User login
Natural disasters leave late heart problems
Hurricanes, earthquakes and other natural disasters cause long-term changes in cardiovascular risk, new data show. Physicians probably should be thinking about how to monitor or even counter these increased risks, according to several speakers at the annual meeting of the American College of Cardiology in San Francisco.
Since Hurricane Katrina hit New Orleans in 2005, the proportion of patients admitted to Tulane Medical Center who were hospitalized because of myocardial infarction more than tripled and has stayed high for a surprising 6 years (so far), one retrospective study of 69,337 patients found. And New Orleans residents started having more heart attacks at night and on weekends instead of the usual pattern of increased MI rates on weekday mornings, especially Mondays, a separate retrospective study showed. A world away, in Japan, the MI rate jumped not only after the massive earthquake in 2011 but also after seismically intense shocks before or after the big one, a third study found.
There’s not much we can do to prevent natural disasters, but can we do something about the cardiac consequences? "The impact is inevitable if we don’t intervene prophylactically," Tulane’s Dr. Anand M. Irimpen said at a press conference featuring these studies.
He and his associates found that the incidence of MI in patients being admitted to the hospital increased from 0.7% in the 2 years preceding Hurricane Katrina to 2.4% in the 6 years after Katrina (P less than .0001). Patients post Katrina were significantly more likely to have known coronary artery disease, a prior coronary artery bypass graft, hyperlipidemia, psychiatric comorbidities, and substance abuse; and to be smokers, unemployed, and uninsured, compared with pre-hurricane patients. Prescriptions for aspirin, beta-blockers, statins, ACE inhibitors, and angiotensin receptor blockers were more likely for post-Katrina patients, but they were less likely to adhere to therapy, compared with pre-hurricane patients (52% vs. 71%, respectively; P less than .0001).
"The fact that this persisted for 6 years speaks volumes" about the far-reaching, long-term destruction caused by the hurricane, he said.
Often, the idea of rebuilding homes gets the most attention after a natural disaster, but maybe rebuilding things like pharmacies or fitness centers should get more attention, he added. Mobile pharmacies might help patients adhere to therapy in the meantime. And clinicians should pay close attention to patients’ stress and anxiety levels long after a disaster, Dr. Irimpen said.
The shift away from weekday morning heart attacks to night and weekend MIs, and the persistence of this pattern, "shows that stress played a big role" and even outweighed some other factors, said Tulane’s Dr. Matthew Peters.
The study of data on 299 MIs before Katrina, 408 in the first 3 years after, and 337 in years 4 and 5 after the hurricane, showed that 44% of heart attacks in years 4 and 5 happened on weekends after Katrina, compared with 30% before. The proportion of weekday heart attacks decreased from 60% before Katrina to 36% in years 4 and 5, and the proportion of morning MIs decreased from 45% to 31%.
The influx of heart attacks in the off-hours of nights and weekends has practical implications. Fewer medical staff are available, and other data suggest that MI patients treated at night are more likely to fail angioplasty, have longer door-to-balloon times, and develop complications in the hospital, he said.
The Japanese study also suggested that stress trumped the usual circadian pattern of heart attacks and the environmental destruction caused by the March 11, 2011 earthquake and tsunami. The intensity of the earth’s shaking – even in pre-shocks leading up to the big one and aftershocks – corresponded with increases in heart attacks, Dr. Motoyuki Nakamura and his associates reported.
They analyzed data for 53 patients in Japan’s Iwate Prefecture who suffered MI or sudden death in the 4 weeks leading up to the big earthquake and 8 weeks after, and compared those with 30 cases of MI or sudden death in the same weeks of 2009 and 2010 in the same geographical area. In 2011, the incidence of MI or sudden death rose and fell according to the seismic intensity of earth shocks and was nearly twice as high as in the previous years.
Preventive measures in earthquake-prone areas should be considered, such as public availability of automated external defibrillators and advanced hospital disaster planning, suggested Dr. Nakamura, professor of medicine at Iwate (Japan) Medical University. Clinicians should keep a supply of medications on hand to help prevent MI in high-risk patients, such as quick-acting calcium channel blockers, beta-blockers, aspirin, and sublingual nitroglycerin, he added.
The cardiac effects of natural disasters can be felt long after a hurricane dies or the ground stops shaking. Pictures of the destruction may feel heartbreaking, but it’s clear that these disasters really do break hearts.
Dr. Nakamura’s study was funded in part by the Takeda Science Foundation. Dr. Irimpen, Dr. Peters, and Dr. Nakamura reported having no financial disclosures.
–By Sherry Boschert
Hurricanes, earthquakes and other natural disasters cause long-term changes in cardiovascular risk, new data show. Physicians probably should be thinking about how to monitor or even counter these increased risks, according to several speakers at the annual meeting of the American College of Cardiology in San Francisco.
Since Hurricane Katrina hit New Orleans in 2005, the proportion of patients admitted to Tulane Medical Center who were hospitalized because of myocardial infarction more than tripled and has stayed high for a surprising 6 years (so far), one retrospective study of 69,337 patients found. And New Orleans residents started having more heart attacks at night and on weekends instead of the usual pattern of increased MI rates on weekday mornings, especially Mondays, a separate retrospective study showed. A world away, in Japan, the MI rate jumped not only after the massive earthquake in 2011 but also after seismically intense shocks before or after the big one, a third study found.
There’s not much we can do to prevent natural disasters, but can we do something about the cardiac consequences? "The impact is inevitable if we don’t intervene prophylactically," Tulane’s Dr. Anand M. Irimpen said at a press conference featuring these studies.
He and his associates found that the incidence of MI in patients being admitted to the hospital increased from 0.7% in the 2 years preceding Hurricane Katrina to 2.4% in the 6 years after Katrina (P less than .0001). Patients post Katrina were significantly more likely to have known coronary artery disease, a prior coronary artery bypass graft, hyperlipidemia, psychiatric comorbidities, and substance abuse; and to be smokers, unemployed, and uninsured, compared with pre-hurricane patients. Prescriptions for aspirin, beta-blockers, statins, ACE inhibitors, and angiotensin receptor blockers were more likely for post-Katrina patients, but they were less likely to adhere to therapy, compared with pre-hurricane patients (52% vs. 71%, respectively; P less than .0001).
"The fact that this persisted for 6 years speaks volumes" about the far-reaching, long-term destruction caused by the hurricane, he said.
Often, the idea of rebuilding homes gets the most attention after a natural disaster, but maybe rebuilding things like pharmacies or fitness centers should get more attention, he added. Mobile pharmacies might help patients adhere to therapy in the meantime. And clinicians should pay close attention to patients’ stress and anxiety levels long after a disaster, Dr. Irimpen said.
The shift away from weekday morning heart attacks to night and weekend MIs, and the persistence of this pattern, "shows that stress played a big role" and even outweighed some other factors, said Tulane’s Dr. Matthew Peters.
The study of data on 299 MIs before Katrina, 408 in the first 3 years after, and 337 in years 4 and 5 after the hurricane, showed that 44% of heart attacks in years 4 and 5 happened on weekends after Katrina, compared with 30% before. The proportion of weekday heart attacks decreased from 60% before Katrina to 36% in years 4 and 5, and the proportion of morning MIs decreased from 45% to 31%.
The influx of heart attacks in the off-hours of nights and weekends has practical implications. Fewer medical staff are available, and other data suggest that MI patients treated at night are more likely to fail angioplasty, have longer door-to-balloon times, and develop complications in the hospital, he said.
The Japanese study also suggested that stress trumped the usual circadian pattern of heart attacks and the environmental destruction caused by the March 11, 2011 earthquake and tsunami. The intensity of the earth’s shaking – even in pre-shocks leading up to the big one and aftershocks – corresponded with increases in heart attacks, Dr. Motoyuki Nakamura and his associates reported.
They analyzed data for 53 patients in Japan’s Iwate Prefecture who suffered MI or sudden death in the 4 weeks leading up to the big earthquake and 8 weeks after, and compared those with 30 cases of MI or sudden death in the same weeks of 2009 and 2010 in the same geographical area. In 2011, the incidence of MI or sudden death rose and fell according to the seismic intensity of earth shocks and was nearly twice as high as in the previous years.
Preventive measures in earthquake-prone areas should be considered, such as public availability of automated external defibrillators and advanced hospital disaster planning, suggested Dr. Nakamura, professor of medicine at Iwate (Japan) Medical University. Clinicians should keep a supply of medications on hand to help prevent MI in high-risk patients, such as quick-acting calcium channel blockers, beta-blockers, aspirin, and sublingual nitroglycerin, he added.
The cardiac effects of natural disasters can be felt long after a hurricane dies or the ground stops shaking. Pictures of the destruction may feel heartbreaking, but it’s clear that these disasters really do break hearts.
Dr. Nakamura’s study was funded in part by the Takeda Science Foundation. Dr. Irimpen, Dr. Peters, and Dr. Nakamura reported having no financial disclosures.
–By Sherry Boschert
Hurricanes, earthquakes and other natural disasters cause long-term changes in cardiovascular risk, new data show. Physicians probably should be thinking about how to monitor or even counter these increased risks, according to several speakers at the annual meeting of the American College of Cardiology in San Francisco.
Since Hurricane Katrina hit New Orleans in 2005, the proportion of patients admitted to Tulane Medical Center who were hospitalized because of myocardial infarction more than tripled and has stayed high for a surprising 6 years (so far), one retrospective study of 69,337 patients found. And New Orleans residents started having more heart attacks at night and on weekends instead of the usual pattern of increased MI rates on weekday mornings, especially Mondays, a separate retrospective study showed. A world away, in Japan, the MI rate jumped not only after the massive earthquake in 2011 but also after seismically intense shocks before or after the big one, a third study found.
There’s not much we can do to prevent natural disasters, but can we do something about the cardiac consequences? "The impact is inevitable if we don’t intervene prophylactically," Tulane’s Dr. Anand M. Irimpen said at a press conference featuring these studies.
He and his associates found that the incidence of MI in patients being admitted to the hospital increased from 0.7% in the 2 years preceding Hurricane Katrina to 2.4% in the 6 years after Katrina (P less than .0001). Patients post Katrina were significantly more likely to have known coronary artery disease, a prior coronary artery bypass graft, hyperlipidemia, psychiatric comorbidities, and substance abuse; and to be smokers, unemployed, and uninsured, compared with pre-hurricane patients. Prescriptions for aspirin, beta-blockers, statins, ACE inhibitors, and angiotensin receptor blockers were more likely for post-Katrina patients, but they were less likely to adhere to therapy, compared with pre-hurricane patients (52% vs. 71%, respectively; P less than .0001).
"The fact that this persisted for 6 years speaks volumes" about the far-reaching, long-term destruction caused by the hurricane, he said.
Often, the idea of rebuilding homes gets the most attention after a natural disaster, but maybe rebuilding things like pharmacies or fitness centers should get more attention, he added. Mobile pharmacies might help patients adhere to therapy in the meantime. And clinicians should pay close attention to patients’ stress and anxiety levels long after a disaster, Dr. Irimpen said.
The shift away from weekday morning heart attacks to night and weekend MIs, and the persistence of this pattern, "shows that stress played a big role" and even outweighed some other factors, said Tulane’s Dr. Matthew Peters.
The study of data on 299 MIs before Katrina, 408 in the first 3 years after, and 337 in years 4 and 5 after the hurricane, showed that 44% of heart attacks in years 4 and 5 happened on weekends after Katrina, compared with 30% before. The proportion of weekday heart attacks decreased from 60% before Katrina to 36% in years 4 and 5, and the proportion of morning MIs decreased from 45% to 31%.
The influx of heart attacks in the off-hours of nights and weekends has practical implications. Fewer medical staff are available, and other data suggest that MI patients treated at night are more likely to fail angioplasty, have longer door-to-balloon times, and develop complications in the hospital, he said.
The Japanese study also suggested that stress trumped the usual circadian pattern of heart attacks and the environmental destruction caused by the March 11, 2011 earthquake and tsunami. The intensity of the earth’s shaking – even in pre-shocks leading up to the big one and aftershocks – corresponded with increases in heart attacks, Dr. Motoyuki Nakamura and his associates reported.
They analyzed data for 53 patients in Japan’s Iwate Prefecture who suffered MI or sudden death in the 4 weeks leading up to the big earthquake and 8 weeks after, and compared those with 30 cases of MI or sudden death in the same weeks of 2009 and 2010 in the same geographical area. In 2011, the incidence of MI or sudden death rose and fell according to the seismic intensity of earth shocks and was nearly twice as high as in the previous years.
Preventive measures in earthquake-prone areas should be considered, such as public availability of automated external defibrillators and advanced hospital disaster planning, suggested Dr. Nakamura, professor of medicine at Iwate (Japan) Medical University. Clinicians should keep a supply of medications on hand to help prevent MI in high-risk patients, such as quick-acting calcium channel blockers, beta-blockers, aspirin, and sublingual nitroglycerin, he added.
The cardiac effects of natural disasters can be felt long after a hurricane dies or the ground stops shaking. Pictures of the destruction may feel heartbreaking, but it’s clear that these disasters really do break hearts.
Dr. Nakamura’s study was funded in part by the Takeda Science Foundation. Dr. Irimpen, Dr. Peters, and Dr. Nakamura reported having no financial disclosures.
–By Sherry Boschert
More insulin resistance, metabolic syndrome with PTSD
SAN FRANCISCO – Posttraumatic stress disorder independently increased the risk of insulin resistance by 80% and metabolic syndrome by 40% in a retrospective study of 207,954 veterans.
The incidence of insulin resistance was 14% higher and the incidence of metabolic syndrome was 12% higher in 11,420 veterans with posttraumatic stress disorder (PTSD), compared with 196,534 without PTSD, after adjusting for the effects of age, gender, ethnicity, high blood pressure, high cholesterol, family history of premature coronary artery disease, and obesity, study coleader Dr. Ramin Ebrahimi reported at the annual meeting of the American College of Cardiology.
Insulin resistance is known to increase atherogenesis and atherosclerotic plaque instability, resulting in greater risk for MI. The cluster of conditions known as metabolic syndrome (hypertension, hyperlipidemia, hyperglycemia, and abnormal cholesterol levels) has been associated with increased risk of heart disease, diabetes, and stroke.
Among veterans with PTSD, 35% had insulin resistance and 53% had metabolic syndrome, compared with 19% and 38%, respectively, in the non-PTSD group before adjusting for other factors, showing relative risk increases of roughly 80% and 40%. PTSD remained independently associated with higher rates of insulin resistance and metabolic syndrome after controlling for other risk factors, said Dr. Ebrahimi of the University of California, Los Angeles.
The investigators analyzed electronic medical records from primary care settings in the Veterans Health Administration for patients in Southern California and Nevada without known diabetes or coronary artery disease who were followed for a median of 2 years.
Although the PTSD and non-PTSD groups were similar at baseline in age, gender, lipid measures, fasting blood sugar measures, and conventional risk factors for insulin resistance and metabolic syndrome, the PTSD group developed significantly higher levels of triglycerides and fasting blood sugar, lower HDL levels, and a higher triglyceride to HDL ratio, compared with the non-PTSD group. At baseline, the veterans had a mean age of 60 years and 93% were male.
The findings may help clinicians identify and treat cardiovascular risks earlier in patients with PTSD, Dr. Ebrahimi suggested at a press conference at the meeting. Early-stage insulin resistance and metabolic syndrome can be reversed with lifestyle modifications in diet and exercise, and a more integrated approach to treating PTSD may be warranted.
The investigators now are studying the relationship of early PTSD treatment and the risk for insulin resistance and metabolic syndrome. They also are examining the effects of combined psychiatric and medical management of PTSD on the risks of metabolic disorders, MI, stroke, and death.
Whether the PTSD itself or something associated with PTSD increases the risks for insulin resistance and metabolic syndrome once PTSD is diagnosed, "these patients must be looked at a little more closely, and the risk factors in general have to be controlled a little bit more vigilantly," said Dr. Ebrahimi, who is also an interventional cardiologist in the Veterans Affairs Greater Los Angeles Healthcare System.
Nearly 8 million U.S. residents have PTSD, which is now recognized to be prevalent not only in veterans but in the broader population. "It happens when you are in an accident or have an experience that confers significant stress, health damage, or death," including rape and other traumatic events, he said.
The study defined insulin resistance as a triglyceride/HDL ratio of 3.8 or greater. Metabolic syndrome was defined by National Cholesterol Education Program Adult Treatment Program III guidelines.
Dr. Ebrahimi disclosed financial relationships with Boehringer Ingelheim, Abbott Vascular, the Medicines Company, Sanofi-Aventis, and Gilead.
*Correction 3/27/2013: An earlier version of this article included different percentages of insulin resistance and metabolic syndrome in the Major Finding section of this page.
SAN FRANCISCO – Posttraumatic stress disorder independently increased the risk of insulin resistance by 80% and metabolic syndrome by 40% in a retrospective study of 207,954 veterans.
The incidence of insulin resistance was 14% higher and the incidence of metabolic syndrome was 12% higher in 11,420 veterans with posttraumatic stress disorder (PTSD), compared with 196,534 without PTSD, after adjusting for the effects of age, gender, ethnicity, high blood pressure, high cholesterol, family history of premature coronary artery disease, and obesity, study coleader Dr. Ramin Ebrahimi reported at the annual meeting of the American College of Cardiology.
Insulin resistance is known to increase atherogenesis and atherosclerotic plaque instability, resulting in greater risk for MI. The cluster of conditions known as metabolic syndrome (hypertension, hyperlipidemia, hyperglycemia, and abnormal cholesterol levels) has been associated with increased risk of heart disease, diabetes, and stroke.
Among veterans with PTSD, 35% had insulin resistance and 53% had metabolic syndrome, compared with 19% and 38%, respectively, in the non-PTSD group before adjusting for other factors, showing relative risk increases of roughly 80% and 40%. PTSD remained independently associated with higher rates of insulin resistance and metabolic syndrome after controlling for other risk factors, said Dr. Ebrahimi of the University of California, Los Angeles.
The investigators analyzed electronic medical records from primary care settings in the Veterans Health Administration for patients in Southern California and Nevada without known diabetes or coronary artery disease who were followed for a median of 2 years.
Although the PTSD and non-PTSD groups were similar at baseline in age, gender, lipid measures, fasting blood sugar measures, and conventional risk factors for insulin resistance and metabolic syndrome, the PTSD group developed significantly higher levels of triglycerides and fasting blood sugar, lower HDL levels, and a higher triglyceride to HDL ratio, compared with the non-PTSD group. At baseline, the veterans had a mean age of 60 years and 93% were male.
The findings may help clinicians identify and treat cardiovascular risks earlier in patients with PTSD, Dr. Ebrahimi suggested at a press conference at the meeting. Early-stage insulin resistance and metabolic syndrome can be reversed with lifestyle modifications in diet and exercise, and a more integrated approach to treating PTSD may be warranted.
The investigators now are studying the relationship of early PTSD treatment and the risk for insulin resistance and metabolic syndrome. They also are examining the effects of combined psychiatric and medical management of PTSD on the risks of metabolic disorders, MI, stroke, and death.
Whether the PTSD itself or something associated with PTSD increases the risks for insulin resistance and metabolic syndrome once PTSD is diagnosed, "these patients must be looked at a little more closely, and the risk factors in general have to be controlled a little bit more vigilantly," said Dr. Ebrahimi, who is also an interventional cardiologist in the Veterans Affairs Greater Los Angeles Healthcare System.
Nearly 8 million U.S. residents have PTSD, which is now recognized to be prevalent not only in veterans but in the broader population. "It happens when you are in an accident or have an experience that confers significant stress, health damage, or death," including rape and other traumatic events, he said.
The study defined insulin resistance as a triglyceride/HDL ratio of 3.8 or greater. Metabolic syndrome was defined by National Cholesterol Education Program Adult Treatment Program III guidelines.
Dr. Ebrahimi disclosed financial relationships with Boehringer Ingelheim, Abbott Vascular, the Medicines Company, Sanofi-Aventis, and Gilead.
*Correction 3/27/2013: An earlier version of this article included different percentages of insulin resistance and metabolic syndrome in the Major Finding section of this page.
SAN FRANCISCO – Posttraumatic stress disorder independently increased the risk of insulin resistance by 80% and metabolic syndrome by 40% in a retrospective study of 207,954 veterans.
The incidence of insulin resistance was 14% higher and the incidence of metabolic syndrome was 12% higher in 11,420 veterans with posttraumatic stress disorder (PTSD), compared with 196,534 without PTSD, after adjusting for the effects of age, gender, ethnicity, high blood pressure, high cholesterol, family history of premature coronary artery disease, and obesity, study coleader Dr. Ramin Ebrahimi reported at the annual meeting of the American College of Cardiology.
Insulin resistance is known to increase atherogenesis and atherosclerotic plaque instability, resulting in greater risk for MI. The cluster of conditions known as metabolic syndrome (hypertension, hyperlipidemia, hyperglycemia, and abnormal cholesterol levels) has been associated with increased risk of heart disease, diabetes, and stroke.
Among veterans with PTSD, 35% had insulin resistance and 53% had metabolic syndrome, compared with 19% and 38%, respectively, in the non-PTSD group before adjusting for other factors, showing relative risk increases of roughly 80% and 40%. PTSD remained independently associated with higher rates of insulin resistance and metabolic syndrome after controlling for other risk factors, said Dr. Ebrahimi of the University of California, Los Angeles.
The investigators analyzed electronic medical records from primary care settings in the Veterans Health Administration for patients in Southern California and Nevada without known diabetes or coronary artery disease who were followed for a median of 2 years.
Although the PTSD and non-PTSD groups were similar at baseline in age, gender, lipid measures, fasting blood sugar measures, and conventional risk factors for insulin resistance and metabolic syndrome, the PTSD group developed significantly higher levels of triglycerides and fasting blood sugar, lower HDL levels, and a higher triglyceride to HDL ratio, compared with the non-PTSD group. At baseline, the veterans had a mean age of 60 years and 93% were male.
The findings may help clinicians identify and treat cardiovascular risks earlier in patients with PTSD, Dr. Ebrahimi suggested at a press conference at the meeting. Early-stage insulin resistance and metabolic syndrome can be reversed with lifestyle modifications in diet and exercise, and a more integrated approach to treating PTSD may be warranted.
The investigators now are studying the relationship of early PTSD treatment and the risk for insulin resistance and metabolic syndrome. They also are examining the effects of combined psychiatric and medical management of PTSD on the risks of metabolic disorders, MI, stroke, and death.
Whether the PTSD itself or something associated with PTSD increases the risks for insulin resistance and metabolic syndrome once PTSD is diagnosed, "these patients must be looked at a little more closely, and the risk factors in general have to be controlled a little bit more vigilantly," said Dr. Ebrahimi, who is also an interventional cardiologist in the Veterans Affairs Greater Los Angeles Healthcare System.
Nearly 8 million U.S. residents have PTSD, which is now recognized to be prevalent not only in veterans but in the broader population. "It happens when you are in an accident or have an experience that confers significant stress, health damage, or death," including rape and other traumatic events, he said.
The study defined insulin resistance as a triglyceride/HDL ratio of 3.8 or greater. Metabolic syndrome was defined by National Cholesterol Education Program Adult Treatment Program III guidelines.
Dr. Ebrahimi disclosed financial relationships with Boehringer Ingelheim, Abbott Vascular, the Medicines Company, Sanofi-Aventis, and Gilead.
*Correction 3/27/2013: An earlier version of this article included different percentages of insulin resistance and metabolic syndrome in the Major Finding section of this page.
AT ACC 13
Major finding: *The incidence of insulin resistance was 14% higher and the incidence of metabolic syndrome was 12% higher in patients with PTSD, compared with those without PTSD.
Data source: Retrospective study of 207,954 veterans in two states.
Disclosures: Dr. Ebrahimi disclosed financial relationships with Boehringer Ingelheim, Abbott Vascular, the Medicines Company, Sanofi-Aventis, and Gilead.
HPS2-THRIVE drives another nail in niacin's coffin
SAN FRANCISCO – The largest-ever randomized trial of niacin for cardiovascular protection has not only failed to show clinical benefit, it spotlighted a hitherto underrecognized and disturbingly high range of serious harms in users of the HDL-raising drug.
The HPS2-THRIVE (Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events) trial found a rate of serious adverse events warranting hospitalization of 30 cases per 1,000 treated patients. Although many of these adverse events were already known to be associated with niacin therapy, two types of serious adverse events not previously recognized as niacin related were also identified: infections and bleeding, Dr. Jane Armitage reported at the annual meeting of the American College of Cardiology.
"The role of extended-release niacin for the treatment and prevention of cardiovascular disease needs to be reconsidered in light of these findings," said Dr. Armitage, professor of clinical trials and epidemiology at the University of Oxford (England).
That’s exactly what is now happening, as the European Medicine Agency has announced it is reviewing niacin’s status and considering its possible withdrawal from the marketplace.
The HPS2-THRIVE study involved 25,673 high-cardiovascular-risk patients in six countries, all of whom were placed on simvastatin 40 mg/day, with ezetimibe added if necessary in order to achieve a target total cholesterol level below 135 mg/dL. On that regimen, their mean LDL was an impressively favorable 63 mg/dL, with an HDL of 44 mg/dL and triglycerides of 125 mg/dL. This was a high-risk population. Roughly 80% of participants had a history of coronary artery disease, one-third had a history of cerebrovascular disease, and one-third had diabetes at baseline. After the investigators established during a run-in period that all participants could tolerate full-dose niacin, they were randomized to 2 g/day of extended-release niacin plus 40 mg of laropiprant to mitigate flushing or to placebo.
The active treatment arm had an average further 10-mg/dL reduction in LDL, a 6-mg/dL rise in HDL, and a 33-mg/dL drop in triglycerides. Extrapolating from earlier studies, Dr. Armitage and his coworkers anticipated that these lipid improvements would lead to an estimated 10%-15% reduction in the primary study endpoint, a composite of cardiac death, MI, stroke, or revascularization. But that’s not what transpired.
Indeed, during a median follow-up of 3.9 years, the primary endpoint occurred in 14.5% of patients on niacin/laropiprant and 15% on placebo, a nonsignificant difference.
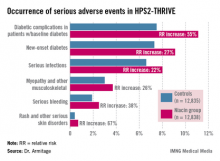
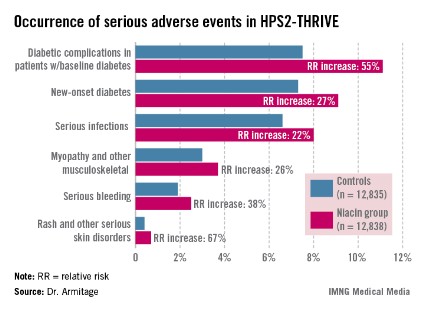
Diabetic complications among the more than 8,200 patients with diabetes at baseline occurred at an absolute 3.7% greater rate and 55% increased relative rate in the niacin group, compared with controls. Most of these diabetic complications led to multiday hospitalizations. Leading the way were major hyperglycemic episodes, which were 3.1-fold more common in the active treatment arm.
Among the other serious events that occurred at a significantly higher rate in the niacin group were serious infections in a variety of different organ systems, with a 22% increased rate; serious bleeding into the brain or gut, with a 38% increase; new-onset diabetes, with a 27% increase; myopathy and other serious musculoskeletal problems, with a 26% rise; and serious rash and other skin issues, which increased by 67%.
On the basis of prior studies, the most plausible culprit in the increased rates of serious adverse events is niacin rather than laropiprant, according to Dr. Armitage.
Discussant Donna Arnett, Ph.D., called HPS2-THRIVE "an exceptionally well-conducted study that definitively tells us the story of this drug combination in the setting of very well-controlled LDL."
"The results call into question the concept that increasing a low HDL in the context of a low LDL is really an important clinical problem," added Dr. Arnett, president of the American Heart Association and professor and chair of the department of epidemiology at the University of Alabama at Birmingham.
But panelist Dr. Christie M. Ballantyne indicated he has a big issue with the study: "If your LDL is 60, how important is your HDL, anyway? If I saw a patient who came to me with an LDL of 63, an HDL of 44, and triglycerides of 125, like the subjects in this trial, I would never even think of adding niacin because I know the drug has some risk and there is in my mind very little likelihood of seeing any benefit. But the majority of people in the U.S. who get niacin have LDLs greater than 70. I have high-risk patients who can only get their LDL down to 110 mg/dL with maximum statin therapy, and they can lower it by a further 30-40 mg/dL with niacin. What do I tell those patients now?
"Niacin has always been known as a drug that’s not easy to use, one with lots of side effects. It’s never been a first- or second-line therapy. It’s always been the drug where, when you can’t get there any other way, then you use it," observed Dr. Ballantyne, professor of medicine and chief of cardiology at Baylor College of Medicine, Houston.
Dr. Spencer B. King III called HPS2-THRIVE "another nail in the coffin for niacin," coming after the negative results of the National Institutes of Health–sponsored AIM-HIGH (Niacin Plus Statin to Prevent Vascular Events) study.
"I think the practice-changing part of this study is that many of us have been piling on a lot of niacin in people with low HDL. What you’ve shown is going to change attitudes among many physicians," predicted Dr. King, president of the Saint Joseph’s Heart and Vascular Institute, Atlanta.
When he asked Dr. Armitage whether any room remains now for individualized niacin therapy in selected patients, she replied that the investigators couldn’t identify any patient subgroup in whom the benefit outweighed the greater risk.
Dr. Rory Collins, chair of the HPS2-THRIVE trial, chimed in, adding: "Is niacin dead? I think in the light of these findings, it’s not healthy. The default position should change. It should be, Is there a good reason for using this therapy in any particular patient when there are additional ways, besides statins, of lowering LDL cholesterol, and where we don’t as yet have evidence that raising HDL cholesterol produces benefit?"
The HPS2-THRIVE trial was funded by Merck. Dr. Armitage, Dr. Collins, Dr. Arnett, and Dr. King reported having no relevant financial conflicts. Dr. Ballantyne serves as a consultant to numerous pharmaceutical companies, including Merck.
SAN FRANCISCO – The largest-ever randomized trial of niacin for cardiovascular protection has not only failed to show clinical benefit, it spotlighted a hitherto underrecognized and disturbingly high range of serious harms in users of the HDL-raising drug.
The HPS2-THRIVE (Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events) trial found a rate of serious adverse events warranting hospitalization of 30 cases per 1,000 treated patients. Although many of these adverse events were already known to be associated with niacin therapy, two types of serious adverse events not previously recognized as niacin related were also identified: infections and bleeding, Dr. Jane Armitage reported at the annual meeting of the American College of Cardiology.
"The role of extended-release niacin for the treatment and prevention of cardiovascular disease needs to be reconsidered in light of these findings," said Dr. Armitage, professor of clinical trials and epidemiology at the University of Oxford (England).
That’s exactly what is now happening, as the European Medicine Agency has announced it is reviewing niacin’s status and considering its possible withdrawal from the marketplace.
The HPS2-THRIVE study involved 25,673 high-cardiovascular-risk patients in six countries, all of whom were placed on simvastatin 40 mg/day, with ezetimibe added if necessary in order to achieve a target total cholesterol level below 135 mg/dL. On that regimen, their mean LDL was an impressively favorable 63 mg/dL, with an HDL of 44 mg/dL and triglycerides of 125 mg/dL. This was a high-risk population. Roughly 80% of participants had a history of coronary artery disease, one-third had a history of cerebrovascular disease, and one-third had diabetes at baseline. After the investigators established during a run-in period that all participants could tolerate full-dose niacin, they were randomized to 2 g/day of extended-release niacin plus 40 mg of laropiprant to mitigate flushing or to placebo.
The active treatment arm had an average further 10-mg/dL reduction in LDL, a 6-mg/dL rise in HDL, and a 33-mg/dL drop in triglycerides. Extrapolating from earlier studies, Dr. Armitage and his coworkers anticipated that these lipid improvements would lead to an estimated 10%-15% reduction in the primary study endpoint, a composite of cardiac death, MI, stroke, or revascularization. But that’s not what transpired.
Indeed, during a median follow-up of 3.9 years, the primary endpoint occurred in 14.5% of patients on niacin/laropiprant and 15% on placebo, a nonsignificant difference.
Diabetic complications among the more than 8,200 patients with diabetes at baseline occurred at an absolute 3.7% greater rate and 55% increased relative rate in the niacin group, compared with controls. Most of these diabetic complications led to multiday hospitalizations. Leading the way were major hyperglycemic episodes, which were 3.1-fold more common in the active treatment arm.
Among the other serious events that occurred at a significantly higher rate in the niacin group were serious infections in a variety of different organ systems, with a 22% increased rate; serious bleeding into the brain or gut, with a 38% increase; new-onset diabetes, with a 27% increase; myopathy and other serious musculoskeletal problems, with a 26% rise; and serious rash and other skin issues, which increased by 67%.
On the basis of prior studies, the most plausible culprit in the increased rates of serious adverse events is niacin rather than laropiprant, according to Dr. Armitage.
Discussant Donna Arnett, Ph.D., called HPS2-THRIVE "an exceptionally well-conducted study that definitively tells us the story of this drug combination in the setting of very well-controlled LDL."
"The results call into question the concept that increasing a low HDL in the context of a low LDL is really an important clinical problem," added Dr. Arnett, president of the American Heart Association and professor and chair of the department of epidemiology at the University of Alabama at Birmingham.
But panelist Dr. Christie M. Ballantyne indicated he has a big issue with the study: "If your LDL is 60, how important is your HDL, anyway? If I saw a patient who came to me with an LDL of 63, an HDL of 44, and triglycerides of 125, like the subjects in this trial, I would never even think of adding niacin because I know the drug has some risk and there is in my mind very little likelihood of seeing any benefit. But the majority of people in the U.S. who get niacin have LDLs greater than 70. I have high-risk patients who can only get their LDL down to 110 mg/dL with maximum statin therapy, and they can lower it by a further 30-40 mg/dL with niacin. What do I tell those patients now?
"Niacin has always been known as a drug that’s not easy to use, one with lots of side effects. It’s never been a first- or second-line therapy. It’s always been the drug where, when you can’t get there any other way, then you use it," observed Dr. Ballantyne, professor of medicine and chief of cardiology at Baylor College of Medicine, Houston.
Dr. Spencer B. King III called HPS2-THRIVE "another nail in the coffin for niacin," coming after the negative results of the National Institutes of Health–sponsored AIM-HIGH (Niacin Plus Statin to Prevent Vascular Events) study.
"I think the practice-changing part of this study is that many of us have been piling on a lot of niacin in people with low HDL. What you’ve shown is going to change attitudes among many physicians," predicted Dr. King, president of the Saint Joseph’s Heart and Vascular Institute, Atlanta.
When he asked Dr. Armitage whether any room remains now for individualized niacin therapy in selected patients, she replied that the investigators couldn’t identify any patient subgroup in whom the benefit outweighed the greater risk.
Dr. Rory Collins, chair of the HPS2-THRIVE trial, chimed in, adding: "Is niacin dead? I think in the light of these findings, it’s not healthy. The default position should change. It should be, Is there a good reason for using this therapy in any particular patient when there are additional ways, besides statins, of lowering LDL cholesterol, and where we don’t as yet have evidence that raising HDL cholesterol produces benefit?"
The HPS2-THRIVE trial was funded by Merck. Dr. Armitage, Dr. Collins, Dr. Arnett, and Dr. King reported having no relevant financial conflicts. Dr. Ballantyne serves as a consultant to numerous pharmaceutical companies, including Merck.
SAN FRANCISCO – The largest-ever randomized trial of niacin for cardiovascular protection has not only failed to show clinical benefit, it spotlighted a hitherto underrecognized and disturbingly high range of serious harms in users of the HDL-raising drug.
The HPS2-THRIVE (Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events) trial found a rate of serious adverse events warranting hospitalization of 30 cases per 1,000 treated patients. Although many of these adverse events were already known to be associated with niacin therapy, two types of serious adverse events not previously recognized as niacin related were also identified: infections and bleeding, Dr. Jane Armitage reported at the annual meeting of the American College of Cardiology.
"The role of extended-release niacin for the treatment and prevention of cardiovascular disease needs to be reconsidered in light of these findings," said Dr. Armitage, professor of clinical trials and epidemiology at the University of Oxford (England).
That’s exactly what is now happening, as the European Medicine Agency has announced it is reviewing niacin’s status and considering its possible withdrawal from the marketplace.
The HPS2-THRIVE study involved 25,673 high-cardiovascular-risk patients in six countries, all of whom were placed on simvastatin 40 mg/day, with ezetimibe added if necessary in order to achieve a target total cholesterol level below 135 mg/dL. On that regimen, their mean LDL was an impressively favorable 63 mg/dL, with an HDL of 44 mg/dL and triglycerides of 125 mg/dL. This was a high-risk population. Roughly 80% of participants had a history of coronary artery disease, one-third had a history of cerebrovascular disease, and one-third had diabetes at baseline. After the investigators established during a run-in period that all participants could tolerate full-dose niacin, they were randomized to 2 g/day of extended-release niacin plus 40 mg of laropiprant to mitigate flushing or to placebo.
The active treatment arm had an average further 10-mg/dL reduction in LDL, a 6-mg/dL rise in HDL, and a 33-mg/dL drop in triglycerides. Extrapolating from earlier studies, Dr. Armitage and his coworkers anticipated that these lipid improvements would lead to an estimated 10%-15% reduction in the primary study endpoint, a composite of cardiac death, MI, stroke, or revascularization. But that’s not what transpired.
Indeed, during a median follow-up of 3.9 years, the primary endpoint occurred in 14.5% of patients on niacin/laropiprant and 15% on placebo, a nonsignificant difference.
Diabetic complications among the more than 8,200 patients with diabetes at baseline occurred at an absolute 3.7% greater rate and 55% increased relative rate in the niacin group, compared with controls. Most of these diabetic complications led to multiday hospitalizations. Leading the way were major hyperglycemic episodes, which were 3.1-fold more common in the active treatment arm.
Among the other serious events that occurred at a significantly higher rate in the niacin group were serious infections in a variety of different organ systems, with a 22% increased rate; serious bleeding into the brain or gut, with a 38% increase; new-onset diabetes, with a 27% increase; myopathy and other serious musculoskeletal problems, with a 26% rise; and serious rash and other skin issues, which increased by 67%.
On the basis of prior studies, the most plausible culprit in the increased rates of serious adverse events is niacin rather than laropiprant, according to Dr. Armitage.
Discussant Donna Arnett, Ph.D., called HPS2-THRIVE "an exceptionally well-conducted study that definitively tells us the story of this drug combination in the setting of very well-controlled LDL."
"The results call into question the concept that increasing a low HDL in the context of a low LDL is really an important clinical problem," added Dr. Arnett, president of the American Heart Association and professor and chair of the department of epidemiology at the University of Alabama at Birmingham.
But panelist Dr. Christie M. Ballantyne indicated he has a big issue with the study: "If your LDL is 60, how important is your HDL, anyway? If I saw a patient who came to me with an LDL of 63, an HDL of 44, and triglycerides of 125, like the subjects in this trial, I would never even think of adding niacin because I know the drug has some risk and there is in my mind very little likelihood of seeing any benefit. But the majority of people in the U.S. who get niacin have LDLs greater than 70. I have high-risk patients who can only get their LDL down to 110 mg/dL with maximum statin therapy, and they can lower it by a further 30-40 mg/dL with niacin. What do I tell those patients now?
"Niacin has always been known as a drug that’s not easy to use, one with lots of side effects. It’s never been a first- or second-line therapy. It’s always been the drug where, when you can’t get there any other way, then you use it," observed Dr. Ballantyne, professor of medicine and chief of cardiology at Baylor College of Medicine, Houston.
Dr. Spencer B. King III called HPS2-THRIVE "another nail in the coffin for niacin," coming after the negative results of the National Institutes of Health–sponsored AIM-HIGH (Niacin Plus Statin to Prevent Vascular Events) study.
"I think the practice-changing part of this study is that many of us have been piling on a lot of niacin in people with low HDL. What you’ve shown is going to change attitudes among many physicians," predicted Dr. King, president of the Saint Joseph’s Heart and Vascular Institute, Atlanta.
When he asked Dr. Armitage whether any room remains now for individualized niacin therapy in selected patients, she replied that the investigators couldn’t identify any patient subgroup in whom the benefit outweighed the greater risk.
Dr. Rory Collins, chair of the HPS2-THRIVE trial, chimed in, adding: "Is niacin dead? I think in the light of these findings, it’s not healthy. The default position should change. It should be, Is there a good reason for using this therapy in any particular patient when there are additional ways, besides statins, of lowering LDL cholesterol, and where we don’t as yet have evidence that raising HDL cholesterol produces benefit?"
The HPS2-THRIVE trial was funded by Merck. Dr. Armitage, Dr. Collins, Dr. Arnett, and Dr. King reported having no relevant financial conflicts. Dr. Ballantyne serves as a consultant to numerous pharmaceutical companies, including Merck.
AT ACC 13
Major Finding: During an average 3.9-year prospective follow-up, the rate of the combined endpoint of coronary death, nonfatal MI, stroke, or revascularization occurred in 14.5% of patients on 2 g of extended-release niacin plus 40 mg of laropiprant per day and 15% of placebo-treated controls, a nonsignificant difference. Serious adverse events warranting hospitalization occurred in the niacin group at a rate of 30/1,000 treated patients.
Data Source: HPS2-THRIVE, which randomized 25,673 high-cardiovascular-risk patients with low baseline LDL levels on simvastatin with or without ezetimibe to niacin/laropiprant or placebo.
Disclosures: The study was sponsored by Merck. The presenter reported having no relevant financial conflicts.
Being postmenopausal doubles hepatic steatosis risk
SAN FRANCISCO – Postmenopausal status is independently associated with a twofold increased risk of hepatic steatosis, the Dallas Heart Study has shown.
Among 1,018 women aged 30-65 years enrolled in the population-based study, 48% were postmenopausal. Their prevalence of hepatic steatosis as defined by greater than 5.5% hepatic fat content measured by magnetic resonance spectroscopy was 34%. In contrast, the prevalence was significantly less at 24% in the premenopausal women, Dr. Monika Sanghavi reported at the annual meeting of the American College of Cardiology.
The absolute hepatic triglyceride content in the postmenopausal cohort was 4.0%, significantly more than the 2.9% value in premenopausal women.
Of note, the prevalence of hepatic steatosis rose with greater time since the last menstrual period (LMP). The prevalence was 22% among women whose LMP was less than 2 months earlier, 31% in those whose LMP was 2-12 months earlier, and 35% in women whose LMP was more than 12 months prior, according to Dr. Sanghavi of the University of Texas Southwestern Medical Center, Dallas.
Women who were postmenopausal were, of course, substantially older on average than premenopausal women. However, they also had significantly higher average systolic blood pressure, 129 compared with 117 mm Hg; a greater prevalence of diabetes, 16% vs. 7%; a mean LDL cholesterol of 113 compared with 98 mg/dL, and an average serum triglyceride level of 107 mg/dL compared with 81 mg/dL in premenopausal women. In a multivariate analysis adjusted for these variables as well as smoking status, body mass index, and C-reactive protein level, being postmenopausal remained independently associated with a twofold increased likelihood of hepatic steatosis (odds ratio, 2.0).
Hepatic steatosis has come under increasing research scrutiny of late because it appears to be a marker of increased atherosclerotic risk. The liver abnormality is associated with the metabolic syndrome, but as the Dallas Heart Study data show, postmenopausal status confers an increased risk of hepatic steatosis through a mechanism independent of obesity, hyperlipidemia, and other conventional cardiovascular risk factors.
Dr. Sanghavi reported having no relevant financial conflicts.
SAN FRANCISCO – Postmenopausal status is independently associated with a twofold increased risk of hepatic steatosis, the Dallas Heart Study has shown.
Among 1,018 women aged 30-65 years enrolled in the population-based study, 48% were postmenopausal. Their prevalence of hepatic steatosis as defined by greater than 5.5% hepatic fat content measured by magnetic resonance spectroscopy was 34%. In contrast, the prevalence was significantly less at 24% in the premenopausal women, Dr. Monika Sanghavi reported at the annual meeting of the American College of Cardiology.
The absolute hepatic triglyceride content in the postmenopausal cohort was 4.0%, significantly more than the 2.9% value in premenopausal women.
Of note, the prevalence of hepatic steatosis rose with greater time since the last menstrual period (LMP). The prevalence was 22% among women whose LMP was less than 2 months earlier, 31% in those whose LMP was 2-12 months earlier, and 35% in women whose LMP was more than 12 months prior, according to Dr. Sanghavi of the University of Texas Southwestern Medical Center, Dallas.
Women who were postmenopausal were, of course, substantially older on average than premenopausal women. However, they also had significantly higher average systolic blood pressure, 129 compared with 117 mm Hg; a greater prevalence of diabetes, 16% vs. 7%; a mean LDL cholesterol of 113 compared with 98 mg/dL, and an average serum triglyceride level of 107 mg/dL compared with 81 mg/dL in premenopausal women. In a multivariate analysis adjusted for these variables as well as smoking status, body mass index, and C-reactive protein level, being postmenopausal remained independently associated with a twofold increased likelihood of hepatic steatosis (odds ratio, 2.0).
Hepatic steatosis has come under increasing research scrutiny of late because it appears to be a marker of increased atherosclerotic risk. The liver abnormality is associated with the metabolic syndrome, but as the Dallas Heart Study data show, postmenopausal status confers an increased risk of hepatic steatosis through a mechanism independent of obesity, hyperlipidemia, and other conventional cardiovascular risk factors.
Dr. Sanghavi reported having no relevant financial conflicts.
SAN FRANCISCO – Postmenopausal status is independently associated with a twofold increased risk of hepatic steatosis, the Dallas Heart Study has shown.
Among 1,018 women aged 30-65 years enrolled in the population-based study, 48% were postmenopausal. Their prevalence of hepatic steatosis as defined by greater than 5.5% hepatic fat content measured by magnetic resonance spectroscopy was 34%. In contrast, the prevalence was significantly less at 24% in the premenopausal women, Dr. Monika Sanghavi reported at the annual meeting of the American College of Cardiology.
The absolute hepatic triglyceride content in the postmenopausal cohort was 4.0%, significantly more than the 2.9% value in premenopausal women.
Of note, the prevalence of hepatic steatosis rose with greater time since the last menstrual period (LMP). The prevalence was 22% among women whose LMP was less than 2 months earlier, 31% in those whose LMP was 2-12 months earlier, and 35% in women whose LMP was more than 12 months prior, according to Dr. Sanghavi of the University of Texas Southwestern Medical Center, Dallas.
Women who were postmenopausal were, of course, substantially older on average than premenopausal women. However, they also had significantly higher average systolic blood pressure, 129 compared with 117 mm Hg; a greater prevalence of diabetes, 16% vs. 7%; a mean LDL cholesterol of 113 compared with 98 mg/dL, and an average serum triglyceride level of 107 mg/dL compared with 81 mg/dL in premenopausal women. In a multivariate analysis adjusted for these variables as well as smoking status, body mass index, and C-reactive protein level, being postmenopausal remained independently associated with a twofold increased likelihood of hepatic steatosis (odds ratio, 2.0).
Hepatic steatosis has come under increasing research scrutiny of late because it appears to be a marker of increased atherosclerotic risk. The liver abnormality is associated with the metabolic syndrome, but as the Dallas Heart Study data show, postmenopausal status confers an increased risk of hepatic steatosis through a mechanism independent of obesity, hyperlipidemia, and other conventional cardiovascular risk factors.
Dr. Sanghavi reported having no relevant financial conflicts.
AT ACC13
Major Finding: Postmenopausal women had a significantly greater prevalence of hepatic steatosis (34%) than did premenopausal women (24%). After multivariate adjustment, the risk of steatosis was doubled in postmenopausal women.
Data Source: The Dallas Heart Study, a multiethnic, population-based study, including 1,018 women aged 30-65.
Disclosures: The presenter reported having no relevant financial conflicts.
Subclinical hypothyroidism predicts cardiovascular mortality in NHANES
SAN FRANCISCO – Subclinical hypothyroidism is a strong independent predictor of cardiovascular mortality in a healthy population at baseline, a national study indicated.
Among 7,883 participants in the National Health and Nutrition Examination Survey III (NHANES III) who were over age 40 years and free of overt hyper- or hypothyroidism, 5.3% had subclinical hypothyroidism as defined by a thyroid-stimulating hormone level of 5-19.99 mIU/L and a thyroxine level of 5-12 mcg/dL. During a mean 12.3 years of follow-up, 25.2% of the subclinical hypothyroid group died of cardiovascular causes, compared with 16.9% of euthyroid controls, Dr. Tushar A. Tuliani reported at the annual meeting of the American College of Cardiology.
Similarly, death due specifically to ischemic heart disease occurred in 15.4% of the subjects with subclinical hypothyroidism, compared with 9.6% of euthyroid controls, added Dr. Tuliani of Wayne State University–Detroit Medical Center.
In a multivariate analysis that adjusted for standard cardiovascular risk factors and demographic variables, individuals with subclinical hypothyroidism had a 20% increased risk of all-cause mortality, a 24% increase in cardiovascular mortality, and a 34% increased risk of death from ischemic heart disease. All of these increases were statistically significant and clinically meaningful, he noted.
NHANES III is an in-depth weighted survey conducted by the Centers for Disease Control and Prevention in a nationally representative population.
Dr. Tuliani reported having no financial conflicts.
SAN FRANCISCO – Subclinical hypothyroidism is a strong independent predictor of cardiovascular mortality in a healthy population at baseline, a national study indicated.
Among 7,883 participants in the National Health and Nutrition Examination Survey III (NHANES III) who were over age 40 years and free of overt hyper- or hypothyroidism, 5.3% had subclinical hypothyroidism as defined by a thyroid-stimulating hormone level of 5-19.99 mIU/L and a thyroxine level of 5-12 mcg/dL. During a mean 12.3 years of follow-up, 25.2% of the subclinical hypothyroid group died of cardiovascular causes, compared with 16.9% of euthyroid controls, Dr. Tushar A. Tuliani reported at the annual meeting of the American College of Cardiology.
Similarly, death due specifically to ischemic heart disease occurred in 15.4% of the subjects with subclinical hypothyroidism, compared with 9.6% of euthyroid controls, added Dr. Tuliani of Wayne State University–Detroit Medical Center.
In a multivariate analysis that adjusted for standard cardiovascular risk factors and demographic variables, individuals with subclinical hypothyroidism had a 20% increased risk of all-cause mortality, a 24% increase in cardiovascular mortality, and a 34% increased risk of death from ischemic heart disease. All of these increases were statistically significant and clinically meaningful, he noted.
NHANES III is an in-depth weighted survey conducted by the Centers for Disease Control and Prevention in a nationally representative population.
Dr. Tuliani reported having no financial conflicts.
SAN FRANCISCO – Subclinical hypothyroidism is a strong independent predictor of cardiovascular mortality in a healthy population at baseline, a national study indicated.
Among 7,883 participants in the National Health and Nutrition Examination Survey III (NHANES III) who were over age 40 years and free of overt hyper- or hypothyroidism, 5.3% had subclinical hypothyroidism as defined by a thyroid-stimulating hormone level of 5-19.99 mIU/L and a thyroxine level of 5-12 mcg/dL. During a mean 12.3 years of follow-up, 25.2% of the subclinical hypothyroid group died of cardiovascular causes, compared with 16.9% of euthyroid controls, Dr. Tushar A. Tuliani reported at the annual meeting of the American College of Cardiology.
Similarly, death due specifically to ischemic heart disease occurred in 15.4% of the subjects with subclinical hypothyroidism, compared with 9.6% of euthyroid controls, added Dr. Tuliani of Wayne State University–Detroit Medical Center.
In a multivariate analysis that adjusted for standard cardiovascular risk factors and demographic variables, individuals with subclinical hypothyroidism had a 20% increased risk of all-cause mortality, a 24% increase in cardiovascular mortality, and a 34% increased risk of death from ischemic heart disease. All of these increases were statistically significant and clinically meaningful, he noted.
NHANES III is an in-depth weighted survey conducted by the Centers for Disease Control and Prevention in a nationally representative population.
Dr. Tuliani reported having no financial conflicts.
AT ACC 13
Major finding: Healthy adults with subclinical hypothyroidism had a 24% greater risk of cardiovascular death than did euthyroid individuals during a mean 12.3 years of follow-up.
Data source: Analysis of 7,883 participants in the National Health and Nutrition Examination Survey III, a weighted, nationally representative sample.
Disclosures: NHANES III is sponsored by the Centers for Disease Control and Prevention. The presenter reported having no relevant financial interests.
Intense statin therapy treats atheromas in diabetes
SAN FRANCISCO – Atheromas regressed to similar degrees in patients with or without diabetes on high-intensity statin therapy for symptomatic coronary artery disease, a post hoc subgroup analysis of 1,039 patients found.
The primary endpoint, percent atheroma volume (PAV), decreased by a mean of 1.04% in 159 diabetic patients and by 1.21% in 880 nondiabetic patients compared with baseline – significant drops in both groups, Dr. Brian Stegman and his associates reported. PAV is the percentage of a single vessel’s volume occupied by atheroma.
The total atheroma volume (TAV), a secondary endpoint, decreased compared with baseline by 5.62 mm3 in the diabetic group and by 7.29 mm3 in the nondiabetic group, both of which also were significant changes, he said at the annual meeting of the American College of Cardiology.
Data came from the SATURN (Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin Versus Atorvastatin) trial, which compared intensive therapy with one of the two statin drugs in patients who underwent serial intravascular ultrasonography to assess disease regression. Patients were treated with either 40 mg/day rosuvastatin or 80 mg/day atorvastatin for 2 years. The two drug groups showed similar reductions in PAV, with a greater reduction in TAV using rosuvastatin (N. Engl. J. Med. 2011;365:2078-87).
Previous studies using have shown that aggressive reductions in LDL levels lead to significant regression in coronary atheromas as measured by intravascular ultrasound, but it has not been clear whether this is true in patients with diabetes as well as those without, said Dr. Stegman of the Cleveland Clinic Foundation.
The current analysis regrouped the SATURN cohort by diabetes status. High-intensity statin therapy produced substantial reductions in LDL in both groups, to a similar degree: a 52-mg/dL drop in diabetics and a 55-mg/dL decrease in nondiabetics. HDL levels increased by 3 mg/dL in diabetics and 5 mg/dL in nondiabetics, a significant difference between groups. Total cholesterol decreased 52 mg/dL in diabetics and 54 mg/dL in nondiabetics, and triglyceride levels decreased 13 mg/dL in diabetics and 12 mg/dL in nondiabetics, measures that were not significantly different between groups.
In both diabetics and nondiabetics, the lower the LDL on treatment, the greater the regression of atheroma, according to a linear regression model constructed by the investigators.
The changes in PAV and TAV in both groups showed that "with high-intensity statin therapy, we saw equal regression of atheroma in diabetics compared with nondiabetics," Dr. Stegman said. Lumen volumes were preserved over the 2-year period to a similar degree in both groups, as measured by change in lumen volume and external elastic membrane volume.
The findings differ from previous results in a pooled analysis of five trials involving intravascular ultrasound measurements of atherosclerosis progression in 2,237 patients, 416 of whom had diabetes, he noted. In that analysis, PAV increased by 0.05% in diabetics and by 0.6% in nondiabetics, compared with decreases of 1.21% and 1.04%, respectively, in the current analysis. *The TAV decreased by 2.7 mm3 in diabetics and 0.6 mm3 in nondiabetics, much less than the 5.62-mm3 and 7.29-mm3decreases, respectively, in the current analysis (J. Am. Coll. Cardiol. 2008;52:255-62).
The different outcomes in the two analyses may be due to less aggressive treatment in the trials pooled in the 2008 analysis, which did not decrease LDL levels as much as the high-intensity regimens in the SATURN trial. "I think our current study indicates that diabetic patients require fairly aggressive lipid therapy to get the same results as in nondiabetic patients," Dr. Stegman said.
Separate, previous trials using intravascular ultrasound have shown that high-dose statin therapy is more effective than moderate- or low dose regimens to halt progression of atherosclerosis, he added.
The analysis is limited by its post hoc nature, differing patient numbers in each group, and differences in baseline characteristics. Patients in the diabetic group were significantly older, with a higher mean body mass index, and more likely to be female and to have a history of hypertension compared with nondiabetics. Baseline lipid levels were significantly different between groups, with lower total cholesterol, LDL, and HDL levels and higher triglyceride levels in patients with diabetes. Intravascular ultrasound measurements also differed significantly, with greater PAV and TAV in the diabetic group.
The PAV and TAV endpoints were surrogate measures of clinical outcomes, another limitation of the analysis, but previous studies with intravascular ultrasound have reported that greater PAV and greater progression of PAV are associated with an increased risk of major adverse cardiovascular events, he said.
Dr. Stegman reported having no relevant financial disclosures. Some of his associates in the study reported financial associations with multiple pharmaceutical companies, several of which market statins.
*Correction, 3/27/13: An earlier version of this story incorrectly reported decreased TAV numbers for diabetics and nondiabetics. This version has been updated to reflect the correct numbers.
On Twitter @sherryboschert
SAN FRANCISCO – Atheromas regressed to similar degrees in patients with or without diabetes on high-intensity statin therapy for symptomatic coronary artery disease, a post hoc subgroup analysis of 1,039 patients found.
The primary endpoint, percent atheroma volume (PAV), decreased by a mean of 1.04% in 159 diabetic patients and by 1.21% in 880 nondiabetic patients compared with baseline – significant drops in both groups, Dr. Brian Stegman and his associates reported. PAV is the percentage of a single vessel’s volume occupied by atheroma.
The total atheroma volume (TAV), a secondary endpoint, decreased compared with baseline by 5.62 mm3 in the diabetic group and by 7.29 mm3 in the nondiabetic group, both of which also were significant changes, he said at the annual meeting of the American College of Cardiology.
Data came from the SATURN (Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin Versus Atorvastatin) trial, which compared intensive therapy with one of the two statin drugs in patients who underwent serial intravascular ultrasonography to assess disease regression. Patients were treated with either 40 mg/day rosuvastatin or 80 mg/day atorvastatin for 2 years. The two drug groups showed similar reductions in PAV, with a greater reduction in TAV using rosuvastatin (N. Engl. J. Med. 2011;365:2078-87).
Previous studies using have shown that aggressive reductions in LDL levels lead to significant regression in coronary atheromas as measured by intravascular ultrasound, but it has not been clear whether this is true in patients with diabetes as well as those without, said Dr. Stegman of the Cleveland Clinic Foundation.
The current analysis regrouped the SATURN cohort by diabetes status. High-intensity statin therapy produced substantial reductions in LDL in both groups, to a similar degree: a 52-mg/dL drop in diabetics and a 55-mg/dL decrease in nondiabetics. HDL levels increased by 3 mg/dL in diabetics and 5 mg/dL in nondiabetics, a significant difference between groups. Total cholesterol decreased 52 mg/dL in diabetics and 54 mg/dL in nondiabetics, and triglyceride levels decreased 13 mg/dL in diabetics and 12 mg/dL in nondiabetics, measures that were not significantly different between groups.
In both diabetics and nondiabetics, the lower the LDL on treatment, the greater the regression of atheroma, according to a linear regression model constructed by the investigators.
The changes in PAV and TAV in both groups showed that "with high-intensity statin therapy, we saw equal regression of atheroma in diabetics compared with nondiabetics," Dr. Stegman said. Lumen volumes were preserved over the 2-year period to a similar degree in both groups, as measured by change in lumen volume and external elastic membrane volume.
The findings differ from previous results in a pooled analysis of five trials involving intravascular ultrasound measurements of atherosclerosis progression in 2,237 patients, 416 of whom had diabetes, he noted. In that analysis, PAV increased by 0.05% in diabetics and by 0.6% in nondiabetics, compared with decreases of 1.21% and 1.04%, respectively, in the current analysis. *The TAV decreased by 2.7 mm3 in diabetics and 0.6 mm3 in nondiabetics, much less than the 5.62-mm3 and 7.29-mm3decreases, respectively, in the current analysis (J. Am. Coll. Cardiol. 2008;52:255-62).
The different outcomes in the two analyses may be due to less aggressive treatment in the trials pooled in the 2008 analysis, which did not decrease LDL levels as much as the high-intensity regimens in the SATURN trial. "I think our current study indicates that diabetic patients require fairly aggressive lipid therapy to get the same results as in nondiabetic patients," Dr. Stegman said.
Separate, previous trials using intravascular ultrasound have shown that high-dose statin therapy is more effective than moderate- or low dose regimens to halt progression of atherosclerosis, he added.
The analysis is limited by its post hoc nature, differing patient numbers in each group, and differences in baseline characteristics. Patients in the diabetic group were significantly older, with a higher mean body mass index, and more likely to be female and to have a history of hypertension compared with nondiabetics. Baseline lipid levels were significantly different between groups, with lower total cholesterol, LDL, and HDL levels and higher triglyceride levels in patients with diabetes. Intravascular ultrasound measurements also differed significantly, with greater PAV and TAV in the diabetic group.
The PAV and TAV endpoints were surrogate measures of clinical outcomes, another limitation of the analysis, but previous studies with intravascular ultrasound have reported that greater PAV and greater progression of PAV are associated with an increased risk of major adverse cardiovascular events, he said.
Dr. Stegman reported having no relevant financial disclosures. Some of his associates in the study reported financial associations with multiple pharmaceutical companies, several of which market statins.
*Correction, 3/27/13: An earlier version of this story incorrectly reported decreased TAV numbers for diabetics and nondiabetics. This version has been updated to reflect the correct numbers.
On Twitter @sherryboschert
SAN FRANCISCO – Atheromas regressed to similar degrees in patients with or without diabetes on high-intensity statin therapy for symptomatic coronary artery disease, a post hoc subgroup analysis of 1,039 patients found.
The primary endpoint, percent atheroma volume (PAV), decreased by a mean of 1.04% in 159 diabetic patients and by 1.21% in 880 nondiabetic patients compared with baseline – significant drops in both groups, Dr. Brian Stegman and his associates reported. PAV is the percentage of a single vessel’s volume occupied by atheroma.
The total atheroma volume (TAV), a secondary endpoint, decreased compared with baseline by 5.62 mm3 in the diabetic group and by 7.29 mm3 in the nondiabetic group, both of which also were significant changes, he said at the annual meeting of the American College of Cardiology.
Data came from the SATURN (Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin Versus Atorvastatin) trial, which compared intensive therapy with one of the two statin drugs in patients who underwent serial intravascular ultrasonography to assess disease regression. Patients were treated with either 40 mg/day rosuvastatin or 80 mg/day atorvastatin for 2 years. The two drug groups showed similar reductions in PAV, with a greater reduction in TAV using rosuvastatin (N. Engl. J. Med. 2011;365:2078-87).
Previous studies using have shown that aggressive reductions in LDL levels lead to significant regression in coronary atheromas as measured by intravascular ultrasound, but it has not been clear whether this is true in patients with diabetes as well as those without, said Dr. Stegman of the Cleveland Clinic Foundation.
The current analysis regrouped the SATURN cohort by diabetes status. High-intensity statin therapy produced substantial reductions in LDL in both groups, to a similar degree: a 52-mg/dL drop in diabetics and a 55-mg/dL decrease in nondiabetics. HDL levels increased by 3 mg/dL in diabetics and 5 mg/dL in nondiabetics, a significant difference between groups. Total cholesterol decreased 52 mg/dL in diabetics and 54 mg/dL in nondiabetics, and triglyceride levels decreased 13 mg/dL in diabetics and 12 mg/dL in nondiabetics, measures that were not significantly different between groups.
In both diabetics and nondiabetics, the lower the LDL on treatment, the greater the regression of atheroma, according to a linear regression model constructed by the investigators.
The changes in PAV and TAV in both groups showed that "with high-intensity statin therapy, we saw equal regression of atheroma in diabetics compared with nondiabetics," Dr. Stegman said. Lumen volumes were preserved over the 2-year period to a similar degree in both groups, as measured by change in lumen volume and external elastic membrane volume.
The findings differ from previous results in a pooled analysis of five trials involving intravascular ultrasound measurements of atherosclerosis progression in 2,237 patients, 416 of whom had diabetes, he noted. In that analysis, PAV increased by 0.05% in diabetics and by 0.6% in nondiabetics, compared with decreases of 1.21% and 1.04%, respectively, in the current analysis. *The TAV decreased by 2.7 mm3 in diabetics and 0.6 mm3 in nondiabetics, much less than the 5.62-mm3 and 7.29-mm3decreases, respectively, in the current analysis (J. Am. Coll. Cardiol. 2008;52:255-62).
The different outcomes in the two analyses may be due to less aggressive treatment in the trials pooled in the 2008 analysis, which did not decrease LDL levels as much as the high-intensity regimens in the SATURN trial. "I think our current study indicates that diabetic patients require fairly aggressive lipid therapy to get the same results as in nondiabetic patients," Dr. Stegman said.
Separate, previous trials using intravascular ultrasound have shown that high-dose statin therapy is more effective than moderate- or low dose regimens to halt progression of atherosclerosis, he added.
The analysis is limited by its post hoc nature, differing patient numbers in each group, and differences in baseline characteristics. Patients in the diabetic group were significantly older, with a higher mean body mass index, and more likely to be female and to have a history of hypertension compared with nondiabetics. Baseline lipid levels were significantly different between groups, with lower total cholesterol, LDL, and HDL levels and higher triglyceride levels in patients with diabetes. Intravascular ultrasound measurements also differed significantly, with greater PAV and TAV in the diabetic group.
The PAV and TAV endpoints were surrogate measures of clinical outcomes, another limitation of the analysis, but previous studies with intravascular ultrasound have reported that greater PAV and greater progression of PAV are associated with an increased risk of major adverse cardiovascular events, he said.
Dr. Stegman reported having no relevant financial disclosures. Some of his associates in the study reported financial associations with multiple pharmaceutical companies, several of which market statins.
*Correction, 3/27/13: An earlier version of this story incorrectly reported decreased TAV numbers for diabetics and nondiabetics. This version has been updated to reflect the correct numbers.
On Twitter @sherryboschert
AT ACC 13
Major finding: The percent atheroma volume after high-intensity statin therapy decreased by 1.04% in diabetic patients and by 1.21% in nondiabetic patients, significant drops in both groups.
Data source: Post hoc analysis of prospective randomized trial in 1,039 patients with symptomatic coronary artery disease who underwent serial intravascular ultrasonography.
Disclosures: Dr. Stegman reported having no disclosures. Some of his associates in the study reported financial associations with multiple pharmaceutical companies, several of which market statins.
Old gout drug learns new cardiac tricks
SAN FRANCISCO – The venerable antihyperuricemic agent allopurinol has shown early promise for two novel cardiovascular applications: prevention of atrial fibrillation in the setting of heart failure and reduction of left ventricular hypertrophy in patients with type 2 diabetes.
Allopurinol is a xanthine oxidase inhibitor and antigout drug. The rationale for the drug’s use in reducing the incidence of atrial fibrillation in patients with heart failure lies in the observation that serum uric acid has emerged as an independent marker of mortality and a predictor of new-onset atrial fibrillation in heart failure. Xanthine oxidase is not only a source of reactive oxygen species that adversely affect myocardial function, but it also catalyzes the conversion of xanthine to uric acid, Dr. Fernando E. Hernandez explained at the annual meeting of the American College of Cardiology.
He presented a retrospective cohort study involving 603 patients enrolled in the Miami Veterans Affairs heart failure clinic. The 103 on allopurinol, and the 500 who were not, matched up well in terms of baseline characteristics including age, prevalence of coronary artery disease, median left ventricular ejection, left atrial size, and use of guideline-recommended ACE inhibitors and beta-blockers.
During up to 5 years of follow-up, the incidence of new-onset atrial fibrillation was 184 cases/1,000 person-years in the allopurinol users compared with 252/1,000 person-years in controls. In a Cox proportional hazards analysis adjusted for small differences in potential confounders, the use of allopurinol was independently associated with a 47% reduction in the risk of atrial fibrillation (P = .04), reported Dr. Hernandez of the University of Miami.
This intriguing finding needs to be confirmed in randomized prospective trials, he noted.
In a separate presentation, Dr. Benjamin R. Szwejkowski noted that left ventricular hypertrophy (LVH) is common in patients with type 2 diabetes and contributes to their elevated risk of cardiovascular morbidity and mortality.
Based on their hypothesis that LVH is related in part to oxidative stress and reducing that stress via xanthine oxidase inhibition using allopurinol can cause LVH regression, the investigators conducted a randomized, double-blind placebo-controlled clinical trial. Sixty-six patients with type 2 diabetes and echocardiographic evidence of LVH were randomized to allopurinol at 600 mg/day or placebo for 9 months.
The primary study endpoint was change in left ventricular mass between baseline and 9 months, as measured by cardiac MRI. Allopurinol resulted in a significant mean 2.65-g reduction in LV mass, while in the control group LV mass increased by 1.21 g. Similarly, LV mass indexed to body surface area fell significantly by 1.32 g/m2 in the allopurinol group while increasing by 0.65 g/m2 in the placebo arm, reported Dr. Szwejkowski of the University of Dundee(Scotland).
"Allopurinol may be a useful therapy to reduce cardiovascular risk in type 2 diabetic patients with LVH," according to the cardiologist.
Flow-mediated dilatation didn’t change significantly over time in either study group.
Dr. Szwejkowski and Dr. Hernandez reported having no relevant financial conflicts.
SAN FRANCISCO – The venerable antihyperuricemic agent allopurinol has shown early promise for two novel cardiovascular applications: prevention of atrial fibrillation in the setting of heart failure and reduction of left ventricular hypertrophy in patients with type 2 diabetes.
Allopurinol is a xanthine oxidase inhibitor and antigout drug. The rationale for the drug’s use in reducing the incidence of atrial fibrillation in patients with heart failure lies in the observation that serum uric acid has emerged as an independent marker of mortality and a predictor of new-onset atrial fibrillation in heart failure. Xanthine oxidase is not only a source of reactive oxygen species that adversely affect myocardial function, but it also catalyzes the conversion of xanthine to uric acid, Dr. Fernando E. Hernandez explained at the annual meeting of the American College of Cardiology.
He presented a retrospective cohort study involving 603 patients enrolled in the Miami Veterans Affairs heart failure clinic. The 103 on allopurinol, and the 500 who were not, matched up well in terms of baseline characteristics including age, prevalence of coronary artery disease, median left ventricular ejection, left atrial size, and use of guideline-recommended ACE inhibitors and beta-blockers.
During up to 5 years of follow-up, the incidence of new-onset atrial fibrillation was 184 cases/1,000 person-years in the allopurinol users compared with 252/1,000 person-years in controls. In a Cox proportional hazards analysis adjusted for small differences in potential confounders, the use of allopurinol was independently associated with a 47% reduction in the risk of atrial fibrillation (P = .04), reported Dr. Hernandez of the University of Miami.
This intriguing finding needs to be confirmed in randomized prospective trials, he noted.
In a separate presentation, Dr. Benjamin R. Szwejkowski noted that left ventricular hypertrophy (LVH) is common in patients with type 2 diabetes and contributes to their elevated risk of cardiovascular morbidity and mortality.
Based on their hypothesis that LVH is related in part to oxidative stress and reducing that stress via xanthine oxidase inhibition using allopurinol can cause LVH regression, the investigators conducted a randomized, double-blind placebo-controlled clinical trial. Sixty-six patients with type 2 diabetes and echocardiographic evidence of LVH were randomized to allopurinol at 600 mg/day or placebo for 9 months.
The primary study endpoint was change in left ventricular mass between baseline and 9 months, as measured by cardiac MRI. Allopurinol resulted in a significant mean 2.65-g reduction in LV mass, while in the control group LV mass increased by 1.21 g. Similarly, LV mass indexed to body surface area fell significantly by 1.32 g/m2 in the allopurinol group while increasing by 0.65 g/m2 in the placebo arm, reported Dr. Szwejkowski of the University of Dundee(Scotland).
"Allopurinol may be a useful therapy to reduce cardiovascular risk in type 2 diabetic patients with LVH," according to the cardiologist.
Flow-mediated dilatation didn’t change significantly over time in either study group.
Dr. Szwejkowski and Dr. Hernandez reported having no relevant financial conflicts.
SAN FRANCISCO – The venerable antihyperuricemic agent allopurinol has shown early promise for two novel cardiovascular applications: prevention of atrial fibrillation in the setting of heart failure and reduction of left ventricular hypertrophy in patients with type 2 diabetes.
Allopurinol is a xanthine oxidase inhibitor and antigout drug. The rationale for the drug’s use in reducing the incidence of atrial fibrillation in patients with heart failure lies in the observation that serum uric acid has emerged as an independent marker of mortality and a predictor of new-onset atrial fibrillation in heart failure. Xanthine oxidase is not only a source of reactive oxygen species that adversely affect myocardial function, but it also catalyzes the conversion of xanthine to uric acid, Dr. Fernando E. Hernandez explained at the annual meeting of the American College of Cardiology.
He presented a retrospective cohort study involving 603 patients enrolled in the Miami Veterans Affairs heart failure clinic. The 103 on allopurinol, and the 500 who were not, matched up well in terms of baseline characteristics including age, prevalence of coronary artery disease, median left ventricular ejection, left atrial size, and use of guideline-recommended ACE inhibitors and beta-blockers.
During up to 5 years of follow-up, the incidence of new-onset atrial fibrillation was 184 cases/1,000 person-years in the allopurinol users compared with 252/1,000 person-years in controls. In a Cox proportional hazards analysis adjusted for small differences in potential confounders, the use of allopurinol was independently associated with a 47% reduction in the risk of atrial fibrillation (P = .04), reported Dr. Hernandez of the University of Miami.
This intriguing finding needs to be confirmed in randomized prospective trials, he noted.
In a separate presentation, Dr. Benjamin R. Szwejkowski noted that left ventricular hypertrophy (LVH) is common in patients with type 2 diabetes and contributes to their elevated risk of cardiovascular morbidity and mortality.
Based on their hypothesis that LVH is related in part to oxidative stress and reducing that stress via xanthine oxidase inhibition using allopurinol can cause LVH regression, the investigators conducted a randomized, double-blind placebo-controlled clinical trial. Sixty-six patients with type 2 diabetes and echocardiographic evidence of LVH were randomized to allopurinol at 600 mg/day or placebo for 9 months.
The primary study endpoint was change in left ventricular mass between baseline and 9 months, as measured by cardiac MRI. Allopurinol resulted in a significant mean 2.65-g reduction in LV mass, while in the control group LV mass increased by 1.21 g. Similarly, LV mass indexed to body surface area fell significantly by 1.32 g/m2 in the allopurinol group while increasing by 0.65 g/m2 in the placebo arm, reported Dr. Szwejkowski of the University of Dundee(Scotland).
"Allopurinol may be a useful therapy to reduce cardiovascular risk in type 2 diabetic patients with LVH," according to the cardiologist.
Flow-mediated dilatation didn’t change significantly over time in either study group.
Dr. Szwejkowski and Dr. Hernandez reported having no relevant financial conflicts.
AT ACC 13
Major finding: At the end of 5 years of allopurinol use, the incidence of new-onset atrial fibrillation was 184 cases/1,000 person-years in the allopurinol users compared with 252/1,000 person-years in controls.
Data source: A retrospective cohort study involving 603 patients with heart failure.
Disclosures: The study presenters reported having no relevant financial conflicts.
Cangrelor's success rose from prior failure
Designing a trial to establish the efficacy and safety of a drug may seem like science, but there is a lot of art as well. And sometimes achieving a positive trial result also requires confidence in and commitment to a concept, and the persistence to try a second time when it doesn’t work at first.
All of that happened in the unusual path that the intravenous antiplatelet drug cangrelor has navigated since researchers first devised trials in 2005 to test its efficacy in acute coronary syndrome patients who undergo coronary stenting. Arguably the most clinically important finding reported at the American College of Cardiology annual meeting earlier this month was from the CHAMPION PHOENIX trial, which showed safety and added efficacy from treatment with cangrelor, compared with clopidogrel, in this setting.
But while CHAMPION PHOENIX was a clear success, it was impossible to forget the failure from which it rose, a background that had everything to do with the trial’s myth-based name.
In 2009, results from a pair of cangrelor phase III trials, CHAMPION PCI and CHAMPION PLATFORM showed no added benefit from cangrelor in studies roughly similar in design to the PHOENIX study. The biggest difference between the success of PHOENIX and the failure of the earlier PCI and PLATFORM studies was that the researchers used a different approach to define and track the myocardial infarctions that patients developed during the studies and that served as primary end points.
Close analysis of the 2009 results from PCI and PLATFORM showed that, because many patients with acute coronary syndrome underwent coronary artery stenting so soon after becoming hospitalized, it was hard to discern new MI compared with prior events, said Dr. Robert Harrington, a Stanford (Calif.) University cardiologist and a coleader of all three studies. He, Dr. Deepak Bhatt (the other coleader), and their associates tweaked their original design three ways to address this problem, Dr. Harrington told me.
They obtained baseline measurements on the level of creatinine kinase MB in all patients, they took more frequent measures of CKMB from patients following baseline to track the development of new MIs, and they required, in addition to a further elevation in CKMB, some other indication of a new myocardial infarction: ischemic symptoms, ECG changes, or angiographic evidence. Along with their revised approach to detecting new-onset MIs, they also used a more refined definition of stent thrombosis within the first 48 hours of the study and set up the study presuming that the comparator drug, clopidogrel, would be more effective than they had anticipated the first time around.
These changes made the PHOENIX trial positive where the first two had failed.
Why did they do all this and keep at it so doggedly? Because they saw clear signals of efficacy in the two negative trials, and because they believed strongly in what a boon to cardiology practice would come from having a new drug available that interventionalists could quickly start in patients undergoing coronary catheterization and stenting but also quickly stop and clear from patients who were headed to coronary surgery following their diagnostic catheterization. That’s possible because the clinical effects from cangrelor persist for about an hour after an infusion stops, as opposed to days following a dose of an oral antiplatelet drug like clopidogrel, prasugrel, or ticagrelor.
"This drug will give us the flexibility to use a short-acting, intravenous agent," Dr. Bhatt said at the meeting. "We now give clopidogrel in the emergency department [to acute coronary syndrome patients] prior to knowing a patient’s coronary anatomy. If the patient has anatomy that needs urgent surgery, the patient has to wait a few days until the medicine clears out. By using cangrelor instead that uncomfortable situation disappears."
"The investigators thought that something was there [in CHAMPION PCI and PLATFORM], something useful for patients," added Dr. Bhatt of the Veterans Administration Boston Healthcare System.
Many interventionalists at the meeting agreed that cangrelor will bring an important new dimension of thrombotic protection and treatment flexibility to acute coronary syndrome patients undergoing coronary angiography.
"This is a blockbuster randomized controlled trial that will alter practice on numerous levels," said Dr. Gregg Stone, an interventional cardiologist at Columbia University in New York.
And it is "incredibly important for the treatment of patients undergoing catheterization who may need to have surgery," said Dr. Roxana Mehran, an interventionalist at Mount Sinai Hospital in New York.
CHAMPION "is a strikingly important study. It has global implications for the way we treat patients. The rapid on and off deals with a lot of clinical scenarios we face all the time," said Dr. Martin Leon, an interventionalist at Columbia University.
"Sometimes we need to wait 5-7 days before a patient can go to surgery because of the increased risk of bleeding" following treatment with an oral antiplatelet drug, said Dr. Cindy Grines, an interventionalist at Detroit Medical Center. "This will have a huge impact."
Dr. Bhatt, Dr. Mehran, and Dr. Grines reported financial ties with several pharmaceutical companies, including the Medicines Company, which is developing cangrelor. Dr. Harrington and Dr. Stone reported ties with numerous drug companies, including receiving a research grant from the Medicines Company. Dr. Leon disclosed having ties to two device makers but no pharmaceutical companies.
Designing a trial to establish the efficacy and safety of a drug may seem like science, but there is a lot of art as well. And sometimes achieving a positive trial result also requires confidence in and commitment to a concept, and the persistence to try a second time when it doesn’t work at first.
All of that happened in the unusual path that the intravenous antiplatelet drug cangrelor has navigated since researchers first devised trials in 2005 to test its efficacy in acute coronary syndrome patients who undergo coronary stenting. Arguably the most clinically important finding reported at the American College of Cardiology annual meeting earlier this month was from the CHAMPION PHOENIX trial, which showed safety and added efficacy from treatment with cangrelor, compared with clopidogrel, in this setting.
But while CHAMPION PHOENIX was a clear success, it was impossible to forget the failure from which it rose, a background that had everything to do with the trial’s myth-based name.
In 2009, results from a pair of cangrelor phase III trials, CHAMPION PCI and CHAMPION PLATFORM showed no added benefit from cangrelor in studies roughly similar in design to the PHOENIX study. The biggest difference between the success of PHOENIX and the failure of the earlier PCI and PLATFORM studies was that the researchers used a different approach to define and track the myocardial infarctions that patients developed during the studies and that served as primary end points.
Close analysis of the 2009 results from PCI and PLATFORM showed that, because many patients with acute coronary syndrome underwent coronary artery stenting so soon after becoming hospitalized, it was hard to discern new MI compared with prior events, said Dr. Robert Harrington, a Stanford (Calif.) University cardiologist and a coleader of all three studies. He, Dr. Deepak Bhatt (the other coleader), and their associates tweaked their original design three ways to address this problem, Dr. Harrington told me.
They obtained baseline measurements on the level of creatinine kinase MB in all patients, they took more frequent measures of CKMB from patients following baseline to track the development of new MIs, and they required, in addition to a further elevation in CKMB, some other indication of a new myocardial infarction: ischemic symptoms, ECG changes, or angiographic evidence. Along with their revised approach to detecting new-onset MIs, they also used a more refined definition of stent thrombosis within the first 48 hours of the study and set up the study presuming that the comparator drug, clopidogrel, would be more effective than they had anticipated the first time around.
These changes made the PHOENIX trial positive where the first two had failed.
Why did they do all this and keep at it so doggedly? Because they saw clear signals of efficacy in the two negative trials, and because they believed strongly in what a boon to cardiology practice would come from having a new drug available that interventionalists could quickly start in patients undergoing coronary catheterization and stenting but also quickly stop and clear from patients who were headed to coronary surgery following their diagnostic catheterization. That’s possible because the clinical effects from cangrelor persist for about an hour after an infusion stops, as opposed to days following a dose of an oral antiplatelet drug like clopidogrel, prasugrel, or ticagrelor.
"This drug will give us the flexibility to use a short-acting, intravenous agent," Dr. Bhatt said at the meeting. "We now give clopidogrel in the emergency department [to acute coronary syndrome patients] prior to knowing a patient’s coronary anatomy. If the patient has anatomy that needs urgent surgery, the patient has to wait a few days until the medicine clears out. By using cangrelor instead that uncomfortable situation disappears."
"The investigators thought that something was there [in CHAMPION PCI and PLATFORM], something useful for patients," added Dr. Bhatt of the Veterans Administration Boston Healthcare System.
Many interventionalists at the meeting agreed that cangrelor will bring an important new dimension of thrombotic protection and treatment flexibility to acute coronary syndrome patients undergoing coronary angiography.
"This is a blockbuster randomized controlled trial that will alter practice on numerous levels," said Dr. Gregg Stone, an interventional cardiologist at Columbia University in New York.
And it is "incredibly important for the treatment of patients undergoing catheterization who may need to have surgery," said Dr. Roxana Mehran, an interventionalist at Mount Sinai Hospital in New York.
CHAMPION "is a strikingly important study. It has global implications for the way we treat patients. The rapid on and off deals with a lot of clinical scenarios we face all the time," said Dr. Martin Leon, an interventionalist at Columbia University.
"Sometimes we need to wait 5-7 days before a patient can go to surgery because of the increased risk of bleeding" following treatment with an oral antiplatelet drug, said Dr. Cindy Grines, an interventionalist at Detroit Medical Center. "This will have a huge impact."
Dr. Bhatt, Dr. Mehran, and Dr. Grines reported financial ties with several pharmaceutical companies, including the Medicines Company, which is developing cangrelor. Dr. Harrington and Dr. Stone reported ties with numerous drug companies, including receiving a research grant from the Medicines Company. Dr. Leon disclosed having ties to two device makers but no pharmaceutical companies.
Designing a trial to establish the efficacy and safety of a drug may seem like science, but there is a lot of art as well. And sometimes achieving a positive trial result also requires confidence in and commitment to a concept, and the persistence to try a second time when it doesn’t work at first.
All of that happened in the unusual path that the intravenous antiplatelet drug cangrelor has navigated since researchers first devised trials in 2005 to test its efficacy in acute coronary syndrome patients who undergo coronary stenting. Arguably the most clinically important finding reported at the American College of Cardiology annual meeting earlier this month was from the CHAMPION PHOENIX trial, which showed safety and added efficacy from treatment with cangrelor, compared with clopidogrel, in this setting.
But while CHAMPION PHOENIX was a clear success, it was impossible to forget the failure from which it rose, a background that had everything to do with the trial’s myth-based name.
In 2009, results from a pair of cangrelor phase III trials, CHAMPION PCI and CHAMPION PLATFORM showed no added benefit from cangrelor in studies roughly similar in design to the PHOENIX study. The biggest difference between the success of PHOENIX and the failure of the earlier PCI and PLATFORM studies was that the researchers used a different approach to define and track the myocardial infarctions that patients developed during the studies and that served as primary end points.
Close analysis of the 2009 results from PCI and PLATFORM showed that, because many patients with acute coronary syndrome underwent coronary artery stenting so soon after becoming hospitalized, it was hard to discern new MI compared with prior events, said Dr. Robert Harrington, a Stanford (Calif.) University cardiologist and a coleader of all three studies. He, Dr. Deepak Bhatt (the other coleader), and their associates tweaked their original design three ways to address this problem, Dr. Harrington told me.
They obtained baseline measurements on the level of creatinine kinase MB in all patients, they took more frequent measures of CKMB from patients following baseline to track the development of new MIs, and they required, in addition to a further elevation in CKMB, some other indication of a new myocardial infarction: ischemic symptoms, ECG changes, or angiographic evidence. Along with their revised approach to detecting new-onset MIs, they also used a more refined definition of stent thrombosis within the first 48 hours of the study and set up the study presuming that the comparator drug, clopidogrel, would be more effective than they had anticipated the first time around.
These changes made the PHOENIX trial positive where the first two had failed.
Why did they do all this and keep at it so doggedly? Because they saw clear signals of efficacy in the two negative trials, and because they believed strongly in what a boon to cardiology practice would come from having a new drug available that interventionalists could quickly start in patients undergoing coronary catheterization and stenting but also quickly stop and clear from patients who were headed to coronary surgery following their diagnostic catheterization. That’s possible because the clinical effects from cangrelor persist for about an hour after an infusion stops, as opposed to days following a dose of an oral antiplatelet drug like clopidogrel, prasugrel, or ticagrelor.
"This drug will give us the flexibility to use a short-acting, intravenous agent," Dr. Bhatt said at the meeting. "We now give clopidogrel in the emergency department [to acute coronary syndrome patients] prior to knowing a patient’s coronary anatomy. If the patient has anatomy that needs urgent surgery, the patient has to wait a few days until the medicine clears out. By using cangrelor instead that uncomfortable situation disappears."
"The investigators thought that something was there [in CHAMPION PCI and PLATFORM], something useful for patients," added Dr. Bhatt of the Veterans Administration Boston Healthcare System.
Many interventionalists at the meeting agreed that cangrelor will bring an important new dimension of thrombotic protection and treatment flexibility to acute coronary syndrome patients undergoing coronary angiography.
"This is a blockbuster randomized controlled trial that will alter practice on numerous levels," said Dr. Gregg Stone, an interventional cardiologist at Columbia University in New York.
And it is "incredibly important for the treatment of patients undergoing catheterization who may need to have surgery," said Dr. Roxana Mehran, an interventionalist at Mount Sinai Hospital in New York.
CHAMPION "is a strikingly important study. It has global implications for the way we treat patients. The rapid on and off deals with a lot of clinical scenarios we face all the time," said Dr. Martin Leon, an interventionalist at Columbia University.
"Sometimes we need to wait 5-7 days before a patient can go to surgery because of the increased risk of bleeding" following treatment with an oral antiplatelet drug, said Dr. Cindy Grines, an interventionalist at Detroit Medical Center. "This will have a huge impact."
Dr. Bhatt, Dr. Mehran, and Dr. Grines reported financial ties with several pharmaceutical companies, including the Medicines Company, which is developing cangrelor. Dr. Harrington and Dr. Stone reported ties with numerous drug companies, including receiving a research grant from the Medicines Company. Dr. Leon disclosed having ties to two device makers but no pharmaceutical companies.
TERISA targets diabetes as potential new market for ranolazine
SAN FRANCISCO – Ranolazine is being groomed as potentially the first drug to provide dual antianginal and glucose-lowering benefits in type 2 diabetic patients with coronary artery disease.
Results of the TERISA (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina) trial presented by Dr. Mikhail Kosiborod at the annual meeting of the American College of Cardiology have established the first part of the sought-after demonstration of dual benefit.
The second step in winning an expanded indication for ranolazine (Ranexa) specifically in patients with type 2 diabetes and stable angina is to demonstrate that the drug also lowers fasting blood glucose and hemoglobin A1c. Three ongoing phase III randomized clinical trials are addressing that issue, according to Dr. Kosiborod, a cardiologist at the Saint Luke’s Mid-America Heart Institute and the University of Missouri, Kansas City.
There is a compelling need for targeted, more effective therapies in patients with angina and type 2 diabetes. They often have more extensive and diffuse coronary artery disease (CAD) than do nondiabetic patients with CAD, and they have worse outcomes as well, including more repeat hospitalizations and higher health care costs, he observed.
TERISA was a randomized, double-blind, placebo-controlled trial in 927 patients with type 2 diabetes, coronary artery disease, and stable angina with symptoms despite treatment with one or two other antianginal agents. The trial was conducted in 105 centers in 14 countries.
The target dose of ranolazine was 1,000 mg twice daily in this 8-week trial. The primary outcome was the average number of anginal episodes during weeks 2-8 as captured by patients using a novel electronic diary with daily entries. Participants had a mean diabetes duration of 7.5 years and a baseline HbA1c of 7.3%.
From a baseline anginal frequency of 6.7 episodes/week, the ranolazine group improved to 3.8 episodes/week. The control group averaged 4.3 episodes/week, demonstrating the profound placebo effect commonly noted in angina clinical trials. The half-episode per week advantage in the ranolazine group, compared with placebo was highly significant (P = .008).
The secondary endpoint in TERISA was change in weekly use of sublingual nitroglycerin. From a baseline mean of 4.3 doses/week, it improved to 1.7 episodes/week in the ranolazine group and 2.1 episodes/week in controls. Once again, the between-group difference was significant.
Ranolazine was extremely well tolerated, said Dr. Kosiborod. The only side effects that were more common than in the placebo arm were dizziness and nausea, each affecting fewer than 4% of patients on ranolazine.
Dr. Kosiborod highlighted two intriguing TERISA subgroup analyses. One showed that ranolazine’s antianginal effect was greater among patients with higher baseline HbA1c values. The potential mechanism is under study. If confirmed in other clinical trials, this finding would suggest that ranolazine is particularly beneficial in type 2 diabetic patients with angina and suboptimal glucose control.
Another prespecified subgroup analysis revealed that ranolazine was significantly more effective than placebo at all study sites except for selected locations in Russia. When the Russian sites were excluded from the study analysis, the average number of weekly anginal episodes in the ranolazine group dropped from 3.8 to 3.1.
"There’s a very active investigation underway to find out what was going on at those Russian sites that created a geographic difference in outcome," according to Dr. Kosiborod.
Discussant C. Michael Gibson, professor of medicine at Harvard Medical School, Boston, said he’d really have like to have seen data on the duration as well as the number of anginal episodes. Objective measurement of total ischemic burden, as by Holter monitoring, is particularly important in diabetes patients because they have notoriously poor anginal warning systems.
Dr. Kosiborod replied that he and his coinvestigators opted to go with change in the number of anginal episodes as a more clinically meaningful, patient-centered outcome.
"I would say the reason we prescribe antianginal medications is based upon patient symptoms, not how long they can go on the treadmill or what their Holter monitor looks like," he added.
Dr. Miguel A. Quinones, cochair of the ACC conference and chairman of the department of cardiology at Methodist Hospital, Houston, commented that ranolazine’s 0.5 episode/week greater decrease in angina episodes than with placebo is a rather modest effect. Dr. Kosiborod was quick to respond that it’s in the same ballpark as has been shown in studies of percutaneous coronary intervention to improve angina, a widely accepted form of treatment.
Simultaneously with Dr. Kosiborod’s presentation at the San Francisco conference, the TERISA findings were published online (J. Am. Coll. Cardiol. 2013 [doi:10.1016/j.jacc.2013.02.011]).
TERISA was sponsored by Gilead Sciences. Dr. Kosiborod reported serving as a consultant to Gilead and several other pharmaceutical and medical device companies. Dr. Gibson reported having financial ties to numerous pharmaceutical and device manufacturers, but not Gilead. Dr. Quinones reported having no relevant industry relationships.
SAN FRANCISCO – Ranolazine is being groomed as potentially the first drug to provide dual antianginal and glucose-lowering benefits in type 2 diabetic patients with coronary artery disease.
Results of the TERISA (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina) trial presented by Dr. Mikhail Kosiborod at the annual meeting of the American College of Cardiology have established the first part of the sought-after demonstration of dual benefit.
The second step in winning an expanded indication for ranolazine (Ranexa) specifically in patients with type 2 diabetes and stable angina is to demonstrate that the drug also lowers fasting blood glucose and hemoglobin A1c. Three ongoing phase III randomized clinical trials are addressing that issue, according to Dr. Kosiborod, a cardiologist at the Saint Luke’s Mid-America Heart Institute and the University of Missouri, Kansas City.
There is a compelling need for targeted, more effective therapies in patients with angina and type 2 diabetes. They often have more extensive and diffuse coronary artery disease (CAD) than do nondiabetic patients with CAD, and they have worse outcomes as well, including more repeat hospitalizations and higher health care costs, he observed.
TERISA was a randomized, double-blind, placebo-controlled trial in 927 patients with type 2 diabetes, coronary artery disease, and stable angina with symptoms despite treatment with one or two other antianginal agents. The trial was conducted in 105 centers in 14 countries.
The target dose of ranolazine was 1,000 mg twice daily in this 8-week trial. The primary outcome was the average number of anginal episodes during weeks 2-8 as captured by patients using a novel electronic diary with daily entries. Participants had a mean diabetes duration of 7.5 years and a baseline HbA1c of 7.3%.
From a baseline anginal frequency of 6.7 episodes/week, the ranolazine group improved to 3.8 episodes/week. The control group averaged 4.3 episodes/week, demonstrating the profound placebo effect commonly noted in angina clinical trials. The half-episode per week advantage in the ranolazine group, compared with placebo was highly significant (P = .008).
The secondary endpoint in TERISA was change in weekly use of sublingual nitroglycerin. From a baseline mean of 4.3 doses/week, it improved to 1.7 episodes/week in the ranolazine group and 2.1 episodes/week in controls. Once again, the between-group difference was significant.
Ranolazine was extremely well tolerated, said Dr. Kosiborod. The only side effects that were more common than in the placebo arm were dizziness and nausea, each affecting fewer than 4% of patients on ranolazine.
Dr. Kosiborod highlighted two intriguing TERISA subgroup analyses. One showed that ranolazine’s antianginal effect was greater among patients with higher baseline HbA1c values. The potential mechanism is under study. If confirmed in other clinical trials, this finding would suggest that ranolazine is particularly beneficial in type 2 diabetic patients with angina and suboptimal glucose control.
Another prespecified subgroup analysis revealed that ranolazine was significantly more effective than placebo at all study sites except for selected locations in Russia. When the Russian sites were excluded from the study analysis, the average number of weekly anginal episodes in the ranolazine group dropped from 3.8 to 3.1.
"There’s a very active investigation underway to find out what was going on at those Russian sites that created a geographic difference in outcome," according to Dr. Kosiborod.
Discussant C. Michael Gibson, professor of medicine at Harvard Medical School, Boston, said he’d really have like to have seen data on the duration as well as the number of anginal episodes. Objective measurement of total ischemic burden, as by Holter monitoring, is particularly important in diabetes patients because they have notoriously poor anginal warning systems.
Dr. Kosiborod replied that he and his coinvestigators opted to go with change in the number of anginal episodes as a more clinically meaningful, patient-centered outcome.
"I would say the reason we prescribe antianginal medications is based upon patient symptoms, not how long they can go on the treadmill or what their Holter monitor looks like," he added.
Dr. Miguel A. Quinones, cochair of the ACC conference and chairman of the department of cardiology at Methodist Hospital, Houston, commented that ranolazine’s 0.5 episode/week greater decrease in angina episodes than with placebo is a rather modest effect. Dr. Kosiborod was quick to respond that it’s in the same ballpark as has been shown in studies of percutaneous coronary intervention to improve angina, a widely accepted form of treatment.
Simultaneously with Dr. Kosiborod’s presentation at the San Francisco conference, the TERISA findings were published online (J. Am. Coll. Cardiol. 2013 [doi:10.1016/j.jacc.2013.02.011]).
TERISA was sponsored by Gilead Sciences. Dr. Kosiborod reported serving as a consultant to Gilead and several other pharmaceutical and medical device companies. Dr. Gibson reported having financial ties to numerous pharmaceutical and device manufacturers, but not Gilead. Dr. Quinones reported having no relevant industry relationships.
SAN FRANCISCO – Ranolazine is being groomed as potentially the first drug to provide dual antianginal and glucose-lowering benefits in type 2 diabetic patients with coronary artery disease.
Results of the TERISA (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina) trial presented by Dr. Mikhail Kosiborod at the annual meeting of the American College of Cardiology have established the first part of the sought-after demonstration of dual benefit.
The second step in winning an expanded indication for ranolazine (Ranexa) specifically in patients with type 2 diabetes and stable angina is to demonstrate that the drug also lowers fasting blood glucose and hemoglobin A1c. Three ongoing phase III randomized clinical trials are addressing that issue, according to Dr. Kosiborod, a cardiologist at the Saint Luke’s Mid-America Heart Institute and the University of Missouri, Kansas City.
There is a compelling need for targeted, more effective therapies in patients with angina and type 2 diabetes. They often have more extensive and diffuse coronary artery disease (CAD) than do nondiabetic patients with CAD, and they have worse outcomes as well, including more repeat hospitalizations and higher health care costs, he observed.
TERISA was a randomized, double-blind, placebo-controlled trial in 927 patients with type 2 diabetes, coronary artery disease, and stable angina with symptoms despite treatment with one or two other antianginal agents. The trial was conducted in 105 centers in 14 countries.
The target dose of ranolazine was 1,000 mg twice daily in this 8-week trial. The primary outcome was the average number of anginal episodes during weeks 2-8 as captured by patients using a novel electronic diary with daily entries. Participants had a mean diabetes duration of 7.5 years and a baseline HbA1c of 7.3%.
From a baseline anginal frequency of 6.7 episodes/week, the ranolazine group improved to 3.8 episodes/week. The control group averaged 4.3 episodes/week, demonstrating the profound placebo effect commonly noted in angina clinical trials. The half-episode per week advantage in the ranolazine group, compared with placebo was highly significant (P = .008).
The secondary endpoint in TERISA was change in weekly use of sublingual nitroglycerin. From a baseline mean of 4.3 doses/week, it improved to 1.7 episodes/week in the ranolazine group and 2.1 episodes/week in controls. Once again, the between-group difference was significant.
Ranolazine was extremely well tolerated, said Dr. Kosiborod. The only side effects that were more common than in the placebo arm were dizziness and nausea, each affecting fewer than 4% of patients on ranolazine.
Dr. Kosiborod highlighted two intriguing TERISA subgroup analyses. One showed that ranolazine’s antianginal effect was greater among patients with higher baseline HbA1c values. The potential mechanism is under study. If confirmed in other clinical trials, this finding would suggest that ranolazine is particularly beneficial in type 2 diabetic patients with angina and suboptimal glucose control.
Another prespecified subgroup analysis revealed that ranolazine was significantly more effective than placebo at all study sites except for selected locations in Russia. When the Russian sites were excluded from the study analysis, the average number of weekly anginal episodes in the ranolazine group dropped from 3.8 to 3.1.
"There’s a very active investigation underway to find out what was going on at those Russian sites that created a geographic difference in outcome," according to Dr. Kosiborod.
Discussant C. Michael Gibson, professor of medicine at Harvard Medical School, Boston, said he’d really have like to have seen data on the duration as well as the number of anginal episodes. Objective measurement of total ischemic burden, as by Holter monitoring, is particularly important in diabetes patients because they have notoriously poor anginal warning systems.
Dr. Kosiborod replied that he and his coinvestigators opted to go with change in the number of anginal episodes as a more clinically meaningful, patient-centered outcome.
"I would say the reason we prescribe antianginal medications is based upon patient symptoms, not how long they can go on the treadmill or what their Holter monitor looks like," he added.
Dr. Miguel A. Quinones, cochair of the ACC conference and chairman of the department of cardiology at Methodist Hospital, Houston, commented that ranolazine’s 0.5 episode/week greater decrease in angina episodes than with placebo is a rather modest effect. Dr. Kosiborod was quick to respond that it’s in the same ballpark as has been shown in studies of percutaneous coronary intervention to improve angina, a widely accepted form of treatment.
Simultaneously with Dr. Kosiborod’s presentation at the San Francisco conference, the TERISA findings were published online (J. Am. Coll. Cardiol. 2013 [doi:10.1016/j.jacc.2013.02.011]).
TERISA was sponsored by Gilead Sciences. Dr. Kosiborod reported serving as a consultant to Gilead and several other pharmaceutical and medical device companies. Dr. Gibson reported having financial ties to numerous pharmaceutical and device manufacturers, but not Gilead. Dr. Quinones reported having no relevant industry relationships.
AT ACC 13
Major finding: Weekly frequency of anginal episodes in type 2 diabetic patients with coronary artery disease and angina despite the use of one or two antianginal agents dropped from 6.7 to 3.8 episodes with ranolazine, a significantly greater benefit than with placebo.
Data source: TERISA, an international, randomized, double-blind trial of ranolazine vs. placebo in 927 patients with type 2 diabetes.
Disclosures: TERISA was sponsored by Gilead Sciences. Dr. Kosiborod reported serving as a consultant to Gilead and several other pharmaceutical and medical device companies.
Sapien XT valve shows improvements over original
SAN FRANCISCO – A new, smaller version of the only transcatheter aortic valve available for U.S. use showed noninferior clinical outcomes and safer periprocedural results in a multicenter U.S. comparison of the new and existing devices in a total of 560 patients, raising expectations that the new valve system – the Sapien XT – will soon be on the U.S. market.
Although treatment using the Sapien XT roughly matched treatment with the existing Sapien transcatheter aortic valve in the incidence of the important adverse effect of residual moderate or severe paravalvular leak, several U.S. operators who perform transcatheter aortic valve repair (TAVR) stressed that the leak problem has begun to resolve recently as interventionalists and surgeons have found ways to minimize the issue. They also noted that availability of the new XT device will move the field a step further toward fewer leak issues, because it will allow the option of a larger, 29-mm-diameter valve, a possibility that has not been available with the original Sapien valve.
"Sapien XT represents a worthwhile advance with incremental clinical value, and it is the preferred balloon-expandable transcatheter aortic valve system," said Dr. Martin B. Leon when he presented the trial data on March 10 at the annual meeting of the American College of Cardiology.
"This second-generation device has demonstrated sufficient clinical benefit for us to say it’s the preferred therapy," said Dr. Leon, professor of medicine and director of the Center for Interventional Vascular Therapy at Columbia University in New York.
"This is an evolutionary technology that is easier to use. The results are superb," commented Dr. Gary S. Mintz, an interventional cardiologist based in Washington and medical director of the Cardiovascular Research Foundation in New York.
The PARTNER II (Placement of Aortic Transcatheter Valves) cohort B trial enrolled 560 patients with aortic stenosis deemed inoperable for surgical valve replacement at 28 U.S. centers during April 2011 through February 2012. The study randomized patients to receive the Sapien XT valve or the approved Sapien valve in a prespecified noninferiority design, with a primary outcome of all-cause death, disabling stroke, or need for repeat hospitalization at 1 year after treatment. Patients averaged 84 years old, they had an average Society of Thoracic Surgeons score of about 10%, 96% had New York Heart Association class III or IV heart failure, and about 60% met the study’s criteria for frailty.
At 1-year follow-up, the combined primary endpoint occurred in 35% of patients treated with the approved valve and in 34% of patients treated with the new XT valve, a result that was not statistically significant and that met the study’s standard for noninferiority. At 1 year, as well as at 30 days after treatment, the two patient groups showed no significant difference for any of the individual components of the clinical endpoints.
However, patients treated with the XT valve had significantly fewer procedural complications, with significantly fewer major vascular events (10% with the XT device, compared with 16% with the approved valve) and significantly fewer disabling bleeding events (8% with the XT device, compared with 13% with the first-generation system), Dr. Leon reported. The category of major vascular events included perforations, dissections, and hematomas. Patients treated with the XT device also required significantly fewer episodes when the initially placed valve had to be replaced (3 replaced valve implants, compared with 10 using the approved valve), and numerically fewer aborted procedures – 2 compared with 8 using the approved valve, although this difference just missed statistical significance (P = .06). Placement of the new valve also required an average of 14 minutes less anesthesia time, a significant difference.
"It’s a smaller device. What you would expect from a smaller device is fewer vascular complications and improved procedural events," Dr. Leon said in an interview. He also highlighted the numerical difference in 30-day mortality – 5.1% with the older valve system, compared with 3.5% using the XT device. While the difference did not reach statistical significance, "to me this difference is not trivial," he said.
The difference in disabling bleeding complications is also important, commented Dr. Mintz, who was not involved in the study. "Bleeding complications are hugely disabling, especially in this older, frail population," he said.
"The big concerns from the first PARTNER trial were vascular complications, strokes, and paravalvular leaks," said Dr. Leon. "The XT device seems to address some of these issues. The vascular complications were reduced, and the stroke rate went down" compared with the first PARTNER trial (N. Engl. J. Med. 2010;363:1597-607). "What is left is the paravalvular leak issue, which is real."
In the new trial, moderate or severe paravalvular leaks, a complication that has previously been linked with worse outcomes and increased mortality, occurred in 17% of the patients who received the approved valve and in 24% of patients who received the XT valve at 30-day follow-up, a difference that did not reach significance.
"Is there a difference in paravalvular leaks between Sapien XT and Sapien? We’re not entirely sure. There was a nonsignificant trend" that will be the subject of further analysis, Dr. Leon said. He stressed that the leak rate is confounded because significantly more patients who received the approved valve underwent a second valve placement, which often occurs because a leak occurred with the first valve. "Putting in a second valve is a big thing and is not good," he said. "No matter how you look at it, there still was a significant leak rate that is not as good as it needs to be." Dr. Leon said that he hopes the issue of paravalvular leaks will be addressed by a third-generation valve system that will enter clinical trials later this year.
But some surgeons and interventionalists involved in the PARTNER II trial noted that the incidence of paravalvular leaks has already begun to come down in the past year or so.
"We are [now] much better at sizing the valve with CT scans, and that is reducing the number of paravalvular leaks, at least anecdotally at our center," said Dr. Michael J. Mack, a cardiothoracic surgeon at the Heart Hospital in Plano, Texas, and a PARTNER II investigator. "We also underfill or overfill our [valve expansion] balloons based on the CT scans, and we’re getting better results with that. We’re fitting the valve better into the space," he said in an interview.
"We do everything we can to minimize patients leaving with a moderate or severe leak by balloon dilatation or putting in a second valve," said Dr. Jeffrey J. Popma, an interventional cardiologist and professor of medicine at Harvard University in Boston and a PARTNER II investigator. He also noted that use of the XT device reduces the number of patients who need transapical valve placement, because the smaller catheter can be more easily manipulated through a patient’s ileofemoral anatomy.
A further advantage of the XT valve for minimizing paravalvular leaks is that it is available in a 29-mm diameter, although the 29-mm valve was not included in the PARTNER II trial. The widest valve available in the original Sapien design was 26 mm.
"The 29-mm valve is a huge advance because we know that [until now] TAVR success is greater in women, probably because about 20% of American men need a larger valve, and their leak rate was higher," said Dr. Joseph E. Bavaria, a professor of surgery at the University of Pennsylvania in Philadelphia and a PARTNER II investigator. "Having the 29-mm valve available will be a big difference," he said in an interview.
The PARTNER II trial has a second cohort, cohort A, that is comparing the Sapien XT valve system against open surgical valve replacement in patients who are deemed eligible by cardiac surgeons for surgical aortic valve replacement and are at "intermediate" risk for undergoing surgery. Enrollment of the roughly 2,000 patients who will enter this study should be complete by this summer, and with a 2-year primary endpoint the results should be available sometime in 2015, Dr. Leon said.
The PARTNER II trial is sponsored by Edwards Lifesciences, which markets the XT transcatheter aortic valve system. The company has not filed an application for approval of the Sapien XT device. Dr. Leon, Dr. Mack, Dr. Popma, and Dr. Bavaria all participated in the trial. Dr. Mintz said that he has been a consultant to Boston Scientific.
SAN FRANCISCO – A new, smaller version of the only transcatheter aortic valve available for U.S. use showed noninferior clinical outcomes and safer periprocedural results in a multicenter U.S. comparison of the new and existing devices in a total of 560 patients, raising expectations that the new valve system – the Sapien XT – will soon be on the U.S. market.
Although treatment using the Sapien XT roughly matched treatment with the existing Sapien transcatheter aortic valve in the incidence of the important adverse effect of residual moderate or severe paravalvular leak, several U.S. operators who perform transcatheter aortic valve repair (TAVR) stressed that the leak problem has begun to resolve recently as interventionalists and surgeons have found ways to minimize the issue. They also noted that availability of the new XT device will move the field a step further toward fewer leak issues, because it will allow the option of a larger, 29-mm-diameter valve, a possibility that has not been available with the original Sapien valve.
"Sapien XT represents a worthwhile advance with incremental clinical value, and it is the preferred balloon-expandable transcatheter aortic valve system," said Dr. Martin B. Leon when he presented the trial data on March 10 at the annual meeting of the American College of Cardiology.
"This second-generation device has demonstrated sufficient clinical benefit for us to say it’s the preferred therapy," said Dr. Leon, professor of medicine and director of the Center for Interventional Vascular Therapy at Columbia University in New York.
"This is an evolutionary technology that is easier to use. The results are superb," commented Dr. Gary S. Mintz, an interventional cardiologist based in Washington and medical director of the Cardiovascular Research Foundation in New York.
The PARTNER II (Placement of Aortic Transcatheter Valves) cohort B trial enrolled 560 patients with aortic stenosis deemed inoperable for surgical valve replacement at 28 U.S. centers during April 2011 through February 2012. The study randomized patients to receive the Sapien XT valve or the approved Sapien valve in a prespecified noninferiority design, with a primary outcome of all-cause death, disabling stroke, or need for repeat hospitalization at 1 year after treatment. Patients averaged 84 years old, they had an average Society of Thoracic Surgeons score of about 10%, 96% had New York Heart Association class III or IV heart failure, and about 60% met the study’s criteria for frailty.
At 1-year follow-up, the combined primary endpoint occurred in 35% of patients treated with the approved valve and in 34% of patients treated with the new XT valve, a result that was not statistically significant and that met the study’s standard for noninferiority. At 1 year, as well as at 30 days after treatment, the two patient groups showed no significant difference for any of the individual components of the clinical endpoints.
However, patients treated with the XT valve had significantly fewer procedural complications, with significantly fewer major vascular events (10% with the XT device, compared with 16% with the approved valve) and significantly fewer disabling bleeding events (8% with the XT device, compared with 13% with the first-generation system), Dr. Leon reported. The category of major vascular events included perforations, dissections, and hematomas. Patients treated with the XT device also required significantly fewer episodes when the initially placed valve had to be replaced (3 replaced valve implants, compared with 10 using the approved valve), and numerically fewer aborted procedures – 2 compared with 8 using the approved valve, although this difference just missed statistical significance (P = .06). Placement of the new valve also required an average of 14 minutes less anesthesia time, a significant difference.
"It’s a smaller device. What you would expect from a smaller device is fewer vascular complications and improved procedural events," Dr. Leon said in an interview. He also highlighted the numerical difference in 30-day mortality – 5.1% with the older valve system, compared with 3.5% using the XT device. While the difference did not reach statistical significance, "to me this difference is not trivial," he said.
The difference in disabling bleeding complications is also important, commented Dr. Mintz, who was not involved in the study. "Bleeding complications are hugely disabling, especially in this older, frail population," he said.
"The big concerns from the first PARTNER trial were vascular complications, strokes, and paravalvular leaks," said Dr. Leon. "The XT device seems to address some of these issues. The vascular complications were reduced, and the stroke rate went down" compared with the first PARTNER trial (N. Engl. J. Med. 2010;363:1597-607). "What is left is the paravalvular leak issue, which is real."
In the new trial, moderate or severe paravalvular leaks, a complication that has previously been linked with worse outcomes and increased mortality, occurred in 17% of the patients who received the approved valve and in 24% of patients who received the XT valve at 30-day follow-up, a difference that did not reach significance.
"Is there a difference in paravalvular leaks between Sapien XT and Sapien? We’re not entirely sure. There was a nonsignificant trend" that will be the subject of further analysis, Dr. Leon said. He stressed that the leak rate is confounded because significantly more patients who received the approved valve underwent a second valve placement, which often occurs because a leak occurred with the first valve. "Putting in a second valve is a big thing and is not good," he said. "No matter how you look at it, there still was a significant leak rate that is not as good as it needs to be." Dr. Leon said that he hopes the issue of paravalvular leaks will be addressed by a third-generation valve system that will enter clinical trials later this year.
But some surgeons and interventionalists involved in the PARTNER II trial noted that the incidence of paravalvular leaks has already begun to come down in the past year or so.
"We are [now] much better at sizing the valve with CT scans, and that is reducing the number of paravalvular leaks, at least anecdotally at our center," said Dr. Michael J. Mack, a cardiothoracic surgeon at the Heart Hospital in Plano, Texas, and a PARTNER II investigator. "We also underfill or overfill our [valve expansion] balloons based on the CT scans, and we’re getting better results with that. We’re fitting the valve better into the space," he said in an interview.
"We do everything we can to minimize patients leaving with a moderate or severe leak by balloon dilatation or putting in a second valve," said Dr. Jeffrey J. Popma, an interventional cardiologist and professor of medicine at Harvard University in Boston and a PARTNER II investigator. He also noted that use of the XT device reduces the number of patients who need transapical valve placement, because the smaller catheter can be more easily manipulated through a patient’s ileofemoral anatomy.
A further advantage of the XT valve for minimizing paravalvular leaks is that it is available in a 29-mm diameter, although the 29-mm valve was not included in the PARTNER II trial. The widest valve available in the original Sapien design was 26 mm.
"The 29-mm valve is a huge advance because we know that [until now] TAVR success is greater in women, probably because about 20% of American men need a larger valve, and their leak rate was higher," said Dr. Joseph E. Bavaria, a professor of surgery at the University of Pennsylvania in Philadelphia and a PARTNER II investigator. "Having the 29-mm valve available will be a big difference," he said in an interview.
The PARTNER II trial has a second cohort, cohort A, that is comparing the Sapien XT valve system against open surgical valve replacement in patients who are deemed eligible by cardiac surgeons for surgical aortic valve replacement and are at "intermediate" risk for undergoing surgery. Enrollment of the roughly 2,000 patients who will enter this study should be complete by this summer, and with a 2-year primary endpoint the results should be available sometime in 2015, Dr. Leon said.
The PARTNER II trial is sponsored by Edwards Lifesciences, which markets the XT transcatheter aortic valve system. The company has not filed an application for approval of the Sapien XT device. Dr. Leon, Dr. Mack, Dr. Popma, and Dr. Bavaria all participated in the trial. Dr. Mintz said that he has been a consultant to Boston Scientific.
SAN FRANCISCO – A new, smaller version of the only transcatheter aortic valve available for U.S. use showed noninferior clinical outcomes and safer periprocedural results in a multicenter U.S. comparison of the new and existing devices in a total of 560 patients, raising expectations that the new valve system – the Sapien XT – will soon be on the U.S. market.
Although treatment using the Sapien XT roughly matched treatment with the existing Sapien transcatheter aortic valve in the incidence of the important adverse effect of residual moderate or severe paravalvular leak, several U.S. operators who perform transcatheter aortic valve repair (TAVR) stressed that the leak problem has begun to resolve recently as interventionalists and surgeons have found ways to minimize the issue. They also noted that availability of the new XT device will move the field a step further toward fewer leak issues, because it will allow the option of a larger, 29-mm-diameter valve, a possibility that has not been available with the original Sapien valve.
"Sapien XT represents a worthwhile advance with incremental clinical value, and it is the preferred balloon-expandable transcatheter aortic valve system," said Dr. Martin B. Leon when he presented the trial data on March 10 at the annual meeting of the American College of Cardiology.
"This second-generation device has demonstrated sufficient clinical benefit for us to say it’s the preferred therapy," said Dr. Leon, professor of medicine and director of the Center for Interventional Vascular Therapy at Columbia University in New York.
"This is an evolutionary technology that is easier to use. The results are superb," commented Dr. Gary S. Mintz, an interventional cardiologist based in Washington and medical director of the Cardiovascular Research Foundation in New York.
The PARTNER II (Placement of Aortic Transcatheter Valves) cohort B trial enrolled 560 patients with aortic stenosis deemed inoperable for surgical valve replacement at 28 U.S. centers during April 2011 through February 2012. The study randomized patients to receive the Sapien XT valve or the approved Sapien valve in a prespecified noninferiority design, with a primary outcome of all-cause death, disabling stroke, or need for repeat hospitalization at 1 year after treatment. Patients averaged 84 years old, they had an average Society of Thoracic Surgeons score of about 10%, 96% had New York Heart Association class III or IV heart failure, and about 60% met the study’s criteria for frailty.
At 1-year follow-up, the combined primary endpoint occurred in 35% of patients treated with the approved valve and in 34% of patients treated with the new XT valve, a result that was not statistically significant and that met the study’s standard for noninferiority. At 1 year, as well as at 30 days after treatment, the two patient groups showed no significant difference for any of the individual components of the clinical endpoints.
However, patients treated with the XT valve had significantly fewer procedural complications, with significantly fewer major vascular events (10% with the XT device, compared with 16% with the approved valve) and significantly fewer disabling bleeding events (8% with the XT device, compared with 13% with the first-generation system), Dr. Leon reported. The category of major vascular events included perforations, dissections, and hematomas. Patients treated with the XT device also required significantly fewer episodes when the initially placed valve had to be replaced (3 replaced valve implants, compared with 10 using the approved valve), and numerically fewer aborted procedures – 2 compared with 8 using the approved valve, although this difference just missed statistical significance (P = .06). Placement of the new valve also required an average of 14 minutes less anesthesia time, a significant difference.
"It’s a smaller device. What you would expect from a smaller device is fewer vascular complications and improved procedural events," Dr. Leon said in an interview. He also highlighted the numerical difference in 30-day mortality – 5.1% with the older valve system, compared with 3.5% using the XT device. While the difference did not reach statistical significance, "to me this difference is not trivial," he said.
The difference in disabling bleeding complications is also important, commented Dr. Mintz, who was not involved in the study. "Bleeding complications are hugely disabling, especially in this older, frail population," he said.
"The big concerns from the first PARTNER trial were vascular complications, strokes, and paravalvular leaks," said Dr. Leon. "The XT device seems to address some of these issues. The vascular complications were reduced, and the stroke rate went down" compared with the first PARTNER trial (N. Engl. J. Med. 2010;363:1597-607). "What is left is the paravalvular leak issue, which is real."
In the new trial, moderate or severe paravalvular leaks, a complication that has previously been linked with worse outcomes and increased mortality, occurred in 17% of the patients who received the approved valve and in 24% of patients who received the XT valve at 30-day follow-up, a difference that did not reach significance.
"Is there a difference in paravalvular leaks between Sapien XT and Sapien? We’re not entirely sure. There was a nonsignificant trend" that will be the subject of further analysis, Dr. Leon said. He stressed that the leak rate is confounded because significantly more patients who received the approved valve underwent a second valve placement, which often occurs because a leak occurred with the first valve. "Putting in a second valve is a big thing and is not good," he said. "No matter how you look at it, there still was a significant leak rate that is not as good as it needs to be." Dr. Leon said that he hopes the issue of paravalvular leaks will be addressed by a third-generation valve system that will enter clinical trials later this year.
But some surgeons and interventionalists involved in the PARTNER II trial noted that the incidence of paravalvular leaks has already begun to come down in the past year or so.
"We are [now] much better at sizing the valve with CT scans, and that is reducing the number of paravalvular leaks, at least anecdotally at our center," said Dr. Michael J. Mack, a cardiothoracic surgeon at the Heart Hospital in Plano, Texas, and a PARTNER II investigator. "We also underfill or overfill our [valve expansion] balloons based on the CT scans, and we’re getting better results with that. We’re fitting the valve better into the space," he said in an interview.
"We do everything we can to minimize patients leaving with a moderate or severe leak by balloon dilatation or putting in a second valve," said Dr. Jeffrey J. Popma, an interventional cardiologist and professor of medicine at Harvard University in Boston and a PARTNER II investigator. He also noted that use of the XT device reduces the number of patients who need transapical valve placement, because the smaller catheter can be more easily manipulated through a patient’s ileofemoral anatomy.
A further advantage of the XT valve for minimizing paravalvular leaks is that it is available in a 29-mm diameter, although the 29-mm valve was not included in the PARTNER II trial. The widest valve available in the original Sapien design was 26 mm.
"The 29-mm valve is a huge advance because we know that [until now] TAVR success is greater in women, probably because about 20% of American men need a larger valve, and their leak rate was higher," said Dr. Joseph E. Bavaria, a professor of surgery at the University of Pennsylvania in Philadelphia and a PARTNER II investigator. "Having the 29-mm valve available will be a big difference," he said in an interview.
The PARTNER II trial has a second cohort, cohort A, that is comparing the Sapien XT valve system against open surgical valve replacement in patients who are deemed eligible by cardiac surgeons for surgical aortic valve replacement and are at "intermediate" risk for undergoing surgery. Enrollment of the roughly 2,000 patients who will enter this study should be complete by this summer, and with a 2-year primary endpoint the results should be available sometime in 2015, Dr. Leon said.
The PARTNER II trial is sponsored by Edwards Lifesciences, which markets the XT transcatheter aortic valve system. The company has not filed an application for approval of the Sapien XT device. Dr. Leon, Dr. Mack, Dr. Popma, and Dr. Bavaria all participated in the trial. Dr. Mintz said that he has been a consultant to Boston Scientific.
AT ACC 13
Major finding: Aortic valve replacement with the Sapien XT valve system was noninferior to replacement with the Sapien valve system and produced fewer complications.
Data source: The PARTNER II trial, which randomized 560 patients at 28 U.S. centers.
Disclosures: The PARTNER II trial is sponsored by Edwards Lifesciences, which markets the XT transcatheter aortic valve system. Dr. Leon, Dr. Mack, Dr. Popma, and Dr. Bavaria all participated in the trial. Dr. Mintz said that he has been a consultant to Boston Scientific.