict risk of amenorrhea in women with breast cancer P-291. Presented at the American Society for Reproductive Medicine 78th annual meeting, Anaheim, Calif. Oct. 22-26, 2022.
22. Oktay KH et al. Fertil Steril. 2020;113(6):1251‐60.e1.
23. Soleimani R et al. PLoS One. 2011 Apr 29;6(4):e19475.
24. Marin L et al. Future aspects of ovarian cryopreservation and transplantation. In: Oktay K (ed.). Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier; 2022;223-30.
25. Oktay KH et al. Trends Mol Med. 2021;27(8):753-61.
26. Oktay K and Marin L. Ovarian tissue cryopreservation for delaying childbearing and menopause. In: Oktay, K. (Ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:195-204.
27. Oktay K et al. JAMA. 2001 Sep 26;286(12):1490-3.
28. Oktay K et al. Lancet. 2004 Mar 13;363(9412):837-40.
From the first obscure reference until the 19th century, the maternal mortality rate from an ectopic pregnancy was nearly 100%. In the past 140 years, because of early detection and prompt surgical management, the mortality rate from an ectopic pregnancy declined from 72%-90% in 1880 to 0.48% from 2004 to 2008.1 Given this remarkable reduction in mortality, the 20th-century approach to ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing conservative treatment with methotrexate and/or tubal surgery.
Why the reference to ectopic pregnancy? Advances in oncology have comparably affected our approach to cancer patients. The increase in survival rates following a cancer diagnosis has fostered revolutionary developments in fertility preservation to obviate the effect of gonadotoxic therapy. We have evolved from shielding and transposing ovaries to ovarian tissue cryopreservation2,3 with rapid implementation.
One of the leaders in the field of female fertility preservation is Kutluk Oktay, MD, of Yale University, New Haven, Conn. I posed the following salient questions to him on the state of fertility preservation as well as expectations for the future.
Q1. What medication/treatment is gonadotoxic that warrants a consultation for fertility preservation?
A: While new drugs for cancer treatment continue to be approved and require testing for gonadotoxicity, evidence is clear on the damaging effects of alkylating agents such as cyclophosphamide, ifosfamide, chlorambucil, and melphalan on primordial follicle reserve.4 A useful tool to determine the risk of alkylating agents affecting fertility is the Cyclophosphamide Equivalent Dose (CED) Calculator. Likewise, topoisomerase inhibitors, such as doxorubicin4 induce ovarian reserve damage by causing double-strand DNA breaks (DSBs) in oocytes.5-7 Contrary to common belief, chemotherapy exposure suppresses the mechanisms that can initiate follicle growth.6 When DSBs occur, some oocytes may be able to repair such damage, otherwise apoptosis is triggered, which results in irreversible ovarian reserve loss.7 Younger individuals have much higher repair capacity, the magnitude of damage can be hard to predict, and it is variable.8,9 So, prior exposure to gonadotoxic drugs does not preclude consideration of fertility preservation.10
In addition, pelvic radiation, in a dose-dependent manner, causes severe DSBs and triggers the same cell suicide mechanisms while also potentially damaging uterine function. Additional information can be found in the American Society of Clinical Oncology Fertility Preservation Guidelines.4
Q2. What are the current options for fertility preservation in patients who will be exposed to gonadotoxic medication/treatment?
A: The current fertility preservation options for female patients faced with gonadotoxic treatments are embryo, oocyte, and ovarian tissue cryopreservation (OTC). Selection of fertility preservation is typically contingent upon the timetable of treatment. Oocyte and embryo cryopreservation have been the standard of care. Recently, OTC had its experimental designation removed by American Society for Reproductive Medicine11 with the advantage of not requiring ovarian stimulation or sexual maturity; and it may to be performed while patients are receiving chemotherapy. If successful, OTC followed by orthotopic transplantation has the potential to restore natural ovarian function, thereby allowing spontaneous conception.10 Especially in young adults, ovarian reserve loss is fractional and can remain at reasonable levels after a few courses of chemotherapy. Ovarian stimulation is risky after the initiation of chemotherapy because of the severe DNA damage to oocytes of developing follicles and the associated poor response.7 Hence, ovarian stimulation should be initiated and completed before the initiation of chemotherapy.
Q3. How successful are the approved fertility preservation options in obtaining oocytes for future utilization by ART?
A: We have decades of experience with embryo cryopreservation and proven success rates that patients can check on the SART.org website for individual clinics. For oocyte cryopreservation, models are used to provide calculation estimates because the technique is less established.12 Although success rates are approaching those with fresh oocytes, they are still not equal.13 OTC followed by orthotopic tissue transplantation has the least outcomes data (approximately 200 reported livebirths to date with a 25% live birth rate per recipient worldwide10 since the first success was reported in 2000.2,14
With our robotic surgical approach to orthotopic and heterotopic ovarian tissue transplantation and the utility of neovascularizing agents, we have found that ovarian graft longevity is extended. Oocytes/embryos can be obtained and has resulted in one to two livebirths in all our recipients to date.10 Unfortunately, if any of the critical steps are not up to standards (freezing, thawing, or transplantation), success rates can dramatically decline. Therefore, providers and patients should seek centers with experience in all three stages of this procedure to maximize outcomes.
Q4. Are there concerns of increasing recurrence/mortality with fertility preservation given hormonal exposure?
A: Yes, this concern exists, at least in theory for estrogen-sensitive cancers, most commonly breast cancer. We developed ovarian stimulation protocols supplemented with anti-estrogen treatments (tamoxifen, an estrogen-receptor antagonist, and letrozole, an aromatase inhibitor) that appear equally effective and reduce estrogen exposure in any susceptible cancer.15,16 Even in estrogen receptor–negative tumors, high estrogen exposure may activate non–estrogen receptor–dependent pathways. In addition, even those tumors that are practically deemed estrogen receptor negative may still contain a small percentage of estrogen receptors, which may become active at high estrogen levels.
Therefore, when we approach women with estrogen-sensitive cancers, e.g., breast and endometrial, we do not alter our approach based on receptor status. One exception occurs in women with BRCA mutations, especially the BRCA1, as they have 25% lower serum anti-müllerian hormone (AMH) levels,8,17 yield fewer oocytes in response to ovarian stimulation,18,19 and have lower fertilization rates and embryo numbers20 compared with those without the mutations.
Q5. Are all reproductive centers capable of offering fertility preservation? If not, how does a patient find a center?
A: All IVF clinics offer embryo and, presumably, oocyte cryopreservation. Pregnancy outcomes vary based on the center’s experience. Globally, major differences exist in the availability and competency of OTC along with the subsequent transplantation approach. A limited number of centers have competency in all aspects of OTC, i.e., cryopreservation, thawing, and transplantation. In general, fertility preservation patients have a multitude of medical issues that necessitate management expertise and the bandwidth to coordinate with cancer health professionals. The reproductive centers offering fertility preservation should be prepared to respond immediately and accommodate patients about to undergo gonadotoxic treatment.
Q6. How should a patient be counseled before proceeding with fertility preservation?
A: The candidate should be counseled on the likelihood of damage from gonadotoxic therapy and all fertility preservation options, on the basis of the urgency of treatment and the woman’s long-term goals. For example, the desire for a large family may compel a patient to undergo multiple cycles of ovarian stimulation or a combination of oocyte/embryo cryopreservation with OTC. In patients who are undergoing embryo cryopreservation, I recommend preimplantation genetic testing for aneuploidies, although there are limitations to its application. Other novel pieces of information we are using in counseling are baseline AMH levels and BRCA mutation status for women with breast cancer. In an 8-year-long NIH-funded prospective longitudinal study we found that women with both baseline AMH < 2 ng/mL and BRCA mutations are at significantly higher risk of losing their ovarian reserve and developing amenorrhea.21 Because the oocytes of women with BRCA mutations are deficient in DNA repair as we have previously shown,19 they are more liable to death upon exposure to DNA-damaging cancer drugs such as cyclophosphamide and doxorubicin.22
Q7. What is the time limit for use of cryopreserved oocytes/tissue?
A: Under optimal storage conditions, cryopreserved oocytes/tissue can be utilized indefinitely without a negative effect on pregnancy outcomes.
Q8. What does the future hold for fertility preservation?
A: The future holds promise for both the medical and nonmedical (planned) utility of fertility preservation. With the former, we will see that the utility of OTC and orthotopic and heterotopic tissue transplantation increase as success rates improve. Improved neovascularizing agents will make the transplants last longer and enhance pregnancy outcomes.23,24 I see planned fertility preservation increasing, based on the experience gained from cancer patients and some preliminary experience with planned OTC, especially for healthy women who wish to consider delaying menopause.25,26
Because of attrition from apoptosis, approximately 2,000 oocytes are wasted per ovulation. Through calculation models, we predict that if an equivalent of one-third of a woman’s ovarian cortex can be cryopreserved (which may not significantly affect the age at natural menopause) before age 40 years, transplantation at perimenopause may provide sufficient primordial follicles to delay menopause for 5 years or longer.26 Because ovarian tissue can also be transplanted subcutaneously under local anesthesia, as we have shown,27,28 repeated heterotopic transplants can be performed in an office setting at reduced cost, invasiveness, and with enhanced effectiveness. We can expect increasing reports and progress on this planned use of OTC and transplantation in the future.
Dr. Oktay is professor of obstetrics & gynecology and reproductive sciences and director of the Laboratory of Molecular Reproduction and Fertility Preservation at Yale University, New Haven, Conn. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Lurie S. Eur J Obstet Gynecol Reprod Biol. 1992 Jan 9;43(1):1-7.
2. Oktay K and Karlikaya G. N Engl J Med. 2000 Jun 22;342(25):1919.
3. Sonmezer and Oktay K. Hum Reprod Update. 2004;10(3):251-66.
4. Oktay K et al. J Clin Oncol. 2018 Jul 1;36(19):1994-2001.
5. Goldfarb SB et al. Breast Cancer Res Treat. 2021;185:165-73.
6. Titus S et al. Sci Rep. 2021 Jan 11;11(1):407.
7. Soleimani R et al. Aging (Albany NY). 2011 Aug;3(8):782-93.
8. Titus S et al. Sci Transl Med. 2013 Feb 13;5(172):172ra21.
9. Oktay KH et al. Fertil Steril. 2022 Jan 5:S0015-0282(21)02293-7.
10. Oktay K et al. Fertil Steril. 2022;117(1):181-92.
11. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2019;112(6):1022–33.
12. Cil A et al. Fertil Steril. 2013 Aug;100(2):492-9.e3.
13. Goldman KN et al. Fertil Steril. 2013 Sep;100(3):712-7.
14. Marin L and Oktay K. Scientific history of ovarian tissue cryopreservation and transplantation. In: Oktay K (ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:1-10.
15. Oktay K et al. J Clin Oncol. 2005 Jul 1;23(19):4347-53.
16. Kim JY et al. J Clin Endocrinol Metab. 2016 Apr;101(4):1364-71.
17. Turan V et al. J Clin Oncol. 2021;39:18.
18. Oktay K et al. J Clin Oncol. 2010 Jan 10;28(2):240-4.
19. Lin W et al. J Clin Endocrinol Metab. 2017;102(10):3839-47.
20. Turan V et al. Reprod Sci. 2018;(25):26-32.
21. Oktay K et al. Presence of BRCA mutations and a pre-chemotherapy AMH level of < 2ng/mL strongly predict risk of amenorrhea in women with breast cancer P-291. Presented at the American Society for Reproductive Medicine 78th annual meeting, Anaheim, Calif. Oct. 22-26, 2022.
22. Oktay KH et al. Fertil Steril. 2020;113(6):1251‐60.e1.
23. Soleimani R et al. PLoS One. 2011 Apr 29;6(4):e19475.
24. Marin L et al. Future aspects of ovarian cryopreservation and transplantation. In: Oktay K (ed.). Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier; 2022;223-30.
25. Oktay KH et al. Trends Mol Med. 2021;27(8):753-61.
26. Oktay K and Marin L. Ovarian tissue cryopreservation for delaying childbearing and menopause. In: Oktay, K. (Ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:195-204.
27. Oktay K et al. JAMA. 2001 Sep 26;286(12):1490-3.
28. Oktay K et al. Lancet. 2004 Mar 13;363(9412):837-40.
From the first obscure reference until the 19th century, the maternal mortality rate from an ectopic pregnancy was nearly 100%. In the past 140 years, because of early detection and prompt surgical management, the mortality rate from an ectopic pregnancy declined from 72%-90% in 1880 to 0.48% from 2004 to 2008.1 Given this remarkable reduction in mortality, the 20th-century approach to ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing conservative treatment with methotrexate and/or tubal surgery.
Why the reference to ectopic pregnancy? Advances in oncology have comparably affected our approach to cancer patients. The increase in survival rates following a cancer diagnosis has fostered revolutionary developments in fertility preservation to obviate the effect of gonadotoxic therapy. We have evolved from shielding and transposing ovaries to ovarian tissue cryopreservation2,3 with rapid implementation.
One of the leaders in the field of female fertility preservation is Kutluk Oktay, MD, of Yale University, New Haven, Conn. I posed the following salient questions to him on the state of fertility preservation as well as expectations for the future.
Q1. What medication/treatment is gonadotoxic that warrants a consultation for fertility preservation?
A: While new drugs for cancer treatment continue to be approved and require testing for gonadotoxicity, evidence is clear on the damaging effects of alkylating agents such as cyclophosphamide, ifosfamide, chlorambucil, and melphalan on primordial follicle reserve.4 A useful tool to determine the risk of alkylating agents affecting fertility is the Cyclophosphamide Equivalent Dose (CED) Calculator. Likewise, topoisomerase inhibitors, such as doxorubicin4 induce ovarian reserve damage by causing double-strand DNA breaks (DSBs) in oocytes.5-7 Contrary to common belief, chemotherapy exposure suppresses the mechanisms that can initiate follicle growth.6 When DSBs occur, some oocytes may be able to repair such damage, otherwise apoptosis is triggered, which results in irreversible ovarian reserve loss.7 Younger individuals have much higher repair capacity, the magnitude of damage can be hard to predict, and it is variable.8,9 So, prior exposure to gonadotoxic drugs does not preclude consideration of fertility preservation.10
In addition, pelvic radiation, in a dose-dependent manner, causes severe DSBs and triggers the same cell suicide mechanisms while also potentially damaging uterine function. Additional information can be found in the American Society of Clinical Oncology Fertility Preservation Guidelines.4
Q2. What are the current options for fertility preservation in patients who will be exposed to gonadotoxic medication/treatment?
A: The current fertility preservation options for female patients faced with gonadotoxic treatments are embryo, oocyte, and ovarian tissue cryopreservation (OTC). Selection of fertility preservation is typically contingent upon the timetable of treatment. Oocyte and embryo cryopreservation have been the standard of care. Recently, OTC had its experimental designation removed by American Society for Reproductive Medicine11 with the advantage of not requiring ovarian stimulation or sexual maturity; and it may to be performed while patients are receiving chemotherapy. If successful, OTC followed by orthotopic transplantation has the potential to restore natural ovarian function, thereby allowing spontaneous conception.10 Especially in young adults, ovarian reserve loss is fractional and can remain at reasonable levels after a few courses of chemotherapy. Ovarian stimulation is risky after the initiation of chemotherapy because of the severe DNA damage to oocytes of developing follicles and the associated poor response.7 Hence, ovarian stimulation should be initiated and completed before the initiation of chemotherapy.
Q3. How successful are the approved fertility preservation options in obtaining oocytes for future utilization by ART?
A: We have decades of experience with embryo cryopreservation and proven success rates that patients can check on the SART.org website for individual clinics. For oocyte cryopreservation, models are used to provide calculation estimates because the technique is less established.12 Although success rates are approaching those with fresh oocytes, they are still not equal.13 OTC followed by orthotopic tissue transplantation has the least outcomes data (approximately 200 reported livebirths to date with a 25% live birth rate per recipient worldwide10 since the first success was reported in 2000.2,14
With our robotic surgical approach to orthotopic and heterotopic ovarian tissue transplantation and the utility of neovascularizing agents, we have found that ovarian graft longevity is extended. Oocytes/embryos can be obtained and has resulted in one to two livebirths in all our recipients to date.10 Unfortunately, if any of the critical steps are not up to standards (freezing, thawing, or transplantation), success rates can dramatically decline. Therefore, providers and patients should seek centers with experience in all three stages of this procedure to maximize outcomes.
Q4. Are there concerns of increasing recurrence/mortality with fertility preservation given hormonal exposure?
A: Yes, this concern exists, at least in theory for estrogen-sensitive cancers, most commonly breast cancer. We developed ovarian stimulation protocols supplemented with anti-estrogen treatments (tamoxifen, an estrogen-receptor antagonist, and letrozole, an aromatase inhibitor) that appear equally effective and reduce estrogen exposure in any susceptible cancer.15,16 Even in estrogen receptor–negative tumors, high estrogen exposure may activate non–estrogen receptor–dependent pathways. In addition, even those tumors that are practically deemed estrogen receptor negative may still contain a small percentage of estrogen receptors, which may become active at high estrogen levels.
Therefore, when we approach women with estrogen-sensitive cancers, e.g., breast and endometrial, we do not alter our approach based on receptor status. One exception occurs in women with BRCA mutations, especially the BRCA1, as they have 25% lower serum anti-müllerian hormone (AMH) levels,8,17 yield fewer oocytes in response to ovarian stimulation,18,19 and have lower fertilization rates and embryo numbers20 compared with those without the mutations.
Q5. Are all reproductive centers capable of offering fertility preservation? If not, how does a patient find a center?
