User login
Pregnancy risks elevated in women with chronic pancreatitis
TOPLINE:
METHODOLOGY:
- A retrospective analysis of hospital discharge records from the National Inpatient Sample database between 2009 and 2019 was conducted.
- The sample included 3,094 pregnancies with chronic pancreatitis and roughly 40.8 million pregnancies without this condition.
- The study focused on primary maternal outcomes and primary perinatal outcomes in pregnancies affected by chronic pancreatitis after accounting for relevant covariates.
TAKEAWAY:
- Chronic pancreatitis pregnancies had elevated rates of gestational diabetes (adjusted odds ratio, 1.63), gestational hypertensive complications (aOR, 2.48), preterm labor (aOR, 3.10), and small size for gestational age (aOR, 2.40).
- Women with chronic pancreatitis and a history of renal failure were more prone to gestational hypertensive complications (aOR, 20.09).
- Women with alcohol-induced chronic pancreatitis had a 17-fold higher risk for fetal death (aOR, 17.15).
- Pregnancies with chronic pancreatitis were associated with longer hospital stays and higher hospital costs.
IN PRACTICE:
“Our study provides novel insights into the impact of chronic pancreatitis on maternal and fetal health. The implications of our findings are critical for health care professionals, particularly those involved in preconception counseling. Pregnant women with chronic pancreatitis should be under the care of a multidisciplinary team of health care providers,” the authors advise.
SOURCE:
The study was led by Chengu Niu, MD, with Rochester General Hospital, Rochester, N.Y. It was published online July 18 in Digestive and Liver Disease. The study had no specific funding.
LIMITATIONS:
The authors note potential inaccuracies because of coding in the National Inpatient Sample database, a lack of detailed information regarding medication use, and a lack of follow-up clinical information. The findings are specific to the United States and may not be applicable to other countries.
DISCLOSURES:
The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A retrospective analysis of hospital discharge records from the National Inpatient Sample database between 2009 and 2019 was conducted.
- The sample included 3,094 pregnancies with chronic pancreatitis and roughly 40.8 million pregnancies without this condition.
- The study focused on primary maternal outcomes and primary perinatal outcomes in pregnancies affected by chronic pancreatitis after accounting for relevant covariates.
TAKEAWAY:
- Chronic pancreatitis pregnancies had elevated rates of gestational diabetes (adjusted odds ratio, 1.63), gestational hypertensive complications (aOR, 2.48), preterm labor (aOR, 3.10), and small size for gestational age (aOR, 2.40).
- Women with chronic pancreatitis and a history of renal failure were more prone to gestational hypertensive complications (aOR, 20.09).
- Women with alcohol-induced chronic pancreatitis had a 17-fold higher risk for fetal death (aOR, 17.15).
- Pregnancies with chronic pancreatitis were associated with longer hospital stays and higher hospital costs.
IN PRACTICE:
“Our study provides novel insights into the impact of chronic pancreatitis on maternal and fetal health. The implications of our findings are critical for health care professionals, particularly those involved in preconception counseling. Pregnant women with chronic pancreatitis should be under the care of a multidisciplinary team of health care providers,” the authors advise.
SOURCE:
The study was led by Chengu Niu, MD, with Rochester General Hospital, Rochester, N.Y. It was published online July 18 in Digestive and Liver Disease. The study had no specific funding.
LIMITATIONS:
The authors note potential inaccuracies because of coding in the National Inpatient Sample database, a lack of detailed information regarding medication use, and a lack of follow-up clinical information. The findings are specific to the United States and may not be applicable to other countries.
DISCLOSURES:
The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A retrospective analysis of hospital discharge records from the National Inpatient Sample database between 2009 and 2019 was conducted.
- The sample included 3,094 pregnancies with chronic pancreatitis and roughly 40.8 million pregnancies without this condition.
- The study focused on primary maternal outcomes and primary perinatal outcomes in pregnancies affected by chronic pancreatitis after accounting for relevant covariates.
TAKEAWAY:
- Chronic pancreatitis pregnancies had elevated rates of gestational diabetes (adjusted odds ratio, 1.63), gestational hypertensive complications (aOR, 2.48), preterm labor (aOR, 3.10), and small size for gestational age (aOR, 2.40).
- Women with chronic pancreatitis and a history of renal failure were more prone to gestational hypertensive complications (aOR, 20.09).
- Women with alcohol-induced chronic pancreatitis had a 17-fold higher risk for fetal death (aOR, 17.15).
- Pregnancies with chronic pancreatitis were associated with longer hospital stays and higher hospital costs.
IN PRACTICE:
“Our study provides novel insights into the impact of chronic pancreatitis on maternal and fetal health. The implications of our findings are critical for health care professionals, particularly those involved in preconception counseling. Pregnant women with chronic pancreatitis should be under the care of a multidisciplinary team of health care providers,” the authors advise.
SOURCE:
The study was led by Chengu Niu, MD, with Rochester General Hospital, Rochester, N.Y. It was published online July 18 in Digestive and Liver Disease. The study had no specific funding.
LIMITATIONS:
The authors note potential inaccuracies because of coding in the National Inpatient Sample database, a lack of detailed information regarding medication use, and a lack of follow-up clinical information. The findings are specific to the United States and may not be applicable to other countries.
DISCLOSURES:
The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
Case series supports targeted drugs in treatment of alopecia in children with AD
in children with AA and concomitant atopy.
It was only a little over a year ago that the JAK inhibitor baricitinib became the first systemic therapy approved by the Food and Drug Administration for AA in adults. In June 2023, the JAK inhibitor ritlecitinib was approved for severe AA in patients as young as 12 years of age, but there is accumulating evidence that dupilumab, which binds to the interleukin-4 receptor, might be an option for even younger children with AA.
Of those who have worked with dupilumab for controlling AA in children, Brittany Craiglow, MD, an adjunct associate professor of dermatology at Yale University, New Haven, Conn., updated a case series at the recent MedscapeLive! Annual Women’s and Pediatric Dermatology Seminar in Baltimore. A series of six children with AA treated with dupilumab was published 2 years ago in JAAD Case Reports.
Even in 2021, her case series was not the first report of benefit from dupilumab in children with AA, but instead contributed to a “growing body of literature” supporting the potential benefit in the setting of concomitant atopy, Dr. Craiglow, one of the authors of the series, said in an interview.
Of the six patients in that series, five had improvement and four had complete regrowth with dupilumab, whether as a monotherapy or in combination with other agents. The children ranged in age from 7 to 12 years. The age range at the time of AA onset was 3-11 years. All had atopic dermatitis (AD) and most had additional atopic conditions, such as food allergies or asthma.
Since publication, Dr. Craiglow has successfully treated many more patients with dupilumab, either as monotherapy or in combination with oral minoxidil, corticosteroids, and/or a topical JAK inhibitor. Dupilumab, which is approved for the treatment of AD in children as young as 6 months of age, has been well tolerated.
“Oral minoxidil is often a great adjuvant treatment in patients with AA and should be used unless there are contraindications,” based on the initial and subsequent experience treating AA with dupilumab, said Dr. Craiglow.
“Topical steroids can be used in combination with dupilumab and minoxidil, but in general dupilumab should not be combined with an oral JAK inhibitor,” she added.
Now, with the approval of ritlecitinib, Dr. Craiglow said this JAK inhibitor will become a first-line therapy in children 12 years or older with severe, persistent AA, but she considers a trial of dupilumab reasonable in younger children, given the controlled studies of safety for atopic diseases.
“I would say that dupilumab could be considered in the following clinical scenarios: children under 12 with AA and concomitant atopy, such as atopic dermatitis, asthma, allergies, and/or elevated IgE; and children over the age of 12 with concomitant atopy who either have a contraindication to a JAK inhibitor or whose families have reservations about or are unwilling to take one,” Dr. Craiglow said.
In older children, she believes that dupilumab has “a much lower chance of being effective” than an oral JAK inhibitor like ritlecitinib, but it circumvents the potential safety issues of JAK inhibitors that have been observed in adults.
With ritlecitinib providing an on-label option for AA in older children, Dr. Craiglow suggested it might be easier to obtain third-party coverage for dupilumab as an alternative to a JAK inhibitor for AA in patients younger than 12, particularly when there is an indication for a concomitant atopic condition and a rationale, such as a concern about relative safety.
Two years ago, when Dr. Craiglow and her coinvestigator published their six-patient case series, a second case series was published about the same time by investigators at the University of Pennsylvania, Philadelphia, in the Journal of the American Academy of Dermatology. This series of 16 pediatric patients with AA on dupilumab was more heterogeneous, but four of six patients with active disease and more than 4 months of follow-up had improvement in AA, including total regrowth. The improvement was concentrated in patients with moderate to severe AD at the time of treatment.
Based on this series, the authors, led by Leslie Castelo-Soccio, MD, PhD, who is now an attending physician in the Dermatology Branch of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, Md., concluded that dupilumab “may be a therapeutic option for AA” when traditional therapies have failed, “especially in patients with concurrent AD or asthma, for which the benefits of dupilumab are clear.”
When contacted about where this therapy might fit on the basis of her case series and the update on Dr. Craiglow’s experience, Dr. Castelo-Soccio, like Dr. Craiglow, stressed the importance of employing this therapy selectively.
“I do think that dupilumab is a reasonable option for AA in children with atopy and IgE levels greater than 200 IU/mL, especially if treatment is for atopic dermatitis or asthma as well,” she said.
Many clinicians, including Dr. Craiglow, have experience with oral JAK inhibitors in children younger than 12. Indeed, a recently published case study associated oral abrocitinib, a JAK inhibitor approved for moderate to severe AD in patients ages 12 and older, with hair regrowth in an 11-year-old child who had persistent AA for more than 6 years despite numerous conventional therapies.
However, the advantage of dupilumab in younger children is the greater evidence of safety, providing a level of reassurance for a treatment that is commonly used for severe atopic diseases but does not have a specific indication for AA, according to Dr. Craiglow.
Dr. Craiglow disclosed being a speaker for AbbVie and a speaker and consultant for Eli Lilly, Incyte, Pfizer, Regeneron, and Sanofi Genzyme. Dr. Castelo-Soccio had no disclosures.
in children with AA and concomitant atopy.
It was only a little over a year ago that the JAK inhibitor baricitinib became the first systemic therapy approved by the Food and Drug Administration for AA in adults. In June 2023, the JAK inhibitor ritlecitinib was approved for severe AA in patients as young as 12 years of age, but there is accumulating evidence that dupilumab, which binds to the interleukin-4 receptor, might be an option for even younger children with AA.
Of those who have worked with dupilumab for controlling AA in children, Brittany Craiglow, MD, an adjunct associate professor of dermatology at Yale University, New Haven, Conn., updated a case series at the recent MedscapeLive! Annual Women’s and Pediatric Dermatology Seminar in Baltimore. A series of six children with AA treated with dupilumab was published 2 years ago in JAAD Case Reports.
Even in 2021, her case series was not the first report of benefit from dupilumab in children with AA, but instead contributed to a “growing body of literature” supporting the potential benefit in the setting of concomitant atopy, Dr. Craiglow, one of the authors of the series, said in an interview.
Of the six patients in that series, five had improvement and four had complete regrowth with dupilumab, whether as a monotherapy or in combination with other agents. The children ranged in age from 7 to 12 years. The age range at the time of AA onset was 3-11 years. All had atopic dermatitis (AD) and most had additional atopic conditions, such as food allergies or asthma.
Since publication, Dr. Craiglow has successfully treated many more patients with dupilumab, either as monotherapy or in combination with oral minoxidil, corticosteroids, and/or a topical JAK inhibitor. Dupilumab, which is approved for the treatment of AD in children as young as 6 months of age, has been well tolerated.
“Oral minoxidil is often a great adjuvant treatment in patients with AA and should be used unless there are contraindications,” based on the initial and subsequent experience treating AA with dupilumab, said Dr. Craiglow.
“Topical steroids can be used in combination with dupilumab and minoxidil, but in general dupilumab should not be combined with an oral JAK inhibitor,” she added.
Now, with the approval of ritlecitinib, Dr. Craiglow said this JAK inhibitor will become a first-line therapy in children 12 years or older with severe, persistent AA, but she considers a trial of dupilumab reasonable in younger children, given the controlled studies of safety for atopic diseases.
“I would say that dupilumab could be considered in the following clinical scenarios: children under 12 with AA and concomitant atopy, such as atopic dermatitis, asthma, allergies, and/or elevated IgE; and children over the age of 12 with concomitant atopy who either have a contraindication to a JAK inhibitor or whose families have reservations about or are unwilling to take one,” Dr. Craiglow said.
In older children, she believes that dupilumab has “a much lower chance of being effective” than an oral JAK inhibitor like ritlecitinib, but it circumvents the potential safety issues of JAK inhibitors that have been observed in adults.
With ritlecitinib providing an on-label option for AA in older children, Dr. Craiglow suggested it might be easier to obtain third-party coverage for dupilumab as an alternative to a JAK inhibitor for AA in patients younger than 12, particularly when there is an indication for a concomitant atopic condition and a rationale, such as a concern about relative safety.
Two years ago, when Dr. Craiglow and her coinvestigator published their six-patient case series, a second case series was published about the same time by investigators at the University of Pennsylvania, Philadelphia, in the Journal of the American Academy of Dermatology. This series of 16 pediatric patients with AA on dupilumab was more heterogeneous, but four of six patients with active disease and more than 4 months of follow-up had improvement in AA, including total regrowth. The improvement was concentrated in patients with moderate to severe AD at the time of treatment.
Based on this series, the authors, led by Leslie Castelo-Soccio, MD, PhD, who is now an attending physician in the Dermatology Branch of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, Md., concluded that dupilumab “may be a therapeutic option for AA” when traditional therapies have failed, “especially in patients with concurrent AD or asthma, for which the benefits of dupilumab are clear.”
When contacted about where this therapy might fit on the basis of her case series and the update on Dr. Craiglow’s experience, Dr. Castelo-Soccio, like Dr. Craiglow, stressed the importance of employing this therapy selectively.
“I do think that dupilumab is a reasonable option for AA in children with atopy and IgE levels greater than 200 IU/mL, especially if treatment is for atopic dermatitis or asthma as well,” she said.
