User login
Predictors may help to spot risk for hydroxychloroquine nonadherence
who are newly prescribed the antimalarial, an administrative claims database study has revealed.
Investigators from Brigham and Women’s Hospital, Boston, used prescription refill data to assess nonadherence, hoping to gain greater insight into predictors of nonadherence to HCQ than past studies of nonadherence to the drug, which have been limited by their small and cross-sectional nature and the fact that they often relied on self-reported measures of adherence that were unable to capture the dynamic nature of adherence behavior over time.
In the current study, Candace H. Feldman, MD, ScD, and her coinvestigators used Medicaid data from 28 U.S. states to identify 10,406 adult HCQ initiators with SLE during 2001-2010. Patients included in the study were required to have more than 365 days of continuous follow-up documented.
The researchers described four distinctive monthly patterns of behavior during the first year of use, in which they defined nonadherence as less than 80% of days covered per month by a HCQ prescription. Group 1 comprised 36% who were “persistent nonadherers” who had very few HCQ refills after the initial dispensing.
Almost half of the cohort (47%) formed two dynamic patterns of partial adherence (groups 2 and 3). The trajectories for these groups were similar until month 5, when they diverged: group 3 improved slightly and then reached a plateau, whereas group 2 became nearly completely nonadherent for the remainder of follow-up. At this 5-month point of divergence, belonging to group 2 was more likely among patients with younger age and antidepressant use. Also, patients in group 3 had more hospitalizations beginning at 4 months and longer hospitalizations at 5 months.
For these two “undecided” groups, 5 months may be a “critical juncture” for physicians to intervene, the authors said.
“Five months might also be the point at which patients feel that they have adequately trialed the medication, and if there is no symptomatic improvement, they discontinue. With the growing body of literature suggesting long-term preventive effects from HCQ, increased provider and patient education at this juncture may be beneficial,” they wrote.
Group 4 had persistent adherence and constituted 17% of the cohort, although this group also experienced a decline in adherence at 9 months.
The mean age of group 4 was about 40 years, which was significantly older than 37 years in groups 1-3. Blacks comprised the highest percentage of patients in groups 1-3 (43%-45%), whereas whites at 40% were the highest proportion in group 4. Individuals in group 4 also had slightly higher average income than did group 1 (mean $46,000 vs. $44,000). The index for SLE risk adjustment was highest for group 4 patients (1.3 vs. 0.9-1.1 for other groups), indicating they may have had more SLE-related comorbidities. Group 4 patients also had a greater average number of medications dispensed and a higher mean daily prednisone-equivalent dose.
Patients aged 18-50 or with black race or Hispanic ethnicity were significantly more likely to be in one of the nonadherent trajectory groups (1, 2, and 3), whereas Asians were less likely to be in group 1 than in group 4, compared with whites.
Diabetes made patients more likely to belong to group 1 than group 4, whereas each unit increase in the SLE risk-adjustment index increased the odds of belonging to group 1 vs. 4. Antidepressant use was associated with greater likelihood of belong to groups 1 or 2 vs. group 4.
Addressing potentially modifiable factors such as ensuring sustained access to health care, particularly for patients with severe disease, might go some way to improving adherence, suggested the researchers, who also noted that increased counseling and support at the time of the first HCQ prescription and throughout the first year of use are also needed in order to “promote more sustained patterns of adherence for all patients.”
The study was funded by a Rheumatology Research Foundation Investigator Award and individual grant awards from the National Institutes of Health. No relevant financial disclosures were declared by the authors.
SOURCE: Feldman C et al. Semin Arthritis Rheum. 2018 Jan 8. doi: 10.1016/j.semarthrit.2018.01.002
who are newly prescribed the antimalarial, an administrative claims database study has revealed.
Investigators from Brigham and Women’s Hospital, Boston, used prescription refill data to assess nonadherence, hoping to gain greater insight into predictors of nonadherence to HCQ than past studies of nonadherence to the drug, which have been limited by their small and cross-sectional nature and the fact that they often relied on self-reported measures of adherence that were unable to capture the dynamic nature of adherence behavior over time.
In the current study, Candace H. Feldman, MD, ScD, and her coinvestigators used Medicaid data from 28 U.S. states to identify 10,406 adult HCQ initiators with SLE during 2001-2010. Patients included in the study were required to have more than 365 days of continuous follow-up documented.
The researchers described four distinctive monthly patterns of behavior during the first year of use, in which they defined nonadherence as less than 80% of days covered per month by a HCQ prescription. Group 1 comprised 36% who were “persistent nonadherers” who had very few HCQ refills after the initial dispensing.
Almost half of the cohort (47%) formed two dynamic patterns of partial adherence (groups 2 and 3). The trajectories for these groups were similar until month 5, when they diverged: group 3 improved slightly and then reached a plateau, whereas group 2 became nearly completely nonadherent for the remainder of follow-up. At this 5-month point of divergence, belonging to group 2 was more likely among patients with younger age and antidepressant use. Also, patients in group 3 had more hospitalizations beginning at 4 months and longer hospitalizations at 5 months.
For these two “undecided” groups, 5 months may be a “critical juncture” for physicians to intervene, the authors said.
“Five months might also be the point at which patients feel that they have adequately trialed the medication, and if there is no symptomatic improvement, they discontinue. With the growing body of literature suggesting long-term preventive effects from HCQ, increased provider and patient education at this juncture may be beneficial,” they wrote.
Group 4 had persistent adherence and constituted 17% of the cohort, although this group also experienced a decline in adherence at 9 months.
The mean age of group 4 was about 40 years, which was significantly older than 37 years in groups 1-3. Blacks comprised the highest percentage of patients in groups 1-3 (43%-45%), whereas whites at 40% were the highest proportion in group 4. Individuals in group 4 also had slightly higher average income than did group 1 (mean $46,000 vs. $44,000). The index for SLE risk adjustment was highest for group 4 patients (1.3 vs. 0.9-1.1 for other groups), indicating they may have had more SLE-related comorbidities. Group 4 patients also had a greater average number of medications dispensed and a higher mean daily prednisone-equivalent dose.
Patients aged 18-50 or with black race or Hispanic ethnicity were significantly more likely to be in one of the nonadherent trajectory groups (1, 2, and 3), whereas Asians were less likely to be in group 1 than in group 4, compared with whites.
Diabetes made patients more likely to belong to group 1 than group 4, whereas each unit increase in the SLE risk-adjustment index increased the odds of belonging to group 1 vs. 4. Antidepressant use was associated with greater likelihood of belong to groups 1 or 2 vs. group 4.
Addressing potentially modifiable factors such as ensuring sustained access to health care, particularly for patients with severe disease, might go some way to improving adherence, suggested the researchers, who also noted that increased counseling and support at the time of the first HCQ prescription and throughout the first year of use are also needed in order to “promote more sustained patterns of adherence for all patients.”
The study was funded by a Rheumatology Research Foundation Investigator Award and individual grant awards from the National Institutes of Health. No relevant financial disclosures were declared by the authors.
SOURCE: Feldman C et al. Semin Arthritis Rheum. 2018 Jan 8. doi: 10.1016/j.semarthrit.2018.01.002
who are newly prescribed the antimalarial, an administrative claims database study has revealed.
Investigators from Brigham and Women’s Hospital, Boston, used prescription refill data to assess nonadherence, hoping to gain greater insight into predictors of nonadherence to HCQ than past studies of nonadherence to the drug, which have been limited by their small and cross-sectional nature and the fact that they often relied on self-reported measures of adherence that were unable to capture the dynamic nature of adherence behavior over time.
In the current study, Candace H. Feldman, MD, ScD, and her coinvestigators used Medicaid data from 28 U.S. states to identify 10,406 adult HCQ initiators with SLE during 2001-2010. Patients included in the study were required to have more than 365 days of continuous follow-up documented.
The researchers described four distinctive monthly patterns of behavior during the first year of use, in which they defined nonadherence as less than 80% of days covered per month by a HCQ prescription. Group 1 comprised 36% who were “persistent nonadherers” who had very few HCQ refills after the initial dispensing.
Almost half of the cohort (47%) formed two dynamic patterns of partial adherence (groups 2 and 3). The trajectories for these groups were similar until month 5, when they diverged: group 3 improved slightly and then reached a plateau, whereas group 2 became nearly completely nonadherent for the remainder of follow-up. At this 5-month point of divergence, belonging to group 2 was more likely among patients with younger age and antidepressant use. Also, patients in group 3 had more hospitalizations beginning at 4 months and longer hospitalizations at 5 months.
For these two “undecided” groups, 5 months may be a “critical juncture” for physicians to intervene, the authors said.
“Five months might also be the point at which patients feel that they have adequately trialed the medication, and if there is no symptomatic improvement, they discontinue. With the growing body of literature suggesting long-term preventive effects from HCQ, increased provider and patient education at this juncture may be beneficial,” they wrote.
Group 4 had persistent adherence and constituted 17% of the cohort, although this group also experienced a decline in adherence at 9 months.
The mean age of group 4 was about 40 years, which was significantly older than 37 years in groups 1-3. Blacks comprised the highest percentage of patients in groups 1-3 (43%-45%), whereas whites at 40% were the highest proportion in group 4. Individuals in group 4 also had slightly higher average income than did group 1 (mean $46,000 vs. $44,000). The index for SLE risk adjustment was highest for group 4 patients (1.3 vs. 0.9-1.1 for other groups), indicating they may have had more SLE-related comorbidities. Group 4 patients also had a greater average number of medications dispensed and a higher mean daily prednisone-equivalent dose.
Patients aged 18-50 or with black race or Hispanic ethnicity were significantly more likely to be in one of the nonadherent trajectory groups (1, 2, and 3), whereas Asians were less likely to be in group 1 than in group 4, compared with whites.
Diabetes made patients more likely to belong to group 1 than group 4, whereas each unit increase in the SLE risk-adjustment index increased the odds of belonging to group 1 vs. 4. Antidepressant use was associated with greater likelihood of belong to groups 1 or 2 vs. group 4.
Addressing potentially modifiable factors such as ensuring sustained access to health care, particularly for patients with severe disease, might go some way to improving adherence, suggested the researchers, who also noted that increased counseling and support at the time of the first HCQ prescription and throughout the first year of use are also needed in order to “promote more sustained patterns of adherence for all patients.”
The study was funded by a Rheumatology Research Foundation Investigator Award and individual grant awards from the National Institutes of Health. No relevant financial disclosures were declared by the authors.
SOURCE: Feldman C et al. Semin Arthritis Rheum. 2018 Jan 8. doi: 10.1016/j.semarthrit.2018.01.002
FROM SEMINARS IN ARTHRITIS & RHEUMATISM
Key clinical point: Five months after the first HCQ prescription might be a critical time point to review and educate patients on the importance of continuing treatment, particularly for patients who are “partial” adherers.
Major finding: Almost half of the cohort (47%) formed two dynamic patterns of partial adherence.
Data source: A longitudinal study of 10,406 Medicaid beneficiaries with SLE who were prescribed hydroxychloroquine for the first time.
Disclosures: The study was funded by a Rheumatology Research Foundation Investigator Award and individual grant awards from the National Institutes of Health. No relevant financial disclosures were declared by the authors.
Source: Feldman C et al. Semin Arthritis Rheum. 2018 Jan 8. doi: 10.1016/j.semarthrit.2018.01.002
Pulmonary Mucormycosis in a Patient With Uncontrolled Diabetes
Mucorales fungi are ubiquitous organisms commonly inhabiting soil and can cause opportunistic infections. The majority of infections are caused by 3 genera: Rhizopus, Mucor, and Rhizomucor.1 Infection occurs by inhalation or by direct contact with damaged skin. Mucorales infections can have cutaneous, rhinocerebral, pulmonary, gastrointestinal, and central nervous system manifestations. Pulmonary mucormycosis is often rapidly progressive with angioinvasion and fulminant necrosis causing acute dyspnea, hemoptysis, and chest pain. More indolent pulmonary Mucorales infections can mimic a pulmonary mass with occasional cavitation found on imaging studies similar to other fungal infections (eg, Aspergillus).2 Risk factors include severe uncontrolled diabetes mellitus (DM), recurrent diabetic ketoacidosis (DKA), immunosuppression due to congenital or acquired causes, hematologic malignancies, and chronic renal failure.3 The authors present a case of a patient with recurrent DKA and pulmonary mucormycosis.
Case Presentation
A 62-year-old male with DM and a more than 30-pack-year smoking history presented to the emergency department with abdominal pain and chest pain ongoing for about 1 week. The patient had a history of frequent admissions with DKA and medication nonadherence.
On admission, the patient was hemodynamically stable. His vital signs were: temperature 97.4° F, heart rate 89 bpm, respirations 24 breathes per minute, blood pressure 146/86 mm Hg, and oxygen saturation 94% on ambient air. The patient appeared ill but the physical examination was otherwise unremarkable. Laboratory results revealed a white blood cell count of 24,400 with neutrophilic predominance, blood glucose 658 mg/dL, creatinine clearance 2.16 mL/min/1.73 m2, sodium level 124 mEq/L, bicarbonate 6 mEq/L, anion gap 27 mEq/L, 6.8 pH, partial pressure of CO2 11 mm Hg, and lactic acid 2.3 mmol/L.
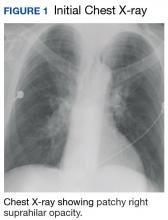
The patient admitted for DKA management and placed on an insulin drip. Although he did not have a fever or cough productive of sputum or hemoptysis, there was concern that pneumonia might have precipitated DKA. A chest X-ray revealed a patchy, right suprahilar opacity (Figure 1).
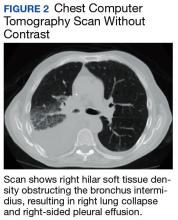
The patient was placed on vancomycin 1,000 mg every 12 hours and cefepime 2,000 mg every 12 hours for possible hospital-acquired pneumonia because of his history of recent DKA hospitalization. Once the patient’s anion gap was closed and metabolic acidosis was resolved, the insulin drip was discontinued, and the patient was transferred to the general medical ward for further management. There, he continued to report having chest pain. A computed tomography (CT) scan without contrast of the chest (contrast was held due to recent acute kidney injury) revealed right hilar soft tissue density obstructing the bronchus intermidius, which had resulted in a right-lung collapse and right-sided pleural effusion (Figure 2). The left lung was clear, and there was no evidence of nodularity.
Given the patient’s extensive smoking history, the initial concern was for pulmonary malignancy. The decision was made to proceed with bronchoscopy with endobronchial ultrasound-guided transbronchial needle biopsy. Endobronchial brushings and biopsies of R11, 7, right bronchus intermedius, and right upper lobe were obtained. Gross inspection of the airway revealed markedly abnormal-appearing mucosa involving the take off to the right upper lobe and the entire bronchus intermedius with friable, cobblestoned, and edematous mucosa. Biopsies and immunostaining for occult carcinoma markers, including CD-56, TTF-1, Synaptophysin A, chromogranin, AE1/AE3, and CK-5/6, were negative for malignancy. Final microbiologic analysis was positive for Mucor. There was no evidence of bacterial or mycobacterial growth.
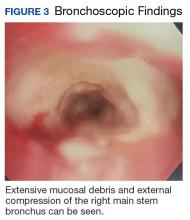
Due to continued suspicion for malignancy and lack of histologic yield, the patient underwent a repeat endobronchial ultrasound-guided needle biopsy. On this occasion, gross inspection revealed significant mucosal necrosis and extensive, extrinsic bronchial compression starting from the right bronchial division and notable throughout the right middle and lower lobes (Figure 3).
