User login
Musculoskeletal ultrasonography basics
Ultrasonography has been used to evaluate musculoskeletal problems for decades but has only recently become more widely available in the United States. Advances in technology and physician familiarity are increasing its role in orthopedic imaging.
No single imaging method can yield all musculoskeletal diagnoses. Like any imaging technique, ultrasonography has strengths and weaknesses specific to orthopedics. Radiography, computed tomography (CT), and magnetic resonance imaging (MRI) play important roles for investigating musculoskeletal problems and are complementary to each other and to ultrasonography.
To help clinicians make informed decisions about ordering musculoskeletal ultrasonography, this article reviews the basic physics underlying ultrasonography, its advantages and disadvantages compared with other imaging methods, and common clinical applications.
CLASSIC TECHNOLOGY MAKING A RESURGENCE
The first reports of the use of musculoskeletal ultrasonography appeared in the 1970s for investigating the rotator cuff,1–3 actually preceding reports of its use in obstetrics and gynecology.4 In the 1980s, reports emerged for evaluating the Achilles tendon.5,6 After that, its popularity in the United States plateaued, likely because of the advent of MRI, lower reimbursement and greater variability in interpretation compared with MRI, as well as a lack of physicians and sonographers trained in its use.7,8
Musculoskeletal ultrasonography is currently experiencing a resurgence. Although it remains a specialized service more commonly available in large hospitals, its use is increasing rapidly, and it will likely become more widely available.
SPECIAL TRAINING REQUIRED
Musculoskeletal ultrasonography is simply an ultrasonographic examination of part of the musculoskeletal system. But because not all ultrasonographic transducers offer sufficient resolution for musculoskeletal evaluation and not all sonographers and imaging physicians are familiar with the specialized techniques, musculoskeletal ultrasonography often has a separate designation (eg, “MSKUS,” “MSUS”). At Cleveland Clinic, it is offered through the department of musculoskeletal imaging by subspecialty-trained musculoskeletal radiologists and specially trained musculoskeletal ultrasonographers with 4 to 5 years of training in the technique.
Musculoskeletal ultrasonography is also performed by physician groups with specialized training, including sports medicine physicians, rheumatologists, physiatrists, neurologists, and orthopedic surgeons. The American Institute of Ultrasound in Medicine offers voluntary accreditation for practice groups using musculoskeletal ultrasonography. Certification in musculoskeletal radiology is offered to sonographers through the American Registry for Diagnostic Medical Sonography.
SONOGRAPHY HAS UNIQUE QUALITIES
Ultrasonography uses high-frequency sound waves to generate images. The transducer (or probe) emits sound from the many piezoelectric elements at its surface, and the sound waves travel through and react with tissues. Sound reflected by tissues is detected by the transducer and converted to an image. Objects that reflect sound appear hyperechoic (brighter), whereas tissues that reflect little or no sound appear hypoechoic.
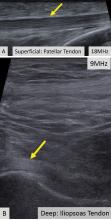
High-resolution imaging of superficial structures
(B, arrow).
Ultrasonography involves a fundamental trade-off between image resolution and imaging depth. Higher-frequency sound waves do not penetrate far into tissues but generate a higher-resolution image; lower-frequency sound waves can penetrate much further but yield a lower-resolution image. Although high-resolution imaging of deep structures with ultrasonography is not possible (Figure 1), many musculoskeletal structures are located superficially and are amenable to ultrasonographic evaluation.
Be aware of artifacts
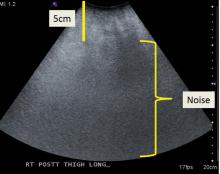
Some materials attenuate sound very little, such as simple fluid. Low attenuation results in artifacts on ultrasonography, making tissues behind the simple fluid appear brighter than neighboring tissues. These artifacts may be reported as “increased through transmission” or “posterior acoustic enhancement.” Conversely, metal and bone reflect all sound waves that reach them, rendering any structures beyond them invisible. This “shadowing” creates a problem for imaging of structures in or near bone. Subcutaneous fat also attenuates sound waves, limiting the use of ultrasonography for patients with obesity (Figure 2).
High-frequency linear transducer sharpens images
High-frequency linear transducers reduce anisotropy because their flat surface keeps sound waves more uniformly perpendicular to the structure of interest.4,7 Their development has allowed imaging of superficial structures that is superior to that of MRI. A high-frequency linear transducer offers more than twice the spatial resolution of a typical 1.5T MRI examination of superficial tissue.12,13
Operator experience is critical
Ultrasonography examinations, more than other imaging tests, are dependent on operator experience. A solid understanding of musculoskeletal anatomy is imperative. Because the probe images only a thin section of tissue (about the thickness of a credit card), referencing adjacent structures for orientation is more difficult with ultrasonography than with CT or MRI.
The accuracy of ultrasonography is highly dependent on acquiring and interpreting images, whereas the accuracy of MRI is dependent primarily on image interpretation.7 Interpreting physicians must check that sonographers capture relevant targets.
STRENGTHS OF MUSCULOSKELETAL ULTRASONOGRAPHY
Ultrasonography has multiple advantages:
No ionizing radiation exposure.
Portability. Unlike CT or MRI, ultrasonography equipment is portable.
Increased patient comfort. Patient positioning for an ultrasonography examination is more flexible than for MRI or CT,14 and the examination does not induce claustrophobia.8
High-resolution imaging. Ultrasonography provides very-high-resolution imaging of superficial soft tissues—in some cases, higher than MRI or CT.
Real-time dynamic examinations are possible with ultrasonography, unlike with CT or MRI, and may increase test sensitivity.4,15–18
Implanted hardware is less of a problem. Although ultrasonography cannot image beyond implanted orthopedic metallic hardware, the hardware does not obscure surrounding soft tissues as it does on CT and MRI.6,19,20 Also, ultrasonography is safe for patients with a pacemaker.8
WEAKNESSES
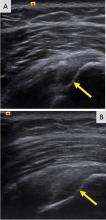
The main disadvantages of musculoskeletal ultrasonography are inherent to its limited field of view, making it inappropriate for a survey examination (eg, for ankle pain, knee pain, hip pain).4 Unlike CT and MRI, ultrasonography does not provide a “bird’s-eye view,” and important abnormalities can be missed during evaluation of large areas (Figure 4).
Ultrasonography also cannot evaluate bone or intra-articular structures such as cartilage, bone marrow, labrum, and intra-articular ligaments; MRI is the standard for evaluating these structures.21
Ultrasonography is time-consuming. To perform a detailed examination of the anterior, posterior, medial, and lateral aspects of the hip, knee, or ankle would require 1.5 to 2 hours of scanning time and an additional 10 to 25 minutes of image checking and interpretation.
CURRENT CLINICAL INDICATIONS
Musculoskeletal ultrasonography is best used for clinical questions regarding limited, superficial musculoskeletal problems.
Fluid collections
Ultrasonography can help evaluate small fluid collections in soft tissue. As is true for a lung opacity on chest radiography, soft-tissue fluid detected on ultrasonography is nonspecific, and results must be correlated with the clinical picture to narrow the differential diagnosis.
Fluid collections can be classified as loculated or nonloculated.
Nonloculated fluid involves more fluid than is simply interposed between tissue planes and has no wall or defined margins. It can be simple or complex in appearance: simple fluid is anechoic, and complex fluid appears more heterogeneous and may contain septations or debris.
Subcutaneous edema, which may occur postoperatively or from trauma, venous insufficiency, or inflammatory or infectious processes, appears on ultrasonography as nonloculated fluid interspersed between subcutaneous fat lobules.
Loculated fluid collections have well-defined margins or a discrete wall that does not follow normal tissue planes. They can also be simple or complex and can be caused by hematoma, abscess, or ganglion. Less commonly, neoplasms can mimic a loculated fluid collection (Figure 4).
A ganglion is a specific type of loculated fluid collection containing synovial fluid arising from a joint or tendon sheath. It tends to occur in specific locations, most commonly around the wrist, most often arising from the dorsal scapholunate ligament and volar wrist between the radial artery and flexor carpi radialis.22 On MRI, it can be difficult to distinguish between small vascular structures and a small ganglion, especially in the hands and feet.23
Ultrasonography can also help identify a Baker cyst, a specific fluid collection arising from the semimembranosus bursa between the medial head of the gastrocnemius tendon and the semimembranosus tendon. Ultrasonography can also detect inflammation, rupture, or leaking associated with a Baker cyst.24
Power Doppler is an ultrasonographic examination that can detect increased blood flow surrounding a fluid collection and determine the likelihood of an acute inflammatory or infectious cause.25
Joint effusion and synovitis
Musculoskeletal ultrasonography can help evaluate joints for effusion and synovitis. It is highly sensitive (94%) and specific (95%) for synovitis, making it superior to contrast-enhanced MRI.26,27 The area of concern should be limited to 1 quadrant of a joint (anterior, posterior, medial, or lateral); for problems beyond that, MRI should be considered.
A joint effusion appears as a distended joint capsule containing hypoechoic (complex) or anechoic (simple) joint fluid.
Complex joint fluid may contain debris and occurs with hemarthrosis, infection, and inflammation.23 Hypertrophied synovium is hypoechoic and can mimic complex joint fluid.
Power Doppler evaluation can help distinguish synovitis from joint fluid by demonstrating blood flow, a feature of synovitis but not of simple joint fluid. Power Doppler is the most sensitive means of detecting blood flow, although it does not show direction of flow.28
Using ultrasonography can help to improve disease control and minimize disabling changes by monitoring synovitis therapy. In addition, subclinical synovitis and enthesitis (inflammation of insertion sites of tendons or ligaments into bone) detected by ultrasonography may predict future disease and disease flares.29–31
Ultrasonographic guidance for a wide range of procedures is increasing rapidly.32–36 Multiple studies have shown the advantage of ultrasonography-guided aspiration and injection compared with techniques without imaging guidance.37,38
Soft-tissue masses
Accurately diagnosing soft-tissue masses can be difficult. A mass may remain indeterminate even after multiple imaging studies, requiring biopsy or surgical referral. However, for a few specific masses, ultrasonography is highly accurate and can eliminate the need for further imaging.
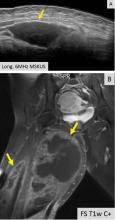
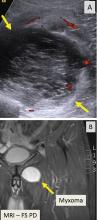
Ultrasonography can help evaluate soft- tissue masses no larger than 5 cm in diameter and no deeper than superficial muscular fascia. If the mass is larger or deeper than that, ultrasonography is less reliable for showing the margins of the mass and its relationship to adjacent structures (Figure 5). Further imaging by MRI may be recommended in such cases.
Fortunately, many of the most common soft-tissue masses can be accurately diagnosed with ultrasonography, including lipomas, ganglion cysts, foreign bodies, and simple fluid collections.4,39 Nerve-sheath tumors can also be diagnosed with ultrasonography if the lesion clearly arises from a nerve. Other soft-tissue masses are likely to be indeterminate with ultrasonography, requiring follow-up with MRI with contrast.
Tendons
Musculoskeletal ultrasonography can be effective for evaluating tendons around joints, especially 1 or a small number of nearby superficial tendons. Tendons particularly well suited for ultrasonographic examination include:
- Upper-extremity tendons located in the rotator cuff or around the elbow, and flexor and extensor tendons of the hands; ultrasonographic evaluation of the rotator cuff is highly accurate, equivalent to that of MRI for partial-thickness and full-thickness tearing40–43
- Lower-extremity tendons of the extensor mechanism of the knee, distal hamstring tendons, tendons around the ankle,44–46 and flexor and extensor tendons of the foot.
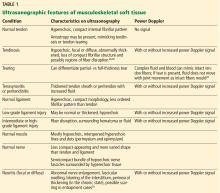
Ultrasonography can help diagnose a variety of tendon abnormalities (Table 1),48,49 including tearing, for which a dynamic examination can be performed.
Many tendons have a tendon sheath containing tenosynovium, while others have surrounding peritenon only; either can become thickened and inflamed. Tenosynovitis is a nonspecific finding and may be inflammatory, infectious, or posttraumatic. The presence of tendon sheath fluid alone on ultrasonography can be a normal finding, and some tendon sheaths that communicate with adjacent joints (eg, the long head biceps tendon, the flexor hallucis longus tendon) commonly contain simple fluid.6 A dynamic examination with ultrasonography can help diagnose snapping related to abnormal tendon movement, for example, in the case of intra-sheath and extra-sheath subluxation of the peroneal tendons.45,50,51
Ligaments
Ultrasonography can detect abnormalities in many superficial ligaments (Table 1).
Ankle. Ankle ligaments are superficial and can be clearly visualized. The diagnostic accuracy of ultrasonography for tearing of the anterior talofibular ligament may be as high as 100%.50,52,53
Elbow and thumb. The larger of the collateral ligaments of the elbow, especially the ulnar collateral ligament, and the ulnar collateral ligament of the thumb can be effectively evaluated with ultrasonography.54,55
Knee. The collateral ligaments of the knee can be seen with ultrasonography, but injuries of the external ligaments of the knee are often associated with intrinsic derangements that cannot be evaluated with ultrasonography.56,57 Intra-articular ligaments such as the anterior cruciate ligament are also not amenable to ultrasonography.
Dynamic examination of a ligament with ultrasonography can help determine the grade of the injury.
Deeply located ligaments (eg, around the hip) and ligaments surrounded by bone, such as the Lisfranc ligament, cannot be completely seen on ultrasonography.
Muscle
Musculoskeletal ultrasonography is useful for small areas of concern within a muscle (Table 1). It can detect muscle strains and tears, intramuscular collections or lesions, and fascial scarring or fascial injuries such as superficial muscle herniation. Although ultrasonography may yield a definitive diagnosis for a muscle problem, further imaging may be needed.
Nerves
Ultrasonography is useful for peripheral nerve investigation but requires a steep learning curve for sonographers and interpreting physicians.58,59 It is best suited for directed questions regarding focal abnormal nerve findings on physical examination.
Ultrasonography can help identify areas of nerve entrapment caused by a mass or dynamic compression. It can detect neuritis (Table 1), lesions of peripheral nerves (eg, nerve-sheath tumors), and neuromas (eg, Morton neuroma of the intermetatarsal space). In a large meta-analysis, ultrasonography and MRI were found to be equally accurate for detecting Morton neuroma.60 Even for nerve-sheath tumors located deep to the muscular fascia, ultrasonography can confirm the diagnosis because of the characteristic appearance of the nerves. Ultrasonography can also demonstrate a large extent of the course of superficial peripheral nerves while keeping the imaging plane appropriately oriented to the nerves.
Acknowledgment: We would like to sincerely thank Megan Griffiths, MA, for her help in the preparation and submission of this manuscript.
- Hamilton JV, Flinn G Jr, Haynie CC, Cefalo RC. Diagnosis of rectus sheath hematoma by B-mode ultrasound: a case report. Am J Obstet Gynecol 1976; 125(4):562–565. doi:10.1016/0002-9378(76)90379-3
- Zweymüller VK, Kratochwil A. Ultrasound diagnosis of bone and soft tissue tumours. Wien Klin Wochenschr 1975; 87(12):397–398. German.
- Mayer V. Ultrasonography of the rotator cuff. J Ultrasound Med 1985; 4(11):608, 607. doi:10.7863/jum.1985.4.11.608
- McNally EG. The development and clinical applications of musculoskeletal ultrasound. Skeletal Radiol 2011; 40(9):1223–1231. doi:10.1007/s00256-011-1220-5
- Ignashin NS, Girshin SG, Tsypin IS. Ultrasonic scanning in subcutaneous rupture of the Achilles tendon. Vestn Khir Im I I Grek 1981; 127(9):82–85. Russian.
- Robinson P. Sonography of common tendon injuries. AJR Am J Roentgenol 2009; 193(3):607–618. doi:10.2214/AJR.09.2808
- Jacobson JA. Musculoskeletal ultrasound: focused impact on MRI. AJR Am J Roentgenol 2009; 193(3):619–627. doi:10.2214/AJR.09.2841
- Nazarian LN. The top 10 reasons musculoskeletal sonography is an important complementary or alternative technique to MRI. AJR Am J Roentgenol 2008; 190(6):1621–1626. doi:10.2214/AJR.07.3385
- AIUM technical bulletin. Transducer manipulation. American Institute of Ultrasound in Medicine. J Ultrasound Med 1999; 18(2):169–175. doi:10.7863/jum.1999.18.2.169
- Connolly DJ, Berman L, McNally EG. The use of beam angulation to overcome anisotropy when viewing human tendon with high frequency linear array ultrasound. Br J Radiol 2001; 74 (878):183–185. doi:10.1259/bjr.74.878.740183
- Crass JR, van de Vegte GL, Harkavy LA. Tendon echogenicity: ex vivo study. Radiology 1988; 167(2):499–501. doi:10.1148/radiology.167.2.3282264
- Erickson SJ. High-resolution imaging of the musculoskeletal system. Radiology 1997; 205(3):593–618. doi:10.1148/radiology.205.3.9393511
- Link TM, Majumdar S, Peterfy C, et al. High resolution MRI of small joints: impact of spatial resolution on diagnostic performance and SNR. Magn Reson Imaging 1998; 16(2):147–155. doi:10.1016/S0730-725X(97)00244-0
- Middleton WD, Payne WT, Teefey SA, Hildebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol 2004; 183(5):1449–1452. doi:10.2214/ajr.183.5.1831449
- Khoury V, Cardinal E, Bureau NJ. Musculoskeletal sonography: a dynamic tool for usual and unusual disorders. AJR Am J Roentgenol 2007; 188(1):W63–W73. doi:10.2214/AJR.06.0579
- Farin PU, Jaroma H, Harju A, Soimakallio S. Medial displacement of the biceps brachii tendon: evaluation with dynamic sonography during maximal external shoulder rotation. Radiology 1995; 195(3):845–848. doi:10.1148/radiology.195.3.7754019
- Miller TT, Adler RS, Friedman L. Sonography of injury of the ulnar collateral ligament of the elbow-initial experience. Skeletal Radiol 2004; 33(7):386–391. doi:10.1007/s00256-004-0788-4
- Nazarian LN, McShane JM, Ciccotti MG, O’Kane PL, Harwood MI. Dynamic US of the anterior band of the ulnar collateral ligament of the elbow in asymptomatic major league baseball pitchers. Radiology 2003; 227(1):149–154. doi:10.1148/radiol.2271020288
- Jacobson JA, Lax MJ. Musculoskeletal sonography of the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6(1):67–77. doi:10.1055/s-2002-23165
- Sofka CM, Adler RS. Original report. Sonographic evaluation of shoulder arthroplasty. AJR Am J Roentgenol 2003; 180(4):1117–1120. doi:10.2214/ajr.180.4.1801117
- Silvestri E, Martinoli C, Derchi LE, Bertolotto M, Chiaramondia M, Rosenberg I. Echotexture of peripheral nerves: correlation between US and histologic findings and criteria to differentiate tendons. Radiology 1995; 197(1):291–296. doi:10.1148/radiology.197.1.7568840
- Cardinal E, Buckwalter KA, Braunstein EM, Mih AD. Occult dorsal carpal ganglion: comparison of US and MR imaging. Radiology 1994; 193(1):259–262. doi:10.1148/radiology.193.1.8090903
- Jacobson JA. Musculoskeletal ultrasound and MRI: which do I choose? Semin Musculoskelet Radiol 2005; 9(2):135–149. doi:10.1055/s-2005-872339
- Ward EE, Jacobson JA, Fessell DP, Hayes CW, van Holsbeeck M. Sonographic detection of Baker’s cysts: comparison with MR imaging. AJR Am J Roentgenol 2001; 176(2):373–380. doi:10.2214/ajr.176.2.1760373
- Bhasin S, Cheung PP. The role of power Doppler ultrasonography as disease activity marker in rheumatoid arthritis. Dis Markers 2015; 2015:325909. doi:10.1155/2015/325909
- Fukuba E, Yoshizako T, Kitagaki H, Murakawa Y, Kondo M, Uchida N. Power Doppler ultrasonography for assessment of rheumatoid synovitis: comparison with dynamic magnetic resonance imaging. Clin Imaging 2013; 37(1):134–137. doi:10.1016/j.clinimag.2012.02.008
- Takase-Minegishi K, Horita N, Kobayashi K, et al. Diagnostic test accuracy of ultrasound for synovitis in rheumatoid arthritis: systematic review and meta-analysis. Rheumatology (Oxford) 2018; 57(1):49–58. doi:10.1093/rheumatology/kex036
- Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet 2009; 373(9664):659–672. doi:10.1016/S0140-6736(09)60008-8
- Ash ZR, Tinazzi I, Gallego CC, et al. Psoriasis patients with nail disease have a greater magnitude of underlying systemic subclinical enthesopathy than those with normal nails. Ann Rheum Dis 2012; 71(4):553–556. doi:10.1136/annrheumdis-2011-200478
- Han J, Geng Y, Deng X, Zhang Z. Subclinical synovitis assessed by ultrasound predicts flare and progressive bone erosion in rheumatoid arthritis patients with clinical remission: a systematic review and metaanalysis. J Rheumatol 2016; 43(11):2010–2018. doi.org/10.3899/jrheum.160193
- Iagnocco A, Finucci A, Ceccarelli F, Perricone C, Iorgoveanu V, Valesini G. Power Doppler ultrasound monitoring of response to anti-tumour necrosis factor alpha treatment in patients with rheumatoid arthritis. Rheumatology (Oxford) 2015; 54(10):1890–1896. doi:10.1093/rheumatology/kev211
- Henning PT. Ultrasound-guided foot and ankle procedures. Phys Med Rehabil Clin N Am 2016; 27(3):649–671. doi:10.1016/j.pmr.2016.04.005
- Lueders DR, Smith J, Sellon JL. Ultrasound-guided knee procedures. Phys Med Rehabil Clin North Am 2016; 27(3):631–648. doi:10.1016/j.pmr.2016.04.010
- Payne JM. Ultrasound-guided hip procedures. Phys Med Rehabil Clin North Am 2016; 27(3):607–629. doi:10.1016/j.pmr.2016.04.004
- Strakowski JA. Ultrasound-guided peripheral nerve procedures. Phys Med Rehabil Clin North Am 2016; 27(3):687–715. doi:10.1016/j.pmr.2016.04.006
- Sussman WI, Williams CJ, Mautner K. Ultrasound-guided elbow procedures. Phys Med Rehabil Clin North Am 2016; 27(3):573–587. doi:10.1016/j.pmr.2016.04.002
- Finnoff JT. The evolution of diagnostic and interventional ultrasound in sports medicine. PM R 2016; 8(suppl 3):S133–S138. doi:10.1016/j.pmrj.2015.09.022
- Wu T, Dong Y, Song H, Fu Y, Li JH. Ultrasound-guided versus landmark in knee arthrocentesis: a systematic review. Semin Arthritis Rheum 2016; 45(5):627–632. doi:10.1016/j.semarthrit.2015.10.011
- Failla JM, van Holsbeeck M, Vanderschueren G. Detection of a 0.5-mm-thick thorn using ultrasound: a case report. J Hand Surg Am 1995; 20(3):456–457.
- Teefey SA, Hasan SA, Middleton WD, Patel M, Wright RW, Yamaguchi K. Ultrasonography of the rotator cuff. A comparison of ultrasonographic and arthroscopic findings in one hundred consecutive cases. J Bone Joint Surg Am 2000; 82(4):498–504.
- van Holsbeeck MT, Kolowich PA, Eyler WR, et al. US depiction of partial-thickness tear of the rotator cuff. Radiology 1995; 197(2):443–446. doi:10.1148/radiology.197.2.7480690
- Balich SM, Sheley RC, Brown TR, Sauser DD, Quinn SF. MR imaging of the rotator cuff tendon: interobserver agreement and analysis of interpretive errors. Radiology 1997; 204(1):191–194. doi:10.1148/radiology.204.1.9205245
- Dinnes J, Loveman E, McIntyre L, Waugh N. The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review. Health Technol Assess 2003; 7(29):1–166. doi:10.3310/hta7290
- Rockett MS, Waitches G, Sudakoff G, Brage M. Use of ultrasonography versus magnetic resonance imaging for tendon abnormalities around the ankle. Foot Ankle Int 1998; 19(9):604–612.
- Grant TH, Kelikian AS, Jereb SE, McCarthy RJ. Ultrasound diagnosis of peroneal tendon tears. A surgical correlation. J Bone Joint Surg Am 2005; 87(8):1788–1794. doi:10.2106/JBJS.D.02450
- Hartgerink P, Fessell DP, Jacobson JA, van Holsbeeck MT. Full- versus partial-thickness Achilles tendon tears: sonographic accuracy and characterization in 26 cases with surgical correlation. Radiology 2001; 220(2):406–412. doi:10.1148/radiology.220.2.r01au41406
- Cho KH, Park BH, Yeon KM. Ultrasound of the adult hip. Semin Ultrasound CT MR 2000; 21(3):214–230.
- Adler RS, Finzel KC. The complementary roles of MR imaging and ultrasound of tendons. Radiol Clin North Am 2005; 43(4):771–807. doi:10.1016/j.rcl.2005.02.011
- Martinoli C, Bianchi S, Derchi LE. Tendon and nerve sonography. Radiol Clin North Am 1999; 37(4):691–711. doi:10.1016/S0033-8389(05)70124-X
- Fessell DP, Vanderschueren GM, Jacobson JA, et al. US of the ankle: technique, anatomy, and diagnosis of pathologic conditions. Radiographics 1998; 18(2):325–340. doi:10.1148/radiographics.18.2.9536481
- Neustadter J, Raikin SM, Nazarian LN. Dynamic sonographic evaluation of peroneal tendon subluxation. AJR Am J Roentgenol 2004; 183(4):985–988. doi:10.2214/ajr.183.4.1830985
- Verhaven EF, Shahabpour M, Handelberg FW, Vaes PH, Opdecam PJ. The accuracy of three-dimensional magnetic resonance imaging in the diagnosis of ruptures of the lateral ligaments of the ankle. Am J Sports Med 1991; 19(6):583–587. doi:10.1177/036354659101900605
- Milz P, Milz S, Steinborn M, Mittlmeier T, Putz R, Reiser M. Lateral ankle ligaments and tibiofibular syndesmosis. 13-MHz high-frequency sonography and MRI compared in 20 patients. Acta Orthop Scand 1998; 69(1):51–55.
- De Smet AA, Winter TC, Best TM, Bernhardt DT. Dynamic sonography with valgus stress to assess elbow ulnar collateral ligament injury in baseball pitchers. Skeletal Radiol 2002; 31(11):671–676. doi:10.1007/s00256-002-0558-0
- Melville DM, Jacobson JA, Fessell DP. Ultrasound of the thumb ulnar collateral ligament: technique and pathology. AJR Am J Roentgenol 2014; 202(2):W168. doi:10.2214/AJR.13.11335
- Court-Payen M. Sonography of the knee: intra-articular pathology. J Clin Ultrasound 2004; 32(9):481–490. doi:10.1002/jcu.20069
- Azzoni R, Cabitza P. Is there a role for sonography in the diagnosis of tears of the knee menisci? J Clin Ultrasound 2002; 30(8):472–476. doi:10.1002/jcu.10106
- Jacobson JA, Wilson TJ, Yang LJ. Sonography of common peripheral nerve disorders with clinical correlation. J Ultrasound Med 2016; 35(4):683–693. doi:10.7863/ultra.15.05061
- Ali ZS, Pisapia JM, Ma TS, Zager EL, Heuer GG, Khoury V. Ultrasonographic evaluation of peripheral nerves. World Neurosurg 2016; 85(1):333–339. doi:10.1016/j.wneu.2015.10.005
- Bignotti B, Signori A, Sormani MP, Molfetta L, Martinoli C, Tagliafico A. Ultrasound versus magnetic resonance imaging for Morton neuroma: systematic review and meta-analysis. Eur Radiol 2015; 25(8):2254–2262. doi:10.1007/s00330-015-3633-3
Ultrasonography has been used to evaluate musculoskeletal problems for decades but has only recently become more widely available in the United States. Advances in technology and physician familiarity are increasing its role in orthopedic imaging.
No single imaging method can yield all musculoskeletal diagnoses. Like any imaging technique, ultrasonography has strengths and weaknesses specific to orthopedics. Radiography, computed tomography (CT), and magnetic resonance imaging (MRI) play important roles for investigating musculoskeletal problems and are complementary to each other and to ultrasonography.
To help clinicians make informed decisions about ordering musculoskeletal ultrasonography, this article reviews the basic physics underlying ultrasonography, its advantages and disadvantages compared with other imaging methods, and common clinical applications.
CLASSIC TECHNOLOGY MAKING A RESURGENCE
The first reports of the use of musculoskeletal ultrasonography appeared in the 1970s for investigating the rotator cuff,1–3 actually preceding reports of its use in obstetrics and gynecology.4 In the 1980s, reports emerged for evaluating the Achilles tendon.5,6 After that, its popularity in the United States plateaued, likely because of the advent of MRI, lower reimbursement and greater variability in interpretation compared with MRI, as well as a lack of physicians and sonographers trained in its use.7,8
Musculoskeletal ultrasonography is currently experiencing a resurgence. Although it remains a specialized service more commonly available in large hospitals, its use is increasing rapidly, and it will likely become more widely available.
SPECIAL TRAINING REQUIRED
Musculoskeletal ultrasonography is simply an ultrasonographic examination of part of the musculoskeletal system. But because not all ultrasonographic transducers offer sufficient resolution for musculoskeletal evaluation and not all sonographers and imaging physicians are familiar with the specialized techniques, musculoskeletal ultrasonography often has a separate designation (eg, “MSKUS,” “MSUS”). At Cleveland Clinic, it is offered through the department of musculoskeletal imaging by subspecialty-trained musculoskeletal radiologists and specially trained musculoskeletal ultrasonographers with 4 to 5 years of training in the technique.
Musculoskeletal ultrasonography is also performed by physician groups with specialized training, including sports medicine physicians, rheumatologists, physiatrists, neurologists, and orthopedic surgeons. The American Institute of Ultrasound in Medicine offers voluntary accreditation for practice groups using musculoskeletal ultrasonography. Certification in musculoskeletal radiology is offered to sonographers through the American Registry for Diagnostic Medical Sonography.
SONOGRAPHY HAS UNIQUE QUALITIES
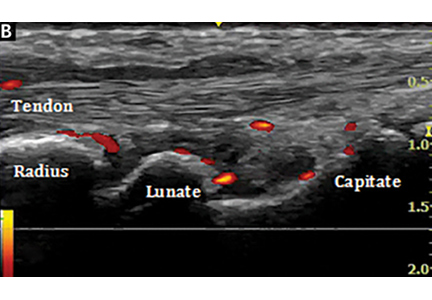
Ultrasonography uses high-frequency sound waves to generate images. The transducer (or probe) emits sound from the many piezoelectric elements at its surface, and the sound waves travel through and react with tissues. Sound reflected by tissues is detected by the transducer and converted to an image. Objects that reflect sound appear hyperechoic (brighter), whereas tissues that reflect little or no sound appear hypoechoic.
High-resolution imaging of superficial structures
(B, arrow).
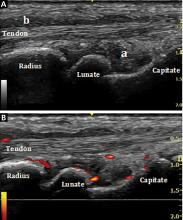
Ultrasonography involves a fundamental trade-off between image resolution and imaging depth. Higher-frequency sound waves do not penetrate far into tissues but generate a higher-resolution image; lower-frequency sound waves can penetrate much further but yield a lower-resolution image. Although high-resolution imaging of deep structures with ultrasonography is not possible (Figure 1), many musculoskeletal structures are located superficially and are amenable to ultrasonographic evaluation.
Be aware of artifacts
Some materials attenuate sound very little, such as simple fluid. Low attenuation results in artifacts on ultrasonography, making tissues behind the simple fluid appear brighter than neighboring tissues. These artifacts may be reported as “increased through transmission” or “posterior acoustic enhancement.” Conversely, metal and bone reflect all sound waves that reach them, rendering any structures beyond them invisible. This “shadowing” creates a problem for imaging of structures in or near bone. Subcutaneous fat also attenuates sound waves, limiting the use of ultrasonography for patients with obesity (Figure 2).
High-frequency linear transducer sharpens images
High-frequency linear transducers reduce anisotropy because their flat surface keeps sound waves more uniformly perpendicular to the structure of interest.4,7 Their development has allowed imaging of superficial structures that is superior to that of MRI. A high-frequency linear transducer offers more than twice the spatial resolution of a typical 1.5T MRI examination of superficial tissue.12,13
Operator experience is critical
Ultrasonography examinations, more than other imaging tests, are dependent on operator experience. A solid understanding of musculoskeletal anatomy is imperative. Because the probe images only a thin section of tissue (about the thickness of a credit card), referencing adjacent structures for orientation is more difficult with ultrasonography than with CT or MRI.
The accuracy of ultrasonography is highly dependent on acquiring and interpreting images, whereas the accuracy of MRI is dependent primarily on image interpretation.7 Interpreting physicians must check that sonographers capture relevant targets.
STRENGTHS OF MUSCULOSKELETAL ULTRASONOGRAPHY
Ultrasonography has multiple advantages:
No ionizing radiation exposure.
Portability. Unlike CT or MRI, ultrasonography equipment is portable.
Increased patient comfort. Patient positioning for an ultrasonography examination is more flexible than for MRI or CT,14 and the examination does not induce claustrophobia.8
High-resolution imaging. Ultrasonography provides very-high-resolution imaging of superficial soft tissues—in some cases, higher than MRI or CT.
Real-time dynamic examinations are possible with ultrasonography, unlike with CT or MRI, and may increase test sensitivity.4,15–18
Implanted hardware is less of a problem. Although ultrasonography cannot image beyond implanted orthopedic metallic hardware, the hardware does not obscure surrounding soft tissues as it does on CT and MRI.6,19,20 Also, ultrasonography is safe for patients with a pacemaker.8
WEAKNESSES
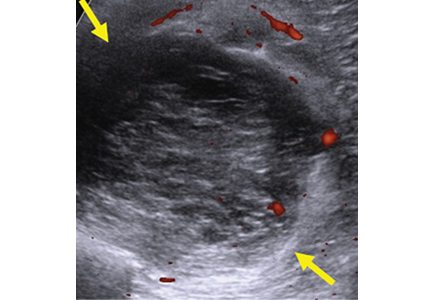
The main disadvantages of musculoskeletal ultrasonography are inherent to its limited field of view, making it inappropriate for a survey examination (eg, for ankle pain, knee pain, hip pain).4 Unlike CT and MRI, ultrasonography does not provide a “bird’s-eye view,” and important abnormalities can be missed during evaluation of large areas (Figure 4).
Ultrasonography also cannot evaluate bone or intra-articular structures such as cartilage, bone marrow, labrum, and intra-articular ligaments; MRI is the standard for evaluating these structures.21
Ultrasonography is time-consuming. To perform a detailed examination of the anterior, posterior, medial, and lateral aspects of the hip, knee, or ankle would require 1.5 to 2 hours of scanning time and an additional 10 to 25 minutes of image checking and interpretation.
CURRENT CLINICAL INDICATIONS
Musculoskeletal ultrasonography is best used for clinical questions regarding limited, superficial musculoskeletal problems.
Fluid collections
Ultrasonography can help evaluate small fluid collections in soft tissue. As is true for a lung opacity on chest radiography, soft-tissue fluid detected on ultrasonography is nonspecific, and results must be correlated with the clinical picture to narrow the differential diagnosis.
Fluid collections can be classified as loculated or nonloculated.
Nonloculated fluid involves more fluid than is simply interposed between tissue planes and has no wall or defined margins. It can be simple or complex in appearance: simple fluid is anechoic, and complex fluid appears more heterogeneous and may contain septations or debris.
Subcutaneous edema, which may occur postoperatively or from trauma, venous insufficiency, or inflammatory or infectious processes, appears on ultrasonography as nonloculated fluid interspersed between subcutaneous fat lobules.
Loculated fluid collections have well-defined margins or a discrete wall that does not follow normal tissue planes. They can also be simple or complex and can be caused by hematoma, abscess, or ganglion. Less commonly, neoplasms can mimic a loculated fluid collection (Figure 4).
A ganglion is a specific type of loculated fluid collection containing synovial fluid arising from a joint or tendon sheath. It tends to occur in specific locations, most commonly around the wrist, most often arising from the dorsal scapholunate ligament and volar wrist between the radial artery and flexor carpi radialis.22 On MRI, it can be difficult to distinguish between small vascular structures and a small ganglion, especially in the hands and feet.23
Ultrasonography can also help identify a Baker cyst, a specific fluid collection arising from the semimembranosus bursa between the medial head of the gastrocnemius tendon and the semimembranosus tendon. Ultrasonography can also detect inflammation, rupture, or leaking associated with a Baker cyst.24
Power Doppler is an ultrasonographic examination that can detect increased blood flow surrounding a fluid collection and determine the likelihood of an acute inflammatory or infectious cause.25
Joint effusion and synovitis
Musculoskeletal ultrasonography can help evaluate joints for effusion and synovitis. It is highly sensitive (94%) and specific (95%) for synovitis, making it superior to contrast-enhanced MRI.26,27 The area of concern should be limited to 1 quadrant of a joint (anterior, posterior, medial, or lateral); for problems beyond that, MRI should be considered.
A joint effusion appears as a distended joint capsule containing hypoechoic (complex) or anechoic (simple) joint fluid.
Complex joint fluid may contain debris and occurs with hemarthrosis, infection, and inflammation.23 Hypertrophied synovium is hypoechoic and can mimic complex joint fluid.
Power Doppler evaluation can help distinguish synovitis from joint fluid by demonstrating blood flow, a feature of synovitis but not of simple joint fluid. Power Doppler is the most sensitive means of detecting blood flow, although it does not show direction of flow.28
Using ultrasonography can help to improve disease control and minimize disabling changes by monitoring synovitis therapy. In addition, subclinical synovitis and enthesitis (inflammation of insertion sites of tendons or ligaments into bone) detected by ultrasonography may predict future disease and disease flares.29–31
Ultrasonographic guidance for a wide range of procedures is increasing rapidly.32–36 Multiple studies have shown the advantage of ultrasonography-guided aspiration and injection compared with techniques without imaging guidance.37,38
Soft-tissue masses
Accurately diagnosing soft-tissue masses can be difficult. A mass may remain indeterminate even after multiple imaging studies, requiring biopsy or surgical referral. However, for a few specific masses, ultrasonography is highly accurate and can eliminate the need for further imaging.
Ultrasonography can help evaluate soft- tissue masses no larger than 5 cm in diameter and no deeper than superficial muscular fascia. If the mass is larger or deeper than that, ultrasonography is less reliable for showing the margins of the mass and its relationship to adjacent structures (Figure 5). Further imaging by MRI may be recommended in such cases.
Fortunately, many of the most common soft-tissue masses can be accurately diagnosed with ultrasonography, including lipomas, ganglion cysts, foreign bodies, and simple fluid collections.4,39 Nerve-sheath tumors can also be diagnosed with ultrasonography if the lesion clearly arises from a nerve. Other soft-tissue masses are likely to be indeterminate with ultrasonography, requiring follow-up with MRI with contrast.
