User login
Marijuana linked to higher PAD risk
But death, intervention rates same
PHOENIX – although there was no greater risk of death from myocardial infarction or other cardiac causes or need for revascularization.
The researchers noted, however, that the study population was young, with an average age of 37.4 years, and that the study period, from 2016 to 2019, predates the legalization of recreational marijuana in a number of states.
Nonetheless, even in this young study population, marijuana users’ risk of developing peripheral artery disease (PAD) was 3.68 times greater (P < .001) than that of nonusers. PAD at a young age could precede worse outcomes later in life, the study authors said.
“Basically, marijuana users were at increased risk of being diagnosed with peripheral artery disease, but there was no increased risk for them requiring any intervention, such as a peripheral vascular intervention, nor were they at increased risk of death from what we found,” said Hirva Vyas, DO, an internal medicine resident at Hackensack University Medical Center in New Jersey, who presented the results at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The study used data on 623,768 marijuana users from the National Inpatient Sample, a nationwide database of inpatient visits covered by all public and commercial payers, then extracted a diagnosis for PAD from all 30 million–plus patient encounters to compare PAD rates between marijuana users and nonusers. Marijuana users were more likely to be White and to have elective rather than emergency admissions (P < .001). The researchers used diagnostic codes to identify marijuana users and PAD patients.
Recreational marijuana is legal in 22 states and the District of Columbia, according to ProCon.org. Since 2019, the last year of the study, 11 states have legalized marijuana for recreational use. “It’s a data point that we studied at one point in time, only from 2016 to 2019,” Dr. Vyas said in an interview.
“As we’ve seen over the past 4-5 years, legalization has skyrocketed and recreational use has become more and more favorable not only among younger folks but older folks,” study coauthor Harsh Jain, MD, a second-year internal medicine resident at Montefiore Medical Center in New York, said in the interview. “It would be really refreshing to see how these data change as we look at endpoints from 2019 to 2023.”
Because of the young age of the study population, Dr. Jain said, these findings may not accurately represent the true cardiovascular risks of marijuana use, especially later in life.
“One of the biggest secondary endpoints that we wanted to study was the development of chronic conditions that lead to multiple rehospitalizations, the most significant one of which would be the development of heart failure,” Dr. Jain said. “However, it was difficult to stratify because, again, many of these patients were very young and so they did not carry the diagnosis for heart failure, so we couldn’t complete that subset analysis.”
The goal is to extend the study period out to 2023, Dr. Jain said. “We know that these are very crude and rudimentary data findings that we presented so far, but we’re hoping that the final paper gives us a chance to flesh out all the details of our study and also gives us a chance to expand going forward,” he said.
The findings are in line with other research into the effects of marijuana and cardiovascular disease, said Carl “Chip” Lavie, MD, medical director for cardiac rehabilitation and prevention at the John Ochsner Heart and Vascular Institute in New Orleans who’s published a number of studies on PAD and substance use, including marijuana.
“It is known that cannabis is associated with more vasoconstriction, has sympathomimetic effects, causes endothelial dysfunction and increased platelet aggregation, and is known to increase the risk of acute myocardial infarction, especially in the hour or so after use,” he said in written comments sent to this news organization.
“It is also well known to be a cause of thromboangiitis obliterans, which is in the PAD family,” he added. “Based on these mechanisms, one would expect an increased PAD and, especially, PAD events. The 3.7-fold increased risk is supportive of this increased PAD.”
One study strength, Dr. Lavie pointed out, is that it’s one of the few studies that found an association between marijuana and PAD, which hasn’t been studied as well as other cardiovascular endpoints. “However,” he said, “the limitation is this is just an inpatient sample, and it is all based on coding – e.g., a patient could have PAD and it may not have been coded.”
But death, intervention rates same
But death, intervention rates same
PHOENIX – although there was no greater risk of death from myocardial infarction or other cardiac causes or need for revascularization.
The researchers noted, however, that the study population was young, with an average age of 37.4 years, and that the study period, from 2016 to 2019, predates the legalization of recreational marijuana in a number of states.
Nonetheless, even in this young study population, marijuana users’ risk of developing peripheral artery disease (PAD) was 3.68 times greater (P < .001) than that of nonusers. PAD at a young age could precede worse outcomes later in life, the study authors said.
“Basically, marijuana users were at increased risk of being diagnosed with peripheral artery disease, but there was no increased risk for them requiring any intervention, such as a peripheral vascular intervention, nor were they at increased risk of death from what we found,” said Hirva Vyas, DO, an internal medicine resident at Hackensack University Medical Center in New Jersey, who presented the results at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The study used data on 623,768 marijuana users from the National Inpatient Sample, a nationwide database of inpatient visits covered by all public and commercial payers, then extracted a diagnosis for PAD from all 30 million–plus patient encounters to compare PAD rates between marijuana users and nonusers. Marijuana users were more likely to be White and to have elective rather than emergency admissions (P < .001). The researchers used diagnostic codes to identify marijuana users and PAD patients.
Recreational marijuana is legal in 22 states and the District of Columbia, according to ProCon.org. Since 2019, the last year of the study, 11 states have legalized marijuana for recreational use. “It’s a data point that we studied at one point in time, only from 2016 to 2019,” Dr. Vyas said in an interview.
“As we’ve seen over the past 4-5 years, legalization has skyrocketed and recreational use has become more and more favorable not only among younger folks but older folks,” study coauthor Harsh Jain, MD, a second-year internal medicine resident at Montefiore Medical Center in New York, said in the interview. “It would be really refreshing to see how these data change as we look at endpoints from 2019 to 2023.”
Because of the young age of the study population, Dr. Jain said, these findings may not accurately represent the true cardiovascular risks of marijuana use, especially later in life.
“One of the biggest secondary endpoints that we wanted to study was the development of chronic conditions that lead to multiple rehospitalizations, the most significant one of which would be the development of heart failure,” Dr. Jain said. “However, it was difficult to stratify because, again, many of these patients were very young and so they did not carry the diagnosis for heart failure, so we couldn’t complete that subset analysis.”
The goal is to extend the study period out to 2023, Dr. Jain said. “We know that these are very crude and rudimentary data findings that we presented so far, but we’re hoping that the final paper gives us a chance to flesh out all the details of our study and also gives us a chance to expand going forward,” he said.
The findings are in line with other research into the effects of marijuana and cardiovascular disease, said Carl “Chip” Lavie, MD, medical director for cardiac rehabilitation and prevention at the John Ochsner Heart and Vascular Institute in New Orleans who’s published a number of studies on PAD and substance use, including marijuana.
“It is known that cannabis is associated with more vasoconstriction, has sympathomimetic effects, causes endothelial dysfunction and increased platelet aggregation, and is known to increase the risk of acute myocardial infarction, especially in the hour or so after use,” he said in written comments sent to this news organization.
“It is also well known to be a cause of thromboangiitis obliterans, which is in the PAD family,” he added. “Based on these mechanisms, one would expect an increased PAD and, especially, PAD events. The 3.7-fold increased risk is supportive of this increased PAD.”
One study strength, Dr. Lavie pointed out, is that it’s one of the few studies that found an association between marijuana and PAD, which hasn’t been studied as well as other cardiovascular endpoints. “However,” he said, “the limitation is this is just an inpatient sample, and it is all based on coding – e.g., a patient could have PAD and it may not have been coded.”
PHOENIX – although there was no greater risk of death from myocardial infarction or other cardiac causes or need for revascularization.
The researchers noted, however, that the study population was young, with an average age of 37.4 years, and that the study period, from 2016 to 2019, predates the legalization of recreational marijuana in a number of states.
Nonetheless, even in this young study population, marijuana users’ risk of developing peripheral artery disease (PAD) was 3.68 times greater (P < .001) than that of nonusers. PAD at a young age could precede worse outcomes later in life, the study authors said.
“Basically, marijuana users were at increased risk of being diagnosed with peripheral artery disease, but there was no increased risk for them requiring any intervention, such as a peripheral vascular intervention, nor were they at increased risk of death from what we found,” said Hirva Vyas, DO, an internal medicine resident at Hackensack University Medical Center in New Jersey, who presented the results at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The study used data on 623,768 marijuana users from the National Inpatient Sample, a nationwide database of inpatient visits covered by all public and commercial payers, then extracted a diagnosis for PAD from all 30 million–plus patient encounters to compare PAD rates between marijuana users and nonusers. Marijuana users were more likely to be White and to have elective rather than emergency admissions (P < .001). The researchers used diagnostic codes to identify marijuana users and PAD patients.
Recreational marijuana is legal in 22 states and the District of Columbia, according to ProCon.org. Since 2019, the last year of the study, 11 states have legalized marijuana for recreational use. “It’s a data point that we studied at one point in time, only from 2016 to 2019,” Dr. Vyas said in an interview.
“As we’ve seen over the past 4-5 years, legalization has skyrocketed and recreational use has become more and more favorable not only among younger folks but older folks,” study coauthor Harsh Jain, MD, a second-year internal medicine resident at Montefiore Medical Center in New York, said in the interview. “It would be really refreshing to see how these data change as we look at endpoints from 2019 to 2023.”
Because of the young age of the study population, Dr. Jain said, these findings may not accurately represent the true cardiovascular risks of marijuana use, especially later in life.
“One of the biggest secondary endpoints that we wanted to study was the development of chronic conditions that lead to multiple rehospitalizations, the most significant one of which would be the development of heart failure,” Dr. Jain said. “However, it was difficult to stratify because, again, many of these patients were very young and so they did not carry the diagnosis for heart failure, so we couldn’t complete that subset analysis.”
The goal is to extend the study period out to 2023, Dr. Jain said. “We know that these are very crude and rudimentary data findings that we presented so far, but we’re hoping that the final paper gives us a chance to flesh out all the details of our study and also gives us a chance to expand going forward,” he said.
The findings are in line with other research into the effects of marijuana and cardiovascular disease, said Carl “Chip” Lavie, MD, medical director for cardiac rehabilitation and prevention at the John Ochsner Heart and Vascular Institute in New Orleans who’s published a number of studies on PAD and substance use, including marijuana.
“It is known that cannabis is associated with more vasoconstriction, has sympathomimetic effects, causes endothelial dysfunction and increased platelet aggregation, and is known to increase the risk of acute myocardial infarction, especially in the hour or so after use,” he said in written comments sent to this news organization.
“It is also well known to be a cause of thromboangiitis obliterans, which is in the PAD family,” he added. “Based on these mechanisms, one would expect an increased PAD and, especially, PAD events. The 3.7-fold increased risk is supportive of this increased PAD.”
One study strength, Dr. Lavie pointed out, is that it’s one of the few studies that found an association between marijuana and PAD, which hasn’t been studied as well as other cardiovascular endpoints. “However,” he said, “the limitation is this is just an inpatient sample, and it is all based on coding – e.g., a patient could have PAD and it may not have been coded.”
AT SCAI 2023
Transcatheter tricuspid valve repair in real-world setting replicates trials
Data support benefit and safety
For the TriClip system (Abbott), the data were drawn from a prospective postmarketing registry, and for the EVOQUE system (Edwards Lifesciences), data were generated by a compassionate use program.
The TriClip system is approved and available in Europe, but neither system has regulatory approval in the United States.
The two sets of data, each presented at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions, are consistent with controlled trials. Each system was associated with high rates of procedural success, low rates of adverse events, and sustained improvements in quality of life.
Real-world backup for TRILUMINATE
Presented just days before the pivotal multinational TRILUMINATE trial was published in the New England Journal of Medicine, the bRIGHT postmarketing study of the TriClip device demonstrated a procedural rate of success and a subsequent reduction in TR that was at least as good but in a substantially sicker patient population.
“To appreciate these results, you have to put into perspective the baseline TR in our population,” reported Philipp Lurz, MD, PhD, of the Heart Center Leipzig, University of Leipzig, Germany. Whereas only 70% of those randomized in TRILUMINATE had grade 4 (massive) or 5 (torrential) TR, the proportion was 90% in bRIGHT.
The proportion with TR of moderate or less severity was 77% when assessed at 30 days in bRIGHT versus 72%, however, when assessed at 1 year in TRILUMINATE. In addition, procedural success was 99% in both studies even though patients in bRIGHT were on average older and had more comorbidities. At baseline, 80% of bRIGHT patients were in New York Heart Association (NYHA) class III or IV heart failure versus 59% of those in TRILUMINATE.
TRILUMINATE data, presented prior to publication at the annual meeting of the American College of Cardiology earlier this year, did not associate the transcatheter TR repair with a reduction in mortality or a reduction in hospitalization for heart failure, which were the first two of three hierarchical endpoints, but it did show benefit on the third, which was quality of life. As measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ), patients in the transcatheter repair group gained 12.3 points versus 0.6 points (P < .001) on medical therapy.
In the bRIGHT registry, patients gained 19 points in the KCCQ score after treatment. By 30 days, the proportion of patients in NHYA class III/IV had fallen from 80% to 20%. The major adverse event rate of 2.5% at 30 days was only modestly higher than the 1.7% rate at 30 days in TRILUMINATE.
“The safety profile remained strong despite the sicker population treated in the registry,” reported Dr. Lurz, whose results were simultaneously published in the Journal of the American College of Cardiology (JACC).
The bRIGHT registry analysis was based on 511 patients treated at 26 sites in Europe. Dr. Lurz characterized it as “the first prospective, single-arm, open-label, multicenter, postmarket registry to evaluate the safety and performance of any transcatheter tricuspid valve repair system.”
In a panel discussion following the presentation, Nicole Karam, MD, PhD, codirector of the heart valve unit, Hospital Georges Pompidou, Paris, praised a study of TEER tricuspid valve device in the real world, but she pointed out that the question of who to treat remains unanswered. Although symptom relief has value for a condition that can impose large deficits in quality of life, she called for more data to identify optimal candidates, particularly in the persistent absence of a major effect on hard endpoints.
Dr. Lurz agreed. In bRIGHT, predictors of a moderate or less TR at discharge included a smaller tethering distance, a smaller right ventricular end diastolic dimension, a smaller right atrial volume, and a smaller tricuspid annular diameter.
Each of these predictors argues for earlier treatment, he said, even if later treatment in a clinical trial provides a greater likelihood of eventually demonstrating benefits on hard endpoints.
‘Remarkable reduction’
The data from the much smaller compassionate use evaluation of the EVOQUE system generated similar evidence of safety and benefit while also making the point that earlier intervention offers a greater opportunity for preventing irreversible progression. With much longer follow up, the compassionate-use analysis, which involved patients even sicker than those included in bRIGHT, suggested these repairs are durable.
In this retrospective analysis of 38 patients treated at eight centers in Europe, the United States, and Canada, the mortality climbed steadily over 2 years of follow-up, reaching 29% at 2 years despite the fact that TR was reduced to < 1% after the procedure and remained durably suppressed at a median follow-up of 520 days.
The tricuspid valve repair with the EVOQUE system “was associated with a remarkable reduction in heart failure symptoms and significant improvement in NYHA functional class up to a maximum of 1,074 days after the intervention,” reported Lukas Stolz, MD, an interventional cardiologist at Ludwig-Maximilians-University, Munich.
In the data he presented at EuroPCR, which was published simultaneously as a letter in JACC, he said that favorable reverse remodeling of the right ventricle, which was observed as early as 30 days after the procedure, was maintained at long-term follow up.
The uncontrolled data from the compassionate analysis, like the bRIGHT registry, could not confirm that tricuspid valve repair changes the trajectory of progressive heart disease, but the favorable effects Dr. Stolz reported on cardiovascular function, not just symptoms, support this idea.
Dr. Lutz has financial relationships with Edwards Lifesciences, ReCor, and Abbott, which funded the bRIGHT registry. Dr. Karam reports financial relationships with Abbott, Edwards Lifesciences, and Medtronic. Dr. Stolz reports no potential conflicts of interest, but other coinvestigators of the retrospective analysis have financial relationships with Edwards Lifesciences, which is developing the EVOQUE system.
Data support benefit and safety
Data support benefit and safety
For the TriClip system (Abbott), the data were drawn from a prospective postmarketing registry, and for the EVOQUE system (Edwards Lifesciences), data were generated by a compassionate use program.
The TriClip system is approved and available in Europe, but neither system has regulatory approval in the United States.
The two sets of data, each presented at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions, are consistent with controlled trials. Each system was associated with high rates of procedural success, low rates of adverse events, and sustained improvements in quality of life.
Real-world backup for TRILUMINATE
Presented just days before the pivotal multinational TRILUMINATE trial was published in the New England Journal of Medicine, the bRIGHT postmarketing study of the TriClip device demonstrated a procedural rate of success and a subsequent reduction in TR that was at least as good but in a substantially sicker patient population.
“To appreciate these results, you have to put into perspective the baseline TR in our population,” reported Philipp Lurz, MD, PhD, of the Heart Center Leipzig, University of Leipzig, Germany. Whereas only 70% of those randomized in TRILUMINATE had grade 4 (massive) or 5 (torrential) TR, the proportion was 90% in bRIGHT.
The proportion with TR of moderate or less severity was 77% when assessed at 30 days in bRIGHT versus 72%, however, when assessed at 1 year in TRILUMINATE. In addition, procedural success was 99% in both studies even though patients in bRIGHT were on average older and had more comorbidities. At baseline, 80% of bRIGHT patients were in New York Heart Association (NYHA) class III or IV heart failure versus 59% of those in TRILUMINATE.
TRILUMINATE data, presented prior to publication at the annual meeting of the American College of Cardiology earlier this year, did not associate the transcatheter TR repair with a reduction in mortality or a reduction in hospitalization for heart failure, which were the first two of three hierarchical endpoints, but it did show benefit on the third, which was quality of life. As measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ), patients in the transcatheter repair group gained 12.3 points versus 0.6 points (P < .001) on medical therapy.
In the bRIGHT registry, patients gained 19 points in the KCCQ score after treatment. By 30 days, the proportion of patients in NHYA class III/IV had fallen from 80% to 20%. The major adverse event rate of 2.5% at 30 days was only modestly higher than the 1.7% rate at 30 days in TRILUMINATE.
“The safety profile remained strong despite the sicker population treated in the registry,” reported Dr. Lurz, whose results were simultaneously published in the Journal of the American College of Cardiology (JACC).
The bRIGHT registry analysis was based on 511 patients treated at 26 sites in Europe. Dr. Lurz characterized it as “the first prospective, single-arm, open-label, multicenter, postmarket registry to evaluate the safety and performance of any transcatheter tricuspid valve repair system.”
In a panel discussion following the presentation, Nicole Karam, MD, PhD, codirector of the heart valve unit, Hospital Georges Pompidou, Paris, praised a study of TEER tricuspid valve device in the real world, but she pointed out that the question of who to treat remains unanswered. Although symptom relief has value for a condition that can impose large deficits in quality of life, she called for more data to identify optimal candidates, particularly in the persistent absence of a major effect on hard endpoints.
Dr. Lurz agreed. In bRIGHT, predictors of a moderate or less TR at discharge included a smaller tethering distance, a smaller right ventricular end diastolic dimension, a smaller right atrial volume, and a smaller tricuspid annular diameter.
Each of these predictors argues for earlier treatment, he said, even if later treatment in a clinical trial provides a greater likelihood of eventually demonstrating benefits on hard endpoints.
‘Remarkable reduction’
The data from the much smaller compassionate use evaluation of the EVOQUE system generated similar evidence of safety and benefit while also making the point that earlier intervention offers a greater opportunity for preventing irreversible progression. With much longer follow up, the compassionate-use analysis, which involved patients even sicker than those included in bRIGHT, suggested these repairs are durable.
In this retrospective analysis of 38 patients treated at eight centers in Europe, the United States, and Canada, the mortality climbed steadily over 2 years of follow-up, reaching 29% at 2 years despite the fact that TR was reduced to < 1% after the procedure and remained durably suppressed at a median follow-up of 520 days.
The tricuspid valve repair with the EVOQUE system “was associated with a remarkable reduction in heart failure symptoms and significant improvement in NYHA functional class up to a maximum of 1,074 days after the intervention,” reported Lukas Stolz, MD, an interventional cardiologist at Ludwig-Maximilians-University, Munich.
In the data he presented at EuroPCR, which was published simultaneously as a letter in JACC, he said that favorable reverse remodeling of the right ventricle, which was observed as early as 30 days after the procedure, was maintained at long-term follow up.
The uncontrolled data from the compassionate analysis, like the bRIGHT registry, could not confirm that tricuspid valve repair changes the trajectory of progressive heart disease, but the favorable effects Dr. Stolz reported on cardiovascular function, not just symptoms, support this idea.
Dr. Lutz has financial relationships with Edwards Lifesciences, ReCor, and Abbott, which funded the bRIGHT registry. Dr. Karam reports financial relationships with Abbott, Edwards Lifesciences, and Medtronic. Dr. Stolz reports no potential conflicts of interest, but other coinvestigators of the retrospective analysis have financial relationships with Edwards Lifesciences, which is developing the EVOQUE system.
For the TriClip system (Abbott), the data were drawn from a prospective postmarketing registry, and for the EVOQUE system (Edwards Lifesciences), data were generated by a compassionate use program.
The TriClip system is approved and available in Europe, but neither system has regulatory approval in the United States.
The two sets of data, each presented at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions, are consistent with controlled trials. Each system was associated with high rates of procedural success, low rates of adverse events, and sustained improvements in quality of life.
Real-world backup for TRILUMINATE
Presented just days before the pivotal multinational TRILUMINATE trial was published in the New England Journal of Medicine, the bRIGHT postmarketing study of the TriClip device demonstrated a procedural rate of success and a subsequent reduction in TR that was at least as good but in a substantially sicker patient population.
“To appreciate these results, you have to put into perspective the baseline TR in our population,” reported Philipp Lurz, MD, PhD, of the Heart Center Leipzig, University of Leipzig, Germany. Whereas only 70% of those randomized in TRILUMINATE had grade 4 (massive) or 5 (torrential) TR, the proportion was 90% in bRIGHT.
The proportion with TR of moderate or less severity was 77% when assessed at 30 days in bRIGHT versus 72%, however, when assessed at 1 year in TRILUMINATE. In addition, procedural success was 99% in both studies even though patients in bRIGHT were on average older and had more comorbidities. At baseline, 80% of bRIGHT patients were in New York Heart Association (NYHA) class III or IV heart failure versus 59% of those in TRILUMINATE.
TRILUMINATE data, presented prior to publication at the annual meeting of the American College of Cardiology earlier this year, did not associate the transcatheter TR repair with a reduction in mortality or a reduction in hospitalization for heart failure, which were the first two of three hierarchical endpoints, but it did show benefit on the third, which was quality of life. As measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ), patients in the transcatheter repair group gained 12.3 points versus 0.6 points (P < .001) on medical therapy.
In the bRIGHT registry, patients gained 19 points in the KCCQ score after treatment. By 30 days, the proportion of patients in NHYA class III/IV had fallen from 80% to 20%. The major adverse event rate of 2.5% at 30 days was only modestly higher than the 1.7% rate at 30 days in TRILUMINATE.
“The safety profile remained strong despite the sicker population treated in the registry,” reported Dr. Lurz, whose results were simultaneously published in the Journal of the American College of Cardiology (JACC).
The bRIGHT registry analysis was based on 511 patients treated at 26 sites in Europe. Dr. Lurz characterized it as “the first prospective, single-arm, open-label, multicenter, postmarket registry to evaluate the safety and performance of any transcatheter tricuspid valve repair system.”
In a panel discussion following the presentation, Nicole Karam, MD, PhD, codirector of the heart valve unit, Hospital Georges Pompidou, Paris, praised a study of TEER tricuspid valve device in the real world, but she pointed out that the question of who to treat remains unanswered. Although symptom relief has value for a condition that can impose large deficits in quality of life, she called for more data to identify optimal candidates, particularly in the persistent absence of a major effect on hard endpoints.
Dr. Lurz agreed. In bRIGHT, predictors of a moderate or less TR at discharge included a smaller tethering distance, a smaller right ventricular end diastolic dimension, a smaller right atrial volume, and a smaller tricuspid annular diameter.
Each of these predictors argues for earlier treatment, he said, even if later treatment in a clinical trial provides a greater likelihood of eventually demonstrating benefits on hard endpoints.
‘Remarkable reduction’
The data from the much smaller compassionate use evaluation of the EVOQUE system generated similar evidence of safety and benefit while also making the point that earlier intervention offers a greater opportunity for preventing irreversible progression. With much longer follow up, the compassionate-use analysis, which involved patients even sicker than those included in bRIGHT, suggested these repairs are durable.
In this retrospective analysis of 38 patients treated at eight centers in Europe, the United States, and Canada, the mortality climbed steadily over 2 years of follow-up, reaching 29% at 2 years despite the fact that TR was reduced to < 1% after the procedure and remained durably suppressed at a median follow-up of 520 days.
The tricuspid valve repair with the EVOQUE system “was associated with a remarkable reduction in heart failure symptoms and significant improvement in NYHA functional class up to a maximum of 1,074 days after the intervention,” reported Lukas Stolz, MD, an interventional cardiologist at Ludwig-Maximilians-University, Munich.
In the data he presented at EuroPCR, which was published simultaneously as a letter in JACC, he said that favorable reverse remodeling of the right ventricle, which was observed as early as 30 days after the procedure, was maintained at long-term follow up.
The uncontrolled data from the compassionate analysis, like the bRIGHT registry, could not confirm that tricuspid valve repair changes the trajectory of progressive heart disease, but the favorable effects Dr. Stolz reported on cardiovascular function, not just symptoms, support this idea.
Dr. Lutz has financial relationships with Edwards Lifesciences, ReCor, and Abbott, which funded the bRIGHT registry. Dr. Karam reports financial relationships with Abbott, Edwards Lifesciences, and Medtronic. Dr. Stolz reports no potential conflicts of interest, but other coinvestigators of the retrospective analysis have financial relationships with Edwards Lifesciences, which is developing the EVOQUE system.
FROM EUROPCR 2023
‘Staggering’ weight loss and benefits in body composition with tirzepatide
DUBLIN – , according to the latest results of the SURMOUNT-1 study.
The new analysis showed that up to 63% of participants achieved a reduction in body weight of at least 20%, and all three tirzepatide doses (5 mg, 10 mg, and 15 mg) led to substantial, clinically meaningful, and sustained body-weight reduction, compared with placebo at 72 weeks of follow-up.
Mean weight loss was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for placebo (all P < .001 vs. placebo). And among participants taking the highest 15-mg dose of tirzepatide, 96%, 90%, and 78% of patients achieved weight reductions of at least 5%, 10%, and 15%.
Tirzepatide is approved in the United States and the European Union for the treatment of type 2 diabetes but is not yet approved for obesity in any country. The manufacturer of tirzepatide, Eli Lilly, intends to seek approval for the drug as an obesity treatment from the U.S. Food and Drug Administration, European Medicines Agency, and in other territories beginning in 2023.
Regardless of baseline BMI category, 9 out of 10 people achieved the greater than or equal to 5% body weight reduction threshold across all doses of tirzepatide, and at the higher doses, over one-third achieved weight loss of 25% or more.
“Similar to lifestyle and surgical treatments, participants on tirzepatide had around a threefold greater percent reduction in fat mass, compared with lean mass, resulting in an overall improvement in body composition,” reported SURMOUNT-1 co-investigator Louis Aronne, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, New York.
“This is staggering weight loss,” remarked Dr. Aronne. “To put it in perspective, mean weight loss in people having Lap-Band surgery is 17%, mean weight loss for sleeve gastrectomy is 25%, and gastric bypass is 33%, which puts the effects of tirzepatide squarely in the realm of bariatric surgery.”
“Something we have sought for decades, we have finally been able to achieve,” he asserted. “I still remember exactly where I was when I saw these results for the first time last April. I knew something big was happening,” declared Dr. Aronne when presenting the latest analyses at the 2023 European Congress on Obesity. Full study results were published in the New England Journal of Medicine.
Moderator Gabriella Lieberman, MD, endocrinologist and head of the Israeli Center for Weight Management, Sheba Medical Center, Ramat-Gan, Israel, welcomed the study but also expressed caution. “It’s very potent, but as we see generally with potent therapies, I think it will change how we look at nutritional advice and the role of the dietician will change. I’m a bit worried the drug is running fast and the support, which is crucial with these treatments, is not keeping up, and we’ll have to deal with some effects later, such as sarcopenia,” she pointed out in an interview.
“We have to treat these drugs as if they are bariatric surgery. I see patients on these types of drugs in clinic and their appetite is so suppressed that they think they can afford to eat things that are unhealthy because they lose weight, and that’s what they want. There has to be a responsible adult looking at what they’re eating, and not just clapping their hands for the weight loss, but ensuring they are not deprived of anything,” she said.
Weight loss and body composition explored
Tirzepatide is a novel glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that works to activate the GIP and GLP-1 receptors, respectively, found in areas of the brain important for appetite regulation, decreasing food intake, and modulating fat utilization.
The phase 3, double-blind, randomized, controlled trial included data from 2,539 adults with a BMI greater than or equal to 30 kg/m2 (class I, II, III obesity) or greater than or equal to 27 kg/m2 (overweight) with one or more weight-related complications, excluding diabetes. At baseline, mean body weight was 104.8 kg, mean BMI was 38.0 kg/m2, and 94.5% of participants had BMI greater than or equal to 30 kg/m2.
Patients were randomized to once-weekly subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 72 weeks. The primary objective was to show that tirzepatide was superior to placebo in terms of percentage change in body weight and proportion of participants with body-weight reduction of greater than or equal to 5%. The percentage change from baseline body weight and proportion of participants with body weight reduction greater than or equal to 5% were also assessed across BMI categories of greater than or equal to 27 to less than 30 kg/m2, greater than or equal to 30 to less than 35 kg/m2 (class 1 obesity), greater than or equal to 35 to less than 40 kg/m2 (class 2 obesity), and greater than or equal to 40 kg/m2 (class 3 obesity).
In addition, in a retrospective subanalysis, body composition was evaluated in a subpopulation that underwent dual-energy x-ray absorptiometry, assessing change from baseline body composition within age subgroups less than 50 years (n = 99), 50-64.9 years (n = 41), and greater than or equal to 65 years (n = 20).
The average weight reduction over the 72 weeks of follow-up was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for participants taking placebo (all P < .001 vs. placebo).
The percentages of participants reaching target weight reductions of greater than or equal to 5%, greater than or equal to 10%, greater than or equal to 15%, greater than or equal to 20%, and greater than or equal to 25% were recorded. Over 90% achieved greater than or equal to 5% weight loss, irrespective of BMI and tirzepatide dose, while 55.5% and 62.9% in the 10-mg and 15-mg groups achieved greater than or equal to 20% weight loss, and 35.0% and 39.7% in the 10-mg and 15-mg groups achieved greater than or equal to 25% weight loss, respectively.
By increasing BMI category, in the 10-mg group, weight loss was –18.2 kg, –21.9 kg, –22.0, and –20.7 kg; and in the 15-mg group, weight loss was –18.1kg, –21.2 kg, –24.5 kg, and –22.8 kg. Weight loss in the 5-mg group ranged from –16.6 kg to –15.9 kg from lowest to highest BMI category.
“In the lower-weight categories, there is less weight to lose, so we see a flattening of the curve [with a] maximum of around 18%, so it may be that as we learn more about a drug that is so potent, we recognize that we don’t need to use such a high dose in people with BMI 27-30 kg/m2,” he explained. “It’s the higher BMI categories where we need the higher dose.”
As with lifestyle and surgical treatments, participants taking tirzepatide had around a three times greater percentage reduction in fat mass than lean mass, resulting in an overall improvement in body composition, reported Dr. Aronne.
“We want loss of fat, not lean mass, and we know that we lose around one part lean to three parts fat mass when on a diet and exercise regimen,” he went on to explain. “We see exactly this [balance of lean-to-fat-mass loss] here with 33.9% total fat mass reduction in the treatment group, compared with 8.2% in the placebo group.”
Visceral fat mass reduction was 40% in the treatment group, compared with 7.3% with placebo. “It’s good to see there’s more loss of visceral fat,” said Dr. Aronne. Lean mass loss was 10.9%. “So around three times greater reduction in fat over lean mass loss, resulting in overall improvement of body composition,” he reported.
Also, in older people (≥ 65 years) there was approximately no difference in fat versus lean mass loss, compared with younger people, despite older people being more likely to lose more lean mass.
With respect to patient-reported outcomes based on the 36-item Short-Form Health Survey (SF-36), Dr. Aronne said that physical functioning scores significantly improved at 72 weeks, compared with placebo, particularly in participants with physical function limitations at baseline.
“In an interesting subanalysis, those with physical limitations at baseline showed a significant improvement versus placebo of over 5% difference [considered significant],” he added.
Safety and tolerability were previously reported in the NEJM article. The most common adverse events with tirzepatide were gastrointestinal, and adverse events causing treatment discontinuation occurred in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5-mg, 10-mg, and 15-mg doses or placebo, respectively.
“A revolution is coming in the treatment of obesity and cardiometabolic disease, and most physicians cannot grasp this. We’re finally getting the efficacy we’ve been looking for that will produce benefits in every realm,” concluded Dr. Aronne. “These data show that we are now hitting all the secondary endpoints and making our patients better.”
“I think this bodes well. I always envisioned a time when the treatment of obesity would come first before the treatment of cardiometabolic complications of obesity, and I think we’re on the verge of that era with semaglutide, tirzepatide, and the very exciting treatments to come.”
The SURMOUNT-1 trial was sponsored by Lilly. Dr. Aronne is cofounder, chief scientific advisor, and a member of the board of directors for Intellihealth. He is also a paid scientific advisory board member for Eli Lilly.
A version of this article first appeared on Medscape.com.
DUBLIN – , according to the latest results of the SURMOUNT-1 study.
The new analysis showed that up to 63% of participants achieved a reduction in body weight of at least 20%, and all three tirzepatide doses (5 mg, 10 mg, and 15 mg) led to substantial, clinically meaningful, and sustained body-weight reduction, compared with placebo at 72 weeks of follow-up.
Mean weight loss was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for placebo (all P < .001 vs. placebo). And among participants taking the highest 15-mg dose of tirzepatide, 96%, 90%, and 78% of patients achieved weight reductions of at least 5%, 10%, and 15%.
Tirzepatide is approved in the United States and the European Union for the treatment of type 2 diabetes but is not yet approved for obesity in any country. The manufacturer of tirzepatide, Eli Lilly, intends to seek approval for the drug as an obesity treatment from the U.S. Food and Drug Administration, European Medicines Agency, and in other territories beginning in 2023.
Regardless of baseline BMI category, 9 out of 10 people achieved the greater than or equal to 5% body weight reduction threshold across all doses of tirzepatide, and at the higher doses, over one-third achieved weight loss of 25% or more.
“Similar to lifestyle and surgical treatments, participants on tirzepatide had around a threefold greater percent reduction in fat mass, compared with lean mass, resulting in an overall improvement in body composition,” reported SURMOUNT-1 co-investigator Louis Aronne, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, New York.
“This is staggering weight loss,” remarked Dr. Aronne. “To put it in perspective, mean weight loss in people having Lap-Band surgery is 17%, mean weight loss for sleeve gastrectomy is 25%, and gastric bypass is 33%, which puts the effects of tirzepatide squarely in the realm of bariatric surgery.”
“Something we have sought for decades, we have finally been able to achieve,” he asserted. “I still remember exactly where I was when I saw these results for the first time last April. I knew something big was happening,” declared Dr. Aronne when presenting the latest analyses at the 2023 European Congress on Obesity. Full study results were published in the New England Journal of Medicine.
Moderator Gabriella Lieberman, MD, endocrinologist and head of the Israeli Center for Weight Management, Sheba Medical Center, Ramat-Gan, Israel, welcomed the study but also expressed caution. “It’s very potent, but as we see generally with potent therapies, I think it will change how we look at nutritional advice and the role of the dietician will change. I’m a bit worried the drug is running fast and the support, which is crucial with these treatments, is not keeping up, and we’ll have to deal with some effects later, such as sarcopenia,” she pointed out in an interview.
“We have to treat these drugs as if they are bariatric surgery. I see patients on these types of drugs in clinic and their appetite is so suppressed that they think they can afford to eat things that are unhealthy because they lose weight, and that’s what they want. There has to be a responsible adult looking at what they’re eating, and not just clapping their hands for the weight loss, but ensuring they are not deprived of anything,” she said.
Weight loss and body composition explored
Tirzepatide is a novel glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that works to activate the GIP and GLP-1 receptors, respectively, found in areas of the brain important for appetite regulation, decreasing food intake, and modulating fat utilization.
The phase 3, double-blind, randomized, controlled trial included data from 2,539 adults with a BMI greater than or equal to 30 kg/m2 (class I, II, III obesity) or greater than or equal to 27 kg/m2 (overweight) with one or more weight-related complications, excluding diabetes. At baseline, mean body weight was 104.8 kg, mean BMI was 38.0 kg/m2, and 94.5% of participants had BMI greater than or equal to 30 kg/m2.
Patients were randomized to once-weekly subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 72 weeks. The primary objective was to show that tirzepatide was superior to placebo in terms of percentage change in body weight and proportion of participants with body-weight reduction of greater than or equal to 5%. The percentage change from baseline body weight and proportion of participants with body weight reduction greater than or equal to 5% were also assessed across BMI categories of greater than or equal to 27 to less than 30 kg/m2, greater than or equal to 30 to less than 35 kg/m2 (class 1 obesity), greater than or equal to 35 to less than 40 kg/m2 (class 2 obesity), and greater than or equal to 40 kg/m2 (class 3 obesity).
In addition, in a retrospective subanalysis, body composition was evaluated in a subpopulation that underwent dual-energy x-ray absorptiometry, assessing change from baseline body composition within age subgroups less than 50 years (n = 99), 50-64.9 years (n = 41), and greater than or equal to 65 years (n = 20).
The average weight reduction over the 72 weeks of follow-up was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for participants taking placebo (all P < .001 vs. placebo).
The percentages of participants reaching target weight reductions of greater than or equal to 5%, greater than or equal to 10%, greater than or equal to 15%, greater than or equal to 20%, and greater than or equal to 25% were recorded. Over 90% achieved greater than or equal to 5% weight loss, irrespective of BMI and tirzepatide dose, while 55.5% and 62.9% in the 10-mg and 15-mg groups achieved greater than or equal to 20% weight loss, and 35.0% and 39.7% in the 10-mg and 15-mg groups achieved greater than or equal to 25% weight loss, respectively.
By increasing BMI category, in the 10-mg group, weight loss was –18.2 kg, –21.9 kg, –22.0, and –20.7 kg; and in the 15-mg group, weight loss was –18.1kg, –21.2 kg, –24.5 kg, and –22.8 kg. Weight loss in the 5-mg group ranged from –16.6 kg to –15.9 kg from lowest to highest BMI category.
“In the lower-weight categories, there is less weight to lose, so we see a flattening of the curve [with a] maximum of around 18%, so it may be that as we learn more about a drug that is so potent, we recognize that we don’t need to use such a high dose in people with BMI 27-30 kg/m2,” he explained. “It’s the higher BMI categories where we need the higher dose.”
As with lifestyle and surgical treatments, participants taking tirzepatide had around a three times greater percentage reduction in fat mass than lean mass, resulting in an overall improvement in body composition, reported Dr. Aronne.
“We want loss of fat, not lean mass, and we know that we lose around one part lean to three parts fat mass when on a diet and exercise regimen,” he went on to explain. “We see exactly this [balance of lean-to-fat-mass loss] here with 33.9% total fat mass reduction in the treatment group, compared with 8.2% in the placebo group.”
Visceral fat mass reduction was 40% in the treatment group, compared with 7.3% with placebo. “It’s good to see there’s more loss of visceral fat,” said Dr. Aronne. Lean mass loss was 10.9%. “So around three times greater reduction in fat over lean mass loss, resulting in overall improvement of body composition,” he reported.
Also, in older people (≥ 65 years) there was approximately no difference in fat versus lean mass loss, compared with younger people, despite older people being more likely to lose more lean mass.
With respect to patient-reported outcomes based on the 36-item Short-Form Health Survey (SF-36), Dr. Aronne said that physical functioning scores significantly improved at 72 weeks, compared with placebo, particularly in participants with physical function limitations at baseline.
“In an interesting subanalysis, those with physical limitations at baseline showed a significant improvement versus placebo of over 5% difference [considered significant],” he added.
Safety and tolerability were previously reported in the NEJM article. The most common adverse events with tirzepatide were gastrointestinal, and adverse events causing treatment discontinuation occurred in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5-mg, 10-mg, and 15-mg doses or placebo, respectively.
“A revolution is coming in the treatment of obesity and cardiometabolic disease, and most physicians cannot grasp this. We’re finally getting the efficacy we’ve been looking for that will produce benefits in every realm,” concluded Dr. Aronne. “These data show that we are now hitting all the secondary endpoints and making our patients better.”
“I think this bodes well. I always envisioned a time when the treatment of obesity would come first before the treatment of cardiometabolic complications of obesity, and I think we’re on the verge of that era with semaglutide, tirzepatide, and the very exciting treatments to come.”
The SURMOUNT-1 trial was sponsored by Lilly. Dr. Aronne is cofounder, chief scientific advisor, and a member of the board of directors for Intellihealth. He is also a paid scientific advisory board member for Eli Lilly.
A version of this article first appeared on Medscape.com.
DUBLIN – , according to the latest results of the SURMOUNT-1 study.
The new analysis showed that up to 63% of participants achieved a reduction in body weight of at least 20%, and all three tirzepatide doses (5 mg, 10 mg, and 15 mg) led to substantial, clinically meaningful, and sustained body-weight reduction, compared with placebo at 72 weeks of follow-up.
Mean weight loss was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for placebo (all P < .001 vs. placebo). And among participants taking the highest 15-mg dose of tirzepatide, 96%, 90%, and 78% of patients achieved weight reductions of at least 5%, 10%, and 15%.
Tirzepatide is approved in the United States and the European Union for the treatment of type 2 diabetes but is not yet approved for obesity in any country. The manufacturer of tirzepatide, Eli Lilly, intends to seek approval for the drug as an obesity treatment from the U.S. Food and Drug Administration, European Medicines Agency, and in other territories beginning in 2023.
Regardless of baseline BMI category, 9 out of 10 people achieved the greater than or equal to 5% body weight reduction threshold across all doses of tirzepatide, and at the higher doses, over one-third achieved weight loss of 25% or more.
“Similar to lifestyle and surgical treatments, participants on tirzepatide had around a threefold greater percent reduction in fat mass, compared with lean mass, resulting in an overall improvement in body composition,” reported SURMOUNT-1 co-investigator Louis Aronne, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, New York.
“This is staggering weight loss,” remarked Dr. Aronne. “To put it in perspective, mean weight loss in people having Lap-Band surgery is 17%, mean weight loss for sleeve gastrectomy is 25%, and gastric bypass is 33%, which puts the effects of tirzepatide squarely in the realm of bariatric surgery.”
“Something we have sought for decades, we have finally been able to achieve,” he asserted. “I still remember exactly where I was when I saw these results for the first time last April. I knew something big was happening,” declared Dr. Aronne when presenting the latest analyses at the 2023 European Congress on Obesity. Full study results were published in the New England Journal of Medicine.
Moderator Gabriella Lieberman, MD, endocrinologist and head of the Israeli Center for Weight Management, Sheba Medical Center, Ramat-Gan, Israel, welcomed the study but also expressed caution. “It’s very potent, but as we see generally with potent therapies, I think it will change how we look at nutritional advice and the role of the dietician will change. I’m a bit worried the drug is running fast and the support, which is crucial with these treatments, is not keeping up, and we’ll have to deal with some effects later, such as sarcopenia,” she pointed out in an interview.
“We have to treat these drugs as if they are bariatric surgery. I see patients on these types of drugs in clinic and their appetite is so suppressed that they think they can afford to eat things that are unhealthy because they lose weight, and that’s what they want. There has to be a responsible adult looking at what they’re eating, and not just clapping their hands for the weight loss, but ensuring they are not deprived of anything,” she said.
Weight loss and body composition explored
Tirzepatide is a novel glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that works to activate the GIP and GLP-1 receptors, respectively, found in areas of the brain important for appetite regulation, decreasing food intake, and modulating fat utilization.
The phase 3, double-blind, randomized, controlled trial included data from 2,539 adults with a BMI greater than or equal to 30 kg/m2 (class I, II, III obesity) or greater than or equal to 27 kg/m2 (overweight) with one or more weight-related complications, excluding diabetes. At baseline, mean body weight was 104.8 kg, mean BMI was 38.0 kg/m2, and 94.5% of participants had BMI greater than or equal to 30 kg/m2.
Patients were randomized to once-weekly subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 72 weeks. The primary objective was to show that tirzepatide was superior to placebo in terms of percentage change in body weight and proportion of participants with body-weight reduction of greater than or equal to 5%. The percentage change from baseline body weight and proportion of participants with body weight reduction greater than or equal to 5% were also assessed across BMI categories of greater than or equal to 27 to less than 30 kg/m2, greater than or equal to 30 to less than 35 kg/m2 (class 1 obesity), greater than or equal to 35 to less than 40 kg/m2 (class 2 obesity), and greater than or equal to 40 kg/m2 (class 3 obesity).
In addition, in a retrospective subanalysis, body composition was evaluated in a subpopulation that underwent dual-energy x-ray absorptiometry, assessing change from baseline body composition within age subgroups less than 50 years (n = 99), 50-64.9 years (n = 41), and greater than or equal to 65 years (n = 20).
The average weight reduction over the 72 weeks of follow-up was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for participants taking placebo (all P < .001 vs. placebo).
The percentages of participants reaching target weight reductions of greater than or equal to 5%, greater than or equal to 10%, greater than or equal to 15%, greater than or equal to 20%, and greater than or equal to 25% were recorded. Over 90% achieved greater than or equal to 5% weight loss, irrespective of BMI and tirzepatide dose, while 55.5% and 62.9% in the 10-mg and 15-mg groups achieved greater than or equal to 20% weight loss, and 35.0% and 39.7% in the 10-mg and 15-mg groups achieved greater than or equal to 25% weight loss, respectively.
By increasing BMI category, in the 10-mg group, weight loss was –18.2 kg, –21.9 kg, –22.0, and –20.7 kg; and in the 15-mg group, weight loss was –18.1kg, –21.2 kg, –24.5 kg, and –22.8 kg. Weight loss in the 5-mg group ranged from –16.6 kg to –15.9 kg from lowest to highest BMI category.
“In the lower-weight categories, there is less weight to lose, so we see a flattening of the curve [with a] maximum of around 18%, so it may be that as we learn more about a drug that is so potent, we recognize that we don’t need to use such a high dose in people with BMI 27-30 kg/m2,” he explained. “It’s the higher BMI categories where we need the higher dose.”
As with lifestyle and surgical treatments, participants taking tirzepatide had around a three times greater percentage reduction in fat mass than lean mass, resulting in an overall improvement in body composition, reported Dr. Aronne.
“We want loss of fat, not lean mass, and we know that we lose around one part lean to three parts fat mass when on a diet and exercise regimen,” he went on to explain. “We see exactly this [balance of lean-to-fat-mass loss] here with 33.9% total fat mass reduction in the treatment group, compared with 8.2% in the placebo group.”
Visceral fat mass reduction was 40% in the treatment group, compared with 7.3% with placebo. “It’s good to see there’s more loss of visceral fat,” said Dr. Aronne. Lean mass loss was 10.9%. “So around three times greater reduction in fat over lean mass loss, resulting in overall improvement of body composition,” he reported.
Also, in older people (≥ 65 years) there was approximately no difference in fat versus lean mass loss, compared with younger people, despite older people being more likely to lose more lean mass.
With respect to patient-reported outcomes based on the 36-item Short-Form Health Survey (SF-36), Dr. Aronne said that physical functioning scores significantly improved at 72 weeks, compared with placebo, particularly in participants with physical function limitations at baseline.
“In an interesting subanalysis, those with physical limitations at baseline showed a significant improvement versus placebo of over 5% difference [considered significant],” he added.
Safety and tolerability were previously reported in the NEJM article. The most common adverse events with tirzepatide were gastrointestinal, and adverse events causing treatment discontinuation occurred in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5-mg, 10-mg, and 15-mg doses or placebo, respectively.
“A revolution is coming in the treatment of obesity and cardiometabolic disease, and most physicians cannot grasp this. We’re finally getting the efficacy we’ve been looking for that will produce benefits in every realm,” concluded Dr. Aronne. “These data show that we are now hitting all the secondary endpoints and making our patients better.”
“I think this bodes well. I always envisioned a time when the treatment of obesity would come first before the treatment of cardiometabolic complications of obesity, and I think we’re on the verge of that era with semaglutide, tirzepatide, and the very exciting treatments to come.”
The SURMOUNT-1 trial was sponsored by Lilly. Dr. Aronne is cofounder, chief scientific advisor, and a member of the board of directors for Intellihealth. He is also a paid scientific advisory board member for Eli Lilly.
A version of this article first appeared on Medscape.com.
Ear acupuncture with diet aids weight loss
DUBLIN – with high levels of visceral fat and overweight/obesity.
Three months of auricular acupuncture stimulation and dietary restriction led to a mean weight loss of nearly 9 kg plus a drop in waist circumference of more than 10 cm.
According to the researchers, acupuncture beads, used in Japan to augment weight loss for more than 30 years, are thought to stimulate nerves and organs that regulate appetite, satiety, hunger, and food cravings.
Findings of the observational study were presented by Takahiro Fujimoto, MD, PhD, Clinic F, Tokyo, at this year’s European Congress on Obesity.
Together with a prior study using the same intervention in women, Dr. Fujimoto and colleagues have now gathered data in more than 1,000 individuals, he said. “We wanted a method that was simple and noninvasive that would serve as a support to exercise and dietary therapy,” Dr. Fujimoto said in an interview.
“We believe there is an effect,” he asserted. “Acupuncture’s effect lies in stimulating the satiety center with benefits in helping individuals to control their food cravings and intake when reducing meals,” he said, pointing out that similar techniques have been used in patients undergoing withdrawal from drug addiction and in smoking cessation. He explained that acupuncture beads are believed to help individuals change their lifestyle habits, and added that “the relapse rate after 6 months is addressed in another paper, and it is very low.”
Professor Jason C.G. Halford, PhD, head of school at the University of Leeds, England, and president of the European Association for the Study of Obesity, commented on the findings. “There is no control group here receiving everything but the acupuncture,” he noted. “As such, it could be other elements of the intervention driving this [effect] including the act of keeping a food diary increasing awareness of one’s diet. A randomized controlled trial would be the next step.”
In women, the technique led to significantly more weight loss than in those who were untreated, and weight loss was maintained for 6 months after the end of treatment.
The researchers added that acupuncture stimulation with beads was a simpler method than traditional use of intradermal needles requiring expert acupuncturists. The stimulation is applied with 1.5-mm metal ear beads on 6 points of the outer ear (shen men, food pipe, upper stomach opening, stomach, lungs, and endocrine system) that correspond to meridian lines, and as such, restores the flow of qi by resolving any blockages or disruption. This may help with a variety of health conditions, according to the researchers. Placed on both ears, surgical tape was used to keep the beads in place to ensure participants continuously received uniform pressure on each of the six acupuncture points.
Dietary guidance was provided to participants to help reduce food intake by half, and nutritional supplements were given to compensate for any deficiencies. Participants attended twice-weekly clinic visits for bead sticking and diet progress monitoring. Body weight, body fat percentage, fat mass, lean mass, muscle mass, body mass index (BMI), and abdominal fat were assessed at the start and end of the study period.
“Since these tiny metal beads are attached to six points on the outer ear that stimulate nerves and organs which regulate appetite, satiety, and hunger, this type of acupuncture does not require complex knowledge or skill,” explained Dr. Fujimoto.
The results of the latest study, in men only, build on a prior study of more than 1,300 women who also received auricular acupuncture stimulation with beads as well as a halving of their food intake. In women, the weight loss program led to total body weight loss of 11.2% over 3 months.
At baseline, the 81 male participants, ages 21-78 years, had a mean BMI of 28.4 kg/m2 and mean waist circumference of 98.4 cm. Body fat percentage was 28.2%.
After 3 months, participants lost a mean of 8.6 kg (P < .001), decreased waist circumference by a mean of 10.4 cm (P < .001), and lost a mean of 4.0% of total body fat (P < .001). Visceral fat levels also fell by 2.2 points (P < .001), from 15.2 points at baseline to 13.0 points after 3 months. (A healthy visceral fat rating is between 1 and 12 points.) BMI decreased by almost 3 kg/m2 (from 28.4 at baseline to 25.5 at 3 months; P < .001).
Improvement in muscle-to-fat ratio was greater in men than women, whereas women had a greater decrease in percentage body fat than men.
“Whilst receiving ear acupuncture, the investigators asked participants to cut their food intake by half. It’s not unreasonable to expect that this major dietary change was the main reason participants lost weight,” remarked Graham Wheeler, PhD, statistical ambassador at the Royal Statistical Society, United Kingdom.
He also commented on the lack of a control group: “This study does not show us the impact of ear acupuncture on weight loss.”
Dr. Fujimoto and Dr. Halford have reported no relevant financial relationships. Dr. Wheeler is a statistical ambassador for the Royal Statistical Society, is employed by GSK, and holds an honorary senior lecturer post at Imperial College London.
A version of this article first appeared on Medscape.com.
DUBLIN – with high levels of visceral fat and overweight/obesity.
Three months of auricular acupuncture stimulation and dietary restriction led to a mean weight loss of nearly 9 kg plus a drop in waist circumference of more than 10 cm.
According to the researchers, acupuncture beads, used in Japan to augment weight loss for more than 30 years, are thought to stimulate nerves and organs that regulate appetite, satiety, hunger, and food cravings.
Findings of the observational study were presented by Takahiro Fujimoto, MD, PhD, Clinic F, Tokyo, at this year’s European Congress on Obesity.
Together with a prior study using the same intervention in women, Dr. Fujimoto and colleagues have now gathered data in more than 1,000 individuals, he said. “We wanted a method that was simple and noninvasive that would serve as a support to exercise and dietary therapy,” Dr. Fujimoto said in an interview.
“We believe there is an effect,” he asserted. “Acupuncture’s effect lies in stimulating the satiety center with benefits in helping individuals to control their food cravings and intake when reducing meals,” he said, pointing out that similar techniques have been used in patients undergoing withdrawal from drug addiction and in smoking cessation. He explained that acupuncture beads are believed to help individuals change their lifestyle habits, and added that “the relapse rate after 6 months is addressed in another paper, and it is very low.”
Professor Jason C.G. Halford, PhD, head of school at the University of Leeds, England, and president of the European Association for the Study of Obesity, commented on the findings. “There is no control group here receiving everything but the acupuncture,” he noted. “As such, it could be other elements of the intervention driving this [effect] including the act of keeping a food diary increasing awareness of one’s diet. A randomized controlled trial would be the next step.”
In women, the technique led to significantly more weight loss than in those who were untreated, and weight loss was maintained for 6 months after the end of treatment.
The researchers added that acupuncture stimulation with beads was a simpler method than traditional use of intradermal needles requiring expert acupuncturists. The stimulation is applied with 1.5-mm metal ear beads on 6 points of the outer ear (shen men, food pipe, upper stomach opening, stomach, lungs, and endocrine system) that correspond to meridian lines, and as such, restores the flow of qi by resolving any blockages or disruption. This may help with a variety of health conditions, according to the researchers. Placed on both ears, surgical tape was used to keep the beads in place to ensure participants continuously received uniform pressure on each of the six acupuncture points.
Dietary guidance was provided to participants to help reduce food intake by half, and nutritional supplements were given to compensate for any deficiencies. Participants attended twice-weekly clinic visits for bead sticking and diet progress monitoring. Body weight, body fat percentage, fat mass, lean mass, muscle mass, body mass index (BMI), and abdominal fat were assessed at the start and end of the study period.
“Since these tiny metal beads are attached to six points on the outer ear that stimulate nerves and organs which regulate appetite, satiety, and hunger, this type of acupuncture does not require complex knowledge or skill,” explained Dr. Fujimoto.
The results of the latest study, in men only, build on a prior study of more than 1,300 women who also received auricular acupuncture stimulation with beads as well as a halving of their food intake. In women, the weight loss program led to total body weight loss of 11.2% over 3 months.
At baseline, the 81 male participants, ages 21-78 years, had a mean BMI of 28.4 kg/m2 and mean waist circumference of 98.4 cm. Body fat percentage was 28.2%.
After 3 months, participants lost a mean of 8.6 kg (P < .001), decreased waist circumference by a mean of 10.4 cm (P < .001), and lost a mean of 4.0% of total body fat (P < .001). Visceral fat levels also fell by 2.2 points (P < .001), from 15.2 points at baseline to 13.0 points after 3 months. (A healthy visceral fat rating is between 1 and 12 points.) BMI decreased by almost 3 kg/m2 (from 28.4 at baseline to 25.5 at 3 months; P < .001).
Improvement in muscle-to-fat ratio was greater in men than women, whereas women had a greater decrease in percentage body fat than men.
“Whilst receiving ear acupuncture, the investigators asked participants to cut their food intake by half. It’s not unreasonable to expect that this major dietary change was the main reason participants lost weight,” remarked Graham Wheeler, PhD, statistical ambassador at the Royal Statistical Society, United Kingdom.
He also commented on the lack of a control group: “This study does not show us the impact of ear acupuncture on weight loss.”
Dr. Fujimoto and Dr. Halford have reported no relevant financial relationships. Dr. Wheeler is a statistical ambassador for the Royal Statistical Society, is employed by GSK, and holds an honorary senior lecturer post at Imperial College London.
A version of this article first appeared on Medscape.com.
DUBLIN – with high levels of visceral fat and overweight/obesity.
Three months of auricular acupuncture stimulation and dietary restriction led to a mean weight loss of nearly 9 kg plus a drop in waist circumference of more than 10 cm.
According to the researchers, acupuncture beads, used in Japan to augment weight loss for more than 30 years, are thought to stimulate nerves and organs that regulate appetite, satiety, hunger, and food cravings.
Findings of the observational study were presented by Takahiro Fujimoto, MD, PhD, Clinic F, Tokyo, at this year’s European Congress on Obesity.
Together with a prior study using the same intervention in women, Dr. Fujimoto and colleagues have now gathered data in more than 1,000 individuals, he said. “We wanted a method that was simple and noninvasive that would serve as a support to exercise and dietary therapy,” Dr. Fujimoto said in an interview.
“We believe there is an effect,” he asserted. “Acupuncture’s effect lies in stimulating the satiety center with benefits in helping individuals to control their food cravings and intake when reducing meals,” he said, pointing out that similar techniques have been used in patients undergoing withdrawal from drug addiction and in smoking cessation. He explained that acupuncture beads are believed to help individuals change their lifestyle habits, and added that “the relapse rate after 6 months is addressed in another paper, and it is very low.”
Professor Jason C.G. Halford, PhD, head of school at the University of Leeds, England, and president of the European Association for the Study of Obesity, commented on the findings. “There is no control group here receiving everything but the acupuncture,” he noted. “As such, it could be other elements of the intervention driving this [effect] including the act of keeping a food diary increasing awareness of one’s diet. A randomized controlled trial would be the next step.”
In women, the technique led to significantly more weight loss than in those who were untreated, and weight loss was maintained for 6 months after the end of treatment.
The researchers added that acupuncture stimulation with beads was a simpler method than traditional use of intradermal needles requiring expert acupuncturists. The stimulation is applied with 1.5-mm metal ear beads on 6 points of the outer ear (shen men, food pipe, upper stomach opening, stomach, lungs, and endocrine system) that correspond to meridian lines, and as such, restores the flow of qi by resolving any blockages or disruption. This may help with a variety of health conditions, according to the researchers. Placed on both ears, surgical tape was used to keep the beads in place to ensure participants continuously received uniform pressure on each of the six acupuncture points.
Dietary guidance was provided to participants to help reduce food intake by half, and nutritional supplements were given to compensate for any deficiencies. Participants attended twice-weekly clinic visits for bead sticking and diet progress monitoring. Body weight, body fat percentage, fat mass, lean mass, muscle mass, body mass index (BMI), and abdominal fat were assessed at the start and end of the study period.
“Since these tiny metal beads are attached to six points on the outer ear that stimulate nerves and organs which regulate appetite, satiety, and hunger, this type of acupuncture does not require complex knowledge or skill,” explained Dr. Fujimoto.
The results of the latest study, in men only, build on a prior study of more than 1,300 women who also received auricular acupuncture stimulation with beads as well as a halving of their food intake. In women, the weight loss program led to total body weight loss of 11.2% over 3 months.
At baseline, the 81 male participants, ages 21-78 years, had a mean BMI of 28.4 kg/m2 and mean waist circumference of 98.4 cm. Body fat percentage was 28.2%.
After 3 months, participants lost a mean of 8.6 kg (P < .001), decreased waist circumference by a mean of 10.4 cm (P < .001), and lost a mean of 4.0% of total body fat (P < .001). Visceral fat levels also fell by 2.2 points (P < .001), from 15.2 points at baseline to 13.0 points after 3 months. (A healthy visceral fat rating is between 1 and 12 points.) BMI decreased by almost 3 kg/m2 (from 28.4 at baseline to 25.5 at 3 months; P < .001).
Improvement in muscle-to-fat ratio was greater in men than women, whereas women had a greater decrease in percentage body fat than men.
“Whilst receiving ear acupuncture, the investigators asked participants to cut their food intake by half. It’s not unreasonable to expect that this major dietary change was the main reason participants lost weight,” remarked Graham Wheeler, PhD, statistical ambassador at the Royal Statistical Society, United Kingdom.
He also commented on the lack of a control group: “This study does not show us the impact of ear acupuncture on weight loss.”
Dr. Fujimoto and Dr. Halford have reported no relevant financial relationships. Dr. Wheeler is a statistical ambassador for the Royal Statistical Society, is employed by GSK, and holds an honorary senior lecturer post at Imperial College London.
A version of this article first appeared on Medscape.com.
AT ECO 2023
Biomarkers for measuring lupus nephritis treatment response gain ground
SEOUL, SOUTH KOREA – A panel of urinary biomarkers may do better than measuring proteinuria in predicting which patients with lupus nephritis are going to respond to treatment, according to a presentation at an international congress on systemic lupus erythematosus.
Physician-scientist Andrea Fava, MD, of the division of rheumatology at Johns Hopkins University, Baltimore, presented data from a study using urine proteomics to identify biomarkers present in the urine of patients with lupus nephritis at 3 months after starting treatment that were linked to better outcomes from that treatment at 1 year.
While proteinuria is the standard measure used to guide decisions about whether to do a kidney biopsy and how to treat lupus nephritis, it doesn’t always correlate with what’s actually going on inside the kidney in terms of histology and inflammation, Dr. Fava said.
He pointed to an earlier study in which researchers did kidney biopsies 6 months after patients with lupus nephritis started treatment with mycophenolate. This suggested that around half of patients who showed a clinical response to treatment – defined as proteinuria below 500 mg/day – still had significant histologic disease activity. Another study suggested that this elevated histologic disease activity is associated with a risk of flare, which can result in significant nephron loss. On the flip side, nearly two-thirds of patients in complete histologic remission still had elevated proteinuria.
Unfortunately, it’s not possible or practical to biopsy patients on a regular basis, Dr. Fava said. “So we need better biomarkers, and to do so, we need better knowledge of the pathophysiology because if we have biomarkers that reflect tissue biology in real-time, that may surely guide personalized treatments,” he said at the congress.
Dr. Fava and colleagues enrolled 225 patients with SLE who were undergoing kidney biopsy and 10 healthy controls and used proteomics to quantify the urinary levels of around 1,200 proteins at baseline, 3, 6, and 12 months after initiating treatment.
The team then analyzed these data to look for protein signatures that correlated with histologic phenotypes – particularly the amount of inflammation in the kidney – and clinical features such as response to treatment.
They found several protein biomarkers that appeared to be linked to histologic activity in the kidney, including interleukin (IL)-16, CD163, and neutrophil granule proteins.
Initially, the team looked at baseline levels of these proteins to see if they predicted who responded to treatment, but found no difference between responders and nonresponders.
However, when they looked at levels at 3 months after treatment, a pattern emerged. “We found that in patients who were not responding, there were no changes after 3 months of treatment in the urine proteome,” Dr. Fava said. Among those who did respond to treatment, the levels of these proteins – IL-16, CD163, galectin-1, and CD206 – decreased significantly.
“So the proteins that are linked to renal activity decrease only in responders, suggesting that effective immunosuppression is effective in reducing intrarenal inflammation, which eventually results in low proteinuria at 1 year.”
The decline in these biomarkers persisted at 1 year, and the study suggested it was a better predictor of which patients would respond to treatment at 1 year than proteinuria.
Dr. Fava said in an interview that better biomarkers could revolutionize the treatment and management of lupus nephritis.
“First of all, it can shift the management strategy from treatment to prevention, because at the very beginning we can nip it in the bud maybe with very gentle treatment,” he said. Different panels of urine biomarkers could identify patients at risk of treatment failure, and also help patients to taper off their immunosuppressive therapy without an increased risk of flare. “If we have a way to tell us there’s still inflammation that needs treatment, that could change the way we do it,” he said.
He acknowledged there are significant challenges to developing these biomarkers for clinical use; one is the decision of how to define disease activity without relying on proteinuria as a measure. “Why do I want a biomarker that can predict another biomarker?” he said.
Another presentation during the same session, by Huihua Ding, MD, of Shanghai Jiao Tong University in Shanghai, China, reported on the use of urinary L-selectin to assess renal disease activity and response to treatment in a multiethnic cohort.
This study, involving 474 patients with SLE with or without renal involvement in the United States and China, found levels of urinary L-selectin were elevated only in patients with active lupus nephritis and showed patterns that correlated with renal histologic characteristics.
Clinical rheumatologist Eric F. Morand, MD, PhD, and head of the School of Clinical Sciences at Monash University in Melbourne said one challenge with using urinary biomarkers was that it was not yet clear what these biomarkers reveal about the kidney. “It will be important to see whether this proteomic data actually link to renal outcomes,” Dr. Morand said in an interview. “I think predicting the response to treatment should be based around GFR [glomerular filtration rate] preservation, and I don’t think I’ve seen data yet that the urine biomarkers are going to tell us how to do that better.”
Dr. Morand is optimistic that urine biomarkers will one day be able to achieve that, but he stressed the importance of having urine biomarker tests available in the field at low cost. “You’re going to be doing the tests repeatedly, so therefore, you’re probably going to need to come down to a smaller list of proteins that you measure.”
Dr. Fava reported receiving support from Sanofi and Annexion Bio.
SEOUL, SOUTH KOREA – A panel of urinary biomarkers may do better than measuring proteinuria in predicting which patients with lupus nephritis are going to respond to treatment, according to a presentation at an international congress on systemic lupus erythematosus.
Physician-scientist Andrea Fava, MD, of the division of rheumatology at Johns Hopkins University, Baltimore, presented data from a study using urine proteomics to identify biomarkers present in the urine of patients with lupus nephritis at 3 months after starting treatment that were linked to better outcomes from that treatment at 1 year.
While proteinuria is the standard measure used to guide decisions about whether to do a kidney biopsy and how to treat lupus nephritis, it doesn’t always correlate with what’s actually going on inside the kidney in terms of histology and inflammation, Dr. Fava said.
He pointed to an earlier study in which researchers did kidney biopsies 6 months after patients with lupus nephritis started treatment with mycophenolate. This suggested that around half of patients who showed a clinical response to treatment – defined as proteinuria below 500 mg/day – still had significant histologic disease activity. Another study suggested that this elevated histologic disease activity is associated with a risk of flare, which can result in significant nephron loss. On the flip side, nearly two-thirds of patients in complete histologic remission still had elevated proteinuria.
Unfortunately, it’s not possible or practical to biopsy patients on a regular basis, Dr. Fava said. “So we need better biomarkers, and to do so, we need better knowledge of the pathophysiology because if we have biomarkers that reflect tissue biology in real-time, that may surely guide personalized treatments,” he said at the congress.
Dr. Fava and colleagues enrolled 225 patients with SLE who were undergoing kidney biopsy and 10 healthy controls and used proteomics to quantify the urinary levels of around 1,200 proteins at baseline, 3, 6, and 12 months after initiating treatment.
The team then analyzed these data to look for protein signatures that correlated with histologic phenotypes – particularly the amount of inflammation in the kidney – and clinical features such as response to treatment.
They found several protein biomarkers that appeared to be linked to histologic activity in the kidney, including interleukin (IL)-16, CD163, and neutrophil granule proteins.
Initially, the team looked at baseline levels of these proteins to see if they predicted who responded to treatment, but found no difference between responders and nonresponders.
However, when they looked at levels at 3 months after treatment, a pattern emerged. “We found that in patients who were not responding, there were no changes after 3 months of treatment in the urine proteome,” Dr. Fava said. Among those who did respond to treatment, the levels of these proteins – IL-16, CD163, galectin-1, and CD206 – decreased significantly.
“So the proteins that are linked to renal activity decrease only in responders, suggesting that effective immunosuppression is effective in reducing intrarenal inflammation, which eventually results in low proteinuria at 1 year.”
The decline in these biomarkers persisted at 1 year, and the study suggested it was a better predictor of which patients would respond to treatment at 1 year than proteinuria.
Dr. Fava said in an interview that better biomarkers could revolutionize the treatment and management of lupus nephritis.
“First of all, it can shift the management strategy from treatment to prevention, because at the very beginning we can nip it in the bud maybe with very gentle treatment,” he said. Different panels of urine biomarkers could identify patients at risk of treatment failure, and also help patients to taper off their immunosuppressive therapy without an increased risk of flare. “If we have a way to tell us there’s still inflammation that needs treatment, that could change the way we do it,” he said.
He acknowledged there are significant challenges to developing these biomarkers for clinical use; one is the decision of how to define disease activity without relying on proteinuria as a measure. “Why do I want a biomarker that can predict another biomarker?” he said.
Another presentation during the same session, by Huihua Ding, MD, of Shanghai Jiao Tong University in Shanghai, China, reported on the use of urinary L-selectin to assess renal disease activity and response to treatment in a multiethnic cohort.
This study, involving 474 patients with SLE with or without renal involvement in the United States and China, found levels of urinary L-selectin were elevated only in patients with active lupus nephritis and showed patterns that correlated with renal histologic characteristics.
Clinical rheumatologist Eric F. Morand, MD, PhD, and head of the School of Clinical Sciences at Monash University in Melbourne said one challenge with using urinary biomarkers was that it was not yet clear what these biomarkers reveal about the kidney. “It will be important to see whether this proteomic data actually link to renal outcomes,” Dr. Morand said in an interview. “I think predicting the response to treatment should be based around GFR [glomerular filtration rate] preservation, and I don’t think I’ve seen data yet that the urine biomarkers are going to tell us how to do that better.”
Dr. Morand is optimistic that urine biomarkers will one day be able to achieve that, but he stressed the importance of having urine biomarker tests available in the field at low cost. “You’re going to be doing the tests repeatedly, so therefore, you’re probably going to need to come down to a smaller list of proteins that you measure.”
Dr. Fava reported receiving support from Sanofi and Annexion Bio.
SEOUL, SOUTH KOREA – A panel of urinary biomarkers may do better than measuring proteinuria in predicting which patients with lupus nephritis are going to respond to treatment, according to a presentation at an international congress on systemic lupus erythematosus.
Physician-scientist Andrea Fava, MD, of the division of rheumatology at Johns Hopkins University, Baltimore, presented data from a study using urine proteomics to identify biomarkers present in the urine of patients with lupus nephritis at 3 months after starting treatment that were linked to better outcomes from that treatment at 1 year.
While proteinuria is the standard measure used to guide decisions about whether to do a kidney biopsy and how to treat lupus nephritis, it doesn’t always correlate with what’s actually going on inside the kidney in terms of histology and inflammation, Dr. Fava said.
He pointed to an earlier study in which researchers did kidney biopsies 6 months after patients with lupus nephritis started treatment with mycophenolate. This suggested that around half of patients who showed a clinical response to treatment – defined as proteinuria below 500 mg/day – still had significant histologic disease activity. Another study suggested that this elevated histologic disease activity is associated with a risk of flare, which can result in significant nephron loss. On the flip side, nearly two-thirds of patients in complete histologic remission still had elevated proteinuria.
Unfortunately, it’s not possible or practical to biopsy patients on a regular basis, Dr. Fava said. “So we need better biomarkers, and to do so, we need better knowledge of the pathophysiology because if we have biomarkers that reflect tissue biology in real-time, that may surely guide personalized treatments,” he said at the congress.
Dr. Fava and colleagues enrolled 225 patients with SLE who were undergoing kidney biopsy and 10 healthy controls and used proteomics to quantify the urinary levels of around 1,200 proteins at baseline, 3, 6, and 12 months after initiating treatment.
The team then analyzed these data to look for protein signatures that correlated with histologic phenotypes – particularly the amount of inflammation in the kidney – and clinical features such as response to treatment.
They found several protein biomarkers that appeared to be linked to histologic activity in the kidney, including interleukin (IL)-16, CD163, and neutrophil granule proteins.
Initially, the team looked at baseline levels of these proteins to see if they predicted who responded to treatment, but found no difference between responders and nonresponders.
However, when they looked at levels at 3 months after treatment, a pattern emerged. “We found that in patients who were not responding, there were no changes after 3 months of treatment in the urine proteome,” Dr. Fava said. Among those who did respond to treatment, the levels of these proteins – IL-16, CD163, galectin-1, and CD206 – decreased significantly.
“So the proteins that are linked to renal activity decrease only in responders, suggesting that effective immunosuppression is effective in reducing intrarenal inflammation, which eventually results in low proteinuria at 1 year.”
The decline in these biomarkers persisted at 1 year, and the study suggested it was a better predictor of which patients would respond to treatment at 1 year than proteinuria.
Dr. Fava said in an interview that better biomarkers could revolutionize the treatment and management of lupus nephritis.
“First of all, it can shift the management strategy from treatment to prevention, because at the very beginning we can nip it in the bud maybe with very gentle treatment,” he said. Different panels of urine biomarkers could identify patients at risk of treatment failure, and also help patients to taper off their immunosuppressive therapy without an increased risk of flare. “If we have a way to tell us there’s still inflammation that needs treatment, that could change the way we do it,” he said.
He acknowledged there are significant challenges to developing these biomarkers for clinical use; one is the decision of how to define disease activity without relying on proteinuria as a measure. “Why do I want a biomarker that can predict another biomarker?” he said.
Another presentation during the same session, by Huihua Ding, MD, of Shanghai Jiao Tong University in Shanghai, China, reported on the use of urinary L-selectin to assess renal disease activity and response to treatment in a multiethnic cohort.
This study, involving 474 patients with SLE with or without renal involvement in the United States and China, found levels of urinary L-selectin were elevated only in patients with active lupus nephritis and showed patterns that correlated with renal histologic characteristics.
Clinical rheumatologist Eric F. Morand, MD, PhD, and head of the School of Clinical Sciences at Monash University in Melbourne said one challenge with using urinary biomarkers was that it was not yet clear what these biomarkers reveal about the kidney. “It will be important to see whether this proteomic data actually link to renal outcomes,” Dr. Morand said in an interview. “I think predicting the response to treatment should be based around GFR [glomerular filtration rate] preservation, and I don’t think I’ve seen data yet that the urine biomarkers are going to tell us how to do that better.”
Dr. Morand is optimistic that urine biomarkers will one day be able to achieve that, but he stressed the importance of having urine biomarker tests available in the field at low cost. “You’re going to be doing the tests repeatedly, so therefore, you’re probably going to need to come down to a smaller list of proteins that you measure.”
Dr. Fava reported receiving support from Sanofi and Annexion Bio.
AT LUPUS 2023
MACE, VTE rates compared between TNF and JAK inhibitors for AxSpA and PsA
CLEVELAND – Patients with axial spondyloarthritis or psoriatic arthritis who used Janus kinase (JAK) inhibitors did not have higher risk of myocardial infarction, stroke, or venous thromboembolism (VTE), compared with those who used tumor necrosis factor inhibitors (TNFi), according to new research.
The information was presented in a poster at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
Patients with axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA) have increased cardiovascular risk compared with the general population. Emerging evidence has suggested that TNFi may protect the cardiovascular system and that there are cardiovascular and thrombotic concerns with JAK inhibitors.
Sali Merjanah, MD, a rheumatology fellow at Boston University, and colleagues, compared how drugs in the two treatment classes affected the likelihood of major adverse cardiovascular events (MACE) or VTE. MACE in this study were myocardial infarction and stroke.
In a search of the Marketscan Database during 2006-2021, the researchers identified 1,621 TNFi and 47 JAK inhibitor users with 273 and 8 cases of MACE, respectively. They identified 2,507 TNFi and 96 JAK users with 452 and 26 cases of VTE, respectively. Patients were aged 18-65 years and had at least one inpatient or two outpatient axSpA or PsA ICD-9 or ICD-10 diagnosis codes separated by at least 7 days.
The likelihood of MACE was 14% lower among JAK inhibitor users than TNFi users (the reference group), whereas the likelihood of VTE was 39% higher for JAK inhibitor users, but neither comparison was statistically significant. JAK/TNFi nonusers had a statistically significant 27% greater likelihood of MACE than did TNFi users. The likelihood for VTE was 12% higher for JAK/TNFi nonusers, compared with TNFi users, but this finding was not statistically significant. The researchers adjusted comparisons for age, medications, and comorbidities.
Small numbers complicate the research
Lianne Gensler, MD, director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco, who was not part of the study, said the limitations the authors list are important to note. The researchers said that the study’s small number of JAK inhibitor users, short duration of exposure, and low event rate limit its precision, and there is potential misclassification of TNF/JAK inhibitor exposure, as well as confounding by indication.
Dr. Gensler noted that these same limitations apply to studies of patients with RA as well that try to answer the question of risk for MACE and malignancy when using these drugs,
“MACE is a rare event, malignancy is a rare event. So it’s like finding a needle in a haystack, and the haystack is really big. You either have to enrich the haystack with more needles or you have to make a smaller haystack,” Dr. Gensler said.
Nevertheless, she said, she credits the researchers for bringing the available information to light.
“I think we have to do this many different ways to try to get at the answer in a partial way,” she said.
The data were drawn from 2006 to 2021, but JAK inhibitors have only been approved for axSpA in the last one and a half years and for PsA at the end of 2017.
Additionally, the people taking JAK inhibitors would have likely already failed TNFis, she said, adding that this can make it hard to tell whether an event was linked with the JAK or the TNFi.
Nonusers may have other risk factors
She pointed out that in this study patients who were not using TNF or JAK inhibitors had slightly higher risk numerically for both MACE and VTE than did those using TNFis.
“There, the assumption is always that this is confounding by indication, meaning it is likely that the people who are nonusers have other risk factors for MACE, which is why we’re not giving them these drugs.”
Having heart failure, for instance, is a contraindication for using a TNF inhibitor, she noted. “So it’s not that these are protective compared to nonusers. It’s probably that the nonuser has higher risk and is not getting treated with these drugs to begin with.”
The authors properly concluded from the data that patients using JAK inhibitors did not have higher risk of MACE or VTE, compared with those who used TNFis, she said, but larger studies with more follow-up are needed.
“No evidence doesn’t mean no effect,” she said. “Part of it depends on the [statistical] power and the population you’re studying.”
Dr. Gensler is a consultant for AbbVie, Acceleron, Eli Lilly, Janssen, Novartis, Pfizer, and UCB; and has received grant support from Novartis and UCB. The authors’ financial relationships were not available.
CLEVELAND – Patients with axial spondyloarthritis or psoriatic arthritis who used Janus kinase (JAK) inhibitors did not have higher risk of myocardial infarction, stroke, or venous thromboembolism (VTE), compared with those who used tumor necrosis factor inhibitors (TNFi), according to new research.
The information was presented in a poster at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
Patients with axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA) have increased cardiovascular risk compared with the general population. Emerging evidence has suggested that TNFi may protect the cardiovascular system and that there are cardiovascular and thrombotic concerns with JAK inhibitors.
Sali Merjanah, MD, a rheumatology fellow at Boston University, and colleagues, compared how drugs in the two treatment classes affected the likelihood of major adverse cardiovascular events (MACE) or VTE. MACE in this study were myocardial infarction and stroke.
In a search of the Marketscan Database during 2006-2021, the researchers identified 1,621 TNFi and 47 JAK inhibitor users with 273 and 8 cases of MACE, respectively. They identified 2,507 TNFi and 96 JAK users with 452 and 26 cases of VTE, respectively. Patients were aged 18-65 years and had at least one inpatient or two outpatient axSpA or PsA ICD-9 or ICD-10 diagnosis codes separated by at least 7 days.
The likelihood of MACE was 14% lower among JAK inhibitor users than TNFi users (the reference group), whereas the likelihood of VTE was 39% higher for JAK inhibitor users, but neither comparison was statistically significant. JAK/TNFi nonusers had a statistically significant 27% greater likelihood of MACE than did TNFi users. The likelihood for VTE was 12% higher for JAK/TNFi nonusers, compared with TNFi users, but this finding was not statistically significant. The researchers adjusted comparisons for age, medications, and comorbidities.
Small numbers complicate the research
Lianne Gensler, MD, director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco, who was not part of the study, said the limitations the authors list are important to note. The researchers said that the study’s small number of JAK inhibitor users, short duration of exposure, and low event rate limit its precision, and there is potential misclassification of TNF/JAK inhibitor exposure, as well as confounding by indication.
Dr. Gensler noted that these same limitations apply to studies of patients with RA as well that try to answer the question of risk for MACE and malignancy when using these drugs,
“MACE is a rare event, malignancy is a rare event. So it’s like finding a needle in a haystack, and the haystack is really big. You either have to enrich the haystack with more needles or you have to make a smaller haystack,” Dr. Gensler said.
Nevertheless, she said, she credits the researchers for bringing the available information to light.
“I think we have to do this many different ways to try to get at the answer in a partial way,” she said.
The data were drawn from 2006 to 2021, but JAK inhibitors have only been approved for axSpA in the last one and a half years and for PsA at the end of 2017.
Additionally, the people taking JAK inhibitors would have likely already failed TNFis, she said, adding that this can make it hard to tell whether an event was linked with the JAK or the TNFi.
Nonusers may have other risk factors
She pointed out that in this study patients who were not using TNF or JAK inhibitors had slightly higher risk numerically for both MACE and VTE than did those using TNFis.
“There, the assumption is always that this is confounding by indication, meaning it is likely that the people who are nonusers have other risk factors for MACE, which is why we’re not giving them these drugs.”
Having heart failure, for instance, is a contraindication for using a TNF inhibitor, she noted. “So it’s not that these are protective compared to nonusers. It’s probably that the nonuser has higher risk and is not getting treated with these drugs to begin with.”
The authors properly concluded from the data that patients using JAK inhibitors did not have higher risk of MACE or VTE, compared with those who used TNFis, she said, but larger studies with more follow-up are needed.
“No evidence doesn’t mean no effect,” she said. “Part of it depends on the [statistical] power and the population you’re studying.”
Dr. Gensler is a consultant for AbbVie, Acceleron, Eli Lilly, Janssen, Novartis, Pfizer, and UCB; and has received grant support from Novartis and UCB. The authors’ financial relationships were not available.
CLEVELAND – Patients with axial spondyloarthritis or psoriatic arthritis who used Janus kinase (JAK) inhibitors did not have higher risk of myocardial infarction, stroke, or venous thromboembolism (VTE), compared with those who used tumor necrosis factor inhibitors (TNFi), according to new research.
The information was presented in a poster at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
Patients with axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA) have increased cardiovascular risk compared with the general population. Emerging evidence has suggested that TNFi may protect the cardiovascular system and that there are cardiovascular and thrombotic concerns with JAK inhibitors.
Sali Merjanah, MD, a rheumatology fellow at Boston University, and colleagues, compared how drugs in the two treatment classes affected the likelihood of major adverse cardiovascular events (MACE) or VTE. MACE in this study were myocardial infarction and stroke.
In a search of the Marketscan Database during 2006-2021, the researchers identified 1,621 TNFi and 47 JAK inhibitor users with 273 and 8 cases of MACE, respectively. They identified 2,507 TNFi and 96 JAK users with 452 and 26 cases of VTE, respectively. Patients were aged 18-65 years and had at least one inpatient or two outpatient axSpA or PsA ICD-9 or ICD-10 diagnosis codes separated by at least 7 days.
The likelihood of MACE was 14% lower among JAK inhibitor users than TNFi users (the reference group), whereas the likelihood of VTE was 39% higher for JAK inhibitor users, but neither comparison was statistically significant. JAK/TNFi nonusers had a statistically significant 27% greater likelihood of MACE than did TNFi users. The likelihood for VTE was 12% higher for JAK/TNFi nonusers, compared with TNFi users, but this finding was not statistically significant. The researchers adjusted comparisons for age, medications, and comorbidities.
Small numbers complicate the research
Lianne Gensler, MD, director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco, who was not part of the study, said the limitations the authors list are important to note. The researchers said that the study’s small number of JAK inhibitor users, short duration of exposure, and low event rate limit its precision, and there is potential misclassification of TNF/JAK inhibitor exposure, as well as confounding by indication.
Dr. Gensler noted that these same limitations apply to studies of patients with RA as well that try to answer the question of risk for MACE and malignancy when using these drugs,
“MACE is a rare event, malignancy is a rare event. So it’s like finding a needle in a haystack, and the haystack is really big. You either have to enrich the haystack with more needles or you have to make a smaller haystack,” Dr. Gensler said.
Nevertheless, she said, she credits the researchers for bringing the available information to light.
“I think we have to do this many different ways to try to get at the answer in a partial way,” she said.
The data were drawn from 2006 to 2021, but JAK inhibitors have only been approved for axSpA in the last one and a half years and for PsA at the end of 2017.
Additionally, the people taking JAK inhibitors would have likely already failed TNFis, she said, adding that this can make it hard to tell whether an event was linked with the JAK or the TNFi.
Nonusers may have other risk factors
She pointed out that in this study patients who were not using TNF or JAK inhibitors had slightly higher risk numerically for both MACE and VTE than did those using TNFis.
“There, the assumption is always that this is confounding by indication, meaning it is likely that the people who are nonusers have other risk factors for MACE, which is why we’re not giving them these drugs.”
Having heart failure, for instance, is a contraindication for using a TNF inhibitor, she noted. “So it’s not that these are protective compared to nonusers. It’s probably that the nonuser has higher risk and is not getting treated with these drugs to begin with.”
The authors properly concluded from the data that patients using JAK inhibitors did not have higher risk of MACE or VTE, compared with those who used TNFis, she said, but larger studies with more follow-up are needed.
“No evidence doesn’t mean no effect,” she said. “Part of it depends on the [statistical] power and the population you’re studying.”
Dr. Gensler is a consultant for AbbVie, Acceleron, Eli Lilly, Janssen, Novartis, Pfizer, and UCB; and has received grant support from Novartis and UCB. The authors’ financial relationships were not available.
AT SPARTAN 2023
FDA approves upadacitinib for Crohn’s disease
whose condition failed to respond adequately or who can’t tolerate one or more tumor necrosis factor (TNF) inhibitors, the company has announced.
Upadacitinib (Rinvoq, AbbVie) is the first oral small molecule approved by the FDA for Crohn’s disease, which is noteworthy, said Kristin E. Burke, MD, MPH, medical director of clinical operations for the Massachusetts General Hospital Crohn’s and Colitis Center, Boston.
“Crohn’s disease is a complex immune-mediated disease for which more effective and fast-acting treatment options are needed. The approval of upadacitinib for anti-TNF refractory Crohn’s disease represents an important milestone in the expansion of treatment options for this disease as the first oral small molecule available,” she said.
The approval for Crohn’s disease was supported by data from two induction studies (U-EXCEED and U-EXCEL) and one maintenance study (U-ENDURE).
In the two induction studies, 857 patients were randomly assigned to receive upadacitinib 45 mg or placebo once daily for 12 weeks. At week 12, a greater proportion of patients who received upadacitinib (vs. those who received placebo) achieved clinical remission, as determined on the basis of the Crohn’s Disease Activity Index (CDAI), and improvement in intestinal inflammation as assessed by colonoscopy.
In the maintenance study, 343 patients who responded to induction therapy with upadacitinib were randomly assigned to receive either a maintenance regimen of 15 or 30 mg once daily or placebo for 52 weeks.
At week 52, a greater proportion of patients who were treated with upadacitinib 15 mg or 30 mg, compared with those who received placebo, achieved clinical remission.
Data from the trials of upadacitinib in Crohn’s disease were presented at the European Crohn’s and Colitis Organisation (ECCO) 2023 Congress in March.
“Symptoms of moderately to severely active Crohn’s disease can be disruptive and uncomfortable for patients, so relief as early as possible is key. Given the progressive nature of the disease, endoscopic response is just as important,” U-EXCEL study investigator Edward V. Loftus Jr., MD, professor of medicine in the division of gastroenterology and hepatology at Mayo Clinic in Rochester, Minn., said in a news release.
“Based on the clinical trial results, treatment with Rinvoq shows both early and long-term symptom relief along with evidence of a visible reduction of damage to the intestinal lining caused by excess inflammation,” he said.
Patients should initially be given 45 mg of upadacitinib once daily for 12 weeks. After 12 weeks, the recommended maintenance dosage is 15 mg once a day. A maintenance dose of 30 mg once daily can be considered for patients with refractory, severe, or extensive Crohn’s disease, the FDA said in a statement announcing approval.
The most common side effects of upadacitinib in patients with Crohn’s disease are upper respiratory tract infection, anemia, fever, acne, herpes zoster, and headache.
Upadacitinib is not recommended for use in combination with other JAK inhibitors, biological therapies for Crohn’s disease, or with strong immunosuppressants, such as azathioprine and cyclosporine.
Serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis have occurred with JAK inhibitors such as upadacitinib.
The indication in Crohn’s disease marks the seventh in the United States for the JAK inhibitor. Other indications include rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis.
Full prescribing information is available online.
Dr. Burke disclosed no conflicts. Dr. Loftus is a consultant and adviser for AbbVie.
A version of this article first appeared on Medscape.com.
whose condition failed to respond adequately or who can’t tolerate one or more tumor necrosis factor (TNF) inhibitors, the company has announced.
Upadacitinib (Rinvoq, AbbVie) is the first oral small molecule approved by the FDA for Crohn’s disease, which is noteworthy, said Kristin E. Burke, MD, MPH, medical director of clinical operations for the Massachusetts General Hospital Crohn’s and Colitis Center, Boston.
“Crohn’s disease is a complex immune-mediated disease for which more effective and fast-acting treatment options are needed. The approval of upadacitinib for anti-TNF refractory Crohn’s disease represents an important milestone in the expansion of treatment options for this disease as the first oral small molecule available,” she said.
The approval for Crohn’s disease was supported by data from two induction studies (U-EXCEED and U-EXCEL) and one maintenance study (U-ENDURE).
In the two induction studies, 857 patients were randomly assigned to receive upadacitinib 45 mg or placebo once daily for 12 weeks. At week 12, a greater proportion of patients who received upadacitinib (vs. those who received placebo) achieved clinical remission, as determined on the basis of the Crohn’s Disease Activity Index (CDAI), and improvement in intestinal inflammation as assessed by colonoscopy.
In the maintenance study, 343 patients who responded to induction therapy with upadacitinib were randomly assigned to receive either a maintenance regimen of 15 or 30 mg once daily or placebo for 52 weeks.
At week 52, a greater proportion of patients who were treated with upadacitinib 15 mg or 30 mg, compared with those who received placebo, achieved clinical remission.
Data from the trials of upadacitinib in Crohn’s disease were presented at the European Crohn’s and Colitis Organisation (ECCO) 2023 Congress in March.
“Symptoms of moderately to severely active Crohn’s disease can be disruptive and uncomfortable for patients, so relief as early as possible is key. Given the progressive nature of the disease, endoscopic response is just as important,” U-EXCEL study investigator Edward V. Loftus Jr., MD, professor of medicine in the division of gastroenterology and hepatology at Mayo Clinic in Rochester, Minn., said in a news release.
“Based on the clinical trial results, treatment with Rinvoq shows both early and long-term symptom relief along with evidence of a visible reduction of damage to the intestinal lining caused by excess inflammation,” he said.
Patients should initially be given 45 mg of upadacitinib once daily for 12 weeks. After 12 weeks, the recommended maintenance dosage is 15 mg once a day. A maintenance dose of 30 mg once daily can be considered for patients with refractory, severe, or extensive Crohn’s disease, the FDA said in a statement announcing approval.
The most common side effects of upadacitinib in patients with Crohn’s disease are upper respiratory tract infection, anemia, fever, acne, herpes zoster, and headache.
Upadacitinib is not recommended for use in combination with other JAK inhibitors, biological therapies for Crohn’s disease, or with strong immunosuppressants, such as azathioprine and cyclosporine.
Serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis have occurred with JAK inhibitors such as upadacitinib.
The indication in Crohn’s disease marks the seventh in the United States for the JAK inhibitor. Other indications include rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis.
Full prescribing information is available online.
Dr. Burke disclosed no conflicts. Dr. Loftus is a consultant and adviser for AbbVie.
A version of this article first appeared on Medscape.com.
whose condition failed to respond adequately or who can’t tolerate one or more tumor necrosis factor (TNF) inhibitors, the company has announced.
Upadacitinib (Rinvoq, AbbVie) is the first oral small molecule approved by the FDA for Crohn’s disease, which is noteworthy, said Kristin E. Burke, MD, MPH, medical director of clinical operations for the Massachusetts General Hospital Crohn’s and Colitis Center, Boston.
“Crohn’s disease is a complex immune-mediated disease for which more effective and fast-acting treatment options are needed. The approval of upadacitinib for anti-TNF refractory Crohn’s disease represents an important milestone in the expansion of treatment options for this disease as the first oral small molecule available,” she said.
The approval for Crohn’s disease was supported by data from two induction studies (U-EXCEED and U-EXCEL) and one maintenance study (U-ENDURE).
In the two induction studies, 857 patients were randomly assigned to receive upadacitinib 45 mg or placebo once daily for 12 weeks. At week 12, a greater proportion of patients who received upadacitinib (vs. those who received placebo) achieved clinical remission, as determined on the basis of the Crohn’s Disease Activity Index (CDAI), and improvement in intestinal inflammation as assessed by colonoscopy.
In the maintenance study, 343 patients who responded to induction therapy with upadacitinib were randomly assigned to receive either a maintenance regimen of 15 or 30 mg once daily or placebo for 52 weeks.
At week 52, a greater proportion of patients who were treated with upadacitinib 15 mg or 30 mg, compared with those who received placebo, achieved clinical remission.
Data from the trials of upadacitinib in Crohn’s disease were presented at the European Crohn’s and Colitis Organisation (ECCO) 2023 Congress in March.
“Symptoms of moderately to severely active Crohn’s disease can be disruptive and uncomfortable for patients, so relief as early as possible is key. Given the progressive nature of the disease, endoscopic response is just as important,” U-EXCEL study investigator Edward V. Loftus Jr., MD, professor of medicine in the division of gastroenterology and hepatology at Mayo Clinic in Rochester, Minn., said in a news release.
“Based on the clinical trial results, treatment with Rinvoq shows both early and long-term symptom relief along with evidence of a visible reduction of damage to the intestinal lining caused by excess inflammation,” he said.
Patients should initially be given 45 mg of upadacitinib once daily for 12 weeks. After 12 weeks, the recommended maintenance dosage is 15 mg once a day. A maintenance dose of 30 mg once daily can be considered for patients with refractory, severe, or extensive Crohn’s disease, the FDA said in a statement announcing approval.
The most common side effects of upadacitinib in patients with Crohn’s disease are upper respiratory tract infection, anemia, fever, acne, herpes zoster, and headache.
Upadacitinib is not recommended for use in combination with other JAK inhibitors, biological therapies for Crohn’s disease, or with strong immunosuppressants, such as azathioprine and cyclosporine.
Serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis have occurred with JAK inhibitors such as upadacitinib.
The indication in Crohn’s disease marks the seventh in the United States for the JAK inhibitor. Other indications include rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis.
Full prescribing information is available online.
Dr. Burke disclosed no conflicts. Dr. Loftus is a consultant and adviser for AbbVie.
A version of this article first appeared on Medscape.com.
The Hospitalist Triage Role for Reducing Admission Delays: Impacts on Throughput, Quality, Interprofessional Practice, and Clinician Experience of Care
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).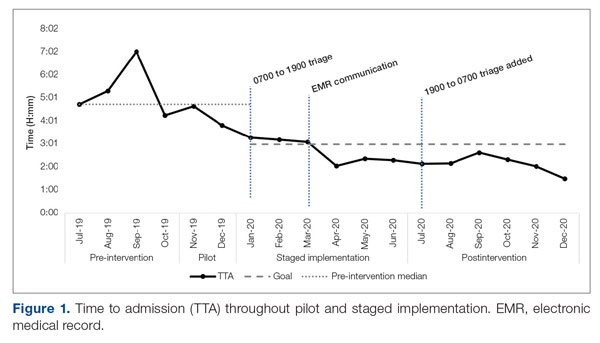
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).
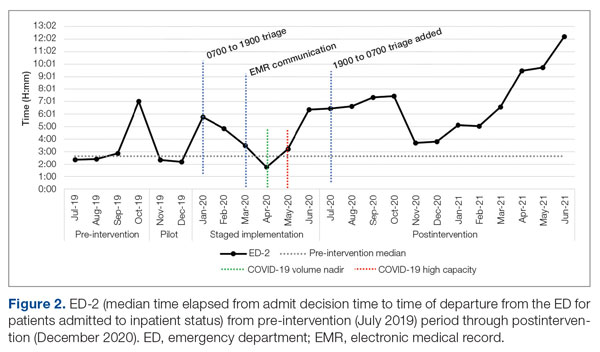
There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.
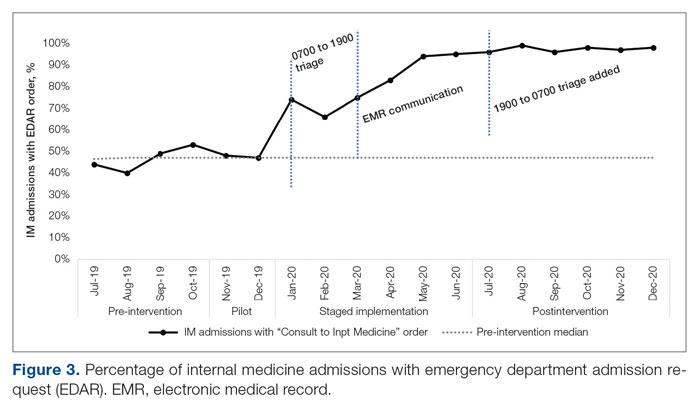
Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions
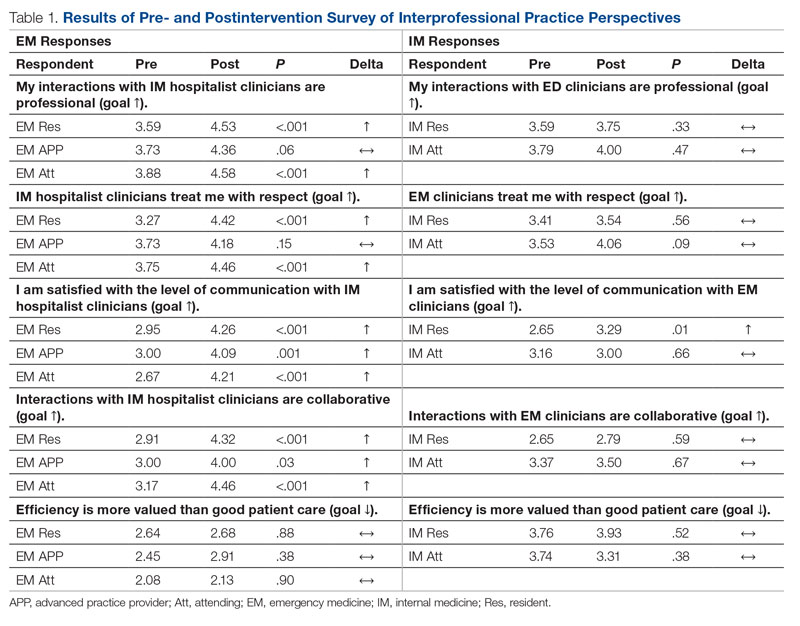
For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.
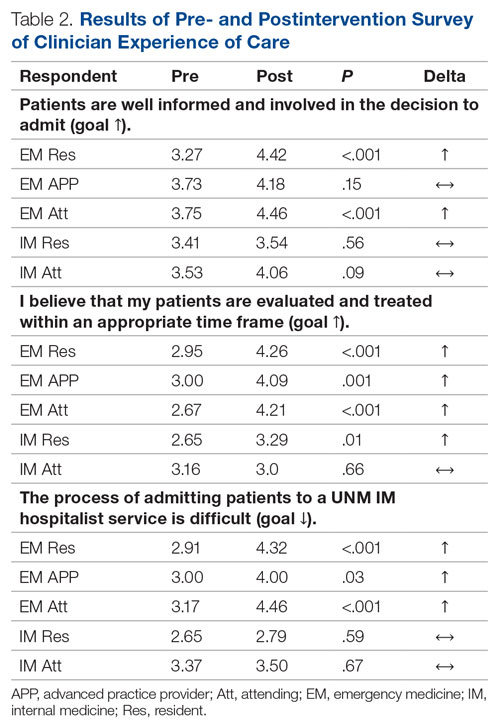
Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected]
Disclosures: None reported.
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).

There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.

Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions

For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.

Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected]
Disclosures: None reported.
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).

There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.

Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions

For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.

Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected]
Disclosures: None reported.
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
Glucagon Prescription Rates for Individuals With Type 1 Diabetes Mellitus Following Implementation of an Electronic Health Records Intervention
From Vanderbilt University School of Medicine, and Vanderbilt University Medical Center, Nashville, TN.
ABSTRACT
Objective: Severe hypoglycemia can alter consciousness and inhibit oral intake, requiring nonoral rescue glucagon administration to raise blood glucose to safe levels. Thus, current guidelines recommend glucagon kit prescriptions for all patients at risk for hypoglycemia, especially patients with type 1 diabetes mellitus (T1DM). At the diabetes outpatient clinic at a tertiary medical center, glucagon prescription rates for T1DM patients remained suboptimal.
Methods: A quality improvement team analyzed patient flow through the endocrinology clinic and identified the lack of a systematic approach to assessing patients for home glucagon prescriptions as a major barrier. The team implemented 2 successive interventions. First, intake staff indicated whether patients lacked an active glucagon prescription on patients’ face sheets. Second, clinical pharmacists reviewed patient prescriptions prior to scheduled visits and pended glucagon orders for patients without active prescriptions. Of note, when a pharmacy pends an order, the pharmacist enters an order into the electronic health record (EHR) but does not sign it. The order is saved for a provider to later access and sign. A statistical process control p-chart tracked monthly prescription rates.
Results: After 7 months, glucagon prescription rates increased from a baseline of 59% to 72% as the new steady state.
Conclusion: This project demonstrates that a series of interventions can improve glucagon prescription rates for patients at risk for hypoglycemia. The project’s success stemmed from combining an EHR-generated report and interdisciplinary staff members’ involvement. Other endocrinology clinics may incorporate this approach to implement similar processes and improve glucagon prescription rates.
Keywords: diabetes, hypoglycemia, glucagon, quality improvement, prescription rates, medical student.
Hypoglycemia limits the management of blood glucose in patients with type 1 diabetes mellitus (T1DM). Severe hypoglycemia, characterized by altered mental status (AMS) or physical status requiring assistance for recovery, can lead to seizure, coma, or death.1 Hypoglycemia in diabetes often occurs iatrogenically, primarily from insulin therapy: 30% to 40% of patients with T1DM and 10% to 30% of patients with insulin-treated type 2 diabetes mellitus experience severe hypoglycemia in a given year.2 One study estimated that nearly 100,000 emergency department visits for hypoglycemia occur in the United States per year, with almost one-third resulting in hospitalization.3
Most patients self-treat mild hypoglycemia with oral intake of carbohydrates. However, since hypoglycemia-induced nausea and AMS can make oral intake more difficult or prevent it entirely, patients require a treatment that family, friends, or coworkers can administer. Rescue glucagon, prescribed as intramuscular injections or intranasal sprays, raises blood glucose to safe levels in 10 to 15 minutes.4 Therefore, the American Diabetes Association (ADA) recommends glucagon for all patients at risk for hypoglycemia, especially patients with T1DM.5 Despite the ADA’s recommendation, current evidence suggests suboptimal glucagon prescription rates, particularly in patients with T1DM. One study reported that, although 85% of US adults with T1DM had formerly been prescribed glucagon, only 68% of these patients (57.8% overall) had a current prescription.4 Few quality improvement efforts have tackled increasing prescription rates. Prior successful studies have attempted to do so via pharmacist-led educational interventions for providers6 and via electronic health record (EHR) notifications for patient risk.7 The project described here aimed to expand upon prior studies with a quality improvement project to increase glucagon prescription rates among patients at risk for severe hypoglycemia.
This study was conducted at a tertiary medical center’s outpatient diabetes clinic; the clinic treats more than 9500 patients with DM annually, more than 2700 of whom have T1DM. In the clinic’s multidisciplinary care model, patients typically follow up every 3 to 6 months, alternating between appointments with fellowship-trained endocrinologists and advanced practice providers (APPs). In addition to having certified diabetes educators, the clinic employs 2 dedicated clinical pharmacists whose duties include assisting providers in prescription management, helping patients identify the most affordable way to obtain their medications, and educating patients regarding their medications.
Patient flow through the clinic involves close coordination with multiple health professionals. Medical assistants (MAs) and licensed practical nurses (LPNs) perform patient intake, document vital signs, and ask screening questions, including dates of patients’ last hemoglobin A1c tests and diabetic eye examination. After intake, the provider (endocrinologist or APP) sees the patient. Once the appointment concludes, patients proceed to the in-house phlebotomy laboratory as indicated and check out with administrative staff to schedule future appointments.
From August 2021 through June 2022, teams of medical students at the tertiary center completed this project as part of a 4-week integrated science course on diabetes. Longitudinal supervision by an endocrinology faculty member ensured project continuity. The project employed the Standards for QUality Improvement Reporting Excellence (SQUIRE 2.0) method for reporting.8
Stakeholder analysis took place in August 2021. Surveyed clinic providers identified patients with T1DM as the most appropriate population and the outpatient setting as the most appropriate site for intervention. A fishbone diagram illustrated stakeholders to interview, impacts of the clinical flow, information technology to leverage, and potential holes contributing to glucagon prescription conversations falling through.
Interviews with T1DM patients, clinical pharmacists, APPs, MAs/LPNs, and endocrinologists identified barriers to glucagon prescription. The interviews and a process map analysis revealed several themes. While patients and providers understood the importance of glucagon prescription, barriers included glucagon cost, prescription fill burden, and, most pervasively, providers forgetting to ask patients whether they have a glucagon prescription and failing to consider glucagon prescriptions.For this study, each team of medical students worked on the project for 1 month. The revolving teams of medical students met approximately once per week for the duration of the project to review data and implementation phases. At the end of each month, the current team recorded the steps they had taken and information they had analyzed in a shared document, prepared short videos summarizing the work completed, and proposed next steps for the incoming team to support knowledge generation and continuity. Students from outgoing teams were available to contact if incoming teams had any questions.
Interventions
In the first implementation phase, which was carried out over 4 months (December 2021 to March 2022), the patient care manager trained MAs/LPNs to write a glucagon reminder on patients’ face sheets. At check-in, MAs/LPNs screened for a current glucagon prescription. If the patient lacked an up-to-date prescription, the MAs/LPNs hand-wrote a reminder on the patient’s face sheet, which was given to the provider immediately prior to seeing the patient. The clinical staff received an email explaining the intervention beforehand; the daily intake staff email included project reminders.
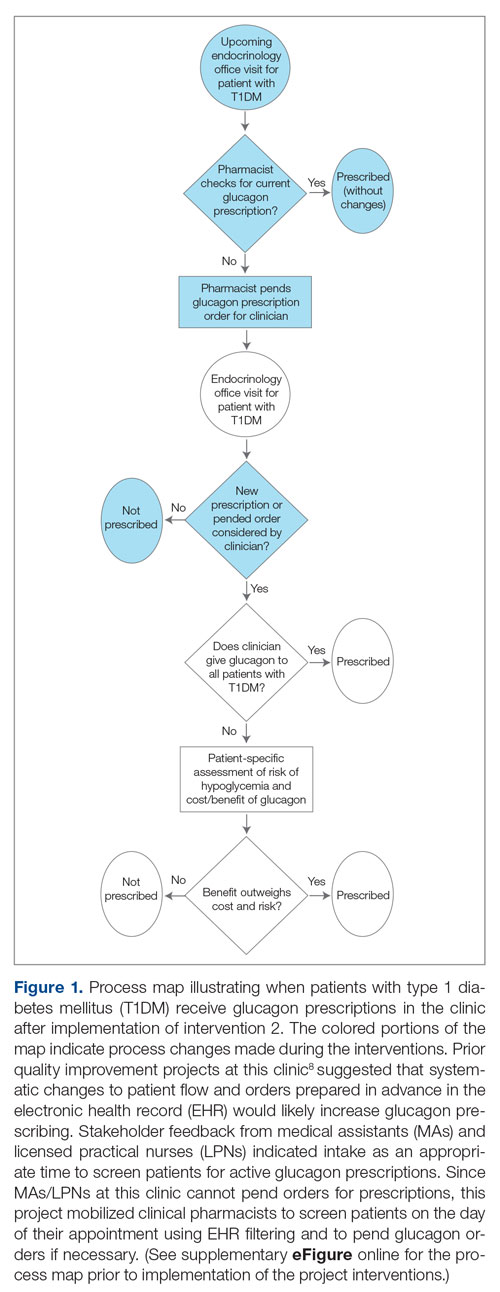
In the second implementation phase, which started in April 2022, had been carried out for 3 months at the time of this report, and is ongoing, clinical pharmacists have been pending glucagon prescriptions ahead of patients’ appointments. Each week, the pharmacists generate an EHR report that includes all patients with T1DM who have attended at least 1 appointment at the clinic within the past year (regardless of whether each patient possessed an active and up-to-date glucagon prescription) and the date of each patient’s next appointment. For patients who have an appointment in the upcoming week and lack an active glucagon prescription, the pharmacists run a benefits investigation to determine the insurance-preferred glucagon formulation and then pend the appropriate order in the EHR. During the patient’s next appointment, the EHR prompts the provider to review and sign the pharmacist’s pended order (Figure 1).
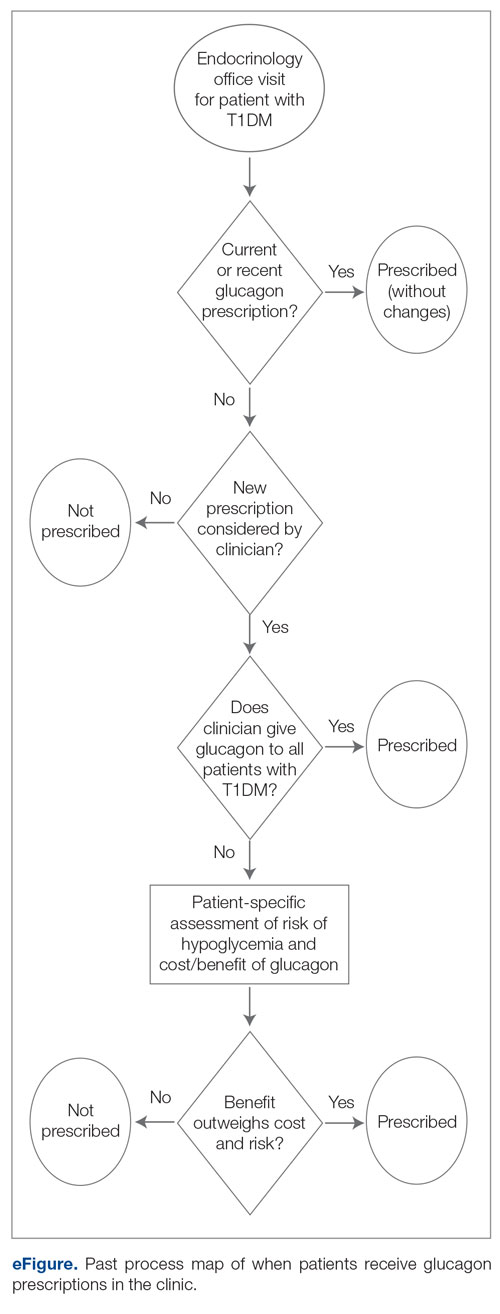
This project used a process measure in its analysis: the percentage of patients with T1DM with an active glucagon prescription at the time of their visit to the clinic. The patient population included all patients with a visit diagnosis of T1DM seen by an APP at the clinic during the time scope of the project. The project’s scope was limited to patients seen by APPs to help standardize appointment comparisons, with the intent to expand to the endocrinologist staff if the interventions proved successful with APPs. Patients seen by APPs were also under the care of endocrinologists and seen by them during this time period. The project excluded no patients.
Each individual patient appointment represented a data point: a time at which an APP could prescribe glucagon for a patient with T1DM. Thus, a single patient who had multiple appointments during the study period would generate multiple data points in this study.
For all T1DM patients at the clinic seen by an APP during the study period, the project aimed to increase the percentage with an active and up-to-date glucagon prescription from 58.8% to 70% over a 6-month period, a relatively modest goal appropriate for the time constraints and that would be similar to the changes seen in previous work in the same clinic.9
This project analyzed de-identified data using a statistical process control chart (specifically, a p-chart) and standard rules for assessing special-cause signals and thus statistical significance.
Results
Baseline data were collected from October 2020 to September 2021. During this time, APPs saw 1959 T1DM patients, of whom 1152 (58.8%) had an active glucagon prescription at the time of visit and 41.2% lacked a glucagon prescription (Figure 2). During the 4 months of implementation phase 1, analysis of the statistical process control chart identified no special cause signal. Therefore, the project moved to a second intervention with implementation phase 2 in April 2022 (3 months of postintervention data are reported). During the entire intervention, 731 of 1080 (67.7%) patients had a glucagon prescription. The average for the last 2 months, with phase 2 fully implemented, was 72.3%, surpassing the 70% threshold identified as the study target (Figure 3).
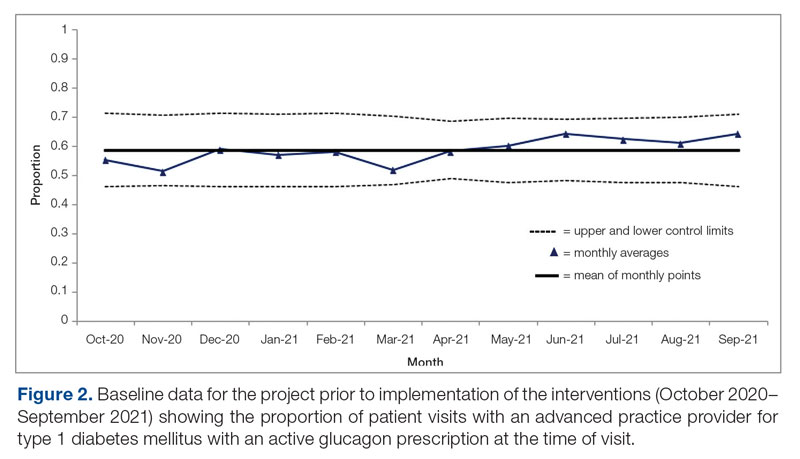
Interviews with clinical pharmacists during implementation phase 2 revealed that generating the EHR report and reviewing patients with glucagon prescription indications resulted in variable daily workload increases ranging from approximately 15 to 45 minutes, depending on the number of patients requiring intervention that day. During the first month of implementation phase 2, the EHR report required repeated modification to fulfill the intervention needs. Staffing changes over the intervention period potentially impacted the pattern of glucagon prescribing. This project excluded the 2 months immediately prior to implementation phase 1, from October 2021 to November 2021, because the staff had begun having discussions about this initiative, which may have influenced glucagon prescription rates.
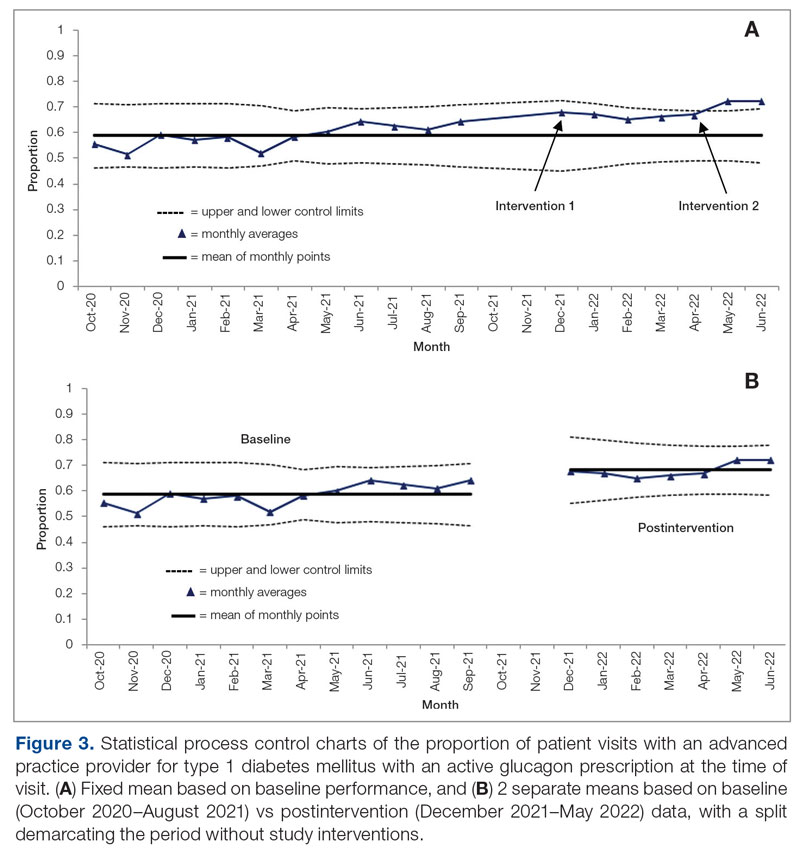
Discussion
This project evaluated 2 interventions over the course of 7 months to determine their efficacy in increasing the frequency of glucagon prescribing for individuals with T1DM in an endocrinology clinic. These interventions were associated with increased prescribing from a baseline of 58.8% to 72.3% over the last 2 months of the project. In the first intervention, performed over 4 months, MAs/LPNs wrote reminders on the appropriate patients’ face sheets, which were given to providers prior to appointments. This project adapted the approach from a successful previous quality improvement study on increasing microalbuminuria screening rates.9 However, glucagon prescription rates did not increase significantly, likely because, unlike with microalbuminuria screenings, MAs/LPNs could not pend glucagon prescriptions.
In the second intervention, performed over 3 months, clinical pharmacists pended glucagon prescriptions for identified eligible patients. Glucagon prescribing rates increased considerably, with rates of 72.3% and 72.4% over May and June 2021, respectively, indicating that the intervention successfully established a new higher steady state of proportion of patient visits with active glucagon prescriptions compared with the baseline rate of 58.8%. Given that the baseline data for this clinic were higher than the baseline glucagon prescription rates reported in other studies (49.3%),10 this intervention could have a major impact in clinics with a baseline more comparable to conditions in that study.
This project demonstrated how a combination of an EHR-generated report and interdisciplinary involvement provides an actionable process to increase glucagon prescription rates for patients with T1DM. Compared to prior studies that implemented passive interventions, such as a note template that relies on provider adherence,7 this project emphasizes the benefit of implementing an active systems-level intervention with a pre-pended order.
Regarding prior studies, 1 large, 2-arm study of clinical pharmacists proactively pending orders for appropriate patients showed a 56% glucagon prescription rate in the intervention group, compared with 0.9% in the control group with no pharmacist intervention.11 Our project had a much higher baseline rate: 58.8% prior to intervention vs 0.9% in the nonintervention group for the previous study—likely due to its chosen location’s status as an endocrinology clinic rather than a general health care setting.
A different study that focused on patient education rather than glucagon prescription rates used similar EHR-generated reports to identify appropriate patients and assessed glucagon prescription needs during check-in. Following the educational interventions in that study, patients reporting self-comfort and education with glucagon administration significantly increased from 66.2% to 83.2%, and household member comfort and education with glucagon administration increased from 50.8% to 79.7%. This suggests the possibility of expanding the use of the EHR-generated report to assist not only with increasing glucagon prescription rates, but also with patient education on glucagon use rates and possibly fill rates.7 While novel glucagon products may change uptake rates, no new glucagon products arose or were prescribed at this clinic during the course of data collection.
Of note, our project increased the workload on clinical pharmacists. The pharmacists agreed to participate, despite the increased work, after a collaborative discussion about how to best address the need to increase glucagon prescriptions or patient safety; the pharmacy department had initially agreed to collaborate specifically to identify and attend to unmet needs such as this one. Although this project greatly benefited from the expertise and enthusiasm of the clinical pharmacists involved, this tradeoff requires further study to determine sustainability.
This project had several limitations. Because of the structure in which this intervention occurred (a year-long course with rotating groups of medical students), there was a necessary component of time constraint, and this project had just 2 implementation phases, for a total of 7 months of postintervention data. The clinic has permanently implemented these changes into its workflow, but subsequent assessments are needed to monitor the effects and assess sustainability.
The specific clinical site chosen for this study benefited from dedicated onsite clinical pharmacists, who are not available at all comparable clinical sites. Due to feasibility, this project only assessed whether the providers prescribed the glucagon, not whether the patients filled the prescriptions and used the glucagon when necessary. Although prescribing rates increased in our study, it cannot be assumed that fill rates increased identically.
Finally, interventions relying on EHR-generated reports carry inherent limitations, such as the risk of misidentification or omission of patients who had indications for a glucagon prescription. The project attempted to mitigate this limitation through random sampling of the EHR report to ensure accuracy. Additionally, EHR-generated reports encourage sustainability and expansion to all clinic patients, with far less required overhead work compared to manually derived data.
Future investigations may focus on expanding this intervention to all patients at risk for hypoglycemia, as well as to study further interventions into prescription fill rates and glucagon use rates.
Conclusion
This project indicates that a proactive, interdisciplinary quality improvement project can increase glucagon prescription rates for patients with T1DM in the outpatient setting. The most effective intervention mobilized clinical pharmacists to identify patients with indications for a glucagon prescription using an integrated EHR-generated report and subsequently pend a glucagon order for the endocrinology provider to sign during the visit. The strengths of the approach included using a multidisciplinary team, minimizing costs to patients by leveraging the pharmacists’ expertise to ensure insurance coverage of specific formulations, and utilizing automatic EHR reporting to streamline patient identification. Ideally, improvements in glucagon prescription rates should ultimately decrease hospitalizations and improve treatment of severe hypoglycemia for at-risk patients.
Corresponding author: Chase D. Hendrickson, MD, MPH; [email protected]
Disclosures: None reported.
1. Weinstock RS, Aleppo G, Bailey TS, et al. The Role of Blood Glucose Monitoring in Diabetes Management. American Diabetes Association; 2020.
2. Lamounier RN, Geloneze B, Leite SO, et al. Hypoglycemia incidence and awareness among insulin-treated patients with diabetes: the HAT study in Brazil. Diabetol Metab Syndr. 2018;10:83. doi:10.1186/s13098-018-0379-5
3. Li P, Geng Z, Ladage VP, et al. Early hypoglycaemia and adherence after basal insulin initiation in a nationally representative sample of Medicare beneficiaries with type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2486-2495. doi:10.1111/dom.13832
4. Haymond MW, Liu J, Bispham J, et al. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37(2):162-166. doi:10.2337/cd18-0028
5. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S83-S96. doi:10.2337/dc22-S006
6. O’Reilly EA, Cross LV, Hayes JS, et al. Impact of pharmacist intervention on glucagon prescribing patterns in an outpatient internal medicine teaching clinic. J Am Pharm Assoc (2003). 2020;60(2):384-390. doi:10.1016/j.japh.2019.04.0097.
7. Cobb EC, Watson NA, Wardian J, et al. Diabetes Center of Excellence Hypoglycemia Emergency Preparedness Project. Clin Diabetes. 2018;36(2):184-186. doi:10.2337/cd17-0040
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
9. Kam S, Angaramo S, Antoun J, et al. Improving annual albuminuria testing for individuals with diabetes. BMJ Open Qual. 2022;11(1):e001591. doi:10.1136/bmjoq-2021-001591
10. Mitchell BD, He X, Sturdy IM, et al. Glucagon prescription patterns in patients with either type 1 or 2 diabetes with newly prescribed insulin. Endocr Pract. 2016;22(2):123-135. doi:10.4158/EP15831.OR
11. Whitfield N, Gregory P, Liu B, et al. Impact of pharmacist outreach on glucagon prescribing. J Am Pharm Assoc. 2022;62(4):1384-1388.e.1. doi:10.1016/j.japh.2022.01.017
From Vanderbilt University School of Medicine, and Vanderbilt University Medical Center, Nashville, TN.
ABSTRACT
Objective: Severe hypoglycemia can alter consciousness and inhibit oral intake, requiring nonoral rescue glucagon administration to raise blood glucose to safe levels. Thus, current guidelines recommend glucagon kit prescriptions for all patients at risk for hypoglycemia, especially patients with type 1 diabetes mellitus (T1DM). At the diabetes outpatient clinic at a tertiary medical center, glucagon prescription rates for T1DM patients remained suboptimal.
Methods: A quality improvement team analyzed patient flow through the endocrinology clinic and identified the lack of a systematic approach to assessing patients for home glucagon prescriptions as a major barrier. The team implemented 2 successive interventions. First, intake staff indicated whether patients lacked an active glucagon prescription on patients’ face sheets. Second, clinical pharmacists reviewed patient prescriptions prior to scheduled visits and pended glucagon orders for patients without active prescriptions. Of note, when a pharmacy pends an order, the pharmacist enters an order into the electronic health record (EHR) but does not sign it. The order is saved for a provider to later access and sign. A statistical process control p-chart tracked monthly prescription rates.
Results: After 7 months, glucagon prescription rates increased from a baseline of 59% to 72% as the new steady state.
Conclusion: This project demonstrates that a series of interventions can improve glucagon prescription rates for patients at risk for hypoglycemia. The project’s success stemmed from combining an EHR-generated report and interdisciplinary staff members’ involvement. Other endocrinology clinics may incorporate this approach to implement similar processes and improve glucagon prescription rates.
Keywords: diabetes, hypoglycemia, glucagon, quality improvement, prescription rates, medical student.
Hypoglycemia limits the management of blood glucose in patients with type 1 diabetes mellitus (T1DM). Severe hypoglycemia, characterized by altered mental status (AMS) or physical status requiring assistance for recovery, can lead to seizure, coma, or death.1 Hypoglycemia in diabetes often occurs iatrogenically, primarily from insulin therapy: 30% to 40% of patients with T1DM and 10% to 30% of patients with insulin-treated type 2 diabetes mellitus experience severe hypoglycemia in a given year.2 One study estimated that nearly 100,000 emergency department visits for hypoglycemia occur in the United States per year, with almost one-third resulting in hospitalization.3
Most patients self-treat mild hypoglycemia with oral intake of carbohydrates. However, since hypoglycemia-induced nausea and AMS can make oral intake more difficult or prevent it entirely, patients require a treatment that family, friends, or coworkers can administer. Rescue glucagon, prescribed as intramuscular injections or intranasal sprays, raises blood glucose to safe levels in 10 to 15 minutes.4 Therefore, the American Diabetes Association (ADA) recommends glucagon for all patients at risk for hypoglycemia, especially patients with T1DM.5 Despite the ADA’s recommendation, current evidence suggests suboptimal glucagon prescription rates, particularly in patients with T1DM. One study reported that, although 85% of US adults with T1DM had formerly been prescribed glucagon, only 68% of these patients (57.8% overall) had a current prescription.4 Few quality improvement efforts have tackled increasing prescription rates. Prior successful studies have attempted to do so via pharmacist-led educational interventions for providers6 and via electronic health record (EHR) notifications for patient risk.7 The project described here aimed to expand upon prior studies with a quality improvement project to increase glucagon prescription rates among patients at risk for severe hypoglycemia.
This study was conducted at a tertiary medical center’s outpatient diabetes clinic; the clinic treats more than 9500 patients with DM annually, more than 2700 of whom have T1DM. In the clinic’s multidisciplinary care model, patients typically follow up every 3 to 6 months, alternating between appointments with fellowship-trained endocrinologists and advanced practice providers (APPs). In addition to having certified diabetes educators, the clinic employs 2 dedicated clinical pharmacists whose duties include assisting providers in prescription management, helping patients identify the most affordable way to obtain their medications, and educating patients regarding their medications.
Patient flow through the clinic involves close coordination with multiple health professionals. Medical assistants (MAs) and licensed practical nurses (LPNs) perform patient intake, document vital signs, and ask screening questions, including dates of patients’ last hemoglobin A1c tests and diabetic eye examination. After intake, the provider (endocrinologist or APP) sees the patient. Once the appointment concludes, patients proceed to the in-house phlebotomy laboratory as indicated and check out with administrative staff to schedule future appointments.
From August 2021 through June 2022, teams of medical students at the tertiary center completed this project as part of a 4-week integrated science course on diabetes. Longitudinal supervision by an endocrinology faculty member ensured project continuity. The project employed the Standards for QUality Improvement Reporting Excellence (SQUIRE 2.0) method for reporting.8
Stakeholder analysis took place in August 2021. Surveyed clinic providers identified patients with T1DM as the most appropriate population and the outpatient setting as the most appropriate site for intervention. A fishbone diagram illustrated stakeholders to interview, impacts of the clinical flow, information technology to leverage, and potential holes contributing to glucagon prescription conversations falling through.
Interviews with T1DM patients, clinical pharmacists, APPs, MAs/LPNs, and endocrinologists identified barriers to glucagon prescription. The interviews and a process map analysis revealed several themes. While patients and providers understood the importance of glucagon prescription, barriers included glucagon cost, prescription fill burden, and, most pervasively, providers forgetting to ask patients whether they have a glucagon prescription and failing to consider glucagon prescriptions.For this study, each team of medical students worked on the project for 1 month. The revolving teams of medical students met approximately once per week for the duration of the project to review data and implementation phases. At the end of each month, the current team recorded the steps they had taken and information they had analyzed in a shared document, prepared short videos summarizing the work completed, and proposed next steps for the incoming team to support knowledge generation and continuity. Students from outgoing teams were available to contact if incoming teams had any questions.
Interventions
In the first implementation phase, which was carried out over 4 months (December 2021 to March 2022), the patient care manager trained MAs/LPNs to write a glucagon reminder on patients’ face sheets. At check-in, MAs/LPNs screened for a current glucagon prescription. If the patient lacked an up-to-date prescription, the MAs/LPNs hand-wrote a reminder on the patient’s face sheet, which was given to the provider immediately prior to seeing the patient. The clinical staff received an email explaining the intervention beforehand; the daily intake staff email included project reminders.

In the second implementation phase, which started in April 2022, had been carried out for 3 months at the time of this report, and is ongoing, clinical pharmacists have been pending glucagon prescriptions ahead of patients’ appointments. Each week, the pharmacists generate an EHR report that includes all patients with T1DM who have attended at least 1 appointment at the clinic within the past year (regardless of whether each patient possessed an active and up-to-date glucagon prescription) and the date of each patient’s next appointment. For patients who have an appointment in the upcoming week and lack an active glucagon prescription, the pharmacists run a benefits investigation to determine the insurance-preferred glucagon formulation and then pend the appropriate order in the EHR. During the patient’s next appointment, the EHR prompts the provider to review and sign the pharmacist’s pended order (Figure 1).

This project used a process measure in its analysis: the percentage of patients with T1DM with an active glucagon prescription at the time of their visit to the clinic. The patient population included all patients with a visit diagnosis of T1DM seen by an APP at the clinic during the time scope of the project. The project’s scope was limited to patients seen by APPs to help standardize appointment comparisons, with the intent to expand to the endocrinologist staff if the interventions proved successful with APPs. Patients seen by APPs were also under the care of endocrinologists and seen by them during this time period. The project excluded no patients.
Each individual patient appointment represented a data point: a time at which an APP could prescribe glucagon for a patient with T1DM. Thus, a single patient who had multiple appointments during the study period would generate multiple data points in this study.
For all T1DM patients at the clinic seen by an APP during the study period, the project aimed to increase the percentage with an active and up-to-date glucagon prescription from 58.8% to 70% over a 6-month period, a relatively modest goal appropriate for the time constraints and that would be similar to the changes seen in previous work in the same clinic.9
This project analyzed de-identified data using a statistical process control chart (specifically, a p-chart) and standard rules for assessing special-cause signals and thus statistical significance.
Results
Baseline data were collected from October 2020 to September 2021. During this time, APPs saw 1959 T1DM patients, of whom 1152 (58.8%) had an active glucagon prescription at the time of visit and 41.2% lacked a glucagon prescription (Figure 2). During the 4 months of implementation phase 1, analysis of the statistical process control chart identified no special cause signal. Therefore, the project moved to a second intervention with implementation phase 2 in April 2022 (3 months of postintervention data are reported). During the entire intervention, 731 of 1080 (67.7%) patients had a glucagon prescription. The average for the last 2 months, with phase 2 fully implemented, was 72.3%, surpassing the 70% threshold identified as the study target (Figure 3).

Interviews with clinical pharmacists during implementation phase 2 revealed that generating the EHR report and reviewing patients with glucagon prescription indications resulted in variable daily workload increases ranging from approximately 15 to 45 minutes, depending on the number of patients requiring intervention that day. During the first month of implementation phase 2, the EHR report required repeated modification to fulfill the intervention needs. Staffing changes over the intervention period potentially impacted the pattern of glucagon prescribing. This project excluded the 2 months immediately prior to implementation phase 1, from October 2021 to November 2021, because the staff had begun having discussions about this initiative, which may have influenced glucagon prescription rates.

Discussion
This project evaluated 2 interventions over the course of 7 months to determine their efficacy in increasing the frequency of glucagon prescribing for individuals with T1DM in an endocrinology clinic. These interventions were associated with increased prescribing from a baseline of 58.8% to 72.3% over the last 2 months of the project. In the first intervention, performed over 4 months, MAs/LPNs wrote reminders on the appropriate patients’ face sheets, which were given to providers prior to appointments. This project adapted the approach from a successful previous quality improvement study on increasing microalbuminuria screening rates.9 However, glucagon prescription rates did not increase significantly, likely because, unlike with microalbuminuria screenings, MAs/LPNs could not pend glucagon prescriptions.
In the second intervention, performed over 3 months, clinical pharmacists pended glucagon prescriptions for identified eligible patients. Glucagon prescribing rates increased considerably, with rates of 72.3% and 72.4% over May and June 2021, respectively, indicating that the intervention successfully established a new higher steady state of proportion of patient visits with active glucagon prescriptions compared with the baseline rate of 58.8%. Given that the baseline data for this clinic were higher than the baseline glucagon prescription rates reported in other studies (49.3%),10 this intervention could have a major impact in clinics with a baseline more comparable to conditions in that study.
This project demonstrated how a combination of an EHR-generated report and interdisciplinary involvement provides an actionable process to increase glucagon prescription rates for patients with T1DM. Compared to prior studies that implemented passive interventions, such as a note template that relies on provider adherence,7 this project emphasizes the benefit of implementing an active systems-level intervention with a pre-pended order.
Regarding prior studies, 1 large, 2-arm study of clinical pharmacists proactively pending orders for appropriate patients showed a 56% glucagon prescription rate in the intervention group, compared with 0.9% in the control group with no pharmacist intervention.11 Our project had a much higher baseline rate: 58.8% prior to intervention vs 0.9% in the nonintervention group for the previous study—likely due to its chosen location’s status as an endocrinology clinic rather than a general health care setting.
A different study that focused on patient education rather than glucagon prescription rates used similar EHR-generated reports to identify appropriate patients and assessed glucagon prescription needs during check-in. Following the educational interventions in that study, patients reporting self-comfort and education with glucagon administration significantly increased from 66.2% to 83.2%, and household member comfort and education with glucagon administration increased from 50.8% to 79.7%. This suggests the possibility of expanding the use of the EHR-generated report to assist not only with increasing glucagon prescription rates, but also with patient education on glucagon use rates and possibly fill rates.7 While novel glucagon products may change uptake rates, no new glucagon products arose or were prescribed at this clinic during the course of data collection.
Of note, our project increased the workload on clinical pharmacists. The pharmacists agreed to participate, despite the increased work, after a collaborative discussion about how to best address the need to increase glucagon prescriptions or patient safety; the pharmacy department had initially agreed to collaborate specifically to identify and attend to unmet needs such as this one. Although this project greatly benefited from the expertise and enthusiasm of the clinical pharmacists involved, this tradeoff requires further study to determine sustainability.
This project had several limitations. Because of the structure in which this intervention occurred (a year-long course with rotating groups of medical students), there was a necessary component of time constraint, and this project had just 2 implementation phases, for a total of 7 months of postintervention data. The clinic has permanently implemented these changes into its workflow, but subsequent assessments are needed to monitor the effects and assess sustainability.
The specific clinical site chosen for this study benefited from dedicated onsite clinical pharmacists, who are not available at all comparable clinical sites. Due to feasibility, this project only assessed whether the providers prescribed the glucagon, not whether the patients filled the prescriptions and used the glucagon when necessary. Although prescribing rates increased in our study, it cannot be assumed that fill rates increased identically.
Finally, interventions relying on EHR-generated reports carry inherent limitations, such as the risk of misidentification or omission of patients who had indications for a glucagon prescription. The project attempted to mitigate this limitation through random sampling of the EHR report to ensure accuracy. Additionally, EHR-generated reports encourage sustainability and expansion to all clinic patients, with far less required overhead work compared to manually derived data.
Future investigations may focus on expanding this intervention to all patients at risk for hypoglycemia, as well as to study further interventions into prescription fill rates and glucagon use rates.
Conclusion
This project indicates that a proactive, interdisciplinary quality improvement project can increase glucagon prescription rates for patients with T1DM in the outpatient setting. The most effective intervention mobilized clinical pharmacists to identify patients with indications for a glucagon prescription using an integrated EHR-generated report and subsequently pend a glucagon order for the endocrinology provider to sign during the visit. The strengths of the approach included using a multidisciplinary team, minimizing costs to patients by leveraging the pharmacists’ expertise to ensure insurance coverage of specific formulations, and utilizing automatic EHR reporting to streamline patient identification. Ideally, improvements in glucagon prescription rates should ultimately decrease hospitalizations and improve treatment of severe hypoglycemia for at-risk patients.
Corresponding author: Chase D. Hendrickson, MD, MPH; [email protected]
Disclosures: None reported.
From Vanderbilt University School of Medicine, and Vanderbilt University Medical Center, Nashville, TN.
ABSTRACT
Objective: Severe hypoglycemia can alter consciousness and inhibit oral intake, requiring nonoral rescue glucagon administration to raise blood glucose to safe levels. Thus, current guidelines recommend glucagon kit prescriptions for all patients at risk for hypoglycemia, especially patients with type 1 diabetes mellitus (T1DM). At the diabetes outpatient clinic at a tertiary medical center, glucagon prescription rates for T1DM patients remained suboptimal.
Methods: A quality improvement team analyzed patient flow through the endocrinology clinic and identified the lack of a systematic approach to assessing patients for home glucagon prescriptions as a major barrier. The team implemented 2 successive interventions. First, intake staff indicated whether patients lacked an active glucagon prescription on patients’ face sheets. Second, clinical pharmacists reviewed patient prescriptions prior to scheduled visits and pended glucagon orders for patients without active prescriptions. Of note, when a pharmacy pends an order, the pharmacist enters an order into the electronic health record (EHR) but does not sign it. The order is saved for a provider to later access and sign. A statistical process control p-chart tracked monthly prescription rates.
Results: After 7 months, glucagon prescription rates increased from a baseline of 59% to 72% as the new steady state.
Conclusion: This project demonstrates that a series of interventions can improve glucagon prescription rates for patients at risk for hypoglycemia. The project’s success stemmed from combining an EHR-generated report and interdisciplinary staff members’ involvement. Other endocrinology clinics may incorporate this approach to implement similar processes and improve glucagon prescription rates.
Keywords: diabetes, hypoglycemia, glucagon, quality improvement, prescription rates, medical student.
Hypoglycemia limits the management of blood glucose in patients with type 1 diabetes mellitus (T1DM). Severe hypoglycemia, characterized by altered mental status (AMS) or physical status requiring assistance for recovery, can lead to seizure, coma, or death.1 Hypoglycemia in diabetes often occurs iatrogenically, primarily from insulin therapy: 30% to 40% of patients with T1DM and 10% to 30% of patients with insulin-treated type 2 diabetes mellitus experience severe hypoglycemia in a given year.2 One study estimated that nearly 100,000 emergency department visits for hypoglycemia occur in the United States per year, with almost one-third resulting in hospitalization.3
Most patients self-treat mild hypoglycemia with oral intake of carbohydrates. However, since hypoglycemia-induced nausea and AMS can make oral intake more difficult or prevent it entirely, patients require a treatment that family, friends, or coworkers can administer. Rescue glucagon, prescribed as intramuscular injections or intranasal sprays, raises blood glucose to safe levels in 10 to 15 minutes.4 Therefore, the American Diabetes Association (ADA) recommends glucagon for all patients at risk for hypoglycemia, especially patients with T1DM.5 Despite the ADA’s recommendation, current evidence suggests suboptimal glucagon prescription rates, particularly in patients with T1DM. One study reported that, although 85% of US adults with T1DM had formerly been prescribed glucagon, only 68% of these patients (57.8% overall) had a current prescription.4 Few quality improvement efforts have tackled increasing prescription rates. Prior successful studies have attempted to do so via pharmacist-led educational interventions for providers6 and via electronic health record (EHR) notifications for patient risk.7 The project described here aimed to expand upon prior studies with a quality improvement project to increase glucagon prescription rates among patients at risk for severe hypoglycemia.
This study was conducted at a tertiary medical center’s outpatient diabetes clinic; the clinic treats more than 9500 patients with DM annually, more than 2700 of whom have T1DM. In the clinic’s multidisciplinary care model, patients typically follow up every 3 to 6 months, alternating between appointments with fellowship-trained endocrinologists and advanced practice providers (APPs). In addition to having certified diabetes educators, the clinic employs 2 dedicated clinical pharmacists whose duties include assisting providers in prescription management, helping patients identify the most affordable way to obtain their medications, and educating patients regarding their medications.
Patient flow through the clinic involves close coordination with multiple health professionals. Medical assistants (MAs) and licensed practical nurses (LPNs) perform patient intake, document vital signs, and ask screening questions, including dates of patients’ last hemoglobin A1c tests and diabetic eye examination. After intake, the provider (endocrinologist or APP) sees the patient. Once the appointment concludes, patients proceed to the in-house phlebotomy laboratory as indicated and check out with administrative staff to schedule future appointments.
From August 2021 through June 2022, teams of medical students at the tertiary center completed this project as part of a 4-week integrated science course on diabetes. Longitudinal supervision by an endocrinology faculty member ensured project continuity. The project employed the Standards for QUality Improvement Reporting Excellence (SQUIRE 2.0) method for reporting.8
Stakeholder analysis took place in August 2021. Surveyed clinic providers identified patients with T1DM as the most appropriate population and the outpatient setting as the most appropriate site for intervention. A fishbone diagram illustrated stakeholders to interview, impacts of the clinical flow, information technology to leverage, and potential holes contributing to glucagon prescription conversations falling through.
Interviews with T1DM patients, clinical pharmacists, APPs, MAs/LPNs, and endocrinologists identified barriers to glucagon prescription. The interviews and a process map analysis revealed several themes. While patients and providers understood the importance of glucagon prescription, barriers included glucagon cost, prescription fill burden, and, most pervasively, providers forgetting to ask patients whether they have a glucagon prescription and failing to consider glucagon prescriptions.For this study, each team of medical students worked on the project for 1 month. The revolving teams of medical students met approximately once per week for the duration of the project to review data and implementation phases. At the end of each month, the current team recorded the steps they had taken and information they had analyzed in a shared document, prepared short videos summarizing the work completed, and proposed next steps for the incoming team to support knowledge generation and continuity. Students from outgoing teams were available to contact if incoming teams had any questions.
Interventions
In the first implementation phase, which was carried out over 4 months (December 2021 to March 2022), the patient care manager trained MAs/LPNs to write a glucagon reminder on patients’ face sheets. At check-in, MAs/LPNs screened for a current glucagon prescription. If the patient lacked an up-to-date prescription, the MAs/LPNs hand-wrote a reminder on the patient’s face sheet, which was given to the provider immediately prior to seeing the patient. The clinical staff received an email explaining the intervention beforehand; the daily intake staff email included project reminders.

In the second implementation phase, which started in April 2022, had been carried out for 3 months at the time of this report, and is ongoing, clinical pharmacists have been pending glucagon prescriptions ahead of patients’ appointments. Each week, the pharmacists generate an EHR report that includes all patients with T1DM who have attended at least 1 appointment at the clinic within the past year (regardless of whether each patient possessed an active and up-to-date glucagon prescription) and the date of each patient’s next appointment. For patients who have an appointment in the upcoming week and lack an active glucagon prescription, the pharmacists run a benefits investigation to determine the insurance-preferred glucagon formulation and then pend the appropriate order in the EHR. During the patient’s next appointment, the EHR prompts the provider to review and sign the pharmacist’s pended order (Figure 1).

This project used a process measure in its analysis: the percentage of patients with T1DM with an active glucagon prescription at the time of their visit to the clinic. The patient population included all patients with a visit diagnosis of T1DM seen by an APP at the clinic during the time scope of the project. The project’s scope was limited to patients seen by APPs to help standardize appointment comparisons, with the intent to expand to the endocrinologist staff if the interventions proved successful with APPs. Patients seen by APPs were also under the care of endocrinologists and seen by them during this time period. The project excluded no patients.
Each individual patient appointment represented a data point: a time at which an APP could prescribe glucagon for a patient with T1DM. Thus, a single patient who had multiple appointments during the study period would generate multiple data points in this study.
For all T1DM patients at the clinic seen by an APP during the study period, the project aimed to increase the percentage with an active and up-to-date glucagon prescription from 58.8% to 70% over a 6-month period, a relatively modest goal appropriate for the time constraints and that would be similar to the changes seen in previous work in the same clinic.9
This project analyzed de-identified data using a statistical process control chart (specifically, a p-chart) and standard rules for assessing special-cause signals and thus statistical significance.
Results
Baseline data were collected from October 2020 to September 2021. During this time, APPs saw 1959 T1DM patients, of whom 1152 (58.8%) had an active glucagon prescription at the time of visit and 41.2% lacked a glucagon prescription (Figure 2). During the 4 months of implementation phase 1, analysis of the statistical process control chart identified no special cause signal. Therefore, the project moved to a second intervention with implementation phase 2 in April 2022 (3 months of postintervention data are reported). During the entire intervention, 731 of 1080 (67.7%) patients had a glucagon prescription. The average for the last 2 months, with phase 2 fully implemented, was 72.3%, surpassing the 70% threshold identified as the study target (Figure 3).

Interviews with clinical pharmacists during implementation phase 2 revealed that generating the EHR report and reviewing patients with glucagon prescription indications resulted in variable daily workload increases ranging from approximately 15 to 45 minutes, depending on the number of patients requiring intervention that day. During the first month of implementation phase 2, the EHR report required repeated modification to fulfill the intervention needs. Staffing changes over the intervention period potentially impacted the pattern of glucagon prescribing. This project excluded the 2 months immediately prior to implementation phase 1, from October 2021 to November 2021, because the staff had begun having discussions about this initiative, which may have influenced glucagon prescription rates.

Discussion
This project evaluated 2 interventions over the course of 7 months to determine their efficacy in increasing the frequency of glucagon prescribing for individuals with T1DM in an endocrinology clinic. These interventions were associated with increased prescribing from a baseline of 58.8% to 72.3% over the last 2 months of the project. In the first intervention, performed over 4 months, MAs/LPNs wrote reminders on the appropriate patients’ face sheets, which were given to providers prior to appointments. This project adapted the approach from a successful previous quality improvement study on increasing microalbuminuria screening rates.9 However, glucagon prescription rates did not increase significantly, likely because, unlike with microalbuminuria screenings, MAs/LPNs could not pend glucagon prescriptions.
In the second intervention, performed over 3 months, clinical pharmacists pended glucagon prescriptions for identified eligible patients. Glucagon prescribing rates increased considerably, with rates of 72.3% and 72.4% over May and June 2021, respectively, indicating that the intervention successfully established a new higher steady state of proportion of patient visits with active glucagon prescriptions compared with the baseline rate of 58.8%. Given that the baseline data for this clinic were higher than the baseline glucagon prescription rates reported in other studies (49.3%),10 this intervention could have a major impact in clinics with a baseline more comparable to conditions in that study.
This project demonstrated how a combination of an EHR-generated report and interdisciplinary involvement provides an actionable process to increase glucagon prescription rates for patients with T1DM. Compared to prior studies that implemented passive interventions, such as a note template that relies on provider adherence,7 this project emphasizes the benefit of implementing an active systems-level intervention with a pre-pended order.
Regarding prior studies, 1 large, 2-arm study of clinical pharmacists proactively pending orders for appropriate patients showed a 56% glucagon prescription rate in the intervention group, compared with 0.9% in the control group with no pharmacist intervention.11 Our project had a much higher baseline rate: 58.8% prior to intervention vs 0.9% in the nonintervention group for the previous study—likely due to its chosen location’s status as an endocrinology clinic rather than a general health care setting.
A different study that focused on patient education rather than glucagon prescription rates used similar EHR-generated reports to identify appropriate patients and assessed glucagon prescription needs during check-in. Following the educational interventions in that study, patients reporting self-comfort and education with glucagon administration significantly increased from 66.2% to 83.2%, and household member comfort and education with glucagon administration increased from 50.8% to 79.7%. This suggests the possibility of expanding the use of the EHR-generated report to assist not only with increasing glucagon prescription rates, but also with patient education on glucagon use rates and possibly fill rates.7 While novel glucagon products may change uptake rates, no new glucagon products arose or were prescribed at this clinic during the course of data collection.
Of note, our project increased the workload on clinical pharmacists. The pharmacists agreed to participate, despite the increased work, after a collaborative discussion about how to best address the need to increase glucagon prescriptions or patient safety; the pharmacy department had initially agreed to collaborate specifically to identify and attend to unmet needs such as this one. Although this project greatly benefited from the expertise and enthusiasm of the clinical pharmacists involved, this tradeoff requires further study to determine sustainability.
This project had several limitations. Because of the structure in which this intervention occurred (a year-long course with rotating groups of medical students), there was a necessary component of time constraint, and this project had just 2 implementation phases, for a total of 7 months of postintervention data. The clinic has permanently implemented these changes into its workflow, but subsequent assessments are needed to monitor the effects and assess sustainability.
The specific clinical site chosen for this study benefited from dedicated onsite clinical pharmacists, who are not available at all comparable clinical sites. Due to feasibility, this project only assessed whether the providers prescribed the glucagon, not whether the patients filled the prescriptions and used the glucagon when necessary. Although prescribing rates increased in our study, it cannot be assumed that fill rates increased identically.
Finally, interventions relying on EHR-generated reports carry inherent limitations, such as the risk of misidentification or omission of patients who had indications for a glucagon prescription. The project attempted to mitigate this limitation through random sampling of the EHR report to ensure accuracy. Additionally, EHR-generated reports encourage sustainability and expansion to all clinic patients, with far less required overhead work compared to manually derived data.
Future investigations may focus on expanding this intervention to all patients at risk for hypoglycemia, as well as to study further interventions into prescription fill rates and glucagon use rates.
Conclusion
This project indicates that a proactive, interdisciplinary quality improvement project can increase glucagon prescription rates for patients with T1DM in the outpatient setting. The most effective intervention mobilized clinical pharmacists to identify patients with indications for a glucagon prescription using an integrated EHR-generated report and subsequently pend a glucagon order for the endocrinology provider to sign during the visit. The strengths of the approach included using a multidisciplinary team, minimizing costs to patients by leveraging the pharmacists’ expertise to ensure insurance coverage of specific formulations, and utilizing automatic EHR reporting to streamline patient identification. Ideally, improvements in glucagon prescription rates should ultimately decrease hospitalizations and improve treatment of severe hypoglycemia for at-risk patients.
Corresponding author: Chase D. Hendrickson, MD, MPH; [email protected]
Disclosures: None reported.
1. Weinstock RS, Aleppo G, Bailey TS, et al. The Role of Blood Glucose Monitoring in Diabetes Management. American Diabetes Association; 2020.
2. Lamounier RN, Geloneze B, Leite SO, et al. Hypoglycemia incidence and awareness among insulin-treated patients with diabetes: the HAT study in Brazil. Diabetol Metab Syndr. 2018;10:83. doi:10.1186/s13098-018-0379-5
3. Li P, Geng Z, Ladage VP, et al. Early hypoglycaemia and adherence after basal insulin initiation in a nationally representative sample of Medicare beneficiaries with type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2486-2495. doi:10.1111/dom.13832
4. Haymond MW, Liu J, Bispham J, et al. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37(2):162-166. doi:10.2337/cd18-0028
5. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S83-S96. doi:10.2337/dc22-S006
6. O’Reilly EA, Cross LV, Hayes JS, et al. Impact of pharmacist intervention on glucagon prescribing patterns in an outpatient internal medicine teaching clinic. J Am Pharm Assoc (2003). 2020;60(2):384-390. doi:10.1016/j.japh.2019.04.0097.
7. Cobb EC, Watson NA, Wardian J, et al. Diabetes Center of Excellence Hypoglycemia Emergency Preparedness Project. Clin Diabetes. 2018;36(2):184-186. doi:10.2337/cd17-0040
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
9. Kam S, Angaramo S, Antoun J, et al. Improving annual albuminuria testing for individuals with diabetes. BMJ Open Qual. 2022;11(1):e001591. doi:10.1136/bmjoq-2021-001591
10. Mitchell BD, He X, Sturdy IM, et al. Glucagon prescription patterns in patients with either type 1 or 2 diabetes with newly prescribed insulin. Endocr Pract. 2016;22(2):123-135. doi:10.4158/EP15831.OR
11. Whitfield N, Gregory P, Liu B, et al. Impact of pharmacist outreach on glucagon prescribing. J Am Pharm Assoc. 2022;62(4):1384-1388.e.1. doi:10.1016/j.japh.2022.01.017
1. Weinstock RS, Aleppo G, Bailey TS, et al. The Role of Blood Glucose Monitoring in Diabetes Management. American Diabetes Association; 2020.
2. Lamounier RN, Geloneze B, Leite SO, et al. Hypoglycemia incidence and awareness among insulin-treated patients with diabetes: the HAT study in Brazil. Diabetol Metab Syndr. 2018;10:83. doi:10.1186/s13098-018-0379-5
3. Li P, Geng Z, Ladage VP, et al. Early hypoglycaemia and adherence after basal insulin initiation in a nationally representative sample of Medicare beneficiaries with type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2486-2495. doi:10.1111/dom.13832
4. Haymond MW, Liu J, Bispham J, et al. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37(2):162-166. doi:10.2337/cd18-0028
5. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S83-S96. doi:10.2337/dc22-S006
6. O’Reilly EA, Cross LV, Hayes JS, et al. Impact of pharmacist intervention on glucagon prescribing patterns in an outpatient internal medicine teaching clinic. J Am Pharm Assoc (2003). 2020;60(2):384-390. doi:10.1016/j.japh.2019.04.0097.
7. Cobb EC, Watson NA, Wardian J, et al. Diabetes Center of Excellence Hypoglycemia Emergency Preparedness Project. Clin Diabetes. 2018;36(2):184-186. doi:10.2337/cd17-0040
8. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
9. Kam S, Angaramo S, Antoun J, et al. Improving annual albuminuria testing for individuals with diabetes. BMJ Open Qual. 2022;11(1):e001591. doi:10.1136/bmjoq-2021-001591
10. Mitchell BD, He X, Sturdy IM, et al. Glucagon prescription patterns in patients with either type 1 or 2 diabetes with newly prescribed insulin. Endocr Pract. 2016;22(2):123-135. doi:10.4158/EP15831.OR
11. Whitfield N, Gregory P, Liu B, et al. Impact of pharmacist outreach on glucagon prescribing. J Am Pharm Assoc. 2022;62(4):1384-1388.e.1. doi:10.1016/j.japh.2022.01.017
Redesign of Health Care Systems to Reduce Diagnostic Errors: Leveraging Human Experience and Artificial Intelligence
From the Institute for Healthcare Improvement, Boston, MA (Dr. Abid); Continuous Quality Improvement and Patient Safety Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Abid); Primary and Secondary Healthcare Department, Government of Punjab, Lahore, Pakistan (Dr. Ahmed); Infection Prevention and Control Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Din); Internal Medicine Department, Greater Baltimore Medical Center, Baltimore, MD (Dr. Abid); Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, Houston, TX (Dr. Ratnani).
Diagnostic errors are defined by the National Academies of Sciences, Engineering, and Medicine (NASEM) as the failure to either establish an accurate and timely explanation of the patient’s health problem(s) or communicate that explanation to the patient.1 According to a report by the Institute of Medicine, diagnostic errors account for a substantial number of adverse events in health care, affecting an estimated 12 million Americans each year.1 Diagnostic errors are a common and serious issue in health care systems, with studies estimating that 5% to 15% of all diagnoses are incorrect.1 Such errors can result in unnecessary treatments, delays in necessary treatments, and harm to patients. The high prevalence of diagnostic errors in primary care has been identified as a global issue.2 While many factors contribute to diagnostic errors, the complex nature of health care systems, the limited processing capacity of human cognition, and deficiencies in interpersonal patient-clinician communication are primary contributors.3,4
Discussions around the redesign of health care systems to reduce diagnostic errors have been at the forefront of medical research for years.2,4 To decrease diagnostic errors in health care, a comprehensive strategy is necessary. This strategy should focus on utilizing both human experience (HX) in health care and artificial intelligence (AI) technologies to transform health care systems into proactive, patient-centered, and safer systems, specifically concerning diagnostic errors.1
Human Experience and Diagnostic Errors
The role of HX in health care cannot be overstated. The HX in health care integrates the sum of all interactions, every encounter among patients, families and care partners, and the health care workforce.5 Patients and their families have a unique perspective on their health care experiences that can provide valuable insight into potential diagnostic errors.6 The new definition of diagnostic errors introduced in the 2015 NASEM report emphasized the significance of effective communication during the diagnostic procedure.1 Engaging patients and their families in the diagnostic process can improve communication, improve diagnostic accuracy, and help to identify errors before they cause harm.7 However, many patients and families feel that they are not listened to or taken seriously by health care providers, and may not feel comfortable sharing information that they feel is important.8 To address this, health care systems can implement programs that encourage patients and families to be more engaged in the diagnostic process, such as shared decision-making, patient portals, and patient and family advisory councils.9 Health care systems must prioritize patient-centered care, teamwork, and communication. Patients and their families must be actively engaged in their care, and health care providers must be willing to work collaboratively and listen to patients’ concerns.6,10
Health care providers also bring their own valuable experiences and expertise to the diagnostic process, as they are often the ones on the front lines of patient care. However, health care providers may not always feel comfortable reporting errors or near misses, and may not have the time or resources to participate in quality improvement initiatives. To address this, health care systems can implement programs that encourage providers to report errors and near misses, such as anonymous reporting systems, just-culture initiatives, and peer review.11 Creating a culture of teamwork and collaboration among health care providers can improve the accuracy of diagnoses and reduce the risk of errors.12
A key factor in utilizing HX to reduce diagnostic errors is effective communication. Communication breakdowns among health care providers, patients, and their families are a common contributing factor resulting in diagnostic errors.2 Strategies to improve communication include using clear and concise language, involving patients and their families in the decision-making process, and utilizing electronic health records (EHRs) to ensure that all health care providers have access to relevant, accurate, and up-to-date patient information.4,13,14
Another important aspect of utilizing HX in health care to reduce diagnostic errors is the need to recognize and address cognitive biases that may influence diagnostic decisions.3 Cognitive biases are common in health care and can lead to errors in diagnosis. For example, confirmation bias, which is the tendency to look for information that confirms preexisting beliefs, can lead providers to overlook important diagnostic information.15 Biases such as anchoring bias, premature closure, and confirmation bias can lead to incorrect diagnoses and can be difficult to recognize and overcome. Addressing cognitive biases requires a commitment to self-reflection and self-awareness among health care providers as well as structured training of health care providers to improve their diagnostic reasoning skills and reduce the risk of cognitive errors.15 By implementing these strategies around HX in health care, health care systems can become more patient-centered and reduce the likelihood of diagnostic errors (Figure).
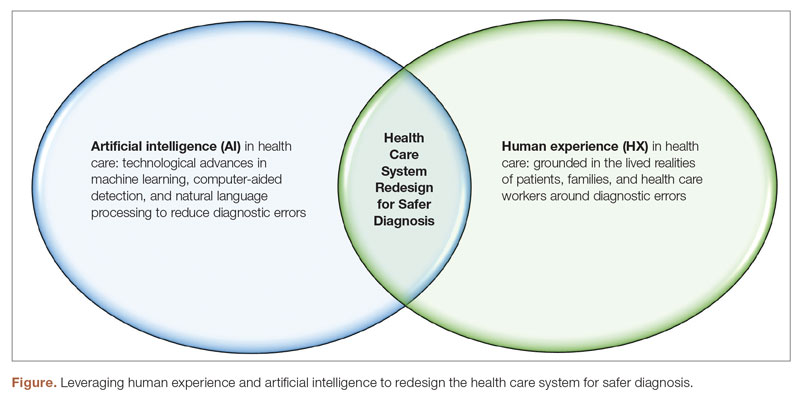
Artificial Intelligence and Diagnostic Errors
Artificial intelligence has the potential to significantly reduce diagnostic errors in health care (Figure), and its role in health care is rapidly expanding. AI technologies such as machine learning (ML) and natural language processing (NLP) have the potential to significantly reduce diagnostic errors by augmenting human cognition and improving access to relevant patient data.1,16 Machine learning algorithms can analyze large amounts of patient data sets to identify patterns and risk factors and predict patient outcomes, which can aid health care providers in making accurate diagnoses.17 Artificial intelligence can also help to address some of the communication breakdowns that contribute to diagnostic errors.18 Natural language processing can improve the accuracy of EHR documentation and reduce the associated clinician burden, making it easier for providers to access relevant patient information and communicate more effectively with each other.18
In health care, AI can be used to analyze medical images, laboratory results, genomic data, and EHRs to identify potential diagnoses and flag patients who may be at risk for diagnostic errors. One of the primary benefits of AI in health care is its ability to process large amounts of data quickly and accurately.19 This can be particularly valuable in diagnosing rare or complex conditions. Machine learning algorithms can analyze patient data to identify subtle patterns that may not be apparent to human providers.16 This can lead to earlier and more accurate diagnoses, which can reduce diagnostic errors and improve patient outcomes.17 One example of the application of AI in health care is the use of computer-aided detection (CAD) software to analyze medical images. This software can help radiologists detect abnormalities in medical images that may be missed by the human eye, such as early-stage breast cancer.20 Another example is the use of NLP and ML to analyze unstructured data in EHRs, such as physician notes, to identify potential diagnoses and flag patients who may be at risk for diagnostic errors.21 A recent study showed that using NLP on EHRs for screening and detecting individuals at risk for psychosis can considerably enhance the prognostic accuracy of psychosis risk calculators.22 This can help identify patients who require assessment and specialized care, facilitating earlier detection and potentially improving patient outcomes. On the same note, ML-based severe sepsis prediction algorithms have been shown to reduce the average length of stay and in-hospital mortality rate.23
However, there are also concerns about the use of AI in health care, including the potential for bias and the risk of overreliance on AI. Bias can occur when AI algorithms are trained on data that is not representative of the population being analyzed, leading to inaccurate or unfair results, hence, perpetuating and exacerbating existing biases in health care.24 Over-reliance on AI can occur when health care providers rely too heavily on AI algorithms and fail to consider other important information, such as the lived experience of patients, families, and health care providers. Addressing these concerns will require ongoing efforts to ensure that AI technologies are developed and implemented in an ethical and responsible manner.25
Conclusion
Reducing diagnostic errors is a critical goal for health care systems, and requires a comprehensive approach that utilizes both HX and AI technologies. Engaging patients and their families in the diagnostic process, promoting teamwork and collaboration among health care providers, addressing cognitive biases, and harnessing the power of AI can all contribute to more accurate diagnoses and better patient outcomes. By integrating the lived experience of patients, families, and health care providers with AI technologies, health care systems can be redesigned to become more proactive, safer, and patient-centered in identifying potential health problems and reducing the risk of diagnostic errors, ensuring that patients receive the care they need and deserve.
Corresponding author: Iqbal Ratnani, Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, 6565 Fannin St, Houston, TX 77030; [email protected]
Disclosures: None reported.
1. National Academy of Medicine. Improving Diagnosis in Health Care. Balogh EP, Miller BT, Ball JR, eds. National Academies Press; 2015. doi:10.17226/21794
2. Singh H, Schiff GD, Graber ML, et al. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
3. Croskerry P, Campbell SG, Petrie DA. The challenge of cognitive science for medical diagnosis. Cogn Res Princ Implic. 2023;8(1):13. doi:10.1186/s41235-022-00460-z
4. Dahm MR, Williams M, Crock C. ‘More than words’ - interpersonal communication, cogntive bias and diagnostic errors. Patient Educ Couns. 2022;105(1):252-256. doi:10.1016/j.pec.2021.05.012
5. Wolf JA, Niederhauser V, Marshburn D, LaVela SL. Reexamining “defining patient experience”: The human experience in Healthcare. Patient Experience J. 2021;8(1):16-29. doi:10.35680/2372-0247.1594
6. Sacco AY, Self QR, Worswick EL, et al. Patients’ perspectives of diagnostic error: A qualitative study. J Patient Saf. 2021;17(8):e1759-e1764. doi:10.1097/PTS.0000000000000642
7. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
8. Austin E, LeRouge C, Hartzler AL, Segal C, Lavallee DC. Capturing the patient voice: implementing patient-reported outcomes across the health system. Qual Life Res. 2020;29(2):347-355. doi:10.1007/s11136-019-02320-8
9. Waddell A, Lennox A, Spassova G, Bragge P. Barriers and facilitators to shared decision-making in hospitals from policy to practice: a systematic review. Implement Sci. 2021;16(1):74. doi: 10.1186/s13012-021-01142-y
10. US Preventive Services Task Force. Collaboration and shared decision-making between patients and clinicians in preventive health care decisions and US Preventive Services Task Force Recommendations. JAMA. 2022;327(12):1171-1176. doi:10.1001/jama.2022.3267
11. Reporting patient safety events. Patient Safety Network. Published September 7, 2019. Accessed April 29, 2023. https://psnet.ahrq.gov/primer/reporting-patient-safety-events
12. McLaney E, Morassaei S, Hughes L, et al. A framework for interprofessional team collaboration in a hospital setting: Advancing team competencies and behaviours. Healthc Manage Forum. 2022;35(2):112-117. doi:10.1177/08404704211063584
13. Abid MH, Abid MM, Shahid R, et al. Patient and family engagement during challenging times: what works and what does not? Cureus. 2021;13(5):e14814. doi:10.7759/cureus.14814
14. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, et al. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
15. Watari T, Tokuda Y, Amano Y, et al. Cognitive bias and diagnostic errors among physicians in Japan: A self-reflection survey. Int J Environ Res Public Health. 2022;19(8):4645. doi:10.3390/ijerph19084645
16. Rajkomar A, Oren E, Chen K et al. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018;1:18. https://doi.org/10.1038/s41746-018-0029-1
17. Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J. 2019;6(2):94-98. doi:10.7861/futurehosp.6-2-94
18. Dymek C, Kim B, Melton GB, et al. Building the evidence-base to reduce electronic health record-related clinician burden. J Am Med Inform Assoc. 2021;28(5):1057-1061. doi:10.1093/jamia/ocaa238
19. Beam AL, Kohane IS. Big data and machine learning in health care. JAMA. 2018;319(13):1317-1318. doi:10.1001/jama.2017.18391
20. Lehman CD, Wellman RD, Buist DS, et al. Diagnostic accuracy of digital screening mammography with and without computer-aided detection. JAMA Intern Med. 2015;175(11):1828-1837. doi:10.1001/jamainternmed.2015.5231
21. Liao KP, Cai T, Savova GK, et al. Development of phenotype algorithms using electronic medical records and incorporating natural language processing. BMJ. 2015;350:h1885. doi:10.1136/bmj.h1885
22. Irving J, Patel R, Oliver D, et al. Using natural language processing on electronic health records to enhance detection and prediction of psychosis risk. Schizophr Bull. 2021;47(2):405-414. doi:10.1093/schbul/sbaa126. Erratum in: Schizophr Bull. 2021;47(2):575.
23. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4(1):e000234. doi:10.1136/bmjresp-2017-000234
24. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. doi:10.1126/science.aax2342
25. Ibrahim SA, Pronovost PJ. Diagnostic errors, health disparities, and artificial intelligence: a combination for health or harm? JAMA Health Forum. 2021;2(9):e212430. doi:10.1001/jamahealthforum.2021.2430
From the Institute for Healthcare Improvement, Boston, MA (Dr. Abid); Continuous Quality Improvement and Patient Safety Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Abid); Primary and Secondary Healthcare Department, Government of Punjab, Lahore, Pakistan (Dr. Ahmed); Infection Prevention and Control Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Din); Internal Medicine Department, Greater Baltimore Medical Center, Baltimore, MD (Dr. Abid); Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, Houston, TX (Dr. Ratnani).
Diagnostic errors are defined by the National Academies of Sciences, Engineering, and Medicine (NASEM) as the failure to either establish an accurate and timely explanation of the patient’s health problem(s) or communicate that explanation to the patient.1 According to a report by the Institute of Medicine, diagnostic errors account for a substantial number of adverse events in health care, affecting an estimated 12 million Americans each year.1 Diagnostic errors are a common and serious issue in health care systems, with studies estimating that 5% to 15% of all diagnoses are incorrect.1 Such errors can result in unnecessary treatments, delays in necessary treatments, and harm to patients. The high prevalence of diagnostic errors in primary care has been identified as a global issue.2 While many factors contribute to diagnostic errors, the complex nature of health care systems, the limited processing capacity of human cognition, and deficiencies in interpersonal patient-clinician communication are primary contributors.3,4
Discussions around the redesign of health care systems to reduce diagnostic errors have been at the forefront of medical research for years.2,4 To decrease diagnostic errors in health care, a comprehensive strategy is necessary. This strategy should focus on utilizing both human experience (HX) in health care and artificial intelligence (AI) technologies to transform health care systems into proactive, patient-centered, and safer systems, specifically concerning diagnostic errors.1
Human Experience and Diagnostic Errors
The role of HX in health care cannot be overstated. The HX in health care integrates the sum of all interactions, every encounter among patients, families and care partners, and the health care workforce.5 Patients and their families have a unique perspective on their health care experiences that can provide valuable insight into potential diagnostic errors.6 The new definition of diagnostic errors introduced in the 2015 NASEM report emphasized the significance of effective communication during the diagnostic procedure.1 Engaging patients and their families in the diagnostic process can improve communication, improve diagnostic accuracy, and help to identify errors before they cause harm.7 However, many patients and families feel that they are not listened to or taken seriously by health care providers, and may not feel comfortable sharing information that they feel is important.8 To address this, health care systems can implement programs that encourage patients and families to be more engaged in the diagnostic process, such as shared decision-making, patient portals, and patient and family advisory councils.9 Health care systems must prioritize patient-centered care, teamwork, and communication. Patients and their families must be actively engaged in their care, and health care providers must be willing to work collaboratively and listen to patients’ concerns.6,10
Health care providers also bring their own valuable experiences and expertise to the diagnostic process, as they are often the ones on the front lines of patient care. However, health care providers may not always feel comfortable reporting errors or near misses, and may not have the time or resources to participate in quality improvement initiatives. To address this, health care systems can implement programs that encourage providers to report errors and near misses, such as anonymous reporting systems, just-culture initiatives, and peer review.11 Creating a culture of teamwork and collaboration among health care providers can improve the accuracy of diagnoses and reduce the risk of errors.12
A key factor in utilizing HX to reduce diagnostic errors is effective communication. Communication breakdowns among health care providers, patients, and their families are a common contributing factor resulting in diagnostic errors.2 Strategies to improve communication include using clear and concise language, involving patients and their families in the decision-making process, and utilizing electronic health records (EHRs) to ensure that all health care providers have access to relevant, accurate, and up-to-date patient information.4,13,14
Another important aspect of utilizing HX in health care to reduce diagnostic errors is the need to recognize and address cognitive biases that may influence diagnostic decisions.3 Cognitive biases are common in health care and can lead to errors in diagnosis. For example, confirmation bias, which is the tendency to look for information that confirms preexisting beliefs, can lead providers to overlook important diagnostic information.15 Biases such as anchoring bias, premature closure, and confirmation bias can lead to incorrect diagnoses and can be difficult to recognize and overcome. Addressing cognitive biases requires a commitment to self-reflection and self-awareness among health care providers as well as structured training of health care providers to improve their diagnostic reasoning skills and reduce the risk of cognitive errors.15 By implementing these strategies around HX in health care, health care systems can become more patient-centered and reduce the likelihood of diagnostic errors (Figure).

Artificial Intelligence and Diagnostic Errors
Artificial intelligence has the potential to significantly reduce diagnostic errors in health care (Figure), and its role in health care is rapidly expanding. AI technologies such as machine learning (ML) and natural language processing (NLP) have the potential to significantly reduce diagnostic errors by augmenting human cognition and improving access to relevant patient data.1,16 Machine learning algorithms can analyze large amounts of patient data sets to identify patterns and risk factors and predict patient outcomes, which can aid health care providers in making accurate diagnoses.17 Artificial intelligence can also help to address some of the communication breakdowns that contribute to diagnostic errors.18 Natural language processing can improve the accuracy of EHR documentation and reduce the associated clinician burden, making it easier for providers to access relevant patient information and communicate more effectively with each other.18
In health care, AI can be used to analyze medical images, laboratory results, genomic data, and EHRs to identify potential diagnoses and flag patients who may be at risk for diagnostic errors. One of the primary benefits of AI in health care is its ability to process large amounts of data quickly and accurately.19 This can be particularly valuable in diagnosing rare or complex conditions. Machine learning algorithms can analyze patient data to identify subtle patterns that may not be apparent to human providers.16 This can lead to earlier and more accurate diagnoses, which can reduce diagnostic errors and improve patient outcomes.17 One example of the application of AI in health care is the use of computer-aided detection (CAD) software to analyze medical images. This software can help radiologists detect abnormalities in medical images that may be missed by the human eye, such as early-stage breast cancer.20 Another example is the use of NLP and ML to analyze unstructured data in EHRs, such as physician notes, to identify potential diagnoses and flag patients who may be at risk for diagnostic errors.21 A recent study showed that using NLP on EHRs for screening and detecting individuals at risk for psychosis can considerably enhance the prognostic accuracy of psychosis risk calculators.22 This can help identify patients who require assessment and specialized care, facilitating earlier detection and potentially improving patient outcomes. On the same note, ML-based severe sepsis prediction algorithms have been shown to reduce the average length of stay and in-hospital mortality rate.23
However, there are also concerns about the use of AI in health care, including the potential for bias and the risk of overreliance on AI. Bias can occur when AI algorithms are trained on data that is not representative of the population being analyzed, leading to inaccurate or unfair results, hence, perpetuating and exacerbating existing biases in health care.24 Over-reliance on AI can occur when health care providers rely too heavily on AI algorithms and fail to consider other important information, such as the lived experience of patients, families, and health care providers. Addressing these concerns will require ongoing efforts to ensure that AI technologies are developed and implemented in an ethical and responsible manner.25
Conclusion
Reducing diagnostic errors is a critical goal for health care systems, and requires a comprehensive approach that utilizes both HX and AI technologies. Engaging patients and their families in the diagnostic process, promoting teamwork and collaboration among health care providers, addressing cognitive biases, and harnessing the power of AI can all contribute to more accurate diagnoses and better patient outcomes. By integrating the lived experience of patients, families, and health care providers with AI technologies, health care systems can be redesigned to become more proactive, safer, and patient-centered in identifying potential health problems and reducing the risk of diagnostic errors, ensuring that patients receive the care they need and deserve.
Corresponding author: Iqbal Ratnani, Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, 6565 Fannin St, Houston, TX 77030; [email protected]
Disclosures: None reported.
From the Institute for Healthcare Improvement, Boston, MA (Dr. Abid); Continuous Quality Improvement and Patient Safety Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Abid); Primary and Secondary Healthcare Department, Government of Punjab, Lahore, Pakistan (Dr. Ahmed); Infection Prevention and Control Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Din); Internal Medicine Department, Greater Baltimore Medical Center, Baltimore, MD (Dr. Abid); Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, Houston, TX (Dr. Ratnani).
Diagnostic errors are defined by the National Academies of Sciences, Engineering, and Medicine (NASEM) as the failure to either establish an accurate and timely explanation of the patient’s health problem(s) or communicate that explanation to the patient.1 According to a report by the Institute of Medicine, diagnostic errors account for a substantial number of adverse events in health care, affecting an estimated 12 million Americans each year.1 Diagnostic errors are a common and serious issue in health care systems, with studies estimating that 5% to 15% of all diagnoses are incorrect.1 Such errors can result in unnecessary treatments, delays in necessary treatments, and harm to patients. The high prevalence of diagnostic errors in primary care has been identified as a global issue.2 While many factors contribute to diagnostic errors, the complex nature of health care systems, the limited processing capacity of human cognition, and deficiencies in interpersonal patient-clinician communication are primary contributors.3,4
Discussions around the redesign of health care systems to reduce diagnostic errors have been at the forefront of medical research for years.2,4 To decrease diagnostic errors in health care, a comprehensive strategy is necessary. This strategy should focus on utilizing both human experience (HX) in health care and artificial intelligence (AI) technologies to transform health care systems into proactive, patient-centered, and safer systems, specifically concerning diagnostic errors.1
Human Experience and Diagnostic Errors
The role of HX in health care cannot be overstated. The HX in health care integrates the sum of all interactions, every encounter among patients, families and care partners, and the health care workforce.5 Patients and their families have a unique perspective on their health care experiences that can provide valuable insight into potential diagnostic errors.6 The new definition of diagnostic errors introduced in the 2015 NASEM report emphasized the significance of effective communication during the diagnostic procedure.1 Engaging patients and their families in the diagnostic process can improve communication, improve diagnostic accuracy, and help to identify errors before they cause harm.7 However, many patients and families feel that they are not listened to or taken seriously by health care providers, and may not feel comfortable sharing information that they feel is important.8 To address this, health care systems can implement programs that encourage patients and families to be more engaged in the diagnostic process, such as shared decision-making, patient portals, and patient and family advisory councils.9 Health care systems must prioritize patient-centered care, teamwork, and communication. Patients and their families must be actively engaged in their care, and health care providers must be willing to work collaboratively and listen to patients’ concerns.6,10
Health care providers also bring their own valuable experiences and expertise to the diagnostic process, as they are often the ones on the front lines of patient care. However, health care providers may not always feel comfortable reporting errors or near misses, and may not have the time or resources to participate in quality improvement initiatives. To address this, health care systems can implement programs that encourage providers to report errors and near misses, such as anonymous reporting systems, just-culture initiatives, and peer review.11 Creating a culture of teamwork and collaboration among health care providers can improve the accuracy of diagnoses and reduce the risk of errors.12
A key factor in utilizing HX to reduce diagnostic errors is effective communication. Communication breakdowns among health care providers, patients, and their families are a common contributing factor resulting in diagnostic errors.2 Strategies to improve communication include using clear and concise language, involving patients and their families in the decision-making process, and utilizing electronic health records (EHRs) to ensure that all health care providers have access to relevant, accurate, and up-to-date patient information.4,13,14
Another important aspect of utilizing HX in health care to reduce diagnostic errors is the need to recognize and address cognitive biases that may influence diagnostic decisions.3 Cognitive biases are common in health care and can lead to errors in diagnosis. For example, confirmation bias, which is the tendency to look for information that confirms preexisting beliefs, can lead providers to overlook important diagnostic information.15 Biases such as anchoring bias, premature closure, and confirmation bias can lead to incorrect diagnoses and can be difficult to recognize and overcome. Addressing cognitive biases requires a commitment to self-reflection and self-awareness among health care providers as well as structured training of health care providers to improve their diagnostic reasoning skills and reduce the risk of cognitive errors.15 By implementing these strategies around HX in health care, health care systems can become more patient-centered and reduce the likelihood of diagnostic errors (Figure).

Artificial Intelligence and Diagnostic Errors
Artificial intelligence has the potential to significantly reduce diagnostic errors in health care (Figure), and its role in health care is rapidly expanding. AI technologies such as machine learning (ML) and natural language processing (NLP) have the potential to significantly reduce diagnostic errors by augmenting human cognition and improving access to relevant patient data.1,16 Machine learning algorithms can analyze large amounts of patient data sets to identify patterns and risk factors and predict patient outcomes, which can aid health care providers in making accurate diagnoses.17 Artificial intelligence can also help to address some of the communication breakdowns that contribute to diagnostic errors.18 Natural language processing can improve the accuracy of EHR documentation and reduce the associated clinician burden, making it easier for providers to access relevant patient information and communicate more effectively with each other.18
In health care, AI can be used to analyze medical images, laboratory results, genomic data, and EHRs to identify potential diagnoses and flag patients who may be at risk for diagnostic errors. One of the primary benefits of AI in health care is its ability to process large amounts of data quickly and accurately.19 This can be particularly valuable in diagnosing rare or complex conditions. Machine learning algorithms can analyze patient data to identify subtle patterns that may not be apparent to human providers.16 This can lead to earlier and more accurate diagnoses, which can reduce diagnostic errors and improve patient outcomes.17 One example of the application of AI in health care is the use of computer-aided detection (CAD) software to analyze medical images. This software can help radiologists detect abnormalities in medical images that may be missed by the human eye, such as early-stage breast cancer.20 Another example is the use of NLP and ML to analyze unstructured data in EHRs, such as physician notes, to identify potential diagnoses and flag patients who may be at risk for diagnostic errors.21 A recent study showed that using NLP on EHRs for screening and detecting individuals at risk for psychosis can considerably enhance the prognostic accuracy of psychosis risk calculators.22 This can help identify patients who require assessment and specialized care, facilitating earlier detection and potentially improving patient outcomes. On the same note, ML-based severe sepsis prediction algorithms have been shown to reduce the average length of stay and in-hospital mortality rate.23
However, there are also concerns about the use of AI in health care, including the potential for bias and the risk of overreliance on AI. Bias can occur when AI algorithms are trained on data that is not representative of the population being analyzed, leading to inaccurate or unfair results, hence, perpetuating and exacerbating existing biases in health care.24 Over-reliance on AI can occur when health care providers rely too heavily on AI algorithms and fail to consider other important information, such as the lived experience of patients, families, and health care providers. Addressing these concerns will require ongoing efforts to ensure that AI technologies are developed and implemented in an ethical and responsible manner.25
Conclusion
Reducing diagnostic errors is a critical goal for health care systems, and requires a comprehensive approach that utilizes both HX and AI technologies. Engaging patients and their families in the diagnostic process, promoting teamwork and collaboration among health care providers, addressing cognitive biases, and harnessing the power of AI can all contribute to more accurate diagnoses and better patient outcomes. By integrating the lived experience of patients, families, and health care providers with AI technologies, health care systems can be redesigned to become more proactive, safer, and patient-centered in identifying potential health problems and reducing the risk of diagnostic errors, ensuring that patients receive the care they need and deserve.
Corresponding author: Iqbal Ratnani, Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, 6565 Fannin St, Houston, TX 77030; [email protected]
Disclosures: None reported.
1. National Academy of Medicine. Improving Diagnosis in Health Care. Balogh EP, Miller BT, Ball JR, eds. National Academies Press; 2015. doi:10.17226/21794
2. Singh H, Schiff GD, Graber ML, et al. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
3. Croskerry P, Campbell SG, Petrie DA. The challenge of cognitive science for medical diagnosis. Cogn Res Princ Implic. 2023;8(1):13. doi:10.1186/s41235-022-00460-z
4. Dahm MR, Williams M, Crock C. ‘More than words’ - interpersonal communication, cogntive bias and diagnostic errors. Patient Educ Couns. 2022;105(1):252-256. doi:10.1016/j.pec.2021.05.012
5. Wolf JA, Niederhauser V, Marshburn D, LaVela SL. Reexamining “defining patient experience”: The human experience in Healthcare. Patient Experience J. 2021;8(1):16-29. doi:10.35680/2372-0247.1594
6. Sacco AY, Self QR, Worswick EL, et al. Patients’ perspectives of diagnostic error: A qualitative study. J Patient Saf. 2021;17(8):e1759-e1764. doi:10.1097/PTS.0000000000000642
7. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
8. Austin E, LeRouge C, Hartzler AL, Segal C, Lavallee DC. Capturing the patient voice: implementing patient-reported outcomes across the health system. Qual Life Res. 2020;29(2):347-355. doi:10.1007/s11136-019-02320-8
9. Waddell A, Lennox A, Spassova G, Bragge P. Barriers and facilitators to shared decision-making in hospitals from policy to practice: a systematic review. Implement Sci. 2021;16(1):74. doi: 10.1186/s13012-021-01142-y
10. US Preventive Services Task Force. Collaboration and shared decision-making between patients and clinicians in preventive health care decisions and US Preventive Services Task Force Recommendations. JAMA. 2022;327(12):1171-1176. doi:10.1001/jama.2022.3267
11. Reporting patient safety events. Patient Safety Network. Published September 7, 2019. Accessed April 29, 2023. https://psnet.ahrq.gov/primer/reporting-patient-safety-events
12. McLaney E, Morassaei S, Hughes L, et al. A framework for interprofessional team collaboration in a hospital setting: Advancing team competencies and behaviours. Healthc Manage Forum. 2022;35(2):112-117. doi:10.1177/08404704211063584
13. Abid MH, Abid MM, Shahid R, et al. Patient and family engagement during challenging times: what works and what does not? Cureus. 2021;13(5):e14814. doi:10.7759/cureus.14814
14. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, et al. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
15. Watari T, Tokuda Y, Amano Y, et al. Cognitive bias and diagnostic errors among physicians in Japan: A self-reflection survey. Int J Environ Res Public Health. 2022;19(8):4645. doi:10.3390/ijerph19084645
16. Rajkomar A, Oren E, Chen K et al. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018;1:18. https://doi.org/10.1038/s41746-018-0029-1
17. Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J. 2019;6(2):94-98. doi:10.7861/futurehosp.6-2-94
18. Dymek C, Kim B, Melton GB, et al. Building the evidence-base to reduce electronic health record-related clinician burden. J Am Med Inform Assoc. 2021;28(5):1057-1061. doi:10.1093/jamia/ocaa238
19. Beam AL, Kohane IS. Big data and machine learning in health care. JAMA. 2018;319(13):1317-1318. doi:10.1001/jama.2017.18391
20. Lehman CD, Wellman RD, Buist DS, et al. Diagnostic accuracy of digital screening mammography with and without computer-aided detection. JAMA Intern Med. 2015;175(11):1828-1837. doi:10.1001/jamainternmed.2015.5231
21. Liao KP, Cai T, Savova GK, et al. Development of phenotype algorithms using electronic medical records and incorporating natural language processing. BMJ. 2015;350:h1885. doi:10.1136/bmj.h1885
22. Irving J, Patel R, Oliver D, et al. Using natural language processing on electronic health records to enhance detection and prediction of psychosis risk. Schizophr Bull. 2021;47(2):405-414. doi:10.1093/schbul/sbaa126. Erratum in: Schizophr Bull. 2021;47(2):575.
23. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4(1):e000234. doi:10.1136/bmjresp-2017-000234
24. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. doi:10.1126/science.aax2342
25. Ibrahim SA, Pronovost PJ. Diagnostic errors, health disparities, and artificial intelligence: a combination for health or harm? JAMA Health Forum. 2021;2(9):e212430. doi:10.1001/jamahealthforum.2021.2430
1. National Academy of Medicine. Improving Diagnosis in Health Care. Balogh EP, Miller BT, Ball JR, eds. National Academies Press; 2015. doi:10.17226/21794
2. Singh H, Schiff GD, Graber ML, et al. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
3. Croskerry P, Campbell SG, Petrie DA. The challenge of cognitive science for medical diagnosis. Cogn Res Princ Implic. 2023;8(1):13. doi:10.1186/s41235-022-00460-z
4. Dahm MR, Williams M, Crock C. ‘More than words’ - interpersonal communication, cogntive bias and diagnostic errors. Patient Educ Couns. 2022;105(1):252-256. doi:10.1016/j.pec.2021.05.012
5. Wolf JA, Niederhauser V, Marshburn D, LaVela SL. Reexamining “defining patient experience”: The human experience in Healthcare. Patient Experience J. 2021;8(1):16-29. doi:10.35680/2372-0247.1594
6. Sacco AY, Self QR, Worswick EL, et al. Patients’ perspectives of diagnostic error: A qualitative study. J Patient Saf. 2021;17(8):e1759-e1764. doi:10.1097/PTS.0000000000000642
7. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
8. Austin E, LeRouge C, Hartzler AL, Segal C, Lavallee DC. Capturing the patient voice: implementing patient-reported outcomes across the health system. Qual Life Res. 2020;29(2):347-355. doi:10.1007/s11136-019-02320-8
9. Waddell A, Lennox A, Spassova G, Bragge P. Barriers and facilitators to shared decision-making in hospitals from policy to practice: a systematic review. Implement Sci. 2021;16(1):74. doi: 10.1186/s13012-021-01142-y
10. US Preventive Services Task Force. Collaboration and shared decision-making between patients and clinicians in preventive health care decisions and US Preventive Services Task Force Recommendations. JAMA. 2022;327(12):1171-1176. doi:10.1001/jama.2022.3267
11. Reporting patient safety events. Patient Safety Network. Published September 7, 2019. Accessed April 29, 2023. https://psnet.ahrq.gov/primer/reporting-patient-safety-events
12. McLaney E, Morassaei S, Hughes L, et al. A framework for interprofessional team collaboration in a hospital setting: Advancing team competencies and behaviours. Healthc Manage Forum. 2022;35(2):112-117. doi:10.1177/08404704211063584
13. Abid MH, Abid MM, Shahid R, et al. Patient and family engagement during challenging times: what works and what does not? Cureus. 2021;13(5):e14814. doi:10.7759/cureus.14814
14. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, et al. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
15. Watari T, Tokuda Y, Amano Y, et al. Cognitive bias and diagnostic errors among physicians in Japan: A self-reflection survey. Int J Environ Res Public Health. 2022;19(8):4645. doi:10.3390/ijerph19084645
16. Rajkomar A, Oren E, Chen K et al. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018;1:18. https://doi.org/10.1038/s41746-018-0029-1
17. Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J. 2019;6(2):94-98. doi:10.7861/futurehosp.6-2-94
18. Dymek C, Kim B, Melton GB, et al. Building the evidence-base to reduce electronic health record-related clinician burden. J Am Med Inform Assoc. 2021;28(5):1057-1061. doi:10.1093/jamia/ocaa238
19. Beam AL, Kohane IS. Big data and machine learning in health care. JAMA. 2018;319(13):1317-1318. doi:10.1001/jama.2017.18391
20. Lehman CD, Wellman RD, Buist DS, et al. Diagnostic accuracy of digital screening mammography with and without computer-aided detection. JAMA Intern Med. 2015;175(11):1828-1837. doi:10.1001/jamainternmed.2015.5231
21. Liao KP, Cai T, Savova GK, et al. Development of phenotype algorithms using electronic medical records and incorporating natural language processing. BMJ. 2015;350:h1885. doi:10.1136/bmj.h1885
22. Irving J, Patel R, Oliver D, et al. Using natural language processing on electronic health records to enhance detection and prediction of psychosis risk. Schizophr Bull. 2021;47(2):405-414. doi:10.1093/schbul/sbaa126. Erratum in: Schizophr Bull. 2021;47(2):575.
23. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4(1):e000234. doi:10.1136/bmjresp-2017-000234
24. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. doi:10.1126/science.aax2342
25. Ibrahim SA, Pronovost PJ. Diagnostic errors, health disparities, and artificial intelligence: a combination for health or harm? JAMA Health Forum. 2021;2(9):e212430. doi:10.1001/jamahealthforum.2021.2430