User login
Healing From Trauma
“You’ll never walk alone.” — Nettie Fowler, Carousel
A few winters ago, a young man and his fiancée were driving on the 91 freeway in southern California during a torrential downpour when their Honda Civic hydroplaned, slamming into the jersey barrier. They were both unhurt. Unsure what to do next, they made the catastrophic decision to exit the vehicle. As the man walked around the back of the car he was nearly hit by a black sedan sliding out of control trying to avoid them. When he came around the car, his fiancé was nowhere to be found. She had been struck at highway speed and lay crushed under the sedan hundreds of feet away.
I know this poor man because he was referred to me. Not as a dermatologist, but as a fellow human healing from trauma. On January 1, 2019, at about 9:30 PM, while we were home together, my beloved wife of 24 years took her own life. Even 5 years on it is difficult to believe that she isn’t proofing this paragraph like she had done for every one of my Derm News columns for years. We had been together since teenagers and had lived a joy-filled life. There isn’t any medical reason to share. But that day I joined the community of those who have carried unbearable heaviness of grief and survived. Sometimes others seek me out for help.
At first, my instinct was to guide them, to give advice, to tell them what to do and where to go. But I’ve learned that people in this dark valley don’t need a guide. They need someone to accompany them. To walk with them for a few minutes on their lonely journey. I recently read David Brooks’s new book, How to Know a Person. I’ve been a fan of his since he joined the New York Times in 2003 and have read almost everything he’s written. I sometimes even imagine how he might approach a column whenever I’m stuck (thank you, David). His The Road to Character book is in my canon of literature for self-growth. This latest book is an interesting digression from that central theme. He argues that our society is in acute need of forming better connections and that an important way we can be moral is to learn, and to practice, how to know each other. He shares an emotional experience of losing a close friend to suicide and writes a poignant explanation of what it means to accompany someone in need. It particularly resonated with me. We are doctors and are wired to find the source of a problem, like quickly rotating through the 4X, 10X, 40X on a microscope. Once identified, we spend most of our time creating and explaining treatments. I see how this makes me a great dermatologist but just an average human.
Brooks tells the story of a woman with a brain tumor who often finds herself on the ground surrounded by well-meaning people trying to help. She explains later that what she really needs in those moments is just for someone to get on the ground and lie with her. To accompany her.
Having crossed the midpoint of life, I see with the benefit of perspective how suffering has afforded me wisdom: I am more sensitive and attuned to others. It also gave me credibility: I know how it feels to walk life’s loneliest journey. I’ve also learned to make myself vulnerable for someone to share their story with me. I won’t be afraid to hear the details. I won’t judge them for weeping too little or for sobbing too much. I don’t answer whys. I won’t say what they should do next. But for a few minutes I can walk beside them as a person who cares.
I do not try to remember the hours and days after Susan’s death, but one moment stands out and makes my eyes well when I think of it. That following day my dear brother flew across the country on the next flight out. I was sitting in a psychiatry waiting room when he came down the hall with his luggage in tow. He hugged me as only a brother could, then looked me in my eyes, which were bloodshot from tears just as his were, and he said, “We’re going to be OK.” And with that he walked with me into the office.
We physicians are blessed to have so many intimate human interactions. This book reminded me that sometimes my most important job is not to be the optimized doctor, but just a good human walking alongside.
I have no conflict of interest and purchased these books.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
“You’ll never walk alone.” — Nettie Fowler, Carousel
A few winters ago, a young man and his fiancée were driving on the 91 freeway in southern California during a torrential downpour when their Honda Civic hydroplaned, slamming into the jersey barrier. They were both unhurt. Unsure what to do next, they made the catastrophic decision to exit the vehicle. As the man walked around the back of the car he was nearly hit by a black sedan sliding out of control trying to avoid them. When he came around the car, his fiancé was nowhere to be found. She had been struck at highway speed and lay crushed under the sedan hundreds of feet away.
I know this poor man because he was referred to me. Not as a dermatologist, but as a fellow human healing from trauma. On January 1, 2019, at about 9:30 PM, while we were home together, my beloved wife of 24 years took her own life. Even 5 years on it is difficult to believe that she isn’t proofing this paragraph like she had done for every one of my Derm News columns for years. We had been together since teenagers and had lived a joy-filled life. There isn’t any medical reason to share. But that day I joined the community of those who have carried unbearable heaviness of grief and survived. Sometimes others seek me out for help.
At first, my instinct was to guide them, to give advice, to tell them what to do and where to go. But I’ve learned that people in this dark valley don’t need a guide. They need someone to accompany them. To walk with them for a few minutes on their lonely journey. I recently read David Brooks’s new book, How to Know a Person. I’ve been a fan of his since he joined the New York Times in 2003 and have read almost everything he’s written. I sometimes even imagine how he might approach a column whenever I’m stuck (thank you, David). His The Road to Character book is in my canon of literature for self-growth. This latest book is an interesting digression from that central theme. He argues that our society is in acute need of forming better connections and that an important way we can be moral is to learn, and to practice, how to know each other. He shares an emotional experience of losing a close friend to suicide and writes a poignant explanation of what it means to accompany someone in need. It particularly resonated with me. We are doctors and are wired to find the source of a problem, like quickly rotating through the 4X, 10X, 40X on a microscope. Once identified, we spend most of our time creating and explaining treatments. I see how this makes me a great dermatologist but just an average human.
Brooks tells the story of a woman with a brain tumor who often finds herself on the ground surrounded by well-meaning people trying to help. She explains later that what she really needs in those moments is just for someone to get on the ground and lie with her. To accompany her.
Having crossed the midpoint of life, I see with the benefit of perspective how suffering has afforded me wisdom: I am more sensitive and attuned to others. It also gave me credibility: I know how it feels to walk life’s loneliest journey. I’ve also learned to make myself vulnerable for someone to share their story with me. I won’t be afraid to hear the details. I won’t judge them for weeping too little or for sobbing too much. I don’t answer whys. I won’t say what they should do next. But for a few minutes I can walk beside them as a person who cares.
I do not try to remember the hours and days after Susan’s death, but one moment stands out and makes my eyes well when I think of it. That following day my dear brother flew across the country on the next flight out. I was sitting in a psychiatry waiting room when he came down the hall with his luggage in tow. He hugged me as only a brother could, then looked me in my eyes, which were bloodshot from tears just as his were, and he said, “We’re going to be OK.” And with that he walked with me into the office.
We physicians are blessed to have so many intimate human interactions. This book reminded me that sometimes my most important job is not to be the optimized doctor, but just a good human walking alongside.
I have no conflict of interest and purchased these books.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
“You’ll never walk alone.” — Nettie Fowler, Carousel
A few winters ago, a young man and his fiancée were driving on the 91 freeway in southern California during a torrential downpour when their Honda Civic hydroplaned, slamming into the jersey barrier. They were both unhurt. Unsure what to do next, they made the catastrophic decision to exit the vehicle. As the man walked around the back of the car he was nearly hit by a black sedan sliding out of control trying to avoid them. When he came around the car, his fiancé was nowhere to be found. She had been struck at highway speed and lay crushed under the sedan hundreds of feet away.
I know this poor man because he was referred to me. Not as a dermatologist, but as a fellow human healing from trauma. On January 1, 2019, at about 9:30 PM, while we were home together, my beloved wife of 24 years took her own life. Even 5 years on it is difficult to believe that she isn’t proofing this paragraph like she had done for every one of my Derm News columns for years. We had been together since teenagers and had lived a joy-filled life. There isn’t any medical reason to share. But that day I joined the community of those who have carried unbearable heaviness of grief and survived. Sometimes others seek me out for help.
At first, my instinct was to guide them, to give advice, to tell them what to do and where to go. But I’ve learned that people in this dark valley don’t need a guide. They need someone to accompany them. To walk with them for a few minutes on their lonely journey. I recently read David Brooks’s new book, How to Know a Person. I’ve been a fan of his since he joined the New York Times in 2003 and have read almost everything he’s written. I sometimes even imagine how he might approach a column whenever I’m stuck (thank you, David). His The Road to Character book is in my canon of literature for self-growth. This latest book is an interesting digression from that central theme. He argues that our society is in acute need of forming better connections and that an important way we can be moral is to learn, and to practice, how to know each other. He shares an emotional experience of losing a close friend to suicide and writes a poignant explanation of what it means to accompany someone in need. It particularly resonated with me. We are doctors and are wired to find the source of a problem, like quickly rotating through the 4X, 10X, 40X on a microscope. Once identified, we spend most of our time creating and explaining treatments. I see how this makes me a great dermatologist but just an average human.
Brooks tells the story of a woman with a brain tumor who often finds herself on the ground surrounded by well-meaning people trying to help. She explains later that what she really needs in those moments is just for someone to get on the ground and lie with her. To accompany her.
Having crossed the midpoint of life, I see with the benefit of perspective how suffering has afforded me wisdom: I am more sensitive and attuned to others. It also gave me credibility: I know how it feels to walk life’s loneliest journey. I’ve also learned to make myself vulnerable for someone to share their story with me. I won’t be afraid to hear the details. I won’t judge them for weeping too little or for sobbing too much. I don’t answer whys. I won’t say what they should do next. But for a few minutes I can walk beside them as a person who cares.
I do not try to remember the hours and days after Susan’s death, but one moment stands out and makes my eyes well when I think of it. That following day my dear brother flew across the country on the next flight out. I was sitting in a psychiatry waiting room when he came down the hall with his luggage in tow. He hugged me as only a brother could, then looked me in my eyes, which were bloodshot from tears just as his were, and he said, “We’re going to be OK.” And with that he walked with me into the office.
We physicians are blessed to have so many intimate human interactions. This book reminded me that sometimes my most important job is not to be the optimized doctor, but just a good human walking alongside.
I have no conflict of interest and purchased these books.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
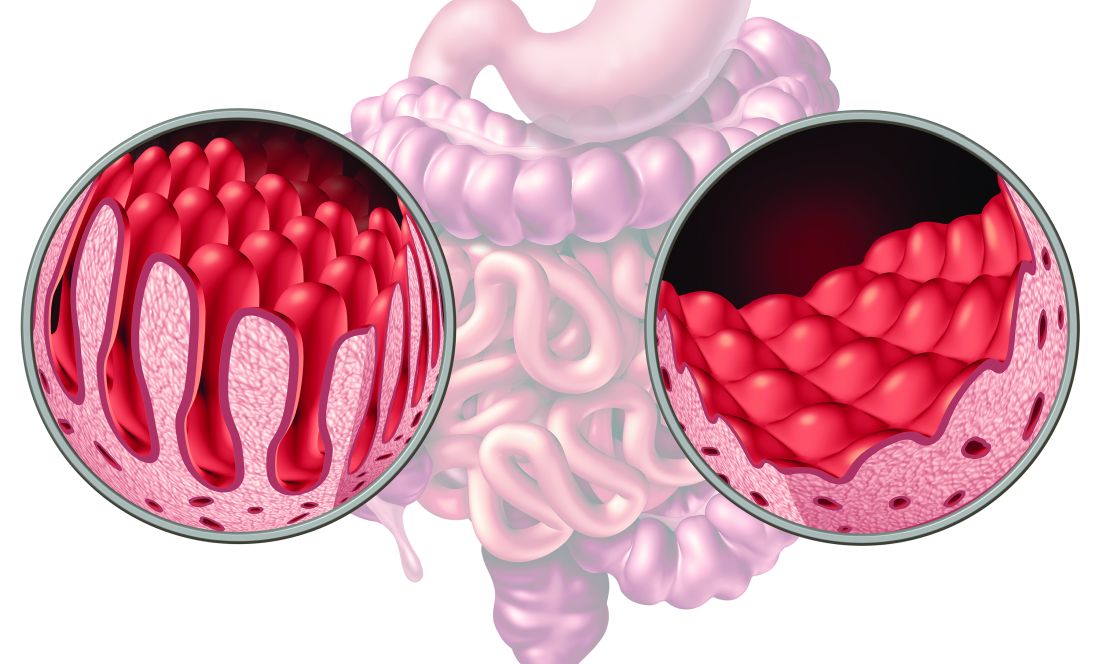
No-Biopsy Approach to Celiac Disease Diagnosis Appears Effective for Select Adult Patients
Select adult patients with immunoglobulin A-tissue transglutaminase antibody levels (IgA-tTG) greater than or equal to 10 times the upper limit of normal (ULN) and a moderate-to-high pretest probability of celiac disease could be diagnosed without undergoing invasive endoscopy and duodenal biopsy, according to a new study.
, the authors wrote.
“Our study confirms the high accuracy of serology-based diagnosis of coeliac disease in select adult patients,” said Mohamed G. Shiha, MBBCh, MRCP, lead author and a clinical research fellow in gastroenterology at Sheffield Teaching Hospitals in the United Kingdom.
“This no-biopsy approach could lead to a shorter time to diagnosis, increased patient satisfaction, and reduced healthcare costs,” he said.
The study was published online in Gastroenterology.
Evaluating the No-Biopsy Approach
Dr. Shiha and colleagues conducted a systematic review and meta-analysis to evaluate to the accuracy of a no-biopsy approach for diagnosing celiac disease in adults. They looked for studies that reported the sensitivity and specificity of IgA-tTG ≥10xULN compared with duodenal biopsies (with a Marsh grade ≥2) in adults with suspected celiac disease.
The research team used a bivariate random-effects model to calculate the summary estimates of sensitivity, specificity, and positive and negative likelihood ratios. Then the positive and negative likelihood ratios were used to calculate the positive predictive value (PPV) of the no-biopsy approach across different pretest probabilities of celiac disease.
Among 18 studies with 12,103 participants from 15 countries, the pooled prevalence of biopsy-proven celiac disease was 62%. The proportion of patients with IgA-tTG ≥10xULN was 32%.
The summary sensitivity of IgA-tTG ≥10xULN was 51%, and the summary specificity was 100% for the diagnosis of celiac disease. The positive and negative likelihood ratios were 183.42 and .49, respectively. The area under the summary receiver operating characteristic curve was .83.
Overall, the PPV of IgA-tTG ≥10xULN to identify patients with celiac disease was 98%, which varied according to pretest probability of celiac disease in the studied population. Specifically, the PPV was 65%, 88%, 95%, and 99% if celiac disease prevalence was 1%, 4%, 10%, and 40%, respectively. The 40% prevalence represents the lower confidence interval of the pooled prevalence from the included studies, the authors noted.
“We provided PPV estimates of IgA-tTG ≥10xULN for common pretest probabilities of coeliac disease to aid clinicians and patients in reaching an informed decision on a no-biopsy diagnosis based on the best available evidence,” the authors wrote.
Considering Additional Factors
Due to the increased accuracy of serological tests, pediatric guidelines have adopted a no-biopsy approach, the authors wrote. Children with IgA-tTG ≥10xULN and positive serum endomysial antibodies (EMA) can be diagnosed with celiac disease without biopsy.
However, the no-biopsy approach remains controversial for diagnosing adult patients and requires additional study, the authors wrote. They noted a limitation that all included studies were conducted in secondary and tertiary care settings and excluded patients with known celiac disease or on a gluten-free diet, so the results may not be generalizable to primary care settings.
In addition, relying on serology testing alone could lead to potential false-positive diagnoses, unnecessary dietary restriction, and negative effects on patients’ quality of life, the authors wrote.
At the same time, duodenal biopsy may not always be accurate due to inadequate sampling and could result in false-negative histology. The no-biopsy approach could mitigate this potential risk, the authors noted.
“This study systematically collates the growing data supporting the accuracy of antibody testing to diagnose celiac disease,” said Benjamin Lebwohl, MD, AGAF, professor of medicine and epidemiology at Columbia University Medical Center and director of clinical research for the Celiac Disease Center at Columbia University, New York. Dr. Lebwohl wasn’t involved with this study.
“We have historically relied on duodenal biopsy to confirm the diagnosis of celiac disease, and the biopsy will still have a central role in most cases in the foreseeable future,” he said. “But as we hone our understanding of antibody testing, one day we may be able to accept or even recommend a biopsy-free approach in select patients.”
Two authors reported grant support from the National Institute for Health and Care Research and National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Shiha reported speaker honorarium from Thermo Fisher. Dr. Lebwohl reported no relevant disclosures.
Select adult patients with immunoglobulin A-tissue transglutaminase antibody levels (IgA-tTG) greater than or equal to 10 times the upper limit of normal (ULN) and a moderate-to-high pretest probability of celiac disease could be diagnosed without undergoing invasive endoscopy and duodenal biopsy, according to a new study.
, the authors wrote.
“Our study confirms the high accuracy of serology-based diagnosis of coeliac disease in select adult patients,” said Mohamed G. Shiha, MBBCh, MRCP, lead author and a clinical research fellow in gastroenterology at Sheffield Teaching Hospitals in the United Kingdom.
“This no-biopsy approach could lead to a shorter time to diagnosis, increased patient satisfaction, and reduced healthcare costs,” he said.
The study was published online in Gastroenterology.
Evaluating the No-Biopsy Approach
Dr. Shiha and colleagues conducted a systematic review and meta-analysis to evaluate to the accuracy of a no-biopsy approach for diagnosing celiac disease in adults. They looked for studies that reported the sensitivity and specificity of IgA-tTG ≥10xULN compared with duodenal biopsies (with a Marsh grade ≥2) in adults with suspected celiac disease.
The research team used a bivariate random-effects model to calculate the summary estimates of sensitivity, specificity, and positive and negative likelihood ratios. Then the positive and negative likelihood ratios were used to calculate the positive predictive value (PPV) of the no-biopsy approach across different pretest probabilities of celiac disease.
Among 18 studies with 12,103 participants from 15 countries, the pooled prevalence of biopsy-proven celiac disease was 62%. The proportion of patients with IgA-tTG ≥10xULN was 32%.
The summary sensitivity of IgA-tTG ≥10xULN was 51%, and the summary specificity was 100% for the diagnosis of celiac disease. The positive and negative likelihood ratios were 183.42 and .49, respectively. The area under the summary receiver operating characteristic curve was .83.
Overall, the PPV of IgA-tTG ≥10xULN to identify patients with celiac disease was 98%, which varied according to pretest probability of celiac disease in the studied population. Specifically, the PPV was 65%, 88%, 95%, and 99% if celiac disease prevalence was 1%, 4%, 10%, and 40%, respectively. The 40% prevalence represents the lower confidence interval of the pooled prevalence from the included studies, the authors noted.
“We provided PPV estimates of IgA-tTG ≥10xULN for common pretest probabilities of coeliac disease to aid clinicians and patients in reaching an informed decision on a no-biopsy diagnosis based on the best available evidence,” the authors wrote.
Considering Additional Factors
Due to the increased accuracy of serological tests, pediatric guidelines have adopted a no-biopsy approach, the authors wrote. Children with IgA-tTG ≥10xULN and positive serum endomysial antibodies (EMA) can be diagnosed with celiac disease without biopsy.
However, the no-biopsy approach remains controversial for diagnosing adult patients and requires additional study, the authors wrote. They noted a limitation that all included studies were conducted in secondary and tertiary care settings and excluded patients with known celiac disease or on a gluten-free diet, so the results may not be generalizable to primary care settings.
In addition, relying on serology testing alone could lead to potential false-positive diagnoses, unnecessary dietary restriction, and negative effects on patients’ quality of life, the authors wrote.
At the same time, duodenal biopsy may not always be accurate due to inadequate sampling and could result in false-negative histology. The no-biopsy approach could mitigate this potential risk, the authors noted.
“This study systematically collates the growing data supporting the accuracy of antibody testing to diagnose celiac disease,” said Benjamin Lebwohl, MD, AGAF, professor of medicine and epidemiology at Columbia University Medical Center and director of clinical research for the Celiac Disease Center at Columbia University, New York. Dr. Lebwohl wasn’t involved with this study.
“We have historically relied on duodenal biopsy to confirm the diagnosis of celiac disease, and the biopsy will still have a central role in most cases in the foreseeable future,” he said. “But as we hone our understanding of antibody testing, one day we may be able to accept or even recommend a biopsy-free approach in select patients.”
Two authors reported grant support from the National Institute for Health and Care Research and National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Shiha reported speaker honorarium from Thermo Fisher. Dr. Lebwohl reported no relevant disclosures.
Select adult patients with immunoglobulin A-tissue transglutaminase antibody levels (IgA-tTG) greater than or equal to 10 times the upper limit of normal (ULN) and a moderate-to-high pretest probability of celiac disease could be diagnosed without undergoing invasive endoscopy and duodenal biopsy, according to a new study.
, the authors wrote.
“Our study confirms the high accuracy of serology-based diagnosis of coeliac disease in select adult patients,” said Mohamed G. Shiha, MBBCh, MRCP, lead author and a clinical research fellow in gastroenterology at Sheffield Teaching Hospitals in the United Kingdom.
“This no-biopsy approach could lead to a shorter time to diagnosis, increased patient satisfaction, and reduced healthcare costs,” he said.
The study was published online in Gastroenterology.
Evaluating the No-Biopsy Approach
Dr. Shiha and colleagues conducted a systematic review and meta-analysis to evaluate to the accuracy of a no-biopsy approach for diagnosing celiac disease in adults. They looked for studies that reported the sensitivity and specificity of IgA-tTG ≥10xULN compared with duodenal biopsies (with a Marsh grade ≥2) in adults with suspected celiac disease.
The research team used a bivariate random-effects model to calculate the summary estimates of sensitivity, specificity, and positive and negative likelihood ratios. Then the positive and negative likelihood ratios were used to calculate the positive predictive value (PPV) of the no-biopsy approach across different pretest probabilities of celiac disease.
Among 18 studies with 12,103 participants from 15 countries, the pooled prevalence of biopsy-proven celiac disease was 62%. The proportion of patients with IgA-tTG ≥10xULN was 32%.
The summary sensitivity of IgA-tTG ≥10xULN was 51%, and the summary specificity was 100% for the diagnosis of celiac disease. The positive and negative likelihood ratios were 183.42 and .49, respectively. The area under the summary receiver operating characteristic curve was .83.
Overall, the PPV of IgA-tTG ≥10xULN to identify patients with celiac disease was 98%, which varied according to pretest probability of celiac disease in the studied population. Specifically, the PPV was 65%, 88%, 95%, and 99% if celiac disease prevalence was 1%, 4%, 10%, and 40%, respectively. The 40% prevalence represents the lower confidence interval of the pooled prevalence from the included studies, the authors noted.
“We provided PPV estimates of IgA-tTG ≥10xULN for common pretest probabilities of coeliac disease to aid clinicians and patients in reaching an informed decision on a no-biopsy diagnosis based on the best available evidence,” the authors wrote.
Considering Additional Factors
Due to the increased accuracy of serological tests, pediatric guidelines have adopted a no-biopsy approach, the authors wrote. Children with IgA-tTG ≥10xULN and positive serum endomysial antibodies (EMA) can be diagnosed with celiac disease without biopsy.
However, the no-biopsy approach remains controversial for diagnosing adult patients and requires additional study, the authors wrote. They noted a limitation that all included studies were conducted in secondary and tertiary care settings and excluded patients with known celiac disease or on a gluten-free diet, so the results may not be generalizable to primary care settings.
In addition, relying on serology testing alone could lead to potential false-positive diagnoses, unnecessary dietary restriction, and negative effects on patients’ quality of life, the authors wrote.
At the same time, duodenal biopsy may not always be accurate due to inadequate sampling and could result in false-negative histology. The no-biopsy approach could mitigate this potential risk, the authors noted.
“This study systematically collates the growing data supporting the accuracy of antibody testing to diagnose celiac disease,” said Benjamin Lebwohl, MD, AGAF, professor of medicine and epidemiology at Columbia University Medical Center and director of clinical research for the Celiac Disease Center at Columbia University, New York. Dr. Lebwohl wasn’t involved with this study.
“We have historically relied on duodenal biopsy to confirm the diagnosis of celiac disease, and the biopsy will still have a central role in most cases in the foreseeable future,” he said. “But as we hone our understanding of antibody testing, one day we may be able to accept or even recommend a biopsy-free approach in select patients.”
Two authors reported grant support from the National Institute for Health and Care Research and National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Shiha reported speaker honorarium from Thermo Fisher. Dr. Lebwohl reported no relevant disclosures.
FROM GASTROENTEROLOGY
Are There Benefits to Taking GLP-1 Receptor Agonists Before Joint Surgery?
Obesity and diabetes increase the risk for complications following joint surgeries like total hip replacement, but can semaglutide and related drugs help?
The question has massive implications. More than 450,000 total hip arthroplasty (THA) procedures are performed annually in the United States, with the number expected to grow to 850,000 by 2030. Obesity is the leading reason for the increase. Semaglutide and other glucagon-like peptide 1 (GLP-1) receptor agonists can lead to dramatic and rapid weight loss, in addition to controlling diabetes, so researchers have wondered if the medications might improve outcomes in patients undergoing joint surgery.
Two studies presented at the 2024 annual meeting of the American Academy of Orthopaedic Surgeons (AAOS) sought to answer the question — but reached different conclusions.
One study of THA patients taking semaglutide found fewer 90-day readmissions for diabetes and fewer prosthetic joint infections at the 2-year mark. Another found similar outcomes on the need for revision surgery, infections, and many other postsurgery metrics in people who took the GLP-1 receptor agonist and those who did not. Neither study had outside funding.
Study: Fewer Infections, Readmissions
For their study, Matthew Magruder, MD, a third-year orthopedic resident at Maimonides Medical Center’s Department of Orthopaedic Surgery and Rehabilitation in New York City, and his colleagues used an administrative claim database (PearlDiver) to identify THA patients who underwent the surgery between January 1, 2020, to October 31, 2021, when semaglutide was approved for the treatment of diabetes but not yet for obesity. The researchers found 9465 patients who had had a primary THA, of whom 1653 had received a prescription for semaglutide.
In total, 84.9% of those on semaglutide had obesity, as did 85.2% of those not on the medication.
Dr. Magruder’s group looked at medical complications such as deep vein thrombosis, myocardial infarction, hypoglycemia, and pulmonary embolism within 90 days of surgery, implant-related complications 2 years after the procedure, rates of readmission within 90 days of the procedure, length of stay in the hospital, and costs of care.
They found that patients taking semaglutide were less likely to be readmitted to the hospital within 90 days of THA (6.2% vs 8.8%; P <.01) and experienced fewer joint infections (1.6% vs 2.9%; P <.01). No significant differences were found in the other outcomes.
Among the potential concerns involving the use of GLP-1 receptor agonists in patients undergoing surgery are their potential to cause hypoglycemia and the risk for aspiration during anesthesia. But those issues did not emerge in the analysis.
“We concluded that this was preliminary evidence that using semaglutide at the time of surgery was safe and potentially effective at reducing complications,” said Dr. Magruder, whose team published their findings in The Journal of Arthroplasty.
Study: Semaglutide Has No Effect on Postop Complications
In another study presented at the AAOS meeting, researchers found that rates of complications after THA were similar in patients with obesity who took semaglutide and those who did not. That information could be helpful for clinicians who have been reluctant to perform THA procedures in patients who also have had bariatric surgery, said Daniel E. Pereira, MD, a resident at Washington University in St. Louis and the first author of the study.
A recent retrospective review found that patients who had bariatric surgery have worse implant survivorship and higher rates of dislocation than do those with a naturally low or high body mass index (BMI).
Pereira and his colleagues used a national database, with deidentified patient records, originally finding 42,410 patients. After matching, they evaluated 616 in each cohort: those who took semaglutide and those who did not. The average age was 62.7 years; average BMI was 35.5.
Both groups had a similar risk for a range of complications including revision surgery, infection of the new joint and surgical site, opioid-related disorders, pulmonary embolism, deep vein thrombosis, and mortality.
“We didn’t observe anything significant [between groups] in terms of the complications,” said David Momtaz, MPH, a fourth-year medical student at the University of Texas Health Science Center at San Antonio, who helped conduct the research.
Dr. Pereira said he hoped the results would end the hesitation he observes, partly due to a lack of research, among some physicians about prescribing semaglutide before THA in appropriate patients. “Our preliminary evidence suggests there is no need to withhold THA in patients who successfully lost weight on semaglutide,” he said.
Expert Perspective: Not Unexpected
Peter Hanson, MD, an orthopedic surgeon and orthopedic medical director at Sharp Grossmont Hospital in La Mesa, California, who specializes in hip and knee replacement, said he was unsurprised by the findings.
The patients he has observed on GLP-1 receptor agonists lose weight, he said, and a few even to the point of not needing a replacement. A recent study found that every 1% decrease in weight was associated with a 2% reduced risk for knee replacement in those with knee osteoarthritis or at risk for it, and every 1% drop in weight was associated with a 3% reduced risk for THA.
“I always advise my overweight patient to lose at least 30 pounds, even if their BMI is less than 40, like many in these studies,” Dr. Hanson said. If a patient’s doctor prescribes semaglutide or another GLP-1 receptor agonist, “I am very supportive, and we postpone surgery until the weight loss is maximized,” he added.
Drs. Magruder, Pereira, Momtaz, and Hanson have no disclosures.
A version of this article appeared on Medscape.com.
Obesity and diabetes increase the risk for complications following joint surgeries like total hip replacement, but can semaglutide and related drugs help?
The question has massive implications. More than 450,000 total hip arthroplasty (THA) procedures are performed annually in the United States, with the number expected to grow to 850,000 by 2030. Obesity is the leading reason for the increase. Semaglutide and other glucagon-like peptide 1 (GLP-1) receptor agonists can lead to dramatic and rapid weight loss, in addition to controlling diabetes, so researchers have wondered if the medications might improve outcomes in patients undergoing joint surgery.
Two studies presented at the 2024 annual meeting of the American Academy of Orthopaedic Surgeons (AAOS) sought to answer the question — but reached different conclusions.
One study of THA patients taking semaglutide found fewer 90-day readmissions for diabetes and fewer prosthetic joint infections at the 2-year mark. Another found similar outcomes on the need for revision surgery, infections, and many other postsurgery metrics in people who took the GLP-1 receptor agonist and those who did not. Neither study had outside funding.
Study: Fewer Infections, Readmissions
For their study, Matthew Magruder, MD, a third-year orthopedic resident at Maimonides Medical Center’s Department of Orthopaedic Surgery and Rehabilitation in New York City, and his colleagues used an administrative claim database (PearlDiver) to identify THA patients who underwent the surgery between January 1, 2020, to October 31, 2021, when semaglutide was approved for the treatment of diabetes but not yet for obesity. The researchers found 9465 patients who had had a primary THA, of whom 1653 had received a prescription for semaglutide.
In total, 84.9% of those on semaglutide had obesity, as did 85.2% of those not on the medication.
Dr. Magruder’s group looked at medical complications such as deep vein thrombosis, myocardial infarction, hypoglycemia, and pulmonary embolism within 90 days of surgery, implant-related complications 2 years after the procedure, rates of readmission within 90 days of the procedure, length of stay in the hospital, and costs of care.
They found that patients taking semaglutide were less likely to be readmitted to the hospital within 90 days of THA (6.2% vs 8.8%; P <.01) and experienced fewer joint infections (1.6% vs 2.9%; P <.01). No significant differences were found in the other outcomes.
Among the potential concerns involving the use of GLP-1 receptor agonists in patients undergoing surgery are their potential to cause hypoglycemia and the risk for aspiration during anesthesia. But those issues did not emerge in the analysis.
“We concluded that this was preliminary evidence that using semaglutide at the time of surgery was safe and potentially effective at reducing complications,” said Dr. Magruder, whose team published their findings in The Journal of Arthroplasty.
Study: Semaglutide Has No Effect on Postop Complications
In another study presented at the AAOS meeting, researchers found that rates of complications after THA were similar in patients with obesity who took semaglutide and those who did not. That information could be helpful for clinicians who have been reluctant to perform THA procedures in patients who also have had bariatric surgery, said Daniel E. Pereira, MD, a resident at Washington University in St. Louis and the first author of the study.
A recent retrospective review found that patients who had bariatric surgery have worse implant survivorship and higher rates of dislocation than do those with a naturally low or high body mass index (BMI).
Pereira and his colleagues used a national database, with deidentified patient records, originally finding 42,410 patients. After matching, they evaluated 616 in each cohort: those who took semaglutide and those who did not. The average age was 62.7 years; average BMI was 35.5.
Both groups had a similar risk for a range of complications including revision surgery, infection of the new joint and surgical site, opioid-related disorders, pulmonary embolism, deep vein thrombosis, and mortality.
“We didn’t observe anything significant [between groups] in terms of the complications,” said David Momtaz, MPH, a fourth-year medical student at the University of Texas Health Science Center at San Antonio, who helped conduct the research.
Dr. Pereira said he hoped the results would end the hesitation he observes, partly due to a lack of research, among some physicians about prescribing semaglutide before THA in appropriate patients. “Our preliminary evidence suggests there is no need to withhold THA in patients who successfully lost weight on semaglutide,” he said.
Expert Perspective: Not Unexpected
Peter Hanson, MD, an orthopedic surgeon and orthopedic medical director at Sharp Grossmont Hospital in La Mesa, California, who specializes in hip and knee replacement, said he was unsurprised by the findings.
The patients he has observed on GLP-1 receptor agonists lose weight, he said, and a few even to the point of not needing a replacement. A recent study found that every 1% decrease in weight was associated with a 2% reduced risk for knee replacement in those with knee osteoarthritis or at risk for it, and every 1% drop in weight was associated with a 3% reduced risk for THA.
“I always advise my overweight patient to lose at least 30 pounds, even if their BMI is less than 40, like many in these studies,” Dr. Hanson said. If a patient’s doctor prescribes semaglutide or another GLP-1 receptor agonist, “I am very supportive, and we postpone surgery until the weight loss is maximized,” he added.
Drs. Magruder, Pereira, Momtaz, and Hanson have no disclosures.
A version of this article appeared on Medscape.com.
Obesity and diabetes increase the risk for complications following joint surgeries like total hip replacement, but can semaglutide and related drugs help?
The question has massive implications. More than 450,000 total hip arthroplasty (THA) procedures are performed annually in the United States, with the number expected to grow to 850,000 by 2030. Obesity is the leading reason for the increase. Semaglutide and other glucagon-like peptide 1 (GLP-1) receptor agonists can lead to dramatic and rapid weight loss, in addition to controlling diabetes, so researchers have wondered if the medications might improve outcomes in patients undergoing joint surgery.
Two studies presented at the 2024 annual meeting of the American Academy of Orthopaedic Surgeons (AAOS) sought to answer the question — but reached different conclusions.
One study of THA patients taking semaglutide found fewer 90-day readmissions for diabetes and fewer prosthetic joint infections at the 2-year mark. Another found similar outcomes on the need for revision surgery, infections, and many other postsurgery metrics in people who took the GLP-1 receptor agonist and those who did not. Neither study had outside funding.
Study: Fewer Infections, Readmissions
For their study, Matthew Magruder, MD, a third-year orthopedic resident at Maimonides Medical Center’s Department of Orthopaedic Surgery and Rehabilitation in New York City, and his colleagues used an administrative claim database (PearlDiver) to identify THA patients who underwent the surgery between January 1, 2020, to October 31, 2021, when semaglutide was approved for the treatment of diabetes but not yet for obesity. The researchers found 9465 patients who had had a primary THA, of whom 1653 had received a prescription for semaglutide.
In total, 84.9% of those on semaglutide had obesity, as did 85.2% of those not on the medication.
Dr. Magruder’s group looked at medical complications such as deep vein thrombosis, myocardial infarction, hypoglycemia, and pulmonary embolism within 90 days of surgery, implant-related complications 2 years after the procedure, rates of readmission within 90 days of the procedure, length of stay in the hospital, and costs of care.
They found that patients taking semaglutide were less likely to be readmitted to the hospital within 90 days of THA (6.2% vs 8.8%; P <.01) and experienced fewer joint infections (1.6% vs 2.9%; P <.01). No significant differences were found in the other outcomes.
Among the potential concerns involving the use of GLP-1 receptor agonists in patients undergoing surgery are their potential to cause hypoglycemia and the risk for aspiration during anesthesia. But those issues did not emerge in the analysis.
“We concluded that this was preliminary evidence that using semaglutide at the time of surgery was safe and potentially effective at reducing complications,” said Dr. Magruder, whose team published their findings in The Journal of Arthroplasty.
Study: Semaglutide Has No Effect on Postop Complications
In another study presented at the AAOS meeting, researchers found that rates of complications after THA were similar in patients with obesity who took semaglutide and those who did not. That information could be helpful for clinicians who have been reluctant to perform THA procedures in patients who also have had bariatric surgery, said Daniel E. Pereira, MD, a resident at Washington University in St. Louis and the first author of the study.
A recent retrospective review found that patients who had bariatric surgery have worse implant survivorship and higher rates of dislocation than do those with a naturally low or high body mass index (BMI).
Pereira and his colleagues used a national database, with deidentified patient records, originally finding 42,410 patients. After matching, they evaluated 616 in each cohort: those who took semaglutide and those who did not. The average age was 62.7 years; average BMI was 35.5.
Both groups had a similar risk for a range of complications including revision surgery, infection of the new joint and surgical site, opioid-related disorders, pulmonary embolism, deep vein thrombosis, and mortality.
“We didn’t observe anything significant [between groups] in terms of the complications,” said David Momtaz, MPH, a fourth-year medical student at the University of Texas Health Science Center at San Antonio, who helped conduct the research.
Dr. Pereira said he hoped the results would end the hesitation he observes, partly due to a lack of research, among some physicians about prescribing semaglutide before THA in appropriate patients. “Our preliminary evidence suggests there is no need to withhold THA in patients who successfully lost weight on semaglutide,” he said.
Expert Perspective: Not Unexpected
Peter Hanson, MD, an orthopedic surgeon and orthopedic medical director at Sharp Grossmont Hospital in La Mesa, California, who specializes in hip and knee replacement, said he was unsurprised by the findings.
The patients he has observed on GLP-1 receptor agonists lose weight, he said, and a few even to the point of not needing a replacement. A recent study found that every 1% decrease in weight was associated with a 2% reduced risk for knee replacement in those with knee osteoarthritis or at risk for it, and every 1% drop in weight was associated with a 3% reduced risk for THA.
“I always advise my overweight patient to lose at least 30 pounds, even if their BMI is less than 40, like many in these studies,” Dr. Hanson said. If a patient’s doctor prescribes semaglutide or another GLP-1 receptor agonist, “I am very supportive, and we postpone surgery until the weight loss is maximized,” he added.
Drs. Magruder, Pereira, Momtaz, and Hanson have no disclosures.
A version of this article appeared on Medscape.com.
FROM AAOS 2024
Management of Tinea Capitis in Children Varies, Survey Finds
TOPLINE:
METHODOLOGY:
- The fungal scalp infection tinea capitis affects an estimated 3%-13% of children.
- While international guidelines exist for the treatment of tinea capitis in infants and children, no such document has been developed in the United States.
- Researchers distributed a survey by email to dermatologists through the and the Society for Pediatric Dermatology in the United States, asking about how they treated and managed pediatric patients with tinea capitis; 56 dermatologists participated.
TAKEAWAY:
- Most respondents (88.2%) said they felt comfortable prescribing oral medications prior to confirmation for those aged 2-18 years ( was the most common choice in 60.4% of cases), compared with 81.6% for those aged 2 months to 2 years ( was the most common treatment choice in 41.5% of cases), and 48.7% for those aged 0-2 months ( was the most common choice in 28.6% of cases).
- When asked what topical medication they would start prior to confirmation, most respondents said shampoo (62.3% for those aged 0-2 months and 75.5% each for those aged 2 months to 2 years and those aged 2-18 years), yet between 11.3% and 13% said they would use none.
- The most common form of confirmatory testing was , followed by potassium hydroxide preparation, trichoscopy, and Wood’s lamp.
- More than half of survey respondents would alter their choice of oral medication based on culture results, but most would not change their topical medication preference.
IN PRACTICE:
“The management of tinea capitis in the United States is currently variable, particularly with the introduction of newer antifungals,” the authors wrote. “Future steps involve establishing evidence-based clinical practice guidelines that consider drug efficacy, safety profiles, and costs.”
SOURCE:
Bernard Cohen, MD, of the Departments of Pediatrics and Dermatology at Johns Hopkins University, Baltimore, Maryland, led the research, which was published in Pediatric Dermatology.
LIMITATIONS:
Lower response rates associated with online surveys and predefined age groups restrict the granularity of responses.
DISCLOSURES:
The authors reported having no financial disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The fungal scalp infection tinea capitis affects an estimated 3%-13% of children.
- While international guidelines exist for the treatment of tinea capitis in infants and children, no such document has been developed in the United States.
- Researchers distributed a survey by email to dermatologists through the and the Society for Pediatric Dermatology in the United States, asking about how they treated and managed pediatric patients with tinea capitis; 56 dermatologists participated.
TAKEAWAY:
- Most respondents (88.2%) said they felt comfortable prescribing oral medications prior to confirmation for those aged 2-18 years ( was the most common choice in 60.4% of cases), compared with 81.6% for those aged 2 months to 2 years ( was the most common treatment choice in 41.5% of cases), and 48.7% for those aged 0-2 months ( was the most common choice in 28.6% of cases).
- When asked what topical medication they would start prior to confirmation, most respondents said shampoo (62.3% for those aged 0-2 months and 75.5% each for those aged 2 months to 2 years and those aged 2-18 years), yet between 11.3% and 13% said they would use none.
- The most common form of confirmatory testing was , followed by potassium hydroxide preparation, trichoscopy, and Wood’s lamp.
- More than half of survey respondents would alter their choice of oral medication based on culture results, but most would not change their topical medication preference.
IN PRACTICE:
“The management of tinea capitis in the United States is currently variable, particularly with the introduction of newer antifungals,” the authors wrote. “Future steps involve establishing evidence-based clinical practice guidelines that consider drug efficacy, safety profiles, and costs.”
SOURCE:
Bernard Cohen, MD, of the Departments of Pediatrics and Dermatology at Johns Hopkins University, Baltimore, Maryland, led the research, which was published in Pediatric Dermatology.
LIMITATIONS:
Lower response rates associated with online surveys and predefined age groups restrict the granularity of responses.
DISCLOSURES:
The authors reported having no financial disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The fungal scalp infection tinea capitis affects an estimated 3%-13% of children.
- While international guidelines exist for the treatment of tinea capitis in infants and children, no such document has been developed in the United States.
- Researchers distributed a survey by email to dermatologists through the and the Society for Pediatric Dermatology in the United States, asking about how they treated and managed pediatric patients with tinea capitis; 56 dermatologists participated.
TAKEAWAY:
- Most respondents (88.2%) said they felt comfortable prescribing oral medications prior to confirmation for those aged 2-18 years ( was the most common choice in 60.4% of cases), compared with 81.6% for those aged 2 months to 2 years ( was the most common treatment choice in 41.5% of cases), and 48.7% for those aged 0-2 months ( was the most common choice in 28.6% of cases).
- When asked what topical medication they would start prior to confirmation, most respondents said shampoo (62.3% for those aged 0-2 months and 75.5% each for those aged 2 months to 2 years and those aged 2-18 years), yet between 11.3% and 13% said they would use none.
- The most common form of confirmatory testing was , followed by potassium hydroxide preparation, trichoscopy, and Wood’s lamp.
- More than half of survey respondents would alter their choice of oral medication based on culture results, but most would not change their topical medication preference.
IN PRACTICE:
“The management of tinea capitis in the United States is currently variable, particularly with the introduction of newer antifungals,” the authors wrote. “Future steps involve establishing evidence-based clinical practice guidelines that consider drug efficacy, safety profiles, and costs.”
SOURCE:
Bernard Cohen, MD, of the Departments of Pediatrics and Dermatology at Johns Hopkins University, Baltimore, Maryland, led the research, which was published in Pediatric Dermatology.
LIMITATIONS:
Lower response rates associated with online surveys and predefined age groups restrict the granularity of responses.
DISCLOSURES:
The authors reported having no financial disclosures.
A version of this article appeared on Medscape.com.
Virus and Booster Apathy Could Be Fueling Long COVID
A celebrity makeup artist, the 55-year-old New Yorker had been boosted and vaccinated at every opportunity since vaccines were approved at the end of 2020, until the fall of 2023, when she skipped the shot.
“I really started subscribing to the mindset that you have an immune system and your immune system is supposed to work for you,” she said. “That was the stupidest thing I’ve ever done.”
Maio was not the only person to skip the latest booster: A recent study reported that while nearly 80% of adults in the United States said they’d received their first series of vaccines, barely 20% were up to date on boosters. Nor was Maio alone in getting long COVID 4 years after the start of the deadliest pandemic in a century.
It’s tempting, this far out from the shutdowns of 2020, to think the virus is over, that we’re immune, and nobody’s going to get sick anymore. But while fewer people are getting COVID, it is still very much a part of our lives. And as Maio and others are learning the hard way, long COVID is, too — and it can be deadly.
For those who have recently contracted long COVID, it can feel as if the whole world has moved on from the pandemic, and they are being left behind.
Too Easy to Let Our Guard Down
“It’s really difficult to prevent exposure to COVID no matter how careful you are and no matter how many times you are vaccinated,” said Akiko Iwasaki, an immunology professor at Yale School of Medicine, New Haven, Connecticut, and pioneer in long COVID research. Iwasaki was quick to point out that “we should never blame anybody for getting long COVID because there is no bulletproof way of preventing long COVID from happening” — although research shows you can increase your protection through vaccination, masking, and increasing ventilation indoors.
Also, just because you didn’t get long COVID after catching the virus once, doesn’t mean you’ll dodge the bullet if you get sick again, as Maio has now learned twice. She had long COVID in 2022 after her second bout with the virus, with breathing problems and brain fog that lasted for several months.
Subsequent long COVID experiences won’t necessarily mimic previous ones. Although Maio developed brain fog again, this time she didn’t have the breathing problems that plagued her in 2022. Instead, she had headaches so excruciating she thought she was dying of a brain aneurysm.
A Journal of the American Medical Association study released in May identified the 37 most common symptoms of long COVID, including symptom subgroups that occurred in 80% of the nearly 10,000 study participants. But the symptoms that patients with long COVID are experiencing now are slightly different from earlier in the pandemic or at least that’s what doctors are finding at the Post-COVID Recovery Clinic affiliated with the University of Pittsburgh Medical Center.
Michael Risbano, MD, the clinic’s codirector, said fewer patients have pulmonary or lung damage now than in the past, but a steady stream report problems with brain fog, forgetfulness, exercise intolerance (shortness of breath and fatigue with exercise and difficulty performing any kind of exertional activity), and post-exertional malaise (feeling wiped out or fatigued for hours or even days after physical or mental activity).
Long COVID Treatments Showing Improvement — Slowly
“There still isn’t a great way to treat any of this,” said Risbano, whose clinic is involved with the National Institute of Health’s RECOVER-VITAL trial, which is evaluating potential treatments including Paxlovid and exercise to treat autonomic dysfunction with similarities to myalgic encephalomyelitis/chronic fatigue syndrome and POTS, exercise intolerance, and neurocognitive effects such as brain fog.
Risbano and colleagues have found that physical therapy and exercise training have helped patients with exercise intolerance and neurocognitive problems. “It’s not a quick thing where they go through one visit and are better the next day,” he stressed. “It takes a little bit of time, a little bit of effort, a little bit of homework — there are no silver bullets, no magic medications.”
A quick fix was definitely not in the cards for Dean Jones, PhD, who could barely move when he developed long COVID in May 2023. A 74-year-old biochemist and professor of medicine at Emory University in Atlanta, Georgia, he’d recovered fully the first time he had COVID, in August 2022, but had a completely different experience the second time. He had been vaccinated four times when he began experiencing chronic fatigue, intense exertion-induced migraines, severe airway congestion, brain fog, and shortness of breath. The symptoms began after Memorial Day and worsened over the next month.
His resting heart rate began racing even when he was sleeping, jumping from 53 to 70 beats per minute. “It was almost as though the virus had hit my heart rather than the lungs alone,” he said.
Doctors prescribed multiple inhalers and glucocorticoids to calm Jones’s immune system. The worst symptoms began to abate after a few weeks. The bad ones continued for fully 2 months, severely limiting Jones’s activity. Although he no longer slept all day, just walking from one room to another was exhausting. A dedicated scientist who typically worked 10-15 hours a day before getting sick, he was lucky to focus on work-related tasks for a fraction of that time.
Although the migraines went away early on, the headaches remained until well into the fall. Jones’s energy level gradually returned, and by Christmas, he was beginning to feel as healthy as he had before getting COVID in May.
Still, he’s not complaining that it took so long to get better. “At 74, there’s a lot of colleagues who have already passed away,” he said. “I respect the realities of my age. There are so many people who died from COVID that I’m thankful I had those vaccines. I’m thankful that I pulled through it and was able to rebound.”
Time Helps Healing — But Prompt Care Still Needed
Recovery is the case for most patients with long COVID, said Lisa Sanders, MD, medical director of the Yale New Haven Health Systems Long COVID Consultation Clinic, which opened in March 2023. Although the clinic has a small segment of patients who have had the condition since 2020, “people who recover, who are most people, move on,” she said. “Even the patients who sometimes have to wait a month or so to see me, some of them say, ‘I’m already starting to get better. I wasn’t sure I should come.’”
Maio, too, is recovering but only after multiple visits to the emergency room and a neurologist in late December and early January. The third emergency room trip was prompted after a brief episode in which she lost the feeling in her legs, which began convulsing. A CAT scan showed severely constricted blood vessels in her brain, leading the medical team to speculate she might have reversible cerebral vasoconstriction syndrome (RCVS), which can trigger the thunderclap headaches that had been causing her such misery.
After her third such headache prompted a fourth emergency room visit, further tests confirmed RCVS, which doctors said was related to inflammation caused by COVID. Maio was then admitted to the hospital, where she spent 4 days starting on a regimen of blood pressure medication, magnesium for the headaches, and oxycodone for pain management.
The TV show Maio works on went back into production after the holidays. She went back at the end of January. She’s still having headaches, though they’re less intense, and she’s still taking medication. She was scheduled for another test to look at her blood vessels in February.
Maio has yet to forgive herself for skipping the last booster, even though there’s no guarantee it would have prevented her from getting sick. Her message for others: it’s better to be safe than to be as sorry as she is.
“I’ll never, ever be persuaded by people who don’t believe in vaccines because I believe in science, and I believe in vaccines — that’s why people don’t die at the age of 30 anymore,” she said. “I really think that people need to know about this and what to expect. Because it is horrendous. It is very painful. I would never want anyone to go through this. Ever.”
A version of this article appeared on Medscape.com.
A celebrity makeup artist, the 55-year-old New Yorker had been boosted and vaccinated at every opportunity since vaccines were approved at the end of 2020, until the fall of 2023, when she skipped the shot.
“I really started subscribing to the mindset that you have an immune system and your immune system is supposed to work for you,” she said. “That was the stupidest thing I’ve ever done.”
Maio was not the only person to skip the latest booster: A recent study reported that while nearly 80% of adults in the United States said they’d received their first series of vaccines, barely 20% were up to date on boosters. Nor was Maio alone in getting long COVID 4 years after the start of the deadliest pandemic in a century.
It’s tempting, this far out from the shutdowns of 2020, to think the virus is over, that we’re immune, and nobody’s going to get sick anymore. But while fewer people are getting COVID, it is still very much a part of our lives. And as Maio and others are learning the hard way, long COVID is, too — and it can be deadly.
For those who have recently contracted long COVID, it can feel as if the whole world has moved on from the pandemic, and they are being left behind.
Too Easy to Let Our Guard Down
“It’s really difficult to prevent exposure to COVID no matter how careful you are and no matter how many times you are vaccinated,” said Akiko Iwasaki, an immunology professor at Yale School of Medicine, New Haven, Connecticut, and pioneer in long COVID research. Iwasaki was quick to point out that “we should never blame anybody for getting long COVID because there is no bulletproof way of preventing long COVID from happening” — although research shows you can increase your protection through vaccination, masking, and increasing ventilation indoors.
Also, just because you didn’t get long COVID after catching the virus once, doesn’t mean you’ll dodge the bullet if you get sick again, as Maio has now learned twice. She had long COVID in 2022 after her second bout with the virus, with breathing problems and brain fog that lasted for several months.
Subsequent long COVID experiences won’t necessarily mimic previous ones. Although Maio developed brain fog again, this time she didn’t have the breathing problems that plagued her in 2022. Instead, she had headaches so excruciating she thought she was dying of a brain aneurysm.
A Journal of the American Medical Association study released in May identified the 37 most common symptoms of long COVID, including symptom subgroups that occurred in 80% of the nearly 10,000 study participants. But the symptoms that patients with long COVID are experiencing now are slightly different from earlier in the pandemic or at least that’s what doctors are finding at the Post-COVID Recovery Clinic affiliated with the University of Pittsburgh Medical Center.
Michael Risbano, MD, the clinic’s codirector, said fewer patients have pulmonary or lung damage now than in the past, but a steady stream report problems with brain fog, forgetfulness, exercise intolerance (shortness of breath and fatigue with exercise and difficulty performing any kind of exertional activity), and post-exertional malaise (feeling wiped out or fatigued for hours or even days after physical or mental activity).
Long COVID Treatments Showing Improvement — Slowly
“There still isn’t a great way to treat any of this,” said Risbano, whose clinic is involved with the National Institute of Health’s RECOVER-VITAL trial, which is evaluating potential treatments including Paxlovid and exercise to treat autonomic dysfunction with similarities to myalgic encephalomyelitis/chronic fatigue syndrome and POTS, exercise intolerance, and neurocognitive effects such as brain fog.
Risbano and colleagues have found that physical therapy and exercise training have helped patients with exercise intolerance and neurocognitive problems. “It’s not a quick thing where they go through one visit and are better the next day,” he stressed. “It takes a little bit of time, a little bit of effort, a little bit of homework — there are no silver bullets, no magic medications.”
A quick fix was definitely not in the cards for Dean Jones, PhD, who could barely move when he developed long COVID in May 2023. A 74-year-old biochemist and professor of medicine at Emory University in Atlanta, Georgia, he’d recovered fully the first time he had COVID, in August 2022, but had a completely different experience the second time. He had been vaccinated four times when he began experiencing chronic fatigue, intense exertion-induced migraines, severe airway congestion, brain fog, and shortness of breath. The symptoms began after Memorial Day and worsened over the next month.
His resting heart rate began racing even when he was sleeping, jumping from 53 to 70 beats per minute. “It was almost as though the virus had hit my heart rather than the lungs alone,” he said.
Doctors prescribed multiple inhalers and glucocorticoids to calm Jones’s immune system. The worst symptoms began to abate after a few weeks. The bad ones continued for fully 2 months, severely limiting Jones’s activity. Although he no longer slept all day, just walking from one room to another was exhausting. A dedicated scientist who typically worked 10-15 hours a day before getting sick, he was lucky to focus on work-related tasks for a fraction of that time.
Although the migraines went away early on, the headaches remained until well into the fall. Jones’s energy level gradually returned, and by Christmas, he was beginning to feel as healthy as he had before getting COVID in May.
Still, he’s not complaining that it took so long to get better. “At 74, there’s a lot of colleagues who have already passed away,” he said. “I respect the realities of my age. There are so many people who died from COVID that I’m thankful I had those vaccines. I’m thankful that I pulled through it and was able to rebound.”
Time Helps Healing — But Prompt Care Still Needed
Recovery is the case for most patients with long COVID, said Lisa Sanders, MD, medical director of the Yale New Haven Health Systems Long COVID Consultation Clinic, which opened in March 2023. Although the clinic has a small segment of patients who have had the condition since 2020, “people who recover, who are most people, move on,” she said. “Even the patients who sometimes have to wait a month or so to see me, some of them say, ‘I’m already starting to get better. I wasn’t sure I should come.’”
Maio, too, is recovering but only after multiple visits to the emergency room and a neurologist in late December and early January. The third emergency room trip was prompted after a brief episode in which she lost the feeling in her legs, which began convulsing. A CAT scan showed severely constricted blood vessels in her brain, leading the medical team to speculate she might have reversible cerebral vasoconstriction syndrome (RCVS), which can trigger the thunderclap headaches that had been causing her such misery.
After her third such headache prompted a fourth emergency room visit, further tests confirmed RCVS, which doctors said was related to inflammation caused by COVID. Maio was then admitted to the hospital, where she spent 4 days starting on a regimen of blood pressure medication, magnesium for the headaches, and oxycodone for pain management.
The TV show Maio works on went back into production after the holidays. She went back at the end of January. She’s still having headaches, though they’re less intense, and she’s still taking medication. She was scheduled for another test to look at her blood vessels in February.
Maio has yet to forgive herself for skipping the last booster, even though there’s no guarantee it would have prevented her from getting sick. Her message for others: it’s better to be safe than to be as sorry as she is.
“I’ll never, ever be persuaded by people who don’t believe in vaccines because I believe in science, and I believe in vaccines — that’s why people don’t die at the age of 30 anymore,” she said. “I really think that people need to know about this and what to expect. Because it is horrendous. It is very painful. I would never want anyone to go through this. Ever.”
A version of this article appeared on Medscape.com.
A celebrity makeup artist, the 55-year-old New Yorker had been boosted and vaccinated at every opportunity since vaccines were approved at the end of 2020, until the fall of 2023, when she skipped the shot.
“I really started subscribing to the mindset that you have an immune system and your immune system is supposed to work for you,” she said. “That was the stupidest thing I’ve ever done.”
Maio was not the only person to skip the latest booster: A recent study reported that while nearly 80% of adults in the United States said they’d received their first series of vaccines, barely 20% were up to date on boosters. Nor was Maio alone in getting long COVID 4 years after the start of the deadliest pandemic in a century.
It’s tempting, this far out from the shutdowns of 2020, to think the virus is over, that we’re immune, and nobody’s going to get sick anymore. But while fewer people are getting COVID, it is still very much a part of our lives. And as Maio and others are learning the hard way, long COVID is, too — and it can be deadly.
For those who have recently contracted long COVID, it can feel as if the whole world has moved on from the pandemic, and they are being left behind.
Too Easy to Let Our Guard Down
“It’s really difficult to prevent exposure to COVID no matter how careful you are and no matter how many times you are vaccinated,” said Akiko Iwasaki, an immunology professor at Yale School of Medicine, New Haven, Connecticut, and pioneer in long COVID research. Iwasaki was quick to point out that “we should never blame anybody for getting long COVID because there is no bulletproof way of preventing long COVID from happening” — although research shows you can increase your protection through vaccination, masking, and increasing ventilation indoors.
Also, just because you didn’t get long COVID after catching the virus once, doesn’t mean you’ll dodge the bullet if you get sick again, as Maio has now learned twice. She had long COVID in 2022 after her second bout with the virus, with breathing problems and brain fog that lasted for several months.
Subsequent long COVID experiences won’t necessarily mimic previous ones. Although Maio developed brain fog again, this time she didn’t have the breathing problems that plagued her in 2022. Instead, she had headaches so excruciating she thought she was dying of a brain aneurysm.
A Journal of the American Medical Association study released in May identified the 37 most common symptoms of long COVID, including symptom subgroups that occurred in 80% of the nearly 10,000 study participants. But the symptoms that patients with long COVID are experiencing now are slightly different from earlier in the pandemic or at least that’s what doctors are finding at the Post-COVID Recovery Clinic affiliated with the University of Pittsburgh Medical Center.
Michael Risbano, MD, the clinic’s codirector, said fewer patients have pulmonary or lung damage now than in the past, but a steady stream report problems with brain fog, forgetfulness, exercise intolerance (shortness of breath and fatigue with exercise and difficulty performing any kind of exertional activity), and post-exertional malaise (feeling wiped out or fatigued for hours or even days after physical or mental activity).
Long COVID Treatments Showing Improvement — Slowly
“There still isn’t a great way to treat any of this,” said Risbano, whose clinic is involved with the National Institute of Health’s RECOVER-VITAL trial, which is evaluating potential treatments including Paxlovid and exercise to treat autonomic dysfunction with similarities to myalgic encephalomyelitis/chronic fatigue syndrome and POTS, exercise intolerance, and neurocognitive effects such as brain fog.
Risbano and colleagues have found that physical therapy and exercise training have helped patients with exercise intolerance and neurocognitive problems. “It’s not a quick thing where they go through one visit and are better the next day,” he stressed. “It takes a little bit of time, a little bit of effort, a little bit of homework — there are no silver bullets, no magic medications.”
A quick fix was definitely not in the cards for Dean Jones, PhD, who could barely move when he developed long COVID in May 2023. A 74-year-old biochemist and professor of medicine at Emory University in Atlanta, Georgia, he’d recovered fully the first time he had COVID, in August 2022, but had a completely different experience the second time. He had been vaccinated four times when he began experiencing chronic fatigue, intense exertion-induced migraines, severe airway congestion, brain fog, and shortness of breath. The symptoms began after Memorial Day and worsened over the next month.
His resting heart rate began racing even when he was sleeping, jumping from 53 to 70 beats per minute. “It was almost as though the virus had hit my heart rather than the lungs alone,” he said.
Doctors prescribed multiple inhalers and glucocorticoids to calm Jones’s immune system. The worst symptoms began to abate after a few weeks. The bad ones continued for fully 2 months, severely limiting Jones’s activity. Although he no longer slept all day, just walking from one room to another was exhausting. A dedicated scientist who typically worked 10-15 hours a day before getting sick, he was lucky to focus on work-related tasks for a fraction of that time.
Although the migraines went away early on, the headaches remained until well into the fall. Jones’s energy level gradually returned, and by Christmas, he was beginning to feel as healthy as he had before getting COVID in May.
Still, he’s not complaining that it took so long to get better. “At 74, there’s a lot of colleagues who have already passed away,” he said. “I respect the realities of my age. There are so many people who died from COVID that I’m thankful I had those vaccines. I’m thankful that I pulled through it and was able to rebound.”
Time Helps Healing — But Prompt Care Still Needed
Recovery is the case for most patients with long COVID, said Lisa Sanders, MD, medical director of the Yale New Haven Health Systems Long COVID Consultation Clinic, which opened in March 2023. Although the clinic has a small segment of patients who have had the condition since 2020, “people who recover, who are most people, move on,” she said. “Even the patients who sometimes have to wait a month or so to see me, some of them say, ‘I’m already starting to get better. I wasn’t sure I should come.’”
Maio, too, is recovering but only after multiple visits to the emergency room and a neurologist in late December and early January. The third emergency room trip was prompted after a brief episode in which she lost the feeling in her legs, which began convulsing. A CAT scan showed severely constricted blood vessels in her brain, leading the medical team to speculate she might have reversible cerebral vasoconstriction syndrome (RCVS), which can trigger the thunderclap headaches that had been causing her such misery.
After her third such headache prompted a fourth emergency room visit, further tests confirmed RCVS, which doctors said was related to inflammation caused by COVID. Maio was then admitted to the hospital, where she spent 4 days starting on a regimen of blood pressure medication, magnesium for the headaches, and oxycodone for pain management.
The TV show Maio works on went back into production after the holidays. She went back at the end of January. She’s still having headaches, though they’re less intense, and she’s still taking medication. She was scheduled for another test to look at her blood vessels in February.
Maio has yet to forgive herself for skipping the last booster, even though there’s no guarantee it would have prevented her from getting sick. Her message for others: it’s better to be safe than to be as sorry as she is.
“I’ll never, ever be persuaded by people who don’t believe in vaccines because I believe in science, and I believe in vaccines — that’s why people don’t die at the age of 30 anymore,” she said. “I really think that people need to know about this and what to expect. Because it is horrendous. It is very painful. I would never want anyone to go through this. Ever.”
A version of this article appeared on Medscape.com.
Plant-Based Diet a Boon for Men With Prostate Cancer
, new research showed.
The findings, published on February 13, 2024, in the journal Cancer, bolster previous research showing plant-based diets can reduce the risk for recurrence and improve survivorship in men with prostate cancer.
“The current study shows for the first time an association between eating more plant-based food with better scores for quality of life among patients diagnosed with prostate cancer,” Stacy Loeb, MD, a urologist in the departments of Urology and Population Health at NYU Langone Health, in New York City, who led the research.
For the new study, Dr. Loeb and her colleagues looked at data from more than 3500 men with prostate cancer in the Health Professionals Follow-Up Study, an ongoing investigation begun in 1986 and sponsored by Harvard T.H. Chan School of Public Health. The dataset included more than 50,000 male dentists, pharmacists, optometrists, osteopaths, podiatrists, and veterinarians.
The median age of prostate cancer diagnosis was 68 years; 48% of patients underwent radical prostatectomy and 35% had radiation as primary therapy. None of the patients were known to have had metastatic disease.
Men in the study answered a questionnaire every 4 years about the kinds of foods they ate and in what proportions. Another survey, administered every 2 years, assessed the frequency of incontinence, difficulties maintaining an erection, and problems with bowels, energy, and mood, among many other health concerns.
Dr. Loeb and her colleagues sorted patients into quintiles based on the proportion of plant vs animal foods the men said they eat. The authors found those who consumed the most plant-based foods scored 8%-11% better in measures of sexual function than the group that consumed the least of these products.
These men also reported up to 14% better scores for urinary health, with fewer instances of incontinence, obstruction, and irritation, and up to 13% better scores in hormonal health, marked by symptoms like low energy, depression, and hot flashes.
Justin Gregg, MD, a urology researcher at the University of Texas MD Anderson Cancer Center, in Houston, Texas, whose research has found the Mediterranean diet can slow tumor progression among men with localized prostate cancer on active surveillance, called the results “not entirely surprising, as prior studies have shown associations between plant-based diet and outcomes like erectile function among men who do not have prostate cancer.”
But Kenneth Jacobsohn, MD, professor of urology and director of lifestyle medicine at the Medical College of Wisconsin, in Milwaukee, said the new findings help establish “the positive role of diet quality and plant-based diets, specifically on quality of life after prostate cancer diagnosis and treatment for men with nonmetastatic prostate cancer.”
Dr. Jacobsohn said the study was limited by its retrospective nature and the manner of the dietary assessment.
“As the authors point out, a plant-based diet may be helpful, though it’s important to keep in mind the strong data for its protective effect in terms of cardiovascular disease risk, which is very important for men who have a history of prostate cancer as many will die of cardiovascular disease,” Dr. Gregg added.
Dr. Loeb, Dr. Gregg, and Dr. Jacobsohn reported no conflicts of interest. Some of the study authors reported a variety of potential conflicts.
A version of this article appeared on Medscape.com .
, new research showed.
The findings, published on February 13, 2024, in the journal Cancer, bolster previous research showing plant-based diets can reduce the risk for recurrence and improve survivorship in men with prostate cancer.
“The current study shows for the first time an association between eating more plant-based food with better scores for quality of life among patients diagnosed with prostate cancer,” Stacy Loeb, MD, a urologist in the departments of Urology and Population Health at NYU Langone Health, in New York City, who led the research.
For the new study, Dr. Loeb and her colleagues looked at data from more than 3500 men with prostate cancer in the Health Professionals Follow-Up Study, an ongoing investigation begun in 1986 and sponsored by Harvard T.H. Chan School of Public Health. The dataset included more than 50,000 male dentists, pharmacists, optometrists, osteopaths, podiatrists, and veterinarians.
The median age of prostate cancer diagnosis was 68 years; 48% of patients underwent radical prostatectomy and 35% had radiation as primary therapy. None of the patients were known to have had metastatic disease.
Men in the study answered a questionnaire every 4 years about the kinds of foods they ate and in what proportions. Another survey, administered every 2 years, assessed the frequency of incontinence, difficulties maintaining an erection, and problems with bowels, energy, and mood, among many other health concerns.
Dr. Loeb and her colleagues sorted patients into quintiles based on the proportion of plant vs animal foods the men said they eat. The authors found those who consumed the most plant-based foods scored 8%-11% better in measures of sexual function than the group that consumed the least of these products.
These men also reported up to 14% better scores for urinary health, with fewer instances of incontinence, obstruction, and irritation, and up to 13% better scores in hormonal health, marked by symptoms like low energy, depression, and hot flashes.
Justin Gregg, MD, a urology researcher at the University of Texas MD Anderson Cancer Center, in Houston, Texas, whose research has found the Mediterranean diet can slow tumor progression among men with localized prostate cancer on active surveillance, called the results “not entirely surprising, as prior studies have shown associations between plant-based diet and outcomes like erectile function among men who do not have prostate cancer.”
But Kenneth Jacobsohn, MD, professor of urology and director of lifestyle medicine at the Medical College of Wisconsin, in Milwaukee, said the new findings help establish “the positive role of diet quality and plant-based diets, specifically on quality of life after prostate cancer diagnosis and treatment for men with nonmetastatic prostate cancer.”
Dr. Jacobsohn said the study was limited by its retrospective nature and the manner of the dietary assessment.
“As the authors point out, a plant-based diet may be helpful, though it’s important to keep in mind the strong data for its protective effect in terms of cardiovascular disease risk, which is very important for men who have a history of prostate cancer as many will die of cardiovascular disease,” Dr. Gregg added.
Dr. Loeb, Dr. Gregg, and Dr. Jacobsohn reported no conflicts of interest. Some of the study authors reported a variety of potential conflicts.
A version of this article appeared on Medscape.com .
, new research showed.
The findings, published on February 13, 2024, in the journal Cancer, bolster previous research showing plant-based diets can reduce the risk for recurrence and improve survivorship in men with prostate cancer.
“The current study shows for the first time an association between eating more plant-based food with better scores for quality of life among patients diagnosed with prostate cancer,” Stacy Loeb, MD, a urologist in the departments of Urology and Population Health at NYU Langone Health, in New York City, who led the research.
For the new study, Dr. Loeb and her colleagues looked at data from more than 3500 men with prostate cancer in the Health Professionals Follow-Up Study, an ongoing investigation begun in 1986 and sponsored by Harvard T.H. Chan School of Public Health. The dataset included more than 50,000 male dentists, pharmacists, optometrists, osteopaths, podiatrists, and veterinarians.
The median age of prostate cancer diagnosis was 68 years; 48% of patients underwent radical prostatectomy and 35% had radiation as primary therapy. None of the patients were known to have had metastatic disease.
Men in the study answered a questionnaire every 4 years about the kinds of foods they ate and in what proportions. Another survey, administered every 2 years, assessed the frequency of incontinence, difficulties maintaining an erection, and problems with bowels, energy, and mood, among many other health concerns.
Dr. Loeb and her colleagues sorted patients into quintiles based on the proportion of plant vs animal foods the men said they eat. The authors found those who consumed the most plant-based foods scored 8%-11% better in measures of sexual function than the group that consumed the least of these products.
These men also reported up to 14% better scores for urinary health, with fewer instances of incontinence, obstruction, and irritation, and up to 13% better scores in hormonal health, marked by symptoms like low energy, depression, and hot flashes.
Justin Gregg, MD, a urology researcher at the University of Texas MD Anderson Cancer Center, in Houston, Texas, whose research has found the Mediterranean diet can slow tumor progression among men with localized prostate cancer on active surveillance, called the results “not entirely surprising, as prior studies have shown associations between plant-based diet and outcomes like erectile function among men who do not have prostate cancer.”
But Kenneth Jacobsohn, MD, professor of urology and director of lifestyle medicine at the Medical College of Wisconsin, in Milwaukee, said the new findings help establish “the positive role of diet quality and plant-based diets, specifically on quality of life after prostate cancer diagnosis and treatment for men with nonmetastatic prostate cancer.”
Dr. Jacobsohn said the study was limited by its retrospective nature and the manner of the dietary assessment.
“As the authors point out, a plant-based diet may be helpful, though it’s important to keep in mind the strong data for its protective effect in terms of cardiovascular disease risk, which is very important for men who have a history of prostate cancer as many will die of cardiovascular disease,” Dr. Gregg added.
Dr. Loeb, Dr. Gregg, and Dr. Jacobsohn reported no conflicts of interest. Some of the study authors reported a variety of potential conflicts.
A version of this article appeared on Medscape.com .
Exercising With Osteoarthritis: Five Things to Know
It’s no secret that regular exercise is important. But for patients with painful joints, it can be the last thing they want to do. Exercise is one of the cornerstones of managing arthritis, yet nearly one third of patients with arthritis are inactive.
This news organization recently spoke to experts on what resources are available, how much exercise is ideal, and how to motivate patients to move more.
What Are the Benefits of Exercise in Osteoarthritis?
Nearly all professional societies agree that exercise is one of the hallmarks of managing osteoarthritis (OA). According to two Cochrane reviews, there is high-equality evidence that exercise can help reduce pain as well as improve physical function in both hip and knee OA. In fact, physical activity can decrease pain and improve function by 40% in adults with arthritis, according to the Centers for Disease Control and Prevention.
Exercise also plays a large role in preventing disability by improving joint range of motion as well as maintaining muscle mass that supports joints.
There is also preliminary evidence that exercise could have a structural benefit to osteoarthritic joints. In a study of about 1200 individuals with knee OA, those who walked for exercise not only had reduced frequent knee pain, compared with non-walkers, but also were 20% less likely to have worsening of medial joint space narrowing.
Beyond symptom and impairment improvements, exercise can also play a role in staving off other chronic diseases linked to OA, such as cardiovascular disease and type 2 diabetes. Physical activity and exercise “are effective in preventing at least 35 chronic conditions and treating at least 26 chronic conditions, with one of the potential working mechanisms being exercise-induced anti-inflammatory effects,” wrote the authors of a commentary in the Journal of Orthopaedic & Sports Physical Therapy.
The known mental health benefits of exercise can also be beneficial for patients, as rates of depression and anxiety can be higher in people with arthritis than in the general population.
What Is the Ideal Amount of Exercise for Patients?
Current guidelines recommend that adults should get 150 minutes of moderate physical activity each week. But for patients with chronic pain, that may seem unachievable, Kelli Allen, PhD, professor of medicine and exercise physiologist at the University of North Carolina School of Medicine in Chapel Hill, said during a presentation at the American College of Rheumatology 2023 annual meeting in San Diego. Promisingly, research has shown that some exercise is better than none.
One study looking at over 1500 adults with lower extremity joint symptoms suggested that approximately 1 hour of physical activity per week increased the likelihood that participants remained disability-free over 4 years. In another analysis looking at 280 studies, researchers concluded that resistance training programs lasting 3-6 months resulted in moderate improvements in pain and physical function, but these benefits did not depend on exercise volume or participant adherence.
“These findings highlight the flexibility available for clinicians in the prescription of resistance exercise for knee and hip OA without compromising improvements in pain and physical function,” the authors wrote.
Step counts can be another way to measure activity, with 10,000 steps being a common target. But fewer steps a day can also yield health benefits. One study found that among nearly 1800 participants with knee OA, each additional 1000 steps per day was associated with a 16%-18% reduced risk of developing functional limitations 2 years later. Walking 6000 steps a day was the threshold that best determined who would develop functional limitations and who would not.
“I think it’s really a helpful message to encourage people with chronic pain that if you can get to 6000, maybe that’s a good goal,” Dr. Allen said.
Going for a 20-minute walk three times a week can be a good place to start, said Grace H. Lo, MD, associate professor in the Division of Immunology, Allergy, and Rheumatology at Baylor College of Medicine in Houston, Texas. For people who currently do not do any activity, Dr. Lo recommends starting small, like walking to get the mail every day. “Do something practical that is something they can sustain and keep in their daily activities that will help to increase their function and hopefully lessen some of their symptoms.”
Are Certain Types of Exercise More Beneficial?
There is no specific type of exercise that is best for OA, so it comes down to patient preference. The best exercise is “whatever somebody is actually going to do,” Dr. Allen noted.
Una Makris, MD, associate professor of internal medicine in the Division of Rheumatic Disease at the University of Texas Southwestern Medical Center and rheumatologist at the North Texas VA Health Care System in Dallas, Texas, said that her practice focuses on a combination of aerobic activity, functional balance, and strength training, as recommended by the World Health Organization.
“It’s not clear to me that one type of exercise is better than another; it’s more about what does this patient enjoy, and how can we make this a routine, so it is a sustainable behavior,” she told this news organization.
Generally, lower-impact exercises like biking, walking, or swimming tend to be better for OA, Dr. Lo added. Several studies have also shown tai chi to be beneficial in patients with OA, she said. More recently, Dr. Lo has conducted research on gardening as an exercise intervention for OA.
“It’s a great way to encourage people to exercise,” she said in an interview. “Besides the fact that they’re physically active, they can also be outside. There are a lot of mental health benefits to doing gardening as well.”
Dr. Allen added that certain exercises should be considered on the basis of an individual’s goals and physical needs. If someone has balance issues, for example, then yoga or tai chi could be useful to add to their exercise program, she said.
What Resources Are Available?
The Osteoarthritis Action Alliance has a list of 23 evidence-based exercise programs that have been shown to improve arthritis symptoms. These arthritis-appropriate, evidence-based interventions vary from instructor-led, in-person sessions to self-directed programs.
Walk with Ease (or Camine Con Gusto in Spanish) is one popular program, noted Dr. Allen. The program can be in-person or self-directed, with a required booklet that costs $11.95. However, there are discounted books for community-based organizations. The My Knee Exercise program, created by the University of Melbourne, Australia, provides a free, self-directed, 6-month strengthening program. The availability and cost of other programs are dependent on the format and location, the guide noted.
But understanding what programs are available in certain communities takes time, which can be a barrier to clinician referrals, noted Katie Huffman, director of education and outreach at OA Action Alliance.
“We would love to see these programs being covered by payers and health plans so that there’s incentive for providers to refer and patients to participate in the programs,” she noted.
While some states do cover a limited number of programs under Medicaid, coverage across states and payers is not yet universal.
In addition to these programs, the alliance has a simple guide to help plan workouts based on current activity level. The guide links to free exercises from CreakyJoints, an online community for people with arthritis, and the Arthritis Foundation.
Dr. Lo noted that the Veterans Affairs program, “VA Whole Health,” has free resources that are available to anyone. The provided videos offer tai chi, chair exercises, and guided meditations.
“It’s thoughtful to people who have some limitations in their physical activity,” she said.
Because the program is online, it could be difficult to access for those who are not comfortable with electronics, she said, “but if you can find a way to pass that, I think that this is an amazing resource,” she said.
How Do You Motivate Patients to Move?
“When it comes to motivation, I don’t think there is a one-size-fits-all approach,” said Dr. Makris. She tries to identify what matters most for each patient as a starting point. “When they can identify something in their day-to-day life that they value, then I like to link a physical activity-based goal to that,” she said. Setting physical activity goals using the mnemonic SMART (Specific, Measurable, Attainable, Realistic, and Timely) can be useful, she advised.
The OA Action Alliance also provides additional tools for clinicians on how to counsel patients on behavior change.
Understanding the patient’s lifestyle is also crucial when discussing physical activity, Dr. Lo added. “You have to give them practical solutions that they can actually incorporate into their lives,” she said.
Discussions around physical activity should be an ongoing part of clinic visits, both Dr. Lo and Dr. Makris agreed, to celebrate achievements and revise goals.
“I’m kind of notorious for being really slow in clinic because I just let people talk,” Dr. Lo said. “I do feel like these extra moments, when you spend time talking about these things, allow your recommendations to be more customized for the patients” and make the biggest impact.
Dr. Allen, Dr. Lo, and Dr. Makris reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
It’s no secret that regular exercise is important. But for patients with painful joints, it can be the last thing they want to do. Exercise is one of the cornerstones of managing arthritis, yet nearly one third of patients with arthritis are inactive.
This news organization recently spoke to experts on what resources are available, how much exercise is ideal, and how to motivate patients to move more.
What Are the Benefits of Exercise in Osteoarthritis?
Nearly all professional societies agree that exercise is one of the hallmarks of managing osteoarthritis (OA). According to two Cochrane reviews, there is high-equality evidence that exercise can help reduce pain as well as improve physical function in both hip and knee OA. In fact, physical activity can decrease pain and improve function by 40% in adults with arthritis, according to the Centers for Disease Control and Prevention.
Exercise also plays a large role in preventing disability by improving joint range of motion as well as maintaining muscle mass that supports joints.
There is also preliminary evidence that exercise could have a structural benefit to osteoarthritic joints. In a study of about 1200 individuals with knee OA, those who walked for exercise not only had reduced frequent knee pain, compared with non-walkers, but also were 20% less likely to have worsening of medial joint space narrowing.
Beyond symptom and impairment improvements, exercise can also play a role in staving off other chronic diseases linked to OA, such as cardiovascular disease and type 2 diabetes. Physical activity and exercise “are effective in preventing at least 35 chronic conditions and treating at least 26 chronic conditions, with one of the potential working mechanisms being exercise-induced anti-inflammatory effects,” wrote the authors of a commentary in the Journal of Orthopaedic & Sports Physical Therapy.
The known mental health benefits of exercise can also be beneficial for patients, as rates of depression and anxiety can be higher in people with arthritis than in the general population.
What Is the Ideal Amount of Exercise for Patients?
Current guidelines recommend that adults should get 150 minutes of moderate physical activity each week. But for patients with chronic pain, that may seem unachievable, Kelli Allen, PhD, professor of medicine and exercise physiologist at the University of North Carolina School of Medicine in Chapel Hill, said during a presentation at the American College of Rheumatology 2023 annual meeting in San Diego. Promisingly, research has shown that some exercise is better than none.
One study looking at over 1500 adults with lower extremity joint symptoms suggested that approximately 1 hour of physical activity per week increased the likelihood that participants remained disability-free over 4 years. In another analysis looking at 280 studies, researchers concluded that resistance training programs lasting 3-6 months resulted in moderate improvements in pain and physical function, but these benefits did not depend on exercise volume or participant adherence.
“These findings highlight the flexibility available for clinicians in the prescription of resistance exercise for knee and hip OA without compromising improvements in pain and physical function,” the authors wrote.
Step counts can be another way to measure activity, with 10,000 steps being a common target. But fewer steps a day can also yield health benefits. One study found that among nearly 1800 participants with knee OA, each additional 1000 steps per day was associated with a 16%-18% reduced risk of developing functional limitations 2 years later. Walking 6000 steps a day was the threshold that best determined who would develop functional limitations and who would not.
“I think it’s really a helpful message to encourage people with chronic pain that if you can get to 6000, maybe that’s a good goal,” Dr. Allen said.
Going for a 20-minute walk three times a week can be a good place to start, said Grace H. Lo, MD, associate professor in the Division of Immunology, Allergy, and Rheumatology at Baylor College of Medicine in Houston, Texas. For people who currently do not do any activity, Dr. Lo recommends starting small, like walking to get the mail every day. “Do something practical that is something they can sustain and keep in their daily activities that will help to increase their function and hopefully lessen some of their symptoms.”
Are Certain Types of Exercise More Beneficial?
There is no specific type of exercise that is best for OA, so it comes down to patient preference. The best exercise is “whatever somebody is actually going to do,” Dr. Allen noted.
Una Makris, MD, associate professor of internal medicine in the Division of Rheumatic Disease at the University of Texas Southwestern Medical Center and rheumatologist at the North Texas VA Health Care System in Dallas, Texas, said that her practice focuses on a combination of aerobic activity, functional balance, and strength training, as recommended by the World Health Organization.
“It’s not clear to me that one type of exercise is better than another; it’s more about what does this patient enjoy, and how can we make this a routine, so it is a sustainable behavior,” she told this news organization.
Generally, lower-impact exercises like biking, walking, or swimming tend to be better for OA, Dr. Lo added. Several studies have also shown tai chi to be beneficial in patients with OA, she said. More recently, Dr. Lo has conducted research on gardening as an exercise intervention for OA.
“It’s a great way to encourage people to exercise,” she said in an interview. “Besides the fact that they’re physically active, they can also be outside. There are a lot of mental health benefits to doing gardening as well.”
Dr. Allen added that certain exercises should be considered on the basis of an individual’s goals and physical needs. If someone has balance issues, for example, then yoga or tai chi could be useful to add to their exercise program, she said.
What Resources Are Available?
The Osteoarthritis Action Alliance has a list of 23 evidence-based exercise programs that have been shown to improve arthritis symptoms. These arthritis-appropriate, evidence-based interventions vary from instructor-led, in-person sessions to self-directed programs.
Walk with Ease (or Camine Con Gusto in Spanish) is one popular program, noted Dr. Allen. The program can be in-person or self-directed, with a required booklet that costs $11.95. However, there are discounted books for community-based organizations. The My Knee Exercise program, created by the University of Melbourne, Australia, provides a free, self-directed, 6-month strengthening program. The availability and cost of other programs are dependent on the format and location, the guide noted.
But understanding what programs are available in certain communities takes time, which can be a barrier to clinician referrals, noted Katie Huffman, director of education and outreach at OA Action Alliance.
“We would love to see these programs being covered by payers and health plans so that there’s incentive for providers to refer and patients to participate in the programs,” she noted.
While some states do cover a limited number of programs under Medicaid, coverage across states and payers is not yet universal.
In addition to these programs, the alliance has a simple guide to help plan workouts based on current activity level. The guide links to free exercises from CreakyJoints, an online community for people with arthritis, and the Arthritis Foundation.
Dr. Lo noted that the Veterans Affairs program, “VA Whole Health,” has free resources that are available to anyone. The provided videos offer tai chi, chair exercises, and guided meditations.
“It’s thoughtful to people who have some limitations in their physical activity,” she said.
Because the program is online, it could be difficult to access for those who are not comfortable with electronics, she said, “but if you can find a way to pass that, I think that this is an amazing resource,” she said.
How Do You Motivate Patients to Move?
“When it comes to motivation, I don’t think there is a one-size-fits-all approach,” said Dr. Makris. She tries to identify what matters most for each patient as a starting point. “When they can identify something in their day-to-day life that they value, then I like to link a physical activity-based goal to that,” she said. Setting physical activity goals using the mnemonic SMART (Specific, Measurable, Attainable, Realistic, and Timely) can be useful, she advised.
The OA Action Alliance also provides additional tools for clinicians on how to counsel patients on behavior change.
Understanding the patient’s lifestyle is also crucial when discussing physical activity, Dr. Lo added. “You have to give them practical solutions that they can actually incorporate into their lives,” she said.
Discussions around physical activity should be an ongoing part of clinic visits, both Dr. Lo and Dr. Makris agreed, to celebrate achievements and revise goals.
“I’m kind of notorious for being really slow in clinic because I just let people talk,” Dr. Lo said. “I do feel like these extra moments, when you spend time talking about these things, allow your recommendations to be more customized for the patients” and make the biggest impact.
Dr. Allen, Dr. Lo, and Dr. Makris reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
It’s no secret that regular exercise is important. But for patients with painful joints, it can be the last thing they want to do. Exercise is one of the cornerstones of managing arthritis, yet nearly one third of patients with arthritis are inactive.
This news organization recently spoke to experts on what resources are available, how much exercise is ideal, and how to motivate patients to move more.
What Are the Benefits of Exercise in Osteoarthritis?
Nearly all professional societies agree that exercise is one of the hallmarks of managing osteoarthritis (OA). According to two Cochrane reviews, there is high-equality evidence that exercise can help reduce pain as well as improve physical function in both hip and knee OA. In fact, physical activity can decrease pain and improve function by 40% in adults with arthritis, according to the Centers for Disease Control and Prevention.
Exercise also plays a large role in preventing disability by improving joint range of motion as well as maintaining muscle mass that supports joints.
There is also preliminary evidence that exercise could have a structural benefit to osteoarthritic joints. In a study of about 1200 individuals with knee OA, those who walked for exercise not only had reduced frequent knee pain, compared with non-walkers, but also were 20% less likely to have worsening of medial joint space narrowing.
Beyond symptom and impairment improvements, exercise can also play a role in staving off other chronic diseases linked to OA, such as cardiovascular disease and type 2 diabetes. Physical activity and exercise “are effective in preventing at least 35 chronic conditions and treating at least 26 chronic conditions, with one of the potential working mechanisms being exercise-induced anti-inflammatory effects,” wrote the authors of a commentary in the Journal of Orthopaedic & Sports Physical Therapy.
The known mental health benefits of exercise can also be beneficial for patients, as rates of depression and anxiety can be higher in people with arthritis than in the general population.
What Is the Ideal Amount of Exercise for Patients?
Current guidelines recommend that adults should get 150 minutes of moderate physical activity each week. But for patients with chronic pain, that may seem unachievable, Kelli Allen, PhD, professor of medicine and exercise physiologist at the University of North Carolina School of Medicine in Chapel Hill, said during a presentation at the American College of Rheumatology 2023 annual meeting in San Diego. Promisingly, research has shown that some exercise is better than none.
One study looking at over 1500 adults with lower extremity joint symptoms suggested that approximately 1 hour of physical activity per week increased the likelihood that participants remained disability-free over 4 years. In another analysis looking at 280 studies, researchers concluded that resistance training programs lasting 3-6 months resulted in moderate improvements in pain and physical function, but these benefits did not depend on exercise volume or participant adherence.
“These findings highlight the flexibility available for clinicians in the prescription of resistance exercise for knee and hip OA without compromising improvements in pain and physical function,” the authors wrote.
Step counts can be another way to measure activity, with 10,000 steps being a common target. But fewer steps a day can also yield health benefits. One study found that among nearly 1800 participants with knee OA, each additional 1000 steps per day was associated with a 16%-18% reduced risk of developing functional limitations 2 years later. Walking 6000 steps a day was the threshold that best determined who would develop functional limitations and who would not.
“I think it’s really a helpful message to encourage people with chronic pain that if you can get to 6000, maybe that’s a good goal,” Dr. Allen said.
Going for a 20-minute walk three times a week can be a good place to start, said Grace H. Lo, MD, associate professor in the Division of Immunology, Allergy, and Rheumatology at Baylor College of Medicine in Houston, Texas. For people who currently do not do any activity, Dr. Lo recommends starting small, like walking to get the mail every day. “Do something practical that is something they can sustain and keep in their daily activities that will help to increase their function and hopefully lessen some of their symptoms.”
Are Certain Types of Exercise More Beneficial?
There is no specific type of exercise that is best for OA, so it comes down to patient preference. The best exercise is “whatever somebody is actually going to do,” Dr. Allen noted.
Una Makris, MD, associate professor of internal medicine in the Division of Rheumatic Disease at the University of Texas Southwestern Medical Center and rheumatologist at the North Texas VA Health Care System in Dallas, Texas, said that her practice focuses on a combination of aerobic activity, functional balance, and strength training, as recommended by the World Health Organization.
“It’s not clear to me that one type of exercise is better than another; it’s more about what does this patient enjoy, and how can we make this a routine, so it is a sustainable behavior,” she told this news organization.
Generally, lower-impact exercises like biking, walking, or swimming tend to be better for OA, Dr. Lo added. Several studies have also shown tai chi to be beneficial in patients with OA, she said. More recently, Dr. Lo has conducted research on gardening as an exercise intervention for OA.
“It’s a great way to encourage people to exercise,” she said in an interview. “Besides the fact that they’re physically active, they can also be outside. There are a lot of mental health benefits to doing gardening as well.”
Dr. Allen added that certain exercises should be considered on the basis of an individual’s goals and physical needs. If someone has balance issues, for example, then yoga or tai chi could be useful to add to their exercise program, she said.
What Resources Are Available?
The Osteoarthritis Action Alliance has a list of 23 evidence-based exercise programs that have been shown to improve arthritis symptoms. These arthritis-appropriate, evidence-based interventions vary from instructor-led, in-person sessions to self-directed programs.
Walk with Ease (or Camine Con Gusto in Spanish) is one popular program, noted Dr. Allen. The program can be in-person or self-directed, with a required booklet that costs $11.95. However, there are discounted books for community-based organizations. The My Knee Exercise program, created by the University of Melbourne, Australia, provides a free, self-directed, 6-month strengthening program. The availability and cost of other programs are dependent on the format and location, the guide noted.
But understanding what programs are available in certain communities takes time, which can be a barrier to clinician referrals, noted Katie Huffman, director of education and outreach at OA Action Alliance.
“We would love to see these programs being covered by payers and health plans so that there’s incentive for providers to refer and patients to participate in the programs,” she noted.
While some states do cover a limited number of programs under Medicaid, coverage across states and payers is not yet universal.
In addition to these programs, the alliance has a simple guide to help plan workouts based on current activity level. The guide links to free exercises from CreakyJoints, an online community for people with arthritis, and the Arthritis Foundation.
Dr. Lo noted that the Veterans Affairs program, “VA Whole Health,” has free resources that are available to anyone. The provided videos offer tai chi, chair exercises, and guided meditations.
“It’s thoughtful to people who have some limitations in their physical activity,” she said.
Because the program is online, it could be difficult to access for those who are not comfortable with electronics, she said, “but if you can find a way to pass that, I think that this is an amazing resource,” she said.
How Do You Motivate Patients to Move?
“When it comes to motivation, I don’t think there is a one-size-fits-all approach,” said Dr. Makris. She tries to identify what matters most for each patient as a starting point. “When they can identify something in their day-to-day life that they value, then I like to link a physical activity-based goal to that,” she said. Setting physical activity goals using the mnemonic SMART (Specific, Measurable, Attainable, Realistic, and Timely) can be useful, she advised.
The OA Action Alliance also provides additional tools for clinicians on how to counsel patients on behavior change.
Understanding the patient’s lifestyle is also crucial when discussing physical activity, Dr. Lo added. “You have to give them practical solutions that they can actually incorporate into their lives,” she said.
Discussions around physical activity should be an ongoing part of clinic visits, both Dr. Lo and Dr. Makris agreed, to celebrate achievements and revise goals.
“I’m kind of notorious for being really slow in clinic because I just let people talk,” Dr. Lo said. “I do feel like these extra moments, when you spend time talking about these things, allow your recommendations to be more customized for the patients” and make the biggest impact.
Dr. Allen, Dr. Lo, and Dr. Makris reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
CAR T-Cell: Do Benefits Still Outweigh Risks?
Importantly, most specialists agree, so far the risk appears no greater than the known risk of secondary primary malignancies that is well established with other cancer therapies.
“The data that we have so far suggest that the risk of secondary T-cell lymphoma in patients treated with CAR T-cells is similar to [that] of patients treated with other cancer therapies, [including] chemotherapy, radiation, transplantation,” Marco Ruella, MD, said in an interview. He reported on a case of a T-cell lymphoma occurring following CAR-T therapy at the University of Pennsylvania.
While his team is still investigating the development of such malignancies, “the FDA notice does not change our clinical practice and patients should be reassured that the benefit of CAR-T therapy significantly outweighs the potential risk of secondary malignancies including T-cell lymphoma,” said Dr. Ruella, scientific director of the Lymphoma Program, Division of Hematology and Oncology and Center for Cellular Immunotherapies, at the University of Pennsylvania, Philadelphia.
FDA: 28 Reports of Malignancies; 3 with Evidence of ‘Likely’ CAR T Involvement
Concerns were raised last November when the FDA announced in a safety communication that it was investigating the “serious risk of T-cell malignancy” following B-cell maturation antigen (BCMA)-directed or CD19-directed CAR T-cell immunotherapies, citing reports from clinical trials and/or postmarketing adverse event data sources. Subsequently, in January, the FDA called for the boxed warning on all approved BCMA- and CD19-targeted genetically modified autologous T-cell immunotherapies, which include: Abecma (idecabtagene vicleucel); Breyanzi (lisocabtagene maraleucel); Carvykti (ciltacabtagene autoleucel); Kymriah (tisagenlecleucel); Tecartus (brexucabtagene autoleucel); and Yescarta (axicabtagene ciloleucel).
“Although the overall benefits of these products continue to outweigh their potential risks for their approved uses, the FDA continues to investigate the identified risk of T-cell malignancy with serious outcomes, including hospitalization and death,” the FDA reported in discussing the safety warnings.
The cases were detailed in a report from FDA researchers published in the New England Journal of Medicine, noting that as of December 31, 2023, the FDA had become aware of 22 cases of T-cell cancers occurring following CAR T-cell treatment, including T-cell lymphoma, T-cell large granular lymphocytosis, peripheral T-cell lymphoma, and cutaneous T-cell lymphoma.
Report coauthor Peter Marks, MD, PhD, of the FDA’s Center for Biologics Evaluation and Research in Silver Spring, Maryland, said in an interview that since the publication of their report, six new cases have emerged.
“As reported in the NEJM Perspective, there were 22 cases of T-cell malignancy after treatment with CAR T-cell immunotherapies as of December 31, 2023, but we have received additional reports and, as of February 9, 2024, FDA has now received 28 reports,” he said. “Note that as new cases are being reported, there will be updates to the total number of cases under ongoing review by FDA.”
The initial 22 cases all occurred relatively soon after treatment. Of 14 cases with sufficient data, all developed within 2 years of the CAR-T therapy, ranging from 1 to 19 months, with about half occurring in the first year after administration.
The cases involved five of the six FDA-approved CAR-T products, with the numbers too low to suggest an association with any particular product.
In three of the cases, the lymphoma was found in genetic testing to contain the CAR construction, “indicating that the CAR-T product was most likely involved in the development of the T-cell cancer,” according to the FDA researchers.
With inadequate genetic sampling in most of the remaining 19 cases, the association is less clear, however “the timing of several of the cases makes association a possibility,” Dr. Marks said. In their report, Dr. Marks and colleagues added that “determination of whether the T-cell cancer is associated with the CAR construct ... most likely won’t be possible for every case reported to date.”
Even if all the reported cases are assumed to be related to CAR-T treatment, the numbers still represent a very small proportion of the more than 27,000 doses of the six CAR-T therapies approved in the United States, the authors noted, but they cautioned that the numbers could indeed be higher than reported.
“Relying on postmarketing reporting may lead to underestimates of such cases,” they said.
Life-Long Monitoring Recommended
In response to the reports, the FDA is urging that clinicians’ monitoring of patients treated with CAR-T therapy should be lifelong.
“Patients and clinical trial participants receiving treatment with these products should be monitored lifelong for new malignancies,” Dr. Marks said.
“In the event that a new malignancy occurs following treatment with these products, contact the manufacturer to report the event and obtain instructions on collection of patient samples for testing for the presence of the CAR transgene.”
In addition, cases should be reported to the FDA, either by calling or through the FDA’s medical product safety reporting program.
T-Cell Malignancy Case Report
In describing the case at their medical center in the report in Nature Medicine, Dr. Ruella and colleagues said a T-cell lymphoma occurred in a patient with non-Hodgkin B-cell lymphoma 3 months after an anti-CD19 CAR T-cell treatment.
As a result, the team conducted a subsequent analysis of 449 patients treated with CAR-T therapy at the University of Pennsylvania center, and with a median follow-up of 10.3 months, 16 patients (3.6%) had developed a secondary primary malignancy, with a median onset time of 26.4 months for solid and 9.7 months for hematological malignancies.
The patient who had developed a T-cell lymphoma tested negative for CAR integration upon diagnosis, and regarding the other cancers, Dr. Ruella noted that “we have no indication that the secondary malignancies are directly caused by the CAR-T therapy.
“We have many patients with a very long follow-up beyond 5 and even 10 years,” he said. “In these patients, we don’t see an increased risk of T-cell lymphoma.”
‘Cautious Reassurance’ Urged in Discussion with Patients
With alarming headlines on the findings suggesting that CAR-T therapy may cause cancer, Rahul Banerjee, MD, and colleagues at the University of Washington, Seattle, recommend the use of “cautious reassurance” in discussing the issue with patients. In a paper published in January in Blood Advances, they suggest a three-part response: underscoring that the benefits of CAR T “far outweigh” the risks in relapsed/refractory malignancies, that the ‘one-and-done’ nature of CAR-T infusions provide meaningful improvements in quality of life, and that the active cancer at hand is “a much larger threat than a hypothetical cancer years later.”
In many cases, patients may only have months to live without CAR-T therapy and will have already had multiple prior lines of therapy, therefore the CAR-T treatment itself may provide time for the secondary primary cancers from any of the treatments to emerge, as experts have noted.
“One has to be alive to be diagnosed with a secondary primary malignancy, and it’s thus very possible that CAR-T may be creating a type of ‘immortal time bias’ wherein patients live long enough to experience the unfortunate sequelae of their previous therapies,” Dr. Banerjee explained in an interview.
Nevertheless, the potential for substantial improvements in quality of life with CAR-T therapy compared with traditional treatments addresses a top priority for patients, he added.
“For most patients with [for instance], myeloma, the ability of CAR-T to put them rapidly into a deep remission without the need for maintenance is an unheard-of potential for them,” Dr. Banerjee said.
“In multiple myeloma, no CAR-T therapy has (yet) demonstrated an overall survival benefit — but I think the substantial quality-of-life benefit stands by itself as a big reason why patients continue to prefer CAR-T.”
Keep Patients In Touch with CAR T Centers
In light of the concerns regarding the secondary malignancies, Dr. Banerjee underscored that CAR-T patients should be kept in close touch with centers that have CAR-T treatment expertise.
With most patients followed primarily at community practices where CAR-T therapy is not administered, “I’d strongly encourage my colleagues in community practices to refer all eligible patients to a CAR-T-capable center for evaluation regardless of what their risk of post-CAR-T secondary primary malignancies may be,” Dr. Banerjee urged.
“Based on the evidence we have currently, which includes the FDA’s updated information, there are many more unknowns about this potential secondary primary malignancy risk than knowns,” he said. “This is of course a much more nuanced issue than any one package insert can convey, and CAR-T experts at treating centers can have these conversations at length with eligible patients who are nervous about these recent updates.”
Dr. Ruella disclosed that he holds patents related to CD19 CAR T cells, as well as relationships with NanoString, Bristol Myers Squibb, GlaxoSmithKline, Scailyte, Bayer, AbClon, Oxford NanoImaging, CURIOX, and Beckman Coulter, and he was the scientific founder of viTToria Biotherapeutics. Dr. Banerjee reported ties with BMS, Caribou Biosciences, Genentech, Janssen, Karyopharm, Pfizer, Sanofi, SparkCures, Novartis, and Pack Health.
Importantly, most specialists agree, so far the risk appears no greater than the known risk of secondary primary malignancies that is well established with other cancer therapies.
“The data that we have so far suggest that the risk of secondary T-cell lymphoma in patients treated with CAR T-cells is similar to [that] of patients treated with other cancer therapies, [including] chemotherapy, radiation, transplantation,” Marco Ruella, MD, said in an interview. He reported on a case of a T-cell lymphoma occurring following CAR-T therapy at the University of Pennsylvania.
While his team is still investigating the development of such malignancies, “the FDA notice does not change our clinical practice and patients should be reassured that the benefit of CAR-T therapy significantly outweighs the potential risk of secondary malignancies including T-cell lymphoma,” said Dr. Ruella, scientific director of the Lymphoma Program, Division of Hematology and Oncology and Center for Cellular Immunotherapies, at the University of Pennsylvania, Philadelphia.
FDA: 28 Reports of Malignancies; 3 with Evidence of ‘Likely’ CAR T Involvement
Concerns were raised last November when the FDA announced in a safety communication that it was investigating the “serious risk of T-cell malignancy” following B-cell maturation antigen (BCMA)-directed or CD19-directed CAR T-cell immunotherapies, citing reports from clinical trials and/or postmarketing adverse event data sources. Subsequently, in January, the FDA called for the boxed warning on all approved BCMA- and CD19-targeted genetically modified autologous T-cell immunotherapies, which include: Abecma (idecabtagene vicleucel); Breyanzi (lisocabtagene maraleucel); Carvykti (ciltacabtagene autoleucel); Kymriah (tisagenlecleucel); Tecartus (brexucabtagene autoleucel); and Yescarta (axicabtagene ciloleucel).
“Although the overall benefits of these products continue to outweigh their potential risks for their approved uses, the FDA continues to investigate the identified risk of T-cell malignancy with serious outcomes, including hospitalization and death,” the FDA reported in discussing the safety warnings.
The cases were detailed in a report from FDA researchers published in the New England Journal of Medicine, noting that as of December 31, 2023, the FDA had become aware of 22 cases of T-cell cancers occurring following CAR T-cell treatment, including T-cell lymphoma, T-cell large granular lymphocytosis, peripheral T-cell lymphoma, and cutaneous T-cell lymphoma.
Report coauthor Peter Marks, MD, PhD, of the FDA’s Center for Biologics Evaluation and Research in Silver Spring, Maryland, said in an interview that since the publication of their report, six new cases have emerged.
“As reported in the NEJM Perspective, there were 22 cases of T-cell malignancy after treatment with CAR T-cell immunotherapies as of December 31, 2023, but we have received additional reports and, as of February 9, 2024, FDA has now received 28 reports,” he said. “Note that as new cases are being reported, there will be updates to the total number of cases under ongoing review by FDA.”
The initial 22 cases all occurred relatively soon after treatment. Of 14 cases with sufficient data, all developed within 2 years of the CAR-T therapy, ranging from 1 to 19 months, with about half occurring in the first year after administration.
The cases involved five of the six FDA-approved CAR-T products, with the numbers too low to suggest an association with any particular product.
In three of the cases, the lymphoma was found in genetic testing to contain the CAR construction, “indicating that the CAR-T product was most likely involved in the development of the T-cell cancer,” according to the FDA researchers.
With inadequate genetic sampling in most of the remaining 19 cases, the association is less clear, however “the timing of several of the cases makes association a possibility,” Dr. Marks said. In their report, Dr. Marks and colleagues added that “determination of whether the T-cell cancer is associated with the CAR construct ... most likely won’t be possible for every case reported to date.”
Even if all the reported cases are assumed to be related to CAR-T treatment, the numbers still represent a very small proportion of the more than 27,000 doses of the six CAR-T therapies approved in the United States, the authors noted, but they cautioned that the numbers could indeed be higher than reported.
“Relying on postmarketing reporting may lead to underestimates of such cases,” they said.
Life-Long Monitoring Recommended
In response to the reports, the FDA is urging that clinicians’ monitoring of patients treated with CAR-T therapy should be lifelong.
“Patients and clinical trial participants receiving treatment with these products should be monitored lifelong for new malignancies,” Dr. Marks said.
“In the event that a new malignancy occurs following treatment with these products, contact the manufacturer to report the event and obtain instructions on collection of patient samples for testing for the presence of the CAR transgene.”
In addition, cases should be reported to the FDA, either by calling or through the FDA’s medical product safety reporting program.
T-Cell Malignancy Case Report
In describing the case at their medical center in the report in Nature Medicine, Dr. Ruella and colleagues said a T-cell lymphoma occurred in a patient with non-Hodgkin B-cell lymphoma 3 months after an anti-CD19 CAR T-cell treatment.
As a result, the team conducted a subsequent analysis of 449 patients treated with CAR-T therapy at the University of Pennsylvania center, and with a median follow-up of 10.3 months, 16 patients (3.6%) had developed a secondary primary malignancy, with a median onset time of 26.4 months for solid and 9.7 months for hematological malignancies.
The patient who had developed a T-cell lymphoma tested negative for CAR integration upon diagnosis, and regarding the other cancers, Dr. Ruella noted that “we have no indication that the secondary malignancies are directly caused by the CAR-T therapy.
“We have many patients with a very long follow-up beyond 5 and even 10 years,” he said. “In these patients, we don’t see an increased risk of T-cell lymphoma.”
‘Cautious Reassurance’ Urged in Discussion with Patients
With alarming headlines on the findings suggesting that CAR-T therapy may cause cancer, Rahul Banerjee, MD, and colleagues at the University of Washington, Seattle, recommend the use of “cautious reassurance” in discussing the issue with patients. In a paper published in January in Blood Advances, they suggest a three-part response: underscoring that the benefits of CAR T “far outweigh” the risks in relapsed/refractory malignancies, that the ‘one-and-done’ nature of CAR-T infusions provide meaningful improvements in quality of life, and that the active cancer at hand is “a much larger threat than a hypothetical cancer years later.”
In many cases, patients may only have months to live without CAR-T therapy and will have already had multiple prior lines of therapy, therefore the CAR-T treatment itself may provide time for the secondary primary cancers from any of the treatments to emerge, as experts have noted.
“One has to be alive to be diagnosed with a secondary primary malignancy, and it’s thus very possible that CAR-T may be creating a type of ‘immortal time bias’ wherein patients live long enough to experience the unfortunate sequelae of their previous therapies,” Dr. Banerjee explained in an interview.
Nevertheless, the potential for substantial improvements in quality of life with CAR-T therapy compared with traditional treatments addresses a top priority for patients, he added.
“For most patients with [for instance], myeloma, the ability of CAR-T to put them rapidly into a deep remission without the need for maintenance is an unheard-of potential for them,” Dr. Banerjee said.
“In multiple myeloma, no CAR-T therapy has (yet) demonstrated an overall survival benefit — but I think the substantial quality-of-life benefit stands by itself as a big reason why patients continue to prefer CAR-T.”
Keep Patients In Touch with CAR T Centers
In light of the concerns regarding the secondary malignancies, Dr. Banerjee underscored that CAR-T patients should be kept in close touch with centers that have CAR-T treatment expertise.
With most patients followed primarily at community practices where CAR-T therapy is not administered, “I’d strongly encourage my colleagues in community practices to refer all eligible patients to a CAR-T-capable center for evaluation regardless of what their risk of post-CAR-T secondary primary malignancies may be,” Dr. Banerjee urged.
“Based on the evidence we have currently, which includes the FDA’s updated information, there are many more unknowns about this potential secondary primary malignancy risk than knowns,” he said. “This is of course a much more nuanced issue than any one package insert can convey, and CAR-T experts at treating centers can have these conversations at length with eligible patients who are nervous about these recent updates.”
Dr. Ruella disclosed that he holds patents related to CD19 CAR T cells, as well as relationships with NanoString, Bristol Myers Squibb, GlaxoSmithKline, Scailyte, Bayer, AbClon, Oxford NanoImaging, CURIOX, and Beckman Coulter, and he was the scientific founder of viTToria Biotherapeutics. Dr. Banerjee reported ties with BMS, Caribou Biosciences, Genentech, Janssen, Karyopharm, Pfizer, Sanofi, SparkCures, Novartis, and Pack Health.
Importantly, most specialists agree, so far the risk appears no greater than the known risk of secondary primary malignancies that is well established with other cancer therapies.
“The data that we have so far suggest that the risk of secondary T-cell lymphoma in patients treated with CAR T-cells is similar to [that] of patients treated with other cancer therapies, [including] chemotherapy, radiation, transplantation,” Marco Ruella, MD, said in an interview. He reported on a case of a T-cell lymphoma occurring following CAR-T therapy at the University of Pennsylvania.
While his team is still investigating the development of such malignancies, “the FDA notice does not change our clinical practice and patients should be reassured that the benefit of CAR-T therapy significantly outweighs the potential risk of secondary malignancies including T-cell lymphoma,” said Dr. Ruella, scientific director of the Lymphoma Program, Division of Hematology and Oncology and Center for Cellular Immunotherapies, at the University of Pennsylvania, Philadelphia.
FDA: 28 Reports of Malignancies; 3 with Evidence of ‘Likely’ CAR T Involvement
Concerns were raised last November when the FDA announced in a safety communication that it was investigating the “serious risk of T-cell malignancy” following B-cell maturation antigen (BCMA)-directed or CD19-directed CAR T-cell immunotherapies, citing reports from clinical trials and/or postmarketing adverse event data sources. Subsequently, in January, the FDA called for the boxed warning on all approved BCMA- and CD19-targeted genetically modified autologous T-cell immunotherapies, which include: Abecma (idecabtagene vicleucel); Breyanzi (lisocabtagene maraleucel); Carvykti (ciltacabtagene autoleucel); Kymriah (tisagenlecleucel); Tecartus (brexucabtagene autoleucel); and Yescarta (axicabtagene ciloleucel).
“Although the overall benefits of these products continue to outweigh their potential risks for their approved uses, the FDA continues to investigate the identified risk of T-cell malignancy with serious outcomes, including hospitalization and death,” the FDA reported in discussing the safety warnings.
The cases were detailed in a report from FDA researchers published in the New England Journal of Medicine, noting that as of December 31, 2023, the FDA had become aware of 22 cases of T-cell cancers occurring following CAR T-cell treatment, including T-cell lymphoma, T-cell large granular lymphocytosis, peripheral T-cell lymphoma, and cutaneous T-cell lymphoma.
Report coauthor Peter Marks, MD, PhD, of the FDA’s Center for Biologics Evaluation and Research in Silver Spring, Maryland, said in an interview that since the publication of their report, six new cases have emerged.
“As reported in the NEJM Perspective, there were 22 cases of T-cell malignancy after treatment with CAR T-cell immunotherapies as of December 31, 2023, but we have received additional reports and, as of February 9, 2024, FDA has now received 28 reports,” he said. “Note that as new cases are being reported, there will be updates to the total number of cases under ongoing review by FDA.”
The initial 22 cases all occurred relatively soon after treatment. Of 14 cases with sufficient data, all developed within 2 years of the CAR-T therapy, ranging from 1 to 19 months, with about half occurring in the first year after administration.
The cases involved five of the six FDA-approved CAR-T products, with the numbers too low to suggest an association with any particular product.
In three of the cases, the lymphoma was found in genetic testing to contain the CAR construction, “indicating that the CAR-T product was most likely involved in the development of the T-cell cancer,” according to the FDA researchers.
With inadequate genetic sampling in most of the remaining 19 cases, the association is less clear, however “the timing of several of the cases makes association a possibility,” Dr. Marks said. In their report, Dr. Marks and colleagues added that “determination of whether the T-cell cancer is associated with the CAR construct ... most likely won’t be possible for every case reported to date.”
Even if all the reported cases are assumed to be related to CAR-T treatment, the numbers still represent a very small proportion of the more than 27,000 doses of the six CAR-T therapies approved in the United States, the authors noted, but they cautioned that the numbers could indeed be higher than reported.
“Relying on postmarketing reporting may lead to underestimates of such cases,” they said.
Life-Long Monitoring Recommended
In response to the reports, the FDA is urging that clinicians’ monitoring of patients treated with CAR-T therapy should be lifelong.
“Patients and clinical trial participants receiving treatment with these products should be monitored lifelong for new malignancies,” Dr. Marks said.
“In the event that a new malignancy occurs following treatment with these products, contact the manufacturer to report the event and obtain instructions on collection of patient samples for testing for the presence of the CAR transgene.”
In addition, cases should be reported to the FDA, either by calling or through the FDA’s medical product safety reporting program.
T-Cell Malignancy Case Report
In describing the case at their medical center in the report in Nature Medicine, Dr. Ruella and colleagues said a T-cell lymphoma occurred in a patient with non-Hodgkin B-cell lymphoma 3 months after an anti-CD19 CAR T-cell treatment.
As a result, the team conducted a subsequent analysis of 449 patients treated with CAR-T therapy at the University of Pennsylvania center, and with a median follow-up of 10.3 months, 16 patients (3.6%) had developed a secondary primary malignancy, with a median onset time of 26.4 months for solid and 9.7 months for hematological malignancies.
The patient who had developed a T-cell lymphoma tested negative for CAR integration upon diagnosis, and regarding the other cancers, Dr. Ruella noted that “we have no indication that the secondary malignancies are directly caused by the CAR-T therapy.
“We have many patients with a very long follow-up beyond 5 and even 10 years,” he said. “In these patients, we don’t see an increased risk of T-cell lymphoma.”
‘Cautious Reassurance’ Urged in Discussion with Patients
With alarming headlines on the findings suggesting that CAR-T therapy may cause cancer, Rahul Banerjee, MD, and colleagues at the University of Washington, Seattle, recommend the use of “cautious reassurance” in discussing the issue with patients. In a paper published in January in Blood Advances, they suggest a three-part response: underscoring that the benefits of CAR T “far outweigh” the risks in relapsed/refractory malignancies, that the ‘one-and-done’ nature of CAR-T infusions provide meaningful improvements in quality of life, and that the active cancer at hand is “a much larger threat than a hypothetical cancer years later.”
In many cases, patients may only have months to live without CAR-T therapy and will have already had multiple prior lines of therapy, therefore the CAR-T treatment itself may provide time for the secondary primary cancers from any of the treatments to emerge, as experts have noted.
“One has to be alive to be diagnosed with a secondary primary malignancy, and it’s thus very possible that CAR-T may be creating a type of ‘immortal time bias’ wherein patients live long enough to experience the unfortunate sequelae of their previous therapies,” Dr. Banerjee explained in an interview.
Nevertheless, the potential for substantial improvements in quality of life with CAR-T therapy compared with traditional treatments addresses a top priority for patients, he added.
“For most patients with [for instance], myeloma, the ability of CAR-T to put them rapidly into a deep remission without the need for maintenance is an unheard-of potential for them,” Dr. Banerjee said.
“In multiple myeloma, no CAR-T therapy has (yet) demonstrated an overall survival benefit — but I think the substantial quality-of-life benefit stands by itself as a big reason why patients continue to prefer CAR-T.”
Keep Patients In Touch with CAR T Centers
In light of the concerns regarding the secondary malignancies, Dr. Banerjee underscored that CAR-T patients should be kept in close touch with centers that have CAR-T treatment expertise.
With most patients followed primarily at community practices where CAR-T therapy is not administered, “I’d strongly encourage my colleagues in community practices to refer all eligible patients to a CAR-T-capable center for evaluation regardless of what their risk of post-CAR-T secondary primary malignancies may be,” Dr. Banerjee urged.
“Based on the evidence we have currently, which includes the FDA’s updated information, there are many more unknowns about this potential secondary primary malignancy risk than knowns,” he said. “This is of course a much more nuanced issue than any one package insert can convey, and CAR-T experts at treating centers can have these conversations at length with eligible patients who are nervous about these recent updates.”
Dr. Ruella disclosed that he holds patents related to CD19 CAR T cells, as well as relationships with NanoString, Bristol Myers Squibb, GlaxoSmithKline, Scailyte, Bayer, AbClon, Oxford NanoImaging, CURIOX, and Beckman Coulter, and he was the scientific founder of viTToria Biotherapeutics. Dr. Banerjee reported ties with BMS, Caribou Biosciences, Genentech, Janssen, Karyopharm, Pfizer, Sanofi, SparkCures, Novartis, and Pack Health.
Expert Hopes to Expand Ohio Model of Melanoma Case Reporting
SAN DIEGO – Soon after Brett M. Coldiron, MD, launched his Cincinnati-based dermatology and Mohs surgery practice more than 20 years ago, he reported his first three cases of thin melanomas to the Ohio Department of Health, as mandated by state law.
“I got sent reams of paperwork to fill out that I did not understand,” Dr. Coldiron, a past president of the American College of Mohs Surgery and the American Academy of Dermatology, recalled at the annual Cutaneous Malignancy Update. “Then, I got chewed out for not reporting sooner and threatened with thousands of dollars in fines if I did not promptly report the forms in the future. It was an obnoxious experience.”
About 15 years later, while testifying at the Ohio Legislature on medical reasons to restrict the use of tanning beds, a lobbyist for the tanning bed industry told him that the melanoma rates had been stable in Ohio for the previous 5 years. “It turns out they were cherry picking certain segments of data to fit their narrative,” Dr. Coldiron said. “I was stunned and it kind of deflated me. I thought about this for a long time, and thought, ‘how do we solve this issue of reporting melanoma cases without adding work to existing staff if you’re a small practice and without spending significant amounts of money? Let’s make this easier.’ ”
In addition to reducing the use of tanning beds, proper reporting of melanoma cases is important for reasons that include efforts to increase sunscreen use and to be counted in ongoing research efforts to obtain a realistic snapshot of melanoma prevalence and incidence, he said.
Quality of melanoma case reporting relies on the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR), and the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) Program, which collects data on the incidence, treatment, staging, and survival for 28% of the US population. All 50 states and US territories require melanoma to be reported to the NPCR, but while most hospital systems have reporting protocols and dedicated data registrars, private practices may not.
Also, many dermatopathology practices operate independently and do not have dedicated registrars and may not report cases. “Melanoma is unique in that it is often completely managed in outpatient settings and these melanomas may never be reported,” said Dr. Coldiron, current president of the Ohio Dermatological Foundation. “That’s the practice gap.” One study published in 2018 found that only 49% of dermatologists knew that melanoma was a reportable disease and only 34% routinely reported newly diagnosed cases to their state’s cancer registry. He characterized melanoma reporting as an unfunded mandate.
“Hospitals are doing the most of them, because they have a registrar,” he said. “Small practices have to assign someone to do this, and it can be difficult to train that person. It’s time consuming. The first time we did it, it took an hour,” but, he said, taking a 2-hour tutorial from the Ohio Department of Health helped.
He noted that there is a lack of awareness and clinicians think it’s the dermatopathologist’s job to report cases, “while the dermatopathologist thinks it’s the clinician’s job,” and many of the entry fields are not applicable to thinner melanomas.
There is also a “patchwork” of ways that state departments of health accept the information, not all electronically, he continued. For example, those in Arizona, Montana, West Virginia, Delaware, Vermont, and Maine accept paper copies only, “meaning you have to download a PDF, fill it out, and fax it back to them,” Dr. Coldiron said at the meeting, which was hosted by Scripps Cancer Center.
“We have them sign a HIPAA form and take the two-hour online tutorial,” he said. They download data that Ohio dermatologists have faxed to a dedicated secure HIPAA-compliant cloud-based fax line that Dr. Coldiron has set up, and the cases are then sent to the Ohio Department of Health.
Dr. Coldiron and colleagues have also partnered with the University of Cincinnati Clermont, which offers a National Cancer Registries Association–accredited certificate program — one of several nationwide. Students in this program are trained to become cancer registrars. “The university staff are gung-ho about it because they are looking for easy cases to train the students on. Also, the Ohio Department of Health staff are keen to help train the students and even help them find jobs or hire them after they complete the degree. Staff from the department of health and college faculty are fully engaged and supervising. It’s a win-win for all.”
According to Dr. Coldiron, in 2023, 8 Ohio dermatology practices were sending their reports to the fax line he set up and 7 more have signed up in recent months, making 15 practices to date. “It’s self-perpetuating at this point,” he said. “The Ohio Department of Health and the University of Cincinnati are invested in this program long-term.” The fax service costs Dr. Coldiron $42 per month — a small price to pay, he said, for being a clearinghouse for private Ohio dermatology practices looking for a practical way to report their melanoma cases. The model has increased melanoma reporting in Ohio by 2.8% in the last 2 years, “which doesn’t seem like that many, but if there are 6500 cases of melanoma, and you can increase reporting by a couple hundred cases, that’s a lot,” he said.
His goal is to expand this model to more states. “Dermatologists, surgical oncologists, and cancer center administrators should embrace this opportunity to make their practices a clearinghouse for their state,” he said. “This is an opportunity to improve state health, quality improvement projects, help providers, and gain recognition as a center of excellence. The increase in incidence of melanoma will lend great clout to public and legislative requests for prevention, treatment, and research dollars.”
In an interview, Hugh Greenway, MD, the head of Mohs and dermatologic surgery at Scripps Clinic in San Diego, also noted that cutaneous melanoma is significantly underreported in spite of individual state requirements. “As Dr. Coldiron reminds us, the main reason is that in many cases the pathology diagnosis and report come from the dermatologist’s/dermatopathologist’s office,” Dr. Greenway said. “With no hospital or large multispecialty laboratory involved, the reporting may be incomplete or not done. This is not the case with almost all other cancers where a hospital laboratory is involved.”
If widespread adoption of Dr. Coldiron’s model can occur, he added, “then we will have much better melanoma reporting data on which to both help our patients and specialty. He is to be applauded for producing a workable solution to the problem of underreporting.”
Dr. Coldiron reported having no relevant disclosures. Dr. Greenway reported that he conducts research for Castle Biosciences. He is also course director of the annual Cutaneous Malignancy Update.
SAN DIEGO – Soon after Brett M. Coldiron, MD, launched his Cincinnati-based dermatology and Mohs surgery practice more than 20 years ago, he reported his first three cases of thin melanomas to the Ohio Department of Health, as mandated by state law.
“I got sent reams of paperwork to fill out that I did not understand,” Dr. Coldiron, a past president of the American College of Mohs Surgery and the American Academy of Dermatology, recalled at the annual Cutaneous Malignancy Update. “Then, I got chewed out for not reporting sooner and threatened with thousands of dollars in fines if I did not promptly report the forms in the future. It was an obnoxious experience.”
About 15 years later, while testifying at the Ohio Legislature on medical reasons to restrict the use of tanning beds, a lobbyist for the tanning bed industry told him that the melanoma rates had been stable in Ohio for the previous 5 years. “It turns out they were cherry picking certain segments of data to fit their narrative,” Dr. Coldiron said. “I was stunned and it kind of deflated me. I thought about this for a long time, and thought, ‘how do we solve this issue of reporting melanoma cases without adding work to existing staff if you’re a small practice and without spending significant amounts of money? Let’s make this easier.’ ”
In addition to reducing the use of tanning beds, proper reporting of melanoma cases is important for reasons that include efforts to increase sunscreen use and to be counted in ongoing research efforts to obtain a realistic snapshot of melanoma prevalence and incidence, he said.
Quality of melanoma case reporting relies on the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR), and the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) Program, which collects data on the incidence, treatment, staging, and survival for 28% of the US population. All 50 states and US territories require melanoma to be reported to the NPCR, but while most hospital systems have reporting protocols and dedicated data registrars, private practices may not.
Also, many dermatopathology practices operate independently and do not have dedicated registrars and may not report cases. “Melanoma is unique in that it is often completely managed in outpatient settings and these melanomas may never be reported,” said Dr. Coldiron, current president of the Ohio Dermatological Foundation. “That’s the practice gap.” One study published in 2018 found that only 49% of dermatologists knew that melanoma was a reportable disease and only 34% routinely reported newly diagnosed cases to their state’s cancer registry. He characterized melanoma reporting as an unfunded mandate.
“Hospitals are doing the most of them, because they have a registrar,” he said. “Small practices have to assign someone to do this, and it can be difficult to train that person. It’s time consuming. The first time we did it, it took an hour,” but, he said, taking a 2-hour tutorial from the Ohio Department of Health helped.
He noted that there is a lack of awareness and clinicians think it’s the dermatopathologist’s job to report cases, “while the dermatopathologist thinks it’s the clinician’s job,” and many of the entry fields are not applicable to thinner melanomas.
There is also a “patchwork” of ways that state departments of health accept the information, not all electronically, he continued. For example, those in Arizona, Montana, West Virginia, Delaware, Vermont, and Maine accept paper copies only, “meaning you have to download a PDF, fill it out, and fax it back to them,” Dr. Coldiron said at the meeting, which was hosted by Scripps Cancer Center.
“We have them sign a HIPAA form and take the two-hour online tutorial,” he said. They download data that Ohio dermatologists have faxed to a dedicated secure HIPAA-compliant cloud-based fax line that Dr. Coldiron has set up, and the cases are then sent to the Ohio Department of Health.
Dr. Coldiron and colleagues have also partnered with the University of Cincinnati Clermont, which offers a National Cancer Registries Association–accredited certificate program — one of several nationwide. Students in this program are trained to become cancer registrars. “The university staff are gung-ho about it because they are looking for easy cases to train the students on. Also, the Ohio Department of Health staff are keen to help train the students and even help them find jobs or hire them after they complete the degree. Staff from the department of health and college faculty are fully engaged and supervising. It’s a win-win for all.”
According to Dr. Coldiron, in 2023, 8 Ohio dermatology practices were sending their reports to the fax line he set up and 7 more have signed up in recent months, making 15 practices to date. “It’s self-perpetuating at this point,” he said. “The Ohio Department of Health and the University of Cincinnati are invested in this program long-term.” The fax service costs Dr. Coldiron $42 per month — a small price to pay, he said, for being a clearinghouse for private Ohio dermatology practices looking for a practical way to report their melanoma cases. The model has increased melanoma reporting in Ohio by 2.8% in the last 2 years, “which doesn’t seem like that many, but if there are 6500 cases of melanoma, and you can increase reporting by a couple hundred cases, that’s a lot,” he said.
His goal is to expand this model to more states. “Dermatologists, surgical oncologists, and cancer center administrators should embrace this opportunity to make their practices a clearinghouse for their state,” he said. “This is an opportunity to improve state health, quality improvement projects, help providers, and gain recognition as a center of excellence. The increase in incidence of melanoma will lend great clout to public and legislative requests for prevention, treatment, and research dollars.”
In an interview, Hugh Greenway, MD, the head of Mohs and dermatologic surgery at Scripps Clinic in San Diego, also noted that cutaneous melanoma is significantly underreported in spite of individual state requirements. “As Dr. Coldiron reminds us, the main reason is that in many cases the pathology diagnosis and report come from the dermatologist’s/dermatopathologist’s office,” Dr. Greenway said. “With no hospital or large multispecialty laboratory involved, the reporting may be incomplete or not done. This is not the case with almost all other cancers where a hospital laboratory is involved.”
If widespread adoption of Dr. Coldiron’s model can occur, he added, “then we will have much better melanoma reporting data on which to both help our patients and specialty. He is to be applauded for producing a workable solution to the problem of underreporting.”
Dr. Coldiron reported having no relevant disclosures. Dr. Greenway reported that he conducts research for Castle Biosciences. He is also course director of the annual Cutaneous Malignancy Update.
SAN DIEGO – Soon after Brett M. Coldiron, MD, launched his Cincinnati-based dermatology and Mohs surgery practice more than 20 years ago, he reported his first three cases of thin melanomas to the Ohio Department of Health, as mandated by state law.
“I got sent reams of paperwork to fill out that I did not understand,” Dr. Coldiron, a past president of the American College of Mohs Surgery and the American Academy of Dermatology, recalled at the annual Cutaneous Malignancy Update. “Then, I got chewed out for not reporting sooner and threatened with thousands of dollars in fines if I did not promptly report the forms in the future. It was an obnoxious experience.”
About 15 years later, while testifying at the Ohio Legislature on medical reasons to restrict the use of tanning beds, a lobbyist for the tanning bed industry told him that the melanoma rates had been stable in Ohio for the previous 5 years. “It turns out they were cherry picking certain segments of data to fit their narrative,” Dr. Coldiron said. “I was stunned and it kind of deflated me. I thought about this for a long time, and thought, ‘how do we solve this issue of reporting melanoma cases without adding work to existing staff if you’re a small practice and without spending significant amounts of money? Let’s make this easier.’ ”
In addition to reducing the use of tanning beds, proper reporting of melanoma cases is important for reasons that include efforts to increase sunscreen use and to be counted in ongoing research efforts to obtain a realistic snapshot of melanoma prevalence and incidence, he said.
Quality of melanoma case reporting relies on the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR), and the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) Program, which collects data on the incidence, treatment, staging, and survival for 28% of the US population. All 50 states and US territories require melanoma to be reported to the NPCR, but while most hospital systems have reporting protocols and dedicated data registrars, private practices may not.
Also, many dermatopathology practices operate independently and do not have dedicated registrars and may not report cases. “Melanoma is unique in that it is often completely managed in outpatient settings and these melanomas may never be reported,” said Dr. Coldiron, current president of the Ohio Dermatological Foundation. “That’s the practice gap.” One study published in 2018 found that only 49% of dermatologists knew that melanoma was a reportable disease and only 34% routinely reported newly diagnosed cases to their state’s cancer registry. He characterized melanoma reporting as an unfunded mandate.
“Hospitals are doing the most of them, because they have a registrar,” he said. “Small practices have to assign someone to do this, and it can be difficult to train that person. It’s time consuming. The first time we did it, it took an hour,” but, he said, taking a 2-hour tutorial from the Ohio Department of Health helped.
He noted that there is a lack of awareness and clinicians think it’s the dermatopathologist’s job to report cases, “while the dermatopathologist thinks it’s the clinician’s job,” and many of the entry fields are not applicable to thinner melanomas.
There is also a “patchwork” of ways that state departments of health accept the information, not all electronically, he continued. For example, those in Arizona, Montana, West Virginia, Delaware, Vermont, and Maine accept paper copies only, “meaning you have to download a PDF, fill it out, and fax it back to them,” Dr. Coldiron said at the meeting, which was hosted by Scripps Cancer Center.
“We have them sign a HIPAA form and take the two-hour online tutorial,” he said. They download data that Ohio dermatologists have faxed to a dedicated secure HIPAA-compliant cloud-based fax line that Dr. Coldiron has set up, and the cases are then sent to the Ohio Department of Health.
Dr. Coldiron and colleagues have also partnered with the University of Cincinnati Clermont, which offers a National Cancer Registries Association–accredited certificate program — one of several nationwide. Students in this program are trained to become cancer registrars. “The university staff are gung-ho about it because they are looking for easy cases to train the students on. Also, the Ohio Department of Health staff are keen to help train the students and even help them find jobs or hire them after they complete the degree. Staff from the department of health and college faculty are fully engaged and supervising. It’s a win-win for all.”
According to Dr. Coldiron, in 2023, 8 Ohio dermatology practices were sending their reports to the fax line he set up and 7 more have signed up in recent months, making 15 practices to date. “It’s self-perpetuating at this point,” he said. “The Ohio Department of Health and the University of Cincinnati are invested in this program long-term.” The fax service costs Dr. Coldiron $42 per month — a small price to pay, he said, for being a clearinghouse for private Ohio dermatology practices looking for a practical way to report their melanoma cases. The model has increased melanoma reporting in Ohio by 2.8% in the last 2 years, “which doesn’t seem like that many, but if there are 6500 cases of melanoma, and you can increase reporting by a couple hundred cases, that’s a lot,” he said.
His goal is to expand this model to more states. “Dermatologists, surgical oncologists, and cancer center administrators should embrace this opportunity to make their practices a clearinghouse for their state,” he said. “This is an opportunity to improve state health, quality improvement projects, help providers, and gain recognition as a center of excellence. The increase in incidence of melanoma will lend great clout to public and legislative requests for prevention, treatment, and research dollars.”
In an interview, Hugh Greenway, MD, the head of Mohs and dermatologic surgery at Scripps Clinic in San Diego, also noted that cutaneous melanoma is significantly underreported in spite of individual state requirements. “As Dr. Coldiron reminds us, the main reason is that in many cases the pathology diagnosis and report come from the dermatologist’s/dermatopathologist’s office,” Dr. Greenway said. “With no hospital or large multispecialty laboratory involved, the reporting may be incomplete or not done. This is not the case with almost all other cancers where a hospital laboratory is involved.”
If widespread adoption of Dr. Coldiron’s model can occur, he added, “then we will have much better melanoma reporting data on which to both help our patients and specialty. He is to be applauded for producing a workable solution to the problem of underreporting.”
Dr. Coldiron reported having no relevant disclosures. Dr. Greenway reported that he conducts research for Castle Biosciences. He is also course director of the annual Cutaneous Malignancy Update.
FROM MELANOMA 2024
Genetic Biomarker May Predict Pancreatic Adenocarcinoma Outcomes
These were the main findings of a new study of more than 300 individuals.
Previous studies have shown an association between widespread disease and loss of SMAD4 immunolabeling, according to the paper. Biomarkers to predict which pancreatic adenocarcinoma patients may benefit from more aggressive therapy are lacking, wrote Emily J. Anstadt, MD, of the University of Pennsylvania, Philadelphia, and colleagues, in their paper published in Cancer.
The human transcription factor and tumor suppressor, mothers against decapentaplegic homolog 4 (SMAD4), “may be a promising biomarker for predicting the likelihood of experiencing distant failure in patients with pancreatic cancer,” the researchers wrote.
“For patients with pancreatic cancer, improving treatments and overall outcomes remains invaluable,” Dr. Anstadt said in an interview. However, the disparate clinical courses make studies of this patient population challenging.
“As with much of medicine and oncology at this time, we feel the key to better outcomes lies in personalizing treatment strategies and relying on tumor genetics to predict tumor behavior and guide us towards individualized optimal treatments,” she added.
Study Methods and Results
The researchers identified 322 patients with resected stage I–III pancreatic adenocarcinoma from The Cancer Genome Atlas (TCGA) and the International Cancer Genome Consortium (ICGC). The study population included 165 patients from the TCGA who served as the training set and 157 patients from the ICGC who served as the validation set.
The primary outcomes were overall survival (OS) and distant metastasis-free survival (DMFS).
A total of 50 patients in the TCGA group (30%) had at least one of the three identified SMAD4 genomic aberrations.
Using the TCGA group, the researchers conducted a regression analysis on the survival outcomes as a function of either the presence of an SMAD4 genomic aberration or the expression of messenger RNA sequencing (RNA-seq). They then used the ICGC to validate whether SMAD4 RNA-seq expression improved risk stratification for OS and DMFS in a separate group of patients.
In the TCGA group, 3-year OS for patients with any SMAD4 aberrations vs no SMAD4 aberrations was 18% vs 36% (hazard ratio, 1.55; P = .048). However, the 3-year DMFS for patients with and without SMAD4 aberrations was 14% vs 23%, a nonsignificant difference (HR, 1.33; P = .19).
In a multivariate analysis, SMAD4 aberrations also were associated with increased risk of stage III disease (HR, 1.89; P = .003). The researchers noted that adjuvant radiotherapy and adjuvant chemotherapy were significantly associated with a decreased risk of death in these patients (HR, 0.53 and HR, 0.28, respectively).
In addition, low SMAD4 RNA-seq expression was associated with worse OS and DMFS, (HR, 1.83 and HR, 1.70, respectively) in the TCGA group.
In the ICGC validation group, increased SMAD4 RNA‐seq expression correlated with improved OS (area under the curve .92) and DMFS (AUC, .84).
Dr. Anstadt said she and her colleagues were not surprised by any of their findings, given earlier research’s suggestions of SMAD4 loss having been associated with poor outcomes for pancreatic cancer.
“Prior studies determined SMAD4 status based on immunohistochemistry and different investigators used different scoring systems,” Dr. Anstadt noted, in an interview. “The results of those studies were conflicting, and consequently SMAD4 has not been adopted clinically as part of the work-up or to aid in treatment decisions.”
“It is essential to find robust, reliable, and cost-effective methods for implementing this in the clinic. As such, we were happy to find that expression of SMAD4 by mRNA sequencing may be that method,” she added.
Not Quite Clinic-Ready
“While we are hopeful that this tool will be a reliable method for use in the clinic, it has yet to be validated in a prospective manner,” Dr. Anstadt said in an interview. “In addition, this study showed that [genetic] expression levels are correlated with worse outcomes and can be of prognostic use; however, we have not directly studied whether expression levels can be predictive of treatment response,” she said.
“Practicing oncologists often have to make difficult decisions in situations where there are no clear answers,” Dr. Anstadt continued. “When considering gray-zone treatment recommendations, we often integrate multiple factors to form an opinion. The reality of cancer medicine is that not all those factors we consider have been validated in prospective studies, but together they produce a picture that is clinically useful. We would submit that SMAD4 status should be one of those factors taken into consideration in forming a comprehensive opinion about suitability for resection or radiotherapy.”
In practice, “if this test is prospectively validated in a future study and will impact clinical decision-making, then this cost will be similar to other genetic tests that have been adopted and have been practice-changing in other oncologic fields,” said Dr. Anstadt. “Being able to individualize treatment can also save overall cost; for instance, predicting which patients would not benefit from local radiation or surgery could decrease use and cost in that population,” she said.
Limitations of the current study included the inability to examine interactions between SMAD4 and radiotherapy because of the sample size and the potential for selection bias, the researchers wrote.
Potential Predictive Value
“A major challenge in the management of patients with pancreatic cancer is the difficulty in predicting which patients will develop metastasis early,” said Jatin Roper, MD, a gastroenterologist at Duke University, Durham, North Carolina, in an interview.
“SMAD4 has previously been evaluated as a prognostic marker in pancreatic cancer, but the association between SMAD4 gene expression, gene mutations, and cancer metastasis has not yet been systematically evaluated in patients, said Dr. Roper, who was not involved in the study.
The new study’s main findings that SMAD4 genomic alterations are associated with worse overall survival, but not distant metastasis-free survival, and that increased SMAD4 expression is associated with improved overall survival and distant metastasis-free survival, suggest that SMAD4 gene expression may be a useful marker in predicting clinical outcomes in pancreatic cancer, Dr. Roper said.
In the future the current study may prompt prospective research to determine a potential association between clinical assessment of SMAD4 gene expression at the time of surgical cancer resection and worse overall survival and distant metastasis-free survival, he said.
The study received no outside funding. Dr. Anstadt and Dr. Roper had no financial conflicts to disclose.
These were the main findings of a new study of more than 300 individuals.
Previous studies have shown an association between widespread disease and loss of SMAD4 immunolabeling, according to the paper. Biomarkers to predict which pancreatic adenocarcinoma patients may benefit from more aggressive therapy are lacking, wrote Emily J. Anstadt, MD, of the University of Pennsylvania, Philadelphia, and colleagues, in their paper published in Cancer.
The human transcription factor and tumor suppressor, mothers against decapentaplegic homolog 4 (SMAD4), “may be a promising biomarker for predicting the likelihood of experiencing distant failure in patients with pancreatic cancer,” the researchers wrote.
“For patients with pancreatic cancer, improving treatments and overall outcomes remains invaluable,” Dr. Anstadt said in an interview. However, the disparate clinical courses make studies of this patient population challenging.
“As with much of medicine and oncology at this time, we feel the key to better outcomes lies in personalizing treatment strategies and relying on tumor genetics to predict tumor behavior and guide us towards individualized optimal treatments,” she added.
Study Methods and Results
The researchers identified 322 patients with resected stage I–III pancreatic adenocarcinoma from The Cancer Genome Atlas (TCGA) and the International Cancer Genome Consortium (ICGC). The study population included 165 patients from the TCGA who served as the training set and 157 patients from the ICGC who served as the validation set.
The primary outcomes were overall survival (OS) and distant metastasis-free survival (DMFS).
A total of 50 patients in the TCGA group (30%) had at least one of the three identified SMAD4 genomic aberrations.
Using the TCGA group, the researchers conducted a regression analysis on the survival outcomes as a function of either the presence of an SMAD4 genomic aberration or the expression of messenger RNA sequencing (RNA-seq). They then used the ICGC to validate whether SMAD4 RNA-seq expression improved risk stratification for OS and DMFS in a separate group of patients.
In the TCGA group, 3-year OS for patients with any SMAD4 aberrations vs no SMAD4 aberrations was 18% vs 36% (hazard ratio, 1.55; P = .048). However, the 3-year DMFS for patients with and without SMAD4 aberrations was 14% vs 23%, a nonsignificant difference (HR, 1.33; P = .19).
In a multivariate analysis, SMAD4 aberrations also were associated with increased risk of stage III disease (HR, 1.89; P = .003). The researchers noted that adjuvant radiotherapy and adjuvant chemotherapy were significantly associated with a decreased risk of death in these patients (HR, 0.53 and HR, 0.28, respectively).
In addition, low SMAD4 RNA-seq expression was associated with worse OS and DMFS, (HR, 1.83 and HR, 1.70, respectively) in the TCGA group.
In the ICGC validation group, increased SMAD4 RNA‐seq expression correlated with improved OS (area under the curve .92) and DMFS (AUC, .84).
Dr. Anstadt said she and her colleagues were not surprised by any of their findings, given earlier research’s suggestions of SMAD4 loss having been associated with poor outcomes for pancreatic cancer.
“Prior studies determined SMAD4 status based on immunohistochemistry and different investigators used different scoring systems,” Dr. Anstadt noted, in an interview. “The results of those studies were conflicting, and consequently SMAD4 has not been adopted clinically as part of the work-up or to aid in treatment decisions.”
“It is essential to find robust, reliable, and cost-effective methods for implementing this in the clinic. As such, we were happy to find that expression of SMAD4 by mRNA sequencing may be that method,” she added.
Not Quite Clinic-Ready
“While we are hopeful that this tool will be a reliable method for use in the clinic, it has yet to be validated in a prospective manner,” Dr. Anstadt said in an interview. “In addition, this study showed that [genetic] expression levels are correlated with worse outcomes and can be of prognostic use; however, we have not directly studied whether expression levels can be predictive of treatment response,” she said.
“Practicing oncologists often have to make difficult decisions in situations where there are no clear answers,” Dr. Anstadt continued. “When considering gray-zone treatment recommendations, we often integrate multiple factors to form an opinion. The reality of cancer medicine is that not all those factors we consider have been validated in prospective studies, but together they produce a picture that is clinically useful. We would submit that SMAD4 status should be one of those factors taken into consideration in forming a comprehensive opinion about suitability for resection or radiotherapy.”
In practice, “if this test is prospectively validated in a future study and will impact clinical decision-making, then this cost will be similar to other genetic tests that have been adopted and have been practice-changing in other oncologic fields,” said Dr. Anstadt. “Being able to individualize treatment can also save overall cost; for instance, predicting which patients would not benefit from local radiation or surgery could decrease use and cost in that population,” she said.
Limitations of the current study included the inability to examine interactions between SMAD4 and radiotherapy because of the sample size and the potential for selection bias, the researchers wrote.
Potential Predictive Value
“A major challenge in the management of patients with pancreatic cancer is the difficulty in predicting which patients will develop metastasis early,” said Jatin Roper, MD, a gastroenterologist at Duke University, Durham, North Carolina, in an interview.
“SMAD4 has previously been evaluated as a prognostic marker in pancreatic cancer, but the association between SMAD4 gene expression, gene mutations, and cancer metastasis has not yet been systematically evaluated in patients, said Dr. Roper, who was not involved in the study.
The new study’s main findings that SMAD4 genomic alterations are associated with worse overall survival, but not distant metastasis-free survival, and that increased SMAD4 expression is associated with improved overall survival and distant metastasis-free survival, suggest that SMAD4 gene expression may be a useful marker in predicting clinical outcomes in pancreatic cancer, Dr. Roper said.
In the future the current study may prompt prospective research to determine a potential association between clinical assessment of SMAD4 gene expression at the time of surgical cancer resection and worse overall survival and distant metastasis-free survival, he said.
The study received no outside funding. Dr. Anstadt and Dr. Roper had no financial conflicts to disclose.
These were the main findings of a new study of more than 300 individuals.
Previous studies have shown an association between widespread disease and loss of SMAD4 immunolabeling, according to the paper. Biomarkers to predict which pancreatic adenocarcinoma patients may benefit from more aggressive therapy are lacking, wrote Emily J. Anstadt, MD, of the University of Pennsylvania, Philadelphia, and colleagues, in their paper published in Cancer.
The human transcription factor and tumor suppressor, mothers against decapentaplegic homolog 4 (SMAD4), “may be a promising biomarker for predicting the likelihood of experiencing distant failure in patients with pancreatic cancer,” the researchers wrote.
“For patients with pancreatic cancer, improving treatments and overall outcomes remains invaluable,” Dr. Anstadt said in an interview. However, the disparate clinical courses make studies of this patient population challenging.
“As with much of medicine and oncology at this time, we feel the key to better outcomes lies in personalizing treatment strategies and relying on tumor genetics to predict tumor behavior and guide us towards individualized optimal treatments,” she added.
Study Methods and Results
The researchers identified 322 patients with resected stage I–III pancreatic adenocarcinoma from The Cancer Genome Atlas (TCGA) and the International Cancer Genome Consortium (ICGC). The study population included 165 patients from the TCGA who served as the training set and 157 patients from the ICGC who served as the validation set.
The primary outcomes were overall survival (OS) and distant metastasis-free survival (DMFS).
A total of 50 patients in the TCGA group (30%) had at least one of the three identified SMAD4 genomic aberrations.
Using the TCGA group, the researchers conducted a regression analysis on the survival outcomes as a function of either the presence of an SMAD4 genomic aberration or the expression of messenger RNA sequencing (RNA-seq). They then used the ICGC to validate whether SMAD4 RNA-seq expression improved risk stratification for OS and DMFS in a separate group of patients.
In the TCGA group, 3-year OS for patients with any SMAD4 aberrations vs no SMAD4 aberrations was 18% vs 36% (hazard ratio, 1.55; P = .048). However, the 3-year DMFS for patients with and without SMAD4 aberrations was 14% vs 23%, a nonsignificant difference (HR, 1.33; P = .19).
In a multivariate analysis, SMAD4 aberrations also were associated with increased risk of stage III disease (HR, 1.89; P = .003). The researchers noted that adjuvant radiotherapy and adjuvant chemotherapy were significantly associated with a decreased risk of death in these patients (HR, 0.53 and HR, 0.28, respectively).
In addition, low SMAD4 RNA-seq expression was associated with worse OS and DMFS, (HR, 1.83 and HR, 1.70, respectively) in the TCGA group.
In the ICGC validation group, increased SMAD4 RNA‐seq expression correlated with improved OS (area under the curve .92) and DMFS (AUC, .84).
Dr. Anstadt said she and her colleagues were not surprised by any of their findings, given earlier research’s suggestions of SMAD4 loss having been associated with poor outcomes for pancreatic cancer.
“Prior studies determined SMAD4 status based on immunohistochemistry and different investigators used different scoring systems,” Dr. Anstadt noted, in an interview. “The results of those studies were conflicting, and consequently SMAD4 has not been adopted clinically as part of the work-up or to aid in treatment decisions.”
“It is essential to find robust, reliable, and cost-effective methods for implementing this in the clinic. As such, we were happy to find that expression of SMAD4 by mRNA sequencing may be that method,” she added.
Not Quite Clinic-Ready
“While we are hopeful that this tool will be a reliable method for use in the clinic, it has yet to be validated in a prospective manner,” Dr. Anstadt said in an interview. “In addition, this study showed that [genetic] expression levels are correlated with worse outcomes and can be of prognostic use; however, we have not directly studied whether expression levels can be predictive of treatment response,” she said.
“Practicing oncologists often have to make difficult decisions in situations where there are no clear answers,” Dr. Anstadt continued. “When considering gray-zone treatment recommendations, we often integrate multiple factors to form an opinion. The reality of cancer medicine is that not all those factors we consider have been validated in prospective studies, but together they produce a picture that is clinically useful. We would submit that SMAD4 status should be one of those factors taken into consideration in forming a comprehensive opinion about suitability for resection or radiotherapy.”
In practice, “if this test is prospectively validated in a future study and will impact clinical decision-making, then this cost will be similar to other genetic tests that have been adopted and have been practice-changing in other oncologic fields,” said Dr. Anstadt. “Being able to individualize treatment can also save overall cost; for instance, predicting which patients would not benefit from local radiation or surgery could decrease use and cost in that population,” she said.
Limitations of the current study included the inability to examine interactions between SMAD4 and radiotherapy because of the sample size and the potential for selection bias, the researchers wrote.
Potential Predictive Value
“A major challenge in the management of patients with pancreatic cancer is the difficulty in predicting which patients will develop metastasis early,” said Jatin Roper, MD, a gastroenterologist at Duke University, Durham, North Carolina, in an interview.
“SMAD4 has previously been evaluated as a prognostic marker in pancreatic cancer, but the association between SMAD4 gene expression, gene mutations, and cancer metastasis has not yet been systematically evaluated in patients, said Dr. Roper, who was not involved in the study.
The new study’s main findings that SMAD4 genomic alterations are associated with worse overall survival, but not distant metastasis-free survival, and that increased SMAD4 expression is associated with improved overall survival and distant metastasis-free survival, suggest that SMAD4 gene expression may be a useful marker in predicting clinical outcomes in pancreatic cancer, Dr. Roper said.
In the future the current study may prompt prospective research to determine a potential association between clinical assessment of SMAD4 gene expression at the time of surgical cancer resection and worse overall survival and distant metastasis-free survival, he said.
The study received no outside funding. Dr. Anstadt and Dr. Roper had no financial conflicts to disclose.
FROM CANCER