User login
2nd-gen BTK inhibitor may be safer, team says

ORLANDO, FL—The second-generation BTK inhibitor acalabrutinib (ACP-196) can elicit durable partial responses in patients with chronic lymphocytic leukemia (CLL) while producing minimal side effects, according to researchers.
They said data suggest that, compared to the first-generation BTK inhibitor ibrutinib, acalabrutinib more selectively blocks the BTK pathway.
And it does so without disrupting other molecular pathways that are important for preserving platelet and immune function, thereby avoiding or minimizing certain side effects.
John C. Byrd, MD, of The Ohio State University Comprehensive Cancer Center in Columbus, and his colleagues reported data from an ongoing phase 1/2 trial of acalabrutinib in NEJM and at the 2015 ASH Annual Meeting (abstract 831). The study was sponsored by Acerta Pharma.
The researchers reported on 61 patients with relapsed CLL. They had a median age of 62 (range, 44-84) and a median of 3 prior therapies (range, 1-13).
Most patients had an ECOG performance status of 1 (59%) or 0 (36%). Most had high-risk (67%) or intermediate-risk disease (31%) according to Rai classification. Forty-six percent of patients had lymph nodes ≥ 5 cm in diameter, and 5% had lymph nodes ≥ 10 cm.
Seventy-five percent of patients had unmutated immunoglobulin variable-region heavy-chain gene, 31% had 17p deletion, 29% had 17q deletion, and 81% had β2-microglobulin > 3.5 mg/liter.
Patients enrolled in the phase 1 portion of the study received escalating doses of acalabrutinib, with a maximum dose of 400 mg once daily. Patients involved in the phase 2 portion of the study were treated with a 100 mg dose twice daily.
Adverse events and discontinuation
At a median follow-up of 14.3 months (range, 0.5 to 20), 53 patients are still receiving treatment.
The primary reasons for treatment discontinuation were investigator or patient decision (n=2), active autoimmune hemolytic anemia that required additional therapy (n=1), fatal pneumonia (n=1), CLL progression, and adverse events of diarrhea (n=1), gastritis (n=1), and dyspnea (n=1).
The most common adverse events of all grades (occurring in at least 20% of patients) were headache (43%), diarrhea (39%), increased weight (26%), pyrexia (23%), upper respiratory tract infection (23%), fatigue (21%), peripheral edema (21%), hypertension (20%), and nausea (20%).
Grade 3/4 adverse events included diarrhea (2%), increased weight (2%), pyrexia (3%), fatigue (3%), hypertension (7%), and arthralgia (2%).
Response
The overall response rate among the 60 evaluable patients was 95%. This included partial responses in 85% of patients and partial responses with lymphocytosis in 10%. The rate of stable disease was 5%.
The researchers noted that responses occurred in all dosing cohorts, and the response rate increased over time.
All 18 patients with 17p deletion experienced a partial response (89%) or partial response with lymphocytosis (11%). But 1 of these patients later progressed.
All 4 patients who previously received idelalisib responded to acalabrutinib, with partial responses in 75% and partial responses with lymphocytosis in 25%.
There were no cases of Richter’s transformation.
In all, 1 patient experienced progression at 16 months, and 1 patient died of pneumonia at 13 months.
“This data is very exciting because it illustrates that acalabrutinib is a highly potent and selective oral BTK inhibitor that can be given safely in patients with relapsed CLL,” Dr Byrd said. “What is particularly remarkable is how well patients are tolerating this therapy.”
Clinical trials of acalabrutinib in CLL are ongoing, including a phase 3 head-to-head comparison of ibrutinib and acalabrutinib. ![]()

ORLANDO, FL—The second-generation BTK inhibitor acalabrutinib (ACP-196) can elicit durable partial responses in patients with chronic lymphocytic leukemia (CLL) while producing minimal side effects, according to researchers.
They said data suggest that, compared to the first-generation BTK inhibitor ibrutinib, acalabrutinib more selectively blocks the BTK pathway.
And it does so without disrupting other molecular pathways that are important for preserving platelet and immune function, thereby avoiding or minimizing certain side effects.
John C. Byrd, MD, of The Ohio State University Comprehensive Cancer Center in Columbus, and his colleagues reported data from an ongoing phase 1/2 trial of acalabrutinib in NEJM and at the 2015 ASH Annual Meeting (abstract 831). The study was sponsored by Acerta Pharma.
The researchers reported on 61 patients with relapsed CLL. They had a median age of 62 (range, 44-84) and a median of 3 prior therapies (range, 1-13).
Most patients had an ECOG performance status of 1 (59%) or 0 (36%). Most had high-risk (67%) or intermediate-risk disease (31%) according to Rai classification. Forty-six percent of patients had lymph nodes ≥ 5 cm in diameter, and 5% had lymph nodes ≥ 10 cm.
Seventy-five percent of patients had unmutated immunoglobulin variable-region heavy-chain gene, 31% had 17p deletion, 29% had 17q deletion, and 81% had β2-microglobulin > 3.5 mg/liter.
Patients enrolled in the phase 1 portion of the study received escalating doses of acalabrutinib, with a maximum dose of 400 mg once daily. Patients involved in the phase 2 portion of the study were treated with a 100 mg dose twice daily.
Adverse events and discontinuation
At a median follow-up of 14.3 months (range, 0.5 to 20), 53 patients are still receiving treatment.
The primary reasons for treatment discontinuation were investigator or patient decision (n=2), active autoimmune hemolytic anemia that required additional therapy (n=1), fatal pneumonia (n=1), CLL progression, and adverse events of diarrhea (n=1), gastritis (n=1), and dyspnea (n=1).
The most common adverse events of all grades (occurring in at least 20% of patients) were headache (43%), diarrhea (39%), increased weight (26%), pyrexia (23%), upper respiratory tract infection (23%), fatigue (21%), peripheral edema (21%), hypertension (20%), and nausea (20%).
Grade 3/4 adverse events included diarrhea (2%), increased weight (2%), pyrexia (3%), fatigue (3%), hypertension (7%), and arthralgia (2%).
Response
The overall response rate among the 60 evaluable patients was 95%. This included partial responses in 85% of patients and partial responses with lymphocytosis in 10%. The rate of stable disease was 5%.
The researchers noted that responses occurred in all dosing cohorts, and the response rate increased over time.
All 18 patients with 17p deletion experienced a partial response (89%) or partial response with lymphocytosis (11%). But 1 of these patients later progressed.
All 4 patients who previously received idelalisib responded to acalabrutinib, with partial responses in 75% and partial responses with lymphocytosis in 25%.
There were no cases of Richter’s transformation.
In all, 1 patient experienced progression at 16 months, and 1 patient died of pneumonia at 13 months.
“This data is very exciting because it illustrates that acalabrutinib is a highly potent and selective oral BTK inhibitor that can be given safely in patients with relapsed CLL,” Dr Byrd said. “What is particularly remarkable is how well patients are tolerating this therapy.”
Clinical trials of acalabrutinib in CLL are ongoing, including a phase 3 head-to-head comparison of ibrutinib and acalabrutinib. ![]()

ORLANDO, FL—The second-generation BTK inhibitor acalabrutinib (ACP-196) can elicit durable partial responses in patients with chronic lymphocytic leukemia (CLL) while producing minimal side effects, according to researchers.
They said data suggest that, compared to the first-generation BTK inhibitor ibrutinib, acalabrutinib more selectively blocks the BTK pathway.
And it does so without disrupting other molecular pathways that are important for preserving platelet and immune function, thereby avoiding or minimizing certain side effects.
John C. Byrd, MD, of The Ohio State University Comprehensive Cancer Center in Columbus, and his colleagues reported data from an ongoing phase 1/2 trial of acalabrutinib in NEJM and at the 2015 ASH Annual Meeting (abstract 831). The study was sponsored by Acerta Pharma.
The researchers reported on 61 patients with relapsed CLL. They had a median age of 62 (range, 44-84) and a median of 3 prior therapies (range, 1-13).
Most patients had an ECOG performance status of 1 (59%) or 0 (36%). Most had high-risk (67%) or intermediate-risk disease (31%) according to Rai classification. Forty-six percent of patients had lymph nodes ≥ 5 cm in diameter, and 5% had lymph nodes ≥ 10 cm.
Seventy-five percent of patients had unmutated immunoglobulin variable-region heavy-chain gene, 31% had 17p deletion, 29% had 17q deletion, and 81% had β2-microglobulin > 3.5 mg/liter.
Patients enrolled in the phase 1 portion of the study received escalating doses of acalabrutinib, with a maximum dose of 400 mg once daily. Patients involved in the phase 2 portion of the study were treated with a 100 mg dose twice daily.
Adverse events and discontinuation
At a median follow-up of 14.3 months (range, 0.5 to 20), 53 patients are still receiving treatment.
The primary reasons for treatment discontinuation were investigator or patient decision (n=2), active autoimmune hemolytic anemia that required additional therapy (n=1), fatal pneumonia (n=1), CLL progression, and adverse events of diarrhea (n=1), gastritis (n=1), and dyspnea (n=1).
The most common adverse events of all grades (occurring in at least 20% of patients) were headache (43%), diarrhea (39%), increased weight (26%), pyrexia (23%), upper respiratory tract infection (23%), fatigue (21%), peripheral edema (21%), hypertension (20%), and nausea (20%).
Grade 3/4 adverse events included diarrhea (2%), increased weight (2%), pyrexia (3%), fatigue (3%), hypertension (7%), and arthralgia (2%).
Response
The overall response rate among the 60 evaluable patients was 95%. This included partial responses in 85% of patients and partial responses with lymphocytosis in 10%. The rate of stable disease was 5%.
The researchers noted that responses occurred in all dosing cohorts, and the response rate increased over time.
All 18 patients with 17p deletion experienced a partial response (89%) or partial response with lymphocytosis (11%). But 1 of these patients later progressed.
All 4 patients who previously received idelalisib responded to acalabrutinib, with partial responses in 75% and partial responses with lymphocytosis in 25%.
There were no cases of Richter’s transformation.
In all, 1 patient experienced progression at 16 months, and 1 patient died of pneumonia at 13 months.
“This data is very exciting because it illustrates that acalabrutinib is a highly potent and selective oral BTK inhibitor that can be given safely in patients with relapsed CLL,” Dr Byrd said. “What is particularly remarkable is how well patients are tolerating this therapy.”
Clinical trials of acalabrutinib in CLL are ongoing, including a phase 3 head-to-head comparison of ibrutinib and acalabrutinib. ![]()
Source of FVIII replacement matters, study shows

Photo courtesy of ASH
ORLANDO, FL—The source of factor VIII (FVIII) replacement therapy affects the risk of inhibitor development in previously untreated patients with severe hemophilia A, according to a prospective, randomized trial.
Results of the SIPPET study indicate that receiving recombinant FVIII is associated with a nearly 2-fold higher risk of developing inhibitory alloantibodies than receiving plasma-derived FVIII.
These results have implications for the choice of therapy for previously untreated patients, as inhibitor development remains a major challenge in the management of hemophilia A, said Flora Peyvandi, MD, PhD, of Angelo Bianchi Bonomi Hemophilia and Thrombosis Center in Milan, Italy.
Dr Peyvandi presented results of the SIPPET study during the plenary session of the 2015 ASH Annual Meeting (abstract 5*).
She noted that 13 previous observational studies indicated an increased risk of inhibitor formation with recombinant FVIII. But 2 consecutive, multicenter, observational trials (CANAL and RODIN) suggested there was no difference in immunogenicity between recombinant and plasma-derived FVIII.
A few meta-analyses showed a higher risk of inhibitors with recombinant FVIII, but the difference between recombinant and plasma-derived FVIII was attenuated after researchers adjusted for confounding factors.
In an attempt to obtain some conclusive results, Dr Peyvandi and her colleagues conducted the SIPPET study. It is the first randomized clinical trial in hemophilia with the goal of comparing the immunogenicity of FVIII product classes—plasma-derived FVIII products with von Willebrand factor and recombinant FVIII products.
Study design
Between 2010 and 2014, the researchers enrolled patients from 42 sites in 14 countries from Africa, the Americas, Asia, and Europe.
The all-male patients were younger than 6 years of age at enrollment. They had severe hemophilia A, negative inhibitor measurement at enrollment, and no or minimal exposure (less than 5 exposure days) to blood products.
The patients were randomized to either a single plasma-derived FVIII product containing von Willebrand factor or a single recombinant FVIII product. The treatment was at the discretion of the local physician.
Patients were treated for 50 exposure days, 3 years, or until inhibitor development.
The primary outcome was any FVIII inhibitor at titers ≥ 0.4 BU/mL. High-titer inhibitors (≥ 5 BU/mL) were a secondary outcome. Transient inhibitors were defined as those that spontaneously disappeared within 6 months.
Patients were assessed every 3 to 5 exposure days in the first 20 exposure days, then every 10 exposure days or every 3 months and every 2 weeks during prophylaxis.
“Every time a clinician had some doubt about the development of inhibitors, this [was] measured and also confirmed at the central lab in Milan,” Dr Peyvandi noted.
Results
In all, 251 patients were analyzed—126 randomized to recombinant FVIII and 125 to plasma-derived FVIII.
Dr Peyvandi pointed out that confounders—such as family history, previous exposure, and surgery—were equally distributed between the treatment arms thanks to the randomization. The same was true for the treatment type—on-demand, standard prophylaxis, etc.
Overall, 76 patients developed inhibitors, for a cumulative incidence of 35.4%. Fifty patients had high-titer inhibitors, for a cumulative incidence of 23.3%.
The cumulative incidence of all inhibitors was 44.5% (n=47) in the recombinant FVIII arm and 26.8% (n=29) in the plasma-derived FVIII arm. The cumulative incidence of high-titer inhibitors was 28.4% (n=30) and 18.6% (n=20), respectively.
More than 73% of all inhibitors were non-transient in both arms.
By univariate Cox regression analysis, recombinant FVIII was associated with an 87% higher incidence of inhibitors than plasma-derived FVIII (hazard ratio [HR]=1.87). And recombinant FVIII was associated with a 69% higher incidence of high-titer inhibitors (HR=1.69).
The researchers also adjusted their analysis for range of potential confounders to see how the randomization worked.
“None of this adjustment made any difference, as you would expect from a randomized study,” Dr Peyvandi said.
She went on to highlight a study published in NEJM in 2013, which suggested that second-generation, full-length FVIII products were associated with an increased risk of inhibitor development when compared to third-generation FVIII products.
Based on this finding, the World Federation of Hemophilia recommended against using second-generation products in previously untreated patients.
So Dr Peyvandi and her colleagues stopped the use of those products during the course of the SIPPET study. And they adjusted their analysis to ensure their observations were not due to any confounding effects of the products.
After excluding second-generation, full-length recombinant FVIII from their analysis, the researchers still observed an increased risk of inhibitor development with recombinant FVIII. The HRs were 1.98 for all inhibitors and 2.59 for high-titer inhibitors.
In closing, Dr Peyvandi said these findings are clinically important because inhibitors are the major therapeutic complication in hemophilia A and can cause a marked increase in morbidity, mortality, and treatment costs. ![]()
*Data in the abstract differ from data presented at the meeting.

Photo courtesy of ASH
ORLANDO, FL—The source of factor VIII (FVIII) replacement therapy affects the risk of inhibitor development in previously untreated patients with severe hemophilia A, according to a prospective, randomized trial.
Results of the SIPPET study indicate that receiving recombinant FVIII is associated with a nearly 2-fold higher risk of developing inhibitory alloantibodies than receiving plasma-derived FVIII.
These results have implications for the choice of therapy for previously untreated patients, as inhibitor development remains a major challenge in the management of hemophilia A, said Flora Peyvandi, MD, PhD, of Angelo Bianchi Bonomi Hemophilia and Thrombosis Center in Milan, Italy.
Dr Peyvandi presented results of the SIPPET study during the plenary session of the 2015 ASH Annual Meeting (abstract 5*).
She noted that 13 previous observational studies indicated an increased risk of inhibitor formation with recombinant FVIII. But 2 consecutive, multicenter, observational trials (CANAL and RODIN) suggested there was no difference in immunogenicity between recombinant and plasma-derived FVIII.
A few meta-analyses showed a higher risk of inhibitors with recombinant FVIII, but the difference between recombinant and plasma-derived FVIII was attenuated after researchers adjusted for confounding factors.
In an attempt to obtain some conclusive results, Dr Peyvandi and her colleagues conducted the SIPPET study. It is the first randomized clinical trial in hemophilia with the goal of comparing the immunogenicity of FVIII product classes—plasma-derived FVIII products with von Willebrand factor and recombinant FVIII products.
Study design
Between 2010 and 2014, the researchers enrolled patients from 42 sites in 14 countries from Africa, the Americas, Asia, and Europe.
The all-male patients were younger than 6 years of age at enrollment. They had severe hemophilia A, negative inhibitor measurement at enrollment, and no or minimal exposure (less than 5 exposure days) to blood products.
The patients were randomized to either a single plasma-derived FVIII product containing von Willebrand factor or a single recombinant FVIII product. The treatment was at the discretion of the local physician.
Patients were treated for 50 exposure days, 3 years, or until inhibitor development.
The primary outcome was any FVIII inhibitor at titers ≥ 0.4 BU/mL. High-titer inhibitors (≥ 5 BU/mL) were a secondary outcome. Transient inhibitors were defined as those that spontaneously disappeared within 6 months.
Patients were assessed every 3 to 5 exposure days in the first 20 exposure days, then every 10 exposure days or every 3 months and every 2 weeks during prophylaxis.
“Every time a clinician had some doubt about the development of inhibitors, this [was] measured and also confirmed at the central lab in Milan,” Dr Peyvandi noted.
Results
In all, 251 patients were analyzed—126 randomized to recombinant FVIII and 125 to plasma-derived FVIII.
Dr Peyvandi pointed out that confounders—such as family history, previous exposure, and surgery—were equally distributed between the treatment arms thanks to the randomization. The same was true for the treatment type—on-demand, standard prophylaxis, etc.
Overall, 76 patients developed inhibitors, for a cumulative incidence of 35.4%. Fifty patients had high-titer inhibitors, for a cumulative incidence of 23.3%.
The cumulative incidence of all inhibitors was 44.5% (n=47) in the recombinant FVIII arm and 26.8% (n=29) in the plasma-derived FVIII arm. The cumulative incidence of high-titer inhibitors was 28.4% (n=30) and 18.6% (n=20), respectively.
More than 73% of all inhibitors were non-transient in both arms.
By univariate Cox regression analysis, recombinant FVIII was associated with an 87% higher incidence of inhibitors than plasma-derived FVIII (hazard ratio [HR]=1.87). And recombinant FVIII was associated with a 69% higher incidence of high-titer inhibitors (HR=1.69).
The researchers also adjusted their analysis for range of potential confounders to see how the randomization worked.
“None of this adjustment made any difference, as you would expect from a randomized study,” Dr Peyvandi said.
She went on to highlight a study published in NEJM in 2013, which suggested that second-generation, full-length FVIII products were associated with an increased risk of inhibitor development when compared to third-generation FVIII products.
Based on this finding, the World Federation of Hemophilia recommended against using second-generation products in previously untreated patients.
So Dr Peyvandi and her colleagues stopped the use of those products during the course of the SIPPET study. And they adjusted their analysis to ensure their observations were not due to any confounding effects of the products.
After excluding second-generation, full-length recombinant FVIII from their analysis, the researchers still observed an increased risk of inhibitor development with recombinant FVIII. The HRs were 1.98 for all inhibitors and 2.59 for high-titer inhibitors.
In closing, Dr Peyvandi said these findings are clinically important because inhibitors are the major therapeutic complication in hemophilia A and can cause a marked increase in morbidity, mortality, and treatment costs. ![]()
*Data in the abstract differ from data presented at the meeting.

Photo courtesy of ASH
ORLANDO, FL—The source of factor VIII (FVIII) replacement therapy affects the risk of inhibitor development in previously untreated patients with severe hemophilia A, according to a prospective, randomized trial.
Results of the SIPPET study indicate that receiving recombinant FVIII is associated with a nearly 2-fold higher risk of developing inhibitory alloantibodies than receiving plasma-derived FVIII.
These results have implications for the choice of therapy for previously untreated patients, as inhibitor development remains a major challenge in the management of hemophilia A, said Flora Peyvandi, MD, PhD, of Angelo Bianchi Bonomi Hemophilia and Thrombosis Center in Milan, Italy.
Dr Peyvandi presented results of the SIPPET study during the plenary session of the 2015 ASH Annual Meeting (abstract 5*).
She noted that 13 previous observational studies indicated an increased risk of inhibitor formation with recombinant FVIII. But 2 consecutive, multicenter, observational trials (CANAL and RODIN) suggested there was no difference in immunogenicity between recombinant and plasma-derived FVIII.
A few meta-analyses showed a higher risk of inhibitors with recombinant FVIII, but the difference between recombinant and plasma-derived FVIII was attenuated after researchers adjusted for confounding factors.
In an attempt to obtain some conclusive results, Dr Peyvandi and her colleagues conducted the SIPPET study. It is the first randomized clinical trial in hemophilia with the goal of comparing the immunogenicity of FVIII product classes—plasma-derived FVIII products with von Willebrand factor and recombinant FVIII products.
Study design
Between 2010 and 2014, the researchers enrolled patients from 42 sites in 14 countries from Africa, the Americas, Asia, and Europe.
The all-male patients were younger than 6 years of age at enrollment. They had severe hemophilia A, negative inhibitor measurement at enrollment, and no or minimal exposure (less than 5 exposure days) to blood products.
The patients were randomized to either a single plasma-derived FVIII product containing von Willebrand factor or a single recombinant FVIII product. The treatment was at the discretion of the local physician.
Patients were treated for 50 exposure days, 3 years, or until inhibitor development.
The primary outcome was any FVIII inhibitor at titers ≥ 0.4 BU/mL. High-titer inhibitors (≥ 5 BU/mL) were a secondary outcome. Transient inhibitors were defined as those that spontaneously disappeared within 6 months.
Patients were assessed every 3 to 5 exposure days in the first 20 exposure days, then every 10 exposure days or every 3 months and every 2 weeks during prophylaxis.
“Every time a clinician had some doubt about the development of inhibitors, this [was] measured and also confirmed at the central lab in Milan,” Dr Peyvandi noted.
Results
In all, 251 patients were analyzed—126 randomized to recombinant FVIII and 125 to plasma-derived FVIII.
Dr Peyvandi pointed out that confounders—such as family history, previous exposure, and surgery—were equally distributed between the treatment arms thanks to the randomization. The same was true for the treatment type—on-demand, standard prophylaxis, etc.
Overall, 76 patients developed inhibitors, for a cumulative incidence of 35.4%. Fifty patients had high-titer inhibitors, for a cumulative incidence of 23.3%.
The cumulative incidence of all inhibitors was 44.5% (n=47) in the recombinant FVIII arm and 26.8% (n=29) in the plasma-derived FVIII arm. The cumulative incidence of high-titer inhibitors was 28.4% (n=30) and 18.6% (n=20), respectively.
More than 73% of all inhibitors were non-transient in both arms.
By univariate Cox regression analysis, recombinant FVIII was associated with an 87% higher incidence of inhibitors than plasma-derived FVIII (hazard ratio [HR]=1.87). And recombinant FVIII was associated with a 69% higher incidence of high-titer inhibitors (HR=1.69).
The researchers also adjusted their analysis for range of potential confounders to see how the randomization worked.
“None of this adjustment made any difference, as you would expect from a randomized study,” Dr Peyvandi said.
She went on to highlight a study published in NEJM in 2013, which suggested that second-generation, full-length FVIII products were associated with an increased risk of inhibitor development when compared to third-generation FVIII products.
Based on this finding, the World Federation of Hemophilia recommended against using second-generation products in previously untreated patients.
So Dr Peyvandi and her colleagues stopped the use of those products during the course of the SIPPET study. And they adjusted their analysis to ensure their observations were not due to any confounding effects of the products.
After excluding second-generation, full-length recombinant FVIII from their analysis, the researchers still observed an increased risk of inhibitor development with recombinant FVIII. The HRs were 1.98 for all inhibitors and 2.59 for high-titer inhibitors.
In closing, Dr Peyvandi said these findings are clinically important because inhibitors are the major therapeutic complication in hemophilia A and can cause a marked increase in morbidity, mortality, and treatment costs. ![]()
*Data in the abstract differ from data presented at the meeting.
PAGS 2015 social highlights
Thanks for joining Co-chairs Tommaso Falcone and Mickey Karram, who gathered top surgeon faculty at Paris in Las Vegas December 10-12, 2015, to discuss the latest advances in all facets of gynecologic surgery. See below for conference highlights. We hope to see next year in Las Vegas for PAGS 2016!
Thanks for joining Co-chairs Tommaso Falcone and Mickey Karram, who gathered top surgeon faculty at Paris in Las Vegas December 10-12, 2015, to discuss the latest advances in all facets of gynecologic surgery. See below for conference highlights. We hope to see next year in Las Vegas for PAGS 2016!
Thanks for joining Co-chairs Tommaso Falcone and Mickey Karram, who gathered top surgeon faculty at Paris in Las Vegas December 10-12, 2015, to discuss the latest advances in all facets of gynecologic surgery. See below for conference highlights. We hope to see next year in Las Vegas for PAGS 2016!
For information about PAGS, visit www.PAGS-cme.org. To sign up for email alerts from PAGS, click here.
FDA approves first recombinant von Willebrand treatment
The first recombinant von Willebrand factor has been approved for von Willebrand disease, the Food and Drug Administration announced.
Dr. Karen Midthun, director of the FDA’s Center for Biologics Evaluation and Research, said in the Dec. 8 statement that the approval “provides an additional therapeutic option for the treatment of bleeding episodes in patients with von Willebrand disease.”
The product was approved for on-demand therapy for bleeding episodes in adults with the disorder, which affects up to 1% of the U.S. population. Current treatment options include desmopressin and plasma-derived von Willebrand factor/factor VIII concentrates. The latter are limited by donor availability and are variable in the factor VIII levels they contain.
The new treatment, marketed as Vonvendi (Baxalta), is administered by infusion. The product contains only trace amounts of factor VIII, allowing for administration of factor VIII only as needed.
In a phase III manufacturer-sponsored nonrandomized trial of 22 subjects, bleeding was controlled with a single infusion in 82% of 192 bleeding episodes, according to a Baxalta press release. Patients with major bleeding required as many as four infusions, but treatment was effective in all subjects and 97% had an excellent efficacy rating and the remaining 3% had a good efficacy rating.
The most common adverse event was pruritus. No thrombotic events or severe allergic reactions were reported. Patients did not develop neutralizing antibodies against von Willebrand factor or factor VIII during the course of the study.
In its news release, the manufacturer said it is building a clinical development program to increase patient access to the treatment and evaluate its use in “prophylaxis, surgical, and pediatric indications.” Vonvendi is expected to be available in late 2016.
The first recombinant von Willebrand factor has been approved for von Willebrand disease, the Food and Drug Administration announced.
Dr. Karen Midthun, director of the FDA’s Center for Biologics Evaluation and Research, said in the Dec. 8 statement that the approval “provides an additional therapeutic option for the treatment of bleeding episodes in patients with von Willebrand disease.”
The product was approved for on-demand therapy for bleeding episodes in adults with the disorder, which affects up to 1% of the U.S. population. Current treatment options include desmopressin and plasma-derived von Willebrand factor/factor VIII concentrates. The latter are limited by donor availability and are variable in the factor VIII levels they contain.
The new treatment, marketed as Vonvendi (Baxalta), is administered by infusion. The product contains only trace amounts of factor VIII, allowing for administration of factor VIII only as needed.
In a phase III manufacturer-sponsored nonrandomized trial of 22 subjects, bleeding was controlled with a single infusion in 82% of 192 bleeding episodes, according to a Baxalta press release. Patients with major bleeding required as many as four infusions, but treatment was effective in all subjects and 97% had an excellent efficacy rating and the remaining 3% had a good efficacy rating.
The most common adverse event was pruritus. No thrombotic events or severe allergic reactions were reported. Patients did not develop neutralizing antibodies against von Willebrand factor or factor VIII during the course of the study.
In its news release, the manufacturer said it is building a clinical development program to increase patient access to the treatment and evaluate its use in “prophylaxis, surgical, and pediatric indications.” Vonvendi is expected to be available in late 2016.
The first recombinant von Willebrand factor has been approved for von Willebrand disease, the Food and Drug Administration announced.
Dr. Karen Midthun, director of the FDA’s Center for Biologics Evaluation and Research, said in the Dec. 8 statement that the approval “provides an additional therapeutic option for the treatment of bleeding episodes in patients with von Willebrand disease.”
The product was approved for on-demand therapy for bleeding episodes in adults with the disorder, which affects up to 1% of the U.S. population. Current treatment options include desmopressin and plasma-derived von Willebrand factor/factor VIII concentrates. The latter are limited by donor availability and are variable in the factor VIII levels they contain.
The new treatment, marketed as Vonvendi (Baxalta), is administered by infusion. The product contains only trace amounts of factor VIII, allowing for administration of factor VIII only as needed.
In a phase III manufacturer-sponsored nonrandomized trial of 22 subjects, bleeding was controlled with a single infusion in 82% of 192 bleeding episodes, according to a Baxalta press release. Patients with major bleeding required as many as four infusions, but treatment was effective in all subjects and 97% had an excellent efficacy rating and the remaining 3% had a good efficacy rating.
The most common adverse event was pruritus. No thrombotic events or severe allergic reactions were reported. Patients did not develop neutralizing antibodies against von Willebrand factor or factor VIII during the course of the study.
In its news release, the manufacturer said it is building a clinical development program to increase patient access to the treatment and evaluate its use in “prophylaxis, surgical, and pediatric indications.” Vonvendi is expected to be available in late 2016.
Study: TKA patients recover faster with periarticular analgesia injection
Patients more often recovered faster from a total knee arthroplasty (TKA) when they received a periarticular injection of analgesic medication than when they received a femoral nerve block for the same surgery on the opposite knee in a study.
The study included 16 recipients of bilateral primary TKA, who received a femoral nerve block at their first TKA operation and a periarticular injection of an extended-release bupivacaine liposome mixture at the second operation. An average of 2.3 years passed between the two procedures, and the same surgeon performed all surgeries, which occurred between March 2009 and August 2013. Two patients were excluded from the study because of subacute rehabilitation admission delay and a third patient was left out of the study because of respiratory failure, resulting in admission to the ICU.
Following the TKA with a periarticular injection of analgesic medication, the average number of inpatient physical therapy sessions a patient completed was 2.3 (standard deviation: 1.0); the average number of inpatient physical therapy sessions a patient completed after having the TKA with femoral nerve block was 3.5 (SD: 1.3). The average number of hospital days following the TKA with periarticular injection was also a smaller number. The mean number of hospital days following the periarticular injection was 1.5 (SD: 0.6 days). compared with 1.9 days (SD: 0.6 days; P is less than .032) following the femoral nerve block.
“Our data demonstrate that periarticular injection of analgesia allowed patients to complete their inpatient physical therapy sessions and to be discharged sooner, compared with femoral nerve block. This finding suggests that patients who receive periarticular injection of analgesia are able to ambulate independently faster because it does not affect postoperative motor function,” according to Dr. Brandon J. Horn and his colleagues.
Read the full study in the Journal of the American Osteopathic Association (doi: 10.7556/jaoa.2015.146).
Patients more often recovered faster from a total knee arthroplasty (TKA) when they received a periarticular injection of analgesic medication than when they received a femoral nerve block for the same surgery on the opposite knee in a study.
The study included 16 recipients of bilateral primary TKA, who received a femoral nerve block at their first TKA operation and a periarticular injection of an extended-release bupivacaine liposome mixture at the second operation. An average of 2.3 years passed between the two procedures, and the same surgeon performed all surgeries, which occurred between March 2009 and August 2013. Two patients were excluded from the study because of subacute rehabilitation admission delay and a third patient was left out of the study because of respiratory failure, resulting in admission to the ICU.
Following the TKA with a periarticular injection of analgesic medication, the average number of inpatient physical therapy sessions a patient completed was 2.3 (standard deviation: 1.0); the average number of inpatient physical therapy sessions a patient completed after having the TKA with femoral nerve block was 3.5 (SD: 1.3). The average number of hospital days following the TKA with periarticular injection was also a smaller number. The mean number of hospital days following the periarticular injection was 1.5 (SD: 0.6 days). compared with 1.9 days (SD: 0.6 days; P is less than .032) following the femoral nerve block.
“Our data demonstrate that periarticular injection of analgesia allowed patients to complete their inpatient physical therapy sessions and to be discharged sooner, compared with femoral nerve block. This finding suggests that patients who receive periarticular injection of analgesia are able to ambulate independently faster because it does not affect postoperative motor function,” according to Dr. Brandon J. Horn and his colleagues.
Read the full study in the Journal of the American Osteopathic Association (doi: 10.7556/jaoa.2015.146).
Patients more often recovered faster from a total knee arthroplasty (TKA) when they received a periarticular injection of analgesic medication than when they received a femoral nerve block for the same surgery on the opposite knee in a study.
The study included 16 recipients of bilateral primary TKA, who received a femoral nerve block at their first TKA operation and a periarticular injection of an extended-release bupivacaine liposome mixture at the second operation. An average of 2.3 years passed between the two procedures, and the same surgeon performed all surgeries, which occurred between March 2009 and August 2013. Two patients were excluded from the study because of subacute rehabilitation admission delay and a third patient was left out of the study because of respiratory failure, resulting in admission to the ICU.
Following the TKA with a periarticular injection of analgesic medication, the average number of inpatient physical therapy sessions a patient completed was 2.3 (standard deviation: 1.0); the average number of inpatient physical therapy sessions a patient completed after having the TKA with femoral nerve block was 3.5 (SD: 1.3). The average number of hospital days following the TKA with periarticular injection was also a smaller number. The mean number of hospital days following the periarticular injection was 1.5 (SD: 0.6 days). compared with 1.9 days (SD: 0.6 days; P is less than .032) following the femoral nerve block.
“Our data demonstrate that periarticular injection of analgesia allowed patients to complete their inpatient physical therapy sessions and to be discharged sooner, compared with femoral nerve block. This finding suggests that patients who receive periarticular injection of analgesia are able to ambulate independently faster because it does not affect postoperative motor function,” according to Dr. Brandon J. Horn and his colleagues.
Read the full study in the Journal of the American Osteopathic Association (doi: 10.7556/jaoa.2015.146).
FROM THE JOURNAL OF THE AMERICAN OSTEOPATHIC ASSOCIATION
Pediatric Dermatology Consult - December 2015
Ancillary testing
Complete blood count showed leukocytosis (13.1 th/mcL), with normal differential. The C-reactive protein was elevated (10 mg/dL). The comprehensive metabolic panel was within normal limits. Mycoplasma pneumoniae antibody, IgM was positive, and herpes virus cultures were negative. The chest x-ray showed a dense right upper lobe airspace opacity, concerning for pneumonia, likely with a component of atelectasis.
Discussion
Mycoplasma pneumoniae–induced rash and mucositis (MIRM) is a syndrome of mucocutaneous involvement in conjunction with an M. pneumoniae infection. M. pneumoniae is a bacteria that commonly causes respiratory tract infections. In children, it is a leading cause of atypical pneumonia.1,2 Extrapulmonary manifestations are common and can involve the skin, heart, kidneys, gastrointestinal system, and nervous system.3 There is a 1-3 week incubation period for infections, which always begin with respiratory symptoms. The most common early symptoms of this infection are cough, malaise, fever, and headaches. Studies show that between 25%-38% of M. pneumoniae infections have mucocutaneous manifestations.3,4
An overwhelming majority of cases have mucosal involvement while the degree of skin involvement varies. Cutaneous manifestations, which are usually polymorphic, present with vesiculobullous lesions, targetoid macules and papules, or morbilliform eruptions.3 MIRM is seen most often in children and adolescents, but a few adult cases have been reported. About 66% of cases are seen in males, but the reason for this gender predilection is not well understood.3 Overall, MIRM has an excellent prognosis, compared with the other disorders on the differential diagnosis.
Differential diagnosis
MIRM used to be considered along the spectrum of bullous diseases that have mucosal involvement including erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrosis (TEN).3-6 The distinction can be difficult to identify, given the similarities that all of these diseases share, and many published articles referencing cases of SJS, TEN, and EM actually fit the criteria for MIRM.6 What sets MIRM apart is the predominance of mucosal involvement with minimal skin involvement and the overwhelming overall good prognosis compared with drug induced SJS.3
Although rare cases have moderate cutaneous involvement, MIRM is most notable for oral lesions, which include ulcers, erosions, and vesiculobullae of the lips and buccal mucosa, as well as ocular and genital mucosal involvement in a lower degree.3 For cases with moderate or even mild involvement of the skin, ruling out other diagnoses becomes more difficult, especially since the dermatologic manifestations of targetoid papules and bullae are common to all of them. Testing for M. pneumoniae will strengthen the diagnosis and can be done in a number of ways. Using polymerase chain reaction to measure for M. pneumoniae DNA offers the benefit of being able to detect an infection early in its course because it does not rely on antibody formation.4 Other testing methods include M. pneumoniae antigen detection, and IgM or IgG titers.4
Treatment
The mainstay of treatment of this bacterial infection is, of course, antibiotics. Azithromycin and erythromycin are the drugs of choice for children. While tetracyclines and fluoroquinolones can be used to treat M. pneumoniae, their use is not recommended in young children.4 Systemic corticosteroids can be used in addition to antibiotics, but there is limited data to support its efficacy in these patients.7 In milder cases, reports have documented that the course can be self-limited and may improve with only supportive care. Because of the severity of mucosal involvement in some cases, such as the one in our patient, supportive care may include intravenous hydration and total parenteral nutrition if the patient is unable to tolerate oral intake. In more severe cases, IVIG can be beneficial.8 This has been shown to induce rapid improvement in refractory mucositis when antibiotics and supportive mucosal care alone have not been sufficient.8
References
- (J Clin Microbiol. 2015 Jan;53[1]:124-30.)
- (Clin Microbiol. 2004;17[4]:697-728.)
- (J Am Acad Dermatol. 2015;72[2]: 239-54.)
- (Int J Dermatol. 2009 Jul;48[7]:673-80.)
- (J Eur Acad Dermatol Venereol. 2015 Mar;29[3]:595-8.)
- (J Am Acad Dermatol. 2005 Feb;52[2]:312-5.)
- (Pediatr Dermatol. 2014 Nov-Dec;31[6]:664-9.)
- (Acta Paediatr. 2011 Nov;100[11]:e238-40.)
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California San Diego and Mr. Ginsberg is a research associate at the hospital. They said they had no relevant financial disclosures.
Email them at [email protected].
Ancillary testing
Complete blood count showed leukocytosis (13.1 th/mcL), with normal differential. The C-reactive protein was elevated (10 mg/dL). The comprehensive metabolic panel was within normal limits. Mycoplasma pneumoniae antibody, IgM was positive, and herpes virus cultures were negative. The chest x-ray showed a dense right upper lobe airspace opacity, concerning for pneumonia, likely with a component of atelectasis.
Discussion
Mycoplasma pneumoniae–induced rash and mucositis (MIRM) is a syndrome of mucocutaneous involvement in conjunction with an M. pneumoniae infection. M. pneumoniae is a bacteria that commonly causes respiratory tract infections. In children, it is a leading cause of atypical pneumonia.1,2 Extrapulmonary manifestations are common and can involve the skin, heart, kidneys, gastrointestinal system, and nervous system.3 There is a 1-3 week incubation period for infections, which always begin with respiratory symptoms. The most common early symptoms of this infection are cough, malaise, fever, and headaches. Studies show that between 25%-38% of M. pneumoniae infections have mucocutaneous manifestations.3,4
An overwhelming majority of cases have mucosal involvement while the degree of skin involvement varies. Cutaneous manifestations, which are usually polymorphic, present with vesiculobullous lesions, targetoid macules and papules, or morbilliform eruptions.3 MIRM is seen most often in children and adolescents, but a few adult cases have been reported. About 66% of cases are seen in males, but the reason for this gender predilection is not well understood.3 Overall, MIRM has an excellent prognosis, compared with the other disorders on the differential diagnosis.
Differential diagnosis
MIRM used to be considered along the spectrum of bullous diseases that have mucosal involvement including erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrosis (TEN).3-6 The distinction can be difficult to identify, given the similarities that all of these diseases share, and many published articles referencing cases of SJS, TEN, and EM actually fit the criteria for MIRM.6 What sets MIRM apart is the predominance of mucosal involvement with minimal skin involvement and the overwhelming overall good prognosis compared with drug induced SJS.3
Although rare cases have moderate cutaneous involvement, MIRM is most notable for oral lesions, which include ulcers, erosions, and vesiculobullae of the lips and buccal mucosa, as well as ocular and genital mucosal involvement in a lower degree.3 For cases with moderate or even mild involvement of the skin, ruling out other diagnoses becomes more difficult, especially since the dermatologic manifestations of targetoid papules and bullae are common to all of them. Testing for M. pneumoniae will strengthen the diagnosis and can be done in a number of ways. Using polymerase chain reaction to measure for M. pneumoniae DNA offers the benefit of being able to detect an infection early in its course because it does not rely on antibody formation.4 Other testing methods include M. pneumoniae antigen detection, and IgM or IgG titers.4
Treatment
The mainstay of treatment of this bacterial infection is, of course, antibiotics. Azithromycin and erythromycin are the drugs of choice for children. While tetracyclines and fluoroquinolones can be used to treat M. pneumoniae, their use is not recommended in young children.4 Systemic corticosteroids can be used in addition to antibiotics, but there is limited data to support its efficacy in these patients.7 In milder cases, reports have documented that the course can be self-limited and may improve with only supportive care. Because of the severity of mucosal involvement in some cases, such as the one in our patient, supportive care may include intravenous hydration and total parenteral nutrition if the patient is unable to tolerate oral intake. In more severe cases, IVIG can be beneficial.8 This has been shown to induce rapid improvement in refractory mucositis when antibiotics and supportive mucosal care alone have not been sufficient.8
References
- (J Clin Microbiol. 2015 Jan;53[1]:124-30.)
- (Clin Microbiol. 2004;17[4]:697-728.)
- (J Am Acad Dermatol. 2015;72[2]: 239-54.)
- (Int J Dermatol. 2009 Jul;48[7]:673-80.)
- (J Eur Acad Dermatol Venereol. 2015 Mar;29[3]:595-8.)
- (J Am Acad Dermatol. 2005 Feb;52[2]:312-5.)
- (Pediatr Dermatol. 2014 Nov-Dec;31[6]:664-9.)
- (Acta Paediatr. 2011 Nov;100[11]:e238-40.)
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California San Diego and Mr. Ginsberg is a research associate at the hospital. They said they had no relevant financial disclosures.
Email them at [email protected].
Ancillary testing
Complete blood count showed leukocytosis (13.1 th/mcL), with normal differential. The C-reactive protein was elevated (10 mg/dL). The comprehensive metabolic panel was within normal limits. Mycoplasma pneumoniae antibody, IgM was positive, and herpes virus cultures were negative. The chest x-ray showed a dense right upper lobe airspace opacity, concerning for pneumonia, likely with a component of atelectasis.
Discussion
Mycoplasma pneumoniae–induced rash and mucositis (MIRM) is a syndrome of mucocutaneous involvement in conjunction with an M. pneumoniae infection. M. pneumoniae is a bacteria that commonly causes respiratory tract infections. In children, it is a leading cause of atypical pneumonia.1,2 Extrapulmonary manifestations are common and can involve the skin, heart, kidneys, gastrointestinal system, and nervous system.3 There is a 1-3 week incubation period for infections, which always begin with respiratory symptoms. The most common early symptoms of this infection are cough, malaise, fever, and headaches. Studies show that between 25%-38% of M. pneumoniae infections have mucocutaneous manifestations.3,4
An overwhelming majority of cases have mucosal involvement while the degree of skin involvement varies. Cutaneous manifestations, which are usually polymorphic, present with vesiculobullous lesions, targetoid macules and papules, or morbilliform eruptions.3 MIRM is seen most often in children and adolescents, but a few adult cases have been reported. About 66% of cases are seen in males, but the reason for this gender predilection is not well understood.3 Overall, MIRM has an excellent prognosis, compared with the other disorders on the differential diagnosis.
Differential diagnosis
MIRM used to be considered along the spectrum of bullous diseases that have mucosal involvement including erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrosis (TEN).3-6 The distinction can be difficult to identify, given the similarities that all of these diseases share, and many published articles referencing cases of SJS, TEN, and EM actually fit the criteria for MIRM.6 What sets MIRM apart is the predominance of mucosal involvement with minimal skin involvement and the overwhelming overall good prognosis compared with drug induced SJS.3
Although rare cases have moderate cutaneous involvement, MIRM is most notable for oral lesions, which include ulcers, erosions, and vesiculobullae of the lips and buccal mucosa, as well as ocular and genital mucosal involvement in a lower degree.3 For cases with moderate or even mild involvement of the skin, ruling out other diagnoses becomes more difficult, especially since the dermatologic manifestations of targetoid papules and bullae are common to all of them. Testing for M. pneumoniae will strengthen the diagnosis and can be done in a number of ways. Using polymerase chain reaction to measure for M. pneumoniae DNA offers the benefit of being able to detect an infection early in its course because it does not rely on antibody formation.4 Other testing methods include M. pneumoniae antigen detection, and IgM or IgG titers.4
Treatment
The mainstay of treatment of this bacterial infection is, of course, antibiotics. Azithromycin and erythromycin are the drugs of choice for children. While tetracyclines and fluoroquinolones can be used to treat M. pneumoniae, their use is not recommended in young children.4 Systemic corticosteroids can be used in addition to antibiotics, but there is limited data to support its efficacy in these patients.7 In milder cases, reports have documented that the course can be self-limited and may improve with only supportive care. Because of the severity of mucosal involvement in some cases, such as the one in our patient, supportive care may include intravenous hydration and total parenteral nutrition if the patient is unable to tolerate oral intake. In more severe cases, IVIG can be beneficial.8 This has been shown to induce rapid improvement in refractory mucositis when antibiotics and supportive mucosal care alone have not been sufficient.8
References
- (J Clin Microbiol. 2015 Jan;53[1]:124-30.)
- (Clin Microbiol. 2004;17[4]:697-728.)
- (J Am Acad Dermatol. 2015;72[2]: 239-54.)
- (Int J Dermatol. 2009 Jul;48[7]:673-80.)
- (J Eur Acad Dermatol Venereol. 2015 Mar;29[3]:595-8.)
- (J Am Acad Dermatol. 2005 Feb;52[2]:312-5.)
- (Pediatr Dermatol. 2014 Nov-Dec;31[6]:664-9.)
- (Acta Paediatr. 2011 Nov;100[11]:e238-40.)
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California San Diego and Mr. Ginsberg is a research associate at the hospital. They said they had no relevant financial disclosures.
Email them at [email protected].
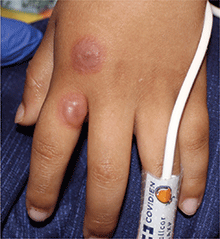
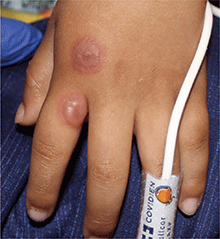
A 4-year-old male with a past history of asthma, presents with a 10-day history of cough, wheezing, rhinorrhea, and fever. He was treated with ibuprofen and albuterol without much improvement. Two days prior to presentation, he developed swollen and cracked lips as well as pink conjunctiva. Since then, he has been hypoactive with poor oral intake. He was evaluated by his pediatrician and was given dexamethasone and diphenhydramine, with no improvement. His oral and ocular symptoms worsen, which is why he was taken to the emergency department. He has no sick contacts. On the day of presentation, he developed several target erythematous lesions on his arms and legs that progressed to bulla (See photo).
Managing menopause symptoms in gynecologic cancer survivors
Due to advancements in surgical treatment, chemotherapy, and radiation therapy, gynecologic cancer survival rates are continuing to improve and quality of life is evolving into an even more significant focus in cancer care.
Roughly 30%-40% of all women with a gynecologic malignancy will experience climacteric symptoms and menopause prior to the anticipated time of natural menopause (J Clin Oncol. 2009 Mar 10;27[8]:1214-9). Cessation of ovarian estrogen and progesterone production can result in short-term as well as long-term sequelae, including vasomotor symptoms, vaginal dryness, osteoporosis, and mood disturbances. Iatrogenic menopause after cancer treatment can be more sudden and severe when compared with the natural course of physiologic menopause. As a result, determination of safe, effective modalities for treating these symptoms is of particular importance for survivor quality of life.
Both combination and estrogen-only hormone replacement therapy (HT) provide greater improvement in these specific symptoms and overall quality of life than placebo as demonstrated in several observational and randomized control trials (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD004143).
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy, with approximately 54,000 new cases anticipated in the United States in 2015. Twenty-five percent of these new cases will be in premenopausal women, and with an ever-increasing obesity rate, this number may continue to climb.
Women with early-stage Type 1 endometrial cancer who have vasomotor symptoms after surgery may be offered a short course of estrogen-based HT at the lowest effective dose following hysterectomy/bilateral salpingo-oophorectomy and staging procedure (J Clin Oncol. 2006 Feb 1;24[4]:587-92). For women with genitourinary symptoms, vaginal moisturizers and/or low-dose vaginal estrogen are reasonable options. Unfortunately, there are no data to guide the use of estrogen replacement therapy in women with Type 2 endometrial cancers (Gynecol Oncol. 2011 Aug;122[2]:447-54).
Ovarian cancer
There is minimal data implicating a hormonal causation to ovarian carcinogenesis. Most women with epithelial ovarian cancer do not express tumor estrogen or progesterone receptors. Treatment will result in abrupt, iatrogenic menopause, raising the question of whether it is safe to use HT in patients with epithelial ovarian cancer.
Multiple studies have failed to demonstrate a difference in 5-year survival rates in women with epithelial cancer using HT for 2 years or less (JAMA. 2009 Jul 15;302[3]:298-305, Eur J Gynaecol Oncol. 2000;21[2]:192-6, Cancer. 1999 Sep 15;86[6]:1013-8). As such, symptomatic patients could be offered a course of HT; however, caution should be exercised in women with estrogen/progesterone–expressing tumors or nonepithelial tumors. As with endometrial cancer patients, the lowest effective doses should be prescribed.
Cervical cancer
Most cervical squamous and adenocarcinomas are not hormone dependent. For women with early-stage squamous cell carcinoma, ovarian conservation may be possible or oophoropexy may be offered. However, for many patients, bilateral salpingo-oophorectomy at the time of hysterectomy is more common, and the local effect of radiation therapy can result in vaginal atrophy with subsequent dyspareunia or ovarian failure from radiation scatter. Even for patients who undergo oophoropexy, radiation scatter may still result in ovarian failure. In a few observational studies, there are no data to infer that cervical cancer is hormonally related or that survival rates are decreased.
Currently, HT use in cervical cancer survivors is considered safe. Of note, for women with more advanced-stage cervical cancer and who received chemoradiation for primary treatment, combination therapy with estrogen and progesterone may be more appropriate if the uterus remains in situ. However, for women who have undergone hysterectomy, combination therapy with progesterone may not be warranted and estrogen alone (orally or vaginally) is acceptable (Gynecol Oncol. 2011 Aug;122[2]:447-54)
Nonhormonal therapies
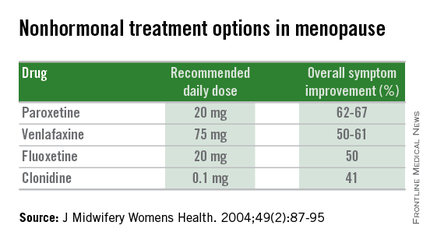
Women presenting with menopausal symptoms in whom estrogen therapy is contraindicated or not desired can also consider using nonhormonal therapies as an alternative. These include selective serotonin reuptake inhibitors (SSRIs) and alpha-2 adrenergic agonists, such as clonidine. Albeit not as effective as HT, these alternative therapies are reasonable options, particularly for management of vasomotor symptoms.
From a limited number of observational studies and a few randomized trials, short-term hormone replacement therapy does not present increased risk to survivors of gynecologic cancers. Additionally, patients have the added option of using nonhormonal therapies, which may provide some benefit. The decision to institute HT should occur after a thorough discussion of the potential to optimize symptom control and the theoretical risk of stimulating quiescent malignant disease.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Due to advancements in surgical treatment, chemotherapy, and radiation therapy, gynecologic cancer survival rates are continuing to improve and quality of life is evolving into an even more significant focus in cancer care.
Roughly 30%-40% of all women with a gynecologic malignancy will experience climacteric symptoms and menopause prior to the anticipated time of natural menopause (J Clin Oncol. 2009 Mar 10;27[8]:1214-9). Cessation of ovarian estrogen and progesterone production can result in short-term as well as long-term sequelae, including vasomotor symptoms, vaginal dryness, osteoporosis, and mood disturbances. Iatrogenic menopause after cancer treatment can be more sudden and severe when compared with the natural course of physiologic menopause. As a result, determination of safe, effective modalities for treating these symptoms is of particular importance for survivor quality of life.
Both combination and estrogen-only hormone replacement therapy (HT) provide greater improvement in these specific symptoms and overall quality of life than placebo as demonstrated in several observational and randomized control trials (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD004143).
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy, with approximately 54,000 new cases anticipated in the United States in 2015. Twenty-five percent of these new cases will be in premenopausal women, and with an ever-increasing obesity rate, this number may continue to climb.
Women with early-stage Type 1 endometrial cancer who have vasomotor symptoms after surgery may be offered a short course of estrogen-based HT at the lowest effective dose following hysterectomy/bilateral salpingo-oophorectomy and staging procedure (J Clin Oncol. 2006 Feb 1;24[4]:587-92). For women with genitourinary symptoms, vaginal moisturizers and/or low-dose vaginal estrogen are reasonable options. Unfortunately, there are no data to guide the use of estrogen replacement therapy in women with Type 2 endometrial cancers (Gynecol Oncol. 2011 Aug;122[2]:447-54).
Ovarian cancer
There is minimal data implicating a hormonal causation to ovarian carcinogenesis. Most women with epithelial ovarian cancer do not express tumor estrogen or progesterone receptors. Treatment will result in abrupt, iatrogenic menopause, raising the question of whether it is safe to use HT in patients with epithelial ovarian cancer.
Multiple studies have failed to demonstrate a difference in 5-year survival rates in women with epithelial cancer using HT for 2 years or less (JAMA. 2009 Jul 15;302[3]:298-305, Eur J Gynaecol Oncol. 2000;21[2]:192-6, Cancer. 1999 Sep 15;86[6]:1013-8). As such, symptomatic patients could be offered a course of HT; however, caution should be exercised in women with estrogen/progesterone–expressing tumors or nonepithelial tumors. As with endometrial cancer patients, the lowest effective doses should be prescribed.
Cervical cancer
Most cervical squamous and adenocarcinomas are not hormone dependent. For women with early-stage squamous cell carcinoma, ovarian conservation may be possible or oophoropexy may be offered. However, for many patients, bilateral salpingo-oophorectomy at the time of hysterectomy is more common, and the local effect of radiation therapy can result in vaginal atrophy with subsequent dyspareunia or ovarian failure from radiation scatter. Even for patients who undergo oophoropexy, radiation scatter may still result in ovarian failure. In a few observational studies, there are no data to infer that cervical cancer is hormonally related or that survival rates are decreased.
Currently, HT use in cervical cancer survivors is considered safe. Of note, for women with more advanced-stage cervical cancer and who received chemoradiation for primary treatment, combination therapy with estrogen and progesterone may be more appropriate if the uterus remains in situ. However, for women who have undergone hysterectomy, combination therapy with progesterone may not be warranted and estrogen alone (orally or vaginally) is acceptable (Gynecol Oncol. 2011 Aug;122[2]:447-54)
Nonhormonal therapies

Women presenting with menopausal symptoms in whom estrogen therapy is contraindicated or not desired can also consider using nonhormonal therapies as an alternative. These include selective serotonin reuptake inhibitors (SSRIs) and alpha-2 adrenergic agonists, such as clonidine. Albeit not as effective as HT, these alternative therapies are reasonable options, particularly for management of vasomotor symptoms.
From a limited number of observational studies and a few randomized trials, short-term hormone replacement therapy does not present increased risk to survivors of gynecologic cancers. Additionally, patients have the added option of using nonhormonal therapies, which may provide some benefit. The decision to institute HT should occur after a thorough discussion of the potential to optimize symptom control and the theoretical risk of stimulating quiescent malignant disease.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Due to advancements in surgical treatment, chemotherapy, and radiation therapy, gynecologic cancer survival rates are continuing to improve and quality of life is evolving into an even more significant focus in cancer care.
Roughly 30%-40% of all women with a gynecologic malignancy will experience climacteric symptoms and menopause prior to the anticipated time of natural menopause (J Clin Oncol. 2009 Mar 10;27[8]:1214-9). Cessation of ovarian estrogen and progesterone production can result in short-term as well as long-term sequelae, including vasomotor symptoms, vaginal dryness, osteoporosis, and mood disturbances. Iatrogenic menopause after cancer treatment can be more sudden and severe when compared with the natural course of physiologic menopause. As a result, determination of safe, effective modalities for treating these symptoms is of particular importance for survivor quality of life.
Both combination and estrogen-only hormone replacement therapy (HT) provide greater improvement in these specific symptoms and overall quality of life than placebo as demonstrated in several observational and randomized control trials (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD004143).
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy, with approximately 54,000 new cases anticipated in the United States in 2015. Twenty-five percent of these new cases will be in premenopausal women, and with an ever-increasing obesity rate, this number may continue to climb.
Women with early-stage Type 1 endometrial cancer who have vasomotor symptoms after surgery may be offered a short course of estrogen-based HT at the lowest effective dose following hysterectomy/bilateral salpingo-oophorectomy and staging procedure (J Clin Oncol. 2006 Feb 1;24[4]:587-92). For women with genitourinary symptoms, vaginal moisturizers and/or low-dose vaginal estrogen are reasonable options. Unfortunately, there are no data to guide the use of estrogen replacement therapy in women with Type 2 endometrial cancers (Gynecol Oncol. 2011 Aug;122[2]:447-54).
Ovarian cancer
There is minimal data implicating a hormonal causation to ovarian carcinogenesis. Most women with epithelial ovarian cancer do not express tumor estrogen or progesterone receptors. Treatment will result in abrupt, iatrogenic menopause, raising the question of whether it is safe to use HT in patients with epithelial ovarian cancer.
Multiple studies have failed to demonstrate a difference in 5-year survival rates in women with epithelial cancer using HT for 2 years or less (JAMA. 2009 Jul 15;302[3]:298-305, Eur J Gynaecol Oncol. 2000;21[2]:192-6, Cancer. 1999 Sep 15;86[6]:1013-8). As such, symptomatic patients could be offered a course of HT; however, caution should be exercised in women with estrogen/progesterone–expressing tumors or nonepithelial tumors. As with endometrial cancer patients, the lowest effective doses should be prescribed.
Cervical cancer
Most cervical squamous and adenocarcinomas are not hormone dependent. For women with early-stage squamous cell carcinoma, ovarian conservation may be possible or oophoropexy may be offered. However, for many patients, bilateral salpingo-oophorectomy at the time of hysterectomy is more common, and the local effect of radiation therapy can result in vaginal atrophy with subsequent dyspareunia or ovarian failure from radiation scatter. Even for patients who undergo oophoropexy, radiation scatter may still result in ovarian failure. In a few observational studies, there are no data to infer that cervical cancer is hormonally related or that survival rates are decreased.
Currently, HT use in cervical cancer survivors is considered safe. Of note, for women with more advanced-stage cervical cancer and who received chemoradiation for primary treatment, combination therapy with estrogen and progesterone may be more appropriate if the uterus remains in situ. However, for women who have undergone hysterectomy, combination therapy with progesterone may not be warranted and estrogen alone (orally or vaginally) is acceptable (Gynecol Oncol. 2011 Aug;122[2]:447-54)
Nonhormonal therapies

Women presenting with menopausal symptoms in whom estrogen therapy is contraindicated or not desired can also consider using nonhormonal therapies as an alternative. These include selective serotonin reuptake inhibitors (SSRIs) and alpha-2 adrenergic agonists, such as clonidine. Albeit not as effective as HT, these alternative therapies are reasonable options, particularly for management of vasomotor symptoms.
From a limited number of observational studies and a few randomized trials, short-term hormone replacement therapy does not present increased risk to survivors of gynecologic cancers. Additionally, patients have the added option of using nonhormonal therapies, which may provide some benefit. The decision to institute HT should occur after a thorough discussion of the potential to optimize symptom control and the theoretical risk of stimulating quiescent malignant disease.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures.
The Patient Who Didn’t Complain Enough?
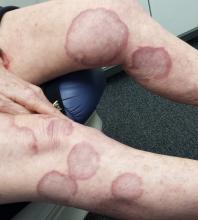
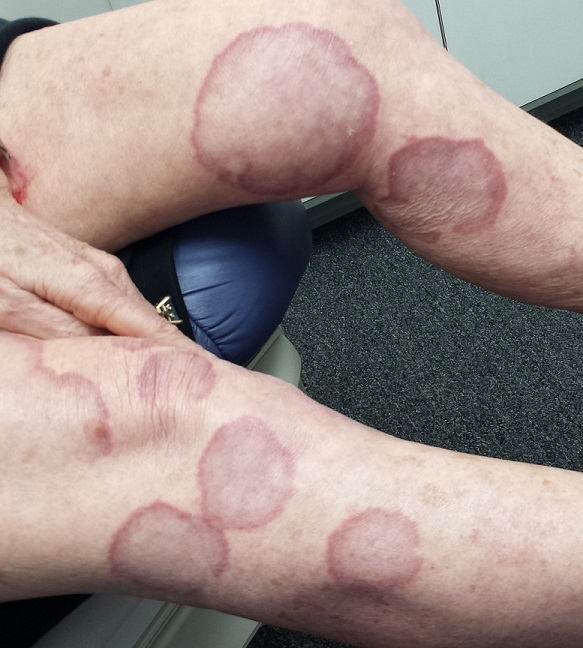
A 70-year-old woman presents to dermatology with asymptomatic lesions on her legs that, she reports, have been there for decades but are now becoming larger and more unsightly. She’s tried several courses of antifungal creams and pills with no success.
In all this time, she hasn’t seen a dermatology provider, because “no one ever felt the need” to refer her since her diagnosis was so “obvious.” When the changes began, however, her children finally convinced her to consult a specialist.
Her medical history is otherwise unremarkable, with no problems directly related to her skin issues. She denies both personal and family history of diabetes.
EXAMINATION
There are numerous impressive, reddish brown, annular plaques with slightly raised borders and clearing centers on the patient’s legs. There are also a few on her arms and one or two on her trunk. There is no epidermal component (scale or other surface disruption) on any of the lesions, which range in size from 2 cm to 15 cm.
What is the diagnosis?
DISCUSSION
It’s hard to believe that any patient could go this long with such a florid condition and never be seen by dermatology. Patients have an obligation to speak up—to complain, as it were—but some are too compliant. This woman’s primary care providers should have attached some significance to the nonresponse to treatment, one explanation for which might have been erroneous diagnosis.
Everybody knows that big round things are fungal, except when they aren’t. That’s where dermatology providers shine. Our training is all about development of differentials, as in: What are the various potential explanations for big round lesions? What would those things look like, and how could we distinguish them from fungal infection?
Here’s one huge clue: Fungal infection commonly refers to a relatively trivial, superficial infection of the outermost layer of the skin, the stratum corneum. By its very nature, it almost always involves scaling; if those scales are examined microscopically, the fungal elements can be seen, thus confirming the diagnosis.
When there is no scale, and the condition fails to respond to antifungal medications, alternative diagnoses must be considered. One major item that fits the bill is granuloma annulare (GA), an asymptomatic condition that presents with reddish brown, distinctly annular papules and plaques with raised (papular) borders and cleared centers. Though GA is extremely common, it is almost always misdiagnosed initially as fungal infection.
Besides the lack of scaling, GA lesions have some other characteristics that aid diagnosis. They’re commonly seen on extremities, especially the dorsa of feet and hands, and are far more common on women (especially young ones) than on men.
Although usually reddish brown, the lesions will be darker on patients with darker skin. It should be mentioned that this patient’s lesions were unusually large and extensive. Most cases are far more modest in size and distribution.
When necessary, punch biopsy shows what is called a palisaded granuloma. This, taken in context with the clinical picture, nails down the diagnosis.
One other piece of information is good to know: GA is quite common. You will see it, regularly.
Since the cause of GA is unknown, treatment can be problematic. Fortunately, the problem almost always resolves, with or without treatment. A few of this patient’s lesions were treated (for cosmetic reasons) with intralesional steroid injection.
TAKE-HOME LEARNING POINTS
• Granuloma annulare (GA) is a benign asymptomatic condition that presents with annular, reddish brown intradermal lesions.
• GA is commonly seen on the dorsa of feet, hands, and arms, especially of young women.
• GA lesions are intradermal, not epidermal, so there is no scaling or other disturbance of the skin surface.
• GA treatment is problematic, but the problem usually resolves on its own.
A 70-year-old woman presents to dermatology with asymptomatic lesions on her legs that, she reports, have been there for decades but are now becoming larger and more unsightly. She’s tried several courses of antifungal creams and pills with no success.
In all this time, she hasn’t seen a dermatology provider, because “no one ever felt the need” to refer her since her diagnosis was so “obvious.” When the changes began, however, her children finally convinced her to consult a specialist.
Her medical history is otherwise unremarkable, with no problems directly related to her skin issues. She denies both personal and family history of diabetes.
EXAMINATION
There are numerous impressive, reddish brown, annular plaques with slightly raised borders and clearing centers on the patient’s legs. There are also a few on her arms and one or two on her trunk. There is no epidermal component (scale or other surface disruption) on any of the lesions, which range in size from 2 cm to 15 cm.
What is the diagnosis?
DISCUSSION
It’s hard to believe that any patient could go this long with such a florid condition and never be seen by dermatology. Patients have an obligation to speak up—to complain, as it were—but some are too compliant. This woman’s primary care providers should have attached some significance to the nonresponse to treatment, one explanation for which might have been erroneous diagnosis.
Everybody knows that big round things are fungal, except when they aren’t. That’s where dermatology providers shine. Our training is all about development of differentials, as in: What are the various potential explanations for big round lesions? What would those things look like, and how could we distinguish them from fungal infection?
Here’s one huge clue: Fungal infection commonly refers to a relatively trivial, superficial infection of the outermost layer of the skin, the stratum corneum. By its very nature, it almost always involves scaling; if those scales are examined microscopically, the fungal elements can be seen, thus confirming the diagnosis.
When there is no scale, and the condition fails to respond to antifungal medications, alternative diagnoses must be considered. One major item that fits the bill is granuloma annulare (GA), an asymptomatic condition that presents with reddish brown, distinctly annular papules and plaques with raised (papular) borders and cleared centers. Though GA is extremely common, it is almost always misdiagnosed initially as fungal infection.
Besides the lack of scaling, GA lesions have some other characteristics that aid diagnosis. They’re commonly seen on extremities, especially the dorsa of feet and hands, and are far more common on women (especially young ones) than on men.
Although usually reddish brown, the lesions will be darker on patients with darker skin. It should be mentioned that this patient’s lesions were unusually large and extensive. Most cases are far more modest in size and distribution.
When necessary, punch biopsy shows what is called a palisaded granuloma. This, taken in context with the clinical picture, nails down the diagnosis.
One other piece of information is good to know: GA is quite common. You will see it, regularly.
Since the cause of GA is unknown, treatment can be problematic. Fortunately, the problem almost always resolves, with or without treatment. A few of this patient’s lesions were treated (for cosmetic reasons) with intralesional steroid injection.
TAKE-HOME LEARNING POINTS
• Granuloma annulare (GA) is a benign asymptomatic condition that presents with annular, reddish brown intradermal lesions.
• GA is commonly seen on the dorsa of feet, hands, and arms, especially of young women.
• GA lesions are intradermal, not epidermal, so there is no scaling or other disturbance of the skin surface.
• GA treatment is problematic, but the problem usually resolves on its own.
A 70-year-old woman presents to dermatology with asymptomatic lesions on her legs that, she reports, have been there for decades but are now becoming larger and more unsightly. She’s tried several courses of antifungal creams and pills with no success.
In all this time, she hasn’t seen a dermatology provider, because “no one ever felt the need” to refer her since her diagnosis was so “obvious.” When the changes began, however, her children finally convinced her to consult a specialist.
Her medical history is otherwise unremarkable, with no problems directly related to her skin issues. She denies both personal and family history of diabetes.
EXAMINATION
There are numerous impressive, reddish brown, annular plaques with slightly raised borders and clearing centers on the patient’s legs. There are also a few on her arms and one or two on her trunk. There is no epidermal component (scale or other surface disruption) on any of the lesions, which range in size from 2 cm to 15 cm.
What is the diagnosis?
DISCUSSION
It’s hard to believe that any patient could go this long with such a florid condition and never be seen by dermatology. Patients have an obligation to speak up—to complain, as it were—but some are too compliant. This woman’s primary care providers should have attached some significance to the nonresponse to treatment, one explanation for which might have been erroneous diagnosis.
Everybody knows that big round things are fungal, except when they aren’t. That’s where dermatology providers shine. Our training is all about development of differentials, as in: What are the various potential explanations for big round lesions? What would those things look like, and how could we distinguish them from fungal infection?
Here’s one huge clue: Fungal infection commonly refers to a relatively trivial, superficial infection of the outermost layer of the skin, the stratum corneum. By its very nature, it almost always involves scaling; if those scales are examined microscopically, the fungal elements can be seen, thus confirming the diagnosis.
When there is no scale, and the condition fails to respond to antifungal medications, alternative diagnoses must be considered. One major item that fits the bill is granuloma annulare (GA), an asymptomatic condition that presents with reddish brown, distinctly annular papules and plaques with raised (papular) borders and cleared centers. Though GA is extremely common, it is almost always misdiagnosed initially as fungal infection.
Besides the lack of scaling, GA lesions have some other characteristics that aid diagnosis. They’re commonly seen on extremities, especially the dorsa of feet and hands, and are far more common on women (especially young ones) than on men.
Although usually reddish brown, the lesions will be darker on patients with darker skin. It should be mentioned that this patient’s lesions were unusually large and extensive. Most cases are far more modest in size and distribution.
When necessary, punch biopsy shows what is called a palisaded granuloma. This, taken in context with the clinical picture, nails down the diagnosis.
One other piece of information is good to know: GA is quite common. You will see it, regularly.
Since the cause of GA is unknown, treatment can be problematic. Fortunately, the problem almost always resolves, with or without treatment. A few of this patient’s lesions were treated (for cosmetic reasons) with intralesional steroid injection.
TAKE-HOME LEARNING POINTS
• Granuloma annulare (GA) is a benign asymptomatic condition that presents with annular, reddish brown intradermal lesions.
• GA is commonly seen on the dorsa of feet, hands, and arms, especially of young women.
• GA lesions are intradermal, not epidermal, so there is no scaling or other disturbance of the skin surface.
• GA treatment is problematic, but the problem usually resolves on its own.
FDA gives nod to rapid-infusion bendamustine for CLL
The Food and Drug Administration has approved a new rapid-infusion form of bendamustine hydrochloride for patients with chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma.
The new formulation of bendamustine (Bendeka) is a 50-mL liquid designed for 10-minute infusion. It was granted orphan drug status for both the leukemia and lymphoma indications.
Bendeka is approved as primary therapy for chronic lymphocytic leukemia (CLL) and also for indolent B-cell non-Hodgkin lymphoma (NHL) that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen.
According to the prescribing information, the recommended dosing regimen for CLL is 100 mg/m2 infused intravenously over 10 minutes on days 1 and 2 of a 28-day cycle, up to six cycles. For NHL, the regimen calls for 120 mg/m2 infused intravenously over 10 minutes on days 1 and 2 of a 21-day cycle, up to eight cycles.
The most common hematologic adverse reactions are lymphopenia, anemia, leukopenia, thrombocytopenia, and neutropenia. Bendamustine has been associated with severe – and even fatal – myelosuppression.
Bendeka is manufactured by Teva Pharmaceutical Industries and succeeds Teva’s previously approved form of bendamustine, Treanda, approved in 2008. “Since 2008, Treanda has played a valuable role in the treatment of patients with CLL or indolent B-cell NHL that has progressed,” Paul Rittman, Teva Oncology’s vice president and general manager, said in a press statement. Treanda’s orphan drug exclusivity status for the NHL indication was set to expire in October, and its pediatric exclusivity status for that indication, next April. Its CLL exclusivity status expired in September.
Teva purchased the new formulation from Eagle Pharmaceuticals last February. The deal was closed with an upfront payment of $30 million, and potential for up to $90 million in additional payments, as well as double-digit royalties on net sales.
At the time of purchase, Eagle had secured orphan drug designations for Bendeka in both CLL and NHL and had submitted the New Drug Application under priority review. Bendeka may be eligible for a 7-year exclusivity status.
The drug is scheduled to be available during the first quarter of 2016.
The Food and Drug Administration has approved a new rapid-infusion form of bendamustine hydrochloride for patients with chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma.
The new formulation of bendamustine (Bendeka) is a 50-mL liquid designed for 10-minute infusion. It was granted orphan drug status for both the leukemia and lymphoma indications.
Bendeka is approved as primary therapy for chronic lymphocytic leukemia (CLL) and also for indolent B-cell non-Hodgkin lymphoma (NHL) that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen.
According to the prescribing information, the recommended dosing regimen for CLL is 100 mg/m2 infused intravenously over 10 minutes on days 1 and 2 of a 28-day cycle, up to six cycles. For NHL, the regimen calls for 120 mg/m2 infused intravenously over 10 minutes on days 1 and 2 of a 21-day cycle, up to eight cycles.
The most common hematologic adverse reactions are lymphopenia, anemia, leukopenia, thrombocytopenia, and neutropenia. Bendamustine has been associated with severe – and even fatal – myelosuppression.
Bendeka is manufactured by Teva Pharmaceutical Industries and succeeds Teva’s previously approved form of bendamustine, Treanda, approved in 2008. “Since 2008, Treanda has played a valuable role in the treatment of patients with CLL or indolent B-cell NHL that has progressed,” Paul Rittman, Teva Oncology’s vice president and general manager, said in a press statement. Treanda’s orphan drug exclusivity status for the NHL indication was set to expire in October, and its pediatric exclusivity status for that indication, next April. Its CLL exclusivity status expired in September.
Teva purchased the new formulation from Eagle Pharmaceuticals last February. The deal was closed with an upfront payment of $30 million, and potential for up to $90 million in additional payments, as well as double-digit royalties on net sales.
At the time of purchase, Eagle had secured orphan drug designations for Bendeka in both CLL and NHL and had submitted the New Drug Application under priority review. Bendeka may be eligible for a 7-year exclusivity status.
The drug is scheduled to be available during the first quarter of 2016.
The Food and Drug Administration has approved a new rapid-infusion form of bendamustine hydrochloride for patients with chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma.
The new formulation of bendamustine (Bendeka) is a 50-mL liquid designed for 10-minute infusion. It was granted orphan drug status for both the leukemia and lymphoma indications.
Bendeka is approved as primary therapy for chronic lymphocytic leukemia (CLL) and also for indolent B-cell non-Hodgkin lymphoma (NHL) that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen.
According to the prescribing information, the recommended dosing regimen for CLL is 100 mg/m2 infused intravenously over 10 minutes on days 1 and 2 of a 28-day cycle, up to six cycles. For NHL, the regimen calls for 120 mg/m2 infused intravenously over 10 minutes on days 1 and 2 of a 21-day cycle, up to eight cycles.
The most common hematologic adverse reactions are lymphopenia, anemia, leukopenia, thrombocytopenia, and neutropenia. Bendamustine has been associated with severe – and even fatal – myelosuppression.
Bendeka is manufactured by Teva Pharmaceutical Industries and succeeds Teva’s previously approved form of bendamustine, Treanda, approved in 2008. “Since 2008, Treanda has played a valuable role in the treatment of patients with CLL or indolent B-cell NHL that has progressed,” Paul Rittman, Teva Oncology’s vice president and general manager, said in a press statement. Treanda’s orphan drug exclusivity status for the NHL indication was set to expire in October, and its pediatric exclusivity status for that indication, next April. Its CLL exclusivity status expired in September.
Teva purchased the new formulation from Eagle Pharmaceuticals last February. The deal was closed with an upfront payment of $30 million, and potential for up to $90 million in additional payments, as well as double-digit royalties on net sales.
At the time of purchase, Eagle had secured orphan drug designations for Bendeka in both CLL and NHL and had submitted the New Drug Application under priority review. Bendeka may be eligible for a 7-year exclusivity status.
The drug is scheduled to be available during the first quarter of 2016.
Naloxone to revive an addict
We are in the midst of an epidemic of heroin and prescription opioid abuse. While the two do not completely explain each other, they are tragically and irrevocably linked.
From 2001 to 2013, we have observed a threefold increase in the total number of overdose deaths from opioid pain relievers (about 16,000 in 2013) and a fivefold increase in the total number of overdose deaths from heroin (about 8,000 in 2013).
Heroin initiation is almost 20 times higher among individuals reporting nonmedical prescription pain reliever use. Among opioid-treatment seekers, the majority of individuals who initiated opioid use in the 1960s were first exposed to heroin. This is in contrast to those who initiated in the 2000s, among whom the majority were exposed to prescription opioids. For young adults, the main sources of opioids are family, friends … and clinicians.
Opioids are powerfully addictive and can be snorted, swallowed, smoked, or shot. Data from the START (Starting Treatment with Agonist Replacement Therapies) trial suggest that individuals who inject opioids are less likely to remain in treatment than noninjectors. This necessarily increases the risk for injectors to inject again and be at risk for overdose.
Opioid overdose can be reversed with the use of naloxone. But naloxone has to be immediately or quickly available for it to be effective. Take-home naloxone programs are located in 30 U.S. states and the District of Columbia. Since 1996, home naloxone programs have reported more than 26,000 drug overdose reversals with naloxone.
On Nov. 18, the Food and Drug Administration announced the approval of a naloxone nasal spray. Prior to this approval, naloxone was only available in the injectable form (syringe or auto-injector), and needle management likely posed a barrier to first responders. The nasal spray can be administered easily without medical training. Naloxone nasal spray administered in one nostril delivered approximately the same levels or higher of naloxone as a single dose of an FDA-approved naloxone intramuscular injection in approximately the same time frame.
It is one thing to save a heroin addict who has just overdosed with nasal naloxone followed by appropriate medical attention. It is entirely another to engage them in an effective drug treatment program.
If naloxone revives them, it is treatment that can save them.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
We are in the midst of an epidemic of heroin and prescription opioid abuse. While the two do not completely explain each other, they are tragically and irrevocably linked.
From 2001 to 2013, we have observed a threefold increase in the total number of overdose deaths from opioid pain relievers (about 16,000 in 2013) and a fivefold increase in the total number of overdose deaths from heroin (about 8,000 in 2013).
Heroin initiation is almost 20 times higher among individuals reporting nonmedical prescription pain reliever use. Among opioid-treatment seekers, the majority of individuals who initiated opioid use in the 1960s were first exposed to heroin. This is in contrast to those who initiated in the 2000s, among whom the majority were exposed to prescription opioids. For young adults, the main sources of opioids are family, friends … and clinicians.
Opioids are powerfully addictive and can be snorted, swallowed, smoked, or shot. Data from the START (Starting Treatment with Agonist Replacement Therapies) trial suggest that individuals who inject opioids are less likely to remain in treatment than noninjectors. This necessarily increases the risk for injectors to inject again and be at risk for overdose.
Opioid overdose can be reversed with the use of naloxone. But naloxone has to be immediately or quickly available for it to be effective. Take-home naloxone programs are located in 30 U.S. states and the District of Columbia. Since 1996, home naloxone programs have reported more than 26,000 drug overdose reversals with naloxone.
On Nov. 18, the Food and Drug Administration announced the approval of a naloxone nasal spray. Prior to this approval, naloxone was only available in the injectable form (syringe or auto-injector), and needle management likely posed a barrier to first responders. The nasal spray can be administered easily without medical training. Naloxone nasal spray administered in one nostril delivered approximately the same levels or higher of naloxone as a single dose of an FDA-approved naloxone intramuscular injection in approximately the same time frame.
It is one thing to save a heroin addict who has just overdosed with nasal naloxone followed by appropriate medical attention. It is entirely another to engage them in an effective drug treatment program.
If naloxone revives them, it is treatment that can save them.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
We are in the midst of an epidemic of heroin and prescription opioid abuse. While the two do not completely explain each other, they are tragically and irrevocably linked.
From 2001 to 2013, we have observed a threefold increase in the total number of overdose deaths from opioid pain relievers (about 16,000 in 2013) and a fivefold increase in the total number of overdose deaths from heroin (about 8,000 in 2013).
Heroin initiation is almost 20 times higher among individuals reporting nonmedical prescription pain reliever use. Among opioid-treatment seekers, the majority of individuals who initiated opioid use in the 1960s were first exposed to heroin. This is in contrast to those who initiated in the 2000s, among whom the majority were exposed to prescription opioids. For young adults, the main sources of opioids are family, friends … and clinicians.
Opioids are powerfully addictive and can be snorted, swallowed, smoked, or shot. Data from the START (Starting Treatment with Agonist Replacement Therapies) trial suggest that individuals who inject opioids are less likely to remain in treatment than noninjectors. This necessarily increases the risk for injectors to inject again and be at risk for overdose.
Opioid overdose can be reversed with the use of naloxone. But naloxone has to be immediately or quickly available for it to be effective. Take-home naloxone programs are located in 30 U.S. states and the District of Columbia. Since 1996, home naloxone programs have reported more than 26,000 drug overdose reversals with naloxone.
On Nov. 18, the Food and Drug Administration announced the approval of a naloxone nasal spray. Prior to this approval, naloxone was only available in the injectable form (syringe or auto-injector), and needle management likely posed a barrier to first responders. The nasal spray can be administered easily without medical training. Naloxone nasal spray administered in one nostril delivered approximately the same levels or higher of naloxone as a single dose of an FDA-approved naloxone intramuscular injection in approximately the same time frame.
It is one thing to save a heroin addict who has just overdosed with nasal naloxone followed by appropriate medical attention. It is entirely another to engage them in an effective drug treatment program.
If naloxone revives them, it is treatment that can save them.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.