User login
New themes emerging about antipsychotic maintenance therapy
COPENHAGEN – Recent studies emphasize the long-term merits of extending antipsychotic therapy beyond 1 year for patients in remission after a first psychotic episode, Mark Weiser, MD, observed at the annual congress of the European College of Neuropsychopharmacology.
Another emerging theme in the research literature, albeit a somewhat heretical one, is the value of antipsychotic polypharmacy, added Dr. Weiser, professor and chairman of the department of psychiatry at Sackler Faculty of Medicine, Tel Aviv University.
“Polypharmacy might not be so bad,” he declared in a keynote lecture in which he highlighted recent major publications addressing issues involving pharmacotherapy of schizophrenia.
“When we were residents, we were all taught that it’s not good to give two antipsychotics together, that all antipsychotics work only through dopamine D2 [receptor] blockade, hence there’s no point in giving more than one antipsychotic because all you’re doing is increasing side effects. But maybe it’s not as simple as that,” the psychiatrist said.
Exhibit 1: A recent Finnish national registry study of 62,250 patients with schizophrenia featuring up to 20-year follow-up showing that any antipsychotic polypharmacy was associated with a significantly lower risk of rehospitalization, compared with any antipsychotic monotherapy. The combination that stood out as having the lowest rehospitalization risk was clozapine plus aripiprazole, with a 14%-23% lower risk than for clozapine alone, which was the most effective monotherapy (JAMA Psychiatry. 2019 May; 76[5]:499-507).
Dr. Weiser pronounced himself a firm believer in the Finnish experience.
“The reason I think that this is true is that it’s exactly what I find in my own VA data,” he said.
Indeed, he and his coinvestigators are preparing to publish the results of their longitudinal study of 37,368 schizophrenia patients in the U.S. Veterans Affairs system. A key finding was that patients on antipsychotic polypharmacy had a longer time to treatment discontinuation than did those on monotherapy.
“Polypharmacy, at least in real-world study designs, seems to be good,” he noted.
Moreover, even in the world of highly selective randomized clinical trials, polypharmacy appears to fare pretty well as a treatment strategy, Dr. Weiser noted. A recent meta-analysis of six RCTs totaling 341 patients showed that switching patients from two antipsychotics to one was associated with a 2.2-fold increased risk of study discontinuation, although the investigators rated the quality of the trials as low to very low (Schizophr Res. 2019 Jul;209:50-7).
“I’m doing more polypharmacy now because of these data,” Dr. Weiser said. “My understanding of the polypharmacy data is that it’s not just blocking dopamine that’s important for treatment efficacy in schizophrenia.”
Getting a handle on first-episode psychosis
Dr. Weiser was a coinvestigator in the European Commission–funded OPTiMiSE study, in which 446 patients with first-episode schizophrenia or schizophreniform disorder in 14 European countries and Israel received 4 weeks of oral amisulpride. Then, if they were not in symptomatic remission, they were randomized double-blind to 6 weeks more of amisulpride or a switch to olanzapine. Those who weren’t in remission at 10 weeks were then placed on 12 weeks of open-label clozapine.
OPTiMiSE validated the clinical utility of a simple treatment algorithm, as 56% of patients achieved remission using stringent criteria by week 4 on amisulpride, and an additional 7% reached that endpoint by week 10, with the switch to olanzapine offering no added value over continued amisulpride. Switching week-10 nonresponders to clozapine enabled 28% of them to achieve remission (Lancet Psychiatry. 2018 Oct 1;5[10]:797-807).
A key take-home message of the trial, Dr. Weiser said, is that a switch to clozapine is justified after 10 weeks of unsuccessful treatment with a first-line antipsychotic; there’s no need to wait until patients have failed on two consecutive antipsychotics, as guidelines now recommend.
He observed that it can be a lot more challenging to keep patients in remission after a first psychotic episode than it is to get them to respond in the first place. For this reason, he found particularly instructive a recent study by psychiatrists at the University of Hong Kong that shined a light on the understudied long-term adverse consequences of stopping antipsychotic therapy after 1 year in patients successfully treated for a first-episode psychosis. At the 1-year point in this randomized trial, the successfully remitted patients were given maintenance therapy or their antipsychotic therapy was discontinued for 12 months. Ten years later, the investigators reported, the rate of a composite endpoint comprising persistent psychotic symptoms, requirement for clozapine, or completed suicide was 21% in the maintenance therapy group and significantly worse at 39% in those who had stopped treatment at 1 year (Lancet Psychiatry. 2018 May 1;5[5]:432-42).
“It’s probably a good idea to keep patients who are stable on maintenance therapy for longer than a year. A lot of first-episode patients don’t want to hear this. A lot of family members don’t want to hear this. And a lot of patients, of course, decide for themselves and stop treatment, although I tell them they should not.
Asked how low his low-dose long-term maintenance therapy is, he replied: “Negotiate with your patient about the lowest dose he or she is willing to take. In my opinion, 1 mg of risperidone is a lot better than 0 mg of risperidone, although that’s not an opinion supported by data.”
Dr. Weiser reported having no financial conflicts regarding his presentation.
COPENHAGEN – Recent studies emphasize the long-term merits of extending antipsychotic therapy beyond 1 year for patients in remission after a first psychotic episode, Mark Weiser, MD, observed at the annual congress of the European College of Neuropsychopharmacology.
Another emerging theme in the research literature, albeit a somewhat heretical one, is the value of antipsychotic polypharmacy, added Dr. Weiser, professor and chairman of the department of psychiatry at Sackler Faculty of Medicine, Tel Aviv University.
“Polypharmacy might not be so bad,” he declared in a keynote lecture in which he highlighted recent major publications addressing issues involving pharmacotherapy of schizophrenia.
“When we were residents, we were all taught that it’s not good to give two antipsychotics together, that all antipsychotics work only through dopamine D2 [receptor] blockade, hence there’s no point in giving more than one antipsychotic because all you’re doing is increasing side effects. But maybe it’s not as simple as that,” the psychiatrist said.
Exhibit 1: A recent Finnish national registry study of 62,250 patients with schizophrenia featuring up to 20-year follow-up showing that any antipsychotic polypharmacy was associated with a significantly lower risk of rehospitalization, compared with any antipsychotic monotherapy. The combination that stood out as having the lowest rehospitalization risk was clozapine plus aripiprazole, with a 14%-23% lower risk than for clozapine alone, which was the most effective monotherapy (JAMA Psychiatry. 2019 May; 76[5]:499-507).
Dr. Weiser pronounced himself a firm believer in the Finnish experience.
“The reason I think that this is true is that it’s exactly what I find in my own VA data,” he said.
Indeed, he and his coinvestigators are preparing to publish the results of their longitudinal study of 37,368 schizophrenia patients in the U.S. Veterans Affairs system. A key finding was that patients on antipsychotic polypharmacy had a longer time to treatment discontinuation than did those on monotherapy.
“Polypharmacy, at least in real-world study designs, seems to be good,” he noted.
Moreover, even in the world of highly selective randomized clinical trials, polypharmacy appears to fare pretty well as a treatment strategy, Dr. Weiser noted. A recent meta-analysis of six RCTs totaling 341 patients showed that switching patients from two antipsychotics to one was associated with a 2.2-fold increased risk of study discontinuation, although the investigators rated the quality of the trials as low to very low (Schizophr Res. 2019 Jul;209:50-7).
“I’m doing more polypharmacy now because of these data,” Dr. Weiser said. “My understanding of the polypharmacy data is that it’s not just blocking dopamine that’s important for treatment efficacy in schizophrenia.”
Getting a handle on first-episode psychosis
Dr. Weiser was a coinvestigator in the European Commission–funded OPTiMiSE study, in which 446 patients with first-episode schizophrenia or schizophreniform disorder in 14 European countries and Israel received 4 weeks of oral amisulpride. Then, if they were not in symptomatic remission, they were randomized double-blind to 6 weeks more of amisulpride or a switch to olanzapine. Those who weren’t in remission at 10 weeks were then placed on 12 weeks of open-label clozapine.
OPTiMiSE validated the clinical utility of a simple treatment algorithm, as 56% of patients achieved remission using stringent criteria by week 4 on amisulpride, and an additional 7% reached that endpoint by week 10, with the switch to olanzapine offering no added value over continued amisulpride. Switching week-10 nonresponders to clozapine enabled 28% of them to achieve remission (Lancet Psychiatry. 2018 Oct 1;5[10]:797-807).
A key take-home message of the trial, Dr. Weiser said, is that a switch to clozapine is justified after 10 weeks of unsuccessful treatment with a first-line antipsychotic; there’s no need to wait until patients have failed on two consecutive antipsychotics, as guidelines now recommend.
He observed that it can be a lot more challenging to keep patients in remission after a first psychotic episode than it is to get them to respond in the first place. For this reason, he found particularly instructive a recent study by psychiatrists at the University of Hong Kong that shined a light on the understudied long-term adverse consequences of stopping antipsychotic therapy after 1 year in patients successfully treated for a first-episode psychosis. At the 1-year point in this randomized trial, the successfully remitted patients were given maintenance therapy or their antipsychotic therapy was discontinued for 12 months. Ten years later, the investigators reported, the rate of a composite endpoint comprising persistent psychotic symptoms, requirement for clozapine, or completed suicide was 21% in the maintenance therapy group and significantly worse at 39% in those who had stopped treatment at 1 year (Lancet Psychiatry. 2018 May 1;5[5]:432-42).
“It’s probably a good idea to keep patients who are stable on maintenance therapy for longer than a year. A lot of first-episode patients don’t want to hear this. A lot of family members don’t want to hear this. And a lot of patients, of course, decide for themselves and stop treatment, although I tell them they should not.
Asked how low his low-dose long-term maintenance therapy is, he replied: “Negotiate with your patient about the lowest dose he or she is willing to take. In my opinion, 1 mg of risperidone is a lot better than 0 mg of risperidone, although that’s not an opinion supported by data.”
Dr. Weiser reported having no financial conflicts regarding his presentation.
COPENHAGEN – Recent studies emphasize the long-term merits of extending antipsychotic therapy beyond 1 year for patients in remission after a first psychotic episode, Mark Weiser, MD, observed at the annual congress of the European College of Neuropsychopharmacology.
Another emerging theme in the research literature, albeit a somewhat heretical one, is the value of antipsychotic polypharmacy, added Dr. Weiser, professor and chairman of the department of psychiatry at Sackler Faculty of Medicine, Tel Aviv University.
“Polypharmacy might not be so bad,” he declared in a keynote lecture in which he highlighted recent major publications addressing issues involving pharmacotherapy of schizophrenia.
“When we were residents, we were all taught that it’s not good to give two antipsychotics together, that all antipsychotics work only through dopamine D2 [receptor] blockade, hence there’s no point in giving more than one antipsychotic because all you’re doing is increasing side effects. But maybe it’s not as simple as that,” the psychiatrist said.
Exhibit 1: A recent Finnish national registry study of 62,250 patients with schizophrenia featuring up to 20-year follow-up showing that any antipsychotic polypharmacy was associated with a significantly lower risk of rehospitalization, compared with any antipsychotic monotherapy. The combination that stood out as having the lowest rehospitalization risk was clozapine plus aripiprazole, with a 14%-23% lower risk than for clozapine alone, which was the most effective monotherapy (JAMA Psychiatry. 2019 May; 76[5]:499-507).
Dr. Weiser pronounced himself a firm believer in the Finnish experience.
“The reason I think that this is true is that it’s exactly what I find in my own VA data,” he said.
Indeed, he and his coinvestigators are preparing to publish the results of their longitudinal study of 37,368 schizophrenia patients in the U.S. Veterans Affairs system. A key finding was that patients on antipsychotic polypharmacy had a longer time to treatment discontinuation than did those on monotherapy.
“Polypharmacy, at least in real-world study designs, seems to be good,” he noted.
Moreover, even in the world of highly selective randomized clinical trials, polypharmacy appears to fare pretty well as a treatment strategy, Dr. Weiser noted. A recent meta-analysis of six RCTs totaling 341 patients showed that switching patients from two antipsychotics to one was associated with a 2.2-fold increased risk of study discontinuation, although the investigators rated the quality of the trials as low to very low (Schizophr Res. 2019 Jul;209:50-7).
“I’m doing more polypharmacy now because of these data,” Dr. Weiser said. “My understanding of the polypharmacy data is that it’s not just blocking dopamine that’s important for treatment efficacy in schizophrenia.”
Getting a handle on first-episode psychosis
Dr. Weiser was a coinvestigator in the European Commission–funded OPTiMiSE study, in which 446 patients with first-episode schizophrenia or schizophreniform disorder in 14 European countries and Israel received 4 weeks of oral amisulpride. Then, if they were not in symptomatic remission, they were randomized double-blind to 6 weeks more of amisulpride or a switch to olanzapine. Those who weren’t in remission at 10 weeks were then placed on 12 weeks of open-label clozapine.
OPTiMiSE validated the clinical utility of a simple treatment algorithm, as 56% of patients achieved remission using stringent criteria by week 4 on amisulpride, and an additional 7% reached that endpoint by week 10, with the switch to olanzapine offering no added value over continued amisulpride. Switching week-10 nonresponders to clozapine enabled 28% of them to achieve remission (Lancet Psychiatry. 2018 Oct 1;5[10]:797-807).
A key take-home message of the trial, Dr. Weiser said, is that a switch to clozapine is justified after 10 weeks of unsuccessful treatment with a first-line antipsychotic; there’s no need to wait until patients have failed on two consecutive antipsychotics, as guidelines now recommend.
He observed that it can be a lot more challenging to keep patients in remission after a first psychotic episode than it is to get them to respond in the first place. For this reason, he found particularly instructive a recent study by psychiatrists at the University of Hong Kong that shined a light on the understudied long-term adverse consequences of stopping antipsychotic therapy after 1 year in patients successfully treated for a first-episode psychosis. At the 1-year point in this randomized trial, the successfully remitted patients were given maintenance therapy or their antipsychotic therapy was discontinued for 12 months. Ten years later, the investigators reported, the rate of a composite endpoint comprising persistent psychotic symptoms, requirement for clozapine, or completed suicide was 21% in the maintenance therapy group and significantly worse at 39% in those who had stopped treatment at 1 year (Lancet Psychiatry. 2018 May 1;5[5]:432-42).
“It’s probably a good idea to keep patients who are stable on maintenance therapy for longer than a year. A lot of first-episode patients don’t want to hear this. A lot of family members don’t want to hear this. And a lot of patients, of course, decide for themselves and stop treatment, although I tell them they should not.
Asked how low his low-dose long-term maintenance therapy is, he replied: “Negotiate with your patient about the lowest dose he or she is willing to take. In my opinion, 1 mg of risperidone is a lot better than 0 mg of risperidone, although that’s not an opinion supported by data.”
Dr. Weiser reported having no financial conflicts regarding his presentation.
REPORTING FROM ECNP 2019
Short Takes
AFM cases continue to rise
Cases of Acute Flaccid Myelitis (AFM) are on the rise, with 210 confirmed cases of AFM in 40 states in 2018, up from 35 confirmed cases in 2017. AFM is a rare but serious condition that usually affects children, causing polio-like symptoms – focal extremity weakness, hyporeflexia, and sometimes cranial nerve dysfunction. The Centers for Disease Control and Prevention encourage all health care providers to contact their local health departments with any suspected cases of AFM.
Citation: Centers for Disease Control and Prevention. AFM Investigation. 2019 Jan. https://www.cdc.gov/acute-flaccid-myelitis/afm-surveillance.html.
HHS recommends prescribing naloxone to patients at high risk for opioid overdose
The U.S. Department of Health & Human Services recommends clinicians strongly consider prescribing or coprescribing naloxone to patients at high risk of opioid overdose. This includes patients who are on relatively high doses of opioids, take other medications which enhance opioid complications, or have underlying health conditions. Clinicians are also advised to educate patients and those likely to respond to an overdose on when and how to use naloxone in its variety of forms.
Citation: U.S. Department of Health & Human Services. HHS recommends prescribing or co-prescribing naloxone to patients at high risk for an opioid overdose. 2018 Dec 18. https://www.hhs.gov/about/news/2018/12/19/hhs-recommends-prescribing-or-co-prescribing-naloxone-to-patients-at-high-risk-for-an-opioid-overdose.html.
Fentanyl tops the list of opioid overdose drugs
The total number of drug overdose deaths per year in the United States increased 54%, from 41,340 deaths in 2011 to 63,632 deaths in 2016. Among opioids, mention of fentanyl increased during 2011-2016; that drug took the lead in 2016 with 29% of all drug overdose deaths. Among the drug overdose deaths involving fentanyl, 69% also involved one or more other drugs.
Citation: Hedegaard H et al. Drugs most frequently involved in drug overdose deaths: United States, 2011–2016. Natl Vital Stat Rep. 2018 Dec;67(9):1-14.
AFM cases continue to rise
Cases of Acute Flaccid Myelitis (AFM) are on the rise, with 210 confirmed cases of AFM in 40 states in 2018, up from 35 confirmed cases in 2017. AFM is a rare but serious condition that usually affects children, causing polio-like symptoms – focal extremity weakness, hyporeflexia, and sometimes cranial nerve dysfunction. The Centers for Disease Control and Prevention encourage all health care providers to contact their local health departments with any suspected cases of AFM.
Citation: Centers for Disease Control and Prevention. AFM Investigation. 2019 Jan. https://www.cdc.gov/acute-flaccid-myelitis/afm-surveillance.html.
HHS recommends prescribing naloxone to patients at high risk for opioid overdose
The U.S. Department of Health & Human Services recommends clinicians strongly consider prescribing or coprescribing naloxone to patients at high risk of opioid overdose. This includes patients who are on relatively high doses of opioids, take other medications which enhance opioid complications, or have underlying health conditions. Clinicians are also advised to educate patients and those likely to respond to an overdose on when and how to use naloxone in its variety of forms.
Citation: U.S. Department of Health & Human Services. HHS recommends prescribing or co-prescribing naloxone to patients at high risk for an opioid overdose. 2018 Dec 18. https://www.hhs.gov/about/news/2018/12/19/hhs-recommends-prescribing-or-co-prescribing-naloxone-to-patients-at-high-risk-for-an-opioid-overdose.html.
Fentanyl tops the list of opioid overdose drugs
The total number of drug overdose deaths per year in the United States increased 54%, from 41,340 deaths in 2011 to 63,632 deaths in 2016. Among opioids, mention of fentanyl increased during 2011-2016; that drug took the lead in 2016 with 29% of all drug overdose deaths. Among the drug overdose deaths involving fentanyl, 69% also involved one or more other drugs.
Citation: Hedegaard H et al. Drugs most frequently involved in drug overdose deaths: United States, 2011–2016. Natl Vital Stat Rep. 2018 Dec;67(9):1-14.
AFM cases continue to rise
Cases of Acute Flaccid Myelitis (AFM) are on the rise, with 210 confirmed cases of AFM in 40 states in 2018, up from 35 confirmed cases in 2017. AFM is a rare but serious condition that usually affects children, causing polio-like symptoms – focal extremity weakness, hyporeflexia, and sometimes cranial nerve dysfunction. The Centers for Disease Control and Prevention encourage all health care providers to contact their local health departments with any suspected cases of AFM.
Citation: Centers for Disease Control and Prevention. AFM Investigation. 2019 Jan. https://www.cdc.gov/acute-flaccid-myelitis/afm-surveillance.html.
HHS recommends prescribing naloxone to patients at high risk for opioid overdose
The U.S. Department of Health & Human Services recommends clinicians strongly consider prescribing or coprescribing naloxone to patients at high risk of opioid overdose. This includes patients who are on relatively high doses of opioids, take other medications which enhance opioid complications, or have underlying health conditions. Clinicians are also advised to educate patients and those likely to respond to an overdose on when and how to use naloxone in its variety of forms.
Citation: U.S. Department of Health & Human Services. HHS recommends prescribing or co-prescribing naloxone to patients at high risk for an opioid overdose. 2018 Dec 18. https://www.hhs.gov/about/news/2018/12/19/hhs-recommends-prescribing-or-co-prescribing-naloxone-to-patients-at-high-risk-for-an-opioid-overdose.html.
Fentanyl tops the list of opioid overdose drugs
The total number of drug overdose deaths per year in the United States increased 54%, from 41,340 deaths in 2011 to 63,632 deaths in 2016. Among opioids, mention of fentanyl increased during 2011-2016; that drug took the lead in 2016 with 29% of all drug overdose deaths. Among the drug overdose deaths involving fentanyl, 69% also involved one or more other drugs.
Citation: Hedegaard H et al. Drugs most frequently involved in drug overdose deaths: United States, 2011–2016. Natl Vital Stat Rep. 2018 Dec;67(9):1-14.
Rash with weakness
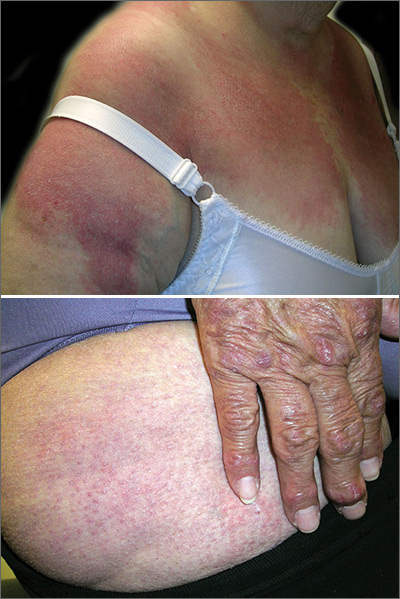
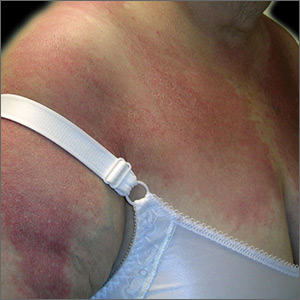
The FP diagnosed dermatomyositis based on the patient’s proximal muscle weakness along with the typical shawl sign (erythema and scale over the shawl distribution) seen in the top image and the Gottron papules (erythematous and scaling papules over the dorsum of the fingers) and holster sign (erythema and scale over the lateral hip region) seen in the bottom image.
The FP started the patient on prednisone 60 mg/d and topical steroids for the affected areas. Laboratory exams showed an elevated creatinine kinase and mildly elevated antinuclear antibodies (typical for dermatomyositis). The FP also started the patient on oral prednisone 40 mg/d while putting in referrals for Dermatology and Rheumatology.
Two weeks later, the patient felt stronger and the rash had faded. Dermatology saw her first and started her on a steroid-sparing agent, methotrexate, with plans to taper her prednisone to lower doses over time. As underlying malignancy may precipitate dermatomyositis, the patient was screened for internal cancers—especially ovarian cancer. Fortunately, the mammogram, Pap smear, colonoscopy, and thoracic, abdominal, and pelvic computed tomography scans all were normal.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Buckley J, Burks M, Allred A, Usatine R. Dermatomyositis. In: Usatine R, Smith M, Mayeaux EJ, et al. eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1194-1203.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/

The FP diagnosed dermatomyositis based on the patient’s proximal muscle weakness along with the typical shawl sign (erythema and scale over the shawl distribution) seen in the top image and the Gottron papules (erythematous and scaling papules over the dorsum of the fingers) and holster sign (erythema and scale over the lateral hip region) seen in the bottom image.
The FP started the patient on prednisone 60 mg/d and topical steroids for the affected areas. Laboratory exams showed an elevated creatinine kinase and mildly elevated antinuclear antibodies (typical for dermatomyositis). The FP also started the patient on oral prednisone 40 mg/d while putting in referrals for Dermatology and Rheumatology.
Two weeks later, the patient felt stronger and the rash had faded. Dermatology saw her first and started her on a steroid-sparing agent, methotrexate, with plans to taper her prednisone to lower doses over time. As underlying malignancy may precipitate dermatomyositis, the patient was screened for internal cancers—especially ovarian cancer. Fortunately, the mammogram, Pap smear, colonoscopy, and thoracic, abdominal, and pelvic computed tomography scans all were normal.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Buckley J, Burks M, Allred A, Usatine R. Dermatomyositis. In: Usatine R, Smith M, Mayeaux EJ, et al. eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1194-1203.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/

The FP diagnosed dermatomyositis based on the patient’s proximal muscle weakness along with the typical shawl sign (erythema and scale over the shawl distribution) seen in the top image and the Gottron papules (erythematous and scaling papules over the dorsum of the fingers) and holster sign (erythema and scale over the lateral hip region) seen in the bottom image.
The FP started the patient on prednisone 60 mg/d and topical steroids for the affected areas. Laboratory exams showed an elevated creatinine kinase and mildly elevated antinuclear antibodies (typical for dermatomyositis). The FP also started the patient on oral prednisone 40 mg/d while putting in referrals for Dermatology and Rheumatology.
Two weeks later, the patient felt stronger and the rash had faded. Dermatology saw her first and started her on a steroid-sparing agent, methotrexate, with plans to taper her prednisone to lower doses over time. As underlying malignancy may precipitate dermatomyositis, the patient was screened for internal cancers—especially ovarian cancer. Fortunately, the mammogram, Pap smear, colonoscopy, and thoracic, abdominal, and pelvic computed tomography scans all were normal.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Buckley J, Burks M, Allred A, Usatine R. Dermatomyositis. In: Usatine R, Smith M, Mayeaux EJ, et al. eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1194-1203.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
Supine sleep in late pregnancy may promote low birth weight
Data from previous studies suggest that impaired uteroplacental flow can affect fetal growth, wrote Ngaire H. Anderson, PhD, of the University of Auckland, N.Z., and colleagues.
“The initial going-to-sleep position is the sleep position that women maintain for the longest duration throughout the night; therefore, going-to-sleep position is likely to have the greatest impact on blood flow to the developing fetus,” they said.
In a study published in JAMA Network Open, the researchers interviewed women with ongoing pregnancies at 28 weeks’ gestation or later to determine their sleeping positions. The mean age of the participants was 30 years. Of the 1,760 women, 3% reported that they usually slept supine during the past 1-4 weeks.
The adjusted mean birth weight was 3,410 g among supine sleepers and 3,554 g among nonsupine sleepers. The primary outcome was an adjusted mean difference in birth weight between infants of supine sleepers and nonsupine sleepers, which was a statistically significant 144 g (P = .009).
The study findings were limited by several factors including the small number of women who were reported supine sleepers, as well as the reliance on self-reports of sleep position, the researchers said.
However, women who had going-to-sleep data for the previous night and the previous month suggest that most women are consistent in their going-to-sleep position, they noted. “It is also biologically plausible that the association of decreased maternal blood flow on birth size with supine maternal position is cumulative over time,” but the researchers were not able to investigate how the duration of supine sleeping might further affect birth weight.
Although it might make additional studies more difficult, a public health campaign to encourage pregnant women to sleep on their side during the third trimester is a safe and easy opportunity to potentially optimize birth weight, they added.
The study was important because of the limited number of high-quality studies on the effects of maternal sleep on perinatal outcomes, Martina Badell, MD of Emory University in Atlanta said in an interview.
“The overall findings suggested a possible small increased risk of small-for-gestational-age babies with supine maternal sleeping, however, the absolute gram difference of 144 grams at term may not be clinically relevant,” she said. In addition, the relatively small number of women who reported supine sleep in late pregnancy suggests that broad public health campaigns or recommendations may not be indicated at this time.
“Also, the percentage of women who are supine sleepers at term is only approximately 3%, and this study didn’t assess reasons for supine sleeping in this small subset of women,” she said. “Further research is needed to assess whether there are specific maternal factors associated with supine sleeping, such as GI symptoms or respiratory difficulties, which could contribute to smaller fetal size rather than the sleep position itself.”
The study was supported by a Trans-Tasman Research Funding Grant by Cure Kids and Red Nose Australia. Six coauthors reported receiving numerous grants from a variety of organizations. Dr. Anderson and the remaining coauthors had no financial conflicts to disclose. Dr. Badell had no relevant financial disclosures.
SOURCE: Anderson NH et al. JAMA Network Open. 2019 Oct 2. doi: 10.1001/jamanetworkopen.2019.12614.
Data from previous studies suggest that impaired uteroplacental flow can affect fetal growth, wrote Ngaire H. Anderson, PhD, of the University of Auckland, N.Z., and colleagues.
“The initial going-to-sleep position is the sleep position that women maintain for the longest duration throughout the night; therefore, going-to-sleep position is likely to have the greatest impact on blood flow to the developing fetus,” they said.
In a study published in JAMA Network Open, the researchers interviewed women with ongoing pregnancies at 28 weeks’ gestation or later to determine their sleeping positions. The mean age of the participants was 30 years. Of the 1,760 women, 3% reported that they usually slept supine during the past 1-4 weeks.
The adjusted mean birth weight was 3,410 g among supine sleepers and 3,554 g among nonsupine sleepers. The primary outcome was an adjusted mean difference in birth weight between infants of supine sleepers and nonsupine sleepers, which was a statistically significant 144 g (P = .009).
The study findings were limited by several factors including the small number of women who were reported supine sleepers, as well as the reliance on self-reports of sleep position, the researchers said.
However, women who had going-to-sleep data for the previous night and the previous month suggest that most women are consistent in their going-to-sleep position, they noted. “It is also biologically plausible that the association of decreased maternal blood flow on birth size with supine maternal position is cumulative over time,” but the researchers were not able to investigate how the duration of supine sleeping might further affect birth weight.
Although it might make additional studies more difficult, a public health campaign to encourage pregnant women to sleep on their side during the third trimester is a safe and easy opportunity to potentially optimize birth weight, they added.
The study was important because of the limited number of high-quality studies on the effects of maternal sleep on perinatal outcomes, Martina Badell, MD of Emory University in Atlanta said in an interview.
“The overall findings suggested a possible small increased risk of small-for-gestational-age babies with supine maternal sleeping, however, the absolute gram difference of 144 grams at term may not be clinically relevant,” she said. In addition, the relatively small number of women who reported supine sleep in late pregnancy suggests that broad public health campaigns or recommendations may not be indicated at this time.
“Also, the percentage of women who are supine sleepers at term is only approximately 3%, and this study didn’t assess reasons for supine sleeping in this small subset of women,” she said. “Further research is needed to assess whether there are specific maternal factors associated with supine sleeping, such as GI symptoms or respiratory difficulties, which could contribute to smaller fetal size rather than the sleep position itself.”
The study was supported by a Trans-Tasman Research Funding Grant by Cure Kids and Red Nose Australia. Six coauthors reported receiving numerous grants from a variety of organizations. Dr. Anderson and the remaining coauthors had no financial conflicts to disclose. Dr. Badell had no relevant financial disclosures.
SOURCE: Anderson NH et al. JAMA Network Open. 2019 Oct 2. doi: 10.1001/jamanetworkopen.2019.12614.
Data from previous studies suggest that impaired uteroplacental flow can affect fetal growth, wrote Ngaire H. Anderson, PhD, of the University of Auckland, N.Z., and colleagues.
“The initial going-to-sleep position is the sleep position that women maintain for the longest duration throughout the night; therefore, going-to-sleep position is likely to have the greatest impact on blood flow to the developing fetus,” they said.
In a study published in JAMA Network Open, the researchers interviewed women with ongoing pregnancies at 28 weeks’ gestation or later to determine their sleeping positions. The mean age of the participants was 30 years. Of the 1,760 women, 3% reported that they usually slept supine during the past 1-4 weeks.
The adjusted mean birth weight was 3,410 g among supine sleepers and 3,554 g among nonsupine sleepers. The primary outcome was an adjusted mean difference in birth weight between infants of supine sleepers and nonsupine sleepers, which was a statistically significant 144 g (P = .009).
The study findings were limited by several factors including the small number of women who were reported supine sleepers, as well as the reliance on self-reports of sleep position, the researchers said.
However, women who had going-to-sleep data for the previous night and the previous month suggest that most women are consistent in their going-to-sleep position, they noted. “It is also biologically plausible that the association of decreased maternal blood flow on birth size with supine maternal position is cumulative over time,” but the researchers were not able to investigate how the duration of supine sleeping might further affect birth weight.
Although it might make additional studies more difficult, a public health campaign to encourage pregnant women to sleep on their side during the third trimester is a safe and easy opportunity to potentially optimize birth weight, they added.
The study was important because of the limited number of high-quality studies on the effects of maternal sleep on perinatal outcomes, Martina Badell, MD of Emory University in Atlanta said in an interview.
“The overall findings suggested a possible small increased risk of small-for-gestational-age babies with supine maternal sleeping, however, the absolute gram difference of 144 grams at term may not be clinically relevant,” she said. In addition, the relatively small number of women who reported supine sleep in late pregnancy suggests that broad public health campaigns or recommendations may not be indicated at this time.
“Also, the percentage of women who are supine sleepers at term is only approximately 3%, and this study didn’t assess reasons for supine sleeping in this small subset of women,” she said. “Further research is needed to assess whether there are specific maternal factors associated with supine sleeping, such as GI symptoms or respiratory difficulties, which could contribute to smaller fetal size rather than the sleep position itself.”
The study was supported by a Trans-Tasman Research Funding Grant by Cure Kids and Red Nose Australia. Six coauthors reported receiving numerous grants from a variety of organizations. Dr. Anderson and the remaining coauthors had no financial conflicts to disclose. Dr. Badell had no relevant financial disclosures.
SOURCE: Anderson NH et al. JAMA Network Open. 2019 Oct 2. doi: 10.1001/jamanetworkopen.2019.12614.
FROM JAMA NETWORK OPEN
Neanderthal otitis media and why you can’t chillax
Dude, just chill out and relax ... or not
It’s been a tough week. You’ve struggled to get all your work done on time, you’ve had to put in long hours, you’re exhausted and stressed out, and you know that next week will be just as tough. But for now, it’s the weekend. Time to relax, right?
According to research from Penn State University published in the Journal of Affective Disorders, if you have anxiety disorder, not only will you have difficulty relaxing (hence the anxiety disorder), you may actually actively resist it, experiencing something called “relaxation-induced anxiety.”
The researchers recruited a group of students, some with anxiety disorder, some with major depressive disorder, and a control group, and administered a series of relaxation exercises both before and after watching a series of potentially upsetting videos. The people with anxiety disorders were more likely to experience spikes of negative emotion after the second relaxation exercise than the other two groups; these spikes were linked to a feeling of anxiety during relaxation exercise.
The researchers theorized that people with anxiety disorder were attempting to avoid large jumps in their stress level by remaining stressed at all times. But the investigators also noted that experiencing a range of emotions is natural and far healthier.
So, as annoying as it is to suffer through constant “stop worrying, just live in the moment” speeches from that one dudebro acquaintance who spends all his free time partying, he does have a point. We don’t recommend participating in the Edward Fortyhands game, though. Leave that to your “friend.” He’s used to it.
A salty surgeon general’s warning
Grab a pack of Marlboro Reds at the corner convenience store, and you’ll find the iconic red and white box adorned with dire warnings from the surgeon general.
Grab a salt shaker at the corner diner, and you’ll find ... salt. In a shaker. Perhaps adorned with the red residue from a prior diner’s ketchup addiction.
The World Hypertension League would like to make that salt shaker look a lot more like those Marlboros.
In a position statement in the Journal of Clinical Hypertension, the league outlined the case for giving sodium chloride the cancer-stick treatment. Exhibit A: “Unhealthy diets are a leading cause of death globally, and excess salt consumption is the biggest culprit, estimated to cause over 3 million deaths globally in 2017.”
Despite that tobacco-rivaling body count, the league says no country has demanded that salt containers wear warning labels.
But isn’t it enough to list sodium levels on food labels? Well, when’s the last time you studied the salty facts before binging on that entire party-size bag of Cheetos, Chester? (You’ll be hotboxing 4,500 mg of sodium, by the way.) The league rests its case.
What’s needed is a message that stops you mid fistful. It’s time to add warning labels to all salt packaging, implores the league. Even to that communal salt shaker. Helpfully, the league’s offering suggested wording for that harbinger-of-doom missive: “Excess sodium can cause high blood pressure and promote stomach cancer. Limit your use.”
Catchy, right? But we at the Bureau of LOTME believe a picture is worth a thousand words of warning. Which is why we’ve taped a simple biohazard logo to our office Cheetos stash. Because of the sodium, you ask? Hardly. But just imagine that much optic-orange food coloring finding its way into the groundwater.
The anatomy of extinction
Barely a day goes by without a new theory about What Killed the Dinosaurs. A meteor killed the dinosaurs. Volcanoes killed the dinosaurs. Donald Trump asked the Ukrainians to kill the dinosaurs. Hilary Clinton’s emails killed the dinosaurs. Donald Trump asked the Australians to say that Robert Mueller killed the dinosaurs. The liberal media are covering up the existence of dinosaurs.
Enough already. What about the primates? We humans are still around – at least for the time being. But when was the last time you heard a good theory about what killed the Neanderthals?
Well, hang on to your earmuffs, because here comes one now.
Researchers have reconstructed the Neanderthal Eustachian tubes and determined that those early rivals of Homo sapiens were done in by … chronic ear infections.
The Neanderthal Eustachian tubes were very similar to those of human infants, and “middle ear infections are nearly ubiquitous among infants because the flat angle of an infant’s Eustachian tubes is prone to retain the otitis media bacteria that cause these infections – the same flat angle we found in Neanderthals,” coauthor Samuel Márquez, PhD, of the State University of New York said in a statement.
Unlike modern humans, however, the Neanderthal eustachian tube did not change with age, so middle ear infections were a lifelong threat.
“It’s not just the threat of dying of an infection,” said Dr. Márquez. “If you are constantly ill, you would not be as fit and effective in competing with your Homo sapiens cousins for food and other resources. In a world of survival of the fittest, it is no wonder that modern man, not Neanderthal, prevailed.”
In other words, it wasn’t brains that beat the big, bad Neanderthals; it was their own baby ears.
H. sapiens, raise a glass: Ears to you, Charles Darwin.

Dude, just chill out and relax ... or not
It’s been a tough week. You’ve struggled to get all your work done on time, you’ve had to put in long hours, you’re exhausted and stressed out, and you know that next week will be just as tough. But for now, it’s the weekend. Time to relax, right?
According to research from Penn State University published in the Journal of Affective Disorders, if you have anxiety disorder, not only will you have difficulty relaxing (hence the anxiety disorder), you may actually actively resist it, experiencing something called “relaxation-induced anxiety.”
The researchers recruited a group of students, some with anxiety disorder, some with major depressive disorder, and a control group, and administered a series of relaxation exercises both before and after watching a series of potentially upsetting videos. The people with anxiety disorders were more likely to experience spikes of negative emotion after the second relaxation exercise than the other two groups; these spikes were linked to a feeling of anxiety during relaxation exercise.
The researchers theorized that people with anxiety disorder were attempting to avoid large jumps in their stress level by remaining stressed at all times. But the investigators also noted that experiencing a range of emotions is natural and far healthier.
So, as annoying as it is to suffer through constant “stop worrying, just live in the moment” speeches from that one dudebro acquaintance who spends all his free time partying, he does have a point. We don’t recommend participating in the Edward Fortyhands game, though. Leave that to your “friend.” He’s used to it.
A salty surgeon general’s warning
Grab a pack of Marlboro Reds at the corner convenience store, and you’ll find the iconic red and white box adorned with dire warnings from the surgeon general.
Grab a salt shaker at the corner diner, and you’ll find ... salt. In a shaker. Perhaps adorned with the red residue from a prior diner’s ketchup addiction.
The World Hypertension League would like to make that salt shaker look a lot more like those Marlboros.
In a position statement in the Journal of Clinical Hypertension, the league outlined the case for giving sodium chloride the cancer-stick treatment. Exhibit A: “Unhealthy diets are a leading cause of death globally, and excess salt consumption is the biggest culprit, estimated to cause over 3 million deaths globally in 2017.”
Despite that tobacco-rivaling body count, the league says no country has demanded that salt containers wear warning labels.
But isn’t it enough to list sodium levels on food labels? Well, when’s the last time you studied the salty facts before binging on that entire party-size bag of Cheetos, Chester? (You’ll be hotboxing 4,500 mg of sodium, by the way.) The league rests its case.
What’s needed is a message that stops you mid fistful. It’s time to add warning labels to all salt packaging, implores the league. Even to that communal salt shaker. Helpfully, the league’s offering suggested wording for that harbinger-of-doom missive: “Excess sodium can cause high blood pressure and promote stomach cancer. Limit your use.”
Catchy, right? But we at the Bureau of LOTME believe a picture is worth a thousand words of warning. Which is why we’ve taped a simple biohazard logo to our office Cheetos stash. Because of the sodium, you ask? Hardly. But just imagine that much optic-orange food coloring finding its way into the groundwater.
The anatomy of extinction
Barely a day goes by without a new theory about What Killed the Dinosaurs. A meteor killed the dinosaurs. Volcanoes killed the dinosaurs. Donald Trump asked the Ukrainians to kill the dinosaurs. Hilary Clinton’s emails killed the dinosaurs. Donald Trump asked the Australians to say that Robert Mueller killed the dinosaurs. The liberal media are covering up the existence of dinosaurs.
Enough already. What about the primates? We humans are still around – at least for the time being. But when was the last time you heard a good theory about what killed the Neanderthals?
Well, hang on to your earmuffs, because here comes one now.
Researchers have reconstructed the Neanderthal Eustachian tubes and determined that those early rivals of Homo sapiens were done in by … chronic ear infections.
The Neanderthal Eustachian tubes were very similar to those of human infants, and “middle ear infections are nearly ubiquitous among infants because the flat angle of an infant’s Eustachian tubes is prone to retain the otitis media bacteria that cause these infections – the same flat angle we found in Neanderthals,” coauthor Samuel Márquez, PhD, of the State University of New York said in a statement.
Unlike modern humans, however, the Neanderthal eustachian tube did not change with age, so middle ear infections were a lifelong threat.
“It’s not just the threat of dying of an infection,” said Dr. Márquez. “If you are constantly ill, you would not be as fit and effective in competing with your Homo sapiens cousins for food and other resources. In a world of survival of the fittest, it is no wonder that modern man, not Neanderthal, prevailed.”
In other words, it wasn’t brains that beat the big, bad Neanderthals; it was their own baby ears.
H. sapiens, raise a glass: Ears to you, Charles Darwin.

Dude, just chill out and relax ... or not
It’s been a tough week. You’ve struggled to get all your work done on time, you’ve had to put in long hours, you’re exhausted and stressed out, and you know that next week will be just as tough. But for now, it’s the weekend. Time to relax, right?
According to research from Penn State University published in the Journal of Affective Disorders, if you have anxiety disorder, not only will you have difficulty relaxing (hence the anxiety disorder), you may actually actively resist it, experiencing something called “relaxation-induced anxiety.”
The researchers recruited a group of students, some with anxiety disorder, some with major depressive disorder, and a control group, and administered a series of relaxation exercises both before and after watching a series of potentially upsetting videos. The people with anxiety disorders were more likely to experience spikes of negative emotion after the second relaxation exercise than the other two groups; these spikes were linked to a feeling of anxiety during relaxation exercise.
The researchers theorized that people with anxiety disorder were attempting to avoid large jumps in their stress level by remaining stressed at all times. But the investigators also noted that experiencing a range of emotions is natural and far healthier.
So, as annoying as it is to suffer through constant “stop worrying, just live in the moment” speeches from that one dudebro acquaintance who spends all his free time partying, he does have a point. We don’t recommend participating in the Edward Fortyhands game, though. Leave that to your “friend.” He’s used to it.
A salty surgeon general’s warning
Grab a pack of Marlboro Reds at the corner convenience store, and you’ll find the iconic red and white box adorned with dire warnings from the surgeon general.
Grab a salt shaker at the corner diner, and you’ll find ... salt. In a shaker. Perhaps adorned with the red residue from a prior diner’s ketchup addiction.
The World Hypertension League would like to make that salt shaker look a lot more like those Marlboros.
In a position statement in the Journal of Clinical Hypertension, the league outlined the case for giving sodium chloride the cancer-stick treatment. Exhibit A: “Unhealthy diets are a leading cause of death globally, and excess salt consumption is the biggest culprit, estimated to cause over 3 million deaths globally in 2017.”
Despite that tobacco-rivaling body count, the league says no country has demanded that salt containers wear warning labels.
But isn’t it enough to list sodium levels on food labels? Well, when’s the last time you studied the salty facts before binging on that entire party-size bag of Cheetos, Chester? (You’ll be hotboxing 4,500 mg of sodium, by the way.) The league rests its case.
What’s needed is a message that stops you mid fistful. It’s time to add warning labels to all salt packaging, implores the league. Even to that communal salt shaker. Helpfully, the league’s offering suggested wording for that harbinger-of-doom missive: “Excess sodium can cause high blood pressure and promote stomach cancer. Limit your use.”
Catchy, right? But we at the Bureau of LOTME believe a picture is worth a thousand words of warning. Which is why we’ve taped a simple biohazard logo to our office Cheetos stash. Because of the sodium, you ask? Hardly. But just imagine that much optic-orange food coloring finding its way into the groundwater.
The anatomy of extinction
Barely a day goes by without a new theory about What Killed the Dinosaurs. A meteor killed the dinosaurs. Volcanoes killed the dinosaurs. Donald Trump asked the Ukrainians to kill the dinosaurs. Hilary Clinton’s emails killed the dinosaurs. Donald Trump asked the Australians to say that Robert Mueller killed the dinosaurs. The liberal media are covering up the existence of dinosaurs.
Enough already. What about the primates? We humans are still around – at least for the time being. But when was the last time you heard a good theory about what killed the Neanderthals?
Well, hang on to your earmuffs, because here comes one now.
Researchers have reconstructed the Neanderthal Eustachian tubes and determined that those early rivals of Homo sapiens were done in by … chronic ear infections.
The Neanderthal Eustachian tubes were very similar to those of human infants, and “middle ear infections are nearly ubiquitous among infants because the flat angle of an infant’s Eustachian tubes is prone to retain the otitis media bacteria that cause these infections – the same flat angle we found in Neanderthals,” coauthor Samuel Márquez, PhD, of the State University of New York said in a statement.
Unlike modern humans, however, the Neanderthal eustachian tube did not change with age, so middle ear infections were a lifelong threat.
“It’s not just the threat of dying of an infection,” said Dr. Márquez. “If you are constantly ill, you would not be as fit and effective in competing with your Homo sapiens cousins for food and other resources. In a world of survival of the fittest, it is no wonder that modern man, not Neanderthal, prevailed.”
In other words, it wasn’t brains that beat the big, bad Neanderthals; it was their own baby ears.
H. sapiens, raise a glass: Ears to you, Charles Darwin.

I have seen the future
Many patients have seen their long-term physicians retire. When I ask how they like their new doctors, they say: “She’s okay, I guess. Quite efficient. Seems thorough. But it’s not the same. It’s just business. Nothing personal.”
Sometimes you have to look backward to look forward. So it’s perhaps fitting that I glimpsed the future at my last colonoscopy.
In recent years, I’ve had such procedures at a local suburban surgicenter. Easy access, plenty of parking.
The woman who checks me in is all business. She scans my insurance cards and hands me a clipboard with a medical history form. Have I ever had cancer? A hernia? Am I pregnant? I wonder whether anyone reads these.
A different young woman brings me inside, the first of many new faces. Their roles are murky.
In a curtained cubby, yet another staff person asks me to pack my clothes in a plastic bag and put on a johnny. Then an older man enters, initiating furious multitasking. A different nursing assistant asks me to confirm my name and date of birth, then inserts an intravenous line in one arm, while the old doctor hands me an anesthesia consent form to sign with the other hand. I check many answers very fast, ignore the small-print boilerplate, and sign.
I am handed two more consent forms to sign, one from each side. The staff makes no pretense of explaining them or even telling me what they are for, and I make none of reading them.
They depart, replaced by still another person, who rolls me into the next room. He confirms my name and date of birth, and which procedure I am there for. The purpose of these multiple checks is clear, along with dispiriting depersonalization. One could mitigate this with some light banter, but no one bothers. No time.
My physician – whom I actually know – enters, says hello, and exchanges pleasantries. The last guy asks me to turn onto my left side. Intravenous sedation flows into my veins. The rest is silence.
Sometime later I wake up, greeted by another staff person. She asks if I am okay and offers me water or juice and saltines. Noting her Boston Red Sox sweatshirt, I say, “Great game last night,” but she does not know what I am talking about. She cares only for football and plans to fly to Nashville, Tenn., to watch her favorites.
Curtains are closed, and I am asked to dress. Another assistant directs me to a chair, where I will await my ride home. Through I try to walk alone, she takes my arm. “We assist everyone,” she explains.
As the sedation wears off, I observe. All around me I see movement, brisk and purposeful. Staff members crisscross before me from all angles, striding from one task to another, from prep room A to cubby D, walking with or pushing patients from procedure room M to holding area 8H. No one I’ve just met recognizes me, or acknowledges having met me before.
At last, the final staff member approaches. She flashes a kind smile as she takes my arm to walk me to the door. I take this for a personal touch, until she explains that she must make sure I don’t fall and that I get into the right car. As we pass, no one in the waiting room, neither staff nor patients, takes any notice.
My wife is outside, idling in the correct car. She’s brought coffee and a chocolate croissant, which – almost – makes last night’s prep worthwhile. She confirms neither my name nor date of birth.
Altogether, I have been in and out in 90 minutes. In the car, I peruse the handout that had been given to me as I exited. Drinking my coffee, I read the postcare instructions and enjoy its full-color pictures. Seldom has my cecum looked more radiant.
In “The Checklist Manifesto,” Atul Gawande described the outcome improvement that systematized practice can achieve. Data analysis confirms the measurably superior efficacy of such a method.
As for me, I feel like output from one of today’s cataract factories: like a car just extruded from an automated wash, with a photo on its front seat of the shiny, Simonized hubcaps included with the premium service package.
Just business, though. Nothing personal.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
Many patients have seen their long-term physicians retire. When I ask how they like their new doctors, they say: “She’s okay, I guess. Quite efficient. Seems thorough. But it’s not the same. It’s just business. Nothing personal.”
Sometimes you have to look backward to look forward. So it’s perhaps fitting that I glimpsed the future at my last colonoscopy.
In recent years, I’ve had such procedures at a local suburban surgicenter. Easy access, plenty of parking.
The woman who checks me in is all business. She scans my insurance cards and hands me a clipboard with a medical history form. Have I ever had cancer? A hernia? Am I pregnant? I wonder whether anyone reads these.
A different young woman brings me inside, the first of many new faces. Their roles are murky.
In a curtained cubby, yet another staff person asks me to pack my clothes in a plastic bag and put on a johnny. Then an older man enters, initiating furious multitasking. A different nursing assistant asks me to confirm my name and date of birth, then inserts an intravenous line in one arm, while the old doctor hands me an anesthesia consent form to sign with the other hand. I check many answers very fast, ignore the small-print boilerplate, and sign.
I am handed two more consent forms to sign, one from each side. The staff makes no pretense of explaining them or even telling me what they are for, and I make none of reading them.
They depart, replaced by still another person, who rolls me into the next room. He confirms my name and date of birth, and which procedure I am there for. The purpose of these multiple checks is clear, along with dispiriting depersonalization. One could mitigate this with some light banter, but no one bothers. No time.
My physician – whom I actually know – enters, says hello, and exchanges pleasantries. The last guy asks me to turn onto my left side. Intravenous sedation flows into my veins. The rest is silence.
Sometime later I wake up, greeted by another staff person. She asks if I am okay and offers me water or juice and saltines. Noting her Boston Red Sox sweatshirt, I say, “Great game last night,” but she does not know what I am talking about. She cares only for football and plans to fly to Nashville, Tenn., to watch her favorites.
Curtains are closed, and I am asked to dress. Another assistant directs me to a chair, where I will await my ride home. Through I try to walk alone, she takes my arm. “We assist everyone,” she explains.
As the sedation wears off, I observe. All around me I see movement, brisk and purposeful. Staff members crisscross before me from all angles, striding from one task to another, from prep room A to cubby D, walking with or pushing patients from procedure room M to holding area 8H. No one I’ve just met recognizes me, or acknowledges having met me before.
At last, the final staff member approaches. She flashes a kind smile as she takes my arm to walk me to the door. I take this for a personal touch, until she explains that she must make sure I don’t fall and that I get into the right car. As we pass, no one in the waiting room, neither staff nor patients, takes any notice.
My wife is outside, idling in the correct car. She’s brought coffee and a chocolate croissant, which – almost – makes last night’s prep worthwhile. She confirms neither my name nor date of birth.
Altogether, I have been in and out in 90 minutes. In the car, I peruse the handout that had been given to me as I exited. Drinking my coffee, I read the postcare instructions and enjoy its full-color pictures. Seldom has my cecum looked more radiant.
In “The Checklist Manifesto,” Atul Gawande described the outcome improvement that systematized practice can achieve. Data analysis confirms the measurably superior efficacy of such a method.
As for me, I feel like output from one of today’s cataract factories: like a car just extruded from an automated wash, with a photo on its front seat of the shiny, Simonized hubcaps included with the premium service package.
Just business, though. Nothing personal.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
Many patients have seen their long-term physicians retire. When I ask how they like their new doctors, they say: “She’s okay, I guess. Quite efficient. Seems thorough. But it’s not the same. It’s just business. Nothing personal.”
Sometimes you have to look backward to look forward. So it’s perhaps fitting that I glimpsed the future at my last colonoscopy.
In recent years, I’ve had such procedures at a local suburban surgicenter. Easy access, plenty of parking.
The woman who checks me in is all business. She scans my insurance cards and hands me a clipboard with a medical history form. Have I ever had cancer? A hernia? Am I pregnant? I wonder whether anyone reads these.
A different young woman brings me inside, the first of many new faces. Their roles are murky.
In a curtained cubby, yet another staff person asks me to pack my clothes in a plastic bag and put on a johnny. Then an older man enters, initiating furious multitasking. A different nursing assistant asks me to confirm my name and date of birth, then inserts an intravenous line in one arm, while the old doctor hands me an anesthesia consent form to sign with the other hand. I check many answers very fast, ignore the small-print boilerplate, and sign.
I am handed two more consent forms to sign, one from each side. The staff makes no pretense of explaining them or even telling me what they are for, and I make none of reading them.
They depart, replaced by still another person, who rolls me into the next room. He confirms my name and date of birth, and which procedure I am there for. The purpose of these multiple checks is clear, along with dispiriting depersonalization. One could mitigate this with some light banter, but no one bothers. No time.
My physician – whom I actually know – enters, says hello, and exchanges pleasantries. The last guy asks me to turn onto my left side. Intravenous sedation flows into my veins. The rest is silence.
Sometime later I wake up, greeted by another staff person. She asks if I am okay and offers me water or juice and saltines. Noting her Boston Red Sox sweatshirt, I say, “Great game last night,” but she does not know what I am talking about. She cares only for football and plans to fly to Nashville, Tenn., to watch her favorites.
Curtains are closed, and I am asked to dress. Another assistant directs me to a chair, where I will await my ride home. Through I try to walk alone, she takes my arm. “We assist everyone,” she explains.
As the sedation wears off, I observe. All around me I see movement, brisk and purposeful. Staff members crisscross before me from all angles, striding from one task to another, from prep room A to cubby D, walking with or pushing patients from procedure room M to holding area 8H. No one I’ve just met recognizes me, or acknowledges having met me before.
At last, the final staff member approaches. She flashes a kind smile as she takes my arm to walk me to the door. I take this for a personal touch, until she explains that she must make sure I don’t fall and that I get into the right car. As we pass, no one in the waiting room, neither staff nor patients, takes any notice.
My wife is outside, idling in the correct car. She’s brought coffee and a chocolate croissant, which – almost – makes last night’s prep worthwhile. She confirms neither my name nor date of birth.
Altogether, I have been in and out in 90 minutes. In the car, I peruse the handout that had been given to me as I exited. Drinking my coffee, I read the postcare instructions and enjoy its full-color pictures. Seldom has my cecum looked more radiant.
In “The Checklist Manifesto,” Atul Gawande described the outcome improvement that systematized practice can achieve. Data analysis confirms the measurably superior efficacy of such a method.
As for me, I feel like output from one of today’s cataract factories: like a car just extruded from an automated wash, with a photo on its front seat of the shiny, Simonized hubcaps included with the premium service package.
Just business, though. Nothing personal.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
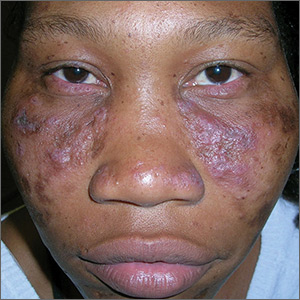
Central centrifugal cicatricial alopecia called epidemic in skin of color
NEW YORK – For unclear reasons, the prevalence of , creating an urgent need for early diagnosis and treatment, according to an expert who described this phenomenon at the Skin of Color Update 2019.
“CCCA has reached epidemic proportions,” contended Susan Taylor, MD, director of diversity, department of dermatology, Penn Medicine, Philadelphia.
Published data place prevalence rates of CCCA somewhere in the range of 3% to 6% among black women, but Dr. Taylor reported that she believes the condition is underdiagnosed. “I am seeing far more patients now than I was 30 years ago,” she maintained.
Others participating in the Skin of Color Update 2019 agreed. Heather Woolery-Lloyd, MD, director of ethnic skin care, department of dermatology and cutaneous surgery, University of Miami, also called the rising incidence of CCCA “an epidemic.” She, like Dr. Taylor, emphasized the critical importance of early diagnosis and treatment.
“I tell patients that if we can prevent hair loss over the next 10 years, this is a treatment success,” Dr. Woolery-Lloyd said. Although hair regrowth can be achieved in a minority of patients with treatments such as minoxidil, “the first goal is to prevent hair loss.”
Upon diagnosis, Dr. Woolery-Lloyd recommends treatment with intralesional triamcinolone and topical steroids immediately, adding other agents, such as oral antibiotics, if needed. Even in cases where CCCA has been identified before hair loss is visible, Dr. Woolery-Lloyd advised immediate therapy. Given that CCCA is a disease of reversible hair loss, she said, “do not take a wait-and-see approach.”
One potential obstacle for early diagnosis of CCCA, shared by other types of alopecia that are common in skin of color, is the failure of many clinicians to employ a standardized diagnosis in this patient population.
“If you do not have tightly coiled hair, it does not mean you cannot understand tightly coiled hair, but you have to learn, and you have to let patients know that you understand and have experience,” said Dr. Taylor, emphasizing the role of reassuring patients so they can be confident in the clinical recommendations.
Part of this reassurance will be derived from “interacting with patients in a culturally competent manner,” Dr. Taylor said. Clinicians must develop comfort and confidence in physically examining the hair and scalp, in asking patients to remove weaves or braids for a thorough inspection, and in avoiding comments that might be misinterpreted. Among these, she advised tactful questions about shampooing to avoid any implication that the clinician is implying inadequate hygiene.
When CCCA is suspected, a “biopsy is important” even in circumstances when the diagnosis seems straightforward. For one reason, a substantial proportion of patients may have a concomitant diagnosis. Dr. Taylor cited data from one study in which nearly 20% of CCCA patients also had traction alopecia and more than 10% had androgenic alopecia. Other coexisting problems identified on biopsy, including infection or seborrheic dermatitis, can help clinicians tailor a more effective intervention.
The initial signs of CCCA are typically hair breakage in the vertex of the scalp, which then expands in a central centrifugal pattern, according to Dr. Taylor. Although not all patients have signs of inflammation, such as itching and pustules, inhibition of inflammation represents the first line of therapy.
Relative to hair in the white population, the hair of black individuals grows more slowly and is more fragile, with greater amounts of breakage, said Dr. Taylor, citing published studies that support these differences. To improve early diagnosis of CCCA, understanding the hair in the black population is the first step for spotting problems in routine physical examinations. It may be this lack of familiarity that is contributing to underdiagnosis of CCCA.
“Almost 70% of African-American patients feel that physicians do not understand their hair,” Dr. Taylor said. “Let’s begin to change that.”
Cautioning that it is too often assumed that hairstyles and hair care, such as relaxants or hot combs, are the source of hair loss in black women, Dr. Taylor advised not to jump to conclusions. As an example, she described a case where weaves, braids, and other strategies were employed to mask alopecia after CCCA developed, not before.
“CCCA is the most important cause of scarring alopecia in African-American women,” Dr. Taylor said. Reiterating that hair loss can be prevented or modified with treatment, she added that this places the emphasis on first obtaining an accurate diagnosis.
Dr. Taylor has a financial relationship with Biersdorf; Dr. Woolery-Lloyd has financial relationships with Allergan, Galderma, Ortho Diagnostics, Pfizer, and Somabella Laboratories.
NEW YORK – For unclear reasons, the prevalence of , creating an urgent need for early diagnosis and treatment, according to an expert who described this phenomenon at the Skin of Color Update 2019.
“CCCA has reached epidemic proportions,” contended Susan Taylor, MD, director of diversity, department of dermatology, Penn Medicine, Philadelphia.
Published data place prevalence rates of CCCA somewhere in the range of 3% to 6% among black women, but Dr. Taylor reported that she believes the condition is underdiagnosed. “I am seeing far more patients now than I was 30 years ago,” she maintained.
Others participating in the Skin of Color Update 2019 agreed. Heather Woolery-Lloyd, MD, director of ethnic skin care, department of dermatology and cutaneous surgery, University of Miami, also called the rising incidence of CCCA “an epidemic.” She, like Dr. Taylor, emphasized the critical importance of early diagnosis and treatment.
“I tell patients that if we can prevent hair loss over the next 10 years, this is a treatment success,” Dr. Woolery-Lloyd said. Although hair regrowth can be achieved in a minority of patients with treatments such as minoxidil, “the first goal is to prevent hair loss.”
Upon diagnosis, Dr. Woolery-Lloyd recommends treatment with intralesional triamcinolone and topical steroids immediately, adding other agents, such as oral antibiotics, if needed. Even in cases where CCCA has been identified before hair loss is visible, Dr. Woolery-Lloyd advised immediate therapy. Given that CCCA is a disease of reversible hair loss, she said, “do not take a wait-and-see approach.”
One potential obstacle for early diagnosis of CCCA, shared by other types of alopecia that are common in skin of color, is the failure of many clinicians to employ a standardized diagnosis in this patient population.
“If you do not have tightly coiled hair, it does not mean you cannot understand tightly coiled hair, but you have to learn, and you have to let patients know that you understand and have experience,” said Dr. Taylor, emphasizing the role of reassuring patients so they can be confident in the clinical recommendations.
Part of this reassurance will be derived from “interacting with patients in a culturally competent manner,” Dr. Taylor said. Clinicians must develop comfort and confidence in physically examining the hair and scalp, in asking patients to remove weaves or braids for a thorough inspection, and in avoiding comments that might be misinterpreted. Among these, she advised tactful questions about shampooing to avoid any implication that the clinician is implying inadequate hygiene.
When CCCA is suspected, a “biopsy is important” even in circumstances when the diagnosis seems straightforward. For one reason, a substantial proportion of patients may have a concomitant diagnosis. Dr. Taylor cited data from one study in which nearly 20% of CCCA patients also had traction alopecia and more than 10% had androgenic alopecia. Other coexisting problems identified on biopsy, including infection or seborrheic dermatitis, can help clinicians tailor a more effective intervention.
The initial signs of CCCA are typically hair breakage in the vertex of the scalp, which then expands in a central centrifugal pattern, according to Dr. Taylor. Although not all patients have signs of inflammation, such as itching and pustules, inhibition of inflammation represents the first line of therapy.
Relative to hair in the white population, the hair of black individuals grows more slowly and is more fragile, with greater amounts of breakage, said Dr. Taylor, citing published studies that support these differences. To improve early diagnosis of CCCA, understanding the hair in the black population is the first step for spotting problems in routine physical examinations. It may be this lack of familiarity that is contributing to underdiagnosis of CCCA.
“Almost 70% of African-American patients feel that physicians do not understand their hair,” Dr. Taylor said. “Let’s begin to change that.”
Cautioning that it is too often assumed that hairstyles and hair care, such as relaxants or hot combs, are the source of hair loss in black women, Dr. Taylor advised not to jump to conclusions. As an example, she described a case where weaves, braids, and other strategies were employed to mask alopecia after CCCA developed, not before.
“CCCA is the most important cause of scarring alopecia in African-American women,” Dr. Taylor said. Reiterating that hair loss can be prevented or modified with treatment, she added that this places the emphasis on first obtaining an accurate diagnosis.
Dr. Taylor has a financial relationship with Biersdorf; Dr. Woolery-Lloyd has financial relationships with Allergan, Galderma, Ortho Diagnostics, Pfizer, and Somabella Laboratories.
NEW YORK – For unclear reasons, the prevalence of , creating an urgent need for early diagnosis and treatment, according to an expert who described this phenomenon at the Skin of Color Update 2019.
“CCCA has reached epidemic proportions,” contended Susan Taylor, MD, director of diversity, department of dermatology, Penn Medicine, Philadelphia.
Published data place prevalence rates of CCCA somewhere in the range of 3% to 6% among black women, but Dr. Taylor reported that she believes the condition is underdiagnosed. “I am seeing far more patients now than I was 30 years ago,” she maintained.
Others participating in the Skin of Color Update 2019 agreed. Heather Woolery-Lloyd, MD, director of ethnic skin care, department of dermatology and cutaneous surgery, University of Miami, also called the rising incidence of CCCA “an epidemic.” She, like Dr. Taylor, emphasized the critical importance of early diagnosis and treatment.
“I tell patients that if we can prevent hair loss over the next 10 years, this is a treatment success,” Dr. Woolery-Lloyd said. Although hair regrowth can be achieved in a minority of patients with treatments such as minoxidil, “the first goal is to prevent hair loss.”
Upon diagnosis, Dr. Woolery-Lloyd recommends treatment with intralesional triamcinolone and topical steroids immediately, adding other agents, such as oral antibiotics, if needed. Even in cases where CCCA has been identified before hair loss is visible, Dr. Woolery-Lloyd advised immediate therapy. Given that CCCA is a disease of reversible hair loss, she said, “do not take a wait-and-see approach.”
One potential obstacle for early diagnosis of CCCA, shared by other types of alopecia that are common in skin of color, is the failure of many clinicians to employ a standardized diagnosis in this patient population.
“If you do not have tightly coiled hair, it does not mean you cannot understand tightly coiled hair, but you have to learn, and you have to let patients know that you understand and have experience,” said Dr. Taylor, emphasizing the role of reassuring patients so they can be confident in the clinical recommendations.
Part of this reassurance will be derived from “interacting with patients in a culturally competent manner,” Dr. Taylor said. Clinicians must develop comfort and confidence in physically examining the hair and scalp, in asking patients to remove weaves or braids for a thorough inspection, and in avoiding comments that might be misinterpreted. Among these, she advised tactful questions about shampooing to avoid any implication that the clinician is implying inadequate hygiene.
When CCCA is suspected, a “biopsy is important” even in circumstances when the diagnosis seems straightforward. For one reason, a substantial proportion of patients may have a concomitant diagnosis. Dr. Taylor cited data from one study in which nearly 20% of CCCA patients also had traction alopecia and more than 10% had androgenic alopecia. Other coexisting problems identified on biopsy, including infection or seborrheic dermatitis, can help clinicians tailor a more effective intervention.
The initial signs of CCCA are typically hair breakage in the vertex of the scalp, which then expands in a central centrifugal pattern, according to Dr. Taylor. Although not all patients have signs of inflammation, such as itching and pustules, inhibition of inflammation represents the first line of therapy.
Relative to hair in the white population, the hair of black individuals grows more slowly and is more fragile, with greater amounts of breakage, said Dr. Taylor, citing published studies that support these differences. To improve early diagnosis of CCCA, understanding the hair in the black population is the first step for spotting problems in routine physical examinations. It may be this lack of familiarity that is contributing to underdiagnosis of CCCA.
“Almost 70% of African-American patients feel that physicians do not understand their hair,” Dr. Taylor said. “Let’s begin to change that.”
Cautioning that it is too often assumed that hairstyles and hair care, such as relaxants or hot combs, are the source of hair loss in black women, Dr. Taylor advised not to jump to conclusions. As an example, she described a case where weaves, braids, and other strategies were employed to mask alopecia after CCCA developed, not before.
“CCCA is the most important cause of scarring alopecia in African-American women,” Dr. Taylor said. Reiterating that hair loss can be prevented or modified with treatment, she added that this places the emphasis on first obtaining an accurate diagnosis.
Dr. Taylor has a financial relationship with Biersdorf; Dr. Woolery-Lloyd has financial relationships with Allergan, Galderma, Ortho Diagnostics, Pfizer, and Somabella Laboratories.
REPORTING FROM SOC 2019
Twitter Chat: Skin Cancer

Join us on Tuesday, October 8, 2019, at 8:00 pm EST on Twitter at #MDedgeChats as we discuss skin cancer, and what’s new in sunscreen, skin of color, and melanoma.
Special guests include physicians with expertise in dermatology and skin cancer, Anthony Rossi, MD (@DrAnthonyRossi), Julie Amthor Croley, MD, 15k followers on IG (@Drskinandsmiles), and Candrice Heath, MD (@DrCandriceHeath). Background information about the chat can be found below.
What will the conversation cover?
Q1: What are the most common types of skin cancer?
Q2: What recent research findings can better inform patients about skin cancer risks?
Q3: What’s the difference between melanoma in fair skin vs. darker skin?
Q4: How does the risk of skin cancer differ in people with darker skin?
Q5: Why should sunscreen be used even in the fall and winter?

Follow us here: @MDedgeDerm | @MDedgeTweets | #MDedgeChats
About Dr. Rossi:
Dr. Anthony Rossi (@DrAnthonyRossi) is a board-certified dermatologist with fellowship training in Mohs micrographic surgery, cosmetic and laser surgery, and advanced cutaneous oncology at the Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College program, both in New York. He specializes in skin cancer surgery, cosmetic dermatologic surgery, and laser surgery.
His research includes quality of life in cancer survivors, the use of noninvasive imaging of the skin, and nonsurgical treatments of skin cancer. Additionally, Dr. Rossi is active in dermatologic organizations and advocacy for medicine.
Research and Publications by Dr. Rossi
About Dr. Heath:
Dr. Candrice Heath (@DrCandriceHeath) is Assistant Professor of Dermatology at the Lewis Katz School of Medicine at Temple University in Philadelphia, Pennsylvania with fellowship training in pediatric dermatology at Johns Hopkins University in Baltimore, Maryland. Dr. Heath is triple board certified in pediatrics, dermatology, and pediatric dermatology. She specializes in adult and pediatric dermatology, skin of color, acne, and eczema. Dr. Heath also enjoys educating primary care physicians on the front lines of health care and delivering easy to understand information to consumers.
Research and publications by Dr. Heath
Guest host of MDedge podcast: A sunscreen update with Dr. Vincent DeLeo.
About Dr. Croley:
Dr. Julie Amthor Croley (@Drskinandsmiles) also known as “Dr. Skin and Smiles” has 15,000 followers on Instagram, and is a Chief Dermatology Resident at the University of Texas Medical Branch in Galveston, Texas. She has a special interest in skin cancer and dermatological surgery and hopes to complete a fellowship in Mohs micrographic surgery after residency. In her free time, Dr. Croley enjoys spending time with her husband (an orthopedic surgeon), running and competing in marathons, and spending time on the beach.
Cutaneous melanoma is the most fatal form of skin cancer and is a considerable public health concern in the United States. Early detection and management of skin cancer can lead to decreased morbidity and mortality from skin cancer. As a result, the American Academy of Dermatology Association supports safe sun-protective practices and diligent self-screening for changing lesions.
Sunscreen use is an essential component of sun protection. New regulations from the US Food and Drug Administration (FDA) have left consumers concerned about the safety of sunscreens. According to a recent Cutis editorial from Vincent A. DeLeo, MD, “There is no question that, as physicians, we want to ‘first, do no harm,’ so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data.”
Patients with skin of color experience disproportionately higher morbidity and mortality when diagnosed with melanoma. “Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis,” according to a recent Cutis article.
Population-based skin cancer screening performed exclusively by dermatologists is not practical. Primary care physicians and other experts in melanoma and public health need to be involved in reducing melanoma mortality.
In this chat, we will provide expert recommendations on the diagnosis of skin cancer, preventive measures, and the latest research discussed among physicians.
- “Doctor, Do I Need a Skin Check?”
- Assessing the effectiveness of knowledge-based interventions in skin of color populations.
- Melanoma in US Hispanics
- Podcast: Sunscreen update from Dr. Vincent DeLeo
- Windshield and UV exposure
- Racial, ethnic minorities often don’t practice sun-protective behaviors.
- Sunscreen regulations and advice for your patients.

Join us on Tuesday, October 8, 2019, at 8:00 pm EST on Twitter at #MDedgeChats as we discuss skin cancer, and what’s new in sunscreen, skin of color, and melanoma.
Special guests include physicians with expertise in dermatology and skin cancer, Anthony Rossi, MD (@DrAnthonyRossi), Julie Amthor Croley, MD, 15k followers on IG (@Drskinandsmiles), and Candrice Heath, MD (@DrCandriceHeath). Background information about the chat can be found below.
What will the conversation cover?
Q1: What are the most common types of skin cancer?
Q2: What recent research findings can better inform patients about skin cancer risks?
Q3: What’s the difference between melanoma in fair skin vs. darker skin?
Q4: How does the risk of skin cancer differ in people with darker skin?
Q5: Why should sunscreen be used even in the fall and winter?

Follow us here: @MDedgeDerm | @MDedgeTweets | #MDedgeChats
About Dr. Rossi:
Dr. Anthony Rossi (@DrAnthonyRossi) is a board-certified dermatologist with fellowship training in Mohs micrographic surgery, cosmetic and laser surgery, and advanced cutaneous oncology at the Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College program, both in New York. He specializes in skin cancer surgery, cosmetic dermatologic surgery, and laser surgery.
His research includes quality of life in cancer survivors, the use of noninvasive imaging of the skin, and nonsurgical treatments of skin cancer. Additionally, Dr. Rossi is active in dermatologic organizations and advocacy for medicine.
Research and Publications by Dr. Rossi
About Dr. Heath:
Dr. Candrice Heath (@DrCandriceHeath) is Assistant Professor of Dermatology at the Lewis Katz School of Medicine at Temple University in Philadelphia, Pennsylvania with fellowship training in pediatric dermatology at Johns Hopkins University in Baltimore, Maryland. Dr. Heath is triple board certified in pediatrics, dermatology, and pediatric dermatology. She specializes in adult and pediatric dermatology, skin of color, acne, and eczema. Dr. Heath also enjoys educating primary care physicians on the front lines of health care and delivering easy to understand information to consumers.
Research and publications by Dr. Heath
Guest host of MDedge podcast: A sunscreen update with Dr. Vincent DeLeo.
About Dr. Croley:
Dr. Julie Amthor Croley (@Drskinandsmiles) also known as “Dr. Skin and Smiles” has 15,000 followers on Instagram, and is a Chief Dermatology Resident at the University of Texas Medical Branch in Galveston, Texas. She has a special interest in skin cancer and dermatological surgery and hopes to complete a fellowship in Mohs micrographic surgery after residency. In her free time, Dr. Croley enjoys spending time with her husband (an orthopedic surgeon), running and competing in marathons, and spending time on the beach.
Cutaneous melanoma is the most fatal form of skin cancer and is a considerable public health concern in the United States. Early detection and management of skin cancer can lead to decreased morbidity and mortality from skin cancer. As a result, the American Academy of Dermatology Association supports safe sun-protective practices and diligent self-screening for changing lesions.
Sunscreen use is an essential component of sun protection. New regulations from the US Food and Drug Administration (FDA) have left consumers concerned about the safety of sunscreens. According to a recent Cutis editorial from Vincent A. DeLeo, MD, “There is no question that, as physicians, we want to ‘first, do no harm,’ so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data.”
Patients with skin of color experience disproportionately higher morbidity and mortality when diagnosed with melanoma. “Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis,” according to a recent Cutis article.
Population-based skin cancer screening performed exclusively by dermatologists is not practical. Primary care physicians and other experts in melanoma and public health need to be involved in reducing melanoma mortality.
In this chat, we will provide expert recommendations on the diagnosis of skin cancer, preventive measures, and the latest research discussed among physicians.
- “Doctor, Do I Need a Skin Check?”
- Assessing the effectiveness of knowledge-based interventions in skin of color populations.
- Melanoma in US Hispanics
- Podcast: Sunscreen update from Dr. Vincent DeLeo
- Windshield and UV exposure
- Racial, ethnic minorities often don’t practice sun-protective behaviors.
- Sunscreen regulations and advice for your patients.

Join us on Tuesday, October 8, 2019, at 8:00 pm EST on Twitter at #MDedgeChats as we discuss skin cancer, and what’s new in sunscreen, skin of color, and melanoma.
Special guests include physicians with expertise in dermatology and skin cancer, Anthony Rossi, MD (@DrAnthonyRossi), Julie Amthor Croley, MD, 15k followers on IG (@Drskinandsmiles), and Candrice Heath, MD (@DrCandriceHeath). Background information about the chat can be found below.
What will the conversation cover?
Q1: What are the most common types of skin cancer?
Q2: What recent research findings can better inform patients about skin cancer risks?
Q3: What’s the difference between melanoma in fair skin vs. darker skin?
Q4: How does the risk of skin cancer differ in people with darker skin?
Q5: Why should sunscreen be used even in the fall and winter?

Follow us here: @MDedgeDerm | @MDedgeTweets | #MDedgeChats
About Dr. Rossi:
Dr. Anthony Rossi (@DrAnthonyRossi) is a board-certified dermatologist with fellowship training in Mohs micrographic surgery, cosmetic and laser surgery, and advanced cutaneous oncology at the Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College program, both in New York. He specializes in skin cancer surgery, cosmetic dermatologic surgery, and laser surgery.
His research includes quality of life in cancer survivors, the use of noninvasive imaging of the skin, and nonsurgical treatments of skin cancer. Additionally, Dr. Rossi is active in dermatologic organizations and advocacy for medicine.
Research and Publications by Dr. Rossi
About Dr. Heath:
Dr. Candrice Heath (@DrCandriceHeath) is Assistant Professor of Dermatology at the Lewis Katz School of Medicine at Temple University in Philadelphia, Pennsylvania with fellowship training in pediatric dermatology at Johns Hopkins University in Baltimore, Maryland. Dr. Heath is triple board certified in pediatrics, dermatology, and pediatric dermatology. She specializes in adult and pediatric dermatology, skin of color, acne, and eczema. Dr. Heath also enjoys educating primary care physicians on the front lines of health care and delivering easy to understand information to consumers.
Research and publications by Dr. Heath
Guest host of MDedge podcast: A sunscreen update with Dr. Vincent DeLeo.
About Dr. Croley:
Dr. Julie Amthor Croley (@Drskinandsmiles) also known as “Dr. Skin and Smiles” has 15,000 followers on Instagram, and is a Chief Dermatology Resident at the University of Texas Medical Branch in Galveston, Texas. She has a special interest in skin cancer and dermatological surgery and hopes to complete a fellowship in Mohs micrographic surgery after residency. In her free time, Dr. Croley enjoys spending time with her husband (an orthopedic surgeon), running and competing in marathons, and spending time on the beach.
Cutaneous melanoma is the most fatal form of skin cancer and is a considerable public health concern in the United States. Early detection and management of skin cancer can lead to decreased morbidity and mortality from skin cancer. As a result, the American Academy of Dermatology Association supports safe sun-protective practices and diligent self-screening for changing lesions.
Sunscreen use is an essential component of sun protection. New regulations from the US Food and Drug Administration (FDA) have left consumers concerned about the safety of sunscreens. According to a recent Cutis editorial from Vincent A. DeLeo, MD, “There is no question that, as physicians, we want to ‘first, do no harm,’ so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data.”
Patients with skin of color experience disproportionately higher morbidity and mortality when diagnosed with melanoma. “Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis,” according to a recent Cutis article.
Population-based skin cancer screening performed exclusively by dermatologists is not practical. Primary care physicians and other experts in melanoma and public health need to be involved in reducing melanoma mortality.
In this chat, we will provide expert recommendations on the diagnosis of skin cancer, preventive measures, and the latest research discussed among physicians.
- “Doctor, Do I Need a Skin Check?”
- Assessing the effectiveness of knowledge-based interventions in skin of color populations.
- Melanoma in US Hispanics
- Podcast: Sunscreen update from Dr. Vincent DeLeo
- Windshield and UV exposure
- Racial, ethnic minorities often don’t practice sun-protective behaviors.
- Sunscreen regulations and advice for your patients.
TAVR, SAVR share same infective endocarditis risk
PARIS – The risk of infective endocarditis following transcatheter aortic valve replacement (TAVR) for the treatment of severe aortic stenosis proved to be the same as after surgical replacement in a French national propensity score–matched study.
This finding from what is believed to be the largest-ever study of infective endocarditis following TAVR will come as a surprise to many physicians. It’s easy to mistakenly assume the risk of this feared complication is lower – and perhaps even negligible – in TAVR patients since the procedure doesn’t involve a significant surgical wound, it’s briefer, the hospital length of stay is shorter, and recovery time is markedly less than with surgical aortic valve replacement (SAVR).
Not so, Laurent Fauchier, MD, PhD, said in presenting the study findings at the annual congress of the European Society of Cardiology.
“Do not think there is a lower risk of infective endocarditis. Be aware, be careful, and provide appropriate antibiotic prophylaxis, just as surgeons do in SAVR. Don’t think, as I did, that with TAVR with no pacemaker implantation there is no risk of infective endocarditis. The TAVR valve is a device, it’s a prosthesis, and the risk is very similar to that of surgery,” advised Dr. Fauchier, a cardiologist at Francois Rabelais University in Tours, France.
He presented a study of all of the nearly 108,000 patients who underwent isolated TAVR or SAVR in France during 2010-2018. The data source was the French national administrative hospital discharge record system. Since the TAVR patients were overall markedly older and sicker than the SAVR patients, especially during the first years of the study, he and his coinvestigators performed propensity score matching using 30 variables, which enabled them to narrow the field of inquiry down to a carefully selected study population of 16,291 TAVR patients and an equal number of closely similar SAVR patients.
A total of 1,070 cases of infective endocarditis occurred during a mean follow-up of just over 2 years. The rate of hospital admission for this complication was 1.89% per year in the TAVR group and similar at 1.71% per year in the SAVR cohort.
Of note, all-cause mortality in TAVR patients who developed infective endocarditis was 1.32-fold greater than it was in SAVR patients with infective endocarditis, a statistically significant difference. The explanation for the increased mortality risk in the TAVR group probably has to do at least in part with an inability on the part of the investigators to fully capture and control for the TAVR group’s greater frailty, according to the cardiologist.
Risk factors for infective endocarditis shared in common by TAVR and SAVR patients included male gender, a higher Charlson Comorbidity Index score, and a greater frailty index. The main predictors unique to the TAVR patients were atrial fibrillation, anemia, and tricuspid regurgitation. And although pacemaker and defibrillator implantation were risk factors for infective endocarditis in the SAVR patients, it wasn’t predictive of increased risk in the TAVR population. Dr. Fauchier called this finding “quite reassuring” given that roughly 20% of the TAVR group received a pacemaker.
The causative microorganisms for infective endocarditis were essentially the same in the TAVR and SAVR groups, simplifying antimicrobial prophylaxis decision making.
Dr. Fauchier reported having no financial conflicts regarding the study, conducted free of commercial support. He serves as a consultant to and/or on speakers’ bureaus for Bayer, BMS Pfizer, Boehringer Ingelheim, Medtronic, and Novartis.
PARIS – The risk of infective endocarditis following transcatheter aortic valve replacement (TAVR) for the treatment of severe aortic stenosis proved to be the same as after surgical replacement in a French national propensity score–matched study.
This finding from what is believed to be the largest-ever study of infective endocarditis following TAVR will come as a surprise to many physicians. It’s easy to mistakenly assume the risk of this feared complication is lower – and perhaps even negligible – in TAVR patients since the procedure doesn’t involve a significant surgical wound, it’s briefer, the hospital length of stay is shorter, and recovery time is markedly less than with surgical aortic valve replacement (SAVR).
Not so, Laurent Fauchier, MD, PhD, said in presenting the study findings at the annual congress of the European Society of Cardiology.
“Do not think there is a lower risk of infective endocarditis. Be aware, be careful, and provide appropriate antibiotic prophylaxis, just as surgeons do in SAVR. Don’t think, as I did, that with TAVR with no pacemaker implantation there is no risk of infective endocarditis. The TAVR valve is a device, it’s a prosthesis, and the risk is very similar to that of surgery,” advised Dr. Fauchier, a cardiologist at Francois Rabelais University in Tours, France.
He presented a study of all of the nearly 108,000 patients who underwent isolated TAVR or SAVR in France during 2010-2018. The data source was the French national administrative hospital discharge record system. Since the TAVR patients were overall markedly older and sicker than the SAVR patients, especially during the first years of the study, he and his coinvestigators performed propensity score matching using 30 variables, which enabled them to narrow the field of inquiry down to a carefully selected study population of 16,291 TAVR patients and an equal number of closely similar SAVR patients.
A total of 1,070 cases of infective endocarditis occurred during a mean follow-up of just over 2 years. The rate of hospital admission for this complication was 1.89% per year in the TAVR group and similar at 1.71% per year in the SAVR cohort.
Of note, all-cause mortality in TAVR patients who developed infective endocarditis was 1.32-fold greater than it was in SAVR patients with infective endocarditis, a statistically significant difference. The explanation for the increased mortality risk in the TAVR group probably has to do at least in part with an inability on the part of the investigators to fully capture and control for the TAVR group’s greater frailty, according to the cardiologist.
Risk factors for infective endocarditis shared in common by TAVR and SAVR patients included male gender, a higher Charlson Comorbidity Index score, and a greater frailty index. The main predictors unique to the TAVR patients were atrial fibrillation, anemia, and tricuspid regurgitation. And although pacemaker and defibrillator implantation were risk factors for infective endocarditis in the SAVR patients, it wasn’t predictive of increased risk in the TAVR population. Dr. Fauchier called this finding “quite reassuring” given that roughly 20% of the TAVR group received a pacemaker.
The causative microorganisms for infective endocarditis were essentially the same in the TAVR and SAVR groups, simplifying antimicrobial prophylaxis decision making.
Dr. Fauchier reported having no financial conflicts regarding the study, conducted free of commercial support. He serves as a consultant to and/or on speakers’ bureaus for Bayer, BMS Pfizer, Boehringer Ingelheim, Medtronic, and Novartis.
PARIS – The risk of infective endocarditis following transcatheter aortic valve replacement (TAVR) for the treatment of severe aortic stenosis proved to be the same as after surgical replacement in a French national propensity score–matched study.
This finding from what is believed to be the largest-ever study of infective endocarditis following TAVR will come as a surprise to many physicians. It’s easy to mistakenly assume the risk of this feared complication is lower – and perhaps even negligible – in TAVR patients since the procedure doesn’t involve a significant surgical wound, it’s briefer, the hospital length of stay is shorter, and recovery time is markedly less than with surgical aortic valve replacement (SAVR).
Not so, Laurent Fauchier, MD, PhD, said in presenting the study findings at the annual congress of the European Society of Cardiology.
“Do not think there is a lower risk of infective endocarditis. Be aware, be careful, and provide appropriate antibiotic prophylaxis, just as surgeons do in SAVR. Don’t think, as I did, that with TAVR with no pacemaker implantation there is no risk of infective endocarditis. The TAVR valve is a device, it’s a prosthesis, and the risk is very similar to that of surgery,” advised Dr. Fauchier, a cardiologist at Francois Rabelais University in Tours, France.
He presented a study of all of the nearly 108,000 patients who underwent isolated TAVR or SAVR in France during 2010-2018. The data source was the French national administrative hospital discharge record system. Since the TAVR patients were overall markedly older and sicker than the SAVR patients, especially during the first years of the study, he and his coinvestigators performed propensity score matching using 30 variables, which enabled them to narrow the field of inquiry down to a carefully selected study population of 16,291 TAVR patients and an equal number of closely similar SAVR patients.
A total of 1,070 cases of infective endocarditis occurred during a mean follow-up of just over 2 years. The rate of hospital admission for this complication was 1.89% per year in the TAVR group and similar at 1.71% per year in the SAVR cohort.
Of note, all-cause mortality in TAVR patients who developed infective endocarditis was 1.32-fold greater than it was in SAVR patients with infective endocarditis, a statistically significant difference. The explanation for the increased mortality risk in the TAVR group probably has to do at least in part with an inability on the part of the investigators to fully capture and control for the TAVR group’s greater frailty, according to the cardiologist.
Risk factors for infective endocarditis shared in common by TAVR and SAVR patients included male gender, a higher Charlson Comorbidity Index score, and a greater frailty index. The main predictors unique to the TAVR patients were atrial fibrillation, anemia, and tricuspid regurgitation. And although pacemaker and defibrillator implantation were risk factors for infective endocarditis in the SAVR patients, it wasn’t predictive of increased risk in the TAVR population. Dr. Fauchier called this finding “quite reassuring” given that roughly 20% of the TAVR group received a pacemaker.
The causative microorganisms for infective endocarditis were essentially the same in the TAVR and SAVR groups, simplifying antimicrobial prophylaxis decision making.
Dr. Fauchier reported having no financial conflicts regarding the study, conducted free of commercial support. He serves as a consultant to and/or on speakers’ bureaus for Bayer, BMS Pfizer, Boehringer Ingelheim, Medtronic, and Novartis.
REPORTING FROM THE ESC CONGRESS 2019
Scars and color changes on face
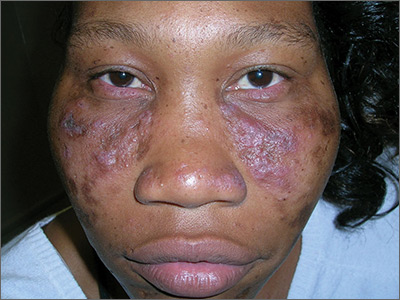
The FP recognized the scarring, skin atrophy, erythema and hyperpigmentation in the malar distribution as discoid lupus erythematosus (DLE). Upon examination, the FP noted similar patterns (with the addition of hypopigmentation) in the conchal bowls of both pinna. This was confirmatory for the DLE diagnosis (AKA chronic cutaneous lupus). (If in doubt, a 4-mm punch biopsy could be performed on the lesions of the face.) Without the lesions in the ears, sarcoidosis might have been considered as part of the differential diagnosis.
DLE lesions are characterized by discrete, erythematous, slightly infiltrated papules or plaques. Hypopigmentation develops in the central area and hyperpigmentation develops at the active border. Resolution of the active lesion results in atrophy and scarring.
In this case, the FP explained the diagnosis of DLE to the patient and ordered testing for antinuclear antibodies (ANA), a complete blood count, and comprehensive metabolic panel. All the labs were normal, and the ANA was negative, which confirmed that this was not a case of systemic lupus erythematosus. Patients with only DLE generally have negative or low-titer ANA titers.
DLE therapy includes corticosteroids (topical or intralesional) and oral antimalarials, such as hydroxychloroquine. The FP in this case started the patient on hydroxychloroquine 200 mg bid and topical triamcinolone 0.1% applied twice daily to the erythematous portions of the cheeks for 2 weeks. At the 2-week follow-up, the patient’s skin looked less red and inflamed and she was happy with the improved appearance of her skin. Patients on hydroxychloroquine need a baseline eye exam by an ophthalmologist and then yearly exams after 5 years of therapy to detect any retinal or visual field problems.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Pye A, Mayeaux, EJ, Mishra V, et al. Lupus. In: Usatine R, Smith M, Mayeaux EJ, et al. eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1183-1193.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/

The FP recognized the scarring, skin atrophy, erythema and hyperpigmentation in the malar distribution as discoid lupus erythematosus (DLE). Upon examination, the FP noted similar patterns (with the addition of hypopigmentation) in the conchal bowls of both pinna. This was confirmatory for the DLE diagnosis (AKA chronic cutaneous lupus). (If in doubt, a 4-mm punch biopsy could be performed on the lesions of the face.) Without the lesions in the ears, sarcoidosis might have been considered as part of the differential diagnosis.
DLE lesions are characterized by discrete, erythematous, slightly infiltrated papules or plaques. Hypopigmentation develops in the central area and hyperpigmentation develops at the active border. Resolution of the active lesion results in atrophy and scarring.
In this case, the FP explained the diagnosis of DLE to the patient and ordered testing for antinuclear antibodies (ANA), a complete blood count, and comprehensive metabolic panel. All the labs were normal, and the ANA was negative, which confirmed that this was not a case of systemic lupus erythematosus. Patients with only DLE generally have negative or low-titer ANA titers.
DLE therapy includes corticosteroids (topical or intralesional) and oral antimalarials, such as hydroxychloroquine. The FP in this case started the patient on hydroxychloroquine 200 mg bid and topical triamcinolone 0.1% applied twice daily to the erythematous portions of the cheeks for 2 weeks. At the 2-week follow-up, the patient’s skin looked less red and inflamed and she was happy with the improved appearance of her skin. Patients on hydroxychloroquine need a baseline eye exam by an ophthalmologist and then yearly exams after 5 years of therapy to detect any retinal or visual field problems.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Pye A, Mayeaux, EJ, Mishra V, et al. Lupus. In: Usatine R, Smith M, Mayeaux EJ, et al. eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1183-1193.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/

The FP recognized the scarring, skin atrophy, erythema and hyperpigmentation in the malar distribution as discoid lupus erythematosus (DLE). Upon examination, the FP noted similar patterns (with the addition of hypopigmentation) in the conchal bowls of both pinna. This was confirmatory for the DLE diagnosis (AKA chronic cutaneous lupus). (If in doubt, a 4-mm punch biopsy could be performed on the lesions of the face.) Without the lesions in the ears, sarcoidosis might have been considered as part of the differential diagnosis.
DLE lesions are characterized by discrete, erythematous, slightly infiltrated papules or plaques. Hypopigmentation develops in the central area and hyperpigmentation develops at the active border. Resolution of the active lesion results in atrophy and scarring.
In this case, the FP explained the diagnosis of DLE to the patient and ordered testing for antinuclear antibodies (ANA), a complete blood count, and comprehensive metabolic panel. All the labs were normal, and the ANA was negative, which confirmed that this was not a case of systemic lupus erythematosus. Patients with only DLE generally have negative or low-titer ANA titers.
DLE therapy includes corticosteroids (topical or intralesional) and oral antimalarials, such as hydroxychloroquine. The FP in this case started the patient on hydroxychloroquine 200 mg bid and topical triamcinolone 0.1% applied twice daily to the erythematous portions of the cheeks for 2 weeks. At the 2-week follow-up, the patient’s skin looked less red and inflamed and she was happy with the improved appearance of her skin. Patients on hydroxychloroquine need a baseline eye exam by an ophthalmologist and then yearly exams after 5 years of therapy to detect any retinal or visual field problems.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Pye A, Mayeaux, EJ, Mishra V, et al. Lupus. In: Usatine R, Smith M, Mayeaux EJ, et al. eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1183-1193.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/