User login
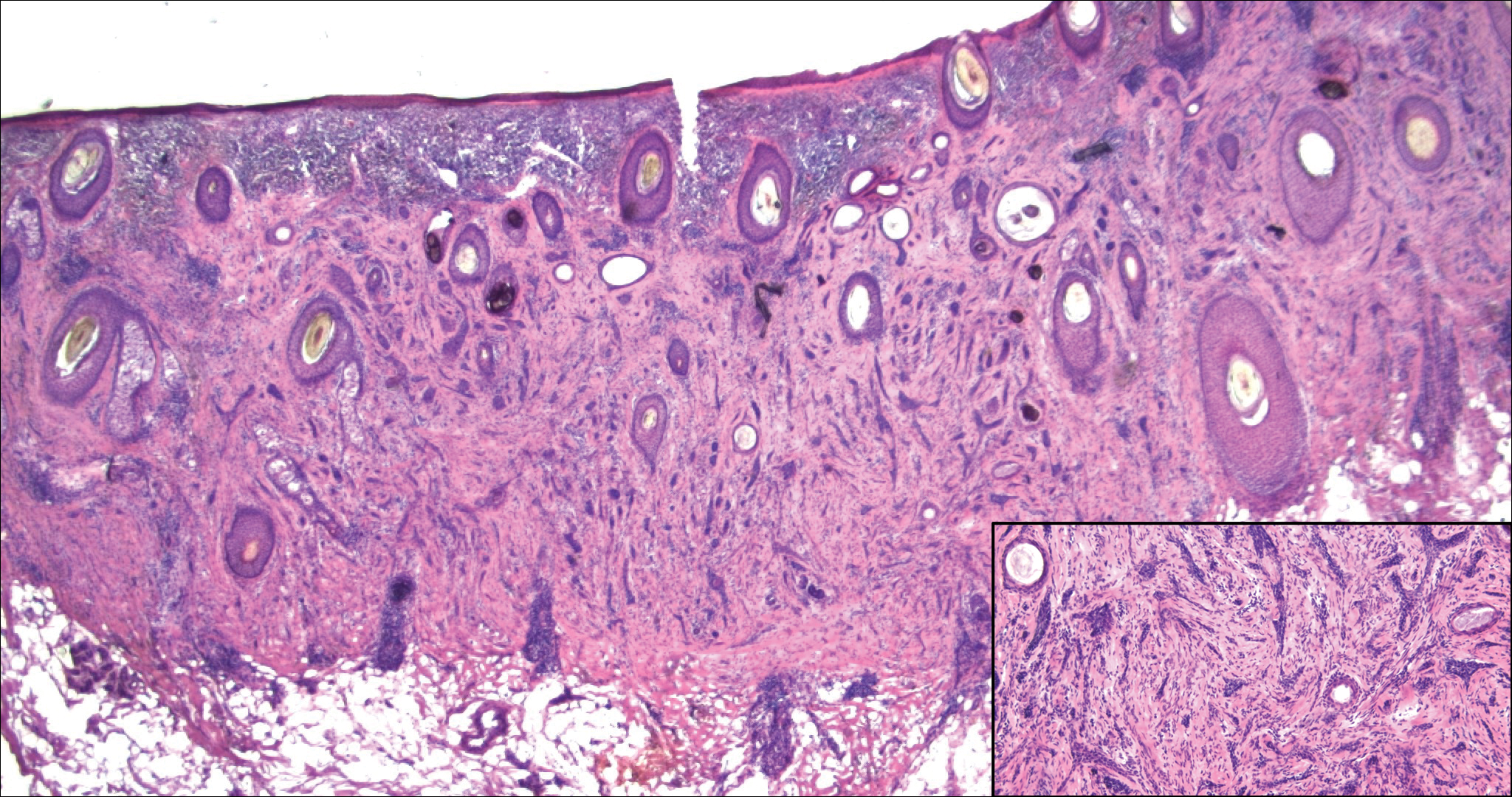
Indurated Plaque on the Eyebrow
The Diagnosis: Microcystic Adnexal Carcinoma
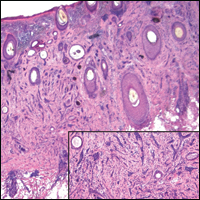
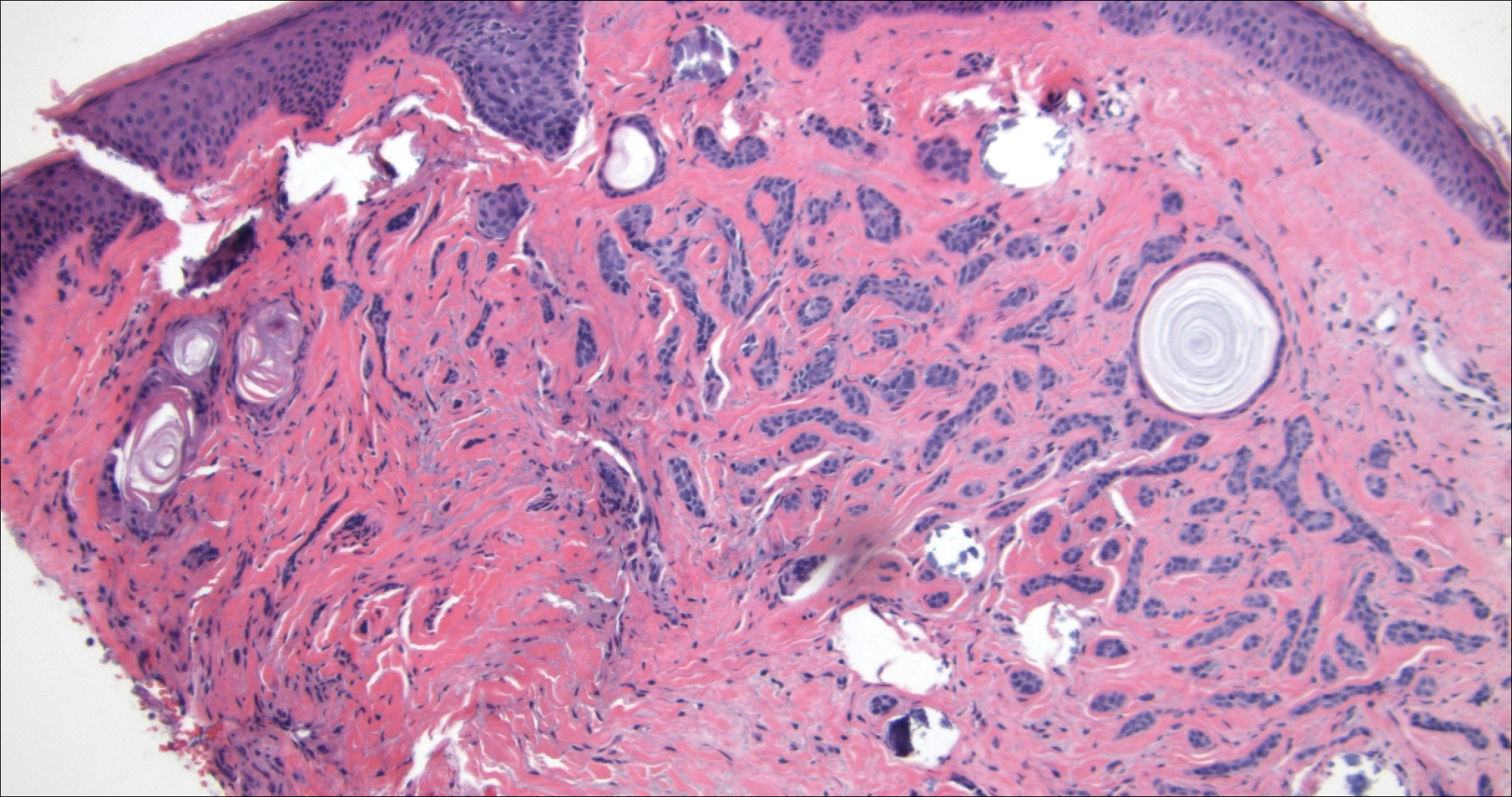
Microcystic adnexal carcinoma (MAC) is a rare, low-grade adnexal carcinoma consisting of both ductal and pilar differentiation.1 It typically presents in young to middle-aged adults as a flesh-colored or yellow indurated plaque on the upper lip, medial cheek, or chin. Histologically, MACs exhibit a biphasic pattern consisting of epithelial islands of cords and lumina creating tadpolelike ducts intermixed with basaloid nests (quiz image). Keratin horn cysts are common superficially. A dense red sclerotic stroma is seen interspersed between the ducts and epithelial islands creating a "paisley tie" appearance. The lesion displays an infiltrative pattern and can be deeply invasive, extending down to the fat and muscle (quiz image, inset). Perineural invasion is common. Atypia, when present, is minimal or mild and mitoses are rare. Although this tumor's histologic pattern appears aggressive in nature, it lacks immunohistochemical staining such as p53, Ki-67, bcl-2, and c-erbB-2 that correlate with malignant behavior.2 A common diagnostic pitfall is examination of a superficial biopsy in which an MAC may be mistakenly identified as another entity.
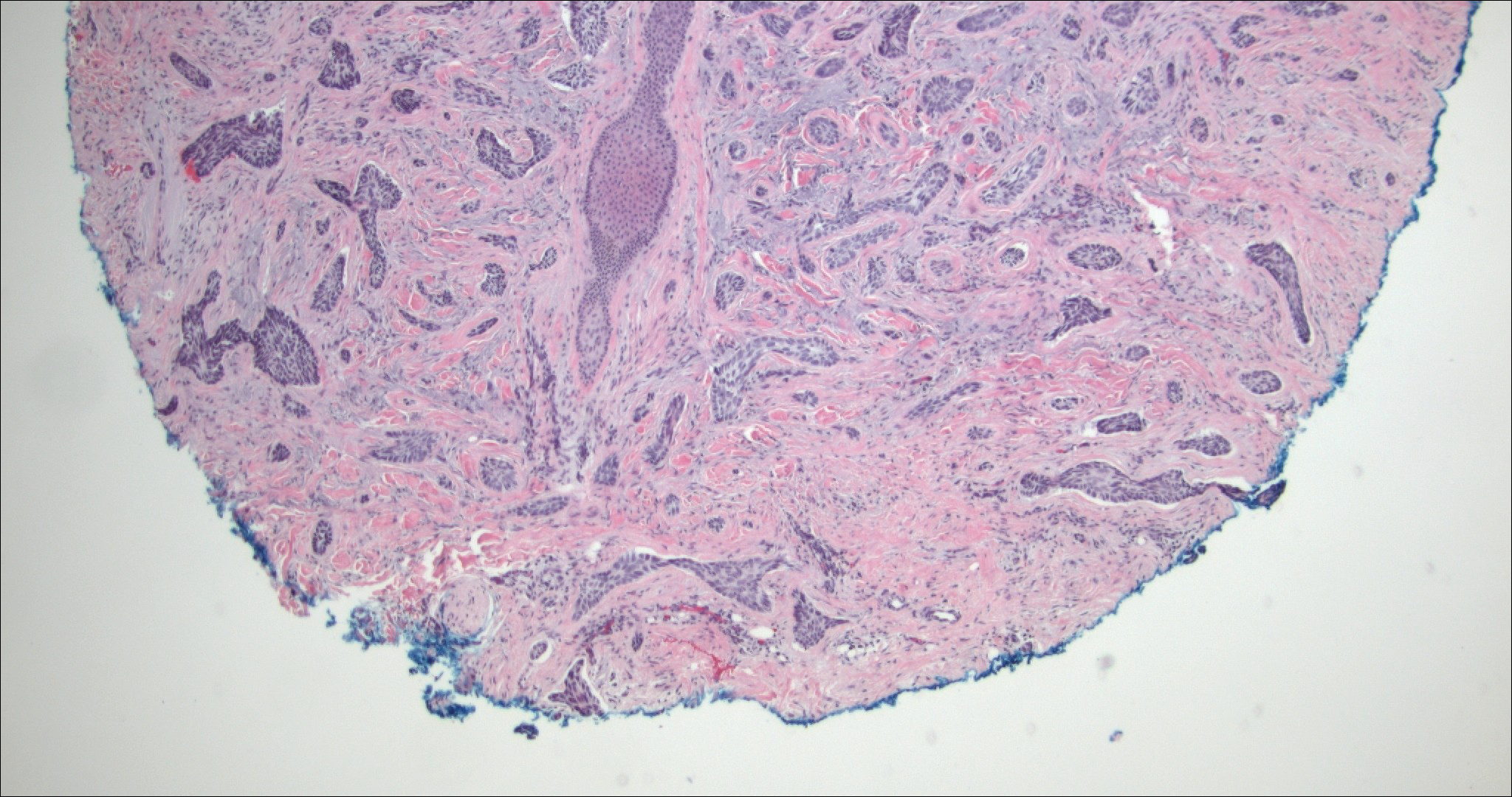
Syringomas are benign adnexal neoplasms with ductal differentiation.3 They are more common in women, especially those of Asian descent, and in patients with Down syndrome. They typically present as multiple small, firm, flesh-colored papules in the periorbital area or upper trunk. Histologically, syringomas also display comma-shaped tubules and ducts with a tadpolelike appearance and a dense red stroma creating a paisley tie-like pattern. Ductal cells have an abundant pink cytoplasm. Syringomas are well-circumscribed and more superficial than MACs without an infiltrative pattern. They lack mitotic activity or perineural invasion (Figure 1).

Desmoplastic trichoepithelioma (DTE) is a benign follicular neoplasm.4 It presents in adulthood with a female predominance. Clinically, it appears as a solitary flesh-colored to yellow annular plaque with raised borders and a depressed central area, often on the medial cheek. Histologically, DTEs are well-circumscribed with narrow branching cords lined with polygonal cells. A dense red stroma in combination with the epithelioid aggregates also creates the paisley tie-like pattern in this lesion. Retraction between collagen bundles within the stroma can be seen, helping distinguish this lesion from a morpheaform basal cell carcinoma (BCC), which has retraction between the epithelium and stroma. Immunohistochemistry also can be a useful tool to help differentiate DTEs from morpheaform BCCs in that sparse cytokeratin 20-positive Merkel cells can be seen within the basaloid islands of DTE but not BCC.5 Also seen with DTEs are numerous keratin horn cysts that commonly are filled with dystrophic calcifications. Cellular atypia and mitoses are not seen (Figure 2). Compared to MACs, DTEs lack abundant ductal structures and also contain papillary mesenchymal bodies and a more fibroblast-rich stroma.
Morpheaform BCC is an aggressive subtype of BCC. It presents as a scarlike plaque that gradually expands. Thin infiltrating strands of basaloid cells are seen haphazardly throughout a pink sclerotic stroma. Tadpolelike basaloid islands and rarely horn cysts can be seen scattered superficially, creating the paisley tie-like pattern. This lesion is more infiltrating than a syringoma or a DTE, and perineural invasion is common. Retraction is uncommon, but when present, it is seen between the epithelial cords and adjacent stroma (Figure 3).
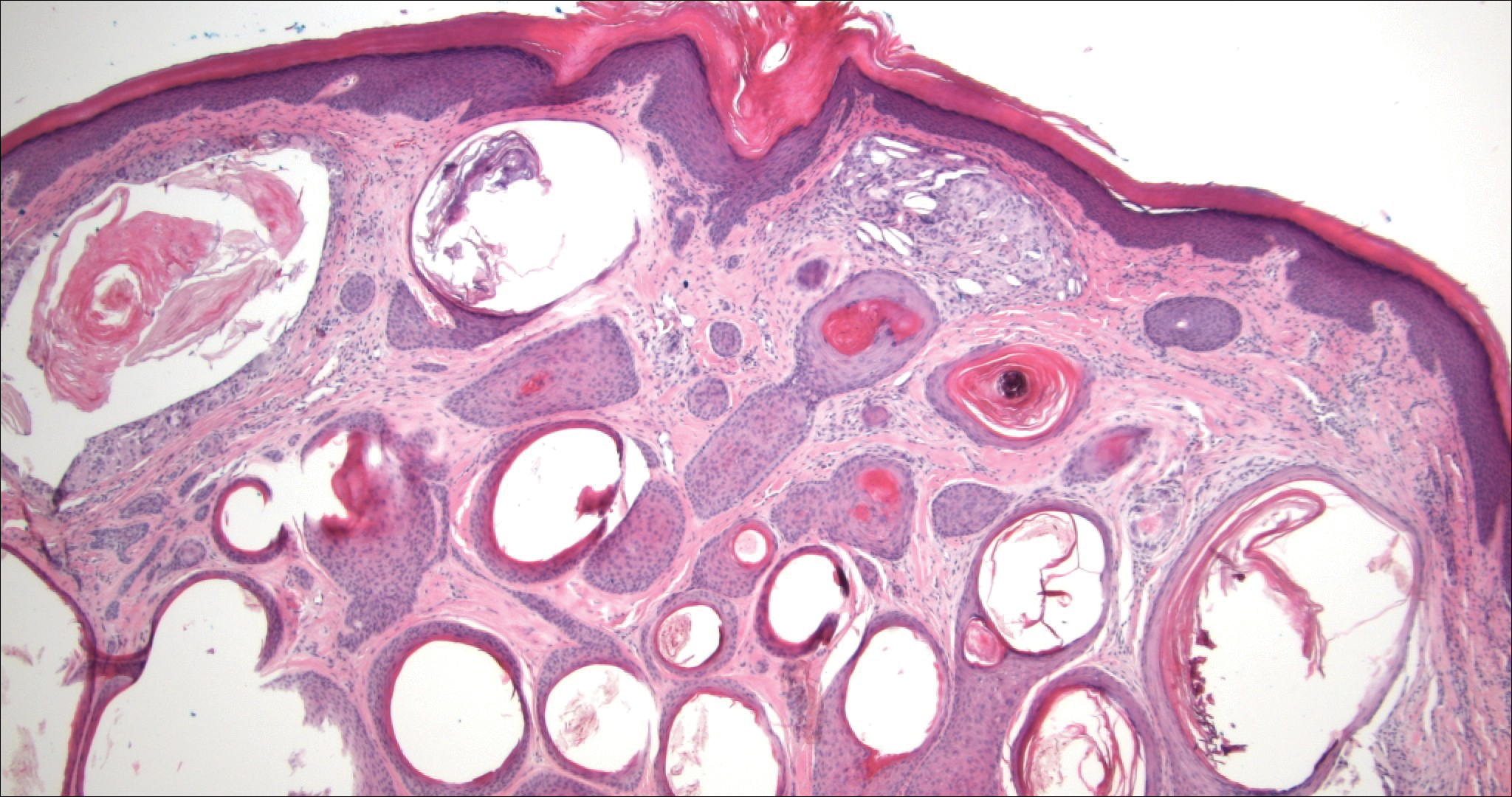
Trichoadenoma is another benign neoplasm of follicular differentiation.6 It typically presents as a dome-shaped papule or plaque on the head or neck. Histologically it displays numerous dilated cystic spaces that reflect its origin from isthmic and infundibular differentiation. There is no attachment to the overlying epidermis. It can be distinguished from MAC, DTE, and syringoma due to a lack of basaloid aggregates and only a small number of non-cyst-forming epithelial cells (Figure 4).
- Nickoloff BJ, Fleischmann HE, Carmel J. Microcystic adnexal carcinoma: immunohistologic observations suggesting dual (pilar and eccrine) differentiation. Arch Dermatol. 1986;122:290-294.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
- Hashimoto K, Lever WF. Histogenesis of skin appendage tumors. Arch Dermatol. 1969;100:356-369.
- Brownstein MH, Shapiro L. Desmoplastic trichoepithelioma. Cancer. 1977;40:2979-2986.
- Hartschuh W, Schulz T. Merkel cells are integral constituents of desmoplastic trichoepithelioma: an immunohistochemical and electron microscopy study. J Cutan Pathol. 1995;22:413-421.
- Rahbari H, Mehregan A, Pinkus A. Trichoadenoma of Nikolowski. J Cutan Pathol. 1977;4:90-98.
The Diagnosis: Microcystic Adnexal Carcinoma
Microcystic adnexal carcinoma (MAC) is a rare, low-grade adnexal carcinoma consisting of both ductal and pilar differentiation.1 It typically presents in young to middle-aged adults as a flesh-colored or yellow indurated plaque on the upper lip, medial cheek, or chin. Histologically, MACs exhibit a biphasic pattern consisting of epithelial islands of cords and lumina creating tadpolelike ducts intermixed with basaloid nests (quiz image). Keratin horn cysts are common superficially. A dense red sclerotic stroma is seen interspersed between the ducts and epithelial islands creating a "paisley tie" appearance. The lesion displays an infiltrative pattern and can be deeply invasive, extending down to the fat and muscle (quiz image, inset). Perineural invasion is common. Atypia, when present, is minimal or mild and mitoses are rare. Although this tumor's histologic pattern appears aggressive in nature, it lacks immunohistochemical staining such as p53, Ki-67, bcl-2, and c-erbB-2 that correlate with malignant behavior.2 A common diagnostic pitfall is examination of a superficial biopsy in which an MAC may be mistakenly identified as another entity.
Syringomas are benign adnexal neoplasms with ductal differentiation.3 They are more common in women, especially those of Asian descent, and in patients with Down syndrome. They typically present as multiple small, firm, flesh-colored papules in the periorbital area or upper trunk. Histologically, syringomas also display comma-shaped tubules and ducts with a tadpolelike appearance and a dense red stroma creating a paisley tie-like pattern. Ductal cells have an abundant pink cytoplasm. Syringomas are well-circumscribed and more superficial than MACs without an infiltrative pattern. They lack mitotic activity or perineural invasion (Figure 1).

Desmoplastic trichoepithelioma (DTE) is a benign follicular neoplasm.4 It presents in adulthood with a female predominance. Clinically, it appears as a solitary flesh-colored to yellow annular plaque with raised borders and a depressed central area, often on the medial cheek. Histologically, DTEs are well-circumscribed with narrow branching cords lined with polygonal cells. A dense red stroma in combination with the epithelioid aggregates also creates the paisley tie-like pattern in this lesion. Retraction between collagen bundles within the stroma can be seen, helping distinguish this lesion from a morpheaform basal cell carcinoma (BCC), which has retraction between the epithelium and stroma. Immunohistochemistry also can be a useful tool to help differentiate DTEs from morpheaform BCCs in that sparse cytokeratin 20-positive Merkel cells can be seen within the basaloid islands of DTE but not BCC.5 Also seen with DTEs are numerous keratin horn cysts that commonly are filled with dystrophic calcifications. Cellular atypia and mitoses are not seen (Figure 2). Compared to MACs, DTEs lack abundant ductal structures and also contain papillary mesenchymal bodies and a more fibroblast-rich stroma.

Morpheaform BCC is an aggressive subtype of BCC. It presents as a scarlike plaque that gradually expands. Thin infiltrating strands of basaloid cells are seen haphazardly throughout a pink sclerotic stroma. Tadpolelike basaloid islands and rarely horn cysts can be seen scattered superficially, creating the paisley tie-like pattern. This lesion is more infiltrating than a syringoma or a DTE, and perineural invasion is common. Retraction is uncommon, but when present, it is seen between the epithelial cords and adjacent stroma (Figure 3).

Trichoadenoma is another benign neoplasm of follicular differentiation.6 It typically presents as a dome-shaped papule or plaque on the head or neck. Histologically it displays numerous dilated cystic spaces that reflect its origin from isthmic and infundibular differentiation. There is no attachment to the overlying epidermis. It can be distinguished from MAC, DTE, and syringoma due to a lack of basaloid aggregates and only a small number of non-cyst-forming epithelial cells (Figure 4).

The Diagnosis: Microcystic Adnexal Carcinoma
Microcystic adnexal carcinoma (MAC) is a rare, low-grade adnexal carcinoma consisting of both ductal and pilar differentiation.1 It typically presents in young to middle-aged adults as a flesh-colored or yellow indurated plaque on the upper lip, medial cheek, or chin. Histologically, MACs exhibit a biphasic pattern consisting of epithelial islands of cords and lumina creating tadpolelike ducts intermixed with basaloid nests (quiz image). Keratin horn cysts are common superficially. A dense red sclerotic stroma is seen interspersed between the ducts and epithelial islands creating a "paisley tie" appearance. The lesion displays an infiltrative pattern and can be deeply invasive, extending down to the fat and muscle (quiz image, inset). Perineural invasion is common. Atypia, when present, is minimal or mild and mitoses are rare. Although this tumor's histologic pattern appears aggressive in nature, it lacks immunohistochemical staining such as p53, Ki-67, bcl-2, and c-erbB-2 that correlate with malignant behavior.2 A common diagnostic pitfall is examination of a superficial biopsy in which an MAC may be mistakenly identified as another entity.
Syringomas are benign adnexal neoplasms with ductal differentiation.3 They are more common in women, especially those of Asian descent, and in patients with Down syndrome. They typically present as multiple small, firm, flesh-colored papules in the periorbital area or upper trunk. Histologically, syringomas also display comma-shaped tubules and ducts with a tadpolelike appearance and a dense red stroma creating a paisley tie-like pattern. Ductal cells have an abundant pink cytoplasm. Syringomas are well-circumscribed and more superficial than MACs without an infiltrative pattern. They lack mitotic activity or perineural invasion (Figure 1).

Desmoplastic trichoepithelioma (DTE) is a benign follicular neoplasm.4 It presents in adulthood with a female predominance. Clinically, it appears as a solitary flesh-colored to yellow annular plaque with raised borders and a depressed central area, often on the medial cheek. Histologically, DTEs are well-circumscribed with narrow branching cords lined with polygonal cells. A dense red stroma in combination with the epithelioid aggregates also creates the paisley tie-like pattern in this lesion. Retraction between collagen bundles within the stroma can be seen, helping distinguish this lesion from a morpheaform basal cell carcinoma (BCC), which has retraction between the epithelium and stroma. Immunohistochemistry also can be a useful tool to help differentiate DTEs from morpheaform BCCs in that sparse cytokeratin 20-positive Merkel cells can be seen within the basaloid islands of DTE but not BCC.5 Also seen with DTEs are numerous keratin horn cysts that commonly are filled with dystrophic calcifications. Cellular atypia and mitoses are not seen (Figure 2). Compared to MACs, DTEs lack abundant ductal structures and also contain papillary mesenchymal bodies and a more fibroblast-rich stroma.

Morpheaform BCC is an aggressive subtype of BCC. It presents as a scarlike plaque that gradually expands. Thin infiltrating strands of basaloid cells are seen haphazardly throughout a pink sclerotic stroma. Tadpolelike basaloid islands and rarely horn cysts can be seen scattered superficially, creating the paisley tie-like pattern. This lesion is more infiltrating than a syringoma or a DTE, and perineural invasion is common. Retraction is uncommon, but when present, it is seen between the epithelial cords and adjacent stroma (Figure 3).

Trichoadenoma is another benign neoplasm of follicular differentiation.6 It typically presents as a dome-shaped papule or plaque on the head or neck. Histologically it displays numerous dilated cystic spaces that reflect its origin from isthmic and infundibular differentiation. There is no attachment to the overlying epidermis. It can be distinguished from MAC, DTE, and syringoma due to a lack of basaloid aggregates and only a small number of non-cyst-forming epithelial cells (Figure 4).

- Nickoloff BJ, Fleischmann HE, Carmel J. Microcystic adnexal carcinoma: immunohistologic observations suggesting dual (pilar and eccrine) differentiation. Arch Dermatol. 1986;122:290-294.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
- Hashimoto K, Lever WF. Histogenesis of skin appendage tumors. Arch Dermatol. 1969;100:356-369.
- Brownstein MH, Shapiro L. Desmoplastic trichoepithelioma. Cancer. 1977;40:2979-2986.
- Hartschuh W, Schulz T. Merkel cells are integral constituents of desmoplastic trichoepithelioma: an immunohistochemical and electron microscopy study. J Cutan Pathol. 1995;22:413-421.
- Rahbari H, Mehregan A, Pinkus A. Trichoadenoma of Nikolowski. J Cutan Pathol. 1977;4:90-98.
- Nickoloff BJ, Fleischmann HE, Carmel J. Microcystic adnexal carcinoma: immunohistologic observations suggesting dual (pilar and eccrine) differentiation. Arch Dermatol. 1986;122:290-294.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
- Hashimoto K, Lever WF. Histogenesis of skin appendage tumors. Arch Dermatol. 1969;100:356-369.
- Brownstein MH, Shapiro L. Desmoplastic trichoepithelioma. Cancer. 1977;40:2979-2986.
- Hartschuh W, Schulz T. Merkel cells are integral constituents of desmoplastic trichoepithelioma: an immunohistochemical and electron microscopy study. J Cutan Pathol. 1995;22:413-421.
- Rahbari H, Mehregan A, Pinkus A. Trichoadenoma of Nikolowski. J Cutan Pathol. 1977;4:90-98.
A 52-year-old woman presented with an indurated plaque on the right lateral eyebrow that had been slowly enlarging over the last 4 months.
DDSEP® 8 Quick Quiz - December 2017 Question 2
Correct Answer: E
Rationale
On serial imaging, two worrisome features have developed in the pancreas cyst, i.e., an enhancing mural nodule and dilation of the main pancreatic duct. These features are high-risk stigmata, and therefore surgical resection is recommended. EUS FNA can be considered but is unlikely to change management if cytology is negative. Radiologic surveillance is not appropriate unless the patient refuses surgery.
Reference
1. Tanaka M., Fernández-del Castillo C., Adsay V., et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12(3):183-97.
Correct Answer: E
Rationale
On serial imaging, two worrisome features have developed in the pancreas cyst, i.e., an enhancing mural nodule and dilation of the main pancreatic duct. These features are high-risk stigmata, and therefore surgical resection is recommended. EUS FNA can be considered but is unlikely to change management if cytology is negative. Radiologic surveillance is not appropriate unless the patient refuses surgery.
Reference
1. Tanaka M., Fernández-del Castillo C., Adsay V., et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12(3):183-97.
Correct Answer: E
Rationale
On serial imaging, two worrisome features have developed in the pancreas cyst, i.e., an enhancing mural nodule and dilation of the main pancreatic duct. These features are high-risk stigmata, and therefore surgical resection is recommended. EUS FNA can be considered but is unlikely to change management if cytology is negative. Radiologic surveillance is not appropriate unless the patient refuses surgery.
Reference
1. Tanaka M., Fernández-del Castillo C., Adsay V., et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12(3):183-97.
A 55-year-old man was diagnosed with a 3.1-cm cyst in the tail of the pancreas 2 years ago. He had an endoscopic ultrasound–guided fine-needle aspiration at that time and approximately 2 cc of mucinous fluid were aspirated; cyst fluid CEA (carcinoembryonic antigen) was 790 ng/mL and cytology showed a paucicellular specimen with abundant extracellular mucin. The patient was asymptomatic and opted for radiologic surveillance with MRI. On his most recent MRI, the cyst size is currently 3.4 cm. In addition, the MRI notes the presence of an enhancing nodule in the wall of the cyst measuring 5 mm and the pancreatic duct in the tail is mildly dilated to 5 mm. He continues to be asymptomatic and in good health.
Early inguinal hernia linked to schizophrenia
PARIS – One of the most unexpected and intriguing new developments in the field of schizophrenia has to be the discovery that the risk of the disease is significantly increased in men who were diagnosed with inguinal hernia before they were 13 years old.
“I think this is interesting because inguinal hernia in boys has to do with fibroblasts producing abnormal collagen structure,” according to Kristina Melkersson, MD, PhD, who presented her study findings at the annual congress of the European College of Neuropsychopharmacology.
She first detected a signal for a potential relationship in an earlier, small interview study in which she noticed that men with schizophrenia were more likely to have a history of inguinal hernia surgery than did men in the general population. This prompted her to try to confirm this preliminary observation in a large Swedish registry-based cohort study.
Among the nearly 1.3 million Swedes born during 1987-1999, there were 20,705 who were diagnosed with inguinal hernia before age 13 years. During a median 9.9 years of follow-up starting at age 13 years, 1,294 of these individuals were diagnosed with schizophrenia or schizoaffective disorder at a mean age of 21.4 years.
Among men, a history of inguinal hernia diagnosed before age 13 years was associated with a 56% increase in subsequent risk of schizophrenia or schizoaffective disorder, compared with men without such a history.
Women with a history of having inguinal hernia before age 13 years were at 16% increased risk; however, this modest increase in risk was not statistically significant, possibly because of small numbers. Inguinal hernia is 25 times more common in men than women.
Dr. Melkersson reported having no financial conflicts of interest regarding her study, which was supported by a grant from the Swedish Society of Medicine.
PARIS – One of the most unexpected and intriguing new developments in the field of schizophrenia has to be the discovery that the risk of the disease is significantly increased in men who were diagnosed with inguinal hernia before they were 13 years old.
“I think this is interesting because inguinal hernia in boys has to do with fibroblasts producing abnormal collagen structure,” according to Kristina Melkersson, MD, PhD, who presented her study findings at the annual congress of the European College of Neuropsychopharmacology.
She first detected a signal for a potential relationship in an earlier, small interview study in which she noticed that men with schizophrenia were more likely to have a history of inguinal hernia surgery than did men in the general population. This prompted her to try to confirm this preliminary observation in a large Swedish registry-based cohort study.
Among the nearly 1.3 million Swedes born during 1987-1999, there were 20,705 who were diagnosed with inguinal hernia before age 13 years. During a median 9.9 years of follow-up starting at age 13 years, 1,294 of these individuals were diagnosed with schizophrenia or schizoaffective disorder at a mean age of 21.4 years.
Among men, a history of inguinal hernia diagnosed before age 13 years was associated with a 56% increase in subsequent risk of schizophrenia or schizoaffective disorder, compared with men without such a history.
Women with a history of having inguinal hernia before age 13 years were at 16% increased risk; however, this modest increase in risk was not statistically significant, possibly because of small numbers. Inguinal hernia is 25 times more common in men than women.
Dr. Melkersson reported having no financial conflicts of interest regarding her study, which was supported by a grant from the Swedish Society of Medicine.
PARIS – One of the most unexpected and intriguing new developments in the field of schizophrenia has to be the discovery that the risk of the disease is significantly increased in men who were diagnosed with inguinal hernia before they were 13 years old.
“I think this is interesting because inguinal hernia in boys has to do with fibroblasts producing abnormal collagen structure,” according to Kristina Melkersson, MD, PhD, who presented her study findings at the annual congress of the European College of Neuropsychopharmacology.
She first detected a signal for a potential relationship in an earlier, small interview study in which she noticed that men with schizophrenia were more likely to have a history of inguinal hernia surgery than did men in the general population. This prompted her to try to confirm this preliminary observation in a large Swedish registry-based cohort study.
Among the nearly 1.3 million Swedes born during 1987-1999, there were 20,705 who were diagnosed with inguinal hernia before age 13 years. During a median 9.9 years of follow-up starting at age 13 years, 1,294 of these individuals were diagnosed with schizophrenia or schizoaffective disorder at a mean age of 21.4 years.
Among men, a history of inguinal hernia diagnosed before age 13 years was associated with a 56% increase in subsequent risk of schizophrenia or schizoaffective disorder, compared with men without such a history.
Women with a history of having inguinal hernia before age 13 years were at 16% increased risk; however, this modest increase in risk was not statistically significant, possibly because of small numbers. Inguinal hernia is 25 times more common in men than women.
Dr. Melkersson reported having no financial conflicts of interest regarding her study, which was supported by a grant from the Swedish Society of Medicine.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Swedish boys diagnosed with inguinal hernia before age 13 years were 56% more likely to be diagnosed with schizophrenia or schizoaffective disorder later in life.
Data source: This retrospective cohort study included nearly 1.3 million Swedes, 20,705 of whom were diagnosed with an inguinal hernia before age 13 years.
Disclosures: The study was supported by a grant from the Swedish Society of Medicine. The presenter reported having no financial conflicts.
DDSEP® 8 Quick quiz - December 2017 Question 1
Correct Answer: C
Rationale
Mixed connective tissue disease can be associated with atrophy of the smooth muscle of the gut, like scleroderma. In the esophagus, this can manifest as a hypotensive lower esophageal sphincter and impaired esophageal smooth muscle peristalsis; in extreme cases, there is absent contractility in the esophagus. This contributes to impaired esophageal clearance of refluxed material, leading to prolonged acid residence times in the esophagus and severe reflux esophagitis. Many patients with mixed connective tissue disease have overlap Sjogren’s syndrome, reducing salivary neutralization of esophageal mucosal acidification and further contributing to esophagitis. While esophageal body motor function can be suboptimal in diabetes mellitus and Barrett’s esophagus, the mechanism of hypomotility is not smooth muscle atrophy and fibrosis. Polymyositis can affect skeletal muscle of the proximal esophagus, but not the smooth muscle. Lichen planus affects mucosa but not muscle.
Reference
1. Savarino E., Mei F., Parodi A., et al. Gastrointestinal motility disorder assessment in systemic sclerosis. Rheumatology (Oxford). 2013 Jun;52(6):1095-100.
2. Langdon P.C., Mulcahy K., Shepherd K.L., et al. Pharyngeal dysphagia in inflammatory muscle diseases resulting from impaired suprahyoid musculature. Dysphagia. 2012 Sep;27(3):408-17.
Correct Answer: C
Rationale
Mixed connective tissue disease can be associated with atrophy of the smooth muscle of the gut, like scleroderma. In the esophagus, this can manifest as a hypotensive lower esophageal sphincter and impaired esophageal smooth muscle peristalsis; in extreme cases, there is absent contractility in the esophagus. This contributes to impaired esophageal clearance of refluxed material, leading to prolonged acid residence times in the esophagus and severe reflux esophagitis. Many patients with mixed connective tissue disease have overlap Sjogren’s syndrome, reducing salivary neutralization of esophageal mucosal acidification and further contributing to esophagitis. While esophageal body motor function can be suboptimal in diabetes mellitus and Barrett’s esophagus, the mechanism of hypomotility is not smooth muscle atrophy and fibrosis. Polymyositis can affect skeletal muscle of the proximal esophagus, but not the smooth muscle. Lichen planus affects mucosa but not muscle.
Reference
1. Savarino E., Mei F., Parodi A., et al. Gastrointestinal motility disorder assessment in systemic sclerosis. Rheumatology (Oxford). 2013 Jun;52(6):1095-100.
2. Langdon P.C., Mulcahy K., Shepherd K.L., et al. Pharyngeal dysphagia in inflammatory muscle diseases resulting from impaired suprahyoid musculature. Dysphagia. 2012 Sep;27(3):408-17.
Correct Answer: C
Rationale
Mixed connective tissue disease can be associated with atrophy of the smooth muscle of the gut, like scleroderma. In the esophagus, this can manifest as a hypotensive lower esophageal sphincter and impaired esophageal smooth muscle peristalsis; in extreme cases, there is absent contractility in the esophagus. This contributes to impaired esophageal clearance of refluxed material, leading to prolonged acid residence times in the esophagus and severe reflux esophagitis. Many patients with mixed connective tissue disease have overlap Sjogren’s syndrome, reducing salivary neutralization of esophageal mucosal acidification and further contributing to esophagitis. While esophageal body motor function can be suboptimal in diabetes mellitus and Barrett’s esophagus, the mechanism of hypomotility is not smooth muscle atrophy and fibrosis. Polymyositis can affect skeletal muscle of the proximal esophagus, but not the smooth muscle. Lichen planus affects mucosa but not muscle.
Reference
1. Savarino E., Mei F., Parodi A., et al. Gastrointestinal motility disorder assessment in systemic sclerosis. Rheumatology (Oxford). 2013 Jun;52(6):1095-100.
2. Langdon P.C., Mulcahy K., Shepherd K.L., et al. Pharyngeal dysphagia in inflammatory muscle diseases resulting from impaired suprahyoid musculature. Dysphagia. 2012 Sep;27(3):408-17.
Which of the following conditions is associated with smooth muscle atrophy impairing esophageal clearance, contributing to prolonged esophageal acid contact and reflux esophagitis?
Self-harm on rise in U.S. among girls aged 10-14
The rate of self-inflicted injuries has increased significantly among young girls since 2009, according to a study of emergency department visits for self-inflicted injuries from 2001 to 2015.
In a research letter, Melissa C. Mercado, PhD, and her associates reached that conclusion based on data from 43,138 emergency department visits for self-inflicted injury among young people aged 10-24 years, which were captured by the National Electronic Injury Surveillance System–All Injury Program (JAMA. 2017;318[19]:1931-3. doi: 10.1001/jama.2017.13317).
From 2001 to 2008, the overall weighted, age-adjusted rate of self-inflicted injury showed no statistically significant trend upward or downward, reported Dr. Mercado of the National Center for Injury Prevention and Control, Atlanta, and her associates. From 2009 to 2015, however, the rate increased by a significant 5.7% per year, reaching 303.7 per 100,000 population in 2015, compared with 201.6 in 2001.
This increase was even more pronounced among girls, rising by 8.4% per year from 2008 to 2015 in all females but by 18.8% per year in those aged 10-14 years. In adolescent females aged 15-19, the rate of self-inflicted injury rose 7.2% per year from 2008 to 2015. In young women aged 20-24 years, the rate rose 2% per year from 2001 to 2015.
Meanwhile, the rates of self-inflicted injury for males were stable across all time periods and age groups.
“Self-inflicted injury is one of the strongest risk factors for suicide – the second-leading cause of death among those aged 10 to 24 years during 2015,” Dr. Mercado and her coauthors wrote.
The most common method of self-inflicted injury for females was poisoning. As with the overall rates of injury in females, the rates of this method of harm were stable until 2007, then increased by 5.3% until 2015. Self-inflicted injuries among females using a sharp object increased by 7.1% each year from 2001 to 2015, but the rates of blunt-object injuries were stable from 2006 to 2015.
The authors wrote that the finding of an increase in self-harm among females was consistent with youth suicide data, which also show an increase after 2006, particularly among girls and female adolescents aged 10-14 years.
Dr. Mercado and her associates called for the implementation of evidence-based, comprehensive suicide and self-harm prevention strategies. “These strategies include strengthening access to and delivery of care for suicidal youth within health systems and creating protective environments, promoting youth connectedness, teaching coping and problem-solving skills, and identifying and supporting at-risk youth within communities.”
The study was conducted under the auspices of the National Center for Injury Prevention and Control, which is part of the Centers for Disease Control and Prevention. The findings, however, do not necessarily represent the views of the CDC. No conflicts of interest were declared.
The rate of self-inflicted injuries has increased significantly among young girls since 2009, according to a study of emergency department visits for self-inflicted injuries from 2001 to 2015.
In a research letter, Melissa C. Mercado, PhD, and her associates reached that conclusion based on data from 43,138 emergency department visits for self-inflicted injury among young people aged 10-24 years, which were captured by the National Electronic Injury Surveillance System–All Injury Program (JAMA. 2017;318[19]:1931-3. doi: 10.1001/jama.2017.13317).
From 2001 to 2008, the overall weighted, age-adjusted rate of self-inflicted injury showed no statistically significant trend upward or downward, reported Dr. Mercado of the National Center for Injury Prevention and Control, Atlanta, and her associates. From 2009 to 2015, however, the rate increased by a significant 5.7% per year, reaching 303.7 per 100,000 population in 2015, compared with 201.6 in 2001.
This increase was even more pronounced among girls, rising by 8.4% per year from 2008 to 2015 in all females but by 18.8% per year in those aged 10-14 years. In adolescent females aged 15-19, the rate of self-inflicted injury rose 7.2% per year from 2008 to 2015. In young women aged 20-24 years, the rate rose 2% per year from 2001 to 2015.
Meanwhile, the rates of self-inflicted injury for males were stable across all time periods and age groups.
“Self-inflicted injury is one of the strongest risk factors for suicide – the second-leading cause of death among those aged 10 to 24 years during 2015,” Dr. Mercado and her coauthors wrote.
The most common method of self-inflicted injury for females was poisoning. As with the overall rates of injury in females, the rates of this method of harm were stable until 2007, then increased by 5.3% until 2015. Self-inflicted injuries among females using a sharp object increased by 7.1% each year from 2001 to 2015, but the rates of blunt-object injuries were stable from 2006 to 2015.
The authors wrote that the finding of an increase in self-harm among females was consistent with youth suicide data, which also show an increase after 2006, particularly among girls and female adolescents aged 10-14 years.
Dr. Mercado and her associates called for the implementation of evidence-based, comprehensive suicide and self-harm prevention strategies. “These strategies include strengthening access to and delivery of care for suicidal youth within health systems and creating protective environments, promoting youth connectedness, teaching coping and problem-solving skills, and identifying and supporting at-risk youth within communities.”
The study was conducted under the auspices of the National Center for Injury Prevention and Control, which is part of the Centers for Disease Control and Prevention. The findings, however, do not necessarily represent the views of the CDC. No conflicts of interest were declared.
The rate of self-inflicted injuries has increased significantly among young girls since 2009, according to a study of emergency department visits for self-inflicted injuries from 2001 to 2015.
In a research letter, Melissa C. Mercado, PhD, and her associates reached that conclusion based on data from 43,138 emergency department visits for self-inflicted injury among young people aged 10-24 years, which were captured by the National Electronic Injury Surveillance System–All Injury Program (JAMA. 2017;318[19]:1931-3. doi: 10.1001/jama.2017.13317).
From 2001 to 2008, the overall weighted, age-adjusted rate of self-inflicted injury showed no statistically significant trend upward or downward, reported Dr. Mercado of the National Center for Injury Prevention and Control, Atlanta, and her associates. From 2009 to 2015, however, the rate increased by a significant 5.7% per year, reaching 303.7 per 100,000 population in 2015, compared with 201.6 in 2001.
This increase was even more pronounced among girls, rising by 8.4% per year from 2008 to 2015 in all females but by 18.8% per year in those aged 10-14 years. In adolescent females aged 15-19, the rate of self-inflicted injury rose 7.2% per year from 2008 to 2015. In young women aged 20-24 years, the rate rose 2% per year from 2001 to 2015.
Meanwhile, the rates of self-inflicted injury for males were stable across all time periods and age groups.
“Self-inflicted injury is one of the strongest risk factors for suicide – the second-leading cause of death among those aged 10 to 24 years during 2015,” Dr. Mercado and her coauthors wrote.
The most common method of self-inflicted injury for females was poisoning. As with the overall rates of injury in females, the rates of this method of harm were stable until 2007, then increased by 5.3% until 2015. Self-inflicted injuries among females using a sharp object increased by 7.1% each year from 2001 to 2015, but the rates of blunt-object injuries were stable from 2006 to 2015.
The authors wrote that the finding of an increase in self-harm among females was consistent with youth suicide data, which also show an increase after 2006, particularly among girls and female adolescents aged 10-14 years.
Dr. Mercado and her associates called for the implementation of evidence-based, comprehensive suicide and self-harm prevention strategies. “These strategies include strengthening access to and delivery of care for suicidal youth within health systems and creating protective environments, promoting youth connectedness, teaching coping and problem-solving skills, and identifying and supporting at-risk youth within communities.”
The study was conducted under the auspices of the National Center for Injury Prevention and Control, which is part of the Centers for Disease Control and Prevention. The findings, however, do not necessarily represent the views of the CDC. No conflicts of interest were declared.
FROM JAMA
Key clinical point: Rates of self-inflicted injury rose significantly in young women between 2009 and 2015, particularly in those aged 10-14 years.
Major finding: The rate of emergency department visits for self-inflicted injury rose 18.8% per year from 2009 to 2015 in females aged 10-14 years.
Data source: Analysis of data from 43,138 emergency department visits of young people aged 10-24 years for self-inflicted injury.
Disclosures: The study was conducted under the auspices of the Centers for Disease Control and Prevention, but the findings do not necessarily represent the views of the CDC. No conflicts of interest were declared.
Delving into the details
Editor’s note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-2018 year, offering two options for students to receive funding and engage in scholarly work during their first, second, and third years of medical school. As a part of the longitudinal (18-month) program, recipients are required to write about their experience on a monthly basis.
For my research project, we are looking to develop a tool that would use data from within 24 hours of a patient’s admission to the hospital to predict whether they will require post-acute care placement after discharge. While I have often been summarizing my project with this broad one-liner, in the last two weeks I have been delving more into the details of what exactly we mean by “data from within 24 hours of a patient’s admission.”
We are going through each of the variables systematically to take into account prior literature on how they were treated in other studies, as well as the practical limitations imposed by the data-gathering within our own system to choose how these values will be selected for each admission. My mentor Dr. Eduard Vasilevskis is helping me with making these decisions, based on the prototype model that was the inspiration for this project. Once we have identified all of the details of each variable we want to track, Dr. Jesse Ehrenfeld will be facilitating our use of the database.
Certainly this project has helped illuminate not only research-specific hurdles, but also underscores the fundamental difficulty of clinical decision-making in the first 24 hours of a patient’s admission. With data changing rapidly and sometimes incomplete data, clinicians need to quickly make care decisions that can impact a lot more than the patient’s post-discharge destination.
We anticipate that once we’ve made these choices, there will be further choices to make about how to treat these variables in the analysis. We hope to have the assistance of an experienced statistician to help guide us in making those decisions.
Monisha Bhatia, a native of Nashville, Tenn., is a fourth-year medical student at Vanderbilt University in Nashville. She is hoping to pursue either a residency in internal medicine or a combined internal medicine/emergency medicine program. Prior to medical school, she completed a JD/MPH program at Boston University, and she hopes to use her legal training in working with regulatory authorities to improve access to health care for all Americans.
Editor’s note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-2018 year, offering two options for students to receive funding and engage in scholarly work during their first, second, and third years of medical school. As a part of the longitudinal (18-month) program, recipients are required to write about their experience on a monthly basis.
For my research project, we are looking to develop a tool that would use data from within 24 hours of a patient’s admission to the hospital to predict whether they will require post-acute care placement after discharge. While I have often been summarizing my project with this broad one-liner, in the last two weeks I have been delving more into the details of what exactly we mean by “data from within 24 hours of a patient’s admission.”
We are going through each of the variables systematically to take into account prior literature on how they were treated in other studies, as well as the practical limitations imposed by the data-gathering within our own system to choose how these values will be selected for each admission. My mentor Dr. Eduard Vasilevskis is helping me with making these decisions, based on the prototype model that was the inspiration for this project. Once we have identified all of the details of each variable we want to track, Dr. Jesse Ehrenfeld will be facilitating our use of the database.
Certainly this project has helped illuminate not only research-specific hurdles, but also underscores the fundamental difficulty of clinical decision-making in the first 24 hours of a patient’s admission. With data changing rapidly and sometimes incomplete data, clinicians need to quickly make care decisions that can impact a lot more than the patient’s post-discharge destination.
We anticipate that once we’ve made these choices, there will be further choices to make about how to treat these variables in the analysis. We hope to have the assistance of an experienced statistician to help guide us in making those decisions.
Monisha Bhatia, a native of Nashville, Tenn., is a fourth-year medical student at Vanderbilt University in Nashville. She is hoping to pursue either a residency in internal medicine or a combined internal medicine/emergency medicine program. Prior to medical school, she completed a JD/MPH program at Boston University, and she hopes to use her legal training in working with regulatory authorities to improve access to health care for all Americans.
Editor’s note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-2018 year, offering two options for students to receive funding and engage in scholarly work during their first, second, and third years of medical school. As a part of the longitudinal (18-month) program, recipients are required to write about their experience on a monthly basis.
For my research project, we are looking to develop a tool that would use data from within 24 hours of a patient’s admission to the hospital to predict whether they will require post-acute care placement after discharge. While I have often been summarizing my project with this broad one-liner, in the last two weeks I have been delving more into the details of what exactly we mean by “data from within 24 hours of a patient’s admission.”
We are going through each of the variables systematically to take into account prior literature on how they were treated in other studies, as well as the practical limitations imposed by the data-gathering within our own system to choose how these values will be selected for each admission. My mentor Dr. Eduard Vasilevskis is helping me with making these decisions, based on the prototype model that was the inspiration for this project. Once we have identified all of the details of each variable we want to track, Dr. Jesse Ehrenfeld will be facilitating our use of the database.
Certainly this project has helped illuminate not only research-specific hurdles, but also underscores the fundamental difficulty of clinical decision-making in the first 24 hours of a patient’s admission. With data changing rapidly and sometimes incomplete data, clinicians need to quickly make care decisions that can impact a lot more than the patient’s post-discharge destination.
We anticipate that once we’ve made these choices, there will be further choices to make about how to treat these variables in the analysis. We hope to have the assistance of an experienced statistician to help guide us in making those decisions.
Monisha Bhatia, a native of Nashville, Tenn., is a fourth-year medical student at Vanderbilt University in Nashville. She is hoping to pursue either a residency in internal medicine or a combined internal medicine/emergency medicine program. Prior to medical school, she completed a JD/MPH program at Boston University, and she hopes to use her legal training in working with regulatory authorities to improve access to health care for all Americans.
Targeted therapies forge ahead in multiple breast cancer subtypes
As our understanding of the biology of breast cancer has improved, treatment has become increasingly personalized. Targeted therapies continue to significantly improve patient outcomes in multiple subtypes, with several recent drug approvals. Here, we discuss some of these latest developments.
A disease of many faces
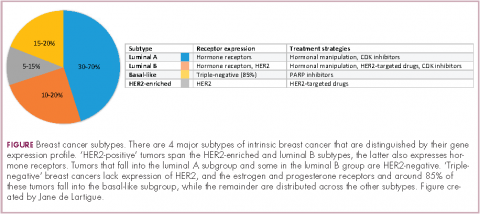
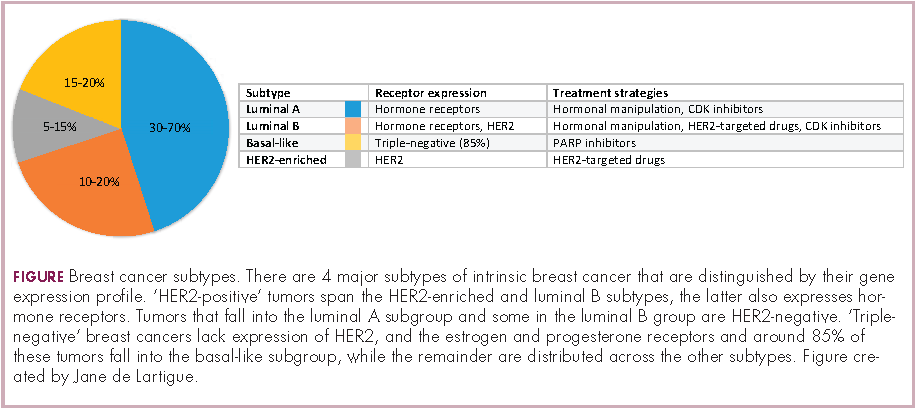
Clinically speaking, breast cancers can be divided into at least 5 subtypes on the basis of the genes they express (Figure 1). The luminal subtypes make up the largest proportion and are characterized by the expression of hormone receptor (HR) genes. Luminal A tumors are negative for human epidermal growth factor receptor 2 (HER2; HER2-negative), whereas luminal B tumors often co-express the HER2 genes.1
The remainder of HER2-positive patients fall into the HER2-enriched category, in which HER2 expression is the defining characteristic. Basal-like tumors, meanwhile, represent the most heterogeneous subtype, overlapping to a large extent with tumors dubbed “triple-negative” because of their lack of either HER2 or ESR1 and PGR gene expression. The fifth subtype is known as normal breast-like and remains poorly characterized.
In recent years, there have been significant advancements in the genomic characterization of breast cancer that have begun to provide a more comprehensive understanding of the driver molecular mechanisms, which has helped to explain some of the limitations of current targeted approaches and to reveal new possible treatments, with a shift toward increasingly personalized strategies.2
HER2: what’s neu?
An estimated 18%-20% of breast tumors are HER2 positive, displaying amplification of the HER2/neu gene or overexpression of its protein product.3 Historically, HER2 positivity correlated with a highly aggressive and metastatic form of disease, conferring poor prognosis.4,5 The HER2-targeted monoclonal antibody (mAb), trastuzumab serves as a prime example of the power of personalized medicine. Evidence suggests that trastuzumab has altered the natural history of HER2-positive breast cancer, such that trastuzumab-treated patients with HER2-positive breast cancer now have a better prognosis than do patients with HER2-negative disease.6,7
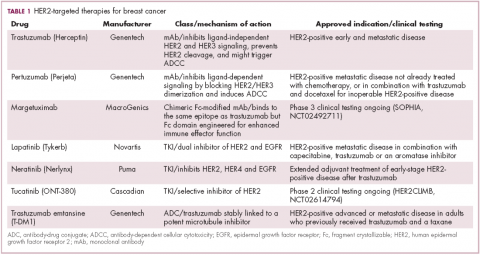
Several additional HER2-targeted drugs have joined trastuzumab on the market, including other mAbs, small molecule tyrosine kinase inhibitors (TKIs), and an antibody–drug conjugate that combines the specificity of a mAb with the anti-tumor potency of a cytotoxic drug. These drugs have further improved patient outcomes in both early and advanced disease settings (Table 1).
The most recent regulatory approval was for neratinib, a potent TKI inhibiting all members of the HER protein family. On the basis of the phase 3 ExteNET study, neratinib was granted approval by the US Food and Drug Administration (FDA) for extended adjuvant treatment of patients with HER2-positive, early-stage breast cancer previously treated with trastuzumab. In a 5-year analysis of the study, invasive disease-free survival (DFS) was 90.4% with neratinib, compared with 87.9% with placebo (hazard ratio [HR], 0.74; P = .017).8,9
The tide of advancements in HER2-targeted therapy looks set to continue in the coming years as potentially practice-changing data emerges from ongoing clinical trials and, as the patent on trastuzumab has expired, a number of biosimilars, such as MYL-1401O have the potential to help patients who may not have access to trastuzumab.10
One of the biggest remaining challenges is identifying drugs that can effectively treat patients with brain metastases because the blood–brain barrier presents an impediment to the delivery of effective concentrations of anticancer drugs. Initially, it was hoped that the small molecule inhibitors lapatinib and neratinib could cross the blood–brain barrier and may be more effective in patients with brain metastases, but that hypothesis has not borne out in randomized clinical trials.11
Tucatinib (ONT-380) has shown significant promise in this respect. In a phase 1 trial, ONT-380 had significant efficacy in patients with and without central nervous system metastases; the overall response rate (ORR) in the CNS was 36%. ONT-380 is also notable for its specificity for HER2, without significant inhibition of HER1 and EGFR, which could translate into a better toxicity profile.12
Doubling down on resistant tumors
Since the success of HER2-targeted therapy is limited by the development of resistance, there has been significant interest in assessing the potential of dual HER2 blockade, exploiting the unique mechanisms of action of different drugs in combination therapy, and ensuring more complete inhibition of the HER2 pathway. Although numerous different combinations have been tested, a double antibody combination has proved most effective.
In fact, dual HER2 targeting with trastuzumab and pertuzumab in combination with chemotherapy has replaced a trastuzumab-chemotherapy regimen as the new standard of care in the metastatic setting. A 6-month improvement in progression-free survival (PFS) sealed FDA approval for the combination and in a recently published final analysis of the trial overall survival (OS) was also improved to a level unprecedented in the first-line setting.13,14The double antibody combination has also been successful in the neoadjuvant setting. Approval followed the results of the phase 2 NeoSphere trial, in which the combination was associated with a significant improvement in pathologic complete response (pCR) rate, a measure that acts as a surrogate for improved survival in the neoadjuvant setting. In a 5-year analysis of the NeoSphere trial, improved pCR did indeed translate into improved PFS and DFS.15,16
The results of the phase 3 APHINITY trial evaluating this combination in the adjuvant setting have been hotly anticipated. In a presentation at the 2017 American Society of Clinical Oncology (ASCO) meeting in June, the study authors reported that in 4,085 patients with operable HER2-positive disease, it significantly reduced the risk of disease recurrence or death compared with trastuzumab and chemotherapy alone.17
There is an ongoing effort to determine if it is possible to de-escalate treatment by removing the chemotherapy component. At least in the neoadjuvant setting, pCR rates in the chemotherapy-free arms of several studies suggest that a proportion of patients might benefit from this strategy15,18,19 and the challenge now is to identify them. To that end, the phase 2 PAMELA trial identified the HER2-enriched subtype as a strong predictor of response to neoadjuvant dual blockade (lapatinib and trastuzumab) without chemotherapy. The pCR rate was 40.6% for the combination in patients with the HER2-enriched subtype of breast cancer and only 10% in patients with non–HER2-enriched tumors.20
Targeting resistance to endocrine therapy
Another coup for personalized medicine in breast cancer is the treatment of hormone receptor–positive cases with endocrine therapy, which has become the cornerstone of treatment in the metastatic and adjuvant settings. Those drugs are designed to block the growth-stimulating effects of the estrogen and progesterone hormones on tumor cells. They include the selective estrogen receptor (ER) modulator tamoxifen, aromatase inhibitors (AIs) such as letrozole, anastrozole, and exemestane, which work by blocking the activity of the aromatase enzyme that converts androgens into estrogens, and the selective estrogen-receptor down-regulator fulvestrant.
As with HER2-targeted therapy, patients treated with endocrine therapy often develop resistance. Activation of alternate signaling cascades, such as the P13K–Akt–mTOR (phosphatidylinositol-3-kinase–Akt–mammalian target of rapamycin) pathway, or downstream targets of ER signaling, including the cyclin-dependent kinases, CDK4 and CDK6, have emerged as important mechanisms of resistance.21,22
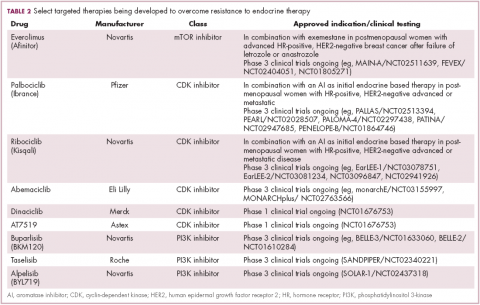
Drugs directed against these secondary targets, aimed to enhance the efficacy of endocrine therapies, have shown significant promise (Table 2). The mTOR inhibitor everolimus received FDA approval in 2012 in combination with exemestane for the treatment of advanced HR-positive, HER2-negative breast cancer.23 More recently, everolimus has also proven effective in combination with either fulvestrant or letrozole, according to the phase 2 PrECOG 0102 and BOLERO-4 studies, both doubling PFS compared with endocrine therapy alone.24,25
Buparlisib is an oral reversible pan-PI3K inhibitor, and the results of the first phase 3 trial of this drug in metastatic breast cancer (MBC) were recently reported. Among 1,147 postmenopausal women with HR-positive, HER2-negative MBC that progressed on or after AI therapy, the combination of buparlisib and fulvestrant prolonged PFS compared with fulvestrant alone (median PFS, 6.9 vs 5 months; HR,0.78; P < .001). However, Novartis, which was developing buparlisib, reported that the combination will not be pursued further due to increased toxicity.26
Two other PI3K inhibitors are currently in phase 3 clinical trials; taselisib and alpelisib, both selective PI3K-alpha inhibitors. The results of a phase 1 dose-escalation study of taselisib were recently published and the ORR among patients with PIK3CA-mutant solid tumors was 36%, including responses in 4 patients with breast cancer.27 Meanwhile, alpelisib has also demonstrated early promise in combination with both letrozole and fulvestrant in patients with ER-positive MBC refractory to endocrine therapy. In combination with letrozole, the clinical benefit rate was 35% overall (44% in patients with PIK3CA mutations, compared with 20% in patients with wild-type PIK3CA status). The combination of alpesilib and fulvestrant produced an ORR of 27%, and both combinations were well tolerated.28,29
Another exciting therapeutic avenue is CDK4 and CDK6 inhibitors. These proteins are critical regulators of cell cycle progression, ensuring transition from G1 to S phase occurs at the appropriate time. The CDK pathway is also a downstream target of ER activation and, unsurprisingly, aberrant expression of the proteins involved in this pathway is commonly observed in breast tumors.
Palbociclib became the first FDA-approved member of this drug class, receiving accelerated approval in patients with HR-positive, HER2-negative metastatic breast cancer, in combination with letrozole in 2015. This became full regulatory approval in combination with any AI earlier this year, following the phase 3 PALOMA-3 study, in which the combination of palbociclib and fulvestrant (accelerated approval was based upon a trial testing palbociclib and letrozole) improved PFS by 5 months (HR, 0.46; P < .0001).30
In addition, a second CDK4/6 inhibitor hit the market this year. Ribociclib demonstrated a significant PFS benefit in combination with letrozole; median PFS was 25.3 months, compared with 16 months for letrozole alone, translating to a 44% reduction in the risk of disease progression or death.31
Abemaciclib, which has greater selectivity for CDK4 than its predecessors, also appears to be heading towards approval. It was granted priority review by the FDA based on data from the MONARCH-2 trial, showing a significant improvement in PFS for the combination of abemaciclib and fulvestrant (median PFS, 16.4 vs 9.3 months for fulvestrant alone; HR, 0.553; P < .001).32
Teasing out ‘HER2-positive’ subtypes
Until recently, “HER2-positive” and “HR-positive” tumors have been treated as separate subtypes, despite the fact that about half of HER2-positive tumors fall into the luminal A subtype and are also HR-positive. Patients were typically treated with HER2-targeted therapy regardless of their endocrine status because of the aggressive nature of HER2-positive disease.
