User login
New drug approved for hepatic veno-occlusive disease
Defibrotide sodium has been approved to treat hepatic veno-occlusive disease (VOD) in patients with renal or pulmonary dysfunction following a hematopoietic stem cell transplantation, the Food and Drug Administration has announced.
The drug, which will be marketed as Defitelio by Jazz Pharmaceuticals, was tested in two prospective clinical trials and an expanded access study that included a total of 528 patients with hepatic VOD and multiorgan dysfunction following a transplantation. All patients received 6.25 mg/kg doses of the drug intravenously, every 6 hours until resolution of VOD. The percentages of patients surviving more than 100 days after receiving a stem cell transplantation in each of the studies were 38%, 44%, and 45%, respectively, according to a statement from the FDA.
Contraindications for taking the drug are concurrent use of anticoagulants or fibrinolytic therapies.
Hypotension, diarrhea, vomiting, nausea, and epistaxis are the most common adverse reactions to the drug.
Full prescribing information is available at the FDA website.
Defibrotide sodium has been approved to treat hepatic veno-occlusive disease (VOD) in patients with renal or pulmonary dysfunction following a hematopoietic stem cell transplantation, the Food and Drug Administration has announced.
The drug, which will be marketed as Defitelio by Jazz Pharmaceuticals, was tested in two prospective clinical trials and an expanded access study that included a total of 528 patients with hepatic VOD and multiorgan dysfunction following a transplantation. All patients received 6.25 mg/kg doses of the drug intravenously, every 6 hours until resolution of VOD. The percentages of patients surviving more than 100 days after receiving a stem cell transplantation in each of the studies were 38%, 44%, and 45%, respectively, according to a statement from the FDA.
Contraindications for taking the drug are concurrent use of anticoagulants or fibrinolytic therapies.
Hypotension, diarrhea, vomiting, nausea, and epistaxis are the most common adverse reactions to the drug.
Full prescribing information is available at the FDA website.
Defibrotide sodium has been approved to treat hepatic veno-occlusive disease (VOD) in patients with renal or pulmonary dysfunction following a hematopoietic stem cell transplantation, the Food and Drug Administration has announced.
The drug, which will be marketed as Defitelio by Jazz Pharmaceuticals, was tested in two prospective clinical trials and an expanded access study that included a total of 528 patients with hepatic VOD and multiorgan dysfunction following a transplantation. All patients received 6.25 mg/kg doses of the drug intravenously, every 6 hours until resolution of VOD. The percentages of patients surviving more than 100 days after receiving a stem cell transplantation in each of the studies were 38%, 44%, and 45%, respectively, according to a statement from the FDA.
Contraindications for taking the drug are concurrent use of anticoagulants or fibrinolytic therapies.
Hypotension, diarrhea, vomiting, nausea, and epistaxis are the most common adverse reactions to the drug.
Full prescribing information is available at the FDA website.
Carpal tunnel syndrome: Guidelines rate evidence for diagnosis, treatment
The American Academy of Orthopaedic Surgeons has adopted clinical practice guidelines that assign evidence-based ratings for common strategies used to diagnose and treat carpal tunnel syndrome (CTS).
The 982-page comprehensive guidelines have been endorsed by the American Society for the Surgery of the Hand and the American College of Radiology. The guidelines address the burden of CTS, the second most common cause of sick days from work, according to AAOS, and its etiology, risk factors, emotional and physical impact, potential benefits, harms, contraindications, and future research. The document is available on the OrthoGuidelines Web-based app at orthoguidelines.org.
The assessments of evidence are based upon a systematic review of the current scientific and clinical information and accepted approaches to treatment and/or diagnosis of carpal tunnel syndrome. In addition to a concise summary, the report includes an exhaustive list of studies used to establish levels of evidence and a summary of the evidence in each. Also included is a list of studies not included, many because of poor study design or very small samples.
The guidelines make recommendations on practices to diagnose and manage CTS based on four levels of evidence:
• Strong: Supported by two or more “high-quality” studies with consistent findings.
• Moderate: Supported by two or more “moderate-quality” studies or one “high-quality” study.
• Limited: Supported by two or more “low-quality” studies or one “moderate-quality” study, or the evidence is considered insufficient or conflicting.
• Consensus: No supporting evidence but the guidelines development group made a recommendation based on clinical opinion.
Diagnosis and risk evidence
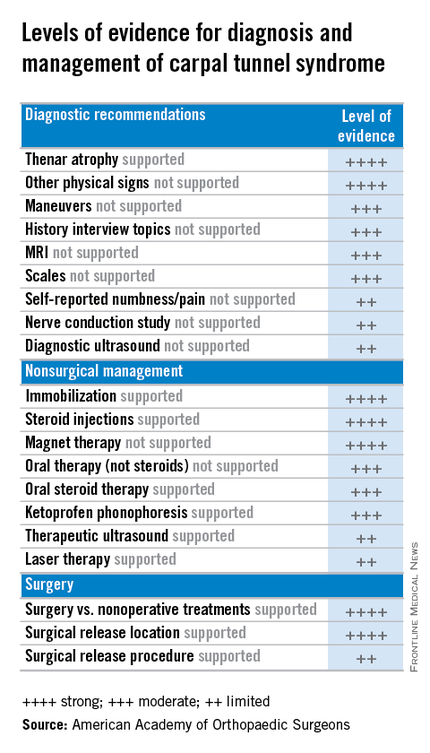
For diagnosis of CTS, the guidelines rate the evidence for the value of both observation and physical signs as strong, but assign ratings of moderate to MRI and limited to ultrasound. Evidence is strong for thenar atrophy, or diminished thumb muscle mass, being associated with CTS, but a lack of thenar atrophy is not enough to rule out a diagnosis. Common evaluation tools such the Phalen test, Tinel sign, Flick sign, or Upper-Limb Neurodynamic/Nerve Tension test (ULNT) are weakly supported as independent physical examination maneuvers to rule in or rule out carpal tunnel and the guidelines suggest that they not be used as sole diagnostic tools.
Moderate evidence supports exercise and physical activity to reduce the risk of developing CTS. The guidelines consider obesity a strong risk factor for CTS, but assign moderate ratings to evidence for a host of other factors, perimenopausal status, wrist ratio/index, rheumatoid arthritis, psychosocial factors, and activities such as gardening and computer use among them.
Treatment evidence
For treatment, the guidelines evaluate evidence for both surgical and nonsurgical strategies. In general, evidence for the efficacy of splinting, steroids (oral or injection), the use of ketoprofen phonophoresis gel, and magnetic therapy is strong. But therapeutic ultrasound and laser therapy are backed up with only limited evidence from the literature.
As might be expected, the evidence is strong for the efficacy of surgery to release the transverse carpal ligament. “Strong evidence supports that surgical treatment of carpal tunnel syndrome should have a greater treatment benefit at 6 and 12 months as compared to splinting, NSAIDs/therapy, and a single steroid injection.” But the value of adjunctive techniques such as epineurotomy, neurolysis, flexor tenosynovectomy, and lengthening/reconstruction of the flexor retinaculum (transverse carpal ligament) is not supported with strong evidence at this point. And the superiority of the endoscopic surgical approach is supported with only limited evidence.
“The impetus for this came from trying to help physicians cull through literally thousands and thousands of published research papers concerning various diagnoses,” said Dr. Allan E. Peljovich, vice-chair of the Guideline Work Group and AAOS representative to the group. It’s a tool to help orthopedic surgeons and other practitioners “understand what our best evidence tells us about diagnosing and treating a variety of conditions,” he said.
The effort to develop the CTS guidelines started February 2013 and involved the Guideline Work Group formulating a set of questions that, as Dr. Peljovich explained, were “the most pertinent questions that anybody interested in a particular diagnosis would want to have answered.” Then a team of statisticians and epidemiologists culled through the “incredible expanse of English language literature” to correlate data to answer those questions.
In May 2015 the work group then met to review the evidence and draft final recommendations. After a period of editing, the draft was submitted for peer review in September. The AAOS board of directors adopted the guidelines in February.
“The guidelines are not intended to be a cookbook on how to treat a condition,” Dr. Peljovich said. “They are really designed to tell you what the best evidence says about a particular set of questions. It helps you to be as updated as you want to be; it’s not designed to tell you this is the only way to do anything. ... It’s an educational tool.”
Members of the Guideline Work Group, AAOS staff, and contributing members submitted their disclosures to the AAOS.
The American Academy of Orthopaedic Surgeons has adopted clinical practice guidelines that assign evidence-based ratings for common strategies used to diagnose and treat carpal tunnel syndrome (CTS).
The 982-page comprehensive guidelines have been endorsed by the American Society for the Surgery of the Hand and the American College of Radiology. The guidelines address the burden of CTS, the second most common cause of sick days from work, according to AAOS, and its etiology, risk factors, emotional and physical impact, potential benefits, harms, contraindications, and future research. The document is available on the OrthoGuidelines Web-based app at orthoguidelines.org.
The assessments of evidence are based upon a systematic review of the current scientific and clinical information and accepted approaches to treatment and/or diagnosis of carpal tunnel syndrome. In addition to a concise summary, the report includes an exhaustive list of studies used to establish levels of evidence and a summary of the evidence in each. Also included is a list of studies not included, many because of poor study design or very small samples.
The guidelines make recommendations on practices to diagnose and manage CTS based on four levels of evidence:
• Strong: Supported by two or more “high-quality” studies with consistent findings.
• Moderate: Supported by two or more “moderate-quality” studies or one “high-quality” study.
• Limited: Supported by two or more “low-quality” studies or one “moderate-quality” study, or the evidence is considered insufficient or conflicting.
• Consensus: No supporting evidence but the guidelines development group made a recommendation based on clinical opinion.
Diagnosis and risk evidence
For diagnosis of CTS, the guidelines rate the evidence for the value of both observation and physical signs as strong, but assign ratings of moderate to MRI and limited to ultrasound. Evidence is strong for thenar atrophy, or diminished thumb muscle mass, being associated with CTS, but a lack of thenar atrophy is not enough to rule out a diagnosis. Common evaluation tools such the Phalen test, Tinel sign, Flick sign, or Upper-Limb Neurodynamic/Nerve Tension test (ULNT) are weakly supported as independent physical examination maneuvers to rule in or rule out carpal tunnel and the guidelines suggest that they not be used as sole diagnostic tools.
Moderate evidence supports exercise and physical activity to reduce the risk of developing CTS. The guidelines consider obesity a strong risk factor for CTS, but assign moderate ratings to evidence for a host of other factors, perimenopausal status, wrist ratio/index, rheumatoid arthritis, psychosocial factors, and activities such as gardening and computer use among them.

Treatment evidence
For treatment, the guidelines evaluate evidence for both surgical and nonsurgical strategies. In general, evidence for the efficacy of splinting, steroids (oral or injection), the use of ketoprofen phonophoresis gel, and magnetic therapy is strong. But therapeutic ultrasound and laser therapy are backed up with only limited evidence from the literature.
As might be expected, the evidence is strong for the efficacy of surgery to release the transverse carpal ligament. “Strong evidence supports that surgical treatment of carpal tunnel syndrome should have a greater treatment benefit at 6 and 12 months as compared to splinting, NSAIDs/therapy, and a single steroid injection.” But the value of adjunctive techniques such as epineurotomy, neurolysis, flexor tenosynovectomy, and lengthening/reconstruction of the flexor retinaculum (transverse carpal ligament) is not supported with strong evidence at this point. And the superiority of the endoscopic surgical approach is supported with only limited evidence.
“The impetus for this came from trying to help physicians cull through literally thousands and thousands of published research papers concerning various diagnoses,” said Dr. Allan E. Peljovich, vice-chair of the Guideline Work Group and AAOS representative to the group. It’s a tool to help orthopedic surgeons and other practitioners “understand what our best evidence tells us about diagnosing and treating a variety of conditions,” he said.
The effort to develop the CTS guidelines started February 2013 and involved the Guideline Work Group formulating a set of questions that, as Dr. Peljovich explained, were “the most pertinent questions that anybody interested in a particular diagnosis would want to have answered.” Then a team of statisticians and epidemiologists culled through the “incredible expanse of English language literature” to correlate data to answer those questions.
In May 2015 the work group then met to review the evidence and draft final recommendations. After a period of editing, the draft was submitted for peer review in September. The AAOS board of directors adopted the guidelines in February.
“The guidelines are not intended to be a cookbook on how to treat a condition,” Dr. Peljovich said. “They are really designed to tell you what the best evidence says about a particular set of questions. It helps you to be as updated as you want to be; it’s not designed to tell you this is the only way to do anything. ... It’s an educational tool.”
Members of the Guideline Work Group, AAOS staff, and contributing members submitted their disclosures to the AAOS.
The American Academy of Orthopaedic Surgeons has adopted clinical practice guidelines that assign evidence-based ratings for common strategies used to diagnose and treat carpal tunnel syndrome (CTS).
The 982-page comprehensive guidelines have been endorsed by the American Society for the Surgery of the Hand and the American College of Radiology. The guidelines address the burden of CTS, the second most common cause of sick days from work, according to AAOS, and its etiology, risk factors, emotional and physical impact, potential benefits, harms, contraindications, and future research. The document is available on the OrthoGuidelines Web-based app at orthoguidelines.org.
The assessments of evidence are based upon a systematic review of the current scientific and clinical information and accepted approaches to treatment and/or diagnosis of carpal tunnel syndrome. In addition to a concise summary, the report includes an exhaustive list of studies used to establish levels of evidence and a summary of the evidence in each. Also included is a list of studies not included, many because of poor study design or very small samples.
The guidelines make recommendations on practices to diagnose and manage CTS based on four levels of evidence:
• Strong: Supported by two or more “high-quality” studies with consistent findings.
• Moderate: Supported by two or more “moderate-quality” studies or one “high-quality” study.
• Limited: Supported by two or more “low-quality” studies or one “moderate-quality” study, or the evidence is considered insufficient or conflicting.
• Consensus: No supporting evidence but the guidelines development group made a recommendation based on clinical opinion.
Diagnosis and risk evidence
For diagnosis of CTS, the guidelines rate the evidence for the value of both observation and physical signs as strong, but assign ratings of moderate to MRI and limited to ultrasound. Evidence is strong for thenar atrophy, or diminished thumb muscle mass, being associated with CTS, but a lack of thenar atrophy is not enough to rule out a diagnosis. Common evaluation tools such the Phalen test, Tinel sign, Flick sign, or Upper-Limb Neurodynamic/Nerve Tension test (ULNT) are weakly supported as independent physical examination maneuvers to rule in or rule out carpal tunnel and the guidelines suggest that they not be used as sole diagnostic tools.
Moderate evidence supports exercise and physical activity to reduce the risk of developing CTS. The guidelines consider obesity a strong risk factor for CTS, but assign moderate ratings to evidence for a host of other factors, perimenopausal status, wrist ratio/index, rheumatoid arthritis, psychosocial factors, and activities such as gardening and computer use among them.

Treatment evidence
For treatment, the guidelines evaluate evidence for both surgical and nonsurgical strategies. In general, evidence for the efficacy of splinting, steroids (oral or injection), the use of ketoprofen phonophoresis gel, and magnetic therapy is strong. But therapeutic ultrasound and laser therapy are backed up with only limited evidence from the literature.
As might be expected, the evidence is strong for the efficacy of surgery to release the transverse carpal ligament. “Strong evidence supports that surgical treatment of carpal tunnel syndrome should have a greater treatment benefit at 6 and 12 months as compared to splinting, NSAIDs/therapy, and a single steroid injection.” But the value of adjunctive techniques such as epineurotomy, neurolysis, flexor tenosynovectomy, and lengthening/reconstruction of the flexor retinaculum (transverse carpal ligament) is not supported with strong evidence at this point. And the superiority of the endoscopic surgical approach is supported with only limited evidence.
“The impetus for this came from trying to help physicians cull through literally thousands and thousands of published research papers concerning various diagnoses,” said Dr. Allan E. Peljovich, vice-chair of the Guideline Work Group and AAOS representative to the group. It’s a tool to help orthopedic surgeons and other practitioners “understand what our best evidence tells us about diagnosing and treating a variety of conditions,” he said.
The effort to develop the CTS guidelines started February 2013 and involved the Guideline Work Group formulating a set of questions that, as Dr. Peljovich explained, were “the most pertinent questions that anybody interested in a particular diagnosis would want to have answered.” Then a team of statisticians and epidemiologists culled through the “incredible expanse of English language literature” to correlate data to answer those questions.
In May 2015 the work group then met to review the evidence and draft final recommendations. After a period of editing, the draft was submitted for peer review in September. The AAOS board of directors adopted the guidelines in February.
“The guidelines are not intended to be a cookbook on how to treat a condition,” Dr. Peljovich said. “They are really designed to tell you what the best evidence says about a particular set of questions. It helps you to be as updated as you want to be; it’s not designed to tell you this is the only way to do anything. ... It’s an educational tool.”
Members of the Guideline Work Group, AAOS staff, and contributing members submitted their disclosures to the AAOS.
Allegations: Current Trends in Medical Malpractice, Part 2
Most medical malpractice cases are still resolved in a courtroom—typically after years of preparation and personal torment. Yet, overall rates of paid medical malpractice claims among all physicians have been steadily decreasing over the past two decades, with reports showing decreases of 30% to 50% in paid claims since 2000.1-3 At the same time, while median payments and insurance premiums continued to increase until the mid-2000s, they now appear to have plateaued.1
None of these changes occurred in isolation. More than 30 states now have caps on noneconomic or total damages.2 As noted in part 1, since 2000, some states have enacted comprehensive tort reform.4 However, whether these changes in malpractice patterns can be attributed directly to specific policy changes remains a hotly contested issue.
Malpractice Risk in Emergency Medicine
To what extent do the trends in medical malpractice apply to emergency medicine (EM)? While emergency physicians’ (EPs’) perception of malpractice risk ranks higher than any other medical specialty,5 in a review of a large sample of malpractice claims from 1991 through 2005, EPs ranked in the middle among specialties with respect to annual risk of a malpractice claim.6 Moreover, the annual risk of a claim for EPs is just under 8%, compared to 7.4% for all physicians. Yet, for neurosurgery and cardiothoracic surgery—the specialties with the highest overall risk of malpractice claims—the annual risk approaches 20%.6 Regarding payout statistics, less than one-fifth of the claims against EPs resulted in payment.6 In a review of a separate insurance database of closed claims, EPs were named as the primary defendant in only 19% of cases.7
Despite the discrepancies between perceived risk and absolute risk of malpractice claims among EPs, malpractice lawsuits continue to affect the practice of EM. This is evidenced in several surveys, in which the majority of EP participants admitted to practicing “defensive medicine” by ordering tests that were felt to be unnecessary and did so in response to perceived malpractice risk.8-10 Perceived risk also accounts for the significant variation in decision-making in the ED with respect to diagnostic testing and hospitalization of patients.11 One would expect that lowering malpractice risk would result in less so-called unnecessary testing, but whether or not this is truly the case remains to be seen.
Effects of Malpractice Reform
A study by Waxman et al12 on the effects of significant malpractice tort reform in ED care in Texas, Georgia, and South Carolina found no difference in rates of imaging studies, charges, or patient admissions. Furthermore, legislation reform did not increase plaintiff onus to prove proximate “gross negligence” rather than simply a breach from “reasonably skillful and careful” medicine.12 These findings suggest that perception of malpractice risk might simply be serving as a proxy for physicians’ underlying risk tolerance, and be less subject to influence by external forces.
Areas Associated With Malpractice Risk
A number of closed-claim databases attempted to identify the characteristics of patient encounters that can lead to malpractice claims, including patient conditions and sources of error. Diagnostic errors have consistently been found to be the leading cause of malpractice claims, accounting for 28% to 65% of claims, followed by inappropriate management of medical treatment and improper performance of a procedure.7,13-16 A January 2016 benchmarking system report by CRICO Strategies found that 30% of 23,658 medical malpractice claims filed between 2009 through 2013 cited failures in communication as a factor.17 The report also revealed that among these failed communications, those that occurred between health care providers are more likely to result in payout compared to miscommunications between providers and patients.17 This report further noted 70% to 80% of claims closed without payment.7,16 Closed claims were significantly more likely to involve serious injuries or death.7,18 Leading conditions that resulted in claims include myocardial infarction, nonspecific chest pain, symptoms involving the abdomen or pelvis, appendicitis, and orthopedic injuries.7,13,16
Diagnostic Errors
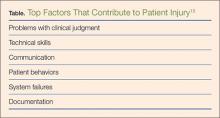
Errors in diagnosis have been attributed to multiple factors in the ED. The two most common factors were failure to order tests and failure to perform an adequate history and physical examination, both of which contribute to rationalization of the practice of defensive medicine under the current tort system.13 Other significant factors associated with errors in diagnosis include misinterpretation of test results or imaging studies and failure to obtain an appropriate consultation. Processes contributing to each of these potential errors include mistakes in judgment, lack of knowledge, miscommunication, and insufficient documentation (Table).15
Strategies for Reducing Malpractice Risk
In part 1, we listed several strategies EPs could adopt to help reduce malpractice risk. In this section, we will discuss in further detail how these strategies help mitigate malpractice claims.
Patient Communication
Open communication with patients is paramount in reducing the risk of a malpractice allegation. Patients are more likely to become angry or frustrated if they sense a physician is not listening to or addressing their concerns. These patients are in turn more likely to file a complaint if they are harmed or experience a bad outcome during their stay in the ED.
Situations in which patients are unable to provide pertinent information also place the EP at significant risk, as the provider must make decisions without full knowledge of the case. Communication with potential resources such as nursing home staff, the patient’s family, and emergency medical service providers to obtain additional information can help reduce risk.
Of course, when evaluating and treating patients, the EP should always take the time to listen to the patient’s concerns during the encounter to ensure his or her needs have been addressed. In the event of a patient allegation or complaint, the EP should make the effort to explore and de-escalate the situation before the patient is discharged.
Discharge Care and Instructions
According to CRICO, premature discharge as a factor in medical malpractice liability results from inadequate assessment and missed opportunities in 41% of diagnosis-related ED cases.16 The following situation illustrates a brief example of such a missed opportunity: A provider makes a diagnosis of urinary tract infection (UTI) in a patient presenting with fever and abdominal pain but whose urinalysis is suspect for contamination and in whom no pelvic examination was performed to rule out other etiologies. When the same patient later returns to the ED with worse abdominal pain, a sterile urine culture invalidates the diagnosis of UTI, and further evaluation leads to a final diagnosis of ruptured appendix.
Prior to discharging any patient, the EP should provide clear and concise at-home care instructions in a manner in which the patient can understand. Clear instructions on how the patient is to manage his or her care after discharge are vital, and failure to do so in terms the patient can understand can create problems if a harmful result occurs. This is especially important in patients with whom there is a communication barrier—eg, language barrier, hearing impairment, cognitive deficit, intoxication, or violent or irrational behavior. In these situations, the EP should always take advantage of available resources and tools such as language lines, interpreters, discharge planners, psychiatric staff, and supportive family members to help reconcile any communication barriers. These measures will in turn optimize patient outcome and reduce the risk of a later malpractice allegation.
Board Certification
All physicians should maintain their respective board certification and specialty training requirements. Efforts in this area help providers to stay up to date in current practice standards and new developments, thus reducing one’s risk of incurring a malpractice claim.
Patient Safety
All members of the care team should engender an environment that is focused on patient safety, including open communication between providers and with nursing staff and technical support teams. Although interruptions can be detrimental to patient care, simply having an understanding of this phenomenon among all staff members can alleviate some of the working stressors in the ED. Effort must be made to create an environment that allows for clarification between nursing staff and physicians without causing undue antagonism. Fostering supportive communication, having a questioning attitude, and seeking clarification can only enhance patient safety.
The importance of the supervisory role of attending physicians to trainees, physician extenders, and nursing staff must be emphasized, and appropriate guidance from the ED attending is germane in keeping patients safe in teaching environments. Additionally, in departments that suffer the burden of high numbers of admitted patient boarders in the ED, attention must be given to the transitional period between decision to admit and termination of ED care and the acquisition of care of the admitting physician. A clear plan of responsibility must be in place for these high-risk situations.
Policies and Procedures
Departmental policies and procedures should be designed to identify and address all late laboratory results data, radiological discrepancies, and culture results in a timely and uniform manner. Since unaddressed results and discrepancies can result in patient harm, patient-callback processes should be designed to reduce risk by addressing these hazards regularly, thoroughly, and in a timely fashion.
Cognitive Biases
An awareness of inherent biases in the medical decision-making process is also helpful to maintain mindfulness in the routine practice of EM and avoid medical errors. The EP should take care not to be influenced by recent events and diagnostic information that is easy to recall or common, and to ensure the differential addresses possibilities beyond the readily available diagnoses. Further, reliance on an existing opinion may be misleading if subsequent judgments are based on this “anchor,” whether it is true or false.
If the data points of the case do not line up as expected, or if there are unexplained outliers, the EP should expand the frame of reference to seek more appropriate possibilities, and avoid attempts to make the data fit a preferred or favored conclusion.
When one fails to recognize that data do not fit the diagnostic presumption, the true diagnosis can be undermined. Such confirmation bias in turn challenges diagnostic success. Hasty judgment without considering and seeking out relevant information can set up diagnostic failure and premature closure.
Remembering the Basics
Finally, providers should follow the basic principles for every patient. Vital signs are vital for a reason, and all abnormal data must be accounted for prior to patient hand off or discharge. Patient turnover is a high-risk occasion, and demands careful attention to case details between the off-going physician, the accepting physician, and the patient.
All patients presenting to the ED for care should leave the ED at their baseline functional level (ie, if they walk independently, they should still walk independently at discharge). If not, the reason should be sought out and clarified with appropriate recommendations for treatment and follow-up.
Patients and staff should always be treated with respect, which in turn will encourage effective communication. Providers should be honest with patients, document truthfully, respect privacy and confidentiality, practice within one’s competence, confirm information, and avoid assumptions. Compassion goes hand in hand with respectful and open communication. Physicians perceived as compassionate and trustworthy are less likely to be the target of a malpractice suit, even when harm has occurred.
Conclusion
Even though the number of paid medical malpractice claims has continued to decrease over the past 20 years, a discrepancy between perceived and absolute risk persists among EPs—one that perpetuates the practice of defensive medicine and continues to affect EM. Despite the current perceptions and climate, EPs can allay their risk of incurring a malpractice claim by employing the strategies outlined above.
1. Mello MM, Studdert DM, Kachalia A. The medical liability climate and prospects for reform. JAMA. 2014;312(20):2146-2155.
2. Paik M, Black B, Hyman DA. The receding tide of medical malpractice: part 1 - national trends. J Empirical Leg Stud. 2013;10(4):612-638.
3. Bishop TF, Ryan AM, Caslino LP. Paid malpractice claims for adverse events in inpatient and outpatient settings. JAMA. 2011;305(23):2427-2431.
4. Kachalia A, Mello MM. New directions in medical liability reform. N Engl J Med. 2011;364(16):
1564-1572.
5. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assured by tort reforms. Health Aff. 2010;29(9):1585-1592.
6. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636.
7. Brown TW, McCarthy ML, Kelen GD, Levy F. An epidemiologic study of closed emergency department malpractice claims in a national database of physician malpractice insurers. Acad Emerg Med. 2010;17(5):553-560.
8. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617.
9. Bishop TF, Federman AD, Keyhani S. Physicians’ views on defensive medicine: a national survey. Arch Intern Med. 2010;170(12):1081-1083.
10. Massachusetts Medical Society. Investigation of defensive medicine in Massachusetts. November 2008. Available at http://www.massmed.org/defensivemedicine. Accessed March 16, 2016.
11. Katz DA, Williams GC, Brown RL, et al. Emergency physicians’ fear of malpractice in evaluating patient with possible acute cardiac ischemia. Ann Emerg Med. 2005;46(6):525-533.
12. Waxman DA, Greenberg MD, Ridgely MS, Kellermann AL, Heaton P. The effect of malpractice reform on emergency department care. N Engl J Med. 2014;371(16):1518-1525.
13. Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
14. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-Year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680.
15. Ross J, Ranum D, Troxel DB. Emergency medicine closed claims study. The Doctors Company. Available at http://www.thedoctors.com/ecm/groups/public/@tdc/@web/@kc/@patientsafety/documents/article/con_id_004776.pdf. Accessed March 16, 2016.
16. Ruoff G, ed. 2011 Annual benchmarking report: malpractice risks in emergency medicine. CRICO strategies. 2012. Available at https://www.rmf.harvard.edu/Strategies/Home/Products-and-Services/Comparative-Data/Annual-Benchmark-Reports. Accessed March 16, 2016.
17. Failures in communication contribute to medical malpractice. January 31, 2016. https://www.rmf.harvard.edu/About-CRICO/Media/Press-Releases/News/2016/February/Failures-in-Communication-Contribute-to-Medical-Malpractice.
18. Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033. Accessed March 16, 2016.
Most medical malpractice cases are still resolved in a courtroom—typically after years of preparation and personal torment. Yet, overall rates of paid medical malpractice claims among all physicians have been steadily decreasing over the past two decades, with reports showing decreases of 30% to 50% in paid claims since 2000.1-3 At the same time, while median payments and insurance premiums continued to increase until the mid-2000s, they now appear to have plateaued.1
None of these changes occurred in isolation. More than 30 states now have caps on noneconomic or total damages.2 As noted in part 1, since 2000, some states have enacted comprehensive tort reform.4 However, whether these changes in malpractice patterns can be attributed directly to specific policy changes remains a hotly contested issue.
Malpractice Risk in Emergency Medicine
To what extent do the trends in medical malpractice apply to emergency medicine (EM)? While emergency physicians’ (EPs’) perception of malpractice risk ranks higher than any other medical specialty,5 in a review of a large sample of malpractice claims from 1991 through 2005, EPs ranked in the middle among specialties with respect to annual risk of a malpractice claim.6 Moreover, the annual risk of a claim for EPs is just under 8%, compared to 7.4% for all physicians. Yet, for neurosurgery and cardiothoracic surgery—the specialties with the highest overall risk of malpractice claims—the annual risk approaches 20%.6 Regarding payout statistics, less than one-fifth of the claims against EPs resulted in payment.6 In a review of a separate insurance database of closed claims, EPs were named as the primary defendant in only 19% of cases.7
Despite the discrepancies between perceived risk and absolute risk of malpractice claims among EPs, malpractice lawsuits continue to affect the practice of EM. This is evidenced in several surveys, in which the majority of EP participants admitted to practicing “defensive medicine” by ordering tests that were felt to be unnecessary and did so in response to perceived malpractice risk.8-10 Perceived risk also accounts for the significant variation in decision-making in the ED with respect to diagnostic testing and hospitalization of patients.11 One would expect that lowering malpractice risk would result in less so-called unnecessary testing, but whether or not this is truly the case remains to be seen.
Effects of Malpractice Reform
A study by Waxman et al12 on the effects of significant malpractice tort reform in ED care in Texas, Georgia, and South Carolina found no difference in rates of imaging studies, charges, or patient admissions. Furthermore, legislation reform did not increase plaintiff onus to prove proximate “gross negligence” rather than simply a breach from “reasonably skillful and careful” medicine.12 These findings suggest that perception of malpractice risk might simply be serving as a proxy for physicians’ underlying risk tolerance, and be less subject to influence by external forces.
Areas Associated With Malpractice Risk
A number of closed-claim databases attempted to identify the characteristics of patient encounters that can lead to malpractice claims, including patient conditions and sources of error. Diagnostic errors have consistently been found to be the leading cause of malpractice claims, accounting for 28% to 65% of claims, followed by inappropriate management of medical treatment and improper performance of a procedure.7,13-16 A January 2016 benchmarking system report by CRICO Strategies found that 30% of 23,658 medical malpractice claims filed between 2009 through 2013 cited failures in communication as a factor.17 The report also revealed that among these failed communications, those that occurred between health care providers are more likely to result in payout compared to miscommunications between providers and patients.17 This report further noted 70% to 80% of claims closed without payment.7,16 Closed claims were significantly more likely to involve serious injuries or death.7,18 Leading conditions that resulted in claims include myocardial infarction, nonspecific chest pain, symptoms involving the abdomen or pelvis, appendicitis, and orthopedic injuries.7,13,16
Diagnostic Errors
Errors in diagnosis have been attributed to multiple factors in the ED. The two most common factors were failure to order tests and failure to perform an adequate history and physical examination, both of which contribute to rationalization of the practice of defensive medicine under the current tort system.13 Other significant factors associated with errors in diagnosis include misinterpretation of test results or imaging studies and failure to obtain an appropriate consultation. Processes contributing to each of these potential errors include mistakes in judgment, lack of knowledge, miscommunication, and insufficient documentation (Table).15
Strategies for Reducing Malpractice Risk
In part 1, we listed several strategies EPs could adopt to help reduce malpractice risk. In this section, we will discuss in further detail how these strategies help mitigate malpractice claims.
Patient Communication
Open communication with patients is paramount in reducing the risk of a malpractice allegation. Patients are more likely to become angry or frustrated if they sense a physician is not listening to or addressing their concerns. These patients are in turn more likely to file a complaint if they are harmed or experience a bad outcome during their stay in the ED.
Situations in which patients are unable to provide pertinent information also place the EP at significant risk, as the provider must make decisions without full knowledge of the case. Communication with potential resources such as nursing home staff, the patient’s family, and emergency medical service providers to obtain additional information can help reduce risk.
Of course, when evaluating and treating patients, the EP should always take the time to listen to the patient’s concerns during the encounter to ensure his or her needs have been addressed. In the event of a patient allegation or complaint, the EP should make the effort to explore and de-escalate the situation before the patient is discharged.
Discharge Care and Instructions
According to CRICO, premature discharge as a factor in medical malpractice liability results from inadequate assessment and missed opportunities in 41% of diagnosis-related ED cases.16 The following situation illustrates a brief example of such a missed opportunity: A provider makes a diagnosis of urinary tract infection (UTI) in a patient presenting with fever and abdominal pain but whose urinalysis is suspect for contamination and in whom no pelvic examination was performed to rule out other etiologies. When the same patient later returns to the ED with worse abdominal pain, a sterile urine culture invalidates the diagnosis of UTI, and further evaluation leads to a final diagnosis of ruptured appendix.
Prior to discharging any patient, the EP should provide clear and concise at-home care instructions in a manner in which the patient can understand. Clear instructions on how the patient is to manage his or her care after discharge are vital, and failure to do so in terms the patient can understand can create problems if a harmful result occurs. This is especially important in patients with whom there is a communication barrier—eg, language barrier, hearing impairment, cognitive deficit, intoxication, or violent or irrational behavior. In these situations, the EP should always take advantage of available resources and tools such as language lines, interpreters, discharge planners, psychiatric staff, and supportive family members to help reconcile any communication barriers. These measures will in turn optimize patient outcome and reduce the risk of a later malpractice allegation.
Board Certification
All physicians should maintain their respective board certification and specialty training requirements. Efforts in this area help providers to stay up to date in current practice standards and new developments, thus reducing one’s risk of incurring a malpractice claim.
Patient Safety
All members of the care team should engender an environment that is focused on patient safety, including open communication between providers and with nursing staff and technical support teams. Although interruptions can be detrimental to patient care, simply having an understanding of this phenomenon among all staff members can alleviate some of the working stressors in the ED. Effort must be made to create an environment that allows for clarification between nursing staff and physicians without causing undue antagonism. Fostering supportive communication, having a questioning attitude, and seeking clarification can only enhance patient safety.
The importance of the supervisory role of attending physicians to trainees, physician extenders, and nursing staff must be emphasized, and appropriate guidance from the ED attending is germane in keeping patients safe in teaching environments. Additionally, in departments that suffer the burden of high numbers of admitted patient boarders in the ED, attention must be given to the transitional period between decision to admit and termination of ED care and the acquisition of care of the admitting physician. A clear plan of responsibility must be in place for these high-risk situations.
Policies and Procedures
Departmental policies and procedures should be designed to identify and address all late laboratory results data, radiological discrepancies, and culture results in a timely and uniform manner. Since unaddressed results and discrepancies can result in patient harm, patient-callback processes should be designed to reduce risk by addressing these hazards regularly, thoroughly, and in a timely fashion.
Cognitive Biases
An awareness of inherent biases in the medical decision-making process is also helpful to maintain mindfulness in the routine practice of EM and avoid medical errors. The EP should take care not to be influenced by recent events and diagnostic information that is easy to recall or common, and to ensure the differential addresses possibilities beyond the readily available diagnoses. Further, reliance on an existing opinion may be misleading if subsequent judgments are based on this “anchor,” whether it is true or false.
If the data points of the case do not line up as expected, or if there are unexplained outliers, the EP should expand the frame of reference to seek more appropriate possibilities, and avoid attempts to make the data fit a preferred or favored conclusion.
When one fails to recognize that data do not fit the diagnostic presumption, the true diagnosis can be undermined. Such confirmation bias in turn challenges diagnostic success. Hasty judgment without considering and seeking out relevant information can set up diagnostic failure and premature closure.
Remembering the Basics
Finally, providers should follow the basic principles for every patient. Vital signs are vital for a reason, and all abnormal data must be accounted for prior to patient hand off or discharge. Patient turnover is a high-risk occasion, and demands careful attention to case details between the off-going physician, the accepting physician, and the patient.
All patients presenting to the ED for care should leave the ED at their baseline functional level (ie, if they walk independently, they should still walk independently at discharge). If not, the reason should be sought out and clarified with appropriate recommendations for treatment and follow-up.
Patients and staff should always be treated with respect, which in turn will encourage effective communication. Providers should be honest with patients, document truthfully, respect privacy and confidentiality, practice within one’s competence, confirm information, and avoid assumptions. Compassion goes hand in hand with respectful and open communication. Physicians perceived as compassionate and trustworthy are less likely to be the target of a malpractice suit, even when harm has occurred.
Conclusion
Even though the number of paid medical malpractice claims has continued to decrease over the past 20 years, a discrepancy between perceived and absolute risk persists among EPs—one that perpetuates the practice of defensive medicine and continues to affect EM. Despite the current perceptions and climate, EPs can allay their risk of incurring a malpractice claim by employing the strategies outlined above.
Most medical malpractice cases are still resolved in a courtroom—typically after years of preparation and personal torment. Yet, overall rates of paid medical malpractice claims among all physicians have been steadily decreasing over the past two decades, with reports showing decreases of 30% to 50% in paid claims since 2000.1-3 At the same time, while median payments and insurance premiums continued to increase until the mid-2000s, they now appear to have plateaued.1
None of these changes occurred in isolation. More than 30 states now have caps on noneconomic or total damages.2 As noted in part 1, since 2000, some states have enacted comprehensive tort reform.4 However, whether these changes in malpractice patterns can be attributed directly to specific policy changes remains a hotly contested issue.
Malpractice Risk in Emergency Medicine
To what extent do the trends in medical malpractice apply to emergency medicine (EM)? While emergency physicians’ (EPs’) perception of malpractice risk ranks higher than any other medical specialty,5 in a review of a large sample of malpractice claims from 1991 through 2005, EPs ranked in the middle among specialties with respect to annual risk of a malpractice claim.6 Moreover, the annual risk of a claim for EPs is just under 8%, compared to 7.4% for all physicians. Yet, for neurosurgery and cardiothoracic surgery—the specialties with the highest overall risk of malpractice claims—the annual risk approaches 20%.6 Regarding payout statistics, less than one-fifth of the claims against EPs resulted in payment.6 In a review of a separate insurance database of closed claims, EPs were named as the primary defendant in only 19% of cases.7
Despite the discrepancies between perceived risk and absolute risk of malpractice claims among EPs, malpractice lawsuits continue to affect the practice of EM. This is evidenced in several surveys, in which the majority of EP participants admitted to practicing “defensive medicine” by ordering tests that were felt to be unnecessary and did so in response to perceived malpractice risk.8-10 Perceived risk also accounts for the significant variation in decision-making in the ED with respect to diagnostic testing and hospitalization of patients.11 One would expect that lowering malpractice risk would result in less so-called unnecessary testing, but whether or not this is truly the case remains to be seen.
Effects of Malpractice Reform
A study by Waxman et al12 on the effects of significant malpractice tort reform in ED care in Texas, Georgia, and South Carolina found no difference in rates of imaging studies, charges, or patient admissions. Furthermore, legislation reform did not increase plaintiff onus to prove proximate “gross negligence” rather than simply a breach from “reasonably skillful and careful” medicine.12 These findings suggest that perception of malpractice risk might simply be serving as a proxy for physicians’ underlying risk tolerance, and be less subject to influence by external forces.
Areas Associated With Malpractice Risk
A number of closed-claim databases attempted to identify the characteristics of patient encounters that can lead to malpractice claims, including patient conditions and sources of error. Diagnostic errors have consistently been found to be the leading cause of malpractice claims, accounting for 28% to 65% of claims, followed by inappropriate management of medical treatment and improper performance of a procedure.7,13-16 A January 2016 benchmarking system report by CRICO Strategies found that 30% of 23,658 medical malpractice claims filed between 2009 through 2013 cited failures in communication as a factor.17 The report also revealed that among these failed communications, those that occurred between health care providers are more likely to result in payout compared to miscommunications between providers and patients.17 This report further noted 70% to 80% of claims closed without payment.7,16 Closed claims were significantly more likely to involve serious injuries or death.7,18 Leading conditions that resulted in claims include myocardial infarction, nonspecific chest pain, symptoms involving the abdomen or pelvis, appendicitis, and orthopedic injuries.7,13,16
Diagnostic Errors
Errors in diagnosis have been attributed to multiple factors in the ED. The two most common factors were failure to order tests and failure to perform an adequate history and physical examination, both of which contribute to rationalization of the practice of defensive medicine under the current tort system.13 Other significant factors associated with errors in diagnosis include misinterpretation of test results or imaging studies and failure to obtain an appropriate consultation. Processes contributing to each of these potential errors include mistakes in judgment, lack of knowledge, miscommunication, and insufficient documentation (Table).15
Strategies for Reducing Malpractice Risk
In part 1, we listed several strategies EPs could adopt to help reduce malpractice risk. In this section, we will discuss in further detail how these strategies help mitigate malpractice claims.
Patient Communication
Open communication with patients is paramount in reducing the risk of a malpractice allegation. Patients are more likely to become angry or frustrated if they sense a physician is not listening to or addressing their concerns. These patients are in turn more likely to file a complaint if they are harmed or experience a bad outcome during their stay in the ED.
Situations in which patients are unable to provide pertinent information also place the EP at significant risk, as the provider must make decisions without full knowledge of the case. Communication with potential resources such as nursing home staff, the patient’s family, and emergency medical service providers to obtain additional information can help reduce risk.
Of course, when evaluating and treating patients, the EP should always take the time to listen to the patient’s concerns during the encounter to ensure his or her needs have been addressed. In the event of a patient allegation or complaint, the EP should make the effort to explore and de-escalate the situation before the patient is discharged.
Discharge Care and Instructions
According to CRICO, premature discharge as a factor in medical malpractice liability results from inadequate assessment and missed opportunities in 41% of diagnosis-related ED cases.16 The following situation illustrates a brief example of such a missed opportunity: A provider makes a diagnosis of urinary tract infection (UTI) in a patient presenting with fever and abdominal pain but whose urinalysis is suspect for contamination and in whom no pelvic examination was performed to rule out other etiologies. When the same patient later returns to the ED with worse abdominal pain, a sterile urine culture invalidates the diagnosis of UTI, and further evaluation leads to a final diagnosis of ruptured appendix.
Prior to discharging any patient, the EP should provide clear and concise at-home care instructions in a manner in which the patient can understand. Clear instructions on how the patient is to manage his or her care after discharge are vital, and failure to do so in terms the patient can understand can create problems if a harmful result occurs. This is especially important in patients with whom there is a communication barrier—eg, language barrier, hearing impairment, cognitive deficit, intoxication, or violent or irrational behavior. In these situations, the EP should always take advantage of available resources and tools such as language lines, interpreters, discharge planners, psychiatric staff, and supportive family members to help reconcile any communication barriers. These measures will in turn optimize patient outcome and reduce the risk of a later malpractice allegation.
Board Certification
All physicians should maintain their respective board certification and specialty training requirements. Efforts in this area help providers to stay up to date in current practice standards and new developments, thus reducing one’s risk of incurring a malpractice claim.
Patient Safety
All members of the care team should engender an environment that is focused on patient safety, including open communication between providers and with nursing staff and technical support teams. Although interruptions can be detrimental to patient care, simply having an understanding of this phenomenon among all staff members can alleviate some of the working stressors in the ED. Effort must be made to create an environment that allows for clarification between nursing staff and physicians without causing undue antagonism. Fostering supportive communication, having a questioning attitude, and seeking clarification can only enhance patient safety.
The importance of the supervisory role of attending physicians to trainees, physician extenders, and nursing staff must be emphasized, and appropriate guidance from the ED attending is germane in keeping patients safe in teaching environments. Additionally, in departments that suffer the burden of high numbers of admitted patient boarders in the ED, attention must be given to the transitional period between decision to admit and termination of ED care and the acquisition of care of the admitting physician. A clear plan of responsibility must be in place for these high-risk situations.
Policies and Procedures
Departmental policies and procedures should be designed to identify and address all late laboratory results data, radiological discrepancies, and culture results in a timely and uniform manner. Since unaddressed results and discrepancies can result in patient harm, patient-callback processes should be designed to reduce risk by addressing these hazards regularly, thoroughly, and in a timely fashion.
Cognitive Biases
An awareness of inherent biases in the medical decision-making process is also helpful to maintain mindfulness in the routine practice of EM and avoid medical errors. The EP should take care not to be influenced by recent events and diagnostic information that is easy to recall or common, and to ensure the differential addresses possibilities beyond the readily available diagnoses. Further, reliance on an existing opinion may be misleading if subsequent judgments are based on this “anchor,” whether it is true or false.
If the data points of the case do not line up as expected, or if there are unexplained outliers, the EP should expand the frame of reference to seek more appropriate possibilities, and avoid attempts to make the data fit a preferred or favored conclusion.
When one fails to recognize that data do not fit the diagnostic presumption, the true diagnosis can be undermined. Such confirmation bias in turn challenges diagnostic success. Hasty judgment without considering and seeking out relevant information can set up diagnostic failure and premature closure.
Remembering the Basics
Finally, providers should follow the basic principles for every patient. Vital signs are vital for a reason, and all abnormal data must be accounted for prior to patient hand off or discharge. Patient turnover is a high-risk occasion, and demands careful attention to case details between the off-going physician, the accepting physician, and the patient.
All patients presenting to the ED for care should leave the ED at their baseline functional level (ie, if they walk independently, they should still walk independently at discharge). If not, the reason should be sought out and clarified with appropriate recommendations for treatment and follow-up.
Patients and staff should always be treated with respect, which in turn will encourage effective communication. Providers should be honest with patients, document truthfully, respect privacy and confidentiality, practice within one’s competence, confirm information, and avoid assumptions. Compassion goes hand in hand with respectful and open communication. Physicians perceived as compassionate and trustworthy are less likely to be the target of a malpractice suit, even when harm has occurred.
Conclusion
Even though the number of paid medical malpractice claims has continued to decrease over the past 20 years, a discrepancy between perceived and absolute risk persists among EPs—one that perpetuates the practice of defensive medicine and continues to affect EM. Despite the current perceptions and climate, EPs can allay their risk of incurring a malpractice claim by employing the strategies outlined above.
1. Mello MM, Studdert DM, Kachalia A. The medical liability climate and prospects for reform. JAMA. 2014;312(20):2146-2155.
2. Paik M, Black B, Hyman DA. The receding tide of medical malpractice: part 1 - national trends. J Empirical Leg Stud. 2013;10(4):612-638.
3. Bishop TF, Ryan AM, Caslino LP. Paid malpractice claims for adverse events in inpatient and outpatient settings. JAMA. 2011;305(23):2427-2431.
4. Kachalia A, Mello MM. New directions in medical liability reform. N Engl J Med. 2011;364(16):
1564-1572.
5. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assured by tort reforms. Health Aff. 2010;29(9):1585-1592.
6. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636.
7. Brown TW, McCarthy ML, Kelen GD, Levy F. An epidemiologic study of closed emergency department malpractice claims in a national database of physician malpractice insurers. Acad Emerg Med. 2010;17(5):553-560.
8. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617.
9. Bishop TF, Federman AD, Keyhani S. Physicians’ views on defensive medicine: a national survey. Arch Intern Med. 2010;170(12):1081-1083.
10. Massachusetts Medical Society. Investigation of defensive medicine in Massachusetts. November 2008. Available at http://www.massmed.org/defensivemedicine. Accessed March 16, 2016.
11. Katz DA, Williams GC, Brown RL, et al. Emergency physicians’ fear of malpractice in evaluating patient with possible acute cardiac ischemia. Ann Emerg Med. 2005;46(6):525-533.
12. Waxman DA, Greenberg MD, Ridgely MS, Kellermann AL, Heaton P. The effect of malpractice reform on emergency department care. N Engl J Med. 2014;371(16):1518-1525.
13. Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
14. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-Year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680.
15. Ross J, Ranum D, Troxel DB. Emergency medicine closed claims study. The Doctors Company. Available at http://www.thedoctors.com/ecm/groups/public/@tdc/@web/@kc/@patientsafety/documents/article/con_id_004776.pdf. Accessed March 16, 2016.
16. Ruoff G, ed. 2011 Annual benchmarking report: malpractice risks in emergency medicine. CRICO strategies. 2012. Available at https://www.rmf.harvard.edu/Strategies/Home/Products-and-Services/Comparative-Data/Annual-Benchmark-Reports. Accessed March 16, 2016.
17. Failures in communication contribute to medical malpractice. January 31, 2016. https://www.rmf.harvard.edu/About-CRICO/Media/Press-Releases/News/2016/February/Failures-in-Communication-Contribute-to-Medical-Malpractice.
18. Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033. Accessed March 16, 2016.
1. Mello MM, Studdert DM, Kachalia A. The medical liability climate and prospects for reform. JAMA. 2014;312(20):2146-2155.
2. Paik M, Black B, Hyman DA. The receding tide of medical malpractice: part 1 - national trends. J Empirical Leg Stud. 2013;10(4):612-638.
3. Bishop TF, Ryan AM, Caslino LP. Paid malpractice claims for adverse events in inpatient and outpatient settings. JAMA. 2011;305(23):2427-2431.
4. Kachalia A, Mello MM. New directions in medical liability reform. N Engl J Med. 2011;364(16):
1564-1572.
5. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assured by tort reforms. Health Aff. 2010;29(9):1585-1592.
6. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636.
7. Brown TW, McCarthy ML, Kelen GD, Levy F. An epidemiologic study of closed emergency department malpractice claims in a national database of physician malpractice insurers. Acad Emerg Med. 2010;17(5):553-560.
8. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617.
9. Bishop TF, Federman AD, Keyhani S. Physicians’ views on defensive medicine: a national survey. Arch Intern Med. 2010;170(12):1081-1083.
10. Massachusetts Medical Society. Investigation of defensive medicine in Massachusetts. November 2008. Available at http://www.massmed.org/defensivemedicine. Accessed March 16, 2016.
11. Katz DA, Williams GC, Brown RL, et al. Emergency physicians’ fear of malpractice in evaluating patient with possible acute cardiac ischemia. Ann Emerg Med. 2005;46(6):525-533.
12. Waxman DA, Greenberg MD, Ridgely MS, Kellermann AL, Heaton P. The effect of malpractice reform on emergency department care. N Engl J Med. 2014;371(16):1518-1525.
13. Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
14. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-Year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680.
15. Ross J, Ranum D, Troxel DB. Emergency medicine closed claims study. The Doctors Company. Available at http://www.thedoctors.com/ecm/groups/public/@tdc/@web/@kc/@patientsafety/documents/article/con_id_004776.pdf. Accessed March 16, 2016.
16. Ruoff G, ed. 2011 Annual benchmarking report: malpractice risks in emergency medicine. CRICO strategies. 2012. Available at https://www.rmf.harvard.edu/Strategies/Home/Products-and-Services/Comparative-Data/Annual-Benchmark-Reports. Accessed March 16, 2016.
17. Failures in communication contribute to medical malpractice. January 31, 2016. https://www.rmf.harvard.edu/About-CRICO/Media/Press-Releases/News/2016/February/Failures-in-Communication-Contribute-to-Medical-Malpractice.
18. Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033. Accessed March 16, 2016.
The Changing Standard of Care for Spinal Immobilization
Prehospital spinal immobilization has long been the standard of care (SOC) to prevent spinal cord injury in trauma patients, but utilizing the best data currently available, some professional societies recently released new recommendations that question this practice. Guidelines released in 2014 from the National Association of EMS Physicians (NAEMSP) and the American College of Surgeons Committee on Trauma (ACS-COT) support limited application of spinal immobilization.1 These guidelines note, “Given the rarity of unstable spinal injuries in EMS trauma patients, the number that might benefit from immobilization to prevent secondary injury is likely extremely small. For each patient who has potential benefit, hundreds to thousands of patients must undergo immobilization with no potential benefit.” Further, they advise “utilization of backboards for spinal immobilization during transport should be judicious, so that potential benefits outweigh risks.”1 Spinal immobilization should not be used at all in patients with penetrating trauma who do not present with obvious neurological injury and should be selective, based on objective findings of injury or the high potential for same.1
Questioning a Long-standing Practice
Fear of the consequences of spinal cord injury from significant vertebral fractures has dictated prehospital spinal immobilization to manage injured trauma patients for decades. For almost 50 years, it has been the SOC. However, increasing evidence that spinal immobilization is not only unnecessary, but may even cause harm has resulted in questioning this paradigm, which has lead to promoting a change in the SOC.
Spinal immobilization dates back to the mid-1960s, when Geisler et al2 reported on a cohort of patients who suffered long-term paralysis from what was believed to be improper handling and failure to discover spinal injuries. Soon after, Farrington3,4 developed and published a systematic approach to spinal immobilization during extrication following blunt force trauma, supporting the widespread acceptance of backboards and cervical collars to immobilize the spine in injured trauma patients. Logic dictated that an unstable spine fracture could be worsened, or a cord injury could result, by unnecessary movement during extrication, transport, and initial evaluation in the ED, resulting in avoidable injury. This fear of potential secondary injury grew as more papers were published examining the link between prehospital handling of blunt force trauma patients and delayed paralysis. This resulted in the use of spinal immobilization on the majority of trauma patients, regardless of mechanism of injury or presenting symptoms.5,6
One review estimated that over 50% of trauma patients with no complaint of neck or back pain were transported with full spinal immobilization.7 This immobilization on uncomfortable long backboards typically continued in the ED for prolonged periods, until the spine could be cleared by physical examination and/or imaging studies. Yet a 2001 Cochrane review found that despite increasing use of spinal immobilization, no prospective, randomized controlled trial of the appropriate use of spinal immobilization or patient outcomes had ever been conducted.8
What the Evidence Says
How much evidence exists that supports the benefits of spinal immobilization? Not much. Studies on healthy volunteers and cadavers evaluating spinal motion with immobilization have been contradictory.9 One study found there was less motion with a cervical collar in place than without,10 whereas others found that the use of a cervical collar did not effectively reduce motion in an unstable spine.11,12 Perry et al13 studied the effectiveness of different head immobilization techniques and found that none could eliminate head and neck motion during emergency medical services (EMS) transport. Still other reports, including two biomechanical studies, demonstrated increased neck motion when using conventional extrication techniques (cervical collar with backboard) versus controlled self-extrication with cervical collar only.14,15
An Abundance of Literature on the Risks
Whereas data regarding the actual benefits of spinal immobilization is lacking, an abundance of literature details the risks. One of the most frequently cited studies is also one of the most controversial. Hauswald et al16 compared the outcomes of two groups of patients with blunt force trauma who were either immobilized during transport (in New Mexico) or non-immobilized (in Malaysia) and found that the risk of disability was higher in the immobilized group (odds ratio, 2.03). Although these environments are very different, the authors noted that mechanism of injury, resources, and the size of the hospitals were similar.16
Studies of spinal immobilization in patients with penetrating trauma report even worse outcomes. In separate studies, Haut et al17 and Vanderlan et al18 demonstrated increased mortality when immobilization led to increased transport times and interference with other resuscitative measures. These and other studies have led the American College of Emergency Physicians, NAEMSP, ACS-COT, the Prehospital Trauma Life Support Executive Committee, and other national organizations to recommend no spinal immobilization in patients with penetrating neck trauma.1,19,20
Many trauma patients arrive with complaints of pain at one or more sites. Some of these complaints, particularly back pain, may be secondary to the use of the backboard itself, especially in cases of prolonged transport.21,22 In a study of healthy volunteers who were immobilized on a backboard for 30 minutes, all of them reported pain, along with headaches, most often involving the occipital and sacral regions.23 A 1996 study compared spinal immobilization utilizing a backboard versus a vacuum mattress in 37 healthy volunteers with no history of back pain or spinal disease.24 Compared to those immobilized with the vacuum mattress, patients immobilized with a backboard for 30 minutes were 3.1 times more likely to have symptoms, 7.9 times more likely to complain of occipital pain, and 4.3 times more likely to have lumbosacral pain.24
Increased pain complaints in the setting of trauma can result in increased imaging, leading to increased costs and unnecessary radiation exposure.25 Prolonged backboard times can also result in sacral pressure ulcers.26 A recent study has shown that patients who undergo computed tomography (CT) scans with automatic tube current modulation (as most modern multidetector row CT systems utilize) while on a backboard may be exposed to a significant increase in radiation dose.27
Spinal immobilization has also been linked to respiratory compromise, particularly with the use of straps across the chest, even when not applied tightly. One study found worse lung function test results in healthy immobilized volunteers.28 Other studies have shown that older patients (even when healthy) and those with lung or chest injury have an even larger degree of restriction and respiratory compromise.29,30
Risks from immobilization are not isolated to backboards. The use of cervical collars alone also carries potential risks. (See “What About Cervical Collars?”8,31-39)
Risk of Secondary Neurological Deterioration Is Low
Many EMS systems have already adopted the new standards calling for less use of spinal immobilization. Though the evidence is compelling, not all EMS systems have adopted these standards due to strongly rooted beliefs and fears of long-term patient disability and subsequent litigation. However, these fears do not appear justified.
A recent review by Oto et al40 found only 42 cases of early secondary neurological deterioration after blunt trauma in all of the indexed medical literature. They noted, “In twelve cases the authors did attribute deterioration to temporally associated precipitants, seven of which were possibly iatrogenic; these included removal of a cervical collar, placement of a halo device, patient agitation, performance of flexion/extension films, ‘unintentional manipulation,’ falling in or near the ED, and forced collar application in patients with ankylosing spondylitis.” Thirteen of these cases occurred during prehospital care, none of them sudden and movement-provoked, and all reported by a single study.” This review highlights the rarity of secondary deterioration.
When Should Immobilization Be Used?
So what’s the next step for spinal immobilization in the field? How do we appropriately protect trauma patients during transport? As always seems to be the case in medicine, more evidence is needed. Oteir et al41 recently published a review of new literature on the epidemiology and current practice of prehospital spine management. They reported that early (8-24 hours) transfer of patients with spinal injury to spinal care units, along with effective resuscitation, was the most important determinant of better neurological outcomes.41 This review reaffirms the need for more data evaluating the relationship between spinal immobilization and neurological outcomes.
Currently, recommendations call for selective spinal immobilization to decrease unnecessary application and potential harm. Use of backboards for spinal immobilization should be limited to the following types of patients:1,20
- Blunt trauma and altered level of consciousness;
- Spinal pain or tenderness;
- Neurological complaint (eg, numbness or motor weakness);
- Anatomic deformity of the spine;
- High-energy mechanism of injury and:
- Drug or alcohol intoxication;
- Inability to communicate; and/or
- Distracting injury.
Patients for whom immobilization on a backboard is not necessary include those with all of the following:
- Normal level of consciousness (GCS 15);
- No spine tenderness or anatomic abnormality;
- No neurological findings or complaints;
- No distracting injury;
- No intoxication.
Cervical collars alone are still recommended for use in patients who do not meet validated clinical rules, such as the NEXUS or Canadian C spine rules.1,20,42,43 As these rules are well validated, they can be safely used to determine who should have a cervical collar placed, with or without a backboard. In a retrospective review, selective spinal immobilization was found to be 99% sensitive in identifying patients with cervical injuries.44
Clearly, there is still work to be done. Due to the relative rarity of actual spinal cord injury with the consequences of neurological injury, prospective trials in this area are rare and very difficult to safely design. However, there is growing confidence that selective spinal protocols, together with the inclusion of validated clinical rules, can effectively limit exposure to unnecessary spinal immobilization. As the current evidence continues to mount for the potential harm in indiscriminate backboard and cervical collar use, it seems clear we should strive to decrease the overuse of prehospital and early spinal immobilization consistent with current position statements and validated clinical rules.
1. White CC, Domeier RM, Millin MG. EMS spinal precautions and the use of the long backboard - resource document to the position statement of the National Association of EMS Physicians and the American College of Surgeons Committee on Trauma. Prehosp Emerg Care. 2014;18(2):306-314.
2. Geisler WO, Wynne-Jones M, Jousse AT. Early management of patients with trauma to the spinal cord. Med Serv J Can. 1966;22(7):512–523.
3. Farrington JD. Death in a ditch. Bulletin of the American College of Surgeons. 1967;52(3):121-130.
4. Farrington JD. Extrication of victims- surgical principles. J Trauma. 1968;8(4):493-512.
5. Riggins RS, Kraus JF. The risk of neurologic damage with fractures of the vertebrae. J Trauma. 1977;17(2):126-133.
6. Soderstrom CA, Brumback RJ. Early care of the patient with cervical spine injury. Orthop Clin North Am. 1986;17(1):3-13.
7. McHugh TP, Taylor JP. Unnecessary out-of-hospital use of full spinal immobilization. Acad Emerg Med. 1998;5(3):278-280.
8. Kwan I, Bunn F, Roberts I. Spinal immobilisation for trauma patients. Cochrane Database Syst Rev. 2001;(2):CD002803.
9. Sundstrøm T, Asbjørnsen H, Habiba S, Sunde GA, Wester K. Prehospital use of cervical collars in trauma patients: a critical review. J Neurotrauma. 2014;31(6):531-540.
10. Conrad BP, Rechtine G, Weight M, Clarke J, Horodyski M. Motion in the unstable cervical spine during hospital bed transfers. J Trauma. 2010;69,432-436.
11. Horodyski M, DiPaola CP, Conrad BP, Rechtine GR. Cervical collars are insufficient for immobilizing an unstable cervical spine injury. J Emerg Med. 2011;41(5):513-519.
12. Hughes SJ. How effective is the Newport/Aspen collar? A prospective radiographic evaluation in healthy adult volunteers. J Trauma. 1998;45(2):374-378.
13. Perry SD, McLellan B, McIlroy WE, Maki BE, Schwartz M, Fernie GR. The efficacy of head immobilization techniques during simulated vehicle motion. Spine (Phil Pa 1976). 1999;24(17):1839-1844.
14. Engsberg JR, Standeven JW, Shurtleff TL, Eggars JL, Shafer JS, Naunheim RS. Cervical spine motion during extrication. J Emerg Med. 2013;44(1):122-127.
15. Dixon M, O’Halloran J, Cummins NM. Biomechanical analysis of spinal immobilization during prehospital extrication—a proof of concept study. Emerg Med J. 2014;31(9):745-749.
16. Hauswald M, Ong G, Tandberg D, Omar Z. Out-of-hospital spinal immobilization: its effect on neurologic injury. Acad Emerg Med. 1998;5(3):214-219.
17. Haut ER, Kalish BT, Efron DT, et al. Spine immobilization in penetrating trauma: more harm than good? J Trauma. 2010;68(1):115-120.
18. Vanderlan WB, Tew BE, McSwain NE. Increased risk of death with cervical spine immobilization in penetrating cervical trauma. Injury. 2009;40(8):880-883.
19. Stuke LE, Pons PT, Guy JS, Chapleau WP, Butler FK, McSwain NE. Prehospital spine immobilization for penetrating trauma—review and recommendations from the Prehospital Trauma Life Support Executive Committee. J Trauma. 2011;71(3):763–769.
20. American College of Emergency Physicians. Policy Statement- EMS Management of Patients with Potential Spinal Injury. 2015. Available at: http://www.acep.org/Physician-Resources/Policies/Policy-Statements/EMS-Management-of-Patients-with-Potential-Spinal-Injury. Accessed February 9, 2016.
21. Barney RN, Cordell WH, Miller E. Pain associated with immobilization on rigid spine boards. Ann Emerg Med. 1989;18:918.
22. Cooney DR, Wallus H, Asaly M, Wojcik S. Backboard time for patients receiving spinal immobilization by emergency medical services. Int J Emerg Med. 2013;6(1):17.
23. Chan D, Goldberg R, Tascone A, Harmon S, Chan L. The effect of spinal immobilization on healthy volunteers. Ann Emerg Med. 1994;23(1):48-51.
24. Chan D, Goldberg RM, Mason J, Chan L. Backboard versus mattress splint immobilization: a comparison of symptoms generated. J Emerg Med, 1996;14(3):293-298.
25. March J, Ausband S, Brown L. Changes in physical examination caused by use of spinal immobilization. Prehosp Emerg Care. 2002;6(4):421-424.
26. Berg G, Nyberg S, Harrison P, Baumchen J, Gurss E, Hennes E. Near-infrared spectroscopy measurement of sacral tissue oxygen saturation in healthy volunteers immobilized on rigid spine boards. Prehosp Emerg Care. 2010;14(4):419-424.
27. Lee AY, Elojeimy S, Kanal KM, Gunn ML. The effect of trauma backboards on computed tomography radiation dose. Clin Radiol. 2016. Epub ahead of print.
28. Bauer D, Kowalski R. Effect of spinal immobilization devices on pulmonary function in the healthy, nonsmoking man. Ann Emerg Med. 1988;17(9):915-918.
29. Walsh M, Grant T, Mickey S. Lung function compromised by spinal immobilization. Ann Emerg Med. 1990;19(5):615-616.
30. Totten VY, Sugarman DB. Respiratory effects of spinal immobilization. Prehosp Emerg Care. 1999; 3(4):347-352.
31. Goutcher CM, Lochhead V. Reduction in mouth opening with semi-rigid cervical collars. Br J Anaesth. 2005;95(3):344-348.
32. Davies G, Deakin C, Wilson A. The effect of a rigid collar on intracranial pressure. Injury. 1996;27(9):647-649.
33. Dunham CM, Brocker BP, Collier BD, Gemmel DJ. Risks associated with magnetic resonance imaging and cervical collar in comatose, blunt trauma patients with negative comprehensive cervical spine computed tomography and no apparent spinal deficit. Crit Care. 2008;12(4):R89.
34. Mobbs RJ, Stoodley MA, Fuller J. Effect of cervical hard collar on intracranial pressure after head injury. ANZ J Surg. 2002;72(6):389-391.
35. Stone MB, Tubridy CM, Curran R. The effect of rigid cervical collars on internal jugular vein dimensions. Acad Emerg Med. 2010;17(1):100-102.
36. Ben-Galim P, Dreiangel N, Mattox KL, Reitman CA, Kalantar SB, Hipp JA. Extrication collars can result in abnormal separation between vertebrae in the presence of a dissociative injury. J Trauma. 2010;69(2):447-450.
37. Podolsky SM, Hoffman JR, Pietrafesa CA. Neurologic complications following immobilization of cervical spine fracture in a patient with ankylosing spondylitis. Ann Emerg Med. 1983;12(9):578-580.
38. Papadopoulos MC, Chakraborty A, Waldron G, Bell BA. Exacerbating cervical spine injury by applying a hard collar. BMJ. 1999;319(7203):171-172.
39. Thumbikat P, Hariharan RP, Ravichandran G, Mcclelland MR, Mathew KM. Spinal cord injury in patients with ankylosing spondylitis: a 10-year review. Spine (Phila Pa 1976). 2007;32(26):2989-2995.
40. Oto B, Corey DJ, Oswald J, Sifford D, Walsh B. Early secondary neurologic deterioration after blunt spinal trauma: a review of the literature. Acad Emerg Med. 2015;22(10):1200-1212.
41. Oteir AO, Smith K, Jennings PA, Stoelwinder JU. The prehospital management of suspected spinal cord injury: an update. Prehosp Disaster Med. 2014;29(4):399-402.
42. Hoffman JR, Mower WR, Wolfson AB, Todd KH, Zucker MI. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. National Emergency X-Radiography Utilization Study Group. N Engl J Med. 2000;343(2):94-99.
43. Stiell IG, Wells GA, Vandemheen KL, et al. The Canadian C-spine rule for radiography in alert and stable trauma patients. JAMA. 2001;286(15):1841–1848.
44. Stroh G, Braude D. Can an out-of-hospital cervical spine clearance protocol identify all patients with injuries? An argument for selective immobilization. Ann Emerg Med. 2001;37(6):609-615.
Prehospital spinal immobilization has long been the standard of care (SOC) to prevent spinal cord injury in trauma patients, but utilizing the best data currently available, some professional societies recently released new recommendations that question this practice. Guidelines released in 2014 from the National Association of EMS Physicians (NAEMSP) and the American College of Surgeons Committee on Trauma (ACS-COT) support limited application of spinal immobilization.1 These guidelines note, “Given the rarity of unstable spinal injuries in EMS trauma patients, the number that might benefit from immobilization to prevent secondary injury is likely extremely small. For each patient who has potential benefit, hundreds to thousands of patients must undergo immobilization with no potential benefit.” Further, they advise “utilization of backboards for spinal immobilization during transport should be judicious, so that potential benefits outweigh risks.”1 Spinal immobilization should not be used at all in patients with penetrating trauma who do not present with obvious neurological injury and should be selective, based on objective findings of injury or the high potential for same.1
Questioning a Long-standing Practice
Fear of the consequences of spinal cord injury from significant vertebral fractures has dictated prehospital spinal immobilization to manage injured trauma patients for decades. For almost 50 years, it has been the SOC. However, increasing evidence that spinal immobilization is not only unnecessary, but may even cause harm has resulted in questioning this paradigm, which has lead to promoting a change in the SOC.
Spinal immobilization dates back to the mid-1960s, when Geisler et al2 reported on a cohort of patients who suffered long-term paralysis from what was believed to be improper handling and failure to discover spinal injuries. Soon after, Farrington3,4 developed and published a systematic approach to spinal immobilization during extrication following blunt force trauma, supporting the widespread acceptance of backboards and cervical collars to immobilize the spine in injured trauma patients. Logic dictated that an unstable spine fracture could be worsened, or a cord injury could result, by unnecessary movement during extrication, transport, and initial evaluation in the ED, resulting in avoidable injury. This fear of potential secondary injury grew as more papers were published examining the link between prehospital handling of blunt force trauma patients and delayed paralysis. This resulted in the use of spinal immobilization on the majority of trauma patients, regardless of mechanism of injury or presenting symptoms.5,6
One review estimated that over 50% of trauma patients with no complaint of neck or back pain were transported with full spinal immobilization.7 This immobilization on uncomfortable long backboards typically continued in the ED for prolonged periods, until the spine could be cleared by physical examination and/or imaging studies. Yet a 2001 Cochrane review found that despite increasing use of spinal immobilization, no prospective, randomized controlled trial of the appropriate use of spinal immobilization or patient outcomes had ever been conducted.8
What the Evidence Says
How much evidence exists that supports the benefits of spinal immobilization? Not much. Studies on healthy volunteers and cadavers evaluating spinal motion with immobilization have been contradictory.9 One study found there was less motion with a cervical collar in place than without,10 whereas others found that the use of a cervical collar did not effectively reduce motion in an unstable spine.11,12 Perry et al13 studied the effectiveness of different head immobilization techniques and found that none could eliminate head and neck motion during emergency medical services (EMS) transport. Still other reports, including two biomechanical studies, demonstrated increased neck motion when using conventional extrication techniques (cervical collar with backboard) versus controlled self-extrication with cervical collar only.14,15
An Abundance of Literature on the Risks
Whereas data regarding the actual benefits of spinal immobilization is lacking, an abundance of literature details the risks. One of the most frequently cited studies is also one of the most controversial. Hauswald et al16 compared the outcomes of two groups of patients with blunt force trauma who were either immobilized during transport (in New Mexico) or non-immobilized (in Malaysia) and found that the risk of disability was higher in the immobilized group (odds ratio, 2.03). Although these environments are very different, the authors noted that mechanism of injury, resources, and the size of the hospitals were similar.16
Studies of spinal immobilization in patients with penetrating trauma report even worse outcomes. In separate studies, Haut et al17 and Vanderlan et al18 demonstrated increased mortality when immobilization led to increased transport times and interference with other resuscitative measures. These and other studies have led the American College of Emergency Physicians, NAEMSP, ACS-COT, the Prehospital Trauma Life Support Executive Committee, and other national organizations to recommend no spinal immobilization in patients with penetrating neck trauma.1,19,20
Many trauma patients arrive with complaints of pain at one or more sites. Some of these complaints, particularly back pain, may be secondary to the use of the backboard itself, especially in cases of prolonged transport.21,22 In a study of healthy volunteers who were immobilized on a backboard for 30 minutes, all of them reported pain, along with headaches, most often involving the occipital and sacral regions.23 A 1996 study compared spinal immobilization utilizing a backboard versus a vacuum mattress in 37 healthy volunteers with no history of back pain or spinal disease.24 Compared to those immobilized with the vacuum mattress, patients immobilized with a backboard for 30 minutes were 3.1 times more likely to have symptoms, 7.9 times more likely to complain of occipital pain, and 4.3 times more likely to have lumbosacral pain.24
Increased pain complaints in the setting of trauma can result in increased imaging, leading to increased costs and unnecessary radiation exposure.25 Prolonged backboard times can also result in sacral pressure ulcers.26 A recent study has shown that patients who undergo computed tomography (CT) scans with automatic tube current modulation (as most modern multidetector row CT systems utilize) while on a backboard may be exposed to a significant increase in radiation dose.27
Spinal immobilization has also been linked to respiratory compromise, particularly with the use of straps across the chest, even when not applied tightly. One study found worse lung function test results in healthy immobilized volunteers.28 Other studies have shown that older patients (even when healthy) and those with lung or chest injury have an even larger degree of restriction and respiratory compromise.29,30
Risks from immobilization are not isolated to backboards. The use of cervical collars alone also carries potential risks. (See “What About Cervical Collars?”8,31-39)
Risk of Secondary Neurological Deterioration Is Low
Many EMS systems have already adopted the new standards calling for less use of spinal immobilization. Though the evidence is compelling, not all EMS systems have adopted these standards due to strongly rooted beliefs and fears of long-term patient disability and subsequent litigation. However, these fears do not appear justified.
A recent review by Oto et al40 found only 42 cases of early secondary neurological deterioration after blunt trauma in all of the indexed medical literature. They noted, “In twelve cases the authors did attribute deterioration to temporally associated precipitants, seven of which were possibly iatrogenic; these included removal of a cervical collar, placement of a halo device, patient agitation, performance of flexion/extension films, ‘unintentional manipulation,’ falling in or near the ED, and forced collar application in patients with ankylosing spondylitis.” Thirteen of these cases occurred during prehospital care, none of them sudden and movement-provoked, and all reported by a single study.” This review highlights the rarity of secondary deterioration.
When Should Immobilization Be Used?
So what’s the next step for spinal immobilization in the field? How do we appropriately protect trauma patients during transport? As always seems to be the case in medicine, more evidence is needed. Oteir et al41 recently published a review of new literature on the epidemiology and current practice of prehospital spine management. They reported that early (8-24 hours) transfer of patients with spinal injury to spinal care units, along with effective resuscitation, was the most important determinant of better neurological outcomes.41 This review reaffirms the need for more data evaluating the relationship between spinal immobilization and neurological outcomes.
Currently, recommendations call for selective spinal immobilization to decrease unnecessary application and potential harm. Use of backboards for spinal immobilization should be limited to the following types of patients:1,20
- Blunt trauma and altered level of consciousness;
- Spinal pain or tenderness;
- Neurological complaint (eg, numbness or motor weakness);
- Anatomic deformity of the spine;
- High-energy mechanism of injury and:
- Drug or alcohol intoxication;
- Inability to communicate; and/or
- Distracting injury.
Patients for whom immobilization on a backboard is not necessary include those with all of the following:
- Normal level of consciousness (GCS 15);
- No spine tenderness or anatomic abnormality;
- No neurological findings or complaints;
- No distracting injury;
- No intoxication.
Cervical collars alone are still recommended for use in patients who do not meet validated clinical rules, such as the NEXUS or Canadian C spine rules.1,20,42,43 As these rules are well validated, they can be safely used to determine who should have a cervical collar placed, with or without a backboard. In a retrospective review, selective spinal immobilization was found to be 99% sensitive in identifying patients with cervical injuries.44
Clearly, there is still work to be done. Due to the relative rarity of actual spinal cord injury with the consequences of neurological injury, prospective trials in this area are rare and very difficult to safely design. However, there is growing confidence that selective spinal protocols, together with the inclusion of validated clinical rules, can effectively limit exposure to unnecessary spinal immobilization. As the current evidence continues to mount for the potential harm in indiscriminate backboard and cervical collar use, it seems clear we should strive to decrease the overuse of prehospital and early spinal immobilization consistent with current position statements and validated clinical rules.
Prehospital spinal immobilization has long been the standard of care (SOC) to prevent spinal cord injury in trauma patients, but utilizing the best data currently available, some professional societies recently released new recommendations that question this practice. Guidelines released in 2014 from the National Association of EMS Physicians (NAEMSP) and the American College of Surgeons Committee on Trauma (ACS-COT) support limited application of spinal immobilization.1 These guidelines note, “Given the rarity of unstable spinal injuries in EMS trauma patients, the number that might benefit from immobilization to prevent secondary injury is likely extremely small. For each patient who has potential benefit, hundreds to thousands of patients must undergo immobilization with no potential benefit.” Further, they advise “utilization of backboards for spinal immobilization during transport should be judicious, so that potential benefits outweigh risks.”1 Spinal immobilization should not be used at all in patients with penetrating trauma who do not present with obvious neurological injury and should be selective, based on objective findings of injury or the high potential for same.1
Questioning a Long-standing Practice
Fear of the consequences of spinal cord injury from significant vertebral fractures has dictated prehospital spinal immobilization to manage injured trauma patients for decades. For almost 50 years, it has been the SOC. However, increasing evidence that spinal immobilization is not only unnecessary, but may even cause harm has resulted in questioning this paradigm, which has lead to promoting a change in the SOC.
Spinal immobilization dates back to the mid-1960s, when Geisler et al2 reported on a cohort of patients who suffered long-term paralysis from what was believed to be improper handling and failure to discover spinal injuries. Soon after, Farrington3,4 developed and published a systematic approach to spinal immobilization during extrication following blunt force trauma, supporting the widespread acceptance of backboards and cervical collars to immobilize the spine in injured trauma patients. Logic dictated that an unstable spine fracture could be worsened, or a cord injury could result, by unnecessary movement during extrication, transport, and initial evaluation in the ED, resulting in avoidable injury. This fear of potential secondary injury grew as more papers were published examining the link between prehospital handling of blunt force trauma patients and delayed paralysis. This resulted in the use of spinal immobilization on the majority of trauma patients, regardless of mechanism of injury or presenting symptoms.5,6
One review estimated that over 50% of trauma patients with no complaint of neck or back pain were transported with full spinal immobilization.7 This immobilization on uncomfortable long backboards typically continued in the ED for prolonged periods, until the spine could be cleared by physical examination and/or imaging studies. Yet a 2001 Cochrane review found that despite increasing use of spinal immobilization, no prospective, randomized controlled trial of the appropriate use of spinal immobilization or patient outcomes had ever been conducted.8
What the Evidence Says
How much evidence exists that supports the benefits of spinal immobilization? Not much. Studies on healthy volunteers and cadavers evaluating spinal motion with immobilization have been contradictory.9 One study found there was less motion with a cervical collar in place than without,10 whereas others found that the use of a cervical collar did not effectively reduce motion in an unstable spine.11,12 Perry et al13 studied the effectiveness of different head immobilization techniques and found that none could eliminate head and neck motion during emergency medical services (EMS) transport. Still other reports, including two biomechanical studies, demonstrated increased neck motion when using conventional extrication techniques (cervical collar with backboard) versus controlled self-extrication with cervical collar only.14,15
An Abundance of Literature on the Risks
Whereas data regarding the actual benefits of spinal immobilization is lacking, an abundance of literature details the risks. One of the most frequently cited studies is also one of the most controversial. Hauswald et al16 compared the outcomes of two groups of patients with blunt force trauma who were either immobilized during transport (in New Mexico) or non-immobilized (in Malaysia) and found that the risk of disability was higher in the immobilized group (odds ratio, 2.03). Although these environments are very different, the authors noted that mechanism of injury, resources, and the size of the hospitals were similar.16
Studies of spinal immobilization in patients with penetrating trauma report even worse outcomes. In separate studies, Haut et al17 and Vanderlan et al18 demonstrated increased mortality when immobilization led to increased transport times and interference with other resuscitative measures. These and other studies have led the American College of Emergency Physicians, NAEMSP, ACS-COT, the Prehospital Trauma Life Support Executive Committee, and other national organizations to recommend no spinal immobilization in patients with penetrating neck trauma.1,19,20
Many trauma patients arrive with complaints of pain at one or more sites. Some of these complaints, particularly back pain, may be secondary to the use of the backboard itself, especially in cases of prolonged transport.21,22 In a study of healthy volunteers who were immobilized on a backboard for 30 minutes, all of them reported pain, along with headaches, most often involving the occipital and sacral regions.23 A 1996 study compared spinal immobilization utilizing a backboard versus a vacuum mattress in 37 healthy volunteers with no history of back pain or spinal disease.24 Compared to those immobilized with the vacuum mattress, patients immobilized with a backboard for 30 minutes were 3.1 times more likely to have symptoms, 7.9 times more likely to complain of occipital pain, and 4.3 times more likely to have lumbosacral pain.24
Increased pain complaints in the setting of trauma can result in increased imaging, leading to increased costs and unnecessary radiation exposure.25 Prolonged backboard times can also result in sacral pressure ulcers.26 A recent study has shown that patients who undergo computed tomography (CT) scans with automatic tube current modulation (as most modern multidetector row CT systems utilize) while on a backboard may be exposed to a significant increase in radiation dose.27
Spinal immobilization has also been linked to respiratory compromise, particularly with the use of straps across the chest, even when not applied tightly. One study found worse lung function test results in healthy immobilized volunteers.28 Other studies have shown that older patients (even when healthy) and those with lung or chest injury have an even larger degree of restriction and respiratory compromise.29,30
Risks from immobilization are not isolated to backboards. The use of cervical collars alone also carries potential risks. (See “What About Cervical Collars?”8,31-39)
Risk of Secondary Neurological Deterioration Is Low
Many EMS systems have already adopted the new standards calling for less use of spinal immobilization. Though the evidence is compelling, not all EMS systems have adopted these standards due to strongly rooted beliefs and fears of long-term patient disability and subsequent litigation. However, these fears do not appear justified.
A recent review by Oto et al40 found only 42 cases of early secondary neurological deterioration after blunt trauma in all of the indexed medical literature. They noted, “In twelve cases the authors did attribute deterioration to temporally associated precipitants, seven of which were possibly iatrogenic; these included removal of a cervical collar, placement of a halo device, patient agitation, performance of flexion/extension films, ‘unintentional manipulation,’ falling in or near the ED, and forced collar application in patients with ankylosing spondylitis.” Thirteen of these cases occurred during prehospital care, none of them sudden and movement-provoked, and all reported by a single study.” This review highlights the rarity of secondary deterioration.
When Should Immobilization Be Used?
So what’s the next step for spinal immobilization in the field? How do we appropriately protect trauma patients during transport? As always seems to be the case in medicine, more evidence is needed. Oteir et al41 recently published a review of new literature on the epidemiology and current practice of prehospital spine management. They reported that early (8-24 hours) transfer of patients with spinal injury to spinal care units, along with effective resuscitation, was the most important determinant of better neurological outcomes.41 This review reaffirms the need for more data evaluating the relationship between spinal immobilization and neurological outcomes.
Currently, recommendations call for selective spinal immobilization to decrease unnecessary application and potential harm. Use of backboards for spinal immobilization should be limited to the following types of patients:1,20
- Blunt trauma and altered level of consciousness;
- Spinal pain or tenderness;
- Neurological complaint (eg, numbness or motor weakness);
- Anatomic deformity of the spine;
- High-energy mechanism of injury and:
- Drug or alcohol intoxication;
- Inability to communicate; and/or
- Distracting injury.
Patients for whom immobilization on a backboard is not necessary include those with all of the following:
- Normal level of consciousness (GCS 15);
- No spine tenderness or anatomic abnormality;
- No neurological findings or complaints;
- No distracting injury;
- No intoxication.
Cervical collars alone are still recommended for use in patients who do not meet validated clinical rules, such as the NEXUS or Canadian C spine rules.1,20,42,43 As these rules are well validated, they can be safely used to determine who should have a cervical collar placed, with or without a backboard. In a retrospective review, selective spinal immobilization was found to be 99% sensitive in identifying patients with cervical injuries.44
Clearly, there is still work to be done. Due to the relative rarity of actual spinal cord injury with the consequences of neurological injury, prospective trials in this area are rare and very difficult to safely design. However, there is growing confidence that selective spinal protocols, together with the inclusion of validated clinical rules, can effectively limit exposure to unnecessary spinal immobilization. As the current evidence continues to mount for the potential harm in indiscriminate backboard and cervical collar use, it seems clear we should strive to decrease the overuse of prehospital and early spinal immobilization consistent with current position statements and validated clinical rules.
1. White CC, Domeier RM, Millin MG. EMS spinal precautions and the use of the long backboard - resource document to the position statement of the National Association of EMS Physicians and the American College of Surgeons Committee on Trauma. Prehosp Emerg Care. 2014;18(2):306-314.
2. Geisler WO, Wynne-Jones M, Jousse AT. Early management of patients with trauma to the spinal cord. Med Serv J Can. 1966;22(7):512–523.
3. Farrington JD. Death in a ditch. Bulletin of the American College of Surgeons. 1967;52(3):121-130.
4. Farrington JD. Extrication of victims- surgical principles. J Trauma. 1968;8(4):493-512.
5. Riggins RS, Kraus JF. The risk of neurologic damage with fractures of the vertebrae. J Trauma. 1977;17(2):126-133.
6. Soderstrom CA, Brumback RJ. Early care of the patient with cervical spine injury. Orthop Clin North Am. 1986;17(1):3-13.
7. McHugh TP, Taylor JP. Unnecessary out-of-hospital use of full spinal immobilization. Acad Emerg Med. 1998;5(3):278-280.
8. Kwan I, Bunn F, Roberts I. Spinal immobilisation for trauma patients. Cochrane Database Syst Rev. 2001;(2):CD002803.
9. Sundstrøm T, Asbjørnsen H, Habiba S, Sunde GA, Wester K. Prehospital use of cervical collars in trauma patients: a critical review. J Neurotrauma. 2014;31(6):531-540.
10. Conrad BP, Rechtine G, Weight M, Clarke J, Horodyski M. Motion in the unstable cervical spine during hospital bed transfers. J Trauma. 2010;69,432-436.
11. Horodyski M, DiPaola CP, Conrad BP, Rechtine GR. Cervical collars are insufficient for immobilizing an unstable cervical spine injury. J Emerg Med. 2011;41(5):513-519.
12. Hughes SJ. How effective is the Newport/Aspen collar? A prospective radiographic evaluation in healthy adult volunteers. J Trauma. 1998;45(2):374-378.
13. Perry SD, McLellan B, McIlroy WE, Maki BE, Schwartz M, Fernie GR. The efficacy of head immobilization techniques during simulated vehicle motion. Spine (Phil Pa 1976). 1999;24(17):1839-1844.
14. Engsberg JR, Standeven JW, Shurtleff TL, Eggars JL, Shafer JS, Naunheim RS. Cervical spine motion during extrication. J Emerg Med. 2013;44(1):122-127.
15. Dixon M, O’Halloran J, Cummins NM. Biomechanical analysis of spinal immobilization during prehospital extrication—a proof of concept study. Emerg Med J. 2014;31(9):745-749.
16. Hauswald M, Ong G, Tandberg D, Omar Z. Out-of-hospital spinal immobilization: its effect on neurologic injury. Acad Emerg Med. 1998;5(3):214-219.
17. Haut ER, Kalish BT, Efron DT, et al. Spine immobilization in penetrating trauma: more harm than good? J Trauma. 2010;68(1):115-120.
18. Vanderlan WB, Tew BE, McSwain NE. Increased risk of death with cervical spine immobilization in penetrating cervical trauma. Injury. 2009;40(8):880-883.
19. Stuke LE, Pons PT, Guy JS, Chapleau WP, Butler FK, McSwain NE. Prehospital spine immobilization for penetrating trauma—review and recommendations from the Prehospital Trauma Life Support Executive Committee. J Trauma. 2011;71(3):763–769.
20. American College of Emergency Physicians. Policy Statement- EMS Management of Patients with Potential Spinal Injury. 2015. Available at: http://www.acep.org/Physician-Resources/Policies/Policy-Statements/EMS-Management-of-Patients-with-Potential-Spinal-Injury. Accessed February 9, 2016.
21. Barney RN, Cordell WH, Miller E. Pain associated with immobilization on rigid spine boards. Ann Emerg Med. 1989;18:918.
22. Cooney DR, Wallus H, Asaly M, Wojcik S. Backboard time for patients receiving spinal immobilization by emergency medical services. Int J Emerg Med. 2013;6(1):17.
23. Chan D, Goldberg R, Tascone A, Harmon S, Chan L. The effect of spinal immobilization on healthy volunteers. Ann Emerg Med. 1994;23(1):48-51.
24. Chan D, Goldberg RM, Mason J, Chan L. Backboard versus mattress splint immobilization: a comparison of symptoms generated. J Emerg Med, 1996;14(3):293-298.
25. March J, Ausband S, Brown L. Changes in physical examination caused by use of spinal immobilization. Prehosp Emerg Care. 2002;6(4):421-424.
26. Berg G, Nyberg S, Harrison P, Baumchen J, Gurss E, Hennes E. Near-infrared spectroscopy measurement of sacral tissue oxygen saturation in healthy volunteers immobilized on rigid spine boards. Prehosp Emerg Care. 2010;14(4):419-424.
27. Lee AY, Elojeimy S, Kanal KM, Gunn ML. The effect of trauma backboards on computed tomography radiation dose. Clin Radiol. 2016. Epub ahead of print.
28. Bauer D, Kowalski R. Effect of spinal immobilization devices on pulmonary function in the healthy, nonsmoking man. Ann Emerg Med. 1988;17(9):915-918.
29. Walsh M, Grant T, Mickey S. Lung function compromised by spinal immobilization. Ann Emerg Med. 1990;19(5):615-616.
30. Totten VY, Sugarman DB. Respiratory effects of spinal immobilization. Prehosp Emerg Care. 1999; 3(4):347-352.
31. Goutcher CM, Lochhead V. Reduction in mouth opening with semi-rigid cervical collars. Br J Anaesth. 2005;95(3):344-348.
32. Davies G, Deakin C, Wilson A. The effect of a rigid collar on intracranial pressure. Injury. 1996;27(9):647-649.
33. Dunham CM, Brocker BP, Collier BD, Gemmel DJ. Risks associated with magnetic resonance imaging and cervical collar in comatose, blunt trauma patients with negative comprehensive cervical spine computed tomography and no apparent spinal deficit. Crit Care. 2008;12(4):R89.
34. Mobbs RJ, Stoodley MA, Fuller J. Effect of cervical hard collar on intracranial pressure after head injury. ANZ J Surg. 2002;72(6):389-391.
35. Stone MB, Tubridy CM, Curran R. The effect of rigid cervical collars on internal jugular vein dimensions. Acad Emerg Med. 2010;17(1):100-102.
36. Ben-Galim P, Dreiangel N, Mattox KL, Reitman CA, Kalantar SB, Hipp JA. Extrication collars can result in abnormal separation between vertebrae in the presence of a dissociative injury. J Trauma. 2010;69(2):447-450.
37. Podolsky SM, Hoffman JR, Pietrafesa CA. Neurologic complications following immobilization of cervical spine fracture in a patient with ankylosing spondylitis. Ann Emerg Med. 1983;12(9):578-580.
38. Papadopoulos MC, Chakraborty A, Waldron G, Bell BA. Exacerbating cervical spine injury by applying a hard collar. BMJ. 1999;319(7203):171-172.
39. Thumbikat P, Hariharan RP, Ravichandran G, Mcclelland MR, Mathew KM. Spinal cord injury in patients with ankylosing spondylitis: a 10-year review. Spine (Phila Pa 1976). 2007;32(26):2989-2995.
40. Oto B, Corey DJ, Oswald J, Sifford D, Walsh B. Early secondary neurologic deterioration after blunt spinal trauma: a review of the literature. Acad Emerg Med. 2015;22(10):1200-1212.
41. Oteir AO, Smith K, Jennings PA, Stoelwinder JU. The prehospital management of suspected spinal cord injury: an update. Prehosp Disaster Med. 2014;29(4):399-402.
42. Hoffman JR, Mower WR, Wolfson AB, Todd KH, Zucker MI. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. National Emergency X-Radiography Utilization Study Group. N Engl J Med. 2000;343(2):94-99.
43. Stiell IG, Wells GA, Vandemheen KL, et al. The Canadian C-spine rule for radiography in alert and stable trauma patients. JAMA. 2001;286(15):1841–1848.
44. Stroh G, Braude D. Can an out-of-hospital cervical spine clearance protocol identify all patients with injuries? An argument for selective immobilization. Ann Emerg Med. 2001;37(6):609-615.
1. White CC, Domeier RM, Millin MG. EMS spinal precautions and the use of the long backboard - resource document to the position statement of the National Association of EMS Physicians and the American College of Surgeons Committee on Trauma. Prehosp Emerg Care. 2014;18(2):306-314.
2. Geisler WO, Wynne-Jones M, Jousse AT. Early management of patients with trauma to the spinal cord. Med Serv J Can. 1966;22(7):512–523.
3. Farrington JD. Death in a ditch. Bulletin of the American College of Surgeons. 1967;52(3):121-130.
4. Farrington JD. Extrication of victims- surgical principles. J Trauma. 1968;8(4):493-512.
5. Riggins RS, Kraus JF. The risk of neurologic damage with fractures of the vertebrae. J Trauma. 1977;17(2):126-133.
6. Soderstrom CA, Brumback RJ. Early care of the patient with cervical spine injury. Orthop Clin North Am. 1986;17(1):3-13.
7. McHugh TP, Taylor JP. Unnecessary out-of-hospital use of full spinal immobilization. Acad Emerg Med. 1998;5(3):278-280.
8. Kwan I, Bunn F, Roberts I. Spinal immobilisation for trauma patients. Cochrane Database Syst Rev. 2001;(2):CD002803.
9. Sundstrøm T, Asbjørnsen H, Habiba S, Sunde GA, Wester K. Prehospital use of cervical collars in trauma patients: a critical review. J Neurotrauma. 2014;31(6):531-540.
10. Conrad BP, Rechtine G, Weight M, Clarke J, Horodyski M. Motion in the unstable cervical spine during hospital bed transfers. J Trauma. 2010;69,432-436.
11. Horodyski M, DiPaola CP, Conrad BP, Rechtine GR. Cervical collars are insufficient for immobilizing an unstable cervical spine injury. J Emerg Med. 2011;41(5):513-519.
12. Hughes SJ. How effective is the Newport/Aspen collar? A prospective radiographic evaluation in healthy adult volunteers. J Trauma. 1998;45(2):374-378.
13. Perry SD, McLellan B, McIlroy WE, Maki BE, Schwartz M, Fernie GR. The efficacy of head immobilization techniques during simulated vehicle motion. Spine (Phil Pa 1976). 1999;24(17):1839-1844.
14. Engsberg JR, Standeven JW, Shurtleff TL, Eggars JL, Shafer JS, Naunheim RS. Cervical spine motion during extrication. J Emerg Med. 2013;44(1):122-127.
15. Dixon M, O’Halloran J, Cummins NM. Biomechanical analysis of spinal immobilization during prehospital extrication—a proof of concept study. Emerg Med J. 2014;31(9):745-749.
16. Hauswald M, Ong G, Tandberg D, Omar Z. Out-of-hospital spinal immobilization: its effect on neurologic injury. Acad Emerg Med. 1998;5(3):214-219.
17. Haut ER, Kalish BT, Efron DT, et al. Spine immobilization in penetrating trauma: more harm than good? J Trauma. 2010;68(1):115-120.
18. Vanderlan WB, Tew BE, McSwain NE. Increased risk of death with cervical spine immobilization in penetrating cervical trauma. Injury. 2009;40(8):880-883.
19. Stuke LE, Pons PT, Guy JS, Chapleau WP, Butler FK, McSwain NE. Prehospital spine immobilization for penetrating trauma—review and recommendations from the Prehospital Trauma Life Support Executive Committee. J Trauma. 2011;71(3):763–769.
20. American College of Emergency Physicians. Policy Statement- EMS Management of Patients with Potential Spinal Injury. 2015. Available at: http://www.acep.org/Physician-Resources/Policies/Policy-Statements/EMS-Management-of-Patients-with-Potential-Spinal-Injury. Accessed February 9, 2016.
21. Barney RN, Cordell WH, Miller E. Pain associated with immobilization on rigid spine boards. Ann Emerg Med. 1989;18:918.
22. Cooney DR, Wallus H, Asaly M, Wojcik S. Backboard time for patients receiving spinal immobilization by emergency medical services. Int J Emerg Med. 2013;6(1):17.
23. Chan D, Goldberg R, Tascone A, Harmon S, Chan L. The effect of spinal immobilization on healthy volunteers. Ann Emerg Med. 1994;23(1):48-51.
24. Chan D, Goldberg RM, Mason J, Chan L. Backboard versus mattress splint immobilization: a comparison of symptoms generated. J Emerg Med, 1996;14(3):293-298.
25. March J, Ausband S, Brown L. Changes in physical examination caused by use of spinal immobilization. Prehosp Emerg Care. 2002;6(4):421-424.
26. Berg G, Nyberg S, Harrison P, Baumchen J, Gurss E, Hennes E. Near-infrared spectroscopy measurement of sacral tissue oxygen saturation in healthy volunteers immobilized on rigid spine boards. Prehosp Emerg Care. 2010;14(4):419-424.
27. Lee AY, Elojeimy S, Kanal KM, Gunn ML. The effect of trauma backboards on computed tomography radiation dose. Clin Radiol. 2016. Epub ahead of print.
28. Bauer D, Kowalski R. Effect of spinal immobilization devices on pulmonary function in the healthy, nonsmoking man. Ann Emerg Med. 1988;17(9):915-918.
29. Walsh M, Grant T, Mickey S. Lung function compromised by spinal immobilization. Ann Emerg Med. 1990;19(5):615-616.
30. Totten VY, Sugarman DB. Respiratory effects of spinal immobilization. Prehosp Emerg Care. 1999; 3(4):347-352.
31. Goutcher CM, Lochhead V. Reduction in mouth opening with semi-rigid cervical collars. Br J Anaesth. 2005;95(3):344-348.
32. Davies G, Deakin C, Wilson A. The effect of a rigid collar on intracranial pressure. Injury. 1996;27(9):647-649.
33. Dunham CM, Brocker BP, Collier BD, Gemmel DJ. Risks associated with magnetic resonance imaging and cervical collar in comatose, blunt trauma patients with negative comprehensive cervical spine computed tomography and no apparent spinal deficit. Crit Care. 2008;12(4):R89.
34. Mobbs RJ, Stoodley MA, Fuller J. Effect of cervical hard collar on intracranial pressure after head injury. ANZ J Surg. 2002;72(6):389-391.
35. Stone MB, Tubridy CM, Curran R. The effect of rigid cervical collars on internal jugular vein dimensions. Acad Emerg Med. 2010;17(1):100-102.
36. Ben-Galim P, Dreiangel N, Mattox KL, Reitman CA, Kalantar SB, Hipp JA. Extrication collars can result in abnormal separation between vertebrae in the presence of a dissociative injury. J Trauma. 2010;69(2):447-450.
37. Podolsky SM, Hoffman JR, Pietrafesa CA. Neurologic complications following immobilization of cervical spine fracture in a patient with ankylosing spondylitis. Ann Emerg Med. 1983;12(9):578-580.
38. Papadopoulos MC, Chakraborty A, Waldron G, Bell BA. Exacerbating cervical spine injury by applying a hard collar. BMJ. 1999;319(7203):171-172.
39. Thumbikat P, Hariharan RP, Ravichandran G, Mcclelland MR, Mathew KM. Spinal cord injury in patients with ankylosing spondylitis: a 10-year review. Spine (Phila Pa 1976). 2007;32(26):2989-2995.
40. Oto B, Corey DJ, Oswald J, Sifford D, Walsh B. Early secondary neurologic deterioration after blunt spinal trauma: a review of the literature. Acad Emerg Med. 2015;22(10):1200-1212.
41. Oteir AO, Smith K, Jennings PA, Stoelwinder JU. The prehospital management of suspected spinal cord injury: an update. Prehosp Disaster Med. 2014;29(4):399-402.
42. Hoffman JR, Mower WR, Wolfson AB, Todd KH, Zucker MI. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. National Emergency X-Radiography Utilization Study Group. N Engl J Med. 2000;343(2):94-99.
43. Stiell IG, Wells GA, Vandemheen KL, et al. The Canadian C-spine rule for radiography in alert and stable trauma patients. JAMA. 2001;286(15):1841–1848.
44. Stroh G, Braude D. Can an out-of-hospital cervical spine clearance protocol identify all patients with injuries? An argument for selective immobilization. Ann Emerg Med. 2001;37(6):609-615.
Phone monitoring program helps cut chemotherapy symptom severity
AMSTERDAM – A telephone-based system to monitor chemotherapy-induced symptoms when patients are home and facilitate interventions as needed led to significant cuts in symptom burden in a single-center, randomized trial with 152 breast cancer patients.
“Symptom care at home gives patients symptom care when and where they need it,” Kathi H. Mooney, Ph.D., said at the European Breast Cancer Conference. “Rarely is symptom monitoring extended to when patients are home, but that is when symptoms are most problematic for patients.”
The 30-day program was linked with significant cuts in the number of days with any of seven chemotherapy-induced symptoms. In addition, overall days with any severe symptom fell by 48% (P = .006) and the number of days with any moderate symptoms fell by 38% (P = .011), reported Dr. Mooney, professor of nursing at the University of Utah, Salt Lake City.
She and her associates designed a telephone-based system to address the usual reticence that chemotherapy patients have to report their symptoms. Prior study results had documented that patients with moderate or severe symptoms initiated calls for about 5% of episodes, even when explicitly told to report symptoms. This led the Utah researchers to develop an automated, interactive system that phoned patients daily.
The enrolled patients spanned the full spectrum of breast cancer stages; the average age was 53 years. All patients received a daily, automated call that prompted them to rate each of 11 chemotherapy symptoms on a scale of 0-10.
The calls also provided automated, self-management coaching for problematic symptoms. The investigational Symptom Care at Home intervention involved a nurse practitioner getting back to patients who reported poorly-controlled symptoms. These follow-up calls averaged just under 7 minutes in length, and on average each patient received 11 calls during the 30 days of the intervention.
After 30 days, the 83 patients in the Symptom Care at Home program had statistically-significant reductions in days with moderate or worse episodes for 7 of the 11 symptoms tallied: numbness or tingling, anxiety, nausea, pain, depressed mood, sore mouth, and fatigue. The most robust effects were a 72% drop in days with moderate or worse numbness or tingling, a 66% reduction in anxiety days, and a 61% cut in nausea days.
“I like the intervention used in this study. I think it shows what can happen when you collect symptom information in a way that is patient friendly,” said Dr. Robert Mansel, professor at the Institute of Cancer & Genetics at Cardiff University, South Wales. “I was delighted to see that this intervention really made a difference. The findings show that patients often suffer a lot more from treatment than they are usually prepared to tell us.”
In addition to the efficacy of this intervention, “patients told us that they liked having someone to talk with about their symptoms,” Dr. Mooney said in an interview. “Patients are concerned about their symptoms.”
One aspect of the Symptom Care at Home program that has not yet been analyzed is its cost efficacy. The University of Utah’s Huntsman Cancer Institute, where the program was developed, is awaiting results from a cost-benefit analysis before deciding whether to make the telephone system part of routine practice, she said.
Dr. Mooney and Dr. Mansel reported having no financial disclosures.
On Twitter @mitchelzoler
AMSTERDAM – A telephone-based system to monitor chemotherapy-induced symptoms when patients are home and facilitate interventions as needed led to significant cuts in symptom burden in a single-center, randomized trial with 152 breast cancer patients.
“Symptom care at home gives patients symptom care when and where they need it,” Kathi H. Mooney, Ph.D., said at the European Breast Cancer Conference. “Rarely is symptom monitoring extended to when patients are home, but that is when symptoms are most problematic for patients.”
The 30-day program was linked with significant cuts in the number of days with any of seven chemotherapy-induced symptoms. In addition, overall days with any severe symptom fell by 48% (P = .006) and the number of days with any moderate symptoms fell by 38% (P = .011), reported Dr. Mooney, professor of nursing at the University of Utah, Salt Lake City.
She and her associates designed a telephone-based system to address the usual reticence that chemotherapy patients have to report their symptoms. Prior study results had documented that patients with moderate or severe symptoms initiated calls for about 5% of episodes, even when explicitly told to report symptoms. This led the Utah researchers to develop an automated, interactive system that phoned patients daily.
The enrolled patients spanned the full spectrum of breast cancer stages; the average age was 53 years. All patients received a daily, automated call that prompted them to rate each of 11 chemotherapy symptoms on a scale of 0-10.
The calls also provided automated, self-management coaching for problematic symptoms. The investigational Symptom Care at Home intervention involved a nurse practitioner getting back to patients who reported poorly-controlled symptoms. These follow-up calls averaged just under 7 minutes in length, and on average each patient received 11 calls during the 30 days of the intervention.
After 30 days, the 83 patients in the Symptom Care at Home program had statistically-significant reductions in days with moderate or worse episodes for 7 of the 11 symptoms tallied: numbness or tingling, anxiety, nausea, pain, depressed mood, sore mouth, and fatigue. The most robust effects were a 72% drop in days with moderate or worse numbness or tingling, a 66% reduction in anxiety days, and a 61% cut in nausea days.
“I like the intervention used in this study. I think it shows what can happen when you collect symptom information in a way that is patient friendly,” said Dr. Robert Mansel, professor at the Institute of Cancer & Genetics at Cardiff University, South Wales. “I was delighted to see that this intervention really made a difference. The findings show that patients often suffer a lot more from treatment than they are usually prepared to tell us.”
In addition to the efficacy of this intervention, “patients told us that they liked having someone to talk with about their symptoms,” Dr. Mooney said in an interview. “Patients are concerned about their symptoms.”
One aspect of the Symptom Care at Home program that has not yet been analyzed is its cost efficacy. The University of Utah’s Huntsman Cancer Institute, where the program was developed, is awaiting results from a cost-benefit analysis before deciding whether to make the telephone system part of routine practice, she said.
Dr. Mooney and Dr. Mansel reported having no financial disclosures.
On Twitter @mitchelzoler
AMSTERDAM – A telephone-based system to monitor chemotherapy-induced symptoms when patients are home and facilitate interventions as needed led to significant cuts in symptom burden in a single-center, randomized trial with 152 breast cancer patients.
“Symptom care at home gives patients symptom care when and where they need it,” Kathi H. Mooney, Ph.D., said at the European Breast Cancer Conference. “Rarely is symptom monitoring extended to when patients are home, but that is when symptoms are most problematic for patients.”
The 30-day program was linked with significant cuts in the number of days with any of seven chemotherapy-induced symptoms. In addition, overall days with any severe symptom fell by 48% (P = .006) and the number of days with any moderate symptoms fell by 38% (P = .011), reported Dr. Mooney, professor of nursing at the University of Utah, Salt Lake City.
She and her associates designed a telephone-based system to address the usual reticence that chemotherapy patients have to report their symptoms. Prior study results had documented that patients with moderate or severe symptoms initiated calls for about 5% of episodes, even when explicitly told to report symptoms. This led the Utah researchers to develop an automated, interactive system that phoned patients daily.
The enrolled patients spanned the full spectrum of breast cancer stages; the average age was 53 years. All patients received a daily, automated call that prompted them to rate each of 11 chemotherapy symptoms on a scale of 0-10.
The calls also provided automated, self-management coaching for problematic symptoms. The investigational Symptom Care at Home intervention involved a nurse practitioner getting back to patients who reported poorly-controlled symptoms. These follow-up calls averaged just under 7 minutes in length, and on average each patient received 11 calls during the 30 days of the intervention.
After 30 days, the 83 patients in the Symptom Care at Home program had statistically-significant reductions in days with moderate or worse episodes for 7 of the 11 symptoms tallied: numbness or tingling, anxiety, nausea, pain, depressed mood, sore mouth, and fatigue. The most robust effects were a 72% drop in days with moderate or worse numbness or tingling, a 66% reduction in anxiety days, and a 61% cut in nausea days.
“I like the intervention used in this study. I think it shows what can happen when you collect symptom information in a way that is patient friendly,” said Dr. Robert Mansel, professor at the Institute of Cancer & Genetics at Cardiff University, South Wales. “I was delighted to see that this intervention really made a difference. The findings show that patients often suffer a lot more from treatment than they are usually prepared to tell us.”
In addition to the efficacy of this intervention, “patients told us that they liked having someone to talk with about their symptoms,” Dr. Mooney said in an interview. “Patients are concerned about their symptoms.”
One aspect of the Symptom Care at Home program that has not yet been analyzed is its cost efficacy. The University of Utah’s Huntsman Cancer Institute, where the program was developed, is awaiting results from a cost-benefit analysis before deciding whether to make the telephone system part of routine practice, she said.
Dr. Mooney and Dr. Mansel reported having no financial disclosures.
On Twitter @mitchelzoler
AT EBCC 10
How to beat apremilast-induced diarrhea
MAUI, HAWAII – If you’re going to prescribe apremilast for psoriasis or psoriatic arthritis – and more and more physicians are doing so because of the drug’s exceptional safety profile – you’d better get familiar with the oral phosphodiesterase-4 inhibitor’s gastrointestinal side effects, Dr. George M. Martin advised at the 2016 Rheumatology Winter Clinical Symposium.
“One of the biggest hurdles we have to deal with when we prescribe apremilast is the fact that there are these GI side effects,” said Dr. Martin, a dermatologist practicing in Maui and codirector of the rheumatology symposium.
Celgene, which markets apremilast (Otezla), sponsored an analysis of the pattern of diarrhea that emerged in the pooled results of the phase III ESTEEM 1 and 2 trials of apremilast at 30 mg twice daily for psoriasis and the PALACE 1-3 phase III psoriatic arthritis trials.
Diarrhea occurred in 16%-18% of patients on apremilast, a rate roughly threefold greater than in placebo-treated controls. Diarrhea onset was usually within the first 14 days of therapy. When it occurred, the duration was typically about 2 weeks.
“This you can relay to your patients so they’re not surprised if it happens,” the dermatologist said.
It’s a secretory diarrhea, and it is believed to be a classwide effect for the phosphodiesterase-4 (PDE-4) inhibitors. For example, roflumilast (Daliresp), an oral PDE-4 inhibitor used in the treatment of chronic obstructive pulmonary disease, has the same diarrhea issues. The mechanism has been worked out: The drug increases intracellular cyclic adenosine monophosphate, with resultant activation of chloride channels in crypts in the small bowel, which in turn leads to secretion of chloride ions. It takes the large bowel a couple of weeks to adapt. Caffeine causes diarrhea in some individuals through a similar mechanism.
Apremilast-related diarrhea often responds to the time-tested OTC remedies, including bismuth salicylate or fiber supplements. Alternatively, Dr. Martin said he is a fan of the oral prescription agent crofelemer (Fulyzaq) because of its exceptional safety, tolerability, and effectiveness. Plus, many residents of the garden islands of Hawaii like the idea of using a botanical derived from the latexlike sap – known as ‘dragon’s blood – of a South American tree. Crofelemer’s approved indication is the treatment of diarrhea associated with anti-HIV agents.
Diphenoxylate/atropine (Lomotil) is another effective prescription option.
Nausea and/or vomiting occurred in 15%-17% of apremilast-treated patients in the phase III trials. As with diarrhea, if nausea and/or vomiting is going to happen, it occurs early, within the first week or two. Dr. Martin said he finds in his own practice that the nausea/vomiting is less bothersome for patients than the diarrhea. Drug discontinuation due to any GI side effects is rarely necessary.
The nausea/vomiting is usually readily managed by encouraging affected patients to make sure that they’re well hydrated, take their apremilast with food, and eat smaller, more frequent meals. OTC diphenhydramine (Benadryl) is often effective, as are the usual prescription antiemetic agents.
Pharmaceutical industry data indicate apremilast has quickly captured a 17% share of the market for systemic psoriasis therapies. There is a good reason for that, according to Dr. Martin: “Dermatologists have historically been risk averse. And apremilast is arguably the safest systemic agent we have to treat psoriasis. The beauty of apremilast is it requires no laboratory monitoring. That makes it attractive to dermatologists who are concerned about systemic therapy. It’s why there has been a huge jump in adoption of apremilast.”
Apremilast is comparable to methotrexate in terms of efficacy as reflected in week 16 PASI-75 response rates of about 35%, meaning 35% of treated patients obtain at least a 75% improvement in Psoriasis Area and Severity Index scores, he continued. Apremilast is particularly effective for scalp and nail psoriasis, making it a good option for patients who have psoriasis at those sites but not extensive involvement elsewhere, which might call for the use of a more potent biologic agent.
Surveys indicate that 20% of dermatologists write 80% of all prescriptions for biologic agents used to treat psoriasis. The thinking was that apremilast would appeal to the 80% of dermatologists who have steered clear of the biologics, and that after becoming comfortable with apremilast, they might become more receptive to using biologics for their patients with an inadequate response to the oral PDE-4 inhibitor. That hasn’t happened yet.
“We’re not seeing apremilast function as the gateway drug we thought it would be. It’s just going to take some time for those prescribers either to refer their patients who aren’t getting a good response to the next doctor who’s more adept at treating with biologic agents, or perhaps they themselves will get more involved,” Dr. Martin predicted.
He reported serving on scientific advisory boards for, and/or as a consultant to, nine pharmaceutical companies.
MAUI, HAWAII – If you’re going to prescribe apremilast for psoriasis or psoriatic arthritis – and more and more physicians are doing so because of the drug’s exceptional safety profile – you’d better get familiar with the oral phosphodiesterase-4 inhibitor’s gastrointestinal side effects, Dr. George M. Martin advised at the 2016 Rheumatology Winter Clinical Symposium.
“One of the biggest hurdles we have to deal with when we prescribe apremilast is the fact that there are these GI side effects,” said Dr. Martin, a dermatologist practicing in Maui and codirector of the rheumatology symposium.
Celgene, which markets apremilast (Otezla), sponsored an analysis of the pattern of diarrhea that emerged in the pooled results of the phase III ESTEEM 1 and 2 trials of apremilast at 30 mg twice daily for psoriasis and the PALACE 1-3 phase III psoriatic arthritis trials.
Diarrhea occurred in 16%-18% of patients on apremilast, a rate roughly threefold greater than in placebo-treated controls. Diarrhea onset was usually within the first 14 days of therapy. When it occurred, the duration was typically about 2 weeks.
“This you can relay to your patients so they’re not surprised if it happens,” the dermatologist said.
It’s a secretory diarrhea, and it is believed to be a classwide effect for the phosphodiesterase-4 (PDE-4) inhibitors. For example, roflumilast (Daliresp), an oral PDE-4 inhibitor used in the treatment of chronic obstructive pulmonary disease, has the same diarrhea issues. The mechanism has been worked out: The drug increases intracellular cyclic adenosine monophosphate, with resultant activation of chloride channels in crypts in the small bowel, which in turn leads to secretion of chloride ions. It takes the large bowel a couple of weeks to adapt. Caffeine causes diarrhea in some individuals through a similar mechanism.
Apremilast-related diarrhea often responds to the time-tested OTC remedies, including bismuth salicylate or fiber supplements. Alternatively, Dr. Martin said he is a fan of the oral prescription agent crofelemer (Fulyzaq) because of its exceptional safety, tolerability, and effectiveness. Plus, many residents of the garden islands of Hawaii like the idea of using a botanical derived from the latexlike sap – known as ‘dragon’s blood – of a South American tree. Crofelemer’s approved indication is the treatment of diarrhea associated with anti-HIV agents.
Diphenoxylate/atropine (Lomotil) is another effective prescription option.
Nausea and/or vomiting occurred in 15%-17% of apremilast-treated patients in the phase III trials. As with diarrhea, if nausea and/or vomiting is going to happen, it occurs early, within the first week or two. Dr. Martin said he finds in his own practice that the nausea/vomiting is less bothersome for patients than the diarrhea. Drug discontinuation due to any GI side effects is rarely necessary.
The nausea/vomiting is usually readily managed by encouraging affected patients to make sure that they’re well hydrated, take their apremilast with food, and eat smaller, more frequent meals. OTC diphenhydramine (Benadryl) is often effective, as are the usual prescription antiemetic agents.
Pharmaceutical industry data indicate apremilast has quickly captured a 17% share of the market for systemic psoriasis therapies. There is a good reason for that, according to Dr. Martin: “Dermatologists have historically been risk averse. And apremilast is arguably the safest systemic agent we have to treat psoriasis. The beauty of apremilast is it requires no laboratory monitoring. That makes it attractive to dermatologists who are concerned about systemic therapy. It’s why there has been a huge jump in adoption of apremilast.”
Apremilast is comparable to methotrexate in terms of efficacy as reflected in week 16 PASI-75 response rates of about 35%, meaning 35% of treated patients obtain at least a 75% improvement in Psoriasis Area and Severity Index scores, he continued. Apremilast is particularly effective for scalp and nail psoriasis, making it a good option for patients who have psoriasis at those sites but not extensive involvement elsewhere, which might call for the use of a more potent biologic agent.
Surveys indicate that 20% of dermatologists write 80% of all prescriptions for biologic agents used to treat psoriasis. The thinking was that apremilast would appeal to the 80% of dermatologists who have steered clear of the biologics, and that after becoming comfortable with apremilast, they might become more receptive to using biologics for their patients with an inadequate response to the oral PDE-4 inhibitor. That hasn’t happened yet.
“We’re not seeing apremilast function as the gateway drug we thought it would be. It’s just going to take some time for those prescribers either to refer their patients who aren’t getting a good response to the next doctor who’s more adept at treating with biologic agents, or perhaps they themselves will get more involved,” Dr. Martin predicted.
He reported serving on scientific advisory boards for, and/or as a consultant to, nine pharmaceutical companies.
MAUI, HAWAII – If you’re going to prescribe apremilast for psoriasis or psoriatic arthritis – and more and more physicians are doing so because of the drug’s exceptional safety profile – you’d better get familiar with the oral phosphodiesterase-4 inhibitor’s gastrointestinal side effects, Dr. George M. Martin advised at the 2016 Rheumatology Winter Clinical Symposium.
“One of the biggest hurdles we have to deal with when we prescribe apremilast is the fact that there are these GI side effects,” said Dr. Martin, a dermatologist practicing in Maui and codirector of the rheumatology symposium.
Celgene, which markets apremilast (Otezla), sponsored an analysis of the pattern of diarrhea that emerged in the pooled results of the phase III ESTEEM 1 and 2 trials of apremilast at 30 mg twice daily for psoriasis and the PALACE 1-3 phase III psoriatic arthritis trials.
Diarrhea occurred in 16%-18% of patients on apremilast, a rate roughly threefold greater than in placebo-treated controls. Diarrhea onset was usually within the first 14 days of therapy. When it occurred, the duration was typically about 2 weeks.
“This you can relay to your patients so they’re not surprised if it happens,” the dermatologist said.
It’s a secretory diarrhea, and it is believed to be a classwide effect for the phosphodiesterase-4 (PDE-4) inhibitors. For example, roflumilast (Daliresp), an oral PDE-4 inhibitor used in the treatment of chronic obstructive pulmonary disease, has the same diarrhea issues. The mechanism has been worked out: The drug increases intracellular cyclic adenosine monophosphate, with resultant activation of chloride channels in crypts in the small bowel, which in turn leads to secretion of chloride ions. It takes the large bowel a couple of weeks to adapt. Caffeine causes diarrhea in some individuals through a similar mechanism.
Apremilast-related diarrhea often responds to the time-tested OTC remedies, including bismuth salicylate or fiber supplements. Alternatively, Dr. Martin said he is a fan of the oral prescription agent crofelemer (Fulyzaq) because of its exceptional safety, tolerability, and effectiveness. Plus, many residents of the garden islands of Hawaii like the idea of using a botanical derived from the latexlike sap – known as ‘dragon’s blood – of a South American tree. Crofelemer’s approved indication is the treatment of diarrhea associated with anti-HIV agents.
Diphenoxylate/atropine (Lomotil) is another effective prescription option.
Nausea and/or vomiting occurred in 15%-17% of apremilast-treated patients in the phase III trials. As with diarrhea, if nausea and/or vomiting is going to happen, it occurs early, within the first week or two. Dr. Martin said he finds in his own practice that the nausea/vomiting is less bothersome for patients than the diarrhea. Drug discontinuation due to any GI side effects is rarely necessary.
The nausea/vomiting is usually readily managed by encouraging affected patients to make sure that they’re well hydrated, take their apremilast with food, and eat smaller, more frequent meals. OTC diphenhydramine (Benadryl) is often effective, as are the usual prescription antiemetic agents.
Pharmaceutical industry data indicate apremilast has quickly captured a 17% share of the market for systemic psoriasis therapies. There is a good reason for that, according to Dr. Martin: “Dermatologists have historically been risk averse. And apremilast is arguably the safest systemic agent we have to treat psoriasis. The beauty of apremilast is it requires no laboratory monitoring. That makes it attractive to dermatologists who are concerned about systemic therapy. It’s why there has been a huge jump in adoption of apremilast.”
Apremilast is comparable to methotrexate in terms of efficacy as reflected in week 16 PASI-75 response rates of about 35%, meaning 35% of treated patients obtain at least a 75% improvement in Psoriasis Area and Severity Index scores, he continued. Apremilast is particularly effective for scalp and nail psoriasis, making it a good option for patients who have psoriasis at those sites but not extensive involvement elsewhere, which might call for the use of a more potent biologic agent.
Surveys indicate that 20% of dermatologists write 80% of all prescriptions for biologic agents used to treat psoriasis. The thinking was that apremilast would appeal to the 80% of dermatologists who have steered clear of the biologics, and that after becoming comfortable with apremilast, they might become more receptive to using biologics for their patients with an inadequate response to the oral PDE-4 inhibitor. That hasn’t happened yet.
“We’re not seeing apremilast function as the gateway drug we thought it would be. It’s just going to take some time for those prescribers either to refer their patients who aren’t getting a good response to the next doctor who’s more adept at treating with biologic agents, or perhaps they themselves will get more involved,” Dr. Martin predicted.
He reported serving on scientific advisory boards for, and/or as a consultant to, nine pharmaceutical companies.
EXPERT ANALYSIS FROM RWCS 2016
How to beat apremilast-induced diarrhea
MAUI, HAWAII – If you’re going to prescribe apremilast for psoriasis or psoriatic arthritis – and more and more physicians are doing so because of the drug’s exceptional safety profile – you’d better get familiar with the oral phosphodiesterase-4 inhibitor’s gastrointestinal side effects, Dr. George M. Martin advised at the 2016 Rheumatology Winter Clinical Symposium.
“One of the biggest hurdles we have to deal with when we prescribe apremilast is the fact that there are these GI side effects,” said Dr. Martin, a dermatologist practicing in Maui and codirector of the rheumatology symposium.
Celgene, which markets apremilast (Otezla), sponsored an analysis of the pattern of diarrhea that emerged in the pooled results of the phase III ESTEEM 1 and 2 trials of apremilast at 30 mg twice daily for psoriasis and the PALACE 1-3 phase III psoriatic arthritis trials.
Diarrhea occurred in 16%-18% of patients on apremilast, a rate roughly threefold greater than in placebo-treated controls. Diarrhea onset was usually within the first 14 days of therapy. When it occurred, the duration was typically about 2 weeks.
“This you can relay to your patients so they’re not surprised if it happens,” the dermatologist said.
It’s a secretory diarrhea, and it is believed to be a classwide effect for the phosphodiesterase-4 (PDE-4) inhibitors. For example, roflumilast (Daliresp), an oral PDE-4 inhibitor used in the treatment of chronic obstructive pulmonary disease, has the same diarrhea issues. The mechanism has been worked out: The drug increases intracellular cyclic adenosine monophosphate, with resultant activation of chloride channels in crypts in the small bowel, which in turn leads to secretion of chloride ions. It takes the large bowel a couple of weeks to adapt. Caffeine causes diarrhea in some individuals through a similar mechanism.
Apremilast-related diarrhea often responds to the time-tested OTC remedies, including bismuth salicylate or fiber supplements. Alternatively, Dr. Martin said he is a fan of the oral prescription agent crofelemer (Fulyzaq) because of its exceptional safety, tolerability, and effectiveness. Plus, many residents of the garden islands of Hawaii like the idea of using a botanical derived from the latexlike sap – known as ‘dragon’s blood – of a South American tree. Crofelemer’s approved indication is the treatment of diarrhea associated with anti-HIV agents.
Diphenoxylate/atropine (Lomotil) is another effective prescription option.
Nausea and/or vomiting occurred in 15%-17% of apremilast-treated patients in the phase III trials. As with diarrhea, if nausea and/or vomiting is going to happen, it occurs early, within the first week or two. Dr. Martin said he finds in his own practice that the nausea/vomiting is less bothersome for patients than the diarrhea. Drug discontinuation due to any GI side effects is rarely necessary.
The nausea/vomiting is usually readily managed by encouraging affected patients to make sure that they’re well hydrated, take their apremilast with food, and eat smaller, more frequent meals. OTC diphenhydramine (Benadryl) is often effective, as are the usual prescription antiemetic agents.
Pharmaceutical industry data indicate apremilast has quickly captured a 17% share of the market for systemic psoriasis therapies. There is a good reason for that, according to Dr. Martin: “Dermatologists have historically been risk averse. And apremilast is arguably the safest systemic agent we have to treat psoriasis. The beauty of apremilast is it requires no laboratory monitoring. That makes it attractive to dermatologists who are concerned about systemic therapy. It’s why there has been a huge jump in adoption of apremilast.”
Apremilast is comparable to methotrexate in terms of efficacy as reflected in week 16 PASI-75 response rates of about 35%, meaning 35% of treated patients obtain at least a 75% improvement in Psoriasis Area and Severity Index scores, he continued. Apremilast is particularly effective for scalp and nail psoriasis, making it a good option for patients who have psoriasis at those sites but not extensive involvement elsewhere, which might call for the use of a more potent biologic agent.
Surveys indicate that 20% of dermatologists write 80% of all prescriptions for biologic agents used to treat psoriasis. The thinking was that apremilast would appeal to the 80% of dermatologists who have steered clear of the biologics, and that after becoming comfortable with apremilast, they might become more receptive to using biologics for their patients with an inadequate response to the oral PDE-4 inhibitor. That hasn’t happened yet.
“We’re not seeing apremilast function as the gateway drug we thought it would be. It’s just going to take some time for those prescribers either to refer their patients who aren’t getting a good response to the next doctor who’s more adept at treating with biologic agents, or perhaps they themselves will get more involved,” Dr. Martin predicted.
He reported serving on scientific advisory boards for, and/or as a consultant to, nine pharmaceutical companies.
MAUI, HAWAII – If you’re going to prescribe apremilast for psoriasis or psoriatic arthritis – and more and more physicians are doing so because of the drug’s exceptional safety profile – you’d better get familiar with the oral phosphodiesterase-4 inhibitor’s gastrointestinal side effects, Dr. George M. Martin advised at the 2016 Rheumatology Winter Clinical Symposium.
“One of the biggest hurdles we have to deal with when we prescribe apremilast is the fact that there are these GI side effects,” said Dr. Martin, a dermatologist practicing in Maui and codirector of the rheumatology symposium.
Celgene, which markets apremilast (Otezla), sponsored an analysis of the pattern of diarrhea that emerged in the pooled results of the phase III ESTEEM 1 and 2 trials of apremilast at 30 mg twice daily for psoriasis and the PALACE 1-3 phase III psoriatic arthritis trials.
Diarrhea occurred in 16%-18% of patients on apremilast, a rate roughly threefold greater than in placebo-treated controls. Diarrhea onset was usually within the first 14 days of therapy. When it occurred, the duration was typically about 2 weeks.
“This you can relay to your patients so they’re not surprised if it happens,” the dermatologist said.
It’s a secretory diarrhea, and it is believed to be a classwide effect for the phosphodiesterase-4 (PDE-4) inhibitors. For example, roflumilast (Daliresp), an oral PDE-4 inhibitor used in the treatment of chronic obstructive pulmonary disease, has the same diarrhea issues. The mechanism has been worked out: The drug increases intracellular cyclic adenosine monophosphate, with resultant activation of chloride channels in crypts in the small bowel, which in turn leads to secretion of chloride ions. It takes the large bowel a couple of weeks to adapt. Caffeine causes diarrhea in some individuals through a similar mechanism.
Apremilast-related diarrhea often responds to the time-tested OTC remedies, including bismuth salicylate or fiber supplements. Alternatively, Dr. Martin said he is a fan of the oral prescription agent crofelemer (Fulyzaq) because of its exceptional safety, tolerability, and effectiveness. Plus, many residents of the garden islands of Hawaii like the idea of using a botanical derived from the latexlike sap – known as ‘dragon’s blood – of a South American tree. Crofelemer’s approved indication is the treatment of diarrhea associated with anti-HIV agents.
Diphenoxylate/atropine (Lomotil) is another effective prescription option.
Nausea and/or vomiting occurred in 15%-17% of apremilast-treated patients in the phase III trials. As with diarrhea, if nausea and/or vomiting is going to happen, it occurs early, within the first week or two. Dr. Martin said he finds in his own practice that the nausea/vomiting is less bothersome for patients than the diarrhea. Drug discontinuation due to any GI side effects is rarely necessary.
The nausea/vomiting is usually readily managed by encouraging affected patients to make sure that they’re well hydrated, take their apremilast with food, and eat smaller, more frequent meals. OTC diphenhydramine (Benadryl) is often effective, as are the usual prescription antiemetic agents.
Pharmaceutical industry data indicate apremilast has quickly captured a 17% share of the market for systemic psoriasis therapies. There is a good reason for that, according to Dr. Martin: “Dermatologists have historically been risk averse. And apremilast is arguably the safest systemic agent we have to treat psoriasis. The beauty of apremilast is it requires no laboratory monitoring. That makes it attractive to dermatologists who are concerned about systemic therapy. It’s why there has been a huge jump in adoption of apremilast.”
Apremilast is comparable to methotrexate in terms of efficacy as reflected in week 16 PASI-75 response rates of about 35%, meaning 35% of treated patients obtain at least a 75% improvement in Psoriasis Area and Severity Index scores, he continued. Apremilast is particularly effective for scalp and nail psoriasis, making it a good option for patients who have psoriasis at those sites but not extensive involvement elsewhere, which might call for the use of a more potent biologic agent.
Surveys indicate that 20% of dermatologists write 80% of all prescriptions for biologic agents used to treat psoriasis. The thinking was that apremilast would appeal to the 80% of dermatologists who have steered clear of the biologics, and that after becoming comfortable with apremilast, they might become more receptive to using biologics for their patients with an inadequate response to the oral PDE-4 inhibitor. That hasn’t happened yet.
“We’re not seeing apremilast function as the gateway drug we thought it would be. It’s just going to take some time for those prescribers either to refer their patients who aren’t getting a good response to the next doctor who’s more adept at treating with biologic agents, or perhaps they themselves will get more involved,” Dr. Martin predicted.
He reported serving on scientific advisory boards for, and/or as a consultant to, nine pharmaceutical companies.
MAUI, HAWAII – If you’re going to prescribe apremilast for psoriasis or psoriatic arthritis – and more and more physicians are doing so because of the drug’s exceptional safety profile – you’d better get familiar with the oral phosphodiesterase-4 inhibitor’s gastrointestinal side effects, Dr. George M. Martin advised at the 2016 Rheumatology Winter Clinical Symposium.
“One of the biggest hurdles we have to deal with when we prescribe apremilast is the fact that there are these GI side effects,” said Dr. Martin, a dermatologist practicing in Maui and codirector of the rheumatology symposium.
Celgene, which markets apremilast (Otezla), sponsored an analysis of the pattern of diarrhea that emerged in the pooled results of the phase III ESTEEM 1 and 2 trials of apremilast at 30 mg twice daily for psoriasis and the PALACE 1-3 phase III psoriatic arthritis trials.
Diarrhea occurred in 16%-18% of patients on apremilast, a rate roughly threefold greater than in placebo-treated controls. Diarrhea onset was usually within the first 14 days of therapy. When it occurred, the duration was typically about 2 weeks.
“This you can relay to your patients so they’re not surprised if it happens,” the dermatologist said.
It’s a secretory diarrhea, and it is believed to be a classwide effect for the phosphodiesterase-4 (PDE-4) inhibitors. For example, roflumilast (Daliresp), an oral PDE-4 inhibitor used in the treatment of chronic obstructive pulmonary disease, has the same diarrhea issues. The mechanism has been worked out: The drug increases intracellular cyclic adenosine monophosphate, with resultant activation of chloride channels in crypts in the small bowel, which in turn leads to secretion of chloride ions. It takes the large bowel a couple of weeks to adapt. Caffeine causes diarrhea in some individuals through a similar mechanism.
Apremilast-related diarrhea often responds to the time-tested OTC remedies, including bismuth salicylate or fiber supplements. Alternatively, Dr. Martin said he is a fan of the oral prescription agent crofelemer (Fulyzaq) because of its exceptional safety, tolerability, and effectiveness. Plus, many residents of the garden islands of Hawaii like the idea of using a botanical derived from the latexlike sap – known as ‘dragon’s blood – of a South American tree. Crofelemer’s approved indication is the treatment of diarrhea associated with anti-HIV agents.
Diphenoxylate/atropine (Lomotil) is another effective prescription option.
Nausea and/or vomiting occurred in 15%-17% of apremilast-treated patients in the phase III trials. As with diarrhea, if nausea and/or vomiting is going to happen, it occurs early, within the first week or two. Dr. Martin said he finds in his own practice that the nausea/vomiting is less bothersome for patients than the diarrhea. Drug discontinuation due to any GI side effects is rarely necessary.
The nausea/vomiting is usually readily managed by encouraging affected patients to make sure that they’re well hydrated, take their apremilast with food, and eat smaller, more frequent meals. OTC diphenhydramine (Benadryl) is often effective, as are the usual prescription antiemetic agents.
Pharmaceutical industry data indicate apremilast has quickly captured a 17% share of the market for systemic psoriasis therapies. There is a good reason for that, according to Dr. Martin: “Dermatologists have historically been risk averse. And apremilast is arguably the safest systemic agent we have to treat psoriasis. The beauty of apremilast is it requires no laboratory monitoring. That makes it attractive to dermatologists who are concerned about systemic therapy. It’s why there has been a huge jump in adoption of apremilast.”
Apremilast is comparable to methotrexate in terms of efficacy as reflected in week 16 PASI-75 response rates of about 35%, meaning 35% of treated patients obtain at least a 75% improvement in Psoriasis Area and Severity Index scores, he continued. Apremilast is particularly effective for scalp and nail psoriasis, making it a good option for patients who have psoriasis at those sites but not extensive involvement elsewhere, which might call for the use of a more potent biologic agent.
Surveys indicate that 20% of dermatologists write 80% of all prescriptions for biologic agents used to treat psoriasis. The thinking was that apremilast would appeal to the 80% of dermatologists who have steered clear of the biologics, and that after becoming comfortable with apremilast, they might become more receptive to using biologics for their patients with an inadequate response to the oral PDE-4 inhibitor. That hasn’t happened yet.
“We’re not seeing apremilast function as the gateway drug we thought it would be. It’s just going to take some time for those prescribers either to refer their patients who aren’t getting a good response to the next doctor who’s more adept at treating with biologic agents, or perhaps they themselves will get more involved,” Dr. Martin predicted.
He reported serving on scientific advisory boards for, and/or as a consultant to, nine pharmaceutical companies.
EXPERT ANALYSIS FROM RWCS 2016
AATS Annual Meeting Registration Packages Still Available
Health Care Professional Package: includes registration for the Saturday Courses, Sunday Symposia and the 96th Annual Meeting (Monday-Wednesday). Registration is $500 (a savings of $400).
Resident/Fellow and Medical Student Package: includes registration for the Saturday Courses, Sunday Symposia and the 96th Annual Meeting (Monday-Wednesday). Registration is $300.
Saturday Courses and Sunday Symposia Registration: Register for a Saturday course and/or a Sunday symposia and have access to all other courses/symposia taking place that same day. Note: Registration for the Saturday courses and/or Sunday symposia is separate from the Annual Meeting fee.
Health Care Professional Package: includes registration for the Saturday Courses, Sunday Symposia and the 96th Annual Meeting (Monday-Wednesday). Registration is $500 (a savings of $400).
Resident/Fellow and Medical Student Package: includes registration for the Saturday Courses, Sunday Symposia and the 96th Annual Meeting (Monday-Wednesday). Registration is $300.
Saturday Courses and Sunday Symposia Registration: Register for a Saturday course and/or a Sunday symposia and have access to all other courses/symposia taking place that same day. Note: Registration for the Saturday courses and/or Sunday symposia is separate from the Annual Meeting fee.
Health Care Professional Package: includes registration for the Saturday Courses, Sunday Symposia and the 96th Annual Meeting (Monday-Wednesday). Registration is $500 (a savings of $400).
Resident/Fellow and Medical Student Package: includes registration for the Saturday Courses, Sunday Symposia and the 96th Annual Meeting (Monday-Wednesday). Registration is $300.
Saturday Courses and Sunday Symposia Registration: Register for a Saturday course and/or a Sunday symposia and have access to all other courses/symposia taking place that same day. Note: Registration for the Saturday courses and/or Sunday symposia is separate from the Annual Meeting fee.
See you at AATS Week 2016!
AATS Week 2016 includes Two Terrific Events
AATS Week 2016 Registration & Housing Open!
Aortic Symposium
May 12–13, 2016
New York, NY
(More information below)
96th Annual Meeting
May 14-18, 2016
Baltimore, MD
(More information below)
Register for AATS Week 2016 today & receive a $100 discount off the AATS Aortic Symposium registration fee.
AATS Aortic Symposium
May 12–13, 2016
New York, NY
Course Directors
Joseph S. Coselli
Steven L. Lansman
The 2016 AATS Aortic Symposium is a two-day symposium focused on the pathophysiology, diagnosis and treatment of aortic aneurysms and dissections. The conference is designed for cardiovascular and thoracic surgeons, residents, perfusionists, ICU and OR nurses and others involved in aortic disease patient care. Faculty members include world leaders in the field who will share their experiences treating difficult aortic disease cases.
Be sure to register for a Friday Morning Breakfast Breakout session.
AATS 96th Annual Meeting
May 14-18, 2016
Baltimore, MD
President & Annual Meeting Chair
Joseph S. Coselli
Annual Meeting Co-Chairs
Charles D. Fraser
David R. Jones
View Preliminary Program, Speakers, Presentations and Full Abstracts
Don’t miss this year’s exciting program including:
Saturday Skills Courses featuring Combined Luncheon Speaker: Denton A. Cooley, followed by Hands-On Sessions
Sunday Postgraduate Symposia with Legends Luncheons featuring Leonard L. Bailey, Joel D. Cooper and John L. Ochsner
New: Survival Guide for the Cardiothoracic Surgical Team course following by a Hands-On Session (Available to Residents, Fellows and Health Care Professionals Only)
Presidential Address: Competition: Perspiration to Inspiration “Aut viam inveniam aut faciam,” Joseph S. Coselli, Baylor College of Medicine
Honored Guest Lecture: Brian Kelly, Notre Dame Head Football Coach and a veteran of 23 seasons as a collegiate head coach. Brian Kelly brings a championship tradition to his fifth year as the 29th head football coach at the University of Notre Dame.
Emerging Technologies & Techniques For: Adult Cardiac and General Thoracic
VAD/ECMO SessionMasters of Surgery Video Sessions
AATS Learning Center: Featuring cutting-edge case videos of novel procedures and surgical techniques.
Check Out the AATS Week Video
Learn more about the exciting program planned for the AATS Aortic Symposium and 2016 Annual Meeting.
AATS Week 2016 includes Two Terrific Events
AATS Week 2016 Registration & Housing Open!
Aortic Symposium
May 12–13, 2016
New York, NY
(More information below)
96th Annual Meeting
May 14-18, 2016
Baltimore, MD
(More information below)
Register for AATS Week 2016 today & receive a $100 discount off the AATS Aortic Symposium registration fee.
AATS Aortic Symposium
May 12–13, 2016
New York, NY
Course Directors
Joseph S. Coselli
Steven L. Lansman
The 2016 AATS Aortic Symposium is a two-day symposium focused on the pathophysiology, diagnosis and treatment of aortic aneurysms and dissections. The conference is designed for cardiovascular and thoracic surgeons, residents, perfusionists, ICU and OR nurses and others involved in aortic disease patient care. Faculty members include world leaders in the field who will share their experiences treating difficult aortic disease cases.
Be sure to register for a Friday Morning Breakfast Breakout session.
AATS 96th Annual Meeting
May 14-18, 2016
Baltimore, MD
President & Annual Meeting Chair
Joseph S. Coselli
Annual Meeting Co-Chairs
Charles D. Fraser
David R. Jones
View Preliminary Program, Speakers, Presentations and Full Abstracts
Don’t miss this year’s exciting program including:
Saturday Skills Courses featuring Combined Luncheon Speaker: Denton A. Cooley, followed by Hands-On Sessions
Sunday Postgraduate Symposia with Legends Luncheons featuring Leonard L. Bailey, Joel D. Cooper and John L. Ochsner
New: Survival Guide for the Cardiothoracic Surgical Team course following by a Hands-On Session (Available to Residents, Fellows and Health Care Professionals Only)
Presidential Address: Competition: Perspiration to Inspiration “Aut viam inveniam aut faciam,” Joseph S. Coselli, Baylor College of Medicine
Honored Guest Lecture: Brian Kelly, Notre Dame Head Football Coach and a veteran of 23 seasons as a collegiate head coach. Brian Kelly brings a championship tradition to his fifth year as the 29th head football coach at the University of Notre Dame.
Emerging Technologies & Techniques For: Adult Cardiac and General Thoracic
VAD/ECMO SessionMasters of Surgery Video Sessions
AATS Learning Center: Featuring cutting-edge case videos of novel procedures and surgical techniques.
Check Out the AATS Week Video
Learn more about the exciting program planned for the AATS Aortic Symposium and 2016 Annual Meeting.
AATS Week 2016 includes Two Terrific Events
AATS Week 2016 Registration & Housing Open!
Aortic Symposium
May 12–13, 2016
New York, NY
(More information below)
96th Annual Meeting
May 14-18, 2016
Baltimore, MD
(More information below)
Register for AATS Week 2016 today & receive a $100 discount off the AATS Aortic Symposium registration fee.
AATS Aortic Symposium
May 12–13, 2016
New York, NY
Course Directors
Joseph S. Coselli
Steven L. Lansman
The 2016 AATS Aortic Symposium is a two-day symposium focused on the pathophysiology, diagnosis and treatment of aortic aneurysms and dissections. The conference is designed for cardiovascular and thoracic surgeons, residents, perfusionists, ICU and OR nurses and others involved in aortic disease patient care. Faculty members include world leaders in the field who will share their experiences treating difficult aortic disease cases.
Be sure to register for a Friday Morning Breakfast Breakout session.
AATS 96th Annual Meeting
May 14-18, 2016
Baltimore, MD
President & Annual Meeting Chair
Joseph S. Coselli
Annual Meeting Co-Chairs
Charles D. Fraser
David R. Jones
View Preliminary Program, Speakers, Presentations and Full Abstracts
Don’t miss this year’s exciting program including:
Saturday Skills Courses featuring Combined Luncheon Speaker: Denton A. Cooley, followed by Hands-On Sessions
Sunday Postgraduate Symposia with Legends Luncheons featuring Leonard L. Bailey, Joel D. Cooper and John L. Ochsner
New: Survival Guide for the Cardiothoracic Surgical Team course following by a Hands-On Session (Available to Residents, Fellows and Health Care Professionals Only)
Presidential Address: Competition: Perspiration to Inspiration “Aut viam inveniam aut faciam,” Joseph S. Coselli, Baylor College of Medicine
Honored Guest Lecture: Brian Kelly, Notre Dame Head Football Coach and a veteran of 23 seasons as a collegiate head coach. Brian Kelly brings a championship tradition to his fifth year as the 29th head football coach at the University of Notre Dame.
Emerging Technologies & Techniques For: Adult Cardiac and General Thoracic
VAD/ECMO SessionMasters of Surgery Video Sessions
AATS Learning Center: Featuring cutting-edge case videos of novel procedures and surgical techniques.
Check Out the AATS Week Video
Learn more about the exciting program planned for the AATS Aortic Symposium and 2016 Annual Meeting.
Congratulations to 2016 “Honoring Our Mentors” Fellows
Winners of the F. Griffith Pearson Fellowships and the Mark R. de Leval Fellowship announced.
F. Griffith Pearson Fellowship
Nestor Villamizar Ortiz, MD
Institution: University of Miami
Host Sponsor: Mark Onaitis, MD
Host Institution: Duke University Medical Center
Fellowship Focus: Robotic Surgery for Malignant and Benign Esophageal Pathology
Xiao Li, MD
Institution: Peking University People’s Hospital, Beijing, China
Host Sponsor: Mark K. Ferguson, MD
Host Institution: Department of Thoracic Surgery, University of Chicago
Fellowship Focus: Advanced Minimally Invasive Thoracic Surgery and Robotic Thoracic Surgery
Marc R. de Leval Fellowship
Jeremy Herrmann, MD
Institution: The Children’s Hospital of Philadelphia
Host Sponsor: David Barron, MD
Host Institution: Birmingham Children’s Hospital, UK
Fellowship Focus: Management of ccTGA
Winners of the F. Griffith Pearson Fellowships and the Mark R. de Leval Fellowship announced.
F. Griffith Pearson Fellowship
Nestor Villamizar Ortiz, MD
Institution: University of Miami
Host Sponsor: Mark Onaitis, MD
Host Institution: Duke University Medical Center
Fellowship Focus: Robotic Surgery for Malignant and Benign Esophageal Pathology
Xiao Li, MD
Institution: Peking University People’s Hospital, Beijing, China
Host Sponsor: Mark K. Ferguson, MD
Host Institution: Department of Thoracic Surgery, University of Chicago
Fellowship Focus: Advanced Minimally Invasive Thoracic Surgery and Robotic Thoracic Surgery
Marc R. de Leval Fellowship
Jeremy Herrmann, MD
Institution: The Children’s Hospital of Philadelphia
Host Sponsor: David Barron, MD
Host Institution: Birmingham Children’s Hospital, UK
Fellowship Focus: Management of ccTGA
Winners of the F. Griffith Pearson Fellowships and the Mark R. de Leval Fellowship announced.
F. Griffith Pearson Fellowship
Nestor Villamizar Ortiz, MD
Institution: University of Miami
Host Sponsor: Mark Onaitis, MD
Host Institution: Duke University Medical Center
Fellowship Focus: Robotic Surgery for Malignant and Benign Esophageal Pathology
Xiao Li, MD
Institution: Peking University People’s Hospital, Beijing, China
Host Sponsor: Mark K. Ferguson, MD
Host Institution: Department of Thoracic Surgery, University of Chicago
Fellowship Focus: Advanced Minimally Invasive Thoracic Surgery and Robotic Thoracic Surgery
Marc R. de Leval Fellowship
Jeremy Herrmann, MD
Institution: The Children’s Hospital of Philadelphia
Host Sponsor: David Barron, MD
Host Institution: Birmingham Children’s Hospital, UK
Fellowship Focus: Management of ccTGA