User login
Hospital-Acquired Conditions & The Hospitalist
Hospitalist Neal Axon, MD, first became aware of an important change in his hospital’s policies last year while attending to an elderly patient the morning after admission to the community hospital where he works part time.
“This new form appeared in the chart requesting a urinalysis for my patient, who’d had a Foley catheter placed,” says Dr. Axon, an assistant professor of medicine at the Medical University of South Carolina in Charleston. “I didn’t know why, so I asked. I was told that it was now necessary to document that there was no UTI present on admission.” He asked the charge nurse, “So what do I do now that the catheter has been in place for 12 hours and has colonization without a true infection?”
The next thing he heard: silence.
The new form Dr. Axon encountered was an outgrowth of the requirements of the Deficit Reduction Act (DRA) of 2005, which ordered Medicare to withhold additional hospital payments for hospital-acquired complications (HAC) developed during a hospital stay. One result of the new rule is that much of a hospital’s response to these initiatives has been placed in the hands of the hospitalist. From accurate documentation of complications already present on admission (POA), to confirming that guidelines for treatment are being followed, to taking the lead on review of staff practices and education, hospitalists are in a position to have a wide-ranging impact on patient care and the financial health of their institutions.
Congress Pushes Reforms
In order for Medicare to not provide a reimbursement, an HAC has to be high-cost and/or high-volume, result in the assignment of the case to a higher payment when present as a secondary diagnosis, and “could reasonably be prevented through the application of evidence-based guidelines,” says Barry Straube, MD, chief medical officer and director of the Office of Clinical Standards and Quality at the Centers for Medicare and Medicaid Services (CMS). “CMS was to implement a process where we would not pay the hospitals additional money for these complications.”
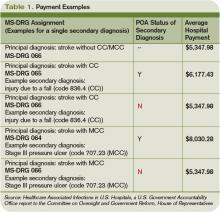
The new rules mean Medicare pays hospitals on the basis of Medicare Severity Diagnostic-Related Groups (MS-DRG), which better reflect the complexity of a patient’s illness. The biggest change was a three-tiered payment schedule: a base level for the diagnosis, a second level adding money to reflect the presence of comorbidities and complications, and a third for major complications and comorbidities (see Table 1, p. 31).
“Instituting HACs means that hospitals would no longer receive the comorbidity and complication payments if the only reason a case qualified for higher payment was the HAC,” Dr. Straube explains. “We did carve out a POA exception for those conditions that were acquired outside of the hospital. HACs only impact additional payments; the hospitals are still paid for the diagnosis that resulted in the hospital admission.”
CMS also identifies three “never events” it won’t reimburse for (see “A Brief History of Never Events,” p. 35): performing the wrong procedure, performing a procedure on the wrong body part, and performing a procedure on the wrong patient. “Neither hospitals nor physicians that are involved in such egregious situations would be paid,” Dr. Straube says.
Preventability: Subject of Controversy
The big questions surrounding HACs: Could they reasonably be prevented through the application of evidence-based guidelines? How preventable are HACs? Who decides if a complication is preventable, and therefore payment for services is withheld?
They’re concerns that are widespread among physicians, hospital administrators, and regulators alike.
“The legislation required the conditions to be ‘reasonably preventable’ using established clinical guidelines,” Dr. Straube says. “We did not have to show 100% prevention. In an imperfect world, they might still take place occasionally, but with good medical care, almost all of these are preventable in this day and age.”
For CMS, the preventable conditions are an either/or situation: Either they existed prior to admission and are subject to payment, or they did not exist at admission and additional payment for the complication will not be made. “HACs do not currently consider a patient’s individual risk for complications,” says Jennifer Meddings, MD, MSc, clinical lecturer and health researcher in the Department of Internal Medicine at the University of Michigan Health System in Ann Arbor. “We know the best strategies to prevent complications in ideal patients, and these are reflected in the HACs. In real life, many of our patients just don’t fit into the guidelines for many reasons—and you have to individualize care.”
Dr. Meddings points to DVT as a prime example. For a certain number of inpatients, the guidelines can be followed to perfection. In other patients (e.g., those with kidney conditions), previous reactions to a medication or an individual’s predisposition to clotting might interfere with treatment. However, CMS doesn’t allow appeals of nonpayment decisions for HACs based on individual circumstances.
Some experts think the rigidness of the payment policy forces physicians to treat patients exactly to guidelines. Even then, payment could be declined if an HAC develops.
“One of the points of most discussion is how preventable some of these are, particularly when choosing those you are no longer going to pay for,” Dr. Meddings says. “Many of the complications currently under review have patients that are at higher risk than others. How much our prevention strategies can alleviate or reduce the risk varies widely among patients.”
Impact on HM Practice
Many of the preventable conditions outlined by CMS do not directly affect hospitalist payment. However, hospitalists often find themselves responsible for properly documenting admission and care.
“The rule changes regarding payment for HACs are only related to hospital payments, and to date, most physicians, including hospitalists, are not directly at financial risk,” says Heidi Wald, MD, MSPH, hospitalist and assistant professor of medicine in the divisions of Health Care Policy Research and General Internal Medicine at the University of Colorado Denver School of Medicine. “Although hospitalists have no financial skin in the game, there are plenty of reasons they would take an interest in addressing HACs in their hospital. In particular, they are often seen as the ‘go-to’ group for quality improvement in their hospitals.”
For example, some HM groups have been active in working with teams of physicians, nurses, and other healthcare providers to address local policies and procedures on prevention of catheter-associated urinary tract infections (UTIs) and DVT.
“This has certainly necessitated a team approach,” says Shaun Frost, MD, FHM, an SHM board member and regional medical director for Cogent Healthcare in St. Paul, Minn. “For many of the HACs that apply to our population of patients, the hospitalist alone cannot be expected to solely execute effective quality improvements. It takes a team effort in that regard, and one that includes many different disciplines.”
The Cogent-affiliated hospitalist group at Temple University Hospital in Philadelphia formed a task force to address issues with catheter-associated UTI. One initiative focused on educating all providers involved in the proper care of the catheters and similar interventions. A secondary focus of the project was an inventory of current practices and procedures.
“It was discovered that we did not have an automatic stop order for Foley catheters, so in some situations, they were likely being left in longer than needed while nursing [staff] tried to contact a physician,” Dr. Frost explains. “We created standardized order sets that include criteria for continuing the catheter. Once the criteria are no longer applicable, nursing will be able to discontinue it.”
Although CMS has only recently turned the spotlight on HACs and never events, hospitalists have been heavily involved in the patient-safety arena for years. “It is not a new phenomenon that hospitalists work for healthcare delivery and healthcare system improvement,” Dr. Meddings says.
Hospital administration at Temple University Hospital recognized the HM group’s quality-improvement (QI) work, and has “specifically charged us with spreading the work we have done in patient safety to the entire house,” Dr. Frost says. “That speaks to the administration’s opinion of the power of the HM program to assist with institution-wide QI initiatives.”
Documentation Is Key
Beyond applying proven methods to avoid HACs, hospitalists can make a difference through documentation. If the hospitalist notes all conditions when the patient first presents to the hospital, additional comorbidity and complication payments should be made.
“The part that probably has the greatest impact on the day-to-day practice of a hospitalist is the increased importance of documentation throughout the hospital episode,” Dr. Meddings says. “If complications are occurring and they are not present in the chart, the coders may not recognize that it has occurred and will not know to include it in the bill. This can have an adverse impact on the hospital and its finances.”
Documentation issues can impact hospital payment in several ways:
- Hospitals might receive additional payment by default if certain HACs are described incorrectly or without sufficient detail (e.g., receiving overpayment because the physician did not indicate a UTI was in fact a catheter-associated UTI);
- As more attention is invested in documenting all conditions POA, hospitals might be coding more comorbidities overall than previously, which also will generate additional payment for hospitals as any POA condition is eligible for increased payment; and
- Hospitals might lose payment when admitting providers fail to adequately document the condition as POA (e.g., a pre-existing decubitus ulcer not detected until the second day of the hospital stay).
The descriptions to be used in coding are very detailed. UTIs, for example, have one code to document the POA assessment, another code to show that a UTI occurred, and a third code to indicate it was catheter-associated. Each code requires appropriate documentation in the chart (see Table 1, above).
The impact hospitalists have on care and payment is not the same across the HAC spectrum. For instance, documenting the presence of pressure ulcers might be easier than distinguishing colonization from infection in those admitted with in-dwelling urinary catheters. Others, such as DVT or vascular catheter-associated infections, are rarely POA unless they are part of the admitting diagnosis.
“This new focus on hospital-acquired conditions may work to the patient’s benefit,” Dr. Meddings says. “The inclusion of pressure ulcers has led to increased attention to skin exams on admission and preventive measures during hospitalizations. In the past, skin exams upon admission may have been given a lower priority, but that has changed.”
Dr. Meddings is concerned that the new rules could force the shifting of resources to areas where the hospital could lose money. If, when, and how many changes will actually take place is still up in the air. “Resource shifting is a concern whenever there is any sort of pay-for-performance attention directed toward one particular complication,” she says. “To balance this, many of the strategies hospitals used to prevent complications are not specific to just the diagnosis that is covered by the HAC.”
Dr. Meddings also hopes the new focus on preventable conditions will have a “halo effect” in the healthcare community. For instance, CMS mandating DVT prevention following orthopedic operations will, hopefully, result in a greater awareness of the problem in other susceptible patients.
POA Indicators
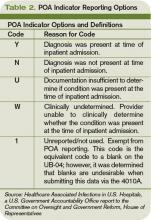
Since hospitalists often perform the initial patient history, physical, and other admission work, they are in the best position to find and document POA indicators (see Table 2, p. below). Proper notes on such things as UTIs present and the state of skin integrity are an important part of making sure the hospital is paid correctly for the care it provides.
Education on the specific definition of each potential HAC is required to help physicians avoid overtreatment of certain conditions, especially UTIs. For example, the Centers for Disease Control and Prevention (CDC) defines all UTIs as symptomatic. Therefore, the screening of all admitted patients, regardless of symptoms, is wasteful and unlikely to help the hospital’s bottom line.
“If you start screening everyone that comes through the door so you don’t miss any pre-existing UTIs, you are going to find a lot of asymptomatic colonization,” Dr. Wald says. “You are also going to spend a lot of money and time on studies and possibly treatments that may not yield many true infections. It is important that physicians know the definition of these HACs to help avoid needless interventions.”
Minimal Loss
Many hospital administrators and physicians were worried when the HAC program was first announced. Much of the stress and concern, however, seems to have dissipated. CMS estimated the HAC program would save Medicare $21 million in fiscal year 2009. Others, such as Peter McNair and colleagues writing in Health Affairs, suggest the actual impact is closer to $1.1 million.1 The CMS-projected impact of the HAC provision in fiscal-year 2009 was $21 million, out of more than $100 billion in payments.
“I think the HACs will not have a major impact because of the way payments are made,” says internist Robert Berenson, MD, a fellow at the Urban Institute in Washington, D.C., who has studied Medicare policy issues extensively, and for two years was in charge of Medicare payment policies at the Health Care Finance Administration, the precursor to CMS. “Patients who have HACs often have another comorbidity that would kick them into a higher payment category regardless of the presence of a hospital-acquired complication. In the end, it is probably more symbolic and unlikely to make a major dent in hospital income—at least at this point.”
Another limitation to CMS nonpayment for HACs is the issue of deciding which conditions are truly preventable. Dr. Berenson questions the ability of the current system to identify many additional complications for which this approach will be feasible.
“CMS has laid out its strategy, suggesting that we should be able to continue increasing the number of conditions for which providers would be paid differently based on quality,” he says. “Many observers question whether there will ever be measurement tools that are robust enough, and there will be a wide agreement on the preventability of enough conditions that this initiative will go very far.”
Although hospitalists might not face a direct financial risk, they still have their hospitals’ best interest—and their reputations—on the line. “Hospitalists care about preventing complications,” Dr. Wald says. “We are very engaged in working with our hospitals to improve care, maximize quality, and minimize cost.” TH
Kurt Ullman is a freelance medical writer based in Indiana.
Reference
- McNair PD, Luft HS, Bindman AB. Medicare’s policy not to pay for treating hospital-acquired conditions: the impact. Health Aff (Millwood). 2009;28(5):1485-1493.
TOP IMAGE SOURCE: KAREEM RIZKHALLA/ISTOCKPHOTO.COM
Hospitalist Neal Axon, MD, first became aware of an important change in his hospital’s policies last year while attending to an elderly patient the morning after admission to the community hospital where he works part time.
“This new form appeared in the chart requesting a urinalysis for my patient, who’d had a Foley catheter placed,” says Dr. Axon, an assistant professor of medicine at the Medical University of South Carolina in Charleston. “I didn’t know why, so I asked. I was told that it was now necessary to document that there was no UTI present on admission.” He asked the charge nurse, “So what do I do now that the catheter has been in place for 12 hours and has colonization without a true infection?”
The next thing he heard: silence.
The new form Dr. Axon encountered was an outgrowth of the requirements of the Deficit Reduction Act (DRA) of 2005, which ordered Medicare to withhold additional hospital payments for hospital-acquired complications (HAC) developed during a hospital stay. One result of the new rule is that much of a hospital’s response to these initiatives has been placed in the hands of the hospitalist. From accurate documentation of complications already present on admission (POA), to confirming that guidelines for treatment are being followed, to taking the lead on review of staff practices and education, hospitalists are in a position to have a wide-ranging impact on patient care and the financial health of their institutions.
Congress Pushes Reforms
In order for Medicare to not provide a reimbursement, an HAC has to be high-cost and/or high-volume, result in the assignment of the case to a higher payment when present as a secondary diagnosis, and “could reasonably be prevented through the application of evidence-based guidelines,” says Barry Straube, MD, chief medical officer and director of the Office of Clinical Standards and Quality at the Centers for Medicare and Medicaid Services (CMS). “CMS was to implement a process where we would not pay the hospitals additional money for these complications.”
The new rules mean Medicare pays hospitals on the basis of Medicare Severity Diagnostic-Related Groups (MS-DRG), which better reflect the complexity of a patient’s illness. The biggest change was a three-tiered payment schedule: a base level for the diagnosis, a second level adding money to reflect the presence of comorbidities and complications, and a third for major complications and comorbidities (see Table 1, p. 31).
“Instituting HACs means that hospitals would no longer receive the comorbidity and complication payments if the only reason a case qualified for higher payment was the HAC,” Dr. Straube explains. “We did carve out a POA exception for those conditions that were acquired outside of the hospital. HACs only impact additional payments; the hospitals are still paid for the diagnosis that resulted in the hospital admission.”
CMS also identifies three “never events” it won’t reimburse for (see “A Brief History of Never Events,” p. 35): performing the wrong procedure, performing a procedure on the wrong body part, and performing a procedure on the wrong patient. “Neither hospitals nor physicians that are involved in such egregious situations would be paid,” Dr. Straube says.
Preventability: Subject of Controversy
The big questions surrounding HACs: Could they reasonably be prevented through the application of evidence-based guidelines? How preventable are HACs? Who decides if a complication is preventable, and therefore payment for services is withheld?
They’re concerns that are widespread among physicians, hospital administrators, and regulators alike.
“The legislation required the conditions to be ‘reasonably preventable’ using established clinical guidelines,” Dr. Straube says. “We did not have to show 100% prevention. In an imperfect world, they might still take place occasionally, but with good medical care, almost all of these are preventable in this day and age.”
For CMS, the preventable conditions are an either/or situation: Either they existed prior to admission and are subject to payment, or they did not exist at admission and additional payment for the complication will not be made. “HACs do not currently consider a patient’s individual risk for complications,” says Jennifer Meddings, MD, MSc, clinical lecturer and health researcher in the Department of Internal Medicine at the University of Michigan Health System in Ann Arbor. “We know the best strategies to prevent complications in ideal patients, and these are reflected in the HACs. In real life, many of our patients just don’t fit into the guidelines for many reasons—and you have to individualize care.”
Dr. Meddings points to DVT as a prime example. For a certain number of inpatients, the guidelines can be followed to perfection. In other patients (e.g., those with kidney conditions), previous reactions to a medication or an individual’s predisposition to clotting might interfere with treatment. However, CMS doesn’t allow appeals of nonpayment decisions for HACs based on individual circumstances.
Some experts think the rigidness of the payment policy forces physicians to treat patients exactly to guidelines. Even then, payment could be declined if an HAC develops.
“One of the points of most discussion is how preventable some of these are, particularly when choosing those you are no longer going to pay for,” Dr. Meddings says. “Many of the complications currently under review have patients that are at higher risk than others. How much our prevention strategies can alleviate or reduce the risk varies widely among patients.”
Impact on HM Practice
Many of the preventable conditions outlined by CMS do not directly affect hospitalist payment. However, hospitalists often find themselves responsible for properly documenting admission and care.
“The rule changes regarding payment for HACs are only related to hospital payments, and to date, most physicians, including hospitalists, are not directly at financial risk,” says Heidi Wald, MD, MSPH, hospitalist and assistant professor of medicine in the divisions of Health Care Policy Research and General Internal Medicine at the University of Colorado Denver School of Medicine. “Although hospitalists have no financial skin in the game, there are plenty of reasons they would take an interest in addressing HACs in their hospital. In particular, they are often seen as the ‘go-to’ group for quality improvement in their hospitals.”
For example, some HM groups have been active in working with teams of physicians, nurses, and other healthcare providers to address local policies and procedures on prevention of catheter-associated urinary tract infections (UTIs) and DVT.
“This has certainly necessitated a team approach,” says Shaun Frost, MD, FHM, an SHM board member and regional medical director for Cogent Healthcare in St. Paul, Minn. “For many of the HACs that apply to our population of patients, the hospitalist alone cannot be expected to solely execute effective quality improvements. It takes a team effort in that regard, and one that includes many different disciplines.”
The Cogent-affiliated hospitalist group at Temple University Hospital in Philadelphia formed a task force to address issues with catheter-associated UTI. One initiative focused on educating all providers involved in the proper care of the catheters and similar interventions. A secondary focus of the project was an inventory of current practices and procedures.
“It was discovered that we did not have an automatic stop order for Foley catheters, so in some situations, they were likely being left in longer than needed while nursing [staff] tried to contact a physician,” Dr. Frost explains. “We created standardized order sets that include criteria for continuing the catheter. Once the criteria are no longer applicable, nursing will be able to discontinue it.”
Although CMS has only recently turned the spotlight on HACs and never events, hospitalists have been heavily involved in the patient-safety arena for years. “It is not a new phenomenon that hospitalists work for healthcare delivery and healthcare system improvement,” Dr. Meddings says.
Hospital administration at Temple University Hospital recognized the HM group’s quality-improvement (QI) work, and has “specifically charged us with spreading the work we have done in patient safety to the entire house,” Dr. Frost says. “That speaks to the administration’s opinion of the power of the HM program to assist with institution-wide QI initiatives.”
Documentation Is Key
Beyond applying proven methods to avoid HACs, hospitalists can make a difference through documentation. If the hospitalist notes all conditions when the patient first presents to the hospital, additional comorbidity and complication payments should be made.
“The part that probably has the greatest impact on the day-to-day practice of a hospitalist is the increased importance of documentation throughout the hospital episode,” Dr. Meddings says. “If complications are occurring and they are not present in the chart, the coders may not recognize that it has occurred and will not know to include it in the bill. This can have an adverse impact on the hospital and its finances.”
Documentation issues can impact hospital payment in several ways:
- Hospitals might receive additional payment by default if certain HACs are described incorrectly or without sufficient detail (e.g., receiving overpayment because the physician did not indicate a UTI was in fact a catheter-associated UTI);
- As more attention is invested in documenting all conditions POA, hospitals might be coding more comorbidities overall than previously, which also will generate additional payment for hospitals as any POA condition is eligible for increased payment; and
- Hospitals might lose payment when admitting providers fail to adequately document the condition as POA (e.g., a pre-existing decubitus ulcer not detected until the second day of the hospital stay).
The descriptions to be used in coding are very detailed. UTIs, for example, have one code to document the POA assessment, another code to show that a UTI occurred, and a third code to indicate it was catheter-associated. Each code requires appropriate documentation in the chart (see Table 1, above).
The impact hospitalists have on care and payment is not the same across the HAC spectrum. For instance, documenting the presence of pressure ulcers might be easier than distinguishing colonization from infection in those admitted with in-dwelling urinary catheters. Others, such as DVT or vascular catheter-associated infections, are rarely POA unless they are part of the admitting diagnosis.
“This new focus on hospital-acquired conditions may work to the patient’s benefit,” Dr. Meddings says. “The inclusion of pressure ulcers has led to increased attention to skin exams on admission and preventive measures during hospitalizations. In the past, skin exams upon admission may have been given a lower priority, but that has changed.”
Dr. Meddings is concerned that the new rules could force the shifting of resources to areas where the hospital could lose money. If, when, and how many changes will actually take place is still up in the air. “Resource shifting is a concern whenever there is any sort of pay-for-performance attention directed toward one particular complication,” she says. “To balance this, many of the strategies hospitals used to prevent complications are not specific to just the diagnosis that is covered by the HAC.”
Dr. Meddings also hopes the new focus on preventable conditions will have a “halo effect” in the healthcare community. For instance, CMS mandating DVT prevention following orthopedic operations will, hopefully, result in a greater awareness of the problem in other susceptible patients.
POA Indicators
Since hospitalists often perform the initial patient history, physical, and other admission work, they are in the best position to find and document POA indicators (see Table 2, p. below). Proper notes on such things as UTIs present and the state of skin integrity are an important part of making sure the hospital is paid correctly for the care it provides.
Education on the specific definition of each potential HAC is required to help physicians avoid overtreatment of certain conditions, especially UTIs. For example, the Centers for Disease Control and Prevention (CDC) defines all UTIs as symptomatic. Therefore, the screening of all admitted patients, regardless of symptoms, is wasteful and unlikely to help the hospital’s bottom line.
“If you start screening everyone that comes through the door so you don’t miss any pre-existing UTIs, you are going to find a lot of asymptomatic colonization,” Dr. Wald says. “You are also going to spend a lot of money and time on studies and possibly treatments that may not yield many true infections. It is important that physicians know the definition of these HACs to help avoid needless interventions.”
Minimal Loss
Many hospital administrators and physicians were worried when the HAC program was first announced. Much of the stress and concern, however, seems to have dissipated. CMS estimated the HAC program would save Medicare $21 million in fiscal year 2009. Others, such as Peter McNair and colleagues writing in Health Affairs, suggest the actual impact is closer to $1.1 million.1 The CMS-projected impact of the HAC provision in fiscal-year 2009 was $21 million, out of more than $100 billion in payments.
“I think the HACs will not have a major impact because of the way payments are made,” says internist Robert Berenson, MD, a fellow at the Urban Institute in Washington, D.C., who has studied Medicare policy issues extensively, and for two years was in charge of Medicare payment policies at the Health Care Finance Administration, the precursor to CMS. “Patients who have HACs often have another comorbidity that would kick them into a higher payment category regardless of the presence of a hospital-acquired complication. In the end, it is probably more symbolic and unlikely to make a major dent in hospital income—at least at this point.”
Another limitation to CMS nonpayment for HACs is the issue of deciding which conditions are truly preventable. Dr. Berenson questions the ability of the current system to identify many additional complications for which this approach will be feasible.
“CMS has laid out its strategy, suggesting that we should be able to continue increasing the number of conditions for which providers would be paid differently based on quality,” he says. “Many observers question whether there will ever be measurement tools that are robust enough, and there will be a wide agreement on the preventability of enough conditions that this initiative will go very far.”
Although hospitalists might not face a direct financial risk, they still have their hospitals’ best interest—and their reputations—on the line. “Hospitalists care about preventing complications,” Dr. Wald says. “We are very engaged in working with our hospitals to improve care, maximize quality, and minimize cost.” TH
Kurt Ullman is a freelance medical writer based in Indiana.
Reference
- McNair PD, Luft HS, Bindman AB. Medicare’s policy not to pay for treating hospital-acquired conditions: the impact. Health Aff (Millwood). 2009;28(5):1485-1493.
TOP IMAGE SOURCE: KAREEM RIZKHALLA/ISTOCKPHOTO.COM
Hospitalist Neal Axon, MD, first became aware of an important change in his hospital’s policies last year while attending to an elderly patient the morning after admission to the community hospital where he works part time.
“This new form appeared in the chart requesting a urinalysis for my patient, who’d had a Foley catheter placed,” says Dr. Axon, an assistant professor of medicine at the Medical University of South Carolina in Charleston. “I didn’t know why, so I asked. I was told that it was now necessary to document that there was no UTI present on admission.” He asked the charge nurse, “So what do I do now that the catheter has been in place for 12 hours and has colonization without a true infection?”
The next thing he heard: silence.
The new form Dr. Axon encountered was an outgrowth of the requirements of the Deficit Reduction Act (DRA) of 2005, which ordered Medicare to withhold additional hospital payments for hospital-acquired complications (HAC) developed during a hospital stay. One result of the new rule is that much of a hospital’s response to these initiatives has been placed in the hands of the hospitalist. From accurate documentation of complications already present on admission (POA), to confirming that guidelines for treatment are being followed, to taking the lead on review of staff practices and education, hospitalists are in a position to have a wide-ranging impact on patient care and the financial health of their institutions.
Congress Pushes Reforms
In order for Medicare to not provide a reimbursement, an HAC has to be high-cost and/or high-volume, result in the assignment of the case to a higher payment when present as a secondary diagnosis, and “could reasonably be prevented through the application of evidence-based guidelines,” says Barry Straube, MD, chief medical officer and director of the Office of Clinical Standards and Quality at the Centers for Medicare and Medicaid Services (CMS). “CMS was to implement a process where we would not pay the hospitals additional money for these complications.”
The new rules mean Medicare pays hospitals on the basis of Medicare Severity Diagnostic-Related Groups (MS-DRG), which better reflect the complexity of a patient’s illness. The biggest change was a three-tiered payment schedule: a base level for the diagnosis, a second level adding money to reflect the presence of comorbidities and complications, and a third for major complications and comorbidities (see Table 1, p. 31).
“Instituting HACs means that hospitals would no longer receive the comorbidity and complication payments if the only reason a case qualified for higher payment was the HAC,” Dr. Straube explains. “We did carve out a POA exception for those conditions that were acquired outside of the hospital. HACs only impact additional payments; the hospitals are still paid for the diagnosis that resulted in the hospital admission.”
CMS also identifies three “never events” it won’t reimburse for (see “A Brief History of Never Events,” p. 35): performing the wrong procedure, performing a procedure on the wrong body part, and performing a procedure on the wrong patient. “Neither hospitals nor physicians that are involved in such egregious situations would be paid,” Dr. Straube says.
Preventability: Subject of Controversy
The big questions surrounding HACs: Could they reasonably be prevented through the application of evidence-based guidelines? How preventable are HACs? Who decides if a complication is preventable, and therefore payment for services is withheld?
They’re concerns that are widespread among physicians, hospital administrators, and regulators alike.
“The legislation required the conditions to be ‘reasonably preventable’ using established clinical guidelines,” Dr. Straube says. “We did not have to show 100% prevention. In an imperfect world, they might still take place occasionally, but with good medical care, almost all of these are preventable in this day and age.”
For CMS, the preventable conditions are an either/or situation: Either they existed prior to admission and are subject to payment, or they did not exist at admission and additional payment for the complication will not be made. “HACs do not currently consider a patient’s individual risk for complications,” says Jennifer Meddings, MD, MSc, clinical lecturer and health researcher in the Department of Internal Medicine at the University of Michigan Health System in Ann Arbor. “We know the best strategies to prevent complications in ideal patients, and these are reflected in the HACs. In real life, many of our patients just don’t fit into the guidelines for many reasons—and you have to individualize care.”
Dr. Meddings points to DVT as a prime example. For a certain number of inpatients, the guidelines can be followed to perfection. In other patients (e.g., those with kidney conditions), previous reactions to a medication or an individual’s predisposition to clotting might interfere with treatment. However, CMS doesn’t allow appeals of nonpayment decisions for HACs based on individual circumstances.
Some experts think the rigidness of the payment policy forces physicians to treat patients exactly to guidelines. Even then, payment could be declined if an HAC develops.
“One of the points of most discussion is how preventable some of these are, particularly when choosing those you are no longer going to pay for,” Dr. Meddings says. “Many of the complications currently under review have patients that are at higher risk than others. How much our prevention strategies can alleviate or reduce the risk varies widely among patients.”
Impact on HM Practice
Many of the preventable conditions outlined by CMS do not directly affect hospitalist payment. However, hospitalists often find themselves responsible for properly documenting admission and care.
“The rule changes regarding payment for HACs are only related to hospital payments, and to date, most physicians, including hospitalists, are not directly at financial risk,” says Heidi Wald, MD, MSPH, hospitalist and assistant professor of medicine in the divisions of Health Care Policy Research and General Internal Medicine at the University of Colorado Denver School of Medicine. “Although hospitalists have no financial skin in the game, there are plenty of reasons they would take an interest in addressing HACs in their hospital. In particular, they are often seen as the ‘go-to’ group for quality improvement in their hospitals.”
For example, some HM groups have been active in working with teams of physicians, nurses, and other healthcare providers to address local policies and procedures on prevention of catheter-associated urinary tract infections (UTIs) and DVT.
“This has certainly necessitated a team approach,” says Shaun Frost, MD, FHM, an SHM board member and regional medical director for Cogent Healthcare in St. Paul, Minn. “For many of the HACs that apply to our population of patients, the hospitalist alone cannot be expected to solely execute effective quality improvements. It takes a team effort in that regard, and one that includes many different disciplines.”
The Cogent-affiliated hospitalist group at Temple University Hospital in Philadelphia formed a task force to address issues with catheter-associated UTI. One initiative focused on educating all providers involved in the proper care of the catheters and similar interventions. A secondary focus of the project was an inventory of current practices and procedures.
“It was discovered that we did not have an automatic stop order for Foley catheters, so in some situations, they were likely being left in longer than needed while nursing [staff] tried to contact a physician,” Dr. Frost explains. “We created standardized order sets that include criteria for continuing the catheter. Once the criteria are no longer applicable, nursing will be able to discontinue it.”
Although CMS has only recently turned the spotlight on HACs and never events, hospitalists have been heavily involved in the patient-safety arena for years. “It is not a new phenomenon that hospitalists work for healthcare delivery and healthcare system improvement,” Dr. Meddings says.
Hospital administration at Temple University Hospital recognized the HM group’s quality-improvement (QI) work, and has “specifically charged us with spreading the work we have done in patient safety to the entire house,” Dr. Frost says. “That speaks to the administration’s opinion of the power of the HM program to assist with institution-wide QI initiatives.”
Documentation Is Key
Beyond applying proven methods to avoid HACs, hospitalists can make a difference through documentation. If the hospitalist notes all conditions when the patient first presents to the hospital, additional comorbidity and complication payments should be made.
“The part that probably has the greatest impact on the day-to-day practice of a hospitalist is the increased importance of documentation throughout the hospital episode,” Dr. Meddings says. “If complications are occurring and they are not present in the chart, the coders may not recognize that it has occurred and will not know to include it in the bill. This can have an adverse impact on the hospital and its finances.”
Documentation issues can impact hospital payment in several ways:
- Hospitals might receive additional payment by default if certain HACs are described incorrectly or without sufficient detail (e.g., receiving overpayment because the physician did not indicate a UTI was in fact a catheter-associated UTI);
- As more attention is invested in documenting all conditions POA, hospitals might be coding more comorbidities overall than previously, which also will generate additional payment for hospitals as any POA condition is eligible for increased payment; and
- Hospitals might lose payment when admitting providers fail to adequately document the condition as POA (e.g., a pre-existing decubitus ulcer not detected until the second day of the hospital stay).
The descriptions to be used in coding are very detailed. UTIs, for example, have one code to document the POA assessment, another code to show that a UTI occurred, and a third code to indicate it was catheter-associated. Each code requires appropriate documentation in the chart (see Table 1, above).
The impact hospitalists have on care and payment is not the same across the HAC spectrum. For instance, documenting the presence of pressure ulcers might be easier than distinguishing colonization from infection in those admitted with in-dwelling urinary catheters. Others, such as DVT or vascular catheter-associated infections, are rarely POA unless they are part of the admitting diagnosis.
“This new focus on hospital-acquired conditions may work to the patient’s benefit,” Dr. Meddings says. “The inclusion of pressure ulcers has led to increased attention to skin exams on admission and preventive measures during hospitalizations. In the past, skin exams upon admission may have been given a lower priority, but that has changed.”
Dr. Meddings is concerned that the new rules could force the shifting of resources to areas where the hospital could lose money. If, when, and how many changes will actually take place is still up in the air. “Resource shifting is a concern whenever there is any sort of pay-for-performance attention directed toward one particular complication,” she says. “To balance this, many of the strategies hospitals used to prevent complications are not specific to just the diagnosis that is covered by the HAC.”
Dr. Meddings also hopes the new focus on preventable conditions will have a “halo effect” in the healthcare community. For instance, CMS mandating DVT prevention following orthopedic operations will, hopefully, result in a greater awareness of the problem in other susceptible patients.
POA Indicators
Since hospitalists often perform the initial patient history, physical, and other admission work, they are in the best position to find and document POA indicators (see Table 2, p. below). Proper notes on such things as UTIs present and the state of skin integrity are an important part of making sure the hospital is paid correctly for the care it provides.
Education on the specific definition of each potential HAC is required to help physicians avoid overtreatment of certain conditions, especially UTIs. For example, the Centers for Disease Control and Prevention (CDC) defines all UTIs as symptomatic. Therefore, the screening of all admitted patients, regardless of symptoms, is wasteful and unlikely to help the hospital’s bottom line.
“If you start screening everyone that comes through the door so you don’t miss any pre-existing UTIs, you are going to find a lot of asymptomatic colonization,” Dr. Wald says. “You are also going to spend a lot of money and time on studies and possibly treatments that may not yield many true infections. It is important that physicians know the definition of these HACs to help avoid needless interventions.”
Minimal Loss
Many hospital administrators and physicians were worried when the HAC program was first announced. Much of the stress and concern, however, seems to have dissipated. CMS estimated the HAC program would save Medicare $21 million in fiscal year 2009. Others, such as Peter McNair and colleagues writing in Health Affairs, suggest the actual impact is closer to $1.1 million.1 The CMS-projected impact of the HAC provision in fiscal-year 2009 was $21 million, out of more than $100 billion in payments.
“I think the HACs will not have a major impact because of the way payments are made,” says internist Robert Berenson, MD, a fellow at the Urban Institute in Washington, D.C., who has studied Medicare policy issues extensively, and for two years was in charge of Medicare payment policies at the Health Care Finance Administration, the precursor to CMS. “Patients who have HACs often have another comorbidity that would kick them into a higher payment category regardless of the presence of a hospital-acquired complication. In the end, it is probably more symbolic and unlikely to make a major dent in hospital income—at least at this point.”
Another limitation to CMS nonpayment for HACs is the issue of deciding which conditions are truly preventable. Dr. Berenson questions the ability of the current system to identify many additional complications for which this approach will be feasible.
“CMS has laid out its strategy, suggesting that we should be able to continue increasing the number of conditions for which providers would be paid differently based on quality,” he says. “Many observers question whether there will ever be measurement tools that are robust enough, and there will be a wide agreement on the preventability of enough conditions that this initiative will go very far.”
Although hospitalists might not face a direct financial risk, they still have their hospitals’ best interest—and their reputations—on the line. “Hospitalists care about preventing complications,” Dr. Wald says. “We are very engaged in working with our hospitals to improve care, maximize quality, and minimize cost.” TH
Kurt Ullman is a freelance medical writer based in Indiana.
Reference
- McNair PD, Luft HS, Bindman AB. Medicare’s policy not to pay for treating hospital-acquired conditions: the impact. Health Aff (Millwood). 2009;28(5):1485-1493.
TOP IMAGE SOURCE: KAREEM RIZKHALLA/ISTOCKPHOTO.COM
HM10 PREVIEW: Center Stage
Timing is everything, and SHM’s 13th annual conference—April 8-11 in Washington, D.C.—is happening at just the right time.
“It’s pretty exciting that we’re coming to Washington this year with all the activity in healthcare reform,” says Larry Wellikson, MD, FHM, CEO of SHM.
SHM officials say HM10, at the Gaylord National Resort & Convention Center in National Harbor, Md., just minutes outside the nation’s capital, is on track to be the best-attended meeting in the group’s history, a tough task given that HM09 in Chicago sold out to the tune of more than 2,000 hospitalists. HM10 will introduce new features for attendees: added pre-courses, the induction of the first classes of Senior Fellows in Hospital Medicine and Master Fellows in Hospital Medicine, an expanded research and innovation platform, and a series of more than 90 educational sessions, including a new focus on limited-seating workshops.
“For the first time since we have been having an annual meeting, we will actually have what we call workshops that were actually peer-reviewed and selected as part of a competitive process,” says Amir Jaffer, MD, FHM, chair of SHM’s annual meeting committee and division chief of hospital medicine at the University of Miami Miller School of Medicine. “We had a competitive submission process for these workshops. We had over 90 submissions and we selected approximately 25 workshops to be presented.”
Paul Levy, president and CEO at Beth Israel Deaconess Medical Center in Boston, will deliver HM10’s keynote address at 9 a.m. Friday, April 9. Levy has a national reputation as a quality-improvement (QI) and patient-safety innovator, and has titled his presentation “The Hospitalist’s Role in the Hospital of the Future.”
“It's a classic discussion on how you do process improvement,” Levy explains in an in-depth Q&A on p. 6. “How do you standardize care? Once you standardize care ... how do you measure that? Hospitalists are in an excellent position to do that because they work on all of the different floors of their hospitals.”
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
Robert Wachter, MD, FHM, chief of the hospitalist division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco, former SHM president and author of the blog Wachter’s World (www.wachtersworld.com), will address the group—as has become custom—at noon Sunday. His focus will be “How Healthcare Reform Changes the Hospitalist Field … And Vice Versa.”
Attendees are encouraged to meet visiting professor Mark Zeidel, MD, chair of the Department of Medicine at Beth Israel. Dr. Zeidel will be a featured part of the Best of the Research, Innovations, and Clinical Vignettes (RIV) presentations. He will lead rounds during the poster sessions.
SHM officials say the speakers, educational opportunities, and new offerings continue to draw larger and larger crowds, despite the financial straits many HM groups face today.
“Even though there are travel budget cuts and education budget cuts, the one meeting that hospitalists continue to go to is SHM’s annual conference,” says Geri Barnes, SHM senior director of education and meetings. “That’s where they get their education, and, probably almost as importantly, is the networking aspect.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTO MATT FENSTERMACHER
Timing is everything, and SHM’s 13th annual conference—April 8-11 in Washington, D.C.—is happening at just the right time.
“It’s pretty exciting that we’re coming to Washington this year with all the activity in healthcare reform,” says Larry Wellikson, MD, FHM, CEO of SHM.
SHM officials say HM10, at the Gaylord National Resort & Convention Center in National Harbor, Md., just minutes outside the nation’s capital, is on track to be the best-attended meeting in the group’s history, a tough task given that HM09 in Chicago sold out to the tune of more than 2,000 hospitalists. HM10 will introduce new features for attendees: added pre-courses, the induction of the first classes of Senior Fellows in Hospital Medicine and Master Fellows in Hospital Medicine, an expanded research and innovation platform, and a series of more than 90 educational sessions, including a new focus on limited-seating workshops.
“For the first time since we have been having an annual meeting, we will actually have what we call workshops that were actually peer-reviewed and selected as part of a competitive process,” says Amir Jaffer, MD, FHM, chair of SHM’s annual meeting committee and division chief of hospital medicine at the University of Miami Miller School of Medicine. “We had a competitive submission process for these workshops. We had over 90 submissions and we selected approximately 25 workshops to be presented.”
Paul Levy, president and CEO at Beth Israel Deaconess Medical Center in Boston, will deliver HM10’s keynote address at 9 a.m. Friday, April 9. Levy has a national reputation as a quality-improvement (QI) and patient-safety innovator, and has titled his presentation “The Hospitalist’s Role in the Hospital of the Future.”
“It's a classic discussion on how you do process improvement,” Levy explains in an in-depth Q&A on p. 6. “How do you standardize care? Once you standardize care ... how do you measure that? Hospitalists are in an excellent position to do that because they work on all of the different floors of their hospitals.”
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
Robert Wachter, MD, FHM, chief of the hospitalist division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco, former SHM president and author of the blog Wachter’s World (www.wachtersworld.com), will address the group—as has become custom—at noon Sunday. His focus will be “How Healthcare Reform Changes the Hospitalist Field … And Vice Versa.”
Attendees are encouraged to meet visiting professor Mark Zeidel, MD, chair of the Department of Medicine at Beth Israel. Dr. Zeidel will be a featured part of the Best of the Research, Innovations, and Clinical Vignettes (RIV) presentations. He will lead rounds during the poster sessions.
SHM officials say the speakers, educational opportunities, and new offerings continue to draw larger and larger crowds, despite the financial straits many HM groups face today.
“Even though there are travel budget cuts and education budget cuts, the one meeting that hospitalists continue to go to is SHM’s annual conference,” says Geri Barnes, SHM senior director of education and meetings. “That’s where they get their education, and, probably almost as importantly, is the networking aspect.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTO MATT FENSTERMACHER
Timing is everything, and SHM’s 13th annual conference—April 8-11 in Washington, D.C.—is happening at just the right time.
“It’s pretty exciting that we’re coming to Washington this year with all the activity in healthcare reform,” says Larry Wellikson, MD, FHM, CEO of SHM.
SHM officials say HM10, at the Gaylord National Resort & Convention Center in National Harbor, Md., just minutes outside the nation’s capital, is on track to be the best-attended meeting in the group’s history, a tough task given that HM09 in Chicago sold out to the tune of more than 2,000 hospitalists. HM10 will introduce new features for attendees: added pre-courses, the induction of the first classes of Senior Fellows in Hospital Medicine and Master Fellows in Hospital Medicine, an expanded research and innovation platform, and a series of more than 90 educational sessions, including a new focus on limited-seating workshops.
“For the first time since we have been having an annual meeting, we will actually have what we call workshops that were actually peer-reviewed and selected as part of a competitive process,” says Amir Jaffer, MD, FHM, chair of SHM’s annual meeting committee and division chief of hospital medicine at the University of Miami Miller School of Medicine. “We had a competitive submission process for these workshops. We had over 90 submissions and we selected approximately 25 workshops to be presented.”
Paul Levy, president and CEO at Beth Israel Deaconess Medical Center in Boston, will deliver HM10’s keynote address at 9 a.m. Friday, April 9. Levy has a national reputation as a quality-improvement (QI) and patient-safety innovator, and has titled his presentation “The Hospitalist’s Role in the Hospital of the Future.”
“It's a classic discussion on how you do process improvement,” Levy explains in an in-depth Q&A on p. 6. “How do you standardize care? Once you standardize care ... how do you measure that? Hospitalists are in an excellent position to do that because they work on all of the different floors of their hospitals.”
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
Robert Wachter, MD, FHM, chief of the hospitalist division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco, former SHM president and author of the blog Wachter’s World (www.wachtersworld.com), will address the group—as has become custom—at noon Sunday. His focus will be “How Healthcare Reform Changes the Hospitalist Field … And Vice Versa.”
Attendees are encouraged to meet visiting professor Mark Zeidel, MD, chair of the Department of Medicine at Beth Israel. Dr. Zeidel will be a featured part of the Best of the Research, Innovations, and Clinical Vignettes (RIV) presentations. He will lead rounds during the poster sessions.
SHM officials say the speakers, educational opportunities, and new offerings continue to draw larger and larger crowds, despite the financial straits many HM groups face today.
“Even though there are travel budget cuts and education budget cuts, the one meeting that hospitalists continue to go to is SHM’s annual conference,” says Geri Barnes, SHM senior director of education and meetings. “That’s where they get their education, and, probably almost as importantly, is the networking aspect.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTO MATT FENSTERMACHER
Perioperative Medicine Summit 2010
Summit Director:
Amir K. Jaffer, MD
Contents
Abstract 1: Venous thromboembolism after total hip and knee replacement in older adults with single and co-occurring comorbidities
Alok Kapoor, MD, MSc; A. Labonte; M. Winter; J.B. Segal; R.A. Silliman; J.N. Katz; E. Losina; and D.R. Berlowitz
Abstract 2: Are there consequences of discontinuing angiotensin system inhibitors preoperatively in ambulatory and same-day admission patients?
Vasudha Goel, MBBS; David Rahmani, BS; Roy Braid, BS; Dmitry Rozin, BS; and Rebecca Twersky, MD, MPH
Abstract 3: Residents’ knowledge of ACC/AHA guidelines for preoperative cardiac evaluation is limited
BobbieJean Sweitzer, MD; Michael Vigoda, MD, MBA; Nikola Milokjic; Ben Boedeker, DVM, MD, PhD, MBA; Kip D. Robinson, MD, FACP; Michael A. Pilla, MD; Robert Gaiser, MD; Angela F. Edwards, MD; Ronald P. Olson, MD; Matthew D. Caldwell, MD; Shawn T. Beaman, MD; Jeffrey A. Green, MD; Jesse M. Ehrenfeld, MD, MPH; Marsha L. Wakefield, MD; Praveen Kalra, MD; David M. Feinstein, MD; Deborah C. Richman, MBChB, FFA(SA); Gail Van Norman; Gary E. Loyd, MD, MMM; Paul W. Kranner, MD; Stevin Dubin, MD; Sunil Eappen, MD; Sergio D. Bergese, MD; Suzanne Karan, MD; James R. Rowbottom, MD, FCCP; and Keith Candiotti, MD
Abstract 4: Descriptive perioperative BNP and CRP in vascular surgery patients
Thomas Barrett, MD, MCR, and Rebecca Duby, BS
Abstract 5: Selective serotonin reuptake inhibitors and risk of intraoperative bleeding
Adriana Oprea, MD, and Paula Zimbrean, MD
Abstract 6: Incidence and nature of postoperative complications in patients with obstructive sleep apnea undergoing noncardiac surgery
Roop Kaw, MD; Vinay Pasupuleti, MBBS, PhD; Esteban Walker, PhD; Anuradha Ramaswamy, MD; Thadeo Catacutan, MD; and Nancy Foldvary, DO
Abstract 7: HMG-CoA reductase inhibitor therapy and the risk of venous thromboembolism in joint replacement surgery
William Ho, MBBS; Brendan Flaim, MBBS, FRACP; and Andrea Chan, MBBS, FRACP
Abstract 8: Risk prediction models for cardiac morbidity and mortality in noncardiac surgery: A systematic review of the literature
Ramani Moonesinghe, MBBS, MRCP, FRCA; Kathy Rowan, PhD; Judith Hulf, CBE, FRCA; Michael G. Mythen, MD, FRCA; and Michael P.W. Grocott, MD, FRCA
Abstract 9: Economic aspects of preoperative testing
Gerhard Fritsch, MD; Maria Flamm, MD; Josef Seer, MD; and Andreas Soennichsen, MD
Abstract 10: Postoperative myocardial infarction and in-hospital mortality predictors in patients undergoing rlective noncardiac surgery
Anitha Rajamanickam, MD; Ali Usmani, MD; Jelica Janicijevic, MD; Preethi Patel, MD; Eric Hixson; Omeed Zardkoohi, MD; Michael Pecic; Changhong Yu; Michael Kattan, PhD; Sagar Kalahasti, MD; and Mina K. Chung, MD
Abstract 11: Incidence and predictors of postoperative heart failure in patients undergoing elective noncardiac surgery
Anitha Rajamanickam, MD; Ali Usmani, MD; Jelica Janicijevic, MD; Preethi Patel, MD; Eric Hixson; Omeed Zardkoohi, MD; Michael Pecic; Changhong Yu; Michael Kattan, PhD; Sagar Kalahasti, MD; and Mina K. Chung, MD
Abstract 12: Predictors of length of stay in patients undergoing total knee replacement surgery
Vishal Sehgal, MD; Pardeep Bansal, MD; Praveen Reddy, MD; Vishal Sharma, MD; Rajendra Palepu, MD; Linda Thomas, MD; and Jeremiah Eagan, MD
Abstract 13: Analysis of surgeon utilization of the Preoperative Assessment Communication Education (PACE) center in the pediatric population
Lisa Price Stevens, MD, and Ezinne Akamiro, BA, MD/MHA
Abstract 14: Use of the BATHE method to increase satisfaction amongst patients undergoing cardiac and major vascular operations
Samuel DeMaria, MD; Anthony P. DeMaria, MA; Menachem Weiner, MD; and George Silvay, MD
Abstract 15: Indication for surgery predicts long-term but not in-hospital mortality in patients undergoing lower extremity bypass vascular surgery
Brigid C. Flynn, MD; Michael Mazzeffi , MD; Carol Bodian, PhD; and Vivek Moitra, MD
Abstract 16: Research and outcomes on analgesia and nociception during surgery
Jinu Kim, MD; Tehila Adams, MD; Deepak Sreedharan, MD; Shanti Raju, MD; and Henry Bennett, PhD
Abstract 17: A snapshot survey of fluid prescribing
Helen Grote, MD; Luke Evans, MRCS; Abdel Omer, MD, PhD, FRCS; and Rob Lewis, MD, FRCA
Abstract 18: Predictors of difficult intubation with the video laryngoscope
Dario Galante, MD
Abstract 19: Use of technology to improve operational efficiency
Lucy Duffy, RN, MA, and Rita Lanaras, RN, BS, CNOR
Abstract 20: The ASA physical status score for the nonanesthesiologist
Adriana Oprea, MD, and David Silverman, MD
Abstract 21: Development of a shared multidisciplinary electronic preanesthetic record
Meghan Tadel, MD; R. Boyer, DO, MS; N. Smith; and P. Kallas, MD
Abstract 22: Development of a patient selection protocol prior to robotic radical prostatectomy (RRP) in the Preoperative Assessment Unit (PAU)
James Dyer, MD
Abstract 23: Protocol-driven preoperative testing in the Preoperative Assessment Unit (PAU): Which patients should receive a resting transthoracic echocardiogram (TTE) prior to elective noncardiac surgery?
James Dyer, MD
Abstract 24: High-risk preoperative assessment for elective orthopedic surgery patients
Terrence Adam, MD, PhD; Connie Parenti, MD; Terence Gioe, MD; Karen Ringsred, MD; and Joseph Wels, MD
Abstract 25: A novel use of web-based software to efficiently triage presurgical patients based on perioperative risk: A pilot
Alicia Kalamas, MD
Abstract 26: Value of a specialized clinic for day admission surgery for cardiac and major vascular operations
George Silvay, MD, PhD; Samuel DeMaria, MD; Marietta dePerio, NP, CCRN; Ellen Hughes, MA, RN; Samantha Silvay; Marina Krol, PhD; Brigid C. Flynn, MD; and David L. Reich, MD
Abstract 27: Preoperative evaluation for parathyroidectomy—rule out pheochromocytoma
Rubin Bahuva, MD; Sudhir Manda, MD; and Saurabh Kandpal, MD
Abstract 28: Should we stop the oral selective estrogen receptor modulator raloxifene prior to surgery?
Vesselin Dimov, MD; Tarek Hamieh, MD; and Ajay Kumar, MD
Abstract 29: Should mesalamine be stopped prior to noncardiac surgery to avoid bleeding complications?
Vesselin Dimov, MD; Tarek Hamieh, MD; and Ajay Kumar, MD
Abstract 30: Thyroidectomy: Perioperative management of acute thyroid storm
Stephen VanHaerents, MD, and Aashish A. Shah, MD
Abstract 31: Core competencies: Not just for the ACGME—but for successful and ethical perioperative management of a young respiratory cripple
Deborah Richman, MBChB, FFA(SA); Misako P. Sakamaki, MD; and Slawomir P. Oleszak, MD
Abstract 32: ‘If I have to be transfused I only want my wwn blood, or blood from family members’—what is best-practice advice to be given in the preoperative clinic?
Deborah Richman, MBChB, FFA(SA), and Joseph L. Conrad, MD
Abstract 33: Prolonged QTc and hypokalemia: A bad combination before surgery
Chadi Alraies, MD, and Abdul Hamid Alraiyes, MD
Abstract 34: Perioperative management of a parturient with neuromyelitis optica
Neeti Sadana, MD; Michael Orosco, MD; Michaela Farber, MD; and Scott Segal, MD
Abstract 35: ‘High’-pertension
Anuradha Ramaswamy, MD, and Franklin A. Michota, Jr., MD
Abstract 36: Perioperative care in neuromuscular scoliosis
Saurabh Basu Kandpal, MD, and Priya Baronia, MD
Summit Director:
Amir K. Jaffer, MD
Contents
Abstract 1: Venous thromboembolism after total hip and knee replacement in older adults with single and co-occurring comorbidities
Alok Kapoor, MD, MSc; A. Labonte; M. Winter; J.B. Segal; R.A. Silliman; J.N. Katz; E. Losina; and D.R. Berlowitz
Abstract 2: Are there consequences of discontinuing angiotensin system inhibitors preoperatively in ambulatory and same-day admission patients?
Vasudha Goel, MBBS; David Rahmani, BS; Roy Braid, BS; Dmitry Rozin, BS; and Rebecca Twersky, MD, MPH
Abstract 3: Residents’ knowledge of ACC/AHA guidelines for preoperative cardiac evaluation is limited
BobbieJean Sweitzer, MD; Michael Vigoda, MD, MBA; Nikola Milokjic; Ben Boedeker, DVM, MD, PhD, MBA; Kip D. Robinson, MD, FACP; Michael A. Pilla, MD; Robert Gaiser, MD; Angela F. Edwards, MD; Ronald P. Olson, MD; Matthew D. Caldwell, MD; Shawn T. Beaman, MD; Jeffrey A. Green, MD; Jesse M. Ehrenfeld, MD, MPH; Marsha L. Wakefield, MD; Praveen Kalra, MD; David M. Feinstein, MD; Deborah C. Richman, MBChB, FFA(SA); Gail Van Norman; Gary E. Loyd, MD, MMM; Paul W. Kranner, MD; Stevin Dubin, MD; Sunil Eappen, MD; Sergio D. Bergese, MD; Suzanne Karan, MD; James R. Rowbottom, MD, FCCP; and Keith Candiotti, MD
Abstract 4: Descriptive perioperative BNP and CRP in vascular surgery patients
Thomas Barrett, MD, MCR, and Rebecca Duby, BS
Abstract 5: Selective serotonin reuptake inhibitors and risk of intraoperative bleeding
Adriana Oprea, MD, and Paula Zimbrean, MD
Abstract 6: Incidence and nature of postoperative complications in patients with obstructive sleep apnea undergoing noncardiac surgery
Roop Kaw, MD; Vinay Pasupuleti, MBBS, PhD; Esteban Walker, PhD; Anuradha Ramaswamy, MD; Thadeo Catacutan, MD; and Nancy Foldvary, DO
Abstract 7: HMG-CoA reductase inhibitor therapy and the risk of venous thromboembolism in joint replacement surgery
William Ho, MBBS; Brendan Flaim, MBBS, FRACP; and Andrea Chan, MBBS, FRACP
Abstract 8: Risk prediction models for cardiac morbidity and mortality in noncardiac surgery: A systematic review of the literature
Ramani Moonesinghe, MBBS, MRCP, FRCA; Kathy Rowan, PhD; Judith Hulf, CBE, FRCA; Michael G. Mythen, MD, FRCA; and Michael P.W. Grocott, MD, FRCA
Abstract 9: Economic aspects of preoperative testing
Gerhard Fritsch, MD; Maria Flamm, MD; Josef Seer, MD; and Andreas Soennichsen, MD
Abstract 10: Postoperative myocardial infarction and in-hospital mortality predictors in patients undergoing rlective noncardiac surgery
Anitha Rajamanickam, MD; Ali Usmani, MD; Jelica Janicijevic, MD; Preethi Patel, MD; Eric Hixson; Omeed Zardkoohi, MD; Michael Pecic; Changhong Yu; Michael Kattan, PhD; Sagar Kalahasti, MD; and Mina K. Chung, MD
Abstract 11: Incidence and predictors of postoperative heart failure in patients undergoing elective noncardiac surgery
Anitha Rajamanickam, MD; Ali Usmani, MD; Jelica Janicijevic, MD; Preethi Patel, MD; Eric Hixson; Omeed Zardkoohi, MD; Michael Pecic; Changhong Yu; Michael Kattan, PhD; Sagar Kalahasti, MD; and Mina K. Chung, MD
Abstract 12: Predictors of length of stay in patients undergoing total knee replacement surgery
Vishal Sehgal, MD; Pardeep Bansal, MD; Praveen Reddy, MD; Vishal Sharma, MD; Rajendra Palepu, MD; Linda Thomas, MD; and Jeremiah Eagan, MD
Abstract 13: Analysis of surgeon utilization of the Preoperative Assessment Communication Education (PACE) center in the pediatric population
Lisa Price Stevens, MD, and Ezinne Akamiro, BA, MD/MHA
Abstract 14: Use of the BATHE method to increase satisfaction amongst patients undergoing cardiac and major vascular operations
Samuel DeMaria, MD; Anthony P. DeMaria, MA; Menachem Weiner, MD; and George Silvay, MD
Abstract 15: Indication for surgery predicts long-term but not in-hospital mortality in patients undergoing lower extremity bypass vascular surgery
Brigid C. Flynn, MD; Michael Mazzeffi , MD; Carol Bodian, PhD; and Vivek Moitra, MD
Abstract 16: Research and outcomes on analgesia and nociception during surgery
Jinu Kim, MD; Tehila Adams, MD; Deepak Sreedharan, MD; Shanti Raju, MD; and Henry Bennett, PhD
Abstract 17: A snapshot survey of fluid prescribing
Helen Grote, MD; Luke Evans, MRCS; Abdel Omer, MD, PhD, FRCS; and Rob Lewis, MD, FRCA
Abstract 18: Predictors of difficult intubation with the video laryngoscope
Dario Galante, MD
Abstract 19: Use of technology to improve operational efficiency
Lucy Duffy, RN, MA, and Rita Lanaras, RN, BS, CNOR
Abstract 20: The ASA physical status score for the nonanesthesiologist
Adriana Oprea, MD, and David Silverman, MD
Abstract 21: Development of a shared multidisciplinary electronic preanesthetic record
Meghan Tadel, MD; R. Boyer, DO, MS; N. Smith; and P. Kallas, MD
Abstract 22: Development of a patient selection protocol prior to robotic radical prostatectomy (RRP) in the Preoperative Assessment Unit (PAU)
James Dyer, MD
Abstract 23: Protocol-driven preoperative testing in the Preoperative Assessment Unit (PAU): Which patients should receive a resting transthoracic echocardiogram (TTE) prior to elective noncardiac surgery?
James Dyer, MD
Abstract 24: High-risk preoperative assessment for elective orthopedic surgery patients
Terrence Adam, MD, PhD; Connie Parenti, MD; Terence Gioe, MD; Karen Ringsred, MD; and Joseph Wels, MD
Abstract 25: A novel use of web-based software to efficiently triage presurgical patients based on perioperative risk: A pilot
Alicia Kalamas, MD
Abstract 26: Value of a specialized clinic for day admission surgery for cardiac and major vascular operations
George Silvay, MD, PhD; Samuel DeMaria, MD; Marietta dePerio, NP, CCRN; Ellen Hughes, MA, RN; Samantha Silvay; Marina Krol, PhD; Brigid C. Flynn, MD; and David L. Reich, MD
Abstract 27: Preoperative evaluation for parathyroidectomy—rule out pheochromocytoma
Rubin Bahuva, MD; Sudhir Manda, MD; and Saurabh Kandpal, MD
Abstract 28: Should we stop the oral selective estrogen receptor modulator raloxifene prior to surgery?
Vesselin Dimov, MD; Tarek Hamieh, MD; and Ajay Kumar, MD
Abstract 29: Should mesalamine be stopped prior to noncardiac surgery to avoid bleeding complications?
Vesselin Dimov, MD; Tarek Hamieh, MD; and Ajay Kumar, MD
Abstract 30: Thyroidectomy: Perioperative management of acute thyroid storm
Stephen VanHaerents, MD, and Aashish A. Shah, MD
Abstract 31: Core competencies: Not just for the ACGME—but for successful and ethical perioperative management of a young respiratory cripple
Deborah Richman, MBChB, FFA(SA); Misako P. Sakamaki, MD; and Slawomir P. Oleszak, MD
Abstract 32: ‘If I have to be transfused I only want my wwn blood, or blood from family members’—what is best-practice advice to be given in the preoperative clinic?
Deborah Richman, MBChB, FFA(SA), and Joseph L. Conrad, MD
Abstract 33: Prolonged QTc and hypokalemia: A bad combination before surgery
Chadi Alraies, MD, and Abdul Hamid Alraiyes, MD
Abstract 34: Perioperative management of a parturient with neuromyelitis optica
Neeti Sadana, MD; Michael Orosco, MD; Michaela Farber, MD; and Scott Segal, MD
Abstract 35: ‘High’-pertension
Anuradha Ramaswamy, MD, and Franklin A. Michota, Jr., MD
Abstract 36: Perioperative care in neuromuscular scoliosis
Saurabh Basu Kandpal, MD, and Priya Baronia, MD
Summit Director:
Amir K. Jaffer, MD
Contents
Abstract 1: Venous thromboembolism after total hip and knee replacement in older adults with single and co-occurring comorbidities
Alok Kapoor, MD, MSc; A. Labonte; M. Winter; J.B. Segal; R.A. Silliman; J.N. Katz; E. Losina; and D.R. Berlowitz
Abstract 2: Are there consequences of discontinuing angiotensin system inhibitors preoperatively in ambulatory and same-day admission patients?
Vasudha Goel, MBBS; David Rahmani, BS; Roy Braid, BS; Dmitry Rozin, BS; and Rebecca Twersky, MD, MPH
Abstract 3: Residents’ knowledge of ACC/AHA guidelines for preoperative cardiac evaluation is limited
BobbieJean Sweitzer, MD; Michael Vigoda, MD, MBA; Nikola Milokjic; Ben Boedeker, DVM, MD, PhD, MBA; Kip D. Robinson, MD, FACP; Michael A. Pilla, MD; Robert Gaiser, MD; Angela F. Edwards, MD; Ronald P. Olson, MD; Matthew D. Caldwell, MD; Shawn T. Beaman, MD; Jeffrey A. Green, MD; Jesse M. Ehrenfeld, MD, MPH; Marsha L. Wakefield, MD; Praveen Kalra, MD; David M. Feinstein, MD; Deborah C. Richman, MBChB, FFA(SA); Gail Van Norman; Gary E. Loyd, MD, MMM; Paul W. Kranner, MD; Stevin Dubin, MD; Sunil Eappen, MD; Sergio D. Bergese, MD; Suzanne Karan, MD; James R. Rowbottom, MD, FCCP; and Keith Candiotti, MD
Abstract 4: Descriptive perioperative BNP and CRP in vascular surgery patients
Thomas Barrett, MD, MCR, and Rebecca Duby, BS
Abstract 5: Selective serotonin reuptake inhibitors and risk of intraoperative bleeding
Adriana Oprea, MD, and Paula Zimbrean, MD
Abstract 6: Incidence and nature of postoperative complications in patients with obstructive sleep apnea undergoing noncardiac surgery
Roop Kaw, MD; Vinay Pasupuleti, MBBS, PhD; Esteban Walker, PhD; Anuradha Ramaswamy, MD; Thadeo Catacutan, MD; and Nancy Foldvary, DO
Abstract 7: HMG-CoA reductase inhibitor therapy and the risk of venous thromboembolism in joint replacement surgery
William Ho, MBBS; Brendan Flaim, MBBS, FRACP; and Andrea Chan, MBBS, FRACP
Abstract 8: Risk prediction models for cardiac morbidity and mortality in noncardiac surgery: A systematic review of the literature
Ramani Moonesinghe, MBBS, MRCP, FRCA; Kathy Rowan, PhD; Judith Hulf, CBE, FRCA; Michael G. Mythen, MD, FRCA; and Michael P.W. Grocott, MD, FRCA
Abstract 9: Economic aspects of preoperative testing
Gerhard Fritsch, MD; Maria Flamm, MD; Josef Seer, MD; and Andreas Soennichsen, MD
Abstract 10: Postoperative myocardial infarction and in-hospital mortality predictors in patients undergoing rlective noncardiac surgery
Anitha Rajamanickam, MD; Ali Usmani, MD; Jelica Janicijevic, MD; Preethi Patel, MD; Eric Hixson; Omeed Zardkoohi, MD; Michael Pecic; Changhong Yu; Michael Kattan, PhD; Sagar Kalahasti, MD; and Mina K. Chung, MD
Abstract 11: Incidence and predictors of postoperative heart failure in patients undergoing elective noncardiac surgery
Anitha Rajamanickam, MD; Ali Usmani, MD; Jelica Janicijevic, MD; Preethi Patel, MD; Eric Hixson; Omeed Zardkoohi, MD; Michael Pecic; Changhong Yu; Michael Kattan, PhD; Sagar Kalahasti, MD; and Mina K. Chung, MD
Abstract 12: Predictors of length of stay in patients undergoing total knee replacement surgery
Vishal Sehgal, MD; Pardeep Bansal, MD; Praveen Reddy, MD; Vishal Sharma, MD; Rajendra Palepu, MD; Linda Thomas, MD; and Jeremiah Eagan, MD
Abstract 13: Analysis of surgeon utilization of the Preoperative Assessment Communication Education (PACE) center in the pediatric population
Lisa Price Stevens, MD, and Ezinne Akamiro, BA, MD/MHA
Abstract 14: Use of the BATHE method to increase satisfaction amongst patients undergoing cardiac and major vascular operations
Samuel DeMaria, MD; Anthony P. DeMaria, MA; Menachem Weiner, MD; and George Silvay, MD
Abstract 15: Indication for surgery predicts long-term but not in-hospital mortality in patients undergoing lower extremity bypass vascular surgery
Brigid C. Flynn, MD; Michael Mazzeffi , MD; Carol Bodian, PhD; and Vivek Moitra, MD
Abstract 16: Research and outcomes on analgesia and nociception during surgery
Jinu Kim, MD; Tehila Adams, MD; Deepak Sreedharan, MD; Shanti Raju, MD; and Henry Bennett, PhD
Abstract 17: A snapshot survey of fluid prescribing
Helen Grote, MD; Luke Evans, MRCS; Abdel Omer, MD, PhD, FRCS; and Rob Lewis, MD, FRCA
Abstract 18: Predictors of difficult intubation with the video laryngoscope
Dario Galante, MD
Abstract 19: Use of technology to improve operational efficiency
Lucy Duffy, RN, MA, and Rita Lanaras, RN, BS, CNOR
Abstract 20: The ASA physical status score for the nonanesthesiologist
Adriana Oprea, MD, and David Silverman, MD
Abstract 21: Development of a shared multidisciplinary electronic preanesthetic record
Meghan Tadel, MD; R. Boyer, DO, MS; N. Smith; and P. Kallas, MD
Abstract 22: Development of a patient selection protocol prior to robotic radical prostatectomy (RRP) in the Preoperative Assessment Unit (PAU)
James Dyer, MD
Abstract 23: Protocol-driven preoperative testing in the Preoperative Assessment Unit (PAU): Which patients should receive a resting transthoracic echocardiogram (TTE) prior to elective noncardiac surgery?
James Dyer, MD
Abstract 24: High-risk preoperative assessment for elective orthopedic surgery patients
Terrence Adam, MD, PhD; Connie Parenti, MD; Terence Gioe, MD; Karen Ringsred, MD; and Joseph Wels, MD
Abstract 25: A novel use of web-based software to efficiently triage presurgical patients based on perioperative risk: A pilot
Alicia Kalamas, MD
Abstract 26: Value of a specialized clinic for day admission surgery for cardiac and major vascular operations
George Silvay, MD, PhD; Samuel DeMaria, MD; Marietta dePerio, NP, CCRN; Ellen Hughes, MA, RN; Samantha Silvay; Marina Krol, PhD; Brigid C. Flynn, MD; and David L. Reich, MD
Abstract 27: Preoperative evaluation for parathyroidectomy—rule out pheochromocytoma
Rubin Bahuva, MD; Sudhir Manda, MD; and Saurabh Kandpal, MD
Abstract 28: Should we stop the oral selective estrogen receptor modulator raloxifene prior to surgery?
Vesselin Dimov, MD; Tarek Hamieh, MD; and Ajay Kumar, MD
Abstract 29: Should mesalamine be stopped prior to noncardiac surgery to avoid bleeding complications?
Vesselin Dimov, MD; Tarek Hamieh, MD; and Ajay Kumar, MD
Abstract 30: Thyroidectomy: Perioperative management of acute thyroid storm
Stephen VanHaerents, MD, and Aashish A. Shah, MD
Abstract 31: Core competencies: Not just for the ACGME—but for successful and ethical perioperative management of a young respiratory cripple
Deborah Richman, MBChB, FFA(SA); Misako P. Sakamaki, MD; and Slawomir P. Oleszak, MD
Abstract 32: ‘If I have to be transfused I only want my wwn blood, or blood from family members’—what is best-practice advice to be given in the preoperative clinic?
Deborah Richman, MBChB, FFA(SA), and Joseph L. Conrad, MD
Abstract 33: Prolonged QTc and hypokalemia: A bad combination before surgery
Chadi Alraies, MD, and Abdul Hamid Alraiyes, MD
Abstract 34: Perioperative management of a parturient with neuromyelitis optica
Neeti Sadana, MD; Michael Orosco, MD; Michaela Farber, MD; and Scott Segal, MD
Abstract 35: ‘High’-pertension
Anuradha Ramaswamy, MD, and Franklin A. Michota, Jr., MD
Abstract 36: Perioperative care in neuromuscular scoliosis
Saurabh Basu Kandpal, MD, and Priya Baronia, MD
Bone and Soft-Tissue Sarcomas
Supplement Co-Editors:
Steven A. Lietman, MD, and Risal Djohan, MD
Contents
Clinical presentation and imaging of bone and soft-tissue sarcomas
Hakan Ilaslan, MD; Jean Schils, MD; William Nageotte, PA-C; Steven A. Lietman, MD; and Murali Sundaram, MD
Bone sarcomas: Overview of management, with a focus on surgical treatment considerations
Steven A. Lietman, MD, and Michael J. Joyce, MD
Soft-tissue sarcomas: Overview of management, with a focus on surgical treatment considerations
Steven A. Lietman, MD
Considerations surrounding reconstruction after resection of musculoskeletal sarcomas
Andrea Moreira-Gonzalez, MD; Risal Djohan, MD; and Robert Lohman, MD
Use of chemotherapy for patients with bone and soft-tissue sarcomas
Robert Wesolowski, MD, and George Thomas Budd, MD
Use of radiation therapy for patients with soft-tissue and bone sarcomas
Lawrence J. Sheplan, MD, and Justin J. Juliano, MD
Supplement Co-Editors:
Steven A. Lietman, MD, and Risal Djohan, MD
Contents
Clinical presentation and imaging of bone and soft-tissue sarcomas
Hakan Ilaslan, MD; Jean Schils, MD; William Nageotte, PA-C; Steven A. Lietman, MD; and Murali Sundaram, MD
Bone sarcomas: Overview of management, with a focus on surgical treatment considerations
Steven A. Lietman, MD, and Michael J. Joyce, MD
Soft-tissue sarcomas: Overview of management, with a focus on surgical treatment considerations
Steven A. Lietman, MD
Considerations surrounding reconstruction after resection of musculoskeletal sarcomas
Andrea Moreira-Gonzalez, MD; Risal Djohan, MD; and Robert Lohman, MD
Use of chemotherapy for patients with bone and soft-tissue sarcomas
Robert Wesolowski, MD, and George Thomas Budd, MD
Use of radiation therapy for patients with soft-tissue and bone sarcomas
Lawrence J. Sheplan, MD, and Justin J. Juliano, MD
Supplement Co-Editors:
Steven A. Lietman, MD, and Risal Djohan, MD
Contents
Clinical presentation and imaging of bone and soft-tissue sarcomas
Hakan Ilaslan, MD; Jean Schils, MD; William Nageotte, PA-C; Steven A. Lietman, MD; and Murali Sundaram, MD
Bone sarcomas: Overview of management, with a focus on surgical treatment considerations
Steven A. Lietman, MD, and Michael J. Joyce, MD
Soft-tissue sarcomas: Overview of management, with a focus on surgical treatment considerations
Steven A. Lietman, MD
Considerations surrounding reconstruction after resection of musculoskeletal sarcomas
Andrea Moreira-Gonzalez, MD; Risal Djohan, MD; and Robert Lohman, MD
Use of chemotherapy for patients with bone and soft-tissue sarcomas
Robert Wesolowski, MD, and George Thomas Budd, MD
Use of radiation therapy for patients with soft-tissue and bone sarcomas
Lawrence J. Sheplan, MD, and Justin J. Juliano, MD
Clinical presentation and imaging of bone and soft-tissue sarcomas
Sarcomas are rare neoplasms arising from connective tissue elements of the body. Approximately 80% arise in soft tissue, while the remainder originate in bone.1 Soft-tissue sarcomas are tumors of the mesenchymal system, and about half develop in the extremities. Bone sarcomas are characterized by their location in bone and sometimes produce osteoid, or immature bone.
The most common types of soft-tissue sarcomas are malignant fibrous histiocytoma (although this term has lost favor with some pathologists) and liposarcoma.
The most common types of bone sarcomas are osteosarcoma (a sarcoma that makes osteoid or bone), Ewing sarcoma (composed of small round blue cells with a characteristic chromosomal translocation), and chondrosarcoma (a sarcoma that makes chondroid tissue, or cartilage).
EPIDEMIOLOGY AND PRIMARY BODY SITES
Approximately 10,600 new cases of soft-tissue sarcoma and 2,570 new cases of bone sarcoma were estimated to have been diagnosed in the United States in 2009.2,3 For perspective, the annual incidence of soft-tissue sarcoma is approximately 5.5% that of breast cancer and approximately 5% that of lung cancer.3
Most sarcomas develop in the lower extremities, although the age groups at greatest risk vary among sarcoma types.4
Soft-tissue sarcomas develop most commonly in the thigh and occur primarily in adults.2
Osteosarcomas are the most common bone sarcoma and develop most frequently in 10- to 20-year-olds; their most common location is the distal femur.5–8 Metastatic osteosarcoma is found in approximately 20% of patients at the time of osteosarcoma diagnosis. Osteosarcomas mainly spread hematogenously, and the lungs are the most common initial site of metastases, being affected in up to 90% of patients with metastatic disease.9
Ewing sarcomas develop most often in the long bones of the extremities or bones of the pelvis. The large majority of cases develop in patients aged 10 to 15 years. 5–8
Chondrosarcomas represent approximately 20% of all bone sarcomas and primarily affect older adults, with a peak incidence in the sixth decade of life.10
OVERVIEW OF PRESENTATION AND EVALUATION
Presentation is highly variable
The clinical presentation of patients with bone or soft-tissue sarcoma is highly variable. Patients often present with a mass, typically one that is increasing in size. In general, bone sarcomas are painful and soft-tissue sarcomas are not, but there are exceptions to this general rule. Constitutional symptoms are rare in patients with bone or soft-tissue sarcomas, but symptoms such as fever, malaise, and weight loss can be seen, especially in patients with Ewing sarcoma.11
Delayed presentation and diagnosis are common
Particularly when a sarcoma is painless, patients sometimes do not seek medical attention until a suspicious mass becomes quite large. Certain tumors, such as synovial sarcoma, a high-grade soft-tissue sarcoma often seen in young adults, may present as a slowly growing or stable-appearing mass over several years. In one study of 33 children with synovial sarcoma, the mean duration of symptoms was 98 weeks (range, 2–364), the mean patient delay before a doctor was seen was 43 weeks (0–156), the mean doctor delay before a correct diagnosis was made was 50 weeks (0–362), and the mean number of doctors seen before referral was 3 (1–6).12 For nearly half the patients in this study (15), the diagnosis was obtained only after unplanned excision, meaning that the surgeon did not expect a malignancy at the time of biopsy. Because delayed presentation is not uncommon in cases of bone or soft-tissue sarcoma, every patient with a mass with indeterminate imaging findings should be referred to or reviewed by an orthopedic or musculoskeletal oncologist.
Biopsy is gold standard for diagnosis
A comprehensive medical history and physical examination are essential at the initial presentation of patients with masses and/or pain suggestive of bone or soft-tissue sarcoma. Sarcoma simulators such as hematoma, metastatic disease, or infection can sometimes be ruled out by careful clinical examination, laboratory work-up, and appropriate imaging, but the gold standard for diagnosis is a biopsy. Moreover, an index of suspicion is required to rule out primary malignancy in any soft-tissue or bone lesion, and this index of suspicion will allow for referral or appropriate selection of the site for biopsy.
Biopsy considerations, as well as further detail on clinical presentation, are provided in the second and third articles in this supplement, which focus, respectively, on bone sarcoma and soft-tissue sarcoma. The remainder of this article reviews the use of imaging for the evaluation of suspected sarcomas, as imaging findings typically prompt or guide biopsy of a suspicious mass. Choosing the right imaging modality is critical to the diagnosis and management of patients with suspected sarcoma.
CONVENTIONAL IMAGING MODALITIES
Despite their utility for evaluating osseous lesions, radiographs have limited to no value in the evaluation of soft-tissue sarcomas but can demonstrate matrix mineralization and erosion or destruction of adjacent bone.
Angiography. In the past, angiography was frequently used to assess the vascularity of sarcomas preoperatively. Diagnostic angiography has been replaced by conventional MRI and magnetic resonance angiography, but some vascular sarcomas may require presurgical embolization to prevent excessive bleeding during surgery.
Radionuclide bone scans have long been a reliable tool for detecting multifocal or disseminated osseous lesions and remain the mainstay for evaluation of osseous metastases. They also are helpful in identifying skip lesions of osteosarcoma (ie, smaller discrete foci of osteosarcoma occurring in the same bone or on the opposing side of a joint).14 Advantages of this modality include whole-body scanning and low radiation at relatively low cost. Radionuclide bone scans demonstrate areas of bony repair and thus could be negative in purely lytic/destructive processes such as renal cell carcinoma metastases and multiple myeloma.
Chest radiographs are typically obtained in the initial stages of patient evaluation and are helpful in demonstrating large nodules or masses resulting from metastatic disease. In a patient with known sarcoma, a negative or equivocal chest radiograph should be followed by chest CT to definitively assess for metastasis.
CROSS-SECTIONAL IMAGING WITH MRI AND CT
MRI preferred for evaluation of most masses
MRI is the examination of choice in the evaluation of soft-tissue masses in light of its superior contrast resolution and ability to demonstrate subtle changes in soft tissues.
Predicting the histology of most soft-tissue masses is difficult, with the exception of some benign vascular lesions (eg, hemangioma), ganglia, neurogenic lesions, and well-differentiated lipomatous lesions. Aggressive features of a soft-tissue neoplasm include size greater than 5 cm,15 deep location, and absence of central enhancement, which is suggestive of necrosis (Figure 1). Yet one third of soft-tissue sarcomas are either superficial or smaller than 5 cm, which highlights the relative nonspecificity of these features.15
MRI is also the preferred modality in the evaluation of the majority of bone sarcomas, given its ability to accurately define the extent of marrow changes and soft-tissue involvement. MRI should be performed prior to a biopsy to prevent misinterpretation of biopsy-related signal changes in the surrounding tissues, which may negate the value of MRI in sarcoma staging.
Several distinct roles for CT
Chest CT should be obtained in all cases of known malignant neoplasms to evaluate for pulmonary nodules, masses, and lymphadenopathy. Despite the recent advances in MRI, CT remains the imaging modality of choice to evaluate the retroperitoneum, abdomen, and pelvis for masses, lymphadenopathy, or other signs of metastatic disease.
Post-treatment monitoring for recurrence
ULTRASONOGRAPHY
Ultrasonography has a limited role in the initial diagnosis and follow-up of musculoskeletal tumors. Its main advantages are a lack of ionizing radiation and dynamic imaging capabilities. Doppler ultrasonography allows direct visualization of tumor vascularity, which may be important for diagnosis and presurgical planning. Unfortunately, bone lesions cannot be evaluated with ultrasonography, owing to the inability of sound waves to penetrate the bony cortex. Poor sound wave penetration may prevent visualization of deep-seated lesions, such as retroperitoneal sarcomas.
Ultrasonography is best used for differentiating solid masses from cystic structures and can provide image guidance in solid tumor biopsy and cyst aspiration. It also may play a role in detecting suspected tumor recurrence in patients in whom artifact from implanted hardware precludes cross-sectional imaging, and it can be reliably used for following up unequivocal soft-tissue masses such as ganglia near joints.
POSITRON EMISSION TOMOGRAPHY
IMAGING-GUIDED INTERVENTIONS
Percutaneous imaging-guided procedures have increasingly replaced open surgical biopsies for bone and soft-tissue tumors. CT guidance is commonly used for percutaneous biopsy, whereas ultrasonographic guidance is sometimes used for superficial soft-tissue lesions. Although the shortest and most direct approach is desirable, this may not be possible in all cases due to the presence of nearby vital structures or the risk of contamination. Seeding of malignant cells along the biopsy tract is a well-known possible complication of image-guided biopsies, and en bloc resection of the needle tract is typically performed at the definitive surgery.
Knowledge of compartmental anatomy is paramount in planning the approach for these biopsies, and consultation with the referring orthopedic surgeon is recommended for optimal management. Expert histopathological interpretation of bone and soft-tissue specimens is essential for the efficacy and high success rates of percutaneous imaging-guided biopsies. Such expertise is integral to the broader interdisciplinary collaboration that is needed to arrive at the most plausible diagnosis, especially in the setting of uncommon or atypical neoplasms.
Currently, MRI-guided interventions are in the initial stage of evolution and could provide valuable guidance for subtle marrow or soft-tissue lesions visible on MRI but not well seen on CT.22 In the future, MRI could play an increasingly important role in imaging-guided procedures because of its lack of ionizing radiation and its ability to demonstrate subtle soft-tissue and bone marrow changes. Imaging-guided therapeutics are growing in their applications in musculoskeletal oncology. CT-guided radiofrequency ablation and cryoablation have been used in the treatment of a variety of tumors23 as well as in the palliation of metastatic bone pain.24
SUMMARY AND CONCLUSION
Bone and soft-tissue sarcomas are rare neoplasms with variable clinical presentations. A high index of suspicion is required for any unexplained mass with indeterminate imaging findings. Recent advances in imaging technology, including cross-sectional MRI and CT, have significantly refined the diagnosis and management of bone and soft-tissue sarcomas. When faced with a possible sarcoma, the clinician’s selection of imaging modalities has a direct impact on diagnosis, staging, and patient management.
- American Cancer Society. Cancer facts & figures 2009. Atlanta, GA: American Cancer Society; 2009.
- Weiss SW, Goldblum JR, Enzinger FM. Enzinger and Weiss’ Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225–249.
- Simon MA, Springfield DS, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Dahlin DC, Unni KK. Bone Tumors: General Aspects and Data on 8,542 Cases. 4th ed. Springfield, IL: Thomas; 1986.
- Unni KK. Bone Tumors. New York, NY: Churchill Livingstone; 1988.
- Unni KK. Atlas of Bone Pathology. New York, NY: Chapman & Hall; 1996:1 computer optical disc.
- Unni KK, Dahlin DC. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
- Kaste SC, Pratt CB, Cain AM, Jones-Wallace DJ, Rao BN. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer 1999; 86:1602–1608.
- Healey JH, Lane JM. Chondrosarcoma. Clin Orthop Relat Res 1986; 204:119–129.
- Kissane JM, Askin FB, Foulkes M, Stratton LB, Shirley SF. Ewing’s sarcoma of bone: clinicopathologic aspects of 303 cases from the Intergroup Ewing’s Sarcoma Study. Hum Pathol 1983; 14:773–779.
- Chotel F, Unnithan A, Chandrasekar CR, et al. Variability in the presentation of synovial sarcoma in children: a plea for greater awareness. J Bone Joint Surg Br 2008; 90:1090–1096.
- Miller TT. Bone tumors and tumorlike conditions: analysis with conventional radiography. Radiology 2008; 246:662–674.
- Richardson ML, Gillespy T. Magnetic resonance imaging. In: Kricun ME, ed. Imaging of Bone Tumors. Philadelphia, PA: WB Saunders; 1993:365.
- Fisher C. Soft tissue sarcomas: diagnosis, classification and prognostic factors. Br J Plast Surg 1996; 49:27–33.
- White LM, Wunder JS, Bell RS, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys 2005; 61:1439–1445.
- White LM, Buckwalter KA. Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6:5–17.
- Blodgett TM, Casagranda B, Townsend DW, Meltzer CC. Issues, controversies, and clinical utility of combined PET/CT imaging: what is the interpreting physician facing? AJR Am J Roentgenol 2005; 184(suppl 5):S138–S145.
- Shin DS, Shon OJ, Han DS, Choi JH, Chun KA, Cho IH. The clinical efficacy of 18F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med 2008; 22:603–609.
- Benz MR, Czernin J, Allen-Auerbach MS, et al. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res 2009; 15:2856–2863.
- Aoki J, Watanabe H, Shinozaki T, et al. FDG-PET for preoperative differential diagnosis between benign and malignant soft tissue masses. Skeletal Radiol 2003; 32:133–138.
- Blanco Sequeiros R, Klemola R, Ojala R, et al. MRI-guided trephine biopsy and fine-needle aspiration in the diagnosis of bone lesions in low-field (0.23 T) MRI system using optical instrument tracking. Eur Radiol 2002; 12:830–835.
- Rosenthal DI. Radiofrequency treatment. Orthop Clin North Am 2006; 37:475–484.
- Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol 2007; 10:120–131.