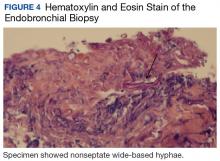
Bronchial washings revealed necrotic material with rare fungal hyphae present. Biopsies yielded necrotic material or lung tissue containing nonseptate hyphae with rare, right-angle branching consistent with Mucor (Figures 4 and 5). Malignancy was not present in the specimens obtained.
Based on the bronchoscopy results, thoracic surgery and infectious disease specialists were consulted. Surgical intervention was not recommended because of concerns for potential postoperative complications. The infectious disease specialists recommended initiation of liposomal amphotericin B at 10 mg/kg/d. Magnetic resonance imaging of the head showed parietal lobe enhancement with restricted diffusion most consistent with prior infarct. Paranasal sinus disease also was demonstrated. The latter findings prompted further evaluation. The patient underwent right and left endoscopic resection of concha bullosa as well as left maxillary endoscopic antrostomy. Gross examination showed thick mucosa in left concha bullosa, polypoid changes anterior to bulla ethmoidalis, and clear left maxillary sinus. The procedure had to be aborted when the patient experienced cardiac arrest secondary to ventricular fibrillation; he was successfully resuscitated.
Samples from the contents of right and left sinuses as well as left concha bullosa were submitted to pathology, showing benign respiratory mucosa with chronic inflammation and foci of bone without fungal elements. There was no other evidence of disseminated mucormycosis. The patient had a prolonged hospital course complicated by progressive hypoxemia, acute kidney injury, and toxic metabolic encephalopathy. Three months after his original diagnosis, he sustained another cardiac arrest in the hospital. Shortly after achieving return of spontaneous circulation and initiation of invasive mechanical ventilation, the family elected to withdraw care. The family declined an autopsy.
Discussion
This article describes a case of subacute pulmonary mucormycosis in a patient with recurrent DKA. Although patients with poorly controlled DM commonly present with the rhinocerebral form of mucormycosis, pulmonary involvement with a subacute course has been described. Determining the final diagnosis for the current patient was challenging due to the subtlety of his respiratory symptoms and the inconsistent initial findings on chest radiography. A pulmonary disease was finally suspected when a mass was found on the CT scan. However, the middle mediastinal mass was more suspicious for malignancy, particularly given the patient’s smoking history and persistent hyponatremia. In fact, the lack of any neoplastic findings on the initial endobronchial biopsy prompted the health care team to pursue a second biopsy that was consistent with mucormycosis.
This case demonstrates the challenges of prompt diagnosis and treatment of this potentially fatal infection. Furthermore, the extent of the disease at diagnosis precluded this patient from having a surgical intervention, which has been associated with better outcomes than those of medical management alone. Finally, it remains unknown whether the patient had an underlying malignancy, which could have increased the likelihood of pulmonary mucormycosis; the biopsy yield may have been confounded by repeated sampling of necrotic material caused by mucormycosis. Further investigation of any potential pulmonary neoplasm was limited by the patient’s clinical condition and the poor prognosis due to the extent of infection.
Mucorales is an order of fungi comprised of 6 main families that have potential to cause a variety of infections. The genera Mucor, Rhizopus, and Rhizomucor cause the majority of infections.1 Mucormycosis (infection with Mucorales) is generally a rare fungal infection with an incidence of about 500 cases per year in the U.S. However, the incidence is increasing with an aging population, higher prevalence of DM and chronic kidney disease, and a growing population of immunocompromised patients due to advances in cancer therapy and transplantation. Risk factors for pulmonary mucormycosis include conditions associated with congenital and acquired immunodeficiency: hematologic malignancies, uncontrolled DM, solid tumors, and organ transplantation.2
Presentation
Notably, there seems to be an association between specific organ system involvement and predisposing conditions. Pulmonary mucormycosis occurs much less frequently than does the rhinocerebral form in patients with DKA but occurs more commonly in patients with neutropenia that is due to chemotherapy or hematopoietic stem cell transplantation (HSCT) for the treatment of hematologic malignancies.2
The mechanisms for preferential site infection are not well understood with current knowledge of mucormycosis pathogenesis. Current research demonstrates monocytes and neutrophils may play a vital role in the body’s defense against Mucor by both phagocytosis and oxidative damage. Chemotaxis and oxidative cell lysis seem to be compromised in states of hyperglycemia and acidosis. Iron metabolism repeatedly has been shown to play a role in the pathogenesis of mucormycosis. Specifically, patients receiving deferoxamine seem to have a predisposition to Mucorales infections, presumably due to the increased iron supply to the fungus.4 Notably, systemic acidosis also facilitates higher concentrations of available serum iron.
One of the main characteristics of mucormycosis is its ability to aggressively invade blood vessels, causing thrombosis and necrosis and subsequently disseminate hematogenously or through the lymphatic system. This property, at least in large part, depends on endothelial cell damage following phagocytosis of fungus by these cells.
Of note, some of the azole class of drugs (eg, voriconazole), which may be used for antifungal prophylaxis in patients with hematologic malignancies accompanied by neutropenia, have been implicated in predisposition to mucormycosis.2 It also is commonly seen in patients undergoing HSCT. Patients with DM and DKA also can present with pulmonary mucormycosis but generally have a more indolent course unless they develop pulmonary hemorrhage.3 Infection usually occurs by inhalation.
Patients may report dyspnea, cough, and chest pain, which is sometimes accompanied by a fever. Presentation is generally indistinguishable from other causes of pneumonia, and the routinely obtained sputum cultures are usually not diagnostically significant.
Radiographic findings are variable and may include pulmonary nodules, consolidations, masses, and cavitary lesions.1 Due to tissue invasion, a CT scan of the chest might demonstrate a mass crossing mediastinal tissue planes. Definitive diagnosis requires a biopsy with a demonstration of characteristic broad-based nonseptate hyphae with tissue invasion as well as a positive culture (Figures 4 and 5).5 Due to nonspecific symptoms as well as laboratory and imaging findings, a biopsy and, therefore, definitive diagnosis are often delayed. However, postponing medical and surgical therapy for mucormycosis has been associated with worse outcomes.6 With the absence of easily available serologic tests and unspecific symptoms in early disease, many mucormycosis cases are diagnosed postmortem.
Treatments
Recently described therapy advancements have indicated improved outcomes.7 Nevertheless, prognosis remains universally poor with 65% to 70% mortality for patients with cases of isolated pulmonary mucormycosis.8 Many of these patients succumb to sepsis, respiratory failure, and hemoptysis. Patients with pulmonary mucormycosis usually die of dissemination rather than of the sequelae of the pulmonary disease. In fact, pulmonary infection seems to have the highest incidence of dissemination in patients with neutropenia. Surgical therapy seems to have more favorable outcomes than treatment with antifungals alone, especially when considering infection primarily affecting 1 lung.8
Amphotericin B remains the first-line agent for treatment of pulmonary mucormycosis. Retrospective studies show that this agent remains one of the few with activity against Mucor with reported successful outcomes. Specifically, the liposomal formulation seems to have greater efficacy.9 Strong prospective data are lacking. An increasing body of evidence supports a potential benefit from adding echinocandins.10 Although these agents have minimal activity against mucormycosis in vitro, adjunctive therapy to amphotericin resulted in better survival. Alternative regimens include the combination of amphotericin with posaconazole or itraconazole. Both these agents seem to have in vitro activity against mucormycosis pathogens, although poor absorption of these agents puts the potential benefit of such combinations in question.
In patients unable to tolerate polyenes due to adverse effects (AEs), the use of posaconazole as monotherapy has been reported with positive results. One retrospective study reported treatment success in up to 60% and stable disease in 21% of patients at 12 weeks. This study included 24 out of 36 patients with pulmonary mucormycosis.11 Significantly fewer AEs and oral administration makes posaconazole an attractive alternative treatment for mucormycosis and needs further prospective evaluation.
Novel therapies have been attempted, though without success thus far. One randomized clinical trial conducted on patients with mucormycosis attempted to determine whether capitalizing on iron metabolism by Mucor by providing adjunctive deferasirox, an iron chelator, would lead to an initial improvement in mortality. However, outcomes did not improve and resulted in higher mortality rates at 90 days in the intervention group.12
Reversal of underlying conditions remains the cornerstone of successful therapy. If possible, it is important to cease immunosuppression by avoiding corticosteroids, correcting acidosis and hyperglycemia, and discontinuing aluminum and iron chelators.13 This approach becomes problematic in patients with DM with poor glucose control due to nonadherence or lack of resources and in situations where the underlying condition is difficult to treat or the treatment puts patients at risk for mucormycosis (eg, malignancies). Surgery in addition to antifungal therapy should be pursued wherever possible for definitive therapy.
1. Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236-301.
2. Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology. 2012;17(6):913-926.
3. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569.
4. Prokopowicz GP, Bradley SF, Kauffman CA. Indolent zygomycosis associated with deferoxamine chelation therapy. Mycoses. 1994;37(11-12):427-431.
5. Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32(6):693-702.
6. Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503-509.
7. Parfrey NA. Improved diagnosis and prognosis of mucormycosis. A clinicopathologic study of 33 cases. Medicine (Baltimore). 1986;65(2):113-1
8. Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044-1050.
9.
10. Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364-371.
11. van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61-e65.
12. Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67(3):715-722.
13. de Locht M, Boelaert JR, Schneider YJ. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem Pharmacol. 1994; 47(10):1843-1850.
Mucorales fungi are ubiquitous organisms commonly inhabiting soil and can cause opportunistic infections. The majority of infections are caused by 3 genera: Rhizopus, Mucor, and Rhizomucor.1 Infection occurs by inhalation or by direct contact with damaged skin. Mucorales infections can have cutaneous, rhinocerebral, pulmonary, gastrointestinal, and central nervous system manifestations. Pulmonary mucormycosis is often rapidly progressive with angioinvasion and fulminant necrosis causing acute dyspnea, hemoptysis, and chest pain. More indolent pulmonary Mucorales infections can mimic a pulmonary mass with occasional cavitation found on imaging studies similar to other fungal infections (eg, Aspergillus).2 Risk factors include severe uncontrolled diabetes mellitus (DM), recurrent diabetic ketoacidosis (DKA), immunosuppression due to congenital or acquired causes, hematologic malignancies, and chronic renal failure.3 The authors present a case of a patient with recurrent DKA and pulmonary mucormycosis.
Case Presentation
A 62-year-old male with DM and a more than 30-pack-year smoking history presented to the emergency department with abdominal pain and chest pain ongoing for about 1 week. The patient had a history of frequent admissions with DKA and medication nonadherence.
On admission, the patient was hemodynamically stable. His vital signs were: temperature 97.4° F, heart rate 89 bpm, respirations 24 breathes per minute, blood pressure 146/86 mm Hg, and oxygen saturation 94% on ambient air. The patient appeared ill but the physical examination was otherwise unremarkable. Laboratory results revealed a white blood cell count of 24,400 with neutrophilic predominance, blood glucose 658 mg/dL, creatinine clearance 2.16 mL/min/1.73 m2, sodium level 124 mEq/L, bicarbonate 6 mEq/L, anion gap 27 mEq/L, 6.8 pH, partial pressure of CO2 11 mm Hg, and lactic acid 2.3 mmol/L.
The patient admitted for DKA management and placed on an insulin drip. Although he did not have a fever or cough productive of sputum or hemoptysis, there was concern that pneumonia might have precipitated DKA. A chest X-ray revealed a patchy, right suprahilar opacity (Figure 1).
The patient was placed on vancomycin 1,000 mg every 12 hours and cefepime 2,000 mg every 12 hours for possible hospital-acquired pneumonia because of his history of recent DKA hospitalization. Once the patient’s anion gap was closed and metabolic acidosis was resolved, the insulin drip was discontinued, and the patient was transferred to the general medical ward for further management. There, he continued to report having chest pain. A computed tomography (CT) scan without contrast of the chest (contrast was held due to recent acute kidney injury) revealed right hilar soft tissue density obstructing the bronchus intermidius, which had resulted in a right-lung collapse and right-sided pleural effusion (Figure 2). The left lung was clear, and there was no evidence of nodularity.
Given the patient’s extensive smoking history, the initial concern was for pulmonary malignancy. The decision was made to proceed with bronchoscopy with endobronchial ultrasound-guided transbronchial needle biopsy. Endobronchial brushings and biopsies of R11, 7, right bronchus intermedius, and right upper lobe were obtained. Gross inspection of the airway revealed markedly abnormal-appearing mucosa involving the take off to the right upper lobe and the entire bronchus intermedius with friable, cobblestoned, and edematous mucosa. Biopsies and immunostaining for occult carcinoma markers, including CD-56, TTF-1, Synaptophysin A, chromogranin, AE1/AE3, and CK-5/6, were negative for malignancy. Final microbiologic analysis was positive for Mucor. There was no evidence of bacterial or mycobacterial growth.
Due to continued suspicion for malignancy and lack of histologic yield, the patient underwent a repeat endobronchial ultrasound-guided needle biopsy. On this occasion, gross inspection revealed significant mucosal necrosis and extensive, extrinsic bronchial compression starting from the right bronchial division and notable throughout the right middle and lower lobes (Figure 3).
Bronchial washings revealed necrotic material with rare fungal hyphae present. Biopsies yielded necrotic material or lung tissue containing nonseptate hyphae with rare, right-angle branching consistent with Mucor (Figures 4 and 5). Malignancy was not present in the specimens obtained.
Based on the bronchoscopy results, thoracic surgery and infectious disease specialists were consulted. Surgical intervention was not recommended because of concerns for potential postoperative complications. The infectious disease specialists recommended initiation of liposomal amphotericin B at 10 mg/kg/d. Magnetic resonance imaging of the head showed parietal lobe enhancement with restricted diffusion most consistent with prior infarct. Paranasal sinus disease also was demonstrated. The latter findings prompted further evaluation. The patient underwent right and left endoscopic resection of concha bullosa as well as left maxillary endoscopic antrostomy. Gross examination showed thick mucosa in left concha bullosa, polypoid changes anterior to bulla ethmoidalis, and clear left maxillary sinus. The procedure had to be aborted when the patient experienced cardiac arrest secondary to ventricular fibrillation; he was successfully resuscitated.
Samples from the contents of right and left sinuses as well as left concha bullosa were submitted to pathology, showing benign respiratory mucosa with chronic inflammation and foci of bone without fungal elements. There was no other evidence of disseminated mucormycosis. The patient had a prolonged hospital course complicated by progressive hypoxemia, acute kidney injury, and toxic metabolic encephalopathy. Three months after his original diagnosis, he sustained another cardiac arrest in the hospital. Shortly after achieving return of spontaneous circulation and initiation of invasive mechanical ventilation, the family elected to withdraw care. The family declined an autopsy.
Discussion
This article describes a case of subacute pulmonary mucormycosis in a patient with recurrent DKA. Although patients with poorly controlled DM commonly present with the rhinocerebral form of mucormycosis, pulmonary involvement with a subacute course has been described. Determining the final diagnosis for the current patient was challenging due to the subtlety of his respiratory symptoms and the inconsistent initial findings on chest radiography. A pulmonary disease was finally suspected when a mass was found on the CT scan. However, the middle mediastinal mass was more suspicious for malignancy, particularly given the patient’s smoking history and persistent hyponatremia. In fact, the lack of any neoplastic findings on the initial endobronchial biopsy prompted the health care team to pursue a second biopsy that was consistent with mucormycosis.
This case demonstrates the challenges of prompt diagnosis and treatment of this potentially fatal infection. Furthermore, the extent of the disease at diagnosis precluded this patient from having a surgical intervention, which has been associated with better outcomes than those of medical management alone. Finally, it remains unknown whether the patient had an underlying malignancy, which could have increased the likelihood of pulmonary mucormycosis; the biopsy yield may have been confounded by repeated sampling of necrotic material caused by mucormycosis. Further investigation of any potential pulmonary neoplasm was limited by the patient’s clinical condition and the poor prognosis due to the extent of infection.
Mucorales is an order of fungi comprised of 6 main families that have potential to cause a variety of infections. The genera Mucor, Rhizopus, and Rhizomucor cause the majority of infections.1 Mucormycosis (infection with Mucorales) is generally a rare fungal infection with an incidence of about 500 cases per year in the U.S. However, the incidence is increasing with an aging population, higher prevalence of DM and chronic kidney disease, and a growing population of immunocompromised patients due to advances in cancer therapy and transplantation. Risk factors for pulmonary mucormycosis include conditions associated with congenital and acquired immunodeficiency: hematologic malignancies, uncontrolled DM, solid tumors, and organ transplantation.2
Presentation
Notably, there seems to be an association between specific organ system involvement and predisposing conditions. Pulmonary mucormycosis occurs much less frequently than does the rhinocerebral form in patients with DKA but occurs more commonly in patients with neutropenia that is due to chemotherapy or hematopoietic stem cell transplantation (HSCT) for the treatment of hematologic malignancies.2
The mechanisms for preferential site infection are not well understood with current knowledge of mucormycosis pathogenesis. Current research demonstrates monocytes and neutrophils may play a vital role in the body’s defense against Mucor by both phagocytosis and oxidative damage. Chemotaxis and oxidative cell lysis seem to be compromised in states of hyperglycemia and acidosis. Iron metabolism repeatedly has been shown to play a role in the pathogenesis of mucormycosis. Specifically, patients receiving deferoxamine seem to have a predisposition to Mucorales infections, presumably due to the increased iron supply to the fungus.4 Notably, systemic acidosis also facilitates higher concentrations of available serum iron.
One of the main characteristics of mucormycosis is its ability to aggressively invade blood vessels, causing thrombosis and necrosis and subsequently disseminate hematogenously or through the lymphatic system. This property, at least in large part, depends on endothelial cell damage following phagocytosis of fungus by these cells.
Of note, some of the azole class of drugs (eg, voriconazole), which may be used for antifungal prophylaxis in patients with hematologic malignancies accompanied by neutropenia, have been implicated in predisposition to mucormycosis.2 It also is commonly seen in patients undergoing HSCT. Patients with DM and DKA also can present with pulmonary mucormycosis but generally have a more indolent course unless they develop pulmonary hemorrhage.3 Infection usually occurs by inhalation.
Patients may report dyspnea, cough, and chest pain, which is sometimes accompanied by a fever. Presentation is generally indistinguishable from other causes of pneumonia, and the routinely obtained sputum cultures are usually not diagnostically significant.
Radiographic findings are variable and may include pulmonary nodules, consolidations, masses, and cavitary lesions.1 Due to tissue invasion, a CT scan of the chest might demonstrate a mass crossing mediastinal tissue planes. Definitive diagnosis requires a biopsy with a demonstration of characteristic broad-based nonseptate hyphae with tissue invasion as well as a positive culture (Figures 4 and 5).5 Due to nonspecific symptoms as well as laboratory and imaging findings, a biopsy and, therefore, definitive diagnosis are often delayed. However, postponing medical and surgical therapy for mucormycosis has been associated with worse outcomes.6 With the absence of easily available serologic tests and unspecific symptoms in early disease, many mucormycosis cases are diagnosed postmortem.
Treatments
Recently described therapy advancements have indicated improved outcomes.7 Nevertheless, prognosis remains universally poor with 65% to 70% mortality for patients with cases of isolated pulmonary mucormycosis.8 Many of these patients succumb to sepsis, respiratory failure, and hemoptysis. Patients with pulmonary mucormycosis usually die of dissemination rather than of the sequelae of the pulmonary disease. In fact, pulmonary infection seems to have the highest incidence of dissemination in patients with neutropenia. Surgical therapy seems to have more favorable outcomes than treatment with antifungals alone, especially when considering infection primarily affecting 1 lung.8
Amphotericin B remains the first-line agent for treatment of pulmonary mucormycosis. Retrospective studies show that this agent remains one of the few with activity against Mucor with reported successful outcomes. Specifically, the liposomal formulation seems to have greater efficacy.9 Strong prospective data are lacking. An increasing body of evidence supports a potential benefit from adding echinocandins.10 Although these agents have minimal activity against mucormycosis in vitro, adjunctive therapy to amphotericin resulted in better survival. Alternative regimens include the combination of amphotericin with posaconazole or itraconazole. Both these agents seem to have in vitro activity against mucormycosis pathogens, although poor absorption of these agents puts the potential benefit of such combinations in question.
In patients unable to tolerate polyenes due to adverse effects (AEs), the use of posaconazole as monotherapy has been reported with positive results. One retrospective study reported treatment success in up to 60% and stable disease in 21% of patients at 12 weeks. This study included 24 out of 36 patients with pulmonary mucormycosis.11 Significantly fewer AEs and oral administration makes posaconazole an attractive alternative treatment for mucormycosis and needs further prospective evaluation.
Novel therapies have been attempted, though without success thus far. One randomized clinical trial conducted on patients with mucormycosis attempted to determine whether capitalizing on iron metabolism by Mucor by providing adjunctive deferasirox, an iron chelator, would lead to an initial improvement in mortality. However, outcomes did not improve and resulted in higher mortality rates at 90 days in the intervention group.12
Reversal of underlying conditions remains the cornerstone of successful therapy. If possible, it is important to cease immunosuppression by avoiding corticosteroids, correcting acidosis and hyperglycemia, and discontinuing aluminum and iron chelators.13 This approach becomes problematic in patients with DM with poor glucose control due to nonadherence or lack of resources and in situations where the underlying condition is difficult to treat or the treatment puts patients at risk for mucormycosis (eg, malignancies). Surgery in addition to antifungal therapy should be pursued wherever possible for definitive therapy.
Mucorales fungi are ubiquitous organisms commonly inhabiting soil and can cause opportunistic infections. The majority of infections are caused by 3 genera: Rhizopus, Mucor, and Rhizomucor.1 Infection occurs by inhalation or by direct contact with damaged skin. Mucorales infections can have cutaneous, rhinocerebral, pulmonary, gastrointestinal, and central nervous system manifestations. Pulmonary mucormycosis is often rapidly progressive with angioinvasion and fulminant necrosis causing acute dyspnea, hemoptysis, and chest pain. More indolent pulmonary Mucorales infections can mimic a pulmonary mass with occasional cavitation found on imaging studies similar to other fungal infections (eg, Aspergillus).2 Risk factors include severe uncontrolled diabetes mellitus (DM), recurrent diabetic ketoacidosis (DKA), immunosuppression due to congenital or acquired causes, hematologic malignancies, and chronic renal failure.3 The authors present a case of a patient with recurrent DKA and pulmonary mucormycosis.
Case Presentation
A 62-year-old male with DM and a more than 30-pack-year smoking history presented to the emergency department with abdominal pain and chest pain ongoing for about 1 week. The patient had a history of frequent admissions with DKA and medication nonadherence.
On admission, the patient was hemodynamically stable. His vital signs were: temperature 97.4° F, heart rate 89 bpm, respirations 24 breathes per minute, blood pressure 146/86 mm Hg, and oxygen saturation 94% on ambient air. The patient appeared ill but the physical examination was otherwise unremarkable. Laboratory results revealed a white blood cell count of 24,400 with neutrophilic predominance, blood glucose 658 mg/dL, creatinine clearance 2.16 mL/min/1.73 m2, sodium level 124 mEq/L, bicarbonate 6 mEq/L, anion gap 27 mEq/L, 6.8 pH, partial pressure of CO2 11 mm Hg, and lactic acid 2.3 mmol/L.
The patient admitted for DKA management and placed on an insulin drip. Although he did not have a fever or cough productive of sputum or hemoptysis, there was concern that pneumonia might have precipitated DKA. A chest X-ray revealed a patchy, right suprahilar opacity (Figure 1).
The patient was placed on vancomycin 1,000 mg every 12 hours and cefepime 2,000 mg every 12 hours for possible hospital-acquired pneumonia because of his history of recent DKA hospitalization. Once the patient’s anion gap was closed and metabolic acidosis was resolved, the insulin drip was discontinued, and the patient was transferred to the general medical ward for further management. There, he continued to report having chest pain. A computed tomography (CT) scan without contrast of the chest (contrast was held due to recent acute kidney injury) revealed right hilar soft tissue density obstructing the bronchus intermidius, which had resulted in a right-lung collapse and right-sided pleural effusion (Figure 2). The left lung was clear, and there was no evidence of nodularity.
Given the patient’s extensive smoking history, the initial concern was for pulmonary malignancy. The decision was made to proceed with bronchoscopy with endobronchial ultrasound-guided transbronchial needle biopsy. Endobronchial brushings and biopsies of R11, 7, right bronchus intermedius, and right upper lobe were obtained. Gross inspection of the airway revealed markedly abnormal-appearing mucosa involving the take off to the right upper lobe and the entire bronchus intermedius with friable, cobblestoned, and edematous mucosa. Biopsies and immunostaining for occult carcinoma markers, including CD-56, TTF-1, Synaptophysin A, chromogranin, AE1/AE3, and CK-5/6, were negative for malignancy. Final microbiologic analysis was positive for Mucor. There was no evidence of bacterial or mycobacterial growth.
Due to continued suspicion for malignancy and lack of histologic yield, the patient underwent a repeat endobronchial ultrasound-guided needle biopsy. On this occasion, gross inspection revealed significant mucosal necrosis and extensive, extrinsic bronchial compression starting from the right bronchial division and notable throughout the right middle and lower lobes (Figure 3).
Bronchial washings revealed necrotic material with rare fungal hyphae present. Biopsies yielded necrotic material or lung tissue containing nonseptate hyphae with rare, right-angle branching consistent with Mucor (Figures 4 and 5). Malignancy was not present in the specimens obtained.
Based on the bronchoscopy results, thoracic surgery and infectious disease specialists were consulted. Surgical intervention was not recommended because of concerns for potential postoperative complications. The infectious disease specialists recommended initiation of liposomal amphotericin B at 10 mg/kg/d. Magnetic resonance imaging of the head showed parietal lobe enhancement with restricted diffusion most consistent with prior infarct. Paranasal sinus disease also was demonstrated. The latter findings prompted further evaluation. The patient underwent right and left endoscopic resection of concha bullosa as well as left maxillary endoscopic antrostomy. Gross examination showed thick mucosa in left concha bullosa, polypoid changes anterior to bulla ethmoidalis, and clear left maxillary sinus. The procedure had to be aborted when the patient experienced cardiac arrest secondary to ventricular fibrillation; he was successfully resuscitated.
Samples from the contents of right and left sinuses as well as left concha bullosa were submitted to pathology, showing benign respiratory mucosa with chronic inflammation and foci of bone without fungal elements. There was no other evidence of disseminated mucormycosis. The patient had a prolonged hospital course complicated by progressive hypoxemia, acute kidney injury, and toxic metabolic encephalopathy. Three months after his original diagnosis, he sustained another cardiac arrest in the hospital. Shortly after achieving return of spontaneous circulation and initiation of invasive mechanical ventilation, the family elected to withdraw care. The family declined an autopsy.
Discussion
This article describes a case of subacute pulmonary mucormycosis in a patient with recurrent DKA. Although patients with poorly controlled DM commonly present with the rhinocerebral form of mucormycosis, pulmonary involvement with a subacute course has been described. Determining the final diagnosis for the current patient was challenging due to the subtlety of his respiratory symptoms and the inconsistent initial findings on chest radiography. A pulmonary disease was finally suspected when a mass was found on the CT scan. However, the middle mediastinal mass was more suspicious for malignancy, particularly given the patient’s smoking history and persistent hyponatremia. In fact, the lack of any neoplastic findings on the initial endobronchial biopsy prompted the health care team to pursue a second biopsy that was consistent with mucormycosis.
This case demonstrates the challenges of prompt diagnosis and treatment of this potentially fatal infection. Furthermore, the extent of the disease at diagnosis precluded this patient from having a surgical intervention, which has been associated with better outcomes than those of medical management alone. Finally, it remains unknown whether the patient had an underlying malignancy, which could have increased the likelihood of pulmonary mucormycosis; the biopsy yield may have been confounded by repeated sampling of necrotic material caused by mucormycosis. Further investigation of any potential pulmonary neoplasm was limited by the patient’s clinical condition and the poor prognosis due to the extent of infection.
Mucorales is an order of fungi comprised of 6 main families that have potential to cause a variety of infections. The genera Mucor, Rhizopus, and Rhizomucor cause the majority of infections.1 Mucormycosis (infection with Mucorales) is generally a rare fungal infection with an incidence of about 500 cases per year in the U.S. However, the incidence is increasing with an aging population, higher prevalence of DM and chronic kidney disease, and a growing population of immunocompromised patients due to advances in cancer therapy and transplantation. Risk factors for pulmonary mucormycosis include conditions associated with congenital and acquired immunodeficiency: hematologic malignancies, uncontrolled DM, solid tumors, and organ transplantation.2
Presentation
Notably, there seems to be an association between specific organ system involvement and predisposing conditions. Pulmonary mucormycosis occurs much less frequently than does the rhinocerebral form in patients with DKA but occurs more commonly in patients with neutropenia that is due to chemotherapy or hematopoietic stem cell transplantation (HSCT) for the treatment of hematologic malignancies.2
The mechanisms for preferential site infection are not well understood with current knowledge of mucormycosis pathogenesis. Current research demonstrates monocytes and neutrophils may play a vital role in the body’s defense against Mucor by both phagocytosis and oxidative damage. Chemotaxis and oxidative cell lysis seem to be compromised in states of hyperglycemia and acidosis. Iron metabolism repeatedly has been shown to play a role in the pathogenesis of mucormycosis. Specifically, patients receiving deferoxamine seem to have a predisposition to Mucorales infections, presumably due to the increased iron supply to the fungus.4 Notably, systemic acidosis also facilitates higher concentrations of available serum iron.
One of the main characteristics of mucormycosis is its ability to aggressively invade blood vessels, causing thrombosis and necrosis and subsequently disseminate hematogenously or through the lymphatic system. This property, at least in large part, depends on endothelial cell damage following phagocytosis of fungus by these cells.
Of note, some of the azole class of drugs (eg, voriconazole), which may be used for antifungal prophylaxis in patients with hematologic malignancies accompanied by neutropenia, have been implicated in predisposition to mucormycosis.2 It also is commonly seen in patients undergoing HSCT. Patients with DM and DKA also can present with pulmonary mucormycosis but generally have a more indolent course unless they develop pulmonary hemorrhage.3 Infection usually occurs by inhalation.
Patients may report dyspnea, cough, and chest pain, which is sometimes accompanied by a fever. Presentation is generally indistinguishable from other causes of pneumonia, and the routinely obtained sputum cultures are usually not diagnostically significant.
Radiographic findings are variable and may include pulmonary nodules, consolidations, masses, and cavitary lesions.1 Due to tissue invasion, a CT scan of the chest might demonstrate a mass crossing mediastinal tissue planes. Definitive diagnosis requires a biopsy with a demonstration of characteristic broad-based nonseptate hyphae with tissue invasion as well as a positive culture (Figures 4 and 5).5 Due to nonspecific symptoms as well as laboratory and imaging findings, a biopsy and, therefore, definitive diagnosis are often delayed. However, postponing medical and surgical therapy for mucormycosis has been associated with worse outcomes.6 With the absence of easily available serologic tests and unspecific symptoms in early disease, many mucormycosis cases are diagnosed postmortem.
Treatments
Recently described therapy advancements have indicated improved outcomes.7 Nevertheless, prognosis remains universally poor with 65% to 70% mortality for patients with cases of isolated pulmonary mucormycosis.8 Many of these patients succumb to sepsis, respiratory failure, and hemoptysis. Patients with pulmonary mucormycosis usually die of dissemination rather than of the sequelae of the pulmonary disease. In fact, pulmonary infection seems to have the highest incidence of dissemination in patients with neutropenia. Surgical therapy seems to have more favorable outcomes than treatment with antifungals alone, especially when considering infection primarily affecting 1 lung.8
Amphotericin B remains the first-line agent for treatment of pulmonary mucormycosis. Retrospective studies show that this agent remains one of the few with activity against Mucor with reported successful outcomes. Specifically, the liposomal formulation seems to have greater efficacy.9 Strong prospective data are lacking. An increasing body of evidence supports a potential benefit from adding echinocandins.10 Although these agents have minimal activity against mucormycosis in vitro, adjunctive therapy to amphotericin resulted in better survival. Alternative regimens include the combination of amphotericin with posaconazole or itraconazole. Both these agents seem to have in vitro activity against mucormycosis pathogens, although poor absorption of these agents puts the potential benefit of such combinations in question.
In patients unable to tolerate polyenes due to adverse effects (AEs), the use of posaconazole as monotherapy has been reported with positive results. One retrospective study reported treatment success in up to 60% and stable disease in 21% of patients at 12 weeks. This study included 24 out of 36 patients with pulmonary mucormycosis.11 Significantly fewer AEs and oral administration makes posaconazole an attractive alternative treatment for mucormycosis and needs further prospective evaluation.
Novel therapies have been attempted, though without success thus far. One randomized clinical trial conducted on patients with mucormycosis attempted to determine whether capitalizing on iron metabolism by Mucor by providing adjunctive deferasirox, an iron chelator, would lead to an initial improvement in mortality. However, outcomes did not improve and resulted in higher mortality rates at 90 days in the intervention group.12
Reversal of underlying conditions remains the cornerstone of successful therapy. If possible, it is important to cease immunosuppression by avoiding corticosteroids, correcting acidosis and hyperglycemia, and discontinuing aluminum and iron chelators.13 This approach becomes problematic in patients with DM with poor glucose control due to nonadherence or lack of resources and in situations where the underlying condition is difficult to treat or the treatment puts patients at risk for mucormycosis (eg, malignancies). Surgery in addition to antifungal therapy should be pursued wherever possible for definitive therapy.
1. Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236-301.
2. Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology. 2012;17(6):913-926.
3. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569.
4. Prokopowicz GP, Bradley SF, Kauffman CA. Indolent zygomycosis associated with deferoxamine chelation therapy. Mycoses. 1994;37(11-12):427-431.
5. Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32(6):693-702.
6. Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503-509.
7. Parfrey NA. Improved diagnosis and prognosis of mucormycosis. A clinicopathologic study of 33 cases. Medicine (Baltimore). 1986;65(2):113-1
8. Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044-1050.
9.
10. Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364-371.
11. van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61-e65.
12. Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67(3):715-722.
13. de Locht M, Boelaert JR, Schneider YJ. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem Pharmacol. 1994; 47(10):1843-1850.
1. Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236-301.
2. Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology. 2012;17(6):913-926.
3. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569.
4. Prokopowicz GP, Bradley SF, Kauffman CA. Indolent zygomycosis associated with deferoxamine chelation therapy. Mycoses. 1994;37(11-12):427-431.
5. Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32(6):693-702.
6. Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503-509.
7. Parfrey NA. Improved diagnosis and prognosis of mucormycosis. A clinicopathologic study of 33 cases. Medicine (Baltimore). 1986;65(2):113-1
8. Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044-1050.
9.
10. Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364-371.
11. van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61-e65.
12. Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67(3):715-722.
13. de Locht M, Boelaert JR, Schneider YJ. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem Pharmacol. 1994; 47(10):1843-1850.
KRd improves OS in relapsed/refractory MM
Adding carfilzomib (K) to treatment with lenalidomide and dexamethasone (Rd) can improve overall survival (OS) in patients with relapsed or refractory multiple myeloma (MM), according to research published in the Journal of Clinical Oncology.
Final results from the phase 3 ASPIRE trial showed that KRd reduced the risk of death by 21% and extended OS by 7.9 months, when compared to Rd.
In patients at first relapse, KRd was associated with an OS improvement of 11.4 months.
“Results from the final analysis of the phase 3 ASPIRE trial . . . are significant, as they further validate carfilzomib, lenalidomide, and dexamethasone as a standard-of-care regimen for patients with relapsed or refractory multiple myeloma,” said study author Keith Stewart, MBChB, of Mayo Clinic in Scottsdale, Arizona.
“Furthermore, these data showed that early use of carfilzomib, lenalidomide, and dexamethasone at first relapse provided nearly 1 additional year of survival for patients, regardless of prior treatment with bortezomib or transplant.”
ASPIRE enrolled 792 MM patients who had received a median of 2 prior therapies (range, 1-3). They were randomized to receive KRd (n=396) or Rd (n=396). Baseline characteristics were well balanced between the treatment arms.
Details on patients and treatment, as well as interim results from ASPIRE, were reported at the 2014 ASH Annual Meeting and published in NEJM in January 2015.
Treatment update
In the final analysis, there were 340 patients in the KRd arm and 358 in the Rd arm who stopped study treatment.
Reasons for discontinuation (in the KRd and Rd arms, respectively) were disease progression (n=188 and 224), adverse events (AEs, n=79 and 85), other reasons (n=61 and 35), withdrawn consent (n=10 and 12), and noncompliance (n=2 and 1).
A total of 182 patients in the KRd arm and 211 in the Rd arm received subsequent treatment for MM. These treatments were generally balanced between the KRd and Rd arms.
The median time to next treatment from the time of randomization was 39.0 months in the KRd arm and 24.4 months in the Rd arm (hazard ratio [HR]=0.65 P<0.001).
Survival
Interim ASPIRE data had shown a significant improvement in progression-free survival (PFS) and a trend toward improved OS in patients who received KRd. Now, researchers have observed a significant improvement in both endpoints with KRd.
The data cutoff for the final analysis was April 28, 2017. For PFS, the median follow-up was 48.8 months in the KRd arm and 48.0 months in the Rd arm.
The median PFS was 26.1 months in the KRd arm and 16.6 months in the Rd arm (9.5-month improvement, HR=0.66; P<0.001). The 3-year PFS rates were 38.2% and 28.4%, respectively. And the 5-year PFS rates were 25.6% and 17.3%, respectively.
The median follow-up for OS was 67.1 months. The median OS was 48.3 months in the KRd arm and 40.4 months in the Rd arm (7.9-month improvement, HR=0.79, P=0.0045).
The researchers also performed subgroup analyses according to prior lines of therapy, prior bortezomib exposure at first relapse, and prior transplant at first relapse.
In patients who had received 1 prior line of therapy, the median OS was 47.3 months in the KRd arm and 35.9 months in the Rd arm (11.4-month improvement, HR=0.81). For patients with 2 or more prior lines of therapy, the median OS was 48.8 months and 42.3 months, respectively (6.5-month improvement, HR=0.79).
Among patients with prior bortezomib exposure at first relapse, the median OS was 45.9 months in the KRd arm and 33.9 months in the Rd arm (12-month improvement, HR=0.82). Among patients without prior bortezomib exposure at first relapse, the median OS was 48.3 months and 40.4 months, respectively (7.9-month improvement, HR=0.80).
Among patients with prior transplant at first relapse, the median OS was 57.2 months in the KRd arm and 38.6 months in the Rd arm (18.6-month improvement, HR=0.71).
Safety
The incidence of treatment-emergent AEs was 98% in the KRd arm and 97.9% in the Rd arm. The incidence of grade 3 or higher AEs was 87% and 83.3%, respectively. The incidence of serious AEs was 65.3% and 56.8%, respectively.
Treatment discontinuation due to an AE occurred in 19.9% of patients in the KRd arm and 21.5% of patients in the Rd arm.
AEs of interest (in the KRd and Rd arms, respectively) included acute renal failure (9.2% and 7.7%), cardiac failure (7.1% and 4.1%), ischemic heart disease (6.9% and 4.6%), hypertension (17.1% and 8.7%), hematopoietic thrombocytopenia (32.7% and 26.2%), and peripheral neuropathy (18.9% and 17.2%).
Fatal AEs were reported in 11.5% of patients in the KRd arm and 10.8% of those in the Rd arm.
Fatal AEs reported in at least 2 patients in the KRd arm included (in the KRd and Rd arms, respectively) cardiac disorders (2.6% and 2.3%), pneumonia (1.5% and 0.8%), sepsis (0.8% for both), myocardial infarction (0.8% and 0.5%), acute respiratory distress syndrome (0.8% and 0%), death (0.5% for both), and cardiac arrest (0.5% and 0.3%).
This trial was funded by Onyx Pharmaceuticals, Inc. ![]()
Adding carfilzomib (K) to treatment with lenalidomide and dexamethasone (Rd) can improve overall survival (OS) in patients with relapsed or refractory multiple myeloma (MM), according to research published in the Journal of Clinical Oncology.
Final results from the phase 3 ASPIRE trial showed that KRd reduced the risk of death by 21% and extended OS by 7.9 months, when compared to Rd.
In patients at first relapse, KRd was associated with an OS improvement of 11.4 months.
“Results from the final analysis of the phase 3 ASPIRE trial . . . are significant, as they further validate carfilzomib, lenalidomide, and dexamethasone as a standard-of-care regimen for patients with relapsed or refractory multiple myeloma,” said study author Keith Stewart, MBChB, of Mayo Clinic in Scottsdale, Arizona.
“Furthermore, these data showed that early use of carfilzomib, lenalidomide, and dexamethasone at first relapse provided nearly 1 additional year of survival for patients, regardless of prior treatment with bortezomib or transplant.”
ASPIRE enrolled 792 MM patients who had received a median of 2 prior therapies (range, 1-3). They were randomized to receive KRd (n=396) or Rd (n=396). Baseline characteristics were well balanced between the treatment arms.
Details on patients and treatment, as well as interim results from ASPIRE, were reported at the 2014 ASH Annual Meeting and published in NEJM in January 2015.
Treatment update
In the final analysis, there were 340 patients in the KRd arm and 358 in the Rd arm who stopped study treatment.
Reasons for discontinuation (in the KRd and Rd arms, respectively) were disease progression (n=188 and 224), adverse events (AEs, n=79 and 85), other reasons (n=61 and 35), withdrawn consent (n=10 and 12), and noncompliance (n=2 and 1).
A total of 182 patients in the KRd arm and 211 in the Rd arm received subsequent treatment for MM. These treatments were generally balanced between the KRd and Rd arms.
The median time to next treatment from the time of randomization was 39.0 months in the KRd arm and 24.4 months in the Rd arm (hazard ratio [HR]=0.65 P<0.001).
Survival
Interim ASPIRE data had shown a significant improvement in progression-free survival (PFS) and a trend toward improved OS in patients who received KRd. Now, researchers have observed a significant improvement in both endpoints with KRd.
The data cutoff for the final analysis was April 28, 2017. For PFS, the median follow-up was 48.8 months in the KRd arm and 48.0 months in the Rd arm.
The median PFS was 26.1 months in the KRd arm and 16.6 months in the Rd arm (9.5-month improvement, HR=0.66; P<0.001). The 3-year PFS rates were 38.2% and 28.4%, respectively. And the 5-year PFS rates were 25.6% and 17.3%, respectively.
The median follow-up for OS was 67.1 months. The median OS was 48.3 months in the KRd arm and 40.4 months in the Rd arm (7.9-month improvement, HR=0.79, P=0.0045).
The researchers also performed subgroup analyses according to prior lines of therapy, prior bortezomib exposure at first relapse, and prior transplant at first relapse.
In patients who had received 1 prior line of therapy, the median OS was 47.3 months in the KRd arm and 35.9 months in the Rd arm (11.4-month improvement, HR=0.81). For patients with 2 or more prior lines of therapy, the median OS was 48.8 months and 42.3 months, respectively (6.5-month improvement, HR=0.79).
Among patients with prior bortezomib exposure at first relapse, the median OS was 45.9 months in the KRd arm and 33.9 months in the Rd arm (12-month improvement, HR=0.82). Among patients without prior bortezomib exposure at first relapse, the median OS was 48.3 months and 40.4 months, respectively (7.9-month improvement, HR=0.80).
Among patients with prior transplant at first relapse, the median OS was 57.2 months in the KRd arm and 38.6 months in the Rd arm (18.6-month improvement, HR=0.71).
Safety
The incidence of treatment-emergent AEs was 98% in the KRd arm and 97.9% in the Rd arm. The incidence of grade 3 or higher AEs was 87% and 83.3%, respectively. The incidence of serious AEs was 65.3% and 56.8%, respectively.
Treatment discontinuation due to an AE occurred in 19.9% of patients in the KRd arm and 21.5% of patients in the Rd arm.
AEs of interest (in the KRd and Rd arms, respectively) included acute renal failure (9.2% and 7.7%), cardiac failure (7.1% and 4.1%), ischemic heart disease (6.9% and 4.6%), hypertension (17.1% and 8.7%), hematopoietic thrombocytopenia (32.7% and 26.2%), and peripheral neuropathy (18.9% and 17.2%).
Fatal AEs were reported in 11.5% of patients in the KRd arm and 10.8% of those in the Rd arm.
Fatal AEs reported in at least 2 patients in the KRd arm included (in the KRd and Rd arms, respectively) cardiac disorders (2.6% and 2.3%), pneumonia (1.5% and 0.8%), sepsis (0.8% for both), myocardial infarction (0.8% and 0.5%), acute respiratory distress syndrome (0.8% and 0%), death (0.5% for both), and cardiac arrest (0.5% and 0.3%).
This trial was funded by Onyx Pharmaceuticals, Inc. ![]()
Adding carfilzomib (K) to treatment with lenalidomide and dexamethasone (Rd) can improve overall survival (OS) in patients with relapsed or refractory multiple myeloma (MM), according to research published in the Journal of Clinical Oncology.
Final results from the phase 3 ASPIRE trial showed that KRd reduced the risk of death by 21% and extended OS by 7.9 months, when compared to Rd.
In patients at first relapse, KRd was associated with an OS improvement of 11.4 months.
“Results from the final analysis of the phase 3 ASPIRE trial . . . are significant, as they further validate carfilzomib, lenalidomide, and dexamethasone as a standard-of-care regimen for patients with relapsed or refractory multiple myeloma,” said study author Keith Stewart, MBChB, of Mayo Clinic in Scottsdale, Arizona.
“Furthermore, these data showed that early use of carfilzomib, lenalidomide, and dexamethasone at first relapse provided nearly 1 additional year of survival for patients, regardless of prior treatment with bortezomib or transplant.”
ASPIRE enrolled 792 MM patients who had received a median of 2 prior therapies (range, 1-3). They were randomized to receive KRd (n=396) or Rd (n=396). Baseline characteristics were well balanced between the treatment arms.
Details on patients and treatment, as well as interim results from ASPIRE, were reported at the 2014 ASH Annual Meeting and published in NEJM in January 2015.
Treatment update
In the final analysis, there were 340 patients in the KRd arm and 358 in the Rd arm who stopped study treatment.
Reasons for discontinuation (in the KRd and Rd arms, respectively) were disease progression (n=188 and 224), adverse events (AEs, n=79 and 85), other reasons (n=61 and 35), withdrawn consent (n=10 and 12), and noncompliance (n=2 and 1).
A total of 182 patients in the KRd arm and 211 in the Rd arm received subsequent treatment for MM. These treatments were generally balanced between the KRd and Rd arms.
The median time to next treatment from the time of randomization was 39.0 months in the KRd arm and 24.4 months in the Rd arm (hazard ratio [HR]=0.65 P<0.001).
Survival
Interim ASPIRE data had shown a significant improvement in progression-free survival (PFS) and a trend toward improved OS in patients who received KRd. Now, researchers have observed a significant improvement in both endpoints with KRd.
The data cutoff for the final analysis was April 28, 2017. For PFS, the median follow-up was 48.8 months in the KRd arm and 48.0 months in the Rd arm.
The median PFS was 26.1 months in the KRd arm and 16.6 months in the Rd arm (9.5-month improvement, HR=0.66; P<0.001). The 3-year PFS rates were 38.2% and 28.4%, respectively. And the 5-year PFS rates were 25.6% and 17.3%, respectively.
The median follow-up for OS was 67.1 months. The median OS was 48.3 months in the KRd arm and 40.4 months in the Rd arm (7.9-month improvement, HR=0.79, P=0.0045).
The researchers also performed subgroup analyses according to prior lines of therapy, prior bortezomib exposure at first relapse, and prior transplant at first relapse.
In patients who had received 1 prior line of therapy, the median OS was 47.3 months in the KRd arm and 35.9 months in the Rd arm (11.4-month improvement, HR=0.81). For patients with 2 or more prior lines of therapy, the median OS was 48.8 months and 42.3 months, respectively (6.5-month improvement, HR=0.79).
Among patients with prior bortezomib exposure at first relapse, the median OS was 45.9 months in the KRd arm and 33.9 months in the Rd arm (12-month improvement, HR=0.82). Among patients without prior bortezomib exposure at first relapse, the median OS was 48.3 months and 40.4 months, respectively (7.9-month improvement, HR=0.80).
Among patients with prior transplant at first relapse, the median OS was 57.2 months in the KRd arm and 38.6 months in the Rd arm (18.6-month improvement, HR=0.71).
Safety
The incidence of treatment-emergent AEs was 98% in the KRd arm and 97.9% in the Rd arm. The incidence of grade 3 or higher AEs was 87% and 83.3%, respectively. The incidence of serious AEs was 65.3% and 56.8%, respectively.
Treatment discontinuation due to an AE occurred in 19.9% of patients in the KRd arm and 21.5% of patients in the Rd arm.
AEs of interest (in the KRd and Rd arms, respectively) included acute renal failure (9.2% and 7.7%), cardiac failure (7.1% and 4.1%), ischemic heart disease (6.9% and 4.6%), hypertension (17.1% and 8.7%), hematopoietic thrombocytopenia (32.7% and 26.2%), and peripheral neuropathy (18.9% and 17.2%).
Fatal AEs were reported in 11.5% of patients in the KRd arm and 10.8% of those in the Rd arm.
Fatal AEs reported in at least 2 patients in the KRd arm included (in the KRd and Rd arms, respectively) cardiac disorders (2.6% and 2.3%), pneumonia (1.5% and 0.8%), sepsis (0.8% for both), myocardial infarction (0.8% and 0.5%), acute respiratory distress syndrome (0.8% and 0%), death (0.5% for both), and cardiac arrest (0.5% and 0.3%).
This trial was funded by Onyx Pharmaceuticals, Inc. ![]()
FDA surveillance shows how rivaroxaban compares to warfarin
The US Food and Drug Administration’s (FDA) Mini-Sentinel surveillance system has revealed no new safety concerns associated with rivaroxaban use in patients with nonvalvular atrial fibrillation.
Results from this surveillance showed the risk of ischemic stroke was lower in patients who received rivaroxaban than in those who received warfarin.
Rivaroxaban was also associated with a lower risk of intracranial hemorrhage but a higher risk of gastrointestinal bleeding compared to warfarin.
These findings were published in Pharmacoepidemiology & Drug Safety.
About the surveillance
The FDA’s Sentinel Initiative began in 2008 as a multi-year effort to create a national electronic system for monitoring the safety of approved and FDA-regulated medical products using existing electronic healthcare data from multiple sources.
The initiative is the FDA’s response to the Food and Drug Administration Amendments Act (FDAAA), and it includes Mini-Sentinel, a working pilot project to develop an active surveillance system and complement existing methods of safety surveillance.
Rivaroxaban results
Researchers analyzed Mini-Sentinel data for patients with nonvalvular atrial fibrillation who initiated treatment with rivaroxaban or warfarin from November 2011 to April 2015.
To examine the safety of both products, methodologies included assessing ICD-9-CM codes from inpatient claims and evaluating rates of gastrointestinal bleeding, ischemic stroke, and intracranial hemorrhage.*
The incidence of ischemic stroke was higher among patients on warfarin (268/80,180) than among those on rivaroxaban (82/36,512). The adjusted incidence rate per 1000 person-years was 9.57 among rivaroxaban users and 17.10 among warfarin users. The adjusted hazard ratio was 0.61 (95% CI, 0.47-0.79).
The incidence of gastrointestinal bleeding was higher among patients on rivaroxaban (423/36,173) than among those on warfarin (651/79,520). The incidence rate was 50.20 among rivaroxaban users and 34.82 among warfarin users. The hazard ratio was 1.47 (95% CI, 1.29-1.67).
The incidence of intracranial hemorrhage was higher among patients on warfarin (143/79,529) than among those on rivaroxaban (46/36,171).The incidence rate was 5.41 among rivaroxaban users and 7.49 among warfarin users. The hazard ratio was 0.71 (95% CI, 0.50-1.01). ![]()
*Total patient numbers differed in the analyses for each endpoint. For example, there were 80,180 warfarin users in the ischemic stroke analysis and 79,520 warfarin users in the analysis of gastrointestinal bleeding.
The US Food and Drug Administration’s (FDA) Mini-Sentinel surveillance system has revealed no new safety concerns associated with rivaroxaban use in patients with nonvalvular atrial fibrillation.
Results from this surveillance showed the risk of ischemic stroke was lower in patients who received rivaroxaban than in those who received warfarin.
Rivaroxaban was also associated with a lower risk of intracranial hemorrhage but a higher risk of gastrointestinal bleeding compared to warfarin.
These findings were published in Pharmacoepidemiology & Drug Safety.
About the surveillance
The FDA’s Sentinel Initiative began in 2008 as a multi-year effort to create a national electronic system for monitoring the safety of approved and FDA-regulated medical products using existing electronic healthcare data from multiple sources.
The initiative is the FDA’s response to the Food and Drug Administration Amendments Act (FDAAA), and it includes Mini-Sentinel, a working pilot project to develop an active surveillance system and complement existing methods of safety surveillance.
Rivaroxaban results
Researchers analyzed Mini-Sentinel data for patients with nonvalvular atrial fibrillation who initiated treatment with rivaroxaban or warfarin from November 2011 to April 2015.
To examine the safety of both products, methodologies included assessing ICD-9-CM codes from inpatient claims and evaluating rates of gastrointestinal bleeding, ischemic stroke, and intracranial hemorrhage.*
The incidence of ischemic stroke was higher among patients on warfarin (268/80,180) than among those on rivaroxaban (82/36,512). The adjusted incidence rate per 1000 person-years was 9.57 among rivaroxaban users and 17.10 among warfarin users. The adjusted hazard ratio was 0.61 (95% CI, 0.47-0.79).
The incidence of gastrointestinal bleeding was higher among patients on rivaroxaban (423/36,173) than among those on warfarin (651/79,520). The incidence rate was 50.20 among rivaroxaban users and 34.82 among warfarin users. The hazard ratio was 1.47 (95% CI, 1.29-1.67).
The incidence of intracranial hemorrhage was higher among patients on warfarin (143/79,529) than among those on rivaroxaban (46/36,171).The incidence rate was 5.41 among rivaroxaban users and 7.49 among warfarin users. The hazard ratio was 0.71 (95% CI, 0.50-1.01). ![]()
*Total patient numbers differed in the analyses for each endpoint. For example, there were 80,180 warfarin users in the ischemic stroke analysis and 79,520 warfarin users in the analysis of gastrointestinal bleeding.
The US Food and Drug Administration’s (FDA) Mini-Sentinel surveillance system has revealed no new safety concerns associated with rivaroxaban use in patients with nonvalvular atrial fibrillation.
Results from this surveillance showed the risk of ischemic stroke was lower in patients who received rivaroxaban than in those who received warfarin.
Rivaroxaban was also associated with a lower risk of intracranial hemorrhage but a higher risk of gastrointestinal bleeding compared to warfarin.
These findings were published in Pharmacoepidemiology & Drug Safety.
About the surveillance
The FDA’s Sentinel Initiative began in 2008 as a multi-year effort to create a national electronic system for monitoring the safety of approved and FDA-regulated medical products using existing electronic healthcare data from multiple sources.
The initiative is the FDA’s response to the Food and Drug Administration Amendments Act (FDAAA), and it includes Mini-Sentinel, a working pilot project to develop an active surveillance system and complement existing methods of safety surveillance.
Rivaroxaban results
Researchers analyzed Mini-Sentinel data for patients with nonvalvular atrial fibrillation who initiated treatment with rivaroxaban or warfarin from November 2011 to April 2015.
To examine the safety of both products, methodologies included assessing ICD-9-CM codes from inpatient claims and evaluating rates of gastrointestinal bleeding, ischemic stroke, and intracranial hemorrhage.*
The incidence of ischemic stroke was higher among patients on warfarin (268/80,180) than among those on rivaroxaban (82/36,512). The adjusted incidence rate per 1000 person-years was 9.57 among rivaroxaban users and 17.10 among warfarin users. The adjusted hazard ratio was 0.61 (95% CI, 0.47-0.79).
The incidence of gastrointestinal bleeding was higher among patients on rivaroxaban (423/36,173) than among those on warfarin (651/79,520). The incidence rate was 50.20 among rivaroxaban users and 34.82 among warfarin users. The hazard ratio was 1.47 (95% CI, 1.29-1.67).
The incidence of intracranial hemorrhage was higher among patients on warfarin (143/79,529) than among those on rivaroxaban (46/36,171).The incidence rate was 5.41 among rivaroxaban users and 7.49 among warfarin users. The hazard ratio was 0.71 (95% CI, 0.50-1.01). ![]()
*Total patient numbers differed in the analyses for each endpoint. For example, there were 80,180 warfarin users in the ischemic stroke analysis and 79,520 warfarin users in the analysis of gastrointestinal bleeding.
Compound may treat drug-resistant malaria
The antimicrobial agent triclosan may be able to fight drug-resistant malaria, according to research published in Scientific Reports.
For this work, researchers used an artificially intelligent “robot scientist” named Eve to perform a high-throughput screen of potential antimalarial compounds.
The screen revealed that triclosan is effective against Plasmodium parasites that have grown resistant to the antimalarial drug pyrimethamine.
Researchers have known for some time that triclosan inhibits in vitro growth of Plasmodium parasites. They assumed this was because triclosan inhibits the enzyme enoyl reductase (ENR).
However, subsequent work showed that improving triclosan’s ability to target ENR had no effect on parasite growth in the blood.
With the current study, researchers discovered that triclosan also affects parasite growth by inhibiting an enzyme called dihydrofolate reductase (DHFR), which is the target of the antimalarial drug pyrimethamine.
The researchers conducted growth competition experiments with 3 yeast strains dependent on a DHFR enzyme from Plasmodium falciparum, a DHFR enzyme from Plasmodium vivax, and human DHFR.
The team used Eve to screen compounds and identify drugs that inhibit the parasite targets but not the human counterpart.
The researchers found that triclosan was able to target and act on DHFR in both wild-type and pyrimethamine-resistant P falciparum and P vivax parasites.
Because triclosan inhibits both ENR and DHFR, the researchers think it may be possible to target Plasmodium parasites at both the liver and blood stages.
“The discovery by our robot ‘colleague’ Eve that triclosan is effective against malaria targets offers hope that we may be able to use it to develop a new drug,” said study author Elizabeth Bilsland, PhD, of the University of Campinas in Brazil.
“We know it is a safe compound, and its ability to target 2 points in the malaria parasite’s life-cycle means the parasite will find it difficult to evolve resistance.”
Eve was developed to speed up the drug discovery process by automatically developing and testing hypotheses to explain observations, run experiments using laboratory robotics, interpret the results to amend hypotheses, and then repeat the cycle.
“Artificial intelligence and machine-learning enables us to create automated scientists that do not just take a ‘brute force’ approach but, rather, take an intelligent approach to science,” said Ross King, PhD, a professor at the University of Manchester in the UK who led the development of Eve.
“This could greatly speed up the drug discovery process and potentially reap huge rewards.” ![]()
The antimicrobial agent triclosan may be able to fight drug-resistant malaria, according to research published in Scientific Reports.
For this work, researchers used an artificially intelligent “robot scientist” named Eve to perform a high-throughput screen of potential antimalarial compounds.
The screen revealed that triclosan is effective against Plasmodium parasites that have grown resistant to the antimalarial drug pyrimethamine.
Researchers have known for some time that triclosan inhibits in vitro growth of Plasmodium parasites. They assumed this was because triclosan inhibits the enzyme enoyl reductase (ENR).
However, subsequent work showed that improving triclosan’s ability to target ENR had no effect on parasite growth in the blood.
With the current study, researchers discovered that triclosan also affects parasite growth by inhibiting an enzyme called dihydrofolate reductase (DHFR), which is the target of the antimalarial drug pyrimethamine.
The researchers conducted growth competition experiments with 3 yeast strains dependent on a DHFR enzyme from Plasmodium falciparum, a DHFR enzyme from Plasmodium vivax, and human DHFR.
The team used Eve to screen compounds and identify drugs that inhibit the parasite targets but not the human counterpart.
The researchers found that triclosan was able to target and act on DHFR in both wild-type and pyrimethamine-resistant P falciparum and P vivax parasites.
Because triclosan inhibits both ENR and DHFR, the researchers think it may be possible to target Plasmodium parasites at both the liver and blood stages.
“The discovery by our robot ‘colleague’ Eve that triclosan is effective against malaria targets offers hope that we may be able to use it to develop a new drug,” said study author Elizabeth Bilsland, PhD, of the University of Campinas in Brazil.
“We know it is a safe compound, and its ability to target 2 points in the malaria parasite’s life-cycle means the parasite will find it difficult to evolve resistance.”
Eve was developed to speed up the drug discovery process by automatically developing and testing hypotheses to explain observations, run experiments using laboratory robotics, interpret the results to amend hypotheses, and then repeat the cycle.
“Artificial intelligence and machine-learning enables us to create automated scientists that do not just take a ‘brute force’ approach but, rather, take an intelligent approach to science,” said Ross King, PhD, a professor at the University of Manchester in the UK who led the development of Eve.
“This could greatly speed up the drug discovery process and potentially reap huge rewards.” ![]()
The antimicrobial agent triclosan may be able to fight drug-resistant malaria, according to research published in Scientific Reports.
For this work, researchers used an artificially intelligent “robot scientist” named Eve to perform a high-throughput screen of potential antimalarial compounds.
The screen revealed that triclosan is effective against Plasmodium parasites that have grown resistant to the antimalarial drug pyrimethamine.
Researchers have known for some time that triclosan inhibits in vitro growth of Plasmodium parasites. They assumed this was because triclosan inhibits the enzyme enoyl reductase (ENR).
However, subsequent work showed that improving triclosan’s ability to target ENR had no effect on parasite growth in the blood.
With the current study, researchers discovered that triclosan also affects parasite growth by inhibiting an enzyme called dihydrofolate reductase (DHFR), which is the target of the antimalarial drug pyrimethamine.
The researchers conducted growth competition experiments with 3 yeast strains dependent on a DHFR enzyme from Plasmodium falciparum, a DHFR enzyme from Plasmodium vivax, and human DHFR.
The team used Eve to screen compounds and identify drugs that inhibit the parasite targets but not the human counterpart.
The researchers found that triclosan was able to target and act on DHFR in both wild-type and pyrimethamine-resistant P falciparum and P vivax parasites.
Because triclosan inhibits both ENR and DHFR, the researchers think it may be possible to target Plasmodium parasites at both the liver and blood stages.
“The discovery by our robot ‘colleague’ Eve that triclosan is effective against malaria targets offers hope that we may be able to use it to develop a new drug,” said study author Elizabeth Bilsland, PhD, of the University of Campinas in Brazil.
“We know it is a safe compound, and its ability to target 2 points in the malaria parasite’s life-cycle means the parasite will find it difficult to evolve resistance.”
Eve was developed to speed up the drug discovery process by automatically developing and testing hypotheses to explain observations, run experiments using laboratory robotics, interpret the results to amend hypotheses, and then repeat the cycle.
“Artificial intelligence and machine-learning enables us to create automated scientists that do not just take a ‘brute force’ approach but, rather, take an intelligent approach to science,” said Ross King, PhD, a professor at the University of Manchester in the UK who led the development of Eve.
“This could greatly speed up the drug discovery process and potentially reap huge rewards.” ![]()
CBT cost effective for depressed teens refusing antidepressants
“In this study, we demonstrate that brief primary care CBT is a cost-effective treatment option for adolescents with depression and likely generates cost savings over 2 years,” said John F. Dickerson, PhD, of Kaiser Permanente Center for Health Research, Portland, Ore., and his associates
Youth randomly assigned to CBT plus TAU had 26.8 more depression-free days (P = .043) and 0.067 more quality-adjusted life-years (P = .043) on average, compared with patients receiving TAU over a 12-month period. Also, patients in the CBT group had fewer hospitalizations, compared with patients in the TAU group (1.1% vs. 8.8% during 12 months, and 4.4% vs. 12.1% during 24 months), reported Dr. Dickerson and his colleagues.
By the end of the 24-month follow-up, average total costs were $2,811 among youth randomly assigned to CBT plus TAU and $7,354 among youth assigned TAU (adjusted to 2008 U.S. dollars).
“Many adolescents with depression choose to not initiate or continue antidepressant therapy, which limits their options for depression treatment,” the investigators said. “In this evaluation, it is demonstrated that brief, primary care–based CBT is a cost-effective option for the treatment of depression among adolescents with depression who decline or quickly discontinue pharmacotherapy.”
Read more at Pediatrics (2018. doi: 10.1542/peds.2017-1969).
“In this study, we demonstrate that brief primary care CBT is a cost-effective treatment option for adolescents with depression and likely generates cost savings over 2 years,” said John F. Dickerson, PhD, of Kaiser Permanente Center for Health Research, Portland, Ore., and his associates
Youth randomly assigned to CBT plus TAU had 26.8 more depression-free days (P = .043) and 0.067 more quality-adjusted life-years (P = .043) on average, compared with patients receiving TAU over a 12-month period. Also, patients in the CBT group had fewer hospitalizations, compared with patients in the TAU group (1.1% vs. 8.8% during 12 months, and 4.4% vs. 12.1% during 24 months), reported Dr. Dickerson and his colleagues.
By the end of the 24-month follow-up, average total costs were $2,811 among youth randomly assigned to CBT plus TAU and $7,354 among youth assigned TAU (adjusted to 2008 U.S. dollars).
“Many adolescents with depression choose to not initiate or continue antidepressant therapy, which limits their options for depression treatment,” the investigators said. “In this evaluation, it is demonstrated that brief, primary care–based CBT is a cost-effective option for the treatment of depression among adolescents with depression who decline or quickly discontinue pharmacotherapy.”
Read more at Pediatrics (2018. doi: 10.1542/peds.2017-1969).
“In this study, we demonstrate that brief primary care CBT is a cost-effective treatment option for adolescents with depression and likely generates cost savings over 2 years,” said John F. Dickerson, PhD, of Kaiser Permanente Center for Health Research, Portland, Ore., and his associates
Youth randomly assigned to CBT plus TAU had 26.8 more depression-free days (P = .043) and 0.067 more quality-adjusted life-years (P = .043) on average, compared with patients receiving TAU over a 12-month period. Also, patients in the CBT group had fewer hospitalizations, compared with patients in the TAU group (1.1% vs. 8.8% during 12 months, and 4.4% vs. 12.1% during 24 months), reported Dr. Dickerson and his colleagues.
By the end of the 24-month follow-up, average total costs were $2,811 among youth randomly assigned to CBT plus TAU and $7,354 among youth assigned TAU (adjusted to 2008 U.S. dollars).
“Many adolescents with depression choose to not initiate or continue antidepressant therapy, which limits their options for depression treatment,” the investigators said. “In this evaluation, it is demonstrated that brief, primary care–based CBT is a cost-effective option for the treatment of depression among adolescents with depression who decline or quickly discontinue pharmacotherapy.”
Read more at Pediatrics (2018. doi: 10.1542/peds.2017-1969).
FROM PEDIATRICS
Handshake
There’s a simple act you’ve done with all your patients that you’ve probably been doing incorrectly. Yes, that is rather a bold assertion, but I’ll bet no one ever taught you the proper way. It’s only recently, after having done it thousands of times, that I came to realize there is a better way to give a handshake.
The first helps establish who you are as a doctor and reassures your patient that you’re both capable and trustworthy. At the end of the visit, it seals the agreement wherein they commit to take your advice (or at least try) and you commit to do whatever necessary to help them.
A poorly executed handshake, or worse, none at all, can erode trust or convey a lack of ability on your part. It’s true that handshakes aren’t always appropriate: For certain patients or disease states, they would be unsuitable. For the majority of patient visits, however, they are key. Here are some secrets to a good handshake:
- As you’ve probably experienced, timing is critical. A handshake requires someone to anticipate your action and to coordinate perfectly with you. When you enter the room, move toward your patient and put your hand forward just as you approach your patient. Too early and you look like an awkward high schooler eager for a Justin Bieber autograph. Too late and you’ll take your patient by surprise. The best position is to have your left foot forward as you reach for their hand. This gives you stability and allows you to convey confidence.
- As you approach your patient, make eye contact. Just a second or two as you cross the room is perfect. Then glance down at their now outstretched hand and connect web to web. Your arm should be tucked in and move straight toward their hand. Swinging out to come back in is great when you’re getting your new NBA jersey from the basketball commissioner, but not for getting patients comfortable with you.
- The grip depends on the patient. For most adults, a firm squeeze with two arm pumps is just right. For the hard-charging, testosterone-replacing ex-Marine, you can reciprocate the extra-firm grasp – let him win the grip contest though, that’s what he wants. For the freezing-in-her-gown great grandmother, an extra long hold, sometimes even double handed, is fine, even appreciated.
- No matter how firm, it is important to convey your enthusiasm and ability to your patient. This is done with a gentle push. As you shake hands, lightly push their arm back into them. This subtle transfer of energy from you to them is a little known tip that will make your handshake much more effective. Never push them off balance or worse, pull them toward you. Your objective is to create trust; making them unsteady will make that impossible.
- Finally, let go after two pumps. If you feel them holding on, then stay until they release. For the majority of patients, that will be a just a couple seconds.
For patients I’ve never met, I often proffer my hand turned slightly upward for our first handshake. This subtle sign of submission shows I’m open and committed to them. For our closing handshake, I have my hand turned slightly downward so that my hand is slightly over theirs. This conveys that I’m confident in what I’ve said and done and that now I want them to uphold their part in our agreement.
I’ve been using the above technique for a few years now with success. It has helped with my patient satisfaction scores, and importantly, has helped me manage difficult patients for whom trust in our relationship is invaluable.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
There’s a simple act you’ve done with all your patients that you’ve probably been doing incorrectly. Yes, that is rather a bold assertion, but I’ll bet no one ever taught you the proper way. It’s only recently, after having done it thousands of times, that I came to realize there is a better way to give a handshake.
The first helps establish who you are as a doctor and reassures your patient that you’re both capable and trustworthy. At the end of the visit, it seals the agreement wherein they commit to take your advice (or at least try) and you commit to do whatever necessary to help them.
A poorly executed handshake, or worse, none at all, can erode trust or convey a lack of ability on your part. It’s true that handshakes aren’t always appropriate: For certain patients or disease states, they would be unsuitable. For the majority of patient visits, however, they are key. Here are some secrets to a good handshake:
- As you’ve probably experienced, timing is critical. A handshake requires someone to anticipate your action and to coordinate perfectly with you. When you enter the room, move toward your patient and put your hand forward just as you approach your patient. Too early and you look like an awkward high schooler eager for a Justin Bieber autograph. Too late and you’ll take your patient by surprise. The best position is to have your left foot forward as you reach for their hand. This gives you stability and allows you to convey confidence.
- As you approach your patient, make eye contact. Just a second or two as you cross the room is perfect. Then glance down at their now outstretched hand and connect web to web. Your arm should be tucked in and move straight toward their hand. Swinging out to come back in is great when you’re getting your new NBA jersey from the basketball commissioner, but not for getting patients comfortable with you.
- The grip depends on the patient. For most adults, a firm squeeze with two arm pumps is just right. For the hard-charging, testosterone-replacing ex-Marine, you can reciprocate the extra-firm grasp – let him win the grip contest though, that’s what he wants. For the freezing-in-her-gown great grandmother, an extra long hold, sometimes even double handed, is fine, even appreciated.
- No matter how firm, it is important to convey your enthusiasm and ability to your patient. This is done with a gentle push. As you shake hands, lightly push their arm back into them. This subtle transfer of energy from you to them is a little known tip that will make your handshake much more effective. Never push them off balance or worse, pull them toward you. Your objective is to create trust; making them unsteady will make that impossible.
- Finally, let go after two pumps. If you feel them holding on, then stay until they release. For the majority of patients, that will be a just a couple seconds.
For patients I’ve never met, I often proffer my hand turned slightly upward for our first handshake. This subtle sign of submission shows I’m open and committed to them. For our closing handshake, I have my hand turned slightly downward so that my hand is slightly over theirs. This conveys that I’m confident in what I’ve said and done and that now I want them to uphold their part in our agreement.
I’ve been using the above technique for a few years now with success. It has helped with my patient satisfaction scores, and importantly, has helped me manage difficult patients for whom trust in our relationship is invaluable.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
There’s a simple act you’ve done with all your patients that you’ve probably been doing incorrectly. Yes, that is rather a bold assertion, but I’ll bet no one ever taught you the proper way. It’s only recently, after having done it thousands of times, that I came to realize there is a better way to give a handshake.
The first helps establish who you are as a doctor and reassures your patient that you’re both capable and trustworthy. At the end of the visit, it seals the agreement wherein they commit to take your advice (or at least try) and you commit to do whatever necessary to help them.
A poorly executed handshake, or worse, none at all, can erode trust or convey a lack of ability on your part. It’s true that handshakes aren’t always appropriate: For certain patients or disease states, they would be unsuitable. For the majority of patient visits, however, they are key. Here are some secrets to a good handshake:
- As you’ve probably experienced, timing is critical. A handshake requires someone to anticipate your action and to coordinate perfectly with you. When you enter the room, move toward your patient and put your hand forward just as you approach your patient. Too early and you look like an awkward high schooler eager for a Justin Bieber autograph. Too late and you’ll take your patient by surprise. The best position is to have your left foot forward as you reach for their hand. This gives you stability and allows you to convey confidence.
- As you approach your patient, make eye contact. Just a second or two as you cross the room is perfect. Then glance down at their now outstretched hand and connect web to web. Your arm should be tucked in and move straight toward their hand. Swinging out to come back in is great when you’re getting your new NBA jersey from the basketball commissioner, but not for getting patients comfortable with you.
- The grip depends on the patient. For most adults, a firm squeeze with two arm pumps is just right. For the hard-charging, testosterone-replacing ex-Marine, you can reciprocate the extra-firm grasp – let him win the grip contest though, that’s what he wants. For the freezing-in-her-gown great grandmother, an extra long hold, sometimes even double handed, is fine, even appreciated.
- No matter how firm, it is important to convey your enthusiasm and ability to your patient. This is done with a gentle push. As you shake hands, lightly push their arm back into them. This subtle transfer of energy from you to them is a little known tip that will make your handshake much more effective. Never push them off balance or worse, pull them toward you. Your objective is to create trust; making them unsteady will make that impossible.
- Finally, let go after two pumps. If you feel them holding on, then stay until they release. For the majority of patients, that will be a just a couple seconds.
For patients I’ve never met, I often proffer my hand turned slightly upward for our first handshake. This subtle sign of submission shows I’m open and committed to them. For our closing handshake, I have my hand turned slightly downward so that my hand is slightly over theirs. This conveys that I’m confident in what I’ve said and done and that now I want them to uphold their part in our agreement.
I’ve been using the above technique for a few years now with success. It has helped with my patient satisfaction scores, and importantly, has helped me manage difficult patients for whom trust in our relationship is invaluable.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Preterm intervention underused in at-risk women
A significant number of woman who delivered ahead of term and were already considered at risk for spontaneous preterm birth did not receive any sort of intervention to prevent the preterm birth, according to Yu Yang Feng and associates.
For the retrospective study, the researchers analyzed the medical records of 31 women who had been admitted to a primary, secondary, or tertiary prenatal care center and who had a history of spontaneous singleton preterm birth and/or cervical shortening before 24 weeks of gestation. Of the 23 women who were referred to a prenatal care center before reaching a gestational age of 24 weeks, only 13 were offered an intervention and only 12 received progesterone or cerclage as an intervention, while none received pessary. One of the 13 was offered and declined progesterone.
Of the 12 women who received an intervention before 24 weeks’ gestational age, 8 received progesterone, 2 received elective cerclage, and 2 received rescue cerclage. An additional woman who was referred to a prenatal care center after 24 weeks received progesterone, the only one in that group to receive an intervention.
Most of the women in the study were referred to a prenatal care center by their family physician, although two were referred by their midwife, one was referred by a nurse practitioner and fertility specialist, and three started their care with an obstetrician.
“Our findings that less than one in three women with a previous preterm birth or current short cervix received progesterone attest to the importance of improving knowledge translation with the latest evidence to encourage referral in time and use of this intervention for the prevention of preterm birth,” the study investigators concluded.
Find the full study in the Journal of Obstetrics and Gynaecology Canada (2018 Jan. doi: 10.1016/j.jogc.2017.08.036).
A significant number of woman who delivered ahead of term and were already considered at risk for spontaneous preterm birth did not receive any sort of intervention to prevent the preterm birth, according to Yu Yang Feng and associates.
For the retrospective study, the researchers analyzed the medical records of 31 women who had been admitted to a primary, secondary, or tertiary prenatal care center and who had a history of spontaneous singleton preterm birth and/or cervical shortening before 24 weeks of gestation. Of the 23 women who were referred to a prenatal care center before reaching a gestational age of 24 weeks, only 13 were offered an intervention and only 12 received progesterone or cerclage as an intervention, while none received pessary. One of the 13 was offered and declined progesterone.
Of the 12 women who received an intervention before 24 weeks’ gestational age, 8 received progesterone, 2 received elective cerclage, and 2 received rescue cerclage. An additional woman who was referred to a prenatal care center after 24 weeks received progesterone, the only one in that group to receive an intervention.
Most of the women in the study were referred to a prenatal care center by their family physician, although two were referred by their midwife, one was referred by a nurse practitioner and fertility specialist, and three started their care with an obstetrician.
“Our findings that less than one in three women with a previous preterm birth or current short cervix received progesterone attest to the importance of improving knowledge translation with the latest evidence to encourage referral in time and use of this intervention for the prevention of preterm birth,” the study investigators concluded.
Find the full study in the Journal of Obstetrics and Gynaecology Canada (2018 Jan. doi: 10.1016/j.jogc.2017.08.036).
A significant number of woman who delivered ahead of term and were already considered at risk for spontaneous preterm birth did not receive any sort of intervention to prevent the preterm birth, according to Yu Yang Feng and associates.
For the retrospective study, the researchers analyzed the medical records of 31 women who had been admitted to a primary, secondary, or tertiary prenatal care center and who had a history of spontaneous singleton preterm birth and/or cervical shortening before 24 weeks of gestation. Of the 23 women who were referred to a prenatal care center before reaching a gestational age of 24 weeks, only 13 were offered an intervention and only 12 received progesterone or cerclage as an intervention, while none received pessary. One of the 13 was offered and declined progesterone.
Of the 12 women who received an intervention before 24 weeks’ gestational age, 8 received progesterone, 2 received elective cerclage, and 2 received rescue cerclage. An additional woman who was referred to a prenatal care center after 24 weeks received progesterone, the only one in that group to receive an intervention.
Most of the women in the study were referred to a prenatal care center by their family physician, although two were referred by their midwife, one was referred by a nurse practitioner and fertility specialist, and three started their care with an obstetrician.
“Our findings that less than one in three women with a previous preterm birth or current short cervix received progesterone attest to the importance of improving knowledge translation with the latest evidence to encourage referral in time and use of this intervention for the prevention of preterm birth,” the study investigators concluded.
Find the full study in the Journal of Obstetrics and Gynaecology Canada (2018 Jan. doi: 10.1016/j.jogc.2017.08.036).
FROM THE JOURNAL OF OBSTETRICS AND GYNAECOLOGY CANADA
Topical retinoid plus benzoyl peroxide beats antibiotics for inflammatory acne
, according to the consensus of an international panel of dermatologists, led by Diane M. Thiboutot, MD, professor of dermatology, Penn State University, Hershey.
Dr. Thiboutot is the chair of the Global Alliance to Improve Outcomes in Acne, an international group of dermatologists interested in acne research and education; they have been meeting regularly since 2001 and continuously evaluate the literature on acne.
A Delphi panel and questionnaire method was used to reach consensus. A panel of 36 dermatologists from 27 countries who were members of the alliance answered a set of questionnaires on selected topics. An online questionnaire on various topics was developed, and panel members were asked to rate agreement with each statement on a 5-point Likert scale. If they selected disagree, strongly disagree, or unable to answer, they were asked to provide a written explanation of what they disagreed with. Ultimately, consensus was reached if 75% of more of the panel members agreed on a statement.
Assessing acne severity
The term “severe acne” currently is perceived to refer to nodular/conglobate acne, which is generally treated with oral isotretinoin. But development of added first-line treatment options means there may be a need for a system of classifying moderately severe, severe, and very severe acne. The 2016 European S3 Acne Guideline uses a 4-point classification system that might help: 1) comedonal acne, 2) mild-moderate papulopustular acne; 3) severe papulopustular acne, moderate nodular acne; and 4) severe nodular acne, conglobate acne. In a similar fashion, the U.S. Food and Drug Administration’s Investigator’s Global Assessment scale considers quality of lesions and quantity, including a grade of severe acne that is separate from nodular/conglobate acne.
“We propose that the designation “very severe” be reserved for cystic and conglobate acne,” Dr. Thiboutot and her associates wrote.
Strategic approach to acne therapy
First-line therapy for most patients with inflammatory acne, comedonal acne, or both, should be a topical retinoid plus topical benzoyl peroxide (BPO), the panelists agreed.
Topical or systemic antibiotics should not be used as monotherapy because of the rapid development of resistance. All strains of Propionibacterium acnes are sensitive to BPhO.
Topical retinoids (with or without BPO) or azelaic acid are the treatment of choice for maintenance, not topical antibiotics, they said.
Oral isotretinoin is indicated as first-line therapy for very severe (cystic and conglobate) acne. A small percentage of patients, perhaps 15%, experience acne flare on oral isotretinoin. This often can be managed by using low-dose therapy, say 0.5 mg/kg, although some panelists said that in some cases inflammatory flare occurs regardless of dose.
Oral tretinoin therapy should continue until full clearance of acne. More studies are needed to define the total cumulative dose that maintains remission, the alliance members said.
Determining the risk-benefit for using systemic antibiotics needs to balance individual need against public interest in preserving antibiotic effectiveness, the authors said. Antibiotics should be avoided when there are effective alternatives.
Oral antibiotics are indicated if inflammatory acne isn’t responding well to topical treatments, and acne involves the trunk or multiple bodily areas. Evaluate response to therapy at 6-8 weeks, and don’t treat for more than 3-4 months. After stopping antibiotics, use a topical retinoid and BPO or azelaic acid, they said.
The article, published in the Journal of the American Academy of Dermatology, provides an acne management algorithm that summarizes a treatment approach on the basis of these consensus recommendations.
Special populations
The panelists largely agreed that azelaic acid cream 20% or gel 15% can be useful to treat acne in pregnant women and patients with acne and postinflammatory hyperpigmention, although some said it can cause irritation and aggravate already inflamed skin.
In patients with inflammatory acne, devices such as laser, intense pulsed light, and photodynamic therapy should not be considered first-line treatment, they concluded.
The article also includes clinical pearls for treating acne and postinflammatory hyperpigmentation, questions to ask when taking an acne scar history, an atrophic acne scar risk-assessment tool, clinical pearls for preventing atrophic acne scars, interventions for treating facial atrophic acne scars, clinical pearls for preventing and managing hypertrophic or keloidal scars, and clinical pearls regarding acne in women.
The study is supported by an unrestricted educational grant from Galderma International SAS, Paris. All authors have served as advisory board members for Galderma and received honoraria. Dr. Thiboutot has received fees and research funding for serving as a consultant and investigator for Allergan, Mimetica, Novan, and Sebacia and as a consultant for Dermira, Galderma, Photosonix, and Xenon. Many of the other alliance members have financial associations with biopharmaceutical companies.
SOURCE: Thiboutot DM et al. J Am Acad Dermatol. 2018 Feb. doi: 10.1016/j.jaad.2017.09.078.
, according to the consensus of an international panel of dermatologists, led by Diane M. Thiboutot, MD, professor of dermatology, Penn State University, Hershey.
Dr. Thiboutot is the chair of the Global Alliance to Improve Outcomes in Acne, an international group of dermatologists interested in acne research and education; they have been meeting regularly since 2001 and continuously evaluate the literature on acne.
A Delphi panel and questionnaire method was used to reach consensus. A panel of 36 dermatologists from 27 countries who were members of the alliance answered a set of questionnaires on selected topics. An online questionnaire on various topics was developed, and panel members were asked to rate agreement with each statement on a 5-point Likert scale. If they selected disagree, strongly disagree, or unable to answer, they were asked to provide a written explanation of what they disagreed with. Ultimately, consensus was reached if 75% of more of the panel members agreed on a statement.
Assessing acne severity
The term “severe acne” currently is perceived to refer to nodular/conglobate acne, which is generally treated with oral isotretinoin. But development of added first-line treatment options means there may be a need for a system of classifying moderately severe, severe, and very severe acne. The 2016 European S3 Acne Guideline uses a 4-point classification system that might help: 1) comedonal acne, 2) mild-moderate papulopustular acne; 3) severe papulopustular acne, moderate nodular acne; and 4) severe nodular acne, conglobate acne. In a similar fashion, the U.S. Food and Drug Administration’s Investigator’s Global Assessment scale considers quality of lesions and quantity, including a grade of severe acne that is separate from nodular/conglobate acne.
“We propose that the designation “very severe” be reserved for cystic and conglobate acne,” Dr. Thiboutot and her associates wrote.
Strategic approach to acne therapy
First-line therapy for most patients with inflammatory acne, comedonal acne, or both, should be a topical retinoid plus topical benzoyl peroxide (BPO), the panelists agreed.
Topical or systemic antibiotics should not be used as monotherapy because of the rapid development of resistance. All strains of Propionibacterium acnes are sensitive to BPhO.
Topical retinoids (with or without BPO) or azelaic acid are the treatment of choice for maintenance, not topical antibiotics, they said.
Oral isotretinoin is indicated as first-line therapy for very severe (cystic and conglobate) acne. A small percentage of patients, perhaps 15%, experience acne flare on oral isotretinoin. This often can be managed by using low-dose therapy, say 0.5 mg/kg, although some panelists said that in some cases inflammatory flare occurs regardless of dose.
Oral tretinoin therapy should continue until full clearance of acne. More studies are needed to define the total cumulative dose that maintains remission, the alliance members said.
Determining the risk-benefit for using systemic antibiotics needs to balance individual need against public interest in preserving antibiotic effectiveness, the authors said. Antibiotics should be avoided when there are effective alternatives.
Oral antibiotics are indicated if inflammatory acne isn’t responding well to topical treatments, and acne involves the trunk or multiple bodily areas. Evaluate response to therapy at 6-8 weeks, and don’t treat for more than 3-4 months. After stopping antibiotics, use a topical retinoid and BPO or azelaic acid, they said.
The article, published in the Journal of the American Academy of Dermatology, provides an acne management algorithm that summarizes a treatment approach on the basis of these consensus recommendations.
Special populations
The panelists largely agreed that azelaic acid cream 20% or gel 15% can be useful to treat acne in pregnant women and patients with acne and postinflammatory hyperpigmention, although some said it can cause irritation and aggravate already inflamed skin.
In patients with inflammatory acne, devices such as laser, intense pulsed light, and photodynamic therapy should not be considered first-line treatment, they concluded.
The article also includes clinical pearls for treating acne and postinflammatory hyperpigmentation, questions to ask when taking an acne scar history, an atrophic acne scar risk-assessment tool, clinical pearls for preventing atrophic acne scars, interventions for treating facial atrophic acne scars, clinical pearls for preventing and managing hypertrophic or keloidal scars, and clinical pearls regarding acne in women.
The study is supported by an unrestricted educational grant from Galderma International SAS, Paris. All authors have served as advisory board members for Galderma and received honoraria. Dr. Thiboutot has received fees and research funding for serving as a consultant and investigator for Allergan, Mimetica, Novan, and Sebacia and as a consultant for Dermira, Galderma, Photosonix, and Xenon. Many of the other alliance members have financial associations with biopharmaceutical companies.
SOURCE: Thiboutot DM et al. J Am Acad Dermatol. 2018 Feb. doi: 10.1016/j.jaad.2017.09.078.
, according to the consensus of an international panel of dermatologists, led by Diane M. Thiboutot, MD, professor of dermatology, Penn State University, Hershey.
Dr. Thiboutot is the chair of the Global Alliance to Improve Outcomes in Acne, an international group of dermatologists interested in acne research and education; they have been meeting regularly since 2001 and continuously evaluate the literature on acne.
A Delphi panel and questionnaire method was used to reach consensus. A panel of 36 dermatologists from 27 countries who were members of the alliance answered a set of questionnaires on selected topics. An online questionnaire on various topics was developed, and panel members were asked to rate agreement with each statement on a 5-point Likert scale. If they selected disagree, strongly disagree, or unable to answer, they were asked to provide a written explanation of what they disagreed with. Ultimately, consensus was reached if 75% of more of the panel members agreed on a statement.
Assessing acne severity
The term “severe acne” currently is perceived to refer to nodular/conglobate acne, which is generally treated with oral isotretinoin. But development of added first-line treatment options means there may be a need for a system of classifying moderately severe, severe, and very severe acne. The 2016 European S3 Acne Guideline uses a 4-point classification system that might help: 1) comedonal acne, 2) mild-moderate papulopustular acne; 3) severe papulopustular acne, moderate nodular acne; and 4) severe nodular acne, conglobate acne. In a similar fashion, the U.S. Food and Drug Administration’s Investigator’s Global Assessment scale considers quality of lesions and quantity, including a grade of severe acne that is separate from nodular/conglobate acne.
“We propose that the designation “very severe” be reserved for cystic and conglobate acne,” Dr. Thiboutot and her associates wrote.
Strategic approach to acne therapy
First-line therapy for most patients with inflammatory acne, comedonal acne, or both, should be a topical retinoid plus topical benzoyl peroxide (BPO), the panelists agreed.
Topical or systemic antibiotics should not be used as monotherapy because of the rapid development of resistance. All strains of Propionibacterium acnes are sensitive to BPhO.
Topical retinoids (with or without BPO) or azelaic acid are the treatment of choice for maintenance, not topical antibiotics, they said.
Oral isotretinoin is indicated as first-line therapy for very severe (cystic and conglobate) acne. A small percentage of patients, perhaps 15%, experience acne flare on oral isotretinoin. This often can be managed by using low-dose therapy, say 0.5 mg/kg, although some panelists said that in some cases inflammatory flare occurs regardless of dose.
Oral tretinoin therapy should continue until full clearance of acne. More studies are needed to define the total cumulative dose that maintains remission, the alliance members said.
Determining the risk-benefit for using systemic antibiotics needs to balance individual need against public interest in preserving antibiotic effectiveness, the authors said. Antibiotics should be avoided when there are effective alternatives.
Oral antibiotics are indicated if inflammatory acne isn’t responding well to topical treatments, and acne involves the trunk or multiple bodily areas. Evaluate response to therapy at 6-8 weeks, and don’t treat for more than 3-4 months. After stopping antibiotics, use a topical retinoid and BPO or azelaic acid, they said.
The article, published in the Journal of the American Academy of Dermatology, provides an acne management algorithm that summarizes a treatment approach on the basis of these consensus recommendations.
Special populations
The panelists largely agreed that azelaic acid cream 20% or gel 15% can be useful to treat acne in pregnant women and patients with acne and postinflammatory hyperpigmention, although some said it can cause irritation and aggravate already inflamed skin.
In patients with inflammatory acne, devices such as laser, intense pulsed light, and photodynamic therapy should not be considered first-line treatment, they concluded.
The article also includes clinical pearls for treating acne and postinflammatory hyperpigmentation, questions to ask when taking an acne scar history, an atrophic acne scar risk-assessment tool, clinical pearls for preventing atrophic acne scars, interventions for treating facial atrophic acne scars, clinical pearls for preventing and managing hypertrophic or keloidal scars, and clinical pearls regarding acne in women.
The study is supported by an unrestricted educational grant from Galderma International SAS, Paris. All authors have served as advisory board members for Galderma and received honoraria. Dr. Thiboutot has received fees and research funding for serving as a consultant and investigator for Allergan, Mimetica, Novan, and Sebacia and as a consultant for Dermira, Galderma, Photosonix, and Xenon. Many of the other alliance members have financial associations with biopharmaceutical companies.
SOURCE: Thiboutot DM et al. J Am Acad Dermatol. 2018 Feb. doi: 10.1016/j.jaad.2017.09.078.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Approach to the asymptomatic adnexal mass: When to operate, refer, or observe
Adnexal masses are common findings in women. While the decision to operate on symptomatic adnexal masses is straightforward, the decision-making process for asymptomatic masses is more complicated. Here we address how to approach an asymptomatic adnexal mass, including how to decide when to operate, when to refer, or how to monitor.
It is important to minimize the number of surgeries for benign, asymptomatic adnexal masses because complications are reported in 2%-15% of surgeries for adnexal masses and these can range from minimal to devastating.1 In addition, unnecessary surgery is associated with a burden of cost to the health care system. Therefore, there is a paradigm shift in the management of asymptomatic adnexal masses trending toward surveillance of any masses that are likely to be benign. What becomes critical in this approach is the ability to accurately classify these masses preoperatively.
Determining the malignant potential of a mass
Guidance is provided by the ACOG Practice Bulletin Number 174, which was published in 2016: “Evaluation and Management of Adnexal Masses.”2 These guidelines remind clinicians that:
- Most adnexal masses are benign, even in postmenopausal patients.
- The recommended imaging modality is quality transvaginal ultrasonography with an ultrasonographer accredited through the American Registry of Diagnostic Medical Sonographers.
- Simple cysts up to 10 cm can be monitored using repeat imaging every 6 months without surgical intervention, even in postmenopausal patients. In prospective studies, no cases of malignancy were diagnosed over 6 years of surveillance and most resolved. Those that persist are likely to be serous cystadenomas.
- Many benign lesions such as endometriomas and cystic teratomas have characteristic radiologic features. Surgery for these lesions is warranted for large size, symptoms, or growth in size.
- Ultrasound characteristics of malignant masses include:
1. Cyst size greater than 10 cm
2. Papillary or solid components
3. Septations
4. Internal blood flow on color Doppler.
An alternative approach that has been proposed is an ultrasound scoring system devised by International Ovarian Tumor Analysis Group. The scoring system uses 10 ultrasound findings that are characteristic of malignant and benign and is designed to characterize masses as either benign or malignant.3 This approach is able to correctly classify 77% of masses. The remaining masses with features that do not fit the “simple rules” are considered potentially malignant and should be referred to an oncology specialist for further decision making.
Decision to operate
After referral to gynecologic oncologists, surgery is not always inevitable, particularly for women with indeterminate masses. The gynecologic oncologist uses a decision-making process that factors in the underlying surgical risks for that patient with the likelihood of malignancy based on the features of the mass. The threshold to operate is higher in women with underlying major comorbidities, such as morbid obesity, complex prior surgical history, or cardiopulmonary disease. Healthier surgical candidates are more likely to be considered for a surgery, even if the suspicion for malignancy is lower. However, low surgical risk does not equate to no surgical risk. Therefore, even in apparently “good” surgical candidates, the suspicion for underlying malignancy needs to be reasonably high in order to justify the cost and risk of surgery in an asymptomatic patient. Sometimes it is patient anxiety and a desire to avoid repeated surveillance that prompts a decision to operate.
How to monitor
The role of surveillance and monitoring is to establish a natural history of the lesion or to allow it to reveal itself to be stable or regressive. Surveillance with serial sonography has shown that most asymptomatic adnexal masses with low risk features will resolve over time. Lack of resolution in the setting of stable findings is not a worrisome feature and is not suggestive of malignancy. The mere persistence of an otherwise benign-appearing lesion is not a reason to intervene with surgery.
Unfortunately, there is no clear guidance on the surveillance intervals. Some experts recommend an initial repeat scan in 3 months. If at that point the morphologic features and size are stable or decreasing, ultrasounds can be repeated at annual intervals for 5 years. In one study, masses that became malignant demonstrated growth by 7 months. Other experts recommend limiting the period of surveillance of cystic lesions to 1 year and lesions with solid components to 2 years.
Conclusions
Many asymptomatic adnexal masses discovered on imaging can be monitored with serial sonography. Lesions with more worrisome morphology that’s suggestive of malignancy should prompt referral to a gynecologic oncologist. Surgery on benign masses can be avoided. Outcome data is needed to advise the optimal timing intervals and the limit of follow-up serial ultrasonography. A caveat of this watch-and-see approach is having to allay the patient’s fears of the malignant potential of the mass. This requires conversations with the patient informing them that the stability of the mass will be shown over time and that surgery can be safely avoided.
References
1. Glanc P et al. J Ultrasound Med. 2017;36:849-63.
2. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins – Gynecology. Obstet Gynecol. 2016 Nov;128(5):e210-26.
3. Timmerman D et al. Ultrasound Obstet Gynecol. 2008 Jun;31(6):681-90.
Dr. Jackson-Moore is an associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Adnexal masses are common findings in women. While the decision to operate on symptomatic adnexal masses is straightforward, the decision-making process for asymptomatic masses is more complicated. Here we address how to approach an asymptomatic adnexal mass, including how to decide when to operate, when to refer, or how to monitor.
It is important to minimize the number of surgeries for benign, asymptomatic adnexal masses because complications are reported in 2%-15% of surgeries for adnexal masses and these can range from minimal to devastating.1 In addition, unnecessary surgery is associated with a burden of cost to the health care system. Therefore, there is a paradigm shift in the management of asymptomatic adnexal masses trending toward surveillance of any masses that are likely to be benign. What becomes critical in this approach is the ability to accurately classify these masses preoperatively.
Determining the malignant potential of a mass
Guidance is provided by the ACOG Practice Bulletin Number 174, which was published in 2016: “Evaluation and Management of Adnexal Masses.”2 These guidelines remind clinicians that:
- Most adnexal masses are benign, even in postmenopausal patients.
- The recommended imaging modality is quality transvaginal ultrasonography with an ultrasonographer accredited through the American Registry of Diagnostic Medical Sonographers.
- Simple cysts up to 10 cm can be monitored using repeat imaging every 6 months without surgical intervention, even in postmenopausal patients. In prospective studies, no cases of malignancy were diagnosed over 6 years of surveillance and most resolved. Those that persist are likely to be serous cystadenomas.
- Many benign lesions such as endometriomas and cystic teratomas have characteristic radiologic features. Surgery for these lesions is warranted for large size, symptoms, or growth in size.
- Ultrasound characteristics of malignant masses include:
1. Cyst size greater than 10 cm
2. Papillary or solid components
3. Septations
4. Internal blood flow on color Doppler.
An alternative approach that has been proposed is an ultrasound scoring system devised by International Ovarian Tumor Analysis Group. The scoring system uses 10 ultrasound findings that are characteristic of malignant and benign and is designed to characterize masses as either benign or malignant.3 This approach is able to correctly classify 77% of masses. The remaining masses with features that do not fit the “simple rules” are considered potentially malignant and should be referred to an oncology specialist for further decision making.
Decision to operate
After referral to gynecologic oncologists, surgery is not always inevitable, particularly for women with indeterminate masses. The gynecologic oncologist uses a decision-making process that factors in the underlying surgical risks for that patient with the likelihood of malignancy based on the features of the mass. The threshold to operate is higher in women with underlying major comorbidities, such as morbid obesity, complex prior surgical history, or cardiopulmonary disease. Healthier surgical candidates are more likely to be considered for a surgery, even if the suspicion for malignancy is lower. However, low surgical risk does not equate to no surgical risk. Therefore, even in apparently “good” surgical candidates, the suspicion for underlying malignancy needs to be reasonably high in order to justify the cost and risk of surgery in an asymptomatic patient. Sometimes it is patient anxiety and a desire to avoid repeated surveillance that prompts a decision to operate.
How to monitor
The role of surveillance and monitoring is to establish a natural history of the lesion or to allow it to reveal itself to be stable or regressive. Surveillance with serial sonography has shown that most asymptomatic adnexal masses with low risk features will resolve over time. Lack of resolution in the setting of stable findings is not a worrisome feature and is not suggestive of malignancy. The mere persistence of an otherwise benign-appearing lesion is not a reason to intervene with surgery.
Unfortunately, there is no clear guidance on the surveillance intervals. Some experts recommend an initial repeat scan in 3 months. If at that point the morphologic features and size are stable or decreasing, ultrasounds can be repeated at annual intervals for 5 years. In one study, masses that became malignant demonstrated growth by 7 months. Other experts recommend limiting the period of surveillance of cystic lesions to 1 year and lesions with solid components to 2 years.
Conclusions
Many asymptomatic adnexal masses discovered on imaging can be monitored with serial sonography. Lesions with more worrisome morphology that’s suggestive of malignancy should prompt referral to a gynecologic oncologist. Surgery on benign masses can be avoided. Outcome data is needed to advise the optimal timing intervals and the limit of follow-up serial ultrasonography. A caveat of this watch-and-see approach is having to allay the patient’s fears of the malignant potential of the mass. This requires conversations with the patient informing them that the stability of the mass will be shown over time and that surgery can be safely avoided.
References
1. Glanc P et al. J Ultrasound Med. 2017;36:849-63.
2. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins – Gynecology. Obstet Gynecol. 2016 Nov;128(5):e210-26.
3. Timmerman D et al. Ultrasound Obstet Gynecol. 2008 Jun;31(6):681-90.
Dr. Jackson-Moore is an associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Adnexal masses are common findings in women. While the decision to operate on symptomatic adnexal masses is straightforward, the decision-making process for asymptomatic masses is more complicated. Here we address how to approach an asymptomatic adnexal mass, including how to decide when to operate, when to refer, or how to monitor.
It is important to minimize the number of surgeries for benign, asymptomatic adnexal masses because complications are reported in 2%-15% of surgeries for adnexal masses and these can range from minimal to devastating.1 In addition, unnecessary surgery is associated with a burden of cost to the health care system. Therefore, there is a paradigm shift in the management of asymptomatic adnexal masses trending toward surveillance of any masses that are likely to be benign. What becomes critical in this approach is the ability to accurately classify these masses preoperatively.
Determining the malignant potential of a mass
Guidance is provided by the ACOG Practice Bulletin Number 174, which was published in 2016: “Evaluation and Management of Adnexal Masses.”2 These guidelines remind clinicians that:
- Most adnexal masses are benign, even in postmenopausal patients.
- The recommended imaging modality is quality transvaginal ultrasonography with an ultrasonographer accredited through the American Registry of Diagnostic Medical Sonographers.
- Simple cysts up to 10 cm can be monitored using repeat imaging every 6 months without surgical intervention, even in postmenopausal patients. In prospective studies, no cases of malignancy were diagnosed over 6 years of surveillance and most resolved. Those that persist are likely to be serous cystadenomas.
- Many benign lesions such as endometriomas and cystic teratomas have characteristic radiologic features. Surgery for these lesions is warranted for large size, symptoms, or growth in size.
- Ultrasound characteristics of malignant masses include:
1. Cyst size greater than 10 cm
2. Papillary or solid components
3. Septations
4. Internal blood flow on color Doppler.
An alternative approach that has been proposed is an ultrasound scoring system devised by International Ovarian Tumor Analysis Group. The scoring system uses 10 ultrasound findings that are characteristic of malignant and benign and is designed to characterize masses as either benign or malignant.3 This approach is able to correctly classify 77% of masses. The remaining masses with features that do not fit the “simple rules” are considered potentially malignant and should be referred to an oncology specialist for further decision making.
Decision to operate
After referral to gynecologic oncologists, surgery is not always inevitable, particularly for women with indeterminate masses. The gynecologic oncologist uses a decision-making process that factors in the underlying surgical risks for that patient with the likelihood of malignancy based on the features of the mass. The threshold to operate is higher in women with underlying major comorbidities, such as morbid obesity, complex prior surgical history, or cardiopulmonary disease. Healthier surgical candidates are more likely to be considered for a surgery, even if the suspicion for malignancy is lower. However, low surgical risk does not equate to no surgical risk. Therefore, even in apparently “good” surgical candidates, the suspicion for underlying malignancy needs to be reasonably high in order to justify the cost and risk of surgery in an asymptomatic patient. Sometimes it is patient anxiety and a desire to avoid repeated surveillance that prompts a decision to operate.
How to monitor
The role of surveillance and monitoring is to establish a natural history of the lesion or to allow it to reveal itself to be stable or regressive. Surveillance with serial sonography has shown that most asymptomatic adnexal masses with low risk features will resolve over time. Lack of resolution in the setting of stable findings is not a worrisome feature and is not suggestive of malignancy. The mere persistence of an otherwise benign-appearing lesion is not a reason to intervene with surgery.
Unfortunately, there is no clear guidance on the surveillance intervals. Some experts recommend an initial repeat scan in 3 months. If at that point the morphologic features and size are stable or decreasing, ultrasounds can be repeated at annual intervals for 5 years. In one study, masses that became malignant demonstrated growth by 7 months. Other experts recommend limiting the period of surveillance of cystic lesions to 1 year and lesions with solid components to 2 years.
Conclusions
Many asymptomatic adnexal masses discovered on imaging can be monitored with serial sonography. Lesions with more worrisome morphology that’s suggestive of malignancy should prompt referral to a gynecologic oncologist. Surgery on benign masses can be avoided. Outcome data is needed to advise the optimal timing intervals and the limit of follow-up serial ultrasonography. A caveat of this watch-and-see approach is having to allay the patient’s fears of the malignant potential of the mass. This requires conversations with the patient informing them that the stability of the mass will be shown over time and that surgery can be safely avoided.
References
1. Glanc P et al. J Ultrasound Med. 2017;36:849-63.
2. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins – Gynecology. Obstet Gynecol. 2016 Nov;128(5):e210-26.
3. Timmerman D et al. Ultrasound Obstet Gynecol. 2008 Jun;31(6):681-90.
Dr. Jackson-Moore is an associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC. They reported having no relevant financial disclosures.