Increasingly, researchers are reconsidering this view, especially as several studies have shown differential response rates to HER2-targeted therapy in HR-positive compared with HR-negative patients and accumulating evidence suggests that there is significant crosstalk between the HER2 and HR pathways, which may be responsible for the development of resistance with both treatment paradigms.
Findings from several studies have shown a benefit to combining HER2-targeted and hormonal therapies in patients with luminal (HR-positive), HER2-positive disease. In the metastatic setting, the results of the phase 2 PERTAIN study, presented at the 2017 ASCO annual meeting suggest that dual HER2 blockade could prove even more effective. The addition of pertuzumab to a combination of trastuzumab and an AI improved PFS by more than 3 months (median PFS, 19.89 vs 15.8 months; HR, 0.65; P = .007).33
The clinical application of these combinations may be limited by the additional cost – several studies have suggested that they are not cost effective – and toxicity, but have served to drive the development of new clinical trial designs as the importance of considering luminal and nonluminal HER2-positive tumors has become increasingly apparent.
PARP inhibitors make a dent in BRCA1/2-mutated cancers
The most renowned breast cancer genes, BRCA1 and BRCA2 are present in about 5%-10% of all breast cancers. They play a central role in the homologous recombination pathway that fixes double-strand breaks in the DNA. Genome sequencing studies have revealed that the presence of the BRCA1/2 genes and other DNA repair defects is highest among patients with the basal-like subtype of breast cancer, in particular those who have triple-negative disease.34,35
This type of breast cancer has proved stubbornly resistant to efforts to improve patient outcomes with targeted therapies. BRCA1/2 mutations and other DNA repair defects that confer a so-called BRCAness phenotype, render tumor cells dependent on other pathways for DNA repair and there has been considerable interest in therapeutically exploiting this through the development of inhibitors of the poly(ADP-ribose) polymerase (PARP) enzyme, which is involved in the repair of single-strand breaks in the DNA. The double damage to DNA repair mechanisms through PARP inhibition in patients with BRCA1/2-mutant tumors proves overwhelming to cancerous cells.
Despite more than a decade of investigation in breast cancer, PARP inhibitors have yet to yield any FDA-approved treatment options. That may be set to change imminently, following the success of olaparib (Table 3). In the first randomized phase 3 trial of a PARP inhibitor in breast cancer (OlympiAD), olaparib was compared with standard chemotherapy in patients with BRCA1/2-mutated MBC who had received up to 2 previous lines of chemotherapy. Olaparib reduced the risk of disease progression by 42% compared with standard chemotherapy and was well tolerated.36
The novel PARP inhibitor talazoparib, which is the most potent to date, is also demonstrating significant efficacy in clinical trials. The results of the phase 2 ABRAZO trial were presented at the ASCO annual meeting. Two cohorts were treated; the first included 49 patients who had responded to their last platinum-containing regimen for metastatic disease and progressed more than 8 weeks after last platinum dose and the other included 35 patients previously treated with 3 or more nonplatinum regimens for metastatic disease. ORR was 28% across the 2 cohorts; 23% and 33% in BRCA1- and BRCA2-mutant carriers, respectively; and 26% in patients with triple-negative breast cancer.37 PARP inhibition is not faring so well in early-stage triple-negative disease; a phase 3 trial of veliparib in combination with chemotherapy did not meet its primary endpoint.38
As our understanding of the biology of breast cancer has improved, treatment has become increasingly personalized. Targeted therapies continue to significantly improve patient outcomes in multiple subtypes, with several recent drug approvals. Here, we discuss some of these latest developments.
A disease of many faces
Clinically speaking, breast cancers can be divided into at least 5 subtypes on the basis of the genes they express (Figure 1). The luminal subtypes make up the largest proportion and are characterized by the expression of hormone receptor (HR) genes. Luminal A tumors are negative for human epidermal growth factor receptor 2 (HER2; HER2-negative), whereas luminal B tumors often co-express the HER2 genes.1
The remainder of HER2-positive patients fall into the HER2-enriched category, in which HER2 expression is the defining characteristic. Basal-like tumors, meanwhile, represent the most heterogeneous subtype, overlapping to a large extent with tumors dubbed “triple-negative” because of their lack of either HER2 or ESR1 and PGR gene expression. The fifth subtype is known as normal breast-like and remains poorly characterized.
In recent years, there have been significant advancements in the genomic characterization of breast cancer that have begun to provide a more comprehensive understanding of the driver molecular mechanisms, which has helped to explain some of the limitations of current targeted approaches and to reveal new possible treatments, with a shift toward increasingly personalized strategies.2
HER2: what’s neu?
An estimated 18%-20% of breast tumors are HER2 positive, displaying amplification of the HER2/neu gene or overexpression of its protein product.3 Historically, HER2 positivity correlated with a highly aggressive and metastatic form of disease, conferring poor prognosis.4,5 The HER2-targeted monoclonal antibody (mAb), trastuzumab serves as a prime example of the power of personalized medicine. Evidence suggests that trastuzumab has altered the natural history of HER2-positive breast cancer, such that trastuzumab-treated patients with HER2-positive breast cancer now have a better prognosis than do patients with HER2-negative disease.6,7
Several additional HER2-targeted drugs have joined trastuzumab on the market, including other mAbs, small molecule tyrosine kinase inhibitors (TKIs), and an antibody–drug conjugate that combines the specificity of a mAb with the anti-tumor potency of a cytotoxic drug. These drugs have further improved patient outcomes in both early and advanced disease settings (Table 1).
The most recent regulatory approval was for neratinib, a potent TKI inhibiting all members of the HER protein family. On the basis of the phase 3 ExteNET study, neratinib was granted approval by the US Food and Drug Administration (FDA) for extended adjuvant treatment of patients with HER2-positive, early-stage breast cancer previously treated with trastuzumab. In a 5-year analysis of the study, invasive disease-free survival (DFS) was 90.4% with neratinib, compared with 87.9% with placebo (hazard ratio [HR], 0.74; P = .017).8,9
The tide of advancements in HER2-targeted therapy looks set to continue in the coming years as potentially practice-changing data emerges from ongoing clinical trials and, as the patent on trastuzumab has expired, a number of biosimilars, such as MYL-1401O have the potential to help patients who may not have access to trastuzumab.10
One of the biggest remaining challenges is identifying drugs that can effectively treat patients with brain metastases because the blood–brain barrier presents an impediment to the delivery of effective concentrations of anticancer drugs. Initially, it was hoped that the small molecule inhibitors lapatinib and neratinib could cross the blood–brain barrier and may be more effective in patients with brain metastases, but that hypothesis has not borne out in randomized clinical trials.11
Tucatinib (ONT-380) has shown significant promise in this respect. In a phase 1 trial, ONT-380 had significant efficacy in patients with and without central nervous system metastases; the overall response rate (ORR) in the CNS was 36%. ONT-380 is also notable for its specificity for HER2, without significant inhibition of HER1 and EGFR, which could translate into a better toxicity profile.12
Doubling down on resistant tumors
Since the success of HER2-targeted therapy is limited by the development of resistance, there has been significant interest in assessing the potential of dual HER2 blockade, exploiting the unique mechanisms of action of different drugs in combination therapy, and ensuring more complete inhibition of the HER2 pathway. Although numerous different combinations have been tested, a double antibody combination has proved most effective.
In fact, dual HER2 targeting with trastuzumab and pertuzumab in combination with chemotherapy has replaced a trastuzumab-chemotherapy regimen as the new standard of care in the metastatic setting. A 6-month improvement in progression-free survival (PFS) sealed FDA approval for the combination and in a recently published final analysis of the trial overall survival (OS) was also improved to a level unprecedented in the first-line setting.13,14The double antibody combination has also been successful in the neoadjuvant setting. Approval followed the results of the phase 2 NeoSphere trial, in which the combination was associated with a significant improvement in pathologic complete response (pCR) rate, a measure that acts as a surrogate for improved survival in the neoadjuvant setting. In a 5-year analysis of the NeoSphere trial, improved pCR did indeed translate into improved PFS and DFS.15,16
The results of the phase 3 APHINITY trial evaluating this combination in the adjuvant setting have been hotly anticipated. In a presentation at the 2017 American Society of Clinical Oncology (ASCO) meeting in June, the study authors reported that in 4,085 patients with operable HER2-positive disease, it significantly reduced the risk of disease recurrence or death compared with trastuzumab and chemotherapy alone.17
There is an ongoing effort to determine if it is possible to de-escalate treatment by removing the chemotherapy component. At least in the neoadjuvant setting, pCR rates in the chemotherapy-free arms of several studies suggest that a proportion of patients might benefit from this strategy15,18,19 and the challenge now is to identify them. To that end, the phase 2 PAMELA trial identified the HER2-enriched subtype as a strong predictor of response to neoadjuvant dual blockade (lapatinib and trastuzumab) without chemotherapy. The pCR rate was 40.6% for the combination in patients with the HER2-enriched subtype of breast cancer and only 10% in patients with non–HER2-enriched tumors.20
Targeting resistance to endocrine therapy
Another coup for personalized medicine in breast cancer is the treatment of hormone receptor–positive cases with endocrine therapy, which has become the cornerstone of treatment in the metastatic and adjuvant settings. Those drugs are designed to block the growth-stimulating effects of the estrogen and progesterone hormones on tumor cells. They include the selective estrogen receptor (ER) modulator tamoxifen, aromatase inhibitors (AIs) such as letrozole, anastrozole, and exemestane, which work by blocking the activity of the aromatase enzyme that converts androgens into estrogens, and the selective estrogen-receptor down-regulator fulvestrant.
As with HER2-targeted therapy, patients treated with endocrine therapy often develop resistance. Activation of alternate signaling cascades, such as the P13K–Akt–mTOR (phosphatidylinositol-3-kinase–Akt–mammalian target of rapamycin) pathway, or downstream targets of ER signaling, including the cyclin-dependent kinases, CDK4 and CDK6, have emerged as important mechanisms of resistance.21,22
Drugs directed against these secondary targets, aimed to enhance the efficacy of endocrine therapies, have shown significant promise (Table 2). The mTOR inhibitor everolimus received FDA approval in 2012 in combination with exemestane for the treatment of advanced HR-positive, HER2-negative breast cancer.23 More recently, everolimus has also proven effective in combination with either fulvestrant or letrozole, according to the phase 2 PrECOG 0102 and BOLERO-4 studies, both doubling PFS compared with endocrine therapy alone.24,25
Buparlisib is an oral reversible pan-PI3K inhibitor, and the results of the first phase 3 trial of this drug in metastatic breast cancer (MBC) were recently reported. Among 1,147 postmenopausal women with HR-positive, HER2-negative MBC that progressed on or after AI therapy, the combination of buparlisib and fulvestrant prolonged PFS compared with fulvestrant alone (median PFS, 6.9 vs 5 months; HR,0.78; P < .001). However, Novartis, which was developing buparlisib, reported that the combination will not be pursued further due to increased toxicity.26
Two other PI3K inhibitors are currently in phase 3 clinical trials; taselisib and alpelisib, both selective PI3K-alpha inhibitors. The results of a phase 1 dose-escalation study of taselisib were recently published and the ORR among patients with PIK3CA-mutant solid tumors was 36%, including responses in 4 patients with breast cancer.27 Meanwhile, alpelisib has also demonstrated early promise in combination with both letrozole and fulvestrant in patients with ER-positive MBC refractory to endocrine therapy. In combination with letrozole, the clinical benefit rate was 35% overall (44% in patients with PIK3CA mutations, compared with 20% in patients with wild-type PIK3CA status). The combination of alpesilib and fulvestrant produced an ORR of 27%, and both combinations were well tolerated.28,29
Another exciting therapeutic avenue is CDK4 and CDK6 inhibitors. These proteins are critical regulators of cell cycle progression, ensuring transition from G1 to S phase occurs at the appropriate time. The CDK pathway is also a downstream target of ER activation and, unsurprisingly, aberrant expression of the proteins involved in this pathway is commonly observed in breast tumors.
Palbociclib became the first FDA-approved member of this drug class, receiving accelerated approval in patients with HR-positive, HER2-negative metastatic breast cancer, in combination with letrozole in 2015. This became full regulatory approval in combination with any AI earlier this year, following the phase 3 PALOMA-3 study, in which the combination of palbociclib and fulvestrant (accelerated approval was based upon a trial testing palbociclib and letrozole) improved PFS by 5 months (HR, 0.46; P < .0001).30
In addition, a second CDK4/6 inhibitor hit the market this year. Ribociclib demonstrated a significant PFS benefit in combination with letrozole; median PFS was 25.3 months, compared with 16 months for letrozole alone, translating to a 44% reduction in the risk of disease progression or death.31
Abemaciclib, which has greater selectivity for CDK4 than its predecessors, also appears to be heading towards approval. It was granted priority review by the FDA based on data from the MONARCH-2 trial, showing a significant improvement in PFS for the combination of abemaciclib and fulvestrant (median PFS, 16.4 vs 9.3 months for fulvestrant alone; HR, 0.553; P < .001).32
Teasing out ‘HER2-positive’ subtypes
Until recently, “HER2-positive” and “HR-positive” tumors have been treated as separate subtypes, despite the fact that about half of HER2-positive tumors fall into the luminal A subtype and are also HR-positive. Patients were typically treated with HER2-targeted therapy regardless of their endocrine status because of the aggressive nature of HER2-positive disease.
Increasingly, researchers are reconsidering this view, especially as several studies have shown differential response rates to HER2-targeted therapy in HR-positive compared with HR-negative patients and accumulating evidence suggests that there is significant crosstalk between the HER2 and HR pathways, which may be responsible for the development of resistance with both treatment paradigms.
Findings from several studies have shown a benefit to combining HER2-targeted and hormonal therapies in patients with luminal (HR-positive), HER2-positive disease. In the metastatic setting, the results of the phase 2 PERTAIN study, presented at the 2017 ASCO annual meeting suggest that dual HER2 blockade could prove even more effective. The addition of pertuzumab to a combination of trastuzumab and an AI improved PFS by more than 3 months (median PFS, 19.89 vs 15.8 months; HR, 0.65; P = .007).33
The clinical application of these combinations may be limited by the additional cost – several studies have suggested that they are not cost effective – and toxicity, but have served to drive the development of new clinical trial designs as the importance of considering luminal and nonluminal HER2-positive tumors has become increasingly apparent.
PARP inhibitors make a dent in BRCA1/2-mutated cancers
The most renowned breast cancer genes, BRCA1 and BRCA2 are present in about 5%-10% of all breast cancers. They play a central role in the homologous recombination pathway that fixes double-strand breaks in the DNA. Genome sequencing studies have revealed that the presence of the BRCA1/2 genes and other DNA repair defects is highest among patients with the basal-like subtype of breast cancer, in particular those who have triple-negative disease.34,35
This type of breast cancer has proved stubbornly resistant to efforts to improve patient outcomes with targeted therapies. BRCA1/2 mutations and other DNA repair defects that confer a so-called BRCAness phenotype, render tumor cells dependent on other pathways for DNA repair and there has been considerable interest in therapeutically exploiting this through the development of inhibitors of the poly(ADP-ribose) polymerase (PARP) enzyme, which is involved in the repair of single-strand breaks in the DNA. The double damage to DNA repair mechanisms through PARP inhibition in patients with BRCA1/2-mutant tumors proves overwhelming to cancerous cells.
Despite more than a decade of investigation in breast cancer, PARP inhibitors have yet to yield any FDA-approved treatment options. That may be set to change imminently, following the success of olaparib (Table 3). In the first randomized phase 3 trial of a PARP inhibitor in breast cancer (OlympiAD), olaparib was compared with standard chemotherapy in patients with BRCA1/2-mutated MBC who had received up to 2 previous lines of chemotherapy. Olaparib reduced the risk of disease progression by 42% compared with standard chemotherapy and was well tolerated.36
The novel PARP inhibitor talazoparib, which is the most potent to date, is also demonstrating significant efficacy in clinical trials. The results of the phase 2 ABRAZO trial were presented at the ASCO annual meeting. Two cohorts were treated; the first included 49 patients who had responded to their last platinum-containing regimen for metastatic disease and progressed more than 8 weeks after last platinum dose and the other included 35 patients previously treated with 3 or more nonplatinum regimens for metastatic disease. ORR was 28% across the 2 cohorts; 23% and 33% in BRCA1- and BRCA2-mutant carriers, respectively; and 26% in patients with triple-negative breast cancer.37 PARP inhibition is not faring so well in early-stage triple-negative disease; a phase 3 trial of veliparib in combination with chemotherapy did not meet its primary endpoint.38
As our understanding of the biology of breast cancer has improved, treatment has become increasingly personalized. Targeted therapies continue to significantly improve patient outcomes in multiple subtypes, with several recent drug approvals. Here, we discuss some of these latest developments.
A disease of many faces
Clinically speaking, breast cancers can be divided into at least 5 subtypes on the basis of the genes they express (Figure 1). The luminal subtypes make up the largest proportion and are characterized by the expression of hormone receptor (HR) genes. Luminal A tumors are negative for human epidermal growth factor receptor 2 (HER2; HER2-negative), whereas luminal B tumors often co-express the HER2 genes.1
The remainder of HER2-positive patients fall into the HER2-enriched category, in which HER2 expression is the defining characteristic. Basal-like tumors, meanwhile, represent the most heterogeneous subtype, overlapping to a large extent with tumors dubbed “triple-negative” because of their lack of either HER2 or ESR1 and PGR gene expression. The fifth subtype is known as normal breast-like and remains poorly characterized.
In recent years, there have been significant advancements in the genomic characterization of breast cancer that have begun to provide a more comprehensive understanding of the driver molecular mechanisms, which has helped to explain some of the limitations of current targeted approaches and to reveal new possible treatments, with a shift toward increasingly personalized strategies.2
HER2: what’s neu?
An estimated 18%-20% of breast tumors are HER2 positive, displaying amplification of the HER2/neu gene or overexpression of its protein product.3 Historically, HER2 positivity correlated with a highly aggressive and metastatic form of disease, conferring poor prognosis.4,5 The HER2-targeted monoclonal antibody (mAb), trastuzumab serves as a prime example of the power of personalized medicine. Evidence suggests that trastuzumab has altered the natural history of HER2-positive breast cancer, such that trastuzumab-treated patients with HER2-positive breast cancer now have a better prognosis than do patients with HER2-negative disease.6,7
Several additional HER2-targeted drugs have joined trastuzumab on the market, including other mAbs, small molecule tyrosine kinase inhibitors (TKIs), and an antibody–drug conjugate that combines the specificity of a mAb with the anti-tumor potency of a cytotoxic drug. These drugs have further improved patient outcomes in both early and advanced disease settings (Table 1).
The most recent regulatory approval was for neratinib, a potent TKI inhibiting all members of the HER protein family. On the basis of the phase 3 ExteNET study, neratinib was granted approval by the US Food and Drug Administration (FDA) for extended adjuvant treatment of patients with HER2-positive, early-stage breast cancer previously treated with trastuzumab. In a 5-year analysis of the study, invasive disease-free survival (DFS) was 90.4% with neratinib, compared with 87.9% with placebo (hazard ratio [HR], 0.74; P = .017).8,9
The tide of advancements in HER2-targeted therapy looks set to continue in the coming years as potentially practice-changing data emerges from ongoing clinical trials and, as the patent on trastuzumab has expired, a number of biosimilars, such as MYL-1401O have the potential to help patients who may not have access to trastuzumab.10
One of the biggest remaining challenges is identifying drugs that can effectively treat patients with brain metastases because the blood–brain barrier presents an impediment to the delivery of effective concentrations of anticancer drugs. Initially, it was hoped that the small molecule inhibitors lapatinib and neratinib could cross the blood–brain barrier and may be more effective in patients with brain metastases, but that hypothesis has not borne out in randomized clinical trials.11
Tucatinib (ONT-380) has shown significant promise in this respect. In a phase 1 trial, ONT-380 had significant efficacy in patients with and without central nervous system metastases; the overall response rate (ORR) in the CNS was 36%. ONT-380 is also notable for its specificity for HER2, without significant inhibition of HER1 and EGFR, which could translate into a better toxicity profile.12
Doubling down on resistant tumors
Since the success of HER2-targeted therapy is limited by the development of resistance, there has been significant interest in assessing the potential of dual HER2 blockade, exploiting the unique mechanisms of action of different drugs in combination therapy, and ensuring more complete inhibition of the HER2 pathway. Although numerous different combinations have been tested, a double antibody combination has proved most effective.
In fact, dual HER2 targeting with trastuzumab and pertuzumab in combination with chemotherapy has replaced a trastuzumab-chemotherapy regimen as the new standard of care in the metastatic setting. A 6-month improvement in progression-free survival (PFS) sealed FDA approval for the combination and in a recently published final analysis of the trial overall survival (OS) was also improved to a level unprecedented in the first-line setting.13,14The double antibody combination has also been successful in the neoadjuvant setting. Approval followed the results of the phase 2 NeoSphere trial, in which the combination was associated with a significant improvement in pathologic complete response (pCR) rate, a measure that acts as a surrogate for improved survival in the neoadjuvant setting. In a 5-year analysis of the NeoSphere trial, improved pCR did indeed translate into improved PFS and DFS.15,16
The results of the phase 3 APHINITY trial evaluating this combination in the adjuvant setting have been hotly anticipated. In a presentation at the 2017 American Society of Clinical Oncology (ASCO) meeting in June, the study authors reported that in 4,085 patients with operable HER2-positive disease, it significantly reduced the risk of disease recurrence or death compared with trastuzumab and chemotherapy alone.17
There is an ongoing effort to determine if it is possible to de-escalate treatment by removing the chemotherapy component. At least in the neoadjuvant setting, pCR rates in the chemotherapy-free arms of several studies suggest that a proportion of patients might benefit from this strategy15,18,19 and the challenge now is to identify them. To that end, the phase 2 PAMELA trial identified the HER2-enriched subtype as a strong predictor of response to neoadjuvant dual blockade (lapatinib and trastuzumab) without chemotherapy. The pCR rate was 40.6% for the combination in patients with the HER2-enriched subtype of breast cancer and only 10% in patients with non–HER2-enriched tumors.20
Targeting resistance to endocrine therapy
Another coup for personalized medicine in breast cancer is the treatment of hormone receptor–positive cases with endocrine therapy, which has become the cornerstone of treatment in the metastatic and adjuvant settings. Those drugs are designed to block the growth-stimulating effects of the estrogen and progesterone hormones on tumor cells. They include the selective estrogen receptor (ER) modulator tamoxifen, aromatase inhibitors (AIs) such as letrozole, anastrozole, and exemestane, which work by blocking the activity of the aromatase enzyme that converts androgens into estrogens, and the selective estrogen-receptor down-regulator fulvestrant.
As with HER2-targeted therapy, patients treated with endocrine therapy often develop resistance. Activation of alternate signaling cascades, such as the P13K–Akt–mTOR (phosphatidylinositol-3-kinase–Akt–mammalian target of rapamycin) pathway, or downstream targets of ER signaling, including the cyclin-dependent kinases, CDK4 and CDK6, have emerged as important mechanisms of resistance.21,22
Drugs directed against these secondary targets, aimed to enhance the efficacy of endocrine therapies, have shown significant promise (Table 2). The mTOR inhibitor everolimus received FDA approval in 2012 in combination with exemestane for the treatment of advanced HR-positive, HER2-negative breast cancer.23 More recently, everolimus has also proven effective in combination with either fulvestrant or letrozole, according to the phase 2 PrECOG 0102 and BOLERO-4 studies, both doubling PFS compared with endocrine therapy alone.24,25
Buparlisib is an oral reversible pan-PI3K inhibitor, and the results of the first phase 3 trial of this drug in metastatic breast cancer (MBC) were recently reported. Among 1,147 postmenopausal women with HR-positive, HER2-negative MBC that progressed on or after AI therapy, the combination of buparlisib and fulvestrant prolonged PFS compared with fulvestrant alone (median PFS, 6.9 vs 5 months; HR,0.78; P < .001). However, Novartis, which was developing buparlisib, reported that the combination will not be pursued further due to increased toxicity.26
Two other PI3K inhibitors are currently in phase 3 clinical trials; taselisib and alpelisib, both selective PI3K-alpha inhibitors. The results of a phase 1 dose-escalation study of taselisib were recently published and the ORR among patients with PIK3CA-mutant solid tumors was 36%, including responses in 4 patients with breast cancer.27 Meanwhile, alpelisib has also demonstrated early promise in combination with both letrozole and fulvestrant in patients with ER-positive MBC refractory to endocrine therapy. In combination with letrozole, the clinical benefit rate was 35% overall (44% in patients with PIK3CA mutations, compared with 20% in patients with wild-type PIK3CA status). The combination of alpesilib and fulvestrant produced an ORR of 27%, and both combinations were well tolerated.28,29
Another exciting therapeutic avenue is CDK4 and CDK6 inhibitors. These proteins are critical regulators of cell cycle progression, ensuring transition from G1 to S phase occurs at the appropriate time. The CDK pathway is also a downstream target of ER activation and, unsurprisingly, aberrant expression of the proteins involved in this pathway is commonly observed in breast tumors.
Palbociclib became the first FDA-approved member of this drug class, receiving accelerated approval in patients with HR-positive, HER2-negative metastatic breast cancer, in combination with letrozole in 2015. This became full regulatory approval in combination with any AI earlier this year, following the phase 3 PALOMA-3 study, in which the combination of palbociclib and fulvestrant (accelerated approval was based upon a trial testing palbociclib and letrozole) improved PFS by 5 months (HR, 0.46; P < .0001).30
In addition, a second CDK4/6 inhibitor hit the market this year. Ribociclib demonstrated a significant PFS benefit in combination with letrozole; median PFS was 25.3 months, compared with 16 months for letrozole alone, translating to a 44% reduction in the risk of disease progression or death.31
Abemaciclib, which has greater selectivity for CDK4 than its predecessors, also appears to be heading towards approval. It was granted priority review by the FDA based on data from the MONARCH-2 trial, showing a significant improvement in PFS for the combination of abemaciclib and fulvestrant (median PFS, 16.4 vs 9.3 months for fulvestrant alone; HR, 0.553; P < .001).32
Teasing out ‘HER2-positive’ subtypes
Until recently, “HER2-positive” and “HR-positive” tumors have been treated as separate subtypes, despite the fact that about half of HER2-positive tumors fall into the luminal A subtype and are also HR-positive. Patients were typically treated with HER2-targeted therapy regardless of their endocrine status because of the aggressive nature of HER2-positive disease.
Increasingly, researchers are reconsidering this view, especially as several studies have shown differential response rates to HER2-targeted therapy in HR-positive compared with HR-negative patients and accumulating evidence suggests that there is significant crosstalk between the HER2 and HR pathways, which may be responsible for the development of resistance with both treatment paradigms.
Findings from several studies have shown a benefit to combining HER2-targeted and hormonal therapies in patients with luminal (HR-positive), HER2-positive disease. In the metastatic setting, the results of the phase 2 PERTAIN study, presented at the 2017 ASCO annual meeting suggest that dual HER2 blockade could prove even more effective. The addition of pertuzumab to a combination of trastuzumab and an AI improved PFS by more than 3 months (median PFS, 19.89 vs 15.8 months; HR, 0.65; P = .007).33
The clinical application of these combinations may be limited by the additional cost – several studies have suggested that they are not cost effective – and toxicity, but have served to drive the development of new clinical trial designs as the importance of considering luminal and nonluminal HER2-positive tumors has become increasingly apparent.
PARP inhibitors make a dent in BRCA1/2-mutated cancers
The most renowned breast cancer genes, BRCA1 and BRCA2 are present in about 5%-10% of all breast cancers. They play a central role in the homologous recombination pathway that fixes double-strand breaks in the DNA. Genome sequencing studies have revealed that the presence of the BRCA1/2 genes and other DNA repair defects is highest among patients with the basal-like subtype of breast cancer, in particular those who have triple-negative disease.34,35
This type of breast cancer has proved stubbornly resistant to efforts to improve patient outcomes with targeted therapies. BRCA1/2 mutations and other DNA repair defects that confer a so-called BRCAness phenotype, render tumor cells dependent on other pathways for DNA repair and there has been considerable interest in therapeutically exploiting this through the development of inhibitors of the poly(ADP-ribose) polymerase (PARP) enzyme, which is involved in the repair of single-strand breaks in the DNA. The double damage to DNA repair mechanisms through PARP inhibition in patients with BRCA1/2-mutant tumors proves overwhelming to cancerous cells.
Despite more than a decade of investigation in breast cancer, PARP inhibitors have yet to yield any FDA-approved treatment options. That may be set to change imminently, following the success of olaparib (Table 3). In the first randomized phase 3 trial of a PARP inhibitor in breast cancer (OlympiAD), olaparib was compared with standard chemotherapy in patients with BRCA1/2-mutated MBC who had received up to 2 previous lines of chemotherapy. Olaparib reduced the risk of disease progression by 42% compared with standard chemotherapy and was well tolerated.36
The novel PARP inhibitor talazoparib, which is the most potent to date, is also demonstrating significant efficacy in clinical trials. The results of the phase 2 ABRAZO trial were presented at the ASCO annual meeting. Two cohorts were treated; the first included 49 patients who had responded to their last platinum-containing regimen for metastatic disease and progressed more than 8 weeks after last platinum dose and the other included 35 patients previously treated with 3 or more nonplatinum regimens for metastatic disease. ORR was 28% across the 2 cohorts; 23% and 33% in BRCA1- and BRCA2-mutant carriers, respectively; and 26% in patients with triple-negative breast cancer.37 PARP inhibition is not faring so well in early-stage triple-negative disease; a phase 3 trial of veliparib in combination with chemotherapy did not meet its primary endpoint.38
The effect of centralizing breast cancer care in an urban public hospital
When cancer care is centralized in a comprehensive fashion, the quality of care and the outcomes improve.1,2 Unfortunately, because of the medical insurance structure in New York City, most patients of lower socioeconomic status do not receive their cancer care in such dedicated cancer centers. In New York City, the majority of the underserved vulnerable populations – that is, those without health insurance – receive their care from the public hospital system known as NYC Health and Hospitals. Cancer care in this system is not centralized and may result in fragmented implementation of various modalities of treatment. In addition, because there is no centralized care, needs such as early screening and prevention programs are often not addressed. This problem was evident in Queens in 2000 and before when many patients with late-stage cancers were presenting for cancer care. Queens, which is one of the 5 boroughs of New York City, has more than 2.3 million residents. It has 2 public hospitals, Elmhurst Hospital Center and Queens Hospital Center (QHC). In 2001, the plan was devised for the establishment of a cancer center at QHC, mainly because of the high rate of late-stage cancers that were being seen at presentation and recognition of the need for more comprehensive care. In 2002, the Queens Cancer Center (QCC) began to see patients. QCC is a single facility that provides medical, surgical, radiation, gynecologic, and urologic oncology all in one area of the QHC.
This study is an investigation of the possible impact on care for breast cancer patients of low socioeconomic status who were treated at a comprehensive cancer center, with specific consideration of the change or improvement in treatment modalities and outcomes. Data on treatment modalities and outcomes of cancer patients who were treated at the QHC during 2000, before the QCC was set up, were compared with data of patients treated during 2008 (2008 was selected because we have 5-year survival data for those patients). The public hospital system treats all patients regardless of their ability to pay, so the majority of patients in the system are of lower socioeconomic status. In addition, 92% of the patients seen QHC are from a minority population. These are the populations that tend to have a worse prognosis and often are not given optimal treatment.3 The payer mix of patients in the public hospital system is different than that of private hospitals. Most of the patients present at the hospital with no insurance and if they are diagnosed with cancer they may be converted to emergency Medicaid. About 10% of patients will not be converted because of their document status.
Patients and methods
We used the Queens Hospital Tumor Registry to identify the patients who had been diagnosed with and treated for breast cancer in 2000 and 2008. The electronic medical records were reviewed, and in the case of the 2000-year patients, the written charts were also reviewed. The study was approved by the Mount Sinai institutional review board. It was not necessary to obtain patient consent because it was a retrospective study.
Only patients diagnosed with stage 0, I, II, or III breast cancer who received their treatment at QHC were included in the study. Patients who were seen in consultation at QHC but not treated there were excluded. Statistics were done using the 2x2 chi-squared SPSS analysis; a P value of .05 was considered significant. The survival data was analyzed using SAS.
Results
There were 24 evaluable patients in 2000 and 78 evaluable patients in 2008 who had stage 0, I, II, or III primary breast cancer and were treated at QHC. The average age of the patients in 2000 was 53.5 years and 54.7 years in 2008. The mean age for both groups was 55 years. The patients were ethnically diverse in both groups with 46% black, 17% Hispanic, 25% ethnic Asian Indian, and 6% white (Figure 1).
The payer mix in 2000 was 9 patients (37.5%) self-pay, 7 (29%) Medicaid, and 8 (33%) Medicare. In 2008, 11 patients (14%) were self-pay, 46 (59%) Medicaid, 11 (14%) Medicare, and 10 (13%) were private insurance. In 2000, there were 3 (12%) patients with stage 0 disease, 5 (21%) with stage I; 9 (37.5%) with stage II, and 7 (29%) with stage III. In 2008 there were 28 (36%) patients with stage 0 disease, 15 (19%) with stage I, 17 (22%) with stage II, and 18 (23%) with stage III (Figure 2).
None of those values are statistically different. In 2000, 2 of the 24 patients had lumpectomies (partial mastectomy) and the rest had mastectomies. In 2008, 39 (50%) patients had mastectomy and 39 (50%) had lumpectomies (Figure 3). This was a statistically significant difference.
Radiation was given to both patients with lumpectomy in the 2000 group. In the 2008 group, all patients with lumpectomies were evaluated for radiation, and 6 of them did not receive radiation for the following reasons: 3 had very small foci of ductal carcinoma in situ (DCIS) and were treated with hormone therapy and no radiation; 1 patient had a lumpectomy for stage 1 cancer and also did not get radiation therapy because of a low oncotype and very small lesion; 2 patients were older than 70 years and had DCIS and were treated with tamoxifen alone as per NCCN Guidelines for women in that age group. The rest of the patients with lumpectomies received postoperative radiation.
Hormone and HER2 (human epidermal growth factor receptor 2) status was obtained on all patients. For the 2000 patients, 71% had 1 hormone receptor–positive (estrogen receptor [ER] or progesterone receptor [PR]), 21% were triple negative (ER-PR and HER2-neu), and 42% had HER2-neu–positive tumors. For the 2008, patients 65% were positive for 1 hormone receptor (ER or PR), 28% were triple negative (ER-PR and HER2-neu), and 7% had HER2-neu-positive tumors.
All patients were offered chemotherapy and hormone therapy if appropriate, as per NCCN guidelines. If a patient’s tumor was found to be HER2-positive, then the chemotherapy regimen would include the use of trastuzumab in both groups.
The 5-year survival for the 2008 stage III patients was 73.7%, compared with 14.2% for the 2000 stage III patients. The only deaths in the 2008 group were in patients with stage III disease. In the 2000 group, 4 of the 5 patients with stage III cancer died, and 33% of patients with stage I or II either died or were lost to follow-up before 5 years. This survival difference is significant by a chi-square and Wilcoxon analysis, with a P value of .01.
In 2000, 86% of patients with cancer were termed self-pay, that is, they had no insurance and they were not converted to emergency Medicaid. In 2008, 16% of patients were self-pay, and the rest were converted to Medicaid. In 2000, fewer than 2% of patients had commercial insurance, compared with 9% in 2008.
Discussion
There have been numerous studies reporting on disparities in the treatment of patients with breast cancer based on race or socioeconomic status.4-18 Many studies have shown inferior survival for black women with breast cancer, but it is not entirely clear whether these differences are the result of the quality of medical care received or biologic differences.14,19 A moderately large study from a metropolitan medical center in Detroit showed no difference in survival in their patients based on race when all of the patients received equal treatments.15 A meta-analysis of survival in black and white breast cancer patients showed that the black women had significantly poorer outcomes.19
Findings from a recent study showed that patients of lower socioeconomic status are more likely to undergo mastectomy than breast conserving therapy.20 The study, which identified 727,927 patients with early-stage breast cancer during 1998-2011, found that the rate of breast conservation increased from 54% to 59% during that time period and that there were significant barriers to women receiving breast-conserving therapy based on their type of insurance and having a lower socioeconomic status.20
The treatment of breast cancer is best delivered in a multimodality setting, but many inner-city public hospitals do not have such a facility for their patients. QHC is the only public hospital in New York City that has established a comprehensive cancer center. The patient population of QHC is overwhelmingly of minority origin (only 5% of patients are white). In addition, it is a safety net hospital, so no patient is turned away because they cannot pay, and most patients are of lower socioeconomic status and do not have insurance. The purpose of the cancer center was to provide a single site at which our patients could receive all their treatment. It was to ensure that our patients had easy access to care and treatment during all phases of their disease trajectory and did not “fall through the cracks” of the system. Those goals were addressed by having all of the center’s physicians in one place. Physicians involved in care included medical, surgical, and radiation oncologists, a gynecologic oncologist, a genitourinary oncologist, and a thoracic surgery oncologist. The support groups organized for the cancer patients included 3 oncology social workers, an oncology navigator, a nutritionist, a pastoral care supporter, and an oncology psychologist, all located in the same area. All of the clerical and financial aspects of care were also placed within the center. This made the experience as seamless as possible for both the patients and the treating physicians. A “survivors clinic” was established so the cancer patients could be seen by integrated primary care providers to address all noncancer-related health issues such as hypertension, diabetes, or heart disease. Finally, a robust clinical oncology research team was established in the same location. The research included several protocols for new drug treatments for breast cancer from pharmaceutical companies as well as the multi-institutional oncology groups.
Part of the mission of the cancer center was to reach out into the community of Queens to provide education about early detection, cancer prevention, and other public health issues such as tobacco cessation. We established a close working relationship with the Queens Public Library System to connect with their users and dispense information about cancer care and early detection. The Queens Library system is the largest in the United States, and everyone who lives in Queens has easy access to one of its 63 branch libraries. We arranged several lectures about breast cancer awareness in some of the branch libraries. We also procured a mobile mammogram unit for free screening events at the lectures, especially in neighborhoods with a large number residents who were of lower socioeconomic status.
To study the possible effect of these changes on our patients with breast cancer, we compared 2 groups of patients. One group was from the year 2000, a year before the cancer center was opened. The other was from the year 2008, the last year we could get real 5-year survival statistics. We explored how establishing the cancer center might have changed the patients’ stage at diagnosis, care, treatment modalities such as type of surgery, and outcomes. It is difficult to compare these 2 groups because of differences in the patients’ cancers, such as their receptor status, as well as differences in treatment options between the two time periods. However, we had no other way to compare the data to see if there were any trends.
There was a migration to earlier-stage cancer at diagnosis during the 6-year period after the cancer center was opened. It is likely that the educational sessions that were done in the community contributed to this migration. We also saw an increase in the number of mammograms done, from 6,300 in 2000 to 8,800 in 2008. This increase in screening also could account for more patients being identified with earlier-stage disease and might be attributable to the community education through the outreach programs.
As a quality control method, the cancer center has been evaluated by the Commission on Cancer every 3 years. At the 2013 evaluation, we received the Gold Commendation – the highest possible recognition for having 8 out of 8 commendations – and a 3-year accreditation.
There was a notable increase in the use of lumpectomy over mastectomy after the establishment of the cancer center, possibly due to the addition of 2 surgical oncologists to the cancer center’s care team. The integration of multimodiality care for each patient may also have increased the use of breast-conserving surgery.
There was a significant increase from 2000 to 2008 in the survival of patients treated for stage III breast cancer. New drugs and new patterns of adjuvant care might have been partly responsible for that change. The establishment of the comprehensive cancer center with access to new protocols ensured that patients received state-of-the-art cancer treatment. Moreover, the facility addressed all aspects of patient care throughout the disease trajectory by including designated social workers, psychologists, a nutritionist, pastoral care, and patient and survivor support groups to ensure that patients would keep coming to the center for their therapy, with no delays and very little loss to follow-up.
Most patients without insurance were able to acquire emergency Medicaid through the cancer center. This was done by having 2 financial counselors who met with every patient and who could facilitate access to Medicaid as needed. As a result of that, the percentage of patients with no coverage went from 86% in 2000 to 16% in 2008. Before this system was set up, patients who were designated self-pay would pay a fee as low as $15 for each visit and received thousands of dollars’ worth of care. Thus, by forming a cancer center and facilitating patient access to Medicaid, we were able to save money for this public institution because of the gain in revenue from Medicaid.
Our findings suggest that the development of comprehensive cancer centers within inner-city health systems can ensure better treatment for patients of lower socioeconomic status. We present evidence that this may result in increased survival, more sophisticated surgical options, and better patient quality of life. Moreover, this can be achieved while effectively increasing revenue for the public hospitals. Correcting the inequality of access to care and better therapeutic options by setting up comprehensive cancer centers could contribute to improved parity of outcomes for underserved populations.
The author acknowledges the statistical help of Brian Altonen, MPH.
1. Kesson EM, Allardice GM, George WD, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ. 2012;344:e2718.
2. Vrijens F, Stordeur S, Beirens K, Devriese S, Van Eycken E, Vlayen J. Effect of hospital volume on processes of care and 5-year survival after breast cancer: a population-based study on 25000 women. Breast. 2012;21(3):261-266.
3. Bradley CJ, Given CW, Roberts C. Race, socioeconomic status and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490-496.
4. Wheeler SB, Hayes-Reeder KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18:986-993.
5. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78-93.
6. Chen F, Puig M, Yermilov I, et al. Using breast cancer quality indicators in a vulnerable population. Cancer. 2011;117:3311-3321.
7. Banerjee M, George J, Yee C, Hryniuk W, Schwartz K. Disentangling the effects of race on breast cancer treatment. Cancer. 2007;110:2169-2177.
8. Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713-719.
9. Bickell NA, Shastri K, Fei K, et al. A tracking and feedback registry to reduce racial disparities in breast cancer care. J Natl Cancer Inst. 2008;100:1717-1723.
19. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357-1362.
11. Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987-2005). Cancer Epidemiol Biomarkers Prev. 2009;18:121-131.
12. Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9-31.
13. Naik AM, Joseph K, Harris M, Davis C, Shapiro R, Hiotis KL. Indigent breast cancer patients among all racial and ethnic groups present with more advanced disease compared with nationally reported date. Am J Surg. 2003;186:400-403.
14. Hersman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of southwest oncology studies S8814/S8897. J Clin Oncol. 2009;27: 2157-2162.
15
16. Brawley OW. Disaggregating the effects of race and poverty on breast cancer outcomes. J Natl Cancer Inst. 2002;94:471-473.
17. Baquet CR, Commiskey P. Socioeconomic factors and breast carcinoma in multicultural women. Cancer. 2000;88:1256-1264.
18. Cross C, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the US. Cancer. 2002;95:1988-1999.
19. Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: Ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342-1349.
20. Lautner M, Lin H, Shen Y, et al. Disparities in the use of breast-conserving therapy among patients with early-stage breast cancer. JAMA. 2015;150:778-786.
When cancer care is centralized in a comprehensive fashion, the quality of care and the outcomes improve.1,2 Unfortunately, because of the medical insurance structure in New York City, most patients of lower socioeconomic status do not receive their cancer care in such dedicated cancer centers. In New York City, the majority of the underserved vulnerable populations – that is, those without health insurance – receive their care from the public hospital system known as NYC Health and Hospitals. Cancer care in this system is not centralized and may result in fragmented implementation of various modalities of treatment. In addition, because there is no centralized care, needs such as early screening and prevention programs are often not addressed. This problem was evident in Queens in 2000 and before when many patients with late-stage cancers were presenting for cancer care. Queens, which is one of the 5 boroughs of New York City, has more than 2.3 million residents. It has 2 public hospitals, Elmhurst Hospital Center and Queens Hospital Center (QHC). In 2001, the plan was devised for the establishment of a cancer center at QHC, mainly because of the high rate of late-stage cancers that were being seen at presentation and recognition of the need for more comprehensive care. In 2002, the Queens Cancer Center (QCC) began to see patients. QCC is a single facility that provides medical, surgical, radiation, gynecologic, and urologic oncology all in one area of the QHC.
This study is an investigation of the possible impact on care for breast cancer patients of low socioeconomic status who were treated at a comprehensive cancer center, with specific consideration of the change or improvement in treatment modalities and outcomes. Data on treatment modalities and outcomes of cancer patients who were treated at the QHC during 2000, before the QCC was set up, were compared with data of patients treated during 2008 (2008 was selected because we have 5-year survival data for those patients). The public hospital system treats all patients regardless of their ability to pay, so the majority of patients in the system are of lower socioeconomic status. In addition, 92% of the patients seen QHC are from a minority population. These are the populations that tend to have a worse prognosis and often are not given optimal treatment.3 The payer mix of patients in the public hospital system is different than that of private hospitals. Most of the patients present at the hospital with no insurance and if they are diagnosed with cancer they may be converted to emergency Medicaid. About 10% of patients will not be converted because of their document status.
Patients and methods
We used the Queens Hospital Tumor Registry to identify the patients who had been diagnosed with and treated for breast cancer in 2000 and 2008. The electronic medical records were reviewed, and in the case of the 2000-year patients, the written charts were also reviewed. The study was approved by the Mount Sinai institutional review board. It was not necessary to obtain patient consent because it was a retrospective study.
Only patients diagnosed with stage 0, I, II, or III breast cancer who received their treatment at QHC were included in the study. Patients who were seen in consultation at QHC but not treated there were excluded. Statistics were done using the 2x2 chi-squared SPSS analysis; a P value of .05 was considered significant. The survival data was analyzed using SAS.
Results
There were 24 evaluable patients in 2000 and 78 evaluable patients in 2008 who had stage 0, I, II, or III primary breast cancer and were treated at QHC. The average age of the patients in 2000 was 53.5 years and 54.7 years in 2008. The mean age for both groups was 55 years. The patients were ethnically diverse in both groups with 46% black, 17% Hispanic, 25% ethnic Asian Indian, and 6% white (Figure 1).
The payer mix in 2000 was 9 patients (37.5%) self-pay, 7 (29%) Medicaid, and 8 (33%) Medicare. In 2008, 11 patients (14%) were self-pay, 46 (59%) Medicaid, 11 (14%) Medicare, and 10 (13%) were private insurance. In 2000, there were 3 (12%) patients with stage 0 disease, 5 (21%) with stage I; 9 (37.5%) with stage II, and 7 (29%) with stage III. In 2008 there were 28 (36%) patients with stage 0 disease, 15 (19%) with stage I, 17 (22%) with stage II, and 18 (23%) with stage III (Figure 2).
None of those values are statistically different. In 2000, 2 of the 24 patients had lumpectomies (partial mastectomy) and the rest had mastectomies. In 2008, 39 (50%) patients had mastectomy and 39 (50%) had lumpectomies (Figure 3). This was a statistically significant difference.
Radiation was given to both patients with lumpectomy in the 2000 group. In the 2008 group, all patients with lumpectomies were evaluated for radiation, and 6 of them did not receive radiation for the following reasons: 3 had very small foci of ductal carcinoma in situ (DCIS) and were treated with hormone therapy and no radiation; 1 patient had a lumpectomy for stage 1 cancer and also did not get radiation therapy because of a low oncotype and very small lesion; 2 patients were older than 70 years and had DCIS and were treated with tamoxifen alone as per NCCN Guidelines for women in that age group. The rest of the patients with lumpectomies received postoperative radiation.
Hormone and HER2 (human epidermal growth factor receptor 2) status was obtained on all patients. For the 2000 patients, 71% had 1 hormone receptor–positive (estrogen receptor [ER] or progesterone receptor [PR]), 21% were triple negative (ER-PR and HER2-neu), and 42% had HER2-neu–positive tumors. For the 2008, patients 65% were positive for 1 hormone receptor (ER or PR), 28% were triple negative (ER-PR and HER2-neu), and 7% had HER2-neu-positive tumors.
All patients were offered chemotherapy and hormone therapy if appropriate, as per NCCN guidelines. If a patient’s tumor was found to be HER2-positive, then the chemotherapy regimen would include the use of trastuzumab in both groups.
The 5-year survival for the 2008 stage III patients was 73.7%, compared with 14.2% for the 2000 stage III patients. The only deaths in the 2008 group were in patients with stage III disease. In the 2000 group, 4 of the 5 patients with stage III cancer died, and 33% of patients with stage I or II either died or were lost to follow-up before 5 years. This survival difference is significant by a chi-square and Wilcoxon analysis, with a P value of .01.
In 2000, 86% of patients with cancer were termed self-pay, that is, they had no insurance and they were not converted to emergency Medicaid. In 2008, 16% of patients were self-pay, and the rest were converted to Medicaid. In 2000, fewer than 2% of patients had commercial insurance, compared with 9% in 2008.
Discussion
There have been numerous studies reporting on disparities in the treatment of patients with breast cancer based on race or socioeconomic status.4-18 Many studies have shown inferior survival for black women with breast cancer, but it is not entirely clear whether these differences are the result of the quality of medical care received or biologic differences.14,19 A moderately large study from a metropolitan medical center in Detroit showed no difference in survival in their patients based on race when all of the patients received equal treatments.15 A meta-analysis of survival in black and white breast cancer patients showed that the black women had significantly poorer outcomes.19
Findings from a recent study showed that patients of lower socioeconomic status are more likely to undergo mastectomy than breast conserving therapy.20 The study, which identified 727,927 patients with early-stage breast cancer during 1998-2011, found that the rate of breast conservation increased from 54% to 59% during that time period and that there were significant barriers to women receiving breast-conserving therapy based on their type of insurance and having a lower socioeconomic status.20
The treatment of breast cancer is best delivered in a multimodality setting, but many inner-city public hospitals do not have such a facility for their patients. QHC is the only public hospital in New York City that has established a comprehensive cancer center. The patient population of QHC is overwhelmingly of minority origin (only 5% of patients are white). In addition, it is a safety net hospital, so no patient is turned away because they cannot pay, and most patients are of lower socioeconomic status and do not have insurance. The purpose of the cancer center was to provide a single site at which our patients could receive all their treatment. It was to ensure that our patients had easy access to care and treatment during all phases of their disease trajectory and did not “fall through the cracks” of the system. Those goals were addressed by having all of the center’s physicians in one place. Physicians involved in care included medical, surgical, and radiation oncologists, a gynecologic oncologist, a genitourinary oncologist, and a thoracic surgery oncologist. The support groups organized for the cancer patients included 3 oncology social workers, an oncology navigator, a nutritionist, a pastoral care supporter, and an oncology psychologist, all located in the same area. All of the clerical and financial aspects of care were also placed within the center. This made the experience as seamless as possible for both the patients and the treating physicians. A “survivors clinic” was established so the cancer patients could be seen by integrated primary care providers to address all noncancer-related health issues such as hypertension, diabetes, or heart disease. Finally, a robust clinical oncology research team was established in the same location. The research included several protocols for new drug treatments for breast cancer from pharmaceutical companies as well as the multi-institutional oncology groups.
Part of the mission of the cancer center was to reach out into the community of Queens to provide education about early detection, cancer prevention, and other public health issues such as tobacco cessation. We established a close working relationship with the Queens Public Library System to connect with their users and dispense information about cancer care and early detection. The Queens Library system is the largest in the United States, and everyone who lives in Queens has easy access to one of its 63 branch libraries. We arranged several lectures about breast cancer awareness in some of the branch libraries. We also procured a mobile mammogram unit for free screening events at the lectures, especially in neighborhoods with a large number residents who were of lower socioeconomic status.
To study the possible effect of these changes on our patients with breast cancer, we compared 2 groups of patients. One group was from the year 2000, a year before the cancer center was opened. The other was from the year 2008, the last year we could get real 5-year survival statistics. We explored how establishing the cancer center might have changed the patients’ stage at diagnosis, care, treatment modalities such as type of surgery, and outcomes. It is difficult to compare these 2 groups because of differences in the patients’ cancers, such as their receptor status, as well as differences in treatment options between the two time periods. However, we had no other way to compare the data to see if there were any trends.
There was a migration to earlier-stage cancer at diagnosis during the 6-year period after the cancer center was opened. It is likely that the educational sessions that were done in the community contributed to this migration. We also saw an increase in the number of mammograms done, from 6,300 in 2000 to 8,800 in 2008. This increase in screening also could account for more patients being identified with earlier-stage disease and might be attributable to the community education through the outreach programs.
As a quality control method, the cancer center has been evaluated by the Commission on Cancer every 3 years. At the 2013 evaluation, we received the Gold Commendation – the highest possible recognition for having 8 out of 8 commendations – and a 3-year accreditation.
There was a notable increase in the use of lumpectomy over mastectomy after the establishment of the cancer center, possibly due to the addition of 2 surgical oncologists to the cancer center’s care team. The integration of multimodiality care for each patient may also have increased the use of breast-conserving surgery.
There was a significant increase from 2000 to 2008 in the survival of patients treated for stage III breast cancer. New drugs and new patterns of adjuvant care might have been partly responsible for that change. The establishment of the comprehensive cancer center with access to new protocols ensured that patients received state-of-the-art cancer treatment. Moreover, the facility addressed all aspects of patient care throughout the disease trajectory by including designated social workers, psychologists, a nutritionist, pastoral care, and patient and survivor support groups to ensure that patients would keep coming to the center for their therapy, with no delays and very little loss to follow-up.
Most patients without insurance were able to acquire emergency Medicaid through the cancer center. This was done by having 2 financial counselors who met with every patient and who could facilitate access to Medicaid as needed. As a result of that, the percentage of patients with no coverage went from 86% in 2000 to 16% in 2008. Before this system was set up, patients who were designated self-pay would pay a fee as low as $15 for each visit and received thousands of dollars’ worth of care. Thus, by forming a cancer center and facilitating patient access to Medicaid, we were able to save money for this public institution because of the gain in revenue from Medicaid.
Our findings suggest that the development of comprehensive cancer centers within inner-city health systems can ensure better treatment for patients of lower socioeconomic status. We present evidence that this may result in increased survival, more sophisticated surgical options, and better patient quality of life. Moreover, this can be achieved while effectively increasing revenue for the public hospitals. Correcting the inequality of access to care and better therapeutic options by setting up comprehensive cancer centers could contribute to improved parity of outcomes for underserved populations.
The author acknowledges the statistical help of Brian Altonen, MPH.
When cancer care is centralized in a comprehensive fashion, the quality of care and the outcomes improve.1,2 Unfortunately, because of the medical insurance structure in New York City, most patients of lower socioeconomic status do not receive their cancer care in such dedicated cancer centers. In New York City, the majority of the underserved vulnerable populations – that is, those without health insurance – receive their care from the public hospital system known as NYC Health and Hospitals. Cancer care in this system is not centralized and may result in fragmented implementation of various modalities of treatment. In addition, because there is no centralized care, needs such as early screening and prevention programs are often not addressed. This problem was evident in Queens in 2000 and before when many patients with late-stage cancers were presenting for cancer care. Queens, which is one of the 5 boroughs of New York City, has more than 2.3 million residents. It has 2 public hospitals, Elmhurst Hospital Center and Queens Hospital Center (QHC). In 2001, the plan was devised for the establishment of a cancer center at QHC, mainly because of the high rate of late-stage cancers that were being seen at presentation and recognition of the need for more comprehensive care. In 2002, the Queens Cancer Center (QCC) began to see patients. QCC is a single facility that provides medical, surgical, radiation, gynecologic, and urologic oncology all in one area of the QHC.
This study is an investigation of the possible impact on care for breast cancer patients of low socioeconomic status who were treated at a comprehensive cancer center, with specific consideration of the change or improvement in treatment modalities and outcomes. Data on treatment modalities and outcomes of cancer patients who were treated at the QHC during 2000, before the QCC was set up, were compared with data of patients treated during 2008 (2008 was selected because we have 5-year survival data for those patients). The public hospital system treats all patients regardless of their ability to pay, so the majority of patients in the system are of lower socioeconomic status. In addition, 92% of the patients seen QHC are from a minority population. These are the populations that tend to have a worse prognosis and often are not given optimal treatment.3 The payer mix of patients in the public hospital system is different than that of private hospitals. Most of the patients present at the hospital with no insurance and if they are diagnosed with cancer they may be converted to emergency Medicaid. About 10% of patients will not be converted because of their document status.
Patients and methods
We used the Queens Hospital Tumor Registry to identify the patients who had been diagnosed with and treated for breast cancer in 2000 and 2008. The electronic medical records were reviewed, and in the case of the 2000-year patients, the written charts were also reviewed. The study was approved by the Mount Sinai institutional review board. It was not necessary to obtain patient consent because it was a retrospective study.
Only patients diagnosed with stage 0, I, II, or III breast cancer who received their treatment at QHC were included in the study. Patients who were seen in consultation at QHC but not treated there were excluded. Statistics were done using the 2x2 chi-squared SPSS analysis; a P value of .05 was considered significant. The survival data was analyzed using SAS.
Results
There were 24 evaluable patients in 2000 and 78 evaluable patients in 2008 who had stage 0, I, II, or III primary breast cancer and were treated at QHC. The average age of the patients in 2000 was 53.5 years and 54.7 years in 2008. The mean age for both groups was 55 years. The patients were ethnically diverse in both groups with 46% black, 17% Hispanic, 25% ethnic Asian Indian, and 6% white (Figure 1).
The payer mix in 2000 was 9 patients (37.5%) self-pay, 7 (29%) Medicaid, and 8 (33%) Medicare. In 2008, 11 patients (14%) were self-pay, 46 (59%) Medicaid, 11 (14%) Medicare, and 10 (13%) were private insurance. In 2000, there were 3 (12%) patients with stage 0 disease, 5 (21%) with stage I; 9 (37.5%) with stage II, and 7 (29%) with stage III. In 2008 there were 28 (36%) patients with stage 0 disease, 15 (19%) with stage I, 17 (22%) with stage II, and 18 (23%) with stage III (Figure 2).
None of those values are statistically different. In 2000, 2 of the 24 patients had lumpectomies (partial mastectomy) and the rest had mastectomies. In 2008, 39 (50%) patients had mastectomy and 39 (50%) had lumpectomies (Figure 3). This was a statistically significant difference.
Radiation was given to both patients with lumpectomy in the 2000 group. In the 2008 group, all patients with lumpectomies were evaluated for radiation, and 6 of them did not receive radiation for the following reasons: 3 had very small foci of ductal carcinoma in situ (DCIS) and were treated with hormone therapy and no radiation; 1 patient had a lumpectomy for stage 1 cancer and also did not get radiation therapy because of a low oncotype and very small lesion; 2 patients were older than 70 years and had DCIS and were treated with tamoxifen alone as per NCCN Guidelines for women in that age group. The rest of the patients with lumpectomies received postoperative radiation.
Hormone and HER2 (human epidermal growth factor receptor 2) status was obtained on all patients. For the 2000 patients, 71% had 1 hormone receptor–positive (estrogen receptor [ER] or progesterone receptor [PR]), 21% were triple negative (ER-PR and HER2-neu), and 42% had HER2-neu–positive tumors. For the 2008, patients 65% were positive for 1 hormone receptor (ER or PR), 28% were triple negative (ER-PR and HER2-neu), and 7% had HER2-neu-positive tumors.
All patients were offered chemotherapy and hormone therapy if appropriate, as per NCCN guidelines. If a patient’s tumor was found to be HER2-positive, then the chemotherapy regimen would include the use of trastuzumab in both groups.
The 5-year survival for the 2008 stage III patients was 73.7%, compared with 14.2% for the 2000 stage III patients. The only deaths in the 2008 group were in patients with stage III disease. In the 2000 group, 4 of the 5 patients with stage III cancer died, and 33% of patients with stage I or II either died or were lost to follow-up before 5 years. This survival difference is significant by a chi-square and Wilcoxon analysis, with a P value of .01.
In 2000, 86% of patients with cancer were termed self-pay, that is, they had no insurance and they were not converted to emergency Medicaid. In 2008, 16% of patients were self-pay, and the rest were converted to Medicaid. In 2000, fewer than 2% of patients had commercial insurance, compared with 9% in 2008.
Discussion
There have been numerous studies reporting on disparities in the treatment of patients with breast cancer based on race or socioeconomic status.4-18 Many studies have shown inferior survival for black women with breast cancer, but it is not entirely clear whether these differences are the result of the quality of medical care received or biologic differences.14,19 A moderately large study from a metropolitan medical center in Detroit showed no difference in survival in their patients based on race when all of the patients received equal treatments.15 A meta-analysis of survival in black and white breast cancer patients showed that the black women had significantly poorer outcomes.19
Findings from a recent study showed that patients of lower socioeconomic status are more likely to undergo mastectomy than breast conserving therapy.20 The study, which identified 727,927 patients with early-stage breast cancer during 1998-2011, found that the rate of breast conservation increased from 54% to 59% during that time period and that there were significant barriers to women receiving breast-conserving therapy based on their type of insurance and having a lower socioeconomic status.20
The treatment of breast cancer is best delivered in a multimodality setting, but many inner-city public hospitals do not have such a facility for their patients. QHC is the only public hospital in New York City that has established a comprehensive cancer center. The patient population of QHC is overwhelmingly of minority origin (only 5% of patients are white). In addition, it is a safety net hospital, so no patient is turned away because they cannot pay, and most patients are of lower socioeconomic status and do not have insurance. The purpose of the cancer center was to provide a single site at which our patients could receive all their treatment. It was to ensure that our patients had easy access to care and treatment during all phases of their disease trajectory and did not “fall through the cracks” of the system. Those goals were addressed by having all of the center’s physicians in one place. Physicians involved in care included medical, surgical, and radiation oncologists, a gynecologic oncologist, a genitourinary oncologist, and a thoracic surgery oncologist. The support groups organized for the cancer patients included 3 oncology social workers, an oncology navigator, a nutritionist, a pastoral care supporter, and an oncology psychologist, all located in the same area. All of the clerical and financial aspects of care were also placed within the center. This made the experience as seamless as possible for both the patients and the treating physicians. A “survivors clinic” was established so the cancer patients could be seen by integrated primary care providers to address all noncancer-related health issues such as hypertension, diabetes, or heart disease. Finally, a robust clinical oncology research team was established in the same location. The research included several protocols for new drug treatments for breast cancer from pharmaceutical companies as well as the multi-institutional oncology groups.
Part of the mission of the cancer center was to reach out into the community of Queens to provide education about early detection, cancer prevention, and other public health issues such as tobacco cessation. We established a close working relationship with the Queens Public Library System to connect with their users and dispense information about cancer care and early detection. The Queens Library system is the largest in the United States, and everyone who lives in Queens has easy access to one of its 63 branch libraries. We arranged several lectures about breast cancer awareness in some of the branch libraries. We also procured a mobile mammogram unit for free screening events at the lectures, especially in neighborhoods with a large number residents who were of lower socioeconomic status.
To study the possible effect of these changes on our patients with breast cancer, we compared 2 groups of patients. One group was from the year 2000, a year before the cancer center was opened. The other was from the year 2008, the last year we could get real 5-year survival statistics. We explored how establishing the cancer center might have changed the patients’ stage at diagnosis, care, treatment modalities such as type of surgery, and outcomes. It is difficult to compare these 2 groups because of differences in the patients’ cancers, such as their receptor status, as well as differences in treatment options between the two time periods. However, we had no other way to compare the data to see if there were any trends.
There was a migration to earlier-stage cancer at diagnosis during the 6-year period after the cancer center was opened. It is likely that the educational sessions that were done in the community contributed to this migration. We also saw an increase in the number of mammograms done, from 6,300 in 2000 to 8,800 in 2008. This increase in screening also could account for more patients being identified with earlier-stage disease and might be attributable to the community education through the outreach programs.
As a quality control method, the cancer center has been evaluated by the Commission on Cancer every 3 years. At the 2013 evaluation, we received the Gold Commendation – the highest possible recognition for having 8 out of 8 commendations – and a 3-year accreditation.
There was a notable increase in the use of lumpectomy over mastectomy after the establishment of the cancer center, possibly due to the addition of 2 surgical oncologists to the cancer center’s care team. The integration of multimodiality care for each patient may also have increased the use of breast-conserving surgery.
There was a significant increase from 2000 to 2008 in the survival of patients treated for stage III breast cancer. New drugs and new patterns of adjuvant care might have been partly responsible for that change. The establishment of the comprehensive cancer center with access to new protocols ensured that patients received state-of-the-art cancer treatment. Moreover, the facility addressed all aspects of patient care throughout the disease trajectory by including designated social workers, psychologists, a nutritionist, pastoral care, and patient and survivor support groups to ensure that patients would keep coming to the center for their therapy, with no delays and very little loss to follow-up.
Most patients without insurance were able to acquire emergency Medicaid through the cancer center. This was done by having 2 financial counselors who met with every patient and who could facilitate access to Medicaid as needed. As a result of that, the percentage of patients with no coverage went from 86% in 2000 to 16% in 2008. Before this system was set up, patients who were designated self-pay would pay a fee as low as $15 for each visit and received thousands of dollars’ worth of care. Thus, by forming a cancer center and facilitating patient access to Medicaid, we were able to save money for this public institution because of the gain in revenue from Medicaid.
Our findings suggest that the development of comprehensive cancer centers within inner-city health systems can ensure better treatment for patients of lower socioeconomic status. We present evidence that this may result in increased survival, more sophisticated surgical options, and better patient quality of life. Moreover, this can be achieved while effectively increasing revenue for the public hospitals. Correcting the inequality of access to care and better therapeutic options by setting up comprehensive cancer centers could contribute to improved parity of outcomes for underserved populations.
The author acknowledges the statistical help of Brian Altonen, MPH.
1. Kesson EM, Allardice GM, George WD, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ. 2012;344:e2718.
2. Vrijens F, Stordeur S, Beirens K, Devriese S, Van Eycken E, Vlayen J. Effect of hospital volume on processes of care and 5-year survival after breast cancer: a population-based study on 25000 women. Breast. 2012;21(3):261-266.
3. Bradley CJ, Given CW, Roberts C. Race, socioeconomic status and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490-496.
4. Wheeler SB, Hayes-Reeder KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18:986-993.
5. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78-93.
6. Chen F, Puig M, Yermilov I, et al. Using breast cancer quality indicators in a vulnerable population. Cancer. 2011;117:3311-3321.
7. Banerjee M, George J, Yee C, Hryniuk W, Schwartz K. Disentangling the effects of race on breast cancer treatment. Cancer. 2007;110:2169-2177.
8. Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713-719.
9. Bickell NA, Shastri K, Fei K, et al. A tracking and feedback registry to reduce racial disparities in breast cancer care. J Natl Cancer Inst. 2008;100:1717-1723.
19. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357-1362.
11. Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987-2005). Cancer Epidemiol Biomarkers Prev. 2009;18:121-131.
12. Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9-31.
13. Naik AM, Joseph K, Harris M, Davis C, Shapiro R, Hiotis KL. Indigent breast cancer patients among all racial and ethnic groups present with more advanced disease compared with nationally reported date. Am J Surg. 2003;186:400-403.
14. Hersman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of southwest oncology studies S8814/S8897. J Clin Oncol. 2009;27: 2157-2162.
15
16. Brawley OW. Disaggregating the effects of race and poverty on breast cancer outcomes. J Natl Cancer Inst. 2002;94:471-473.
17. Baquet CR, Commiskey P. Socioeconomic factors and breast carcinoma in multicultural women. Cancer. 2000;88:1256-1264.
18. Cross C, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the US. Cancer. 2002;95:1988-1999.
19. Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: Ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342-1349.
20. Lautner M, Lin H, Shen Y, et al. Disparities in the use of breast-conserving therapy among patients with early-stage breast cancer. JAMA. 2015;150:778-786.
1. Kesson EM, Allardice GM, George WD, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ. 2012;344:e2718.
2. Vrijens F, Stordeur S, Beirens K, Devriese S, Van Eycken E, Vlayen J. Effect of hospital volume on processes of care and 5-year survival after breast cancer: a population-based study on 25000 women. Breast. 2012;21(3):261-266.
3. Bradley CJ, Given CW, Roberts C. Race, socioeconomic status and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490-496.
4. Wheeler SB, Hayes-Reeder KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18:986-993.
5. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78-93.
6. Chen F, Puig M, Yermilov I, et al. Using breast cancer quality indicators in a vulnerable population. Cancer. 2011;117:3311-3321.
7. Banerjee M, George J, Yee C, Hryniuk W, Schwartz K. Disentangling the effects of race on breast cancer treatment. Cancer. 2007;110:2169-2177.
8. Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713-719.
9. Bickell NA, Shastri K, Fei K, et al. A tracking and feedback registry to reduce racial disparities in breast cancer care. J Natl Cancer Inst. 2008;100:1717-1723.
19. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357-1362.
11. Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987-2005). Cancer Epidemiol Biomarkers Prev. 2009;18:121-131.
12. Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9-31.
13. Naik AM, Joseph K, Harris M, Davis C, Shapiro R, Hiotis KL. Indigent breast cancer patients among all racial and ethnic groups present with more advanced disease compared with nationally reported date. Am J Surg. 2003;186:400-403.
14. Hersman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of southwest oncology studies S8814/S8897. J Clin Oncol. 2009;27: 2157-2162.
15
16. Brawley OW. Disaggregating the effects of race and poverty on breast cancer outcomes. J Natl Cancer Inst. 2002;94:471-473.
17. Baquet CR, Commiskey P. Socioeconomic factors and breast carcinoma in multicultural women. Cancer. 2000;88:1256-1264.
18. Cross C, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the US. Cancer. 2002;95:1988-1999.
19. Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: Ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342-1349.
20. Lautner M, Lin H, Shen Y, et al. Disparities in the use of breast-conserving therapy among patients with early-stage breast cancer. JAMA. 2015;150:778-786.
Diagnosis and treatment of hyperkalemia
Hyperkalemia is common in patients with cardiovascular disease. Its consequences can be severe and life-threatening, and its management and prevention require a multidisciplinary approach that entails reducing intake of high-potassium foods, adjusting medications that cause hyperkalemia, and adding medications that reduce the plasma potassium concentration. With this approach, patients at high risk can receive the cardiorenal benefits of drugs that block the renin-angiotensin-aldosterone system without developing hyperkalemia.
98% OF POTASSIUM IS INSIDE CELLS
The body of a typical 70-kg man contains about 3,500 mmol of potassium, 98% of which is in the intracellular space; the remaining 2% is in the extracellular space. This large intracellular-to-extracellular gradient determines the cell voltage and explains why disorders in plasma potassium give rise to manifestations in excitable tissues such as the heart and nervous system.
The most important determinants of potassium distribution between the intracellular and extracellular space are insulin and beta-adrenergic receptor stimulation.
Maintenance of total-body potassium content is primarily the job of the kidneys, with a small contribution by the gastrointestinal tract.1,2 Hyperkalemia is most commonly encountered in patients with decreased kidney function.
The normal kidney can secrete a large amount of potassium, making hyperkalemia uncommon in the absence of kidney disease. This large capacity may have evolved to handle the diet of Paleolithic humans, which contained 4 times as much potassium as contemporary diets.3,4 With the onset of agriculture, dietary intake of potassium has progressively declined while sodium intake has risen. A popular theory suggests this mismatch between the modern diet and the nutritional requirements encoded in the human genome during evolution may contribute to chronic diseases such as hypertension, stroke, kidney stones, and bone disease.5
MANY POTENTIAL CAUSES OF HYPERKALEMIA
Causes of hyperkalemia are outlined in Table 1. Shifting of potassium from the cells to the extracellular space is a cause of transient hyperkalemia, while chronic hyperkalemia indicates an impairment in renal potassium secretion. The following discussion is a guide to the approach to the hyperkalemic patient.
Is the patient’s hyperkalemia really pseudohyperkalemia?
Pseudohyperkalemia, an artifact of measurement, occurs due to mechanical release of potassium from cells during phlebotomy or specimen processing.6 This diagnosis is made when the serum potassium concentration exceeds the plasma potassium concentration by more than 0.5 mmol/L, and should be considered when hyperkalemia occurs in the absence of a clinical risk factor. Fist-clenching, application of a tight-fitting tourniquet, or use of small-bore needles during phlebotomy can all cause pseudohyperkalemia.
Mechanism of pseudohyperkalemia. Since serum is the liquid part of blood remaining after coagulation, release of potassium from cells injured during the process of coagulation raises the potassium level in the serum. Plasma is the cell-free part of blood that has been treated with anticoagulants; it has no cells that can be injured and release potassium. Thus, the serum potassium level will be higher than that in the plasma.
Reverse pseudohyperkalemia, in contrast, occurs when the plasma potassium level is falsely elevated but the serum value is normal. This situation has been described in hematologic disorders characterized by pronounced leukocytosis in which malignant cells are prone to lysis with minimal mechanical stress due to increased fragility or altered sodium-potassium ATPase pump activity.7 This phenomenon is unusual but occurs because the cells are so fragile.
A spurious increase in plasma potassium concentration along with a low plasma calcium concentration raises the possibility of calcium chelation and release of potassium in a sample tube contaminated with the anticoagulant ethylenediaminetetraacetic acid.
Is there increased potassium intake?
Increased potassium intake is a potential cause of hyperkalemia in patients with decreased kidney function or adrenal disease.
Foods naturally rich in potassium include bananas (a medium-sized banana contains 451 mg or 12 mmol of potassium) and potatoes (844 mg or 22 mmol in a large baked potato with skin). Other potassium-rich foods are melons, citrus juice, and avocados. Less-obvious food sources include raw coconut juice (potassium concentration 44.3 mmol/L) and noni juice (56 mmol/L).
Salt substitutes, recommended to hypertensive patients with chronic kidney disease, can be a hidden source of dietary potassium.
Clay ingestion is a potential cause of dyskalemia. White clay consumption causes hypokalemia due to potassium binding in the gastrointestinal tract. Red clay or river bed clay, on the other hand, is enriched in potassium (100 mmol of potassium in 100 g of clay) and can cause life-threatening hyperkalemia in patients with chronic kidney disease.8
Eating burnt match heads. Some individuals chew and ingest burnt match heads, a condition called cautopyreiophagia. In one reported case,9 this activity contributed an additional 80 mmol of daily potassium intake in a dialysis patient, resulting in a plasma potassium concentration of 8 mmol/L.
Is the hyperkalemia the result of a cellular shift?
Acute hyperkalemia can be the result of redistribution of cellular potassium. Shifting of as little as 2% of the body’s potassium from the intracellular to the extracellular space can double the plasma potassium concentration.
Tissue injury. Hyperkalemia frequently occurs in diseases that cause tissue injury such as rhabdomyolysis, trauma, massive hemolysis, and tumor lysis.
Insulin deficiency. Insulin and catecholamines are major regulators of potassium distribution within the body. After a meal, release of insulin not only regulates the plasma glucose concentration, it also causes potassium to move into cells until the kidneys have had sufficient time to excrete the dietary potassium load and reestablish total-body potassium content.
Exercise, beta-blockers. During exercise, potassium is released from skeletal muscle cells and accumulates in the interstitial compartment, where it exerts a vasodilatory effect. The simultaneous increase in circulating catecholamines regulates this release by promoting cell potassium uptake through beta-adrenergic receptor stimulation.
Metabolic acidosis can facilitate exit (ie, shift) of potassium from cells, but this effect depends on the type of acidosis. Hyperchloremic normal anion gap acidosis (mineral acidosis) most commonly causes this effect due to the relative impermeability of the cell membrane to the chloride anion. As hydrogen ions move into the cell due to accumulation of ammonium chloride or hydrogen chloride, electrical neutrality is maintained by potassium exit.
In contrast, organic acidosis (due to lactic, beta-hydroxybutyric, or methylmalonic acid) tends not to cause a potassium shift, since most organic anions readily cross the cell membrane along with hydrogen. Lactic acidosis is often associated with potassium shift, but this effect is due to loss of cell integrity as a result of cell ischemia. The hyperkalemia typically present on admission in patients with diabetic ketoacidosis is the result of insulin deficiency and hypertonicity and not the underlying organic acidosis.10
Hypertonic states can cause hyperkalemia due to cell shift. For example, hyperglycemia, as in diabetic ketoacidosis, pulls water from the intracellular into the extracellular compartment, thereby concentrating intracellular potassium and creating a more favorable gradient for potassium efflux through membrane channels. This same effect can occur in neurosurgical patients given large amounts of hypertonic mannitol. Repetitive doses of immunoglobulin can lead to extracellular accumulation of sorbitol, maltose, or sucrose, since these sugars are added to the preparations to prevent immunoglobulin aggregation.11
Is a disturbance in renal potassium excretion present?
Sustained hyperkalemia is more commonly associated with decreases in renal potassium excretion than with a cellular shift. In most instances the clinician can distinguish between cell shift and impaired renal excretion based on the available clinical data.
The transtubular potassium gradient has been used to determine whether there is a disturbance in renal potassium excretion and to assess renal potassium handling.12
This calculation is based on the assumption that only water is reabsorbed past the cortical collecting duct, and not solutes. It has fallen out of favor since we have found this assumption to be incorrect; a large amount of urea is reabsorbed daily in the downstream medullary collecting duct as a result of intrarenal recycling of urea.
The one situation in which the transtubular potassium gradient may be of use is determining whether hyperkalemia is a result of low aldosterone levels as opposed to aldosterone resistance. One can compare the transtubular potassium gradient before and after a physiologic dose (0.05 mg) of 9-alpha fludrocortisone. An increase of more than 6 over a 4-hour period favors aldosterone deficiency, whereas smaller changes would indicate aldosterone resistance.
24-hour potassium excretion, spot urine potassium-creatinine ratio. A better way to assess renal potassium handling is to measure the amount of potassium in a 24-hour urine collection or determine a spot urine potassium-creatinine ratio. A 24-hour urinary potassium excretion of less than 15 mmol or a potassium-creatinine ratio less than 1 suggests an extrarenal cause of hypokalemia. A ratio greater than 20 would be an appropriate renal response to hyperkalemia.
One or more of 3 abnormalities should be considered in the hyperkalemic patient with impaired renal excretion of potassium:
- Decreased distal delivery of sodium
- Mineralocorticoid deficiency
- Abnormal cortical collecting tubule function.13
Decreased distal delivery of sodium
Under normal circumstances, potassium is freely filtered across the glomerulus and then mostly reabsorbed in the proximal tubule and thick ascending limb. Potassium secretion begins in the distal convoluted tubule and increases in magnitude into the collecting duct. Tubular secretion is the component of potassium handling that varies and is regulated according to physiologic needs.
In acute kidney injury, the rapid decline in glomerular filtration rate and reduction in functioning nephron mass lead to decreased distal potassium secretion.
Hyperkalemia is a frequent problem when oliguria is present, since the reduction in distal delivery of sodium and water further impairs potassium secretion. Patients with oliguric acute kidney injury are more likely to have a more severe underlying disease state, and therefore tissue breakdown and catabolism further increase the risk of hyperkalemia.
In contrast, in nonoliguric patients, the renal injury tends to be less severe, and enough sodium and water are usually delivered distally to prevent hyperkalemia.
In chronic kidney disease, nephron dropout and reduction in collecting tubule mass also lead to a global decline in distal potassium secretion. However, this is countered by an increased capacity of the remaining individual nephrons for potassium secretion. High flow, increased distal sodium delivery, and increased activity and number of sodium-potassium ATPase pumps in the remaining nephrons account for this increased secretory capacity.14 As renal function declines over time, colonic potassium secretion progressively increases.15
These adaptive changes help to keep the plasma potassium concentration within the normal range until the glomerular filtration rate falls to less than 10 or 15 mL/min. Development of hyperkalemia with more modest reductions in the glomerular filtration rate suggest decreased mineralocorticoid activity or a specific lesion of the tubule.
Mineralocorticoid deficiency
Aldosterone deficiency can occur alone or in combination with decreased cortisol levels. Destruction of the adrenal glands is suggested when both hormones are reduced. Enzyme defects in cortisol metabolism can result in either isolated deficiency of aldosterone or adrenogenital syndromes associated with decreased mineralocorticoid activity.
Heparin administration leads to a reversible defect in adrenal synthesis of aldosterone. Drugs that block the stimulatory effect of angiotensin II on the zona glomerulosa cells of the adrenal gland will lower aldosterone.
Renin-angiotensin-aldosterone system blockers. Angiotensin-converting enzyme inhibitors block the formation of angiotensin II, whereas angiotensin II receptor blockers prevent angiotensin II from binding to its adrenal receptor. The direct renin inhibitor aliskiren lowers angiotensin II levels by blocking the enzymatic activity of renin and lowers the circulating levels of both angiotensin I and II.16
The syndrome of hyporeninemic hypoaldosteronism is a common cause of hyperkalemia in patients who have a glomerular filtration rate between 40 and 60 mL/min. Diabetic nephropathy and interstitial renal disease are the most common clinical entities associated with this syndrome.10 Other causes include analgesic nephropathy, urinary tract obstruction, sickle cell disease, systemic lupus erythematosus, and amyloidosis.
Nonsteroidal anti-inflammatory drugs can cause hyperkalemia by suppressing renin release and reducing delivery of sodium to the distal nephron.18
Calcineurin inhibitors impair potassium secretion by suppressing renin release and by direct tubular effects.19
Beta-blockers. Beta-1 and to a lesser extent beta-2 receptor blockade can also result in a hyporeninemic state.
Distal tubular defect
Hyperkalemia can result from interstitial renal diseases that specifically affect the distal nephron. In this setting, the glomerular filtration rate is only mildly reduced, and circulating aldosterone levels are normal.
Renal transplant, lupus erythematosus, amyloidosis, urinary obstruction, and sickle cell disease are conditions in which an impairment in renin release may coexist with a defect in tubular secretion.
Potassium-sparing diuretics impair the ability of the cortical collecting tubule to secrete potassium. Specifically, amiloride and triamterene inhibit sodium reabsorption mediated by the epithelial sodium channel located on the apical membrane of the principal cell. This effect abolishes the lumen’s negative potential and thereby removes a driving force for potassium secretion.
Trimethoprim and pentamidine cause similar effects.
Spironolactone and eplerenone compete with aldosterone at the level of the mineralocorticoid receptor and can result in hyperkalemia.
Drospirenone, a non-testosterone-derived progestin contained in certain oral contraceptives, possesses mineralocorticoid-blocking effects similar to those of spironolactone.
The plasma potassium level should be monitored when these drugs are prescribed in patients receiving potassium supplements, renin-angiotensin-aldosterone system blockers, or nonsteroidal anti-inflammatory drugs.20
CLINICAL FEATURES OF HYPERKALEMIA
Neuromuscular manifestations of hyperkalemia include paresthesias and fasciculations in the arms and legs. Severe elevation in potassium can give rise to an ascending paralysis with eventual flaccid quadriplegia. Typically, the trunk, head, and respiratory muscles are spared, and respiratory failure is rare.
Cardiac signs
Hyperkalemia has depolarizing effects on the heart that are manifested by changes in the electrocardiogram (Figure 2). The progressive changes of hyperkalemia are classically listed as:
- Peaked T waves that are tall, narrow, and symmetrical and can occasionally be confused with the hyperacute T-wave change associated with an ST-segment elevation myocardial infarction.21 However, in the latter condition, the T waves tend to be more broad-based and asymmetric in shape.
- ST-segment depression
- Widening of the PR interval
- Widening of the QRS interval
- Loss of the P wave
- A sine-wave pattern—an ominous development and a harbinger of impending ventricular fibrillation and asystole.
The plasma potassium concentration often correlates poorly with cardiac manifestations. In a retrospective review, only 16 of 90 cases met strict criteria for electrocardiographic changes reflective of hyperkalemia (defined as new peaked and symmetric T waves that resolved on follow-up).22 In 13 of these cases, the electrocardiogram was interpreted as showing no T-wave changes even when read by a cardiologist. In addition, electrocardiographic criteria for hyperkalemia were noted in only 1 of 14 patients who manifested arrhythmias or cardiac arrest attributed to increased plasma potassium concentration.
TREATMENT OF ACUTE HYPERKALEMIA
The treatment of hyperkalemia depends on the magnitude of increase in the plasma potassium concentration and the presence or absence of electrocardiographic changes or neuromuscular symptoms.23 Acute treatment is indicated for marked electrocardiographic changes and severe muscle weakness.
Intravenous calcium rapidly normalizes membrane excitability by antagonizing the potassium-induced decrease in membrane excitability but does not alter the plasma potassium concentration.
Insulin lowers the plasma potassium concentration by promoting its entry into cells. To avoid hypoglycemia, 10 units of short-acting insulin should be accompanied by a 50-g infusion of glucose, increased to 60 g if 20 units of insulin are given.24
Beta-2 receptor agonists produce a similar effect. The shift of potassium into cells with insulin and beta-2-adrenergic receptor stimulation is brought about by increases in sodium-potassium ATPase pump activity, primarily in skeletal muscle cells.
Sodium bicarbonate, in the absence of acidosis, lowers the plasma potassium concentration only slightly. It should be reserved for hyperkalemic patients who have coexisting metabolic acidosis after the patient has received insulin and glucose, an adrenergic agent, and calcium.
These acute treatments need to be followed by therapies designed to lower the total body potassium content such as diuretics, potassium-binding drugs, and dialysis.
TREATMENT OF CHRONIC HYPERKALEMIA
Review medications. Once the diagnosis of hyperkalemia has been made, the initial approach should be to review the patient’s medications and make every effort to discontinue drugs that can impair renal potassium excretion.16 Patients should be asked about their use of over-the-counter nonsteroidal anti-inflammatory drugs and herbal remedies, since herbs may be a hidden source of dietary potassium.
Dietary counseling. Patients should be instructed to reduce their dietary intake of potassium and to avoid salt substitutes that contain potassium.
Diuretic therapy is beneficial in minimizing hyperkalemia in patients with chronic kidney disease. Thiazide and loop diuretics enhance renal potassium excretion by increasing flow and delivery of sodium to the collecting duct. Thiazide diuretics are effective when the estimated glomerular filtration rate is greater than 30 mL/min, while loop diuretics should be used in patients with more severe renal insufficiency (Table 2).
Sodium bicarbonate is an effective agent to minimize increases in the plasma potassium concentration in patients with chronic kidney disease and metabolic acidosis. This drug increases renal potassium excretion by increasing distal sodium delivery and shifts potassium into cells as the acidosis is corrected. The likelihood of developing volume overload as a complication of sodium bicarbonate administration can be minimized with effective diuretic therapy.
Avoiding hyperkalemia if renin-angiotensin-aldosterone system blockers are needed
Renin-angiotensin-aldosterone system blockers can be problematic, as these drugs cause hyperkalemia, often in the very patients who derive the greatest cardiovascular benefit from them.16 A number of steps can reduce the risk of hyperkalemia and allow these drugs to be used.
The initial dose should be low and the plasma potassium should be measured within 1 to 2 weeks after drug initiation. If the potassium level is normal, the dose can be titrated upwards with remeasurement of the plasma potassium after each dose titration. If the plasma potassium concentration rises to 5.5 mmol/L, in some cases lowering the dose will reduce the potassium concentration and allow the patient to remain on the drug.
In patients at risk of hyperkalemia, angiotensin II receptor blockers and direct renin inhibitors should be used with the same caution as angiotensin-converting enzyme inhibitors.
If the plasma potassium concentration exceeds 5.5 mmol/L despite the above precautions, one can consider using a potassium-binding drug (see below) before deciding to avoid renin-angiotensin-aldosterone system blockers.
Sodium polystyrene sulfonate binds potassium in the gastrointestinal tract in exchange for sodium and has been used to manage hyperkalemia. This drug is most commonly given along with sorbitol as a therapy for acute hyperkalemia. Although the drug is widely used, most of the potassium-lowering effect is due to an increase in stool volume caused by sorbitol.25,26 In addition, long-term use is poorly tolerated, and the drug has been linked to gastrointestinal toxicity in rare cases.
Patiromer and sodium zirconium cyclosilicate are two new potassium-binding drugs that have been shown to be effective in reducing plasma potassium concentration in the setting of ongoing use of renin-angiotensin-aldosterone system blockers.
Patiromer is a nonabsorbed polymer approved for clinical use to treat hyperkalemia. The drug binds potassium in exchange for calcium in the gastrointestinal tract, predominantly in the colon, and lowers the plasma potassium concentration in a dose-dependent manner, with the greatest reduction in those with higher starting values.27,28
Patiromer effectively controlled plasma potassium concentrations in a 1-year randomized trial in high-risk patients on renin-angiotensin-aldosterone system blockers.29 The main adverse events in clinical trials have been constipation and hypomagnesemia, which required magnesium replacement in a small number of patients, but overall, the drug is well tolerated.
Sodium zirconium cyclosilicate is a nonabsorbed microporous compound that binds potassium in exchange for sodium throughout the gastrointestinal tract. It has been found effective in lowering plasma potassium concentration in a dose-dependent fashion in high-risk patients, most of whom were receiving renin-angiotensin-aldosterone system blockers.30–32 Adverse events were generally comparable to those with placebo in clinical trials; however, edema occurred more frequently when higher doses were used. This drug is not yet approved for clinical use.
- Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ 2016; 40:480–490.
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10:1050–1060.
- Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med 1985; 312:283–289.
- Sebastian A, Frassetto LA, Sellmeyer DE, Morris RC Jr. The evolution-informed optimal dietary potassium intake of human beings greatly exceeds current and recommended intakes. Semin Nephrol 2006; 26:447–453.
- Palmer BF, Clegg DJ. Achieving the benefits of a high potassium, Paleolithic diet, without the toxicity. Mayo Clin Proc 2016; 91:496–508.
- Liamis G, Liberopoulos E, Barkas F, Elisaf M. Spurious electrolyte disorders: a diagnostic challenge for clinicians. Am J Nephrol 2013; 38:50–57.
- Mansoor S, Holtzman N, Emadi A. Reverse pseudohyperkalemia: an important clinical entity in chronic lymphocytic leukemia. Case Rep Hematol 2015; 2015:930379.
- Gelfand M, Zarate A, Knepshield J. Geophagia. A cause of life-threatening hyperkalemia in patients with chronic renal failure. JAMA 1975; 234:738–740.
- Abu-Hamdan D, Sondheimer J, Mahajan S. Cautopyreiophagia. Cause of life-threatening hyperkalemia in a patient undergoing hemodialysis. Am J Med 1985; 79:517–519.
- Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med 2015; 373:548–559.
- Daphnis E, Stylianou K, Alexandrakis M, et al. Acute renal failure, translocational hyponatremia and hyperkalemia following intravenous immunoglobulin therapy. Nephron Clin Pract 2007; 106:c143–c148.
- Choi M, Ziyadeh F. The utility of the transtubular potassium gradient in the evaluation of hyperkalemia. J Am Soc Nephrol 2008; 19:424–426.
- Palmer BF. A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am J Kidney Dis 2010; 56:387–393.
- Stanton BA. Renal potassium transport: morphological and functional adaptations. Am J Physiol 1989; 257:R989–R997.
- Hayes CP Jr, McLeod ME, Robinson RR. An extravenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physicians 1967; 80:207–216.
- Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med 2004; 351:585–592.
- Palmer BF. Renal dysfunction complicating treatment of hypertension. N Engl J Med 2002; 347:1256–1261.
- Palmer BF. Renal complications associated with use of nonsteroidal anti-inflammatory agents. J Investig Med 1995; 43:516–533.
- Hoorn E, Walsh S, McCormick J, et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med 2011; 17:1304–1309.
- Bird ST, Pepe SR, Etminan M, Liu X, Brophy JM, Delaney JA. The association between drospirenone and hyperkalemia: a comparative-safety study. BMC Clin Pharmacol 2011; 11:23.
- Wang K. Images in clinical medicine. “Pseudoinfarction” pattern due to hyperkalemia. N Engl J Med 2004; 351:593.
- Montague BT, Ouellette JR, Buller GK. Retrospective review of the frequency of ECG changes in hyperkalemia. Clin J Am Soc Nephrol 2008; 3:324–330.
- Weisberg LS. Management of severe hyperkalemia. Crit Care Med 2008; 36:3246–3251.
- Harel Z, Kamel KS. Optimal dose and method of administration of intravenous insulin in the management of emergency hyperkalemia: a systematic review. PLoS One 2016; 11:e0154963.
- Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol 2010; 21:733–735.
- Emmett M, Hootkins RE, Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Effect of three laxatives and a cation exchange resin on fecal sodium and potassium excretion. Gastroenterology 1995; 108:752–760.
- Bushinsky DA, Spiegel DM, Gross C, et al. Effect of patiromer on urinary ion excretion in healthy adults. Clin J Am Soc Nephrol 2016; 11:1769–1776.
- Weir MR, Bakris GL, Bushinsky DA, et al; OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015; 372:211–221.
- Bakris GL, Pitt B, Weir MR, et al; AMETHYST-DN Investigators. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA 2015; 314:151–161.
- Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia. The HARMONIZE randomized clinical trial. JAMA 2014; 312:2223–2233.
- Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 2015; 372:222–231.
- Anker SD, Kosiborod M, Zannad F, et al. Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial. Eur J Heart Fail 2015; 17:1050–1056.
Hyperkalemia is common in patients with cardiovascular disease. Its consequences can be severe and life-threatening, and its management and prevention require a multidisciplinary approach that entails reducing intake of high-potassium foods, adjusting medications that cause hyperkalemia, and adding medications that reduce the plasma potassium concentration. With this approach, patients at high risk can receive the cardiorenal benefits of drugs that block the renin-angiotensin-aldosterone system without developing hyperkalemia.
98% OF POTASSIUM IS INSIDE CELLS
The body of a typical 70-kg man contains about 3,500 mmol of potassium, 98% of which is in the intracellular space; the remaining 2% is in the extracellular space. This large intracellular-to-extracellular gradient determines the cell voltage and explains why disorders in plasma potassium give rise to manifestations in excitable tissues such as the heart and nervous system.
The most important determinants of potassium distribution between the intracellular and extracellular space are insulin and beta-adrenergic receptor stimulation.
Maintenance of total-body potassium content is primarily the job of the kidneys, with a small contribution by the gastrointestinal tract.1,2 Hyperkalemia is most commonly encountered in patients with decreased kidney function.
The normal kidney can secrete a large amount of potassium, making hyperkalemia uncommon in the absence of kidney disease. This large capacity may have evolved to handle the diet of Paleolithic humans, which contained 4 times as much potassium as contemporary diets.3,4 With the onset of agriculture, dietary intake of potassium has progressively declined while sodium intake has risen. A popular theory suggests this mismatch between the modern diet and the nutritional requirements encoded in the human genome during evolution may contribute to chronic diseases such as hypertension, stroke, kidney stones, and bone disease.5
MANY POTENTIAL CAUSES OF HYPERKALEMIA
Causes of hyperkalemia are outlined in Table 1. Shifting of potassium from the cells to the extracellular space is a cause of transient hyperkalemia, while chronic hyperkalemia indicates an impairment in renal potassium secretion. The following discussion is a guide to the approach to the hyperkalemic patient.
Is the patient’s hyperkalemia really pseudohyperkalemia?
Pseudohyperkalemia, an artifact of measurement, occurs due to mechanical release of potassium from cells during phlebotomy or specimen processing.6 This diagnosis is made when the serum potassium concentration exceeds the plasma potassium concentration by more than 0.5 mmol/L, and should be considered when hyperkalemia occurs in the absence of a clinical risk factor. Fist-clenching, application of a tight-fitting tourniquet, or use of small-bore needles during phlebotomy can all cause pseudohyperkalemia.
Mechanism of pseudohyperkalemia. Since serum is the liquid part of blood remaining after coagulation, release of potassium from cells injured during the process of coagulation raises the potassium level in the serum. Plasma is the cell-free part of blood that has been treated with anticoagulants; it has no cells that can be injured and release potassium. Thus, the serum potassium level will be higher than that in the plasma.
Reverse pseudohyperkalemia, in contrast, occurs when the plasma potassium level is falsely elevated but the serum value is normal. This situation has been described in hematologic disorders characterized by pronounced leukocytosis in which malignant cells are prone to lysis with minimal mechanical stress due to increased fragility or altered sodium-potassium ATPase pump activity.7 This phenomenon is unusual but occurs because the cells are so fragile.
A spurious increase in plasma potassium concentration along with a low plasma calcium concentration raises the possibility of calcium chelation and release of potassium in a sample tube contaminated with the anticoagulant ethylenediaminetetraacetic acid.
Is there increased potassium intake?
Increased potassium intake is a potential cause of hyperkalemia in patients with decreased kidney function or adrenal disease.
Foods naturally rich in potassium include bananas (a medium-sized banana contains 451 mg or 12 mmol of potassium) and potatoes (844 mg or 22 mmol in a large baked potato with skin). Other potassium-rich foods are melons, citrus juice, and avocados. Less-obvious food sources include raw coconut juice (potassium concentration 44.3 mmol/L) and noni juice (56 mmol/L).
Salt substitutes, recommended to hypertensive patients with chronic kidney disease, can be a hidden source of dietary potassium.
Clay ingestion is a potential cause of dyskalemia. White clay consumption causes hypokalemia due to potassium binding in the gastrointestinal tract. Red clay or river bed clay, on the other hand, is enriched in potassium (100 mmol of potassium in 100 g of clay) and can cause life-threatening hyperkalemia in patients with chronic kidney disease.8
Eating burnt match heads. Some individuals chew and ingest burnt match heads, a condition called cautopyreiophagia. In one reported case,9 this activity contributed an additional 80 mmol of daily potassium intake in a dialysis patient, resulting in a plasma potassium concentration of 8 mmol/L.
Is the hyperkalemia the result of a cellular shift?
Acute hyperkalemia can be the result of redistribution of cellular potassium. Shifting of as little as 2% of the body’s potassium from the intracellular to the extracellular space can double the plasma potassium concentration.
Tissue injury. Hyperkalemia frequently occurs in diseases that cause tissue injury such as rhabdomyolysis, trauma, massive hemolysis, and tumor lysis.
Insulin deficiency. Insulin and catecholamines are major regulators of potassium distribution within the body. After a meal, release of insulin not only regulates the plasma glucose concentration, it also causes potassium to move into cells until the kidneys have had sufficient time to excrete the dietary potassium load and reestablish total-body potassium content.
Exercise, beta-blockers. During exercise, potassium is released from skeletal muscle cells and accumulates in the interstitial compartment, where it exerts a vasodilatory effect. The simultaneous increase in circulating catecholamines regulates this release by promoting cell potassium uptake through beta-adrenergic receptor stimulation.
Metabolic acidosis can facilitate exit (ie, shift) of potassium from cells, but this effect depends on the type of acidosis. Hyperchloremic normal anion gap acidosis (mineral acidosis) most commonly causes this effect due to the relative impermeability of the cell membrane to the chloride anion. As hydrogen ions move into the cell due to accumulation of ammonium chloride or hydrogen chloride, electrical neutrality is maintained by potassium exit.
In contrast, organic acidosis (due to lactic, beta-hydroxybutyric, or methylmalonic acid) tends not to cause a potassium shift, since most organic anions readily cross the cell membrane along with hydrogen. Lactic acidosis is often associated with potassium shift, but this effect is due to loss of cell integrity as a result of cell ischemia. The hyperkalemia typically present on admission in patients with diabetic ketoacidosis is the result of insulin deficiency and hypertonicity and not the underlying organic acidosis.10
Hypertonic states can cause hyperkalemia due to cell shift. For example, hyperglycemia, as in diabetic ketoacidosis, pulls water from the intracellular into the extracellular compartment, thereby concentrating intracellular potassium and creating a more favorable gradient for potassium efflux through membrane channels. This same effect can occur in neurosurgical patients given large amounts of hypertonic mannitol. Repetitive doses of immunoglobulin can lead to extracellular accumulation of sorbitol, maltose, or sucrose, since these sugars are added to the preparations to prevent immunoglobulin aggregation.11
Is a disturbance in renal potassium excretion present?
Sustained hyperkalemia is more commonly associated with decreases in renal potassium excretion than with a cellular shift. In most instances the clinician can distinguish between cell shift and impaired renal excretion based on the available clinical data.
The transtubular potassium gradient has been used to determine whether there is a disturbance in renal potassium excretion and to assess renal potassium handling.12
This calculation is based on the assumption that only water is reabsorbed past the cortical collecting duct, and not solutes. It has fallen out of favor since we have found this assumption to be incorrect; a large amount of urea is reabsorbed daily in the downstream medullary collecting duct as a result of intrarenal recycling of urea.
The one situation in which the transtubular potassium gradient may be of use is determining whether hyperkalemia is a result of low aldosterone levels as opposed to aldosterone resistance. One can compare the transtubular potassium gradient before and after a physiologic dose (0.05 mg) of 9-alpha fludrocortisone. An increase of more than 6 over a 4-hour period favors aldosterone deficiency, whereas smaller changes would indicate aldosterone resistance.
24-hour potassium excretion, spot urine potassium-creatinine ratio. A better way to assess renal potassium handling is to measure the amount of potassium in a 24-hour urine collection or determine a spot urine potassium-creatinine ratio. A 24-hour urinary potassium excretion of less than 15 mmol or a potassium-creatinine ratio less than 1 suggests an extrarenal cause of hypokalemia. A ratio greater than 20 would be an appropriate renal response to hyperkalemia.
One or more of 3 abnormalities should be considered in the hyperkalemic patient with impaired renal excretion of potassium:
- Decreased distal delivery of sodium
- Mineralocorticoid deficiency
- Abnormal cortical collecting tubule function.13
Decreased distal delivery of sodium
Under normal circumstances, potassium is freely filtered across the glomerulus and then mostly reabsorbed in the proximal tubule and thick ascending limb. Potassium secretion begins in the distal convoluted tubule and increases in magnitude into the collecting duct. Tubular secretion is the component of potassium handling that varies and is regulated according to physiologic needs.
In acute kidney injury, the rapid decline in glomerular filtration rate and reduction in functioning nephron mass lead to decreased distal potassium secretion.
Hyperkalemia is a frequent problem when oliguria is present, since the reduction in distal delivery of sodium and water further impairs potassium secretion. Patients with oliguric acute kidney injury are more likely to have a more severe underlying disease state, and therefore tissue breakdown and catabolism further increase the risk of hyperkalemia.
In contrast, in nonoliguric patients, the renal injury tends to be less severe, and enough sodium and water are usually delivered distally to prevent hyperkalemia.
In chronic kidney disease, nephron dropout and reduction in collecting tubule mass also lead to a global decline in distal potassium secretion. However, this is countered by an increased capacity of the remaining individual nephrons for potassium secretion. High flow, increased distal sodium delivery, and increased activity and number of sodium-potassium ATPase pumps in the remaining nephrons account for this increased secretory capacity.14 As renal function declines over time, colonic potassium secretion progressively increases.15
These adaptive changes help to keep the plasma potassium concentration within the normal range until the glomerular filtration rate falls to less than 10 or 15 mL/min. Development of hyperkalemia with more modest reductions in the glomerular filtration rate suggest decreased mineralocorticoid activity or a specific lesion of the tubule.
Mineralocorticoid deficiency
Aldosterone deficiency can occur alone or in combination with decreased cortisol levels. Destruction of the adrenal glands is suggested when both hormones are reduced. Enzyme defects in cortisol metabolism can result in either isolated deficiency of aldosterone or adrenogenital syndromes associated with decreased mineralocorticoid activity.
Heparin administration leads to a reversible defect in adrenal synthesis of aldosterone. Drugs that block the stimulatory effect of angiotensin II on the zona glomerulosa cells of the adrenal gland will lower aldosterone.
Renin-angiotensin-aldosterone system blockers. Angiotensin-converting enzyme inhibitors block the formation of angiotensin II, whereas angiotensin II receptor blockers prevent angiotensin II from binding to its adrenal receptor. The direct renin inhibitor aliskiren lowers angiotensin II levels by blocking the enzymatic activity of renin and lowers the circulating levels of both angiotensin I and II.16
The syndrome of hyporeninemic hypoaldosteronism is a common cause of hyperkalemia in patients who have a glomerular filtration rate between 40 and 60 mL/min. Diabetic nephropathy and interstitial renal disease are the most common clinical entities associated with this syndrome.10 Other causes include analgesic nephropathy, urinary tract obstruction, sickle cell disease, systemic lupus erythematosus, and amyloidosis.
Nonsteroidal anti-inflammatory drugs can cause hyperkalemia by suppressing renin release and reducing delivery of sodium to the distal nephron.18
Calcineurin inhibitors impair potassium secretion by suppressing renin release and by direct tubular effects.19
Beta-blockers. Beta-1 and to a lesser extent beta-2 receptor blockade can also result in a hyporeninemic state.
Distal tubular defect
Hyperkalemia can result from interstitial renal diseases that specifically affect the distal nephron. In this setting, the glomerular filtration rate is only mildly reduced, and circulating aldosterone levels are normal.
Renal transplant, lupus erythematosus, amyloidosis, urinary obstruction, and sickle cell disease are conditions in which an impairment in renin release may coexist with a defect in tubular secretion.
Potassium-sparing diuretics impair the ability of the cortical collecting tubule to secrete potassium. Specifically, amiloride and triamterene inhibit sodium reabsorption mediated by the epithelial sodium channel located on the apical membrane of the principal cell. This effect abolishes the lumen’s negative potential and thereby removes a driving force for potassium secretion.
Trimethoprim and pentamidine cause similar effects.
Spironolactone and eplerenone compete with aldosterone at the level of the mineralocorticoid receptor and can result in hyperkalemia.
Drospirenone, a non-testosterone-derived progestin contained in certain oral contraceptives, possesses mineralocorticoid-blocking effects similar to those of spironolactone.
The plasma potassium level should be monitored when these drugs are prescribed in patients receiving potassium supplements, renin-angiotensin-aldosterone system blockers, or nonsteroidal anti-inflammatory drugs.20
CLINICAL FEATURES OF HYPERKALEMIA
Neuromuscular manifestations of hyperkalemia include paresthesias and fasciculations in the arms and legs. Severe elevation in potassium can give rise to an ascending paralysis with eventual flaccid quadriplegia. Typically, the trunk, head, and respiratory muscles are spared, and respiratory failure is rare.
Cardiac signs
Hyperkalemia has depolarizing effects on the heart that are manifested by changes in the electrocardiogram (Figure 2). The progressive changes of hyperkalemia are classically listed as:
- Peaked T waves that are tall, narrow, and symmetrical and can occasionally be confused with the hyperacute T-wave change associated with an ST-segment elevation myocardial infarction.21 However, in the latter condition, the T waves tend to be more broad-based and asymmetric in shape.
- ST-segment depression
- Widening of the PR interval
- Widening of the QRS interval
- Loss of the P wave
- A sine-wave pattern—an ominous development and a harbinger of impending ventricular fibrillation and asystole.
The plasma potassium concentration often correlates poorly with cardiac manifestations. In a retrospective review, only 16 of 90 cases met strict criteria for electrocardiographic changes reflective of hyperkalemia (defined as new peaked and symmetric T waves that resolved on follow-up).22 In 13 of these cases, the electrocardiogram was interpreted as showing no T-wave changes even when read by a cardiologist. In addition, electrocardiographic criteria for hyperkalemia were noted in only 1 of 14 patients who manifested arrhythmias or cardiac arrest attributed to increased plasma potassium concentration.
TREATMENT OF ACUTE HYPERKALEMIA
The treatment of hyperkalemia depends on the magnitude of increase in the plasma potassium concentration and the presence or absence of electrocardiographic changes or neuromuscular symptoms.23 Acute treatment is indicated for marked electrocardiographic changes and severe muscle weakness.
Intravenous calcium rapidly normalizes membrane excitability by antagonizing the potassium-induced decrease in membrane excitability but does not alter the plasma potassium concentration.
Insulin lowers the plasma potassium concentration by promoting its entry into cells. To avoid hypoglycemia, 10 units of short-acting insulin should be accompanied by a 50-g infusion of glucose, increased to 60 g if 20 units of insulin are given.24
Beta-2 receptor agonists produce a similar effect. The shift of potassium into cells with insulin and beta-2-adrenergic receptor stimulation is brought about by increases in sodium-potassium ATPase pump activity, primarily in skeletal muscle cells.
Sodium bicarbonate, in the absence of acidosis, lowers the plasma potassium concentration only slightly. It should be reserved for hyperkalemic patients who have coexisting metabolic acidosis after the patient has received insulin and glucose, an adrenergic agent, and calcium.
These acute treatments need to be followed by therapies designed to lower the total body potassium content such as diuretics, potassium-binding drugs, and dialysis.
TREATMENT OF CHRONIC HYPERKALEMIA
Review medications. Once the diagnosis of hyperkalemia has been made, the initial approach should be to review the patient’s medications and make every effort to discontinue drugs that can impair renal potassium excretion.16 Patients should be asked about their use of over-the-counter nonsteroidal anti-inflammatory drugs and herbal remedies, since herbs may be a hidden source of dietary potassium.
Dietary counseling. Patients should be instructed to reduce their dietary intake of potassium and to avoid salt substitutes that contain potassium.
Diuretic therapy is beneficial in minimizing hyperkalemia in patients with chronic kidney disease. Thiazide and loop diuretics enhance renal potassium excretion by increasing flow and delivery of sodium to the collecting duct. Thiazide diuretics are effective when the estimated glomerular filtration rate is greater than 30 mL/min, while loop diuretics should be used in patients with more severe renal insufficiency (Table 2).
Sodium bicarbonate is an effective agent to minimize increases in the plasma potassium concentration in patients with chronic kidney disease and metabolic acidosis. This drug increases renal potassium excretion by increasing distal sodium delivery and shifts potassium into cells as the acidosis is corrected. The likelihood of developing volume overload as a complication of sodium bicarbonate administration can be minimized with effective diuretic therapy.
Avoiding hyperkalemia if renin-angiotensin-aldosterone system blockers are needed
Renin-angiotensin-aldosterone system blockers can be problematic, as these drugs cause hyperkalemia, often in the very patients who derive the greatest cardiovascular benefit from them.16 A number of steps can reduce the risk of hyperkalemia and allow these drugs to be used.
The initial dose should be low and the plasma potassium should be measured within 1 to 2 weeks after drug initiation. If the potassium level is normal, the dose can be titrated upwards with remeasurement of the plasma potassium after each dose titration. If the plasma potassium concentration rises to 5.5 mmol/L, in some cases lowering the dose will reduce the potassium concentration and allow the patient to remain on the drug.
In patients at risk of hyperkalemia, angiotensin II receptor blockers and direct renin inhibitors should be used with the same caution as angiotensin-converting enzyme inhibitors.
If the plasma potassium concentration exceeds 5.5 mmol/L despite the above precautions, one can consider using a potassium-binding drug (see below) before deciding to avoid renin-angiotensin-aldosterone system blockers.
Sodium polystyrene sulfonate binds potassium in the gastrointestinal tract in exchange for sodium and has been used to manage hyperkalemia. This drug is most commonly given along with sorbitol as a therapy for acute hyperkalemia. Although the drug is widely used, most of the potassium-lowering effect is due to an increase in stool volume caused by sorbitol.25,26 In addition, long-term use is poorly tolerated, and the drug has been linked to gastrointestinal toxicity in rare cases.
Patiromer and sodium zirconium cyclosilicate are two new potassium-binding drugs that have been shown to be effective in reducing plasma potassium concentration in the setting of ongoing use of renin-angiotensin-aldosterone system blockers.
Patiromer is a nonabsorbed polymer approved for clinical use to treat hyperkalemia. The drug binds potassium in exchange for calcium in the gastrointestinal tract, predominantly in the colon, and lowers the plasma potassium concentration in a dose-dependent manner, with the greatest reduction in those with higher starting values.27,28
Patiromer effectively controlled plasma potassium concentrations in a 1-year randomized trial in high-risk patients on renin-angiotensin-aldosterone system blockers.29 The main adverse events in clinical trials have been constipation and hypomagnesemia, which required magnesium replacement in a small number of patients, but overall, the drug is well tolerated.
Sodium zirconium cyclosilicate is a nonabsorbed microporous compound that binds potassium in exchange for sodium throughout the gastrointestinal tract. It has been found effective in lowering plasma potassium concentration in a dose-dependent fashion in high-risk patients, most of whom were receiving renin-angiotensin-aldosterone system blockers.30–32 Adverse events were generally comparable to those with placebo in clinical trials; however, edema occurred more frequently when higher doses were used. This drug is not yet approved for clinical use.
Hyperkalemia is common in patients with cardiovascular disease. Its consequences can be severe and life-threatening, and its management and prevention require a multidisciplinary approach that entails reducing intake of high-potassium foods, adjusting medications that cause hyperkalemia, and adding medications that reduce the plasma potassium concentration. With this approach, patients at high risk can receive the cardiorenal benefits of drugs that block the renin-angiotensin-aldosterone system without developing hyperkalemia.
98% OF POTASSIUM IS INSIDE CELLS
The body of a typical 70-kg man contains about 3,500 mmol of potassium, 98% of which is in the intracellular space; the remaining 2% is in the extracellular space. This large intracellular-to-extracellular gradient determines the cell voltage and explains why disorders in plasma potassium give rise to manifestations in excitable tissues such as the heart and nervous system.
The most important determinants of potassium distribution between the intracellular and extracellular space are insulin and beta-adrenergic receptor stimulation.
Maintenance of total-body potassium content is primarily the job of the kidneys, with a small contribution by the gastrointestinal tract.1,2 Hyperkalemia is most commonly encountered in patients with decreased kidney function.
The normal kidney can secrete a large amount of potassium, making hyperkalemia uncommon in the absence of kidney disease. This large capacity may have evolved to handle the diet of Paleolithic humans, which contained 4 times as much potassium as contemporary diets.3,4 With the onset of agriculture, dietary intake of potassium has progressively declined while sodium intake has risen. A popular theory suggests this mismatch between the modern diet and the nutritional requirements encoded in the human genome during evolution may contribute to chronic diseases such as hypertension, stroke, kidney stones, and bone disease.5
MANY POTENTIAL CAUSES OF HYPERKALEMIA
Causes of hyperkalemia are outlined in Table 1. Shifting of potassium from the cells to the extracellular space is a cause of transient hyperkalemia, while chronic hyperkalemia indicates an impairment in renal potassium secretion. The following discussion is a guide to the approach to the hyperkalemic patient.
Is the patient’s hyperkalemia really pseudohyperkalemia?
Pseudohyperkalemia, an artifact of measurement, occurs due to mechanical release of potassium from cells during phlebotomy or specimen processing.6 This diagnosis is made when the serum potassium concentration exceeds the plasma potassium concentration by more than 0.5 mmol/L, and should be considered when hyperkalemia occurs in the absence of a clinical risk factor. Fist-clenching, application of a tight-fitting tourniquet, or use of small-bore needles during phlebotomy can all cause pseudohyperkalemia.
Mechanism of pseudohyperkalemia. Since serum is the liquid part of blood remaining after coagulation, release of potassium from cells injured during the process of coagulation raises the potassium level in the serum. Plasma is the cell-free part of blood that has been treated with anticoagulants; it has no cells that can be injured and release potassium. Thus, the serum potassium level will be higher than that in the plasma.
Reverse pseudohyperkalemia, in contrast, occurs when the plasma potassium level is falsely elevated but the serum value is normal. This situation has been described in hematologic disorders characterized by pronounced leukocytosis in which malignant cells are prone to lysis with minimal mechanical stress due to increased fragility or altered sodium-potassium ATPase pump activity.7 This phenomenon is unusual but occurs because the cells are so fragile.
A spurious increase in plasma potassium concentration along with a low plasma calcium concentration raises the possibility of calcium chelation and release of potassium in a sample tube contaminated with the anticoagulant ethylenediaminetetraacetic acid.
Is there increased potassium intake?
Increased potassium intake is a potential cause of hyperkalemia in patients with decreased kidney function or adrenal disease.
Foods naturally rich in potassium include bananas (a medium-sized banana contains 451 mg or 12 mmol of potassium) and potatoes (844 mg or 22 mmol in a large baked potato with skin). Other potassium-rich foods are melons, citrus juice, and avocados. Less-obvious food sources include raw coconut juice (potassium concentration 44.3 mmol/L) and noni juice (56 mmol/L).
Salt substitutes, recommended to hypertensive patients with chronic kidney disease, can be a hidden source of dietary potassium.
Clay ingestion is a potential cause of dyskalemia. White clay consumption causes hypokalemia due to potassium binding in the gastrointestinal tract. Red clay or river bed clay, on the other hand, is enriched in potassium (100 mmol of potassium in 100 g of clay) and can cause life-threatening hyperkalemia in patients with chronic kidney disease.8
Eating burnt match heads. Some individuals chew and ingest burnt match heads, a condition called cautopyreiophagia. In one reported case,9 this activity contributed an additional 80 mmol of daily potassium intake in a dialysis patient, resulting in a plasma potassium concentration of 8 mmol/L.
Is the hyperkalemia the result of a cellular shift?
Acute hyperkalemia can be the result of redistribution of cellular potassium. Shifting of as little as 2% of the body’s potassium from the intracellular to the extracellular space can double the plasma potassium concentration.
Tissue injury. Hyperkalemia frequently occurs in diseases that cause tissue injury such as rhabdomyolysis, trauma, massive hemolysis, and tumor lysis.
Insulin deficiency. Insulin and catecholamines are major regulators of potassium distribution within the body. After a meal, release of insulin not only regulates the plasma glucose concentration, it also causes potassium to move into cells until the kidneys have had sufficient time to excrete the dietary potassium load and reestablish total-body potassium content.
Exercise, beta-blockers. During exercise, potassium is released from skeletal muscle cells and accumulates in the interstitial compartment, where it exerts a vasodilatory effect. The simultaneous increase in circulating catecholamines regulates this release by promoting cell potassium uptake through beta-adrenergic receptor stimulation.
Metabolic acidosis can facilitate exit (ie, shift) of potassium from cells, but this effect depends on the type of acidosis. Hyperchloremic normal anion gap acidosis (mineral acidosis) most commonly causes this effect due to the relative impermeability of the cell membrane to the chloride anion. As hydrogen ions move into the cell due to accumulation of ammonium chloride or hydrogen chloride, electrical neutrality is maintained by potassium exit.
In contrast, organic acidosis (due to lactic, beta-hydroxybutyric, or methylmalonic acid) tends not to cause a potassium shift, since most organic anions readily cross the cell membrane along with hydrogen. Lactic acidosis is often associated with potassium shift, but this effect is due to loss of cell integrity as a result of cell ischemia. The hyperkalemia typically present on admission in patients with diabetic ketoacidosis is the result of insulin deficiency and hypertonicity and not the underlying organic acidosis.10
Hypertonic states can cause hyperkalemia due to cell shift. For example, hyperglycemia, as in diabetic ketoacidosis, pulls water from the intracellular into the extracellular compartment, thereby concentrating intracellular potassium and creating a more favorable gradient for potassium efflux through membrane channels. This same effect can occur in neurosurgical patients given large amounts of hypertonic mannitol. Repetitive doses of immunoglobulin can lead to extracellular accumulation of sorbitol, maltose, or sucrose, since these sugars are added to the preparations to prevent immunoglobulin aggregation.11
Is a disturbance in renal potassium excretion present?
Sustained hyperkalemia is more commonly associated with decreases in renal potassium excretion than with a cellular shift. In most instances the clinician can distinguish between cell shift and impaired renal excretion based on the available clinical data.
The transtubular potassium gradient has been used to determine whether there is a disturbance in renal potassium excretion and to assess renal potassium handling.12
This calculation is based on the assumption that only water is reabsorbed past the cortical collecting duct, and not solutes. It has fallen out of favor since we have found this assumption to be incorrect; a large amount of urea is reabsorbed daily in the downstream medullary collecting duct as a result of intrarenal recycling of urea.
The one situation in which the transtubular potassium gradient may be of use is determining whether hyperkalemia is a result of low aldosterone levels as opposed to aldosterone resistance. One can compare the transtubular potassium gradient before and after a physiologic dose (0.05 mg) of 9-alpha fludrocortisone. An increase of more than 6 over a 4-hour period favors aldosterone deficiency, whereas smaller changes would indicate aldosterone resistance.
24-hour potassium excretion, spot urine potassium-creatinine ratio. A better way to assess renal potassium handling is to measure the amount of potassium in a 24-hour urine collection or determine a spot urine potassium-creatinine ratio. A 24-hour urinary potassium excretion of less than 15 mmol or a potassium-creatinine ratio less than 1 suggests an extrarenal cause of hypokalemia. A ratio greater than 20 would be an appropriate renal response to hyperkalemia.
One or more of 3 abnormalities should be considered in the hyperkalemic patient with impaired renal excretion of potassium:
- Decreased distal delivery of sodium
- Mineralocorticoid deficiency
- Abnormal cortical collecting tubule function.13
Decreased distal delivery of sodium
Under normal circumstances, potassium is freely filtered across the glomerulus and then mostly reabsorbed in the proximal tubule and thick ascending limb. Potassium secretion begins in the distal convoluted tubule and increases in magnitude into the collecting duct. Tubular secretion is the component of potassium handling that varies and is regulated according to physiologic needs.
In acute kidney injury, the rapid decline in glomerular filtration rate and reduction in functioning nephron mass lead to decreased distal potassium secretion.
Hyperkalemia is a frequent problem when oliguria is present, since the reduction in distal delivery of sodium and water further impairs potassium secretion. Patients with oliguric acute kidney injury are more likely to have a more severe underlying disease state, and therefore tissue breakdown and catabolism further increase the risk of hyperkalemia.
In contrast, in nonoliguric patients, the renal injury tends to be less severe, and enough sodium and water are usually delivered distally to prevent hyperkalemia.
In chronic kidney disease, nephron dropout and reduction in collecting tubule mass also lead to a global decline in distal potassium secretion. However, this is countered by an increased capacity of the remaining individual nephrons for potassium secretion. High flow, increased distal sodium delivery, and increased activity and number of sodium-potassium ATPase pumps in the remaining nephrons account for this increased secretory capacity.14 As renal function declines over time, colonic potassium secretion progressively increases.15
These adaptive changes help to keep the plasma potassium concentration within the normal range until the glomerular filtration rate falls to less than 10 or 15 mL/min. Development of hyperkalemia with more modest reductions in the glomerular filtration rate suggest decreased mineralocorticoid activity or a specific lesion of the tubule.
Mineralocorticoid deficiency
Aldosterone deficiency can occur alone or in combination with decreased cortisol levels. Destruction of the adrenal glands is suggested when both hormones are reduced. Enzyme defects in cortisol metabolism can result in either isolated deficiency of aldosterone or adrenogenital syndromes associated with decreased mineralocorticoid activity.
Heparin administration leads to a reversible defect in adrenal synthesis of aldosterone. Drugs that block the stimulatory effect of angiotensin II on the zona glomerulosa cells of the adrenal gland will lower aldosterone.
Renin-angiotensin-aldosterone system blockers. Angiotensin-converting enzyme inhibitors block the formation of angiotensin II, whereas angiotensin II receptor blockers prevent angiotensin II from binding to its adrenal receptor. The direct renin inhibitor aliskiren lowers angiotensin II levels by blocking the enzymatic activity of renin and lowers the circulating levels of both angiotensin I and II.16
The syndrome of hyporeninemic hypoaldosteronism is a common cause of hyperkalemia in patients who have a glomerular filtration rate between 40 and 60 mL/min. Diabetic nephropathy and interstitial renal disease are the most common clinical entities associated with this syndrome.10 Other causes include analgesic nephropathy, urinary tract obstruction, sickle cell disease, systemic lupus erythematosus, and amyloidosis.
Nonsteroidal anti-inflammatory drugs can cause hyperkalemia by suppressing renin release and reducing delivery of sodium to the distal nephron.18
Calcineurin inhibitors impair potassium secretion by suppressing renin release and by direct tubular effects.19
Beta-blockers. Beta-1 and to a lesser extent beta-2 receptor blockade can also result in a hyporeninemic state.
Distal tubular defect
Hyperkalemia can result from interstitial renal diseases that specifically affect the distal nephron. In this setting, the glomerular filtration rate is only mildly reduced, and circulating aldosterone levels are normal.
Renal transplant, lupus erythematosus, amyloidosis, urinary obstruction, and sickle cell disease are conditions in which an impairment in renin release may coexist with a defect in tubular secretion.
Potassium-sparing diuretics impair the ability of the cortical collecting tubule to secrete potassium. Specifically, amiloride and triamterene inhibit sodium reabsorption mediated by the epithelial sodium channel located on the apical membrane of the principal cell. This effect abolishes the lumen’s negative potential and thereby removes a driving force for potassium secretion.
Trimethoprim and pentamidine cause similar effects.
Spironolactone and eplerenone compete with aldosterone at the level of the mineralocorticoid receptor and can result in hyperkalemia.
Drospirenone, a non-testosterone-derived progestin contained in certain oral contraceptives, possesses mineralocorticoid-blocking effects similar to those of spironolactone.
The plasma potassium level should be monitored when these drugs are prescribed in patients receiving potassium supplements, renin-angiotensin-aldosterone system blockers, or nonsteroidal anti-inflammatory drugs.20
CLINICAL FEATURES OF HYPERKALEMIA
Neuromuscular manifestations of hyperkalemia include paresthesias and fasciculations in the arms and legs. Severe elevation in potassium can give rise to an ascending paralysis with eventual flaccid quadriplegia. Typically, the trunk, head, and respiratory muscles are spared, and respiratory failure is rare.
Cardiac signs
Hyperkalemia has depolarizing effects on the heart that are manifested by changes in the electrocardiogram (Figure 2). The progressive changes of hyperkalemia are classically listed as:
- Peaked T waves that are tall, narrow, and symmetrical and can occasionally be confused with the hyperacute T-wave change associated with an ST-segment elevation myocardial infarction.21 However, in the latter condition, the T waves tend to be more broad-based and asymmetric in shape.
- ST-segment depression
- Widening of the PR interval
- Widening of the QRS interval
- Loss of the P wave
- A sine-wave pattern—an ominous development and a harbinger of impending ventricular fibrillation and asystole.
The plasma potassium concentration often correlates poorly with cardiac manifestations. In a retrospective review, only 16 of 90 cases met strict criteria for electrocardiographic changes reflective of hyperkalemia (defined as new peaked and symmetric T waves that resolved on follow-up).22 In 13 of these cases, the electrocardiogram was interpreted as showing no T-wave changes even when read by a cardiologist. In addition, electrocardiographic criteria for hyperkalemia were noted in only 1 of 14 patients who manifested arrhythmias or cardiac arrest attributed to increased plasma potassium concentration.
TREATMENT OF ACUTE HYPERKALEMIA
The treatment of hyperkalemia depends on the magnitude of increase in the plasma potassium concentration and the presence or absence of electrocardiographic changes or neuromuscular symptoms.23 Acute treatment is indicated for marked electrocardiographic changes and severe muscle weakness.
Intravenous calcium rapidly normalizes membrane excitability by antagonizing the potassium-induced decrease in membrane excitability but does not alter the plasma potassium concentration.
Insulin lowers the plasma potassium concentration by promoting its entry into cells. To avoid hypoglycemia, 10 units of short-acting insulin should be accompanied by a 50-g infusion of glucose, increased to 60 g if 20 units of insulin are given.24
Beta-2 receptor agonists produce a similar effect. The shift of potassium into cells with insulin and beta-2-adrenergic receptor stimulation is brought about by increases in sodium-potassium ATPase pump activity, primarily in skeletal muscle cells.
Sodium bicarbonate, in the absence of acidosis, lowers the plasma potassium concentration only slightly. It should be reserved for hyperkalemic patients who have coexisting metabolic acidosis after the patient has received insulin and glucose, an adrenergic agent, and calcium.
These acute treatments need to be followed by therapies designed to lower the total body potassium content such as diuretics, potassium-binding drugs, and dialysis.
TREATMENT OF CHRONIC HYPERKALEMIA
Review medications. Once the diagnosis of hyperkalemia has been made, the initial approach should be to review the patient’s medications and make every effort to discontinue drugs that can impair renal potassium excretion.16 Patients should be asked about their use of over-the-counter nonsteroidal anti-inflammatory drugs and herbal remedies, since herbs may be a hidden source of dietary potassium.
Dietary counseling. Patients should be instructed to reduce their dietary intake of potassium and to avoid salt substitutes that contain potassium.
Diuretic therapy is beneficial in minimizing hyperkalemia in patients with chronic kidney disease. Thiazide and loop diuretics enhance renal potassium excretion by increasing flow and delivery of sodium to the collecting duct. Thiazide diuretics are effective when the estimated glomerular filtration rate is greater than 30 mL/min, while loop diuretics should be used in patients with more severe renal insufficiency (Table 2).
Sodium bicarbonate is an effective agent to minimize increases in the plasma potassium concentration in patients with chronic kidney disease and metabolic acidosis. This drug increases renal potassium excretion by increasing distal sodium delivery and shifts potassium into cells as the acidosis is corrected. The likelihood of developing volume overload as a complication of sodium bicarbonate administration can be minimized with effective diuretic therapy.
Avoiding hyperkalemia if renin-angiotensin-aldosterone system blockers are needed
Renin-angiotensin-aldosterone system blockers can be problematic, as these drugs cause hyperkalemia, often in the very patients who derive the greatest cardiovascular benefit from them.16 A number of steps can reduce the risk of hyperkalemia and allow these drugs to be used.
The initial dose should be low and the plasma potassium should be measured within 1 to 2 weeks after drug initiation. If the potassium level is normal, the dose can be titrated upwards with remeasurement of the plasma potassium after each dose titration. If the plasma potassium concentration rises to 5.5 mmol/L, in some cases lowering the dose will reduce the potassium concentration and allow the patient to remain on the drug.
In patients at risk of hyperkalemia, angiotensin II receptor blockers and direct renin inhibitors should be used with the same caution as angiotensin-converting enzyme inhibitors.
If the plasma potassium concentration exceeds 5.5 mmol/L despite the above precautions, one can consider using a potassium-binding drug (see below) before deciding to avoid renin-angiotensin-aldosterone system blockers.
Sodium polystyrene sulfonate binds potassium in the gastrointestinal tract in exchange for sodium and has been used to manage hyperkalemia. This drug is most commonly given along with sorbitol as a therapy for acute hyperkalemia. Although the drug is widely used, most of the potassium-lowering effect is due to an increase in stool volume caused by sorbitol.25,26 In addition, long-term use is poorly tolerated, and the drug has been linked to gastrointestinal toxicity in rare cases.
Patiromer and sodium zirconium cyclosilicate are two new potassium-binding drugs that have been shown to be effective in reducing plasma potassium concentration in the setting of ongoing use of renin-angiotensin-aldosterone system blockers.
Patiromer is a nonabsorbed polymer approved for clinical use to treat hyperkalemia. The drug binds potassium in exchange for calcium in the gastrointestinal tract, predominantly in the colon, and lowers the plasma potassium concentration in a dose-dependent manner, with the greatest reduction in those with higher starting values.27,28
Patiromer effectively controlled plasma potassium concentrations in a 1-year randomized trial in high-risk patients on renin-angiotensin-aldosterone system blockers.29 The main adverse events in clinical trials have been constipation and hypomagnesemia, which required magnesium replacement in a small number of patients, but overall, the drug is well tolerated.
Sodium zirconium cyclosilicate is a nonabsorbed microporous compound that binds potassium in exchange for sodium throughout the gastrointestinal tract. It has been found effective in lowering plasma potassium concentration in a dose-dependent fashion in high-risk patients, most of whom were receiving renin-angiotensin-aldosterone system blockers.30–32 Adverse events were generally comparable to those with placebo in clinical trials; however, edema occurred more frequently when higher doses were used. This drug is not yet approved for clinical use.
- Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ 2016; 40:480–490.
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10:1050–1060.
- Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med 1985; 312:283–289.
- Sebastian A, Frassetto LA, Sellmeyer DE, Morris RC Jr. The evolution-informed optimal dietary potassium intake of human beings greatly exceeds current and recommended intakes. Semin Nephrol 2006; 26:447–453.
- Palmer BF, Clegg DJ. Achieving the benefits of a high potassium, Paleolithic diet, without the toxicity. Mayo Clin Proc 2016; 91:496–508.
- Liamis G, Liberopoulos E, Barkas F, Elisaf M. Spurious electrolyte disorders: a diagnostic challenge for clinicians. Am J Nephrol 2013; 38:50–57.
- Mansoor S, Holtzman N, Emadi A. Reverse pseudohyperkalemia: an important clinical entity in chronic lymphocytic leukemia. Case Rep Hematol 2015; 2015:930379.
- Gelfand M, Zarate A, Knepshield J. Geophagia. A cause of life-threatening hyperkalemia in patients with chronic renal failure. JAMA 1975; 234:738–740.
- Abu-Hamdan D, Sondheimer J, Mahajan S. Cautopyreiophagia. Cause of life-threatening hyperkalemia in a patient undergoing hemodialysis. Am J Med 1985; 79:517–519.
- Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med 2015; 373:548–559.
- Daphnis E, Stylianou K, Alexandrakis M, et al. Acute renal failure, translocational hyponatremia and hyperkalemia following intravenous immunoglobulin therapy. Nephron Clin Pract 2007; 106:c143–c148.
- Choi M, Ziyadeh F. The utility of the transtubular potassium gradient in the evaluation of hyperkalemia. J Am Soc Nephrol 2008; 19:424–426.
- Palmer BF. A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am J Kidney Dis 2010; 56:387–393.
- Stanton BA. Renal potassium transport: morphological and functional adaptations. Am J Physiol 1989; 257:R989–R997.
- Hayes CP Jr, McLeod ME, Robinson RR. An extravenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physicians 1967; 80:207–216.
- Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med 2004; 351:585–592.
- Palmer BF. Renal dysfunction complicating treatment of hypertension. N Engl J Med 2002; 347:1256–1261.
- Palmer BF. Renal complications associated with use of nonsteroidal anti-inflammatory agents. J Investig Med 1995; 43:516–533.
- Hoorn E, Walsh S, McCormick J, et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med 2011; 17:1304–1309.
- Bird ST, Pepe SR, Etminan M, Liu X, Brophy JM, Delaney JA. The association between drospirenone and hyperkalemia: a comparative-safety study. BMC Clin Pharmacol 2011; 11:23.
- Wang K. Images in clinical medicine. “Pseudoinfarction” pattern due to hyperkalemia. N Engl J Med 2004; 351:593.
- Montague BT, Ouellette JR, Buller GK. Retrospective review of the frequency of ECG changes in hyperkalemia. Clin J Am Soc Nephrol 2008; 3:324–330.
- Weisberg LS. Management of severe hyperkalemia. Crit Care Med 2008; 36:3246–3251.
- Harel Z, Kamel KS. Optimal dose and method of administration of intravenous insulin in the management of emergency hyperkalemia: a systematic review. PLoS One 2016; 11:e0154963.
- Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol 2010; 21:733–735.
- Emmett M, Hootkins RE, Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Effect of three laxatives and a cation exchange resin on fecal sodium and potassium excretion. Gastroenterology 1995; 108:752–760.
- Bushinsky DA, Spiegel DM, Gross C, et al. Effect of patiromer on urinary ion excretion in healthy adults. Clin J Am Soc Nephrol 2016; 11:1769–1776.
- Weir MR, Bakris GL, Bushinsky DA, et al; OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015; 372:211–221.
- Bakris GL, Pitt B, Weir MR, et al; AMETHYST-DN Investigators. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA 2015; 314:151–161.
- Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia. The HARMONIZE randomized clinical trial. JAMA 2014; 312:2223–2233.
- Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 2015; 372:222–231.
- Anker SD, Kosiborod M, Zannad F, et al. Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial. Eur J Heart Fail 2015; 17:1050–1056.
- Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ 2016; 40:480–490.
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10:1050–1060.
- Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med 1985; 312:283–289.
- Sebastian A, Frassetto LA, Sellmeyer DE, Morris RC Jr. The evolution-informed optimal dietary potassium intake of human beings greatly exceeds current and recommended intakes. Semin Nephrol 2006; 26:447–453.
- Palmer BF, Clegg DJ. Achieving the benefits of a high potassium, Paleolithic diet, without the toxicity. Mayo Clin Proc 2016; 91:496–508.
- Liamis G, Liberopoulos E, Barkas F, Elisaf M. Spurious electrolyte disorders: a diagnostic challenge for clinicians. Am J Nephrol 2013; 38:50–57.
- Mansoor S, Holtzman N, Emadi A. Reverse pseudohyperkalemia: an important clinical entity in chronic lymphocytic leukemia. Case Rep Hematol 2015; 2015:930379.
- Gelfand M, Zarate A, Knepshield J. Geophagia. A cause of life-threatening hyperkalemia in patients with chronic renal failure. JAMA 1975; 234:738–740.
- Abu-Hamdan D, Sondheimer J, Mahajan S. Cautopyreiophagia. Cause of life-threatening hyperkalemia in a patient undergoing hemodialysis. Am J Med 1985; 79:517–519.
- Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med 2015; 373:548–559.
- Daphnis E, Stylianou K, Alexandrakis M, et al. Acute renal failure, translocational hyponatremia and hyperkalemia following intravenous immunoglobulin therapy. Nephron Clin Pract 2007; 106:c143–c148.
- Choi M, Ziyadeh F. The utility of the transtubular potassium gradient in the evaluation of hyperkalemia. J Am Soc Nephrol 2008; 19:424–426.
- Palmer BF. A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am J Kidney Dis 2010; 56:387–393.
- Stanton BA. Renal potassium transport: morphological and functional adaptations. Am J Physiol 1989; 257:R989–R997.
- Hayes CP Jr, McLeod ME, Robinson RR. An extravenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physicians 1967; 80:207–216.
- Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med 2004; 351:585–592.
- Palmer BF. Renal dysfunction complicating treatment of hypertension. N Engl J Med 2002; 347:1256–1261.
- Palmer BF. Renal complications associated with use of nonsteroidal anti-inflammatory agents. J Investig Med 1995; 43:516–533.
- Hoorn E, Walsh S, McCormick J, et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med 2011; 17:1304–1309.
- Bird ST, Pepe SR, Etminan M, Liu X, Brophy JM, Delaney JA. The association between drospirenone and hyperkalemia: a comparative-safety study. BMC Clin Pharmacol 2011; 11:23.
- Wang K. Images in clinical medicine. “Pseudoinfarction” pattern due to hyperkalemia. N Engl J Med 2004; 351:593.
- Montague BT, Ouellette JR, Buller GK. Retrospective review of the frequency of ECG changes in hyperkalemia. Clin J Am Soc Nephrol 2008; 3:324–330.
- Weisberg LS. Management of severe hyperkalemia. Crit Care Med 2008; 36:3246–3251.
- Harel Z, Kamel KS. Optimal dose and method of administration of intravenous insulin in the management of emergency hyperkalemia: a systematic review. PLoS One 2016; 11:e0154963.
- Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol 2010; 21:733–735.
- Emmett M, Hootkins RE, Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Effect of three laxatives and a cation exchange resin on fecal sodium and potassium excretion. Gastroenterology 1995; 108:752–760.
- Bushinsky DA, Spiegel DM, Gross C, et al. Effect of patiromer on urinary ion excretion in healthy adults. Clin J Am Soc Nephrol 2016; 11:1769–1776.
- Weir MR, Bakris GL, Bushinsky DA, et al; OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015; 372:211–221.
- Bakris GL, Pitt B, Weir MR, et al; AMETHYST-DN Investigators. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA 2015; 314:151–161.
- Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia. The HARMONIZE randomized clinical trial. JAMA 2014; 312:2223–2233.
- Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 2015; 372:222–231.
- Anker SD, Kosiborod M, Zannad F, et al. Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial. Eur J Heart Fail 2015; 17:1050–1056.
KEY POINTS
- Exclude pseudohyperkalemia in patients who have a normal electrocardiogram and no risk factors for the development of hyperkalemia.
- Decreased distal delivery of sodium, reduced mineralocorticoid levels or activity, and a distal tubular defect are causes of impaired renal potassium secretion.
- Medical conditions and medications that alter the renin-angiotensin-aldosterone system can give rise to hyperkalemia.
Diagnostic value of the physical examination in patients with dyspnea
Laennec’s stethoscope has survived more than 200 years, much longer than some of his contemporaries predicted. But will it survive the challenge of bedside ultrasonography and other technologic advances?
The physical examination, with its roots extending at least as far back as Hippocrates, may be at a crossroads as the mainstay of diagnosis. Physical signs can be subjective and lack sensitivity and specificity. Modern imaging and laboratory studies may already be more trusted.
If the physical examination is to survive, it must be accurate, reproducible, and efficient. Needed is a simple, evidence-based approach to the physical examination that enhances its diagnostic accuracy while maintaining bedside efficiency.
Here, we analyze the accuracy of the physical signs that are most effective in the clinical diagnosis of 4 common cardiopulmonary conditions that often present with dyspnea: pneumonia, pleural effusion, chronic obstructive pulmonary disease (COPD), and congestive heart failure.
LIKELIHOOD RATIOS
To grasp the significance of physical findings, it is necessary to understand the concept of likelihood ratios, which are widely accepted measures of the accuracy of a test or clinical finding.1,2 The positive likelihood ratio is the probability of a disease being present when the test is positive or the clinical finding is present, while the negative likelihood ratio is the probability that the disease is present when the test is negative or the clinical finding is absent. They are calculated as follows1:
Positive likelihood ratio = sensitivity / (1 – specificity)
Negative likelihood ratio = (1 – sensitivity) / specificity
Table 1 shows how the likelihood ratio of a test changes the posttest probability that a condition is present or absent, according to an analysis by McGee.2
STANDARDIZED TERMINOLOGY
PNEUMONIA
Pneumonia is a common disease, with more than 2 million cases annually in the United States. It is most often diagnosed by standard chest radiography, although computed tomography can identify it earlier and with higher sensitivity and specificity.5 The amount of published data on physical examination findings in pneumonia is surprisingly small.
Asymmetry in chest expansion: Specific, reproducible, but not sensitive
The physical finding with the highest positive likelihood ratio for diagnosing pneumonia is asymmetry in chest expansion.6,7
In a 1984 study of 1,819 patients presenting to an emergency department with acute cough, Diehr et al6 evaluated several physical signs of pneumonia. Asymmetric chest expansion had a specificity and positive predictive value of 100%, but its sensitivity was only 4.3%. Thus, it is not a good screening test, but it is a good diagnostic or confirmatory test. From these numbers, Metlay et al8 calculated that the positive likelihood ratio was infinity and the negative likelihood ratio was 0.96.
McGee,7 on the other hand, calculated the positive likelihood ratio of asymmetric chest expansion at 44.1. McGee also found chest expansion to be a highly reproducible finding, with an interobserver agreement kappa score of 0.85.7 (A kappa score of 1.0 would indicate perfect interobserver agreement.) Interestingly, chest radiographs interpreted for pulmonary infiltrates have an interobserver kappa score of only 0.38.7 Further studies of this physical sign could shed more light upon this area of uncertainty.
Other signs of pneumonia
None of the other physical signs studied for the diagnosis of pneumonia has as high a positive likelihood ratio as asymmetric chest expansion.6–12
Egophony is a high-pitched or nasal quality of the patient’s voice heard on auscultation over lung tissue that is consolidated or fibrosed, due to enhanced transmission of high-frequency sound across fluid. It is often described as the “E-to-A change.” Although listening for egophony is widely done and easy to do, we calculate that this sign has a positive likelihood ratio of only 6.8 based on pooled data from 3 trials with a total of 3,245 patients.6,10,11
Faring less favorably, in descending order of diagnostic accuracy, are:
Percussion dullness (positive likelihood ratio 5.7 based on 4 studies with 3,653 patients)6,10–12
Bronchophony or bronchial breath sounds (positive likelihood ratio 3.3 based on 1,118 patients)10
Crackles have long been taught as a common physical finding in pneumonia. Bohadana et al pointed out that “crackle” can be defined acoustically but does not suggest any means or site of generation.4 Pooled data from 4 studies in 3,647 patients6,10–12 result in a positive likelihood ratio for crackles in the diagnosis of pneumonia of only 3.2.
Diminished breath sounds (positive likelihood ratio 2.5 based on 3 studies with 1,828 patients).10–12
Consider pneumonia signs in combination
These physical examination maneuvers are time-honored and part of the rite of training for medical students and residents. As we have shown, they are not extremely helpful as individual tests in diagnosing pneumonia; however, they may be useful when used in combination as a clinical prediction rule or diagnostic algorithm. These rules often have higher diagnostic accuracy but drawbacks of taking more time and not being easily reproducible.
PLEURAL EFFUSION
Pleural effusion commonly occurs in patients with congestive heart failure, pneumonia, and malignancies. The following are signs of effusion.
Dullness to percussion had a positive likelihood ratio of 5.7 from pooled data from 3 studies analyzed by Wong et al.13
Asymmetric chest expansion, in a study by Kalantri et al,14 had a positive likelihood ratio of 8.1 and a negative likelihood ratio of 0.29, the latter making it a reasonably good test to help rule out a pleural effusion.
Negative signs. Since a pleural effusion is an abnormal fluid collection in the pleural space and not the lung parenchyma, one would not expect it to cause loud breath sounds, adventitious sounds, or vocal resonance. Since these 3 findings emanate from the lung, their absence would be expected to support the presence of a pleural effusion.
Tactile fremitus, also known as vocal fremitus, is the vibration felt on the chest wall while the patient is speaking. Traditionally, the patient says “ninety-nine” as the examiner feels for asymmetry in vibration. A consolidation such as pneumonia increases the vibration, while fluid in a pleural effusion diminishes it.
DIAGNOSTIC ALGORITHM FOR PNEUMONIA OR PLEURAL EFFUSION
Patients presenting with cough or dyspnea will most likely be evaluated for pneumonia and pleural effusion, among other diagnoses. We propose the following physical examination strategy in this setting.
First, evaluate the patient for asymmetric chest expansion. The positive likelihood ratio for this sign is excellent for pneumonia (44.1) and moderate for pleural effusion (8.1); therefore, both conditions are possible with a positive test.
Second, percuss the chest. Dullness to percussion has a low positive likelihood ratio for pneumonia but a moderate one for pleural effusion.13 The absence of this sign is only modest in excluding a pleural effusion (negative likelihood ratio 0.31 in pooled data analyzed by Wong et al).13
Third, auscultate the chest to elicit normal, diminished, or adventitious breath sounds. Diminished breath sounds may be noted in both conditions, but vocal resonance (egophony or bronchophony) and tactile fremitus should not be present directly over a pleural effusion. Either vocal resonance or tactile fremitus in a patient with asymmetric chest expansion would strongly support the diagnosis of pneumonia.
Figure 2 summarizes our proposed diagnostic algorithm for pneumonia and pleural effusion.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
COPD imposes a heavy burden on public health worldwide in terms of cost and mortality. It is the third leading cause of death in the United States, after heart disease and cancer.15
Spirometry remains the gold standard for diagnosis. The Global Initiative for Chronic Obstructive Lung Disease standard for diagnosing COPD was the better of 2 spirometry test results, showing a forced expiratory volume in 1 second (FEV1) and FEV1/forced vital capacity ratio less than 70%.16
Unfortunately, there is little evidence that physical signs aid in the early diagnosis of COPD, as physical signs of airflow limitation may not manifest until lung function is substantially impaired.17,18
Early inspiratory crackles had a positive likelihood ratio of 14.6 based on 2 small studies.19,20
Percussion dullness over the left sternal border in the fifth intercostal space should be present in the normal situation and is known as cardiac dullness. Absent cardiac dullness had a positive likelihood ratio of 16 and a negative likelihood ratio of 0.8 for diagnosing COPD in a study in 92 patients with a history of smoking or self-reported COPD.21 The kappa score was 0.49, signifying moderate interobserver agreement.
A combined strategy using the history and physical examination may have the highest diagnostic accuracy. Many of these combinations are too cumbersome for practical clinical use. However, 1 of them is based on only 3 questions21:
- Has the patient smoked for more than 70-pack years?
- Has the patient been previously diagnosed with chronic bronchitis or emphysema?
- Are breath sounds diminished in intensity?
Answering yes to 2 of these questions gives a positive likelihood ratio of a diagnosis of COPD of 33.5.
Early detection of COPD may improve outcomes and lower healthcare costs and thus would be clinically useful. Unfortunately, a diagnostic approach using the history and physical in the early diagnosis of COPD remains uncertain at this time.
CONGESTIVE HEART FAILURE
The clinical presentation of acute congestive heart failure has much in common with pneumonia, pleural effusion, and COPD.
Echocardiography, the gold standard for diagnosis, is costly and may not be immediately available for most patients evaluated for cardiorespiratory complaints. The American College of Cardiology reports the cost of standard echocardiography to be between $1,000 and $2,000.22 A physical examination approach in the assessment of dyspnea can be very useful.
Height of jugular venous distention approximates central venous pressure
Assessing the central venous pressure by estimating the vertical height of distention of the right internal or external jugular vein is validated and easily reproducible.23,24 The use of the external jugular vein is supported by correlation with catheter-measured central venous pressure in critically ill patients.25,26 The central venous pressure reflects the right atrial pressure, and in the absence of tricuspid stenosis, the right ventricular end-diastolic pressure. An elevation in central venous pressure can be seen in patients with congestive heart failure, pulmonary hypertension, and pulmonary valve stenosis.
The right side is preferred due to its anatomically direct route to the heart. In contrast, the left internal jugular vein crosses the mediastinum and can be compressed by the aorta, causing a false elevation.
In summary, an elevated jugular venous pressure on examination is a good test to rule in an elevated central venous pressure, and its absence is a good sign in ruling out an elevated central venous pressure. When using jugular venous pressure specifically for the diagnosis of congestive heart failure with reduced ejection fraction (ie, ejection fraction < 50%), the positive likelihood ratio is 6.3 based on 3 studies.25–27
Heart failure with preserved ejection fraction has not been well studied for physical examination. The Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve)28 looked only at the sensitivity of elevated jugular venous pressure in 4,128 patients, which was 8%. Specificity was not reported.
The abdominojugular reflux
Another way to gauge the jugular venous pressure is to examine the neck veins while firmly pressing on the mid-abdomen for 10 to 15 seconds to look for the abdominojugular reflux, also known as the hepatojugular reflux. An increase in the jugular venous pressure of 3 cm from baseline constitutes a positive abdominojugular reflux. It has a positive likelihood ratio of 8.0 and a negative likelihood ratio of 0.3 for the diagnosis of congestive heart failure by the assessment of end-diastolic pressure of the left ventricle (Table 5).29–31
The abdominojugular reflux is a much more reliable test than examination of neck veins for jugular venous pressure. The interobserver agreement for examining neck veins has a wide range of kappa scores (0.08–0.81), whereas the abdominojugular reflux has a very high kappa score of 0.92.7 Interestingly, chest radiography showing interstitial edema has a kappa of 0.83.7
Displaced apical impulse
An evaluation of the apical impulse of the heart is also a very good and quick test in the examination of patients suspected of having congestive heart failure. An abnormal finding is defined by an apical impulse displaced laterally (to the left of the midclavicular line).
Using data from several studies,32–35 a displaced apical impulse has a positive likelihood ratio of 10.3. The absence of this finding, however, is not very good for ruling out congestive heart failure, with a negative likelihood ratio of 0.7. Interobserver agreement is moderate to excellent (kappa score 0.43–0.86).7
A third heart sound
Auscultation to assess the third heart sound is much more difficult. A systematic review found that likelihood ratios vary widely and confidence intervals are wide.36 Interobserver agreement also varies widely (kappa scores –0.17 to 0.84).7 In a primary care study,37 a third heart sound had a very low sensitivity (4.3%) but a specificity of 99.8%.
Therefore, we are uncertain about a conclusion for this physical finding based on the concern for wide ranges in likelihood ratio and poor interobserver reliability.
PHYSICAL EXAMINATION STILL HAS A FUTURE
The physical examination has a long and distinguished place in the history of medicine. Technologic advances have changed the manner in which clinicians practice the art of healing. Modern technology in US healthcare has become a double-edged sword, with many benefits as well as detriments.3 Reproducibility and accuracy are paramount for the physical examination to remain a core component of medical diagnosis. Advances in the diagnostic accuracy of laboratory and imaging studies challenge the importance of the physical examination. However, we firmly believe that the traditional techniques have stood the test of time and have a future in the clinical practice of medicine.
Acknowledgments: The authors thank Ruby Marr, MD, Mohammed Nabhan, MD, Rajiv Doddamani, MD, and Sohaib Galani, MD, for their important contributions to this article, which included research assistance and editorial advice.
- Lang TA, Secic M. Chapter 10. Determining the presence or absence of disease. Reporting the characteristics of diagnostic tests. In: Lang TC, Secic M. How to Report Statistics in Medicine. Annotated Guidelines for Authors, Editors, and Reviewers. Philadelphia, PA, American College of Physicians, 1997:147–169.
- McGee S. Simplifying likelihood ratios. J Gen Intern Med 2002; 17:647–650.
- Mikami R, Murao M, Cugell DW, et al. International symposium on lung sounds. Synopsis of proceedings. Chest 1987; 92:342–345.
- Bohadana A, Izbicki G, Kraman SS. Fundamentals of lung auscultation. N Engl J Med 2014; 370:744–751.
- Heussel CP, Kauczor HU, Ullmann AJ. Pneumonia in neutropenic patients. Eur Radiol 2004; 14:256–271.
- Diehr P, Wood RW, Bushyhead J, Krueger L, Wolcott B, Tompkins RK. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis 1984; 37:215–225.
- McGee S. Evidence-Based Physical Diagnosis. 4th ed. Philadelphia, PA: Elsevier; 2017.
- Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997; 278:1440–1445.
- Melbye H, Straume B, Aasebo U, Brox J. The diagnosis of adult pneumonia in general practice. The diagnostic value of history, physical examination and some blood tests. Scand J Prim Health Care 1988; 6:111–117.
- Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med 1990; 113:664–670.
- Gennis P, Gallagher J, Falvo C, Baker S, Than W. Clinical criteria for the detection of pneumonia in adults: guidelines for ordering chest roentgenograms in the emergency department. J Emerg Med 1989; 7:263–268.
- Melbye H, Straume B, Aasebo U, Dale K. Diagnosis of pneumonia in adults in general practice. Relative importance of typical symptoms and abnormal chest signs evaluated against a radiographic reference standard. Scand J Prim Health Care 1992; 10:226–233.
- Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA 2009; 301:309–317.
- Kalantri S, Joshi R, Lokhande T, et al. Accuracy and reliability of physical signs in the diagnosis of pleural effusion. Respir Med 2007; 101:431–438.
- Heron M. Deaths: leading causes for 2014. National Vital Statistics Reports 2016; 65(5) June 30, 2016. www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_05.pdf. Accessed October 20, 2017.
- Global Initiative for Chronic Obstructive Lung Disease. Pocket guide to COPD diagnosis, management, and prevention. http://goldcopd.org/wp-content/uploads/2016/12/wms-GOLD-2017-Pocket-Guide.pdf. Accessed November 13, 2017.
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004; 364:613–620.
- Oshaug K, Halvorsen PA, Melbye H. Should chest examination be reinstated in the early diagnosis of chronic obstructive pulmonary disease? Int J Chron Obstruct Pulmon Dis 2013; 8:369–377.
- Bettencourt PE, Del Bono EA, Spiegelman D, Hertzmark E, Murphy RL Jr. Clinical utility of chest auscultation in common pulmonary diseases. Am J Respir Crit Care Med 1994; 150:1291–1297.
- Nath AR, Capel LH. Inspiratory crackles and mechanical events of breathing. Thorax 1974; 29:695–698.
- Badgett RG, Tanaka DJ, Hunt DK, et al. Can moderate chronic obstructive pulmonary disease be diagnosed by historical and physical findings alone? Am J Med 1993; 94:188–196.
- ABIM Foundation. Choosing wisely. Echocardiograms for heart valve disease. www.choosingwisely.org. Accessed November 13, 2017.
- Davison R, Cannon R. Estimation of central venous pressure by examination of jugular veins. Am Heart J 1974; 87:279–282.
- Ducas J, Magder S, McGregor M. Validity of the hepatojugular reflux as a clinical test for congestive heart failure. Am J Cardiol 1983; 52:1299–1303.
- Vinayak AG, Levitt J, Gehlbach B, Pohlman AS, Hall JB, Kress JP. Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients. Arch Intern Med 2006; 166:2132–2137.
- Sankoff J, Zidulka A. Non-invasive method for the rapid assessment of central venous pressure: description and validation by a single examiner. West J Emerg Med 2008; 9:201–205.
- Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJ. Assessing diagnosis in heart failure: which features are any use? QJM 1997; 90:335–339.
- Kristensen SL, Mogensen UM, Jhund PS, et al. Clinical and echocardiographic characeristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction. A report from the Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve). Circulation 2017; https://doi.org/10.1161/CIRCULATIONAHA.116.024593. Accessed November 1, 2017.
- Butman SM, Ewy GA, Standen JR, Kern KB, Hahn E. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distension. J Am Coll Cardiol 1993; 22:968–974.
- Sochowski RA, Dubbin JD, Naqvi SZ. Clinical and hemodynamic assessment of the hepatojugular reflux. Am J Cardiol 1990; 66:1002–1006.
- Ewy GA. The abdominojugular test: technique and hemodynamic correlates. Ann Intern Med 1988; 109:456–460.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Symptoms and signs of heart failure in patients with myocardial infarction: reproducibility and relationship to chest X-ray, radionuclide ventriculography and right heart catheterization. Eur Heart J 1989; 10:1017–1028.
- Fahey T, Jeyaseelan S, McCowan C, et al. Diagnosis of left ventricular systolic dysfunction (LVSD): development and validation of a clinical prediction rule in primary care. Fam Pract 2007; 24:628–635.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Interobserver agreement and accuracy of bedside estimation of right and left ventricular ejection fraction in acute myocardial infarction. Am J Cardiol 1989; 63:1301–1307.
- Mattleman SJ, Hakki AH, Iskandrian AS, Segal BL, Kane SA. Reliability of bedside evaluation in determining left ventricular function: correlation with left ventricular ejection fraction determined by radionuclide ventriculography. J Am Coll Cardiol 1983; 1:417–420.
- Madhok V, Falk G, Rogers A, Struthers AD, Sullivan FM, Fahey T. The accuracy of symptoms, signs and diagnostic tests in the diagnosis of left ventricular dysfunction in primary care: a diagnostic accuracy systematic review. BMC Fam Pract 2008; 9:56.
- Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation 2011; 124:2865–2873.
Laennec’s stethoscope has survived more than 200 years, much longer than some of his contemporaries predicted. But will it survive the challenge of bedside ultrasonography and other technologic advances?
The physical examination, with its roots extending at least as far back as Hippocrates, may be at a crossroads as the mainstay of diagnosis. Physical signs can be subjective and lack sensitivity and specificity. Modern imaging and laboratory studies may already be more trusted.
If the physical examination is to survive, it must be accurate, reproducible, and efficient. Needed is a simple, evidence-based approach to the physical examination that enhances its diagnostic accuracy while maintaining bedside efficiency.
Here, we analyze the accuracy of the physical signs that are most effective in the clinical diagnosis of 4 common cardiopulmonary conditions that often present with dyspnea: pneumonia, pleural effusion, chronic obstructive pulmonary disease (COPD), and congestive heart failure.
LIKELIHOOD RATIOS
To grasp the significance of physical findings, it is necessary to understand the concept of likelihood ratios, which are widely accepted measures of the accuracy of a test or clinical finding.1,2 The positive likelihood ratio is the probability of a disease being present when the test is positive or the clinical finding is present, while the negative likelihood ratio is the probability that the disease is present when the test is negative or the clinical finding is absent. They are calculated as follows1:
Positive likelihood ratio = sensitivity / (1 – specificity)
Negative likelihood ratio = (1 – sensitivity) / specificity
Table 1 shows how the likelihood ratio of a test changes the posttest probability that a condition is present or absent, according to an analysis by McGee.2
STANDARDIZED TERMINOLOGY
PNEUMONIA
Pneumonia is a common disease, with more than 2 million cases annually in the United States. It is most often diagnosed by standard chest radiography, although computed tomography can identify it earlier and with higher sensitivity and specificity.5 The amount of published data on physical examination findings in pneumonia is surprisingly small.
Asymmetry in chest expansion: Specific, reproducible, but not sensitive
The physical finding with the highest positive likelihood ratio for diagnosing pneumonia is asymmetry in chest expansion.6,7
In a 1984 study of 1,819 patients presenting to an emergency department with acute cough, Diehr et al6 evaluated several physical signs of pneumonia. Asymmetric chest expansion had a specificity and positive predictive value of 100%, but its sensitivity was only 4.3%. Thus, it is not a good screening test, but it is a good diagnostic or confirmatory test. From these numbers, Metlay et al8 calculated that the positive likelihood ratio was infinity and the negative likelihood ratio was 0.96.
McGee,7 on the other hand, calculated the positive likelihood ratio of asymmetric chest expansion at 44.1. McGee also found chest expansion to be a highly reproducible finding, with an interobserver agreement kappa score of 0.85.7 (A kappa score of 1.0 would indicate perfect interobserver agreement.) Interestingly, chest radiographs interpreted for pulmonary infiltrates have an interobserver kappa score of only 0.38.7 Further studies of this physical sign could shed more light upon this area of uncertainty.
Other signs of pneumonia
None of the other physical signs studied for the diagnosis of pneumonia has as high a positive likelihood ratio as asymmetric chest expansion.6–12
Egophony is a high-pitched or nasal quality of the patient’s voice heard on auscultation over lung tissue that is consolidated or fibrosed, due to enhanced transmission of high-frequency sound across fluid. It is often described as the “E-to-A change.” Although listening for egophony is widely done and easy to do, we calculate that this sign has a positive likelihood ratio of only 6.8 based on pooled data from 3 trials with a total of 3,245 patients.6,10,11
Faring less favorably, in descending order of diagnostic accuracy, are:
Percussion dullness (positive likelihood ratio 5.7 based on 4 studies with 3,653 patients)6,10–12
Bronchophony or bronchial breath sounds (positive likelihood ratio 3.3 based on 1,118 patients)10
Crackles have long been taught as a common physical finding in pneumonia. Bohadana et al pointed out that “crackle” can be defined acoustically but does not suggest any means or site of generation.4 Pooled data from 4 studies in 3,647 patients6,10–12 result in a positive likelihood ratio for crackles in the diagnosis of pneumonia of only 3.2.
Diminished breath sounds (positive likelihood ratio 2.5 based on 3 studies with 1,828 patients).10–12
Consider pneumonia signs in combination
These physical examination maneuvers are time-honored and part of the rite of training for medical students and residents. As we have shown, they are not extremely helpful as individual tests in diagnosing pneumonia; however, they may be useful when used in combination as a clinical prediction rule or diagnostic algorithm. These rules often have higher diagnostic accuracy but drawbacks of taking more time and not being easily reproducible.
PLEURAL EFFUSION
Pleural effusion commonly occurs in patients with congestive heart failure, pneumonia, and malignancies. The following are signs of effusion.
Dullness to percussion had a positive likelihood ratio of 5.7 from pooled data from 3 studies analyzed by Wong et al.13
Asymmetric chest expansion, in a study by Kalantri et al,14 had a positive likelihood ratio of 8.1 and a negative likelihood ratio of 0.29, the latter making it a reasonably good test to help rule out a pleural effusion.
Negative signs. Since a pleural effusion is an abnormal fluid collection in the pleural space and not the lung parenchyma, one would not expect it to cause loud breath sounds, adventitious sounds, or vocal resonance. Since these 3 findings emanate from the lung, their absence would be expected to support the presence of a pleural effusion.
Tactile fremitus, also known as vocal fremitus, is the vibration felt on the chest wall while the patient is speaking. Traditionally, the patient says “ninety-nine” as the examiner feels for asymmetry in vibration. A consolidation such as pneumonia increases the vibration, while fluid in a pleural effusion diminishes it.
DIAGNOSTIC ALGORITHM FOR PNEUMONIA OR PLEURAL EFFUSION
Patients presenting with cough or dyspnea will most likely be evaluated for pneumonia and pleural effusion, among other diagnoses. We propose the following physical examination strategy in this setting.
First, evaluate the patient for asymmetric chest expansion. The positive likelihood ratio for this sign is excellent for pneumonia (44.1) and moderate for pleural effusion (8.1); therefore, both conditions are possible with a positive test.
Second, percuss the chest. Dullness to percussion has a low positive likelihood ratio for pneumonia but a moderate one for pleural effusion.13 The absence of this sign is only modest in excluding a pleural effusion (negative likelihood ratio 0.31 in pooled data analyzed by Wong et al).13
Third, auscultate the chest to elicit normal, diminished, or adventitious breath sounds. Diminished breath sounds may be noted in both conditions, but vocal resonance (egophony or bronchophony) and tactile fremitus should not be present directly over a pleural effusion. Either vocal resonance or tactile fremitus in a patient with asymmetric chest expansion would strongly support the diagnosis of pneumonia.
Figure 2 summarizes our proposed diagnostic algorithm for pneumonia and pleural effusion.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
COPD imposes a heavy burden on public health worldwide in terms of cost and mortality. It is the third leading cause of death in the United States, after heart disease and cancer.15
Spirometry remains the gold standard for diagnosis. The Global Initiative for Chronic Obstructive Lung Disease standard for diagnosing COPD was the better of 2 spirometry test results, showing a forced expiratory volume in 1 second (FEV1) and FEV1/forced vital capacity ratio less than 70%.16
Unfortunately, there is little evidence that physical signs aid in the early diagnosis of COPD, as physical signs of airflow limitation may not manifest until lung function is substantially impaired.17,18
Early inspiratory crackles had a positive likelihood ratio of 14.6 based on 2 small studies.19,20
Percussion dullness over the left sternal border in the fifth intercostal space should be present in the normal situation and is known as cardiac dullness. Absent cardiac dullness had a positive likelihood ratio of 16 and a negative likelihood ratio of 0.8 for diagnosing COPD in a study in 92 patients with a history of smoking or self-reported COPD.21 The kappa score was 0.49, signifying moderate interobserver agreement.
A combined strategy using the history and physical examination may have the highest diagnostic accuracy. Many of these combinations are too cumbersome for practical clinical use. However, 1 of them is based on only 3 questions21:
- Has the patient smoked for more than 70-pack years?
- Has the patient been previously diagnosed with chronic bronchitis or emphysema?
- Are breath sounds diminished in intensity?
Answering yes to 2 of these questions gives a positive likelihood ratio of a diagnosis of COPD of 33.5.
Early detection of COPD may improve outcomes and lower healthcare costs and thus would be clinically useful. Unfortunately, a diagnostic approach using the history and physical in the early diagnosis of COPD remains uncertain at this time.
CONGESTIVE HEART FAILURE
The clinical presentation of acute congestive heart failure has much in common with pneumonia, pleural effusion, and COPD.
Echocardiography, the gold standard for diagnosis, is costly and may not be immediately available for most patients evaluated for cardiorespiratory complaints. The American College of Cardiology reports the cost of standard echocardiography to be between $1,000 and $2,000.22 A physical examination approach in the assessment of dyspnea can be very useful.
Height of jugular venous distention approximates central venous pressure
Assessing the central venous pressure by estimating the vertical height of distention of the right internal or external jugular vein is validated and easily reproducible.23,24 The use of the external jugular vein is supported by correlation with catheter-measured central venous pressure in critically ill patients.25,26 The central venous pressure reflects the right atrial pressure, and in the absence of tricuspid stenosis, the right ventricular end-diastolic pressure. An elevation in central venous pressure can be seen in patients with congestive heart failure, pulmonary hypertension, and pulmonary valve stenosis.
The right side is preferred due to its anatomically direct route to the heart. In contrast, the left internal jugular vein crosses the mediastinum and can be compressed by the aorta, causing a false elevation.
In summary, an elevated jugular venous pressure on examination is a good test to rule in an elevated central venous pressure, and its absence is a good sign in ruling out an elevated central venous pressure. When using jugular venous pressure specifically for the diagnosis of congestive heart failure with reduced ejection fraction (ie, ejection fraction < 50%), the positive likelihood ratio is 6.3 based on 3 studies.25–27
Heart failure with preserved ejection fraction has not been well studied for physical examination. The Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve)28 looked only at the sensitivity of elevated jugular venous pressure in 4,128 patients, which was 8%. Specificity was not reported.
The abdominojugular reflux
Another way to gauge the jugular venous pressure is to examine the neck veins while firmly pressing on the mid-abdomen for 10 to 15 seconds to look for the abdominojugular reflux, also known as the hepatojugular reflux. An increase in the jugular venous pressure of 3 cm from baseline constitutes a positive abdominojugular reflux. It has a positive likelihood ratio of 8.0 and a negative likelihood ratio of 0.3 for the diagnosis of congestive heart failure by the assessment of end-diastolic pressure of the left ventricle (Table 5).29–31
The abdominojugular reflux is a much more reliable test than examination of neck veins for jugular venous pressure. The interobserver agreement for examining neck veins has a wide range of kappa scores (0.08–0.81), whereas the abdominojugular reflux has a very high kappa score of 0.92.7 Interestingly, chest radiography showing interstitial edema has a kappa of 0.83.7
Displaced apical impulse
An evaluation of the apical impulse of the heart is also a very good and quick test in the examination of patients suspected of having congestive heart failure. An abnormal finding is defined by an apical impulse displaced laterally (to the left of the midclavicular line).
Using data from several studies,32–35 a displaced apical impulse has a positive likelihood ratio of 10.3. The absence of this finding, however, is not very good for ruling out congestive heart failure, with a negative likelihood ratio of 0.7. Interobserver agreement is moderate to excellent (kappa score 0.43–0.86).7
A third heart sound
Auscultation to assess the third heart sound is much more difficult. A systematic review found that likelihood ratios vary widely and confidence intervals are wide.36 Interobserver agreement also varies widely (kappa scores –0.17 to 0.84).7 In a primary care study,37 a third heart sound had a very low sensitivity (4.3%) but a specificity of 99.8%.
Therefore, we are uncertain about a conclusion for this physical finding based on the concern for wide ranges in likelihood ratio and poor interobserver reliability.
PHYSICAL EXAMINATION STILL HAS A FUTURE
The physical examination has a long and distinguished place in the history of medicine. Technologic advances have changed the manner in which clinicians practice the art of healing. Modern technology in US healthcare has become a double-edged sword, with many benefits as well as detriments.3 Reproducibility and accuracy are paramount for the physical examination to remain a core component of medical diagnosis. Advances in the diagnostic accuracy of laboratory and imaging studies challenge the importance of the physical examination. However, we firmly believe that the traditional techniques have stood the test of time and have a future in the clinical practice of medicine.
Acknowledgments: The authors thank Ruby Marr, MD, Mohammed Nabhan, MD, Rajiv Doddamani, MD, and Sohaib Galani, MD, for their important contributions to this article, which included research assistance and editorial advice.
Laennec’s stethoscope has survived more than 200 years, much longer than some of his contemporaries predicted. But will it survive the challenge of bedside ultrasonography and other technologic advances?
The physical examination, with its roots extending at least as far back as Hippocrates, may be at a crossroads as the mainstay of diagnosis. Physical signs can be subjective and lack sensitivity and specificity. Modern imaging and laboratory studies may already be more trusted.
If the physical examination is to survive, it must be accurate, reproducible, and efficient. Needed is a simple, evidence-based approach to the physical examination that enhances its diagnostic accuracy while maintaining bedside efficiency.
Here, we analyze the accuracy of the physical signs that are most effective in the clinical diagnosis of 4 common cardiopulmonary conditions that often present with dyspnea: pneumonia, pleural effusion, chronic obstructive pulmonary disease (COPD), and congestive heart failure.
LIKELIHOOD RATIOS
To grasp the significance of physical findings, it is necessary to understand the concept of likelihood ratios, which are widely accepted measures of the accuracy of a test or clinical finding.1,2 The positive likelihood ratio is the probability of a disease being present when the test is positive or the clinical finding is present, while the negative likelihood ratio is the probability that the disease is present when the test is negative or the clinical finding is absent. They are calculated as follows1:
Positive likelihood ratio = sensitivity / (1 – specificity)
Negative likelihood ratio = (1 – sensitivity) / specificity
Table 1 shows how the likelihood ratio of a test changes the posttest probability that a condition is present or absent, according to an analysis by McGee.2
STANDARDIZED TERMINOLOGY
PNEUMONIA
Pneumonia is a common disease, with more than 2 million cases annually in the United States. It is most often diagnosed by standard chest radiography, although computed tomography can identify it earlier and with higher sensitivity and specificity.5 The amount of published data on physical examination findings in pneumonia is surprisingly small.
Asymmetry in chest expansion: Specific, reproducible, but not sensitive
The physical finding with the highest positive likelihood ratio for diagnosing pneumonia is asymmetry in chest expansion.6,7
In a 1984 study of 1,819 patients presenting to an emergency department with acute cough, Diehr et al6 evaluated several physical signs of pneumonia. Asymmetric chest expansion had a specificity and positive predictive value of 100%, but its sensitivity was only 4.3%. Thus, it is not a good screening test, but it is a good diagnostic or confirmatory test. From these numbers, Metlay et al8 calculated that the positive likelihood ratio was infinity and the negative likelihood ratio was 0.96.
McGee,7 on the other hand, calculated the positive likelihood ratio of asymmetric chest expansion at 44.1. McGee also found chest expansion to be a highly reproducible finding, with an interobserver agreement kappa score of 0.85.7 (A kappa score of 1.0 would indicate perfect interobserver agreement.) Interestingly, chest radiographs interpreted for pulmonary infiltrates have an interobserver kappa score of only 0.38.7 Further studies of this physical sign could shed more light upon this area of uncertainty.
Other signs of pneumonia
None of the other physical signs studied for the diagnosis of pneumonia has as high a positive likelihood ratio as asymmetric chest expansion.6–12
Egophony is a high-pitched or nasal quality of the patient’s voice heard on auscultation over lung tissue that is consolidated or fibrosed, due to enhanced transmission of high-frequency sound across fluid. It is often described as the “E-to-A change.” Although listening for egophony is widely done and easy to do, we calculate that this sign has a positive likelihood ratio of only 6.8 based on pooled data from 3 trials with a total of 3,245 patients.6,10,11
Faring less favorably, in descending order of diagnostic accuracy, are:
Percussion dullness (positive likelihood ratio 5.7 based on 4 studies with 3,653 patients)6,10–12
Bronchophony or bronchial breath sounds (positive likelihood ratio 3.3 based on 1,118 patients)10
Crackles have long been taught as a common physical finding in pneumonia. Bohadana et al pointed out that “crackle” can be defined acoustically but does not suggest any means or site of generation.4 Pooled data from 4 studies in 3,647 patients6,10–12 result in a positive likelihood ratio for crackles in the diagnosis of pneumonia of only 3.2.
Diminished breath sounds (positive likelihood ratio 2.5 based on 3 studies with 1,828 patients).10–12
Consider pneumonia signs in combination
These physical examination maneuvers are time-honored and part of the rite of training for medical students and residents. As we have shown, they are not extremely helpful as individual tests in diagnosing pneumonia; however, they may be useful when used in combination as a clinical prediction rule or diagnostic algorithm. These rules often have higher diagnostic accuracy but drawbacks of taking more time and not being easily reproducible.
PLEURAL EFFUSION
Pleural effusion commonly occurs in patients with congestive heart failure, pneumonia, and malignancies. The following are signs of effusion.
Dullness to percussion had a positive likelihood ratio of 5.7 from pooled data from 3 studies analyzed by Wong et al.13
Asymmetric chest expansion, in a study by Kalantri et al,14 had a positive likelihood ratio of 8.1 and a negative likelihood ratio of 0.29, the latter making it a reasonably good test to help rule out a pleural effusion.
Negative signs. Since a pleural effusion is an abnormal fluid collection in the pleural space and not the lung parenchyma, one would not expect it to cause loud breath sounds, adventitious sounds, or vocal resonance. Since these 3 findings emanate from the lung, their absence would be expected to support the presence of a pleural effusion.
Tactile fremitus, also known as vocal fremitus, is the vibration felt on the chest wall while the patient is speaking. Traditionally, the patient says “ninety-nine” as the examiner feels for asymmetry in vibration. A consolidation such as pneumonia increases the vibration, while fluid in a pleural effusion diminishes it.
DIAGNOSTIC ALGORITHM FOR PNEUMONIA OR PLEURAL EFFUSION
Patients presenting with cough or dyspnea will most likely be evaluated for pneumonia and pleural effusion, among other diagnoses. We propose the following physical examination strategy in this setting.
First, evaluate the patient for asymmetric chest expansion. The positive likelihood ratio for this sign is excellent for pneumonia (44.1) and moderate for pleural effusion (8.1); therefore, both conditions are possible with a positive test.
Second, percuss the chest. Dullness to percussion has a low positive likelihood ratio for pneumonia but a moderate one for pleural effusion.13 The absence of this sign is only modest in excluding a pleural effusion (negative likelihood ratio 0.31 in pooled data analyzed by Wong et al).13
Third, auscultate the chest to elicit normal, diminished, or adventitious breath sounds. Diminished breath sounds may be noted in both conditions, but vocal resonance (egophony or bronchophony) and tactile fremitus should not be present directly over a pleural effusion. Either vocal resonance or tactile fremitus in a patient with asymmetric chest expansion would strongly support the diagnosis of pneumonia.
Figure 2 summarizes our proposed diagnostic algorithm for pneumonia and pleural effusion.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
COPD imposes a heavy burden on public health worldwide in terms of cost and mortality. It is the third leading cause of death in the United States, after heart disease and cancer.15
Spirometry remains the gold standard for diagnosis. The Global Initiative for Chronic Obstructive Lung Disease standard for diagnosing COPD was the better of 2 spirometry test results, showing a forced expiratory volume in 1 second (FEV1) and FEV1/forced vital capacity ratio less than 70%.16
Unfortunately, there is little evidence that physical signs aid in the early diagnosis of COPD, as physical signs of airflow limitation may not manifest until lung function is substantially impaired.17,18
Early inspiratory crackles had a positive likelihood ratio of 14.6 based on 2 small studies.19,20
Percussion dullness over the left sternal border in the fifth intercostal space should be present in the normal situation and is known as cardiac dullness. Absent cardiac dullness had a positive likelihood ratio of 16 and a negative likelihood ratio of 0.8 for diagnosing COPD in a study in 92 patients with a history of smoking or self-reported COPD.21 The kappa score was 0.49, signifying moderate interobserver agreement.
A combined strategy using the history and physical examination may have the highest diagnostic accuracy. Many of these combinations are too cumbersome for practical clinical use. However, 1 of them is based on only 3 questions21:
- Has the patient smoked for more than 70-pack years?
- Has the patient been previously diagnosed with chronic bronchitis or emphysema?
- Are breath sounds diminished in intensity?
Answering yes to 2 of these questions gives a positive likelihood ratio of a diagnosis of COPD of 33.5.
Early detection of COPD may improve outcomes and lower healthcare costs and thus would be clinically useful. Unfortunately, a diagnostic approach using the history and physical in the early diagnosis of COPD remains uncertain at this time.
CONGESTIVE HEART FAILURE
The clinical presentation of acute congestive heart failure has much in common with pneumonia, pleural effusion, and COPD.
Echocardiography, the gold standard for diagnosis, is costly and may not be immediately available for most patients evaluated for cardiorespiratory complaints. The American College of Cardiology reports the cost of standard echocardiography to be between $1,000 and $2,000.22 A physical examination approach in the assessment of dyspnea can be very useful.
Height of jugular venous distention approximates central venous pressure
Assessing the central venous pressure by estimating the vertical height of distention of the right internal or external jugular vein is validated and easily reproducible.23,24 The use of the external jugular vein is supported by correlation with catheter-measured central venous pressure in critically ill patients.25,26 The central venous pressure reflects the right atrial pressure, and in the absence of tricuspid stenosis, the right ventricular end-diastolic pressure. An elevation in central venous pressure can be seen in patients with congestive heart failure, pulmonary hypertension, and pulmonary valve stenosis.
The right side is preferred due to its anatomically direct route to the heart. In contrast, the left internal jugular vein crosses the mediastinum and can be compressed by the aorta, causing a false elevation.
In summary, an elevated jugular venous pressure on examination is a good test to rule in an elevated central venous pressure, and its absence is a good sign in ruling out an elevated central venous pressure. When using jugular venous pressure specifically for the diagnosis of congestive heart failure with reduced ejection fraction (ie, ejection fraction < 50%), the positive likelihood ratio is 6.3 based on 3 studies.25–27
Heart failure with preserved ejection fraction has not been well studied for physical examination. The Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve)28 looked only at the sensitivity of elevated jugular venous pressure in 4,128 patients, which was 8%. Specificity was not reported.
The abdominojugular reflux
Another way to gauge the jugular venous pressure is to examine the neck veins while firmly pressing on the mid-abdomen for 10 to 15 seconds to look for the abdominojugular reflux, also known as the hepatojugular reflux. An increase in the jugular venous pressure of 3 cm from baseline constitutes a positive abdominojugular reflux. It has a positive likelihood ratio of 8.0 and a negative likelihood ratio of 0.3 for the diagnosis of congestive heart failure by the assessment of end-diastolic pressure of the left ventricle (Table 5).29–31
The abdominojugular reflux is a much more reliable test than examination of neck veins for jugular venous pressure. The interobserver agreement for examining neck veins has a wide range of kappa scores (0.08–0.81), whereas the abdominojugular reflux has a very high kappa score of 0.92.7 Interestingly, chest radiography showing interstitial edema has a kappa of 0.83.7
Displaced apical impulse
An evaluation of the apical impulse of the heart is also a very good and quick test in the examination of patients suspected of having congestive heart failure. An abnormal finding is defined by an apical impulse displaced laterally (to the left of the midclavicular line).
Using data from several studies,32–35 a displaced apical impulse has a positive likelihood ratio of 10.3. The absence of this finding, however, is not very good for ruling out congestive heart failure, with a negative likelihood ratio of 0.7. Interobserver agreement is moderate to excellent (kappa score 0.43–0.86).7
A third heart sound
Auscultation to assess the third heart sound is much more difficult. A systematic review found that likelihood ratios vary widely and confidence intervals are wide.36 Interobserver agreement also varies widely (kappa scores –0.17 to 0.84).7 In a primary care study,37 a third heart sound had a very low sensitivity (4.3%) but a specificity of 99.8%.
Therefore, we are uncertain about a conclusion for this physical finding based on the concern for wide ranges in likelihood ratio and poor interobserver reliability.
PHYSICAL EXAMINATION STILL HAS A FUTURE
The physical examination has a long and distinguished place in the history of medicine. Technologic advances have changed the manner in which clinicians practice the art of healing. Modern technology in US healthcare has become a double-edged sword, with many benefits as well as detriments.3 Reproducibility and accuracy are paramount for the physical examination to remain a core component of medical diagnosis. Advances in the diagnostic accuracy of laboratory and imaging studies challenge the importance of the physical examination. However, we firmly believe that the traditional techniques have stood the test of time and have a future in the clinical practice of medicine.
Acknowledgments: The authors thank Ruby Marr, MD, Mohammed Nabhan, MD, Rajiv Doddamani, MD, and Sohaib Galani, MD, for their important contributions to this article, which included research assistance and editorial advice.
- Lang TA, Secic M. Chapter 10. Determining the presence or absence of disease. Reporting the characteristics of diagnostic tests. In: Lang TC, Secic M. How to Report Statistics in Medicine. Annotated Guidelines for Authors, Editors, and Reviewers. Philadelphia, PA, American College of Physicians, 1997:147–169.
- McGee S. Simplifying likelihood ratios. J Gen Intern Med 2002; 17:647–650.
- Mikami R, Murao M, Cugell DW, et al. International symposium on lung sounds. Synopsis of proceedings. Chest 1987; 92:342–345.
- Bohadana A, Izbicki G, Kraman SS. Fundamentals of lung auscultation. N Engl J Med 2014; 370:744–751.
- Heussel CP, Kauczor HU, Ullmann AJ. Pneumonia in neutropenic patients. Eur Radiol 2004; 14:256–271.
- Diehr P, Wood RW, Bushyhead J, Krueger L, Wolcott B, Tompkins RK. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis 1984; 37:215–225.
- McGee S. Evidence-Based Physical Diagnosis. 4th ed. Philadelphia, PA: Elsevier; 2017.
- Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997; 278:1440–1445.
- Melbye H, Straume B, Aasebo U, Brox J. The diagnosis of adult pneumonia in general practice. The diagnostic value of history, physical examination and some blood tests. Scand J Prim Health Care 1988; 6:111–117.
- Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med 1990; 113:664–670.
- Gennis P, Gallagher J, Falvo C, Baker S, Than W. Clinical criteria for the detection of pneumonia in adults: guidelines for ordering chest roentgenograms in the emergency department. J Emerg Med 1989; 7:263–268.
- Melbye H, Straume B, Aasebo U, Dale K. Diagnosis of pneumonia in adults in general practice. Relative importance of typical symptoms and abnormal chest signs evaluated against a radiographic reference standard. Scand J Prim Health Care 1992; 10:226–233.
- Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA 2009; 301:309–317.
- Kalantri S, Joshi R, Lokhande T, et al. Accuracy and reliability of physical signs in the diagnosis of pleural effusion. Respir Med 2007; 101:431–438.
- Heron M. Deaths: leading causes for 2014. National Vital Statistics Reports 2016; 65(5) June 30, 2016. www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_05.pdf. Accessed October 20, 2017.
- Global Initiative for Chronic Obstructive Lung Disease. Pocket guide to COPD diagnosis, management, and prevention. http://goldcopd.org/wp-content/uploads/2016/12/wms-GOLD-2017-Pocket-Guide.pdf. Accessed November 13, 2017.
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004; 364:613–620.
- Oshaug K, Halvorsen PA, Melbye H. Should chest examination be reinstated in the early diagnosis of chronic obstructive pulmonary disease? Int J Chron Obstruct Pulmon Dis 2013; 8:369–377.
- Bettencourt PE, Del Bono EA, Spiegelman D, Hertzmark E, Murphy RL Jr. Clinical utility of chest auscultation in common pulmonary diseases. Am J Respir Crit Care Med 1994; 150:1291–1297.
- Nath AR, Capel LH. Inspiratory crackles and mechanical events of breathing. Thorax 1974; 29:695–698.
- Badgett RG, Tanaka DJ, Hunt DK, et al. Can moderate chronic obstructive pulmonary disease be diagnosed by historical and physical findings alone? Am J Med 1993; 94:188–196.
- ABIM Foundation. Choosing wisely. Echocardiograms for heart valve disease. www.choosingwisely.org. Accessed November 13, 2017.
- Davison R, Cannon R. Estimation of central venous pressure by examination of jugular veins. Am Heart J 1974; 87:279–282.
- Ducas J, Magder S, McGregor M. Validity of the hepatojugular reflux as a clinical test for congestive heart failure. Am J Cardiol 1983; 52:1299–1303.
- Vinayak AG, Levitt J, Gehlbach B, Pohlman AS, Hall JB, Kress JP. Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients. Arch Intern Med 2006; 166:2132–2137.
- Sankoff J, Zidulka A. Non-invasive method for the rapid assessment of central venous pressure: description and validation by a single examiner. West J Emerg Med 2008; 9:201–205.
- Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJ. Assessing diagnosis in heart failure: which features are any use? QJM 1997; 90:335–339.
- Kristensen SL, Mogensen UM, Jhund PS, et al. Clinical and echocardiographic characeristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction. A report from the Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve). Circulation 2017; https://doi.org/10.1161/CIRCULATIONAHA.116.024593. Accessed November 1, 2017.
- Butman SM, Ewy GA, Standen JR, Kern KB, Hahn E. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distension. J Am Coll Cardiol 1993; 22:968–974.
- Sochowski RA, Dubbin JD, Naqvi SZ. Clinical and hemodynamic assessment of the hepatojugular reflux. Am J Cardiol 1990; 66:1002–1006.
- Ewy GA. The abdominojugular test: technique and hemodynamic correlates. Ann Intern Med 1988; 109:456–460.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Symptoms and signs of heart failure in patients with myocardial infarction: reproducibility and relationship to chest X-ray, radionuclide ventriculography and right heart catheterization. Eur Heart J 1989; 10:1017–1028.
- Fahey T, Jeyaseelan S, McCowan C, et al. Diagnosis of left ventricular systolic dysfunction (LVSD): development and validation of a clinical prediction rule in primary care. Fam Pract 2007; 24:628–635.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Interobserver agreement and accuracy of bedside estimation of right and left ventricular ejection fraction in acute myocardial infarction. Am J Cardiol 1989; 63:1301–1307.
- Mattleman SJ, Hakki AH, Iskandrian AS, Segal BL, Kane SA. Reliability of bedside evaluation in determining left ventricular function: correlation with left ventricular ejection fraction determined by radionuclide ventriculography. J Am Coll Cardiol 1983; 1:417–420.
- Madhok V, Falk G, Rogers A, Struthers AD, Sullivan FM, Fahey T. The accuracy of symptoms, signs and diagnostic tests in the diagnosis of left ventricular dysfunction in primary care: a diagnostic accuracy systematic review. BMC Fam Pract 2008; 9:56.
- Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation 2011; 124:2865–2873.
- Lang TA, Secic M. Chapter 10. Determining the presence or absence of disease. Reporting the characteristics of diagnostic tests. In: Lang TC, Secic M. How to Report Statistics in Medicine. Annotated Guidelines for Authors, Editors, and Reviewers. Philadelphia, PA, American College of Physicians, 1997:147–169.
- McGee S. Simplifying likelihood ratios. J Gen Intern Med 2002; 17:647–650.
- Mikami R, Murao M, Cugell DW, et al. International symposium on lung sounds. Synopsis of proceedings. Chest 1987; 92:342–345.
- Bohadana A, Izbicki G, Kraman SS. Fundamentals of lung auscultation. N Engl J Med 2014; 370:744–751.
- Heussel CP, Kauczor HU, Ullmann AJ. Pneumonia in neutropenic patients. Eur Radiol 2004; 14:256–271.
- Diehr P, Wood RW, Bushyhead J, Krueger L, Wolcott B, Tompkins RK. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis 1984; 37:215–225.
- McGee S. Evidence-Based Physical Diagnosis. 4th ed. Philadelphia, PA: Elsevier; 2017.
- Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997; 278:1440–1445.
- Melbye H, Straume B, Aasebo U, Brox J. The diagnosis of adult pneumonia in general practice. The diagnostic value of history, physical examination and some blood tests. Scand J Prim Health Care 1988; 6:111–117.
- Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med 1990; 113:664–670.
- Gennis P, Gallagher J, Falvo C, Baker S, Than W. Clinical criteria for the detection of pneumonia in adults: guidelines for ordering chest roentgenograms in the emergency department. J Emerg Med 1989; 7:263–268.
- Melbye H, Straume B, Aasebo U, Dale K. Diagnosis of pneumonia in adults in general practice. Relative importance of typical symptoms and abnormal chest signs evaluated against a radiographic reference standard. Scand J Prim Health Care 1992; 10:226–233.
- Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA 2009; 301:309–317.
- Kalantri S, Joshi R, Lokhande T, et al. Accuracy and reliability of physical signs in the diagnosis of pleural effusion. Respir Med 2007; 101:431–438.
- Heron M. Deaths: leading causes for 2014. National Vital Statistics Reports 2016; 65(5) June 30, 2016. www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_05.pdf. Accessed October 20, 2017.
- Global Initiative for Chronic Obstructive Lung Disease. Pocket guide to COPD diagnosis, management, and prevention. http://goldcopd.org/wp-content/uploads/2016/12/wms-GOLD-2017-Pocket-Guide.pdf. Accessed November 13, 2017.
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004; 364:613–620.
- Oshaug K, Halvorsen PA, Melbye H. Should chest examination be reinstated in the early diagnosis of chronic obstructive pulmonary disease? Int J Chron Obstruct Pulmon Dis 2013; 8:369–377.
- Bettencourt PE, Del Bono EA, Spiegelman D, Hertzmark E, Murphy RL Jr. Clinical utility of chest auscultation in common pulmonary diseases. Am J Respir Crit Care Med 1994; 150:1291–1297.
- Nath AR, Capel LH. Inspiratory crackles and mechanical events of breathing. Thorax 1974; 29:695–698.
- Badgett RG, Tanaka DJ, Hunt DK, et al. Can moderate chronic obstructive pulmonary disease be diagnosed by historical and physical findings alone? Am J Med 1993; 94:188–196.
- ABIM Foundation. Choosing wisely. Echocardiograms for heart valve disease. www.choosingwisely.org. Accessed November 13, 2017.
- Davison R, Cannon R. Estimation of central venous pressure by examination of jugular veins. Am Heart J 1974; 87:279–282.
- Ducas J, Magder S, McGregor M. Validity of the hepatojugular reflux as a clinical test for congestive heart failure. Am J Cardiol 1983; 52:1299–1303.
- Vinayak AG, Levitt J, Gehlbach B, Pohlman AS, Hall JB, Kress JP. Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients. Arch Intern Med 2006; 166:2132–2137.
- Sankoff J, Zidulka A. Non-invasive method for the rapid assessment of central venous pressure: description and validation by a single examiner. West J Emerg Med 2008; 9:201–205.
- Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJ. Assessing diagnosis in heart failure: which features are any use? QJM 1997; 90:335–339.
- Kristensen SL, Mogensen UM, Jhund PS, et al. Clinical and echocardiographic characeristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction. A report from the Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve). Circulation 2017; https://doi.org/10.1161/CIRCULATIONAHA.116.024593. Accessed November 1, 2017.
- Butman SM, Ewy GA, Standen JR, Kern KB, Hahn E. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distension. J Am Coll Cardiol 1993; 22:968–974.
- Sochowski RA, Dubbin JD, Naqvi SZ. Clinical and hemodynamic assessment of the hepatojugular reflux. Am J Cardiol 1990; 66:1002–1006.
- Ewy GA. The abdominojugular test: technique and hemodynamic correlates. Ann Intern Med 1988; 109:456–460.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Symptoms and signs of heart failure in patients with myocardial infarction: reproducibility and relationship to chest X-ray, radionuclide ventriculography and right heart catheterization. Eur Heart J 1989; 10:1017–1028.
- Fahey T, Jeyaseelan S, McCowan C, et al. Diagnosis of left ventricular systolic dysfunction (LVSD): development and validation of a clinical prediction rule in primary care. Fam Pract 2007; 24:628–635.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Interobserver agreement and accuracy of bedside estimation of right and left ventricular ejection fraction in acute myocardial infarction. Am J Cardiol 1989; 63:1301–1307.
- Mattleman SJ, Hakki AH, Iskandrian AS, Segal BL, Kane SA. Reliability of bedside evaluation in determining left ventricular function: correlation with left ventricular ejection fraction determined by radionuclide ventriculography. J Am Coll Cardiol 1983; 1:417–420.
- Madhok V, Falk G, Rogers A, Struthers AD, Sullivan FM, Fahey T. The accuracy of symptoms, signs and diagnostic tests in the diagnosis of left ventricular dysfunction in primary care: a diagnostic accuracy systematic review. BMC Fam Pract 2008; 9:56.
- Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation 2011; 124:2865–2873.
KEY POINTS
- Asymmetrical chest expansion, diminished breath sounds, egophony, bronchophony, and tactile fremitus can be used in combination to accurately diagnose pneumonia and pleural effusion.
- No physical sign performs with a high degree of accuracy for diagnosing early-stage chronic obstructive pulmonary disease.
- Inspiratory crackles, diminished breath sounds, and cardiac dullness have high diagnostic value for advanced obstructive airway disease.
- Congestive heart failure can be diagnosed at the bedside by examining the jugular veins and palpating the point of maximal intensity.