Sarcomas are rare neoplasms arising from connective tissue elements of the body. Approximately 80% arise in soft tissue, while the remainder originate in bone.1 Soft-tissue sarcomas are tumors of the mesenchymal system, and about half develop in the extremities. Bone sarcomas are characterized by their location in bone and sometimes produce osteoid, or immature bone.
The most common types of soft-tissue sarcomas are malignant fibrous histiocytoma (although this term has lost favor with some pathologists) and liposarcoma.
The most common types of bone sarcomas are osteosarcoma (a sarcoma that makes osteoid or bone), Ewing sarcoma (composed of small round blue cells with a characteristic chromosomal translocation), and chondrosarcoma (a sarcoma that makes chondroid tissue, or cartilage).
EPIDEMIOLOGY AND PRIMARY BODY SITES
Approximately 10,600 new cases of soft-tissue sarcoma and 2,570 new cases of bone sarcoma were estimated to have been diagnosed in the United States in 2009.2,3 For perspective, the annual incidence of soft-tissue sarcoma is approximately 5.5% that of breast cancer and approximately 5% that of lung cancer.3
Most sarcomas develop in the lower extremities, although the age groups at greatest risk vary among sarcoma types.4
Soft-tissue sarcomas develop most commonly in the thigh and occur primarily in adults.2
Osteosarcomas are the most common bone sarcoma and develop most frequently in 10- to 20-year-olds; their most common location is the distal femur.5–8 Metastatic osteosarcoma is found in approximately 20% of patients at the time of osteosarcoma diagnosis. Osteosarcomas mainly spread hematogenously, and the lungs are the most common initial site of metastases, being affected in up to 90% of patients with metastatic disease.9
Ewing sarcomas develop most often in the long bones of the extremities or bones of the pelvis. The large majority of cases develop in patients aged 10 to 15 years. 5–8
Chondrosarcomas represent approximately 20% of all bone sarcomas and primarily affect older adults, with a peak incidence in the sixth decade of life.10
OVERVIEW OF PRESENTATION AND EVALUATION
Presentation is highly variable
The clinical presentation of patients with bone or soft-tissue sarcoma is highly variable. Patients often present with a mass, typically one that is increasing in size. In general, bone sarcomas are painful and soft-tissue sarcomas are not, but there are exceptions to this general rule. Constitutional symptoms are rare in patients with bone or soft-tissue sarcomas, but symptoms such as fever, malaise, and weight loss can be seen, especially in patients with Ewing sarcoma.11
Delayed presentation and diagnosis are common
Particularly when a sarcoma is painless, patients sometimes do not seek medical attention until a suspicious mass becomes quite large. Certain tumors, such as synovial sarcoma, a high-grade soft-tissue sarcoma often seen in young adults, may present as a slowly growing or stable-appearing mass over several years. In one study of 33 children with synovial sarcoma, the mean duration of symptoms was 98 weeks (range, 2–364), the mean patient delay before a doctor was seen was 43 weeks (0–156), the mean doctor delay before a correct diagnosis was made was 50 weeks (0–362), and the mean number of doctors seen before referral was 3 (1–6).12 For nearly half the patients in this study (15), the diagnosis was obtained only after unplanned excision, meaning that the surgeon did not expect a malignancy at the time of biopsy. Because delayed presentation is not uncommon in cases of bone or soft-tissue sarcoma, every patient with a mass with indeterminate imaging findings should be referred to or reviewed by an orthopedic or musculoskeletal oncologist.
Biopsy is gold standard for diagnosis
A comprehensive medical history and physical examination are essential at the initial presentation of patients with masses and/or pain suggestive of bone or soft-tissue sarcoma. Sarcoma simulators such as hematoma, metastatic disease, or infection can sometimes be ruled out by careful clinical examination, laboratory work-up, and appropriate imaging, but the gold standard for diagnosis is a biopsy. Moreover, an index of suspicion is required to rule out primary malignancy in any soft-tissue or bone lesion, and this index of suspicion will allow for referral or appropriate selection of the site for biopsy.
Biopsy considerations, as well as further detail on clinical presentation, are provided in the second and third articles in this supplement, which focus, respectively, on bone sarcoma and soft-tissue sarcoma. The remainder of this article reviews the use of imaging for the evaluation of suspected sarcomas, as imaging findings typically prompt or guide biopsy of a suspicious mass. Choosing the right imaging modality is critical to the diagnosis and management of patients with suspected sarcoma.
CONVENTIONAL IMAGING MODALITIES
Despite their utility for evaluating osseous lesions, radiographs have limited to no value in the evaluation of soft-tissue sarcomas but can demonstrate matrix mineralization and erosion or destruction of adjacent bone.
Angiography. In the past, angiography was frequently used to assess the vascularity of sarcomas preoperatively. Diagnostic angiography has been replaced by conventional MRI and magnetic resonance angiography, but some vascular sarcomas may require presurgical embolization to prevent excessive bleeding during surgery.
Radionuclide bone scans have long been a reliable tool for detecting multifocal or disseminated osseous lesions and remain the mainstay for evaluation of osseous metastases. They also are helpful in identifying skip lesions of osteosarcoma (ie, smaller discrete foci of osteosarcoma occurring in the same bone or on the opposing side of a joint).14 Advantages of this modality include whole-body scanning and low radiation at relatively low cost. Radionuclide bone scans demonstrate areas of bony repair and thus could be negative in purely lytic/destructive processes such as renal cell carcinoma metastases and multiple myeloma.
Chest radiographs are typically obtained in the initial stages of patient evaluation and are helpful in demonstrating large nodules or masses resulting from metastatic disease. In a patient with known sarcoma, a negative or equivocal chest radiograph should be followed by chest CT to definitively assess for metastasis.
CROSS-SECTIONAL IMAGING WITH MRI AND CT
MRI preferred for evaluation of most masses
MRI is the examination of choice in the evaluation of soft-tissue masses in light of its superior contrast resolution and ability to demonstrate subtle changes in soft tissues.
Predicting the histology of most soft-tissue masses is difficult, with the exception of some benign vascular lesions (eg, hemangioma), ganglia, neurogenic lesions, and well-differentiated lipomatous lesions. Aggressive features of a soft-tissue neoplasm include size greater than 5 cm,15 deep location, and absence of central enhancement, which is suggestive of necrosis (Figure 1). Yet one third of soft-tissue sarcomas are either superficial or smaller than 5 cm, which highlights the relative nonspecificity of these features.15
MRI is also the preferred modality in the evaluation of the majority of bone sarcomas, given its ability to accurately define the extent of marrow changes and soft-tissue involvement. MRI should be performed prior to a biopsy to prevent misinterpretation of biopsy-related signal changes in the surrounding tissues, which may negate the value of MRI in sarcoma staging.
Several distinct roles for CT
Chest CT should be obtained in all cases of known malignant neoplasms to evaluate for pulmonary nodules, masses, and lymphadenopathy. Despite the recent advances in MRI, CT remains the imaging modality of choice to evaluate the retroperitoneum, abdomen, and pelvis for masses, lymphadenopathy, or other signs of metastatic disease.
Post-treatment monitoring for recurrence
ULTRASONOGRAPHY
Ultrasonography has a limited role in the initial diagnosis and follow-up of musculoskeletal tumors. Its main advantages are a lack of ionizing radiation and dynamic imaging capabilities. Doppler ultrasonography allows direct visualization of tumor vascularity, which may be important for diagnosis and presurgical planning. Unfortunately, bone lesions cannot be evaluated with ultrasonography, owing to the inability of sound waves to penetrate the bony cortex. Poor sound wave penetration may prevent visualization of deep-seated lesions, such as retroperitoneal sarcomas.
Ultrasonography is best used for differentiating solid masses from cystic structures and can provide image guidance in solid tumor biopsy and cyst aspiration. It also may play a role in detecting suspected tumor recurrence in patients in whom artifact from implanted hardware precludes cross-sectional imaging, and it can be reliably used for following up unequivocal soft-tissue masses such as ganglia near joints.
POSITRON EMISSION TOMOGRAPHY
IMAGING-GUIDED INTERVENTIONS
Percutaneous imaging-guided procedures have increasingly replaced open surgical biopsies for bone and soft-tissue tumors. CT guidance is commonly used for percutaneous biopsy, whereas ultrasonographic guidance is sometimes used for superficial soft-tissue lesions. Although the shortest and most direct approach is desirable, this may not be possible in all cases due to the presence of nearby vital structures or the risk of contamination. Seeding of malignant cells along the biopsy tract is a well-known possible complication of image-guided biopsies, and en bloc resection of the needle tract is typically performed at the definitive surgery.
Knowledge of compartmental anatomy is paramount in planning the approach for these biopsies, and consultation with the referring orthopedic surgeon is recommended for optimal management. Expert histopathological interpretation of bone and soft-tissue specimens is essential for the efficacy and high success rates of percutaneous imaging-guided biopsies. Such expertise is integral to the broader interdisciplinary collaboration that is needed to arrive at the most plausible diagnosis, especially in the setting of uncommon or atypical neoplasms.
Currently, MRI-guided interventions are in the initial stage of evolution and could provide valuable guidance for subtle marrow or soft-tissue lesions visible on MRI but not well seen on CT.22 In the future, MRI could play an increasingly important role in imaging-guided procedures because of its lack of ionizing radiation and its ability to demonstrate subtle soft-tissue and bone marrow changes. Imaging-guided therapeutics are growing in their applications in musculoskeletal oncology. CT-guided radiofrequency ablation and cryoablation have been used in the treatment of a variety of tumors23 as well as in the palliation of metastatic bone pain.24
SUMMARY AND CONCLUSION
Bone and soft-tissue sarcomas are rare neoplasms with variable clinical presentations. A high index of suspicion is required for any unexplained mass with indeterminate imaging findings. Recent advances in imaging technology, including cross-sectional MRI and CT, have significantly refined the diagnosis and management of bone and soft-tissue sarcomas. When faced with a possible sarcoma, the clinician’s selection of imaging modalities has a direct impact on diagnosis, staging, and patient management.
Sarcomas are rare neoplasms arising from connective tissue elements of the body. Approximately 80% arise in soft tissue, while the remainder originate in bone.1 Soft-tissue sarcomas are tumors of the mesenchymal system, and about half develop in the extremities. Bone sarcomas are characterized by their location in bone and sometimes produce osteoid, or immature bone.
The most common types of soft-tissue sarcomas are malignant fibrous histiocytoma (although this term has lost favor with some pathologists) and liposarcoma.
The most common types of bone sarcomas are osteosarcoma (a sarcoma that makes osteoid or bone), Ewing sarcoma (composed of small round blue cells with a characteristic chromosomal translocation), and chondrosarcoma (a sarcoma that makes chondroid tissue, or cartilage).
EPIDEMIOLOGY AND PRIMARY BODY SITES
Approximately 10,600 new cases of soft-tissue sarcoma and 2,570 new cases of bone sarcoma were estimated to have been diagnosed in the United States in 2009.2,3 For perspective, the annual incidence of soft-tissue sarcoma is approximately 5.5% that of breast cancer and approximately 5% that of lung cancer.3
Most sarcomas develop in the lower extremities, although the age groups at greatest risk vary among sarcoma types.4
Soft-tissue sarcomas develop most commonly in the thigh and occur primarily in adults.2
Osteosarcomas are the most common bone sarcoma and develop most frequently in 10- to 20-year-olds; their most common location is the distal femur.5–8 Metastatic osteosarcoma is found in approximately 20% of patients at the time of osteosarcoma diagnosis. Osteosarcomas mainly spread hematogenously, and the lungs are the most common initial site of metastases, being affected in up to 90% of patients with metastatic disease.9
Ewing sarcomas develop most often in the long bones of the extremities or bones of the pelvis. The large majority of cases develop in patients aged 10 to 15 years. 5–8
Chondrosarcomas represent approximately 20% of all bone sarcomas and primarily affect older adults, with a peak incidence in the sixth decade of life.10
OVERVIEW OF PRESENTATION AND EVALUATION
Presentation is highly variable
The clinical presentation of patients with bone or soft-tissue sarcoma is highly variable. Patients often present with a mass, typically one that is increasing in size. In general, bone sarcomas are painful and soft-tissue sarcomas are not, but there are exceptions to this general rule. Constitutional symptoms are rare in patients with bone or soft-tissue sarcomas, but symptoms such as fever, malaise, and weight loss can be seen, especially in patients with Ewing sarcoma.11
Delayed presentation and diagnosis are common
Particularly when a sarcoma is painless, patients sometimes do not seek medical attention until a suspicious mass becomes quite large. Certain tumors, such as synovial sarcoma, a high-grade soft-tissue sarcoma often seen in young adults, may present as a slowly growing or stable-appearing mass over several years. In one study of 33 children with synovial sarcoma, the mean duration of symptoms was 98 weeks (range, 2–364), the mean patient delay before a doctor was seen was 43 weeks (0–156), the mean doctor delay before a correct diagnosis was made was 50 weeks (0–362), and the mean number of doctors seen before referral was 3 (1–6).12 For nearly half the patients in this study (15), the diagnosis was obtained only after unplanned excision, meaning that the surgeon did not expect a malignancy at the time of biopsy. Because delayed presentation is not uncommon in cases of bone or soft-tissue sarcoma, every patient with a mass with indeterminate imaging findings should be referred to or reviewed by an orthopedic or musculoskeletal oncologist.
Biopsy is gold standard for diagnosis
A comprehensive medical history and physical examination are essential at the initial presentation of patients with masses and/or pain suggestive of bone or soft-tissue sarcoma. Sarcoma simulators such as hematoma, metastatic disease, or infection can sometimes be ruled out by careful clinical examination, laboratory work-up, and appropriate imaging, but the gold standard for diagnosis is a biopsy. Moreover, an index of suspicion is required to rule out primary malignancy in any soft-tissue or bone lesion, and this index of suspicion will allow for referral or appropriate selection of the site for biopsy.
Biopsy considerations, as well as further detail on clinical presentation, are provided in the second and third articles in this supplement, which focus, respectively, on bone sarcoma and soft-tissue sarcoma. The remainder of this article reviews the use of imaging for the evaluation of suspected sarcomas, as imaging findings typically prompt or guide biopsy of a suspicious mass. Choosing the right imaging modality is critical to the diagnosis and management of patients with suspected sarcoma.
CONVENTIONAL IMAGING MODALITIES
Despite their utility for evaluating osseous lesions, radiographs have limited to no value in the evaluation of soft-tissue sarcomas but can demonstrate matrix mineralization and erosion or destruction of adjacent bone.
Angiography. In the past, angiography was frequently used to assess the vascularity of sarcomas preoperatively. Diagnostic angiography has been replaced by conventional MRI and magnetic resonance angiography, but some vascular sarcomas may require presurgical embolization to prevent excessive bleeding during surgery.
Radionuclide bone scans have long been a reliable tool for detecting multifocal or disseminated osseous lesions and remain the mainstay for evaluation of osseous metastases. They also are helpful in identifying skip lesions of osteosarcoma (ie, smaller discrete foci of osteosarcoma occurring in the same bone or on the opposing side of a joint).14 Advantages of this modality include whole-body scanning and low radiation at relatively low cost. Radionuclide bone scans demonstrate areas of bony repair and thus could be negative in purely lytic/destructive processes such as renal cell carcinoma metastases and multiple myeloma.
Chest radiographs are typically obtained in the initial stages of patient evaluation and are helpful in demonstrating large nodules or masses resulting from metastatic disease. In a patient with known sarcoma, a negative or equivocal chest radiograph should be followed by chest CT to definitively assess for metastasis.
CROSS-SECTIONAL IMAGING WITH MRI AND CT
MRI preferred for evaluation of most masses
MRI is the examination of choice in the evaluation of soft-tissue masses in light of its superior contrast resolution and ability to demonstrate subtle changes in soft tissues.
Predicting the histology of most soft-tissue masses is difficult, with the exception of some benign vascular lesions (eg, hemangioma), ganglia, neurogenic lesions, and well-differentiated lipomatous lesions. Aggressive features of a soft-tissue neoplasm include size greater than 5 cm,15 deep location, and absence of central enhancement, which is suggestive of necrosis (Figure 1). Yet one third of soft-tissue sarcomas are either superficial or smaller than 5 cm, which highlights the relative nonspecificity of these features.15
MRI is also the preferred modality in the evaluation of the majority of bone sarcomas, given its ability to accurately define the extent of marrow changes and soft-tissue involvement. MRI should be performed prior to a biopsy to prevent misinterpretation of biopsy-related signal changes in the surrounding tissues, which may negate the value of MRI in sarcoma staging.
Several distinct roles for CT
Chest CT should be obtained in all cases of known malignant neoplasms to evaluate for pulmonary nodules, masses, and lymphadenopathy. Despite the recent advances in MRI, CT remains the imaging modality of choice to evaluate the retroperitoneum, abdomen, and pelvis for masses, lymphadenopathy, or other signs of metastatic disease.
Post-treatment monitoring for recurrence
ULTRASONOGRAPHY
Ultrasonography has a limited role in the initial diagnosis and follow-up of musculoskeletal tumors. Its main advantages are a lack of ionizing radiation and dynamic imaging capabilities. Doppler ultrasonography allows direct visualization of tumor vascularity, which may be important for diagnosis and presurgical planning. Unfortunately, bone lesions cannot be evaluated with ultrasonography, owing to the inability of sound waves to penetrate the bony cortex. Poor sound wave penetration may prevent visualization of deep-seated lesions, such as retroperitoneal sarcomas.
Ultrasonography is best used for differentiating solid masses from cystic structures and can provide image guidance in solid tumor biopsy and cyst aspiration. It also may play a role in detecting suspected tumor recurrence in patients in whom artifact from implanted hardware precludes cross-sectional imaging, and it can be reliably used for following up unequivocal soft-tissue masses such as ganglia near joints.
POSITRON EMISSION TOMOGRAPHY
IMAGING-GUIDED INTERVENTIONS
Percutaneous imaging-guided procedures have increasingly replaced open surgical biopsies for bone and soft-tissue tumors. CT guidance is commonly used for percutaneous biopsy, whereas ultrasonographic guidance is sometimes used for superficial soft-tissue lesions. Although the shortest and most direct approach is desirable, this may not be possible in all cases due to the presence of nearby vital structures or the risk of contamination. Seeding of malignant cells along the biopsy tract is a well-known possible complication of image-guided biopsies, and en bloc resection of the needle tract is typically performed at the definitive surgery.
Knowledge of compartmental anatomy is paramount in planning the approach for these biopsies, and consultation with the referring orthopedic surgeon is recommended for optimal management. Expert histopathological interpretation of bone and soft-tissue specimens is essential for the efficacy and high success rates of percutaneous imaging-guided biopsies. Such expertise is integral to the broader interdisciplinary collaboration that is needed to arrive at the most plausible diagnosis, especially in the setting of uncommon or atypical neoplasms.
Currently, MRI-guided interventions are in the initial stage of evolution and could provide valuable guidance for subtle marrow or soft-tissue lesions visible on MRI but not well seen on CT.22 In the future, MRI could play an increasingly important role in imaging-guided procedures because of its lack of ionizing radiation and its ability to demonstrate subtle soft-tissue and bone marrow changes. Imaging-guided therapeutics are growing in their applications in musculoskeletal oncology. CT-guided radiofrequency ablation and cryoablation have been used in the treatment of a variety of tumors23 as well as in the palliation of metastatic bone pain.24
SUMMARY AND CONCLUSION
Bone and soft-tissue sarcomas are rare neoplasms with variable clinical presentations. A high index of suspicion is required for any unexplained mass with indeterminate imaging findings. Recent advances in imaging technology, including cross-sectional MRI and CT, have significantly refined the diagnosis and management of bone and soft-tissue sarcomas. When faced with a possible sarcoma, the clinician’s selection of imaging modalities has a direct impact on diagnosis, staging, and patient management.
- American Cancer Society. Cancer facts & figures 2009. Atlanta, GA: American Cancer Society; 2009.
- Weiss SW, Goldblum JR, Enzinger FM. Enzinger and Weiss’ Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225–249.
- Simon MA, Springfield DS, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Dahlin DC, Unni KK. Bone Tumors: General Aspects and Data on 8,542 Cases. 4th ed. Springfield, IL: Thomas; 1986.
- Unni KK. Bone Tumors. New York, NY: Churchill Livingstone; 1988.
- Unni KK. Atlas of Bone Pathology. New York, NY: Chapman & Hall; 1996:1 computer optical disc.
- Unni KK, Dahlin DC. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
- Kaste SC, Pratt CB, Cain AM, Jones-Wallace DJ, Rao BN. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer 1999; 86:1602–1608.
- Healey JH, Lane JM. Chondrosarcoma. Clin Orthop Relat Res 1986; 204:119–129.
- Kissane JM, Askin FB, Foulkes M, Stratton LB, Shirley SF. Ewing’s sarcoma of bone: clinicopathologic aspects of 303 cases from the Intergroup Ewing’s Sarcoma Study. Hum Pathol 1983; 14:773–779.
- Chotel F, Unnithan A, Chandrasekar CR, et al. Variability in the presentation of synovial sarcoma in children: a plea for greater awareness. J Bone Joint Surg Br 2008; 90:1090–1096.
- Miller TT. Bone tumors and tumorlike conditions: analysis with conventional radiography. Radiology 2008; 246:662–674.
- Richardson ML, Gillespy T. Magnetic resonance imaging. In: Kricun ME, ed. Imaging of Bone Tumors. Philadelphia, PA: WB Saunders; 1993:365.
- Fisher C. Soft tissue sarcomas: diagnosis, classification and prognostic factors. Br J Plast Surg 1996; 49:27–33.
- White LM, Wunder JS, Bell RS, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys 2005; 61:1439–1445.
- White LM, Buckwalter KA. Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6:5–17.
- Blodgett TM, Casagranda B, Townsend DW, Meltzer CC. Issues, controversies, and clinical utility of combined PET/CT imaging: what is the interpreting physician facing? AJR Am J Roentgenol 2005; 184(suppl 5):S138–S145.
- Shin DS, Shon OJ, Han DS, Choi JH, Chun KA, Cho IH. The clinical efficacy of 18F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med 2008; 22:603–609.
- Benz MR, Czernin J, Allen-Auerbach MS, et al. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res 2009; 15:2856–2863.
- Aoki J, Watanabe H, Shinozaki T, et al. FDG-PET for preoperative differential diagnosis between benign and malignant soft tissue masses. Skeletal Radiol 2003; 32:133–138.
- Blanco Sequeiros R, Klemola R, Ojala R, et al. MRI-guided trephine biopsy and fine-needle aspiration in the diagnosis of bone lesions in low-field (0.23 T) MRI system using optical instrument tracking. Eur Radiol 2002; 12:830–835.
- Rosenthal DI. Radiofrequency treatment. Orthop Clin North Am 2006; 37:475–484.
- Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol 2007; 10:120–131.
- American Cancer Society. Cancer facts & figures 2009. Atlanta, GA: American Cancer Society; 2009.
- Weiss SW, Goldblum JR, Enzinger FM. Enzinger and Weiss’ Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225–249.
- Simon MA, Springfield DS, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Dahlin DC, Unni KK. Bone Tumors: General Aspects and Data on 8,542 Cases. 4th ed. Springfield, IL: Thomas; 1986.
- Unni KK. Bone Tumors. New York, NY: Churchill Livingstone; 1988.
- Unni KK. Atlas of Bone Pathology. New York, NY: Chapman & Hall; 1996:1 computer optical disc.
- Unni KK, Dahlin DC. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
- Kaste SC, Pratt CB, Cain AM, Jones-Wallace DJ, Rao BN. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer 1999; 86:1602–1608.
- Healey JH, Lane JM. Chondrosarcoma. Clin Orthop Relat Res 1986; 204:119–129.
- Kissane JM, Askin FB, Foulkes M, Stratton LB, Shirley SF. Ewing’s sarcoma of bone: clinicopathologic aspects of 303 cases from the Intergroup Ewing’s Sarcoma Study. Hum Pathol 1983; 14:773–779.
- Chotel F, Unnithan A, Chandrasekar CR, et al. Variability in the presentation of synovial sarcoma in children: a plea for greater awareness. J Bone Joint Surg Br 2008; 90:1090–1096.
- Miller TT. Bone tumors and tumorlike conditions: analysis with conventional radiography. Radiology 2008; 246:662–674.
- Richardson ML, Gillespy T. Magnetic resonance imaging. In: Kricun ME, ed. Imaging of Bone Tumors. Philadelphia, PA: WB Saunders; 1993:365.
- Fisher C. Soft tissue sarcomas: diagnosis, classification and prognostic factors. Br J Plast Surg 1996; 49:27–33.
- White LM, Wunder JS, Bell RS, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys 2005; 61:1439–1445.
- White LM, Buckwalter KA. Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6:5–17.
- Blodgett TM, Casagranda B, Townsend DW, Meltzer CC. Issues, controversies, and clinical utility of combined PET/CT imaging: what is the interpreting physician facing? AJR Am J Roentgenol 2005; 184(suppl 5):S138–S145.
- Shin DS, Shon OJ, Han DS, Choi JH, Chun KA, Cho IH. The clinical efficacy of 18F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med 2008; 22:603–609.
- Benz MR, Czernin J, Allen-Auerbach MS, et al. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res 2009; 15:2856–2863.
- Aoki J, Watanabe H, Shinozaki T, et al. FDG-PET for preoperative differential diagnosis between benign and malignant soft tissue masses. Skeletal Radiol 2003; 32:133–138.
- Blanco Sequeiros R, Klemola R, Ojala R, et al. MRI-guided trephine biopsy and fine-needle aspiration in the diagnosis of bone lesions in low-field (0.23 T) MRI system using optical instrument tracking. Eur Radiol 2002; 12:830–835.
- Rosenthal DI. Radiofrequency treatment. Orthop Clin North Am 2006; 37:475–484.
- Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol 2007; 10:120–131.
Bone sarcomas: Overview of management, with a focus on surgical treatment considerations
Prior to the 1970s, bone sarcomas were routinely treated with amputation, yet most patients still died from metastatic disease.1 The advent of the use of chemotherapy for bone sarcomas in the 1970s was shown to increase long-term survival,2–5 contributing in part to tremendous subsequent advances in the treatment of the most common bone sarcomas—osteosarcoma and Ewing sarcoma. Today, long-term disease-free survival rates of about 60% to 80% are observed for patients with Ewing sarcoma or osteosarcoma with no metastasis at presentation.6,7 In addition to the chemotherapy advances, modular metallic prosthetic limb reconstruction systems are now readily available, eliminating the need to wait for custom reconstructive hardware. Moreover, these systems can be used in combination with large bone allografts or vascularized bone flaps.
The majority of patients with bone sarcomas require multimodal treatment, primarily with surgery and chemotherapy. Patients with chondrosarcomas are the primary exception, as chondrosarcomas are generally treated with resection alone. Thus, management of most patients with bone sarcomas requires a multidisciplinary team that includes orthopedic, medical, and radiation oncologists as well as plastic and reconstructive surgeons, physical therapy specialists, pathologists, and radiologists with expertise in bone tumors.
Despite this broad need for multimodal therapy, surgical resection is fundamental to the management of virtually all bone sarcomas and is the primary focus of this article. The roles of chemotherapy and radiation therapy for bone sarcomas are detailed in the final two articles in this supplement.
INITIAL EVALUATION OF SUSPICIOUS BONE MASSES
History and physical examination
As noted in the preceding article in this supplement, most bone sarcomas (particularly osteosarcomas and Ewing sarcomas) occur in pediatric patients and young adults and develop in the extremities (especially the distal femur) or pelvis.
In terms of history, most patients with a bone sarcoma will report pain, but pain is not a good indicator of malignancy, as some patients with no pain or an improvement in pain have sarcomas while many patients with pain do not have malignancies.1
The other most common finding in patients with a bone sarcoma is an enlarging mass. The presence of a mass, as well as its location, depth, size, and overlying skin quality, can be determined on physical examination. An accurate neurovascular exam should be performed as well, although damage to neurovascular structures is a late finding in sarcoma patients.
Imaging
Radiographs are important in any patient with prolonged unexplained bone pain and will almost always reveal an aggressive lesion in the patient with a bone sarcoma. Lengthy delays in the diagnosis of a bone sarcoma are nearly always explained by failure to obtain a radiograph.
Magnetic resonance imaging (MRI). Questions about whether a radiograph of a lesion is determinate or not are best resolved by MRI, which is the primary imaging method for evaluating bone lesions, their exact location, and their proximity to neurovascular structures. While “determinate” and “indeterminate” are most precisely used to refer to imaging studies of a lesion, these terms are often used in clinical parlance to refer to the lesions themselves. As such, “determinate lesions” by imaging are those that can be accurately judged malignant or benign with a high level of certainty. Determinate benign inactive lesions such as enchondromas and osteochondromas, if asymptomatic and without severe bony destruction, do not require a bone biopsy. “Indeterminate lesions” by imaging are those whose imaging findings are not clearly consistent with a single diagnosis, and nearly all of these lesions require a biopsy.
In general, any patient with a bone mass with indeterminate imaging results should be referred to an orthopedic oncologist.
Staging
When imaging findings are highly suggestive of bone sarcoma, efforts should be made to delineate how far the tumor extends and whether systemic disease is present. Bone sarcomas can metastasize to other bones, but their most common site for metastasis is the lung.
MRI of the lesion without gadolinium is indicated, and the entire bone is imaged to determine the extent of the external mass outside the bone and to look for medullary extension and skip lesions (eg, smaller foci of sarcoma occurring in the same bone or on the opposing side of a joint). The precision offered by MRI has dramatically increased surgeons’ ability to achieve negative margins during resection.
Radiography or computed tomography of the chest is required to accurately assess the lungs for metastasis. A nuclear medicine technetium scan can be obtained to look for other similar bone lesions (metachronous lesions) or metastatic bony disease.
Laboratory tests are not helpful in the staging of bone sarcomas.
BIOPSY
Biopsy is the gold standard for diagnosis of bone sarcoma (Figure 1). The primary biopsy methods used are needle or open biopsy techniques, and Tru-cut needles or core bone biopsy needles are increasingly used. If the core needle biopsy is diagnostically inconclusive, an open biopsy can promptly be performed. Biopsies yielding specimens that are too small can result in inconclusive pathology reports. Regardless of the biopsy technique, hemostasis is of paramount importance, and patients are generally advised to not use the affected limb for at least several days after the procedure to reduce the risk of a cancer cell–laden hematoma.
If a needle biopsy is performed, 2 to 10 minutes of gentle pressure is applied to the site. In an open biopsy, electrocauterization is used extensively. Aggressive hemostasis is achieved, and if a drain is placed it should be in proximity to the incision site itself so that the drain site will be resected with the specimen at the time of definitive resection. Open biopsies are performed in the operating room with regional or general anesthesia. Incisions are made longitudinally and never transversely.
Ideally, the biopsy should be performed or supervised by a physician experienced with limb salvage for bone sarcomas. Otherwise there is risk for an inappropriate biopsy tract or approach, misinterpretation of the radiographic studies, misinterpretation of the pathology, or biopsy complications. These errors may lead to undertreatment or even unnecessary amputation.8,9
RESECTION
For some bone sarcomas, such as osteosarcoma and Ewing sarcoma, there is a preference to treat the potential micrometastatic disease at the beginning of the course, prior to surgical treatment. This may result in reduction of the soft-tissue mass about the bone tumor and/or maturing of the mass, allowing for easier resection.
Importance of margins
The goal of resection is to achieve a margin or normal cuff of tissue around the pseudocapsule of the tumor. In general, the larger the margin, the less the chance of recurrence.10–12 Ideally, the tumor and pseudocapsule should not be violated or exposed and a margin of at least 1 cm should be obtained. It has been postulated that margins of less than 1 cm may be associated with a very low rate of recurrence, although no well-controlled study has proven this and such a study would be difficult to perform given the rarity and heterogeneity of bone sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery is generally to be avoided
Intralesional surgery should not be performed on high-grade bone sarcomas because it will lead to a high risk of local recurrence regardless of whether the patient receives perioperative radiation therapy or chemotherapy. If intralesional surgery has been performed for a high-grade sarcoma at an outside institution, re-excision of the tumor bed is recommended, as it has reduced the rate of recurrence following intralesional surgery.13 For low-grade chondrosarcomas, intralesional curettage (ie, violating the margin of the tumor by scraping it out thoroughly) with use of an adjuvant (freezing, phenol, methylmethacrylate, or argon beam) may be adequate and has been reported to have a low rate of recurrence.14
Preoperative planning
The resection procedure involves careful preoperative planning, typically guided by an MRI reviewed by a musculoskeletal tumor radiologist. General anesthesia is usually preferred because it can be used for a lengthy procedure, ensures complete muscle relaxation over the duration of the procedure, and allows for immediate postoperative nerve assessment. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), these structures are spared. If arteries are encased, arterial resection with reverse interpositional vein graft, synthetic graft, or vein allograft allows for bypass of the vessel and leaves the encased structure with the resection specimen for en bloc resection. In Ewing sarcoma, if the tumor is adjacent to but not encasing the neurovascular structures, the radiation oncologist is consulted about whether there is a preference for pre- or postoperative radiation therapy.
Limb salvage for Ewing sarcoma was originally with radiation only, but subsequently limb-salvaging surgery has been shown in several studies to have lower rates of local failure.6,15–18 Whether primary radiation or surgery is performed after the initiation of chemotherapy is generally determined by a discussion between the surgeon and radiation oncologist about the feasibility of a negative margin with surgery and the inherent functional loss with resection. There are particular concerns about radiation in younger patients, who have a relatively high rate of postradiation sarcoma.18
In osteosarcoma and chondrosarcoma, radiation has been found not to be effective, so resection with a negative margin is especially important for preventing local recurrence.
RECONSTRUCTION
Allograft or metallic prosthesis?
In the proximal and distal femur, modular metallic replacement prosthetic joint devices are used. Often a wafer of greater trochanter bone (if uninvolved in the tumor process) can be preserved and a “cable-claw” attachment to the metal component can be accomplished instead of using an allograft.
Since the proximal humerus is not weight-bearing and because of the importance of the rotator cuff, use of an APC in the proximal humerus can be most helpful. Function is not good with a metallic proximal humerus implant alone, and the dislocation rate is high over long-term follow-up, owing to lack of healing of the rotator cuff remnant to the metal prosthesis.
In patients with scapular sarcomas, allograft or prosthetic reconstruction has not been consistently better than simply repairing the remaining muscles to each other, so we generally do not use allografts or prostheses after sarcoma resection in these patients.
Growing bones of youth pose special challenges
In growing children, who represent a large share of bone sarcoma patients, reconstruction after resection in the lower extremity is challenging, particularly in terms of addressing leg length inequality. In general, a prosthesis is used and if the end growth discrepancy will be greater than 3 cm, use of an expandable prosthesis is considered. Use of these expandable prostheses has been fraught with complications, however, and by their nature they require revision because of breakage. An alternative is reoperation to disconnect the modular prosthesis and insert an additional 1- to 2-cm segment to increase length when necessary. Allograft bones are a common method of reconstruction when the resection does not involve the joint.
Rotationplasty
Rotationplasty—which involves saving the portion of the extremity distal to the resection site and reattaching it after being rotated 180 degrees—is rarely performed for leg reconstruction, in light of the disfiguring nature of the surgery as a result of the 180-degree rotation.
When rotationplasty is performed, the lower tibia and foot generally are brought up to the middle or proximal femoral area and attached to the short proximal femur. Rather than a short above-knee amputation, the reversed foot functions as a knee, allowing for better prosthetic function (ideally similar to a short below-knee prosthesis), and adds length to a short above-knee amputation.
Another alternative is a tibial turn-up to add length to a very short above-knee amputation if the vessels are not involved with the tumor and limb salvage is otherwise not practical. In this procedure the ankle can be turned up to the hip and the proximal tibia ends up distal to the ankle.
AMPUTATION
When curative surgery is possible and limb-salvaging resection is unlikely to obtain a negative margin or a functionally viable extremity, amputations are still performed. For example, amputation is recommended in a patient with a high-grade calcaneal (heel bone) sarcoma with a large soft-tissue mass. However, amputation is not the usual approach for most bone sarcomas today and it is not benign in outcome. Notably, phantom limb pain and stump pain have been reported after amputation in the typically sensate tumor patient.
Meticulous hemostasis is necessary in all amputations, and myodesis, or direct suturing of muscle to the distal end of the bone, is important for soft-tissue coverage over the distal stump. In general, a fish-mouth incision is used for the upper extremity and thigh, and a posterior flap is used, when possible, below the knee. However, the choice of technique depends on factors such as the presence or absence of a biopsy incision and the location of tumor soft-tissue mass, so local tissue rearrangement or flaps may need to be used for stable coverage or closure.
For all amputation patients, early involvement of an acute pain specialist reduces the incidence of phantom limb pain.
SURVEILLANCE AND FOLLOW-UP
Post-therapy follow-up of patients with bone sarcomas is critical. Even among patients who receive appropriate surgery with negative margins there is a recurrence rate of approximately 9% (personal communication from Dr. Dempsey Springfield), and previously undetectable metastatic disease may become detectable in the postoperative period. In general, patients are followed at 3-month intervals for the first 2 years, at 6-month intervals for the next 3 years, and at yearly intervals thereafter. Follow-up evaluations must include examination of the the involved extremity and imaging of the chest, with radiography or computed tomography, to assess for metastasis.
Rehabilitation is specific to the site of resection and the reconstruction. In general, range of motion is important around the knee, whereas in patients with resection and reconstruction involving the shoulder, hip, or pelvis, it is more important that the affected muscles be given time to heal (6–12 weeks) before aggressive rehabilitation is begun.
Many patients limp postoperatively, particularly in the initial period, and the degree of limp depends primarily on the amount of muscle and the bony insertion sites that are resected with the tumor. Improvements in function are common over time, even at several years after surgery.
FUTURE DIRECTIONS
Despite the advances in bone sarcoma outcomes in recent decades, sarcomas of the pelvis continue to carry a worse prognosis than those of the extremities and thus represent an opportunity for improvement. Among the improvements hoped for is an ability to accomplish partial pelvic resections—eg, of the wing, ischium, or ramus—without need for reconstruction for these smaller localized tumors. Options include amputation (hemipelvectomy) with loss of leg; internal hemipelvectomy (where the pelvis is resected but the leg is left attached without reconstruction of the defect); or resection of the pelvic/acetabular area but with reconstruction using pelvic allografts/total hip composites or large metallic prostheses.
- Simon MA, Springfield DS. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Cortes EP, Holland JF, Wang JJ, et al. Amputation and adriamycin in primary osteosarcoma. N Engl J Med 1974; 291:998–1000.
- Goorin AM, Abelson HT, Frei E III. Osteosarcoma: fifteen years later. N Engl J Med 1985; 313:1637–1643.
- Goorin AM, Frei E, Abelson HT. Adjuvant chemotherapy for osteosarcoma: a decade of experience. Surg Clin North Am 1981; 61:1379–1389.
- Jaffe N, Goorin A, Link M, et al. High-dose methotrexate in osteogenic sarcoma adjuvant chemotherapy and limb salvage results. Cancer Treat Rep 1981; 65(suppl 1):99–106.
- Rodriguez-Galindo C, Navid F, Liu T, et al. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol 2008; 19:814–820.
- Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival: a report from the Children’s Oncology Group. J Clin Oncol 2008; 26:633–638.
- Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am 1996; 78:656–663.
- Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am 1982; 64:1121–1127.
- Blakely ML, Spurbeck WW, Pappo AS, et al. The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg 1999; 34:672–675.
- Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol 1997; 66:81–87.
- Gupta GR, Yasko AW, Lewis VO, et al. Risk of local recurrence after deltoid-sparing resection for osteosarcoma of the proximal humerus. Cancer 2009; 115:3767–3773.
- Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 2008; 90:203–208.
- Bauer HC, Brosjö O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities: 80 patients followed for 2–25 years. Acta Orthop Scand 1995; 66:283–288.
- Graham-Pole J. Ewing sarcoma: treatment with high dose radiation and adjuvant chemotherapy. Med Pediatr Oncol 1979; 7:1–8.
- Merchant TE, Kushner BH, Sheldon JM, LaQuaglia M, Healey JH. Effect of low-dose radiation therapy when combined with surgical resection for Ewing sarcoma. Med Pediatr Oncol 1999; 33:65–70.
- Rosito P, Mancini AF, Rondelli R, et al. Italian Cooperative Study for the treatment of children and young adults with localized Ewing sarcoma of bone: a preliminary report of 6 years of experience. Cancer 1999; 86:421–428.
- Goldsby R, Burke C, Nagarajan R, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children’s Oncology Group cohort. Cancer 2008; 113:2597–2604.
Prior to the 1970s, bone sarcomas were routinely treated with amputation, yet most patients still died from metastatic disease.1 The advent of the use of chemotherapy for bone sarcomas in the 1970s was shown to increase long-term survival,2–5 contributing in part to tremendous subsequent advances in the treatment of the most common bone sarcomas—osteosarcoma and Ewing sarcoma. Today, long-term disease-free survival rates of about 60% to 80% are observed for patients with Ewing sarcoma or osteosarcoma with no metastasis at presentation.6,7 In addition to the chemotherapy advances, modular metallic prosthetic limb reconstruction systems are now readily available, eliminating the need to wait for custom reconstructive hardware. Moreover, these systems can be used in combination with large bone allografts or vascularized bone flaps.
The majority of patients with bone sarcomas require multimodal treatment, primarily with surgery and chemotherapy. Patients with chondrosarcomas are the primary exception, as chondrosarcomas are generally treated with resection alone. Thus, management of most patients with bone sarcomas requires a multidisciplinary team that includes orthopedic, medical, and radiation oncologists as well as plastic and reconstructive surgeons, physical therapy specialists, pathologists, and radiologists with expertise in bone tumors.
Despite this broad need for multimodal therapy, surgical resection is fundamental to the management of virtually all bone sarcomas and is the primary focus of this article. The roles of chemotherapy and radiation therapy for bone sarcomas are detailed in the final two articles in this supplement.
INITIAL EVALUATION OF SUSPICIOUS BONE MASSES
History and physical examination
As noted in the preceding article in this supplement, most bone sarcomas (particularly osteosarcomas and Ewing sarcomas) occur in pediatric patients and young adults and develop in the extremities (especially the distal femur) or pelvis.
In terms of history, most patients with a bone sarcoma will report pain, but pain is not a good indicator of malignancy, as some patients with no pain or an improvement in pain have sarcomas while many patients with pain do not have malignancies.1
The other most common finding in patients with a bone sarcoma is an enlarging mass. The presence of a mass, as well as its location, depth, size, and overlying skin quality, can be determined on physical examination. An accurate neurovascular exam should be performed as well, although damage to neurovascular structures is a late finding in sarcoma patients.
Imaging
Radiographs are important in any patient with prolonged unexplained bone pain and will almost always reveal an aggressive lesion in the patient with a bone sarcoma. Lengthy delays in the diagnosis of a bone sarcoma are nearly always explained by failure to obtain a radiograph.