Many clinicians, including Dr. Craiglow, have experience with oral JAK inhibitors in children younger than 12. Indeed, a recently published case study associated oral abrocitinib, a JAK inhibitor approved for moderate to severe AD in patients ages 12 and older, with hair regrowth in an 11-year-old child who had persistent AA for more than 6 years despite numerous conventional therapies.
However, the advantage of dupilumab in younger children is the greater evidence of safety, providing a level of reassurance for a treatment that is commonly used for severe atopic diseases but does not have a specific indication for AA, according to Dr. Craiglow.
Dr. Craiglow disclosed being a speaker for AbbVie and a speaker and consultant for Eli Lilly, Incyte, Pfizer, Regeneron, and Sanofi Genzyme. Dr. Castelo-Soccio had no disclosures.
in children with AA and concomitant atopy.
It was only a little over a year ago that the JAK inhibitor baricitinib became the first systemic therapy approved by the Food and Drug Administration for AA in adults. In June 2023, the JAK inhibitor ritlecitinib was approved for severe AA in patients as young as 12 years of age, but there is accumulating evidence that dupilumab, which binds to the interleukin-4 receptor, might be an option for even younger children with AA.
Of those who have worked with dupilumab for controlling AA in children, Brittany Craiglow, MD, an adjunct associate professor of dermatology at Yale University, New Haven, Conn., updated a case series at the recent MedscapeLive! Annual Women’s and Pediatric Dermatology Seminar in Baltimore. A series of six children with AA treated with dupilumab was published 2 years ago in JAAD Case Reports.
Even in 2021, her case series was not the first report of benefit from dupilumab in children with AA, but instead contributed to a “growing body of literature” supporting the potential benefit in the setting of concomitant atopy, Dr. Craiglow, one of the authors of the series, said in an interview.
Of the six patients in that series, five had improvement and four had complete regrowth with dupilumab, whether as a monotherapy or in combination with other agents. The children ranged in age from 7 to 12 years. The age range at the time of AA onset was 3-11 years. All had atopic dermatitis (AD) and most had additional atopic conditions, such as food allergies or asthma.
Since publication, Dr. Craiglow has successfully treated many more patients with dupilumab, either as monotherapy or in combination with oral minoxidil, corticosteroids, and/or a topical JAK inhibitor. Dupilumab, which is approved for the treatment of AD in children as young as 6 months of age, has been well tolerated.
“Oral minoxidil is often a great adjuvant treatment in patients with AA and should be used unless there are contraindications,” based on the initial and subsequent experience treating AA with dupilumab, said Dr. Craiglow.
“Topical steroids can be used in combination with dupilumab and minoxidil, but in general dupilumab should not be combined with an oral JAK inhibitor,” she added.
Now, with the approval of ritlecitinib, Dr. Craiglow said this JAK inhibitor will become a first-line therapy in children 12 years or older with severe, persistent AA, but she considers a trial of dupilumab reasonable in younger children, given the controlled studies of safety for atopic diseases.
“I would say that dupilumab could be considered in the following clinical scenarios: children under 12 with AA and concomitant atopy, such as atopic dermatitis, asthma, allergies, and/or elevated IgE; and children over the age of 12 with concomitant atopy who either have a contraindication to a JAK inhibitor or whose families have reservations about or are unwilling to take one,” Dr. Craiglow said.
In older children, she believes that dupilumab has “a much lower chance of being effective” than an oral JAK inhibitor like ritlecitinib, but it circumvents the potential safety issues of JAK inhibitors that have been observed in adults.
With ritlecitinib providing an on-label option for AA in older children, Dr. Craiglow suggested it might be easier to obtain third-party coverage for dupilumab as an alternative to a JAK inhibitor for AA in patients younger than 12, particularly when there is an indication for a concomitant atopic condition and a rationale, such as a concern about relative safety.
Two years ago, when Dr. Craiglow and her coinvestigator published their six-patient case series, a second case series was published about the same time by investigators at the University of Pennsylvania, Philadelphia, in the Journal of the American Academy of Dermatology. This series of 16 pediatric patients with AA on dupilumab was more heterogeneous, but four of six patients with active disease and more than 4 months of follow-up had improvement in AA, including total regrowth. The improvement was concentrated in patients with moderate to severe AD at the time of treatment.
Based on this series, the authors, led by Leslie Castelo-Soccio, MD, PhD, who is now an attending physician in the Dermatology Branch of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, Md., concluded that dupilumab “may be a therapeutic option for AA” when traditional therapies have failed, “especially in patients with concurrent AD or asthma, for which the benefits of dupilumab are clear.”
When contacted about where this therapy might fit on the basis of her case series and the update on Dr. Craiglow’s experience, Dr. Castelo-Soccio, like Dr. Craiglow, stressed the importance of employing this therapy selectively.
“I do think that dupilumab is a reasonable option for AA in children with atopy and IgE levels greater than 200 IU/mL, especially if treatment is for atopic dermatitis or asthma as well,” she said.
Many clinicians, including Dr. Craiglow, have experience with oral JAK inhibitors in children younger than 12. Indeed, a recently published case study associated oral abrocitinib, a JAK inhibitor approved for moderate to severe AD in patients ages 12 and older, with hair regrowth in an 11-year-old child who had persistent AA for more than 6 years despite numerous conventional therapies.
However, the advantage of dupilumab in younger children is the greater evidence of safety, providing a level of reassurance for a treatment that is commonly used for severe atopic diseases but does not have a specific indication for AA, according to Dr. Craiglow.
Dr. Craiglow disclosed being a speaker for AbbVie and a speaker and consultant for Eli Lilly, Incyte, Pfizer, Regeneron, and Sanofi Genzyme. Dr. Castelo-Soccio had no disclosures.
Pending Legislation Puts VA Health Care at Risk
“What if VA health care goes away?” That was the headline of a July 6, 2023, Disabled American Veterans news article to its members. The question was not hypothetical. Legislation currently under consideration by the US Congress may make it a strong probability.
The US Senate Committee on Veterans’ Affairs recently held a hearing to discuss 2 bills that would drastically reshape the provision of private health care services through the Veterans Community Care Program. An unprecedented coalition of 10 organizations—made up of US Department of Veterans Affairs (VA) nurses, psychologists, physicians, dentists, social workers, optometrists, physician assistants, and nurse anesthetists, as well as the American Psychological Association, the Military and Veterans Committee of the Group for the Advancement of Psychiatry and the Veterans Healthcare Policy Institute—came together in a unified statement for the record highlighting how these proposed policies would open a Pandora’s box that could forever eliminate the Veterans Health Administration as we know it.
Over the past decade—and especially following the passage of the Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act—there has been a surge of veterans gaining eligibility for private care if a VA medical facility is too far away, does not offer the needed care, or the wait time for an appointment is too long.
Testifying before the House Committee on Veterans’ Affairs hearing last year, Miguel LaPuz, MD, MBA, then the acting Deputy Under Secretary for Health at the VA, warned that “VA is rapidly approaching a point where half of all care available in both settings is provided through community care.” He cautioned that leaders were bracing for “the potential of a spiral effect.”
Care that is rendered to veterans in the community must, of course, be paid for. When those community costs began to soar at the start of the Community Care program, Congress bailed out the VA by allocating extra funds. Today, escalating costs are drawn from local VA facility budgets. And to guarantee that private sector care is paid for out of VA facility funds, legislators are introducing language, such as in the Veterans Healthcare Freedom Act, which states: “No additional funds are authorized to be appropriated to carry out this section and the amendments made by this section, and this section and the amendments made by this section shall be carried out using amounts otherwise made available to the Veterans Health Administration.” The anticipated vicious cycle looms. More money pouring into the private sector will force reductions and closures of in-house VA staff, programs, clinics, and units. This will cause more veterans to obtain care in the community, which will further drain more money out of VA facilities, leading to more reductions, etc. Rural areas will likely be hit hardest.
The VA is nearing the tipping point of this ever-descending spiral. And that is even without expanding eligibility further. Three provisions in this pair of bills could, on their own, drastically open eligibility, eliminate remaining guardrails, and push VA over the edge:
(1) Veteran preference. Tucked into the HEALTH Act, introduced by the ranking Republican Member, Jerry Moran of Kansas, is language which would require VA to consider a “veteran’s preference” for obtaining their health care in the private sector.
This stipulation violates the intent of the VA MISSION Act. When MISSION passed, there was bipartisan agreement that the Community Care Program was meant, in numerous Senators’ words, to “supplement, not supplant” VA health care. A veteran would be offered the option of receiving health care outside of the VA under 6 narrowly defined criteria. Legislators understood that veterans would get the option to choose whether to receive care in the private sector or the VA if, and only if, they qualified under the 6 eligibility rules. As a well-researched document coauthored by Disabled American Veterans, Paralyzed Veterans of America, and the Veterans of Foreign Wars stated, “veteran convenience or preference” should never be used as a sole reason for referral.
Explicitly adding preference for the first time will create the expectation among veterans and lawmakers of a new allowance. Were this to pass, every veteran—100%—would become eligible for referral to the private sector, kicking off an unstoppable drainage of VA budget resources and threatening its viability. Hopefully, the Senate will follow the lead of the US House Committee on Veterans’ Affairs which, last week, amended its its own community care bill by deleting “veteran preference” as a possible new eligibility criterion.
(2) Self-referral. Also being deliberated is the Making Community Care Work for Veterans Act, a draft bill authored by the US Senate Committee on Veterans’ Affairs Democratic Chairman Senator Jon Tester of Montana. It calls for allowing self-initiated routine vaccinations and routine vision/hearing services in the community.
On the surface, Tester’s bill focuses on only a tiny sliver of care. But once self-referral is permitted for a few services, private sector interests will, in no time, push the door wide open and add more services to which veterans can self-refer. Testimony at the hearing confirmed that prediction, as the Veterans of Foreign Wars and America’s Warrior Partnership stated there is no reason to limit self-referral to only eye and ear examinations. They proposed that self-referral should extend to mental health, substance use, podiatry, prosthetics, laboratory services, dermatology, and diabetes. Like other perilous sections of these bills, seemingly innocuous language would quickly lead to crippling impacts.
(3) Pilot program for unfettered access. The HEALTH Act contains another provision in which veterans would be allowed to receive outpatient care without VA referral, authorization, or oversight. An enrolled veteran could simply make an appointment with any Veterans Community Care Program mental health or substance use disorder practitioner for care for any duration of time. VA’s only role would be to pay the invoice. Private sector interests have been pressing this sort of program for years, and when it was carefully studied by the Commission on Care, the costs were estimated to be 2 to 3 times the existing system. That would come from a combination of fee-for-service reimbursement structures that abet overuse and higher overall costs in the private sector. Were the pilot to pass, VA would convert from its primary role as a system providing health care to an insurance carrier.
In the name of offering more choices, health care options will diminish for veterans. When VA programs/clinics/facilities close, veterans—especially service-connected veterans who depend on VA for high-quality care tailored to their needs—will lose those choices. Moreover, a downsized VA will make it nearly impossible for the VA to continue to research veterans’ complex health conditions, educate future health care professionals (the majority of whom train at VA medical centers), or fulfill its Fourth Mission as a backup for national emergencies.
Senate Committee members indicated their intention to combine provisions of the Moran and Tester bills into a larger compromise bill in September. Legislators must slow down, contemplate the ramifications, and set aside the stipulations noted above. What is needed first is a projection of future veterans’ authorizations for community care (under current eligibility criteria and also with these new allowances), how much money would that pull out of VA facilities, and what is the tipping point of a doom cycle.
In the meantime, there are smart solutions to ensure veterans can access high-quality care, as Disabled American Veterans testified at the hearing: By “investing in VA's health care infrastructure and staffing… this is particularly true for veterans who live in rural and remote areas where VA is most likely to be a stable, long-term health care option for veterans.”
The VA administers the most successful health care system in the country. As a recent summary of research confirmed yet again, the quality of care delivered by the VA is as good as or better than the care veterans receive from VA-paid community care or the general public obtains through private care. There will always be a supplemental role for the community to play when VA cannot provide care in a timely or convenient manner. But community care must be fixed in ways that never starves VA facilities of essential funding. If there ever were a time to stand up for the sake of our veterans and the long-term viability of the VA, it is now.
“What if VA health care goes away?” That was the headline of a July 6, 2023, Disabled American Veterans news article to its members. The question was not hypothetical. Legislation currently under consideration by the US Congress may make it a strong probability.
The US Senate Committee on Veterans’ Affairs recently held a hearing to discuss 2 bills that would drastically reshape the provision of private health care services through the Veterans Community Care Program. An unprecedented coalition of 10 organizations—made up of US Department of Veterans Affairs (VA) nurses, psychologists, physicians, dentists, social workers, optometrists, physician assistants, and nurse anesthetists, as well as the American Psychological Association, the Military and Veterans Committee of the Group for the Advancement of Psychiatry and the Veterans Healthcare Policy Institute—came together in a unified statement for the record highlighting how these proposed policies would open a Pandora’s box that could forever eliminate the Veterans Health Administration as we know it.
Over the past decade—and especially following the passage of the Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act—there has been a surge of veterans gaining eligibility for private care if a VA medical facility is too far away, does not offer the needed care, or the wait time for an appointment is too long.
Testifying before the House Committee on Veterans’ Affairs hearing last year, Miguel LaPuz, MD, MBA, then the acting Deputy Under Secretary for Health at the VA, warned that “VA is rapidly approaching a point where half of all care available in both settings is provided through community care.” He cautioned that leaders were bracing for “the potential of a spiral effect.”
Care that is rendered to veterans in the community must, of course, be paid for. When those community costs began to soar at the start of the Community Care program, Congress bailed out the VA by allocating extra funds. Today, escalating costs are drawn from local VA facility budgets. And to guarantee that private sector care is paid for out of VA facility funds, legislators are introducing language, such as in the Veterans Healthcare Freedom Act, which states: “No additional funds are authorized to be appropriated to carry out this section and the amendments made by this section, and this section and the amendments made by this section shall be carried out using amounts otherwise made available to the Veterans Health Administration.” The anticipated vicious cycle looms. More money pouring into the private sector will force reductions and closures of in-house VA staff, programs, clinics, and units. This will cause more veterans to obtain care in the community, which will further drain more money out of VA facilities, leading to more reductions, etc. Rural areas will likely be hit hardest.
The VA is nearing the tipping point of this ever-descending spiral. And that is even without expanding eligibility further. Three provisions in this pair of bills could, on their own, drastically open eligibility, eliminate remaining guardrails, and push VA over the edge:
(1) Veteran preference. Tucked into the HEALTH Act, introduced by the ranking Republican Member, Jerry Moran of Kansas, is language which would require VA to consider a “veteran’s preference” for obtaining their health care in the private sector.
This stipulation violates the intent of the VA MISSION Act. When MISSION passed, there was bipartisan agreement that the Community Care Program was meant, in numerous Senators’ words, to “supplement, not supplant” VA health care. A veteran would be offered the option of receiving health care outside of the VA under 6 narrowly defined criteria. Legislators understood that veterans would get the option to choose whether to receive care in the private sector or the VA if, and only if, they qualified under the 6 eligibility rules. As a well-researched document coauthored by Disabled American Veterans, Paralyzed Veterans of America, and the Veterans of Foreign Wars stated, “veteran convenience or preference” should never be used as a sole reason for referral.
Explicitly adding preference for the first time will create the expectation among veterans and lawmakers of a new allowance. Were this to pass, every veteran—100%—would become eligible for referral to the private sector, kicking off an unstoppable drainage of VA budget resources and threatening its viability. Hopefully, the Senate will follow the lead of the US House Committee on Veterans’ Affairs which, last week, amended its its own community care bill by deleting “veteran preference” as a possible new eligibility criterion.
(2) Self-referral. Also being deliberated is the Making Community Care Work for Veterans Act, a draft bill authored by the US Senate Committee on Veterans’ Affairs Democratic Chairman Senator Jon Tester of Montana. It calls for allowing self-initiated routine vaccinations and routine vision/hearing services in the community.
On the surface, Tester’s bill focuses on only a tiny sliver of care. But once self-referral is permitted for a few services, private sector interests will, in no time, push the door wide open and add more services to which veterans can self-refer. Testimony at the hearing confirmed that prediction, as the Veterans of Foreign Wars and America’s Warrior Partnership stated there is no reason to limit self-referral to only eye and ear examinations. They proposed that self-referral should extend to mental health, substance use, podiatry, prosthetics, laboratory services, dermatology, and diabetes. Like other perilous sections of these bills, seemingly innocuous language would quickly lead to crippling impacts.
(3) Pilot program for unfettered access. The HEALTH Act contains another provision in which veterans would be allowed to receive outpatient care without VA referral, authorization, or oversight. An enrolled veteran could simply make an appointment with any Veterans Community Care Program mental health or substance use disorder practitioner for care for any duration of time. VA’s only role would be to pay the invoice. Private sector interests have been pressing this sort of program for years, and when it was carefully studied by the Commission on Care, the costs were estimated to be 2 to 3 times the existing system. That would come from a combination of fee-for-service reimbursement structures that abet overuse and higher overall costs in the private sector. Were the pilot to pass, VA would convert from its primary role as a system providing health care to an insurance carrier.
In the name of offering more choices, health care options will diminish for veterans. When VA programs/clinics/facilities close, veterans—especially service-connected veterans who depend on VA for high-quality care tailored to their needs—will lose those choices. Moreover, a downsized VA will make it nearly impossible for the VA to continue to research veterans’ complex health conditions, educate future health care professionals (the majority of whom train at VA medical centers), or fulfill its Fourth Mission as a backup for national emergencies.
Senate Committee members indicated their intention to combine provisions of the Moran and Tester bills into a larger compromise bill in September. Legislators must slow down, contemplate the ramifications, and set aside the stipulations noted above. What is needed first is a projection of future veterans’ authorizations for community care (under current eligibility criteria and also with these new allowances), how much money would that pull out of VA facilities, and what is the tipping point of a doom cycle.
In the meantime, there are smart solutions to ensure veterans can access high-quality care, as Disabled American Veterans testified at the hearing: By “investing in VA's health care infrastructure and staffing… this is particularly true for veterans who live in rural and remote areas where VA is most likely to be a stable, long-term health care option for veterans.”
The VA administers the most successful health care system in the country. As a recent summary of research confirmed yet again, the quality of care delivered by the VA is as good as or better than the care veterans receive from VA-paid community care or the general public obtains through private care. There will always be a supplemental role for the community to play when VA cannot provide care in a timely or convenient manner. But community care must be fixed in ways that never starves VA facilities of essential funding. If there ever were a time to stand up for the sake of our veterans and the long-term viability of the VA, it is now.
“What if VA health care goes away?” That was the headline of a July 6, 2023, Disabled American Veterans news article to its members. The question was not hypothetical. Legislation currently under consideration by the US Congress may make it a strong probability.
The US Senate Committee on Veterans’ Affairs recently held a hearing to discuss 2 bills that would drastically reshape the provision of private health care services through the Veterans Community Care Program. An unprecedented coalition of 10 organizations—made up of US Department of Veterans Affairs (VA) nurses, psychologists, physicians, dentists, social workers, optometrists, physician assistants, and nurse anesthetists, as well as the American Psychological Association, the Military and Veterans Committee of the Group for the Advancement of Psychiatry and the Veterans Healthcare Policy Institute—came together in a unified statement for the record highlighting how these proposed policies would open a Pandora’s box that could forever eliminate the Veterans Health Administration as we know it.
Over the past decade—and especially following the passage of the Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act—there has been a surge of veterans gaining eligibility for private care if a VA medical facility is too far away, does not offer the needed care, or the wait time for an appointment is too long.
Testifying before the House Committee on Veterans’ Affairs hearing last year, Miguel LaPuz, MD, MBA, then the acting Deputy Under Secretary for Health at the VA, warned that “VA is rapidly approaching a point where half of all care available in both settings is provided through community care.” He cautioned that leaders were bracing for “the potential of a spiral effect.”
Care that is rendered to veterans in the community must, of course, be paid for. When those community costs began to soar at the start of the Community Care program, Congress bailed out the VA by allocating extra funds. Today, escalating costs are drawn from local VA facility budgets. And to guarantee that private sector care is paid for out of VA facility funds, legislators are introducing language, such as in the Veterans Healthcare Freedom Act, which states: “No additional funds are authorized to be appropriated to carry out this section and the amendments made by this section, and this section and the amendments made by this section shall be carried out using amounts otherwise made available to the Veterans Health Administration.” The anticipated vicious cycle looms. More money pouring into the private sector will force reductions and closures of in-house VA staff, programs, clinics, and units. This will cause more veterans to obtain care in the community, which will further drain more money out of VA facilities, leading to more reductions, etc. Rural areas will likely be hit hardest.
The VA is nearing the tipping point of this ever-descending spiral. And that is even without expanding eligibility further. Three provisions in this pair of bills could, on their own, drastically open eligibility, eliminate remaining guardrails, and push VA over the edge:
(1) Veteran preference. Tucked into the HEALTH Act, introduced by the ranking Republican Member, Jerry Moran of Kansas, is language which would require VA to consider a “veteran’s preference” for obtaining their health care in the private sector.
This stipulation violates the intent of the VA MISSION Act. When MISSION passed, there was bipartisan agreement that the Community Care Program was meant, in numerous Senators’ words, to “supplement, not supplant” VA health care. A veteran would be offered the option of receiving health care outside of the VA under 6 narrowly defined criteria. Legislators understood that veterans would get the option to choose whether to receive care in the private sector or the VA if, and only if, they qualified under the 6 eligibility rules. As a well-researched document coauthored by Disabled American Veterans, Paralyzed Veterans of America, and the Veterans of Foreign Wars stated, “veteran convenience or preference” should never be used as a sole reason for referral.
Explicitly adding preference for the first time will create the expectation among veterans and lawmakers of a new allowance. Were this to pass, every veteran—100%—would become eligible for referral to the private sector, kicking off an unstoppable drainage of VA budget resources and threatening its viability. Hopefully, the Senate will follow the lead of the US House Committee on Veterans’ Affairs which, last week, amended its its own community care bill by deleting “veteran preference” as a possible new eligibility criterion.
(2) Self-referral. Also being deliberated is the Making Community Care Work for Veterans Act, a draft bill authored by the US Senate Committee on Veterans’ Affairs Democratic Chairman Senator Jon Tester of Montana. It calls for allowing self-initiated routine vaccinations and routine vision/hearing services in the community.
On the surface, Tester’s bill focuses on only a tiny sliver of care. But once self-referral is permitted for a few services, private sector interests will, in no time, push the door wide open and add more services to which veterans can self-refer. Testimony at the hearing confirmed that prediction, as the Veterans of Foreign Wars and America’s Warrior Partnership stated there is no reason to limit self-referral to only eye and ear examinations. They proposed that self-referral should extend to mental health, substance use, podiatry, prosthetics, laboratory services, dermatology, and diabetes. Like other perilous sections of these bills, seemingly innocuous language would quickly lead to crippling impacts.
(3) Pilot program for unfettered access. The HEALTH Act contains another provision in which veterans would be allowed to receive outpatient care without VA referral, authorization, or oversight. An enrolled veteran could simply make an appointment with any Veterans Community Care Program mental health or substance use disorder practitioner for care for any duration of time. VA’s only role would be to pay the invoice. Private sector interests have been pressing this sort of program for years, and when it was carefully studied by the Commission on Care, the costs were estimated to be 2 to 3 times the existing system. That would come from a combination of fee-for-service reimbursement structures that abet overuse and higher overall costs in the private sector. Were the pilot to pass, VA would convert from its primary role as a system providing health care to an insurance carrier.
In the name of offering more choices, health care options will diminish for veterans. When VA programs/clinics/facilities close, veterans—especially service-connected veterans who depend on VA for high-quality care tailored to their needs—will lose those choices. Moreover, a downsized VA will make it nearly impossible for the VA to continue to research veterans’ complex health conditions, educate future health care professionals (the majority of whom train at VA medical centers), or fulfill its Fourth Mission as a backup for national emergencies.
Senate Committee members indicated their intention to combine provisions of the Moran and Tester bills into a larger compromise bill in September. Legislators must slow down, contemplate the ramifications, and set aside the stipulations noted above. What is needed first is a projection of future veterans’ authorizations for community care (under current eligibility criteria and also with these new allowances), how much money would that pull out of VA facilities, and what is the tipping point of a doom cycle.
In the meantime, there are smart solutions to ensure veterans can access high-quality care, as Disabled American Veterans testified at the hearing: By “investing in VA's health care infrastructure and staffing… this is particularly true for veterans who live in rural and remote areas where VA is most likely to be a stable, long-term health care option for veterans.”
The VA administers the most successful health care system in the country. As a recent summary of research confirmed yet again, the quality of care delivered by the VA is as good as or better than the care veterans receive from VA-paid community care or the general public obtains through private care. There will always be a supplemental role for the community to play when VA cannot provide care in a timely or convenient manner. But community care must be fixed in ways that never starves VA facilities of essential funding. If there ever were a time to stand up for the sake of our veterans and the long-term viability of the VA, it is now.
Off-label meds: Promising long COVID treatments?
Those with long COVID for years now are engaging in robust online conversations about a range of treatments not formally approved by the Food and Drug Administration for the condition, reporting good and bad results.
High on the current list: low-dose naltrexone (LDN). A version of drug developed to help addicts has been shown to help some long COVID patients.
But evidence is building for other treatments, many of them targeted to treat brain fog or one of the other long-term symptoms in individuals 3 months or more after acute COVID infection.
Some patients are taking metformin, which studies have found to be effective at lowering long COVID risk. Paxlovid is being tested for long COVID. Antivirals are also on the list.
Alba Azola, MD, said she has treated long COVID patients with brain fog and dizziness who have postural orthostatic tachycardia syndrome (POTS).
Dr. Azola said she asked the staff at Johns Hopkins Medicine in Baltimore, where she is a rehabilitation specialist, to teach her how to treat the condition. Since there is no approved treatment for POTS, that meant using off-label drugs, she said.
“It was super scary as a provider to start doing that, but my patients were suffering so much,” she said, noting the wait for patients to get into the POTS clinic at Hopkins was 2 years.
Dr. Azola was the lead author on guidelines published by the American Academy of Physical Medicine and Rehabilitation (AAPM&R) last September on how to treat autonomic dysfunction, a common symptom of long COVID.
The guidelines she helped write include drugs designed for blood pressure – such as midodrine – and steroids.
Dr. Azola noted the medications are prescribed on a case-by-case basis because the same drug that works for one patient may have awful side effects for another patient, she said. At the same time, some of these drugs have helped her patients go back to living relatively normal lives.
The first time JD Davids of Brooklyn, N.Y., took LDN, it was for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and he couldn’t tolerate it. He had nightmares. But when he took it for long COVID, he started out at a low dose and worked his way up, at his doctor’s advice.
“It’s been a game-changer,” said Mr. Davids, cofounder of Long COVID Justice, an activist group. He has ME/CFS and several other chronic conditions, including long COVID. But, since he started taking LDN for long COVID, Mr. Davids said he has more energy and less pain.
Technically, evidence is required to show off-label drug use could be effective in treating conditions for which the drug is not formally approved. Research suggests that 20%-30% of drugs are prescribed off-label.
No formal data exist on how widespread the use of off-label drugs for long COVID may be. But LDN is a major topic of discussion on public patient groups on Facebook.
A recent study in The Lancet Infectious Diseases suggested that the diabetes drug metformin could be helpful. (The same study found no benefit from ivermectin, a drug since dismissed as a possible COVID treatment.)
Patients who testified at a virtual FDA hearing on drug development in April reported using vitamins, herbal supplements, over-the-counter medications and off-label drugs such as gabapentin and beta-blockers. Both of those drugs were on a list of potential treatments published in a January Nature Review article, along with LDN and Paxlovid.
Currently, Paxlovid is approved for acute COVID, and is in clinical trials as a treatment for long COVID as part of the federal government’s RECOVER Initiative. While only small studies of LDN have been conducted for long COVID, doctors are already prescribing the treatment. Mr. Davids said his primary care doctor recommended it.
Some doctors, such as Michael Peluso, MD, are comparing the trend to the early days of the AIDS epidemic, when the federal government was slow to recognize the viral disease. Patients banded together to protest and gain access to experimental treatments.
Dr. Peluso, who treats long COVID patients at the University of California, San Francisco, said that without any approved treatment, patients are turning to one another to find out what works.
“A lot of people experiencing long COVID are looking for ways to feel better now, rather than waiting for the science or the guidelines to catch up,” he said in an interview.
In some cases, the drugs are backed by small studies, he said.
“While we still need clinical trials to prove what will work, the drugs tested in these trials are also being informed by anecdotes shared by patients,” Dr. Peluso said.
Gail Van Norman, MD, of the University of Washington, Seattle, also said the long COVID situation today is reminiscent of the AIDS movement, which was “one of the times in history where we saw a real response to patient advocacy groups in terms of access to drugs.” Since then, the FDA has set up multiple programs to expand access to experimental drugs, added Dr. Van Norman, author of a recent study on off-label drug use.
But off-label use needs to be supervised by a physician, she and others said. Many patients get their information from social media, which Dr. Van Norman sees as a double-edged sword. Patients can share information, do their own research online, and alert practitioners to new findings, she said. But social media also promotes misinformation.
“People with no expertise have the same level of voice, and they are magnified,” Dr. Van Norman said.
The FDA requires doctors to have some evidence to support off-label use, she said. Doctors should talk to patients who want to try-off label drugs about what has been studied and what has not been studied.
“If I had [long COVID], I would be asking questions about all these drugs,” Dr. Van Norman said.
Mr. Davids has been asking questions like this for years. Diagnosed in 2019 with ME/CFS, he developed long COVID during the pandemic. Once he began started taking LDN, he started feeling better.
As someone with multiple chronic illnesses, Mr. Davids has tried a lot of treatments – he’s currently on two intravenous drugs and two compounded drugs, including LDN. But when his doctor first suggested it, he was wary.
“I’ve worked with her to help increase the dosage slowly over time,” he said. “It’s very important for many people to start low and slow and work their way up.”
He hears stories of people who can’t get it from their physicians. Some, he said, think it may be because of the association of the drug with opioid abuse.
Mr. Davids said long COVID patients have no other choice but to turn to alternative treatments.
“I think we’ve been ill-served by our research establishment,” he said. “It is not set up for complex chronic conditions.”
Mr. Davids said he doesn’t know if LDN helps with underlying conditions or treats the symptoms – such as pain and fatigue – that keep him from doing things such as typing.
“My understanding is that it may be doing both,” he said. “I sure am happy that it allows me to do things like keep my job.”
Dr. Azola and others said patients need to be monitored closely if they are taking an off-label drug. She recommends primary care doctors become familiar with them so they can offer patients some relief.
“It’s about the relationship between the patient and the provider and the provider being comfortable,” she said. “l was very transparent with my patients.”
A version of this article appeared on Medscape.com.
Those with long COVID for years now are engaging in robust online conversations about a range of treatments not formally approved by the Food and Drug Administration for the condition, reporting good and bad results.
High on the current list: low-dose naltrexone (LDN). A version of drug developed to help addicts has been shown to help some long COVID patients.
But evidence is building for other treatments, many of them targeted to treat brain fog or one of the other long-term symptoms in individuals 3 months or more after acute COVID infection.
Some patients are taking metformin, which studies have found to be effective at lowering long COVID risk. Paxlovid is being tested for long COVID. Antivirals are also on the list.
Alba Azola, MD, said she has treated long COVID patients with brain fog and dizziness who have postural orthostatic tachycardia syndrome (POTS).
Dr. Azola said she asked the staff at Johns Hopkins Medicine in Baltimore, where she is a rehabilitation specialist, to teach her how to treat the condition. Since there is no approved treatment for POTS, that meant using off-label drugs, she said.
“It was super scary as a provider to start doing that, but my patients were suffering so much,” she said, noting the wait for patients to get into the POTS clinic at Hopkins was 2 years.
Dr. Azola was the lead author on guidelines published by the American Academy of Physical Medicine and Rehabilitation (AAPM&R) last September on how to treat autonomic dysfunction, a common symptom of long COVID.
The guidelines she helped write include drugs designed for blood pressure – such as midodrine – and steroids.
Dr. Azola noted the medications are prescribed on a case-by-case basis because the same drug that works for one patient may have awful side effects for another patient, she said. At the same time, some of these drugs have helped her patients go back to living relatively normal lives.
The first time JD Davids of Brooklyn, N.Y., took LDN, it was for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and he couldn’t tolerate it. He had nightmares. But when he took it for long COVID, he started out at a low dose and worked his way up, at his doctor’s advice.
“It’s been a game-changer,” said Mr. Davids, cofounder of Long COVID Justice, an activist group. He has ME/CFS and several other chronic conditions, including long COVID. But, since he started taking LDN for long COVID, Mr. Davids said he has more energy and less pain.
Technically, evidence is required to show off-label drug use could be effective in treating conditions for which the drug is not formally approved. Research suggests that 20%-30% of drugs are prescribed off-label.
No formal data exist on how widespread the use of off-label drugs for long COVID may be. But LDN is a major topic of discussion on public patient groups on Facebook.
A recent study in The Lancet Infectious Diseases suggested that the diabetes drug metformin could be helpful. (The same study found no benefit from ivermectin, a drug since dismissed as a possible COVID treatment.)
Patients who testified at a virtual FDA hearing on drug development in April reported using vitamins, herbal supplements, over-the-counter medications and off-label drugs such as gabapentin and beta-blockers. Both of those drugs were on a list of potential treatments published in a January Nature Review article, along with LDN and Paxlovid.
Currently, Paxlovid is approved for acute COVID, and is in clinical trials as a treatment for long COVID as part of the federal government’s RECOVER Initiative. While only small studies of LDN have been conducted for long COVID, doctors are already prescribing the treatment. Mr. Davids said his primary care doctor recommended it.
Some doctors, such as Michael Peluso, MD, are comparing the trend to the early days of the AIDS epidemic, when the federal government was slow to recognize the viral disease. Patients banded together to protest and gain access to experimental treatments.
Dr. Peluso, who treats long COVID patients at the University of California, San Francisco, said that without any approved treatment, patients are turning to one another to find out what works.
“A lot of people experiencing long COVID are looking for ways to feel better now, rather than waiting for the science or the guidelines to catch up,” he said in an interview.
In some cases, the drugs are backed by small studies, he said.
“While we still need clinical trials to prove what will work, the drugs tested in these trials are also being informed by anecdotes shared by patients,” Dr. Peluso said.
Gail Van Norman, MD, of the University of Washington, Seattle, also said the long COVID situation today is reminiscent of the AIDS movement, which was “one of the times in history where we saw a real response to patient advocacy groups in terms of access to drugs.” Since then, the FDA has set up multiple programs to expand access to experimental drugs, added Dr. Van Norman, author of a recent study on off-label drug use.
But off-label use needs to be supervised by a physician, she and others said. Many patients get their information from social media, which Dr. Van Norman sees as a double-edged sword. Patients can share information, do their own research online, and alert practitioners to new findings, she said. But social media also promotes misinformation.
“People with no expertise have the same level of voice, and they are magnified,” Dr. Van Norman said.
The FDA requires doctors to have some evidence to support off-label use, she said. Doctors should talk to patients who want to try-off label drugs about what has been studied and what has not been studied.
“If I had [long COVID], I would be asking questions about all these drugs,” Dr. Van Norman said.
Mr. Davids has been asking questions like this for years. Diagnosed in 2019 with ME/CFS, he developed long COVID during the pandemic. Once he began started taking LDN, he started feeling better.
As someone with multiple chronic illnesses, Mr. Davids has tried a lot of treatments – he’s currently on two intravenous drugs and two compounded drugs, including LDN. But when his doctor first suggested it, he was wary.
“I’ve worked with her to help increase the dosage slowly over time,” he said. “It’s very important for many people to start low and slow and work their way up.”
He hears stories of people who can’t get it from their physicians. Some, he said, think it may be because of the association of the drug with opioid abuse.
Mr. Davids said long COVID patients have no other choice but to turn to alternative treatments.
“I think we’ve been ill-served by our research establishment,” he said. “It is not set up for complex chronic conditions.”
Mr. Davids said he doesn’t know if LDN helps with underlying conditions or treats the symptoms – such as pain and fatigue – that keep him from doing things such as typing.
“My understanding is that it may be doing both,” he said. “I sure am happy that it allows me to do things like keep my job.”
Dr. Azola and others said patients need to be monitored closely if they are taking an off-label drug. She recommends primary care doctors become familiar with them so they can offer patients some relief.
“It’s about the relationship between the patient and the provider and the provider being comfortable,” she said. “l was very transparent with my patients.”
A version of this article appeared on Medscape.com.
Those with long COVID for years now are engaging in robust online conversations about a range of treatments not formally approved by the Food and Drug Administration for the condition, reporting good and bad results.
High on the current list: low-dose naltrexone (LDN). A version of drug developed to help addicts has been shown to help some long COVID patients.
But evidence is building for other treatments, many of them targeted to treat brain fog or one of the other long-term symptoms in individuals 3 months or more after acute COVID infection.
Some patients are taking metformin, which studies have found to be effective at lowering long COVID risk. Paxlovid is being tested for long COVID. Antivirals are also on the list.
Alba Azola, MD, said she has treated long COVID patients with brain fog and dizziness who have postural orthostatic tachycardia syndrome (POTS).
Dr. Azola said she asked the staff at Johns Hopkins Medicine in Baltimore, where she is a rehabilitation specialist, to teach her how to treat the condition. Since there is no approved treatment for POTS, that meant using off-label drugs, she said.
“It was super scary as a provider to start doing that, but my patients were suffering so much,” she said, noting the wait for patients to get into the POTS clinic at Hopkins was 2 years.
Dr. Azola was the lead author on guidelines published by the American Academy of Physical Medicine and Rehabilitation (AAPM&R) last September on how to treat autonomic dysfunction, a common symptom of long COVID.
The guidelines she helped write include drugs designed for blood pressure – such as midodrine – and steroids.
Dr. Azola noted the medications are prescribed on a case-by-case basis because the same drug that works for one patient may have awful side effects for another patient, she said. At the same time, some of these drugs have helped her patients go back to living relatively normal lives.
The first time JD Davids of Brooklyn, N.Y., took LDN, it was for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and he couldn’t tolerate it. He had nightmares. But when he took it for long COVID, he started out at a low dose and worked his way up, at his doctor’s advice.
“It’s been a game-changer,” said Mr. Davids, cofounder of Long COVID Justice, an activist group. He has ME/CFS and several other chronic conditions, including long COVID. But, since he started taking LDN for long COVID, Mr. Davids said he has more energy and less pain.
Technically, evidence is required to show off-label drug use could be effective in treating conditions for which the drug is not formally approved. Research suggests that 20%-30% of drugs are prescribed off-label.
No formal data exist on how widespread the use of off-label drugs for long COVID may be. But LDN is a major topic of discussion on public patient groups on Facebook.
A recent study in The Lancet Infectious Diseases suggested that the diabetes drug metformin could be helpful. (The same study found no benefit from ivermectin, a drug since dismissed as a possible COVID treatment.)
Patients who testified at a virtual FDA hearing on drug development in April reported using vitamins, herbal supplements, over-the-counter medications and off-label drugs such as gabapentin and beta-blockers. Both of those drugs were on a list of potential treatments published in a January Nature Review article, along with LDN and Paxlovid.
Currently, Paxlovid is approved for acute COVID, and is in clinical trials as a treatment for long COVID as part of the federal government’s RECOVER Initiative. While only small studies of LDN have been conducted for long COVID, doctors are already prescribing the treatment. Mr. Davids said his primary care doctor recommended it.
Some doctors, such as Michael Peluso, MD, are comparing the trend to the early days of the AIDS epidemic, when the federal government was slow to recognize the viral disease. Patients banded together to protest and gain access to experimental treatments.
Dr. Peluso, who treats long COVID patients at the University of California, San Francisco, said that without any approved treatment, patients are turning to one another to find out what works.
“A lot of people experiencing long COVID are looking for ways to feel better now, rather than waiting for the science or the guidelines to catch up,” he said in an interview.
In some cases, the drugs are backed by small studies, he said.
“While we still need clinical trials to prove what will work, the drugs tested in these trials are also being informed by anecdotes shared by patients,” Dr. Peluso said.
Gail Van Norman, MD, of the University of Washington, Seattle, also said the long COVID situation today is reminiscent of the AIDS movement, which was “one of the times in history where we saw a real response to patient advocacy groups in terms of access to drugs.” Since then, the FDA has set up multiple programs to expand access to experimental drugs, added Dr. Van Norman, author of a recent study on off-label drug use.
But off-label use needs to be supervised by a physician, she and others said. Many patients get their information from social media, which Dr. Van Norman sees as a double-edged sword. Patients can share information, do their own research online, and alert practitioners to new findings, she said. But social media also promotes misinformation.
“People with no expertise have the same level of voice, and they are magnified,” Dr. Van Norman said.
The FDA requires doctors to have some evidence to support off-label use, she said. Doctors should talk to patients who want to try-off label drugs about what has been studied and what has not been studied.
“If I had [long COVID], I would be asking questions about all these drugs,” Dr. Van Norman said.
Mr. Davids has been asking questions like this for years. Diagnosed in 2019 with ME/CFS, he developed long COVID during the pandemic. Once he began started taking LDN, he started feeling better.
As someone with multiple chronic illnesses, Mr. Davids has tried a lot of treatments – he’s currently on two intravenous drugs and two compounded drugs, including LDN. But when his doctor first suggested it, he was wary.
“I’ve worked with her to help increase the dosage slowly over time,” he said. “It’s very important for many people to start low and slow and work their way up.”
He hears stories of people who can’t get it from their physicians. Some, he said, think it may be because of the association of the drug with opioid abuse.
Mr. Davids said long COVID patients have no other choice but to turn to alternative treatments.
“I think we’ve been ill-served by our research establishment,” he said. “It is not set up for complex chronic conditions.”
Mr. Davids said he doesn’t know if LDN helps with underlying conditions or treats the symptoms – such as pain and fatigue – that keep him from doing things such as typing.
“My understanding is that it may be doing both,” he said. “I sure am happy that it allows me to do things like keep my job.”
Dr. Azola and others said patients need to be monitored closely if they are taking an off-label drug. She recommends primary care doctors become familiar with them so they can offer patients some relief.
“It’s about the relationship between the patient and the provider and the provider being comfortable,” she said. “l was very transparent with my patients.”
A version of this article appeared on Medscape.com.
New air monitor can detect COVID virus in 5 minutes
The project was a collaboration among researchers from the university’s engineering and medical schools; the results were published in Nature Communications.
One of the challenges the team had to overcome is that detecting the virus in a roomful of air “is like finding a needle in a haystack,” researcher and associate engineering professor Rajan Chakrabarty, PhD, said in a statement.
The team overcame that challenge using a technology called wet cyclone that samples the equivalent of 176 cubic feet of air in 5 minutes. A light on the device turns from green to red when the virus is detected, which the researchers said indicates that increased air circulation is needed.
The device stands just 10 inches tall and 1 foot wide and is considered a proof of concept. The next step would be to implement the technology into a prototype to see how a commercial or household design could be achieved. The researchers foresee potential for the device to be used in hospitals and schools, as well as to be able to detect other respiratory viruses such as influenza and respiratory syncytial virus.
Current methods used for detecting viruses in the air take between 1 and 24 hours to collect and analyze samples. The existing methods usually require skilled labor, resulting in a process that doesn’t allow for real-time information that could translate into reducing risk or the spread of the virus, the researchers wrote.
The team tested their device both in laboratory experiments where they released aerosolized SARS-CoV-2 into a room-sized chamber, as well as in the apartments of two people who were COVID positive.
“There is nothing at the moment that tells us how safe a room is,” Washington University neurology professor John Cirrito, PhD, said in a statement. “If you are in a room with 100 people, you don’t want to find out 5 days later whether you could be sick or not. The idea with this device is that you can know essentially in real time, or every 5 minutes, if there is a live virus in the air.”
Their goal is to develop a commercially available air quality monitor, the researchers said.
The study authors reported that they had no conflicts of interest.
A version of this article appeared on WebMD.com.
The project was a collaboration among researchers from the university’s engineering and medical schools; the results were published in Nature Communications.
One of the challenges the team had to overcome is that detecting the virus in a roomful of air “is like finding a needle in a haystack,” researcher and associate engineering professor Rajan Chakrabarty, PhD, said in a statement.
The team overcame that challenge using a technology called wet cyclone that samples the equivalent of 176 cubic feet of air in 5 minutes. A light on the device turns from green to red when the virus is detected, which the researchers said indicates that increased air circulation is needed.
The device stands just 10 inches tall and 1 foot wide and is considered a proof of concept. The next step would be to implement the technology into a prototype to see how a commercial or household design could be achieved. The researchers foresee potential for the device to be used in hospitals and schools, as well as to be able to detect other respiratory viruses such as influenza and respiratory syncytial virus.
Current methods used for detecting viruses in the air take between 1 and 24 hours to collect and analyze samples. The existing methods usually require skilled labor, resulting in a process that doesn’t allow for real-time information that could translate into reducing risk or the spread of the virus, the researchers wrote.
The team tested their device both in laboratory experiments where they released aerosolized SARS-CoV-2 into a room-sized chamber, as well as in the apartments of two people who were COVID positive.
“There is nothing at the moment that tells us how safe a room is,” Washington University neurology professor John Cirrito, PhD, said in a statement. “If you are in a room with 100 people, you don’t want to find out 5 days later whether you could be sick or not. The idea with this device is that you can know essentially in real time, or every 5 minutes, if there is a live virus in the air.”
Their goal is to develop a commercially available air quality monitor, the researchers said.
The study authors reported that they had no conflicts of interest.
A version of this article appeared on WebMD.com.
The project was a collaboration among researchers from the university’s engineering and medical schools; the results were published in Nature Communications.
One of the challenges the team had to overcome is that detecting the virus in a roomful of air “is like finding a needle in a haystack,” researcher and associate engineering professor Rajan Chakrabarty, PhD, said in a statement.
The team overcame that challenge using a technology called wet cyclone that samples the equivalent of 176 cubic feet of air in 5 minutes. A light on the device turns from green to red when the virus is detected, which the researchers said indicates that increased air circulation is needed.
The device stands just 10 inches tall and 1 foot wide and is considered a proof of concept. The next step would be to implement the technology into a prototype to see how a commercial or household design could be achieved. The researchers foresee potential for the device to be used in hospitals and schools, as well as to be able to detect other respiratory viruses such as influenza and respiratory syncytial virus.
Current methods used for detecting viruses in the air take between 1 and 24 hours to collect and analyze samples. The existing methods usually require skilled labor, resulting in a process that doesn’t allow for real-time information that could translate into reducing risk or the spread of the virus, the researchers wrote.
The team tested their device both in laboratory experiments where they released aerosolized SARS-CoV-2 into a room-sized chamber, as well as in the apartments of two people who were COVID positive.
“There is nothing at the moment that tells us how safe a room is,” Washington University neurology professor John Cirrito, PhD, said in a statement. “If you are in a room with 100 people, you don’t want to find out 5 days later whether you could be sick or not. The idea with this device is that you can know essentially in real time, or every 5 minutes, if there is a live virus in the air.”
Their goal is to develop a commercially available air quality monitor, the researchers said.
The study authors reported that they had no conflicts of interest.
A version of this article appeared on WebMD.com.
FROM NATURE COMMUNICATIONS
New guidelines for laser treatment of cutaneous vascular anomalies
A new practice guideline is setting a standard for doctors who use lasers to treat cutaneous vascular anomalies.
Poor treatment has been an issue in this field because no uniform guidelines existed to inform practice, according to a press release from the American Society for Laser Medicine and Surgery.
The laser treatment settings can vary based on the type and location of the birthmark and also the patient’s skin type, which has resulted in an inconsistent approach from clinicians, according to the release.
“For decades, I have observed adverse outcomes from the improper laser treatment of vascular birthmarks,” Linda Rozell-Shannon, PhD, president and founder of the Vascular Birthmarks Foundation said in a statement from ASLMS. “As a result of these guidelines, patient outcomes will be improved.”
The guideline, published on the ASLMS website along with supporting videos, was jointly developed by ASLMS, VBF, and an international group of clinicians, marking the first consensus guideline on laser treatments for cutaneous vascular anomalies. It details 32 best practice directives for various scenarios, including advice on safety considerations, additional testing, and when to refer.
“It is important to realize that just because someone is board certified does not mean they are skilled in treating all conditions or using all lasers,” Paul Friedman, MD, a dermatologist in Houston, and former president of ASLMS, said in the ASLMS statement.
Vascular birthmarks are a common condition affecting up to 14% of children, according to VBF. Most are hemangiomas, a buildup of blood vessels that usually appears at birth or within a month after birth. Laser therapy reduces the size and color of the anomalies.
Support for this initiative was provided by Candela Medical.
A version of this article first appeared on Medscape.com.
A new practice guideline is setting a standard for doctors who use lasers to treat cutaneous vascular anomalies.
Poor treatment has been an issue in this field because no uniform guidelines existed to inform practice, according to a press release from the American Society for Laser Medicine and Surgery.
The laser treatment settings can vary based on the type and location of the birthmark and also the patient’s skin type, which has resulted in an inconsistent approach from clinicians, according to the release.
“For decades, I have observed adverse outcomes from the improper laser treatment of vascular birthmarks,” Linda Rozell-Shannon, PhD, president and founder of the Vascular Birthmarks Foundation said in a statement from ASLMS. “As a result of these guidelines, patient outcomes will be improved.”
The guideline, published on the ASLMS website along with supporting videos, was jointly developed by ASLMS, VBF, and an international group of clinicians, marking the first consensus guideline on laser treatments for cutaneous vascular anomalies. It details 32 best practice directives for various scenarios, including advice on safety considerations, additional testing, and when to refer.
“It is important to realize that just because someone is board certified does not mean they are skilled in treating all conditions or using all lasers,” Paul Friedman, MD, a dermatologist in Houston, and former president of ASLMS, said in the ASLMS statement.
Vascular birthmarks are a common condition affecting up to 14% of children, according to VBF. Most are hemangiomas, a buildup of blood vessels that usually appears at birth or within a month after birth. Laser therapy reduces the size and color of the anomalies.
Support for this initiative was provided by Candela Medical.
A version of this article first appeared on Medscape.com.
A new practice guideline is setting a standard for doctors who use lasers to treat cutaneous vascular anomalies.
Poor treatment has been an issue in this field because no uniform guidelines existed to inform practice, according to a press release from the American Society for Laser Medicine and Surgery.
The laser treatment settings can vary based on the type and location of the birthmark and also the patient’s skin type, which has resulted in an inconsistent approach from clinicians, according to the release.
“For decades, I have observed adverse outcomes from the improper laser treatment of vascular birthmarks,” Linda Rozell-Shannon, PhD, president and founder of the Vascular Birthmarks Foundation said in a statement from ASLMS. “As a result of these guidelines, patient outcomes will be improved.”
The guideline, published on the ASLMS website along with supporting videos, was jointly developed by ASLMS, VBF, and an international group of clinicians, marking the first consensus guideline on laser treatments for cutaneous vascular anomalies. It details 32 best practice directives for various scenarios, including advice on safety considerations, additional testing, and when to refer.
“It is important to realize that just because someone is board certified does not mean they are skilled in treating all conditions or using all lasers,” Paul Friedman, MD, a dermatologist in Houston, and former president of ASLMS, said in the ASLMS statement.
Vascular birthmarks are a common condition affecting up to 14% of children, according to VBF. Most are hemangiomas, a buildup of blood vessels that usually appears at birth or within a month after birth. Laser therapy reduces the size and color of the anomalies.
Support for this initiative was provided by Candela Medical.
A version of this article first appeared on Medscape.com.
Progress seen on five fronts for substantially improving treatment of epidermolysis bullosa
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
AT SPD 2023
A Special Supplement on Hot Topics in Primary Care 2023

Hot Topics in Primary Care 2023 is a resource that explores the newest developments in primary care topics that impact your daily clinical practice.
Click on the link below to access the entire supplement. You can also click on the video panes below to view brief summaries of individual chapters. Titles above the video panes link directly to each article.
- A Patient-Centered Approach to Managing IBS-C and CIC
- Acute Pain in Perspective
- Continuous Glucose Monitoring in Practice
- Early Intervention by Family Physicians to Delay Type 1 Diabetes
- Early Life Nutrition and the Developing Brain
- Insomnia Management: A Review and Update
- New Paradigms for CKD Management in Patients With T2D
- Optimized Management of Cardio-Renal-Metabolic (CRM) Conditions in Patients with T2D
- Reducing Cardiopulmonary Risk and Exacerbations in COPD
- Reducing Ischemic Stroke in Diabetes: The Role of GLP-1 RAs
- Use of ICS and Fast-Acting Bronchodilators in Asthma: Past, Present, and Future
This supplement offers the opportunity to earn a total of 4 continuing medical education (CME) credits. Credit is awarded for successful completion of the evaluation after reading the article. The links can be found within the supplement on the first page of each article where CME credits are offered.
Click here to read Hot Topics in Primary Care 2023
This supplement to The Journal of Family Practice was sponsored by the Primary Care Education Consortium and Primary Care Metabolic Group.
Check out these short video segments, which were prepared by the supplement authors and summarize the individual articles.
The title above each video links to the related article.
A Patient-Centered Approach to Managing IBS-C and CIC, Brian E. Lacy, MD, PhD, FACG
Acute Pain in Perspective, Bill McCarberg, MD
Continuous Glucose Monitoring in Practice, Eden M. Miller, DO
Early Intervention by Family Physicians to Delay Type 1 Diabetes, Steven Edelman, MD
Early Life Nutrition and the Developing Brain, Danielle Christifano, PhD; Lara Bennett, MS, RD, LD
Insomnia Management: A Review and Update, David P. Shaha, MD
New Paradigms for CKD Management in Patients With T2D, Joshua J. Neumiller, PharmD, CDCES, FADCES, FASCP; Stephen A. Brunton, MD, FAAFP, CDCES
Optimized Management of Cardio-Renal-Metabolic (CRM) Conditions in Patients with T2D, Jay H. Shubrook, DO; Joshua J. Neumiller, PharmD, CDCES, FADCES, FASCP
Reducing Cardiopulmonary Risk and Exacerbations in COPD, Barbara Yawn, MD, MSc, FAAFP
Reducing Ischemic Stroke in Diabetes: The Role of GLP-1 Ras, John E. Anderson, MD; Javed Butler, MD, MPH, MBA; Andrei V. Alexandrov, MD
Use of ICS and Fast-Acting Bronchodilators in Asthma: Past, Present, and Future, Neil Skolnik, MD; Marissa Norden, DO; Njira Lugogo, MD: Wendy Wright, DNP, ANP-BC, FNP-BC, FAANP, FAAN, FNAP

Hot Topics in Primary Care 2023 is a resource that explores the newest developments in primary care topics that impact your daily clinical practice.
Click on the link below to access the entire supplement. You can also click on the video panes below to view brief summaries of individual chapters. Titles above the video panes link directly to each article.
- A Patient-Centered Approach to Managing IBS-C and CIC
- Acute Pain in Perspective
- Continuous Glucose Monitoring in Practice
- Early Intervention by Family Physicians to Delay Type 1 Diabetes
- Early Life Nutrition and the Developing Brain
- Insomnia Management: A Review and Update
- New Paradigms for CKD Management in Patients With T2D
- Optimized Management of Cardio-Renal-Metabolic (CRM) Conditions in Patients with T2D
- Reducing Cardiopulmonary Risk and Exacerbations in COPD
- Reducing Ischemic Stroke in Diabetes: The Role of GLP-1 RAs
- Use of ICS and Fast-Acting Bronchodilators in Asthma: Past, Present, and Future
This supplement offers the opportunity to earn a total of 4 continuing medical education (CME) credits. Credit is awarded for successful completion of the evaluation after reading the article. The links can be found within the supplement on the first page of each article where CME credits are offered.
Click here to read Hot Topics in Primary Care 2023
This supplement to The Journal of Family Practice was sponsored by the Primary Care Education Consortium and Primary Care Metabolic Group.
Check out these short video segments, which were prepared by the supplement authors and summarize the individual articles.
The title above each video links to the related article.