A: All IVF clinics offer embryo and, presumably, oocyte cryopreservation. Pregnancy outcomes vary based on the center’s experience. Globally, major differences exist in the availability and competency of OTC along with the subsequent transplantation approach. A limited number of centers have competency in all aspects of OTC, i.e., cryopreservation, thawing, and transplantation. In general, fertility preservation patients have a multitude of medical issues that necessitate management expertise and the bandwidth to coordinate with cancer health professionals. The reproductive centers offering fertility preservation should be prepared to respond immediately and accommodate patients about to undergo gonadotoxic treatment.
Q6. How should a patient be counseled before proceeding with fertility preservation?
A: The candidate should be counseled on the likelihood of damage from gonadotoxic therapy and all fertility preservation options, on the basis of the urgency of treatment and the woman’s long-term goals. For example, the desire for a large family may compel a patient to undergo multiple cycles of ovarian stimulation or a combination of oocyte/embryo cryopreservation with OTC. In patients who are undergoing embryo cryopreservation, I recommend preimplantation genetic testing for aneuploidies, although there are limitations to its application. Other novel pieces of information we are using in counseling are baseline AMH levels and BRCA mutation status for women with breast cancer. In an 8-year-long NIH-funded prospective longitudinal study we found that women with both baseline AMH < 2 ng/mL and BRCA mutations are at significantly higher risk of losing their ovarian reserve and developing amenorrhea.21 Because the oocytes of women with BRCA mutations are deficient in DNA repair as we have previously shown,19 they are more liable to death upon exposure to DNA-damaging cancer drugs such as cyclophosphamide and doxorubicin.22
Q7. What is the time limit for use of cryopreserved oocytes/tissue?
A: Under optimal storage conditions, cryopreserved oocytes/tissue can be utilized indefinitely without a negative effect on pregnancy outcomes.
Q8. What does the future hold for fertility preservation?
A: The future holds promise for both the medical and nonmedical (planned) utility of fertility preservation. With the former, we will see that the utility of OTC and orthotopic and heterotopic tissue transplantation increase as success rates improve. Improved neovascularizing agents will make the transplants last longer and enhance pregnancy outcomes.23,24 I see planned fertility preservation increasing, based on the experience gained from cancer patients and some preliminary experience with planned OTC, especially for healthy women who wish to consider delaying menopause.25,26
Because of attrition from apoptosis, approximately 2,000 oocytes are wasted per ovulation. Through calculation models, we predict that if an equivalent of one-third of a woman’s ovarian cortex can be cryopreserved (which may not significantly affect the age at natural menopause) before age 40 years, transplantation at perimenopause may provide sufficient primordial follicles to delay menopause for 5 years or longer.26 Because ovarian tissue can also be transplanted subcutaneously under local anesthesia, as we have shown,27,28 repeated heterotopic transplants can be performed in an office setting at reduced cost, invasiveness, and with enhanced effectiveness. We can expect increasing reports and progress on this planned use of OTC and transplantation in the future.
Dr. Oktay is professor of obstetrics & gynecology and reproductive sciences and director of the Laboratory of Molecular Reproduction and Fertility Preservation at Yale University, New Haven, Conn. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Lurie S. Eur J Obstet Gynecol Reprod Biol. 1992 Jan 9;43(1):1-7.
2. Oktay K and Karlikaya G. N Engl J Med. 2000 Jun 22;342(25):1919.
3. Sonmezer and Oktay K. Hum Reprod Update. 2004;10(3):251-66.
4. Oktay K et al. J Clin Oncol. 2018 Jul 1;36(19):1994-2001.
5. Goldfarb SB et al. Breast Cancer Res Treat. 2021;185:165-73.
6. Titus S et al. Sci Rep. 2021 Jan 11;11(1):407.
7. Soleimani R et al. Aging (Albany NY). 2011 Aug;3(8):782-93.
8. Titus S et al. Sci Transl Med. 2013 Feb 13;5(172):172ra21.
9. Oktay KH et al. Fertil Steril. 2022 Jan 5:S0015-0282(21)02293-7.
10. Oktay K et al. Fertil Steril. 2022;117(1):181-92.
11. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2019;112(6):1022–33.
12. Cil A et al. Fertil Steril. 2013 Aug;100(2):492-9.e3.
13. Goldman KN et al. Fertil Steril. 2013 Sep;100(3):712-7.
14. Marin L and Oktay K. Scientific history of ovarian tissue cryopreservation and transplantation. In: Oktay K (ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:1-10.
15. Oktay K et al. J Clin Oncol. 2005 Jul 1;23(19):4347-53.
16. Kim JY et al. J Clin Endocrinol Metab. 2016 Apr;101(4):1364-71.
17. Turan V et al. J Clin Oncol. 2021;39:18.
18. Oktay K et al. J Clin Oncol. 2010 Jan 10;28(2):240-4.
19. Lin W et al. J Clin Endocrinol Metab. 2017;102(10):3839-47.
20. Turan V et al. Reprod Sci. 2018;(25):26-32.
21. Oktay K et al. Presence of BRCA mutations and a pre-chemotherapy AMH level of < 2ng/mL strongly predict risk of amenorrhea in women with breast cancer P-291. Presented at the American Society for Reproductive Medicine 78th annual meeting, Anaheim, Calif. Oct. 22-26, 2022.
22. Oktay KH et al. Fertil Steril. 2020;113(6):1251‐60.e1.
23. Soleimani R et al. PLoS One. 2011 Apr 29;6(4):e19475.
24. Marin L et al. Future aspects of ovarian cryopreservation and transplantation. In: Oktay K (ed.). Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier; 2022;223-30.
25. Oktay KH et al. Trends Mol Med. 2021;27(8):753-61.
26. Oktay K and Marin L. Ovarian tissue cryopreservation for delaying childbearing and menopause. In: Oktay, K. (Ed.), Principles and Practice of Ovarian Tissue Cryopreservation and Transplantation. Elsevier;2022:195-204.
27. Oktay K et al. JAMA. 2001 Sep 26;286(12):1490-3.
28. Oktay K et al. Lancet. 2004 Mar 13;363(9412):837-40.
Doctors’ happiness has not rebounded as pandemic drags on
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
A patient named ‘Settle’ decides to sue instead
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
Reversing abortion drug’s approval would harm public interest, FDA says
(Reuters) – President Joe Biden’s administration is urging a judge to reject a request by abortion opponents for a court order withdrawing federal approval for the drug used in medication abortions – which account for more than half of U.S. abortions – citing potential dangers to women seeking to end their pregnancies.
The U.S. Food and Drug Administration’s filing to U.S. District Judge Matthew Kacsmaryk, made available online on Tuesday, came in a lawsuit in Texas by antiabortion groups challenging the agency’s approval of the drug mifepristone in 2000 for medication abortion.
“The public interest would be dramatically harmed by effectively withdrawing from the marketplace a safe and effective drug that has lawfully been on the market for 22 years,” lawyers for the FDA said in the filing to Mr. Kacsmaryk, who is based in Amarillo.
Mifepristone is available under the brand name Mifeprex and as a generic. Used in conjunction with another drug, it is approved to terminate a pregnancy within the first 10 weeks of a pregnancy. The FDA on Jan. 3 said the government for the first time will allow mifepristone to be dispensed at retail pharmacies.
Medication abortion has drawn increasing attention since the U.S. Supreme Court last June overturned its landmark 1973 Roe v. Wade decision that had legalized abortion nationwide. Nearly all abortions, including medication abortions, are now banned in 12 states, and 16 states that permit some abortions also had laws restricting medication abortion as of November, according to the Guttmacher Institute, a research group that supports abortion rights.
“No abortion is safe, and chemical abortions are particularly dangerous,” said Julie Blake, senior counsel at the conservative legal group Alliance Defending Freedom, which represents the plaintiffs in the lawsuit. “The FDA, by approving chemical abortion drugs for home use, puts a woman or girl’s life at risk.”
The American College of Obstetricians and Gynecologists and the American Medical Association said in a joint letter to the Biden administration last June that “robust evidence exists regarding the safety of mifepristone for medication-induced abortion.”
Antiabortion groups including the Alliance for Hippocratic Medicine and the American Association of Pro-Life Obstetricians and Gynecologists sued the FDA in November, saying the agency improperly used an accelerated process to approve mifepristone and failed to study its risks for minors adequately.
In its court filing, the FDA said there was no basis for second-guessing the FDA’s judgment. The FDA said that pulling the drug would force patients seeking abortions in many cases to undergo unnecessary and more invasive surgical abortion. That would result in longer wait times and would carry risks for some patients including those intolerant to anesthesia, the FDA added.
In support of its position, the agency submitted declarations from abortion providers. For example, nonprofit Maine Family Planning said it would have to eliminate abortion services at 17 of its 18 clinics if mifepristone were no longer available.
Mifeprex maker Danco Laboratories on Friday also asked to intervene in the lawsuit to protect its ability to sell the drug.
A version of this article first appeared on Medscape.com.
(Reuters) – President Joe Biden’s administration is urging a judge to reject a request by abortion opponents for a court order withdrawing federal approval for the drug used in medication abortions – which account for more than half of U.S. abortions – citing potential dangers to women seeking to end their pregnancies.
The U.S. Food and Drug Administration’s filing to U.S. District Judge Matthew Kacsmaryk, made available online on Tuesday, came in a lawsuit in Texas by antiabortion groups challenging the agency’s approval of the drug mifepristone in 2000 for medication abortion.
“The public interest would be dramatically harmed by effectively withdrawing from the marketplace a safe and effective drug that has lawfully been on the market for 22 years,” lawyers for the FDA said in the filing to Mr. Kacsmaryk, who is based in Amarillo.
Mifepristone is available under the brand name Mifeprex and as a generic. Used in conjunction with another drug, it is approved to terminate a pregnancy within the first 10 weeks of a pregnancy. The FDA on Jan. 3 said the government for the first time will allow mifepristone to be dispensed at retail pharmacies.
Medication abortion has drawn increasing attention since the U.S. Supreme Court last June overturned its landmark 1973 Roe v. Wade decision that had legalized abortion nationwide. Nearly all abortions, including medication abortions, are now banned in 12 states, and 16 states that permit some abortions also had laws restricting medication abortion as of November, according to the Guttmacher Institute, a research group that supports abortion rights.
“No abortion is safe, and chemical abortions are particularly dangerous,” said Julie Blake, senior counsel at the conservative legal group Alliance Defending Freedom, which represents the plaintiffs in the lawsuit. “The FDA, by approving chemical abortion drugs for home use, puts a woman or girl’s life at risk.”
The American College of Obstetricians and Gynecologists and the American Medical Association said in a joint letter to the Biden administration last June that “robust evidence exists regarding the safety of mifepristone for medication-induced abortion.”
Antiabortion groups including the Alliance for Hippocratic Medicine and the American Association of Pro-Life Obstetricians and Gynecologists sued the FDA in November, saying the agency improperly used an accelerated process to approve mifepristone and failed to study its risks for minors adequately.
In its court filing, the FDA said there was no basis for second-guessing the FDA’s judgment. The FDA said that pulling the drug would force patients seeking abortions in many cases to undergo unnecessary and more invasive surgical abortion. That would result in longer wait times and would carry risks for some patients including those intolerant to anesthesia, the FDA added.
In support of its position, the agency submitted declarations from abortion providers. For example, nonprofit Maine Family Planning said it would have to eliminate abortion services at 17 of its 18 clinics if mifepristone were no longer available.
Mifeprex maker Danco Laboratories on Friday also asked to intervene in the lawsuit to protect its ability to sell the drug.
A version of this article first appeared on Medscape.com.
(Reuters) – President Joe Biden’s administration is urging a judge to reject a request by abortion opponents for a court order withdrawing federal approval for the drug used in medication abortions – which account for more than half of U.S. abortions – citing potential dangers to women seeking to end their pregnancies.
The U.S. Food and Drug Administration’s filing to U.S. District Judge Matthew Kacsmaryk, made available online on Tuesday, came in a lawsuit in Texas by antiabortion groups challenging the agency’s approval of the drug mifepristone in 2000 for medication abortion.
“The public interest would be dramatically harmed by effectively withdrawing from the marketplace a safe and effective drug that has lawfully been on the market for 22 years,” lawyers for the FDA said in the filing to Mr. Kacsmaryk, who is based in Amarillo.
Mifepristone is available under the brand name Mifeprex and as a generic. Used in conjunction with another drug, it is approved to terminate a pregnancy within the first 10 weeks of a pregnancy. The FDA on Jan. 3 said the government for the first time will allow mifepristone to be dispensed at retail pharmacies.
Medication abortion has drawn increasing attention since the U.S. Supreme Court last June overturned its landmark 1973 Roe v. Wade decision that had legalized abortion nationwide. Nearly all abortions, including medication abortions, are now banned in 12 states, and 16 states that permit some abortions also had laws restricting medication abortion as of November, according to the Guttmacher Institute, a research group that supports abortion rights.
“No abortion is safe, and chemical abortions are particularly dangerous,” said Julie Blake, senior counsel at the conservative legal group Alliance Defending Freedom, which represents the plaintiffs in the lawsuit. “The FDA, by approving chemical abortion drugs for home use, puts a woman or girl’s life at risk.”
The American College of Obstetricians and Gynecologists and the American Medical Association said in a joint letter to the Biden administration last June that “robust evidence exists regarding the safety of mifepristone for medication-induced abortion.”
Antiabortion groups including the Alliance for Hippocratic Medicine and the American Association of Pro-Life Obstetricians and Gynecologists sued the FDA in November, saying the agency improperly used an accelerated process to approve mifepristone and failed to study its risks for minors adequately.
In its court filing, the FDA said there was no basis for second-guessing the FDA’s judgment. The FDA said that pulling the drug would force patients seeking abortions in many cases to undergo unnecessary and more invasive surgical abortion. That would result in longer wait times and would carry risks for some patients including those intolerant to anesthesia, the FDA added.
In support of its position, the agency submitted declarations from abortion providers. For example, nonprofit Maine Family Planning said it would have to eliminate abortion services at 17 of its 18 clinics if mifepristone were no longer available.
Mifeprex maker Danco Laboratories on Friday also asked to intervene in the lawsuit to protect its ability to sell the drug.
A version of this article first appeared on Medscape.com.
How the Dobbs decision shapes the ObGyn workforce and training landscape

Six months after the Supreme Court decision that overturned the constitutional right to abortion, trainees across the United States are asking a critical question in the current resident recruitment season: How will the restrictions on abortion access affect my training as an obstetrician-gynecologist, and will they impact my ability to be the kind of provider I want to be in the future?
Among the myriad of downstream effects to patient care, the Dobbs decision will indisputably impact the scope of residency training for those that provide reproductive health services. Almost half of ObGyn residents train in states that have abortion restrictions in place.1 New educational milestones for abortion training, which are a requirement by the Accreditation Council for Graduate Medical Education (ACGME), were proposed quickly after Dobbs, guiding programs to offer opportunities for training in nonrestricted areas or the “combination of didactic activities, including simulation” to meet the training requirement in abortion care.2
Like many providers, residents already are grappling with precarious and risky circumstances, balancing patient safety and patient-driven care amidst pre-existing and newly enforced abortion restrictions. Whether managing a patient with an undesired pregnancy, severe medical comorbidities, unexpected pregnancy complications such as preterm premature rupture of membranes, or bleeding, or substantial fetal anomalies, ObGyn residents cannot gain the experience of providing the full scope of reproductive health care without the ability to offer all possible management options. While some enacted abortion restrictions have exceptions for the health of or life-saving measures for the mother, there is no standard guidance for timing of interventions, leaving providers confused and in fear of legal retribution. At a time when trainees should be learning to provide patient-centered, evidence-based care, they are instead paralyzed by the legal or professional consequences they may face for offering their best medical judgements.
Furthermore, the lack of exposure to dilation and evacuation procedures for residents in restricted practice areas will undoubtably decrease their confidence in managing acute complications, which is one of the critical facets of residency training. In a surgical field where repetition is crucial for technical competence, highlighted by ACGME minimum case requirements, the decreased volume of abortion procedures is a disadvantage for trainees and a disservice for patients. While anti-choice promoters may argue that involvement in surgical management of early pregnancy loss should suffice for ObGyn training in family planning, this piecemeal approach will leave gaps in technical skills.
The fear of legal ramifications, moral injury, and inadequate surgical training may lead to the siphoning of talented trainees to areas in the country with fewer restrictions.3 Dobbs already has demonstrated how limiting abortion access will deepen inequities in reproductive health care service delivery. Approximately 55% of ObGyn trainees and nearly two-thirds of maternal-fetal medicine graduates join the workforce in the state where they received their training.4 Medical students will seek opportunities for high-quality ObGyn training in areas that will help them to be well-prepared, competent physicians—and more often than not, stay in the area or region that they trained in. This will lead to provider shortages in areas where access to reproductive health care and subspecialist providers already is limited, further exacerbating existing health disparities.
During this recruitment season, trainees and residency programs alike will need to reckon with how the ramifications of Dobbs will alter both the immediate and long-term training in comprehensive reproductive health care for the ObGyn workforce. ObGyn trainees have taken a stand in response to the Dobbs decision, and nearly 750 current residents signed onto the statement below as a commitment to high-quality training and patient-centered care. Clinical experience in performing abortions is essential to the provision of comprehensive evidence-based reproductive health care, and access to these procedures is as important for physicians-in-training as it is for patients.
Actions to take to ensure high-quality abortion training in ObGyn residencies include the following:
- Connect with and stay involved with organizations such as the American College of Obstetricians and Gynecologists (ACOG), Physicians for Reproductive Health (PRH), and Medical Students for Choice (MSFC) for initiatives, toolkits, and resources for training at your institutions.
- Seek specific abortion training opportunities through the Leadership Training Academy (offered through PRH) or the Abortion Training Institute (offered through MSFC).
- Ensure that your residency program meets the ACGME criteria of providing opportunities for clinical experiences for abortion care and work with program leadership at a program, state, or regional level to enforce these competencies.
- Reach out to your local American Civil Liberties Union or other local reproductive legal rights organizations if you want to be involved with advocacy around abortion access and training but have concerns about legal protections.
- Have a voice at the table for empowering training opportunities by seeking leadership positions through ACOG, ACGME, Council on Resident Education in Obstetrics and Gynecology and the Association of Professors of Gynecology and Obstetrics, American Medical Association, Student National Medical Association, and subspecialty organizations.
- Vote in every election and promote voting registration and access to your patients, colleagues, and communities. ●
Continue to: The implications of the Dobbs v Jackson Women’s Health Organization decision on the health care and wellbeing of our patients...
On June 24, 2022, the Supreme Court of the United States ruled in a 6-3 majority decision to overturn the constitutional right to abortion protected by Roe v Wade since 1973. As health care providers, we are outraged at the Court’s disregard for an individual’s right to make reproductive decisions for themselves and their families and are deeply concerned about the devastating consequences to reproductive care and outcomes in this country for all people. Reproductive health decisions, including growing a family and whether or not to continue a pregnancy, are complex and incredibly personal. Our role as health care providers is to help guide those decisions with empathy and evidencebased clinical recommendations. This ruling undermines a patient’s right to bodily autonomy, free of impositions from government and political pressures, and it threatens the sanctity of complex medical decision-making between a patient, their family, and their medical team.
As medical professionals, we know that every patient’s situation is unique—banning abortion procedures ties the hands of physicians trying to provide the most medically appropriate options in a compassionate manner. We know that both medical and surgical abortions are safe and can save lives. These procedures can help patients with potentially life-threatening conditions worsened by pregnancy, a poor prognosis for the fetus, or a complication from the pregnancy itself. Physicians use scientific research and individualized approaches to help patients in unique situations, and attempts to legislate personal health decisions compromise the practice of evidence-based medicine.
We also know that this decision will impact some communities more than others. Access to safe abortion care will become dependent on which region of the country a person lives in and whether or not a person has resources to seek this care. Due to continued systemic racism and oppression, patients of color will be disproportionately impacted and likely will suffer worse health outcomes from unsafe abortions. Those that rely on public insurance or who are uninsured will face overwhelming barriers in seeking abortion services. These disparities in reproductive care, which contribute to our nation’s health crises in maternal morbidity and mortality, unintended pregnancy, and neonatal complications, will further entrench health inequities, and patient lives and livelihoods will suffer.
We acknowledge the impact that this decision will have on restricting access to reproductive care. We stand by the fact that abortion care is health care. We vow to uphold the tenets of our profession to place patient autonomy and provision of safe quality medical care at the forefront of our practices.
We, as health care providers and physician trainees, hereby pledge:
- To continue to provide evidence-based, nonjudgmental counseling for all pregnancy options, including abortion, and support our patients through all reproductive health decisions
- To promote equity in providing comprehensive reproductive health care, recognizing the impacts of systemic racism and oppression
- To promote high quality training in providing safe reproductive care in our respective institutions
- To use our voices in our communities to advocate for all our patients to have the freedom to access the safe and compassionate health care they deserve.
Sincerely,
The undersigned 747 ObGyn resident physicians
Please note that we sign this statement on our own behalf as individuals and not on behalf of our respective institutions.
Orchideh Abar, MD
Laurel S. Aberle, MD
Kathleen E. Ackert, DO
Lauryn Adams, MD
Temiloluwa Adejuyigbe, MD
Oluwatoyosi M. Adeoye, MD
Hufriya Y. Aderianwalla, MD
Fareeza Afzal, MD
Adelaide Agyepong, MD
Erin R. Ahart, MD
Noha T. Ahmed, DO
Faria Ahmed, MD
Tracey O. Akanbi, MD
Eloho E. Akpovi, MD
Austin H. Allen, DO
Amanda M. Allen, MD
Alexis L. Allihien, MD
Jorge L. Alsina, MD
Paulina C. Altshuler, DO
Sivani Aluru, MD
Amal Amir, DO
Jon Anderson, DO
Andreas Antono, MD
Annie N. Apple, MD
Janine Appleton, DO
Aarthi Arab, MD
Sydney R. Archer, MD
Youngeun C. Armbuster, MD
Kara Arnold, MD
Blessing C. Aroh, MD
Savannah Pearson Ayala, MD
Archana K. Ayyar, MD
Ann-Sophie Van Backle, DO
Connor R. Baker, MD
Japjot K. Bal, MD
Abigail E. Barger, MD
Kathryn E. Barron, MD
Silvia Bastea, MD
Samantha V.H. Bayer, MD
Kristen Beierwaltes, MD
Gisel Bello, MD
Michelle A. Benassai, MD
Dana Benyas, MD
Alice F. Berenson, MD
Hanna P. Berlin, MD
Abigail L. Bernard, MD
Eli H. Bernstein, MD
Julia T. Berry, MD
Bryce L. Beyer, MD
Caroline Bilbe, MD
Grace E. Binter, DO
Erin E. Bishop, MD
Sierra G. Bishop, MD
Stephanie S. Bista, MD
Tara E. Bjorklund, DO
Alyssa N. Black, MD
Continue to: Kelsey Boghean, DO...
Kelsey Boghean, DO
Areta Bojko, MD
Grace E. Bommarito, DO
Aditi R. Bommireddy, MD
Genna C. Bonfiglio, MD
Mary E. Booker, MD
Kayce L. Booth, MD
Samantha T. Boothe, DO
William Borenzweig, MD
Rebecca M. Borneman, MD
Alexander L. Boscia, MD
Gina M. Botsko, MD
Glenn P. Boyles, MD
Avery C. Bramnik, MD
Sophia N. Brancazio, MD
Katarina M. Braun, MD
Anthony Brausch, MD
Emily L. Brekke, MD
Sara E. Brenner, MD
Bailey A. Brown, DO
Kathryn S. Brown, MD
Denese C. Brown, MD
Abena Bruce, MD
Sabrina C. Brunozzi, MD
Madison Buchman, DO
Deirdre G. Buckley, MD
Rachel L. Budker, MD
Leeann M. Bui, MD
Anthony H. Bui, MD
Jessie Bujouves, MD
Kimberley A. Bullard, MD
Sophia G. Bunde, MD
Emily R. Burdette, MD
Iris Burgard, DO
Korbi M. Burkey, MD
Lindsey K. Burleson, MD
Lindsay M. Burton, MD
Brianna N. Byers, MD
Stephanie Cai, MD
Alexandra S. Calderon, MD
Alexandra G. Caldwell, MD
Natalia Calzada, MD
Tamara Cameo, MD
Arielle Caplin, MD
Angela M. Carracino, DO
Anna L. Carroll, MD
Leigha M. Carryl, MD
Ashlie S. Carter, MD
Stephanie Casey, DO
Chase W. Cataline, DO
Carson L. Catasus, MD
Alena R. Cave, MD
Kelly M. Chacon, MD
Avis L. Chan, MD
Shruthi Chandra, MD
Jennifer Chang, MD
Shannon Chang, DO
Gillian Chase, MD
Cindy Chen, MD
Jessie C. Chen, MD
Jessica T. Chen, MD
Wenjin Cheng, MB
Laura J. Cheng, MD
Lucy Cheng, MD
Monica S. Choo, MD
Jody S. Chou, MD
Hannah C. Christopher, DO
Continue to: David J. Chromey, DO...
David J. Chromey, DO
Grace V. Clark, MD
Celeste Colegrove, MD
Sarah C. Combs, MD
Victoria L. Conniff, MD
Hannah C. Connor, MD
Angela J. Conway, MD
Steffany A. Conyers, MD
Alexandra Cooke, MD
Ashley A. Cooney, MD
Anna Cornelius-Schecter, MD
Alexa M. Corso, DO
Krysten A. Costley, MD
Madeline Coulter, MD
Kelsey Cramer, MD
Anna E. Cronin, MD
Bethany N. Croyle, DO
Carmen A. Cueto, MD
Nicole Cumbo, MD
Mackenzie A. Cummings, MD
Carrie Cummiskey, MD
Hannah M. Cunningham, MD
Sarah D’Souza, DO
Rachael M. D’Auria, MD
Caitlin Dane, MD
Rachel N. Dang, MD
Talin R. Darian, MD
Abigail C. Davies, MD
Berkley Davis, MD
Lois A. Davis, MD
Jennie J. DeBlanc, MD
Ayana G.R. DeGaia, MD, MPH
Katerina N. DeHaan, MD
Rebekka M. Delgado, MD
Brettany C. DeMier, MD
Bonnie W. DePaso, MD
Hemaxi H. Desai, DO
Amberly T. Diep, MD
Abigail K. Dillaha, MD
Sarah K. Dominguez, MD
Abbey P. Donahue, MD
Allan C. Dong, MD
James Doss, MD
Taylor B. Douglas, MD
Abigail G. Downey, MD
Janelle M. Driscoll, MD
Emily Du, MD
Leslie V. Dunmire, MD
Jennifer Duong, DO
Leigh C. Durudogan, MD
Mai N. Dyer, MD, MPH
Rebecca A. Ebbott, MD
Lindsey P. Eck, MD
Molly C. Eckman, MD
Alex Ede, MD, ScM
Claire E. Edelman, MD
Sara E. Edwards, MD
David J. Eggert, DO
Michelle Eide, MD
Etoroabasi Ekpe, MD
Tressa L. Ellett, MD
Laura Peyton Ellis, MD
Kaitlin H. Ellis, MD
Mariah G. Elly, MD
Jennifer Embry, MD
Claire Englert, MD
Brenna Espelien, MD
Kamilah Evans, MD
Joshua A. Ewy, MD
Elana D. Fackler, MD
Lauren E. Falk, MD
Brianna A. Farley, MD
Amanda Stephanie R. Farrell, MD
Sara Fassio, DO
Daniela A. Febres-Cordero, MD
Jasmin E. Feliciano, MD
Alayna H. Feng, MD
Amanda M. Ferraro, MD
Brittany A. Fickau, MD
Brittany H. File, MD
Shannon M. Finner, DO
Mia E. Fischbein, DO
Briah Fischer, MD
Shira Fishbach, MD
Alison C. Fitzgerald, MD
Evan R. Fitzgerald, MD
Margaret R. Flanigan, MD
Kevin C. Flatley, MD
Jordan A. Fletcher, MD
Claudia E. Flores, MD
Lauren A. Forbes, MD
Rana K. Fowlkes, MD
Jennifer M. Franks, MD, MPH
Christina M. Frasik, MD
Haven N. Frazier, DO
Sarah W. Freeman, MD
Emilie O. Fromm, DO
Anna R. Fuchss, MD
Emma K. Gaboury, MD
Madeline H. Ganz, MD
Lex J. Gardner, MD
Keri-Lee Garel, MD
Hailey B. Gaskamp, DO
Brittney A. Gaudet, MD
Gabrielle M. Gear, MD
Eleanor R. Germano, MD
Lauren G. Gernon, MD
Allen Ghareeb, MD
Patricia Giglio Ayers, MD
Jordana L. Gilman, MD
Mianna M. Gilmore, DO
Brian W. Goddard, MD
Julia L. Goldberg, MD
M. Isabel Gonzaga, MD
Fred P. Gonzales, MD
Lillian H. Goodman, MD, MPH
Ashley Goreshnik, MD
Lauren E. Gottshall, MD
Lindsay L. Gould, MD
Kelsea R. Grant, MD
Dorender A. Gray, MD
Sophie Green, MD
Erica A. Green, MD
Danielle C. Greenberg, MD
Kalin J. Gregory-Davis, MD
David M. Greiner, MD
Tyler M. Gresham, MD
Continue to: Nelly Grigorian, MD...
Nelly Grigorian, MD
Erin L. Grimes, MD
Whitney Grither, MD
Jared M. Grootwassink, MD
Maya E. Gross, MD
Paoula Gueorguieva, MD
Margot M. Gurganus, DO
Rachel L. Gutfreund, MD
Andres Gutierrez, MD
Dorothy L. Hakimian, DO
Ashley N. Hamati, DO
Marie M. Hanna-Wagner, MD
Katie Hansen, MD
Courtney Hargreaves, MD
Stephanie Harlow, MD
Kelsey B. Harper, MD
Devon A. Harris, MD
Lauren E. Harris, MD
Emily S. Hart, DO
Sarah A. Hartley, MD
Becky K. Hartman, MD
Abigail K. Hartmann, MD
Charlotte V. Hastings, MD
Cherise Hatch, DO
Jordan Hauck, DO
Sarena Hayer, MD
Jenna M. Heath, MD
Eric D. Helm, MD
Julie A. Hemphill, MD
Ric A.S. Henderson, MD
Nicola A. Hendricks, MD
Andrea A. Henricks, MD
Jesse M. Herman, DO
Alyssa M. Hernandez, DO
Melissa Hernandez, MD
Alyssa R. Hersh, MD
Alexandra Herweck, MD
Brianna Hickey, MD
Allix M. Hillebrand, MD
Alessandra I. Hirsch, MD
Emily A. Hoffberg, MD
Chloe L. Holmes, DO
Cameron M. Holmes, MD
Helena Y. Hong, MD
Wakako Horiuchi, MD
Shweta Hosakoppal, MD
Jaycee E. Housh, MD
Shannon M. Howard, MD
Meredith C. Huszagh, MD
Yihharn P. Hwang, MD
Emma C. Hyde, MD
Brooke Hyman, MD
Hala Ali Ibrahim, MD
Gnendy Indig, MD
Erin E. Isaacson, MD
Shruti S. Iyer, DO
Audrey J. Jaeger, DO
Shobha Jagannatham, MD
Cyrus M. Jalai, MD
Emma V. James, MD
Isabel Janmey, MD
Phoebe Jen, DO
Corey L. Johnson, MD
Crystal J. Johnson, MD
Andrea M. Johnson, MD
Nat C. Jones, MD
Briana L. Jones, DO
Rebecca J. Josephson, MD
Sarah Natasha Jost-Haynes, MD
Continue to: Hannah S. Juhel, MD...
Hannah S. Juhel, MD
Erin Jun, DO
Katherine B. Kaak, MD
Dhara N. Kadakia, MD
Amanda D. Kadesh, MD
Riana K. Kahlon, MD
Nadi N. Kaonga, MD
Moli Karsalia, MD
Stephanie L. Kass, MD
Amanda M. Katz, MD
Chelsea S. Katz, MD
Virginia Kaufman, MD
Gurpinder Kaur, MD
Jessica A. Keesee, MD
Cassandra N. Kelly, MD
Whitney Kelly, DO
Hannah V. Kennedy, MD
Bethany H. Kette, MD
Iman Khan, MD
Maryam M. Khan, MD
Alisa Jion Kim, MD
Tesia G. Kim, MD
Anne E. Kim, MD
Emily H. King, MD
Tarynne E. Kinghorn, MD
Holly T. Kiper, DO
Thomas Kishkovich, MD
Quinn M. Kistenfeger, MD
Sofia E. Klar, DO
Jessica B. Klugman, MD
Hope E. Knochenhauer, MD
Kathleen J. Koenigs, MD
Olga Kontarovich, DO
Alison Kosmacki, MD
Ana E. Kouri, MD
Olga M. Kovalenko, MD
Leigh T. Kowalski, MD
Kayla A. Krajick, MD
Elizabeth S. Kravitz, MD
Shruti Rani Kumar, MD
Alyssa Kurtz, DO
Lauren H. Kus, MD
Arkadiy Kusayev, DO
Amanda E. Lacue, MD
Nava Lalehzari, MD
Amber Lalla, MD
Allie C. Lamari, DO
Kelly L. Lamiman, MD
Stephen Lammers, MD
Monet Lane, MD
Madeline L. Lang, MD
Liana Langdon-Embry, MD
Carolyn Larkins, MD
Leah E. Larson, MD
Matthew W. Lee, MD
Eunjae Lee, MD
Alice Lee, MD
Jared Z. Lee, MD
Charlotte M. Lee, MD
Nicole R. Legro, MD
Aurora Leibold, MD
Rosiris Leon-Rivera, MD, PhD
Anna M. Leone, MD
Keiko M. Leong, MD
Lindsey M. LePoidevin, MD
Molly E. Levine, MD
Khrystyna Levytska, MD
Dana L. Lewis, DO
Jessica L. Li, MD
Kristina Lilja, MD
Deanna M. Lines, DO
Annalise Littman, MD
Julia F. Liu, MD
Tyler B. Lloyd, MD
Alyssa Lo, MD
K’ara A. Locke, MD
Minica Long, MD
Melissa Lopez, MD
Wilfredo A. Lopez, MD
Connie F. Lu, MD
Tyler J. Lueck, MD
Katherine L. Lukas, MD
Davlyn L. Luke, MD
Shani Ma, MD
Colton Mabis, MD
Lauren T. MacNeill, MD
Rachel Madding, MD
Mona Makhamreh, MD
Francesca R. Mancuso, MD
Kelsey L. Manfredi, MD
Valeria Mantilla, MD
Kaitlin M. Mar, MD
Starcher R. Margaret, MD
Audrey M. Marinelli, MD
Brittany A. Marinelli, MD
Emily S. Markovic, MD
Hannah L. Marshall, MD
Aaron Masjedi, MD
Isabelle M. Mason, MD
Akailah T. Mason-Otey, MD
Nicole Massad, MD
Megan M. Masten, MD
Stephanie M. Masters, MD
Anastasia Matthews, MD
Natalia del Mazo, MD
Sara A. McAllaster, MD
Continue to: Nicole McAndrew, DO...
Nicole McAndrew, DO
Madeline G. McCosker, MD
Jamie L. McDowell, DO
Christine E. McGough, MD
Mackenzi R. McHugh, MD
Madeline M. McIntire, MD
Cynthia R. McKinney, MD
Kirsten D. McLane, MD
Shian F. McLeish, MD
Megan I. McNitt, MD
Sarah R. McShane, MD
Grace R. Meade, MD
Nikki Ann R. Medina, DO
Tiffany L. Mei, MD
Jenna Meiman, MD
Anna M. Melicher, MD
Rosa M. Mendez, MD
Riley Mickelsen, MD
Sage A. Mikami, MD
Aletheia B. Millien, MD
Hannah C. Milthorpe, MD
Caroline J. Min, MD
Julie A. Mina, MD
Annie G. Minns, MD
Natalie Mironov, DO
Elizabeth L. Mirsky, MD
Astha Mittal, MD
Rachel E. Mnuk, MD
Silki Modi, MD
Sudarshan J. Mohan, MD
Roxana Mohhebali-Solis, MD
Mugdha V. Mokashi, MD
Jessica A. Montgomery, MD
Ellen Moore, MD
Savannah J. Morehouse, MD
Kristen L. Moriarty, MD
Alexa P. Morrison, MD
Bijan Morshedi, MD
Matthew H. Mossayebi, MD
Kathy Mostajeran, DO
Sharan Mullen, DO
Ellen C. Murphy, MD
Emma Chew Murphy, MD
Lauren M. Murphy, MD
Bria Murray, MD
Erin C. Nacev, MD
Preetha Nandi, MD
Blaire E. Nasstrom, DO
Hallie N. Nelson, MD
Katherine A. Nelson, MD
Margaret S. Nemetz, MD
Daniela Ben Neriah, DO
Cosima M. Neumann, MD
Mollie H. Newbern, DO
Gisella M. Newbery, MD
Stephanie Nguyen, MD
Christine G.T. Nguyen, MD
Desiree Nguyen, MD
Jacqueline W. Nichols, MD
Annika M. Nilsen, MD
Margaret A. Nixon, MD
Emily M. Norkett, MD
Allison N. Nostrant, DO
Susan E. Nourse, MD
Aliya S. Nurani, MD
Emily E. Nuss, MD
Jeanne O. Nwagwu, DO
Kelsey E. O’Hagan, MD
Margaret O’Neill, MD
Emily A. O’Brien, MD
Carly M. O’Connor-Terry, MD, MS
Madison O. Odom, MD
Cynthia I. Okot-Kotber, MD
Sarah P. Oliver, MD
Leanne P. Ondreicka, MD
Ngozika G. Onyiuke, MD
Erika Gonzalez Osorio, MD
Marika L. Osterbur Badhey, MD
Linda A. Otieno, MD
Claire H. Packer, MD
Chloe W. Page, DO
Marissa Palmor, MD
Rishitha Panditi, MD
Katherine A. Panushka, MD
Kelsey J. Pape, MD
Rachel R. Paquette, DO
Hillary C. Park, DO
Kendall M. Parrott, MD
Ekta Partani, MD
Karishma Patel, MD
Shivani Patel, MD
Continue to: Priya Patel, MD...
Priya Patel, MD
Jenna M. Patterson, MD
Ashleigh Pavlovic, MD
Katie M. Peagler, MD
Katherine T. Pellino, MD
Nicholas Per, MD
Elana Perry, MD
Emily J. Peters, MD
Sara E. Peterson, MD
Michelle R. Petrich, MD
Destiny L. Phillips, MD
Chloe Phillips, MD
Megan E. Piacquadio, DO
Sara C. Pierpoint, MD
Celeste M. Pilato, MD
Emma Pindra, MD
Minerva L.R. Pineda, MD
Rebecca Pisan, MD
Alessandra R. Piscina, MD
Rachael Piver, MD
Andrew J. Polio, MD
Hector S. Porragas, MD
Natalie Posever, MD
Allison R. Powell, MD
Mahima V. Prasad, MD
Angelina D. Prat, DO
Rebecca L. Purvis, MD
Teresa L. Qi, MD
Nicholas R. Quam, MD
Candice A. Quarella, MD
Nicholas W. Racchi, DO
Jeannie G. Radoc, MD
Samuel Raine, MD
Anna C. Raines, MD
Stephanie A. Rains, MD
Nicole M. Rainville, DO
Karissa Rajagopal, DO
Kristian R. Ramage, MD
Praveen Ramesh, MD
Tia M. Ramirez, MD
Jania Ramos, MD
Neel K. Rana, MD
Urvi Rana, DO
Indira Ranaweera, MD
Sindhuja Ranganathan, DO
Chloe R. Rasmussen, MD
Laura P. Reguero-Cadilla, MD
Devin M. Reilly, MD
Kimberly E. Reimold, MD
Cory R. Reiter, MD, PhD
Maya E. Reuven, DO
Jessica Reyes-Peterson, MD
Jacqueline Rice, MD
Rebecca L. Richardson, MD
Mikaela J. Rico, DO
Katelyn Rittenhouse, MD
Giuliana A. Rivera Casul, MD
Jill N.T. Roberts, MD
Luke N. Roberts, MD
Esther Robin, MD
Marcella Israel Rocha, MD
Zoe A. Roecker, MD
Hilary E. Rogers, MD
Kelsey A. Roof, MD
Zarah Rosen, MD
Cecilia M. Rossi, MD
Eva S. Rostonics, MD
Felix Rubio, MD
Amela Rugova, MD
Anna J. Rujan, MD
Erika T. Russ, MD
Colin Russell, MD
Ruby L. Russell, MD
Isabella A. Sabatina, MD
Gouri Sadananda, MD
Aashna Saini, MD
Salomeh M. Salari, MD
Ndeye N. Sall, MD
Nicole M. Salvador, MD
Aayushi Sardana, MD
Kendall M. Sarson, MD
Rita Abigail Sartor, MD
Continue to: Haley A. Scarbrough, MD...
Haley A. Scarbrough, MD
Kimberly Schaefer, MD
Demetra Schermerhorn, MD
Ellen C. Schleckman, MD
Maura A. Schlussel, MD
Ellie Schmidt, MD
Alison M. Schmidt, MD
Evan A. Schrader, MD
Morgan A. Schriever, MD
Brianna L. Schumaker Nguyen, DO
Whitney E. Scott, MD
Claire Scrivani, MD
Catherine E. Seaman, MD
Rachel D. Seaman, MD
Danielle J. Seltzer, MD
Joshua R. Shaffer, MD
Emily A. Shaffer, MD
Delia S. Shash, MD
Ishana P. Shetty, MD
Tushar Shetty, MD
Carol Shi, MD
Sarah P. Shim, MD
Emma C. Siewert, MD
Seth M. Sigler, DO
Rebecca L. SigourneyTennyck, MD
Daniella D. Silvino, DO
Andrea M. Simi, MD
Amelia R. Simmons, MD
Amy E. Skeels, DO
Ashley E.S. Keith, MD
Hannah C. Smerker, DO
Katarina Smigoc, MD
Madeline I. Smith, MD
Jessica D. Smith, MD
Melanie R. Smith, MD
Alicia L. Smith, MD
Chloe Smith, MD
Ayanna Smith, MD
Melanie R. Smith, MD
Megan M. Smith, MD
Haverly J. Snyder, MD
Beatrice R. Soderholm, DO
Brianna C. Sohl, MD
Samantha A. Solaru, MD
Michael Solotke, MD
Dara A.H. Som, MD
Alexandra R. Sotiros-Lowry, MD
Melanie Spall, DO
Alicia C. Speak, DO
Lisa M. Spencer, MD
Prakrithi Srinand, MD
Sierra M. Starr, MD
Kathryne E. Staudinger, MD
Emily K. Steele, MD
Morgan R. Steffen, DO
Tricia R. Stepanek, MD
Taylor P. Stewart, MD
Kelsey A. Stewart, MD
Alyssa M. Stiff, MD
Alexandra B. Stiles, MD
Nairi K. Strauch, MD
Margaret J. Stroup, DO
Sean C. Stuart, DO
Hannah M. Stump, MD
Shalini B. Subbarao, MD
Lakshmi Subramani, MD
Heather E. Sweeney, MD
Kristin I. Swope, MD
Suha Syed, MD
Mireya P. Taboada, MD
Eneti S. Tagaloa, MD
Rachel Tang, DO
Adam R. Taylor, MD
Simone R. Thibault, MD
Kimberly A. Thill, MD
Dhanu Thiyag, MD
Andrew T. Thornton, MD
Wendy Tian, MD
Stephanie Tilberry, MD
Amanda L. Tillett, MD
Amanda M. Tjitro, MD
Logan P. Todhunter, DO
David Toffey, MD
Maris K. Toland, MD
Rachel E. Tomassi, MD
Sarah Tounsi, MD
Antonia K. Traina, MD
Taylor Tran, MD
Diem Samantha Tran, DO
Emily C. Trautner, MD
Emma Trawick, MD
Continue to: Elissa Trieu, MD...
Elissa Trieu, MD
Ariel Trilling, MD
Samantha Truong, MD
Mary M. Tsaturian, MD
Athena Tudino, MD
Kati A. Turner, MD
Nicole-Marie Tuzinkiewicz, MD
Gayathri D. Vadlamudi, MD
Stylianos Vagios, MD
Pauline V. Van Dijck, DO
Kaylee A. VanDommelen, MD
Isha B. Vasudeva, MD
Shivani J. Vasudeva, DO
Diana Q. Vazquez Parker, MD
Ridhima Vemula, MD
Elena C. Vinopal, MD
Caroline J. Violette, MD
Pascal T. Vo, DO
Michelle H. Vu, MD
Macy M. Walz, MD
Angelia Wang, MD
Eileen Wang, MD
Courtney Y. Wang, MD
Joyce Wang, MD
Meryl G. Warshafsky, MD
Sophie E.N. Weinstein, MD
Sarah H. Weinstein, MD
Annalyn M. Welp, MD
Shannon M. Wentworth, MD
Erika M. Wert, MD
Rachel C. White, MBchB
Morgan N. Wilhoite, DO
Mercedes Williams, MD
Hayley Williams, MD
Jacquelyn D. Williams, MD
Mary H. Williamson, MD
Elise Wilson, MD
Lauren M. Witchey, MD
Emily A. Wolverton, MD
Stephanie Y. Wong, MD
Jenny Wu, MD
Jackie Xiang, MD
Nancy S. Yang, MD
Kevin P. Yeagle, MD
Halina M. Yee, MD
Alyssa M. Yeung, MD
Samuel K. Yost, MD
Megan Yuen, MD
Nayab Zafar, DO
Cindy X. Zhang, DO
Yingao Zhang, MD
Helen Zhao, MD
Chelsea Zhu, MD
Billie E. Zidel, MD
Ryan A. Zoldowski, MD
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- ACGME program requirements for graduate medical education in obstetrics and gynecology summary and impact of interim requirement revisions. ACGME website. Accessed December 18, 2022. https://www.acgme.org/globalassets/pfassets/reviewandcomment/220_obstetricsandgynecology_2022-06-24_impact.pdf
- Crear-Perry J, Hassan A, Daniel S. Advancing birth equity in a post-Dobbs US. JAMA. 2022;328:1689-1690.
- Report on residents. AAMC website. Accessed December 18, 2022. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2021/table-c4-physician-reten tion-state-residency-training-last-completed-gme

Six months after the Supreme Court decision that overturned the constitutional right to abortion, trainees across the United States are asking a critical question in the current resident recruitment season: How will the restrictions on abortion access affect my training as an obstetrician-gynecologist, and will they impact my ability to be the kind of provider I want to be in the future?
Among the myriad of downstream effects to patient care, the Dobbs decision will indisputably impact the scope of residency training for those that provide reproductive health services. Almost half of ObGyn residents train in states that have abortion restrictions in place.1 New educational milestones for abortion training, which are a requirement by the Accreditation Council for Graduate Medical Education (ACGME), were proposed quickly after Dobbs, guiding programs to offer opportunities for training in nonrestricted areas or the “combination of didactic activities, including simulation” to meet the training requirement in abortion care.2
Like many providers, residents already are grappling with precarious and risky circumstances, balancing patient safety and patient-driven care amidst pre-existing and newly enforced abortion restrictions. Whether managing a patient with an undesired pregnancy, severe medical comorbidities, unexpected pregnancy complications such as preterm premature rupture of membranes, or bleeding, or substantial fetal anomalies, ObGyn residents cannot gain the experience of providing the full scope of reproductive health care without the ability to offer all possible management options. While some enacted abortion restrictions have exceptions for the health of or life-saving measures for the mother, there is no standard guidance for timing of interventions, leaving providers confused and in fear of legal retribution. At a time when trainees should be learning to provide patient-centered, evidence-based care, they are instead paralyzed by the legal or professional consequences they may face for offering their best medical judgements.
Furthermore, the lack of exposure to dilation and evacuation procedures for residents in restricted practice areas will undoubtably decrease their confidence in managing acute complications, which is one of the critical facets of residency training. In a surgical field where repetition is crucial for technical competence, highlighted by ACGME minimum case requirements, the decreased volume of abortion procedures is a disadvantage for trainees and a disservice for patients. While anti-choice promoters may argue that involvement in surgical management of early pregnancy loss should suffice for ObGyn training in family planning, this piecemeal approach will leave gaps in technical skills.
The fear of legal ramifications, moral injury, and inadequate surgical training may lead to the siphoning of talented trainees to areas in the country with fewer restrictions.3 Dobbs already has demonstrated how limiting abortion access will deepen inequities in reproductive health care service delivery. Approximately 55% of ObGyn trainees and nearly two-thirds of maternal-fetal medicine graduates join the workforce in the state where they received their training.4 Medical students will seek opportunities for high-quality ObGyn training in areas that will help them to be well-prepared, competent physicians—and more often than not, stay in the area or region that they trained in. This will lead to provider shortages in areas where access to reproductive health care and subspecialist providers already is limited, further exacerbating existing health disparities.
During this recruitment season, trainees and residency programs alike will need to reckon with how the ramifications of Dobbs will alter both the immediate and long-term training in comprehensive reproductive health care for the ObGyn workforce. ObGyn trainees have taken a stand in response to the Dobbs decision, and nearly 750 current residents signed onto the statement below as a commitment to high-quality training and patient-centered care. Clinical experience in performing abortions is essential to the provision of comprehensive evidence-based reproductive health care, and access to these procedures is as important for physicians-in-training as it is for patients.
Actions to take to ensure high-quality abortion training in ObGyn residencies include the following:
- Connect with and stay involved with organizations such as the American College of Obstetricians and Gynecologists (ACOG), Physicians for Reproductive Health (PRH), and Medical Students for Choice (MSFC) for initiatives, toolkits, and resources for training at your institutions.
- Seek specific abortion training opportunities through the Leadership Training Academy (offered through PRH) or the Abortion Training Institute (offered through MSFC).
- Ensure that your residency program meets the ACGME criteria of providing opportunities for clinical experiences for abortion care and work with program leadership at a program, state, or regional level to enforce these competencies.
- Reach out to your local American Civil Liberties Union or other local reproductive legal rights organizations if you want to be involved with advocacy around abortion access and training but have concerns about legal protections.
- Have a voice at the table for empowering training opportunities by seeking leadership positions through ACOG, ACGME, Council on Resident Education in Obstetrics and Gynecology and the Association of Professors of Gynecology and Obstetrics, American Medical Association, Student National Medical Association, and subspecialty organizations.
- Vote in every election and promote voting registration and access to your patients, colleagues, and communities. ●
Continue to: The implications of the Dobbs v Jackson Women’s Health Organization decision on the health care and wellbeing of our patients...
On June 24, 2022, the Supreme Court of the United States ruled in a 6-3 majority decision to overturn the constitutional right to abortion protected by Roe v Wade since 1973. As health care providers, we are outraged at the Court’s disregard for an individual’s right to make reproductive decisions for themselves and their families and are deeply concerned about the devastating consequences to reproductive care and outcomes in this country for all people. Reproductive health decisions, including growing a family and whether or not to continue a pregnancy, are complex and incredibly personal. Our role as health care providers is to help guide those decisions with empathy and evidencebased clinical recommendations. This ruling undermines a patient’s right to bodily autonomy, free of impositions from government and political pressures, and it threatens the sanctity of complex medical decision-making between a patient, their family, and their medical team.
As medical professionals, we know that every patient’s situation is unique—banning abortion procedures ties the hands of physicians trying to provide the most medically appropriate options in a compassionate manner. We know that both medical and surgical abortions are safe and can save lives. These procedures can help patients with potentially life-threatening conditions worsened by pregnancy, a poor prognosis for the fetus, or a complication from the pregnancy itself. Physicians use scientific research and individualized approaches to help patients in unique situations, and attempts to legislate personal health decisions compromise the practice of evidence-based medicine.
We also know that this decision will impact some communities more than others. Access to safe abortion care will become dependent on which region of the country a person lives in and whether or not a person has resources to seek this care. Due to continued systemic racism and oppression, patients of color will be disproportionately impacted and likely will suffer worse health outcomes from unsafe abortions. Those that rely on public insurance or who are uninsured will face overwhelming barriers in seeking abortion services. These disparities in reproductive care, which contribute to our nation’s health crises in maternal morbidity and mortality, unintended pregnancy, and neonatal complications, will further entrench health inequities, and patient lives and livelihoods will suffer.
We acknowledge the impact that this decision will have on restricting access to reproductive care. We stand by the fact that abortion care is health care. We vow to uphold the tenets of our profession to place patient autonomy and provision of safe quality medical care at the forefront of our practices.
We, as health care providers and physician trainees, hereby pledge:
- To continue to provide evidence-based, nonjudgmental counseling for all pregnancy options, including abortion, and support our patients through all reproductive health decisions
- To promote equity in providing comprehensive reproductive health care, recognizing the impacts of systemic racism and oppression
- To promote high quality training in providing safe reproductive care in our respective institutions
- To use our voices in our communities to advocate for all our patients to have the freedom to access the safe and compassionate health care they deserve.
Sincerely,
The undersigned 747 ObGyn resident physicians
Please note that we sign this statement on our own behalf as individuals and not on behalf of our respective institutions.
Orchideh Abar, MD
Laurel S. Aberle, MD
Kathleen E. Ackert, DO
Lauryn Adams, MD
Temiloluwa Adejuyigbe, MD
Oluwatoyosi M. Adeoye, MD
Hufriya Y. Aderianwalla, MD
Fareeza Afzal, MD
Adelaide Agyepong, MD
Erin R. Ahart, MD
Noha T. Ahmed, DO
Faria Ahmed, MD
Tracey O. Akanbi, MD
Eloho E. Akpovi, MD
Austin H. Allen, DO
Amanda M. Allen, MD
Alexis L. Allihien, MD
Jorge L. Alsina, MD
Paulina C. Altshuler, DO
Sivani Aluru, MD
Amal Amir, DO
Jon Anderson, DO
Andreas Antono, MD
Annie N. Apple, MD
Janine Appleton, DO
Aarthi Arab, MD
Sydney R. Archer, MD
Youngeun C. Armbuster, MD
Kara Arnold, MD
Blessing C. Aroh, MD
Savannah Pearson Ayala, MD
Archana K. Ayyar, MD
Ann-Sophie Van Backle, DO
Connor R. Baker, MD
Japjot K. Bal, MD
Abigail E. Barger, MD
Kathryn E. Barron, MD
Silvia Bastea, MD
Samantha V.H. Bayer, MD
Kristen Beierwaltes, MD
Gisel Bello, MD
Michelle A. Benassai, MD
Dana Benyas, MD
Alice F. Berenson, MD
Hanna P. Berlin, MD
Abigail L. Bernard, MD
Eli H. Bernstein, MD
Julia T. Berry, MD
Bryce L. Beyer, MD
Caroline Bilbe, MD
Grace E. Binter, DO
Erin E. Bishop, MD
Sierra G. Bishop, MD
Stephanie S. Bista, MD
Tara E. Bjorklund, DO
Alyssa N. Black, MD
Continue to: Kelsey Boghean, DO...
Kelsey Boghean, DO
Areta Bojko, MD
Grace E. Bommarito, DO
Aditi R. Bommireddy, MD
Genna C. Bonfiglio, MD
Mary E. Booker, MD
Kayce L. Booth, MD
Samantha T. Boothe, DO
William Borenzweig, MD
Rebecca M. Borneman, MD
Alexander L. Boscia, MD
Gina M. Botsko, MD
Glenn P. Boyles, MD
Avery C. Bramnik, MD
Sophia N. Brancazio, MD
Katarina M. Braun, MD
Anthony Brausch, MD
Emily L. Brekke, MD
Sara E. Brenner, MD
Bailey A. Brown, DO
Kathryn S. Brown, MD
Denese C. Brown, MD
Abena Bruce, MD
Sabrina C. Brunozzi, MD
Madison Buchman, DO
Deirdre G. Buckley, MD
Rachel L. Budker, MD
Leeann M. Bui, MD
Anthony H. Bui, MD
Jessie Bujouves, MD
Kimberley A. Bullard, MD
Sophia G. Bunde, MD
Emily R. Burdette, MD
Iris Burgard, DO
Korbi M. Burkey, MD
Lindsey K. Burleson, MD
Lindsay M. Burton, MD
Brianna N. Byers, MD
Stephanie Cai, MD
Alexandra S. Calderon, MD
Alexandra G. Caldwell, MD
Natalia Calzada, MD
Tamara Cameo, MD
Arielle Caplin, MD
Angela M. Carracino, DO
Anna L. Carroll, MD
Leigha M. Carryl, MD
Ashlie S. Carter, MD
Stephanie Casey, DO
Chase W. Cataline, DO
Carson L. Catasus, MD
Alena R. Cave, MD
Kelly M. Chacon, MD
Avis L. Chan, MD
Shruthi Chandra, MD
Jennifer Chang, MD
Shannon Chang, DO
Gillian Chase, MD
Cindy Chen, MD
Jessie C. Chen, MD
Jessica T. Chen, MD
Wenjin Cheng, MB
Laura J. Cheng, MD
Lucy Cheng, MD
Monica S. Choo, MD
Jody S. Chou, MD
Hannah C. Christopher, DO
Continue to: David J. Chromey, DO...
David J. Chromey, DO
Grace V. Clark, MD
Celeste Colegrove, MD
Sarah C. Combs, MD
Victoria L. Conniff, MD
Hannah C. Connor, MD
Angela J. Conway, MD
Steffany A. Conyers, MD
Alexandra Cooke, MD
Ashley A. Cooney, MD
Anna Cornelius-Schecter, MD
Alexa M. Corso, DO
Krysten A. Costley, MD
Madeline Coulter, MD
Kelsey Cramer, MD
Anna E. Cronin, MD
Bethany N. Croyle, DO
Carmen A. Cueto, MD
Nicole Cumbo, MD
Mackenzie A. Cummings, MD
Carrie Cummiskey, MD
Hannah M. Cunningham, MD
Sarah D’Souza, DO
Rachael M. D’Auria, MD
Caitlin Dane, MD
Rachel N. Dang, MD
Talin R. Darian, MD
Abigail C. Davies, MD
Berkley Davis, MD
Lois A. Davis, MD
Jennie J. DeBlanc, MD
Ayana G.R. DeGaia, MD, MPH
Katerina N. DeHaan, MD
Rebekka M. Delgado, MD
Brettany C. DeMier, MD
Bonnie W. DePaso, MD
Hemaxi H. Desai, DO
Amberly T. Diep, MD
Abigail K. Dillaha, MD
Sarah K. Dominguez, MD
Abbey P. Donahue, MD
Allan C. Dong, MD
James Doss, MD
Taylor B. Douglas, MD
Abigail G. Downey, MD
Janelle M. Driscoll, MD
Emily Du, MD
Leslie V. Dunmire, MD
Jennifer Duong, DO
Leigh C. Durudogan, MD
Mai N. Dyer, MD, MPH
Rebecca A. Ebbott, MD
Lindsey P. Eck, MD
Molly C. Eckman, MD
Alex Ede, MD, ScM
Claire E. Edelman, MD
Sara E. Edwards, MD
David J. Eggert, DO
Michelle Eide, MD
Etoroabasi Ekpe, MD
Tressa L. Ellett, MD
Laura Peyton Ellis, MD
Kaitlin H. Ellis, MD
Mariah G. Elly, MD
Jennifer Embry, MD
Claire Englert, MD
Brenna Espelien, MD
Kamilah Evans, MD
Joshua A. Ewy, MD
Elana D. Fackler, MD
Lauren E. Falk, MD
Brianna A. Farley, MD
Amanda Stephanie R. Farrell, MD
Sara Fassio, DO
Daniela A. Febres-Cordero, MD
Jasmin E. Feliciano, MD
Alayna H. Feng, MD
Amanda M. Ferraro, MD
Brittany A. Fickau, MD
Brittany H. File, MD
Shannon M. Finner, DO
Mia E. Fischbein, DO
Briah Fischer, MD
Shira Fishbach, MD
Alison C. Fitzgerald, MD
Evan R. Fitzgerald, MD
Margaret R. Flanigan, MD
Kevin C. Flatley, MD
Jordan A. Fletcher, MD
Claudia E. Flores, MD
Lauren A. Forbes, MD
Rana K. Fowlkes, MD
Jennifer M. Franks, MD, MPH
Christina M. Frasik, MD
Haven N. Frazier, DO
Sarah W. Freeman, MD
Emilie O. Fromm, DO
Anna R. Fuchss, MD
Emma K. Gaboury, MD
Madeline H. Ganz, MD
Lex J. Gardner, MD
Keri-Lee Garel, MD
Hailey B. Gaskamp, DO
Brittney A. Gaudet, MD
Gabrielle M. Gear, MD
Eleanor R. Germano, MD
Lauren G. Gernon, MD
Allen Ghareeb, MD
Patricia Giglio Ayers, MD
Jordana L. Gilman, MD
Mianna M. Gilmore, DO
Brian W. Goddard, MD
Julia L. Goldberg, MD
M. Isabel Gonzaga, MD
Fred P. Gonzales, MD
Lillian H. Goodman, MD, MPH
Ashley Goreshnik, MD
Lauren E. Gottshall, MD
Lindsay L. Gould, MD
Kelsea R. Grant, MD
Dorender A. Gray, MD
Sophie Green, MD
Erica A. Green, MD
Danielle C. Greenberg, MD
Kalin J. Gregory-Davis, MD
David M. Greiner, MD
Tyler M. Gresham, MD
Continue to: Nelly Grigorian, MD...
Nelly Grigorian, MD
Erin L. Grimes, MD
Whitney Grither, MD
Jared M. Grootwassink, MD
Maya E. Gross, MD
Paoula Gueorguieva, MD
Margot M. Gurganus, DO
Rachel L. Gutfreund, MD
Andres Gutierrez, MD
Dorothy L. Hakimian, DO
Ashley N. Hamati, DO
Marie M. Hanna-Wagner, MD
Katie Hansen, MD
Courtney Hargreaves, MD
Stephanie Harlow, MD
Kelsey B. Harper, MD
Devon A. Harris, MD
Lauren E. Harris, MD
Emily S. Hart, DO
Sarah A. Hartley, MD
Becky K. Hartman, MD
Abigail K. Hartmann, MD
Charlotte V. Hastings, MD
Cherise Hatch, DO
Jordan Hauck, DO
Sarena Hayer, MD
Jenna M. Heath, MD
Eric D. Helm, MD
Julie A. Hemphill, MD
Ric A.S. Henderson, MD
Nicola A. Hendricks, MD
Andrea A. Henricks, MD
Jesse M. Herman, DO
Alyssa M. Hernandez, DO
Melissa Hernandez, MD
Alyssa R. Hersh, MD
Alexandra Herweck, MD
Brianna Hickey, MD
Allix M. Hillebrand, MD
Alessandra I. Hirsch, MD
Emily A. Hoffberg, MD
Chloe L. Holmes, DO
Cameron M. Holmes, MD
Helena Y. Hong, MD
Wakako Horiuchi, MD
Shweta Hosakoppal, MD
Jaycee E. Housh, MD
Shannon M. Howard, MD
Meredith C. Huszagh, MD
Yihharn P. Hwang, MD
Emma C. Hyde, MD
Brooke Hyman, MD
Hala Ali Ibrahim, MD
Gnendy Indig, MD
Erin E. Isaacson, MD
Shruti S. Iyer, DO
Audrey J. Jaeger, DO
Shobha Jagannatham, MD
Cyrus M. Jalai, MD
Emma V. James, MD
Isabel Janmey, MD
Phoebe Jen, DO
Corey L. Johnson, MD
Crystal J. Johnson, MD
Andrea M. Johnson, MD
Nat C. Jones, MD
Briana L. Jones, DO
Rebecca J. Josephson, MD
Sarah Natasha Jost-Haynes, MD
Continue to: Hannah S. Juhel, MD...
Hannah S. Juhel, MD
Erin Jun, DO
Katherine B. Kaak, MD
Dhara N. Kadakia, MD
Amanda D. Kadesh, MD
Riana K. Kahlon, MD
Nadi N. Kaonga, MD
Moli Karsalia, MD
Stephanie L. Kass, MD
Amanda M. Katz, MD
Chelsea S. Katz, MD
Virginia Kaufman, MD
Gurpinder Kaur, MD
Jessica A. Keesee, MD
Cassandra N. Kelly, MD
Whitney Kelly, DO
Hannah V. Kennedy, MD
Bethany H. Kette, MD
Iman Khan, MD
Maryam M. Khan, MD
Alisa Jion Kim, MD
Tesia G. Kim, MD
Anne E. Kim, MD
Emily H. King, MD
Tarynne E. Kinghorn, MD
Holly T. Kiper, DO
Thomas Kishkovich, MD
Quinn M. Kistenfeger, MD
Sofia E. Klar, DO
Jessica B. Klugman, MD
Hope E. Knochenhauer, MD
Kathleen J. Koenigs, MD
Olga Kontarovich, DO
Alison Kosmacki, MD
Ana E. Kouri, MD
Olga M. Kovalenko, MD
Leigh T. Kowalski, MD
Kayla A. Krajick, MD
Elizabeth S. Kravitz, MD
Shruti Rani Kumar, MD
Alyssa Kurtz, DO
Lauren H. Kus, MD
Arkadiy Kusayev, DO
Amanda E. Lacue, MD
Nava Lalehzari, MD
Amber Lalla, MD
Allie C. Lamari, DO
Kelly L. Lamiman, MD
Stephen Lammers, MD
Monet Lane, MD
Madeline L. Lang, MD
Liana Langdon-Embry, MD
Carolyn Larkins, MD
Leah E. Larson, MD
Matthew W. Lee, MD
Eunjae Lee, MD
Alice Lee, MD
Jared Z. Lee, MD
Charlotte M. Lee, MD
Nicole R. Legro, MD
Aurora Leibold, MD
Rosiris Leon-Rivera, MD, PhD
Anna M. Leone, MD
Keiko M. Leong, MD
Lindsey M. LePoidevin, MD
Molly E. Levine, MD
Khrystyna Levytska, MD
Dana L. Lewis, DO
Jessica L. Li, MD
Kristina Lilja, MD
Deanna M. Lines, DO
Annalise Littman, MD
Julia F. Liu, MD
Tyler B. Lloyd, MD
Alyssa Lo, MD
K’ara A. Locke, MD
Minica Long, MD
Melissa Lopez, MD
Wilfredo A. Lopez, MD
Connie F. Lu, MD
Tyler J. Lueck, MD
Katherine L. Lukas, MD
Davlyn L. Luke, MD
Shani Ma, MD
Colton Mabis, MD
Lauren T. MacNeill, MD
Rachel Madding, MD
Mona Makhamreh, MD
Francesca R. Mancuso, MD
Kelsey L. Manfredi, MD
Valeria Mantilla, MD
Kaitlin M. Mar, MD
Starcher R. Margaret, MD
Audrey M. Marinelli, MD
Brittany A. Marinelli, MD
Emily S. Markovic, MD
Hannah L. Marshall, MD
Aaron Masjedi, MD
Isabelle M. Mason, MD
Akailah T. Mason-Otey, MD
Nicole Massad, MD
Megan M. Masten, MD
Stephanie M. Masters, MD
Anastasia Matthews, MD
Natalia del Mazo, MD
Sara A. McAllaster, MD
Continue to: Nicole McAndrew, DO...
Nicole McAndrew, DO
Madeline G. McCosker, MD
Jamie L. McDowell, DO
Christine E. McGough, MD
Mackenzi R. McHugh, MD
Madeline M. McIntire, MD
Cynthia R. McKinney, MD
Kirsten D. McLane, MD
Shian F. McLeish, MD
Megan I. McNitt, MD
Sarah R. McShane, MD
Grace R. Meade, MD
Nikki Ann R. Medina, DO
Tiffany L. Mei, MD
Jenna Meiman, MD
Anna M. Melicher, MD
Rosa M. Mendez, MD
Riley Mickelsen, MD
Sage A. Mikami, MD
Aletheia B. Millien, MD
Hannah C. Milthorpe, MD
Caroline J. Min, MD
Julie A. Mina, MD
Annie G. Minns, MD
Natalie Mironov, DO
Elizabeth L. Mirsky, MD
Astha Mittal, MD
Rachel E. Mnuk, MD
Silki Modi, MD
Sudarshan J. Mohan, MD
Roxana Mohhebali-Solis, MD
Mugdha V. Mokashi, MD
Jessica A. Montgomery, MD
Ellen Moore, MD
Savannah J. Morehouse, MD
Kristen L. Moriarty, MD
Alexa P. Morrison, MD
Bijan Morshedi, MD
Matthew H. Mossayebi, MD
Kathy Mostajeran, DO
Sharan Mullen, DO
Ellen C. Murphy, MD
Emma Chew Murphy, MD
Lauren M. Murphy, MD
Bria Murray, MD
Erin C. Nacev, MD
Preetha Nandi, MD
Blaire E. Nasstrom, DO
Hallie N. Nelson, MD
Katherine A. Nelson, MD
Margaret S. Nemetz, MD
Daniela Ben Neriah, DO
Cosima M. Neumann, MD
Mollie H. Newbern, DO
Gisella M. Newbery, MD
Stephanie Nguyen, MD
Christine G.T. Nguyen, MD
Desiree Nguyen, MD
Jacqueline W. Nichols, MD
Annika M. Nilsen, MD
Margaret A. Nixon, MD
Emily M. Norkett, MD
Allison N. Nostrant, DO
Susan E. Nourse, MD
Aliya S. Nurani, MD
Emily E. Nuss, MD
Jeanne O. Nwagwu, DO
Kelsey E. O’Hagan, MD
Margaret O’Neill, MD
Emily A. O’Brien, MD
Carly M. O’Connor-Terry, MD, MS
Madison O. Odom, MD
Cynthia I. Okot-Kotber, MD
Sarah P. Oliver, MD
Leanne P. Ondreicka, MD
Ngozika G. Onyiuke, MD
Erika Gonzalez Osorio, MD
Marika L. Osterbur Badhey, MD
Linda A. Otieno, MD
Claire H. Packer, MD
Chloe W. Page, DO
Marissa Palmor, MD
Rishitha Panditi, MD
Katherine A. Panushka, MD
Kelsey J. Pape, MD
Rachel R. Paquette, DO
Hillary C. Park, DO
Kendall M. Parrott, MD
Ekta Partani, MD
Karishma Patel, MD
Shivani Patel, MD
Continue to: Priya Patel, MD...
Priya Patel, MD
Jenna M. Patterson, MD
Ashleigh Pavlovic, MD
Katie M. Peagler, MD
Katherine T. Pellino, MD
Nicholas Per, MD
Elana Perry, MD
Emily J. Peters, MD
Sara E. Peterson, MD
Michelle R. Petrich, MD
Destiny L. Phillips, MD
Chloe Phillips, MD
Megan E. Piacquadio, DO
Sara C. Pierpoint, MD
Celeste M. Pilato, MD
Emma Pindra, MD
Minerva L.R. Pineda, MD
Rebecca Pisan, MD
Alessandra R. Piscina, MD
Rachael Piver, MD
Andrew J. Polio, MD
Hector S. Porragas, MD
Natalie Posever, MD
Allison R. Powell, MD
Mahima V. Prasad, MD
Angelina D. Prat, DO
Rebecca L. Purvis, MD
Teresa L. Qi, MD
Nicholas R. Quam, MD
Candice A. Quarella, MD
Nicholas W. Racchi, DO
Jeannie G. Radoc, MD
Samuel Raine, MD
Anna C. Raines, MD
Stephanie A. Rains, MD
Nicole M. Rainville, DO
Karissa Rajagopal, DO
Kristian R. Ramage, MD
Praveen Ramesh, MD
Tia M. Ramirez, MD
Jania Ramos, MD
Neel K. Rana, MD
Urvi Rana, DO
Indira Ranaweera, MD
Sindhuja Ranganathan, DO
Chloe R. Rasmussen, MD
Laura P. Reguero-Cadilla, MD
Devin M. Reilly, MD
Kimberly E. Reimold, MD
Cory R. Reiter, MD, PhD
Maya E. Reuven, DO
Jessica Reyes-Peterson, MD
Jacqueline Rice, MD
Rebecca L. Richardson, MD
Mikaela J. Rico, DO
Katelyn Rittenhouse, MD
Giuliana A. Rivera Casul, MD
Jill N.T. Roberts, MD
Luke N. Roberts, MD
Esther Robin, MD
Marcella Israel Rocha, MD
Zoe A. Roecker, MD
Hilary E. Rogers, MD
Kelsey A. Roof, MD
Zarah Rosen, MD
Cecilia M. Rossi, MD
Eva S. Rostonics, MD
Felix Rubio, MD
Amela Rugova, MD
Anna J. Rujan, MD
Erika T. Russ, MD
Colin Russell, MD
Ruby L. Russell, MD
Isabella A. Sabatina, MD
Gouri Sadananda, MD
Aashna Saini, MD
Salomeh M. Salari, MD
Ndeye N. Sall, MD
Nicole M. Salvador, MD
Aayushi Sardana, MD
Kendall M. Sarson, MD
Rita Abigail Sartor, MD
Continue to: Haley A. Scarbrough, MD...
Haley A. Scarbrough, MD
Kimberly Schaefer, MD
Demetra Schermerhorn, MD
Ellen C. Schleckman, MD
Maura A. Schlussel, MD
Ellie Schmidt, MD
Alison M. Schmidt, MD
Evan A. Schrader, MD
Morgan A. Schriever, MD
Brianna L. Schumaker Nguyen, DO
Whitney E. Scott, MD
Claire Scrivani, MD
Catherine E. Seaman, MD
Rachel D. Seaman, MD
Danielle J. Seltzer, MD
Joshua R. Shaffer, MD
Emily A. Shaffer, MD
Delia S. Shash, MD
Ishana P. Shetty, MD
Tushar Shetty, MD
Carol Shi, MD
Sarah P. Shim, MD
Emma C. Siewert, MD
Seth M. Sigler, DO
Rebecca L. SigourneyTennyck, MD
Daniella D. Silvino, DO
Andrea M. Simi, MD
Amelia R. Simmons, MD
Amy E. Skeels, DO
Ashley E.S. Keith, MD
Hannah C. Smerker, DO
Katarina Smigoc, MD
Madeline I. Smith, MD
Jessica D. Smith, MD
Melanie R. Smith, MD
Alicia L. Smith, MD
Chloe Smith, MD
Ayanna Smith, MD
Melanie R. Smith, MD
Megan M. Smith, MD
Haverly J. Snyder, MD
Beatrice R. Soderholm, DO
Brianna C. Sohl, MD
Samantha A. Solaru, MD
Michael Solotke, MD
Dara A.H. Som, MD
Alexandra R. Sotiros-Lowry, MD
Melanie Spall, DO
Alicia C. Speak, DO
Lisa M. Spencer, MD
Prakrithi Srinand, MD
Sierra M. Starr, MD
Kathryne E. Staudinger, MD
Emily K. Steele, MD
Morgan R. Steffen, DO
Tricia R. Stepanek, MD
Taylor P. Stewart, MD
Kelsey A. Stewart, MD
Alyssa M. Stiff, MD
Alexandra B. Stiles, MD
Nairi K. Strauch, MD
Margaret J. Stroup, DO
Sean C. Stuart, DO
Hannah M. Stump, MD
Shalini B. Subbarao, MD
Lakshmi Subramani, MD
Heather E. Sweeney, MD
Kristin I. Swope, MD
Suha Syed, MD
Mireya P. Taboada, MD
Eneti S. Tagaloa, MD
Rachel Tang, DO
Adam R. Taylor, MD
Simone R. Thibault, MD
Kimberly A. Thill, MD
Dhanu Thiyag, MD
Andrew T. Thornton, MD
Wendy Tian, MD
Stephanie Tilberry, MD
Amanda L. Tillett, MD
Amanda M. Tjitro, MD
Logan P. Todhunter, DO
David Toffey, MD
Maris K. Toland, MD
Rachel E. Tomassi, MD
Sarah Tounsi, MD
Antonia K. Traina, MD
Taylor Tran, MD
Diem Samantha Tran, DO
Emily C. Trautner, MD
Emma Trawick, MD
Continue to: Elissa Trieu, MD...
Elissa Trieu, MD
Ariel Trilling, MD
Samantha Truong, MD
Mary M. Tsaturian, MD
Athena Tudino, MD
Kati A. Turner, MD
Nicole-Marie Tuzinkiewicz, MD
Gayathri D. Vadlamudi, MD
Stylianos Vagios, MD
Pauline V. Van Dijck, DO
Kaylee A. VanDommelen, MD
Isha B. Vasudeva, MD
Shivani J. Vasudeva, DO
Diana Q. Vazquez Parker, MD
Ridhima Vemula, MD
Elena C. Vinopal, MD
Caroline J. Violette, MD
Pascal T. Vo, DO
Michelle H. Vu, MD
Macy M. Walz, MD
Angelia Wang, MD
Eileen Wang, MD
Courtney Y. Wang, MD
Joyce Wang, MD
Meryl G. Warshafsky, MD
Sophie E.N. Weinstein, MD
Sarah H. Weinstein, MD
Annalyn M. Welp, MD
Shannon M. Wentworth, MD
Erika M. Wert, MD
Rachel C. White, MBchB
Morgan N. Wilhoite, DO
Mercedes Williams, MD
Hayley Williams, MD
Jacquelyn D. Williams, MD
Mary H. Williamson, MD
Elise Wilson, MD
Lauren M. Witchey, MD
Emily A. Wolverton, MD
Stephanie Y. Wong, MD
Jenny Wu, MD
Jackie Xiang, MD
Nancy S. Yang, MD
Kevin P. Yeagle, MD
Halina M. Yee, MD
Alyssa M. Yeung, MD
Samuel K. Yost, MD
Megan Yuen, MD
Nayab Zafar, DO
Cindy X. Zhang, DO
Yingao Zhang, MD
Helen Zhao, MD
Chelsea Zhu, MD
Billie E. Zidel, MD
Ryan A. Zoldowski, MD

Six months after the Supreme Court decision that overturned the constitutional right to abortion, trainees across the United States are asking a critical question in the current resident recruitment season: How will the restrictions on abortion access affect my training as an obstetrician-gynecologist, and will they impact my ability to be the kind of provider I want to be in the future?
Among the myriad of downstream effects to patient care, the Dobbs decision will indisputably impact the scope of residency training for those that provide reproductive health services. Almost half of ObGyn residents train in states that have abortion restrictions in place.1 New educational milestones for abortion training, which are a requirement by the Accreditation Council for Graduate Medical Education (ACGME), were proposed quickly after Dobbs, guiding programs to offer opportunities for training in nonrestricted areas or the “combination of didactic activities, including simulation” to meet the training requirement in abortion care.2
Like many providers, residents already are grappling with precarious and risky circumstances, balancing patient safety and patient-driven care amidst pre-existing and newly enforced abortion restrictions. Whether managing a patient with an undesired pregnancy, severe medical comorbidities, unexpected pregnancy complications such as preterm premature rupture of membranes, or bleeding, or substantial fetal anomalies, ObGyn residents cannot gain the experience of providing the full scope of reproductive health care without the ability to offer all possible management options. While some enacted abortion restrictions have exceptions for the health of or life-saving measures for the mother, there is no standard guidance for timing of interventions, leaving providers confused and in fear of legal retribution. At a time when trainees should be learning to provide patient-centered, evidence-based care, they are instead paralyzed by the legal or professional consequences they may face for offering their best medical judgements.
Furthermore, the lack of exposure to dilation and evacuation procedures for residents in restricted practice areas will undoubtably decrease their confidence in managing acute complications, which is one of the critical facets of residency training. In a surgical field where repetition is crucial for technical competence, highlighted by ACGME minimum case requirements, the decreased volume of abortion procedures is a disadvantage for trainees and a disservice for patients. While anti-choice promoters may argue that involvement in surgical management of early pregnancy loss should suffice for ObGyn training in family planning, this piecemeal approach will leave gaps in technical skills.
The fear of legal ramifications, moral injury, and inadequate surgical training may lead to the siphoning of talented trainees to areas in the country with fewer restrictions.3 Dobbs already has demonstrated how limiting abortion access will deepen inequities in reproductive health care service delivery. Approximately 55% of ObGyn trainees and nearly two-thirds of maternal-fetal medicine graduates join the workforce in the state where they received their training.4 Medical students will seek opportunities for high-quality ObGyn training in areas that will help them to be well-prepared, competent physicians—and more often than not, stay in the area or region that they trained in. This will lead to provider shortages in areas where access to reproductive health care and subspecialist providers already is limited, further exacerbating existing health disparities.
During this recruitment season, trainees and residency programs alike will need to reckon with how the ramifications of Dobbs will alter both the immediate and long-term training in comprehensive reproductive health care for the ObGyn workforce. ObGyn trainees have taken a stand in response to the Dobbs decision, and nearly 750 current residents signed onto the statement below as a commitment to high-quality training and patient-centered care. Clinical experience in performing abortions is essential to the provision of comprehensive evidence-based reproductive health care, and access to these procedures is as important for physicians-in-training as it is for patients.
Actions to take to ensure high-quality abortion training in ObGyn residencies include the following:
- Connect with and stay involved with organizations such as the American College of Obstetricians and Gynecologists (ACOG), Physicians for Reproductive Health (PRH), and Medical Students for Choice (MSFC) for initiatives, toolkits, and resources for training at your institutions.
- Seek specific abortion training opportunities through the Leadership Training Academy (offered through PRH) or the Abortion Training Institute (offered through MSFC).
- Ensure that your residency program meets the ACGME criteria of providing opportunities for clinical experiences for abortion care and work with program leadership at a program, state, or regional level to enforce these competencies.
- Reach out to your local American Civil Liberties Union or other local reproductive legal rights organizations if you want to be involved with advocacy around abortion access and training but have concerns about legal protections.
- Have a voice at the table for empowering training opportunities by seeking leadership positions through ACOG, ACGME, Council on Resident Education in Obstetrics and Gynecology and the Association of Professors of Gynecology and Obstetrics, American Medical Association, Student National Medical Association, and subspecialty organizations.
- Vote in every election and promote voting registration and access to your patients, colleagues, and communities. ●
Continue to: The implications of the Dobbs v Jackson Women’s Health Organization decision on the health care and wellbeing of our patients...
On June 24, 2022, the Supreme Court of the United States ruled in a 6-3 majority decision to overturn the constitutional right to abortion protected by Roe v Wade since 1973. As health care providers, we are outraged at the Court’s disregard for an individual’s right to make reproductive decisions for themselves and their families and are deeply concerned about the devastating consequences to reproductive care and outcomes in this country for all people. Reproductive health decisions, including growing a family and whether or not to continue a pregnancy, are complex and incredibly personal. Our role as health care providers is to help guide those decisions with empathy and evidencebased clinical recommendations. This ruling undermines a patient’s right to bodily autonomy, free of impositions from government and political pressures, and it threatens the sanctity of complex medical decision-making between a patient, their family, and their medical team.
As medical professionals, we know that every patient’s situation is unique—banning abortion procedures ties the hands of physicians trying to provide the most medically appropriate options in a compassionate manner. We know that both medical and surgical abortions are safe and can save lives. These procedures can help patients with potentially life-threatening conditions worsened by pregnancy, a poor prognosis for the fetus, or a complication from the pregnancy itself. Physicians use scientific research and individualized approaches to help patients in unique situations, and attempts to legislate personal health decisions compromise the practice of evidence-based medicine.
We also know that this decision will impact some communities more than others. Access to safe abortion care will become dependent on which region of the country a person lives in and whether or not a person has resources to seek this care. Due to continued systemic racism and oppression, patients of color will be disproportionately impacted and likely will suffer worse health outcomes from unsafe abortions. Those that rely on public insurance or who are uninsured will face overwhelming barriers in seeking abortion services. These disparities in reproductive care, which contribute to our nation’s health crises in maternal morbidity and mortality, unintended pregnancy, and neonatal complications, will further entrench health inequities, and patient lives and livelihoods will suffer.
We acknowledge the impact that this decision will have on restricting access to reproductive care. We stand by the fact that abortion care is health care. We vow to uphold the tenets of our profession to place patient autonomy and provision of safe quality medical care at the forefront of our practices.
We, as health care providers and physician trainees, hereby pledge:
- To continue to provide evidence-based, nonjudgmental counseling for all pregnancy options, including abortion, and support our patients through all reproductive health decisions
- To promote equity in providing comprehensive reproductive health care, recognizing the impacts of systemic racism and oppression
- To promote high quality training in providing safe reproductive care in our respective institutions
- To use our voices in our communities to advocate for all our patients to have the freedom to access the safe and compassionate health care they deserve.
Sincerely,
The undersigned 747 ObGyn resident physicians
Please note that we sign this statement on our own behalf as individuals and not on behalf of our respective institutions.
Orchideh Abar, MD
Laurel S. Aberle, MD
Kathleen E. Ackert, DO
Lauryn Adams, MD
Temiloluwa Adejuyigbe, MD
Oluwatoyosi M. Adeoye, MD
Hufriya Y. Aderianwalla, MD
Fareeza Afzal, MD
Adelaide Agyepong, MD
Erin R. Ahart, MD
Noha T. Ahmed, DO
Faria Ahmed, MD
Tracey O. Akanbi, MD
Eloho E. Akpovi, MD
Austin H. Allen, DO
Amanda M. Allen, MD
Alexis L. Allihien, MD
Jorge L. Alsina, MD
Paulina C. Altshuler, DO
Sivani Aluru, MD
Amal Amir, DO
Jon Anderson, DO
Andreas Antono, MD
Annie N. Apple, MD
Janine Appleton, DO
Aarthi Arab, MD
Sydney R. Archer, MD
Youngeun C. Armbuster, MD
Kara Arnold, MD
Blessing C. Aroh, MD
Savannah Pearson Ayala, MD
Archana K. Ayyar, MD
Ann-Sophie Van Backle, DO
Connor R. Baker, MD
Japjot K. Bal, MD
Abigail E. Barger, MD
Kathryn E. Barron, MD
Silvia Bastea, MD
Samantha V.H. Bayer, MD
Kristen Beierwaltes, MD
Gisel Bello, MD
Michelle A. Benassai, MD
Dana Benyas, MD
Alice F. Berenson, MD
Hanna P. Berlin, MD
Abigail L. Bernard, MD
Eli H. Bernstein, MD
Julia T. Berry, MD
Bryce L. Beyer, MD
Caroline Bilbe, MD
Grace E. Binter, DO
Erin E. Bishop, MD
Sierra G. Bishop, MD
Stephanie S. Bista, MD
Tara E. Bjorklund, DO
Alyssa N. Black, MD
Continue to: Kelsey Boghean, DO...
Kelsey Boghean, DO
Areta Bojko, MD
Grace E. Bommarito, DO
Aditi R. Bommireddy, MD
Genna C. Bonfiglio, MD
Mary E. Booker, MD
Kayce L. Booth, MD
Samantha T. Boothe, DO
William Borenzweig, MD
Rebecca M. Borneman, MD
Alexander L. Boscia, MD
Gina M. Botsko, MD
Glenn P. Boyles, MD
Avery C. Bramnik, MD
Sophia N. Brancazio, MD
Katarina M. Braun, MD
Anthony Brausch, MD
Emily L. Brekke, MD
Sara E. Brenner, MD
Bailey A. Brown, DO
Kathryn S. Brown, MD
Denese C. Brown, MD
Abena Bruce, MD
Sabrina C. Brunozzi, MD
Madison Buchman, DO
Deirdre G. Buckley, MD
Rachel L. Budker, MD
Leeann M. Bui, MD
Anthony H. Bui, MD
Jessie Bujouves, MD
Kimberley A. Bullard, MD
Sophia G. Bunde, MD
Emily R. Burdette, MD
Iris Burgard, DO
Korbi M. Burkey, MD
Lindsey K. Burleson, MD
Lindsay M. Burton, MD
Brianna N. Byers, MD
Stephanie Cai, MD
Alexandra S. Calderon, MD
Alexandra G. Caldwell, MD
Natalia Calzada, MD
Tamara Cameo, MD
Arielle Caplin, MD
Angela M. Carracino, DO
Anna L. Carroll, MD
Leigha M. Carryl, MD
Ashlie S. Carter, MD
Stephanie Casey, DO
Chase W. Cataline, DO
Carson L. Catasus, MD
Alena R. Cave, MD
Kelly M. Chacon, MD
Avis L. Chan, MD
Shruthi Chandra, MD
Jennifer Chang, MD
Shannon Chang, DO
Gillian Chase, MD
Cindy Chen, MD
Jessie C. Chen, MD
Jessica T. Chen, MD
Wenjin Cheng, MB
Laura J. Cheng, MD
Lucy Cheng, MD
Monica S. Choo, MD
Jody S. Chou, MD
Hannah C. Christopher, DO
Continue to: David J. Chromey, DO...
David J. Chromey, DO
Grace V. Clark, MD
Celeste Colegrove, MD
Sarah C. Combs, MD
Victoria L. Conniff, MD
Hannah C. Connor, MD
Angela J. Conway, MD
Steffany A. Conyers, MD
Alexandra Cooke, MD
Ashley A. Cooney, MD
Anna Cornelius-Schecter, MD
Alexa M. Corso, DO
Krysten A. Costley, MD
Madeline Coulter, MD
Kelsey Cramer, MD
Anna E. Cronin, MD
Bethany N. Croyle, DO
Carmen A. Cueto, MD
Nicole Cumbo, MD
Mackenzie A. Cummings, MD
Carrie Cummiskey, MD
Hannah M. Cunningham, MD
Sarah D’Souza, DO
Rachael M. D’Auria, MD
Caitlin Dane, MD
Rachel N. Dang, MD
Talin R. Darian, MD
Abigail C. Davies, MD
Berkley Davis, MD
Lois A. Davis, MD
Jennie J. DeBlanc, MD
Ayana G.R. DeGaia, MD, MPH
Katerina N. DeHaan, MD
Rebekka M. Delgado, MD
Brettany C. DeMier, MD
Bonnie W. DePaso, MD
Hemaxi H. Desai, DO
Amberly T. Diep, MD
Abigail K. Dillaha, MD
Sarah K. Dominguez, MD
Abbey P. Donahue, MD
Allan C. Dong, MD
James Doss, MD
Taylor B. Douglas, MD
Abigail G. Downey, MD
Janelle M. Driscoll, MD
Emily Du, MD
Leslie V. Dunmire, MD
Jennifer Duong, DO
Leigh C. Durudogan, MD
Mai N. Dyer, MD, MPH
Rebecca A. Ebbott, MD
Lindsey P. Eck, MD
Molly C. Eckman, MD
Alex Ede, MD, ScM
Claire E. Edelman, MD
Sara E. Edwards, MD
David J. Eggert, DO
Michelle Eide, MD
Etoroabasi Ekpe, MD
Tressa L. Ellett, MD
Laura Peyton Ellis, MD
Kaitlin H. Ellis, MD
Mariah G. Elly, MD
Jennifer Embry, MD
Claire Englert, MD
Brenna Espelien, MD
Kamilah Evans, MD
Joshua A. Ewy, MD
Elana D. Fackler, MD
Lauren E. Falk, MD
Brianna A. Farley, MD
Amanda Stephanie R. Farrell, MD
Sara Fassio, DO
Daniela A. Febres-Cordero, MD
Jasmin E. Feliciano, MD
Alayna H. Feng, MD
Amanda M. Ferraro, MD
Brittany A. Fickau, MD
Brittany H. File, MD
Shannon M. Finner, DO
Mia E. Fischbein, DO
Briah Fischer, MD
Shira Fishbach, MD
Alison C. Fitzgerald, MD
Evan R. Fitzgerald, MD
Margaret R. Flanigan, MD
Kevin C. Flatley, MD
Jordan A. Fletcher, MD
Claudia E. Flores, MD
Lauren A. Forbes, MD
Rana K. Fowlkes, MD
Jennifer M. Franks, MD, MPH
Christina M. Frasik, MD
Haven N. Frazier, DO
Sarah W. Freeman, MD
Emilie O. Fromm, DO
Anna R. Fuchss, MD
Emma K. Gaboury, MD
Madeline H. Ganz, MD
Lex J. Gardner, MD
Keri-Lee Garel, MD
Hailey B. Gaskamp, DO
Brittney A. Gaudet, MD
Gabrielle M. Gear, MD
Eleanor R. Germano, MD
Lauren G. Gernon, MD
Allen Ghareeb, MD
Patricia Giglio Ayers, MD
Jordana L. Gilman, MD
Mianna M. Gilmore, DO
Brian W. Goddard, MD
Julia L. Goldberg, MD
M. Isabel Gonzaga, MD
Fred P. Gonzales, MD
Lillian H. Goodman, MD, MPH
Ashley Goreshnik, MD
Lauren E. Gottshall, MD
Lindsay L. Gould, MD
Kelsea R. Grant, MD
Dorender A. Gray, MD
Sophie Green, MD
Erica A. Green, MD
Danielle C. Greenberg, MD
Kalin J. Gregory-Davis, MD
David M. Greiner, MD
Tyler M. Gresham, MD
Continue to: Nelly Grigorian, MD...
Nelly Grigorian, MD
Erin L. Grimes, MD
Whitney Grither, MD
Jared M. Grootwassink, MD
Maya E. Gross, MD
Paoula Gueorguieva, MD
Margot M. Gurganus, DO
Rachel L. Gutfreund, MD
Andres Gutierrez, MD
Dorothy L. Hakimian, DO
Ashley N. Hamati, DO
Marie M. Hanna-Wagner, MD
Katie Hansen, MD
Courtney Hargreaves, MD
Stephanie Harlow, MD
Kelsey B. Harper, MD
Devon A. Harris, MD
Lauren E. Harris, MD
Emily S. Hart, DO
Sarah A. Hartley, MD
Becky K. Hartman, MD
Abigail K. Hartmann, MD
Charlotte V. Hastings, MD
Cherise Hatch, DO
Jordan Hauck, DO
Sarena Hayer, MD
Jenna M. Heath, MD
Eric D. Helm, MD
Julie A. Hemphill, MD
Ric A.S. Henderson, MD
Nicola A. Hendricks, MD
Andrea A. Henricks, MD
Jesse M. Herman, DO
Alyssa M. Hernandez, DO
Melissa Hernandez, MD
Alyssa R. Hersh, MD
Alexandra Herweck, MD
Brianna Hickey, MD
Allix M. Hillebrand, MD
Alessandra I. Hirsch, MD
Emily A. Hoffberg, MD
Chloe L. Holmes, DO
Cameron M. Holmes, MD
Helena Y. Hong, MD
Wakako Horiuchi, MD
Shweta Hosakoppal, MD
Jaycee E. Housh, MD
Shannon M. Howard, MD
Meredith C. Huszagh, MD
Yihharn P. Hwang, MD
Emma C. Hyde, MD
Brooke Hyman, MD
Hala Ali Ibrahim, MD
Gnendy Indig, MD
Erin E. Isaacson, MD
Shruti S. Iyer, DO
Audrey J. Jaeger, DO
Shobha Jagannatham, MD
Cyrus M. Jalai, MD
Emma V. James, MD
Isabel Janmey, MD
Phoebe Jen, DO
Corey L. Johnson, MD
Crystal J. Johnson, MD
Andrea M. Johnson, MD
Nat C. Jones, MD
Briana L. Jones, DO
Rebecca J. Josephson, MD
Sarah Natasha Jost-Haynes, MD
Continue to: Hannah S. Juhel, MD...
Hannah S. Juhel, MD
Erin Jun, DO
Katherine B. Kaak, MD
Dhara N. Kadakia, MD
Amanda D. Kadesh, MD
Riana K. Kahlon, MD
Nadi N. Kaonga, MD
Moli Karsalia, MD
Stephanie L. Kass, MD
Amanda M. Katz, MD
Chelsea S. Katz, MD
Virginia Kaufman, MD
Gurpinder Kaur, MD
Jessica A. Keesee, MD
Cassandra N. Kelly, MD
Whitney Kelly, DO
Hannah V. Kennedy, MD
Bethany H. Kette, MD
Iman Khan, MD
Maryam M. Khan, MD
Alisa Jion Kim, MD
Tesia G. Kim, MD
Anne E. Kim, MD
Emily H. King, MD
Tarynne E. Kinghorn, MD
Holly T. Kiper, DO
Thomas Kishkovich, MD
Quinn M. Kistenfeger, MD
Sofia E. Klar, DO
Jessica B. Klugman, MD
Hope E. Knochenhauer, MD
Kathleen J. Koenigs, MD
Olga Kontarovich, DO
Alison Kosmacki, MD
Ana E. Kouri, MD
Olga M. Kovalenko, MD
Leigh T. Kowalski, MD
Kayla A. Krajick, MD
Elizabeth S. Kravitz, MD
Shruti Rani Kumar, MD
Alyssa Kurtz, DO
Lauren H. Kus, MD
Arkadiy Kusayev, DO
Amanda E. Lacue, MD
Nava Lalehzari, MD
Amber Lalla, MD
Allie C. Lamari, DO
Kelly L. Lamiman, MD
Stephen Lammers, MD
Monet Lane, MD
Madeline L. Lang, MD
Liana Langdon-Embry, MD
Carolyn Larkins, MD
Leah E. Larson, MD
Matthew W. Lee, MD
Eunjae Lee, MD
Alice Lee, MD
Jared Z. Lee, MD
Charlotte M. Lee, MD
Nicole R. Legro, MD
Aurora Leibold, MD
Rosiris Leon-Rivera, MD, PhD
Anna M. Leone, MD
Keiko M. Leong, MD
Lindsey M. LePoidevin, MD
Molly E. Levine, MD
Khrystyna Levytska, MD
Dana L. Lewis, DO
Jessica L. Li, MD
Kristina Lilja, MD
Deanna M. Lines, DO
Annalise Littman, MD
Julia F. Liu, MD
Tyler B. Lloyd, MD
Alyssa Lo, MD
K’ara A. Locke, MD
Minica Long, MD
Melissa Lopez, MD
Wilfredo A. Lopez, MD
Connie F. Lu, MD
Tyler J. Lueck, MD
Katherine L. Lukas, MD
Davlyn L. Luke, MD
Shani Ma, MD
Colton Mabis, MD
Lauren T. MacNeill, MD
Rachel Madding, MD
Mona Makhamreh, MD
Francesca R. Mancuso, MD
Kelsey L. Manfredi, MD
Valeria Mantilla, MD
Kaitlin M. Mar, MD
Starcher R. Margaret, MD
Audrey M. Marinelli, MD
Brittany A. Marinelli, MD
Emily S. Markovic, MD
Hannah L. Marshall, MD
Aaron Masjedi, MD
Isabelle M. Mason, MD
Akailah T. Mason-Otey, MD
Nicole Massad, MD
Megan M. Masten, MD
Stephanie M. Masters, MD
Anastasia Matthews, MD
Natalia del Mazo, MD
Sara A. McAllaster, MD
Continue to: Nicole McAndrew, DO...
Nicole McAndrew, DO
Madeline G. McCosker, MD
Jamie L. McDowell, DO
Christine E. McGough, MD
Mackenzi R. McHugh, MD
Madeline M. McIntire, MD
Cynthia R. McKinney, MD
Kirsten D. McLane, MD
Shian F. McLeish, MD
Megan I. McNitt, MD
Sarah R. McShane, MD
Grace R. Meade, MD
Nikki Ann R. Medina, DO
Tiffany L. Mei, MD
Jenna Meiman, MD
Anna M. Melicher, MD
Rosa M. Mendez, MD
Riley Mickelsen, MD
Sage A. Mikami, MD
Aletheia B. Millien, MD
Hannah C. Milthorpe, MD
Caroline J. Min, MD
Julie A. Mina, MD
Annie G. Minns, MD
Natalie Mironov, DO
Elizabeth L. Mirsky, MD
Astha Mittal, MD
Rachel E. Mnuk, MD
Silki Modi, MD
Sudarshan J. Mohan, MD
Roxana Mohhebali-Solis, MD
Mugdha V. Mokashi, MD
Jessica A. Montgomery, MD
Ellen Moore, MD
Savannah J. Morehouse, MD
Kristen L. Moriarty, MD
Alexa P. Morrison, MD
Bijan Morshedi, MD
Matthew H. Mossayebi, MD
Kathy Mostajeran, DO
Sharan Mullen, DO
Ellen C. Murphy, MD
Emma Chew Murphy, MD
Lauren M. Murphy, MD
Bria Murray, MD
Erin C. Nacev, MD
Preetha Nandi, MD
Blaire E. Nasstrom, DO
Hallie N. Nelson, MD
Katherine A. Nelson, MD
Margaret S. Nemetz, MD
Daniela Ben Neriah, DO
Cosima M. Neumann, MD
Mollie H. Newbern, DO
Gisella M. Newbery, MD
Stephanie Nguyen, MD
Christine G.T. Nguyen, MD
Desiree Nguyen, MD
Jacqueline W. Nichols, MD
Annika M. Nilsen, MD
Margaret A. Nixon, MD
Emily M. Norkett, MD
Allison N. Nostrant, DO
Susan E. Nourse, MD
Aliya S. Nurani, MD
Emily E. Nuss, MD
Jeanne O. Nwagwu, DO
Kelsey E. O’Hagan, MD
Margaret O’Neill, MD
Emily A. O’Brien, MD
Carly M. O’Connor-Terry, MD, MS
Madison O. Odom, MD
Cynthia I. Okot-Kotber, MD
Sarah P. Oliver, MD
Leanne P. Ondreicka, MD
Ngozika G. Onyiuke, MD
Erika Gonzalez Osorio, MD
Marika L. Osterbur Badhey, MD
Linda A. Otieno, MD
Claire H. Packer, MD
Chloe W. Page, DO
Marissa Palmor, MD
Rishitha Panditi, MD
Katherine A. Panushka, MD
Kelsey J. Pape, MD
Rachel R. Paquette, DO
Hillary C. Park, DO
Kendall M. Parrott, MD
Ekta Partani, MD
Karishma Patel, MD
Shivani Patel, MD
Continue to: Priya Patel, MD...
Priya Patel, MD
Jenna M. Patterson, MD
Ashleigh Pavlovic, MD
Katie M. Peagler, MD
Katherine T. Pellino, MD
Nicholas Per, MD
Elana Perry, MD
Emily J. Peters, MD
Sara E. Peterson, MD
Michelle R. Petrich, MD
Destiny L. Phillips, MD
Chloe Phillips, MD
Megan E. Piacquadio, DO
Sara C. Pierpoint, MD
Celeste M. Pilato, MD
Emma Pindra, MD
Minerva L.R. Pineda, MD
Rebecca Pisan, MD
Alessandra R. Piscina, MD
Rachael Piver, MD
Andrew J. Polio, MD
Hector S. Porragas, MD
Natalie Posever, MD
Allison R. Powell, MD
Mahima V. Prasad, MD
Angelina D. Prat, DO
Rebecca L. Purvis, MD
Teresa L. Qi, MD
Nicholas R. Quam, MD
Candice A. Quarella, MD
Nicholas W. Racchi, DO
Jeannie G. Radoc, MD
Samuel Raine, MD
Anna C. Raines, MD
Stephanie A. Rains, MD
Nicole M. Rainville, DO
Karissa Rajagopal, DO
Kristian R. Ramage, MD
Praveen Ramesh, MD
Tia M. Ramirez, MD
Jania Ramos, MD
Neel K. Rana, MD
Urvi Rana, DO
Indira Ranaweera, MD
Sindhuja Ranganathan, DO
Chloe R. Rasmussen, MD
Laura P. Reguero-Cadilla, MD
Devin M. Reilly, MD
Kimberly E. Reimold, MD
Cory R. Reiter, MD, PhD
Maya E. Reuven, DO
Jessica Reyes-Peterson, MD
Jacqueline Rice, MD
Rebecca L. Richardson, MD
Mikaela J. Rico, DO
Katelyn Rittenhouse, MD
Giuliana A. Rivera Casul, MD
Jill N.T. Roberts, MD
Luke N. Roberts, MD
Esther Robin, MD
Marcella Israel Rocha, MD
Zoe A. Roecker, MD
Hilary E. Rogers, MD
Kelsey A. Roof, MD
Zarah Rosen, MD
Cecilia M. Rossi, MD
Eva S. Rostonics, MD
Felix Rubio, MD
Amela Rugova, MD
Anna J. Rujan, MD
Erika T. Russ, MD
Colin Russell, MD
Ruby L. Russell, MD
Isabella A. Sabatina, MD
Gouri Sadananda, MD
Aashna Saini, MD
Salomeh M. Salari, MD
Ndeye N. Sall, MD
Nicole M. Salvador, MD
Aayushi Sardana, MD
Kendall M. Sarson, MD
Rita Abigail Sartor, MD
Continue to: Haley A. Scarbrough, MD...
Haley A. Scarbrough, MD
Kimberly Schaefer, MD
Demetra Schermerhorn, MD
Ellen C. Schleckman, MD
Maura A. Schlussel, MD
Ellie Schmidt, MD
Alison M. Schmidt, MD
Evan A. Schrader, MD
Morgan A. Schriever, MD
Brianna L. Schumaker Nguyen, DO
Whitney E. Scott, MD
Claire Scrivani, MD
Catherine E. Seaman, MD
Rachel D. Seaman, MD
Danielle J. Seltzer, MD
Joshua R. Shaffer, MD
Emily A. Shaffer, MD
Delia S. Shash, MD
Ishana P. Shetty, MD
Tushar Shetty, MD
Carol Shi, MD
Sarah P. Shim, MD
Emma C. Siewert, MD
Seth M. Sigler, DO
Rebecca L. SigourneyTennyck, MD
Daniella D. Silvino, DO
Andrea M. Simi, MD
Amelia R. Simmons, MD
Amy E. Skeels, DO
Ashley E.S. Keith, MD
Hannah C. Smerker, DO
Katarina Smigoc, MD
Madeline I. Smith, MD
Jessica D. Smith, MD
Melanie R. Smith, MD
Alicia L. Smith, MD
Chloe Smith, MD
Ayanna Smith, MD
Melanie R. Smith, MD
Megan M. Smith, MD
Haverly J. Snyder, MD
Beatrice R. Soderholm, DO
Brianna C. Sohl, MD
Samantha A. Solaru, MD
Michael Solotke, MD
Dara A.H. Som, MD
Alexandra R. Sotiros-Lowry, MD
Melanie Spall, DO
Alicia C. Speak, DO
Lisa M. Spencer, MD
Prakrithi Srinand, MD
Sierra M. Starr, MD
Kathryne E. Staudinger, MD
Emily K. Steele, MD
Morgan R. Steffen, DO
Tricia R. Stepanek, MD
Taylor P. Stewart, MD
Kelsey A. Stewart, MD
Alyssa M. Stiff, MD
Alexandra B. Stiles, MD
Nairi K. Strauch, MD
Margaret J. Stroup, DO
Sean C. Stuart, DO
Hannah M. Stump, MD
Shalini B. Subbarao, MD
Lakshmi Subramani, MD
Heather E. Sweeney, MD
Kristin I. Swope, MD
Suha Syed, MD
Mireya P. Taboada, MD
Eneti S. Tagaloa, MD
Rachel Tang, DO
Adam R. Taylor, MD
Simone R. Thibault, MD
Kimberly A. Thill, MD
Dhanu Thiyag, MD
Andrew T. Thornton, MD
Wendy Tian, MD
Stephanie Tilberry, MD
Amanda L. Tillett, MD
Amanda M. Tjitro, MD
Logan P. Todhunter, DO
David Toffey, MD
Maris K. Toland, MD
Rachel E. Tomassi, MD
Sarah Tounsi, MD
Antonia K. Traina, MD
Taylor Tran, MD
Diem Samantha Tran, DO
Emily C. Trautner, MD
Emma Trawick, MD
Continue to: Elissa Trieu, MD...
Elissa Trieu, MD
Ariel Trilling, MD
Samantha Truong, MD
Mary M. Tsaturian, MD
Athena Tudino, MD
Kati A. Turner, MD
Nicole-Marie Tuzinkiewicz, MD
Gayathri D. Vadlamudi, MD
Stylianos Vagios, MD
Pauline V. Van Dijck, DO
Kaylee A. VanDommelen, MD
Isha B. Vasudeva, MD
Shivani J. Vasudeva, DO
Diana Q. Vazquez Parker, MD
Ridhima Vemula, MD
Elena C. Vinopal, MD
Caroline J. Violette, MD
Pascal T. Vo, DO
Michelle H. Vu, MD
Macy M. Walz, MD
Angelia Wang, MD
Eileen Wang, MD
Courtney Y. Wang, MD
Joyce Wang, MD
Meryl G. Warshafsky, MD
Sophie E.N. Weinstein, MD
Sarah H. Weinstein, MD
Annalyn M. Welp, MD
Shannon M. Wentworth, MD
Erika M. Wert, MD
Rachel C. White, MBchB
Morgan N. Wilhoite, DO
Mercedes Williams, MD
Hayley Williams, MD
Jacquelyn D. Williams, MD
Mary H. Williamson, MD
Elise Wilson, MD
Lauren M. Witchey, MD
Emily A. Wolverton, MD
Stephanie Y. Wong, MD
Jenny Wu, MD
Jackie Xiang, MD
Nancy S. Yang, MD
Kevin P. Yeagle, MD
Halina M. Yee, MD
Alyssa M. Yeung, MD
Samuel K. Yost, MD
Megan Yuen, MD
Nayab Zafar, DO
Cindy X. Zhang, DO
Yingao Zhang, MD
Helen Zhao, MD
Chelsea Zhu, MD
Billie E. Zidel, MD
Ryan A. Zoldowski, MD
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- ACGME program requirements for graduate medical education in obstetrics and gynecology summary and impact of interim requirement revisions. ACGME website. Accessed December 18, 2022. https://www.acgme.org/globalassets/pfassets/reviewandcomment/220_obstetricsandgynecology_2022-06-24_impact.pdf
- Crear-Perry J, Hassan A, Daniel S. Advancing birth equity in a post-Dobbs US. JAMA. 2022;328:1689-1690.
- Report on residents. AAMC website. Accessed December 18, 2022. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2021/table-c4-physician-reten tion-state-residency-training-last-completed-gme
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- ACGME program requirements for graduate medical education in obstetrics and gynecology summary and impact of interim requirement revisions. ACGME website. Accessed December 18, 2022. https://www.acgme.org/globalassets/pfassets/reviewandcomment/220_obstetricsandgynecology_2022-06-24_impact.pdf
- Crear-Perry J, Hassan A, Daniel S. Advancing birth equity in a post-Dobbs US. JAMA. 2022;328:1689-1690.
- Report on residents. AAMC website. Accessed December 18, 2022. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2021/table-c4-physician-reten tion-state-residency-training-last-completed-gme
Have investigators reached the first steps for redefining a diagnostic definition of preeclampsia that includes morbidity?
Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med. 2022;1. DOI: 10.1056/EVIDoa2200161
EXPERT COMMENTARY
The standard core lecture on preeclampsia given to all medical students frequently begins with an epic, if not potentially apocryphal, statement regarding how this disease has been noted in the annals of medical history since the time of the Ancients. Although contemporary diagnostic criteria for preeclampsia are not that far out of date, they are close. The increased urinary protein loss and hypertension preceding eclamptic seizures was first noted at the end of the 19th century. The blood pressure and proteinuria criteria used for diagnosis was codified in its contemporary form in the late 1940s. Since then, “tweak” rather than “overhaul” probably best describes the updates of the obstetrical community to the definition of preeclampsia. This has just changed.
Details of the study
Thadhani and colleagues prospectively recruited a nationally representative observational cohort of patients hospitalized for hypertension during pregnancy, then followed the patients until either the diagnosis of preeclampsia with severe features or for 2 weeks, whichever came first. At enrollment, circulating levels of the soluble fms-like tyrosine kinase 1 (sFlt-1) and placental growth factor (PlGF) were measured. In a 2-phased design, the first 219 participants were used to define a sFlt-1/PlGF ratio that would predict progression to severe preeclampsia within 2 weeks. The next 556 enrollees served to validate the predictive properties of the ratio. The authors found that a sFlt-1/PlGF ratio of ≥40 predicted progression to preeclampsia with severe features with an area under the curve (AUC) of 0.92.
As products of the trophoblasts, both sFlt-1 and PlGF have been mooted for almost 2 decades as potential predictive, if not diagnostic, aids with respect to preeclampsia. Indeed, both analytes are commercially available in Europe for specifically this purpose and many maternal-fetal medicine practitioners working in the European equivalent American tertiary referral centers use an sFlt-1/PlGF ratio as their primary criteria for a diagnosis of preeclampsia. Within the United States, there was an initial flurry of interest in and an infusion of corporate and federal research support for sFlt-1 and PlGF as diagnostic aids for preeclampsia in the mid-2000s. However, at present, the US Food and Drug Administration (FDA) has not sanctioned these (or any) biomarkers to aid in the diagnosis of preeclampsia. As Thermo-Fisher Scientific (Waltham, Massachusetts) is a supporting partner in this study, it is almost certain that these data will be submitted for review by the FDA as part of an application for a preeclampsia diagnostic. At some point in the near future, American practitioners will potentially be able to join their European colleagues in utilizing these biomarkers in the diagnosis of preeclampsia with severe features. ●
Thadhani and colleagues observed that the majority of both maternal and neonatal morbidity in their study, including 8 of the 9 neonatal deaths and both cases of eclampsia, occurred among patients with a ratio ≥40 at admission. There was an inverse relation between the sFlt-1/PlGF ratio and the admission to delivery interval. Where only 17% of patients in the highest quartile of ratios remained pregnant at 14 days post-enrollment, more than 79% of the lowest quartile were still pregnant. If not a causal relationship, sFlt-1 and PlGF are clearly associated with not only the occurrence of preeclampsia with severe features but also the degree of morbidity.
The implication for the disposition of patient care resources is clear. Patients at higher risk for preeclampsia could be seen in specialty high-risk clinics with an emphasis on increased monitoring. In situations where tertiary care is more remote, plans could be developed should they need to be transported to centers able to provide the appropriate level of care. Conversely, patients screening at lower ratios may be more appropriately managed as outpatients, or at least in less clinically involved accommodations.
Thadhani et al do note that there were false negative cases in which the sFlt-1/PlGF ratio at admission was <40 but patients nonetheless progressed to preeclampsia with severe features. The majority of these cases had concurrent pre-pregnancy, chronic hypertension. This observation suggests not only the potential for insights into the pathophysiology of the hypertensive diseases in pregnancy but also that the interpretation of the sFlt/PlGF ratio may eventually need to be stratified by preexisting conditions.
The final implications for the observations of this study are perhaps the most tantalizing. If there is a causal relation between the level of the sFlt-1/PlGF ratio and the morbidity of preeclampsia with severe features, then lowering the circulating concentration of sFlt-1 would ameliorate not only the morbidity but also the risk of preeclampsia. Work with plasma phoresies has suggested that this might be possible, albeit via a clinical intervention demanding more intensive resources. The potential for a targeted pharmacologic moderation of sFlt-1 levels would hold great promise in that those identified as at increased risk could be offered an intervention widely available to all.
Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med. 2022;1. DOI: 10.1056/EVIDoa2200161
EXPERT COMMENTARY
The standard core lecture on preeclampsia given to all medical students frequently begins with an epic, if not potentially apocryphal, statement regarding how this disease has been noted in the annals of medical history since the time of the Ancients. Although contemporary diagnostic criteria for preeclampsia are not that far out of date, they are close. The increased urinary protein loss and hypertension preceding eclamptic seizures was first noted at the end of the 19th century. The blood pressure and proteinuria criteria used for diagnosis was codified in its contemporary form in the late 1940s. Since then, “tweak” rather than “overhaul” probably best describes the updates of the obstetrical community to the definition of preeclampsia. This has just changed.
Details of the study
Thadhani and colleagues prospectively recruited a nationally representative observational cohort of patients hospitalized for hypertension during pregnancy, then followed the patients until either the diagnosis of preeclampsia with severe features or for 2 weeks, whichever came first. At enrollment, circulating levels of the soluble fms-like tyrosine kinase 1 (sFlt-1) and placental growth factor (PlGF) were measured. In a 2-phased design, the first 219 participants were used to define a sFlt-1/PlGF ratio that would predict progression to severe preeclampsia within 2 weeks. The next 556 enrollees served to validate the predictive properties of the ratio. The authors found that a sFlt-1/PlGF ratio of ≥40 predicted progression to preeclampsia with severe features with an area under the curve (AUC) of 0.92.

As products of the trophoblasts, both sFlt-1 and PlGF have been mooted for almost 2 decades as potential predictive, if not diagnostic, aids with respect to preeclampsia. Indeed, both analytes are commercially available in Europe for specifically this purpose and many maternal-fetal medicine practitioners working in the European equivalent American tertiary referral centers use an sFlt-1/PlGF ratio as their primary criteria for a diagnosis of preeclampsia. Within the United States, there was an initial flurry of interest in and an infusion of corporate and federal research support for sFlt-1 and PlGF as diagnostic aids for preeclampsia in the mid-2000s. However, at present, the US Food and Drug Administration (FDA) has not sanctioned these (or any) biomarkers to aid in the diagnosis of preeclampsia. As Thermo-Fisher Scientific (Waltham, Massachusetts) is a supporting partner in this study, it is almost certain that these data will be submitted for review by the FDA as part of an application for a preeclampsia diagnostic. At some point in the near future, American practitioners will potentially be able to join their European colleagues in utilizing these biomarkers in the diagnosis of preeclampsia with severe features. ●
Thadhani and colleagues observed that the majority of both maternal and neonatal morbidity in their study, including 8 of the 9 neonatal deaths and both cases of eclampsia, occurred among patients with a ratio ≥40 at admission. There was an inverse relation between the sFlt-1/PlGF ratio and the admission to delivery interval. Where only 17% of patients in the highest quartile of ratios remained pregnant at 14 days post-enrollment, more than 79% of the lowest quartile were still pregnant. If not a causal relationship, sFlt-1 and PlGF are clearly associated with not only the occurrence of preeclampsia with severe features but also the degree of morbidity.
The implication for the disposition of patient care resources is clear. Patients at higher risk for preeclampsia could be seen in specialty high-risk clinics with an emphasis on increased monitoring. In situations where tertiary care is more remote, plans could be developed should they need to be transported to centers able to provide the appropriate level of care. Conversely, patients screening at lower ratios may be more appropriately managed as outpatients, or at least in less clinically involved accommodations.
Thadhani et al do note that there were false negative cases in which the sFlt-1/PlGF ratio at admission was <40 but patients nonetheless progressed to preeclampsia with severe features. The majority of these cases had concurrent pre-pregnancy, chronic hypertension. This observation suggests not only the potential for insights into the pathophysiology of the hypertensive diseases in pregnancy but also that the interpretation of the sFlt/PlGF ratio may eventually need to be stratified by preexisting conditions.
The final implications for the observations of this study are perhaps the most tantalizing. If there is a causal relation between the level of the sFlt-1/PlGF ratio and the morbidity of preeclampsia with severe features, then lowering the circulating concentration of sFlt-1 would ameliorate not only the morbidity but also the risk of preeclampsia. Work with plasma phoresies has suggested that this might be possible, albeit via a clinical intervention demanding more intensive resources. The potential for a targeted pharmacologic moderation of sFlt-1 levels would hold great promise in that those identified as at increased risk could be offered an intervention widely available to all.
Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med. 2022;1. DOI: 10.1056/EVIDoa2200161
EXPERT COMMENTARY
The standard core lecture on preeclampsia given to all medical students frequently begins with an epic, if not potentially apocryphal, statement regarding how this disease has been noted in the annals of medical history since the time of the Ancients. Although contemporary diagnostic criteria for preeclampsia are not that far out of date, they are close. The increased urinary protein loss and hypertension preceding eclamptic seizures was first noted at the end of the 19th century. The blood pressure and proteinuria criteria used for diagnosis was codified in its contemporary form in the late 1940s. Since then, “tweak” rather than “overhaul” probably best describes the updates of the obstetrical community to the definition of preeclampsia. This has just changed.
Details of the study
Thadhani and colleagues prospectively recruited a nationally representative observational cohort of patients hospitalized for hypertension during pregnancy, then followed the patients until either the diagnosis of preeclampsia with severe features or for 2 weeks, whichever came first. At enrollment, circulating levels of the soluble fms-like tyrosine kinase 1 (sFlt-1) and placental growth factor (PlGF) were measured. In a 2-phased design, the first 219 participants were used to define a sFlt-1/PlGF ratio that would predict progression to severe preeclampsia within 2 weeks. The next 556 enrollees served to validate the predictive properties of the ratio. The authors found that a sFlt-1/PlGF ratio of ≥40 predicted progression to preeclampsia with severe features with an area under the curve (AUC) of 0.92.

As products of the trophoblasts, both sFlt-1 and PlGF have been mooted for almost 2 decades as potential predictive, if not diagnostic, aids with respect to preeclampsia. Indeed, both analytes are commercially available in Europe for specifically this purpose and many maternal-fetal medicine practitioners working in the European equivalent American tertiary referral centers use an sFlt-1/PlGF ratio as their primary criteria for a diagnosis of preeclampsia. Within the United States, there was an initial flurry of interest in and an infusion of corporate and federal research support for sFlt-1 and PlGF as diagnostic aids for preeclampsia in the mid-2000s. However, at present, the US Food and Drug Administration (FDA) has not sanctioned these (or any) biomarkers to aid in the diagnosis of preeclampsia. As Thermo-Fisher Scientific (Waltham, Massachusetts) is a supporting partner in this study, it is almost certain that these data will be submitted for review by the FDA as part of an application for a preeclampsia diagnostic. At some point in the near future, American practitioners will potentially be able to join their European colleagues in utilizing these biomarkers in the diagnosis of preeclampsia with severe features. ●
Thadhani and colleagues observed that the majority of both maternal and neonatal morbidity in their study, including 8 of the 9 neonatal deaths and both cases of eclampsia, occurred among patients with a ratio ≥40 at admission. There was an inverse relation between the sFlt-1/PlGF ratio and the admission to delivery interval. Where only 17% of patients in the highest quartile of ratios remained pregnant at 14 days post-enrollment, more than 79% of the lowest quartile were still pregnant. If not a causal relationship, sFlt-1 and PlGF are clearly associated with not only the occurrence of preeclampsia with severe features but also the degree of morbidity.
The implication for the disposition of patient care resources is clear. Patients at higher risk for preeclampsia could be seen in specialty high-risk clinics with an emphasis on increased monitoring. In situations where tertiary care is more remote, plans could be developed should they need to be transported to centers able to provide the appropriate level of care. Conversely, patients screening at lower ratios may be more appropriately managed as outpatients, or at least in less clinically involved accommodations.
Thadhani et al do note that there were false negative cases in which the sFlt-1/PlGF ratio at admission was <40 but patients nonetheless progressed to preeclampsia with severe features. The majority of these cases had concurrent pre-pregnancy, chronic hypertension. This observation suggests not only the potential for insights into the pathophysiology of the hypertensive diseases in pregnancy but also that the interpretation of the sFlt/PlGF ratio may eventually need to be stratified by preexisting conditions.
The final implications for the observations of this study are perhaps the most tantalizing. If there is a causal relation between the level of the sFlt-1/PlGF ratio and the morbidity of preeclampsia with severe features, then lowering the circulating concentration of sFlt-1 would ameliorate not only the morbidity but also the risk of preeclampsia. Work with plasma phoresies has suggested that this might be possible, albeit via a clinical intervention demanding more intensive resources. The potential for a targeted pharmacologic moderation of sFlt-1 levels would hold great promise in that those identified as at increased risk could be offered an intervention widely available to all.