Magnetic resonance imaging (MRI). Questions about whether a radiograph of a lesion is determinate or not are best resolved by MRI, which is the primary imaging method for evaluating bone lesions, their exact location, and their proximity to neurovascular structures. While “determinate” and “indeterminate” are most precisely used to refer to imaging studies of a lesion, these terms are often used in clinical parlance to refer to the lesions themselves. As such, “determinate lesions” by imaging are those that can be accurately judged malignant or benign with a high level of certainty. Determinate benign inactive lesions such as enchondromas and osteochondromas, if asymptomatic and without severe bony destruction, do not require a bone biopsy. “Indeterminate lesions” by imaging are those whose imaging findings are not clearly consistent with a single diagnosis, and nearly all of these lesions require a biopsy.
In general, any patient with a bone mass with indeterminate imaging results should be referred to an orthopedic oncologist.
Staging
When imaging findings are highly suggestive of bone sarcoma, efforts should be made to delineate how far the tumor extends and whether systemic disease is present. Bone sarcomas can metastasize to other bones, but their most common site for metastasis is the lung.
MRI of the lesion without gadolinium is indicated, and the entire bone is imaged to determine the extent of the external mass outside the bone and to look for medullary extension and skip lesions (eg, smaller foci of sarcoma occurring in the same bone or on the opposing side of a joint). The precision offered by MRI has dramatically increased surgeons’ ability to achieve negative margins during resection.
Radiography or computed tomography of the chest is required to accurately assess the lungs for metastasis. A nuclear medicine technetium scan can be obtained to look for other similar bone lesions (metachronous lesions) or metastatic bony disease.
Laboratory tests are not helpful in the staging of bone sarcomas.
BIOPSY
Biopsy is the gold standard for diagnosis of bone sarcoma (Figure 1). The primary biopsy methods used are needle or open biopsy techniques, and Tru-cut needles or core bone biopsy needles are increasingly used. If the core needle biopsy is diagnostically inconclusive, an open biopsy can promptly be performed. Biopsies yielding specimens that are too small can result in inconclusive pathology reports. Regardless of the biopsy technique, hemostasis is of paramount importance, and patients are generally advised to not use the affected limb for at least several days after the procedure to reduce the risk of a cancer cell–laden hematoma.
If a needle biopsy is performed, 2 to 10 minutes of gentle pressure is applied to the site. In an open biopsy, electrocauterization is used extensively. Aggressive hemostasis is achieved, and if a drain is placed it should be in proximity to the incision site itself so that the drain site will be resected with the specimen at the time of definitive resection. Open biopsies are performed in the operating room with regional or general anesthesia. Incisions are made longitudinally and never transversely.
Ideally, the biopsy should be performed or supervised by a physician experienced with limb salvage for bone sarcomas. Otherwise there is risk for an inappropriate biopsy tract or approach, misinterpretation of the radiographic studies, misinterpretation of the pathology, or biopsy complications. These errors may lead to undertreatment or even unnecessary amputation.8,9
RESECTION
For some bone sarcomas, such as osteosarcoma and Ewing sarcoma, there is a preference to treat the potential micrometastatic disease at the beginning of the course, prior to surgical treatment. This may result in reduction of the soft-tissue mass about the bone tumor and/or maturing of the mass, allowing for easier resection.
Importance of margins
The goal of resection is to achieve a margin or normal cuff of tissue around the pseudocapsule of the tumor. In general, the larger the margin, the less the chance of recurrence.10–12 Ideally, the tumor and pseudocapsule should not be violated or exposed and a margin of at least 1 cm should be obtained. It has been postulated that margins of less than 1 cm may be associated with a very low rate of recurrence, although no well-controlled study has proven this and such a study would be difficult to perform given the rarity and heterogeneity of bone sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery is generally to be avoided
Intralesional surgery should not be performed on high-grade bone sarcomas because it will lead to a high risk of local recurrence regardless of whether the patient receives perioperative radiation therapy or chemotherapy. If intralesional surgery has been performed for a high-grade sarcoma at an outside institution, re-excision of the tumor bed is recommended, as it has reduced the rate of recurrence following intralesional surgery.13 For low-grade chondrosarcomas, intralesional curettage (ie, violating the margin of the tumor by scraping it out thoroughly) with use of an adjuvant (freezing, phenol, methylmethacrylate, or argon beam) may be adequate and has been reported to have a low rate of recurrence.14
Preoperative planning
The resection procedure involves careful preoperative planning, typically guided by an MRI reviewed by a musculoskeletal tumor radiologist. General anesthesia is usually preferred because it can be used for a lengthy procedure, ensures complete muscle relaxation over the duration of the procedure, and allows for immediate postoperative nerve assessment. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), these structures are spared. If arteries are encased, arterial resection with reverse interpositional vein graft, synthetic graft, or vein allograft allows for bypass of the vessel and leaves the encased structure with the resection specimen for en bloc resection. In Ewing sarcoma, if the tumor is adjacent to but not encasing the neurovascular structures, the radiation oncologist is consulted about whether there is a preference for pre- or postoperative radiation therapy.
Limb salvage for Ewing sarcoma was originally with radiation only, but subsequently limb-salvaging surgery has been shown in several studies to have lower rates of local failure.6,15–18 Whether primary radiation or surgery is performed after the initiation of chemotherapy is generally determined by a discussion between the surgeon and radiation oncologist about the feasibility of a negative margin with surgery and the inherent functional loss with resection. There are particular concerns about radiation in younger patients, who have a relatively high rate of postradiation sarcoma.18
In osteosarcoma and chondrosarcoma, radiation has been found not to be effective, so resection with a negative margin is especially important for preventing local recurrence.
RECONSTRUCTION
Allograft or metallic prosthesis?
In the proximal and distal femur, modular metallic replacement prosthetic joint devices are used. Often a wafer of greater trochanter bone (if uninvolved in the tumor process) can be preserved and a “cable-claw” attachment to the metal component can be accomplished instead of using an allograft.
Since the proximal humerus is not weight-bearing and because of the importance of the rotator cuff, use of an APC in the proximal humerus can be most helpful. Function is not good with a metallic proximal humerus implant alone, and the dislocation rate is high over long-term follow-up, owing to lack of healing of the rotator cuff remnant to the metal prosthesis.
In patients with scapular sarcomas, allograft or prosthetic reconstruction has not been consistently better than simply repairing the remaining muscles to each other, so we generally do not use allografts or prostheses after sarcoma resection in these patients.
Growing bones of youth pose special challenges
In growing children, who represent a large share of bone sarcoma patients, reconstruction after resection in the lower extremity is challenging, particularly in terms of addressing leg length inequality. In general, a prosthesis is used and if the end growth discrepancy will be greater than 3 cm, use of an expandable prosthesis is considered. Use of these expandable prostheses has been fraught with complications, however, and by their nature they require revision because of breakage. An alternative is reoperation to disconnect the modular prosthesis and insert an additional 1- to 2-cm segment to increase length when necessary. Allograft bones are a common method of reconstruction when the resection does not involve the joint.
Rotationplasty
Rotationplasty—which involves saving the portion of the extremity distal to the resection site and reattaching it after being rotated 180 degrees—is rarely performed for leg reconstruction, in light of the disfiguring nature of the surgery as a result of the 180-degree rotation.
When rotationplasty is performed, the lower tibia and foot generally are brought up to the middle or proximal femoral area and attached to the short proximal femur. Rather than a short above-knee amputation, the reversed foot functions as a knee, allowing for better prosthetic function (ideally similar to a short below-knee prosthesis), and adds length to a short above-knee amputation.
Another alternative is a tibial turn-up to add length to a very short above-knee amputation if the vessels are not involved with the tumor and limb salvage is otherwise not practical. In this procedure the ankle can be turned up to the hip and the proximal tibia ends up distal to the ankle.
AMPUTATION
When curative surgery is possible and limb-salvaging resection is unlikely to obtain a negative margin or a functionally viable extremity, amputations are still performed. For example, amputation is recommended in a patient with a high-grade calcaneal (heel bone) sarcoma with a large soft-tissue mass. However, amputation is not the usual approach for most bone sarcomas today and it is not benign in outcome. Notably, phantom limb pain and stump pain have been reported after amputation in the typically sensate tumor patient.
Meticulous hemostasis is necessary in all amputations, and myodesis, or direct suturing of muscle to the distal end of the bone, is important for soft-tissue coverage over the distal stump. In general, a fish-mouth incision is used for the upper extremity and thigh, and a posterior flap is used, when possible, below the knee. However, the choice of technique depends on factors such as the presence or absence of a biopsy incision and the location of tumor soft-tissue mass, so local tissue rearrangement or flaps may need to be used for stable coverage or closure.
For all amputation patients, early involvement of an acute pain specialist reduces the incidence of phantom limb pain.
SURVEILLANCE AND FOLLOW-UP
Post-therapy follow-up of patients with bone sarcomas is critical. Even among patients who receive appropriate surgery with negative margins there is a recurrence rate of approximately 9% (personal communication from Dr. Dempsey Springfield), and previously undetectable metastatic disease may become detectable in the postoperative period. In general, patients are followed at 3-month intervals for the first 2 years, at 6-month intervals for the next 3 years, and at yearly intervals thereafter. Follow-up evaluations must include examination of the the involved extremity and imaging of the chest, with radiography or computed tomography, to assess for metastasis.
Rehabilitation is specific to the site of resection and the reconstruction. In general, range of motion is important around the knee, whereas in patients with resection and reconstruction involving the shoulder, hip, or pelvis, it is more important that the affected muscles be given time to heal (6–12 weeks) before aggressive rehabilitation is begun.
Many patients limp postoperatively, particularly in the initial period, and the degree of limp depends primarily on the amount of muscle and the bony insertion sites that are resected with the tumor. Improvements in function are common over time, even at several years after surgery.
FUTURE DIRECTIONS
Despite the advances in bone sarcoma outcomes in recent decades, sarcomas of the pelvis continue to carry a worse prognosis than those of the extremities and thus represent an opportunity for improvement. Among the improvements hoped for is an ability to accomplish partial pelvic resections—eg, of the wing, ischium, or ramus—without need for reconstruction for these smaller localized tumors. Options include amputation (hemipelvectomy) with loss of leg; internal hemipelvectomy (where the pelvis is resected but the leg is left attached without reconstruction of the defect); or resection of the pelvic/acetabular area but with reconstruction using pelvic allografts/total hip composites or large metallic prostheses.
Prior to the 1970s, bone sarcomas were routinely treated with amputation, yet most patients still died from metastatic disease.1 The advent of the use of chemotherapy for bone sarcomas in the 1970s was shown to increase long-term survival,2–5 contributing in part to tremendous subsequent advances in the treatment of the most common bone sarcomas—osteosarcoma and Ewing sarcoma. Today, long-term disease-free survival rates of about 60% to 80% are observed for patients with Ewing sarcoma or osteosarcoma with no metastasis at presentation.6,7 In addition to the chemotherapy advances, modular metallic prosthetic limb reconstruction systems are now readily available, eliminating the need to wait for custom reconstructive hardware. Moreover, these systems can be used in combination with large bone allografts or vascularized bone flaps.
The majority of patients with bone sarcomas require multimodal treatment, primarily with surgery and chemotherapy. Patients with chondrosarcomas are the primary exception, as chondrosarcomas are generally treated with resection alone. Thus, management of most patients with bone sarcomas requires a multidisciplinary team that includes orthopedic, medical, and radiation oncologists as well as plastic and reconstructive surgeons, physical therapy specialists, pathologists, and radiologists with expertise in bone tumors.
Despite this broad need for multimodal therapy, surgical resection is fundamental to the management of virtually all bone sarcomas and is the primary focus of this article. The roles of chemotherapy and radiation therapy for bone sarcomas are detailed in the final two articles in this supplement.
INITIAL EVALUATION OF SUSPICIOUS BONE MASSES
History and physical examination
As noted in the preceding article in this supplement, most bone sarcomas (particularly osteosarcomas and Ewing sarcomas) occur in pediatric patients and young adults and develop in the extremities (especially the distal femur) or pelvis.
In terms of history, most patients with a bone sarcoma will report pain, but pain is not a good indicator of malignancy, as some patients with no pain or an improvement in pain have sarcomas while many patients with pain do not have malignancies.1
The other most common finding in patients with a bone sarcoma is an enlarging mass. The presence of a mass, as well as its location, depth, size, and overlying skin quality, can be determined on physical examination. An accurate neurovascular exam should be performed as well, although damage to neurovascular structures is a late finding in sarcoma patients.
Imaging
Radiographs are important in any patient with prolonged unexplained bone pain and will almost always reveal an aggressive lesion in the patient with a bone sarcoma. Lengthy delays in the diagnosis of a bone sarcoma are nearly always explained by failure to obtain a radiograph.
Magnetic resonance imaging (MRI). Questions about whether a radiograph of a lesion is determinate or not are best resolved by MRI, which is the primary imaging method for evaluating bone lesions, their exact location, and their proximity to neurovascular structures. While “determinate” and “indeterminate” are most precisely used to refer to imaging studies of a lesion, these terms are often used in clinical parlance to refer to the lesions themselves. As such, “determinate lesions” by imaging are those that can be accurately judged malignant or benign with a high level of certainty. Determinate benign inactive lesions such as enchondromas and osteochondromas, if asymptomatic and without severe bony destruction, do not require a bone biopsy. “Indeterminate lesions” by imaging are those whose imaging findings are not clearly consistent with a single diagnosis, and nearly all of these lesions require a biopsy.
In general, any patient with a bone mass with indeterminate imaging results should be referred to an orthopedic oncologist.
Staging
When imaging findings are highly suggestive of bone sarcoma, efforts should be made to delineate how far the tumor extends and whether systemic disease is present. Bone sarcomas can metastasize to other bones, but their most common site for metastasis is the lung.
MRI of the lesion without gadolinium is indicated, and the entire bone is imaged to determine the extent of the external mass outside the bone and to look for medullary extension and skip lesions (eg, smaller foci of sarcoma occurring in the same bone or on the opposing side of a joint). The precision offered by MRI has dramatically increased surgeons’ ability to achieve negative margins during resection.
Radiography or computed tomography of the chest is required to accurately assess the lungs for metastasis. A nuclear medicine technetium scan can be obtained to look for other similar bone lesions (metachronous lesions) or metastatic bony disease.
Laboratory tests are not helpful in the staging of bone sarcomas.
BIOPSY
Biopsy is the gold standard for diagnosis of bone sarcoma (Figure 1). The primary biopsy methods used are needle or open biopsy techniques, and Tru-cut needles or core bone biopsy needles are increasingly used. If the core needle biopsy is diagnostically inconclusive, an open biopsy can promptly be performed. Biopsies yielding specimens that are too small can result in inconclusive pathology reports. Regardless of the biopsy technique, hemostasis is of paramount importance, and patients are generally advised to not use the affected limb for at least several days after the procedure to reduce the risk of a cancer cell–laden hematoma.
If a needle biopsy is performed, 2 to 10 minutes of gentle pressure is applied to the site. In an open biopsy, electrocauterization is used extensively. Aggressive hemostasis is achieved, and if a drain is placed it should be in proximity to the incision site itself so that the drain site will be resected with the specimen at the time of definitive resection. Open biopsies are performed in the operating room with regional or general anesthesia. Incisions are made longitudinally and never transversely.
Ideally, the biopsy should be performed or supervised by a physician experienced with limb salvage for bone sarcomas. Otherwise there is risk for an inappropriate biopsy tract or approach, misinterpretation of the radiographic studies, misinterpretation of the pathology, or biopsy complications. These errors may lead to undertreatment or even unnecessary amputation.8,9
RESECTION
For some bone sarcomas, such as osteosarcoma and Ewing sarcoma, there is a preference to treat the potential micrometastatic disease at the beginning of the course, prior to surgical treatment. This may result in reduction of the soft-tissue mass about the bone tumor and/or maturing of the mass, allowing for easier resection.
Importance of margins
The goal of resection is to achieve a margin or normal cuff of tissue around the pseudocapsule of the tumor. In general, the larger the margin, the less the chance of recurrence.10–12 Ideally, the tumor and pseudocapsule should not be violated or exposed and a margin of at least 1 cm should be obtained. It has been postulated that margins of less than 1 cm may be associated with a very low rate of recurrence, although no well-controlled study has proven this and such a study would be difficult to perform given the rarity and heterogeneity of bone sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery is generally to be avoided
Intralesional surgery should not be performed on high-grade bone sarcomas because it will lead to a high risk of local recurrence regardless of whether the patient receives perioperative radiation therapy or chemotherapy. If intralesional surgery has been performed for a high-grade sarcoma at an outside institution, re-excision of the tumor bed is recommended, as it has reduced the rate of recurrence following intralesional surgery.13 For low-grade chondrosarcomas, intralesional curettage (ie, violating the margin of the tumor by scraping it out thoroughly) with use of an adjuvant (freezing, phenol, methylmethacrylate, or argon beam) may be adequate and has been reported to have a low rate of recurrence.14
Preoperative planning
The resection procedure involves careful preoperative planning, typically guided by an MRI reviewed by a musculoskeletal tumor radiologist. General anesthesia is usually preferred because it can be used for a lengthy procedure, ensures complete muscle relaxation over the duration of the procedure, and allows for immediate postoperative nerve assessment. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), these structures are spared. If arteries are encased, arterial resection with reverse interpositional vein graft, synthetic graft, or vein allograft allows for bypass of the vessel and leaves the encased structure with the resection specimen for en bloc resection. In Ewing sarcoma, if the tumor is adjacent to but not encasing the neurovascular structures, the radiation oncologist is consulted about whether there is a preference for pre- or postoperative radiation therapy.
Limb salvage for Ewing sarcoma was originally with radiation only, but subsequently limb-salvaging surgery has been shown in several studies to have lower rates of local failure.6,15–18 Whether primary radiation or surgery is performed after the initiation of chemotherapy is generally determined by a discussion between the surgeon and radiation oncologist about the feasibility of a negative margin with surgery and the inherent functional loss with resection. There are particular concerns about radiation in younger patients, who have a relatively high rate of postradiation sarcoma.18
In osteosarcoma and chondrosarcoma, radiation has been found not to be effective, so resection with a negative margin is especially important for preventing local recurrence.
RECONSTRUCTION
Allograft or metallic prosthesis?
In the proximal and distal femur, modular metallic replacement prosthetic joint devices are used. Often a wafer of greater trochanter bone (if uninvolved in the tumor process) can be preserved and a “cable-claw” attachment to the metal component can be accomplished instead of using an allograft.
Since the proximal humerus is not weight-bearing and because of the importance of the rotator cuff, use of an APC in the proximal humerus can be most helpful. Function is not good with a metallic proximal humerus implant alone, and the dislocation rate is high over long-term follow-up, owing to lack of healing of the rotator cuff remnant to the metal prosthesis.
In patients with scapular sarcomas, allograft or prosthetic reconstruction has not been consistently better than simply repairing the remaining muscles to each other, so we generally do not use allografts or prostheses after sarcoma resection in these patients.
Growing bones of youth pose special challenges
In growing children, who represent a large share of bone sarcoma patients, reconstruction after resection in the lower extremity is challenging, particularly in terms of addressing leg length inequality. In general, a prosthesis is used and if the end growth discrepancy will be greater than 3 cm, use of an expandable prosthesis is considered. Use of these expandable prostheses has been fraught with complications, however, and by their nature they require revision because of breakage. An alternative is reoperation to disconnect the modular prosthesis and insert an additional 1- to 2-cm segment to increase length when necessary. Allograft bones are a common method of reconstruction when the resection does not involve the joint.
Rotationplasty
Rotationplasty—which involves saving the portion of the extremity distal to the resection site and reattaching it after being rotated 180 degrees—is rarely performed for leg reconstruction, in light of the disfiguring nature of the surgery as a result of the 180-degree rotation.
When rotationplasty is performed, the lower tibia and foot generally are brought up to the middle or proximal femoral area and attached to the short proximal femur. Rather than a short above-knee amputation, the reversed foot functions as a knee, allowing for better prosthetic function (ideally similar to a short below-knee prosthesis), and adds length to a short above-knee amputation.
Another alternative is a tibial turn-up to add length to a very short above-knee amputation if the vessels are not involved with the tumor and limb salvage is otherwise not practical. In this procedure the ankle can be turned up to the hip and the proximal tibia ends up distal to the ankle.
AMPUTATION
When curative surgery is possible and limb-salvaging resection is unlikely to obtain a negative margin or a functionally viable extremity, amputations are still performed. For example, amputation is recommended in a patient with a high-grade calcaneal (heel bone) sarcoma with a large soft-tissue mass. However, amputation is not the usual approach for most bone sarcomas today and it is not benign in outcome. Notably, phantom limb pain and stump pain have been reported after amputation in the typically sensate tumor patient.
Meticulous hemostasis is necessary in all amputations, and myodesis, or direct suturing of muscle to the distal end of the bone, is important for soft-tissue coverage over the distal stump. In general, a fish-mouth incision is used for the upper extremity and thigh, and a posterior flap is used, when possible, below the knee. However, the choice of technique depends on factors such as the presence or absence of a biopsy incision and the location of tumor soft-tissue mass, so local tissue rearrangement or flaps may need to be used for stable coverage or closure.
For all amputation patients, early involvement of an acute pain specialist reduces the incidence of phantom limb pain.
SURVEILLANCE AND FOLLOW-UP
Post-therapy follow-up of patients with bone sarcomas is critical. Even among patients who receive appropriate surgery with negative margins there is a recurrence rate of approximately 9% (personal communication from Dr. Dempsey Springfield), and previously undetectable metastatic disease may become detectable in the postoperative period. In general, patients are followed at 3-month intervals for the first 2 years, at 6-month intervals for the next 3 years, and at yearly intervals thereafter. Follow-up evaluations must include examination of the the involved extremity and imaging of the chest, with radiography or computed tomography, to assess for metastasis.
Rehabilitation is specific to the site of resection and the reconstruction. In general, range of motion is important around the knee, whereas in patients with resection and reconstruction involving the shoulder, hip, or pelvis, it is more important that the affected muscles be given time to heal (6–12 weeks) before aggressive rehabilitation is begun.
Many patients limp postoperatively, particularly in the initial period, and the degree of limp depends primarily on the amount of muscle and the bony insertion sites that are resected with the tumor. Improvements in function are common over time, even at several years after surgery.
FUTURE DIRECTIONS
Despite the advances in bone sarcoma outcomes in recent decades, sarcomas of the pelvis continue to carry a worse prognosis than those of the extremities and thus represent an opportunity for improvement. Among the improvements hoped for is an ability to accomplish partial pelvic resections—eg, of the wing, ischium, or ramus—without need for reconstruction for these smaller localized tumors. Options include amputation (hemipelvectomy) with loss of leg; internal hemipelvectomy (where the pelvis is resected but the leg is left attached without reconstruction of the defect); or resection of the pelvic/acetabular area but with reconstruction using pelvic allografts/total hip composites or large metallic prostheses.
- Simon MA, Springfield DS. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Cortes EP, Holland JF, Wang JJ, et al. Amputation and adriamycin in primary osteosarcoma. N Engl J Med 1974; 291:998–1000.
- Goorin AM, Abelson HT, Frei E III. Osteosarcoma: fifteen years later. N Engl J Med 1985; 313:1637–1643.
- Goorin AM, Frei E, Abelson HT. Adjuvant chemotherapy for osteosarcoma: a decade of experience. Surg Clin North Am 1981; 61:1379–1389.
- Jaffe N, Goorin A, Link M, et al. High-dose methotrexate in osteogenic sarcoma adjuvant chemotherapy and limb salvage results. Cancer Treat Rep 1981; 65(suppl 1):99–106.
- Rodriguez-Galindo C, Navid F, Liu T, et al. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol 2008; 19:814–820.
- Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival: a report from the Children’s Oncology Group. J Clin Oncol 2008; 26:633–638.
- Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am 1996; 78:656–663.
- Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am 1982; 64:1121–1127.
- Blakely ML, Spurbeck WW, Pappo AS, et al. The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg 1999; 34:672–675.
- Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol 1997; 66:81–87.
- Gupta GR, Yasko AW, Lewis VO, et al. Risk of local recurrence after deltoid-sparing resection for osteosarcoma of the proximal humerus. Cancer 2009; 115:3767–3773.
- Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 2008; 90:203–208.
- Bauer HC, Brosjö O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities: 80 patients followed for 2–25 years. Acta Orthop Scand 1995; 66:283–288.
- Graham-Pole J. Ewing sarcoma: treatment with high dose radiation and adjuvant chemotherapy. Med Pediatr Oncol 1979; 7:1–8.
- Merchant TE, Kushner BH, Sheldon JM, LaQuaglia M, Healey JH. Effect of low-dose radiation therapy when combined with surgical resection for Ewing sarcoma. Med Pediatr Oncol 1999; 33:65–70.
- Rosito P, Mancini AF, Rondelli R, et al. Italian Cooperative Study for the treatment of children and young adults with localized Ewing sarcoma of bone: a preliminary report of 6 years of experience. Cancer 1999; 86:421–428.
- Goldsby R, Burke C, Nagarajan R, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children’s Oncology Group cohort. Cancer 2008; 113:2597–2604.
- Simon MA, Springfield DS. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Cortes EP, Holland JF, Wang JJ, et al. Amputation and adriamycin in primary osteosarcoma. N Engl J Med 1974; 291:998–1000.
- Goorin AM, Abelson HT, Frei E III. Osteosarcoma: fifteen years later. N Engl J Med 1985; 313:1637–1643.
- Goorin AM, Frei E, Abelson HT. Adjuvant chemotherapy for osteosarcoma: a decade of experience. Surg Clin North Am 1981; 61:1379–1389.
- Jaffe N, Goorin A, Link M, et al. High-dose methotrexate in osteogenic sarcoma adjuvant chemotherapy and limb salvage results. Cancer Treat Rep 1981; 65(suppl 1):99–106.
- Rodriguez-Galindo C, Navid F, Liu T, et al. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol 2008; 19:814–820.
- Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival: a report from the Children’s Oncology Group. J Clin Oncol 2008; 26:633–638.
- Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am 1996; 78:656–663.
- Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am 1982; 64:1121–1127.
- Blakely ML, Spurbeck WW, Pappo AS, et al. The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg 1999; 34:672–675.
- Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol 1997; 66:81–87.
- Gupta GR, Yasko AW, Lewis VO, et al. Risk of local recurrence after deltoid-sparing resection for osteosarcoma of the proximal humerus. Cancer 2009; 115:3767–3773.
- Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 2008; 90:203–208.
- Bauer HC, Brosjö O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities: 80 patients followed for 2–25 years. Acta Orthop Scand 1995; 66:283–288.
- Graham-Pole J. Ewing sarcoma: treatment with high dose radiation and adjuvant chemotherapy. Med Pediatr Oncol 1979; 7:1–8.
- Merchant TE, Kushner BH, Sheldon JM, LaQuaglia M, Healey JH. Effect of low-dose radiation therapy when combined with surgical resection for Ewing sarcoma. Med Pediatr Oncol 1999; 33:65–70.
- Rosito P, Mancini AF, Rondelli R, et al. Italian Cooperative Study for the treatment of children and young adults with localized Ewing sarcoma of bone: a preliminary report of 6 years of experience. Cancer 1999; 86:421–428.
- Goldsby R, Burke C, Nagarajan R, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children’s Oncology Group cohort. Cancer 2008; 113:2597–2604.
Soft-tissue sarcomas: Overview of management, with a focus on surgical treatment considerations
Soft-tissue sarcomas are tumors of the mesenchymal system, and half develop in the extremities.1 Although patients with soft-tissue sarcomas have been treated with a combination of surgery, radiation therapy, and chemotherapy, it remains unclear whether either radiation or chemotherapy improves outcomes for these patients. Soft-tissue sarcomas are therefore currently treated with surgical resection when possible, with or without chemotherapy or radiation.
Even though multimodal therapy for patients with these tumors is controversial, a multidisciplinary conference among the many providers who may be involved in the management these patients—orthopedic, medical, and radiation oncologists, as well as the referring primary care physician, plastic and reconstructive surgeons, physical therapists, and radiologists and pathologists with expertise in these tumors—is helpful.2 This article presents an overview of the management of these patients, with a focus on the mainstay treatment, surgical resection. The roles of chemotherapy and radiation therapy for soft-tissue sarcomas, while touched upon here, are detailed in the final two articles in this supplement.
HISTOLOGIC GRADING AND THERAPY IMPLICATIONS
The prognosis of soft-tissue sarcomas correlates with histopathologic grade, and a three-grade system appears to be more accurate than a two-grade system.3 In general, low-grade lesions (grade 1) are unlikely to metastasize and are therefore less likely to need treatment with chemotherapy or radiation, as the risks of these therapies would most likely outweigh any benefit in terms of local control.
Specifically, the risk of radiation involves debilitation of local wound healing and the chance of dedifferentiation of low-grade lesions to higher-grade lesions with more metastatic potential. Grade 2 and 3 lesions are usually considered high-grade and are more likely to be treated with radiation and chemotherapy. Radiation is frequently used in patients with high-grade lesions when anticipated margins or actual margins are less than 1 cm.4–6
Chemotherapy’s lack of proven efficacy for soft-tissue sarcomas likely stems from poor understanding of the pathophysiology, molecular biology, and even some aspects of the natural history of these uncommon and heterogeneous tumors. There are more than 50 subtypes of soft-tissue sarcoma,7,8 and this heterogeneity has likely contributed to the difficulty of identifying chemotherapeutic agents that are highly active against these diseases.9
THE ROLE OF FAMILIAL GENETICS
Developing effective chemotherapeutic strategies may depend on grouping soft-tissue sarcomas more homogeneously. To compare like lesions with like lesions, molecular analysis and even molecular signatures may be of assistance. Along these lines, critical mutations and translocations have been described for several soft-tissue sarcoma subtypes.
Li-Fraumeni syndrome is an autosomal dominant cancer predisposition syndrome caused by germline mutations (ie, in every cell) in the p53 gene.10 Patients with Li-Fraumeni syndrome have an increased risk of developing soft-tissue sarcomas.1,11
Neurofibromatosis type 1 is caused by germline mutations in the NF1 gene, and malignant peripheral nerve sheath tumors occur within neurofibromas in neurofibromatosis patients and typically have additional mutations in CDKN2A or p53.9 INI1 loss is seen in all cases of extrarenal rhabdoid tumors and has been reported in a subset of epithelioid sarcomas (those occurring in proximal/axial regions).9,12 Delineation and greater understanding of these genetic abnormalities may lead to more effective medical therapy.
EVALUATION OF SUSPICIOUS SOFT-TISSUE MASSES
Figure 1 presents in flow chart form our general approach to the evaluation and management of patients with a soft-tissue mass suspicious for sarcoma—an approach detailed in the text below.
History and physical examination
Patients with soft-tissue sarcomas present with a mass that generally is increasing in size. The location and depth of the mass can be assessed on physical examination. In general, the deeper the mass, the more likely it is to be a sarcoma.13 Unlike bone sarcomas, soft-tissue sarcomas frequently are not associated with pain, so lack of pain does not make a mass more likely to be benign. In general, the only way to be sure that a mass is not malignant is to biopsy it. However, there are certain symptoms and signs that make a benign diagnosis much more likely. For example, very soft superficial masses that have not changed in size in years tend to be benign lipomas, and discolored lesions that go away with elevation of the affected body part tend to be hemangiomas.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is the primary imaging method for soft-tissue sarcomas. The benignity of a lesion such as a lipoma or hemangioma may be able to be determined with high certainty on MRI, in which case we call the imaging of the lesion “determinate.” Such lesions with determinate imaging (often referred to as “determinate lesions”) usually do not require a biopsy. However, the nature and identity of most lesions cannot be determined by MRI; although the MRI is still useful to help plan the biopsy in these cases, these lesions are termed “indeterminate” by MRI and should usually be biopsied.
Lesions that can be deemed determinate and usually be diagnosed as benign based on MRI findings include lipomas, hemangiomas, granuloma annulare, and ganglion cysts. However, most other soft-tissue lesions are indeterminate on MRI and, except in rare circumstances, require a biopsy to determine what they are and how they should be treated.
BIOPSY
The primary biopsy procedures for soft-tissue sarcomas are needle or open biopsy techniques and, in general, are similar to those for bone sarcomas, as reviewed in the previous article in this supplement. Regardless of the biopsy technique, hemostasis must be meticulous and patients are generally advised to not use the affected limb for at least several days after the biopsy to reduce the risk of a cancer cell–laden hematoma. It is preferable for the biopsy to be performed by or in consultation with the surgeon who will do the resection, if required.
Avoid transverse incisions
Lymph node biopsies
Lymph node biopsies are not generally indicated in patients with soft-tissue sarcoma. However, lymph node assessment and management should be considered in cases of clear cell sarcoma, epithelioid sarcoma, angiosarcoma, and embryonal/alveolar rhabdomyosarcoma, each of which has a greater than 10% incidence of lymph node metastasis.14 In this subset of soft-tissue sarcomas, a 5-year survival rate of 46% has been reported with therapeutic lymphadenectomy with curative intent versus nearly 0% with no lymphadenectomy or noncurative lymphadenectomy.14
Our approach in these sarcomas that go to the lymph nodes with increased relative frequency has been to first resect the sarcoma and then, after the margin is determined to be negative on the permanent pathology report, to schedule a nuclear medicine radiotracer study to analyze the drainage of the surgical bed. With this information we take the patient to the operating room and assess the location of the sentinel node (ie, node with the highest level of activity) through the skin using a radioactive counter with a sterile probe. We then make an incision in this area and find the lymph node. Upon removal of the “hot” lymph node, we reassess the radioactivity of the resected node and its node bed to be sure that we have the sentinel node. If this node or any node in the dissection has tumor in it, we do a therapeutic lymphadenectomy to remove all the lymph nodes in the area. For example, in the lower leg the lymphatic drainage is to the popliteal area, the inguinal area, or both. In the lower arm the lymphatic drainage is to the epitrochlear area and the axilla.
RESECTION
The resection surgery involves careful preoperative planning, almost always with an MRI and subsequent review by musculoskeletal tumor radiologists. In the operating room, general anesthesia is preferred to avoid ineffective blocks or overly effective blocks, which prevent neurologic examination immediately after the operation. If the functional loss is not too great, resection of the entire muscle or muscles involved is performed. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), then these structures are spared. If arteries are encased, the vessels are bypassed and the encased structure is left with the resection specimen. If the tumor is adjacent to but not encasing the neurovascular structures, the best course is to discuss with the radiation oncology team whether they prefer preoperative or postoperative radiation therapy. In general, for a high-grade lesion with adjacent neurovascular structues and no plane between the tumor and these structures, we ask our radiation oncologist colleagues to see the patient and discuss preoperative or perioperative (brachytherapy) radiation therapy. Postoperatively, where there is less than a 1-cm margin with no fascial boundary, we generally recommend radiation.
Margins
In our experience, margins of 1 cm or greater or resections with a fascial boundary are adequate and will leave patients with a much lower than 10% risk of recurrence. Others have postulated that margins that are smaller than this can have a very low rate of recurrence if perioperative (preoperative, intraoperative, or postoperative) radiation is given (personal communication from Drs. Jeffrey Eckardt and Dempsey Springfield). However, no well-controlled study has demonstrated how close the margin can be while still achieving an acceptable recurrence rate, and such a study would be very hard to perform given the rarity and heterogeneity of soft-tissue sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery leads to recurrence
Intralesional surgery will always lead to recurrence if the lesion is truly a soft-tissue sarcoma, even in spite of radiation therapy, chemotherapy, or both. Myomectomy and compartmental resections are frequently necessary to achieve a negative margin (normal tissue around the entire resection specimen). If intralesional surgery has been performed at an outside institution, we have generally recommended resection of the tumor bed, and in our experience this has reduced the recurrence rate after intralesional surgery to levels near those obtained when we perform the biopsy. In our experience, intralesional surgery without tumor bed resection will result in recurrence in nearly every case.
Reconstruction
Postoperative reconstruction of the defect involves closure of the fascia and skin with minimal tension, if possible. If there is tension, a vacuum-assisted closure dressing is placed on the wound and the patient returns for definitive closure, usually with a muscle flap. If the flap is a straightforward rotational flap, such as a medial gastrocnemius, or if only a split-thickness skin graft is required because there is healthy muscle in the floor of the open wound, this can be performed by experienced orthopedic surgeons. If these straightforward solutions are not possible, consultation with plastic surgeons is required, and they will cover the area with a complex rotational flap or, occasionally, with a free flap. For split-thickness skin grafts, it is prudent to make certain that the width of a #15 knife blade can pass between the blade and the housing of the Padgett dermatome and to take the skin from the extremity ipsilateral to the sarcoma (even with negative margins) to ensure that skin will not be contaminated with errant sarcoma cells.
Reconstruction following sarcoma resection is discussed in further detail in the next article in this supplement.
OUTCOMES AND FOLLOW-UP
The recurrence rate for soft-tissue sarcomas resected at Cleveland Clinic over the past 15 years has been less than 10%. This rate is comparable to the rates at other institutions that perform a high volume of sarcoma resections, but at institutions without a group dedicated to these procedures or without substantial experience in them, the recurrence rate is much higher, particularly with positive margins.15
Cure for soft-tissue sarcomas depends on being disease-free not only locally but also systemically. Most metastases from soft-tissue sarcomas are to the lung and, less commonly (as noted above), the lymph nodes. We assess local recurrence and metastatic disease at 3-month intervals for the first 2 years. Among patients who are disease-free at 2 years after the definitive surgery, the cure rate is 80% to 85%. After 2 years, we assess patients for presence of disease at 6-month intervals for the next 3 years and at yearly intervals thereafter.
Patients who have a recurrence are at increased risk for metastatic disease, and it is often very hard to achieve local control, as these patients frequently have had tumor contamination of the wound. At that point, unless the entire wound is excised or an amputation is performed, recurrences will continue. A nomogram has been validated for evaluating 10-year soft-tissue sarcoma–specific survival16 and is freely available at www.nomograms.org.
FUTURE DIRECTIONS
Future research challenges in this area include breaking down soft-tissue sarcoma subgroups more homogeneously, possibly with genetic markers, to better determine which lesions might benefit from chemotherapy. The goal of improved subtyping is to decrease the metastatic rate of soft-tissue sarcomas in much the same manner that directed chemotherapy has improved the metastasis and cure rates for patients with Ewing sarcoma and osteosarcoma.
- Simon MA, Springfield D, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Glencross J, Balasubramanian SP, Bacon J, Robinson MH, Reed MW. An audit of the management of soft tissue sarcoma within a health region in the UK. Eur J Surg Oncol 2003; 29:670–675.
- Kandel RA, Bell RS, Wunder JS, et al. Comparison between a 2- and 3-grade system in predicting metastatic-free survival in extremity soft-tissue sarcoma. J Surg Oncol 1999; 72:77–82.
- Suit HD, Spiro I. Role of radiation in the management of adult patients with sarcoma of soft tissue. Semin Surg Oncol 1994; 10:347–356.
- Suit H, Spiro I. Preoperative radiation therapy for patients with sarcoma of the soft tissues. Cancer Treat Res 1993; 67:99–105.
- Suit HD, Mankin HJ, Wood WC, et al. Treatment of the patient with stage M0 soft tissue sarcoma. J Clin Oncol 1988; 6:854–862.
- Fletcher CDM, Unni KK, Mertens F. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002.
- Brennan MF, Singer S, Maki RG, O’Sullivan B. Sarcomas of the soft tissue and bone. In: DeVita Jr VT, Lawrence TS, Rosenbert SA, eds. Cancer: Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
- Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Gonzalez KD, Buzin CH, Noltner KA, et al. High frequency of de novo mutations in Li-Fraumeni syndrome. J Med Genet 2009; 46:689–693.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- Modena P, Lualdi E, Facchinetti F, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res 2005; 65:4012–4019.
- Peabody TD, Simon MA. Principles of staging of soft-tissue sarcomas. Clin Orthop Relat Res 1993; 289:19–31.
- Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults: analysis of data from a prospective database of 1,772 sarcoma patients. Ann Surg 1993; 217:72–77.
- Potter BK, Adams SC, Pitcher JD Jr, Temple HT. Local recurrence of disease after unplanned excisions of high-grade soft tissue sarcomas. Clin Orthop Relat Res 2008; 466:3093–3100.
- Mariani L, Miceli R, Kattan MW, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer 2005; 103:402–408.
Soft-tissue sarcomas are tumors of the mesenchymal system, and half develop in the extremities.1 Although patients with soft-tissue sarcomas have been treated with a combination of surgery, radiation therapy, and chemotherapy, it remains unclear whether either radiation or chemotherapy improves outcomes for these patients. Soft-tissue sarcomas are therefore currently treated with surgical resection when possible, with or without chemotherapy or radiation.
Even though multimodal therapy for patients with these tumors is controversial, a multidisciplinary conference among the many providers who may be involved in the management these patients—orthopedic, medical, and radiation oncologists, as well as the referring primary care physician, plastic and reconstructive surgeons, physical therapists, and radiologists and pathologists with expertise in these tumors—is helpful.2 This article presents an overview of the management of these patients, with a focus on the mainstay treatment, surgical resection. The roles of chemotherapy and radiation therapy for soft-tissue sarcomas, while touched upon here, are detailed in the final two articles in this supplement.
HISTOLOGIC GRADING AND THERAPY IMPLICATIONS
The prognosis of soft-tissue sarcomas correlates with histopathologic grade, and a three-grade system appears to be more accurate than a two-grade system.3 In general, low-grade lesions (grade 1) are unlikely to metastasize and are therefore less likely to need treatment with chemotherapy or radiation, as the risks of these therapies would most likely outweigh any benefit in terms of local control.
Specifically, the risk of radiation involves debilitation of local wound healing and the chance of dedifferentiation of low-grade lesions to higher-grade lesions with more metastatic potential. Grade 2 and 3 lesions are usually considered high-grade and are more likely to be treated with radiation and chemotherapy. Radiation is frequently used in patients with high-grade lesions when anticipated margins or actual margins are less than 1 cm.4–6
Chemotherapy’s lack of proven efficacy for soft-tissue sarcomas likely stems from poor understanding of the pathophysiology, molecular biology, and even some aspects of the natural history of these uncommon and heterogeneous tumors. There are more than 50 subtypes of soft-tissue sarcoma,7,8 and this heterogeneity has likely contributed to the difficulty of identifying chemotherapeutic agents that are highly active against these diseases.9
THE ROLE OF FAMILIAL GENETICS
Developing effective chemotherapeutic strategies may depend on grouping soft-tissue sarcomas more homogeneously. To compare like lesions with like lesions, molecular analysis and even molecular signatures may be of assistance. Along these lines, critical mutations and translocations have been described for several soft-tissue sarcoma subtypes.
Li-Fraumeni syndrome is an autosomal dominant cancer predisposition syndrome caused by germline mutations (ie, in every cell) in the p53 gene.10 Patients with Li-Fraumeni syndrome have an increased risk of developing soft-tissue sarcomas.1,11
Neurofibromatosis type 1 is caused by germline mutations in the NF1 gene, and malignant peripheral nerve sheath tumors occur within neurofibromas in neurofibromatosis patients and typically have additional mutations in CDKN2A or p53.9 INI1 loss is seen in all cases of extrarenal rhabdoid tumors and has been reported in a subset of epithelioid sarcomas (those occurring in proximal/axial regions).9,12 Delineation and greater understanding of these genetic abnormalities may lead to more effective medical therapy.
EVALUATION OF SUSPICIOUS SOFT-TISSUE MASSES
Figure 1 presents in flow chart form our general approach to the evaluation and management of patients with a soft-tissue mass suspicious for sarcoma—an approach detailed in the text below.
History and physical examination
Patients with soft-tissue sarcomas present with a mass that generally is increasing in size. The location and depth of the mass can be assessed on physical examination. In general, the deeper the mass, the more likely it is to be a sarcoma.13 Unlike bone sarcomas, soft-tissue sarcomas frequently are not associated with pain, so lack of pain does not make a mass more likely to be benign. In general, the only way to be sure that a mass is not malignant is to biopsy it. However, there are certain symptoms and signs that make a benign diagnosis much more likely. For example, very soft superficial masses that have not changed in size in years tend to be benign lipomas, and discolored lesions that go away with elevation of the affected body part tend to be hemangiomas.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is the primary imaging method for soft-tissue sarcomas. The benignity of a lesion such as a lipoma or hemangioma may be able to be determined with high certainty on MRI, in which case we call the imaging of the lesion “determinate.” Such lesions with determinate imaging (often referred to as “determinate lesions”) usually do not require a biopsy. However, the nature and identity of most lesions cannot be determined by MRI; although the MRI is still useful to help plan the biopsy in these cases, these lesions are termed “indeterminate” by MRI and should usually be biopsied.
Lesions that can be deemed determinate and usually be diagnosed as benign based on MRI findings include lipomas, hemangiomas, granuloma annulare, and ganglion cysts. However, most other soft-tissue lesions are indeterminate on MRI and, except in rare circumstances, require a biopsy to determine what they are and how they should be treated.
BIOPSY
The primary biopsy procedures for soft-tissue sarcomas are needle or open biopsy techniques and, in general, are similar to those for bone sarcomas, as reviewed in the previous article in this supplement. Regardless of the biopsy technique, hemostasis must be meticulous and patients are generally advised to not use the affected limb for at least several days after the biopsy to reduce the risk of a cancer cell–laden hematoma. It is preferable for the biopsy to be performed by or in consultation with the surgeon who will do the resection, if required.
Avoid transverse incisions
Lymph node biopsies
Lymph node biopsies are not generally indicated in patients with soft-tissue sarcoma. However, lymph node assessment and management should be considered in cases of clear cell sarcoma, epithelioid sarcoma, angiosarcoma, and embryonal/alveolar rhabdomyosarcoma, each of which has a greater than 10% incidence of lymph node metastasis.14 In this subset of soft-tissue sarcomas, a 5-year survival rate of 46% has been reported with therapeutic lymphadenectomy with curative intent versus nearly 0% with no lymphadenectomy or noncurative lymphadenectomy.14
Our approach in these sarcomas that go to the lymph nodes with increased relative frequency has been to first resect the sarcoma and then, after the margin is determined to be negative on the permanent pathology report, to schedule a nuclear medicine radiotracer study to analyze the drainage of the surgical bed. With this information we take the patient to the operating room and assess the location of the sentinel node (ie, node with the highest level of activity) through the skin using a radioactive counter with a sterile probe. We then make an incision in this area and find the lymph node. Upon removal of the “hot” lymph node, we reassess the radioactivity of the resected node and its node bed to be sure that we have the sentinel node. If this node or any node in the dissection has tumor in it, we do a therapeutic lymphadenectomy to remove all the lymph nodes in the area. For example, in the lower leg the lymphatic drainage is to the popliteal area, the inguinal area, or both. In the lower arm the lymphatic drainage is to the epitrochlear area and the axilla.
RESECTION
The resection surgery involves careful preoperative planning, almost always with an MRI and subsequent review by musculoskeletal tumor radiologists. In the operating room, general anesthesia is preferred to avoid ineffective blocks or overly effective blocks, which prevent neurologic examination immediately after the operation. If the functional loss is not too great, resection of the entire muscle or muscles involved is performed. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), then these structures are spared. If arteries are encased, the vessels are bypassed and the encased structure is left with the resection specimen. If the tumor is adjacent to but not encasing the neurovascular structures, the best course is to discuss with the radiation oncology team whether they prefer preoperative or postoperative radiation therapy. In general, for a high-grade lesion with adjacent neurovascular structues and no plane between the tumor and these structures, we ask our radiation oncologist colleagues to see the patient and discuss preoperative or perioperative (brachytherapy) radiation therapy. Postoperatively, where there is less than a 1-cm margin with no fascial boundary, we generally recommend radiation.
Margins
In our experience, margins of 1 cm or greater or resections with a fascial boundary are adequate and will leave patients with a much lower than 10% risk of recurrence. Others have postulated that margins that are smaller than this can have a very low rate of recurrence if perioperative (preoperative, intraoperative, or postoperative) radiation is given (personal communication from Drs. Jeffrey Eckardt and Dempsey Springfield). However, no well-controlled study has demonstrated how close the margin can be while still achieving an acceptable recurrence rate, and such a study would be very hard to perform given the rarity and heterogeneity of soft-tissue sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery leads to recurrence
Intralesional surgery will always lead to recurrence if the lesion is truly a soft-tissue sarcoma, even in spite of radiation therapy, chemotherapy, or both. Myomectomy and compartmental resections are frequently necessary to achieve a negative margin (normal tissue around the entire resection specimen). If intralesional surgery has been performed at an outside institution, we have generally recommended resection of the tumor bed, and in our experience this has reduced the recurrence rate after intralesional surgery to levels near those obtained when we perform the biopsy. In our experience, intralesional surgery without tumor bed resection will result in recurrence in nearly every case.
Reconstruction
Postoperative reconstruction of the defect involves closure of the fascia and skin with minimal tension, if possible. If there is tension, a vacuum-assisted closure dressing is placed on the wound and the patient returns for definitive closure, usually with a muscle flap. If the flap is a straightforward rotational flap, such as a medial gastrocnemius, or if only a split-thickness skin graft is required because there is healthy muscle in the floor of the open wound, this can be performed by experienced orthopedic surgeons. If these straightforward solutions are not possible, consultation with plastic surgeons is required, and they will cover the area with a complex rotational flap or, occasionally, with a free flap. For split-thickness skin grafts, it is prudent to make certain that the width of a #15 knife blade can pass between the blade and the housing of the Padgett dermatome and to take the skin from the extremity ipsilateral to the sarcoma (even with negative margins) to ensure that skin will not be contaminated with errant sarcoma cells.
Reconstruction following sarcoma resection is discussed in further detail in the next article in this supplement.
OUTCOMES AND FOLLOW-UP
The recurrence rate for soft-tissue sarcomas resected at Cleveland Clinic over the past 15 years has been less than 10%. This rate is comparable to the rates at other institutions that perform a high volume of sarcoma resections, but at institutions without a group dedicated to these procedures or without substantial experience in them, the recurrence rate is much higher, particularly with positive margins.15
Cure for soft-tissue sarcomas depends on being disease-free not only locally but also systemically. Most metastases from soft-tissue sarcomas are to the lung and, less commonly (as noted above), the lymph nodes. We assess local recurrence and metastatic disease at 3-month intervals for the first 2 years. Among patients who are disease-free at 2 years after the definitive surgery, the cure rate is 80% to 85%. After 2 years, we assess patients for presence of disease at 6-month intervals for the next 3 years and at yearly intervals thereafter.
Patients who have a recurrence are at increased risk for metastatic disease, and it is often very hard to achieve local control, as these patients frequently have had tumor contamination of the wound. At that point, unless the entire wound is excised or an amputation is performed, recurrences will continue. A nomogram has been validated for evaluating 10-year soft-tissue sarcoma–specific survival16 and is freely available at www.nomograms.org.
FUTURE DIRECTIONS
Future research challenges in this area include breaking down soft-tissue sarcoma subgroups more homogeneously, possibly with genetic markers, to better determine which lesions might benefit from chemotherapy. The goal of improved subtyping is to decrease the metastatic rate of soft-tissue sarcomas in much the same manner that directed chemotherapy has improved the metastasis and cure rates for patients with Ewing sarcoma and osteosarcoma.
Soft-tissue sarcomas are tumors of the mesenchymal system, and half develop in the extremities.1 Although patients with soft-tissue sarcomas have been treated with a combination of surgery, radiation therapy, and chemotherapy, it remains unclear whether either radiation or chemotherapy improves outcomes for these patients. Soft-tissue sarcomas are therefore currently treated with surgical resection when possible, with or without chemotherapy or radiation.
Even though multimodal therapy for patients with these tumors is controversial, a multidisciplinary conference among the many providers who may be involved in the management these patients—orthopedic, medical, and radiation oncologists, as well as the referring primary care physician, plastic and reconstructive surgeons, physical therapists, and radiologists and pathologists with expertise in these tumors—is helpful.2 This article presents an overview of the management of these patients, with a focus on the mainstay treatment, surgical resection. The roles of chemotherapy and radiation therapy for soft-tissue sarcomas, while touched upon here, are detailed in the final two articles in this supplement.
HISTOLOGIC GRADING AND THERAPY IMPLICATIONS
The prognosis of soft-tissue sarcomas correlates with histopathologic grade, and a three-grade system appears to be more accurate than a two-grade system.3 In general, low-grade lesions (grade 1) are unlikely to metastasize and are therefore less likely to need treatment with chemotherapy or radiation, as the risks of these therapies would most likely outweigh any benefit in terms of local control.
Specifically, the risk of radiation involves debilitation of local wound healing and the chance of dedifferentiation of low-grade lesions to higher-grade lesions with more metastatic potential. Grade 2 and 3 lesions are usually considered high-grade and are more likely to be treated with radiation and chemotherapy. Radiation is frequently used in patients with high-grade lesions when anticipated margins or actual margins are less than 1 cm.4–6
Chemotherapy’s lack of proven efficacy for soft-tissue sarcomas likely stems from poor understanding of the pathophysiology, molecular biology, and even some aspects of the natural history of these uncommon and heterogeneous tumors. There are more than 50 subtypes of soft-tissue sarcoma,7,8 and this heterogeneity has likely contributed to the difficulty of identifying chemotherapeutic agents that are highly active against these diseases.9
THE ROLE OF FAMILIAL GENETICS
Developing effective chemotherapeutic strategies may depend on grouping soft-tissue sarcomas more homogeneously. To compare like lesions with like lesions, molecular analysis and even molecular signatures may be of assistance. Along these lines, critical mutations and translocations have been described for several soft-tissue sarcoma subtypes.
Li-Fraumeni syndrome is an autosomal dominant cancer predisposition syndrome caused by germline mutations (ie, in every cell) in the p53 gene.10 Patients with Li-Fraumeni syndrome have an increased risk of developing soft-tissue sarcomas.1,11
Neurofibromatosis type 1 is caused by germline mutations in the NF1 gene, and malignant peripheral nerve sheath tumors occur within neurofibromas in neurofibromatosis patients and typically have additional mutations in CDKN2A or p53.9 INI1 loss is seen in all cases of extrarenal rhabdoid tumors and has been reported in a subset of epithelioid sarcomas (those occurring in proximal/axial regions).9,12 Delineation and greater understanding of these genetic abnormalities may lead to more effective medical therapy.
EVALUATION OF SUSPICIOUS SOFT-TISSUE MASSES
Figure 1 presents in flow chart form our general approach to the evaluation and management of patients with a soft-tissue mass suspicious for sarcoma—an approach detailed in the text below.
History and physical examination
Patients with soft-tissue sarcomas present with a mass that generally is increasing in size. The location and depth of the mass can be assessed on physical examination. In general, the deeper the mass, the more likely it is to be a sarcoma.13 Unlike bone sarcomas, soft-tissue sarcomas frequently are not associated with pain, so lack of pain does not make a mass more likely to be benign. In general, the only way to be sure that a mass is not malignant is to biopsy it. However, there are certain symptoms and signs that make a benign diagnosis much more likely. For example, very soft superficial masses that have not changed in size in years tend to be benign lipomas, and discolored lesions that go away with elevation of the affected body part tend to be hemangiomas.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is the primary imaging method for soft-tissue sarcomas. The benignity of a lesion such as a lipoma or hemangioma may be able to be determined with high certainty on MRI, in which case we call the imaging of the lesion “determinate.” Such lesions with determinate imaging (often referred to as “determinate lesions”) usually do not require a biopsy. However, the nature and identity of most lesions cannot be determined by MRI; although the MRI is still useful to help plan the biopsy in these cases, these lesions are termed “indeterminate” by MRI and should usually be biopsied.
Lesions that can be deemed determinate and usually be diagnosed as benign based on MRI findings include lipomas, hemangiomas, granuloma annulare, and ganglion cysts. However, most other soft-tissue lesions are indeterminate on MRI and, except in rare circumstances, require a biopsy to determine what they are and how they should be treated.
BIOPSY
The primary biopsy procedures for soft-tissue sarcomas are needle or open biopsy techniques and, in general, are similar to those for bone sarcomas, as reviewed in the previous article in this supplement. Regardless of the biopsy technique, hemostasis must be meticulous and patients are generally advised to not use the affected limb for at least several days after the biopsy to reduce the risk of a cancer cell–laden hematoma. It is preferable for the biopsy to be performed by or in consultation with the surgeon who will do the resection, if required.
Avoid transverse incisions
Lymph node biopsies
Lymph node biopsies are not generally indicated in patients with soft-tissue sarcoma. However, lymph node assessment and management should be considered in cases of clear cell sarcoma, epithelioid sarcoma, angiosarcoma, and embryonal/alveolar rhabdomyosarcoma, each of which has a greater than 10% incidence of lymph node metastasis.14 In this subset of soft-tissue sarcomas, a 5-year survival rate of 46% has been reported with therapeutic lymphadenectomy with curative intent versus nearly 0% with no lymphadenectomy or noncurative lymphadenectomy.14
Our approach in these sarcomas that go to the lymph nodes with increased relative frequency has been to first resect the sarcoma and then, after the margin is determined to be negative on the permanent pathology report, to schedule a nuclear medicine radiotracer study to analyze the drainage of the surgical bed. With this information we take the patient to the operating room and assess the location of the sentinel node (ie, node with the highest level of activity) through the skin using a radioactive counter with a sterile probe. We then make an incision in this area and find the lymph node. Upon removal of the “hot” lymph node, we reassess the radioactivity of the resected node and its node bed to be sure that we have the sentinel node. If this node or any node in the dissection has tumor in it, we do a therapeutic lymphadenectomy to remove all the lymph nodes in the area. For example, in the lower leg the lymphatic drainage is to the popliteal area, the inguinal area, or both. In the lower arm the lymphatic drainage is to the epitrochlear area and the axilla.
RESECTION
The resection surgery involves careful preoperative planning, almost always with an MRI and subsequent review by musculoskeletal tumor radiologists. In the operating room, general anesthesia is preferred to avoid ineffective blocks or overly effective blocks, which prevent neurologic examination immediately after the operation. If the functional loss is not too great, resection of the entire muscle or muscles involved is performed. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), then these structures are spared. If arteries are encased, the vessels are bypassed and the encased structure is left with the resection specimen. If the tumor is adjacent to but not encasing the neurovascular structures, the best course is to discuss with the radiation oncology team whether they prefer preoperative or postoperative radiation therapy. In general, for a high-grade lesion with adjacent neurovascular structues and no plane between the tumor and these structures, we ask our radiation oncologist colleagues to see the patient and discuss preoperative or perioperative (brachytherapy) radiation therapy. Postoperatively, where there is less than a 1-cm margin with no fascial boundary, we generally recommend radiation.
Margins
In our experience, margins of 1 cm or greater or resections with a fascial boundary are adequate and will leave patients with a much lower than 10% risk of recurrence. Others have postulated that margins that are smaller than this can have a very low rate of recurrence if perioperative (preoperative, intraoperative, or postoperative) radiation is given (personal communication from Drs. Jeffrey Eckardt and Dempsey Springfield). However, no well-controlled study has demonstrated how close the margin can be while still achieving an acceptable recurrence rate, and such a study would be very hard to perform given the rarity and heterogeneity of soft-tissue sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery leads to recurrence
Intralesional surgery will always lead to recurrence if the lesion is truly a soft-tissue sarcoma, even in spite of radiation therapy, chemotherapy, or both. Myomectomy and compartmental resections are frequently necessary to achieve a negative margin (normal tissue around the entire resection specimen). If intralesional surgery has been performed at an outside institution, we have generally recommended resection of the tumor bed, and in our experience this has reduced the recurrence rate after intralesional surgery to levels near those obtained when we perform the biopsy. In our experience, intralesional surgery without tumor bed resection will result in recurrence in nearly every case.
Reconstruction
Postoperative reconstruction of the defect involves closure of the fascia and skin with minimal tension, if possible. If there is tension, a vacuum-assisted closure dressing is placed on the wound and the patient returns for definitive closure, usually with a muscle flap. If the flap is a straightforward rotational flap, such as a medial gastrocnemius, or if only a split-thickness skin graft is required because there is healthy muscle in the floor of the open wound, this can be performed by experienced orthopedic surgeons. If these straightforward solutions are not possible, consultation with plastic surgeons is required, and they will cover the area with a complex rotational flap or, occasionally, with a free flap. For split-thickness skin grafts, it is prudent to make certain that the width of a #15 knife blade can pass between the blade and the housing of the Padgett dermatome and to take the skin from the extremity ipsilateral to the sarcoma (even with negative margins) to ensure that skin will not be contaminated with errant sarcoma cells.
Reconstruction following sarcoma resection is discussed in further detail in the next article in this supplement.
OUTCOMES AND FOLLOW-UP
The recurrence rate for soft-tissue sarcomas resected at Cleveland Clinic over the past 15 years has been less than 10%. This rate is comparable to the rates at other institutions that perform a high volume of sarcoma resections, but at institutions without a group dedicated to these procedures or without substantial experience in them, the recurrence rate is much higher, particularly with positive margins.15
Cure for soft-tissue sarcomas depends on being disease-free not only locally but also systemically. Most metastases from soft-tissue sarcomas are to the lung and, less commonly (as noted above), the lymph nodes. We assess local recurrence and metastatic disease at 3-month intervals for the first 2 years. Among patients who are disease-free at 2 years after the definitive surgery, the cure rate is 80% to 85%. After 2 years, we assess patients for presence of disease at 6-month intervals for the next 3 years and at yearly intervals thereafter.
Patients who have a recurrence are at increased risk for metastatic disease, and it is often very hard to achieve local control, as these patients frequently have had tumor contamination of the wound. At that point, unless the entire wound is excised or an amputation is performed, recurrences will continue. A nomogram has been validated for evaluating 10-year soft-tissue sarcoma–specific survival16 and is freely available at www.nomograms.org.
FUTURE DIRECTIONS
Future research challenges in this area include breaking down soft-tissue sarcoma subgroups more homogeneously, possibly with genetic markers, to better determine which lesions might benefit from chemotherapy. The goal of improved subtyping is to decrease the metastatic rate of soft-tissue sarcomas in much the same manner that directed chemotherapy has improved the metastasis and cure rates for patients with Ewing sarcoma and osteosarcoma.
- Simon MA, Springfield D, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Glencross J, Balasubramanian SP, Bacon J, Robinson MH, Reed MW. An audit of the management of soft tissue sarcoma within a health region in the UK. Eur J Surg Oncol 2003; 29:670–675.
- Kandel RA, Bell RS, Wunder JS, et al. Comparison between a 2- and 3-grade system in predicting metastatic-free survival in extremity soft-tissue sarcoma. J Surg Oncol 1999; 72:77–82.
- Suit HD, Spiro I. Role of radiation in the management of adult patients with sarcoma of soft tissue. Semin Surg Oncol 1994; 10:347–356.
- Suit H, Spiro I. Preoperative radiation therapy for patients with sarcoma of the soft tissues. Cancer Treat Res 1993; 67:99–105.
- Suit HD, Mankin HJ, Wood WC, et al. Treatment of the patient with stage M0 soft tissue sarcoma. J Clin Oncol 1988; 6:854–862.
- Fletcher CDM, Unni KK, Mertens F. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002.
- Brennan MF, Singer S, Maki RG, O’Sullivan B. Sarcomas of the soft tissue and bone. In: DeVita Jr VT, Lawrence TS, Rosenbert SA, eds. Cancer: Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
- Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Gonzalez KD, Buzin CH, Noltner KA, et al. High frequency of de novo mutations in Li-Fraumeni syndrome. J Med Genet 2009; 46:689–693.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- Modena P, Lualdi E, Facchinetti F, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res 2005; 65:4012–4019.
- Peabody TD, Simon MA. Principles of staging of soft-tissue sarcomas. Clin Orthop Relat Res 1993; 289:19–31.
- Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults: analysis of data from a prospective database of 1,772 sarcoma patients. Ann Surg 1993; 217:72–77.
- Potter BK, Adams SC, Pitcher JD Jr, Temple HT. Local recurrence of disease after unplanned excisions of high-grade soft tissue sarcomas. Clin Orthop Relat Res 2008; 466:3093–3100.
- Mariani L, Miceli R, Kattan MW, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer 2005; 103:402–408.
- Simon MA, Springfield D, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Glencross J, Balasubramanian SP, Bacon J, Robinson MH, Reed MW. An audit of the management of soft tissue sarcoma within a health region in the UK. Eur J Surg Oncol 2003; 29:670–675.
- Kandel RA, Bell RS, Wunder JS, et al. Comparison between a 2- and 3-grade system in predicting metastatic-free survival in extremity soft-tissue sarcoma. J Surg Oncol 1999; 72:77–82.
- Suit HD, Spiro I. Role of radiation in the management of adult patients with sarcoma of soft tissue. Semin Surg Oncol 1994; 10:347–356.
- Suit H, Spiro I. Preoperative radiation therapy for patients with sarcoma of the soft tissues. Cancer Treat Res 1993; 67:99–105.
- Suit HD, Mankin HJ, Wood WC, et al. Treatment of the patient with stage M0 soft tissue sarcoma. J Clin Oncol 1988; 6:854–862.
- Fletcher CDM, Unni KK, Mertens F. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002.
- Brennan MF, Singer S, Maki RG, O’Sullivan B. Sarcomas of the soft tissue and bone. In: DeVita Jr VT, Lawrence TS, Rosenbert SA, eds. Cancer: Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
- Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Gonzalez KD, Buzin CH, Noltner KA, et al. High frequency of de novo mutations in Li-Fraumeni syndrome. J Med Genet 2009; 46:689–693.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- Modena P, Lualdi E, Facchinetti F, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res 2005; 65:4012–4019.
- Peabody TD, Simon MA. Principles of staging of soft-tissue sarcomas. Clin Orthop Relat Res 1993; 289:19–31.
- Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults: analysis of data from a prospective database of 1,772 sarcoma patients. Ann Surg 1993; 217:72–77.
- Potter BK, Adams SC, Pitcher JD Jr, Temple HT. Local recurrence of disease after unplanned excisions of high-grade soft tissue sarcomas. Clin Orthop Relat Res 2008; 466:3093–3100.
- Mariani L, Miceli R, Kattan MW, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer 2005; 103:402–408.
Considerations surrounding reconstruction after resection of musculoskeletal sarcomas
Advances in the management of soft-tissue and bone sarcomas—referred to collectively as “musculoskeletal sarcomas” hereafter—have resulted in significant improvements in survival and quality of life.1–3 Several factors have likely contributed to these advances, including improved surgical technique and the development of referral centers for sarcoma treatment that have embraced a multidisciplinary approach.1,2
The goal of treatment for musculoskeletal sarcomas is to optimize oncologic outcome and maximize functional restoration.2,3 Surgical resection has been the mainstay of therapy,1–7 as detailed earlier in this supplement. In patients with musculoskeletal sarcomas of the extremities, limb-sparing resection has been shown to be significantly superior to amputation.1,7–9 Wide local excision of the tumor along with its muscle compartment, followed by adjuvant chemotherapy and radiation therapy, has allowed limb salvage without an increased risk of recurrence in many patients.3 However, wide tumor resection can leave large defects that are not amenable to coverage by mobilization of the surrounding tissues, particularly if those tissues have been irradiated. As a result, resection can expose neurovascular structures, bone without periosteum, alloplastic materials, and internal fixation devices.
GOALS OF RECONSTRUCTION
Reconstructive surgery after musculoskeletal sarcoma resection aims to provide adequate wound coverage, preserve function, and optimize the cosmetic outcome.1–3 Tumors can be found on areas crucial to limb movement or may involve tissues vital to limb function. Reconstruction to repair these deficits can take many forms. In certain situations, amputation is still inevitable. In those cases, the reconstruction should provide stable stump coverage with durability and the ability to fit well with an external prosthesis.3
TIMING OF RECONSTRUCTION
Immediate reconstruction should be pursued if possible
Immediate reconstruction after a negative margin should always be considered and should be attempted when possible. Immediate reconstruction allows the reconstructive surgeon to benefit from better evaluation of the defect and exposed structures, as no scar tissue is present to distort the anatomy. Likewise, patients benefit from faster recovery and can receive adjuvant treatment (if necessary) sooner, as well as earlier rehabilitation. Patients may also benefit psychologically from immediate reconstruction.1,3
Indications for delayed reconstruction
Delayed reconstruction is primarily indicated when there are wound healing problems or there is uncertainty about the tumor margins. Secondary indications for delayed reconstruction are wound dehiscence and unstable soft-tissue coverage. If hardware is exposed, the recommendation is for early intervention and wound coverage with well-vascularized tissue to protect and cover the implant or prosthesis.
What about radiation therapy?
A very important consideration in reconstruction is the need for neoadjuvant or adjuvant radiation therapy.3,10,11 Irradiated wounds have a higher incidence of complications, including a tendency to dehisce. In patients who have been previously irradiated, the best practice is to perform immediate reconstruction with well-vascularized tissue, most likely a free tissue transfer.4,6,11,12 This practice reduces hospital stay, costs, and morbidity and increases limb salvage and patient satisfaction.13
SYSTEMATIC PREOPERATIVE PLANNING NEEDED
Reconstruction after musculoskeletal sarcoma resection should be planned systematically within a process that involves preoperative anticipation of the defect size and the resulting functional and cosmetic deficits that might need to be addressed. A preoperative visit to the reconstructive surgeon can be very helpful for presurgical planning.
During surgery it is usually preferable to allow the surgeon doing the tumor resection (eg, surgical oncologist or orthopedic oncologist) to complete the resection because the dimensions of the defect are not certain until negative margins are obtained.14 If tumor margins are unclear at the time of initial resection, the surgeon should consider delaying the definitive reconstruction until the permanent sections confirm negative margins. Temporary closure can be achieved with wound dressings, skin grafts (either allograft or autograft), or negative-pressure wound therapy. In the same context, if neurovascular structures are exposed it is reasonable to use a muscle flap without “tailoring” the flap to the defect. This approach allows the flap to be advanced or repositioned in case of positive margins, and the skin graft can be applied to the muscle surface in a second procedure.3
RECONSTRUCTIVE METHODS: A BRIEF OVERVIEW
Several methods can be used to close musculoskeletal sarcoma excision defects. Smaller defects can be closed primarily, although most defects are large and not amenable to primary closure. If fascia or muscle is preserved with only the skin coverage missing, the wound can be covered with either split-thickness or full-thickness skin grafts.1,4,6 Split-thickness skin grafts can be obtained in larger amounts and often heal faster than full-thickness skin grafts. However, most resections will require durable tissue coverage, particularly if adjuvant radiation therapy is planned.
In the case of long bone sarcoma resection, the resulting defect is usually large and complex and the traditional reconstruction is based on avascular allografts and local tissue flaps. However, allografts are associated with high rates of infection, nonunion, and fracture, leading to failure in about 50% of cases. Microvascular free flaps that contain bone, such as free fibula flaps, have been used instead of allografts with good success rates.2
Lately there has been growing interest in the use of the vacuum-assisted closure device (a form of negative-pressure wound therapy) to promote wound healing. It has been shown to improve the granulation and healing of open wounds by absorbing moisture, as well as to promote adherence after skin grafting, thereby reducing the risk of graft displacement.1,3 This device can be used immediately after musculoskeletal sarcoma resection while definitive tumor margin results are pending. It also can be used to prepare the wound bed for grafting in high-risk patients who would not tolerate more complex reconstructions.
Local or adjacent fascial, fasciocutaneous, and dermal flaps can also be used in lower-extremity reconstruction. However, muscle or musculocutaneous flaps are the mainstay of reconstruction after resection of musculoskeletal sarcomas. This group also includes perforator flaps, which have grown in popularity in the last few years.1,3
LOCATION-BASED WOUND RECONSTRUCTION
Musculoskeletal sarcomas can occur in virtually any region of the body, and myriad reconstructive options are available for various body sites. Since lower-extremity musculoskeletal sarcomas represent about 75% of cases,1 we will focus mainly on reconstruction of the lower extremity.
Factors driving choice of flap
Selection of an appropriate flap is essential to an optimal outcome. Flaps should be chosen with regard to donor site morbidity, functional requirements, length and diameter of the vascular pedicle, and aesthetic outcome.3 Usually physical examination, palpation of peripheral pulses, and Doppler ultrasonography are sufficient to evaluate the circulation. A preoperative angiogram should be considered in patients with severe peripheral vascular disease or previous trauma, which can potentially compromise the reconstructive outcome.15
Each region of the lower extremity possesses unique anatomic and functional characteristics that must be evaluated. It is useful to categorize the thigh, lower leg, and foot into separate anatomic units when planning reconstruction. We further divided these units into several subunits, as previously proposed by Sherman and Law15 and as outlined below.
Thigh
The thigh is usually well perfused and has several muscle groups, which facilitates reconstruction. Primary closure, skin grafts, or local flaps are acceptable options in most cases. The remaining musculature can be rotated or advanced to cover defects in the anterior or posterior thigh, providing bulk and adequate blood supply.
Hip and proximal/lateral thigh. Local muscle or myocutaneous flap options include tensor fascia lata, vastus lateralis, and rectus femoris flaps, all of which are based on the lateral circumflex femoral artery.
The tensor fascia lata flap is thin but has a long fascia extension that can be elevated from above the knee and can include a large skin paddle that is innervated by the lateral femoral cutaneous nerve. Some patients may experience knee instability after tensor fascia lata harvest.
The vastus lateralis muscle flap provides good bulk. Its arc of rotation reaches most of the inferior and posterior pelvis. It has little effect on ambulation.
The rectus femoris muscle flap is not so bulky, is easily mobilized, and has a wide arc of rotation. The donor site can be closed primarily. Harvest of this muscle can be associated with some strength loss during knee extension. For large defects of the upper third of the leg, a pedicled rectus abdominis muscle flap based on the deep inferior epigastric artery can be used. A vertically oriented skin island can be extended up to the costal margin, improving the reach. When the nature of the wound precludes use of pedicle flaps, free tissue transfer is indicated, with the latissimus dorsi muscle flap being used most commonly.15,16
Mid-thigh. Wounds in this location often can be closed with skin grafts or fasciocutaneous flaps. If the femur is exposed, however, a muscle flap will be required. As above, the tensor fascia lata, vastus lateralis, and rectus femoris can be used as flap options. If the lateral circumflex artery is unavailable, other flap options include the gracilis, vastus medialis, and rectus abdominis muscles. The gracilis muscle flap is based on the medial circumflex femoral artery and is useful for covering the medial aspect of the mid-thigh. Although this is a thin muscle, it can be used to cover long defects. The vastus medialis muscle flap is supplied by perforators from the profunda femoris and superficial femoral arteries. It can be rotated medially and advanced distally to cover patellar defects.
Supracondylar knee. The knee is a location where sarcoma resection is particularly likely to leave a defect with exposed bone, tendons, or ligaments that will need coverage. The gastrocnemius muscle flap combined with a split-thickness skin graft remains a consistent and reliable reconstructive option for this area. Other options are an extended medial gastrocnemius muscle flap or myocutaneous flap, which incorporates a random fasciocutaneous extension. For larger defects, free flaps should be considered, such as the anterior thigh flap, rectus abdominis muscle flap, or latissimus dorsi muscle flap. If tendons or ligaments need to be reconstructed, we favor autologous tissue, such as the fascia lata and plantaris tendons. These are easy to harvest and provide long-lasting joint stability.
Lower leg
Proximal third of the tibia. Defects here can usually be covered with a medial or lateral gastrocnemius muscle or myocutaneous flap, or a combination of the two. These muscles have a dominant vascular pedicle—the medial and lateral sural arteries. They can be harvested as an island for better reach, and they are reliable and have minimal donor site morbidity.15 The soleus muscle flap is another option that can be used alone or in combination with the medial or lateral gastrocnemius. Defects that are not amenable to closure by these flaps will most likely require free tissue transfer. The rectus abdominis or latissimus dorsi muscles are the first options. The latter can be combined with the serratus muscle if more bulk is needed.
Middle and lower thirds of the tibia. The soleus flap is frequently used for small or medium-sized mid-tibial defects. It is based on branches of the popliteal artery and posterior tibial artery. Larger defects require a combination of soleus and gastrocnemius muscle flaps or free tissue transfer.
Foot
Ideal reconstruction of the foot should provide thin and durable skin that will tolerate mechanical stress, and achieving this can be quite difficult. Skin grafts are seldom used for the foot, and are limited to non–weight-bearing portions with good underlying soft tissue.
Proximal non–weight-bearing areas (Achilles tendon and malleolar area). Local fasciocutaneous flaps are preferred. The lateral calcaneal artery flap, which is based on the peroneal artery branch, can cover exposed Achilles tendon, providing sensate coverage (sural nerve). The dorsalis pedis flap can be mobilized to cover the malleolar region and distal Achilles tendon, but donor site morbidity limits its use. Free tissue transfer is required for larger defects, and the the main options are flaps from the radial forearm, temporoparietal fascia, or lateral arm.
Heel and midplantar area. For heel reconstruction, the medial plantar artery flap, dorsalis pedis flap, abductor myocutaneous flap, peroneal artery flap, or anterior tibial artery flap can be used. The most versatile flap of the foot is the medial plantar artery flap, which is available only when the posterior tibial artery is intact. If local flaps are not suitable, microvascular tissue transfer is indicated. The radial forearm flap, scapular flap, lateral arm flap, or anterolateral thigh flap can be used. The radial forearm flap is usually the first choice because it is thin, has a long pedicle, and is easy to harvest.
If the foot defect is associated with a large cavity, muscle flaps are the first choices, specifically the gracilis or anterior serratus. A split latissimus muscle can also be applied. The full latissimus or the rectus abdominis are often too large for the type of defects observed.
Distal plantar area and forefoot. Most wounds in this region will require free tissue transfer. Free muscle flaps with split-thickness skin grafts provide the most stable and durable coverage.
Amputation vs limb salvage
It is important to evaluate the effects of lower-extremity salvage on ambulation. Salvage of a nonfunctional limb is of little value for the patient. Likewise, patients with severe medical problems may not be good candidates for limb salvage procedures. In those situations, amputation of the lower extremity is indicated. Adequate soft-tissue coverage and good distal perfusion are necessary to ensure healing of an amputation. If possible, local tissue rearrangement may be enough to provide a good amputation stump to fit an external prosthesis. In the case of radiation damage to the tissue, a free tissue transfer is necessary. The calcaneal-plantar unit from the amputated limb is frequently used as a free flap. Other flaps from the amputated limb, called fillet flaps, are harvested immediately and converted to flaps transferred to the defect site. Studies show that they are oncologically safe and reliable.17 Other flaps that provide good coverage for amputation defects are the latissimus dorsi muscle flap, the radial forearm flap, and the anterolateral thigh flap.
Upper extremities
POSTOPERATIVE CARE
Postoperative care following reconstruction after sarcoma resection requires a dedicated and trained team, particularly if a free flap is used for reconstruction.
Clinical evaluation of flaps includes color, temperature, and capillary refill. In cases of microsurgical reconstruction, postoperative care should include hourly examination of audible Doppler signals, at least for the first 36 hours. Free flap complications develop primarily in the first 24 hours, but they can occur during initial mobilization of the patient after a long period of bed rest. The surgical team should be aware of the potential problems and be able to act fast if necessary to reestablish blood flow to the flap.
In addition to flap monitoring, immobilization of the patient after surgery is extremely important. Postoperative swelling to the extremity should be avoided. Patients should be placed on bed rest until the postoperative swelling has subsided and the flap has adhered to the wound bed. Our protocol includes strict bed rest for about 7 days, followed by several days of dangling the extremity for short periods to ensure that dependent positioning will not alter the blood supply. A physical therapist should be involved to assist with crutches or a wheelchair. The patient should receive prophylactic anticoagulation during the resting period, in light of the high risk of deep vein thrombosis and pulmonary embolism. A compressive garment should be used to prevent lymphedema.
COMPLICATIONS ASSOCIATED WITH FLAPS
Once the flap is raised, it can still fail as a result of tension at insetting, inadequate blood flow, twisting of the pedicle, hematoma and/or infection, or the patient’s condition (eg, coagulopathy, poor nutritional status, anemia). Failure to correctly evaluate the direction of arterial flow, whether anterograde or retrograde, can cause flap loss. Instruments such as Doppler ultrasonographic equipment can be used to help to determine the flow. Partial or complete occlusion of the vascular pedicle can occur for several reasons (eg, twisting of the pedicle), and the consequences are disastrous if not recognized in time. If a pedicle problem is suspected in the case of a free flap, the patient should be taken to the operating room immediately and the flap should be explored. Rupture of the vascular anastomosis can occur as a result of technical problems, tension, and (in rare cases) infection.
Hematomas can cause mass effect, limit the venous return, and lead to flap necrosis. Hematoma formation also releases free radicals that can contribute to flap necrosis. Prevention is achieved through meticulous hemostasis. If a hematoma is suspected, the wound should be explored and the hematoma evacuated and washed out with normal saline.
The presence of an infected wound bed can also damage a flap by increasing its metabolic demand and causing the flap to be compromised by the infection itself. It is usually best to wait until the infection is controlled before planning the reconstruction.
Partial flap losses, skin graft losses, and wound dehiscence also are possible. Most of the time these require wound care, and patients’ nutrition and general health should be optimized to help the healing process. In the case of partial or complete flap loss, a new flap is often required and should be planned at a proper time.
CONCLUSIONS
Soft-tissue reconstruction following musculoskeletal sarcoma resection can be as simple as allowing the wound to heal by itself, which is less ideal, or as complex as coverage with a microsurgical osteocutaneous free flap. Limb salvage for sarcomas of the lower extremity has demonstrated good final functional outcomes without adversely affecting the oncologic results. Moreover, patients feel better psychologically and have higher quality of life.18,19
We believe that soft-tissue coverage after a wide resection is the most critical factor for avoiding postoperative complications of the tumor resection, such as infection or fractures. For this reason, we recommend the use of well-vascularized coverage at the time of the initial operation, if possible. Careful preoperative planning is especially important. We believe that reconstruction following musculoskeletal sarcoma resection can be done effectively only by using a team approach. Every such team should include, at minimum, an orthopedic surgeon and a reconstructive surgeon, with the mix of other providers dictated by the individual case.
- Misra A, Mistry N, Grimer R, Peart F. The management of soft tissue sarcoma. J Plast Reconstr Aesthet Surg 2009; 62:161–174.
- Morii T, Mochizuki K, Takushima A, Okazaki M, Satomi K. Soft tissue reconstruction using vascularized tissue transplantation following resection of musculoskeletal sarcoma: evaluation of oncologic and functional outcomes in 55 cases. Ann Plast Surg 2009; 62:252–257.
- Heller L, Kronowitz SJ. Lower extremity reconstruction. J Surg Oncol 2006; 94:479–489.
- Bannasch H, Haivas I, Momeni A, Stark GB. Oncosurgical and reconstructive concepts in the treatment of soft tissue sarcomas: a retrospective analysis. Arch Orthop Trauma Surg 2009; 129:43–49.
- Muramatsu K, Ihara K, Doi K, Hashimoto T, Taguchi T. Sarcoma in the forearm and hand: clinical outcomes and microsurgical reconstruction for limb salvage. Ann Plast Surg 2009; 62:28–33.
- Tukiainen E, Böhling T, Huuhtanen R. Soft tissue sarcoma of the trunk and extremities. Scand J Surg 2003; 92:257–263.
- Adelani MA, Holt GE, Dittus RS, Passman MA, Schwartz HS. Revascularization after segmental resection of lower extremity soft tissue sarcomas. J Surg Oncol 2007; 95:455–460.
- Lohman RF, Nabawi AS, Reece GP, Pollock RE, Evans GR. Soft tissue sarcoma of the upper extremity: a 5-year experience at two institutions emphasizing the role of soft tissue flap reconstruction. Cancer 2002; 94:2256–2264.
- Davis AM, Sennik S, Griffin AM, et al. Predictors of functional outcomes following limb salvage surgery for lower-extremity soft tissue sarcoma. J Surg Oncol 2000; 73:206–211.
- Heller L, Ballo MT, Cormier JN, Oates SD, Butler CE. Staged reconstruction for resection wounds in sarcoma patients treated with brachytherapy. Ann Plast Surg 2008; 60:58–63.
- Evans GR, Black JJ, Robb GL, et al. Adjuvant therapy: the effects on microvascular lower extremity reconstruction. Ann Plast Surg 1997; 39:141–144.
- Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg 1994; 93:980–987.
- Barwick WJ, Goldberg JA, Scully SP, Harrelson JM. Vascularized tissue transfer for closure of irradiated wounds after soft tissue sarcoma resection. Ann Surg 1992; 216:591–595.
- Masquelet AC, Romana MC. The medialis pedis flap: a new fasciocutaneous flap. Plast Reconstr Surg 1990; 85:765–772.
- Sherman R, Law M. Lower extremity reconstruction. In: Achauer BM, Eriksson E, Guyuron B, Coleman III JJ, Russell RC, Vander Kolk CA, eds. Plastic Surgery: Indications, Operations, and Outcomes. Vol 1. St. Louis, MO: Mosby; 2000:475–496.
- Innocenti M, Abed YY, Beltrami G, Delcroix L, Balatri A, Capanna R. Quadriceps muscle reconstruction with free functioning latissimus dorsi muscle flap after oncological resection. Microsurgery 2009; 29:189–198.
- Chiang YC, Wei FC, Wang JW, Chen WS. Reconstruction of below-knee stump using the salvaged foot fillet flap. Plast Reconstr Surg 1995; 96:731–738.
- Serletti JM, Carras AJ, O’Keefe RJ, Rosier RN. Functional outcome after soft-tissue reconstruction for limb salvage after sarcoma surgery. Plast Reconstr Surg 1998; 102:1576–1583.
- Niimi R, Matsumine A, Kusuzaki K, et al. Usefulness of limb salvage surgery for bone and soft tissue sarcomas of the distal lower leg. J Cancer Res Clin Oncol 2008; 134:1087–1095.
