User login
Current Considerations for Recognizing and Treating Iron Deficiency Anemia in Women
Click here to read the supplement.
Iron deficiency anemia (IDA) is one of the most common causes of anemia in women, and affects women of all ages. In this supplement, you will read about:
- How to identify IDA in women
- How to treat IDA
- A patient case study
Click here to read the supplement.
Click here to read the supplement.
Iron deficiency anemia (IDA) is one of the most common causes of anemia in women, and affects women of all ages. In this supplement, you will read about:
- How to identify IDA in women
- How to treat IDA
- A patient case study
Click here to read the supplement.
Click here to read the supplement.
Iron deficiency anemia (IDA) is one of the most common causes of anemia in women, and affects women of all ages. In this supplement, you will read about:
- How to identify IDA in women
- How to treat IDA
- A patient case study
Click here to read the supplement.
DBS may improve nonmotor symptoms in Parkinson’s disease
LAS VEGAS – , according to a small study presented at the annual meeting of the North American Neuromodulation Society. DBS of the subthalamic nucleus (STN), however, does not significantly improve these symptoms.
“Further work will be needed to confirm whether DBS needs to be bilateral ... and whether demographic differences are significant,” said Michael Gillogly, RN, clinical research nurse in the department of neurosurgery at Albany (New York) Medical Center. “The pilot data suggest that, if all else is equal, and the patient has significant urinary dysfunction as a major complaint, GPI DBS may be preferentially considered.”
The benefits of DBS on motor symptoms in Parkinson’s disease are well documented in the literature, but the technique’s effects on nonmotor symptoms are less clear. Nonmotor symptoms – such as cognitive deficits, gastrointestinal dysfunction, genitourinary dysfunction, and sleep disturbance – are common in all stages of Parkinson’s disease and significantly impair quality of life. Data indicate that speech and neuropsychological symptoms worsen with DBS of the STN, but research into the effect of DBS of the GPI on nonmotor symptoms is limited.
Mr. Gillogly and his colleagues considered all surgical candidates at their facility for enrollment into a study evaluating nonmotor outcomes in Parkinson’s disease at baseline, before implantation, and at 6 months after DBS. Study outcomes were patient perception of urinary, swallowing, and gastrointestinal function at 6 months after DBS of the GPI, compared with DBS of the STN.
The researchers chose two tools each to measure sialorrhea, dysphagia, and genitourinary dysfunction. These tools included the Drooling Severity and Frequency Scale (DSFS), the Swallowing Disturbance Questionnaire, and the International Prostate Symptom Score (IPSS). The investigators also collected demographic information, including sex, age at the time of surgery, duration of illness, neuropsychological profile, and medication inventory.
In all, 34 patients (12 women) were enrolled in the study and completed each outcome measure preoperatively and at 6 months postoperatively. The mean age of our subjects at the time of surgery was 64 years. Eight received DBS of the GPI, and 26 received DBS of the STN. Mr. Gillogly and his colleagues observed a significant 31% improvement in DSFS score and a significant 24% improvement on the IPSS among GPI-targeted patients. They found no significant improvements among patients who had STN targeting. When the investigators compared patients with unilateral lead placement and those with bilateral lead placement, they observed that all of the significant improvement among patients with GPI targeting occurred when treatment was bilateral.
The small sample size is a notable limitation of the study, and subset analyses were limited, said Mr. Gillogly. In addition, it was difficult to determine whether the symptoms studied were directly related to Parkinson’s disease, because they often arise as part of the natural aging process. “Other limitations of the study include lack of objective measurements, as these are all patient perception, and the innate limitations of self-reported questionnaires,” said Mr. Gillogly.
Two of the researchers reported having consulted for Medtronic, which markets a DBS system. One author received grant funding and consulting fees from Boston Scientific, Medtronic, and Abbott, all of which make DBS devices.
LAS VEGAS – , according to a small study presented at the annual meeting of the North American Neuromodulation Society. DBS of the subthalamic nucleus (STN), however, does not significantly improve these symptoms.
“Further work will be needed to confirm whether DBS needs to be bilateral ... and whether demographic differences are significant,” said Michael Gillogly, RN, clinical research nurse in the department of neurosurgery at Albany (New York) Medical Center. “The pilot data suggest that, if all else is equal, and the patient has significant urinary dysfunction as a major complaint, GPI DBS may be preferentially considered.”
The benefits of DBS on motor symptoms in Parkinson’s disease are well documented in the literature, but the technique’s effects on nonmotor symptoms are less clear. Nonmotor symptoms – such as cognitive deficits, gastrointestinal dysfunction, genitourinary dysfunction, and sleep disturbance – are common in all stages of Parkinson’s disease and significantly impair quality of life. Data indicate that speech and neuropsychological symptoms worsen with DBS of the STN, but research into the effect of DBS of the GPI on nonmotor symptoms is limited.
Mr. Gillogly and his colleagues considered all surgical candidates at their facility for enrollment into a study evaluating nonmotor outcomes in Parkinson’s disease at baseline, before implantation, and at 6 months after DBS. Study outcomes were patient perception of urinary, swallowing, and gastrointestinal function at 6 months after DBS of the GPI, compared with DBS of the STN.
The researchers chose two tools each to measure sialorrhea, dysphagia, and genitourinary dysfunction. These tools included the Drooling Severity and Frequency Scale (DSFS), the Swallowing Disturbance Questionnaire, and the International Prostate Symptom Score (IPSS). The investigators also collected demographic information, including sex, age at the time of surgery, duration of illness, neuropsychological profile, and medication inventory.
In all, 34 patients (12 women) were enrolled in the study and completed each outcome measure preoperatively and at 6 months postoperatively. The mean age of our subjects at the time of surgery was 64 years. Eight received DBS of the GPI, and 26 received DBS of the STN. Mr. Gillogly and his colleagues observed a significant 31% improvement in DSFS score and a significant 24% improvement on the IPSS among GPI-targeted patients. They found no significant improvements among patients who had STN targeting. When the investigators compared patients with unilateral lead placement and those with bilateral lead placement, they observed that all of the significant improvement among patients with GPI targeting occurred when treatment was bilateral.
The small sample size is a notable limitation of the study, and subset analyses were limited, said Mr. Gillogly. In addition, it was difficult to determine whether the symptoms studied were directly related to Parkinson’s disease, because they often arise as part of the natural aging process. “Other limitations of the study include lack of objective measurements, as these are all patient perception, and the innate limitations of self-reported questionnaires,” said Mr. Gillogly.
Two of the researchers reported having consulted for Medtronic, which markets a DBS system. One author received grant funding and consulting fees from Boston Scientific, Medtronic, and Abbott, all of which make DBS devices.
LAS VEGAS – , according to a small study presented at the annual meeting of the North American Neuromodulation Society. DBS of the subthalamic nucleus (STN), however, does not significantly improve these symptoms.
“Further work will be needed to confirm whether DBS needs to be bilateral ... and whether demographic differences are significant,” said Michael Gillogly, RN, clinical research nurse in the department of neurosurgery at Albany (New York) Medical Center. “The pilot data suggest that, if all else is equal, and the patient has significant urinary dysfunction as a major complaint, GPI DBS may be preferentially considered.”
The benefits of DBS on motor symptoms in Parkinson’s disease are well documented in the literature, but the technique’s effects on nonmotor symptoms are less clear. Nonmotor symptoms – such as cognitive deficits, gastrointestinal dysfunction, genitourinary dysfunction, and sleep disturbance – are common in all stages of Parkinson’s disease and significantly impair quality of life. Data indicate that speech and neuropsychological symptoms worsen with DBS of the STN, but research into the effect of DBS of the GPI on nonmotor symptoms is limited.
Mr. Gillogly and his colleagues considered all surgical candidates at their facility for enrollment into a study evaluating nonmotor outcomes in Parkinson’s disease at baseline, before implantation, and at 6 months after DBS. Study outcomes were patient perception of urinary, swallowing, and gastrointestinal function at 6 months after DBS of the GPI, compared with DBS of the STN.
The researchers chose two tools each to measure sialorrhea, dysphagia, and genitourinary dysfunction. These tools included the Drooling Severity and Frequency Scale (DSFS), the Swallowing Disturbance Questionnaire, and the International Prostate Symptom Score (IPSS). The investigators also collected demographic information, including sex, age at the time of surgery, duration of illness, neuropsychological profile, and medication inventory.
In all, 34 patients (12 women) were enrolled in the study and completed each outcome measure preoperatively and at 6 months postoperatively. The mean age of our subjects at the time of surgery was 64 years. Eight received DBS of the GPI, and 26 received DBS of the STN. Mr. Gillogly and his colleagues observed a significant 31% improvement in DSFS score and a significant 24% improvement on the IPSS among GPI-targeted patients. They found no significant improvements among patients who had STN targeting. When the investigators compared patients with unilateral lead placement and those with bilateral lead placement, they observed that all of the significant improvement among patients with GPI targeting occurred when treatment was bilateral.
The small sample size is a notable limitation of the study, and subset analyses were limited, said Mr. Gillogly. In addition, it was difficult to determine whether the symptoms studied were directly related to Parkinson’s disease, because they often arise as part of the natural aging process. “Other limitations of the study include lack of objective measurements, as these are all patient perception, and the innate limitations of self-reported questionnaires,” said Mr. Gillogly.
Two of the researchers reported having consulted for Medtronic, which markets a DBS system. One author received grant funding and consulting fees from Boston Scientific, Medtronic, and Abbott, all of which make DBS devices.
REPORTING FROM NANS 2019
Key clinical point: Bilateral stimulation of the globus pallidus internus reduces sialorrhea and improves genitourinary symptoms.
Major finding: Patients reported 31% improvement in sialorrhea and 24% improvement in urinary function.
Study details: A prospective study of 34 patients receiving DBS of the STN or GPI.
Disclosures: No funding was reported.
Cancer vaccine fails in CRC but trial yields lessons
SAN FRANCISCO – , according to final results of the German and Austrian phase 2 randomized LICC trial. However, information gleaned from the results, which were reported at the 2019 GI Cancers Symposium, will help inform future research.
“Hepatic metastectomy … is deemed the only potential curative treatment for stage IV colorectal cancer with limited liver disease. However, high recurrence rates after resection remain a major challenge: They range up to 50%-75% within the first 2 years,” said lead investigator Carl C. Schimanski, MD, PhD, of the Klinikum Darmstadt GmbH in Darmstadt, Germany.
Tecemotide is a liposome carrying mucin 1 (MUC1) antigen and an adjuvant that is taken up by antigen-presenting cells, ultimately leading to production of MUC1-specific cytotoxic T lymphocytes that target tumors. “MUC1 has been described to be expressed in up to 100% of colorectal cancer metastasis, so we thought this might be a good target,” Dr. Schimanski explained.
All 121 patients in the LICC trial had recently undergone primary or secondary resection, with either R0 or R1 outcome, for liver-only metastases of colorectal cancer. They were treated on a double-blind basis with a single dose of cyclophosphamide to reduce regulatory T cells, followed by tecemotide (weekly for 8 weeks, then every 6 weeks for up to 2 years) or with placebo.
Results showed that recurrence-free survival was actually shorter, by more than 5 months, with the vaccine versus placebo. In addition, the 3-year rate of overall survival was lower by an absolute 10%. Interestingly, tumor expression of MUC1 did not influence benefit from the vaccine.
But Dr. Schimanski noted that survival was better than expected at the trial’s outset. For example, the 65-month median overall survival among all patients in LICC undergoing secondary resection was about a year longer than that of similar patients in the CELIM trial (54 months) and the FIRE-3 trial (56 months).
“The LICC trial failed to meet its primary endpoint of significantly improving recurrence-free survival or overall survival with tecemotide. We had unexpectedly high overall survival in both arms, highlighting the critical importance of accurate staging and intensive surveillance, in our eyes,” he concluded. “We have further analysis of a very large translational program, and we hope to learn a lot about recurrence independent of tecemotide.”
A good space for testing immune therapies
In 2009, a consensus panel of immunologists ranked MUC1 as the second-best cancer antigen for translational research, “so there was clearly a feeling that this was a good target at that time for going forward,” noted invited discussant Michael J. Overman, MD, a professor in the department of gastrointestinal medical oncology, division of cancer medicine, University of Texas MD Anderson Cancer Center, Houston.
He agreed with the LICC investigators’ conclusions that the trial was negative and that MUC1 expression does not appear to predict outcome. “Whether that’s the wrong target, or whether it was the wrong formulation in regards to cancer vaccine, I think we do not know. I do think that survival was encouraging,” he said.
“There’s many unanswered questions in regards to the LICC study and in regards to cancer vaccines in general,” Dr. Overman noted. Among them, what are the optimal antigens to target, what are the optimal vaccine formulations and adjuvant agents, what is the best way to address the immunosuppressive tumor microenvironment, and what is the correct disease setting for vaccine testing?
“The LICC study is very impressive in demonstrating that we can enroll in this posthepatectomy space, postmetastectomy space. It’s a very increasingly interesting space for, potentially, drug development and immunologic exploration,” he maintained. “One of the benefits of this space when we talk about a minimal residual disease setting is that you potentially do not have the suppressive effects from the tumor microenvironment that potentially are hindering success in regards to having immune therapy response. So I would say that this is a space we should consider for drug development going forward.”
Study details
In the LICC trial, tecemotide and placebo yielded a respective median recurrence-free survival of 6.1 months and 11.4 months (P = .1754) and a respective overall survival of 62.8 months and not reached (P = .2141), Dr. Schimanski reported at the symposium, sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology. The 3-year overall survival rate was 69.1% with tecemotide and 79.1% with placebo.
That survival “was astonishing for us,” Dr. Schimanski said. “We think – but we cannot prove it – that has resulted from careful staging due to the retrospective radiological review and the initial staging, and a very tight surveillance program.”
Findings were similar regardless of whether patients had low, medium, or high tumor MUC1 expression; therefore, “we have to conclude that the target is not really validated.”
Patients in the tecemotide arm had higher rates of any-grade nausea, fatigue, diarrhea, and viral upper respiratory tract infections, at least some of which was likely attributable to the single dose of cyclophosphamide, according to Dr. Schimanski. They also had higher (but still low) rates of grade 3 or 4 back pain, anemia, ileus, cholestatic jaundice, and increased blood uric acid levels (2.5% for each). There was a single death in that arm from Merkel cell carcinoma that was deemed potentially related to the vaccine.
Dr. Schimanski disclosed that an immediate family member is employed by Merck and that he receives research funding from Merck KGaA (institutional). The trial was funded by Merck KGaA.
SOURCE: Schimanski CC et al. GI Cancers Symposium, Abstract 480.
SAN FRANCISCO – , according to final results of the German and Austrian phase 2 randomized LICC trial. However, information gleaned from the results, which were reported at the 2019 GI Cancers Symposium, will help inform future research.
“Hepatic metastectomy … is deemed the only potential curative treatment for stage IV colorectal cancer with limited liver disease. However, high recurrence rates after resection remain a major challenge: They range up to 50%-75% within the first 2 years,” said lead investigator Carl C. Schimanski, MD, PhD, of the Klinikum Darmstadt GmbH in Darmstadt, Germany.
Tecemotide is a liposome carrying mucin 1 (MUC1) antigen and an adjuvant that is taken up by antigen-presenting cells, ultimately leading to production of MUC1-specific cytotoxic T lymphocytes that target tumors. “MUC1 has been described to be expressed in up to 100% of colorectal cancer metastasis, so we thought this might be a good target,” Dr. Schimanski explained.
All 121 patients in the LICC trial had recently undergone primary or secondary resection, with either R0 or R1 outcome, for liver-only metastases of colorectal cancer. They were treated on a double-blind basis with a single dose of cyclophosphamide to reduce regulatory T cells, followed by tecemotide (weekly for 8 weeks, then every 6 weeks for up to 2 years) or with placebo.
Results showed that recurrence-free survival was actually shorter, by more than 5 months, with the vaccine versus placebo. In addition, the 3-year rate of overall survival was lower by an absolute 10%. Interestingly, tumor expression of MUC1 did not influence benefit from the vaccine.
But Dr. Schimanski noted that survival was better than expected at the trial’s outset. For example, the 65-month median overall survival among all patients in LICC undergoing secondary resection was about a year longer than that of similar patients in the CELIM trial (54 months) and the FIRE-3 trial (56 months).
“The LICC trial failed to meet its primary endpoint of significantly improving recurrence-free survival or overall survival with tecemotide. We had unexpectedly high overall survival in both arms, highlighting the critical importance of accurate staging and intensive surveillance, in our eyes,” he concluded. “We have further analysis of a very large translational program, and we hope to learn a lot about recurrence independent of tecemotide.”
A good space for testing immune therapies
In 2009, a consensus panel of immunologists ranked MUC1 as the second-best cancer antigen for translational research, “so there was clearly a feeling that this was a good target at that time for going forward,” noted invited discussant Michael J. Overman, MD, a professor in the department of gastrointestinal medical oncology, division of cancer medicine, University of Texas MD Anderson Cancer Center, Houston.
He agreed with the LICC investigators’ conclusions that the trial was negative and that MUC1 expression does not appear to predict outcome. “Whether that’s the wrong target, or whether it was the wrong formulation in regards to cancer vaccine, I think we do not know. I do think that survival was encouraging,” he said.
“There’s many unanswered questions in regards to the LICC study and in regards to cancer vaccines in general,” Dr. Overman noted. Among them, what are the optimal antigens to target, what are the optimal vaccine formulations and adjuvant agents, what is the best way to address the immunosuppressive tumor microenvironment, and what is the correct disease setting for vaccine testing?
“The LICC study is very impressive in demonstrating that we can enroll in this posthepatectomy space, postmetastectomy space. It’s a very increasingly interesting space for, potentially, drug development and immunologic exploration,” he maintained. “One of the benefits of this space when we talk about a minimal residual disease setting is that you potentially do not have the suppressive effects from the tumor microenvironment that potentially are hindering success in regards to having immune therapy response. So I would say that this is a space we should consider for drug development going forward.”
Study details
In the LICC trial, tecemotide and placebo yielded a respective median recurrence-free survival of 6.1 months and 11.4 months (P = .1754) and a respective overall survival of 62.8 months and not reached (P = .2141), Dr. Schimanski reported at the symposium, sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology. The 3-year overall survival rate was 69.1% with tecemotide and 79.1% with placebo.
That survival “was astonishing for us,” Dr. Schimanski said. “We think – but we cannot prove it – that has resulted from careful staging due to the retrospective radiological review and the initial staging, and a very tight surveillance program.”
Findings were similar regardless of whether patients had low, medium, or high tumor MUC1 expression; therefore, “we have to conclude that the target is not really validated.”
Patients in the tecemotide arm had higher rates of any-grade nausea, fatigue, diarrhea, and viral upper respiratory tract infections, at least some of which was likely attributable to the single dose of cyclophosphamide, according to Dr. Schimanski. They also had higher (but still low) rates of grade 3 or 4 back pain, anemia, ileus, cholestatic jaundice, and increased blood uric acid levels (2.5% for each). There was a single death in that arm from Merkel cell carcinoma that was deemed potentially related to the vaccine.
Dr. Schimanski disclosed that an immediate family member is employed by Merck and that he receives research funding from Merck KGaA (institutional). The trial was funded by Merck KGaA.
SOURCE: Schimanski CC et al. GI Cancers Symposium, Abstract 480.
SAN FRANCISCO – , according to final results of the German and Austrian phase 2 randomized LICC trial. However, information gleaned from the results, which were reported at the 2019 GI Cancers Symposium, will help inform future research.
“Hepatic metastectomy … is deemed the only potential curative treatment for stage IV colorectal cancer with limited liver disease. However, high recurrence rates after resection remain a major challenge: They range up to 50%-75% within the first 2 years,” said lead investigator Carl C. Schimanski, MD, PhD, of the Klinikum Darmstadt GmbH in Darmstadt, Germany.
Tecemotide is a liposome carrying mucin 1 (MUC1) antigen and an adjuvant that is taken up by antigen-presenting cells, ultimately leading to production of MUC1-specific cytotoxic T lymphocytes that target tumors. “MUC1 has been described to be expressed in up to 100% of colorectal cancer metastasis, so we thought this might be a good target,” Dr. Schimanski explained.
All 121 patients in the LICC trial had recently undergone primary or secondary resection, with either R0 or R1 outcome, for liver-only metastases of colorectal cancer. They were treated on a double-blind basis with a single dose of cyclophosphamide to reduce regulatory T cells, followed by tecemotide (weekly for 8 weeks, then every 6 weeks for up to 2 years) or with placebo.
Results showed that recurrence-free survival was actually shorter, by more than 5 months, with the vaccine versus placebo. In addition, the 3-year rate of overall survival was lower by an absolute 10%. Interestingly, tumor expression of MUC1 did not influence benefit from the vaccine.
But Dr. Schimanski noted that survival was better than expected at the trial’s outset. For example, the 65-month median overall survival among all patients in LICC undergoing secondary resection was about a year longer than that of similar patients in the CELIM trial (54 months) and the FIRE-3 trial (56 months).
“The LICC trial failed to meet its primary endpoint of significantly improving recurrence-free survival or overall survival with tecemotide. We had unexpectedly high overall survival in both arms, highlighting the critical importance of accurate staging and intensive surveillance, in our eyes,” he concluded. “We have further analysis of a very large translational program, and we hope to learn a lot about recurrence independent of tecemotide.”
A good space for testing immune therapies
In 2009, a consensus panel of immunologists ranked MUC1 as the second-best cancer antigen for translational research, “so there was clearly a feeling that this was a good target at that time for going forward,” noted invited discussant Michael J. Overman, MD, a professor in the department of gastrointestinal medical oncology, division of cancer medicine, University of Texas MD Anderson Cancer Center, Houston.
He agreed with the LICC investigators’ conclusions that the trial was negative and that MUC1 expression does not appear to predict outcome. “Whether that’s the wrong target, or whether it was the wrong formulation in regards to cancer vaccine, I think we do not know. I do think that survival was encouraging,” he said.
“There’s many unanswered questions in regards to the LICC study and in regards to cancer vaccines in general,” Dr. Overman noted. Among them, what are the optimal antigens to target, what are the optimal vaccine formulations and adjuvant agents, what is the best way to address the immunosuppressive tumor microenvironment, and what is the correct disease setting for vaccine testing?
“The LICC study is very impressive in demonstrating that we can enroll in this posthepatectomy space, postmetastectomy space. It’s a very increasingly interesting space for, potentially, drug development and immunologic exploration,” he maintained. “One of the benefits of this space when we talk about a minimal residual disease setting is that you potentially do not have the suppressive effects from the tumor microenvironment that potentially are hindering success in regards to having immune therapy response. So I would say that this is a space we should consider for drug development going forward.”
Study details
In the LICC trial, tecemotide and placebo yielded a respective median recurrence-free survival of 6.1 months and 11.4 months (P = .1754) and a respective overall survival of 62.8 months and not reached (P = .2141), Dr. Schimanski reported at the symposium, sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology. The 3-year overall survival rate was 69.1% with tecemotide and 79.1% with placebo.
That survival “was astonishing for us,” Dr. Schimanski said. “We think – but we cannot prove it – that has resulted from careful staging due to the retrospective radiological review and the initial staging, and a very tight surveillance program.”
Findings were similar regardless of whether patients had low, medium, or high tumor MUC1 expression; therefore, “we have to conclude that the target is not really validated.”
Patients in the tecemotide arm had higher rates of any-grade nausea, fatigue, diarrhea, and viral upper respiratory tract infections, at least some of which was likely attributable to the single dose of cyclophosphamide, according to Dr. Schimanski. They also had higher (but still low) rates of grade 3 or 4 back pain, anemia, ileus, cholestatic jaundice, and increased blood uric acid levels (2.5% for each). There was a single death in that arm from Merkel cell carcinoma that was deemed potentially related to the vaccine.
Dr. Schimanski disclosed that an immediate family member is employed by Merck and that he receives research funding from Merck KGaA (institutional). The trial was funded by Merck KGaA.
SOURCE: Schimanski CC et al. GI Cancers Symposium, Abstract 480.
REPORTING FROM THE 2019 GI CANCERS SYMPOSIUM
Key clinical point: Tecemotide did not improve outcomes among patients with resected liver-only metastases of CRC.
Major finding: Tecemotide was not superior to placebo with respect to median recurrence-free survival (6.1 vs. 11.4 months; P = .1754) or overall survival (62.8 months vs. not reached; P = .2141).
Study details: A phase 2 randomized controlled trial among 121 patients having had R0/R1 resection of isolated liver CRC metastases (LICC trial).
Disclosures: Dr. Schimanski disclosed that an immediate family member is employed by Merck and that he receives research funding from Merck KGaA (institutional). The trial was funded by Merck KGaA.
Source: Schimanski CC et al. GI Cancers Symposium, Abstract 480.
Texas launches website in fight against opioid abuse; Gen Z’ers report more mental health problems
Officials in Texas see their new “Dose of Reality” website as a tool that might help address the opioid crisis in their state.
Dose of Reality, an initiative of the state attorney general, the Texas Department of State Health Services, and Texas Health and Human Services, offers for download material on opioids. People also can learn about risk factors of opioid abuse and how to safely store the medications. Drug disposal sites statewide also are included, according to an article published by the Dallas Morning News.
“The misuse of prescription opioids costs lives and devastates Texas families in every corner of our state. Dose of Reality is a one-stop shop of information on the opioid epidemic in Texas. [It] will pull back the curtain on opioids, educate Texans and save, hopefully, many lives,” Texas Attorney General Ken Paxton reportedly said at a press conference announcing the website launch.
Of the 42,249 deaths tied to opioid overdoses reported nationwide by the National Institute on Drug Abuse in 2016, 1,375 of those deaths reportedly occurred in Texas. According to Mr. Paxton, deceptive marketing and promotion by pharmaceutical companies have been part of the problem.
Generation Z and mental health
Gen Z’ers – young people born from the mid-1990s to the early 2000s – are the most likely age group to report mental health problems, according to a report from the American Psychological Association.
The findings from the group’s 12th annual Stress in America survey of 3,458 Americans aged 18 years or older and 300 teens aged 15-17 years showed that issues such as sexual harassment and gun violence are significant stressors for Gen Z. America’s youngest adults are most likely of all generations to report poor mental health, and Gen Z also is significantly more likely to seek professional help for mental health issues, the study authors wrote.
Adolescents and young adults aged 15-21 years are more concerned than are other generations about the state of the United States, and overall, 71% of the Gen Z’ers are more positive about the country’s future. About 60% had gotten politically involved in the past year.
But that optimism did not extend to Gen Z’ers of color. “For around 4 in 10 Gen Zs of color, personal debt [41%] and housing instability (40%) are significant sources of stress, while 3 in 10 white Gen Zs [30%] say the same about personal debt and less than one-quarter [24%] of this demographic cite housing instability,” the authors wrote.
“Solutions” center in the works
A new facility to be built in a Denver neighborhood will enable offenders with mental health issues to receive treatment instead of incarceration. Once up and running, the facility, dubbed a “solutions” or “stabilization” center, will be a go-to option for police officers who have picked up someone judged to be in the throes of a mental health crisis, instead of a trip to the police station and booking, the Denver Post reported.
People referred to the center will be eligible to stay for up to 5 days and referrals will be available for continued counseling. Walk-ins will not be admitted.
“In my heart, I’m committed to making this an addition to the neighborhood that will make the neighborhood a safer place and not a more difficult place,” said Jay Flynn, a vice president of the Mental Health Center of Denver, which helped spearhead the initiative.
Not everyone is on board. Residents near the center site have voiced their concern about neighborhood safety. “It’s not that we don’t understand the needs of homelessness in our community,” said one resident at a community meeting held to discuss the center. “The fact is that our community is extremely stressed and we need to preserve a safe environment.”
The center is scheduled to open in 2020.
Is masculinity really toxic?
A new ad by Gillette raises questions about what it means to be male. The ad initially presents a more traditional view of men as boors, bullies, and sexual oppressors, then morphs into a call for a sea change to males with empathy, compassion, and a need to help. The ad came a few months after the American Psychological Association issued new practice guidelines for boys and men, in which traditional masculinity ideology was conceptualized as limiting.
Those developments prompted an op-ed piece in the Los Angeles Times that considered whether masculinity really is toxic.
“Some of the angry responses to the [Gillette] ad were over the top, and yet the detractors have a point. Take the way the ad exhorts men to start doing and saying ‘the right thing,’ and then continues, ‘Some already are. But some is not enough.’ This suggests decent men are a minority while brutes are the norm,” wrote Cathy Young, a contributing editor at Reason magazine.
“What’s more, some of the ‘toxic’ behavior shown is pretty innocuous, such as teenage boys ogling bikini-clad babes on television. (Should we shame girls who drool over cute male pop stars?) The ad also blurs the line between fighting and roughhousing, implicitly condemning the physical play styles more common among boys,” she wrote.
Meanwhile, the psychologists pointed out that, in light of many factors, including higher death rates in the United States for boys and men – compared with those of girls and women – understanding “how boys and men experience masculinity is an important cultural competency.”
Dementia and an aging workforce
As the American workforce continues to age, employers are having tough conversations about dementia and other cognitive issues, according an article from the Associated Press.
“And it’s not just managing missed deadlines,” Sarah Wood, director of global work-life services at an organization called Workplace Options, said in the piece. “If this person has been a dependable employee for 40 years and is now missing meetings, they’ll be beating themselves up over this.”
According to the Bureau of Labor Statistics, the number of U.S. workers aged 65-74 years was expected to skyrocket by 55% between 2014 and 2024.
Those aged 65 years and older are more likely to face dementia diagnoses. Because of the Americans with Disabilities Act, employers with dementia – including Alzheimer’s – are protected, “depending on the employee’s position and level of impairment,” according to the article.
Employers can accommodate employees by taking steps such as writing instructions rather than communicating verbally and reassigning employees who operate heavy machines to desk work, according to David K. Fram, director of the Americans with Disabilities Act equal opportunity services at the National Employment Law Institute. But employees must be able to do the “essential functions of the job,” he said.
Officials in Texas see their new “Dose of Reality” website as a tool that might help address the opioid crisis in their state.
Dose of Reality, an initiative of the state attorney general, the Texas Department of State Health Services, and Texas Health and Human Services, offers for download material on opioids. People also can learn about risk factors of opioid abuse and how to safely store the medications. Drug disposal sites statewide also are included, according to an article published by the Dallas Morning News.
“The misuse of prescription opioids costs lives and devastates Texas families in every corner of our state. Dose of Reality is a one-stop shop of information on the opioid epidemic in Texas. [It] will pull back the curtain on opioids, educate Texans and save, hopefully, many lives,” Texas Attorney General Ken Paxton reportedly said at a press conference announcing the website launch.
Of the 42,249 deaths tied to opioid overdoses reported nationwide by the National Institute on Drug Abuse in 2016, 1,375 of those deaths reportedly occurred in Texas. According to Mr. Paxton, deceptive marketing and promotion by pharmaceutical companies have been part of the problem.
Generation Z and mental health
Gen Z’ers – young people born from the mid-1990s to the early 2000s – are the most likely age group to report mental health problems, according to a report from the American Psychological Association.
The findings from the group’s 12th annual Stress in America survey of 3,458 Americans aged 18 years or older and 300 teens aged 15-17 years showed that issues such as sexual harassment and gun violence are significant stressors for Gen Z. America’s youngest adults are most likely of all generations to report poor mental health, and Gen Z also is significantly more likely to seek professional help for mental health issues, the study authors wrote.
Adolescents and young adults aged 15-21 years are more concerned than are other generations about the state of the United States, and overall, 71% of the Gen Z’ers are more positive about the country’s future. About 60% had gotten politically involved in the past year.
But that optimism did not extend to Gen Z’ers of color. “For around 4 in 10 Gen Zs of color, personal debt [41%] and housing instability (40%) are significant sources of stress, while 3 in 10 white Gen Zs [30%] say the same about personal debt and less than one-quarter [24%] of this demographic cite housing instability,” the authors wrote.
“Solutions” center in the works
A new facility to be built in a Denver neighborhood will enable offenders with mental health issues to receive treatment instead of incarceration. Once up and running, the facility, dubbed a “solutions” or “stabilization” center, will be a go-to option for police officers who have picked up someone judged to be in the throes of a mental health crisis, instead of a trip to the police station and booking, the Denver Post reported.
People referred to the center will be eligible to stay for up to 5 days and referrals will be available for continued counseling. Walk-ins will not be admitted.
“In my heart, I’m committed to making this an addition to the neighborhood that will make the neighborhood a safer place and not a more difficult place,” said Jay Flynn, a vice president of the Mental Health Center of Denver, which helped spearhead the initiative.
Not everyone is on board. Residents near the center site have voiced their concern about neighborhood safety. “It’s not that we don’t understand the needs of homelessness in our community,” said one resident at a community meeting held to discuss the center. “The fact is that our community is extremely stressed and we need to preserve a safe environment.”
The center is scheduled to open in 2020.
Is masculinity really toxic?
A new ad by Gillette raises questions about what it means to be male. The ad initially presents a more traditional view of men as boors, bullies, and sexual oppressors, then morphs into a call for a sea change to males with empathy, compassion, and a need to help. The ad came a few months after the American Psychological Association issued new practice guidelines for boys and men, in which traditional masculinity ideology was conceptualized as limiting.
Those developments prompted an op-ed piece in the Los Angeles Times that considered whether masculinity really is toxic.
“Some of the angry responses to the [Gillette] ad were over the top, and yet the detractors have a point. Take the way the ad exhorts men to start doing and saying ‘the right thing,’ and then continues, ‘Some already are. But some is not enough.’ This suggests decent men are a minority while brutes are the norm,” wrote Cathy Young, a contributing editor at Reason magazine.
“What’s more, some of the ‘toxic’ behavior shown is pretty innocuous, such as teenage boys ogling bikini-clad babes on television. (Should we shame girls who drool over cute male pop stars?) The ad also blurs the line between fighting and roughhousing, implicitly condemning the physical play styles more common among boys,” she wrote.
Meanwhile, the psychologists pointed out that, in light of many factors, including higher death rates in the United States for boys and men – compared with those of girls and women – understanding “how boys and men experience masculinity is an important cultural competency.”
Dementia and an aging workforce
As the American workforce continues to age, employers are having tough conversations about dementia and other cognitive issues, according an article from the Associated Press.
“And it’s not just managing missed deadlines,” Sarah Wood, director of global work-life services at an organization called Workplace Options, said in the piece. “If this person has been a dependable employee for 40 years and is now missing meetings, they’ll be beating themselves up over this.”
According to the Bureau of Labor Statistics, the number of U.S. workers aged 65-74 years was expected to skyrocket by 55% between 2014 and 2024.
Those aged 65 years and older are more likely to face dementia diagnoses. Because of the Americans with Disabilities Act, employers with dementia – including Alzheimer’s – are protected, “depending on the employee’s position and level of impairment,” according to the article.
Employers can accommodate employees by taking steps such as writing instructions rather than communicating verbally and reassigning employees who operate heavy machines to desk work, according to David K. Fram, director of the Americans with Disabilities Act equal opportunity services at the National Employment Law Institute. But employees must be able to do the “essential functions of the job,” he said.
Officials in Texas see their new “Dose of Reality” website as a tool that might help address the opioid crisis in their state.
Dose of Reality, an initiative of the state attorney general, the Texas Department of State Health Services, and Texas Health and Human Services, offers for download material on opioids. People also can learn about risk factors of opioid abuse and how to safely store the medications. Drug disposal sites statewide also are included, according to an article published by the Dallas Morning News.
“The misuse of prescription opioids costs lives and devastates Texas families in every corner of our state. Dose of Reality is a one-stop shop of information on the opioid epidemic in Texas. [It] will pull back the curtain on opioids, educate Texans and save, hopefully, many lives,” Texas Attorney General Ken Paxton reportedly said at a press conference announcing the website launch.
Of the 42,249 deaths tied to opioid overdoses reported nationwide by the National Institute on Drug Abuse in 2016, 1,375 of those deaths reportedly occurred in Texas. According to Mr. Paxton, deceptive marketing and promotion by pharmaceutical companies have been part of the problem.
Generation Z and mental health
Gen Z’ers – young people born from the mid-1990s to the early 2000s – are the most likely age group to report mental health problems, according to a report from the American Psychological Association.
The findings from the group’s 12th annual Stress in America survey of 3,458 Americans aged 18 years or older and 300 teens aged 15-17 years showed that issues such as sexual harassment and gun violence are significant stressors for Gen Z. America’s youngest adults are most likely of all generations to report poor mental health, and Gen Z also is significantly more likely to seek professional help for mental health issues, the study authors wrote.
Adolescents and young adults aged 15-21 years are more concerned than are other generations about the state of the United States, and overall, 71% of the Gen Z’ers are more positive about the country’s future. About 60% had gotten politically involved in the past year.
But that optimism did not extend to Gen Z’ers of color. “For around 4 in 10 Gen Zs of color, personal debt [41%] and housing instability (40%) are significant sources of stress, while 3 in 10 white Gen Zs [30%] say the same about personal debt and less than one-quarter [24%] of this demographic cite housing instability,” the authors wrote.
“Solutions” center in the works
A new facility to be built in a Denver neighborhood will enable offenders with mental health issues to receive treatment instead of incarceration. Once up and running, the facility, dubbed a “solutions” or “stabilization” center, will be a go-to option for police officers who have picked up someone judged to be in the throes of a mental health crisis, instead of a trip to the police station and booking, the Denver Post reported.
People referred to the center will be eligible to stay for up to 5 days and referrals will be available for continued counseling. Walk-ins will not be admitted.
“In my heart, I’m committed to making this an addition to the neighborhood that will make the neighborhood a safer place and not a more difficult place,” said Jay Flynn, a vice president of the Mental Health Center of Denver, which helped spearhead the initiative.
Not everyone is on board. Residents near the center site have voiced their concern about neighborhood safety. “It’s not that we don’t understand the needs of homelessness in our community,” said one resident at a community meeting held to discuss the center. “The fact is that our community is extremely stressed and we need to preserve a safe environment.”
The center is scheduled to open in 2020.
Is masculinity really toxic?
A new ad by Gillette raises questions about what it means to be male. The ad initially presents a more traditional view of men as boors, bullies, and sexual oppressors, then morphs into a call for a sea change to males with empathy, compassion, and a need to help. The ad came a few months after the American Psychological Association issued new practice guidelines for boys and men, in which traditional masculinity ideology was conceptualized as limiting.
Those developments prompted an op-ed piece in the Los Angeles Times that considered whether masculinity really is toxic.
“Some of the angry responses to the [Gillette] ad were over the top, and yet the detractors have a point. Take the way the ad exhorts men to start doing and saying ‘the right thing,’ and then continues, ‘Some already are. But some is not enough.’ This suggests decent men are a minority while brutes are the norm,” wrote Cathy Young, a contributing editor at Reason magazine.
“What’s more, some of the ‘toxic’ behavior shown is pretty innocuous, such as teenage boys ogling bikini-clad babes on television. (Should we shame girls who drool over cute male pop stars?) The ad also blurs the line between fighting and roughhousing, implicitly condemning the physical play styles more common among boys,” she wrote.
Meanwhile, the psychologists pointed out that, in light of many factors, including higher death rates in the United States for boys and men – compared with those of girls and women – understanding “how boys and men experience masculinity is an important cultural competency.”
Dementia and an aging workforce
As the American workforce continues to age, employers are having tough conversations about dementia and other cognitive issues, according an article from the Associated Press.
“And it’s not just managing missed deadlines,” Sarah Wood, director of global work-life services at an organization called Workplace Options, said in the piece. “If this person has been a dependable employee for 40 years and is now missing meetings, they’ll be beating themselves up over this.”
According to the Bureau of Labor Statistics, the number of U.S. workers aged 65-74 years was expected to skyrocket by 55% between 2014 and 2024.
Those aged 65 years and older are more likely to face dementia diagnoses. Because of the Americans with Disabilities Act, employers with dementia – including Alzheimer’s – are protected, “depending on the employee’s position and level of impairment,” according to the article.
Employers can accommodate employees by taking steps such as writing instructions rather than communicating verbally and reassigning employees who operate heavy machines to desk work, according to David K. Fram, director of the Americans with Disabilities Act equal opportunity services at the National Employment Law Institute. But employees must be able to do the “essential functions of the job,” he said.
Insulin may be toxic to the placenta in early pregnancy
, according to findings from an experimental in vitro study published in Fertility and Sterility.
“Collectively these results demonstrate that insulin itself may be directly toxic to the early human placenta but that metformin can prevent these deleterious effects,” wrote Mario Vega, MD, of Columbia University Fertility Center, New York, and his colleagues. “If confirmed in animal and human studies, this would indicate that screening and treatment for insulin resistance should focus on hyperinsulinemia.”
Dr. Vega and his colleagues cultivated trophoblast cells from three healthy women scheduled for manual vacuum aspiration during the first trimester of pregnancy to study the effects of insulin exposure alone, while trophoblast cells were cultured from a different set of women for the insulin and metformin follow-up experiments. The researchers tested each experiment against a control group of cultivated lung fibroblast cells. Insulin was measured in doses of 0.2 nmol, 1 nmol, and 5 nmol, while metformin was measured at 10 micromol. The primary outcome measures examined were gamma-H2AX for DNA damage, cell proliferation assay for cell survival, and cleaved caspase-3 for apoptosis.
Within 48 hours, the cultures showed DNA damage and induction of apoptosis when exposed to 1 nmol of insulin, but researchers said pretreatment with metformin prevented these effects. Exposing cells to metformin after insulin reduced but did not eliminate the effects of insulin.
The researchers noted the study is limited because the effects of insulin and metformin have not been examined in vivo, and it is not known at what level insulin causes damage. In addition, they suggested downregulation of genes in trophoblasts caused by insulin could cause apoptosis and DNA damage to trophoblast cells.
“Although studies performed on kidney and colon cells suggest that one possible mechanism of action for insulin-mediated genotoxicity is through AKT activation of mitochondria and subsequent reactive oxygen species production, the exact mechanism is poorly understood,” Dr. Vega and colleagues said. “Future studies will be necessary to determine variability among subjects, as well as mechanisms of action through which insulin exerts its cytotoxicity and genotoxicity.”
This study was funded by a grant from the National Institutes of Health Human Placenta Project. The authors reported no relevant financial disclosures.
SOURCE: Vega M et al. Fertil Steril. 2019. doi: 10.1016/j.fertnstert.2018.11.032.
, according to findings from an experimental in vitro study published in Fertility and Sterility.
“Collectively these results demonstrate that insulin itself may be directly toxic to the early human placenta but that metformin can prevent these deleterious effects,” wrote Mario Vega, MD, of Columbia University Fertility Center, New York, and his colleagues. “If confirmed in animal and human studies, this would indicate that screening and treatment for insulin resistance should focus on hyperinsulinemia.”
Dr. Vega and his colleagues cultivated trophoblast cells from three healthy women scheduled for manual vacuum aspiration during the first trimester of pregnancy to study the effects of insulin exposure alone, while trophoblast cells were cultured from a different set of women for the insulin and metformin follow-up experiments. The researchers tested each experiment against a control group of cultivated lung fibroblast cells. Insulin was measured in doses of 0.2 nmol, 1 nmol, and 5 nmol, while metformin was measured at 10 micromol. The primary outcome measures examined were gamma-H2AX for DNA damage, cell proliferation assay for cell survival, and cleaved caspase-3 for apoptosis.
Within 48 hours, the cultures showed DNA damage and induction of apoptosis when exposed to 1 nmol of insulin, but researchers said pretreatment with metformin prevented these effects. Exposing cells to metformin after insulin reduced but did not eliminate the effects of insulin.
The researchers noted the study is limited because the effects of insulin and metformin have not been examined in vivo, and it is not known at what level insulin causes damage. In addition, they suggested downregulation of genes in trophoblasts caused by insulin could cause apoptosis and DNA damage to trophoblast cells.
“Although studies performed on kidney and colon cells suggest that one possible mechanism of action for insulin-mediated genotoxicity is through AKT activation of mitochondria and subsequent reactive oxygen species production, the exact mechanism is poorly understood,” Dr. Vega and colleagues said. “Future studies will be necessary to determine variability among subjects, as well as mechanisms of action through which insulin exerts its cytotoxicity and genotoxicity.”
This study was funded by a grant from the National Institutes of Health Human Placenta Project. The authors reported no relevant financial disclosures.
SOURCE: Vega M et al. Fertil Steril. 2019. doi: 10.1016/j.fertnstert.2018.11.032.
, according to findings from an experimental in vitro study published in Fertility and Sterility.
“Collectively these results demonstrate that insulin itself may be directly toxic to the early human placenta but that metformin can prevent these deleterious effects,” wrote Mario Vega, MD, of Columbia University Fertility Center, New York, and his colleagues. “If confirmed in animal and human studies, this would indicate that screening and treatment for insulin resistance should focus on hyperinsulinemia.”
Dr. Vega and his colleagues cultivated trophoblast cells from three healthy women scheduled for manual vacuum aspiration during the first trimester of pregnancy to study the effects of insulin exposure alone, while trophoblast cells were cultured from a different set of women for the insulin and metformin follow-up experiments. The researchers tested each experiment against a control group of cultivated lung fibroblast cells. Insulin was measured in doses of 0.2 nmol, 1 nmol, and 5 nmol, while metformin was measured at 10 micromol. The primary outcome measures examined were gamma-H2AX for DNA damage, cell proliferation assay for cell survival, and cleaved caspase-3 for apoptosis.
Within 48 hours, the cultures showed DNA damage and induction of apoptosis when exposed to 1 nmol of insulin, but researchers said pretreatment with metformin prevented these effects. Exposing cells to metformin after insulin reduced but did not eliminate the effects of insulin.
The researchers noted the study is limited because the effects of insulin and metformin have not been examined in vivo, and it is not known at what level insulin causes damage. In addition, they suggested downregulation of genes in trophoblasts caused by insulin could cause apoptosis and DNA damage to trophoblast cells.
“Although studies performed on kidney and colon cells suggest that one possible mechanism of action for insulin-mediated genotoxicity is through AKT activation of mitochondria and subsequent reactive oxygen species production, the exact mechanism is poorly understood,” Dr. Vega and colleagues said. “Future studies will be necessary to determine variability among subjects, as well as mechanisms of action through which insulin exerts its cytotoxicity and genotoxicity.”
This study was funded by a grant from the National Institutes of Health Human Placenta Project. The authors reported no relevant financial disclosures.
SOURCE: Vega M et al. Fertil Steril. 2019. doi: 10.1016/j.fertnstert.2018.11.032.
FROM FERTILITY & STERILITY
Key clinical point: Trophoblasts cultured during the first trimester of pregnancy exposed to insulin were more likely to have increased apoptosis, DNA damage, and decreased cell survival, while pretreatment with metformin prior to exposure with insulin prevented these effects.
Major finding: DNA damage and rate of apoptosis increased in trophoblast cells exposed to 1 nmol of insulin, and cell survival decreased, compared with primary lung fibroblast cells; treating the cells with metformin prior to exposure with insulin resulted in prevention of these effects.
Study details: An experimental in vitro study of first trimester trophoblast cells exposed to insulin and metformin.
Disclosures: This study was funded by a grant from the National Institutes of Health Human Placenta Project. The authors reported they had no relevant financial disclosures.
Source: Vega M et al. Fertil Steril. 2019. doi: 10.1016/j.fertnstert.2018.11.032.
Paraneoplastic Dermatomyositis Presenting With Interesting Cutaneous Findings
To the Editor:
We report an interesting clinical case of dermatomyositis (DM) that presented with an associated malignancy (small cell lung cancer). This patient also had an unusual clinical finding of predominantly unilateral, confluent, erythematous papules on the knee, a cutaneous sign that is seldom described in the DM literature. This case serves to reinforce the classic findings and associations of DM, in addition to the uncommon manifestation of predominantly unilateral papules on the knee.
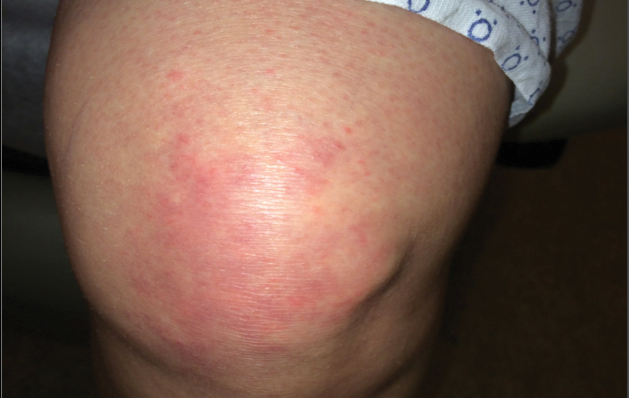
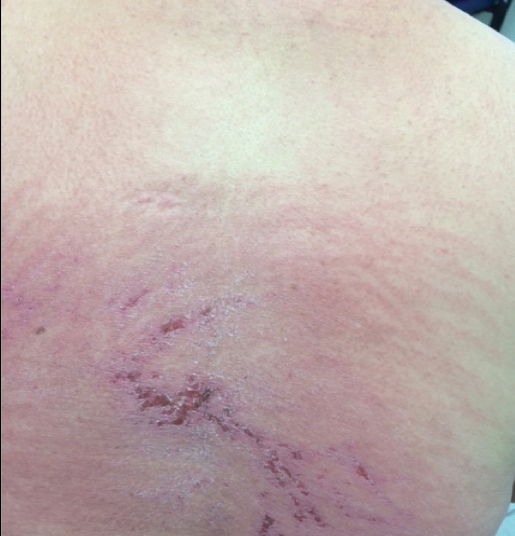
A 68-year-old woman presented with several cutaneous manifestations including the classic findings of photo distributed erythema on the arms and face, a heliotrope rash, Gottron papules, and confluent pink papules on the left knee (Figure 1). The patient also had one of the more rare manifestations of DM, flagellate erythema on the back (Figure 2). She had a history of breast cancer and was found to have metastatic small cell lung cancer at the time of the DM diagnosis.
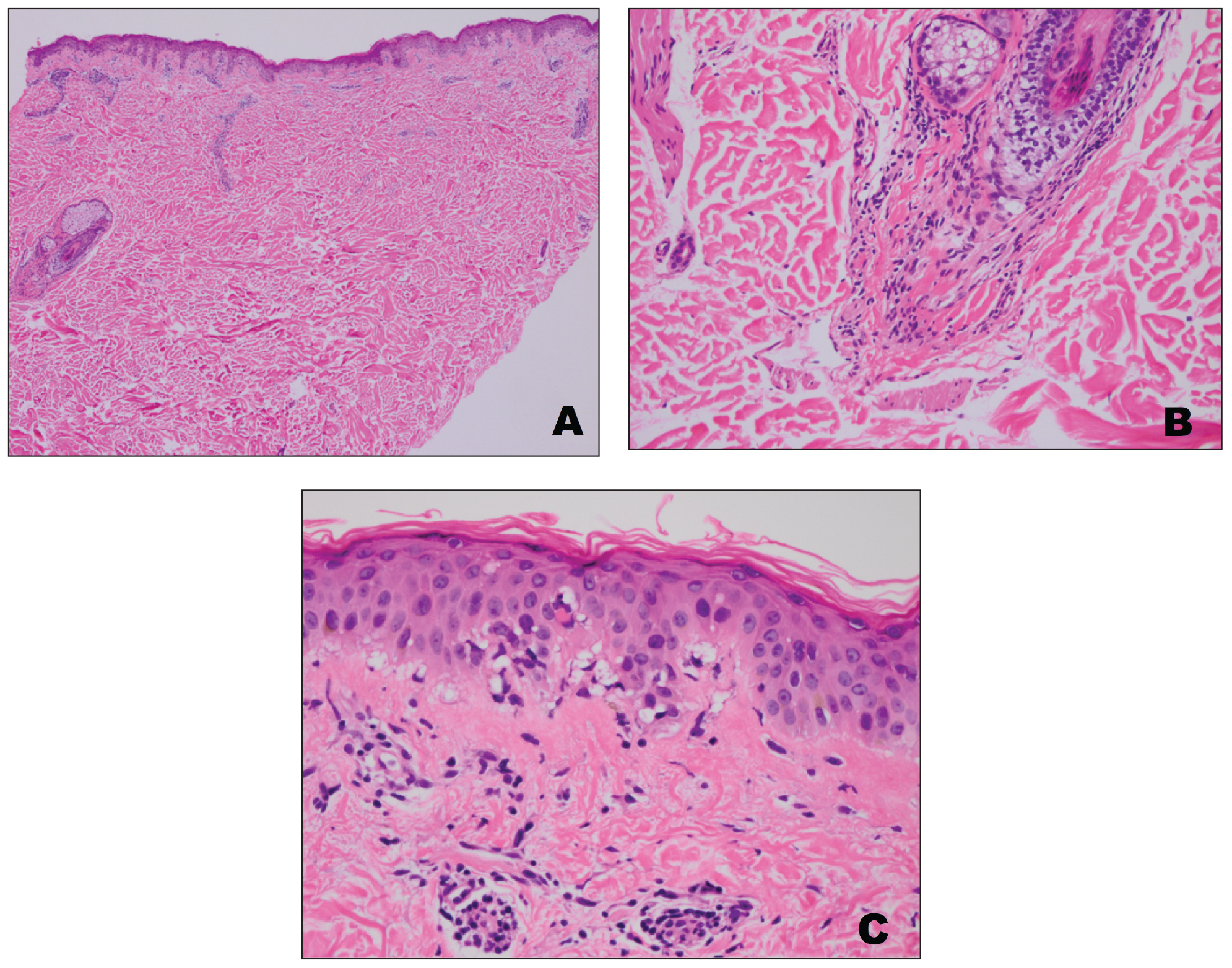
A punch biopsy from an area of flagellate erythema on the back revealed an interface dermatitis with a superficial, perivascular, lymphocyte-predominant inflammatory infiltrate (Figure 3). Alcian blue and colloidal iron stains revealed a marked increase in papillary dermal mucin. With the characteristic changes on skin biopsy and the classic skin findings present in our patient, we felt confident diagnosing her with DM. At the time of diagnosis, the patient also was found to have metastatic small cell lung cancer, suggesting a true paraneoplastic relationship.
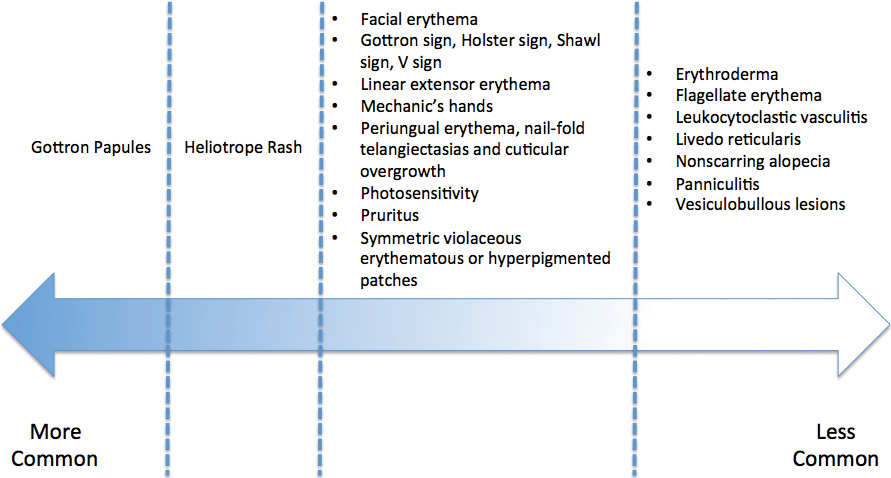
The association of DM and amyopathic DM with internal malignancy is well known. Bohan and Peter1 noted an overall figure ranging from 15% to 34% with an increased frequency in patients with skin and muscle involvement.1 Hill et al5 examined this link in a population-based study that identified corresponding malignancies. Specifically, they noted cancers to arise most frequently in the airway (eg, lung, trachea, bronchus), ovaries, breasts, colorectal region, and stomach.5 There also has been work performed to identify if certain dermatologic findings may be associated with a higher risk of malignancy.6,7 A meta-analysis by Wang et al6 showed that Gottron sign did not have an association with cancer, but findings of cutaneous necrosis did have an association. It is unknown if the specific cutaneous findings in our patient, including the predominantly unilateral papules on the knee, may have been a clue to the underlying malignancy.
In summary, we believe that our patient presented with the classic manifestations of DM in addition to the curious cutaneous sign of predominantly unilateral, confluent, erythematous papules on the knee, a clinical finding that may aid in the diagnosis of DM and also may alert the clinician to a possible underlying malignancy.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Santmyire-Rosenberger B, Dugan EM. Skin involvement in dermatomyositis. Curr Opin Rheumatol. 2003;15:714-722.
- Callen JP. Dermatomyositis. Lancet. 2000;355:53-57.
- Lister RK, Cooper ES, Paige DG. Papules and pustules of the elbows and knees: an uncommon clinical sign of dermatomyositis in oriental children. Pediatr Dermatol. 2000;17:37-40.
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100.
- Wang J, Guo G, Chen G, et al. Meta‐analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847.
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case–control study. Br J Dermatol. 2001;144:825-831.
To the Editor:
We report an interesting clinical case of dermatomyositis (DM) that presented with an associated malignancy (small cell lung cancer). This patient also had an unusual clinical finding of predominantly unilateral, confluent, erythematous papules on the knee, a cutaneous sign that is seldom described in the DM literature. This case serves to reinforce the classic findings and associations of DM, in addition to the uncommon manifestation of predominantly unilateral papules on the knee.
A 68-year-old woman presented with several cutaneous manifestations including the classic findings of photo distributed erythema on the arms and face, a heliotrope rash, Gottron papules, and confluent pink papules on the left knee (Figure 1). The patient also had one of the more rare manifestations of DM, flagellate erythema on the back (Figure 2). She had a history of breast cancer and was found to have metastatic small cell lung cancer at the time of the DM diagnosis.


A punch biopsy from an area of flagellate erythema on the back revealed an interface dermatitis with a superficial, perivascular, lymphocyte-predominant inflammatory infiltrate (Figure 3). Alcian blue and colloidal iron stains revealed a marked increase in papillary dermal mucin. With the characteristic changes on skin biopsy and the classic skin findings present in our patient, we felt confident diagnosing her with DM. At the time of diagnosis, the patient also was found to have metastatic small cell lung cancer, suggesting a true paraneoplastic relationship.


The association of DM and amyopathic DM with internal malignancy is well known. Bohan and Peter1 noted an overall figure ranging from 15% to 34% with an increased frequency in patients with skin and muscle involvement.1 Hill et al5 examined this link in a population-based study that identified corresponding malignancies. Specifically, they noted cancers to arise most frequently in the airway (eg, lung, trachea, bronchus), ovaries, breasts, colorectal region, and stomach.5 There also has been work performed to identify if certain dermatologic findings may be associated with a higher risk of malignancy.6,7 A meta-analysis by Wang et al6 showed that Gottron sign did not have an association with cancer, but findings of cutaneous necrosis did have an association. It is unknown if the specific cutaneous findings in our patient, including the predominantly unilateral papules on the knee, may have been a clue to the underlying malignancy.
In summary, we believe that our patient presented with the classic manifestations of DM in addition to the curious cutaneous sign of predominantly unilateral, confluent, erythematous papules on the knee, a clinical finding that may aid in the diagnosis of DM and also may alert the clinician to a possible underlying malignancy.
To the Editor:
We report an interesting clinical case of dermatomyositis (DM) that presented with an associated malignancy (small cell lung cancer). This patient also had an unusual clinical finding of predominantly unilateral, confluent, erythematous papules on the knee, a cutaneous sign that is seldom described in the DM literature. This case serves to reinforce the classic findings and associations of DM, in addition to the uncommon manifestation of predominantly unilateral papules on the knee.
A 68-year-old woman presented with several cutaneous manifestations including the classic findings of photo distributed erythema on the arms and face, a heliotrope rash, Gottron papules, and confluent pink papules on the left knee (Figure 1). The patient also had one of the more rare manifestations of DM, flagellate erythema on the back (Figure 2). She had a history of breast cancer and was found to have metastatic small cell lung cancer at the time of the DM diagnosis.


A punch biopsy from an area of flagellate erythema on the back revealed an interface dermatitis with a superficial, perivascular, lymphocyte-predominant inflammatory infiltrate (Figure 3). Alcian blue and colloidal iron stains revealed a marked increase in papillary dermal mucin. With the characteristic changes on skin biopsy and the classic skin findings present in our patient, we felt confident diagnosing her with DM. At the time of diagnosis, the patient also was found to have metastatic small cell lung cancer, suggesting a true paraneoplastic relationship.


The association of DM and amyopathic DM with internal malignancy is well known. Bohan and Peter1 noted an overall figure ranging from 15% to 34% with an increased frequency in patients with skin and muscle involvement.1 Hill et al5 examined this link in a population-based study that identified corresponding malignancies. Specifically, they noted cancers to arise most frequently in the airway (eg, lung, trachea, bronchus), ovaries, breasts, colorectal region, and stomach.5 There also has been work performed to identify if certain dermatologic findings may be associated with a higher risk of malignancy.6,7 A meta-analysis by Wang et al6 showed that Gottron sign did not have an association with cancer, but findings of cutaneous necrosis did have an association. It is unknown if the specific cutaneous findings in our patient, including the predominantly unilateral papules on the knee, may have been a clue to the underlying malignancy.
In summary, we believe that our patient presented with the classic manifestations of DM in addition to the curious cutaneous sign of predominantly unilateral, confluent, erythematous papules on the knee, a clinical finding that may aid in the diagnosis of DM and also may alert the clinician to a possible underlying malignancy.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Santmyire-Rosenberger B, Dugan EM. Skin involvement in dermatomyositis. Curr Opin Rheumatol. 2003;15:714-722.
- Callen JP. Dermatomyositis. Lancet. 2000;355:53-57.
- Lister RK, Cooper ES, Paige DG. Papules and pustules of the elbows and knees: an uncommon clinical sign of dermatomyositis in oriental children. Pediatr Dermatol. 2000;17:37-40.
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100.
- Wang J, Guo G, Chen G, et al. Meta‐analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847.
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case–control study. Br J Dermatol. 2001;144:825-831.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Santmyire-Rosenberger B, Dugan EM. Skin involvement in dermatomyositis. Curr Opin Rheumatol. 2003;15:714-722.
- Callen JP. Dermatomyositis. Lancet. 2000;355:53-57.
- Lister RK, Cooper ES, Paige DG. Papules and pustules of the elbows and knees: an uncommon clinical sign of dermatomyositis in oriental children. Pediatr Dermatol. 2000;17:37-40.
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100.
- Wang J, Guo G, Chen G, et al. Meta‐analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847.
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case–control study. Br J Dermatol. 2001;144:825-831.
Practice Points
- Dermatomyositis has myriad cutaneous features including the shawl sign, the heliotrope sign, and Gottron papules.
- Less commonly, patients can present with the Holster sign (poikiloderma of the lateral thighs).
- Even less commonly, as in this report, patients can present with a psoriasiform papular eruption on the knees or with flagellate erythema on the back.
Revised U.S. A fib guidelines revamp anticoagulation
The first update to U.S. medical-society guidelines for managing atrial fibrillation since 2014 raised the threshold for starting anticoagulant therapy in women, pegged the direct-acting oral anticoagulants (DOACs) as preferred over warfarin, and introduced for the first time weight loss as an important intervention tool for treating patients with an atrial arrhythmia.

On January 28, the American College of Cardiology, American Heart Association, and Heart Rhythm Society posted online a 2019 focused update (Circulation. 2019 Jan 28. doi: 10.1161/CIR.0000000000000665) to the 2014 atrial fibrillation (AF) management guidelines that the groups had previously published (J Am Coll Cardiol. 2014 Dec 2;64[21]:2246-80).
Perhaps the two most important changes, as well as the two that lead off the new document, were a pair of class I recommendations on using oral anticoagulation in AF patients.
This brought U.S. guidelines in line with European guidelines, set by the European Society of Cardiology in 2016 (Eur Heart J. 2016 Oct 7;37[38]:2893-962). It will now also mean that, because of the way the CHA2DS2-VASc score is calculated, women with AF who are at least 65 years old will no longer automatically get flagged as needing oral anticoagulant therapy.
“This is a really important shift. It’s recognition that female sex is not as important a risk factor [for AF-associated stroke] as once was thought,” commented Hugh Calkins, MD, professor of medicine at Johns Hopkins Medicine in Baltimore and a member of the panel that wrote the update. “This will change the number of women with AF who go on anticoagulation,” predicted Dr. Calkins, who directs the cardiac arrhythmia service at his center. “We have been struggling with the notion that all women 65 or older with AF had to be on an anticoagulant. Now a clinician has more leeway. In general, patients with AF remain underanticoagulated, but this clarifies practice and brings us in line with the European guidelines.”
The second important change to the anticoagulation recommendations was to specify the DOACs as recommended over warfarin in AF patients eligible for oral anticoagulation and without moderate to severe mitral stenosis or a mechanical heart valve, which also matches the 2016 European guidelines and updates the prior, 2014, U.S. guidelines, which didn’t even mention DOACs.
Prescribing a DOAC preferentially to AF patients has already become routine among electrophysiologists, but possibly not as routine among primary care physicians, so this change has the potential to shift practice, said Dr. Calkins. But the higher price for DOACs, compared with warfarin, can pose problems. “The cost of DOACs remains an issue that can be a serious limitation to some patients,” said Craig T. January, MD, professor of medicine at the University of Wisconsin in Madison and chair of the guideline-writing panel. He also bemoaned the absence of head-to-head comparisons of individual DOACs that could inform selecting among apixaban, dabigatran, edoxaban, and rivaroxaban.
Another notable change in the 2019 update was inclusion for the first time of weight loss as a recommended intervention, along with other risk factor modification, an addition that Dr. Calkins called “long overdue.”

“This is a new recommendation, and it will potentially be important,” said Dr. January, although the guidelines do not spell out how aggressive clinicians should be about having patients achieve weight loss, how much loss patients should achieve, or how they should do it. “There are a lot of observational data and basic science data suggesting the importance of weight loss. Most electrophysiologists already address weight loss. The problem is how to get patients to do it,” commented Vivek Reddy, MD, professor of medicine and director of cardiac arrhythmia services at Mount Sinai Hospital in New York.
Dr. Reddy expressed surprise over two other features of the updated guidelines. For the first time, the guidelines now address percutaneous left atrial appendage (LAA) occlusion and say: “Percutaneous LAA occlusion may be considered in patients with AF at increased risk of stroke who have contraindications to long-term anticoagulation.” The guidelines’ text acknowledges that this runs counter to the Food and Drug Administration labeling for the Watchman LAA occlusion device, which restricts the device to patients “deemed suitable for long-term warfarin (mirroring the inclusion criteria for enrollment in the clinical trials) but had an appropriate rationale to seek a nonpharmacological alternative to warfarin.”
“We do not take a position on the FDA’s” actions, Dr. January said in an interview.
“The ACC, AHA, and HRS guidelines should reflect what the FDA decided,” Dr. Reddy said in an interview. “I’m a little surprised the guidelines said that anticoagulation had to be contraindicated.
The 2019 update also added a class IIb, “may be reasonable” recommendation for catheter ablation of AF in patients with heart failure with reduced ejection fraction.
“I think a IIb recommendation is unfair; I think it should be a IIa recommendation because there have been positive results from two large, randomized, multicenter trials – CASTLE-AF [Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF; N Engl J Med. 2018 Feb 1;378(5):417-27] and AATAC [Ablation vs Amiodarone for Treatment of AFib in Patients With CHF and an ICD; Circulation. 2016 Apr 26;133(7):1637-44], as well as positive results from several smaller randomized studies,” Dr. Reddy said. “I’m really surprised” that the recommendation was not stronger.
Dr. Calkins has been a consultant to Abbott, Altathera, AtriCare, Boehringer-Ingelheim, King, Medtronic, and St. Jude and has received research funding from Boehringer-Ingelheim, Boston Scientific, and St. Jude. Dr. January had no disclosures. Dr. Reddy has been a consultant to, received research funding from, or has an equity interest in more than three dozen companies.
The first update to U.S. medical-society guidelines for managing atrial fibrillation since 2014 raised the threshold for starting anticoagulant therapy in women, pegged the direct-acting oral anticoagulants (DOACs) as preferred over warfarin, and introduced for the first time weight loss as an important intervention tool for treating patients with an atrial arrhythmia.

On January 28, the American College of Cardiology, American Heart Association, and Heart Rhythm Society posted online a 2019 focused update (Circulation. 2019 Jan 28. doi: 10.1161/CIR.0000000000000665) to the 2014 atrial fibrillation (AF) management guidelines that the groups had previously published (J Am Coll Cardiol. 2014 Dec 2;64[21]:2246-80).
Perhaps the two most important changes, as well as the two that lead off the new document, were a pair of class I recommendations on using oral anticoagulation in AF patients.
This brought U.S. guidelines in line with European guidelines, set by the European Society of Cardiology in 2016 (Eur Heart J. 2016 Oct 7;37[38]:2893-962). It will now also mean that, because of the way the CHA2DS2-VASc score is calculated, women with AF who are at least 65 years old will no longer automatically get flagged as needing oral anticoagulant therapy.
“This is a really important shift. It’s recognition that female sex is not as important a risk factor [for AF-associated stroke] as once was thought,” commented Hugh Calkins, MD, professor of medicine at Johns Hopkins Medicine in Baltimore and a member of the panel that wrote the update. “This will change the number of women with AF who go on anticoagulation,” predicted Dr. Calkins, who directs the cardiac arrhythmia service at his center. “We have been struggling with the notion that all women 65 or older with AF had to be on an anticoagulant. Now a clinician has more leeway. In general, patients with AF remain underanticoagulated, but this clarifies practice and brings us in line with the European guidelines.”
The second important change to the anticoagulation recommendations was to specify the DOACs as recommended over warfarin in AF patients eligible for oral anticoagulation and without moderate to severe mitral stenosis or a mechanical heart valve, which also matches the 2016 European guidelines and updates the prior, 2014, U.S. guidelines, which didn’t even mention DOACs.
Prescribing a DOAC preferentially to AF patients has already become routine among electrophysiologists, but possibly not as routine among primary care physicians, so this change has the potential to shift practice, said Dr. Calkins. But the higher price for DOACs, compared with warfarin, can pose problems. “The cost of DOACs remains an issue that can be a serious limitation to some patients,” said Craig T. January, MD, professor of medicine at the University of Wisconsin in Madison and chair of the guideline-writing panel. He also bemoaned the absence of head-to-head comparisons of individual DOACs that could inform selecting among apixaban, dabigatran, edoxaban, and rivaroxaban.
Another notable change in the 2019 update was inclusion for the first time of weight loss as a recommended intervention, along with other risk factor modification, an addition that Dr. Calkins called “long overdue.”

“This is a new recommendation, and it will potentially be important,” said Dr. January, although the guidelines do not spell out how aggressive clinicians should be about having patients achieve weight loss, how much loss patients should achieve, or how they should do it. “There are a lot of observational data and basic science data suggesting the importance of weight loss. Most electrophysiologists already address weight loss. The problem is how to get patients to do it,” commented Vivek Reddy, MD, professor of medicine and director of cardiac arrhythmia services at Mount Sinai Hospital in New York.
Dr. Reddy expressed surprise over two other features of the updated guidelines. For the first time, the guidelines now address percutaneous left atrial appendage (LAA) occlusion and say: “Percutaneous LAA occlusion may be considered in patients with AF at increased risk of stroke who have contraindications to long-term anticoagulation.” The guidelines’ text acknowledges that this runs counter to the Food and Drug Administration labeling for the Watchman LAA occlusion device, which restricts the device to patients “deemed suitable for long-term warfarin (mirroring the inclusion criteria for enrollment in the clinical trials) but had an appropriate rationale to seek a nonpharmacological alternative to warfarin.”
“We do not take a position on the FDA’s” actions, Dr. January said in an interview.
“The ACC, AHA, and HRS guidelines should reflect what the FDA decided,” Dr. Reddy said in an interview. “I’m a little surprised the guidelines said that anticoagulation had to be contraindicated.
The 2019 update also added a class IIb, “may be reasonable” recommendation for catheter ablation of AF in patients with heart failure with reduced ejection fraction.
“I think a IIb recommendation is unfair; I think it should be a IIa recommendation because there have been positive results from two large, randomized, multicenter trials – CASTLE-AF [Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF; N Engl J Med. 2018 Feb 1;378(5):417-27] and AATAC [Ablation vs Amiodarone for Treatment of AFib in Patients With CHF and an ICD; Circulation. 2016 Apr 26;133(7):1637-44], as well as positive results from several smaller randomized studies,” Dr. Reddy said. “I’m really surprised” that the recommendation was not stronger.
Dr. Calkins has been a consultant to Abbott, Altathera, AtriCare, Boehringer-Ingelheim, King, Medtronic, and St. Jude and has received research funding from Boehringer-Ingelheim, Boston Scientific, and St. Jude. Dr. January had no disclosures. Dr. Reddy has been a consultant to, received research funding from, or has an equity interest in more than three dozen companies.
The first update to U.S. medical-society guidelines for managing atrial fibrillation since 2014 raised the threshold for starting anticoagulant therapy in women, pegged the direct-acting oral anticoagulants (DOACs) as preferred over warfarin, and introduced for the first time weight loss as an important intervention tool for treating patients with an atrial arrhythmia.

On January 28, the American College of Cardiology, American Heart Association, and Heart Rhythm Society posted online a 2019 focused update (Circulation. 2019 Jan 28. doi: 10.1161/CIR.0000000000000665) to the 2014 atrial fibrillation (AF) management guidelines that the groups had previously published (J Am Coll Cardiol. 2014 Dec 2;64[21]:2246-80).
Perhaps the two most important changes, as well as the two that lead off the new document, were a pair of class I recommendations on using oral anticoagulation in AF patients.
This brought U.S. guidelines in line with European guidelines, set by the European Society of Cardiology in 2016 (Eur Heart J. 2016 Oct 7;37[38]:2893-962). It will now also mean that, because of the way the CHA2DS2-VASc score is calculated, women with AF who are at least 65 years old will no longer automatically get flagged as needing oral anticoagulant therapy.
“This is a really important shift. It’s recognition that female sex is not as important a risk factor [for AF-associated stroke] as once was thought,” commented Hugh Calkins, MD, professor of medicine at Johns Hopkins Medicine in Baltimore and a member of the panel that wrote the update. “This will change the number of women with AF who go on anticoagulation,” predicted Dr. Calkins, who directs the cardiac arrhythmia service at his center. “We have been struggling with the notion that all women 65 or older with AF had to be on an anticoagulant. Now a clinician has more leeway. In general, patients with AF remain underanticoagulated, but this clarifies practice and brings us in line with the European guidelines.”
The second important change to the anticoagulation recommendations was to specify the DOACs as recommended over warfarin in AF patients eligible for oral anticoagulation and without moderate to severe mitral stenosis or a mechanical heart valve, which also matches the 2016 European guidelines and updates the prior, 2014, U.S. guidelines, which didn’t even mention DOACs.
Prescribing a DOAC preferentially to AF patients has already become routine among electrophysiologists, but possibly not as routine among primary care physicians, so this change has the potential to shift practice, said Dr. Calkins. But the higher price for DOACs, compared with warfarin, can pose problems. “The cost of DOACs remains an issue that can be a serious limitation to some patients,” said Craig T. January, MD, professor of medicine at the University of Wisconsin in Madison and chair of the guideline-writing panel. He also bemoaned the absence of head-to-head comparisons of individual DOACs that could inform selecting among apixaban, dabigatran, edoxaban, and rivaroxaban.
Another notable change in the 2019 update was inclusion for the first time of weight loss as a recommended intervention, along with other risk factor modification, an addition that Dr. Calkins called “long overdue.”

“This is a new recommendation, and it will potentially be important,” said Dr. January, although the guidelines do not spell out how aggressive clinicians should be about having patients achieve weight loss, how much loss patients should achieve, or how they should do it. “There are a lot of observational data and basic science data suggesting the importance of weight loss. Most electrophysiologists already address weight loss. The problem is how to get patients to do it,” commented Vivek Reddy, MD, professor of medicine and director of cardiac arrhythmia services at Mount Sinai Hospital in New York.
Dr. Reddy expressed surprise over two other features of the updated guidelines. For the first time, the guidelines now address percutaneous left atrial appendage (LAA) occlusion and say: “Percutaneous LAA occlusion may be considered in patients with AF at increased risk of stroke who have contraindications to long-term anticoagulation.” The guidelines’ text acknowledges that this runs counter to the Food and Drug Administration labeling for the Watchman LAA occlusion device, which restricts the device to patients “deemed suitable for long-term warfarin (mirroring the inclusion criteria for enrollment in the clinical trials) but had an appropriate rationale to seek a nonpharmacological alternative to warfarin.”
“We do not take a position on the FDA’s” actions, Dr. January said in an interview.
“The ACC, AHA, and HRS guidelines should reflect what the FDA decided,” Dr. Reddy said in an interview. “I’m a little surprised the guidelines said that anticoagulation had to be contraindicated.
The 2019 update also added a class IIb, “may be reasonable” recommendation for catheter ablation of AF in patients with heart failure with reduced ejection fraction.
“I think a IIb recommendation is unfair; I think it should be a IIa recommendation because there have been positive results from two large, randomized, multicenter trials – CASTLE-AF [Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF; N Engl J Med. 2018 Feb 1;378(5):417-27] and AATAC [Ablation vs Amiodarone for Treatment of AFib in Patients With CHF and an ICD; Circulation. 2016 Apr 26;133(7):1637-44], as well as positive results from several smaller randomized studies,” Dr. Reddy said. “I’m really surprised” that the recommendation was not stronger.
Dr. Calkins has been a consultant to Abbott, Altathera, AtriCare, Boehringer-Ingelheim, King, Medtronic, and St. Jude and has received research funding from Boehringer-Ingelheim, Boston Scientific, and St. Jude. Dr. January had no disclosures. Dr. Reddy has been a consultant to, received research funding from, or has an equity interest in more than three dozen companies.
Pediatric hospitalist and researcher: Dr. Samir Shah
Stoking collaboration between adult and pediatric clinicians
Samir S. Shah, MD, MSCE, director of the division of hospital medicine at Cincinnati Children’s Hospital Medical Center, believes that pediatric and adult hospitalists have much to learn from each other. And he aims to promote that mutual education in his new role as editor in chief of the Journal of Hospital Medicine.
Dr. Shah is the first pediatric hospitalist to hold this position for JHM, the official journal of the Society of Hospital Medicine. He says his new position, which became effective Jan. 1, is primed for fostering interaction between pediatric and adult hospitalists. “Pediatric hospital medicine is such a vibrant community of its own. There are many opportunities for partnership and collaboration between adult and pediatric hospitalists,” he said.
The field of pediatric hospital medicine has started down the path toward becoming recognized as a board-certified subspecialty.1 “That will place a greater emphasis on our role in fellowship training, which is important to ensure that pediatric hospitalists have a clearly defined skill set,” Dr. Shah said. “So much of what we learn in medical school is oriented to the medical care of adults. If you go into pediatrics, you’ve already had a fair amount of grounding in the healthy physiology and common diseases of adults. Pediatric hospital medicine fellowships offer an opportunity to refine clinical skill sets, as well as develop new skills in domains such as research and leadership.”
An emphasis on diversity
Although he has praised the innovative work of his predecessors, Mark Williams, MD, MHM, and Andrew Auerbach, MD, MPH, MHM, in shepherding the journal to its current strong position, Dr. Shah brings ideas for new features and directions.
“We as a field really benefit from a diversity of skill sets and perspectives. I’m excited to create processes to ensure equity and diversity in everything we do, starting with adding more women and more pediatric hospitalists to the journal’s leadership team, as well as purposefully developing a diverse leadership pipeline for the journal and for the field,” he said.
“We are intentionally reaching out to pediatricians to emphasize the extent to which JHM is invested in their field. For example, we have increased by seven the number of pediatricians as part of the JHM leadership team.” But pediatric hospitalists have always seen JHM as a home for their work, and Dr. Shah himself has published a couple dozen research papers in the journal. “It has always felt to me like a welcoming place,” he said.
“The great thing for me is that I’m not doing this alone. We have a marvelous crew of senior deputy editors, deputy editors, associate editors, and advisors. The opportunity I have is to leverage the phenomenal expertise and enthusiasm of this exceptional team.”
The journal under Dr. Auerbach’s lead created an editorial fellowship program offering opportunities for 1-year mentored exposure to the publication of academic scholarship and to different aspects of how a medical journal works. “We’re excited to continue investing in this program and included an editorial about it and an application form in the January 2019 issue of the Journal,” Dr. Shah said. He encourages editorial fellowship applications from physicians who historically have been underrepresented in academic medicine leadership.
“We’re also creating a column on leadership and professional development so that leaders in different fields can share their perspective and wisdom with our readers. We’ll be presenting a new, shorter review format; distilling clinical practice guidelines; and working on redesigning the journal’s web presence. We believe that our readers interact with the journal differently than they did five years ago, and increasingly are leveraging social media,” he said.
“I’m eager to broaden the scope of the journal. In the past, we focused on quality, value in health care and transitions of care in and out of the hospital, which are important topics. But I’m also excited about the adoption of new technologies, how to evaluate them and incorporate them into medical practice – things like Apple Watch for measuring heart rhythm,” Dr. Shah.
He wants to explore other technology-related topics like alarm fatigue and the use of monitors. Another big subject is the management of health of populations under new, emerging, risk-based payment models, with their pressures on health systems to take greater responsibility for risk. JHM is a medical journal and an official society journal, Dr. Shah said. “But our readership and submitters are not limited to hospitalists. As editor in chief, I’m here to make sure the journal is relevant to our members and to our other constituencies.”
Dr. Shah joined JHM’s editorial leadership team in 2009, then he became its deputy editor in 2012 and its senior deputy editor in 2015. A founding associate editor of the Journal of the Pediatric Infectious Diseases Society, he has also served on the editorial board of JAMA Pediatrics. He is editor or coeditor of 12 books in the fields of pediatrics and infectious diseases, including coauthoring “The Philadelphia Guide: Inpatient Pediatrics for McGraw-Hill Education” while still a fellow in academic general pediatrics and pediatric infectious diseases at Children’s Hospital of Philadelphia (CHOP) and, more recently, “Pediatric Infectious Diseases: Essentials for Practice,” a textbook for the pediatric generalist.
Broad scope of activities
Dr. Shah started practicing pediatric hospital medicine in 2001 during his fellowship training. He joined the faculty at CHOP and the University of Pennsylvania, also in Philadelphia, in 2005. In 2011 he arrived at Cincinnati Children’s Hospital, a facility with more than 600 beds that’s affiliated with the University of Cincinnati, where he is professor in the department of pediatrics and holds the James M. Ewell Endowed Chair, to lead a newly created division of hospital medicine. That division now includes more than 55 physician faculty members, 10 nurse practitioners, and nine 3-year fellows.
Collectively the staff represent a broad scope of clinical and research activities along with consulting and surgical comanagement roles and a unique service staffed by med/peds hospitalists for adult patients who have been followed at the hospital since they were children. “Years ago, those patients would not have survived beyond childhood, but with medical advances, they have. Although they continue to benefit from pediatric expertise, these adults also require internal medicine expertise for their adult health needs,” he explained. Examples include patients with neurologic impairments, dependence on medical technology, or congenital heart defects.
Dr. Shah’s own schedule is 28% clinical. He also serves as the hospital’s chief metrics officer, and his research interests include serious infectious diseases, such as pneumonia and meningitis. He is studying the comparative effectiveness of different antibiotic treatments for community-acquired pneumonia and how to improve outcomes for hospital-acquired pneumonia.
Dr. Shah has tried to be deliberate in leading efforts to grow researchers within the field, both nationally and locally. He serves as the chair of the National Childhood Pneumonia Guidelines Committee of the Infectious Diseases Society of America and the Pediatric Infectious Diseases Society, and he also is vice chair of the Pediatric Research in Inpatient Settings (PRIS) Network, which facilitates multicenter cost-effectiveness studies among its 120 hospital members. For example, a series of studies funded by the Patient- Centered Outcomes Research Institute has demonstrated the comparable effectiveness of oral and intravenous antibiotics for osteomyelitis and complicated pneumonia.
Sustainable positions
When he was asked whether he felt pediatric hospitalists face particular challenges in trying to take their place in the burgeoning field of hospital medicine, Dr. Shah said he and his colleagues don’t really think of it in those terms. “Hospital medicine is such a dynamic field. For example, pediatric hospital medicine has charted its own course by pursuing subspecialty certification and fellowship training. Yet support from the field broadly has been quite strong, and SHM has embraced pediatricians, who serve on its board of directors and on numerous committees.”
SHM’s commitment to supporting pediatric hospital medicine practice and research includes its cosponsorship, with the Academic Pediatric Association and the American Academy of Pediatrics, an annual pediatric hospital medicine educational and research conference, which will next be held July 25-28, 2019, in Seattle. “In my recent meetings with society leaders I have seen exceptional enthusiasm for increasing the presence of pediatric hospitalists in the society’s work. Many pediatric hospitalists already attend SHM’s annual meeting and submit their research, but we all recognize that a strong pediatric presence is important for the society.”
Dr. Shah credits Cincinnati Children’s Hospital for supporting a sustainable work schedule for its hospitalists and for a team-oriented culture that emphasizes both professional and personal development and encourages a diversity of skill sets and perspectives, skills development, and additional training. “Individuals are recognized for their achievements within and beyond the confines of the hospital. The mentorship structure we set up here is incredible. Each faculty member has a primary mentor, a peer mentor, and access to a career development committee. Additionally, there is broad participation in clinical operations, educational scholarship, research, and quality improvement.”
Dr. Shah’s professional interests in academics, research, and infectious diseases trace back in part to a thesis project he did on neonatal infections while in medical school at Yale University, New Haven, Conn. “I was working with basic sciences in a hematology lab under the direction of the neonatologist Dr. Patrick Gallagher, whose research focused on pediatric blood cell membrane disorders.” Dr. Gallagher, who directs the Yale Center for Blood Disorders, had a keen interest in infections in infants, Dr. Shah recalled.
“He would share with me interesting cases from his practice. What particularly captured my attention was realizing how the research I could do might have a direct impact on patients and families.” Thus inspired to do an additional year of medical school training at Yale before graduating in 1998, Dr. Shah used that year to focus on research, including a placement at the Centers for Disease Control and Prevention to investigate infectious disease outbreaks, which offered real-world mysteries to solve.
“When I was a resident, pediatric hospital medicine had not yet been recognized as a specialty. But during my fellowships, most of my work was focused on the inpatient side of medicine,” he said. That made hospital medicine a natural career path.
Dr. Shah describes himself as a devoted soccer fan with season tickets for himself, his wife, and their three children to the Major League Soccer team FC Cincinnati. He’s also a movie buff and a former avid bicyclist who’s now trying to get back into cycling. He encourages readers of The Hospitalist to contact him with input on any aspect of the Journal of Hospital Medicine. Email him at [email protected] and follow him on Twitter: @samirshahmd.
Reference
1. Barrett DJ et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017 March;139(3):e20161823.
Stoking collaboration between adult and pediatric clinicians
Stoking collaboration between adult and pediatric clinicians
Samir S. Shah, MD, MSCE, director of the division of hospital medicine at Cincinnati Children’s Hospital Medical Center, believes that pediatric and adult hospitalists have much to learn from each other. And he aims to promote that mutual education in his new role as editor in chief of the Journal of Hospital Medicine.
Dr. Shah is the first pediatric hospitalist to hold this position for JHM, the official journal of the Society of Hospital Medicine. He says his new position, which became effective Jan. 1, is primed for fostering interaction between pediatric and adult hospitalists. “Pediatric hospital medicine is such a vibrant community of its own. There are many opportunities for partnership and collaboration between adult and pediatric hospitalists,” he said.
The field of pediatric hospital medicine has started down the path toward becoming recognized as a board-certified subspecialty.1 “That will place a greater emphasis on our role in fellowship training, which is important to ensure that pediatric hospitalists have a clearly defined skill set,” Dr. Shah said. “So much of what we learn in medical school is oriented to the medical care of adults. If you go into pediatrics, you’ve already had a fair amount of grounding in the healthy physiology and common diseases of adults. Pediatric hospital medicine fellowships offer an opportunity to refine clinical skill sets, as well as develop new skills in domains such as research and leadership.”
An emphasis on diversity
Although he has praised the innovative work of his predecessors, Mark Williams, MD, MHM, and Andrew Auerbach, MD, MPH, MHM, in shepherding the journal to its current strong position, Dr. Shah brings ideas for new features and directions.
“We as a field really benefit from a diversity of skill sets and perspectives. I’m excited to create processes to ensure equity and diversity in everything we do, starting with adding more women and more pediatric hospitalists to the journal’s leadership team, as well as purposefully developing a diverse leadership pipeline for the journal and for the field,” he said.
“We are intentionally reaching out to pediatricians to emphasize the extent to which JHM is invested in their field. For example, we have increased by seven the number of pediatricians as part of the JHM leadership team.” But pediatric hospitalists have always seen JHM as a home for their work, and Dr. Shah himself has published a couple dozen research papers in the journal. “It has always felt to me like a welcoming place,” he said.
“The great thing for me is that I’m not doing this alone. We have a marvelous crew of senior deputy editors, deputy editors, associate editors, and advisors. The opportunity I have is to leverage the phenomenal expertise and enthusiasm of this exceptional team.”
The journal under Dr. Auerbach’s lead created an editorial fellowship program offering opportunities for 1-year mentored exposure to the publication of academic scholarship and to different aspects of how a medical journal works. “We’re excited to continue investing in this program and included an editorial about it and an application form in the January 2019 issue of the Journal,” Dr. Shah said. He encourages editorial fellowship applications from physicians who historically have been underrepresented in academic medicine leadership.
“We’re also creating a column on leadership and professional development so that leaders in different fields can share their perspective and wisdom with our readers. We’ll be presenting a new, shorter review format; distilling clinical practice guidelines; and working on redesigning the journal’s web presence. We believe that our readers interact with the journal differently than they did five years ago, and increasingly are leveraging social media,” he said.
“I’m eager to broaden the scope of the journal. In the past, we focused on quality, value in health care and transitions of care in and out of the hospital, which are important topics. But I’m also excited about the adoption of new technologies, how to evaluate them and incorporate them into medical practice – things like Apple Watch for measuring heart rhythm,” Dr. Shah.
He wants to explore other technology-related topics like alarm fatigue and the use of monitors. Another big subject is the management of health of populations under new, emerging, risk-based payment models, with their pressures on health systems to take greater responsibility for risk. JHM is a medical journal and an official society journal, Dr. Shah said. “But our readership and submitters are not limited to hospitalists. As editor in chief, I’m here to make sure the journal is relevant to our members and to our other constituencies.”
Dr. Shah joined JHM’s editorial leadership team in 2009, then he became its deputy editor in 2012 and its senior deputy editor in 2015. A founding associate editor of the Journal of the Pediatric Infectious Diseases Society, he has also served on the editorial board of JAMA Pediatrics. He is editor or coeditor of 12 books in the fields of pediatrics and infectious diseases, including coauthoring “The Philadelphia Guide: Inpatient Pediatrics for McGraw-Hill Education” while still a fellow in academic general pediatrics and pediatric infectious diseases at Children’s Hospital of Philadelphia (CHOP) and, more recently, “Pediatric Infectious Diseases: Essentials for Practice,” a textbook for the pediatric generalist.
Broad scope of activities
Dr. Shah started practicing pediatric hospital medicine in 2001 during his fellowship training. He joined the faculty at CHOP and the University of Pennsylvania, also in Philadelphia, in 2005. In 2011 he arrived at Cincinnati Children’s Hospital, a facility with more than 600 beds that’s affiliated with the University of Cincinnati, where he is professor in the department of pediatrics and holds the James M. Ewell Endowed Chair, to lead a newly created division of hospital medicine. That division now includes more than 55 physician faculty members, 10 nurse practitioners, and nine 3-year fellows.
Collectively the staff represent a broad scope of clinical and research activities along with consulting and surgical comanagement roles and a unique service staffed by med/peds hospitalists for adult patients who have been followed at the hospital since they were children. “Years ago, those patients would not have survived beyond childhood, but with medical advances, they have. Although they continue to benefit from pediatric expertise, these adults also require internal medicine expertise for their adult health needs,” he explained. Examples include patients with neurologic impairments, dependence on medical technology, or congenital heart defects.
Dr. Shah’s own schedule is 28% clinical. He also serves as the hospital’s chief metrics officer, and his research interests include serious infectious diseases, such as pneumonia and meningitis. He is studying the comparative effectiveness of different antibiotic treatments for community-acquired pneumonia and how to improve outcomes for hospital-acquired pneumonia.
Dr. Shah has tried to be deliberate in leading efforts to grow researchers within the field, both nationally and locally. He serves as the chair of the National Childhood Pneumonia Guidelines Committee of the Infectious Diseases Society of America and the Pediatric Infectious Diseases Society, and he also is vice chair of the Pediatric Research in Inpatient Settings (PRIS) Network, which facilitates multicenter cost-effectiveness studies among its 120 hospital members. For example, a series of studies funded by the Patient- Centered Outcomes Research Institute has demonstrated the comparable effectiveness of oral and intravenous antibiotics for osteomyelitis and complicated pneumonia.
Sustainable positions
When he was asked whether he felt pediatric hospitalists face particular challenges in trying to take their place in the burgeoning field of hospital medicine, Dr. Shah said he and his colleagues don’t really think of it in those terms. “Hospital medicine is such a dynamic field. For example, pediatric hospital medicine has charted its own course by pursuing subspecialty certification and fellowship training. Yet support from the field broadly has been quite strong, and SHM has embraced pediatricians, who serve on its board of directors and on numerous committees.”
SHM’s commitment to supporting pediatric hospital medicine practice and research includes its cosponsorship, with the Academic Pediatric Association and the American Academy of Pediatrics, an annual pediatric hospital medicine educational and research conference, which will next be held July 25-28, 2019, in Seattle. “In my recent meetings with society leaders I have seen exceptional enthusiasm for increasing the presence of pediatric hospitalists in the society’s work. Many pediatric hospitalists already attend SHM’s annual meeting and submit their research, but we all recognize that a strong pediatric presence is important for the society.”
Dr. Shah credits Cincinnati Children’s Hospital for supporting a sustainable work schedule for its hospitalists and for a team-oriented culture that emphasizes both professional and personal development and encourages a diversity of skill sets and perspectives, skills development, and additional training. “Individuals are recognized for their achievements within and beyond the confines of the hospital. The mentorship structure we set up here is incredible. Each faculty member has a primary mentor, a peer mentor, and access to a career development committee. Additionally, there is broad participation in clinical operations, educational scholarship, research, and quality improvement.”
Dr. Shah’s professional interests in academics, research, and infectious diseases trace back in part to a thesis project he did on neonatal infections while in medical school at Yale University, New Haven, Conn. “I was working with basic sciences in a hematology lab under the direction of the neonatologist Dr. Patrick Gallagher, whose research focused on pediatric blood cell membrane disorders.” Dr. Gallagher, who directs the Yale Center for Blood Disorders, had a keen interest in infections in infants, Dr. Shah recalled.
“He would share with me interesting cases from his practice. What particularly captured my attention was realizing how the research I could do might have a direct impact on patients and families.” Thus inspired to do an additional year of medical school training at Yale before graduating in 1998, Dr. Shah used that year to focus on research, including a placement at the Centers for Disease Control and Prevention to investigate infectious disease outbreaks, which offered real-world mysteries to solve.
“When I was a resident, pediatric hospital medicine had not yet been recognized as a specialty. But during my fellowships, most of my work was focused on the inpatient side of medicine,” he said. That made hospital medicine a natural career path.
Dr. Shah describes himself as a devoted soccer fan with season tickets for himself, his wife, and their three children to the Major League Soccer team FC Cincinnati. He’s also a movie buff and a former avid bicyclist who’s now trying to get back into cycling. He encourages readers of The Hospitalist to contact him with input on any aspect of the Journal of Hospital Medicine. Email him at [email protected] and follow him on Twitter: @samirshahmd.
Reference
1. Barrett DJ et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017 March;139(3):e20161823.
Samir S. Shah, MD, MSCE, director of the division of hospital medicine at Cincinnati Children’s Hospital Medical Center, believes that pediatric and adult hospitalists have much to learn from each other. And he aims to promote that mutual education in his new role as editor in chief of the Journal of Hospital Medicine.
Dr. Shah is the first pediatric hospitalist to hold this position for JHM, the official journal of the Society of Hospital Medicine. He says his new position, which became effective Jan. 1, is primed for fostering interaction between pediatric and adult hospitalists. “Pediatric hospital medicine is such a vibrant community of its own. There are many opportunities for partnership and collaboration between adult and pediatric hospitalists,” he said.
The field of pediatric hospital medicine has started down the path toward becoming recognized as a board-certified subspecialty.1 “That will place a greater emphasis on our role in fellowship training, which is important to ensure that pediatric hospitalists have a clearly defined skill set,” Dr. Shah said. “So much of what we learn in medical school is oriented to the medical care of adults. If you go into pediatrics, you’ve already had a fair amount of grounding in the healthy physiology and common diseases of adults. Pediatric hospital medicine fellowships offer an opportunity to refine clinical skill sets, as well as develop new skills in domains such as research and leadership.”
An emphasis on diversity
Although he has praised the innovative work of his predecessors, Mark Williams, MD, MHM, and Andrew Auerbach, MD, MPH, MHM, in shepherding the journal to its current strong position, Dr. Shah brings ideas for new features and directions.
“We as a field really benefit from a diversity of skill sets and perspectives. I’m excited to create processes to ensure equity and diversity in everything we do, starting with adding more women and more pediatric hospitalists to the journal’s leadership team, as well as purposefully developing a diverse leadership pipeline for the journal and for the field,” he said.
“We are intentionally reaching out to pediatricians to emphasize the extent to which JHM is invested in their field. For example, we have increased by seven the number of pediatricians as part of the JHM leadership team.” But pediatric hospitalists have always seen JHM as a home for their work, and Dr. Shah himself has published a couple dozen research papers in the journal. “It has always felt to me like a welcoming place,” he said.
“The great thing for me is that I’m not doing this alone. We have a marvelous crew of senior deputy editors, deputy editors, associate editors, and advisors. The opportunity I have is to leverage the phenomenal expertise and enthusiasm of this exceptional team.”
The journal under Dr. Auerbach’s lead created an editorial fellowship program offering opportunities for 1-year mentored exposure to the publication of academic scholarship and to different aspects of how a medical journal works. “We’re excited to continue investing in this program and included an editorial about it and an application form in the January 2019 issue of the Journal,” Dr. Shah said. He encourages editorial fellowship applications from physicians who historically have been underrepresented in academic medicine leadership.
“We’re also creating a column on leadership and professional development so that leaders in different fields can share their perspective and wisdom with our readers. We’ll be presenting a new, shorter review format; distilling clinical practice guidelines; and working on redesigning the journal’s web presence. We believe that our readers interact with the journal differently than they did five years ago, and increasingly are leveraging social media,” he said.
“I’m eager to broaden the scope of the journal. In the past, we focused on quality, value in health care and transitions of care in and out of the hospital, which are important topics. But I’m also excited about the adoption of new technologies, how to evaluate them and incorporate them into medical practice – things like Apple Watch for measuring heart rhythm,” Dr. Shah.
He wants to explore other technology-related topics like alarm fatigue and the use of monitors. Another big subject is the management of health of populations under new, emerging, risk-based payment models, with their pressures on health systems to take greater responsibility for risk. JHM is a medical journal and an official society journal, Dr. Shah said. “But our readership and submitters are not limited to hospitalists. As editor in chief, I’m here to make sure the journal is relevant to our members and to our other constituencies.”
Dr. Shah joined JHM’s editorial leadership team in 2009, then he became its deputy editor in 2012 and its senior deputy editor in 2015. A founding associate editor of the Journal of the Pediatric Infectious Diseases Society, he has also served on the editorial board of JAMA Pediatrics. He is editor or coeditor of 12 books in the fields of pediatrics and infectious diseases, including coauthoring “The Philadelphia Guide: Inpatient Pediatrics for McGraw-Hill Education” while still a fellow in academic general pediatrics and pediatric infectious diseases at Children’s Hospital of Philadelphia (CHOP) and, more recently, “Pediatric Infectious Diseases: Essentials for Practice,” a textbook for the pediatric generalist.
Broad scope of activities
Dr. Shah started practicing pediatric hospital medicine in 2001 during his fellowship training. He joined the faculty at CHOP and the University of Pennsylvania, also in Philadelphia, in 2005. In 2011 he arrived at Cincinnati Children’s Hospital, a facility with more than 600 beds that’s affiliated with the University of Cincinnati, where he is professor in the department of pediatrics and holds the James M. Ewell Endowed Chair, to lead a newly created division of hospital medicine. That division now includes more than 55 physician faculty members, 10 nurse practitioners, and nine 3-year fellows.
Collectively the staff represent a broad scope of clinical and research activities along with consulting and surgical comanagement roles and a unique service staffed by med/peds hospitalists for adult patients who have been followed at the hospital since they were children. “Years ago, those patients would not have survived beyond childhood, but with medical advances, they have. Although they continue to benefit from pediatric expertise, these adults also require internal medicine expertise for their adult health needs,” he explained. Examples include patients with neurologic impairments, dependence on medical technology, or congenital heart defects.
Dr. Shah’s own schedule is 28% clinical. He also serves as the hospital’s chief metrics officer, and his research interests include serious infectious diseases, such as pneumonia and meningitis. He is studying the comparative effectiveness of different antibiotic treatments for community-acquired pneumonia and how to improve outcomes for hospital-acquired pneumonia.
Dr. Shah has tried to be deliberate in leading efforts to grow researchers within the field, both nationally and locally. He serves as the chair of the National Childhood Pneumonia Guidelines Committee of the Infectious Diseases Society of America and the Pediatric Infectious Diseases Society, and he also is vice chair of the Pediatric Research in Inpatient Settings (PRIS) Network, which facilitates multicenter cost-effectiveness studies among its 120 hospital members. For example, a series of studies funded by the Patient- Centered Outcomes Research Institute has demonstrated the comparable effectiveness of oral and intravenous antibiotics for osteomyelitis and complicated pneumonia.
Sustainable positions
When he was asked whether he felt pediatric hospitalists face particular challenges in trying to take their place in the burgeoning field of hospital medicine, Dr. Shah said he and his colleagues don’t really think of it in those terms. “Hospital medicine is such a dynamic field. For example, pediatric hospital medicine has charted its own course by pursuing subspecialty certification and fellowship training. Yet support from the field broadly has been quite strong, and SHM has embraced pediatricians, who serve on its board of directors and on numerous committees.”
SHM’s commitment to supporting pediatric hospital medicine practice and research includes its cosponsorship, with the Academic Pediatric Association and the American Academy of Pediatrics, an annual pediatric hospital medicine educational and research conference, which will next be held July 25-28, 2019, in Seattle. “In my recent meetings with society leaders I have seen exceptional enthusiasm for increasing the presence of pediatric hospitalists in the society’s work. Many pediatric hospitalists already attend SHM’s annual meeting and submit their research, but we all recognize that a strong pediatric presence is important for the society.”
Dr. Shah credits Cincinnati Children’s Hospital for supporting a sustainable work schedule for its hospitalists and for a team-oriented culture that emphasizes both professional and personal development and encourages a diversity of skill sets and perspectives, skills development, and additional training. “Individuals are recognized for their achievements within and beyond the confines of the hospital. The mentorship structure we set up here is incredible. Each faculty member has a primary mentor, a peer mentor, and access to a career development committee. Additionally, there is broad participation in clinical operations, educational scholarship, research, and quality improvement.”
Dr. Shah’s professional interests in academics, research, and infectious diseases trace back in part to a thesis project he did on neonatal infections while in medical school at Yale University, New Haven, Conn. “I was working with basic sciences in a hematology lab under the direction of the neonatologist Dr. Patrick Gallagher, whose research focused on pediatric blood cell membrane disorders.” Dr. Gallagher, who directs the Yale Center for Blood Disorders, had a keen interest in infections in infants, Dr. Shah recalled.
“He would share with me interesting cases from his practice. What particularly captured my attention was realizing how the research I could do might have a direct impact on patients and families.” Thus inspired to do an additional year of medical school training at Yale before graduating in 1998, Dr. Shah used that year to focus on research, including a placement at the Centers for Disease Control and Prevention to investigate infectious disease outbreaks, which offered real-world mysteries to solve.
“When I was a resident, pediatric hospital medicine had not yet been recognized as a specialty. But during my fellowships, most of my work was focused on the inpatient side of medicine,” he said. That made hospital medicine a natural career path.
Dr. Shah describes himself as a devoted soccer fan with season tickets for himself, his wife, and their three children to the Major League Soccer team FC Cincinnati. He’s also a movie buff and a former avid bicyclist who’s now trying to get back into cycling. He encourages readers of The Hospitalist to contact him with input on any aspect of the Journal of Hospital Medicine. Email him at [email protected] and follow him on Twitter: @samirshahmd.
Reference
1. Barrett DJ et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017 March;139(3):e20161823.
Stopping TNF inhibitors before 20 weeks’ gestation not linked to worsening RA, JIA
In pregnant women with arthritis, discontinuing tumor necrosis factor inhibitors prior to gestational week 20 seems feasible without an increased risk of disease worsening, particularly in those with well-controlled disease, according to authors of a recent analysis of a prospective cohort study.
Stopping tumor necrosis factor inhibitor (TNFi) treatment at that point in the second trimester was not linked to any clinically important worsening of patient-reported outcomes later in pregnancy for women with rheumatoid arthritis (RA) or juvenile idiopathic arthritis (JIA), the researchers said.
However, continuing a TNFi past gestational week 20 may also be warranted for some patients, according to the researchers, led by Frauke Förger, MD, of the University of Bern (Switzerland).
“In case of active disease, the continuation of TNF inhibitors beyond gestational week 20 seems reasonable from the standpoint of improved disease activity in the third trimester, which may in turn lead to improved pregnancy outcomes,” Dr. Förger and her coinvestigators wrote in Arthritis & Rheumatology.
These findings stand in contrast to those of another recent study, in which stopping a TNF inhibitor after a positive pregnancy test was linked to disease flares in women with RA, according to the authors.
“The timing of drug discontinuation during pregnancy may be of importance,” they said in their report.
Beyond these studies, there are very limited data on the effects of discontinuing TNF inhibitors in pregnant women with rheumatoid arthritis, and “a lack of any data” in pregnant women with JIA, they said.
The current investigation by Dr. Förger and her colleagues included 490 pregnant women in the United States or Canada who were enrolled in the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project, a prospective cohort study. Of those women, 397 had RA and 93 had JIA.
About one-quarter of the women (122, or 24.9%) discontinued TNF inhibitor therapy prior to gestational week 20, while 41% continued on TNF inhibitors beyond that point, and 34.1% did not use a TNF inhibitor in pregnancy.
For those women who discontinued TNF inhibitors before gestational week 20, scores on the Patient Activity Scale (PAS) were stable over time, Dr. Förger and her colleagues reported.
Women who continued TNF inhibitor treatment past gestational week 20 had improved PAS scores in the third trimester, according to results of a univariate analysis (P = .02). However, the improvement appeared to be attenuated after adjustment for factors including race, smoking, use of prednisone or disease-modifying antirheumatic drugs, and gestational age, the investigators said.
They were unable to analyze the effects of ongoing TNFi treatment or discontinuation on patients with JIA separately because of the limited number of such patients in each group.
Another limitation of the study is that a high proportion of women – nearly three-quarters – had low disease activity at the start of pregnancy, according to the investigators, who said that group of women might expect some degree of improvement in the third trimester with or without TNF inhibitor discontinuation.
“In this context, the ameliorating effect of pregnancy on RA and JIA, which is most pronounced in the third trimester, may play a role,” they explained.
A certain proportion of women choose to discontinue certain arthritis treatments during pregnancy because of concerns that the medication may lead to fetal harm, but that may be changing, the investigators noted in their report.
“In recent years, more patients requiring treatment have been continuing on effective TNF inhibitors beyond conception as the available data on the safety of TNF inhibitors during pregnancy has increased,” they wrote.
Dr. Förger and her coauthors reported no financial disclosures or conflicts of interest. Financial support for the OTIS Collaborative Research Group comes from industry sources including AbbVie, Bristol-Myers Squibb, Celgene, Hoffman La Roche-Genentech, Janssen, Pfizer, Regeneron, Sandoz, and UCB, among others.
SOURCE: Förger F et al. Arthritis Rheumatol. 2019 Jan 21. doi: 10.1002/art.40821
In pregnant women with arthritis, discontinuing tumor necrosis factor inhibitors prior to gestational week 20 seems feasible without an increased risk of disease worsening, particularly in those with well-controlled disease, according to authors of a recent analysis of a prospective cohort study.
Stopping tumor necrosis factor inhibitor (TNFi) treatment at that point in the second trimester was not linked to any clinically important worsening of patient-reported outcomes later in pregnancy for women with rheumatoid arthritis (RA) or juvenile idiopathic arthritis (JIA), the researchers said.
However, continuing a TNFi past gestational week 20 may also be warranted for some patients, according to the researchers, led by Frauke Förger, MD, of the University of Bern (Switzerland).
“In case of active disease, the continuation of TNF inhibitors beyond gestational week 20 seems reasonable from the standpoint of improved disease activity in the third trimester, which may in turn lead to improved pregnancy outcomes,” Dr. Förger and her coinvestigators wrote in Arthritis & Rheumatology.
These findings stand in contrast to those of another recent study, in which stopping a TNF inhibitor after a positive pregnancy test was linked to disease flares in women with RA, according to the authors.
“The timing of drug discontinuation during pregnancy may be of importance,” they said in their report.
Beyond these studies, there are very limited data on the effects of discontinuing TNF inhibitors in pregnant women with rheumatoid arthritis, and “a lack of any data” in pregnant women with JIA, they said.
The current investigation by Dr. Förger and her colleagues included 490 pregnant women in the United States or Canada who were enrolled in the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project, a prospective cohort study. Of those women, 397 had RA and 93 had JIA.
About one-quarter of the women (122, or 24.9%) discontinued TNF inhibitor therapy prior to gestational week 20, while 41% continued on TNF inhibitors beyond that point, and 34.1% did not use a TNF inhibitor in pregnancy.
For those women who discontinued TNF inhibitors before gestational week 20, scores on the Patient Activity Scale (PAS) were stable over time, Dr. Förger and her colleagues reported.
Women who continued TNF inhibitor treatment past gestational week 20 had improved PAS scores in the third trimester, according to results of a univariate analysis (P = .02). However, the improvement appeared to be attenuated after adjustment for factors including race, smoking, use of prednisone or disease-modifying antirheumatic drugs, and gestational age, the investigators said.
They were unable to analyze the effects of ongoing TNFi treatment or discontinuation on patients with JIA separately because of the limited number of such patients in each group.
Another limitation of the study is that a high proportion of women – nearly three-quarters – had low disease activity at the start of pregnancy, according to the investigators, who said that group of women might expect some degree of improvement in the third trimester with or without TNF inhibitor discontinuation.
“In this context, the ameliorating effect of pregnancy on RA and JIA, which is most pronounced in the third trimester, may play a role,” they explained.
A certain proportion of women choose to discontinue certain arthritis treatments during pregnancy because of concerns that the medication may lead to fetal harm, but that may be changing, the investigators noted in their report.
“In recent years, more patients requiring treatment have been continuing on effective TNF inhibitors beyond conception as the available data on the safety of TNF inhibitors during pregnancy has increased,” they wrote.
Dr. Förger and her coauthors reported no financial disclosures or conflicts of interest. Financial support for the OTIS Collaborative Research Group comes from industry sources including AbbVie, Bristol-Myers Squibb, Celgene, Hoffman La Roche-Genentech, Janssen, Pfizer, Regeneron, Sandoz, and UCB, among others.
SOURCE: Förger F et al. Arthritis Rheumatol. 2019 Jan 21. doi: 10.1002/art.40821
In pregnant women with arthritis, discontinuing tumor necrosis factor inhibitors prior to gestational week 20 seems feasible without an increased risk of disease worsening, particularly in those with well-controlled disease, according to authors of a recent analysis of a prospective cohort study.
Stopping tumor necrosis factor inhibitor (TNFi) treatment at that point in the second trimester was not linked to any clinically important worsening of patient-reported outcomes later in pregnancy for women with rheumatoid arthritis (RA) or juvenile idiopathic arthritis (JIA), the researchers said.
However, continuing a TNFi past gestational week 20 may also be warranted for some patients, according to the researchers, led by Frauke Förger, MD, of the University of Bern (Switzerland).
“In case of active disease, the continuation of TNF inhibitors beyond gestational week 20 seems reasonable from the standpoint of improved disease activity in the third trimester, which may in turn lead to improved pregnancy outcomes,” Dr. Förger and her coinvestigators wrote in Arthritis & Rheumatology.
These findings stand in contrast to those of another recent study, in which stopping a TNF inhibitor after a positive pregnancy test was linked to disease flares in women with RA, according to the authors.
“The timing of drug discontinuation during pregnancy may be of importance,” they said in their report.
Beyond these studies, there are very limited data on the effects of discontinuing TNF inhibitors in pregnant women with rheumatoid arthritis, and “a lack of any data” in pregnant women with JIA, they said.
The current investigation by Dr. Förger and her colleagues included 490 pregnant women in the United States or Canada who were enrolled in the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project, a prospective cohort study. Of those women, 397 had RA and 93 had JIA.
About one-quarter of the women (122, or 24.9%) discontinued TNF inhibitor therapy prior to gestational week 20, while 41% continued on TNF inhibitors beyond that point, and 34.1% did not use a TNF inhibitor in pregnancy.
For those women who discontinued TNF inhibitors before gestational week 20, scores on the Patient Activity Scale (PAS) were stable over time, Dr. Förger and her colleagues reported.
Women who continued TNF inhibitor treatment past gestational week 20 had improved PAS scores in the third trimester, according to results of a univariate analysis (P = .02). However, the improvement appeared to be attenuated after adjustment for factors including race, smoking, use of prednisone or disease-modifying antirheumatic drugs, and gestational age, the investigators said.
They were unable to analyze the effects of ongoing TNFi treatment or discontinuation on patients with JIA separately because of the limited number of such patients in each group.
Another limitation of the study is that a high proportion of women – nearly three-quarters – had low disease activity at the start of pregnancy, according to the investigators, who said that group of women might expect some degree of improvement in the third trimester with or without TNF inhibitor discontinuation.
“In this context, the ameliorating effect of pregnancy on RA and JIA, which is most pronounced in the third trimester, may play a role,” they explained.
A certain proportion of women choose to discontinue certain arthritis treatments during pregnancy because of concerns that the medication may lead to fetal harm, but that may be changing, the investigators noted in their report.
“In recent years, more patients requiring treatment have been continuing on effective TNF inhibitors beyond conception as the available data on the safety of TNF inhibitors during pregnancy has increased,” they wrote.
Dr. Förger and her coauthors reported no financial disclosures or conflicts of interest. Financial support for the OTIS Collaborative Research Group comes from industry sources including AbbVie, Bristol-Myers Squibb, Celgene, Hoffman La Roche-Genentech, Janssen, Pfizer, Regeneron, Sandoz, and UCB, among others.
SOURCE: Förger F et al. Arthritis Rheumatol. 2019 Jan 21. doi: 10.1002/art.40821
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: In contrast to a previous report,
Major finding: Patient Activity Scale (PAS) scores were stable over time in women who discontinued TNF inhibitors before gestational week 20. Those who continued past week 20 had improved PAS scores in the third trimester (univariate analysis; P = .02).
Study details: Analysis including 490 pregnant women in the United States or Canada who enrolled in the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project, a prospective cohort study.
Disclosures: Dr. Förger and her coauthors reported no financial disclosures or conflicts of interest. Financial support for the OTIS Collaborative Research Group comes from industry sources including AbbVie, Bristol-Myers Squibb, Celgene, Hoffman La Roche-Genentech, Janssen, Pfizer, Regeneron, Sandoz, and UCB, among others.
Source: Förger F et al. Arthritis Rheumatol. 2019 Jan 21. doi: 10.1002/art.40821.
The fog may be lifting
One of the common symptoms described by postconcussion patients is that their heads feel a bit foggy. It may not be simply by chance that “foggy” is the best word to describe the atmosphere surrounding the entire field of concussion diagnosis and management.
Back in the Dark Ages, when the diagnosis of concussion was a simpler binary call, the issue of management seldom created much discussion. If the patient lost consciousness or was amnesic, he (it was less frequently she) could return to activity when his headache was gone and he could remember what he was supposed to do when the quarterback called for a “Red 34, Drive Right Smash” play. That may have even been during the second half of the game in which he was injured.
As it became more widely understood that the diagnosis of concussion didn’t require loss of consciousness and that repeated concussions could have serious sequelae, management became a bit fuzzier. No one had thought much about the recuperative process. Into this vacuum came a wide variety of researchers and providers. Not surprisingly, much of their advice was based on unproven assumptions, including the concept of “brain rest.”
It has taken time, but fortunately, folks with patience and wisdom have questioned these assumptions and begun collecting data. The result of these investigations and others has prompted the American Academy of Pediatrics to publish an updated set of guidelines on concussion management that includes the observation that extended school absence may slow the rehabilitation process (Pediatrics. 2018 Dec. doi: 10.1542/peds.2018-3074).
It is becoming clear that management of concussion can be rather complex and must be individualized to each patient. In my experience, the postconcussion period can unmask behavioral, cognitive, and emotional problems that were preexisting but had received little or no attention. For example, the trauma of the event may trigger anxiety about further injury or exacerbate depression that had been building for years. The student who “couldn’t do algebra” following a head injury may have had a lifelong learning disability that had gone unnoticed. The student athlete with prolonged postconcussion symptoms may indeed have another more serious problem. Hopefully, the new guidelines from the AAP will be a first step toward a more thoughtful and scientifically driven approach to concussion management.
It would be nice if that approach could filter down to the management of the more common but less dramatic pediatric injuries. There is hope. Choosing Wisely – a patient/parent–targeted initiative by the American Board of Internal Medicine Foundation in cooperation with the AAP – points out that, although half of the pediatric head injury patients seen in emergency departments received CT scan, only a third of those studies were indicated. Parents are encouraged to learn more about the risks of CT scans and question the physician when one is recommended.
But, doctors’ habits and old wives’ tales die slowly. I hope that you no longer recommend that parents keep their children awake after a head injury, or wake them every hour to check their pupils. Those counterproductive recommendations make about as much sense as staying out of the swimming pool for an hour after eating a chocolate chip cookie.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
One of the common symptoms described by postconcussion patients is that their heads feel a bit foggy. It may not be simply by chance that “foggy” is the best word to describe the atmosphere surrounding the entire field of concussion diagnosis and management.
Back in the Dark Ages, when the diagnosis of concussion was a simpler binary call, the issue of management seldom created much discussion. If the patient lost consciousness or was amnesic, he (it was less frequently she) could return to activity when his headache was gone and he could remember what he was supposed to do when the quarterback called for a “Red 34, Drive Right Smash” play. That may have even been during the second half of the game in which he was injured.
As it became more widely understood that the diagnosis of concussion didn’t require loss of consciousness and that repeated concussions could have serious sequelae, management became a bit fuzzier. No one had thought much about the recuperative process. Into this vacuum came a wide variety of researchers and providers. Not surprisingly, much of their advice was based on unproven assumptions, including the concept of “brain rest.”
It has taken time, but fortunately, folks with patience and wisdom have questioned these assumptions and begun collecting data. The result of these investigations and others has prompted the American Academy of Pediatrics to publish an updated set of guidelines on concussion management that includes the observation that extended school absence may slow the rehabilitation process (Pediatrics. 2018 Dec. doi: 10.1542/peds.2018-3074).
It is becoming clear that management of concussion can be rather complex and must be individualized to each patient. In my experience, the postconcussion period can unmask behavioral, cognitive, and emotional problems that were preexisting but had received little or no attention. For example, the trauma of the event may trigger anxiety about further injury or exacerbate depression that had been building for years. The student who “couldn’t do algebra” following a head injury may have had a lifelong learning disability that had gone unnoticed. The student athlete with prolonged postconcussion symptoms may indeed have another more serious problem. Hopefully, the new guidelines from the AAP will be a first step toward a more thoughtful and scientifically driven approach to concussion management.
It would be nice if that approach could filter down to the management of the more common but less dramatic pediatric injuries. There is hope. Choosing Wisely – a patient/parent–targeted initiative by the American Board of Internal Medicine Foundation in cooperation with the AAP – points out that, although half of the pediatric head injury patients seen in emergency departments received CT scan, only a third of those studies were indicated. Parents are encouraged to learn more about the risks of CT scans and question the physician when one is recommended.
But, doctors’ habits and old wives’ tales die slowly. I hope that you no longer recommend that parents keep their children awake after a head injury, or wake them every hour to check their pupils. Those counterproductive recommendations make about as much sense as staying out of the swimming pool for an hour after eating a chocolate chip cookie.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
One of the common symptoms described by postconcussion patients is that their heads feel a bit foggy. It may not be simply by chance that “foggy” is the best word to describe the atmosphere surrounding the entire field of concussion diagnosis and management.
Back in the Dark Ages, when the diagnosis of concussion was a simpler binary call, the issue of management seldom created much discussion. If the patient lost consciousness or was amnesic, he (it was less frequently she) could return to activity when his headache was gone and he could remember what he was supposed to do when the quarterback called for a “Red 34, Drive Right Smash” play. That may have even been during the second half of the game in which he was injured.
As it became more widely understood that the diagnosis of concussion didn’t require loss of consciousness and that repeated concussions could have serious sequelae, management became a bit fuzzier. No one had thought much about the recuperative process. Into this vacuum came a wide variety of researchers and providers. Not surprisingly, much of their advice was based on unproven assumptions, including the concept of “brain rest.”
It has taken time, but fortunately, folks with patience and wisdom have questioned these assumptions and begun collecting data. The result of these investigations and others has prompted the American Academy of Pediatrics to publish an updated set of guidelines on concussion management that includes the observation that extended school absence may slow the rehabilitation process (Pediatrics. 2018 Dec. doi: 10.1542/peds.2018-3074).
It is becoming clear that management of concussion can be rather complex and must be individualized to each patient. In my experience, the postconcussion period can unmask behavioral, cognitive, and emotional problems that were preexisting but had received little or no attention. For example, the trauma of the event may trigger anxiety about further injury or exacerbate depression that had been building for years. The student who “couldn’t do algebra” following a head injury may have had a lifelong learning disability that had gone unnoticed. The student athlete with prolonged postconcussion symptoms may indeed have another more serious problem. Hopefully, the new guidelines from the AAP will be a first step toward a more thoughtful and scientifically driven approach to concussion management.
It would be nice if that approach could filter down to the management of the more common but less dramatic pediatric injuries. There is hope. Choosing Wisely – a patient/parent–targeted initiative by the American Board of Internal Medicine Foundation in cooperation with the AAP – points out that, although half of the pediatric head injury patients seen in emergency departments received CT scan, only a third of those studies were indicated. Parents are encouraged to learn more about the risks of CT scans and question the physician when one is recommended.
But, doctors’ habits and old wives’ tales die slowly. I hope that you no longer recommend that parents keep their children awake after a head injury, or wake them every hour to check their pupils. Those counterproductive recommendations make about as much sense as staying out of the swimming pool for an hour after eating a chocolate chip cookie.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].