User login
Neurology Reviews covers innovative and emerging news in neurology and neuroscience every month, with a focus on practical approaches to treating Parkinson's disease, epilepsy, headache, stroke, multiple sclerosis, Alzheimer's disease, and other neurologic disorders.
PML
Progressive multifocal leukoencephalopathy
Rituxan
The leading independent newspaper covering neurology news and commentary.
Telehealth effective in managing patients with movement disorders
Researchers presented their findings at the International Congress of Parkinson’s Disease and Movement Disorders.
Serving the underserved
One of the studies – from Kenya, Africa – documented a 2-year experience with telemedicine in a rural patient population.
Kenya suffers from a dearth of neurologists and movement disorder specialists. Most are based in the capital city of Nairobi, “leaving regions with a population of more than 30 million without access to their care,” wrote the study’s investigators. Internists with an interest in neurology usually manage the bulk of these patients.
Telemedicine has helped to bridge gaps between providers in this part of Africa.
Investigators in their study reviewed all cases of movement disorders at Meru Teaching and Referral Hospital and an affiliated clinic, Oregon Health Services, Meru, Kenya, during 2020 and 2021.
They also reviewed WhatsApp messaging, video calls via WhatsApp, patient videos, and phone calls to see how final diagnoses were arrived at using these platforms.
“For instance, a relative would send a video of a patient experiencing a tremor,” explained lead study author Bundi Karau, MD, a consultant physician. “We also shared the diagnostic challenges with experienced neurologists in Kenya and abroad by forwarding WhatsApp and recorded videos of the patients,” he added.
Telemedicine bridged the gap between rural doctors and patients in several ways. It enabled physicians to discuss cases with neurologists in and out of Kenya. “We were able to advise on medical management or further investigations in a more structured pattern and without spending months to make a diagnosis,” said Dr. Karau.
Patients no longer had to travel to Nairobi for care. “Where a direct link could be expensive or out of reach, we bridged this and consequently brought care closer to the patient,” he added.
More than 100 patients were diagnosed with a movement disorder and enrolled in care and follow-up during this 2-year time. Patients averaged about 62 years of age and more than 60% were male. Parkinson’s disease was the most common diagnosed condition (38.9%) followed by drug-induced movement disorders (30.6%), dystonia (11.1%), and functional movement disorders (11.1%).
Investigators found 3 cases of diabetic striatopathy, 8 cases of myoclonus, and 2 cases of Sydenham’s chorea.
Looking ahead, Dr. Karau and colleagues plan to do a cost benefit analysis vis-a-vis traditional physician visits and a trial model for follow-up visits for other neurological diseases.
Wearable devices and apps improve care
Moving from Africa to Greece, investigators in another study assessed the feasibility of using wearable devices to monitor symptoms in patients with Parkinson’s disease.
Such devices may enhance physical exams during virtual visits. Studies have shown that patients can commit to using such devices or mobile apps. What’s lacking is real-world data from everyday device usage, noted lead author George Rigas, PhD, and colleagues.
Fifty-two private physicians instructed a total of 133 patients to wear a device for Parkinson’s disease motor symptom telemonitoring for 1 week per month during waking hours.
Patients used a mobile app to report symptoms, medication, and nutrition adherence and to message their doctor.
The study team noticed that adherence rates stayed above 70% over a 12-month period. Medication and nutrition were among the most popular app features, an encouraging finding given that patients averaged 67 years of age.
“The high adherence percentage is significant, considering the target population and the early stage of telemedicine in Greece,” they concluded. Additional real-world data could help better inform longer-term adherence.
“These studies from all over the world demonstrate that we are only scratching the surface of the telehealth’s potential to improve care and the lives of individuals with Parkinson’s disease,” said Ray Dorsey, MD, a professor of neurology with the Center for Health + Technology at the University of Rochester (N.Y.).
Dr. Dorsey was not involved with the studies but has written and researched extensively on this topic.
Dr. Dorsey is a consultant for and has equity interests in Mediflix and Included Health, two digital health companies.
Researchers presented their findings at the International Congress of Parkinson’s Disease and Movement Disorders.
Serving the underserved
One of the studies – from Kenya, Africa – documented a 2-year experience with telemedicine in a rural patient population.
Kenya suffers from a dearth of neurologists and movement disorder specialists. Most are based in the capital city of Nairobi, “leaving regions with a population of more than 30 million without access to their care,” wrote the study’s investigators. Internists with an interest in neurology usually manage the bulk of these patients.
Telemedicine has helped to bridge gaps between providers in this part of Africa.
Investigators in their study reviewed all cases of movement disorders at Meru Teaching and Referral Hospital and an affiliated clinic, Oregon Health Services, Meru, Kenya, during 2020 and 2021.
They also reviewed WhatsApp messaging, video calls via WhatsApp, patient videos, and phone calls to see how final diagnoses were arrived at using these platforms.
“For instance, a relative would send a video of a patient experiencing a tremor,” explained lead study author Bundi Karau, MD, a consultant physician. “We also shared the diagnostic challenges with experienced neurologists in Kenya and abroad by forwarding WhatsApp and recorded videos of the patients,” he added.
Telemedicine bridged the gap between rural doctors and patients in several ways. It enabled physicians to discuss cases with neurologists in and out of Kenya. “We were able to advise on medical management or further investigations in a more structured pattern and without spending months to make a diagnosis,” said Dr. Karau.
Patients no longer had to travel to Nairobi for care. “Where a direct link could be expensive or out of reach, we bridged this and consequently brought care closer to the patient,” he added.
More than 100 patients were diagnosed with a movement disorder and enrolled in care and follow-up during this 2-year time. Patients averaged about 62 years of age and more than 60% were male. Parkinson’s disease was the most common diagnosed condition (38.9%) followed by drug-induced movement disorders (30.6%), dystonia (11.1%), and functional movement disorders (11.1%).
Investigators found 3 cases of diabetic striatopathy, 8 cases of myoclonus, and 2 cases of Sydenham’s chorea.
Looking ahead, Dr. Karau and colleagues plan to do a cost benefit analysis vis-a-vis traditional physician visits and a trial model for follow-up visits for other neurological diseases.
Wearable devices and apps improve care
Moving from Africa to Greece, investigators in another study assessed the feasibility of using wearable devices to monitor symptoms in patients with Parkinson’s disease.
Such devices may enhance physical exams during virtual visits. Studies have shown that patients can commit to using such devices or mobile apps. What’s lacking is real-world data from everyday device usage, noted lead author George Rigas, PhD, and colleagues.
Fifty-two private physicians instructed a total of 133 patients to wear a device for Parkinson’s disease motor symptom telemonitoring for 1 week per month during waking hours.
Patients used a mobile app to report symptoms, medication, and nutrition adherence and to message their doctor.
The study team noticed that adherence rates stayed above 70% over a 12-month period. Medication and nutrition were among the most popular app features, an encouraging finding given that patients averaged 67 years of age.
“The high adherence percentage is significant, considering the target population and the early stage of telemedicine in Greece,” they concluded. Additional real-world data could help better inform longer-term adherence.
“These studies from all over the world demonstrate that we are only scratching the surface of the telehealth’s potential to improve care and the lives of individuals with Parkinson’s disease,” said Ray Dorsey, MD, a professor of neurology with the Center for Health + Technology at the University of Rochester (N.Y.).
Dr. Dorsey was not involved with the studies but has written and researched extensively on this topic.
Dr. Dorsey is a consultant for and has equity interests in Mediflix and Included Health, two digital health companies.
Researchers presented their findings at the International Congress of Parkinson’s Disease and Movement Disorders.
Serving the underserved
One of the studies – from Kenya, Africa – documented a 2-year experience with telemedicine in a rural patient population.
Kenya suffers from a dearth of neurologists and movement disorder specialists. Most are based in the capital city of Nairobi, “leaving regions with a population of more than 30 million without access to their care,” wrote the study’s investigators. Internists with an interest in neurology usually manage the bulk of these patients.
Telemedicine has helped to bridge gaps between providers in this part of Africa.
Investigators in their study reviewed all cases of movement disorders at Meru Teaching and Referral Hospital and an affiliated clinic, Oregon Health Services, Meru, Kenya, during 2020 and 2021.
They also reviewed WhatsApp messaging, video calls via WhatsApp, patient videos, and phone calls to see how final diagnoses were arrived at using these platforms.
“For instance, a relative would send a video of a patient experiencing a tremor,” explained lead study author Bundi Karau, MD, a consultant physician. “We also shared the diagnostic challenges with experienced neurologists in Kenya and abroad by forwarding WhatsApp and recorded videos of the patients,” he added.
Telemedicine bridged the gap between rural doctors and patients in several ways. It enabled physicians to discuss cases with neurologists in and out of Kenya. “We were able to advise on medical management or further investigations in a more structured pattern and without spending months to make a diagnosis,” said Dr. Karau.
Patients no longer had to travel to Nairobi for care. “Where a direct link could be expensive or out of reach, we bridged this and consequently brought care closer to the patient,” he added.
More than 100 patients were diagnosed with a movement disorder and enrolled in care and follow-up during this 2-year time. Patients averaged about 62 years of age and more than 60% were male. Parkinson’s disease was the most common diagnosed condition (38.9%) followed by drug-induced movement disorders (30.6%), dystonia (11.1%), and functional movement disorders (11.1%).
Investigators found 3 cases of diabetic striatopathy, 8 cases of myoclonus, and 2 cases of Sydenham’s chorea.
Looking ahead, Dr. Karau and colleagues plan to do a cost benefit analysis vis-a-vis traditional physician visits and a trial model for follow-up visits for other neurological diseases.
Wearable devices and apps improve care
Moving from Africa to Greece, investigators in another study assessed the feasibility of using wearable devices to monitor symptoms in patients with Parkinson’s disease.
Such devices may enhance physical exams during virtual visits. Studies have shown that patients can commit to using such devices or mobile apps. What’s lacking is real-world data from everyday device usage, noted lead author George Rigas, PhD, and colleagues.
Fifty-two private physicians instructed a total of 133 patients to wear a device for Parkinson’s disease motor symptom telemonitoring for 1 week per month during waking hours.
Patients used a mobile app to report symptoms, medication, and nutrition adherence and to message their doctor.
The study team noticed that adherence rates stayed above 70% over a 12-month period. Medication and nutrition were among the most popular app features, an encouraging finding given that patients averaged 67 years of age.
“The high adherence percentage is significant, considering the target population and the early stage of telemedicine in Greece,” they concluded. Additional real-world data could help better inform longer-term adherence.
“These studies from all over the world demonstrate that we are only scratching the surface of the telehealth’s potential to improve care and the lives of individuals with Parkinson’s disease,” said Ray Dorsey, MD, a professor of neurology with the Center for Health + Technology at the University of Rochester (N.Y.).
Dr. Dorsey was not involved with the studies but has written and researched extensively on this topic.
Dr. Dorsey is a consultant for and has equity interests in Mediflix and Included Health, two digital health companies.
From MDS 2022
Medical cannabis appears safe for patients with movement disorders
, two Israeli research teams reported.
The practice calls for careful monitoring of patients and additional study, said the researchers, who presented their findings at the International Congress of Parkinson’s Disease and Movement Disorders.
Cannabis for Parkinson’s disease
One retrospective study focused on Parkinson’s disease, evaluating the safety and effects of long-term treatment with medical cannabis, which has become a widely available treatment for controlling symptoms in Parkinson’s disease and other pain disorders. Studies have demonstrated its efficacy in patients with Parkinson’s disease, but long-term safety has never been examined in Parkinson’s disease compared with untreated patients.
Their study included 152 patients with idiopathic Parkinson’s disease (mean age at diagnosis: 55.6 plus or minus 9.5 years) from the Sheba Medical Center Movement Disorders Institute who had been issued a license for medical cannabis. Seventy-six patients treated with cannabis were compared with 76 patients with similar characteristics who were not treated with cannabis.
Investigators collected data on patients who were followed at the institute between 2008 and 2022. Average follow-up period was 3.6 years.
Specifically, they collected data on levodopa equivalent daily dose (LEDD), Hoehn and Yahr scale progression, and patient-reported outcome measures on cognitive impairment, depressive, and psychotic symptoms, at baseline and at follow-up.
The Hoehn and Yahr scale allows for the quantification of different disease stages and LEDD provides a summary of the total daily medication a patient is receiving, explained Tomer Goldberg, BSc, the study’s lead author. Both are widely accepted motor severity and progression measures for Parkinson’s disease. “We wanted to check whether cannabis treatment influences these two motor parameters,” said Mr. Goldberg, who is affiliated with Tel Aviv University and the Movement Disorders Institute at Sheba Medical Center.
The medical cannabis–treated group and the untreated group had no significant differences in the mean annual change in LEDD or Hoehn and Yahr score. At 1, 2, and 3 years of follow-up, the treated group showed no signs of psychotic, depressive, or cognitive deterioration (P = .10-.68). The groups in Kaplan-Meier analyses also exhibited no differences in these nonmotor symptoms over time (P = .27-.93).
The findings suggest that cannabis treatment appears to be safe and has no negative effect on disease progression, said Mr. Goldberg. “It is important to note that we did not investigate all of the potential side effects of this treatment, and that prescribing medical cannabis for patients with Parkinson’s disease should be done with careful monitoring of each patient’s individual response to the treatment,” he added.
Cannabis for Huntington’s disease
Another study, targeting Huntington’s disease, drew similar conclusions. Psychiatric symptoms and cognitive decline are often present in Huntington’s disease patients, who have few treatment options. “An overall improvement in chorea and in neuropsychiatric symptoms was reported following cannabis treatment in several studies both in humans and in murine models,” wrote the study authors.
In this study, a certified Huntington’s disease specialist reviewed the medical records of 150 patients who were being followed in an Huntington’s disease clinic. Study metrics included the Unified Huntington’s Disease Rating Scale and Montreal Cognitive Assessment scores, indications for treatment, and adverse events related to treatment. Among the 150 patients, 19 had received cannabis treatment for indications such as sleep disorders, behavioral anomalies, and chorea. All but one patient reported an improvement in symptoms (94%). No adverse events were recorded, although one patient died from a COVID-19 infection.
Overall, medical cannabis appeared to safely relieve symptoms in patients with Huntington’s disease. A double-blind randomized controlled trial should further examine efficacy of these findings, the study authors recommended.
Mr. Goldberg had no disclosures or conflicts of interest in reporting his research.
, two Israeli research teams reported.
The practice calls for careful monitoring of patients and additional study, said the researchers, who presented their findings at the International Congress of Parkinson’s Disease and Movement Disorders.
Cannabis for Parkinson’s disease
One retrospective study focused on Parkinson’s disease, evaluating the safety and effects of long-term treatment with medical cannabis, which has become a widely available treatment for controlling symptoms in Parkinson’s disease and other pain disorders. Studies have demonstrated its efficacy in patients with Parkinson’s disease, but long-term safety has never been examined in Parkinson’s disease compared with untreated patients.
Their study included 152 patients with idiopathic Parkinson’s disease (mean age at diagnosis: 55.6 plus or minus 9.5 years) from the Sheba Medical Center Movement Disorders Institute who had been issued a license for medical cannabis. Seventy-six patients treated with cannabis were compared with 76 patients with similar characteristics who were not treated with cannabis.
Investigators collected data on patients who were followed at the institute between 2008 and 2022. Average follow-up period was 3.6 years.
Specifically, they collected data on levodopa equivalent daily dose (LEDD), Hoehn and Yahr scale progression, and patient-reported outcome measures on cognitive impairment, depressive, and psychotic symptoms, at baseline and at follow-up.
The Hoehn and Yahr scale allows for the quantification of different disease stages and LEDD provides a summary of the total daily medication a patient is receiving, explained Tomer Goldberg, BSc, the study’s lead author. Both are widely accepted motor severity and progression measures for Parkinson’s disease. “We wanted to check whether cannabis treatment influences these two motor parameters,” said Mr. Goldberg, who is affiliated with Tel Aviv University and the Movement Disorders Institute at Sheba Medical Center.
The medical cannabis–treated group and the untreated group had no significant differences in the mean annual change in LEDD or Hoehn and Yahr score. At 1, 2, and 3 years of follow-up, the treated group showed no signs of psychotic, depressive, or cognitive deterioration (P = .10-.68). The groups in Kaplan-Meier analyses also exhibited no differences in these nonmotor symptoms over time (P = .27-.93).
The findings suggest that cannabis treatment appears to be safe and has no negative effect on disease progression, said Mr. Goldberg. “It is important to note that we did not investigate all of the potential side effects of this treatment, and that prescribing medical cannabis for patients with Parkinson’s disease should be done with careful monitoring of each patient’s individual response to the treatment,” he added.
Cannabis for Huntington’s disease
Another study, targeting Huntington’s disease, drew similar conclusions. Psychiatric symptoms and cognitive decline are often present in Huntington’s disease patients, who have few treatment options. “An overall improvement in chorea and in neuropsychiatric symptoms was reported following cannabis treatment in several studies both in humans and in murine models,” wrote the study authors.
In this study, a certified Huntington’s disease specialist reviewed the medical records of 150 patients who were being followed in an Huntington’s disease clinic. Study metrics included the Unified Huntington’s Disease Rating Scale and Montreal Cognitive Assessment scores, indications for treatment, and adverse events related to treatment. Among the 150 patients, 19 had received cannabis treatment for indications such as sleep disorders, behavioral anomalies, and chorea. All but one patient reported an improvement in symptoms (94%). No adverse events were recorded, although one patient died from a COVID-19 infection.
Overall, medical cannabis appeared to safely relieve symptoms in patients with Huntington’s disease. A double-blind randomized controlled trial should further examine efficacy of these findings, the study authors recommended.
Mr. Goldberg had no disclosures or conflicts of interest in reporting his research.
, two Israeli research teams reported.
The practice calls for careful monitoring of patients and additional study, said the researchers, who presented their findings at the International Congress of Parkinson’s Disease and Movement Disorders.
Cannabis for Parkinson’s disease
One retrospective study focused on Parkinson’s disease, evaluating the safety and effects of long-term treatment with medical cannabis, which has become a widely available treatment for controlling symptoms in Parkinson’s disease and other pain disorders. Studies have demonstrated its efficacy in patients with Parkinson’s disease, but long-term safety has never been examined in Parkinson’s disease compared with untreated patients.
Their study included 152 patients with idiopathic Parkinson’s disease (mean age at diagnosis: 55.6 plus or minus 9.5 years) from the Sheba Medical Center Movement Disorders Institute who had been issued a license for medical cannabis. Seventy-six patients treated with cannabis were compared with 76 patients with similar characteristics who were not treated with cannabis.
Investigators collected data on patients who were followed at the institute between 2008 and 2022. Average follow-up period was 3.6 years.
Specifically, they collected data on levodopa equivalent daily dose (LEDD), Hoehn and Yahr scale progression, and patient-reported outcome measures on cognitive impairment, depressive, and psychotic symptoms, at baseline and at follow-up.
The Hoehn and Yahr scale allows for the quantification of different disease stages and LEDD provides a summary of the total daily medication a patient is receiving, explained Tomer Goldberg, BSc, the study’s lead author. Both are widely accepted motor severity and progression measures for Parkinson’s disease. “We wanted to check whether cannabis treatment influences these two motor parameters,” said Mr. Goldberg, who is affiliated with Tel Aviv University and the Movement Disorders Institute at Sheba Medical Center.
The medical cannabis–treated group and the untreated group had no significant differences in the mean annual change in LEDD or Hoehn and Yahr score. At 1, 2, and 3 years of follow-up, the treated group showed no signs of psychotic, depressive, or cognitive deterioration (P = .10-.68). The groups in Kaplan-Meier analyses also exhibited no differences in these nonmotor symptoms over time (P = .27-.93).
The findings suggest that cannabis treatment appears to be safe and has no negative effect on disease progression, said Mr. Goldberg. “It is important to note that we did not investigate all of the potential side effects of this treatment, and that prescribing medical cannabis for patients with Parkinson’s disease should be done with careful monitoring of each patient’s individual response to the treatment,” he added.
Cannabis for Huntington’s disease
Another study, targeting Huntington’s disease, drew similar conclusions. Psychiatric symptoms and cognitive decline are often present in Huntington’s disease patients, who have few treatment options. “An overall improvement in chorea and in neuropsychiatric symptoms was reported following cannabis treatment in several studies both in humans and in murine models,” wrote the study authors.
In this study, a certified Huntington’s disease specialist reviewed the medical records of 150 patients who were being followed in an Huntington’s disease clinic. Study metrics included the Unified Huntington’s Disease Rating Scale and Montreal Cognitive Assessment scores, indications for treatment, and adverse events related to treatment. Among the 150 patients, 19 had received cannabis treatment for indications such as sleep disorders, behavioral anomalies, and chorea. All but one patient reported an improvement in symptoms (94%). No adverse events were recorded, although one patient died from a COVID-19 infection.
Overall, medical cannabis appeared to safely relieve symptoms in patients with Huntington’s disease. A double-blind randomized controlled trial should further examine efficacy of these findings, the study authors recommended.
Mr. Goldberg had no disclosures or conflicts of interest in reporting his research.
FROM MDS 2022
Early bird gets the worm, night owl gets the diabetes
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
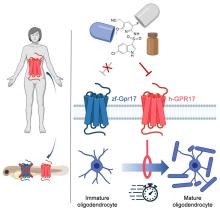
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.
A history of head trauma may predict Parkinson’s disease progression
, new research suggests.
In a longitudinal online study, among patients with Parkinson’s disease who had a history of head injury, motor impairment developed 25% faster and cognitive impairment developed 45% faster than among those without such a history.
In addition, severe head injuries were associated with an even more rapid onset of impairment. The results give weight to the idea that “it’s head injuries themselves” prior to the development of Parkinson’s disease that might exacerbate motor and cognitive symptoms, said study investigator Ethan Brown, MD, assistant professor, Weill Institute of Neurosciences, department of neurology, University of California, San Francisco.
The findings emphasize the importance of “doing everything we can” to prevent falls and head injuries for patients with Parkinson’s disease, Dr. Brown said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Reverse causality concerns
Head injury is a risk factor for Parkinson’s disease, but its relationship to Parkinson’s disease progression is not well established. “There has always been this concern in Parkinson’s disease that maybe it’s problems with motor impairment that lead to head injuries, so reverse causality is an issue,” said Dr. Brown. “We wanted to look at whether risk factors we know relate to the development of Parkinson’s disease can also have a bearing on its progression,” he added.
The analysis was part of the online Fox Insight study that is evaluating motor and nonmotor symptoms in individuals with and those without Parkinson’s disease. The study included participants who had completed questionnaires on such things as head trauma.
The study included 1,065 patients (47% women; mean age, 63 years) with Parkinson’s disease who reported having had a head injury at least 5 years prior to their diagnosis. Among the participants, the mean duration of Parkinson’s disease was 7.5 years.
The investigators employed a 5-year lag time in their study to exclude head injuries caused by early motor dysfunction, they noted. “We wanted to look at people who had these head injuries we think might be part of the cause of Parkinson’s disease as opposed to a result of them,” Dr. Brown said.
In this head injury group, 51% had received one head injury, 28% had received two injuries, and 22% had received more than two injuries.
The study also included 1,457 participants (56% women; mean age, 65 years) with Parkinson’s disease who had not had a head injury prior to their diagnosis. Of these patients, the mean time with a Parkinson’s disease diagnosis was 8 years.
Dr. Brown noted that the age and sex distribution of the study group was “probably representative” of the general Parkinson’s disease population. However, because the participants had to be able to go online and complete questionnaires, it is unlikely that, among these patients, Parkinson’s disease was far advanced, he said.
The investigators adjusted for age, sex, years of education, and Parkinson’s disease duration.
Two-hit hypothesis?
The researchers compared time from diagnosis to the development of significant motor impairment, such as the need for assistance with walking, and cognitive impairment, such as having a score of less than 43 on the Penn Daily Activities Questionnaire.
They also examined the role of more severe head injuries. In the head injury group, over half (54%) had had a severe head injury, including 543 who had lost consciousness and others who had suffered a fracture or had had a seizure.
Results showed that the adjusted hazard ratio for developing motor impairment among those with a head injury, compared with those who had not had a head injury was 1.24 (95% confidence interval, 1.01-1.53; P = .037). For severe injuries, the aHR for motor impairment was 1.44 (95% CI, 1.13-1.83; P = .003).
For cognitive impairment, the aHR for those with versus without head injuries was 1.45 (95% CI, 1.14-1.86; P = .003); and for severe injuries, the aHR was 1.49 (95% CI, 1.11-2.0; P = .008).
Aside from severity, the researchers did not examine subgroups. However, Dr. Brown reported that his team would like to stratify results by sex and other variables in the future.
He noted that various mechanisms may explain why Parkinson’s disease progression is faster for patients who have a history of head injury, compared with others. Chronic inflammation due to the injury and “co-pathology” might play some role, he said. He noted that head injuries are associated with cognitive impairment in other conditions, including Alzheimer’s disease.
There is also the “two hit” hypothesis, Dr. Brown said. “A head injury could cause such broad damage that once people develop Parkinson’s disease, it’s harder for them to compensate.”
Dr. Brown also noted there might have been a “higher magnitude” of a difference between groups had the study captured participants with more severe symptoms.
‘Provocative’ findings
Michael S. Okun, MD, medical advisor at the Parkinson’s Foundation and professor and director at the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the new data are “provocative.”
“The idea that a head injury may be important in predicting how quickly and how severely deficits will manifest could be important to the treating clinician,” said Dr. Okun, who was not involved with the research.
He noted that the results suggest clinicians should elicit more information from patients about head trauma. “They should be seeking more than a binary ‘yes or no’ answer to head injury when questioning patients,” he added.
Dr. Okun reiterated that head injury is a “known and important risk factor” not only for Parkinson’s disease but also for other neurodegenerative diseases. “It’s important to counsel patients about the association,” he said.
The study was supported by the Michael J. Fox Foundation. Dr. Brown reports having received grant support from the Michael J. Fox Foundation. Dr. Okun has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a longitudinal online study, among patients with Parkinson’s disease who had a history of head injury, motor impairment developed 25% faster and cognitive impairment developed 45% faster than among those without such a history.
In addition, severe head injuries were associated with an even more rapid onset of impairment. The results give weight to the idea that “it’s head injuries themselves” prior to the development of Parkinson’s disease that might exacerbate motor and cognitive symptoms, said study investigator Ethan Brown, MD, assistant professor, Weill Institute of Neurosciences, department of neurology, University of California, San Francisco.
The findings emphasize the importance of “doing everything we can” to prevent falls and head injuries for patients with Parkinson’s disease, Dr. Brown said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Reverse causality concerns
Head injury is a risk factor for Parkinson’s disease, but its relationship to Parkinson’s disease progression is not well established. “There has always been this concern in Parkinson’s disease that maybe it’s problems with motor impairment that lead to head injuries, so reverse causality is an issue,” said Dr. Brown. “We wanted to look at whether risk factors we know relate to the development of Parkinson’s disease can also have a bearing on its progression,” he added.
The analysis was part of the online Fox Insight study that is evaluating motor and nonmotor symptoms in individuals with and those without Parkinson’s disease. The study included participants who had completed questionnaires on such things as head trauma.
The study included 1,065 patients (47% women; mean age, 63 years) with Parkinson’s disease who reported having had a head injury at least 5 years prior to their diagnosis. Among the participants, the mean duration of Parkinson’s disease was 7.5 years.
The investigators employed a 5-year lag time in their study to exclude head injuries caused by early motor dysfunction, they noted. “We wanted to look at people who had these head injuries we think might be part of the cause of Parkinson’s disease as opposed to a result of them,” Dr. Brown said.
In this head injury group, 51% had received one head injury, 28% had received two injuries, and 22% had received more than two injuries.
The study also included 1,457 participants (56% women; mean age, 65 years) with Parkinson’s disease who had not had a head injury prior to their diagnosis. Of these patients, the mean time with a Parkinson’s disease diagnosis was 8 years.
Dr. Brown noted that the age and sex distribution of the study group was “probably representative” of the general Parkinson’s disease population. However, because the participants had to be able to go online and complete questionnaires, it is unlikely that, among these patients, Parkinson’s disease was far advanced, he said.
The investigators adjusted for age, sex, years of education, and Parkinson’s disease duration.
Two-hit hypothesis?
The researchers compared time from diagnosis to the development of significant motor impairment, such as the need for assistance with walking, and cognitive impairment, such as having a score of less than 43 on the Penn Daily Activities Questionnaire.
They also examined the role of more severe head injuries. In the head injury group, over half (54%) had had a severe head injury, including 543 who had lost consciousness and others who had suffered a fracture or had had a seizure.
Results showed that the adjusted hazard ratio for developing motor impairment among those with a head injury, compared with those who had not had a head injury was 1.24 (95% confidence interval, 1.01-1.53; P = .037). For severe injuries, the aHR for motor impairment was 1.44 (95% CI, 1.13-1.83; P = .003).
For cognitive impairment, the aHR for those with versus without head injuries was 1.45 (95% CI, 1.14-1.86; P = .003); and for severe injuries, the aHR was 1.49 (95% CI, 1.11-2.0; P = .008).
Aside from severity, the researchers did not examine subgroups. However, Dr. Brown reported that his team would like to stratify results by sex and other variables in the future.
He noted that various mechanisms may explain why Parkinson’s disease progression is faster for patients who have a history of head injury, compared with others. Chronic inflammation due to the injury and “co-pathology” might play some role, he said. He noted that head injuries are associated with cognitive impairment in other conditions, including Alzheimer’s disease.
There is also the “two hit” hypothesis, Dr. Brown said. “A head injury could cause such broad damage that once people develop Parkinson’s disease, it’s harder for them to compensate.”
Dr. Brown also noted there might have been a “higher magnitude” of a difference between groups had the study captured participants with more severe symptoms.
‘Provocative’ findings
Michael S. Okun, MD, medical advisor at the Parkinson’s Foundation and professor and director at the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the new data are “provocative.”
“The idea that a head injury may be important in predicting how quickly and how severely deficits will manifest could be important to the treating clinician,” said Dr. Okun, who was not involved with the research.
He noted that the results suggest clinicians should elicit more information from patients about head trauma. “They should be seeking more than a binary ‘yes or no’ answer to head injury when questioning patients,” he added.
Dr. Okun reiterated that head injury is a “known and important risk factor” not only for Parkinson’s disease but also for other neurodegenerative diseases. “It’s important to counsel patients about the association,” he said.
The study was supported by the Michael J. Fox Foundation. Dr. Brown reports having received grant support from the Michael J. Fox Foundation. Dr. Okun has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a longitudinal online study, among patients with Parkinson’s disease who had a history of head injury, motor impairment developed 25% faster and cognitive impairment developed 45% faster than among those without such a history.
In addition, severe head injuries were associated with an even more rapid onset of impairment. The results give weight to the idea that “it’s head injuries themselves” prior to the development of Parkinson’s disease that might exacerbate motor and cognitive symptoms, said study investigator Ethan Brown, MD, assistant professor, Weill Institute of Neurosciences, department of neurology, University of California, San Francisco.
The findings emphasize the importance of “doing everything we can” to prevent falls and head injuries for patients with Parkinson’s disease, Dr. Brown said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Reverse causality concerns
Head injury is a risk factor for Parkinson’s disease, but its relationship to Parkinson’s disease progression is not well established. “There has always been this concern in Parkinson’s disease that maybe it’s problems with motor impairment that lead to head injuries, so reverse causality is an issue,” said Dr. Brown. “We wanted to look at whether risk factors we know relate to the development of Parkinson’s disease can also have a bearing on its progression,” he added.
The analysis was part of the online Fox Insight study that is evaluating motor and nonmotor symptoms in individuals with and those without Parkinson’s disease. The study included participants who had completed questionnaires on such things as head trauma.
The study included 1,065 patients (47% women; mean age, 63 years) with Parkinson’s disease who reported having had a head injury at least 5 years prior to their diagnosis. Among the participants, the mean duration of Parkinson’s disease was 7.5 years.
The investigators employed a 5-year lag time in their study to exclude head injuries caused by early motor dysfunction, they noted. “We wanted to look at people who had these head injuries we think might be part of the cause of Parkinson’s disease as opposed to a result of them,” Dr. Brown said.
In this head injury group, 51% had received one head injury, 28% had received two injuries, and 22% had received more than two injuries.
The study also included 1,457 participants (56% women; mean age, 65 years) with Parkinson’s disease who had not had a head injury prior to their diagnosis. Of these patients, the mean time with a Parkinson’s disease diagnosis was 8 years.
Dr. Brown noted that the age and sex distribution of the study group was “probably representative” of the general Parkinson’s disease population. However, because the participants had to be able to go online and complete questionnaires, it is unlikely that, among these patients, Parkinson’s disease was far advanced, he said.
The investigators adjusted for age, sex, years of education, and Parkinson’s disease duration.
Two-hit hypothesis?
The researchers compared time from diagnosis to the development of significant motor impairment, such as the need for assistance with walking, and cognitive impairment, such as having a score of less than 43 on the Penn Daily Activities Questionnaire.
They also examined the role of more severe head injuries. In the head injury group, over half (54%) had had a severe head injury, including 543 who had lost consciousness and others who had suffered a fracture or had had a seizure.
Results showed that the adjusted hazard ratio for developing motor impairment among those with a head injury, compared with those who had not had a head injury was 1.24 (95% confidence interval, 1.01-1.53; P = .037). For severe injuries, the aHR for motor impairment was 1.44 (95% CI, 1.13-1.83; P = .003).
For cognitive impairment, the aHR for those with versus without head injuries was 1.45 (95% CI, 1.14-1.86; P = .003); and for severe injuries, the aHR was 1.49 (95% CI, 1.11-2.0; P = .008).
Aside from severity, the researchers did not examine subgroups. However, Dr. Brown reported that his team would like to stratify results by sex and other variables in the future.
He noted that various mechanisms may explain why Parkinson’s disease progression is faster for patients who have a history of head injury, compared with others. Chronic inflammation due to the injury and “co-pathology” might play some role, he said. He noted that head injuries are associated with cognitive impairment in other conditions, including Alzheimer’s disease.
There is also the “two hit” hypothesis, Dr. Brown said. “A head injury could cause such broad damage that once people develop Parkinson’s disease, it’s harder for them to compensate.”
Dr. Brown also noted there might have been a “higher magnitude” of a difference between groups had the study captured participants with more severe symptoms.
‘Provocative’ findings
Michael S. Okun, MD, medical advisor at the Parkinson’s Foundation and professor and director at the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the new data are “provocative.”
“The idea that a head injury may be important in predicting how quickly and how severely deficits will manifest could be important to the treating clinician,” said Dr. Okun, who was not involved with the research.
He noted that the results suggest clinicians should elicit more information from patients about head trauma. “They should be seeking more than a binary ‘yes or no’ answer to head injury when questioning patients,” he added.
Dr. Okun reiterated that head injury is a “known and important risk factor” not only for Parkinson’s disease but also for other neurodegenerative diseases. “It’s important to counsel patients about the association,” he said.
The study was supported by the Michael J. Fox Foundation. Dr. Brown reports having received grant support from the Michael J. Fox Foundation. Dr. Okun has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From MDS 2022
Is acetaminophen really safer than NSAIDs in heart disease?
New research calls into question the assumption that acetaminophen is safer than NSAIDs for patients with known cardiovascular disease (CVD) or CVD risk factors.
The analysis found a significant correlation between the use of acetaminophen and elevated systolic blood pressure.
While acetaminophen may still be safer than NSAIDs from a bleeding risk standpoint, or in patients with known kidney disease, “the gap may not be as large as once thought,” Rahul Gupta, MD, cardiologist with Lehigh Valley Health Network, Allentown, Pa., said in an interview.
“Cautious use is recommended over the long term, especially in patients with pre-existing hypertension or cardiovascular risk factors,” Dr. Gupta said.
The study was presented at the Hypertension Scientific Sessions, San Diego, sponsored by the American Heart Association.
Acetaminophen is one of the most widely used over-the-counter medications, as it is considered a safer medication for long-term use since it lacks the anti-inflammatory effects of NSAIDs, Dr. Gupta explained.
NSAIDs have been known to raise blood pressure, but the effect of acetaminophen in this regard has not been well studied. Observational studies have shown contradictory results in terms of its effect on blood pressure, he noted.
To investigate further, Dr. Gupta and colleagues did a meta-analysis of three studies that compared the effect of acetaminophen (3-4 g/day) versus placebo on systolic and diastolic ambulatory blood pressure in patients with heart disease or hypertension. Together, the studies included 172 adults (mean age, 60 years; 73% male).
They found that patients receiving acetaminophen had significantly higher systolic blood pressure, compared with those receiving placebo (standard mean difference [SMD] = 0.35; 95% confidence interval, 0.08-0.63; P = .01).
Subgroup analysis of the effect on hypertensive patients showed significant change in systolic blood pressure as well (SMD = 0.38; 95% CI, 0.05-0.71; P = .02).
“Interestingly, there was no significant difference in the effect on diastolic blood pressure,” Dr. Gupta commented.
Reached for comment, Timothy S. Anderson, MD, clinical investigator in the Division of General Medicine at Beth Israel Deaconess Medical Center and assistant professor of medicine at the Harvard Medical School, both in Boston, said this is “an interesting and not particularly well-known issue.”
“However, most of the trials look at very high doses of acetaminophen use (for example, six to eight of the 500 mg pills each day) so we don’t really know whether the more common patterns of using one to two acetaminophen pills every once in a while is problematic,” Dr. Anderson told this news organization.
“We also don’t have data showing a direct harm from these medications with regards to strokes or heart attacks or other downstream consequences of high blood pressure. Ideally we would need a head-to-head trial comparing ibuprofen-type medications to acetaminophen-type medications,” Dr. Anderson said.
The study had no specific funding. Dr. Gupta and Dr. Anderson reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
New research calls into question the assumption that acetaminophen is safer than NSAIDs for patients with known cardiovascular disease (CVD) or CVD risk factors.
The analysis found a significant correlation between the use of acetaminophen and elevated systolic blood pressure.
While acetaminophen may still be safer than NSAIDs from a bleeding risk standpoint, or in patients with known kidney disease, “the gap may not be as large as once thought,” Rahul Gupta, MD, cardiologist with Lehigh Valley Health Network, Allentown, Pa., said in an interview.
“Cautious use is recommended over the long term, especially in patients with pre-existing hypertension or cardiovascular risk factors,” Dr. Gupta said.
The study was presented at the Hypertension Scientific Sessions, San Diego, sponsored by the American Heart Association.
Acetaminophen is one of the most widely used over-the-counter medications, as it is considered a safer medication for long-term use since it lacks the anti-inflammatory effects of NSAIDs, Dr. Gupta explained.
NSAIDs have been known to raise blood pressure, but the effect of acetaminophen in this regard has not been well studied. Observational studies have shown contradictory results in terms of its effect on blood pressure, he noted.
To investigate further, Dr. Gupta and colleagues did a meta-analysis of three studies that compared the effect of acetaminophen (3-4 g/day) versus placebo on systolic and diastolic ambulatory blood pressure in patients with heart disease or hypertension. Together, the studies included 172 adults (mean age, 60 years; 73% male).
They found that patients receiving acetaminophen had significantly higher systolic blood pressure, compared with those receiving placebo (standard mean difference [SMD] = 0.35; 95% confidence interval, 0.08-0.63; P = .01).
Subgroup analysis of the effect on hypertensive patients showed significant change in systolic blood pressure as well (SMD = 0.38; 95% CI, 0.05-0.71; P = .02).
“Interestingly, there was no significant difference in the effect on diastolic blood pressure,” Dr. Gupta commented.
Reached for comment, Timothy S. Anderson, MD, clinical investigator in the Division of General Medicine at Beth Israel Deaconess Medical Center and assistant professor of medicine at the Harvard Medical School, both in Boston, said this is “an interesting and not particularly well-known issue.”
“However, most of the trials look at very high doses of acetaminophen use (for example, six to eight of the 500 mg pills each day) so we don’t really know whether the more common patterns of using one to two acetaminophen pills every once in a while is problematic,” Dr. Anderson told this news organization.
“We also don’t have data showing a direct harm from these medications with regards to strokes or heart attacks or other downstream consequences of high blood pressure. Ideally we would need a head-to-head trial comparing ibuprofen-type medications to acetaminophen-type medications,” Dr. Anderson said.
The study had no specific funding. Dr. Gupta and Dr. Anderson reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
New research calls into question the assumption that acetaminophen is safer than NSAIDs for patients with known cardiovascular disease (CVD) or CVD risk factors.
The analysis found a significant correlation between the use of acetaminophen and elevated systolic blood pressure.
While acetaminophen may still be safer than NSAIDs from a bleeding risk standpoint, or in patients with known kidney disease, “the gap may not be as large as once thought,” Rahul Gupta, MD, cardiologist with Lehigh Valley Health Network, Allentown, Pa., said in an interview.
“Cautious use is recommended over the long term, especially in patients with pre-existing hypertension or cardiovascular risk factors,” Dr. Gupta said.
The study was presented at the Hypertension Scientific Sessions, San Diego, sponsored by the American Heart Association.
Acetaminophen is one of the most widely used over-the-counter medications, as it is considered a safer medication for long-term use since it lacks the anti-inflammatory effects of NSAIDs, Dr. Gupta explained.
NSAIDs have been known to raise blood pressure, but the effect of acetaminophen in this regard has not been well studied. Observational studies have shown contradictory results in terms of its effect on blood pressure, he noted.
To investigate further, Dr. Gupta and colleagues did a meta-analysis of three studies that compared the effect of acetaminophen (3-4 g/day) versus placebo on systolic and diastolic ambulatory blood pressure in patients with heart disease or hypertension. Together, the studies included 172 adults (mean age, 60 years; 73% male).
They found that patients receiving acetaminophen had significantly higher systolic blood pressure, compared with those receiving placebo (standard mean difference [SMD] = 0.35; 95% confidence interval, 0.08-0.63; P = .01).
Subgroup analysis of the effect on hypertensive patients showed significant change in systolic blood pressure as well (SMD = 0.38; 95% CI, 0.05-0.71; P = .02).
“Interestingly, there was no significant difference in the effect on diastolic blood pressure,” Dr. Gupta commented.
Reached for comment, Timothy S. Anderson, MD, clinical investigator in the Division of General Medicine at Beth Israel Deaconess Medical Center and assistant professor of medicine at the Harvard Medical School, both in Boston, said this is “an interesting and not particularly well-known issue.”
“However, most of the trials look at very high doses of acetaminophen use (for example, six to eight of the 500 mg pills each day) so we don’t really know whether the more common patterns of using one to two acetaminophen pills every once in a while is problematic,” Dr. Anderson told this news organization.
“We also don’t have data showing a direct harm from these medications with regards to strokes or heart attacks or other downstream consequences of high blood pressure. Ideally we would need a head-to-head trial comparing ibuprofen-type medications to acetaminophen-type medications,” Dr. Anderson said.
The study had no specific funding. Dr. Gupta and Dr. Anderson reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION 2022
House passes prior authorization bill, Senate path unclear
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
WHO releases six ‘action steps’ to combat global disparities in Parkinson’s disease
Since 2000, Parkinson’s disease has increased 81% and related deaths have increased 100% globally. In addition, many patients affected by Parkinson’s disease live in low- and middle-income countries and experience large inequalities in access to neurologic care and essential medicines.
To address these issues, the Brain Health Unit at the WHO developed six “action steps” it says are urgently required to combat global disparities in Parkinson’s disease.
The need for action is great, said lead author Nicoline Schiess, MD, MPH, a neurologist and technical officer in the WHO’s Brain Health Unit in Geneva.
“In adults, disorders of the nervous system are the leading cause of disability adjusted life years, or DALYs, and the second leading cause of death globally, accounting for 9 million deaths per year,” Dr. Schiess said.
The WHO’s recommendations were published online recently as a “Special Communication” in JAMA Neurology.
Serious public health challenge
Parkinson’s disease is the fastest growing disorder in terms of death and disability, and it is estimated that it caused 329,000 deaths in 2019 – an increase of more than 100% since 2000.
“The rise in cases is thought to be multifactorial and is likely affected by factors such as aging populations and environmental exposures, such as certain pesticides. With these rapidly increasing numbers, compounded by a lack of specialists and medicines in low- and middle-income countries, Parkinson’s disease presents a serious public health challenge,” Dr. Schiess said.
The publication of the six action steps is targeted toward clinicians and researchers who work in Parkinson’s disease, she added. The steps address the following areas:
- 1. Disease burden
- 2. Advocacy and awareness
- 3. Prevention and risk reduction
- 4. Diagnosis, treatment, and care
- 5. Caregiver support
- 6. Research
Dr. Schiess noted that data on disease burden are lacking in certain areas of the world, such as low- and middle-income countries, and information “based on race and ethnicity are inconsistent. Studies are needed to establish more representative epidemiological data.”
She said that advocacy and awareness are particularly important since young people may not be aware they can also develop Parkinson’s disease, and sex and race differences can factor in to the potential for delays in diagnosis and care. “This is often due to the incorrect perception that Parkinson’s disease only affects older people,” she noted.
In addition, “a substantial need exists to identify risks for Parkinson’s disease – in particular the risks we can mitigate,” said Dr. Schiess, citing pesticide exposure as one example. “The evidence linking pesticide exposure, for example paraquat and chlorpyrifos, with the risk of developing Parkinson’s disease is substantial. And yet in many countries, these products are still being used.”
Under the heading of diagnosis, treatment, and care, Dr. Schiess noted that patients with Parkinson’s disease in “low resource settings” and low- to middle-income countries are unable to obtain “even the most basic medications” to treat Parkinson’s disease.
“Strengthening health and social systems, and building capacity to improve medical care, including rehabilitation and palliative care and medication access, are vital. Also, education and training of primary health care professionals, growing the neurological workforce, and increasing the use of digital technology such as telemedicine, are key mechanisms to improving diagnosis and sustainability of care,” she said.
For caregiver support, Dr. Schiess pointed out that the progressive nature of the disease and timing of onset are contributors to increased caregiver burden. Other contributors, as the disease advances in a patient, include the development of cognitive impairment, psychiatric manifestations, and sleep disruption.
“Solutions that could decrease the burden on caregivers include providing an accurate and timely diagnosis and training and education to caregivers, such as the WHO iSUPPORT program, as well as psychosocial, financial, and community-based support,” said Dr. Schiess.
For research, she noted that the amount of studies in the field of Parkinson’s disease has grown because of increased funding and a greater number of initiatives over the past 2 decades.
“Continuing to build on this momentum is important in order to generate new treatment options, better care, and research capacity, especially in low- and middle-income countries,” she said.
Dr. Schiess emphasized the urgency for adopting these measures as cases of Parkinson’s disease continue to rise.
“The take-away message for clinicians is that Parkinson disease is a growing global public health issue. There is a pressing need for a global public health response to address health and social requirements for people with Parkinson’s disease,” she said.
Dr. Schiess reports having received grants from the Edmond J. Safra Foundation paid to her institution during the conduct of the study.
A version of this article first appeared on Medscape.com.
Since 2000, Parkinson’s disease has increased 81% and related deaths have increased 100% globally. In addition, many patients affected by Parkinson’s disease live in low- and middle-income countries and experience large inequalities in access to neurologic care and essential medicines.
To address these issues, the Brain Health Unit at the WHO developed six “action steps” it says are urgently required to combat global disparities in Parkinson’s disease.
The need for action is great, said lead author Nicoline Schiess, MD, MPH, a neurologist and technical officer in the WHO’s Brain Health Unit in Geneva.
“In adults, disorders of the nervous system are the leading cause of disability adjusted life years, or DALYs, and the second leading cause of death globally, accounting for 9 million deaths per year,” Dr. Schiess said.
The WHO’s recommendations were published online recently as a “Special Communication” in JAMA Neurology.
Serious public health challenge
Parkinson’s disease is the fastest growing disorder in terms of death and disability, and it is estimated that it caused 329,000 deaths in 2019 – an increase of more than 100% since 2000.
“The rise in cases is thought to be multifactorial and is likely affected by factors such as aging populations and environmental exposures, such as certain pesticides. With these rapidly increasing numbers, compounded by a lack of specialists and medicines in low- and middle-income countries, Parkinson’s disease presents a serious public health challenge,” Dr. Schiess said.
The publication of the six action steps is targeted toward clinicians and researchers who work in Parkinson’s disease, she added. The steps address the following areas:
- 1. Disease burden
- 2. Advocacy and awareness
- 3. Prevention and risk reduction
- 4. Diagnosis, treatment, and care
- 5. Caregiver support
- 6. Research
Dr. Schiess noted that data on disease burden are lacking in certain areas of the world, such as low- and middle-income countries, and information “based on race and ethnicity are inconsistent. Studies are needed to establish more representative epidemiological data.”
She said that advocacy and awareness are particularly important since young people may not be aware they can also develop Parkinson’s disease, and sex and race differences can factor in to the potential for delays in diagnosis and care. “This is often due to the incorrect perception that Parkinson’s disease only affects older people,” she noted.
In addition, “a substantial need exists to identify risks for Parkinson’s disease – in particular the risks we can mitigate,” said Dr. Schiess, citing pesticide exposure as one example. “The evidence linking pesticide exposure, for example paraquat and chlorpyrifos, with the risk of developing Parkinson’s disease is substantial. And yet in many countries, these products are still being used.”
Under the heading of diagnosis, treatment, and care, Dr. Schiess noted that patients with Parkinson’s disease in “low resource settings” and low- to middle-income countries are unable to obtain “even the most basic medications” to treat Parkinson’s disease.
“Strengthening health and social systems, and building capacity to improve medical care, including rehabilitation and palliative care and medication access, are vital. Also, education and training of primary health care professionals, growing the neurological workforce, and increasing the use of digital technology such as telemedicine, are key mechanisms to improving diagnosis and sustainability of care,” she said.
For caregiver support, Dr. Schiess pointed out that the progressive nature of the disease and timing of onset are contributors to increased caregiver burden. Other contributors, as the disease advances in a patient, include the development of cognitive impairment, psychiatric manifestations, and sleep disruption.
“Solutions that could decrease the burden on caregivers include providing an accurate and timely diagnosis and training and education to caregivers, such as the WHO iSUPPORT program, as well as psychosocial, financial, and community-based support,” said Dr. Schiess.
For research, she noted that the amount of studies in the field of Parkinson’s disease has grown because of increased funding and a greater number of initiatives over the past 2 decades.
“Continuing to build on this momentum is important in order to generate new treatment options, better care, and research capacity, especially in low- and middle-income countries,” she said.
Dr. Schiess emphasized the urgency for adopting these measures as cases of Parkinson’s disease continue to rise.
“The take-away message for clinicians is that Parkinson disease is a growing global public health issue. There is a pressing need for a global public health response to address health and social requirements for people with Parkinson’s disease,” she said.
Dr. Schiess reports having received grants from the Edmond J. Safra Foundation paid to her institution during the conduct of the study.
A version of this article first appeared on Medscape.com.
Since 2000, Parkinson’s disease has increased 81% and related deaths have increased 100% globally. In addition, many patients affected by Parkinson’s disease live in low- and middle-income countries and experience large inequalities in access to neurologic care and essential medicines.
To address these issues, the Brain Health Unit at the WHO developed six “action steps” it says are urgently required to combat global disparities in Parkinson’s disease.
The need for action is great, said lead author Nicoline Schiess, MD, MPH, a neurologist and technical officer in the WHO’s Brain Health Unit in Geneva.
“In adults, disorders of the nervous system are the leading cause of disability adjusted life years, or DALYs, and the second leading cause of death globally, accounting for 9 million deaths per year,” Dr. Schiess said.
The WHO’s recommendations were published online recently as a “Special Communication” in JAMA Neurology.
Serious public health challenge
Parkinson’s disease is the fastest growing disorder in terms of death and disability, and it is estimated that it caused 329,000 deaths in 2019 – an increase of more than 100% since 2000.
“The rise in cases is thought to be multifactorial and is likely affected by factors such as aging populations and environmental exposures, such as certain pesticides. With these rapidly increasing numbers, compounded by a lack of specialists and medicines in low- and middle-income countries, Parkinson’s disease presents a serious public health challenge,” Dr. Schiess said.
The publication of the six action steps is targeted toward clinicians and researchers who work in Parkinson’s disease, she added. The steps address the following areas:
- 1. Disease burden
- 2. Advocacy and awareness
- 3. Prevention and risk reduction
- 4. Diagnosis, treatment, and care
- 5. Caregiver support
- 6. Research
Dr. Schiess noted that data on disease burden are lacking in certain areas of the world, such as low- and middle-income countries, and information “based on race and ethnicity are inconsistent. Studies are needed to establish more representative epidemiological data.”
She said that advocacy and awareness are particularly important since young people may not be aware they can also develop Parkinson’s disease, and sex and race differences can factor in to the potential for delays in diagnosis and care. “This is often due to the incorrect perception that Parkinson’s disease only affects older people,” she noted.
In addition, “a substantial need exists to identify risks for Parkinson’s disease – in particular the risks we can mitigate,” said Dr. Schiess, citing pesticide exposure as one example. “The evidence linking pesticide exposure, for example paraquat and chlorpyrifos, with the risk of developing Parkinson’s disease is substantial. And yet in many countries, these products are still being used.”
Under the heading of diagnosis, treatment, and care, Dr. Schiess noted that patients with Parkinson’s disease in “low resource settings” and low- to middle-income countries are unable to obtain “even the most basic medications” to treat Parkinson’s disease.
“Strengthening health and social systems, and building capacity to improve medical care, including rehabilitation and palliative care and medication access, are vital. Also, education and training of primary health care professionals, growing the neurological workforce, and increasing the use of digital technology such as telemedicine, are key mechanisms to improving diagnosis and sustainability of care,” she said.
For caregiver support, Dr. Schiess pointed out that the progressive nature of the disease and timing of onset are contributors to increased caregiver burden. Other contributors, as the disease advances in a patient, include the development of cognitive impairment, psychiatric manifestations, and sleep disruption.
“Solutions that could decrease the burden on caregivers include providing an accurate and timely diagnosis and training and education to caregivers, such as the WHO iSUPPORT program, as well as psychosocial, financial, and community-based support,” said Dr. Schiess.
For research, she noted that the amount of studies in the field of Parkinson’s disease has grown because of increased funding and a greater number of initiatives over the past 2 decades.
“Continuing to build on this momentum is important in order to generate new treatment options, better care, and research capacity, especially in low- and middle-income countries,” she said.
Dr. Schiess emphasized the urgency for adopting these measures as cases of Parkinson’s disease continue to rise.
“The take-away message for clinicians is that Parkinson disease is a growing global public health issue. There is a pressing need for a global public health response to address health and social requirements for people with Parkinson’s disease,” she said.
Dr. Schiess reports having received grants from the Edmond J. Safra Foundation paid to her institution during the conduct of the study.
A version of this article first appeared on Medscape.com.
Horse hockey notwithstanding
He’s 24 years younger than I am, recently married, no kids. Just starting out as a neurologist. He also has a full head of hair, something I’m admittedly jealous of.
He’s always in a sweater, something that seems oddly out of place in Phoenix, Arizona.
He’s the picture on my hospital ID.
I don’t go to the hospital much anymore, but he still sits in my car, greeting me whenever I open the center console to get my sunglasses or phone charger. He looks very enthusiastic about starting his career. I clearly remember the day I had the picture taken, as a newly-minted attending getting his first hospital privileges.
Sometimes I talk to him. Usually it’s just silly advice (“bet on the ’16 Cubs”). Other times I wonder what he’d do in certain situations, with all his youthful enthusiasm. I’m sure he wonders the same about me, with my 24 years of experience.
To a large extent we are the same people we started out as, but time changes us, in ways besides the obvious (like my hairs jumping off like lemmings).
Looking back at him (or even the older pic on my medical school application) I have no complaints about where life and my career have taken me. Would there be a few things I might have changed if I could go back?
Realistically, maybe one or two, both involving my father, but neither of them would likely change where I am.
But as far as medicine goes? Not really. The things I liked then, that got me into the field? I still enjoy them. The horse hockey? Yeah, it’s always there, probably has gotten worse over time, and it still bothers me. But there isn’t a job that doesn’t have its share of cow patties. It’s just a matter of trying not to step in them more than necessary as you do the parts you enjoy.
Sometimes I look at my younger self, and wonder what I’d really say to him if we actually met.
Probably just “good luck, enjoy the ride, and ditch the sweater.”
We measure our gains out in luck and coincidence
Lanterns to turn back the night.
And put our defeats down to chance or experience
And try once again for the light.
– Al Stewart
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
He’s 24 years younger than I am, recently married, no kids. Just starting out as a neurologist. He also has a full head of hair, something I’m admittedly jealous of.
He’s always in a sweater, something that seems oddly out of place in Phoenix, Arizona.
He’s the picture on my hospital ID.
I don’t go to the hospital much anymore, but he still sits in my car, greeting me whenever I open the center console to get my sunglasses or phone charger. He looks very enthusiastic about starting his career. I clearly remember the day I had the picture taken, as a newly-minted attending getting his first hospital privileges.
Sometimes I talk to him. Usually it’s just silly advice (“bet on the ’16 Cubs”). Other times I wonder what he’d do in certain situations, with all his youthful enthusiasm. I’m sure he wonders the same about me, with my 24 years of experience.
To a large extent we are the same people we started out as, but time changes us, in ways besides the obvious (like my hairs jumping off like lemmings).
Looking back at him (or even the older pic on my medical school application) I have no complaints about where life and my career have taken me. Would there be a few things I might have changed if I could go back?
Realistically, maybe one or two, both involving my father, but neither of them would likely change where I am.
But as far as medicine goes? Not really. The things I liked then, that got me into the field? I still enjoy them. The horse hockey? Yeah, it’s always there, probably has gotten worse over time, and it still bothers me. But there isn’t a job that doesn’t have its share of cow patties. It’s just a matter of trying not to step in them more than necessary as you do the parts you enjoy.
Sometimes I look at my younger self, and wonder what I’d really say to him if we actually met.
Probably just “good luck, enjoy the ride, and ditch the sweater.”
We measure our gains out in luck and coincidence
Lanterns to turn back the night.
And put our defeats down to chance or experience
And try once again for the light.
– Al Stewart
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
He’s 24 years younger than I am, recently married, no kids. Just starting out as a neurologist. He also has a full head of hair, something I’m admittedly jealous of.
He’s always in a sweater, something that seems oddly out of place in Phoenix, Arizona.
He’s the picture on my hospital ID.
I don’t go to the hospital much anymore, but he still sits in my car, greeting me whenever I open the center console to get my sunglasses or phone charger. He looks very enthusiastic about starting his career. I clearly remember the day I had the picture taken, as a newly-minted attending getting his first hospital privileges.
Sometimes I talk to him. Usually it’s just silly advice (“bet on the ’16 Cubs”). Other times I wonder what he’d do in certain situations, with all his youthful enthusiasm. I’m sure he wonders the same about me, with my 24 years of experience.
To a large extent we are the same people we started out as, but time changes us, in ways besides the obvious (like my hairs jumping off like lemmings).
Looking back at him (or even the older pic on my medical school application) I have no complaints about where life and my career have taken me. Would there be a few things I might have changed if I could go back?
Realistically, maybe one or two, both involving my father, but neither of them would likely change where I am.
But as far as medicine goes? Not really. The things I liked then, that got me into the field? I still enjoy them. The horse hockey? Yeah, it’s always there, probably has gotten worse over time, and it still bothers me. But there isn’t a job that doesn’t have its share of cow patties. It’s just a matter of trying not to step in them more than necessary as you do the parts you enjoy.
Sometimes I look at my younger self, and wonder what I’d really say to him if we actually met.
Probably just “good luck, enjoy the ride, and ditch the sweater.”
We measure our gains out in luck and coincidence
Lanterns to turn back the night.
And put our defeats down to chance or experience
And try once again for the light.
– Al Stewart
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Obstructive sleep apnea linked to unprovoked VTE
Add unprovoked venous thromboembolic events to the list of potential consequences of severe obstructive sleep apnea.
That conclusion comes from a study showing that patients with obstructive sleep apnea (OSA) who had the longest nocturnal hypoxemia episodes had a twofold risk for venous thromboembolic events.
systems, reported Wojciech Trzepizur, MD, of Angers University Hospital, France.
Previous studies have suggested links between OSA and both cancer and cognitive decline, but this is the first study to investigate the association between OSA and the incidence of unprovoked VTE, he reported in an oral abstract session at the annual congress of the European Respiratory Society.
“We found that those who spent more than 6% of their nighttime with levels of oxygen in their blood below 90% of normal had an almost twofold risk of developing VTEs compared to patients without oxygen deprivation,” he said.
Dr. Trzepizur and colleagues conducted a retrospective study linking cohort data to an administrative health database. They identified unprovoked VTE in patients with a suspicion for OSA and no previous VTE.
They created Cox proportional hazard models to assess the association of unprovoked VTE with apnea hypopnea index (AHI) measures and nocturnal hypoxemia markers, including the time patients spent below 90% oxygen saturation (T90), oxygen desaturation index (ODI), and hypoxic burden, defined as the total area under the respiratory event-related desaturation curve.
They found that after a median follow-up of 6.3 years, 104 out of 7,355 patients had an unprovoked VTE. In an unadjusted hazard model, there were significant associations between VTE and T90, as well as with hypoxic burden, but not with either AHI or ODI.
However, in an analysis adjusted for age, gender, body mass index, alcohol intake, hypertension, depression, history of cardiovascular disease, statin use, type of sleep study, study site, and CPAP adherence, the investigators found that only T90 remained a significant independent predictor of VTE, with a hazard ratio of 1.06, P = .02.
The association between T90 and VTE strengthened as the time spent below 90% saturation increased. Patients in the highest tercile, who spent more than 6% of the time undersaturated, had an HR for VTE of 1.95 (P = .02), compared with patients with a T90 less than 1%.
There were no significant differences in VTE risk between patients who used CPAP for more than 4 hours per night and those who either used the devices for less than 4 hours or refused CPAP.
“We see that T90 seems to be a strong parameter,” said session comoderator Raphael Heinzer, MD, MPH, of Lausanne University Hospital, Switzerland.
Dr. Heinzer’s comoderator, Silke Ryan, MD, of University College Dublin, pointed out that although T90 was the main predictor of responses, Dr. Trzepizur and colleagues did not control for other pulmonary diseases.
“Obviously, there could be an influence of other hypoxic-related diseases,” she said, and recommended controlling for this in future studies.
Winfried Randerath, MD, of the Bethanien Hospital at the University of Cologne, Germany, head of the ERS specialist group on sleep disordered breathing, said that this study and others presented at the meeting “show worrying associations between obstructive sleep apnea and important diseases that affect survival and quality of life.
“While they cannot prove that OSA causes any of these health problems, people should be made aware of these links and should try to make lifestyle changes in order to reduce their risk of OSA, for instance, by maintaining a healthy weight. However, if OSA is suspected, definite diagnosis and treatment should be initiated. We look forward to further research that may help to clarify whether OSA may be causing some of the health problems seen in these studies,” said Dr. Randerath, who was not involved with the study.
The study was supported by a grant from Institut de Recherche en Santé Respiratoire des Pays de la Loire (IRSR), Beaucouzé, France. Dr. Trzepizur, Dr. Heinzer, Dr. Ryan and Dr. Randerath reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Add unprovoked venous thromboembolic events to the list of potential consequences of severe obstructive sleep apnea.
That conclusion comes from a study showing that patients with obstructive sleep apnea (OSA) who had the longest nocturnal hypoxemia episodes had a twofold risk for venous thromboembolic events.
systems, reported Wojciech Trzepizur, MD, of Angers University Hospital, France.
Previous studies have suggested links between OSA and both cancer and cognitive decline, but this is the first study to investigate the association between OSA and the incidence of unprovoked VTE, he reported in an oral abstract session at the annual congress of the European Respiratory Society.
“We found that those who spent more than 6% of their nighttime with levels of oxygen in their blood below 90% of normal had an almost twofold risk of developing VTEs compared to patients without oxygen deprivation,” he said.
Dr. Trzepizur and colleagues conducted a retrospective study linking cohort data to an administrative health database. They identified unprovoked VTE in patients with a suspicion for OSA and no previous VTE.
They created Cox proportional hazard models to assess the association of unprovoked VTE with apnea hypopnea index (AHI) measures and nocturnal hypoxemia markers, including the time patients spent below 90% oxygen saturation (T90), oxygen desaturation index (ODI), and hypoxic burden, defined as the total area under the respiratory event-related desaturation curve.
They found that after a median follow-up of 6.3 years, 104 out of 7,355 patients had an unprovoked VTE. In an unadjusted hazard model, there were significant associations between VTE and T90, as well as with hypoxic burden, but not with either AHI or ODI.
However, in an analysis adjusted for age, gender, body mass index, alcohol intake, hypertension, depression, history of cardiovascular disease, statin use, type of sleep study, study site, and CPAP adherence, the investigators found that only T90 remained a significant independent predictor of VTE, with a hazard ratio of 1.06, P = .02.
The association between T90 and VTE strengthened as the time spent below 90% saturation increased. Patients in the highest tercile, who spent more than 6% of the time undersaturated, had an HR for VTE of 1.95 (P = .02), compared with patients with a T90 less than 1%.
There were no significant differences in VTE risk between patients who used CPAP for more than 4 hours per night and those who either used the devices for less than 4 hours or refused CPAP.
“We see that T90 seems to be a strong parameter,” said session comoderator Raphael Heinzer, MD, MPH, of Lausanne University Hospital, Switzerland.
Dr. Heinzer’s comoderator, Silke Ryan, MD, of University College Dublin, pointed out that although T90 was the main predictor of responses, Dr. Trzepizur and colleagues did not control for other pulmonary diseases.
“Obviously, there could be an influence of other hypoxic-related diseases,” she said, and recommended controlling for this in future studies.
Winfried Randerath, MD, of the Bethanien Hospital at the University of Cologne, Germany, head of the ERS specialist group on sleep disordered breathing, said that this study and others presented at the meeting “show worrying associations between obstructive sleep apnea and important diseases that affect survival and quality of life.
“While they cannot prove that OSA causes any of these health problems, people should be made aware of these links and should try to make lifestyle changes in order to reduce their risk of OSA, for instance, by maintaining a healthy weight. However, if OSA is suspected, definite diagnosis and treatment should be initiated. We look forward to further research that may help to clarify whether OSA may be causing some of the health problems seen in these studies,” said Dr. Randerath, who was not involved with the study.
The study was supported by a grant from Institut de Recherche en Santé Respiratoire des Pays de la Loire (IRSR), Beaucouzé, France. Dr. Trzepizur, Dr. Heinzer, Dr. Ryan and Dr. Randerath reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Add unprovoked venous thromboembolic events to the list of potential consequences of severe obstructive sleep apnea.
That conclusion comes from a study showing that patients with obstructive sleep apnea (OSA) who had the longest nocturnal hypoxemia episodes had a twofold risk for venous thromboembolic events.
systems, reported Wojciech Trzepizur, MD, of Angers University Hospital, France.
Previous studies have suggested links between OSA and both cancer and cognitive decline, but this is the first study to investigate the association between OSA and the incidence of unprovoked VTE, he reported in an oral abstract session at the annual congress of the European Respiratory Society.
“We found that those who spent more than 6% of their nighttime with levels of oxygen in their blood below 90% of normal had an almost twofold risk of developing VTEs compared to patients without oxygen deprivation,” he said.
Dr. Trzepizur and colleagues conducted a retrospective study linking cohort data to an administrative health database. They identified unprovoked VTE in patients with a suspicion for OSA and no previous VTE.
They created Cox proportional hazard models to assess the association of unprovoked VTE with apnea hypopnea index (AHI) measures and nocturnal hypoxemia markers, including the time patients spent below 90% oxygen saturation (T90), oxygen desaturation index (ODI), and hypoxic burden, defined as the total area under the respiratory event-related desaturation curve.
They found that after a median follow-up of 6.3 years, 104 out of 7,355 patients had an unprovoked VTE. In an unadjusted hazard model, there were significant associations between VTE and T90, as well as with hypoxic burden, but not with either AHI or ODI.
However, in an analysis adjusted for age, gender, body mass index, alcohol intake, hypertension, depression, history of cardiovascular disease, statin use, type of sleep study, study site, and CPAP adherence, the investigators found that only T90 remained a significant independent predictor of VTE, with a hazard ratio of 1.06, P = .02.
The association between T90 and VTE strengthened as the time spent below 90% saturation increased. Patients in the highest tercile, who spent more than 6% of the time undersaturated, had an HR for VTE of 1.95 (P = .02), compared with patients with a T90 less than 1%.
There were no significant differences in VTE risk between patients who used CPAP for more than 4 hours per night and those who either used the devices for less than 4 hours or refused CPAP.
“We see that T90 seems to be a strong parameter,” said session comoderator Raphael Heinzer, MD, MPH, of Lausanne University Hospital, Switzerland.
Dr. Heinzer’s comoderator, Silke Ryan, MD, of University College Dublin, pointed out that although T90 was the main predictor of responses, Dr. Trzepizur and colleagues did not control for other pulmonary diseases.
“Obviously, there could be an influence of other hypoxic-related diseases,” she said, and recommended controlling for this in future studies.
Winfried Randerath, MD, of the Bethanien Hospital at the University of Cologne, Germany, head of the ERS specialist group on sleep disordered breathing, said that this study and others presented at the meeting “show worrying associations between obstructive sleep apnea and important diseases that affect survival and quality of life.
“While they cannot prove that OSA causes any of these health problems, people should be made aware of these links and should try to make lifestyle changes in order to reduce their risk of OSA, for instance, by maintaining a healthy weight. However, if OSA is suspected, definite diagnosis and treatment should be initiated. We look forward to further research that may help to clarify whether OSA may be causing some of the health problems seen in these studies,” said Dr. Randerath, who was not involved with the study.
The study was supported by a grant from Institut de Recherche en Santé Respiratoire des Pays de la Loire (IRSR), Beaucouzé, France. Dr. Trzepizur, Dr. Heinzer, Dr. Ryan and Dr. Randerath reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ERS 2022
COVID-19 linked to increased Alzheimer’s risk
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE