User login
Finding the Best Match for MASLD Management
, according to the authors of clinical reviews who offered guidance on the pros and cons of resmetirom and semaglutide.
MASLD has become one of the most common causes of chronic liver disease due to the increased prevalence of diabetes, obesity, and other metabolic disorders, Joanne Lin, DO, an internist in the Division of Gastroenterology and Hepatology at the University of California, San Francisco, and colleagues wrote, in a review published in the Journal of Clinical Gastroenterology.
Its complexity makes MASLD challenging to manage. Metabolic, genetic, and environmental factors are involved in the disease, so patients require multidisciplinary and individualized care, Lin told GI & Hepatology News.
Weight loss, dietary changes, and exercise had long been the only treatment approach clinicians could offer patients. But the approval of two drugs — the thyroid hormone receptor-beta agonist resmetirom and the GLP-1 receptor agonist (RA) semaglutide — for patients whose MASLD has advanced to metabolic dysfunction-associated steatohepatitis (MASH) gives physicians new options for patients with severe disease.
In the review, published online before the official approval of semaglutide, Lin and colleagues proposed an algorithm to guide clinicians in choosing a pharmacological therapy for MASLD. “Resmetirom should be primarily used to reverse fibrosis for patients with MASLD and F2-F3 stages, while GLP-1 RAs are beneficial in managing metabolic comorbidities and weight loss in patients with MASLD,” the researchers concluded.
GLP-1 Power and Potential
In August 2025, the FDA approved semaglutide for MASH and cited evidence from the ESSENCE trial in its decision.
The ESSENCE study, published in The New England Journal of Medicine, showed significantly higher rates of resolution of steatohepatitis without worsening of fibrosis and reduction in liver fibrosis without worsening steatohepatitis in patients with MASH and moderate or advanced liver fibrosis who received 2.4 mg of once-weekly semaglutide compared with patients who received placebo.
The most common adverse events reported with GLP-1 RAs are gastrointestinal-related, including nausea, diarrhea, vomiting, and constipation, and are mainly mild-to-moderate and dose dependent, Lin and colleagues noted in their review.
GLP-1s have some limitations, Lin said. “GLP-1s are great for weight loss and metabolic risk reduction, but studies are still ongoing to determine their effect on liver histology and reversing fibrosis/cirrhosis,” she said. Some patients seeking these medications also have trouble obtaining them because of their popularity for weight loss, she noted.
Resmetirom Shows Success
Resmetirom has demonstrated ability to target hepatocytes and increase the hepatic metabolism of lipids, Lin and colleagues wrote in their review.
Several trials have examined resmetirom as a treatment for MASH, notably the landmark MAESTRO-NASH study , a randomized, placebo-controlled trial of nearly 1000 adults with biopsy-confirmed MASH and stage F2 or F3 fibrosis, which was the basis for the FDA’s approval of the drug in 2024. In the study, 25.9% of the patients treated with 80 mg of resmetirom and 29.9% treated with 100 mg resmetirom achieved MASH resolution with no increase in fibrosis compared with 9.7% of patients treated with placebo. In addition, 24.2% of the patients in the 80-mg resmetirom group and 25.9% of those in the 100-mg resmetirom group achieved fibrosis improvement by at least one stage without worsening of MASLD activity scores compared with 14.2% of patients treated with placebo.
The most common reported side effects from resmetirom are diarrhea or constipation, nausea or vomiting, and abdominal pain.
“The limitations of resmetirom include the absence of validated predictors for individual patient response, and no societal guidelines are available to determine when to stop the medication if ineffective,” Lin told GI & Hepatology News. In addition, resmetirom is currently only recommended for a subset of patients with F2-F3 fibrosis, based on the existing trial, she said.
Other limitations include its high cost, which restricts access to the drug for some patients, and lack of long-term safety and efficacy data, Lin added.
Weighing the Options
Comparing the emerging agents in the context of MASLD/MASH is important to help clinicians understand how different patient populations respond and guide evidence-based treatment decisions, said Hazem Ayesh, MD, an endocrinologist at Deaconess Health System, Evansville, Indiana, in an interview.
“The choice of therapy should be individualized based on comorbidities,” said Ayesh, the lead author of a 2024 review published in Biomedicines that compared resmetirom, GLP-1 agonists, and fibroblast growth factor 21 analogs.
“For example, a GLP-1 receptor agonist may be more appropriate for patients with coexisting diabetes or obesity, while resmetirom may be better suited for patients with more advanced liver disease or minimal metabolic comorbidities,” he said.
GLP-1 RAs, such as semaglutide, offer benefits for diabetes, obesity, and metabolic dysfunction in patients with MASLD/MASH and may be more accessible and cost effective, Ayesh told GI & Hepatology News. However, some patients may experience gastrointestinal side effects or be unable to tolerate GLP-1 RAs, he noted.
By contrast, resmetirom may be preferable for patients with low BMI, advanced fibrosis, or an inability to tolerate GLP-1s, as resmetirom directly targets hepatic pathways involved in MASLD/MASH progression, Ayesh said.
Next Steps to Inform Practice
“More research is needed to validate noninvasive biomarkers to monitor response to these medications, determine predictors of efficacy, and evaluate the additive effects, safety, and drug-drug interactions of combination therapy,” Lin said.
Studies are needed to determine both medications’ effects on patients with advanced fibrosis/cirrhosis and special populations, such as individuals with advanced renal disease or posttransplant patients, she added. More studies are expected to inform clinical practice and proper guidelines for the treatment of MASLD, as has been the case with chronic diseases such as hypertension and diabetes, Lin said.
Long-term safety and efficacy data are critical, as most trials of the newly approved medications have had relatively short follow-up periods of approximately 1 year, Ayesh said. “We need real-world evidence and longitudinal studies spanning 3-5 years to confirm sustained efficacy and safety,” he said. Research on cost effectiveness and health-system impacts will be essential to guide policy and ensure equitable access to the medications, he added.
The study by Lin and colleagues received no outside funding. The researchers had no financial conflicts to disclose. Ayesh had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
, according to the authors of clinical reviews who offered guidance on the pros and cons of resmetirom and semaglutide.
MASLD has become one of the most common causes of chronic liver disease due to the increased prevalence of diabetes, obesity, and other metabolic disorders, Joanne Lin, DO, an internist in the Division of Gastroenterology and Hepatology at the University of California, San Francisco, and colleagues wrote, in a review published in the Journal of Clinical Gastroenterology.
Its complexity makes MASLD challenging to manage. Metabolic, genetic, and environmental factors are involved in the disease, so patients require multidisciplinary and individualized care, Lin told GI & Hepatology News.
Weight loss, dietary changes, and exercise had long been the only treatment approach clinicians could offer patients. But the approval of two drugs — the thyroid hormone receptor-beta agonist resmetirom and the GLP-1 receptor agonist (RA) semaglutide — for patients whose MASLD has advanced to metabolic dysfunction-associated steatohepatitis (MASH) gives physicians new options for patients with severe disease.
In the review, published online before the official approval of semaglutide, Lin and colleagues proposed an algorithm to guide clinicians in choosing a pharmacological therapy for MASLD. “Resmetirom should be primarily used to reverse fibrosis for patients with MASLD and F2-F3 stages, while GLP-1 RAs are beneficial in managing metabolic comorbidities and weight loss in patients with MASLD,” the researchers concluded.
GLP-1 Power and Potential
In August 2025, the FDA approved semaglutide for MASH and cited evidence from the ESSENCE trial in its decision.
The ESSENCE study, published in The New England Journal of Medicine, showed significantly higher rates of resolution of steatohepatitis without worsening of fibrosis and reduction in liver fibrosis without worsening steatohepatitis in patients with MASH and moderate or advanced liver fibrosis who received 2.4 mg of once-weekly semaglutide compared with patients who received placebo.
The most common adverse events reported with GLP-1 RAs are gastrointestinal-related, including nausea, diarrhea, vomiting, and constipation, and are mainly mild-to-moderate and dose dependent, Lin and colleagues noted in their review.
GLP-1s have some limitations, Lin said. “GLP-1s are great for weight loss and metabolic risk reduction, but studies are still ongoing to determine their effect on liver histology and reversing fibrosis/cirrhosis,” she said. Some patients seeking these medications also have trouble obtaining them because of their popularity for weight loss, she noted.
Resmetirom Shows Success
Resmetirom has demonstrated ability to target hepatocytes and increase the hepatic metabolism of lipids, Lin and colleagues wrote in their review.
Several trials have examined resmetirom as a treatment for MASH, notably the landmark MAESTRO-NASH study , a randomized, placebo-controlled trial of nearly 1000 adults with biopsy-confirmed MASH and stage F2 or F3 fibrosis, which was the basis for the FDA’s approval of the drug in 2024. In the study, 25.9% of the patients treated with 80 mg of resmetirom and 29.9% treated with 100 mg resmetirom achieved MASH resolution with no increase in fibrosis compared with 9.7% of patients treated with placebo. In addition, 24.2% of the patients in the 80-mg resmetirom group and 25.9% of those in the 100-mg resmetirom group achieved fibrosis improvement by at least one stage without worsening of MASLD activity scores compared with 14.2% of patients treated with placebo.
The most common reported side effects from resmetirom are diarrhea or constipation, nausea or vomiting, and abdominal pain.
“The limitations of resmetirom include the absence of validated predictors for individual patient response, and no societal guidelines are available to determine when to stop the medication if ineffective,” Lin told GI & Hepatology News. In addition, resmetirom is currently only recommended for a subset of patients with F2-F3 fibrosis, based on the existing trial, she said.
Other limitations include its high cost, which restricts access to the drug for some patients, and lack of long-term safety and efficacy data, Lin added.
Weighing the Options
Comparing the emerging agents in the context of MASLD/MASH is important to help clinicians understand how different patient populations respond and guide evidence-based treatment decisions, said Hazem Ayesh, MD, an endocrinologist at Deaconess Health System, Evansville, Indiana, in an interview.
“The choice of therapy should be individualized based on comorbidities,” said Ayesh, the lead author of a 2024 review published in Biomedicines that compared resmetirom, GLP-1 agonists, and fibroblast growth factor 21 analogs.
“For example, a GLP-1 receptor agonist may be more appropriate for patients with coexisting diabetes or obesity, while resmetirom may be better suited for patients with more advanced liver disease or minimal metabolic comorbidities,” he said.
GLP-1 RAs, such as semaglutide, offer benefits for diabetes, obesity, and metabolic dysfunction in patients with MASLD/MASH and may be more accessible and cost effective, Ayesh told GI & Hepatology News. However, some patients may experience gastrointestinal side effects or be unable to tolerate GLP-1 RAs, he noted.
By contrast, resmetirom may be preferable for patients with low BMI, advanced fibrosis, or an inability to tolerate GLP-1s, as resmetirom directly targets hepatic pathways involved in MASLD/MASH progression, Ayesh said.
Next Steps to Inform Practice
“More research is needed to validate noninvasive biomarkers to monitor response to these medications, determine predictors of efficacy, and evaluate the additive effects, safety, and drug-drug interactions of combination therapy,” Lin said.
Studies are needed to determine both medications’ effects on patients with advanced fibrosis/cirrhosis and special populations, such as individuals with advanced renal disease or posttransplant patients, she added. More studies are expected to inform clinical practice and proper guidelines for the treatment of MASLD, as has been the case with chronic diseases such as hypertension and diabetes, Lin said.
Long-term safety and efficacy data are critical, as most trials of the newly approved medications have had relatively short follow-up periods of approximately 1 year, Ayesh said. “We need real-world evidence and longitudinal studies spanning 3-5 years to confirm sustained efficacy and safety,” he said. Research on cost effectiveness and health-system impacts will be essential to guide policy and ensure equitable access to the medications, he added.
The study by Lin and colleagues received no outside funding. The researchers had no financial conflicts to disclose. Ayesh had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
, according to the authors of clinical reviews who offered guidance on the pros and cons of resmetirom and semaglutide.
MASLD has become one of the most common causes of chronic liver disease due to the increased prevalence of diabetes, obesity, and other metabolic disorders, Joanne Lin, DO, an internist in the Division of Gastroenterology and Hepatology at the University of California, San Francisco, and colleagues wrote, in a review published in the Journal of Clinical Gastroenterology.
Its complexity makes MASLD challenging to manage. Metabolic, genetic, and environmental factors are involved in the disease, so patients require multidisciplinary and individualized care, Lin told GI & Hepatology News.
Weight loss, dietary changes, and exercise had long been the only treatment approach clinicians could offer patients. But the approval of two drugs — the thyroid hormone receptor-beta agonist resmetirom and the GLP-1 receptor agonist (RA) semaglutide — for patients whose MASLD has advanced to metabolic dysfunction-associated steatohepatitis (MASH) gives physicians new options for patients with severe disease.
In the review, published online before the official approval of semaglutide, Lin and colleagues proposed an algorithm to guide clinicians in choosing a pharmacological therapy for MASLD. “Resmetirom should be primarily used to reverse fibrosis for patients with MASLD and F2-F3 stages, while GLP-1 RAs are beneficial in managing metabolic comorbidities and weight loss in patients with MASLD,” the researchers concluded.
GLP-1 Power and Potential
In August 2025, the FDA approved semaglutide for MASH and cited evidence from the ESSENCE trial in its decision.
The ESSENCE study, published in The New England Journal of Medicine, showed significantly higher rates of resolution of steatohepatitis without worsening of fibrosis and reduction in liver fibrosis without worsening steatohepatitis in patients with MASH and moderate or advanced liver fibrosis who received 2.4 mg of once-weekly semaglutide compared with patients who received placebo.
The most common adverse events reported with GLP-1 RAs are gastrointestinal-related, including nausea, diarrhea, vomiting, and constipation, and are mainly mild-to-moderate and dose dependent, Lin and colleagues noted in their review.
GLP-1s have some limitations, Lin said. “GLP-1s are great for weight loss and metabolic risk reduction, but studies are still ongoing to determine their effect on liver histology and reversing fibrosis/cirrhosis,” she said. Some patients seeking these medications also have trouble obtaining them because of their popularity for weight loss, she noted.
Resmetirom Shows Success
Resmetirom has demonstrated ability to target hepatocytes and increase the hepatic metabolism of lipids, Lin and colleagues wrote in their review.
Several trials have examined resmetirom as a treatment for MASH, notably the landmark MAESTRO-NASH study , a randomized, placebo-controlled trial of nearly 1000 adults with biopsy-confirmed MASH and stage F2 or F3 fibrosis, which was the basis for the FDA’s approval of the drug in 2024. In the study, 25.9% of the patients treated with 80 mg of resmetirom and 29.9% treated with 100 mg resmetirom achieved MASH resolution with no increase in fibrosis compared with 9.7% of patients treated with placebo. In addition, 24.2% of the patients in the 80-mg resmetirom group and 25.9% of those in the 100-mg resmetirom group achieved fibrosis improvement by at least one stage without worsening of MASLD activity scores compared with 14.2% of patients treated with placebo.
The most common reported side effects from resmetirom are diarrhea or constipation, nausea or vomiting, and abdominal pain.
“The limitations of resmetirom include the absence of validated predictors for individual patient response, and no societal guidelines are available to determine when to stop the medication if ineffective,” Lin told GI & Hepatology News. In addition, resmetirom is currently only recommended for a subset of patients with F2-F3 fibrosis, based on the existing trial, she said.
Other limitations include its high cost, which restricts access to the drug for some patients, and lack of long-term safety and efficacy data, Lin added.
Weighing the Options
Comparing the emerging agents in the context of MASLD/MASH is important to help clinicians understand how different patient populations respond and guide evidence-based treatment decisions, said Hazem Ayesh, MD, an endocrinologist at Deaconess Health System, Evansville, Indiana, in an interview.
“The choice of therapy should be individualized based on comorbidities,” said Ayesh, the lead author of a 2024 review published in Biomedicines that compared resmetirom, GLP-1 agonists, and fibroblast growth factor 21 analogs.
“For example, a GLP-1 receptor agonist may be more appropriate for patients with coexisting diabetes or obesity, while resmetirom may be better suited for patients with more advanced liver disease or minimal metabolic comorbidities,” he said.
GLP-1 RAs, such as semaglutide, offer benefits for diabetes, obesity, and metabolic dysfunction in patients with MASLD/MASH and may be more accessible and cost effective, Ayesh told GI & Hepatology News. However, some patients may experience gastrointestinal side effects or be unable to tolerate GLP-1 RAs, he noted.
By contrast, resmetirom may be preferable for patients with low BMI, advanced fibrosis, or an inability to tolerate GLP-1s, as resmetirom directly targets hepatic pathways involved in MASLD/MASH progression, Ayesh said.
Next Steps to Inform Practice
“More research is needed to validate noninvasive biomarkers to monitor response to these medications, determine predictors of efficacy, and evaluate the additive effects, safety, and drug-drug interactions of combination therapy,” Lin said.
Studies are needed to determine both medications’ effects on patients with advanced fibrosis/cirrhosis and special populations, such as individuals with advanced renal disease or posttransplant patients, she added. More studies are expected to inform clinical practice and proper guidelines for the treatment of MASLD, as has been the case with chronic diseases such as hypertension and diabetes, Lin said.
Long-term safety and efficacy data are critical, as most trials of the newly approved medications have had relatively short follow-up periods of approximately 1 year, Ayesh said. “We need real-world evidence and longitudinal studies spanning 3-5 years to confirm sustained efficacy and safety,” he said. Research on cost effectiveness and health-system impacts will be essential to guide policy and ensure equitable access to the medications, he added.
The study by Lin and colleagues received no outside funding. The researchers had no financial conflicts to disclose. Ayesh had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Vaping Increases Peptic Ulcer Disease Risk
PHOENIX — , a cross-sectional study found.
The study also found increased risk of PUD among former users of e-cigarettes, reported Albert E. Ohrin, MBChB, MHS, of Ascension Saint Agnes Hospital, Baltimore, Maryland, who presented the study here at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
While cigarette smoking is a known risk factor for PUD, there was little in the literature investigating whether vaping has a similar risk profile, said Ohrin, a first-year internal medicine resident. He told GI & Hepatology News he found e-cigarette users on Reddit discussing worsening PUD and decided to investigate further, especially since vaping is so popular among young people.
E-cigarettes are the most-used tobacco product among middle and high school students. The National Youth Tobacco Survey in the US reported that 1.6 million students (5.9%) vaped in 2024, a decline from 7.7% in 2023. And the number of adults using e-cigarettes is increasing, according to the US CDC. In 2023, 6.5% of adults over age 18 used e-cigarettes, up from 3.7% in 2020.
Ohrin and colleagues conducted a cross-sectional analysis of adults enrolled in the National Institutes of Health All of Us Research Program. Participants self-reported e-cigarette use. PUD was defined using validated electronic health record diagnosis codes.
Among the 371,398 participants, 29,373 (8%) reported using e-cigarettes, including 21,277 current users and 8096 former users. E-cigarette users were significantly younger (mean age 45.3 vs 59.3 years; P < .001), more likely to be female, and more likely to report lower education and income (P < .001).
Current e-cigarette users had 27% higher odds of PUD (adjusted odds ratio [aOR], 1.27; 95% CI, 1.12-1.45), compared to never-users. This was greater than the risk with traditional combustible cigarettes (aOR, 1.19) that was seen in the study.
Former e-cig users had 13% higher odds (aOR, 1.13; 95% CI, 1.04-1.24) compared to never-users, and any e-cigarette use was associated with higher odds of PUD (aOR, 1.17; 95% CI, 1.09-1.26) compared to never-use.
Use of non-steroidal anti-inflammatories (aOR, 2.15) and having gastroesophageal reflux disease (aOR, 4.45) presented the most significant PUD risk.
Ohrin said he and his colleagues were surprised to see that people who had stopped using e-cigarettes still had higher odds of PUD, although he pointed out that the researchers did not know the frequency of use or how long users had stopped.
“Now that we know there’s an association, we are going to do more studies on e-cigarettes” to see what the potential harms are, especially on the gastrointestinal system, he told GI & Hepatology News.
“One of the things we are looking to elicit is — is there a dose response?” he said, noting it would take a prospective trial to determine that effect.
‘Opens a Door’ to Looking at the GI System
Laura Crotty Alexander, MD, a professor of medicine and associate division chief of pulmonary, critical care, sleep medicine, and physiology at the University of California, San Diego, said she found the study novel and interesting.
“It’s the first I’ve heard of an association between e-cigarette vaping and peptic ulcer disease,” said Crotty Alexander, who has studied the health effects of e-cigarettes for a decade.
Previous studies have shown that nicotine itself can drive an increase in gastric acid production and decrease healing, which can contribute to PUD, Crotty Alexander told GI & Hepatology News. With combustible cigarettes, it is thought that “the larger drivers of that association are the other things in tobacco smoke, such as tar and carbon monoxide and a million other horrible chemicals,” she said.
Crotty Alexander and her colleagues have conducted studies in vitro and in mice that show that e-cigarette aerosols are irritants and cause oxidative stress, which can drive PUD.
While many studies have shown vaping impacts various organs, Ohrin’s study “opens a door” to start looking at the gastrointestinal system, she said.
The study is also a signal to clinicians to “take an accurate inhalant history,” which means asking about vaping, she added.
Ohrin and Crotty Alexander reported no conflicts.
A version of this article first appeared on Medscape.com.
PHOENIX — , a cross-sectional study found.
The study also found increased risk of PUD among former users of e-cigarettes, reported Albert E. Ohrin, MBChB, MHS, of Ascension Saint Agnes Hospital, Baltimore, Maryland, who presented the study here at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
While cigarette smoking is a known risk factor for PUD, there was little in the literature investigating whether vaping has a similar risk profile, said Ohrin, a first-year internal medicine resident. He told GI & Hepatology News he found e-cigarette users on Reddit discussing worsening PUD and decided to investigate further, especially since vaping is so popular among young people.
E-cigarettes are the most-used tobacco product among middle and high school students. The National Youth Tobacco Survey in the US reported that 1.6 million students (5.9%) vaped in 2024, a decline from 7.7% in 2023. And the number of adults using e-cigarettes is increasing, according to the US CDC. In 2023, 6.5% of adults over age 18 used e-cigarettes, up from 3.7% in 2020.
Ohrin and colleagues conducted a cross-sectional analysis of adults enrolled in the National Institutes of Health All of Us Research Program. Participants self-reported e-cigarette use. PUD was defined using validated electronic health record diagnosis codes.
Among the 371,398 participants, 29,373 (8%) reported using e-cigarettes, including 21,277 current users and 8096 former users. E-cigarette users were significantly younger (mean age 45.3 vs 59.3 years; P < .001), more likely to be female, and more likely to report lower education and income (P < .001).
Current e-cigarette users had 27% higher odds of PUD (adjusted odds ratio [aOR], 1.27; 95% CI, 1.12-1.45), compared to never-users. This was greater than the risk with traditional combustible cigarettes (aOR, 1.19) that was seen in the study.
Former e-cig users had 13% higher odds (aOR, 1.13; 95% CI, 1.04-1.24) compared to never-users, and any e-cigarette use was associated with higher odds of PUD (aOR, 1.17; 95% CI, 1.09-1.26) compared to never-use.
Use of non-steroidal anti-inflammatories (aOR, 2.15) and having gastroesophageal reflux disease (aOR, 4.45) presented the most significant PUD risk.
Ohrin said he and his colleagues were surprised to see that people who had stopped using e-cigarettes still had higher odds of PUD, although he pointed out that the researchers did not know the frequency of use or how long users had stopped.
“Now that we know there’s an association, we are going to do more studies on e-cigarettes” to see what the potential harms are, especially on the gastrointestinal system, he told GI & Hepatology News.
“One of the things we are looking to elicit is — is there a dose response?” he said, noting it would take a prospective trial to determine that effect.
‘Opens a Door’ to Looking at the GI System
Laura Crotty Alexander, MD, a professor of medicine and associate division chief of pulmonary, critical care, sleep medicine, and physiology at the University of California, San Diego, said she found the study novel and interesting.
“It’s the first I’ve heard of an association between e-cigarette vaping and peptic ulcer disease,” said Crotty Alexander, who has studied the health effects of e-cigarettes for a decade.
Previous studies have shown that nicotine itself can drive an increase in gastric acid production and decrease healing, which can contribute to PUD, Crotty Alexander told GI & Hepatology News. With combustible cigarettes, it is thought that “the larger drivers of that association are the other things in tobacco smoke, such as tar and carbon monoxide and a million other horrible chemicals,” she said.
Crotty Alexander and her colleagues have conducted studies in vitro and in mice that show that e-cigarette aerosols are irritants and cause oxidative stress, which can drive PUD.
While many studies have shown vaping impacts various organs, Ohrin’s study “opens a door” to start looking at the gastrointestinal system, she said.
The study is also a signal to clinicians to “take an accurate inhalant history,” which means asking about vaping, she added.
Ohrin and Crotty Alexander reported no conflicts.
A version of this article first appeared on Medscape.com.
PHOENIX — , a cross-sectional study found.
The study also found increased risk of PUD among former users of e-cigarettes, reported Albert E. Ohrin, MBChB, MHS, of Ascension Saint Agnes Hospital, Baltimore, Maryland, who presented the study here at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
While cigarette smoking is a known risk factor for PUD, there was little in the literature investigating whether vaping has a similar risk profile, said Ohrin, a first-year internal medicine resident. He told GI & Hepatology News he found e-cigarette users on Reddit discussing worsening PUD and decided to investigate further, especially since vaping is so popular among young people.
E-cigarettes are the most-used tobacco product among middle and high school students. The National Youth Tobacco Survey in the US reported that 1.6 million students (5.9%) vaped in 2024, a decline from 7.7% in 2023. And the number of adults using e-cigarettes is increasing, according to the US CDC. In 2023, 6.5% of adults over age 18 used e-cigarettes, up from 3.7% in 2020.
Ohrin and colleagues conducted a cross-sectional analysis of adults enrolled in the National Institutes of Health All of Us Research Program. Participants self-reported e-cigarette use. PUD was defined using validated electronic health record diagnosis codes.
Among the 371,398 participants, 29,373 (8%) reported using e-cigarettes, including 21,277 current users and 8096 former users. E-cigarette users were significantly younger (mean age 45.3 vs 59.3 years; P < .001), more likely to be female, and more likely to report lower education and income (P < .001).
Current e-cigarette users had 27% higher odds of PUD (adjusted odds ratio [aOR], 1.27; 95% CI, 1.12-1.45), compared to never-users. This was greater than the risk with traditional combustible cigarettes (aOR, 1.19) that was seen in the study.
Former e-cig users had 13% higher odds (aOR, 1.13; 95% CI, 1.04-1.24) compared to never-users, and any e-cigarette use was associated with higher odds of PUD (aOR, 1.17; 95% CI, 1.09-1.26) compared to never-use.
Use of non-steroidal anti-inflammatories (aOR, 2.15) and having gastroesophageal reflux disease (aOR, 4.45) presented the most significant PUD risk.
Ohrin said he and his colleagues were surprised to see that people who had stopped using e-cigarettes still had higher odds of PUD, although he pointed out that the researchers did not know the frequency of use or how long users had stopped.
“Now that we know there’s an association, we are going to do more studies on e-cigarettes” to see what the potential harms are, especially on the gastrointestinal system, he told GI & Hepatology News.
“One of the things we are looking to elicit is — is there a dose response?” he said, noting it would take a prospective trial to determine that effect.
‘Opens a Door’ to Looking at the GI System
Laura Crotty Alexander, MD, a professor of medicine and associate division chief of pulmonary, critical care, sleep medicine, and physiology at the University of California, San Diego, said she found the study novel and interesting.
“It’s the first I’ve heard of an association between e-cigarette vaping and peptic ulcer disease,” said Crotty Alexander, who has studied the health effects of e-cigarettes for a decade.
Previous studies have shown that nicotine itself can drive an increase in gastric acid production and decrease healing, which can contribute to PUD, Crotty Alexander told GI & Hepatology News. With combustible cigarettes, it is thought that “the larger drivers of that association are the other things in tobacco smoke, such as tar and carbon monoxide and a million other horrible chemicals,” she said.
Crotty Alexander and her colleagues have conducted studies in vitro and in mice that show that e-cigarette aerosols are irritants and cause oxidative stress, which can drive PUD.
While many studies have shown vaping impacts various organs, Ohrin’s study “opens a door” to start looking at the gastrointestinal system, she said.
The study is also a signal to clinicians to “take an accurate inhalant history,” which means asking about vaping, she added.
Ohrin and Crotty Alexander reported no conflicts.
A version of this article first appeared on Medscape.com.
FROM ACG 2025
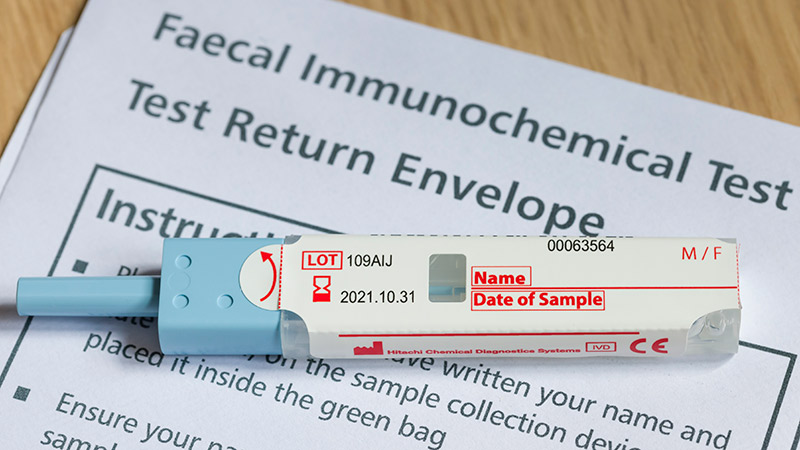
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
PHOENIX -- Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
"In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy," said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings this week at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
"These findings reinforce the importance of colonoscopy following positive interval FIT, given the high risk of advanced neoplasia and colorectal cancer, regardless of polypectomy history," Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, due to the higher risk.
Reasons patients may instead turn to FIT include cost or other factors.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2004.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the nonpolypectomy group).
Among the FIT screenings, results were positive in 17.2% of postpolypectomy patients and 14.1% of patients who no prior polypectomy, indicating a history of polypectomy to be predictive of positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result -- and having a previous polypectomy should add further emergency to the matter -- the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the nonpolypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the postcolonoscopy interval FIT.
The findings underscore that "positive results carried a high risk of advanced neoplasia or cancer, irrespective or prior polypectomy history," Wilson said.
Commenting on the study, William D. Chey, MD, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, Michigan, noted that the study "addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy."
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
"Other data suggest that the rate might even be significantly higher -- at 70% to 80%, depending upon the population and the test," Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies "should raise questions about whether there might be a role for FIT testing in addition to colonoscopy." However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is "how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy," he said.
"I think a lot of this is going to come down to how it's down at the primary care level."
Chey added that in that, and any other setting, "the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it's positive, a follow-up colonoscopy must be performed.
"Otherwise, the stool-based test is of no value."
Wilson had no disclosures to report. Chey's disclosures include consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestle, Phathom, Redhill, Salix/Valean, Takeda, and Vibrant.
A version of this article first appeared on Medscape.com.
PHOENIX -- Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
"In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy," said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings this week at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
"These findings reinforce the importance of colonoscopy following positive interval FIT, given the high risk of advanced neoplasia and colorectal cancer, regardless of polypectomy history," Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, due to the higher risk.
Reasons patients may instead turn to FIT include cost or other factors.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2004.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the nonpolypectomy group).
Among the FIT screenings, results were positive in 17.2% of postpolypectomy patients and 14.1% of patients who no prior polypectomy, indicating a history of polypectomy to be predictive of positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result -- and having a previous polypectomy should add further emergency to the matter -- the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the nonpolypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the postcolonoscopy interval FIT.
The findings underscore that "positive results carried a high risk of advanced neoplasia or cancer, irrespective or prior polypectomy history," Wilson said.
Commenting on the study, William D. Chey, MD, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, Michigan, noted that the study "addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy."
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
"Other data suggest that the rate might even be significantly higher -- at 70% to 80%, depending upon the population and the test," Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies "should raise questions about whether there might be a role for FIT testing in addition to colonoscopy." However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is "how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy," he said.
"I think a lot of this is going to come down to how it's down at the primary care level."
Chey added that in that, and any other setting, "the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it's positive, a follow-up colonoscopy must be performed.
"Otherwise, the stool-based test is of no value."
Wilson had no disclosures to report. Chey's disclosures include consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestle, Phathom, Redhill, Salix/Valean, Takeda, and Vibrant.
A version of this article first appeared on Medscape.com.
PHOENIX -- Patients with or without polyp removal in an index colonoscopy commonly receive follow-up surveillance with a fecal immunochemical test (FIT), yet many of these patients do not receive a recommended colonoscopy after a positive FIT.
"In this large US study, we found interval FITs are frequently performed in patients with and without prior polypectomy," said first author Natalie J. Wilson, MD, of the University of Minnesota in Minneapolis, while presenting the findings this week at the American College of Gastroenterology (ACG) 2025 Annual Scientific Meeting.
"These findings reinforce the importance of colonoscopy following positive interval FIT, given the high risk of advanced neoplasia and colorectal cancer, regardless of polypectomy history," Wilson said.
Guideline recommendations stress the need for follow-up surveillance with a colonoscopy, particularly in patients who have had a prior polypectomy, due to the higher risk.
Reasons patients may instead turn to FIT include cost or other factors.
To determine just how often that happens, how having a previous polypectomy affects FIT results, and how adherent patients are to follow up if a FIT result is positive, Wilson and her colleagues evaluated data from nearly 4.8 million individuals in the Veterans Health Administration Corporate Data Warehouse who underwent colonoscopy between 2000 and 2004.
Of the patients, 10.9% were found to have subsequently received interval FIT within 10 years of the index colonoscopy, and of those patients, nearly half (49.9%) had received a polypectomy at the index colonoscopy.
The average time from the colonoscopy/polypectomy to the interval FIT was 5.9 years (5.6 years in the polypectomy group vs 6.2 years in the nonpolypectomy group).
Among the FIT screenings, results were positive in 17.2% of postpolypectomy patients and 14.1% of patients who no prior polypectomy, indicating a history of polypectomy to be predictive of positive interval FIT (odds ratio [OR], 1.12; P < .0001).
Notably, while a follow-up colonoscopy is considered essential following a positive FIT result -- and having a previous polypectomy should add further emergency to the matter -- the study showed only 50.4% of those who had an earlier polypectomy went on to receive the recommended follow-up colonoscopy after a positive follow-up FIT, and the rate was 49.3% among those who had not received a polypectomy (P = .001).
For those who did receive a follow-up colonoscopy after a positive FIT, the duration of time to receiving the colonoscopy was longer among those who had a prior polypectomy, at 2.9 months compared with 2.5 months in the nonpolypectomy group (P < .001).
Colonoscopy results following a positive FIT showed higher rates of detections among patients who had prior polypectomies than among those with no prior polypectomy, including tubular adenomas (54.7% vs 45.8%), tubulovillous adenomas (5.6% vs 4.7%), adenomas with high-grade dysplasia (0.8% vs 0.7%), sessile serrated lesions (3.52% vs 2.4%), advanced colorectal neoplasia (9.2% vs 7.9%), and colorectal cancer (3.3% vs 3.0%).
However, a prior polypectomy was not independently predictive of colorectal cancer (OR, 0.96; P = .65) or advanced colorectal neoplasia (OR, 0.97; P = .57) in the postcolonoscopy interval FIT.
The findings underscore that "positive results carried a high risk of advanced neoplasia or cancer, irrespective or prior polypectomy history," Wilson said.
Commenting on the study, William D. Chey, MD, chief of the Division of Gastroenterology & Hepatology at the University of Michigan in Ann Arbor, Michigan, noted that the study "addresses one of the biggest challenges we face as a profession, which is making sure that patients who have a positive stool test get a colonoscopy."
He noted that the low rate of just 50% of recipients of positive FITs going on to receive a colonoscopy is consistent with what is observed in other trials.
"Other data suggest that the rate might even be significantly higher -- at 70% to 80%, depending upon the population and the test," Chey told Medscape Medical News.
Reasons for the failure to receive the follow-up testing range from income restrictions (due to the high cost of a colonoscopy, especially if not covered by insurance), education, speaking a foreign language, and other factors, he said.
The relatively high rates of colon cancers detected by FIT in the study, in those with and without a prior polypectomy, along with findings from other studies "should raise questions about whether there might be a role for FIT testing in addition to colonoscopy." However, much stronger evidence would be needed, Chey noted.
In the meantime, a key issue is "how do we do a better job of making sure that individuals who have a positive FIT test get a colonoscopy," he said.
"I think a lot of this is going to come down to how it's down at the primary care level."
Chey added that in that, and any other setting, "the main message that needs to get out to people who are undergoing stool-based screening is that the stool test is only the first part of the screening process, and if it's positive, a follow-up colonoscopy must be performed.
"Otherwise, the stool-based test is of no value."
Wilson had no disclosures to report. Chey's disclosures include consulting and/or other relationships with Ardelyx, Atmo, Biomerica, Commonwealth Diagnostics International, Corprata, Dieta, Evinature, Food Marble, Gemelli, Kiwi BioScience, Modify Health, Nestle, Phathom, Redhill, Salix/Valean, Takeda, and Vibrant.
A version of this article first appeared on Medscape.com.
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
Patients With a Positive FIT Fail to Get Follow-Up Colonoscopies
As Federal Cuts Deepen Mental Health Crisis, Philanthropy Scrambles to Fill the Gap
As Federal Cuts Deepen Mental Health Crisis, Philanthropy Scrambles to Fill the Gap
It's hardly news that the United States is experiencing a mental health crisis -- the CDC says as much. But experts in the field say that the current administration has severely compounded the problem by eliminating agency funding and national programs, slashing research grants and data resources, and creating new barriers to behavioral health care.
Philanthropic foundations aim to do what they can to address the shortfall. The numbers, however, just don't add up.
"Some big foundations and philanthropies have said they're going to increase what they give out in the next 4 years, but they'll never be able to fill the gap," said Morgan F. McDonald, MD, national director of population health at the Milbank Memorial Fund in New York City, which works with states on health policy. "Even if every one of them were to spend down their endowments, they still couldn't."
Given the financial limitations, some foundations are taking a different tack. While looking for ways to join forces with fellow nonprofits, they are providing emergency grants to bridge funding in the short term to keep research from grinding to a halt.
Budget Cuts Reach Far and Wide
Mental health research certainly didn't escape the extensive grant cancellations at the National Institutes of Health and the National Science Foundation.
"It's already affecting our ability to stay on the cutting edge of research, best practices, and treatment approaches," said Zainab Okolo, EdD, senior vice president of policy, advocacy, and government relations at The Jed Foundation in New York City, which focuses on the emotional health of teens and young adults.
The upheaval is evident in an array of government agencies. The Health Resources and Services Administration, which last year awarded $12 billion in grants to community health centers and addiction treatment services, has seen > one-fourth of its staff eliminated. The Substance Abuse and Mental Health Services Administration has lost more than a third of its staff as federal cuts took a $1 billion bite out of its operating budget. The Education Department has halted $1 billion in grants used to hire mental health workers in school districts nationwide.
"We're very, very concerned about cuts to behavioral health systems," said Alonzo Plough, PhD, chief science officer at the Robert Wood Johnson Foundation in Princeton, New Jersey. "Doctors and nurses working in safety-net clinics are seeing tremendous reductions."
All in all, the new tax and spending law means $1 trillion in cuts to health care programs including Medicaid -- the nation's largest payer for mental health services -- Medicare, and Affordable Care Act insurance. An estimated 10 million Americans are expected to lose their health coverage as a result.
"When accessibility to care goes down, there's a chance that more people will die by suicide," said Jill Harkavy-Friedman, PhD, senior vice president of research at the American Foundation for Suicide Prevention. "But it also means people will come into care later in the course of their difficulties. Health professionals will be dealing with worse problems."
Foundations Take Emergency Measures
Even if private dollars can't replace what's been lost, philanthropic and medical foundations are stepping up.
We're seeing a lot of foundations and funders that are shifting their funding," said Alyson Niemann, CEO of Mindful Philanthropy, an organization that works with > 1000 private funders to marshal resources for mental health. This year, in response to federal cuts, "many increased funding to health and well-being, doubling or even tripling it," Niemann noted.
"They're making a great deal of effort to respond with emergency funds, really getting in the trenches and being good partners to their grantees," she said. "We've seen them asking deliberate questions, thinking about where their funding can have the most impact."
The American Psychological Foundation (APF), a longtime supporter of research and innovation, is addressing the current crisis with 2 initiatives, Michelle Quist Ryder, PhD, the organization's CEO, explained in an email. The first is APF Director Action, which funds innovative interventions at the community level. The second, Direct Action Crisis Funding Grants, will help continue research that is at risk of stalling because of budget cuts.
"Studies that are 'paused' or lose funding often cannot simply pick back up where they left off. Having to halt progress on a project can invalidate the work already completed," Ryder wrote. "These Direct Action Crisis Grants help bridge funding gaps and keep research viable."
At the same time, collaboration between foundations is becoming more widespread as they seek to maximize their impact. Philanthropic organizations are sharing ideas and best practices as well as pooling fundings.
"The goal of philanthropy is to help people," Harkavy-Friedman said. "There's strength in numbers and more dollars in numbers."
Some See Hope in Raised Voices
Despite the emergency scrambling, many of those in the trenches remain surprisingly optimistic. Some point out that the current turmoil has put a helpful spotlight on behavioral health care. Practitioners, meanwhile, have an essential role to play.
"There's a reason that things were the way they were: People advocated for many years to get where we've gotten," Harkavy-Friedman said, citing veterans' mental health care, the national violent death reporting system, and 988 as examples. "We have to raise our voices louder -- professionals in particular, because they know the impact a person in the general public many not fully grasp."
As a growing numbers of health professionals call attention to the damage wrought by deep cuts in the federal budget, foundation executives see an opportunity.
"In the mental health field, there's a deficit in the narrative, where there's a lot of focus on crisis. What we're hoping to do is shift the narrative toward 'How do we flourish together?'" Niemann said. "Sometimes deficits are where the most incredible innovations appear."
Debbie Koenig is a health writer whose work has been published by WebMD, The New York Times, and The Washington Post.
A version of this article first appeared on Medscape.com.
It's hardly news that the United States is experiencing a mental health crisis -- the CDC says as much. But experts in the field say that the current administration has severely compounded the problem by eliminating agency funding and national programs, slashing research grants and data resources, and creating new barriers to behavioral health care.
Philanthropic foundations aim to do what they can to address the shortfall. The numbers, however, just don't add up.
"Some big foundations and philanthropies have said they're going to increase what they give out in the next 4 years, but they'll never be able to fill the gap," said Morgan F. McDonald, MD, national director of population health at the Milbank Memorial Fund in New York City, which works with states on health policy. "Even if every one of them were to spend down their endowments, they still couldn't."
Given the financial limitations, some foundations are taking a different tack. While looking for ways to join forces with fellow nonprofits, they are providing emergency grants to bridge funding in the short term to keep research from grinding to a halt.
Budget Cuts Reach Far and Wide
Mental health research certainly didn't escape the extensive grant cancellations at the National Institutes of Health and the National Science Foundation.
"It's already affecting our ability to stay on the cutting edge of research, best practices, and treatment approaches," said Zainab Okolo, EdD, senior vice president of policy, advocacy, and government relations at The Jed Foundation in New York City, which focuses on the emotional health of teens and young adults.
The upheaval is evident in an array of government agencies. The Health Resources and Services Administration, which last year awarded $12 billion in grants to community health centers and addiction treatment services, has seen > one-fourth of its staff eliminated. The Substance Abuse and Mental Health Services Administration has lost more than a third of its staff as federal cuts took a $1 billion bite out of its operating budget. The Education Department has halted $1 billion in grants used to hire mental health workers in school districts nationwide.
"We're very, very concerned about cuts to behavioral health systems," said Alonzo Plough, PhD, chief science officer at the Robert Wood Johnson Foundation in Princeton, New Jersey. "Doctors and nurses working in safety-net clinics are seeing tremendous reductions."
All in all, the new tax and spending law means $1 trillion in cuts to health care programs including Medicaid -- the nation's largest payer for mental health services -- Medicare, and Affordable Care Act insurance. An estimated 10 million Americans are expected to lose their health coverage as a result.
"When accessibility to care goes down, there's a chance that more people will die by suicide," said Jill Harkavy-Friedman, PhD, senior vice president of research at the American Foundation for Suicide Prevention. "But it also means people will come into care later in the course of their difficulties. Health professionals will be dealing with worse problems."
Foundations Take Emergency Measures
Even if private dollars can't replace what's been lost, philanthropic and medical foundations are stepping up.
We're seeing a lot of foundations and funders that are shifting their funding," said Alyson Niemann, CEO of Mindful Philanthropy, an organization that works with > 1000 private funders to marshal resources for mental health. This year, in response to federal cuts, "many increased funding to health and well-being, doubling or even tripling it," Niemann noted.
"They're making a great deal of effort to respond with emergency funds, really getting in the trenches and being good partners to their grantees," she said. "We've seen them asking deliberate questions, thinking about where their funding can have the most impact."
The American Psychological Foundation (APF), a longtime supporter of research and innovation, is addressing the current crisis with 2 initiatives, Michelle Quist Ryder, PhD, the organization's CEO, explained in an email. The first is APF Director Action, which funds innovative interventions at the community level. The second, Direct Action Crisis Funding Grants, will help continue research that is at risk of stalling because of budget cuts.
"Studies that are 'paused' or lose funding often cannot simply pick back up where they left off. Having to halt progress on a project can invalidate the work already completed," Ryder wrote. "These Direct Action Crisis Grants help bridge funding gaps and keep research viable."
At the same time, collaboration between foundations is becoming more widespread as they seek to maximize their impact. Philanthropic organizations are sharing ideas and best practices as well as pooling fundings.
"The goal of philanthropy is to help people," Harkavy-Friedman said. "There's strength in numbers and more dollars in numbers."
Some See Hope in Raised Voices
Despite the emergency scrambling, many of those in the trenches remain surprisingly optimistic. Some point out that the current turmoil has put a helpful spotlight on behavioral health care. Practitioners, meanwhile, have an essential role to play.
"There's a reason that things were the way they were: People advocated for many years to get where we've gotten," Harkavy-Friedman said, citing veterans' mental health care, the national violent death reporting system, and 988 as examples. "We have to raise our voices louder -- professionals in particular, because they know the impact a person in the general public many not fully grasp."
As a growing numbers of health professionals call attention to the damage wrought by deep cuts in the federal budget, foundation executives see an opportunity.
"In the mental health field, there's a deficit in the narrative, where there's a lot of focus on crisis. What we're hoping to do is shift the narrative toward 'How do we flourish together?'" Niemann said. "Sometimes deficits are where the most incredible innovations appear."
Debbie Koenig is a health writer whose work has been published by WebMD, The New York Times, and The Washington Post.
A version of this article first appeared on Medscape.com.
It's hardly news that the United States is experiencing a mental health crisis -- the CDC says as much. But experts in the field say that the current administration has severely compounded the problem by eliminating agency funding and national programs, slashing research grants and data resources, and creating new barriers to behavioral health care.
Philanthropic foundations aim to do what they can to address the shortfall. The numbers, however, just don't add up.
"Some big foundations and philanthropies have said they're going to increase what they give out in the next 4 years, but they'll never be able to fill the gap," said Morgan F. McDonald, MD, national director of population health at the Milbank Memorial Fund in New York City, which works with states on health policy. "Even if every one of them were to spend down their endowments, they still couldn't."
Given the financial limitations, some foundations are taking a different tack. While looking for ways to join forces with fellow nonprofits, they are providing emergency grants to bridge funding in the short term to keep research from grinding to a halt.
Budget Cuts Reach Far and Wide
Mental health research certainly didn't escape the extensive grant cancellations at the National Institutes of Health and the National Science Foundation.
"It's already affecting our ability to stay on the cutting edge of research, best practices, and treatment approaches," said Zainab Okolo, EdD, senior vice president of policy, advocacy, and government relations at The Jed Foundation in New York City, which focuses on the emotional health of teens and young adults.
The upheaval is evident in an array of government agencies. The Health Resources and Services Administration, which last year awarded $12 billion in grants to community health centers and addiction treatment services, has seen > one-fourth of its staff eliminated. The Substance Abuse and Mental Health Services Administration has lost more than a third of its staff as federal cuts took a $1 billion bite out of its operating budget. The Education Department has halted $1 billion in grants used to hire mental health workers in school districts nationwide.
"We're very, very concerned about cuts to behavioral health systems," said Alonzo Plough, PhD, chief science officer at the Robert Wood Johnson Foundation in Princeton, New Jersey. "Doctors and nurses working in safety-net clinics are seeing tremendous reductions."
All in all, the new tax and spending law means $1 trillion in cuts to health care programs including Medicaid -- the nation's largest payer for mental health services -- Medicare, and Affordable Care Act insurance. An estimated 10 million Americans are expected to lose their health coverage as a result.
"When accessibility to care goes down, there's a chance that more people will die by suicide," said Jill Harkavy-Friedman, PhD, senior vice president of research at the American Foundation for Suicide Prevention. "But it also means people will come into care later in the course of their difficulties. Health professionals will be dealing with worse problems."
Foundations Take Emergency Measures
Even if private dollars can't replace what's been lost, philanthropic and medical foundations are stepping up.
We're seeing a lot of foundations and funders that are shifting their funding," said Alyson Niemann, CEO of Mindful Philanthropy, an organization that works with > 1000 private funders to marshal resources for mental health. This year, in response to federal cuts, "many increased funding to health and well-being, doubling or even tripling it," Niemann noted.
"They're making a great deal of effort to respond with emergency funds, really getting in the trenches and being good partners to their grantees," she said. "We've seen them asking deliberate questions, thinking about where their funding can have the most impact."
The American Psychological Foundation (APF), a longtime supporter of research and innovation, is addressing the current crisis with 2 initiatives, Michelle Quist Ryder, PhD, the organization's CEO, explained in an email. The first is APF Director Action, which funds innovative interventions at the community level. The second, Direct Action Crisis Funding Grants, will help continue research that is at risk of stalling because of budget cuts.
"Studies that are 'paused' or lose funding often cannot simply pick back up where they left off. Having to halt progress on a project can invalidate the work already completed," Ryder wrote. "These Direct Action Crisis Grants help bridge funding gaps and keep research viable."
At the same time, collaboration between foundations is becoming more widespread as they seek to maximize their impact. Philanthropic organizations are sharing ideas and best practices as well as pooling fundings.
"The goal of philanthropy is to help people," Harkavy-Friedman said. "There's strength in numbers and more dollars in numbers."
Some See Hope in Raised Voices
Despite the emergency scrambling, many of those in the trenches remain surprisingly optimistic. Some point out that the current turmoil has put a helpful spotlight on behavioral health care. Practitioners, meanwhile, have an essential role to play.
"There's a reason that things were the way they were: People advocated for many years to get where we've gotten," Harkavy-Friedman said, citing veterans' mental health care, the national violent death reporting system, and 988 as examples. "We have to raise our voices louder -- professionals in particular, because they know the impact a person in the general public many not fully grasp."
As a growing numbers of health professionals call attention to the damage wrought by deep cuts in the federal budget, foundation executives see an opportunity.
"In the mental health field, there's a deficit in the narrative, where there's a lot of focus on crisis. What we're hoping to do is shift the narrative toward 'How do we flourish together?'" Niemann said. "Sometimes deficits are where the most incredible innovations appear."
Debbie Koenig is a health writer whose work has been published by WebMD, The New York Times, and The Washington Post.
A version of this article first appeared on Medscape.com.
As Federal Cuts Deepen Mental Health Crisis, Philanthropy Scrambles to Fill the Gap
As Federal Cuts Deepen Mental Health Crisis, Philanthropy Scrambles to Fill the Gap
Taking Therapy Home With Mobile Mental Health Apps
For Kelly, a retired Navy operations specialist, coping with depression and anxiety hindered her ability to enjoy everyday life. Then she elected to enter therapy, a decision she calls “transformative.”
“When I started doing therapy, it was like releasing the toxins, releasing the buildup of the fear or the rage or the overwhelming feelings of shame,” she says. “We can’t just hold on to it. Just telling the truth, it helps me every single day. It is so worth it.”
Kurt, an Army veteran, tried to power through his anxiety, depression, and survivor guilt. He didn’t have much faith in mental health therapy, thinking no one could relate to him. He was surprised, though, once he started treatment, how much his life improved. He now encourages other veterans to face their own mental health challenges, be it through virtual/mental health apps or in-person care.
“From getting help, every day of my life is better,” he says, “and I couldn’t be more grateful for it.”
Stories from Kelly and Kurt are 2 of 7 the US Department of Veterans Affairs (VA) highlighted during National Recovery Month, outlining how their lives were forever changed with the support of mental health care.
But for every Kelly and Kurt, there are thousands of individuals reluctant to seek mental health care. A analysis of 2019-2020 data from the National Health and Resilience in Veterans Study found that 924 (26%) of 4069 veterans met criteria for ≥ 1 psychological disorders, but only 12% reported engagement in mental health care. The researchers considered the role of protective psychosocial characteristics, such as grit (ie, “trait perseverance that extends to one’s decision or commitment to address mental health needs on one’s own; dispositional optimism; and purpose in life”). Veterans who reported mental dysfunction but scored highly on grit were less likely to be engaged in treatment. This pattern suggests higher levels of grit may reduce the likelihood of seeking treatment, “even in the presence of clinically meaningful distress.”
A 2004 study found only 23% to 40% of service members who screened positive for a mental disorder sought care. They often believed they would be seen as weak, or their unit leadership might treat them differently, and unit members would have less confidence in them.
Given that military members and veterans are at increased risk of posttraumatic stress disorder (PTSD) in addition to mood, anxiety, and substance use disorders, any alternatives that increase their access to support and services are crucial. For those who aren’t disposed to office visits and group therapy, the answer may lie in mobile apps.
In a recent randomized controlled trial, 201 veterans who screened positive for PTSD and alcohol use disorder were divided into 2 groups: a mobile mindfulness-based intervention group enhanced with brief alcohol intervention content (Mind Guide), and an active stress management program group. Mind Guide engagement was excellent, according to the study, with averages of > 31 logins and 5 hours of app use. At 16 weeks, the Mind Guide group showed significant reductions in PTSD symptoms (no differences emerged for alcohol use frequency). Mind Guide may be a valuable adjunct to more intensive in-person PTSD treatment by facilitating interest in services, integration into care, and/or sustainment of posttreatment improvements. The VA currently offers 16 apps, including MHA for Veterans, an app designed for patients to complete mental health assessments after their clinician assigned them. Other apps address a variety of issues, such as anger management, insomnia, chronic pain, and PTSD.
Two apps were created with an eye toward specific communities. One, Veterans Wellness Path, was designed for American Indians and Alaska Natives with input from those veterans, their family members, and health care practitioners. It supports the transition from military service to home and encourages balance and connection with self, family, community, and environment. Similarly, WellWithin Coach was designed by the VA National Center for PTSD with input from women veterans and subject matter experts in women’s mental health.
Whatever form it takes—in-person or virtual—finding support that works can make all the difference for veterans. Kelly founded and serves as the executive director of Acta Non Verba: Youth Urban Farm Project, an organization that brings together > 3000 low-income youth and families annually to learn about urban farming, aiming to fill a gap in an area known as a food desert: “We do have the power and the right to wake up the next day and try to do something different,” she said.
For Kelly, a retired Navy operations specialist, coping with depression and anxiety hindered her ability to enjoy everyday life. Then she elected to enter therapy, a decision she calls “transformative.”
“When I started doing therapy, it was like releasing the toxins, releasing the buildup of the fear or the rage or the overwhelming feelings of shame,” she says. “We can’t just hold on to it. Just telling the truth, it helps me every single day. It is so worth it.”
Kurt, an Army veteran, tried to power through his anxiety, depression, and survivor guilt. He didn’t have much faith in mental health therapy, thinking no one could relate to him. He was surprised, though, once he started treatment, how much his life improved. He now encourages other veterans to face their own mental health challenges, be it through virtual/mental health apps or in-person care.
“From getting help, every day of my life is better,” he says, “and I couldn’t be more grateful for it.”
Stories from Kelly and Kurt are 2 of 7 the US Department of Veterans Affairs (VA) highlighted during National Recovery Month, outlining how their lives were forever changed with the support of mental health care.
But for every Kelly and Kurt, there are thousands of individuals reluctant to seek mental health care. A analysis of 2019-2020 data from the National Health and Resilience in Veterans Study found that 924 (26%) of 4069 veterans met criteria for ≥ 1 psychological disorders, but only 12% reported engagement in mental health care. The researchers considered the role of protective psychosocial characteristics, such as grit (ie, “trait perseverance that extends to one’s decision or commitment to address mental health needs on one’s own; dispositional optimism; and purpose in life”). Veterans who reported mental dysfunction but scored highly on grit were less likely to be engaged in treatment. This pattern suggests higher levels of grit may reduce the likelihood of seeking treatment, “even in the presence of clinically meaningful distress.”
A 2004 study found only 23% to 40% of service members who screened positive for a mental disorder sought care. They often believed they would be seen as weak, or their unit leadership might treat them differently, and unit members would have less confidence in them.
Given that military members and veterans are at increased risk of posttraumatic stress disorder (PTSD) in addition to mood, anxiety, and substance use disorders, any alternatives that increase their access to support and services are crucial. For those who aren’t disposed to office visits and group therapy, the answer may lie in mobile apps.
In a recent randomized controlled trial, 201 veterans who screened positive for PTSD and alcohol use disorder were divided into 2 groups: a mobile mindfulness-based intervention group enhanced with brief alcohol intervention content (Mind Guide), and an active stress management program group. Mind Guide engagement was excellent, according to the study, with averages of > 31 logins and 5 hours of app use. At 16 weeks, the Mind Guide group showed significant reductions in PTSD symptoms (no differences emerged for alcohol use frequency). Mind Guide may be a valuable adjunct to more intensive in-person PTSD treatment by facilitating interest in services, integration into care, and/or sustainment of posttreatment improvements. The VA currently offers 16 apps, including MHA for Veterans, an app designed for patients to complete mental health assessments after their clinician assigned them. Other apps address a variety of issues, such as anger management, insomnia, chronic pain, and PTSD.
Two apps were created with an eye toward specific communities. One, Veterans Wellness Path, was designed for American Indians and Alaska Natives with input from those veterans, their family members, and health care practitioners. It supports the transition from military service to home and encourages balance and connection with self, family, community, and environment. Similarly, WellWithin Coach was designed by the VA National Center for PTSD with input from women veterans and subject matter experts in women’s mental health.
Whatever form it takes—in-person or virtual—finding support that works can make all the difference for veterans. Kelly founded and serves as the executive director of Acta Non Verba: Youth Urban Farm Project, an organization that brings together > 3000 low-income youth and families annually to learn about urban farming, aiming to fill a gap in an area known as a food desert: “We do have the power and the right to wake up the next day and try to do something different,” she said.
For Kelly, a retired Navy operations specialist, coping with depression and anxiety hindered her ability to enjoy everyday life. Then she elected to enter therapy, a decision she calls “transformative.”
“When I started doing therapy, it was like releasing the toxins, releasing the buildup of the fear or the rage or the overwhelming feelings of shame,” she says. “We can’t just hold on to it. Just telling the truth, it helps me every single day. It is so worth it.”
Kurt, an Army veteran, tried to power through his anxiety, depression, and survivor guilt. He didn’t have much faith in mental health therapy, thinking no one could relate to him. He was surprised, though, once he started treatment, how much his life improved. He now encourages other veterans to face their own mental health challenges, be it through virtual/mental health apps or in-person care.
“From getting help, every day of my life is better,” he says, “and I couldn’t be more grateful for it.”
Stories from Kelly and Kurt are 2 of 7 the US Department of Veterans Affairs (VA) highlighted during National Recovery Month, outlining how their lives were forever changed with the support of mental health care.
But for every Kelly and Kurt, there are thousands of individuals reluctant to seek mental health care. A analysis of 2019-2020 data from the National Health and Resilience in Veterans Study found that 924 (26%) of 4069 veterans met criteria for ≥ 1 psychological disorders, but only 12% reported engagement in mental health care. The researchers considered the role of protective psychosocial characteristics, such as grit (ie, “trait perseverance that extends to one’s decision or commitment to address mental health needs on one’s own; dispositional optimism; and purpose in life”). Veterans who reported mental dysfunction but scored highly on grit were less likely to be engaged in treatment. This pattern suggests higher levels of grit may reduce the likelihood of seeking treatment, “even in the presence of clinically meaningful distress.”
A 2004 study found only 23% to 40% of service members who screened positive for a mental disorder sought care. They often believed they would be seen as weak, or their unit leadership might treat them differently, and unit members would have less confidence in them.
Given that military members and veterans are at increased risk of posttraumatic stress disorder (PTSD) in addition to mood, anxiety, and substance use disorders, any alternatives that increase their access to support and services are crucial. For those who aren’t disposed to office visits and group therapy, the answer may lie in mobile apps.
In a recent randomized controlled trial, 201 veterans who screened positive for PTSD and alcohol use disorder were divided into 2 groups: a mobile mindfulness-based intervention group enhanced with brief alcohol intervention content (Mind Guide), and an active stress management program group. Mind Guide engagement was excellent, according to the study, with averages of > 31 logins and 5 hours of app use. At 16 weeks, the Mind Guide group showed significant reductions in PTSD symptoms (no differences emerged for alcohol use frequency). Mind Guide may be a valuable adjunct to more intensive in-person PTSD treatment by facilitating interest in services, integration into care, and/or sustainment of posttreatment improvements. The VA currently offers 16 apps, including MHA for Veterans, an app designed for patients to complete mental health assessments after their clinician assigned them. Other apps address a variety of issues, such as anger management, insomnia, chronic pain, and PTSD.
Two apps were created with an eye toward specific communities. One, Veterans Wellness Path, was designed for American Indians and Alaska Natives with input from those veterans, their family members, and health care practitioners. It supports the transition from military service to home and encourages balance and connection with self, family, community, and environment. Similarly, WellWithin Coach was designed by the VA National Center for PTSD with input from women veterans and subject matter experts in women’s mental health.
Whatever form it takes—in-person or virtual—finding support that works can make all the difference for veterans. Kelly founded and serves as the executive director of Acta Non Verba: Youth Urban Farm Project, an organization that brings together > 3000 low-income youth and families annually to learn about urban farming, aiming to fill a gap in an area known as a food desert: “We do have the power and the right to wake up the next day and try to do something different,” she said.
Novel Agent Promising for Refractory Ulcerative Colitis
The findings, from the ABTECT-1 and ABTECT-2 phase 3 induction trials, were presented in two separate late-breaking presentations at United European Gastroenterology (UEG) Week 2025 in Berlin, Germany.
“These trials enrolled a broad spectrum of participants, including one of the most severe and refractory populations evaluated to date in a phase 3 UC trial, with about 60% of patients across the pooled dataset having a Mayo endoscopic subscore of 3 — the highest level of UC endoscopic disease activity,” study investigator Marla Dubinsky, MD, gastroenterologist and co-director of the IBD Center at Mount Sinai in New York City, told GI & Hepatology News.
“Even within this challenging population, obefazimod achieved the primary endpoint of clinical remission and all key secondary endpoints, including endoscopic improvement, after just 8 weeks of therapy,” Dubinsky said.
This suggests that obefazimod may serve as both an early advanced therapy option and a much-needed alternative for patients with moderately to severely active UC who have failed multiple biologics and JAK inhibitors, with few choices left short of colectomy, she added.
Study Details
Obefazimod is an investigational oral, potentially first-in-class drug that enhances expression of microRNA-124, resulting in regulation of the inflammatory response and restoring mucosal homeostasis in UC.
The ABTECT-1 and ABTECT-2 were identically designed induction trials enrolling a total of 1272 patients with moderately to severely active UC who had inadequate response, loss of response, or intolerance to at least one prior therapy (with no upper limit), including corticosteroids, immunosuppressants, biologics, S1P receptor modulators, and/or JAK inhibitors. Participants were randomly assigned in a 2:1:1 ratio to receive obefazimod 50 mg or 25 mg or placebo once daily for 8 weeks.
In ABTECT-1, obefazimod 50 mg and 25 mg met the primary endpoint of clinical remission, with 22% of patients in the 50-mg group and 24% in the 25-mg group achieving clinical remission at 8 weeks compared with 2.5% of the placebo group.
The effect sizes for clinical remission were 21% for the 25-mg dose and 19% for the 50-mg dose, reported Bruce E. Sands, MD, MS, AGAF, professor of medicine at Icahn School of Medicine at Mount Sinai and chief in the Division of Gastroenterology at Mount Sinai Health System in New York City.
In ABTECT-2, the 50-mg dose met the primary endpoint of clinical remission, with 20% of patients achieving remission compared with 11% in the 25-mg group and 6.3% in the placebo group.
The effect sizes for clinical remission in ABTECT-2 were “a bit smaller” (13% for the 50-mg dose and 5% for the 25-mg dose) “because the absolute efficacy of 50 mg in this study was a little bit lower, and the placebo response rate was a little bit higher at 6.3%, and so accordingly, the 25-mg dose did not achieve statistical significance,” Sands explained.
Both doses of obefazimod met all secondary endpoints in ABTECT-1 and the 50-mg dose achieved all secondary endpoints in ABTECT-2. Secondary endpoints included clinical response, endoscopic improvement, symptomatic remission, and histo-endoscopic mucosal improvement.
Pooled data across the two studies showed that both doses achieved “clinically meaningful improvements across all efficacy points,” Sands noted.
Notably, obefazimod 50 mg once daily achieved “consistent and clinically meaningful improvements” regardless of prior failure of advanced therapy, and both doses performed similarly well in the subgroup with no prior failure of advanced therapy, Silvio Danese, MD, PhD, with Vita-Salute San Raffaele University, Milan, Italy, reported in a separate presentation.
Adverse Events ‘Not a Barrier to Treatment’
Pooled data across the two studies showed no signal for serious, severe, or opportunistic infections or malignancies.
The most commonly reported treatment-emergent adverse event was headache, reported in 24% and 16% of patients taking obefazimod 50 mg and 25 mg, respectively, vs 6% of those taking placebo. Headaches were mild, transient, and short-lasting and “not a barrier to treatment, as evidenced by the low discontinuation (< 1%),” Sands noted.
“Because this is a safe agent and it’s an oral agent and convenient, I think the drug could be used early in the course of the disease, before advanced therapy or after failure of advanced therapies, even multiple advanced therapies,” Sands said.
“Of course, we’ll have to see what the maintenance data show. But we have a long experience from the phase 2a and 2b long-term extension treatments, and the durability seems to be quite good,” Sands cautioned.
Abivax CEO Marc de Garidel, MBA, told GI & Hepatology News that the company will share “top-line data” from the 44-week maintenance study evaluating obefazimod in UC in the second quarter of 2026.
“If positive, the data will support a potential NDA [New Drug Application] submission in the second half of 2026,” de Garidel said.
‘Promising Data’
Ashwin Ananthakrishnan, MBBS, MPH, AGAF, associate professor of medicine at Harvard Medical School and a gastroenterologist at Massachusetts General Hospital, Boston, who wasn’t involved in the study, was impressed.
“I think this is very promising data from an important study. This is an entirely novel mechanism of action in ulcerative colitis,” Ananthakrishnan told GI & Hepatology News.
“While we have many treatments available, there are still a large number of patients who do not respond to existing treatment mechanisms,” he said. These trials “consisted of a large number of very refractory patients (severe endoscopic disease or multiple prior mechanism failures). That it works well in this population is very promising (and clinically impactful).”
It would be a “welcome addition to the armamentarium,” he added.
The study was funded by Abivax. Several study authors disclosed having financial relationships with the company. Ananthakrishnan reported having no disclosures.
A version of this article appeared on Medscape.com.
The findings, from the ABTECT-1 and ABTECT-2 phase 3 induction trials, were presented in two separate late-breaking presentations at United European Gastroenterology (UEG) Week 2025 in Berlin, Germany.
“These trials enrolled a broad spectrum of participants, including one of the most severe and refractory populations evaluated to date in a phase 3 UC trial, with about 60% of patients across the pooled dataset having a Mayo endoscopic subscore of 3 — the highest level of UC endoscopic disease activity,” study investigator Marla Dubinsky, MD, gastroenterologist and co-director of the IBD Center at Mount Sinai in New York City, told GI & Hepatology News.
“Even within this challenging population, obefazimod achieved the primary endpoint of clinical remission and all key secondary endpoints, including endoscopic improvement, after just 8 weeks of therapy,” Dubinsky said.
This suggests that obefazimod may serve as both an early advanced therapy option and a much-needed alternative for patients with moderately to severely active UC who have failed multiple biologics and JAK inhibitors, with few choices left short of colectomy, she added.
Study Details
Obefazimod is an investigational oral, potentially first-in-class drug that enhances expression of microRNA-124, resulting in regulation of the inflammatory response and restoring mucosal homeostasis in UC.
The ABTECT-1 and ABTECT-2 were identically designed induction trials enrolling a total of 1272 patients with moderately to severely active UC who had inadequate response, loss of response, or intolerance to at least one prior therapy (with no upper limit), including corticosteroids, immunosuppressants, biologics, S1P receptor modulators, and/or JAK inhibitors. Participants were randomly assigned in a 2:1:1 ratio to receive obefazimod 50 mg or 25 mg or placebo once daily for 8 weeks.
In ABTECT-1, obefazimod 50 mg and 25 mg met the primary endpoint of clinical remission, with 22% of patients in the 50-mg group and 24% in the 25-mg group achieving clinical remission at 8 weeks compared with 2.5% of the placebo group.
The effect sizes for clinical remission were 21% for the 25-mg dose and 19% for the 50-mg dose, reported Bruce E. Sands, MD, MS, AGAF, professor of medicine at Icahn School of Medicine at Mount Sinai and chief in the Division of Gastroenterology at Mount Sinai Health System in New York City.
In ABTECT-2, the 50-mg dose met the primary endpoint of clinical remission, with 20% of patients achieving remission compared with 11% in the 25-mg group and 6.3% in the placebo group.
The effect sizes for clinical remission in ABTECT-2 were “a bit smaller” (13% for the 50-mg dose and 5% for the 25-mg dose) “because the absolute efficacy of 50 mg in this study was a little bit lower, and the placebo response rate was a little bit higher at 6.3%, and so accordingly, the 25-mg dose did not achieve statistical significance,” Sands explained.
Both doses of obefazimod met all secondary endpoints in ABTECT-1 and the 50-mg dose achieved all secondary endpoints in ABTECT-2. Secondary endpoints included clinical response, endoscopic improvement, symptomatic remission, and histo-endoscopic mucosal improvement.
Pooled data across the two studies showed that both doses achieved “clinically meaningful improvements across all efficacy points,” Sands noted.
Notably, obefazimod 50 mg once daily achieved “consistent and clinically meaningful improvements” regardless of prior failure of advanced therapy, and both doses performed similarly well in the subgroup with no prior failure of advanced therapy, Silvio Danese, MD, PhD, with Vita-Salute San Raffaele University, Milan, Italy, reported in a separate presentation.
Adverse Events ‘Not a Barrier to Treatment’
Pooled data across the two studies showed no signal for serious, severe, or opportunistic infections or malignancies.
The most commonly reported treatment-emergent adverse event was headache, reported in 24% and 16% of patients taking obefazimod 50 mg and 25 mg, respectively, vs 6% of those taking placebo. Headaches were mild, transient, and short-lasting and “not a barrier to treatment, as evidenced by the low discontinuation (< 1%),” Sands noted.
“Because this is a safe agent and it’s an oral agent and convenient, I think the drug could be used early in the course of the disease, before advanced therapy or after failure of advanced therapies, even multiple advanced therapies,” Sands said.
“Of course, we’ll have to see what the maintenance data show. But we have a long experience from the phase 2a and 2b long-term extension treatments, and the durability seems to be quite good,” Sands cautioned.
Abivax CEO Marc de Garidel, MBA, told GI & Hepatology News that the company will share “top-line data” from the 44-week maintenance study evaluating obefazimod in UC in the second quarter of 2026.
“If positive, the data will support a potential NDA [New Drug Application] submission in the second half of 2026,” de Garidel said.
‘Promising Data’
Ashwin Ananthakrishnan, MBBS, MPH, AGAF, associate professor of medicine at Harvard Medical School and a gastroenterologist at Massachusetts General Hospital, Boston, who wasn’t involved in the study, was impressed.
“I think this is very promising data from an important study. This is an entirely novel mechanism of action in ulcerative colitis,” Ananthakrishnan told GI & Hepatology News.
“While we have many treatments available, there are still a large number of patients who do not respond to existing treatment mechanisms,” he said. These trials “consisted of a large number of very refractory patients (severe endoscopic disease or multiple prior mechanism failures). That it works well in this population is very promising (and clinically impactful).”
It would be a “welcome addition to the armamentarium,” he added.
The study was funded by Abivax. Several study authors disclosed having financial relationships with the company. Ananthakrishnan reported having no disclosures.
A version of this article appeared on Medscape.com.
The findings, from the ABTECT-1 and ABTECT-2 phase 3 induction trials, were presented in two separate late-breaking presentations at United European Gastroenterology (UEG) Week 2025 in Berlin, Germany.
“These trials enrolled a broad spectrum of participants, including one of the most severe and refractory populations evaluated to date in a phase 3 UC trial, with about 60% of patients across the pooled dataset having a Mayo endoscopic subscore of 3 — the highest level of UC endoscopic disease activity,” study investigator Marla Dubinsky, MD, gastroenterologist and co-director of the IBD Center at Mount Sinai in New York City, told GI & Hepatology News.
“Even within this challenging population, obefazimod achieved the primary endpoint of clinical remission and all key secondary endpoints, including endoscopic improvement, after just 8 weeks of therapy,” Dubinsky said.
This suggests that obefazimod may serve as both an early advanced therapy option and a much-needed alternative for patients with moderately to severely active UC who have failed multiple biologics and JAK inhibitors, with few choices left short of colectomy, she added.
Study Details
Obefazimod is an investigational oral, potentially first-in-class drug that enhances expression of microRNA-124, resulting in regulation of the inflammatory response and restoring mucosal homeostasis in UC.
The ABTECT-1 and ABTECT-2 were identically designed induction trials enrolling a total of 1272 patients with moderately to severely active UC who had inadequate response, loss of response, or intolerance to at least one prior therapy (with no upper limit), including corticosteroids, immunosuppressants, biologics, S1P receptor modulators, and/or JAK inhibitors. Participants were randomly assigned in a 2:1:1 ratio to receive obefazimod 50 mg or 25 mg or placebo once daily for 8 weeks.
In ABTECT-1, obefazimod 50 mg and 25 mg met the primary endpoint of clinical remission, with 22% of patients in the 50-mg group and 24% in the 25-mg group achieving clinical remission at 8 weeks compared with 2.5% of the placebo group.
The effect sizes for clinical remission were 21% for the 25-mg dose and 19% for the 50-mg dose, reported Bruce E. Sands, MD, MS, AGAF, professor of medicine at Icahn School of Medicine at Mount Sinai and chief in the Division of Gastroenterology at Mount Sinai Health System in New York City.
In ABTECT-2, the 50-mg dose met the primary endpoint of clinical remission, with 20% of patients achieving remission compared with 11% in the 25-mg group and 6.3% in the placebo group.
The effect sizes for clinical remission in ABTECT-2 were “a bit smaller” (13% for the 50-mg dose and 5% for the 25-mg dose) “because the absolute efficacy of 50 mg in this study was a little bit lower, and the placebo response rate was a little bit higher at 6.3%, and so accordingly, the 25-mg dose did not achieve statistical significance,” Sands explained.
Both doses of obefazimod met all secondary endpoints in ABTECT-1 and the 50-mg dose achieved all secondary endpoints in ABTECT-2. Secondary endpoints included clinical response, endoscopic improvement, symptomatic remission, and histo-endoscopic mucosal improvement.
Pooled data across the two studies showed that both doses achieved “clinically meaningful improvements across all efficacy points,” Sands noted.
Notably, obefazimod 50 mg once daily achieved “consistent and clinically meaningful improvements” regardless of prior failure of advanced therapy, and both doses performed similarly well in the subgroup with no prior failure of advanced therapy, Silvio Danese, MD, PhD, with Vita-Salute San Raffaele University, Milan, Italy, reported in a separate presentation.
Adverse Events ‘Not a Barrier to Treatment’
Pooled data across the two studies showed no signal for serious, severe, or opportunistic infections or malignancies.
The most commonly reported treatment-emergent adverse event was headache, reported in 24% and 16% of patients taking obefazimod 50 mg and 25 mg, respectively, vs 6% of those taking placebo. Headaches were mild, transient, and short-lasting and “not a barrier to treatment, as evidenced by the low discontinuation (< 1%),” Sands noted.
“Because this is a safe agent and it’s an oral agent and convenient, I think the drug could be used early in the course of the disease, before advanced therapy or after failure of advanced therapies, even multiple advanced therapies,” Sands said.
“Of course, we’ll have to see what the maintenance data show. But we have a long experience from the phase 2a and 2b long-term extension treatments, and the durability seems to be quite good,” Sands cautioned.
Abivax CEO Marc de Garidel, MBA, told GI & Hepatology News that the company will share “top-line data” from the 44-week maintenance study evaluating obefazimod in UC in the second quarter of 2026.
“If positive, the data will support a potential NDA [New Drug Application] submission in the second half of 2026,” de Garidel said.
‘Promising Data’
Ashwin Ananthakrishnan, MBBS, MPH, AGAF, associate professor of medicine at Harvard Medical School and a gastroenterologist at Massachusetts General Hospital, Boston, who wasn’t involved in the study, was impressed.
“I think this is very promising data from an important study. This is an entirely novel mechanism of action in ulcerative colitis,” Ananthakrishnan told GI & Hepatology News.
“While we have many treatments available, there are still a large number of patients who do not respond to existing treatment mechanisms,” he said. These trials “consisted of a large number of very refractory patients (severe endoscopic disease or multiple prior mechanism failures). That it works well in this population is very promising (and clinically impactful).”
It would be a “welcome addition to the armamentarium,” he added.
The study was funded by Abivax. Several study authors disclosed having financial relationships with the company. Ananthakrishnan reported having no disclosures.
A version of this article appeared on Medscape.com.
Half of Patients Skip Repeat Stool Tests for CRC Screening
A large real-world study found that
Among those who did repeat the test, the average delay was 3 months before COVID and increased to 5 months during the pandemic, the authors reported in BMJ Public Health.
“Stool tests are relatively easy to complete at home and mailed for testing, and they are inexpensive, but they must be completed annually. In contrast, colonoscopies are more invasive and require more time away from work but only need to be repeated every 5-10 years,” Staci J Wendt, PhD, director, health research accelerator, Providence Research Network, Providence, Rhode Island, told GI & Hepatology News.
In the end, “the best colorectal cancer screening test is the one that gets done,” Wendt said.
“This is why we stress the importance of patients and their doctor having these discussions together and deciding which screening is the most preferred method for the individual patient,” she added.
Stool Tests Gaining Traction
Adults are increasingly turning to at-home stool tests for CRC screening — a trend that accelerated during the pandemic. Yet, there is limited data on whether patients undergo repeat stool tests following initial negative test results.
Wendt and her colleagues documented rates of repeat preventative stool tests by analyzing electronic medical records from Providence St Joseph Health, a large health system with 51 hospitals and over 1000 clinics across seven western US states.
They divided their analysis into two periods based on the onset of the pandemic. The pre-COVID onset period spanned January 2018 to February 2020 and the post-COVID period spanned March 2020 to February 2022.
“The pandemic is a salient time to conduct this study because it resulted in a dramatic decrease in colonoscopies, which were partially replaced by stool tests. This partial replacement of colonoscopies by stool tests has led other studies to conclude that stool tests mitigated gaps in CRC screening during the pandemic. But gaps may persist if patients do not undergo repeat testing,” the study team explained.
Their sample included 403,085 patients. Among those with an initial negative stool test, the share who obtained a timely repeat screening ranged from 38% to 49% across the study years, confirming that “most patients do not undergo the recommended repeat screening after their initial stool test,” the researchers said.
Among adults who do a repeat test, delays were common. The average lag to the follow-up test was 3months on average, increasing to about 5 months amid COVID — almost half as long as the preventative screening period of stool tests (12 months).
“These gaps could delay detection of CRC and subsequent treatment, potentially resulting in higher mortality. These gaps are particularly important as more and more patients use stool tests instead of colonoscopes for CRC screening,” the researchers wrote.
Screening patterns shifted markedly during the pandemic.
Not surprisingly, the volume of colonoscopies declined substantially after the onset of the pandemic and stayed low through the study’s end. In contrast, the volume of at-home stool tests was increasing before the pandemic and accelerated during the pandemic.
“Given this increase in stool tests, it will be increasingly important to focus on improving long-term adherence to screening through outreach, policies and programs,” the researchers said.
A Multilevel Approach
Wendt said health systems that are incorporating proactive measures like sending stool kits to patients who are eligible for screening, should ensure that these screening kits and information are sent annually and that it is stressed that the screening must happen every year.
Reached for comment, Aasma Shaukat, MD, MPH, AGAF, director of outcomes research, Division of Gastroenterology and Hepatology, NYU Langone Health, New York City, who wasn’t involved in the study, said the poor adherence to repeat stool tests for CRC screening seen in this study is “not surprising.”
“We know that adherence goes down with each consecutive screening round and what is really needed is an organized program to keep the level of adherence up,” Shaukat told GI & Hepatology News.
Shaukat agreed that boosting adherence to stool tests requires a “multilevel approach.”
She cited the success of the CRC screening program implemented across Kaiser Permanente Northern California. The program includes proactive and targeted outreach to members who are overdue for screening and mailed fecal immunochemical test kits for at-home use.
As reported previously by GI & Hepatology News, the program has made a huge difference in CRC incidence, deaths, and racial disparities.
The program has doubled the proportion of people up to date with screening. And, within about 10 years, cancer rates were cut by a third, deaths were halved and largely eliminated long-standing differences by race and ethnicity.
The study had no commercial funding. Wendt and Shaukat declared having no relevant disclosures.
A version of this article appeared on Medscape.com.
A large real-world study found that
Among those who did repeat the test, the average delay was 3 months before COVID and increased to 5 months during the pandemic, the authors reported in BMJ Public Health.
“Stool tests are relatively easy to complete at home and mailed for testing, and they are inexpensive, but they must be completed annually. In contrast, colonoscopies are more invasive and require more time away from work but only need to be repeated every 5-10 years,” Staci J Wendt, PhD, director, health research accelerator, Providence Research Network, Providence, Rhode Island, told GI & Hepatology News.
In the end, “the best colorectal cancer screening test is the one that gets done,” Wendt said.
“This is why we stress the importance of patients and their doctor having these discussions together and deciding which screening is the most preferred method for the individual patient,” she added.
Stool Tests Gaining Traction
Adults are increasingly turning to at-home stool tests for CRC screening — a trend that accelerated during the pandemic. Yet, there is limited data on whether patients undergo repeat stool tests following initial negative test results.
Wendt and her colleagues documented rates of repeat preventative stool tests by analyzing electronic medical records from Providence St Joseph Health, a large health system with 51 hospitals and over 1000 clinics across seven western US states.
They divided their analysis into two periods based on the onset of the pandemic. The pre-COVID onset period spanned January 2018 to February 2020 and the post-COVID period spanned March 2020 to February 2022.
“The pandemic is a salient time to conduct this study because it resulted in a dramatic decrease in colonoscopies, which were partially replaced by stool tests. This partial replacement of colonoscopies by stool tests has led other studies to conclude that stool tests mitigated gaps in CRC screening during the pandemic. But gaps may persist if patients do not undergo repeat testing,” the study team explained.
Their sample included 403,085 patients. Among those with an initial negative stool test, the share who obtained a timely repeat screening ranged from 38% to 49% across the study years, confirming that “most patients do not undergo the recommended repeat screening after their initial stool test,” the researchers said.
Among adults who do a repeat test, delays were common. The average lag to the follow-up test was 3months on average, increasing to about 5 months amid COVID — almost half as long as the preventative screening period of stool tests (12 months).
“These gaps could delay detection of CRC and subsequent treatment, potentially resulting in higher mortality. These gaps are particularly important as more and more patients use stool tests instead of colonoscopes for CRC screening,” the researchers wrote.
Screening patterns shifted markedly during the pandemic.
Not surprisingly, the volume of colonoscopies declined substantially after the onset of the pandemic and stayed low through the study’s end. In contrast, the volume of at-home stool tests was increasing before the pandemic and accelerated during the pandemic.
“Given this increase in stool tests, it will be increasingly important to focus on improving long-term adherence to screening through outreach, policies and programs,” the researchers said.
A Multilevel Approach
Wendt said health systems that are incorporating proactive measures like sending stool kits to patients who are eligible for screening, should ensure that these screening kits and information are sent annually and that it is stressed that the screening must happen every year.
Reached for comment, Aasma Shaukat, MD, MPH, AGAF, director of outcomes research, Division of Gastroenterology and Hepatology, NYU Langone Health, New York City, who wasn’t involved in the study, said the poor adherence to repeat stool tests for CRC screening seen in this study is “not surprising.”
“We know that adherence goes down with each consecutive screening round and what is really needed is an organized program to keep the level of adherence up,” Shaukat told GI & Hepatology News.
Shaukat agreed that boosting adherence to stool tests requires a “multilevel approach.”
She cited the success of the CRC screening program implemented across Kaiser Permanente Northern California. The program includes proactive and targeted outreach to members who are overdue for screening and mailed fecal immunochemical test kits for at-home use.
As reported previously by GI & Hepatology News, the program has made a huge difference in CRC incidence, deaths, and racial disparities.
The program has doubled the proportion of people up to date with screening. And, within about 10 years, cancer rates were cut by a third, deaths were halved and largely eliminated long-standing differences by race and ethnicity.
The study had no commercial funding. Wendt and Shaukat declared having no relevant disclosures.
A version of this article appeared on Medscape.com.
A large real-world study found that
Among those who did repeat the test, the average delay was 3 months before COVID and increased to 5 months during the pandemic, the authors reported in BMJ Public Health.
“Stool tests are relatively easy to complete at home and mailed for testing, and they are inexpensive, but they must be completed annually. In contrast, colonoscopies are more invasive and require more time away from work but only need to be repeated every 5-10 years,” Staci J Wendt, PhD, director, health research accelerator, Providence Research Network, Providence, Rhode Island, told GI & Hepatology News.
In the end, “the best colorectal cancer screening test is the one that gets done,” Wendt said.
“This is why we stress the importance of patients and their doctor having these discussions together and deciding which screening is the most preferred method for the individual patient,” she added.
Stool Tests Gaining Traction
Adults are increasingly turning to at-home stool tests for CRC screening — a trend that accelerated during the pandemic. Yet, there is limited data on whether patients undergo repeat stool tests following initial negative test results.
Wendt and her colleagues documented rates of repeat preventative stool tests by analyzing electronic medical records from Providence St Joseph Health, a large health system with 51 hospitals and over 1000 clinics across seven western US states.
They divided their analysis into two periods based on the onset of the pandemic. The pre-COVID onset period spanned January 2018 to February 2020 and the post-COVID period spanned March 2020 to February 2022.
“The pandemic is a salient time to conduct this study because it resulted in a dramatic decrease in colonoscopies, which were partially replaced by stool tests. This partial replacement of colonoscopies by stool tests has led other studies to conclude that stool tests mitigated gaps in CRC screening during the pandemic. But gaps may persist if patients do not undergo repeat testing,” the study team explained.
Their sample included 403,085 patients. Among those with an initial negative stool test, the share who obtained a timely repeat screening ranged from 38% to 49% across the study years, confirming that “most patients do not undergo the recommended repeat screening after their initial stool test,” the researchers said.
Among adults who do a repeat test, delays were common. The average lag to the follow-up test was 3months on average, increasing to about 5 months amid COVID — almost half as long as the preventative screening period of stool tests (12 months).
“These gaps could delay detection of CRC and subsequent treatment, potentially resulting in higher mortality. These gaps are particularly important as more and more patients use stool tests instead of colonoscopes for CRC screening,” the researchers wrote.
Screening patterns shifted markedly during the pandemic.
Not surprisingly, the volume of colonoscopies declined substantially after the onset of the pandemic and stayed low through the study’s end. In contrast, the volume of at-home stool tests was increasing before the pandemic and accelerated during the pandemic.
“Given this increase in stool tests, it will be increasingly important to focus on improving long-term adherence to screening through outreach, policies and programs,” the researchers said.
A Multilevel Approach
Wendt said health systems that are incorporating proactive measures like sending stool kits to patients who are eligible for screening, should ensure that these screening kits and information are sent annually and that it is stressed that the screening must happen every year.
Reached for comment, Aasma Shaukat, MD, MPH, AGAF, director of outcomes research, Division of Gastroenterology and Hepatology, NYU Langone Health, New York City, who wasn’t involved in the study, said the poor adherence to repeat stool tests for CRC screening seen in this study is “not surprising.”
“We know that adherence goes down with each consecutive screening round and what is really needed is an organized program to keep the level of adherence up,” Shaukat told GI & Hepatology News.
Shaukat agreed that boosting adherence to stool tests requires a “multilevel approach.”
She cited the success of the CRC screening program implemented across Kaiser Permanente Northern California. The program includes proactive and targeted outreach to members who are overdue for screening and mailed fecal immunochemical test kits for at-home use.
As reported previously by GI & Hepatology News, the program has made a huge difference in CRC incidence, deaths, and racial disparities.
The program has doubled the proportion of people up to date with screening. And, within about 10 years, cancer rates were cut by a third, deaths were halved and largely eliminated long-standing differences by race and ethnicity.
The study had no commercial funding. Wendt and Shaukat declared having no relevant disclosures.
A version of this article appeared on Medscape.com.
Why Veterans May Conceal Suicidal Thoughts
Veterans at risk of suicide may not share their suicidal ideation with their psychotherapists or may choose not to disclose enough detail to illustrate the depths of those thoughts due to feelings of shame or embarrassment, according to a newly published study. These individuals may view suicidal thoughts as a sign of weakness, fear involuntary hospitalization or prescriptions, or belong to marginalized groups who do not feel comfortable (or safe) to reveal their thoughts or intentions. This can make it difficult for mental health professionals to identify the exact details of a patient’s mindset and provide appropriate care.
A veteran’s first—and sometimes only—stop may be their primary care practitioner (PCPs) rather than a mental health professional. A review of 40 studies found that although 45% of individuals who died by suicide had contact with PCPs within 1 month of their death, only 19% had contact with mental health services. Studies have also found that veterans disclose suicidal ideation during primary care visits closest to the actual suicide less than half the time.
Patients may have an appointment for medical, but not psychological reasons. In a study conducted at Portland Veterans Affairs Medical Center (VAMC), researchers reviewed the medical records of 112 veterans who died by suicide and had contact with a VAMC within 1 year prior to death. Of those last contacts, 32% were patient-initiated for new or exacerbated medical concerns, and 68% were follow-ups.
In that study, health care professionals (HCPs) noted that 41 patients (37%) were experiencing emotional distress at the last contact, but 13 of 18 patients (72%) who were assessed for suicidal ideation at their last contact denied such thoughts. The study says this finding “highlights the complexity of addressing suicidal ideation and associated risk factors in health care settings.” Additionally, a number of veterans who died by suicide either did not have suicidal thoughts at the time of their last contact with HCPs or denied such thoughts even when questioned.
In 2018, the Veterans Health Administration (VHA) implemented the Suicide Risk Identification Strategy (Risk ID), an evidence-informed assessment that includes initial screening and subsequent evaluation. Veterans receiving VHA care are screened annually for suicidal ideation and behaviors. Most screening takes place in primary care and mental health specialty settings, but timely screening may not be enough to assess who is at risk if the patients aren’t being forthcoming about their thoughts and plans.
A recent cross-sectional national survey examined the frequency of self-reported “inaccurate disclosure” of suicidal ideation during initial screening and subsequent evaluation among 734 VHA patients screened in primary care.
Using the Risk ID process with the Columbia Suicide Severity Rating Scale Screener (C-SSRS), the study asked respondents about their previous suicide screening in 2021. Of the 734 respondents, 306 screened positive and 428 screened negative. One survey item asked about the extent to which veterans had accurately responded to the HCP when asked about suicidal thoughts, while another asked how likely they would discuss when they felt suicidal with their PCP.
The study found that inaccurate disclosure is not uncommon: When asked about suicidal thoughts, about one-fifth of screen-negative participants and two-fifths of screen-positive participants said they responded, “less than very accurately.”
In the screen-positive group, women and those who reported more barriers to care were less likely to discuss feeling suicidal. Veterans who had lower ratings of satisfaction with the screening process, patient-staff communication, and the therapeutic relationship reported being less likely to discuss times they were suicidal. Notably, among C-SSRS-negative patients, Black, American Indian/Alaska Native, Hispanic, Asian, and multiracial veterans were more likely than White veterans to inaccurately report suicidal thoughts.
This is consistent with studies on medical mistrust and other research suggesting that veterans who have experienced identity-based discrimination may be less inclined to discuss suicidal thoughts with VHA HCPs. A large 2023 study surveyed veterans about why they might hold back such information. One Gulf War-era veteran, a Black woman, had encountered discrimination when filing her VA benefits claim, leading her to feel like the care system was not interested in helping her.
“It’s one of the main reasons why when I do go in, they don’t get an honest response,” she wrote in her survey response. “I feel that you’re not for me, you’re not trying to help me, you don’t wanna help me, and why even go through it, go through the motions it seems. So, I can come in feeling suicidal and I leave out feeling suicidal then.”
Veterans typically welcome screening for suicidal risk. In a 2023 study, > 90% of veterans reported that it is appropriate to be asked about thoughts of suicide during primary care visits, and about one-half agreed that veterans should be asked about suicidal thoughts at every visit.
For many, though, the level of trust they have with HCPs makes or breaks whether they discuss their suicidal ideation. Higher ratings of the therapeutic relationship with clinicians are associated with more frequent disclosure. However, the screen-positive group demonstrated higher rates of inaccurate disclosure than the screen-negative group. While this may seem counterintuitive, it is possible that screen-positive individuals did not fully disclose their thoughts on the initial screen, or did not fully disclose the severity of their thoughts during follow-up evaluations. Individuals who disclose suicidal thoughts during initial screening may be ambivalent about disclosure and/or become more concerned about consequences of disclosure as additional evaluation ensues.
A 2013 study of 34 Operation Enduring Freedom/Operation Iraqi Freedom veterans found that veterans felt trying to suppress and avoid thoughts of suicide was “burdensome and exhausting.” Despite this, they often failed to disclose severe and pervasive suicidal thoughts when screened. Among the reasons was that they perceived the templated computer reminder process as “perfunctory and disrespectful.”
Research has found that HCPs who focuses on building relationships, demonstrates genuineness and empathy, and uses straightforward and understandable language promotes the trust that can result in more honest disclosure of suicidal thoughts. In the “inaccurate disclosure” study, some veterans reported they did not understand the screening questions, or the questions did not make sense to them. This aligns with prior research, which demonstrates that how HCPs and researchers conceptualize suicidal thoughts may not fit with patients’ experiences. A lack of shared terminology, they note, “may confound how we think about ‘under-disclosure,’ such that perhaps patients may not be trying to hide their thoughts so much as not finding screening questions applicable to their unique situations or experiences.”
Veterans at risk of suicide may not share their suicidal ideation with their psychotherapists or may choose not to disclose enough detail to illustrate the depths of those thoughts due to feelings of shame or embarrassment, according to a newly published study. These individuals may view suicidal thoughts as a sign of weakness, fear involuntary hospitalization or prescriptions, or belong to marginalized groups who do not feel comfortable (or safe) to reveal their thoughts or intentions. This can make it difficult for mental health professionals to identify the exact details of a patient’s mindset and provide appropriate care.
A veteran’s first—and sometimes only—stop may be their primary care practitioner (PCPs) rather than a mental health professional. A review of 40 studies found that although 45% of individuals who died by suicide had contact with PCPs within 1 month of their death, only 19% had contact with mental health services. Studies have also found that veterans disclose suicidal ideation during primary care visits closest to the actual suicide less than half the time.
Patients may have an appointment for medical, but not psychological reasons. In a study conducted at Portland Veterans Affairs Medical Center (VAMC), researchers reviewed the medical records of 112 veterans who died by suicide and had contact with a VAMC within 1 year prior to death. Of those last contacts, 32% were patient-initiated for new or exacerbated medical concerns, and 68% were follow-ups.
In that study, health care professionals (HCPs) noted that 41 patients (37%) were experiencing emotional distress at the last contact, but 13 of 18 patients (72%) who were assessed for suicidal ideation at their last contact denied such thoughts. The study says this finding “highlights the complexity of addressing suicidal ideation and associated risk factors in health care settings.” Additionally, a number of veterans who died by suicide either did not have suicidal thoughts at the time of their last contact with HCPs or denied such thoughts even when questioned.
In 2018, the Veterans Health Administration (VHA) implemented the Suicide Risk Identification Strategy (Risk ID), an evidence-informed assessment that includes initial screening and subsequent evaluation. Veterans receiving VHA care are screened annually for suicidal ideation and behaviors. Most screening takes place in primary care and mental health specialty settings, but timely screening may not be enough to assess who is at risk if the patients aren’t being forthcoming about their thoughts and plans.
A recent cross-sectional national survey examined the frequency of self-reported “inaccurate disclosure” of suicidal ideation during initial screening and subsequent evaluation among 734 VHA patients screened in primary care.
Using the Risk ID process with the Columbia Suicide Severity Rating Scale Screener (C-SSRS), the study asked respondents about their previous suicide screening in 2021. Of the 734 respondents, 306 screened positive and 428 screened negative. One survey item asked about the extent to which veterans had accurately responded to the HCP when asked about suicidal thoughts, while another asked how likely they would discuss when they felt suicidal with their PCP.
The study found that inaccurate disclosure is not uncommon: When asked about suicidal thoughts, about one-fifth of screen-negative participants and two-fifths of screen-positive participants said they responded, “less than very accurately.”
In the screen-positive group, women and those who reported more barriers to care were less likely to discuss feeling suicidal. Veterans who had lower ratings of satisfaction with the screening process, patient-staff communication, and the therapeutic relationship reported being less likely to discuss times they were suicidal. Notably, among C-SSRS-negative patients, Black, American Indian/Alaska Native, Hispanic, Asian, and multiracial veterans were more likely than White veterans to inaccurately report suicidal thoughts.
This is consistent with studies on medical mistrust and other research suggesting that veterans who have experienced identity-based discrimination may be less inclined to discuss suicidal thoughts with VHA HCPs. A large 2023 study surveyed veterans about why they might hold back such information. One Gulf War-era veteran, a Black woman, had encountered discrimination when filing her VA benefits claim, leading her to feel like the care system was not interested in helping her.
“It’s one of the main reasons why when I do go in, they don’t get an honest response,” she wrote in her survey response. “I feel that you’re not for me, you’re not trying to help me, you don’t wanna help me, and why even go through it, go through the motions it seems. So, I can come in feeling suicidal and I leave out feeling suicidal then.”
Veterans typically welcome screening for suicidal risk. In a 2023 study, > 90% of veterans reported that it is appropriate to be asked about thoughts of suicide during primary care visits, and about one-half agreed that veterans should be asked about suicidal thoughts at every visit.
For many, though, the level of trust they have with HCPs makes or breaks whether they discuss their suicidal ideation. Higher ratings of the therapeutic relationship with clinicians are associated with more frequent disclosure. However, the screen-positive group demonstrated higher rates of inaccurate disclosure than the screen-negative group. While this may seem counterintuitive, it is possible that screen-positive individuals did not fully disclose their thoughts on the initial screen, or did not fully disclose the severity of their thoughts during follow-up evaluations. Individuals who disclose suicidal thoughts during initial screening may be ambivalent about disclosure and/or become more concerned about consequences of disclosure as additional evaluation ensues.
A 2013 study of 34 Operation Enduring Freedom/Operation Iraqi Freedom veterans found that veterans felt trying to suppress and avoid thoughts of suicide was “burdensome and exhausting.” Despite this, they often failed to disclose severe and pervasive suicidal thoughts when screened. Among the reasons was that they perceived the templated computer reminder process as “perfunctory and disrespectful.”
Research has found that HCPs who focuses on building relationships, demonstrates genuineness and empathy, and uses straightforward and understandable language promotes the trust that can result in more honest disclosure of suicidal thoughts. In the “inaccurate disclosure” study, some veterans reported they did not understand the screening questions, or the questions did not make sense to them. This aligns with prior research, which demonstrates that how HCPs and researchers conceptualize suicidal thoughts may not fit with patients’ experiences. A lack of shared terminology, they note, “may confound how we think about ‘under-disclosure,’ such that perhaps patients may not be trying to hide their thoughts so much as not finding screening questions applicable to their unique situations or experiences.”
Veterans at risk of suicide may not share their suicidal ideation with their psychotherapists or may choose not to disclose enough detail to illustrate the depths of those thoughts due to feelings of shame or embarrassment, according to a newly published study. These individuals may view suicidal thoughts as a sign of weakness, fear involuntary hospitalization or prescriptions, or belong to marginalized groups who do not feel comfortable (or safe) to reveal their thoughts or intentions. This can make it difficult for mental health professionals to identify the exact details of a patient’s mindset and provide appropriate care.
A veteran’s first—and sometimes only—stop may be their primary care practitioner (PCPs) rather than a mental health professional. A review of 40 studies found that although 45% of individuals who died by suicide had contact with PCPs within 1 month of their death, only 19% had contact with mental health services. Studies have also found that veterans disclose suicidal ideation during primary care visits closest to the actual suicide less than half the time.
Patients may have an appointment for medical, but not psychological reasons. In a study conducted at Portland Veterans Affairs Medical Center (VAMC), researchers reviewed the medical records of 112 veterans who died by suicide and had contact with a VAMC within 1 year prior to death. Of those last contacts, 32% were patient-initiated for new or exacerbated medical concerns, and 68% were follow-ups.
In that study, health care professionals (HCPs) noted that 41 patients (37%) were experiencing emotional distress at the last contact, but 13 of 18 patients (72%) who were assessed for suicidal ideation at their last contact denied such thoughts. The study says this finding “highlights the complexity of addressing suicidal ideation and associated risk factors in health care settings.” Additionally, a number of veterans who died by suicide either did not have suicidal thoughts at the time of their last contact with HCPs or denied such thoughts even when questioned.
In 2018, the Veterans Health Administration (VHA) implemented the Suicide Risk Identification Strategy (Risk ID), an evidence-informed assessment that includes initial screening and subsequent evaluation. Veterans receiving VHA care are screened annually for suicidal ideation and behaviors. Most screening takes place in primary care and mental health specialty settings, but timely screening may not be enough to assess who is at risk if the patients aren’t being forthcoming about their thoughts and plans.
A recent cross-sectional national survey examined the frequency of self-reported “inaccurate disclosure” of suicidal ideation during initial screening and subsequent evaluation among 734 VHA patients screened in primary care.
Using the Risk ID process with the Columbia Suicide Severity Rating Scale Screener (C-SSRS), the study asked respondents about their previous suicide screening in 2021. Of the 734 respondents, 306 screened positive and 428 screened negative. One survey item asked about the extent to which veterans had accurately responded to the HCP when asked about suicidal thoughts, while another asked how likely they would discuss when they felt suicidal with their PCP.
The study found that inaccurate disclosure is not uncommon: When asked about suicidal thoughts, about one-fifth of screen-negative participants and two-fifths of screen-positive participants said they responded, “less than very accurately.”
In the screen-positive group, women and those who reported more barriers to care were less likely to discuss feeling suicidal. Veterans who had lower ratings of satisfaction with the screening process, patient-staff communication, and the therapeutic relationship reported being less likely to discuss times they were suicidal. Notably, among C-SSRS-negative patients, Black, American Indian/Alaska Native, Hispanic, Asian, and multiracial veterans were more likely than White veterans to inaccurately report suicidal thoughts.
This is consistent with studies on medical mistrust and other research suggesting that veterans who have experienced identity-based discrimination may be less inclined to discuss suicidal thoughts with VHA HCPs. A large 2023 study surveyed veterans about why they might hold back such information. One Gulf War-era veteran, a Black woman, had encountered discrimination when filing her VA benefits claim, leading her to feel like the care system was not interested in helping her.
“It’s one of the main reasons why when I do go in, they don’t get an honest response,” she wrote in her survey response. “I feel that you’re not for me, you’re not trying to help me, you don’t wanna help me, and why even go through it, go through the motions it seems. So, I can come in feeling suicidal and I leave out feeling suicidal then.”
Veterans typically welcome screening for suicidal risk. In a 2023 study, > 90% of veterans reported that it is appropriate to be asked about thoughts of suicide during primary care visits, and about one-half agreed that veterans should be asked about suicidal thoughts at every visit.
For many, though, the level of trust they have with HCPs makes or breaks whether they discuss their suicidal ideation. Higher ratings of the therapeutic relationship with clinicians are associated with more frequent disclosure. However, the screen-positive group demonstrated higher rates of inaccurate disclosure than the screen-negative group. While this may seem counterintuitive, it is possible that screen-positive individuals did not fully disclose their thoughts on the initial screen, or did not fully disclose the severity of their thoughts during follow-up evaluations. Individuals who disclose suicidal thoughts during initial screening may be ambivalent about disclosure and/or become more concerned about consequences of disclosure as additional evaluation ensues.
A 2013 study of 34 Operation Enduring Freedom/Operation Iraqi Freedom veterans found that veterans felt trying to suppress and avoid thoughts of suicide was “burdensome and exhausting.” Despite this, they often failed to disclose severe and pervasive suicidal thoughts when screened. Among the reasons was that they perceived the templated computer reminder process as “perfunctory and disrespectful.”
Research has found that HCPs who focuses on building relationships, demonstrates genuineness and empathy, and uses straightforward and understandable language promotes the trust that can result in more honest disclosure of suicidal thoughts. In the “inaccurate disclosure” study, some veterans reported they did not understand the screening questions, or the questions did not make sense to them. This aligns with prior research, which demonstrates that how HCPs and researchers conceptualize suicidal thoughts may not fit with patients’ experiences. A lack of shared terminology, they note, “may confound how we think about ‘under-disclosure,’ such that perhaps patients may not be trying to hide their thoughts so much as not finding screening questions applicable to their unique situations or experiences.”
Updates in Multiple Sclerosis Imaging
Updates in Multiple Sclerosis Imaging
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
Updates in Multiple Sclerosis Imaging
Updates in Multiple Sclerosis Imaging