User login
More children with high-risk brain cancer now surviving
A practice-changing study that used molecular testing to distinguish between subtypes of medulloblastoma has shown a significant improvement in survival for children with high-risk disease who underwent treatment intensification with carboplatin.
“Each of the four subgroups of medulloblastoma has a different prognosis, but for this particular subgroup, 20 fewer children out of every 100 would have survived prior to this study,” James Olson, MD, professor of medicine, French Hutchinson Cancer Research Center, University of Washington, Seattle, said in an interview.
“This is the reason for celebration – for now and forevermore, we can expect 20 more children with high-risk, group 3 medulloblastoma to survive,” he said.
“We recommend that all children with high-risk, group 3 medulloblastoma receive carboplatin and all children in the other subgroups do not, because we don’t want them to experience the toxicity without benefit,” Dr. Olson said.
The study was published online July 22, 2021, in JAMA Oncology.
Hematologic toxicity was more pronounced in the carboplatin arm in the induction phase of the protocol, and toxicity persisted into the first cycles of maintenance therapy. On the other hand, “there weren’t enough additional side effects to recommend children not get carboplatin if they would benefit from it,” Dr. Olson noted.
At least 75% of children with newly diagnosed medulloblastoma survive, although those with high-risk, group 3 disease have a substantially poorer prognosis than those with other molecular subtypes.
However, if a child with medulloblastoma experiences relapse, “the likelihood of survival is near zero, so it’s important to get it right the first time,” Dr. Olson said.
One of the patients who took part in this trial, Sammy Loch of Seattle, is now 27 years old and has been cancer free for 11 years.
She was diagnosed with medulloblastoma when in high school. At the time of her diagnosis, she was asked by her pediatric oncologist at Seattle Children’s Hospital about taking part in the study. After careful consideration, she agreed.
“Participating in research was my way to give back and pay it forward,” Ms. Loch said in a statement. “It’s really exciting to know more people will survive because of the research I was involved in,” she added. She continues to pay her debt forward, serving as a therapist for people with chronic health conditions and raising funds for pediatric cancer research.
Patients had high-risk features
The study involved 261 evaluable patients (median age, 8.6 years). All patients had high-risk features, including metastatic disease (72.4% of the group), diffuse anaplastic histologic characteristics (22.2%), and incomplete surgical resection (5.4%), defined as residual tumor greater than 1.5 cm2.
“All patients received 36 Gy craniospinal radiotherapy with boost to the posterior fossa of 55.8 Gy cumulative dose with conventional fractionation of 1.8 Gy/d,” Dr. Olson and colleagues explain. Patients also received six doses of vincristine 1.5 mg/m2 weekly during radiotherapy and were randomly assigned to receive carboplatin 35 mg/m2 for a total of 30 doses given daily prior to radiotherapy or placebo.
This regimen was followed by maintenance therapy, which consisted of six 28-day cycles of the combination of cisplatin 75 mg/m2 on day 1; cyclophosphamide 1,000 mg/m2 on days 2 and 3; and vincristine 1.5 mg/m2 on days 1 and 8.
Patients were originally assigned to receive an additional 12 cycles of isotretinoin or placebo, to be given during and after maintenance therapy. However, randomization to isotretinoin was discontinued early because of futility.
The study was initially powered to evaluate medulloblastoma as a single disease. However, as a result of biologic insights gained after the study was conceived, it was amended to include a molecular subgroup analysis to better distinguish patients who might truly benefit from intensified therapy, the authors explained.
Study results
The World Health Organization categorizes tumors of the central nervous system into four groups. The authors followed this system of categorization for their patients with medulloblastoma. The four groups are WNT, in which WNT signaling pathway is activated; SHH, in which the SHH signaling pathway is activated; with or without TP53 mutation (provisionally designated group 3); and non-WNT/non-SHH (provisionally designed group 4)
The primary endpoint of the trial was event-free survival (EFS). In the patient population overall, there was no significant difference regarding this endpoint among those who received carboplatin and those who did not (EFS at 5 years, 66.4% vs. 59.2%).
However, there was a significant improvement among the patients in subgroup 3. Among those patients, EFS at 5 years was 73.2% with carboplatin versus 53.7% without (P = .047).
Similarly, in the overall group, there was no significant improvement in overall survival (OS) at 5 years from the addition of carboplatin (77.6% vs. 68.8% without carboplatin). However, the OS at 5 years varied widely between the different subtypes. There was again a significant improvement in OS at 5 years among the patients in subgroup 3 (82% with carboplation vs 63.7% without).
The beneficial effects from the addition of carboplatin on both endpoints were seen exclusively in patients in group 3, the authors emphasized.
“The WNT group does really well with less therapy, so if we treated all children the same, we would likely be overtreating WNT children and undertreating group 3 children,” Dr. Olson observed. “Genetic analysis is essential.”
In an earlier study, Dr. Olson and colleagues found that 70% of children with primitive neuroectodermal tumor of the CNS and pineoblastoma had been misdiagnosed even by outstanding children’s oncology centers because clinicians were relying on microscopic diagnosis.
“With molecular diagnosis, we were able to learn that many of these children had completely different diseases that require complexly different treatments, so doing diagnosis by molecular classification is absolutely essential,” he reemphasized.
“Glimmers of hope”
This study provides clinicians with “glimmers of hope” that children with high-risk, group 3 medulloblastoma will experience improvements in survival, wrote Allison Martin, MD, Albert Einstein College of Medicine, New York, and Sadhana Jackson, MD, National Institutes of Health, Bethesda, M.d., in an accompanying editorial.
The editorialists hope that “the treatment paradigm for all patients with high-risk disease can be improved through incorporation of detailed molecular analyses.”
However, they pointed out that DNA methylation and other advanced testing methods used to distinguish subgroups 3 and 4 in this study are not widely available, even at most Children’s Oncology Group member institutions. (Dr. Olson countered that, even if these sophisticated tests are not available at all pediatric oncology centers, tests will be performed if clinicians send tissue to the few sites that are equipped to conduct them.)
The editorialists also noted that therapy intensification with carboplatin is associated with an increased risk for adverse effects – “underscoring the importance of correctly identifying patients who could benefit from this intervention and avoid unnecessary toxic effects.”
The study was funded by the National Cancer Institute. Dr. Olson has disclosed no relevant financial relationships. Dr. Martin reported that she previously owed shares in Celgene, which she has subsequently sold.
A version of this article first appeared on Medscape.com.
A practice-changing study that used molecular testing to distinguish between subtypes of medulloblastoma has shown a significant improvement in survival for children with high-risk disease who underwent treatment intensification with carboplatin.
“Each of the four subgroups of medulloblastoma has a different prognosis, but for this particular subgroup, 20 fewer children out of every 100 would have survived prior to this study,” James Olson, MD, professor of medicine, French Hutchinson Cancer Research Center, University of Washington, Seattle, said in an interview.
“This is the reason for celebration – for now and forevermore, we can expect 20 more children with high-risk, group 3 medulloblastoma to survive,” he said.
“We recommend that all children with high-risk, group 3 medulloblastoma receive carboplatin and all children in the other subgroups do not, because we don’t want them to experience the toxicity without benefit,” Dr. Olson said.
The study was published online July 22, 2021, in JAMA Oncology.
Hematologic toxicity was more pronounced in the carboplatin arm in the induction phase of the protocol, and toxicity persisted into the first cycles of maintenance therapy. On the other hand, “there weren’t enough additional side effects to recommend children not get carboplatin if they would benefit from it,” Dr. Olson noted.
At least 75% of children with newly diagnosed medulloblastoma survive, although those with high-risk, group 3 disease have a substantially poorer prognosis than those with other molecular subtypes.
However, if a child with medulloblastoma experiences relapse, “the likelihood of survival is near zero, so it’s important to get it right the first time,” Dr. Olson said.
One of the patients who took part in this trial, Sammy Loch of Seattle, is now 27 years old and has been cancer free for 11 years.
She was diagnosed with medulloblastoma when in high school. At the time of her diagnosis, she was asked by her pediatric oncologist at Seattle Children’s Hospital about taking part in the study. After careful consideration, she agreed.
“Participating in research was my way to give back and pay it forward,” Ms. Loch said in a statement. “It’s really exciting to know more people will survive because of the research I was involved in,” she added. She continues to pay her debt forward, serving as a therapist for people with chronic health conditions and raising funds for pediatric cancer research.
Patients had high-risk features
The study involved 261 evaluable patients (median age, 8.6 years). All patients had high-risk features, including metastatic disease (72.4% of the group), diffuse anaplastic histologic characteristics (22.2%), and incomplete surgical resection (5.4%), defined as residual tumor greater than 1.5 cm2.
“All patients received 36 Gy craniospinal radiotherapy with boost to the posterior fossa of 55.8 Gy cumulative dose with conventional fractionation of 1.8 Gy/d,” Dr. Olson and colleagues explain. Patients also received six doses of vincristine 1.5 mg/m2 weekly during radiotherapy and were randomly assigned to receive carboplatin 35 mg/m2 for a total of 30 doses given daily prior to radiotherapy or placebo.
This regimen was followed by maintenance therapy, which consisted of six 28-day cycles of the combination of cisplatin 75 mg/m2 on day 1; cyclophosphamide 1,000 mg/m2 on days 2 and 3; and vincristine 1.5 mg/m2 on days 1 and 8.
Patients were originally assigned to receive an additional 12 cycles of isotretinoin or placebo, to be given during and after maintenance therapy. However, randomization to isotretinoin was discontinued early because of futility.
The study was initially powered to evaluate medulloblastoma as a single disease. However, as a result of biologic insights gained after the study was conceived, it was amended to include a molecular subgroup analysis to better distinguish patients who might truly benefit from intensified therapy, the authors explained.
Study results
The World Health Organization categorizes tumors of the central nervous system into four groups. The authors followed this system of categorization for their patients with medulloblastoma. The four groups are WNT, in which WNT signaling pathway is activated; SHH, in which the SHH signaling pathway is activated; with or without TP53 mutation (provisionally designated group 3); and non-WNT/non-SHH (provisionally designed group 4)
The primary endpoint of the trial was event-free survival (EFS). In the patient population overall, there was no significant difference regarding this endpoint among those who received carboplatin and those who did not (EFS at 5 years, 66.4% vs. 59.2%).
However, there was a significant improvement among the patients in subgroup 3. Among those patients, EFS at 5 years was 73.2% with carboplatin versus 53.7% without (P = .047).
Similarly, in the overall group, there was no significant improvement in overall survival (OS) at 5 years from the addition of carboplatin (77.6% vs. 68.8% without carboplatin). However, the OS at 5 years varied widely between the different subtypes. There was again a significant improvement in OS at 5 years among the patients in subgroup 3 (82% with carboplation vs 63.7% without).
The beneficial effects from the addition of carboplatin on both endpoints were seen exclusively in patients in group 3, the authors emphasized.
“The WNT group does really well with less therapy, so if we treated all children the same, we would likely be overtreating WNT children and undertreating group 3 children,” Dr. Olson observed. “Genetic analysis is essential.”
In an earlier study, Dr. Olson and colleagues found that 70% of children with primitive neuroectodermal tumor of the CNS and pineoblastoma had been misdiagnosed even by outstanding children’s oncology centers because clinicians were relying on microscopic diagnosis.
“With molecular diagnosis, we were able to learn that many of these children had completely different diseases that require complexly different treatments, so doing diagnosis by molecular classification is absolutely essential,” he reemphasized.
“Glimmers of hope”
This study provides clinicians with “glimmers of hope” that children with high-risk, group 3 medulloblastoma will experience improvements in survival, wrote Allison Martin, MD, Albert Einstein College of Medicine, New York, and Sadhana Jackson, MD, National Institutes of Health, Bethesda, M.d., in an accompanying editorial.
The editorialists hope that “the treatment paradigm for all patients with high-risk disease can be improved through incorporation of detailed molecular analyses.”
However, they pointed out that DNA methylation and other advanced testing methods used to distinguish subgroups 3 and 4 in this study are not widely available, even at most Children’s Oncology Group member institutions. (Dr. Olson countered that, even if these sophisticated tests are not available at all pediatric oncology centers, tests will be performed if clinicians send tissue to the few sites that are equipped to conduct them.)
The editorialists also noted that therapy intensification with carboplatin is associated with an increased risk for adverse effects – “underscoring the importance of correctly identifying patients who could benefit from this intervention and avoid unnecessary toxic effects.”
The study was funded by the National Cancer Institute. Dr. Olson has disclosed no relevant financial relationships. Dr. Martin reported that she previously owed shares in Celgene, which she has subsequently sold.
A version of this article first appeared on Medscape.com.
A practice-changing study that used molecular testing to distinguish between subtypes of medulloblastoma has shown a significant improvement in survival for children with high-risk disease who underwent treatment intensification with carboplatin.
“Each of the four subgroups of medulloblastoma has a different prognosis, but for this particular subgroup, 20 fewer children out of every 100 would have survived prior to this study,” James Olson, MD, professor of medicine, French Hutchinson Cancer Research Center, University of Washington, Seattle, said in an interview.
“This is the reason for celebration – for now and forevermore, we can expect 20 more children with high-risk, group 3 medulloblastoma to survive,” he said.
“We recommend that all children with high-risk, group 3 medulloblastoma receive carboplatin and all children in the other subgroups do not, because we don’t want them to experience the toxicity without benefit,” Dr. Olson said.
The study was published online July 22, 2021, in JAMA Oncology.
Hematologic toxicity was more pronounced in the carboplatin arm in the induction phase of the protocol, and toxicity persisted into the first cycles of maintenance therapy. On the other hand, “there weren’t enough additional side effects to recommend children not get carboplatin if they would benefit from it,” Dr. Olson noted.
At least 75% of children with newly diagnosed medulloblastoma survive, although those with high-risk, group 3 disease have a substantially poorer prognosis than those with other molecular subtypes.
However, if a child with medulloblastoma experiences relapse, “the likelihood of survival is near zero, so it’s important to get it right the first time,” Dr. Olson said.
One of the patients who took part in this trial, Sammy Loch of Seattle, is now 27 years old and has been cancer free for 11 years.
She was diagnosed with medulloblastoma when in high school. At the time of her diagnosis, she was asked by her pediatric oncologist at Seattle Children’s Hospital about taking part in the study. After careful consideration, she agreed.
“Participating in research was my way to give back and pay it forward,” Ms. Loch said in a statement. “It’s really exciting to know more people will survive because of the research I was involved in,” she added. She continues to pay her debt forward, serving as a therapist for people with chronic health conditions and raising funds for pediatric cancer research.
Patients had high-risk features
The study involved 261 evaluable patients (median age, 8.6 years). All patients had high-risk features, including metastatic disease (72.4% of the group), diffuse anaplastic histologic characteristics (22.2%), and incomplete surgical resection (5.4%), defined as residual tumor greater than 1.5 cm2.
“All patients received 36 Gy craniospinal radiotherapy with boost to the posterior fossa of 55.8 Gy cumulative dose with conventional fractionation of 1.8 Gy/d,” Dr. Olson and colleagues explain. Patients also received six doses of vincristine 1.5 mg/m2 weekly during radiotherapy and were randomly assigned to receive carboplatin 35 mg/m2 for a total of 30 doses given daily prior to radiotherapy or placebo.
This regimen was followed by maintenance therapy, which consisted of six 28-day cycles of the combination of cisplatin 75 mg/m2 on day 1; cyclophosphamide 1,000 mg/m2 on days 2 and 3; and vincristine 1.5 mg/m2 on days 1 and 8.
Patients were originally assigned to receive an additional 12 cycles of isotretinoin or placebo, to be given during and after maintenance therapy. However, randomization to isotretinoin was discontinued early because of futility.
The study was initially powered to evaluate medulloblastoma as a single disease. However, as a result of biologic insights gained after the study was conceived, it was amended to include a molecular subgroup analysis to better distinguish patients who might truly benefit from intensified therapy, the authors explained.
Study results
The World Health Organization categorizes tumors of the central nervous system into four groups. The authors followed this system of categorization for their patients with medulloblastoma. The four groups are WNT, in which WNT signaling pathway is activated; SHH, in which the SHH signaling pathway is activated; with or without TP53 mutation (provisionally designated group 3); and non-WNT/non-SHH (provisionally designed group 4)
The primary endpoint of the trial was event-free survival (EFS). In the patient population overall, there was no significant difference regarding this endpoint among those who received carboplatin and those who did not (EFS at 5 years, 66.4% vs. 59.2%).
However, there was a significant improvement among the patients in subgroup 3. Among those patients, EFS at 5 years was 73.2% with carboplatin versus 53.7% without (P = .047).
Similarly, in the overall group, there was no significant improvement in overall survival (OS) at 5 years from the addition of carboplatin (77.6% vs. 68.8% without carboplatin). However, the OS at 5 years varied widely between the different subtypes. There was again a significant improvement in OS at 5 years among the patients in subgroup 3 (82% with carboplation vs 63.7% without).
The beneficial effects from the addition of carboplatin on both endpoints were seen exclusively in patients in group 3, the authors emphasized.
“The WNT group does really well with less therapy, so if we treated all children the same, we would likely be overtreating WNT children and undertreating group 3 children,” Dr. Olson observed. “Genetic analysis is essential.”
In an earlier study, Dr. Olson and colleagues found that 70% of children with primitive neuroectodermal tumor of the CNS and pineoblastoma had been misdiagnosed even by outstanding children’s oncology centers because clinicians were relying on microscopic diagnosis.
“With molecular diagnosis, we were able to learn that many of these children had completely different diseases that require complexly different treatments, so doing diagnosis by molecular classification is absolutely essential,” he reemphasized.
“Glimmers of hope”
This study provides clinicians with “glimmers of hope” that children with high-risk, group 3 medulloblastoma will experience improvements in survival, wrote Allison Martin, MD, Albert Einstein College of Medicine, New York, and Sadhana Jackson, MD, National Institutes of Health, Bethesda, M.d., in an accompanying editorial.
The editorialists hope that “the treatment paradigm for all patients with high-risk disease can be improved through incorporation of detailed molecular analyses.”
However, they pointed out that DNA methylation and other advanced testing methods used to distinguish subgroups 3 and 4 in this study are not widely available, even at most Children’s Oncology Group member institutions. (Dr. Olson countered that, even if these sophisticated tests are not available at all pediatric oncology centers, tests will be performed if clinicians send tissue to the few sites that are equipped to conduct them.)
The editorialists also noted that therapy intensification with carboplatin is associated with an increased risk for adverse effects – “underscoring the importance of correctly identifying patients who could benefit from this intervention and avoid unnecessary toxic effects.”
The study was funded by the National Cancer Institute. Dr. Olson has disclosed no relevant financial relationships. Dr. Martin reported that she previously owed shares in Celgene, which she has subsequently sold.
A version of this article first appeared on Medscape.com.
Prostate Cancer: Small Cell Prostate Carcinoma-Pathology
Occipital nerve stimulation offers relief for patients with intractable chronic cluster headache
Occipital nerve stimulation may help safely prevent attacks of medically intractable chronic cluster headache, according to a new study.
Medically intractable chronic cluster headaches are unilateral headaches that cause excruciating pain during attacks, which may happen as frequently as eight times per day. They are refractory to, or intolerant of, preventive medications typically used in chronic cluster headaches.
“ONS was associated with a major, rapid, and sustained improvement of severe and long-lasting medically intractable chronic cluster headache, both at high and low intensity,” Leopoldine A. Wilbrink, MD, of Leiden (the Netherlands) University Medical Centre, and coauthors wrote in their paper.
The findings were published online.
The multicenter, randomized, double-blind, phase 3 clinical trial was carried out at seven hospitals in the Netherlands, Belgium, Germany, and Hungary. A total of 150 patients with suspected medically intractable chronic cluster headache were enrolled between October 2010 and December 2017, and observed for 12 weeks at baseline. Of those initially enrolled, 131 patients with at least four medically intractable chronic cluster headache attacks per week and a history of nonresponsiveness to at least three standard preventive medications were randomly allocated to one of two groups: Sixty-five patients received 24 weeks of ONS at high intensity (100% intensity, or the intensity 10% below the threshold of discomfort as reported by the patient) while 66 received low-intensity (30%) ONS. At 25-48 weeks, the patients received open-label ONS.
Safe and well tolerated
“Because ONS causes paraesthesia, preventing masked comparison versus placebo, we compared high-intensity versus low-intensity ONS, which are hypothesised to cause similar paraesthesia, but with different efficacy,” wrote Dr. Wilbrink and colleagues.
From baseline to weeks 21-24, the median weekly mean attack frequencies decreased to 7.38 (95% confidence interval [CI]: 2.5-18.5, P < .0001). A median decrease in 5.21 attacks per week (–11.18 to –0.19, P < .0001) was observed.
The 100% ONS group saw a decrease in mean attack frequency from 17.58 at baseline (range, 9.83-29.33) to 9.5 (3-21.25) at 21-24 weeks with a median change of –4.08 (–11.92 to –0.25). In the 30% ONS group, the mean attack frequency decreased from 15 (9.25 to 22.33) to 6.75 (1.5-16.5) with a median change of –6.5 (–10.83 to –0.08).
At weeks 21-24, the difference in median weekly mean attack frequency between the groups was –2.42 (–5.17 to 3.33).
The authors stated that, in both groups, ONS was “safe and well tolerated.” A total of 129 adverse events were reported in the 100% ONS group and 95 in the 30% ONS group, of which 17 and 9 were considered serious, respectively. The serious adverse events required a short hospital stay to resolve minor hardware issues. The adverse events most frequently observed were local pain, impaired wound healing, neck stiffness, and hardware damage.
Low intensity stimulation may be best
“The main limitation of the study comes from the difficulty in defining the electrical dose, which was not constant across patients within each group, but individually adjusted depending on the perception of the ONS-induced paraesthesia,” Denys Fontaine, MD, and Michel Lanteri-Minet, MD, both from Université Cote D’Azur in France, wrote in an accompanying editorial.
Given that the primary outcome did not differ significantly between the treatment groups, the editorialists stated that “the lowest stimulation intensity that induces paraesthesia is sufficient to obtain an effect in the patients who respond. Increasing the electrical dose or intensity does not seem to bring better efficacy and might even induce discomfort (painful paraesthesia or shock-like sensations) that might substantially reduce the tolerance of this approach.”
While the trial did not provide convincing evidence of high intensity ONS in medically intractable chronic cluster headache, the editorialists are otherwise optimistic about the findings: “… considering the significant difference between baseline and the end of the randomised stimulation phase in both groups (about half of the patients showed a 50% decrease in attack frequency), the findings of this study support the favourable results of previous real-world studies, and indicate that a substantial proportion of patients with intractable chronic cluster headache, although not all, could have their condition substantially improved by ONS.” Dr. Fontaine and Dr. Lanteri-Minet added that they hope that “these data will help health authorities to recognise the efficacy of ONS and consider its approval for use in patients with intractable chronic cluster headache.”
Priorities for future research in this area should “focus on optimising stimulation protocols and disentangling the underlying mechanism of action,” Dr. Wilbrink and colleagues wrote.
The study was funded by the Spinoza 2009 Lifetime Scientific Research Achievement Premium, the Netherlands Organisation for Scientific Research, the Dutch Ministry of Health (as part of a national provisional reimbursement program for promising new treatments), the NutsOhra Foundation from the Dutch Health Insurance Companies, and an unrestricted grant from Medtronic, all to Dr. Ferrari.
Occipital nerve stimulation may help safely prevent attacks of medically intractable chronic cluster headache, according to a new study.
Medically intractable chronic cluster headaches are unilateral headaches that cause excruciating pain during attacks, which may happen as frequently as eight times per day. They are refractory to, or intolerant of, preventive medications typically used in chronic cluster headaches.
“ONS was associated with a major, rapid, and sustained improvement of severe and long-lasting medically intractable chronic cluster headache, both at high and low intensity,” Leopoldine A. Wilbrink, MD, of Leiden (the Netherlands) University Medical Centre, and coauthors wrote in their paper.
The findings were published online.
The multicenter, randomized, double-blind, phase 3 clinical trial was carried out at seven hospitals in the Netherlands, Belgium, Germany, and Hungary. A total of 150 patients with suspected medically intractable chronic cluster headache were enrolled between October 2010 and December 2017, and observed for 12 weeks at baseline. Of those initially enrolled, 131 patients with at least four medically intractable chronic cluster headache attacks per week and a history of nonresponsiveness to at least three standard preventive medications were randomly allocated to one of two groups: Sixty-five patients received 24 weeks of ONS at high intensity (100% intensity, or the intensity 10% below the threshold of discomfort as reported by the patient) while 66 received low-intensity (30%) ONS. At 25-48 weeks, the patients received open-label ONS.
Safe and well tolerated
“Because ONS causes paraesthesia, preventing masked comparison versus placebo, we compared high-intensity versus low-intensity ONS, which are hypothesised to cause similar paraesthesia, but with different efficacy,” wrote Dr. Wilbrink and colleagues.
From baseline to weeks 21-24, the median weekly mean attack frequencies decreased to 7.38 (95% confidence interval [CI]: 2.5-18.5, P < .0001). A median decrease in 5.21 attacks per week (–11.18 to –0.19, P < .0001) was observed.
The 100% ONS group saw a decrease in mean attack frequency from 17.58 at baseline (range, 9.83-29.33) to 9.5 (3-21.25) at 21-24 weeks with a median change of –4.08 (–11.92 to –0.25). In the 30% ONS group, the mean attack frequency decreased from 15 (9.25 to 22.33) to 6.75 (1.5-16.5) with a median change of –6.5 (–10.83 to –0.08).
At weeks 21-24, the difference in median weekly mean attack frequency between the groups was –2.42 (–5.17 to 3.33).
The authors stated that, in both groups, ONS was “safe and well tolerated.” A total of 129 adverse events were reported in the 100% ONS group and 95 in the 30% ONS group, of which 17 and 9 were considered serious, respectively. The serious adverse events required a short hospital stay to resolve minor hardware issues. The adverse events most frequently observed were local pain, impaired wound healing, neck stiffness, and hardware damage.
Low intensity stimulation may be best
“The main limitation of the study comes from the difficulty in defining the electrical dose, which was not constant across patients within each group, but individually adjusted depending on the perception of the ONS-induced paraesthesia,” Denys Fontaine, MD, and Michel Lanteri-Minet, MD, both from Université Cote D’Azur in France, wrote in an accompanying editorial.
Given that the primary outcome did not differ significantly between the treatment groups, the editorialists stated that “the lowest stimulation intensity that induces paraesthesia is sufficient to obtain an effect in the patients who respond. Increasing the electrical dose or intensity does not seem to bring better efficacy and might even induce discomfort (painful paraesthesia or shock-like sensations) that might substantially reduce the tolerance of this approach.”
While the trial did not provide convincing evidence of high intensity ONS in medically intractable chronic cluster headache, the editorialists are otherwise optimistic about the findings: “… considering the significant difference between baseline and the end of the randomised stimulation phase in both groups (about half of the patients showed a 50% decrease in attack frequency), the findings of this study support the favourable results of previous real-world studies, and indicate that a substantial proportion of patients with intractable chronic cluster headache, although not all, could have their condition substantially improved by ONS.” Dr. Fontaine and Dr. Lanteri-Minet added that they hope that “these data will help health authorities to recognise the efficacy of ONS and consider its approval for use in patients with intractable chronic cluster headache.”
Priorities for future research in this area should “focus on optimising stimulation protocols and disentangling the underlying mechanism of action,” Dr. Wilbrink and colleagues wrote.
The study was funded by the Spinoza 2009 Lifetime Scientific Research Achievement Premium, the Netherlands Organisation for Scientific Research, the Dutch Ministry of Health (as part of a national provisional reimbursement program for promising new treatments), the NutsOhra Foundation from the Dutch Health Insurance Companies, and an unrestricted grant from Medtronic, all to Dr. Ferrari.
Occipital nerve stimulation may help safely prevent attacks of medically intractable chronic cluster headache, according to a new study.
Medically intractable chronic cluster headaches are unilateral headaches that cause excruciating pain during attacks, which may happen as frequently as eight times per day. They are refractory to, or intolerant of, preventive medications typically used in chronic cluster headaches.
“ONS was associated with a major, rapid, and sustained improvement of severe and long-lasting medically intractable chronic cluster headache, both at high and low intensity,” Leopoldine A. Wilbrink, MD, of Leiden (the Netherlands) University Medical Centre, and coauthors wrote in their paper.
The findings were published online.
The multicenter, randomized, double-blind, phase 3 clinical trial was carried out at seven hospitals in the Netherlands, Belgium, Germany, and Hungary. A total of 150 patients with suspected medically intractable chronic cluster headache were enrolled between October 2010 and December 2017, and observed for 12 weeks at baseline. Of those initially enrolled, 131 patients with at least four medically intractable chronic cluster headache attacks per week and a history of nonresponsiveness to at least three standard preventive medications were randomly allocated to one of two groups: Sixty-five patients received 24 weeks of ONS at high intensity (100% intensity, or the intensity 10% below the threshold of discomfort as reported by the patient) while 66 received low-intensity (30%) ONS. At 25-48 weeks, the patients received open-label ONS.
Safe and well tolerated
“Because ONS causes paraesthesia, preventing masked comparison versus placebo, we compared high-intensity versus low-intensity ONS, which are hypothesised to cause similar paraesthesia, but with different efficacy,” wrote Dr. Wilbrink and colleagues.
From baseline to weeks 21-24, the median weekly mean attack frequencies decreased to 7.38 (95% confidence interval [CI]: 2.5-18.5, P < .0001). A median decrease in 5.21 attacks per week (–11.18 to –0.19, P < .0001) was observed.
The 100% ONS group saw a decrease in mean attack frequency from 17.58 at baseline (range, 9.83-29.33) to 9.5 (3-21.25) at 21-24 weeks with a median change of –4.08 (–11.92 to –0.25). In the 30% ONS group, the mean attack frequency decreased from 15 (9.25 to 22.33) to 6.75 (1.5-16.5) with a median change of –6.5 (–10.83 to –0.08).
At weeks 21-24, the difference in median weekly mean attack frequency between the groups was –2.42 (–5.17 to 3.33).
The authors stated that, in both groups, ONS was “safe and well tolerated.” A total of 129 adverse events were reported in the 100% ONS group and 95 in the 30% ONS group, of which 17 and 9 were considered serious, respectively. The serious adverse events required a short hospital stay to resolve minor hardware issues. The adverse events most frequently observed were local pain, impaired wound healing, neck stiffness, and hardware damage.
Low intensity stimulation may be best
“The main limitation of the study comes from the difficulty in defining the electrical dose, which was not constant across patients within each group, but individually adjusted depending on the perception of the ONS-induced paraesthesia,” Denys Fontaine, MD, and Michel Lanteri-Minet, MD, both from Université Cote D’Azur in France, wrote in an accompanying editorial.
Given that the primary outcome did not differ significantly between the treatment groups, the editorialists stated that “the lowest stimulation intensity that induces paraesthesia is sufficient to obtain an effect in the patients who respond. Increasing the electrical dose or intensity does not seem to bring better efficacy and might even induce discomfort (painful paraesthesia or shock-like sensations) that might substantially reduce the tolerance of this approach.”
While the trial did not provide convincing evidence of high intensity ONS in medically intractable chronic cluster headache, the editorialists are otherwise optimistic about the findings: “… considering the significant difference between baseline and the end of the randomised stimulation phase in both groups (about half of the patients showed a 50% decrease in attack frequency), the findings of this study support the favourable results of previous real-world studies, and indicate that a substantial proportion of patients with intractable chronic cluster headache, although not all, could have their condition substantially improved by ONS.” Dr. Fontaine and Dr. Lanteri-Minet added that they hope that “these data will help health authorities to recognise the efficacy of ONS and consider its approval for use in patients with intractable chronic cluster headache.”
Priorities for future research in this area should “focus on optimising stimulation protocols and disentangling the underlying mechanism of action,” Dr. Wilbrink and colleagues wrote.
The study was funded by the Spinoza 2009 Lifetime Scientific Research Achievement Premium, the Netherlands Organisation for Scientific Research, the Dutch Ministry of Health (as part of a national provisional reimbursement program for promising new treatments), the NutsOhra Foundation from the Dutch Health Insurance Companies, and an unrestricted grant from Medtronic, all to Dr. Ferrari.
FROM LANCET NEUROLOGY
Atopic Dermatitis: Pediatric AD
Study estimates carbon footprint reduction of virtual isotretinoin visits
: A reduction of 5,137 kg of greenhouse gas emissions in carbon dioxide equivalents.
In what they say is “one of the first studies to evaluate the environmental impact of any aspect of dermatology,” the authors of the retrospective cross-sectional study identified patients who had virtual visits for isotretinoin management between March 25 and May 29, 2020, – the period during which all such visits were conducted virtually in keeping with hospital recommendations to minimize the spread of COVID-19.
The investigators, from the department of dermatology and the department of civil and environmental engineering at West Virginia University, Morgantown, then counted the number of virtual visits that occurred during this period and through Dec. 1, 2020, (175 virtual visits), calculated the distance patients would have traveled round-trip had these visits been in-person, and converted miles saved into the environmental impact using U.S. Environmental Protection Agency and Federal Highway Administration data and relevant EPA standards.
Most patients had elected to continue virtual visits after May 29, the point at which patients were given the option to return to the WVUH clinic. (Patients who initiated treatment during the 2-month identification period were not included.)
The investigators determined that virtual management of isotretinoin saved a median of 37.8 miles per visit during the study period of March 25 to Dec. 1, and estimated that the virtual visits reduced total travel by 14,450 miles. For the analysis, patients were assumed to use light-duty vehicles.
In addition to calculating the reduction in emissions during the study period (5,137 kg of CO2equivalents) they used patient census data from 2019 to 2020 and data from the study period to project the mileage – and the associated emissions – that would be saved annually if all in-person visits for isotretinoin management occurred virtually.
Their calculation for a projected emissions reduction with 1 year of all-virtual isotretinoin management was 49,400 kg of greenhouse gas emissions in CO2equivalents. This is the emission load released when 24,690 kg of coal are burned or 6.3 million smartphones are charged, the researchers wrote.
“Considering that more than 1,000,000 prescriptions of isotretinoin are authorized annually in the United States, the environmental impact could be magnified if virtual delivery of isotretinoin care is adopted on a national scale,” they commented.“Given the serious consequences of global climate change, analysis of the environmental impact of all fields of medicine, including dermatology, is warranted,” they added.
The reduced greenhouse gas emissions are “definitely [being taken] into consideration for future decisions about virtual visits” in the department of dermatology, said Zachary Zinn, MD, residency director and associate professor in the department of dermatology at West Virginia University, Morgantown, who is the senior author of the study. “The main benefit of virtual care in my opinion,” he said in an interview, “is the potential to reduce our carbon footprint.”
Justin Lee, MD, an intern at WVU and the study’s first author, said that the research team was motivated to think about how they “could reduce the negative environmental impact of practicing dermatology” after they read a paper about the environmental impact of endoscopy, written by a gastroenterologist.
In the study, no pregnancies occurred and monthly tests were performed, but “formal assessment of pregnancy risk with virtual isotretinoin management would be warranted,” Dr. Lee and coauthors wrote, noting too that, while no differences were seen with respect to isotretinoin side effects, these were not formally analyzed.
Dr. Zinn said that he and colleagues at WVUH are currently conducting clinical trials to assess the quality and efficacy of virtual care for patients with acne, atopic dermatitis, and psoriasis. Delivering care virtually “will be easier to do if there are data supporting [its] quality and efficacy,” he said. Rosacea is another condition that may be amendable to virtual care, he noted.
Meanwhile, he said, isotretinoin management is “well suited” for virtual visits. When initiating isotretinoin treatment, Dr. Zinn now “proactively inquires” if patients would like to pursue their follow-up visits virtually. “I’ll note that it will save the time and decrease the burden of travel, including the financial cost as well as the environmental cost of travel,” he said, estimating that about half of their management visits are currently virtual.
Asked about the study, Misha Rosenbach, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said the reduced carbon footprint calculated by the researchers and its downstream health benefits “should be taken into consideration by [dermatology] departments, insurers and policymakers” when making decisions about teledermatology.
While environmental impact is “not something I think most institutions are considering for virtual versus in-person care, they should be. And some are,” said Dr. Rosenbach, a founder and cochair of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues.
Limitations of the study include the generalizability of the results. The impact of virtual isotretinoin management “may be less in predominantly urban areas” than it is in predominately rural West Virginia, the study authors note. And in the case of West Virginia, travel to a local laboratory and pharmacy offsets some of the environmental benefits for the virtual care, they noted. Such travel wasn’t accounted for in the study, but it was found to be a fraction of travel to the WVU hospital clinic. (Patients traveled a median of 5.8 miles to a lab 2.4 times from March 25 to Dec. 1, 2020.)
Dr. Lee will start his dermatology residency at WVU next year. The study was funded by a grant from the U.S. National Science Foundation. The authors have no relevant conflicts of interest, according to Dr. Lee.
: A reduction of 5,137 kg of greenhouse gas emissions in carbon dioxide equivalents.
In what they say is “one of the first studies to evaluate the environmental impact of any aspect of dermatology,” the authors of the retrospective cross-sectional study identified patients who had virtual visits for isotretinoin management between March 25 and May 29, 2020, – the period during which all such visits were conducted virtually in keeping with hospital recommendations to minimize the spread of COVID-19.
The investigators, from the department of dermatology and the department of civil and environmental engineering at West Virginia University, Morgantown, then counted the number of virtual visits that occurred during this period and through Dec. 1, 2020, (175 virtual visits), calculated the distance patients would have traveled round-trip had these visits been in-person, and converted miles saved into the environmental impact using U.S. Environmental Protection Agency and Federal Highway Administration data and relevant EPA standards.
Most patients had elected to continue virtual visits after May 29, the point at which patients were given the option to return to the WVUH clinic. (Patients who initiated treatment during the 2-month identification period were not included.)
The investigators determined that virtual management of isotretinoin saved a median of 37.8 miles per visit during the study period of March 25 to Dec. 1, and estimated that the virtual visits reduced total travel by 14,450 miles. For the analysis, patients were assumed to use light-duty vehicles.
In addition to calculating the reduction in emissions during the study period (5,137 kg of CO2equivalents) they used patient census data from 2019 to 2020 and data from the study period to project the mileage – and the associated emissions – that would be saved annually if all in-person visits for isotretinoin management occurred virtually.
Their calculation for a projected emissions reduction with 1 year of all-virtual isotretinoin management was 49,400 kg of greenhouse gas emissions in CO2equivalents. This is the emission load released when 24,690 kg of coal are burned or 6.3 million smartphones are charged, the researchers wrote.
“Considering that more than 1,000,000 prescriptions of isotretinoin are authorized annually in the United States, the environmental impact could be magnified if virtual delivery of isotretinoin care is adopted on a national scale,” they commented.“Given the serious consequences of global climate change, analysis of the environmental impact of all fields of medicine, including dermatology, is warranted,” they added.
The reduced greenhouse gas emissions are “definitely [being taken] into consideration for future decisions about virtual visits” in the department of dermatology, said Zachary Zinn, MD, residency director and associate professor in the department of dermatology at West Virginia University, Morgantown, who is the senior author of the study. “The main benefit of virtual care in my opinion,” he said in an interview, “is the potential to reduce our carbon footprint.”
Justin Lee, MD, an intern at WVU and the study’s first author, said that the research team was motivated to think about how they “could reduce the negative environmental impact of practicing dermatology” after they read a paper about the environmental impact of endoscopy, written by a gastroenterologist.
In the study, no pregnancies occurred and monthly tests were performed, but “formal assessment of pregnancy risk with virtual isotretinoin management would be warranted,” Dr. Lee and coauthors wrote, noting too that, while no differences were seen with respect to isotretinoin side effects, these were not formally analyzed.
Dr. Zinn said that he and colleagues at WVUH are currently conducting clinical trials to assess the quality and efficacy of virtual care for patients with acne, atopic dermatitis, and psoriasis. Delivering care virtually “will be easier to do if there are data supporting [its] quality and efficacy,” he said. Rosacea is another condition that may be amendable to virtual care, he noted.
Meanwhile, he said, isotretinoin management is “well suited” for virtual visits. When initiating isotretinoin treatment, Dr. Zinn now “proactively inquires” if patients would like to pursue their follow-up visits virtually. “I’ll note that it will save the time and decrease the burden of travel, including the financial cost as well as the environmental cost of travel,” he said, estimating that about half of their management visits are currently virtual.
Asked about the study, Misha Rosenbach, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said the reduced carbon footprint calculated by the researchers and its downstream health benefits “should be taken into consideration by [dermatology] departments, insurers and policymakers” when making decisions about teledermatology.
While environmental impact is “not something I think most institutions are considering for virtual versus in-person care, they should be. And some are,” said Dr. Rosenbach, a founder and cochair of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues.
Limitations of the study include the generalizability of the results. The impact of virtual isotretinoin management “may be less in predominantly urban areas” than it is in predominately rural West Virginia, the study authors note. And in the case of West Virginia, travel to a local laboratory and pharmacy offsets some of the environmental benefits for the virtual care, they noted. Such travel wasn’t accounted for in the study, but it was found to be a fraction of travel to the WVU hospital clinic. (Patients traveled a median of 5.8 miles to a lab 2.4 times from March 25 to Dec. 1, 2020.)
Dr. Lee will start his dermatology residency at WVU next year. The study was funded by a grant from the U.S. National Science Foundation. The authors have no relevant conflicts of interest, according to Dr. Lee.
: A reduction of 5,137 kg of greenhouse gas emissions in carbon dioxide equivalents.
In what they say is “one of the first studies to evaluate the environmental impact of any aspect of dermatology,” the authors of the retrospective cross-sectional study identified patients who had virtual visits for isotretinoin management between March 25 and May 29, 2020, – the period during which all such visits were conducted virtually in keeping with hospital recommendations to minimize the spread of COVID-19.
The investigators, from the department of dermatology and the department of civil and environmental engineering at West Virginia University, Morgantown, then counted the number of virtual visits that occurred during this period and through Dec. 1, 2020, (175 virtual visits), calculated the distance patients would have traveled round-trip had these visits been in-person, and converted miles saved into the environmental impact using U.S. Environmental Protection Agency and Federal Highway Administration data and relevant EPA standards.
Most patients had elected to continue virtual visits after May 29, the point at which patients were given the option to return to the WVUH clinic. (Patients who initiated treatment during the 2-month identification period were not included.)
The investigators determined that virtual management of isotretinoin saved a median of 37.8 miles per visit during the study period of March 25 to Dec. 1, and estimated that the virtual visits reduced total travel by 14,450 miles. For the analysis, patients were assumed to use light-duty vehicles.
In addition to calculating the reduction in emissions during the study period (5,137 kg of CO2equivalents) they used patient census data from 2019 to 2020 and data from the study period to project the mileage – and the associated emissions – that would be saved annually if all in-person visits for isotretinoin management occurred virtually.
Their calculation for a projected emissions reduction with 1 year of all-virtual isotretinoin management was 49,400 kg of greenhouse gas emissions in CO2equivalents. This is the emission load released when 24,690 kg of coal are burned or 6.3 million smartphones are charged, the researchers wrote.
“Considering that more than 1,000,000 prescriptions of isotretinoin are authorized annually in the United States, the environmental impact could be magnified if virtual delivery of isotretinoin care is adopted on a national scale,” they commented.“Given the serious consequences of global climate change, analysis of the environmental impact of all fields of medicine, including dermatology, is warranted,” they added.
The reduced greenhouse gas emissions are “definitely [being taken] into consideration for future decisions about virtual visits” in the department of dermatology, said Zachary Zinn, MD, residency director and associate professor in the department of dermatology at West Virginia University, Morgantown, who is the senior author of the study. “The main benefit of virtual care in my opinion,” he said in an interview, “is the potential to reduce our carbon footprint.”
Justin Lee, MD, an intern at WVU and the study’s first author, said that the research team was motivated to think about how they “could reduce the negative environmental impact of practicing dermatology” after they read a paper about the environmental impact of endoscopy, written by a gastroenterologist.
In the study, no pregnancies occurred and monthly tests were performed, but “formal assessment of pregnancy risk with virtual isotretinoin management would be warranted,” Dr. Lee and coauthors wrote, noting too that, while no differences were seen with respect to isotretinoin side effects, these were not formally analyzed.
Dr. Zinn said that he and colleagues at WVUH are currently conducting clinical trials to assess the quality and efficacy of virtual care for patients with acne, atopic dermatitis, and psoriasis. Delivering care virtually “will be easier to do if there are data supporting [its] quality and efficacy,” he said. Rosacea is another condition that may be amendable to virtual care, he noted.
Meanwhile, he said, isotretinoin management is “well suited” for virtual visits. When initiating isotretinoin treatment, Dr. Zinn now “proactively inquires” if patients would like to pursue their follow-up visits virtually. “I’ll note that it will save the time and decrease the burden of travel, including the financial cost as well as the environmental cost of travel,” he said, estimating that about half of their management visits are currently virtual.
Asked about the study, Misha Rosenbach, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said the reduced carbon footprint calculated by the researchers and its downstream health benefits “should be taken into consideration by [dermatology] departments, insurers and policymakers” when making decisions about teledermatology.
While environmental impact is “not something I think most institutions are considering for virtual versus in-person care, they should be. And some are,” said Dr. Rosenbach, a founder and cochair of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues.
Limitations of the study include the generalizability of the results. The impact of virtual isotretinoin management “may be less in predominantly urban areas” than it is in predominately rural West Virginia, the study authors note. And in the case of West Virginia, travel to a local laboratory and pharmacy offsets some of the environmental benefits for the virtual care, they noted. Such travel wasn’t accounted for in the study, but it was found to be a fraction of travel to the WVU hospital clinic. (Patients traveled a median of 5.8 miles to a lab 2.4 times from March 25 to Dec. 1, 2020.)
Dr. Lee will start his dermatology residency at WVU next year. The study was funded by a grant from the U.S. National Science Foundation. The authors have no relevant conflicts of interest, according to Dr. Lee.
FROM PEDIATRIC DERMATOLOGY
ARBs equal ACE inhibitors for hypertension, and better tolerated
In the largest comparison of angiotensin receptor blockers (ARBs) and ACE inhibitors to date, a study of nearly 2.3 million patients starting the drugs as monotherapy shows no significant differences between the two in the long-term prevention of hypertension-related cardiovascular events.
However, side effects were notably lower with ARBs.
“This is a very large, well-executed observational study that confirms that ARBs appear to have fewer side effects than ACE inhibitors, and no unexpected ARB side effects were detected,” senior author George Hripcsak, MD, professor and chair of biomedical informatics at Columbia University, New York, told this news organization.
“Despite being equally guideline-recommended first-line therapies for hypertension, these results support preferentially starting ARBs rather than ACE inhibitors when initiating treatment for hypertension for physicians and patients considering renin-angiotensin system (RAS) inhibition,” the authors added in the study, published online July 26, 2021, in the journal Hypertension.
They noted that both drug classes have been on the market a long time, with proven efficacy in hypertension and “a wide availability of inexpensive generic forms.”
They also stressed that their findings only apply to patients with hypertension for whom a RAS inhibitor would be the best choice of therapy.
Commenting on the research, George Bakris, MD, of the American Heart Association’s Comprehensive Hypertension Center at the University of Chicago, said the findings were consistent with his experience in prescribing as well as researching the two drug classes.
“I have been in practice for over 30 years and studied both classes, including head-to-head prospective trials to assess blood pressure, and found in many cases better blood pressure lowering by some ARBs and always better tolerability,” he told this news organization. “I think this study confirms and extends my thoughts between the two classes of blood pressure–lowering agents.”
Head-to-head comparisons of ACE inhibitors and ARBs limited to date
ACE inhibitors and ARBs each have extensive evidence supporting their roles as first-line medications in the treatment of hypertension, and each have the strongest recommendations in international guidelines.
However, ACE inhibitors are prescribed more commonly than ARBs as the first-line drug for lowering blood pressure, and head-to-head comparisons of the two are limited, with conflicting results.
For the study, Dr. Hripcsak and colleagues evaluated data on almost 3 million patients starting monotherapy with an ACE inhibitor or ARB for the first time between 1996 and 2018 in the United States, Germany, and South Korea, who had no history of heart disease or stroke.
They identified a total of 2,297,881 patients initiating ACE inhibitors and 673,938 starting ARBs. Among new users of ACE inhibitors, most received lisinopril (80%), followed by ramipril and enalapril, while most patients prescribed ARBs received losartan (45%), followed by valsartan and olmesartan.
With follow-up times ranging from about 4 months to more than 18 months, the data show no statistically significant differences between ACE inhibitors versus ARBs in the primary outcomes of acute myocardial infarction (hazard ratio, 1.11), heart failure (HR, 1.03), stroke (HR, 1.07), or composite cardiovascular events (HR, 1.06).
For secondary and safety outcomes, including an analysis of 51 possible side effects, ACE inhibitors, compared with ARBs, were associated with a significantly higher risk of angioedema (HR, 3.31; P < .01), cough (HR, 1.32; P < .01), acute pancreatitis (HR, 1.32; P = .02), gastrointestinal bleeding (HR, 1.18; P = .04), and abnormal weight loss (HR, 1.18; P = .04).
While the link between ACE inhibitors and pancreatitis has been previously reported, the association with GI bleeding may be a novel finding, with no prior studies comparing those effects in the two drug classes, the authors noted.
Despite most patients taking just a couple of drugs in either class, Dr. Hripcsak said, “we don’t expect that other drugs from those classes will have fewer differences. It is possible, of course, but that is not our expectation.”
Results only applicable to those starting therapy with RAS inhibitors
First author RuiJun Chen, MD, added that, importantly, the results may not apply to patients switching therapies or adding on therapy, “such as for the patient whose hypertension is not effectively controlled with one drug and requires the addition of a second medication,” he said in an interview.
“Also, the suggestion of preferentially prescribing ARBs only applies to those patients and providers intending to control blood pressure through RAS inhibition,” said Dr. Chen, an assistant professor in translational data science and informatics at Geisinger Medical Center in Danville, Pa., who was a National Library of Medicine postdoctoral fellow at Columbia University at the time of the study.
Hence, he stressed the results do not extend to other classes of recommended first-line blood pressure medications.
“Essentially, since this is an ACE inhibitor versus ARB study, we would not claim that ARBs are preferred over all other types of hypertension medications which were not studied here,” the researchers emphasize.
In addition to ARBs and ACE inhibitors, other medications recommended by the AHA/American College of Cardiology in the 2017 “Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults” for the primary treatment of hypertension include thiazide diuretics and calcium channel blockers.
The study received support from the National Library of Medicine and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health; the National Science Foundation; and the Ministries of Health & Welfare and of Trade, Industry & Energy of the Republic of Korea. Dr. Hripcsak reported receiving grants from the National Library of Medicine during the study and grants from Janssen Research outside the submitted work. Dr. Bakris reported being a consultant for Merck, KBP Biosciences, and Ionis.
A version of this article first appeared on Medscape.com.
In the largest comparison of angiotensin receptor blockers (ARBs) and ACE inhibitors to date, a study of nearly 2.3 million patients starting the drugs as monotherapy shows no significant differences between the two in the long-term prevention of hypertension-related cardiovascular events.
However, side effects were notably lower with ARBs.
“This is a very large, well-executed observational study that confirms that ARBs appear to have fewer side effects than ACE inhibitors, and no unexpected ARB side effects were detected,” senior author George Hripcsak, MD, professor and chair of biomedical informatics at Columbia University, New York, told this news organization.
“Despite being equally guideline-recommended first-line therapies for hypertension, these results support preferentially starting ARBs rather than ACE inhibitors when initiating treatment for hypertension for physicians and patients considering renin-angiotensin system (RAS) inhibition,” the authors added in the study, published online July 26, 2021, in the journal Hypertension.
They noted that both drug classes have been on the market a long time, with proven efficacy in hypertension and “a wide availability of inexpensive generic forms.”
They also stressed that their findings only apply to patients with hypertension for whom a RAS inhibitor would be the best choice of therapy.
Commenting on the research, George Bakris, MD, of the American Heart Association’s Comprehensive Hypertension Center at the University of Chicago, said the findings were consistent with his experience in prescribing as well as researching the two drug classes.
“I have been in practice for over 30 years and studied both classes, including head-to-head prospective trials to assess blood pressure, and found in many cases better blood pressure lowering by some ARBs and always better tolerability,” he told this news organization. “I think this study confirms and extends my thoughts between the two classes of blood pressure–lowering agents.”
Head-to-head comparisons of ACE inhibitors and ARBs limited to date
ACE inhibitors and ARBs each have extensive evidence supporting their roles as first-line medications in the treatment of hypertension, and each have the strongest recommendations in international guidelines.
However, ACE inhibitors are prescribed more commonly than ARBs as the first-line drug for lowering blood pressure, and head-to-head comparisons of the two are limited, with conflicting results.
For the study, Dr. Hripcsak and colleagues evaluated data on almost 3 million patients starting monotherapy with an ACE inhibitor or ARB for the first time between 1996 and 2018 in the United States, Germany, and South Korea, who had no history of heart disease or stroke.
They identified a total of 2,297,881 patients initiating ACE inhibitors and 673,938 starting ARBs. Among new users of ACE inhibitors, most received lisinopril (80%), followed by ramipril and enalapril, while most patients prescribed ARBs received losartan (45%), followed by valsartan and olmesartan.
With follow-up times ranging from about 4 months to more than 18 months, the data show no statistically significant differences between ACE inhibitors versus ARBs in the primary outcomes of acute myocardial infarction (hazard ratio, 1.11), heart failure (HR, 1.03), stroke (HR, 1.07), or composite cardiovascular events (HR, 1.06).
For secondary and safety outcomes, including an analysis of 51 possible side effects, ACE inhibitors, compared with ARBs, were associated with a significantly higher risk of angioedema (HR, 3.31; P < .01), cough (HR, 1.32; P < .01), acute pancreatitis (HR, 1.32; P = .02), gastrointestinal bleeding (HR, 1.18; P = .04), and abnormal weight loss (HR, 1.18; P = .04).
While the link between ACE inhibitors and pancreatitis has been previously reported, the association with GI bleeding may be a novel finding, with no prior studies comparing those effects in the two drug classes, the authors noted.
Despite most patients taking just a couple of drugs in either class, Dr. Hripcsak said, “we don’t expect that other drugs from those classes will have fewer differences. It is possible, of course, but that is not our expectation.”
Results only applicable to those starting therapy with RAS inhibitors
First author RuiJun Chen, MD, added that, importantly, the results may not apply to patients switching therapies or adding on therapy, “such as for the patient whose hypertension is not effectively controlled with one drug and requires the addition of a second medication,” he said in an interview.
“Also, the suggestion of preferentially prescribing ARBs only applies to those patients and providers intending to control blood pressure through RAS inhibition,” said Dr. Chen, an assistant professor in translational data science and informatics at Geisinger Medical Center in Danville, Pa., who was a National Library of Medicine postdoctoral fellow at Columbia University at the time of the study.
Hence, he stressed the results do not extend to other classes of recommended first-line blood pressure medications.
“Essentially, since this is an ACE inhibitor versus ARB study, we would not claim that ARBs are preferred over all other types of hypertension medications which were not studied here,” the researchers emphasize.
In addition to ARBs and ACE inhibitors, other medications recommended by the AHA/American College of Cardiology in the 2017 “Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults” for the primary treatment of hypertension include thiazide diuretics and calcium channel blockers.
The study received support from the National Library of Medicine and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health; the National Science Foundation; and the Ministries of Health & Welfare and of Trade, Industry & Energy of the Republic of Korea. Dr. Hripcsak reported receiving grants from the National Library of Medicine during the study and grants from Janssen Research outside the submitted work. Dr. Bakris reported being a consultant for Merck, KBP Biosciences, and Ionis.
A version of this article first appeared on Medscape.com.
In the largest comparison of angiotensin receptor blockers (ARBs) and ACE inhibitors to date, a study of nearly 2.3 million patients starting the drugs as monotherapy shows no significant differences between the two in the long-term prevention of hypertension-related cardiovascular events.
However, side effects were notably lower with ARBs.
“This is a very large, well-executed observational study that confirms that ARBs appear to have fewer side effects than ACE inhibitors, and no unexpected ARB side effects were detected,” senior author George Hripcsak, MD, professor and chair of biomedical informatics at Columbia University, New York, told this news organization.
“Despite being equally guideline-recommended first-line therapies for hypertension, these results support preferentially starting ARBs rather than ACE inhibitors when initiating treatment for hypertension for physicians and patients considering renin-angiotensin system (RAS) inhibition,” the authors added in the study, published online July 26, 2021, in the journal Hypertension.
They noted that both drug classes have been on the market a long time, with proven efficacy in hypertension and “a wide availability of inexpensive generic forms.”
They also stressed that their findings only apply to patients with hypertension for whom a RAS inhibitor would be the best choice of therapy.
Commenting on the research, George Bakris, MD, of the American Heart Association’s Comprehensive Hypertension Center at the University of Chicago, said the findings were consistent with his experience in prescribing as well as researching the two drug classes.
“I have been in practice for over 30 years and studied both classes, including head-to-head prospective trials to assess blood pressure, and found in many cases better blood pressure lowering by some ARBs and always better tolerability,” he told this news organization. “I think this study confirms and extends my thoughts between the two classes of blood pressure–lowering agents.”
Head-to-head comparisons of ACE inhibitors and ARBs limited to date
ACE inhibitors and ARBs each have extensive evidence supporting their roles as first-line medications in the treatment of hypertension, and each have the strongest recommendations in international guidelines.
However, ACE inhibitors are prescribed more commonly than ARBs as the first-line drug for lowering blood pressure, and head-to-head comparisons of the two are limited, with conflicting results.
For the study, Dr. Hripcsak and colleagues evaluated data on almost 3 million patients starting monotherapy with an ACE inhibitor or ARB for the first time between 1996 and 2018 in the United States, Germany, and South Korea, who had no history of heart disease or stroke.
They identified a total of 2,297,881 patients initiating ACE inhibitors and 673,938 starting ARBs. Among new users of ACE inhibitors, most received lisinopril (80%), followed by ramipril and enalapril, while most patients prescribed ARBs received losartan (45%), followed by valsartan and olmesartan.
With follow-up times ranging from about 4 months to more than 18 months, the data show no statistically significant differences between ACE inhibitors versus ARBs in the primary outcomes of acute myocardial infarction (hazard ratio, 1.11), heart failure (HR, 1.03), stroke (HR, 1.07), or composite cardiovascular events (HR, 1.06).
For secondary and safety outcomes, including an analysis of 51 possible side effects, ACE inhibitors, compared with ARBs, were associated with a significantly higher risk of angioedema (HR, 3.31; P < .01), cough (HR, 1.32; P < .01), acute pancreatitis (HR, 1.32; P = .02), gastrointestinal bleeding (HR, 1.18; P = .04), and abnormal weight loss (HR, 1.18; P = .04).
While the link between ACE inhibitors and pancreatitis has been previously reported, the association with GI bleeding may be a novel finding, with no prior studies comparing those effects in the two drug classes, the authors noted.
Despite most patients taking just a couple of drugs in either class, Dr. Hripcsak said, “we don’t expect that other drugs from those classes will have fewer differences. It is possible, of course, but that is not our expectation.”
Results only applicable to those starting therapy with RAS inhibitors
First author RuiJun Chen, MD, added that, importantly, the results may not apply to patients switching therapies or adding on therapy, “such as for the patient whose hypertension is not effectively controlled with one drug and requires the addition of a second medication,” he said in an interview.
“Also, the suggestion of preferentially prescribing ARBs only applies to those patients and providers intending to control blood pressure through RAS inhibition,” said Dr. Chen, an assistant professor in translational data science and informatics at Geisinger Medical Center in Danville, Pa., who was a National Library of Medicine postdoctoral fellow at Columbia University at the time of the study.
Hence, he stressed the results do not extend to other classes of recommended first-line blood pressure medications.
“Essentially, since this is an ACE inhibitor versus ARB study, we would not claim that ARBs are preferred over all other types of hypertension medications which were not studied here,” the researchers emphasize.
In addition to ARBs and ACE inhibitors, other medications recommended by the AHA/American College of Cardiology in the 2017 “Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults” for the primary treatment of hypertension include thiazide diuretics and calcium channel blockers.
The study received support from the National Library of Medicine and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health; the National Science Foundation; and the Ministries of Health & Welfare and of Trade, Industry & Energy of the Republic of Korea. Dr. Hripcsak reported receiving grants from the National Library of Medicine during the study and grants from Janssen Research outside the submitted work. Dr. Bakris reported being a consultant for Merck, KBP Biosciences, and Ionis.
A version of this article first appeared on Medscape.com.
Formaldehyde-Induced Contact Dermatitis From an N95 Respirator Mask
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19

Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19

Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19

Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
Practice Points
- Prolonged wearing of N95 respirator masks has been associated with causing or complicating a number of facial inflammatory dermatoses.
- Consider the possibility of contact dermatitis secondary to formaldehyde exposure in individuals wearing N95 masks for prolonged periods.
- Information on the chemical components of N95 masks would be useful for clinicians tasked with evaluating patients with facial inflammatory dermatoses.
Phototherapy: Safe and Effective for Challenging Skin Conditions in Older Adults
Identifying safe, effective, and affordable evidence-based dermatologic treatments for older adults can be challenging because of age-related changes in the skin, comorbidities, polypharmacy, mobility issues, and cognitive changes. Phototherapy has been shown to be an effective nonpharmacologic treatment option for multiple challenging dermatologic conditions1-8; however, few studies have specifically examined its effectiveness in older adults. The challenge for older patients with psoriasis and dermatitis is that the conditions can be difficult to control and often require multiple treatment modalities.9,10 Patients with psoriasis also have a higher risk for diabetes, dyslipidemia, and cardiovascular disease compared to other older patients,11,12 which poses treatment challenges and makes nonpharmacologic treatments even more appealing.
Recent studies show that phototherapy can help decrease the use of dermatologic medications. Foerster and colleagues2 found that adults with psoriasis who were treated with phototherapy significantly decreased their use of topical steroids (24.5% fewer patients required steroid creams and 31.1% fewer patients required psoriasis-specific topicals)(P<.01) while their use of non–psoriasis-specific medications did not change. Click and colleagues13 identified a decrease in medication costs, health care utilization, and risk for immunosuppression in patients treated with phototherapy when compared to those treated with biologics and apremilast. Methotrexate is a common dermatologic medication that is highly associated with increased risks in elderly patients because of impaired immune system function and the presence of comorbidities (eg, kidney disease, obesity, diabetes, fatty liver),14 which increase in prevalence with age. Combining phototherapy with methotrexate can substantially decrease the amount of methotrexate needed to achieve disease control,15 thereby decreasing the methotrexate-associated risks. Findings from these studies suggest that a safe, effective, cost-effective, and well-tolerated nonpharmacologic alternative, such as phototherapy, is highly desirable and should be optimized. Unfortunately, most studies that report the effectiveness of phototherapy are in younger populations.
This retrospective study aimed to (1) identify the most common dermatologic conditions treated with phototherapy in older adults, (2) examine the effectiveness and safety of phototherapy in older adults
Methods
Design, Setting, Sample, and Statistical Analysis
The institutional review boards of Kaiser Permanente Washington Health Research Institute, Seattle, and the University of Washington, Seattle, approved this study. It was conducted in a large US multispecialty health care system (Group Health, Seattle, Washington [now Kaiser Permanente Washington]) serving approximately 600,000 patients, using billing records to identify all patients treated with phototherapy between January 1, 2015, and December 31, 2015, all who received narrowband UVB (NB-UVB) phototherapy. All adults 65 years and older who received phototherapy treatment during the 12-month study period were included. Patients were included regardless of comorbidities and other dermatologic treatments to maintain as much uniformity as possible between the present study and 2 prior studies examining phototherapy in older adult populations in the United Kingdom16 and Turkey.17 Demographic and clinical factors were presented using frequencies (percentages) or means and medians as appropriate. Comparisons of dermatologic conditions and clearance levels used a Fisher exact test. The number of phototherapy treatments to clearance and total number of treatments were compared between groups of patients using independent sample t tests.
Phototherapy Protocol
All patients received treatments administered by specially trained phototherapy nurses using a Daavlin UV Series (The Daavlin Company) or an Ultralite unit (Ultralite Enterprises, Inc), both with 48 lamps. All phototherapy nurses had been previously trained to provide treatments based on standardized protocols (Table 1) and to determine the patient’s level of disease clearance using a high to low clearance scale (Table 2). Daavlin’s treatment protocols were built into the software that accompanied the units and were developed based on the American Academy of Dermatology guidelines. The starting dose for an individual patient was determined based on the estimated
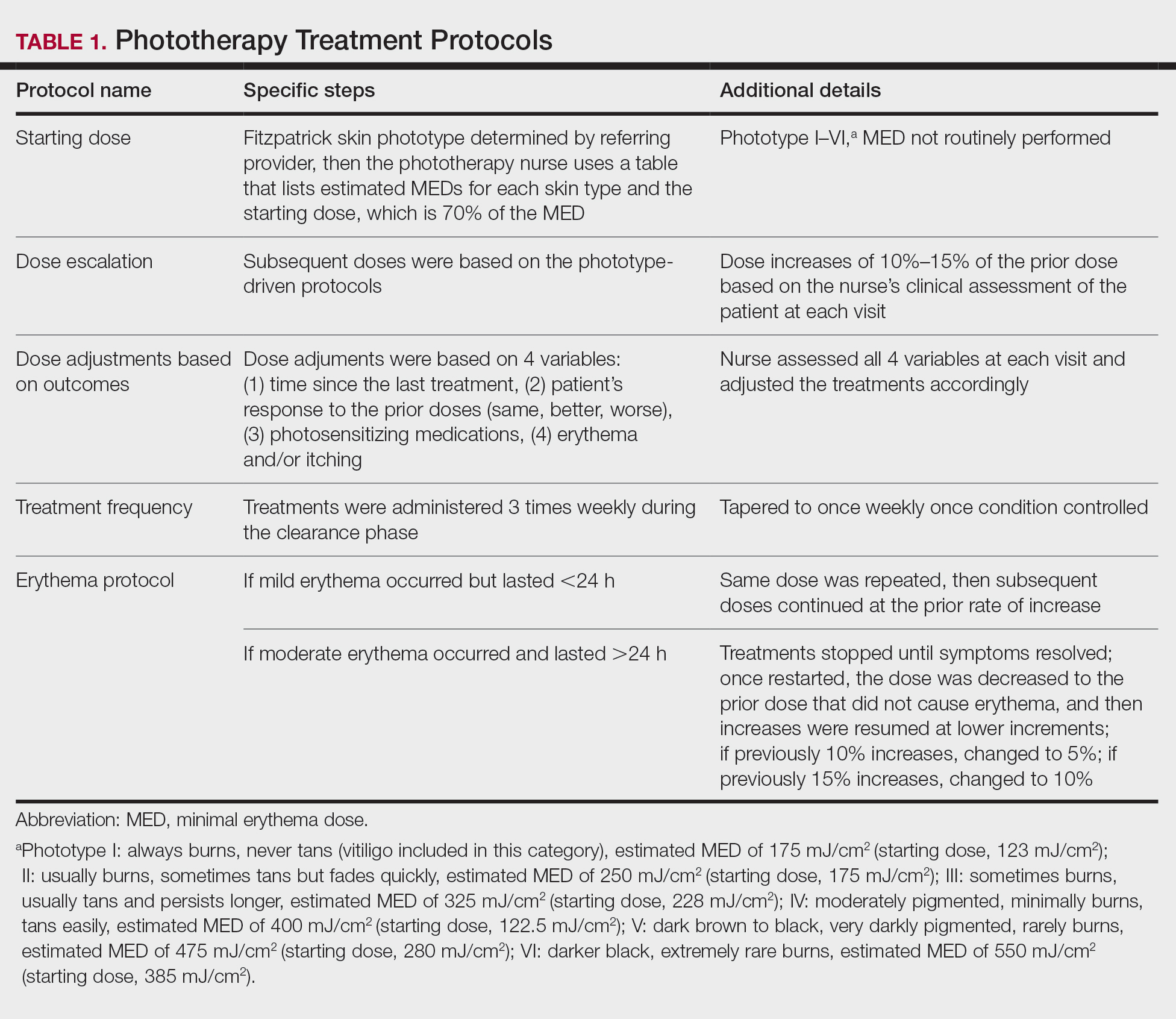
Results
Patients
Billing records identified 229 total patients who received phototherapy in 2015, of whom 52 (22.7%) were at least 65 years old. The median age was 70 years (range, 65–91 years). Twenty-nine (56%) were men and 35 (67%) had previously received phototherapy treatments.
Dermatologic Conditions Treated With Phototherapy
Our primary aim was to identify the most common dermatologic conditions treated with phototherapy in older adults. Psoriasis and dermatitis were the most common conditions treated in the sample (50% [26/52] and 21% [11/52], respectively), with mycosis fungoides being the third most common (10% [5/52]) and vitiligo tied with prurigo nodularis as fourth most common (6% [3/52])(Figure 1).

Effectiveness and Safety of Phototherapy
Our secondary aim was to examine the effectiveness and safety of phototherapy in older adults. Phototherapy was effective in this population, with 50 of 52 patients (96%) achieving a high or medium level of clearance. The degree of clearance for each of the dermatologic conditions is shown in Figure 2. Psoriasis and dermatitis achieved high clearance rates in 81% (21/26) and 82% (9/11) of patients, respectively. Overall, conditions did not have significant differences in clearances rates (Fisher exact test, P=.10). On average, it took patients 33 treatments to achieve medium or high rates of clearance. Psoriasis cleared more quickly, with an average of 30.4 treatments vs 36.1 treatments for other conditions, but the difference was not significant (t test, P=.26). Patients received an average of 98 total phototherapy treatments; the median number of treatments was 81 due to many being on maintenance therapy over several months. There was no relationship between a history of treatment with phototherapy and the total number of treatments needed to achieve clearance (t test, P=.40), but interestingly, those who had a history of phototherapy took approximately 5 more treatments to achieve clearance. The present study found that a slightly larger number of men were being treated for psoriasis (15 men vs 11 women), but there was no significant difference in response rate based on gender.
Side effects from phototherapy were minimal; 24 patients (46%) experienced grade 1 (mild) erythema at some point during their treatment course. Thirteen (25%) patients experienced grade 2 erythema, but this was a rare event for most patients. Only 1 (2%) patient experienced grade 3 erythema 1 time. Three patients experienced increased itching (6%). Thirteen (25%) patients had no side effects. None developed severe erythema or blisters, and none discontinued phototherapy because of side effects. Over the course of the study year, we found a high degree of acceptance of phototherapy treatments by older patients: 22 (42%) completed therapy after achieving clearance, 10 (19%) were continuing ongoing treatments (maintenance), and 15 (29%) stopped because of life circumstances (eg, other health issues, moving out of the area). Only 4 (8%) stopped because of a lack of effectiveness, and 1 (2%) patient because the treatments were burdensome.
Comparison of Outcomes
Our third aim was to compare the outcomes with similar studies in the United Kingdom16 and Turkey.17 This study confirmed that phototherapy is being used in older adults (22.7% of this study’s total patients) and is an effective treatment for older patients experiencing a range of challenging inflammatory and proliferative skin diseases similar to studies in the general population. Prior phototherapy studies in elderly patients also found psoriasis to be the most common skin condition treated, with 1 study finding that 51% (19/37) of older phototherapy patients had psoriasis,16 while another reported 58% (37/95) of older phototherapy patients had psoriasis.17 These numbers are similar to those in our study, which showed 50% (26/52) of elderly phototherapy patients had psoriasis. Psoriasis is the main indication for treatment with NB-UVB phototherapy in the general population,19 and because the risk for psoriasis increases with age,20 it is not surprising that all 3 studies found psoriasis to be the most common indication in elderly phototherapy patients. Table 3 provides further details on conditions treated in all 3 studies.
Comment
Our study found that 94% of patients with psoriasis achieved clearance with an average of 30.4 treatments, which is comparable to the reported 91% response rate with an average of 30 treatments in the United Kingdom.16 The other similar study in Turkey17 reported 73.7% of psoriasis patients achieved a 75% or more improvement from baseline with an average of 42 treatments, which may reflect underlying differences in regional skin type. Of note, the scatter chart (Figure 3) shows that several patients in the present study’s analysis are listed as not clear, but many of those patients had low treatment numbers below the mean time to clearance. Thus, the present study’s response rate may have been underestimated.
In the general population, studies show that psoriasis treated with standardized phototherapy protocols typically clears with an average of 20.6 treatments.21 The levels of clearance were similar in our study’s older population, but more treatments were required to achieve those results, with an average of 10 more treatments needed (an additional 3.3 weeks). Similar results were found in this sample for dermatitis and mycosis fungoides, indicating comparable clearance rates and levels but a need for more treatments to achieve similar results compared to the general population.
Additionally, in the current study more patients experienced grade 1 (mild) erythema (46%) and grade 2 erythema (25%) at some point in their treatment compared with the United Kingdom16 (1.89%) and Turkey17 (35%) studies, though these side effects did not impact the clearance rate. Interestingly, the current study’s scatter chart (Figure 3) illustrates that this side effect did not seem to increase with aging in this population. If anything, the erythema response was more prevalent in the median or younger patients in the sample. Erythema may have been due to the frequent use of photosensitizing medications in older adults in the United States, some of which typically get discontinued in patients 75 years and older (eg, statins). Other potential causes might include the use of phototype vs minimal erythema dose–driven protocols, the standard utilization of protocols originally designed for psoriasis vs other condition-specific protocols, missed treatments leading to increased sensitivity, or possibly shielding mishaps (eg, not wearing a prescribed face shield). Given the number of potential causes and the possibility of overlapping factors, careful analysis is important. With NB-UVB phototherapy, near-erythemogenic doses are optimal to achieve effective treatments, but this delicate balance may be more problematic for older adults. Future studies are needed to fully determine the factors at play for this population. In the interim, it is important for phototherapy-trained nurses to consider this risk carefully in the older population. They must follow the prescribed protocols that guide them to query patients about their responses to the prior treatment (eg, erythema, tenderness, itching), photosensitizing medications, missed treatments, and placement of shielding, and then adjust the treatment dosing accordingly.
Limitations
This study had several limitations. Although clinical outcomes were recorded prospectively, the analysis was retrospective, unblinded, and not placebo controlled. It was conducted in a single organization (Group Health [now Kaiser Permanente Washington]) but did analyze data from 4 medical centers in different cities with diverse demographics and a variety of nursing staff providing the treatments. Although the vitiligo treatment protocol likely slowed the response rate for those patients with vitiligo, the numbers were small (ie, only 3 of 52 patients), so the researchers chose to include them in the current study. The sample population was relatively small, but when these data are evaluated alongside the studies in the United Kingdom16 and Turkey,17 they show a consistent picture illustrating the effectiveness and safety of phototherapy in the older population. Further epidemiologic studies could be helpful to further describe the usefulness of this modality compared with other treatments for a variety of dermatoses in this age group. Supplementary analysis specifically examining the relationship between the number and type of photosensitizing medications, frequency of erythema, and time to clearance also could be useful.
Conclusion
Older adults with a variety of dermatoses respond well to phototherapy and should have the opportunity to use it, particularly considering the potential for increased complications and costs from other treatment modalities, such as commonly used immunosuppressive pharmaceuticals. However, the current study and the comparison studies indicate that it is important to carefully consider the slower clearance rates and the potential risk for increased erythema in this population and adjust patient education and treatment dosing accordingly.
Unfortunately, many dermatology centers do not offer phototherapy because of infrastructure limitations such as space and specially trained nursing staff. Increasing accessibility of phototherapy for older adults through home treatments may be an alternative, given its effectiveness in the general population.22,23 In addition, home phototherapy may be worth pursuing for the older population considering the challenges they may face with transportation to the clinic setting and their increased risk for serious illness if exposed to infections such as COVID-19. The COVID-19 pandemic has brought to light the need for reliable, safe, and effective treatments that can be utilized in the safety of patients’ homes and should therefore be considered as an option for older adults. Issues such as mobility and cognitive decline could pose some complicating factors, but with the help of a well-trained family member or caregiver, home phototherapy could be a viable option that improves accessibility for older patients. Future research opportunities include further examination of the slower but ultimately equivalent response to phototherapy in the older population, the influence of photosensitizing medications on phototherapy effects, and the impact of phototherapy on utilization of immunosuppressive pharmaceuticals in older adults.
- British Photodermatology Group. An appraisal of narrowband (TL-01) UVB phototherapy. British Photodermatology Group Workshop Report (April 1996). Br J Dermatol. 1997;137:327-330.
Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS ONE. 2017;12:e0181813. doi:10.1371/journal.pone.0181813 - Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2015;29:19-23.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593. - Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Martin JA, Laube S, Edwards C, et al. Rate of acute adverse events for narrow-band UVB and psoralen-UVA phototherapy. Photodermatol Photoimmunol Photomed. 2007;23:68-72.
- Mokos ZB, Jovic A, Ceovic R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Exp Opin Biol Ther. 2018;18:897-903.
- Napolitano M, Balato N, Ayala F, et al. Psoriasis in elderly and non-elderly population: clinical and molecular features. G Ital Dermatol Venereol. 2016;151:587-595.
- Grozdev IS, Van Voorhees AS, Gottlieb AB, et al. Psoriasis in the elderly: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2011;65:537-545.
- Click J, Alabaster A, Postlethwaite D, et al. Effect of availability of at-home phototherapy on the use of systemic medications for psoriasis.
Photodermatol Photoimmunol Photomed. 2017;33:345-346. - Piaserico S, Conti A, Lo Console F, et al.
Efficacy and safety of systemic treatments for psoriasis in elderly. Acta Derm Venereol. 2014;94:293-297. - Soliman A, Nofal E, Nofal A, et al. Combination therapy of methotrexate plus NB-UVB phototherapy is more effective than methotrexate monotherapy in the treatment of chronic plaque psoriasis. J Dermatol Treat. 2015;26:528-534.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Madigan LM, Al-Jamal M, Hamzavi I. Exploring the gaps in the evidence-based application of narrowband UVB for the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2016;32:66-80.
- Ibbotson SH. A perspective on the use of NB-UVB phototherapy vs. PUVA photochemotherapy. Front Med (Lausanne). 2018;5:184.
- Bell LM, Sedlack R, Beard CM, et al. Incidence of psoriasis in Rochester, Minn, 1980-1983. Arch Dermatol. 1991;127:1184-1187.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413.
- Cameron H, Yule S, Dawe RS, et al. Review of an established UK home phototherapy service 1998-2011: improving access to a cost-effective treatment for chronic skin disease. Public Health. 2014;128:317-324.
- Matthews SW, Simmer M, Williams L, et al. Transition of patients with psoriasis from office-based phototherapy to nurse-supported home phototherapy: a pilot study. JDNA. 2018;10:29-41.
Identifying safe, effective, and affordable evidence-based dermatologic treatments for older adults can be challenging because of age-related changes in the skin, comorbidities, polypharmacy, mobility issues, and cognitive changes. Phototherapy has been shown to be an effective nonpharmacologic treatment option for multiple challenging dermatologic conditions1-8; however, few studies have specifically examined its effectiveness in older adults. The challenge for older patients with psoriasis and dermatitis is that the conditions can be difficult to control and often require multiple treatment modalities.9,10 Patients with psoriasis also have a higher risk for diabetes, dyslipidemia, and cardiovascular disease compared to other older patients,11,12 which poses treatment challenges and makes nonpharmacologic treatments even more appealing.
Recent studies show that phototherapy can help decrease the use of dermatologic medications. Foerster and colleagues2 found that adults with psoriasis who were treated with phototherapy significantly decreased their use of topical steroids (24.5% fewer patients required steroid creams and 31.1% fewer patients required psoriasis-specific topicals)(P<.01) while their use of non–psoriasis-specific medications did not change. Click and colleagues13 identified a decrease in medication costs, health care utilization, and risk for immunosuppression in patients treated with phototherapy when compared to those treated with biologics and apremilast. Methotrexate is a common dermatologic medication that is highly associated with increased risks in elderly patients because of impaired immune system function and the presence of comorbidities (eg, kidney disease, obesity, diabetes, fatty liver),14 which increase in prevalence with age. Combining phototherapy with methotrexate can substantially decrease the amount of methotrexate needed to achieve disease control,15 thereby decreasing the methotrexate-associated risks. Findings from these studies suggest that a safe, effective, cost-effective, and well-tolerated nonpharmacologic alternative, such as phototherapy, is highly desirable and should be optimized. Unfortunately, most studies that report the effectiveness of phototherapy are in younger populations.
This retrospective study aimed to (1) identify the most common dermatologic conditions treated with phototherapy in older adults, (2) examine the effectiveness and safety of phototherapy in older adults
Methods
Design, Setting, Sample, and Statistical Analysis
The institutional review boards of Kaiser Permanente Washington Health Research Institute, Seattle, and the University of Washington, Seattle, approved this study. It was conducted in a large US multispecialty health care system (Group Health, Seattle, Washington [now Kaiser Permanente Washington]) serving approximately 600,000 patients, using billing records to identify all patients treated with phototherapy between January 1, 2015, and December 31, 2015, all who received narrowband UVB (NB-UVB) phototherapy. All adults 65 years and older who received phototherapy treatment during the 12-month study period were included. Patients were included regardless of comorbidities and other dermatologic treatments to maintain as much uniformity as possible between the present study and 2 prior studies examining phototherapy in older adult populations in the United Kingdom16 and Turkey.17 Demographic and clinical factors were presented using frequencies (percentages) or means and medians as appropriate. Comparisons of dermatologic conditions and clearance levels used a Fisher exact test. The number of phototherapy treatments to clearance and total number of treatments were compared between groups of patients using independent sample t tests.
Phototherapy Protocol
All patients received treatments administered by specially trained phototherapy nurses using a Daavlin UV Series (The Daavlin Company) or an Ultralite unit (Ultralite Enterprises, Inc), both with 48 lamps. All phototherapy nurses had been previously trained to provide treatments based on standardized protocols (Table 1) and to determine the patient’s level of disease clearance using a high to low clearance scale (Table 2). Daavlin’s treatment protocols were built into the software that accompanied the units and were developed based on the American Academy of Dermatology guidelines. The starting dose for an individual patient was determined based on the estimated


Results
Patients
Billing records identified 229 total patients who received phototherapy in 2015, of whom 52 (22.7%) were at least 65 years old. The median age was 70 years (range, 65–91 years). Twenty-nine (56%) were men and 35 (67%) had previously received phototherapy treatments.
Dermatologic Conditions Treated With Phototherapy
Our primary aim was to identify the most common dermatologic conditions treated with phototherapy in older adults. Psoriasis and dermatitis were the most common conditions treated in the sample (50% [26/52] and 21% [11/52], respectively), with mycosis fungoides being the third most common (10% [5/52]) and vitiligo tied with prurigo nodularis as fourth most common (6% [3/52])(Figure 1).

Effectiveness and Safety of Phototherapy
Our secondary aim was to examine the effectiveness and safety of phototherapy in older adults. Phototherapy was effective in this population, with 50 of 52 patients (96%) achieving a high or medium level of clearance. The degree of clearance for each of the dermatologic conditions is shown in Figure 2. Psoriasis and dermatitis achieved high clearance rates in 81% (21/26) and 82% (9/11) of patients, respectively. Overall, conditions did not have significant differences in clearances rates (Fisher exact test, P=.10). On average, it took patients 33 treatments to achieve medium or high rates of clearance. Psoriasis cleared more quickly, with an average of 30.4 treatments vs 36.1 treatments for other conditions, but the difference was not significant (t test, P=.26). Patients received an average of 98 total phototherapy treatments; the median number of treatments was 81 due to many being on maintenance therapy over several months. There was no relationship between a history of treatment with phototherapy and the total number of treatments needed to achieve clearance (t test, P=.40), but interestingly, those who had a history of phototherapy took approximately 5 more treatments to achieve clearance. The present study found that a slightly larger number of men were being treated for psoriasis (15 men vs 11 women), but there was no significant difference in response rate based on gender.

Side effects from phototherapy were minimal; 24 patients (46%) experienced grade 1 (mild) erythema at some point during their treatment course. Thirteen (25%) patients experienced grade 2 erythema, but this was a rare event for most patients. Only 1 (2%) patient experienced grade 3 erythema 1 time. Three patients experienced increased itching (6%). Thirteen (25%) patients had no side effects. None developed severe erythema or blisters, and none discontinued phototherapy because of side effects. Over the course of the study year, we found a high degree of acceptance of phototherapy treatments by older patients: 22 (42%) completed therapy after achieving clearance, 10 (19%) were continuing ongoing treatments (maintenance), and 15 (29%) stopped because of life circumstances (eg, other health issues, moving out of the area). Only 4 (8%) stopped because of a lack of effectiveness, and 1 (2%) patient because the treatments were burdensome.
Comparison of Outcomes
Our third aim was to compare the outcomes with similar studies in the United Kingdom16 and Turkey.17 This study confirmed that phototherapy is being used in older adults (22.7% of this study’s total patients) and is an effective treatment for older patients experiencing a range of challenging inflammatory and proliferative skin diseases similar to studies in the general population. Prior phototherapy studies in elderly patients also found psoriasis to be the most common skin condition treated, with 1 study finding that 51% (19/37) of older phototherapy patients had psoriasis,16 while another reported 58% (37/95) of older phototherapy patients had psoriasis.17 These numbers are similar to those in our study, which showed 50% (26/52) of elderly phototherapy patients had psoriasis. Psoriasis is the main indication for treatment with NB-UVB phototherapy in the general population,19 and because the risk for psoriasis increases with age,20 it is not surprising that all 3 studies found psoriasis to be the most common indication in elderly phototherapy patients. Table 3 provides further details on conditions treated in all 3 studies.

Comment
Our study found that 94% of patients with psoriasis achieved clearance with an average of 30.4 treatments, which is comparable to the reported 91% response rate with an average of 30 treatments in the United Kingdom.16 The other similar study in Turkey17 reported 73.7% of psoriasis patients achieved a 75% or more improvement from baseline with an average of 42 treatments, which may reflect underlying differences in regional skin type. Of note, the scatter chart (Figure 3) shows that several patients in the present study’s analysis are listed as not clear, but many of those patients had low treatment numbers below the mean time to clearance. Thus, the present study’s response rate may have been underestimated.

In the general population, studies show that psoriasis treated with standardized phototherapy protocols typically clears with an average of 20.6 treatments.21 The levels of clearance were similar in our study’s older population, but more treatments were required to achieve those results, with an average of 10 more treatments needed (an additional 3.3 weeks). Similar results were found in this sample for dermatitis and mycosis fungoides, indicating comparable clearance rates and levels but a need for more treatments to achieve similar results compared to the general population.
Additionally, in the current study more patients experienced grade 1 (mild) erythema (46%) and grade 2 erythema (25%) at some point in their treatment compared with the United Kingdom16 (1.89%) and Turkey17 (35%) studies, though these side effects did not impact the clearance rate. Interestingly, the current study’s scatter chart (Figure 3) illustrates that this side effect did not seem to increase with aging in this population. If anything, the erythema response was more prevalent in the median or younger patients in the sample. Erythema may have been due to the frequent use of photosensitizing medications in older adults in the United States, some of which typically get discontinued in patients 75 years and older (eg, statins). Other potential causes might include the use of phototype vs minimal erythema dose–driven protocols, the standard utilization of protocols originally designed for psoriasis vs other condition-specific protocols, missed treatments leading to increased sensitivity, or possibly shielding mishaps (eg, not wearing a prescribed face shield). Given the number of potential causes and the possibility of overlapping factors, careful analysis is important. With NB-UVB phototherapy, near-erythemogenic doses are optimal to achieve effective treatments, but this delicate balance may be more problematic for older adults. Future studies are needed to fully determine the factors at play for this population. In the interim, it is important for phototherapy-trained nurses to consider this risk carefully in the older population. They must follow the prescribed protocols that guide them to query patients about their responses to the prior treatment (eg, erythema, tenderness, itching), photosensitizing medications, missed treatments, and placement of shielding, and then adjust the treatment dosing accordingly.
Limitations
This study had several limitations. Although clinical outcomes were recorded prospectively, the analysis was retrospective, unblinded, and not placebo controlled. It was conducted in a single organization (Group Health [now Kaiser Permanente Washington]) but did analyze data from 4 medical centers in different cities with diverse demographics and a variety of nursing staff providing the treatments. Although the vitiligo treatment protocol likely slowed the response rate for those patients with vitiligo, the numbers were small (ie, only 3 of 52 patients), so the researchers chose to include them in the current study. The sample population was relatively small, but when these data are evaluated alongside the studies in the United Kingdom16 and Turkey,17 they show a consistent picture illustrating the effectiveness and safety of phototherapy in the older population. Further epidemiologic studies could be helpful to further describe the usefulness of this modality compared with other treatments for a variety of dermatoses in this age group. Supplementary analysis specifically examining the relationship between the number and type of photosensitizing medications, frequency of erythema, and time to clearance also could be useful.
Conclusion
Older adults with a variety of dermatoses respond well to phototherapy and should have the opportunity to use it, particularly considering the potential for increased complications and costs from other treatment modalities, such as commonly used immunosuppressive pharmaceuticals. However, the current study and the comparison studies indicate that it is important to carefully consider the slower clearance rates and the potential risk for increased erythema in this population and adjust patient education and treatment dosing accordingly.
Unfortunately, many dermatology centers do not offer phototherapy because of infrastructure limitations such as space and specially trained nursing staff. Increasing accessibility of phototherapy for older adults through home treatments may be an alternative, given its effectiveness in the general population.22,23 In addition, home phototherapy may be worth pursuing for the older population considering the challenges they may face with transportation to the clinic setting and their increased risk for serious illness if exposed to infections such as COVID-19. The COVID-19 pandemic has brought to light the need for reliable, safe, and effective treatments that can be utilized in the safety of patients’ homes and should therefore be considered as an option for older adults. Issues such as mobility and cognitive decline could pose some complicating factors, but with the help of a well-trained family member or caregiver, home phototherapy could be a viable option that improves accessibility for older patients. Future research opportunities include further examination of the slower but ultimately equivalent response to phototherapy in the older population, the influence of photosensitizing medications on phototherapy effects, and the impact of phototherapy on utilization of immunosuppressive pharmaceuticals in older adults.
Identifying safe, effective, and affordable evidence-based dermatologic treatments for older adults can be challenging because of age-related changes in the skin, comorbidities, polypharmacy, mobility issues, and cognitive changes. Phototherapy has been shown to be an effective nonpharmacologic treatment option for multiple challenging dermatologic conditions1-8; however, few studies have specifically examined its effectiveness in older adults. The challenge for older patients with psoriasis and dermatitis is that the conditions can be difficult to control and often require multiple treatment modalities.9,10 Patients with psoriasis also have a higher risk for diabetes, dyslipidemia, and cardiovascular disease compared to other older patients,11,12 which poses treatment challenges and makes nonpharmacologic treatments even more appealing.
Recent studies show that phototherapy can help decrease the use of dermatologic medications. Foerster and colleagues2 found that adults with psoriasis who were treated with phototherapy significantly decreased their use of topical steroids (24.5% fewer patients required steroid creams and 31.1% fewer patients required psoriasis-specific topicals)(P<.01) while their use of non–psoriasis-specific medications did not change. Click and colleagues13 identified a decrease in medication costs, health care utilization, and risk for immunosuppression in patients treated with phototherapy when compared to those treated with biologics and apremilast. Methotrexate is a common dermatologic medication that is highly associated with increased risks in elderly patients because of impaired immune system function and the presence of comorbidities (eg, kidney disease, obesity, diabetes, fatty liver),14 which increase in prevalence with age. Combining phototherapy with methotrexate can substantially decrease the amount of methotrexate needed to achieve disease control,15 thereby decreasing the methotrexate-associated risks. Findings from these studies suggest that a safe, effective, cost-effective, and well-tolerated nonpharmacologic alternative, such as phototherapy, is highly desirable and should be optimized. Unfortunately, most studies that report the effectiveness of phototherapy are in younger populations.
This retrospective study aimed to (1) identify the most common dermatologic conditions treated with phototherapy in older adults, (2) examine the effectiveness and safety of phototherapy in older adults
Methods
Design, Setting, Sample, and Statistical Analysis
The institutional review boards of Kaiser Permanente Washington Health Research Institute, Seattle, and the University of Washington, Seattle, approved this study. It was conducted in a large US multispecialty health care system (Group Health, Seattle, Washington [now Kaiser Permanente Washington]) serving approximately 600,000 patients, using billing records to identify all patients treated with phototherapy between January 1, 2015, and December 31, 2015, all who received narrowband UVB (NB-UVB) phototherapy. All adults 65 years and older who received phototherapy treatment during the 12-month study period were included. Patients were included regardless of comorbidities and other dermatologic treatments to maintain as much uniformity as possible between the present study and 2 prior studies examining phototherapy in older adult populations in the United Kingdom16 and Turkey.17 Demographic and clinical factors were presented using frequencies (percentages) or means and medians as appropriate. Comparisons of dermatologic conditions and clearance levels used a Fisher exact test. The number of phototherapy treatments to clearance and total number of treatments were compared between groups of patients using independent sample t tests.
Phototherapy Protocol
All patients received treatments administered by specially trained phototherapy nurses using a Daavlin UV Series (The Daavlin Company) or an Ultralite unit (Ultralite Enterprises, Inc), both with 48 lamps. All phototherapy nurses had been previously trained to provide treatments based on standardized protocols (Table 1) and to determine the patient’s level of disease clearance using a high to low clearance scale (Table 2). Daavlin’s treatment protocols were built into the software that accompanied the units and were developed based on the American Academy of Dermatology guidelines. The starting dose for an individual patient was determined based on the estimated


Results
Patients
Billing records identified 229 total patients who received phototherapy in 2015, of whom 52 (22.7%) were at least 65 years old. The median age was 70 years (range, 65–91 years). Twenty-nine (56%) were men and 35 (67%) had previously received phototherapy treatments.
Dermatologic Conditions Treated With Phototherapy
Our primary aim was to identify the most common dermatologic conditions treated with phototherapy in older adults. Psoriasis and dermatitis were the most common conditions treated in the sample (50% [26/52] and 21% [11/52], respectively), with mycosis fungoides being the third most common (10% [5/52]) and vitiligo tied with prurigo nodularis as fourth most common (6% [3/52])(Figure 1).

Effectiveness and Safety of Phototherapy
Our secondary aim was to examine the effectiveness and safety of phototherapy in older adults. Phototherapy was effective in this population, with 50 of 52 patients (96%) achieving a high or medium level of clearance. The degree of clearance for each of the dermatologic conditions is shown in Figure 2. Psoriasis and dermatitis achieved high clearance rates in 81% (21/26) and 82% (9/11) of patients, respectively. Overall, conditions did not have significant differences in clearances rates (Fisher exact test, P=.10). On average, it took patients 33 treatments to achieve medium or high rates of clearance. Psoriasis cleared more quickly, with an average of 30.4 treatments vs 36.1 treatments for other conditions, but the difference was not significant (t test, P=.26). Patients received an average of 98 total phototherapy treatments; the median number of treatments was 81 due to many being on maintenance therapy over several months. There was no relationship between a history of treatment with phototherapy and the total number of treatments needed to achieve clearance (t test, P=.40), but interestingly, those who had a history of phototherapy took approximately 5 more treatments to achieve clearance. The present study found that a slightly larger number of men were being treated for psoriasis (15 men vs 11 women), but there was no significant difference in response rate based on gender.

Side effects from phototherapy were minimal; 24 patients (46%) experienced grade 1 (mild) erythema at some point during their treatment course. Thirteen (25%) patients experienced grade 2 erythema, but this was a rare event for most patients. Only 1 (2%) patient experienced grade 3 erythema 1 time. Three patients experienced increased itching (6%). Thirteen (25%) patients had no side effects. None developed severe erythema or blisters, and none discontinued phototherapy because of side effects. Over the course of the study year, we found a high degree of acceptance of phototherapy treatments by older patients: 22 (42%) completed therapy after achieving clearance, 10 (19%) were continuing ongoing treatments (maintenance), and 15 (29%) stopped because of life circumstances (eg, other health issues, moving out of the area). Only 4 (8%) stopped because of a lack of effectiveness, and 1 (2%) patient because the treatments were burdensome.
Comparison of Outcomes
Our third aim was to compare the outcomes with similar studies in the United Kingdom16 and Turkey.17 This study confirmed that phototherapy is being used in older adults (22.7% of this study’s total patients) and is an effective treatment for older patients experiencing a range of challenging inflammatory and proliferative skin diseases similar to studies in the general population. Prior phototherapy studies in elderly patients also found psoriasis to be the most common skin condition treated, with 1 study finding that 51% (19/37) of older phototherapy patients had psoriasis,16 while another reported 58% (37/95) of older phototherapy patients had psoriasis.17 These numbers are similar to those in our study, which showed 50% (26/52) of elderly phototherapy patients had psoriasis. Psoriasis is the main indication for treatment with NB-UVB phototherapy in the general population,19 and because the risk for psoriasis increases with age,20 it is not surprising that all 3 studies found psoriasis to be the most common indication in elderly phototherapy patients. Table 3 provides further details on conditions treated in all 3 studies.

Comment
Our study found that 94% of patients with psoriasis achieved clearance with an average of 30.4 treatments, which is comparable to the reported 91% response rate with an average of 30 treatments in the United Kingdom.16 The other similar study in Turkey17 reported 73.7% of psoriasis patients achieved a 75% or more improvement from baseline with an average of 42 treatments, which may reflect underlying differences in regional skin type. Of note, the scatter chart (Figure 3) shows that several patients in the present study’s analysis are listed as not clear, but many of those patients had low treatment numbers below the mean time to clearance. Thus, the present study’s response rate may have been underestimated.

In the general population, studies show that psoriasis treated with standardized phototherapy protocols typically clears with an average of 20.6 treatments.21 The levels of clearance were similar in our study’s older population, but more treatments were required to achieve those results, with an average of 10 more treatments needed (an additional 3.3 weeks). Similar results were found in this sample for dermatitis and mycosis fungoides, indicating comparable clearance rates and levels but a need for more treatments to achieve similar results compared to the general population.
Additionally, in the current study more patients experienced grade 1 (mild) erythema (46%) and grade 2 erythema (25%) at some point in their treatment compared with the United Kingdom16 (1.89%) and Turkey17 (35%) studies, though these side effects did not impact the clearance rate. Interestingly, the current study’s scatter chart (Figure 3) illustrates that this side effect did not seem to increase with aging in this population. If anything, the erythema response was more prevalent in the median or younger patients in the sample. Erythema may have been due to the frequent use of photosensitizing medications in older adults in the United States, some of which typically get discontinued in patients 75 years and older (eg, statins). Other potential causes might include the use of phototype vs minimal erythema dose–driven protocols, the standard utilization of protocols originally designed for psoriasis vs other condition-specific protocols, missed treatments leading to increased sensitivity, or possibly shielding mishaps (eg, not wearing a prescribed face shield). Given the number of potential causes and the possibility of overlapping factors, careful analysis is important. With NB-UVB phototherapy, near-erythemogenic doses are optimal to achieve effective treatments, but this delicate balance may be more problematic for older adults. Future studies are needed to fully determine the factors at play for this population. In the interim, it is important for phototherapy-trained nurses to consider this risk carefully in the older population. They must follow the prescribed protocols that guide them to query patients about their responses to the prior treatment (eg, erythema, tenderness, itching), photosensitizing medications, missed treatments, and placement of shielding, and then adjust the treatment dosing accordingly.
Limitations
This study had several limitations. Although clinical outcomes were recorded prospectively, the analysis was retrospective, unblinded, and not placebo controlled. It was conducted in a single organization (Group Health [now Kaiser Permanente Washington]) but did analyze data from 4 medical centers in different cities with diverse demographics and a variety of nursing staff providing the treatments. Although the vitiligo treatment protocol likely slowed the response rate for those patients with vitiligo, the numbers were small (ie, only 3 of 52 patients), so the researchers chose to include them in the current study. The sample population was relatively small, but when these data are evaluated alongside the studies in the United Kingdom16 and Turkey,17 they show a consistent picture illustrating the effectiveness and safety of phototherapy in the older population. Further epidemiologic studies could be helpful to further describe the usefulness of this modality compared with other treatments for a variety of dermatoses in this age group. Supplementary analysis specifically examining the relationship between the number and type of photosensitizing medications, frequency of erythema, and time to clearance also could be useful.
Conclusion
Older adults with a variety of dermatoses respond well to phototherapy and should have the opportunity to use it, particularly considering the potential for increased complications and costs from other treatment modalities, such as commonly used immunosuppressive pharmaceuticals. However, the current study and the comparison studies indicate that it is important to carefully consider the slower clearance rates and the potential risk for increased erythema in this population and adjust patient education and treatment dosing accordingly.
Unfortunately, many dermatology centers do not offer phototherapy because of infrastructure limitations such as space and specially trained nursing staff. Increasing accessibility of phototherapy for older adults through home treatments may be an alternative, given its effectiveness in the general population.22,23 In addition, home phototherapy may be worth pursuing for the older population considering the challenges they may face with transportation to the clinic setting and their increased risk for serious illness if exposed to infections such as COVID-19. The COVID-19 pandemic has brought to light the need for reliable, safe, and effective treatments that can be utilized in the safety of patients’ homes and should therefore be considered as an option for older adults. Issues such as mobility and cognitive decline could pose some complicating factors, but with the help of a well-trained family member or caregiver, home phototherapy could be a viable option that improves accessibility for older patients. Future research opportunities include further examination of the slower but ultimately equivalent response to phototherapy in the older population, the influence of photosensitizing medications on phototherapy effects, and the impact of phototherapy on utilization of immunosuppressive pharmaceuticals in older adults.
- British Photodermatology Group. An appraisal of narrowband (TL-01) UVB phototherapy. British Photodermatology Group Workshop Report (April 1996). Br J Dermatol. 1997;137:327-330.
Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS ONE. 2017;12:e0181813. doi:10.1371/journal.pone.0181813 - Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2015;29:19-23.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593. - Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Martin JA, Laube S, Edwards C, et al. Rate of acute adverse events for narrow-band UVB and psoralen-UVA phototherapy. Photodermatol Photoimmunol Photomed. 2007;23:68-72.
- Mokos ZB, Jovic A, Ceovic R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Exp Opin Biol Ther. 2018;18:897-903.
- Napolitano M, Balato N, Ayala F, et al. Psoriasis in elderly and non-elderly population: clinical and molecular features. G Ital Dermatol Venereol. 2016;151:587-595.
- Grozdev IS, Van Voorhees AS, Gottlieb AB, et al. Psoriasis in the elderly: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2011;65:537-545.
- Click J, Alabaster A, Postlethwaite D, et al. Effect of availability of at-home phototherapy on the use of systemic medications for psoriasis.
Photodermatol Photoimmunol Photomed. 2017;33:345-346. - Piaserico S, Conti A, Lo Console F, et al.
Efficacy and safety of systemic treatments for psoriasis in elderly. Acta Derm Venereol. 2014;94:293-297. - Soliman A, Nofal E, Nofal A, et al. Combination therapy of methotrexate plus NB-UVB phototherapy is more effective than methotrexate monotherapy in the treatment of chronic plaque psoriasis. J Dermatol Treat. 2015;26:528-534.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Madigan LM, Al-Jamal M, Hamzavi I. Exploring the gaps in the evidence-based application of narrowband UVB for the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2016;32:66-80.
- Ibbotson SH. A perspective on the use of NB-UVB phototherapy vs. PUVA photochemotherapy. Front Med (Lausanne). 2018;5:184.
- Bell LM, Sedlack R, Beard CM, et al. Incidence of psoriasis in Rochester, Minn, 1980-1983. Arch Dermatol. 1991;127:1184-1187.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413.
- Cameron H, Yule S, Dawe RS, et al. Review of an established UK home phototherapy service 1998-2011: improving access to a cost-effective treatment for chronic skin disease. Public Health. 2014;128:317-324.
- Matthews SW, Simmer M, Williams L, et al. Transition of patients with psoriasis from office-based phototherapy to nurse-supported home phototherapy: a pilot study. JDNA. 2018;10:29-41.
- British Photodermatology Group. An appraisal of narrowband (TL-01) UVB phototherapy. British Photodermatology Group Workshop Report (April 1996). Br J Dermatol. 1997;137:327-330.
Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS ONE. 2017;12:e0181813. doi:10.1371/journal.pone.0181813 - Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2015;29:19-23.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593. - Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Martin JA, Laube S, Edwards C, et al. Rate of acute adverse events for narrow-band UVB and psoralen-UVA phototherapy. Photodermatol Photoimmunol Photomed. 2007;23:68-72.
- Mokos ZB, Jovic A, Ceovic R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Exp Opin Biol Ther. 2018;18:897-903.
- Napolitano M, Balato N, Ayala F, et al. Psoriasis in elderly and non-elderly population: clinical and molecular features. G Ital Dermatol Venereol. 2016;151:587-595.
- Grozdev IS, Van Voorhees AS, Gottlieb AB, et al. Psoriasis in the elderly: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2011;65:537-545.
- Click J, Alabaster A, Postlethwaite D, et al. Effect of availability of at-home phototherapy on the use of systemic medications for psoriasis.
Photodermatol Photoimmunol Photomed. 2017;33:345-346. - Piaserico S, Conti A, Lo Console F, et al.
Efficacy and safety of systemic treatments for psoriasis in elderly. Acta Derm Venereol. 2014;94:293-297. - Soliman A, Nofal E, Nofal A, et al. Combination therapy of methotrexate plus NB-UVB phototherapy is more effective than methotrexate monotherapy in the treatment of chronic plaque psoriasis. J Dermatol Treat. 2015;26:528-534.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Madigan LM, Al-Jamal M, Hamzavi I. Exploring the gaps in the evidence-based application of narrowband UVB for the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2016;32:66-80.
- Ibbotson SH. A perspective on the use of NB-UVB phototherapy vs. PUVA photochemotherapy. Front Med (Lausanne). 2018;5:184.
- Bell LM, Sedlack R, Beard CM, et al. Incidence of psoriasis in Rochester, Minn, 1980-1983. Arch Dermatol. 1991;127:1184-1187.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413.
- Cameron H, Yule S, Dawe RS, et al. Review of an established UK home phototherapy service 1998-2011: improving access to a cost-effective treatment for chronic skin disease. Public Health. 2014;128:317-324.
- Matthews SW, Simmer M, Williams L, et al. Transition of patients with psoriasis from office-based phototherapy to nurse-supported home phototherapy: a pilot study. JDNA. 2018;10:29-41.
Practice Points
- With appropriate nursing care, phototherapy can be safe and effective for a variety of conditions in elderly patients.
- Compared to younger patients, elderly patients may need more sessions to achieve comparable clearance rates.
- The increased prevalence of photosensitizing medications in the elderly population will require careful adjustments in dosing.
CDC calls for masks in schools, hard-hit areas, even if vaccinated
The agency has called for masks in K-12 school settings and in areas of the United States experiencing high or substantial SARS-CoV-2 transmission, even for the fully vaccinated.
The move reverses a controversial announcement the agency made in May 2021 that fully vaccinated Americans could skip wearing a mask in most settings.
Unlike the increasing vaccination rates and decreasing case numbers reported in May, however, some regions of the United States are now reporting large jumps in COVID-19 case numbers. And the Delta variant as well as new evidence of transmission from breakthrough cases are largely driving these changes.
“Today we have new science related to the [D]elta variant that requires us to update the guidance on what you can do when you are fully vaccinated,” CDC Director Rochelle Walensky, MD, MPH, said during a media briefing July 27.
New evidence has emerged on breakthrough-case transmission risk, for example. “Information on the [D]elta variant from several states and other countries indicates that in rare cases, some people infected with the [D]elta variant after vaccination may be contagious and spread virus to others,” Dr. Walensky said, adding that the viral loads appear to be about the same in vaccinated and unvaccinated individuals.
“This new science is worrisome,” she said.
Even though unvaccinated people represent the vast majority of cases of transmission, Dr. Walensky said, “we thought it was important for [vaccinated] people to understand they have the potential to transmit the virus to others.”
As a result, in addition to continuing to strongly encourage everyone to get vaccinated, the CDC recommends that fully vaccinated people wear masks in public indoor settings to help prevent the spread of the Delta variant in areas with substantial or high transmission, Dr. Walensky said. “This includes schools.”
Masks in schools
The CDC is now recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status. Their goal is to optimize safety and allow children to return to full-time in-person learning in the fall.
The CDC tracks substantial and high transmission rates through the agency’s COVID Data Tracker site. Substantial transmission means between 50 and 100 cases per 100,000 people reported over 7 days and high means more than 100 cases per 100,000 people.
The B.1.617.2, or Delta, variant is believed to be responsible for COVID-19 cases increasing more than 300% nationally from June 19 to July 23, 2021.
“A prudent move”
“I think it’s a prudent move. Given the dominance of the [D]elta variant and the caseloads that we are seeing rising in many locations across the United States, including in my backyard here in San Francisco,” Joe DeRisi, PhD, copresident of the Chan Zuckerberg Biohub and professor of biochemistry and biophysics at the University of California San Francisco, said in an interview.
Dr. DeRisi said he was not surprised that vaccinated people with breakthrough infections could be capable of transmitting the virus. He added that clinical testing done by the Biohub and UCSF produced a lot of data on viral load levels, “and they cover an enormous range.”
What was unexpected to him was the rapid rise of the dominant variant. “The rise of the [D]elta strain is astonishing. It’s happened so fast,” he said.
“I know it’s difficult”
Reacting to the news, Colleen Kraft, MD, said, “One of the things that we’re learning is that if we’re going to have low vaccine uptake or we have a number of people that can’t be vaccinated yet, such as children, that we really need to go back to stopping transmission, which involves mask wearing.”
“I know that it’s very difficult and people feel like we’re sliding backward,” Dr. Kraft said during a media briefing sponsored by Emory University held shortly after the CDC announcement.
She added that the CDC updated guidance seems appropriate. “I don’t think any of us really want to be in this position or want to go back to masking but…we’re finding ourselves in the same place we were a year ago, in July 2020.
“In general we just don’t want anybody to be infected even if there’s a small chance for you to be infected and there’s a small chance for you to transmit it,” said Dr. Kraft, who’s an assistant professor in the department of pathology and associate professor in the department of medicine, division of infectious diseases at Emory University School of Medicine in Atlanta.
Breakthrough transmissions
“The good news is you’re still unlikely to get critically ill if you’re vaccinated. But what has changed with the [D]elta variant is instead of being 90% plus protected from getting the virus at all, you’re probably more in the 70% to 80% range,” James T. McDeavitt, MD, told this news organization.
“So we’re seeing breakthrough infections,” said Dr. McDeavitt, executive vice president and dean of clinical affairs at Baylor College of Medicine in Houston. “We are starting to see [such people] are potentially infectious.” Even if a vaccinated person is individually much less likely to experience serious COVID-19 outcomes, “they can spread it to someone else who spreads it to someone else who is more vulnerable. It puts the more at-risk populations at further risk.”
It breaks down to individual and public health concerns. “I am fully vaccinated. I am very confident I am not going to end up in a hospital,” he said. “Now if I were unvaccinated, with the prevalence of the virus around the country, I’m probably in more danger than I’ve ever been in the course of the pandemic. The unvaccinated are really at risk right now.”
IDSA and AMA support mask change
The Infectious Diseases Society of America (IDSA) has released a statement supporting the new CDC recommendations. “To stay ahead of the spread of the highly transmissible Delta variant, IDSA also urges that in communities with moderate transmission rates, all individuals, even those who are vaccinated, wear masks in indoor public places,” stated IDSA President Barbara D. Alexander, MD, MHS.
“IDSA also supports CDC’s guidance recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status, until vaccines are authorized and widely available to all children and vaccination rates are sufficient to control transmission.”
“Mask wearing will help reduce infections, prevent serious illnesses and death, limit strain on local hospitals and stave off the development of even more troubling variants,” she added.
The American Medical Association (AMA) also released a statement supporting the CDC’s policy changes.
“According to the CDC, emerging data indicates that vaccinated individuals infected with the Delta variant have similar viral loads as those who are unvaccinated and are capable of transmission,” AMA President Gerald E. Harmon, MD said in the statement.
“However, the science remains clear, the authorized vaccines remain safe and effective in preventing severe complications from COVID-19, including hospitalization and death,” he stated. “We strongly support the updated recommendations, which call for universal masking in areas of high or substantial COVID-19 transmission and in K-12 schools, to help reduce transmission of the virus. Wearing a mask is a small but important protective measure that can help us all stay safer.”
“The highest spread of cases and [most] severe outcomes are happening in places with low vaccination rates and among unvaccinated people,” Dr. Walensky said. “With the [D]elta variant, vaccinating more Americans now is more urgent than ever.”
“This moment, and the associated suffering, illness, and death, could have been avoided with higher vaccination coverage in this country,” she said.
A version of this article first appeared on Medscape.com.
The agency has called for masks in K-12 school settings and in areas of the United States experiencing high or substantial SARS-CoV-2 transmission, even for the fully vaccinated.
The move reverses a controversial announcement the agency made in May 2021 that fully vaccinated Americans could skip wearing a mask in most settings.
Unlike the increasing vaccination rates and decreasing case numbers reported in May, however, some regions of the United States are now reporting large jumps in COVID-19 case numbers. And the Delta variant as well as new evidence of transmission from breakthrough cases are largely driving these changes.
“Today we have new science related to the [D]elta variant that requires us to update the guidance on what you can do when you are fully vaccinated,” CDC Director Rochelle Walensky, MD, MPH, said during a media briefing July 27.
New evidence has emerged on breakthrough-case transmission risk, for example. “Information on the [D]elta variant from several states and other countries indicates that in rare cases, some people infected with the [D]elta variant after vaccination may be contagious and spread virus to others,” Dr. Walensky said, adding that the viral loads appear to be about the same in vaccinated and unvaccinated individuals.
“This new science is worrisome,” she said.
Even though unvaccinated people represent the vast majority of cases of transmission, Dr. Walensky said, “we thought it was important for [vaccinated] people to understand they have the potential to transmit the virus to others.”
As a result, in addition to continuing to strongly encourage everyone to get vaccinated, the CDC recommends that fully vaccinated people wear masks in public indoor settings to help prevent the spread of the Delta variant in areas with substantial or high transmission, Dr. Walensky said. “This includes schools.”
Masks in schools
The CDC is now recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status. Their goal is to optimize safety and allow children to return to full-time in-person learning in the fall.
The CDC tracks substantial and high transmission rates through the agency’s COVID Data Tracker site. Substantial transmission means between 50 and 100 cases per 100,000 people reported over 7 days and high means more than 100 cases per 100,000 people.
The B.1.617.2, or Delta, variant is believed to be responsible for COVID-19 cases increasing more than 300% nationally from June 19 to July 23, 2021.
“A prudent move”
“I think it’s a prudent move. Given the dominance of the [D]elta variant and the caseloads that we are seeing rising in many locations across the United States, including in my backyard here in San Francisco,” Joe DeRisi, PhD, copresident of the Chan Zuckerberg Biohub and professor of biochemistry and biophysics at the University of California San Francisco, said in an interview.
Dr. DeRisi said he was not surprised that vaccinated people with breakthrough infections could be capable of transmitting the virus. He added that clinical testing done by the Biohub and UCSF produced a lot of data on viral load levels, “and they cover an enormous range.”
What was unexpected to him was the rapid rise of the dominant variant. “The rise of the [D]elta strain is astonishing. It’s happened so fast,” he said.
“I know it’s difficult”
Reacting to the news, Colleen Kraft, MD, said, “One of the things that we’re learning is that if we’re going to have low vaccine uptake or we have a number of people that can’t be vaccinated yet, such as children, that we really need to go back to stopping transmission, which involves mask wearing.”
“I know that it’s very difficult and people feel like we’re sliding backward,” Dr. Kraft said during a media briefing sponsored by Emory University held shortly after the CDC announcement.
She added that the CDC updated guidance seems appropriate. “I don’t think any of us really want to be in this position or want to go back to masking but…we’re finding ourselves in the same place we were a year ago, in July 2020.
“In general we just don’t want anybody to be infected even if there’s a small chance for you to be infected and there’s a small chance for you to transmit it,” said Dr. Kraft, who’s an assistant professor in the department of pathology and associate professor in the department of medicine, division of infectious diseases at Emory University School of Medicine in Atlanta.
Breakthrough transmissions
“The good news is you’re still unlikely to get critically ill if you’re vaccinated. But what has changed with the [D]elta variant is instead of being 90% plus protected from getting the virus at all, you’re probably more in the 70% to 80% range,” James T. McDeavitt, MD, told this news organization.
“So we’re seeing breakthrough infections,” said Dr. McDeavitt, executive vice president and dean of clinical affairs at Baylor College of Medicine in Houston. “We are starting to see [such people] are potentially infectious.” Even if a vaccinated person is individually much less likely to experience serious COVID-19 outcomes, “they can spread it to someone else who spreads it to someone else who is more vulnerable. It puts the more at-risk populations at further risk.”
It breaks down to individual and public health concerns. “I am fully vaccinated. I am very confident I am not going to end up in a hospital,” he said. “Now if I were unvaccinated, with the prevalence of the virus around the country, I’m probably in more danger than I’ve ever been in the course of the pandemic. The unvaccinated are really at risk right now.”
IDSA and AMA support mask change
The Infectious Diseases Society of America (IDSA) has released a statement supporting the new CDC recommendations. “To stay ahead of the spread of the highly transmissible Delta variant, IDSA also urges that in communities with moderate transmission rates, all individuals, even those who are vaccinated, wear masks in indoor public places,” stated IDSA President Barbara D. Alexander, MD, MHS.
“IDSA also supports CDC’s guidance recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status, until vaccines are authorized and widely available to all children and vaccination rates are sufficient to control transmission.”
“Mask wearing will help reduce infections, prevent serious illnesses and death, limit strain on local hospitals and stave off the development of even more troubling variants,” she added.
The American Medical Association (AMA) also released a statement supporting the CDC’s policy changes.
“According to the CDC, emerging data indicates that vaccinated individuals infected with the Delta variant have similar viral loads as those who are unvaccinated and are capable of transmission,” AMA President Gerald E. Harmon, MD said in the statement.
“However, the science remains clear, the authorized vaccines remain safe and effective in preventing severe complications from COVID-19, including hospitalization and death,” he stated. “We strongly support the updated recommendations, which call for universal masking in areas of high or substantial COVID-19 transmission and in K-12 schools, to help reduce transmission of the virus. Wearing a mask is a small but important protective measure that can help us all stay safer.”
“The highest spread of cases and [most] severe outcomes are happening in places with low vaccination rates and among unvaccinated people,” Dr. Walensky said. “With the [D]elta variant, vaccinating more Americans now is more urgent than ever.”
“This moment, and the associated suffering, illness, and death, could have been avoided with higher vaccination coverage in this country,” she said.
A version of this article first appeared on Medscape.com.
The agency has called for masks in K-12 school settings and in areas of the United States experiencing high or substantial SARS-CoV-2 transmission, even for the fully vaccinated.
The move reverses a controversial announcement the agency made in May 2021 that fully vaccinated Americans could skip wearing a mask in most settings.
Unlike the increasing vaccination rates and decreasing case numbers reported in May, however, some regions of the United States are now reporting large jumps in COVID-19 case numbers. And the Delta variant as well as new evidence of transmission from breakthrough cases are largely driving these changes.
“Today we have new science related to the [D]elta variant that requires us to update the guidance on what you can do when you are fully vaccinated,” CDC Director Rochelle Walensky, MD, MPH, said during a media briefing July 27.
New evidence has emerged on breakthrough-case transmission risk, for example. “Information on the [D]elta variant from several states and other countries indicates that in rare cases, some people infected with the [D]elta variant after vaccination may be contagious and spread virus to others,” Dr. Walensky said, adding that the viral loads appear to be about the same in vaccinated and unvaccinated individuals.
“This new science is worrisome,” she said.
Even though unvaccinated people represent the vast majority of cases of transmission, Dr. Walensky said, “we thought it was important for [vaccinated] people to understand they have the potential to transmit the virus to others.”
As a result, in addition to continuing to strongly encourage everyone to get vaccinated, the CDC recommends that fully vaccinated people wear masks in public indoor settings to help prevent the spread of the Delta variant in areas with substantial or high transmission, Dr. Walensky said. “This includes schools.”
Masks in schools
The CDC is now recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status. Their goal is to optimize safety and allow children to return to full-time in-person learning in the fall.
The CDC tracks substantial and high transmission rates through the agency’s COVID Data Tracker site. Substantial transmission means between 50 and 100 cases per 100,000 people reported over 7 days and high means more than 100 cases per 100,000 people.
The B.1.617.2, or Delta, variant is believed to be responsible for COVID-19 cases increasing more than 300% nationally from June 19 to July 23, 2021.
“A prudent move”
“I think it’s a prudent move. Given the dominance of the [D]elta variant and the caseloads that we are seeing rising in many locations across the United States, including in my backyard here in San Francisco,” Joe DeRisi, PhD, copresident of the Chan Zuckerberg Biohub and professor of biochemistry and biophysics at the University of California San Francisco, said in an interview.
Dr. DeRisi said he was not surprised that vaccinated people with breakthrough infections could be capable of transmitting the virus. He added that clinical testing done by the Biohub and UCSF produced a lot of data on viral load levels, “and they cover an enormous range.”
What was unexpected to him was the rapid rise of the dominant variant. “The rise of the [D]elta strain is astonishing. It’s happened so fast,” he said.
“I know it’s difficult”
Reacting to the news, Colleen Kraft, MD, said, “One of the things that we’re learning is that if we’re going to have low vaccine uptake or we have a number of people that can’t be vaccinated yet, such as children, that we really need to go back to stopping transmission, which involves mask wearing.”
“I know that it’s very difficult and people feel like we’re sliding backward,” Dr. Kraft said during a media briefing sponsored by Emory University held shortly after the CDC announcement.
She added that the CDC updated guidance seems appropriate. “I don’t think any of us really want to be in this position or want to go back to masking but…we’re finding ourselves in the same place we were a year ago, in July 2020.
“In general we just don’t want anybody to be infected even if there’s a small chance for you to be infected and there’s a small chance for you to transmit it,” said Dr. Kraft, who’s an assistant professor in the department of pathology and associate professor in the department of medicine, division of infectious diseases at Emory University School of Medicine in Atlanta.
Breakthrough transmissions
“The good news is you’re still unlikely to get critically ill if you’re vaccinated. But what has changed with the [D]elta variant is instead of being 90% plus protected from getting the virus at all, you’re probably more in the 70% to 80% range,” James T. McDeavitt, MD, told this news organization.
“So we’re seeing breakthrough infections,” said Dr. McDeavitt, executive vice president and dean of clinical affairs at Baylor College of Medicine in Houston. “We are starting to see [such people] are potentially infectious.” Even if a vaccinated person is individually much less likely to experience serious COVID-19 outcomes, “they can spread it to someone else who spreads it to someone else who is more vulnerable. It puts the more at-risk populations at further risk.”
It breaks down to individual and public health concerns. “I am fully vaccinated. I am very confident I am not going to end up in a hospital,” he said. “Now if I were unvaccinated, with the prevalence of the virus around the country, I’m probably in more danger than I’ve ever been in the course of the pandemic. The unvaccinated are really at risk right now.”
IDSA and AMA support mask change
The Infectious Diseases Society of America (IDSA) has released a statement supporting the new CDC recommendations. “To stay ahead of the spread of the highly transmissible Delta variant, IDSA also urges that in communities with moderate transmission rates, all individuals, even those who are vaccinated, wear masks in indoor public places,” stated IDSA President Barbara D. Alexander, MD, MHS.
“IDSA also supports CDC’s guidance recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status, until vaccines are authorized and widely available to all children and vaccination rates are sufficient to control transmission.”
“Mask wearing will help reduce infections, prevent serious illnesses and death, limit strain on local hospitals and stave off the development of even more troubling variants,” she added.
The American Medical Association (AMA) also released a statement supporting the CDC’s policy changes.
“According to the CDC, emerging data indicates that vaccinated individuals infected with the Delta variant have similar viral loads as those who are unvaccinated and are capable of transmission,” AMA President Gerald E. Harmon, MD said in the statement.
“However, the science remains clear, the authorized vaccines remain safe and effective in preventing severe complications from COVID-19, including hospitalization and death,” he stated. “We strongly support the updated recommendations, which call for universal masking in areas of high or substantial COVID-19 transmission and in K-12 schools, to help reduce transmission of the virus. Wearing a mask is a small but important protective measure that can help us all stay safer.”
“The highest spread of cases and [most] severe outcomes are happening in places with low vaccination rates and among unvaccinated people,” Dr. Walensky said. “With the [D]elta variant, vaccinating more Americans now is more urgent than ever.”
“This moment, and the associated suffering, illness, and death, could have been avoided with higher vaccination coverage in this country,” she said.
A version of this article first appeared on Medscape.com.
Designing Quality Programs for Rural Hospitals
Population-based hospital payments provide incentives to reduce unnecessary healthcare use and a mechanism to finance population health investments. For hospitals, these payments provide stable revenue and flexibility in exchange for increased financial risk. The COVID-19 pandemic significantly reduced fee-for-service revenues, which has spurred provider interest in population-based payments, particularly from cash-strapped rural hospitals.
The Centers for Medicare & Medicaid Services (CMS) recently announced the launch of the Community Health Access and Rural Transformation (CHART) Model to test whether up-front, population-based payments improve access to high-quality care in rural communities and protect the financial stability of rural providers. This model follows the ongoing Pennsylvania Rural Health Model (PARHM), which offers similar payments to Pennsylvania’s rural hospitals. Prospective population-based hospital reimbursement appears to have helped Maryland’s hospitals survive the financial stress of the COVID-19 pandemic,1 and it is likely that the PARHM did the same for rural hospitals in Pennsylvania. Both the PARHM and the CHART Model place quality measurement and improvement at the core of payment reform, and for good reason. Capitation generates incentives for care stinting; linking prospective payments to quality measurement helps to ensure accountability. However, measuring the quality of rural healthcare is challenging. Rural health is different: Hospital size, payment mechanisms, and community health priorities are all distinct from those of metropolitan areas, which is why CMS exempts Critical Access Hospitals from Medicare’s core quality programs. Rural quality reporting programs could be established that address the unique aspects of rural healthcare.
As designers (JEF, DTL) of, and an advisor (ALS) for, a proposed pay-for-performance (P4P) program for the PARHM,2 we identified three central challenges in constructing and implementing P4P programs for rural hospitals, along with potential solutions. We hope that the lessons we learned can inform similar policy efforts.
First, many rural hospitals serve as stewards of community health resources. While metropolitan hospital systems can make targeted investments in population health, assigning accountability for health outcomes is challenging in cities where geographically overlapping provider systems compete for patients. In contrast, a rural hospital system with few or no competing providers is more naturally accountable for community health outcomes, especially if it owns most ambulatory clinics in its community. P4P programs could therefore reward rural hospitals for improving healthcare quality or health outcomes within their catchment areas. Like an accountable care organization (ACO), a rural hospital or hospital-based health system could be held accountable for appropriate screening for, and treatment of, uncontrolled hypertension, diabetes, or asthma, even without a network of community-based primary care providers that ACOs usually possess. Participants in the CHART Model’s Community Transformation Track, for example, select three community-level population health measures from four domains: substance use, chronic conditions, maternal health, and prevention. Accountability for community health outcomes is increasingly feasible because many larger rural hospitals have merged or been acquired.3
Second, small rural hospital patient volumes obscure the signal of true quality with statistical noise. Many common quality indicators, like risk-standardized mortality rates, are unreliable in rural settings with low patient volumes; in 2012-2013, the mean rural hospital daily census was seven inpatients.4,5 Payers and regulators have addressed this challenge by exempting rural hospitals from quality-reporting programs or by employing statistical techniques that diminish incentives to invest in improvement. CMS, for example, uses “shrinkage” estimators that adjust a hospital’s quality score toward a program-wide average, which makes it difficult to detect and reward performance improvement.4 Instead, rural P4P programs should use measures that are resistant to low patient volumes, such as the Measure Application Partnership’s (MAP) Core Set of Rural-Relevant Measures.6 Low volume–resistant measures include process and population-health outcome measures with naturally large denominators (eg, medication reconciliation), structural measures for which sample size is irrelevant (eg, nurse staffing ratios), and qualitative assessments of hospital adherence to best practices. CMS and other measure developers should also prioritize the creation of other rural-relevant, cross-cutting, low volume–resistant measures, like avoidance of deliriogenic medications in the elderly or initiation of treatment for substance use disorders, in consultation with rural stakeholders and the MAP Rural Health Workgroup. When extensive measurement noise is inevitable, public and private policymakers should eschew downside risk in rural P4P contracts.
Third, many rural hospitals have limited resources for measurement and improvement.7 While many well-resourced community hospitals have dedicated quality departments, quality directors in rural hospitals often have at least one other full-time job. Well-intentioned exemptions from P4P programs have left rural hospitals with limited experience with basic data collection and reporting, a handicap compounded by redundant and misaligned payor quality reporting requirements. To engage rural hospitals in quality improvement work, payors should coordinate to make participation in rural P4P programs as easy as possible. The adoption of a locally aligned set of healthcare quality measures by all payors in a region, like the PARHM’s proposed “all-payer quality program,” could substantially reduce administrative burden and motivate rural hospitals to enhance patient care and improve community health. In the CHART Model’s Community Transformation Track, for example, all public and private participating payers in each region must report on six quality measures: inpatient and emergency department visits for ambulatory care sensitive conditions, hospital-wide all-cause unplanned readmissions, and the Hospital Consumer Assessment of Health Care survey, as well as three community-chosen measures from the domains of substance use, maternal health, and prevention.8 As with all P4P programs, rural P4P programs should focus on a small number of meaningful measures, such as functional and clinical outcomes, complications, and patient experience, and feature relatively large rewards for improvement.9 The National Quality Forum recommends that rural programs avoid downside risk, reward improvement as well as achievement, and permit virtual provider groups.10 We would add that programs in rural communities ought to pair economic rewards with social recognition and comparison, offer technical assistance and opportunities for shared learning, and account for social as well as medical risk.
Many challenges to the adoption of rural P4P programs have been targeted through multi-stakeholder collaborations like the PARHM. Careful allocation of technical assistance resources may help address barriers such as comparing the performance of heterogeneous rural hospitals that vary in characteristics like size, affiliation with large health systems, or integration of ambulatory care services, which may affect hospital measurement capabilities and performance. Quality improvement efforts could be further bolstered through direct allocation of funds to the creation of virtual shared learning platforms, and by providing performance bonuses to groups of small hospitals that elect to engage in shared reporting.
The stakes are high for designing robust quality programs for rural hospitals. Although one in five Americans rely on them for healthcare, their rate of closure has accelerated in the past decade.11 CMS has made it clear that a sustainable system for financing rural health must be built around a commitment to quality measurement and improvement. While some rural provider organizations might be best served by participating in voluntary rural health networks and preexisting federal programs like the Medicare Beneficiary Quality Improvement Project, they should also have the opportunity to accept payments tied to quality, especially as growing numbers of rural hospitals are absorbed into larger healthcare systems. Adopting aligned sets of reliable and meaningful quality measures alongside population-based payments will help to create a sustainable future for rural hospitals.
Acknowledgment
We thank Mark Friedberg, MD, MPP, for his helpful comments on an earlier draft of this manuscript.
1. Peterson CL, Schumacher DN. How Maryland’s Total Cost of Care Model has helped hospitals manage the COVID-19 stress test. Health Affairs blog. October 7, 2020. Accessed July 15, 2021. https://www.healthaffairs.org/doi/10.1377/hblog20201005.677034/full/
2. Herzog MB, Fried JE, Liebers DT, MacKinney AC. Development of an all-payer quality program for the Pennsylvania Rural Health Model. J Rural Health. Published online December 4, 2020. https://doi.org/10.1111/jrh.12547
3. Williams D Jr, Reiter KL, Pink GH, Holmes GM, Song PH. Rural hospital mergers increased between 2005 and 2016—what did those hospitals look like? Inquiry. 2020;57:46958020935666. https://doi.org/10.1177/0046958020935666
4. Schwartz AL. Accuracy vs. incentives: a tradeoff for performance measurement. Am J Health Econ. Accepted February 8, 2021. https://doi.org/10.1086/714374
5. Freeman V, Thompson K, Howard HA, et al. The 21st Century Rural Hospital: A Chart Book. Cecil G. Sheps Center for Health Services Research. March 2015. https://www.shepscenter.unc.edu/product/21st-century-rural-hospital-chart-book/https://www.shepscenter.unc.edu/programs-projects/rural-health/projects/north-carolina-rural-health-research-and-policy-analysis-center/publications/
6. National Quality Forum. A core set of rural-relevant measures and measuring and improving access to care: 2018 recommendations from the MAP Rural Health Workgroup. August 31, 2018.
7. US Government Accountability Office. Medicare value-based payment models: participation challenges and available assistance for small and rural practices. December 9, 2016. Accessed July 15, 2021. https://www.gao.gov/products/gao-17-55
8. US Department of Health & Human Services. Community Health Access and Rural Transformation (CHART). Funding Opportunity Number: CMS-2G2-21-001. March 5, 2021. Accessed July 15, 2021. https://www.grants.gov/web/grants/view-opportunity.html?oppId=329062
9. Jha AK. Time to get serious about pay for performance. JAMA. 2013;309(4):347-348. https://doi.org/10.1001/jama.2012.196646
10. National Quality Forum. Performance measurement for rural low-volume providers. September 14, 2015. https://www.qualityforum.org/Publications/2015/09/Rural_Health_Final_Report.aspx
11. US Government Accountability Office. Rural hospital closures: number and characteristics of affected hospitals and contributing factors. GAO-18-634. August 29, 2018. https://www.gao.gov/assets/gao-18-634.pdf
Population-based hospital payments provide incentives to reduce unnecessary healthcare use and a mechanism to finance population health investments. For hospitals, these payments provide stable revenue and flexibility in exchange for increased financial risk. The COVID-19 pandemic significantly reduced fee-for-service revenues, which has spurred provider interest in population-based payments, particularly from cash-strapped rural hospitals.
The Centers for Medicare & Medicaid Services (CMS) recently announced the launch of the Community Health Access and Rural Transformation (CHART) Model to test whether up-front, population-based payments improve access to high-quality care in rural communities and protect the financial stability of rural providers. This model follows the ongoing Pennsylvania Rural Health Model (PARHM), which offers similar payments to Pennsylvania’s rural hospitals. Prospective population-based hospital reimbursement appears to have helped Maryland’s hospitals survive the financial stress of the COVID-19 pandemic,1 and it is likely that the PARHM did the same for rural hospitals in Pennsylvania. Both the PARHM and the CHART Model place quality measurement and improvement at the core of payment reform, and for good reason. Capitation generates incentives for care stinting; linking prospective payments to quality measurement helps to ensure accountability. However, measuring the quality of rural healthcare is challenging. Rural health is different: Hospital size, payment mechanisms, and community health priorities are all distinct from those of metropolitan areas, which is why CMS exempts Critical Access Hospitals from Medicare’s core quality programs. Rural quality reporting programs could be established that address the unique aspects of rural healthcare.
As designers (JEF, DTL) of, and an advisor (ALS) for, a proposed pay-for-performance (P4P) program for the PARHM,2 we identified three central challenges in constructing and implementing P4P programs for rural hospitals, along with potential solutions. We hope that the lessons we learned can inform similar policy efforts.
First, many rural hospitals serve as stewards of community health resources. While metropolitan hospital systems can make targeted investments in population health, assigning accountability for health outcomes is challenging in cities where geographically overlapping provider systems compete for patients. In contrast, a rural hospital system with few or no competing providers is more naturally accountable for community health outcomes, especially if it owns most ambulatory clinics in its community. P4P programs could therefore reward rural hospitals for improving healthcare quality or health outcomes within their catchment areas. Like an accountable care organization (ACO), a rural hospital or hospital-based health system could be held accountable for appropriate screening for, and treatment of, uncontrolled hypertension, diabetes, or asthma, even without a network of community-based primary care providers that ACOs usually possess. Participants in the CHART Model’s Community Transformation Track, for example, select three community-level population health measures from four domains: substance use, chronic conditions, maternal health, and prevention. Accountability for community health outcomes is increasingly feasible because many larger rural hospitals have merged or been acquired.3
Second, small rural hospital patient volumes obscure the signal of true quality with statistical noise. Many common quality indicators, like risk-standardized mortality rates, are unreliable in rural settings with low patient volumes; in 2012-2013, the mean rural hospital daily census was seven inpatients.4,5 Payers and regulators have addressed this challenge by exempting rural hospitals from quality-reporting programs or by employing statistical techniques that diminish incentives to invest in improvement. CMS, for example, uses “shrinkage” estimators that adjust a hospital’s quality score toward a program-wide average, which makes it difficult to detect and reward performance improvement.4 Instead, rural P4P programs should use measures that are resistant to low patient volumes, such as the Measure Application Partnership’s (MAP) Core Set of Rural-Relevant Measures.6 Low volume–resistant measures include process and population-health outcome measures with naturally large denominators (eg, medication reconciliation), structural measures for which sample size is irrelevant (eg, nurse staffing ratios), and qualitative assessments of hospital adherence to best practices. CMS and other measure developers should also prioritize the creation of other rural-relevant, cross-cutting, low volume–resistant measures, like avoidance of deliriogenic medications in the elderly or initiation of treatment for substance use disorders, in consultation with rural stakeholders and the MAP Rural Health Workgroup. When extensive measurement noise is inevitable, public and private policymakers should eschew downside risk in rural P4P contracts.
Third, many rural hospitals have limited resources for measurement and improvement.7 While many well-resourced community hospitals have dedicated quality departments, quality directors in rural hospitals often have at least one other full-time job. Well-intentioned exemptions from P4P programs have left rural hospitals with limited experience with basic data collection and reporting, a handicap compounded by redundant and misaligned payor quality reporting requirements. To engage rural hospitals in quality improvement work, payors should coordinate to make participation in rural P4P programs as easy as possible. The adoption of a locally aligned set of healthcare quality measures by all payors in a region, like the PARHM’s proposed “all-payer quality program,” could substantially reduce administrative burden and motivate rural hospitals to enhance patient care and improve community health. In the CHART Model’s Community Transformation Track, for example, all public and private participating payers in each region must report on six quality measures: inpatient and emergency department visits for ambulatory care sensitive conditions, hospital-wide all-cause unplanned readmissions, and the Hospital Consumer Assessment of Health Care survey, as well as three community-chosen measures from the domains of substance use, maternal health, and prevention.8 As with all P4P programs, rural P4P programs should focus on a small number of meaningful measures, such as functional and clinical outcomes, complications, and patient experience, and feature relatively large rewards for improvement.9 The National Quality Forum recommends that rural programs avoid downside risk, reward improvement as well as achievement, and permit virtual provider groups.10 We would add that programs in rural communities ought to pair economic rewards with social recognition and comparison, offer technical assistance and opportunities for shared learning, and account for social as well as medical risk.
Many challenges to the adoption of rural P4P programs have been targeted through multi-stakeholder collaborations like the PARHM. Careful allocation of technical assistance resources may help address barriers such as comparing the performance of heterogeneous rural hospitals that vary in characteristics like size, affiliation with large health systems, or integration of ambulatory care services, which may affect hospital measurement capabilities and performance. Quality improvement efforts could be further bolstered through direct allocation of funds to the creation of virtual shared learning platforms, and by providing performance bonuses to groups of small hospitals that elect to engage in shared reporting.
The stakes are high for designing robust quality programs for rural hospitals. Although one in five Americans rely on them for healthcare, their rate of closure has accelerated in the past decade.11 CMS has made it clear that a sustainable system for financing rural health must be built around a commitment to quality measurement and improvement. While some rural provider organizations might be best served by participating in voluntary rural health networks and preexisting federal programs like the Medicare Beneficiary Quality Improvement Project, they should also have the opportunity to accept payments tied to quality, especially as growing numbers of rural hospitals are absorbed into larger healthcare systems. Adopting aligned sets of reliable and meaningful quality measures alongside population-based payments will help to create a sustainable future for rural hospitals.
Acknowledgment
We thank Mark Friedberg, MD, MPP, for his helpful comments on an earlier draft of this manuscript.
Population-based hospital payments provide incentives to reduce unnecessary healthcare use and a mechanism to finance population health investments. For hospitals, these payments provide stable revenue and flexibility in exchange for increased financial risk. The COVID-19 pandemic significantly reduced fee-for-service revenues, which has spurred provider interest in population-based payments, particularly from cash-strapped rural hospitals.
The Centers for Medicare & Medicaid Services (CMS) recently announced the launch of the Community Health Access and Rural Transformation (CHART) Model to test whether up-front, population-based payments improve access to high-quality care in rural communities and protect the financial stability of rural providers. This model follows the ongoing Pennsylvania Rural Health Model (PARHM), which offers similar payments to Pennsylvania’s rural hospitals. Prospective population-based hospital reimbursement appears to have helped Maryland’s hospitals survive the financial stress of the COVID-19 pandemic,1 and it is likely that the PARHM did the same for rural hospitals in Pennsylvania. Both the PARHM and the CHART Model place quality measurement and improvement at the core of payment reform, and for good reason. Capitation generates incentives for care stinting; linking prospective payments to quality measurement helps to ensure accountability. However, measuring the quality of rural healthcare is challenging. Rural health is different: Hospital size, payment mechanisms, and community health priorities are all distinct from those of metropolitan areas, which is why CMS exempts Critical Access Hospitals from Medicare’s core quality programs. Rural quality reporting programs could be established that address the unique aspects of rural healthcare.
As designers (JEF, DTL) of, and an advisor (ALS) for, a proposed pay-for-performance (P4P) program for the PARHM,2 we identified three central challenges in constructing and implementing P4P programs for rural hospitals, along with potential solutions. We hope that the lessons we learned can inform similar policy efforts.
First, many rural hospitals serve as stewards of community health resources. While metropolitan hospital systems can make targeted investments in population health, assigning accountability for health outcomes is challenging in cities where geographically overlapping provider systems compete for patients. In contrast, a rural hospital system with few or no competing providers is more naturally accountable for community health outcomes, especially if it owns most ambulatory clinics in its community. P4P programs could therefore reward rural hospitals for improving healthcare quality or health outcomes within their catchment areas. Like an accountable care organization (ACO), a rural hospital or hospital-based health system could be held accountable for appropriate screening for, and treatment of, uncontrolled hypertension, diabetes, or asthma, even without a network of community-based primary care providers that ACOs usually possess. Participants in the CHART Model’s Community Transformation Track, for example, select three community-level population health measures from four domains: substance use, chronic conditions, maternal health, and prevention. Accountability for community health outcomes is increasingly feasible because many larger rural hospitals have merged or been acquired.3
Second, small rural hospital patient volumes obscure the signal of true quality with statistical noise. Many common quality indicators, like risk-standardized mortality rates, are unreliable in rural settings with low patient volumes; in 2012-2013, the mean rural hospital daily census was seven inpatients.4,5 Payers and regulators have addressed this challenge by exempting rural hospitals from quality-reporting programs or by employing statistical techniques that diminish incentives to invest in improvement. CMS, for example, uses “shrinkage” estimators that adjust a hospital’s quality score toward a program-wide average, which makes it difficult to detect and reward performance improvement.4 Instead, rural P4P programs should use measures that are resistant to low patient volumes, such as the Measure Application Partnership’s (MAP) Core Set of Rural-Relevant Measures.6 Low volume–resistant measures include process and population-health outcome measures with naturally large denominators (eg, medication reconciliation), structural measures for which sample size is irrelevant (eg, nurse staffing ratios), and qualitative assessments of hospital adherence to best practices. CMS and other measure developers should also prioritize the creation of other rural-relevant, cross-cutting, low volume–resistant measures, like avoidance of deliriogenic medications in the elderly or initiation of treatment for substance use disorders, in consultation with rural stakeholders and the MAP Rural Health Workgroup. When extensive measurement noise is inevitable, public and private policymakers should eschew downside risk in rural P4P contracts.
Third, many rural hospitals have limited resources for measurement and improvement.7 While many well-resourced community hospitals have dedicated quality departments, quality directors in rural hospitals often have at least one other full-time job. Well-intentioned exemptions from P4P programs have left rural hospitals with limited experience with basic data collection and reporting, a handicap compounded by redundant and misaligned payor quality reporting requirements. To engage rural hospitals in quality improvement work, payors should coordinate to make participation in rural P4P programs as easy as possible. The adoption of a locally aligned set of healthcare quality measures by all payors in a region, like the PARHM’s proposed “all-payer quality program,” could substantially reduce administrative burden and motivate rural hospitals to enhance patient care and improve community health. In the CHART Model’s Community Transformation Track, for example, all public and private participating payers in each region must report on six quality measures: inpatient and emergency department visits for ambulatory care sensitive conditions, hospital-wide all-cause unplanned readmissions, and the Hospital Consumer Assessment of Health Care survey, as well as three community-chosen measures from the domains of substance use, maternal health, and prevention.8 As with all P4P programs, rural P4P programs should focus on a small number of meaningful measures, such as functional and clinical outcomes, complications, and patient experience, and feature relatively large rewards for improvement.9 The National Quality Forum recommends that rural programs avoid downside risk, reward improvement as well as achievement, and permit virtual provider groups.10 We would add that programs in rural communities ought to pair economic rewards with social recognition and comparison, offer technical assistance and opportunities for shared learning, and account for social as well as medical risk.
Many challenges to the adoption of rural P4P programs have been targeted through multi-stakeholder collaborations like the PARHM. Careful allocation of technical assistance resources may help address barriers such as comparing the performance of heterogeneous rural hospitals that vary in characteristics like size, affiliation with large health systems, or integration of ambulatory care services, which may affect hospital measurement capabilities and performance. Quality improvement efforts could be further bolstered through direct allocation of funds to the creation of virtual shared learning platforms, and by providing performance bonuses to groups of small hospitals that elect to engage in shared reporting.
The stakes are high for designing robust quality programs for rural hospitals. Although one in five Americans rely on them for healthcare, their rate of closure has accelerated in the past decade.11 CMS has made it clear that a sustainable system for financing rural health must be built around a commitment to quality measurement and improvement. While some rural provider organizations might be best served by participating in voluntary rural health networks and preexisting federal programs like the Medicare Beneficiary Quality Improvement Project, they should also have the opportunity to accept payments tied to quality, especially as growing numbers of rural hospitals are absorbed into larger healthcare systems. Adopting aligned sets of reliable and meaningful quality measures alongside population-based payments will help to create a sustainable future for rural hospitals.
Acknowledgment
We thank Mark Friedberg, MD, MPP, for his helpful comments on an earlier draft of this manuscript.
1. Peterson CL, Schumacher DN. How Maryland’s Total Cost of Care Model has helped hospitals manage the COVID-19 stress test. Health Affairs blog. October 7, 2020. Accessed July 15, 2021. https://www.healthaffairs.org/doi/10.1377/hblog20201005.677034/full/
2. Herzog MB, Fried JE, Liebers DT, MacKinney AC. Development of an all-payer quality program for the Pennsylvania Rural Health Model. J Rural Health. Published online December 4, 2020. https://doi.org/10.1111/jrh.12547
3. Williams D Jr, Reiter KL, Pink GH, Holmes GM, Song PH. Rural hospital mergers increased between 2005 and 2016—what did those hospitals look like? Inquiry. 2020;57:46958020935666. https://doi.org/10.1177/0046958020935666
4. Schwartz AL. Accuracy vs. incentives: a tradeoff for performance measurement. Am J Health Econ. Accepted February 8, 2021. https://doi.org/10.1086/714374
5. Freeman V, Thompson K, Howard HA, et al. The 21st Century Rural Hospital: A Chart Book. Cecil G. Sheps Center for Health Services Research. March 2015. https://www.shepscenter.unc.edu/product/21st-century-rural-hospital-chart-book/https://www.shepscenter.unc.edu/programs-projects/rural-health/projects/north-carolina-rural-health-research-and-policy-analysis-center/publications/
6. National Quality Forum. A core set of rural-relevant measures and measuring and improving access to care: 2018 recommendations from the MAP Rural Health Workgroup. August 31, 2018.
7. US Government Accountability Office. Medicare value-based payment models: participation challenges and available assistance for small and rural practices. December 9, 2016. Accessed July 15, 2021. https://www.gao.gov/products/gao-17-55
8. US Department of Health & Human Services. Community Health Access and Rural Transformation (CHART). Funding Opportunity Number: CMS-2G2-21-001. March 5, 2021. Accessed July 15, 2021. https://www.grants.gov/web/grants/view-opportunity.html?oppId=329062
9. Jha AK. Time to get serious about pay for performance. JAMA. 2013;309(4):347-348. https://doi.org/10.1001/jama.2012.196646
10. National Quality Forum. Performance measurement for rural low-volume providers. September 14, 2015. https://www.qualityforum.org/Publications/2015/09/Rural_Health_Final_Report.aspx
11. US Government Accountability Office. Rural hospital closures: number and characteristics of affected hospitals and contributing factors. GAO-18-634. August 29, 2018. https://www.gao.gov/assets/gao-18-634.pdf
1. Peterson CL, Schumacher DN. How Maryland’s Total Cost of Care Model has helped hospitals manage the COVID-19 stress test. Health Affairs blog. October 7, 2020. Accessed July 15, 2021. https://www.healthaffairs.org/doi/10.1377/hblog20201005.677034/full/
2. Herzog MB, Fried JE, Liebers DT, MacKinney AC. Development of an all-payer quality program for the Pennsylvania Rural Health Model. J Rural Health. Published online December 4, 2020. https://doi.org/10.1111/jrh.12547
3. Williams D Jr, Reiter KL, Pink GH, Holmes GM, Song PH. Rural hospital mergers increased between 2005 and 2016—what did those hospitals look like? Inquiry. 2020;57:46958020935666. https://doi.org/10.1177/0046958020935666
4. Schwartz AL. Accuracy vs. incentives: a tradeoff for performance measurement. Am J Health Econ. Accepted February 8, 2021. https://doi.org/10.1086/714374
5. Freeman V, Thompson K, Howard HA, et al. The 21st Century Rural Hospital: A Chart Book. Cecil G. Sheps Center for Health Services Research. March 2015. https://www.shepscenter.unc.edu/product/21st-century-rural-hospital-chart-book/https://www.shepscenter.unc.edu/programs-projects/rural-health/projects/north-carolina-rural-health-research-and-policy-analysis-center/publications/
6. National Quality Forum. A core set of rural-relevant measures and measuring and improving access to care: 2018 recommendations from the MAP Rural Health Workgroup. August 31, 2018.
7. US Government Accountability Office. Medicare value-based payment models: participation challenges and available assistance for small and rural practices. December 9, 2016. Accessed July 15, 2021. https://www.gao.gov/products/gao-17-55
8. US Department of Health & Human Services. Community Health Access and Rural Transformation (CHART). Funding Opportunity Number: CMS-2G2-21-001. March 5, 2021. Accessed July 15, 2021. https://www.grants.gov/web/grants/view-opportunity.html?oppId=329062
9. Jha AK. Time to get serious about pay for performance. JAMA. 2013;309(4):347-348. https://doi.org/10.1001/jama.2012.196646
10. National Quality Forum. Performance measurement for rural low-volume providers. September 14, 2015. https://www.qualityforum.org/Publications/2015/09/Rural_Health_Final_Report.aspx
11. US Government Accountability Office. Rural hospital closures: number and characteristics of affected hospitals and contributing factors. GAO-18-634. August 29, 2018. https://www.gao.gov/assets/gao-18-634.pdf
© 2021 Society of Hospital Medicine