A Patient-Centered Approach to Managing IBS-C and CIC, Brian E. Lacy, MD, PhD, FACG
Acute Pain in Perspective, Bill McCarberg, MD
Continuous Glucose Monitoring in Practice, Eden M. Miller, DO
Early Intervention by Family Physicians to Delay Type 1 Diabetes, Steven Edelman, MD
Early Life Nutrition and the Developing Brain, Danielle Christifano, PhD; Lara Bennett, MS, RD, LD
Insomnia Management: A Review and Update, David P. Shaha, MD
New Paradigms for CKD Management in Patients With T2D, Joshua J. Neumiller, PharmD, CDCES, FADCES, FASCP; Stephen A. Brunton, MD, FAAFP, CDCES
Optimized Management of Cardio-Renal-Metabolic (CRM) Conditions in Patients with T2D, Jay H. Shubrook, DO; Joshua J. Neumiller, PharmD, CDCES, FADCES, FASCP
Reducing Cardiopulmonary Risk and Exacerbations in COPD, Barbara Yawn, MD, MSc, FAAFP
Reducing Ischemic Stroke in Diabetes: The Role of GLP-1 Ras, John E. Anderson, MD; Javed Butler, MD, MPH, MBA; Andrei V. Alexandrov, MD
Use of ICS and Fast-Acting Bronchodilators in Asthma: Past, Present, and Future, Neil Skolnik, MD; Marissa Norden, DO; Njira Lugogo, MD: Wendy Wright, DNP, ANP-BC, FNP-BC, FAANP, FAAN, FNAP

Hot Topics in Primary Care 2023 is a resource that explores the newest developments in primary care topics that impact your daily clinical practice.
Click on the link below to access the entire supplement. You can also click on the video panes below to view brief summaries of individual chapters. Titles above the video panes link directly to each article.
- A Patient-Centered Approach to Managing IBS-C and CIC
- Acute Pain in Perspective
- Continuous Glucose Monitoring in Practice
- Early Intervention by Family Physicians to Delay Type 1 Diabetes
- Early Life Nutrition and the Developing Brain
- Insomnia Management: A Review and Update
- New Paradigms for CKD Management in Patients With T2D
- Optimized Management of Cardio-Renal-Metabolic (CRM) Conditions in Patients with T2D
- Reducing Cardiopulmonary Risk and Exacerbations in COPD
- Reducing Ischemic Stroke in Diabetes: The Role of GLP-1 RAs
- Use of ICS and Fast-Acting Bronchodilators in Asthma: Past, Present, and Future
This supplement offers the opportunity to earn a total of 4 continuing medical education (CME) credits. Credit is awarded for successful completion of the evaluation after reading the article. The links can be found within the supplement on the first page of each article where CME credits are offered.
Click here to read Hot Topics in Primary Care 2023
This supplement to The Journal of Family Practice was sponsored by the Primary Care Education Consortium and Primary Care Metabolic Group.
Check out these short video segments, which were prepared by the supplement authors and summarize the individual articles.
The title above each video links to the related article.
A Patient-Centered Approach to Managing IBS-C and CIC, Brian E. Lacy, MD, PhD, FACG
Acute Pain in Perspective, Bill McCarberg, MD
Continuous Glucose Monitoring in Practice, Eden M. Miller, DO
Early Intervention by Family Physicians to Delay Type 1 Diabetes, Steven Edelman, MD
Early Life Nutrition and the Developing Brain, Danielle Christifano, PhD; Lara Bennett, MS, RD, LD
Insomnia Management: A Review and Update, David P. Shaha, MD
New Paradigms for CKD Management in Patients With T2D, Joshua J. Neumiller, PharmD, CDCES, FADCES, FASCP; Stephen A. Brunton, MD, FAAFP, CDCES
Optimized Management of Cardio-Renal-Metabolic (CRM) Conditions in Patients with T2D, Jay H. Shubrook, DO; Joshua J. Neumiller, PharmD, CDCES, FADCES, FASCP
Reducing Cardiopulmonary Risk and Exacerbations in COPD, Barbara Yawn, MD, MSc, FAAFP
Reducing Ischemic Stroke in Diabetes: The Role of GLP-1 Ras, John E. Anderson, MD; Javed Butler, MD, MPH, MBA; Andrei V. Alexandrov, MD
Use of ICS and Fast-Acting Bronchodilators in Asthma: Past, Present, and Future, Neil Skolnik, MD; Marissa Norden, DO; Njira Lugogo, MD: Wendy Wright, DNP, ANP-BC, FNP-BC, FAANP, FAAN, FNAP
Analysis of a Pilot Curriculum for Business Education in Dermatology Residency
To the Editor:
With health care constituting one of the larger segments of the US economy, medical practice is increasingly subject to business considerations.1 Patients, providers, and organizations are all required to make decisions that reflect choices beyond clinical needs alone. Given the impact of market forces, clinicians often are asked to navigate operational and business decisions. Accordingly, education about the policy and systems that shape care delivery can improve quality and help patients.2
The ability to understand the ecosystem of health care is of utmost importance for medical providers and can be achieved through resident education. Teaching fundamental business concepts enables residents to deliver care that is responsive to the constraints and opportunities encountered by patients and organizations, which ultimately will better prepare them to serve as advocates in alignment with their principal duties as physicians.
Despite the recognizable relationship between business and medicine, training has not yet been standardized to include topics in business education, and clinicians in dermatology are remarkably positioned to benefit because of the variety of practice settings and services they can provide. In dermatology, the diversity of services provided gives rise to complex coding and use of modifiers. Proper utilization of coding and billing is critical to create accurate documentation and receive appropriate reimbursement.3 Furthermore, clinicians in dermatology have to contend with the influence of insurance at many points of care, such as with coverage of pharmaceuticals. Formularies often have wide variability in coverage and are changing as new drugs come to market in the dermatologic space.4
The landscape of practice structure also has undergone change with increasing consolidation and mergers. The acquisition of practices by private equity firms has induced changes in practice infrastructure. The impact of changing organizational and managerial influences continues to be a topic of debate, with disparate opinions on how these developments shape standards of physician satisfaction and patient care.5
The convergence of these factors points to an important question that is gaining popularity: How will young dermatologists work within the context of all these parameters to best advocate and care for their patients? These questions are garnering more attention and were recently investigated through a survey of participants in a pilot program to evaluate the importance of business education in dermatology residency.
A survey of residency program directors was created by Patrinley and Dewan,6 which found that business education during residency was important and additional training should be implemented. Despite the perceived importance of business education, only half of the programs represented by survey respondents offered any structured educational opportunities, revealing a discrepancy between believed importance and practical implementation of business training, which suggests the need to develop a standardized, dermatology-specific curriculum that could be accessed by all residents in training.6
We performed a search of the medical literature to identify models of business education in residency programs. Only a few programs were identified, in which courses were predominantly instructed to trainees in primary care–based fields. According to course graduates, the programs were beneficial.7,8 Programs that had descriptive information about curriculum structure and content were chosen for further investigation and included internal medicine programs at the University of California San Francisco (UCSF) and Columbia University Vagelos College of Physicians and Surgeons (New York, New York). UCSF implemented a Program in Residency Investigation Methods and Epidemiology (PRIME program) to deliver seven 90-minute sessions dedicated to introducing residents to medical economics. Sessions were constructed with the intent of being interactive seminars that took on a variety of forms, including reading-based discussions, case-based analysis, and simulation-based learning.7 Columbia University developed a pilot program of week-long didactic sessions that were delivered to third-year internal medicine residents. These seminars featured discussions on health policy and economics, health insurance, technology and cost assessment, legal medicine, public health, community-oriented primary care, and local health department initiatives.8 We drew on both courses to build a lecture series focused on the business of dermatology that was delivered to dermatology residents at UMass Chan Medical School (Worcester, Massachusetts). Topic selection also was informed by qualitative input collected via email from recent graduates of the UMass dermatology residency program, focusing on the following areas: the US medical economy and health care costs; billing, coding, and claims processing; quality, relative value units (RVUs), reimbursement, and the merit-based incentive payment system; coverage of pharmaceuticals and teledermatology; and management. Residents were not required to prepare for any of the sessions; they were provided with handouts and slideshow presentations for reference to review at their convenience if desired. Five seminars were virtually conducted by an MD/MBA candidate at the institution (E.H.). They were recorded over the course of an academic year at 1- to 2-month intervals. Each 45-minute session was conducted in a lecture-discussion format and included case examples to help illustrate key principles and stimulate conversation. For example, the lecture on reimbursement incorporated a fee schedule calculation for a shave biopsy, using RVU and geographic pricing cost index (GCPI) multipliers. This demonstrated the variation in Centers for Medicare & Medicaid Services reimbursement in relation to (1) constituents of the RVU calculation (ie, work, practice expense, and malpractice) and (2) practice in a particular location (ie, the GCPI). Following this example, a conversation ensued among participants regarding the factors that drive valuation, with particular interest in variation based on urban vs suburban locations across the United States. Participants also found it of interest to examine the percentage of the valuation dedicated to each constituent and how features such as lesion size informed the final assessment of the charge. Another stylistic choice in developing the model was to include prompts for further consideration prior to transitioning topics in the lectures. For example: when examining the burden of skin disease, the audience was prompted to consider: “What is driving cost escalations, and how will services of the clinical domain meet these evolving needs?” At another point in the introductory lecture, residents were asked: “How do different types of insurance plans impact the management of patients with dermatologic concerns?” These questions were intended to transition residents to the next topic of discussion and highlight take-home points of consideration for medical practice. The project was reviewed by the UMass institutional review board and met criteria for exemption.
Residents who participated in at least 1 lecture (N=10) were surveyed after attendance; there were 7 responses (70% response rate). Residents were asked to rate a series of statements on a scale of 1 (strongly disagree) to 5 (strongly agree) and to provide commentary via an online form. Respondents indicated that the course was enjoyable (average score, 4.00), provided an appropriate level of detail (average score, 4.00), would be beneficial to integrate into a dermatology residency curriculum (average score, 3.86), and informed how they would practice as a clinician (average score, 3.86)(Figure). The respondents agreed that the course met the main goals of this initiative: it helped them develop knowledge about the interface between business and dermatology (4.14) and exposed residents to topics they had not learned about previously (4.71).
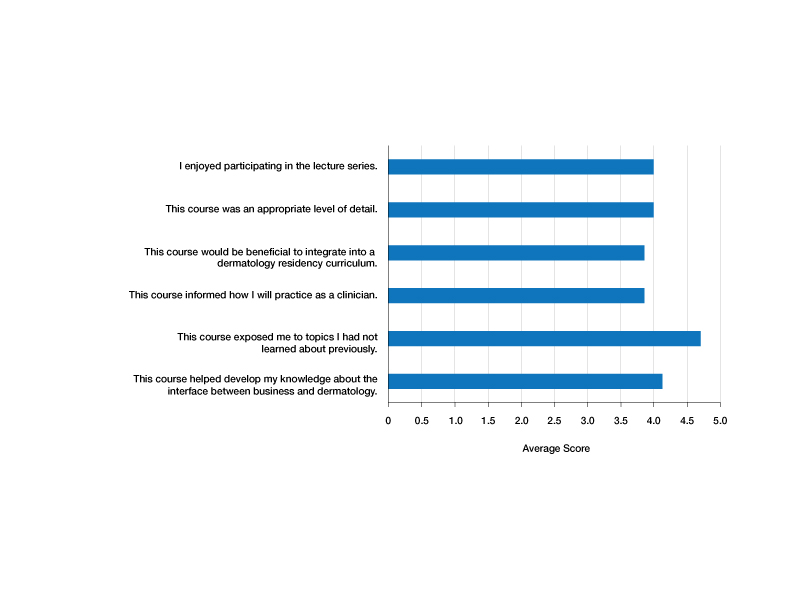
Although the course generally was well received, areas for improvement were identified from respondents’ comments, relating to audience engagement and refining the level of detail in the lectures. Recommendations included “less technical jargon and more focus on ‘big picture’ concepts, given audience’s low baseline knowledge”; “more case examples in each module”; and “more diagrams or interactive activities (polls, quizzes, break-out rooms) because the lectures were a bit dense.” This input was taken into consideration when revising the lectures for future use; they were reconstructed to have more case-based examples and prompts to encourage participation.
Resident commentary also demonstrated appreciation for education in this subject material. Statements such as “this is an important topic for future dermatologists” and “thank you so much for taking the time to implement this course” reflected the perceived value of this material during critical academic time. Another resident remarked: “This was great, thanks for putting it together.”
Given the positive experience of the residents and successful implementation of the series, this course was made available to all dermatology trainees on a network server with accompanying written documents. It is planned to be offered on a 3-year cycle in the future and will be updated to reflect inevitable changes in health care.
Although the relationship between business and medicine is increasingly important, teaching business principles has not become standardized or required in medical training. Despite the perception that this content is of value, implementation of programming has lagged behind that recognition, likely due to challenges in designing the curriculum and diffusing content into an already-saturated schedule. A model course that can be replicated in other residency programs would be valuable. We introduced a dermatology-specific lecture series to help prepare trainees for dermatology practice in a variety of clinical settings and train them with the language of business and operations that will equip them to respond to the needs of their patients, their practice, and the medical environment. Findings of this pilot study may not be generalizable to all dermatology residency programs because the sample size was small; the study was conducted at a single institution; and the content was delivered entirely online.
1. Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021. doi:10.1001/jamadermatol.2019.1634
2. The business of health care in the United States. Harvard Online [Internet]. June 27, 2022. Accessed July 24, 2023. https://www.harvardonline.harvard.edu/blog/business-health-care-united-states
3. Ranpariya V, Cull D, Feldman SR, et al. Evaluation and management 2021 coding guidelines: key changes and implications. The Dermatologist. December 2020. Accessed July 24, 2023. https://www.hmpgloballearningnetwork.com/site/thederm/article/evaluation-and-management-2021-coding-guidelines-key-changes-and-implications?key=Ranpariya&elastic%5B0%5D=brand%3A73468
4. Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
5. Resneck JS Jr. Dermatology practice consolidation fueled by private equity investment: potential consequences for the specialty and patients. JAMA Dermatol. 2018;154:13-14. doi:10.1001/jamadermatol.2017.5558
6. Patrinely JR Jr, Dewan AK. Business education in dermatology residency: a survey of program directors. Cutis. 2021;108:E7-E19. doi:10.12788/cutis.0331
7. Kohlwes RJ, Chou CL. A curriculum in medical economics for residents. Acad Med. 2002;77:465-466. doi:10.1097/00001888-200205000-00040
8. Fiebach NH, Rao D, Hamm ME. A curriculum in health systems and public health for internal medicine residents. Am J Prev Med. 2011;41(4 suppl 3):S264-S269. doi:10.1016/j.amepre.2011.05.025
To the Editor:
With health care constituting one of the larger segments of the US economy, medical practice is increasingly subject to business considerations.1 Patients, providers, and organizations are all required to make decisions that reflect choices beyond clinical needs alone. Given the impact of market forces, clinicians often are asked to navigate operational and business decisions. Accordingly, education about the policy and systems that shape care delivery can improve quality and help patients.2
The ability to understand the ecosystem of health care is of utmost importance for medical providers and can be achieved through resident education. Teaching fundamental business concepts enables residents to deliver care that is responsive to the constraints and opportunities encountered by patients and organizations, which ultimately will better prepare them to serve as advocates in alignment with their principal duties as physicians.
Despite the recognizable relationship between business and medicine, training has not yet been standardized to include topics in business education, and clinicians in dermatology are remarkably positioned to benefit because of the variety of practice settings and services they can provide. In dermatology, the diversity of services provided gives rise to complex coding and use of modifiers. Proper utilization of coding and billing is critical to create accurate documentation and receive appropriate reimbursement.3 Furthermore, clinicians in dermatology have to contend with the influence of insurance at many points of care, such as with coverage of pharmaceuticals. Formularies often have wide variability in coverage and are changing as new drugs come to market in the dermatologic space.4
The landscape of practice structure also has undergone change with increasing consolidation and mergers. The acquisition of practices by private equity firms has induced changes in practice infrastructure. The impact of changing organizational and managerial influences continues to be a topic of debate, with disparate opinions on how these developments shape standards of physician satisfaction and patient care.5
The convergence of these factors points to an important question that is gaining popularity: How will young dermatologists work within the context of all these parameters to best advocate and care for their patients? These questions are garnering more attention and were recently investigated through a survey of participants in a pilot program to evaluate the importance of business education in dermatology residency.
A survey of residency program directors was created by Patrinley and Dewan,6 which found that business education during residency was important and additional training should be implemented. Despite the perceived importance of business education, only half of the programs represented by survey respondents offered any structured educational opportunities, revealing a discrepancy between believed importance and practical implementation of business training, which suggests the need to develop a standardized, dermatology-specific curriculum that could be accessed by all residents in training.6
We performed a search of the medical literature to identify models of business education in residency programs. Only a few programs were identified, in which courses were predominantly instructed to trainees in primary care–based fields. According to course graduates, the programs were beneficial.7,8 Programs that had descriptive information about curriculum structure and content were chosen for further investigation and included internal medicine programs at the University of California San Francisco (UCSF) and Columbia University Vagelos College of Physicians and Surgeons (New York, New York). UCSF implemented a Program in Residency Investigation Methods and Epidemiology (PRIME program) to deliver seven 90-minute sessions dedicated to introducing residents to medical economics. Sessions were constructed with the intent of being interactive seminars that took on a variety of forms, including reading-based discussions, case-based analysis, and simulation-based learning.7 Columbia University developed a pilot program of week-long didactic sessions that were delivered to third-year internal medicine residents. These seminars featured discussions on health policy and economics, health insurance, technology and cost assessment, legal medicine, public health, community-oriented primary care, and local health department initiatives.8 We drew on both courses to build a lecture series focused on the business of dermatology that was delivered to dermatology residents at UMass Chan Medical School (Worcester, Massachusetts). Topic selection also was informed by qualitative input collected via email from recent graduates of the UMass dermatology residency program, focusing on the following areas: the US medical economy and health care costs; billing, coding, and claims processing; quality, relative value units (RVUs), reimbursement, and the merit-based incentive payment system; coverage of pharmaceuticals and teledermatology; and management. Residents were not required to prepare for any of the sessions; they were provided with handouts and slideshow presentations for reference to review at their convenience if desired. Five seminars were virtually conducted by an MD/MBA candidate at the institution (E.H.). They were recorded over the course of an academic year at 1- to 2-month intervals. Each 45-minute session was conducted in a lecture-discussion format and included case examples to help illustrate key principles and stimulate conversation. For example, the lecture on reimbursement incorporated a fee schedule calculation for a shave biopsy, using RVU and geographic pricing cost index (GCPI) multipliers. This demonstrated the variation in Centers for Medicare & Medicaid Services reimbursement in relation to (1) constituents of the RVU calculation (ie, work, practice expense, and malpractice) and (2) practice in a particular location (ie, the GCPI). Following this example, a conversation ensued among participants regarding the factors that drive valuation, with particular interest in variation based on urban vs suburban locations across the United States. Participants also found it of interest to examine the percentage of the valuation dedicated to each constituent and how features such as lesion size informed the final assessment of the charge. Another stylistic choice in developing the model was to include prompts for further consideration prior to transitioning topics in the lectures. For example: when examining the burden of skin disease, the audience was prompted to consider: “What is driving cost escalations, and how will services of the clinical domain meet these evolving needs?” At another point in the introductory lecture, residents were asked: “How do different types of insurance plans impact the management of patients with dermatologic concerns?” These questions were intended to transition residents to the next topic of discussion and highlight take-home points of consideration for medical practice. The project was reviewed by the UMass institutional review board and met criteria for exemption.
Residents who participated in at least 1 lecture (N=10) were surveyed after attendance; there were 7 responses (70% response rate). Residents were asked to rate a series of statements on a scale of 1 (strongly disagree) to 5 (strongly agree) and to provide commentary via an online form. Respondents indicated that the course was enjoyable (average score, 4.00), provided an appropriate level of detail (average score, 4.00), would be beneficial to integrate into a dermatology residency curriculum (average score, 3.86), and informed how they would practice as a clinician (average score, 3.86)(Figure). The respondents agreed that the course met the main goals of this initiative: it helped them develop knowledge about the interface between business and dermatology (4.14) and exposed residents to topics they had not learned about previously (4.71).

Although the course generally was well received, areas for improvement were identified from respondents’ comments, relating to audience engagement and refining the level of detail in the lectures. Recommendations included “less technical jargon and more focus on ‘big picture’ concepts, given audience’s low baseline knowledge”; “more case examples in each module”; and “more diagrams or interactive activities (polls, quizzes, break-out rooms) because the lectures were a bit dense.” This input was taken into consideration when revising the lectures for future use; they were reconstructed to have more case-based examples and prompts to encourage participation.
Resident commentary also demonstrated appreciation for education in this subject material. Statements such as “this is an important topic for future dermatologists” and “thank you so much for taking the time to implement this course” reflected the perceived value of this material during critical academic time. Another resident remarked: “This was great, thanks for putting it together.”
Given the positive experience of the residents and successful implementation of the series, this course was made available to all dermatology trainees on a network server with accompanying written documents. It is planned to be offered on a 3-year cycle in the future and will be updated to reflect inevitable changes in health care.
Although the relationship between business and medicine is increasingly important, teaching business principles has not become standardized or required in medical training. Despite the perception that this content is of value, implementation of programming has lagged behind that recognition, likely due to challenges in designing the curriculum and diffusing content into an already-saturated schedule. A model course that can be replicated in other residency programs would be valuable. We introduced a dermatology-specific lecture series to help prepare trainees for dermatology practice in a variety of clinical settings and train them with the language of business and operations that will equip them to respond to the needs of their patients, their practice, and the medical environment. Findings of this pilot study may not be generalizable to all dermatology residency programs because the sample size was small; the study was conducted at a single institution; and the content was delivered entirely online.
To the Editor:
With health care constituting one of the larger segments of the US economy, medical practice is increasingly subject to business considerations.1 Patients, providers, and organizations are all required to make decisions that reflect choices beyond clinical needs alone. Given the impact of market forces, clinicians often are asked to navigate operational and business decisions. Accordingly, education about the policy and systems that shape care delivery can improve quality and help patients.2
The ability to understand the ecosystem of health care is of utmost importance for medical providers and can be achieved through resident education. Teaching fundamental business concepts enables residents to deliver care that is responsive to the constraints and opportunities encountered by patients and organizations, which ultimately will better prepare them to serve as advocates in alignment with their principal duties as physicians.
Despite the recognizable relationship between business and medicine, training has not yet been standardized to include topics in business education, and clinicians in dermatology are remarkably positioned to benefit because of the variety of practice settings and services they can provide. In dermatology, the diversity of services provided gives rise to complex coding and use of modifiers. Proper utilization of coding and billing is critical to create accurate documentation and receive appropriate reimbursement.3 Furthermore, clinicians in dermatology have to contend with the influence of insurance at many points of care, such as with coverage of pharmaceuticals. Formularies often have wide variability in coverage and are changing as new drugs come to market in the dermatologic space.4
The landscape of practice structure also has undergone change with increasing consolidation and mergers. The acquisition of practices by private equity firms has induced changes in practice infrastructure. The impact of changing organizational and managerial influences continues to be a topic of debate, with disparate opinions on how these developments shape standards of physician satisfaction and patient care.5
The convergence of these factors points to an important question that is gaining popularity: How will young dermatologists work within the context of all these parameters to best advocate and care for their patients? These questions are garnering more attention and were recently investigated through a survey of participants in a pilot program to evaluate the importance of business education in dermatology residency.
A survey of residency program directors was created by Patrinley and Dewan,6 which found that business education during residency was important and additional training should be implemented. Despite the perceived importance of business education, only half of the programs represented by survey respondents offered any structured educational opportunities, revealing a discrepancy between believed importance and practical implementation of business training, which suggests the need to develop a standardized, dermatology-specific curriculum that could be accessed by all residents in training.6
We performed a search of the medical literature to identify models of business education in residency programs. Only a few programs were identified, in which courses were predominantly instructed to trainees in primary care–based fields. According to course graduates, the programs were beneficial.7,8 Programs that had descriptive information about curriculum structure and content were chosen for further investigation and included internal medicine programs at the University of California San Francisco (UCSF) and Columbia University Vagelos College of Physicians and Surgeons (New York, New York). UCSF implemented a Program in Residency Investigation Methods and Epidemiology (PRIME program) to deliver seven 90-minute sessions dedicated to introducing residents to medical economics. Sessions were constructed with the intent of being interactive seminars that took on a variety of forms, including reading-based discussions, case-based analysis, and simulation-based learning.7 Columbia University developed a pilot program of week-long didactic sessions that were delivered to third-year internal medicine residents. These seminars featured discussions on health policy and economics, health insurance, technology and cost assessment, legal medicine, public health, community-oriented primary care, and local health department initiatives.8 We drew on both courses to build a lecture series focused on the business of dermatology that was delivered to dermatology residents at UMass Chan Medical School (Worcester, Massachusetts). Topic selection also was informed by qualitative input collected via email from recent graduates of the UMass dermatology residency program, focusing on the following areas: the US medical economy and health care costs; billing, coding, and claims processing; quality, relative value units (RVUs), reimbursement, and the merit-based incentive payment system; coverage of pharmaceuticals and teledermatology; and management. Residents were not required to prepare for any of the sessions; they were provided with handouts and slideshow presentations for reference to review at their convenience if desired. Five seminars were virtually conducted by an MD/MBA candidate at the institution (E.H.). They were recorded over the course of an academic year at 1- to 2-month intervals. Each 45-minute session was conducted in a lecture-discussion format and included case examples to help illustrate key principles and stimulate conversation. For example, the lecture on reimbursement incorporated a fee schedule calculation for a shave biopsy, using RVU and geographic pricing cost index (GCPI) multipliers. This demonstrated the variation in Centers for Medicare & Medicaid Services reimbursement in relation to (1) constituents of the RVU calculation (ie, work, practice expense, and malpractice) and (2) practice in a particular location (ie, the GCPI). Following this example, a conversation ensued among participants regarding the factors that drive valuation, with particular interest in variation based on urban vs suburban locations across the United States. Participants also found it of interest to examine the percentage of the valuation dedicated to each constituent and how features such as lesion size informed the final assessment of the charge. Another stylistic choice in developing the model was to include prompts for further consideration prior to transitioning topics in the lectures. For example: when examining the burden of skin disease, the audience was prompted to consider: “What is driving cost escalations, and how will services of the clinical domain meet these evolving needs?” At another point in the introductory lecture, residents were asked: “How do different types of insurance plans impact the management of patients with dermatologic concerns?” These questions were intended to transition residents to the next topic of discussion and highlight take-home points of consideration for medical practice. The project was reviewed by the UMass institutional review board and met criteria for exemption.
Residents who participated in at least 1 lecture (N=10) were surveyed after attendance; there were 7 responses (70% response rate). Residents were asked to rate a series of statements on a scale of 1 (strongly disagree) to 5 (strongly agree) and to provide commentary via an online form. Respondents indicated that the course was enjoyable (average score, 4.00), provided an appropriate level of detail (average score, 4.00), would be beneficial to integrate into a dermatology residency curriculum (average score, 3.86), and informed how they would practice as a clinician (average score, 3.86)(Figure). The respondents agreed that the course met the main goals of this initiative: it helped them develop knowledge about the interface between business and dermatology (4.14) and exposed residents to topics they had not learned about previously (4.71).

Although the course generally was well received, areas for improvement were identified from respondents’ comments, relating to audience engagement and refining the level of detail in the lectures. Recommendations included “less technical jargon and more focus on ‘big picture’ concepts, given audience’s low baseline knowledge”; “more case examples in each module”; and “more diagrams or interactive activities (polls, quizzes, break-out rooms) because the lectures were a bit dense.” This input was taken into consideration when revising the lectures for future use; they were reconstructed to have more case-based examples and prompts to encourage participation.
Resident commentary also demonstrated appreciation for education in this subject material. Statements such as “this is an important topic for future dermatologists” and “thank you so much for taking the time to implement this course” reflected the perceived value of this material during critical academic time. Another resident remarked: “This was great, thanks for putting it together.”
Given the positive experience of the residents and successful implementation of the series, this course was made available to all dermatology trainees on a network server with accompanying written documents. It is planned to be offered on a 3-year cycle in the future and will be updated to reflect inevitable changes in health care.
Although the relationship between business and medicine is increasingly important, teaching business principles has not become standardized or required in medical training. Despite the perception that this content is of value, implementation of programming has lagged behind that recognition, likely due to challenges in designing the curriculum and diffusing content into an already-saturated schedule. A model course that can be replicated in other residency programs would be valuable. We introduced a dermatology-specific lecture series to help prepare trainees for dermatology practice in a variety of clinical settings and train them with the language of business and operations that will equip them to respond to the needs of their patients, their practice, and the medical environment. Findings of this pilot study may not be generalizable to all dermatology residency programs because the sample size was small; the study was conducted at a single institution; and the content was delivered entirely online.
1. Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021. doi:10.1001/jamadermatol.2019.1634
2. The business of health care in the United States. Harvard Online [Internet]. June 27, 2022. Accessed July 24, 2023. https://www.harvardonline.harvard.edu/blog/business-health-care-united-states
3. Ranpariya V, Cull D, Feldman SR, et al. Evaluation and management 2021 coding guidelines: key changes and implications. The Dermatologist. December 2020. Accessed July 24, 2023. https://www.hmpgloballearningnetwork.com/site/thederm/article/evaluation-and-management-2021-coding-guidelines-key-changes-and-implications?key=Ranpariya&elastic%5B0%5D=brand%3A73468
4. Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
5. Resneck JS Jr. Dermatology practice consolidation fueled by private equity investment: potential consequences for the specialty and patients. JAMA Dermatol. 2018;154:13-14. doi:10.1001/jamadermatol.2017.5558
6. Patrinely JR Jr, Dewan AK. Business education in dermatology residency: a survey of program directors. Cutis. 2021;108:E7-E19. doi:10.12788/cutis.0331
7. Kohlwes RJ, Chou CL. A curriculum in medical economics for residents. Acad Med. 2002;77:465-466. doi:10.1097/00001888-200205000-00040
8. Fiebach NH, Rao D, Hamm ME. A curriculum in health systems and public health for internal medicine residents. Am J Prev Med. 2011;41(4 suppl 3):S264-S269. doi:10.1016/j.amepre.2011.05.025
1. Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021. doi:10.1001/jamadermatol.2019.1634
2. The business of health care in the United States. Harvard Online [Internet]. June 27, 2022. Accessed July 24, 2023. https://www.harvardonline.harvard.edu/blog/business-health-care-united-states
3. Ranpariya V, Cull D, Feldman SR, et al. Evaluation and management 2021 coding guidelines: key changes and implications. The Dermatologist. December 2020. Accessed July 24, 2023. https://www.hmpgloballearningnetwork.com/site/thederm/article/evaluation-and-management-2021-coding-guidelines-key-changes-and-implications?key=Ranpariya&elastic%5B0%5D=brand%3A73468
4. Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
5. Resneck JS Jr. Dermatology practice consolidation fueled by private equity investment: potential consequences for the specialty and patients. JAMA Dermatol. 2018;154:13-14. doi:10.1001/jamadermatol.2017.5558
6. Patrinely JR Jr, Dewan AK. Business education in dermatology residency: a survey of program directors. Cutis. 2021;108:E7-E19. doi:10.12788/cutis.0331
7. Kohlwes RJ, Chou CL. A curriculum in medical economics for residents. Acad Med. 2002;77:465-466. doi:10.1097/00001888-200205000-00040
8. Fiebach NH, Rao D, Hamm ME. A curriculum in health systems and public health for internal medicine residents. Am J Prev Med. 2011;41(4 suppl 3):S264-S269. doi:10.1016/j.amepre.2011.05.025
Practice Points
- Business education in dermatology residency promotes understanding of the health care ecosystem and can enable residents to more effectively deliver care that is responsive to the needs of their patients.
- Teaching fundamental business principles to residents can inform decision-making on patient, provider, and systems levels.
- A pilot curriculum supports implementation of business education teaching and will be particularly helpful in dermatology.
Tooth loss, gum disease tied to hippocampal atrophy
Gum disease and tooth loss are linked to hippocampal atrophy and may have a more negative impact on the brain than aging, new research suggests.
Investigators found that in a late middle-aged and older cohort, among patients with mild periodontitis, having fewer teeth was linked to a faster rate of left hippocampal atrophy. For those with severe gum disease, each additional lost tooth was associated with a faster rate of brain shrinkage, equivalent to 1.3 years of brain aging.
“Tooth loss and gum disease, which is inflammation of the tissue around the teeth that can cause shrinkage of the gums and loosening of the teeth, are very common, so evaluating a potential link with dementia is incredibly important,” study investigator Satoshi Yamaguchi, PhD, DDS, of Tohoku University, in Sendai, Japan, said in a release.
“Our study found that these conditions may play a role in the health of the brain area that controls thinking and memory, giving people another reason to take better care of their teeth,” Dr. Yamaguchi noted.
The findings were published online in Neurology.
Greater effect than aging
Although previous research suggests that tooth loss and periodontitis are risk factors for Alzheimer’s disease, longitudinal research has not shown a significant correlation between these conditions and hippocampal atrophy.
To clarify this association, the investigators followed 172 men and women (average age, 67 years) who had undergone two MRI brain scans 4 years apart and had had a dental examination. None of the participants had any signs of cognitive decline at baseline.
At study outset, information on cerebrovascular and cardiovascular disease, alcohol consumption, smoking, depression history, and cognitive function was gathered. The Mini Mental State Exam and dental exams were administered at baseline and at 4-year follow-up.
For each participant, the number of teeth was counted, and all participants were assessed for gum disease via periodontal probing depth (PD).
Healthy gums typically measure between 1 and 3 mm in depth. Mild gum disease is signified by measurements of 3-4 mm in several areas. Severe gum disease involves measurements of 5-6 mm and is accompanied by greater bone loss, leading to potential tooth loss.
Multiple regression analysis was performed, with the annual symmetric percentage change (SPC) of hippocampal volume as the dependent variable. The analysis included an interaction term between the number of teeth present (NTP) and mean PD.
Over the 4-year study period, the investigators found that the qualitative interaction between NTP and mean PD was significant for the annual SPC in the left hippocampus.
Among those with mild periodontitis, having fewer teeth correlated with more rapid atrophy of the left hippocampus, such that every tooth lost was equivalent to nearly 1 year of brain aging.
In contrast, having more teeth was associated with a faster rate of left hippocampal atrophy among those with severe periodontitis and was equivalent to 1.3 years of brain aging.
For those with severe gum disease, each additional lost tooth corresponded to a faster rate of brain shrinkage, equivalent to 1.3 years of brain aging.
“This finding indicates that periodontitis may have a greater association with left hippocampal atrophy than the association exhibited by age. Furthermore, in cases of mild periodontitis, fewer teeth may be associated with a subsequent decline in cognitive function,” the investigators write.
The study’s results, they add, highlight the importance of preserving oral health, not just the retaining of teeth. “These findings suggest that retaining teeth with severe gum disease is associated with brain atrophy,” said Dr. Yamaguchi.
“Controlling the progression of gum disease through regular dental visits is crucial, and teeth with severe gum disease may need to be extracted and replaced with appropriate prosthetic devices,” he added.
The researchers note that further studies are needed to confirm these findings.
The study was supported the Japanese Ministry of Education, Culture, Sports, Science, and Technology; Kelo University; Japan Arteriosclerosis Prevention Fund; Japanese Ministry of Health, Labor, and Welfare; Teiko University; Pfizer Japan; Bayer Yakuhin; Chugai Pharmaceutical; Daiichi Sankyo; Astrellas Pharma; Takeda Pharmaceutical; the Health Care Science Institute; the Health Science Center; and the Takeda Science Foundation. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gum disease and tooth loss are linked to hippocampal atrophy and may have a more negative impact on the brain than aging, new research suggests.
Investigators found that in a late middle-aged and older cohort, among patients with mild periodontitis, having fewer teeth was linked to a faster rate of left hippocampal atrophy. For those with severe gum disease, each additional lost tooth was associated with a faster rate of brain shrinkage, equivalent to 1.3 years of brain aging.
“Tooth loss and gum disease, which is inflammation of the tissue around the teeth that can cause shrinkage of the gums and loosening of the teeth, are very common, so evaluating a potential link with dementia is incredibly important,” study investigator Satoshi Yamaguchi, PhD, DDS, of Tohoku University, in Sendai, Japan, said in a release.
“Our study found that these conditions may play a role in the health of the brain area that controls thinking and memory, giving people another reason to take better care of their teeth,” Dr. Yamaguchi noted.
The findings were published online in Neurology.
Greater effect than aging
Although previous research suggests that tooth loss and periodontitis are risk factors for Alzheimer’s disease, longitudinal research has not shown a significant correlation between these conditions and hippocampal atrophy.
To clarify this association, the investigators followed 172 men and women (average age, 67 years) who had undergone two MRI brain scans 4 years apart and had had a dental examination. None of the participants had any signs of cognitive decline at baseline.
At study outset, information on cerebrovascular and cardiovascular disease, alcohol consumption, smoking, depression history, and cognitive function was gathered. The Mini Mental State Exam and dental exams were administered at baseline and at 4-year follow-up.
For each participant, the number of teeth was counted, and all participants were assessed for gum disease via periodontal probing depth (PD).
Healthy gums typically measure between 1 and 3 mm in depth. Mild gum disease is signified by measurements of 3-4 mm in several areas. Severe gum disease involves measurements of 5-6 mm and is accompanied by greater bone loss, leading to potential tooth loss.
Multiple regression analysis was performed, with the annual symmetric percentage change (SPC) of hippocampal volume as the dependent variable. The analysis included an interaction term between the number of teeth present (NTP) and mean PD.
Over the 4-year study period, the investigators found that the qualitative interaction between NTP and mean PD was significant for the annual SPC in the left hippocampus.
Among those with mild periodontitis, having fewer teeth correlated with more rapid atrophy of the left hippocampus, such that every tooth lost was equivalent to nearly 1 year of brain aging.
In contrast, having more teeth was associated with a faster rate of left hippocampal atrophy among those with severe periodontitis and was equivalent to 1.3 years of brain aging.
For those with severe gum disease, each additional lost tooth corresponded to a faster rate of brain shrinkage, equivalent to 1.3 years of brain aging.
“This finding indicates that periodontitis may have a greater association with left hippocampal atrophy than the association exhibited by age. Furthermore, in cases of mild periodontitis, fewer teeth may be associated with a subsequent decline in cognitive function,” the investigators write.
The study’s results, they add, highlight the importance of preserving oral health, not just the retaining of teeth. “These findings suggest that retaining teeth with severe gum disease is associated with brain atrophy,” said Dr. Yamaguchi.
“Controlling the progression of gum disease through regular dental visits is crucial, and teeth with severe gum disease may need to be extracted and replaced with appropriate prosthetic devices,” he added.
The researchers note that further studies are needed to confirm these findings.
The study was supported the Japanese Ministry of Education, Culture, Sports, Science, and Technology; Kelo University; Japan Arteriosclerosis Prevention Fund; Japanese Ministry of Health, Labor, and Welfare; Teiko University; Pfizer Japan; Bayer Yakuhin; Chugai Pharmaceutical; Daiichi Sankyo; Astrellas Pharma; Takeda Pharmaceutical; the Health Care Science Institute; the Health Science Center; and the Takeda Science Foundation. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gum disease and tooth loss are linked to hippocampal atrophy and may have a more negative impact on the brain than aging, new research suggests.
Investigators found that in a late middle-aged and older cohort, among patients with mild periodontitis, having fewer teeth was linked to a faster rate of left hippocampal atrophy. For those with severe gum disease, each additional lost tooth was associated with a faster rate of brain shrinkage, equivalent to 1.3 years of brain aging.
“Tooth loss and gum disease, which is inflammation of the tissue around the teeth that can cause shrinkage of the gums and loosening of the teeth, are very common, so evaluating a potential link with dementia is incredibly important,” study investigator Satoshi Yamaguchi, PhD, DDS, of Tohoku University, in Sendai, Japan, said in a release.
“Our study found that these conditions may play a role in the health of the brain area that controls thinking and memory, giving people another reason to take better care of their teeth,” Dr. Yamaguchi noted.
The findings were published online in Neurology.
Greater effect than aging
Although previous research suggests that tooth loss and periodontitis are risk factors for Alzheimer’s disease, longitudinal research has not shown a significant correlation between these conditions and hippocampal atrophy.
To clarify this association, the investigators followed 172 men and women (average age, 67 years) who had undergone two MRI brain scans 4 years apart and had had a dental examination. None of the participants had any signs of cognitive decline at baseline.
At study outset, information on cerebrovascular and cardiovascular disease, alcohol consumption, smoking, depression history, and cognitive function was gathered. The Mini Mental State Exam and dental exams were administered at baseline and at 4-year follow-up.
For each participant, the number of teeth was counted, and all participants were assessed for gum disease via periodontal probing depth (PD).
Healthy gums typically measure between 1 and 3 mm in depth. Mild gum disease is signified by measurements of 3-4 mm in several areas. Severe gum disease involves measurements of 5-6 mm and is accompanied by greater bone loss, leading to potential tooth loss.
Multiple regression analysis was performed, with the annual symmetric percentage change (SPC) of hippocampal volume as the dependent variable. The analysis included an interaction term between the number of teeth present (NTP) and mean PD.
Over the 4-year study period, the investigators found that the qualitative interaction between NTP and mean PD was significant for the annual SPC in the left hippocampus.
Among those with mild periodontitis, having fewer teeth correlated with more rapid atrophy of the left hippocampus, such that every tooth lost was equivalent to nearly 1 year of brain aging.
In contrast, having more teeth was associated with a faster rate of left hippocampal atrophy among those with severe periodontitis and was equivalent to 1.3 years of brain aging.
For those with severe gum disease, each additional lost tooth corresponded to a faster rate of brain shrinkage, equivalent to 1.3 years of brain aging.
“This finding indicates that periodontitis may have a greater association with left hippocampal atrophy than the association exhibited by age. Furthermore, in cases of mild periodontitis, fewer teeth may be associated with a subsequent decline in cognitive function,” the investigators write.
The study’s results, they add, highlight the importance of preserving oral health, not just the retaining of teeth. “These findings suggest that retaining teeth with severe gum disease is associated with brain atrophy,” said Dr. Yamaguchi.
“Controlling the progression of gum disease through regular dental visits is crucial, and teeth with severe gum disease may need to be extracted and replaced with appropriate prosthetic devices,” he added.
The researchers note that further studies are needed to confirm these findings.
The study was supported the Japanese Ministry of Education, Culture, Sports, Science, and Technology; Kelo University; Japan Arteriosclerosis Prevention Fund; Japanese Ministry of Health, Labor, and Welfare; Teiko University; Pfizer Japan; Bayer Yakuhin; Chugai Pharmaceutical; Daiichi Sankyo; Astrellas Pharma; Takeda Pharmaceutical; the Health Care Science Institute; the Health Science Center; and the Takeda Science Foundation. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY

