User login
Treating psychosis in patients with HIV/AIDS
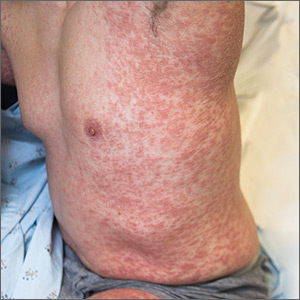
Mr. S, age 56, has human immunodeficiency virus (HIV) and schizoaffective disorder. He presents to your clinic with increased auditory hallucinations, disorganized behavior, and worsened tremors that have begun to seriously disrupt his daily life. Mr. S is prescribed risperidone; however, he reports that he has not been taking it lately due to the tremor despite being controlled on his medication regimen for nearly 1 year. His Abnormal Involuntary Movement Scale (AIMS) score reveals an increased wrist rigidity compared with previous clinic visits. Mr. S has a 40 pack-year history of smoking and history of IV drug use. Furthermore, he has a medical history of type 2 diabetes mellitus, hypertension, and hyperlipidemia.
His medication regimen includes atazanavir sulfate, 200 mg/d, ritonavir, 100 mg/d, efavirenz/emtricitabine/tenofovir disoproxil fumarate, 600/200/300 mg/d, risperidone, 6 mg/d, bupropion extended-release, 300 mg/d, gabapentin, 600 mg/d, amlodipine, 5 mg/d, pravastatin, 40 mg/d, metformin, 1000 mg twice daily, and glipizide, 10 mg twice daily. Today, his laboratory findings show that his CD4 count is 405 cell/mm3, and his viral load is <40 copies/mL, indicating his HIV is well managed. A hepatitis C virus antibody test result is negative and serum creatinine level is 1.0 mg/dL. Total cholesterol is 212 mg/dL, high-density lipoprotein cholesterol is 43 mg/dL, low-density lipoprotein cholesterol is 121 mg/dL, and triglycerides level is 238 mg/dL. Electrocardiography reveals a QTc interval of 426 ms. Mr. S’s blood pressure is 105/65 mm Hg. Based on this clinic visit, the treatment team decides to change Mr. S’s antipsychotic.
Psychiatric illness and HIV/AIDS
There is a strong link between mental illness and HIV/AIDS; 50% or more of patients with HIV/AIDS have a comorbid psychiatric disorder.1 The prevalence of mental illness in patients with HIV/AIDS is reported to be 8 times higher than in those without HIV/AIDS.2 Depression, bipolar disorder, anxiety disorders, delirium, substance abuse, and schizophrenia have all been identified in persons receiving highly active antiretroviral therapy (HAART). Patients with HIV/AIDS and psychiatric illness have a decreased quality of life, poor adherence to medications, faster disease progression, and increased mortality. Care of these individuals is complicated by the stigma of HIV/AIDS and the prevalence of the illness in underserved populations, as well as the need for complex medication regimens and the possibility of drug–drug interactions (DDIs).1,2 If left untreated, psychiatric illness in patients with HIV/AIDS may lead to further transmission of HIV as a result of patients engaging in high-risk behaviors, along with poor adherence to HAART.3
Individuals diagnosed with schizophrenia, schizoaffective disorder, and bipolar disorder are at greater risk for HIV infection.3 Patients with HIV/AIDS with primary psychosis may have poor medication adherence rates due to illness-related confusion or paranoia about medications. Furthermore, they may lack the resources to manage the complications and stress related to living with HIV/AIDS.
New-onset, or secondary psychosis, has been reported in individuals with late-stage HIV/AIDS with CD4 counts <200 who have not been diagnosed with a psychotic disorder previously.3 These patients may experience more persecutory and grandiose delusions rather than hallucinations. Neuropsychiatric symptoms in patients with HIV/AIDS may be due to the presence of HIV or other infections in the CNS, tumors, or other inflammatory illnesses. Medications that have been implicated in neuropsychiatric symptoms include efavirenz, rilpivirine, and other HAART regimens; interferon; metoclopramide; corticosteroids; muscle relaxants; and clonidine. It is possible that symptoms may continue even after the medications are discontinued.3
Antipsychotics remain the treatment of choice for psychosis in HIV/AIDS, regardless of the cause of the symptoms. Many factors must be taken into consideration when choosing an antipsychotic, such as DDIs, adverse effect profiles, patient history of antipsychotic use, cost, and patient preference. Here we focus primarily on DDIs and adverse effect profiles.
When treating psychosis in patients with HIV/AIDS, it is crucial to consider potential DDIs. Many antipsychotics and antiretroviral medications utilize cytochrome P450 (CYP) enzymes for their metabolism. The CYP enzyme system is responsible for the oxidative reactions that constitute the phase I reactions necessary for the metabolism of most drugs. Inhibition and induction of CYP enzymes are among the most common causes of pharmacokinetic DDIs. Antipsychotics are predominately metabolized by CYP3A4, CYP1A2, and CYP2D6.4
Continue to: The DDIs arise because...
The DDIs arise because many antiretroviral medications inhibit, or in some cases, induce, these CYP enzymes, thereby altering substrate-drug metabolism. Inhibiting a CYP enzyme pathway can decrease substrate-drug clearance and lead to increased levels of that drug. This, in turn, can cause an increased risk of adverse effects, such as extrapyramidal symptoms (EPS) or QTc prolongation, which are both types of pharmacodynamic DDIs.4-28 However, because antipsychotics often have more than one pathway of metabolism, it can be challenging to understand the full effect of CYP-related DDIs. Furthermore, CYP enzyme inducers can decrease drug levels, and in the case of antipsychotics, lead to subtherapeutic responses.
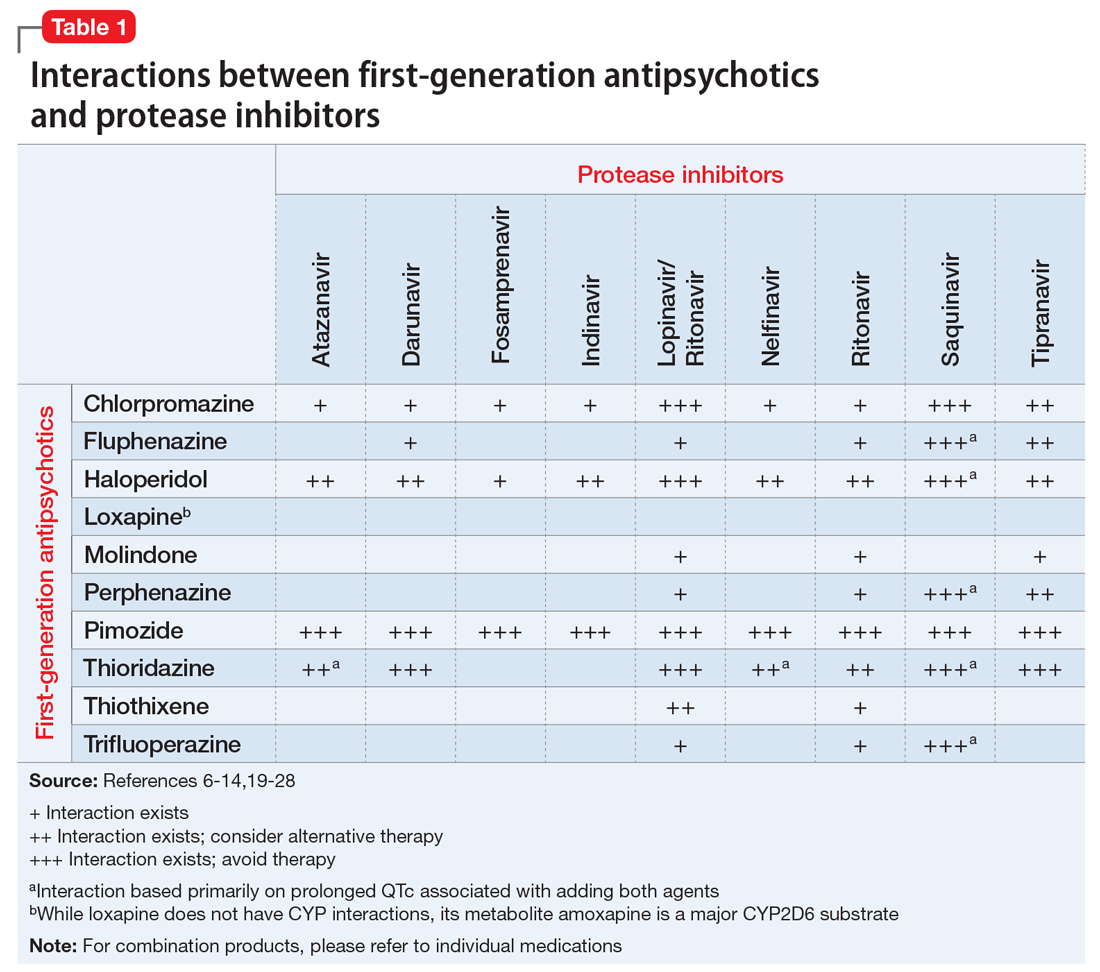
Table 1,6-14,19-28 Table 2,15-28 Table 3,6-14,19-28 and Table 415-28 list many of the known CYP enzyme-related DDIs that may occur with combination antipsychotic and antiretroviral medication therapy and aim to predict CYP induction or inhibition based on a particular combination. The following antiretroviral medications do not have any CYP-related interactions and therefore are not included in the Tables: abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir disoproxil, zidovudine, enfuvirtide, maraviroc, and raltegravir.
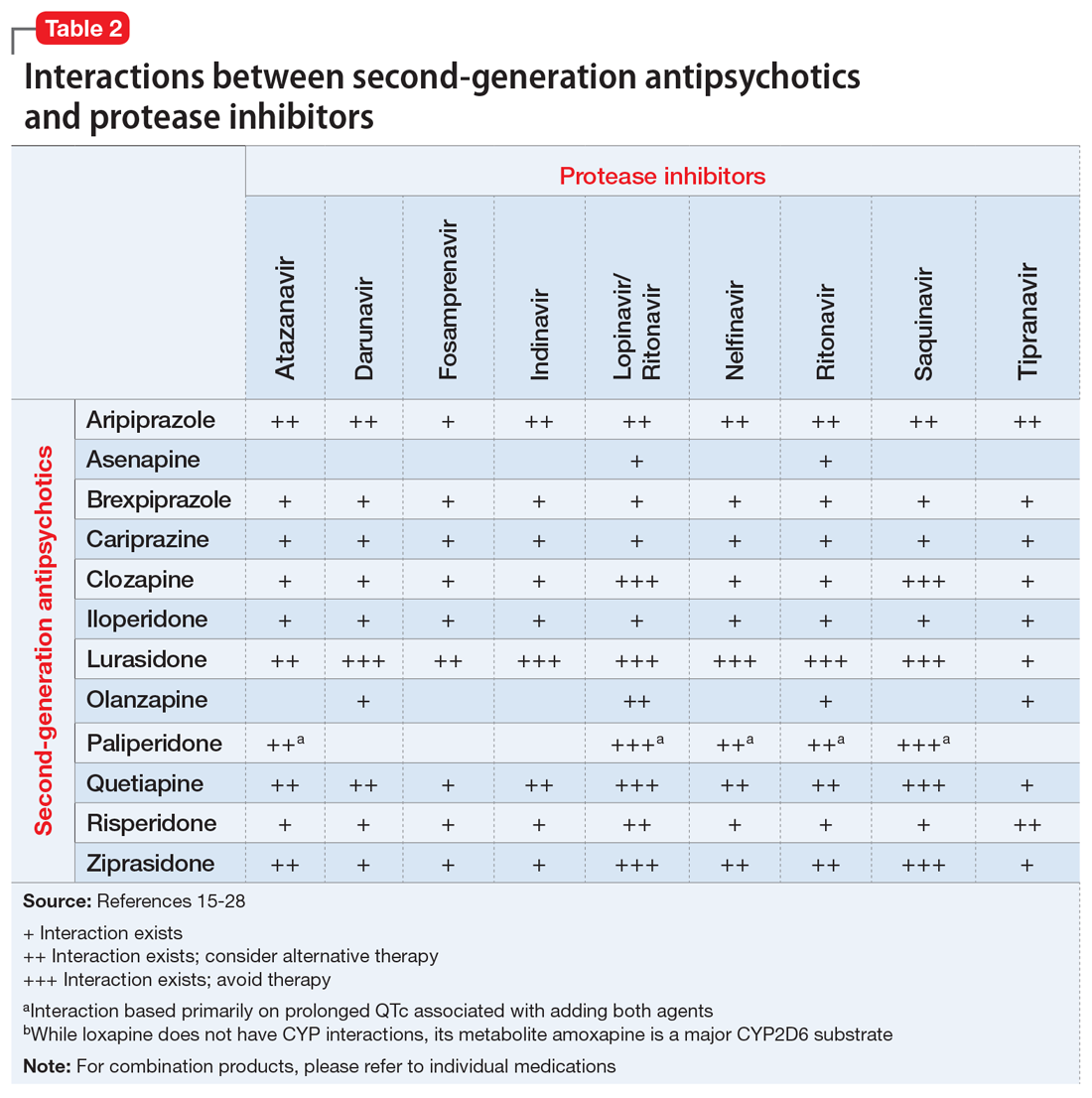
These Tables include the risk ratings for all D-rated (consider alternative therapy) and X-rated (avoid therapy) combinations. The majority of D-rated interactions are caused by CYP inhibition or induction that could potentially lead to altered antipsychotic levels. The majority of X-rated interactions are caused by increased QTc prolongation that may or may not be due to CYP-related DDIs. For example, paliperidone is not believed to be affected by the CYP enzyme system, but it does present a high risk of QTc prolongation on its own. When combined with an antiretroviral that also has a high risk of QTc prolongation, such as lopinavir, then the risk further increases.
Non-nucleoside reverse transcriptase inhibitors and protease inhibitors (PIs) are the antiretroviral medications most likely to cause DDIs with antipsychotics. Other antiretroviral classes, such as nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), fusion inhibitors, chemokine receptor 5 inhibitors, and integrase inhibitors, are not associated with CYP-related DDIs.19-28 For the most part, the severity of the CYP-related DDIs have not been well studied; therefore, most recommendations call for closer patient monitoring when combining antiretroviral medications and antipsychotics.6-18 The goal is to monitor for any changes in medication efficacy or adverse effects.
Continue to: Consider adverse effect profiles
Consider adverse effect profiles
When selecting an antipsychotic agent for a patient receiving HIV therapy, also consider adverse effect profiles. The emergence of adverse effects can greatly impact patients’ quality of life, leading to consequences of medication nonadherence and exacerbation of mental illness.
Extrapyramidal symptoms. Patients with HIV have a higher sensitivity to treatment-emergent EPS from antipsychotics.2 This sensitivity is generally thought to arise from the involvement of HIV on the basal ganglia. Historically, psychotic symptoms in HIV have been managed with second-generation antipsychotics (SGAs) at the lowest effective dose because these medications are less likely to cause EPS.1,29 The antipsychotic with the lowest rate of EPS is clozapine, followed by quetiapine, olanzapine, ziprasidone, and aripiprazole. Conversely, high-potency first-generation antipsychotics (FGAs) have the highest rates of EPS, followed by intermediate-potency FGAs and risperidone.30
Metabolic disturbances are another concern with concomitant antipsychotic/antiretroviral therapy. Patients with HIV who are receiving NRTIs or PIs can present with drug-induced lipodystrophy syndrome, which is associated with hyperglycemia, hyperinsulinemia, hyperlipidemia, and hypertension, and ultimately may cause metabolic syndrome.29 The prevalence of metabolic syndrome in patients receiving PI therapy has a vast range—2% to 84%—which can be attributed to inconsistent definitions, criteria, and assessment methodology.29 Use of a PI is considered to be the most prominent risk factor for developing lipodystrophy.29 Among the PIs, metabolic disturbances in regards to lipids are most often seen with lopinavir/ritonavir (LPV/r), saquinavir/ritonavir, tipranavir/ritonavir, and fosamprenavir/ritonavir.31 In comparison with LPV/r, darunavir showed improvement in lipids.32 Atazanavir (ATV) boosted with ritonavir has not shown clinically significant adverse effects on lipids.31 Additionally, amprenavir, LPV/r, and ritonavir demonstrated more glucose uptake inhibition via blockade of the glucose transporter type 4 than ATV.31 Of the NRTIs, lipodystrophy syndrome is most commonly seen with stavudine, which is used minimally in practice.2
The rates of metabolic disturbance with antipsychotic use range from 2% to 36%.2 The American Psychiatric Association recommends selecting one of the SGAs least likely to affect metabolic parameters.29 Aripiprazole and ziprasidone are associated with the lowest risk of weight gain, hyperglycemia, and hyperlipidemia. They are followed by risperidone and quetiapine, which are associated with moderate risk, and then clozapine and olanzapine, which are associated with high risk.2,30,33
Continue to: Management of metabolic adverse effects involves...
Management of metabolic adverse effects involves switching the antiretroviral agent and/or antipsychotic agent to an alternative associated with lower metabolic risk. Antipsychotics with low metabolic risk include aripiprazole, lurasidone, and ziprasidone. Lifestyle modifications are encouraged. Additionally, medication interventions, such as metformin, are also recommended in patients meeting criteria for pre-diabetes or type 2 diabetes mellitus.2 Lipid panels and metabolic parameters should be monitored periodically, according to guidelines.25,34
Bone marrow toxicity and blood dyscrasias. Lastly, consider the risk of bone marrow suppression. Patients receiving clozapine for treatment-resistant schizophrenia should be closely monitored for neutropenia and agranulocytosis. Although zidovudine is rarely used, its use is associated with adverse myelosuppressive effects, and the combination of clozapine and zidovudine could pose danger to the patient.2,35,36
CASE CONTINUED
Because Mr. S’s diagnosis of HIV puts him at a higher risk of developing EPS, and because he is already experiencing increased wrist rigidity, the treatment team decides to switch his antipsychotic therapy to an agent with a lower risk of EPS. His comorbidities, including type 2 diabetes mellitus, hypertension, and hyperlipidemia, are taken into account, and an SGA with a benign metabolic profile is considered. Aripiprazole and ziprasidone are favorable options. However, because efavirenz, ATZ, and ritonavir may cause QTc prolongation, ziprasidone, the SGA with the highest rate of QTc prolongation, is not the preferred option.
Mr. S’s SGA therapy is switched from risperidone to aripiprazole. Because potential CYP-related interactions between aripiprazole and Mr. S’s current antiretroviral therapy could lead to increased aripiprazole levels. Mr. S is started on a low dose (5 mg/d) with the goal to titrate based on response and tolerability. Increased levels of aripiprazole may increase the risk of akathisia, drowsiness, headaches, and fatigue. Mr. S is monitored closely for improvement of EPS, adverse effects of medication, and metabolic parameters. Furthermore, if the treatment team believes there is a more preferred antipsychotic for the patient that it did not prescribe because of the risk of DDIs, it may be worthwhile to consider discussing the HAART regimen with the patient’s infectious disease treatment team.
Continue to: Acknowledgements
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the Chillicothe Veterans Affairs Medical Center in Chillicothe, Ohio. The contents of this paper do not represent the views of the U.S. Department of Veterans Affairs or the U.S. government.
Related Resources
- Cohen MA. HIV: How to provide compassionate care. Current Psychiatry. 2013;12(6):19-23,A,B.
- Khan AY, Zaidi SN. Reducing morbidity and mortality from common medical conditions in schizophrenia. Current Psychiatry. 2016;15(3):30-32,34-38,40.
Drug Brand Names
Abacavir • Ziagen
Amlodipine • Norvasc
Amprenavir • Agenerase
Aripiprazole • Abilify
Asenapine • Saphris
Atazanavir • Reyataz
Brexpiprazole • Rexulti
Bupropion ER • Wellbutrin SR
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Darunavir • Prezista
Delavirdine • Rescriptor
Didanosine • Videx EC
Efavirenz • Sustiva
Efavirenz/emtricitabine/tenofovir disoproxil fumarate • Atripla
Enfuvirtide • Fuzeon
Emtricitabine • Emtriva
Etravirine • Intelence
Fluphenazine • Prolixin
Fosamprenavir • Lexiva
Gabapentin • Neurontin
Glipizide • Glucotrol
Haloperidol • Haldol
Iloperidone • Fanapt
Indinavir • Crixivan
Lamivudine • Epivir
Lopinavir/ritonavir • Kaletra
Loxapine • Loxitane
Lurasidone • Latuda
Maraviroc • Selzentry
Metformin • Glucophage
Metoclopramide • Reglan
Molindone • Moban
Nelfinavir • Viracept
Nevirapine • Viramune
Olanzapine • Zyprexa
Paliperidone • Invega
Perphenazine • Trilafon
Pimozide • Orap
Pravastatin • Pravachol
Quetiapine • Seroquel
Raltegravir • Isentress
Rilpivirine • Edurant
Risperidone • Risperdal
Ritonavir • Norvir
Saquinavir • Invirase
Stavudine • Zerit
Tenofovir disoproxil • Viread
Thioridazine • Mellaril
Thiothixene • Navane
Tipranavir • Aptivus
Trifluoperazine • Stelazine
Zidovudine • Retrovir
Ziprasidone • Geodon
1. Freudenreich O, Goforth HW, Cozza KL, et al. Psychiatric treatment of persons with HIV/AIDS: An HIV-psychiatry consensus survey of current practices. Psychosomatics. 2010;51(6):480-488.
2. Hill L, Lee KC. Pharmacotherapy considerations in patients with HIV and psychiatric disorders: Focus on antidepressants and antipsychotics. Ann Pharmacother. 2013;47(1):75-89.
3. Watkins CC, Treisman GJ. Neuropsychiatric complications of aging with HIV. J Neurovirol. 2012;18(4):277-290.
4. Prior TI, Baker GB. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci. 2003;28(2):99-112.
5. Ponte ML, Keller GA, Di Girolamo G. Mechanisms of drug induced QT interval prolongation. Curr Drug Saf. 2010;5(1):44-53
6. Reyataz [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2017.
7. Prezista [package insert]. Toronto, ON: Janssen Inc.; 2017.
8. Lexiva [package insert]. Research Triangle Park, NC: Viiv Healthcare; 2017
9. Crixivan [package insert]. Whitehouse Station, NJ; Merck; 2016.
10. Kaletra [package insert]. North Chicago, IL: AbbVie Inc; 2017
11. Viracept [package insert]. Kirkland, QC: Pfizer Canada Inc.; 201
12. Norvir tablets and oral solution [package insert]. North Chicago, IL: AbbVie Inc; 2017
13. Invirase [package insert]. South San Francisco, CA: Genentech USA, Inc.; 2016.
14. Aptivus [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc.; 2016.
15. Sustiva [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2017
16. Intelence [package insert]. Titusville, NJ: Tibotec Pharmaceuticals; 2014.
17. Viramune [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc.; 2017.
18. Rescriptor [package insert]. Laval, QC: ViiV Healthcare ULC; 2013.
19. Ziagen [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
20. Videx EC [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2015.
21. Emtriva [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2017.
22. Epivir [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2017.
23. Zerit [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2017.
24. Viread [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2017.
25. Retrovir [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2015.
26. Fuzeon [package insert]. South San Francisco, CA: Genentech USA, Inc; 2017.
27. Selzentry [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2016.
28. Isentress [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2017.
29. American Psychiatry Association. Practice guidelines for treatment of patients with HIV/AIDS. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/hivaids.pdf. Published 2010. Accessed March 1, 2018.
30. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 Schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
31. Hughes PJ, Cretton-Scott E, Teague A, et al. Protease inhibitors for patients with HIV-1 infection. P T. 2011;36(6):332-336,341-345.
32. Ortiz R, Dejesus E, Khanlou H, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS. 2008;22(12):1389-1397.
33. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962.
34. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013;12(9):51-54.
35. Singh D, Goodkin K. Choice of antipsychotic in HIV-infected patients. J Clin Psychiatry. 2007;68(3):479-480.
36. Max B, Sherer R. Management of the adverse effects of antiretroviral therapy and medication adherence. Clin Infect Dis. 2000;30(suppl 2):S96-S116.
Mr. S, age 56, has human immunodeficiency virus (HIV) and schizoaffective disorder. He presents to your clinic with increased auditory hallucinations, disorganized behavior, and worsened tremors that have begun to seriously disrupt his daily life. Mr. S is prescribed risperidone; however, he reports that he has not been taking it lately due to the tremor despite being controlled on his medication regimen for nearly 1 year. His Abnormal Involuntary Movement Scale (AIMS) score reveals an increased wrist rigidity compared with previous clinic visits. Mr. S has a 40 pack-year history of smoking and history of IV drug use. Furthermore, he has a medical history of type 2 diabetes mellitus, hypertension, and hyperlipidemia.
His medication regimen includes atazanavir sulfate, 200 mg/d, ritonavir, 100 mg/d, efavirenz/emtricitabine/tenofovir disoproxil fumarate, 600/200/300 mg/d, risperidone, 6 mg/d, bupropion extended-release, 300 mg/d, gabapentin, 600 mg/d, amlodipine, 5 mg/d, pravastatin, 40 mg/d, metformin, 1000 mg twice daily, and glipizide, 10 mg twice daily. Today, his laboratory findings show that his CD4 count is 405 cell/mm3, and his viral load is <40 copies/mL, indicating his HIV is well managed. A hepatitis C virus antibody test result is negative and serum creatinine level is 1.0 mg/dL. Total cholesterol is 212 mg/dL, high-density lipoprotein cholesterol is 43 mg/dL, low-density lipoprotein cholesterol is 121 mg/dL, and triglycerides level is 238 mg/dL. Electrocardiography reveals a QTc interval of 426 ms. Mr. S’s blood pressure is 105/65 mm Hg. Based on this clinic visit, the treatment team decides to change Mr. S’s antipsychotic.
Psychiatric illness and HIV/AIDS
There is a strong link between mental illness and HIV/AIDS; 50% or more of patients with HIV/AIDS have a comorbid psychiatric disorder.1 The prevalence of mental illness in patients with HIV/AIDS is reported to be 8 times higher than in those without HIV/AIDS.2 Depression, bipolar disorder, anxiety disorders, delirium, substance abuse, and schizophrenia have all been identified in persons receiving highly active antiretroviral therapy (HAART). Patients with HIV/AIDS and psychiatric illness have a decreased quality of life, poor adherence to medications, faster disease progression, and increased mortality. Care of these individuals is complicated by the stigma of HIV/AIDS and the prevalence of the illness in underserved populations, as well as the need for complex medication regimens and the possibility of drug–drug interactions (DDIs).1,2 If left untreated, psychiatric illness in patients with HIV/AIDS may lead to further transmission of HIV as a result of patients engaging in high-risk behaviors, along with poor adherence to HAART.3
Individuals diagnosed with schizophrenia, schizoaffective disorder, and bipolar disorder are at greater risk for HIV infection.3 Patients with HIV/AIDS with primary psychosis may have poor medication adherence rates due to illness-related confusion or paranoia about medications. Furthermore, they may lack the resources to manage the complications and stress related to living with HIV/AIDS.
New-onset, or secondary psychosis, has been reported in individuals with late-stage HIV/AIDS with CD4 counts <200 who have not been diagnosed with a psychotic disorder previously.3 These patients may experience more persecutory and grandiose delusions rather than hallucinations. Neuropsychiatric symptoms in patients with HIV/AIDS may be due to the presence of HIV or other infections in the CNS, tumors, or other inflammatory illnesses. Medications that have been implicated in neuropsychiatric symptoms include efavirenz, rilpivirine, and other HAART regimens; interferon; metoclopramide; corticosteroids; muscle relaxants; and clonidine. It is possible that symptoms may continue even after the medications are discontinued.3
Antipsychotics remain the treatment of choice for psychosis in HIV/AIDS, regardless of the cause of the symptoms. Many factors must be taken into consideration when choosing an antipsychotic, such as DDIs, adverse effect profiles, patient history of antipsychotic use, cost, and patient preference. Here we focus primarily on DDIs and adverse effect profiles.
When treating psychosis in patients with HIV/AIDS, it is crucial to consider potential DDIs. Many antipsychotics and antiretroviral medications utilize cytochrome P450 (CYP) enzymes for their metabolism. The CYP enzyme system is responsible for the oxidative reactions that constitute the phase I reactions necessary for the metabolism of most drugs. Inhibition and induction of CYP enzymes are among the most common causes of pharmacokinetic DDIs. Antipsychotics are predominately metabolized by CYP3A4, CYP1A2, and CYP2D6.4
Continue to: The DDIs arise because...
The DDIs arise because many antiretroviral medications inhibit, or in some cases, induce, these CYP enzymes, thereby altering substrate-drug metabolism. Inhibiting a CYP enzyme pathway can decrease substrate-drug clearance and lead to increased levels of that drug. This, in turn, can cause an increased risk of adverse effects, such as extrapyramidal symptoms (EPS) or QTc prolongation, which are both types of pharmacodynamic DDIs.4-28 However, because antipsychotics often have more than one pathway of metabolism, it can be challenging to understand the full effect of CYP-related DDIs. Furthermore, CYP enzyme inducers can decrease drug levels, and in the case of antipsychotics, lead to subtherapeutic responses.

Table 1,6-14,19-28 Table 2,15-28 Table 3,6-14,19-28 and Table 415-28 list many of the known CYP enzyme-related DDIs that may occur with combination antipsychotic and antiretroviral medication therapy and aim to predict CYP induction or inhibition based on a particular combination. The following antiretroviral medications do not have any CYP-related interactions and therefore are not included in the Tables: abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir disoproxil, zidovudine, enfuvirtide, maraviroc, and raltegravir.

These Tables include the risk ratings for all D-rated (consider alternative therapy) and X-rated (avoid therapy) combinations. The majority of D-rated interactions are caused by CYP inhibition or induction that could potentially lead to altered antipsychotic levels. The majority of X-rated interactions are caused by increased QTc prolongation that may or may not be due to CYP-related DDIs. For example, paliperidone is not believed to be affected by the CYP enzyme system, but it does present a high risk of QTc prolongation on its own. When combined with an antiretroviral that also has a high risk of QTc prolongation, such as lopinavir, then the risk further increases.
Non-nucleoside reverse transcriptase inhibitors and protease inhibitors (PIs) are the antiretroviral medications most likely to cause DDIs with antipsychotics. Other antiretroviral classes, such as nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), fusion inhibitors, chemokine receptor 5 inhibitors, and integrase inhibitors, are not associated with CYP-related DDIs.19-28 For the most part, the severity of the CYP-related DDIs have not been well studied; therefore, most recommendations call for closer patient monitoring when combining antiretroviral medications and antipsychotics.6-18 The goal is to monitor for any changes in medication efficacy or adverse effects.
Continue to: Consider adverse effect profiles
Consider adverse effect profiles
When selecting an antipsychotic agent for a patient receiving HIV therapy, also consider adverse effect profiles. The emergence of adverse effects can greatly impact patients’ quality of life, leading to consequences of medication nonadherence and exacerbation of mental illness.
Extrapyramidal symptoms. Patients with HIV have a higher sensitivity to treatment-emergent EPS from antipsychotics.2 This sensitivity is generally thought to arise from the involvement of HIV on the basal ganglia. Historically, psychotic symptoms in HIV have been managed with second-generation antipsychotics (SGAs) at the lowest effective dose because these medications are less likely to cause EPS.1,29 The antipsychotic with the lowest rate of EPS is clozapine, followed by quetiapine, olanzapine, ziprasidone, and aripiprazole. Conversely, high-potency first-generation antipsychotics (FGAs) have the highest rates of EPS, followed by intermediate-potency FGAs and risperidone.30
Metabolic disturbances are another concern with concomitant antipsychotic/antiretroviral therapy. Patients with HIV who are receiving NRTIs or PIs can present with drug-induced lipodystrophy syndrome, which is associated with hyperglycemia, hyperinsulinemia, hyperlipidemia, and hypertension, and ultimately may cause metabolic syndrome.29 The prevalence of metabolic syndrome in patients receiving PI therapy has a vast range—2% to 84%—which can be attributed to inconsistent definitions, criteria, and assessment methodology.29 Use of a PI is considered to be the most prominent risk factor for developing lipodystrophy.29 Among the PIs, metabolic disturbances in regards to lipids are most often seen with lopinavir/ritonavir (LPV/r), saquinavir/ritonavir, tipranavir/ritonavir, and fosamprenavir/ritonavir.31 In comparison with LPV/r, darunavir showed improvement in lipids.32 Atazanavir (ATV) boosted with ritonavir has not shown clinically significant adverse effects on lipids.31 Additionally, amprenavir, LPV/r, and ritonavir demonstrated more glucose uptake inhibition via blockade of the glucose transporter type 4 than ATV.31 Of the NRTIs, lipodystrophy syndrome is most commonly seen with stavudine, which is used minimally in practice.2
The rates of metabolic disturbance with antipsychotic use range from 2% to 36%.2 The American Psychiatric Association recommends selecting one of the SGAs least likely to affect metabolic parameters.29 Aripiprazole and ziprasidone are associated with the lowest risk of weight gain, hyperglycemia, and hyperlipidemia. They are followed by risperidone and quetiapine, which are associated with moderate risk, and then clozapine and olanzapine, which are associated with high risk.2,30,33
Continue to: Management of metabolic adverse effects involves...
Management of metabolic adverse effects involves switching the antiretroviral agent and/or antipsychotic agent to an alternative associated with lower metabolic risk. Antipsychotics with low metabolic risk include aripiprazole, lurasidone, and ziprasidone. Lifestyle modifications are encouraged. Additionally, medication interventions, such as metformin, are also recommended in patients meeting criteria for pre-diabetes or type 2 diabetes mellitus.2 Lipid panels and metabolic parameters should be monitored periodically, according to guidelines.25,34
Bone marrow toxicity and blood dyscrasias. Lastly, consider the risk of bone marrow suppression. Patients receiving clozapine for treatment-resistant schizophrenia should be closely monitored for neutropenia and agranulocytosis. Although zidovudine is rarely used, its use is associated with adverse myelosuppressive effects, and the combination of clozapine and zidovudine could pose danger to the patient.2,35,36
CASE CONTINUED
Because Mr. S’s diagnosis of HIV puts him at a higher risk of developing EPS, and because he is already experiencing increased wrist rigidity, the treatment team decides to switch his antipsychotic therapy to an agent with a lower risk of EPS. His comorbidities, including type 2 diabetes mellitus, hypertension, and hyperlipidemia, are taken into account, and an SGA with a benign metabolic profile is considered. Aripiprazole and ziprasidone are favorable options. However, because efavirenz, ATZ, and ritonavir may cause QTc prolongation, ziprasidone, the SGA with the highest rate of QTc prolongation, is not the preferred option.
Mr. S’s SGA therapy is switched from risperidone to aripiprazole. Because potential CYP-related interactions between aripiprazole and Mr. S’s current antiretroviral therapy could lead to increased aripiprazole levels. Mr. S is started on a low dose (5 mg/d) with the goal to titrate based on response and tolerability. Increased levels of aripiprazole may increase the risk of akathisia, drowsiness, headaches, and fatigue. Mr. S is monitored closely for improvement of EPS, adverse effects of medication, and metabolic parameters. Furthermore, if the treatment team believes there is a more preferred antipsychotic for the patient that it did not prescribe because of the risk of DDIs, it may be worthwhile to consider discussing the HAART regimen with the patient’s infectious disease treatment team.
Continue to: Acknowledgements
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the Chillicothe Veterans Affairs Medical Center in Chillicothe, Ohio. The contents of this paper do not represent the views of the U.S. Department of Veterans Affairs or the U.S. government.
Related Resources
- Cohen MA. HIV: How to provide compassionate care. Current Psychiatry. 2013;12(6):19-23,A,B.
- Khan AY, Zaidi SN. Reducing morbidity and mortality from common medical conditions in schizophrenia. Current Psychiatry. 2016;15(3):30-32,34-38,40.
Drug Brand Names
Abacavir • Ziagen
Amlodipine • Norvasc
Amprenavir • Agenerase
Aripiprazole • Abilify
Asenapine • Saphris
Atazanavir • Reyataz
Brexpiprazole • Rexulti
Bupropion ER • Wellbutrin SR
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Darunavir • Prezista
Delavirdine • Rescriptor
Didanosine • Videx EC
Efavirenz • Sustiva
Efavirenz/emtricitabine/tenofovir disoproxil fumarate • Atripla
Enfuvirtide • Fuzeon
Emtricitabine • Emtriva
Etravirine • Intelence
Fluphenazine • Prolixin
Fosamprenavir • Lexiva
Gabapentin • Neurontin
Glipizide • Glucotrol
Haloperidol • Haldol
Iloperidone • Fanapt
Indinavir • Crixivan
Lamivudine • Epivir
Lopinavir/ritonavir • Kaletra
Loxapine • Loxitane
Lurasidone • Latuda
Maraviroc • Selzentry
Metformin • Glucophage
Metoclopramide • Reglan
Molindone • Moban
Nelfinavir • Viracept
Nevirapine • Viramune
Olanzapine • Zyprexa
Paliperidone • Invega
Perphenazine • Trilafon
Pimozide • Orap
Pravastatin • Pravachol
Quetiapine • Seroquel
Raltegravir • Isentress
Rilpivirine • Edurant
Risperidone • Risperdal
Ritonavir • Norvir
Saquinavir • Invirase
Stavudine • Zerit
Tenofovir disoproxil • Viread
Thioridazine • Mellaril
Thiothixene • Navane
Tipranavir • Aptivus
Trifluoperazine • Stelazine
Zidovudine • Retrovir
Ziprasidone • Geodon
Mr. S, age 56, has human immunodeficiency virus (HIV) and schizoaffective disorder. He presents to your clinic with increased auditory hallucinations, disorganized behavior, and worsened tremors that have begun to seriously disrupt his daily life. Mr. S is prescribed risperidone; however, he reports that he has not been taking it lately due to the tremor despite being controlled on his medication regimen for nearly 1 year. His Abnormal Involuntary Movement Scale (AIMS) score reveals an increased wrist rigidity compared with previous clinic visits. Mr. S has a 40 pack-year history of smoking and history of IV drug use. Furthermore, he has a medical history of type 2 diabetes mellitus, hypertension, and hyperlipidemia.
His medication regimen includes atazanavir sulfate, 200 mg/d, ritonavir, 100 mg/d, efavirenz/emtricitabine/tenofovir disoproxil fumarate, 600/200/300 mg/d, risperidone, 6 mg/d, bupropion extended-release, 300 mg/d, gabapentin, 600 mg/d, amlodipine, 5 mg/d, pravastatin, 40 mg/d, metformin, 1000 mg twice daily, and glipizide, 10 mg twice daily. Today, his laboratory findings show that his CD4 count is 405 cell/mm3, and his viral load is <40 copies/mL, indicating his HIV is well managed. A hepatitis C virus antibody test result is negative and serum creatinine level is 1.0 mg/dL. Total cholesterol is 212 mg/dL, high-density lipoprotein cholesterol is 43 mg/dL, low-density lipoprotein cholesterol is 121 mg/dL, and triglycerides level is 238 mg/dL. Electrocardiography reveals a QTc interval of 426 ms. Mr. S’s blood pressure is 105/65 mm Hg. Based on this clinic visit, the treatment team decides to change Mr. S’s antipsychotic.
Psychiatric illness and HIV/AIDS
There is a strong link between mental illness and HIV/AIDS; 50% or more of patients with HIV/AIDS have a comorbid psychiatric disorder.1 The prevalence of mental illness in patients with HIV/AIDS is reported to be 8 times higher than in those without HIV/AIDS.2 Depression, bipolar disorder, anxiety disorders, delirium, substance abuse, and schizophrenia have all been identified in persons receiving highly active antiretroviral therapy (HAART). Patients with HIV/AIDS and psychiatric illness have a decreased quality of life, poor adherence to medications, faster disease progression, and increased mortality. Care of these individuals is complicated by the stigma of HIV/AIDS and the prevalence of the illness in underserved populations, as well as the need for complex medication regimens and the possibility of drug–drug interactions (DDIs).1,2 If left untreated, psychiatric illness in patients with HIV/AIDS may lead to further transmission of HIV as a result of patients engaging in high-risk behaviors, along with poor adherence to HAART.3
Individuals diagnosed with schizophrenia, schizoaffective disorder, and bipolar disorder are at greater risk for HIV infection.3 Patients with HIV/AIDS with primary psychosis may have poor medication adherence rates due to illness-related confusion or paranoia about medications. Furthermore, they may lack the resources to manage the complications and stress related to living with HIV/AIDS.
New-onset, or secondary psychosis, has been reported in individuals with late-stage HIV/AIDS with CD4 counts <200 who have not been diagnosed with a psychotic disorder previously.3 These patients may experience more persecutory and grandiose delusions rather than hallucinations. Neuropsychiatric symptoms in patients with HIV/AIDS may be due to the presence of HIV or other infections in the CNS, tumors, or other inflammatory illnesses. Medications that have been implicated in neuropsychiatric symptoms include efavirenz, rilpivirine, and other HAART regimens; interferon; metoclopramide; corticosteroids; muscle relaxants; and clonidine. It is possible that symptoms may continue even after the medications are discontinued.3
Antipsychotics remain the treatment of choice for psychosis in HIV/AIDS, regardless of the cause of the symptoms. Many factors must be taken into consideration when choosing an antipsychotic, such as DDIs, adverse effect profiles, patient history of antipsychotic use, cost, and patient preference. Here we focus primarily on DDIs and adverse effect profiles.
When treating psychosis in patients with HIV/AIDS, it is crucial to consider potential DDIs. Many antipsychotics and antiretroviral medications utilize cytochrome P450 (CYP) enzymes for their metabolism. The CYP enzyme system is responsible for the oxidative reactions that constitute the phase I reactions necessary for the metabolism of most drugs. Inhibition and induction of CYP enzymes are among the most common causes of pharmacokinetic DDIs. Antipsychotics are predominately metabolized by CYP3A4, CYP1A2, and CYP2D6.4
Continue to: The DDIs arise because...
The DDIs arise because many antiretroviral medications inhibit, or in some cases, induce, these CYP enzymes, thereby altering substrate-drug metabolism. Inhibiting a CYP enzyme pathway can decrease substrate-drug clearance and lead to increased levels of that drug. This, in turn, can cause an increased risk of adverse effects, such as extrapyramidal symptoms (EPS) or QTc prolongation, which are both types of pharmacodynamic DDIs.4-28 However, because antipsychotics often have more than one pathway of metabolism, it can be challenging to understand the full effect of CYP-related DDIs. Furthermore, CYP enzyme inducers can decrease drug levels, and in the case of antipsychotics, lead to subtherapeutic responses.

Table 1,6-14,19-28 Table 2,15-28 Table 3,6-14,19-28 and Table 415-28 list many of the known CYP enzyme-related DDIs that may occur with combination antipsychotic and antiretroviral medication therapy and aim to predict CYP induction or inhibition based on a particular combination. The following antiretroviral medications do not have any CYP-related interactions and therefore are not included in the Tables: abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir disoproxil, zidovudine, enfuvirtide, maraviroc, and raltegravir.

These Tables include the risk ratings for all D-rated (consider alternative therapy) and X-rated (avoid therapy) combinations. The majority of D-rated interactions are caused by CYP inhibition or induction that could potentially lead to altered antipsychotic levels. The majority of X-rated interactions are caused by increased QTc prolongation that may or may not be due to CYP-related DDIs. For example, paliperidone is not believed to be affected by the CYP enzyme system, but it does present a high risk of QTc prolongation on its own. When combined with an antiretroviral that also has a high risk of QTc prolongation, such as lopinavir, then the risk further increases.
Non-nucleoside reverse transcriptase inhibitors and protease inhibitors (PIs) are the antiretroviral medications most likely to cause DDIs with antipsychotics. Other antiretroviral classes, such as nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), fusion inhibitors, chemokine receptor 5 inhibitors, and integrase inhibitors, are not associated with CYP-related DDIs.19-28 For the most part, the severity of the CYP-related DDIs have not been well studied; therefore, most recommendations call for closer patient monitoring when combining antiretroviral medications and antipsychotics.6-18 The goal is to monitor for any changes in medication efficacy or adverse effects.
Continue to: Consider adverse effect profiles
Consider adverse effect profiles
When selecting an antipsychotic agent for a patient receiving HIV therapy, also consider adverse effect profiles. The emergence of adverse effects can greatly impact patients’ quality of life, leading to consequences of medication nonadherence and exacerbation of mental illness.
Extrapyramidal symptoms. Patients with HIV have a higher sensitivity to treatment-emergent EPS from antipsychotics.2 This sensitivity is generally thought to arise from the involvement of HIV on the basal ganglia. Historically, psychotic symptoms in HIV have been managed with second-generation antipsychotics (SGAs) at the lowest effective dose because these medications are less likely to cause EPS.1,29 The antipsychotic with the lowest rate of EPS is clozapine, followed by quetiapine, olanzapine, ziprasidone, and aripiprazole. Conversely, high-potency first-generation antipsychotics (FGAs) have the highest rates of EPS, followed by intermediate-potency FGAs and risperidone.30
Metabolic disturbances are another concern with concomitant antipsychotic/antiretroviral therapy. Patients with HIV who are receiving NRTIs or PIs can present with drug-induced lipodystrophy syndrome, which is associated with hyperglycemia, hyperinsulinemia, hyperlipidemia, and hypertension, and ultimately may cause metabolic syndrome.29 The prevalence of metabolic syndrome in patients receiving PI therapy has a vast range—2% to 84%—which can be attributed to inconsistent definitions, criteria, and assessment methodology.29 Use of a PI is considered to be the most prominent risk factor for developing lipodystrophy.29 Among the PIs, metabolic disturbances in regards to lipids are most often seen with lopinavir/ritonavir (LPV/r), saquinavir/ritonavir, tipranavir/ritonavir, and fosamprenavir/ritonavir.31 In comparison with LPV/r, darunavir showed improvement in lipids.32 Atazanavir (ATV) boosted with ritonavir has not shown clinically significant adverse effects on lipids.31 Additionally, amprenavir, LPV/r, and ritonavir demonstrated more glucose uptake inhibition via blockade of the glucose transporter type 4 than ATV.31 Of the NRTIs, lipodystrophy syndrome is most commonly seen with stavudine, which is used minimally in practice.2
The rates of metabolic disturbance with antipsychotic use range from 2% to 36%.2 The American Psychiatric Association recommends selecting one of the SGAs least likely to affect metabolic parameters.29 Aripiprazole and ziprasidone are associated with the lowest risk of weight gain, hyperglycemia, and hyperlipidemia. They are followed by risperidone and quetiapine, which are associated with moderate risk, and then clozapine and olanzapine, which are associated with high risk.2,30,33
Continue to: Management of metabolic adverse effects involves...
Management of metabolic adverse effects involves switching the antiretroviral agent and/or antipsychotic agent to an alternative associated with lower metabolic risk. Antipsychotics with low metabolic risk include aripiprazole, lurasidone, and ziprasidone. Lifestyle modifications are encouraged. Additionally, medication interventions, such as metformin, are also recommended in patients meeting criteria for pre-diabetes or type 2 diabetes mellitus.2 Lipid panels and metabolic parameters should be monitored periodically, according to guidelines.25,34
Bone marrow toxicity and blood dyscrasias. Lastly, consider the risk of bone marrow suppression. Patients receiving clozapine for treatment-resistant schizophrenia should be closely monitored for neutropenia and agranulocytosis. Although zidovudine is rarely used, its use is associated with adverse myelosuppressive effects, and the combination of clozapine and zidovudine could pose danger to the patient.2,35,36
CASE CONTINUED
Because Mr. S’s diagnosis of HIV puts him at a higher risk of developing EPS, and because he is already experiencing increased wrist rigidity, the treatment team decides to switch his antipsychotic therapy to an agent with a lower risk of EPS. His comorbidities, including type 2 diabetes mellitus, hypertension, and hyperlipidemia, are taken into account, and an SGA with a benign metabolic profile is considered. Aripiprazole and ziprasidone are favorable options. However, because efavirenz, ATZ, and ritonavir may cause QTc prolongation, ziprasidone, the SGA with the highest rate of QTc prolongation, is not the preferred option.
Mr. S’s SGA therapy is switched from risperidone to aripiprazole. Because potential CYP-related interactions between aripiprazole and Mr. S’s current antiretroviral therapy could lead to increased aripiprazole levels. Mr. S is started on a low dose (5 mg/d) with the goal to titrate based on response and tolerability. Increased levels of aripiprazole may increase the risk of akathisia, drowsiness, headaches, and fatigue. Mr. S is monitored closely for improvement of EPS, adverse effects of medication, and metabolic parameters. Furthermore, if the treatment team believes there is a more preferred antipsychotic for the patient that it did not prescribe because of the risk of DDIs, it may be worthwhile to consider discussing the HAART regimen with the patient’s infectious disease treatment team.
Continue to: Acknowledgements
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the Chillicothe Veterans Affairs Medical Center in Chillicothe, Ohio. The contents of this paper do not represent the views of the U.S. Department of Veterans Affairs or the U.S. government.
Related Resources
- Cohen MA. HIV: How to provide compassionate care. Current Psychiatry. 2013;12(6):19-23,A,B.
- Khan AY, Zaidi SN. Reducing morbidity and mortality from common medical conditions in schizophrenia. Current Psychiatry. 2016;15(3):30-32,34-38,40.
Drug Brand Names
Abacavir • Ziagen
Amlodipine • Norvasc
Amprenavir • Agenerase
Aripiprazole • Abilify
Asenapine • Saphris
Atazanavir • Reyataz
Brexpiprazole • Rexulti
Bupropion ER • Wellbutrin SR
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Darunavir • Prezista
Delavirdine • Rescriptor
Didanosine • Videx EC
Efavirenz • Sustiva
Efavirenz/emtricitabine/tenofovir disoproxil fumarate • Atripla
Enfuvirtide • Fuzeon
Emtricitabine • Emtriva
Etravirine • Intelence
Fluphenazine • Prolixin
Fosamprenavir • Lexiva
Gabapentin • Neurontin
Glipizide • Glucotrol
Haloperidol • Haldol
Iloperidone • Fanapt
Indinavir • Crixivan
Lamivudine • Epivir
Lopinavir/ritonavir • Kaletra
Loxapine • Loxitane
Lurasidone • Latuda
Maraviroc • Selzentry
Metformin • Glucophage
Metoclopramide • Reglan
Molindone • Moban
Nelfinavir • Viracept
Nevirapine • Viramune
Olanzapine • Zyprexa
Paliperidone • Invega
Perphenazine • Trilafon
Pimozide • Orap
Pravastatin • Pravachol
Quetiapine • Seroquel
Raltegravir • Isentress
Rilpivirine • Edurant
Risperidone • Risperdal
Ritonavir • Norvir
Saquinavir • Invirase
Stavudine • Zerit
Tenofovir disoproxil • Viread
Thioridazine • Mellaril
Thiothixene • Navane
Tipranavir • Aptivus
Trifluoperazine • Stelazine
Zidovudine • Retrovir
Ziprasidone • Geodon
1. Freudenreich O, Goforth HW, Cozza KL, et al. Psychiatric treatment of persons with HIV/AIDS: An HIV-psychiatry consensus survey of current practices. Psychosomatics. 2010;51(6):480-488.
2. Hill L, Lee KC. Pharmacotherapy considerations in patients with HIV and psychiatric disorders: Focus on antidepressants and antipsychotics. Ann Pharmacother. 2013;47(1):75-89.
3. Watkins CC, Treisman GJ. Neuropsychiatric complications of aging with HIV. J Neurovirol. 2012;18(4):277-290.
4. Prior TI, Baker GB. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci. 2003;28(2):99-112.
5. Ponte ML, Keller GA, Di Girolamo G. Mechanisms of drug induced QT interval prolongation. Curr Drug Saf. 2010;5(1):44-53
6. Reyataz [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2017.
7. Prezista [package insert]. Toronto, ON: Janssen Inc.; 2017.
8. Lexiva [package insert]. Research Triangle Park, NC: Viiv Healthcare; 2017
9. Crixivan [package insert]. Whitehouse Station, NJ; Merck; 2016.
10. Kaletra [package insert]. North Chicago, IL: AbbVie Inc; 2017
11. Viracept [package insert]. Kirkland, QC: Pfizer Canada Inc.; 201
12. Norvir tablets and oral solution [package insert]. North Chicago, IL: AbbVie Inc; 2017
13. Invirase [package insert]. South San Francisco, CA: Genentech USA, Inc.; 2016.
14. Aptivus [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc.; 2016.
15. Sustiva [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2017
16. Intelence [package insert]. Titusville, NJ: Tibotec Pharmaceuticals; 2014.
17. Viramune [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc.; 2017.
18. Rescriptor [package insert]. Laval, QC: ViiV Healthcare ULC; 2013.
19. Ziagen [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
20. Videx EC [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2015.
21. Emtriva [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2017.
22. Epivir [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2017.
23. Zerit [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2017.
24. Viread [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2017.
25. Retrovir [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2015.
26. Fuzeon [package insert]. South San Francisco, CA: Genentech USA, Inc; 2017.
27. Selzentry [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2016.
28. Isentress [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2017.
29. American Psychiatry Association. Practice guidelines for treatment of patients with HIV/AIDS. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/hivaids.pdf. Published 2010. Accessed March 1, 2018.
30. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 Schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
31. Hughes PJ, Cretton-Scott E, Teague A, et al. Protease inhibitors for patients with HIV-1 infection. P T. 2011;36(6):332-336,341-345.
32. Ortiz R, Dejesus E, Khanlou H, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS. 2008;22(12):1389-1397.
33. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962.
34. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013;12(9):51-54.
35. Singh D, Goodkin K. Choice of antipsychotic in HIV-infected patients. J Clin Psychiatry. 2007;68(3):479-480.
36. Max B, Sherer R. Management of the adverse effects of antiretroviral therapy and medication adherence. Clin Infect Dis. 2000;30(suppl 2):S96-S116.
1. Freudenreich O, Goforth HW, Cozza KL, et al. Psychiatric treatment of persons with HIV/AIDS: An HIV-psychiatry consensus survey of current practices. Psychosomatics. 2010;51(6):480-488.
2. Hill L, Lee KC. Pharmacotherapy considerations in patients with HIV and psychiatric disorders: Focus on antidepressants and antipsychotics. Ann Pharmacother. 2013;47(1):75-89.
3. Watkins CC, Treisman GJ. Neuropsychiatric complications of aging with HIV. J Neurovirol. 2012;18(4):277-290.
4. Prior TI, Baker GB. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci. 2003;28(2):99-112.
5. Ponte ML, Keller GA, Di Girolamo G. Mechanisms of drug induced QT interval prolongation. Curr Drug Saf. 2010;5(1):44-53
6. Reyataz [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2017.
7. Prezista [package insert]. Toronto, ON: Janssen Inc.; 2017.
8. Lexiva [package insert]. Research Triangle Park, NC: Viiv Healthcare; 2017
9. Crixivan [package insert]. Whitehouse Station, NJ; Merck; 2016.
10. Kaletra [package insert]. North Chicago, IL: AbbVie Inc; 2017
11. Viracept [package insert]. Kirkland, QC: Pfizer Canada Inc.; 201
12. Norvir tablets and oral solution [package insert]. North Chicago, IL: AbbVie Inc; 2017
13. Invirase [package insert]. South San Francisco, CA: Genentech USA, Inc.; 2016.
14. Aptivus [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc.; 2016.
15. Sustiva [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2017
16. Intelence [package insert]. Titusville, NJ: Tibotec Pharmaceuticals; 2014.
17. Viramune [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc.; 2017.
18. Rescriptor [package insert]. Laval, QC: ViiV Healthcare ULC; 2013.
19. Ziagen [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
20. Videx EC [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2015.
21. Emtriva [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2017.
22. Epivir [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2017.
23. Zerit [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2017.
24. Viread [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2017.
25. Retrovir [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2015.
26. Fuzeon [package insert]. South San Francisco, CA: Genentech USA, Inc; 2017.
27. Selzentry [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2016.
28. Isentress [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2017.
29. American Psychiatry Association. Practice guidelines for treatment of patients with HIV/AIDS. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/hivaids.pdf. Published 2010. Accessed March 1, 2018.
30. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 Schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
31. Hughes PJ, Cretton-Scott E, Teague A, et al. Protease inhibitors for patients with HIV-1 infection. P T. 2011;36(6):332-336,341-345.
32. Ortiz R, Dejesus E, Khanlou H, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS. 2008;22(12):1389-1397.
33. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962.
34. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013;12(9):51-54.
35. Singh D, Goodkin K. Choice of antipsychotic in HIV-infected patients. J Clin Psychiatry. 2007;68(3):479-480.
36. Max B, Sherer R. Management of the adverse effects of antiretroviral therapy and medication adherence. Clin Infect Dis. 2000;30(suppl 2):S96-S116.
Career Choices: Directorship/leadership
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Cornel Stanciu, MD, talked with Thomas Penders, MS, MD. For most of his career, Dr. Penders has practiced in directorship roles. He currently serves as the leader of an addiction consultation service at the Walter B. Jones Center in Greenville, North Carolina, as well as working at the state level with federally qualified health centers to develop collaborative care models.
Dr. Stanciu: What led you to decide to pursue a director role?
Dr. Penders: Early in my career, I was offered opportunities to provide leadership for an organization in its efforts to assure quality and availability of appropriate medical and psychiatric care.
Dr. Stanciu: How has the director role evolved over the years?
Dr. Penders: Thirty years ago, when I got started, hospital administrations depended heavily on medical directors to provide advice on new service initiates. Medical directors were frequently provided with support by health care organizations when recommendations were made based on patient and community need as perceived by medical staff providers. There has been a dramatic shift in the relationship and role of medical directorship, particularly over the past decade. Budgetary constraints have influenced planning and operational decisions to the extent that these decisions are much more likely to be made based on financial analyses rather than on clinical needs identified by physicians. As a result, medical directors are encouraged to be mindful of the effect of their suggestions on the bottom line of the organization. This has resulted in a very significant shift away from programs that are needed but not funded, and toward programs that are revenue-positive or at least neutral.
Medical directors who do not conform in this way are unlikely to be part of the administration for very long in the present environment.
Continue to: What training qualifications are required or desirable to assume a medical leadership role (post residency fellowship, MBA, etc.)?
Dr. Stanciu: What training qualifications are required or desirable to assume a medical leadership role (post-residency fellowship, MBA, etc.)?
Dr. Penders: In addition to a foundation in evidence-based practices and knowledge of regulatory requirements, general leadership skills are probably the most important qualities for medical leadership. Hospitals are complex organizations with confusing reporting relationships. Negotiation skills and communication skills are critical to success. Because most modern health care organizations are well staffed with administrative personnel trained in business and finance, I would not suggest that an MBA is necessary or even important to a medical director’s success. Having said that, there are an increasing number of physicians assuming the role of chief executive officer in complex health care systems. In this case, MBA training will likely be advantageous.
I would suggest that the focus of training that occurs in MPH programs would provide more relevant tools for those in positions of medical leadership. Skills such as biostatistics and epidemiology provide those in such positions with the perspective required to understand the effectiveness of health care systems, and to relate to changes that might be beneficial to the populations they serve. A firm foundation in information systems and data analysis is becoming increasingly important as the payment system moves toward one that is value-based. Increasingly, health care systems decisions will be guided by the analysis of aggregated information gathered from electronic medical records.
Dr. Stanciu: What personal qualities makes a psychiatric physician well-suited for the role of a medical director?
Dr. Penders: Medical directors will confront a variety of difficult situations with colleagues, administrative staff, patients, and family members. A calm demeanor with an ability to reflect rather than react is important. As I previously mentioned, an ability to communicate, including strength as a listener, is another personality trait valued in this position.
Continue to: What are some of the challenges you face on a daily basis?
Dr. Stanciu: What are some of the challenges you face on a daily basis?
Dr. Penders: There are challenges in multiple areas. First and foremost, medical leadership is responsible for maintaining and improving the quality of patient care and experience. One can expect frequent conflicts to arise when providers vary from established standards or disagree with established policies.
Additionally, there appears to be an increasing lack of a distinct line between administrative and patient care decisions. It is often a challenge to manage the conflicting incentives involved when cost containment and quality care are seen to diverge.
Dr. Stanciu: What are the metrics that measure success by a medical administrator?
Dr. Penders: Some would say that the financial status of the organization is an important metric. Measures such as length of stay, patient satisfaction, and numbers of clinically relevant adverse events are how the success of medical leadership is assessed.
I would argue that patient outcomes as measured by standard clinical tools are the true measure of the success of the efforts of medical providers led by a medical director. Increasingly, measures of population health will likely be used to measure the overall success of health care organizations.
Continue to: How do you keep up-to-date on the latest rules and regulations to ensure facility compliance?
Dr. Stanciu: How do you keep up-to-date on the latest rules and regulations to ensure facility compliance?
Dr. Penders: Medical directors attend many professional meetings, both within their organizations and outside, which assures that information is provided on regulatory initiatives from government bodies and organizations such as the Joint Commission.
Hospital risk managers and attorneys also play a part in keeping everyone honest when it comes to changes in laws governing our work.
Dr. Stanciu: How is it working in a supervisory capacity with other physicians and the growing number of mid-level providers and their expanding scope of practice?
Dr. Penders: There is a variety of opinions today about the relationship between physicians and mid-level providers. Fairly recently, nurse practitioners and physician assistants were known as “extenders.” We don’t hear that term as much anymore, as these providers are becoming increasingly independent in their practice roles.
The supervisory challenge varies with each situation. Most hospital organizations have medical staff rules and regulations that define the relationships within hospitals. Efforts in outpatient care are often less well defined, and supervisory relationships can be tailored to the specific effort involved.
Continue to: Is there a stipend or additional compensation for administrative duties?
Dr. Stanciu: Is there a stipend or additional compensation for administrative duties?
Dr. Penders: Always. There is considerable time and effort needed on a flexibly “as needed” basis that serves as a justification for administrative compensation.
Dr. Stanciu: Any major differences when working in an independent facility vs a large corporation?
Dr. Penders: As health care organizations become larger and more complex, the role of medical directorships in the larger systems are generally defined by policies that can be restrictive. Small organizations may have less formal rules and allow some flexibility for the role of medical leadership in general.
Dr. Stanciu: What preparation do you suggest for trainees and early career psychiatrists who are contemplating such a role?
Dr. Penders: Become involved in quality and organizational initiatives whenever they are available. Generally, organizations will invite and value the input trainees can provide to these efforts. Functioning as a chief resident is real-world experience that can be invaluable.
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Cornel Stanciu, MD, talked with Thomas Penders, MS, MD. For most of his career, Dr. Penders has practiced in directorship roles. He currently serves as the leader of an addiction consultation service at the Walter B. Jones Center in Greenville, North Carolina, as well as working at the state level with federally qualified health centers to develop collaborative care models.
Dr. Stanciu: What led you to decide to pursue a director role?
Dr. Penders: Early in my career, I was offered opportunities to provide leadership for an organization in its efforts to assure quality and availability of appropriate medical and psychiatric care.
Dr. Stanciu: How has the director role evolved over the years?
Dr. Penders: Thirty years ago, when I got started, hospital administrations depended heavily on medical directors to provide advice on new service initiates. Medical directors were frequently provided with support by health care organizations when recommendations were made based on patient and community need as perceived by medical staff providers. There has been a dramatic shift in the relationship and role of medical directorship, particularly over the past decade. Budgetary constraints have influenced planning and operational decisions to the extent that these decisions are much more likely to be made based on financial analyses rather than on clinical needs identified by physicians. As a result, medical directors are encouraged to be mindful of the effect of their suggestions on the bottom line of the organization. This has resulted in a very significant shift away from programs that are needed but not funded, and toward programs that are revenue-positive or at least neutral.
Medical directors who do not conform in this way are unlikely to be part of the administration for very long in the present environment.
Continue to: What training qualifications are required or desirable to assume a medical leadership role (post residency fellowship, MBA, etc.)?
Dr. Stanciu: What training qualifications are required or desirable to assume a medical leadership role (post-residency fellowship, MBA, etc.)?
Dr. Penders: In addition to a foundation in evidence-based practices and knowledge of regulatory requirements, general leadership skills are probably the most important qualities for medical leadership. Hospitals are complex organizations with confusing reporting relationships. Negotiation skills and communication skills are critical to success. Because most modern health care organizations are well staffed with administrative personnel trained in business and finance, I would not suggest that an MBA is necessary or even important to a medical director’s success. Having said that, there are an increasing number of physicians assuming the role of chief executive officer in complex health care systems. In this case, MBA training will likely be advantageous.
I would suggest that the focus of training that occurs in MPH programs would provide more relevant tools for those in positions of medical leadership. Skills such as biostatistics and epidemiology provide those in such positions with the perspective required to understand the effectiveness of health care systems, and to relate to changes that might be beneficial to the populations they serve. A firm foundation in information systems and data analysis is becoming increasingly important as the payment system moves toward one that is value-based. Increasingly, health care systems decisions will be guided by the analysis of aggregated information gathered from electronic medical records.
Dr. Stanciu: What personal qualities makes a psychiatric physician well-suited for the role of a medical director?
Dr. Penders: Medical directors will confront a variety of difficult situations with colleagues, administrative staff, patients, and family members. A calm demeanor with an ability to reflect rather than react is important. As I previously mentioned, an ability to communicate, including strength as a listener, is another personality trait valued in this position.
Continue to: What are some of the challenges you face on a daily basis?
Dr. Stanciu: What are some of the challenges you face on a daily basis?
Dr. Penders: There are challenges in multiple areas. First and foremost, medical leadership is responsible for maintaining and improving the quality of patient care and experience. One can expect frequent conflicts to arise when providers vary from established standards or disagree with established policies.
Additionally, there appears to be an increasing lack of a distinct line between administrative and patient care decisions. It is often a challenge to manage the conflicting incentives involved when cost containment and quality care are seen to diverge.
Dr. Stanciu: What are the metrics that measure success by a medical administrator?
Dr. Penders: Some would say that the financial status of the organization is an important metric. Measures such as length of stay, patient satisfaction, and numbers of clinically relevant adverse events are how the success of medical leadership is assessed.
I would argue that patient outcomes as measured by standard clinical tools are the true measure of the success of the efforts of medical providers led by a medical director. Increasingly, measures of population health will likely be used to measure the overall success of health care organizations.
Continue to: How do you keep up-to-date on the latest rules and regulations to ensure facility compliance?
Dr. Stanciu: How do you keep up-to-date on the latest rules and regulations to ensure facility compliance?
Dr. Penders: Medical directors attend many professional meetings, both within their organizations and outside, which assures that information is provided on regulatory initiatives from government bodies and organizations such as the Joint Commission.
Hospital risk managers and attorneys also play a part in keeping everyone honest when it comes to changes in laws governing our work.
Dr. Stanciu: How is it working in a supervisory capacity with other physicians and the growing number of mid-level providers and their expanding scope of practice?
Dr. Penders: There is a variety of opinions today about the relationship between physicians and mid-level providers. Fairly recently, nurse practitioners and physician assistants were known as “extenders.” We don’t hear that term as much anymore, as these providers are becoming increasingly independent in their practice roles.
The supervisory challenge varies with each situation. Most hospital organizations have medical staff rules and regulations that define the relationships within hospitals. Efforts in outpatient care are often less well defined, and supervisory relationships can be tailored to the specific effort involved.
Continue to: Is there a stipend or additional compensation for administrative duties?
Dr. Stanciu: Is there a stipend or additional compensation for administrative duties?
Dr. Penders: Always. There is considerable time and effort needed on a flexibly “as needed” basis that serves as a justification for administrative compensation.
Dr. Stanciu: Any major differences when working in an independent facility vs a large corporation?
Dr. Penders: As health care organizations become larger and more complex, the role of medical directorships in the larger systems are generally defined by policies that can be restrictive. Small organizations may have less formal rules and allow some flexibility for the role of medical leadership in general.
Dr. Stanciu: What preparation do you suggest for trainees and early career psychiatrists who are contemplating such a role?
Dr. Penders: Become involved in quality and organizational initiatives whenever they are available. Generally, organizations will invite and value the input trainees can provide to these efforts. Functioning as a chief resident is real-world experience that can be invaluable.
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Cornel Stanciu, MD, talked with Thomas Penders, MS, MD. For most of his career, Dr. Penders has practiced in directorship roles. He currently serves as the leader of an addiction consultation service at the Walter B. Jones Center in Greenville, North Carolina, as well as working at the state level with federally qualified health centers to develop collaborative care models.
Dr. Stanciu: What led you to decide to pursue a director role?
Dr. Penders: Early in my career, I was offered opportunities to provide leadership for an organization in its efforts to assure quality and availability of appropriate medical and psychiatric care.
Dr. Stanciu: How has the director role evolved over the years?
Dr. Penders: Thirty years ago, when I got started, hospital administrations depended heavily on medical directors to provide advice on new service initiates. Medical directors were frequently provided with support by health care organizations when recommendations were made based on patient and community need as perceived by medical staff providers. There has been a dramatic shift in the relationship and role of medical directorship, particularly over the past decade. Budgetary constraints have influenced planning and operational decisions to the extent that these decisions are much more likely to be made based on financial analyses rather than on clinical needs identified by physicians. As a result, medical directors are encouraged to be mindful of the effect of their suggestions on the bottom line of the organization. This has resulted in a very significant shift away from programs that are needed but not funded, and toward programs that are revenue-positive or at least neutral.
Medical directors who do not conform in this way are unlikely to be part of the administration for very long in the present environment.
Continue to: What training qualifications are required or desirable to assume a medical leadership role (post residency fellowship, MBA, etc.)?
Dr. Stanciu: What training qualifications are required or desirable to assume a medical leadership role (post-residency fellowship, MBA, etc.)?
Dr. Penders: In addition to a foundation in evidence-based practices and knowledge of regulatory requirements, general leadership skills are probably the most important qualities for medical leadership. Hospitals are complex organizations with confusing reporting relationships. Negotiation skills and communication skills are critical to success. Because most modern health care organizations are well staffed with administrative personnel trained in business and finance, I would not suggest that an MBA is necessary or even important to a medical director’s success. Having said that, there are an increasing number of physicians assuming the role of chief executive officer in complex health care systems. In this case, MBA training will likely be advantageous.
I would suggest that the focus of training that occurs in MPH programs would provide more relevant tools for those in positions of medical leadership. Skills such as biostatistics and epidemiology provide those in such positions with the perspective required to understand the effectiveness of health care systems, and to relate to changes that might be beneficial to the populations they serve. A firm foundation in information systems and data analysis is becoming increasingly important as the payment system moves toward one that is value-based. Increasingly, health care systems decisions will be guided by the analysis of aggregated information gathered from electronic medical records.
Dr. Stanciu: What personal qualities makes a psychiatric physician well-suited for the role of a medical director?
Dr. Penders: Medical directors will confront a variety of difficult situations with colleagues, administrative staff, patients, and family members. A calm demeanor with an ability to reflect rather than react is important. As I previously mentioned, an ability to communicate, including strength as a listener, is another personality trait valued in this position.
Continue to: What are some of the challenges you face on a daily basis?
Dr. Stanciu: What are some of the challenges you face on a daily basis?
Dr. Penders: There are challenges in multiple areas. First and foremost, medical leadership is responsible for maintaining and improving the quality of patient care and experience. One can expect frequent conflicts to arise when providers vary from established standards or disagree with established policies.
Additionally, there appears to be an increasing lack of a distinct line between administrative and patient care decisions. It is often a challenge to manage the conflicting incentives involved when cost containment and quality care are seen to diverge.
Dr. Stanciu: What are the metrics that measure success by a medical administrator?
Dr. Penders: Some would say that the financial status of the organization is an important metric. Measures such as length of stay, patient satisfaction, and numbers of clinically relevant adverse events are how the success of medical leadership is assessed.
I would argue that patient outcomes as measured by standard clinical tools are the true measure of the success of the efforts of medical providers led by a medical director. Increasingly, measures of population health will likely be used to measure the overall success of health care organizations.
Continue to: How do you keep up-to-date on the latest rules and regulations to ensure facility compliance?
Dr. Stanciu: How do you keep up-to-date on the latest rules and regulations to ensure facility compliance?
Dr. Penders: Medical directors attend many professional meetings, both within their organizations and outside, which assures that information is provided on regulatory initiatives from government bodies and organizations such as the Joint Commission.
Hospital risk managers and attorneys also play a part in keeping everyone honest when it comes to changes in laws governing our work.
Dr. Stanciu: How is it working in a supervisory capacity with other physicians and the growing number of mid-level providers and their expanding scope of practice?
Dr. Penders: There is a variety of opinions today about the relationship between physicians and mid-level providers. Fairly recently, nurse practitioners and physician assistants were known as “extenders.” We don’t hear that term as much anymore, as these providers are becoming increasingly independent in their practice roles.
The supervisory challenge varies with each situation. Most hospital organizations have medical staff rules and regulations that define the relationships within hospitals. Efforts in outpatient care are often less well defined, and supervisory relationships can be tailored to the specific effort involved.
Continue to: Is there a stipend or additional compensation for administrative duties?
Dr. Stanciu: Is there a stipend or additional compensation for administrative duties?
Dr. Penders: Always. There is considerable time and effort needed on a flexibly “as needed” basis that serves as a justification for administrative compensation.
Dr. Stanciu: Any major differences when working in an independent facility vs a large corporation?
Dr. Penders: As health care organizations become larger and more complex, the role of medical directorships in the larger systems are generally defined by policies that can be restrictive. Small organizations may have less formal rules and allow some flexibility for the role of medical leadership in general.
Dr. Stanciu: What preparation do you suggest for trainees and early career psychiatrists who are contemplating such a role?
Dr. Penders: Become involved in quality and organizational initiatives whenever they are available. Generally, organizations will invite and value the input trainees can provide to these efforts. Functioning as a chief resident is real-world experience that can be invaluable.
‘Robotripping’: What residents need to know
Dextromethorphan (DXM) is commonly found in over-the-counter (OTC) cold and cough preparations. When used at the therapeutic doses DXM has cough-suppressant properties through its action on the medulla. However, OTC preparations containing DXM are being increasingly used recreationally for the drug’s psychoactive effects, a practice referred to as “robotripping.” Such use can result in a toxidrome of delirium with agitation, paranoia, and hallucinations.1 Residents need to be able to recognize the signs of DXM abuse and manage its potentially serious complications.
How DXM works
DXM has a wide therapeutic window. A typical therapeutic dose for cough is up to 120 mg/d. The most common adverse effects are mild (fever, diaphoresis, dizziness, nausea). At higher dosages, it acts as a nonselective serotonin reuptake inhibitor, a sigma-1 receptor agonist, and an N-methyl-
Adverse effects include hallucinations, disorientation, mania, and aggression with delusions of supernatural abilities and insensitivity to pain; these effects are similar to those produced by phencyclidine (PCP).2-4 Physiologically, diaphoresis, hyperthermia, and tachycardia are often observed.3,5 These presentations carry a significant risk of mortality, and appropriate recognition and management is needed.
4 Phases of intoxication
DXM users have described 4 progressive behavioral phases that vary with dosage.3,6,7 First, at 1.5 to 2.5 mg/kg, users report stimulating effects with perceptual alterations similar to those produced by 3,4-methylenedioxymethamphetamine (“ecstasy”). The second phase, reached at 2.5 to 7.5 mg/kg, is similar to alcohol and marijuana intoxication but includes more pronounced dysfunction in motor, cognitive, and perceptual skills, and perhaps visual hallucinations.3,6,7 The third phase, noted at 7.5 to 15 mg/kg, resembles ketamine intoxication, with strong dissociation and hallucinations.3,6,7 At greater doses, out-of-body, trance-like experiences may occur. Delirious misperceptions often lead to violent behavior and limited perception of pain. Users may experience a long course of any of these phases, with presentations lasting for up to 1 to 2 weeks after discontinuing use.8
Management is mainly supportive
Early recognition of DXM use is essential for treatment. Unfortunately, without collateral reports, this can be challenging because specialized toxicology screens are needed to detect DXM. Basic screens sometimes show a false positive for PCP. Take an inventory of all substances in the patient’s possession, either by examining the patient’s belongings or by obtaining collateral information from the patient’s family or friends.
Supportive care should be implemented, with a primary goal of controlling agitation. Short-acting benzodiazepines are helpful. Low-dose, short-term antipsychotics have shown benefit when hallucinations and paranoia are prominent.3 Decreasing stimulation and avoiding physical restraints while attempting to control aggression and psychosis with these medications is recommended. Using physical restraints on an individual who is in a state of agitated delirium can lead to severe injuries, cardiac and respiratory arrest, and death.9-11
Patients typically experience rapid and complete remission of symptoms after discontinuing DXM use. However, evidence suggests DXM users can develop tolerance as well as psychological and physiological dependence. DXM withdrawal can be quite protracted and may include anxiety, dysphoria, insomnia, and suicidality.
1. Stanciu CN, Penders TM, Rouse EM. Recreational use of dextromethorphan,“Robotripping”-A brief review. Am J Addict. 2016;25(5):374-377.
2. Martinak B, Bolis RA, Black JR, et al. Dextromethorphan in cough syrup: The poor man’s psychosis. Psychopharmacol Bull. 2017;47(4):59-63.
3. Logan BK, Yeakel JK, Goldfogel G, et al. Dextromethorphan abuse leading to assault, suicide, or homicide. J Forensic Sci. 2012;57(5):1388-1394.
4. Dextromethorphan (Street names: DXM, CCC, Triple C, Skittles, Robo, Poor Man’s PCP). Drug Enforcement Administration. Office of Diversion Control. Drug & Chemical Evaluation Section. https://www.deadiversion.usdoj.gov/drug_chem_info/dextro_m.pdf. Published March 2014. Accessed April 22, 2018.
5. Reissig CJ, Carter LP, Johnson MW, et al. High doses of dextromethorphan, an NMDA antagonist, produce effects similar to classic hallucinogens. Psychopharmacology (Berl). 2012;223(1):1-15.
6. Boyer EW. Dextromethorphan abuse. Pediatr Emerg Care. 2004;20(12):858-863.
7. Drug Fact Sheet: Dextromethorphan (DXM). Drug Enforcement Administration. https://www.dea.gov/druginfo/drug_data_sheets/Detromethorphan.pdf. Accessed April 22, 2018.
8. Jacob R, Nicholapillai JN. Dextromethorphan induced bipolar disorder. Int Clin Psychopharmacol. 2012;28:e37-e38.
9. Hick JL, Smith SW, Lynch MT. Metabolic acidosis in restraint-associated cardiac arrest: a case series. Acad Emerg Med. 1999;6(3):239-243.
10. Mohr WK, Petti TA, Mohr BD. Adverse effects associated with physical restraint. Can J Psychiatry. 2003;48(5):330-337.
11. Otahbachi M, Cevik C, Bagdure S, et al. Excited delirium, restraints, and unexpected death: a review of pathogenesis. Am J Forensic Med Pathol. 2010;31(2):107-112.
Dextromethorphan (DXM) is commonly found in over-the-counter (OTC) cold and cough preparations. When used at the therapeutic doses DXM has cough-suppressant properties through its action on the medulla. However, OTC preparations containing DXM are being increasingly used recreationally for the drug’s psychoactive effects, a practice referred to as “robotripping.” Such use can result in a toxidrome of delirium with agitation, paranoia, and hallucinations.1 Residents need to be able to recognize the signs of DXM abuse and manage its potentially serious complications.
How DXM works
DXM has a wide therapeutic window. A typical therapeutic dose for cough is up to 120 mg/d. The most common adverse effects are mild (fever, diaphoresis, dizziness, nausea). At higher dosages, it acts as a nonselective serotonin reuptake inhibitor, a sigma-1 receptor agonist, and an N-methyl-
Adverse effects include hallucinations, disorientation, mania, and aggression with delusions of supernatural abilities and insensitivity to pain; these effects are similar to those produced by phencyclidine (PCP).2-4 Physiologically, diaphoresis, hyperthermia, and tachycardia are often observed.3,5 These presentations carry a significant risk of mortality, and appropriate recognition and management is needed.
4 Phases of intoxication
DXM users have described 4 progressive behavioral phases that vary with dosage.3,6,7 First, at 1.5 to 2.5 mg/kg, users report stimulating effects with perceptual alterations similar to those produced by 3,4-methylenedioxymethamphetamine (“ecstasy”). The second phase, reached at 2.5 to 7.5 mg/kg, is similar to alcohol and marijuana intoxication but includes more pronounced dysfunction in motor, cognitive, and perceptual skills, and perhaps visual hallucinations.3,6,7 The third phase, noted at 7.5 to 15 mg/kg, resembles ketamine intoxication, with strong dissociation and hallucinations.3,6,7 At greater doses, out-of-body, trance-like experiences may occur. Delirious misperceptions often lead to violent behavior and limited perception of pain. Users may experience a long course of any of these phases, with presentations lasting for up to 1 to 2 weeks after discontinuing use.8
Management is mainly supportive
Early recognition of DXM use is essential for treatment. Unfortunately, without collateral reports, this can be challenging because specialized toxicology screens are needed to detect DXM. Basic screens sometimes show a false positive for PCP. Take an inventory of all substances in the patient’s possession, either by examining the patient’s belongings or by obtaining collateral information from the patient’s family or friends.
Supportive care should be implemented, with a primary goal of controlling agitation. Short-acting benzodiazepines are helpful. Low-dose, short-term antipsychotics have shown benefit when hallucinations and paranoia are prominent.3 Decreasing stimulation and avoiding physical restraints while attempting to control aggression and psychosis with these medications is recommended. Using physical restraints on an individual who is in a state of agitated delirium can lead to severe injuries, cardiac and respiratory arrest, and death.9-11
Patients typically experience rapid and complete remission of symptoms after discontinuing DXM use. However, evidence suggests DXM users can develop tolerance as well as psychological and physiological dependence. DXM withdrawal can be quite protracted and may include anxiety, dysphoria, insomnia, and suicidality.
Dextromethorphan (DXM) is commonly found in over-the-counter (OTC) cold and cough preparations. When used at the therapeutic doses DXM has cough-suppressant properties through its action on the medulla. However, OTC preparations containing DXM are being increasingly used recreationally for the drug’s psychoactive effects, a practice referred to as “robotripping.” Such use can result in a toxidrome of delirium with agitation, paranoia, and hallucinations.1 Residents need to be able to recognize the signs of DXM abuse and manage its potentially serious complications.
How DXM works
DXM has a wide therapeutic window. A typical therapeutic dose for cough is up to 120 mg/d. The most common adverse effects are mild (fever, diaphoresis, dizziness, nausea). At higher dosages, it acts as a nonselective serotonin reuptake inhibitor, a sigma-1 receptor agonist, and an N-methyl-
Adverse effects include hallucinations, disorientation, mania, and aggression with delusions of supernatural abilities and insensitivity to pain; these effects are similar to those produced by phencyclidine (PCP).2-4 Physiologically, diaphoresis, hyperthermia, and tachycardia are often observed.3,5 These presentations carry a significant risk of mortality, and appropriate recognition and management is needed.
4 Phases of intoxication
DXM users have described 4 progressive behavioral phases that vary with dosage.3,6,7 First, at 1.5 to 2.5 mg/kg, users report stimulating effects with perceptual alterations similar to those produced by 3,4-methylenedioxymethamphetamine (“ecstasy”). The second phase, reached at 2.5 to 7.5 mg/kg, is similar to alcohol and marijuana intoxication but includes more pronounced dysfunction in motor, cognitive, and perceptual skills, and perhaps visual hallucinations.3,6,7 The third phase, noted at 7.5 to 15 mg/kg, resembles ketamine intoxication, with strong dissociation and hallucinations.3,6,7 At greater doses, out-of-body, trance-like experiences may occur. Delirious misperceptions often lead to violent behavior and limited perception of pain. Users may experience a long course of any of these phases, with presentations lasting for up to 1 to 2 weeks after discontinuing use.8
Management is mainly supportive
Early recognition of DXM use is essential for treatment. Unfortunately, without collateral reports, this can be challenging because specialized toxicology screens are needed to detect DXM. Basic screens sometimes show a false positive for PCP. Take an inventory of all substances in the patient’s possession, either by examining the patient’s belongings or by obtaining collateral information from the patient’s family or friends.
Supportive care should be implemented, with a primary goal of controlling agitation. Short-acting benzodiazepines are helpful. Low-dose, short-term antipsychotics have shown benefit when hallucinations and paranoia are prominent.3 Decreasing stimulation and avoiding physical restraints while attempting to control aggression and psychosis with these medications is recommended. Using physical restraints on an individual who is in a state of agitated delirium can lead to severe injuries, cardiac and respiratory arrest, and death.9-11
Patients typically experience rapid and complete remission of symptoms after discontinuing DXM use. However, evidence suggests DXM users can develop tolerance as well as psychological and physiological dependence. DXM withdrawal can be quite protracted and may include anxiety, dysphoria, insomnia, and suicidality.
1. Stanciu CN, Penders TM, Rouse EM. Recreational use of dextromethorphan,“Robotripping”-A brief review. Am J Addict. 2016;25(5):374-377.
2. Martinak B, Bolis RA, Black JR, et al. Dextromethorphan in cough syrup: The poor man’s psychosis. Psychopharmacol Bull. 2017;47(4):59-63.
3. Logan BK, Yeakel JK, Goldfogel G, et al. Dextromethorphan abuse leading to assault, suicide, or homicide. J Forensic Sci. 2012;57(5):1388-1394.
4. Dextromethorphan (Street names: DXM, CCC, Triple C, Skittles, Robo, Poor Man’s PCP). Drug Enforcement Administration. Office of Diversion Control. Drug & Chemical Evaluation Section. https://www.deadiversion.usdoj.gov/drug_chem_info/dextro_m.pdf. Published March 2014. Accessed April 22, 2018.
5. Reissig CJ, Carter LP, Johnson MW, et al. High doses of dextromethorphan, an NMDA antagonist, produce effects similar to classic hallucinogens. Psychopharmacology (Berl). 2012;223(1):1-15.
6. Boyer EW. Dextromethorphan abuse. Pediatr Emerg Care. 2004;20(12):858-863.
7. Drug Fact Sheet: Dextromethorphan (DXM). Drug Enforcement Administration. https://www.dea.gov/druginfo/drug_data_sheets/Detromethorphan.pdf. Accessed April 22, 2018.
8. Jacob R, Nicholapillai JN. Dextromethorphan induced bipolar disorder. Int Clin Psychopharmacol. 2012;28:e37-e38.
9. Hick JL, Smith SW, Lynch MT. Metabolic acidosis in restraint-associated cardiac arrest: a case series. Acad Emerg Med. 1999;6(3):239-243.
10. Mohr WK, Petti TA, Mohr BD. Adverse effects associated with physical restraint. Can J Psychiatry. 2003;48(5):330-337.
11. Otahbachi M, Cevik C, Bagdure S, et al. Excited delirium, restraints, and unexpected death: a review of pathogenesis. Am J Forensic Med Pathol. 2010;31(2):107-112.
1. Stanciu CN, Penders TM, Rouse EM. Recreational use of dextromethorphan,“Robotripping”-A brief review. Am J Addict. 2016;25(5):374-377.
2. Martinak B, Bolis RA, Black JR, et al. Dextromethorphan in cough syrup: The poor man’s psychosis. Psychopharmacol Bull. 2017;47(4):59-63.
3. Logan BK, Yeakel JK, Goldfogel G, et al. Dextromethorphan abuse leading to assault, suicide, or homicide. J Forensic Sci. 2012;57(5):1388-1394.
4. Dextromethorphan (Street names: DXM, CCC, Triple C, Skittles, Robo, Poor Man’s PCP). Drug Enforcement Administration. Office of Diversion Control. Drug & Chemical Evaluation Section. https://www.deadiversion.usdoj.gov/drug_chem_info/dextro_m.pdf. Published March 2014. Accessed April 22, 2018.
5. Reissig CJ, Carter LP, Johnson MW, et al. High doses of dextromethorphan, an NMDA antagonist, produce effects similar to classic hallucinogens. Psychopharmacology (Berl). 2012;223(1):1-15.
6. Boyer EW. Dextromethorphan abuse. Pediatr Emerg Care. 2004;20(12):858-863.
7. Drug Fact Sheet: Dextromethorphan (DXM). Drug Enforcement Administration. https://www.dea.gov/druginfo/drug_data_sheets/Detromethorphan.pdf. Accessed April 22, 2018.
8. Jacob R, Nicholapillai JN. Dextromethorphan induced bipolar disorder. Int Clin Psychopharmacol. 2012;28:e37-e38.
9. Hick JL, Smith SW, Lynch MT. Metabolic acidosis in restraint-associated cardiac arrest: a case series. Acad Emerg Med. 1999;6(3):239-243.
10. Mohr WK, Petti TA, Mohr BD. Adverse effects associated with physical restraint. Can J Psychiatry. 2003;48(5):330-337.
11. Otahbachi M, Cevik C, Bagdure S, et al. Excited delirium, restraints, and unexpected death: a review of pathogenesis. Am J Forensic Med Pathol. 2010;31(2):107-112.
Tardive dyskinesia: Screening and management
The DNA of psychiatric practice: A covenant with our patients
As the end of the academic year approaches, I always think of one last message to send to the freshly minted psychiatrists who will complete their 4 years of post-MD training. This year, I thought of emphasizing the principles of psychiatric practice, which the graduates will deliver for the next 4 to 5 decades of their professional lives. Those essential principles are coded in the DNA of psychiatric practice, just as the construction of all organs in the human body is coded within the DNA of the 22,000 genes that comprise our 23 chromosomes.
So here are the principles of psychiatry that I propose govern the relationship of psychiatrists with their patients, encrypted within the DNA of our esteemed medical specialty:
- Provide total dedication to helping psychiatric patients recover from their illness and regain their wellness.
- Maintain total and unimpeachable confidentiality.
- Demonstrate unconditional acceptance and respect to every patient.
- Adopt a nonjudgmental stance toward all patients.
- Establish a strong therapeutic alliance as early as possible. It is the center of the doctor–patient relationship.
- Provide the same standard of care to all patients—the same care you would want your family members to receive.
- Provide evidence-based treatments first, and if no response, use unapproved treatments judiciously, but above all, do no harm.
- Educate patients, and their families, about the illness, and discuss the benefits and risks of various treatments.
- Do not practice “naked psychopharmacology.” Psychotherapy must always be provided side-by-side with medications.
- Support the patient’s family. Their burden often is very heavy.
- Emphasize adherence as a key patient responsibility, and address it at every visit.
- Do not hesitate to consult a seasoned colleague about your complex clinical cases.
- Deal effectively with negative countertransference. Recognize it, and refer the patient to another colleague if you cannot resolve it.
- Always inquire about thoughts of harming self or others and act accordingly.
- Always ask about alcohol and substance use, and about over-the-counter drugs as well. They all can complicate your patient’s treatment course and outcome.
- Never breach boundaries with your patient, and firmly guide the patient about breaching boundaries with you.
- Uphold the medical tenet that all “mental” disorders of thought, mood, affect, behavior, and cognition are generated by disruptions of brain structure and/or function, whether molecular, cellular, or connectomic, caused by various combinations of genetic and/or environmental etiologies.
- Check your patients’ physical health status, including all treatments they received from other specialists, and always rule out iatrogenesis and disruptive pharmacokinetic interactions that may trigger or exacerbate psychiatric symptoms.
- Learn and use clinical rating scales to quantify symptom severity and adverse effects at baseline and at each visit. Measuring the severity of psychosis, depression, or anxiety in psychiatry is like measuring fasting glucose, triglycerides, or blood pressure in internal medicine.
- Use rational adjunctive and augmentation therapies when indicated, but avoid irrational and hazardous polypharmacy.
- Document your clinical findings, diagnosis, and treatment plan conscientiously and accurately. The medical record is a clinical, billing, legal, and research document.
- Advocate tirelessly for psychiatric patients to increase their access to care, and fight the unfair and hurtful stigma vigorously until it is completely erased. A psychiatric disorder should have no more stigma than a broken leg or peptic ulcer, and insurance parity must be identical as well.
- Establish collaborative care for each of your patients and link them to a primary care provider if they do not already have one. Disorders of the body and the brain are bidirectional in their effects and psychiatric patients often suffer from multiple organ diseases.
- Do some pro bono care for indigent or uninsured patients, and actively ask companies to provide free drugs to patients who cannot afford the medication you believe they need.
- Recognize that every treatment you use as the current standard of care was at one time a research project. Know that the research of today is the treatment of tomorrow. So support the creation of new medical knowledge by referring patients to FDA clinical trials or to National Institutes of Health–funded biologic investigations.
- No matter how busy you are, write a case report or a letter to the editor about an unusual response or adverse effect. This generates hypotheses that researchers can pursue and test.
- Volunteer to serve as a clinical supervisor for medical students and residents from your local medical school. Most academic departments of psychiatry appreciate their community-based volunteer faculty.
You, the readers of
As the end of the academic year approaches, I always think of one last message to send to the freshly minted psychiatrists who will complete their 4 years of post-MD training. This year, I thought of emphasizing the principles of psychiatric practice, which the graduates will deliver for the next 4 to 5 decades of their professional lives. Those essential principles are coded in the DNA of psychiatric practice, just as the construction of all organs in the human body is coded within the DNA of the 22,000 genes that comprise our 23 chromosomes.
So here are the principles of psychiatry that I propose govern the relationship of psychiatrists with their patients, encrypted within the DNA of our esteemed medical specialty:
- Provide total dedication to helping psychiatric patients recover from their illness and regain their wellness.
- Maintain total and unimpeachable confidentiality.
- Demonstrate unconditional acceptance and respect to every patient.
- Adopt a nonjudgmental stance toward all patients.
- Establish a strong therapeutic alliance as early as possible. It is the center of the doctor–patient relationship.
- Provide the same standard of care to all patients—the same care you would want your family members to receive.
- Provide evidence-based treatments first, and if no response, use unapproved treatments judiciously, but above all, do no harm.
- Educate patients, and their families, about the illness, and discuss the benefits and risks of various treatments.
- Do not practice “naked psychopharmacology.” Psychotherapy must always be provided side-by-side with medications.
- Support the patient’s family. Their burden often is very heavy.
- Emphasize adherence as a key patient responsibility, and address it at every visit.
- Do not hesitate to consult a seasoned colleague about your complex clinical cases.
- Deal effectively with negative countertransference. Recognize it, and refer the patient to another colleague if you cannot resolve it.
- Always inquire about thoughts of harming self or others and act accordingly.
- Always ask about alcohol and substance use, and about over-the-counter drugs as well. They all can complicate your patient’s treatment course and outcome.
- Never breach boundaries with your patient, and firmly guide the patient about breaching boundaries with you.
- Uphold the medical tenet that all “mental” disorders of thought, mood, affect, behavior, and cognition are generated by disruptions of brain structure and/or function, whether molecular, cellular, or connectomic, caused by various combinations of genetic and/or environmental etiologies.
- Check your patients’ physical health status, including all treatments they received from other specialists, and always rule out iatrogenesis and disruptive pharmacokinetic interactions that may trigger or exacerbate psychiatric symptoms.
- Learn and use clinical rating scales to quantify symptom severity and adverse effects at baseline and at each visit. Measuring the severity of psychosis, depression, or anxiety in psychiatry is like measuring fasting glucose, triglycerides, or blood pressure in internal medicine.
- Use rational adjunctive and augmentation therapies when indicated, but avoid irrational and hazardous polypharmacy.
- Document your clinical findings, diagnosis, and treatment plan conscientiously and accurately. The medical record is a clinical, billing, legal, and research document.
- Advocate tirelessly for psychiatric patients to increase their access to care, and fight the unfair and hurtful stigma vigorously until it is completely erased. A psychiatric disorder should have no more stigma than a broken leg or peptic ulcer, and insurance parity must be identical as well.
- Establish collaborative care for each of your patients and link them to a primary care provider if they do not already have one. Disorders of the body and the brain are bidirectional in their effects and psychiatric patients often suffer from multiple organ diseases.
- Do some pro bono care for indigent or uninsured patients, and actively ask companies to provide free drugs to patients who cannot afford the medication you believe they need.
- Recognize that every treatment you use as the current standard of care was at one time a research project. Know that the research of today is the treatment of tomorrow. So support the creation of new medical knowledge by referring patients to FDA clinical trials or to National Institutes of Health–funded biologic investigations.
- No matter how busy you are, write a case report or a letter to the editor about an unusual response or adverse effect. This generates hypotheses that researchers can pursue and test.
- Volunteer to serve as a clinical supervisor for medical students and residents from your local medical school. Most academic departments of psychiatry appreciate their community-based volunteer faculty.
You, the readers of
As the end of the academic year approaches, I always think of one last message to send to the freshly minted psychiatrists who will complete their 4 years of post-MD training. This year, I thought of emphasizing the principles of psychiatric practice, which the graduates will deliver for the next 4 to 5 decades of their professional lives. Those essential principles are coded in the DNA of psychiatric practice, just as the construction of all organs in the human body is coded within the DNA of the 22,000 genes that comprise our 23 chromosomes.
So here are the principles of psychiatry that I propose govern the relationship of psychiatrists with their patients, encrypted within the DNA of our esteemed medical specialty:
- Provide total dedication to helping psychiatric patients recover from their illness and regain their wellness.
- Maintain total and unimpeachable confidentiality.
- Demonstrate unconditional acceptance and respect to every patient.
- Adopt a nonjudgmental stance toward all patients.
- Establish a strong therapeutic alliance as early as possible. It is the center of the doctor–patient relationship.
- Provide the same standard of care to all patients—the same care you would want your family members to receive.
- Provide evidence-based treatments first, and if no response, use unapproved treatments judiciously, but above all, do no harm.
- Educate patients, and their families, about the illness, and discuss the benefits and risks of various treatments.
- Do not practice “naked psychopharmacology.” Psychotherapy must always be provided side-by-side with medications.
- Support the patient’s family. Their burden often is very heavy.
- Emphasize adherence as a key patient responsibility, and address it at every visit.
- Do not hesitate to consult a seasoned colleague about your complex clinical cases.
- Deal effectively with negative countertransference. Recognize it, and refer the patient to another colleague if you cannot resolve it.
- Always inquire about thoughts of harming self or others and act accordingly.
- Always ask about alcohol and substance use, and about over-the-counter drugs as well. They all can complicate your patient’s treatment course and outcome.
- Never breach boundaries with your patient, and firmly guide the patient about breaching boundaries with you.
- Uphold the medical tenet that all “mental” disorders of thought, mood, affect, behavior, and cognition are generated by disruptions of brain structure and/or function, whether molecular, cellular, or connectomic, caused by various combinations of genetic and/or environmental etiologies.
- Check your patients’ physical health status, including all treatments they received from other specialists, and always rule out iatrogenesis and disruptive pharmacokinetic interactions that may trigger or exacerbate psychiatric symptoms.
- Learn and use clinical rating scales to quantify symptom severity and adverse effects at baseline and at each visit. Measuring the severity of psychosis, depression, or anxiety in psychiatry is like measuring fasting glucose, triglycerides, or blood pressure in internal medicine.
- Use rational adjunctive and augmentation therapies when indicated, but avoid irrational and hazardous polypharmacy.
- Document your clinical findings, diagnosis, and treatment plan conscientiously and accurately. The medical record is a clinical, billing, legal, and research document.
- Advocate tirelessly for psychiatric patients to increase their access to care, and fight the unfair and hurtful stigma vigorously until it is completely erased. A psychiatric disorder should have no more stigma than a broken leg or peptic ulcer, and insurance parity must be identical as well.
- Establish collaborative care for each of your patients and link them to a primary care provider if they do not already have one. Disorders of the body and the brain are bidirectional in their effects and psychiatric patients often suffer from multiple organ diseases.
- Do some pro bono care for indigent or uninsured patients, and actively ask companies to provide free drugs to patients who cannot afford the medication you believe they need.
- Recognize that every treatment you use as the current standard of care was at one time a research project. Know that the research of today is the treatment of tomorrow. So support the creation of new medical knowledge by referring patients to FDA clinical trials or to National Institutes of Health–funded biologic investigations.
- No matter how busy you are, write a case report or a letter to the editor about an unusual response or adverse effect. This generates hypotheses that researchers can pursue and test.
- Volunteer to serve as a clinical supervisor for medical students and residents from your local medical school. Most academic departments of psychiatry appreciate their community-based volunteer faculty.
You, the readers of
The Goldwater Rule and free speech, the current 'political morass', and more
In his editorial, “The toxic zeitgeist of hyper-partisanship: A psychiatric perspective” (From the Editor,
One’s political views do not inform us of his or her mental health status. This appreciation can be obtained only by a thorough psychological assessment. This is the basis of the Goldwater Rule, coupled with the ethical responsibility not to discuss patients’ private communications.
Today, this rule is tested by the behavior and actions of President Donald Trump. Proponents of the Goldwater Rule state that a psychiatrist cannot diagnose someone without performing a face-to-face diagnostic evaluation. This assumes psychiatrists diagnose patients only by interviewing them. However, any psychiatrist who has worked in an emergency room has signed involuntary commitment papers for a patient who refuses to talk to them. This clinical action typically is based on reports of the patient’s potential dangerousness from family, friends, or the police.
The diagnostic criteria for some personality disorders are based only on observed or reported behavior. They do not indicate a need for an interview. The diagnosis of a personality disorder cannot be made solely by interviewing an individual without knowledge of his or her behavior. Interviewing Bernie Madoff would not have revealed his sociopathic behavior.
The critical question may not be whether one could ethically make a psychiatric diagnosis of the President (I believe you can), but rather would it indicate or imply that he is dangerous? History informs us that the existence of a psychiatric disorder does not determine a politician’s fitness for office or if they are dangerous. Behavioral accounts of President Abraham Lincoln and his self-reports seem to confirm that at times he was depressed, but he clearly served our country with distinction.
Finally, it is not clear whether the Goldwater Rule is legal. It arguably interferes with a psychiatrist’s right of free speech without the risk of being accused of unethical behavior. I wonder what would happen if it were tested in court. Does the First Amendment of the U.S. Constitution protect a psychiatrist’s right to speak freely?
Sidney Weissman, MD
Clinical Professor of Psychiatry and Behavioral Science
Feinberg School of Medicine
Northwestern University
Chicago, Illinois
The current ‘political morass’
Thank you, Dr. Nasrallah, for the wonderful synopsis of the current political morass in your editorial (From the Editor,
James Gallagher, MD
Private psychiatric practice
Des Moines, Iowa
Continue to: The biological etiology of compulsive sexual behavior
The biological etiology of compulsive sexual behavior
Dr. Grant’s article, “Compulsive sexual behavior: A nonjudgmental approach” (Evidence-Based Reviews,
Mukesh Sanghadia, MD, MRCPsych (UK), Diplomate ABPN
PsychiatristCommunity Research Foundation
San Diego, California
The author responds
Dr. Sanghadia highlights the lack of possible biological etiology of compulsive sexual behavior (CSB) in my article. This is a fair comment. The lack of agreed-upon diagnostic criteria, however, has resulted in a vast literature discussing sexual behaviors that may or may not be related to each other, and even suggest that what is currently referred to as CSB may in fact be quite heterogeneous. My article mentions the few neuroimaging and neurocognitive studies that address a more rigorously defined CSB. Other possible etiologies have been suggested for a range of out-of-control sexual behaviors, but have not been studied with a focus on this formal diagnostic category. For example, endocrine issues have been explored to some extent in individuals with paraphilic sexual behaviors (behaviors that appear to many to have no relationship to CSB as discussed in my article), and in those cases of paraphilic sexual behavior, a range of endocrine hormones have been examined—gonadotropin-releasing hormone, follicle-stimulating hormone, luteinizing hormone, testosterone/dihydrotestosterone, and estrogen/progesterone. But these studies have yielded no conclusive outcomes in terms of findings or treatments.
In summary, the biology of CSB lags far behind that of other mental health disorders (and even other psychiatric disorders lack conclusive biological etiologies). Establishing this behavior as a legitimate diagnostic entity with agreed-upon criteria may be the first step in furthering our understanding of its possible biology.
Jon E. Grant, JD, MD, MPH
Professor
Department of Psychiatry and Behavioral Neuroscience
University of Chicago, Pritzker School of Medicine
Chicago, Illinois
Continue to: A different view of patients with schizophrenia
A different view of patients with schizophrenia
After treating patients with schizophrenia for more than 30 years, I’ve observed a continuous flood of information about them. This overload has been consistent since my residency back in the 1980s. Theories ranging from the psychoanalytic to the biologic are numerous and valuable additions to our understanding of those who suffer with this malady, yet they provide no summation or overview with which to understand it.
For instance, we know that schizophrenia usually begins in the late teens or early twenties. We know that antidopaminergic medications usually help to varying degrees. Psychosocial interventions may contribute greatly to the ultimate outcome. Substance use invariably makes it worse. Establishing a connection with the patient can often be helpful. Medication compliance is crucial.
It is more or less accepted that there is deterioration of higher brain functions, hypofrontality, as well as so-called dysconnectivity of white matter. There is a genetic vulnerability, and there seems to be an excess of inflammation and changes in mitochondria. Most patients have low functioning, poor compensation, and a lack of social adeptness. However, some patients can recover quite nicely. Although most of us would agree that this is not dementia, we’d also concede that these patients’ cognitive functioning is not what it used to be. Electroconvulsive therapy also can sometimes be helpful.
So, how are we to view our patients with schizophrenia in a way that can be illuminating and give us a deeper sense of understanding this quizzical disorder? It has been helpful to me to regard these individuals as a people whose brain function has been usurped by a more primitive organization that is characterized by:
- a reduction in mental development, where patients function in a more childlike way with magical thinking and impaired reality-testing
- atrophy of higher brain structures, leading to hallucinatory experiences
- a hyper-dominergic state
- a usually gradual onset with some evidence of struggle between the old and new brain organizations
- impaired prepulse inhibition that’s likely secondary to diffuseness of thought
- eventual demise of higher brain structures with an inability to respond to anti-dopaminergics. (Antipsychotics can push the brain organization closer to the adult structure attained before the onset of the disease, at least initially.)
The list goes on. Thinking about patients with schizophrenia in this way allows me to appreciate what I feel is a more encompassing view of who they are and how they got there. I have some theories about where this more primitive organization may have originated, but whatever its origin, in a small percentage of people it is there, ready to assume control of their thinking just as they are reaching reproductive age. Early intervention and medication compliance may minimize damage.
If a theory helps us gain a greater understanding of our patients, then it’s worth considering. This proposition fits much of what we know about schizophrenia. Reading patients’ firsthand accounts of the illness helps confirm, in my opinion, this point of view.
Steven Lesk, MD
Private psychiatric practice
Fridley, Minnesota
Continue to: Cognitive impairment in schizophrenia
Cognitive impairment in schizophrenia
The authors of “Suspicious, sleepless, and smoking” (Cases That Test Your Skills,
During the past 15 years, I have routinely measured cognitive functioning in patients with schizophrenia. Some have no impairment, some have severe impairment, and some fall in between these extremes. Most often, impairment occurs in the area of executive function, which can lead to significant disability. Indeed, positive symptoms can clear up completely with treatment, but the deficits in executive functioning can remain.
I think it is fair to say that cognitive impairment is a common, although not nearly universal, feature of schizophrenia that sometimes improves with antipsychotic medication. I look forward to the advent of more clinicians paying attention to the issue of cognition in schizophrenia and, hopefully, better treatments for it.
John M. Mahoney, PhD
Shasta Psychiatric Hospital
Redding, California
The authors respond
We thank Dr. Mahoney for his thoughtful letter and queries into the case of Mr. F.
First, regarding the prevalence of cognitive impairment in schizophrenia, it is our opinion that cognitive impairment is a distinct, core, and nearly universal feature of schizophrenia. This also is the conclusion of many clinicians and researchers based on their significant work in the field; still, just as in our initial case study, we concede that these symptoms are not part of the DSM-5’s formal diagnostic criteria.
The core question Dr. Mahoney seems to pose is whether we contradicted ourselves. We assert that cognitive impairment in schizophrenia is not effectively treated with existing medications, and yet we described Mr. F’s cognitive improvement after he received risperidone, 2 mg/d, titrated up to 2 mg twice daily. We first pointed out that part of our treatment strategy was to target comorbid depression in this patient; nonetheless, Dr. Mahoney’s question remains valid, and we will attempt to answer.
Dr. Mahoney has observed that his patients with schizophrenia variably experience improved cognition, and notes that executive function is a particularly common lingering impairment. On this we wholly agree; this is a helpful point of clarification, and a useful distinction in light of the above question. Improvement in positive and negative symptoms of schizophrenia, as psychosis resolves, is a well-known and studied effect of antipsychotic therapy. As a result, the sensorium becomes more congruent with external reality, and one would expect the patient to display improved orientation. This then might be reasonably expected to produce mental status improvements; however, while some improvement is frequently observed, this is neither consistent nor complete improvement. In the case of Mr. F, we document improvement, but also significant continued impairment. Thus, we maintain that treating the cognitive symptoms of schizophrenia with antipsychotics has been largely ineffective.
We do not see this as a slight distinction or an argument of minutiae. That patients frequently experience some degree of lingering impairment is a salient point. Neurocognitive impairment is a strong contributor to and predictor of disability in schizophrenia, and neurocognitive abilities most strongly predict functional outcomes. From a patient’s point of view, these symptoms have real-world consequences. Thus, we believe they should be evaluated and treated as aggressively and consistently as other schizophrenia symptoms.
In our case, we attempted to convey one primary message: Despite the challenges of treatment, there are viable options that should be pursued in the treatment of schizophrenia-related cognitive impairments. Nonpharmacologic modalities have shown encouraging results. Cognitive remediation therapy produces durable cognitive improvement—especially when combined with adjunctive therapies, such as small group therapy and vocational rehabilitation, and when comorbid conditions (major depressive disorder in Mr. F’s case) are treated.
In summary, we reiterate that cognitive impairments in schizophrenia represent a strong predictor of patient-oriented outcomes; we maintain our assertion regarding their inadequate treatment with existing medications; and we suggest that future trials attempt to find effective alternative strategies. We encourage psychiatric clinicians to approach treatment of this facet of pathology with an open mind, and to utilize alternative multi-modal therapies for the benefit of their patients with schizophrenia while waiting for new safe and effective pharmaceutical regimens.
Jarrett Dawson, MD
Family medicine resident
Department of Psychiatry
Saint Louis University
St. Louis, Missouri
Catalina Belean, MD
Assistant Professor
Department of Psychiatry
Saint Louis University
St. Louis, Missouri
In his editorial, “The toxic zeitgeist of hyper-partisanship: A psychiatric perspective” (From the Editor,
One’s political views do not inform us of his or her mental health status. This appreciation can be obtained only by a thorough psychological assessment. This is the basis of the Goldwater Rule, coupled with the ethical responsibility not to discuss patients’ private communications.
Today, this rule is tested by the behavior and actions of President Donald Trump. Proponents of the Goldwater Rule state that a psychiatrist cannot diagnose someone without performing a face-to-face diagnostic evaluation. This assumes psychiatrists diagnose patients only by interviewing them. However, any psychiatrist who has worked in an emergency room has signed involuntary commitment papers for a patient who refuses to talk to them. This clinical action typically is based on reports of the patient’s potential dangerousness from family, friends, or the police.
The diagnostic criteria for some personality disorders are based only on observed or reported behavior. They do not indicate a need for an interview. The diagnosis of a personality disorder cannot be made solely by interviewing an individual without knowledge of his or her behavior. Interviewing Bernie Madoff would not have revealed his sociopathic behavior.
The critical question may not be whether one could ethically make a psychiatric diagnosis of the President (I believe you can), but rather would it indicate or imply that he is dangerous? History informs us that the existence of a psychiatric disorder does not determine a politician’s fitness for office or if they are dangerous. Behavioral accounts of President Abraham Lincoln and his self-reports seem to confirm that at times he was depressed, but he clearly served our country with distinction.
Finally, it is not clear whether the Goldwater Rule is legal. It arguably interferes with a psychiatrist’s right of free speech without the risk of being accused of unethical behavior. I wonder what would happen if it were tested in court. Does the First Amendment of the U.S. Constitution protect a psychiatrist’s right to speak freely?
Sidney Weissman, MD
Clinical Professor of Psychiatry and Behavioral Science
Feinberg School of Medicine
Northwestern University
Chicago, Illinois
The current ‘political morass’
Thank you, Dr. Nasrallah, for the wonderful synopsis of the current political morass in your editorial (From the Editor,
James Gallagher, MD
Private psychiatric practice
Des Moines, Iowa
Continue to: The biological etiology of compulsive sexual behavior
The biological etiology of compulsive sexual behavior
Dr. Grant’s article, “Compulsive sexual behavior: A nonjudgmental approach” (Evidence-Based Reviews,
Mukesh Sanghadia, MD, MRCPsych (UK), Diplomate ABPN
PsychiatristCommunity Research Foundation
San Diego, California
The author responds
Dr. Sanghadia highlights the lack of possible biological etiology of compulsive sexual behavior (CSB) in my article. This is a fair comment. The lack of agreed-upon diagnostic criteria, however, has resulted in a vast literature discussing sexual behaviors that may or may not be related to each other, and even suggest that what is currently referred to as CSB may in fact be quite heterogeneous. My article mentions the few neuroimaging and neurocognitive studies that address a more rigorously defined CSB. Other possible etiologies have been suggested for a range of out-of-control sexual behaviors, but have not been studied with a focus on this formal diagnostic category. For example, endocrine issues have been explored to some extent in individuals with paraphilic sexual behaviors (behaviors that appear to many to have no relationship to CSB as discussed in my article), and in those cases of paraphilic sexual behavior, a range of endocrine hormones have been examined—gonadotropin-releasing hormone, follicle-stimulating hormone, luteinizing hormone, testosterone/dihydrotestosterone, and estrogen/progesterone. But these studies have yielded no conclusive outcomes in terms of findings or treatments.
In summary, the biology of CSB lags far behind that of other mental health disorders (and even other psychiatric disorders lack conclusive biological etiologies). Establishing this behavior as a legitimate diagnostic entity with agreed-upon criteria may be the first step in furthering our understanding of its possible biology.
Jon E. Grant, JD, MD, MPH
Professor
Department of Psychiatry and Behavioral Neuroscience
University of Chicago, Pritzker School of Medicine
Chicago, Illinois
Continue to: A different view of patients with schizophrenia
A different view of patients with schizophrenia
After treating patients with schizophrenia for more than 30 years, I’ve observed a continuous flood of information about them. This overload has been consistent since my residency back in the 1980s. Theories ranging from the psychoanalytic to the biologic are numerous and valuable additions to our understanding of those who suffer with this malady, yet they provide no summation or overview with which to understand it.
For instance, we know that schizophrenia usually begins in the late teens or early twenties. We know that antidopaminergic medications usually help to varying degrees. Psychosocial interventions may contribute greatly to the ultimate outcome. Substance use invariably makes it worse. Establishing a connection with the patient can often be helpful. Medication compliance is crucial.
It is more or less accepted that there is deterioration of higher brain functions, hypofrontality, as well as so-called dysconnectivity of white matter. There is a genetic vulnerability, and there seems to be an excess of inflammation and changes in mitochondria. Most patients have low functioning, poor compensation, and a lack of social adeptness. However, some patients can recover quite nicely. Although most of us would agree that this is not dementia, we’d also concede that these patients’ cognitive functioning is not what it used to be. Electroconvulsive therapy also can sometimes be helpful.
So, how are we to view our patients with schizophrenia in a way that can be illuminating and give us a deeper sense of understanding this quizzical disorder? It has been helpful to me to regard these individuals as a people whose brain function has been usurped by a more primitive organization that is characterized by:
- a reduction in mental development, where patients function in a more childlike way with magical thinking and impaired reality-testing
- atrophy of higher brain structures, leading to hallucinatory experiences
- a hyper-dominergic state
- a usually gradual onset with some evidence of struggle between the old and new brain organizations
- impaired prepulse inhibition that’s likely secondary to diffuseness of thought
- eventual demise of higher brain structures with an inability to respond to anti-dopaminergics. (Antipsychotics can push the brain organization closer to the adult structure attained before the onset of the disease, at least initially.)
The list goes on. Thinking about patients with schizophrenia in this way allows me to appreciate what I feel is a more encompassing view of who they are and how they got there. I have some theories about where this more primitive organization may have originated, but whatever its origin, in a small percentage of people it is there, ready to assume control of their thinking just as they are reaching reproductive age. Early intervention and medication compliance may minimize damage.
If a theory helps us gain a greater understanding of our patients, then it’s worth considering. This proposition fits much of what we know about schizophrenia. Reading patients’ firsthand accounts of the illness helps confirm, in my opinion, this point of view.
Steven Lesk, MD
Private psychiatric practice
Fridley, Minnesota
Continue to: Cognitive impairment in schizophrenia
Cognitive impairment in schizophrenia
The authors of “Suspicious, sleepless, and smoking” (Cases That Test Your Skills,
During the past 15 years, I have routinely measured cognitive functioning in patients with schizophrenia. Some have no impairment, some have severe impairment, and some fall in between these extremes. Most often, impairment occurs in the area of executive function, which can lead to significant disability. Indeed, positive symptoms can clear up completely with treatment, but the deficits in executive functioning can remain.
I think it is fair to say that cognitive impairment is a common, although not nearly universal, feature of schizophrenia that sometimes improves with antipsychotic medication. I look forward to the advent of more clinicians paying attention to the issue of cognition in schizophrenia and, hopefully, better treatments for it.
John M. Mahoney, PhD
Shasta Psychiatric Hospital
Redding, California
The authors respond
We thank Dr. Mahoney for his thoughtful letter and queries into the case of Mr. F.
First, regarding the prevalence of cognitive impairment in schizophrenia, it is our opinion that cognitive impairment is a distinct, core, and nearly universal feature of schizophrenia. This also is the conclusion of many clinicians and researchers based on their significant work in the field; still, just as in our initial case study, we concede that these symptoms are not part of the DSM-5’s formal diagnostic criteria.
The core question Dr. Mahoney seems to pose is whether we contradicted ourselves. We assert that cognitive impairment in schizophrenia is not effectively treated with existing medications, and yet we described Mr. F’s cognitive improvement after he received risperidone, 2 mg/d, titrated up to 2 mg twice daily. We first pointed out that part of our treatment strategy was to target comorbid depression in this patient; nonetheless, Dr. Mahoney’s question remains valid, and we will attempt to answer.
Dr. Mahoney has observed that his patients with schizophrenia variably experience improved cognition, and notes that executive function is a particularly common lingering impairment. On this we wholly agree; this is a helpful point of clarification, and a useful distinction in light of the above question. Improvement in positive and negative symptoms of schizophrenia, as psychosis resolves, is a well-known and studied effect of antipsychotic therapy. As a result, the sensorium becomes more congruent with external reality, and one would expect the patient to display improved orientation. This then might be reasonably expected to produce mental status improvements; however, while some improvement is frequently observed, this is neither consistent nor complete improvement. In the case of Mr. F, we document improvement, but also significant continued impairment. Thus, we maintain that treating the cognitive symptoms of schizophrenia with antipsychotics has been largely ineffective.
We do not see this as a slight distinction or an argument of minutiae. That patients frequently experience some degree of lingering impairment is a salient point. Neurocognitive impairment is a strong contributor to and predictor of disability in schizophrenia, and neurocognitive abilities most strongly predict functional outcomes. From a patient’s point of view, these symptoms have real-world consequences. Thus, we believe they should be evaluated and treated as aggressively and consistently as other schizophrenia symptoms.
In our case, we attempted to convey one primary message: Despite the challenges of treatment, there are viable options that should be pursued in the treatment of schizophrenia-related cognitive impairments. Nonpharmacologic modalities have shown encouraging results. Cognitive remediation therapy produces durable cognitive improvement—especially when combined with adjunctive therapies, such as small group therapy and vocational rehabilitation, and when comorbid conditions (major depressive disorder in Mr. F’s case) are treated.
In summary, we reiterate that cognitive impairments in schizophrenia represent a strong predictor of patient-oriented outcomes; we maintain our assertion regarding their inadequate treatment with existing medications; and we suggest that future trials attempt to find effective alternative strategies. We encourage psychiatric clinicians to approach treatment of this facet of pathology with an open mind, and to utilize alternative multi-modal therapies for the benefit of their patients with schizophrenia while waiting for new safe and effective pharmaceutical regimens.
Jarrett Dawson, MD
Family medicine resident
Department of Psychiatry
Saint Louis University
St. Louis, Missouri
Catalina Belean, MD
Assistant Professor
Department of Psychiatry
Saint Louis University
St. Louis, Missouri
In his editorial, “The toxic zeitgeist of hyper-partisanship: A psychiatric perspective” (From the Editor,
One’s political views do not inform us of his or her mental health status. This appreciation can be obtained only by a thorough psychological assessment. This is the basis of the Goldwater Rule, coupled with the ethical responsibility not to discuss patients’ private communications.
Today, this rule is tested by the behavior and actions of President Donald Trump. Proponents of the Goldwater Rule state that a psychiatrist cannot diagnose someone without performing a face-to-face diagnostic evaluation. This assumes psychiatrists diagnose patients only by interviewing them. However, any psychiatrist who has worked in an emergency room has signed involuntary commitment papers for a patient who refuses to talk to them. This clinical action typically is based on reports of the patient’s potential dangerousness from family, friends, or the police.
The diagnostic criteria for some personality disorders are based only on observed or reported behavior. They do not indicate a need for an interview. The diagnosis of a personality disorder cannot be made solely by interviewing an individual without knowledge of his or her behavior. Interviewing Bernie Madoff would not have revealed his sociopathic behavior.
The critical question may not be whether one could ethically make a psychiatric diagnosis of the President (I believe you can), but rather would it indicate or imply that he is dangerous? History informs us that the existence of a psychiatric disorder does not determine a politician’s fitness for office or if they are dangerous. Behavioral accounts of President Abraham Lincoln and his self-reports seem to confirm that at times he was depressed, but he clearly served our country with distinction.
Finally, it is not clear whether the Goldwater Rule is legal. It arguably interferes with a psychiatrist’s right of free speech without the risk of being accused of unethical behavior. I wonder what would happen if it were tested in court. Does the First Amendment of the U.S. Constitution protect a psychiatrist’s right to speak freely?
Sidney Weissman, MD
Clinical Professor of Psychiatry and Behavioral Science
Feinberg School of Medicine
Northwestern University
Chicago, Illinois
The current ‘political morass’
Thank you, Dr. Nasrallah, for the wonderful synopsis of the current political morass in your editorial (From the Editor,
James Gallagher, MD
Private psychiatric practice
Des Moines, Iowa
Continue to: The biological etiology of compulsive sexual behavior
The biological etiology of compulsive sexual behavior
Dr. Grant’s article, “Compulsive sexual behavior: A nonjudgmental approach” (Evidence-Based Reviews,
Mukesh Sanghadia, MD, MRCPsych (UK), Diplomate ABPN
PsychiatristCommunity Research Foundation
San Diego, California
The author responds
Dr. Sanghadia highlights the lack of possible biological etiology of compulsive sexual behavior (CSB) in my article. This is a fair comment. The lack of agreed-upon diagnostic criteria, however, has resulted in a vast literature discussing sexual behaviors that may or may not be related to each other, and even suggest that what is currently referred to as CSB may in fact be quite heterogeneous. My article mentions the few neuroimaging and neurocognitive studies that address a more rigorously defined CSB. Other possible etiologies have been suggested for a range of out-of-control sexual behaviors, but have not been studied with a focus on this formal diagnostic category. For example, endocrine issues have been explored to some extent in individuals with paraphilic sexual behaviors (behaviors that appear to many to have no relationship to CSB as discussed in my article), and in those cases of paraphilic sexual behavior, a range of endocrine hormones have been examined—gonadotropin-releasing hormone, follicle-stimulating hormone, luteinizing hormone, testosterone/dihydrotestosterone, and estrogen/progesterone. But these studies have yielded no conclusive outcomes in terms of findings or treatments.
In summary, the biology of CSB lags far behind that of other mental health disorders (and even other psychiatric disorders lack conclusive biological etiologies). Establishing this behavior as a legitimate diagnostic entity with agreed-upon criteria may be the first step in furthering our understanding of its possible biology.
Jon E. Grant, JD, MD, MPH
Professor
Department of Psychiatry and Behavioral Neuroscience
University of Chicago, Pritzker School of Medicine
Chicago, Illinois
Continue to: A different view of patients with schizophrenia
A different view of patients with schizophrenia
After treating patients with schizophrenia for more than 30 years, I’ve observed a continuous flood of information about them. This overload has been consistent since my residency back in the 1980s. Theories ranging from the psychoanalytic to the biologic are numerous and valuable additions to our understanding of those who suffer with this malady, yet they provide no summation or overview with which to understand it.
For instance, we know that schizophrenia usually begins in the late teens or early twenties. We know that antidopaminergic medications usually help to varying degrees. Psychosocial interventions may contribute greatly to the ultimate outcome. Substance use invariably makes it worse. Establishing a connection with the patient can often be helpful. Medication compliance is crucial.
It is more or less accepted that there is deterioration of higher brain functions, hypofrontality, as well as so-called dysconnectivity of white matter. There is a genetic vulnerability, and there seems to be an excess of inflammation and changes in mitochondria. Most patients have low functioning, poor compensation, and a lack of social adeptness. However, some patients can recover quite nicely. Although most of us would agree that this is not dementia, we’d also concede that these patients’ cognitive functioning is not what it used to be. Electroconvulsive therapy also can sometimes be helpful.
So, how are we to view our patients with schizophrenia in a way that can be illuminating and give us a deeper sense of understanding this quizzical disorder? It has been helpful to me to regard these individuals as a people whose brain function has been usurped by a more primitive organization that is characterized by:
- a reduction in mental development, where patients function in a more childlike way with magical thinking and impaired reality-testing
- atrophy of higher brain structures, leading to hallucinatory experiences
- a hyper-dominergic state
- a usually gradual onset with some evidence of struggle between the old and new brain organizations
- impaired prepulse inhibition that’s likely secondary to diffuseness of thought
- eventual demise of higher brain structures with an inability to respond to anti-dopaminergics. (Antipsychotics can push the brain organization closer to the adult structure attained before the onset of the disease, at least initially.)
The list goes on. Thinking about patients with schizophrenia in this way allows me to appreciate what I feel is a more encompassing view of who they are and how they got there. I have some theories about where this more primitive organization may have originated, but whatever its origin, in a small percentage of people it is there, ready to assume control of their thinking just as they are reaching reproductive age. Early intervention and medication compliance may minimize damage.
If a theory helps us gain a greater understanding of our patients, then it’s worth considering. This proposition fits much of what we know about schizophrenia. Reading patients’ firsthand accounts of the illness helps confirm, in my opinion, this point of view.
Steven Lesk, MD
Private psychiatric practice
Fridley, Minnesota
Continue to: Cognitive impairment in schizophrenia
Cognitive impairment in schizophrenia
The authors of “Suspicious, sleepless, and smoking” (Cases That Test Your Skills,
During the past 15 years, I have routinely measured cognitive functioning in patients with schizophrenia. Some have no impairment, some have severe impairment, and some fall in between these extremes. Most often, impairment occurs in the area of executive function, which can lead to significant disability. Indeed, positive symptoms can clear up completely with treatment, but the deficits in executive functioning can remain.
I think it is fair to say that cognitive impairment is a common, although not nearly universal, feature of schizophrenia that sometimes improves with antipsychotic medication. I look forward to the advent of more clinicians paying attention to the issue of cognition in schizophrenia and, hopefully, better treatments for it.
John M. Mahoney, PhD
Shasta Psychiatric Hospital
Redding, California
The authors respond
We thank Dr. Mahoney for his thoughtful letter and queries into the case of Mr. F.
First, regarding the prevalence of cognitive impairment in schizophrenia, it is our opinion that cognitive impairment is a distinct, core, and nearly universal feature of schizophrenia. This also is the conclusion of many clinicians and researchers based on their significant work in the field; still, just as in our initial case study, we concede that these symptoms are not part of the DSM-5’s formal diagnostic criteria.
The core question Dr. Mahoney seems to pose is whether we contradicted ourselves. We assert that cognitive impairment in schizophrenia is not effectively treated with existing medications, and yet we described Mr. F’s cognitive improvement after he received risperidone, 2 mg/d, titrated up to 2 mg twice daily. We first pointed out that part of our treatment strategy was to target comorbid depression in this patient; nonetheless, Dr. Mahoney’s question remains valid, and we will attempt to answer.
Dr. Mahoney has observed that his patients with schizophrenia variably experience improved cognition, and notes that executive function is a particularly common lingering impairment. On this we wholly agree; this is a helpful point of clarification, and a useful distinction in light of the above question. Improvement in positive and negative symptoms of schizophrenia, as psychosis resolves, is a well-known and studied effect of antipsychotic therapy. As a result, the sensorium becomes more congruent with external reality, and one would expect the patient to display improved orientation. This then might be reasonably expected to produce mental status improvements; however, while some improvement is frequently observed, this is neither consistent nor complete improvement. In the case of Mr. F, we document improvement, but also significant continued impairment. Thus, we maintain that treating the cognitive symptoms of schizophrenia with antipsychotics has been largely ineffective.
We do not see this as a slight distinction or an argument of minutiae. That patients frequently experience some degree of lingering impairment is a salient point. Neurocognitive impairment is a strong contributor to and predictor of disability in schizophrenia, and neurocognitive abilities most strongly predict functional outcomes. From a patient’s point of view, these symptoms have real-world consequences. Thus, we believe they should be evaluated and treated as aggressively and consistently as other schizophrenia symptoms.
In our case, we attempted to convey one primary message: Despite the challenges of treatment, there are viable options that should be pursued in the treatment of schizophrenia-related cognitive impairments. Nonpharmacologic modalities have shown encouraging results. Cognitive remediation therapy produces durable cognitive improvement—especially when combined with adjunctive therapies, such as small group therapy and vocational rehabilitation, and when comorbid conditions (major depressive disorder in Mr. F’s case) are treated.
In summary, we reiterate that cognitive impairments in schizophrenia represent a strong predictor of patient-oriented outcomes; we maintain our assertion regarding their inadequate treatment with existing medications; and we suggest that future trials attempt to find effective alternative strategies. We encourage psychiatric clinicians to approach treatment of this facet of pathology with an open mind, and to utilize alternative multi-modal therapies for the benefit of their patients with schizophrenia while waiting for new safe and effective pharmaceutical regimens.
Jarrett Dawson, MD
Family medicine resident
Department of Psychiatry
Saint Louis University
St. Louis, Missouri
Catalina Belean, MD
Assistant Professor
Department of Psychiatry
Saint Louis University
St. Louis, Missouri
Aggressive outbursts and emotional lability in a 16-year-old boy
Mr. X, age 16, has cerebral palsy (CP), idiopathic normal pressure hydrocephalus (iNPH), and a history of impulse control disorder and behavioral instability, including episodes of aggression or combativeness. Mr. X’s mother reports that these episodes are almost always preceded by inappropriate laughing or crying. His outbursts and emotional lability have gotten worse during the last 6 months. Due to his disruptive behaviors, Mr. X has been unable to attend school, and his parents are considering group home placement. Although they were previously able to control their son’s aggressive behaviors, they fear for his safety, and after one such episode, they call 911. Mr. X is transported by police in handcuffs to the comprehensive psychiatric emergency room (CPEP) for evaluation.
While in CPEP, Mr. X remains uncooperative and disruptive; subsequently, he is placed in 4-point restraints and given
[polldaddy:9991896]
The authors’ observations
Pseudobulbar affect (PBA) is a disorder characterized by sporadic episodes of inappropriate laughing and/or crying that are incongruent with situational context and are frequently exaggerated in comparison with the actual feelings of the patient. The duration of PBA episodes can last seconds to minutes and arise unpredictably.
PBA typically develops secondary to a neurologic disorder, most commonly Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), Parkinson’s disease (PD), stroke, or traumatic brain injury (TBI).1 PBA symptoms are present in an estimated 29.3% of patients with AD, 44.8% of patients with ALS, 45.8% of patients with MS, 26% of patients with PD, 37.8% of patients with stroke, and 52.4% of patients with TBI.2 Although PBA appears far more frequently in patients with MS or ALS compared with those with PD, PD represents an under-recognized and larger patient population. A small fraction of patients also develops PBA secondary to hyperthyroidism, hypothyroidism, Graves’ disease, Wilson’s disease, brain tumors, and a multitude of encephalopathies.3 These neurologic disorders cause dysregulation of the corticopontine-cerebellar circuitry, resulting in functional impediment to the normal affect modulator action of the cerebellum.4
The neurologic insults that can result in PBA may include CP or iNPH. Cerebellar injury is a frequent pathological finding in CP.5 In patients with iNPH, in addition to altered CSF flow, enlarged ventricles compress the corticospinal tracts in the lateral ventricles,6 which is theorized to induce PBA symptoms.
PBA is diagnosed by subjective clinical evaluation and by using the Center for Neurologic Study–Lability Scale (CNS-LS). The CNS-LS is a 7-question survey that addresses the severity of affect lability (Table 17). It may be completed by the patient or caregiver. Each question ranges in score from 1 to 5, with the total score ranging from 7 to 35. The minimum score required for the diagnosis of PBA is 13.7
PBA is frequently misdiagnosed as depression, although the 2 disorders can occur simultaneously (Table 21,8). A crucial distinguishing factor between depression and PBA is the extent of symptoms. Depression presents as feelings of sadness associated with crying and disinterest that occur for weeks to months. In contrast, PBA presents as brief, uncontrollable episodes of laughing and/or crying that last seconds to minutes. Unlike depression, the behaviors associated with PBA are exaggerated or do not match the patient’s feelings. Furthermore, a neurologic disease or brain injury is always present in a patient with PBA, but is not imperative for the diagnosis of depression.
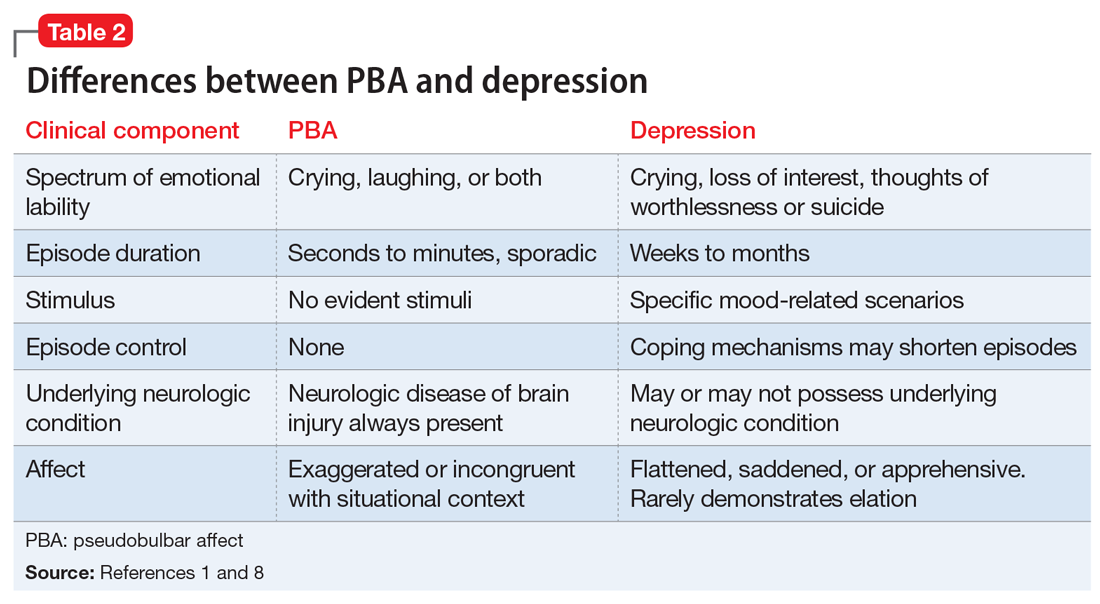
Continue to: Compared with individuals without PBA...
Compared with individuals without PBA, patients with PBA also experience more distress, embarrassment, and social disability, and are consequently more likely to suffer from other psychiatric conditions, including depression, anxiety/panic attacks, bipolar disorder, posttraumatic stress disorder, psychotic disorder, and schizophrenia.1 The Patient Health Questionnaire (PHQ-9), a tool for measuring depression severity, can be used in addition to the CNS-LS to determine if the patient has both depression and PBA.
HISTORY Poor response to anxiolytics and antipsychotics
Mr. X previously received a ventriculoperitoneal shunt for treating iNPH. He was not taking any medications for CP. To address his impulse control disorder, he was prescribed olanzapine, 20 mg/d, risperidone, 2 mg/d, and diazepam, 5 mg three times a day. Mr. X is uncontrolled on these medications, experiencing frequent behavioral outbursts at home. His mother completes a CNS-LS for him. He receives a score of 20, which suggests a diagnosis of PBA. His PHQ-9 score is 8, indicating mild depression.
[polldaddy:9991899]
TREATMENT Introducing a new medication
Mr. X is started on
Continue to: The authors' observations
The authors’ observations
Decreasing the severity and frequency of episodes constitutes the mainstay of treating PBA. In the past, off-label treatments, including selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants, were prescribed to reduce PBA symptoms.5 Currently, dextromethorphan/quinidine is the only FDA-approved medication for treating PBA; however, its use in patients younger than age 18 is considered investigational.
Atypical antipsychotics, such as olanzapine and risperidone, have more warnings and precautions than dextromethorphan/quinidine. Risperidone has a “black-box” warning for QT prolongation, in addition to death and stroke in elderly patients.10 Although dextromethorphan/quinidine does not have a black-box warning, it does increase the risk of QT prolongation, and patients with cardiac risk factors should undergo an electrocardiogram before starting this medication. Additionally, risperidone and olanzapine are known to cause significant weight gain, which can increase the risk of developing hyperlipidemia, metabolic syndrome, and type 2 diabetes mellitus.10,11 Neuroleptic malignant syndrome (NMS) is a potentially life-threatening adverse effect of all antipsychotics. NMS is characterized by fever, rigidity, altered consciousness, and increased heart and respiratory rates.12
Quinidine increases the bioavailability of dextromethorphan by inhibiting CYP2D6. When dextromethorphan/quinidine is simultaneously used with an SSRI that also inhibits CYP2D6, such as paroxetine or fluoxetine, the patient may be at increased risk for developing adverse effects such as respiratory depression and serotonin syndrome.13
[polldaddy:9991902]
Continue to: The authors' observations
The authors’ observations
Although the exact pathophysiology of PBA is unknown, multiple theories may explain the principle elements of the condition. In the absence of a neurologic insult, the cerebellum acts as an affect regulator, inhibiting laughter and crying at times in which they are considered inappropriate. Parvizi et al4 have theorized that the lesions involved in PBA disrupt the corticopontine-cerebellar circuitry, which impedes the ability of the cerebellum to function as an affect modulator.3 In addition to the dysregulation of cerebellar circuitry, altered serotonin and glutamate levels are believed to contribute to the deficient affect regulation observed in PBA; therefore, adding dextromethorphan/quinidine potentiates serotonin and glutamate levels in the synaptic cleft, resulting in a reduction in PBA episodes.4
OUTCOME Affect stability
Seven months after beginning dextromethorphan/quinidine, Mr. X has experienced resolution of his PBA episodes. His PHQ-9 score was reduced to 0 (no clinical signs of depression) within 1 month of starting this medication and his PHQ-9 scores remain below 5, representing minimal depressive severity. The CNS-LS scale is not conducted at further visits because the patient’s mother reported no further PBA episodes. Mr. X no longer exhibits episodes of aggression. These episodes seemed to have been a manifestation of his frustration and difficulty in controlling his PBA episodes. Furthermore, his dosage of diazepam was reduced, and he was weaned off risperidone. Mr. X’s parents report that he has a drastically improved affect. He continues to tolerate his medication well and no longer demonstrates any exacerbations of his psychiatric symptoms.
Bottom Line
Pseudobulbar affect (PBA) may occur secondary to various neurologic insults, including cerebral palsy and idiopathic normal pressure hydrocephalus. The condition is diagnosed by a subjective clinical evaluation and use of the Center for Neurologic Study–Lability Scale. Dextromethorphan/quinidine can significantly reduce PBA symptoms.
Acknowledgements
The authors thank Anthony S. Graziano and Rachel M. Watt, both Physician Assistant students, Daemen College, Amherst, New York.
Related Resources
- Frock B, Williams A, Caplan JP. Pseudobulbar affect: when patients laugh or cry, but don’t know why. Current Psychiatry. 2016;15(9):56-60,63.
- Crumpacker DW. Enhancing approaches to the identification and management of pseudobulbar affect. J Clin Psychiatry. 2016;77(9):e1155.
Drug Brand Names
Dextromethorphan/quinidine • Nuedexta
Diazepam • Valium
Fluoxetine • Prozac
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Paroxetine • Paxil
Risperidone • Risperdal
1. Colamonico J, Formella A, Bradley W. Pseudobulbar affect: burden of illness in the USA. Adv Ther. 2012;29(9):775-798.
2. Brooks BR, Crumpacker D, Fellus J, et al. PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One. 2013;8(8):e72232. doi: 10.1371/journal.pone.0072232.
3. Schiffer R, Pope LE. Review of pseudobulbar affect including a novel and potential therapy. J Neuropsychiatry Clin Neurosci. 2005;17(4):447-454.
4. Parvizi J, Anderson SW, Martin CO, et al. Pathological laughter and crying: a link to the cerebellum. Brain. 2001;124(pt 9):1708-1719.
5. Johnsen SD, Bodensteiner JB, Lotze TE. Frequency and nature of cerebellar injury in the extremely premature survivor with cerebral palsy. J Child Neurol. 2005;20(1):60-64.
6. Kamiya K, Hori M, Miyajima M, et al. Axon diameter and intra-axonal volume fraction of the corticospinal tract in idiopathic normal pressure hydrocephalus measured by Q-Space imaging. PLoS One. 2014;9(8):e103842. doi: 10.1371/journal.pone.0103842.
7. Moore SR, Gresham LS, Bromberg MB, et al. A self report measuredextromethorphan of affective lability. J Neurol Neurosurg Psychiatry. 1997;63(1):89-93.
8. Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clinical Risk Manag. 2013;9:483-489.
9. Cruz MP. Nuedexta for the treatment of pseudobulbar affect. A condition of involuntary crying or laughing. P T. 2013;38(6):325-328.
10. Goëb JL, Marco S, Duhamel A, et al. Metabolic side effects of risperidone in children and adolescents with early onset schizophrenia. Prim Care Companion J Clin Psychiatry. 2008;10(6):486-487.
11. Nemeroff CB. Dosing the antipsychotic medication olanzapine. J Clin Psychiatry. 1997;58(suppl 10):45-49.
12. Troller JN, Chen X, Sachdev PS. Neuroleptic malignant syndrome associated with atypical antipsychotic drugs. CNS Drugs. 2009;23(6):477-492.
13. Schoedel KA, Pope LE, Sellers EM. Randomized open-label drug-drug interaction trial of dextromethorphan/quinidine and paroxetine in healthy volunteers. Clin Drug Investig. 2012;32(3):157-169.
Mr. X, age 16, has cerebral palsy (CP), idiopathic normal pressure hydrocephalus (iNPH), and a history of impulse control disorder and behavioral instability, including episodes of aggression or combativeness. Mr. X’s mother reports that these episodes are almost always preceded by inappropriate laughing or crying. His outbursts and emotional lability have gotten worse during the last 6 months. Due to his disruptive behaviors, Mr. X has been unable to attend school, and his parents are considering group home placement. Although they were previously able to control their son’s aggressive behaviors, they fear for his safety, and after one such episode, they call 911. Mr. X is transported by police in handcuffs to the comprehensive psychiatric emergency room (CPEP) for evaluation.
While in CPEP, Mr. X remains uncooperative and disruptive; subsequently, he is placed in 4-point restraints and given
[polldaddy:9991896]
The authors’ observations
Pseudobulbar affect (PBA) is a disorder characterized by sporadic episodes of inappropriate laughing and/or crying that are incongruent with situational context and are frequently exaggerated in comparison with the actual feelings of the patient. The duration of PBA episodes can last seconds to minutes and arise unpredictably.
PBA typically develops secondary to a neurologic disorder, most commonly Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), Parkinson’s disease (PD), stroke, or traumatic brain injury (TBI).1 PBA symptoms are present in an estimated 29.3% of patients with AD, 44.8% of patients with ALS, 45.8% of patients with MS, 26% of patients with PD, 37.8% of patients with stroke, and 52.4% of patients with TBI.2 Although PBA appears far more frequently in patients with MS or ALS compared with those with PD, PD represents an under-recognized and larger patient population. A small fraction of patients also develops PBA secondary to hyperthyroidism, hypothyroidism, Graves’ disease, Wilson’s disease, brain tumors, and a multitude of encephalopathies.3 These neurologic disorders cause dysregulation of the corticopontine-cerebellar circuitry, resulting in functional impediment to the normal affect modulator action of the cerebellum.4
The neurologic insults that can result in PBA may include CP or iNPH. Cerebellar injury is a frequent pathological finding in CP.5 In patients with iNPH, in addition to altered CSF flow, enlarged ventricles compress the corticospinal tracts in the lateral ventricles,6 which is theorized to induce PBA symptoms.
PBA is diagnosed by subjective clinical evaluation and by using the Center for Neurologic Study–Lability Scale (CNS-LS). The CNS-LS is a 7-question survey that addresses the severity of affect lability (Table 17). It may be completed by the patient or caregiver. Each question ranges in score from 1 to 5, with the total score ranging from 7 to 35. The minimum score required for the diagnosis of PBA is 13.7

PBA is frequently misdiagnosed as depression, although the 2 disorders can occur simultaneously (Table 21,8). A crucial distinguishing factor between depression and PBA is the extent of symptoms. Depression presents as feelings of sadness associated with crying and disinterest that occur for weeks to months. In contrast, PBA presents as brief, uncontrollable episodes of laughing and/or crying that last seconds to minutes. Unlike depression, the behaviors associated with PBA are exaggerated or do not match the patient’s feelings. Furthermore, a neurologic disease or brain injury is always present in a patient with PBA, but is not imperative for the diagnosis of depression.

Continue to: Compared with individuals without PBA...
Compared with individuals without PBA, patients with PBA also experience more distress, embarrassment, and social disability, and are consequently more likely to suffer from other psychiatric conditions, including depression, anxiety/panic attacks, bipolar disorder, posttraumatic stress disorder, psychotic disorder, and schizophrenia.1 The Patient Health Questionnaire (PHQ-9), a tool for measuring depression severity, can be used in addition to the CNS-LS to determine if the patient has both depression and PBA.
HISTORY Poor response to anxiolytics and antipsychotics
Mr. X previously received a ventriculoperitoneal shunt for treating iNPH. He was not taking any medications for CP. To address his impulse control disorder, he was prescribed olanzapine, 20 mg/d, risperidone, 2 mg/d, and diazepam, 5 mg three times a day. Mr. X is uncontrolled on these medications, experiencing frequent behavioral outbursts at home. His mother completes a CNS-LS for him. He receives a score of 20, which suggests a diagnosis of PBA. His PHQ-9 score is 8, indicating mild depression.
[polldaddy:9991899]
TREATMENT Introducing a new medication
Mr. X is started on
Continue to: The authors' observations
The authors’ observations
Decreasing the severity and frequency of episodes constitutes the mainstay of treating PBA. In the past, off-label treatments, including selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants, were prescribed to reduce PBA symptoms.5 Currently, dextromethorphan/quinidine is the only FDA-approved medication for treating PBA; however, its use in patients younger than age 18 is considered investigational.
Atypical antipsychotics, such as olanzapine and risperidone, have more warnings and precautions than dextromethorphan/quinidine. Risperidone has a “black-box” warning for QT prolongation, in addition to death and stroke in elderly patients.10 Although dextromethorphan/quinidine does not have a black-box warning, it does increase the risk of QT prolongation, and patients with cardiac risk factors should undergo an electrocardiogram before starting this medication. Additionally, risperidone and olanzapine are known to cause significant weight gain, which can increase the risk of developing hyperlipidemia, metabolic syndrome, and type 2 diabetes mellitus.10,11 Neuroleptic malignant syndrome (NMS) is a potentially life-threatening adverse effect of all antipsychotics. NMS is characterized by fever, rigidity, altered consciousness, and increased heart and respiratory rates.12
Quinidine increases the bioavailability of dextromethorphan by inhibiting CYP2D6. When dextromethorphan/quinidine is simultaneously used with an SSRI that also inhibits CYP2D6, such as paroxetine or fluoxetine, the patient may be at increased risk for developing adverse effects such as respiratory depression and serotonin syndrome.13
[polldaddy:9991902]
Continue to: The authors' observations
The authors’ observations
Although the exact pathophysiology of PBA is unknown, multiple theories may explain the principle elements of the condition. In the absence of a neurologic insult, the cerebellum acts as an affect regulator, inhibiting laughter and crying at times in which they are considered inappropriate. Parvizi et al4 have theorized that the lesions involved in PBA disrupt the corticopontine-cerebellar circuitry, which impedes the ability of the cerebellum to function as an affect modulator.3 In addition to the dysregulation of cerebellar circuitry, altered serotonin and glutamate levels are believed to contribute to the deficient affect regulation observed in PBA; therefore, adding dextromethorphan/quinidine potentiates serotonin and glutamate levels in the synaptic cleft, resulting in a reduction in PBA episodes.4
OUTCOME Affect stability
Seven months after beginning dextromethorphan/quinidine, Mr. X has experienced resolution of his PBA episodes. His PHQ-9 score was reduced to 0 (no clinical signs of depression) within 1 month of starting this medication and his PHQ-9 scores remain below 5, representing minimal depressive severity. The CNS-LS scale is not conducted at further visits because the patient’s mother reported no further PBA episodes. Mr. X no longer exhibits episodes of aggression. These episodes seemed to have been a manifestation of his frustration and difficulty in controlling his PBA episodes. Furthermore, his dosage of diazepam was reduced, and he was weaned off risperidone. Mr. X’s parents report that he has a drastically improved affect. He continues to tolerate his medication well and no longer demonstrates any exacerbations of his psychiatric symptoms.
Bottom Line
Pseudobulbar affect (PBA) may occur secondary to various neurologic insults, including cerebral palsy and idiopathic normal pressure hydrocephalus. The condition is diagnosed by a subjective clinical evaluation and use of the Center for Neurologic Study–Lability Scale. Dextromethorphan/quinidine can significantly reduce PBA symptoms.
Acknowledgements
The authors thank Anthony S. Graziano and Rachel M. Watt, both Physician Assistant students, Daemen College, Amherst, New York.
Related Resources
- Frock B, Williams A, Caplan JP. Pseudobulbar affect: when patients laugh or cry, but don’t know why. Current Psychiatry. 2016;15(9):56-60,63.
- Crumpacker DW. Enhancing approaches to the identification and management of pseudobulbar affect. J Clin Psychiatry. 2016;77(9):e1155.
Drug Brand Names
Dextromethorphan/quinidine • Nuedexta
Diazepam • Valium
Fluoxetine • Prozac
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Paroxetine • Paxil
Risperidone • Risperdal
Mr. X, age 16, has cerebral palsy (CP), idiopathic normal pressure hydrocephalus (iNPH), and a history of impulse control disorder and behavioral instability, including episodes of aggression or combativeness. Mr. X’s mother reports that these episodes are almost always preceded by inappropriate laughing or crying. His outbursts and emotional lability have gotten worse during the last 6 months. Due to his disruptive behaviors, Mr. X has been unable to attend school, and his parents are considering group home placement. Although they were previously able to control their son’s aggressive behaviors, they fear for his safety, and after one such episode, they call 911. Mr. X is transported by police in handcuffs to the comprehensive psychiatric emergency room (CPEP) for evaluation.
While in CPEP, Mr. X remains uncooperative and disruptive; subsequently, he is placed in 4-point restraints and given
[polldaddy:9991896]
The authors’ observations
Pseudobulbar affect (PBA) is a disorder characterized by sporadic episodes of inappropriate laughing and/or crying that are incongruent with situational context and are frequently exaggerated in comparison with the actual feelings of the patient. The duration of PBA episodes can last seconds to minutes and arise unpredictably.
PBA typically develops secondary to a neurologic disorder, most commonly Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), Parkinson’s disease (PD), stroke, or traumatic brain injury (TBI).1 PBA symptoms are present in an estimated 29.3% of patients with AD, 44.8% of patients with ALS, 45.8% of patients with MS, 26% of patients with PD, 37.8% of patients with stroke, and 52.4% of patients with TBI.2 Although PBA appears far more frequently in patients with MS or ALS compared with those with PD, PD represents an under-recognized and larger patient population. A small fraction of patients also develops PBA secondary to hyperthyroidism, hypothyroidism, Graves’ disease, Wilson’s disease, brain tumors, and a multitude of encephalopathies.3 These neurologic disorders cause dysregulation of the corticopontine-cerebellar circuitry, resulting in functional impediment to the normal affect modulator action of the cerebellum.4
The neurologic insults that can result in PBA may include CP or iNPH. Cerebellar injury is a frequent pathological finding in CP.5 In patients with iNPH, in addition to altered CSF flow, enlarged ventricles compress the corticospinal tracts in the lateral ventricles,6 which is theorized to induce PBA symptoms.
PBA is diagnosed by subjective clinical evaluation and by using the Center for Neurologic Study–Lability Scale (CNS-LS). The CNS-LS is a 7-question survey that addresses the severity of affect lability (Table 17). It may be completed by the patient or caregiver. Each question ranges in score from 1 to 5, with the total score ranging from 7 to 35. The minimum score required for the diagnosis of PBA is 13.7

PBA is frequently misdiagnosed as depression, although the 2 disorders can occur simultaneously (Table 21,8). A crucial distinguishing factor between depression and PBA is the extent of symptoms. Depression presents as feelings of sadness associated with crying and disinterest that occur for weeks to months. In contrast, PBA presents as brief, uncontrollable episodes of laughing and/or crying that last seconds to minutes. Unlike depression, the behaviors associated with PBA are exaggerated or do not match the patient’s feelings. Furthermore, a neurologic disease or brain injury is always present in a patient with PBA, but is not imperative for the diagnosis of depression.

Continue to: Compared with individuals without PBA...
Compared with individuals without PBA, patients with PBA also experience more distress, embarrassment, and social disability, and are consequently more likely to suffer from other psychiatric conditions, including depression, anxiety/panic attacks, bipolar disorder, posttraumatic stress disorder, psychotic disorder, and schizophrenia.1 The Patient Health Questionnaire (PHQ-9), a tool for measuring depression severity, can be used in addition to the CNS-LS to determine if the patient has both depression and PBA.
HISTORY Poor response to anxiolytics and antipsychotics
Mr. X previously received a ventriculoperitoneal shunt for treating iNPH. He was not taking any medications for CP. To address his impulse control disorder, he was prescribed olanzapine, 20 mg/d, risperidone, 2 mg/d, and diazepam, 5 mg three times a day. Mr. X is uncontrolled on these medications, experiencing frequent behavioral outbursts at home. His mother completes a CNS-LS for him. He receives a score of 20, which suggests a diagnosis of PBA. His PHQ-9 score is 8, indicating mild depression.
[polldaddy:9991899]
TREATMENT Introducing a new medication
Mr. X is started on
Continue to: The authors' observations
The authors’ observations
Decreasing the severity and frequency of episodes constitutes the mainstay of treating PBA. In the past, off-label treatments, including selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants, were prescribed to reduce PBA symptoms.5 Currently, dextromethorphan/quinidine is the only FDA-approved medication for treating PBA; however, its use in patients younger than age 18 is considered investigational.
Atypical antipsychotics, such as olanzapine and risperidone, have more warnings and precautions than dextromethorphan/quinidine. Risperidone has a “black-box” warning for QT prolongation, in addition to death and stroke in elderly patients.10 Although dextromethorphan/quinidine does not have a black-box warning, it does increase the risk of QT prolongation, and patients with cardiac risk factors should undergo an electrocardiogram before starting this medication. Additionally, risperidone and olanzapine are known to cause significant weight gain, which can increase the risk of developing hyperlipidemia, metabolic syndrome, and type 2 diabetes mellitus.10,11 Neuroleptic malignant syndrome (NMS) is a potentially life-threatening adverse effect of all antipsychotics. NMS is characterized by fever, rigidity, altered consciousness, and increased heart and respiratory rates.12
Quinidine increases the bioavailability of dextromethorphan by inhibiting CYP2D6. When dextromethorphan/quinidine is simultaneously used with an SSRI that also inhibits CYP2D6, such as paroxetine or fluoxetine, the patient may be at increased risk for developing adverse effects such as respiratory depression and serotonin syndrome.13
[polldaddy:9991902]
Continue to: The authors' observations
The authors’ observations
Although the exact pathophysiology of PBA is unknown, multiple theories may explain the principle elements of the condition. In the absence of a neurologic insult, the cerebellum acts as an affect regulator, inhibiting laughter and crying at times in which they are considered inappropriate. Parvizi et al4 have theorized that the lesions involved in PBA disrupt the corticopontine-cerebellar circuitry, which impedes the ability of the cerebellum to function as an affect modulator.3 In addition to the dysregulation of cerebellar circuitry, altered serotonin and glutamate levels are believed to contribute to the deficient affect regulation observed in PBA; therefore, adding dextromethorphan/quinidine potentiates serotonin and glutamate levels in the synaptic cleft, resulting in a reduction in PBA episodes.4
OUTCOME Affect stability
Seven months after beginning dextromethorphan/quinidine, Mr. X has experienced resolution of his PBA episodes. His PHQ-9 score was reduced to 0 (no clinical signs of depression) within 1 month of starting this medication and his PHQ-9 scores remain below 5, representing minimal depressive severity. The CNS-LS scale is not conducted at further visits because the patient’s mother reported no further PBA episodes. Mr. X no longer exhibits episodes of aggression. These episodes seemed to have been a manifestation of his frustration and difficulty in controlling his PBA episodes. Furthermore, his dosage of diazepam was reduced, and he was weaned off risperidone. Mr. X’s parents report that he has a drastically improved affect. He continues to tolerate his medication well and no longer demonstrates any exacerbations of his psychiatric symptoms.
Bottom Line
Pseudobulbar affect (PBA) may occur secondary to various neurologic insults, including cerebral palsy and idiopathic normal pressure hydrocephalus. The condition is diagnosed by a subjective clinical evaluation and use of the Center for Neurologic Study–Lability Scale. Dextromethorphan/quinidine can significantly reduce PBA symptoms.
Acknowledgements
The authors thank Anthony S. Graziano and Rachel M. Watt, both Physician Assistant students, Daemen College, Amherst, New York.
Related Resources
- Frock B, Williams A, Caplan JP. Pseudobulbar affect: when patients laugh or cry, but don’t know why. Current Psychiatry. 2016;15(9):56-60,63.
- Crumpacker DW. Enhancing approaches to the identification and management of pseudobulbar affect. J Clin Psychiatry. 2016;77(9):e1155.
Drug Brand Names
Dextromethorphan/quinidine • Nuedexta
Diazepam • Valium
Fluoxetine • Prozac
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Paroxetine • Paxil
Risperidone • Risperdal
1. Colamonico J, Formella A, Bradley W. Pseudobulbar affect: burden of illness in the USA. Adv Ther. 2012;29(9):775-798.
2. Brooks BR, Crumpacker D, Fellus J, et al. PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One. 2013;8(8):e72232. doi: 10.1371/journal.pone.0072232.
3. Schiffer R, Pope LE. Review of pseudobulbar affect including a novel and potential therapy. J Neuropsychiatry Clin Neurosci. 2005;17(4):447-454.
4. Parvizi J, Anderson SW, Martin CO, et al. Pathological laughter and crying: a link to the cerebellum. Brain. 2001;124(pt 9):1708-1719.
5. Johnsen SD, Bodensteiner JB, Lotze TE. Frequency and nature of cerebellar injury in the extremely premature survivor with cerebral palsy. J Child Neurol. 2005;20(1):60-64.
6. Kamiya K, Hori M, Miyajima M, et al. Axon diameter and intra-axonal volume fraction of the corticospinal tract in idiopathic normal pressure hydrocephalus measured by Q-Space imaging. PLoS One. 2014;9(8):e103842. doi: 10.1371/journal.pone.0103842.
7. Moore SR, Gresham LS, Bromberg MB, et al. A self report measuredextromethorphan of affective lability. J Neurol Neurosurg Psychiatry. 1997;63(1):89-93.
8. Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clinical Risk Manag. 2013;9:483-489.
9. Cruz MP. Nuedexta for the treatment of pseudobulbar affect. A condition of involuntary crying or laughing. P T. 2013;38(6):325-328.
10. Goëb JL, Marco S, Duhamel A, et al. Metabolic side effects of risperidone in children and adolescents with early onset schizophrenia. Prim Care Companion J Clin Psychiatry. 2008;10(6):486-487.
11. Nemeroff CB. Dosing the antipsychotic medication olanzapine. J Clin Psychiatry. 1997;58(suppl 10):45-49.
12. Troller JN, Chen X, Sachdev PS. Neuroleptic malignant syndrome associated with atypical antipsychotic drugs. CNS Drugs. 2009;23(6):477-492.
13. Schoedel KA, Pope LE, Sellers EM. Randomized open-label drug-drug interaction trial of dextromethorphan/quinidine and paroxetine in healthy volunteers. Clin Drug Investig. 2012;32(3):157-169.
1. Colamonico J, Formella A, Bradley W. Pseudobulbar affect: burden of illness in the USA. Adv Ther. 2012;29(9):775-798.
2. Brooks BR, Crumpacker D, Fellus J, et al. PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One. 2013;8(8):e72232. doi: 10.1371/journal.pone.0072232.
3. Schiffer R, Pope LE. Review of pseudobulbar affect including a novel and potential therapy. J Neuropsychiatry Clin Neurosci. 2005;17(4):447-454.
4. Parvizi J, Anderson SW, Martin CO, et al. Pathological laughter and crying: a link to the cerebellum. Brain. 2001;124(pt 9):1708-1719.
5. Johnsen SD, Bodensteiner JB, Lotze TE. Frequency and nature of cerebellar injury in the extremely premature survivor with cerebral palsy. J Child Neurol. 2005;20(1):60-64.
6. Kamiya K, Hori M, Miyajima M, et al. Axon diameter and intra-axonal volume fraction of the corticospinal tract in idiopathic normal pressure hydrocephalus measured by Q-Space imaging. PLoS One. 2014;9(8):e103842. doi: 10.1371/journal.pone.0103842.
7. Moore SR, Gresham LS, Bromberg MB, et al. A self report measuredextromethorphan of affective lability. J Neurol Neurosurg Psychiatry. 1997;63(1):89-93.
8. Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clinical Risk Manag. 2013;9:483-489.
9. Cruz MP. Nuedexta for the treatment of pseudobulbar affect. A condition of involuntary crying or laughing. P T. 2013;38(6):325-328.
10. Goëb JL, Marco S, Duhamel A, et al. Metabolic side effects of risperidone in children and adolescents with early onset schizophrenia. Prim Care Companion J Clin Psychiatry. 2008;10(6):486-487.
11. Nemeroff CB. Dosing the antipsychotic medication olanzapine. J Clin Psychiatry. 1997;58(suppl 10):45-49.
12. Troller JN, Chen X, Sachdev PS. Neuroleptic malignant syndrome associated with atypical antipsychotic drugs. CNS Drugs. 2009;23(6):477-492.
13. Schoedel KA, Pope LE, Sellers EM. Randomized open-label drug-drug interaction trial of dextromethorphan/quinidine and paroxetine in healthy volunteers. Clin Drug Investig. 2012;32(3):157-169.
Strategies for working with patients with personality disorders
Patients with personality disorders can disrupt the treatment relationship, and may leave us feeling angry, ineffective, inadequate, and defeated. Although their behaviors may appear volitional and purposeful, they often are the result of a dysfunctional personality structure.1 These patients’ unbending patterns of viewing themselves, interacting with others, and navigating the world can be problematic in an inpatient or outpatient setting, causing distress for both the staff and patient. Because no 2 personalities are identical, there is no algorithm for managing patients with personality disorders. However, there are strategies that we can apply to provide effective clinical care.1,2
Discuss the responses the patient evokes. Patients with personality disorders can elicit strong responses from the treatment team. Each clinician can have a different response to the same patient, ranging from feeling the need to protect the patient to strongly disliking him or her. Because cohesion among staff is essential for effective patient care, we need to discuss these responses in an open forum with our team members so we can effectively manage our responses and provide the patient with consistent interactions. Limiting the delivery of inconsistent or conflicting messages will decrease staff splitting and increase team unity.
Reinforce appropriate behaviors. Patients with personality disorders usually have negative interpersonal interactions, such as acting out, misinterpreting neutral social cues, and seeking constant attention. However, when they are not engaging in detrimental behaviors, we should provide positive reinforcement for appropriate behaviors, such as remaining composed, that help maintain the treatment relationship. When a patient displays disruptive behaviors, take a neutral approach by stating, “You appear upset. I will come back later when you are feeling better.”1
Set limits. These patients are likely to have difficulty conforming to appropriate social boundaries. Our reflex reaction may be to set concrete rules that fit our preferences. This could lead to a power struggle between us and our patients, which is not helpful. Rather than a “one-size-fits-all” approach to rules, it may be prudent to tailor boundaries according to each patient’s unique personality. Also, allowing the patient to help set these limits could increase the chances that he or she will follow your treatment plan and reinforce the more positive aspects of his or her personality structure.
Offer empathy. Empathy can be conceptualized as a step further than sympathy; in addition to expressing concern and compassion, empathy involves recognizing and sharing the patient’s emotions. Seek to comprehend the reasons behind a patient’s negative reactions by
1. Riddle M, Meeks T, Alvarez C, et al. When personality is the problem: managing patients with difficult personalitie s on the acute care unit. J Hosp Med. 2016;11(12):873-878.
2. Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
Patients with personality disorders can disrupt the treatment relationship, and may leave us feeling angry, ineffective, inadequate, and defeated. Although their behaviors may appear volitional and purposeful, they often are the result of a dysfunctional personality structure.1 These patients’ unbending patterns of viewing themselves, interacting with others, and navigating the world can be problematic in an inpatient or outpatient setting, causing distress for both the staff and patient. Because no 2 personalities are identical, there is no algorithm for managing patients with personality disorders. However, there are strategies that we can apply to provide effective clinical care.1,2
Discuss the responses the patient evokes. Patients with personality disorders can elicit strong responses from the treatment team. Each clinician can have a different response to the same patient, ranging from feeling the need to protect the patient to strongly disliking him or her. Because cohesion among staff is essential for effective patient care, we need to discuss these responses in an open forum with our team members so we can effectively manage our responses and provide the patient with consistent interactions. Limiting the delivery of inconsistent or conflicting messages will decrease staff splitting and increase team unity.
Reinforce appropriate behaviors. Patients with personality disorders usually have negative interpersonal interactions, such as acting out, misinterpreting neutral social cues, and seeking constant attention. However, when they are not engaging in detrimental behaviors, we should provide positive reinforcement for appropriate behaviors, such as remaining composed, that help maintain the treatment relationship. When a patient displays disruptive behaviors, take a neutral approach by stating, “You appear upset. I will come back later when you are feeling better.”1
Set limits. These patients are likely to have difficulty conforming to appropriate social boundaries. Our reflex reaction may be to set concrete rules that fit our preferences. This could lead to a power struggle between us and our patients, which is not helpful. Rather than a “one-size-fits-all” approach to rules, it may be prudent to tailor boundaries according to each patient’s unique personality. Also, allowing the patient to help set these limits could increase the chances that he or she will follow your treatment plan and reinforce the more positive aspects of his or her personality structure.
Offer empathy. Empathy can be conceptualized as a step further than sympathy; in addition to expressing concern and compassion, empathy involves recognizing and sharing the patient’s emotions. Seek to comprehend the reasons behind a patient’s negative reactions by
Patients with personality disorders can disrupt the treatment relationship, and may leave us feeling angry, ineffective, inadequate, and defeated. Although their behaviors may appear volitional and purposeful, they often are the result of a dysfunctional personality structure.1 These patients’ unbending patterns of viewing themselves, interacting with others, and navigating the world can be problematic in an inpatient or outpatient setting, causing distress for both the staff and patient. Because no 2 personalities are identical, there is no algorithm for managing patients with personality disorders. However, there are strategies that we can apply to provide effective clinical care.1,2
Discuss the responses the patient evokes. Patients with personality disorders can elicit strong responses from the treatment team. Each clinician can have a different response to the same patient, ranging from feeling the need to protect the patient to strongly disliking him or her. Because cohesion among staff is essential for effective patient care, we need to discuss these responses in an open forum with our team members so we can effectively manage our responses and provide the patient with consistent interactions. Limiting the delivery of inconsistent or conflicting messages will decrease staff splitting and increase team unity.
Reinforce appropriate behaviors. Patients with personality disorders usually have negative interpersonal interactions, such as acting out, misinterpreting neutral social cues, and seeking constant attention. However, when they are not engaging in detrimental behaviors, we should provide positive reinforcement for appropriate behaviors, such as remaining composed, that help maintain the treatment relationship. When a patient displays disruptive behaviors, take a neutral approach by stating, “You appear upset. I will come back later when you are feeling better.”1
Set limits. These patients are likely to have difficulty conforming to appropriate social boundaries. Our reflex reaction may be to set concrete rules that fit our preferences. This could lead to a power struggle between us and our patients, which is not helpful. Rather than a “one-size-fits-all” approach to rules, it may be prudent to tailor boundaries according to each patient’s unique personality. Also, allowing the patient to help set these limits could increase the chances that he or she will follow your treatment plan and reinforce the more positive aspects of his or her personality structure.
Offer empathy. Empathy can be conceptualized as a step further than sympathy; in addition to expressing concern and compassion, empathy involves recognizing and sharing the patient’s emotions. Seek to comprehend the reasons behind a patient’s negative reactions by
1. Riddle M, Meeks T, Alvarez C, et al. When personality is the problem: managing patients with difficult personalitie s on the acute care unit. J Hosp Med. 2016;11(12):873-878.
2. Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
1. Riddle M, Meeks T, Alvarez C, et al. When personality is the problem: managing patients with difficult personalitie s on the acute care unit. J Hosp Med. 2016;11(12):873-878.
2. Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
‘Nocebo’ effects: Address these 4 psychosocial factors
Sorting out the causes of unexplained adverse effects from psychotropic medications can be challenging. Treatment may be further complicated by ‘nocebo’ effects, which are adverse effects based on the patient’s conscious and unconscious expectations of harm. Having strategies for managing nocebo effects can help clinicians better understand and treat patients who have complex medication complaints. When your patient experiences nocebo effects, consider the following 4 psychosocial factors.1
Pills. The impact of a medication is not solely based on its chemical makeup. For example, the appearance of a medication can affect treatment outcomes. Substituting generic medications for branded ones has been shown to negatively impact patient adherence and increase reports of adverse effects that have no physiologic cause.2 Educating patients about medication manufacturing and distribution practices may decrease such consequences.
Patient. A sense of powerlessness is fertile ground for nocebo effects. Patients with an external locus of control may unconsciously employ nocebo effects to express themselves when other outlets are limited. Having a psychosocial formulation of your patient can help you anticipate pitfalls, offer pertinent insights, and mobilize the patient’s adaptive coping mechanisms. Also, clinicians can bolster their patients’ self-agency by encouraging them to participate in healthy activities.
Provider. Irrational factors in the clinician, such as countertransference, may also affect medication outcomes. Unprocessed countertransference can contribute to clinician burnout and impact the therapeutic relationship negatively. Nocebo effects may indicate that the clinician is not “tuned in” to the patient or is acting out harmful unconscious thoughts. Additionally, countertransference can lead to unnecessary prescribing and polypharmacy that confounds nocebo effects. Therefore self-care, consultation, and supervision may be vital in promoting therapeutic outcomes.
Partnership. The doctor–patient relationship can contribute to nocebo effects. A 2016 Gallup Poll found that Americans had low confidence in the honesty and ethics of psychiatrists compared with other healthcare professionals.3 It is important to have conversations with your patients about their reservations and perceived stigma of mental health. Such conversations can bring a patient’s ambivalence into treatment so that it can be further explored and addressed. Psychoeducation about treatment limitations, motivational interviewing techniques, and involving patients in decision-making can be useful tools for fostering a therapeutic alliance and positive outcomes.
Take an active approach
Evidence demonstrates that psychosocial factors significantly impact treatment outcomes.1 Incorporating this evidence into practice and attending to the 4 factors discussed here can enhance a clinician’s ability to flexibly respond to their patients’ complaints, especially in relation to nocebo effects.
1. Mallo CJ, Mintz DL. Teaching all the evidence bases: reintegrating psychodynamic aspects of prescribing into psychopharmacology training. Psychodyn Psychiatry. 2013;41(1):13-37.
2. Weissenfeld J, Stock S, Lüngen M, et al. The nocebo effect: a reason for patients’ non-adherence to generic substitution? Pharmazie. 2010;65(7):451-456.
3. Norman J. Americans rate healthcare providers high on honesty, ethics. Gallup. http://news.gallup.com/poll/200057/americans-rate-healthcare-providers-high-honesty-ethics.aspx. Published December 19, 2016. Accessed October 22, 2017.
Sorting out the causes of unexplained adverse effects from psychotropic medications can be challenging. Treatment may be further complicated by ‘nocebo’ effects, which are adverse effects based on the patient’s conscious and unconscious expectations of harm. Having strategies for managing nocebo effects can help clinicians better understand and treat patients who have complex medication complaints. When your patient experiences nocebo effects, consider the following 4 psychosocial factors.1
Pills. The impact of a medication is not solely based on its chemical makeup. For example, the appearance of a medication can affect treatment outcomes. Substituting generic medications for branded ones has been shown to negatively impact patient adherence and increase reports of adverse effects that have no physiologic cause.2 Educating patients about medication manufacturing and distribution practices may decrease such consequences.
Patient. A sense of powerlessness is fertile ground for nocebo effects. Patients with an external locus of control may unconsciously employ nocebo effects to express themselves when other outlets are limited. Having a psychosocial formulation of your patient can help you anticipate pitfalls, offer pertinent insights, and mobilize the patient’s adaptive coping mechanisms. Also, clinicians can bolster their patients’ self-agency by encouraging them to participate in healthy activities.
Provider. Irrational factors in the clinician, such as countertransference, may also affect medication outcomes. Unprocessed countertransference can contribute to clinician burnout and impact the therapeutic relationship negatively. Nocebo effects may indicate that the clinician is not “tuned in” to the patient or is acting out harmful unconscious thoughts. Additionally, countertransference can lead to unnecessary prescribing and polypharmacy that confounds nocebo effects. Therefore self-care, consultation, and supervision may be vital in promoting therapeutic outcomes.
Partnership. The doctor–patient relationship can contribute to nocebo effects. A 2016 Gallup Poll found that Americans had low confidence in the honesty and ethics of psychiatrists compared with other healthcare professionals.3 It is important to have conversations with your patients about their reservations and perceived stigma of mental health. Such conversations can bring a patient’s ambivalence into treatment so that it can be further explored and addressed. Psychoeducation about treatment limitations, motivational interviewing techniques, and involving patients in decision-making can be useful tools for fostering a therapeutic alliance and positive outcomes.
Take an active approach
Evidence demonstrates that psychosocial factors significantly impact treatment outcomes.1 Incorporating this evidence into practice and attending to the 4 factors discussed here can enhance a clinician’s ability to flexibly respond to their patients’ complaints, especially in relation to nocebo effects.
Sorting out the causes of unexplained adverse effects from psychotropic medications can be challenging. Treatment may be further complicated by ‘nocebo’ effects, which are adverse effects based on the patient’s conscious and unconscious expectations of harm. Having strategies for managing nocebo effects can help clinicians better understand and treat patients who have complex medication complaints. When your patient experiences nocebo effects, consider the following 4 psychosocial factors.1
Pills. The impact of a medication is not solely based on its chemical makeup. For example, the appearance of a medication can affect treatment outcomes. Substituting generic medications for branded ones has been shown to negatively impact patient adherence and increase reports of adverse effects that have no physiologic cause.2 Educating patients about medication manufacturing and distribution practices may decrease such consequences.
Patient. A sense of powerlessness is fertile ground for nocebo effects. Patients with an external locus of control may unconsciously employ nocebo effects to express themselves when other outlets are limited. Having a psychosocial formulation of your patient can help you anticipate pitfalls, offer pertinent insights, and mobilize the patient’s adaptive coping mechanisms. Also, clinicians can bolster their patients’ self-agency by encouraging them to participate in healthy activities.
Provider. Irrational factors in the clinician, such as countertransference, may also affect medication outcomes. Unprocessed countertransference can contribute to clinician burnout and impact the therapeutic relationship negatively. Nocebo effects may indicate that the clinician is not “tuned in” to the patient or is acting out harmful unconscious thoughts. Additionally, countertransference can lead to unnecessary prescribing and polypharmacy that confounds nocebo effects. Therefore self-care, consultation, and supervision may be vital in promoting therapeutic outcomes.
Partnership. The doctor–patient relationship can contribute to nocebo effects. A 2016 Gallup Poll found that Americans had low confidence in the honesty and ethics of psychiatrists compared with other healthcare professionals.3 It is important to have conversations with your patients about their reservations and perceived stigma of mental health. Such conversations can bring a patient’s ambivalence into treatment so that it can be further explored and addressed. Psychoeducation about treatment limitations, motivational interviewing techniques, and involving patients in decision-making can be useful tools for fostering a therapeutic alliance and positive outcomes.
Take an active approach
Evidence demonstrates that psychosocial factors significantly impact treatment outcomes.1 Incorporating this evidence into practice and attending to the 4 factors discussed here can enhance a clinician’s ability to flexibly respond to their patients’ complaints, especially in relation to nocebo effects.
1. Mallo CJ, Mintz DL. Teaching all the evidence bases: reintegrating psychodynamic aspects of prescribing into psychopharmacology training. Psychodyn Psychiatry. 2013;41(1):13-37.
2. Weissenfeld J, Stock S, Lüngen M, et al. The nocebo effect: a reason for patients’ non-adherence to generic substitution? Pharmazie. 2010;65(7):451-456.
3. Norman J. Americans rate healthcare providers high on honesty, ethics. Gallup. http://news.gallup.com/poll/200057/americans-rate-healthcare-providers-high-honesty-ethics.aspx. Published December 19, 2016. Accessed October 22, 2017.
1. Mallo CJ, Mintz DL. Teaching all the evidence bases: reintegrating psychodynamic aspects of prescribing into psychopharmacology training. Psychodyn Psychiatry. 2013;41(1):13-37.
2. Weissenfeld J, Stock S, Lüngen M, et al. The nocebo effect: a reason for patients’ non-adherence to generic substitution? Pharmazie. 2010;65(7):451-456.
3. Norman J. Americans rate healthcare providers high on honesty, ethics. Gallup. http://news.gallup.com/poll/200057/americans-rate-healthcare-providers-high-honesty-ethics.aspx. Published December 19, 2016. Accessed October 22, 2017.
Generalized pustular eruption
A 38-year-old man sought care in the emergency department for an acute, pruritic, generalized cutaneous eruption that manifested in the intertriginous areas of the inner thighs, antecubital fossae, and axilla (FIGURE 1A). He reported associated chills, a 15-pound weight gain, and swelling of his inner thighs. Two weeks before presentation, he had received azithromycin for an upper respiratory tract infection. He was unsure if the rash developed prior to or after taking the medication. He was not taking any other medications and had no history of skin conditions.
On examination, the patient was afebrile and had bilateral thigh edema. Skin examination revealed background erythema with morbilliform papules, plaques, and patches on the bilateral flanks, back, buttocks, arms, legs, and central neck. Pinpoint pustules were present in the intertriginous sites and on the low back and buttocks. The laboratory evaluation revealed leukocytosis (11.0 × 109 cells/L), increased levels of neutrophils and eosinophils, and an elevated C-reactive protein level (12.8 mg/L). The remaining laboratory results were unremarkable. The patient was referred to Dermatology.
An examination by the dermatologist 3 days later revealed small areas of annular desquamation with a few pinpoint pustules, mostly located on the inner thighs and buttocks (FIGURE 1B). Skin biopsies were taken from the anterior hip region. The histopathology revealed subacute dermatitis with mixed dermal inflammatory cells, including neutrophils and eosinophils, and discrete subcorneal spongiform pustules.
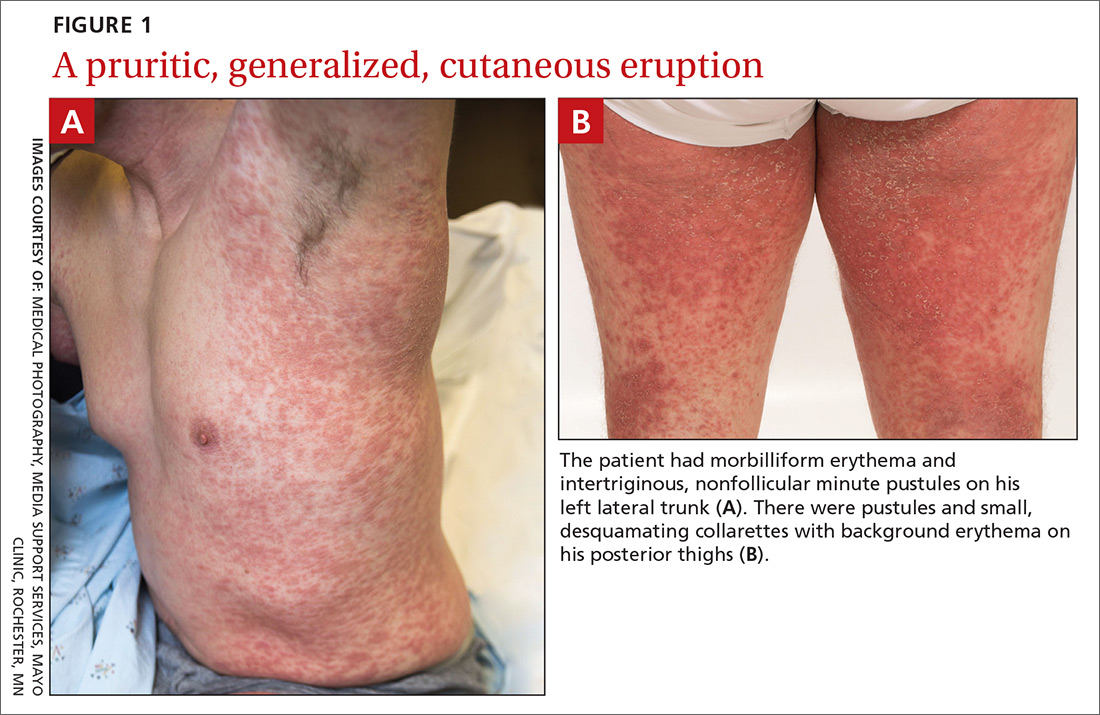
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute generalized exanthematous pustulosis (AGEP)
The acute rash with minute pustules and associated leukocytosis with neutrophilia and eosinophilia led to an early diagnosis of AGEP, which may have been triggered by azithromycin—the patient’s only recent medication. AGEP is a severe cutaneous eruption that may be associated with systemic involvement. Medications are usually implicated, and patients often seek urgent evaluation.
AGEP typically begins as an acute eruption in the intertriginous sites of the axilla, groin, and neck, but often becomes more generalized.1,2 The diagnosis is strongly suggested by the condition’s key features: fever (97% of cases) and leukocytosis (87%) with neutrophilia (91%) and eosinophilia (30%); leukocytosis peaks 4 days after pustulosis occurs and lasts for about 12 days.1 Although common, fever is not always documented in patients with AGEP. 3 (Our patient was a case in point.) While not a key characteristic of AGEP, our patient’s weight gain was likely explained by the severe edema secondary to his inflammatory skin eruption.
Medications are implicated, but pathophysiology is unknown
In approximately 90% of AGEP cases, medications such as antibiotics and calcium channel blockers are implicated; however, the lack of such an association does not preclude the diagnosis.1,4 In cases of drug reactions, the eruption typically develops 1 to 2 days after a medication is begun, and the pustules typically resolve in fewer than 15 days.5 In 17% of patients, systemic involvement can occur and can include the liver, kidneys, bone marrow, and lungs.6 A physical exam, review of systems, and a laboratory evaluation can help rule out systemic involvement and guide additional testing.
AGEP has an incidence of 1 to 5 cases per million people per year, affecting women slightly more frequently than men.7 While the pathophysiology is not well understood, AGEP and its differential diagnoses are categorized as T cell-related inflammatory responses.4,7
Distinguishing AGEP from some look-alikes
There are at least 4 severe cutaneous eruptions that might be confused with AGEP, all of which may be associated with fever. They include: drug reaction with eosinophilia and systemic symptoms (DRESS), also known as drug-induced hypersensitivity syndrome; Stevens-Johnson syndrome (SJS); toxic epidermal necrolysis (TEN); and pustular psoriasis.8-10 The clinical features that may help differentiate these conditions from AGEP include timeline, mucocutaneous features, organ system involvement, and histopathologic findings.4,8
DRESS occurs 2 to 6 weeks after drug exposure, rather than a few days, as is seen with AGEP. It often involves morbilliform erythema and facial edema with substantial eosinophilia and possible nephritis, pneumonitis, myocarditis, and thyroiditis.9 Unlike AGEP, DRESS does not have a predilection for intertriginous anatomic locations.
SJS and TEN occur 1 to 3 weeks after drug exposure. These conditions manifest with the development of bullae, atypical targetoid lesions, painful dusky erythema, epidermal necrosis, and mucosal involvement at multiple sites. Tubular nephritis, tracheobronchial necrosis, and multisystem organ failure can occur, with reported mortality rates of 5% to 35%.8,11
Pustular psoriasis is frequently confused with AGEP. However, AGEP usually develops fewer than 2 days after drug exposure, with pustules that begin in intertriginous sites, and there is associated neutrophilia and possible organ involvement.1,8 Patients who have AGEP typically do not have a history of psoriasis, while patients with pustular psoriasis often do.7 A history of drug reaction is uncommon with pustular psoriasis (although rapid tapering of systemic corticosteroids in patients with psoriasis can trigger the development of pustular psoriasis), whereas a previous history of drug reaction is common in AGEP.3,7
Discontinue medication, treat with corticosteroids
Patients who have AGEP, including those with systemic involvement, generally improve after the offending drug is discontinued and treatment with topical corticosteroids is initiated.6 A brief course of systemic corticosteroids can also be considered for patients with severe skin involvement or systemic involvement.3
Our patient was prescribed topical corticosteroid wet dressing treatments twice daily for 2 weeks. At the 2-week follow-up visit, the rash had completely cleared, and only minimal residual erythema was noted (FIGURE 2). The patient was instructed to avoid azithromycin.
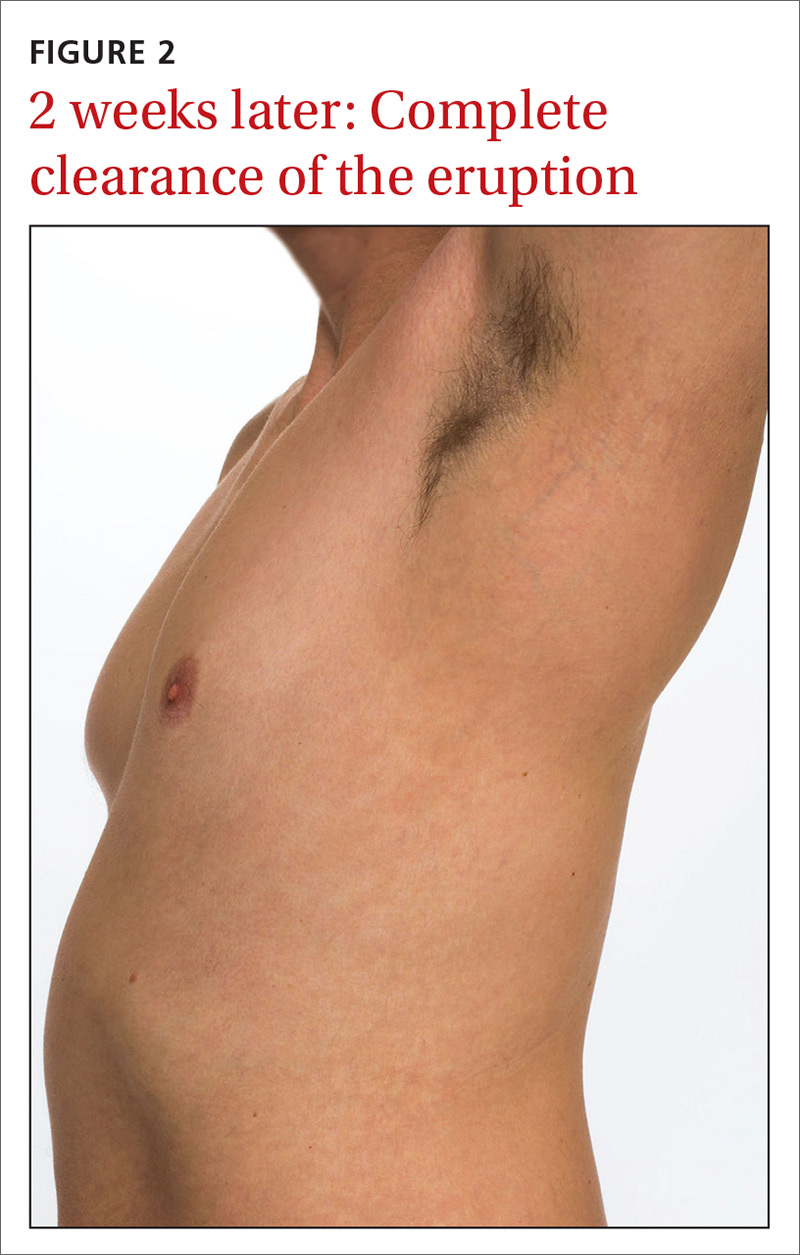
CORRESPONDENCE
David A. Wetter, MD, Department of Dermatology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; [email protected].
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Lee HY, Chou D, Pang SM, et al. Acute generalized exanthematous pustulosis: analysis of cases managed in a tertiary hospital in Singapore. Int J Dermatol. 2010;49:507-512.
3. Alniemi DT, Wetter DA, Bridges AG, et al. Acute generalized exanthematous pustulosis: clinical characteristics, etiologic associations, treatments, and outcomes in a series of 28 patients at Mayo Clinic, 1996-2013. Int J Dermatol. 2017;56:405-414.
4. Bouvresse S, Valeyrie-Allanore L, Ortonne N, et al. Toxic epidermal necrolysis, DRESS, AGEP: do overlap cases exist? Orphanet J Rare Dis. 2012;7:72.
5. Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
6. Hotz C, Valeyrie-Allanore L, Haddad C, et al. Systemic involvement of acute generalized exanthematous pustulosis: a retrospective study on 58 patients. Br J Dermatol. 2013;169:1223-1232.
7. Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:E1214.
8. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part II. Management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9.
9. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-e14.
10. Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92-96.
11. Roujeau JC. The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: a clinical classification. J Invest Dermatol. 1994;102:28S-30S.
A 38-year-old man sought care in the emergency department for an acute, pruritic, generalized cutaneous eruption that manifested in the intertriginous areas of the inner thighs, antecubital fossae, and axilla (FIGURE 1A). He reported associated chills, a 15-pound weight gain, and swelling of his inner thighs. Two weeks before presentation, he had received azithromycin for an upper respiratory tract infection. He was unsure if the rash developed prior to or after taking the medication. He was not taking any other medications and had no history of skin conditions.
On examination, the patient was afebrile and had bilateral thigh edema. Skin examination revealed background erythema with morbilliform papules, plaques, and patches on the bilateral flanks, back, buttocks, arms, legs, and central neck. Pinpoint pustules were present in the intertriginous sites and on the low back and buttocks. The laboratory evaluation revealed leukocytosis (11.0 × 109 cells/L), increased levels of neutrophils and eosinophils, and an elevated C-reactive protein level (12.8 mg/L). The remaining laboratory results were unremarkable. The patient was referred to Dermatology.
An examination by the dermatologist 3 days later revealed small areas of annular desquamation with a few pinpoint pustules, mostly located on the inner thighs and buttocks (FIGURE 1B). Skin biopsies were taken from the anterior hip region. The histopathology revealed subacute dermatitis with mixed dermal inflammatory cells, including neutrophils and eosinophils, and discrete subcorneal spongiform pustules.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute generalized exanthematous pustulosis (AGEP)
The acute rash with minute pustules and associated leukocytosis with neutrophilia and eosinophilia led to an early diagnosis of AGEP, which may have been triggered by azithromycin—the patient’s only recent medication. AGEP is a severe cutaneous eruption that may be associated with systemic involvement. Medications are usually implicated, and patients often seek urgent evaluation.
AGEP typically begins as an acute eruption in the intertriginous sites of the axilla, groin, and neck, but often becomes more generalized.1,2 The diagnosis is strongly suggested by the condition’s key features: fever (97% of cases) and leukocytosis (87%) with neutrophilia (91%) and eosinophilia (30%); leukocytosis peaks 4 days after pustulosis occurs and lasts for about 12 days.1 Although common, fever is not always documented in patients with AGEP. 3 (Our patient was a case in point.) While not a key characteristic of AGEP, our patient’s weight gain was likely explained by the severe edema secondary to his inflammatory skin eruption.
Medications are implicated, but pathophysiology is unknown
In approximately 90% of AGEP cases, medications such as antibiotics and calcium channel blockers are implicated; however, the lack of such an association does not preclude the diagnosis.1,4 In cases of drug reactions, the eruption typically develops 1 to 2 days after a medication is begun, and the pustules typically resolve in fewer than 15 days.5 In 17% of patients, systemic involvement can occur and can include the liver, kidneys, bone marrow, and lungs.6 A physical exam, review of systems, and a laboratory evaluation can help rule out systemic involvement and guide additional testing.
AGEP has an incidence of 1 to 5 cases per million people per year, affecting women slightly more frequently than men.7 While the pathophysiology is not well understood, AGEP and its differential diagnoses are categorized as T cell-related inflammatory responses.4,7
Distinguishing AGEP from some look-alikes
There are at least 4 severe cutaneous eruptions that might be confused with AGEP, all of which may be associated with fever. They include: drug reaction with eosinophilia and systemic symptoms (DRESS), also known as drug-induced hypersensitivity syndrome; Stevens-Johnson syndrome (SJS); toxic epidermal necrolysis (TEN); and pustular psoriasis.8-10 The clinical features that may help differentiate these conditions from AGEP include timeline, mucocutaneous features, organ system involvement, and histopathologic findings.4,8
DRESS occurs 2 to 6 weeks after drug exposure, rather than a few days, as is seen with AGEP. It often involves morbilliform erythema and facial edema with substantial eosinophilia and possible nephritis, pneumonitis, myocarditis, and thyroiditis.9 Unlike AGEP, DRESS does not have a predilection for intertriginous anatomic locations.
SJS and TEN occur 1 to 3 weeks after drug exposure. These conditions manifest with the development of bullae, atypical targetoid lesions, painful dusky erythema, epidermal necrosis, and mucosal involvement at multiple sites. Tubular nephritis, tracheobronchial necrosis, and multisystem organ failure can occur, with reported mortality rates of 5% to 35%.8,11
Pustular psoriasis is frequently confused with AGEP. However, AGEP usually develops fewer than 2 days after drug exposure, with pustules that begin in intertriginous sites, and there is associated neutrophilia and possible organ involvement.1,8 Patients who have AGEP typically do not have a history of psoriasis, while patients with pustular psoriasis often do.7 A history of drug reaction is uncommon with pustular psoriasis (although rapid tapering of systemic corticosteroids in patients with psoriasis can trigger the development of pustular psoriasis), whereas a previous history of drug reaction is common in AGEP.3,7
Discontinue medication, treat with corticosteroids
Patients who have AGEP, including those with systemic involvement, generally improve after the offending drug is discontinued and treatment with topical corticosteroids is initiated.6 A brief course of systemic corticosteroids can also be considered for patients with severe skin involvement or systemic involvement.3
Our patient was prescribed topical corticosteroid wet dressing treatments twice daily for 2 weeks. At the 2-week follow-up visit, the rash had completely cleared, and only minimal residual erythema was noted (FIGURE 2). The patient was instructed to avoid azithromycin.

CORRESPONDENCE
David A. Wetter, MD, Department of Dermatology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; [email protected].
A 38-year-old man sought care in the emergency department for an acute, pruritic, generalized cutaneous eruption that manifested in the intertriginous areas of the inner thighs, antecubital fossae, and axilla (FIGURE 1A). He reported associated chills, a 15-pound weight gain, and swelling of his inner thighs. Two weeks before presentation, he had received azithromycin for an upper respiratory tract infection. He was unsure if the rash developed prior to or after taking the medication. He was not taking any other medications and had no history of skin conditions.
On examination, the patient was afebrile and had bilateral thigh edema. Skin examination revealed background erythema with morbilliform papules, plaques, and patches on the bilateral flanks, back, buttocks, arms, legs, and central neck. Pinpoint pustules were present in the intertriginous sites and on the low back and buttocks. The laboratory evaluation revealed leukocytosis (11.0 × 109 cells/L), increased levels of neutrophils and eosinophils, and an elevated C-reactive protein level (12.8 mg/L). The remaining laboratory results were unremarkable. The patient was referred to Dermatology.
An examination by the dermatologist 3 days later revealed small areas of annular desquamation with a few pinpoint pustules, mostly located on the inner thighs and buttocks (FIGURE 1B). Skin biopsies were taken from the anterior hip region. The histopathology revealed subacute dermatitis with mixed dermal inflammatory cells, including neutrophils and eosinophils, and discrete subcorneal spongiform pustules.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute generalized exanthematous pustulosis (AGEP)
The acute rash with minute pustules and associated leukocytosis with neutrophilia and eosinophilia led to an early diagnosis of AGEP, which may have been triggered by azithromycin—the patient’s only recent medication. AGEP is a severe cutaneous eruption that may be associated with systemic involvement. Medications are usually implicated, and patients often seek urgent evaluation.
AGEP typically begins as an acute eruption in the intertriginous sites of the axilla, groin, and neck, but often becomes more generalized.1,2 The diagnosis is strongly suggested by the condition’s key features: fever (97% of cases) and leukocytosis (87%) with neutrophilia (91%) and eosinophilia (30%); leukocytosis peaks 4 days after pustulosis occurs and lasts for about 12 days.1 Although common, fever is not always documented in patients with AGEP. 3 (Our patient was a case in point.) While not a key characteristic of AGEP, our patient’s weight gain was likely explained by the severe edema secondary to his inflammatory skin eruption.
Medications are implicated, but pathophysiology is unknown
In approximately 90% of AGEP cases, medications such as antibiotics and calcium channel blockers are implicated; however, the lack of such an association does not preclude the diagnosis.1,4 In cases of drug reactions, the eruption typically develops 1 to 2 days after a medication is begun, and the pustules typically resolve in fewer than 15 days.5 In 17% of patients, systemic involvement can occur and can include the liver, kidneys, bone marrow, and lungs.6 A physical exam, review of systems, and a laboratory evaluation can help rule out systemic involvement and guide additional testing.
AGEP has an incidence of 1 to 5 cases per million people per year, affecting women slightly more frequently than men.7 While the pathophysiology is not well understood, AGEP and its differential diagnoses are categorized as T cell-related inflammatory responses.4,7
Distinguishing AGEP from some look-alikes
There are at least 4 severe cutaneous eruptions that might be confused with AGEP, all of which may be associated with fever. They include: drug reaction with eosinophilia and systemic symptoms (DRESS), also known as drug-induced hypersensitivity syndrome; Stevens-Johnson syndrome (SJS); toxic epidermal necrolysis (TEN); and pustular psoriasis.8-10 The clinical features that may help differentiate these conditions from AGEP include timeline, mucocutaneous features, organ system involvement, and histopathologic findings.4,8
DRESS occurs 2 to 6 weeks after drug exposure, rather than a few days, as is seen with AGEP. It often involves morbilliform erythema and facial edema with substantial eosinophilia and possible nephritis, pneumonitis, myocarditis, and thyroiditis.9 Unlike AGEP, DRESS does not have a predilection for intertriginous anatomic locations.
SJS and TEN occur 1 to 3 weeks after drug exposure. These conditions manifest with the development of bullae, atypical targetoid lesions, painful dusky erythema, epidermal necrosis, and mucosal involvement at multiple sites. Tubular nephritis, tracheobronchial necrosis, and multisystem organ failure can occur, with reported mortality rates of 5% to 35%.8,11
Pustular psoriasis is frequently confused with AGEP. However, AGEP usually develops fewer than 2 days after drug exposure, with pustules that begin in intertriginous sites, and there is associated neutrophilia and possible organ involvement.1,8 Patients who have AGEP typically do not have a history of psoriasis, while patients with pustular psoriasis often do.7 A history of drug reaction is uncommon with pustular psoriasis (although rapid tapering of systemic corticosteroids in patients with psoriasis can trigger the development of pustular psoriasis), whereas a previous history of drug reaction is common in AGEP.3,7
Discontinue medication, treat with corticosteroids
Patients who have AGEP, including those with systemic involvement, generally improve after the offending drug is discontinued and treatment with topical corticosteroids is initiated.6 A brief course of systemic corticosteroids can also be considered for patients with severe skin involvement or systemic involvement.3
Our patient was prescribed topical corticosteroid wet dressing treatments twice daily for 2 weeks. At the 2-week follow-up visit, the rash had completely cleared, and only minimal residual erythema was noted (FIGURE 2). The patient was instructed to avoid azithromycin.

CORRESPONDENCE
David A. Wetter, MD, Department of Dermatology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; [email protected].
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Lee HY, Chou D, Pang SM, et al. Acute generalized exanthematous pustulosis: analysis of cases managed in a tertiary hospital in Singapore. Int J Dermatol. 2010;49:507-512.
3. Alniemi DT, Wetter DA, Bridges AG, et al. Acute generalized exanthematous pustulosis: clinical characteristics, etiologic associations, treatments, and outcomes in a series of 28 patients at Mayo Clinic, 1996-2013. Int J Dermatol. 2017;56:405-414.
4. Bouvresse S, Valeyrie-Allanore L, Ortonne N, et al. Toxic epidermal necrolysis, DRESS, AGEP: do overlap cases exist? Orphanet J Rare Dis. 2012;7:72.
5. Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
6. Hotz C, Valeyrie-Allanore L, Haddad C, et al. Systemic involvement of acute generalized exanthematous pustulosis: a retrospective study on 58 patients. Br J Dermatol. 2013;169:1223-1232.
7. Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:E1214.
8. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part II. Management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9.
9. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-e14.
10. Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92-96.
11. Roujeau JC. The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: a clinical classification. J Invest Dermatol. 1994;102:28S-30S.
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Lee HY, Chou D, Pang SM, et al. Acute generalized exanthematous pustulosis: analysis of cases managed in a tertiary hospital in Singapore. Int J Dermatol. 2010;49:507-512.
3. Alniemi DT, Wetter DA, Bridges AG, et al. Acute generalized exanthematous pustulosis: clinical characteristics, etiologic associations, treatments, and outcomes in a series of 28 patients at Mayo Clinic, 1996-2013. Int J Dermatol. 2017;56:405-414.
4. Bouvresse S, Valeyrie-Allanore L, Ortonne N, et al. Toxic epidermal necrolysis, DRESS, AGEP: do overlap cases exist? Orphanet J Rare Dis. 2012;7:72.
5. Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
6. Hotz C, Valeyrie-Allanore L, Haddad C, et al. Systemic involvement of acute generalized exanthematous pustulosis: a retrospective study on 58 patients. Br J Dermatol. 2013;169:1223-1232.
7. Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:E1214.
8. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part II. Management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9.
9. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-e14.
10. Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92-96.
11. Roujeau JC. The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: a clinical classification. J Invest Dermatol. 1994;102:28S-30S.