User login
Ibrutinib preserves immune memory while fighting cGVHD
SALT LAKE CITY – Ibrutinib’s effectiveness in inhibiting chronic graft versus host disease (cGVHD) may hinge in part on inhibition of pre–germinal center B cells and follicular helper cells, according to a new analysis of clinical trial data.
The analysis also showed that ibrutinib preserved immune memory and type 1 T-helper cells.
Bita Sahaf, PhD, presented results of a “comprehensive and high dimensional proteomic approach” to data from 42 patients who were enrolled in a phase 1/2 clinical trial of ibrutinib for cGVHD (NCT02195869).
In that study, 80% of patients who had two or more organs affected by cGVHD responded in at least two organs; overall, two-thirds of patients had a complete or partial response with ibrutinib. The highest response rates were seen in disease affecting the skin, mouth, and gastrointestinal tract.
The new analysis used blood samples from trial participants collected before and during ibrutinib therapy to look for soluble plasma factors known to be related to inflammation, fibrosis, and cGVHD.
“A heat map of cytokines, chemokines, and factors associated with fibrosis shows a significant decrease following ibrutinib treatment,” Dr. Sahaf said during a top abstracts session at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
In addition, inflammatory gene expression was reduced with ibrutinib use, with reductions in the chemokines nuclear factor kappa-B-1, CXCL10, CCL7, and CCL3 dropping by 2.6-fold, 2.3-fold, 25-fold, and 1.8-fold, respectively, after 3 months of ibrutinib therapy, Dr. Sahaf and her colleagues reported.
The investigators used several different techniques to tease apart the mechanisms behind ibrutinib’s effectiveness. Immunophenotyping was accomplished with cytometry by time of flight (CyTOF), a technique that uses transition element isotopes to tag antibodies, which are then analyzed on a cell-by-cell basis by a time-of-flight mass spectrometer.
Ibrutinib inhibits CD19+CD38+CD27+IgD+ pre–germinal center B cells as well as pathogenic CD4+ T follicular helper cells, both implicated in cGVHD, the investigators found. However, Th1 T cells were preserved in a patient-by-patient analysis.
The CyTOF technique also allowed a phosphorylation analysis showing ibrutinib’s blocking effect on Bruton’s tyrosine kinase (BTK) as well as IL-2 inducible T-cell kinase (ITK), with subsequent effects on the signaling molecule PLCgamma2. In individual patients, this inhibition was confirmed when BTK-activated B-cell populations were eliminated after ibrutinib therapy, Dr. Sahaf said.
Ibrutinib also decreased phosphorylation of ITK, with subsequent depletion of CD4+, CD185+, and BCL6+ follicular helper T cells, and of other T cell populations still to be characterized. However, neither CD4+Tbet+Th1 cells nor CD4+CD25+CD127dim Treg cells saw depletion.
Importantly, “CD8+ cytotoxic T cells persist,” said Dr. Sahaf. Phosphorylation of ITK, she said, “appears heterogeneous across most T-cell populations.
“These data support the clinical efficacy of ibrutinib in cGVHD and highlight ibrutinib’s multifactorial mechanism of action in this disease,” Dr. Sahaf, of Stanford (Calif.) University, and her collaborators wrote in the abstract accompanying the presentation.
In August 2017, ibrutinib became the first treatment approved by the Food and Drug Administration for cGVHD. It is indicated for adults who have failed at least one other therapy.
“These correlative studies suggest that ibrutinib impacts a number of the immunologic mechanisms underlying the development of chronic graft versus host disease,” Dr. Sahaf said. Taken together, her team’s work has shown a reduction in expression of inflammatory genes and cytokines, and a decrease in plasma levels of chemotactic, inflammatory, and fibrotic cytokines that all have been implicated in cGVHD pathogenesis. The selective inhibition of pre–germinal center B cells and the trend toward reduced follicular helper T cells also plays a role in ibrutinib’s effectiveness, she said.
Ibrutinib’s efficacy in damping down inflammatory pathways that lead to cGVHD does not come at the expense of other immune function, however. Immune memory and Th 1 cells were not affected by ibrutinib administration in the study population, Dr. Sahaf said. Comparing 33 ibrutinib-receiving patients who received intravenous immune globulin with three patients who did not, the investigators saw no differences in relative antibody concentrations for tetanus or Epstein-Barr virus between the two groups.
“Protective antibodies against tetanus and Epstein-Barr virus persist following ibrutinib therapy,” Dr. Sahaf said.
Next up is the iNTEGRATE trial (NCT02959944), a phase 3 study that will test ibrutinib plus prednisone as first-line therapy for cGVHD, Dr. Sahaf said. The research team will continue its extensive proteomics work in this study as well, she said.
Dr. Sahaf reported research funding from Pharmacyclics LLC, an AbbVie company, which markets ibrutinib. She also reported having patent, royalty, or intellectual property arrangements with Stanford University.
SOURCE: Sahaf, B et al. BMT Tandem Meetings, Abstract 2.
SALT LAKE CITY – Ibrutinib’s effectiveness in inhibiting chronic graft versus host disease (cGVHD) may hinge in part on inhibition of pre–germinal center B cells and follicular helper cells, according to a new analysis of clinical trial data.
The analysis also showed that ibrutinib preserved immune memory and type 1 T-helper cells.
Bita Sahaf, PhD, presented results of a “comprehensive and high dimensional proteomic approach” to data from 42 patients who were enrolled in a phase 1/2 clinical trial of ibrutinib for cGVHD (NCT02195869).
In that study, 80% of patients who had two or more organs affected by cGVHD responded in at least two organs; overall, two-thirds of patients had a complete or partial response with ibrutinib. The highest response rates were seen in disease affecting the skin, mouth, and gastrointestinal tract.
The new analysis used blood samples from trial participants collected before and during ibrutinib therapy to look for soluble plasma factors known to be related to inflammation, fibrosis, and cGVHD.
“A heat map of cytokines, chemokines, and factors associated with fibrosis shows a significant decrease following ibrutinib treatment,” Dr. Sahaf said during a top abstracts session at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
In addition, inflammatory gene expression was reduced with ibrutinib use, with reductions in the chemokines nuclear factor kappa-B-1, CXCL10, CCL7, and CCL3 dropping by 2.6-fold, 2.3-fold, 25-fold, and 1.8-fold, respectively, after 3 months of ibrutinib therapy, Dr. Sahaf and her colleagues reported.
The investigators used several different techniques to tease apart the mechanisms behind ibrutinib’s effectiveness. Immunophenotyping was accomplished with cytometry by time of flight (CyTOF), a technique that uses transition element isotopes to tag antibodies, which are then analyzed on a cell-by-cell basis by a time-of-flight mass spectrometer.
Ibrutinib inhibits CD19+CD38+CD27+IgD+ pre–germinal center B cells as well as pathogenic CD4+ T follicular helper cells, both implicated in cGVHD, the investigators found. However, Th1 T cells were preserved in a patient-by-patient analysis.
The CyTOF technique also allowed a phosphorylation analysis showing ibrutinib’s blocking effect on Bruton’s tyrosine kinase (BTK) as well as IL-2 inducible T-cell kinase (ITK), with subsequent effects on the signaling molecule PLCgamma2. In individual patients, this inhibition was confirmed when BTK-activated B-cell populations were eliminated after ibrutinib therapy, Dr. Sahaf said.
Ibrutinib also decreased phosphorylation of ITK, with subsequent depletion of CD4+, CD185+, and BCL6+ follicular helper T cells, and of other T cell populations still to be characterized. However, neither CD4+Tbet+Th1 cells nor CD4+CD25+CD127dim Treg cells saw depletion.
Importantly, “CD8+ cytotoxic T cells persist,” said Dr. Sahaf. Phosphorylation of ITK, she said, “appears heterogeneous across most T-cell populations.
“These data support the clinical efficacy of ibrutinib in cGVHD and highlight ibrutinib’s multifactorial mechanism of action in this disease,” Dr. Sahaf, of Stanford (Calif.) University, and her collaborators wrote in the abstract accompanying the presentation.
In August 2017, ibrutinib became the first treatment approved by the Food and Drug Administration for cGVHD. It is indicated for adults who have failed at least one other therapy.
“These correlative studies suggest that ibrutinib impacts a number of the immunologic mechanisms underlying the development of chronic graft versus host disease,” Dr. Sahaf said. Taken together, her team’s work has shown a reduction in expression of inflammatory genes and cytokines, and a decrease in plasma levels of chemotactic, inflammatory, and fibrotic cytokines that all have been implicated in cGVHD pathogenesis. The selective inhibition of pre–germinal center B cells and the trend toward reduced follicular helper T cells also plays a role in ibrutinib’s effectiveness, she said.
Ibrutinib’s efficacy in damping down inflammatory pathways that lead to cGVHD does not come at the expense of other immune function, however. Immune memory and Th 1 cells were not affected by ibrutinib administration in the study population, Dr. Sahaf said. Comparing 33 ibrutinib-receiving patients who received intravenous immune globulin with three patients who did not, the investigators saw no differences in relative antibody concentrations for tetanus or Epstein-Barr virus between the two groups.
“Protective antibodies against tetanus and Epstein-Barr virus persist following ibrutinib therapy,” Dr. Sahaf said.
Next up is the iNTEGRATE trial (NCT02959944), a phase 3 study that will test ibrutinib plus prednisone as first-line therapy for cGVHD, Dr. Sahaf said. The research team will continue its extensive proteomics work in this study as well, she said.
Dr. Sahaf reported research funding from Pharmacyclics LLC, an AbbVie company, which markets ibrutinib. She also reported having patent, royalty, or intellectual property arrangements with Stanford University.
SOURCE: Sahaf, B et al. BMT Tandem Meetings, Abstract 2.
SALT LAKE CITY – Ibrutinib’s effectiveness in inhibiting chronic graft versus host disease (cGVHD) may hinge in part on inhibition of pre–germinal center B cells and follicular helper cells, according to a new analysis of clinical trial data.
The analysis also showed that ibrutinib preserved immune memory and type 1 T-helper cells.
Bita Sahaf, PhD, presented results of a “comprehensive and high dimensional proteomic approach” to data from 42 patients who were enrolled in a phase 1/2 clinical trial of ibrutinib for cGVHD (NCT02195869).
In that study, 80% of patients who had two or more organs affected by cGVHD responded in at least two organs; overall, two-thirds of patients had a complete or partial response with ibrutinib. The highest response rates were seen in disease affecting the skin, mouth, and gastrointestinal tract.
The new analysis used blood samples from trial participants collected before and during ibrutinib therapy to look for soluble plasma factors known to be related to inflammation, fibrosis, and cGVHD.
“A heat map of cytokines, chemokines, and factors associated with fibrosis shows a significant decrease following ibrutinib treatment,” Dr. Sahaf said during a top abstracts session at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
In addition, inflammatory gene expression was reduced with ibrutinib use, with reductions in the chemokines nuclear factor kappa-B-1, CXCL10, CCL7, and CCL3 dropping by 2.6-fold, 2.3-fold, 25-fold, and 1.8-fold, respectively, after 3 months of ibrutinib therapy, Dr. Sahaf and her colleagues reported.
The investigators used several different techniques to tease apart the mechanisms behind ibrutinib’s effectiveness. Immunophenotyping was accomplished with cytometry by time of flight (CyTOF), a technique that uses transition element isotopes to tag antibodies, which are then analyzed on a cell-by-cell basis by a time-of-flight mass spectrometer.
Ibrutinib inhibits CD19+CD38+CD27+IgD+ pre–germinal center B cells as well as pathogenic CD4+ T follicular helper cells, both implicated in cGVHD, the investigators found. However, Th1 T cells were preserved in a patient-by-patient analysis.
The CyTOF technique also allowed a phosphorylation analysis showing ibrutinib’s blocking effect on Bruton’s tyrosine kinase (BTK) as well as IL-2 inducible T-cell kinase (ITK), with subsequent effects on the signaling molecule PLCgamma2. In individual patients, this inhibition was confirmed when BTK-activated B-cell populations were eliminated after ibrutinib therapy, Dr. Sahaf said.
Ibrutinib also decreased phosphorylation of ITK, with subsequent depletion of CD4+, CD185+, and BCL6+ follicular helper T cells, and of other T cell populations still to be characterized. However, neither CD4+Tbet+Th1 cells nor CD4+CD25+CD127dim Treg cells saw depletion.
Importantly, “CD8+ cytotoxic T cells persist,” said Dr. Sahaf. Phosphorylation of ITK, she said, “appears heterogeneous across most T-cell populations.
“These data support the clinical efficacy of ibrutinib in cGVHD and highlight ibrutinib’s multifactorial mechanism of action in this disease,” Dr. Sahaf, of Stanford (Calif.) University, and her collaborators wrote in the abstract accompanying the presentation.
In August 2017, ibrutinib became the first treatment approved by the Food and Drug Administration for cGVHD. It is indicated for adults who have failed at least one other therapy.
“These correlative studies suggest that ibrutinib impacts a number of the immunologic mechanisms underlying the development of chronic graft versus host disease,” Dr. Sahaf said. Taken together, her team’s work has shown a reduction in expression of inflammatory genes and cytokines, and a decrease in plasma levels of chemotactic, inflammatory, and fibrotic cytokines that all have been implicated in cGVHD pathogenesis. The selective inhibition of pre–germinal center B cells and the trend toward reduced follicular helper T cells also plays a role in ibrutinib’s effectiveness, she said.
Ibrutinib’s efficacy in damping down inflammatory pathways that lead to cGVHD does not come at the expense of other immune function, however. Immune memory and Th 1 cells were not affected by ibrutinib administration in the study population, Dr. Sahaf said. Comparing 33 ibrutinib-receiving patients who received intravenous immune globulin with three patients who did not, the investigators saw no differences in relative antibody concentrations for tetanus or Epstein-Barr virus between the two groups.
“Protective antibodies against tetanus and Epstein-Barr virus persist following ibrutinib therapy,” Dr. Sahaf said.
Next up is the iNTEGRATE trial (NCT02959944), a phase 3 study that will test ibrutinib plus prednisone as first-line therapy for cGVHD, Dr. Sahaf said. The research team will continue its extensive proteomics work in this study as well, she said.
Dr. Sahaf reported research funding from Pharmacyclics LLC, an AbbVie company, which markets ibrutinib. She also reported having patent, royalty, or intellectual property arrangements with Stanford University.
SOURCE: Sahaf, B et al. BMT Tandem Meetings, Abstract 2.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Inflammatory gene expression dropped between 1.8-fold and 25-fold for individual chemokines after ibrutinib treatment.
Study details: Comprehensive proteomics analysis of data from a phase 1/2 clinical trial of ibrutinib as second-line therapy for cGVHD.
Disclosures: The clinical trial was sponsored by Pharmacyclics LLC, an Abbvie company. Dr. Sahaf reported having patent, royalty, or intellectual property arrangements with Stanford University.
Source: Sahaf B et al. 2018 BMT Tandem Meetings. Abstract 2.
21-gene assay predicts survival in male and female breast cancer
A study of the molecular and genomic features of breast cancer in men, compared with those in women, highlights the prognostic value of a 21-gene breast recurrence score in both sexes, investigators say.
Men and women with estrogen receptor (ER)–positive breast cancer who had recurrence scores (RS) of 0 to 30 on the 21-gene assay (Oncotype DX) had excellent breast cancer–specific survival rates, which suggests that such patients could be spared from more aggressive treatments, such as chemotherapy, according to Suleiman Alfred Massarweh, MD, of Stanford (Calif.) University and his colleagues.
“Future adjuvant trials in ER-positive breast cancer may need to focus on targeting endocrine resistance in those patients with RS greater than 31 and may need to consider the weight of competing mortality risk when investigating the value of any additional treatment beyond endocrine therapy,” they wrote in the Journal of Clinical Oncology.
In 2016, an estimated 2,600 men were diagnosed with breast cancer in the United States.
“Approximately 95% of breast cancers diagnosed in men express the estrogen receptor and progesterone receptor (PR), which is a higher percentage than in women and suggests a key role for ER in the biology of breast cancer in men,” the investigators noted.
Although treatment of men with breast cancer has traditionally been extrapolated from treatment of women with breast cancer, genomic studies have suggested some key differences, the investigators noted, citing a study of the genomic landscape of male breast cancers presented at the 2014 San Antonio Breast Cancer Symposium.
In that study, investigators from the Memorial Sloan Kettering Cancer Center in New York and other institutions found that all male breast cancers in their sample of 64 patients were ER+ and human epidermal growth factor receptor 2 (HER2)–negative, predominantly of the luminal B subtype, and that the genetic alterations seen in male breast cancers frequently target DNA-repair fibroblast growth factor pathways. However, the pathways that are known to drive luminal cancers when mutated in women are seen less often among men, said Salvatore Piscuoglio, PhD, then a research fellow at MSKCC.
The current study helps to confirm and expand on the findings from that study, commented Steven J. Isakoff, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, who was not involved in either study.
“I think it’s helpful to see in a larger dataset what the spectrum of oncotypes [Oncotype DX] looks like in men. In general, as the study described, we have a real lack of large-scale data in men and certainly no prospective data with oncotypes,” he said in an interview.
To get a better idea of the molecular characteristics of breast cancer in men and how they relate to breast cancer–specific mortality, Dr. Massarweh and his associates looked at deidentified 21-gene assay data from the Genomic Health Laboratory database on 3,806 men and 571,115 women with breast cancer with either no nodal involvement, micrometastases only, or one to three involved lymph nodes.
They also looked at survival data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) population of patients with breast cancer diagnosed during 2004-2012, which included data on 332 men and 55,842 women with ER-positive and/or PR-positive invasive breast cancer.
Among the entire 21-gene assay sample, they found that men were significantly older than women at the time of diagnosis, at a mean age of 64.2 vs. 59.1 years (P less than .001).
Both men and women had infiltrating ductal carcinoma as the most common histology; the prevalence was slightly higher among men at 87.6% versus 81.3% for women.
The average recurrence score in men was 16.8 versus 17.0 in women, a difference that was not statistically significant. A majority of both men and women had RS scores below 18 (65.8% and 58.2%, respectively), although significantly more men than women had RS scores of 31 or higher (12.4% vs. 7.4%; P less than .001).
“This relative predominance of high RS results in men was encountered across age groups but was most prominent in men younger than 40 years of age,” the investigators wrote.
At the other end of the scale, RS lower than 11, especially RS 0, were seen more frequently in men than in women, except among those younger than 40 years.
Looking at individual gene expression profiles, the authors found that mean gene expression was higher in men for genes associated with ER, proliferation, and invasion. ER expression was lowest and PR expression was highest in women younger than 50 years, but ER expression increased progressively with age.
Among men, those younger than 50 years had slightly lower ER and PR expression than did older men.
In the analysis of SEER survival data, they found that 5-year breast cancer severity score (BCSS) was 99% for men with RS below 18, 95.7% for those with RS between18 and 30, and 81% for those with RS of 31 or higher. Among women, 5-year BCSS was 99.5% for those with RS under 18, 98.6% for those with RS between 18 and 30, and 94.9% for those with RS of 31 or higher.
Five-year overall survival estimates were 92.6% for men with RS below 18, 86% for those with RS between 18 and 30, and 69% for those with RS of 31 or higher. Respective 5-year OS rates for women were 95%, 94.2%, and 89.9%.
“The 21-gene RS provided clear prognostic information in our cohort, with a significantly different 5-year BCSS determined by RS in both men and women,” the investigators wrote.
They noted that patients with low and intermediate RS have excellent prognoses regardless of nodal status, which suggests that these patients have more indolent disease and better outcomes than do patients with higher RS.
The more frequent use of adjuvant chemotherapy in the RS 31 and higher group indicates that “the prognostic utility of RS results is evident despite adjuvant chemotherapy use,” they wrote.
Dr. Isakoff pointed out, however, that the population in the study is from a registry of patients eligible for the 21-gene assay, which can only be used for patients with ER-positive and HER2-negative tumors.
“In other words, this is not a random sample. This is a sample of patients for whom the treating physician was on the fence about chemotherapy and in some way thought that getting an oncotype might be helpful,” he said.
He added that although the study findings “don’t change anything we have been doing, they provide reassurance that oncotype is a reasonable test to consider in patients with male breast cancer for whom we’re considering including or avoiding chemotherapy,” he said.
A funding source for the study was not reported. Dr. Massarweh disclosed stock or ownership in Radius Health, consulting for Novartis, and institutional research funding from multiple companies. Three coauthors are employees and stockholders of Genomic Health, maker of the Oncotype DX assay used in the study. Dr. Isakoff reported no conflicts of interest related to the study
SOURCE: Massarweh SA et al. 2018 Mar 27. doi: 10.1200/JCO.2017.76.8861.
A study of the molecular and genomic features of breast cancer in men, compared with those in women, highlights the prognostic value of a 21-gene breast recurrence score in both sexes, investigators say.
Men and women with estrogen receptor (ER)–positive breast cancer who had recurrence scores (RS) of 0 to 30 on the 21-gene assay (Oncotype DX) had excellent breast cancer–specific survival rates, which suggests that such patients could be spared from more aggressive treatments, such as chemotherapy, according to Suleiman Alfred Massarweh, MD, of Stanford (Calif.) University and his colleagues.
“Future adjuvant trials in ER-positive breast cancer may need to focus on targeting endocrine resistance in those patients with RS greater than 31 and may need to consider the weight of competing mortality risk when investigating the value of any additional treatment beyond endocrine therapy,” they wrote in the Journal of Clinical Oncology.
In 2016, an estimated 2,600 men were diagnosed with breast cancer in the United States.
“Approximately 95% of breast cancers diagnosed in men express the estrogen receptor and progesterone receptor (PR), which is a higher percentage than in women and suggests a key role for ER in the biology of breast cancer in men,” the investigators noted.
Although treatment of men with breast cancer has traditionally been extrapolated from treatment of women with breast cancer, genomic studies have suggested some key differences, the investigators noted, citing a study of the genomic landscape of male breast cancers presented at the 2014 San Antonio Breast Cancer Symposium.
In that study, investigators from the Memorial Sloan Kettering Cancer Center in New York and other institutions found that all male breast cancers in their sample of 64 patients were ER+ and human epidermal growth factor receptor 2 (HER2)–negative, predominantly of the luminal B subtype, and that the genetic alterations seen in male breast cancers frequently target DNA-repair fibroblast growth factor pathways. However, the pathways that are known to drive luminal cancers when mutated in women are seen less often among men, said Salvatore Piscuoglio, PhD, then a research fellow at MSKCC.
The current study helps to confirm and expand on the findings from that study, commented Steven J. Isakoff, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, who was not involved in either study.
“I think it’s helpful to see in a larger dataset what the spectrum of oncotypes [Oncotype DX] looks like in men. In general, as the study described, we have a real lack of large-scale data in men and certainly no prospective data with oncotypes,” he said in an interview.
To get a better idea of the molecular characteristics of breast cancer in men and how they relate to breast cancer–specific mortality, Dr. Massarweh and his associates looked at deidentified 21-gene assay data from the Genomic Health Laboratory database on 3,806 men and 571,115 women with breast cancer with either no nodal involvement, micrometastases only, or one to three involved lymph nodes.
They also looked at survival data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) population of patients with breast cancer diagnosed during 2004-2012, which included data on 332 men and 55,842 women with ER-positive and/or PR-positive invasive breast cancer.
Among the entire 21-gene assay sample, they found that men were significantly older than women at the time of diagnosis, at a mean age of 64.2 vs. 59.1 years (P less than .001).
Both men and women had infiltrating ductal carcinoma as the most common histology; the prevalence was slightly higher among men at 87.6% versus 81.3% for women.
The average recurrence score in men was 16.8 versus 17.0 in women, a difference that was not statistically significant. A majority of both men and women had RS scores below 18 (65.8% and 58.2%, respectively), although significantly more men than women had RS scores of 31 or higher (12.4% vs. 7.4%; P less than .001).
“This relative predominance of high RS results in men was encountered across age groups but was most prominent in men younger than 40 years of age,” the investigators wrote.
At the other end of the scale, RS lower than 11, especially RS 0, were seen more frequently in men than in women, except among those younger than 40 years.
Looking at individual gene expression profiles, the authors found that mean gene expression was higher in men for genes associated with ER, proliferation, and invasion. ER expression was lowest and PR expression was highest in women younger than 50 years, but ER expression increased progressively with age.
Among men, those younger than 50 years had slightly lower ER and PR expression than did older men.
In the analysis of SEER survival data, they found that 5-year breast cancer severity score (BCSS) was 99% for men with RS below 18, 95.7% for those with RS between18 and 30, and 81% for those with RS of 31 or higher. Among women, 5-year BCSS was 99.5% for those with RS under 18, 98.6% for those with RS between 18 and 30, and 94.9% for those with RS of 31 or higher.
Five-year overall survival estimates were 92.6% for men with RS below 18, 86% for those with RS between 18 and 30, and 69% for those with RS of 31 or higher. Respective 5-year OS rates for women were 95%, 94.2%, and 89.9%.
“The 21-gene RS provided clear prognostic information in our cohort, with a significantly different 5-year BCSS determined by RS in both men and women,” the investigators wrote.
They noted that patients with low and intermediate RS have excellent prognoses regardless of nodal status, which suggests that these patients have more indolent disease and better outcomes than do patients with higher RS.
The more frequent use of adjuvant chemotherapy in the RS 31 and higher group indicates that “the prognostic utility of RS results is evident despite adjuvant chemotherapy use,” they wrote.
Dr. Isakoff pointed out, however, that the population in the study is from a registry of patients eligible for the 21-gene assay, which can only be used for patients with ER-positive and HER2-negative tumors.
“In other words, this is not a random sample. This is a sample of patients for whom the treating physician was on the fence about chemotherapy and in some way thought that getting an oncotype might be helpful,” he said.
He added that although the study findings “don’t change anything we have been doing, they provide reassurance that oncotype is a reasonable test to consider in patients with male breast cancer for whom we’re considering including or avoiding chemotherapy,” he said.
A funding source for the study was not reported. Dr. Massarweh disclosed stock or ownership in Radius Health, consulting for Novartis, and institutional research funding from multiple companies. Three coauthors are employees and stockholders of Genomic Health, maker of the Oncotype DX assay used in the study. Dr. Isakoff reported no conflicts of interest related to the study
SOURCE: Massarweh SA et al. 2018 Mar 27. doi: 10.1200/JCO.2017.76.8861.
A study of the molecular and genomic features of breast cancer in men, compared with those in women, highlights the prognostic value of a 21-gene breast recurrence score in both sexes, investigators say.
Men and women with estrogen receptor (ER)–positive breast cancer who had recurrence scores (RS) of 0 to 30 on the 21-gene assay (Oncotype DX) had excellent breast cancer–specific survival rates, which suggests that such patients could be spared from more aggressive treatments, such as chemotherapy, according to Suleiman Alfred Massarweh, MD, of Stanford (Calif.) University and his colleagues.
“Future adjuvant trials in ER-positive breast cancer may need to focus on targeting endocrine resistance in those patients with RS greater than 31 and may need to consider the weight of competing mortality risk when investigating the value of any additional treatment beyond endocrine therapy,” they wrote in the Journal of Clinical Oncology.
In 2016, an estimated 2,600 men were diagnosed with breast cancer in the United States.
“Approximately 95% of breast cancers diagnosed in men express the estrogen receptor and progesterone receptor (PR), which is a higher percentage than in women and suggests a key role for ER in the biology of breast cancer in men,” the investigators noted.
Although treatment of men with breast cancer has traditionally been extrapolated from treatment of women with breast cancer, genomic studies have suggested some key differences, the investigators noted, citing a study of the genomic landscape of male breast cancers presented at the 2014 San Antonio Breast Cancer Symposium.
In that study, investigators from the Memorial Sloan Kettering Cancer Center in New York and other institutions found that all male breast cancers in their sample of 64 patients were ER+ and human epidermal growth factor receptor 2 (HER2)–negative, predominantly of the luminal B subtype, and that the genetic alterations seen in male breast cancers frequently target DNA-repair fibroblast growth factor pathways. However, the pathways that are known to drive luminal cancers when mutated in women are seen less often among men, said Salvatore Piscuoglio, PhD, then a research fellow at MSKCC.
The current study helps to confirm and expand on the findings from that study, commented Steven J. Isakoff, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, who was not involved in either study.
“I think it’s helpful to see in a larger dataset what the spectrum of oncotypes [Oncotype DX] looks like in men. In general, as the study described, we have a real lack of large-scale data in men and certainly no prospective data with oncotypes,” he said in an interview.
To get a better idea of the molecular characteristics of breast cancer in men and how they relate to breast cancer–specific mortality, Dr. Massarweh and his associates looked at deidentified 21-gene assay data from the Genomic Health Laboratory database on 3,806 men and 571,115 women with breast cancer with either no nodal involvement, micrometastases only, or one to three involved lymph nodes.
They also looked at survival data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) population of patients with breast cancer diagnosed during 2004-2012, which included data on 332 men and 55,842 women with ER-positive and/or PR-positive invasive breast cancer.
Among the entire 21-gene assay sample, they found that men were significantly older than women at the time of diagnosis, at a mean age of 64.2 vs. 59.1 years (P less than .001).
Both men and women had infiltrating ductal carcinoma as the most common histology; the prevalence was slightly higher among men at 87.6% versus 81.3% for women.
The average recurrence score in men was 16.8 versus 17.0 in women, a difference that was not statistically significant. A majority of both men and women had RS scores below 18 (65.8% and 58.2%, respectively), although significantly more men than women had RS scores of 31 or higher (12.4% vs. 7.4%; P less than .001).
“This relative predominance of high RS results in men was encountered across age groups but was most prominent in men younger than 40 years of age,” the investigators wrote.
At the other end of the scale, RS lower than 11, especially RS 0, were seen more frequently in men than in women, except among those younger than 40 years.
Looking at individual gene expression profiles, the authors found that mean gene expression was higher in men for genes associated with ER, proliferation, and invasion. ER expression was lowest and PR expression was highest in women younger than 50 years, but ER expression increased progressively with age.
Among men, those younger than 50 years had slightly lower ER and PR expression than did older men.
In the analysis of SEER survival data, they found that 5-year breast cancer severity score (BCSS) was 99% for men with RS below 18, 95.7% for those with RS between18 and 30, and 81% for those with RS of 31 or higher. Among women, 5-year BCSS was 99.5% for those with RS under 18, 98.6% for those with RS between 18 and 30, and 94.9% for those with RS of 31 or higher.
Five-year overall survival estimates were 92.6% for men with RS below 18, 86% for those with RS between 18 and 30, and 69% for those with RS of 31 or higher. Respective 5-year OS rates for women were 95%, 94.2%, and 89.9%.
“The 21-gene RS provided clear prognostic information in our cohort, with a significantly different 5-year BCSS determined by RS in both men and women,” the investigators wrote.
They noted that patients with low and intermediate RS have excellent prognoses regardless of nodal status, which suggests that these patients have more indolent disease and better outcomes than do patients with higher RS.
The more frequent use of adjuvant chemotherapy in the RS 31 and higher group indicates that “the prognostic utility of RS results is evident despite adjuvant chemotherapy use,” they wrote.
Dr. Isakoff pointed out, however, that the population in the study is from a registry of patients eligible for the 21-gene assay, which can only be used for patients with ER-positive and HER2-negative tumors.
“In other words, this is not a random sample. This is a sample of patients for whom the treating physician was on the fence about chemotherapy and in some way thought that getting an oncotype might be helpful,” he said.
He added that although the study findings “don’t change anything we have been doing, they provide reassurance that oncotype is a reasonable test to consider in patients with male breast cancer for whom we’re considering including or avoiding chemotherapy,” he said.
A funding source for the study was not reported. Dr. Massarweh disclosed stock or ownership in Radius Health, consulting for Novartis, and institutional research funding from multiple companies. Three coauthors are employees and stockholders of Genomic Health, maker of the Oncotype DX assay used in the study. Dr. Isakoff reported no conflicts of interest related to the study
SOURCE: Massarweh SA et al. 2018 Mar 27. doi: 10.1200/JCO.2017.76.8861.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: A 21-gene assay provides useful information about survival odds for men and women with breast cancer.
Major finding: A recurrence score of 31 or greater was associated with worse survival, particularly in men.
Study details: Retrospective review of genomic and surveillance data on 3,806 men and 571,115 women with breast cancer.
Disclosures: A funding source for the study was not reported. Dr. Massarweh disclosed stock or ownership in Radius Health, consulting for Novartis, and institutional research funding from multiple companies. Three coauthors are employees and stockholders of Genomic Health, maker of the Oncotype DX assay used in the study. Dr. Isakoff reported no conflicts of interest related to the study.
Source: Massarweh SA et al. 2018 Mar 27. doi: 10.1200/JCO.2017.76.8861.
FDA recalls kratom products for salmonella contamination
The Food and Drug Administration on April 3 recalled all products containing kratom manufactured by Triangle Pharmanaturals LLC, after a number of supplements tested positive for salmonella.
The FDA advises consumers to get rid of products including Raw Form Organics Maeng Da Kratom Emerald Green, Raw Form Organics Maeng Da Kratom Ivory White, and Raw Form Organics Maeng Da Kratom Ruby Red.
Evidence of the contamination was found after two samples were collected from a retail store in Oregon by the Oregon Public Health Division and tested positive for salmonella.
The recall was ordered after Triangle Pharmanaturals did not comply with a March 30 formal request from the FDA to voluntarily recall their products.
“This action is based on the imminent health risk posed by the contamination of this product with salmonella, and the refusal of this company to voluntarily act to protect its customers and issue a recall, despite our repeated requests and actions,” said FDA Commissioner Scott Gottlieb, M.D., said in a statement. “The action today is based on the risks posed by the contamination of this particular product with a potentially dangerous pathogen.”
At press time, Triangle Pharmanaturals did not respond to a request for comment.
This is the most recent in a list recalls of kratom products as part of an ongoing investigation of a salmonella outbreak by the FDA; however Triangle Pharmanaturals’ noncompliance is unique to the agency, according to an FDA representative.
“This is the first time the agency has issued a mandatory recall order to protect Americans from contaminated food products,” Michael Felberbaum, an FDA press officer, said in an interview. “This is the third time the FDA has invoked its mandatory recall authority, but the first time the agency ordered a mandatory recall because a company has opted not to voluntarily recall after the FDA’s notification of an opportunity to initiate a voluntary recall.”
Earlier in March, the CDC reported 87 people in 35 states infected with either Salmonella Javiana, Salmonela Okatie, or Salmonella Thompson, which have been associated with the outbreak.
While salmonella was identified in Triangle Pharmanaturals’ products, the strains identified are not currently linked to the outbreak.
Kratom, a plant that commonly grows in South East Asian countries like Thailand, Malaysia, Indonesia, and Papua New Guinea, has recently been used to produce food supplements and marketed as an alternative to addictive pain medication like opioids, as well as used to help treat opioid withdrawal symptoms.
Use of the food supplement has fired debate among physicians, patients, and public officials as all sides continue to determine its efficacy and how, or whether, it should be given a drug classification.
The Food and Drug Administration on April 3 recalled all products containing kratom manufactured by Triangle Pharmanaturals LLC, after a number of supplements tested positive for salmonella.
The FDA advises consumers to get rid of products including Raw Form Organics Maeng Da Kratom Emerald Green, Raw Form Organics Maeng Da Kratom Ivory White, and Raw Form Organics Maeng Da Kratom Ruby Red.
Evidence of the contamination was found after two samples were collected from a retail store in Oregon by the Oregon Public Health Division and tested positive for salmonella.
The recall was ordered after Triangle Pharmanaturals did not comply with a March 30 formal request from the FDA to voluntarily recall their products.
“This action is based on the imminent health risk posed by the contamination of this product with salmonella, and the refusal of this company to voluntarily act to protect its customers and issue a recall, despite our repeated requests and actions,” said FDA Commissioner Scott Gottlieb, M.D., said in a statement. “The action today is based on the risks posed by the contamination of this particular product with a potentially dangerous pathogen.”
At press time, Triangle Pharmanaturals did not respond to a request for comment.
This is the most recent in a list recalls of kratom products as part of an ongoing investigation of a salmonella outbreak by the FDA; however Triangle Pharmanaturals’ noncompliance is unique to the agency, according to an FDA representative.
“This is the first time the agency has issued a mandatory recall order to protect Americans from contaminated food products,” Michael Felberbaum, an FDA press officer, said in an interview. “This is the third time the FDA has invoked its mandatory recall authority, but the first time the agency ordered a mandatory recall because a company has opted not to voluntarily recall after the FDA’s notification of an opportunity to initiate a voluntary recall.”
Earlier in March, the CDC reported 87 people in 35 states infected with either Salmonella Javiana, Salmonela Okatie, or Salmonella Thompson, which have been associated with the outbreak.
While salmonella was identified in Triangle Pharmanaturals’ products, the strains identified are not currently linked to the outbreak.
Kratom, a plant that commonly grows in South East Asian countries like Thailand, Malaysia, Indonesia, and Papua New Guinea, has recently been used to produce food supplements and marketed as an alternative to addictive pain medication like opioids, as well as used to help treat opioid withdrawal symptoms.
Use of the food supplement has fired debate among physicians, patients, and public officials as all sides continue to determine its efficacy and how, or whether, it should be given a drug classification.
The Food and Drug Administration on April 3 recalled all products containing kratom manufactured by Triangle Pharmanaturals LLC, after a number of supplements tested positive for salmonella.
The FDA advises consumers to get rid of products including Raw Form Organics Maeng Da Kratom Emerald Green, Raw Form Organics Maeng Da Kratom Ivory White, and Raw Form Organics Maeng Da Kratom Ruby Red.
Evidence of the contamination was found after two samples were collected from a retail store in Oregon by the Oregon Public Health Division and tested positive for salmonella.
The recall was ordered after Triangle Pharmanaturals did not comply with a March 30 formal request from the FDA to voluntarily recall their products.
“This action is based on the imminent health risk posed by the contamination of this product with salmonella, and the refusal of this company to voluntarily act to protect its customers and issue a recall, despite our repeated requests and actions,” said FDA Commissioner Scott Gottlieb, M.D., said in a statement. “The action today is based on the risks posed by the contamination of this particular product with a potentially dangerous pathogen.”
At press time, Triangle Pharmanaturals did not respond to a request for comment.
This is the most recent in a list recalls of kratom products as part of an ongoing investigation of a salmonella outbreak by the FDA; however Triangle Pharmanaturals’ noncompliance is unique to the agency, according to an FDA representative.
“This is the first time the agency has issued a mandatory recall order to protect Americans from contaminated food products,” Michael Felberbaum, an FDA press officer, said in an interview. “This is the third time the FDA has invoked its mandatory recall authority, but the first time the agency ordered a mandatory recall because a company has opted not to voluntarily recall after the FDA’s notification of an opportunity to initiate a voluntary recall.”
Earlier in March, the CDC reported 87 people in 35 states infected with either Salmonella Javiana, Salmonela Okatie, or Salmonella Thompson, which have been associated with the outbreak.
While salmonella was identified in Triangle Pharmanaturals’ products, the strains identified are not currently linked to the outbreak.
Kratom, a plant that commonly grows in South East Asian countries like Thailand, Malaysia, Indonesia, and Papua New Guinea, has recently been used to produce food supplements and marketed as an alternative to addictive pain medication like opioids, as well as used to help treat opioid withdrawal symptoms.
Use of the food supplement has fired debate among physicians, patients, and public officials as all sides continue to determine its efficacy and how, or whether, it should be given a drug classification.
Developing a Pediatric Epilepsy Self-Management Protocol
Pediatric patients with epilepsy would benefit from a comprehensive self-management system that includes several modifiable targets such as adherence, self-sufficiency, attitudes about the disorder, and family variables, according to a review published in Epilepsia.
- To reach that conclusion, investigators studied English-based literature from 1985 to 2014, concentrating on countries with a very high human development index.
- They identified 25 studies related to self-management of epilepsy and found that individual and family-focused factors were most often analyzed for their ability to predict successful self-management.
- Psychosocial care needs and self-efficiency were identified as key factors linked to pediatric epilepsy self-management.
- Researchers identified adherence, self-efficacy for seizure management, attitudes toward epilepsy, and family variables as skills to concentrate on when developing a self-management model.
Smith G, Modi AC, Johnson EK, et al. Measurement in pediatric epilepsy self-management: a critical review. Epilepsia. 2018;59(3):509-522.
Pediatric patients with epilepsy would benefit from a comprehensive self-management system that includes several modifiable targets such as adherence, self-sufficiency, attitudes about the disorder, and family variables, according to a review published in Epilepsia.
- To reach that conclusion, investigators studied English-based literature from 1985 to 2014, concentrating on countries with a very high human development index.
- They identified 25 studies related to self-management of epilepsy and found that individual and family-focused factors were most often analyzed for their ability to predict successful self-management.
- Psychosocial care needs and self-efficiency were identified as key factors linked to pediatric epilepsy self-management.
- Researchers identified adherence, self-efficacy for seizure management, attitudes toward epilepsy, and family variables as skills to concentrate on when developing a self-management model.
Smith G, Modi AC, Johnson EK, et al. Measurement in pediatric epilepsy self-management: a critical review. Epilepsia. 2018;59(3):509-522.
Pediatric patients with epilepsy would benefit from a comprehensive self-management system that includes several modifiable targets such as adherence, self-sufficiency, attitudes about the disorder, and family variables, according to a review published in Epilepsia.
- To reach that conclusion, investigators studied English-based literature from 1985 to 2014, concentrating on countries with a very high human development index.
- They identified 25 studies related to self-management of epilepsy and found that individual and family-focused factors were most often analyzed for their ability to predict successful self-management.
- Psychosocial care needs and self-efficiency were identified as key factors linked to pediatric epilepsy self-management.
- Researchers identified adherence, self-efficacy for seizure management, attitudes toward epilepsy, and family variables as skills to concentrate on when developing a self-management model.
Smith G, Modi AC, Johnson EK, et al. Measurement in pediatric epilepsy self-management: a critical review. Epilepsia. 2018;59(3):509-522.
Linking EEG Oscillations to Epileptic Spasms
The occurrence rate of high-frequency oscillations is higher in children with drug-resistant multilobar epilepsy who experience epileptic spasms, when compared with those who do not, according to a study published in Epilepsia.
- Researchers found that the number of electrodes with high-rate fast ripple (FR) amplitude and the modulation index of coupling between slow and fast oscillations in all electrodes were significantly higher in children with epileptic spasms.
- The investigation involved 24 pediatric patients with drug-resistant multilobar onset epilepsy who had intracranial video EEGs done before multilobar resection.
- The modulation index was highest in 5 frequency bands among both epileptic spasm and nonepileptic spasm prone children.
- Researchers concluded that the greater number of high-rate FR electrodes provided evidence that there was more widespread epileptogenicity in the spasming patients, compared to those without spasms.
- Children with epileptic spasms who were free of seizures after surgery had strong coupling between slow oscillations and FRs.
Iimura Y, Jones K, Takada L, et al. Strong coupling between slow oscillations and wide fast ripples in children with epileptic spasms: Investigation of modulation index and occurrence rate. Epilepsia. 2018;59(3):544-554.
The occurrence rate of high-frequency oscillations is higher in children with drug-resistant multilobar epilepsy who experience epileptic spasms, when compared with those who do not, according to a study published in Epilepsia.
- Researchers found that the number of electrodes with high-rate fast ripple (FR) amplitude and the modulation index of coupling between slow and fast oscillations in all electrodes were significantly higher in children with epileptic spasms.
- The investigation involved 24 pediatric patients with drug-resistant multilobar onset epilepsy who had intracranial video EEGs done before multilobar resection.
- The modulation index was highest in 5 frequency bands among both epileptic spasm and nonepileptic spasm prone children.
- Researchers concluded that the greater number of high-rate FR electrodes provided evidence that there was more widespread epileptogenicity in the spasming patients, compared to those without spasms.
- Children with epileptic spasms who were free of seizures after surgery had strong coupling between slow oscillations and FRs.
Iimura Y, Jones K, Takada L, et al. Strong coupling between slow oscillations and wide fast ripples in children with epileptic spasms: Investigation of modulation index and occurrence rate. Epilepsia. 2018;59(3):544-554.
The occurrence rate of high-frequency oscillations is higher in children with drug-resistant multilobar epilepsy who experience epileptic spasms, when compared with those who do not, according to a study published in Epilepsia.
- Researchers found that the number of electrodes with high-rate fast ripple (FR) amplitude and the modulation index of coupling between slow and fast oscillations in all electrodes were significantly higher in children with epileptic spasms.
- The investigation involved 24 pediatric patients with drug-resistant multilobar onset epilepsy who had intracranial video EEGs done before multilobar resection.
- The modulation index was highest in 5 frequency bands among both epileptic spasm and nonepileptic spasm prone children.
- Researchers concluded that the greater number of high-rate FR electrodes provided evidence that there was more widespread epileptogenicity in the spasming patients, compared to those without spasms.
- Children with epileptic spasms who were free of seizures after surgery had strong coupling between slow oscillations and FRs.
Iimura Y, Jones K, Takada L, et al. Strong coupling between slow oscillations and wide fast ripples in children with epileptic spasms: Investigation of modulation index and occurrence rate. Epilepsia. 2018;59(3):544-554.
Angelman Syndrome Accompanied by Nonepileptic Myoclonus
Angelman syndrome, a neurogenetic disorder brought on by loss of the Ube3a gene, is often accompanied by nonepileptic myoclonus according to a study of 200 patients reported by the Massachusetts General Hospital and the Lurie Center for Autism.
- Myoclonus seizures were reported in 14% of patients with Angelman syndrome, with the first episode beginning before 8 years of age.
- The seizures were usually brief, unless the patient was experiencing myoclonic status, and EEGs showed interictal generalized spike and wave activity.
- 40% of patients older than 10 years had nonepileptic myoclonus.
- Nonepileptic myoclonus typically started during puberty or later.
- The nonepileptic myoclonus lasted from seconds to hours and always began in patients’ hands and, in some cases, spread to face and all extremities.
Pollack SF, Grocott OR, Parkin KA, et al. Myoclonus in Angelman syndrome [Published online ahead of print March 17, 2018]. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2018.02.006.
Angelman syndrome, a neurogenetic disorder brought on by loss of the Ube3a gene, is often accompanied by nonepileptic myoclonus according to a study of 200 patients reported by the Massachusetts General Hospital and the Lurie Center for Autism.
- Myoclonus seizures were reported in 14% of patients with Angelman syndrome, with the first episode beginning before 8 years of age.
- The seizures were usually brief, unless the patient was experiencing myoclonic status, and EEGs showed interictal generalized spike and wave activity.
- 40% of patients older than 10 years had nonepileptic myoclonus.
- Nonepileptic myoclonus typically started during puberty or later.
- The nonepileptic myoclonus lasted from seconds to hours and always began in patients’ hands and, in some cases, spread to face and all extremities.
Pollack SF, Grocott OR, Parkin KA, et al. Myoclonus in Angelman syndrome [Published online ahead of print March 17, 2018]. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2018.02.006.
Angelman syndrome, a neurogenetic disorder brought on by loss of the Ube3a gene, is often accompanied by nonepileptic myoclonus according to a study of 200 patients reported by the Massachusetts General Hospital and the Lurie Center for Autism.
- Myoclonus seizures were reported in 14% of patients with Angelman syndrome, with the first episode beginning before 8 years of age.
- The seizures were usually brief, unless the patient was experiencing myoclonic status, and EEGs showed interictal generalized spike and wave activity.
- 40% of patients older than 10 years had nonepileptic myoclonus.
- Nonepileptic myoclonus typically started during puberty or later.
- The nonepileptic myoclonus lasted from seconds to hours and always began in patients’ hands and, in some cases, spread to face and all extremities.
Pollack SF, Grocott OR, Parkin KA, et al. Myoclonus in Angelman syndrome [Published online ahead of print March 17, 2018]. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2018.02.006.
Evaluating fever in the first 90 days of life
Fever in the youngest of infants creates a challenge for the pediatric clinician. Fever is a common presentation for serious bacterial infection (SBI) although most fevers are due to viral infection. However, the clinical presentation does not necessarily differ, and the risk for a poor outcome in this age group is substantial.
In the early stages of my pediatric career, most febrile infants less than 90 days of age were evaluated for sepsis, admitted, and treated with antibiotics pending culture results. Group B streptococcal sepsis or Escherichia coli sepsis were common in the first month of life, and Haemophilus influenza type B or Streptococcus pneumoniae in the second and third months of life. The approach to fever in the first 90 days has changed following both the introduction of haemophilus and pneumococcal conjugate vaccines, the experience with risk stratification criteria for identifying infants at low risk for SBI, and the recognition of urinary tract infection (UTI) as a common source of infection in this age group as well as development of criteria for diagnosis.
A further nuance was subsequently added with the introduction of rapid diagnostics for viral infection. Byington et al. found that the majority of febrile infants less than 90 days of age had viral infection with enterovirus, respiratory syncytial virus (RSV), influenza or rotavirus.1 Using the Rochester risk stratification and the presence or absence of viral infection, she demonstrated that the risk of SBI was reduced in both high- and low-risk infants in the presence of viral infection; in low risk infants with viral infection, SBI was identified in 1.8%, compared with 3.1% in those without viral infection, and in high-risk infants. 5.5% has SBI when viral infection was found, compared to 16.7% in the absence of viral infection. She also proposed risk features to identify those infected with herpes simplex virus; age less than 42 days, vesicular rash, elevated alanine transaminase (ALT) and aspartate aminotransferase (AST), CSF pleocytosis, and seizure or twitching.
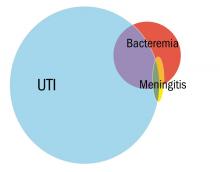
Greenhow et al. reported on the experience with “serious” bacterial infection in infants less than 90 days of age receiving care at Northern California Kaiser Permanente during the period 2005-2011.2 As pictured, the majority of children have UTI, and smaller numbers have bacteremia or meningitis. A small group of children with UTI have urosepsis as well; those with urosepsis can be differentiated from those with only UTI by age (less than 21 days), clinical exam (ill appearing), and elevated C reactive protein (greater than 20 mg/L) or elevated procalcitonin (greater than 0.5 ng/mL).3 Further evaluation of procalcitonin by other groups appears to validate its role in identifying children at low risk of SBI (procalcitonin less than 0.3 ng/mL).4
Currently, studies of febrile infants less than 90 days of age demonstrate that E. coli dominates in bacteremia, UTI, and meningitis, with Group B streptococcus as the next most frequent pathogen identified.2 Increasingly ampicillin resistance has been reported among E. coli isolates from both early- and late-onset disease as well as rare isolates that are resistant to third generation cephalosporins or gentamicin. Surveillance to identify changes in antimicrobial susceptibility will need to be ongoing to ensure that current approaches for initial therapy in high-risk infants aligns with current susceptibility patterns.
Dr. Pelton is chief of the section of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Pediatrics. 2004 Jun;113(6):1662-6.
2. Pediatr Infect Dis J. 2014 Jun;33(6):595-9.
3. Pediatr Infect Dis J. 2015 Jan;34(1):17-21.
4. JAMA Pediatr. 2016;170(1):17-18.
5. “AAP Proposes Update to Evaluating, Managing Febrile Infants Guideline,” The Hospitalist, 2016.
Fever in the youngest of infants creates a challenge for the pediatric clinician. Fever is a common presentation for serious bacterial infection (SBI) although most fevers are due to viral infection. However, the clinical presentation does not necessarily differ, and the risk for a poor outcome in this age group is substantial.
In the early stages of my pediatric career, most febrile infants less than 90 days of age were evaluated for sepsis, admitted, and treated with antibiotics pending culture results. Group B streptococcal sepsis or Escherichia coli sepsis were common in the first month of life, and Haemophilus influenza type B or Streptococcus pneumoniae in the second and third months of life. The approach to fever in the first 90 days has changed following both the introduction of haemophilus and pneumococcal conjugate vaccines, the experience with risk stratification criteria for identifying infants at low risk for SBI, and the recognition of urinary tract infection (UTI) as a common source of infection in this age group as well as development of criteria for diagnosis.
A further nuance was subsequently added with the introduction of rapid diagnostics for viral infection. Byington et al. found that the majority of febrile infants less than 90 days of age had viral infection with enterovirus, respiratory syncytial virus (RSV), influenza or rotavirus.1 Using the Rochester risk stratification and the presence or absence of viral infection, she demonstrated that the risk of SBI was reduced in both high- and low-risk infants in the presence of viral infection; in low risk infants with viral infection, SBI was identified in 1.8%, compared with 3.1% in those without viral infection, and in high-risk infants. 5.5% has SBI when viral infection was found, compared to 16.7% in the absence of viral infection. She also proposed risk features to identify those infected with herpes simplex virus; age less than 42 days, vesicular rash, elevated alanine transaminase (ALT) and aspartate aminotransferase (AST), CSF pleocytosis, and seizure or twitching.
Greenhow et al. reported on the experience with “serious” bacterial infection in infants less than 90 days of age receiving care at Northern California Kaiser Permanente during the period 2005-2011.2 As pictured, the majority of children have UTI, and smaller numbers have bacteremia or meningitis. A small group of children with UTI have urosepsis as well; those with urosepsis can be differentiated from those with only UTI by age (less than 21 days), clinical exam (ill appearing), and elevated C reactive protein (greater than 20 mg/L) or elevated procalcitonin (greater than 0.5 ng/mL).3 Further evaluation of procalcitonin by other groups appears to validate its role in identifying children at low risk of SBI (procalcitonin less than 0.3 ng/mL).4
Currently, studies of febrile infants less than 90 days of age demonstrate that E. coli dominates in bacteremia, UTI, and meningitis, with Group B streptococcus as the next most frequent pathogen identified.2 Increasingly ampicillin resistance has been reported among E. coli isolates from both early- and late-onset disease as well as rare isolates that are resistant to third generation cephalosporins or gentamicin. Surveillance to identify changes in antimicrobial susceptibility will need to be ongoing to ensure that current approaches for initial therapy in high-risk infants aligns with current susceptibility patterns.
Dr. Pelton is chief of the section of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Pediatrics. 2004 Jun;113(6):1662-6.
2. Pediatr Infect Dis J. 2014 Jun;33(6):595-9.
3. Pediatr Infect Dis J. 2015 Jan;34(1):17-21.
4. JAMA Pediatr. 2016;170(1):17-18.
5. “AAP Proposes Update to Evaluating, Managing Febrile Infants Guideline,” The Hospitalist, 2016.
Fever in the youngest of infants creates a challenge for the pediatric clinician. Fever is a common presentation for serious bacterial infection (SBI) although most fevers are due to viral infection. However, the clinical presentation does not necessarily differ, and the risk for a poor outcome in this age group is substantial.
In the early stages of my pediatric career, most febrile infants less than 90 days of age were evaluated for sepsis, admitted, and treated with antibiotics pending culture results. Group B streptococcal sepsis or Escherichia coli sepsis were common in the first month of life, and Haemophilus influenza type B or Streptococcus pneumoniae in the second and third months of life. The approach to fever in the first 90 days has changed following both the introduction of haemophilus and pneumococcal conjugate vaccines, the experience with risk stratification criteria for identifying infants at low risk for SBI, and the recognition of urinary tract infection (UTI) as a common source of infection in this age group as well as development of criteria for diagnosis.
A further nuance was subsequently added with the introduction of rapid diagnostics for viral infection. Byington et al. found that the majority of febrile infants less than 90 days of age had viral infection with enterovirus, respiratory syncytial virus (RSV), influenza or rotavirus.1 Using the Rochester risk stratification and the presence or absence of viral infection, she demonstrated that the risk of SBI was reduced in both high- and low-risk infants in the presence of viral infection; in low risk infants with viral infection, SBI was identified in 1.8%, compared with 3.1% in those without viral infection, and in high-risk infants. 5.5% has SBI when viral infection was found, compared to 16.7% in the absence of viral infection. She also proposed risk features to identify those infected with herpes simplex virus; age less than 42 days, vesicular rash, elevated alanine transaminase (ALT) and aspartate aminotransferase (AST), CSF pleocytosis, and seizure or twitching.
Greenhow et al. reported on the experience with “serious” bacterial infection in infants less than 90 days of age receiving care at Northern California Kaiser Permanente during the period 2005-2011.2 As pictured, the majority of children have UTI, and smaller numbers have bacteremia or meningitis. A small group of children with UTI have urosepsis as well; those with urosepsis can be differentiated from those with only UTI by age (less than 21 days), clinical exam (ill appearing), and elevated C reactive protein (greater than 20 mg/L) or elevated procalcitonin (greater than 0.5 ng/mL).3 Further evaluation of procalcitonin by other groups appears to validate its role in identifying children at low risk of SBI (procalcitonin less than 0.3 ng/mL).4
Currently, studies of febrile infants less than 90 days of age demonstrate that E. coli dominates in bacteremia, UTI, and meningitis, with Group B streptococcus as the next most frequent pathogen identified.2 Increasingly ampicillin resistance has been reported among E. coli isolates from both early- and late-onset disease as well as rare isolates that are resistant to third generation cephalosporins or gentamicin. Surveillance to identify changes in antimicrobial susceptibility will need to be ongoing to ensure that current approaches for initial therapy in high-risk infants aligns with current susceptibility patterns.
Dr. Pelton is chief of the section of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Pediatrics. 2004 Jun;113(6):1662-6.
2. Pediatr Infect Dis J. 2014 Jun;33(6):595-9.
3. Pediatr Infect Dis J. 2015 Jan;34(1):17-21.
4. JAMA Pediatr. 2016;170(1):17-18.
5. “AAP Proposes Update to Evaluating, Managing Febrile Infants Guideline,” The Hospitalist, 2016.
What Are the Therapeutic Considerations of Antiseizure Drugs in the Elderly?
WASHINGTON, DC—When initiating antiseizure therapy for elderly patients with epilepsy, drug interactions and side effects, especially impaired cognition and falls, are important treatment considerations, according to an overview given at the 71st Annual Meeting of the American Epilepsy Society.
In addition, neurologists should take into account comorbidities and concomitant medications that could be relevant in the future—similar to considering the possibility of pregnancy when treating a patient of childbearing potential, said Ilo Leppik, MD, Professor of Neurology and Pharmacy at the University of Minnesota in Minneapolis. An elderly patient with poststroke seizures, for instance, may not be on warfarin at an initial visit. “But they might be … the next time you see them,” he said. “In the next six months, what could this person have in terms of comorbidities?”
Interactions and Side Effects
“In general, most antiseizure drugs are effective for epilepsy encountered in the elderly,” Dr. Leppik said. “Drug interactions and cognitive and behavioral side effects are key.”
Research indicates that a drug’s side effect profile, rather than its efficacy, determines how long patients stay on a medication. Dr. Leppik tries to see elderly patients about a month after starting treatment and asks family members to report cognitive or behavioral changes.
“My preferred approach is not to use drugs that are strong enzyme inducers because of the issues with drug–drug interactions,” Dr. Leppik said. Blood levels of simvastatin, for example, are markedly lower in patients who receive carbamazepine and are likely to be so with other inducing antiseizure drugs.
Hyponatremia may be a concern. Xiaoming Dong, MD, Dr. Leppik, and colleagues found that oxcarbazepine is a significant cause of hyponatremia in people over age 40, and the risk is greater in people over age 65, he said.
In addition, many older patients have cognitive issues, and antiseizure drugs (eg, topiramate, zonisamide, valproic acid, phenobarbital, and phenytoin) may cause cognitive side effects. One clinical question that neurologists may encounter is whether to treat a patient with end-stage Alzheimer’s disease who has a single convulsion. Families may want to initiate antiseizure treatment. “But my experience is that if you start them on any of our antiseizure medications, [these patients] are much more sensitive to side effects,” Dr. Leppik said. “I have seen them deteriorate cognitively once they are started on an antiseizure medication.”
The risk of falls increases with age, and studies have found that higher levels of antiseizure drugs are associated with falls and fractures. Falls may be related to the adverse effects of antiseizure drugs, such as dizziness or ataxia. Prescribing an antiseizure drug with a long half-life once daily at bedtime may help to avoid daytime imbalance, Dr. Leppik said.
Therapeutic Drug Monitoring
Monitoring serum concentrations of antiseizure drugs may help guide patient management. When a therapeutic response has been reached, measuring the drug level can provide a target benchmark. When treatment is not working, in most cases it is because blood levels are not at the target level, Dr. Leppik added. Monitoring also may be useful when a drug is added or removed, or when there are concerns about toxicity or compliance.
“Most breakthrough seizures are d
—Jake Remaly
Suggested Reading
Dong X, Leppik IE, White J, Rarick J. Hyponatremia from oxcarbazepine and carbamazepine. Neurology. 2005;65(12):1976-1978.
Leppik IE, Birnbaum AK. Epilepsy in the elderly. Ann N Y Acad Sci. 2010;1184:208-224.
Leppik IE, Walczak TS, Birnbaum AK. Challenges ofepilepsy in elderly people. Lancet. 2012;380(9848):1128-1130.
Patsalos PN, Berry DJ, Bourgeois BF, et al. Antiepileptic drugs--best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia. 2008;49(7):1239-1276.
WASHINGTON, DC—When initiating antiseizure therapy for elderly patients with epilepsy, drug interactions and side effects, especially impaired cognition and falls, are important treatment considerations, according to an overview given at the 71st Annual Meeting of the American Epilepsy Society.
In addition, neurologists should take into account comorbidities and concomitant medications that could be relevant in the future—similar to considering the possibility of pregnancy when treating a patient of childbearing potential, said Ilo Leppik, MD, Professor of Neurology and Pharmacy at the University of Minnesota in Minneapolis. An elderly patient with poststroke seizures, for instance, may not be on warfarin at an initial visit. “But they might be … the next time you see them,” he said. “In the next six months, what could this person have in terms of comorbidities?”
Interactions and Side Effects
“In general, most antiseizure drugs are effective for epilepsy encountered in the elderly,” Dr. Leppik said. “Drug interactions and cognitive and behavioral side effects are key.”
Research indicates that a drug’s side effect profile, rather than its efficacy, determines how long patients stay on a medication. Dr. Leppik tries to see elderly patients about a month after starting treatment and asks family members to report cognitive or behavioral changes.
“My preferred approach is not to use drugs that are strong enzyme inducers because of the issues with drug–drug interactions,” Dr. Leppik said. Blood levels of simvastatin, for example, are markedly lower in patients who receive carbamazepine and are likely to be so with other inducing antiseizure drugs.
Hyponatremia may be a concern. Xiaoming Dong, MD, Dr. Leppik, and colleagues found that oxcarbazepine is a significant cause of hyponatremia in people over age 40, and the risk is greater in people over age 65, he said.
In addition, many older patients have cognitive issues, and antiseizure drugs (eg, topiramate, zonisamide, valproic acid, phenobarbital, and phenytoin) may cause cognitive side effects. One clinical question that neurologists may encounter is whether to treat a patient with end-stage Alzheimer’s disease who has a single convulsion. Families may want to initiate antiseizure treatment. “But my experience is that if you start them on any of our antiseizure medications, [these patients] are much more sensitive to side effects,” Dr. Leppik said. “I have seen them deteriorate cognitively once they are started on an antiseizure medication.”
The risk of falls increases with age, and studies have found that higher levels of antiseizure drugs are associated with falls and fractures. Falls may be related to the adverse effects of antiseizure drugs, such as dizziness or ataxia. Prescribing an antiseizure drug with a long half-life once daily at bedtime may help to avoid daytime imbalance, Dr. Leppik said.
Therapeutic Drug Monitoring
Monitoring serum concentrations of antiseizure drugs may help guide patient management. When a therapeutic response has been reached, measuring the drug level can provide a target benchmark. When treatment is not working, in most cases it is because blood levels are not at the target level, Dr. Leppik added. Monitoring also may be useful when a drug is added or removed, or when there are concerns about toxicity or compliance.
“Most breakthrough seizures are d
—Jake Remaly
Suggested Reading
Dong X, Leppik IE, White J, Rarick J. Hyponatremia from oxcarbazepine and carbamazepine. Neurology. 2005;65(12):1976-1978.
Leppik IE, Birnbaum AK. Epilepsy in the elderly. Ann N Y Acad Sci. 2010;1184:208-224.
Leppik IE, Walczak TS, Birnbaum AK. Challenges ofepilepsy in elderly people. Lancet. 2012;380(9848):1128-1130.
Patsalos PN, Berry DJ, Bourgeois BF, et al. Antiepileptic drugs--best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia. 2008;49(7):1239-1276.
WASHINGTON, DC—When initiating antiseizure therapy for elderly patients with epilepsy, drug interactions and side effects, especially impaired cognition and falls, are important treatment considerations, according to an overview given at the 71st Annual Meeting of the American Epilepsy Society.
In addition, neurologists should take into account comorbidities and concomitant medications that could be relevant in the future—similar to considering the possibility of pregnancy when treating a patient of childbearing potential, said Ilo Leppik, MD, Professor of Neurology and Pharmacy at the University of Minnesota in Minneapolis. An elderly patient with poststroke seizures, for instance, may not be on warfarin at an initial visit. “But they might be … the next time you see them,” he said. “In the next six months, what could this person have in terms of comorbidities?”
Interactions and Side Effects
“In general, most antiseizure drugs are effective for epilepsy encountered in the elderly,” Dr. Leppik said. “Drug interactions and cognitive and behavioral side effects are key.”
Research indicates that a drug’s side effect profile, rather than its efficacy, determines how long patients stay on a medication. Dr. Leppik tries to see elderly patients about a month after starting treatment and asks family members to report cognitive or behavioral changes.
“My preferred approach is not to use drugs that are strong enzyme inducers because of the issues with drug–drug interactions,” Dr. Leppik said. Blood levels of simvastatin, for example, are markedly lower in patients who receive carbamazepine and are likely to be so with other inducing antiseizure drugs.
Hyponatremia may be a concern. Xiaoming Dong, MD, Dr. Leppik, and colleagues found that oxcarbazepine is a significant cause of hyponatremia in people over age 40, and the risk is greater in people over age 65, he said.
In addition, many older patients have cognitive issues, and antiseizure drugs (eg, topiramate, zonisamide, valproic acid, phenobarbital, and phenytoin) may cause cognitive side effects. One clinical question that neurologists may encounter is whether to treat a patient with end-stage Alzheimer’s disease who has a single convulsion. Families may want to initiate antiseizure treatment. “But my experience is that if you start them on any of our antiseizure medications, [these patients] are much more sensitive to side effects,” Dr. Leppik said. “I have seen them deteriorate cognitively once they are started on an antiseizure medication.”
The risk of falls increases with age, and studies have found that higher levels of antiseizure drugs are associated with falls and fractures. Falls may be related to the adverse effects of antiseizure drugs, such as dizziness or ataxia. Prescribing an antiseizure drug with a long half-life once daily at bedtime may help to avoid daytime imbalance, Dr. Leppik said.
Therapeutic Drug Monitoring
Monitoring serum concentrations of antiseizure drugs may help guide patient management. When a therapeutic response has been reached, measuring the drug level can provide a target benchmark. When treatment is not working, in most cases it is because blood levels are not at the target level, Dr. Leppik added. Monitoring also may be useful when a drug is added or removed, or when there are concerns about toxicity or compliance.
“Most breakthrough seizures are d
—Jake Remaly
Suggested Reading
Dong X, Leppik IE, White J, Rarick J. Hyponatremia from oxcarbazepine and carbamazepine. Neurology. 2005;65(12):1976-1978.
Leppik IE, Birnbaum AK. Epilepsy in the elderly. Ann N Y Acad Sci. 2010;1184:208-224.
Leppik IE, Walczak TS, Birnbaum AK. Challenges ofepilepsy in elderly people. Lancet. 2012;380(9848):1128-1130.
Patsalos PN, Berry DJ, Bourgeois BF, et al. Antiepileptic drugs--best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia. 2008;49(7):1239-1276.
Causes, Mimics, and Pitfalls of Epilepsy in the Elderly
WASHINGTON, DC—The diagnosis of epilepsy in elderly patients may be challenging due to the disease’s many mimics, a patient’s cognitive impairment, unwitnessed events, and the potential for false positives on imaging or EEG, according to a lecture delivered at the 71st Annual Meeting of the American Epilepsy Society.
“The onus is on us to rigorously explore the histories of these patients,” said Colin Josephson, MD, Assistant Professor of Neurology and Community Health Sciences at the University of Calgary in Alberta. “Ninety percent of our diagnosis is in the history.”
The Differential Diagnosis
Preictal premonitory symptoms, ictal semiology, and postictal symptoms may be key to distinguishing between epilepsy and its mimics, which include cardiac arrhythmia, hypoglycemia, hyperglycemia, syncope, transient ischemic attack, stroke, transient global amnesia, delirium, sleep disorders, and psychogenic nonepileptic attacks. Abrupt loss of consciousness, for example, may suggest a diagnosis of cardiac arrhythmia. In cases involving impaired awareness, neurologists should have a high level of suspicion for hypoglycemia or hyperglycemia. “Delirium in and of itself can mimic nonconvulsive status epilepticus,” Dr. Josephson said.
Medications may cause glucose or electrolyte abnormalities that trigger seizures or similar events. One study found that about 40% of patients older than 65 were taking five to nine medications, and 18% were taking 10 or more medications. A 2011 study by Budnitz et al found that, based on a sample of 58 hospitals, adverse drug events may result in about 1.5% of emergency hospitalizations among older adults. In particular, endocrine, cardiovascular, neurologic, and anti-infectious agents can cause provoked acute seizures and seizure mimics, Dr. Josephson said.
Causes of Epilepsy in the Elderly
The etiology of epilepsy in the elderly may influence the disease’s comorbidities, psychosocial impact, functional status, and treatment. The largest group of cases—about 30% to 50% of the total—has cerebrovascular etiology. Neurodegenerative conditions may be the etiology in 10% to 20% of cases, and other conditions (eg, tumor, trauma, or autoimmune epilepsy) may cause 5% to 15% of cases. The cause may be unknown in 30% to 50% of cases.
After ischemic stroke, the incidence of epilepsy ranges from about 2.5% to 15% up to one year, and from 7% to 10% up to five years. After hemorrhagic stroke, the incidence of epilepsy ranges from 10% to 20%. A recent systematic review found that the likelihood of developing a seizure or seizure disorder is about 1.4- to 1.5-fold greater after a hemorrhagic stroke, compared with after an ischemic stroke. A review by Ferlazzo et al identified three main risk factors for poststroke epilepsy: cerebral hemorrhage, early-onset seizures, and cortical involvement.
About 5% of patients with dementia develop epilepsy. “It can be hard to distinguish [epilepsy] in this population due to the fact that a lot of seizures would involve altered levels of awareness or confusion, which is a feature of dementias, as well,” Dr. Josephson said.
Research suggests that elderly-onset epilepsy may be a unique disease entity with etiologies, seizure characteristics, and comorbidities that differ from those of younger-onset epilepsy. Using a prospective cohort database, Dr. Josephson and colleagues examined differences in patients according to age of epilepsy onset and identified 14 clinical variables that predict whether a patient will have elderly-onset epilepsy or young-onset epilepsy. “We may be able to develop targeted therapy or precision medicine for this population,” he said.
The new operational definition of epilepsy allows neurologists to diagnose epilepsy based on one unprovoked seizure when the 10-year risk of having a second seizure is at least 60%. Many elderly patients have focal lesional epilepsy, which suggests that many patients’ 10-year risk of a second seizure is greater than 50%. “This is a paradigm shift that we continue to take into account when evaluating this patient population,” Dr. Josephson said. Further research is needed to determine “whether the vast majority of these patients, if they are 65 or older, actually have a diagnosis of epilepsy.”
Nonspecific Findings
Neurologists should be aware of potential pitfalls when investigating suspected epilepsy in the elderly. Incidental imaging findings are common and more likely with age. “Video-EEG telemetry may be just as useful for this population as it is for younger adults or children,” however, Dr. Josephson said.
A systematic review by Morris et al found that among patients ages 70 to 89 with no neurologic symptoms who were evaluated for screening purposes with MRI, between 15% and 25% had nonspecific white matter hyperintensities. Likewise, non-neoplastic incidental brain findings occurred in as much as 10% of patients. “Silent infarcts were relatively common in 2% to 3% of patients, as well,” Dr. Josephson said. “You do run the risk, if you start a scattershot approach for these patients, of running down the black hole of finding nonspecific findings and then trying to explain them or going to further investigations … when they may in fact be red herrings. In this population, we have to be careful about what we order, why we order it, and how we interpret it.”
Elderly patients may have high rates of nonspecific EEG findings, as well. Generalized slowing was reported in about 31% of elderly patients examined by Widdess-Walsh et al. Focal slowing, especially within the temporal lobes, was seen in about 10% of patients, and about 5% of these patients had epileptiform discharges.
McBride et al studied 94 patients ages 60 and older who were admitted to a seizure monitoring unit. About 26% of patients with nonepileptic events nevertheless had epileptiform discharges. Of the patients with epileptic events, 76% had interictal epileptiform discharges. “Overreliance on the EEG when making a diagnosis in this population is a major mistake,” and neurologists should not solely rely on the interictal EEG, Dr. Josephson said. “The sensitivity and the specificity are not high enough…. You want to capture events.”
In McBride’s eight-year study, about 42% of the patients had epilepsy. “But interestingly, about 14% had nonepileptic attacks.... This is not necessarily a rare consideration in these patients,” said Dr. Josephson.
About 30% of the patients did not have an event during their admissions to the unit, but for about 60%, neurologists were able to establish a diagnosis of epilepsy or epilepsy mimic. Video-EEG monitoring may be useful “in those patients in which there are diagnostic conundrums,” Dr. Josephson said. Establishing the correct diagnosis is important because even appropriate therapy entails additional risk in elderly patients.
—Jake Remaly
Suggested Reading
Brodie MJ, Kwan P. Epilepsy in elderly people. BMJ. 2005;331(7528):1317-1322.
Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002-2012.
Ferlazzo E, Gasparini S, Beghi E, et al. Epilepsy in cerebrovascular diseases: Review of experimental and clinical data with meta-analysis of risk factors. Epilepsia. 2016;57(8):1205-1214.
Josephson CB, Engbers JD, Sajobi TT, et al. Towards a clinically informed, data-driven definition of elderly onset epilepsy. Epilepsia. 2016;57(2):298-305.
McBride AE, Shih TT, Hirsch LJ. Video-EEG monitoring in the elderly: a review of 94 patients. Epilepsia. 2002; 43(2):165-169.
Morris Z, Whiteley WN, Longstreth WT Jr, et al. Incidental findings on brain magnetic resonanceimaging: systematic review and meta-analysis. BMJ. 2009; 339:b3016.
Subota A, Pham T, Jetté N, et al. The association between dementia and epilepsy: A systematic review and meta-analysis. Epilepsia. 2017;58(6):962-972.
Widdess-Walsh P, Sweeney BJ, Galvin R, McNamara B. Utilization and yield of EEG in the elderly population. J Clin Neurophysiol. 2005;22(4):253-255.
WASHINGTON, DC—The diagnosis of epilepsy in elderly patients may be challenging due to the disease’s many mimics, a patient’s cognitive impairment, unwitnessed events, and the potential for false positives on imaging or EEG, according to a lecture delivered at the 71st Annual Meeting of the American Epilepsy Society.
“The onus is on us to rigorously explore the histories of these patients,” said Colin Josephson, MD, Assistant Professor of Neurology and Community Health Sciences at the University of Calgary in Alberta. “Ninety percent of our diagnosis is in the history.”
The Differential Diagnosis
Preictal premonitory symptoms, ictal semiology, and postictal symptoms may be key to distinguishing between epilepsy and its mimics, which include cardiac arrhythmia, hypoglycemia, hyperglycemia, syncope, transient ischemic attack, stroke, transient global amnesia, delirium, sleep disorders, and psychogenic nonepileptic attacks. Abrupt loss of consciousness, for example, may suggest a diagnosis of cardiac arrhythmia. In cases involving impaired awareness, neurologists should have a high level of suspicion for hypoglycemia or hyperglycemia. “Delirium in and of itself can mimic nonconvulsive status epilepticus,” Dr. Josephson said.
Medications may cause glucose or electrolyte abnormalities that trigger seizures or similar events. One study found that about 40% of patients older than 65 were taking five to nine medications, and 18% were taking 10 or more medications. A 2011 study by Budnitz et al found that, based on a sample of 58 hospitals, adverse drug events may result in about 1.5% of emergency hospitalizations among older adults. In particular, endocrine, cardiovascular, neurologic, and anti-infectious agents can cause provoked acute seizures and seizure mimics, Dr. Josephson said.
Causes of Epilepsy in the Elderly
The etiology of epilepsy in the elderly may influence the disease’s comorbidities, psychosocial impact, functional status, and treatment. The largest group of cases—about 30% to 50% of the total—has cerebrovascular etiology. Neurodegenerative conditions may be the etiology in 10% to 20% of cases, and other conditions (eg, tumor, trauma, or autoimmune epilepsy) may cause 5% to 15% of cases. The cause may be unknown in 30% to 50% of cases.
After ischemic stroke, the incidence of epilepsy ranges from about 2.5% to 15% up to one year, and from 7% to 10% up to five years. After hemorrhagic stroke, the incidence of epilepsy ranges from 10% to 20%. A recent systematic review found that the likelihood of developing a seizure or seizure disorder is about 1.4- to 1.5-fold greater after a hemorrhagic stroke, compared with after an ischemic stroke. A review by Ferlazzo et al identified three main risk factors for poststroke epilepsy: cerebral hemorrhage, early-onset seizures, and cortical involvement.
About 5% of patients with dementia develop epilepsy. “It can be hard to distinguish [epilepsy] in this population due to the fact that a lot of seizures would involve altered levels of awareness or confusion, which is a feature of dementias, as well,” Dr. Josephson said.
Research suggests that elderly-onset epilepsy may be a unique disease entity with etiologies, seizure characteristics, and comorbidities that differ from those of younger-onset epilepsy. Using a prospective cohort database, Dr. Josephson and colleagues examined differences in patients according to age of epilepsy onset and identified 14 clinical variables that predict whether a patient will have elderly-onset epilepsy or young-onset epilepsy. “We may be able to develop targeted therapy or precision medicine for this population,” he said.
The new operational definition of epilepsy allows neurologists to diagnose epilepsy based on one unprovoked seizure when the 10-year risk of having a second seizure is at least 60%. Many elderly patients have focal lesional epilepsy, which suggests that many patients’ 10-year risk of a second seizure is greater than 50%. “This is a paradigm shift that we continue to take into account when evaluating this patient population,” Dr. Josephson said. Further research is needed to determine “whether the vast majority of these patients, if they are 65 or older, actually have a diagnosis of epilepsy.”
Nonspecific Findings
Neurologists should be aware of potential pitfalls when investigating suspected epilepsy in the elderly. Incidental imaging findings are common and more likely with age. “Video-EEG telemetry may be just as useful for this population as it is for younger adults or children,” however, Dr. Josephson said.
A systematic review by Morris et al found that among patients ages 70 to 89 with no neurologic symptoms who were evaluated for screening purposes with MRI, between 15% and 25% had nonspecific white matter hyperintensities. Likewise, non-neoplastic incidental brain findings occurred in as much as 10% of patients. “Silent infarcts were relatively common in 2% to 3% of patients, as well,” Dr. Josephson said. “You do run the risk, if you start a scattershot approach for these patients, of running down the black hole of finding nonspecific findings and then trying to explain them or going to further investigations … when they may in fact be red herrings. In this population, we have to be careful about what we order, why we order it, and how we interpret it.”
Elderly patients may have high rates of nonspecific EEG findings, as well. Generalized slowing was reported in about 31% of elderly patients examined by Widdess-Walsh et al. Focal slowing, especially within the temporal lobes, was seen in about 10% of patients, and about 5% of these patients had epileptiform discharges.
McBride et al studied 94 patients ages 60 and older who were admitted to a seizure monitoring unit. About 26% of patients with nonepileptic events nevertheless had epileptiform discharges. Of the patients with epileptic events, 76% had interictal epileptiform discharges. “Overreliance on the EEG when making a diagnosis in this population is a major mistake,” and neurologists should not solely rely on the interictal EEG, Dr. Josephson said. “The sensitivity and the specificity are not high enough…. You want to capture events.”
In McBride’s eight-year study, about 42% of the patients had epilepsy. “But interestingly, about 14% had nonepileptic attacks.... This is not necessarily a rare consideration in these patients,” said Dr. Josephson.
About 30% of the patients did not have an event during their admissions to the unit, but for about 60%, neurologists were able to establish a diagnosis of epilepsy or epilepsy mimic. Video-EEG monitoring may be useful “in those patients in which there are diagnostic conundrums,” Dr. Josephson said. Establishing the correct diagnosis is important because even appropriate therapy entails additional risk in elderly patients.
—Jake Remaly
Suggested Reading
Brodie MJ, Kwan P. Epilepsy in elderly people. BMJ. 2005;331(7528):1317-1322.
Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002-2012.
Ferlazzo E, Gasparini S, Beghi E, et al. Epilepsy in cerebrovascular diseases: Review of experimental and clinical data with meta-analysis of risk factors. Epilepsia. 2016;57(8):1205-1214.
Josephson CB, Engbers JD, Sajobi TT, et al. Towards a clinically informed, data-driven definition of elderly onset epilepsy. Epilepsia. 2016;57(2):298-305.
McBride AE, Shih TT, Hirsch LJ. Video-EEG monitoring in the elderly: a review of 94 patients. Epilepsia. 2002; 43(2):165-169.
Morris Z, Whiteley WN, Longstreth WT Jr, et al. Incidental findings on brain magnetic resonanceimaging: systematic review and meta-analysis. BMJ. 2009; 339:b3016.
Subota A, Pham T, Jetté N, et al. The association between dementia and epilepsy: A systematic review and meta-analysis. Epilepsia. 2017;58(6):962-972.
Widdess-Walsh P, Sweeney BJ, Galvin R, McNamara B. Utilization and yield of EEG in the elderly population. J Clin Neurophysiol. 2005;22(4):253-255.
WASHINGTON, DC—The diagnosis of epilepsy in elderly patients may be challenging due to the disease’s many mimics, a patient’s cognitive impairment, unwitnessed events, and the potential for false positives on imaging or EEG, according to a lecture delivered at the 71st Annual Meeting of the American Epilepsy Society.
“The onus is on us to rigorously explore the histories of these patients,” said Colin Josephson, MD, Assistant Professor of Neurology and Community Health Sciences at the University of Calgary in Alberta. “Ninety percent of our diagnosis is in the history.”
The Differential Diagnosis
Preictal premonitory symptoms, ictal semiology, and postictal symptoms may be key to distinguishing between epilepsy and its mimics, which include cardiac arrhythmia, hypoglycemia, hyperglycemia, syncope, transient ischemic attack, stroke, transient global amnesia, delirium, sleep disorders, and psychogenic nonepileptic attacks. Abrupt loss of consciousness, for example, may suggest a diagnosis of cardiac arrhythmia. In cases involving impaired awareness, neurologists should have a high level of suspicion for hypoglycemia or hyperglycemia. “Delirium in and of itself can mimic nonconvulsive status epilepticus,” Dr. Josephson said.
Medications may cause glucose or electrolyte abnormalities that trigger seizures or similar events. One study found that about 40% of patients older than 65 were taking five to nine medications, and 18% were taking 10 or more medications. A 2011 study by Budnitz et al found that, based on a sample of 58 hospitals, adverse drug events may result in about 1.5% of emergency hospitalizations among older adults. In particular, endocrine, cardiovascular, neurologic, and anti-infectious agents can cause provoked acute seizures and seizure mimics, Dr. Josephson said.
Causes of Epilepsy in the Elderly
The etiology of epilepsy in the elderly may influence the disease’s comorbidities, psychosocial impact, functional status, and treatment. The largest group of cases—about 30% to 50% of the total—has cerebrovascular etiology. Neurodegenerative conditions may be the etiology in 10% to 20% of cases, and other conditions (eg, tumor, trauma, or autoimmune epilepsy) may cause 5% to 15% of cases. The cause may be unknown in 30% to 50% of cases.
After ischemic stroke, the incidence of epilepsy ranges from about 2.5% to 15% up to one year, and from 7% to 10% up to five years. After hemorrhagic stroke, the incidence of epilepsy ranges from 10% to 20%. A recent systematic review found that the likelihood of developing a seizure or seizure disorder is about 1.4- to 1.5-fold greater after a hemorrhagic stroke, compared with after an ischemic stroke. A review by Ferlazzo et al identified three main risk factors for poststroke epilepsy: cerebral hemorrhage, early-onset seizures, and cortical involvement.
About 5% of patients with dementia develop epilepsy. “It can be hard to distinguish [epilepsy] in this population due to the fact that a lot of seizures would involve altered levels of awareness or confusion, which is a feature of dementias, as well,” Dr. Josephson said.
Research suggests that elderly-onset epilepsy may be a unique disease entity with etiologies, seizure characteristics, and comorbidities that differ from those of younger-onset epilepsy. Using a prospective cohort database, Dr. Josephson and colleagues examined differences in patients according to age of epilepsy onset and identified 14 clinical variables that predict whether a patient will have elderly-onset epilepsy or young-onset epilepsy. “We may be able to develop targeted therapy or precision medicine for this population,” he said.
The new operational definition of epilepsy allows neurologists to diagnose epilepsy based on one unprovoked seizure when the 10-year risk of having a second seizure is at least 60%. Many elderly patients have focal lesional epilepsy, which suggests that many patients’ 10-year risk of a second seizure is greater than 50%. “This is a paradigm shift that we continue to take into account when evaluating this patient population,” Dr. Josephson said. Further research is needed to determine “whether the vast majority of these patients, if they are 65 or older, actually have a diagnosis of epilepsy.”
Nonspecific Findings
Neurologists should be aware of potential pitfalls when investigating suspected epilepsy in the elderly. Incidental imaging findings are common and more likely with age. “Video-EEG telemetry may be just as useful for this population as it is for younger adults or children,” however, Dr. Josephson said.
A systematic review by Morris et al found that among patients ages 70 to 89 with no neurologic symptoms who were evaluated for screening purposes with MRI, between 15% and 25% had nonspecific white matter hyperintensities. Likewise, non-neoplastic incidental brain findings occurred in as much as 10% of patients. “Silent infarcts were relatively common in 2% to 3% of patients, as well,” Dr. Josephson said. “You do run the risk, if you start a scattershot approach for these patients, of running down the black hole of finding nonspecific findings and then trying to explain them or going to further investigations … when they may in fact be red herrings. In this population, we have to be careful about what we order, why we order it, and how we interpret it.”
Elderly patients may have high rates of nonspecific EEG findings, as well. Generalized slowing was reported in about 31% of elderly patients examined by Widdess-Walsh et al. Focal slowing, especially within the temporal lobes, was seen in about 10% of patients, and about 5% of these patients had epileptiform discharges.
McBride et al studied 94 patients ages 60 and older who were admitted to a seizure monitoring unit. About 26% of patients with nonepileptic events nevertheless had epileptiform discharges. Of the patients with epileptic events, 76% had interictal epileptiform discharges. “Overreliance on the EEG when making a diagnosis in this population is a major mistake,” and neurologists should not solely rely on the interictal EEG, Dr. Josephson said. “The sensitivity and the specificity are not high enough…. You want to capture events.”
In McBride’s eight-year study, about 42% of the patients had epilepsy. “But interestingly, about 14% had nonepileptic attacks.... This is not necessarily a rare consideration in these patients,” said Dr. Josephson.
About 30% of the patients did not have an event during their admissions to the unit, but for about 60%, neurologists were able to establish a diagnosis of epilepsy or epilepsy mimic. Video-EEG monitoring may be useful “in those patients in which there are diagnostic conundrums,” Dr. Josephson said. Establishing the correct diagnosis is important because even appropriate therapy entails additional risk in elderly patients.
—Jake Remaly
Suggested Reading
Brodie MJ, Kwan P. Epilepsy in elderly people. BMJ. 2005;331(7528):1317-1322.
Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002-2012.
Ferlazzo E, Gasparini S, Beghi E, et al. Epilepsy in cerebrovascular diseases: Review of experimental and clinical data with meta-analysis of risk factors. Epilepsia. 2016;57(8):1205-1214.
Josephson CB, Engbers JD, Sajobi TT, et al. Towards a clinically informed, data-driven definition of elderly onset epilepsy. Epilepsia. 2016;57(2):298-305.
McBride AE, Shih TT, Hirsch LJ. Video-EEG monitoring in the elderly: a review of 94 patients. Epilepsia. 2002; 43(2):165-169.
Morris Z, Whiteley WN, Longstreth WT Jr, et al. Incidental findings on brain magnetic resonanceimaging: systematic review and meta-analysis. BMJ. 2009; 339:b3016.
Subota A, Pham T, Jetté N, et al. The association between dementia and epilepsy: A systematic review and meta-analysis. Epilepsia. 2017;58(6):962-972.
Widdess-Walsh P, Sweeney BJ, Galvin R, McNamara B. Utilization and yield of EEG in the elderly population. J Clin Neurophysiol. 2005;22(4):253-255.
Recent Studies Elucidate the Advantages of the Newest AEDs
NAPLES, FL—The new antiepileptic drugs (AEDs) perampanel, eslicarbazepine, and brivaracetam have been the subjects of much research in recent years. Investigators have shed light on areas such as drug interactions, tolerability, and drug response in different patient populations, according to Bassel W. Abou-Khalil, MD, Professor of Neurology and Director of the Epilepsy Center at Vanderbilt University Medical Center in Nashville.
Perampanel
Perampanel is a noncompetitive AMPA glutamate receptor antagonist that was originally approved in 2012 as adjunctive therapy for patients with partial-onset seizures, said Dr. Abou-Khalil at the 45th Annual Meeting of the Southern Clinical Neurological Society. The drug was approved as adjunctive treatment for primary generalized tonic-clonic seizures in 2015. In 2017, the FDA extrapolated from the drug’s efficacy and safety as adjunctive therapy and granted it additional approval as monotherapy for partial-onset seizures, making it the first AED to be approved via this regulatory pathway.
Although perampanel does not appear to have significant interactions with other new AEDs, it has been shown that the 12-mg dose, but not the 8-mg dose, reduces levels of the oral contraceptive levonorgestrel by approximately 40%. Enzyme inducers such as phenytoin, carbamazepine, and phenobarbital decrease perampanel levels, Dr. Abou-Khalil said. Perampanel has a black box warning about aggression and hostility, particularly in patients taking the 12-mg dose. “Research suggests that severe psychiatric adverse effects are rare and appear to be more common in patients with intellectual disability,” Dr. Abou-Khalil said.
Two case reports have documented significant responses to adjunctive perampanel in patients with Lafora disease, a fatal autosomal-recessive genetic disorder. These patients had significantly reduced or remitted myoclonus and seizures and regained the ability to ambulate, Dr. Abou-Khalil said. Similarly, in a small study involving 10 patients with Lafora disease taking adjunctive perampanel, four had a 74% or greater reduction in seizures from baseline, and seven had major improvements in myoclonus. “In this study, there were no significant improvements in the progressive disability and cognitive decline that Lafora disease patients experience. However, it still is an exceptional drug for this very difficult type of epilepsy,” Dr. Abou-Khalil said.
In another study, perampanel also had significantly beneficial effects on myoclonus and conferred seizure freedom in 12 patients with Unverricht-Lundborg disease, a rare, inherited form of epilepsy that can interfere with walking and performance of everyday activities.
Eslicarbazepine Acetate
“Eslicarbazepine acetate, which has the same mechanism of action as oxcarbazepine, blocks sodium channels and stabilizes the inactive state of the voltage-gated sodium channel,” Dr. Abou-Khalil said. Oxcarbazepine is metabolized to both S-licarbazepine and the inactive R-licarbazepine and is detectible in serum as the parent compound, while eslicarbazepine acetate is rapidly converted primarily to S-licarbazepine and is generally undetectable in serum as the parent compound. “With eslicarbazepine, we are giving the patient more of the active metabolite of oxcarbazepine, while avoiding the adverse effects of the parent drug and the inactive element,” said Dr. Abou-Khalil.
Eslicarbazepine acetate was first approved in 2013 as add-on therapy, then in 2015 as monotherapy to treat partial-onset seizures in adults. In 2017, eslicarbazepine’s indication was expanded to include treatment of partial-onset seizures in children and adolescents age 4 and older.
Risk of common adverse effects, which include dizziness, somnolence, vomiting, blurred vision, and vertigo, are increased with concomitant use of other sodium-channel blockers, particularly carbamazepine. “However, the way the drug is titrated seems to be more important than the final dose,” Dr. Abou-Khalil said. One study demonstrated that the incidence of adverse events was lower in patients who initiated eslicarbazepine at 400 mg/day versus 800 mg/day. As much as 3% of patients taking 1,200 mg/day may develop rash.
Furthermore, research shows that elderly patients have a higher incidence of adverse effects than younger patients. “This suggests that eslicarbazepine acetate may not be an ideal drug in the older age group.”
Eslicarbazepine acetate also may have beneficial effects on lipid levels. In a retrospective cohort study of 36 adults with epilepsy taking eslicarbazepine acetate as add-on treatment, total and LDL cholesterol values were significantly decreased after at least six months of treatment, compared with the period before treatment (191.3 mg/dL vs 179.7 mg/dL, and 114.58 mg/dL vs 103.11mg/dL, respectively). HDL values were significantly increased (57.5 mg/dL vs 63.9 mg/dL before treatment).
Brivaracetam
Brivaracetam, approved in 2016 as adjunctive treatment for partial-onset seizures and in 2017 as monotherapy, binds to synaptic vesicle glycoprotein 2A with a 20-fold higher affinity than levetiracetam and higher brain permeability. “As far as drug interactions are concerned, enzyme inducers will reduce brivaracetam levels. In turn, brivaracetam may increase 10,11-carbamazepine epoxide and phenytoin concentrations,” Dr. Abou-Khalil explained.
Clinical studies have demonstrated that adjunctive brivaracetam at doses of 100 mg and 200 mg is more effective than placebo for all outcome measures, with 50% responder rates of 38.9% and 37.8%, respectively. “Yet there does not appear to be a clear dose–response effect,” Dr. Abou-Khalil said. “In addition, the efficacy of the 20-mg and 50-mg doses is inconsistent across studies, although in a pooled analysis, the 50-mg dose was more effective than placebo.”
Generally, brivaracetam does not appear to be effective when taken concomitantly with levetiracetam. In addition, brivaracetam’s efficacy is greater in levetiracetam-naïve patients than in patients who have failed levetiracetam, but that could be because the latter patients have greater drug resistance, he added.
“Switching to brivaracetam may be a consideration for patients who have seizure control with levetiracetam, but cannot tolerate it due to adverse effects,” Dr. Abou-Khalil said. In one study, patients who switched overnight from levetiracetam 1–3 g/day to brivaracetam 200 mg/day had clinically meaningful reductions in nonpsychotic behavioral adverse events. Health-related quality of life scores were improved, as well.
In an analysis of data from three phase III trials, adjunctive brivaracetam effectively reduced the frequency of generalized tonic-clonic seizures in 409 patients, with around one-third achieving seizure freedom at 100 mg and 200 mg per day. Another study showed that brivaracetam may be more effective in patients age 65 and older than in the general population of epilepsy patients, Dr. Abou-Khalil said. In that study, the median reduction from baseline in focal seizure frequency was 14.0% for placebo versus 25.5%, 49.6%, and 74.9% for brivaracetam 50 mg/day, 100 mg/day, and 200 mg/day, respectively.
—Adriene Marshall
NAPLES, FL—The new antiepileptic drugs (AEDs) perampanel, eslicarbazepine, and brivaracetam have been the subjects of much research in recent years. Investigators have shed light on areas such as drug interactions, tolerability, and drug response in different patient populations, according to Bassel W. Abou-Khalil, MD, Professor of Neurology and Director of the Epilepsy Center at Vanderbilt University Medical Center in Nashville.
Perampanel
Perampanel is a noncompetitive AMPA glutamate receptor antagonist that was originally approved in 2012 as adjunctive therapy for patients with partial-onset seizures, said Dr. Abou-Khalil at the 45th Annual Meeting of the Southern Clinical Neurological Society. The drug was approved as adjunctive treatment for primary generalized tonic-clonic seizures in 2015. In 2017, the FDA extrapolated from the drug’s efficacy and safety as adjunctive therapy and granted it additional approval as monotherapy for partial-onset seizures, making it the first AED to be approved via this regulatory pathway.
Although perampanel does not appear to have significant interactions with other new AEDs, it has been shown that the 12-mg dose, but not the 8-mg dose, reduces levels of the oral contraceptive levonorgestrel by approximately 40%. Enzyme inducers such as phenytoin, carbamazepine, and phenobarbital decrease perampanel levels, Dr. Abou-Khalil said. Perampanel has a black box warning about aggression and hostility, particularly in patients taking the 12-mg dose. “Research suggests that severe psychiatric adverse effects are rare and appear to be more common in patients with intellectual disability,” Dr. Abou-Khalil said.
Two case reports have documented significant responses to adjunctive perampanel in patients with Lafora disease, a fatal autosomal-recessive genetic disorder. These patients had significantly reduced or remitted myoclonus and seizures and regained the ability to ambulate, Dr. Abou-Khalil said. Similarly, in a small study involving 10 patients with Lafora disease taking adjunctive perampanel, four had a 74% or greater reduction in seizures from baseline, and seven had major improvements in myoclonus. “In this study, there were no significant improvements in the progressive disability and cognitive decline that Lafora disease patients experience. However, it still is an exceptional drug for this very difficult type of epilepsy,” Dr. Abou-Khalil said.
In another study, perampanel also had significantly beneficial effects on myoclonus and conferred seizure freedom in 12 patients with Unverricht-Lundborg disease, a rare, inherited form of epilepsy that can interfere with walking and performance of everyday activities.
Eslicarbazepine Acetate
“Eslicarbazepine acetate, which has the same mechanism of action as oxcarbazepine, blocks sodium channels and stabilizes the inactive state of the voltage-gated sodium channel,” Dr. Abou-Khalil said. Oxcarbazepine is metabolized to both S-licarbazepine and the inactive R-licarbazepine and is detectible in serum as the parent compound, while eslicarbazepine acetate is rapidly converted primarily to S-licarbazepine and is generally undetectable in serum as the parent compound. “With eslicarbazepine, we are giving the patient more of the active metabolite of oxcarbazepine, while avoiding the adverse effects of the parent drug and the inactive element,” said Dr. Abou-Khalil.
Eslicarbazepine acetate was first approved in 2013 as add-on therapy, then in 2015 as monotherapy to treat partial-onset seizures in adults. In 2017, eslicarbazepine’s indication was expanded to include treatment of partial-onset seizures in children and adolescents age 4 and older.
Risk of common adverse effects, which include dizziness, somnolence, vomiting, blurred vision, and vertigo, are increased with concomitant use of other sodium-channel blockers, particularly carbamazepine. “However, the way the drug is titrated seems to be more important than the final dose,” Dr. Abou-Khalil said. One study demonstrated that the incidence of adverse events was lower in patients who initiated eslicarbazepine at 400 mg/day versus 800 mg/day. As much as 3% of patients taking 1,200 mg/day may develop rash.
Furthermore, research shows that elderly patients have a higher incidence of adverse effects than younger patients. “This suggests that eslicarbazepine acetate may not be an ideal drug in the older age group.”
Eslicarbazepine acetate also may have beneficial effects on lipid levels. In a retrospective cohort study of 36 adults with epilepsy taking eslicarbazepine acetate as add-on treatment, total and LDL cholesterol values were significantly decreased after at least six months of treatment, compared with the period before treatment (191.3 mg/dL vs 179.7 mg/dL, and 114.58 mg/dL vs 103.11mg/dL, respectively). HDL values were significantly increased (57.5 mg/dL vs 63.9 mg/dL before treatment).
Brivaracetam
Brivaracetam, approved in 2016 as adjunctive treatment for partial-onset seizures and in 2017 as monotherapy, binds to synaptic vesicle glycoprotein 2A with a 20-fold higher affinity than levetiracetam and higher brain permeability. “As far as drug interactions are concerned, enzyme inducers will reduce brivaracetam levels. In turn, brivaracetam may increase 10,11-carbamazepine epoxide and phenytoin concentrations,” Dr. Abou-Khalil explained.
Clinical studies have demonstrated that adjunctive brivaracetam at doses of 100 mg and 200 mg is more effective than placebo for all outcome measures, with 50% responder rates of 38.9% and 37.8%, respectively. “Yet there does not appear to be a clear dose–response effect,” Dr. Abou-Khalil said. “In addition, the efficacy of the 20-mg and 50-mg doses is inconsistent across studies, although in a pooled analysis, the 50-mg dose was more effective than placebo.”
Generally, brivaracetam does not appear to be effective when taken concomitantly with levetiracetam. In addition, brivaracetam’s efficacy is greater in levetiracetam-naïve patients than in patients who have failed levetiracetam, but that could be because the latter patients have greater drug resistance, he added.
“Switching to brivaracetam may be a consideration for patients who have seizure control with levetiracetam, but cannot tolerate it due to adverse effects,” Dr. Abou-Khalil said. In one study, patients who switched overnight from levetiracetam 1–3 g/day to brivaracetam 200 mg/day had clinically meaningful reductions in nonpsychotic behavioral adverse events. Health-related quality of life scores were improved, as well.
In an analysis of data from three phase III trials, adjunctive brivaracetam effectively reduced the frequency of generalized tonic-clonic seizures in 409 patients, with around one-third achieving seizure freedom at 100 mg and 200 mg per day. Another study showed that brivaracetam may be more effective in patients age 65 and older than in the general population of epilepsy patients, Dr. Abou-Khalil said. In that study, the median reduction from baseline in focal seizure frequency was 14.0% for placebo versus 25.5%, 49.6%, and 74.9% for brivaracetam 50 mg/day, 100 mg/day, and 200 mg/day, respectively.
—Adriene Marshall
NAPLES, FL—The new antiepileptic drugs (AEDs) perampanel, eslicarbazepine, and brivaracetam have been the subjects of much research in recent years. Investigators have shed light on areas such as drug interactions, tolerability, and drug response in different patient populations, according to Bassel W. Abou-Khalil, MD, Professor of Neurology and Director of the Epilepsy Center at Vanderbilt University Medical Center in Nashville.
Perampanel
Perampanel is a noncompetitive AMPA glutamate receptor antagonist that was originally approved in 2012 as adjunctive therapy for patients with partial-onset seizures, said Dr. Abou-Khalil at the 45th Annual Meeting of the Southern Clinical Neurological Society. The drug was approved as adjunctive treatment for primary generalized tonic-clonic seizures in 2015. In 2017, the FDA extrapolated from the drug’s efficacy and safety as adjunctive therapy and granted it additional approval as monotherapy for partial-onset seizures, making it the first AED to be approved via this regulatory pathway.
Although perampanel does not appear to have significant interactions with other new AEDs, it has been shown that the 12-mg dose, but not the 8-mg dose, reduces levels of the oral contraceptive levonorgestrel by approximately 40%. Enzyme inducers such as phenytoin, carbamazepine, and phenobarbital decrease perampanel levels, Dr. Abou-Khalil said. Perampanel has a black box warning about aggression and hostility, particularly in patients taking the 12-mg dose. “Research suggests that severe psychiatric adverse effects are rare and appear to be more common in patients with intellectual disability,” Dr. Abou-Khalil said.
Two case reports have documented significant responses to adjunctive perampanel in patients with Lafora disease, a fatal autosomal-recessive genetic disorder. These patients had significantly reduced or remitted myoclonus and seizures and regained the ability to ambulate, Dr. Abou-Khalil said. Similarly, in a small study involving 10 patients with Lafora disease taking adjunctive perampanel, four had a 74% or greater reduction in seizures from baseline, and seven had major improvements in myoclonus. “In this study, there were no significant improvements in the progressive disability and cognitive decline that Lafora disease patients experience. However, it still is an exceptional drug for this very difficult type of epilepsy,” Dr. Abou-Khalil said.
In another study, perampanel also had significantly beneficial effects on myoclonus and conferred seizure freedom in 12 patients with Unverricht-Lundborg disease, a rare, inherited form of epilepsy that can interfere with walking and performance of everyday activities.
Eslicarbazepine Acetate
“Eslicarbazepine acetate, which has the same mechanism of action as oxcarbazepine, blocks sodium channels and stabilizes the inactive state of the voltage-gated sodium channel,” Dr. Abou-Khalil said. Oxcarbazepine is metabolized to both S-licarbazepine and the inactive R-licarbazepine and is detectible in serum as the parent compound, while eslicarbazepine acetate is rapidly converted primarily to S-licarbazepine and is generally undetectable in serum as the parent compound. “With eslicarbazepine, we are giving the patient more of the active metabolite of oxcarbazepine, while avoiding the adverse effects of the parent drug and the inactive element,” said Dr. Abou-Khalil.
Eslicarbazepine acetate was first approved in 2013 as add-on therapy, then in 2015 as monotherapy to treat partial-onset seizures in adults. In 2017, eslicarbazepine’s indication was expanded to include treatment of partial-onset seizures in children and adolescents age 4 and older.
Risk of common adverse effects, which include dizziness, somnolence, vomiting, blurred vision, and vertigo, are increased with concomitant use of other sodium-channel blockers, particularly carbamazepine. “However, the way the drug is titrated seems to be more important than the final dose,” Dr. Abou-Khalil said. One study demonstrated that the incidence of adverse events was lower in patients who initiated eslicarbazepine at 400 mg/day versus 800 mg/day. As much as 3% of patients taking 1,200 mg/day may develop rash.
Furthermore, research shows that elderly patients have a higher incidence of adverse effects than younger patients. “This suggests that eslicarbazepine acetate may not be an ideal drug in the older age group.”
Eslicarbazepine acetate also may have beneficial effects on lipid levels. In a retrospective cohort study of 36 adults with epilepsy taking eslicarbazepine acetate as add-on treatment, total and LDL cholesterol values were significantly decreased after at least six months of treatment, compared with the period before treatment (191.3 mg/dL vs 179.7 mg/dL, and 114.58 mg/dL vs 103.11mg/dL, respectively). HDL values were significantly increased (57.5 mg/dL vs 63.9 mg/dL before treatment).
Brivaracetam
Brivaracetam, approved in 2016 as adjunctive treatment for partial-onset seizures and in 2017 as monotherapy, binds to synaptic vesicle glycoprotein 2A with a 20-fold higher affinity than levetiracetam and higher brain permeability. “As far as drug interactions are concerned, enzyme inducers will reduce brivaracetam levels. In turn, brivaracetam may increase 10,11-carbamazepine epoxide and phenytoin concentrations,” Dr. Abou-Khalil explained.
Clinical studies have demonstrated that adjunctive brivaracetam at doses of 100 mg and 200 mg is more effective than placebo for all outcome measures, with 50% responder rates of 38.9% and 37.8%, respectively. “Yet there does not appear to be a clear dose–response effect,” Dr. Abou-Khalil said. “In addition, the efficacy of the 20-mg and 50-mg doses is inconsistent across studies, although in a pooled analysis, the 50-mg dose was more effective than placebo.”
Generally, brivaracetam does not appear to be effective when taken concomitantly with levetiracetam. In addition, brivaracetam’s efficacy is greater in levetiracetam-naïve patients than in patients who have failed levetiracetam, but that could be because the latter patients have greater drug resistance, he added.
“Switching to brivaracetam may be a consideration for patients who have seizure control with levetiracetam, but cannot tolerate it due to adverse effects,” Dr. Abou-Khalil said. In one study, patients who switched overnight from levetiracetam 1–3 g/day to brivaracetam 200 mg/day had clinically meaningful reductions in nonpsychotic behavioral adverse events. Health-related quality of life scores were improved, as well.
In an analysis of data from three phase III trials, adjunctive brivaracetam effectively reduced the frequency of generalized tonic-clonic seizures in 409 patients, with around one-third achieving seizure freedom at 100 mg and 200 mg per day. Another study showed that brivaracetam may be more effective in patients age 65 and older than in the general population of epilepsy patients, Dr. Abou-Khalil said. In that study, the median reduction from baseline in focal seizure frequency was 14.0% for placebo versus 25.5%, 49.6%, and 74.9% for brivaracetam 50 mg/day, 100 mg/day, and 200 mg/day, respectively.
—Adriene Marshall