User login
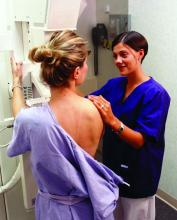
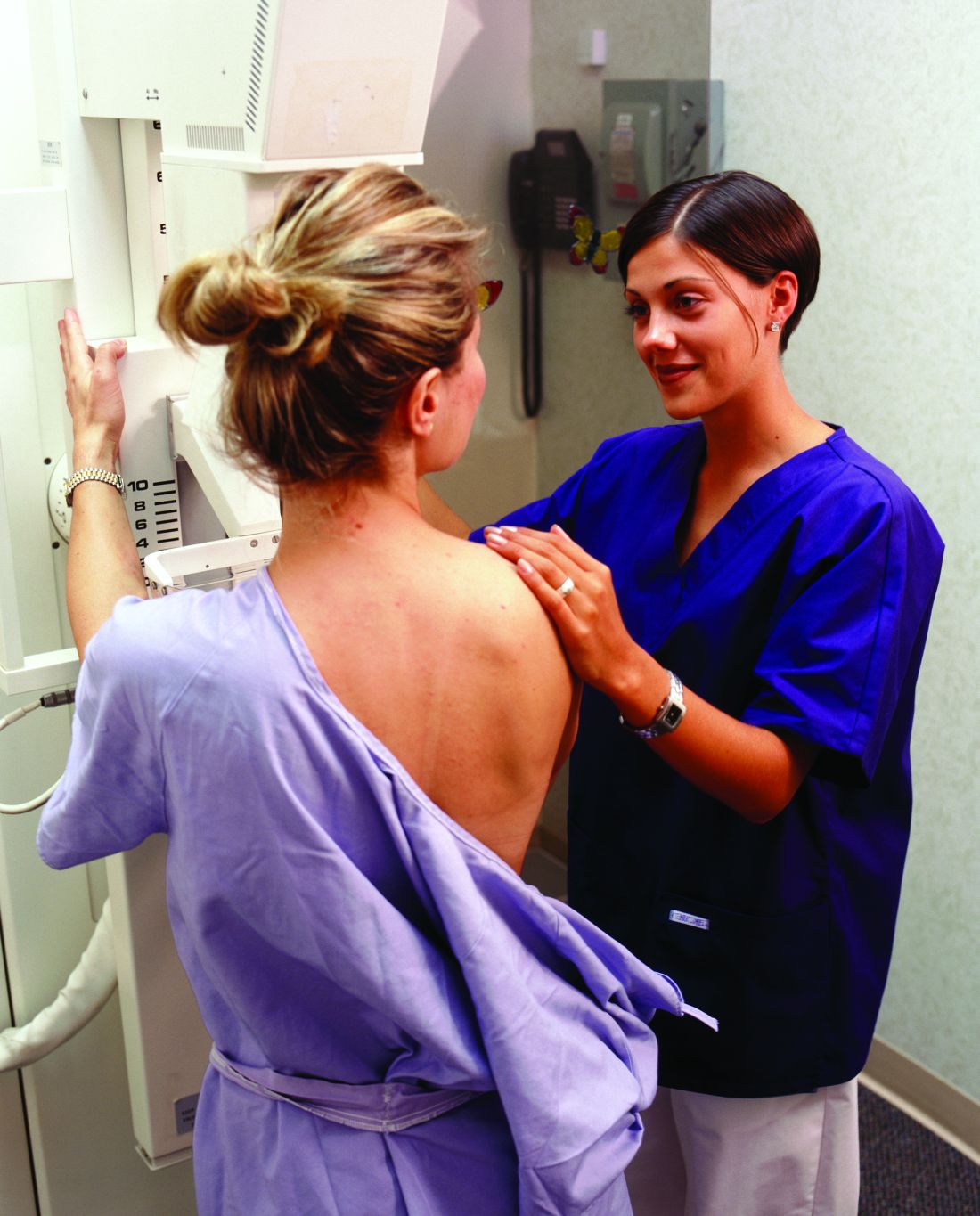
STUDY: More mammograms after cost-sharing elimination
More women received recommended mammograms after cost sharing for the service was eliminated under the Affordable Care Act, a study shows.
In health plans that eliminated cost sharing, such as copays, deductibles, or other out-of-pocket costs, the rate of biennial screening mammography increased from 60% in the 2-year period before the cost-sharing elimination to 65% in the 2-year period following the new regulation, according to an analysis in the New England Journal of Medicine.
In addition to the increased rate of mammograms in the first group, results showed the rates of biennial mammography in the second group were 73.1% (95% confidence interval, 69.2-77.0) and 72.8% (95% CI, 69.7-76.0) during the same periods, yielding a difference in differences of 5.7 percentage points. Investigators also found the difference in differences was 9.8 percentage points among women living in areas with the highest quartile of educational attainment, compared with 4.3 percentage points among women in the lowest quartile. After the elimination of cost sharing, the rate of biennial mammography rose by 6.5 percentage points for white women and 8.4 percentage points for black women, the study found. The rate was nearly unchanged for Hispanic women.
The findings extend that of past studies that show older women who need mammograms are sensitive to out-of-pocket costs and the presence of supplemental coverage, the authors conclude. If the cost-sharing provisions of the ACA are rescinded, the results also “raise concern that fewer older women will receive recommended breast-cancer screening.”
However, the authors also note that since mammogram rates in the health plans that eliminated cost sharing remained below those in plans with full coverage and less than three-quarters of women in control plans received biennial breast cancer screenings, that “the removal of out-of-pocket payments alone may not raise screening rates to desired levels.”
SOURCE: Trivedi A et al. N Engl J Med. 2018 Jan 18;378:262-9.
More women received recommended mammograms after cost sharing for the service was eliminated under the Affordable Care Act, a study shows.
In health plans that eliminated cost sharing, such as copays, deductibles, or other out-of-pocket costs, the rate of biennial screening mammography increased from 60% in the 2-year period before the cost-sharing elimination to 65% in the 2-year period following the new regulation, according to an analysis in the New England Journal of Medicine.
In addition to the increased rate of mammograms in the first group, results showed the rates of biennial mammography in the second group were 73.1% (95% confidence interval, 69.2-77.0) and 72.8% (95% CI, 69.7-76.0) during the same periods, yielding a difference in differences of 5.7 percentage points. Investigators also found the difference in differences was 9.8 percentage points among women living in areas with the highest quartile of educational attainment, compared with 4.3 percentage points among women in the lowest quartile. After the elimination of cost sharing, the rate of biennial mammography rose by 6.5 percentage points for white women and 8.4 percentage points for black women, the study found. The rate was nearly unchanged for Hispanic women.
The findings extend that of past studies that show older women who need mammograms are sensitive to out-of-pocket costs and the presence of supplemental coverage, the authors conclude. If the cost-sharing provisions of the ACA are rescinded, the results also “raise concern that fewer older women will receive recommended breast-cancer screening.”
However, the authors also note that since mammogram rates in the health plans that eliminated cost sharing remained below those in plans with full coverage and less than three-quarters of women in control plans received biennial breast cancer screenings, that “the removal of out-of-pocket payments alone may not raise screening rates to desired levels.”
SOURCE: Trivedi A et al. N Engl J Med. 2018 Jan 18;378:262-9.
More women received recommended mammograms after cost sharing for the service was eliminated under the Affordable Care Act, a study shows.
In health plans that eliminated cost sharing, such as copays, deductibles, or other out-of-pocket costs, the rate of biennial screening mammography increased from 60% in the 2-year period before the cost-sharing elimination to 65% in the 2-year period following the new regulation, according to an analysis in the New England Journal of Medicine.
In addition to the increased rate of mammograms in the first group, results showed the rates of biennial mammography in the second group were 73.1% (95% confidence interval, 69.2-77.0) and 72.8% (95% CI, 69.7-76.0) during the same periods, yielding a difference in differences of 5.7 percentage points. Investigators also found the difference in differences was 9.8 percentage points among women living in areas with the highest quartile of educational attainment, compared with 4.3 percentage points among women in the lowest quartile. After the elimination of cost sharing, the rate of biennial mammography rose by 6.5 percentage points for white women and 8.4 percentage points for black women, the study found. The rate was nearly unchanged for Hispanic women.
The findings extend that of past studies that show older women who need mammograms are sensitive to out-of-pocket costs and the presence of supplemental coverage, the authors conclude. If the cost-sharing provisions of the ACA are rescinded, the results also “raise concern that fewer older women will receive recommended breast-cancer screening.”
However, the authors also note that since mammogram rates in the health plans that eliminated cost sharing remained below those in plans with full coverage and less than three-quarters of women in control plans received biennial breast cancer screenings, that “the removal of out-of-pocket payments alone may not raise screening rates to desired levels.”
SOURCE: Trivedi A et al. N Engl J Med. 2018 Jan 18;378:262-9.
Key clinical point:
Major finding: In plans that eliminated cost sharing, the rate of biennial screening mammography increased from 60% to 65% in the 2-year period thereafter.
Study details: A difference-in-differences study of biennial screening mammography among 15,085 women aged 65-74 years in 24 Medicare Advantage plans.
Disclosures: Dr. Trivedi reported personal fees from Merck Foundation outside the submitted work. Authors reported no other disclosures.
Source: Trivedi A et al. N Engl J Med. 2018 Jan 18;378:262-9.
Surgery or Medical Management for Refractory Pediatric Epilepsy?
Children and adolescents with drug-resistant epilepsy who undergo surgery appear to have significantly higher rates of seizure freedom and better quality of life and behavior scores at 12 months than those who receive medical therapy alone, according to a study published in the October 26, 2017, issue of the New England Journal of Medicine. Serious anticipated adverse events may occur after surgery, however.
“The improvements that were observed in other cognitive, behavioral, and quality of life scores in the surgery group may have been due to a reduction in the frequency of seizures; conversely, the deterioration in these measures in the medical-therapy group may be attributed to a continuation of seizures,” said Rekha Dwivedi, PhD, a postdoctoral fellow at the All India Institute of Medical Sciences in New Delhi, and colleagues.
Comparing Methods Intended to Improve Outcomes
Children and adolescents with drug-resistant epilepsy have an increased risk of poor long-term intellectual and psychosocial outcomes, along with a poor health-related quality of life. Neurosurgical treatment may improve seizures in children and adolescents with drug-resistant epilepsy, but evidence of benefit from randomized trials in this age group is limited.
A meta-analysis of uncontrolled studies comparing seizure outcomes of surgeries in children indicated that 74% of patients with brain lesions and 45% without lesions had become seizure-free at one year of follow-up. In a retrospective analysis involving 142 children and adolescents with drug-resistant epilepsy who had undergone surgery, 79.3% of patients were free from disabling seizures after a mean follow-up of approximately four years.
To investigate the effects of surgery further, Dr. Dwivedi and colleagues performed a single-center trial. They sought to compare epilepsy surgery with continued medical therapy alone in patients on a waiting list for surgery.
Researchers randomized 116 patients age 18 or younger with drug-resistant epilepsy to brain surgery appropriate to the underlying cause of epilepsy, along with appropriate medical therapy, or to medical therapy alone. Patients for whom there was no consensus regarding the location of an epileptic focus, patients who had any other systemic illness, and patients with a history of status epilepticus were excluded.
Participants assigned to the surgery group underwent the procedure within a month after randomization. Those assigned to the medical-therapy group remained on a waiting list. Surgery for these patients was scheduled for one year or longer after randomization. The primary outcome was seizure freedom at 12 months. Secondary outcomes included the Hague Seizure Severity scale score, the Binet–Kamat intelligence quotient or the social quotient on the Vineland Social Maturity Scale, the T score on the Child Behavior Checklist, and the Pediatric Quality of Life Inventory score.
Most of the Surgery Group Became Seizure-Free at 12 Months
Median age was 9 in the surgery group and 10 in the medical-therapy group. In all, 14 patients had temporal lobe resections, 12 patients had resection of a lesion in a lobe other than the temporal lobe, 15 patients had hemispherotomy, 10 patients had a corpus callosotomy, and six patients had a disconnection or resection of hypothalamic hamartoma.
At 12 months, 44 of 57 patients (77%) in the surgery group became seizure-free, compared with four of 59 patients (7%) in the medical-therapy group. Furthermore, 21 patients (37%) in the surgery group were completely seizure-free during the entire 12-month period.
All patients who had undergone temporal lobectomy or hypothalamic hamartoma surgeries were seizure-free at the last follow-up. Of the patients who had undergone extratemporal resection or hemispherotomy, 11 of 12 patients (92%) and 13 of 15 (87%), had complete freedom from seizures, respectively.
Two of 15 patients (13%) in the medical-therapy group who were on the waiting list for a temporal lobectomy were seizure-free at 12 months, along with one of 19 patients (5%) who were on a waiting list for extratemporal resection and one of 16 patients (6%) who were waiting for a corpus callostomy. Patients with a planned hemispherotomy or intervention for hypothalamic hamartoma were not seizure-free at 12 months.
In addition, between-group differences in the change from baseline to 12 months significantly favored surgery with respect to the Hague Seizure Severity scale score, the Child Behavior Checklist, the Pediatric Quality of Life Inventory, and the Vineland Social Maturity Scale, but not the Binet–Kamat intelligence quotient, said the researchers.
Adverse Events and Study Limitations
Serious adverse events occurred in 19 patients (33%) in the surgery group and no patients in the medical-therapy group. Monoparesis occurred in two patients who had undergone temporal lobectomy or resection of parietal focal cortical dysplasia. Hemiparesis occurred in 15 patients who had undergone hemispherotomy. Finally, generalized hypotonia and language deficits occurred in one patient who had undergone frontal lobectomy.
Ten patients in the medical-therapy group had physical injuries associated with seizures (eg, cuts, burns, and fractures). One patient had an adverse event associated with an antiepileptic drug, and autistic features developed in another patient. No deaths occurred in either group.
One study limitation was that patients included in this trial underwent many types of epilepsy surgeries to treat various underlying pathologic causes of seizures. Another limitation was that there was an overrepresentation of hypothalamic hamartomas, compared with some other series, said the researchers.
—Erica Tricarico
Suggested Reading
Dwivedi R, Ramanujam B, Chandra PS, et al. Surgery for drug-resistant epilepsy in children. N Engl J Med. 2017;377(17):1639-1647.
Children and adolescents with drug-resistant epilepsy who undergo surgery appear to have significantly higher rates of seizure freedom and better quality of life and behavior scores at 12 months than those who receive medical therapy alone, according to a study published in the October 26, 2017, issue of the New England Journal of Medicine. Serious anticipated adverse events may occur after surgery, however.
“The improvements that were observed in other cognitive, behavioral, and quality of life scores in the surgery group may have been due to a reduction in the frequency of seizures; conversely, the deterioration in these measures in the medical-therapy group may be attributed to a continuation of seizures,” said Rekha Dwivedi, PhD, a postdoctoral fellow at the All India Institute of Medical Sciences in New Delhi, and colleagues.
Comparing Methods Intended to Improve Outcomes
Children and adolescents with drug-resistant epilepsy have an increased risk of poor long-term intellectual and psychosocial outcomes, along with a poor health-related quality of life. Neurosurgical treatment may improve seizures in children and adolescents with drug-resistant epilepsy, but evidence of benefit from randomized trials in this age group is limited.
A meta-analysis of uncontrolled studies comparing seizure outcomes of surgeries in children indicated that 74% of patients with brain lesions and 45% without lesions had become seizure-free at one year of follow-up. In a retrospective analysis involving 142 children and adolescents with drug-resistant epilepsy who had undergone surgery, 79.3% of patients were free from disabling seizures after a mean follow-up of approximately four years.
To investigate the effects of surgery further, Dr. Dwivedi and colleagues performed a single-center trial. They sought to compare epilepsy surgery with continued medical therapy alone in patients on a waiting list for surgery.
Researchers randomized 116 patients age 18 or younger with drug-resistant epilepsy to brain surgery appropriate to the underlying cause of epilepsy, along with appropriate medical therapy, or to medical therapy alone. Patients for whom there was no consensus regarding the location of an epileptic focus, patients who had any other systemic illness, and patients with a history of status epilepticus were excluded.
Participants assigned to the surgery group underwent the procedure within a month after randomization. Those assigned to the medical-therapy group remained on a waiting list. Surgery for these patients was scheduled for one year or longer after randomization. The primary outcome was seizure freedom at 12 months. Secondary outcomes included the Hague Seizure Severity scale score, the Binet–Kamat intelligence quotient or the social quotient on the Vineland Social Maturity Scale, the T score on the Child Behavior Checklist, and the Pediatric Quality of Life Inventory score.
Most of the Surgery Group Became Seizure-Free at 12 Months
Median age was 9 in the surgery group and 10 in the medical-therapy group. In all, 14 patients had temporal lobe resections, 12 patients had resection of a lesion in a lobe other than the temporal lobe, 15 patients had hemispherotomy, 10 patients had a corpus callosotomy, and six patients had a disconnection or resection of hypothalamic hamartoma.
At 12 months, 44 of 57 patients (77%) in the surgery group became seizure-free, compared with four of 59 patients (7%) in the medical-therapy group. Furthermore, 21 patients (37%) in the surgery group were completely seizure-free during the entire 12-month period.
All patients who had undergone temporal lobectomy or hypothalamic hamartoma surgeries were seizure-free at the last follow-up. Of the patients who had undergone extratemporal resection or hemispherotomy, 11 of 12 patients (92%) and 13 of 15 (87%), had complete freedom from seizures, respectively.
Two of 15 patients (13%) in the medical-therapy group who were on the waiting list for a temporal lobectomy were seizure-free at 12 months, along with one of 19 patients (5%) who were on a waiting list for extratemporal resection and one of 16 patients (6%) who were waiting for a corpus callostomy. Patients with a planned hemispherotomy or intervention for hypothalamic hamartoma were not seizure-free at 12 months.
In addition, between-group differences in the change from baseline to 12 months significantly favored surgery with respect to the Hague Seizure Severity scale score, the Child Behavior Checklist, the Pediatric Quality of Life Inventory, and the Vineland Social Maturity Scale, but not the Binet–Kamat intelligence quotient, said the researchers.
Adverse Events and Study Limitations
Serious adverse events occurred in 19 patients (33%) in the surgery group and no patients in the medical-therapy group. Monoparesis occurred in two patients who had undergone temporal lobectomy or resection of parietal focal cortical dysplasia. Hemiparesis occurred in 15 patients who had undergone hemispherotomy. Finally, generalized hypotonia and language deficits occurred in one patient who had undergone frontal lobectomy.
Ten patients in the medical-therapy group had physical injuries associated with seizures (eg, cuts, burns, and fractures). One patient had an adverse event associated with an antiepileptic drug, and autistic features developed in another patient. No deaths occurred in either group.
One study limitation was that patients included in this trial underwent many types of epilepsy surgeries to treat various underlying pathologic causes of seizures. Another limitation was that there was an overrepresentation of hypothalamic hamartomas, compared with some other series, said the researchers.
—Erica Tricarico
Suggested Reading
Dwivedi R, Ramanujam B, Chandra PS, et al. Surgery for drug-resistant epilepsy in children. N Engl J Med. 2017;377(17):1639-1647.
Children and adolescents with drug-resistant epilepsy who undergo surgery appear to have significantly higher rates of seizure freedom and better quality of life and behavior scores at 12 months than those who receive medical therapy alone, according to a study published in the October 26, 2017, issue of the New England Journal of Medicine. Serious anticipated adverse events may occur after surgery, however.
“The improvements that were observed in other cognitive, behavioral, and quality of life scores in the surgery group may have been due to a reduction in the frequency of seizures; conversely, the deterioration in these measures in the medical-therapy group may be attributed to a continuation of seizures,” said Rekha Dwivedi, PhD, a postdoctoral fellow at the All India Institute of Medical Sciences in New Delhi, and colleagues.
Comparing Methods Intended to Improve Outcomes
Children and adolescents with drug-resistant epilepsy have an increased risk of poor long-term intellectual and psychosocial outcomes, along with a poor health-related quality of life. Neurosurgical treatment may improve seizures in children and adolescents with drug-resistant epilepsy, but evidence of benefit from randomized trials in this age group is limited.
A meta-analysis of uncontrolled studies comparing seizure outcomes of surgeries in children indicated that 74% of patients with brain lesions and 45% without lesions had become seizure-free at one year of follow-up. In a retrospective analysis involving 142 children and adolescents with drug-resistant epilepsy who had undergone surgery, 79.3% of patients were free from disabling seizures after a mean follow-up of approximately four years.
To investigate the effects of surgery further, Dr. Dwivedi and colleagues performed a single-center trial. They sought to compare epilepsy surgery with continued medical therapy alone in patients on a waiting list for surgery.
Researchers randomized 116 patients age 18 or younger with drug-resistant epilepsy to brain surgery appropriate to the underlying cause of epilepsy, along with appropriate medical therapy, or to medical therapy alone. Patients for whom there was no consensus regarding the location of an epileptic focus, patients who had any other systemic illness, and patients with a history of status epilepticus were excluded.
Participants assigned to the surgery group underwent the procedure within a month after randomization. Those assigned to the medical-therapy group remained on a waiting list. Surgery for these patients was scheduled for one year or longer after randomization. The primary outcome was seizure freedom at 12 months. Secondary outcomes included the Hague Seizure Severity scale score, the Binet–Kamat intelligence quotient or the social quotient on the Vineland Social Maturity Scale, the T score on the Child Behavior Checklist, and the Pediatric Quality of Life Inventory score.
Most of the Surgery Group Became Seizure-Free at 12 Months
Median age was 9 in the surgery group and 10 in the medical-therapy group. In all, 14 patients had temporal lobe resections, 12 patients had resection of a lesion in a lobe other than the temporal lobe, 15 patients had hemispherotomy, 10 patients had a corpus callosotomy, and six patients had a disconnection or resection of hypothalamic hamartoma.
At 12 months, 44 of 57 patients (77%) in the surgery group became seizure-free, compared with four of 59 patients (7%) in the medical-therapy group. Furthermore, 21 patients (37%) in the surgery group were completely seizure-free during the entire 12-month period.
All patients who had undergone temporal lobectomy or hypothalamic hamartoma surgeries were seizure-free at the last follow-up. Of the patients who had undergone extratemporal resection or hemispherotomy, 11 of 12 patients (92%) and 13 of 15 (87%), had complete freedom from seizures, respectively.
Two of 15 patients (13%) in the medical-therapy group who were on the waiting list for a temporal lobectomy were seizure-free at 12 months, along with one of 19 patients (5%) who were on a waiting list for extratemporal resection and one of 16 patients (6%) who were waiting for a corpus callostomy. Patients with a planned hemispherotomy or intervention for hypothalamic hamartoma were not seizure-free at 12 months.
In addition, between-group differences in the change from baseline to 12 months significantly favored surgery with respect to the Hague Seizure Severity scale score, the Child Behavior Checklist, the Pediatric Quality of Life Inventory, and the Vineland Social Maturity Scale, but not the Binet–Kamat intelligence quotient, said the researchers.
Adverse Events and Study Limitations
Serious adverse events occurred in 19 patients (33%) in the surgery group and no patients in the medical-therapy group. Monoparesis occurred in two patients who had undergone temporal lobectomy or resection of parietal focal cortical dysplasia. Hemiparesis occurred in 15 patients who had undergone hemispherotomy. Finally, generalized hypotonia and language deficits occurred in one patient who had undergone frontal lobectomy.
Ten patients in the medical-therapy group had physical injuries associated with seizures (eg, cuts, burns, and fractures). One patient had an adverse event associated with an antiepileptic drug, and autistic features developed in another patient. No deaths occurred in either group.
One study limitation was that patients included in this trial underwent many types of epilepsy surgeries to treat various underlying pathologic causes of seizures. Another limitation was that there was an overrepresentation of hypothalamic hamartomas, compared with some other series, said the researchers.
—Erica Tricarico
Suggested Reading
Dwivedi R, Ramanujam B, Chandra PS, et al. Surgery for drug-resistant epilepsy in children. N Engl J Med. 2017;377(17):1639-1647.
Flu season takes another turn for the worse
By one measure at least – the proportion of outpatient visits for influenza-like illness (ILI) – this flu season is now the worst in almost a decade, according to data from the Centers for Disease Control and Prevention.
which hit an early peak of 7.7% in October of 2009. The slight pause that occurred in the first week of January as the rate only rose from 5.7% to 5.8% now looks more like the earlier trend from December, when the level of outpatient visits more than doubled over a 3-week period, data from the CDC FluView website show.
“The geographic spread of influenza in Puerto Rico and 49 states was reported as widespread” for the week ending Jan. 13, and 24 states had the highest level of ILI activity on the CDC’s 1-10 scale, the CDC influenza division reported Jan 19.
There were 10 flu-related pediatric deaths reported during the week, with two occurring in the week ending Jan. 13. A total of 30 deaths in children have been associated with influenza so far for the 2017-2018 season, the CDC said.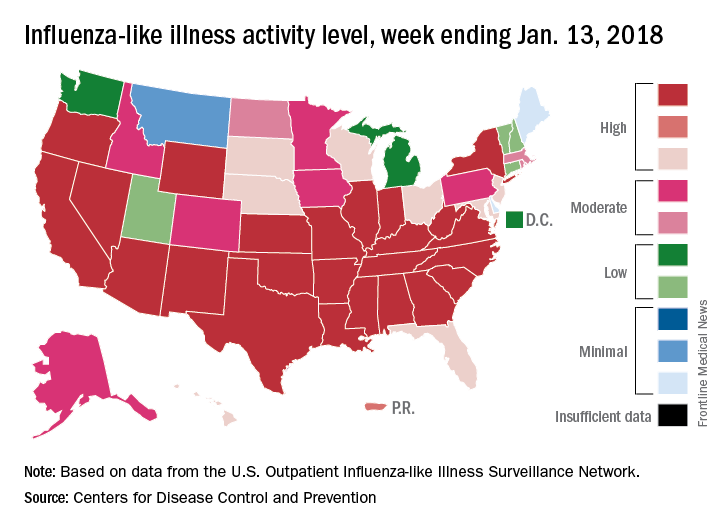
By one measure at least – the proportion of outpatient visits for influenza-like illness (ILI) – this flu season is now the worst in almost a decade, according to data from the Centers for Disease Control and Prevention.
which hit an early peak of 7.7% in October of 2009. The slight pause that occurred in the first week of January as the rate only rose from 5.7% to 5.8% now looks more like the earlier trend from December, when the level of outpatient visits more than doubled over a 3-week period, data from the CDC FluView website show.
“The geographic spread of influenza in Puerto Rico and 49 states was reported as widespread” for the week ending Jan. 13, and 24 states had the highest level of ILI activity on the CDC’s 1-10 scale, the CDC influenza division reported Jan 19.
There were 10 flu-related pediatric deaths reported during the week, with two occurring in the week ending Jan. 13. A total of 30 deaths in children have been associated with influenza so far for the 2017-2018 season, the CDC said.
By one measure at least – the proportion of outpatient visits for influenza-like illness (ILI) – this flu season is now the worst in almost a decade, according to data from the Centers for Disease Control and Prevention.
which hit an early peak of 7.7% in October of 2009. The slight pause that occurred in the first week of January as the rate only rose from 5.7% to 5.8% now looks more like the earlier trend from December, when the level of outpatient visits more than doubled over a 3-week period, data from the CDC FluView website show.
“The geographic spread of influenza in Puerto Rico and 49 states was reported as widespread” for the week ending Jan. 13, and 24 states had the highest level of ILI activity on the CDC’s 1-10 scale, the CDC influenza division reported Jan 19.
There were 10 flu-related pediatric deaths reported during the week, with two occurring in the week ending Jan. 13. A total of 30 deaths in children have been associated with influenza so far for the 2017-2018 season, the CDC said.
New multi-analyte blood test shows promise in screening for several common solid tumors
Imagine a single blood test that would cost less than $500 and could screen for at least eight cancer types.
It’s early days for the technology, called CancerSEEK, but the test had a sensitivity of 69%-98%, depending on the cancer type, and a specificity of 99% in a cohort of 1,005 patients with stage I-III cancers and 850 healthy controls, wrote Joshua D. Cohen of the Ludwig Center for Cancer Genetics and Therapeutics at Johns Hopkins University, Baltimore, and his colleagues. The report was published in Science.
CancerSEEK tests for mutations in 2,001 genomic positions and eight proteins. The researchers examined a 61-amplicon panel with each amplicon analyzing an average of 33 base pairs within a gene. They theorized the test could detect between 41% and 95% of the cancers in the Catalog of Somatic Mutations in Cancer dataset. They next used multiplex-PCR techniques to minimize errors associated with large sequencing and identified protein biomarkers for early stage cancers that may not release detectable ctDNA.
The researchers used the technology to examine blood samples from 1,005 patients with stage I (20%), stage II (49%), or stage III (31%) cancers of the ovary, liver, stomach, pancreas, esophagus, colorectum, lung, or breast prior to undergoing neoadjuvant chemotherapy. Participants had a median age of 64 years (range of 22-93 years). The healthy controls did not have a history of cancer, chronic kidney disease, autoimmune disease, or high-grade dysplasia.
The sensitivity of the test ranged from 98% in ovarian cancer to 33% in breast cancer, but the specificity was greater than 99% with only 7 of 812 control participants having a positive result. “We could not be certain that the few ‘false positive’ individuals identified among the healthy cohort did not actually have an as-yet undetected cancer, but classifying them as false positives provided the most conservative approach to classification and interpretation of the data,” the authors wrote.
Based on cancer stage, sensitivity for stage I cancers was 43%, for stage II 73%, and for stage III 78%. Again, sensitivity varied depending on cancer type, with 100% sensitivity for stage I liver cancer and 20% sensitivity for stage I esophageal cancer.
When tumor tissue samples from 153 patients with statistically significant ctDNA levels were analyzed, identical mutations were found in the plasma and tumor in 90% (138) of all cases.
The protein markers in the CancerSEEK test might also be able to anatomically locate malignancies. Using machine learning to analyze patients testing positive with CancerSEEK, the results narrowed the source of the cancer to two possible anatomical sites in approximately 83% of patients and to one anatomical site in approximately 63% of patients. Accuracy was highest for colorectal cancer and lowest for lung cancer.
As the study included otherwise healthy patients with known malignancies, the results need to be confirmed with prospective studies of incidence cancer types in a large population. Patients in the screening setting may have less advanced disease and other comorbidities that could impact the sensitivity and specificity of the CancerSEEK test, the researchers wrote.
The study was funded by multiple sources including grants from the National Institutes of Health. The authors reported various disclosures involving diagnostics and pharmaceutical companies.
SOURCE: Cohen JD et al., Science 2018 Jan 18. doi: 10.1126/science.aar3247.
Molecular panels are here to stay – and the GI community will in some shape or form be impacted, be it in performing diagnostic procedures on test-positive patients, or risk-stratifying patients prior to testing.
The conceptual challenge is that it is not about what any given test measures – various panels use separate combination of markers from epigenetics to DNA mutations as well as whole or truncated proteins – but how well a specific test with its somewhat arbitrarily chosen components and cutoffs performs. And, more importantly, what the clinical implications of positive or negative test results are. And no one knows that. At least for now.
A recent report in Science from a group from the Ludwig Center for Cancer Genetics at Johns Hopkins proposes a new cancer blood test based on a very systematic and thoughtful approach to include select mutations in cell-free DNA and circulating proteins associated with various solid organ tumors. For validation, they used healthy and advanced but nonmetastatic cancer cohorts. Through stringent controls and a series of validations, the authors present a range of sensitivities for the various cancer types with an impressive specificity. This is a technically very strong approach with many nifty and thoughtful additions to give this test a very promising first foray – did anybody watch CNN?
While not ready for prime time, which is a tall order for a first report, the authors dutifully point out the need for a prospective real life cohort validation. In the meantime, regardless of the outcome of this particular test, it is a repeated reminder that we need to stay abreast of the advances and the details of each molecular test, especially with a likely very diverse and distinct group of tests to choose from.
Many of us will be part of interpreting results and determining further management. Just as with hereditary cancer genetic panel testing, our technical ability may have stretched beyond our ability to fully understand the implications. Many questions will arise: What about true false positives? False negatives? Intervals? Can such tests replace other screening? How to choose any given test over the other? Should tests be combined or alternated? The tests will be technically refined and are here to stay – we need to get to work on finding answers to the clinically relevant questions.
Barbara Jung, MD, AGAF, is the Thomas J. Layden Endowed Professor and chief of the division of gastroenterology and hepatology, University of Chicago.
Molecular panels are here to stay – and the GI community will in some shape or form be impacted, be it in performing diagnostic procedures on test-positive patients, or risk-stratifying patients prior to testing.
The conceptual challenge is that it is not about what any given test measures – various panels use separate combination of markers from epigenetics to DNA mutations as well as whole or truncated proteins – but how well a specific test with its somewhat arbitrarily chosen components and cutoffs performs. And, more importantly, what the clinical implications of positive or negative test results are. And no one knows that. At least for now.
A recent report in Science from a group from the Ludwig Center for Cancer Genetics at Johns Hopkins proposes a new cancer blood test based on a very systematic and thoughtful approach to include select mutations in cell-free DNA and circulating proteins associated with various solid organ tumors. For validation, they used healthy and advanced but nonmetastatic cancer cohorts. Through stringent controls and a series of validations, the authors present a range of sensitivities for the various cancer types with an impressive specificity. This is a technically very strong approach with many nifty and thoughtful additions to give this test a very promising first foray – did anybody watch CNN?
While not ready for prime time, which is a tall order for a first report, the authors dutifully point out the need for a prospective real life cohort validation. In the meantime, regardless of the outcome of this particular test, it is a repeated reminder that we need to stay abreast of the advances and the details of each molecular test, especially with a likely very diverse and distinct group of tests to choose from.
Many of us will be part of interpreting results and determining further management. Just as with hereditary cancer genetic panel testing, our technical ability may have stretched beyond our ability to fully understand the implications. Many questions will arise: What about true false positives? False negatives? Intervals? Can such tests replace other screening? How to choose any given test over the other? Should tests be combined or alternated? The tests will be technically refined and are here to stay – we need to get to work on finding answers to the clinically relevant questions.
Barbara Jung, MD, AGAF, is the Thomas J. Layden Endowed Professor and chief of the division of gastroenterology and hepatology, University of Chicago.
Molecular panels are here to stay – and the GI community will in some shape or form be impacted, be it in performing diagnostic procedures on test-positive patients, or risk-stratifying patients prior to testing.
The conceptual challenge is that it is not about what any given test measures – various panels use separate combination of markers from epigenetics to DNA mutations as well as whole or truncated proteins – but how well a specific test with its somewhat arbitrarily chosen components and cutoffs performs. And, more importantly, what the clinical implications of positive or negative test results are. And no one knows that. At least for now.
A recent report in Science from a group from the Ludwig Center for Cancer Genetics at Johns Hopkins proposes a new cancer blood test based on a very systematic and thoughtful approach to include select mutations in cell-free DNA and circulating proteins associated with various solid organ tumors. For validation, they used healthy and advanced but nonmetastatic cancer cohorts. Through stringent controls and a series of validations, the authors present a range of sensitivities for the various cancer types with an impressive specificity. This is a technically very strong approach with many nifty and thoughtful additions to give this test a very promising first foray – did anybody watch CNN?
While not ready for prime time, which is a tall order for a first report, the authors dutifully point out the need for a prospective real life cohort validation. In the meantime, regardless of the outcome of this particular test, it is a repeated reminder that we need to stay abreast of the advances and the details of each molecular test, especially with a likely very diverse and distinct group of tests to choose from.
Many of us will be part of interpreting results and determining further management. Just as with hereditary cancer genetic panel testing, our technical ability may have stretched beyond our ability to fully understand the implications. Many questions will arise: What about true false positives? False negatives? Intervals? Can such tests replace other screening? How to choose any given test over the other? Should tests be combined or alternated? The tests will be technically refined and are here to stay – we need to get to work on finding answers to the clinically relevant questions.
Barbara Jung, MD, AGAF, is the Thomas J. Layden Endowed Professor and chief of the division of gastroenterology and hepatology, University of Chicago.
Imagine a single blood test that would cost less than $500 and could screen for at least eight cancer types.
It’s early days for the technology, called CancerSEEK, but the test had a sensitivity of 69%-98%, depending on the cancer type, and a specificity of 99% in a cohort of 1,005 patients with stage I-III cancers and 850 healthy controls, wrote Joshua D. Cohen of the Ludwig Center for Cancer Genetics and Therapeutics at Johns Hopkins University, Baltimore, and his colleagues. The report was published in Science.
CancerSEEK tests for mutations in 2,001 genomic positions and eight proteins. The researchers examined a 61-amplicon panel with each amplicon analyzing an average of 33 base pairs within a gene. They theorized the test could detect between 41% and 95% of the cancers in the Catalog of Somatic Mutations in Cancer dataset. They next used multiplex-PCR techniques to minimize errors associated with large sequencing and identified protein biomarkers for early stage cancers that may not release detectable ctDNA.
The researchers used the technology to examine blood samples from 1,005 patients with stage I (20%), stage II (49%), or stage III (31%) cancers of the ovary, liver, stomach, pancreas, esophagus, colorectum, lung, or breast prior to undergoing neoadjuvant chemotherapy. Participants had a median age of 64 years (range of 22-93 years). The healthy controls did not have a history of cancer, chronic kidney disease, autoimmune disease, or high-grade dysplasia.
The sensitivity of the test ranged from 98% in ovarian cancer to 33% in breast cancer, but the specificity was greater than 99% with only 7 of 812 control participants having a positive result. “We could not be certain that the few ‘false positive’ individuals identified among the healthy cohort did not actually have an as-yet undetected cancer, but classifying them as false positives provided the most conservative approach to classification and interpretation of the data,” the authors wrote.
Based on cancer stage, sensitivity for stage I cancers was 43%, for stage II 73%, and for stage III 78%. Again, sensitivity varied depending on cancer type, with 100% sensitivity for stage I liver cancer and 20% sensitivity for stage I esophageal cancer.
When tumor tissue samples from 153 patients with statistically significant ctDNA levels were analyzed, identical mutations were found in the plasma and tumor in 90% (138) of all cases.
The protein markers in the CancerSEEK test might also be able to anatomically locate malignancies. Using machine learning to analyze patients testing positive with CancerSEEK, the results narrowed the source of the cancer to two possible anatomical sites in approximately 83% of patients and to one anatomical site in approximately 63% of patients. Accuracy was highest for colorectal cancer and lowest for lung cancer.
As the study included otherwise healthy patients with known malignancies, the results need to be confirmed with prospective studies of incidence cancer types in a large population. Patients in the screening setting may have less advanced disease and other comorbidities that could impact the sensitivity and specificity of the CancerSEEK test, the researchers wrote.
The study was funded by multiple sources including grants from the National Institutes of Health. The authors reported various disclosures involving diagnostics and pharmaceutical companies.
SOURCE: Cohen JD et al., Science 2018 Jan 18. doi: 10.1126/science.aar3247.
Imagine a single blood test that would cost less than $500 and could screen for at least eight cancer types.
It’s early days for the technology, called CancerSEEK, but the test had a sensitivity of 69%-98%, depending on the cancer type, and a specificity of 99% in a cohort of 1,005 patients with stage I-III cancers and 850 healthy controls, wrote Joshua D. Cohen of the Ludwig Center for Cancer Genetics and Therapeutics at Johns Hopkins University, Baltimore, and his colleagues. The report was published in Science.
CancerSEEK tests for mutations in 2,001 genomic positions and eight proteins. The researchers examined a 61-amplicon panel with each amplicon analyzing an average of 33 base pairs within a gene. They theorized the test could detect between 41% and 95% of the cancers in the Catalog of Somatic Mutations in Cancer dataset. They next used multiplex-PCR techniques to minimize errors associated with large sequencing and identified protein biomarkers for early stage cancers that may not release detectable ctDNA.
The researchers used the technology to examine blood samples from 1,005 patients with stage I (20%), stage II (49%), or stage III (31%) cancers of the ovary, liver, stomach, pancreas, esophagus, colorectum, lung, or breast prior to undergoing neoadjuvant chemotherapy. Participants had a median age of 64 years (range of 22-93 years). The healthy controls did not have a history of cancer, chronic kidney disease, autoimmune disease, or high-grade dysplasia.
The sensitivity of the test ranged from 98% in ovarian cancer to 33% in breast cancer, but the specificity was greater than 99% with only 7 of 812 control participants having a positive result. “We could not be certain that the few ‘false positive’ individuals identified among the healthy cohort did not actually have an as-yet undetected cancer, but classifying them as false positives provided the most conservative approach to classification and interpretation of the data,” the authors wrote.
Based on cancer stage, sensitivity for stage I cancers was 43%, for stage II 73%, and for stage III 78%. Again, sensitivity varied depending on cancer type, with 100% sensitivity for stage I liver cancer and 20% sensitivity for stage I esophageal cancer.
When tumor tissue samples from 153 patients with statistically significant ctDNA levels were analyzed, identical mutations were found in the plasma and tumor in 90% (138) of all cases.
The protein markers in the CancerSEEK test might also be able to anatomically locate malignancies. Using machine learning to analyze patients testing positive with CancerSEEK, the results narrowed the source of the cancer to two possible anatomical sites in approximately 83% of patients and to one anatomical site in approximately 63% of patients. Accuracy was highest for colorectal cancer and lowest for lung cancer.
As the study included otherwise healthy patients with known malignancies, the results need to be confirmed with prospective studies of incidence cancer types in a large population. Patients in the screening setting may have less advanced disease and other comorbidities that could impact the sensitivity and specificity of the CancerSEEK test, the researchers wrote.
The study was funded by multiple sources including grants from the National Institutes of Health. The authors reported various disclosures involving diagnostics and pharmaceutical companies.
SOURCE: Cohen JD et al., Science 2018 Jan 18. doi: 10.1126/science.aar3247.
FROM SCIENCE
Key clinical point: New blood test demonstrates ability to identify presence of eight common cancers.
Major finding: CancerSEEK demonstrated a mean sensitivity of 70% for the eight cancer types and a specificity of greater than 99%.
Data source: Retrospective study of 1,005 patients with known malignancy and 812 healthy controls.
Disclosures: The study was funded by multiple sources including grants from the National Institutes of Health. The authors reported several disclosures involving diagnostics and pharmaceutical companies.
Source: Cohen JD et al. Science 2018 Jan 18. doi: 10.1126/science.aar3247.
HHS creates new religious freedoms division
The Trump administration has announced a new division within the U.S. Health & Human Services Department that aims to protect health care providers who have religious or moral objections to performing medical services, such as abortion.
In a Jan. 18 announcement, HHS Office for Civil Rights Director Roger Severino said the new Conscience and Religious Freedom Division will focus on outreach, policy making, and vigorously enforcing existing federal laws that protect conscience and religious freedom rights. The new branch will enable health providers to file conscience and religious freedom complaints, which will then be investigated by the division.
Opinions on the new division from physicians and medical associations were mixed. The American College of Physicians (ACP) cautioned that the division’s creation must not lead to discrimination against any category of class of patients, as guided by medical professions’ ethical obligations.
“ACP would be particularly concerned if the new HHS division takes any actions that would result in denial of access to appropriate health care based on gender, gender identity, sexual orientation, race, ethnicity, or other personal characteristics,” ACP President Jack Ende, MD, said in a statement. “ACP will evaluate the newly formed division as it begins operating, informed by our ethics and public policy positions. Those state that physicians have a professional obligation to not discriminate against any class of patients, but also that a physician may have a conscientious objection to providing a specific medical service to a patient.”
The Catholic Medical Association (CMA) applauded the new HHS division, saying it’s about time that the religious and conscience rights of health providers were protected by the federal government. In the past, health providers could be drawn into medical activities in a hospital or health care system that they found moral objectionable with no recourse, said Kathleen Raviele, MD, a board member for the Catholic Medical Association and a Decatur, Ga.–based ob.gyn.
“The fact that individual health care providers are going to have a place to go if they feel they have valid complaint is really momentous,” Dr. Raviele said in an interview. “It’s respecting the rights of all of us and not just some special interest groups ... I also think [the new division] will make states [and] hospital systems less likely to try to infringe on people’s conscientious rights.”
The HIV Medicine Association called the formation of the new HHS division appalling, and said the office appears to protect health care providers who discriminate against vulnerable patients, such as women, LGBTQ patients, and minorities.
“The new division, designed to ‘protect’ health care providers who discriminate in the care and services they provide, defies the fundamental medical ethic to first do no harm,” HIV Medicine Association Chair Melanie Thompson, MD, said in a statement. “Using federal dollars to shield providers who choose to discriminate rather than protect vulnerable patients and provide services to improve their health is counter to the mission of HHS, wasteful of scarce federal funds, and will result in delayed or lack of care for vulnerable individuals, threatening their health and lives.”
The HHS Conscience and Religious Freedom Division is necessary to address the “sustained attack on conscience rights,” said Jane Orient, MD, executive director for the conservative Association of American Physicians and Surgeons.
“Many powerful and influential forces are attempting to impose their views on Americans, predominantly on Christians, forcing them to perform acts they believe to be immoral, [or risk] losing their job, their business, their livelihood, or even their life savings,” Dr. Orient said in an interview. “...We hope that the OCR will help to return our nation to its foundational principles of freedom and respect for people of conscience.”
The Trump administration has announced a new division within the U.S. Health & Human Services Department that aims to protect health care providers who have religious or moral objections to performing medical services, such as abortion.
In a Jan. 18 announcement, HHS Office for Civil Rights Director Roger Severino said the new Conscience and Religious Freedom Division will focus on outreach, policy making, and vigorously enforcing existing federal laws that protect conscience and religious freedom rights. The new branch will enable health providers to file conscience and religious freedom complaints, which will then be investigated by the division.
Opinions on the new division from physicians and medical associations were mixed. The American College of Physicians (ACP) cautioned that the division’s creation must not lead to discrimination against any category of class of patients, as guided by medical professions’ ethical obligations.
“ACP would be particularly concerned if the new HHS division takes any actions that would result in denial of access to appropriate health care based on gender, gender identity, sexual orientation, race, ethnicity, or other personal characteristics,” ACP President Jack Ende, MD, said in a statement. “ACP will evaluate the newly formed division as it begins operating, informed by our ethics and public policy positions. Those state that physicians have a professional obligation to not discriminate against any class of patients, but also that a physician may have a conscientious objection to providing a specific medical service to a patient.”
The Catholic Medical Association (CMA) applauded the new HHS division, saying it’s about time that the religious and conscience rights of health providers were protected by the federal government. In the past, health providers could be drawn into medical activities in a hospital or health care system that they found moral objectionable with no recourse, said Kathleen Raviele, MD, a board member for the Catholic Medical Association and a Decatur, Ga.–based ob.gyn.
“The fact that individual health care providers are going to have a place to go if they feel they have valid complaint is really momentous,” Dr. Raviele said in an interview. “It’s respecting the rights of all of us and not just some special interest groups ... I also think [the new division] will make states [and] hospital systems less likely to try to infringe on people’s conscientious rights.”
The HIV Medicine Association called the formation of the new HHS division appalling, and said the office appears to protect health care providers who discriminate against vulnerable patients, such as women, LGBTQ patients, and minorities.
“The new division, designed to ‘protect’ health care providers who discriminate in the care and services they provide, defies the fundamental medical ethic to first do no harm,” HIV Medicine Association Chair Melanie Thompson, MD, said in a statement. “Using federal dollars to shield providers who choose to discriminate rather than protect vulnerable patients and provide services to improve their health is counter to the mission of HHS, wasteful of scarce federal funds, and will result in delayed or lack of care for vulnerable individuals, threatening their health and lives.”
The HHS Conscience and Religious Freedom Division is necessary to address the “sustained attack on conscience rights,” said Jane Orient, MD, executive director for the conservative Association of American Physicians and Surgeons.
“Many powerful and influential forces are attempting to impose their views on Americans, predominantly on Christians, forcing them to perform acts they believe to be immoral, [or risk] losing their job, their business, their livelihood, or even their life savings,” Dr. Orient said in an interview. “...We hope that the OCR will help to return our nation to its foundational principles of freedom and respect for people of conscience.”
The Trump administration has announced a new division within the U.S. Health & Human Services Department that aims to protect health care providers who have religious or moral objections to performing medical services, such as abortion.
In a Jan. 18 announcement, HHS Office for Civil Rights Director Roger Severino said the new Conscience and Religious Freedom Division will focus on outreach, policy making, and vigorously enforcing existing federal laws that protect conscience and religious freedom rights. The new branch will enable health providers to file conscience and religious freedom complaints, which will then be investigated by the division.
Opinions on the new division from physicians and medical associations were mixed. The American College of Physicians (ACP) cautioned that the division’s creation must not lead to discrimination against any category of class of patients, as guided by medical professions’ ethical obligations.
“ACP would be particularly concerned if the new HHS division takes any actions that would result in denial of access to appropriate health care based on gender, gender identity, sexual orientation, race, ethnicity, or other personal characteristics,” ACP President Jack Ende, MD, said in a statement. “ACP will evaluate the newly formed division as it begins operating, informed by our ethics and public policy positions. Those state that physicians have a professional obligation to not discriminate against any class of patients, but also that a physician may have a conscientious objection to providing a specific medical service to a patient.”
The Catholic Medical Association (CMA) applauded the new HHS division, saying it’s about time that the religious and conscience rights of health providers were protected by the federal government. In the past, health providers could be drawn into medical activities in a hospital or health care system that they found moral objectionable with no recourse, said Kathleen Raviele, MD, a board member for the Catholic Medical Association and a Decatur, Ga.–based ob.gyn.
“The fact that individual health care providers are going to have a place to go if they feel they have valid complaint is really momentous,” Dr. Raviele said in an interview. “It’s respecting the rights of all of us and not just some special interest groups ... I also think [the new division] will make states [and] hospital systems less likely to try to infringe on people’s conscientious rights.”
The HIV Medicine Association called the formation of the new HHS division appalling, and said the office appears to protect health care providers who discriminate against vulnerable patients, such as women, LGBTQ patients, and minorities.
“The new division, designed to ‘protect’ health care providers who discriminate in the care and services they provide, defies the fundamental medical ethic to first do no harm,” HIV Medicine Association Chair Melanie Thompson, MD, said in a statement. “Using federal dollars to shield providers who choose to discriminate rather than protect vulnerable patients and provide services to improve their health is counter to the mission of HHS, wasteful of scarce federal funds, and will result in delayed or lack of care for vulnerable individuals, threatening their health and lives.”
The HHS Conscience and Religious Freedom Division is necessary to address the “sustained attack on conscience rights,” said Jane Orient, MD, executive director for the conservative Association of American Physicians and Surgeons.
“Many powerful and influential forces are attempting to impose their views on Americans, predominantly on Christians, forcing them to perform acts they believe to be immoral, [or risk] losing their job, their business, their livelihood, or even their life savings,” Dr. Orient said in an interview. “...We hope that the OCR will help to return our nation to its foundational principles of freedom and respect for people of conscience.”
Are two drugs as good as three in maintaining HIV suppression?
Phase 3 clinical results show an oral, two-drug antiretroviral therapy (ART) of dolutegravir and rilpivirine to be an effective and safe alternative to a triple-drug current antiretroviral regimen (CAR) for maintaining virologic suppression of HIV-1 in adults.
Josep M Llibre, MD, from the department of infectious diseases at the Pujol University Hospital Germans Trias in Barcelona and his colleagues conducted a phase 3, randomized, parallel-group (SWORD-1 and SWORD-2), multicenter, noninferiority investigation. The pooled, open-label study reported on a total of 1,028 individuals, median age 43 years, who met a base criteria of stable viral suppression (viral load fewer than 50 copies/mL) by a first or second CAR regimen consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) plus a third drug (nonnucleoside reverse transcriptase inhibitors, integrase strand transfer inhibitors, or a protease inhibitor) for a minimum of 6 months at time of screening. The overall population was 80% white, with women making up 22% of the pooled populations. Random assignment with stratification according to class, age, and planned substudy participation resulted in 512 participants continuing their CAR regimen and an intention-to-treat population of 516 participants switching to the oral, once-daily, two-drug dolutegravir/rilpivirine regimen (dolutegravir 50 mg plus rilpivirine 25 mg).
Analysis of pooled SWORD1 and SWORD2 trials at the primary 48-week endpoint showed 95% of the participants for both the intent-to-treat, two-drug dolutegravir/rilpivirine therapy and the population maintaining their standard triple-drug CAR therapy continued to have successful viral suppression as evidenced by viral loads of fewer than 50 copies/mL. This result together with an adjusted treatment difference of –2.2% (95% confidence interval, –3.0 to 2.5) supports a noninferiority with a predefined margin of –8% of the dolutegravir/rilpivirine therapy to CAR for viral suppression as reported in the Lancet.
Reports of at least one adverse event by week 48 were slightly higher for the dolutegravir/rilpivirine therapy group (77%) when compared with those in the CAR group (71%). The most commonly experienced adverse effects were nasopharyngitis (10% each for dolutegravir/rilpivirine and CAR) and headache (8% for dolutegravir/rilpivirine vs. 5% for CAR). However, adverse effects were associated with higher withdrawals from the dolutegravir/rilpivirine therapy group, compared with the CAR group (3% vs. less than 1%, respectively) by the primary 48-week endpoint.
“Once-daily oral dolutegravir/rilpivirine therapy would be the first oral two-drug regimen that provides patients with an alternative to guideline-preferred triple-drug regimens, avoids major NRTI [nucleoside reverse transcriptase inhibitor] toxicities, has limited potential for drug-drug interactions, and does not increase lipid concentrations or inflammatory biomarkers,” according to Dr. Llibre and his colleagues.
The authors disclosed associations with financial and regulatory sponsor ViiV Healthcare and partner participation by Janssen Pharmaceutica NV in the development of the dolutegravir/rilpivirine two-drug regimen.
Source: Llibre JM et al. Lancet. 2018 Jan 10. doi: 10.1016/S0140-6736(17)33095-7.
In an accompanying commentary (Lancet. 2018 Jan 10. doi: 10.1016/S0140-6736[18]30008-4), Mark A Boyd, MD, and David A Cooper, MD, of the University of Adelaide, Australia, wrote that studies done during the first 10 years of antiretroviral therapy led to the conclusion that triple therapy was the minimum required to fully maintain suppression of HIV replication. The results of this study and others make a convincing case for using dual therapy instead. “The potential pipeline for a variety of effective dual-therapy options appears rich,” they concluded.
Dr. Boyd reported receiving personal fees from Janssen-Cilag and ViiV Healthcare, while Dr. Cooper had no disclosures.
In an accompanying commentary (Lancet. 2018 Jan 10. doi: 10.1016/S0140-6736[18]30008-4), Mark A Boyd, MD, and David A Cooper, MD, of the University of Adelaide, Australia, wrote that studies done during the first 10 years of antiretroviral therapy led to the conclusion that triple therapy was the minimum required to fully maintain suppression of HIV replication. The results of this study and others make a convincing case for using dual therapy instead. “The potential pipeline for a variety of effective dual-therapy options appears rich,” they concluded.
Dr. Boyd reported receiving personal fees from Janssen-Cilag and ViiV Healthcare, while Dr. Cooper had no disclosures.
In an accompanying commentary (Lancet. 2018 Jan 10. doi: 10.1016/S0140-6736[18]30008-4), Mark A Boyd, MD, and David A Cooper, MD, of the University of Adelaide, Australia, wrote that studies done during the first 10 years of antiretroviral therapy led to the conclusion that triple therapy was the minimum required to fully maintain suppression of HIV replication. The results of this study and others make a convincing case for using dual therapy instead. “The potential pipeline for a variety of effective dual-therapy options appears rich,” they concluded.
Dr. Boyd reported receiving personal fees from Janssen-Cilag and ViiV Healthcare, while Dr. Cooper had no disclosures.
Phase 3 clinical results show an oral, two-drug antiretroviral therapy (ART) of dolutegravir and rilpivirine to be an effective and safe alternative to a triple-drug current antiretroviral regimen (CAR) for maintaining virologic suppression of HIV-1 in adults.
Josep M Llibre, MD, from the department of infectious diseases at the Pujol University Hospital Germans Trias in Barcelona and his colleagues conducted a phase 3, randomized, parallel-group (SWORD-1 and SWORD-2), multicenter, noninferiority investigation. The pooled, open-label study reported on a total of 1,028 individuals, median age 43 years, who met a base criteria of stable viral suppression (viral load fewer than 50 copies/mL) by a first or second CAR regimen consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) plus a third drug (nonnucleoside reverse transcriptase inhibitors, integrase strand transfer inhibitors, or a protease inhibitor) for a minimum of 6 months at time of screening. The overall population was 80% white, with women making up 22% of the pooled populations. Random assignment with stratification according to class, age, and planned substudy participation resulted in 512 participants continuing their CAR regimen and an intention-to-treat population of 516 participants switching to the oral, once-daily, two-drug dolutegravir/rilpivirine regimen (dolutegravir 50 mg plus rilpivirine 25 mg).
Analysis of pooled SWORD1 and SWORD2 trials at the primary 48-week endpoint showed 95% of the participants for both the intent-to-treat, two-drug dolutegravir/rilpivirine therapy and the population maintaining their standard triple-drug CAR therapy continued to have successful viral suppression as evidenced by viral loads of fewer than 50 copies/mL. This result together with an adjusted treatment difference of –2.2% (95% confidence interval, –3.0 to 2.5) supports a noninferiority with a predefined margin of –8% of the dolutegravir/rilpivirine therapy to CAR for viral suppression as reported in the Lancet.
Reports of at least one adverse event by week 48 were slightly higher for the dolutegravir/rilpivirine therapy group (77%) when compared with those in the CAR group (71%). The most commonly experienced adverse effects were nasopharyngitis (10% each for dolutegravir/rilpivirine and CAR) and headache (8% for dolutegravir/rilpivirine vs. 5% for CAR). However, adverse effects were associated with higher withdrawals from the dolutegravir/rilpivirine therapy group, compared with the CAR group (3% vs. less than 1%, respectively) by the primary 48-week endpoint.
“Once-daily oral dolutegravir/rilpivirine therapy would be the first oral two-drug regimen that provides patients with an alternative to guideline-preferred triple-drug regimens, avoids major NRTI [nucleoside reverse transcriptase inhibitor] toxicities, has limited potential for drug-drug interactions, and does not increase lipid concentrations or inflammatory biomarkers,” according to Dr. Llibre and his colleagues.
The authors disclosed associations with financial and regulatory sponsor ViiV Healthcare and partner participation by Janssen Pharmaceutica NV in the development of the dolutegravir/rilpivirine two-drug regimen.
Source: Llibre JM et al. Lancet. 2018 Jan 10. doi: 10.1016/S0140-6736(17)33095-7.
Phase 3 clinical results show an oral, two-drug antiretroviral therapy (ART) of dolutegravir and rilpivirine to be an effective and safe alternative to a triple-drug current antiretroviral regimen (CAR) for maintaining virologic suppression of HIV-1 in adults.
Josep M Llibre, MD, from the department of infectious diseases at the Pujol University Hospital Germans Trias in Barcelona and his colleagues conducted a phase 3, randomized, parallel-group (SWORD-1 and SWORD-2), multicenter, noninferiority investigation. The pooled, open-label study reported on a total of 1,028 individuals, median age 43 years, who met a base criteria of stable viral suppression (viral load fewer than 50 copies/mL) by a first or second CAR regimen consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) plus a third drug (nonnucleoside reverse transcriptase inhibitors, integrase strand transfer inhibitors, or a protease inhibitor) for a minimum of 6 months at time of screening. The overall population was 80% white, with women making up 22% of the pooled populations. Random assignment with stratification according to class, age, and planned substudy participation resulted in 512 participants continuing their CAR regimen and an intention-to-treat population of 516 participants switching to the oral, once-daily, two-drug dolutegravir/rilpivirine regimen (dolutegravir 50 mg plus rilpivirine 25 mg).
Analysis of pooled SWORD1 and SWORD2 trials at the primary 48-week endpoint showed 95% of the participants for both the intent-to-treat, two-drug dolutegravir/rilpivirine therapy and the population maintaining their standard triple-drug CAR therapy continued to have successful viral suppression as evidenced by viral loads of fewer than 50 copies/mL. This result together with an adjusted treatment difference of –2.2% (95% confidence interval, –3.0 to 2.5) supports a noninferiority with a predefined margin of –8% of the dolutegravir/rilpivirine therapy to CAR for viral suppression as reported in the Lancet.
Reports of at least one adverse event by week 48 were slightly higher for the dolutegravir/rilpivirine therapy group (77%) when compared with those in the CAR group (71%). The most commonly experienced adverse effects were nasopharyngitis (10% each for dolutegravir/rilpivirine and CAR) and headache (8% for dolutegravir/rilpivirine vs. 5% for CAR). However, adverse effects were associated with higher withdrawals from the dolutegravir/rilpivirine therapy group, compared with the CAR group (3% vs. less than 1%, respectively) by the primary 48-week endpoint.
“Once-daily oral dolutegravir/rilpivirine therapy would be the first oral two-drug regimen that provides patients with an alternative to guideline-preferred triple-drug regimens, avoids major NRTI [nucleoside reverse transcriptase inhibitor] toxicities, has limited potential for drug-drug interactions, and does not increase lipid concentrations or inflammatory biomarkers,” according to Dr. Llibre and his colleagues.
The authors disclosed associations with financial and regulatory sponsor ViiV Healthcare and partner participation by Janssen Pharmaceutica NV in the development of the dolutegravir/rilpivirine two-drug regimen.
Source: Llibre JM et al. Lancet. 2018 Jan 10. doi: 10.1016/S0140-6736(17)33095-7.
FROM THE LANCET
Key clinical point: No difference was seen in maintaining virologic suppression of HIV-1 in adults switching from a traditional triple-drug regimen to a dolutegravir/rilpivirine dual-drug antiretroviral therapy.
Major finding: Of the participants in both the dual- and triple-drug groups, 95% maintained suppression to a viral load of fewer than 50 copies/mL.Study details: Two identical parallel-group studies (SWORD1 and SWORD2) of 1,024 adults participants randomly assigned in a 1:1 ratio comparing two-drug dolutegravir/rilpivirine with the active-control current triple-drug group.
Disclosures: The authors disclosed associations with financial and regulatory sponsor ViiV Healthcare and partner participation by Janssen Pharmaceutica NV in the development of the dolutegravir/rilpivirine two-drug regimen.
Source: Llibre JM et al. Lancet. 2018 Jan 10. doi: 10.1016/S0140-6736(17)33095-7.
FDA issues safety measures for all gadolinium-based contrast agents for MRI
A US Food & Drug Administration (FDA) Drug Safety Communication concerning a New Class Warning for all gadolinium-based contrast agents (GBCAs) for magnetic resonance imaging (MRI) has been released. Gadolinium has been found to remain in patients’ bodies, including the brain, for months to years.1
The FDA concluded that the benefit of all approved GBCAs outweighs any potential risks because gadolinium retention has not been directly linked to adverse health effects in patients with normal kidney function. To date, the only known adverse health effect related to gadolinium retention is a rare condition called nephrogenic systemic fibrosis that occurs in a small subgroup of patients with preexisting kidney failure. However, the FDA has recently received reports of adverse events involving multiple organ systems in patients with normal kidney function.1
After a review by the Medical Imaging Drugs Advisory Committee, the FDA is requiring several actions1:
- the development of a new Patient Medication Guide for GBCAs
- a requirement that every patient must read educational information before receiving a GBCA
- manufacturers of GBCAs must conduct human and animal studies to further assess the safety of these contrast agents.
FDA recommendations for your practice
The FDA advises that health care professionals should consider the retention characteristics of each agent when choosing a GBCA for patients who might be of higher risk for gadolinium retention.1,2 These patients include1:
- those requiring multiple lifetime doses
- pregnant women
- children
- patients with inflammatory conditions.
There are 2 types of GBCAs based on chemical structure: linear and macrocyclic. Linear GBCAs result in more retention and retention for a longer time than macrocyclic GBCAs.2 A list of FDA-approved GBCAs with their chemical structures is found here: https://www.fda.gov/Drugs/DrugSafety/ucm589213.htm.2
Recommendations also state that repeated GBCA imaging studies be minimized when possible, particularly closely spaced MRI studies. However, necessary GBCA MRI scans should not be avoided or deferred.1
Report adverse effects
Health care professionals and patients are encouraged to report adverse effects or side effects related to the use of GBCAs to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program found here: https://www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.home.
- US Food & Drug Administration. Safety: Gadolinium-based Contrast Agents (GBCAs): Drug Safety Communication - Retained in Body; New Class Warnings. https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm589580.htm. Published December 19, 2017. Accessed January 10, 2018.
- US Food & Drug Administration. Drugs: FDA Drug Safety Communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warning. https://www.fda.gov/Drugs/DrugSafety/ucm589213.htm. Published December 19, 2017. Accessed January 10, 2018.
A US Food & Drug Administration (FDA) Drug Safety Communication concerning a New Class Warning for all gadolinium-based contrast agents (GBCAs) for magnetic resonance imaging (MRI) has been released. Gadolinium has been found to remain in patients’ bodies, including the brain, for months to years.1
The FDA concluded that the benefit of all approved GBCAs outweighs any potential risks because gadolinium retention has not been directly linked to adverse health effects in patients with normal kidney function. To date, the only known adverse health effect related to gadolinium retention is a rare condition called nephrogenic systemic fibrosis that occurs in a small subgroup of patients with preexisting kidney failure. However, the FDA has recently received reports of adverse events involving multiple organ systems in patients with normal kidney function.1
After a review by the Medical Imaging Drugs Advisory Committee, the FDA is requiring several actions1:
- the development of a new Patient Medication Guide for GBCAs
- a requirement that every patient must read educational information before receiving a GBCA
- manufacturers of GBCAs must conduct human and animal studies to further assess the safety of these contrast agents.
FDA recommendations for your practice
The FDA advises that health care professionals should consider the retention characteristics of each agent when choosing a GBCA for patients who might be of higher risk for gadolinium retention.1,2 These patients include1:
- those requiring multiple lifetime doses
- pregnant women
- children
- patients with inflammatory conditions.
There are 2 types of GBCAs based on chemical structure: linear and macrocyclic. Linear GBCAs result in more retention and retention for a longer time than macrocyclic GBCAs.2 A list of FDA-approved GBCAs with their chemical structures is found here: https://www.fda.gov/Drugs/DrugSafety/ucm589213.htm.2
Recommendations also state that repeated GBCA imaging studies be minimized when possible, particularly closely spaced MRI studies. However, necessary GBCA MRI scans should not be avoided or deferred.1
Report adverse effects
Health care professionals and patients are encouraged to report adverse effects or side effects related to the use of GBCAs to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program found here: https://www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.home.
A US Food & Drug Administration (FDA) Drug Safety Communication concerning a New Class Warning for all gadolinium-based contrast agents (GBCAs) for magnetic resonance imaging (MRI) has been released. Gadolinium has been found to remain in patients’ bodies, including the brain, for months to years.1
The FDA concluded that the benefit of all approved GBCAs outweighs any potential risks because gadolinium retention has not been directly linked to adverse health effects in patients with normal kidney function. To date, the only known adverse health effect related to gadolinium retention is a rare condition called nephrogenic systemic fibrosis that occurs in a small subgroup of patients with preexisting kidney failure. However, the FDA has recently received reports of adverse events involving multiple organ systems in patients with normal kidney function.1
After a review by the Medical Imaging Drugs Advisory Committee, the FDA is requiring several actions1:
- the development of a new Patient Medication Guide for GBCAs
- a requirement that every patient must read educational information before receiving a GBCA
- manufacturers of GBCAs must conduct human and animal studies to further assess the safety of these contrast agents.
FDA recommendations for your practice
The FDA advises that health care professionals should consider the retention characteristics of each agent when choosing a GBCA for patients who might be of higher risk for gadolinium retention.1,2 These patients include1:
- those requiring multiple lifetime doses
- pregnant women
- children
- patients with inflammatory conditions.
There are 2 types of GBCAs based on chemical structure: linear and macrocyclic. Linear GBCAs result in more retention and retention for a longer time than macrocyclic GBCAs.2 A list of FDA-approved GBCAs with their chemical structures is found here: https://www.fda.gov/Drugs/DrugSafety/ucm589213.htm.2
Recommendations also state that repeated GBCA imaging studies be minimized when possible, particularly closely spaced MRI studies. However, necessary GBCA MRI scans should not be avoided or deferred.1
Report adverse effects
Health care professionals and patients are encouraged to report adverse effects or side effects related to the use of GBCAs to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program found here: https://www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.home.
- US Food & Drug Administration. Safety: Gadolinium-based Contrast Agents (GBCAs): Drug Safety Communication - Retained in Body; New Class Warnings. https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm589580.htm. Published December 19, 2017. Accessed January 10, 2018.
- US Food & Drug Administration. Drugs: FDA Drug Safety Communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warning. https://www.fda.gov/Drugs/DrugSafety/ucm589213.htm. Published December 19, 2017. Accessed January 10, 2018.
- US Food & Drug Administration. Safety: Gadolinium-based Contrast Agents (GBCAs): Drug Safety Communication - Retained in Body; New Class Warnings. https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm589580.htm. Published December 19, 2017. Accessed January 10, 2018.
- US Food & Drug Administration. Drugs: FDA Drug Safety Communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warning. https://www.fda.gov/Drugs/DrugSafety/ucm589213.htm. Published December 19, 2017. Accessed January 10, 2018.
NSAIDs reduce spinal pain but are not "clinically important"
Background: Although neck and low back pain are leading causes of pain and disability, there is no consensus first-line pharmacologic therapy for treatment. Recent research has pointed to acetaminophen as being ineffective, which – in combination with increased awareness of opioid dependency and adverse risks – could lead to greater use of NSAIDs.
Study design: Systematic review and meta-analysis.
Setting: Randomized controlled trials.
Synopsis: Researchers used MEDLINE, EMBASE, CINAHL, CENTRAL, and LILACS to select 35 randomized, placebo-controlled trials evaluating the impact of NSAIDs on reducing spinal pain and disability from a total of 302 full-text articles. Trial data were pooled based on follow-up time and outcomes. Pain and disability outcomes were converted to a 100-point scale with a 10-point difference between groups defined as “clinically important.” NSAIDs were found to offer greater pain reduction than placebo in the immediate (number needed to treat, 5; 95% confidence interval, 4-6) and short (NNT, 6; 95% CI, 4-10) range. However, this effect did not meet the specified 10-point difference to support “clinical importance,” despite having favorable numbers needed to treat. Limited corresponding safety analysis did not find significant adverse event rate differences other than increased reporting of gastrointestinal symptoms.
Bottom line: NSAIDs reduce spinal pain, compared with placebo, with low numbers needed to treat, but nevertheless were not determined to have a “clinically important” effect.
Citation: Machado GC et al. Nonsteroidal anti-inflammatory drugs for spinal pain: A systematic review and meta-analysis. Ann Rheum Dis. 2017 Jul;76(7):1269-78.
Background: Although neck and low back pain are leading causes of pain and disability, there is no consensus first-line pharmacologic therapy for treatment. Recent research has pointed to acetaminophen as being ineffective, which – in combination with increased awareness of opioid dependency and adverse risks – could lead to greater use of NSAIDs.
Study design: Systematic review and meta-analysis.
Setting: Randomized controlled trials.
Synopsis: Researchers used MEDLINE, EMBASE, CINAHL, CENTRAL, and LILACS to select 35 randomized, placebo-controlled trials evaluating the impact of NSAIDs on reducing spinal pain and disability from a total of 302 full-text articles. Trial data were pooled based on follow-up time and outcomes. Pain and disability outcomes were converted to a 100-point scale with a 10-point difference between groups defined as “clinically important.” NSAIDs were found to offer greater pain reduction than placebo in the immediate (number needed to treat, 5; 95% confidence interval, 4-6) and short (NNT, 6; 95% CI, 4-10) range. However, this effect did not meet the specified 10-point difference to support “clinical importance,” despite having favorable numbers needed to treat. Limited corresponding safety analysis did not find significant adverse event rate differences other than increased reporting of gastrointestinal symptoms.
Bottom line: NSAIDs reduce spinal pain, compared with placebo, with low numbers needed to treat, but nevertheless were not determined to have a “clinically important” effect.
Citation: Machado GC et al. Nonsteroidal anti-inflammatory drugs for spinal pain: A systematic review and meta-analysis. Ann Rheum Dis. 2017 Jul;76(7):1269-78.
Background: Although neck and low back pain are leading causes of pain and disability, there is no consensus first-line pharmacologic therapy for treatment. Recent research has pointed to acetaminophen as being ineffective, which – in combination with increased awareness of opioid dependency and adverse risks – could lead to greater use of NSAIDs.
Study design: Systematic review and meta-analysis.
Setting: Randomized controlled trials.
Synopsis: Researchers used MEDLINE, EMBASE, CINAHL, CENTRAL, and LILACS to select 35 randomized, placebo-controlled trials evaluating the impact of NSAIDs on reducing spinal pain and disability from a total of 302 full-text articles. Trial data were pooled based on follow-up time and outcomes. Pain and disability outcomes were converted to a 100-point scale with a 10-point difference between groups defined as “clinically important.” NSAIDs were found to offer greater pain reduction than placebo in the immediate (number needed to treat, 5; 95% confidence interval, 4-6) and short (NNT, 6; 95% CI, 4-10) range. However, this effect did not meet the specified 10-point difference to support “clinical importance,” despite having favorable numbers needed to treat. Limited corresponding safety analysis did not find significant adverse event rate differences other than increased reporting of gastrointestinal symptoms.
Bottom line: NSAIDs reduce spinal pain, compared with placebo, with low numbers needed to treat, but nevertheless were not determined to have a “clinically important” effect.
Citation: Machado GC et al. Nonsteroidal anti-inflammatory drugs for spinal pain: A systematic review and meta-analysis. Ann Rheum Dis. 2017 Jul;76(7):1269-78.
Malpractice premiums dip again
Malpractice premiums continue to inch down but wide disparities in total cost still linger across states.
Internists, general surgeons, and obstetrician-gynecologists experienced a respective 1% drop in their medical liability premiums last year, according to the 2017 Medical Liability Monitor Annual Rate Survey. The rate drop follows an ongoing trend of decreasing premiums over the last decade.
“The takeaways for doctors are really all good ones in that the rates remain very stable,” said Paul A. Greve Jr. senior vice president/senior consultant for Willis Towers Watson Health Care Practice and coauthor of the 2017 MLM Survey report. “The market for physician coverage remains very competitive because there are so many players involved for what is really a shrinking number of buyers, so the groups and individual physicians that are buying are seeing favorable pricing.”
Premiums differed vastly across geographic area, consistent with previous years. Southern Florida internists for example, paid $47,707 for malpractice insurance last year, while their Minnesota colleagues paid $3,375. For ob.gyns., premiums ranged from $214,999 in southern New York to $16,240 in central California. General surgeons in Southern Florida paid $190,829 in 2016, while those in Wisconsin paid $10,868.
Overall, no states experienced a premium rate change in the double digits, and physicians in only five states – Hawaii, Kansas, Michigan, Montana, and Ohio – saw premium decreases of more than 5%. No states experienced rate increases of more than 5%.
Fewer claims filed by plaintiffs’ attorneys is one factor contributing to the continued stability of malpractice premiums, according to analysts. However, there are signs that high verdicts are on the rise, said Michael Matray, editor of the Medical Liability Monitor and chief content officer for Cunningham Group. Survey data show claims closing at greater than $1 million are increasing.
“There is data that indicates claim severity has experienced a slight uptick,” Mr. Matray said in an interview. “It obviously hasn’t affected rates, yet. This could be due to the positive effect state-level tort reforms have had – where plaintiff attorneys are only bringing cases that are a slam dunk and carry a larger dollar value.”
Continued practice consolidation and the increase in employed physicians also helped keep premiums steady, Mr. Greve said in an interview. Consolidation means fewer buyers and a more competitive market, which helps keep premiums low and stable.
The jury is still out on how the move to value-based care might impact medical malpractice insurance payments. There is concern that the methods required to determine health care value could unwittingly increase malpractice risk, Mr. Matray said.
“To support value-based reimbursement models, a health care system must manage a vast network of public and private data used by various entities in order to monitor quality and cost,” Mr. Matray said. “The collection of that data requires using electronic health record technology that many physicians find onerous. This leads to physician burnout and dangerous EHR workarounds, such as copy-and-paste practices where previous EHR entries are cloned and inserted into a new progress note, as well as disabling or overriding burdensome safety alerts, to save time and increase efficiency. You can see how this would increase medical liability claim risk.”
The MLM survey is published yearly based on July 1 premium data from the major malpractice insurers and examines premium rates for mature, claims-made policies with $1 million/$3 million limits for internists, general surgeons, and ob.gyns.
Malpractice premiums continue to inch down but wide disparities in total cost still linger across states.
Internists, general surgeons, and obstetrician-gynecologists experienced a respective 1% drop in their medical liability premiums last year, according to the 2017 Medical Liability Monitor Annual Rate Survey. The rate drop follows an ongoing trend of decreasing premiums over the last decade.
“The takeaways for doctors are really all good ones in that the rates remain very stable,” said Paul A. Greve Jr. senior vice president/senior consultant for Willis Towers Watson Health Care Practice and coauthor of the 2017 MLM Survey report. “The market for physician coverage remains very competitive because there are so many players involved for what is really a shrinking number of buyers, so the groups and individual physicians that are buying are seeing favorable pricing.”
Premiums differed vastly across geographic area, consistent with previous years. Southern Florida internists for example, paid $47,707 for malpractice insurance last year, while their Minnesota colleagues paid $3,375. For ob.gyns., premiums ranged from $214,999 in southern New York to $16,240 in central California. General surgeons in Southern Florida paid $190,829 in 2016, while those in Wisconsin paid $10,868.
Overall, no states experienced a premium rate change in the double digits, and physicians in only five states – Hawaii, Kansas, Michigan, Montana, and Ohio – saw premium decreases of more than 5%. No states experienced rate increases of more than 5%.
Fewer claims filed by plaintiffs’ attorneys is one factor contributing to the continued stability of malpractice premiums, according to analysts. However, there are signs that high verdicts are on the rise, said Michael Matray, editor of the Medical Liability Monitor and chief content officer for Cunningham Group. Survey data show claims closing at greater than $1 million are increasing.
“There is data that indicates claim severity has experienced a slight uptick,” Mr. Matray said in an interview. “It obviously hasn’t affected rates, yet. This could be due to the positive effect state-level tort reforms have had – where plaintiff attorneys are only bringing cases that are a slam dunk and carry a larger dollar value.”
Continued practice consolidation and the increase in employed physicians also helped keep premiums steady, Mr. Greve said in an interview. Consolidation means fewer buyers and a more competitive market, which helps keep premiums low and stable.
The jury is still out on how the move to value-based care might impact medical malpractice insurance payments. There is concern that the methods required to determine health care value could unwittingly increase malpractice risk, Mr. Matray said.
“To support value-based reimbursement models, a health care system must manage a vast network of public and private data used by various entities in order to monitor quality and cost,” Mr. Matray said. “The collection of that data requires using electronic health record technology that many physicians find onerous. This leads to physician burnout and dangerous EHR workarounds, such as copy-and-paste practices where previous EHR entries are cloned and inserted into a new progress note, as well as disabling or overriding burdensome safety alerts, to save time and increase efficiency. You can see how this would increase medical liability claim risk.”
The MLM survey is published yearly based on July 1 premium data from the major malpractice insurers and examines premium rates for mature, claims-made policies with $1 million/$3 million limits for internists, general surgeons, and ob.gyns.
Malpractice premiums continue to inch down but wide disparities in total cost still linger across states.
Internists, general surgeons, and obstetrician-gynecologists experienced a respective 1% drop in their medical liability premiums last year, according to the 2017 Medical Liability Monitor Annual Rate Survey. The rate drop follows an ongoing trend of decreasing premiums over the last decade.
“The takeaways for doctors are really all good ones in that the rates remain very stable,” said Paul A. Greve Jr. senior vice president/senior consultant for Willis Towers Watson Health Care Practice and coauthor of the 2017 MLM Survey report. “The market for physician coverage remains very competitive because there are so many players involved for what is really a shrinking number of buyers, so the groups and individual physicians that are buying are seeing favorable pricing.”
Premiums differed vastly across geographic area, consistent with previous years. Southern Florida internists for example, paid $47,707 for malpractice insurance last year, while their Minnesota colleagues paid $3,375. For ob.gyns., premiums ranged from $214,999 in southern New York to $16,240 in central California. General surgeons in Southern Florida paid $190,829 in 2016, while those in Wisconsin paid $10,868.
Overall, no states experienced a premium rate change in the double digits, and physicians in only five states – Hawaii, Kansas, Michigan, Montana, and Ohio – saw premium decreases of more than 5%. No states experienced rate increases of more than 5%.
Fewer claims filed by plaintiffs’ attorneys is one factor contributing to the continued stability of malpractice premiums, according to analysts. However, there are signs that high verdicts are on the rise, said Michael Matray, editor of the Medical Liability Monitor and chief content officer for Cunningham Group. Survey data show claims closing at greater than $1 million are increasing.
“There is data that indicates claim severity has experienced a slight uptick,” Mr. Matray said in an interview. “It obviously hasn’t affected rates, yet. This could be due to the positive effect state-level tort reforms have had – where plaintiff attorneys are only bringing cases that are a slam dunk and carry a larger dollar value.”
Continued practice consolidation and the increase in employed physicians also helped keep premiums steady, Mr. Greve said in an interview. Consolidation means fewer buyers and a more competitive market, which helps keep premiums low and stable.
The jury is still out on how the move to value-based care might impact medical malpractice insurance payments. There is concern that the methods required to determine health care value could unwittingly increase malpractice risk, Mr. Matray said.
“To support value-based reimbursement models, a health care system must manage a vast network of public and private data used by various entities in order to monitor quality and cost,” Mr. Matray said. “The collection of that data requires using electronic health record technology that many physicians find onerous. This leads to physician burnout and dangerous EHR workarounds, such as copy-and-paste practices where previous EHR entries are cloned and inserted into a new progress note, as well as disabling or overriding burdensome safety alerts, to save time and increase efficiency. You can see how this would increase medical liability claim risk.”
The MLM survey is published yearly based on July 1 premium data from the major malpractice insurers and examines premium rates for mature, claims-made policies with $1 million/$3 million limits for internists, general surgeons, and ob.gyns.
AAD guidelines favor surgery for nonmelanoma skin cancers
, according to new practice guidelines issued by the American Academy of Dermatology.
Nonsurgical approaches such as cryotherapy, photodynamic therapy, and radiation may be considered for low-risk cancers if surgery is contraindicated, but these methods have lower cure rates, according to the guidelines. Christopher K. Bichakjian, MD, professor of dermatology, University of Michigan, Ann Arbor, and Murad Alam, MD, professor of dermatology, Northwestern University, Chicago, cochaired the work groups that developed the guidelines.
The guidelines for BCC and cSCC, published online in two separate papers (J Am Acad Dermatol. 2018 Jan. 10. doi: 10.1016/j.jaad.2017.10.006; J Am Acad Dermatol. 2018 Jan. 10. doi: 10.1016/j.jaad.2017.10.007), also discuss biopsy techniques, tumor staging, and prevention of recurrence of nonmelanoma skin cancers.
The most suitable stratification for localized BCC and cSCC is the framework provided by the National Comprehensive Cancer Network, the authors said in the guidelines.
For suspected BCC and cSCC, recommended biopsy techniques are punch biopsy, shave biopsy, and excisional biopsy. Biopsy technique is “contingent on the clinical characteristics of the suspected tumor, including morphology, expected histologic subtype and depth, natural history, and anatomic location; patient-specific factors, such as bleeding and wound healing diatheses; and patient preference and physician judgment,” the guidelines state. If the initial biopsy proves insufficient for diagnosis, a repeat biopsy may be considered.
For surgical treatment of BCC, curettage and electrodessication may be considered for low-risk tumors in nonterminal hair-bearing locations. Surgical excision with 4-mm clinical margins and histologic margin assessment is recommended for low-risk primary BCC. For high-risk BCC, Mohs micrographic surgery is recommended, the authors said.
Surgical options for cSCC also include curettage and electrodessication and standard excision for low-risk disease, and Mohs micrographic surgery for high-risk cSCC. In both BCC and cSCC, standard excision may be considered for high-risk tumors in some cases, but “strong caution is advised when selecting a treatment modality” for high-risk tumors “without complete margin assessment,” the guidelines state.
Nonsurgical therapies are generally not recommended as first-line treatment, especially in cSCC because of possible recurrence and metastasis. In cases where nonsurgical therapies are preferred, options may include cryosurgery, topical therapy, photodynamic therapy, radiation, or laser therapy, “with the understanding that the cure rate may be lower,” the authors wrote.
Patients with diagnosed nonmelanoma skin cancer should continue to undergo screening for new primary skin cancers (including BCC, cSCC, and melanoma) at least once per year, the guideline states. They should also be counseled on sun protection, tanning bed avoidance, and regular use of broad-spectrum sunscreen.
Although the new guidelines mainly “reaffirm common knowledge and current practice,” they offer a reminder of “alternative therapeutic or preventive options when insufficient evidence is available to support new therapies or previously dogmatic practice patterns,” the authors said.
These are the first guidelines of care for BCC and cSCC published by the AAD. Commonly used guidelines for the management of BCC and cSCC are published by the National Comprehensive Cancer Network, which are frequently referenced throughout the new AAD guidelines, Dr. Bichakjian said in an interview. While the aim of the cancer network is to develop multidisciplinary guidelines, reflected by the composition of the panel members, “AAD guidelines of care are established primarily by dermatologists for dermatologists,” he pointed out. “However, the work group recognizes that a variety of health care providers outside of dermatology take care of patients with BCC and cSCC, and acknowledges the importance of multidisciplinary care,” he added. “With these considerations in mind, reviewers from specialties outside of dermatology, including plastic surgery, otolaryngology/head and neck surgery, medical oncology, radiation oncology, and family medicine, were invited to critically review the current guidelines.”
The guidelines do not cover the management of actinic keratosis and cSCC in situ, he said. “The work group acknowledges the importance of appropriate management of premalignant and in situ lesions in the prevention of their potential progression to cSCC. However, additional data to provide comprehensive evidence-based recommendations were deemed too extensive to include in the current guidelines and will need to addressed separately.”
In an interview, David J. Leffell, MD, who was not an author of the guidelines, said that the new guidelines do an effective job of “highlighting where valid outcomes data exist and areas where they do not” for a wide range of therapies. They also “attempt to standardize approaches to diagnosis and care of nonmelanoma skin cancer and in general are consistent with established practice patterns,” he added. “Those contemporary approaches have developed in largely empirical fashion over many decades, but bear clarification and reinforcement,” said Dr. Leffell, professor of dermatology and surgery and chief of the section of dermatologic surgery and cutaneous oncology at Yale University, New Haven, Conn.
The guidelines “thoroughly summarize evidence-based recommendations for the entire spectrum of disease management,” Daniel D. Bennett, MD, of the department of dermatology at the University of Wisconsin – Madison, said in an interview. “While surgery remains the mainstay of treatment for BCC and cutaneous SCC, these guidelines include excellent reviews of nonsurgical management options,” he said.
Dr. Bichakjian, who is also chief of the division of cutaneous surgery and oncology at the University of Michigan, had no relevant financial disclosures to report. Dr. Alam, who is also chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern, disclosed relationships with Amway, OptMed, and 3M. Dr. Bennett and Dr. Leffell had no relevant disclosures.
, according to new practice guidelines issued by the American Academy of Dermatology.
Nonsurgical approaches such as cryotherapy, photodynamic therapy, and radiation may be considered for low-risk cancers if surgery is contraindicated, but these methods have lower cure rates, according to the guidelines. Christopher K. Bichakjian, MD, professor of dermatology, University of Michigan, Ann Arbor, and Murad Alam, MD, professor of dermatology, Northwestern University, Chicago, cochaired the work groups that developed the guidelines.
The guidelines for BCC and cSCC, published online in two separate papers (J Am Acad Dermatol. 2018 Jan. 10. doi: 10.1016/j.jaad.2017.10.006; J Am Acad Dermatol. 2018 Jan. 10. doi: 10.1016/j.jaad.2017.10.007), also discuss biopsy techniques, tumor staging, and prevention of recurrence of nonmelanoma skin cancers.
The most suitable stratification for localized BCC and cSCC is the framework provided by the National Comprehensive Cancer Network, the authors said in the guidelines.
For suspected BCC and cSCC, recommended biopsy techniques are punch biopsy, shave biopsy, and excisional biopsy. Biopsy technique is “contingent on the clinical characteristics of the suspected tumor, including morphology, expected histologic subtype and depth, natural history, and anatomic location; patient-specific factors, such as bleeding and wound healing diatheses; and patient preference and physician judgment,” the guidelines state. If the initial biopsy proves insufficient for diagnosis, a repeat biopsy may be considered.
For surgical treatment of BCC, curettage and electrodessication may be considered for low-risk tumors in nonterminal hair-bearing locations. Surgical excision with 4-mm clinical margins and histologic margin assessment is recommended for low-risk primary BCC. For high-risk BCC, Mohs micrographic surgery is recommended, the authors said.
Surgical options for cSCC also include curettage and electrodessication and standard excision for low-risk disease, and Mohs micrographic surgery for high-risk cSCC. In both BCC and cSCC, standard excision may be considered for high-risk tumors in some cases, but “strong caution is advised when selecting a treatment modality” for high-risk tumors “without complete margin assessment,” the guidelines state.
Nonsurgical therapies are generally not recommended as first-line treatment, especially in cSCC because of possible recurrence and metastasis. In cases where nonsurgical therapies are preferred, options may include cryosurgery, topical therapy, photodynamic therapy, radiation, or laser therapy, “with the understanding that the cure rate may be lower,” the authors wrote.
Patients with diagnosed nonmelanoma skin cancer should continue to undergo screening for new primary skin cancers (including BCC, cSCC, and melanoma) at least once per year, the guideline states. They should also be counseled on sun protection, tanning bed avoidance, and regular use of broad-spectrum sunscreen.
Although the new guidelines mainly “reaffirm common knowledge and current practice,” they offer a reminder of “alternative therapeutic or preventive options when insufficient evidence is available to support new therapies or previously dogmatic practice patterns,” the authors said.
These are the first guidelines of care for BCC and cSCC published by the AAD. Commonly used guidelines for the management of BCC and cSCC are published by the National Comprehensive Cancer Network, which are frequently referenced throughout the new AAD guidelines, Dr. Bichakjian said in an interview. While the aim of the cancer network is to develop multidisciplinary guidelines, reflected by the composition of the panel members, “AAD guidelines of care are established primarily by dermatologists for dermatologists,” he pointed out. “However, the work group recognizes that a variety of health care providers outside of dermatology take care of patients with BCC and cSCC, and acknowledges the importance of multidisciplinary care,” he added. “With these considerations in mind, reviewers from specialties outside of dermatology, including plastic surgery, otolaryngology/head and neck surgery, medical oncology, radiation oncology, and family medicine, were invited to critically review the current guidelines.”
The guidelines do not cover the management of actinic keratosis and cSCC in situ, he said. “The work group acknowledges the importance of appropriate management of premalignant and in situ lesions in the prevention of their potential progression to cSCC. However, additional data to provide comprehensive evidence-based recommendations were deemed too extensive to include in the current guidelines and will need to addressed separately.”
In an interview, David J. Leffell, MD, who was not an author of the guidelines, said that the new guidelines do an effective job of “highlighting where valid outcomes data exist and areas where they do not” for a wide range of therapies. They also “attempt to standardize approaches to diagnosis and care of nonmelanoma skin cancer and in general are consistent with established practice patterns,” he added. “Those contemporary approaches have developed in largely empirical fashion over many decades, but bear clarification and reinforcement,” said Dr. Leffell, professor of dermatology and surgery and chief of the section of dermatologic surgery and cutaneous oncology at Yale University, New Haven, Conn.
The guidelines “thoroughly summarize evidence-based recommendations for the entire spectrum of disease management,” Daniel D. Bennett, MD, of the department of dermatology at the University of Wisconsin – Madison, said in an interview. “While surgery remains the mainstay of treatment for BCC and cutaneous SCC, these guidelines include excellent reviews of nonsurgical management options,” he said.
Dr. Bichakjian, who is also chief of the division of cutaneous surgery and oncology at the University of Michigan, had no relevant financial disclosures to report. Dr. Alam, who is also chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern, disclosed relationships with Amway, OptMed, and 3M. Dr. Bennett and Dr. Leffell had no relevant disclosures.
, according to new practice guidelines issued by the American Academy of Dermatology.
Nonsurgical approaches such as cryotherapy, photodynamic therapy, and radiation may be considered for low-risk cancers if surgery is contraindicated, but these methods have lower cure rates, according to the guidelines. Christopher K. Bichakjian, MD, professor of dermatology, University of Michigan, Ann Arbor, and Murad Alam, MD, professor of dermatology, Northwestern University, Chicago, cochaired the work groups that developed the guidelines.
The guidelines for BCC and cSCC, published online in two separate papers (J Am Acad Dermatol. 2018 Jan. 10. doi: 10.1016/j.jaad.2017.10.006; J Am Acad Dermatol. 2018 Jan. 10. doi: 10.1016/j.jaad.2017.10.007), also discuss biopsy techniques, tumor staging, and prevention of recurrence of nonmelanoma skin cancers.
The most suitable stratification for localized BCC and cSCC is the framework provided by the National Comprehensive Cancer Network, the authors said in the guidelines.
For suspected BCC and cSCC, recommended biopsy techniques are punch biopsy, shave biopsy, and excisional biopsy. Biopsy technique is “contingent on the clinical characteristics of the suspected tumor, including morphology, expected histologic subtype and depth, natural history, and anatomic location; patient-specific factors, such as bleeding and wound healing diatheses; and patient preference and physician judgment,” the guidelines state. If the initial biopsy proves insufficient for diagnosis, a repeat biopsy may be considered.
For surgical treatment of BCC, curettage and electrodessication may be considered for low-risk tumors in nonterminal hair-bearing locations. Surgical excision with 4-mm clinical margins and histologic margin assessment is recommended for low-risk primary BCC. For high-risk BCC, Mohs micrographic surgery is recommended, the authors said.
Surgical options for cSCC also include curettage and electrodessication and standard excision for low-risk disease, and Mohs micrographic surgery for high-risk cSCC. In both BCC and cSCC, standard excision may be considered for high-risk tumors in some cases, but “strong caution is advised when selecting a treatment modality” for high-risk tumors “without complete margin assessment,” the guidelines state.
Nonsurgical therapies are generally not recommended as first-line treatment, especially in cSCC because of possible recurrence and metastasis. In cases where nonsurgical therapies are preferred, options may include cryosurgery, topical therapy, photodynamic therapy, radiation, or laser therapy, “with the understanding that the cure rate may be lower,” the authors wrote.
Patients with diagnosed nonmelanoma skin cancer should continue to undergo screening for new primary skin cancers (including BCC, cSCC, and melanoma) at least once per year, the guideline states. They should also be counseled on sun protection, tanning bed avoidance, and regular use of broad-spectrum sunscreen.
Although the new guidelines mainly “reaffirm common knowledge and current practice,” they offer a reminder of “alternative therapeutic or preventive options when insufficient evidence is available to support new therapies or previously dogmatic practice patterns,” the authors said.
These are the first guidelines of care for BCC and cSCC published by the AAD. Commonly used guidelines for the management of BCC and cSCC are published by the National Comprehensive Cancer Network, which are frequently referenced throughout the new AAD guidelines, Dr. Bichakjian said in an interview. While the aim of the cancer network is to develop multidisciplinary guidelines, reflected by the composition of the panel members, “AAD guidelines of care are established primarily by dermatologists for dermatologists,” he pointed out. “However, the work group recognizes that a variety of health care providers outside of dermatology take care of patients with BCC and cSCC, and acknowledges the importance of multidisciplinary care,” he added. “With these considerations in mind, reviewers from specialties outside of dermatology, including plastic surgery, otolaryngology/head and neck surgery, medical oncology, radiation oncology, and family medicine, were invited to critically review the current guidelines.”
The guidelines do not cover the management of actinic keratosis and cSCC in situ, he said. “The work group acknowledges the importance of appropriate management of premalignant and in situ lesions in the prevention of their potential progression to cSCC. However, additional data to provide comprehensive evidence-based recommendations were deemed too extensive to include in the current guidelines and will need to addressed separately.”
In an interview, David J. Leffell, MD, who was not an author of the guidelines, said that the new guidelines do an effective job of “highlighting where valid outcomes data exist and areas where they do not” for a wide range of therapies. They also “attempt to standardize approaches to diagnosis and care of nonmelanoma skin cancer and in general are consistent with established practice patterns,” he added. “Those contemporary approaches have developed in largely empirical fashion over many decades, but bear clarification and reinforcement,” said Dr. Leffell, professor of dermatology and surgery and chief of the section of dermatologic surgery and cutaneous oncology at Yale University, New Haven, Conn.
The guidelines “thoroughly summarize evidence-based recommendations for the entire spectrum of disease management,” Daniel D. Bennett, MD, of the department of dermatology at the University of Wisconsin – Madison, said in an interview. “While surgery remains the mainstay of treatment for BCC and cutaneous SCC, these guidelines include excellent reviews of nonsurgical management options,” he said.
Dr. Bichakjian, who is also chief of the division of cutaneous surgery and oncology at the University of Michigan, had no relevant financial disclosures to report. Dr. Alam, who is also chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern, disclosed relationships with Amway, OptMed, and 3M. Dr. Bennett and Dr. Leffell had no relevant disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY