User login
Experts say teledermatology’s postpandemic role is unclear
over those provided prior to the COVID-19 pandemic. But it is not clear whether the current rate will fall further, be sustained, or even climb again, according to data presented and opinions expressed in a forum on this topic at the American Academy of Dermatology Virtual Meeting Experience.
There are many unknowns, not least of which is future reimbursement from the Centers for Medicare & Medicaid Services and other third-party payers, according to several participants in a scientific session devoted to this topic. The CARES Act, which was passed in the early stages of the pandemic, provided only a temporary increase in reimbursement for telehealth. Postpandemic payments for telehealth services are yet undetermined.
Many of the assembled experts are convinced that teledermatology will continue to be offered at far higher rates than prior to the pandemic, but many issues, including physician acceptance of this approach remain unresolved. This was reflected in an AAD survey of members conducted in June 2020.
“Seventy percent of dermatologists responded that teledermatology will continue, but only 58% reported that they intend to offer it,” after the pandemic, reported Jules Lipoff, MD, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who is one of the authors of the paper that reported the results.
The low relative proportion of dermatologists planning to participate in teledermatology might at least in part reflect uncertainty about reimbursement, according to Dr. Lipoff, who is the outgoing chair of the AAD teledermatology task force
Many dermatologists might find it difficult to opt out of telehealth. In some types of care, such as follow-up visits, a combination of patient demand and institutional policy, particularly if reimbursement is adequate, might compel or at least strongly incentivize teledermatology services.
“Now that telemedicine has gotten out there, we will never go back to what once was normal practice,” Dr. Lipoff predicted. According to Dr. Lipoff, there was a great deal of data even prior to the pandemic to conclude that mobile dermatology is “an acceptable equivalent” for delivering many types of dermatologic care.
The rapid evolution in telemedicine is remarkable. According to the results of the AAD survey, 14.1% of dermatologists had experience with teledermatology prior to the COVID-19 pandemic, which increased to 96.9% by June, 2020, when the survey was conducted. Nearly 600 dermatologists completed the survey, for a 13.6% response rate.
At the beginning of the pandemic, the CARES act, along with other pandemic legislation and policy changes, changed the landscape of telemedicine by providing reimbursement commensurate with in-office visits, modifying HIPAA regulations, and permitting reciprocal licensing to allow physicians to provide care to patients who had moved out of the state. While these were among the factors that facilitated the phenomenal growth in telemedicine, nearly all of these changes were temporary or are subject to revision.
“Reimbursement [for telehealth] was very low prior to the pandemic,” noted Elizabeth K. Jones, MD, assistant professor of dermatology, Thomas Jefferson University, Philadelphia. While many physicians and policy makers were convinced that reimbursement levels had to be increased temporarily to provide medical care when in-office visits were unsafe, Dr. Jones said it is unlikely that pandemic reimbursement rates will be preserved. But recent statements from the CMS foreshadow lower rates for most video and telephone consults, she added.
Reimbursement is not the only consideration. George Han, MD, PhD, chief of teledermatology at the Icahn School of Medicine at Mount Sinai, New York, spoke about the frustration of using imperfect tools. He, like many dermatologists, have become familiar with the difficulty of making a definitive diagnosis from transmitted images of skin lesions.
As long as patients communicate with personal computers and phones under variable lighting conditions, this problem might never go away, but suboptimal quality images do not necessarily preclude other types of consults, particularly follow-up visits, according to Dr. Han, who is also system medical director for dermatology at Mount Sinai Health System.
“Now that patients know about teledermatology, they are for it,” he said. He suggested that increased efficiency of follow-up using telemedicine for both patients and physicians might increase the frequency with which these types of visits are scheduled. Citing evidence that follow-up visits increase patient retention rates, Dr. Han sees these visits among the routine uses of telemedicine when the pandemic is over.
At the height of the pandemic, teledermatology was employed broadly, but after the pandemic, Dr. Han and others predict a narrower focus. Some consults, such as those for acne or other conditions reasonably treated on the basis of patient history, appear to lend themselves to telemedicine. Others, such as a skin check for malignancy, might not.
As the role of telemedicine is sorted out and finds its equilibrium in a postpandemic world, Dr. Lipoff pointed out the need to consider populations without good-quality internet access. Without specific strategies to ensure these patients are not forgotten, he warned of “wealthier patients consuming more than their fair share” of health care resources, further widening an existing disparity.
“Is telemedicine here to stay? It is clear that, yes, it is in some way,” said Dr. Lipoff, who sees no reason for dermatology to be an exception.
Dr. Lipoff reported a financial relationship with AcneAway. The other investigators reported no potential conflicts of interest related to telemedicine.
over those provided prior to the COVID-19 pandemic. But it is not clear whether the current rate will fall further, be sustained, or even climb again, according to data presented and opinions expressed in a forum on this topic at the American Academy of Dermatology Virtual Meeting Experience.
There are many unknowns, not least of which is future reimbursement from the Centers for Medicare & Medicaid Services and other third-party payers, according to several participants in a scientific session devoted to this topic. The CARES Act, which was passed in the early stages of the pandemic, provided only a temporary increase in reimbursement for telehealth. Postpandemic payments for telehealth services are yet undetermined.
Many of the assembled experts are convinced that teledermatology will continue to be offered at far higher rates than prior to the pandemic, but many issues, including physician acceptance of this approach remain unresolved. This was reflected in an AAD survey of members conducted in June 2020.
“Seventy percent of dermatologists responded that teledermatology will continue, but only 58% reported that they intend to offer it,” after the pandemic, reported Jules Lipoff, MD, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who is one of the authors of the paper that reported the results.
The low relative proportion of dermatologists planning to participate in teledermatology might at least in part reflect uncertainty about reimbursement, according to Dr. Lipoff, who is the outgoing chair of the AAD teledermatology task force
Many dermatologists might find it difficult to opt out of telehealth. In some types of care, such as follow-up visits, a combination of patient demand and institutional policy, particularly if reimbursement is adequate, might compel or at least strongly incentivize teledermatology services.
“Now that telemedicine has gotten out there, we will never go back to what once was normal practice,” Dr. Lipoff predicted. According to Dr. Lipoff, there was a great deal of data even prior to the pandemic to conclude that mobile dermatology is “an acceptable equivalent” for delivering many types of dermatologic care.
The rapid evolution in telemedicine is remarkable. According to the results of the AAD survey, 14.1% of dermatologists had experience with teledermatology prior to the COVID-19 pandemic, which increased to 96.9% by June, 2020, when the survey was conducted. Nearly 600 dermatologists completed the survey, for a 13.6% response rate.
At the beginning of the pandemic, the CARES act, along with other pandemic legislation and policy changes, changed the landscape of telemedicine by providing reimbursement commensurate with in-office visits, modifying HIPAA regulations, and permitting reciprocal licensing to allow physicians to provide care to patients who had moved out of the state. While these were among the factors that facilitated the phenomenal growth in telemedicine, nearly all of these changes were temporary or are subject to revision.
“Reimbursement [for telehealth] was very low prior to the pandemic,” noted Elizabeth K. Jones, MD, assistant professor of dermatology, Thomas Jefferson University, Philadelphia. While many physicians and policy makers were convinced that reimbursement levels had to be increased temporarily to provide medical care when in-office visits were unsafe, Dr. Jones said it is unlikely that pandemic reimbursement rates will be preserved. But recent statements from the CMS foreshadow lower rates for most video and telephone consults, she added.
Reimbursement is not the only consideration. George Han, MD, PhD, chief of teledermatology at the Icahn School of Medicine at Mount Sinai, New York, spoke about the frustration of using imperfect tools. He, like many dermatologists, have become familiar with the difficulty of making a definitive diagnosis from transmitted images of skin lesions.
As long as patients communicate with personal computers and phones under variable lighting conditions, this problem might never go away, but suboptimal quality images do not necessarily preclude other types of consults, particularly follow-up visits, according to Dr. Han, who is also system medical director for dermatology at Mount Sinai Health System.
“Now that patients know about teledermatology, they are for it,” he said. He suggested that increased efficiency of follow-up using telemedicine for both patients and physicians might increase the frequency with which these types of visits are scheduled. Citing evidence that follow-up visits increase patient retention rates, Dr. Han sees these visits among the routine uses of telemedicine when the pandemic is over.
At the height of the pandemic, teledermatology was employed broadly, but after the pandemic, Dr. Han and others predict a narrower focus. Some consults, such as those for acne or other conditions reasonably treated on the basis of patient history, appear to lend themselves to telemedicine. Others, such as a skin check for malignancy, might not.
As the role of telemedicine is sorted out and finds its equilibrium in a postpandemic world, Dr. Lipoff pointed out the need to consider populations without good-quality internet access. Without specific strategies to ensure these patients are not forgotten, he warned of “wealthier patients consuming more than their fair share” of health care resources, further widening an existing disparity.
“Is telemedicine here to stay? It is clear that, yes, it is in some way,” said Dr. Lipoff, who sees no reason for dermatology to be an exception.
Dr. Lipoff reported a financial relationship with AcneAway. The other investigators reported no potential conflicts of interest related to telemedicine.
over those provided prior to the COVID-19 pandemic. But it is not clear whether the current rate will fall further, be sustained, or even climb again, according to data presented and opinions expressed in a forum on this topic at the American Academy of Dermatology Virtual Meeting Experience.
There are many unknowns, not least of which is future reimbursement from the Centers for Medicare & Medicaid Services and other third-party payers, according to several participants in a scientific session devoted to this topic. The CARES Act, which was passed in the early stages of the pandemic, provided only a temporary increase in reimbursement for telehealth. Postpandemic payments for telehealth services are yet undetermined.
Many of the assembled experts are convinced that teledermatology will continue to be offered at far higher rates than prior to the pandemic, but many issues, including physician acceptance of this approach remain unresolved. This was reflected in an AAD survey of members conducted in June 2020.
“Seventy percent of dermatologists responded that teledermatology will continue, but only 58% reported that they intend to offer it,” after the pandemic, reported Jules Lipoff, MD, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who is one of the authors of the paper that reported the results.
The low relative proportion of dermatologists planning to participate in teledermatology might at least in part reflect uncertainty about reimbursement, according to Dr. Lipoff, who is the outgoing chair of the AAD teledermatology task force
Many dermatologists might find it difficult to opt out of telehealth. In some types of care, such as follow-up visits, a combination of patient demand and institutional policy, particularly if reimbursement is adequate, might compel or at least strongly incentivize teledermatology services.
“Now that telemedicine has gotten out there, we will never go back to what once was normal practice,” Dr. Lipoff predicted. According to Dr. Lipoff, there was a great deal of data even prior to the pandemic to conclude that mobile dermatology is “an acceptable equivalent” for delivering many types of dermatologic care.
The rapid evolution in telemedicine is remarkable. According to the results of the AAD survey, 14.1% of dermatologists had experience with teledermatology prior to the COVID-19 pandemic, which increased to 96.9% by June, 2020, when the survey was conducted. Nearly 600 dermatologists completed the survey, for a 13.6% response rate.
At the beginning of the pandemic, the CARES act, along with other pandemic legislation and policy changes, changed the landscape of telemedicine by providing reimbursement commensurate with in-office visits, modifying HIPAA regulations, and permitting reciprocal licensing to allow physicians to provide care to patients who had moved out of the state. While these were among the factors that facilitated the phenomenal growth in telemedicine, nearly all of these changes were temporary or are subject to revision.
“Reimbursement [for telehealth] was very low prior to the pandemic,” noted Elizabeth K. Jones, MD, assistant professor of dermatology, Thomas Jefferson University, Philadelphia. While many physicians and policy makers were convinced that reimbursement levels had to be increased temporarily to provide medical care when in-office visits were unsafe, Dr. Jones said it is unlikely that pandemic reimbursement rates will be preserved. But recent statements from the CMS foreshadow lower rates for most video and telephone consults, she added.
Reimbursement is not the only consideration. George Han, MD, PhD, chief of teledermatology at the Icahn School of Medicine at Mount Sinai, New York, spoke about the frustration of using imperfect tools. He, like many dermatologists, have become familiar with the difficulty of making a definitive diagnosis from transmitted images of skin lesions.
As long as patients communicate with personal computers and phones under variable lighting conditions, this problem might never go away, but suboptimal quality images do not necessarily preclude other types of consults, particularly follow-up visits, according to Dr. Han, who is also system medical director for dermatology at Mount Sinai Health System.
“Now that patients know about teledermatology, they are for it,” he said. He suggested that increased efficiency of follow-up using telemedicine for both patients and physicians might increase the frequency with which these types of visits are scheduled. Citing evidence that follow-up visits increase patient retention rates, Dr. Han sees these visits among the routine uses of telemedicine when the pandemic is over.
At the height of the pandemic, teledermatology was employed broadly, but after the pandemic, Dr. Han and others predict a narrower focus. Some consults, such as those for acne or other conditions reasonably treated on the basis of patient history, appear to lend themselves to telemedicine. Others, such as a skin check for malignancy, might not.
As the role of telemedicine is sorted out and finds its equilibrium in a postpandemic world, Dr. Lipoff pointed out the need to consider populations without good-quality internet access. Without specific strategies to ensure these patients are not forgotten, he warned of “wealthier patients consuming more than their fair share” of health care resources, further widening an existing disparity.
“Is telemedicine here to stay? It is clear that, yes, it is in some way,” said Dr. Lipoff, who sees no reason for dermatology to be an exception.
Dr. Lipoff reported a financial relationship with AcneAway. The other investigators reported no potential conflicts of interest related to telemedicine.
FROM AAD VMX 2021
More reassurance for certain antiseizure drugs in pregnancy
Further evidence supporting the safety of two antiseizure medications in pregnancy has come from a new study. Most of the women with epilepsy in the study took either lamotrigine or levetiracetam, or a combination of the two, during their pregnancy.
However, a secondary analysis suggested a possible signal of exposure-dependent effects on child outcomes – worse outcomes with higher exposure levels – with levetiracetam.
The results were presented at the American Academy of Neurology’s 2021 annual meeting.
Additional reassurance
“Our new study adds confidence to the use of lamotrigine and levetiracetam during pregnancy, adding larger numbers with a new cohort. In addition, it provides some preliminary data on some of the other new antiseizure medications, and it is the first study to address the effects of clearance in pregnancy to better assess exposure,” said lead investigator, Kimford J. Meador, MD. “Overall, I am reassured by this data, but there is still a lot that is unknown,” he added.
“Our main results show no difference in verbal index or general conceptual ability scores in children born to women with epilepsy compared to children born to healthy women. This is a big positive message,” Dr. Meador said.
In terms of secondary analysis focusing on exposure levels (dose and blood levels of antiseizure medications), there was no overall signal of harm when looking at the whole group, but when the researchers analyzed the data on individual drugs, they found a “slight signal” toward reduced verbal index scores with increasing exposure levels with levetiracetam. No differences were seen on general conceptual ability.
“In the secondary analysis, there was a marginal signal for exposure levels with levetiracetam, with increased blood levels of the drug associated with reduced verbal index scores,” reported Dr. Meador, professor of neurology and neurological sciences at Stanford (Calif.) University. “We saw some signal in the children when they were 2 years old, and this was still there but not as striking at 3 years old.”
He said these secondary results should be interpreted with extreme caution. “We don’t want to overemphasize these secondary findings, as the primary outcome showed no difference, and there was no effect on exposure levels when looking at all the drugs together. I don’t want to oversimplify this, as I am still not sure whether this is a real association or not,” Dr. Meador commented.
He explained that conducting neurobehavioral tests on 2- and 3-year-olds was very difficult. “It is more of an art form than science, and as the children get older these signals often dissipate. We will know more by the time they are 6, when these tests become easier to conduct,” he said. He also noted that the results would need to be replicated in a different cohort.
“I don’t think these results would change how we manage women during pregnancy in terms of using levetiracetam. It is still a safe drug during pregnancy,” Dr. Meador said.
He pointed out that data on safety in pregnancy is only available for very few antiseizure drugs. “There are over 30 antiseizure medications, but we have adequate data in pregnancy on only a handful. We have data suggesting lamotrigine, levetiracetam, and carbamazepine appear to be relatively safe, and evidence showing phenobarbital and valproate are not safe.”
Antiseizure medications as a class are among the most commonly prescribed teratogenic drugs given to women of childbearing age, Dr. Meador noted. They are used not only for epilepsy but also for many other psychiatric and pain indications, so these results are applicable to quite a broad population, he added.
He pointed out that previous studies did not assess exposure using blood levels, which is important, as clearance of drug increases during pregnancy but varies across antiseizure medications and across individuals on the same drug. “Thus, it is unclear if these changes could obscure exposure-dependent effects. Our present studies assessed blood levels to better measure fetal exposure.”
Advice for pregnant patients with epilepsy
Dr. Meador explained that risk for adverse effects with antiseizure medication always needs to be balanced with risk for seizures if the medication was not used.
“In women planning a pregnancy, we recommend that they plan ahead with their physician to try and use the safest antiseizure medication and gain good control beforehand and then maintain the same blood levels of whichever drug is being used during pregnancy,” Dr. Meador said. “At present, lamotrigine and levetiracetam are the two safest drugs to use in pregnancy. They both look generally very safe compared with some other epilepsy drugs – such as valproate, which poses a serious risk to cognitive and behavioral development.”
He also advised that women should be taking folic acid regularly, as this has been shown to be related to improved cognitive and behavioral outcomes. “Since half of pregnancies are not planned, it is important to take these actions before pregnancy,” he added.
The current study involved 289 women with epilepsy and 89 women without epilepsy, all of whom enrolled in the study during pregnancy. Use of antiseizure medications was recorded. Of the women with epilepsy, 74% were on monotherapy, with 43% on lamotrigine and 37% on levetiracetam. There were 4% who took no drug and 22% took more than one drug. Of those who took more than one drug, close to half took a combination of lamotrigine and levetiracetam. Levels of medications in the blood of the women with epilepsy were measured in the third trimester.
Assessment of neurobehavioral development
For the current analysis, the children were evaluated at age 3 with a series of cognitive and developmental tests that measured vocabulary, listening comprehension, number recall, and pattern recognition, and results were adjusted for mother’s IQ, education level, age at enrollment, postbirth average BAI (Beck Anxiety Inventory score), and child’s ethnicity, sex, and breastfeeding status.
The primary outcome showed that verbal Index scores at age 3 did not differ for children of women with epilepsy versus those for children of women without epilepsy (LS mean 102.7 vs. 102.1).
Antiseizure medication exposure as evident by the maximum third trimester blood levels was not related to verbal index scores (n = 265; adjusted parameter estimate, -1.9; 95% confidence interval, -6.8 to 3.1).
General conceptual ability scores also did not differ between the two groups: 105.1 for children of women with epilepsy versus 103.5 for children of healthy women.
In terms of exposure levels, the third trimester maximum observed ratio of antiseizure medication blood levels was not significantly associated with adjusted general conceptual ability scores for children of women with epilepsy; neither were monotherapies or polytherapies evaluated separately, Dr. Meador reported.
However, when the verbal index scores for the main antiepileptic drug groups were analyzed separately, exposure level to levetiracetam was the only one that was significant, with a P value of .028. But Dr. Meador again stressed that this finding should be interpreted with caution given that it is a secondary exploratory analysis without control for multiple comparisons.
The researchers plan to assess these children at older ages where evaluations are more sensitive to ultimate outcomes.
“Information on use in pregnancy for most antiseizure medications is still unknown, so further studies to assess risks for the newer antiseizure medications are needed,” Dr. Meador added. “Further, additional research is needed on the underlying mechanisms including genetic predispositions, since teratogens act on a susceptible genotype.”
The study was supported by the National Institutes of Health, the National Institute of Neurological Disorders and Stroke, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
A version of this article first appeared on Medscape.com.
Further evidence supporting the safety of two antiseizure medications in pregnancy has come from a new study. Most of the women with epilepsy in the study took either lamotrigine or levetiracetam, or a combination of the two, during their pregnancy.
However, a secondary analysis suggested a possible signal of exposure-dependent effects on child outcomes – worse outcomes with higher exposure levels – with levetiracetam.
The results were presented at the American Academy of Neurology’s 2021 annual meeting.
Additional reassurance
“Our new study adds confidence to the use of lamotrigine and levetiracetam during pregnancy, adding larger numbers with a new cohort. In addition, it provides some preliminary data on some of the other new antiseizure medications, and it is the first study to address the effects of clearance in pregnancy to better assess exposure,” said lead investigator, Kimford J. Meador, MD. “Overall, I am reassured by this data, but there is still a lot that is unknown,” he added.
“Our main results show no difference in verbal index or general conceptual ability scores in children born to women with epilepsy compared to children born to healthy women. This is a big positive message,” Dr. Meador said.
In terms of secondary analysis focusing on exposure levels (dose and blood levels of antiseizure medications), there was no overall signal of harm when looking at the whole group, but when the researchers analyzed the data on individual drugs, they found a “slight signal” toward reduced verbal index scores with increasing exposure levels with levetiracetam. No differences were seen on general conceptual ability.
“In the secondary analysis, there was a marginal signal for exposure levels with levetiracetam, with increased blood levels of the drug associated with reduced verbal index scores,” reported Dr. Meador, professor of neurology and neurological sciences at Stanford (Calif.) University. “We saw some signal in the children when they were 2 years old, and this was still there but not as striking at 3 years old.”
He said these secondary results should be interpreted with extreme caution. “We don’t want to overemphasize these secondary findings, as the primary outcome showed no difference, and there was no effect on exposure levels when looking at all the drugs together. I don’t want to oversimplify this, as I am still not sure whether this is a real association or not,” Dr. Meador commented.
He explained that conducting neurobehavioral tests on 2- and 3-year-olds was very difficult. “It is more of an art form than science, and as the children get older these signals often dissipate. We will know more by the time they are 6, when these tests become easier to conduct,” he said. He also noted that the results would need to be replicated in a different cohort.
“I don’t think these results would change how we manage women during pregnancy in terms of using levetiracetam. It is still a safe drug during pregnancy,” Dr. Meador said.
He pointed out that data on safety in pregnancy is only available for very few antiseizure drugs. “There are over 30 antiseizure medications, but we have adequate data in pregnancy on only a handful. We have data suggesting lamotrigine, levetiracetam, and carbamazepine appear to be relatively safe, and evidence showing phenobarbital and valproate are not safe.”
Antiseizure medications as a class are among the most commonly prescribed teratogenic drugs given to women of childbearing age, Dr. Meador noted. They are used not only for epilepsy but also for many other psychiatric and pain indications, so these results are applicable to quite a broad population, he added.
He pointed out that previous studies did not assess exposure using blood levels, which is important, as clearance of drug increases during pregnancy but varies across antiseizure medications and across individuals on the same drug. “Thus, it is unclear if these changes could obscure exposure-dependent effects. Our present studies assessed blood levels to better measure fetal exposure.”
Advice for pregnant patients with epilepsy
Dr. Meador explained that risk for adverse effects with antiseizure medication always needs to be balanced with risk for seizures if the medication was not used.
“In women planning a pregnancy, we recommend that they plan ahead with their physician to try and use the safest antiseizure medication and gain good control beforehand and then maintain the same blood levels of whichever drug is being used during pregnancy,” Dr. Meador said. “At present, lamotrigine and levetiracetam are the two safest drugs to use in pregnancy. They both look generally very safe compared with some other epilepsy drugs – such as valproate, which poses a serious risk to cognitive and behavioral development.”
He also advised that women should be taking folic acid regularly, as this has been shown to be related to improved cognitive and behavioral outcomes. “Since half of pregnancies are not planned, it is important to take these actions before pregnancy,” he added.
The current study involved 289 women with epilepsy and 89 women without epilepsy, all of whom enrolled in the study during pregnancy. Use of antiseizure medications was recorded. Of the women with epilepsy, 74% were on monotherapy, with 43% on lamotrigine and 37% on levetiracetam. There were 4% who took no drug and 22% took more than one drug. Of those who took more than one drug, close to half took a combination of lamotrigine and levetiracetam. Levels of medications in the blood of the women with epilepsy were measured in the third trimester.
Assessment of neurobehavioral development
For the current analysis, the children were evaluated at age 3 with a series of cognitive and developmental tests that measured vocabulary, listening comprehension, number recall, and pattern recognition, and results were adjusted for mother’s IQ, education level, age at enrollment, postbirth average BAI (Beck Anxiety Inventory score), and child’s ethnicity, sex, and breastfeeding status.
The primary outcome showed that verbal Index scores at age 3 did not differ for children of women with epilepsy versus those for children of women without epilepsy (LS mean 102.7 vs. 102.1).
Antiseizure medication exposure as evident by the maximum third trimester blood levels was not related to verbal index scores (n = 265; adjusted parameter estimate, -1.9; 95% confidence interval, -6.8 to 3.1).
General conceptual ability scores also did not differ between the two groups: 105.1 for children of women with epilepsy versus 103.5 for children of healthy women.
In terms of exposure levels, the third trimester maximum observed ratio of antiseizure medication blood levels was not significantly associated with adjusted general conceptual ability scores for children of women with epilepsy; neither were monotherapies or polytherapies evaluated separately, Dr. Meador reported.
However, when the verbal index scores for the main antiepileptic drug groups were analyzed separately, exposure level to levetiracetam was the only one that was significant, with a P value of .028. But Dr. Meador again stressed that this finding should be interpreted with caution given that it is a secondary exploratory analysis without control for multiple comparisons.
The researchers plan to assess these children at older ages where evaluations are more sensitive to ultimate outcomes.
“Information on use in pregnancy for most antiseizure medications is still unknown, so further studies to assess risks for the newer antiseizure medications are needed,” Dr. Meador added. “Further, additional research is needed on the underlying mechanisms including genetic predispositions, since teratogens act on a susceptible genotype.”
The study was supported by the National Institutes of Health, the National Institute of Neurological Disorders and Stroke, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
A version of this article first appeared on Medscape.com.
Further evidence supporting the safety of two antiseizure medications in pregnancy has come from a new study. Most of the women with epilepsy in the study took either lamotrigine or levetiracetam, or a combination of the two, during their pregnancy.
However, a secondary analysis suggested a possible signal of exposure-dependent effects on child outcomes – worse outcomes with higher exposure levels – with levetiracetam.
The results were presented at the American Academy of Neurology’s 2021 annual meeting.
Additional reassurance
“Our new study adds confidence to the use of lamotrigine and levetiracetam during pregnancy, adding larger numbers with a new cohort. In addition, it provides some preliminary data on some of the other new antiseizure medications, and it is the first study to address the effects of clearance in pregnancy to better assess exposure,” said lead investigator, Kimford J. Meador, MD. “Overall, I am reassured by this data, but there is still a lot that is unknown,” he added.
“Our main results show no difference in verbal index or general conceptual ability scores in children born to women with epilepsy compared to children born to healthy women. This is a big positive message,” Dr. Meador said.
In terms of secondary analysis focusing on exposure levels (dose and blood levels of antiseizure medications), there was no overall signal of harm when looking at the whole group, but when the researchers analyzed the data on individual drugs, they found a “slight signal” toward reduced verbal index scores with increasing exposure levels with levetiracetam. No differences were seen on general conceptual ability.
“In the secondary analysis, there was a marginal signal for exposure levels with levetiracetam, with increased blood levels of the drug associated with reduced verbal index scores,” reported Dr. Meador, professor of neurology and neurological sciences at Stanford (Calif.) University. “We saw some signal in the children when they were 2 years old, and this was still there but not as striking at 3 years old.”
He said these secondary results should be interpreted with extreme caution. “We don’t want to overemphasize these secondary findings, as the primary outcome showed no difference, and there was no effect on exposure levels when looking at all the drugs together. I don’t want to oversimplify this, as I am still not sure whether this is a real association or not,” Dr. Meador commented.
He explained that conducting neurobehavioral tests on 2- and 3-year-olds was very difficult. “It is more of an art form than science, and as the children get older these signals often dissipate. We will know more by the time they are 6, when these tests become easier to conduct,” he said. He also noted that the results would need to be replicated in a different cohort.
“I don’t think these results would change how we manage women during pregnancy in terms of using levetiracetam. It is still a safe drug during pregnancy,” Dr. Meador said.
He pointed out that data on safety in pregnancy is only available for very few antiseizure drugs. “There are over 30 antiseizure medications, but we have adequate data in pregnancy on only a handful. We have data suggesting lamotrigine, levetiracetam, and carbamazepine appear to be relatively safe, and evidence showing phenobarbital and valproate are not safe.”
Antiseizure medications as a class are among the most commonly prescribed teratogenic drugs given to women of childbearing age, Dr. Meador noted. They are used not only for epilepsy but also for many other psychiatric and pain indications, so these results are applicable to quite a broad population, he added.
He pointed out that previous studies did not assess exposure using blood levels, which is important, as clearance of drug increases during pregnancy but varies across antiseizure medications and across individuals on the same drug. “Thus, it is unclear if these changes could obscure exposure-dependent effects. Our present studies assessed blood levels to better measure fetal exposure.”
Advice for pregnant patients with epilepsy
Dr. Meador explained that risk for adverse effects with antiseizure medication always needs to be balanced with risk for seizures if the medication was not used.
“In women planning a pregnancy, we recommend that they plan ahead with their physician to try and use the safest antiseizure medication and gain good control beforehand and then maintain the same blood levels of whichever drug is being used during pregnancy,” Dr. Meador said. “At present, lamotrigine and levetiracetam are the two safest drugs to use in pregnancy. They both look generally very safe compared with some other epilepsy drugs – such as valproate, which poses a serious risk to cognitive and behavioral development.”
He also advised that women should be taking folic acid regularly, as this has been shown to be related to improved cognitive and behavioral outcomes. “Since half of pregnancies are not planned, it is important to take these actions before pregnancy,” he added.
The current study involved 289 women with epilepsy and 89 women without epilepsy, all of whom enrolled in the study during pregnancy. Use of antiseizure medications was recorded. Of the women with epilepsy, 74% were on monotherapy, with 43% on lamotrigine and 37% on levetiracetam. There were 4% who took no drug and 22% took more than one drug. Of those who took more than one drug, close to half took a combination of lamotrigine and levetiracetam. Levels of medications in the blood of the women with epilepsy were measured in the third trimester.
Assessment of neurobehavioral development
For the current analysis, the children were evaluated at age 3 with a series of cognitive and developmental tests that measured vocabulary, listening comprehension, number recall, and pattern recognition, and results were adjusted for mother’s IQ, education level, age at enrollment, postbirth average BAI (Beck Anxiety Inventory score), and child’s ethnicity, sex, and breastfeeding status.
The primary outcome showed that verbal Index scores at age 3 did not differ for children of women with epilepsy versus those for children of women without epilepsy (LS mean 102.7 vs. 102.1).
Antiseizure medication exposure as evident by the maximum third trimester blood levels was not related to verbal index scores (n = 265; adjusted parameter estimate, -1.9; 95% confidence interval, -6.8 to 3.1).
General conceptual ability scores also did not differ between the two groups: 105.1 for children of women with epilepsy versus 103.5 for children of healthy women.
In terms of exposure levels, the third trimester maximum observed ratio of antiseizure medication blood levels was not significantly associated with adjusted general conceptual ability scores for children of women with epilepsy; neither were monotherapies or polytherapies evaluated separately, Dr. Meador reported.
However, when the verbal index scores for the main antiepileptic drug groups were analyzed separately, exposure level to levetiracetam was the only one that was significant, with a P value of .028. But Dr. Meador again stressed that this finding should be interpreted with caution given that it is a secondary exploratory analysis without control for multiple comparisons.
The researchers plan to assess these children at older ages where evaluations are more sensitive to ultimate outcomes.
“Information on use in pregnancy for most antiseizure medications is still unknown, so further studies to assess risks for the newer antiseizure medications are needed,” Dr. Meador added. “Further, additional research is needed on the underlying mechanisms including genetic predispositions, since teratogens act on a susceptible genotype.”
The study was supported by the National Institutes of Health, the National Institute of Neurological Disorders and Stroke, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
A version of this article first appeared on Medscape.com.
From AAN 2021
Pediatric bronchiolitis: Less is more
A common cause of infant morbidity and hospitalization in developed countries, infant viral bronchiolitis, has long been bedeviled by treatment uncertainty beyond supportive care.
Rationales for most pharmacologic treatments continue to be debated, and clinical practice guidelines generally advise respiratory and hydration support, discouraging the use of chest radiography, albuterol, glucocorticoids, antibiotics, and epinephrine.
Despite evidence that the latter interventions are ineffective, they are still too often applied, according to two recent studies, one in Pediatrics, the other in JAMA Pediatrics.
“The pull of the therapeutic vacuum surrounding this disease has been noted in the pages of this journal for at least 50 years, with Wright and Beem writing in 1965 that ‘energies should not be frittered away by the annoyance of unnecessary or futile medications and procedures’ for the child with bronchiolitis,” said emergency physicians Matthew J. Lipshaw, MD, MS, of the Cincinnati Children’s Hospital Medical Center, and Todd A. Florin, MD, MSCE, of Ann and Robert H. Lurie Children’s Hospital of Chicago.
These remarks came in their editorial in Pediatrics wryly titled: “Don’t Just Do Something, Stand There” and published online to accompany a recent study of three network meta-analyses.
Led by Sarah A. Elliott, PhD, of the Alberta Research Centre for Health Evidence at the University of Alberta in Edmonton, this analysis amalgamated 150 randomized, controlled trials comparing a placebo or active comparator with any bronchodilator, glucocorticoid steroid, hypertonic saline solution, antibiotic, helium-oxygen therapy, or high-flow oxygen therapy. It then looked at the following outcomes in children aged 2 years and younger: hospital admission rate on day 1, hospital admission rate within 7 days, and total hospital length of stay.
Few treatments seemed more effective than nebulized placebo (0.9% saline) for short-term outcomes, the authors found. While nebulized epinephrine and nebulized hypertonic saline plus salbutamol appeared to reduce admission rates during the index ED presentation, and hypertonic saline, alone or in combination with epinephrine, seemed to reduce hospital stays, such treatment had no effect on admissions within 7 days of initial presentation. Furthermore, most benefits disappeared in higher-quality studies.
Concluding, albeit with weak evidence and low confidence, that some benefit might accrue with hypertonic saline with salbutamol to reduce admission rates on initial presentation to the ED, the authors called for well-designed studies on treatments in inpatients and outpatients.
According to Dr. Lipshaw, assistant professor of clinical pediatrics, the lack of benefit observed in superior studies limits the applicability of Dr. Elliott and colleagues’ results to immediate clinical practice. “These findings could be used, however, to target future high-quality studies toward the medications that they found might be useful,” he said in an interview.
For the present, other recent research augurs well for strategically reducing unnecessary care. In a paper published online in JAMA Pediatrics, Libby Haskell, MN, of the ED at Starship Children’s Hospital in Auckland, New Zealand, and associates reported on a cluster-randomized, controlled trial of targeted interventions.
Conducted in 2017 at 26 hospitals and with 3,727 babies in New Zealand and Australia, the study addressed drivers of non–evidence-based approaches with behavior-modifying approaches such as on-site clinical leads, stakeholder meetings, a train-the-trainer workshop, education, and audit and feedback.
The authors reported a 14.1% difference in rates of compliance during the first 24 hours of hospitalization favoring the intervention group for all five bronchiolitis guideline recommendations. The greatest change was seen in albuterol and chest radiography use, with other improvements in ED visits, inpatient consultations, and throughout hospitalization.
“These results provide clinicians and hospitals with clear implementation strategies to address unnecessary treatment of infants with bronchiolitis,” Dr. Haskell’s group wrote. Dr. Lipshaw agreed that multifaceted deimplementation packages including clinician and family education, audit and feedback, and clinical decision support have been successful. “Haskell et al. demonstrated that it is possible to successfully deimplement non–evidence-based practices for bronchiolitis with targeted inventions,” he said. “It would be wonderful to see their success replicated in the U.S.”
Why the slow adoption of guidelines?
The American Academy of Pediatrics issued bronchiolitis guidelines for babies to 23 months in 2014 and updated them in 2018. Why, then, has care in some centers been seemingly all over the map and counter to guidelines? “Both parents and clinicians are acting in what they believe to be the best interests of the child, and in the absence of high-value interventions, can feel the need to do something, even if that something is not supported by evidence,” Dr. Lipshaw said.
Furthermore, with children in obvious distress, breathing fast and with difficulty, and sometimes unable to eat or drink, “we feel like we should have some way to make them feel better quicker. Unfortunately, none of the medications we have tried seem to be useful for most children, and we are left with supportive care measures such as suctioning their noses, giving them oxygen if their oxygen is low, and giving them fluids if they are dehydrated.”
Other physicians agree that taking a less-is-more approach can be challenging and even counterintuitive. “To families, seeing their child’s doctor ‘doing less’ can be frustrating,” admitted Diana S. Lee, MD, assistant professor of pediatrics at Icahn School of Medicine at Mount Sinai, New York.
Beyond that, altering practice behavior will need more than guidelines, Dr. Lee said in an interview. “Haskell et al. showed targeted behavior-change interventions improved compliance with bronchiolitis guidelines, but such change requires motivation and resources, and the sustainability of this effect over time remains to be seen.”
At Dr. Lipshaw’s institution, treatment depends on the attending physician, “but we have an emergency department care algorithm, which does not recommend any inhaled medications or steroids in accordance with the 2014 AAP guidelines,” he said.
Similarly at Mount Sinai, practitioners strive to follow the AAP guidelines, although their implementation has not been immediate, Dr. Lee said. “This is a situation where we must make the effort to choose not to do more, given current evidence.”
But Michelle Dunn, MD, an attending physician in the division of general pediatrics at the Children’s Hospital of Philadelphia, said the American practice norm already tends more to the observance than the breach of the guidelines, noting that since 2014 quality improvement efforts have been made throughout the country. “At our institution, we have effectively reduced the use of albuterol in patients with bronchiolitis and we use evidence-based therapy as much as possible, which in the case of bronchiolitis generally involves supportive management alone,” she said in an interview.
Still, Dr. Dunn added, many patients receive unnecessary diagnostic testing and ineffective therapies, with some providers facing psychological barriers to doing less. “However, with more and more evidence to support this, hopefully, physicians will become more comfortable with this.”
To that end, Dr. Lipshaw’s editorial urges physicians to “curb the rampant use of therapies repeatedly revealed to be ineffective,” citing team engagement, clear practice guidelines, and information technology as key factors in deimplementation. In the meantime, his mantra remains: “Don’t just do something, stand there.”
The study by Dr. Elliot and colleagues was supported by the Canadian Institutes of Health Research Knowledge Synthesis grant program. One coauthor is supported by a University of Ottawa Tier I Research Chair in Pediatric Emergency Medicine. Another is supported by a Tier 1 Canada Research Chair in Knowledge Synthesis and Translation and the Stollery Science Laboratory. Dr. Lipshaw and Dr. Florin disclosed no financial relationships relevant to their commentary. Dr. Haskell and colleagues were supported, variously, by the National Health and Medical Research Council of New Zealand, the Center of Research Excellence for Pediatric Emergency Medicine, the Victorian Government’s Operational Infrastructure Support Program, Cure Kids New Zealand, the Royal Children’s Hospital Foundation, and the Starship Foundation. Dr. Lee and Dr. Dunn had no competing interests to disclose with regard to their comments.
A common cause of infant morbidity and hospitalization in developed countries, infant viral bronchiolitis, has long been bedeviled by treatment uncertainty beyond supportive care.
Rationales for most pharmacologic treatments continue to be debated, and clinical practice guidelines generally advise respiratory and hydration support, discouraging the use of chest radiography, albuterol, glucocorticoids, antibiotics, and epinephrine.
Despite evidence that the latter interventions are ineffective, they are still too often applied, according to two recent studies, one in Pediatrics, the other in JAMA Pediatrics.
“The pull of the therapeutic vacuum surrounding this disease has been noted in the pages of this journal for at least 50 years, with Wright and Beem writing in 1965 that ‘energies should not be frittered away by the annoyance of unnecessary or futile medications and procedures’ for the child with bronchiolitis,” said emergency physicians Matthew J. Lipshaw, MD, MS, of the Cincinnati Children’s Hospital Medical Center, and Todd A. Florin, MD, MSCE, of Ann and Robert H. Lurie Children’s Hospital of Chicago.
These remarks came in their editorial in Pediatrics wryly titled: “Don’t Just Do Something, Stand There” and published online to accompany a recent study of three network meta-analyses.
Led by Sarah A. Elliott, PhD, of the Alberta Research Centre for Health Evidence at the University of Alberta in Edmonton, this analysis amalgamated 150 randomized, controlled trials comparing a placebo or active comparator with any bronchodilator, glucocorticoid steroid, hypertonic saline solution, antibiotic, helium-oxygen therapy, or high-flow oxygen therapy. It then looked at the following outcomes in children aged 2 years and younger: hospital admission rate on day 1, hospital admission rate within 7 days, and total hospital length of stay.
Few treatments seemed more effective than nebulized placebo (0.9% saline) for short-term outcomes, the authors found. While nebulized epinephrine and nebulized hypertonic saline plus salbutamol appeared to reduce admission rates during the index ED presentation, and hypertonic saline, alone or in combination with epinephrine, seemed to reduce hospital stays, such treatment had no effect on admissions within 7 days of initial presentation. Furthermore, most benefits disappeared in higher-quality studies.
Concluding, albeit with weak evidence and low confidence, that some benefit might accrue with hypertonic saline with salbutamol to reduce admission rates on initial presentation to the ED, the authors called for well-designed studies on treatments in inpatients and outpatients.
According to Dr. Lipshaw, assistant professor of clinical pediatrics, the lack of benefit observed in superior studies limits the applicability of Dr. Elliott and colleagues’ results to immediate clinical practice. “These findings could be used, however, to target future high-quality studies toward the medications that they found might be useful,” he said in an interview.
For the present, other recent research augurs well for strategically reducing unnecessary care. In a paper published online in JAMA Pediatrics, Libby Haskell, MN, of the ED at Starship Children’s Hospital in Auckland, New Zealand, and associates reported on a cluster-randomized, controlled trial of targeted interventions.
Conducted in 2017 at 26 hospitals and with 3,727 babies in New Zealand and Australia, the study addressed drivers of non–evidence-based approaches with behavior-modifying approaches such as on-site clinical leads, stakeholder meetings, a train-the-trainer workshop, education, and audit and feedback.
The authors reported a 14.1% difference in rates of compliance during the first 24 hours of hospitalization favoring the intervention group for all five bronchiolitis guideline recommendations. The greatest change was seen in albuterol and chest radiography use, with other improvements in ED visits, inpatient consultations, and throughout hospitalization.
“These results provide clinicians and hospitals with clear implementation strategies to address unnecessary treatment of infants with bronchiolitis,” Dr. Haskell’s group wrote. Dr. Lipshaw agreed that multifaceted deimplementation packages including clinician and family education, audit and feedback, and clinical decision support have been successful. “Haskell et al. demonstrated that it is possible to successfully deimplement non–evidence-based practices for bronchiolitis with targeted inventions,” he said. “It would be wonderful to see their success replicated in the U.S.”
Why the slow adoption of guidelines?
The American Academy of Pediatrics issued bronchiolitis guidelines for babies to 23 months in 2014 and updated them in 2018. Why, then, has care in some centers been seemingly all over the map and counter to guidelines? “Both parents and clinicians are acting in what they believe to be the best interests of the child, and in the absence of high-value interventions, can feel the need to do something, even if that something is not supported by evidence,” Dr. Lipshaw said.
Furthermore, with children in obvious distress, breathing fast and with difficulty, and sometimes unable to eat or drink, “we feel like we should have some way to make them feel better quicker. Unfortunately, none of the medications we have tried seem to be useful for most children, and we are left with supportive care measures such as suctioning their noses, giving them oxygen if their oxygen is low, and giving them fluids if they are dehydrated.”
Other physicians agree that taking a less-is-more approach can be challenging and even counterintuitive. “To families, seeing their child’s doctor ‘doing less’ can be frustrating,” admitted Diana S. Lee, MD, assistant professor of pediatrics at Icahn School of Medicine at Mount Sinai, New York.
Beyond that, altering practice behavior will need more than guidelines, Dr. Lee said in an interview. “Haskell et al. showed targeted behavior-change interventions improved compliance with bronchiolitis guidelines, but such change requires motivation and resources, and the sustainability of this effect over time remains to be seen.”
At Dr. Lipshaw’s institution, treatment depends on the attending physician, “but we have an emergency department care algorithm, which does not recommend any inhaled medications or steroids in accordance with the 2014 AAP guidelines,” he said.
Similarly at Mount Sinai, practitioners strive to follow the AAP guidelines, although their implementation has not been immediate, Dr. Lee said. “This is a situation where we must make the effort to choose not to do more, given current evidence.”
But Michelle Dunn, MD, an attending physician in the division of general pediatrics at the Children’s Hospital of Philadelphia, said the American practice norm already tends more to the observance than the breach of the guidelines, noting that since 2014 quality improvement efforts have been made throughout the country. “At our institution, we have effectively reduced the use of albuterol in patients with bronchiolitis and we use evidence-based therapy as much as possible, which in the case of bronchiolitis generally involves supportive management alone,” she said in an interview.
Still, Dr. Dunn added, many patients receive unnecessary diagnostic testing and ineffective therapies, with some providers facing psychological barriers to doing less. “However, with more and more evidence to support this, hopefully, physicians will become more comfortable with this.”
To that end, Dr. Lipshaw’s editorial urges physicians to “curb the rampant use of therapies repeatedly revealed to be ineffective,” citing team engagement, clear practice guidelines, and information technology as key factors in deimplementation. In the meantime, his mantra remains: “Don’t just do something, stand there.”
The study by Dr. Elliot and colleagues was supported by the Canadian Institutes of Health Research Knowledge Synthesis grant program. One coauthor is supported by a University of Ottawa Tier I Research Chair in Pediatric Emergency Medicine. Another is supported by a Tier 1 Canada Research Chair in Knowledge Synthesis and Translation and the Stollery Science Laboratory. Dr. Lipshaw and Dr. Florin disclosed no financial relationships relevant to their commentary. Dr. Haskell and colleagues were supported, variously, by the National Health and Medical Research Council of New Zealand, the Center of Research Excellence for Pediatric Emergency Medicine, the Victorian Government’s Operational Infrastructure Support Program, Cure Kids New Zealand, the Royal Children’s Hospital Foundation, and the Starship Foundation. Dr. Lee and Dr. Dunn had no competing interests to disclose with regard to their comments.
A common cause of infant morbidity and hospitalization in developed countries, infant viral bronchiolitis, has long been bedeviled by treatment uncertainty beyond supportive care.
Rationales for most pharmacologic treatments continue to be debated, and clinical practice guidelines generally advise respiratory and hydration support, discouraging the use of chest radiography, albuterol, glucocorticoids, antibiotics, and epinephrine.
Despite evidence that the latter interventions are ineffective, they are still too often applied, according to two recent studies, one in Pediatrics, the other in JAMA Pediatrics.
“The pull of the therapeutic vacuum surrounding this disease has been noted in the pages of this journal for at least 50 years, with Wright and Beem writing in 1965 that ‘energies should not be frittered away by the annoyance of unnecessary or futile medications and procedures’ for the child with bronchiolitis,” said emergency physicians Matthew J. Lipshaw, MD, MS, of the Cincinnati Children’s Hospital Medical Center, and Todd A. Florin, MD, MSCE, of Ann and Robert H. Lurie Children’s Hospital of Chicago.
These remarks came in their editorial in Pediatrics wryly titled: “Don’t Just Do Something, Stand There” and published online to accompany a recent study of three network meta-analyses.
Led by Sarah A. Elliott, PhD, of the Alberta Research Centre for Health Evidence at the University of Alberta in Edmonton, this analysis amalgamated 150 randomized, controlled trials comparing a placebo or active comparator with any bronchodilator, glucocorticoid steroid, hypertonic saline solution, antibiotic, helium-oxygen therapy, or high-flow oxygen therapy. It then looked at the following outcomes in children aged 2 years and younger: hospital admission rate on day 1, hospital admission rate within 7 days, and total hospital length of stay.
Few treatments seemed more effective than nebulized placebo (0.9% saline) for short-term outcomes, the authors found. While nebulized epinephrine and nebulized hypertonic saline plus salbutamol appeared to reduce admission rates during the index ED presentation, and hypertonic saline, alone or in combination with epinephrine, seemed to reduce hospital stays, such treatment had no effect on admissions within 7 days of initial presentation. Furthermore, most benefits disappeared in higher-quality studies.
Concluding, albeit with weak evidence and low confidence, that some benefit might accrue with hypertonic saline with salbutamol to reduce admission rates on initial presentation to the ED, the authors called for well-designed studies on treatments in inpatients and outpatients.
According to Dr. Lipshaw, assistant professor of clinical pediatrics, the lack of benefit observed in superior studies limits the applicability of Dr. Elliott and colleagues’ results to immediate clinical practice. “These findings could be used, however, to target future high-quality studies toward the medications that they found might be useful,” he said in an interview.
For the present, other recent research augurs well for strategically reducing unnecessary care. In a paper published online in JAMA Pediatrics, Libby Haskell, MN, of the ED at Starship Children’s Hospital in Auckland, New Zealand, and associates reported on a cluster-randomized, controlled trial of targeted interventions.
Conducted in 2017 at 26 hospitals and with 3,727 babies in New Zealand and Australia, the study addressed drivers of non–evidence-based approaches with behavior-modifying approaches such as on-site clinical leads, stakeholder meetings, a train-the-trainer workshop, education, and audit and feedback.
The authors reported a 14.1% difference in rates of compliance during the first 24 hours of hospitalization favoring the intervention group for all five bronchiolitis guideline recommendations. The greatest change was seen in albuterol and chest radiography use, with other improvements in ED visits, inpatient consultations, and throughout hospitalization.
“These results provide clinicians and hospitals with clear implementation strategies to address unnecessary treatment of infants with bronchiolitis,” Dr. Haskell’s group wrote. Dr. Lipshaw agreed that multifaceted deimplementation packages including clinician and family education, audit and feedback, and clinical decision support have been successful. “Haskell et al. demonstrated that it is possible to successfully deimplement non–evidence-based practices for bronchiolitis with targeted inventions,” he said. “It would be wonderful to see their success replicated in the U.S.”
Why the slow adoption of guidelines?
The American Academy of Pediatrics issued bronchiolitis guidelines for babies to 23 months in 2014 and updated them in 2018. Why, then, has care in some centers been seemingly all over the map and counter to guidelines? “Both parents and clinicians are acting in what they believe to be the best interests of the child, and in the absence of high-value interventions, can feel the need to do something, even if that something is not supported by evidence,” Dr. Lipshaw said.
Furthermore, with children in obvious distress, breathing fast and with difficulty, and sometimes unable to eat or drink, “we feel like we should have some way to make them feel better quicker. Unfortunately, none of the medications we have tried seem to be useful for most children, and we are left with supportive care measures such as suctioning their noses, giving them oxygen if their oxygen is low, and giving them fluids if they are dehydrated.”
Other physicians agree that taking a less-is-more approach can be challenging and even counterintuitive. “To families, seeing their child’s doctor ‘doing less’ can be frustrating,” admitted Diana S. Lee, MD, assistant professor of pediatrics at Icahn School of Medicine at Mount Sinai, New York.
Beyond that, altering practice behavior will need more than guidelines, Dr. Lee said in an interview. “Haskell et al. showed targeted behavior-change interventions improved compliance with bronchiolitis guidelines, but such change requires motivation and resources, and the sustainability of this effect over time remains to be seen.”
At Dr. Lipshaw’s institution, treatment depends on the attending physician, “but we have an emergency department care algorithm, which does not recommend any inhaled medications or steroids in accordance with the 2014 AAP guidelines,” he said.
Similarly at Mount Sinai, practitioners strive to follow the AAP guidelines, although their implementation has not been immediate, Dr. Lee said. “This is a situation where we must make the effort to choose not to do more, given current evidence.”
But Michelle Dunn, MD, an attending physician in the division of general pediatrics at the Children’s Hospital of Philadelphia, said the American practice norm already tends more to the observance than the breach of the guidelines, noting that since 2014 quality improvement efforts have been made throughout the country. “At our institution, we have effectively reduced the use of albuterol in patients with bronchiolitis and we use evidence-based therapy as much as possible, which in the case of bronchiolitis generally involves supportive management alone,” she said in an interview.
Still, Dr. Dunn added, many patients receive unnecessary diagnostic testing and ineffective therapies, with some providers facing psychological barriers to doing less. “However, with more and more evidence to support this, hopefully, physicians will become more comfortable with this.”
To that end, Dr. Lipshaw’s editorial urges physicians to “curb the rampant use of therapies repeatedly revealed to be ineffective,” citing team engagement, clear practice guidelines, and information technology as key factors in deimplementation. In the meantime, his mantra remains: “Don’t just do something, stand there.”
The study by Dr. Elliot and colleagues was supported by the Canadian Institutes of Health Research Knowledge Synthesis grant program. One coauthor is supported by a University of Ottawa Tier I Research Chair in Pediatric Emergency Medicine. Another is supported by a Tier 1 Canada Research Chair in Knowledge Synthesis and Translation and the Stollery Science Laboratory. Dr. Lipshaw and Dr. Florin disclosed no financial relationships relevant to their commentary. Dr. Haskell and colleagues were supported, variously, by the National Health and Medical Research Council of New Zealand, the Center of Research Excellence for Pediatric Emergency Medicine, the Victorian Government’s Operational Infrastructure Support Program, Cure Kids New Zealand, the Royal Children’s Hospital Foundation, and the Starship Foundation. Dr. Lee and Dr. Dunn had no competing interests to disclose with regard to their comments.
Are psychiatric disorders a ‘canary in a coal mine’ for Alzheimer’s disease?
, according to findings from a review of 1,500 patients with Alzheimer’s disease from a single-center population.
“Could psychosis symptoms be the proverbial canary in a coal mine?” Emily Eijansantos, a medical student at the University of California, San Francisco, said in reporting results of the chart review at the 2021 annual meeting of the American Academy of Neurology. “Previously in this cohort it was found that neurodevelopmental factors as well as chronic insults such as autoimmunity and seizure were also associated with an early age of onset in Alzheimer’s disease.”
The link between depression and autoimmunity, and anxiety and seizure “beg more questions about underlying pathophysiology,” she said. The study included 750 patients with early-onset Alzheimer’s disease and a similar number of late-onset patients from the UCSF Memory and Aging Center.
An inverse correlation between psychiatric disorders and age of Alzheimer’s onset
In the total study population, 43.5% (n = 652) had a previous diagnosis of depression and 32.3% (n = 485) had been diagnosed with anxiety. That, Ms. Eijansantos said, falls into similar ranges that other studies have reported.
“When we look at individual psychiatric disorders, we find that those with depression, anxiety, or PTSD are younger on average,” she said. “Patients with depression and anxiety are more [likely] female and have less vascular risk factors, and we observed an association between depression and autoimmunity, anxiety, and seizures.”
Specifically, patients with a history of depression were 2.2 years younger, on average, at the age of onset than patients without such history (P = .01); those with anxiety were 3 years younger on average (P = .01); and those with PTSD were 6.8 years younger on average, although only 1% (n = 15) of study subjects had PTSD, making for a small sample to study. These age-of-onset disparities didn’t appear among patients with previously diagnosed bipolar disorder (BPD) or schizophrenia.
Ms. Eijansantos noted that there were no differences in education attained or apolipoprotein-E gene status between the patients with and without a history of psychosis, and, within the subgroups of individual psychiatric disorders, there were no differences between patients with past and current or formal and informal diagnoses.
“When we split the cohort into quintiles based on age of Alzheimer’s disease onset, we find an inverse correlation between the amount of depression, anxiety, and PTSD endorsed and their ages of onset,” Ms. Eijansantos said. For example, the youngest quintile had a greater than 50% rate of depression while the oldest quintile had a depression rate around 36%. A similar spread was found with anxiety: a rate around 46% in the youngest quantile versus around 25% in the oldest, whereas rates of PTSD, BPD, and schizophrenia were similar across the five age-of-onset groups.
Patients with a history of multiple psychiatric disorders had an even younger age of onset. “We see that those with two psychiatric disorder are younger than those with one, and those with three psychiatric disorders are younger still,” Ms. Eijansantos said. “And we find that the Alzheimer’s disease age-at-onset reduction doubles with each additional psychiatric disorder.” Multiple disorders also adversely impacted survival, she said.
Because they found no difference between patients with past versus active symptoms and informal versus formal diagnosis, Ms. Eijansantos explained that they further studied the National Alzheimer’s Coordinating Center cohort of 8,267 patients with Alzheimer’s disease and found a similar relationship between psychiatric history and age of onset among patients with depression or anxiety or both. This cohort also documented symptom severity, she noted. “So when we look at depression and anxiety we find similar reductions in the Alzheimer’s disease age of onset with each increasing degree of symptom severity,” she said.
“Does this mean that psychiatric disease is a risk factor for Alzheimr’s disease?” Ms. Eijansantos said. “We can’t answer that with this study because it was only designed to see if the psychiatric factors modulate the age of onset in those that have Alzheimer’s disease, but taken together we believe that these results fit the framework that there are pathophysiological and profound differences between earlier and later presentations of Alzheimer’s disease.”
She pointed to reports that early-onset Alzheimer’s disease is associated with more aggressive tau pathology and that depression is associated with tau. However, the evidence supporting a link between amyloid and psychiatric disease is less certain, she said.
Preliminary and speculative findings
Senior study author Zachary Miller, MD, an assistant professor in the UCSF Memory and Aging Center, explained the significance of the study findings of potential links between depression and autoimmunity, and anxiety and seizure. “There may be distinct underlying pathophysiological mechanisms in patients with Alzheimer’s disease who have symptoms of depression versus anxiety,” he said, acknowledging the findings “are quite preliminary and our interpretations quite speculative.”
The findings raise the question that the symptomatic presentation of greater amounts of depression in early-onset Alzheimer’s disease may be moderated by an underlying neuroinflammatory insult, he said. “If so, depression symptomatology could then be seen as a possible clinical marker of this inflammatory response and possibly be used in testing clinical endpoints for future intervention trials,” Dr. Miller said. “Similarly, if neuronal hyperexcitability in Alzheimer’s disease manifests itself as either seizure and/or anxiety, this would have significant impact for therapeutic monitoring and treatment.”
He said a multicenter study of Alzheimer’s disease cohorts would validate the findings. “At the same time, we are also interested in looking deeper into these findings, investigating the potential cognitive and neuroanatomical correlates associated with these conditions,” Dr. Miller said.
Clinical phenotyping may provide more insight into the relationship between psychosis and age of Alzheimer’s disease onset, said Vijay K. Ramanan, MD, PhD, an assistant professor of neurology at Mayo Clinic in Rochester, Minn.
“Less typical presentations of Alzheimer’s disease, such as posterior cortical atrophy or dysexecutive Alzheimer’s disease, are associated with younger age of onset and are sometimes misdiagnosed as having pure psychiatric disease,” he said. “It is also possible that, in some cases with psychiatric disease, a younger age of onset of cognitive symptoms is charted, even though there are fundamentally two distinct processes at play – a psychiatric disease and a separate neurodegenerative disease – each having independent but additive impacts on cognition.”
Dr. Ramanan added, “This work is also a good reminder to be on the lookout for neuropsychiatric symptoms, treat where indicated, and be open to the possibility that psychiatric symptoms and Alzheimer’s disease can coexist.”
Ms. Eijansantos, Dr. Miller, and Dr. Ramanan have no relevant financial relationships to disclose.
, according to findings from a review of 1,500 patients with Alzheimer’s disease from a single-center population.
“Could psychosis symptoms be the proverbial canary in a coal mine?” Emily Eijansantos, a medical student at the University of California, San Francisco, said in reporting results of the chart review at the 2021 annual meeting of the American Academy of Neurology. “Previously in this cohort it was found that neurodevelopmental factors as well as chronic insults such as autoimmunity and seizure were also associated with an early age of onset in Alzheimer’s disease.”
The link between depression and autoimmunity, and anxiety and seizure “beg more questions about underlying pathophysiology,” she said. The study included 750 patients with early-onset Alzheimer’s disease and a similar number of late-onset patients from the UCSF Memory and Aging Center.
An inverse correlation between psychiatric disorders and age of Alzheimer’s onset
In the total study population, 43.5% (n = 652) had a previous diagnosis of depression and 32.3% (n = 485) had been diagnosed with anxiety. That, Ms. Eijansantos said, falls into similar ranges that other studies have reported.
“When we look at individual psychiatric disorders, we find that those with depression, anxiety, or PTSD are younger on average,” she said. “Patients with depression and anxiety are more [likely] female and have less vascular risk factors, and we observed an association between depression and autoimmunity, anxiety, and seizures.”
Specifically, patients with a history of depression were 2.2 years younger, on average, at the age of onset than patients without such history (P = .01); those with anxiety were 3 years younger on average (P = .01); and those with PTSD were 6.8 years younger on average, although only 1% (n = 15) of study subjects had PTSD, making for a small sample to study. These age-of-onset disparities didn’t appear among patients with previously diagnosed bipolar disorder (BPD) or schizophrenia.
Ms. Eijansantos noted that there were no differences in education attained or apolipoprotein-E gene status between the patients with and without a history of psychosis, and, within the subgroups of individual psychiatric disorders, there were no differences between patients with past and current or formal and informal diagnoses.
“When we split the cohort into quintiles based on age of Alzheimer’s disease onset, we find an inverse correlation between the amount of depression, anxiety, and PTSD endorsed and their ages of onset,” Ms. Eijansantos said. For example, the youngest quintile had a greater than 50% rate of depression while the oldest quintile had a depression rate around 36%. A similar spread was found with anxiety: a rate around 46% in the youngest quantile versus around 25% in the oldest, whereas rates of PTSD, BPD, and schizophrenia were similar across the five age-of-onset groups.
Patients with a history of multiple psychiatric disorders had an even younger age of onset. “We see that those with two psychiatric disorder are younger than those with one, and those with three psychiatric disorders are younger still,” Ms. Eijansantos said. “And we find that the Alzheimer’s disease age-at-onset reduction doubles with each additional psychiatric disorder.” Multiple disorders also adversely impacted survival, she said.
Because they found no difference between patients with past versus active symptoms and informal versus formal diagnosis, Ms. Eijansantos explained that they further studied the National Alzheimer’s Coordinating Center cohort of 8,267 patients with Alzheimer’s disease and found a similar relationship between psychiatric history and age of onset among patients with depression or anxiety or both. This cohort also documented symptom severity, she noted. “So when we look at depression and anxiety we find similar reductions in the Alzheimer’s disease age of onset with each increasing degree of symptom severity,” she said.
“Does this mean that psychiatric disease is a risk factor for Alzheimr’s disease?” Ms. Eijansantos said. “We can’t answer that with this study because it was only designed to see if the psychiatric factors modulate the age of onset in those that have Alzheimer’s disease, but taken together we believe that these results fit the framework that there are pathophysiological and profound differences between earlier and later presentations of Alzheimer’s disease.”
She pointed to reports that early-onset Alzheimer’s disease is associated with more aggressive tau pathology and that depression is associated with tau. However, the evidence supporting a link between amyloid and psychiatric disease is less certain, she said.
Preliminary and speculative findings
Senior study author Zachary Miller, MD, an assistant professor in the UCSF Memory and Aging Center, explained the significance of the study findings of potential links between depression and autoimmunity, and anxiety and seizure. “There may be distinct underlying pathophysiological mechanisms in patients with Alzheimer’s disease who have symptoms of depression versus anxiety,” he said, acknowledging the findings “are quite preliminary and our interpretations quite speculative.”
The findings raise the question that the symptomatic presentation of greater amounts of depression in early-onset Alzheimer’s disease may be moderated by an underlying neuroinflammatory insult, he said. “If so, depression symptomatology could then be seen as a possible clinical marker of this inflammatory response and possibly be used in testing clinical endpoints for future intervention trials,” Dr. Miller said. “Similarly, if neuronal hyperexcitability in Alzheimer’s disease manifests itself as either seizure and/or anxiety, this would have significant impact for therapeutic monitoring and treatment.”
He said a multicenter study of Alzheimer’s disease cohorts would validate the findings. “At the same time, we are also interested in looking deeper into these findings, investigating the potential cognitive and neuroanatomical correlates associated with these conditions,” Dr. Miller said.
Clinical phenotyping may provide more insight into the relationship between psychosis and age of Alzheimer’s disease onset, said Vijay K. Ramanan, MD, PhD, an assistant professor of neurology at Mayo Clinic in Rochester, Minn.
“Less typical presentations of Alzheimer’s disease, such as posterior cortical atrophy or dysexecutive Alzheimer’s disease, are associated with younger age of onset and are sometimes misdiagnosed as having pure psychiatric disease,” he said. “It is also possible that, in some cases with psychiatric disease, a younger age of onset of cognitive symptoms is charted, even though there are fundamentally two distinct processes at play – a psychiatric disease and a separate neurodegenerative disease – each having independent but additive impacts on cognition.”
Dr. Ramanan added, “This work is also a good reminder to be on the lookout for neuropsychiatric symptoms, treat where indicated, and be open to the possibility that psychiatric symptoms and Alzheimer’s disease can coexist.”
Ms. Eijansantos, Dr. Miller, and Dr. Ramanan have no relevant financial relationships to disclose.
, according to findings from a review of 1,500 patients with Alzheimer’s disease from a single-center population.
“Could psychosis symptoms be the proverbial canary in a coal mine?” Emily Eijansantos, a medical student at the University of California, San Francisco, said in reporting results of the chart review at the 2021 annual meeting of the American Academy of Neurology. “Previously in this cohort it was found that neurodevelopmental factors as well as chronic insults such as autoimmunity and seizure were also associated with an early age of onset in Alzheimer’s disease.”
The link between depression and autoimmunity, and anxiety and seizure “beg more questions about underlying pathophysiology,” she said. The study included 750 patients with early-onset Alzheimer’s disease and a similar number of late-onset patients from the UCSF Memory and Aging Center.
An inverse correlation between psychiatric disorders and age of Alzheimer’s onset
In the total study population, 43.5% (n = 652) had a previous diagnosis of depression and 32.3% (n = 485) had been diagnosed with anxiety. That, Ms. Eijansantos said, falls into similar ranges that other studies have reported.
“When we look at individual psychiatric disorders, we find that those with depression, anxiety, or PTSD are younger on average,” she said. “Patients with depression and anxiety are more [likely] female and have less vascular risk factors, and we observed an association between depression and autoimmunity, anxiety, and seizures.”
Specifically, patients with a history of depression were 2.2 years younger, on average, at the age of onset than patients without such history (P = .01); those with anxiety were 3 years younger on average (P = .01); and those with PTSD were 6.8 years younger on average, although only 1% (n = 15) of study subjects had PTSD, making for a small sample to study. These age-of-onset disparities didn’t appear among patients with previously diagnosed bipolar disorder (BPD) or schizophrenia.
Ms. Eijansantos noted that there were no differences in education attained or apolipoprotein-E gene status between the patients with and without a history of psychosis, and, within the subgroups of individual psychiatric disorders, there were no differences between patients with past and current or formal and informal diagnoses.
“When we split the cohort into quintiles based on age of Alzheimer’s disease onset, we find an inverse correlation between the amount of depression, anxiety, and PTSD endorsed and their ages of onset,” Ms. Eijansantos said. For example, the youngest quintile had a greater than 50% rate of depression while the oldest quintile had a depression rate around 36%. A similar spread was found with anxiety: a rate around 46% in the youngest quantile versus around 25% in the oldest, whereas rates of PTSD, BPD, and schizophrenia were similar across the five age-of-onset groups.
Patients with a history of multiple psychiatric disorders had an even younger age of onset. “We see that those with two psychiatric disorder are younger than those with one, and those with three psychiatric disorders are younger still,” Ms. Eijansantos said. “And we find that the Alzheimer’s disease age-at-onset reduction doubles with each additional psychiatric disorder.” Multiple disorders also adversely impacted survival, she said.
Because they found no difference between patients with past versus active symptoms and informal versus formal diagnosis, Ms. Eijansantos explained that they further studied the National Alzheimer’s Coordinating Center cohort of 8,267 patients with Alzheimer’s disease and found a similar relationship between psychiatric history and age of onset among patients with depression or anxiety or both. This cohort also documented symptom severity, she noted. “So when we look at depression and anxiety we find similar reductions in the Alzheimer’s disease age of onset with each increasing degree of symptom severity,” she said.
“Does this mean that psychiatric disease is a risk factor for Alzheimr’s disease?” Ms. Eijansantos said. “We can’t answer that with this study because it was only designed to see if the psychiatric factors modulate the age of onset in those that have Alzheimer’s disease, but taken together we believe that these results fit the framework that there are pathophysiological and profound differences between earlier and later presentations of Alzheimer’s disease.”
She pointed to reports that early-onset Alzheimer’s disease is associated with more aggressive tau pathology and that depression is associated with tau. However, the evidence supporting a link between amyloid and psychiatric disease is less certain, she said.
Preliminary and speculative findings
Senior study author Zachary Miller, MD, an assistant professor in the UCSF Memory and Aging Center, explained the significance of the study findings of potential links between depression and autoimmunity, and anxiety and seizure. “There may be distinct underlying pathophysiological mechanisms in patients with Alzheimer’s disease who have symptoms of depression versus anxiety,” he said, acknowledging the findings “are quite preliminary and our interpretations quite speculative.”
The findings raise the question that the symptomatic presentation of greater amounts of depression in early-onset Alzheimer’s disease may be moderated by an underlying neuroinflammatory insult, he said. “If so, depression symptomatology could then be seen as a possible clinical marker of this inflammatory response and possibly be used in testing clinical endpoints for future intervention trials,” Dr. Miller said. “Similarly, if neuronal hyperexcitability in Alzheimer’s disease manifests itself as either seizure and/or anxiety, this would have significant impact for therapeutic monitoring and treatment.”
He said a multicenter study of Alzheimer’s disease cohorts would validate the findings. “At the same time, we are also interested in looking deeper into these findings, investigating the potential cognitive and neuroanatomical correlates associated with these conditions,” Dr. Miller said.
Clinical phenotyping may provide more insight into the relationship between psychosis and age of Alzheimer’s disease onset, said Vijay K. Ramanan, MD, PhD, an assistant professor of neurology at Mayo Clinic in Rochester, Minn.
“Less typical presentations of Alzheimer’s disease, such as posterior cortical atrophy or dysexecutive Alzheimer’s disease, are associated with younger age of onset and are sometimes misdiagnosed as having pure psychiatric disease,” he said. “It is also possible that, in some cases with psychiatric disease, a younger age of onset of cognitive symptoms is charted, even though there are fundamentally two distinct processes at play – a psychiatric disease and a separate neurodegenerative disease – each having independent but additive impacts on cognition.”
Dr. Ramanan added, “This work is also a good reminder to be on the lookout for neuropsychiatric symptoms, treat where indicated, and be open to the possibility that psychiatric symptoms and Alzheimer’s disease can coexist.”
Ms. Eijansantos, Dr. Miller, and Dr. Ramanan have no relevant financial relationships to disclose.
FROM AAN 2021
Trend reversed: New cases of COVID-19 decline in children
New cases of COVID-19 dropped among children for just the second time in the past 6 weeks, but that was not enough to reverse the trend in children’s share of the weekly total, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

of all COVID-19 cases reported for the week, surpassing the pandemic-high 20.6% seen just a week earlier, the AAP/CHA report shows.
The total number of cases in children is now over 3.7 million – that’s 13.7% of cases in all ages – since the start of the pandemic, and the cumulative rate of infection has reached 4,931 per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Cases of more severe illness in children continue to trend lower. The cumulative number of hospitalizations in children (15,187) is only 2.0% of the total of almost 760,000 in the 25 jurisdictions (24 states and New York City) that report such data, and deaths in children now number 296, which is just 0.06% of all COVID-19–related mortality in 43 states, New York City, Puerto Rico, and Guam, the AAP and CHA said in their report.
Among those 46 jurisdictions, Texas has reported the most deaths (51) in children, followed by Arizona (29) and New York City (23), while 9 states and the District of Columbia have reported no deaths so far. Children represent the highest proportion of deaths (0.19%) in Colorado, but Guam, with 2 child deaths among its total of 136, has by far the highest rate at 1.47%, the AAP/CHA data show.
Data from the 25 reporting jurisdictions show that children make up the largest share of hospitalizations (3.1%) in Colorado and Minnesota, while New York City (1.9%), Georgia (1.3%), and Rhode Island (1.3%) have the highest hospitalization rates among children diagnosed with SARS-CoV-2 infection, the two groups reported.
New cases of COVID-19 dropped among children for just the second time in the past 6 weeks, but that was not enough to reverse the trend in children’s share of the weekly total, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

of all COVID-19 cases reported for the week, surpassing the pandemic-high 20.6% seen just a week earlier, the AAP/CHA report shows.
The total number of cases in children is now over 3.7 million – that’s 13.7% of cases in all ages – since the start of the pandemic, and the cumulative rate of infection has reached 4,931 per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Cases of more severe illness in children continue to trend lower. The cumulative number of hospitalizations in children (15,187) is only 2.0% of the total of almost 760,000 in the 25 jurisdictions (24 states and New York City) that report such data, and deaths in children now number 296, which is just 0.06% of all COVID-19–related mortality in 43 states, New York City, Puerto Rico, and Guam, the AAP and CHA said in their report.
Among those 46 jurisdictions, Texas has reported the most deaths (51) in children, followed by Arizona (29) and New York City (23), while 9 states and the District of Columbia have reported no deaths so far. Children represent the highest proportion of deaths (0.19%) in Colorado, but Guam, with 2 child deaths among its total of 136, has by far the highest rate at 1.47%, the AAP/CHA data show.
Data from the 25 reporting jurisdictions show that children make up the largest share of hospitalizations (3.1%) in Colorado and Minnesota, while New York City (1.9%), Georgia (1.3%), and Rhode Island (1.3%) have the highest hospitalization rates among children diagnosed with SARS-CoV-2 infection, the two groups reported.
New cases of COVID-19 dropped among children for just the second time in the past 6 weeks, but that was not enough to reverse the trend in children’s share of the weekly total, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

of all COVID-19 cases reported for the week, surpassing the pandemic-high 20.6% seen just a week earlier, the AAP/CHA report shows.
The total number of cases in children is now over 3.7 million – that’s 13.7% of cases in all ages – since the start of the pandemic, and the cumulative rate of infection has reached 4,931 per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Cases of more severe illness in children continue to trend lower. The cumulative number of hospitalizations in children (15,187) is only 2.0% of the total of almost 760,000 in the 25 jurisdictions (24 states and New York City) that report such data, and deaths in children now number 296, which is just 0.06% of all COVID-19–related mortality in 43 states, New York City, Puerto Rico, and Guam, the AAP and CHA said in their report.
Among those 46 jurisdictions, Texas has reported the most deaths (51) in children, followed by Arizona (29) and New York City (23), while 9 states and the District of Columbia have reported no deaths so far. Children represent the highest proportion of deaths (0.19%) in Colorado, but Guam, with 2 child deaths among its total of 136, has by far the highest rate at 1.47%, the AAP/CHA data show.
Data from the 25 reporting jurisdictions show that children make up the largest share of hospitalizations (3.1%) in Colorado and Minnesota, while New York City (1.9%), Georgia (1.3%), and Rhode Island (1.3%) have the highest hospitalization rates among children diagnosed with SARS-CoV-2 infection, the two groups reported.
Thyroid hormone analogues can reverse NASH
Background: Fat toxicity results in inflammation of the liver and eventual hepatic fibrosis and cirrhosis. Thyroid hormones can greatly reduce this hepatic steatosis by restoring metabolic pathways in damaged liver, prevent fibrosis progression, and have broad atherogenic lipid-lowering actions by activating hepatic thyroid beta-receptors.
However, hyperthyroidism also leads to osteoporosis, tachyarrhythmias, muscle wasting, and psychiatric side effects, mediated by the alpha-thyroid receptor. Resmetirom (MGL-3196) is a novel, highly selective thyroid beta-agonist, with a minimal side-effect profile, which avoids the alpha–side effects.
Study design: Randomized, double-blind, placebo-controlled study.
Setting: 25 centers in the United States.
Synopsis: Of 125 adults with NASH fibrosis 1-3 and greater than 10% hepatic fat, 84 received resmetirom and 41 received placebo. Resmetirom resulted in a nearly 30% decrease over placebo in hepatic fat, compared with baseline, significant improvement in lipid profile, improvement in liver enzymes, fibrosis markers, and histologic resolution of NASH in some patients.
While the study showed resolution of inflammation, the 36-week study was likely not long enough to show improvement of fibrosis. The relatively small sample size also limited results. Placebo patients who lost significant weight also showed improvement and were discarded from analysis, suggesting that weight loss itself is also an excellent alternative to reverse NASH. Resmetirom use in NASH is now moving into a large phase 3 trial.
Bottom line: Resmetirom results in major liver and cardiovascular benefits in patients with NASH.
Citation: Harrison SA et al. Resmetirom (MGL-3196) for the treatment of nonalcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019 Nov 11;394(10213):2012-24.
Dr. Raghavan is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Fat toxicity results in inflammation of the liver and eventual hepatic fibrosis and cirrhosis. Thyroid hormones can greatly reduce this hepatic steatosis by restoring metabolic pathways in damaged liver, prevent fibrosis progression, and have broad atherogenic lipid-lowering actions by activating hepatic thyroid beta-receptors.
However, hyperthyroidism also leads to osteoporosis, tachyarrhythmias, muscle wasting, and psychiatric side effects, mediated by the alpha-thyroid receptor. Resmetirom (MGL-3196) is a novel, highly selective thyroid beta-agonist, with a minimal side-effect profile, which avoids the alpha–side effects.
Study design: Randomized, double-blind, placebo-controlled study.
Setting: 25 centers in the United States.
Synopsis: Of 125 adults with NASH fibrosis 1-3 and greater than 10% hepatic fat, 84 received resmetirom and 41 received placebo. Resmetirom resulted in a nearly 30% decrease over placebo in hepatic fat, compared with baseline, significant improvement in lipid profile, improvement in liver enzymes, fibrosis markers, and histologic resolution of NASH in some patients.
While the study showed resolution of inflammation, the 36-week study was likely not long enough to show improvement of fibrosis. The relatively small sample size also limited results. Placebo patients who lost significant weight also showed improvement and were discarded from analysis, suggesting that weight loss itself is also an excellent alternative to reverse NASH. Resmetirom use in NASH is now moving into a large phase 3 trial.
Bottom line: Resmetirom results in major liver and cardiovascular benefits in patients with NASH.
Citation: Harrison SA et al. Resmetirom (MGL-3196) for the treatment of nonalcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019 Nov 11;394(10213):2012-24.
Dr. Raghavan is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Fat toxicity results in inflammation of the liver and eventual hepatic fibrosis and cirrhosis. Thyroid hormones can greatly reduce this hepatic steatosis by restoring metabolic pathways in damaged liver, prevent fibrosis progression, and have broad atherogenic lipid-lowering actions by activating hepatic thyroid beta-receptors.
However, hyperthyroidism also leads to osteoporosis, tachyarrhythmias, muscle wasting, and psychiatric side effects, mediated by the alpha-thyroid receptor. Resmetirom (MGL-3196) is a novel, highly selective thyroid beta-agonist, with a minimal side-effect profile, which avoids the alpha–side effects.
Study design: Randomized, double-blind, placebo-controlled study.
Setting: 25 centers in the United States.
Synopsis: Of 125 adults with NASH fibrosis 1-3 and greater than 10% hepatic fat, 84 received resmetirom and 41 received placebo. Resmetirom resulted in a nearly 30% decrease over placebo in hepatic fat, compared with baseline, significant improvement in lipid profile, improvement in liver enzymes, fibrosis markers, and histologic resolution of NASH in some patients.
While the study showed resolution of inflammation, the 36-week study was likely not long enough to show improvement of fibrosis. The relatively small sample size also limited results. Placebo patients who lost significant weight also showed improvement and were discarded from analysis, suggesting that weight loss itself is also an excellent alternative to reverse NASH. Resmetirom use in NASH is now moving into a large phase 3 trial.
Bottom line: Resmetirom results in major liver and cardiovascular benefits in patients with NASH.
Citation: Harrison SA et al. Resmetirom (MGL-3196) for the treatment of nonalcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019 Nov 11;394(10213):2012-24.
Dr. Raghavan is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
A Practical Guide to Treatment of Hair Loss Beyond Standard Therapy
When I was a medical student rotating in dermatology, a patient with extensive alopecia looked at my long thick hair and said tearfully, “I just wish I could have hair like yours.”
I smiled, removed my wig, and replied, “You can have hair like mine.”
Determination and Perseverance
I was 2 years old when I was given a diagnosis of alopecia areata. Bald spots on my scalp would come and go for years but were not overly burdensome until I turned 12. At that point, my hair loss escalated despite frequent intralesional injections of triamcinolone; every 2 steps forward were followed by 3 steps backward.
As a freshman in high school, I finally took control of my condition and emotions, shaved my head, and purchased a wig—actions that confronted my hair loss and awoke a determination and perseverance that I did not think I would ever gain while living with this condition. As McGettigan1 wrote in the Journal of the American Academy of Dermatology in 2004, “Being diagnosed with [alopecia areata] does not mean one cannot have a full and meaningful life. By choosing to confront the condition and turn its negative aspects into positive actions, one can succeed in life.”1
As a Provider, Another Perspective
Now, as a dermatology resident, I have the distinct perspective of being patient and provider. Patients often want to know, “Why is this happening?”, “Is my hair going to grow back?”, and “What treatments are available?”
They want to feel supported, understood, and heard.
As health care providers, we must understand that hair loss can result in overwhelming fear, hopelessness, and loss of self-esteem. Although we can give good news and offer helpful treatment options to some patients, there are those for whom medical treatment fails, and we can offer no more than a supportive hand and warm smile.
But can we do even more than that? The answer is: “Yes.”
Management Options
I recommend that all patients with hair loss should receive a copy of the aforementioned McGettigan1 article, “Ahead With No Hair,” which is geared toward patients with alopecia areata but offers inspiring words to any patient struggling to cope with hair loss. Dermatologists also can offer management options for patients with hair loss, including camouflage, wigs, and cosmetic replacement of eyelashes and eyebrows. Of note, several companies offer wigs and brow replacement options for men and children.
Camouflage
We can offer creative and readily available camouflage options for patients with hair loss. For small bald spots and thinning hair on the scalp, keratin hair-building fibers can be extremely useful. This over-the-counter product comes in a variety of natural hair colors, conceals the underlying skin, and adds fullness to hair. The keratin fibers have an innate static charge that allows them to adhere to the hair shaft. Daily application typically is necessary; duration can be maximized if hair spray or other brand-specific bonding spray is used following application of the fibers. A simple online search using the term keratin hair building fibers will reveal many online and in-store options with 4- or 5-star reviews. Most negative reviews pertain to sweating or moisture that causes clumping, but overall this is an easy and affordable option for mild hair loss.
Wigs
For patients hoping to mask moderate or severe hair loss, I recommend wigs, which can be made from synthetic fibers or human hair. In order to effectively guide patients, it is helpful for providers to have some knowledge about the 2 types of wigs. Synthetic wigs are of variable quality, ranging from costume-grade to top-quality products that look and feel like human hair. They are more affordable and often are easier to maintain than human-hair wigs, and hairstyles hold up better after washing. Many synthetic wigs cannot withstand heat from a hot iron and have a slightly shorter lifespan (6–12 months) than human-hair wigs (1–2 years).
Human hair wigs are made of real human hair, so they look and feel natural. These wigs can be made from European, African, Indian, Malaysian, Chinese, or other ethnic hair. Patients can choose the texture of the hair, including silky (smooth), kinky (mimicking natural blow-dried Black hair), and yaki (mimicking relaxed Black hair), as well as the curl pattern (straight, wavy, or curly), length, color, density, and cap construction.
The cap of a wig is what the hair is tied to. The construction of wig caps varies to allow for realistic hair lines as well as security for active use or up-dos. Among the many cap-construction options, the most realistic-appearing are hand-tied monofilament, lace-front, and full-lace wigs, all of which may require tape or glue to keep them in place. Some wig companies offer nonslip so-called “alopecia caps” for patients with no scalp hair. Patients who find their wig irritating to the scalp should consider wearing a nylon wig cap or liner.
Wigs can be purchased in store or online and can be pre-made or custom-built to be tailored to the patient’s specific desires and expectations. The cost depends on the type and quality of hair, cap construction, and length; prices can range from less than $100 to more than $5000.
When choosing a wig, which option—synthetic or human hair—is better for a given patient? Synthetic wigs are rather inexpensive and easy to care for, making them great for new users and those who want to try different styles and colors. Human-hair wigs can be custom-made to match the patient’s natural hair; however, they require extra care to maintain their longevity. Both types of wigs have pros and cons depending on the patient’s budget, time required for maintenance and styling, and needs (Table 1). I encourage patients to have fun with all wig options: Now is the time, I tell them, to try out the cute or daring hair style they have always wanted. The great thing is that if the patient does not like their wig, they can readily change it.
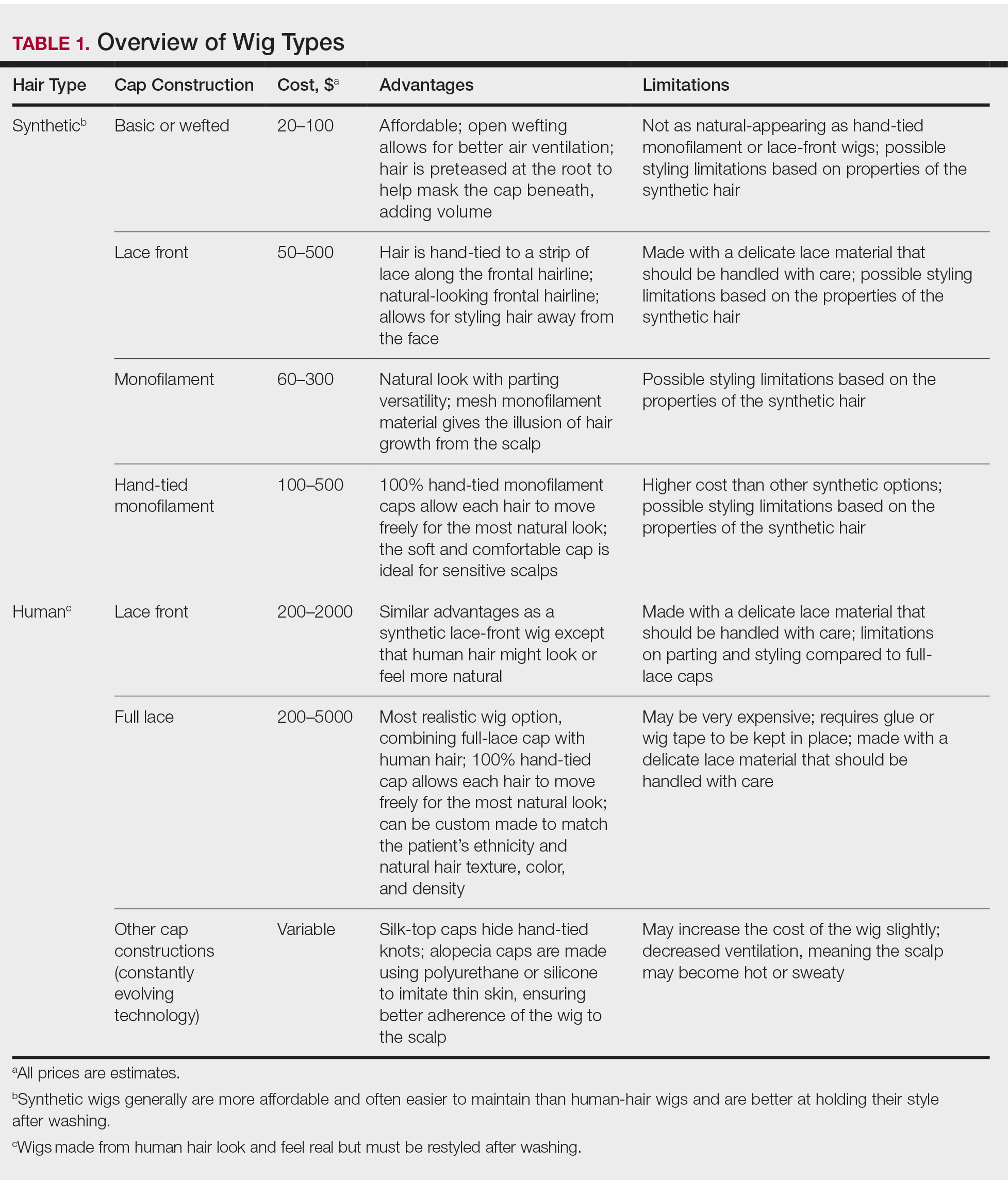
Good-quality wigs are expensive but sometimes are necessary to regain self-confidence and improve one’s quality of life. Advise patients to call their health insurance company to find out if a cranial or scalp prosthesis is covered by their policy. Coverage might require a written prescription for a cranial prosthesis, listing the diagnosis, diagnosis code, and letter of medical necessity. Patients can then purchase the wig online or through a certified distributor depending on their insurance requirements and obtain reimbursement (partial or full coverage). If a wig is not covered by insurance, a cranial prosthesis might be a flexible spending account–eligible expense. For guidance on the reimbursability of wigs, visit the National Alopecia Areata Foundation (NAAF) website (www.naaf.org/AccessHealthcare).
Eyelashes and Eyebrows
Cosmetic replacement of eyelashes (Table 2) and eyebrows (Table 3) is another treatment option that physicians can offer to hair-loss patients. For patients who desire false eyelashes, strip lashes that are glued to the eyelid margin are easiest to apply (but with caution—do not get glue in the eyes!). There are magnetic lashes, but these require natural lashes on which to adhere them. Eyebrows can be hand-drawn using brow pencils or powders with or without a stencil to maintain symmetry. There are even brow wigs and temporary brow tattoos that can last 1 to several days. Semi-permanent tattooing, including microblading, is an option that has amazing results but can be painful and expensive, often requiring touch-ups every 6 to 18 months.
Resources Abound
Experiencing and treating hair loss can be overwhelming, but there are countless resources available for patients. The NAAF has utility beyond the concerns of alopecia areata patients; there also is useful information on YouTube and social media, and support groups exist for hair-loss patients. I recommend starting with the NAAF website, which offers many helpful resources and support groups for patients and their families, including tips on applying for insurance reimbursement and drafting an appeal letter. Lastly, several nonprofit organizations serve the hair-replacement needs of children and adults with hair loss (Table 4).
Final Thoughts
My experience as a patient with alopecia has been long and initially was challenging; however, I found the silver lining after choosing to confront my literal and figurative “losses” and move forward—to grow, so to speak. With the use of custom-made human-hair wigs, false strip eyelashes, and a mix of eyebrow replacement options, I have been able to regain my confidence and self-esteem. Now, my goal as a physician—a goal that I hope you will share—is to be knowledgeable about hair-replacement options and provide information and resources to patients to help them feel empowered, brave, and beautiful.
- McGettigan ML. Ahead with no hair. J Am Acad Dermatol. 2004;51(1 suppl):18-19.
When I was a medical student rotating in dermatology, a patient with extensive alopecia looked at my long thick hair and said tearfully, “I just wish I could have hair like yours.”
I smiled, removed my wig, and replied, “You can have hair like mine.”
Determination and Perseverance
I was 2 years old when I was given a diagnosis of alopecia areata. Bald spots on my scalp would come and go for years but were not overly burdensome until I turned 12. At that point, my hair loss escalated despite frequent intralesional injections of triamcinolone; every 2 steps forward were followed by 3 steps backward.
As a freshman in high school, I finally took control of my condition and emotions, shaved my head, and purchased a wig—actions that confronted my hair loss and awoke a determination and perseverance that I did not think I would ever gain while living with this condition. As McGettigan1 wrote in the Journal of the American Academy of Dermatology in 2004, “Being diagnosed with [alopecia areata] does not mean one cannot have a full and meaningful life. By choosing to confront the condition and turn its negative aspects into positive actions, one can succeed in life.”1
As a Provider, Another Perspective
Now, as a dermatology resident, I have the distinct perspective of being patient and provider. Patients often want to know, “Why is this happening?”, “Is my hair going to grow back?”, and “What treatments are available?”
They want to feel supported, understood, and heard.
As health care providers, we must understand that hair loss can result in overwhelming fear, hopelessness, and loss of self-esteem. Although we can give good news and offer helpful treatment options to some patients, there are those for whom medical treatment fails, and we can offer no more than a supportive hand and warm smile.
But can we do even more than that? The answer is: “Yes.”
Management Options
I recommend that all patients with hair loss should receive a copy of the aforementioned McGettigan1 article, “Ahead With No Hair,” which is geared toward patients with alopecia areata but offers inspiring words to any patient struggling to cope with hair loss. Dermatologists also can offer management options for patients with hair loss, including camouflage, wigs, and cosmetic replacement of eyelashes and eyebrows. Of note, several companies offer wigs and brow replacement options for men and children.
Camouflage
We can offer creative and readily available camouflage options for patients with hair loss. For small bald spots and thinning hair on the scalp, keratin hair-building fibers can be extremely useful. This over-the-counter product comes in a variety of natural hair colors, conceals the underlying skin, and adds fullness to hair. The keratin fibers have an innate static charge that allows them to adhere to the hair shaft. Daily application typically is necessary; duration can be maximized if hair spray or other brand-specific bonding spray is used following application of the fibers. A simple online search using the term keratin hair building fibers will reveal many online and in-store options with 4- or 5-star reviews. Most negative reviews pertain to sweating or moisture that causes clumping, but overall this is an easy and affordable option for mild hair loss.
Wigs
For patients hoping to mask moderate or severe hair loss, I recommend wigs, which can be made from synthetic fibers or human hair. In order to effectively guide patients, it is helpful for providers to have some knowledge about the 2 types of wigs. Synthetic wigs are of variable quality, ranging from costume-grade to top-quality products that look and feel like human hair. They are more affordable and often are easier to maintain than human-hair wigs, and hairstyles hold up better after washing. Many synthetic wigs cannot withstand heat from a hot iron and have a slightly shorter lifespan (6–12 months) than human-hair wigs (1–2 years).
Human hair wigs are made of real human hair, so they look and feel natural. These wigs can be made from European, African, Indian, Malaysian, Chinese, or other ethnic hair. Patients can choose the texture of the hair, including silky (smooth), kinky (mimicking natural blow-dried Black hair), and yaki (mimicking relaxed Black hair), as well as the curl pattern (straight, wavy, or curly), length, color, density, and cap construction.
The cap of a wig is what the hair is tied to. The construction of wig caps varies to allow for realistic hair lines as well as security for active use or up-dos. Among the many cap-construction options, the most realistic-appearing are hand-tied monofilament, lace-front, and full-lace wigs, all of which may require tape or glue to keep them in place. Some wig companies offer nonslip so-called “alopecia caps” for patients with no scalp hair. Patients who find their wig irritating to the scalp should consider wearing a nylon wig cap or liner.
Wigs can be purchased in store or online and can be pre-made or custom-built to be tailored to the patient’s specific desires and expectations. The cost depends on the type and quality of hair, cap construction, and length; prices can range from less than $100 to more than $5000.
When choosing a wig, which option—synthetic or human hair—is better for a given patient? Synthetic wigs are rather inexpensive and easy to care for, making them great for new users and those who want to try different styles and colors. Human-hair wigs can be custom-made to match the patient’s natural hair; however, they require extra care to maintain their longevity. Both types of wigs have pros and cons depending on the patient’s budget, time required for maintenance and styling, and needs (Table 1). I encourage patients to have fun with all wig options: Now is the time, I tell them, to try out the cute or daring hair style they have always wanted. The great thing is that if the patient does not like their wig, they can readily change it.

Good-quality wigs are expensive but sometimes are necessary to regain self-confidence and improve one’s quality of life. Advise patients to call their health insurance company to find out if a cranial or scalp prosthesis is covered by their policy. Coverage might require a written prescription for a cranial prosthesis, listing the diagnosis, diagnosis code, and letter of medical necessity. Patients can then purchase the wig online or through a certified distributor depending on their insurance requirements and obtain reimbursement (partial or full coverage). If a wig is not covered by insurance, a cranial prosthesis might be a flexible spending account–eligible expense. For guidance on the reimbursability of wigs, visit the National Alopecia Areata Foundation (NAAF) website (www.naaf.org/AccessHealthcare).
Eyelashes and Eyebrows
Cosmetic replacement of eyelashes (Table 2) and eyebrows (Table 3) is another treatment option that physicians can offer to hair-loss patients. For patients who desire false eyelashes, strip lashes that are glued to the eyelid margin are easiest to apply (but with caution—do not get glue in the eyes!). There are magnetic lashes, but these require natural lashes on which to adhere them. Eyebrows can be hand-drawn using brow pencils or powders with or without a stencil to maintain symmetry. There are even brow wigs and temporary brow tattoos that can last 1 to several days. Semi-permanent tattooing, including microblading, is an option that has amazing results but can be painful and expensive, often requiring touch-ups every 6 to 18 months.


Resources Abound
Experiencing and treating hair loss can be overwhelming, but there are countless resources available for patients. The NAAF has utility beyond the concerns of alopecia areata patients; there also is useful information on YouTube and social media, and support groups exist for hair-loss patients. I recommend starting with the NAAF website, which offers many helpful resources and support groups for patients and their families, including tips on applying for insurance reimbursement and drafting an appeal letter. Lastly, several nonprofit organizations serve the hair-replacement needs of children and adults with hair loss (Table 4).

Final Thoughts
My experience as a patient with alopecia has been long and initially was challenging; however, I found the silver lining after choosing to confront my literal and figurative “losses” and move forward—to grow, so to speak. With the use of custom-made human-hair wigs, false strip eyelashes, and a mix of eyebrow replacement options, I have been able to regain my confidence and self-esteem. Now, my goal as a physician—a goal that I hope you will share—is to be knowledgeable about hair-replacement options and provide information and resources to patients to help them feel empowered, brave, and beautiful.
When I was a medical student rotating in dermatology, a patient with extensive alopecia looked at my long thick hair and said tearfully, “I just wish I could have hair like yours.”
I smiled, removed my wig, and replied, “You can have hair like mine.”
Determination and Perseverance
I was 2 years old when I was given a diagnosis of alopecia areata. Bald spots on my scalp would come and go for years but were not overly burdensome until I turned 12. At that point, my hair loss escalated despite frequent intralesional injections of triamcinolone; every 2 steps forward were followed by 3 steps backward.
As a freshman in high school, I finally took control of my condition and emotions, shaved my head, and purchased a wig—actions that confronted my hair loss and awoke a determination and perseverance that I did not think I would ever gain while living with this condition. As McGettigan1 wrote in the Journal of the American Academy of Dermatology in 2004, “Being diagnosed with [alopecia areata] does not mean one cannot have a full and meaningful life. By choosing to confront the condition and turn its negative aspects into positive actions, one can succeed in life.”1
As a Provider, Another Perspective
Now, as a dermatology resident, I have the distinct perspective of being patient and provider. Patients often want to know, “Why is this happening?”, “Is my hair going to grow back?”, and “What treatments are available?”
They want to feel supported, understood, and heard.
As health care providers, we must understand that hair loss can result in overwhelming fear, hopelessness, and loss of self-esteem. Although we can give good news and offer helpful treatment options to some patients, there are those for whom medical treatment fails, and we can offer no more than a supportive hand and warm smile.
But can we do even more than that? The answer is: “Yes.”
Management Options
I recommend that all patients with hair loss should receive a copy of the aforementioned McGettigan1 article, “Ahead With No Hair,” which is geared toward patients with alopecia areata but offers inspiring words to any patient struggling to cope with hair loss. Dermatologists also can offer management options for patients with hair loss, including camouflage, wigs, and cosmetic replacement of eyelashes and eyebrows. Of note, several companies offer wigs and brow replacement options for men and children.
Camouflage
We can offer creative and readily available camouflage options for patients with hair loss. For small bald spots and thinning hair on the scalp, keratin hair-building fibers can be extremely useful. This over-the-counter product comes in a variety of natural hair colors, conceals the underlying skin, and adds fullness to hair. The keratin fibers have an innate static charge that allows them to adhere to the hair shaft. Daily application typically is necessary; duration can be maximized if hair spray or other brand-specific bonding spray is used following application of the fibers. A simple online search using the term keratin hair building fibers will reveal many online and in-store options with 4- or 5-star reviews. Most negative reviews pertain to sweating or moisture that causes clumping, but overall this is an easy and affordable option for mild hair loss.
Wigs
For patients hoping to mask moderate or severe hair loss, I recommend wigs, which can be made from synthetic fibers or human hair. In order to effectively guide patients, it is helpful for providers to have some knowledge about the 2 types of wigs. Synthetic wigs are of variable quality, ranging from costume-grade to top-quality products that look and feel like human hair. They are more affordable and often are easier to maintain than human-hair wigs, and hairstyles hold up better after washing. Many synthetic wigs cannot withstand heat from a hot iron and have a slightly shorter lifespan (6–12 months) than human-hair wigs (1–2 years).
Human hair wigs are made of real human hair, so they look and feel natural. These wigs can be made from European, African, Indian, Malaysian, Chinese, or other ethnic hair. Patients can choose the texture of the hair, including silky (smooth), kinky (mimicking natural blow-dried Black hair), and yaki (mimicking relaxed Black hair), as well as the curl pattern (straight, wavy, or curly), length, color, density, and cap construction.
The cap of a wig is what the hair is tied to. The construction of wig caps varies to allow for realistic hair lines as well as security for active use or up-dos. Among the many cap-construction options, the most realistic-appearing are hand-tied monofilament, lace-front, and full-lace wigs, all of which may require tape or glue to keep them in place. Some wig companies offer nonslip so-called “alopecia caps” for patients with no scalp hair. Patients who find their wig irritating to the scalp should consider wearing a nylon wig cap or liner.
Wigs can be purchased in store or online and can be pre-made or custom-built to be tailored to the patient’s specific desires and expectations. The cost depends on the type and quality of hair, cap construction, and length; prices can range from less than $100 to more than $5000.
When choosing a wig, which option—synthetic or human hair—is better for a given patient? Synthetic wigs are rather inexpensive and easy to care for, making them great for new users and those who want to try different styles and colors. Human-hair wigs can be custom-made to match the patient’s natural hair; however, they require extra care to maintain their longevity. Both types of wigs have pros and cons depending on the patient’s budget, time required for maintenance and styling, and needs (Table 1). I encourage patients to have fun with all wig options: Now is the time, I tell them, to try out the cute or daring hair style they have always wanted. The great thing is that if the patient does not like their wig, they can readily change it.

Good-quality wigs are expensive but sometimes are necessary to regain self-confidence and improve one’s quality of life. Advise patients to call their health insurance company to find out if a cranial or scalp prosthesis is covered by their policy. Coverage might require a written prescription for a cranial prosthesis, listing the diagnosis, diagnosis code, and letter of medical necessity. Patients can then purchase the wig online or through a certified distributor depending on their insurance requirements and obtain reimbursement (partial or full coverage). If a wig is not covered by insurance, a cranial prosthesis might be a flexible spending account–eligible expense. For guidance on the reimbursability of wigs, visit the National Alopecia Areata Foundation (NAAF) website (www.naaf.org/AccessHealthcare).
Eyelashes and Eyebrows
Cosmetic replacement of eyelashes (Table 2) and eyebrows (Table 3) is another treatment option that physicians can offer to hair-loss patients. For patients who desire false eyelashes, strip lashes that are glued to the eyelid margin are easiest to apply (but with caution—do not get glue in the eyes!). There are magnetic lashes, but these require natural lashes on which to adhere them. Eyebrows can be hand-drawn using brow pencils or powders with or without a stencil to maintain symmetry. There are even brow wigs and temporary brow tattoos that can last 1 to several days. Semi-permanent tattooing, including microblading, is an option that has amazing results but can be painful and expensive, often requiring touch-ups every 6 to 18 months.


Resources Abound
Experiencing and treating hair loss can be overwhelming, but there are countless resources available for patients. The NAAF has utility beyond the concerns of alopecia areata patients; there also is useful information on YouTube and social media, and support groups exist for hair-loss patients. I recommend starting with the NAAF website, which offers many helpful resources and support groups for patients and their families, including tips on applying for insurance reimbursement and drafting an appeal letter. Lastly, several nonprofit organizations serve the hair-replacement needs of children and adults with hair loss (Table 4).

Final Thoughts
My experience as a patient with alopecia has been long and initially was challenging; however, I found the silver lining after choosing to confront my literal and figurative “losses” and move forward—to grow, so to speak. With the use of custom-made human-hair wigs, false strip eyelashes, and a mix of eyebrow replacement options, I have been able to regain my confidence and self-esteem. Now, my goal as a physician—a goal that I hope you will share—is to be knowledgeable about hair-replacement options and provide information and resources to patients to help them feel empowered, brave, and beautiful.
- McGettigan ML. Ahead with no hair. J Am Acad Dermatol. 2004;51(1 suppl):18-19.
- McGettigan ML. Ahead with no hair. J Am Acad Dermatol. 2004;51(1 suppl):18-19.
Practice Points
- Keratin hair-building fibers can help thinning hair appear thick and full.
- Wigs are useful in masking moderate to severe hair loss.
- False eyelashes, eyebrow wigs, temporary eyebrow tattoos, microblading, and other semipermanent makeup can disguise the loss of eyelashes and eyebrows.
Erythema Multiforme–like Dermatitis Due to Isoniazid Hypersensitivity in a Patient With Psoriasis
To the Editor:
Psoriasis vulgaris is a chronic autoimmune inflammatory disease and biologic agents, such as anti–tumor necrosis factor α (TNF-α), are alternative drugs in case of resistance or adverse events to conventional ones.1 The limitation of these agents is immunosuppression that may cause infections such as tuberculosis (TB). Prophylaxis is indicated to latent TB diseases if the purified protein derivative (tuberculin) skin test is higher than 5 mm before starting these treatments. The challenge in TB treatment is adverse drug reactions (ADRs) that are reported in 4% to 6% of cases.2,3
Erythema multiforme–like dermatitis is a rare skin rash that develops due to isoniazid (INH). The clinical presentation includes erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH. Skin biopsy and patch tests are the supportive diagnostic methods. Isoniazid-associated skin rashes rarely are reported and generally are not severe enough to terminate the drug. We present a patient with psoriasis who received TB prophylaxis before anti–TNF-α use. He presented with erythema multiforme–like dermatitis due to INH. Withdrawal of the drug and treatment of the lesions were the first steps of intolerance, followed by a patch test with the culprit drug after recovery. We discuss the diagnostic drug allergy evaluation and treatment approach.
A 37-year-old man presented with a 15-year history of severe psoriasis with frequent flares. He was treated with various topical and systemic agents including acitretin and methotrexate at 4-year intervals. Despite the addition of phototherapy, he underwent a new treatment with anti–TNF-α, as the disease control with other treatments was insufficient. Before starting anti–TNF-α, preventive treatment against TB with INH (300 mg/d) was indicated with 20 mm of purified protein derivative. On approximately the 20th day of treatment, he developed pruritic erythema with desquamation and exfoliation localized to the hands and feet (Figure 1). Isoniazid was discontinued and a topical steroid was initiated. After 3 weeks, the skin lesions were completely improved and INH was reinitiated at the same dose with antihistamine prophylaxis (oral levocetirizine 5 mg/d). Seven days later, similar skin lesions presented that were more extensive on the arms and legs (Figure 2). Complete blood cell counts, renal and hepatic function tests, and hepatitis markers were within reference range in consultation with the allergy division. To distinguish the lesions from a psoriasis attack, a punch biopsy of the eruptive dermatitis showed erythema multiforme–like dermatitis including dermal edema and perivascular lymphocytic infiltration with no relation to psoriasis but consistent with a drug eruption. Isoniazid was discontinued, and the skin lesions resolved after 4 weeks of topical steroid and oral antihistamine use (Figure 3). There was no other drug use except INH, and a skin patch test with INH was positive at 72 hours (Figure 4). Skin tests with INH were done to 5 healthy lesions that were negative. Finally, TB prophylaxis was performed with rifampicin (10 mg/kg/d [600 mg/d]) for 4 months with no ADRs. The patient’s psoriasis lesions improved with anti–TNF-α that was initiated 1 month after starting TB prevention with rifampicin.
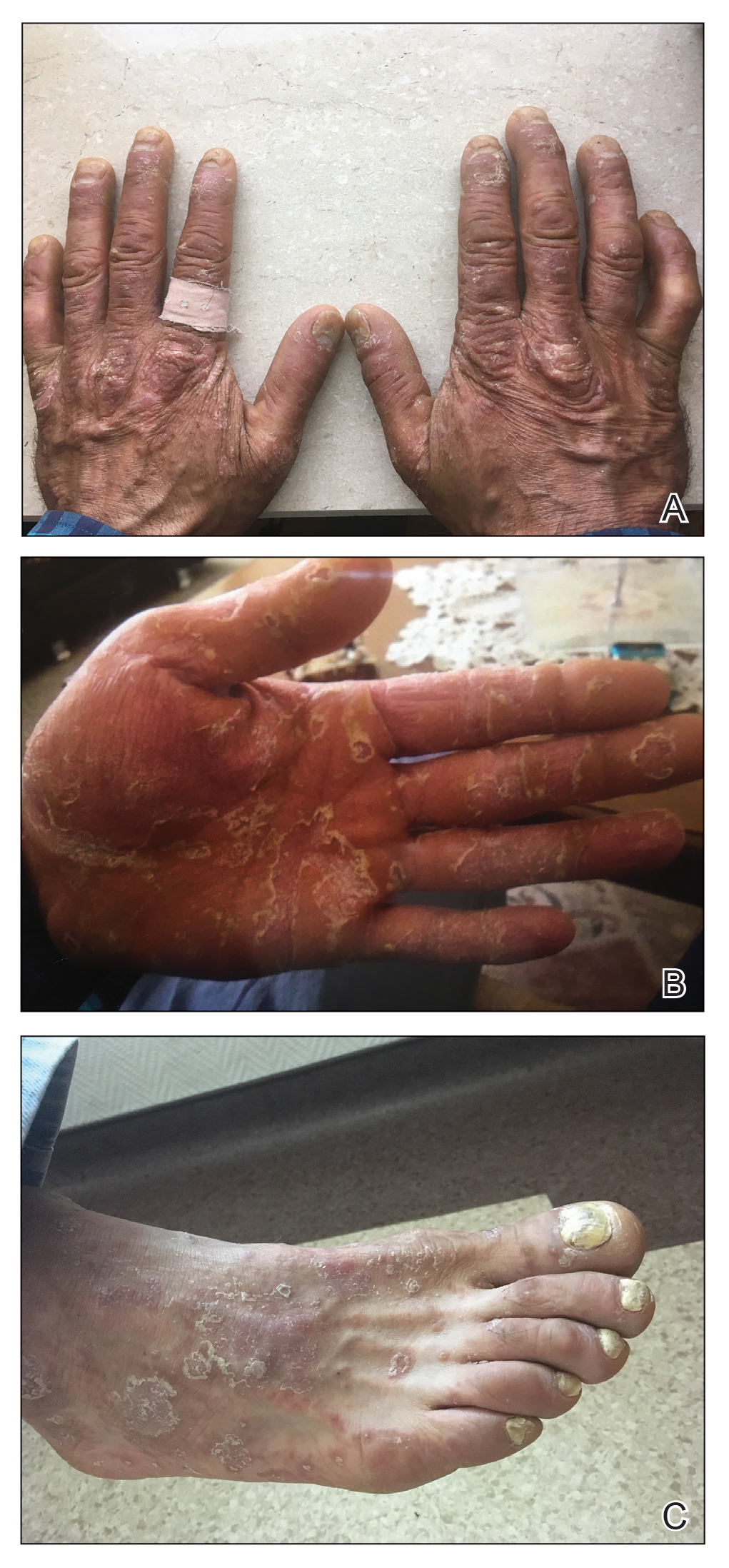
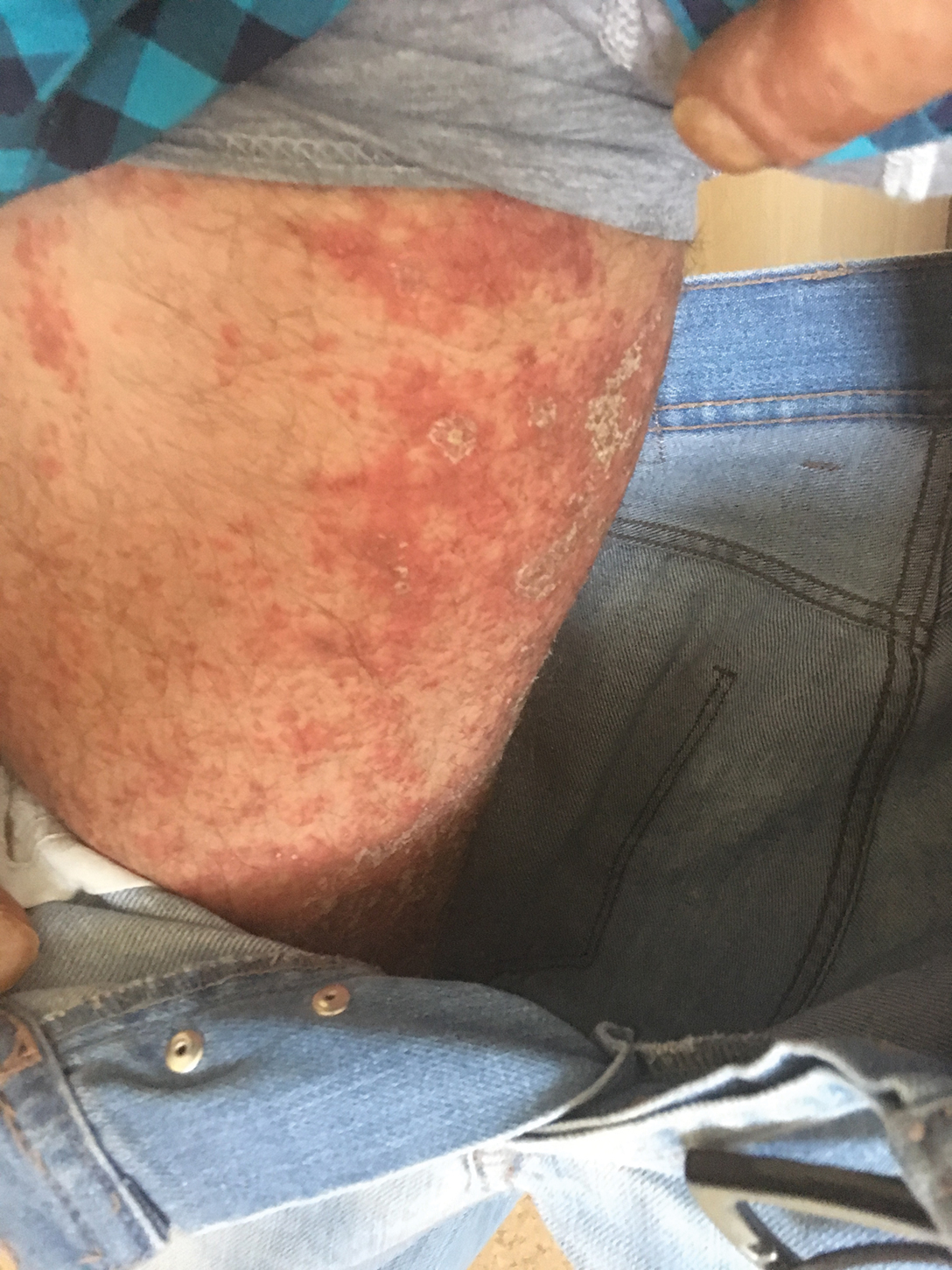
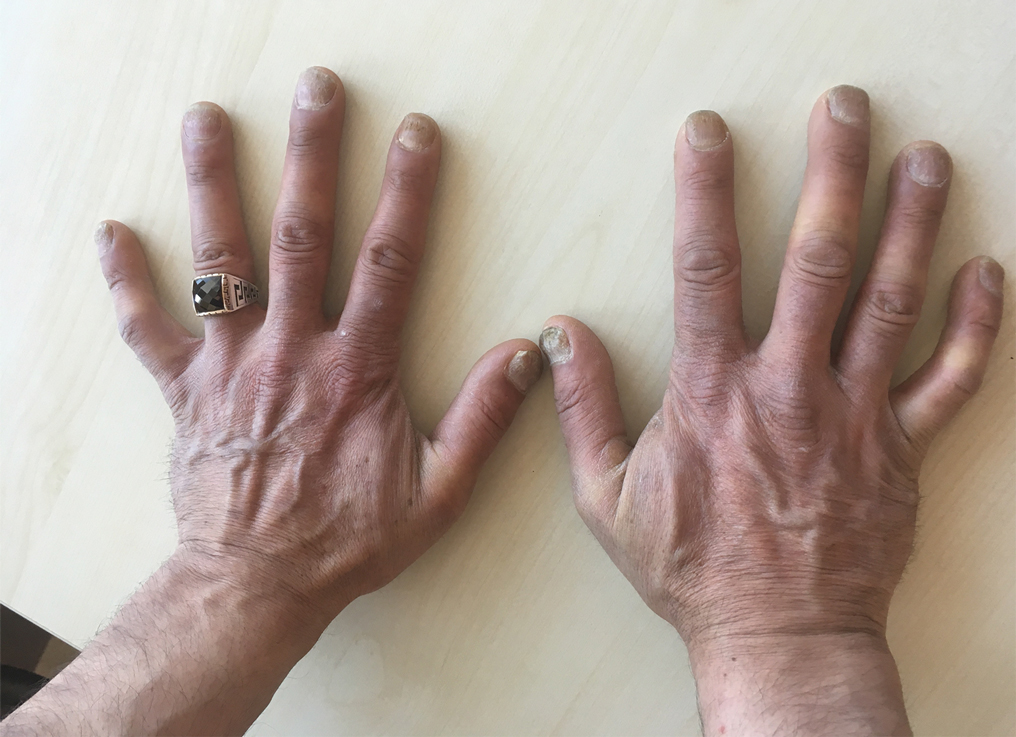
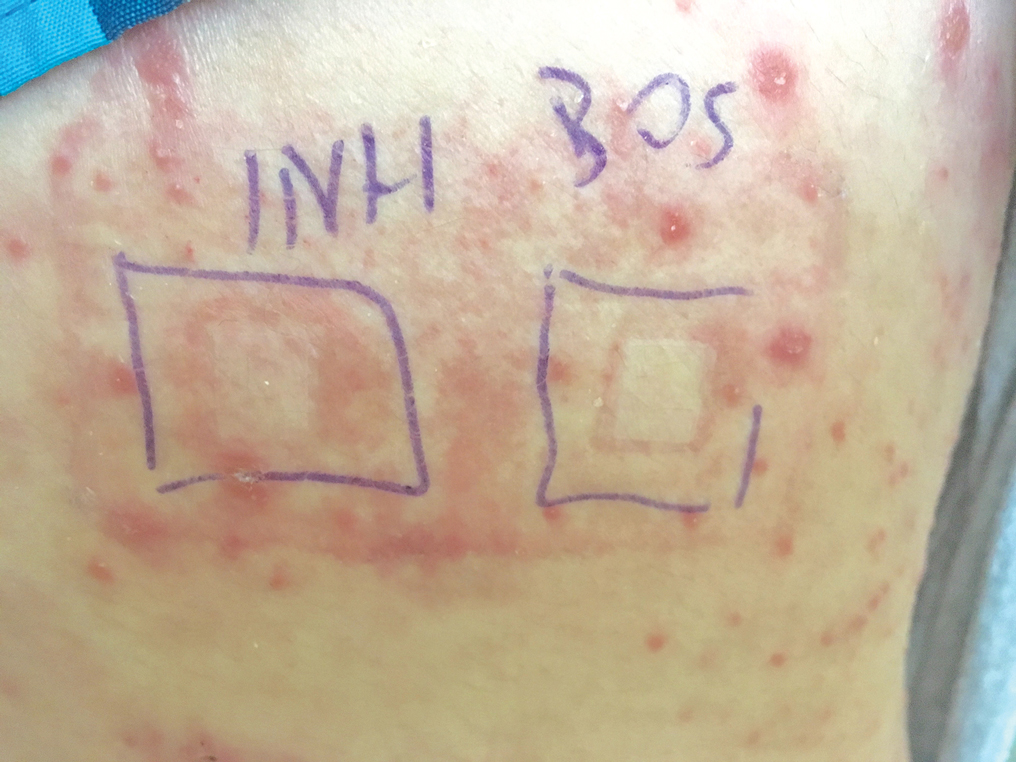
This case of erythema multiforme–like dermatitis was diagnosed with acral involvement, a positive patch test to INH, and lymphocytic inflammation in a skin biopsy. It was a drug-induced reaction, as skin lesions developed during INH intake and improved after drug withdrawal.
Isoniazid, also known as isonicotinylhydrazide, is an oral antibiotic used for the treatment of TB and other mycobacteria. Protective treatment against latent TB primarily is done with daily INH for 6 or 9 months; alternatively, INH may be taken weekly with rifapentine for 3 months or daily with rifampicin for 4 months. Daily rifampicin alone for 4 months also is an option. In general, these regimens have similar efficacy; however, in terms of safety, the rifampicin and rifapentine combination regimens have fewer hepatotoxicity events compared to the INH alone regimen, but there are more cutaneous and flulike reactions and gastrointestinal intolerance.4 Cutaneous ADRs to TB treatment such as mild itchiness and cutaneous eruptions usually are observed within 2 months of drug initiation. Pyrazinamide was reported as the most common drug associated with cutaneous ADRs, and INH was the rarest offending drug.5
The frequency of ADRs to INH is approximately 5.4%, and the most prevalent ADRs include asymptomatic elevation of serum liver enzyme concentrations, peripheral neuropathy, and hepatotoxicity, and skin lesions are less common.2 Our patient’s laboratory test results excluded vitamin B deficiency, hepatic and renal dysfunction, and neuropathy.
Previously reported skin reactions related to INH were late-type reactions such as maculopapular rash, dermatitis, erythema multiforme, drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, Stevens-Johnson syndrome, and toxic epidermal necrolysis.5,6 The concerning prediagnosis of psoriatic exacerbation in our patient was ruled out by the absence of typical skin lesions such as well-defined, erythematous plaques and pustules and atypical localization such as the dorsal hands and feet rather than the knees, elbows, lumbosacral region, scalp, and abdomen, which is typical of psoriasis. DRESS syndrome was unlikely with the absence of fever, lymphadenopathy, hypereosinophilia, leukocytosis, and renal and hepatic dysfunction.7 There were no widespread blisters, epidermal detachment, or mucosal involvement on the trunk or face typically associated with Stevens-Johnson syndrome and toxic epidermal necrolysis.7,8 A possible diagnosis of contact dermatitis was suspected with likely skin lesions as exfoliation and chapping, typical localization on the hands and feet, and positive patch test that supported sensitization to the drug. However, the patient’s skin lesions were not eczematous (characterized by erythema, vesiculation, exudation, or bullous edema in the acute phase), and were not localized to areas of irritant exposure.3 In our patient, erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH was compatible with erythema multiforme, whereas the absence of target appearance, positive patch test, and late appearance were incompatible with erythema multiforme.8
Because the clinical picture did not fit contact dermatitis or erythema multiforme, a diagnosis of erythema multiforme–like noneczematous dermatitis was suggested. Noneczematous dermatitis has subtypes that include purpuric, lichenoid, pustular, lymphomatoid, dyshidrosiform, and pigmented, as well as erythema multiforme–like contact eruptions.9 These clinical entities are not associated with contact exposure, but are related to systemic exposure, as seen in our patient.10 The patch test positivity and skin biopsy report also supported the diagnosis of erythema multiforme–like dermatitis. Erythema multiforme–like dermatitis is thought to be caused by medications or infections inducing immunocomplexes and lymphocytic infiltration in the dermis and subepidermis. Nevertheless, the prognosis was self-limiting in both.8 The clinical polymorphism caused by INH in this patient was suggested to be related with individual susceptibility, variability of contact-activating modalities, and the targeted cutaneous structures. Furthermore, among the risk factors for cutaneous ADRs—HIV, polypharmacy, older age, and preexisting renal and liver impairment—the only notable factor in this patient was psoriasis as an autoimmune disorder.
Patients with skin diseases such as psoriasis should be followed up by closer monitoring during INH use. Withdrawal of the drug and symptomatic treatment of the lesions with corticosteroid and antihistamine are the first steps of drug intolerance. After complete recovery and termination of antiallergic drugs, diagnostic tests are recommended if the drug reaction was not life-threatening. Skin prick and intradermal tests are useful in early-type drug reactions, whereas patch testing and late evaluation of an intradermal test may be helpful in the diagnosis of delayed-type reactions. The full dose of INH is avoided in an intradermal test against irritation. A patch test with INH was performed by diluting a 100-mg tablet with 1 mL of distilled water, and used as 1/100, 1/10, and 1/1 dilutions.8 Patch testing with INH also was done in 5 healthy control patients to exclude the irritation effect in this case. The rechallenge of INH was done in a controlled manner in our patient to rule out psoriasis activation since it was a localized skin reaction with no serious ADR. An oral provocation test with the culprit drug is the gold standard of drug allergy diagnosis that should be done in a tertiary hospital with an intensive care unit.
This case of erythema multiforme–like dermatitis due to INH is interesting due to systemic intake of INH, which resulted in dermatitis with localized involvement similar to erythema multiforme but with no immunologic processes or prior sensitization. With the increasing use of anti–TNF-α treatment, INH use will be more prevalent than in the past for the treatment of latent TB. Even though the skin-restricted ADRs of INH are rare and minor, particular attention should be paid to patients with dermatologic diseases. In our case, diagnostic drug allergy evaluation was performed to optimize the second-line treatment of TB infection, in addition to early withdrawal of the culprit drug.
- Vide J, Magina S. Moderate to severe psoriasis treatment challenges through the era of biological drugs.An Bras Dermatol. 2017;92:668-674.
- Gülbay BE, Gürkan OU, Yildiz OA, et al. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir Med. 2006;100:1834-1842.
- Holdiness MR. Contact dermatitis to antituberculosis drugs. Contact Dermatitis. 1986;15:282-288.
- Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46:1563-1576.
- Tan WC, Ong CK, Kang SC, et al. Two years review of cutaneous adverse drug reaction from first line anti-tuberculous drugs. Med J Malaysia. 2007;62:143-146.
- Özkaya E.Eczematous-type multiple drug allergy from isoniazid and ethambutol with positive patch test results. Cutis. 2013;92:121-124.
- Fernando SL. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas J Dermatol. 2014;55:15-23.
- Rebollo S, Sanchez P, Vega JM, et al. Hypersensitivity syndrome from isoniazid with positive patch test. Contact Dermatitis. 2001;45:306.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Bonamonte D, Foti C, Vestita M, et al. Nummular eczema and contact allergy: a retrospective study. Dermatitis. 2012;23:153-157.
To the Editor:
Psoriasis vulgaris is a chronic autoimmune inflammatory disease and biologic agents, such as anti–tumor necrosis factor α (TNF-α), are alternative drugs in case of resistance or adverse events to conventional ones.1 The limitation of these agents is immunosuppression that may cause infections such as tuberculosis (TB). Prophylaxis is indicated to latent TB diseases if the purified protein derivative (tuberculin) skin test is higher than 5 mm before starting these treatments. The challenge in TB treatment is adverse drug reactions (ADRs) that are reported in 4% to 6% of cases.2,3
Erythema multiforme–like dermatitis is a rare skin rash that develops due to isoniazid (INH). The clinical presentation includes erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH. Skin biopsy and patch tests are the supportive diagnostic methods. Isoniazid-associated skin rashes rarely are reported and generally are not severe enough to terminate the drug. We present a patient with psoriasis who received TB prophylaxis before anti–TNF-α use. He presented with erythema multiforme–like dermatitis due to INH. Withdrawal of the drug and treatment of the lesions were the first steps of intolerance, followed by a patch test with the culprit drug after recovery. We discuss the diagnostic drug allergy evaluation and treatment approach.
A 37-year-old man presented with a 15-year history of severe psoriasis with frequent flares. He was treated with various topical and systemic agents including acitretin and methotrexate at 4-year intervals. Despite the addition of phototherapy, he underwent a new treatment with anti–TNF-α, as the disease control with other treatments was insufficient. Before starting anti–TNF-α, preventive treatment against TB with INH (300 mg/d) was indicated with 20 mm of purified protein derivative. On approximately the 20th day of treatment, he developed pruritic erythema with desquamation and exfoliation localized to the hands and feet (Figure 1). Isoniazid was discontinued and a topical steroid was initiated. After 3 weeks, the skin lesions were completely improved and INH was reinitiated at the same dose with antihistamine prophylaxis (oral levocetirizine 5 mg/d). Seven days later, similar skin lesions presented that were more extensive on the arms and legs (Figure 2). Complete blood cell counts, renal and hepatic function tests, and hepatitis markers were within reference range in consultation with the allergy division. To distinguish the lesions from a psoriasis attack, a punch biopsy of the eruptive dermatitis showed erythema multiforme–like dermatitis including dermal edema and perivascular lymphocytic infiltration with no relation to psoriasis but consistent with a drug eruption. Isoniazid was discontinued, and the skin lesions resolved after 4 weeks of topical steroid and oral antihistamine use (Figure 3). There was no other drug use except INH, and a skin patch test with INH was positive at 72 hours (Figure 4). Skin tests with INH were done to 5 healthy lesions that were negative. Finally, TB prophylaxis was performed with rifampicin (10 mg/kg/d [600 mg/d]) for 4 months with no ADRs. The patient’s psoriasis lesions improved with anti–TNF-α that was initiated 1 month after starting TB prevention with rifampicin.




This case of erythema multiforme–like dermatitis was diagnosed with acral involvement, a positive patch test to INH, and lymphocytic inflammation in a skin biopsy. It was a drug-induced reaction, as skin lesions developed during INH intake and improved after drug withdrawal.
Isoniazid, also known as isonicotinylhydrazide, is an oral antibiotic used for the treatment of TB and other mycobacteria. Protective treatment against latent TB primarily is done with daily INH for 6 or 9 months; alternatively, INH may be taken weekly with rifapentine for 3 months or daily with rifampicin for 4 months. Daily rifampicin alone for 4 months also is an option. In general, these regimens have similar efficacy; however, in terms of safety, the rifampicin and rifapentine combination regimens have fewer hepatotoxicity events compared to the INH alone regimen, but there are more cutaneous and flulike reactions and gastrointestinal intolerance.4 Cutaneous ADRs to TB treatment such as mild itchiness and cutaneous eruptions usually are observed within 2 months of drug initiation. Pyrazinamide was reported as the most common drug associated with cutaneous ADRs, and INH was the rarest offending drug.5
The frequency of ADRs to INH is approximately 5.4%, and the most prevalent ADRs include asymptomatic elevation of serum liver enzyme concentrations, peripheral neuropathy, and hepatotoxicity, and skin lesions are less common.2 Our patient’s laboratory test results excluded vitamin B deficiency, hepatic and renal dysfunction, and neuropathy.
Previously reported skin reactions related to INH were late-type reactions such as maculopapular rash, dermatitis, erythema multiforme, drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, Stevens-Johnson syndrome, and toxic epidermal necrolysis.5,6 The concerning prediagnosis of psoriatic exacerbation in our patient was ruled out by the absence of typical skin lesions such as well-defined, erythematous plaques and pustules and atypical localization such as the dorsal hands and feet rather than the knees, elbows, lumbosacral region, scalp, and abdomen, which is typical of psoriasis. DRESS syndrome was unlikely with the absence of fever, lymphadenopathy, hypereosinophilia, leukocytosis, and renal and hepatic dysfunction.7 There were no widespread blisters, epidermal detachment, or mucosal involvement on the trunk or face typically associated with Stevens-Johnson syndrome and toxic epidermal necrolysis.7,8 A possible diagnosis of contact dermatitis was suspected with likely skin lesions as exfoliation and chapping, typical localization on the hands and feet, and positive patch test that supported sensitization to the drug. However, the patient’s skin lesions were not eczematous (characterized by erythema, vesiculation, exudation, or bullous edema in the acute phase), and were not localized to areas of irritant exposure.3 In our patient, erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH was compatible with erythema multiforme, whereas the absence of target appearance, positive patch test, and late appearance were incompatible with erythema multiforme.8
Because the clinical picture did not fit contact dermatitis or erythema multiforme, a diagnosis of erythema multiforme–like noneczematous dermatitis was suggested. Noneczematous dermatitis has subtypes that include purpuric, lichenoid, pustular, lymphomatoid, dyshidrosiform, and pigmented, as well as erythema multiforme–like contact eruptions.9 These clinical entities are not associated with contact exposure, but are related to systemic exposure, as seen in our patient.10 The patch test positivity and skin biopsy report also supported the diagnosis of erythema multiforme–like dermatitis. Erythema multiforme–like dermatitis is thought to be caused by medications or infections inducing immunocomplexes and lymphocytic infiltration in the dermis and subepidermis. Nevertheless, the prognosis was self-limiting in both.8 The clinical polymorphism caused by INH in this patient was suggested to be related with individual susceptibility, variability of contact-activating modalities, and the targeted cutaneous structures. Furthermore, among the risk factors for cutaneous ADRs—HIV, polypharmacy, older age, and preexisting renal and liver impairment—the only notable factor in this patient was psoriasis as an autoimmune disorder.
Patients with skin diseases such as psoriasis should be followed up by closer monitoring during INH use. Withdrawal of the drug and symptomatic treatment of the lesions with corticosteroid and antihistamine are the first steps of drug intolerance. After complete recovery and termination of antiallergic drugs, diagnostic tests are recommended if the drug reaction was not life-threatening. Skin prick and intradermal tests are useful in early-type drug reactions, whereas patch testing and late evaluation of an intradermal test may be helpful in the diagnosis of delayed-type reactions. The full dose of INH is avoided in an intradermal test against irritation. A patch test with INH was performed by diluting a 100-mg tablet with 1 mL of distilled water, and used as 1/100, 1/10, and 1/1 dilutions.8 Patch testing with INH also was done in 5 healthy control patients to exclude the irritation effect in this case. The rechallenge of INH was done in a controlled manner in our patient to rule out psoriasis activation since it was a localized skin reaction with no serious ADR. An oral provocation test with the culprit drug is the gold standard of drug allergy diagnosis that should be done in a tertiary hospital with an intensive care unit.
This case of erythema multiforme–like dermatitis due to INH is interesting due to systemic intake of INH, which resulted in dermatitis with localized involvement similar to erythema multiforme but with no immunologic processes or prior sensitization. With the increasing use of anti–TNF-α treatment, INH use will be more prevalent than in the past for the treatment of latent TB. Even though the skin-restricted ADRs of INH are rare and minor, particular attention should be paid to patients with dermatologic diseases. In our case, diagnostic drug allergy evaluation was performed to optimize the second-line treatment of TB infection, in addition to early withdrawal of the culprit drug.
To the Editor:
Psoriasis vulgaris is a chronic autoimmune inflammatory disease and biologic agents, such as anti–tumor necrosis factor α (TNF-α), are alternative drugs in case of resistance or adverse events to conventional ones.1 The limitation of these agents is immunosuppression that may cause infections such as tuberculosis (TB). Prophylaxis is indicated to latent TB diseases if the purified protein derivative (tuberculin) skin test is higher than 5 mm before starting these treatments. The challenge in TB treatment is adverse drug reactions (ADRs) that are reported in 4% to 6% of cases.2,3
Erythema multiforme–like dermatitis is a rare skin rash that develops due to isoniazid (INH). The clinical presentation includes erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH. Skin biopsy and patch tests are the supportive diagnostic methods. Isoniazid-associated skin rashes rarely are reported and generally are not severe enough to terminate the drug. We present a patient with psoriasis who received TB prophylaxis before anti–TNF-α use. He presented with erythema multiforme–like dermatitis due to INH. Withdrawal of the drug and treatment of the lesions were the first steps of intolerance, followed by a patch test with the culprit drug after recovery. We discuss the diagnostic drug allergy evaluation and treatment approach.
A 37-year-old man presented with a 15-year history of severe psoriasis with frequent flares. He was treated with various topical and systemic agents including acitretin and methotrexate at 4-year intervals. Despite the addition of phototherapy, he underwent a new treatment with anti–TNF-α, as the disease control with other treatments was insufficient. Before starting anti–TNF-α, preventive treatment against TB with INH (300 mg/d) was indicated with 20 mm of purified protein derivative. On approximately the 20th day of treatment, he developed pruritic erythema with desquamation and exfoliation localized to the hands and feet (Figure 1). Isoniazid was discontinued and a topical steroid was initiated. After 3 weeks, the skin lesions were completely improved and INH was reinitiated at the same dose with antihistamine prophylaxis (oral levocetirizine 5 mg/d). Seven days later, similar skin lesions presented that were more extensive on the arms and legs (Figure 2). Complete blood cell counts, renal and hepatic function tests, and hepatitis markers were within reference range in consultation with the allergy division. To distinguish the lesions from a psoriasis attack, a punch biopsy of the eruptive dermatitis showed erythema multiforme–like dermatitis including dermal edema and perivascular lymphocytic infiltration with no relation to psoriasis but consistent with a drug eruption. Isoniazid was discontinued, and the skin lesions resolved after 4 weeks of topical steroid and oral antihistamine use (Figure 3). There was no other drug use except INH, and a skin patch test with INH was positive at 72 hours (Figure 4). Skin tests with INH were done to 5 healthy lesions that were negative. Finally, TB prophylaxis was performed with rifampicin (10 mg/kg/d [600 mg/d]) for 4 months with no ADRs. The patient’s psoriasis lesions improved with anti–TNF-α that was initiated 1 month after starting TB prevention with rifampicin.




This case of erythema multiforme–like dermatitis was diagnosed with acral involvement, a positive patch test to INH, and lymphocytic inflammation in a skin biopsy. It was a drug-induced reaction, as skin lesions developed during INH intake and improved after drug withdrawal.
Isoniazid, also known as isonicotinylhydrazide, is an oral antibiotic used for the treatment of TB and other mycobacteria. Protective treatment against latent TB primarily is done with daily INH for 6 or 9 months; alternatively, INH may be taken weekly with rifapentine for 3 months or daily with rifampicin for 4 months. Daily rifampicin alone for 4 months also is an option. In general, these regimens have similar efficacy; however, in terms of safety, the rifampicin and rifapentine combination regimens have fewer hepatotoxicity events compared to the INH alone regimen, but there are more cutaneous and flulike reactions and gastrointestinal intolerance.4 Cutaneous ADRs to TB treatment such as mild itchiness and cutaneous eruptions usually are observed within 2 months of drug initiation. Pyrazinamide was reported as the most common drug associated with cutaneous ADRs, and INH was the rarest offending drug.5
The frequency of ADRs to INH is approximately 5.4%, and the most prevalent ADRs include asymptomatic elevation of serum liver enzyme concentrations, peripheral neuropathy, and hepatotoxicity, and skin lesions are less common.2 Our patient’s laboratory test results excluded vitamin B deficiency, hepatic and renal dysfunction, and neuropathy.
Previously reported skin reactions related to INH were late-type reactions such as maculopapular rash, dermatitis, erythema multiforme, drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, Stevens-Johnson syndrome, and toxic epidermal necrolysis.5,6 The concerning prediagnosis of psoriatic exacerbation in our patient was ruled out by the absence of typical skin lesions such as well-defined, erythematous plaques and pustules and atypical localization such as the dorsal hands and feet rather than the knees, elbows, lumbosacral region, scalp, and abdomen, which is typical of psoriasis. DRESS syndrome was unlikely with the absence of fever, lymphadenopathy, hypereosinophilia, leukocytosis, and renal and hepatic dysfunction.7 There were no widespread blisters, epidermal detachment, or mucosal involvement on the trunk or face typically associated with Stevens-Johnson syndrome and toxic epidermal necrolysis.7,8 A possible diagnosis of contact dermatitis was suspected with likely skin lesions as exfoliation and chapping, typical localization on the hands and feet, and positive patch test that supported sensitization to the drug. However, the patient’s skin lesions were not eczematous (characterized by erythema, vesiculation, exudation, or bullous edema in the acute phase), and were not localized to areas of irritant exposure.3 In our patient, erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH was compatible with erythema multiforme, whereas the absence of target appearance, positive patch test, and late appearance were incompatible with erythema multiforme.8
Because the clinical picture did not fit contact dermatitis or erythema multiforme, a diagnosis of erythema multiforme–like noneczematous dermatitis was suggested. Noneczematous dermatitis has subtypes that include purpuric, lichenoid, pustular, lymphomatoid, dyshidrosiform, and pigmented, as well as erythema multiforme–like contact eruptions.9 These clinical entities are not associated with contact exposure, but are related to systemic exposure, as seen in our patient.10 The patch test positivity and skin biopsy report also supported the diagnosis of erythema multiforme–like dermatitis. Erythema multiforme–like dermatitis is thought to be caused by medications or infections inducing immunocomplexes and lymphocytic infiltration in the dermis and subepidermis. Nevertheless, the prognosis was self-limiting in both.8 The clinical polymorphism caused by INH in this patient was suggested to be related with individual susceptibility, variability of contact-activating modalities, and the targeted cutaneous structures. Furthermore, among the risk factors for cutaneous ADRs—HIV, polypharmacy, older age, and preexisting renal and liver impairment—the only notable factor in this patient was psoriasis as an autoimmune disorder.
Patients with skin diseases such as psoriasis should be followed up by closer monitoring during INH use. Withdrawal of the drug and symptomatic treatment of the lesions with corticosteroid and antihistamine are the first steps of drug intolerance. After complete recovery and termination of antiallergic drugs, diagnostic tests are recommended if the drug reaction was not life-threatening. Skin prick and intradermal tests are useful in early-type drug reactions, whereas patch testing and late evaluation of an intradermal test may be helpful in the diagnosis of delayed-type reactions. The full dose of INH is avoided in an intradermal test against irritation. A patch test with INH was performed by diluting a 100-mg tablet with 1 mL of distilled water, and used as 1/100, 1/10, and 1/1 dilutions.8 Patch testing with INH also was done in 5 healthy control patients to exclude the irritation effect in this case. The rechallenge of INH was done in a controlled manner in our patient to rule out psoriasis activation since it was a localized skin reaction with no serious ADR. An oral provocation test with the culprit drug is the gold standard of drug allergy diagnosis that should be done in a tertiary hospital with an intensive care unit.
This case of erythema multiforme–like dermatitis due to INH is interesting due to systemic intake of INH, which resulted in dermatitis with localized involvement similar to erythema multiforme but with no immunologic processes or prior sensitization. With the increasing use of anti–TNF-α treatment, INH use will be more prevalent than in the past for the treatment of latent TB. Even though the skin-restricted ADRs of INH are rare and minor, particular attention should be paid to patients with dermatologic diseases. In our case, diagnostic drug allergy evaluation was performed to optimize the second-line treatment of TB infection, in addition to early withdrawal of the culprit drug.
- Vide J, Magina S. Moderate to severe psoriasis treatment challenges through the era of biological drugs.An Bras Dermatol. 2017;92:668-674.
- Gülbay BE, Gürkan OU, Yildiz OA, et al. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir Med. 2006;100:1834-1842.
- Holdiness MR. Contact dermatitis to antituberculosis drugs. Contact Dermatitis. 1986;15:282-288.
- Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46:1563-1576.
- Tan WC, Ong CK, Kang SC, et al. Two years review of cutaneous adverse drug reaction from first line anti-tuberculous drugs. Med J Malaysia. 2007;62:143-146.
- Özkaya E.Eczematous-type multiple drug allergy from isoniazid and ethambutol with positive patch test results. Cutis. 2013;92:121-124.
- Fernando SL. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas J Dermatol. 2014;55:15-23.
- Rebollo S, Sanchez P, Vega JM, et al. Hypersensitivity syndrome from isoniazid with positive patch test. Contact Dermatitis. 2001;45:306.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Bonamonte D, Foti C, Vestita M, et al. Nummular eczema and contact allergy: a retrospective study. Dermatitis. 2012;23:153-157.
- Vide J, Magina S. Moderate to severe psoriasis treatment challenges through the era of biological drugs.An Bras Dermatol. 2017;92:668-674.
- Gülbay BE, Gürkan OU, Yildiz OA, et al. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir Med. 2006;100:1834-1842.
- Holdiness MR. Contact dermatitis to antituberculosis drugs. Contact Dermatitis. 1986;15:282-288.
- Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46:1563-1576.
- Tan WC, Ong CK, Kang SC, et al. Two years review of cutaneous adverse drug reaction from first line anti-tuberculous drugs. Med J Malaysia. 2007;62:143-146.
- Özkaya E.Eczematous-type multiple drug allergy from isoniazid and ethambutol with positive patch test results. Cutis. 2013;92:121-124.
- Fernando SL. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas J Dermatol. 2014;55:15-23.
- Rebollo S, Sanchez P, Vega JM, et al. Hypersensitivity syndrome from isoniazid with positive patch test. Contact Dermatitis. 2001;45:306.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Bonamonte D, Foti C, Vestita M, et al. Nummular eczema and contact allergy: a retrospective study. Dermatitis. 2012;23:153-157.
Practice Points
- Hypersensitivity skin reactions to antituberculosis (TB) drugs are on the rise due to the increasing use of anti–tumor necrosis factor α. Isoniazid (INH) use will be more prevalent than in the past for the treatment of latent TB.
- Even though the skin-restricted adverse events to INH are rare and minor, particular attention should be paid to patients with dermatologic diseases such as psoriasis.
Tinea Incognito Mimicking Pustular Psoriasis in a Patient With Psoriasis and Cushing Syndrome
To the Editor:
The term tinea incognito was introduced by Ive and Marks1 in 1968 and refers to unusual clinical presentations of tinea due to the application of topical corticosteroids. Tinea incognito, which does not feature the classical clinical characteristics of tinea corporis such as well-defined, erythematous, scaly patches and elevated borders, is regularly misdiagnosed as inflammatory dermatosis.2 Immunosuppression caused by topical and/or systemic steroids predisposes patients to the development of tinea.3 Herein, a case of widespread pustular tinea incognito mimicking pustular psoriasis along with failure of tumor necrosis factor (TNF) inhibitor treatment is reported in a patient with chronic plaque psoriasis and steroid-induced Cushing syndrome.
A 46-year-old man with a 25-year history of psoriasis was referred to the dermatologic outpatient clinic with a severe flare-up of chronic plaque psoriasis. Prior treatments included methotrexate and acitretin without response. Narrowband UVB treatment was discontinued due to claustrophobia. Topical treatment with calcipotriol 0.005%–betamethasone dipropionate 0.05% gel was reported to be ineffective. The patient was administered prednisone over several months in a primary care setting at a dosage of 35 mg daily when he presented to the dermatology clinic. Physical examination revealed widespread chronic plaque psoriasis of the trunk and extremities, and a psoriasis area and severity index score of 15 was calculated. The patient had onychodystrophy with subungual hyperkeratosis of all toenails. Signs of prednisone-induced Cushing syndrome, including central obesity, lipodystrophy, and red striae, were noted.
Treatment was started by dermatology with the TNF inhibitor adalimumab at an initial dose of 80 mg, followed by subsequent 40-mg doses every other week; prednisone was tapered off. Topical treatment with a 4-week course of clobetasol propionate cream 0.05% daily for psoriatic lesions was initiated.
Six weeks after the initial consultation, the patient presented to the hospital’s emergency department with worsening symptoms of itchy, burning, and painful skin after good initial improvement. The patient’s skin started to burn upon application of clobetasol and the rash worsened. The patient did not use emollients. At that point, the patient was on a daily dose of 15 mg of prednisone. On dermatologic review, multiple partially annular lesions with subtle scaling and multiple pustules on the arms and legs as well as the buttocks and groin were noticed. These lesions were confined to sites of prior psoriasis as marked by postinflammatory hyperpigmentation (Figure 1). Widespread tinea was assumed, and treatment with fluconazole 50 mg daily was administered for 4 weeks. Direct examination of skin scrapings from the patient’s thigh showed hyphae, and fungal culture was positive for Trichophyton rubrum. Scrapings from the patient’s hallux nail remained inconclusive due to bacterial overgrowth. At 4-week follow-up, the patient’s skin had cleared entirely and showed only postinflammatory changes (Figure 2). Healthy proximal nail growth was observed. Fluconazole was continued at a once-weekly dose of 150 mg together with adalimumab at a dose of 40 mg every 2 weeks and a prednisone tapering schedule.
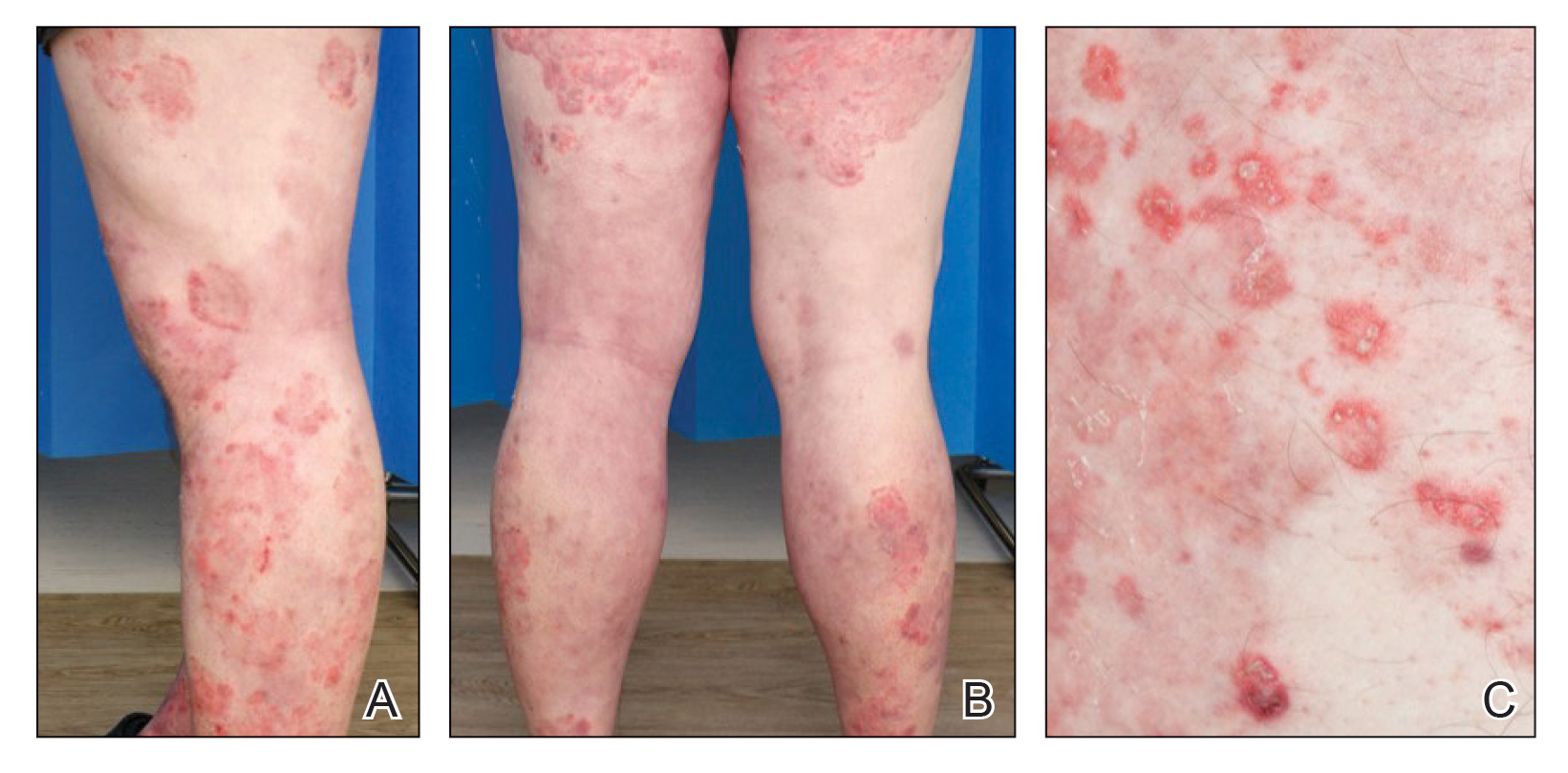
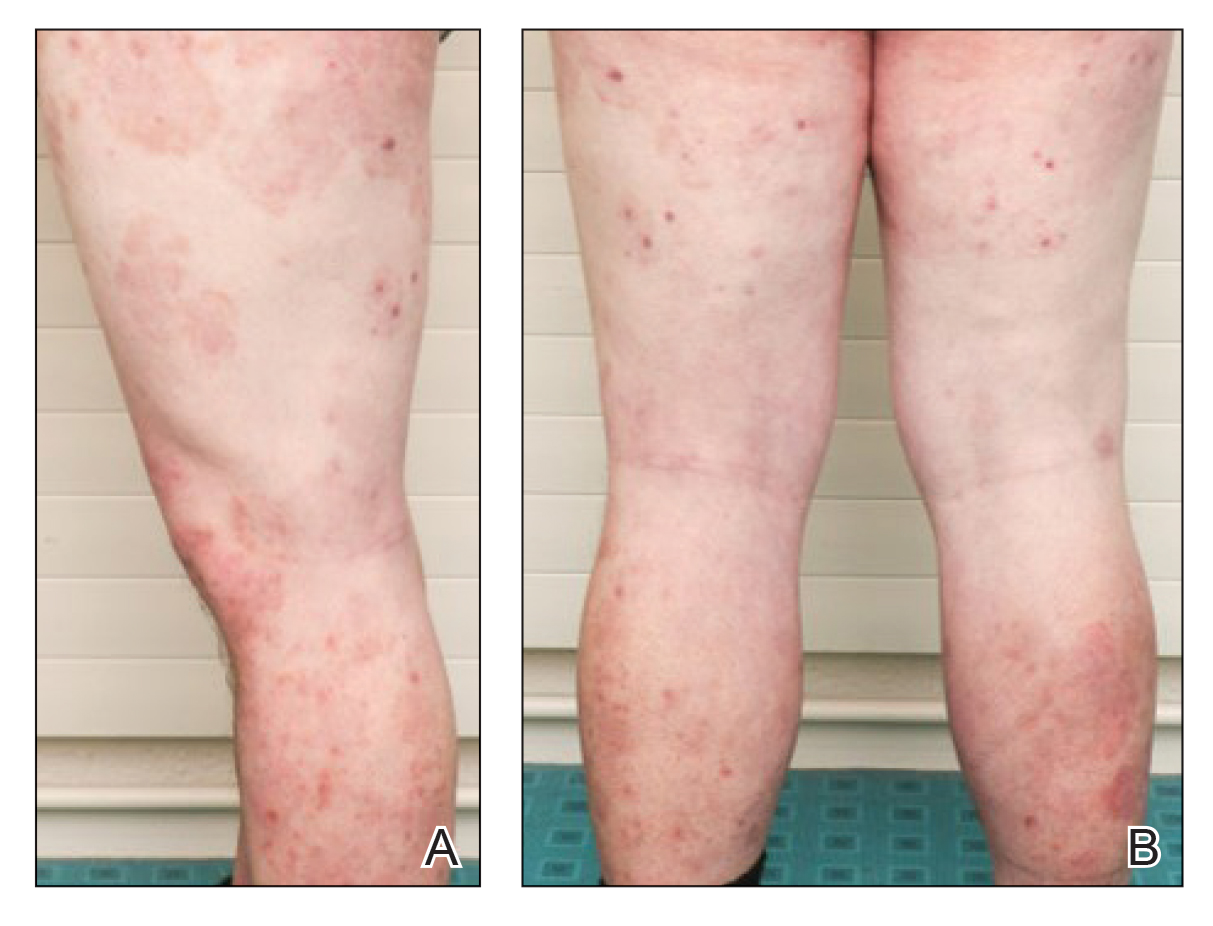
This case describes pustular tinea incognito in a patient with chronic plaque psoriasis. As the name indicates, tinea incognito can mimic other skin conditions and classically is linked to topical application of corticosteroids.1 Tinea incognito can be a diagnostic challenge. Kim et al4 reported a diagnostic delay of 15 months and the frequent requirement for the involvement of a second physician or dermatologist. Treatment with topical or systemic corticosteroids is a risk factor for dermatophyte infections because of their immunosuppressive action.3,5 Although recommended by current guidelines, a large number of psoriatic patients are treated with systemic steroids, predominantly prescribed in primary care, that can lead to iatrogenic Cushing syndrome, as demonstrated in this patient.6
In addition to systemic and topical steroids, the reported patient was started on the TNF inhibitor adalimumab prior to the onset of the tinea. Cases of patients on TNF inhibitors with widespread tinea are scarce. Bardazzi et al7 reported 2 cases of widespread nonpustular tinea in patients with psoriasis on TNF inhibitor treatment without further immunomodulating treatment. They hypothesized that TNF-α could be an important cytokine in the defense against dermatophytes.7
Whether psoriasis itself is a risk factor for tinea is still under debate, but tinea pedum and onychomycosis seem to have higher prevalence among psoriatic patients.8,9 As in this patient, bacterial overgrowth of hyperkeratotic nail samples can confound the culture’s clinical significance, thereby hindering the diagnosis of onychomycosis in patients with psoriasis.10 Alteras et al8 hypothesized that autoinoculation from preexisting onychomycosis or tinea pedum was the underlying mechanism of tinea incognito.
This patient’s hyperkeratotic nails showed healthy regrowth after initiation of both fluconazole and adalimumab, though it remained unclear whether preexisting onychomycosis was a possible source of tinea incognito. The finding that the patient’s tinea was almost exclusively limited to the sites of prior psoriatic lesions argues for autoinoculation and spreading accelerated by application of topical steroids triggered by the immunosuppressive effects of both topical and systemic steroids. The TNF inhibitor treatment may have helped to unmask the dermatophyte infection rather than contributing to it, as it cleared the psoriatic plaques.
Apart from psoriasis, tinea incognito most commonly is mistaken for other inflammatory conditions such as eczema, folliculitis, rosacea, granuloma annulare, and discoid lupus erythematosus.2 Inflammatory tinea can present with pustules due to the increased occurrence of neutrophil invasion.11This patient’s symptoms worsened 4 weeks after the initiation of TNF inhibitor treatment, which suggested treatment failure. However, clearance of the preexisting psoriatic lesions with remnant hyperpigmentation only argued for good response to TNF inhibitor treatment. The main differential diagnosis of this case of tinea incognito was generalized pustular psoriasis. The patient also was being treated with systemic and topical steroids, both known for their potential to trigger pustular psoriasis.12,13 Furthermore, TNF inhibitors have been described as a trigger for predominantly palmoplantar pustulosis but also are additionally associated with generalized pustular psoriasis.14
This case aims to raise awareness that tinea incognito can imitate both pustular psoriasis and TNF inhibitor treatment failure. Furthermore, the presented findings highlight risks associated with the treatment of psoriasis with systemic steroids. Pustular tinea incognito should be considered in the differential diagnosis of pustular psoriasis, especially in the setting of immunosuppression. After initial improvement, worsening of symptoms such as itching and burning as well as extension of the lesions upon application of topical steroids are regularly described in tinea incognito and can be present in addition to the more typical annular presentation of lesions as a clue to the diagnosis.
- Ive FA, Marks R. Tinea incognito. Br Med J. 1968;3:149-152.
- Arenas R, Moreno-Coutiño G, Vera L, et al. Tinea incognito. Clin Dermatol. 2010;28:137-139.
- Rouzaud C, Chosidow O, Brocard A, et al. Severe dermatophytosis in solid organ transplant recipients: a French retrospective series and literature review [published online January 25, 2018]. Transpl Infect Dis. doi:10.1111/tid.12799
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and its risk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Ohta Y, Saitoh N, Tanuma H, et al. Local cytokine expression in steroid-modified tinea faciei. J Dermatol. 1998;25:362-366.
- Augustin M, Schäfer I, Reich K, et al. Systemic treatment with corticosteroids in psoriasis-health care provision far beyond the S3-guidelines. J Dtsch Dermatol Ges. 2011;9:833-838.
- Bardazzi F, Balestri R, Rech G, et al. Dermatophytosis during anti-TNF-α monoclonal antibody therapy. Mycoses. 2011;54:E619-E620.
- Alteras I, Ingberg A, Segal R, et al. The incidence of skin manifestations by dermatophytes in patients with psoriasis. Mycopathologia. 1986;95:37-39.
- Leibovici V, Ramot Y, Siam R, et al. Prevalence of tinea pedis in psoriasis, compared to atopic dermatitis and normal controls—a prospective study. Mycoses. 2014;57:754-758.
- Tsentemeidou A, Vyzantiadis TA, Kyriakou A, et al. Prevalence of onychomycosis amongst patients with nail psoriasis who are not receiving immunosuppressive agents: results of a pilot study. Mycoses. 2017;60:830-835.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Brenner M, Molin S, Ruebsam K, et al. Generalized pustular psoriasis induced by systemic glucocorticosteroids: four cases and recommendations for treatment. Br J Dermatol. 2009;161:964-966.
- Boxley JD, Dawber RP, Summerly R. Generalized pustular psoriasis on withdrawal of clobetasol propionate ointment. Br Med J. 1975;2:255-256.
- Kucharekova M, Winnepenninckx V, Frank J, et al. Generalized pustulosis induced by adalimumab in a patient with rheumatoid arthritis—a therapeutic challenge. Int J Dermatol. 2008;47:25-28.
To the Editor:
The term tinea incognito was introduced by Ive and Marks1 in 1968 and refers to unusual clinical presentations of tinea due to the application of topical corticosteroids. Tinea incognito, which does not feature the classical clinical characteristics of tinea corporis such as well-defined, erythematous, scaly patches and elevated borders, is regularly misdiagnosed as inflammatory dermatosis.2 Immunosuppression caused by topical and/or systemic steroids predisposes patients to the development of tinea.3 Herein, a case of widespread pustular tinea incognito mimicking pustular psoriasis along with failure of tumor necrosis factor (TNF) inhibitor treatment is reported in a patient with chronic plaque psoriasis and steroid-induced Cushing syndrome.
A 46-year-old man with a 25-year history of psoriasis was referred to the dermatologic outpatient clinic with a severe flare-up of chronic plaque psoriasis. Prior treatments included methotrexate and acitretin without response. Narrowband UVB treatment was discontinued due to claustrophobia. Topical treatment with calcipotriol 0.005%–betamethasone dipropionate 0.05% gel was reported to be ineffective. The patient was administered prednisone over several months in a primary care setting at a dosage of 35 mg daily when he presented to the dermatology clinic. Physical examination revealed widespread chronic plaque psoriasis of the trunk and extremities, and a psoriasis area and severity index score of 15 was calculated. The patient had onychodystrophy with subungual hyperkeratosis of all toenails. Signs of prednisone-induced Cushing syndrome, including central obesity, lipodystrophy, and red striae, were noted.
Treatment was started by dermatology with the TNF inhibitor adalimumab at an initial dose of 80 mg, followed by subsequent 40-mg doses every other week; prednisone was tapered off. Topical treatment with a 4-week course of clobetasol propionate cream 0.05% daily for psoriatic lesions was initiated.
Six weeks after the initial consultation, the patient presented to the hospital’s emergency department with worsening symptoms of itchy, burning, and painful skin after good initial improvement. The patient’s skin started to burn upon application of clobetasol and the rash worsened. The patient did not use emollients. At that point, the patient was on a daily dose of 15 mg of prednisone. On dermatologic review, multiple partially annular lesions with subtle scaling and multiple pustules on the arms and legs as well as the buttocks and groin were noticed. These lesions were confined to sites of prior psoriasis as marked by postinflammatory hyperpigmentation (Figure 1). Widespread tinea was assumed, and treatment with fluconazole 50 mg daily was administered for 4 weeks. Direct examination of skin scrapings from the patient’s thigh showed hyphae, and fungal culture was positive for Trichophyton rubrum. Scrapings from the patient’s hallux nail remained inconclusive due to bacterial overgrowth. At 4-week follow-up, the patient’s skin had cleared entirely and showed only postinflammatory changes (Figure 2). Healthy proximal nail growth was observed. Fluconazole was continued at a once-weekly dose of 150 mg together with adalimumab at a dose of 40 mg every 2 weeks and a prednisone tapering schedule.


This case describes pustular tinea incognito in a patient with chronic plaque psoriasis. As the name indicates, tinea incognito can mimic other skin conditions and classically is linked to topical application of corticosteroids.1 Tinea incognito can be a diagnostic challenge. Kim et al4 reported a diagnostic delay of 15 months and the frequent requirement for the involvement of a second physician or dermatologist. Treatment with topical or systemic corticosteroids is a risk factor for dermatophyte infections because of their immunosuppressive action.3,5 Although recommended by current guidelines, a large number of psoriatic patients are treated with systemic steroids, predominantly prescribed in primary care, that can lead to iatrogenic Cushing syndrome, as demonstrated in this patient.6
In addition to systemic and topical steroids, the reported patient was started on the TNF inhibitor adalimumab prior to the onset of the tinea. Cases of patients on TNF inhibitors with widespread tinea are scarce. Bardazzi et al7 reported 2 cases of widespread nonpustular tinea in patients with psoriasis on TNF inhibitor treatment without further immunomodulating treatment. They hypothesized that TNF-α could be an important cytokine in the defense against dermatophytes.7
Whether psoriasis itself is a risk factor for tinea is still under debate, but tinea pedum and onychomycosis seem to have higher prevalence among psoriatic patients.8,9 As in this patient, bacterial overgrowth of hyperkeratotic nail samples can confound the culture’s clinical significance, thereby hindering the diagnosis of onychomycosis in patients with psoriasis.10 Alteras et al8 hypothesized that autoinoculation from preexisting onychomycosis or tinea pedum was the underlying mechanism of tinea incognito.
This patient’s hyperkeratotic nails showed healthy regrowth after initiation of both fluconazole and adalimumab, though it remained unclear whether preexisting onychomycosis was a possible source of tinea incognito. The finding that the patient’s tinea was almost exclusively limited to the sites of prior psoriatic lesions argues for autoinoculation and spreading accelerated by application of topical steroids triggered by the immunosuppressive effects of both topical and systemic steroids. The TNF inhibitor treatment may have helped to unmask the dermatophyte infection rather than contributing to it, as it cleared the psoriatic plaques.
Apart from psoriasis, tinea incognito most commonly is mistaken for other inflammatory conditions such as eczema, folliculitis, rosacea, granuloma annulare, and discoid lupus erythematosus.2 Inflammatory tinea can present with pustules due to the increased occurrence of neutrophil invasion.11This patient’s symptoms worsened 4 weeks after the initiation of TNF inhibitor treatment, which suggested treatment failure. However, clearance of the preexisting psoriatic lesions with remnant hyperpigmentation only argued for good response to TNF inhibitor treatment. The main differential diagnosis of this case of tinea incognito was generalized pustular psoriasis. The patient also was being treated with systemic and topical steroids, both known for their potential to trigger pustular psoriasis.12,13 Furthermore, TNF inhibitors have been described as a trigger for predominantly palmoplantar pustulosis but also are additionally associated with generalized pustular psoriasis.14
This case aims to raise awareness that tinea incognito can imitate both pustular psoriasis and TNF inhibitor treatment failure. Furthermore, the presented findings highlight risks associated with the treatment of psoriasis with systemic steroids. Pustular tinea incognito should be considered in the differential diagnosis of pustular psoriasis, especially in the setting of immunosuppression. After initial improvement, worsening of symptoms such as itching and burning as well as extension of the lesions upon application of topical steroids are regularly described in tinea incognito and can be present in addition to the more typical annular presentation of lesions as a clue to the diagnosis.
To the Editor:
The term tinea incognito was introduced by Ive and Marks1 in 1968 and refers to unusual clinical presentations of tinea due to the application of topical corticosteroids. Tinea incognito, which does not feature the classical clinical characteristics of tinea corporis such as well-defined, erythematous, scaly patches and elevated borders, is regularly misdiagnosed as inflammatory dermatosis.2 Immunosuppression caused by topical and/or systemic steroids predisposes patients to the development of tinea.3 Herein, a case of widespread pustular tinea incognito mimicking pustular psoriasis along with failure of tumor necrosis factor (TNF) inhibitor treatment is reported in a patient with chronic plaque psoriasis and steroid-induced Cushing syndrome.
A 46-year-old man with a 25-year history of psoriasis was referred to the dermatologic outpatient clinic with a severe flare-up of chronic plaque psoriasis. Prior treatments included methotrexate and acitretin without response. Narrowband UVB treatment was discontinued due to claustrophobia. Topical treatment with calcipotriol 0.005%–betamethasone dipropionate 0.05% gel was reported to be ineffective. The patient was administered prednisone over several months in a primary care setting at a dosage of 35 mg daily when he presented to the dermatology clinic. Physical examination revealed widespread chronic plaque psoriasis of the trunk and extremities, and a psoriasis area and severity index score of 15 was calculated. The patient had onychodystrophy with subungual hyperkeratosis of all toenails. Signs of prednisone-induced Cushing syndrome, including central obesity, lipodystrophy, and red striae, were noted.
Treatment was started by dermatology with the TNF inhibitor adalimumab at an initial dose of 80 mg, followed by subsequent 40-mg doses every other week; prednisone was tapered off. Topical treatment with a 4-week course of clobetasol propionate cream 0.05% daily for psoriatic lesions was initiated.
Six weeks after the initial consultation, the patient presented to the hospital’s emergency department with worsening symptoms of itchy, burning, and painful skin after good initial improvement. The patient’s skin started to burn upon application of clobetasol and the rash worsened. The patient did not use emollients. At that point, the patient was on a daily dose of 15 mg of prednisone. On dermatologic review, multiple partially annular lesions with subtle scaling and multiple pustules on the arms and legs as well as the buttocks and groin were noticed. These lesions were confined to sites of prior psoriasis as marked by postinflammatory hyperpigmentation (Figure 1). Widespread tinea was assumed, and treatment with fluconazole 50 mg daily was administered for 4 weeks. Direct examination of skin scrapings from the patient’s thigh showed hyphae, and fungal culture was positive for Trichophyton rubrum. Scrapings from the patient’s hallux nail remained inconclusive due to bacterial overgrowth. At 4-week follow-up, the patient’s skin had cleared entirely and showed only postinflammatory changes (Figure 2). Healthy proximal nail growth was observed. Fluconazole was continued at a once-weekly dose of 150 mg together with adalimumab at a dose of 40 mg every 2 weeks and a prednisone tapering schedule.


This case describes pustular tinea incognito in a patient with chronic plaque psoriasis. As the name indicates, tinea incognito can mimic other skin conditions and classically is linked to topical application of corticosteroids.1 Tinea incognito can be a diagnostic challenge. Kim et al4 reported a diagnostic delay of 15 months and the frequent requirement for the involvement of a second physician or dermatologist. Treatment with topical or systemic corticosteroids is a risk factor for dermatophyte infections because of their immunosuppressive action.3,5 Although recommended by current guidelines, a large number of psoriatic patients are treated with systemic steroids, predominantly prescribed in primary care, that can lead to iatrogenic Cushing syndrome, as demonstrated in this patient.6
In addition to systemic and topical steroids, the reported patient was started on the TNF inhibitor adalimumab prior to the onset of the tinea. Cases of patients on TNF inhibitors with widespread tinea are scarce. Bardazzi et al7 reported 2 cases of widespread nonpustular tinea in patients with psoriasis on TNF inhibitor treatment without further immunomodulating treatment. They hypothesized that TNF-α could be an important cytokine in the defense against dermatophytes.7
Whether psoriasis itself is a risk factor for tinea is still under debate, but tinea pedum and onychomycosis seem to have higher prevalence among psoriatic patients.8,9 As in this patient, bacterial overgrowth of hyperkeratotic nail samples can confound the culture’s clinical significance, thereby hindering the diagnosis of onychomycosis in patients with psoriasis.10 Alteras et al8 hypothesized that autoinoculation from preexisting onychomycosis or tinea pedum was the underlying mechanism of tinea incognito.
This patient’s hyperkeratotic nails showed healthy regrowth after initiation of both fluconazole and adalimumab, though it remained unclear whether preexisting onychomycosis was a possible source of tinea incognito. The finding that the patient’s tinea was almost exclusively limited to the sites of prior psoriatic lesions argues for autoinoculation and spreading accelerated by application of topical steroids triggered by the immunosuppressive effects of both topical and systemic steroids. The TNF inhibitor treatment may have helped to unmask the dermatophyte infection rather than contributing to it, as it cleared the psoriatic plaques.
Apart from psoriasis, tinea incognito most commonly is mistaken for other inflammatory conditions such as eczema, folliculitis, rosacea, granuloma annulare, and discoid lupus erythematosus.2 Inflammatory tinea can present with pustules due to the increased occurrence of neutrophil invasion.11This patient’s symptoms worsened 4 weeks after the initiation of TNF inhibitor treatment, which suggested treatment failure. However, clearance of the preexisting psoriatic lesions with remnant hyperpigmentation only argued for good response to TNF inhibitor treatment. The main differential diagnosis of this case of tinea incognito was generalized pustular psoriasis. The patient also was being treated with systemic and topical steroids, both known for their potential to trigger pustular psoriasis.12,13 Furthermore, TNF inhibitors have been described as a trigger for predominantly palmoplantar pustulosis but also are additionally associated with generalized pustular psoriasis.14
This case aims to raise awareness that tinea incognito can imitate both pustular psoriasis and TNF inhibitor treatment failure. Furthermore, the presented findings highlight risks associated with the treatment of psoriasis with systemic steroids. Pustular tinea incognito should be considered in the differential diagnosis of pustular psoriasis, especially in the setting of immunosuppression. After initial improvement, worsening of symptoms such as itching and burning as well as extension of the lesions upon application of topical steroids are regularly described in tinea incognito and can be present in addition to the more typical annular presentation of lesions as a clue to the diagnosis.
- Ive FA, Marks R. Tinea incognito. Br Med J. 1968;3:149-152.
- Arenas R, Moreno-Coutiño G, Vera L, et al. Tinea incognito. Clin Dermatol. 2010;28:137-139.
- Rouzaud C, Chosidow O, Brocard A, et al. Severe dermatophytosis in solid organ transplant recipients: a French retrospective series and literature review [published online January 25, 2018]. Transpl Infect Dis. doi:10.1111/tid.12799
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and its risk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Ohta Y, Saitoh N, Tanuma H, et al. Local cytokine expression in steroid-modified tinea faciei. J Dermatol. 1998;25:362-366.
- Augustin M, Schäfer I, Reich K, et al. Systemic treatment with corticosteroids in psoriasis-health care provision far beyond the S3-guidelines. J Dtsch Dermatol Ges. 2011;9:833-838.
- Bardazzi F, Balestri R, Rech G, et al. Dermatophytosis during anti-TNF-α monoclonal antibody therapy. Mycoses. 2011;54:E619-E620.
- Alteras I, Ingberg A, Segal R, et al. The incidence of skin manifestations by dermatophytes in patients with psoriasis. Mycopathologia. 1986;95:37-39.
- Leibovici V, Ramot Y, Siam R, et al. Prevalence of tinea pedis in psoriasis, compared to atopic dermatitis and normal controls—a prospective study. Mycoses. 2014;57:754-758.
- Tsentemeidou A, Vyzantiadis TA, Kyriakou A, et al. Prevalence of onychomycosis amongst patients with nail psoriasis who are not receiving immunosuppressive agents: results of a pilot study. Mycoses. 2017;60:830-835.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Brenner M, Molin S, Ruebsam K, et al. Generalized pustular psoriasis induced by systemic glucocorticosteroids: four cases and recommendations for treatment. Br J Dermatol. 2009;161:964-966.
- Boxley JD, Dawber RP, Summerly R. Generalized pustular psoriasis on withdrawal of clobetasol propionate ointment. Br Med J. 1975;2:255-256.
- Kucharekova M, Winnepenninckx V, Frank J, et al. Generalized pustulosis induced by adalimumab in a patient with rheumatoid arthritis—a therapeutic challenge. Int J Dermatol. 2008;47:25-28.
- Ive FA, Marks R. Tinea incognito. Br Med J. 1968;3:149-152.
- Arenas R, Moreno-Coutiño G, Vera L, et al. Tinea incognito. Clin Dermatol. 2010;28:137-139.
- Rouzaud C, Chosidow O, Brocard A, et al. Severe dermatophytosis in solid organ transplant recipients: a French retrospective series and literature review [published online January 25, 2018]. Transpl Infect Dis. doi:10.1111/tid.12799
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and its risk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Ohta Y, Saitoh N, Tanuma H, et al. Local cytokine expression in steroid-modified tinea faciei. J Dermatol. 1998;25:362-366.
- Augustin M, Schäfer I, Reich K, et al. Systemic treatment with corticosteroids in psoriasis-health care provision far beyond the S3-guidelines. J Dtsch Dermatol Ges. 2011;9:833-838.
- Bardazzi F, Balestri R, Rech G, et al. Dermatophytosis during anti-TNF-α monoclonal antibody therapy. Mycoses. 2011;54:E619-E620.
- Alteras I, Ingberg A, Segal R, et al. The incidence of skin manifestations by dermatophytes in patients with psoriasis. Mycopathologia. 1986;95:37-39.
- Leibovici V, Ramot Y, Siam R, et al. Prevalence of tinea pedis in psoriasis, compared to atopic dermatitis and normal controls—a prospective study. Mycoses. 2014;57:754-758.
- Tsentemeidou A, Vyzantiadis TA, Kyriakou A, et al. Prevalence of onychomycosis amongst patients with nail psoriasis who are not receiving immunosuppressive agents: results of a pilot study. Mycoses. 2017;60:830-835.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Brenner M, Molin S, Ruebsam K, et al. Generalized pustular psoriasis induced by systemic glucocorticosteroids: four cases and recommendations for treatment. Br J Dermatol. 2009;161:964-966.
- Boxley JD, Dawber RP, Summerly R. Generalized pustular psoriasis on withdrawal of clobetasol propionate ointment. Br Med J. 1975;2:255-256.
- Kucharekova M, Winnepenninckx V, Frank J, et al. Generalized pustulosis induced by adalimumab in a patient with rheumatoid arthritis—a therapeutic challenge. Int J Dermatol. 2008;47:25-28.
Practice Points
- Tinea incognito and its altered clinical presentation can provide clinical challenges and often is diagnosed with delay.
- Immunosuppression, such as iatrogenic Cushing syndrome, is a risk factor for tinea incognito.
- Pustular tinea incognito is a differential diagnosis of pustular psoriasis that can mimic tumor necrosis factor inhibitor treatment failure in patients with psoriasis.
Two studies add to knowledge base of biosimilar use in psoriasis, HS
And another study, a small, single-center retrospective study of patients with hidradenitis suppurativa (HS), found that administration of infliximab and biosimilar infliximab were associated with similar and significant improvement in disease.
Both studies were published online in April in JAMA Dermatology and add to mounting evidence that biosimilars may be interchangeable in certain dermatologic conditions.
“Biosimilars are an exciting innovation in the field,” Joseph Zahn, MD, assistant professor of dermatology at George Washington University, Washington, said in an interview. “Their efficacy and price point will allow patients greater access to effective treatment.” To date, biosimilars approved in the United States that could be prescribed by dermatologists include those for rituximab, etanercept, adalimumab, and infliximab.
In the trial from Denmark, Nikolai Loft, MD, of the University of Copenhagen and colleagues evaluated outcomes following a mandatory medical switch from the brand name adalimumab, referred to as adalimumab originator, to adalimumab biosimilars among 726 individuals who were enrolled in a Danish nationwide registry of patients treated with biologics since 2007. The primary outcome was 1-year drug retention in patients switching to adalimumab biosimilars compared with patients treated with adalimumab originator.
The study population consisted of 348 patients with at least 2 years of exposure to adalimumab who had switched from originator to adalimumab biosimilars (a mean age of 52 and 72% male) and 378 patients who served as the adalimumab cohort (a mean age of 51 and 71% male). When the researchers compared the 1-year drug retention rates between the adalimumab biosimilar cohort and the adalimumab originator cohort, the rates were similar (92% vs. 92.1%, respectively).
The hazard ratios for other outcomes were similar as well. Specifically, the crude hazard ratios were 1.02 (P = .94) for all causes of drug discontinuation, 0.82 (P = .60) for insufficient effect, and 1.41 (P = .50) for adverse events (AEs) in the adalimumab biosimilar cohort, compared with the adalimumab originator cohort.
“Overall, results for any AEs were contradicting, but certain AEs were more prevalent in the adalimumab biosimilar cohort,” the authors wrote. Dermatologic AEs and AEs in the “other” category “were more prevalent, which could be attributable to more patients experiencing injection site reactions as a result of larger volumes and differences in excipients and syringes in the adalimumab biosimilars and the adalimumab originator.” Other potential explanations they offered were the nocebo effect and greater awareness of AEs among practitioners and patients.
“This study concludes that, when switched to a biosimilar medication, patients do not have worse control of their psoriasis nor do they switch to other medications,” Dr. Zahn, who was asked to comment about these results, said in the interview. “However, there was a trend toward a higher number of side effects in the biosimilar group. The main takeaway point from this study is that biosimilars of adalimumab seem to be relatively interchangeable in patients with psoriasis without loss of efficacy or significant increase in side effects that lead to a medication change for the patient.”
The researchers acknowledged certain limitations of their study, including the fact that it was limited to Danish patients and that individual AEs could not be examined. “Moreover, the surveillance of AEs is not as vigilant as in clinical trials, and AEs are most likely underreported,” they wrote. “Although no major differences were found when switching from adalimumab originator to adalimumab biosimilar versions, it was not possible to assess the performance of individual adalimumab biosimilar versions in this study.”
In the second study, Christopher Sayed, MD, associate professor of dermatology, University of North Carolina, Chapel Hill, and colleagues retrospectively evaluated the effectiveness of infliximab-abda versus infliximab administration in the treatment of 34 patients with HS who were cared for at the university’s dermatology clinic. Patients were treated with either agent for at least 10 weeks. The infliximab treatment group consisted of 20 patients with a mean age of 42 years who were mostly female (17; 85%), while the infliximab-abda treatment group included 14 patients with a mean age of 36 years who also were mostly female (13; 93%).
Both groups received loading doses of 10 mg/kg at weeks 0, 2, and 6, and treatment was continued with a maintenance dose administered every 4-8 weeks. The patients were followed between February 2016 and June 2020 and the primary outcome measure was Hidradenitis Suppurative Clinical Response (HiSCR), which was defined as at least a 50% decrease in inflammatory nodule count without any increase in the number of abscesses or draining sinuses.
The researchers found that 71% of patients in the infliximab-abda treatment group achieved a HiSCR, compared with 60% of their counterparts in the infliximab treatment group, a difference that did not reach statistical significance (P = .47). Three patients in the infliximab treatment group experienced AEs, compared with none in the infliximab-abda treatment group.
“The data are promising,” Dr. Zahn said. “Although this is a small study with a limited number of patients, it suggests that this particular biosimilar may be a reasonable or possibly even equivalent alternative to infliximab. A larger, prospective trial will be needed before we can be sure the results are equivalent.”
Dr. Sayed and colleagues noted certain limitations of their study, including the retrospective design and the use of concomitant medications by some participants. “There is also a risk of selection bias because copay and medication assistance programs are not available for infliximab-abda for patients with HS,” they wrote.
In an editorial accompanying the two studies, Mark Lebwohl, MD, professor of dermatology, Icahn School of Medicine at Mount Sinai, New York, wrote that the introduction of biosimilars have been justified by “the hope that lower costs” will increase availability of treatments to patients with moderate to severe psoriasis. “Inroads in the U.S. market, however, have been limited,” he added, and there is concern that they “may be used to prevent access to newer interleukin-17 blockers and interleukin-23 blockers for which biosimilars are available and that do not carry the boxed warnings found on tumor necrosis factor blockers.”
Dr. Loft reported receiving personal fees from Eli Lilly and Janssen outside of the submitted work. Many of his coauthors reporting having numerous financial conflicts of interest with the pharmaceutical industry. The HS study was supported by a public health service research award from the National Institutes of Health. Dr. Sayed reported receiving personal fees or personal fees paid to the institution from AbbVie, Novartis, Chemocentryx, GlaxoSmithKline, Incyte, InflaRx, and UCB. No other disclosures were reported. Dr. Lebwohl disclosed receiving research funds from companies including AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, and Incyte; and receiving personal fees from multiple companies, outside of the submitted work. Dr. Zahn reported having no disclosures.
And another study, a small, single-center retrospective study of patients with hidradenitis suppurativa (HS), found that administration of infliximab and biosimilar infliximab were associated with similar and significant improvement in disease.
Both studies were published online in April in JAMA Dermatology and add to mounting evidence that biosimilars may be interchangeable in certain dermatologic conditions.
“Biosimilars are an exciting innovation in the field,” Joseph Zahn, MD, assistant professor of dermatology at George Washington University, Washington, said in an interview. “Their efficacy and price point will allow patients greater access to effective treatment.” To date, biosimilars approved in the United States that could be prescribed by dermatologists include those for rituximab, etanercept, adalimumab, and infliximab.
In the trial from Denmark, Nikolai Loft, MD, of the University of Copenhagen and colleagues evaluated outcomes following a mandatory medical switch from the brand name adalimumab, referred to as adalimumab originator, to adalimumab biosimilars among 726 individuals who were enrolled in a Danish nationwide registry of patients treated with biologics since 2007. The primary outcome was 1-year drug retention in patients switching to adalimumab biosimilars compared with patients treated with adalimumab originator.
The study population consisted of 348 patients with at least 2 years of exposure to adalimumab who had switched from originator to adalimumab biosimilars (a mean age of 52 and 72% male) and 378 patients who served as the adalimumab cohort (a mean age of 51 and 71% male). When the researchers compared the 1-year drug retention rates between the adalimumab biosimilar cohort and the adalimumab originator cohort, the rates were similar (92% vs. 92.1%, respectively).
The hazard ratios for other outcomes were similar as well. Specifically, the crude hazard ratios were 1.02 (P = .94) for all causes of drug discontinuation, 0.82 (P = .60) for insufficient effect, and 1.41 (P = .50) for adverse events (AEs) in the adalimumab biosimilar cohort, compared with the adalimumab originator cohort.
“Overall, results for any AEs were contradicting, but certain AEs were more prevalent in the adalimumab biosimilar cohort,” the authors wrote. Dermatologic AEs and AEs in the “other” category “were more prevalent, which could be attributable to more patients experiencing injection site reactions as a result of larger volumes and differences in excipients and syringes in the adalimumab biosimilars and the adalimumab originator.” Other potential explanations they offered were the nocebo effect and greater awareness of AEs among practitioners and patients.
“This study concludes that, when switched to a biosimilar medication, patients do not have worse control of their psoriasis nor do they switch to other medications,” Dr. Zahn, who was asked to comment about these results, said in the interview. “However, there was a trend toward a higher number of side effects in the biosimilar group. The main takeaway point from this study is that biosimilars of adalimumab seem to be relatively interchangeable in patients with psoriasis without loss of efficacy or significant increase in side effects that lead to a medication change for the patient.”
The researchers acknowledged certain limitations of their study, including the fact that it was limited to Danish patients and that individual AEs could not be examined. “Moreover, the surveillance of AEs is not as vigilant as in clinical trials, and AEs are most likely underreported,” they wrote. “Although no major differences were found when switching from adalimumab originator to adalimumab biosimilar versions, it was not possible to assess the performance of individual adalimumab biosimilar versions in this study.”
In the second study, Christopher Sayed, MD, associate professor of dermatology, University of North Carolina, Chapel Hill, and colleagues retrospectively evaluated the effectiveness of infliximab-abda versus infliximab administration in the treatment of 34 patients with HS who were cared for at the university’s dermatology clinic. Patients were treated with either agent for at least 10 weeks. The infliximab treatment group consisted of 20 patients with a mean age of 42 years who were mostly female (17; 85%), while the infliximab-abda treatment group included 14 patients with a mean age of 36 years who also were mostly female (13; 93%).
Both groups received loading doses of 10 mg/kg at weeks 0, 2, and 6, and treatment was continued with a maintenance dose administered every 4-8 weeks. The patients were followed between February 2016 and June 2020 and the primary outcome measure was Hidradenitis Suppurative Clinical Response (HiSCR), which was defined as at least a 50% decrease in inflammatory nodule count without any increase in the number of abscesses or draining sinuses.
The researchers found that 71% of patients in the infliximab-abda treatment group achieved a HiSCR, compared with 60% of their counterparts in the infliximab treatment group, a difference that did not reach statistical significance (P = .47). Three patients in the infliximab treatment group experienced AEs, compared with none in the infliximab-abda treatment group.
“The data are promising,” Dr. Zahn said. “Although this is a small study with a limited number of patients, it suggests that this particular biosimilar may be a reasonable or possibly even equivalent alternative to infliximab. A larger, prospective trial will be needed before we can be sure the results are equivalent.”
Dr. Sayed and colleagues noted certain limitations of their study, including the retrospective design and the use of concomitant medications by some participants. “There is also a risk of selection bias because copay and medication assistance programs are not available for infliximab-abda for patients with HS,” they wrote.
In an editorial accompanying the two studies, Mark Lebwohl, MD, professor of dermatology, Icahn School of Medicine at Mount Sinai, New York, wrote that the introduction of biosimilars have been justified by “the hope that lower costs” will increase availability of treatments to patients with moderate to severe psoriasis. “Inroads in the U.S. market, however, have been limited,” he added, and there is concern that they “may be used to prevent access to newer interleukin-17 blockers and interleukin-23 blockers for which biosimilars are available and that do not carry the boxed warnings found on tumor necrosis factor blockers.”
Dr. Loft reported receiving personal fees from Eli Lilly and Janssen outside of the submitted work. Many of his coauthors reporting having numerous financial conflicts of interest with the pharmaceutical industry. The HS study was supported by a public health service research award from the National Institutes of Health. Dr. Sayed reported receiving personal fees or personal fees paid to the institution from AbbVie, Novartis, Chemocentryx, GlaxoSmithKline, Incyte, InflaRx, and UCB. No other disclosures were reported. Dr. Lebwohl disclosed receiving research funds from companies including AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, and Incyte; and receiving personal fees from multiple companies, outside of the submitted work. Dr. Zahn reported having no disclosures.
And another study, a small, single-center retrospective study of patients with hidradenitis suppurativa (HS), found that administration of infliximab and biosimilar infliximab were associated with similar and significant improvement in disease.
Both studies were published online in April in JAMA Dermatology and add to mounting evidence that biosimilars may be interchangeable in certain dermatologic conditions.
“Biosimilars are an exciting innovation in the field,” Joseph Zahn, MD, assistant professor of dermatology at George Washington University, Washington, said in an interview. “Their efficacy and price point will allow patients greater access to effective treatment.” To date, biosimilars approved in the United States that could be prescribed by dermatologists include those for rituximab, etanercept, adalimumab, and infliximab.
In the trial from Denmark, Nikolai Loft, MD, of the University of Copenhagen and colleagues evaluated outcomes following a mandatory medical switch from the brand name adalimumab, referred to as adalimumab originator, to adalimumab biosimilars among 726 individuals who were enrolled in a Danish nationwide registry of patients treated with biologics since 2007. The primary outcome was 1-year drug retention in patients switching to adalimumab biosimilars compared with patients treated with adalimumab originator.
The study population consisted of 348 patients with at least 2 years of exposure to adalimumab who had switched from originator to adalimumab biosimilars (a mean age of 52 and 72% male) and 378 patients who served as the adalimumab cohort (a mean age of 51 and 71% male). When the researchers compared the 1-year drug retention rates between the adalimumab biosimilar cohort and the adalimumab originator cohort, the rates were similar (92% vs. 92.1%, respectively).
The hazard ratios for other outcomes were similar as well. Specifically, the crude hazard ratios were 1.02 (P = .94) for all causes of drug discontinuation, 0.82 (P = .60) for insufficient effect, and 1.41 (P = .50) for adverse events (AEs) in the adalimumab biosimilar cohort, compared with the adalimumab originator cohort.
“Overall, results for any AEs were contradicting, but certain AEs were more prevalent in the adalimumab biosimilar cohort,” the authors wrote. Dermatologic AEs and AEs in the “other” category “were more prevalent, which could be attributable to more patients experiencing injection site reactions as a result of larger volumes and differences in excipients and syringes in the adalimumab biosimilars and the adalimumab originator.” Other potential explanations they offered were the nocebo effect and greater awareness of AEs among practitioners and patients.
“This study concludes that, when switched to a biosimilar medication, patients do not have worse control of their psoriasis nor do they switch to other medications,” Dr. Zahn, who was asked to comment about these results, said in the interview. “However, there was a trend toward a higher number of side effects in the biosimilar group. The main takeaway point from this study is that biosimilars of adalimumab seem to be relatively interchangeable in patients with psoriasis without loss of efficacy or significant increase in side effects that lead to a medication change for the patient.”
The researchers acknowledged certain limitations of their study, including the fact that it was limited to Danish patients and that individual AEs could not be examined. “Moreover, the surveillance of AEs is not as vigilant as in clinical trials, and AEs are most likely underreported,” they wrote. “Although no major differences were found when switching from adalimumab originator to adalimumab biosimilar versions, it was not possible to assess the performance of individual adalimumab biosimilar versions in this study.”
In the second study, Christopher Sayed, MD, associate professor of dermatology, University of North Carolina, Chapel Hill, and colleagues retrospectively evaluated the effectiveness of infliximab-abda versus infliximab administration in the treatment of 34 patients with HS who were cared for at the university’s dermatology clinic. Patients were treated with either agent for at least 10 weeks. The infliximab treatment group consisted of 20 patients with a mean age of 42 years who were mostly female (17; 85%), while the infliximab-abda treatment group included 14 patients with a mean age of 36 years who also were mostly female (13; 93%).
Both groups received loading doses of 10 mg/kg at weeks 0, 2, and 6, and treatment was continued with a maintenance dose administered every 4-8 weeks. The patients were followed between February 2016 and June 2020 and the primary outcome measure was Hidradenitis Suppurative Clinical Response (HiSCR), which was defined as at least a 50% decrease in inflammatory nodule count without any increase in the number of abscesses or draining sinuses.
The researchers found that 71% of patients in the infliximab-abda treatment group achieved a HiSCR, compared with 60% of their counterparts in the infliximab treatment group, a difference that did not reach statistical significance (P = .47). Three patients in the infliximab treatment group experienced AEs, compared with none in the infliximab-abda treatment group.
“The data are promising,” Dr. Zahn said. “Although this is a small study with a limited number of patients, it suggests that this particular biosimilar may be a reasonable or possibly even equivalent alternative to infliximab. A larger, prospective trial will be needed before we can be sure the results are equivalent.”
Dr. Sayed and colleagues noted certain limitations of their study, including the retrospective design and the use of concomitant medications by some participants. “There is also a risk of selection bias because copay and medication assistance programs are not available for infliximab-abda for patients with HS,” they wrote.
In an editorial accompanying the two studies, Mark Lebwohl, MD, professor of dermatology, Icahn School of Medicine at Mount Sinai, New York, wrote that the introduction of biosimilars have been justified by “the hope that lower costs” will increase availability of treatments to patients with moderate to severe psoriasis. “Inroads in the U.S. market, however, have been limited,” he added, and there is concern that they “may be used to prevent access to newer interleukin-17 blockers and interleukin-23 blockers for which biosimilars are available and that do not carry the boxed warnings found on tumor necrosis factor blockers.”
Dr. Loft reported receiving personal fees from Eli Lilly and Janssen outside of the submitted work. Many of his coauthors reporting having numerous financial conflicts of interest with the pharmaceutical industry. The HS study was supported by a public health service research award from the National Institutes of Health. Dr. Sayed reported receiving personal fees or personal fees paid to the institution from AbbVie, Novartis, Chemocentryx, GlaxoSmithKline, Incyte, InflaRx, and UCB. No other disclosures were reported. Dr. Lebwohl disclosed receiving research funds from companies including AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, and Incyte; and receiving personal fees from multiple companies, outside of the submitted work. Dr. Zahn reported having no disclosures.
FROM JAMA DERMATOLOGY