Tendons
Musculoskeletal ultrasonography can be effective for evaluating tendons around joints, especially 1 or a small number of nearby superficial tendons. Tendons particularly well suited for ultrasonographic examination include:
- Upper-extremity tendons located in the rotator cuff or around the elbow, and flexor and extensor tendons of the hands; ultrasonographic evaluation of the rotator cuff is highly accurate, equivalent to that of MRI for partial-thickness and full-thickness tearing40–43
- Lower-extremity tendons of the extensor mechanism of the knee, distal hamstring tendons, tendons around the ankle,44–46 and flexor and extensor tendons of the foot.
Ultrasonography can help diagnose a variety of tendon abnormalities (Table 1),48,49 including tearing, for which a dynamic examination can be performed.
Many tendons have a tendon sheath containing tenosynovium, while others have surrounding peritenon only; either can become thickened and inflamed. Tenosynovitis is a nonspecific finding and may be inflammatory, infectious, or posttraumatic. The presence of tendon sheath fluid alone on ultrasonography can be a normal finding, and some tendon sheaths that communicate with adjacent joints (eg, the long head biceps tendon, the flexor hallucis longus tendon) commonly contain simple fluid.6 A dynamic examination with ultrasonography can help diagnose snapping related to abnormal tendon movement, for example, in the case of intra-sheath and extra-sheath subluxation of the peroneal tendons.45,50,51
Ligaments
Ultrasonography can detect abnormalities in many superficial ligaments (Table 1).
Ankle. Ankle ligaments are superficial and can be clearly visualized. The diagnostic accuracy of ultrasonography for tearing of the anterior talofibular ligament may be as high as 100%.50,52,53
Elbow and thumb. The larger of the collateral ligaments of the elbow, especially the ulnar collateral ligament, and the ulnar collateral ligament of the thumb can be effectively evaluated with ultrasonography.54,55
Knee. The collateral ligaments of the knee can be seen with ultrasonography, but injuries of the external ligaments of the knee are often associated with intrinsic derangements that cannot be evaluated with ultrasonography.56,57 Intra-articular ligaments such as the anterior cruciate ligament are also not amenable to ultrasonography.
Dynamic examination of a ligament with ultrasonography can help determine the grade of the injury.
Deeply located ligaments (eg, around the hip) and ligaments surrounded by bone, such as the Lisfranc ligament, cannot be completely seen on ultrasonography.
Muscle
Musculoskeletal ultrasonography is useful for small areas of concern within a muscle (Table 1). It can detect muscle strains and tears, intramuscular collections or lesions, and fascial scarring or fascial injuries such as superficial muscle herniation. Although ultrasonography may yield a definitive diagnosis for a muscle problem, further imaging may be needed.
Nerves
Ultrasonography is useful for peripheral nerve investigation but requires a steep learning curve for sonographers and interpreting physicians.58,59 It is best suited for directed questions regarding focal abnormal nerve findings on physical examination.
Ultrasonography can help identify areas of nerve entrapment caused by a mass or dynamic compression. It can detect neuritis (Table 1), lesions of peripheral nerves (eg, nerve-sheath tumors), and neuromas (eg, Morton neuroma of the intermetatarsal space). In a large meta-analysis, ultrasonography and MRI were found to be equally accurate for detecting Morton neuroma.60 Even for nerve-sheath tumors located deep to the muscular fascia, ultrasonography can confirm the diagnosis because of the characteristic appearance of the nerves. Ultrasonography can also demonstrate a large extent of the course of superficial peripheral nerves while keeping the imaging plane appropriately oriented to the nerves.
Acknowledgment: We would like to sincerely thank Megan Griffiths, MA, for her help in the preparation and submission of this manuscript.
Ultrasonography has been used to evaluate musculoskeletal problems for decades but has only recently become more widely available in the United States. Advances in technology and physician familiarity are increasing its role in orthopedic imaging.
No single imaging method can yield all musculoskeletal diagnoses. Like any imaging technique, ultrasonography has strengths and weaknesses specific to orthopedics. Radiography, computed tomography (CT), and magnetic resonance imaging (MRI) play important roles for investigating musculoskeletal problems and are complementary to each other and to ultrasonography.
To help clinicians make informed decisions about ordering musculoskeletal ultrasonography, this article reviews the basic physics underlying ultrasonography, its advantages and disadvantages compared with other imaging methods, and common clinical applications.
CLASSIC TECHNOLOGY MAKING A RESURGENCE
The first reports of the use of musculoskeletal ultrasonography appeared in the 1970s for investigating the rotator cuff,1–3 actually preceding reports of its use in obstetrics and gynecology.4 In the 1980s, reports emerged for evaluating the Achilles tendon.5,6 After that, its popularity in the United States plateaued, likely because of the advent of MRI, lower reimbursement and greater variability in interpretation compared with MRI, as well as a lack of physicians and sonographers trained in its use.7,8
Musculoskeletal ultrasonography is currently experiencing a resurgence. Although it remains a specialized service more commonly available in large hospitals, its use is increasing rapidly, and it will likely become more widely available.
SPECIAL TRAINING REQUIRED
Musculoskeletal ultrasonography is simply an ultrasonographic examination of part of the musculoskeletal system. But because not all ultrasonographic transducers offer sufficient resolution for musculoskeletal evaluation and not all sonographers and imaging physicians are familiar with the specialized techniques, musculoskeletal ultrasonography often has a separate designation (eg, “MSKUS,” “MSUS”). At Cleveland Clinic, it is offered through the department of musculoskeletal imaging by subspecialty-trained musculoskeletal radiologists and specially trained musculoskeletal ultrasonographers with 4 to 5 years of training in the technique.
Musculoskeletal ultrasonography is also performed by physician groups with specialized training, including sports medicine physicians, rheumatologists, physiatrists, neurologists, and orthopedic surgeons. The American Institute of Ultrasound in Medicine offers voluntary accreditation for practice groups using musculoskeletal ultrasonography. Certification in musculoskeletal radiology is offered to sonographers through the American Registry for Diagnostic Medical Sonography.
SONOGRAPHY HAS UNIQUE QUALITIES
Ultrasonography uses high-frequency sound waves to generate images. The transducer (or probe) emits sound from the many piezoelectric elements at its surface, and the sound waves travel through and react with tissues. Sound reflected by tissues is detected by the transducer and converted to an image. Objects that reflect sound appear hyperechoic (brighter), whereas tissues that reflect little or no sound appear hypoechoic.
High-resolution imaging of superficial structures
(B, arrow).
Ultrasonography involves a fundamental trade-off between image resolution and imaging depth. Higher-frequency sound waves do not penetrate far into tissues but generate a higher-resolution image; lower-frequency sound waves can penetrate much further but yield a lower-resolution image. Although high-resolution imaging of deep structures with ultrasonography is not possible (Figure 1), many musculoskeletal structures are located superficially and are amenable to ultrasonographic evaluation.
Be aware of artifacts
Some materials attenuate sound very little, such as simple fluid. Low attenuation results in artifacts on ultrasonography, making tissues behind the simple fluid appear brighter than neighboring tissues. These artifacts may be reported as “increased through transmission” or “posterior acoustic enhancement.” Conversely, metal and bone reflect all sound waves that reach them, rendering any structures beyond them invisible. This “shadowing” creates a problem for imaging of structures in or near bone. Subcutaneous fat also attenuates sound waves, limiting the use of ultrasonography for patients with obesity (Figure 2).
High-frequency linear transducer sharpens images
High-frequency linear transducers reduce anisotropy because their flat surface keeps sound waves more uniformly perpendicular to the structure of interest.4,7 Their development has allowed imaging of superficial structures that is superior to that of MRI. A high-frequency linear transducer offers more than twice the spatial resolution of a typical 1.5T MRI examination of superficial tissue.12,13
Operator experience is critical
Ultrasonography examinations, more than other imaging tests, are dependent on operator experience. A solid understanding of musculoskeletal anatomy is imperative. Because the probe images only a thin section of tissue (about the thickness of a credit card), referencing adjacent structures for orientation is more difficult with ultrasonography than with CT or MRI.
The accuracy of ultrasonography is highly dependent on acquiring and interpreting images, whereas the accuracy of MRI is dependent primarily on image interpretation.7 Interpreting physicians must check that sonographers capture relevant targets.
STRENGTHS OF MUSCULOSKELETAL ULTRASONOGRAPHY
Ultrasonography has multiple advantages:
No ionizing radiation exposure.
Portability. Unlike CT or MRI, ultrasonography equipment is portable.
Increased patient comfort. Patient positioning for an ultrasonography examination is more flexible than for MRI or CT,14 and the examination does not induce claustrophobia.8
High-resolution imaging. Ultrasonography provides very-high-resolution imaging of superficial soft tissues—in some cases, higher than MRI or CT.
Real-time dynamic examinations are possible with ultrasonography, unlike with CT or MRI, and may increase test sensitivity.4,15–18
Implanted hardware is less of a problem. Although ultrasonography cannot image beyond implanted orthopedic metallic hardware, the hardware does not obscure surrounding soft tissues as it does on CT and MRI.6,19,20 Also, ultrasonography is safe for patients with a pacemaker.8
WEAKNESSES
The main disadvantages of musculoskeletal ultrasonography are inherent to its limited field of view, making it inappropriate for a survey examination (eg, for ankle pain, knee pain, hip pain).4 Unlike CT and MRI, ultrasonography does not provide a “bird’s-eye view,” and important abnormalities can be missed during evaluation of large areas (Figure 4).
Ultrasonography also cannot evaluate bone or intra-articular structures such as cartilage, bone marrow, labrum, and intra-articular ligaments; MRI is the standard for evaluating these structures.21
Ultrasonography is time-consuming. To perform a detailed examination of the anterior, posterior, medial, and lateral aspects of the hip, knee, or ankle would require 1.5 to 2 hours of scanning time and an additional 10 to 25 minutes of image checking and interpretation.
CURRENT CLINICAL INDICATIONS
Musculoskeletal ultrasonography is best used for clinical questions regarding limited, superficial musculoskeletal problems.
Fluid collections
Ultrasonography can help evaluate small fluid collections in soft tissue. As is true for a lung opacity on chest radiography, soft-tissue fluid detected on ultrasonography is nonspecific, and results must be correlated with the clinical picture to narrow the differential diagnosis.
Fluid collections can be classified as loculated or nonloculated.
Nonloculated fluid involves more fluid than is simply interposed between tissue planes and has no wall or defined margins. It can be simple or complex in appearance: simple fluid is anechoic, and complex fluid appears more heterogeneous and may contain septations or debris.
Subcutaneous edema, which may occur postoperatively or from trauma, venous insufficiency, or inflammatory or infectious processes, appears on ultrasonography as nonloculated fluid interspersed between subcutaneous fat lobules.
Loculated fluid collections have well-defined margins or a discrete wall that does not follow normal tissue planes. They can also be simple or complex and can be caused by hematoma, abscess, or ganglion. Less commonly, neoplasms can mimic a loculated fluid collection (Figure 4).
A ganglion is a specific type of loculated fluid collection containing synovial fluid arising from a joint or tendon sheath. It tends to occur in specific locations, most commonly around the wrist, most often arising from the dorsal scapholunate ligament and volar wrist between the radial artery and flexor carpi radialis.22 On MRI, it can be difficult to distinguish between small vascular structures and a small ganglion, especially in the hands and feet.23
Ultrasonography can also help identify a Baker cyst, a specific fluid collection arising from the semimembranosus bursa between the medial head of the gastrocnemius tendon and the semimembranosus tendon. Ultrasonography can also detect inflammation, rupture, or leaking associated with a Baker cyst.24
Power Doppler is an ultrasonographic examination that can detect increased blood flow surrounding a fluid collection and determine the likelihood of an acute inflammatory or infectious cause.25
Joint effusion and synovitis
Musculoskeletal ultrasonography can help evaluate joints for effusion and synovitis. It is highly sensitive (94%) and specific (95%) for synovitis, making it superior to contrast-enhanced MRI.26,27 The area of concern should be limited to 1 quadrant of a joint (anterior, posterior, medial, or lateral); for problems beyond that, MRI should be considered.
A joint effusion appears as a distended joint capsule containing hypoechoic (complex) or anechoic (simple) joint fluid.
Complex joint fluid may contain debris and occurs with hemarthrosis, infection, and inflammation.23 Hypertrophied synovium is hypoechoic and can mimic complex joint fluid.
Power Doppler evaluation can help distinguish synovitis from joint fluid by demonstrating blood flow, a feature of synovitis but not of simple joint fluid. Power Doppler is the most sensitive means of detecting blood flow, although it does not show direction of flow.28
Using ultrasonography can help to improve disease control and minimize disabling changes by monitoring synovitis therapy. In addition, subclinical synovitis and enthesitis (inflammation of insertion sites of tendons or ligaments into bone) detected by ultrasonography may predict future disease and disease flares.29–31
Ultrasonographic guidance for a wide range of procedures is increasing rapidly.32–36 Multiple studies have shown the advantage of ultrasonography-guided aspiration and injection compared with techniques without imaging guidance.37,38
Soft-tissue masses
Accurately diagnosing soft-tissue masses can be difficult. A mass may remain indeterminate even after multiple imaging studies, requiring biopsy or surgical referral. However, for a few specific masses, ultrasonography is highly accurate and can eliminate the need for further imaging.
Ultrasonography can help evaluate soft- tissue masses no larger than 5 cm in diameter and no deeper than superficial muscular fascia. If the mass is larger or deeper than that, ultrasonography is less reliable for showing the margins of the mass and its relationship to adjacent structures (Figure 5). Further imaging by MRI may be recommended in such cases.
Fortunately, many of the most common soft-tissue masses can be accurately diagnosed with ultrasonography, including lipomas, ganglion cysts, foreign bodies, and simple fluid collections.4,39 Nerve-sheath tumors can also be diagnosed with ultrasonography if the lesion clearly arises from a nerve. Other soft-tissue masses are likely to be indeterminate with ultrasonography, requiring follow-up with MRI with contrast.
Tendons
Musculoskeletal ultrasonography can be effective for evaluating tendons around joints, especially 1 or a small number of nearby superficial tendons. Tendons particularly well suited for ultrasonographic examination include:
- Upper-extremity tendons located in the rotator cuff or around the elbow, and flexor and extensor tendons of the hands; ultrasonographic evaluation of the rotator cuff is highly accurate, equivalent to that of MRI for partial-thickness and full-thickness tearing40–43
- Lower-extremity tendons of the extensor mechanism of the knee, distal hamstring tendons, tendons around the ankle,44–46 and flexor and extensor tendons of the foot.
Ultrasonography can help diagnose a variety of tendon abnormalities (Table 1),48,49 including tearing, for which a dynamic examination can be performed.
Many tendons have a tendon sheath containing tenosynovium, while others have surrounding peritenon only; either can become thickened and inflamed. Tenosynovitis is a nonspecific finding and may be inflammatory, infectious, or posttraumatic. The presence of tendon sheath fluid alone on ultrasonography can be a normal finding, and some tendon sheaths that communicate with adjacent joints (eg, the long head biceps tendon, the flexor hallucis longus tendon) commonly contain simple fluid.6 A dynamic examination with ultrasonography can help diagnose snapping related to abnormal tendon movement, for example, in the case of intra-sheath and extra-sheath subluxation of the peroneal tendons.45,50,51
Ligaments
Ultrasonography can detect abnormalities in many superficial ligaments (Table 1).
Ankle. Ankle ligaments are superficial and can be clearly visualized. The diagnostic accuracy of ultrasonography for tearing of the anterior talofibular ligament may be as high as 100%.50,52,53
Elbow and thumb. The larger of the collateral ligaments of the elbow, especially the ulnar collateral ligament, and the ulnar collateral ligament of the thumb can be effectively evaluated with ultrasonography.54,55
Knee. The collateral ligaments of the knee can be seen with ultrasonography, but injuries of the external ligaments of the knee are often associated with intrinsic derangements that cannot be evaluated with ultrasonography.56,57 Intra-articular ligaments such as the anterior cruciate ligament are also not amenable to ultrasonography.
Dynamic examination of a ligament with ultrasonography can help determine the grade of the injury.
Deeply located ligaments (eg, around the hip) and ligaments surrounded by bone, such as the Lisfranc ligament, cannot be completely seen on ultrasonography.
Muscle
Musculoskeletal ultrasonography is useful for small areas of concern within a muscle (Table 1). It can detect muscle strains and tears, intramuscular collections or lesions, and fascial scarring or fascial injuries such as superficial muscle herniation. Although ultrasonography may yield a definitive diagnosis for a muscle problem, further imaging may be needed.
Nerves
Ultrasonography is useful for peripheral nerve investigation but requires a steep learning curve for sonographers and interpreting physicians.58,59 It is best suited for directed questions regarding focal abnormal nerve findings on physical examination.
Ultrasonography can help identify areas of nerve entrapment caused by a mass or dynamic compression. It can detect neuritis (Table 1), lesions of peripheral nerves (eg, nerve-sheath tumors), and neuromas (eg, Morton neuroma of the intermetatarsal space). In a large meta-analysis, ultrasonography and MRI were found to be equally accurate for detecting Morton neuroma.60 Even for nerve-sheath tumors located deep to the muscular fascia, ultrasonography can confirm the diagnosis because of the characteristic appearance of the nerves. Ultrasonography can also demonstrate a large extent of the course of superficial peripheral nerves while keeping the imaging plane appropriately oriented to the nerves.
Acknowledgment: We would like to sincerely thank Megan Griffiths, MA, for her help in the preparation and submission of this manuscript.
- Hamilton JV, Flinn G Jr, Haynie CC, Cefalo RC. Diagnosis of rectus sheath hematoma by B-mode ultrasound: a case report. Am J Obstet Gynecol 1976; 125(4):562–565. doi:10.1016/0002-9378(76)90379-3
- Zweymüller VK, Kratochwil A. Ultrasound diagnosis of bone and soft tissue tumours. Wien Klin Wochenschr 1975; 87(12):397–398. German.
- Mayer V. Ultrasonography of the rotator cuff. J Ultrasound Med 1985; 4(11):608, 607. doi:10.7863/jum.1985.4.11.608
- McNally EG. The development and clinical applications of musculoskeletal ultrasound. Skeletal Radiol 2011; 40(9):1223–1231. doi:10.1007/s00256-011-1220-5
- Ignashin NS, Girshin SG, Tsypin IS. Ultrasonic scanning in subcutaneous rupture of the Achilles tendon. Vestn Khir Im I I Grek 1981; 127(9):82–85. Russian.
- Robinson P. Sonography of common tendon injuries. AJR Am J Roentgenol 2009; 193(3):607–618. doi:10.2214/AJR.09.2808
- Jacobson JA. Musculoskeletal ultrasound: focused impact on MRI. AJR Am J Roentgenol 2009; 193(3):619–627. doi:10.2214/AJR.09.2841
- Nazarian LN. The top 10 reasons musculoskeletal sonography is an important complementary or alternative technique to MRI. AJR Am J Roentgenol 2008; 190(6):1621–1626. doi:10.2214/AJR.07.3385
- AIUM technical bulletin. Transducer manipulation. American Institute of Ultrasound in Medicine. J Ultrasound Med 1999; 18(2):169–175. doi:10.7863/jum.1999.18.2.169
- Connolly DJ, Berman L, McNally EG. The use of beam angulation to overcome anisotropy when viewing human tendon with high frequency linear array ultrasound. Br J Radiol 2001; 74 (878):183–185. doi:10.1259/bjr.74.878.740183
- Crass JR, van de Vegte GL, Harkavy LA. Tendon echogenicity: ex vivo study. Radiology 1988; 167(2):499–501. doi:10.1148/radiology.167.2.3282264
- Erickson SJ. High-resolution imaging of the musculoskeletal system. Radiology 1997; 205(3):593–618. doi:10.1148/radiology.205.3.9393511
- Link TM, Majumdar S, Peterfy C, et al. High resolution MRI of small joints: impact of spatial resolution on diagnostic performance and SNR. Magn Reson Imaging 1998; 16(2):147–155. doi:10.1016/S0730-725X(97)00244-0
- Middleton WD, Payne WT, Teefey SA, Hildebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol 2004; 183(5):1449–1452. doi:10.2214/ajr.183.5.1831449
- Khoury V, Cardinal E, Bureau NJ. Musculoskeletal sonography: a dynamic tool for usual and unusual disorders. AJR Am J Roentgenol 2007; 188(1):W63–W73. doi:10.2214/AJR.06.0579
- Farin PU, Jaroma H, Harju A, Soimakallio S. Medial displacement of the biceps brachii tendon: evaluation with dynamic sonography during maximal external shoulder rotation. Radiology 1995; 195(3):845–848. doi:10.1148/radiology.195.3.7754019
- Miller TT, Adler RS, Friedman L. Sonography of injury of the ulnar collateral ligament of the elbow-initial experience. Skeletal Radiol 2004; 33(7):386–391. doi:10.1007/s00256-004-0788-4
- Nazarian LN, McShane JM, Ciccotti MG, O’Kane PL, Harwood MI. Dynamic US of the anterior band of the ulnar collateral ligament of the elbow in asymptomatic major league baseball pitchers. Radiology 2003; 227(1):149–154. doi:10.1148/radiol.2271020288
- Jacobson JA, Lax MJ. Musculoskeletal sonography of the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6(1):67–77. doi:10.1055/s-2002-23165
- Sofka CM, Adler RS. Original report. Sonographic evaluation of shoulder arthroplasty. AJR Am J Roentgenol 2003; 180(4):1117–1120. doi:10.2214/ajr.180.4.1801117
- Silvestri E, Martinoli C, Derchi LE, Bertolotto M, Chiaramondia M, Rosenberg I. Echotexture of peripheral nerves: correlation between US and histologic findings and criteria to differentiate tendons. Radiology 1995; 197(1):291–296. doi:10.1148/radiology.197.1.7568840
- Cardinal E, Buckwalter KA, Braunstein EM, Mih AD. Occult dorsal carpal ganglion: comparison of US and MR imaging. Radiology 1994; 193(1):259–262. doi:10.1148/radiology.193.1.8090903
- Jacobson JA. Musculoskeletal ultrasound and MRI: which do I choose? Semin Musculoskelet Radiol 2005; 9(2):135–149. doi:10.1055/s-2005-872339
- Ward EE, Jacobson JA, Fessell DP, Hayes CW, van Holsbeeck M. Sonographic detection of Baker’s cysts: comparison with MR imaging. AJR Am J Roentgenol 2001; 176(2):373–380. doi:10.2214/ajr.176.2.1760373
- Bhasin S, Cheung PP. The role of power Doppler ultrasonography as disease activity marker in rheumatoid arthritis. Dis Markers 2015; 2015:325909. doi:10.1155/2015/325909
- Fukuba E, Yoshizako T, Kitagaki H, Murakawa Y, Kondo M, Uchida N. Power Doppler ultrasonography for assessment of rheumatoid synovitis: comparison with dynamic magnetic resonance imaging. Clin Imaging 2013; 37(1):134–137. doi:10.1016/j.clinimag.2012.02.008
- Takase-Minegishi K, Horita N, Kobayashi K, et al. Diagnostic test accuracy of ultrasound for synovitis in rheumatoid arthritis: systematic review and meta-analysis. Rheumatology (Oxford) 2018; 57(1):49–58. doi:10.1093/rheumatology/kex036
- Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet 2009; 373(9664):659–672. doi:10.1016/S0140-6736(09)60008-8
- Ash ZR, Tinazzi I, Gallego CC, et al. Psoriasis patients with nail disease have a greater magnitude of underlying systemic subclinical enthesopathy than those with normal nails. Ann Rheum Dis 2012; 71(4):553–556. doi:10.1136/annrheumdis-2011-200478
- Han J, Geng Y, Deng X, Zhang Z. Subclinical synovitis assessed by ultrasound predicts flare and progressive bone erosion in rheumatoid arthritis patients with clinical remission: a systematic review and metaanalysis. J Rheumatol 2016; 43(11):2010–2018. doi.org/10.3899/jrheum.160193
- Iagnocco A, Finucci A, Ceccarelli F, Perricone C, Iorgoveanu V, Valesini G. Power Doppler ultrasound monitoring of response to anti-tumour necrosis factor alpha treatment in patients with rheumatoid arthritis. Rheumatology (Oxford) 2015; 54(10):1890–1896. doi:10.1093/rheumatology/kev211
- Henning PT. Ultrasound-guided foot and ankle procedures. Phys Med Rehabil Clin N Am 2016; 27(3):649–671. doi:10.1016/j.pmr.2016.04.005
- Lueders DR, Smith J, Sellon JL. Ultrasound-guided knee procedures. Phys Med Rehabil Clin North Am 2016; 27(3):631–648. doi:10.1016/j.pmr.2016.04.010
- Payne JM. Ultrasound-guided hip procedures. Phys Med Rehabil Clin North Am 2016; 27(3):607–629. doi:10.1016/j.pmr.2016.04.004
- Strakowski JA. Ultrasound-guided peripheral nerve procedures. Phys Med Rehabil Clin North Am 2016; 27(3):687–715. doi:10.1016/j.pmr.2016.04.006
- Sussman WI, Williams CJ, Mautner K. Ultrasound-guided elbow procedures. Phys Med Rehabil Clin North Am 2016; 27(3):573–587. doi:10.1016/j.pmr.2016.04.002
- Finnoff JT. The evolution of diagnostic and interventional ultrasound in sports medicine. PM R 2016; 8(suppl 3):S133–S138. doi:10.1016/j.pmrj.2015.09.022
- Wu T, Dong Y, Song H, Fu Y, Li JH. Ultrasound-guided versus landmark in knee arthrocentesis: a systematic review. Semin Arthritis Rheum 2016; 45(5):627–632. doi:10.1016/j.semarthrit.2015.10.011
- Failla JM, van Holsbeeck M, Vanderschueren G. Detection of a 0.5-mm-thick thorn using ultrasound: a case report. J Hand Surg Am 1995; 20(3):456–457.
- Teefey SA, Hasan SA, Middleton WD, Patel M, Wright RW, Yamaguchi K. Ultrasonography of the rotator cuff. A comparison of ultrasonographic and arthroscopic findings in one hundred consecutive cases. J Bone Joint Surg Am 2000; 82(4):498–504.
- van Holsbeeck MT, Kolowich PA, Eyler WR, et al. US depiction of partial-thickness tear of the rotator cuff. Radiology 1995; 197(2):443–446. doi:10.1148/radiology.197.2.7480690
- Balich SM, Sheley RC, Brown TR, Sauser DD, Quinn SF. MR imaging of the rotator cuff tendon: interobserver agreement and analysis of interpretive errors. Radiology 1997; 204(1):191–194. doi:10.1148/radiology.204.1.9205245
- Dinnes J, Loveman E, McIntyre L, Waugh N. The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review. Health Technol Assess 2003; 7(29):1–166. doi:10.3310/hta7290
- Rockett MS, Waitches G, Sudakoff G, Brage M. Use of ultrasonography versus magnetic resonance imaging for tendon abnormalities around the ankle. Foot Ankle Int 1998; 19(9):604–612.
- Grant TH, Kelikian AS, Jereb SE, McCarthy RJ. Ultrasound diagnosis of peroneal tendon tears. A surgical correlation. J Bone Joint Surg Am 2005; 87(8):1788–1794. doi:10.2106/JBJS.D.02450
- Hartgerink P, Fessell DP, Jacobson JA, van Holsbeeck MT. Full- versus partial-thickness Achilles tendon tears: sonographic accuracy and characterization in 26 cases with surgical correlation. Radiology 2001; 220(2):406–412. doi:10.1148/radiology.220.2.r01au41406
- Cho KH, Park BH, Yeon KM. Ultrasound of the adult hip. Semin Ultrasound CT MR 2000; 21(3):214–230.
- Adler RS, Finzel KC. The complementary roles of MR imaging and ultrasound of tendons. Radiol Clin North Am 2005; 43(4):771–807. doi:10.1016/j.rcl.2005.02.011
- Martinoli C, Bianchi S, Derchi LE. Tendon and nerve sonography. Radiol Clin North Am 1999; 37(4):691–711. doi:10.1016/S0033-8389(05)70124-X
- Fessell DP, Vanderschueren GM, Jacobson JA, et al. US of the ankle: technique, anatomy, and diagnosis of pathologic conditions. Radiographics 1998; 18(2):325–340. doi:10.1148/radiographics.18.2.9536481
- Neustadter J, Raikin SM, Nazarian LN. Dynamic sonographic evaluation of peroneal tendon subluxation. AJR Am J Roentgenol 2004; 183(4):985–988. doi:10.2214/ajr.183.4.1830985
- Verhaven EF, Shahabpour M, Handelberg FW, Vaes PH, Opdecam PJ. The accuracy of three-dimensional magnetic resonance imaging in the diagnosis of ruptures of the lateral ligaments of the ankle. Am J Sports Med 1991; 19(6):583–587. doi:10.1177/036354659101900605
- Milz P, Milz S, Steinborn M, Mittlmeier T, Putz R, Reiser M. Lateral ankle ligaments and tibiofibular syndesmosis. 13-MHz high-frequency sonography and MRI compared in 20 patients. Acta Orthop Scand 1998; 69(1):51–55.
- De Smet AA, Winter TC, Best TM, Bernhardt DT. Dynamic sonography with valgus stress to assess elbow ulnar collateral ligament injury in baseball pitchers. Skeletal Radiol 2002; 31(11):671–676. doi:10.1007/s00256-002-0558-0
- Melville DM, Jacobson JA, Fessell DP. Ultrasound of the thumb ulnar collateral ligament: technique and pathology. AJR Am J Roentgenol 2014; 202(2):W168. doi:10.2214/AJR.13.11335
- Court-Payen M. Sonography of the knee: intra-articular pathology. J Clin Ultrasound 2004; 32(9):481–490. doi:10.1002/jcu.20069
- Azzoni R, Cabitza P. Is there a role for sonography in the diagnosis of tears of the knee menisci? J Clin Ultrasound 2002; 30(8):472–476. doi:10.1002/jcu.10106
- Jacobson JA, Wilson TJ, Yang LJ. Sonography of common peripheral nerve disorders with clinical correlation. J Ultrasound Med 2016; 35(4):683–693. doi:10.7863/ultra.15.05061
- Ali ZS, Pisapia JM, Ma TS, Zager EL, Heuer GG, Khoury V. Ultrasonographic evaluation of peripheral nerves. World Neurosurg 2016; 85(1):333–339. doi:10.1016/j.wneu.2015.10.005
- Bignotti B, Signori A, Sormani MP, Molfetta L, Martinoli C, Tagliafico A. Ultrasound versus magnetic resonance imaging for Morton neuroma: systematic review and meta-analysis. Eur Radiol 2015; 25(8):2254–2262. doi:10.1007/s00330-015-3633-3
- Hamilton JV, Flinn G Jr, Haynie CC, Cefalo RC. Diagnosis of rectus sheath hematoma by B-mode ultrasound: a case report. Am J Obstet Gynecol 1976; 125(4):562–565. doi:10.1016/0002-9378(76)90379-3
- Zweymüller VK, Kratochwil A. Ultrasound diagnosis of bone and soft tissue tumours. Wien Klin Wochenschr 1975; 87(12):397–398. German.
- Mayer V. Ultrasonography of the rotator cuff. J Ultrasound Med 1985; 4(11):608, 607. doi:10.7863/jum.1985.4.11.608
- McNally EG. The development and clinical applications of musculoskeletal ultrasound. Skeletal Radiol 2011; 40(9):1223–1231. doi:10.1007/s00256-011-1220-5
- Ignashin NS, Girshin SG, Tsypin IS. Ultrasonic scanning in subcutaneous rupture of the Achilles tendon. Vestn Khir Im I I Grek 1981; 127(9):82–85. Russian.
- Robinson P. Sonography of common tendon injuries. AJR Am J Roentgenol 2009; 193(3):607–618. doi:10.2214/AJR.09.2808
- Jacobson JA. Musculoskeletal ultrasound: focused impact on MRI. AJR Am J Roentgenol 2009; 193(3):619–627. doi:10.2214/AJR.09.2841
- Nazarian LN. The top 10 reasons musculoskeletal sonography is an important complementary or alternative technique to MRI. AJR Am J Roentgenol 2008; 190(6):1621–1626. doi:10.2214/AJR.07.3385
- AIUM technical bulletin. Transducer manipulation. American Institute of Ultrasound in Medicine. J Ultrasound Med 1999; 18(2):169–175. doi:10.7863/jum.1999.18.2.169
- Connolly DJ, Berman L, McNally EG. The use of beam angulation to overcome anisotropy when viewing human tendon with high frequency linear array ultrasound. Br J Radiol 2001; 74 (878):183–185. doi:10.1259/bjr.74.878.740183
- Crass JR, van de Vegte GL, Harkavy LA. Tendon echogenicity: ex vivo study. Radiology 1988; 167(2):499–501. doi:10.1148/radiology.167.2.3282264
- Erickson SJ. High-resolution imaging of the musculoskeletal system. Radiology 1997; 205(3):593–618. doi:10.1148/radiology.205.3.9393511
- Link TM, Majumdar S, Peterfy C, et al. High resolution MRI of small joints: impact of spatial resolution on diagnostic performance and SNR. Magn Reson Imaging 1998; 16(2):147–155. doi:10.1016/S0730-725X(97)00244-0
- Middleton WD, Payne WT, Teefey SA, Hildebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol 2004; 183(5):1449–1452. doi:10.2214/ajr.183.5.1831449
- Khoury V, Cardinal E, Bureau NJ. Musculoskeletal sonography: a dynamic tool for usual and unusual disorders. AJR Am J Roentgenol 2007; 188(1):W63–W73. doi:10.2214/AJR.06.0579
- Farin PU, Jaroma H, Harju A, Soimakallio S. Medial displacement of the biceps brachii tendon: evaluation with dynamic sonography during maximal external shoulder rotation. Radiology 1995; 195(3):845–848. doi:10.1148/radiology.195.3.7754019
- Miller TT, Adler RS, Friedman L. Sonography of injury of the ulnar collateral ligament of the elbow-initial experience. Skeletal Radiol 2004; 33(7):386–391. doi:10.1007/s00256-004-0788-4
- Nazarian LN, McShane JM, Ciccotti MG, O’Kane PL, Harwood MI. Dynamic US of the anterior band of the ulnar collateral ligament of the elbow in asymptomatic major league baseball pitchers. Radiology 2003; 227(1):149–154. doi:10.1148/radiol.2271020288
- Jacobson JA, Lax MJ. Musculoskeletal sonography of the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6(1):67–77. doi:10.1055/s-2002-23165
- Sofka CM, Adler RS. Original report. Sonographic evaluation of shoulder arthroplasty. AJR Am J Roentgenol 2003; 180(4):1117–1120. doi:10.2214/ajr.180.4.1801117
- Silvestri E, Martinoli C, Derchi LE, Bertolotto M, Chiaramondia M, Rosenberg I. Echotexture of peripheral nerves: correlation between US and histologic findings and criteria to differentiate tendons. Radiology 1995; 197(1):291–296. doi:10.1148/radiology.197.1.7568840
- Cardinal E, Buckwalter KA, Braunstein EM, Mih AD. Occult dorsal carpal ganglion: comparison of US and MR imaging. Radiology 1994; 193(1):259–262. doi:10.1148/radiology.193.1.8090903
- Jacobson JA. Musculoskeletal ultrasound and MRI: which do I choose? Semin Musculoskelet Radiol 2005; 9(2):135–149. doi:10.1055/s-2005-872339
- Ward EE, Jacobson JA, Fessell DP, Hayes CW, van Holsbeeck M. Sonographic detection of Baker’s cysts: comparison with MR imaging. AJR Am J Roentgenol 2001; 176(2):373–380. doi:10.2214/ajr.176.2.1760373
- Bhasin S, Cheung PP. The role of power Doppler ultrasonography as disease activity marker in rheumatoid arthritis. Dis Markers 2015; 2015:325909. doi:10.1155/2015/325909
- Fukuba E, Yoshizako T, Kitagaki H, Murakawa Y, Kondo M, Uchida N. Power Doppler ultrasonography for assessment of rheumatoid synovitis: comparison with dynamic magnetic resonance imaging. Clin Imaging 2013; 37(1):134–137. doi:10.1016/j.clinimag.2012.02.008
- Takase-Minegishi K, Horita N, Kobayashi K, et al. Diagnostic test accuracy of ultrasound for synovitis in rheumatoid arthritis: systematic review and meta-analysis. Rheumatology (Oxford) 2018; 57(1):49–58. doi:10.1093/rheumatology/kex036
- Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet 2009; 373(9664):659–672. doi:10.1016/S0140-6736(09)60008-8
- Ash ZR, Tinazzi I, Gallego CC, et al. Psoriasis patients with nail disease have a greater magnitude of underlying systemic subclinical enthesopathy than those with normal nails. Ann Rheum Dis 2012; 71(4):553–556. doi:10.1136/annrheumdis-2011-200478
- Han J, Geng Y, Deng X, Zhang Z. Subclinical synovitis assessed by ultrasound predicts flare and progressive bone erosion in rheumatoid arthritis patients with clinical remission: a systematic review and metaanalysis. J Rheumatol 2016; 43(11):2010–2018. doi.org/10.3899/jrheum.160193
- Iagnocco A, Finucci A, Ceccarelli F, Perricone C, Iorgoveanu V, Valesini G. Power Doppler ultrasound monitoring of response to anti-tumour necrosis factor alpha treatment in patients with rheumatoid arthritis. Rheumatology (Oxford) 2015; 54(10):1890–1896. doi:10.1093/rheumatology/kev211
- Henning PT. Ultrasound-guided foot and ankle procedures. Phys Med Rehabil Clin N Am 2016; 27(3):649–671. doi:10.1016/j.pmr.2016.04.005
- Lueders DR, Smith J, Sellon JL. Ultrasound-guided knee procedures. Phys Med Rehabil Clin North Am 2016; 27(3):631–648. doi:10.1016/j.pmr.2016.04.010
- Payne JM. Ultrasound-guided hip procedures. Phys Med Rehabil Clin North Am 2016; 27(3):607–629. doi:10.1016/j.pmr.2016.04.004
- Strakowski JA. Ultrasound-guided peripheral nerve procedures. Phys Med Rehabil Clin North Am 2016; 27(3):687–715. doi:10.1016/j.pmr.2016.04.006
- Sussman WI, Williams CJ, Mautner K. Ultrasound-guided elbow procedures. Phys Med Rehabil Clin North Am 2016; 27(3):573–587. doi:10.1016/j.pmr.2016.04.002
- Finnoff JT. The evolution of diagnostic and interventional ultrasound in sports medicine. PM R 2016; 8(suppl 3):S133–S138. doi:10.1016/j.pmrj.2015.09.022
- Wu T, Dong Y, Song H, Fu Y, Li JH. Ultrasound-guided versus landmark in knee arthrocentesis: a systematic review. Semin Arthritis Rheum 2016; 45(5):627–632. doi:10.1016/j.semarthrit.2015.10.011
- Failla JM, van Holsbeeck M, Vanderschueren G. Detection of a 0.5-mm-thick thorn using ultrasound: a case report. J Hand Surg Am 1995; 20(3):456–457.
- Teefey SA, Hasan SA, Middleton WD, Patel M, Wright RW, Yamaguchi K. Ultrasonography of the rotator cuff. A comparison of ultrasonographic and arthroscopic findings in one hundred consecutive cases. J Bone Joint Surg Am 2000; 82(4):498–504.
- van Holsbeeck MT, Kolowich PA, Eyler WR, et al. US depiction of partial-thickness tear of the rotator cuff. Radiology 1995; 197(2):443–446. doi:10.1148/radiology.197.2.7480690
- Balich SM, Sheley RC, Brown TR, Sauser DD, Quinn SF. MR imaging of the rotator cuff tendon: interobserver agreement and analysis of interpretive errors. Radiology 1997; 204(1):191–194. doi:10.1148/radiology.204.1.9205245
- Dinnes J, Loveman E, McIntyre L, Waugh N. The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review. Health Technol Assess 2003; 7(29):1–166. doi:10.3310/hta7290
- Rockett MS, Waitches G, Sudakoff G, Brage M. Use of ultrasonography versus magnetic resonance imaging for tendon abnormalities around the ankle. Foot Ankle Int 1998; 19(9):604–612.
- Grant TH, Kelikian AS, Jereb SE, McCarthy RJ. Ultrasound diagnosis of peroneal tendon tears. A surgical correlation. J Bone Joint Surg Am 2005; 87(8):1788–1794. doi:10.2106/JBJS.D.02450
- Hartgerink P, Fessell DP, Jacobson JA, van Holsbeeck MT. Full- versus partial-thickness Achilles tendon tears: sonographic accuracy and characterization in 26 cases with surgical correlation. Radiology 2001; 220(2):406–412. doi:10.1148/radiology.220.2.r01au41406
- Cho KH, Park BH, Yeon KM. Ultrasound of the adult hip. Semin Ultrasound CT MR 2000; 21(3):214–230.
- Adler RS, Finzel KC. The complementary roles of MR imaging and ultrasound of tendons. Radiol Clin North Am 2005; 43(4):771–807. doi:10.1016/j.rcl.2005.02.011
- Martinoli C, Bianchi S, Derchi LE. Tendon and nerve sonography. Radiol Clin North Am 1999; 37(4):691–711. doi:10.1016/S0033-8389(05)70124-X
- Fessell DP, Vanderschueren GM, Jacobson JA, et al. US of the ankle: technique, anatomy, and diagnosis of pathologic conditions. Radiographics 1998; 18(2):325–340. doi:10.1148/radiographics.18.2.9536481
- Neustadter J, Raikin SM, Nazarian LN. Dynamic sonographic evaluation of peroneal tendon subluxation. AJR Am J Roentgenol 2004; 183(4):985–988. doi:10.2214/ajr.183.4.1830985
- Verhaven EF, Shahabpour M, Handelberg FW, Vaes PH, Opdecam PJ. The accuracy of three-dimensional magnetic resonance imaging in the diagnosis of ruptures of the lateral ligaments of the ankle. Am J Sports Med 1991; 19(6):583–587. doi:10.1177/036354659101900605
- Milz P, Milz S, Steinborn M, Mittlmeier T, Putz R, Reiser M. Lateral ankle ligaments and tibiofibular syndesmosis. 13-MHz high-frequency sonography and MRI compared in 20 patients. Acta Orthop Scand 1998; 69(1):51–55.
- De Smet AA, Winter TC, Best TM, Bernhardt DT. Dynamic sonography with valgus stress to assess elbow ulnar collateral ligament injury in baseball pitchers. Skeletal Radiol 2002; 31(11):671–676. doi:10.1007/s00256-002-0558-0
- Melville DM, Jacobson JA, Fessell DP. Ultrasound of the thumb ulnar collateral ligament: technique and pathology. AJR Am J Roentgenol 2014; 202(2):W168. doi:10.2214/AJR.13.11335
- Court-Payen M. Sonography of the knee: intra-articular pathology. J Clin Ultrasound 2004; 32(9):481–490. doi:10.1002/jcu.20069
- Azzoni R, Cabitza P. Is there a role for sonography in the diagnosis of tears of the knee menisci? J Clin Ultrasound 2002; 30(8):472–476. doi:10.1002/jcu.10106
- Jacobson JA, Wilson TJ, Yang LJ. Sonography of common peripheral nerve disorders with clinical correlation. J Ultrasound Med 2016; 35(4):683–693. doi:10.7863/ultra.15.05061
- Ali ZS, Pisapia JM, Ma TS, Zager EL, Heuer GG, Khoury V. Ultrasonographic evaluation of peripheral nerves. World Neurosurg 2016; 85(1):333–339. doi:10.1016/j.wneu.2015.10.005
- Bignotti B, Signori A, Sormani MP, Molfetta L, Martinoli C, Tagliafico A. Ultrasound versus magnetic resonance imaging for Morton neuroma: systematic review and meta-analysis. Eur Radiol 2015; 25(8):2254–2262. doi:10.1007/s00330-015-3633-3
KEY POINTS
- Ultrasonography can be used to evaluate small fluid collections in soft tissue; joint effusions and synovitis; soft tissue masses (≤ 5 cm in diameter); tendon, ligament and muscle injuries; and peripheral nerve entrapment and lesions.
- Ultrasonography is not appropriate for survey examinations of vague or diffuse symptoms or for evaluating soft-tissue areas more than a few centimeters in diameter or more than a few centimeters deep.
- Musculoskeletal ultrasonography requires specially trained sonographers and interpreting physicians.
The female athlete triad: It takes a team
Striving for athletic excellence, many young women—and some young men—create an energy deficit from increased exercise, decreased intake, or both. In women, the resulting energy deficit can suppress the menstrual cycle and in turn lead to bone demineralization in a syndrome called the female athlete triad.
Primary care physicians should be aware of this syndrome because it can lead to short-term and long-term health complications, and they are in a good position to screen for, diagnose, and treat it. However, a study of 931 US physicians in 2015 found that only 37% had heard of it.1
DEFINITION HAS CHANGED: ONLY 1 OF 3 COMPONENTS NEEDED
In 1972, Title IX of the Education Amendment Act was passed, prohibiting sex discrimination in any higher education program or activity receiving federal financial aid. Since then, female athletic participation in the United States has increased more than 10-fold.2
Also increasing has been awareness of the link between athletics, eating disorders, and amenorrhea. The American College of Sports Medicine coined the term female athlete triad in 1992, describing it as the constellation of disordered eating, amenorrhea, and osteoporosis (all 3 needed to be present).3 They broadened the definition in 2007 so that the syndrome can be diagnosed if any of the following is present4:
- Low energy availability (with or without an eating disorder)
- Menstrual dysfunction
- Decreased bone mineral density.
Recognizing that low energy availability can affect athletes of either sex and have consequences beyond the female reproductive system and skeleton, in 2014 the International Olympic Committee introduced a broader term called relative energy deficiency in sport.5,6 Like the triad, this condition occurs when energy intake falls below energy output to the point that it negatively affects an athlete’s physical and mental health.
THE COMPONENTS ARE COMMON
The female athlete triad can be seen in high school, collegiate, and elite athletes7 and is especially common in sports with subjective judging (gymnastics, figure skating) or endurance sports that emphasize leanness (eg, running).8
In a review of 65 studies, Gibbs et al9 found that the prevalence of any one of the triad conditions in exercising women and female athletes ranged from 16.0% to 60.0%, the prevalence of any 2 ranged from 2.7% to 27.0%, and the prevalence of all 3 ranged from 0% to 15.9%.
Low energy availability is categorized as either intentional (ie, due to disordered eating) or unintentional (ie, due to activities not associated with eating). Sustained low energy availability is often associated with eating disorders and subsequent low self-esteem, depression, and anxiety disorders.4
The prevalence of eating disorders is high in female athletes—31% and 20% in 2 large studies of elite female athletes, compared with 5.5% and 9%, respectively, in the general population.10,11 Another study found that the prevalence of disordered eating was 46.7% in sports that emphasize leanness, such as track and gymnastics, compared with 19.8% in sports that did not, such as basketball and soccer.12
Calorie restriction is common. In a study of 15 elite ballet dancers and 15 matched controls, the dancers were found to consume only about 3/4 as many calories per day as the controls (1,577 vs 2,075 kcal/day, P ≤ .01).13
Menstrual dysfunction. In small studies, the prevalence of secondary amenorrhea was as high as 69% in dancers and 65% in long-distance runners.4,14–16
Decreased bone mineral density. According to a systematic review, the prevalence of osteopenia in amenorrheic athletes ranged between 22% and 50% and the prevalence of osteoporosis was 0% to 13%, compared with 12% and 2.3%, respectively, in the general population.17
THE COMPONENTS ARE LINKED
dysfunction play causative roles in bone mineral density pathology. Within each component of the triad a spectrum of dysfunction exists, with all 3 components exhibiting serious health end points including low energy availability, functional hypothalamic amenorrhea, and osteoporosis.
The 3 components of the female athlete triad—low energy availability, menstrual dysfunction, and decreased bone mineral density—are linked, and each exists on a spectrum (Figure 1). The long-term consequences are far-reaching and can affect the cardiovascular, endocrine, reproductive, skeletal, gastrointestinal, renal, and central nervous systems.
Low energy availability is the driving force of the triad, causing menstrual irregularity and subsequent low bone mineral density.
Menstrual dysfunction. Low energy availability can contribute to menstrual disturbances because the body suppresses reproductive function to prevent pregnancy. Functional hypothalamic amenorrhea results from decreased gonadotropin-releasing hormone leading to decreased gonadotropin release from the pituitary gland and, ultimately, to low circulating estrogen levels.18 Menstrual irregularities related to the triad include:
- Primary amenorrhea (a delay in menarche)
- Oligomenorrhea (menstrual cycles occurring at intervals greater than every 35 days)
- Secondary amenorrhea (cessation of menstruation for 3 consecutive months).
(Primary amenorrhea is defined as no menses by age 15 in the presence of normal secondary sexual development or within 5 years after breast development if that occurs before the age of 10. Secondary amenorrhea is defined as the loss of menses for 90 or more days after menarche.19)
In animal studies, reducing dietary intake by more than 30% resulted in infertility.4 Menstrual abnormalities can present as early as 5 days after a patient enters a state of low energy availability.20 Symptoms of menstrual dysfunction are largely indicative of hypogonadism and include vaginal dryness, infertility, and impaired bone health.
Bone health in women with the female athlete triad can range from optimal to osteoporosis.
Low bone mineral density is a result of low energy availability and menstrual dysfunction leading to estrogen deficiency.21,22 Specifically, menstrual abnormalities can result in low estrogen and overactivity of osteoclasts, while low energy availability alters the metabolic environment, inducing changes in insulinlike growth factor 1, leptin, and peptide YY, resulting in deficiencies in vitamin D and calcium—nutrients necessary for bone mineralization. In turn, bone health and density are compromised.21,22
Ninety percent of peak bone mass is attained by age 18. Those who have low bone mineral density as part of the female athlete triad can suffer from long-lasting effects on their bone health.
SCREENING
Untreated, the triad can lead to fatigue, poor sports performance, and a number of serious comorbid conditions such as osteopenia and osteoporosis (leading to stress fractures) anemia, heart arrhythmias, and amenorrhea. Therefore, it is important for primary care providers to screen female athletes for the triad during routine office visits.
In 2014, the Triad Consensus Panel recommended screening female athletes at the high school and collegiate levels during a preparticipation physical evaluation and then every year by a primary care physician, athletic trainer, team physician, or coach.23
Risk factors include signs of dietary restriction, low body mass index, delayed menarche, oligomenorrhea or amenorrhea, and bone stress reactions or fractures.23 Athletes should be questioned about their menstrual history (age of menarche, frequency, and duration of menstrual cycles), history of stress fractures, medication history, family history (osteoporosis, eating disorders, and fractures),24,25 and dietary habits.
Physical findings include low body mass index, recent weight loss, orthostatic hypotension, lanugo, hypercarotenemia, and signs of eating disorders (restrictive, binging, purging) (Table 1).25–27
Additionally, it is important to ascertain if the patient receives critical comments regarding performance or body image from coaches, parents, or teammates and if sport-specific training began early in life.
Certain personality factors and behaviors are clues, such as perfectionism, obsessiveness, frequent weight cycling, and overtraining.4,25 If any of the triad components are apparent, a deeper evaluation can be completed.
Specific screening questions
The Female Athlete Triad Coalition recommends asking 11 screening questions and having prompt discussions regarding the athlete’s nutritional status and body image.23 If the patient gives a worrisome response to a screening question, further workup for a formal diagnosis should be initiated.
Questions about nutritional status.
- Do you worry about your weight?
- Are you trying to gain or lose weight, or has anyone recommended that you do so?
- Are you on a special diet or do you avoid certain types of foods or food groups?
- Have you ever had an eating disorder?
Questions about menstrual function.
- Have you ever had a menstrual period?
- How old were you when you had your first menstrual period?
- When was your most recent menstrual period?
- How many periods have you had in the last 12 months?
- Are you presently taking any female hormones (estrogen, progesterone, birth control pills)?
Questions about bone health.
- Have you ever had a stress fracture?
- Have you ever been told you have low bone density (osteopenia or osteoporosis)?
Along similar lines, the American Academy of Pediatrics, American Academy of Family Physicians, and American College of Sports Medicine28 have a list of 7 questions:
- Do you worry about your weight?
- Do you limit the foods you eat?
- Do you lose weight to meet image requirements for sports?
- Have you ever suffered from an eating disorder?
- How old were you when you had your first menstrual period?
- How many menstrual cycles have you had in the past 12 months?
- Have you ever had a stress fracture?
These questions are not being widely used. A study of the National Collegiate Athletic Association Division I universities found that only 9% of universities included 9 or more of the recommended 12 questions that the Female Athlete Triad Coalition was recommending at that time, and 22% asked only 1 or 2 of the questions. None of the universities included all 12.29 These findings are not surprising, given that screening for the triad is not state-mandated. Screening discrepancies among providers largely stem from knowledge gaps, nonstandardized questionnaires, lack of time at appointments, and the sensitive nature of the questioning (eg, disordered eating).30
DIAGNOSING THE TRIAD
Given that the signs of low energy availability and menstrual dysfunction are often subtle, the diagnosis of the triad for those at risk requires input from a multidisciplinary team including a physician, sports dietitian, mental health professional, exercise physiologist, and other medical consultants.
Table 2 lists diagnostic tests the primary care provider should consider.
Diagnosing low energy availability
Energy availability is the dietary energy remaining after exercise energy expenditure; it is normalized to fat-free (lean) mass to account for resting energy expenditure. It is a product of energy intake, energy expenditure, and stored energy, and is calculated as:
An optimal value is at least 45 kcal/kg/day, while physiologic changes start to occur at less than 30 kcal/kg/day.4,31 Low energy is often seen in adult patients with a body mass index less than 17.5 kg/m2 and adolescent patients who are less than 80% of expected body weight.
Energy availability is hard to calculate, but certain assessments can be performed in a primary care setting to approximate it. To assess dietary intake, patients can bring in a 3-, 4-, or 7-day dietary log or complete a 24-hour food recall or food-frequency questionnaire in the office. To objectively document energy expenditure, patients can use heart rate monitors, accelerometers, an exercise diary, and web-based calculators. The fat-free mass can be calculated using a bioelectric impedance scale and skinfold caliper measurements.26
Those with chronic energy deficiency states may have reduced resting metabolic rates, with measured rates less than 90% of predicted and low triiodothyronine (T3) levels.31
Diagnosing menstrual dysfunction
When evaluating patients with menstrual dysfunction, it is important to first rule out pregnancy and endocrinopathies. These include thyroid dysfunction, hyperprolactinemia, primary ovarian insufficiency, other hypothalamic and pituitary disorders, and hyperandrogenic conditions such as polycystic ovarian syndrome, ovarian tumor, adrenal tumor, nonclassic congenital adrenal hyperplasia, and Cushing syndrome.
Depending on the patient’s age, laboratory tests can include follicle-stimulating hormone, luteinizing hormone, prolactin, serum estradiol, and a progesterone challenge.32 For hyperandrogenic symptoms, measuring total and free testosterone, dehydroepiandrosterone sulfate, 24-hour urine cortisol, and 17-hydroxyprogesterone levels may be helpful.
An endocrinologist should be consulted to evaluate the underlying cause of amenorrhea and address any associated hormonal imbalances. Attributing menstrual dysfunction to low energy availability is generally a diagnosis of exclusion. Additionally, outflow tract obstruction should be considered and ruled out with transvaginal ultrasonography in patients with primary amenorrhea.
A patient with hypoestrogenemia and amenorrhea may have the same steroid hormone profile as that of a menopausal woman. Lack of estrogen results in impaired endothelial cell function and arterial dilation, with accelerated development of atherosclerosis and subsequent cardiovascular events.33,34 Further, low energy availability has been linked to negative cardiovascular effects such as decreased vessel dilation leading to decreased tissue perfusion and hastened development of atherosclerosis.33 Female athletes with hypoestrogenism may show reduced perfusion of working muscle, impaired aerobic metabolism in skeletal muscle, elevated low-density lipoprotein cholesterol, and vaginal dryness.4
Diagnosing low bone mineral density
The most common clinical manifestations of low bone mineral density in female athletes are bone stress reactions such as stress fractures. In a study of 311 female high school athletes, 65.6% suffered from musculoskeletal injury from trauma or overuse including stress fractures and the patellofemoral syndrome.35 Many athletes seek medical attention from their primary care physician for stress reactions, providing an opportunity for triad screening.36
In postmenopausal women, osteopenia and osteoporosis are defined using the T score. However, in premenopausal women and adolescents, the International Society for Clinical Densitometry recommends using the Z score. A Z score less than –2.0 is described as “low bone density for chronological age.”14 For the diagnosis of osteoporosis in children and premenopausal women, the Society recommends using a Z score less than –2.0 along with the presence of a secondary risk factor for fracture such as undernutrition, hypogonadism, or a history of fracture.
Table 2 summarizes the diagnosis of low bone mineral density and osteoporosis in premenopausal women, adolescents, and children as well as when to order dual-energy x-ray absorptiometry (DEXA).37,38
Adolescents with low bone mineral density should have an annual DEXA scan of the total hip and lumbar spine.22 Amenorrheic athletes typically present with low areal density at the lumbar spine, reduced trabecular volumetric bone mineral density and bone strength index at the distal radius, and deterioration of the distal tibia.39
EARLY INTERVENTION IS ESSENTIAL
Early intervention is essential in patients with any component of the female athlete triad to prevent long-term adverse health effects. Successful treatment is strongly correlated with a trusting relationship between the athlete and the multidisciplinary team involved in her treatment.40
If needed, selective serotonin reuptake inhibitors and other psychotropic medications can be prescribed for comorbid conditions including bulimia nervosa, anxiety, depression, and obsessive-compulsive disorder. Primary providers can identify risk factors that prompt the evaluation and diagnosis of the triad, as well as support the goals of treatment and help manage comorbid conditions.
Eat more, exercise less
The primary goal is to restore body weight, maximizing nutritional and energy status by modifying the diet and adjusting exercise behavior to increase energy availability.41 Creating an energy-positive state by increasing intake, decreasing energy expenditure, or both increases energy availability, subsequently improves bone mineral density, and normalizes menstrual function.40
To sustain normal physiologic function, an energy availability of at least 45 kcal/kg/day is recommended.42 Patients should consume a minimum of 2,000 kcal/day, although energy needs may far exceed that, depending on energy expenditure. Olympic athletes participating in women’s crew and other sports have been anecdotally known to require over 12,000 kcal a day to maintain weight and performance. Goals include a body mass index of at least 18.5 kg/m2 in adults and a body weight of at least 90% of predicted in adolescents.
Involving a dietitian in the care team can help ensure that the patient consumes an adequate amount of macronutrients and micronutrients necessary for bone growth; these include calcium, vitamin D, iron, zinc, and vitamin K.4,32 For patients with disordered eating, referral to a mental health professional is important to help them avoid pathologic eating behaviors, reduce dieting attempts, and alter negative emotions associated with food and body image.
Once treatment begins, patients must undergo standardized periodic monitoring of their body weight. Although positive effects such as normalization of metabolic hormones (eg, insulinlike growth factor 1) may be seen in days to weeks by reversing low energy availability, it may take several months for menstrual function to improve and years for measurable improvement in bone mineral density to occur.23
Menstrual function should improve with weight gain
Normalizing menses in patients with the female athlete triad depends on improving the low energy availability and inducing weight gain.
Pharmacotherapy such as combined oral contraceptives can treat symptoms of hypogonadism.25 However, combined oral contraceptives do not restore spontaneous menses but rather induce withdrawal bleeding, which can lead to a false sense of security.23 While there are some benefits to prescribing combined oral contraceptives to treat hypogonadism, nonpharmacologic methods should be tried initially to restore menses, including increasing caloric intake and body weight. Golden et al showed that hormone replacement with combined oral contraceptives did not improve bone density in women with low estrogen states (eg, anorexia nervosa, osteopenia).43 Further, combined oral contraceptives may worsen bone health, as oral estrogen suppresses hepatic production of insulinlike growth factor 1, a bone trophic hormone.23
Treating low bone mineral density
Improving energy availability and menstrual function can help improve bone mineral density. Nutritional enhancement is recommended for mineralization of trabecular bone and growth of cortical bone. Supplemental calcium (1,000–1,500 mg daily) and vitamin D (600–1,000 IU daily) should be incorporated into the treatment of low bone mineral density.15,19
Additionally, weight gain has a positive effect on bone mineral density independent of its effect on the resumption of menses. However, weight gain alone does not normalize bone mineral density. Resuming normal physiologic production of hormones with estrogen-dependent effects on bone health is integral to normalization as well.23
Resistance training is encouraged to increase lean mass, although caution must be used to prevent fractures during high-impact activity. Bone mineral density may take up to several years to improve and may not be fully reversible.4,25,39
Pharmacologic therapy for low bone mineral density has unclear outcomes in women under age 50. The decision to treat should be based on bone mineral density along with fracture history. Given their unknown effects on the human fetal skeleton, bisphosphonates and denosumab should be used with caution in women of childbearing potential.24 No studies have used denosumab or teriparatide in women with the female athlete triad. Despite concerns regarding use of these drugs in premenopausal women, drug therapy should be strongly considered in women with a history of fracture and those with a high risk of subsequent fracture. The decision to treat can be made in conjunction with the athlete’s endocrinologist.
RETURN TO PLAY
If an athlete is noted to be at risk for or diagnosed with the female athlete triad, it is important to formulate a plan for her to return to play once her health improves.
De Souza et al provided a cumulative risk assessment for risk stratification and made recommendations on when an athlete should return to play depending on her level of risk.23 Using this grading system, primary care physicians can risk-stratify their patients. Those at low risk may be fully cleared to return to play, while those at moderate to high risk must first follow up with a multidisciplinary team to develop treatment strategies for improving their health.
Once a patient reaches her established goals, she may provisionally return to play under the close supervision of a team physician or primary care physician. A written treatment contract, including the goals set by the multidisciplinary team, should be followed closely as the athlete continues to participate in the sport.23;
- Curry EJ, Logan C, Ackerman K, McInnis KC, Matzkin EG. Female athlete triad awareness among multispecialty physicians. Sports Med Open 2015; 1(1):38. doi:10.1186/s40798-015-0037-5
- National Federation of State High School Associations. 2012–13 high school athletics participation survey. http://old.nfhs.org/content.aspx?id=3282. Accessed February 1, 2018.
- Otis CL, Drinkwater B, Johnson M, Loucks A, Wilmore J. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 1997; 29(5):i–ix.
- Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 2007; 39(10):1867–1882. doi:10.1249/mss.0b013e318149f111
- Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sport (RED-S). Br J Sports Med 2014; 48(7):491–497. doi:10.1136/bjsports-2014-093502
- Tenforde AS, Barrack MT, Nattiv A, Fredericson M. Parallels with the female athlete triad in male athletes. Sports Med 2016; 46(2):171–182. doi:10.1007/s40279-015-0411-y
- Thein-Nissenbaum JM, Carr KE. Female athlete triad syndrome in the high school athlete. Phys Ther Sport 2011; 12(3):108–116. doi:10.1016/j.ptsp.2011.04.002
- Matzkin E, Curry EJ, Whitlock K. Female athlete triad: past, present, and future. J Am Acad Orthop Surg 2015; 23(7):424–432. doi:10.5435/JAAOS-D-14-00168
- Gibbs JC, Williams NI, De Souza MJ. Prevalence of individual and combined components of the female athlete triad. Med Sci Sports Exerc 2013; 45(5):985–996. doi:10.1249/MSS.0b013e31827e1bdc
- Byrne S, McLean N. Elite athletes: effects of the pressure to be thin. J Sci Med Sport 2002; 5(2):80–94. doi:10.1016/S1440-2440(02)80029-9
- Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med 2004; 14(1):25–32.
- Lynch SL, Hoch AZ. The female runner: gender specifics. Clin Sports Med 2010; 29(3):477–498. doi:10.1016/j.csm.2010.03.003
- Doyle-Lucas AF, Akers JD, Davy BM. Energetic efficiency, menstrual irregularity, and bone mineral density in elite professional female ballet dancers. J Dance Med Sci 2010; 14(4):146–154.
- Thein-Nissenbaum J. Long term consequences of the female athlete triad. Maturitas 2013; 75(2):107–112. doi:10.1016/j.maturitas.2013.02.010
- Hilibrand MJ, Hammoud S, Bishop M, Woods D, Fredrick RW, Dodson CC. Common injuries and ailments of the female athlete; pathophysiology, treatment and prevention. Phys Sportsmed 2015; 43(4):403–411. doi:10.1080/00913847.2015.1092856
- Demorest RA, Hergenroeder AC. Preface. Sports medicine and sports injuries. Adolesc Med State Art Rev 2015; 26(1):xv–xvi.
- Khan KM, Liu-Ambrose T, Sran MM, Ashe MC, Donaldson MG, Wark JD. New criteria for female athlete triad syndrome? Br J Sports Med 2002; 36(1):10–13. doi:10.1136/bjsm.36.1.10
- Fontana R, Della Torre S. The deep correlation between energy metabolism and reproduction: a view of the effects of nutrition for women fertility. Nutrients 2016; 8:87. www.ncbi.nlm.nih.gov/pmc/articles/PMC4772050/. Accessed February 2, 2018.
- Nazem TG, Ackerman K. The female athlete triad. Sports Health 2012; 4(4):302–311. doi:10.1177/1941738112439685
- Pantano KJ. Coaching concerns in physically active girls and young women—part 1: the female athlete triad. Strength Conditioning J 2009; 31(6):38–43. doi:10.1519/SSC.0b013e3181c105dd
- Micklesfield LK, Hugo J, Johnson C, Noakes TD, Lambert EV. Factors associated with menstrual dysfunction and self-reported bone stress injuries in female runners in the ultra- and half-marathons of the Two Oceans. Br J Sports Med 2007; 41(10):679–683. doi:10.1136/bjsm.2007.037077
- Lambrinoudaki I, Papadimitriou D. Pathophysiology of bone loss in the female athlete. Ann N Y Acad Sci 2010; 1205:45–50. doi:10.1111/j.1749-6632.2010.05681.x
- De Souza MJ, Nattiv A, Joy E, et al. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad: 1st International Conference held in San Francisco, CA, May 2012, and 2nd International Conference held in Indianapolis, IN, May 2013. Clin J Sport Med 2014; 24(2):96–119. doi:10.1136/bjsports-2013-093218
- Horn E, Gergen N, McGarry KA. The female athlete triad. R I Med J (2013) 2014; 97(11):18–21.
- Joy E, De Souza MJ, Nattiv A, et al. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad. Curr Sports Med Rep 2014; 13(4):219–232. doi:10.1249/JSR.0000000000000077
- Temme KE, Hoch AZ. Recognition and rehabilitation of the female athlete triad/tetrad: a multidisciplinary approach. Curr Sports Med Rep 2013; 12(3):190–199. doi:10.1249/JSR.0b013e318296190b
- Pritts SD, Susman J. Diagnosis of eating disorders in primary care. Am Fam Physician 2003; 67(2):297–304.
- Berhardt DR, Roberts WO, editors. Preparticipation Physical Evaluation, 4th Ed. American Academy of Pediatrics, Elk Grove Village, IL, 2010.
- Mencias T, Noon M, Hoch AZ. Female athlete triad screening in National Collegiate Athletic Association Division I athletes: is the preparticipation evaluation form effective? Clin J Sport Med 2012; 22(2):122–125. doi:10.1097/JSM.0b013e3182425aee
- Javed A, Tebben PJ, Fischer PR, Lteif AN. Female athlete triad and its components: toward improved screening and management. Mayo Clin Proc 2013; 88(9):996–1009. doi:10.1016/j.mayocp.2013.07.001
- Melin A, Tornberg AB, Skouby S, et al. Energy availability and the female athlete triad in elite endurance athletes. Scand J Med Sci Sports 2015; 25(5):610–622. doi:10.1111/sms.12261
- Warr BJ, Woolf K. The female athlete triad: patients do best with a team approach to care. JAAPA 2011; 24(4):50–55.
- Hoch AZ, Lal S, Jurva JW, Gutterman DD. The female athlete triad and cardiovascular dysfunction. Phys Med Rehabil Clin North Am 2007; 18(3):385–400. doi:10.1016/j.pmr.2007.05.001
- Lanser EM, Zach KN, Hoch AZ. The female athlete triad and endothelial dysfunction. PM R 2011; 3(5):458–465. doi:10.1016/j.pmrj.2010.12.024
- Thein-Nissenbaum JM, Rauh MJ, Carr KE, Loud KJ, McGuine TA. Associations between disordered eating, menstrual dysfunction, and musculoskeletal injury among high school athletes. J Orthop Sports Phys Ther 2011; 41(2):60–69. doi:10.2519/jospt.2011.3312
- Ducher G, Turner AI, Kukuljan S, et al. Obstacles in the optimization of bone health outcomes in the female athlete triad. Sports Med 2011; 41(7):587–607. doi:10.2165/11588770-000000000-00000
- Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin North Am 2010; 39(1):155–167. doi:10.1016/j.ecl.2009.11.002
- House S, Loud K, Shubkin C. Female athlete triad for the primary care pediatrician. Curr Opin Pediatr 2013; 25(6):755–761. doi:10.1097/MOP.0000000000000033
- Mallinson RJ, De Souza MJ. Current perspectives on the etiology and manifestation of the “silent” component of the female athlete triad. Int J Womens Health 2014; 6:451–467. doi:10.2147/IJWH.S38603
- Deimel JF, Dunlap BJ. The female athlete triad. Clin Sports Med 2012; 31(2):247–254. doi:10.1016/j.csm.2011.09.007
- Manore MM, Kam LC, Loucks AB; International Association of Athletics Federations. The female athlete triad: components, nutrition issues, and health consequences. J Sports Sci 2007; 25(suppl 1):S61–S71. doi:10.1080/02640410701607320
- Witkop CT, Warren MP. Understanding the spectrum of the female athlete triad. Obstet Gynecol 2010; 116(6):1444–1448. doi:10.1097/AOG.0b013e3181fbed40
- Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002; 15(3):135–143. doi:10.1016/S1083-3188(02)00145-6
Striving for athletic excellence, many young women—and some young men—create an energy deficit from increased exercise, decreased intake, or both. In women, the resulting energy deficit can suppress the menstrual cycle and in turn lead to bone demineralization in a syndrome called the female athlete triad.
Primary care physicians should be aware of this syndrome because it can lead to short-term and long-term health complications, and they are in a good position to screen for, diagnose, and treat it. However, a study of 931 US physicians in 2015 found that only 37% had heard of it.1
DEFINITION HAS CHANGED: ONLY 1 OF 3 COMPONENTS NEEDED
In 1972, Title IX of the Education Amendment Act was passed, prohibiting sex discrimination in any higher education program or activity receiving federal financial aid. Since then, female athletic participation in the United States has increased more than 10-fold.2
Also increasing has been awareness of the link between athletics, eating disorders, and amenorrhea. The American College of Sports Medicine coined the term female athlete triad in 1992, describing it as the constellation of disordered eating, amenorrhea, and osteoporosis (all 3 needed to be present).3 They broadened the definition in 2007 so that the syndrome can be diagnosed if any of the following is present4:
- Low energy availability (with or without an eating disorder)
- Menstrual dysfunction
- Decreased bone mineral density.
Recognizing that low energy availability can affect athletes of either sex and have consequences beyond the female reproductive system and skeleton, in 2014 the International Olympic Committee introduced a broader term called relative energy deficiency in sport.5,6 Like the triad, this condition occurs when energy intake falls below energy output to the point that it negatively affects an athlete’s physical and mental health.
THE COMPONENTS ARE COMMON
The female athlete triad can be seen in high school, collegiate, and elite athletes7 and is especially common in sports with subjective judging (gymnastics, figure skating) or endurance sports that emphasize leanness (eg, running).8
In a review of 65 studies, Gibbs et al9 found that the prevalence of any one of the triad conditions in exercising women and female athletes ranged from 16.0% to 60.0%, the prevalence of any 2 ranged from 2.7% to 27.0%, and the prevalence of all 3 ranged from 0% to 15.9%.
Low energy availability is categorized as either intentional (ie, due to disordered eating) or unintentional (ie, due to activities not associated with eating). Sustained low energy availability is often associated with eating disorders and subsequent low self-esteem, depression, and anxiety disorders.4
The prevalence of eating disorders is high in female athletes—31% and 20% in 2 large studies of elite female athletes, compared with 5.5% and 9%, respectively, in the general population.10,11 Another study found that the prevalence of disordered eating was 46.7% in sports that emphasize leanness, such as track and gymnastics, compared with 19.8% in sports that did not, such as basketball and soccer.12
Calorie restriction is common. In a study of 15 elite ballet dancers and 15 matched controls, the dancers were found to consume only about 3/4 as many calories per day as the controls (1,577 vs 2,075 kcal/day, P ≤ .01).13
Menstrual dysfunction. In small studies, the prevalence of secondary amenorrhea was as high as 69% in dancers and 65% in long-distance runners.4,14–16
Decreased bone mineral density. According to a systematic review, the prevalence of osteopenia in amenorrheic athletes ranged between 22% and 50% and the prevalence of osteoporosis was 0% to 13%, compared with 12% and 2.3%, respectively, in the general population.17
THE COMPONENTS ARE LINKED
dysfunction play causative roles in bone mineral density pathology. Within each component of the triad a spectrum of dysfunction exists, with all 3 components exhibiting serious health end points including low energy availability, functional hypothalamic amenorrhea, and osteoporosis.
The 3 components of the female athlete triad—low energy availability, menstrual dysfunction, and decreased bone mineral density—are linked, and each exists on a spectrum (Figure 1). The long-term consequences are far-reaching and can affect the cardiovascular, endocrine, reproductive, skeletal, gastrointestinal, renal, and central nervous systems.
Low energy availability is the driving force of the triad, causing menstrual irregularity and subsequent low bone mineral density.
Menstrual dysfunction. Low energy availability can contribute to menstrual disturbances because the body suppresses reproductive function to prevent pregnancy. Functional hypothalamic amenorrhea results from decreased gonadotropin-releasing hormone leading to decreased gonadotropin release from the pituitary gland and, ultimately, to low circulating estrogen levels.18 Menstrual irregularities related to the triad include:
- Primary amenorrhea (a delay in menarche)
- Oligomenorrhea (menstrual cycles occurring at intervals greater than every 35 days)
- Secondary amenorrhea (cessation of menstruation for 3 consecutive months).
(Primary amenorrhea is defined as no menses by age 15 in the presence of normal secondary sexual development or within 5 years after breast development if that occurs before the age of 10. Secondary amenorrhea is defined as the loss of menses for 90 or more days after menarche.19)
In animal studies, reducing dietary intake by more than 30% resulted in infertility.4 Menstrual abnormalities can present as early as 5 days after a patient enters a state of low energy availability.20 Symptoms of menstrual dysfunction are largely indicative of hypogonadism and include vaginal dryness, infertility, and impaired bone health.
Bone health in women with the female athlete triad can range from optimal to osteoporosis.
Low bone mineral density is a result of low energy availability and menstrual dysfunction leading to estrogen deficiency.21,22 Specifically, menstrual abnormalities can result in low estrogen and overactivity of osteoclasts, while low energy availability alters the metabolic environment, inducing changes in insulinlike growth factor 1, leptin, and peptide YY, resulting in deficiencies in vitamin D and calcium—nutrients necessary for bone mineralization. In turn, bone health and density are compromised.21,22
Ninety percent of peak bone mass is attained by age 18. Those who have low bone mineral density as part of the female athlete triad can suffer from long-lasting effects on their bone health.
SCREENING
Untreated, the triad can lead to fatigue, poor sports performance, and a number of serious comorbid conditions such as osteopenia and osteoporosis (leading to stress fractures) anemia, heart arrhythmias, and amenorrhea. Therefore, it is important for primary care providers to screen female athletes for the triad during routine office visits.
In 2014, the Triad Consensus Panel recommended screening female athletes at the high school and collegiate levels during a preparticipation physical evaluation and then every year by a primary care physician, athletic trainer, team physician, or coach.23
Risk factors include signs of dietary restriction, low body mass index, delayed menarche, oligomenorrhea or amenorrhea, and bone stress reactions or fractures.23 Athletes should be questioned about their menstrual history (age of menarche, frequency, and duration of menstrual cycles), history of stress fractures, medication history, family history (osteoporosis, eating disorders, and fractures),24,25 and dietary habits.
Physical findings include low body mass index, recent weight loss, orthostatic hypotension, lanugo, hypercarotenemia, and signs of eating disorders (restrictive, binging, purging) (Table 1).25–27
Additionally, it is important to ascertain if the patient receives critical comments regarding performance or body image from coaches, parents, or teammates and if sport-specific training began early in life.
Certain personality factors and behaviors are clues, such as perfectionism, obsessiveness, frequent weight cycling, and overtraining.4,25 If any of the triad components are apparent, a deeper evaluation can be completed.
Specific screening questions
The Female Athlete Triad Coalition recommends asking 11 screening questions and having prompt discussions regarding the athlete’s nutritional status and body image.23 If the patient gives a worrisome response to a screening question, further workup for a formal diagnosis should be initiated.
Questions about nutritional status.
- Do you worry about your weight?
- Are you trying to gain or lose weight, or has anyone recommended that you do so?
- Are you on a special diet or do you avoid certain types of foods or food groups?
- Have you ever had an eating disorder?
Questions about menstrual function.
- Have you ever had a menstrual period?
- How old were you when you had your first menstrual period?
- When was your most recent menstrual period?
- How many periods have you had in the last 12 months?
- Are you presently taking any female hormones (estrogen, progesterone, birth control pills)?
Questions about bone health.
- Have you ever had a stress fracture?
- Have you ever been told you have low bone density (osteopenia or osteoporosis)?
Along similar lines, the American Academy of Pediatrics, American Academy of Family Physicians, and American College of Sports Medicine28 have a list of 7 questions:
- Do you worry about your weight?
- Do you limit the foods you eat?
- Do you lose weight to meet image requirements for sports?
- Have you ever suffered from an eating disorder?
- How old were you when you had your first menstrual period?
- How many menstrual cycles have you had in the past 12 months?
- Have you ever had a stress fracture?
These questions are not being widely used. A study of the National Collegiate Athletic Association Division I universities found that only 9% of universities included 9 or more of the recommended 12 questions that the Female Athlete Triad Coalition was recommending at that time, and 22% asked only 1 or 2 of the questions. None of the universities included all 12.29 These findings are not surprising, given that screening for the triad is not state-mandated. Screening discrepancies among providers largely stem from knowledge gaps, nonstandardized questionnaires, lack of time at appointments, and the sensitive nature of the questioning (eg, disordered eating).30
DIAGNOSING THE TRIAD
Given that the signs of low energy availability and menstrual dysfunction are often subtle, the diagnosis of the triad for those at risk requires input from a multidisciplinary team including a physician, sports dietitian, mental health professional, exercise physiologist, and other medical consultants.
Table 2 lists diagnostic tests the primary care provider should consider.
Diagnosing low energy availability
Energy availability is the dietary energy remaining after exercise energy expenditure; it is normalized to fat-free (lean) mass to account for resting energy expenditure. It is a product of energy intake, energy expenditure, and stored energy, and is calculated as:
An optimal value is at least 45 kcal/kg/day, while physiologic changes start to occur at less than 30 kcal/kg/day.4,31 Low energy is often seen in adult patients with a body mass index less than 17.5 kg/m2 and adolescent patients who are less than 80% of expected body weight.
Energy availability is hard to calculate, but certain assessments can be performed in a primary care setting to approximate it. To assess dietary intake, patients can bring in a 3-, 4-, or 7-day dietary log or complete a 24-hour food recall or food-frequency questionnaire in the office. To objectively document energy expenditure, patients can use heart rate monitors, accelerometers, an exercise diary, and web-based calculators. The fat-free mass can be calculated using a bioelectric impedance scale and skinfold caliper measurements.26
Those with chronic energy deficiency states may have reduced resting metabolic rates, with measured rates less than 90% of predicted and low triiodothyronine (T3) levels.31
Diagnosing menstrual dysfunction
When evaluating patients with menstrual dysfunction, it is important to first rule out pregnancy and endocrinopathies. These include thyroid dysfunction, hyperprolactinemia, primary ovarian insufficiency, other hypothalamic and pituitary disorders, and hyperandrogenic conditions such as polycystic ovarian syndrome, ovarian tumor, adrenal tumor, nonclassic congenital adrenal hyperplasia, and Cushing syndrome.
Depending on the patient’s age, laboratory tests can include follicle-stimulating hormone, luteinizing hormone, prolactin, serum estradiol, and a progesterone challenge.32 For hyperandrogenic symptoms, measuring total and free testosterone, dehydroepiandrosterone sulfate, 24-hour urine cortisol, and 17-hydroxyprogesterone levels may be helpful.
An endocrinologist should be consulted to evaluate the underlying cause of amenorrhea and address any associated hormonal imbalances. Attributing menstrual dysfunction to low energy availability is generally a diagnosis of exclusion. Additionally, outflow tract obstruction should be considered and ruled out with transvaginal ultrasonography in patients with primary amenorrhea.
A patient with hypoestrogenemia and amenorrhea may have the same steroid hormone profile as that of a menopausal woman. Lack of estrogen results in impaired endothelial cell function and arterial dilation, with accelerated development of atherosclerosis and subsequent cardiovascular events.33,34 Further, low energy availability has been linked to negative cardiovascular effects such as decreased vessel dilation leading to decreased tissue perfusion and hastened development of atherosclerosis.33 Female athletes with hypoestrogenism may show reduced perfusion of working muscle, impaired aerobic metabolism in skeletal muscle, elevated low-density lipoprotein cholesterol, and vaginal dryness.4
Diagnosing low bone mineral density
The most common clinical manifestations of low bone mineral density in female athletes are bone stress reactions such as stress fractures. In a study of 311 female high school athletes, 65.6% suffered from musculoskeletal injury from trauma or overuse including stress fractures and the patellofemoral syndrome.35 Many athletes seek medical attention from their primary care physician for stress reactions, providing an opportunity for triad screening.36
In postmenopausal women, osteopenia and osteoporosis are defined using the T score. However, in premenopausal women and adolescents, the International Society for Clinical Densitometry recommends using the Z score. A Z score less than –2.0 is described as “low bone density for chronological age.”14 For the diagnosis of osteoporosis in children and premenopausal women, the Society recommends using a Z score less than –2.0 along with the presence of a secondary risk factor for fracture such as undernutrition, hypogonadism, or a history of fracture.
Table 2 summarizes the diagnosis of low bone mineral density and osteoporosis in premenopausal women, adolescents, and children as well as when to order dual-energy x-ray absorptiometry (DEXA).37,38
Adolescents with low bone mineral density should have an annual DEXA scan of the total hip and lumbar spine.22 Amenorrheic athletes typically present with low areal density at the lumbar spine, reduced trabecular volumetric bone mineral density and bone strength index at the distal radius, and deterioration of the distal tibia.39
EARLY INTERVENTION IS ESSENTIAL
Early intervention is essential in patients with any component of the female athlete triad to prevent long-term adverse health effects. Successful treatment is strongly correlated with a trusting relationship between the athlete and the multidisciplinary team involved in her treatment.40
If needed, selective serotonin reuptake inhibitors and other psychotropic medications can be prescribed for comorbid conditions including bulimia nervosa, anxiety, depression, and obsessive-compulsive disorder. Primary providers can identify risk factors that prompt the evaluation and diagnosis of the triad, as well as support the goals of treatment and help manage comorbid conditions.
Eat more, exercise less
The primary goal is to restore body weight, maximizing nutritional and energy status by modifying the diet and adjusting exercise behavior to increase energy availability.41 Creating an energy-positive state by increasing intake, decreasing energy expenditure, or both increases energy availability, subsequently improves bone mineral density, and normalizes menstrual function.40
To sustain normal physiologic function, an energy availability of at least 45 kcal/kg/day is recommended.42 Patients should consume a minimum of 2,000 kcal/day, although energy needs may far exceed that, depending on energy expenditure. Olympic athletes participating in women’s crew and other sports have been anecdotally known to require over 12,000 kcal a day to maintain weight and performance. Goals include a body mass index of at least 18.5 kg/m2 in adults and a body weight of at least 90% of predicted in adolescents.
Involving a dietitian in the care team can help ensure that the patient consumes an adequate amount of macronutrients and micronutrients necessary for bone growth; these include calcium, vitamin D, iron, zinc, and vitamin K.4,32 For patients with disordered eating, referral to a mental health professional is important to help them avoid pathologic eating behaviors, reduce dieting attempts, and alter negative emotions associated with food and body image.
Once treatment begins, patients must undergo standardized periodic monitoring of their body weight. Although positive effects such as normalization of metabolic hormones (eg, insulinlike growth factor 1) may be seen in days to weeks by reversing low energy availability, it may take several months for menstrual function to improve and years for measurable improvement in bone mineral density to occur.23
Menstrual function should improve with weight gain
Normalizing menses in patients with the female athlete triad depends on improving the low energy availability and inducing weight gain.
Pharmacotherapy such as combined oral contraceptives can treat symptoms of hypogonadism.25 However, combined oral contraceptives do not restore spontaneous menses but rather induce withdrawal bleeding, which can lead to a false sense of security.23 While there are some benefits to prescribing combined oral contraceptives to treat hypogonadism, nonpharmacologic methods should be tried initially to restore menses, including increasing caloric intake and body weight. Golden et al showed that hormone replacement with combined oral contraceptives did not improve bone density in women with low estrogen states (eg, anorexia nervosa, osteopenia).43 Further, combined oral contraceptives may worsen bone health, as oral estrogen suppresses hepatic production of insulinlike growth factor 1, a bone trophic hormone.23
Treating low bone mineral density
Improving energy availability and menstrual function can help improve bone mineral density. Nutritional enhancement is recommended for mineralization of trabecular bone and growth of cortical bone. Supplemental calcium (1,000–1,500 mg daily) and vitamin D (600–1,000 IU daily) should be incorporated into the treatment of low bone mineral density.15,19
Additionally, weight gain has a positive effect on bone mineral density independent of its effect on the resumption of menses. However, weight gain alone does not normalize bone mineral density. Resuming normal physiologic production of hormones with estrogen-dependent effects on bone health is integral to normalization as well.23
Resistance training is encouraged to increase lean mass, although caution must be used to prevent fractures during high-impact activity. Bone mineral density may take up to several years to improve and may not be fully reversible.4,25,39
Pharmacologic therapy for low bone mineral density has unclear outcomes in women under age 50. The decision to treat should be based on bone mineral density along with fracture history. Given their unknown effects on the human fetal skeleton, bisphosphonates and denosumab should be used with caution in women of childbearing potential.24 No studies have used denosumab or teriparatide in women with the female athlete triad. Despite concerns regarding use of these drugs in premenopausal women, drug therapy should be strongly considered in women with a history of fracture and those with a high risk of subsequent fracture. The decision to treat can be made in conjunction with the athlete’s endocrinologist.
RETURN TO PLAY
If an athlete is noted to be at risk for or diagnosed with the female athlete triad, it is important to formulate a plan for her to return to play once her health improves.
De Souza et al provided a cumulative risk assessment for risk stratification and made recommendations on when an athlete should return to play depending on her level of risk.23 Using this grading system, primary care physicians can risk-stratify their patients. Those at low risk may be fully cleared to return to play, while those at moderate to high risk must first follow up with a multidisciplinary team to develop treatment strategies for improving their health.
Once a patient reaches her established goals, she may provisionally return to play under the close supervision of a team physician or primary care physician. A written treatment contract, including the goals set by the multidisciplinary team, should be followed closely as the athlete continues to participate in the sport.23;
Striving for athletic excellence, many young women—and some young men—create an energy deficit from increased exercise, decreased intake, or both. In women, the resulting energy deficit can suppress the menstrual cycle and in turn lead to bone demineralization in a syndrome called the female athlete triad.
Primary care physicians should be aware of this syndrome because it can lead to short-term and long-term health complications, and they are in a good position to screen for, diagnose, and treat it. However, a study of 931 US physicians in 2015 found that only 37% had heard of it.1
DEFINITION HAS CHANGED: ONLY 1 OF 3 COMPONENTS NEEDED
In 1972, Title IX of the Education Amendment Act was passed, prohibiting sex discrimination in any higher education program or activity receiving federal financial aid. Since then, female athletic participation in the United States has increased more than 10-fold.2
Also increasing has been awareness of the link between athletics, eating disorders, and amenorrhea. The American College of Sports Medicine coined the term female athlete triad in 1992, describing it as the constellation of disordered eating, amenorrhea, and osteoporosis (all 3 needed to be present).3 They broadened the definition in 2007 so that the syndrome can be diagnosed if any of the following is present4:
- Low energy availability (with or without an eating disorder)
- Menstrual dysfunction
- Decreased bone mineral density.
Recognizing that low energy availability can affect athletes of either sex and have consequences beyond the female reproductive system and skeleton, in 2014 the International Olympic Committee introduced a broader term called relative energy deficiency in sport.5,6 Like the triad, this condition occurs when energy intake falls below energy output to the point that it negatively affects an athlete’s physical and mental health.
THE COMPONENTS ARE COMMON
The female athlete triad can be seen in high school, collegiate, and elite athletes7 and is especially common in sports with subjective judging (gymnastics, figure skating) or endurance sports that emphasize leanness (eg, running).8
In a review of 65 studies, Gibbs et al9 found that the prevalence of any one of the triad conditions in exercising women and female athletes ranged from 16.0% to 60.0%, the prevalence of any 2 ranged from 2.7% to 27.0%, and the prevalence of all 3 ranged from 0% to 15.9%.
Low energy availability is categorized as either intentional (ie, due to disordered eating) or unintentional (ie, due to activities not associated with eating). Sustained low energy availability is often associated with eating disorders and subsequent low self-esteem, depression, and anxiety disorders.4
The prevalence of eating disorders is high in female athletes—31% and 20% in 2 large studies of elite female athletes, compared with 5.5% and 9%, respectively, in the general population.10,11 Another study found that the prevalence of disordered eating was 46.7% in sports that emphasize leanness, such as track and gymnastics, compared with 19.8% in sports that did not, such as basketball and soccer.12
Calorie restriction is common. In a study of 15 elite ballet dancers and 15 matched controls, the dancers were found to consume only about 3/4 as many calories per day as the controls (1,577 vs 2,075 kcal/day, P ≤ .01).13
Menstrual dysfunction. In small studies, the prevalence of secondary amenorrhea was as high as 69% in dancers and 65% in long-distance runners.4,14–16
Decreased bone mineral density. According to a systematic review, the prevalence of osteopenia in amenorrheic athletes ranged between 22% and 50% and the prevalence of osteoporosis was 0% to 13%, compared with 12% and 2.3%, respectively, in the general population.17
THE COMPONENTS ARE LINKED
dysfunction play causative roles in bone mineral density pathology. Within each component of the triad a spectrum of dysfunction exists, with all 3 components exhibiting serious health end points including low energy availability, functional hypothalamic amenorrhea, and osteoporosis.
The 3 components of the female athlete triad—low energy availability, menstrual dysfunction, and decreased bone mineral density—are linked, and each exists on a spectrum (Figure 1). The long-term consequences are far-reaching and can affect the cardiovascular, endocrine, reproductive, skeletal, gastrointestinal, renal, and central nervous systems.
Low energy availability is the driving force of the triad, causing menstrual irregularity and subsequent low bone mineral density.
Menstrual dysfunction. Low energy availability can contribute to menstrual disturbances because the body suppresses reproductive function to prevent pregnancy. Functional hypothalamic amenorrhea results from decreased gonadotropin-releasing hormone leading to decreased gonadotropin release from the pituitary gland and, ultimately, to low circulating estrogen levels.18 Menstrual irregularities related to the triad include:
- Primary amenorrhea (a delay in menarche)
- Oligomenorrhea (menstrual cycles occurring at intervals greater than every 35 days)
- Secondary amenorrhea (cessation of menstruation for 3 consecutive months).
(Primary amenorrhea is defined as no menses by age 15 in the presence of normal secondary sexual development or within 5 years after breast development if that occurs before the age of 10. Secondary amenorrhea is defined as the loss of menses for 90 or more days after menarche.19)
In animal studies, reducing dietary intake by more than 30% resulted in infertility.4 Menstrual abnormalities can present as early as 5 days after a patient enters a state of low energy availability.20 Symptoms of menstrual dysfunction are largely indicative of hypogonadism and include vaginal dryness, infertility, and impaired bone health.
Bone health in women with the female athlete triad can range from optimal to osteoporosis.
Low bone mineral density is a result of low energy availability and menstrual dysfunction leading to estrogen deficiency.21,22 Specifically, menstrual abnormalities can result in low estrogen and overactivity of osteoclasts, while low energy availability alters the metabolic environment, inducing changes in insulinlike growth factor 1, leptin, and peptide YY, resulting in deficiencies in vitamin D and calcium—nutrients necessary for bone mineralization. In turn, bone health and density are compromised.21,22
Ninety percent of peak bone mass is attained by age 18. Those who have low bone mineral density as part of the female athlete triad can suffer from long-lasting effects on their bone health.
SCREENING
Untreated, the triad can lead to fatigue, poor sports performance, and a number of serious comorbid conditions such as osteopenia and osteoporosis (leading to stress fractures) anemia, heart arrhythmias, and amenorrhea. Therefore, it is important for primary care providers to screen female athletes for the triad during routine office visits.
In 2014, the Triad Consensus Panel recommended screening female athletes at the high school and collegiate levels during a preparticipation physical evaluation and then every year by a primary care physician, athletic trainer, team physician, or coach.23
Risk factors include signs of dietary restriction, low body mass index, delayed menarche, oligomenorrhea or amenorrhea, and bone stress reactions or fractures.23 Athletes should be questioned about their menstrual history (age of menarche, frequency, and duration of menstrual cycles), history of stress fractures, medication history, family history (osteoporosis, eating disorders, and fractures),24,25 and dietary habits.
Physical findings include low body mass index, recent weight loss, orthostatic hypotension, lanugo, hypercarotenemia, and signs of eating disorders (restrictive, binging, purging) (Table 1).25–27
Additionally, it is important to ascertain if the patient receives critical comments regarding performance or body image from coaches, parents, or teammates and if sport-specific training began early in life.
Certain personality factors and behaviors are clues, such as perfectionism, obsessiveness, frequent weight cycling, and overtraining.4,25 If any of the triad components are apparent, a deeper evaluation can be completed.
Specific screening questions
The Female Athlete Triad Coalition recommends asking 11 screening questions and having prompt discussions regarding the athlete’s nutritional status and body image.23 If the patient gives a worrisome response to a screening question, further workup for a formal diagnosis should be initiated.
Questions about nutritional status.
- Do you worry about your weight?
- Are you trying to gain or lose weight, or has anyone recommended that you do so?
- Are you on a special diet or do you avoid certain types of foods or food groups?
- Have you ever had an eating disorder?
Questions about menstrual function.
- Have you ever had a menstrual period?
- How old were you when you had your first menstrual period?
- When was your most recent menstrual period?
- How many periods have you had in the last 12 months?
- Are you presently taking any female hormones (estrogen, progesterone, birth control pills)?
Questions about bone health.
- Have you ever had a stress fracture?
- Have you ever been told you have low bone density (osteopenia or osteoporosis)?
Along similar lines, the American Academy of Pediatrics, American Academy of Family Physicians, and American College of Sports Medicine28 have a list of 7 questions:
- Do you worry about your weight?
- Do you limit the foods you eat?
- Do you lose weight to meet image requirements for sports?
- Have you ever suffered from an eating disorder?
- How old were you when you had your first menstrual period?
- How many menstrual cycles have you had in the past 12 months?
- Have you ever had a stress fracture?
These questions are not being widely used. A study of the National Collegiate Athletic Association Division I universities found that only 9% of universities included 9 or more of the recommended 12 questions that the Female Athlete Triad Coalition was recommending at that time, and 22% asked only 1 or 2 of the questions. None of the universities included all 12.29 These findings are not surprising, given that screening for the triad is not state-mandated. Screening discrepancies among providers largely stem from knowledge gaps, nonstandardized questionnaires, lack of time at appointments, and the sensitive nature of the questioning (eg, disordered eating).30
DIAGNOSING THE TRIAD
Given that the signs of low energy availability and menstrual dysfunction are often subtle, the diagnosis of the triad for those at risk requires input from a multidisciplinary team including a physician, sports dietitian, mental health professional, exercise physiologist, and other medical consultants.
Table 2 lists diagnostic tests the primary care provider should consider.
Diagnosing low energy availability
Energy availability is the dietary energy remaining after exercise energy expenditure; it is normalized to fat-free (lean) mass to account for resting energy expenditure. It is a product of energy intake, energy expenditure, and stored energy, and is calculated as:
An optimal value is at least 45 kcal/kg/day, while physiologic changes start to occur at less than 30 kcal/kg/day.4,31 Low energy is often seen in adult patients with a body mass index less than 17.5 kg/m2 and adolescent patients who are less than 80% of expected body weight.
Energy availability is hard to calculate, but certain assessments can be performed in a primary care setting to approximate it. To assess dietary intake, patients can bring in a 3-, 4-, or 7-day dietary log or complete a 24-hour food recall or food-frequency questionnaire in the office. To objectively document energy expenditure, patients can use heart rate monitors, accelerometers, an exercise diary, and web-based calculators. The fat-free mass can be calculated using a bioelectric impedance scale and skinfold caliper measurements.26
Those with chronic energy deficiency states may have reduced resting metabolic rates, with measured rates less than 90% of predicted and low triiodothyronine (T3) levels.31
Diagnosing menstrual dysfunction
When evaluating patients with menstrual dysfunction, it is important to first rule out pregnancy and endocrinopathies. These include thyroid dysfunction, hyperprolactinemia, primary ovarian insufficiency, other hypothalamic and pituitary disorders, and hyperandrogenic conditions such as polycystic ovarian syndrome, ovarian tumor, adrenal tumor, nonclassic congenital adrenal hyperplasia, and Cushing syndrome.
Depending on the patient’s age, laboratory tests can include follicle-stimulating hormone, luteinizing hormone, prolactin, serum estradiol, and a progesterone challenge.32 For hyperandrogenic symptoms, measuring total and free testosterone, dehydroepiandrosterone sulfate, 24-hour urine cortisol, and 17-hydroxyprogesterone levels may be helpful.
An endocrinologist should be consulted to evaluate the underlying cause of amenorrhea and address any associated hormonal imbalances. Attributing menstrual dysfunction to low energy availability is generally a diagnosis of exclusion. Additionally, outflow tract obstruction should be considered and ruled out with transvaginal ultrasonography in patients with primary amenorrhea.
A patient with hypoestrogenemia and amenorrhea may have the same steroid hormone profile as that of a menopausal woman. Lack of estrogen results in impaired endothelial cell function and arterial dilation, with accelerated development of atherosclerosis and subsequent cardiovascular events.33,34 Further, low energy availability has been linked to negative cardiovascular effects such as decreased vessel dilation leading to decreased tissue perfusion and hastened development of atherosclerosis.33 Female athletes with hypoestrogenism may show reduced perfusion of working muscle, impaired aerobic metabolism in skeletal muscle, elevated low-density lipoprotein cholesterol, and vaginal dryness.4
Diagnosing low bone mineral density
The most common clinical manifestations of low bone mineral density in female athletes are bone stress reactions such as stress fractures. In a study of 311 female high school athletes, 65.6% suffered from musculoskeletal injury from trauma or overuse including stress fractures and the patellofemoral syndrome.35 Many athletes seek medical attention from their primary care physician for stress reactions, providing an opportunity for triad screening.36
In postmenopausal women, osteopenia and osteoporosis are defined using the T score. However, in premenopausal women and adolescents, the International Society for Clinical Densitometry recommends using the Z score. A Z score less than –2.0 is described as “low bone density for chronological age.”14 For the diagnosis of osteoporosis in children and premenopausal women, the Society recommends using a Z score less than –2.0 along with the presence of a secondary risk factor for fracture such as undernutrition, hypogonadism, or a history of fracture.
Table 2 summarizes the diagnosis of low bone mineral density and osteoporosis in premenopausal women, adolescents, and children as well as when to order dual-energy x-ray absorptiometry (DEXA).37,38
Adolescents with low bone mineral density should have an annual DEXA scan of the total hip and lumbar spine.22 Amenorrheic athletes typically present with low areal density at the lumbar spine, reduced trabecular volumetric bone mineral density and bone strength index at the distal radius, and deterioration of the distal tibia.39
EARLY INTERVENTION IS ESSENTIAL
Early intervention is essential in patients with any component of the female athlete triad to prevent long-term adverse health effects. Successful treatment is strongly correlated with a trusting relationship between the athlete and the multidisciplinary team involved in her treatment.40
If needed, selective serotonin reuptake inhibitors and other psychotropic medications can be prescribed for comorbid conditions including bulimia nervosa, anxiety, depression, and obsessive-compulsive disorder. Primary providers can identify risk factors that prompt the evaluation and diagnosis of the triad, as well as support the goals of treatment and help manage comorbid conditions.
Eat more, exercise less
The primary goal is to restore body weight, maximizing nutritional and energy status by modifying the diet and adjusting exercise behavior to increase energy availability.41 Creating an energy-positive state by increasing intake, decreasing energy expenditure, or both increases energy availability, subsequently improves bone mineral density, and normalizes menstrual function.40
To sustain normal physiologic function, an energy availability of at least 45 kcal/kg/day is recommended.42 Patients should consume a minimum of 2,000 kcal/day, although energy needs may far exceed that, depending on energy expenditure. Olympic athletes participating in women’s crew and other sports have been anecdotally known to require over 12,000 kcal a day to maintain weight and performance. Goals include a body mass index of at least 18.5 kg/m2 in adults and a body weight of at least 90% of predicted in adolescents.
Involving a dietitian in the care team can help ensure that the patient consumes an adequate amount of macronutrients and micronutrients necessary for bone growth; these include calcium, vitamin D, iron, zinc, and vitamin K.4,32 For patients with disordered eating, referral to a mental health professional is important to help them avoid pathologic eating behaviors, reduce dieting attempts, and alter negative emotions associated with food and body image.
Once treatment begins, patients must undergo standardized periodic monitoring of their body weight. Although positive effects such as normalization of metabolic hormones (eg, insulinlike growth factor 1) may be seen in days to weeks by reversing low energy availability, it may take several months for menstrual function to improve and years for measurable improvement in bone mineral density to occur.23
Menstrual function should improve with weight gain
Normalizing menses in patients with the female athlete triad depends on improving the low energy availability and inducing weight gain.
Pharmacotherapy such as combined oral contraceptives can treat symptoms of hypogonadism.25 However, combined oral contraceptives do not restore spontaneous menses but rather induce withdrawal bleeding, which can lead to a false sense of security.23 While there are some benefits to prescribing combined oral contraceptives to treat hypogonadism, nonpharmacologic methods should be tried initially to restore menses, including increasing caloric intake and body weight. Golden et al showed that hormone replacement with combined oral contraceptives did not improve bone density in women with low estrogen states (eg, anorexia nervosa, osteopenia).43 Further, combined oral contraceptives may worsen bone health, as oral estrogen suppresses hepatic production of insulinlike growth factor 1, a bone trophic hormone.23
Treating low bone mineral density
Improving energy availability and menstrual function can help improve bone mineral density. Nutritional enhancement is recommended for mineralization of trabecular bone and growth of cortical bone. Supplemental calcium (1,000–1,500 mg daily) and vitamin D (600–1,000 IU daily) should be incorporated into the treatment of low bone mineral density.15,19
Additionally, weight gain has a positive effect on bone mineral density independent of its effect on the resumption of menses. However, weight gain alone does not normalize bone mineral density. Resuming normal physiologic production of hormones with estrogen-dependent effects on bone health is integral to normalization as well.23
Resistance training is encouraged to increase lean mass, although caution must be used to prevent fractures during high-impact activity. Bone mineral density may take up to several years to improve and may not be fully reversible.4,25,39
Pharmacologic therapy for low bone mineral density has unclear outcomes in women under age 50. The decision to treat should be based on bone mineral density along with fracture history. Given their unknown effects on the human fetal skeleton, bisphosphonates and denosumab should be used with caution in women of childbearing potential.24 No studies have used denosumab or teriparatide in women with the female athlete triad. Despite concerns regarding use of these drugs in premenopausal women, drug therapy should be strongly considered in women with a history of fracture and those with a high risk of subsequent fracture. The decision to treat can be made in conjunction with the athlete’s endocrinologist.
RETURN TO PLAY
If an athlete is noted to be at risk for or diagnosed with the female athlete triad, it is important to formulate a plan for her to return to play once her health improves.
De Souza et al provided a cumulative risk assessment for risk stratification and made recommendations on when an athlete should return to play depending on her level of risk.23 Using this grading system, primary care physicians can risk-stratify their patients. Those at low risk may be fully cleared to return to play, while those at moderate to high risk must first follow up with a multidisciplinary team to develop treatment strategies for improving their health.
Once a patient reaches her established goals, she may provisionally return to play under the close supervision of a team physician or primary care physician. A written treatment contract, including the goals set by the multidisciplinary team, should be followed closely as the athlete continues to participate in the sport.23;
- Curry EJ, Logan C, Ackerman K, McInnis KC, Matzkin EG. Female athlete triad awareness among multispecialty physicians. Sports Med Open 2015; 1(1):38. doi:10.1186/s40798-015-0037-5
- National Federation of State High School Associations. 2012–13 high school athletics participation survey. http://old.nfhs.org/content.aspx?id=3282. Accessed February 1, 2018.
- Otis CL, Drinkwater B, Johnson M, Loucks A, Wilmore J. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 1997; 29(5):i–ix.
- Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 2007; 39(10):1867–1882. doi:10.1249/mss.0b013e318149f111
- Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sport (RED-S). Br J Sports Med 2014; 48(7):491–497. doi:10.1136/bjsports-2014-093502
- Tenforde AS, Barrack MT, Nattiv A, Fredericson M. Parallels with the female athlete triad in male athletes. Sports Med 2016; 46(2):171–182. doi:10.1007/s40279-015-0411-y
- Thein-Nissenbaum JM, Carr KE. Female athlete triad syndrome in the high school athlete. Phys Ther Sport 2011; 12(3):108–116. doi:10.1016/j.ptsp.2011.04.002
- Matzkin E, Curry EJ, Whitlock K. Female athlete triad: past, present, and future. J Am Acad Orthop Surg 2015; 23(7):424–432. doi:10.5435/JAAOS-D-14-00168
- Gibbs JC, Williams NI, De Souza MJ. Prevalence of individual and combined components of the female athlete triad. Med Sci Sports Exerc 2013; 45(5):985–996. doi:10.1249/MSS.0b013e31827e1bdc
- Byrne S, McLean N. Elite athletes: effects of the pressure to be thin. J Sci Med Sport 2002; 5(2):80–94. doi:10.1016/S1440-2440(02)80029-9
- Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med 2004; 14(1):25–32.
- Lynch SL, Hoch AZ. The female runner: gender specifics. Clin Sports Med 2010; 29(3):477–498. doi:10.1016/j.csm.2010.03.003
- Doyle-Lucas AF, Akers JD, Davy BM. Energetic efficiency, menstrual irregularity, and bone mineral density in elite professional female ballet dancers. J Dance Med Sci 2010; 14(4):146–154.
- Thein-Nissenbaum J. Long term consequences of the female athlete triad. Maturitas 2013; 75(2):107–112. doi:10.1016/j.maturitas.2013.02.010
- Hilibrand MJ, Hammoud S, Bishop M, Woods D, Fredrick RW, Dodson CC. Common injuries and ailments of the female athlete; pathophysiology, treatment and prevention. Phys Sportsmed 2015; 43(4):403–411. doi:10.1080/00913847.2015.1092856
- Demorest RA, Hergenroeder AC. Preface. Sports medicine and sports injuries. Adolesc Med State Art Rev 2015; 26(1):xv–xvi.
- Khan KM, Liu-Ambrose T, Sran MM, Ashe MC, Donaldson MG, Wark JD. New criteria for female athlete triad syndrome? Br J Sports Med 2002; 36(1):10–13. doi:10.1136/bjsm.36.1.10
- Fontana R, Della Torre S. The deep correlation between energy metabolism and reproduction: a view of the effects of nutrition for women fertility. Nutrients 2016; 8:87. www.ncbi.nlm.nih.gov/pmc/articles/PMC4772050/. Accessed February 2, 2018.
- Nazem TG, Ackerman K. The female athlete triad. Sports Health 2012; 4(4):302–311. doi:10.1177/1941738112439685
- Pantano KJ. Coaching concerns in physically active girls and young women—part 1: the female athlete triad. Strength Conditioning J 2009; 31(6):38–43. doi:10.1519/SSC.0b013e3181c105dd
- Micklesfield LK, Hugo J, Johnson C, Noakes TD, Lambert EV. Factors associated with menstrual dysfunction and self-reported bone stress injuries in female runners in the ultra- and half-marathons of the Two Oceans. Br J Sports Med 2007; 41(10):679–683. doi:10.1136/bjsm.2007.037077
- Lambrinoudaki I, Papadimitriou D. Pathophysiology of bone loss in the female athlete. Ann N Y Acad Sci 2010; 1205:45–50. doi:10.1111/j.1749-6632.2010.05681.x
- De Souza MJ, Nattiv A, Joy E, et al. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad: 1st International Conference held in San Francisco, CA, May 2012, and 2nd International Conference held in Indianapolis, IN, May 2013. Clin J Sport Med 2014; 24(2):96–119. doi:10.1136/bjsports-2013-093218
- Horn E, Gergen N, McGarry KA. The female athlete triad. R I Med J (2013) 2014; 97(11):18–21.
- Joy E, De Souza MJ, Nattiv A, et al. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad. Curr Sports Med Rep 2014; 13(4):219–232. doi:10.1249/JSR.0000000000000077
- Temme KE, Hoch AZ. Recognition and rehabilitation of the female athlete triad/tetrad: a multidisciplinary approach. Curr Sports Med Rep 2013; 12(3):190–199. doi:10.1249/JSR.0b013e318296190b
- Pritts SD, Susman J. Diagnosis of eating disorders in primary care. Am Fam Physician 2003; 67(2):297–304.
- Berhardt DR, Roberts WO, editors. Preparticipation Physical Evaluation, 4th Ed. American Academy of Pediatrics, Elk Grove Village, IL, 2010.
- Mencias T, Noon M, Hoch AZ. Female athlete triad screening in National Collegiate Athletic Association Division I athletes: is the preparticipation evaluation form effective? Clin J Sport Med 2012; 22(2):122–125. doi:10.1097/JSM.0b013e3182425aee
- Javed A, Tebben PJ, Fischer PR, Lteif AN. Female athlete triad and its components: toward improved screening and management. Mayo Clin Proc 2013; 88(9):996–1009. doi:10.1016/j.mayocp.2013.07.001
- Melin A, Tornberg AB, Skouby S, et al. Energy availability and the female athlete triad in elite endurance athletes. Scand J Med Sci Sports 2015; 25(5):610–622. doi:10.1111/sms.12261
- Warr BJ, Woolf K. The female athlete triad: patients do best with a team approach to care. JAAPA 2011; 24(4):50–55.
- Hoch AZ, Lal S, Jurva JW, Gutterman DD. The female athlete triad and cardiovascular dysfunction. Phys Med Rehabil Clin North Am 2007; 18(3):385–400. doi:10.1016/j.pmr.2007.05.001
- Lanser EM, Zach KN, Hoch AZ. The female athlete triad and endothelial dysfunction. PM R 2011; 3(5):458–465. doi:10.1016/j.pmrj.2010.12.024
- Thein-Nissenbaum JM, Rauh MJ, Carr KE, Loud KJ, McGuine TA. Associations between disordered eating, menstrual dysfunction, and musculoskeletal injury among high school athletes. J Orthop Sports Phys Ther 2011; 41(2):60–69. doi:10.2519/jospt.2011.3312
- Ducher G, Turner AI, Kukuljan S, et al. Obstacles in the optimization of bone health outcomes in the female athlete triad. Sports Med 2011; 41(7):587–607. doi:10.2165/11588770-000000000-00000
- Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin North Am 2010; 39(1):155–167. doi:10.1016/j.ecl.2009.11.002
- House S, Loud K, Shubkin C. Female athlete triad for the primary care pediatrician. Curr Opin Pediatr 2013; 25(6):755–761. doi:10.1097/MOP.0000000000000033
- Mallinson RJ, De Souza MJ. Current perspectives on the etiology and manifestation of the “silent” component of the female athlete triad. Int J Womens Health 2014; 6:451–467. doi:10.2147/IJWH.S38603
- Deimel JF, Dunlap BJ. The female athlete triad. Clin Sports Med 2012; 31(2):247–254. doi:10.1016/j.csm.2011.09.007
- Manore MM, Kam LC, Loucks AB; International Association of Athletics Federations. The female athlete triad: components, nutrition issues, and health consequences. J Sports Sci 2007; 25(suppl 1):S61–S71. doi:10.1080/02640410701607320
- Witkop CT, Warren MP. Understanding the spectrum of the female athlete triad. Obstet Gynecol 2010; 116(6):1444–1448. doi:10.1097/AOG.0b013e3181fbed40
- Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002; 15(3):135–143. doi:10.1016/S1083-3188(02)00145-6
- Curry EJ, Logan C, Ackerman K, McInnis KC, Matzkin EG. Female athlete triad awareness among multispecialty physicians. Sports Med Open 2015; 1(1):38. doi:10.1186/s40798-015-0037-5
- National Federation of State High School Associations. 2012–13 high school athletics participation survey. http://old.nfhs.org/content.aspx?id=3282. Accessed February 1, 2018.
- Otis CL, Drinkwater B, Johnson M, Loucks A, Wilmore J. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 1997; 29(5):i–ix.
- Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 2007; 39(10):1867–1882. doi:10.1249/mss.0b013e318149f111
- Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sport (RED-S). Br J Sports Med 2014; 48(7):491–497. doi:10.1136/bjsports-2014-093502
- Tenforde AS, Barrack MT, Nattiv A, Fredericson M. Parallels with the female athlete triad in male athletes. Sports Med 2016; 46(2):171–182. doi:10.1007/s40279-015-0411-y
- Thein-Nissenbaum JM, Carr KE. Female athlete triad syndrome in the high school athlete. Phys Ther Sport 2011; 12(3):108–116. doi:10.1016/j.ptsp.2011.04.002
- Matzkin E, Curry EJ, Whitlock K. Female athlete triad: past, present, and future. J Am Acad Orthop Surg 2015; 23(7):424–432. doi:10.5435/JAAOS-D-14-00168
- Gibbs JC, Williams NI, De Souza MJ. Prevalence of individual and combined components of the female athlete triad. Med Sci Sports Exerc 2013; 45(5):985–996. doi:10.1249/MSS.0b013e31827e1bdc
- Byrne S, McLean N. Elite athletes: effects of the pressure to be thin. J Sci Med Sport 2002; 5(2):80–94. doi:10.1016/S1440-2440(02)80029-9
- Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med 2004; 14(1):25–32.
- Lynch SL, Hoch AZ. The female runner: gender specifics. Clin Sports Med 2010; 29(3):477–498. doi:10.1016/j.csm.2010.03.003
- Doyle-Lucas AF, Akers JD, Davy BM. Energetic efficiency, menstrual irregularity, and bone mineral density in elite professional female ballet dancers. J Dance Med Sci 2010; 14(4):146–154.
- Thein-Nissenbaum J. Long term consequences of the female athlete triad. Maturitas 2013; 75(2):107–112. doi:10.1016/j.maturitas.2013.02.010
- Hilibrand MJ, Hammoud S, Bishop M, Woods D, Fredrick RW, Dodson CC. Common injuries and ailments of the female athlete; pathophysiology, treatment and prevention. Phys Sportsmed 2015; 43(4):403–411. doi:10.1080/00913847.2015.1092856
- Demorest RA, Hergenroeder AC. Preface. Sports medicine and sports injuries. Adolesc Med State Art Rev 2015; 26(1):xv–xvi.
- Khan KM, Liu-Ambrose T, Sran MM, Ashe MC, Donaldson MG, Wark JD. New criteria for female athlete triad syndrome? Br J Sports Med 2002; 36(1):10–13. doi:10.1136/bjsm.36.1.10
- Fontana R, Della Torre S. The deep correlation between energy metabolism and reproduction: a view of the effects of nutrition for women fertility. Nutrients 2016; 8:87. www.ncbi.nlm.nih.gov/pmc/articles/PMC4772050/. Accessed February 2, 2018.
- Nazem TG, Ackerman K. The female athlete triad. Sports Health 2012; 4(4):302–311. doi:10.1177/1941738112439685
- Pantano KJ. Coaching concerns in physically active girls and young women—part 1: the female athlete triad. Strength Conditioning J 2009; 31(6):38–43. doi:10.1519/SSC.0b013e3181c105dd
- Micklesfield LK, Hugo J, Johnson C, Noakes TD, Lambert EV. Factors associated with menstrual dysfunction and self-reported bone stress injuries in female runners in the ultra- and half-marathons of the Two Oceans. Br J Sports Med 2007; 41(10):679–683. doi:10.1136/bjsm.2007.037077
- Lambrinoudaki I, Papadimitriou D. Pathophysiology of bone loss in the female athlete. Ann N Y Acad Sci 2010; 1205:45–50. doi:10.1111/j.1749-6632.2010.05681.x
- De Souza MJ, Nattiv A, Joy E, et al. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad: 1st International Conference held in San Francisco, CA, May 2012, and 2nd International Conference held in Indianapolis, IN, May 2013. Clin J Sport Med 2014; 24(2):96–119. doi:10.1136/bjsports-2013-093218
- Horn E, Gergen N, McGarry KA. The female athlete triad. R I Med J (2013) 2014; 97(11):18–21.
- Joy E, De Souza MJ, Nattiv A, et al. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad. Curr Sports Med Rep 2014; 13(4):219–232. doi:10.1249/JSR.0000000000000077
- Temme KE, Hoch AZ. Recognition and rehabilitation of the female athlete triad/tetrad: a multidisciplinary approach. Curr Sports Med Rep 2013; 12(3):190–199. doi:10.1249/JSR.0b013e318296190b
- Pritts SD, Susman J. Diagnosis of eating disorders in primary care. Am Fam Physician 2003; 67(2):297–304.
- Berhardt DR, Roberts WO, editors. Preparticipation Physical Evaluation, 4th Ed. American Academy of Pediatrics, Elk Grove Village, IL, 2010.
- Mencias T, Noon M, Hoch AZ. Female athlete triad screening in National Collegiate Athletic Association Division I athletes: is the preparticipation evaluation form effective? Clin J Sport Med 2012; 22(2):122–125. doi:10.1097/JSM.0b013e3182425aee
- Javed A, Tebben PJ, Fischer PR, Lteif AN. Female athlete triad and its components: toward improved screening and management. Mayo Clin Proc 2013; 88(9):996–1009. doi:10.1016/j.mayocp.2013.07.001
- Melin A, Tornberg AB, Skouby S, et al. Energy availability and the female athlete triad in elite endurance athletes. Scand J Med Sci Sports 2015; 25(5):610–622. doi:10.1111/sms.12261
- Warr BJ, Woolf K. The female athlete triad: patients do best with a team approach to care. JAAPA 2011; 24(4):50–55.
- Hoch AZ, Lal S, Jurva JW, Gutterman DD. The female athlete triad and cardiovascular dysfunction. Phys Med Rehabil Clin North Am 2007; 18(3):385–400. doi:10.1016/j.pmr.2007.05.001
- Lanser EM, Zach KN, Hoch AZ. The female athlete triad and endothelial dysfunction. PM R 2011; 3(5):458–465. doi:10.1016/j.pmrj.2010.12.024
- Thein-Nissenbaum JM, Rauh MJ, Carr KE, Loud KJ, McGuine TA. Associations between disordered eating, menstrual dysfunction, and musculoskeletal injury among high school athletes. J Orthop Sports Phys Ther 2011; 41(2):60–69. doi:10.2519/jospt.2011.3312
- Ducher G, Turner AI, Kukuljan S, et al. Obstacles in the optimization of bone health outcomes in the female athlete triad. Sports Med 2011; 41(7):587–607. doi:10.2165/11588770-000000000-00000
- Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin North Am 2010; 39(1):155–167. doi:10.1016/j.ecl.2009.11.002
- House S, Loud K, Shubkin C. Female athlete triad for the primary care pediatrician. Curr Opin Pediatr 2013; 25(6):755–761. doi:10.1097/MOP.0000000000000033
- Mallinson RJ, De Souza MJ. Current perspectives on the etiology and manifestation of the “silent” component of the female athlete triad. Int J Womens Health 2014; 6:451–467. doi:10.2147/IJWH.S38603
- Deimel JF, Dunlap BJ. The female athlete triad. Clin Sports Med 2012; 31(2):247–254. doi:10.1016/j.csm.2011.09.007
- Manore MM, Kam LC, Loucks AB; International Association of Athletics Federations. The female athlete triad: components, nutrition issues, and health consequences. J Sports Sci 2007; 25(suppl 1):S61–S71. doi:10.1080/02640410701607320
- Witkop CT, Warren MP. Understanding the spectrum of the female athlete triad. Obstet Gynecol 2010; 116(6):1444–1448. doi:10.1097/AOG.0b013e3181fbed40
- Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002; 15(3):135–143. doi:10.1016/S1083-3188(02)00145-6
KEY POINTS
- Low energy availability is the driving force of the triad, causing menstrual irregularity and subsequent low bone mineral density.
- Recognizing that men as well as women can suffer from energy deficiency and that it can affect more than the female reproductive system and skeleton, the International Olympic Committee has proposed calling the disorder relative energy deficiency in sport.
- Screening for the triad with a specific set of questions is recommended during the preparticipation assessment.
- Early intervention and treatment prevents serious health consequences including life-threatening arrhythmias, amenorrhea, and osteoporosis.
Make no bones about it!
Make no bones about it!
We just celebrated 45 years since the passing of Title IX, which opened the floodgates for women’s participation in sports. According to a report by the National Collegiate Athletic Association,1 in this interval, the participation rate of high school girls increased 1,000 percent, and Division I colleges have the highest female athletic participation rate, with women accounting for 46.7% of athletes.
Participating in competitive sports is especially important for women because it increases self-confidence. Indeed, there is a direct relationship between athletics and women in leadership roles. A 2013 Ernst and Young survey of 821 high-level executives demonstrated that 90% of the women and 96% of the women in chief executive positions had played sports.2
Twenty years after Title IX was passed, physicians identified a triad of symptoms commonly seen in female athletes. The original definition of the female athletic triad consisted of eating disorders, irregular menstrual cycles, and reduced bone mineral density (BMD).3 Malnutrition led to abnormalities in the menstrual cycle, which in turn affected bone density. The triad was thought to most commonly affect women participating in weight-dependent or judging sports, such as gymnastics, ice-skating, and endurance running. However, many athletes remained undiagnosed because specific criteria for the triad diagnosis remained elusive.
In 2007, the American College of Sports Medicine updated the diagnostic guidelines, defining the female athlete triad as a constellation of abnormalities in energy availability, menstrual function, and BMD.4 Each of these 3 components is part of a spectrum ranging from normal to varying degrees of pathology. Thus, the female athlete no longer needs to demonstrate pathology in all 3 components of the triad to be diagnosed with the syndrome. The presence of 1 or 2 of the components on the pathologic side of the spectrum falls under the umbrella of the triad and may meet the criteria for diagnosis, prompting further assessment.
In 2014, the International Olympic Committee (IOC) coined the term relative energy deficiency in sport (RED-S).5 The IOC authors intended for RED-S to be a more comprehensive and broader definition for the triad. As defined in the IOC consensus statement, RED-S is “impaired physiological function including, but not limited to, metabolic rate, menstrual function, bone health, immunity, protein synthesis, [and] cardiovascular health caused by relative energy deficiency.”5 The statement indicates that the underlying problem is “energy deficiency relative to the balance between dietary energy intake and energy expenditure required for health and activities of daily living, growth, and sporting activities.”5 The IOC consensus statement also expands the vulnerable population, discussing the susceptibility of male athletes, athletes of nonwhite ethnicity, and athletes with a disability.
Although there is some contention as to how we should refer to this syndrome, the most important facet is that it can be identified in an office setting. Awareness is key to prevention. Unfortunately, in a 2015 survey of 931 physicians, only 37% could identify the 3 components of the triad.6 If you do not ask your patients about their nutrition, eating habits, and menstrual cycle, it is not possible to identify any potential problems.
WHY IS THIS SO IMPORTANT?
Simply stated, it is important to diagnose and treat the triad syndrome to maintain optimal bone health. About 90% of peak bone mass is acquired by age 18. Bone density continues to build until about the age of 25, after which it is only possible to maintain. If young athletes are losing bone density, it cannot be replaced. Athletes who have a relative energy deficiency and are not maximizing bone growth will potentially struggle with lower bone mineral density later in life. Early awareness of bone health is paramount to sustaining it as we age.
Osteoporosis, often called a silent disease, affects more than 75 million men and women in the United States, Europe, and Japan.7 According to the International Osteoporosis Foundation, more than 8.9 million fractures are secondary to osteoporosis worldwide each year. Astoundingly, this epidemic equates to an osteoporotic fracture every 3 seconds.8
As physicians, we need to do what we can to prevent this, and the easiest prevention is maintaining bone health and adequate nutrition early in life. We know that weight-bearing exercise is important for bone health, but it is counterintuitive to think that active patients who are running and playing sports may be negatively affecting bone health if they have a relative energy deficiency.
Many females (professional athletes, competitive athletes, or just women who want to stay active) exercise excessively and restrict calories to lose or maintain weight. This can be a formula for disaster resulting in a sidelining bone stress injury. A stress fracture occurs when bone experiences more stress or impact than it can handle from overtraining or increasing training too quickly, not providing enough time for the bone to strengthen. Stress fractures can also occur when bone mineral density declines, lowering the impact threshold. This is most often a direct result of a relative energy deficiency or poor nutrition. The incidence of stress fractures in female athletes is 2 to 3 times higher than that in male athletes, and female athletes with missed menstrual cycles have a 2 to 4 times higher risk of stress fractures than females with a normal monthly menstrual cycle.9
The triad syndrome is seen not only in women but in all athletes—including men and Paralympic athletes.10,11 According to Tenforde et al,10 male athletes, particularly those who participate in sports requiring leanness such as cycling and running, can exhibit nutritional deficits, reduced sex hormone levels, and impaired bone health.10
If we can educate patients on the importance of maintaining a healthy diet and supplying active bodies with the energy they need, then many of these injuries could be prevented.
Make no bones about it.
- Wilson AS; NCAA Research Department. 45 years of Title IX: the status of women in intercollegiate athletics. www.ncaa.org/sites/default/files/TitleIX45-295-FINAL_WEB.pdf. Accessed February 5, 2018.
- Ernst and Young; Women Athletes Business Network. Perspectives on sport and teams. www.ey.com/br/pt/about-us/our-sponsorships-and-programs/women-athletes-global-leadership-network---perspectives-on-sport-and-teams. Accessed February 5, 2018.
- Matzkin E, Curry EJ, Whitlock K. Female athlete triad: past, present, and future. J Am Acad Orthop Surg 2015; 23:424–432. doi:10.5435/JAAOS-D-14-00168
- Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 2007; 39:1867–1882. doi:10.1249/mss.0b013e318149f111
- Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sport (RED-S). Br J Sports Med 2014; 48:491–497. doi:10.1136/bjsports-2014-093502
- Curry EJ, Logan C, Ackerman K, McInnis KC, Matzkin EG. Female athlete triad awareness among multispecialty physicians. Sports Med Open 2015; 1:38. doi:10.1186/s40798-015-0037-5
- Who are candidates for prevention and treatment for osteoporosis? Osteoporos Int 1997; 7:1–6.
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17:1726–1733. doi:10.1007/s00198-006-0172-4
- Wentz L, Liu PY, Haymes E, Ilich JZ. Females have a greater incidence of stress fractures than males in both military and athletic populations: a systemic review. Mil Med 2011; 176:420–430.
- Tenforde AS, Barrack MT, Nattiv A, Fredericson M. Parallels with the female athlete triad in male athletes. Sports Med 2016; 46:171–182. doi:10.1007/s40279-015-0411-y
- Blauwet CA, Brook EM, Tenforde AS, et al. Low energy availability, menstrual dysfunction, and low bone mineral density in individuals with a disability: implications for the para athlete population. Sports Med 2017; 47(9)1697–1708. doi:10.1007/s40279-017-0696-0
Make no bones about it!
We just celebrated 45 years since the passing of Title IX, which opened the floodgates for women’s participation in sports. According to a report by the National Collegiate Athletic Association,1 in this interval, the participation rate of high school girls increased 1,000 percent, and Division I colleges have the highest female athletic participation rate, with women accounting for 46.7% of athletes.
Participating in competitive sports is especially important for women because it increases self-confidence. Indeed, there is a direct relationship between athletics and women in leadership roles. A 2013 Ernst and Young survey of 821 high-level executives demonstrated that 90% of the women and 96% of the women in chief executive positions had played sports.2
Twenty years after Title IX was passed, physicians identified a triad of symptoms commonly seen in female athletes. The original definition of the female athletic triad consisted of eating disorders, irregular menstrual cycles, and reduced bone mineral density (BMD).3 Malnutrition led to abnormalities in the menstrual cycle, which in turn affected bone density. The triad was thought to most commonly affect women participating in weight-dependent or judging sports, such as gymnastics, ice-skating, and endurance running. However, many athletes remained undiagnosed because specific criteria for the triad diagnosis remained elusive.
In 2007, the American College of Sports Medicine updated the diagnostic guidelines, defining the female athlete triad as a constellation of abnormalities in energy availability, menstrual function, and BMD.4 Each of these 3 components is part of a spectrum ranging from normal to varying degrees of pathology. Thus, the female athlete no longer needs to demonstrate pathology in all 3 components of the triad to be diagnosed with the syndrome. The presence of 1 or 2 of the components on the pathologic side of the spectrum falls under the umbrella of the triad and may meet the criteria for diagnosis, prompting further assessment.
In 2014, the International Olympic Committee (IOC) coined the term relative energy deficiency in sport (RED-S).5 The IOC authors intended for RED-S to be a more comprehensive and broader definition for the triad. As defined in the IOC consensus statement, RED-S is “impaired physiological function including, but not limited to, metabolic rate, menstrual function, bone health, immunity, protein synthesis, [and] cardiovascular health caused by relative energy deficiency.”5 The statement indicates that the underlying problem is “energy deficiency relative to the balance between dietary energy intake and energy expenditure required for health and activities of daily living, growth, and sporting activities.”5 The IOC consensus statement also expands the vulnerable population, discussing the susceptibility of male athletes, athletes of nonwhite ethnicity, and athletes with a disability.
Although there is some contention as to how we should refer to this syndrome, the most important facet is that it can be identified in an office setting. Awareness is key to prevention. Unfortunately, in a 2015 survey of 931 physicians, only 37% could identify the 3 components of the triad.6 If you do not ask your patients about their nutrition, eating habits, and menstrual cycle, it is not possible to identify any potential problems.
WHY IS THIS SO IMPORTANT?
Simply stated, it is important to diagnose and treat the triad syndrome to maintain optimal bone health. About 90% of peak bone mass is acquired by age 18. Bone density continues to build until about the age of 25, after which it is only possible to maintain. If young athletes are losing bone density, it cannot be replaced. Athletes who have a relative energy deficiency and are not maximizing bone growth will potentially struggle with lower bone mineral density later in life. Early awareness of bone health is paramount to sustaining it as we age.
Osteoporosis, often called a silent disease, affects more than 75 million men and women in the United States, Europe, and Japan.7 According to the International Osteoporosis Foundation, more than 8.9 million fractures are secondary to osteoporosis worldwide each year. Astoundingly, this epidemic equates to an osteoporotic fracture every 3 seconds.8
As physicians, we need to do what we can to prevent this, and the easiest prevention is maintaining bone health and adequate nutrition early in life. We know that weight-bearing exercise is important for bone health, but it is counterintuitive to think that active patients who are running and playing sports may be negatively affecting bone health if they have a relative energy deficiency.
Many females (professional athletes, competitive athletes, or just women who want to stay active) exercise excessively and restrict calories to lose or maintain weight. This can be a formula for disaster resulting in a sidelining bone stress injury. A stress fracture occurs when bone experiences more stress or impact than it can handle from overtraining or increasing training too quickly, not providing enough time for the bone to strengthen. Stress fractures can also occur when bone mineral density declines, lowering the impact threshold. This is most often a direct result of a relative energy deficiency or poor nutrition. The incidence of stress fractures in female athletes is 2 to 3 times higher than that in male athletes, and female athletes with missed menstrual cycles have a 2 to 4 times higher risk of stress fractures than females with a normal monthly menstrual cycle.9
The triad syndrome is seen not only in women but in all athletes—including men and Paralympic athletes.10,11 According to Tenforde et al,10 male athletes, particularly those who participate in sports requiring leanness such as cycling and running, can exhibit nutritional deficits, reduced sex hormone levels, and impaired bone health.10
If we can educate patients on the importance of maintaining a healthy diet and supplying active bodies with the energy they need, then many of these injuries could be prevented.
Make no bones about it.
Make no bones about it!
We just celebrated 45 years since the passing of Title IX, which opened the floodgates for women’s participation in sports. According to a report by the National Collegiate Athletic Association,1 in this interval, the participation rate of high school girls increased 1,000 percent, and Division I colleges have the highest female athletic participation rate, with women accounting for 46.7% of athletes.
Participating in competitive sports is especially important for women because it increases self-confidence. Indeed, there is a direct relationship between athletics and women in leadership roles. A 2013 Ernst and Young survey of 821 high-level executives demonstrated that 90% of the women and 96% of the women in chief executive positions had played sports.2
Twenty years after Title IX was passed, physicians identified a triad of symptoms commonly seen in female athletes. The original definition of the female athletic triad consisted of eating disorders, irregular menstrual cycles, and reduced bone mineral density (BMD).3 Malnutrition led to abnormalities in the menstrual cycle, which in turn affected bone density. The triad was thought to most commonly affect women participating in weight-dependent or judging sports, such as gymnastics, ice-skating, and endurance running. However, many athletes remained undiagnosed because specific criteria for the triad diagnosis remained elusive.
In 2007, the American College of Sports Medicine updated the diagnostic guidelines, defining the female athlete triad as a constellation of abnormalities in energy availability, menstrual function, and BMD.4 Each of these 3 components is part of a spectrum ranging from normal to varying degrees of pathology. Thus, the female athlete no longer needs to demonstrate pathology in all 3 components of the triad to be diagnosed with the syndrome. The presence of 1 or 2 of the components on the pathologic side of the spectrum falls under the umbrella of the triad and may meet the criteria for diagnosis, prompting further assessment.
In 2014, the International Olympic Committee (IOC) coined the term relative energy deficiency in sport (RED-S).5 The IOC authors intended for RED-S to be a more comprehensive and broader definition for the triad. As defined in the IOC consensus statement, RED-S is “impaired physiological function including, but not limited to, metabolic rate, menstrual function, bone health, immunity, protein synthesis, [and] cardiovascular health caused by relative energy deficiency.”5 The statement indicates that the underlying problem is “energy deficiency relative to the balance between dietary energy intake and energy expenditure required for health and activities of daily living, growth, and sporting activities.”5 The IOC consensus statement also expands the vulnerable population, discussing the susceptibility of male athletes, athletes of nonwhite ethnicity, and athletes with a disability.
Although there is some contention as to how we should refer to this syndrome, the most important facet is that it can be identified in an office setting. Awareness is key to prevention. Unfortunately, in a 2015 survey of 931 physicians, only 37% could identify the 3 components of the triad.6 If you do not ask your patients about their nutrition, eating habits, and menstrual cycle, it is not possible to identify any potential problems.
WHY IS THIS SO IMPORTANT?
Simply stated, it is important to diagnose and treat the triad syndrome to maintain optimal bone health. About 90% of peak bone mass is acquired by age 18. Bone density continues to build until about the age of 25, after which it is only possible to maintain. If young athletes are losing bone density, it cannot be replaced. Athletes who have a relative energy deficiency and are not maximizing bone growth will potentially struggle with lower bone mineral density later in life. Early awareness of bone health is paramount to sustaining it as we age.
Osteoporosis, often called a silent disease, affects more than 75 million men and women in the United States, Europe, and Japan.7 According to the International Osteoporosis Foundation, more than 8.9 million fractures are secondary to osteoporosis worldwide each year. Astoundingly, this epidemic equates to an osteoporotic fracture every 3 seconds.8
As physicians, we need to do what we can to prevent this, and the easiest prevention is maintaining bone health and adequate nutrition early in life. We know that weight-bearing exercise is important for bone health, but it is counterintuitive to think that active patients who are running and playing sports may be negatively affecting bone health if they have a relative energy deficiency.
Many females (professional athletes, competitive athletes, or just women who want to stay active) exercise excessively and restrict calories to lose or maintain weight. This can be a formula for disaster resulting in a sidelining bone stress injury. A stress fracture occurs when bone experiences more stress or impact than it can handle from overtraining or increasing training too quickly, not providing enough time for the bone to strengthen. Stress fractures can also occur when bone mineral density declines, lowering the impact threshold. This is most often a direct result of a relative energy deficiency or poor nutrition. The incidence of stress fractures in female athletes is 2 to 3 times higher than that in male athletes, and female athletes with missed menstrual cycles have a 2 to 4 times higher risk of stress fractures than females with a normal monthly menstrual cycle.9
The triad syndrome is seen not only in women but in all athletes—including men and Paralympic athletes.10,11 According to Tenforde et al,10 male athletes, particularly those who participate in sports requiring leanness such as cycling and running, can exhibit nutritional deficits, reduced sex hormone levels, and impaired bone health.10
If we can educate patients on the importance of maintaining a healthy diet and supplying active bodies with the energy they need, then many of these injuries could be prevented.
Make no bones about it.
- Wilson AS; NCAA Research Department. 45 years of Title IX: the status of women in intercollegiate athletics. www.ncaa.org/sites/default/files/TitleIX45-295-FINAL_WEB.pdf. Accessed February 5, 2018.
- Ernst and Young; Women Athletes Business Network. Perspectives on sport and teams. www.ey.com/br/pt/about-us/our-sponsorships-and-programs/women-athletes-global-leadership-network---perspectives-on-sport-and-teams. Accessed February 5, 2018.
- Matzkin E, Curry EJ, Whitlock K. Female athlete triad: past, present, and future. J Am Acad Orthop Surg 2015; 23:424–432. doi:10.5435/JAAOS-D-14-00168
- Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 2007; 39:1867–1882. doi:10.1249/mss.0b013e318149f111
- Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sport (RED-S). Br J Sports Med 2014; 48:491–497. doi:10.1136/bjsports-2014-093502
- Curry EJ, Logan C, Ackerman K, McInnis KC, Matzkin EG. Female athlete triad awareness among multispecialty physicians. Sports Med Open 2015; 1:38. doi:10.1186/s40798-015-0037-5
- Who are candidates for prevention and treatment for osteoporosis? Osteoporos Int 1997; 7:1–6.
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17:1726–1733. doi:10.1007/s00198-006-0172-4
- Wentz L, Liu PY, Haymes E, Ilich JZ. Females have a greater incidence of stress fractures than males in both military and athletic populations: a systemic review. Mil Med 2011; 176:420–430.
- Tenforde AS, Barrack MT, Nattiv A, Fredericson M. Parallels with the female athlete triad in male athletes. Sports Med 2016; 46:171–182. doi:10.1007/s40279-015-0411-y
- Blauwet CA, Brook EM, Tenforde AS, et al. Low energy availability, menstrual dysfunction, and low bone mineral density in individuals with a disability: implications for the para athlete population. Sports Med 2017; 47(9)1697–1708. doi:10.1007/s40279-017-0696-0
- Wilson AS; NCAA Research Department. 45 years of Title IX: the status of women in intercollegiate athletics. www.ncaa.org/sites/default/files/TitleIX45-295-FINAL_WEB.pdf. Accessed February 5, 2018.
- Ernst and Young; Women Athletes Business Network. Perspectives on sport and teams. www.ey.com/br/pt/about-us/our-sponsorships-and-programs/women-athletes-global-leadership-network---perspectives-on-sport-and-teams. Accessed February 5, 2018.
- Matzkin E, Curry EJ, Whitlock K. Female athlete triad: past, present, and future. J Am Acad Orthop Surg 2015; 23:424–432. doi:10.5435/JAAOS-D-14-00168
- Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 2007; 39:1867–1882. doi:10.1249/mss.0b013e318149f111
- Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sport (RED-S). Br J Sports Med 2014; 48:491–497. doi:10.1136/bjsports-2014-093502
- Curry EJ, Logan C, Ackerman K, McInnis KC, Matzkin EG. Female athlete triad awareness among multispecialty physicians. Sports Med Open 2015; 1:38. doi:10.1186/s40798-015-0037-5
- Who are candidates for prevention and treatment for osteoporosis? Osteoporos Int 1997; 7:1–6.
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17:1726–1733. doi:10.1007/s00198-006-0172-4
- Wentz L, Liu PY, Haymes E, Ilich JZ. Females have a greater incidence of stress fractures than males in both military and athletic populations: a systemic review. Mil Med 2011; 176:420–430.
- Tenforde AS, Barrack MT, Nattiv A, Fredericson M. Parallels with the female athlete triad in male athletes. Sports Med 2016; 46:171–182. doi:10.1007/s40279-015-0411-y
- Blauwet CA, Brook EM, Tenforde AS, et al. Low energy availability, menstrual dysfunction, and low bone mineral density in individuals with a disability: implications for the para athlete population. Sports Med 2017; 47(9)1697–1708. doi:10.1007/s40279-017-0696-0
Hyperkeratotic fissured plaques on both hands: Mechanic’s hands
Nail fold capillaroscopy did not reveal telangiectasia or ragged cuticles. Further examination of the skin showed confluent macular violaceous erythema on the eyelids (suggestive of the heliotrope sign), V area of the neck, upper arms, and back.
She also had a low-grade intermittent fever for the past 2 months, as well as difficulty in getting up from a squatting position and combing her hair, dyspnea on exertion, blue discoloration of the fingers on exposure to cold, and intermittent pain, stiffness, and swelling in the small joints of both hands that was worse in the morning and seemed to be relieved by activity. She had no history of dysphagia or nasal regurgitation of food. Strength against resistance was reduced in both arms and knee extensors. A diagnosis of dermatomyositis with “mechanic’s hands” was considered.
Laboratory testing was negative for antinuclear antibodies and showed elevated creatine kinase and positive anti-Jo-1 antibodies. High-resolution computed tomography of the chest showed evidence of interstitial lung disease. Features were consistent with antisynthetase syndrome and dermatomyositis. An age-appropriate malignancy screen was normal.
The patient was started on oral prednisolone 60 mg, hydroxychloroquine 300 mg, and azathioprine 100 mg. For her hands, topical clobetasol propionate 0.05% with 3% salicyclic acid and emollients were advised. Her muscle weakness improved considerably after 2 months, but the photosensitivity and mechanic’s hands improved only minimally.
ANTISYNTHETASE SYNDROME
Antisynthetase syndrome is a subset of idiopathic inflammatory myopathies characterized by fever, Raynaud phenomenon, arthritis, myositis, interstitial lung disease, and mechanic’s hands. It is associated with myositis-specific antibodies directed against aminoacyl-tRNA synthetases, of which anti-Jo-1 is the most common. Other antibodies including anti-PL-7 and anti-PL-12 may be present, whereas antinuclear antibodies may be negative.
Mechanic’s hands is seen in about 30% of patients with antisynthetase syndrome and is an important physical sign, as its presence in a patient with myositis and arthritis prompts an evaluation to exclude interstitial lung disease. Its onset later in the disease course may herald the flare-up of interstitial lung disease.1 A similar hyperkeratosis may also affect the feet, and the importance of a careful cutaneous examination of the hands and feet should be stressed in patients presenting with polymyositis and dermatomyositis.
In contrast, hyperkeratotic eczema of the hand is usually pruritic, and involvement of the tips and palmar aspects of the fingers and palms is characteristic.2,3 Vesicles (pompholyx) and coarse pitting of the nails may also be seen in eczema. Other features that help rule out eczema are the development of these features over a short period of time, asymptomatic nature, presence of systemic symptoms, and involvement of only the lateral margins of the index fingers, with no involvement of the palmar aspects and other fingers.
Degenerative collagenous plaque, closely resembling mechanic’s hands, is common in elderly people with photodamaged skin. It is asymptomatic, is not associated with systemic illness, and features clumping and thickening of elastic fibers on histopathology.
Association with cancer risk
Though antisynthetase syndrome was not previously considered to be associated with an increased risk of malignancy, a retrospective review of 124 patients with antisynthetase syndrome recently showed a malignancy risk of 6.5%.4 Overall, the data regarding the association of malignancy and antisynthetase syndrome are conflicting, and this needs further study. Therefore, an age-appropriate malignancy screen is recommended.4–6 Also, the presence of malignancy and interstitial lung disease is associated with a poor prognosis in these patients.
Treatment
Glucocorticoids are the mainstay of treatment, and azathioprine and methotrexate are important steroid-sparing agents.5,7 Use of methotrexate warrants caution in patients with interstitial lung disease, since methotrexate itself can cause pulmonary fibrosis.
In our patient, prednisolone was slowly tapered to 20 mg/day, and hydroxychloroquine and azathioprine were continued at 300 mg/day and 100 mg/day, respectively. Topical treatment for mechanic’s hands was continued, with only minimal improvement.
- Bartoloni E, Gonzalez-Gay MA, Scire C, et al. Clinical follow-up predictors of disease pattern change in anti-Jo1 positive anti-synthetase syndrome: results from a multicenter, international and retrospective study. Autoimmun Rev 2017; 16(3):253–257. doi:10.1016/j.autrev.2017.01.008
- Bachmeyer C, Tillie-Leblond I, Lacert A, Cadranel J, Aractingi S. “Mechanic's hands”: a misleading cutaneous sign of the antisynthetase syndrome. Br J Dermatol 2007; 156(1):192–194. doi:10.1111/j.1365-2133.2006.07593.x
- Mii S, Kobayashi R, Nakano T, et al. A histopathologic study of mechanic's hands associated with dermatomyositis: a report of five cases. Int J Dermatol 2009; 48(11):1177–1182. doi:10.1111/j.1365-4632.2009.04164.x
- Shi J, Li S, Yang H, et al. Clinical profiles and prognosis of patients with distinct antisynthetase autoantibodies. J Rheumatol 2017; 44(7):1051–1057. doi:10.3899/jrheum.161480
- Chatterjee S, Prayson R, Farver C. Antisynthetase syndrome: not just an inflammatory myopathy. Cleve Clin J Med 2013; 80(10):655–666. doi:10.3949/ccjm.80a.12171
- Boleto G, Perotin JM, Eschard JP, Salmon JH. Squamous cell carcinoma of the lung associated with anti-Jo1 antisynthetase syndrome: a case report and review of the literature. Rheumatol Int 2017; 37(7):1203–1206. doi:10.1007/s00296-017-3728-z
- Mirrakhimov AE. Antisynthetase syndrome: a review of etiopathogenesis, diagnosis and management. Curr Med Chem 2015; 22(16):1963–1975. doi:10.2174/0929867322666150514094935
Nail fold capillaroscopy did not reveal telangiectasia or ragged cuticles. Further examination of the skin showed confluent macular violaceous erythema on the eyelids (suggestive of the heliotrope sign), V area of the neck, upper arms, and back.
She also had a low-grade intermittent fever for the past 2 months, as well as difficulty in getting up from a squatting position and combing her hair, dyspnea on exertion, blue discoloration of the fingers on exposure to cold, and intermittent pain, stiffness, and swelling in the small joints of both hands that was worse in the morning and seemed to be relieved by activity. She had no history of dysphagia or nasal regurgitation of food. Strength against resistance was reduced in both arms and knee extensors. A diagnosis of dermatomyositis with “mechanic’s hands” was considered.
Laboratory testing was negative for antinuclear antibodies and showed elevated creatine kinase and positive anti-Jo-1 antibodies. High-resolution computed tomography of the chest showed evidence of interstitial lung disease. Features were consistent with antisynthetase syndrome and dermatomyositis. An age-appropriate malignancy screen was normal.
The patient was started on oral prednisolone 60 mg, hydroxychloroquine 300 mg, and azathioprine 100 mg. For her hands, topical clobetasol propionate 0.05% with 3% salicyclic acid and emollients were advised. Her muscle weakness improved considerably after 2 months, but the photosensitivity and mechanic’s hands improved only minimally.
ANTISYNTHETASE SYNDROME
Antisynthetase syndrome is a subset of idiopathic inflammatory myopathies characterized by fever, Raynaud phenomenon, arthritis, myositis, interstitial lung disease, and mechanic’s hands. It is associated with myositis-specific antibodies directed against aminoacyl-tRNA synthetases, of which anti-Jo-1 is the most common. Other antibodies including anti-PL-7 and anti-PL-12 may be present, whereas antinuclear antibodies may be negative.
Mechanic’s hands is seen in about 30% of patients with antisynthetase syndrome and is an important physical sign, as its presence in a patient with myositis and arthritis prompts an evaluation to exclude interstitial lung disease. Its onset later in the disease course may herald the flare-up of interstitial lung disease.1 A similar hyperkeratosis may also affect the feet, and the importance of a careful cutaneous examination of the hands and feet should be stressed in patients presenting with polymyositis and dermatomyositis.
In contrast, hyperkeratotic eczema of the hand is usually pruritic, and involvement of the tips and palmar aspects of the fingers and palms is characteristic.2,3 Vesicles (pompholyx) and coarse pitting of the nails may also be seen in eczema. Other features that help rule out eczema are the development of these features over a short period of time, asymptomatic nature, presence of systemic symptoms, and involvement of only the lateral margins of the index fingers, with no involvement of the palmar aspects and other fingers.
Degenerative collagenous plaque, closely resembling mechanic’s hands, is common in elderly people with photodamaged skin. It is asymptomatic, is not associated with systemic illness, and features clumping and thickening of elastic fibers on histopathology.
Association with cancer risk
Though antisynthetase syndrome was not previously considered to be associated with an increased risk of malignancy, a retrospective review of 124 patients with antisynthetase syndrome recently showed a malignancy risk of 6.5%.4 Overall, the data regarding the association of malignancy and antisynthetase syndrome are conflicting, and this needs further study. Therefore, an age-appropriate malignancy screen is recommended.4–6 Also, the presence of malignancy and interstitial lung disease is associated with a poor prognosis in these patients.
Treatment
Glucocorticoids are the mainstay of treatment, and azathioprine and methotrexate are important steroid-sparing agents.5,7 Use of methotrexate warrants caution in patients with interstitial lung disease, since methotrexate itself can cause pulmonary fibrosis.
In our patient, prednisolone was slowly tapered to 20 mg/day, and hydroxychloroquine and azathioprine were continued at 300 mg/day and 100 mg/day, respectively. Topical treatment for mechanic’s hands was continued, with only minimal improvement.
Nail fold capillaroscopy did not reveal telangiectasia or ragged cuticles. Further examination of the skin showed confluent macular violaceous erythema on the eyelids (suggestive of the heliotrope sign), V area of the neck, upper arms, and back.
She also had a low-grade intermittent fever for the past 2 months, as well as difficulty in getting up from a squatting position and combing her hair, dyspnea on exertion, blue discoloration of the fingers on exposure to cold, and intermittent pain, stiffness, and swelling in the small joints of both hands that was worse in the morning and seemed to be relieved by activity. She had no history of dysphagia or nasal regurgitation of food. Strength against resistance was reduced in both arms and knee extensors. A diagnosis of dermatomyositis with “mechanic’s hands” was considered.
Laboratory testing was negative for antinuclear antibodies and showed elevated creatine kinase and positive anti-Jo-1 antibodies. High-resolution computed tomography of the chest showed evidence of interstitial lung disease. Features were consistent with antisynthetase syndrome and dermatomyositis. An age-appropriate malignancy screen was normal.
The patient was started on oral prednisolone 60 mg, hydroxychloroquine 300 mg, and azathioprine 100 mg. For her hands, topical clobetasol propionate 0.05% with 3% salicyclic acid and emollients were advised. Her muscle weakness improved considerably after 2 months, but the photosensitivity and mechanic’s hands improved only minimally.
ANTISYNTHETASE SYNDROME
Antisynthetase syndrome is a subset of idiopathic inflammatory myopathies characterized by fever, Raynaud phenomenon, arthritis, myositis, interstitial lung disease, and mechanic’s hands. It is associated with myositis-specific antibodies directed against aminoacyl-tRNA synthetases, of which anti-Jo-1 is the most common. Other antibodies including anti-PL-7 and anti-PL-12 may be present, whereas antinuclear antibodies may be negative.
Mechanic’s hands is seen in about 30% of patients with antisynthetase syndrome and is an important physical sign, as its presence in a patient with myositis and arthritis prompts an evaluation to exclude interstitial lung disease. Its onset later in the disease course may herald the flare-up of interstitial lung disease.1 A similar hyperkeratosis may also affect the feet, and the importance of a careful cutaneous examination of the hands and feet should be stressed in patients presenting with polymyositis and dermatomyositis.
In contrast, hyperkeratotic eczema of the hand is usually pruritic, and involvement of the tips and palmar aspects of the fingers and palms is characteristic.2,3 Vesicles (pompholyx) and coarse pitting of the nails may also be seen in eczema. Other features that help rule out eczema are the development of these features over a short period of time, asymptomatic nature, presence of systemic symptoms, and involvement of only the lateral margins of the index fingers, with no involvement of the palmar aspects and other fingers.
Degenerative collagenous plaque, closely resembling mechanic’s hands, is common in elderly people with photodamaged skin. It is asymptomatic, is not associated with systemic illness, and features clumping and thickening of elastic fibers on histopathology.
Association with cancer risk
Though antisynthetase syndrome was not previously considered to be associated with an increased risk of malignancy, a retrospective review of 124 patients with antisynthetase syndrome recently showed a malignancy risk of 6.5%.4 Overall, the data regarding the association of malignancy and antisynthetase syndrome are conflicting, and this needs further study. Therefore, an age-appropriate malignancy screen is recommended.4–6 Also, the presence of malignancy and interstitial lung disease is associated with a poor prognosis in these patients.
Treatment
Glucocorticoids are the mainstay of treatment, and azathioprine and methotrexate are important steroid-sparing agents.5,7 Use of methotrexate warrants caution in patients with interstitial lung disease, since methotrexate itself can cause pulmonary fibrosis.
In our patient, prednisolone was slowly tapered to 20 mg/day, and hydroxychloroquine and azathioprine were continued at 300 mg/day and 100 mg/day, respectively. Topical treatment for mechanic’s hands was continued, with only minimal improvement.
- Bartoloni E, Gonzalez-Gay MA, Scire C, et al. Clinical follow-up predictors of disease pattern change in anti-Jo1 positive anti-synthetase syndrome: results from a multicenter, international and retrospective study. Autoimmun Rev 2017; 16(3):253–257. doi:10.1016/j.autrev.2017.01.008
- Bachmeyer C, Tillie-Leblond I, Lacert A, Cadranel J, Aractingi S. “Mechanic's hands”: a misleading cutaneous sign of the antisynthetase syndrome. Br J Dermatol 2007; 156(1):192–194. doi:10.1111/j.1365-2133.2006.07593.x
- Mii S, Kobayashi R, Nakano T, et al. A histopathologic study of mechanic's hands associated with dermatomyositis: a report of five cases. Int J Dermatol 2009; 48(11):1177–1182. doi:10.1111/j.1365-4632.2009.04164.x
- Shi J, Li S, Yang H, et al. Clinical profiles and prognosis of patients with distinct antisynthetase autoantibodies. J Rheumatol 2017; 44(7):1051–1057. doi:10.3899/jrheum.161480
- Chatterjee S, Prayson R, Farver C. Antisynthetase syndrome: not just an inflammatory myopathy. Cleve Clin J Med 2013; 80(10):655–666. doi:10.3949/ccjm.80a.12171
- Boleto G, Perotin JM, Eschard JP, Salmon JH. Squamous cell carcinoma of the lung associated with anti-Jo1 antisynthetase syndrome: a case report and review of the literature. Rheumatol Int 2017; 37(7):1203–1206. doi:10.1007/s00296-017-3728-z
- Mirrakhimov AE. Antisynthetase syndrome: a review of etiopathogenesis, diagnosis and management. Curr Med Chem 2015; 22(16):1963–1975. doi:10.2174/0929867322666150514094935
- Bartoloni E, Gonzalez-Gay MA, Scire C, et al. Clinical follow-up predictors of disease pattern change in anti-Jo1 positive anti-synthetase syndrome: results from a multicenter, international and retrospective study. Autoimmun Rev 2017; 16(3):253–257. doi:10.1016/j.autrev.2017.01.008
- Bachmeyer C, Tillie-Leblond I, Lacert A, Cadranel J, Aractingi S. “Mechanic's hands”: a misleading cutaneous sign of the antisynthetase syndrome. Br J Dermatol 2007; 156(1):192–194. doi:10.1111/j.1365-2133.2006.07593.x
- Mii S, Kobayashi R, Nakano T, et al. A histopathologic study of mechanic's hands associated with dermatomyositis: a report of five cases. Int J Dermatol 2009; 48(11):1177–1182. doi:10.1111/j.1365-4632.2009.04164.x
- Shi J, Li S, Yang H, et al. Clinical profiles and prognosis of patients with distinct antisynthetase autoantibodies. J Rheumatol 2017; 44(7):1051–1057. doi:10.3899/jrheum.161480
- Chatterjee S, Prayson R, Farver C. Antisynthetase syndrome: not just an inflammatory myopathy. Cleve Clin J Med 2013; 80(10):655–666. doi:10.3949/ccjm.80a.12171
- Boleto G, Perotin JM, Eschard JP, Salmon JH. Squamous cell carcinoma of the lung associated with anti-Jo1 antisynthetase syndrome: a case report and review of the literature. Rheumatol Int 2017; 37(7):1203–1206. doi:10.1007/s00296-017-3728-z
- Mirrakhimov AE. Antisynthetase syndrome: a review of etiopathogenesis, diagnosis and management. Curr Med Chem 2015; 22(16):1963–1975. doi:10.2174/0929867322666150514094935
Musculoskeletal ultrasonography has arrived
A 50-year-old woman with hypertension presents with a history of polyarticular small-joint pain for the last 3 months. Her pain is worse in the morning, and it affects her metacarpal, proximal, and distal phalangeal joints. She describes intermittent swelling of her hands and morning stiffness lasting 15 to 30 minutes.
Her physical examination is unremarkable, with no evidence of active inflammation (synovitis), joint tenderness, restrictions in movement, or deformity. Her description of her symptoms raises suspicion for an inflammatory arthritis, but her physical examination does not support this diagnosis.
Bedside musculoskeletal ultrasonography of her wrists reveals synovial hypertrophy, and power Doppler shows active inflammation, findings consistent with synovitis (Figure 1).
This scenario illustrates how musculoskeletal ultrasonography can prevent delayed diagnosis, thus directing the ordering of appropriate laboratory studies and allowing treatment for pain relief to be started promptly.
ULTRASONOGRAPHY HAS GAINED A SOLID ROLE
Ultrasonography has gained a solid role in the care of patients with musculoskeletal conditions.
Using obtained images, as well as power Doppler to assess inflammation, the clinician can visualize superficial anatomic structures, including the skin, muscles, joints, nerves, and the cortical layer of bone. Combining the dynamic assessment with the clinical history and findings of the physical examination makes musculoskeletal ultrasonography a powerful tool for diagnosis and management.1
In this issue, Forney and Delzell2 review the clinical use of ultrasonography of the muscles and bones and its advantages and disadvantages compared with other imaging methods. They describe its gain in popularity over the last decade and its incorporation into clinical care in multiple medical subspecialties.
Musculoskeletal ultrasonography is performed and interpreted by specially trained sonographers. It should be viewed as a complementary procedure, not as a replacement for a thorough and systematic clinical examination.3
ADVANTAGES ARE MANY
A major advantage of musculoskeletal ultrasonography over other imaging techniques is its capacity to dynamically assess joint and tendon movements4 and to immediately interpret them in real time.
In rheumatology, where it has made the biggest impact, it can help evaluate inflammatory and noninflammatory rheumatic diseases, assess treatment response, and guide joint injections.1 It has been demonstrated to significantly improve timely diagnosis and management,5 decrease dependence on other imaging modalities, and reduce healthcare costs.6
With its easy portability, ultrasonography has also been integrated into orthopedics, podiatry, physical medicine and rehabilitation, sports medicine, and emergency medicine. Its role is expanding to include the assessment of the skin in systemic sclerosis, parotid and submandibular glands in Sjögren syndrome, nails in patients with psoriasis, and temporal arteries in giant cell arteritis.
A ROLE IN MEDICAL EDUCATION
Musculoskeletal ultrasonography has entered into medical education, with an increasing number of medical schools incorporating it into their curriculum over the last few years.7 It enhances student learning of anatomy, the physical examination, and pathologic findings of rheumatic diseases.7,8 Some internal medicine residency programs have added ultrasonography to help identify anatomic structures for invasive procedures, increasing patient safety and reducing procedural complications.9
It has been incorporated into the core curriculum in many rheumatology fellowship training programs.10 Rheumatologists can now also take additional courses to enhance their skills and become certified sonographers.
Musculoskeletal ultrasonography has proven to be a useful adjunct to the physical examination. With its many advantages, it has gained acceptance and is now a mainstay in many subspecialties.
- Cannella AC, Kissin EY, Torralba KD, Higgs JB, Kaeley GS. Evolution of musculoskeletal ultrasound in the United States: implementation and practice in rheumatology. Arthritis Care Res (Hoboken) 2014; 66(1):7–13. doi:10.1002/acr.22183
- Forney MC, Delzell PB. Musculoskeletal ultrasonography basics. Cleve Clin J Med 2018; 85(4):283–300. doi:10.3949/ccjm.85a.17014
- McAlindon T, Kissin E, Nazarian L, et al. American College of Rheumatology report on reasonable use of musculoskeletal ultrasonography in rheumatology clinical practice. Arthritis Care Res (Hoboken) 2012; 64(11):1625–1640. doi:10.1002/acr.21836
- Backhaus M, Burmester GR, Gerber T, et al; Working Group for Musculoskeletal Ultrasound in the EULAR Standing Committee on International Clinical Studies including Therapeutic Trials. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis 2001; 60(7):641–649.
- Micu MC, Alcalde M, Saenz JI, et al. Impact of musculoskeletal ultrasound in an outpatient rheumatology clinic. Arthritis Care Res (Hoboken) 2013; 65(4):615–621. doi:10.1002/acr.21853
- Kay JC, Higgs JB, Battafarano DF. Utility of musculoskeletal ultrasound in a Department of Defense rheumatology practice: a four-year retrospective experience. Arthritis Care Res (Hoboken) 2014; 66(1):14–18. doi:10.1002/acr.22127
- Dinh VA, Fu JY, Lu S, Chiem A, Fox JC, Blaivas M. Integration of ultrasound in medical education at United States medical schools. J Ultrasound Med 2016; 35(2):413–419. doi:10.7863/ultra.15.05073
- Wright SA, Bell AL. Enhancement of undergraduate rheumatology teaching through the use of musculoskeletal ultrasound. Rheumatology (Oxford) 2008; 47(10):1564–1566. doi:10.1093/rheumatology/ken324
- Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ 2011; 11:75. doi:0.1186/1472-6920-11-75
- Torralba K, Cannella AC, Kissin EY, et al. Musculoskeletal ultrasound instruction in adult rheumatology fellowship programs. Arthritis Care Res (Hoboken) 2017. Epub ahead of print. doi:10.1002/acr.23336
A 50-year-old woman with hypertension presents with a history of polyarticular small-joint pain for the last 3 months. Her pain is worse in the morning, and it affects her metacarpal, proximal, and distal phalangeal joints. She describes intermittent swelling of her hands and morning stiffness lasting 15 to 30 minutes.
Her physical examination is unremarkable, with no evidence of active inflammation (synovitis), joint tenderness, restrictions in movement, or deformity. Her description of her symptoms raises suspicion for an inflammatory arthritis, but her physical examination does not support this diagnosis.
Bedside musculoskeletal ultrasonography of her wrists reveals synovial hypertrophy, and power Doppler shows active inflammation, findings consistent with synovitis (Figure 1).
This scenario illustrates how musculoskeletal ultrasonography can prevent delayed diagnosis, thus directing the ordering of appropriate laboratory studies and allowing treatment for pain relief to be started promptly.
ULTRASONOGRAPHY HAS GAINED A SOLID ROLE
Ultrasonography has gained a solid role in the care of patients with musculoskeletal conditions.
Using obtained images, as well as power Doppler to assess inflammation, the clinician can visualize superficial anatomic structures, including the skin, muscles, joints, nerves, and the cortical layer of bone. Combining the dynamic assessment with the clinical history and findings of the physical examination makes musculoskeletal ultrasonography a powerful tool for diagnosis and management.1
In this issue, Forney and Delzell2 review the clinical use of ultrasonography of the muscles and bones and its advantages and disadvantages compared with other imaging methods. They describe its gain in popularity over the last decade and its incorporation into clinical care in multiple medical subspecialties.
Musculoskeletal ultrasonography is performed and interpreted by specially trained sonographers. It should be viewed as a complementary procedure, not as a replacement for a thorough and systematic clinical examination.3
ADVANTAGES ARE MANY
A major advantage of musculoskeletal ultrasonography over other imaging techniques is its capacity to dynamically assess joint and tendon movements4 and to immediately interpret them in real time.
In rheumatology, where it has made the biggest impact, it can help evaluate inflammatory and noninflammatory rheumatic diseases, assess treatment response, and guide joint injections.1 It has been demonstrated to significantly improve timely diagnosis and management,5 decrease dependence on other imaging modalities, and reduce healthcare costs.6
With its easy portability, ultrasonography has also been integrated into orthopedics, podiatry, physical medicine and rehabilitation, sports medicine, and emergency medicine. Its role is expanding to include the assessment of the skin in systemic sclerosis, parotid and submandibular glands in Sjögren syndrome, nails in patients with psoriasis, and temporal arteries in giant cell arteritis.
A ROLE IN MEDICAL EDUCATION
Musculoskeletal ultrasonography has entered into medical education, with an increasing number of medical schools incorporating it into their curriculum over the last few years.7 It enhances student learning of anatomy, the physical examination, and pathologic findings of rheumatic diseases.7,8 Some internal medicine residency programs have added ultrasonography to help identify anatomic structures for invasive procedures, increasing patient safety and reducing procedural complications.9
It has been incorporated into the core curriculum in many rheumatology fellowship training programs.10 Rheumatologists can now also take additional courses to enhance their skills and become certified sonographers.
Musculoskeletal ultrasonography has proven to be a useful adjunct to the physical examination. With its many advantages, it has gained acceptance and is now a mainstay in many subspecialties.
A 50-year-old woman with hypertension presents with a history of polyarticular small-joint pain for the last 3 months. Her pain is worse in the morning, and it affects her metacarpal, proximal, and distal phalangeal joints. She describes intermittent swelling of her hands and morning stiffness lasting 15 to 30 minutes.
Her physical examination is unremarkable, with no evidence of active inflammation (synovitis), joint tenderness, restrictions in movement, or deformity. Her description of her symptoms raises suspicion for an inflammatory arthritis, but her physical examination does not support this diagnosis.
Bedside musculoskeletal ultrasonography of her wrists reveals synovial hypertrophy, and power Doppler shows active inflammation, findings consistent with synovitis (Figure 1).
This scenario illustrates how musculoskeletal ultrasonography can prevent delayed diagnosis, thus directing the ordering of appropriate laboratory studies and allowing treatment for pain relief to be started promptly.
ULTRASONOGRAPHY HAS GAINED A SOLID ROLE
Ultrasonography has gained a solid role in the care of patients with musculoskeletal conditions.
Using obtained images, as well as power Doppler to assess inflammation, the clinician can visualize superficial anatomic structures, including the skin, muscles, joints, nerves, and the cortical layer of bone. Combining the dynamic assessment with the clinical history and findings of the physical examination makes musculoskeletal ultrasonography a powerful tool for diagnosis and management.1
In this issue, Forney and Delzell2 review the clinical use of ultrasonography of the muscles and bones and its advantages and disadvantages compared with other imaging methods. They describe its gain in popularity over the last decade and its incorporation into clinical care in multiple medical subspecialties.
Musculoskeletal ultrasonography is performed and interpreted by specially trained sonographers. It should be viewed as a complementary procedure, not as a replacement for a thorough and systematic clinical examination.3
ADVANTAGES ARE MANY
A major advantage of musculoskeletal ultrasonography over other imaging techniques is its capacity to dynamically assess joint and tendon movements4 and to immediately interpret them in real time.
In rheumatology, where it has made the biggest impact, it can help evaluate inflammatory and noninflammatory rheumatic diseases, assess treatment response, and guide joint injections.1 It has been demonstrated to significantly improve timely diagnosis and management,5 decrease dependence on other imaging modalities, and reduce healthcare costs.6
With its easy portability, ultrasonography has also been integrated into orthopedics, podiatry, physical medicine and rehabilitation, sports medicine, and emergency medicine. Its role is expanding to include the assessment of the skin in systemic sclerosis, parotid and submandibular glands in Sjögren syndrome, nails in patients with psoriasis, and temporal arteries in giant cell arteritis.
A ROLE IN MEDICAL EDUCATION
Musculoskeletal ultrasonography has entered into medical education, with an increasing number of medical schools incorporating it into their curriculum over the last few years.7 It enhances student learning of anatomy, the physical examination, and pathologic findings of rheumatic diseases.7,8 Some internal medicine residency programs have added ultrasonography to help identify anatomic structures for invasive procedures, increasing patient safety and reducing procedural complications.9
It has been incorporated into the core curriculum in many rheumatology fellowship training programs.10 Rheumatologists can now also take additional courses to enhance their skills and become certified sonographers.
Musculoskeletal ultrasonography has proven to be a useful adjunct to the physical examination. With its many advantages, it has gained acceptance and is now a mainstay in many subspecialties.
- Cannella AC, Kissin EY, Torralba KD, Higgs JB, Kaeley GS. Evolution of musculoskeletal ultrasound in the United States: implementation and practice in rheumatology. Arthritis Care Res (Hoboken) 2014; 66(1):7–13. doi:10.1002/acr.22183
- Forney MC, Delzell PB. Musculoskeletal ultrasonography basics. Cleve Clin J Med 2018; 85(4):283–300. doi:10.3949/ccjm.85a.17014
- McAlindon T, Kissin E, Nazarian L, et al. American College of Rheumatology report on reasonable use of musculoskeletal ultrasonography in rheumatology clinical practice. Arthritis Care Res (Hoboken) 2012; 64(11):1625–1640. doi:10.1002/acr.21836
- Backhaus M, Burmester GR, Gerber T, et al; Working Group for Musculoskeletal Ultrasound in the EULAR Standing Committee on International Clinical Studies including Therapeutic Trials. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis 2001; 60(7):641–649.
- Micu MC, Alcalde M, Saenz JI, et al. Impact of musculoskeletal ultrasound in an outpatient rheumatology clinic. Arthritis Care Res (Hoboken) 2013; 65(4):615–621. doi:10.1002/acr.21853
- Kay JC, Higgs JB, Battafarano DF. Utility of musculoskeletal ultrasound in a Department of Defense rheumatology practice: a four-year retrospective experience. Arthritis Care Res (Hoboken) 2014; 66(1):14–18. doi:10.1002/acr.22127
- Dinh VA, Fu JY, Lu S, Chiem A, Fox JC, Blaivas M. Integration of ultrasound in medical education at United States medical schools. J Ultrasound Med 2016; 35(2):413–419. doi:10.7863/ultra.15.05073
- Wright SA, Bell AL. Enhancement of undergraduate rheumatology teaching through the use of musculoskeletal ultrasound. Rheumatology (Oxford) 2008; 47(10):1564–1566. doi:10.1093/rheumatology/ken324
- Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ 2011; 11:75. doi:0.1186/1472-6920-11-75
- Torralba K, Cannella AC, Kissin EY, et al. Musculoskeletal ultrasound instruction in adult rheumatology fellowship programs. Arthritis Care Res (Hoboken) 2017. Epub ahead of print. doi:10.1002/acr.23336
- Cannella AC, Kissin EY, Torralba KD, Higgs JB, Kaeley GS. Evolution of musculoskeletal ultrasound in the United States: implementation and practice in rheumatology. Arthritis Care Res (Hoboken) 2014; 66(1):7–13. doi:10.1002/acr.22183
- Forney MC, Delzell PB. Musculoskeletal ultrasonography basics. Cleve Clin J Med 2018; 85(4):283–300. doi:10.3949/ccjm.85a.17014
- McAlindon T, Kissin E, Nazarian L, et al. American College of Rheumatology report on reasonable use of musculoskeletal ultrasonography in rheumatology clinical practice. Arthritis Care Res (Hoboken) 2012; 64(11):1625–1640. doi:10.1002/acr.21836
- Backhaus M, Burmester GR, Gerber T, et al; Working Group for Musculoskeletal Ultrasound in the EULAR Standing Committee on International Clinical Studies including Therapeutic Trials. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis 2001; 60(7):641–649.
- Micu MC, Alcalde M, Saenz JI, et al. Impact of musculoskeletal ultrasound in an outpatient rheumatology clinic. Arthritis Care Res (Hoboken) 2013; 65(4):615–621. doi:10.1002/acr.21853
- Kay JC, Higgs JB, Battafarano DF. Utility of musculoskeletal ultrasound in a Department of Defense rheumatology practice: a four-year retrospective experience. Arthritis Care Res (Hoboken) 2014; 66(1):14–18. doi:10.1002/acr.22127
- Dinh VA, Fu JY, Lu S, Chiem A, Fox JC, Blaivas M. Integration of ultrasound in medical education at United States medical schools. J Ultrasound Med 2016; 35(2):413–419. doi:10.7863/ultra.15.05073
- Wright SA, Bell AL. Enhancement of undergraduate rheumatology teaching through the use of musculoskeletal ultrasound. Rheumatology (Oxford) 2008; 47(10):1564–1566. doi:10.1093/rheumatology/ken324
- Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ 2011; 11:75. doi:0.1186/1472-6920-11-75
- Torralba K, Cannella AC, Kissin EY, et al. Musculoskeletal ultrasound instruction in adult rheumatology fellowship programs. Arthritis Care Res (Hoboken) 2017. Epub ahead of print. doi:10.1002/acr.23336
A 71-year-old woman with shock and a high INR
A 71-year-old woman is brought to the emergency department by her neighbor after complaining of fatigue and light-headedness for the last 8 hours. The patient lives alone and was feeling well when she woke up this morning, but then began to feel nauseated and vomited twice.
The patient appears drowsy and confused and cannot provide any further history. Her medical records show that she was seen in the cardiology clinic 6 months ago but has not kept her appointments since then.
Her medical history includes atrial fibrillation, hypertension, type 2 diabetes mellitus, and osteoarthritis. Her medications are daily warfarin, atenolol, aspirin, candesartan, and metformin, and she takes acetaminophen as needed. She is neither a smoker nor a drug user, but she drinks alcohol occasionally. Her family history is significant for her mother’s death from breast cancer at age 55.
The neighbor confirms that the patient appeared well this morning and has not had any recent illnesses except for a minor cold last week that improved over 5 days with acetaminophen only.
INITIAL EVALUATION AND MANAGEMENT
Physical examination
On physical examination, her blood pressure is 80/40 mm Hg, respiratory rate 25 breaths per minute, oral temperature 38.3°C (100.9°F), and heart rate 130 beats per minute and irregular.
Her neck veins are flat, and her chest is clear to auscultation with normal heart sounds. Abdominal palpation elicits discomfort in the middle segments, voluntary withdrawal, and abdominal wall rigidity. Her skin feels dry and cool, with decreased turgor.
Initial treatment
The patient is given 1 L of 0.9% saline intravenously over the first hour and then is transferred to the intensive care unit, where a norepinephrine drip is started to treat her ongoing hypotension. Normal saline is continued at a rate of 500 mL per hour for the next 4 hours.
Cardiac monitoring and 12-lead electrocardiography show atrial fibrillation with a rapid ventricular response of 138 beats per minute, but electrical cardioversion is not done.
Initial laboratory tests
Of note, her international normalized ratio (INR) is 6.13, while the therapeutic range for a patient taking warfarin because of atrial fibrillation is 2.0 to 3.0.
Her blood pH is 7.34 (reference range 7.35–7.45), and her bicarbonate level is 18 mmol/L (22–26); a low pH and low bicarbonate together indicate metabolic acidosis. Her sodium level is 128 mmol/L (135–145), her chloride level is 100 mmol/L (97–107), and, as mentioned, her bicarbonate level is 18 mmol/L; therefore, her anion gap is 128 – (100 + 18) = 10 mmol/L, which is normal (≤ 10).1
Her serum creatinine level is 1.3 mg/dL (0.5–1.1), and her blood urea nitrogen level is 35 mg/dL (7–20).
Her potassium level is 5.8 mmol/L, which is consistent with hyperkalemia (reference range 3.5–5.2).
DIFFERENTIAL DIAGNOSIS
1. Which of the following is the most likely cause of this patient’s symptoms?
- Adrenal crisis
- Cardiogenic shock due to decreased cardiac contractility
- Intracranial hemorrhage
- Acute abdomen due to small bowel obstruction
- Septic shock due to bacterial toxin-induced loss of vascular tone
Our patient is presenting with shock. Given our inability to obtain a meaningful history, the differential diagnosis is broad and includes all of the above.
Adrenal crisis
The sudden onset and laboratory results that include hyperkalemia, hyponatremia, and normal anion gap metabolic acidosis raise suspicion of adrenal crisis resulting in acute mineralocorticoid and glucocorticoid insufficiency.1
The patient’s elevated serum creatinine and high blood urea nitrogen-to-creatinine ratio of 26.9 (reference range 10–20) also suggest intravascular volume contraction. Her low hemoglobin level and supratherapeutic INR, possibly due to an interaction between warfarin and acetaminophen combined with poor medical follow-up, raise suspicion of acute bilateral adrenal necrosis due to hemorrhage.
Bilateral adrenal hemorrhage is one cause of adrenal crisis resulting in bilateral adrenal necrosis. Risk factors for adrenal hemorrhage include anticoagulation therapy, underlying coagulopathy, postoperative states, and certain infections such as meningococcemia and Haemophilus influenzae infection.2–5 Nevertheless, in most cases the INR is in the therapeutic range and the patient has no bleeding elsewhere.4 Other causes of adrenal necrosis include emboli, sepsis, and blunt trauma.6,7
Other causes of adrenal crisis are listed in Table 3.
Cardiogenic shock
Intracranial hemorrhage
Intracranial hemorrhage can present with a decreased level of consciousness, but it is less likely to cause hypotension, as the cranial space is limited. If massive intracranial hemorrhage would occur, the increase in intracranial pressure would more likely cause hypertension by the Cushing reflex than hypotension.
Acute abdomen
Abdominal pain and rigidity along with fever can be presenting symptoms of both adrenal insufficiency and an acute abdomen due to intestinal obstruction.4 However, intestinal obstruction typically causes a high anion gap metabolic acidosis due to lactic acidosis, instead of the normal anion gap metabolic acidosis present in this patient.8 Moreover, her deranged electrolytes, supratherapeutic INR, and absence of previous gastroenterologic conditions make adrenal crisis a more likely diagnosis.
Septic shock
Septic shock would also cause fever and hypotension as bacterial toxins induce a pyrexic response and vasodilation. However, at such an early stage of sepsis, the patient would be expected to be warm and hyperemic, whereas this patient’s skin is cool and dry due to volume depletion secondary to adrenal insufficiency.9 Sepsis would also cause a high anion gap metabolic acidosis due to lactic acidosis, as opposed to this patient’s normal anion gap metabolic acidosis. These findings, along with the metabolic derangements and the absence of a focus of infection, make sepsis a less likely possibility.
CASE CONTINUED: CARDIOMEGALY, PERSISTENT HYPOTENSION
Blood is drawn for cultures and measurement of troponins and lactic acid, and urine samples are taken for culture and biochemical analysis. Chest radiography shows mild cardiomegaly. The patient is started empirically on vancomycin and cefepime, and her warfarin is discontinued.
Five hours after presenting to the emergency department, her blood pressure remains at 80/40 mm Hg even after receiving 3 L of normal saline intravenously.
PROMPT MANAGEMENT OF ADRENAL CRISIS
2. Which of the following is the most appropriate next step in managing this patient?
- Draw samples for serum cortisol and plasma adrenocorticotropic hormone (ACTH) levels, then give hydrocortisone 100 mg intravenously
- Perform abdominal computed tomography (CT) without contrast
- Perform transthoracic echocardiography
- Increase the norepinephrine infusion
- Immediately give fludrocortisone
First give fluids
The first step in managing a patient with suspected adrenal crisis is liberal intravenous fluid administration to replenish the depleted intravascular space. The amount and choice of fluid is empiric, but a recommendation is 1 L of normal saline or dextrose 5% in normal saline, infused quickly over the first hour and then titrated according to the patient’s fluid status.10
Measure cortisol and ACTH; start corticosteroids immediately
Immediate therapy with an appropriate stress dose of intravenous corticosteroids (eg, hydrocortisone 100 mg) is essential. However, this should be done after drawing blood for cortisol and ACTH measurements.10
Do not delay corticosteroid therapy while awaiting the results of the diagnostic tests.
In addition, in the early phase of evolving primary adrenal insufficiency, measurement of plasma renin and aldosterone levels may be beneficial, as mineralocorticoid deficiency may predominate.10,12,13
One of the most important aims of early corticosteroid supplementation is to prevent further hyponatremia by reducing a reactive increase in antidiuretic hormone secretion caused by cortisol deficiency. Corticosteroids also help to restore normal blood pressure by increasing vascular tone, as glucocorticoid receptor activation potentiates the vasoconstrictor actions of norepinephrine, angiotensin II, and other vasoconstrictors.14,15
Which corticosteroid to use?
Which corticosteroid to use in previously undiagnosed adrenal insufficiency is controversial. The Endocrine Society10 and Japan Endocrine Society16 clinical practice guidelines recommend hydrocortisone in a 100-mg intravenous bolus followed by 200 mg over 24 hours.
The choice of hydrocortisone is justified by its superior mineralocorticoid activity.10,16 Further, hydrocortisone is preferred over dexamethasone if the patient is known to have primary adrenal insufficiency, or if the serum potassium level is higher than 6.0 mmol/L.
Some clinicians, on the other hand, recommend dexamethasone, given as a 4-mg intravenous bolus followed by 4-mg boluses every 12 hours. Their rationale is that dexamethasone, unlike hydrocortisone, does not interfere with subsequent serum cortisol assays if the patient later undergoes ACTH stimulation testing.17 Dexamethasone may also be preferred to minimize unwanted mineralocorticoid effects, such as in neurosurgical patients at risk of brain edema.
If hydrocortisone is used, ACTH stimulation testing can be done after withholding hydrocortisone for 24 hours once the patient is stable. (It should be restarted after the test if the results are abnormal.)
Other possible steps
Abdominal CT should be done in our patient to address the possibility of bilateral adrenal hemorrhage. However, it is preferable to wait until the patient is stabilized.
Echocardiography. Our patient is likely to have an element of cardiac failure, given her hypertension and cardiomegaly. However, decompensated heart failure is probably not the cause of her presentation. Thus, the first priority is to treat her adrenal crisis, and echocardiography should be deferred.
Increasing the norepinephrine infusion is unlikely to improve her blood pressure very much, as she is significantly volume-depleted. Further, low cortisol decreases the vascular response to norepinephrine.15
Mineralocorticoids such as fludrocortisone are used to treat primary adrenal insufficiency. However, they are not required during acute management of adrenal crisis, as 40 mg of hydrocortisone offers mineralocorticoid activity equivalent to 100 µg of fludrocortisone. Thus, the high doses of hydrocortisone used to treat adrenal crisis provide adequate mineralocorticoid therapy.10,18
If dexamethasone is used, its effect along with normal saline supplementation would be sufficient to replete the intravenous space and bring the sodium level back up to normal in the acute setting.
CASE RESUMED: IMPROVEMENT WITH HYDROCORTISONE
The patient’s blood is drawn for serum cortisol and plasma ACTH measurements. A 100-mg intravenous bolus of hydrocortisone is given, followed by a 50-mg bolus every 6 hours until the patient stabilizes.
Twenty-four hours later, the patient states that she has more energy, and her appetite has improved. The norepinephrine infusion is stopped 48 hours after presentation, at which time her blood pressure is 120/70 mm Hg, heart rate 85 beats per minute and irregular, and temperature 36.7°C (98.1°F). Her current laboratory values include the following:
- Serum sodium 137 mmolL
- Serum potassium 4.3 mmol/L
- Hemoglobin 9.3 g/dL
- Serum cortisol (random) 7.2 μg/dL
- Plasma ACTH 752 pg/mL (10–60 pg/mL).
ESTABLISHING THE DIAGNOSIS OF ADRENAL INSUFFICIENCY
3. Which of the following is the most appropriate test to establish the diagnosis of adrenal insufficiency?
- 7 am total serum cortisol measurement
- Random serum cortisol measurement
- 7 am salivary cortisol measurement
- 24-hour urinary free cortisol measurement
- ACTH stimulation test for cortisol
- Insulin tolerance test for cortisol
These tests also help determine the type of adrenal insufficiency (primary, secondary, or tertiary) and guide further management. Secondary adrenal insufficiency is caused by inadequate pituitary ACTH secretion and subsequent inadequate cortisol production, whereas tertiary adrenal insufficiency is caused by inadequate hypothalamic corticotropin-releasing hormone secretion and subsequent inadequate ACTH and cortisol production. The diagnosis of adrenal insufficiency relies first on demonstrating inappropriately low total serum cortisol production. Subsequently, serum ACTH helps to differentiate between primary (high ACTH) and secondary or tertiary (low or inappropriately normal ACTH) adrenal insufficiency.
Each test listed above may demonstrate a low cortisol level. However, in a nonacute setting, safety concerns (especially regarding insulin tolerance testing), poor diagnostic value, feasibility (ie, the difficulty of 24-hour tests), and poor sensitivity of 7 am cortisol make the ACTH stimulation test the most appropriate test in clinical practice to establish the diagnosis of adrenal insufficiency.
7 am serum cortisol measurement
Measuring the serum cortisol level early in the morning in the nonacute setting could be of diagnostic value, as an extremely low value (< 3–5 μg/dL) is almost 100% specific for adrenal insufficiency in the absence of concurrent exogenous steroid intake. However, the very low cutoff for this test causes poor sensitivity (about 33%), as many patients have partial adrenal insufficiency and hence have higher serum cortisol levels that may even be in the normal physiologic range.19–22
Random serum cortisol measurements
Random serum cortisol measurements are not very useful in a nonacute setting, since cortisol levels are affected by factors such as stress and hydration status. Moreover, they fluctuate during the day in a circadian rhythm.
On the other hand, random serum cortisol is a very good test to evaluate for adrenal insufficiency in the acute setting. A random value higher than 15 to 18 μg/dL is almost always associated with adequate adrenal function and generally rules out adrenal insufficiency.11,23,24
7 am salivary cortisol measurement
The same principle applies to early morning salivary cortisol. Only extremely low values (< 2.65 ng/mL) may distinguish patients with adrenal insufficiency from healthy individuals, with 97.1% sensitivity and 93.3% specificity.25
Of note, early morning salivary cortisol is not routinely measured in most clinical practices for evaluation of adrenal function. Hence, morning serum and morning salivary cortisol are useful screening tools and have meaningful results when their values are in the extremes of the spectrum, but they are not reliable as a single test, as they may overlook patients with partial adrenal insufficiency.
Urinary cortisol measurement
Urinary cortisol measurement is not used to diagnose adrenal insufficiency, as values can be normal in patients with partial adrenal insufficiency.
The ACTH stimulation test
The ACTH stimulation test involves an intramuscular or intravenous injection of cosyntropin (a synthetic analogue of ACTH fragment 1–24 that has the full activity of native ACTH) and measuring total serum cortisol at baseline, 30 minutes, and 60 minutes to assess the response of the adrenal glands.
The test can be done using a high or low dose of cosyntropin. The Endocrine Society’s 2016 guidelines recommend the high dose (250 μg) for most patients.10 The standard high-dose stimulation test can be done at any time during the day.26 If the cosyntropin is injected intravenously, any value higher than 18 to 20 μg/dL indicates normal adrenal function and excludes adrenal insufficiency.27,28 If intramuscular injection is used, any value higher than 16 to 18 μg/dL at 30 minutes post-consyntropin excludes adrenal insufficiency.29
The ACTH stimulation test may not exclude acute secondary or tertiary adrenal insufficiency.
Insulin tolerance testing
Insulin tolerance testing remains the gold standard for diagnosing adrenal insufficiency and assessing the integrity of the pituitary-adrenal axis. However, given its difficulty to perform, safety concerns, and the availability of other reliable tests, its use in clinical practice is limited. It is nonetheless useful in assessing patients with recent onset of ACTH deficiency.30,31
CASE RESUMED: PATIENT DISCHARGED, LOST TO FOLLOW-UP
Abdominal CT without contrast is done and demonstrates bilateral adrenal hemorrhage. Thus, the patient is diagnosed with primary acute adrenal insufficiency due to adrenal necrosis.
She is started on oral hydrocortisone and fludrocortisone after intravenous hydrocortisone is discontinued. She is counseled about adhering to medications, wearing a medical alert bracelet, giving herself emergency cortisol injections, taking higher doses of hydrocortisone if she is ill, and monitoring her INR. She is discharged home after her symptoms resolve.
The patient does not keep her scheduled appointment and is lost to follow-up. She returns 2 years later complaining of fatigue and feeling unwell. She admits that she stopped taking hydrocortisone 1 year ago after reading an online article about corticosteroid side effects. She has continued to take fludrocortisone.
MINERALOCORTICOID VS CORTICOSTEROID DEFICIENCY
4. Which of the following is least likely to be present in this patient at this time?
- Intravascular volume depletion
- Hyponatremia
- Skin hyperpigmentation
- Normokalemia
- Elevated serum ACTH level
Intravascular volume depletion
Intravascular volume depletion is the least likely to be present. This is because intravascular volume depletion is mainly secondary to mineralocorticoid deficiency rather than corticosteroid deficiency, which is not present in this patient, as she is compliant with her mineralocorticoid replacement therapy.32,33 However, even with sufficient mineralocorticoid replacement, mild hypotension may be present in this patient due to corticosteroid deficiency-induced loss of vascular tone.
Hyponatremia
Hyponatremia in adrenal insufficiency is not due only to mineralocorticoid deficiency. Patients with secondary or tertiary adrenal insufficiency may also exhibit hyponatremia.34 ACTH deficiency in such patients is not expected to cause mineralocorticoid deficiency, as ACTH has only a minor role in aldosterone production.
It has been proposed that hyponatremia in secondary adrenal insufficiency is due to cortisol deficiency resulting in an increase of antidiuretic hormone secretion.35,36 The mechanisms for increased antidiuretic hormone include cortisol deficiency resulting in an increased corticotropin-releasing hormone level, which acts as an antidiuretic hormone secretagogue,37,38 and cortisol directly suppressing antidiuretic hormone secretion.39
In our patient, volume expansion and hyponatremia are expected due to increased antidiuretic hormone secretion as a result of corticosteroid insufficiency.
Hyperpigmentation
Hyperpigmentation of the skin is present only in long-standing primary adrenal insufficiency. This is due to chronic cortisol deficiency causing an increased secretion of pro-opiomelanocortin, a prohormone that is cleaved into ACTH, melanocyte-stimulating hormone, and other hormones. Melanocyte-stimulating hormone causes skin hyperpigmentation due to increased melanin synthesis.40 The hyperpigmentation is seen in sun-exposed areas, pressure areas, palmar creases, nipples, and mucous membranes.
This patient has long-standing corticosteroid deficiency due to noncompliance and primary adrenal insufficiency, and as a result she is expected to have elevated serum ACTH and hyperpigmentation.
Normokalemia
Mineralocorticoid deficiency results in hyperkalemia and metabolic acidosis by impairing renal excretion of potassium and acid.41 This patient is compliant with her mineralocorticoid replacement regimen; thus, potassium levels and pH are expected to be normal.
TAKE-HOME POINTS
- Suspect adrenal crisis in any patient who presents with shock.
- Acute abdomen or unexplained fever could be among the manifestations.
- Initial management requires liberal normal saline intravenous fluid administration to replete the intravascular space.
- Draw blood samples for serum chemistry, cortisol, and ACTH, followed immediately by intravenous hydrocortisone supplementation.
- In critically ill patients, evaluate adrenal function with random serum cortisol; in a nonacute setting use the ACTH stimulation test.
- Chronic management of primary adrenal insufficiency requires corticosteroid and mineralocorticoid therapy.
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84(1):27–34. doi:10.3949/ccjm.84a.16042
- Almiani M, Gorthi J, Subbiah S, Firoz M. Quiz page November 2012: an unusual case of acute hyponatremia and normal anion gap metabolic acidosis. Am J Kidney Dis 2012; 60(5):xxxiii–xxxvi. doi:10.1053/j.ajkd.2012.05.026
- Migeon CJ, Kenny FM, Hung W, Voorhess ML. Study of adrenal function in children with meningitis. Pediatrics 1967; 40(2):163–183.
- Rao RH, Vagnucci AH, Amico JA. Bilateral massive adrenal hemorrhage: early recognition and treatment. Ann Intern Med 1989; 110(3):227–235.
- Shimizu S, Tahara Y, Atsumi T, et al. Waterhouse-Friderichsen syndrome caused by invasive Haemophilus influenzae type B infection in a previously healthy young man. Anaesth Intensive Care 2010; 38(1):214–215.
- Castaldo ET, Guillamondegui OD, Greco JA 3rd, Feurer ID, Miller RS, Morris JA Jr. Are adrenal injuries predictive of adrenal insufficiency in patients sustaining blunt trauma? Am Surg 2008; 74(3):262–266.
- Xarli VP, Steele AA, Davis PJ, Buescher ES, Rios CN, Garcia-Bunuel R. Adrenal hemorrhage in the adult. Medicine (Baltimore) 1978; 57(3):211–221.
- Takeuchi K, Tsuzuki Y, Ando T, et al. Clinical studies of strangulating small bowel obstruction. Am Surg 2004; 70(1):40–44.
- MacKenzie IM. The haemodynamics of human septic shock. Anaesthesia 2001; 56(2):130–144.
- Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2016; 101(2):364–389. doi:10.1210/jc.2015-1710
- Hamrahian AH, Fleseriu M; AACE Adrenal Scientific Committee. Evaluation and management of adrenal insufficiency in critically ill patients: disease state review. Endocr Pract 2017; 23(6):716–725. doi:10.4158/EP161720.RA
- Saenger P, Levine LS, Irvine WJ, et al. Progressive adrenal failure in polyglandular autoimmune disease. J Clin Endocrinol Metab 1982; 54(4):863–867.
- Coco G, Dal Pra C, Presotto F, et al. Estimated risk for developing autoimmune Addison's disease in patients with adrenal cortex autoantibodies. J Clin Endocrinol Metab 2006; 91(5):1637–1645. doi:10.1210/jc.2005-0860
- Ullian ME. The role of corticosteroids in the regulation of vascular tone. Cardiovasc Res 1999; 41(1):55–64.
- Yang S, Zhang L. Glucocorticoids and vascular reactivity. Curr Vasc Pharmacol 2004; 2(1):1–12.
- Yanase T, Tajima T, Katabami T, et al. Diagnosis and treatment of adrenal insufficiency including adrenal crisis: a Japan Endocrine Society clinical practice guideline [Opinion]. Endocr J 2016; 63(9):765–784. doi:10.1507/endocrj.EJ16-0242
- Taylor RL, Grebe SK, Singh RJ. Quantitative, highly sensitive liquid chromatography-tandem mass spectrometry method for detection of synthetic corticosteroids. Clin Chem 2004; 50(10):2345–2352. doi:10.1373/clinchem.2004.033605
- Goldfien A, Laidlaw JC, Haydar NA, Renold AE, Thorn GW. Fluorohydrocortisone and chlorohydrocortisone, highly potent derivatives of compound F. N Engl J Med 1955; 252(11):415–421. doi:10.1056/NEJM195503172521101
- Jenkins D, Forsham PH, Laidlaw JC, Reddy WJ, Thorn GW. Use of ACTH in the diagnosis of adrenal cortical insufficiency. Am J Med 1955; 18(1):3–14.
- Hägg E, Asplund K, Lithner F. Value of basal plasma cortisol assays in the assessment of pituitary-adrenal insufficiency. Clin Endocrinol (Oxf) 1987; 26(2):221–226.
- Deutschbein T, Unger N, Mann K, Petersenn S. Diagnosis of secondary adrenal insufficiency: unstimulated early morning cortisol in saliva and serum in comparison with the insulin tolerance test. Horm Metab Res 2009; 41(4):834–839. doi:10.1055/s-0029-1225630
- Erturk E, Jaffe CA, Barkan AL. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab 1998; 83(7):2350–2354.
- Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med 2003; 348(8):727–734. doi:10.1056/NEJMra020529
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39(2):165–228. doi:10.1007/s00134-012-2769-8
- Ceccato F, Barbot M, Zilio M, et al. Performance of salivary cortisol in the diagnosis of Cushing's syndrome, adrenal incidentaloma, and adrenal insufficiency. Eur J Endocrinol 2013; 169(1):31–36. doi:10.1530/EJE-13-0159
- Dickstein G, Shechner C, Nicholson WE, et al. Adrenocorticotropin stimulation test: effects of basal cortisol level, time of day, and suggested new sensitive low dose test. J Clin Endocrinol Metab 1991; 72(4):773–778. doi:10.1210/jcem-72-4-773
- May ME, Carey RM. Rapid adrenocorticotropic hormone test in practice. Retrospective review. Am J Med 1985; 79(6):679–884.
- Speckart PF, Nicoloff JT, Bethune JE. Screening for adrenocortical insufficiency with cosyntropin (synthetic ACTH). Arch Intern Med 1971; 128(5):761–763.
- Peechakara S, Bena J, Clarke NJ, et al. Total and free cortisol levels during 1 μg, 25 μg, and 250 μg cosyntropin stimulation tests compared to insulin tolerance test: results of a randomized, prospective, pilot study. Endocrine 2017; 57(3):388–393. doi:10.1007/s12020-017-1371-9
- Finucane FM, Liew A, Thornton E, Rogers B, Tormey W, Agha A. Clinical insights into the safety and utility of the insulin tolerance test (ITT) in the assessment of the hypothalamo-pituitary-adrenal axis. Clin Endocrinol (Oxf) 2008; 69(4):603–607. doi:10.1111/j.1365-2265.2008.03240.x
- Lindholm J, Kehlet H. Re-evaluation of the clinical value of the 30 min ACTH test in assessing the hypothalamic-pituitary-adrenocortical function. Clin Endocrinol (Oxf) 1987; 26(1):53–59.
- Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet 2014; 383(9935):2152–2167. doi:10.1016/S0140-6736(13)61684-0
- Burke CW. Adrenocortical insufficiency. Clin Endocrinol Metab 1985; 14(4):947–976.
- Jessani N, Jehangir W, Behman D, Yousif A, Spiler IJ. Secondary adrenal insufficiency: an overlooked cause of hyponatremia. J Clin Med Res 2015; 7(4):286–288. doi:10.14740/jocmr2041w
- Oelkers W. Hyponatremia and inappropriate secretion of vasopressin (antidiuretic hormone) in patients with hypopituitarism. N Engl J Med 1989; 321(8):492–496. doi:10.1056/NEJM198908243210802
- Ishikawa S, Schrier RW. Effect of arginine vasopressin antagonist on renal water excretion in glucocorticoid and mineralocorticoid deficient rats. Kidney Int 1982; 22(6):587–593.
- Wolfson B, Manning RW, Davis LG, Arentzen R, Baldino F Jr. Co-localization of corticotropin releasing factor and vasopressin mRNA in neurones after adrenalectomy. Nature 1985; 315(6014):59–61.
- Kalogeras KT, Nieman LK, Friedman TC, et al. Inferior petrosal sinus sampling in healthy subjects reveals a unilateral corticotropin-releasing hormone-induced arginine vasopressin release associated with ipsilateral adrenocorticotropin secretion. J Clin Invest 1996; 97:2045–2050.
- Kovacs KJ, Foldes A, Sawchenko PE. Glucocorticoid negative feedback selectively targets vasopressin transcription in parvocellular neurosecretory neurons. J Neurosci 2000; 20:3843–3852.
- Sarkar SB, Sarkar S, Ghosh S, Bandyopadhyay S. Addison's disease. Contemp Clin Dent 2012; 3(4):484–486. doi:10.4103/0976-237X.107450
- Szylman P, Better OS, Chaimowitz C, Rosler A. Role of hyperkalemia in the metabolic acidosis of isolated hypoaldosteronism. N Engl J Med 1976; 294(7):361–365. doi:10.1056/NEJM197602122940703
A 71-year-old woman is brought to the emergency department by her neighbor after complaining of fatigue and light-headedness for the last 8 hours. The patient lives alone and was feeling well when she woke up this morning, but then began to feel nauseated and vomited twice.
The patient appears drowsy and confused and cannot provide any further history. Her medical records show that she was seen in the cardiology clinic 6 months ago but has not kept her appointments since then.
Her medical history includes atrial fibrillation, hypertension, type 2 diabetes mellitus, and osteoarthritis. Her medications are daily warfarin, atenolol, aspirin, candesartan, and metformin, and she takes acetaminophen as needed. She is neither a smoker nor a drug user, but she drinks alcohol occasionally. Her family history is significant for her mother’s death from breast cancer at age 55.
The neighbor confirms that the patient appeared well this morning and has not had any recent illnesses except for a minor cold last week that improved over 5 days with acetaminophen only.
INITIAL EVALUATION AND MANAGEMENT
Physical examination
On physical examination, her blood pressure is 80/40 mm Hg, respiratory rate 25 breaths per minute, oral temperature 38.3°C (100.9°F), and heart rate 130 beats per minute and irregular.
Her neck veins are flat, and her chest is clear to auscultation with normal heart sounds. Abdominal palpation elicits discomfort in the middle segments, voluntary withdrawal, and abdominal wall rigidity. Her skin feels dry and cool, with decreased turgor.
Initial treatment
The patient is given 1 L of 0.9% saline intravenously over the first hour and then is transferred to the intensive care unit, where a norepinephrine drip is started to treat her ongoing hypotension. Normal saline is continued at a rate of 500 mL per hour for the next 4 hours.
Cardiac monitoring and 12-lead electrocardiography show atrial fibrillation with a rapid ventricular response of 138 beats per minute, but electrical cardioversion is not done.
Initial laboratory tests
Of note, her international normalized ratio (INR) is 6.13, while the therapeutic range for a patient taking warfarin because of atrial fibrillation is 2.0 to 3.0.
Her blood pH is 7.34 (reference range 7.35–7.45), and her bicarbonate level is 18 mmol/L (22–26); a low pH and low bicarbonate together indicate metabolic acidosis. Her sodium level is 128 mmol/L (135–145), her chloride level is 100 mmol/L (97–107), and, as mentioned, her bicarbonate level is 18 mmol/L; therefore, her anion gap is 128 – (100 + 18) = 10 mmol/L, which is normal (≤ 10).1
Her serum creatinine level is 1.3 mg/dL (0.5–1.1), and her blood urea nitrogen level is 35 mg/dL (7–20).
Her potassium level is 5.8 mmol/L, which is consistent with hyperkalemia (reference range 3.5–5.2).
DIFFERENTIAL DIAGNOSIS
1. Which of the following is the most likely cause of this patient’s symptoms?
- Adrenal crisis
- Cardiogenic shock due to decreased cardiac contractility
- Intracranial hemorrhage
- Acute abdomen due to small bowel obstruction
- Septic shock due to bacterial toxin-induced loss of vascular tone
Our patient is presenting with shock. Given our inability to obtain a meaningful history, the differential diagnosis is broad and includes all of the above.
Adrenal crisis
The sudden onset and laboratory results that include hyperkalemia, hyponatremia, and normal anion gap metabolic acidosis raise suspicion of adrenal crisis resulting in acute mineralocorticoid and glucocorticoid insufficiency.1
The patient’s elevated serum creatinine and high blood urea nitrogen-to-creatinine ratio of 26.9 (reference range 10–20) also suggest intravascular volume contraction. Her low hemoglobin level and supratherapeutic INR, possibly due to an interaction between warfarin and acetaminophen combined with poor medical follow-up, raise suspicion of acute bilateral adrenal necrosis due to hemorrhage.
Bilateral adrenal hemorrhage is one cause of adrenal crisis resulting in bilateral adrenal necrosis. Risk factors for adrenal hemorrhage include anticoagulation therapy, underlying coagulopathy, postoperative states, and certain infections such as meningococcemia and Haemophilus influenzae infection.2–5 Nevertheless, in most cases the INR is in the therapeutic range and the patient has no bleeding elsewhere.4 Other causes of adrenal necrosis include emboli, sepsis, and blunt trauma.6,7
Other causes of adrenal crisis are listed in Table 3.
Cardiogenic shock
Intracranial hemorrhage
Intracranial hemorrhage can present with a decreased level of consciousness, but it is less likely to cause hypotension, as the cranial space is limited. If massive intracranial hemorrhage would occur, the increase in intracranial pressure would more likely cause hypertension by the Cushing reflex than hypotension.
Acute abdomen
Abdominal pain and rigidity along with fever can be presenting symptoms of both adrenal insufficiency and an acute abdomen due to intestinal obstruction.4 However, intestinal obstruction typically causes a high anion gap metabolic acidosis due to lactic acidosis, instead of the normal anion gap metabolic acidosis present in this patient.8 Moreover, her deranged electrolytes, supratherapeutic INR, and absence of previous gastroenterologic conditions make adrenal crisis a more likely diagnosis.
Septic shock
Septic shock would also cause fever and hypotension as bacterial toxins induce a pyrexic response and vasodilation. However, at such an early stage of sepsis, the patient would be expected to be warm and hyperemic, whereas this patient’s skin is cool and dry due to volume depletion secondary to adrenal insufficiency.9 Sepsis would also cause a high anion gap metabolic acidosis due to lactic acidosis, as opposed to this patient’s normal anion gap metabolic acidosis. These findings, along with the metabolic derangements and the absence of a focus of infection, make sepsis a less likely possibility.
CASE CONTINUED: CARDIOMEGALY, PERSISTENT HYPOTENSION
Blood is drawn for cultures and measurement of troponins and lactic acid, and urine samples are taken for culture and biochemical analysis. Chest radiography shows mild cardiomegaly. The patient is started empirically on vancomycin and cefepime, and her warfarin is discontinued.
Five hours after presenting to the emergency department, her blood pressure remains at 80/40 mm Hg even after receiving 3 L of normal saline intravenously.
PROMPT MANAGEMENT OF ADRENAL CRISIS
2. Which of the following is the most appropriate next step in managing this patient?
- Draw samples for serum cortisol and plasma adrenocorticotropic hormone (ACTH) levels, then give hydrocortisone 100 mg intravenously
- Perform abdominal computed tomography (CT) without contrast
- Perform transthoracic echocardiography
- Increase the norepinephrine infusion
- Immediately give fludrocortisone
First give fluids
The first step in managing a patient with suspected adrenal crisis is liberal intravenous fluid administration to replenish the depleted intravascular space. The amount and choice of fluid is empiric, but a recommendation is 1 L of normal saline or dextrose 5% in normal saline, infused quickly over the first hour and then titrated according to the patient’s fluid status.10
Measure cortisol and ACTH; start corticosteroids immediately
Immediate therapy with an appropriate stress dose of intravenous corticosteroids (eg, hydrocortisone 100 mg) is essential. However, this should be done after drawing blood for cortisol and ACTH measurements.10
Do not delay corticosteroid therapy while awaiting the results of the diagnostic tests.
In addition, in the early phase of evolving primary adrenal insufficiency, measurement of plasma renin and aldosterone levels may be beneficial, as mineralocorticoid deficiency may predominate.10,12,13
One of the most important aims of early corticosteroid supplementation is to prevent further hyponatremia by reducing a reactive increase in antidiuretic hormone secretion caused by cortisol deficiency. Corticosteroids also help to restore normal blood pressure by increasing vascular tone, as glucocorticoid receptor activation potentiates the vasoconstrictor actions of norepinephrine, angiotensin II, and other vasoconstrictors.14,15
Which corticosteroid to use?
Which corticosteroid to use in previously undiagnosed adrenal insufficiency is controversial. The Endocrine Society10 and Japan Endocrine Society16 clinical practice guidelines recommend hydrocortisone in a 100-mg intravenous bolus followed by 200 mg over 24 hours.
The choice of hydrocortisone is justified by its superior mineralocorticoid activity.10,16 Further, hydrocortisone is preferred over dexamethasone if the patient is known to have primary adrenal insufficiency, or if the serum potassium level is higher than 6.0 mmol/L.
Some clinicians, on the other hand, recommend dexamethasone, given as a 4-mg intravenous bolus followed by 4-mg boluses every 12 hours. Their rationale is that dexamethasone, unlike hydrocortisone, does not interfere with subsequent serum cortisol assays if the patient later undergoes ACTH stimulation testing.17 Dexamethasone may also be preferred to minimize unwanted mineralocorticoid effects, such as in neurosurgical patients at risk of brain edema.
If hydrocortisone is used, ACTH stimulation testing can be done after withholding hydrocortisone for 24 hours once the patient is stable. (It should be restarted after the test if the results are abnormal.)
Other possible steps
Abdominal CT should be done in our patient to address the possibility of bilateral adrenal hemorrhage. However, it is preferable to wait until the patient is stabilized.
Echocardiography. Our patient is likely to have an element of cardiac failure, given her hypertension and cardiomegaly. However, decompensated heart failure is probably not the cause of her presentation. Thus, the first priority is to treat her adrenal crisis, and echocardiography should be deferred.
Increasing the norepinephrine infusion is unlikely to improve her blood pressure very much, as she is significantly volume-depleted. Further, low cortisol decreases the vascular response to norepinephrine.15
Mineralocorticoids such as fludrocortisone are used to treat primary adrenal insufficiency. However, they are not required during acute management of adrenal crisis, as 40 mg of hydrocortisone offers mineralocorticoid activity equivalent to 100 µg of fludrocortisone. Thus, the high doses of hydrocortisone used to treat adrenal crisis provide adequate mineralocorticoid therapy.10,18
If dexamethasone is used, its effect along with normal saline supplementation would be sufficient to replete the intravenous space and bring the sodium level back up to normal in the acute setting.
CASE RESUMED: IMPROVEMENT WITH HYDROCORTISONE
The patient’s blood is drawn for serum cortisol and plasma ACTH measurements. A 100-mg intravenous bolus of hydrocortisone is given, followed by a 50-mg bolus every 6 hours until the patient stabilizes.
Twenty-four hours later, the patient states that she has more energy, and her appetite has improved. The norepinephrine infusion is stopped 48 hours after presentation, at which time her blood pressure is 120/70 mm Hg, heart rate 85 beats per minute and irregular, and temperature 36.7°C (98.1°F). Her current laboratory values include the following:
- Serum sodium 137 mmolL
- Serum potassium 4.3 mmol/L
- Hemoglobin 9.3 g/dL
- Serum cortisol (random) 7.2 μg/dL
- Plasma ACTH 752 pg/mL (10–60 pg/mL).
ESTABLISHING THE DIAGNOSIS OF ADRENAL INSUFFICIENCY
3. Which of the following is the most appropriate test to establish the diagnosis of adrenal insufficiency?
- 7 am total serum cortisol measurement
- Random serum cortisol measurement
- 7 am salivary cortisol measurement
- 24-hour urinary free cortisol measurement
- ACTH stimulation test for cortisol
- Insulin tolerance test for cortisol
These tests also help determine the type of adrenal insufficiency (primary, secondary, or tertiary) and guide further management. Secondary adrenal insufficiency is caused by inadequate pituitary ACTH secretion and subsequent inadequate cortisol production, whereas tertiary adrenal insufficiency is caused by inadequate hypothalamic corticotropin-releasing hormone secretion and subsequent inadequate ACTH and cortisol production. The diagnosis of adrenal insufficiency relies first on demonstrating inappropriately low total serum cortisol production. Subsequently, serum ACTH helps to differentiate between primary (high ACTH) and secondary or tertiary (low or inappropriately normal ACTH) adrenal insufficiency.
Each test listed above may demonstrate a low cortisol level. However, in a nonacute setting, safety concerns (especially regarding insulin tolerance testing), poor diagnostic value, feasibility (ie, the difficulty of 24-hour tests), and poor sensitivity of 7 am cortisol make the ACTH stimulation test the most appropriate test in clinical practice to establish the diagnosis of adrenal insufficiency.
7 am serum cortisol measurement
Measuring the serum cortisol level early in the morning in the nonacute setting could be of diagnostic value, as an extremely low value (< 3–5 μg/dL) is almost 100% specific for adrenal insufficiency in the absence of concurrent exogenous steroid intake. However, the very low cutoff for this test causes poor sensitivity (about 33%), as many patients have partial adrenal insufficiency and hence have higher serum cortisol levels that may even be in the normal physiologic range.19–22
Random serum cortisol measurements
Random serum cortisol measurements are not very useful in a nonacute setting, since cortisol levels are affected by factors such as stress and hydration status. Moreover, they fluctuate during the day in a circadian rhythm.
On the other hand, random serum cortisol is a very good test to evaluate for adrenal insufficiency in the acute setting. A random value higher than 15 to 18 μg/dL is almost always associated with adequate adrenal function and generally rules out adrenal insufficiency.11,23,24
7 am salivary cortisol measurement
The same principle applies to early morning salivary cortisol. Only extremely low values (< 2.65 ng/mL) may distinguish patients with adrenal insufficiency from healthy individuals, with 97.1% sensitivity and 93.3% specificity.25
Of note, early morning salivary cortisol is not routinely measured in most clinical practices for evaluation of adrenal function. Hence, morning serum and morning salivary cortisol are useful screening tools and have meaningful results when their values are in the extremes of the spectrum, but they are not reliable as a single test, as they may overlook patients with partial adrenal insufficiency.
Urinary cortisol measurement
Urinary cortisol measurement is not used to diagnose adrenal insufficiency, as values can be normal in patients with partial adrenal insufficiency.
The ACTH stimulation test
The ACTH stimulation test involves an intramuscular or intravenous injection of cosyntropin (a synthetic analogue of ACTH fragment 1–24 that has the full activity of native ACTH) and measuring total serum cortisol at baseline, 30 minutes, and 60 minutes to assess the response of the adrenal glands.
The test can be done using a high or low dose of cosyntropin. The Endocrine Society’s 2016 guidelines recommend the high dose (250 μg) for most patients.10 The standard high-dose stimulation test can be done at any time during the day.26 If the cosyntropin is injected intravenously, any value higher than 18 to 20 μg/dL indicates normal adrenal function and excludes adrenal insufficiency.27,28 If intramuscular injection is used, any value higher than 16 to 18 μg/dL at 30 minutes post-consyntropin excludes adrenal insufficiency.29
The ACTH stimulation test may not exclude acute secondary or tertiary adrenal insufficiency.
Insulin tolerance testing
Insulin tolerance testing remains the gold standard for diagnosing adrenal insufficiency and assessing the integrity of the pituitary-adrenal axis. However, given its difficulty to perform, safety concerns, and the availability of other reliable tests, its use in clinical practice is limited. It is nonetheless useful in assessing patients with recent onset of ACTH deficiency.30,31
CASE RESUMED: PATIENT DISCHARGED, LOST TO FOLLOW-UP
Abdominal CT without contrast is done and demonstrates bilateral adrenal hemorrhage. Thus, the patient is diagnosed with primary acute adrenal insufficiency due to adrenal necrosis.
She is started on oral hydrocortisone and fludrocortisone after intravenous hydrocortisone is discontinued. She is counseled about adhering to medications, wearing a medical alert bracelet, giving herself emergency cortisol injections, taking higher doses of hydrocortisone if she is ill, and monitoring her INR. She is discharged home after her symptoms resolve.
The patient does not keep her scheduled appointment and is lost to follow-up. She returns 2 years later complaining of fatigue and feeling unwell. She admits that she stopped taking hydrocortisone 1 year ago after reading an online article about corticosteroid side effects. She has continued to take fludrocortisone.
MINERALOCORTICOID VS CORTICOSTEROID DEFICIENCY
4. Which of the following is least likely to be present in this patient at this time?
- Intravascular volume depletion
- Hyponatremia
- Skin hyperpigmentation
- Normokalemia
- Elevated serum ACTH level
Intravascular volume depletion
Intravascular volume depletion is the least likely to be present. This is because intravascular volume depletion is mainly secondary to mineralocorticoid deficiency rather than corticosteroid deficiency, which is not present in this patient, as she is compliant with her mineralocorticoid replacement therapy.32,33 However, even with sufficient mineralocorticoid replacement, mild hypotension may be present in this patient due to corticosteroid deficiency-induced loss of vascular tone.
Hyponatremia
Hyponatremia in adrenal insufficiency is not due only to mineralocorticoid deficiency. Patients with secondary or tertiary adrenal insufficiency may also exhibit hyponatremia.34 ACTH deficiency in such patients is not expected to cause mineralocorticoid deficiency, as ACTH has only a minor role in aldosterone production.
It has been proposed that hyponatremia in secondary adrenal insufficiency is due to cortisol deficiency resulting in an increase of antidiuretic hormone secretion.35,36 The mechanisms for increased antidiuretic hormone include cortisol deficiency resulting in an increased corticotropin-releasing hormone level, which acts as an antidiuretic hormone secretagogue,37,38 and cortisol directly suppressing antidiuretic hormone secretion.39
In our patient, volume expansion and hyponatremia are expected due to increased antidiuretic hormone secretion as a result of corticosteroid insufficiency.
Hyperpigmentation
Hyperpigmentation of the skin is present only in long-standing primary adrenal insufficiency. This is due to chronic cortisol deficiency causing an increased secretion of pro-opiomelanocortin, a prohormone that is cleaved into ACTH, melanocyte-stimulating hormone, and other hormones. Melanocyte-stimulating hormone causes skin hyperpigmentation due to increased melanin synthesis.40 The hyperpigmentation is seen in sun-exposed areas, pressure areas, palmar creases, nipples, and mucous membranes.
This patient has long-standing corticosteroid deficiency due to noncompliance and primary adrenal insufficiency, and as a result she is expected to have elevated serum ACTH and hyperpigmentation.
Normokalemia
Mineralocorticoid deficiency results in hyperkalemia and metabolic acidosis by impairing renal excretion of potassium and acid.41 This patient is compliant with her mineralocorticoid replacement regimen; thus, potassium levels and pH are expected to be normal.
TAKE-HOME POINTS
- Suspect adrenal crisis in any patient who presents with shock.
- Acute abdomen or unexplained fever could be among the manifestations.
- Initial management requires liberal normal saline intravenous fluid administration to replete the intravascular space.
- Draw blood samples for serum chemistry, cortisol, and ACTH, followed immediately by intravenous hydrocortisone supplementation.
- In critically ill patients, evaluate adrenal function with random serum cortisol; in a nonacute setting use the ACTH stimulation test.
- Chronic management of primary adrenal insufficiency requires corticosteroid and mineralocorticoid therapy.
A 71-year-old woman is brought to the emergency department by her neighbor after complaining of fatigue and light-headedness for the last 8 hours. The patient lives alone and was feeling well when she woke up this morning, but then began to feel nauseated and vomited twice.
The patient appears drowsy and confused and cannot provide any further history. Her medical records show that she was seen in the cardiology clinic 6 months ago but has not kept her appointments since then.
Her medical history includes atrial fibrillation, hypertension, type 2 diabetes mellitus, and osteoarthritis. Her medications are daily warfarin, atenolol, aspirin, candesartan, and metformin, and she takes acetaminophen as needed. She is neither a smoker nor a drug user, but she drinks alcohol occasionally. Her family history is significant for her mother’s death from breast cancer at age 55.
The neighbor confirms that the patient appeared well this morning and has not had any recent illnesses except for a minor cold last week that improved over 5 days with acetaminophen only.
INITIAL EVALUATION AND MANAGEMENT
Physical examination
On physical examination, her blood pressure is 80/40 mm Hg, respiratory rate 25 breaths per minute, oral temperature 38.3°C (100.9°F), and heart rate 130 beats per minute and irregular.
Her neck veins are flat, and her chest is clear to auscultation with normal heart sounds. Abdominal palpation elicits discomfort in the middle segments, voluntary withdrawal, and abdominal wall rigidity. Her skin feels dry and cool, with decreased turgor.
Initial treatment
The patient is given 1 L of 0.9% saline intravenously over the first hour and then is transferred to the intensive care unit, where a norepinephrine drip is started to treat her ongoing hypotension. Normal saline is continued at a rate of 500 mL per hour for the next 4 hours.
Cardiac monitoring and 12-lead electrocardiography show atrial fibrillation with a rapid ventricular response of 138 beats per minute, but electrical cardioversion is not done.
Initial laboratory tests
Of note, her international normalized ratio (INR) is 6.13, while the therapeutic range for a patient taking warfarin because of atrial fibrillation is 2.0 to 3.0.
Her blood pH is 7.34 (reference range 7.35–7.45), and her bicarbonate level is 18 mmol/L (22–26); a low pH and low bicarbonate together indicate metabolic acidosis. Her sodium level is 128 mmol/L (135–145), her chloride level is 100 mmol/L (97–107), and, as mentioned, her bicarbonate level is 18 mmol/L; therefore, her anion gap is 128 – (100 + 18) = 10 mmol/L, which is normal (≤ 10).1
Her serum creatinine level is 1.3 mg/dL (0.5–1.1), and her blood urea nitrogen level is 35 mg/dL (7–20).
Her potassium level is 5.8 mmol/L, which is consistent with hyperkalemia (reference range 3.5–5.2).
DIFFERENTIAL DIAGNOSIS
1. Which of the following is the most likely cause of this patient’s symptoms?
- Adrenal crisis
- Cardiogenic shock due to decreased cardiac contractility
- Intracranial hemorrhage
- Acute abdomen due to small bowel obstruction
- Septic shock due to bacterial toxin-induced loss of vascular tone
Our patient is presenting with shock. Given our inability to obtain a meaningful history, the differential diagnosis is broad and includes all of the above.
Adrenal crisis
The sudden onset and laboratory results that include hyperkalemia, hyponatremia, and normal anion gap metabolic acidosis raise suspicion of adrenal crisis resulting in acute mineralocorticoid and glucocorticoid insufficiency.1
The patient’s elevated serum creatinine and high blood urea nitrogen-to-creatinine ratio of 26.9 (reference range 10–20) also suggest intravascular volume contraction. Her low hemoglobin level and supratherapeutic INR, possibly due to an interaction between warfarin and acetaminophen combined with poor medical follow-up, raise suspicion of acute bilateral adrenal necrosis due to hemorrhage.
Bilateral adrenal hemorrhage is one cause of adrenal crisis resulting in bilateral adrenal necrosis. Risk factors for adrenal hemorrhage include anticoagulation therapy, underlying coagulopathy, postoperative states, and certain infections such as meningococcemia and Haemophilus influenzae infection.2–5 Nevertheless, in most cases the INR is in the therapeutic range and the patient has no bleeding elsewhere.4 Other causes of adrenal necrosis include emboli, sepsis, and blunt trauma.6,7
Other causes of adrenal crisis are listed in Table 3.
Cardiogenic shock
Intracranial hemorrhage
Intracranial hemorrhage can present with a decreased level of consciousness, but it is less likely to cause hypotension, as the cranial space is limited. If massive intracranial hemorrhage would occur, the increase in intracranial pressure would more likely cause hypertension by the Cushing reflex than hypotension.
Acute abdomen
Abdominal pain and rigidity along with fever can be presenting symptoms of both adrenal insufficiency and an acute abdomen due to intestinal obstruction.4 However, intestinal obstruction typically causes a high anion gap metabolic acidosis due to lactic acidosis, instead of the normal anion gap metabolic acidosis present in this patient.8 Moreover, her deranged electrolytes, supratherapeutic INR, and absence of previous gastroenterologic conditions make adrenal crisis a more likely diagnosis.
Septic shock
Septic shock would also cause fever and hypotension as bacterial toxins induce a pyrexic response and vasodilation. However, at such an early stage of sepsis, the patient would be expected to be warm and hyperemic, whereas this patient’s skin is cool and dry due to volume depletion secondary to adrenal insufficiency.9 Sepsis would also cause a high anion gap metabolic acidosis due to lactic acidosis, as opposed to this patient’s normal anion gap metabolic acidosis. These findings, along with the metabolic derangements and the absence of a focus of infection, make sepsis a less likely possibility.
CASE CONTINUED: CARDIOMEGALY, PERSISTENT HYPOTENSION
Blood is drawn for cultures and measurement of troponins and lactic acid, and urine samples are taken for culture and biochemical analysis. Chest radiography shows mild cardiomegaly. The patient is started empirically on vancomycin and cefepime, and her warfarin is discontinued.
Five hours after presenting to the emergency department, her blood pressure remains at 80/40 mm Hg even after receiving 3 L of normal saline intravenously.
PROMPT MANAGEMENT OF ADRENAL CRISIS
2. Which of the following is the most appropriate next step in managing this patient?
- Draw samples for serum cortisol and plasma adrenocorticotropic hormone (ACTH) levels, then give hydrocortisone 100 mg intravenously
- Perform abdominal computed tomography (CT) without contrast
- Perform transthoracic echocardiography
- Increase the norepinephrine infusion
- Immediately give fludrocortisone
First give fluids
The first step in managing a patient with suspected adrenal crisis is liberal intravenous fluid administration to replenish the depleted intravascular space. The amount and choice of fluid is empiric, but a recommendation is 1 L of normal saline or dextrose 5% in normal saline, infused quickly over the first hour and then titrated according to the patient’s fluid status.10
Measure cortisol and ACTH; start corticosteroids immediately
Immediate therapy with an appropriate stress dose of intravenous corticosteroids (eg, hydrocortisone 100 mg) is essential. However, this should be done after drawing blood for cortisol and ACTH measurements.10
Do not delay corticosteroid therapy while awaiting the results of the diagnostic tests.
In addition, in the early phase of evolving primary adrenal insufficiency, measurement of plasma renin and aldosterone levels may be beneficial, as mineralocorticoid deficiency may predominate.10,12,13
One of the most important aims of early corticosteroid supplementation is to prevent further hyponatremia by reducing a reactive increase in antidiuretic hormone secretion caused by cortisol deficiency. Corticosteroids also help to restore normal blood pressure by increasing vascular tone, as glucocorticoid receptor activation potentiates the vasoconstrictor actions of norepinephrine, angiotensin II, and other vasoconstrictors.14,15
Which corticosteroid to use?
Which corticosteroid to use in previously undiagnosed adrenal insufficiency is controversial. The Endocrine Society10 and Japan Endocrine Society16 clinical practice guidelines recommend hydrocortisone in a 100-mg intravenous bolus followed by 200 mg over 24 hours.
The choice of hydrocortisone is justified by its superior mineralocorticoid activity.10,16 Further, hydrocortisone is preferred over dexamethasone if the patient is known to have primary adrenal insufficiency, or if the serum potassium level is higher than 6.0 mmol/L.
Some clinicians, on the other hand, recommend dexamethasone, given as a 4-mg intravenous bolus followed by 4-mg boluses every 12 hours. Their rationale is that dexamethasone, unlike hydrocortisone, does not interfere with subsequent serum cortisol assays if the patient later undergoes ACTH stimulation testing.17 Dexamethasone may also be preferred to minimize unwanted mineralocorticoid effects, such as in neurosurgical patients at risk of brain edema.
If hydrocortisone is used, ACTH stimulation testing can be done after withholding hydrocortisone for 24 hours once the patient is stable. (It should be restarted after the test if the results are abnormal.)
Other possible steps
Abdominal CT should be done in our patient to address the possibility of bilateral adrenal hemorrhage. However, it is preferable to wait until the patient is stabilized.
Echocardiography. Our patient is likely to have an element of cardiac failure, given her hypertension and cardiomegaly. However, decompensated heart failure is probably not the cause of her presentation. Thus, the first priority is to treat her adrenal crisis, and echocardiography should be deferred.
Increasing the norepinephrine infusion is unlikely to improve her blood pressure very much, as she is significantly volume-depleted. Further, low cortisol decreases the vascular response to norepinephrine.15
Mineralocorticoids such as fludrocortisone are used to treat primary adrenal insufficiency. However, they are not required during acute management of adrenal crisis, as 40 mg of hydrocortisone offers mineralocorticoid activity equivalent to 100 µg of fludrocortisone. Thus, the high doses of hydrocortisone used to treat adrenal crisis provide adequate mineralocorticoid therapy.10,18
If dexamethasone is used, its effect along with normal saline supplementation would be sufficient to replete the intravenous space and bring the sodium level back up to normal in the acute setting.
CASE RESUMED: IMPROVEMENT WITH HYDROCORTISONE
The patient’s blood is drawn for serum cortisol and plasma ACTH measurements. A 100-mg intravenous bolus of hydrocortisone is given, followed by a 50-mg bolus every 6 hours until the patient stabilizes.
Twenty-four hours later, the patient states that she has more energy, and her appetite has improved. The norepinephrine infusion is stopped 48 hours after presentation, at which time her blood pressure is 120/70 mm Hg, heart rate 85 beats per minute and irregular, and temperature 36.7°C (98.1°F). Her current laboratory values include the following:
- Serum sodium 137 mmolL
- Serum potassium 4.3 mmol/L
- Hemoglobin 9.3 g/dL
- Serum cortisol (random) 7.2 μg/dL
- Plasma ACTH 752 pg/mL (10–60 pg/mL).
ESTABLISHING THE DIAGNOSIS OF ADRENAL INSUFFICIENCY
3. Which of the following is the most appropriate test to establish the diagnosis of adrenal insufficiency?
- 7 am total serum cortisol measurement
- Random serum cortisol measurement
- 7 am salivary cortisol measurement
- 24-hour urinary free cortisol measurement
- ACTH stimulation test for cortisol
- Insulin tolerance test for cortisol
These tests also help determine the type of adrenal insufficiency (primary, secondary, or tertiary) and guide further management. Secondary adrenal insufficiency is caused by inadequate pituitary ACTH secretion and subsequent inadequate cortisol production, whereas tertiary adrenal insufficiency is caused by inadequate hypothalamic corticotropin-releasing hormone secretion and subsequent inadequate ACTH and cortisol production. The diagnosis of adrenal insufficiency relies first on demonstrating inappropriately low total serum cortisol production. Subsequently, serum ACTH helps to differentiate between primary (high ACTH) and secondary or tertiary (low or inappropriately normal ACTH) adrenal insufficiency.
Each test listed above may demonstrate a low cortisol level. However, in a nonacute setting, safety concerns (especially regarding insulin tolerance testing), poor diagnostic value, feasibility (ie, the difficulty of 24-hour tests), and poor sensitivity of 7 am cortisol make the ACTH stimulation test the most appropriate test in clinical practice to establish the diagnosis of adrenal insufficiency.
7 am serum cortisol measurement
Measuring the serum cortisol level early in the morning in the nonacute setting could be of diagnostic value, as an extremely low value (< 3–5 μg/dL) is almost 100% specific for adrenal insufficiency in the absence of concurrent exogenous steroid intake. However, the very low cutoff for this test causes poor sensitivity (about 33%), as many patients have partial adrenal insufficiency and hence have higher serum cortisol levels that may even be in the normal physiologic range.19–22
Random serum cortisol measurements
Random serum cortisol measurements are not very useful in a nonacute setting, since cortisol levels are affected by factors such as stress and hydration status. Moreover, they fluctuate during the day in a circadian rhythm.
On the other hand, random serum cortisol is a very good test to evaluate for adrenal insufficiency in the acute setting. A random value higher than 15 to 18 μg/dL is almost always associated with adequate adrenal function and generally rules out adrenal insufficiency.11,23,24
7 am salivary cortisol measurement
The same principle applies to early morning salivary cortisol. Only extremely low values (< 2.65 ng/mL) may distinguish patients with adrenal insufficiency from healthy individuals, with 97.1% sensitivity and 93.3% specificity.25
Of note, early morning salivary cortisol is not routinely measured in most clinical practices for evaluation of adrenal function. Hence, morning serum and morning salivary cortisol are useful screening tools and have meaningful results when their values are in the extremes of the spectrum, but they are not reliable as a single test, as they may overlook patients with partial adrenal insufficiency.
Urinary cortisol measurement
Urinary cortisol measurement is not used to diagnose adrenal insufficiency, as values can be normal in patients with partial adrenal insufficiency.
The ACTH stimulation test
The ACTH stimulation test involves an intramuscular or intravenous injection of cosyntropin (a synthetic analogue of ACTH fragment 1–24 that has the full activity of native ACTH) and measuring total serum cortisol at baseline, 30 minutes, and 60 minutes to assess the response of the adrenal glands.
The test can be done using a high or low dose of cosyntropin. The Endocrine Society’s 2016 guidelines recommend the high dose (250 μg) for most patients.10 The standard high-dose stimulation test can be done at any time during the day.26 If the cosyntropin is injected intravenously, any value higher than 18 to 20 μg/dL indicates normal adrenal function and excludes adrenal insufficiency.27,28 If intramuscular injection is used, any value higher than 16 to 18 μg/dL at 30 minutes post-consyntropin excludes adrenal insufficiency.29
The ACTH stimulation test may not exclude acute secondary or tertiary adrenal insufficiency.
Insulin tolerance testing
Insulin tolerance testing remains the gold standard for diagnosing adrenal insufficiency and assessing the integrity of the pituitary-adrenal axis. However, given its difficulty to perform, safety concerns, and the availability of other reliable tests, its use in clinical practice is limited. It is nonetheless useful in assessing patients with recent onset of ACTH deficiency.30,31
CASE RESUMED: PATIENT DISCHARGED, LOST TO FOLLOW-UP
Abdominal CT without contrast is done and demonstrates bilateral adrenal hemorrhage. Thus, the patient is diagnosed with primary acute adrenal insufficiency due to adrenal necrosis.
She is started on oral hydrocortisone and fludrocortisone after intravenous hydrocortisone is discontinued. She is counseled about adhering to medications, wearing a medical alert bracelet, giving herself emergency cortisol injections, taking higher doses of hydrocortisone if she is ill, and monitoring her INR. She is discharged home after her symptoms resolve.
The patient does not keep her scheduled appointment and is lost to follow-up. She returns 2 years later complaining of fatigue and feeling unwell. She admits that she stopped taking hydrocortisone 1 year ago after reading an online article about corticosteroid side effects. She has continued to take fludrocortisone.
MINERALOCORTICOID VS CORTICOSTEROID DEFICIENCY
4. Which of the following is least likely to be present in this patient at this time?
- Intravascular volume depletion
- Hyponatremia
- Skin hyperpigmentation
- Normokalemia
- Elevated serum ACTH level
Intravascular volume depletion
Intravascular volume depletion is the least likely to be present. This is because intravascular volume depletion is mainly secondary to mineralocorticoid deficiency rather than corticosteroid deficiency, which is not present in this patient, as she is compliant with her mineralocorticoid replacement therapy.32,33 However, even with sufficient mineralocorticoid replacement, mild hypotension may be present in this patient due to corticosteroid deficiency-induced loss of vascular tone.
Hyponatremia
Hyponatremia in adrenal insufficiency is not due only to mineralocorticoid deficiency. Patients with secondary or tertiary adrenal insufficiency may also exhibit hyponatremia.34 ACTH deficiency in such patients is not expected to cause mineralocorticoid deficiency, as ACTH has only a minor role in aldosterone production.
It has been proposed that hyponatremia in secondary adrenal insufficiency is due to cortisol deficiency resulting in an increase of antidiuretic hormone secretion.35,36 The mechanisms for increased antidiuretic hormone include cortisol deficiency resulting in an increased corticotropin-releasing hormone level, which acts as an antidiuretic hormone secretagogue,37,38 and cortisol directly suppressing antidiuretic hormone secretion.39
In our patient, volume expansion and hyponatremia are expected due to increased antidiuretic hormone secretion as a result of corticosteroid insufficiency.
Hyperpigmentation
Hyperpigmentation of the skin is present only in long-standing primary adrenal insufficiency. This is due to chronic cortisol deficiency causing an increased secretion of pro-opiomelanocortin, a prohormone that is cleaved into ACTH, melanocyte-stimulating hormone, and other hormones. Melanocyte-stimulating hormone causes skin hyperpigmentation due to increased melanin synthesis.40 The hyperpigmentation is seen in sun-exposed areas, pressure areas, palmar creases, nipples, and mucous membranes.
This patient has long-standing corticosteroid deficiency due to noncompliance and primary adrenal insufficiency, and as a result she is expected to have elevated serum ACTH and hyperpigmentation.
Normokalemia
Mineralocorticoid deficiency results in hyperkalemia and metabolic acidosis by impairing renal excretion of potassium and acid.41 This patient is compliant with her mineralocorticoid replacement regimen; thus, potassium levels and pH are expected to be normal.
TAKE-HOME POINTS
- Suspect adrenal crisis in any patient who presents with shock.
- Acute abdomen or unexplained fever could be among the manifestations.
- Initial management requires liberal normal saline intravenous fluid administration to replete the intravascular space.
- Draw blood samples for serum chemistry, cortisol, and ACTH, followed immediately by intravenous hydrocortisone supplementation.
- In critically ill patients, evaluate adrenal function with random serum cortisol; in a nonacute setting use the ACTH stimulation test.
- Chronic management of primary adrenal insufficiency requires corticosteroid and mineralocorticoid therapy.
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84(1):27–34. doi:10.3949/ccjm.84a.16042
- Almiani M, Gorthi J, Subbiah S, Firoz M. Quiz page November 2012: an unusual case of acute hyponatremia and normal anion gap metabolic acidosis. Am J Kidney Dis 2012; 60(5):xxxiii–xxxvi. doi:10.1053/j.ajkd.2012.05.026
- Migeon CJ, Kenny FM, Hung W, Voorhess ML. Study of adrenal function in children with meningitis. Pediatrics 1967; 40(2):163–183.
- Rao RH, Vagnucci AH, Amico JA. Bilateral massive adrenal hemorrhage: early recognition and treatment. Ann Intern Med 1989; 110(3):227–235.
- Shimizu S, Tahara Y, Atsumi T, et al. Waterhouse-Friderichsen syndrome caused by invasive Haemophilus influenzae type B infection in a previously healthy young man. Anaesth Intensive Care 2010; 38(1):214–215.
- Castaldo ET, Guillamondegui OD, Greco JA 3rd, Feurer ID, Miller RS, Morris JA Jr. Are adrenal injuries predictive of adrenal insufficiency in patients sustaining blunt trauma? Am Surg 2008; 74(3):262–266.
- Xarli VP, Steele AA, Davis PJ, Buescher ES, Rios CN, Garcia-Bunuel R. Adrenal hemorrhage in the adult. Medicine (Baltimore) 1978; 57(3):211–221.
- Takeuchi K, Tsuzuki Y, Ando T, et al. Clinical studies of strangulating small bowel obstruction. Am Surg 2004; 70(1):40–44.
- MacKenzie IM. The haemodynamics of human septic shock. Anaesthesia 2001; 56(2):130–144.
- Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2016; 101(2):364–389. doi:10.1210/jc.2015-1710
- Hamrahian AH, Fleseriu M; AACE Adrenal Scientific Committee. Evaluation and management of adrenal insufficiency in critically ill patients: disease state review. Endocr Pract 2017; 23(6):716–725. doi:10.4158/EP161720.RA
- Saenger P, Levine LS, Irvine WJ, et al. Progressive adrenal failure in polyglandular autoimmune disease. J Clin Endocrinol Metab 1982; 54(4):863–867.
- Coco G, Dal Pra C, Presotto F, et al. Estimated risk for developing autoimmune Addison's disease in patients with adrenal cortex autoantibodies. J Clin Endocrinol Metab 2006; 91(5):1637–1645. doi:10.1210/jc.2005-0860
- Ullian ME. The role of corticosteroids in the regulation of vascular tone. Cardiovasc Res 1999; 41(1):55–64.
- Yang S, Zhang L. Glucocorticoids and vascular reactivity. Curr Vasc Pharmacol 2004; 2(1):1–12.
- Yanase T, Tajima T, Katabami T, et al. Diagnosis and treatment of adrenal insufficiency including adrenal crisis: a Japan Endocrine Society clinical practice guideline [Opinion]. Endocr J 2016; 63(9):765–784. doi:10.1507/endocrj.EJ16-0242
- Taylor RL, Grebe SK, Singh RJ. Quantitative, highly sensitive liquid chromatography-tandem mass spectrometry method for detection of synthetic corticosteroids. Clin Chem 2004; 50(10):2345–2352. doi:10.1373/clinchem.2004.033605
- Goldfien A, Laidlaw JC, Haydar NA, Renold AE, Thorn GW. Fluorohydrocortisone and chlorohydrocortisone, highly potent derivatives of compound F. N Engl J Med 1955; 252(11):415–421. doi:10.1056/NEJM195503172521101
- Jenkins D, Forsham PH, Laidlaw JC, Reddy WJ, Thorn GW. Use of ACTH in the diagnosis of adrenal cortical insufficiency. Am J Med 1955; 18(1):3–14.
- Hägg E, Asplund K, Lithner F. Value of basal plasma cortisol assays in the assessment of pituitary-adrenal insufficiency. Clin Endocrinol (Oxf) 1987; 26(2):221–226.
- Deutschbein T, Unger N, Mann K, Petersenn S. Diagnosis of secondary adrenal insufficiency: unstimulated early morning cortisol in saliva and serum in comparison with the insulin tolerance test. Horm Metab Res 2009; 41(4):834–839. doi:10.1055/s-0029-1225630
- Erturk E, Jaffe CA, Barkan AL. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab 1998; 83(7):2350–2354.
- Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med 2003; 348(8):727–734. doi:10.1056/NEJMra020529
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39(2):165–228. doi:10.1007/s00134-012-2769-8
- Ceccato F, Barbot M, Zilio M, et al. Performance of salivary cortisol in the diagnosis of Cushing's syndrome, adrenal incidentaloma, and adrenal insufficiency. Eur J Endocrinol 2013; 169(1):31–36. doi:10.1530/EJE-13-0159
- Dickstein G, Shechner C, Nicholson WE, et al. Adrenocorticotropin stimulation test: effects of basal cortisol level, time of day, and suggested new sensitive low dose test. J Clin Endocrinol Metab 1991; 72(4):773–778. doi:10.1210/jcem-72-4-773
- May ME, Carey RM. Rapid adrenocorticotropic hormone test in practice. Retrospective review. Am J Med 1985; 79(6):679–884.
- Speckart PF, Nicoloff JT, Bethune JE. Screening for adrenocortical insufficiency with cosyntropin (synthetic ACTH). Arch Intern Med 1971; 128(5):761–763.
- Peechakara S, Bena J, Clarke NJ, et al. Total and free cortisol levels during 1 μg, 25 μg, and 250 μg cosyntropin stimulation tests compared to insulin tolerance test: results of a randomized, prospective, pilot study. Endocrine 2017; 57(3):388–393. doi:10.1007/s12020-017-1371-9
- Finucane FM, Liew A, Thornton E, Rogers B, Tormey W, Agha A. Clinical insights into the safety and utility of the insulin tolerance test (ITT) in the assessment of the hypothalamo-pituitary-adrenal axis. Clin Endocrinol (Oxf) 2008; 69(4):603–607. doi:10.1111/j.1365-2265.2008.03240.x
- Lindholm J, Kehlet H. Re-evaluation of the clinical value of the 30 min ACTH test in assessing the hypothalamic-pituitary-adrenocortical function. Clin Endocrinol (Oxf) 1987; 26(1):53–59.
- Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet 2014; 383(9935):2152–2167. doi:10.1016/S0140-6736(13)61684-0
- Burke CW. Adrenocortical insufficiency. Clin Endocrinol Metab 1985; 14(4):947–976.
- Jessani N, Jehangir W, Behman D, Yousif A, Spiler IJ. Secondary adrenal insufficiency: an overlooked cause of hyponatremia. J Clin Med Res 2015; 7(4):286–288. doi:10.14740/jocmr2041w
- Oelkers W. Hyponatremia and inappropriate secretion of vasopressin (antidiuretic hormone) in patients with hypopituitarism. N Engl J Med 1989; 321(8):492–496. doi:10.1056/NEJM198908243210802
- Ishikawa S, Schrier RW. Effect of arginine vasopressin antagonist on renal water excretion in glucocorticoid and mineralocorticoid deficient rats. Kidney Int 1982; 22(6):587–593.
- Wolfson B, Manning RW, Davis LG, Arentzen R, Baldino F Jr. Co-localization of corticotropin releasing factor and vasopressin mRNA in neurones after adrenalectomy. Nature 1985; 315(6014):59–61.
- Kalogeras KT, Nieman LK, Friedman TC, et al. Inferior petrosal sinus sampling in healthy subjects reveals a unilateral corticotropin-releasing hormone-induced arginine vasopressin release associated with ipsilateral adrenocorticotropin secretion. J Clin Invest 1996; 97:2045–2050.
- Kovacs KJ, Foldes A, Sawchenko PE. Glucocorticoid negative feedback selectively targets vasopressin transcription in parvocellular neurosecretory neurons. J Neurosci 2000; 20:3843–3852.
- Sarkar SB, Sarkar S, Ghosh S, Bandyopadhyay S. Addison's disease. Contemp Clin Dent 2012; 3(4):484–486. doi:10.4103/0976-237X.107450
- Szylman P, Better OS, Chaimowitz C, Rosler A. Role of hyperkalemia in the metabolic acidosis of isolated hypoaldosteronism. N Engl J Med 1976; 294(7):361–365. doi:10.1056/NEJM197602122940703
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84(1):27–34. doi:10.3949/ccjm.84a.16042
- Almiani M, Gorthi J, Subbiah S, Firoz M. Quiz page November 2012: an unusual case of acute hyponatremia and normal anion gap metabolic acidosis. Am J Kidney Dis 2012; 60(5):xxxiii–xxxvi. doi:10.1053/j.ajkd.2012.05.026
- Migeon CJ, Kenny FM, Hung W, Voorhess ML. Study of adrenal function in children with meningitis. Pediatrics 1967; 40(2):163–183.
- Rao RH, Vagnucci AH, Amico JA. Bilateral massive adrenal hemorrhage: early recognition and treatment. Ann Intern Med 1989; 110(3):227–235.
- Shimizu S, Tahara Y, Atsumi T, et al. Waterhouse-Friderichsen syndrome caused by invasive Haemophilus influenzae type B infection in a previously healthy young man. Anaesth Intensive Care 2010; 38(1):214–215.
- Castaldo ET, Guillamondegui OD, Greco JA 3rd, Feurer ID, Miller RS, Morris JA Jr. Are adrenal injuries predictive of adrenal insufficiency in patients sustaining blunt trauma? Am Surg 2008; 74(3):262–266.
- Xarli VP, Steele AA, Davis PJ, Buescher ES, Rios CN, Garcia-Bunuel R. Adrenal hemorrhage in the adult. Medicine (Baltimore) 1978; 57(3):211–221.
- Takeuchi K, Tsuzuki Y, Ando T, et al. Clinical studies of strangulating small bowel obstruction. Am Surg 2004; 70(1):40–44.
- MacKenzie IM. The haemodynamics of human septic shock. Anaesthesia 2001; 56(2):130–144.
- Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2016; 101(2):364–389. doi:10.1210/jc.2015-1710
- Hamrahian AH, Fleseriu M; AACE Adrenal Scientific Committee. Evaluation and management of adrenal insufficiency in critically ill patients: disease state review. Endocr Pract 2017; 23(6):716–725. doi:10.4158/EP161720.RA
- Saenger P, Levine LS, Irvine WJ, et al. Progressive adrenal failure in polyglandular autoimmune disease. J Clin Endocrinol Metab 1982; 54(4):863–867.
- Coco G, Dal Pra C, Presotto F, et al. Estimated risk for developing autoimmune Addison's disease in patients with adrenal cortex autoantibodies. J Clin Endocrinol Metab 2006; 91(5):1637–1645. doi:10.1210/jc.2005-0860
- Ullian ME. The role of corticosteroids in the regulation of vascular tone. Cardiovasc Res 1999; 41(1):55–64.
- Yang S, Zhang L. Glucocorticoids and vascular reactivity. Curr Vasc Pharmacol 2004; 2(1):1–12.
- Yanase T, Tajima T, Katabami T, et al. Diagnosis and treatment of adrenal insufficiency including adrenal crisis: a Japan Endocrine Society clinical practice guideline [Opinion]. Endocr J 2016; 63(9):765–784. doi:10.1507/endocrj.EJ16-0242
- Taylor RL, Grebe SK, Singh RJ. Quantitative, highly sensitive liquid chromatography-tandem mass spectrometry method for detection of synthetic corticosteroids. Clin Chem 2004; 50(10):2345–2352. doi:10.1373/clinchem.2004.033605
- Goldfien A, Laidlaw JC, Haydar NA, Renold AE, Thorn GW. Fluorohydrocortisone and chlorohydrocortisone, highly potent derivatives of compound F. N Engl J Med 1955; 252(11):415–421. doi:10.1056/NEJM195503172521101
- Jenkins D, Forsham PH, Laidlaw JC, Reddy WJ, Thorn GW. Use of ACTH in the diagnosis of adrenal cortical insufficiency. Am J Med 1955; 18(1):3–14.
- Hägg E, Asplund K, Lithner F. Value of basal plasma cortisol assays in the assessment of pituitary-adrenal insufficiency. Clin Endocrinol (Oxf) 1987; 26(2):221–226.
- Deutschbein T, Unger N, Mann K, Petersenn S. Diagnosis of secondary adrenal insufficiency: unstimulated early morning cortisol in saliva and serum in comparison with the insulin tolerance test. Horm Metab Res 2009; 41(4):834–839. doi:10.1055/s-0029-1225630
- Erturk E, Jaffe CA, Barkan AL. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab 1998; 83(7):2350–2354.
- Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med 2003; 348(8):727–734. doi:10.1056/NEJMra020529
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39(2):165–228. doi:10.1007/s00134-012-2769-8
- Ceccato F, Barbot M, Zilio M, et al. Performance of salivary cortisol in the diagnosis of Cushing's syndrome, adrenal incidentaloma, and adrenal insufficiency. Eur J Endocrinol 2013; 169(1):31–36. doi:10.1530/EJE-13-0159
- Dickstein G, Shechner C, Nicholson WE, et al. Adrenocorticotropin stimulation test: effects of basal cortisol level, time of day, and suggested new sensitive low dose test. J Clin Endocrinol Metab 1991; 72(4):773–778. doi:10.1210/jcem-72-4-773
- May ME, Carey RM. Rapid adrenocorticotropic hormone test in practice. Retrospective review. Am J Med 1985; 79(6):679–884.
- Speckart PF, Nicoloff JT, Bethune JE. Screening for adrenocortical insufficiency with cosyntropin (synthetic ACTH). Arch Intern Med 1971; 128(5):761–763.
- Peechakara S, Bena J, Clarke NJ, et al. Total and free cortisol levels during 1 μg, 25 μg, and 250 μg cosyntropin stimulation tests compared to insulin tolerance test: results of a randomized, prospective, pilot study. Endocrine 2017; 57(3):388–393. doi:10.1007/s12020-017-1371-9
- Finucane FM, Liew A, Thornton E, Rogers B, Tormey W, Agha A. Clinical insights into the safety and utility of the insulin tolerance test (ITT) in the assessment of the hypothalamo-pituitary-adrenal axis. Clin Endocrinol (Oxf) 2008; 69(4):603–607. doi:10.1111/j.1365-2265.2008.03240.x
- Lindholm J, Kehlet H. Re-evaluation of the clinical value of the 30 min ACTH test in assessing the hypothalamic-pituitary-adrenocortical function. Clin Endocrinol (Oxf) 1987; 26(1):53–59.
- Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet 2014; 383(9935):2152–2167. doi:10.1016/S0140-6736(13)61684-0
- Burke CW. Adrenocortical insufficiency. Clin Endocrinol Metab 1985; 14(4):947–976.
- Jessani N, Jehangir W, Behman D, Yousif A, Spiler IJ. Secondary adrenal insufficiency: an overlooked cause of hyponatremia. J Clin Med Res 2015; 7(4):286–288. doi:10.14740/jocmr2041w
- Oelkers W. Hyponatremia and inappropriate secretion of vasopressin (antidiuretic hormone) in patients with hypopituitarism. N Engl J Med 1989; 321(8):492–496. doi:10.1056/NEJM198908243210802
- Ishikawa S, Schrier RW. Effect of arginine vasopressin antagonist on renal water excretion in glucocorticoid and mineralocorticoid deficient rats. Kidney Int 1982; 22(6):587–593.
- Wolfson B, Manning RW, Davis LG, Arentzen R, Baldino F Jr. Co-localization of corticotropin releasing factor and vasopressin mRNA in neurones after adrenalectomy. Nature 1985; 315(6014):59–61.
- Kalogeras KT, Nieman LK, Friedman TC, et al. Inferior petrosal sinus sampling in healthy subjects reveals a unilateral corticotropin-releasing hormone-induced arginine vasopressin release associated with ipsilateral adrenocorticotropin secretion. J Clin Invest 1996; 97:2045–2050.
- Kovacs KJ, Foldes A, Sawchenko PE. Glucocorticoid negative feedback selectively targets vasopressin transcription in parvocellular neurosecretory neurons. J Neurosci 2000; 20:3843–3852.
- Sarkar SB, Sarkar S, Ghosh S, Bandyopadhyay S. Addison's disease. Contemp Clin Dent 2012; 3(4):484–486. doi:10.4103/0976-237X.107450
- Szylman P, Better OS, Chaimowitz C, Rosler A. Role of hyperkalemia in the metabolic acidosis of isolated hypoaldosteronism. N Engl J Med 1976; 294(7):361–365. doi:10.1056/NEJM197602122940703
Perioperative interruption of dual antiplatelet therapy
To the Editor: We read with great interest the article by Munyon et al1 addressing recent developments in perioperative medicine. We would like to comment on the perioperative interruption of dual antiplatelet therapy, a common clinical problem.
Several registry analyses have shown that, with second-generation drug-eluting stents, interruption of 1 antiplatelet agent after the first month is safe.2,3 These registries included a substantial proportion of patients whose index stenting procedure was performed for acute coronary syndrome (up to 60%).2 On average, antiplatelet therapy interruption was brief (about 6 to 7 days).
Additional registry analyses have shown that surgery may be safely performed beyond the first month after drug-eluting stent placement.4,5 Specifically, a large Danish analysis of patients with a drug-eluting stent who underwent noncardiac surgery, matched to control patients without ischemic heart disease, showed that the risk of perioperative myocardial infarction and death was not increased beyond the first month after drug-eluting stent implantation. Specifically, the risk was not increased at the 1- to 2-month and 2- to 12-month postimplantation intervals. Acute coronary syndrome was the indication for stenting in 56% of the patients.
Therefore, while surgery is preferably delayed 6 months after drug-eluting stent implantation (class I recommendation in the European Society of Cardiology guidelines), surgery may be selectively performed 1 to 6 months after drug-eluting stent implantation with an acceptable risk. This is particularly so if the index stenting was performed in the setting of stable coronary arterial disease (class IIa recommendation if stenting was performed in the setting of stable coronary arterial disease without complex procedural features; class IIb recommendation if stenting was performed in the setting of acute coronary syndrome or complex procedural features).6 After drug-eluting stent implantation, the earliest cutpoint for considering surgery is 1 month rather than 3 months.
When surgery is performed within this 1- to 6-month interval, thienopyridine interruption should be kept brief and dual antiplatelet therapy reinitiated as soon as possible postoperatively. In fact, when thienopyridine therapy is interrupted 1 to 6 months after drug-eluting stent implantation, stent thrombosis typically occurs more than 6 or 7 days after interruption.7
- Munyon R, Cohn SL, Slawski B, Smetana GW, Pfeifer K. 2017 update in perioperative medicine: 6 questions answered. Cleve Clin J Med 2017; 84(11):863–872. doi:10.3949/ccjm.84a.17068
- Ferreira-Gonzáles, Marsal JR, Ribera A, et al. Double antiplatelet therapy after drug-eluting stent implantation: risk associated with discontinuation within the first year. J Am Coll Cardiol 2012; 60(15):1333–1339. doi:10.1016/j.jacc.2012.04.057
- Naidu SS, Krucoff MW, Rutledge DR, et al. Contemporary incidence and predictors of stent thrombosis and other major adverse cardiac events in the year after XIENCE V implantation: results from the 8,061-patient XIENCE V United States study. JACC Cardiovasc Interv 2012; 5(5):626–635. doi:10.1016/j.jcin.2012.02.014
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
- Singla S, Sachdeva R, Uretsky BF. The risk of adverse cardiac and bleeding events following noncardiac surgery relative to antiplatelet therapy in patients with prior percutaneous coronary intervention. J Am Coll Cardiol 2012; 60(20):2005–2016. doi:10.1016/j.jacc.2012.04.062
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Airoldi F, Colombo A, Morici N, et al. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation 2007; 116(7):745–754. doi:10.1161/CIRCULATIONAHA.106.686048
To the Editor: We read with great interest the article by Munyon et al1 addressing recent developments in perioperative medicine. We would like to comment on the perioperative interruption of dual antiplatelet therapy, a common clinical problem.
Several registry analyses have shown that, with second-generation drug-eluting stents, interruption of 1 antiplatelet agent after the first month is safe.2,3 These registries included a substantial proportion of patients whose index stenting procedure was performed for acute coronary syndrome (up to 60%).2 On average, antiplatelet therapy interruption was brief (about 6 to 7 days).
Additional registry analyses have shown that surgery may be safely performed beyond the first month after drug-eluting stent placement.4,5 Specifically, a large Danish analysis of patients with a drug-eluting stent who underwent noncardiac surgery, matched to control patients without ischemic heart disease, showed that the risk of perioperative myocardial infarction and death was not increased beyond the first month after drug-eluting stent implantation. Specifically, the risk was not increased at the 1- to 2-month and 2- to 12-month postimplantation intervals. Acute coronary syndrome was the indication for stenting in 56% of the patients.
Therefore, while surgery is preferably delayed 6 months after drug-eluting stent implantation (class I recommendation in the European Society of Cardiology guidelines), surgery may be selectively performed 1 to 6 months after drug-eluting stent implantation with an acceptable risk. This is particularly so if the index stenting was performed in the setting of stable coronary arterial disease (class IIa recommendation if stenting was performed in the setting of stable coronary arterial disease without complex procedural features; class IIb recommendation if stenting was performed in the setting of acute coronary syndrome or complex procedural features).6 After drug-eluting stent implantation, the earliest cutpoint for considering surgery is 1 month rather than 3 months.
When surgery is performed within this 1- to 6-month interval, thienopyridine interruption should be kept brief and dual antiplatelet therapy reinitiated as soon as possible postoperatively. In fact, when thienopyridine therapy is interrupted 1 to 6 months after drug-eluting stent implantation, stent thrombosis typically occurs more than 6 or 7 days after interruption.7
To the Editor: We read with great interest the article by Munyon et al1 addressing recent developments in perioperative medicine. We would like to comment on the perioperative interruption of dual antiplatelet therapy, a common clinical problem.
Several registry analyses have shown that, with second-generation drug-eluting stents, interruption of 1 antiplatelet agent after the first month is safe.2,3 These registries included a substantial proportion of patients whose index stenting procedure was performed for acute coronary syndrome (up to 60%).2 On average, antiplatelet therapy interruption was brief (about 6 to 7 days).
Additional registry analyses have shown that surgery may be safely performed beyond the first month after drug-eluting stent placement.4,5 Specifically, a large Danish analysis of patients with a drug-eluting stent who underwent noncardiac surgery, matched to control patients without ischemic heart disease, showed that the risk of perioperative myocardial infarction and death was not increased beyond the first month after drug-eluting stent implantation. Specifically, the risk was not increased at the 1- to 2-month and 2- to 12-month postimplantation intervals. Acute coronary syndrome was the indication for stenting in 56% of the patients.
Therefore, while surgery is preferably delayed 6 months after drug-eluting stent implantation (class I recommendation in the European Society of Cardiology guidelines), surgery may be selectively performed 1 to 6 months after drug-eluting stent implantation with an acceptable risk. This is particularly so if the index stenting was performed in the setting of stable coronary arterial disease (class IIa recommendation if stenting was performed in the setting of stable coronary arterial disease without complex procedural features; class IIb recommendation if stenting was performed in the setting of acute coronary syndrome or complex procedural features).6 After drug-eluting stent implantation, the earliest cutpoint for considering surgery is 1 month rather than 3 months.
When surgery is performed within this 1- to 6-month interval, thienopyridine interruption should be kept brief and dual antiplatelet therapy reinitiated as soon as possible postoperatively. In fact, when thienopyridine therapy is interrupted 1 to 6 months after drug-eluting stent implantation, stent thrombosis typically occurs more than 6 or 7 days after interruption.7
- Munyon R, Cohn SL, Slawski B, Smetana GW, Pfeifer K. 2017 update in perioperative medicine: 6 questions answered. Cleve Clin J Med 2017; 84(11):863–872. doi:10.3949/ccjm.84a.17068
- Ferreira-Gonzáles, Marsal JR, Ribera A, et al. Double antiplatelet therapy after drug-eluting stent implantation: risk associated with discontinuation within the first year. J Am Coll Cardiol 2012; 60(15):1333–1339. doi:10.1016/j.jacc.2012.04.057
- Naidu SS, Krucoff MW, Rutledge DR, et al. Contemporary incidence and predictors of stent thrombosis and other major adverse cardiac events in the year after XIENCE V implantation: results from the 8,061-patient XIENCE V United States study. JACC Cardiovasc Interv 2012; 5(5):626–635. doi:10.1016/j.jcin.2012.02.014
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
- Singla S, Sachdeva R, Uretsky BF. The risk of adverse cardiac and bleeding events following noncardiac surgery relative to antiplatelet therapy in patients with prior percutaneous coronary intervention. J Am Coll Cardiol 2012; 60(20):2005–2016. doi:10.1016/j.jacc.2012.04.062
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Airoldi F, Colombo A, Morici N, et al. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation 2007; 116(7):745–754. doi:10.1161/CIRCULATIONAHA.106.686048
- Munyon R, Cohn SL, Slawski B, Smetana GW, Pfeifer K. 2017 update in perioperative medicine: 6 questions answered. Cleve Clin J Med 2017; 84(11):863–872. doi:10.3949/ccjm.84a.17068
- Ferreira-Gonzáles, Marsal JR, Ribera A, et al. Double antiplatelet therapy after drug-eluting stent implantation: risk associated with discontinuation within the first year. J Am Coll Cardiol 2012; 60(15):1333–1339. doi:10.1016/j.jacc.2012.04.057
- Naidu SS, Krucoff MW, Rutledge DR, et al. Contemporary incidence and predictors of stent thrombosis and other major adverse cardiac events in the year after XIENCE V implantation: results from the 8,061-patient XIENCE V United States study. JACC Cardiovasc Interv 2012; 5(5):626–635. doi:10.1016/j.jcin.2012.02.014
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
- Singla S, Sachdeva R, Uretsky BF. The risk of adverse cardiac and bleeding events following noncardiac surgery relative to antiplatelet therapy in patients with prior percutaneous coronary intervention. J Am Coll Cardiol 2012; 60(20):2005–2016. doi:10.1016/j.jacc.2012.04.062
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Airoldi F, Colombo A, Morici N, et al. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation 2007; 116(7):745–754. doi:10.1161/CIRCULATIONAHA.106.686048
In reply: Perioperative interruption of dual antiplatelet therapy
In Reply: We reported on publications from 2016–2017 and, unfortunately, at the time we were writing our paper, the European Society of Cardiology (ESC) update on dual antiplatelet therapy1 had not yet been published. We presented the recommendations from the American College of Cardiology (ACC) and American Heart Association (AHA),2 which differ from the recently published ESC guidelines. The ESC suggests that the minimum waiting period after drug-eluting stent placement before noncardiac surgery should be 1 month rather than 3 months but acknowledges that in the setting of complex stenting or recent acute coronary syndrome, 6 months is preferred. The recommendation in this latter scenario is a class IIb C recommendation—essentially expert consensus opinion.
Further, in the study by Egholm et al,3 the event rates in patients undergoing noncardiac surgery in the 1- to 2-month period were numerically higher than in the control group, and no adjusted odds ratios were given. The numbers of events were very low, and a change of only 1 or 2 events in the other direction in the groups would likely make it statistically significant.
All of these recommendations are based on observational studies and registry data, as there are no randomized controlled trials to address this issue. There are many complexities to be accounted for including the type of stent, timing, circumstances surrounding stenting, anatomy, number of stents, patient comorbidities (particularly age, diabetes mellitus, cardiac disease), type of surgery and anesthesia, and perioperative management of antiplatelet therapy. While we acknowledge the ESC recommendation, we would urge caution in the recommendation to wait only 1 month, and in the United States most would prefer to wait 3 months if possible.
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
In Reply: We reported on publications from 2016–2017 and, unfortunately, at the time we were writing our paper, the European Society of Cardiology (ESC) update on dual antiplatelet therapy1 had not yet been published. We presented the recommendations from the American College of Cardiology (ACC) and American Heart Association (AHA),2 which differ from the recently published ESC guidelines. The ESC suggests that the minimum waiting period after drug-eluting stent placement before noncardiac surgery should be 1 month rather than 3 months but acknowledges that in the setting of complex stenting or recent acute coronary syndrome, 6 months is preferred. The recommendation in this latter scenario is a class IIb C recommendation—essentially expert consensus opinion.
Further, in the study by Egholm et al,3 the event rates in patients undergoing noncardiac surgery in the 1- to 2-month period were numerically higher than in the control group, and no adjusted odds ratios were given. The numbers of events were very low, and a change of only 1 or 2 events in the other direction in the groups would likely make it statistically significant.
All of these recommendations are based on observational studies and registry data, as there are no randomized controlled trials to address this issue. There are many complexities to be accounted for including the type of stent, timing, circumstances surrounding stenting, anatomy, number of stents, patient comorbidities (particularly age, diabetes mellitus, cardiac disease), type of surgery and anesthesia, and perioperative management of antiplatelet therapy. While we acknowledge the ESC recommendation, we would urge caution in the recommendation to wait only 1 month, and in the United States most would prefer to wait 3 months if possible.
In Reply: We reported on publications from 2016–2017 and, unfortunately, at the time we were writing our paper, the European Society of Cardiology (ESC) update on dual antiplatelet therapy1 had not yet been published. We presented the recommendations from the American College of Cardiology (ACC) and American Heart Association (AHA),2 which differ from the recently published ESC guidelines. The ESC suggests that the minimum waiting period after drug-eluting stent placement before noncardiac surgery should be 1 month rather than 3 months but acknowledges that in the setting of complex stenting or recent acute coronary syndrome, 6 months is preferred. The recommendation in this latter scenario is a class IIb C recommendation—essentially expert consensus opinion.
Further, in the study by Egholm et al,3 the event rates in patients undergoing noncardiac surgery in the 1- to 2-month period were numerically higher than in the control group, and no adjusted odds ratios were given. The numbers of events were very low, and a change of only 1 or 2 events in the other direction in the groups would likely make it statistically significant.
All of these recommendations are based on observational studies and registry data, as there are no randomized controlled trials to address this issue. There are many complexities to be accounted for including the type of stent, timing, circumstances surrounding stenting, anatomy, number of stents, patient comorbidities (particularly age, diabetes mellitus, cardiac disease), type of surgery and anesthesia, and perioperative management of antiplatelet therapy. While we acknowledge the ESC recommendation, we would urge caution in the recommendation to wait only 1 month, and in the United States most would prefer to wait 3 months if possible.
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39(3):213–260. doi:10.1093/eurheartj/ehx419
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404
- Egholm G, Kristensen SD, Thim T, et al. Risk associated with surgery within 12 months after coronary drug-eluting stent implantation. J Am Coll Cardiol 2016; 68(24):2622–2632. doi:10.1016/j.jacc.2016.09.967
Correction: Gas under the right diagphragm
In the article “Gas under the right diaphragm” (Matsuura H, Hata H. Cleve Clin J Med 2018; 85[2]:98–100), Figure 2 appeared upside down. It should have appeared as follows:
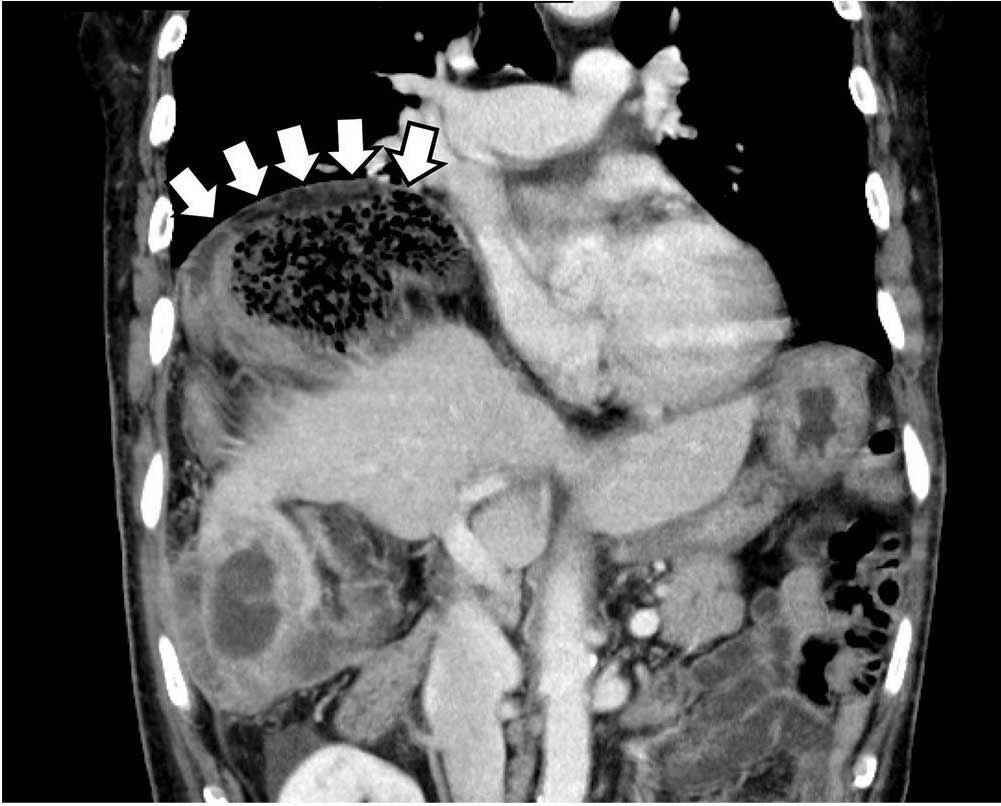
This correction has been made to the online version.
In the article “Gas under the right diaphragm” (Matsuura H, Hata H. Cleve Clin J Med 2018; 85[2]:98–100), Figure 2 appeared upside down. It should have appeared as follows:

This correction has been made to the online version.
In the article “Gas under the right diaphragm” (Matsuura H, Hata H. Cleve Clin J Med 2018; 85[2]:98–100), Figure 2 appeared upside down. It should have appeared as follows:

This correction has been made to the online version.
Correction: Physical examination in dyspnea
On page 949 of the article “Diagnostic value of the physical examination in patients with dyspnea” (Shellenberger RA, Balakrishnan B, Avula S, Ebel A, Shaik S. Cleve Clin J Med 2017; 84[12]:943–950), the terms “abdominojugular reflex” and “hepatojugular reflex” should have been “abdominojugular reflux” and “hepatojugular reflux.” This error also occurred in Table 5 on that page.
On page 949 of the article “Diagnostic value of the physical examination in patients with dyspnea” (Shellenberger RA, Balakrishnan B, Avula S, Ebel A, Shaik S. Cleve Clin J Med 2017; 84[12]:943–950), the terms “abdominojugular reflex” and “hepatojugular reflex” should have been “abdominojugular reflux” and “hepatojugular reflux.” This error also occurred in Table 5 on that page.
On page 949 of the article “Diagnostic value of the physical examination in patients with dyspnea” (Shellenberger RA, Balakrishnan B, Avula S, Ebel A, Shaik S. Cleve Clin J Med 2017; 84[12]:943–950), the terms “abdominojugular reflex” and “hepatojugular reflex” should have been “abdominojugular reflux” and “hepatojugular reflux.” This error also occurred in Table 5 on that page.