User login
FDA approves first duodenoscope with disposable distal cap
, according to an FDA press release.
A disposable distal cap will improve the ability to clean and reprocess the duodenoscope. Without being thoroughly cleaned and disinfected, contaminated tissue can remain and potentially can be transmitted to other patients.
A previous version of the Pentax duodenoscope, the ED-3490TK, was subject to a January 2017 FDA Safety Alert, because of the potential for cracks and gaps to develop in the adhesive sealing the duodenoscope’s distal cap.
“Since the issue of duodenoscope-associated transmission of infections first received widespread attention in 2015, the AGA Center for GI Innovation and Technology has been working closely with regulators and endoscope manufacturers to identify and address problems in scope design and develop a path forward to ensure zero device-associated infections,” said V. Raman Muthusamy, MD, AGAF, FACG, FASGE, chair, AGA Center for GI Innovation and Technology. “We applaud Pentax for answering our call for innovation to improve patient safety for this common and life-saving GI procedure. We encourage all device manufacturers to continue on a path of innovation to better support gastroenterologists and, most importantly, the patients we serve.”
, according to an FDA press release.
A disposable distal cap will improve the ability to clean and reprocess the duodenoscope. Without being thoroughly cleaned and disinfected, contaminated tissue can remain and potentially can be transmitted to other patients.
A previous version of the Pentax duodenoscope, the ED-3490TK, was subject to a January 2017 FDA Safety Alert, because of the potential for cracks and gaps to develop in the adhesive sealing the duodenoscope’s distal cap.
“Since the issue of duodenoscope-associated transmission of infections first received widespread attention in 2015, the AGA Center for GI Innovation and Technology has been working closely with regulators and endoscope manufacturers to identify and address problems in scope design and develop a path forward to ensure zero device-associated infections,” said V. Raman Muthusamy, MD, AGAF, FACG, FASGE, chair, AGA Center for GI Innovation and Technology. “We applaud Pentax for answering our call for innovation to improve patient safety for this common and life-saving GI procedure. We encourage all device manufacturers to continue on a path of innovation to better support gastroenterologists and, most importantly, the patients we serve.”
, according to an FDA press release.
A disposable distal cap will improve the ability to clean and reprocess the duodenoscope. Without being thoroughly cleaned and disinfected, contaminated tissue can remain and potentially can be transmitted to other patients.
A previous version of the Pentax duodenoscope, the ED-3490TK, was subject to a January 2017 FDA Safety Alert, because of the potential for cracks and gaps to develop in the adhesive sealing the duodenoscope’s distal cap.
“Since the issue of duodenoscope-associated transmission of infections first received widespread attention in 2015, the AGA Center for GI Innovation and Technology has been working closely with regulators and endoscope manufacturers to identify and address problems in scope design and develop a path forward to ensure zero device-associated infections,” said V. Raman Muthusamy, MD, AGAF, FACG, FASGE, chair, AGA Center for GI Innovation and Technology. “We applaud Pentax for answering our call for innovation to improve patient safety for this common and life-saving GI procedure. We encourage all device manufacturers to continue on a path of innovation to better support gastroenterologists and, most importantly, the patients we serve.”
Caffeine offers no perks for Parkinson’s patients
Caffeine consumption has no significant impact on motor skills in patients with Parkinson’s disease, based on data from a double-blind, randomized, placebo-controlled trial of 121 adults. The findings were published online Sept. 27 in Neurology.
Data from previous studies have suggested a link between caffeine consumption and reduced risk of Parkinson’s disease, wrote Ronald B. Postuma, MD, of McGill University in Montreal, and his colleagues (Neurology. 2017 Sep 27. doi: 10.1212/WNL.0000000000004568). In addition, Dr. Postuma and his coauthors found a small impact of caffeine on motor skills in patients with existing Parkinson’s as a secondary outcome in a 2012 study on the role of caffeine on daytime sleepiness. Based on these findings, they designed a multicenter, randomized, controlled trial of 121 adults with Parkinson’s to assess the impact of caffeine. The average age of the patients was 62 years and the average disease duration was 4 years.
Motor skills worsened in the caffeine group by an average of 0.16 points on the Movement Disorder Society–sponsored Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III during patients’ “on” state and by 0.64 points in the placebo group; the difference was not significant.
The researchers found no differences between groups in the clinical global impression of change based on both patient and examiner assessment. In addition, no differences appeared in depression or anxiety, or in quality of life.
The study findings included long-term follow up data from 88 patients assessed at 12 months and 66 patients assessed at 18 months. The results were similar to the 6-month results, and the study was stopped, although the original design included a 4-year extension.
A total of 29 patients in the caffeine group and 31 patients in the placebo group reported adverse events, and 7 patients in the caffeine group and 5 in the placebo group discontinued the study because of side effects. A serious adverse event was reported by one patient in each group, but neither was deemed related to the interventions.
The findings contrast with the effects of caffeine in the 6-week study in 2012 that showed a significant, 3.2-point improvement in motor skills on the MDS-UPDRS part III with caffeine use, as well as reduced daytime sleepiness, the researchers said (Neurology. 2012;79:651-8). Interpretations of the different findings between the trials may be constrained by factors including differences in the study populations, speed of dose escalation, and trial duration and the possible short-term nature of caffeine’s impact, they noted. “Regardless, our core finding is that caffeine cannot be recommended as symptomatic therapy for parkinsonism.” However, “since caffeine is safe and generally well tolerated, it seems reasonable to empirically try intermittent moderate doses of caffeine for somnolence, and repeat if improvement is seen,” they added.
Dr. Postuma disclosed grant funding from the Canadian Institute of Health Research, the Webster Foundation, and Fonds de Recherche du Québec-Santé for this study.
The appeal of an inexpensive, well-tolerated intervention such as caffeine to improve motor symptoms in Parkinson’s patients gave Dr. Postuma and his associates a good reason to try to replicate the positive results they found in an earlier study of caffeine.
The investigators found no benefit from caffeine, although their study was designed to have more than four times as many participants as the pilot study, was adequately powered to detect the same level of improvement as before, and was planned to have an extended follow-up period to look for persistent effects. The trial ended early and enrolled approximately half of its intended total, but was well run and did not suffer from differential compliance or loss to follow-up.
Although the small number of participants resulted in a wide confidence interval and cannot exclude a small effect, this trial suggests that caffeine does not significantly improve Parkinson’s disease symptoms and that it should not be a priority for future Parkinson’s disease intervention studies.
Charles B. Hall, PhD, is affiliated with the department of epidemiology and population health at Albert Einstein College of Medicine, New York. He disclosed salary support from the National Institute of Occupational Safety and Health, National Institute of Aging, National Cancer Institute, and National Center for Advancing Translational Sciences. His comments are derived from an editorial that accompanied Dr. Postuma and colleagues’ study (Neurology. 2017 Sep 27. doi: 10.1212/WNL.0000000000004584).
The appeal of an inexpensive, well-tolerated intervention such as caffeine to improve motor symptoms in Parkinson’s patients gave Dr. Postuma and his associates a good reason to try to replicate the positive results they found in an earlier study of caffeine.
The investigators found no benefit from caffeine, although their study was designed to have more than four times as many participants as the pilot study, was adequately powered to detect the same level of improvement as before, and was planned to have an extended follow-up period to look for persistent effects. The trial ended early and enrolled approximately half of its intended total, but was well run and did not suffer from differential compliance or loss to follow-up.
Although the small number of participants resulted in a wide confidence interval and cannot exclude a small effect, this trial suggests that caffeine does not significantly improve Parkinson’s disease symptoms and that it should not be a priority for future Parkinson’s disease intervention studies.
Charles B. Hall, PhD, is affiliated with the department of epidemiology and population health at Albert Einstein College of Medicine, New York. He disclosed salary support from the National Institute of Occupational Safety and Health, National Institute of Aging, National Cancer Institute, and National Center for Advancing Translational Sciences. His comments are derived from an editorial that accompanied Dr. Postuma and colleagues’ study (Neurology. 2017 Sep 27. doi: 10.1212/WNL.0000000000004584).
The appeal of an inexpensive, well-tolerated intervention such as caffeine to improve motor symptoms in Parkinson’s patients gave Dr. Postuma and his associates a good reason to try to replicate the positive results they found in an earlier study of caffeine.
The investigators found no benefit from caffeine, although their study was designed to have more than four times as many participants as the pilot study, was adequately powered to detect the same level of improvement as before, and was planned to have an extended follow-up period to look for persistent effects. The trial ended early and enrolled approximately half of its intended total, but was well run and did not suffer from differential compliance or loss to follow-up.
Although the small number of participants resulted in a wide confidence interval and cannot exclude a small effect, this trial suggests that caffeine does not significantly improve Parkinson’s disease symptoms and that it should not be a priority for future Parkinson’s disease intervention studies.
Charles B. Hall, PhD, is affiliated with the department of epidemiology and population health at Albert Einstein College of Medicine, New York. He disclosed salary support from the National Institute of Occupational Safety and Health, National Institute of Aging, National Cancer Institute, and National Center for Advancing Translational Sciences. His comments are derived from an editorial that accompanied Dr. Postuma and colleagues’ study (Neurology. 2017 Sep 27. doi: 10.1212/WNL.0000000000004584).
Caffeine consumption has no significant impact on motor skills in patients with Parkinson’s disease, based on data from a double-blind, randomized, placebo-controlled trial of 121 adults. The findings were published online Sept. 27 in Neurology.
Data from previous studies have suggested a link between caffeine consumption and reduced risk of Parkinson’s disease, wrote Ronald B. Postuma, MD, of McGill University in Montreal, and his colleagues (Neurology. 2017 Sep 27. doi: 10.1212/WNL.0000000000004568). In addition, Dr. Postuma and his coauthors found a small impact of caffeine on motor skills in patients with existing Parkinson’s as a secondary outcome in a 2012 study on the role of caffeine on daytime sleepiness. Based on these findings, they designed a multicenter, randomized, controlled trial of 121 adults with Parkinson’s to assess the impact of caffeine. The average age of the patients was 62 years and the average disease duration was 4 years.
Motor skills worsened in the caffeine group by an average of 0.16 points on the Movement Disorder Society–sponsored Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III during patients’ “on” state and by 0.64 points in the placebo group; the difference was not significant.
The researchers found no differences between groups in the clinical global impression of change based on both patient and examiner assessment. In addition, no differences appeared in depression or anxiety, or in quality of life.
The study findings included long-term follow up data from 88 patients assessed at 12 months and 66 patients assessed at 18 months. The results were similar to the 6-month results, and the study was stopped, although the original design included a 4-year extension.
A total of 29 patients in the caffeine group and 31 patients in the placebo group reported adverse events, and 7 patients in the caffeine group and 5 in the placebo group discontinued the study because of side effects. A serious adverse event was reported by one patient in each group, but neither was deemed related to the interventions.
The findings contrast with the effects of caffeine in the 6-week study in 2012 that showed a significant, 3.2-point improvement in motor skills on the MDS-UPDRS part III with caffeine use, as well as reduced daytime sleepiness, the researchers said (Neurology. 2012;79:651-8). Interpretations of the different findings between the trials may be constrained by factors including differences in the study populations, speed of dose escalation, and trial duration and the possible short-term nature of caffeine’s impact, they noted. “Regardless, our core finding is that caffeine cannot be recommended as symptomatic therapy for parkinsonism.” However, “since caffeine is safe and generally well tolerated, it seems reasonable to empirically try intermittent moderate doses of caffeine for somnolence, and repeat if improvement is seen,” they added.
Dr. Postuma disclosed grant funding from the Canadian Institute of Health Research, the Webster Foundation, and Fonds de Recherche du Québec-Santé for this study.
Caffeine consumption has no significant impact on motor skills in patients with Parkinson’s disease, based on data from a double-blind, randomized, placebo-controlled trial of 121 adults. The findings were published online Sept. 27 in Neurology.
Data from previous studies have suggested a link between caffeine consumption and reduced risk of Parkinson’s disease, wrote Ronald B. Postuma, MD, of McGill University in Montreal, and his colleagues (Neurology. 2017 Sep 27. doi: 10.1212/WNL.0000000000004568). In addition, Dr. Postuma and his coauthors found a small impact of caffeine on motor skills in patients with existing Parkinson’s as a secondary outcome in a 2012 study on the role of caffeine on daytime sleepiness. Based on these findings, they designed a multicenter, randomized, controlled trial of 121 adults with Parkinson’s to assess the impact of caffeine. The average age of the patients was 62 years and the average disease duration was 4 years.
Motor skills worsened in the caffeine group by an average of 0.16 points on the Movement Disorder Society–sponsored Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III during patients’ “on” state and by 0.64 points in the placebo group; the difference was not significant.
The researchers found no differences between groups in the clinical global impression of change based on both patient and examiner assessment. In addition, no differences appeared in depression or anxiety, or in quality of life.
The study findings included long-term follow up data from 88 patients assessed at 12 months and 66 patients assessed at 18 months. The results were similar to the 6-month results, and the study was stopped, although the original design included a 4-year extension.
A total of 29 patients in the caffeine group and 31 patients in the placebo group reported adverse events, and 7 patients in the caffeine group and 5 in the placebo group discontinued the study because of side effects. A serious adverse event was reported by one patient in each group, but neither was deemed related to the interventions.
The findings contrast with the effects of caffeine in the 6-week study in 2012 that showed a significant, 3.2-point improvement in motor skills on the MDS-UPDRS part III with caffeine use, as well as reduced daytime sleepiness, the researchers said (Neurology. 2012;79:651-8). Interpretations of the different findings between the trials may be constrained by factors including differences in the study populations, speed of dose escalation, and trial duration and the possible short-term nature of caffeine’s impact, they noted. “Regardless, our core finding is that caffeine cannot be recommended as symptomatic therapy for parkinsonism.” However, “since caffeine is safe and generally well tolerated, it seems reasonable to empirically try intermittent moderate doses of caffeine for somnolence, and repeat if improvement is seen,” they added.
Dr. Postuma disclosed grant funding from the Canadian Institute of Health Research, the Webster Foundation, and Fonds de Recherche du Québec-Santé for this study.
FROM NEUROLOGY
Key clinical point:
Major finding: Motor skills declined by an average of 0.16 points in the caffeine group and 0.64 points in the placebo group; the difference was not significant.
Data source: A double-blind, multicenter, placebo-controlled trial of 121 adults with Parkinson’s.
Disclosures: Dr. Postuma disclosed grant funding from the Canadian Institute of Health Research, the Webster Foundation, and Fonds de Recherche du Québec-Santé for this study.
LUME-Mesa trial: Nintedanib improves PFS in mesothelioma
CHICAGO – Adding the oral kinase inhibitor nintedanib to pemetrexed/cisplatin resulted in substantial improvements in outcomes in patients with unresectable malignant pleural mesothelioma in phase 2 of the randomized, placebo-controlled phase 2/3 LUME-Meso study.
The effects were particularly pronounced among those with epithelioid histology, Jose Barrueco, PhD, of Boehringer Ingelheim Pharmaceuticals, Ridgefield, Conn., reported at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
Progression-free survival – the primary endpoint of the study – was improved in 44 patients randomized to receive up to six cycles of pemetrexed/cisplatin plus nintedanib, compared with 43 patients who received pemetrexed/cisplatin plus placebo (median 9.4 vs. 5.7 months; hazard ratio, 0.54), Dr. Barrueco said.
In the 89% of patients with epithelioid malignant pleural mesothelioma, progression-free survival was a median of 9.7 vs. 5.7 months with nintedanib vs. placebo (HR, 0.49).
There was a trend toward improved overall survival in the nintedanib group vs. the placebo group, (median 18.3 vs. 14.2 months; HR, 0.77; P = .319), and overall survival was slightly better in those with epithelioid histology (median 20.6 vs. 15.2 months ; HR, 0.70; P = .197).
Consistent with these results, the adjusted mean change in forced vital capacity at cycle eight also favored nintedanib over placebo (+10.0 vs. +2.8 for a mean treatment difference of 7.2 overall, and +14.1 vs. +4.2 for a mean treatment difference of 9.9 in those with epithelioid histology).
“Overall frequency of adverse events was consistent with the known safety profile of nintedanib,” Dr. Barrueco said, noting that most adverse events were reversible with dose reduction.
Study participants were chemotherapy-naive patients with a mean age of 67 years and Eastern Cooperative Oncology Group performance status of 0-1. They received pemetrexed at a dose of 500 mg/m2 and cisplatin at a dose of 75 mg/m2 on day 1 plus either nintedanib at a dose of 200 mg twice daily on days 2-21 or placebo, followed by monotherapy with nintedanib or placebo until progression or unacceptable toxicity.
“In conclusion, nintedanib plus pemetrexed/cisplatin demonstrated a signal for clinical benefit in the first-time treatment of patients with malignant pleural mesothelioma. This was evident in all endpoints of the trial, and consistently showed benefit for the nintedanib group,” Mr. Barrueco said, noting that phase 3 of the LUME-Meso study is now recruiting.
CHICAGO – Adding the oral kinase inhibitor nintedanib to pemetrexed/cisplatin resulted in substantial improvements in outcomes in patients with unresectable malignant pleural mesothelioma in phase 2 of the randomized, placebo-controlled phase 2/3 LUME-Meso study.
The effects were particularly pronounced among those with epithelioid histology, Jose Barrueco, PhD, of Boehringer Ingelheim Pharmaceuticals, Ridgefield, Conn., reported at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
Progression-free survival – the primary endpoint of the study – was improved in 44 patients randomized to receive up to six cycles of pemetrexed/cisplatin plus nintedanib, compared with 43 patients who received pemetrexed/cisplatin plus placebo (median 9.4 vs. 5.7 months; hazard ratio, 0.54), Dr. Barrueco said.
In the 89% of patients with epithelioid malignant pleural mesothelioma, progression-free survival was a median of 9.7 vs. 5.7 months with nintedanib vs. placebo (HR, 0.49).
There was a trend toward improved overall survival in the nintedanib group vs. the placebo group, (median 18.3 vs. 14.2 months; HR, 0.77; P = .319), and overall survival was slightly better in those with epithelioid histology (median 20.6 vs. 15.2 months ; HR, 0.70; P = .197).
Consistent with these results, the adjusted mean change in forced vital capacity at cycle eight also favored nintedanib over placebo (+10.0 vs. +2.8 for a mean treatment difference of 7.2 overall, and +14.1 vs. +4.2 for a mean treatment difference of 9.9 in those with epithelioid histology).
“Overall frequency of adverse events was consistent with the known safety profile of nintedanib,” Dr. Barrueco said, noting that most adverse events were reversible with dose reduction.
Study participants were chemotherapy-naive patients with a mean age of 67 years and Eastern Cooperative Oncology Group performance status of 0-1. They received pemetrexed at a dose of 500 mg/m2 and cisplatin at a dose of 75 mg/m2 on day 1 plus either nintedanib at a dose of 200 mg twice daily on days 2-21 or placebo, followed by monotherapy with nintedanib or placebo until progression or unacceptable toxicity.
“In conclusion, nintedanib plus pemetrexed/cisplatin demonstrated a signal for clinical benefit in the first-time treatment of patients with malignant pleural mesothelioma. This was evident in all endpoints of the trial, and consistently showed benefit for the nintedanib group,” Mr. Barrueco said, noting that phase 3 of the LUME-Meso study is now recruiting.
CHICAGO – Adding the oral kinase inhibitor nintedanib to pemetrexed/cisplatin resulted in substantial improvements in outcomes in patients with unresectable malignant pleural mesothelioma in phase 2 of the randomized, placebo-controlled phase 2/3 LUME-Meso study.
The effects were particularly pronounced among those with epithelioid histology, Jose Barrueco, PhD, of Boehringer Ingelheim Pharmaceuticals, Ridgefield, Conn., reported at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
Progression-free survival – the primary endpoint of the study – was improved in 44 patients randomized to receive up to six cycles of pemetrexed/cisplatin plus nintedanib, compared with 43 patients who received pemetrexed/cisplatin plus placebo (median 9.4 vs. 5.7 months; hazard ratio, 0.54), Dr. Barrueco said.
In the 89% of patients with epithelioid malignant pleural mesothelioma, progression-free survival was a median of 9.7 vs. 5.7 months with nintedanib vs. placebo (HR, 0.49).
There was a trend toward improved overall survival in the nintedanib group vs. the placebo group, (median 18.3 vs. 14.2 months; HR, 0.77; P = .319), and overall survival was slightly better in those with epithelioid histology (median 20.6 vs. 15.2 months ; HR, 0.70; P = .197).
Consistent with these results, the adjusted mean change in forced vital capacity at cycle eight also favored nintedanib over placebo (+10.0 vs. +2.8 for a mean treatment difference of 7.2 overall, and +14.1 vs. +4.2 for a mean treatment difference of 9.9 in those with epithelioid histology).
“Overall frequency of adverse events was consistent with the known safety profile of nintedanib,” Dr. Barrueco said, noting that most adverse events were reversible with dose reduction.
Study participants were chemotherapy-naive patients with a mean age of 67 years and Eastern Cooperative Oncology Group performance status of 0-1. They received pemetrexed at a dose of 500 mg/m2 and cisplatin at a dose of 75 mg/m2 on day 1 plus either nintedanib at a dose of 200 mg twice daily on days 2-21 or placebo, followed by monotherapy with nintedanib or placebo until progression or unacceptable toxicity.
“In conclusion, nintedanib plus pemetrexed/cisplatin demonstrated a signal for clinical benefit in the first-time treatment of patients with malignant pleural mesothelioma. This was evident in all endpoints of the trial, and consistently showed benefit for the nintedanib group,” Mr. Barrueco said, noting that phase 3 of the LUME-Meso study is now recruiting.
AT A SYMPOSIUM IN THORACIC ONCOLOGY
Key clinical point:
Major finding: Overall median PFS with nintedanib vs. placebo was 9.4 vs. 5.7 months (HR, 0.54).
Data source: Phase 2 of the LUME-Meso trial with 87 patients.
Disclosures: Dr. Barrueco is an employee of Boehringer Ingelheim.
Risk of Osteoporotic Fracture After Steroid Injections in Patients With Medicare
Take-Home Points
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
Although statistically significant, this may not be clinically relevant.
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
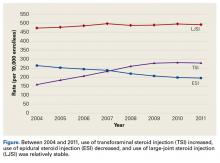
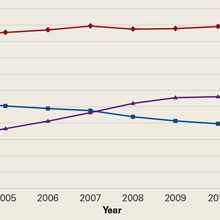
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
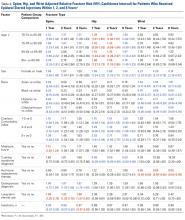
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
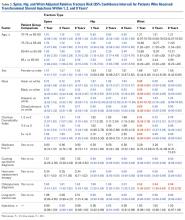
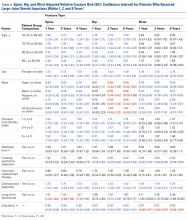
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.
Take-Home Points
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
Although statistically significant, this may not be clinically relevant.
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
Take-Home Points
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
Although statistically significant, this may not be clinically relevant.
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.
Robot-assisted prostatectomy providing better outcomes
Robot-assisted radical prostatectomy shows better early postoperative outcomes than does laparoscopic radical prostatectomy, but the differences between the two surgical approaches disappeared by the 6-month follow-up.
Dr. Hiroyuki Koike and his colleagues at Wakayama (Japan) Medical University Hospital conducted a study of two groups of patients treated for localized prostate cancer. One group of 229 patients underwent laparoscopic radical prostatectomy (LRP) between July 2007 and July 2013. The other group of 115 patients had robot-assisted radical prostatectomy (RARP) between December 2012 and August 2014 (J Robot Surg. 2017;11[3]:325-31).
The patients were given health-related quality of life self-assessment surveys prior to surgery and at 3, 6, and 12 months post surgery. In addition, a generic questionnaire, the eight-item Short-Form Health Survey, was used to assess a physical component summary (PCS) and a mental component summary (MCS). The Expanded Prostate Cancer Index of Prostate, which covers four domains – urinary, sexual, bowel, and hormonal – was used as a disease-specific measure, and the response rates for both LRP and RARP at each follow-up interval were over 80%.
“The RARP group showed significantly better scores in urinary summary and all urinary subscales at postoperative 3-month follow-up. However, these differences disappeared at postoperative 6 and 12-month follow-up,” the investigators wrote. For the urinary summary score, LRP significantly underperformed, compared with RARP, with scores of 63.3 vs. 75.8, respectively, after 3 months. In addition, the bowel function score was superior for RARP, compared with LRP, at 96.9 vs. 92.9, respectively. Sexual function results were similar, with RARP and LRP scores of 2.8 vs. 0.
The general measures of the PCS and MCS also favored RARP. At the 3-month follow-up, PCS (51.3 vs. 48.1) and MCS (50 vs. 47.8) scores were higher for RARP, compared with LRP.
“It is unclear why our superiority of urinary function in RARP was observed only in early period. However, we can speculate several reasons for better urinary function in RARP group. First, we were able to treat the apex area more delicately with RARP. Second, some of the new techniques which we employed after the introduction of RARP could influence the urinary continence recovery,” the investigators wrote.
The authors had no relevant financial disclosures.
Robot-assisted radical prostatectomy shows better early postoperative outcomes than does laparoscopic radical prostatectomy, but the differences between the two surgical approaches disappeared by the 6-month follow-up.
Dr. Hiroyuki Koike and his colleagues at Wakayama (Japan) Medical University Hospital conducted a study of two groups of patients treated for localized prostate cancer. One group of 229 patients underwent laparoscopic radical prostatectomy (LRP) between July 2007 and July 2013. The other group of 115 patients had robot-assisted radical prostatectomy (RARP) between December 2012 and August 2014 (J Robot Surg. 2017;11[3]:325-31).
The patients were given health-related quality of life self-assessment surveys prior to surgery and at 3, 6, and 12 months post surgery. In addition, a generic questionnaire, the eight-item Short-Form Health Survey, was used to assess a physical component summary (PCS) and a mental component summary (MCS). The Expanded Prostate Cancer Index of Prostate, which covers four domains – urinary, sexual, bowel, and hormonal – was used as a disease-specific measure, and the response rates for both LRP and RARP at each follow-up interval were over 80%.
“The RARP group showed significantly better scores in urinary summary and all urinary subscales at postoperative 3-month follow-up. However, these differences disappeared at postoperative 6 and 12-month follow-up,” the investigators wrote. For the urinary summary score, LRP significantly underperformed, compared with RARP, with scores of 63.3 vs. 75.8, respectively, after 3 months. In addition, the bowel function score was superior for RARP, compared with LRP, at 96.9 vs. 92.9, respectively. Sexual function results were similar, with RARP and LRP scores of 2.8 vs. 0.
The general measures of the PCS and MCS also favored RARP. At the 3-month follow-up, PCS (51.3 vs. 48.1) and MCS (50 vs. 47.8) scores were higher for RARP, compared with LRP.
“It is unclear why our superiority of urinary function in RARP was observed only in early period. However, we can speculate several reasons for better urinary function in RARP group. First, we were able to treat the apex area more delicately with RARP. Second, some of the new techniques which we employed after the introduction of RARP could influence the urinary continence recovery,” the investigators wrote.
The authors had no relevant financial disclosures.
Robot-assisted radical prostatectomy shows better early postoperative outcomes than does laparoscopic radical prostatectomy, but the differences between the two surgical approaches disappeared by the 6-month follow-up.
Dr. Hiroyuki Koike and his colleagues at Wakayama (Japan) Medical University Hospital conducted a study of two groups of patients treated for localized prostate cancer. One group of 229 patients underwent laparoscopic radical prostatectomy (LRP) between July 2007 and July 2013. The other group of 115 patients had robot-assisted radical prostatectomy (RARP) between December 2012 and August 2014 (J Robot Surg. 2017;11[3]:325-31).
The patients were given health-related quality of life self-assessment surveys prior to surgery and at 3, 6, and 12 months post surgery. In addition, a generic questionnaire, the eight-item Short-Form Health Survey, was used to assess a physical component summary (PCS) and a mental component summary (MCS). The Expanded Prostate Cancer Index of Prostate, which covers four domains – urinary, sexual, bowel, and hormonal – was used as a disease-specific measure, and the response rates for both LRP and RARP at each follow-up interval were over 80%.
“The RARP group showed significantly better scores in urinary summary and all urinary subscales at postoperative 3-month follow-up. However, these differences disappeared at postoperative 6 and 12-month follow-up,” the investigators wrote. For the urinary summary score, LRP significantly underperformed, compared with RARP, with scores of 63.3 vs. 75.8, respectively, after 3 months. In addition, the bowel function score was superior for RARP, compared with LRP, at 96.9 vs. 92.9, respectively. Sexual function results were similar, with RARP and LRP scores of 2.8 vs. 0.
The general measures of the PCS and MCS also favored RARP. At the 3-month follow-up, PCS (51.3 vs. 48.1) and MCS (50 vs. 47.8) scores were higher for RARP, compared with LRP.
“It is unclear why our superiority of urinary function in RARP was observed only in early period. However, we can speculate several reasons for better urinary function in RARP group. First, we were able to treat the apex area more delicately with RARP. Second, some of the new techniques which we employed after the introduction of RARP could influence the urinary continence recovery,” the investigators wrote.
The authors had no relevant financial disclosures.
FROM JOURNAL OF ROBOTIC SURGERY
Key clinical point:
Major finding: Quality-of-life score for robotic-assisted radical prostatectomy was higher in all urinary categories after 3 months.
Data source: Postop survey results from patients with localized prostate cancer who underwent laparoscopic radical prostatectomy (n = 229) or robot-assisted radical prostatectomy (n = 115).
Disclosures: The investigators had no financial disclosures to report.
FDA approves pembrolizumab for gastric and GEJ adenocarcinoma
The Food and Drug Administration has approved pembrolizumab (Keytruda) for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma, in cases where tests confirm that the tumors contain programmed death–ligand 1 and where the disease is progressing on or after two or more prior lines of therapy.
Pembrolizumab has been approved in the United States since 2014 for the treatment of melanoma, with subsequent approvals for treatment of non–small-cell lung cancer, head and neck squamous cell carcinoma, Hodgkin lymphoma, and several other advanced cancers.
The approval comes on the basis of the nonrandomized, open label KEYNOTE-059 trial, which enrolled 259 patients with gastric or GEJ adenocarcinoma that progressed on at least two prior systemic treatments for advanced disease. Of the enrollees, 143 patients had tumors with a PD-L1 Combined Positive Score of 1 or greater. The primary trial outcome, the objective response rate for these 143 patients, was 13.3% (95% confidence interval; 8.2-20), with a complete response rate of 1.4% and a partial response rate of 11.9%. The duration of response ranged from at least 2.8 months to at least 19.4 months.
Continued approval for the new indication will depend upon further demonstration of a clinical benefit in confirmatory trials.
The Food and Drug Administration has approved pembrolizumab (Keytruda) for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma, in cases where tests confirm that the tumors contain programmed death–ligand 1 and where the disease is progressing on or after two or more prior lines of therapy.
Pembrolizumab has been approved in the United States since 2014 for the treatment of melanoma, with subsequent approvals for treatment of non–small-cell lung cancer, head and neck squamous cell carcinoma, Hodgkin lymphoma, and several other advanced cancers.
The approval comes on the basis of the nonrandomized, open label KEYNOTE-059 trial, which enrolled 259 patients with gastric or GEJ adenocarcinoma that progressed on at least two prior systemic treatments for advanced disease. Of the enrollees, 143 patients had tumors with a PD-L1 Combined Positive Score of 1 or greater. The primary trial outcome, the objective response rate for these 143 patients, was 13.3% (95% confidence interval; 8.2-20), with a complete response rate of 1.4% and a partial response rate of 11.9%. The duration of response ranged from at least 2.8 months to at least 19.4 months.
Continued approval for the new indication will depend upon further demonstration of a clinical benefit in confirmatory trials.
The Food and Drug Administration has approved pembrolizumab (Keytruda) for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma, in cases where tests confirm that the tumors contain programmed death–ligand 1 and where the disease is progressing on or after two or more prior lines of therapy.
Pembrolizumab has been approved in the United States since 2014 for the treatment of melanoma, with subsequent approvals for treatment of non–small-cell lung cancer, head and neck squamous cell carcinoma, Hodgkin lymphoma, and several other advanced cancers.
The approval comes on the basis of the nonrandomized, open label KEYNOTE-059 trial, which enrolled 259 patients with gastric or GEJ adenocarcinoma that progressed on at least two prior systemic treatments for advanced disease. Of the enrollees, 143 patients had tumors with a PD-L1 Combined Positive Score of 1 or greater. The primary trial outcome, the objective response rate for these 143 patients, was 13.3% (95% confidence interval; 8.2-20), with a complete response rate of 1.4% and a partial response rate of 11.9%. The duration of response ranged from at least 2.8 months to at least 19.4 months.
Continued approval for the new indication will depend upon further demonstration of a clinical benefit in confirmatory trials.
Commentary—A Credible Case for Neuroprotection
To date, efforts to translate exciting laboratory findings into clinical trials for Parkinson’s disease have not met with much success. However, the latest exenatide study results offer another chance for improving this situation. This randomized, placebo-controlled trial also highlights how a medication already in human use might be repurposed for a novel application.
Whether this study actually proved its concept will require confirmation by further clinical investigation. The biggest challenge presented by this study has to do with studying patients with Parkinson’s disease who already are receiving symptomatic therapy with dopaminergic drugs. The study plan was to eliminate this effect by an overnight washout of Parkinson’s disease drugs. But waiting for a day or two after stopping medication, even for a short-acting drug like levodopa, does not necessarily abolish all of its symptomatic effects. If exenatide acted by enhancing the temporary relief of parkinsonism offered by medications rather than by protecting against further neurodegeneration, study ratings would not be able to discern this difference. Similarly, if exenatide exerts a trophic effect on striatal dopamine transporters, the observed reduction of decline in dopamine transporter (DAT) scan readings would not necessarily reflect sparing of further dopaminergic neuron loss.
Another potentially confounding factor was that during the course of the trial, the exenatide group increased its concomitant dopaminergic therapy more than the placebo-treated controls did. In a study of only 62 subjects who were already advanced in the course of Parkinson’s disease and experiencing motor fluctuations, any imbalance between treatment and placebo groups might impart uncertainties for which it may be difficult to correct.
Despite all the reasonable objections that might detract from a definitive interpretation of this study, its results make a credible argument for a neuroprotection outcome. The happenstance of two seemingly independent indicators of disease modification—in this case, the washout ratings of parkinsonian features and the DAT scan results—provides grounds for optimism. In the quest for halting the advance of Parkinson’s disease, the perfect study has yet to emerge. The exenatide investigators’ careful attention to detail and collection of other study information and biomarker specimens will serve well the next researchers who explore the potential for glucagon-like peptide-1 treatments.
—Peter A. LeWitt, MD
Professor of Neurology
Wayne State University School of Medicine
Director, Parkinson's Disease and Movement Disorders Program
Henry Ford Hospital West Bloomfield
West Bloomfield, Michigan
To date, efforts to translate exciting laboratory findings into clinical trials for Parkinson’s disease have not met with much success. However, the latest exenatide study results offer another chance for improving this situation. This randomized, placebo-controlled trial also highlights how a medication already in human use might be repurposed for a novel application.
Whether this study actually proved its concept will require confirmation by further clinical investigation. The biggest challenge presented by this study has to do with studying patients with Parkinson’s disease who already are receiving symptomatic therapy with dopaminergic drugs. The study plan was to eliminate this effect by an overnight washout of Parkinson’s disease drugs. But waiting for a day or two after stopping medication, even for a short-acting drug like levodopa, does not necessarily abolish all of its symptomatic effects. If exenatide acted by enhancing the temporary relief of parkinsonism offered by medications rather than by protecting against further neurodegeneration, study ratings would not be able to discern this difference. Similarly, if exenatide exerts a trophic effect on striatal dopamine transporters, the observed reduction of decline in dopamine transporter (DAT) scan readings would not necessarily reflect sparing of further dopaminergic neuron loss.
Another potentially confounding factor was that during the course of the trial, the exenatide group increased its concomitant dopaminergic therapy more than the placebo-treated controls did. In a study of only 62 subjects who were already advanced in the course of Parkinson’s disease and experiencing motor fluctuations, any imbalance between treatment and placebo groups might impart uncertainties for which it may be difficult to correct.
Despite all the reasonable objections that might detract from a definitive interpretation of this study, its results make a credible argument for a neuroprotection outcome. The happenstance of two seemingly independent indicators of disease modification—in this case, the washout ratings of parkinsonian features and the DAT scan results—provides grounds for optimism. In the quest for halting the advance of Parkinson’s disease, the perfect study has yet to emerge. The exenatide investigators’ careful attention to detail and collection of other study information and biomarker specimens will serve well the next researchers who explore the potential for glucagon-like peptide-1 treatments.
—Peter A. LeWitt, MD
Professor of Neurology
Wayne State University School of Medicine
Director, Parkinson's Disease and Movement Disorders Program
Henry Ford Hospital West Bloomfield
West Bloomfield, Michigan
To date, efforts to translate exciting laboratory findings into clinical trials for Parkinson’s disease have not met with much success. However, the latest exenatide study results offer another chance for improving this situation. This randomized, placebo-controlled trial also highlights how a medication already in human use might be repurposed for a novel application.
Whether this study actually proved its concept will require confirmation by further clinical investigation. The biggest challenge presented by this study has to do with studying patients with Parkinson’s disease who already are receiving symptomatic therapy with dopaminergic drugs. The study plan was to eliminate this effect by an overnight washout of Parkinson’s disease drugs. But waiting for a day or two after stopping medication, even for a short-acting drug like levodopa, does not necessarily abolish all of its symptomatic effects. If exenatide acted by enhancing the temporary relief of parkinsonism offered by medications rather than by protecting against further neurodegeneration, study ratings would not be able to discern this difference. Similarly, if exenatide exerts a trophic effect on striatal dopamine transporters, the observed reduction of decline in dopamine transporter (DAT) scan readings would not necessarily reflect sparing of further dopaminergic neuron loss.
Another potentially confounding factor was that during the course of the trial, the exenatide group increased its concomitant dopaminergic therapy more than the placebo-treated controls did. In a study of only 62 subjects who were already advanced in the course of Parkinson’s disease and experiencing motor fluctuations, any imbalance between treatment and placebo groups might impart uncertainties for which it may be difficult to correct.
Despite all the reasonable objections that might detract from a definitive interpretation of this study, its results make a credible argument for a neuroprotection outcome. The happenstance of two seemingly independent indicators of disease modification—in this case, the washout ratings of parkinsonian features and the DAT scan results—provides grounds for optimism. In the quest for halting the advance of Parkinson’s disease, the perfect study has yet to emerge. The exenatide investigators’ careful attention to detail and collection of other study information and biomarker specimens will serve well the next researchers who explore the potential for glucagon-like peptide-1 treatments.
—Peter A. LeWitt, MD
Professor of Neurology
Wayne State University School of Medicine
Director, Parkinson's Disease and Movement Disorders Program
Henry Ford Hospital West Bloomfield
West Bloomfield, Michigan
Exenatide Aids Motor Function in Parkinson’s Disease
Exenatide improves motor function in patients with Parkinson’s disease, according to research published online ahead of print August 3 in Lancet. The improvements may persist for months after treatment exposure.
Exenatide, an analogue of glucagon-like peptide-1, is used to treat type 2 diabetes. In rodent models of Parkinson’s disease, exenatide had neuroprotective effects and improved motor performance, behavior, learning, and memory. The drug also provided motor and cognitive benefits in a proof-of-concept study including patients with Parkinson’s disease.
Active Group Was Slightly Older
Dilan Athauda, MBBS, Senior Clinical Research Associate at University College London, and colleagues conducted a double-blind study to assess exenatide’s potential disease-modifying effects. At screening for study entry, patients with idiopathic Parkinson’s disease underwent physical and neurologic examinations, assessments of mood and cognition, and blood sampling. The investigators randomized eligible participants to subcutaneous injections of exenatide (2 mg) or placebo once weekly for 48 weeks. Participants continued to take their regular medications. Investigators examined patients in an off-medication state and collected blood and urine at baseline and weeks 12, 24, 36, and 48. Study drugs were withdrawn after 48 weeks, and the final follow-up visit was at week 60.
The primary outcome was change in Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part 3 score at 60 weeks. Secondary outcomes included differences between exenatide and placebo in each subsection of the MDS-UPDRS in the on-medication state, and the Mattis Dementia Rating Scale at weeks 48 and 60.
In all, 62 participants were randomized. Patients assigned to exenatide were slightly older, had higher baseline MDS-UPDRS part 3 scores, and had lower levodopa equivalent dose than did controls. Average age was about 62 in the exenatide group and about 58 among controls. About 26% of the population was female, and the mean disease duration at baseline was 6.4 years. Approximately 97% of the population were between Hoehn and Yahr stage 1 and 2 at baseline.
Exenatide Yielded Motor Improvement
At week 60, off-medication MDS-UPDRS part 3 scores had worsened by 2.1 points in the placebo group and improved by 1.0 point in the exenatide group, yielding a significant adjusted difference of –3.5 points. At week 48, scores among controls had deteriorated by 1.7 points, and those in the exenatide group had improved by 2.3 points, resulting in a significant adjusted between-group difference of –4.3 points.
On-medication scores on MDS-UPDRS parts 1 through 4 did not differ significantly between groups at weeks 48 or 60. The researchers also did not observe a significant difference between groups in Mattis Dementia Rating Scale score at those time points. The frequency of adverse events was similar between groups.
“Exenatide could have a longer-lasting effect on disease severity beyond conventional drug effects on dopaminergic receptors,” said the researchers. “Whether exenatide affects the underlying disease pathophysiology or simply induces long-lasting symptomatic effects is uncertain. Exenatide represents a major new avenue for investigation in Parkinson’s disease, and effects on everyday symptoms should be examined in longer-term trials.”
—Erik Greb
Suggested Reading
Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017 Aug 3 [Epub ahead of print].
Exenatide improves motor function in patients with Parkinson’s disease, according to research published online ahead of print August 3 in Lancet. The improvements may persist for months after treatment exposure.
Exenatide, an analogue of glucagon-like peptide-1, is used to treat type 2 diabetes. In rodent models of Parkinson’s disease, exenatide had neuroprotective effects and improved motor performance, behavior, learning, and memory. The drug also provided motor and cognitive benefits in a proof-of-concept study including patients with Parkinson’s disease.
Active Group Was Slightly Older
Dilan Athauda, MBBS, Senior Clinical Research Associate at University College London, and colleagues conducted a double-blind study to assess exenatide’s potential disease-modifying effects. At screening for study entry, patients with idiopathic Parkinson’s disease underwent physical and neurologic examinations, assessments of mood and cognition, and blood sampling. The investigators randomized eligible participants to subcutaneous injections of exenatide (2 mg) or placebo once weekly for 48 weeks. Participants continued to take their regular medications. Investigators examined patients in an off-medication state and collected blood and urine at baseline and weeks 12, 24, 36, and 48. Study drugs were withdrawn after 48 weeks, and the final follow-up visit was at week 60.
The primary outcome was change in Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part 3 score at 60 weeks. Secondary outcomes included differences between exenatide and placebo in each subsection of the MDS-UPDRS in the on-medication state, and the Mattis Dementia Rating Scale at weeks 48 and 60.
In all, 62 participants were randomized. Patients assigned to exenatide were slightly older, had higher baseline MDS-UPDRS part 3 scores, and had lower levodopa equivalent dose than did controls. Average age was about 62 in the exenatide group and about 58 among controls. About 26% of the population was female, and the mean disease duration at baseline was 6.4 years. Approximately 97% of the population were between Hoehn and Yahr stage 1 and 2 at baseline.
Exenatide Yielded Motor Improvement
At week 60, off-medication MDS-UPDRS part 3 scores had worsened by 2.1 points in the placebo group and improved by 1.0 point in the exenatide group, yielding a significant adjusted difference of –3.5 points. At week 48, scores among controls had deteriorated by 1.7 points, and those in the exenatide group had improved by 2.3 points, resulting in a significant adjusted between-group difference of –4.3 points.
On-medication scores on MDS-UPDRS parts 1 through 4 did not differ significantly between groups at weeks 48 or 60. The researchers also did not observe a significant difference between groups in Mattis Dementia Rating Scale score at those time points. The frequency of adverse events was similar between groups.
“Exenatide could have a longer-lasting effect on disease severity beyond conventional drug effects on dopaminergic receptors,” said the researchers. “Whether exenatide affects the underlying disease pathophysiology or simply induces long-lasting symptomatic effects is uncertain. Exenatide represents a major new avenue for investigation in Parkinson’s disease, and effects on everyday symptoms should be examined in longer-term trials.”
—Erik Greb
Suggested Reading
Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017 Aug 3 [Epub ahead of print].
Exenatide improves motor function in patients with Parkinson’s disease, according to research published online ahead of print August 3 in Lancet. The improvements may persist for months after treatment exposure.
Exenatide, an analogue of glucagon-like peptide-1, is used to treat type 2 diabetes. In rodent models of Parkinson’s disease, exenatide had neuroprotective effects and improved motor performance, behavior, learning, and memory. The drug also provided motor and cognitive benefits in a proof-of-concept study including patients with Parkinson’s disease.
Active Group Was Slightly Older
Dilan Athauda, MBBS, Senior Clinical Research Associate at University College London, and colleagues conducted a double-blind study to assess exenatide’s potential disease-modifying effects. At screening for study entry, patients with idiopathic Parkinson’s disease underwent physical and neurologic examinations, assessments of mood and cognition, and blood sampling. The investigators randomized eligible participants to subcutaneous injections of exenatide (2 mg) or placebo once weekly for 48 weeks. Participants continued to take their regular medications. Investigators examined patients in an off-medication state and collected blood and urine at baseline and weeks 12, 24, 36, and 48. Study drugs were withdrawn after 48 weeks, and the final follow-up visit was at week 60.
The primary outcome was change in Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part 3 score at 60 weeks. Secondary outcomes included differences between exenatide and placebo in each subsection of the MDS-UPDRS in the on-medication state, and the Mattis Dementia Rating Scale at weeks 48 and 60.
In all, 62 participants were randomized. Patients assigned to exenatide were slightly older, had higher baseline MDS-UPDRS part 3 scores, and had lower levodopa equivalent dose than did controls. Average age was about 62 in the exenatide group and about 58 among controls. About 26% of the population was female, and the mean disease duration at baseline was 6.4 years. Approximately 97% of the population were between Hoehn and Yahr stage 1 and 2 at baseline.
Exenatide Yielded Motor Improvement
At week 60, off-medication MDS-UPDRS part 3 scores had worsened by 2.1 points in the placebo group and improved by 1.0 point in the exenatide group, yielding a significant adjusted difference of –3.5 points. At week 48, scores among controls had deteriorated by 1.7 points, and those in the exenatide group had improved by 2.3 points, resulting in a significant adjusted between-group difference of –4.3 points.
On-medication scores on MDS-UPDRS parts 1 through 4 did not differ significantly between groups at weeks 48 or 60. The researchers also did not observe a significant difference between groups in Mattis Dementia Rating Scale score at those time points. The frequency of adverse events was similar between groups.
“Exenatide could have a longer-lasting effect on disease severity beyond conventional drug effects on dopaminergic receptors,” said the researchers. “Whether exenatide affects the underlying disease pathophysiology or simply induces long-lasting symptomatic effects is uncertain. Exenatide represents a major new avenue for investigation in Parkinson’s disease, and effects on everyday symptoms should be examined in longer-term trials.”
—Erik Greb
Suggested Reading
Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017 Aug 3 [Epub ahead of print].
Peer-to-peer learning about robotic surgery is happening on social media
Social media is now being used by surgeons for interaction among peers, informal learning, exchange of technical information, and diffusion of ideas.
A study has examined posting and membership data from a closed Facebook page, the “Robotic Surgery Collaboration,” created by surgeons who practice robotic-assisted procedures. Overall, the findings show exponential growth in membership in January 2015 through August 2016, some signs of stagnating engagement, and use of the platform for peer-to-peer learning and discussion.
“The growth in this group over time suggests that surgeons found it useful for engaging in informal interactions and learning vicariously from one another, but also reveals that not all users were actively engaged in these interactions each month and that growth in active membership differed from growth in overall group membership (as evident in the stagnating growth of active members, despite continued growth in total members),” the investigators concluded. Read the full study at Ann Surg. 2017 Aug 29. doi: 10.1097/SLA.0000000000002479. (Epub ahead of print).
Social media is now being used by surgeons for interaction among peers, informal learning, exchange of technical information, and diffusion of ideas.
A study has examined posting and membership data from a closed Facebook page, the “Robotic Surgery Collaboration,” created by surgeons who practice robotic-assisted procedures. Overall, the findings show exponential growth in membership in January 2015 through August 2016, some signs of stagnating engagement, and use of the platform for peer-to-peer learning and discussion.
“The growth in this group over time suggests that surgeons found it useful for engaging in informal interactions and learning vicariously from one another, but also reveals that not all users were actively engaged in these interactions each month and that growth in active membership differed from growth in overall group membership (as evident in the stagnating growth of active members, despite continued growth in total members),” the investigators concluded. Read the full study at Ann Surg. 2017 Aug 29. doi: 10.1097/SLA.0000000000002479. (Epub ahead of print).
Social media is now being used by surgeons for interaction among peers, informal learning, exchange of technical information, and diffusion of ideas.
A study has examined posting and membership data from a closed Facebook page, the “Robotic Surgery Collaboration,” created by surgeons who practice robotic-assisted procedures. Overall, the findings show exponential growth in membership in January 2015 through August 2016, some signs of stagnating engagement, and use of the platform for peer-to-peer learning and discussion.
“The growth in this group over time suggests that surgeons found it useful for engaging in informal interactions and learning vicariously from one another, but also reveals that not all users were actively engaged in these interactions each month and that growth in active membership differed from growth in overall group membership (as evident in the stagnating growth of active members, despite continued growth in total members),” the investigators concluded. Read the full study at Ann Surg. 2017 Aug 29. doi: 10.1097/SLA.0000000000002479. (Epub ahead of print).
Immunogenicity concerns for biosimilars so far don’t go beyond originator biologics
A key factor that impacts the efficacy and the toxicity of biologics used for rheumatic diseases is their immunogenicity, and this factor doesn’t appear to be any different for biosimilars in studies conducted so far.
In a meta-analysis of 63 studies of tumor necrosis factor (TNF) inhibitors, investigators including Daniel E. Furst, MD, professor of rheumatology at the University of Washington, Seattle, who also is affiliated with the University of California, Los Angeles, and the University of Florence, Italy, found that antidrug antibodies developed in 17% of patients (BioDrugs 2015;29:241-58).
“That doesn’t sound too bad, but does it differ by medication?” asked Dr. Furst, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “For infliximab, 30% of the time, there are antidrug antibodies. So if that has a clinical effect, that’s a big deal. For certolizumab ... it’s only 6%, so there is a huge difference within the TNF inhibitors.” At the same time, antidrug antibodies developed in patients on adalimumab 23% of the time, followed by certolizumab (6%), golimumab (4%), and etanercept (2%).
One approach to circumventing the impact of antidrug antibodies on clinical response is by using immunosuppression, which in the meta-analysis had a 70% probability for decreasing antidrug antibodies. But this approach comes with a hitch, Dr. Furst said. The effect of immunosuppression on antidrug antibody positivity differs by disease. Immunosuppression had a 78% probability for decreasing antidrug antibody positivity in RA, 63% probability in inflammatory bowel disease, and 32% probability in ankylosing spondylitis. “That’s a wild mix of immunogenicity and the possibility that it’s going to affect the underlying response,” he said. The best studies in this area are of infliximab and adalimumab, which showed that antidrug antibodies in patients on infliximab reduced the probability of a clinical response by 54% and for adalimumab by 65%.
The same meta-analysis found that there were more antibodies to adalimumab than to certolizumab, golimumab, or etanercept, and also more antibodies to infliximab than to certolizumab, golimumab, or etanercept, but no difference between adalimumab and infliximab. “You’d think that maybe there is a difference between adalimumab and infliximab, but that’s not true,” Dr. Furst said. The apparent effect on the percentage of antidrug antibodies depends on what type of assay is used. For example, radioimmunoassay measures about 11% more antidrug antibodies, compared with enzyme-linked immunosorbent assay. Disease duration also matters. Each year of disease duration increases the antidrug antibodies by 1%.
What about the non-TNF inhibitors? A meta-analysis of five core trials of tocilizumab for RA found that the antidrug antibodies ranged from 1.7% at baseline to 2.3% during the respective trials (Clin Ther. 2010;32:1597-1609). “Anywhere along the way, the percentage of antidrug antibodies was about 1.5%,” said Dr. Furst, who was not involved with the study. “So as opposed to the TNF inhibitors, when you look at tocilizumab, this whole immunity question is probably a non-issue. The same is true for abatacept. With rituximab, the question is a little bit different. In one randomized, controlled trial, immunogenicity was 2.9%, while in the next it was 7.9%. In an open-label trial, it was 11.5%. So I think for rituximab we have to assume that you have some potentially important antidrug antibodies that might affect response.”
Studies have shown that antidrug antibodies do affect the pharmacokinetics of a biologic (and thereby clinical response) by producing lower trough levels of the biologic through an increase in its clearance. However, studies of infliximab and its biosimilars have not yielded any significant differences in clinical responses rates despite small differences in rates of antidrug antibodies or in rates of treatment-emergent adverse events, he said.
There has also been no difference in disease worsening in the only reported double-blind, randomized, switching study for infliximab and an infliximab biosimilar, the NOR-SWITCH study. However, the small numbers of patients in the study with certain diseases for which infliximab is indicated do not allow for conclusions to be drawn for specific diseases, Dr. Furst said.
Dr. Furst disclosed that he has received grant/research support from AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, and Roche/Genentech. He is also a consultant for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Cytori, Novartis, Pfizer, and Roche/Genentech. Global Academy for Medical Education and this news organization are owned by the same parent company.
A key factor that impacts the efficacy and the toxicity of biologics used for rheumatic diseases is their immunogenicity, and this factor doesn’t appear to be any different for biosimilars in studies conducted so far.
In a meta-analysis of 63 studies of tumor necrosis factor (TNF) inhibitors, investigators including Daniel E. Furst, MD, professor of rheumatology at the University of Washington, Seattle, who also is affiliated with the University of California, Los Angeles, and the University of Florence, Italy, found that antidrug antibodies developed in 17% of patients (BioDrugs 2015;29:241-58).
“That doesn’t sound too bad, but does it differ by medication?” asked Dr. Furst, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “For infliximab, 30% of the time, there are antidrug antibodies. So if that has a clinical effect, that’s a big deal. For certolizumab ... it’s only 6%, so there is a huge difference within the TNF inhibitors.” At the same time, antidrug antibodies developed in patients on adalimumab 23% of the time, followed by certolizumab (6%), golimumab (4%), and etanercept (2%).
One approach to circumventing the impact of antidrug antibodies on clinical response is by using immunosuppression, which in the meta-analysis had a 70% probability for decreasing antidrug antibodies. But this approach comes with a hitch, Dr. Furst said. The effect of immunosuppression on antidrug antibody positivity differs by disease. Immunosuppression had a 78% probability for decreasing antidrug antibody positivity in RA, 63% probability in inflammatory bowel disease, and 32% probability in ankylosing spondylitis. “That’s a wild mix of immunogenicity and the possibility that it’s going to affect the underlying response,” he said. The best studies in this area are of infliximab and adalimumab, which showed that antidrug antibodies in patients on infliximab reduced the probability of a clinical response by 54% and for adalimumab by 65%.
The same meta-analysis found that there were more antibodies to adalimumab than to certolizumab, golimumab, or etanercept, and also more antibodies to infliximab than to certolizumab, golimumab, or etanercept, but no difference between adalimumab and infliximab. “You’d think that maybe there is a difference between adalimumab and infliximab, but that’s not true,” Dr. Furst said. The apparent effect on the percentage of antidrug antibodies depends on what type of assay is used. For example, radioimmunoassay measures about 11% more antidrug antibodies, compared with enzyme-linked immunosorbent assay. Disease duration also matters. Each year of disease duration increases the antidrug antibodies by 1%.
What about the non-TNF inhibitors? A meta-analysis of five core trials of tocilizumab for RA found that the antidrug antibodies ranged from 1.7% at baseline to 2.3% during the respective trials (Clin Ther. 2010;32:1597-1609). “Anywhere along the way, the percentage of antidrug antibodies was about 1.5%,” said Dr. Furst, who was not involved with the study. “So as opposed to the TNF inhibitors, when you look at tocilizumab, this whole immunity question is probably a non-issue. The same is true for abatacept. With rituximab, the question is a little bit different. In one randomized, controlled trial, immunogenicity was 2.9%, while in the next it was 7.9%. In an open-label trial, it was 11.5%. So I think for rituximab we have to assume that you have some potentially important antidrug antibodies that might affect response.”
Studies have shown that antidrug antibodies do affect the pharmacokinetics of a biologic (and thereby clinical response) by producing lower trough levels of the biologic through an increase in its clearance. However, studies of infliximab and its biosimilars have not yielded any significant differences in clinical responses rates despite small differences in rates of antidrug antibodies or in rates of treatment-emergent adverse events, he said.
There has also been no difference in disease worsening in the only reported double-blind, randomized, switching study for infliximab and an infliximab biosimilar, the NOR-SWITCH study. However, the small numbers of patients in the study with certain diseases for which infliximab is indicated do not allow for conclusions to be drawn for specific diseases, Dr. Furst said.
Dr. Furst disclosed that he has received grant/research support from AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, and Roche/Genentech. He is also a consultant for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Cytori, Novartis, Pfizer, and Roche/Genentech. Global Academy for Medical Education and this news organization are owned by the same parent company.
A key factor that impacts the efficacy and the toxicity of biologics used for rheumatic diseases is their immunogenicity, and this factor doesn’t appear to be any different for biosimilars in studies conducted so far.
In a meta-analysis of 63 studies of tumor necrosis factor (TNF) inhibitors, investigators including Daniel E. Furst, MD, professor of rheumatology at the University of Washington, Seattle, who also is affiliated with the University of California, Los Angeles, and the University of Florence, Italy, found that antidrug antibodies developed in 17% of patients (BioDrugs 2015;29:241-58).
“That doesn’t sound too bad, but does it differ by medication?” asked Dr. Furst, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “For infliximab, 30% of the time, there are antidrug antibodies. So if that has a clinical effect, that’s a big deal. For certolizumab ... it’s only 6%, so there is a huge difference within the TNF inhibitors.” At the same time, antidrug antibodies developed in patients on adalimumab 23% of the time, followed by certolizumab (6%), golimumab (4%), and etanercept (2%).
One approach to circumventing the impact of antidrug antibodies on clinical response is by using immunosuppression, which in the meta-analysis had a 70% probability for decreasing antidrug antibodies. But this approach comes with a hitch, Dr. Furst said. The effect of immunosuppression on antidrug antibody positivity differs by disease. Immunosuppression had a 78% probability for decreasing antidrug antibody positivity in RA, 63% probability in inflammatory bowel disease, and 32% probability in ankylosing spondylitis. “That’s a wild mix of immunogenicity and the possibility that it’s going to affect the underlying response,” he said. The best studies in this area are of infliximab and adalimumab, which showed that antidrug antibodies in patients on infliximab reduced the probability of a clinical response by 54% and for adalimumab by 65%.
The same meta-analysis found that there were more antibodies to adalimumab than to certolizumab, golimumab, or etanercept, and also more antibodies to infliximab than to certolizumab, golimumab, or etanercept, but no difference between adalimumab and infliximab. “You’d think that maybe there is a difference between adalimumab and infliximab, but that’s not true,” Dr. Furst said. The apparent effect on the percentage of antidrug antibodies depends on what type of assay is used. For example, radioimmunoassay measures about 11% more antidrug antibodies, compared with enzyme-linked immunosorbent assay. Disease duration also matters. Each year of disease duration increases the antidrug antibodies by 1%.
What about the non-TNF inhibitors? A meta-analysis of five core trials of tocilizumab for RA found that the antidrug antibodies ranged from 1.7% at baseline to 2.3% during the respective trials (Clin Ther. 2010;32:1597-1609). “Anywhere along the way, the percentage of antidrug antibodies was about 1.5%,” said Dr. Furst, who was not involved with the study. “So as opposed to the TNF inhibitors, when you look at tocilizumab, this whole immunity question is probably a non-issue. The same is true for abatacept. With rituximab, the question is a little bit different. In one randomized, controlled trial, immunogenicity was 2.9%, while in the next it was 7.9%. In an open-label trial, it was 11.5%. So I think for rituximab we have to assume that you have some potentially important antidrug antibodies that might affect response.”
Studies have shown that antidrug antibodies do affect the pharmacokinetics of a biologic (and thereby clinical response) by producing lower trough levels of the biologic through an increase in its clearance. However, studies of infliximab and its biosimilars have not yielded any significant differences in clinical responses rates despite small differences in rates of antidrug antibodies or in rates of treatment-emergent adverse events, he said.
There has also been no difference in disease worsening in the only reported double-blind, randomized, switching study for infliximab and an infliximab biosimilar, the NOR-SWITCH study. However, the small numbers of patients in the study with certain diseases for which infliximab is indicated do not allow for conclusions to be drawn for specific diseases, Dr. Furst said.
Dr. Furst disclosed that he has received grant/research support from AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, and Roche/Genentech. He is also a consultant for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Cytori, Novartis, Pfizer, and Roche/Genentech. Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES