User login
Product updates and reviews
REVIEW
Butterfly iQ+: Offering day-to-day portable ultrasound tech
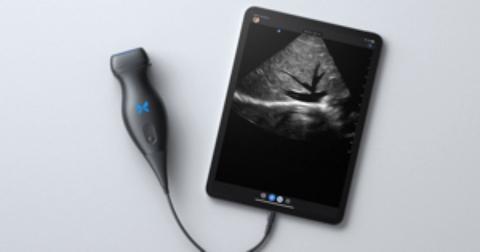
The Butterfly iQ+ app with an ultrasound probe and cable is available from Butterfly Network, Inc, in Guilford, Connecticut.
Background. It could be reasonably argued that ultrasonography has surpassed the speculum as the single most important tool in ObGyn. From its origins in 1949 with the pioneering work of George Ludwig using A-mode (amplitude-mode) ultrasound and the first publication of its use in pregnancy using B-mode (brightness-mode) ultrasound in Lancet in 1958 by Donald and colleagues, this technology has become so ingrained into ObGyn that it is often frustrating to practice comfortably without it. Thus, today, the biggest question facing most practitioners is not whether or not to have an ultrasound in their practice but which one to have.
Given the wide range of quality, functionality, and price within the ultrasound device space, choosing the right technology can feel as daunting as choosing the perfect restaurant in New York City. That said, when looking for entry-level ultrasound technology to address the day-to-day basic needs of your average ObGyn, the Butterfly iQ+ may be an easy choice.
Design/Functionality. The Butterfly iQ+ app does not come with a screen. Rather, the device is compatible with both iOS and Android systems and readily connects to a vast array of easily purchased devices, with either lightening or USB-C ports. In our office, we use an iPad mini. The probe is lightweight (309 g) and contains a rechargeable 2600 mAh lithium ion battery, so that its power source is independent of the device to which it is attached. The probe is a 2D array with 9000 micro-machined sensors. It allows for imaging using M-mode, B-mode, Color Doppler, Power Doppler, and Pulsed Wave Doppler. (I don’t know what the last two are or what they are used for, but they sound important.) It has a scan depth range of 1 cm to 30 cm. The downloadable Butterfly iQ+ app that has the software that makes the probe functional has more tools, controls, and presets than anyone could ever need. But that’s not all. The App has data encrypted HIPAA/HITECH-compliant Cloud-based connectivity that offers unlimited image storage, access to reports, and embedded CPT codes should billing capabilities be needed.
The true beauty of the Butterfly iQ+ is that the image quality is awesome and it is really easy to use. The software is mostly intuitive and takes only a minimal effort to learn. The device holds its charge more than adequately for a day in the office and the recharging process is fast and easy. When it comes to the device’s design and functionality–as a Capricorn–I am still looking for its flaws.
Innovation. The real innovations of the Butterfly iQ+ are its “ultrasound-on-a-chip”™ technology and its incorporation of a rechargeable battery into the probe. This combination allows for crystal clear imaging in a cordless, portable device. While most other similar technologies waste their time, technology, space, and cost on the screen, the Butterfly iQ+ punted on that challenge and put all their efforts into the probe and the software. It was a great choice.
Summary. In our office, the Butterfly iQ+ has changed the way we practice. Our trusty fetal dopplers are mostly gone, having been replaced by the Butterfly iQ+. At almost every prenatal visit, patients can now see their baby rather than just hear the heartbeat (and they can hear it too if they want by using the M-mode functionality on the device). Patients love it, and so do the doctors. Instead of just hearing heart beats, fetal position and quick fluid checks are now routine, so we think our care is actually a little better than it was. The Butterfly iQ+ is also great for confirming IUD locations after placement or when the strings are not visible. All-in-all, I love this product. Who doesn’t love butterflies?!
For more information, visit https://www.butterflynetwork.com
The views of the author are personal opinions and do not necessarily represent the views of OBG
References
- Kaproth-Joslin KA, Nicola R, Dogra VS. The History of US: from bats and boats to the bedside and beyond: RSNA centennial article. Radiographics. 2015;35:960-970.
- Donald I, MacVicar J, Brown TG. Investigation of abdominal masses by pulsed ultrasound. Lancet. 1958;1:1188-1195.
REVIEW
Butterfly iQ+: Offering day-to-day portable ultrasound tech
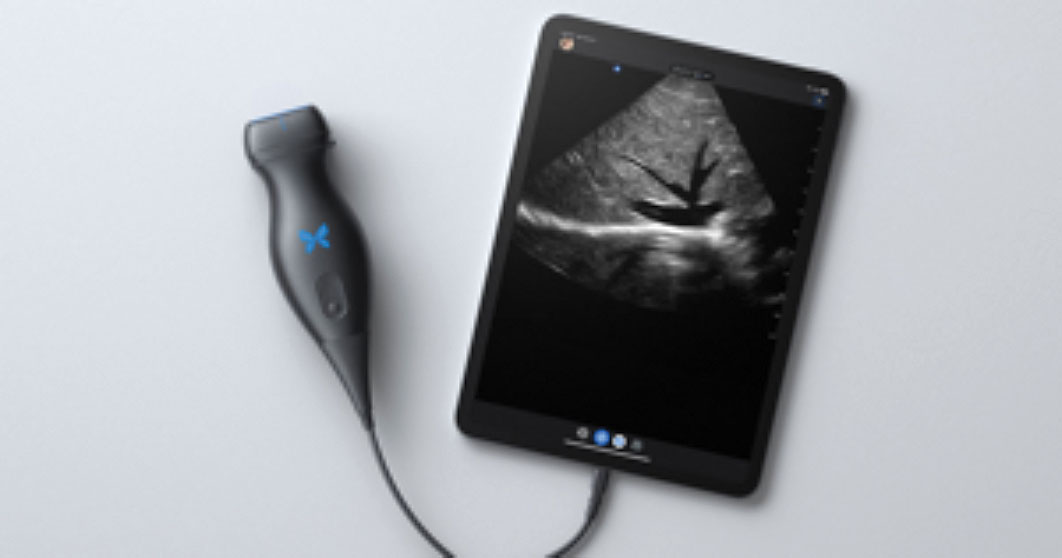
The Butterfly iQ+ app with an ultrasound probe and cable is available from Butterfly Network, Inc, in Guilford, Connecticut.
Background. It could be reasonably argued that ultrasonography has surpassed the speculum as the single most important tool in ObGyn. From its origins in 1949 with the pioneering work of George Ludwig using A-mode (amplitude-mode) ultrasound and the first publication of its use in pregnancy using B-mode (brightness-mode) ultrasound in Lancet in 1958 by Donald and colleagues, this technology has become so ingrained into ObGyn that it is often frustrating to practice comfortably without it. Thus, today, the biggest question facing most practitioners is not whether or not to have an ultrasound in their practice but which one to have.
Given the wide range of quality, functionality, and price within the ultrasound device space, choosing the right technology can feel as daunting as choosing the perfect restaurant in New York City. That said, when looking for entry-level ultrasound technology to address the day-to-day basic needs of your average ObGyn, the Butterfly iQ+ may be an easy choice.
Design/Functionality. The Butterfly iQ+ app does not come with a screen. Rather, the device is compatible with both iOS and Android systems and readily connects to a vast array of easily purchased devices, with either lightening or USB-C ports. In our office, we use an iPad mini. The probe is lightweight (309 g) and contains a rechargeable 2600 mAh lithium ion battery, so that its power source is independent of the device to which it is attached. The probe is a 2D array with 9000 micro-machined sensors. It allows for imaging using M-mode, B-mode, Color Doppler, Power Doppler, and Pulsed Wave Doppler. (I don’t know what the last two are or what they are used for, but they sound important.) It has a scan depth range of 1 cm to 30 cm. The downloadable Butterfly iQ+ app that has the software that makes the probe functional has more tools, controls, and presets than anyone could ever need. But that’s not all. The App has data encrypted HIPAA/HITECH-compliant Cloud-based connectivity that offers unlimited image storage, access to reports, and embedded CPT codes should billing capabilities be needed.
The true beauty of the Butterfly iQ+ is that the image quality is awesome and it is really easy to use. The software is mostly intuitive and takes only a minimal effort to learn. The device holds its charge more than adequately for a day in the office and the recharging process is fast and easy. When it comes to the device’s design and functionality–as a Capricorn–I am still looking for its flaws.
Innovation. The real innovations of the Butterfly iQ+ are its “ultrasound-on-a-chip”™ technology and its incorporation of a rechargeable battery into the probe. This combination allows for crystal clear imaging in a cordless, portable device. While most other similar technologies waste their time, technology, space, and cost on the screen, the Butterfly iQ+ punted on that challenge and put all their efforts into the probe and the software. It was a great choice.
Summary. In our office, the Butterfly iQ+ has changed the way we practice. Our trusty fetal dopplers are mostly gone, having been replaced by the Butterfly iQ+. At almost every prenatal visit, patients can now see their baby rather than just hear the heartbeat (and they can hear it too if they want by using the M-mode functionality on the device). Patients love it, and so do the doctors. Instead of just hearing heart beats, fetal position and quick fluid checks are now routine, so we think our care is actually a little better than it was. The Butterfly iQ+ is also great for confirming IUD locations after placement or when the strings are not visible. All-in-all, I love this product. Who doesn’t love butterflies?!
For more information, visit https://www.butterflynetwork.com
The views of the author are personal opinions and do not necessarily represent the views of OBG
References
- Kaproth-Joslin KA, Nicola R, Dogra VS. The History of US: from bats and boats to the bedside and beyond: RSNA centennial article. Radiographics. 2015;35:960-970.
- Donald I, MacVicar J, Brown TG. Investigation of abdominal masses by pulsed ultrasound. Lancet. 1958;1:1188-1195.
REVIEW
Butterfly iQ+: Offering day-to-day portable ultrasound tech
The Butterfly iQ+ app with an ultrasound probe and cable is available from Butterfly Network, Inc, in Guilford, Connecticut.
Background. It could be reasonably argued that ultrasonography has surpassed the speculum as the single most important tool in ObGyn. From its origins in 1949 with the pioneering work of George Ludwig using A-mode (amplitude-mode) ultrasound and the first publication of its use in pregnancy using B-mode (brightness-mode) ultrasound in Lancet in 1958 by Donald and colleagues, this technology has become so ingrained into ObGyn that it is often frustrating to practice comfortably without it. Thus, today, the biggest question facing most practitioners is not whether or not to have an ultrasound in their practice but which one to have.
Given the wide range of quality, functionality, and price within the ultrasound device space, choosing the right technology can feel as daunting as choosing the perfect restaurant in New York City. That said, when looking for entry-level ultrasound technology to address the day-to-day basic needs of your average ObGyn, the Butterfly iQ+ may be an easy choice.
Design/Functionality. The Butterfly iQ+ app does not come with a screen. Rather, the device is compatible with both iOS and Android systems and readily connects to a vast array of easily purchased devices, with either lightening or USB-C ports. In our office, we use an iPad mini. The probe is lightweight (309 g) and contains a rechargeable 2600 mAh lithium ion battery, so that its power source is independent of the device to which it is attached. The probe is a 2D array with 9000 micro-machined sensors. It allows for imaging using M-mode, B-mode, Color Doppler, Power Doppler, and Pulsed Wave Doppler. (I don’t know what the last two are or what they are used for, but they sound important.) It has a scan depth range of 1 cm to 30 cm. The downloadable Butterfly iQ+ app that has the software that makes the probe functional has more tools, controls, and presets than anyone could ever need. But that’s not all. The App has data encrypted HIPAA/HITECH-compliant Cloud-based connectivity that offers unlimited image storage, access to reports, and embedded CPT codes should billing capabilities be needed.
The true beauty of the Butterfly iQ+ is that the image quality is awesome and it is really easy to use. The software is mostly intuitive and takes only a minimal effort to learn. The device holds its charge more than adequately for a day in the office and the recharging process is fast and easy. When it comes to the device’s design and functionality–as a Capricorn–I am still looking for its flaws.
Innovation. The real innovations of the Butterfly iQ+ are its “ultrasound-on-a-chip”™ technology and its incorporation of a rechargeable battery into the probe. This combination allows for crystal clear imaging in a cordless, portable device. While most other similar technologies waste their time, technology, space, and cost on the screen, the Butterfly iQ+ punted on that challenge and put all their efforts into the probe and the software. It was a great choice.
Summary. In our office, the Butterfly iQ+ has changed the way we practice. Our trusty fetal dopplers are mostly gone, having been replaced by the Butterfly iQ+. At almost every prenatal visit, patients can now see their baby rather than just hear the heartbeat (and they can hear it too if they want by using the M-mode functionality on the device). Patients love it, and so do the doctors. Instead of just hearing heart beats, fetal position and quick fluid checks are now routine, so we think our care is actually a little better than it was. The Butterfly iQ+ is also great for confirming IUD locations after placement or when the strings are not visible. All-in-all, I love this product. Who doesn’t love butterflies?!
For more information, visit https://www.butterflynetwork.com
The views of the author are personal opinions and do not necessarily represent the views of OBG
References
- Kaproth-Joslin KA, Nicola R, Dogra VS. The History of US: from bats and boats to the bedside and beyond: RSNA centennial article. Radiographics. 2015;35:960-970.
- Donald I, MacVicar J, Brown TG. Investigation of abdominal masses by pulsed ultrasound. Lancet. 1958;1:1188-1195.
Commentary: Topical treatments, dupilumab, and long-term treatment of AD, July 2023
Silverberg and colleagues described a very well-designed, vehicle-controlled, randomized 8-week study of a topical formulation of a purified strain of Nitrosomonas eutropha, an ammonia-oxidizing bacterium. In theory, this bacterium may reduce Staphylococcus aureus. The study compared two concentrations of the bacterium vs vehicle delivered as a spray twice per day. Study participants were adults with AD affecting 10%-40% of body surface area.
The study found "meaningful" improvements in itch and objective signs of disease, with clear separation between both doses of the bacterial spray compared with vehicle. At week 4, about 23% of participants treated with the bacterium were clear or almost clear (with a 2-point improvement) compared with 12% in the vehicle group (for comparison, in a phase 2 study comparing topical ruxolitinib with 0.1% triamcinolone cream, there was a 25% clear or almost clear rate [with 2-point improvement] in the triamcinolone-treated individuals).
Though an "all-natural" bacterial approach to managing AD may be appealing to some, it sounded like magic to me. But this well-done study makes it seem like the bacterial approach could be more promising than I had thought. This study also reported about twice as many adverse events (including gastrointestinal issues) with the bacterium-treated participants compared with those who received vehicle, adding to my belief that the bacterial product has efficacy. Whether any other topical will be more effective and safer than is topical triamcinolone remains to be seen. I'm still pessimistic about topicals because of patients' poor adherence to topical treatment, but perhaps an easy-to-use spray that isn't associated with patients' fear of "steroids" will be helpful.
Chen and colleagues. They analyzed data on hundreds of thousands of patients with and without AD. Adults with AD had a "significantly increased risk" of developing venous thromboembolism compared with adults without AD. The huge sample size of their study seems compelling. That huge sample size allows detection of effects so small that they may be clinically insignificant.
They report that patients with AD had a venous thromboembolism at a rate of 1.05/1000 patients-years; the rate was 0.82 for patients without AD. From that, we can calculate that there would be an additional 23 patients with venous thromboembolism for every 100,000 patient-years or about one more venous thromboembolism in the AD group in every 4000 patient-years. Though the finding was statistically significant, I don't think it is clinically meaningful.
The authors correctly conclude that "vascular examination and consultation with the emergency department, cardiologists, or pulmonologists are indicated for patients with AD who present with relevant symptoms (eg, unexplained dyspnea, chest tightness, and limb swelling)." But it is probably also true that vascular examination and consultation with the emergency department, cardiologists, or pulmonologists are indicated for patients without AD who present with those symptoms. I think the authors might have been on solid ground if they had concluded that there was a statistically significant but clinically insignificant increased risk for venous thromboembolism in patients with AD.
Eichenfeld and colleagues examined the use of topical crisaborole once per day as a maintenance treatment for patients with mild to moderate AD. The study compared patients given topical crisaborole with those randomly assigned to vehicle. The active treatment was effective because topical crisaborole treated patients had longer times to the first flare following treatment and fewer flares over the 1 year of treatment. The differences were not huge, but I think they were clinically meaningful. I'm guessing that the topical crisaborole maintenance treatment would have been even more effective had it been used regularly. The study did not, as far as I could tell, assess how well the treatment was used.
An interesting aspect of this study is that it began with nearly 500 participants who started on twice daily topical crisaborole. The 270 patients who responded to the treatment (achieving clear or almost clear with at least a 2-point improvement) were enrolled in the 1-year maintenance phase. Thus, the participants in the maintenance phase were preselected for patients who respond to topical crisaborole. We don't know why they were responders (I, of course, expect it is because they selected for patients who are better than others are at using a topical treatment), but it may be best not to try to generalize these results and assume this form of maintenance treatment would work equally well in a population who achieve initial success with an oral therapy regimen (for example, a quick course of oral prednisone).
Dupilumab was a revolutionary treatment for AD. I didn't think that I'd ever see a more effective treatment. It's so safe too! It has been a first-line treatment for AD since its introduction. Now, we also have oral Janus kinase inhibitor options. Blauvelt and colleagues examined what happens when patients who have been on dupilumab are switched to a high dose (30 mg/d) of upadacitinib (the standard starting dose of upadacitinib is 15 mg/d). Though dupilumab is very effective, upadacitinib is more so. After 4 weeks of switching to upadacitinib, nearly half the patients were completely clear of AD compared with only 16.0% after 24 weeks of dupilumab! The authors point out, optimistically, that "No new safety risks were observed." Though there were no cancers, gastrointestinal perforations, major adverse cardiovascular events, or venous thromboembolic events, there were cases of eczema herpeticum and zoster in patients treated with upadacitinib. Having upadacitinib available for patients who fail dupilumab is a clear benefit; the role of upadacitinib before dupilumab seems less clear.
Patients doing great on dupilumab for AD may be wondering: Do I still need to take it every 2 weeks? Spekhorst and colleagues may have the answer. They describe the response to tapering dupilumab in patients who had been on the drug for at least 1 year with well-controlled disease for at least 6 months. Patients in the study then continued dupilumab with the longest possible dosing interval while maintaining control of their AD.
Generally, patients maintained good control of their AD, with only a small increase in mean disease severity and in concomitant use of topical steroids. For the patients who attempted prolongation, 83% successfully continued dupilumab treatment with a prolonged interval. Not at all surprisingly, the authors calculated that prolonging the interval between dosing led to large savings in cost.
One of the nice features of dupilumab treatment is that loss of response over time seems unusual. Perhaps there is a low propensity for forming antidrug antibodies when dupilumab is used in the standard every 2-week dosing regimen. I don't know whether antidrug antibodies would be more likely with the intermittent dosing regimen. But now that we have other good systemic treatment options for AD, losing dupilumab efficacy would not be as critical a problem as it used to be. I also want to point out that patients' adherence to injection treatment, though better than adherence to topicals, is far from perfect. It's likely that many patients have already been prolonging the interval between taking their treatments. If you want to know, just ask them. The way I like to phrase the question is: "Are you keeping the extra injectors you've accumulated refrigerated like you are supposed to?"
Silverberg and colleagues described a very well-designed, vehicle-controlled, randomized 8-week study of a topical formulation of a purified strain of Nitrosomonas eutropha, an ammonia-oxidizing bacterium. In theory, this bacterium may reduce Staphylococcus aureus. The study compared two concentrations of the bacterium vs vehicle delivered as a spray twice per day. Study participants were adults with AD affecting 10%-40% of body surface area.
The study found "meaningful" improvements in itch and objective signs of disease, with clear separation between both doses of the bacterial spray compared with vehicle. At week 4, about 23% of participants treated with the bacterium were clear or almost clear (with a 2-point improvement) compared with 12% in the vehicle group (for comparison, in a phase 2 study comparing topical ruxolitinib with 0.1% triamcinolone cream, there was a 25% clear or almost clear rate [with 2-point improvement] in the triamcinolone-treated individuals).
Though an "all-natural" bacterial approach to managing AD may be appealing to some, it sounded like magic to me. But this well-done study makes it seem like the bacterial approach could be more promising than I had thought. This study also reported about twice as many adverse events (including gastrointestinal issues) with the bacterium-treated participants compared with those who received vehicle, adding to my belief that the bacterial product has efficacy. Whether any other topical will be more effective and safer than is topical triamcinolone remains to be seen. I'm still pessimistic about topicals because of patients' poor adherence to topical treatment, but perhaps an easy-to-use spray that isn't associated with patients' fear of "steroids" will be helpful.
Chen and colleagues. They analyzed data on hundreds of thousands of patients with and without AD. Adults with AD had a "significantly increased risk" of developing venous thromboembolism compared with adults without AD. The huge sample size of their study seems compelling. That huge sample size allows detection of effects so small that they may be clinically insignificant.
They report that patients with AD had a venous thromboembolism at a rate of 1.05/1000 patients-years; the rate was 0.82 for patients without AD. From that, we can calculate that there would be an additional 23 patients with venous thromboembolism for every 100,000 patient-years or about one more venous thromboembolism in the AD group in every 4000 patient-years. Though the finding was statistically significant, I don't think it is clinically meaningful.
The authors correctly conclude that "vascular examination and consultation with the emergency department, cardiologists, or pulmonologists are indicated for patients with AD who present with relevant symptoms (eg, unexplained dyspnea, chest tightness, and limb swelling)." But it is probably also true that vascular examination and consultation with the emergency department, cardiologists, or pulmonologists are indicated for patients without AD who present with those symptoms. I think the authors might have been on solid ground if they had concluded that there was a statistically significant but clinically insignificant increased risk for venous thromboembolism in patients with AD.
Eichenfeld and colleagues examined the use of topical crisaborole once per day as a maintenance treatment for patients with mild to moderate AD. The study compared patients given topical crisaborole with those randomly assigned to vehicle. The active treatment was effective because topical crisaborole treated patients had longer times to the first flare following treatment and fewer flares over the 1 year of treatment. The differences were not huge, but I think they were clinically meaningful. I'm guessing that the topical crisaborole maintenance treatment would have been even more effective had it been used regularly. The study did not, as far as I could tell, assess how well the treatment was used.
An interesting aspect of this study is that it began with nearly 500 participants who started on twice daily topical crisaborole. The 270 patients who responded to the treatment (achieving clear or almost clear with at least a 2-point improvement) were enrolled in the 1-year maintenance phase. Thus, the participants in the maintenance phase were preselected for patients who respond to topical crisaborole. We don't know why they were responders (I, of course, expect it is because they selected for patients who are better than others are at using a topical treatment), but it may be best not to try to generalize these results and assume this form of maintenance treatment would work equally well in a population who achieve initial success with an oral therapy regimen (for example, a quick course of oral prednisone).
Dupilumab was a revolutionary treatment for AD. I didn't think that I'd ever see a more effective treatment. It's so safe too! It has been a first-line treatment for AD since its introduction. Now, we also have oral Janus kinase inhibitor options. Blauvelt and colleagues examined what happens when patients who have been on dupilumab are switched to a high dose (30 mg/d) of upadacitinib (the standard starting dose of upadacitinib is 15 mg/d). Though dupilumab is very effective, upadacitinib is more so. After 4 weeks of switching to upadacitinib, nearly half the patients were completely clear of AD compared with only 16.0% after 24 weeks of dupilumab! The authors point out, optimistically, that "No new safety risks were observed." Though there were no cancers, gastrointestinal perforations, major adverse cardiovascular events, or venous thromboembolic events, there were cases of eczema herpeticum and zoster in patients treated with upadacitinib. Having upadacitinib available for patients who fail dupilumab is a clear benefit; the role of upadacitinib before dupilumab seems less clear.
Patients doing great on dupilumab for AD may be wondering: Do I still need to take it every 2 weeks? Spekhorst and colleagues may have the answer. They describe the response to tapering dupilumab in patients who had been on the drug for at least 1 year with well-controlled disease for at least 6 months. Patients in the study then continued dupilumab with the longest possible dosing interval while maintaining control of their AD.
Generally, patients maintained good control of their AD, with only a small increase in mean disease severity and in concomitant use of topical steroids. For the patients who attempted prolongation, 83% successfully continued dupilumab treatment with a prolonged interval. Not at all surprisingly, the authors calculated that prolonging the interval between dosing led to large savings in cost.
One of the nice features of dupilumab treatment is that loss of response over time seems unusual. Perhaps there is a low propensity for forming antidrug antibodies when dupilumab is used in the standard every 2-week dosing regimen. I don't know whether antidrug antibodies would be more likely with the intermittent dosing regimen. But now that we have other good systemic treatment options for AD, losing dupilumab efficacy would not be as critical a problem as it used to be. I also want to point out that patients' adherence to injection treatment, though better than adherence to topicals, is far from perfect. It's likely that many patients have already been prolonging the interval between taking their treatments. If you want to know, just ask them. The way I like to phrase the question is: "Are you keeping the extra injectors you've accumulated refrigerated like you are supposed to?"
Silverberg and colleagues described a very well-designed, vehicle-controlled, randomized 8-week study of a topical formulation of a purified strain of Nitrosomonas eutropha, an ammonia-oxidizing bacterium. In theory, this bacterium may reduce Staphylococcus aureus. The study compared two concentrations of the bacterium vs vehicle delivered as a spray twice per day. Study participants were adults with AD affecting 10%-40% of body surface area.
The study found "meaningful" improvements in itch and objective signs of disease, with clear separation between both doses of the bacterial spray compared with vehicle. At week 4, about 23% of participants treated with the bacterium were clear or almost clear (with a 2-point improvement) compared with 12% in the vehicle group (for comparison, in a phase 2 study comparing topical ruxolitinib with 0.1% triamcinolone cream, there was a 25% clear or almost clear rate [with 2-point improvement] in the triamcinolone-treated individuals).
Though an "all-natural" bacterial approach to managing AD may be appealing to some, it sounded like magic to me. But this well-done study makes it seem like the bacterial approach could be more promising than I had thought. This study also reported about twice as many adverse events (including gastrointestinal issues) with the bacterium-treated participants compared with those who received vehicle, adding to my belief that the bacterial product has efficacy. Whether any other topical will be more effective and safer than is topical triamcinolone remains to be seen. I'm still pessimistic about topicals because of patients' poor adherence to topical treatment, but perhaps an easy-to-use spray that isn't associated with patients' fear of "steroids" will be helpful.
Chen and colleagues. They analyzed data on hundreds of thousands of patients with and without AD. Adults with AD had a "significantly increased risk" of developing venous thromboembolism compared with adults without AD. The huge sample size of their study seems compelling. That huge sample size allows detection of effects so small that they may be clinically insignificant.
They report that patients with AD had a venous thromboembolism at a rate of 1.05/1000 patients-years; the rate was 0.82 for patients without AD. From that, we can calculate that there would be an additional 23 patients with venous thromboembolism for every 100,000 patient-years or about one more venous thromboembolism in the AD group in every 4000 patient-years. Though the finding was statistically significant, I don't think it is clinically meaningful.
The authors correctly conclude that "vascular examination and consultation with the emergency department, cardiologists, or pulmonologists are indicated for patients with AD who present with relevant symptoms (eg, unexplained dyspnea, chest tightness, and limb swelling)." But it is probably also true that vascular examination and consultation with the emergency department, cardiologists, or pulmonologists are indicated for patients without AD who present with those symptoms. I think the authors might have been on solid ground if they had concluded that there was a statistically significant but clinically insignificant increased risk for venous thromboembolism in patients with AD.
Eichenfeld and colleagues examined the use of topical crisaborole once per day as a maintenance treatment for patients with mild to moderate AD. The study compared patients given topical crisaborole with those randomly assigned to vehicle. The active treatment was effective because topical crisaborole treated patients had longer times to the first flare following treatment and fewer flares over the 1 year of treatment. The differences were not huge, but I think they were clinically meaningful. I'm guessing that the topical crisaborole maintenance treatment would have been even more effective had it been used regularly. The study did not, as far as I could tell, assess how well the treatment was used.
An interesting aspect of this study is that it began with nearly 500 participants who started on twice daily topical crisaborole. The 270 patients who responded to the treatment (achieving clear or almost clear with at least a 2-point improvement) were enrolled in the 1-year maintenance phase. Thus, the participants in the maintenance phase were preselected for patients who respond to topical crisaborole. We don't know why they were responders (I, of course, expect it is because they selected for patients who are better than others are at using a topical treatment), but it may be best not to try to generalize these results and assume this form of maintenance treatment would work equally well in a population who achieve initial success with an oral therapy regimen (for example, a quick course of oral prednisone).
Dupilumab was a revolutionary treatment for AD. I didn't think that I'd ever see a more effective treatment. It's so safe too! It has been a first-line treatment for AD since its introduction. Now, we also have oral Janus kinase inhibitor options. Blauvelt and colleagues examined what happens when patients who have been on dupilumab are switched to a high dose (30 mg/d) of upadacitinib (the standard starting dose of upadacitinib is 15 mg/d). Though dupilumab is very effective, upadacitinib is more so. After 4 weeks of switching to upadacitinib, nearly half the patients were completely clear of AD compared with only 16.0% after 24 weeks of dupilumab! The authors point out, optimistically, that "No new safety risks were observed." Though there were no cancers, gastrointestinal perforations, major adverse cardiovascular events, or venous thromboembolic events, there were cases of eczema herpeticum and zoster in patients treated with upadacitinib. Having upadacitinib available for patients who fail dupilumab is a clear benefit; the role of upadacitinib before dupilumab seems less clear.
Patients doing great on dupilumab for AD may be wondering: Do I still need to take it every 2 weeks? Spekhorst and colleagues may have the answer. They describe the response to tapering dupilumab in patients who had been on the drug for at least 1 year with well-controlled disease for at least 6 months. Patients in the study then continued dupilumab with the longest possible dosing interval while maintaining control of their AD.
Generally, patients maintained good control of their AD, with only a small increase in mean disease severity and in concomitant use of topical steroids. For the patients who attempted prolongation, 83% successfully continued dupilumab treatment with a prolonged interval. Not at all surprisingly, the authors calculated that prolonging the interval between dosing led to large savings in cost.
One of the nice features of dupilumab treatment is that loss of response over time seems unusual. Perhaps there is a low propensity for forming antidrug antibodies when dupilumab is used in the standard every 2-week dosing regimen. I don't know whether antidrug antibodies would be more likely with the intermittent dosing regimen. But now that we have other good systemic treatment options for AD, losing dupilumab efficacy would not be as critical a problem as it used to be. I also want to point out that patients' adherence to injection treatment, though better than adherence to topicals, is far from perfect. It's likely that many patients have already been prolonging the interval between taking their treatments. If you want to know, just ask them. The way I like to phrase the question is: "Are you keeping the extra injectors you've accumulated refrigerated like you are supposed to?"
Commentary: Topical treatments, dupilumab, and long-term treatment of AD, July 2023
There is a tremendous amount of atopic dermatitis (AD) research underway. This month, we have several interesting articles to present.
Silverberg and colleagues described a very well-designed, vehicle-controlled, randomized 8-week study of a topical formulation of a purified strain of Nitrosomonas eutropha, an ammonia-oxidizing bacterium. In theory, this bacterium may reduce Staphylococcus aureus. The study compared two concentrations of the bacterium vs vehicle delivered as a spray twice per day. Study participants were adults with AD affecting 10%-40% of body surface area.
The study found "meaningful" improvements in itch and objective signs of disease, with clear separation between both doses of the bacterial spray compared with vehicle. At week 4, about 23% of participants treated with the bacterium were clear or almost clear (with a 2-point improvement) compared with 12% in the vehicle group (for comparison, in a phase 2 study comparing topical ruxolitinib with 0.1% triamcinolone cream, there was a 25% clear or almost clear rate [with 2-point improvement] in the triamcinolone-treated individuals).
Though an "all-natural" bacterial approach to managing AD may be appealing to some, it sounded like magic to me. But this well-done study makes it seem like the bacterial approach could be more promising than I had thought. This study also reported about twice as many adverse events (including gastrointestinal issues) with the bacterium-treated participants compared with those who received vehicle, adding to my belief that the bacterial product has efficacy. Whether any other topical will be more effective and safer than is topical triamcinolone remains to be seen. I'm still pessimistic about topicals because of patients' poor adherence to topical treatment, but perhaps an easy-to-use spray that isn't associated with patients' fear of "steroids" will be helpful.
I love articles like this one from Chen and colleagues. They analyzed data on hundreds of thousands of patients with and without AD. Adults with AD had a "significantly increased risk" of developing venous thromboembolism compared with adults without AD. The huge sample size of their study seems compelling. That huge sample size allows detection of effects so small that they may be clinically insignificant.
They report that patients with AD had a venous thromboembolism at a rate of 1.05/1000 patients-years; the rate was 0.82 for patients without AD. From that, we can calculate that there would be an additional 23 patients with venous thromboembolism for every 100,000 patient-years or about one more venous thromboembolism in the AD group in every 4000 patient-years. Though the finding was statistically significant, I don't think it is clinically meaningful.
The authors correctly conclude that "vascular examination and consultation with the emergency department, cardiologists, or pulmonologists are indicated for patients with AD who present with relevant symptoms (eg, unexplained dyspnea, chest tightness, and limb swelling)." But it is probably also true that vascular examination and consultation with the emergency department, cardiologists, or pulmonologists are indicated for patients without AD who present with those symptoms. I think the authors might have been on solid ground if they had concluded that there was a statistically significant but clinically insignificant increased risk for venous thromboembolism in patients with AD.
Eichenfeld and colleagues examined the use of topical crisaborole once per day as a maintenance treatment for patients with mild to moderate AD. The study compared patients given topical crisaborole with those randomly assigned to vehicle. The active treatment was effective because topical crisaborole treated patients had longer times to the first flare following treatment and fewer flares over the 1 year of treatment. The differences were not huge, but I think they were clinically meaningful. I'm guessing that the topical crisaborole maintenance treatment would have been even more effective had it been used regularly. The study did not, as far as I could tell, assess how well the treatment was used.
An interesting aspect of this study is that it began with nearly 500 participants who started on twice daily topical crisaborole. The 270 patients who responded to the treatment (achieving clear or almost clear with at least a 2-point improvement) were enrolled in the 1-year maintenance phase. Thus, the participants in the maintenance phase were preselected for patients who respond to topical crisaborole. We don't know why they were responders (I, of course, expect it is because they selected for patients who are better than others are at using a topical treatment), but it may be best not to try to generalize these results and assume this form of maintenance treatment would work equally well in a population who achieve initial success with an oral therapy regimen (for example, a quick course of oral prednisone).
Dupilumab was a revolutionary treatment for AD. I didn't think that I'd ever see a more effective treatment. It's so safe too! It has been a first-line treatment for AD since its introduction. Now, we also have oral Janus kinase inhibitor options. Blauvelt and colleagues examined what happens when patients who have been on dupilumab are switched to a high dose (30 mg/d) of upadacitinib (the standard starting dose of upadacitinib is 15 mg/d). Though dupilumab is very effective, upadacitinib is more so. After 4 weeks of switching to upadacitinib, nearly half the patients were completely clear of AD compared with only 16.0% after 24 weeks of dupilumab! The authors point out, optimistically, that "No new safety risks were observed." Though there were no cancers, gastrointestinal perforations, major adverse cardiovascular events, or venous thromboembolic events, there were cases of eczema herpeticum and zoster in patients treated with upadacitinib. Having upadacitinib available for patients who fail dupilumab is a clear benefit; the role of upadacitinib before dupilumab seems less clear.
Patients doing great on dupilumab for AD may be wondering: Do I still need to take it every 2 weeks? Spekhorst and colleagues may have the answer. They describe the response to tapering dupilumab in patients who had been on the drug for at least 1 year with well-controlled disease for at least 6 months. Patients in the study then continued dupilumab with the longest possible dosing interval while maintaining control of their AD.
Generally, patients maintained good control of their AD, with only a small increase in mean disease severity and in concomitant use of topical steroids. For the patients who attempted prolongation, 83% successfully continued dupilumab treatment with a prolonged interval. Not at all surprisingly, the authors calculated that prolonging the interval between dosing led to large savings in cost.
One of the nice features of dupilumab treatment is that loss of response over time seems unusual. Perhaps there is a low propensity for forming antidrug antibodies when dupilumab is used in the standard every 2-week dosing regimen. I don't know whether antidrug antibodies would be more likely with the intermittent dosing regimen. But now that we have other good systemic treatment options for AD, losing dupilumab efficacy would not be as critical a problem as it used to be. I also want to point out that patients' adherence to injection treatment, though better than adherence to topicals, is far from perfect. It's likely that many patients have already been prolonging the interval between taking their treatments. If you want to know, just ask them. The way I like to phrase the question is: "Are you keeping the extra injectors you've accumulated refrigerated like you are supposed to?"
There is a tremendous amount of atopic dermatitis (AD) research underway. This month, we have several interesting articles to present.
Silverberg and colleagues described a very well-designed, vehicle-controlled, randomized 8-week study of a topical formulation of a purified strain of Nitrosomonas eutropha, an ammonia-oxidizing bacterium. In theory, this bacterium may reduce Staphylococcus aureus. The study compared two concentrations of the bacterium vs vehicle delivered as a spray twice per day. Study participants were adults with AD affecting 10%-40% of body surface area.
The study found "meaningful" improvements in itch and objective signs of disease, with clear separation between both doses of the bacterial spray compared with vehicle. At week 4, about 23% of participants treated with the bacterium were clear or almost clear (with a 2-point improvement) compared with 12% in the vehicle group (for comparison, in a phase 2 study comparing topical ruxolitinib with 0.1% triamcinolone cream, there was a 25% clear or almost clear rate [with 2-point improvement] in the triamcinolone-treated individuals).
Though an "all-natural" bacterial approach to managing AD may be appealing to some, it sounded like magic to me. But this well-done study makes it seem like the bacterial approach could be more promising than I had thought. This study also reported about twice as many adverse events (including gastrointestinal issues) with the bacterium-treated participants compared with those who received vehicle, adding to my belief that the bacterial product has efficacy. Whether any other topical will be more effective and safer than is topical triamcinolone remains to be seen. I'm still pessimistic about topicals because of patients' poor adherence to topical treatment, but perhaps an easy-to-use spray that isn't associated with patients' fear of "steroids" will be helpful.
I love articles like this one from Chen and colleagues. They analyzed data on hundreds of thousands of patients with and without AD. Adults with AD had a "significantly increased risk" of developing venous thromboembolism compared with adults without AD. The huge sample size of their study seems compelling. That huge sample size allows detection of effects so small that they may be clinically insignificant.
They report that patients with AD had a venous thromboembolism at a rate of 1.05/1000 patients-years; the rate was 0.82 for patients without AD. From that, we can calculate that there would be an additional 23 patients with venous thromboembolism for every 100,000 patient-years or about one more venous thromboembolism in the AD group in every 4000 patient-years. Though the finding was statistically significant, I don't think it is clinically meaningful.
The authors correctly conclude that "vascular examination and consultation with the emergency department, cardiologists, or pulmonologists are indicated for patients with AD who present with relevant symptoms (eg, unexplained dyspnea, chest tightness, and limb swelling)." But it is probably also true that vascular examination and consultation with the emergency department, cardiologists, or pulmonologists are indicated for patients without AD who present with those symptoms. I think the authors might have been on solid ground if they had concluded that there was a statistically significant but clinically insignificant increased risk for venous thromboembolism in patients with AD.
Eichenfeld and colleagues examined the use of topical crisaborole once per day as a maintenance treatment for patients with mild to moderate AD. The study compared patients given topical crisaborole with those randomly assigned to vehicle. The active treatment was effective because topical crisaborole treated patients had longer times to the first flare following treatment and fewer flares over the 1 year of treatment. The differences were not huge, but I think they were clinically meaningful. I'm guessing that the topical crisaborole maintenance treatment would have been even more effective had it been used regularly. The study did not, as far as I could tell, assess how well the treatment was used.
An interesting aspect of this study is that it began with nearly 500 participants who started on twice daily topical crisaborole. The 270 patients who responded to the treatment (achieving clear or almost clear with at least a 2-point improvement) were enrolled in the 1-year maintenance phase. Thus, the participants in the maintenance phase were preselected for patients who respond to topical crisaborole. We don't know why they were responders (I, of course, expect it is because they selected for patients who are better than others are at using a topical treatment), but it may be best not to try to generalize these results and assume this form of maintenance treatment would work equally well in a population who achieve initial success with an oral therapy regimen (for example, a quick course of oral prednisone).
Dupilumab was a revolutionary treatment for AD. I didn't think that I'd ever see a more effective treatment. It's so safe too! It has been a first-line treatment for AD since its introduction. Now, we also have oral Janus kinase inhibitor options. Blauvelt and colleagues examined what happens when patients who have been on dupilumab are switched to a high dose (30 mg/d) of upadacitinib (the standard starting dose of upadacitinib is 15 mg/d). Though dupilumab is very effective, upadacitinib is more so. After 4 weeks of switching to upadacitinib, nearly half the patients were completely clear of AD compared with only 16.0% after 24 weeks of dupilumab! The authors point out, optimistically, that "No new safety risks were observed." Though there were no cancers, gastrointestinal perforations, major adverse cardiovascular events, or venous thromboembolic events, there were cases of eczema herpeticum and zoster in patients treated with upadacitinib. Having upadacitinib available for patients who fail dupilumab is a clear benefit; the role of upadacitinib before dupilumab seems less clear.
Patients doing great on dupilumab for AD may be wondering: Do I still need to take it every 2 weeks? Spekhorst and colleagues may have the answer. They describe the response to tapering dupilumab in patients who had been on the drug for at least 1 year with well-controlled disease for at least 6 months. Patients in the study then continued dupilumab with the longest possible dosing interval while maintaining control of their AD.
Generally, patients maintained good control of their AD, with only a small increase in mean disease severity and in concomitant use of topical steroids. For the patients who attempted prolongation, 83% successfully continued dupilumab treatment with a prolonged interval. Not at all surprisingly, the authors calculated that prolonging the interval between dosing led to large savings in cost.
One of the nice features of dupilumab treatment is that loss of response over time seems unusual. Perhaps there is a low propensity for forming antidrug antibodies when dupilumab is used in the standard every 2-week dosing regimen. I don't know whether antidrug antibodies would be more likely with the intermittent dosing regimen. But now that we have other good systemic treatment options for AD, losing dupilumab efficacy would not be as critical a problem as it used to be. I also want to point out that patients' adherence to injection treatment, though better than adherence to topicals, is far from perfect. It's likely that many patients have already been prolonging the interval between taking their treatments. If you want to know, just ask them. The way I like to phrase the question is: "Are you keeping the extra injectors you've accumulated refrigerated like you are supposed to?"
There is a tremendous amount of atopic dermatitis (AD) research underway. This month, we have several interesting articles to present.
Silverberg and colleagues described a very well-designed, vehicle-controlled, randomized 8-week study of a topical formulation of a purified strain of Nitrosomonas eutropha, an ammonia-oxidizing bacterium. In theory, this bacterium may reduce Staphylococcus aureus. The study compared two concentrations of the bacterium vs vehicle delivered as a spray twice per day. Study participants were adults with AD affecting 10%-40% of body surface area.
The study found "meaningful" improvements in itch and objective signs of disease, with clear separation between both doses of the bacterial spray compared with vehicle. At week 4, about 23% of participants treated with the bacterium were clear or almost clear (with a 2-point improvement) compared with 12% in the vehicle group (for comparison, in a phase 2 study comparing topical ruxolitinib with 0.1% triamcinolone cream, there was a 25% clear or almost clear rate [with 2-point improvement] in the triamcinolone-treated individuals).
Though an "all-natural" bacterial approach to managing AD may be appealing to some, it sounded like magic to me. But this well-done study makes it seem like the bacterial approach could be more promising than I had thought. This study also reported about twice as many adverse events (including gastrointestinal issues) with the bacterium-treated participants compared with those who received vehicle, adding to my belief that the bacterial product has efficacy. Whether any other topical will be more effective and safer than is topical triamcinolone remains to be seen. I'm still pessimistic about topicals because of patients' poor adherence to topical treatment, but perhaps an easy-to-use spray that isn't associated with patients' fear of "steroids" will be helpful.
I love articles like this one from Chen and colleagues. They analyzed data on hundreds of thousands of patients with and without AD. Adults with AD had a "significantly increased risk" of developing venous thromboembolism compared with adults without AD. The huge sample size of their study seems compelling. That huge sample size allows detection of effects so small that they may be clinically insignificant.
They report that patients with AD had a venous thromboembolism at a rate of 1.05/1000 patients-years; the rate was 0.82 for patients without AD. From that, we can calculate that there would be an additional 23 patients with venous thromboembolism for every 100,000 patient-years or about one more venous thromboembolism in the AD group in every 4000 patient-years. Though the finding was statistically significant, I don't think it is clinically meaningful.
The authors correctly conclude that "vascular examination and consultation with the emergency department, cardiologists, or pulmonologists are indicated for patients with AD who present with relevant symptoms (eg, unexplained dyspnea, chest tightness, and limb swelling)." But it is probably also true that vascular examination and consultation with the emergency department, cardiologists, or pulmonologists are indicated for patients without AD who present with those symptoms. I think the authors might have been on solid ground if they had concluded that there was a statistically significant but clinically insignificant increased risk for venous thromboembolism in patients with AD.
Eichenfeld and colleagues examined the use of topical crisaborole once per day as a maintenance treatment for patients with mild to moderate AD. The study compared patients given topical crisaborole with those randomly assigned to vehicle. The active treatment was effective because topical crisaborole treated patients had longer times to the first flare following treatment and fewer flares over the 1 year of treatment. The differences were not huge, but I think they were clinically meaningful. I'm guessing that the topical crisaborole maintenance treatment would have been even more effective had it been used regularly. The study did not, as far as I could tell, assess how well the treatment was used.
An interesting aspect of this study is that it began with nearly 500 participants who started on twice daily topical crisaborole. The 270 patients who responded to the treatment (achieving clear or almost clear with at least a 2-point improvement) were enrolled in the 1-year maintenance phase. Thus, the participants in the maintenance phase were preselected for patients who respond to topical crisaborole. We don't know why they were responders (I, of course, expect it is because they selected for patients who are better than others are at using a topical treatment), but it may be best not to try to generalize these results and assume this form of maintenance treatment would work equally well in a population who achieve initial success with an oral therapy regimen (for example, a quick course of oral prednisone).
Dupilumab was a revolutionary treatment for AD. I didn't think that I'd ever see a more effective treatment. It's so safe too! It has been a first-line treatment for AD since its introduction. Now, we also have oral Janus kinase inhibitor options. Blauvelt and colleagues examined what happens when patients who have been on dupilumab are switched to a high dose (30 mg/d) of upadacitinib (the standard starting dose of upadacitinib is 15 mg/d). Though dupilumab is very effective, upadacitinib is more so. After 4 weeks of switching to upadacitinib, nearly half the patients were completely clear of AD compared with only 16.0% after 24 weeks of dupilumab! The authors point out, optimistically, that "No new safety risks were observed." Though there were no cancers, gastrointestinal perforations, major adverse cardiovascular events, or venous thromboembolic events, there were cases of eczema herpeticum and zoster in patients treated with upadacitinib. Having upadacitinib available for patients who fail dupilumab is a clear benefit; the role of upadacitinib before dupilumab seems less clear.
Patients doing great on dupilumab for AD may be wondering: Do I still need to take it every 2 weeks? Spekhorst and colleagues may have the answer. They describe the response to tapering dupilumab in patients who had been on the drug for at least 1 year with well-controlled disease for at least 6 months. Patients in the study then continued dupilumab with the longest possible dosing interval while maintaining control of their AD.
Generally, patients maintained good control of their AD, with only a small increase in mean disease severity and in concomitant use of topical steroids. For the patients who attempted prolongation, 83% successfully continued dupilumab treatment with a prolonged interval. Not at all surprisingly, the authors calculated that prolonging the interval between dosing led to large savings in cost.
One of the nice features of dupilumab treatment is that loss of response over time seems unusual. Perhaps there is a low propensity for forming antidrug antibodies when dupilumab is used in the standard every 2-week dosing regimen. I don't know whether antidrug antibodies would be more likely with the intermittent dosing regimen. But now that we have other good systemic treatment options for AD, losing dupilumab efficacy would not be as critical a problem as it used to be. I also want to point out that patients' adherence to injection treatment, though better than adherence to topicals, is far from perfect. It's likely that many patients have already been prolonging the interval between taking their treatments. If you want to know, just ask them. The way I like to phrase the question is: "Are you keeping the extra injectors you've accumulated refrigerated like you are supposed to?"
Can a repurposed Parkinson’s drug slow ALS progression?
However, at least one expert believes the study has “significant flaws.”
Investigators randomly assigned 20 individuals with sporadic ALS to receive either ropinirole or placebo for 24 weeks. During the double-blind period, there was no difference between the groups in terms of decline in functional status.
However, during a further open-label extension period, the ropinirole group showed significant suppression of functional decline and an average of an additional 7 months of progression-free survival.
The researchers were able to predict clinical responsiveness to ropinirole in vitro by analyzing motor neurons derived from participants’ stem cells.
“We found that ropinirole is safe and tolerable for ALS patients and shows therapeutic promise at helping them sustain daily activity and muscle strength,” first author Satoru Morimoto, MD, of the department of physiology, Keio University School of Medicine, Tokyo, said in a news release.
The study was published online in Cell Stem Cell.
Feasibility study
“ALS is totally incurable and it’s a very difficult disease to treat,” senior author Hideyuki Okano, MD, PhD, professor, department of physiology, Keio University, said in the news release.
Preclinical animal models have “limited translational potential” for identifying drug candidates, but induced pluripotent stem cell (iPSC)–derived motor neurons (MNs) from ALS patients can “overcome these limitations for drug screening,” the authors write.
“We previously identified ropinirole [a dopamine D2 receptor agonist] as a potential anti-ALS drug in vitro by iPSC drug discovery,” Dr. Okano said.
The current trial was a randomized, placebo-controlled phase 1/2a feasibility trial that evaluated the safety, tolerability, and efficacy of ropinirole in patients with ALS, using several parameters:
- The revised ALS functional rating scale (ALSFRS-R) score.
- Composite functional endpoints.
- Event-free survival.
- Time to ≤ 50% forced vital capacity (FVC).
The trial consisted of a 12-week run-in period, a 24-week double-blind period, an open-label extension period that lasted from 4 to 24 weeks, and a 4-week follow-up period after administration.
Thirteen patients were assigned to receive ropinirole (23.1% women; mean age, 65.2 ± 12.6 years; 7.7% with clinically definite and 76.9% with clinically probable ALS); seven were assigned to receive placebo (57.1% women; mean age, 66.3 ± 7.5 years; 14.3% with clinically definite and 85.7% with clinically probable ALS).
Of the treatment group, 30.8% had a bulbar onset lesion vs. 57.1% in the placebo group. At baseline, the mean FVC was 94.4% ± 14.9 and 81.5% ± 23.2 in the ropinirole and placebo groups, respectively. The mean body mass index (BMI) was 22.91 ± 3.82 and 19.69 ± 2.63, respectively.
Of the participants,12 in the ropinirole and six in the control group completed the full 24-week treatment protocol; 12 in the ropinirole and five in the placebo group completed the open-label extension (participants who had received placebo were switched to the active drug).
However only seven participants in the ropinirole group and one participant in the placebo group completed the full 1-year trial.
‘Striking correlation’
“During the double-blind period, muscle strength and daily activity were maintained, but a decline in the ALSFRS-R … was not different from that in the placebo group,” the researchers write.
In the open-label extension period, the ropinirole group showed “significant suppression of ALSFRS-R decline,” with an ALSFRS-R score change of only 7.75 (95% confidence interval, 10.66-4.63) for the treatment group vs. 17.51 (95% CI, 22.46-12.56) for the placebo group.
The researchers used the assessment of function and survival (CAFS) score, which adjusts the ALSFRS-R score against mortality, to see whether functional benefits translated into improved survival.
The score “favored ropinirole” in the open-extension period and the entire treatment period but not in the double-blind period.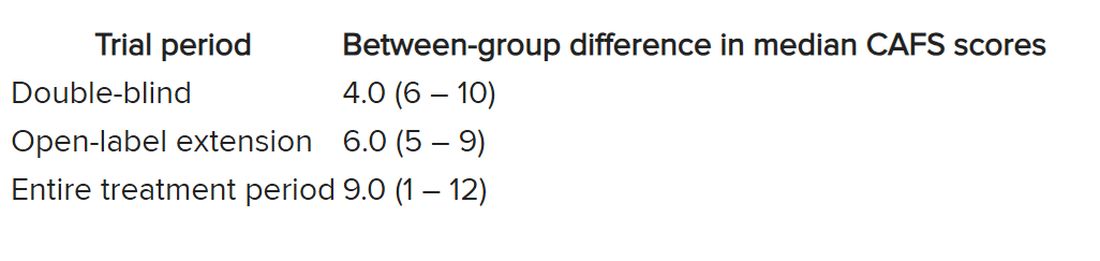
Disease progression events occurred in 7 of 7 (100%) participants in the placebo group and 7 of 13 (54%) in the ropinirole group, “suggesting a twofold decrease in disease progression” in the treatment group.
The ropinirole group experienced an additional 27.9 weeks of disease progression–free survival, compared with the placebo group.
“No participant discontinued treatment because of adverse experiences in either treatment group,” the authors report.
The analysis of iPSC-derived motor neurons from participants showed dopamine D2 receptor expression, as well as the potential involvement of the cholesterol pathway SREBP2 in the therapeutic effects of ropinirole. Lipid peroxide was also identified as a good “surrogate clinical marker to assess disease progression and drug efficacy.”
“We found a very striking correlation between a patient’s clinical response and the response of their motor neurons in vitro,” said Dr. Morimoto. “Patients whose motor neurons responded robustly to ropinirole in vitro had a much slower clinical disease progression with ropinirole treatment, while suboptimal responders showed much more rapid disease progression, despite taking ropinirole.”
Limitations include “small sample sizes and high attrition rates in the open-label extension period,” so “further validation” is required, the authors state.
Significant flaws
Commenting for this article, Carmel Armon, MD, MHS, professor of neurology, Loma Linda (Calif.) University, said the study “falls short of being a credible 1/2a clinical trial.”
Although the “intentions were good and the design not unusual,” the two groups were not “balanced on risk factors for faster progressing disease.” Rather, the placebo group was “tilted towards faster progressing disease” because there were more clinically definite and probable ALS patients in the placebo group than the treatment group, and there were more patients with bulbar onset.
Participants in the placebo group also had shorter median disease duration, lower BMI, and lower FVC, noted Dr. Armon, who was not involved with the study.
And only 1 in 7 control patients completed the open-label extension, compared with 7 of 13 patients in the intervention group.
“With these limitations, I would be disinclined to rely on the findings to justify a larger clinical trial,” Dr. Armon concluded.
The trial was sponsored by K Pharma. The study drug, active drugs, and placebo were supplied free of charge by GlaxoSmithKline K.K. Dr. Okano received grants from JSPS and AMED and grants and personal fees from K Pharma during the conduct of the study and personal fees from Sanbio, outside the submitted work. Dr. Okano has a patent on a therapeutic agent for ALS and composition for treatment licensed to K Pharma. The other authors’ disclosures and additional information are available in the original article. Dr. Armon reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
However, at least one expert believes the study has “significant flaws.”
Investigators randomly assigned 20 individuals with sporadic ALS to receive either ropinirole or placebo for 24 weeks. During the double-blind period, there was no difference between the groups in terms of decline in functional status.
However, during a further open-label extension period, the ropinirole group showed significant suppression of functional decline and an average of an additional 7 months of progression-free survival.
The researchers were able to predict clinical responsiveness to ropinirole in vitro by analyzing motor neurons derived from participants’ stem cells.
“We found that ropinirole is safe and tolerable for ALS patients and shows therapeutic promise at helping them sustain daily activity and muscle strength,” first author Satoru Morimoto, MD, of the department of physiology, Keio University School of Medicine, Tokyo, said in a news release.
The study was published online in Cell Stem Cell.
Feasibility study
“ALS is totally incurable and it’s a very difficult disease to treat,” senior author Hideyuki Okano, MD, PhD, professor, department of physiology, Keio University, said in the news release.
Preclinical animal models have “limited translational potential” for identifying drug candidates, but induced pluripotent stem cell (iPSC)–derived motor neurons (MNs) from ALS patients can “overcome these limitations for drug screening,” the authors write.
“We previously identified ropinirole [a dopamine D2 receptor agonist] as a potential anti-ALS drug in vitro by iPSC drug discovery,” Dr. Okano said.
The current trial was a randomized, placebo-controlled phase 1/2a feasibility trial that evaluated the safety, tolerability, and efficacy of ropinirole in patients with ALS, using several parameters:
- The revised ALS functional rating scale (ALSFRS-R) score.
- Composite functional endpoints.
- Event-free survival.
- Time to ≤ 50% forced vital capacity (FVC).
The trial consisted of a 12-week run-in period, a 24-week double-blind period, an open-label extension period that lasted from 4 to 24 weeks, and a 4-week follow-up period after administration.
Thirteen patients were assigned to receive ropinirole (23.1% women; mean age, 65.2 ± 12.6 years; 7.7% with clinically definite and 76.9% with clinically probable ALS); seven were assigned to receive placebo (57.1% women; mean age, 66.3 ± 7.5 years; 14.3% with clinically definite and 85.7% with clinically probable ALS).
Of the treatment group, 30.8% had a bulbar onset lesion vs. 57.1% in the placebo group. At baseline, the mean FVC was 94.4% ± 14.9 and 81.5% ± 23.2 in the ropinirole and placebo groups, respectively. The mean body mass index (BMI) was 22.91 ± 3.82 and 19.69 ± 2.63, respectively.
Of the participants,12 in the ropinirole and six in the control group completed the full 24-week treatment protocol; 12 in the ropinirole and five in the placebo group completed the open-label extension (participants who had received placebo were switched to the active drug).
However only seven participants in the ropinirole group and one participant in the placebo group completed the full 1-year trial.
‘Striking correlation’
“During the double-blind period, muscle strength and daily activity were maintained, but a decline in the ALSFRS-R … was not different from that in the placebo group,” the researchers write.
In the open-label extension period, the ropinirole group showed “significant suppression of ALSFRS-R decline,” with an ALSFRS-R score change of only 7.75 (95% confidence interval, 10.66-4.63) for the treatment group vs. 17.51 (95% CI, 22.46-12.56) for the placebo group.
The researchers used the assessment of function and survival (CAFS) score, which adjusts the ALSFRS-R score against mortality, to see whether functional benefits translated into improved survival.
The score “favored ropinirole” in the open-extension period and the entire treatment period but not in the double-blind period.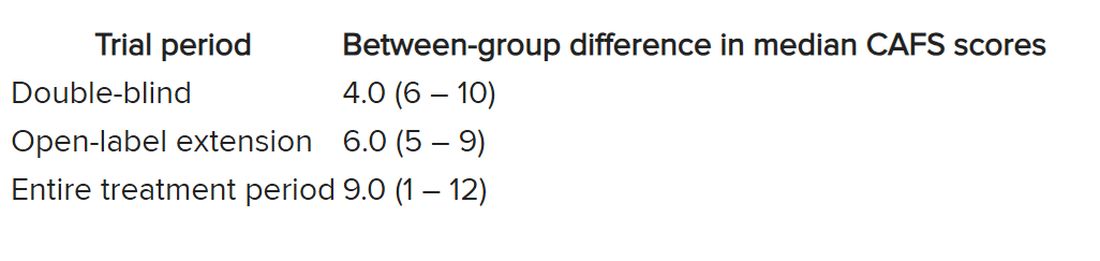
Disease progression events occurred in 7 of 7 (100%) participants in the placebo group and 7 of 13 (54%) in the ropinirole group, “suggesting a twofold decrease in disease progression” in the treatment group.
The ropinirole group experienced an additional 27.9 weeks of disease progression–free survival, compared with the placebo group.
“No participant discontinued treatment because of adverse experiences in either treatment group,” the authors report.
The analysis of iPSC-derived motor neurons from participants showed dopamine D2 receptor expression, as well as the potential involvement of the cholesterol pathway SREBP2 in the therapeutic effects of ropinirole. Lipid peroxide was also identified as a good “surrogate clinical marker to assess disease progression and drug efficacy.”
“We found a very striking correlation between a patient’s clinical response and the response of their motor neurons in vitro,” said Dr. Morimoto. “Patients whose motor neurons responded robustly to ropinirole in vitro had a much slower clinical disease progression with ropinirole treatment, while suboptimal responders showed much more rapid disease progression, despite taking ropinirole.”
Limitations include “small sample sizes and high attrition rates in the open-label extension period,” so “further validation” is required, the authors state.
Significant flaws
Commenting for this article, Carmel Armon, MD, MHS, professor of neurology, Loma Linda (Calif.) University, said the study “falls short of being a credible 1/2a clinical trial.”
Although the “intentions were good and the design not unusual,” the two groups were not “balanced on risk factors for faster progressing disease.” Rather, the placebo group was “tilted towards faster progressing disease” because there were more clinically definite and probable ALS patients in the placebo group than the treatment group, and there were more patients with bulbar onset.
Participants in the placebo group also had shorter median disease duration, lower BMI, and lower FVC, noted Dr. Armon, who was not involved with the study.
And only 1 in 7 control patients completed the open-label extension, compared with 7 of 13 patients in the intervention group.
“With these limitations, I would be disinclined to rely on the findings to justify a larger clinical trial,” Dr. Armon concluded.
The trial was sponsored by K Pharma. The study drug, active drugs, and placebo were supplied free of charge by GlaxoSmithKline K.K. Dr. Okano received grants from JSPS and AMED and grants and personal fees from K Pharma during the conduct of the study and personal fees from Sanbio, outside the submitted work. Dr. Okano has a patent on a therapeutic agent for ALS and composition for treatment licensed to K Pharma. The other authors’ disclosures and additional information are available in the original article. Dr. Armon reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
However, at least one expert believes the study has “significant flaws.”
Investigators randomly assigned 20 individuals with sporadic ALS to receive either ropinirole or placebo for 24 weeks. During the double-blind period, there was no difference between the groups in terms of decline in functional status.
However, during a further open-label extension period, the ropinirole group showed significant suppression of functional decline and an average of an additional 7 months of progression-free survival.
The researchers were able to predict clinical responsiveness to ropinirole in vitro by analyzing motor neurons derived from participants’ stem cells.
“We found that ropinirole is safe and tolerable for ALS patients and shows therapeutic promise at helping them sustain daily activity and muscle strength,” first author Satoru Morimoto, MD, of the department of physiology, Keio University School of Medicine, Tokyo, said in a news release.
The study was published online in Cell Stem Cell.
Feasibility study
“ALS is totally incurable and it’s a very difficult disease to treat,” senior author Hideyuki Okano, MD, PhD, professor, department of physiology, Keio University, said in the news release.
Preclinical animal models have “limited translational potential” for identifying drug candidates, but induced pluripotent stem cell (iPSC)–derived motor neurons (MNs) from ALS patients can “overcome these limitations for drug screening,” the authors write.
“We previously identified ropinirole [a dopamine D2 receptor agonist] as a potential anti-ALS drug in vitro by iPSC drug discovery,” Dr. Okano said.
The current trial was a randomized, placebo-controlled phase 1/2a feasibility trial that evaluated the safety, tolerability, and efficacy of ropinirole in patients with ALS, using several parameters:
- The revised ALS functional rating scale (ALSFRS-R) score.
- Composite functional endpoints.
- Event-free survival.
- Time to ≤ 50% forced vital capacity (FVC).
The trial consisted of a 12-week run-in period, a 24-week double-blind period, an open-label extension period that lasted from 4 to 24 weeks, and a 4-week follow-up period after administration.
Thirteen patients were assigned to receive ropinirole (23.1% women; mean age, 65.2 ± 12.6 years; 7.7% with clinically definite and 76.9% with clinically probable ALS); seven were assigned to receive placebo (57.1% women; mean age, 66.3 ± 7.5 years; 14.3% with clinically definite and 85.7% with clinically probable ALS).
Of the treatment group, 30.8% had a bulbar onset lesion vs. 57.1% in the placebo group. At baseline, the mean FVC was 94.4% ± 14.9 and 81.5% ± 23.2 in the ropinirole and placebo groups, respectively. The mean body mass index (BMI) was 22.91 ± 3.82 and 19.69 ± 2.63, respectively.
Of the participants,12 in the ropinirole and six in the control group completed the full 24-week treatment protocol; 12 in the ropinirole and five in the placebo group completed the open-label extension (participants who had received placebo were switched to the active drug).
However only seven participants in the ropinirole group and one participant in the placebo group completed the full 1-year trial.
‘Striking correlation’
“During the double-blind period, muscle strength and daily activity were maintained, but a decline in the ALSFRS-R … was not different from that in the placebo group,” the researchers write.
In the open-label extension period, the ropinirole group showed “significant suppression of ALSFRS-R decline,” with an ALSFRS-R score change of only 7.75 (95% confidence interval, 10.66-4.63) for the treatment group vs. 17.51 (95% CI, 22.46-12.56) for the placebo group.
The researchers used the assessment of function and survival (CAFS) score, which adjusts the ALSFRS-R score against mortality, to see whether functional benefits translated into improved survival.
The score “favored ropinirole” in the open-extension period and the entire treatment period but not in the double-blind period.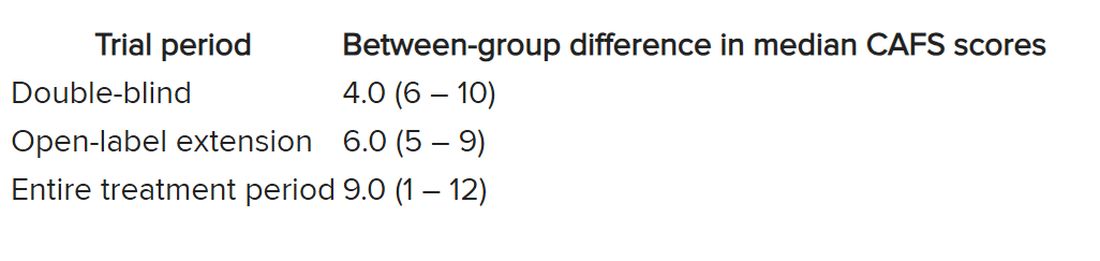
Disease progression events occurred in 7 of 7 (100%) participants in the placebo group and 7 of 13 (54%) in the ropinirole group, “suggesting a twofold decrease in disease progression” in the treatment group.
The ropinirole group experienced an additional 27.9 weeks of disease progression–free survival, compared with the placebo group.
“No participant discontinued treatment because of adverse experiences in either treatment group,” the authors report.
The analysis of iPSC-derived motor neurons from participants showed dopamine D2 receptor expression, as well as the potential involvement of the cholesterol pathway SREBP2 in the therapeutic effects of ropinirole. Lipid peroxide was also identified as a good “surrogate clinical marker to assess disease progression and drug efficacy.”
“We found a very striking correlation between a patient’s clinical response and the response of their motor neurons in vitro,” said Dr. Morimoto. “Patients whose motor neurons responded robustly to ropinirole in vitro had a much slower clinical disease progression with ropinirole treatment, while suboptimal responders showed much more rapid disease progression, despite taking ropinirole.”
Limitations include “small sample sizes and high attrition rates in the open-label extension period,” so “further validation” is required, the authors state.
Significant flaws
Commenting for this article, Carmel Armon, MD, MHS, professor of neurology, Loma Linda (Calif.) University, said the study “falls short of being a credible 1/2a clinical trial.”
Although the “intentions were good and the design not unusual,” the two groups were not “balanced on risk factors for faster progressing disease.” Rather, the placebo group was “tilted towards faster progressing disease” because there were more clinically definite and probable ALS patients in the placebo group than the treatment group, and there were more patients with bulbar onset.
Participants in the placebo group also had shorter median disease duration, lower BMI, and lower FVC, noted Dr. Armon, who was not involved with the study.
And only 1 in 7 control patients completed the open-label extension, compared with 7 of 13 patients in the intervention group.
“With these limitations, I would be disinclined to rely on the findings to justify a larger clinical trial,” Dr. Armon concluded.
The trial was sponsored by K Pharma. The study drug, active drugs, and placebo were supplied free of charge by GlaxoSmithKline K.K. Dr. Okano received grants from JSPS and AMED and grants and personal fees from K Pharma during the conduct of the study and personal fees from Sanbio, outside the submitted work. Dr. Okano has a patent on a therapeutic agent for ALS and composition for treatment licensed to K Pharma. The other authors’ disclosures and additional information are available in the original article. Dr. Armon reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CELL STEM CELL
Starting indicated heart failure meds in-hospital: Progress, opportunities
Most patients aren’t receiving all the medications they should based on guidelines, nor are they getting them at the most effective time in their disease course, suggests a registry study of patients in the United States hospitalized with heart failure with reduced ejection fraction (HFrEF).
On average, one such medication was initiated per patient for every 6 days in the hospital.
Shortfalls in predischarge GDMT initiation disproportionately landed on women, patients at rural centers, and those with renal failure or other comorbidities. But they didn’t seem related to patient race or ethnicity in the study reported in JACC: Heart Failure.
The analysis covers the 3 years preceding the May 2020 first-time approval of a sodium-glucose cotransporter 2 (SGLT2) inhibitor for nondiabetic patients with HFrEF, and therefore doesn’t cover such drugs for that indication. The SGLT2 inhibitors would later join beta-blockers, renin-angiotensin system (RAS) inhibitors, and mineralocorticoid receptor antagonists (MRAs) in the quartet of core GDMT medications broadly indicated for HFrEF.
In-hospital initiation of GDMT for HFrEF is considered a predictor of being on those medications after discharge and is itself guideline recommended. There’s clear evidence that treatment with the four core medications boosts survival and cuts rehospitalization risk, and that “getting those on board as soon as possible will eventually benefit many patients,” Paul L. Hess, MD, MHS, said in an interview.
Dr. Hess, University of Colorado Anschutz Medical Campus, Aurora, is senior author on the report from the Get With The Guidelines–Heart Failure (GWTG-HF) quality improvement program of the American Heart Association.
Broad uptake of new medical therapies into practice may sometimes take 15 or more years from first publication, Dr. Hess said, so, “I find it encouraging in the study that over a shorter time period, 2017-2020, there was improvement.”
Indeed, the odds of in-hospital initiation of an indicated med during that period on average climbed a significant 8% every 3 months, the report states.
The finding suggests that “heart failure hospitalization is, in and of itself, an important intervention for getting folks on the appropriate medications,” Dr. Hess said. It also means “we’re getting better at it,” at least at the study’s 160 GWTG-HF participating hospitals nationwide.
Those centers, the report acknowledges, varied in size, geography, and teaching status but were not necessarily representative of all U.S. hospitals. In another potential limitation, the study couldn’t account for patients who weren’t prescribed all indicated medications for clinically valid reasons. It excluded patients with “clear contraindications,” Dr. Hess said. But there could have been “legitimate reasons” some indicated medications weren’t always prescribed, including patient frailty, hemodynamic intolerance, renal dysfunction, or polypharmacy concerns.
“Positive takeaways” from the analysis, notes an accompanying editorial, include improved prescription rates for key GDMT categories across more than 3 years of data, and evidence that in-hospital initiation “was feasible and, at least for some medications, reliably undertaken.”
Of note, new GDMT prescriptions from admission to discharge went from 70% to almost 98% for beta-blockers, from 59% to about 91% for RAS inhibitors, from about 26% to 56% for MRAs, and from 15.5% to 27.4% for hydralazine/nitrates, wrote Karen E. Joynt Maddox, MD, MPH, and Daniel K. Fox, MD, PhD, both of Washington University in St. Louis.
“Key areas for improvement,” they noted, include prescriptions for women, who were 12% less likely than men to have appropriate GDMT initiated during hospitalization (P < .001); and practice at rural hospitals, which were 40% less likely than urban centers to have patients on full GDMT by discharge (P = .017).
Although only 2.6% of the GWTG-HF centers were in rural locations, “rural hospitals make up approximately one-third of general acute care hospitals in this country,” the editorial states. They therefore “represent a key source of health disparity” in the United States in need of further study.
The analysis of 50,170 patients hospitalized with HFrEF compared the number of GDMT medications for which they were eligible, on at-hospital admission, and by discharge.
The drug categories included “evidence based beta blockers,” that is, bisoprolol, carvedilol, or sustained-release metoprolol; RAS inhibitors, specifically ACE inhibitors, angiotensin receptor blockers, or sacubitril/valsartan (Entresto); MRAs; SGLT2 inhibitors in patients with diabetes; diuretics for congestion; oral anticoagulants for atrial fibrillation; and hydralazine/nitrates in African Americans.
About 15% of the patients at hospital admission were on all indicated HFrEF medications for which they were eligible. The proportion more than doubled to 32.8% by discharge.
Factors significantly associated with reduced odds for in-hospital GDMT initiation include older age (odds ratio, 0.94 per 5-year increment), being female versus male (OR, 0.88), rural location (OR, 0.60), Medicaid versus Medicare or private insurance (OR, 0.93), stroke history (OR, 0.91), peripheral artery disease (OR, 0.93), chronic obstructive pulmonary disease or asthma (OR, 0.86), and renal insufficiency (OR, 0.77).
The findings suggest that there has been at least some progress in getting hospitalized patients “on the right meds” by discharge, Dr. Hess observed. To help address shortfalls in some patient groups, “there is interest in engaging pharmacists in helping us encourage providers on the front lines to initiate and titrate medications.”
The GWTG-HF program is sponsored, in part, by Novartis, Boehringer Ingelheim, Novo Nordisk, AstraZeneca, Bayer, Tylenol, and Alnylam Pharmaceuticals. Dr. Hess disclosed no relevant financial relationships. Dr. Maddox disclosed serving on the Health Policy Advisory Council for Centene. Dr. Fox reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most patients aren’t receiving all the medications they should based on guidelines, nor are they getting them at the most effective time in their disease course, suggests a registry study of patients in the United States hospitalized with heart failure with reduced ejection fraction (HFrEF).
On average, one such medication was initiated per patient for every 6 days in the hospital.
Shortfalls in predischarge GDMT initiation disproportionately landed on women, patients at rural centers, and those with renal failure or other comorbidities. But they didn’t seem related to patient race or ethnicity in the study reported in JACC: Heart Failure.
The analysis covers the 3 years preceding the May 2020 first-time approval of a sodium-glucose cotransporter 2 (SGLT2) inhibitor for nondiabetic patients with HFrEF, and therefore doesn’t cover such drugs for that indication. The SGLT2 inhibitors would later join beta-blockers, renin-angiotensin system (RAS) inhibitors, and mineralocorticoid receptor antagonists (MRAs) in the quartet of core GDMT medications broadly indicated for HFrEF.
In-hospital initiation of GDMT for HFrEF is considered a predictor of being on those medications after discharge and is itself guideline recommended. There’s clear evidence that treatment with the four core medications boosts survival and cuts rehospitalization risk, and that “getting those on board as soon as possible will eventually benefit many patients,” Paul L. Hess, MD, MHS, said in an interview.
Dr. Hess, University of Colorado Anschutz Medical Campus, Aurora, is senior author on the report from the Get With The Guidelines–Heart Failure (GWTG-HF) quality improvement program of the American Heart Association.
Broad uptake of new medical therapies into practice may sometimes take 15 or more years from first publication, Dr. Hess said, so, “I find it encouraging in the study that over a shorter time period, 2017-2020, there was improvement.”
Indeed, the odds of in-hospital initiation of an indicated med during that period on average climbed a significant 8% every 3 months, the report states.
The finding suggests that “heart failure hospitalization is, in and of itself, an important intervention for getting folks on the appropriate medications,” Dr. Hess said. It also means “we’re getting better at it,” at least at the study’s 160 GWTG-HF participating hospitals nationwide.
Those centers, the report acknowledges, varied in size, geography, and teaching status but were not necessarily representative of all U.S. hospitals. In another potential limitation, the study couldn’t account for patients who weren’t prescribed all indicated medications for clinically valid reasons. It excluded patients with “clear contraindications,” Dr. Hess said. But there could have been “legitimate reasons” some indicated medications weren’t always prescribed, including patient frailty, hemodynamic intolerance, renal dysfunction, or polypharmacy concerns.
“Positive takeaways” from the analysis, notes an accompanying editorial, include improved prescription rates for key GDMT categories across more than 3 years of data, and evidence that in-hospital initiation “was feasible and, at least for some medications, reliably undertaken.”
Of note, new GDMT prescriptions from admission to discharge went from 70% to almost 98% for beta-blockers, from 59% to about 91% for RAS inhibitors, from about 26% to 56% for MRAs, and from 15.5% to 27.4% for hydralazine/nitrates, wrote Karen E. Joynt Maddox, MD, MPH, and Daniel K. Fox, MD, PhD, both of Washington University in St. Louis.
“Key areas for improvement,” they noted, include prescriptions for women, who were 12% less likely than men to have appropriate GDMT initiated during hospitalization (P < .001); and practice at rural hospitals, which were 40% less likely than urban centers to have patients on full GDMT by discharge (P = .017).
Although only 2.6% of the GWTG-HF centers were in rural locations, “rural hospitals make up approximately one-third of general acute care hospitals in this country,” the editorial states. They therefore “represent a key source of health disparity” in the United States in need of further study.
The analysis of 50,170 patients hospitalized with HFrEF compared the number of GDMT medications for which they were eligible, on at-hospital admission, and by discharge.
The drug categories included “evidence based beta blockers,” that is, bisoprolol, carvedilol, or sustained-release metoprolol; RAS inhibitors, specifically ACE inhibitors, angiotensin receptor blockers, or sacubitril/valsartan (Entresto); MRAs; SGLT2 inhibitors in patients with diabetes; diuretics for congestion; oral anticoagulants for atrial fibrillation; and hydralazine/nitrates in African Americans.
About 15% of the patients at hospital admission were on all indicated HFrEF medications for which they were eligible. The proportion more than doubled to 32.8% by discharge.
Factors significantly associated with reduced odds for in-hospital GDMT initiation include older age (odds ratio, 0.94 per 5-year increment), being female versus male (OR, 0.88), rural location (OR, 0.60), Medicaid versus Medicare or private insurance (OR, 0.93), stroke history (OR, 0.91), peripheral artery disease (OR, 0.93), chronic obstructive pulmonary disease or asthma (OR, 0.86), and renal insufficiency (OR, 0.77).
The findings suggest that there has been at least some progress in getting hospitalized patients “on the right meds” by discharge, Dr. Hess observed. To help address shortfalls in some patient groups, “there is interest in engaging pharmacists in helping us encourage providers on the front lines to initiate and titrate medications.”
The GWTG-HF program is sponsored, in part, by Novartis, Boehringer Ingelheim, Novo Nordisk, AstraZeneca, Bayer, Tylenol, and Alnylam Pharmaceuticals. Dr. Hess disclosed no relevant financial relationships. Dr. Maddox disclosed serving on the Health Policy Advisory Council for Centene. Dr. Fox reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most patients aren’t receiving all the medications they should based on guidelines, nor are they getting them at the most effective time in their disease course, suggests a registry study of patients in the United States hospitalized with heart failure with reduced ejection fraction (HFrEF).
On average, one such medication was initiated per patient for every 6 days in the hospital.
Shortfalls in predischarge GDMT initiation disproportionately landed on women, patients at rural centers, and those with renal failure or other comorbidities. But they didn’t seem related to patient race or ethnicity in the study reported in JACC: Heart Failure.
The analysis covers the 3 years preceding the May 2020 first-time approval of a sodium-glucose cotransporter 2 (SGLT2) inhibitor for nondiabetic patients with HFrEF, and therefore doesn’t cover such drugs for that indication. The SGLT2 inhibitors would later join beta-blockers, renin-angiotensin system (RAS) inhibitors, and mineralocorticoid receptor antagonists (MRAs) in the quartet of core GDMT medications broadly indicated for HFrEF.
In-hospital initiation of GDMT for HFrEF is considered a predictor of being on those medications after discharge and is itself guideline recommended. There’s clear evidence that treatment with the four core medications boosts survival and cuts rehospitalization risk, and that “getting those on board as soon as possible will eventually benefit many patients,” Paul L. Hess, MD, MHS, said in an interview.
Dr. Hess, University of Colorado Anschutz Medical Campus, Aurora, is senior author on the report from the Get With The Guidelines–Heart Failure (GWTG-HF) quality improvement program of the American Heart Association.
Broad uptake of new medical therapies into practice may sometimes take 15 or more years from first publication, Dr. Hess said, so, “I find it encouraging in the study that over a shorter time period, 2017-2020, there was improvement.”
Indeed, the odds of in-hospital initiation of an indicated med during that period on average climbed a significant 8% every 3 months, the report states.
The finding suggests that “heart failure hospitalization is, in and of itself, an important intervention for getting folks on the appropriate medications,” Dr. Hess said. It also means “we’re getting better at it,” at least at the study’s 160 GWTG-HF participating hospitals nationwide.
Those centers, the report acknowledges, varied in size, geography, and teaching status but were not necessarily representative of all U.S. hospitals. In another potential limitation, the study couldn’t account for patients who weren’t prescribed all indicated medications for clinically valid reasons. It excluded patients with “clear contraindications,” Dr. Hess said. But there could have been “legitimate reasons” some indicated medications weren’t always prescribed, including patient frailty, hemodynamic intolerance, renal dysfunction, or polypharmacy concerns.
“Positive takeaways” from the analysis, notes an accompanying editorial, include improved prescription rates for key GDMT categories across more than 3 years of data, and evidence that in-hospital initiation “was feasible and, at least for some medications, reliably undertaken.”
Of note, new GDMT prescriptions from admission to discharge went from 70% to almost 98% for beta-blockers, from 59% to about 91% for RAS inhibitors, from about 26% to 56% for MRAs, and from 15.5% to 27.4% for hydralazine/nitrates, wrote Karen E. Joynt Maddox, MD, MPH, and Daniel K. Fox, MD, PhD, both of Washington University in St. Louis.
“Key areas for improvement,” they noted, include prescriptions for women, who were 12% less likely than men to have appropriate GDMT initiated during hospitalization (P < .001); and practice at rural hospitals, which were 40% less likely than urban centers to have patients on full GDMT by discharge (P = .017).
Although only 2.6% of the GWTG-HF centers were in rural locations, “rural hospitals make up approximately one-third of general acute care hospitals in this country,” the editorial states. They therefore “represent a key source of health disparity” in the United States in need of further study.
The analysis of 50,170 patients hospitalized with HFrEF compared the number of GDMT medications for which they were eligible, on at-hospital admission, and by discharge.
The drug categories included “evidence based beta blockers,” that is, bisoprolol, carvedilol, or sustained-release metoprolol; RAS inhibitors, specifically ACE inhibitors, angiotensin receptor blockers, or sacubitril/valsartan (Entresto); MRAs; SGLT2 inhibitors in patients with diabetes; diuretics for congestion; oral anticoagulants for atrial fibrillation; and hydralazine/nitrates in African Americans.
About 15% of the patients at hospital admission were on all indicated HFrEF medications for which they were eligible. The proportion more than doubled to 32.8% by discharge.
Factors significantly associated with reduced odds for in-hospital GDMT initiation include older age (odds ratio, 0.94 per 5-year increment), being female versus male (OR, 0.88), rural location (OR, 0.60), Medicaid versus Medicare or private insurance (OR, 0.93), stroke history (OR, 0.91), peripheral artery disease (OR, 0.93), chronic obstructive pulmonary disease or asthma (OR, 0.86), and renal insufficiency (OR, 0.77).
The findings suggest that there has been at least some progress in getting hospitalized patients “on the right meds” by discharge, Dr. Hess observed. To help address shortfalls in some patient groups, “there is interest in engaging pharmacists in helping us encourage providers on the front lines to initiate and titrate medications.”
The GWTG-HF program is sponsored, in part, by Novartis, Boehringer Ingelheim, Novo Nordisk, AstraZeneca, Bayer, Tylenol, and Alnylam Pharmaceuticals. Dr. Hess disclosed no relevant financial relationships. Dr. Maddox disclosed serving on the Health Policy Advisory Council for Centene. Dr. Fox reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JACC: HEART FAILURE
Regular napping linked to greater brain volume
Investigators at University College London, and the University of the Republic of Uruguay, Montevideo, found individuals genetically predisposed to regular napping had larger total brain volume, a surrogate of better cognitive health.
“Our results suggest that napping may improve brain health,” first author Valentina Paz, MSc, a PhD candidate at the University of the Republic of Uruguay said in an interview. “Specifically, our work revealed a 15.8 cubic cm increase in total brain volume with more frequent daytime napping,” she said.
The findings were published online in Sleep Health.
Higher brain volume
Previous studies examining the potential link between napping and cognition in older adults have yielded conflicting results.
To clarify this association, Ms. Paz and colleagues used Mendelian randomization to study DNA samples, cognitive outcomes, and functional magnetic resonance imaging data in participants from the ongoing UK Biobank Study.
Starting with data from 378,932 study participants (mean age 57), investigators compared measures of brain health and cognition of those who are more genetically programmed to nap with people who did not have these genetic variations.
More specifically, the investigators examined 97 sections of genetic code previously linked to the likelihood of regular napping and correlated these results with fMRI and cognitive outcomes between those genetically predisposed to take regular naps and those who weren’t.
Study outcomes included total brain volume, hippocampal volume, reaction time, and visual memory.
The final study sample included 35,080 with neuroimaging, cognitive assessment, and genotype data.
The researchers estimated that the average difference in brain volume between individuals genetically programmed to be habitual nappers and those who were not was equivalent to 15.8 cubic cm, or 2.6-6.5 years of aging.
However, there was no difference in the other three outcomes – hippocampal volume, reaction time, and visual processing – between the two study groups.
Since investigators did not have information on the length of time participants napped, Ms. Paz suggested that “taking a short nap in the early afternoon may help cognition in those needing it.”
However, she added, the study’s findings need to be replicated before any firm conclusions can be made.
“More work is needed to examine the associations between napping and cognition, and the replication of these findings using other datasets and methods,” she said.
The investigators note that the study’s findings augment the knowledge of the “impact of habitual daytime napping on brain health, which is essential to understanding cognitive impairment in the aging population. The lack of evidence for an association between napping and hippocampal volume and cognitive outcomes (for example, alertness) may be affected by habitual daytime napping and should be studied in the future.”
Strengths, limitations
Tara Spires-Jones, PhD, president of the British Neuroscience Association and group leader at the UK Dementia Research Institute, said, “the study shows a small but significant increase in brain volume in people who have a genetic signature associated with taking daytime naps.”
Dr. Spires-Jones, who was not involved in the research, noted that while the study is well-conducted, it has limitations. Because Mendelian randomization uses a genetic signature, she noted, outcomes depend on the accuracy of the signature.
“The napping habits of UK Biobank participants were self-reported, which might not be entirely accurate, and the ‘napping’ signature overlapped substantially with the signature for cognitive outcomes in the study, which makes the causal link weaker,” she said.
“Even with those limitations, this study is interesting because it adds to the data indicating that sleep is important for brain health,” said Dr. Spires-Jones.
The study was supported by Diabetes UK, the British Heart Foundation, and the Diabetes Research and Wellness Foundation. In Uruguay, it was supported by Programa de Desarrollo de las Ciencias Básicas, Agencia Nacional de Investigación e Innovación, Comisión Sectorial de Investigación Científica, and Comisión Académica de Posgrado. In the United States it was supported by the National Heart, Lung, and Blood Institute. There were no disclosures reported.
A version of this article first appeared on Medscape.com.
Investigators at University College London, and the University of the Republic of Uruguay, Montevideo, found individuals genetically predisposed to regular napping had larger total brain volume, a surrogate of better cognitive health.
“Our results suggest that napping may improve brain health,” first author Valentina Paz, MSc, a PhD candidate at the University of the Republic of Uruguay said in an interview. “Specifically, our work revealed a 15.8 cubic cm increase in total brain volume with more frequent daytime napping,” she said.
The findings were published online in Sleep Health.
Higher brain volume
Previous studies examining the potential link between napping and cognition in older adults have yielded conflicting results.
To clarify this association, Ms. Paz and colleagues used Mendelian randomization to study DNA samples, cognitive outcomes, and functional magnetic resonance imaging data in participants from the ongoing UK Biobank Study.
Starting with data from 378,932 study participants (mean age 57), investigators compared measures of brain health and cognition of those who are more genetically programmed to nap with people who did not have these genetic variations.
More specifically, the investigators examined 97 sections of genetic code previously linked to the likelihood of regular napping and correlated these results with fMRI and cognitive outcomes between those genetically predisposed to take regular naps and those who weren’t.
Study outcomes included total brain volume, hippocampal volume, reaction time, and visual memory.
The final study sample included 35,080 with neuroimaging, cognitive assessment, and genotype data.
The researchers estimated that the average difference in brain volume between individuals genetically programmed to be habitual nappers and those who were not was equivalent to 15.8 cubic cm, or 2.6-6.5 years of aging.
However, there was no difference in the other three outcomes – hippocampal volume, reaction time, and visual processing – between the two study groups.
Since investigators did not have information on the length of time participants napped, Ms. Paz suggested that “taking a short nap in the early afternoon may help cognition in those needing it.”
However, she added, the study’s findings need to be replicated before any firm conclusions can be made.
“More work is needed to examine the associations between napping and cognition, and the replication of these findings using other datasets and methods,” she said.
The investigators note that the study’s findings augment the knowledge of the “impact of habitual daytime napping on brain health, which is essential to understanding cognitive impairment in the aging population. The lack of evidence for an association between napping and hippocampal volume and cognitive outcomes (for example, alertness) may be affected by habitual daytime napping and should be studied in the future.”
Strengths, limitations
Tara Spires-Jones, PhD, president of the British Neuroscience Association and group leader at the UK Dementia Research Institute, said, “the study shows a small but significant increase in brain volume in people who have a genetic signature associated with taking daytime naps.”
Dr. Spires-Jones, who was not involved in the research, noted that while the study is well-conducted, it has limitations. Because Mendelian randomization uses a genetic signature, she noted, outcomes depend on the accuracy of the signature.
“The napping habits of UK Biobank participants were self-reported, which might not be entirely accurate, and the ‘napping’ signature overlapped substantially with the signature for cognitive outcomes in the study, which makes the causal link weaker,” she said.
“Even with those limitations, this study is interesting because it adds to the data indicating that sleep is important for brain health,” said Dr. Spires-Jones.
The study was supported by Diabetes UK, the British Heart Foundation, and the Diabetes Research and Wellness Foundation. In Uruguay, it was supported by Programa de Desarrollo de las Ciencias Básicas, Agencia Nacional de Investigación e Innovación, Comisión Sectorial de Investigación Científica, and Comisión Académica de Posgrado. In the United States it was supported by the National Heart, Lung, and Blood Institute. There were no disclosures reported.
A version of this article first appeared on Medscape.com.
Investigators at University College London, and the University of the Republic of Uruguay, Montevideo, found individuals genetically predisposed to regular napping had larger total brain volume, a surrogate of better cognitive health.
“Our results suggest that napping may improve brain health,” first author Valentina Paz, MSc, a PhD candidate at the University of the Republic of Uruguay said in an interview. “Specifically, our work revealed a 15.8 cubic cm increase in total brain volume with more frequent daytime napping,” she said.
The findings were published online in Sleep Health.
Higher brain volume
Previous studies examining the potential link between napping and cognition in older adults have yielded conflicting results.
To clarify this association, Ms. Paz and colleagues used Mendelian randomization to study DNA samples, cognitive outcomes, and functional magnetic resonance imaging data in participants from the ongoing UK Biobank Study.
Starting with data from 378,932 study participants (mean age 57), investigators compared measures of brain health and cognition of those who are more genetically programmed to nap with people who did not have these genetic variations.
More specifically, the investigators examined 97 sections of genetic code previously linked to the likelihood of regular napping and correlated these results with fMRI and cognitive outcomes between those genetically predisposed to take regular naps and those who weren’t.
Study outcomes included total brain volume, hippocampal volume, reaction time, and visual memory.
The final study sample included 35,080 with neuroimaging, cognitive assessment, and genotype data.
The researchers estimated that the average difference in brain volume between individuals genetically programmed to be habitual nappers and those who were not was equivalent to 15.8 cubic cm, or 2.6-6.5 years of aging.
However, there was no difference in the other three outcomes – hippocampal volume, reaction time, and visual processing – between the two study groups.
Since investigators did not have information on the length of time participants napped, Ms. Paz suggested that “taking a short nap in the early afternoon may help cognition in those needing it.”
However, she added, the study’s findings need to be replicated before any firm conclusions can be made.
“More work is needed to examine the associations between napping and cognition, and the replication of these findings using other datasets and methods,” she said.
The investigators note that the study’s findings augment the knowledge of the “impact of habitual daytime napping on brain health, which is essential to understanding cognitive impairment in the aging population. The lack of evidence for an association between napping and hippocampal volume and cognitive outcomes (for example, alertness) may be affected by habitual daytime napping and should be studied in the future.”
Strengths, limitations
Tara Spires-Jones, PhD, president of the British Neuroscience Association and group leader at the UK Dementia Research Institute, said, “the study shows a small but significant increase in brain volume in people who have a genetic signature associated with taking daytime naps.”
Dr. Spires-Jones, who was not involved in the research, noted that while the study is well-conducted, it has limitations. Because Mendelian randomization uses a genetic signature, she noted, outcomes depend on the accuracy of the signature.
“The napping habits of UK Biobank participants were self-reported, which might not be entirely accurate, and the ‘napping’ signature overlapped substantially with the signature for cognitive outcomes in the study, which makes the causal link weaker,” she said.
“Even with those limitations, this study is interesting because it adds to the data indicating that sleep is important for brain health,” said Dr. Spires-Jones.
The study was supported by Diabetes UK, the British Heart Foundation, and the Diabetes Research and Wellness Foundation. In Uruguay, it was supported by Programa de Desarrollo de las Ciencias Básicas, Agencia Nacional de Investigación e Innovación, Comisión Sectorial de Investigación Científica, and Comisión Académica de Posgrado. In the United States it was supported by the National Heart, Lung, and Blood Institute. There were no disclosures reported.
A version of this article first appeared on Medscape.com.
FROM SLEEP HEALTH
Girls with congenital diaphragmatic hernia have lower survival rates
TOPLINE:
, a study published in the Journal of Pediatrics has found.
METHODOLOGY:
- The retrospective cohort study used demographic, clinical, and outcomes data from the registry to investigate the role of biological sex for babies with CDH.
- The study included more than 7,200 newborns at 105 hospitals across 17 countries from January 2007 to December 2018.
- The primary outcomes were 30-day, 60-day, and in-hospital mortality.
- The secondary outcomes included weight gain during admission, feeding, and oxygen status at 30 days and at discharge.
TAKEAWAY:
- After controlling for markers of disease severity, girls with CDH had a 32% increased risk for death at 30-days (adjusted hazard ratio, 1.32; P = .02).
- Girls had lower survival rates at 30 days (77.3%), compared with boys (80.1%).
- The disparity remained at 60 days, with the survival rate for girls with CDH (73.5%) modestly lower than that for boys (77.2%), and also at discharge – 70.2%, compared with 74.2%, respectively.
- The survival differences between boys and girls primarily affected babies who did not undergo cannulation; in this group, the survival rate for girls was 77.5%, compared with 82.1% for boys.
- Girls with CDH weighed less at birth (2.8 kg) than boys with the condition (3 kg); birth weight is a for babies with CDH, .
IN PRACTICE:
“Although racial and ethnic outcome disparities have been documented in CDH, disparities between males and females are not well known,” Shaun Kunisaki, MD, MSc, a senior author of the study and professor of surgery at Johns Hopkins University in Baltimore, said in a press release about the findings. “It is really important to understand if those disparities exist, because it may change how we can better manage these patients.”
STUDY DETAILS:
The authors include seven from the division of general pediatric surgery and the division of pediatric respiratory sciences at Johns Hopkins University. Two authors are from the department of pediatric surgery at the University of Texas and Children’s Memorial Hermann Hospital, both in Houston.
LIMITATIONS:
The authors point to potential challenges with data collection, including diagnosis miscoding and missing data. They acknowledge that MRI fetal lung volumes, degree of liver herniation, and emerging markers of socioeconomic status were not available in the database. The study also could not examine long-term outcomes because data were limited to in-hospital variables.
DISCLOSURES:
The authors report no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, a study published in the Journal of Pediatrics has found.
METHODOLOGY:
- The retrospective cohort study used demographic, clinical, and outcomes data from the registry to investigate the role of biological sex for babies with CDH.
- The study included more than 7,200 newborns at 105 hospitals across 17 countries from January 2007 to December 2018.
- The primary outcomes were 30-day, 60-day, and in-hospital mortality.
- The secondary outcomes included weight gain during admission, feeding, and oxygen status at 30 days and at discharge.
TAKEAWAY:
- After controlling for markers of disease severity, girls with CDH had a 32% increased risk for death at 30-days (adjusted hazard ratio, 1.32; P = .02).
- Girls had lower survival rates at 30 days (77.3%), compared with boys (80.1%).
- The disparity remained at 60 days, with the survival rate for girls with CDH (73.5%) modestly lower than that for boys (77.2%), and also at discharge – 70.2%, compared with 74.2%, respectively.
- The survival differences between boys and girls primarily affected babies who did not undergo cannulation; in this group, the survival rate for girls was 77.5%, compared with 82.1% for boys.
- Girls with CDH weighed less at birth (2.8 kg) than boys with the condition (3 kg); birth weight is a for babies with CDH, .
IN PRACTICE:
“Although racial and ethnic outcome disparities have been documented in CDH, disparities between males and females are not well known,” Shaun Kunisaki, MD, MSc, a senior author of the study and professor of surgery at Johns Hopkins University in Baltimore, said in a press release about the findings. “It is really important to understand if those disparities exist, because it may change how we can better manage these patients.”
STUDY DETAILS:
The authors include seven from the division of general pediatric surgery and the division of pediatric respiratory sciences at Johns Hopkins University. Two authors are from the department of pediatric surgery at the University of Texas and Children’s Memorial Hermann Hospital, both in Houston.
LIMITATIONS:
The authors point to potential challenges with data collection, including diagnosis miscoding and missing data. They acknowledge that MRI fetal lung volumes, degree of liver herniation, and emerging markers of socioeconomic status were not available in the database. The study also could not examine long-term outcomes because data were limited to in-hospital variables.
DISCLOSURES:
The authors report no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, a study published in the Journal of Pediatrics has found.
METHODOLOGY:
- The retrospective cohort study used demographic, clinical, and outcomes data from the registry to investigate the role of biological sex for babies with CDH.
- The study included more than 7,200 newborns at 105 hospitals across 17 countries from January 2007 to December 2018.
- The primary outcomes were 30-day, 60-day, and in-hospital mortality.
- The secondary outcomes included weight gain during admission, feeding, and oxygen status at 30 days and at discharge.
TAKEAWAY:
- After controlling for markers of disease severity, girls with CDH had a 32% increased risk for death at 30-days (adjusted hazard ratio, 1.32; P = .02).
- Girls had lower survival rates at 30 days (77.3%), compared with boys (80.1%).
- The disparity remained at 60 days, with the survival rate for girls with CDH (73.5%) modestly lower than that for boys (77.2%), and also at discharge – 70.2%, compared with 74.2%, respectively.
- The survival differences between boys and girls primarily affected babies who did not undergo cannulation; in this group, the survival rate for girls was 77.5%, compared with 82.1% for boys.
- Girls with CDH weighed less at birth (2.8 kg) than boys with the condition (3 kg); birth weight is a for babies with CDH, .
IN PRACTICE:
“Although racial and ethnic outcome disparities have been documented in CDH, disparities between males and females are not well known,” Shaun Kunisaki, MD, MSc, a senior author of the study and professor of surgery at Johns Hopkins University in Baltimore, said in a press release about the findings. “It is really important to understand if those disparities exist, because it may change how we can better manage these patients.”
STUDY DETAILS:
The authors include seven from the division of general pediatric surgery and the division of pediatric respiratory sciences at Johns Hopkins University. Two authors are from the department of pediatric surgery at the University of Texas and Children’s Memorial Hermann Hospital, both in Houston.
LIMITATIONS:
The authors point to potential challenges with data collection, including diagnosis miscoding and missing data. They acknowledge that MRI fetal lung volumes, degree of liver herniation, and emerging markers of socioeconomic status were not available in the database. The study also could not examine long-term outcomes because data were limited to in-hospital variables.
DISCLOSURES:
The authors report no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
No link between PPIs and dementia in new study
TOPLINE:
A new study provides reassurance about the long-term safety of proton pump inhibitors (PPIs) and histamine-2 receptor antagonist (H2RA) use in older adults, finding no increased risk for dementia or cognitive changes.
METHODOLOGY:
- Post hoc observational study within the Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial.
- 18,934 adults aged 65+ from the United States and Australia without dementia at baseline.
- 4,667 (25%) PPI users and 368 (2%) H2RA users at baseline.
- PPI and H2RA use, dementia incidence, and cognitive changes were tracked.
TAKEAWAY:
- In multivariable analysis, baseline PPI use was not associated with incident dementia (hazard ratio, 0.88) or cognitive impairment (HR, 1.00).
- PPI use was not linked to changes in overall cognitive test scores over time (beta –0.002).
- No associations were found between H2RA use and cognitive endpoints.
IN PRACTICE:
“Long-term use of PPIs in older adults is unlikely to have negative effects on cognition,” the study team concludes.
STUDY DETAILS:
The study was led by Raaj Mehta, MD, PhD, with Massachusetts General Hospital and Harvard Medical School in Boston. The study was published online in Gastroenterology. Funding was provided by grants from the National Institute on Aging, the National Cancer Institute, and other institutions.
LIMITATIONS:
Potential for residual confounding and underestimation of PPI and H2RA use, lack of data on medication dose and duration, and the absence of ApoE4 allele status.
DISCLOSURES:
Dr. Mehta has disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
A new study provides reassurance about the long-term safety of proton pump inhibitors (PPIs) and histamine-2 receptor antagonist (H2RA) use in older adults, finding no increased risk for dementia or cognitive changes.
METHODOLOGY:
- Post hoc observational study within the Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial.
- 18,934 adults aged 65+ from the United States and Australia without dementia at baseline.
- 4,667 (25%) PPI users and 368 (2%) H2RA users at baseline.
- PPI and H2RA use, dementia incidence, and cognitive changes were tracked.
TAKEAWAY:
- In multivariable analysis, baseline PPI use was not associated with incident dementia (hazard ratio, 0.88) or cognitive impairment (HR, 1.00).
- PPI use was not linked to changes in overall cognitive test scores over time (beta –0.002).
- No associations were found between H2RA use and cognitive endpoints.
IN PRACTICE:
“Long-term use of PPIs in older adults is unlikely to have negative effects on cognition,” the study team concludes.
STUDY DETAILS:
The study was led by Raaj Mehta, MD, PhD, with Massachusetts General Hospital and Harvard Medical School in Boston. The study was published online in Gastroenterology. Funding was provided by grants from the National Institute on Aging, the National Cancer Institute, and other institutions.
LIMITATIONS:
Potential for residual confounding and underestimation of PPI and H2RA use, lack of data on medication dose and duration, and the absence of ApoE4 allele status.
DISCLOSURES:
Dr. Mehta has disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
A new study provides reassurance about the long-term safety of proton pump inhibitors (PPIs) and histamine-2 receptor antagonist (H2RA) use in older adults, finding no increased risk for dementia or cognitive changes.
METHODOLOGY:
- Post hoc observational study within the Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial.
- 18,934 adults aged 65+ from the United States and Australia without dementia at baseline.
- 4,667 (25%) PPI users and 368 (2%) H2RA users at baseline.
- PPI and H2RA use, dementia incidence, and cognitive changes were tracked.
TAKEAWAY:
- In multivariable analysis, baseline PPI use was not associated with incident dementia (hazard ratio, 0.88) or cognitive impairment (HR, 1.00).
- PPI use was not linked to changes in overall cognitive test scores over time (beta –0.002).
- No associations were found between H2RA use and cognitive endpoints.
IN PRACTICE:
“Long-term use of PPIs in older adults is unlikely to have negative effects on cognition,” the study team concludes.
STUDY DETAILS:
The study was led by Raaj Mehta, MD, PhD, with Massachusetts General Hospital and Harvard Medical School in Boston. The study was published online in Gastroenterology. Funding was provided by grants from the National Institute on Aging, the National Cancer Institute, and other institutions.
LIMITATIONS:
Potential for residual confounding and underestimation of PPI and H2RA use, lack of data on medication dose and duration, and the absence of ApoE4 allele status.
DISCLOSURES:
Dr. Mehta has disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New cannabis laws, higher binge drinking rates linked
TOPLINE:
METHODOLOGY:
Among adolescents, binge drinking, defined as having five or more drinks for men and four or more drinks for women at one time, is associated with poor academic performance, sexual risk, and injury in the short term, as well as the development of alcohol use disorder and academic disengagement in the long term.
Current evidence regarding the association between recreational cannabis laws (RCLs) and binge drinking is limited.
States in which RCLs have been implemented include Colorado, Washington, Alaska, Oregon, Nevada, California, Massachusetts, and Vermont, as well as the District of Columbia.
The study included 817,359 people aged 12 and older who participated in the 2008-2019 National Survey on Drug Use and Health (NSDUH), a nationally representative survey of the U.S. population.
TAKEAWAY:
Overall, states that have not enacted cannabis laws showed consistently lower rates of binge drinking over time among all age groups.
In all states, there were substantial declines in reporting of past-month binge drinking in some age groups – from 17.5% (95% confidence interval, 16.9-18.2) in 2008 to 11.1% (10.4-11.8) in 2019 among those aged 12-20 and a drop from 43.7% (42.4-44.9) to 40.2% (39.1-41.1) among those aged 21-30.
There were overall increases in binge drinking in all states regardless of cannabis laws among individuals aged 31 and older. The most extensive increases were among people aged 31-40 (from 28.1% [95% CI, 26.6-29.6] to 33.3% [32.1-34.6]), followed by participants aged 51 and over (from 13.3% [95% CI, 12.2-14.4] to 16.8% [15.8-17.7]).
IN PRACTICE:
“Our findings support calls to reinforce health care providers’ discussions about alcohol use with older adults,” particularly in RCL states, the researchers write.
STUDY DETAILS:
The study was conducted out by Priscila Dib Gonçalves, PhD, department of epidemiology, Columbia University School of Public Health, New York, and colleagues. It was published in the International Journal of Drug Policy.
LIMITATIONS:
Alcohol-related measures, including binge drinking, were self-reported, which may introduce recall bias and underreporting. NSDUH binge drinking measures were not adjusted for sex differences from 2008 to 2014, which may result in underreporting of binge drinking in females before 2015. The researchers did not examine cannabis policy provisions, such as cultivation restrictions, pricing control, the tax imposed, and consumption restrictions.
DISCLOSURES:
The study received support from the National Institutes of Health, the National Institute on Drug Abuse, the National Center for Injury Prevention and Control, and the Centers for Disease Control and Prevention. The authors report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
Among adolescents, binge drinking, defined as having five or more drinks for men and four or more drinks for women at one time, is associated with poor academic performance, sexual risk, and injury in the short term, as well as the development of alcohol use disorder and academic disengagement in the long term.
Current evidence regarding the association between recreational cannabis laws (RCLs) and binge drinking is limited.
States in which RCLs have been implemented include Colorado, Washington, Alaska, Oregon, Nevada, California, Massachusetts, and Vermont, as well as the District of Columbia.
The study included 817,359 people aged 12 and older who participated in the 2008-2019 National Survey on Drug Use and Health (NSDUH), a nationally representative survey of the U.S. population.
TAKEAWAY:
Overall, states that have not enacted cannabis laws showed consistently lower rates of binge drinking over time among all age groups.
In all states, there were substantial declines in reporting of past-month binge drinking in some age groups – from 17.5% (95% confidence interval, 16.9-18.2) in 2008 to 11.1% (10.4-11.8) in 2019 among those aged 12-20 and a drop from 43.7% (42.4-44.9) to 40.2% (39.1-41.1) among those aged 21-30.
There were overall increases in binge drinking in all states regardless of cannabis laws among individuals aged 31 and older. The most extensive increases were among people aged 31-40 (from 28.1% [95% CI, 26.6-29.6] to 33.3% [32.1-34.6]), followed by participants aged 51 and over (from 13.3% [95% CI, 12.2-14.4] to 16.8% [15.8-17.7]).
IN PRACTICE:
“Our findings support calls to reinforce health care providers’ discussions about alcohol use with older adults,” particularly in RCL states, the researchers write.
STUDY DETAILS:
The study was conducted out by Priscila Dib Gonçalves, PhD, department of epidemiology, Columbia University School of Public Health, New York, and colleagues. It was published in the International Journal of Drug Policy.
LIMITATIONS:
Alcohol-related measures, including binge drinking, were self-reported, which may introduce recall bias and underreporting. NSDUH binge drinking measures were not adjusted for sex differences from 2008 to 2014, which may result in underreporting of binge drinking in females before 2015. The researchers did not examine cannabis policy provisions, such as cultivation restrictions, pricing control, the tax imposed, and consumption restrictions.
DISCLOSURES:
The study received support from the National Institutes of Health, the National Institute on Drug Abuse, the National Center for Injury Prevention and Control, and the Centers for Disease Control and Prevention. The authors report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
Among adolescents, binge drinking, defined as having five or more drinks for men and four or more drinks for women at one time, is associated with poor academic performance, sexual risk, and injury in the short term, as well as the development of alcohol use disorder and academic disengagement in the long term.
Current evidence regarding the association between recreational cannabis laws (RCLs) and binge drinking is limited.
States in which RCLs have been implemented include Colorado, Washington, Alaska, Oregon, Nevada, California, Massachusetts, and Vermont, as well as the District of Columbia.
The study included 817,359 people aged 12 and older who participated in the 2008-2019 National Survey on Drug Use and Health (NSDUH), a nationally representative survey of the U.S. population.
TAKEAWAY:
Overall, states that have not enacted cannabis laws showed consistently lower rates of binge drinking over time among all age groups.
In all states, there were substantial declines in reporting of past-month binge drinking in some age groups – from 17.5% (95% confidence interval, 16.9-18.2) in 2008 to 11.1% (10.4-11.8) in 2019 among those aged 12-20 and a drop from 43.7% (42.4-44.9) to 40.2% (39.1-41.1) among those aged 21-30.
There were overall increases in binge drinking in all states regardless of cannabis laws among individuals aged 31 and older. The most extensive increases were among people aged 31-40 (from 28.1% [95% CI, 26.6-29.6] to 33.3% [32.1-34.6]), followed by participants aged 51 and over (from 13.3% [95% CI, 12.2-14.4] to 16.8% [15.8-17.7]).
IN PRACTICE:
“Our findings support calls to reinforce health care providers’ discussions about alcohol use with older adults,” particularly in RCL states, the researchers write.
STUDY DETAILS:
The study was conducted out by Priscila Dib Gonçalves, PhD, department of epidemiology, Columbia University School of Public Health, New York, and colleagues. It was published in the International Journal of Drug Policy.
LIMITATIONS:
Alcohol-related measures, including binge drinking, were self-reported, which may introduce recall bias and underreporting. NSDUH binge drinking measures were not adjusted for sex differences from 2008 to 2014, which may result in underreporting of binge drinking in females before 2015. The researchers did not examine cannabis policy provisions, such as cultivation restrictions, pricing control, the tax imposed, and consumption restrictions.
DISCLOSURES:
The study received support from the National Institutes of Health, the National Institute on Drug Abuse, the National Center for Injury Prevention and Control, and the Centers for Disease Control and Prevention. The authors report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Low copays drive better adherence to new diabetes drugs
TOPLINE:
The less U.S. patients pay out of pocket for drugs that often have high copays, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors or glucagon-like peptide-1 (GLP-1) agonists, the more adherent they are.
METHODOLOGY:
- Review of 90,041 U.S. adults who started a GLP-1 agonist (n = 39,149) or SGLT2 inhibitor (n = 50,892) in 2014-2020.
- Participants had type 2 diabetes, heart failure, or both.
- Data are from Clinformatics Data Mart, including both commercial and Medicare health insurance plans.
- Primary outcome: 12-month adherence to prescribed GLP-1 agonist or SGLT2 inhibitor.
TAKEAWAYS:
- U.S. adults with a lower drug copay had significantly higher odds of 12-month adherence to GLP-1 agonists and SGLT2 inhibitors, compared with those with a higher copay.
- These differences persisted after controlling for patient demographic, clinical, and socioeconomic covariates.
- After full adjustment, patients with a high copay (≥ $50/month) were, after 12 months, 53% less likely to adhere to an SGLT2 inhibitor and 32% less likely to adhere to a GLP-1 agonist, compared with patients with a low copay (< $10/month) for these agents.
IN PRACTICE:
“Lowering high out-of-pocket prescription costs may be key to improving adherence to guideline-recommended therapies and advancing overall quality of care in patients with type 2 diabetes and heart failure,” say the authors.
STUDY DETAILS:
The study was led by Utibe R. Essien, MD, from the University of California, Los Angeles, and Balvindar Singh, MD, PhD, from the University of Pittsburgh, and included several authors from other U.S. centers.
LIMITATIONS:
Study could not exclude residual confounding.
Generalizability uncertain for those without health insurance or with public insurance.
Study did not have information on patient preferences associated with medication use, including specific reasons for poor adherence.
Possible misclassifications of type 2 diabetes and heart failure diagnoses or medical comorbidities.
Study could not assess how copayments influenced initial prescription receipt or abandonment at the pharmacy, nor other factors including possible price inflation.
DISCLOSURES:
The study received no commercial funding. One author (not a lead author) is an advisor to several drug companies, including ones that market SGLT2 inhibitors or GLP-1 agonists.
A version of this article first appeared on Medscape.com.
TOPLINE:
The less U.S. patients pay out of pocket for drugs that often have high copays, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors or glucagon-like peptide-1 (GLP-1) agonists, the more adherent they are.
METHODOLOGY:
- Review of 90,041 U.S. adults who started a GLP-1 agonist (n = 39,149) or SGLT2 inhibitor (n = 50,892) in 2014-2020.
- Participants had type 2 diabetes, heart failure, or both.
- Data are from Clinformatics Data Mart, including both commercial and Medicare health insurance plans.
- Primary outcome: 12-month adherence to prescribed GLP-1 agonist or SGLT2 inhibitor.
TAKEAWAYS:
- U.S. adults with a lower drug copay had significantly higher odds of 12-month adherence to GLP-1 agonists and SGLT2 inhibitors, compared with those with a higher copay.
- These differences persisted after controlling for patient demographic, clinical, and socioeconomic covariates.
- After full adjustment, patients with a high copay (≥ $50/month) were, after 12 months, 53% less likely to adhere to an SGLT2 inhibitor and 32% less likely to adhere to a GLP-1 agonist, compared with patients with a low copay (< $10/month) for these agents.
IN PRACTICE:
“Lowering high out-of-pocket prescription costs may be key to improving adherence to guideline-recommended therapies and advancing overall quality of care in patients with type 2 diabetes and heart failure,” say the authors.
STUDY DETAILS:
The study was led by Utibe R. Essien, MD, from the University of California, Los Angeles, and Balvindar Singh, MD, PhD, from the University of Pittsburgh, and included several authors from other U.S. centers.
LIMITATIONS:
Study could not exclude residual confounding.
Generalizability uncertain for those without health insurance or with public insurance.
Study did not have information on patient preferences associated with medication use, including specific reasons for poor adherence.
Possible misclassifications of type 2 diabetes and heart failure diagnoses or medical comorbidities.
Study could not assess how copayments influenced initial prescription receipt or abandonment at the pharmacy, nor other factors including possible price inflation.
DISCLOSURES:
The study received no commercial funding. One author (not a lead author) is an advisor to several drug companies, including ones that market SGLT2 inhibitors or GLP-1 agonists.
A version of this article first appeared on Medscape.com.
TOPLINE:
The less U.S. patients pay out of pocket for drugs that often have high copays, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors or glucagon-like peptide-1 (GLP-1) agonists, the more adherent they are.
METHODOLOGY:
- Review of 90,041 U.S. adults who started a GLP-1 agonist (n = 39,149) or SGLT2 inhibitor (n = 50,892) in 2014-2020.
- Participants had type 2 diabetes, heart failure, or both.
- Data are from Clinformatics Data Mart, including both commercial and Medicare health insurance plans.
- Primary outcome: 12-month adherence to prescribed GLP-1 agonist or SGLT2 inhibitor.
TAKEAWAYS:
- U.S. adults with a lower drug copay had significantly higher odds of 12-month adherence to GLP-1 agonists and SGLT2 inhibitors, compared with those with a higher copay.
- These differences persisted after controlling for patient demographic, clinical, and socioeconomic covariates.
- After full adjustment, patients with a high copay (≥ $50/month) were, after 12 months, 53% less likely to adhere to an SGLT2 inhibitor and 32% less likely to adhere to a GLP-1 agonist, compared with patients with a low copay (< $10/month) for these agents.
IN PRACTICE:
“Lowering high out-of-pocket prescription costs may be key to improving adherence to guideline-recommended therapies and advancing overall quality of care in patients with type 2 diabetes and heart failure,” say the authors.
STUDY DETAILS:
The study was led by Utibe R. Essien, MD, from the University of California, Los Angeles, and Balvindar Singh, MD, PhD, from the University of Pittsburgh, and included several authors from other U.S. centers.
LIMITATIONS:
Study could not exclude residual confounding.
Generalizability uncertain for those without health insurance or with public insurance.
Study did not have information on patient preferences associated with medication use, including specific reasons for poor adherence.
Possible misclassifications of type 2 diabetes and heart failure diagnoses or medical comorbidities.
Study could not assess how copayments influenced initial prescription receipt or abandonment at the pharmacy, nor other factors including possible price inflation.
DISCLOSURES:
The study received no commercial funding. One author (not a lead author) is an advisor to several drug companies, including ones that market SGLT2 inhibitors or GLP-1 agonists.
A version of this article first appeared on Medscape.com.