Advances in the management of soft-tissue and bone sarcomas—referred to collectively as “musculoskeletal sarcomas” hereafter—have resulted in significant improvements in survival and quality of life.1–3 Several factors have likely contributed to these advances, including improved surgical technique and the development of referral centers for sarcoma treatment that have embraced a multidisciplinary approach.1,2
The goal of treatment for musculoskeletal sarcomas is to optimize oncologic outcome and maximize functional restoration.2,3 Surgical resection has been the mainstay of therapy,1–7 as detailed earlier in this supplement. In patients with musculoskeletal sarcomas of the extremities, limb-sparing resection has been shown to be significantly superior to amputation.1,7–9 Wide local excision of the tumor along with its muscle compartment, followed by adjuvant chemotherapy and radiation therapy, has allowed limb salvage without an increased risk of recurrence in many patients.3 However, wide tumor resection can leave large defects that are not amenable to coverage by mobilization of the surrounding tissues, particularly if those tissues have been irradiated. As a result, resection can expose neurovascular structures, bone without periosteum, alloplastic materials, and internal fixation devices.
GOALS OF RECONSTRUCTION
Reconstructive surgery after musculoskeletal sarcoma resection aims to provide adequate wound coverage, preserve function, and optimize the cosmetic outcome.1–3 Tumors can be found on areas crucial to limb movement or may involve tissues vital to limb function. Reconstruction to repair these deficits can take many forms. In certain situations, amputation is still inevitable. In those cases, the reconstruction should provide stable stump coverage with durability and the ability to fit well with an external prosthesis.3
TIMING OF RECONSTRUCTION
Immediate reconstruction should be pursued if possible
Immediate reconstruction after a negative margin should always be considered and should be attempted when possible. Immediate reconstruction allows the reconstructive surgeon to benefit from better evaluation of the defect and exposed structures, as no scar tissue is present to distort the anatomy. Likewise, patients benefit from faster recovery and can receive adjuvant treatment (if necessary) sooner, as well as earlier rehabilitation. Patients may also benefit psychologically from immediate reconstruction.1,3
Indications for delayed reconstruction
Delayed reconstruction is primarily indicated when there are wound healing problems or there is uncertainty about the tumor margins. Secondary indications for delayed reconstruction are wound dehiscence and unstable soft-tissue coverage. If hardware is exposed, the recommendation is for early intervention and wound coverage with well-vascularized tissue to protect and cover the implant or prosthesis.
What about radiation therapy?
A very important consideration in reconstruction is the need for neoadjuvant or adjuvant radiation therapy.3,10,11 Irradiated wounds have a higher incidence of complications, including a tendency to dehisce. In patients who have been previously irradiated, the best practice is to perform immediate reconstruction with well-vascularized tissue, most likely a free tissue transfer.4,6,11,12 This practice reduces hospital stay, costs, and morbidity and increases limb salvage and patient satisfaction.13
SYSTEMATIC PREOPERATIVE PLANNING NEEDED
Reconstruction after musculoskeletal sarcoma resection should be planned systematically within a process that involves preoperative anticipation of the defect size and the resulting functional and cosmetic deficits that might need to be addressed. A preoperative visit to the reconstructive surgeon can be very helpful for presurgical planning.
During surgery it is usually preferable to allow the surgeon doing the tumor resection (eg, surgical oncologist or orthopedic oncologist) to complete the resection because the dimensions of the defect are not certain until negative margins are obtained.14 If tumor margins are unclear at the time of initial resection, the surgeon should consider delaying the definitive reconstruction until the permanent sections confirm negative margins. Temporary closure can be achieved with wound dressings, skin grafts (either allograft or autograft), or negative-pressure wound therapy. In the same context, if neurovascular structures are exposed it is reasonable to use a muscle flap without “tailoring” the flap to the defect. This approach allows the flap to be advanced or repositioned in case of positive margins, and the skin graft can be applied to the muscle surface in a second procedure.3
RECONSTRUCTIVE METHODS: A BRIEF OVERVIEW
Several methods can be used to close musculoskeletal sarcoma excision defects. Smaller defects can be closed primarily, although most defects are large and not amenable to primary closure. If fascia or muscle is preserved with only the skin coverage missing, the wound can be covered with either split-thickness or full-thickness skin grafts.1,4,6 Split-thickness skin grafts can be obtained in larger amounts and often heal faster than full-thickness skin grafts. However, most resections will require durable tissue coverage, particularly if adjuvant radiation therapy is planned.
In the case of long bone sarcoma resection, the resulting defect is usually large and complex and the traditional reconstruction is based on avascular allografts and local tissue flaps. However, allografts are associated with high rates of infection, nonunion, and fracture, leading to failure in about 50% of cases. Microvascular free flaps that contain bone, such as free fibula flaps, have been used instead of allografts with good success rates.2
Lately there has been growing interest in the use of the vacuum-assisted closure device (a form of negative-pressure wound therapy) to promote wound healing. It has been shown to improve the granulation and healing of open wounds by absorbing moisture, as well as to promote adherence after skin grafting, thereby reducing the risk of graft displacement.1,3 This device can be used immediately after musculoskeletal sarcoma resection while definitive tumor margin results are pending. It also can be used to prepare the wound bed for grafting in high-risk patients who would not tolerate more complex reconstructions.
Local or adjacent fascial, fasciocutaneous, and dermal flaps can also be used in lower-extremity reconstruction. However, muscle or musculocutaneous flaps are the mainstay of reconstruction after resection of musculoskeletal sarcomas. This group also includes perforator flaps, which have grown in popularity in the last few years.1,3
LOCATION-BASED WOUND RECONSTRUCTION
Musculoskeletal sarcomas can occur in virtually any region of the body, and myriad reconstructive options are available for various body sites. Since lower-extremity musculoskeletal sarcomas represent about 75% of cases,1 we will focus mainly on reconstruction of the lower extremity.
Factors driving choice of flap
Selection of an appropriate flap is essential to an optimal outcome. Flaps should be chosen with regard to donor site morbidity, functional requirements, length and diameter of the vascular pedicle, and aesthetic outcome.3 Usually physical examination, palpation of peripheral pulses, and Doppler ultrasonography are sufficient to evaluate the circulation. A preoperative angiogram should be considered in patients with severe peripheral vascular disease or previous trauma, which can potentially compromise the reconstructive outcome.15
Each region of the lower extremity possesses unique anatomic and functional characteristics that must be evaluated. It is useful to categorize the thigh, lower leg, and foot into separate anatomic units when planning reconstruction. We further divided these units into several subunits, as previously proposed by Sherman and Law15 and as outlined below.
Thigh
The thigh is usually well perfused and has several muscle groups, which facilitates reconstruction. Primary closure, skin grafts, or local flaps are acceptable options in most cases. The remaining musculature can be rotated or advanced to cover defects in the anterior or posterior thigh, providing bulk and adequate blood supply.
Hip and proximal/lateral thigh. Local muscle or myocutaneous flap options include tensor fascia lata, vastus lateralis, and rectus femoris flaps, all of which are based on the lateral circumflex femoral artery.
The tensor fascia lata flap is thin but has a long fascia extension that can be elevated from above the knee and can include a large skin paddle that is innervated by the lateral femoral cutaneous nerve. Some patients may experience knee instability after tensor fascia lata harvest.
The vastus lateralis muscle flap provides good bulk. Its arc of rotation reaches most of the inferior and posterior pelvis. It has little effect on ambulation.
The rectus femoris muscle flap is not so bulky, is easily mobilized, and has a wide arc of rotation. The donor site can be closed primarily. Harvest of this muscle can be associated with some strength loss during knee extension. For large defects of the upper third of the leg, a pedicled rectus abdominis muscle flap based on the deep inferior epigastric artery can be used. A vertically oriented skin island can be extended up to the costal margin, improving the reach. When the nature of the wound precludes use of pedicle flaps, free tissue transfer is indicated, with the latissimus dorsi muscle flap being used most commonly.15,16
Mid-thigh. Wounds in this location often can be closed with skin grafts or fasciocutaneous flaps. If the femur is exposed, however, a muscle flap will be required. As above, the tensor fascia lata, vastus lateralis, and rectus femoris can be used as flap options. If the lateral circumflex artery is unavailable, other flap options include the gracilis, vastus medialis, and rectus abdominis muscles. The gracilis muscle flap is based on the medial circumflex femoral artery and is useful for covering the medial aspect of the mid-thigh. Although this is a thin muscle, it can be used to cover long defects. The vastus medialis muscle flap is supplied by perforators from the profunda femoris and superficial femoral arteries. It can be rotated medially and advanced distally to cover patellar defects.
Supracondylar knee. The knee is a location where sarcoma resection is particularly likely to leave a defect with exposed bone, tendons, or ligaments that will need coverage. The gastrocnemius muscle flap combined with a split-thickness skin graft remains a consistent and reliable reconstructive option for this area. Other options are an extended medial gastrocnemius muscle flap or myocutaneous flap, which incorporates a random fasciocutaneous extension. For larger defects, free flaps should be considered, such as the anterior thigh flap, rectus abdominis muscle flap, or latissimus dorsi muscle flap. If tendons or ligaments need to be reconstructed, we favor autologous tissue, such as the fascia lata and plantaris tendons. These are easy to harvest and provide long-lasting joint stability.
Lower leg
Proximal third of the tibia. Defects here can usually be covered with a medial or lateral gastrocnemius muscle or myocutaneous flap, or a combination of the two. These muscles have a dominant vascular pedicle—the medial and lateral sural arteries. They can be harvested as an island for better reach, and they are reliable and have minimal donor site morbidity.15 The soleus muscle flap is another option that can be used alone or in combination with the medial or lateral gastrocnemius. Defects that are not amenable to closure by these flaps will most likely require free tissue transfer. The rectus abdominis or latissimus dorsi muscles are the first options. The latter can be combined with the serratus muscle if more bulk is needed.
Middle and lower thirds of the tibia. The soleus flap is frequently used for small or medium-sized mid-tibial defects. It is based on branches of the popliteal artery and posterior tibial artery. Larger defects require a combination of soleus and gastrocnemius muscle flaps or free tissue transfer.
Foot
Ideal reconstruction of the foot should provide thin and durable skin that will tolerate mechanical stress, and achieving this can be quite difficult. Skin grafts are seldom used for the foot, and are limited to non–weight-bearing portions with good underlying soft tissue.
Proximal non–weight-bearing areas (Achilles tendon and malleolar area). Local fasciocutaneous flaps are preferred. The lateral calcaneal artery flap, which is based on the peroneal artery branch, can cover exposed Achilles tendon, providing sensate coverage (sural nerve). The dorsalis pedis flap can be mobilized to cover the malleolar region and distal Achilles tendon, but donor site morbidity limits its use. Free tissue transfer is required for larger defects, and the the main options are flaps from the radial forearm, temporoparietal fascia, or lateral arm.
Heel and midplantar area. For heel reconstruction, the medial plantar artery flap, dorsalis pedis flap, abductor myocutaneous flap, peroneal artery flap, or anterior tibial artery flap can be used. The most versatile flap of the foot is the medial plantar artery flap, which is available only when the posterior tibial artery is intact. If local flaps are not suitable, microvascular tissue transfer is indicated. The radial forearm flap, scapular flap, lateral arm flap, or anterolateral thigh flap can be used. The radial forearm flap is usually the first choice because it is thin, has a long pedicle, and is easy to harvest.
If the foot defect is associated with a large cavity, muscle flaps are the first choices, specifically the gracilis or anterior serratus. A split latissimus muscle can also be applied. The full latissimus or the rectus abdominis are often too large for the type of defects observed.
Distal plantar area and forefoot. Most wounds in this region will require free tissue transfer. Free muscle flaps with split-thickness skin grafts provide the most stable and durable coverage.
Amputation vs limb salvage
It is important to evaluate the effects of lower-extremity salvage on ambulation. Salvage of a nonfunctional limb is of little value for the patient. Likewise, patients with severe medical problems may not be good candidates for limb salvage procedures. In those situations, amputation of the lower extremity is indicated. Adequate soft-tissue coverage and good distal perfusion are necessary to ensure healing of an amputation. If possible, local tissue rearrangement may be enough to provide a good amputation stump to fit an external prosthesis. In the case of radiation damage to the tissue, a free tissue transfer is necessary. The calcaneal-plantar unit from the amputated limb is frequently used as a free flap. Other flaps from the amputated limb, called fillet flaps, are harvested immediately and converted to flaps transferred to the defect site. Studies show that they are oncologically safe and reliable.17 Other flaps that provide good coverage for amputation defects are the latissimus dorsi muscle flap, the radial forearm flap, and the anterolateral thigh flap.
Upper extremities
POSTOPERATIVE CARE
Postoperative care following reconstruction after sarcoma resection requires a dedicated and trained team, particularly if a free flap is used for reconstruction.
Clinical evaluation of flaps includes color, temperature, and capillary refill. In cases of microsurgical reconstruction, postoperative care should include hourly examination of audible Doppler signals, at least for the first 36 hours. Free flap complications develop primarily in the first 24 hours, but they can occur during initial mobilization of the patient after a long period of bed rest. The surgical team should be aware of the potential problems and be able to act fast if necessary to reestablish blood flow to the flap.
In addition to flap monitoring, immobilization of the patient after surgery is extremely important. Postoperative swelling to the extremity should be avoided. Patients should be placed on bed rest until the postoperative swelling has subsided and the flap has adhered to the wound bed. Our protocol includes strict bed rest for about 7 days, followed by several days of dangling the extremity for short periods to ensure that dependent positioning will not alter the blood supply. A physical therapist should be involved to assist with crutches or a wheelchair. The patient should receive prophylactic anticoagulation during the resting period, in light of the high risk of deep vein thrombosis and pulmonary embolism. A compressive garment should be used to prevent lymphedema.
COMPLICATIONS ASSOCIATED WITH FLAPS
Once the flap is raised, it can still fail as a result of tension at insetting, inadequate blood flow, twisting of the pedicle, hematoma and/or infection, or the patient’s condition (eg, coagulopathy, poor nutritional status, anemia). Failure to correctly evaluate the direction of arterial flow, whether anterograde or retrograde, can cause flap loss. Instruments such as Doppler ultrasonographic equipment can be used to help to determine the flow. Partial or complete occlusion of the vascular pedicle can occur for several reasons (eg, twisting of the pedicle), and the consequences are disastrous if not recognized in time. If a pedicle problem is suspected in the case of a free flap, the patient should be taken to the operating room immediately and the flap should be explored. Rupture of the vascular anastomosis can occur as a result of technical problems, tension, and (in rare cases) infection.
Hematomas can cause mass effect, limit the venous return, and lead to flap necrosis. Hematoma formation also releases free radicals that can contribute to flap necrosis. Prevention is achieved through meticulous hemostasis. If a hematoma is suspected, the wound should be explored and the hematoma evacuated and washed out with normal saline.
The presence of an infected wound bed can also damage a flap by increasing its metabolic demand and causing the flap to be compromised by the infection itself. It is usually best to wait until the infection is controlled before planning the reconstruction.
Partial flap losses, skin graft losses, and wound dehiscence also are possible. Most of the time these require wound care, and patients’ nutrition and general health should be optimized to help the healing process. In the case of partial or complete flap loss, a new flap is often required and should be planned at a proper time.
CONCLUSIONS
Soft-tissue reconstruction following musculoskeletal sarcoma resection can be as simple as allowing the wound to heal by itself, which is less ideal, or as complex as coverage with a microsurgical osteocutaneous free flap. Limb salvage for sarcomas of the lower extremity has demonstrated good final functional outcomes without adversely affecting the oncologic results. Moreover, patients feel better psychologically and have higher quality of life.18,19
We believe that soft-tissue coverage after a wide resection is the most critical factor for avoiding postoperative complications of the tumor resection, such as infection or fractures. For this reason, we recommend the use of well-vascularized coverage at the time of the initial operation, if possible. Careful preoperative planning is especially important. We believe that reconstruction following musculoskeletal sarcoma resection can be done effectively only by using a team approach. Every such team should include, at minimum, an orthopedic surgeon and a reconstructive surgeon, with the mix of other providers dictated by the individual case.
Advances in the management of soft-tissue and bone sarcomas—referred to collectively as “musculoskeletal sarcomas” hereafter—have resulted in significant improvements in survival and quality of life.1–3 Several factors have likely contributed to these advances, including improved surgical technique and the development of referral centers for sarcoma treatment that have embraced a multidisciplinary approach.1,2
The goal of treatment for musculoskeletal sarcomas is to optimize oncologic outcome and maximize functional restoration.2,3 Surgical resection has been the mainstay of therapy,1–7 as detailed earlier in this supplement. In patients with musculoskeletal sarcomas of the extremities, limb-sparing resection has been shown to be significantly superior to amputation.1,7–9 Wide local excision of the tumor along with its muscle compartment, followed by adjuvant chemotherapy and radiation therapy, has allowed limb salvage without an increased risk of recurrence in many patients.3 However, wide tumor resection can leave large defects that are not amenable to coverage by mobilization of the surrounding tissues, particularly if those tissues have been irradiated. As a result, resection can expose neurovascular structures, bone without periosteum, alloplastic materials, and internal fixation devices.
GOALS OF RECONSTRUCTION
Reconstructive surgery after musculoskeletal sarcoma resection aims to provide adequate wound coverage, preserve function, and optimize the cosmetic outcome.1–3 Tumors can be found on areas crucial to limb movement or may involve tissues vital to limb function. Reconstruction to repair these deficits can take many forms. In certain situations, amputation is still inevitable. In those cases, the reconstruction should provide stable stump coverage with durability and the ability to fit well with an external prosthesis.3
TIMING OF RECONSTRUCTION
Immediate reconstruction should be pursued if possible
Immediate reconstruction after a negative margin should always be considered and should be attempted when possible. Immediate reconstruction allows the reconstructive surgeon to benefit from better evaluation of the defect and exposed structures, as no scar tissue is present to distort the anatomy. Likewise, patients benefit from faster recovery and can receive adjuvant treatment (if necessary) sooner, as well as earlier rehabilitation. Patients may also benefit psychologically from immediate reconstruction.1,3
Indications for delayed reconstruction
Delayed reconstruction is primarily indicated when there are wound healing problems or there is uncertainty about the tumor margins. Secondary indications for delayed reconstruction are wound dehiscence and unstable soft-tissue coverage. If hardware is exposed, the recommendation is for early intervention and wound coverage with well-vascularized tissue to protect and cover the implant or prosthesis.
What about radiation therapy?
A very important consideration in reconstruction is the need for neoadjuvant or adjuvant radiation therapy.3,10,11 Irradiated wounds have a higher incidence of complications, including a tendency to dehisce. In patients who have been previously irradiated, the best practice is to perform immediate reconstruction with well-vascularized tissue, most likely a free tissue transfer.4,6,11,12 This practice reduces hospital stay, costs, and morbidity and increases limb salvage and patient satisfaction.13
SYSTEMATIC PREOPERATIVE PLANNING NEEDED
Reconstruction after musculoskeletal sarcoma resection should be planned systematically within a process that involves preoperative anticipation of the defect size and the resulting functional and cosmetic deficits that might need to be addressed. A preoperative visit to the reconstructive surgeon can be very helpful for presurgical planning.
During surgery it is usually preferable to allow the surgeon doing the tumor resection (eg, surgical oncologist or orthopedic oncologist) to complete the resection because the dimensions of the defect are not certain until negative margins are obtained.14 If tumor margins are unclear at the time of initial resection, the surgeon should consider delaying the definitive reconstruction until the permanent sections confirm negative margins. Temporary closure can be achieved with wound dressings, skin grafts (either allograft or autograft), or negative-pressure wound therapy. In the same context, if neurovascular structures are exposed it is reasonable to use a muscle flap without “tailoring” the flap to the defect. This approach allows the flap to be advanced or repositioned in case of positive margins, and the skin graft can be applied to the muscle surface in a second procedure.3
RECONSTRUCTIVE METHODS: A BRIEF OVERVIEW
Several methods can be used to close musculoskeletal sarcoma excision defects. Smaller defects can be closed primarily, although most defects are large and not amenable to primary closure. If fascia or muscle is preserved with only the skin coverage missing, the wound can be covered with either split-thickness or full-thickness skin grafts.1,4,6 Split-thickness skin grafts can be obtained in larger amounts and often heal faster than full-thickness skin grafts. However, most resections will require durable tissue coverage, particularly if adjuvant radiation therapy is planned.
In the case of long bone sarcoma resection, the resulting defect is usually large and complex and the traditional reconstruction is based on avascular allografts and local tissue flaps. However, allografts are associated with high rates of infection, nonunion, and fracture, leading to failure in about 50% of cases. Microvascular free flaps that contain bone, such as free fibula flaps, have been used instead of allografts with good success rates.2
Lately there has been growing interest in the use of the vacuum-assisted closure device (a form of negative-pressure wound therapy) to promote wound healing. It has been shown to improve the granulation and healing of open wounds by absorbing moisture, as well as to promote adherence after skin grafting, thereby reducing the risk of graft displacement.1,3 This device can be used immediately after musculoskeletal sarcoma resection while definitive tumor margin results are pending. It also can be used to prepare the wound bed for grafting in high-risk patients who would not tolerate more complex reconstructions.
Local or adjacent fascial, fasciocutaneous, and dermal flaps can also be used in lower-extremity reconstruction. However, muscle or musculocutaneous flaps are the mainstay of reconstruction after resection of musculoskeletal sarcomas. This group also includes perforator flaps, which have grown in popularity in the last few years.1,3
LOCATION-BASED WOUND RECONSTRUCTION
Musculoskeletal sarcomas can occur in virtually any region of the body, and myriad reconstructive options are available for various body sites. Since lower-extremity musculoskeletal sarcomas represent about 75% of cases,1 we will focus mainly on reconstruction of the lower extremity.
Factors driving choice of flap
Selection of an appropriate flap is essential to an optimal outcome. Flaps should be chosen with regard to donor site morbidity, functional requirements, length and diameter of the vascular pedicle, and aesthetic outcome.3 Usually physical examination, palpation of peripheral pulses, and Doppler ultrasonography are sufficient to evaluate the circulation. A preoperative angiogram should be considered in patients with severe peripheral vascular disease or previous trauma, which can potentially compromise the reconstructive outcome.15
Each region of the lower extremity possesses unique anatomic and functional characteristics that must be evaluated. It is useful to categorize the thigh, lower leg, and foot into separate anatomic units when planning reconstruction. We further divided these units into several subunits, as previously proposed by Sherman and Law15 and as outlined below.
Thigh
The thigh is usually well perfused and has several muscle groups, which facilitates reconstruction. Primary closure, skin grafts, or local flaps are acceptable options in most cases. The remaining musculature can be rotated or advanced to cover defects in the anterior or posterior thigh, providing bulk and adequate blood supply.
Hip and proximal/lateral thigh. Local muscle or myocutaneous flap options include tensor fascia lata, vastus lateralis, and rectus femoris flaps, all of which are based on the lateral circumflex femoral artery.
The tensor fascia lata flap is thin but has a long fascia extension that can be elevated from above the knee and can include a large skin paddle that is innervated by the lateral femoral cutaneous nerve. Some patients may experience knee instability after tensor fascia lata harvest.
The vastus lateralis muscle flap provides good bulk. Its arc of rotation reaches most of the inferior and posterior pelvis. It has little effect on ambulation.
The rectus femoris muscle flap is not so bulky, is easily mobilized, and has a wide arc of rotation. The donor site can be closed primarily. Harvest of this muscle can be associated with some strength loss during knee extension. For large defects of the upper third of the leg, a pedicled rectus abdominis muscle flap based on the deep inferior epigastric artery can be used. A vertically oriented skin island can be extended up to the costal margin, improving the reach. When the nature of the wound precludes use of pedicle flaps, free tissue transfer is indicated, with the latissimus dorsi muscle flap being used most commonly.15,16
Mid-thigh. Wounds in this location often can be closed with skin grafts or fasciocutaneous flaps. If the femur is exposed, however, a muscle flap will be required. As above, the tensor fascia lata, vastus lateralis, and rectus femoris can be used as flap options. If the lateral circumflex artery is unavailable, other flap options include the gracilis, vastus medialis, and rectus abdominis muscles. The gracilis muscle flap is based on the medial circumflex femoral artery and is useful for covering the medial aspect of the mid-thigh. Although this is a thin muscle, it can be used to cover long defects. The vastus medialis muscle flap is supplied by perforators from the profunda femoris and superficial femoral arteries. It can be rotated medially and advanced distally to cover patellar defects.
Supracondylar knee. The knee is a location where sarcoma resection is particularly likely to leave a defect with exposed bone, tendons, or ligaments that will need coverage. The gastrocnemius muscle flap combined with a split-thickness skin graft remains a consistent and reliable reconstructive option for this area. Other options are an extended medial gastrocnemius muscle flap or myocutaneous flap, which incorporates a random fasciocutaneous extension. For larger defects, free flaps should be considered, such as the anterior thigh flap, rectus abdominis muscle flap, or latissimus dorsi muscle flap. If tendons or ligaments need to be reconstructed, we favor autologous tissue, such as the fascia lata and plantaris tendons. These are easy to harvest and provide long-lasting joint stability.
Lower leg
Proximal third of the tibia. Defects here can usually be covered with a medial or lateral gastrocnemius muscle or myocutaneous flap, or a combination of the two. These muscles have a dominant vascular pedicle—the medial and lateral sural arteries. They can be harvested as an island for better reach, and they are reliable and have minimal donor site morbidity.15 The soleus muscle flap is another option that can be used alone or in combination with the medial or lateral gastrocnemius. Defects that are not amenable to closure by these flaps will most likely require free tissue transfer. The rectus abdominis or latissimus dorsi muscles are the first options. The latter can be combined with the serratus muscle if more bulk is needed.
Middle and lower thirds of the tibia. The soleus flap is frequently used for small or medium-sized mid-tibial defects. It is based on branches of the popliteal artery and posterior tibial artery. Larger defects require a combination of soleus and gastrocnemius muscle flaps or free tissue transfer.
Foot
Ideal reconstruction of the foot should provide thin and durable skin that will tolerate mechanical stress, and achieving this can be quite difficult. Skin grafts are seldom used for the foot, and are limited to non–weight-bearing portions with good underlying soft tissue.
Proximal non–weight-bearing areas (Achilles tendon and malleolar area). Local fasciocutaneous flaps are preferred. The lateral calcaneal artery flap, which is based on the peroneal artery branch, can cover exposed Achilles tendon, providing sensate coverage (sural nerve). The dorsalis pedis flap can be mobilized to cover the malleolar region and distal Achilles tendon, but donor site morbidity limits its use. Free tissue transfer is required for larger defects, and the the main options are flaps from the radial forearm, temporoparietal fascia, or lateral arm.
Heel and midplantar area. For heel reconstruction, the medial plantar artery flap, dorsalis pedis flap, abductor myocutaneous flap, peroneal artery flap, or anterior tibial artery flap can be used. The most versatile flap of the foot is the medial plantar artery flap, which is available only when the posterior tibial artery is intact. If local flaps are not suitable, microvascular tissue transfer is indicated. The radial forearm flap, scapular flap, lateral arm flap, or anterolateral thigh flap can be used. The radial forearm flap is usually the first choice because it is thin, has a long pedicle, and is easy to harvest.
If the foot defect is associated with a large cavity, muscle flaps are the first choices, specifically the gracilis or anterior serratus. A split latissimus muscle can also be applied. The full latissimus or the rectus abdominis are often too large for the type of defects observed.
Distal plantar area and forefoot. Most wounds in this region will require free tissue transfer. Free muscle flaps with split-thickness skin grafts provide the most stable and durable coverage.
Amputation vs limb salvage
It is important to evaluate the effects of lower-extremity salvage on ambulation. Salvage of a nonfunctional limb is of little value for the patient. Likewise, patients with severe medical problems may not be good candidates for limb salvage procedures. In those situations, amputation of the lower extremity is indicated. Adequate soft-tissue coverage and good distal perfusion are necessary to ensure healing of an amputation. If possible, local tissue rearrangement may be enough to provide a good amputation stump to fit an external prosthesis. In the case of radiation damage to the tissue, a free tissue transfer is necessary. The calcaneal-plantar unit from the amputated limb is frequently used as a free flap. Other flaps from the amputated limb, called fillet flaps, are harvested immediately and converted to flaps transferred to the defect site. Studies show that they are oncologically safe and reliable.17 Other flaps that provide good coverage for amputation defects are the latissimus dorsi muscle flap, the radial forearm flap, and the anterolateral thigh flap.
Upper extremities
POSTOPERATIVE CARE
Postoperative care following reconstruction after sarcoma resection requires a dedicated and trained team, particularly if a free flap is used for reconstruction.
Clinical evaluation of flaps includes color, temperature, and capillary refill. In cases of microsurgical reconstruction, postoperative care should include hourly examination of audible Doppler signals, at least for the first 36 hours. Free flap complications develop primarily in the first 24 hours, but they can occur during initial mobilization of the patient after a long period of bed rest. The surgical team should be aware of the potential problems and be able to act fast if necessary to reestablish blood flow to the flap.
In addition to flap monitoring, immobilization of the patient after surgery is extremely important. Postoperative swelling to the extremity should be avoided. Patients should be placed on bed rest until the postoperative swelling has subsided and the flap has adhered to the wound bed. Our protocol includes strict bed rest for about 7 days, followed by several days of dangling the extremity for short periods to ensure that dependent positioning will not alter the blood supply. A physical therapist should be involved to assist with crutches or a wheelchair. The patient should receive prophylactic anticoagulation during the resting period, in light of the high risk of deep vein thrombosis and pulmonary embolism. A compressive garment should be used to prevent lymphedema.
COMPLICATIONS ASSOCIATED WITH FLAPS
Once the flap is raised, it can still fail as a result of tension at insetting, inadequate blood flow, twisting of the pedicle, hematoma and/or infection, or the patient’s condition (eg, coagulopathy, poor nutritional status, anemia). Failure to correctly evaluate the direction of arterial flow, whether anterograde or retrograde, can cause flap loss. Instruments such as Doppler ultrasonographic equipment can be used to help to determine the flow. Partial or complete occlusion of the vascular pedicle can occur for several reasons (eg, twisting of the pedicle), and the consequences are disastrous if not recognized in time. If a pedicle problem is suspected in the case of a free flap, the patient should be taken to the operating room immediately and the flap should be explored. Rupture of the vascular anastomosis can occur as a result of technical problems, tension, and (in rare cases) infection.
Hematomas can cause mass effect, limit the venous return, and lead to flap necrosis. Hematoma formation also releases free radicals that can contribute to flap necrosis. Prevention is achieved through meticulous hemostasis. If a hematoma is suspected, the wound should be explored and the hematoma evacuated and washed out with normal saline.
The presence of an infected wound bed can also damage a flap by increasing its metabolic demand and causing the flap to be compromised by the infection itself. It is usually best to wait until the infection is controlled before planning the reconstruction.
Partial flap losses, skin graft losses, and wound dehiscence also are possible. Most of the time these require wound care, and patients’ nutrition and general health should be optimized to help the healing process. In the case of partial or complete flap loss, a new flap is often required and should be planned at a proper time.
CONCLUSIONS
Soft-tissue reconstruction following musculoskeletal sarcoma resection can be as simple as allowing the wound to heal by itself, which is less ideal, or as complex as coverage with a microsurgical osteocutaneous free flap. Limb salvage for sarcomas of the lower extremity has demonstrated good final functional outcomes without adversely affecting the oncologic results. Moreover, patients feel better psychologically and have higher quality of life.18,19
We believe that soft-tissue coverage after a wide resection is the most critical factor for avoiding postoperative complications of the tumor resection, such as infection or fractures. For this reason, we recommend the use of well-vascularized coverage at the time of the initial operation, if possible. Careful preoperative planning is especially important. We believe that reconstruction following musculoskeletal sarcoma resection can be done effectively only by using a team approach. Every such team should include, at minimum, an orthopedic surgeon and a reconstructive surgeon, with the mix of other providers dictated by the individual case.
- Misra A, Mistry N, Grimer R, Peart F. The management of soft tissue sarcoma. J Plast Reconstr Aesthet Surg 2009; 62:161–174.
- Morii T, Mochizuki K, Takushima A, Okazaki M, Satomi K. Soft tissue reconstruction using vascularized tissue transplantation following resection of musculoskeletal sarcoma: evaluation of oncologic and functional outcomes in 55 cases. Ann Plast Surg 2009; 62:252–257.
- Heller L, Kronowitz SJ. Lower extremity reconstruction. J Surg Oncol 2006; 94:479–489.
- Bannasch H, Haivas I, Momeni A, Stark GB. Oncosurgical and reconstructive concepts in the treatment of soft tissue sarcomas: a retrospective analysis. Arch Orthop Trauma Surg 2009; 129:43–49.
- Muramatsu K, Ihara K, Doi K, Hashimoto T, Taguchi T. Sarcoma in the forearm and hand: clinical outcomes and microsurgical reconstruction for limb salvage. Ann Plast Surg 2009; 62:28–33.
- Tukiainen E, Böhling T, Huuhtanen R. Soft tissue sarcoma of the trunk and extremities. Scand J Surg 2003; 92:257–263.
- Adelani MA, Holt GE, Dittus RS, Passman MA, Schwartz HS. Revascularization after segmental resection of lower extremity soft tissue sarcomas. J Surg Oncol 2007; 95:455–460.
- Lohman RF, Nabawi AS, Reece GP, Pollock RE, Evans GR. Soft tissue sarcoma of the upper extremity: a 5-year experience at two institutions emphasizing the role of soft tissue flap reconstruction. Cancer 2002; 94:2256–2264.
- Davis AM, Sennik S, Griffin AM, et al. Predictors of functional outcomes following limb salvage surgery for lower-extremity soft tissue sarcoma. J Surg Oncol 2000; 73:206–211.
- Heller L, Ballo MT, Cormier JN, Oates SD, Butler CE. Staged reconstruction for resection wounds in sarcoma patients treated with brachytherapy. Ann Plast Surg 2008; 60:58–63.
- Evans GR, Black JJ, Robb GL, et al. Adjuvant therapy: the effects on microvascular lower extremity reconstruction. Ann Plast Surg 1997; 39:141–144.
- Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg 1994; 93:980–987.
- Barwick WJ, Goldberg JA, Scully SP, Harrelson JM. Vascularized tissue transfer for closure of irradiated wounds after soft tissue sarcoma resection. Ann Surg 1992; 216:591–595.
- Masquelet AC, Romana MC. The medialis pedis flap: a new fasciocutaneous flap. Plast Reconstr Surg 1990; 85:765–772.
- Sherman R, Law M. Lower extremity reconstruction. In: Achauer BM, Eriksson E, Guyuron B, Coleman III JJ, Russell RC, Vander Kolk CA, eds. Plastic Surgery: Indications, Operations, and Outcomes. Vol 1. St. Louis, MO: Mosby; 2000:475–496.
- Innocenti M, Abed YY, Beltrami G, Delcroix L, Balatri A, Capanna R. Quadriceps muscle reconstruction with free functioning latissimus dorsi muscle flap after oncological resection. Microsurgery 2009; 29:189–198.
- Chiang YC, Wei FC, Wang JW, Chen WS. Reconstruction of below-knee stump using the salvaged foot fillet flap. Plast Reconstr Surg 1995; 96:731–738.
- Serletti JM, Carras AJ, O’Keefe RJ, Rosier RN. Functional outcome after soft-tissue reconstruction for limb salvage after sarcoma surgery. Plast Reconstr Surg 1998; 102:1576–1583.
- Niimi R, Matsumine A, Kusuzaki K, et al. Usefulness of limb salvage surgery for bone and soft tissue sarcomas of the distal lower leg. J Cancer Res Clin Oncol 2008; 134:1087–1095.
- Misra A, Mistry N, Grimer R, Peart F. The management of soft tissue sarcoma. J Plast Reconstr Aesthet Surg 2009; 62:161–174.
- Morii T, Mochizuki K, Takushima A, Okazaki M, Satomi K. Soft tissue reconstruction using vascularized tissue transplantation following resection of musculoskeletal sarcoma: evaluation of oncologic and functional outcomes in 55 cases. Ann Plast Surg 2009; 62:252–257.
- Heller L, Kronowitz SJ. Lower extremity reconstruction. J Surg Oncol 2006; 94:479–489.
- Bannasch H, Haivas I, Momeni A, Stark GB. Oncosurgical and reconstructive concepts in the treatment of soft tissue sarcomas: a retrospective analysis. Arch Orthop Trauma Surg 2009; 129:43–49.
- Muramatsu K, Ihara K, Doi K, Hashimoto T, Taguchi T. Sarcoma in the forearm and hand: clinical outcomes and microsurgical reconstruction for limb salvage. Ann Plast Surg 2009; 62:28–33.
- Tukiainen E, Böhling T, Huuhtanen R. Soft tissue sarcoma of the trunk and extremities. Scand J Surg 2003; 92:257–263.
- Adelani MA, Holt GE, Dittus RS, Passman MA, Schwartz HS. Revascularization after segmental resection of lower extremity soft tissue sarcomas. J Surg Oncol 2007; 95:455–460.
- Lohman RF, Nabawi AS, Reece GP, Pollock RE, Evans GR. Soft tissue sarcoma of the upper extremity: a 5-year experience at two institutions emphasizing the role of soft tissue flap reconstruction. Cancer 2002; 94:2256–2264.
- Davis AM, Sennik S, Griffin AM, et al. Predictors of functional outcomes following limb salvage surgery for lower-extremity soft tissue sarcoma. J Surg Oncol 2000; 73:206–211.
- Heller L, Ballo MT, Cormier JN, Oates SD, Butler CE. Staged reconstruction for resection wounds in sarcoma patients treated with brachytherapy. Ann Plast Surg 2008; 60:58–63.
- Evans GR, Black JJ, Robb GL, et al. Adjuvant therapy: the effects on microvascular lower extremity reconstruction. Ann Plast Surg 1997; 39:141–144.
- Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg 1994; 93:980–987.
- Barwick WJ, Goldberg JA, Scully SP, Harrelson JM. Vascularized tissue transfer for closure of irradiated wounds after soft tissue sarcoma resection. Ann Surg 1992; 216:591–595.
- Masquelet AC, Romana MC. The medialis pedis flap: a new fasciocutaneous flap. Plast Reconstr Surg 1990; 85:765–772.
- Sherman R, Law M. Lower extremity reconstruction. In: Achauer BM, Eriksson E, Guyuron B, Coleman III JJ, Russell RC, Vander Kolk CA, eds. Plastic Surgery: Indications, Operations, and Outcomes. Vol 1. St. Louis, MO: Mosby; 2000:475–496.
- Innocenti M, Abed YY, Beltrami G, Delcroix L, Balatri A, Capanna R. Quadriceps muscle reconstruction with free functioning latissimus dorsi muscle flap after oncological resection. Microsurgery 2009; 29:189–198.
- Chiang YC, Wei FC, Wang JW, Chen WS. Reconstruction of below-knee stump using the salvaged foot fillet flap. Plast Reconstr Surg 1995; 96:731–738.
- Serletti JM, Carras AJ, O’Keefe RJ, Rosier RN. Functional outcome after soft-tissue reconstruction for limb salvage after sarcoma surgery. Plast Reconstr Surg 1998; 102:1576–1583.
- Niimi R, Matsumine A, Kusuzaki K, et al. Usefulness of limb salvage surgery for bone and soft tissue sarcomas of the distal lower leg. J Cancer Res Clin Oncol 2008; 134:1087–1095.
Use of chemotherapy for patients with bone and soft-tissue sarcomas
Surgical resection is the mainstay of treatment for musculoskeletal sarcomas, as detailed earlier in this supplement, but chemotherapy also has a proven role in the primary therapy of most bone sarcomas and a potential role for some patients with soft-tissue sarcomas. This article provides an overview of the roles of chemotherapy for patients with bone and soft-tissue sarcomas and addresses key considerations surrounding chemotherapy in the context of overall patient management.
BONE SARCOMAS
Because most bone sarcomas occur in pediatric patients and young adults, studies of chemotherapy in this disease have often enrolled predominantly young subjects. As a result, very limited data are available in older adults. Single-institution experiences indicate that adults with bone sarcomas have inferior outcomes compared with their pediatric and adolescent counterparts,1 but the literature on these tumors in adults is scant. Therefore, the following discussion on chemotherapy for bone sarcomas incorporates data from trials conducted predominantly in children and young adults (ie, generally younger than 30 years and with a very large majority younger than 20 years).
Chemotherapy for osteosarcoma
At present, neoadjuvant (preoperative) chemotherapy followed by definitive resection with subsequent adjuvant (postoperative) chemotherapy is the well-established approach to treatment of localized osteosarcomas. Chemotherapy can eradicate the micrometastatic disease that is believed to be present in the majority of patients with clinically resectable cancer.2
Efficacy. Historically, prior to the institution of effective chemotherapy, metastatic disease developed in 80% to 90% of patients who underwent curative resection with or without radiation therapy, which resulted in a long-term survival rate of less than 20%.3 In the 1980s, clinical trials that randomized patients with resectable osteosarcoma to surgery alone or to surgery plus chemotherapy found that the addition of perioperative chemotherapy led to significant improvements in recurrence rates and survival.4,5 More recent randomized trials have shown that treatment of such patients with modern multiagent chemotherapy regimens results in a 5-year survival rate of approximately 70%.6 Additionally, response to neoadjuvant (preoperative) treatment has become the most important predictor of outcome, as the median survival of osteosarcoma patients who have greater than 90% necrosis in the resected specimen following neoadjuvant chemotherapy is about 90% at 5 years.7,8
Toxicity. Current chemotherapy regimens are based on high doses of methotrexate and leucovorin in combination with doxorubicin, ifosfamide, and platinum. Long-term effects of such regimens include the following3:
- Azospermia (in 100% of patients who received a total ifosfamide dose > 75 g/m2)
- Subclinical renal impairment (in 48% of patients treated with high doses of ifosfamide)
- Hearing impairment (in 40% of patients treated with cisplatin)
- Second malignancies (in 2.1%)
- Cardiomyopathy (in 1.7%).3
In light of this, the development of equally effective but less intensive regimens for patients whose disease carries a better prognosis is highly desirable. Ongoing clinical trials are investigating this strategy.
Metastatic disease. Metastatic osteosarcoma is found in approximately 20% of patients at the time of diagnosis. Sarcoma mainly spreads hematogenously, and the lungs are the most common initial site of metastases, being affected in more than 60% of patients who develop metastatic disease.9 Patients with metachronous lung lesions are initially considered for aggressive treatment with neoadjuvant chemotherapy and subsequent resection of clinically apparent disease, which results in event-free survival rates of 20% to 30%.3
Patients with disease limited to the primary tumor and no more than one or two bone lesions fare best. The presence of multiple metastases is associated with the poorest prognosis, as few patients with this profile live past 2 years.10 In a review of 202 pediatric and adult patients with documented metastases at the time of osteosarcoma diagnosis, the presence of more than 5 metastatic lesions (which was reported in 91 patients) was associated with a 5-year overall survival rate of 19%.9
Chemotherapy for Ewing sarcoma
Perioperative chemotherapy in patients with localized Ewing sarcoma is believed to reduce the burden of micrometastasis that is thought to be present in most patients with early-stage disease. Five-year survival rates of 50% to 72% have been reported among patients with resectable Ewing sarcoma treated perioperatively with multiagent chemotherapy.11,12 Notably, randomized trials that studied intense multiagent chemotherapy regimens (consisting of doxorubicin, cyclophosphamide, vincristine, and dactinomycin alternating with etoposide and ifosfamide) reported the best outcomes despite significant but acceptable toxicity. In a large randomized trial involving 398 patients with resectable disease, a 5-year survival rate of 72% was achieved with the above regimen, compared with 61% in patients treated with a less intense regimen that did not contain ifosfamide and etoposide (p = .01).12
Compressing these standard regimens to an every-14-day instead of every-21-day schedule improved event-free survival at 3 years from 65% to 76% (p = .028) without any significant increase in toxicity in a randomized trial involving 568 patients.13 Data on overall survival from this trial are not yet published.
Metastatic disease. Metastatic Ewing sarcoma is found in 15% to 35% of patients with newly diagnosed disease and is treated with multiagent chemotherapy; resection of residual disease is considered in good responders.3 This approach produces objective responses to therapy, but long-term survival is rare.
Toxicity. Myelodysplastic syndrome and acute myeloid leukemia are the most dreaded long-term complications of intensive multiagent chemotherapy for Ewing sarcoma and develop in up to 8% of patients.14 Additionally, ifosfamide can lead to hematuria (~12% incidence), encephalopathy (mild somnolence and hallucinations to coma), chronic renal impairment (6% incidence), and hemorrhagic cystitis (though administration of mesna and generous intravenous hydration can minimize this latter complication).15 Recent efforts are therefore focused on testing less-intensive regimens in patients who have good prognostic features.
Chondrosarcoma: No role for chemotherapy
Chondrosarcoma, which represents approximately 20% of all bone sarcomas and has a peak incidence in older adults (ie, in the sixth decade of life), is insensitive to chemotherapy. Radiotherapy is also of limited value and is reserved for patients treated in the palliative setting.16 Definitive management of chondrosarcoma involves adequate surgical resection alone.
SOFT-TISSUE SARCOMAS
Aside from recent advances in the treatment of gastrointestinal stromal tumors with the small-molecule tyrosine kinase inhibitors imatinib and sunitinib (which are beyond the scope of this article), an overall survival advantage with chemotherapy has not been demonstrated in adults with soft-tissue sarcoma.17
Resectable disease
The decision to use chemotherapy needs to be weighed against the magnitude of potential clinical benefit and the acute and chronic toxicities that can develop.
Toxicity. Chemotherapy regimens with activity against soft-tissue sarcomas often contain anthracyclines, alkylating agents, and taxanes. These agents can produce serious long-term toxicities, which is especially important in patients treated with curative intent. Doxorubicin and other anthracyclines, for example, may result in cardiomyopathy, the risk of which rises with increasing cumulative dose.18 In addition, acute myeloid leukemia may develop in 2% to 12% of patients treated with anthracyclines or alkylating agents such as ifosfamide and dacarbazine.3,19 Renal failure and an elevated risk of bladder carcinoma are uncommonly reported in patients with a history of ifosfamide treatment.15 Sensory neuropathy associated with the use of taxanes (eg, paclitaxel and docetaxel) is dose dependent and reversible in more than half of patients. However, some patients treated with high doses of these agents can have persistent symptoms of paresthesias, burning, and decreased reflexes, which can be debilitating.20
Efficacy of adjuvant chemotherapy. Because chemotherapy puts patients at risk of such serious chronic toxicities, its use can be justified only if it results in significant benefit, such as prolongation of survival. A 1997 meta-analysis of 14 clinical trials evaluating adjuvant chemotherapy in patients with resectable soft-tissue sarcomas found chemotherapy to have an absolute benefit of 10% in recurrence-free survival at 10 years (ie, from 45% survival to 55% survival), with a hazard ratio of 0.75 (95% confidence interval [CI], 0.64–0.87; p = .0001) for recurrence or death.21 However, when the analysis was limited to overall survival at 10 years, the survival difference between patients who received adjuvant chemotherapy and those who did not (54% vs 50%, respectively) was not statistically significant (hazard ratio = 0.89; 95% CI, 0.76–1.03, p = .12).21
The concept of adjuvant therapy has been revisited since the antisarcoma activity of ifosfamide was established. A large European trial randomized 351 patients with resected soft-tissue sarcoma either to placebo or to doxorubicin and ifosfamide given every 21 days.22 The preliminary results, reported in abstract form at the 2007 annual meeting of the American Society of Clinical Oncology, showed a higher 5-year survival rate in the placebo arm (69%) compared with the chemotherapy arm (64%).22 This and other trials using ifosfamide in various drug combinations showed no difference in survival, suggesting that adjuvant chemotherapy should not be considered to be standard practice outside of a clinical trial.
Efficacy of neoadjuvant chemotherapy. Neoadjuvant chemotherapy also has been studied in patients with soft-tissue sarcomas. A retrospective analysis found that the greatest benefit is derived in patients with primary tumors larger than 10 cm, in whom neoadjuvant chemotherapy increased 3-year disease-specific survival from 62% to 83%.23 However, differing results came from a prospective multicenter trial that randomized patients with large primary and recurrent tumors to either surgery alone or surgery preceded by three cycles of neoadjuvant doxorubicin and ifosfamide (all patients could also receive adjuvant radiation therapy, depending on grade and adequacy of resection).24 The trial suffered from slow accrual, and only 150 patients were enrolled. At 5 years, survival was similar between the groups with and without neoadjuvant chemotherapy.24 Therefore, neoadjuvant chemotherapy is not yet recommended pending results of larger randomized trials.
No clear role for recurrent disease. Local recurrence of the primary tumor after resection occurs in 10% to 50% of cases of soft-tissue sarcoma, with the specific rate depending on the primary tumor location. The highest incidence of recurrence is found in patients with retroperitoneal and head and neck sarcoma (40% and 50%, respectively), mainly because of the difficulty of obtaining clear margins. Chemotherapy has not been well studied in this setting and is of uncertain value.3
Metastatic disease
Metastatic soft-tissue sarcomas may respond to chemotherapy, but there is a lack of evidence that chemotherapy improves overall survival. Pulmonary lesions are the most common site of distant recurrence, and resection of such metastases is sometimes undertaken in well-selected patients. However, there is no level 1 evidence supporting chemotherapy in this clinical setting despite its common preoperative use. There is a paucity of randomized phase 3 trials that compare established palliative chemotherapy regimens to best supportive care. It is believed that some groups of patients do benefit, however, including those who are young and have good performance status, low tumor grade, absence of liver metastasis or pulmonary metastasis only, and a long interval between treatment of the primary tumor and development of metastatic disease.3 Some histologies, such as uterine leiomyosarcomas and facial/scalp angiosarcomas, respond better to chemotherapy.17
Drugs found to have activity against metastatic sarcoma include doxorubicin, ifosfamide, platinum agents, gemcitabine, taxanes, and dacarbazine. Used either alone or in combinations, these drugs produce responses (ie, shrink metastatic tumors) in about 13% to 33% of patients.3 Use of chemotherapy is frequently curtailed by the acute toxicity of these agents, which includes pancytopenia, transfusion requirements, febrile neutropenia, nausea, alopecia, and significant fatigue, as well as renal failure with ifosfamide or cisplatin and peripheral neuropathy with platinum agents or taxanes. Appropriate patient selection for chemotherapy and exclusion of those who should be managed solely with best supportive care is an important challenge that oncologists often face when managing patients with metastatic soft-tissue sarcoma.
Future directions
Trabectedin (ET-743) is a novel compound with promising activity against soft-tissue sarcomas that acts by inhibiting cell-cycle transition from the G2 to M stages. The drug covalently binds to the minor grove of the DNA molecule, changing its three-dimensional structure and impairing transcription and possibly DNA repair.25 Phase 2 studies showed durable responses to trabectedin in 3% to 8% of heavily pretreated patients26–28 and in 17% of treatment-naïve patients with advanced soft-tissue sarcomas.25 Time to progression of up to 20 months has been reported in patients who respond or develop stable disease.3
Toxic effects of trabectedin include myelosuppression, fever, edema, arthralgias, hepatotoxicity, and (rarely) rhabdomyolysis. To date, these toxicities have been self-limiting. Larger clinical trials and longer follow-up is needed to assess whether this agent has any significant long-term toxicities.
Trabectedin has already been approved in Europe for treatment of chemotherapy-refractory soft-tissue sarcoma when given as a 24-hour infusion every 21 days.
More broadly, an active effort is under way to better understand the molecular derangements in a variety of soft-tissue sarcoma subtypes. The hope is that this understanding will lead to improved therapies that target aberrant proliferation, angiogenesis, and other biologic processes that drive the growth and metastasis of soft-tissue and bone sarcomas.
- Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol 1992; 10:5–15.
- Bruland OS, Høifødt H, Saeter G, Smeland S, Fodstad O. Hematogenous micrometastases in osteosarcoma patients. Clin Cancer Res 2005; 11:4666–4673.
- Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ, eds. Cancer Management: A Multidisciplinary Approach. 11th ed. Manhasset, NY: CMP Medica; 2009.
- Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med 1986; 314:1600–1606.
- Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol 1987; 5:21–26.
- Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol 2005; 23:2004–2011.
- Winkler K, Beron G, Delling G, et al. Neoadjuvant chemotherapy of osteosarcomas: results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol 1988; 6:329–337.
- Bramwell VH, Steward WP, Nooij M, et al. Neoadjuvant chemotherapy with doxorubicin and cisplatin in malignant fibrous histiocytoma of bone: a European Osteosarcoma Intergroup study. J Clin Oncol 1999; 17:3260–3269.
- Kager L, Zoubek A, Pötschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on Neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol 2003; 21:2011–2018.
- Longhi A, Fabbri N, Donati D, et al. Neoadjuvant chemotherapy for patients with synchronous multifocal osteosarcoma: results in eleven cases. J Chemother 2001; 13:324–330.
- Nesbit ME Jr, Gehan EA, Burgert EO Jr, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing’s sarcoma of bone: a long-term follow-up of the First Intergroup study. J Clin Oncol 1990; 8:1664–1674.
- Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003; 348:694–701.
- Womer RB, West DC, Krailo MD, et al; for the Children’s Oncology Group AEWS0031 Committee. Randomized comparison of every-two-week v. every-three-week chemotherapy in Ewing sarcoma family tumors. J Clin Oncol 2008; 26(May 20 suppl):10504. Abstract.
- Rodriguez-Galindo C, Poquette CA, Marina NM, et al. Hematologic abnormalities and acute myeloid leukemia in children and adolescents administered intensified chemotherapy for the Ewing sarcoma family of tumors. J Pediatr Hematol Oncol 2000; 22:321–329.
- Brade WP, Herdrich, K, Kachel-Fischer U, Araujo CE. Dosing and side-effects of ifosfamide plus mesna. J Cancer Res Clin Oncol 1991; 117(suppl 4):S164–S186.
- Healey JH, Lane JM. Chondrosarcoma. Clin Orthop Relat Res 1986; 204:119–129.
- Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med 2005; 353:701–711.
- Alexander J, Dainiak N, Berger HJ, et al. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. N Engl J Med 1979; 300:278–283.
- Felix CA. Secondary leukemias induced by topoisomerase-targeted drugs. Biochim Biophys Acta 1998; 1400:233–255.
- Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol 1995; 6:489–494.
- Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet 1997; 350:1647–1654.
- Woll PJ, van Glabbeke M, Hohenberger P, et al. Adjuvant chemotherapy (CT) with doxorubicin and ifosfamide in resected soft tissue sarcoma (STS): interim analysis of a randomised phase III trial. J Clin Oncol 2007; 25(June 20 suppl):10008. Abstract.
- Grobmyer SR, Maki RG, Demetri GD, et al. Neo-adjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Ann Oncol 2004; 15:1667–1672.
- Gortzak E, Azzarelli A, Buesa J, et al. A randomised phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer 2001; 37:1096–1103.
- Garcia-Carbonero R, Supko JG, Maki RG, et al. Ecteinascidin-743 (ET-743) for chemotherapy-naive patients with advanced soft tissue sarcomas: multicenter phase II and pharmacokinetic study. J Clin Oncol 2005; 23:5484–5492.
- Yovine A, Riofrio M, Blay JY, et al. Phase II study of ecteinascidin-743 in advanced pretreated soft tissue sarcoma patients. J Clin Oncol 2004; 22:890–899.
- Garcia-Carbonero R, Supko JG, Manola J, et al. Phase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. J Clin Oncol 2004; 22:1480–1490.
- Le Cesne A, Blay JY, Judson I, et al. Phase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol 2005; 23:576–584.
Surgical resection is the mainstay of treatment for musculoskeletal sarcomas, as detailed earlier in this supplement, but chemotherapy also has a proven role in the primary therapy of most bone sarcomas and a potential role for some patients with soft-tissue sarcomas. This article provides an overview of the roles of chemotherapy for patients with bone and soft-tissue sarcomas and addresses key considerations surrounding chemotherapy in the context of overall patient management.
BONE SARCOMAS
Because most bone sarcomas occur in pediatric patients and young adults, studies of chemotherapy in this disease have often enrolled predominantly young subjects. As a result, very limited data are available in older adults. Single-institution experiences indicate that adults with bone sarcomas have inferior outcomes compared with their pediatric and adolescent counterparts,1 but the literature on these tumors in adults is scant. Therefore, the following discussion on chemotherapy for bone sarcomas incorporates data from trials conducted predominantly in children and young adults (ie, generally younger than 30 years and with a very large majority younger than 20 years).
Chemotherapy for osteosarcoma
At present, neoadjuvant (preoperative) chemotherapy followed by definitive resection with subsequent adjuvant (postoperative) chemotherapy is the well-established approach to treatment of localized osteosarcomas. Chemotherapy can eradicate the micrometastatic disease that is believed to be present in the majority of patients with clinically resectable cancer.2
Efficacy. Historically, prior to the institution of effective chemotherapy, metastatic disease developed in 80% to 90% of patients who underwent curative resection with or without radiation therapy, which resulted in a long-term survival rate of less than 20%.3 In the 1980s, clinical trials that randomized patients with resectable osteosarcoma to surgery alone or to surgery plus chemotherapy found that the addition of perioperative chemotherapy led to significant improvements in recurrence rates and survival.4,5 More recent randomized trials have shown that treatment of such patients with modern multiagent chemotherapy regimens results in a 5-year survival rate of approximately 70%.6 Additionally, response to neoadjuvant (preoperative) treatment has become the most important predictor of outcome, as the median survival of osteosarcoma patients who have greater than 90% necrosis in the resected specimen following neoadjuvant chemotherapy is about 90% at 5 years.7,8
Toxicity. Current chemotherapy regimens are based on high doses of methotrexate and leucovorin in combination with doxorubicin, ifosfamide, and platinum. Long-term effects of such regimens include the following3:
- Azospermia (in 100% of patients who received a total ifosfamide dose > 75 g/m2)
- Subclinical renal impairment (in 48% of patients treated with high doses of ifosfamide)
- Hearing impairment (in 40% of patients treated with cisplatin)
- Second malignancies (in 2.1%)
- Cardiomyopathy (in 1.7%).3
In light of this, the development of equally effective but less intensive regimens for patients whose disease carries a better prognosis is highly desirable. Ongoing clinical trials are investigating this strategy.
Metastatic disease. Metastatic osteosarcoma is found in approximately 20% of patients at the time of diagnosis. Sarcoma mainly spreads hematogenously, and the lungs are the most common initial site of metastases, being affected in more than 60% of patients who develop metastatic disease.9 Patients with metachronous lung lesions are initially considered for aggressive treatment with neoadjuvant chemotherapy and subsequent resection of clinically apparent disease, which results in event-free survival rates of 20% to 30%.3
Patients with disease limited to the primary tumor and no more than one or two bone lesions fare best. The presence of multiple metastases is associated with the poorest prognosis, as few patients with this profile live past 2 years.10 In a review of 202 pediatric and adult patients with documented metastases at the time of osteosarcoma diagnosis, the presence of more than 5 metastatic lesions (which was reported in 91 patients) was associated with a 5-year overall survival rate of 19%.9
Chemotherapy for Ewing sarcoma
Perioperative chemotherapy in patients with localized Ewing sarcoma is believed to reduce the burden of micrometastasis that is thought to be present in most patients with early-stage disease. Five-year survival rates of 50% to 72% have been reported among patients with resectable Ewing sarcoma treated perioperatively with multiagent chemotherapy.11,12 Notably, randomized trials that studied intense multiagent chemotherapy regimens (consisting of doxorubicin, cyclophosphamide, vincristine, and dactinomycin alternating with etoposide and ifosfamide) reported the best outcomes despite significant but acceptable toxicity. In a large randomized trial involving 398 patients with resectable disease, a 5-year survival rate of 72% was achieved with the above regimen, compared with 61% in patients treated with a less intense regimen that did not contain ifosfamide and etoposide (p = .01).12
Compressing these standard regimens to an every-14-day instead of every-21-day schedule improved event-free survival at 3 years from 65% to 76% (p = .028) without any significant increase in toxicity in a randomized trial involving 568 patients.13 Data on overall survival from this trial are not yet published.
Metastatic disease. Metastatic Ewing sarcoma is found in 15% to 35% of patients with newly diagnosed disease and is treated with multiagent chemotherapy; resection of residual disease is considered in good responders.3 This approach produces objective responses to therapy, but long-term survival is rare.
Toxicity. Myelodysplastic syndrome and acute myeloid leukemia are the most dreaded long-term complications of intensive multiagent chemotherapy for Ewing sarcoma and develop in up to 8% of patients.14 Additionally, ifosfamide can lead to hematuria (~12% incidence), encephalopathy (mild somnolence and hallucinations to coma), chronic renal impairment (6% incidence), and hemorrhagic cystitis (though administration of mesna and generous intravenous hydration can minimize this latter complication).15 Recent efforts are therefore focused on testing less-intensive regimens in patients who have good prognostic features.
Chondrosarcoma: No role for chemotherapy
Chondrosarcoma, which represents approximately 20% of all bone sarcomas and has a peak incidence in older adults (ie, in the sixth decade of life), is insensitive to chemotherapy. Radiotherapy is also of limited value and is reserved for patients treated in the palliative setting.16 Definitive management of chondrosarcoma involves adequate surgical resection alone.
SOFT-TISSUE SARCOMAS
Aside from recent advances in the treatment of gastrointestinal stromal tumors with the small-molecule tyrosine kinase inhibitors imatinib and sunitinib (which are beyond the scope of this article), an overall survival advantage with chemotherapy has not been demonstrated in adults with soft-tissue sarcoma.17
Resectable disease
The decision to use chemotherapy needs to be weighed against the magnitude of potential clinical benefit and the acute and chronic toxicities that can develop.
Toxicity. Chemotherapy regimens with activity against soft-tissue sarcomas often contain anthracyclines, alkylating agents, and taxanes. These agents can produce serious long-term toxicities, which is especially important in patients treated with curative intent. Doxorubicin and other anthracyclines, for example, may result in cardiomyopathy, the risk of which rises with increasing cumulative dose.18 In addition, acute myeloid leukemia may develop in 2% to 12% of patients treated with anthracyclines or alkylating agents such as ifosfamide and dacarbazine.3,19 Renal failure and an elevated risk of bladder carcinoma are uncommonly reported in patients with a history of ifosfamide treatment.15 Sensory neuropathy associated with the use of taxanes (eg, paclitaxel and docetaxel) is dose dependent and reversible in more than half of patients. However, some patients treated with high doses of these agents can have persistent symptoms of paresthesias, burning, and decreased reflexes, which can be debilitating.20
Efficacy of adjuvant chemotherapy. Because chemotherapy puts patients at risk of such serious chronic toxicities, its use can be justified only if it results in significant benefit, such as prolongation of survival. A 1997 meta-analysis of 14 clinical trials evaluating adjuvant chemotherapy in patients with resectable soft-tissue sarcomas found chemotherapy to have an absolute benefit of 10% in recurrence-free survival at 10 years (ie, from 45% survival to 55% survival), with a hazard ratio of 0.75 (95% confidence interval [CI], 0.64–0.87; p = .0001) for recurrence or death.21 However, when the analysis was limited to overall survival at 10 years, the survival difference between patients who received adjuvant chemotherapy and those who did not (54% vs 50%, respectively) was not statistically significant (hazard ratio = 0.89; 95% CI, 0.76–1.03, p = .12).21
The concept of adjuvant therapy has been revisited since the antisarcoma activity of ifosfamide was established. A large European trial randomized 351 patients with resected soft-tissue sarcoma either to placebo or to doxorubicin and ifosfamide given every 21 days.22 The preliminary results, reported in abstract form at the 2007 annual meeting of the American Society of Clinical Oncology, showed a higher 5-year survival rate in the placebo arm (69%) compared with the chemotherapy arm (64%).22 This and other trials using ifosfamide in various drug combinations showed no difference in survival, suggesting that adjuvant chemotherapy should not be considered to be standard practice outside of a clinical trial.
Efficacy of neoadjuvant chemotherapy. Neoadjuvant chemotherapy also has been studied in patients with soft-tissue sarcomas. A retrospective analysis found that the greatest benefit is derived in patients with primary tumors larger than 10 cm, in whom neoadjuvant chemotherapy increased 3-year disease-specific survival from 62% to 83%.23 However, differing results came from a prospective multicenter trial that randomized patients with large primary and recurrent tumors to either surgery alone or surgery preceded by three cycles of neoadjuvant doxorubicin and ifosfamide (all patients could also receive adjuvant radiation therapy, depending on grade and adequacy of resection).24 The trial suffered from slow accrual, and only 150 patients were enrolled. At 5 years, survival was similar between the groups with and without neoadjuvant chemotherapy.24 Therefore, neoadjuvant chemotherapy is not yet recommended pending results of larger randomized trials.
No clear role for recurrent disease. Local recurrence of the primary tumor after resection occurs in 10% to 50% of cases of soft-tissue sarcoma, with the specific rate depending on the primary tumor location. The highest incidence of recurrence is found in patients with retroperitoneal and head and neck sarcoma (40% and 50%, respectively), mainly because of the difficulty of obtaining clear margins. Chemotherapy has not been well studied in this setting and is of uncertain value.3
Metastatic disease
Metastatic soft-tissue sarcomas may respond to chemotherapy, but there is a lack of evidence that chemotherapy improves overall survival. Pulmonary lesions are the most common site of distant recurrence, and resection of such metastases is sometimes undertaken in well-selected patients. However, there is no level 1 evidence supporting chemotherapy in this clinical setting despite its common preoperative use. There is a paucity of randomized phase 3 trials that compare established palliative chemotherapy regimens to best supportive care. It is believed that some groups of patients do benefit, however, including those who are young and have good performance status, low tumor grade, absence of liver metastasis or pulmonary metastasis only, and a long interval between treatment of the primary tumor and development of metastatic disease.3 Some histologies, such as uterine leiomyosarcomas and facial/scalp angiosarcomas, respond better to chemotherapy.17
Drugs found to have activity against metastatic sarcoma include doxorubicin, ifosfamide, platinum agents, gemcitabine, taxanes, and dacarbazine. Used either alone or in combinations, these drugs produce responses (ie, shrink metastatic tumors) in about 13% to 33% of patients.3 Use of chemotherapy is frequently curtailed by the acute toxicity of these agents, which includes pancytopenia, transfusion requirements, febrile neutropenia, nausea, alopecia, and significant fatigue, as well as renal failure with ifosfamide or cisplatin and peripheral neuropathy with platinum agents or taxanes. Appropriate patient selection for chemotherapy and exclusion of those who should be managed solely with best supportive care is an important challenge that oncologists often face when managing patients with metastatic soft-tissue sarcoma.
Future directions
Trabectedin (ET-743) is a novel compound with promising activity against soft-tissue sarcomas that acts by inhibiting cell-cycle transition from the G2 to M stages. The drug covalently binds to the minor grove of the DNA molecule, changing its three-dimensional structure and impairing transcription and possibly DNA repair.25 Phase 2 studies showed durable responses to trabectedin in 3% to 8% of heavily pretreated patients26–28 and in 17% of treatment-naïve patients with advanced soft-tissue sarcomas.25 Time to progression of up to 20 months has been reported in patients who respond or develop stable disease.3
Toxic effects of trabectedin include myelosuppression, fever, edema, arthralgias, hepatotoxicity, and (rarely) rhabdomyolysis. To date, these toxicities have been self-limiting. Larger clinical trials and longer follow-up is needed to assess whether this agent has any significant long-term toxicities.
Trabectedin has already been approved in Europe for treatment of chemotherapy-refractory soft-tissue sarcoma when given as a 24-hour infusion every 21 days.
More broadly, an active effort is under way to better understand the molecular derangements in a variety of soft-tissue sarcoma subtypes. The hope is that this understanding will lead to improved therapies that target aberrant proliferation, angiogenesis, and other biologic processes that drive the growth and metastasis of soft-tissue and bone sarcomas.
Surgical resection is the mainstay of treatment for musculoskeletal sarcomas, as detailed earlier in this supplement, but chemotherapy also has a proven role in the primary therapy of most bone sarcomas and a potential role for some patients with soft-tissue sarcomas. This article provides an overview of the roles of chemotherapy for patients with bone and soft-tissue sarcomas and addresses key considerations surrounding chemotherapy in the context of overall patient management.
BONE SARCOMAS
Because most bone sarcomas occur in pediatric patients and young adults, studies of chemotherapy in this disease have often enrolled predominantly young subjects. As a result, very limited data are available in older adults. Single-institution experiences indicate that adults with bone sarcomas have inferior outcomes compared with their pediatric and adolescent counterparts,1 but the literature on these tumors in adults is scant. Therefore, the following discussion on chemotherapy for bone sarcomas incorporates data from trials conducted predominantly in children and young adults (ie, generally younger than 30 years and with a very large majority younger than 20 years).
Chemotherapy for osteosarcoma
At present, neoadjuvant (preoperative) chemotherapy followed by definitive resection with subsequent adjuvant (postoperative) chemotherapy is the well-established approach to treatment of localized osteosarcomas. Chemotherapy can eradicate the micrometastatic disease that is believed to be present in the majority of patients with clinically resectable cancer.2
Efficacy. Historically, prior to the institution of effective chemotherapy, metastatic disease developed in 80% to 90% of patients who underwent curative resection with or without radiation therapy, which resulted in a long-term survival rate of less than 20%.3 In the 1980s, clinical trials that randomized patients with resectable osteosarcoma to surgery alone or to surgery plus chemotherapy found that the addition of perioperative chemotherapy led to significant improvements in recurrence rates and survival.4,5 More recent randomized trials have shown that treatment of such patients with modern multiagent chemotherapy regimens results in a 5-year survival rate of approximately 70%.6 Additionally, response to neoadjuvant (preoperative) treatment has become the most important predictor of outcome, as the median survival of osteosarcoma patients who have greater than 90% necrosis in the resected specimen following neoadjuvant chemotherapy is about 90% at 5 years.7,8
Toxicity. Current chemotherapy regimens are based on high doses of methotrexate and leucovorin in combination with doxorubicin, ifosfamide, and platinum. Long-term effects of such regimens include the following3:
- Azospermia (in 100% of patients who received a total ifosfamide dose > 75 g/m2)
- Subclinical renal impairment (in 48% of patients treated with high doses of ifosfamide)
- Hearing impairment (in 40% of patients treated with cisplatin)
- Second malignancies (in 2.1%)
- Cardiomyopathy (in 1.7%).3
In light of this, the development of equally effective but less intensive regimens for patients whose disease carries a better prognosis is highly desirable. Ongoing clinical trials are investigating this strategy.
Metastatic disease. Metastatic osteosarcoma is found in approximately 20% of patients at the time of diagnosis. Sarcoma mainly spreads hematogenously, and the lungs are the most common initial site of metastases, being affected in more than 60% of patients who develop metastatic disease.9 Patients with metachronous lung lesions are initially considered for aggressive treatment with neoadjuvant chemotherapy and subsequent resection of clinically apparent disease, which results in event-free survival rates of 20% to 30%.3
Patients with disease limited to the primary tumor and no more than one or two bone lesions fare best. The presence of multiple metastases is associated with the poorest prognosis, as few patients with this profile live past 2 years.10 In a review of 202 pediatric and adult patients with documented metastases at the time of osteosarcoma diagnosis, the presence of more than 5 metastatic lesions (which was reported in 91 patients) was associated with a 5-year overall survival rate of 19%.9
Chemotherapy for Ewing sarcoma
Perioperative chemotherapy in patients with localized Ewing sarcoma is believed to reduce the burden of micrometastasis that is thought to be present in most patients with early-stage disease. Five-year survival rates of 50% to 72% have been reported among patients with resectable Ewing sarcoma treated perioperatively with multiagent chemotherapy.11,12 Notably, randomized trials that studied intense multiagent chemotherapy regimens (consisting of doxorubicin, cyclophosphamide, vincristine, and dactinomycin alternating with etoposide and ifosfamide) reported the best outcomes despite significant but acceptable toxicity. In a large randomized trial involving 398 patients with resectable disease, a 5-year survival rate of 72% was achieved with the above regimen, compared with 61% in patients treated with a less intense regimen that did not contain ifosfamide and etoposide (p = .01).12
Compressing these standard regimens to an every-14-day instead of every-21-day schedule improved event-free survival at 3 years from 65% to 76% (p = .028) without any significant increase in toxicity in a randomized trial involving 568 patients.13 Data on overall survival from this trial are not yet published.
Metastatic disease. Metastatic Ewing sarcoma is found in 15% to 35% of patients with newly diagnosed disease and is treated with multiagent chemotherapy; resection of residual disease is considered in good responders.3 This approach produces objective responses to therapy, but long-term survival is rare.
Toxicity. Myelodysplastic syndrome and acute myeloid leukemia are the most dreaded long-term complications of intensive multiagent chemotherapy for Ewing sarcoma and develop in up to 8% of patients.14 Additionally, ifosfamide can lead to hematuria (~12% incidence), encephalopathy (mild somnolence and hallucinations to coma), chronic renal impairment (6% incidence), and hemorrhagic cystitis (though administration of mesna and generous intravenous hydration can minimize this latter complication).15 Recent efforts are therefore focused on testing less-intensive regimens in patients who have good prognostic features.
Chondrosarcoma: No role for chemotherapy
Chondrosarcoma, which represents approximately 20% of all bone sarcomas and has a peak incidence in older adults (ie, in the sixth decade of life), is insensitive to chemotherapy. Radiotherapy is also of limited value and is reserved for patients treated in the palliative setting.16 Definitive management of chondrosarcoma involves adequate surgical resection alone.
SOFT-TISSUE SARCOMAS
Aside from recent advances in the treatment of gastrointestinal stromal tumors with the small-molecule tyrosine kinase inhibitors imatinib and sunitinib (which are beyond the scope of this article), an overall survival advantage with chemotherapy has not been demonstrated in adults with soft-tissue sarcoma.17
Resectable disease
The decision to use chemotherapy needs to be weighed against the magnitude of potential clinical benefit and the acute and chronic toxicities that can develop.
Toxicity. Chemotherapy regimens with activity against soft-tissue sarcomas often contain anthracyclines, alkylating agents, and taxanes. These agents can produce serious long-term toxicities, which is especially important in patients treated with curative intent. Doxorubicin and other anthracyclines, for example, may result in cardiomyopathy, the risk of which rises with increasing cumulative dose.18 In addition, acute myeloid leukemia may develop in 2% to 12% of patients treated with anthracyclines or alkylating agents such as ifosfamide and dacarbazine.3,19 Renal failure and an elevated risk of bladder carcinoma are uncommonly reported in patients with a history of ifosfamide treatment.15 Sensory neuropathy associated with the use of taxanes (eg, paclitaxel and docetaxel) is dose dependent and reversible in more than half of patients. However, some patients treated with high doses of these agents can have persistent symptoms of paresthesias, burning, and decreased reflexes, which can be debilitating.20
Efficacy of adjuvant chemotherapy. Because chemotherapy puts patients at risk of such serious chronic toxicities, its use can be justified only if it results in significant benefit, such as prolongation of survival. A 1997 meta-analysis of 14 clinical trials evaluating adjuvant chemotherapy in patients with resectable soft-tissue sarcomas found chemotherapy to have an absolute benefit of 10% in recurrence-free survival at 10 years (ie, from 45% survival to 55% survival), with a hazard ratio of 0.75 (95% confidence interval [CI], 0.64–0.87; p = .0001) for recurrence or death.21 However, when the analysis was limited to overall survival at 10 years, the survival difference between patients who received adjuvant chemotherapy and those who did not (54% vs 50%, respectively) was not statistically significant (hazard ratio = 0.89; 95% CI, 0.76–1.03, p = .12).21
The concept of adjuvant therapy has been revisited since the antisarcoma activity of ifosfamide was established. A large European trial randomized 351 patients with resected soft-tissue sarcoma either to placebo or to doxorubicin and ifosfamide given every 21 days.22 The preliminary results, reported in abstract form at the 2007 annual meeting of the American Society of Clinical Oncology, showed a higher 5-year survival rate in the placebo arm (69%) compared with the chemotherapy arm (64%).22 This and other trials using ifosfamide in various drug combinations showed no difference in survival, suggesting that adjuvant chemotherapy should not be considered to be standard practice outside of a clinical trial.
Efficacy of neoadjuvant chemotherapy. Neoadjuvant chemotherapy also has been studied in patients with soft-tissue sarcomas. A retrospective analysis found that the greatest benefit is derived in patients with primary tumors larger than 10 cm, in whom neoadjuvant chemotherapy increased 3-year disease-specific survival from 62% to 83%.23 However, differing results came from a prospective multicenter trial that randomized patients with large primary and recurrent tumors to either surgery alone or surgery preceded by three cycles of neoadjuvant doxorubicin and ifosfamide (all patients could also receive adjuvant radiation therapy, depending on grade and adequacy of resection).24 The trial suffered from slow accrual, and only 150 patients were enrolled. At 5 years, survival was similar between the groups with and without neoadjuvant chemotherapy.24 Therefore, neoadjuvant chemotherapy is not yet recommended pending results of larger randomized trials.
No clear role for recurrent disease. Local recurrence of the primary tumor after resection occurs in 10% to 50% of cases of soft-tissue sarcoma, with the specific rate depending on the primary tumor location. The highest incidence of recurrence is found in patients with retroperitoneal and head and neck sarcoma (40% and 50%, respectively), mainly because of the difficulty of obtaining clear margins. Chemotherapy has not been well studied in this setting and is of uncertain value.3
Metastatic disease
Metastatic soft-tissue sarcomas may respond to chemotherapy, but there is a lack of evidence that chemotherapy improves overall survival. Pulmonary lesions are the most common site of distant recurrence, and resection of such metastases is sometimes undertaken in well-selected patients. However, there is no level 1 evidence supporting chemotherapy in this clinical setting despite its common preoperative use. There is a paucity of randomized phase 3 trials that compare established palliative chemotherapy regimens to best supportive care. It is believed that some groups of patients do benefit, however, including those who are young and have good performance status, low tumor grade, absence of liver metastasis or pulmonary metastasis only, and a long interval between treatment of the primary tumor and development of metastatic disease.3 Some histologies, such as uterine leiomyosarcomas and facial/scalp angiosarcomas, respond better to chemotherapy.17
Drugs found to have activity against metastatic sarcoma include doxorubicin, ifosfamide, platinum agents, gemcitabine, taxanes, and dacarbazine. Used either alone or in combinations, these drugs produce responses (ie, shrink metastatic tumors) in about 13% to 33% of patients.3 Use of chemotherapy is frequently curtailed by the acute toxicity of these agents, which includes pancytopenia, transfusion requirements, febrile neutropenia, nausea, alopecia, and significant fatigue, as well as renal failure with ifosfamide or cisplatin and peripheral neuropathy with platinum agents or taxanes. Appropriate patient selection for chemotherapy and exclusion of those who should be managed solely with best supportive care is an important challenge that oncologists often face when managing patients with metastatic soft-tissue sarcoma.
Future directions
Trabectedin (ET-743) is a novel compound with promising activity against soft-tissue sarcomas that acts by inhibiting cell-cycle transition from the G2 to M stages. The drug covalently binds to the minor grove of the DNA molecule, changing its three-dimensional structure and impairing transcription and possibly DNA repair.25 Phase 2 studies showed durable responses to trabectedin in 3% to 8% of heavily pretreated patients26–28 and in 17% of treatment-naïve patients with advanced soft-tissue sarcomas.25 Time to progression of up to 20 months has been reported in patients who respond or develop stable disease.3
Toxic effects of trabectedin include myelosuppression, fever, edema, arthralgias, hepatotoxicity, and (rarely) rhabdomyolysis. To date, these toxicities have been self-limiting. Larger clinical trials and longer follow-up is needed to assess whether this agent has any significant long-term toxicities.
Trabectedin has already been approved in Europe for treatment of chemotherapy-refractory soft-tissue sarcoma when given as a 24-hour infusion every 21 days.
More broadly, an active effort is under way to better understand the molecular derangements in a variety of soft-tissue sarcoma subtypes. The hope is that this understanding will lead to improved therapies that target aberrant proliferation, angiogenesis, and other biologic processes that drive the growth and metastasis of soft-tissue and bone sarcomas.
- Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol 1992; 10:5–15.
- Bruland OS, Høifødt H, Saeter G, Smeland S, Fodstad O. Hematogenous micrometastases in osteosarcoma patients. Clin Cancer Res 2005; 11:4666–4673.
- Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ, eds. Cancer Management: A Multidisciplinary Approach. 11th ed. Manhasset, NY: CMP Medica; 2009.
- Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med 1986; 314:1600–1606.
- Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol 1987; 5:21–26.
- Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol 2005; 23:2004–2011.
- Winkler K, Beron G, Delling G, et al. Neoadjuvant chemotherapy of osteosarcomas: results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol 1988; 6:329–337.
- Bramwell VH, Steward WP, Nooij M, et al. Neoadjuvant chemotherapy with doxorubicin and cisplatin in malignant fibrous histiocytoma of bone: a European Osteosarcoma Intergroup study. J Clin Oncol 1999; 17:3260–3269.
- Kager L, Zoubek A, Pötschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on Neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol 2003; 21:2011–2018.
- Longhi A, Fabbri N, Donati D, et al. Neoadjuvant chemotherapy for patients with synchronous multifocal osteosarcoma: results in eleven cases. J Chemother 2001; 13:324–330.
- Nesbit ME Jr, Gehan EA, Burgert EO Jr, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing’s sarcoma of bone: a long-term follow-up of the First Intergroup study. J Clin Oncol 1990; 8:1664–1674.
- Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003; 348:694–701.
- Womer RB, West DC, Krailo MD, et al; for the Children’s Oncology Group AEWS0031 Committee. Randomized comparison of every-two-week v. every-three-week chemotherapy in Ewing sarcoma family tumors. J Clin Oncol 2008; 26(May 20 suppl):10504. Abstract.
- Rodriguez-Galindo C, Poquette CA, Marina NM, et al. Hematologic abnormalities and acute myeloid leukemia in children and adolescents administered intensified chemotherapy for the Ewing sarcoma family of tumors. J Pediatr Hematol Oncol 2000; 22:321–329.
- Brade WP, Herdrich, K, Kachel-Fischer U, Araujo CE. Dosing and side-effects of ifosfamide plus mesna. J Cancer Res Clin Oncol 1991; 117(suppl 4):S164–S186.
- Healey JH, Lane JM. Chondrosarcoma. Clin Orthop Relat Res 1986; 204:119–129.
- Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med 2005; 353:701–711.
- Alexander J, Dainiak N, Berger HJ, et al. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. N Engl J Med 1979; 300:278–283.
- Felix CA. Secondary leukemias induced by topoisomerase-targeted drugs. Biochim Biophys Acta 1998; 1400:233–255.
- Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol 1995; 6:489–494.
- Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet 1997; 350:1647–1654.
- Woll PJ, van Glabbeke M, Hohenberger P, et al. Adjuvant chemotherapy (CT) with doxorubicin and ifosfamide in resected soft tissue sarcoma (STS): interim analysis of a randomised phase III trial. J Clin Oncol 2007; 25(June 20 suppl):10008. Abstract.
- Grobmyer SR, Maki RG, Demetri GD, et al. Neo-adjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Ann Oncol 2004; 15:1667–1672.
- Gortzak E, Azzarelli A, Buesa J, et al. A randomised phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer 2001; 37:1096–1103.
- Garcia-Carbonero R, Supko JG, Maki RG, et al. Ecteinascidin-743 (ET-743) for chemotherapy-naive patients with advanced soft tissue sarcomas: multicenter phase II and pharmacokinetic study. J Clin Oncol 2005; 23:5484–5492.
- Yovine A, Riofrio M, Blay JY, et al. Phase II study of ecteinascidin-743 in advanced pretreated soft tissue sarcoma patients. J Clin Oncol 2004; 22:890–899.
- Garcia-Carbonero R, Supko JG, Manola J, et al. Phase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. J Clin Oncol 2004; 22:1480–1490.
- Le Cesne A, Blay JY, Judson I, et al. Phase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol 2005; 23:576–584.
- Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol 1992; 10:5–15.
- Bruland OS, Høifødt H, Saeter G, Smeland S, Fodstad O. Hematogenous micrometastases in osteosarcoma patients. Clin Cancer Res 2005; 11:4666–4673.
- Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ, eds. Cancer Management: A Multidisciplinary Approach. 11th ed. Manhasset, NY: CMP Medica; 2009.
- Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med 1986; 314:1600–1606.
- Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol 1987; 5:21–26.
- Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol 2005; 23:2004–2011.
- Winkler K, Beron G, Delling G, et al. Neoadjuvant chemotherapy of osteosarcomas: results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol 1988; 6:329–337.
- Bramwell VH, Steward WP, Nooij M, et al. Neoadjuvant chemotherapy with doxorubicin and cisplatin in malignant fibrous histiocytoma of bone: a European Osteosarcoma Intergroup study. J Clin Oncol 1999; 17:3260–3269.
- Kager L, Zoubek A, Pötschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on Neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol 2003; 21:2011–2018.
- Longhi A, Fabbri N, Donati D, et al. Neoadjuvant chemotherapy for patients with synchronous multifocal osteosarcoma: results in eleven cases. J Chemother 2001; 13:324–330.
- Nesbit ME Jr, Gehan EA, Burgert EO Jr, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing’s sarcoma of bone: a long-term follow-up of the First Intergroup study. J Clin Oncol 1990; 8:1664–1674.
- Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003; 348:694–701.
- Womer RB, West DC, Krailo MD, et al; for the Children’s Oncology Group AEWS0031 Committee. Randomized comparison of every-two-week v. every-three-week chemotherapy in Ewing sarcoma family tumors. J Clin Oncol 2008; 26(May 20 suppl):10504. Abstract.
- Rodriguez-Galindo C, Poquette CA, Marina NM, et al. Hematologic abnormalities and acute myeloid leukemia in children and adolescents administered intensified chemotherapy for the Ewing sarcoma family of tumors. J Pediatr Hematol Oncol 2000; 22:321–329.
- Brade WP, Herdrich, K, Kachel-Fischer U, Araujo CE. Dosing and side-effects of ifosfamide plus mesna. J Cancer Res Clin Oncol 1991; 117(suppl 4):S164–S186.
- Healey JH, Lane JM. Chondrosarcoma. Clin Orthop Relat Res 1986; 204:119–129.
- Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med 2005; 353:701–711.
- Alexander J, Dainiak N, Berger HJ, et al. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. N Engl J Med 1979; 300:278–283.
- Felix CA. Secondary leukemias induced by topoisomerase-targeted drugs. Biochim Biophys Acta 1998; 1400:233–255.
- Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol 1995; 6:489–494.
- Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet 1997; 350:1647–1654.
- Woll PJ, van Glabbeke M, Hohenberger P, et al. Adjuvant chemotherapy (CT) with doxorubicin and ifosfamide in resected soft tissue sarcoma (STS): interim analysis of a randomised phase III trial. J Clin Oncol 2007; 25(June 20 suppl):10008. Abstract.
- Grobmyer SR, Maki RG, Demetri GD, et al. Neo-adjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Ann Oncol 2004; 15:1667–1672.
- Gortzak E, Azzarelli A, Buesa J, et al. A randomised phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer 2001; 37:1096–1103.
- Garcia-Carbonero R, Supko JG, Maki RG, et al. Ecteinascidin-743 (ET-743) for chemotherapy-naive patients with advanced soft tissue sarcomas: multicenter phase II and pharmacokinetic study. J Clin Oncol 2005; 23:5484–5492.
- Yovine A, Riofrio M, Blay JY, et al. Phase II study of ecteinascidin-743 in advanced pretreated soft tissue sarcoma patients. J Clin Oncol 2004; 22:890–899.
- Garcia-Carbonero R, Supko JG, Manola J, et al. Phase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. J Clin Oncol 2004; 22:1480–1490.
- Le Cesne A, Blay JY, Judson I, et al. Phase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol 2005; 23:576–584.
Use of radiation therapy for patients with soft-tissue and bone sarcomas
While radiation therapy (RT) has an integral role in the management of soft-tissue sarcoma, it has a limited role in that of bone sarcoma, with few exceptions (ie, Ewing sarcoma). In keeping with the rarity of these tumors, it has been demonstrated that patients treated at high-volume centers have significantly better survival and functional outcomes.1–3 Accordingly, treatment should be delivered by a multidisciplinary team including orthopedic, medical, and radiation oncologists, as well as plastic and reconstructive surgeons, physical therapy specialists, and pathologists and radiologists with expertise in musculoskeletal sarcomas.4 As the preceding articles in this supplement have addressed the major modalities in the treatment of sarcomas other than RT, this article will focus on how RT fits into the overall management mix, with a focus on soft-tissue sarcomas, where it figures most prominently.
BONE SARCOMAS: A LIMITED ROLE FOR RADIATION
The role of RT in the management of bone sarcomas is limited. Its primary application appears to be in Ewing sarcoma, for which curative treatment requires combined local and systemic therapy. For definitive therapy, limb-salvage surgery is preferable over amputation, but amputation may be an option for younger patients with lesions of the fibula, tibia, and foot. Based on the available data, postoperative RT is probably of benefit for all patients with Ewing sarcoma with close margins and/or those with a poor histologic response.5 Further discussion of Ewing sarcoma management is beyond the scope of this article (see the second and fifth articles in this supplement).
For osteosarcoma, the current standard of care is surgical resection combined with neoadjuvant and adjuvant chemotherapy. RT had been used years ago, prior to the advent of effective chemotherapy regimens, but its use for osteosarcoma has now been relegated to a few select situations. These include lesions not amenable to surgical resection and reconstruction, cases in which the patient refuses surgery, cases where there are positive margins after resection, and cases where palliation is needed for symptomatic lesions.
SOFT-TISSUE SARCOMAS: RADIATION HAS A CLEAR ADJUVANT ROLE
The primary management of localized soft-tissue sarcomas is surgical resection to achieve a negative margin when feasible. Historically, local excision of soft-tissue sarcomas resulted in local failure rates of 50% to 70%, even when a margin of normal tissue around the tumor was excised. As a result, amputation became standard treatment.6 In a landmark National Cancer Institute study 3 decades ago, patients were randomized to amputation or to limb-sparing surgery with the addition of RT.7 Notably, disease-free and overall survival were not compromised by limb-sparing surgery plus RT, demonstrating that although lesser surgery in the absence of RT may be insufficient, limb-sparing surgery with RT was equal to amputation. Consequently, limb-sparing approaches have become the favored surgery for the majority of cases of soft-tissue sarcoma, as advocated in a consensus statement from the National Institutes of Health.1
Indications vary by lesion grade
In general, adjuvant RT is recommended for all intermediate- and high-grade soft-tissue sarcoma lesions. A potential exception is a superficial tumor smaller than 5 cm with widely negative margins after resection. For low-grade lesions, re-excision is favored over adjuvant RT for positive or close margins, and RT is avoided in the setting of negative margins.
Optimal timing of radiation remains unclear
The optimal timing of adjuvant RT—preoperative versus postoperative—remains unknown. The relative advantages of preoperative RT include smaller and well-defined treatment volume, ability to use a lower dose, lack of tissue hypoxia, increased tumor resectability (smaller surgery), and improved limb function with less late fibrosis and edema. The disadvantages include inability to precisely stage patients and higher risk of acute wound-healing complications.
The National Cancer Institute of Canada compared outcomes with preoperative versus postoperative RT among 190 patients with soft-tissue sarcoma in a prospective randomized trial.8 Patients were stratified by tumor size (≤ 10 cm or > 10cm) and then randomized to preoperative RT (50 Gy in 25 fractions) or postoperative RT (66 Gy in 33 fractions).8 There was no difference between the groups in local control, distant control, or survival rates, but a higher rate of late complications, including fibrosis and edema, was observed with postoperative RT.8,9 On the other hand, the incidence of wound complications was higher in the preoperative group (35%) than in the postoperative group (17%).8
Likewise, the optimal sequencing and benefits of systemic therapy (chemotherapy) with relation to local therapy (surgery with pre- or postoperative RT) remain unclear. More than a dozen individual randomized trials of adjuvant chemotherapy, as well as a meta-analysis of 14 trials of doxorubicin-based adjuvant chemotherapy, have failed to demonstrate significant improvement in overall survival in patients with soft-tissue sarcomas.10 With regard to neoadjuvant chemotherapy for soft-tissue sarcomas, there are studies suggesting improvement in local control but no consistent survival benefit.11 Chemotherapy may yield a benefit in select cases, as detailed elsewhere in this supplement.
MECHANISMS OF ACTION: DIRECT AND INDIRECT
In simplified terms, radiation kills cancer cells through two basic mechanisms: indirect and direct.
The indirect effect (the most common mechanism) results from the generation of free radicals in the intracellular medium via ionization by photons. Free radicals, in turn, deposit large amounts of energy that damage DNA or some other vital component of the cell, resulting in cell death.
The direct effect is a consequence of photons themselves interacting directly with the cell in a lethal manner.
The goal of RT is to kill tumor cells selectively, without irreversibly injuring adjacent normal tissue. This is done by exploiting two abnormal aspects of tumor behavior: decreased ability for repair and increased susceptibility to ionizing radiation damage. Tumors are generally less able than normal tissue to repair DNA damage, owing to defective repair mechanisms. Tumor cells are also comparatively more radiosensitive than normal tissues, as they are more frequently in radiosensitive cell-cycle phases. Thus, dividing the radiation dose into a number of treatment fractions provides two advantages that further exploit the biologic differences between tumor and normal tissue: it allows DNA repair to take place within the normal tissues, and it allows proliferating tumor cells to redistribute through the cell cycle and move into the more radiosensitive phases.
TREATMENT PLANNING
Treatment simulation
Following initial consultation with a radiation oncologist, the eligible patient undergoes a simulation, or a treatment planning session in which he or she is positioned so as to allow treatment to be carefully designed and subsequently delivered with precision. This typically requires fabrication of a customized immobilization device to allow for consistent positioning over the treatment course. Sarcomas require that special care be taken to properly immobilize both the proximal and distal joints. Additionally, radiopaque wires are used to delineate the anatomic boundaries of the tumor or scar. Computed tomographic (CT) scans are then obtained to enable image-based three-dimensional treatment planning. The patient setup is photographed, and setup indicators are recorded and marked on the patient’s skin, some with freckle-size tattoos and some with indelible marker.
The treatment fields are then designed on the CT-simulation data set with the aid of virtual reality–type techniques. In addition to delineation of tumor volumes, three-dimensional treatment planning is used to contour all nearby normal structures on each slice. The resulting structures can then be used to specify dose constraints and help determine the optimal beam geometries to ensure proper tumor coverage and minimize the potential for side effects by reducing the dose to organs at risk. In the case of sarcomas, several strategies for reducing the risk of side effects are especially relevant: (1) carefully sparing a portion of the circumference of uninvolved bone to minimize the risk of fractures; (2) carefully sparing a strip of normal tissue to minimize edema by permitting undisrupted lymphatic drainage from the extremity; and (3) keeping dosing to joint spaces and other adjacent organs below tissue tolerances as defined by Emami et al.12
Determining target volume
The target volume for RT is determined on the basis of physical examination, radiologic studies, anatomical considerations, and the natural history of the sarcoma.
In the preoperative setting, longitudinal margins of 5 cm beyond the tumor and tumor-associated edema and radial margins of 2 cm are treated to 50 Gy in 25 fractions. Surgery is undertaken approximately 4 weeks after completion of RT to allow for repair in normal tissues and minimize operative and postoperative complications. Following surgery, an RT boost may be added for positive margins (16 Gy) or gross residual disease (25 Gy).
In the postoperative setting, details on the extent of dissection or observations from the surgeons themselves must be considered. Information regarding the surgical approach must be noted and can influence the effectiveness of postoperative RT as well as the incidence of late side effects. When experienced surgeons are involved, scars and drain sites, which are at risk for subclinical disease, can be planned so that their inclusion in the RT portal allows for sparing a strip of skin to minimize complications. Surgical clip placement at the boundaries of the tumor bed also facilitates RT planning.13 Finally, prophylactic bone stabilization may reduce the risk of subsequent fracture in cases where circumferential bone radiation in high-risk sites is anticipated.
Recommendations on the volume that must be treated vary among different authorities. Some advocate treating the entire compartment because of the risk for microscopic seeding.14 Others recommend margins around the tumor or tumor bed ranging from less than 5 cm up to 15 cm.15 Most often the postoperative approach is to include the resection bed with a 2-cm radial margin, the incision, and any drain sites in the initial treatment volume and to base the longitudinal margin on the grade and size of the primary tumor (5–15 cm). This volume is treated to 50 Gy in 25 fractions followed by two sequential reductions in field size, with the total dose determined by the extent of resection: 60 Gy for negative margins, 66 Gy for microscopically positive margins, and 75 Gy for gross residual disease.
TREATMENT DELIVERY
Once treatment planning is completed, treatments begin and are given daily Monday through Friday. Each day, the patient is positioned in the immobilization device, the field measurements are set, and positioning is checked with measurement tools and external marking of the field borders on the skin. Daily image guidance techniques may be used to increase setup reproducibility. Typical treatment times, including setup and actual delivery, are roughly 20 to 30 minutes daily.
While external beam RT is most commonly delivered as described above, brachytherapy, or intraoperative electron beam techniques, as well as proton or other charged-particle therapies, are also applied in selected cases.16–18
SIDE EFFECTS
Side effects of RT in the setting of sarcomas can be divided according to their onset—ie, acute versus delayed.
Acute effects. Skin changes ranging from erythema to moist desquamation in the skin overlying the high-dose volume are common. Major wound complications (delayed wound healing or need for surgical intervention) occur in approximately 17% of patients after surgical resection with postoperative RT, and perhaps more commonly (35%) with preoperative RT,8 though these rates vary widely in the literature. Another frequently reported acute side effect is fatigue.
Delayed sequelae after conservative resection and RT of extremity lesions include a reduction in range of motion secondary to joint contracture, edema, and fibrosis, as well as pain and bone fractures, all of which can significantly limit function of the preserved limb. In centers treating high volumes of patients with soft-tissue sarcoma, the incidence of moderate to severe late effects is less than 10%.19 In contrast to acute wound complications, a higher rate of late complications, including fibrosis and edema, have been observed with postoperative RT relative to preoperative RT.9 When necessary, high-dose RT does not appear to compromise the viability of skin grafts used to repair defects after sarcoma surgery if adequate time is allowed for healing.20
Regardless of the management approach, intensive rehabilitation led by physical therapy specialists is imperative in minimizing disabilities after treatment of soft-tissue sarcomas.
CONCLUSION
Outcomes of patients with musculoskeletal sarcomas are optimized at specialized sarcoma centers. For patients with soft-tissue sarcomas, effectively implementing an approach that combines conservative surgery and RT—and, in select cases, chemotherapy—achieves excellent local control rates while minimizing morbidity and maximizing long-term extremity function relative to aggressive surgery alone.
- Consensus conference. Limb-sparing treatment of adult soft-tissue sarcomas and osteosarcomas. JAMA 1985; 254:1791–1794.
- Gutierrez JC, Perez EA, Moffat FL, et al. Should soft tissue sarcomas be treated at high-volume centers? An analysis of 4,205 patients. Ann Surg 2007; 245:952–958.
- Halperin EC, Perez CA, Brady LW, eds. Perez and Brady’s Principles and Practice of Radiation Oncology. 5th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008.
- Glencross J, Balasubramanian SP, Bacon J, Robinson MH, Reed MW. An audit of the management of soft tissue sarcoma within a health region in the UK. Eur J Surg Oncol 2003; 29:670–675.
- Dunst J, Schuck A. Role of radiotherapy in Ewing tumors. Pediatr Blood Cancer 2004; 42:465–470.
- Schwartz SI, Brunicardi FC, eds. Schwartz’s Principles of Surgery. 9th ed. New York: McGraw-Hill, Medical Pub. Division; 2010.
- Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982; 196:305–315.
- O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 2002; 359:2235–2241.
- Davis AM, O’Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 2005; 75:48–53.
- Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised respectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet 1997; 350:1647–1654.
- Eilber FC, Tap WD, Nelson SD, Eckardt JJ, Eilber FR. Advances in chemotherapy for patients with extremity soft tissue sarcoma. Orthop Clin North Am 2006; 37:15–22.
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991; 21:109–122.
- Tepper J, Rosenberg SA, Glatstein E. Radiation therapy technique in soft tissue sarcomas of the extremity: policies of treatment at the National Cancer Institute. Int J Radiat Oncol Biol Phys 1982; 8:263–273.
- DeVita VT Jr, Lawrence TS, Rosenberg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology. 8th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2008.
- Suit HD, Spiro I. Role of radiation in the management of adult patients with sarcoma of soft tissue. Semin Surg Oncol 1994; 10:347–356.
- DeLaney TF, Trofimov AV, Engelsman M, Suit HD. Advanced-technology radiation therapy in the management of bone and soft tissue sarcomas. Cancer Control 2005; 12:27–35.
- DeLaney TF, Liebsch NJ, Pedlow FX, et al. Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys 2009; 74:732–739.
- Ishigami N, Suzuki K, Takahashi T, et al. Intimal sarcoma of aortic arch treated with proton therapy following surgery. Asian Cardiovasc Thorac Ann 2008; 16:e12–e14.
- Pollack A, Zagars GK, Goswitz MS, et al. Preoperative vs. postoperative radiotherapy in the treatment of soft tissue sarcomas: a matter of presentation. Int J Radiat Oncol Biol Phys 1998; 42:563–572.
- Lawrence WT, Zabell A, McDonald HD. The tolerance of skin grafts to postoperative radiation therapy in patients with soft-tissue sarcoma. Ann Plast Surg 1986; 16:204–210.
While radiation therapy (RT) has an integral role in the management of soft-tissue sarcoma, it has a limited role in that of bone sarcoma, with few exceptions (ie, Ewing sarcoma). In keeping with the rarity of these tumors, it has been demonstrated that patients treated at high-volume centers have significantly better survival and functional outcomes.1–3 Accordingly, treatment should be delivered by a multidisciplinary team including orthopedic, medical, and radiation oncologists, as well as plastic and reconstructive surgeons, physical therapy specialists, and pathologists and radiologists with expertise in musculoskeletal sarcomas.4 As the preceding articles in this supplement have addressed the major modalities in the treatment of sarcomas other than RT, this article will focus on how RT fits into the overall management mix, with a focus on soft-tissue sarcomas, where it figures most prominently.
BONE SARCOMAS: A LIMITED ROLE FOR RADIATION
The role of RT in the management of bone sarcomas is limited. Its primary application appears to be in Ewing sarcoma, for which curative treatment requires combined local and systemic therapy. For definitive therapy, limb-salvage surgery is preferable over amputation, but amputation may be an option for younger patients with lesions of the fibula, tibia, and foot. Based on the available data, postoperative RT is probably of benefit for all patients with Ewing sarcoma with close margins and/or those with a poor histologic response.5 Further discussion of Ewing sarcoma management is beyond the scope of this article (see the second and fifth articles in this supplement).
For osteosarcoma, the current standard of care is surgical resection combined with neoadjuvant and adjuvant chemotherapy. RT had been used years ago, prior to the advent of effective chemotherapy regimens, but its use for osteosarcoma has now been relegated to a few select situations. These include lesions not amenable to surgical resection and reconstruction, cases in which the patient refuses surgery, cases where there are positive margins after resection, and cases where palliation is needed for symptomatic lesions.
SOFT-TISSUE SARCOMAS: RADIATION HAS A CLEAR ADJUVANT ROLE
The primary management of localized soft-tissue sarcomas is surgical resection to achieve a negative margin when feasible. Historically, local excision of soft-tissue sarcomas resulted in local failure rates of 50% to 70%, even when a margin of normal tissue around the tumor was excised. As a result, amputation became standard treatment.6 In a landmark National Cancer Institute study 3 decades ago, patients were randomized to amputation or to limb-sparing surgery with the addition of RT.7 Notably, disease-free and overall survival were not compromised by limb-sparing surgery plus RT, demonstrating that although lesser surgery in the absence of RT may be insufficient, limb-sparing surgery with RT was equal to amputation. Consequently, limb-sparing approaches have become the favored surgery for the majority of cases of soft-tissue sarcoma, as advocated in a consensus statement from the National Institutes of Health.1
Indications vary by lesion grade
In general, adjuvant RT is recommended for all intermediate- and high-grade soft-tissue sarcoma lesions. A potential exception is a superficial tumor smaller than 5 cm with widely negative margins after resection. For low-grade lesions, re-excision is favored over adjuvant RT for positive or close margins, and RT is avoided in the setting of negative margins.
Optimal timing of radiation remains unclear
The optimal timing of adjuvant RT—preoperative versus postoperative—remains unknown. The relative advantages of preoperative RT include smaller and well-defined treatment volume, ability to use a lower dose, lack of tissue hypoxia, increased tumor resectability (smaller surgery), and improved limb function with less late fibrosis and edema. The disadvantages include inability to precisely stage patients and higher risk of acute wound-healing complications.
The National Cancer Institute of Canada compared outcomes with preoperative versus postoperative RT among 190 patients with soft-tissue sarcoma in a prospective randomized trial.8 Patients were stratified by tumor size (≤ 10 cm or > 10cm) and then randomized to preoperative RT (50 Gy in 25 fractions) or postoperative RT (66 Gy in 33 fractions).8 There was no difference between the groups in local control, distant control, or survival rates, but a higher rate of late complications, including fibrosis and edema, was observed with postoperative RT.8,9 On the other hand, the incidence of wound complications was higher in the preoperative group (35%) than in the postoperative group (17%).8
Likewise, the optimal sequencing and benefits of systemic therapy (chemotherapy) with relation to local therapy (surgery with pre- or postoperative RT) remain unclear. More than a dozen individual randomized trials of adjuvant chemotherapy, as well as a meta-analysis of 14 trials of doxorubicin-based adjuvant chemotherapy, have failed to demonstrate significant improvement in overall survival in patients with soft-tissue sarcomas.10 With regard to neoadjuvant chemotherapy for soft-tissue sarcomas, there are studies suggesting improvement in local control but no consistent survival benefit.11 Chemotherapy may yield a benefit in select cases, as detailed elsewhere in this supplement.
MECHANISMS OF ACTION: DIRECT AND INDIRECT
In simplified terms, radiation kills cancer cells through two basic mechanisms: indirect and direct.
The indirect effect (the most common mechanism) results from the generation of free radicals in the intracellular medium via ionization by photons. Free radicals, in turn, deposit large amounts of energy that damage DNA or some other vital component of the cell, resulting in cell death.
The direct effect is a consequence of photons themselves interacting directly with the cell in a lethal manner.
The goal of RT is to kill tumor cells selectively, without irreversibly injuring adjacent normal tissue. This is done by exploiting two abnormal aspects of tumor behavior: decreased ability for repair and increased susceptibility to ionizing radiation damage. Tumors are generally less able than normal tissue to repair DNA damage, owing to defective repair mechanisms. Tumor cells are also comparatively more radiosensitive than normal tissues, as they are more frequently in radiosensitive cell-cycle phases. Thus, dividing the radiation dose into a number of treatment fractions provides two advantages that further exploit the biologic differences between tumor and normal tissue: it allows DNA repair to take place within the normal tissues, and it allows proliferating tumor cells to redistribute through the cell cycle and move into the more radiosensitive phases.
TREATMENT PLANNING
Treatment simulation
Following initial consultation with a radiation oncologist, the eligible patient undergoes a simulation, or a treatment planning session in which he or she is positioned so as to allow treatment to be carefully designed and subsequently delivered with precision. This typically requires fabrication of a customized immobilization device to allow for consistent positioning over the treatment course. Sarcomas require that special care be taken to properly immobilize both the proximal and distal joints. Additionally, radiopaque wires are used to delineate the anatomic boundaries of the tumor or scar. Computed tomographic (CT) scans are then obtained to enable image-based three-dimensional treatment planning. The patient setup is photographed, and setup indicators are recorded and marked on the patient’s skin, some with freckle-size tattoos and some with indelible marker.
The treatment fields are then designed on the CT-simulation data set with the aid of virtual reality–type techniques. In addition to delineation of tumor volumes, three-dimensional treatment planning is used to contour all nearby normal structures on each slice. The resulting structures can then be used to specify dose constraints and help determine the optimal beam geometries to ensure proper tumor coverage and minimize the potential for side effects by reducing the dose to organs at risk. In the case of sarcomas, several strategies for reducing the risk of side effects are especially relevant: (1) carefully sparing a portion of the circumference of uninvolved bone to minimize the risk of fractures; (2) carefully sparing a strip of normal tissue to minimize edema by permitting undisrupted lymphatic drainage from the extremity; and (3) keeping dosing to joint spaces and other adjacent organs below tissue tolerances as defined by Emami et al.12
Determining target volume
The target volume for RT is determined on the basis of physical examination, radiologic studies, anatomical considerations, and the natural history of the sarcoma.
In the preoperative setting, longitudinal margins of 5 cm beyond the tumor and tumor-associated edema and radial margins of 2 cm are treated to 50 Gy in 25 fractions. Surgery is undertaken approximately 4 weeks after completion of RT to allow for repair in normal tissues and minimize operative and postoperative complications. Following surgery, an RT boost may be added for positive margins (16 Gy) or gross residual disease (25 Gy).
In the postoperative setting, details on the extent of dissection or observations from the surgeons themselves must be considered. Information regarding the surgical approach must be noted and can influence the effectiveness of postoperative RT as well as the incidence of late side effects. When experienced surgeons are involved, scars and drain sites, which are at risk for subclinical disease, can be planned so that their inclusion in the RT portal allows for sparing a strip of skin to minimize complications. Surgical clip placement at the boundaries of the tumor bed also facilitates RT planning.13 Finally, prophylactic bone stabilization may reduce the risk of subsequent fracture in cases where circumferential bone radiation in high-risk sites is anticipated.
Recommendations on the volume that must be treated vary among different authorities. Some advocate treating the entire compartment because of the risk for microscopic seeding.14 Others recommend margins around the tumor or tumor bed ranging from less than 5 cm up to 15 cm.15 Most often the postoperative approach is to include the resection bed with a 2-cm radial margin, the incision, and any drain sites in the initial treatment volume and to base the longitudinal margin on the grade and size of the primary tumor (5–15 cm). This volume is treated to 50 Gy in 25 fractions followed by two sequential reductions in field size, with the total dose determined by the extent of resection: 60 Gy for negative margins, 66 Gy for microscopically positive margins, and 75 Gy for gross residual disease.
TREATMENT DELIVERY
Once treatment planning is completed, treatments begin and are given daily Monday through Friday. Each day, the patient is positioned in the immobilization device, the field measurements are set, and positioning is checked with measurement tools and external marking of the field borders on the skin. Daily image guidance techniques may be used to increase setup reproducibility. Typical treatment times, including setup and actual delivery, are roughly 20 to 30 minutes daily.
While external beam RT is most commonly delivered as described above, brachytherapy, or intraoperative electron beam techniques, as well as proton or other charged-particle therapies, are also applied in selected cases.16–18
SIDE EFFECTS
Side effects of RT in the setting of sarcomas can be divided according to their onset—ie, acute versus delayed.
Acute effects. Skin changes ranging from erythema to moist desquamation in the skin overlying the high-dose volume are common. Major wound complications (delayed wound healing or need for surgical intervention) occur in approximately 17% of patients after surgical resection with postoperative RT, and perhaps more commonly (35%) with preoperative RT,8 though these rates vary widely in the literature. Another frequently reported acute side effect is fatigue.
Delayed sequelae after conservative resection and RT of extremity lesions include a reduction in range of motion secondary to joint contracture, edema, and fibrosis, as well as pain and bone fractures, all of which can significantly limit function of the preserved limb. In centers treating high volumes of patients with soft-tissue sarcoma, the incidence of moderate to severe late effects is less than 10%.19 In contrast to acute wound complications, a higher rate of late complications, including fibrosis and edema, have been observed with postoperative RT relative to preoperative RT.9 When necessary, high-dose RT does not appear to compromise the viability of skin grafts used to repair defects after sarcoma surgery if adequate time is allowed for healing.20
Regardless of the management approach, intensive rehabilitation led by physical therapy specialists is imperative in minimizing disabilities after treatment of soft-tissue sarcomas.
CONCLUSION
Outcomes of patients with musculoskeletal sarcomas are optimized at specialized sarcoma centers. For patients with soft-tissue sarcomas, effectively implementing an approach that combines conservative surgery and RT—and, in select cases, chemotherapy—achieves excellent local control rates while minimizing morbidity and maximizing long-term extremity function relative to aggressive surgery alone.
While radiation therapy (RT) has an integral role in the management of soft-tissue sarcoma, it has a limited role in that of bone sarcoma, with few exceptions (ie, Ewing sarcoma). In keeping with the rarity of these tumors, it has been demonstrated that patients treated at high-volume centers have significantly better survival and functional outcomes.1–3 Accordingly, treatment should be delivered by a multidisciplinary team including orthopedic, medical, and radiation oncologists, as well as plastic and reconstructive surgeons, physical therapy specialists, and pathologists and radiologists with expertise in musculoskeletal sarcomas.4 As the preceding articles in this supplement have addressed the major modalities in the treatment of sarcomas other than RT, this article will focus on how RT fits into the overall management mix, with a focus on soft-tissue sarcomas, where it figures most prominently.
BONE SARCOMAS: A LIMITED ROLE FOR RADIATION
The role of RT in the management of bone sarcomas is limited. Its primary application appears to be in Ewing sarcoma, for which curative treatment requires combined local and systemic therapy. For definitive therapy, limb-salvage surgery is preferable over amputation, but amputation may be an option for younger patients with lesions of the fibula, tibia, and foot. Based on the available data, postoperative RT is probably of benefit for all patients with Ewing sarcoma with close margins and/or those with a poor histologic response.5 Further discussion of Ewing sarcoma management is beyond the scope of this article (see the second and fifth articles in this supplement).
For osteosarcoma, the current standard of care is surgical resection combined with neoadjuvant and adjuvant chemotherapy. RT had been used years ago, prior to the advent of effective chemotherapy regimens, but its use for osteosarcoma has now been relegated to a few select situations. These include lesions not amenable to surgical resection and reconstruction, cases in which the patient refuses surgery, cases where there are positive margins after resection, and cases where palliation is needed for symptomatic lesions.
SOFT-TISSUE SARCOMAS: RADIATION HAS A CLEAR ADJUVANT ROLE
The primary management of localized soft-tissue sarcomas is surgical resection to achieve a negative margin when feasible. Historically, local excision of soft-tissue sarcomas resulted in local failure rates of 50% to 70%, even when a margin of normal tissue around the tumor was excised. As a result, amputation became standard treatment.6 In a landmark National Cancer Institute study 3 decades ago, patients were randomized to amputation or to limb-sparing surgery with the addition of RT.7 Notably, disease-free and overall survival were not compromised by limb-sparing surgery plus RT, demonstrating that although lesser surgery in the absence of RT may be insufficient, limb-sparing surgery with RT was equal to amputation. Consequently, limb-sparing approaches have become the favored surgery for the majority of cases of soft-tissue sarcoma, as advocated in a consensus statement from the National Institutes of Health.1
Indications vary by lesion grade
In general, adjuvant RT is recommended for all intermediate- and high-grade soft-tissue sarcoma lesions. A potential exception is a superficial tumor smaller than 5 cm with widely negative margins after resection. For low-grade lesions, re-excision is favored over adjuvant RT for positive or close margins, and RT is avoided in the setting of negative margins.
Optimal timing of radiation remains unclear
The optimal timing of adjuvant RT—preoperative versus postoperative—remains unknown. The relative advantages of preoperative RT include smaller and well-defined treatment volume, ability to use a lower dose, lack of tissue hypoxia, increased tumor resectability (smaller surgery), and improved limb function with less late fibrosis and edema. The disadvantages include inability to precisely stage patients and higher risk of acute wound-healing complications.
The National Cancer Institute of Canada compared outcomes with preoperative versus postoperative RT among 190 patients with soft-tissue sarcoma in a prospective randomized trial.8 Patients were stratified by tumor size (≤ 10 cm or > 10cm) and then randomized to preoperative RT (50 Gy in 25 fractions) or postoperative RT (66 Gy in 33 fractions).8 There was no difference between the groups in local control, distant control, or survival rates, but a higher rate of late complications, including fibrosis and edema, was observed with postoperative RT.8,9 On the other hand, the incidence of wound complications was higher in the preoperative group (35%) than in the postoperative group (17%).8
Likewise, the optimal sequencing and benefits of systemic therapy (chemotherapy) with relation to local therapy (surgery with pre- or postoperative RT) remain unclear. More than a dozen individual randomized trials of adjuvant chemotherapy, as well as a meta-analysis of 14 trials of doxorubicin-based adjuvant chemotherapy, have failed to demonstrate significant improvement in overall survival in patients with soft-tissue sarcomas.10 With regard to neoadjuvant chemotherapy for soft-tissue sarcomas, there are studies suggesting improvement in local control but no consistent survival benefit.11 Chemotherapy may yield a benefit in select cases, as detailed elsewhere in this supplement.
MECHANISMS OF ACTION: DIRECT AND INDIRECT
In simplified terms, radiation kills cancer cells through two basic mechanisms: indirect and direct.
The indirect effect (the most common mechanism) results from the generation of free radicals in the intracellular medium via ionization by photons. Free radicals, in turn, deposit large amounts of energy that damage DNA or some other vital component of the cell, resulting in cell death.
The direct effect is a consequence of photons themselves interacting directly with the cell in a lethal manner.
The goal of RT is to kill tumor cells selectively, without irreversibly injuring adjacent normal tissue. This is done by exploiting two abnormal aspects of tumor behavior: decreased ability for repair and increased susceptibility to ionizing radiation damage. Tumors are generally less able than normal tissue to repair DNA damage, owing to defective repair mechanisms. Tumor cells are also comparatively more radiosensitive than normal tissues, as they are more frequently in radiosensitive cell-cycle phases. Thus, dividing the radiation dose into a number of treatment fractions provides two advantages that further exploit the biologic differences between tumor and normal tissue: it allows DNA repair to take place within the normal tissues, and it allows proliferating tumor cells to redistribute through the cell cycle and move into the more radiosensitive phases.
TREATMENT PLANNING
Treatment simulation
Following initial consultation with a radiation oncologist, the eligible patient undergoes a simulation, or a treatment planning session in which he or she is positioned so as to allow treatment to be carefully designed and subsequently delivered with precision. This typically requires fabrication of a customized immobilization device to allow for consistent positioning over the treatment course. Sarcomas require that special care be taken to properly immobilize both the proximal and distal joints. Additionally, radiopaque wires are used to delineate the anatomic boundaries of the tumor or scar. Computed tomographic (CT) scans are then obtained to enable image-based three-dimensional treatment planning. The patient setup is photographed, and setup indicators are recorded and marked on the patient’s skin, some with freckle-size tattoos and some with indelible marker.
The treatment fields are then designed on the CT-simulation data set with the aid of virtual reality–type techniques. In addition to delineation of tumor volumes, three-dimensional treatment planning is used to contour all nearby normal structures on each slice. The resulting structures can then be used to specify dose constraints and help determine the optimal beam geometries to ensure proper tumor coverage and minimize the potential for side effects by reducing the dose to organs at risk. In the case of sarcomas, several strategies for reducing the risk of side effects are especially relevant: (1) carefully sparing a portion of the circumference of uninvolved bone to minimize the risk of fractures; (2) carefully sparing a strip of normal tissue to minimize edema by permitting undisrupted lymphatic drainage from the extremity; and (3) keeping dosing to joint spaces and other adjacent organs below tissue tolerances as defined by Emami et al.12
Determining target volume
The target volume for RT is determined on the basis of physical examination, radiologic studies, anatomical considerations, and the natural history of the sarcoma.
In the preoperative setting, longitudinal margins of 5 cm beyond the tumor and tumor-associated edema and radial margins of 2 cm are treated to 50 Gy in 25 fractions. Surgery is undertaken approximately 4 weeks after completion of RT to allow for repair in normal tissues and minimize operative and postoperative complications. Following surgery, an RT boost may be added for positive margins (16 Gy) or gross residual disease (25 Gy).
In the postoperative setting, details on the extent of dissection or observations from the surgeons themselves must be considered. Information regarding the surgical approach must be noted and can influence the effectiveness of postoperative RT as well as the incidence of late side effects. When experienced surgeons are involved, scars and drain sites, which are at risk for subclinical disease, can be planned so that their inclusion in the RT portal allows for sparing a strip of skin to minimize complications. Surgical clip placement at the boundaries of the tumor bed also facilitates RT planning.13 Finally, prophylactic bone stabilization may reduce the risk of subsequent fracture in cases where circumferential bone radiation in high-risk sites is anticipated.
Recommendations on the volume that must be treated vary among different authorities. Some advocate treating the entire compartment because of the risk for microscopic seeding.14 Others recommend margins around the tumor or tumor bed ranging from less than 5 cm up to 15 cm.15 Most often the postoperative approach is to include the resection bed with a 2-cm radial margin, the incision, and any drain sites in the initial treatment volume and to base the longitudinal margin on the grade and size of the primary tumor (5–15 cm). This volume is treated to 50 Gy in 25 fractions followed by two sequential reductions in field size, with the total dose determined by the extent of resection: 60 Gy for negative margins, 66 Gy for microscopically positive margins, and 75 Gy for gross residual disease.
TREATMENT DELIVERY
Once treatment planning is completed, treatments begin and are given daily Monday through Friday. Each day, the patient is positioned in the immobilization device, the field measurements are set, and positioning is checked with measurement tools and external marking of the field borders on the skin. Daily image guidance techniques may be used to increase setup reproducibility. Typical treatment times, including setup and actual delivery, are roughly 20 to 30 minutes daily.
While external beam RT is most commonly delivered as described above, brachytherapy, or intraoperative electron beam techniques, as well as proton or other charged-particle therapies, are also applied in selected cases.16–18
SIDE EFFECTS
Side effects of RT in the setting of sarcomas can be divided according to their onset—ie, acute versus delayed.
Acute effects. Skin changes ranging from erythema to moist desquamation in the skin overlying the high-dose volume are common. Major wound complications (delayed wound healing or need for surgical intervention) occur in approximately 17% of patients after surgical resection with postoperative RT, and perhaps more commonly (35%) with preoperative RT,8 though these rates vary widely in the literature. Another frequently reported acute side effect is fatigue.
Delayed sequelae after conservative resection and RT of extremity lesions include a reduction in range of motion secondary to joint contracture, edema, and fibrosis, as well as pain and bone fractures, all of which can significantly limit function of the preserved limb. In centers treating high volumes of patients with soft-tissue sarcoma, the incidence of moderate to severe late effects is less than 10%.19 In contrast to acute wound complications, a higher rate of late complications, including fibrosis and edema, have been observed with postoperative RT relative to preoperative RT.9 When necessary, high-dose RT does not appear to compromise the viability of skin grafts used to repair defects after sarcoma surgery if adequate time is allowed for healing.20
Regardless of the management approach, intensive rehabilitation led by physical therapy specialists is imperative in minimizing disabilities after treatment of soft-tissue sarcomas.
CONCLUSION
Outcomes of patients with musculoskeletal sarcomas are optimized at specialized sarcoma centers. For patients with soft-tissue sarcomas, effectively implementing an approach that combines conservative surgery and RT—and, in select cases, chemotherapy—achieves excellent local control rates while minimizing morbidity and maximizing long-term extremity function relative to aggressive surgery alone.
- Consensus conference. Limb-sparing treatment of adult soft-tissue sarcomas and osteosarcomas. JAMA 1985; 254:1791–1794.
- Gutierrez JC, Perez EA, Moffat FL, et al. Should soft tissue sarcomas be treated at high-volume centers? An analysis of 4,205 patients. Ann Surg 2007; 245:952–958.
- Halperin EC, Perez CA, Brady LW, eds. Perez and Brady’s Principles and Practice of Radiation Oncology. 5th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008.
- Glencross J, Balasubramanian SP, Bacon J, Robinson MH, Reed MW. An audit of the management of soft tissue sarcoma within a health region in the UK. Eur J Surg Oncol 2003; 29:670–675.
- Dunst J, Schuck A. Role of radiotherapy in Ewing tumors. Pediatr Blood Cancer 2004; 42:465–470.
- Schwartz SI, Brunicardi FC, eds. Schwartz’s Principles of Surgery. 9th ed. New York: McGraw-Hill, Medical Pub. Division; 2010.
- Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982; 196:305–315.
- O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 2002; 359:2235–2241.
- Davis AM, O’Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 2005; 75:48–53.
- Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised respectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet 1997; 350:1647–1654.
- Eilber FC, Tap WD, Nelson SD, Eckardt JJ, Eilber FR. Advances in chemotherapy for patients with extremity soft tissue sarcoma. Orthop Clin North Am 2006; 37:15–22.
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991; 21:109–122.
- Tepper J, Rosenberg SA, Glatstein E. Radiation therapy technique in soft tissue sarcomas of the extremity: policies of treatment at the National Cancer Institute. Int J Radiat Oncol Biol Phys 1982; 8:263–273.
- DeVita VT Jr, Lawrence TS, Rosenberg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology. 8th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2008.
- Suit HD, Spiro I. Role of radiation in the management of adult patients with sarcoma of soft tissue. Semin Surg Oncol 1994; 10:347–356.
- DeLaney TF, Trofimov AV, Engelsman M, Suit HD. Advanced-technology radiation therapy in the management of bone and soft tissue sarcomas. Cancer Control 2005; 12:27–35.
- DeLaney TF, Liebsch NJ, Pedlow FX, et al. Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys 2009; 74:732–739.
- Ishigami N, Suzuki K, Takahashi T, et al. Intimal sarcoma of aortic arch treated with proton therapy following surgery. Asian Cardiovasc Thorac Ann 2008; 16:e12–e14.
- Pollack A, Zagars GK, Goswitz MS, et al. Preoperative vs. postoperative radiotherapy in the treatment of soft tissue sarcomas: a matter of presentation. Int J Radiat Oncol Biol Phys 1998; 42:563–572.
- Lawrence WT, Zabell A, McDonald HD. The tolerance of skin grafts to postoperative radiation therapy in patients with soft-tissue sarcoma. Ann Plast Surg 1986; 16:204–210.
- Consensus conference. Limb-sparing treatment of adult soft-tissue sarcomas and osteosarcomas. JAMA 1985; 254:1791–1794.
- Gutierrez JC, Perez EA, Moffat FL, et al. Should soft tissue sarcomas be treated at high-volume centers? An analysis of 4,205 patients. Ann Surg 2007; 245:952–958.
- Halperin EC, Perez CA, Brady LW, eds. Perez and Brady’s Principles and Practice of Radiation Oncology. 5th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008.
- Glencross J, Balasubramanian SP, Bacon J, Robinson MH, Reed MW. An audit of the management of soft tissue sarcoma within a health region in the UK. Eur J Surg Oncol 2003; 29:670–675.
- Dunst J, Schuck A. Role of radiotherapy in Ewing tumors. Pediatr Blood Cancer 2004; 42:465–470.
- Schwartz SI, Brunicardi FC, eds. Schwartz’s Principles of Surgery. 9th ed. New York: McGraw-Hill, Medical Pub. Division; 2010.
- Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982; 196:305–315.
- O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 2002; 359:2235–2241.
- Davis AM, O’Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 2005; 75:48–53.
- Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised respectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet 1997; 350:1647–1654.
- Eilber FC, Tap WD, Nelson SD, Eckardt JJ, Eilber FR. Advances in chemotherapy for patients with extremity soft tissue sarcoma. Orthop Clin North Am 2006; 37:15–22.
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991; 21:109–122.
- Tepper J, Rosenberg SA, Glatstein E. Radiation therapy technique in soft tissue sarcomas of the extremity: policies of treatment at the National Cancer Institute. Int J Radiat Oncol Biol Phys 1982; 8:263–273.
- DeVita VT Jr, Lawrence TS, Rosenberg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology. 8th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2008.
- Suit HD, Spiro I. Role of radiation in the management of adult patients with sarcoma of soft tissue. Semin Surg Oncol 1994; 10:347–356.
- DeLaney TF, Trofimov AV, Engelsman M, Suit HD. Advanced-technology radiation therapy in the management of bone and soft tissue sarcomas. Cancer Control 2005; 12:27–35.
- DeLaney TF, Liebsch NJ, Pedlow FX, et al. Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys 2009; 74:732–739.
- Ishigami N, Suzuki K, Takahashi T, et al. Intimal sarcoma of aortic arch treated with proton therapy following surgery. Asian Cardiovasc Thorac Ann 2008; 16:e12–e14.
- Pollack A, Zagars GK, Goswitz MS, et al. Preoperative vs. postoperative radiotherapy in the treatment of soft tissue sarcomas: a matter of presentation. Int J Radiat Oncol Biol Phys 1998; 42:563–572.
- Lawrence WT, Zabell A, McDonald HD. The tolerance of skin grafts to postoperative radiation therapy in patients with soft-tissue sarcoma. Ann Plast Surg 1986; 16:204–210.