User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Surgery offers best chance in cancer but needs more ‘support’
warns a European expert.
In addition, there are many obstacles to the delivery of optimal cancer surgery, says Domenico M. D’Ugo, MD, professor of surgery at the Catholic University of Rome – A. Gemelli Medical School, Rome, Italy.
Dr. D’Ugo, who is president of the European Society of Surgical Oncology (ESSO), calls for a range of measures to improve the quality of cancer surgery and patient access in Europe.
These measures include recognition of surgical oncology as a specialist discipline, greater support for surgical research and innovation, and a greater role for surgery in multidisciplinary care.
The demands were made in open letter that was published by ESSO on Nov. 9 to coincide with the society’s annual meeting, held in Lisbon, Portugal.
The theme of this year’s meeting was the future of cancer surgery in Europe – a future that “holds many promises to make surgical oncology safer, more efficient and minimally invasive,” writes Dr. D’Ugo.
However, ESSO needs the support of European leaders to bring the recommendations to life and, ultimately, to help provide high-quality cancer treatment, he adds. This is particularly important given the upcoming implementation of Europe’s Beating Cancer Plan.
The open letter is addressed to Stella Kyriakides, European commissioner for health and food safety, and Bartosz Arłukowicz, chair of the European Parliament Special Committee on Beating Cancer, among others.
Best chance of cure
“High-quality surgery remains the best chance to cure solid cancer when diagnosed early,” Dr. D’Ugo notes in his letter. It is also the most cost-effective treatment for the majority of nonmetastasized tumors, he writes.
In addition, surgery is “fundamental” to the prevention of cancer in patients with inherited susceptibility and to the diagnosis and staging of cancer, as well as to the treatment of metastatic disease, the preservation of quality of life, and the alleviation of cancer symptoms, he writes.
There is thus a substantial and steadily growing demand for surgical oncology.
It is estimated that approximately 80% of cancer patients will require surgical intervention at some point during the course of their disease, and 45 million surgical procedures will be needed worldwide by 2030.
Dr. D’Ugo says that at present, fewer than a quarter of cancer patients receive safe, affordable, or timely surgery.
It is time to give surgical oncology the political and financial attention it deserves, he argues. He outlines a four-point plan to achieve this.
The first point is to enhance recognition of surgical oncology as a specialist discipline through, for example, the global curriculum proposed by ESSO and the Society of Surgical Oncology in 2016.
At present, only eight countries in Europe recognize surgical oncology as a specialty, and the lack of harmonization is “causing disparities in training, qualifications and practices,” as well as in patient access, Dr. D’Ugo says.
Next is a call to support research and innovation. Despite recent advances, research in cancer surgery “remains highly underfunded in Europe when compared with pharmaceutical research,” he says.
Improved screening and early detection of cancer are the next key area, because when the disease is diagnosed at an early stage, curative surgery has “a greater chance to be successful.”
At present, screening programs in Europe address only colorectal, breast, and cervical cancers, and the uptake remains “low,” he writes.
Lastly, he emphasizes that surgery is “integral” to multidisciplinary care and that outcomes for patients are better in comprehensive cancer centers that support patients throughout the disease pathway.
Dr. D’Ugo suggests that surgical oncologists take on a “bigger role” in multidisciplinary care, and he calls for the certification and accreditation of cancer units to increase and unify standards of care across the region.
D’Ugo has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
warns a European expert.
In addition, there are many obstacles to the delivery of optimal cancer surgery, says Domenico M. D’Ugo, MD, professor of surgery at the Catholic University of Rome – A. Gemelli Medical School, Rome, Italy.
Dr. D’Ugo, who is president of the European Society of Surgical Oncology (ESSO), calls for a range of measures to improve the quality of cancer surgery and patient access in Europe.
These measures include recognition of surgical oncology as a specialist discipline, greater support for surgical research and innovation, and a greater role for surgery in multidisciplinary care.
The demands were made in open letter that was published by ESSO on Nov. 9 to coincide with the society’s annual meeting, held in Lisbon, Portugal.
The theme of this year’s meeting was the future of cancer surgery in Europe – a future that “holds many promises to make surgical oncology safer, more efficient and minimally invasive,” writes Dr. D’Ugo.
However, ESSO needs the support of European leaders to bring the recommendations to life and, ultimately, to help provide high-quality cancer treatment, he adds. This is particularly important given the upcoming implementation of Europe’s Beating Cancer Plan.
The open letter is addressed to Stella Kyriakides, European commissioner for health and food safety, and Bartosz Arłukowicz, chair of the European Parliament Special Committee on Beating Cancer, among others.
Best chance of cure
“High-quality surgery remains the best chance to cure solid cancer when diagnosed early,” Dr. D’Ugo notes in his letter. It is also the most cost-effective treatment for the majority of nonmetastasized tumors, he writes.
In addition, surgery is “fundamental” to the prevention of cancer in patients with inherited susceptibility and to the diagnosis and staging of cancer, as well as to the treatment of metastatic disease, the preservation of quality of life, and the alleviation of cancer symptoms, he writes.
There is thus a substantial and steadily growing demand for surgical oncology.
It is estimated that approximately 80% of cancer patients will require surgical intervention at some point during the course of their disease, and 45 million surgical procedures will be needed worldwide by 2030.
Dr. D’Ugo says that at present, fewer than a quarter of cancer patients receive safe, affordable, or timely surgery.
It is time to give surgical oncology the political and financial attention it deserves, he argues. He outlines a four-point plan to achieve this.
The first point is to enhance recognition of surgical oncology as a specialist discipline through, for example, the global curriculum proposed by ESSO and the Society of Surgical Oncology in 2016.
At present, only eight countries in Europe recognize surgical oncology as a specialty, and the lack of harmonization is “causing disparities in training, qualifications and practices,” as well as in patient access, Dr. D’Ugo says.
Next is a call to support research and innovation. Despite recent advances, research in cancer surgery “remains highly underfunded in Europe when compared with pharmaceutical research,” he says.
Improved screening and early detection of cancer are the next key area, because when the disease is diagnosed at an early stage, curative surgery has “a greater chance to be successful.”
At present, screening programs in Europe address only colorectal, breast, and cervical cancers, and the uptake remains “low,” he writes.
Lastly, he emphasizes that surgery is “integral” to multidisciplinary care and that outcomes for patients are better in comprehensive cancer centers that support patients throughout the disease pathway.
Dr. D’Ugo suggests that surgical oncologists take on a “bigger role” in multidisciplinary care, and he calls for the certification and accreditation of cancer units to increase and unify standards of care across the region.
D’Ugo has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
warns a European expert.
In addition, there are many obstacles to the delivery of optimal cancer surgery, says Domenico M. D’Ugo, MD, professor of surgery at the Catholic University of Rome – A. Gemelli Medical School, Rome, Italy.
Dr. D’Ugo, who is president of the European Society of Surgical Oncology (ESSO), calls for a range of measures to improve the quality of cancer surgery and patient access in Europe.
These measures include recognition of surgical oncology as a specialist discipline, greater support for surgical research and innovation, and a greater role for surgery in multidisciplinary care.
The demands were made in open letter that was published by ESSO on Nov. 9 to coincide with the society’s annual meeting, held in Lisbon, Portugal.
The theme of this year’s meeting was the future of cancer surgery in Europe – a future that “holds many promises to make surgical oncology safer, more efficient and minimally invasive,” writes Dr. D’Ugo.
However, ESSO needs the support of European leaders to bring the recommendations to life and, ultimately, to help provide high-quality cancer treatment, he adds. This is particularly important given the upcoming implementation of Europe’s Beating Cancer Plan.
The open letter is addressed to Stella Kyriakides, European commissioner for health and food safety, and Bartosz Arłukowicz, chair of the European Parliament Special Committee on Beating Cancer, among others.
Best chance of cure
“High-quality surgery remains the best chance to cure solid cancer when diagnosed early,” Dr. D’Ugo notes in his letter. It is also the most cost-effective treatment for the majority of nonmetastasized tumors, he writes.
In addition, surgery is “fundamental” to the prevention of cancer in patients with inherited susceptibility and to the diagnosis and staging of cancer, as well as to the treatment of metastatic disease, the preservation of quality of life, and the alleviation of cancer symptoms, he writes.
There is thus a substantial and steadily growing demand for surgical oncology.
It is estimated that approximately 80% of cancer patients will require surgical intervention at some point during the course of their disease, and 45 million surgical procedures will be needed worldwide by 2030.
Dr. D’Ugo says that at present, fewer than a quarter of cancer patients receive safe, affordable, or timely surgery.
It is time to give surgical oncology the political and financial attention it deserves, he argues. He outlines a four-point plan to achieve this.
The first point is to enhance recognition of surgical oncology as a specialist discipline through, for example, the global curriculum proposed by ESSO and the Society of Surgical Oncology in 2016.
At present, only eight countries in Europe recognize surgical oncology as a specialty, and the lack of harmonization is “causing disparities in training, qualifications and practices,” as well as in patient access, Dr. D’Ugo says.
Next is a call to support research and innovation. Despite recent advances, research in cancer surgery “remains highly underfunded in Europe when compared with pharmaceutical research,” he says.
Improved screening and early detection of cancer are the next key area, because when the disease is diagnosed at an early stage, curative surgery has “a greater chance to be successful.”
At present, screening programs in Europe address only colorectal, breast, and cervical cancers, and the uptake remains “low,” he writes.
Lastly, he emphasizes that surgery is “integral” to multidisciplinary care and that outcomes for patients are better in comprehensive cancer centers that support patients throughout the disease pathway.
Dr. D’Ugo suggests that surgical oncologists take on a “bigger role” in multidisciplinary care, and he calls for the certification and accreditation of cancer units to increase and unify standards of care across the region.
D’Ugo has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Shock, disbelief as NCCN changes prostate cancer guidance
For over a decade, the influential National Comprehensive Cancer Network (NCCN) has been recommending that men with low-risk prostate cancer be offered active surveillance as the lone “preferred” initial treatment option.
But the NCCN has now reversed this long-standing recommendation in the latest revision of its prostate cancer guideline.
The organization now recommends that low-risk disease be managed with either active surveillance or radiation therapy or surgery, with equal weight given to all three of these initial options.
The complaints were voiced in unusually blunt and strong language for physicians.
“This is a terrible step back that impacts every urologist,” commented John Griffith, MD, of Hartford Healthcare, who practices in New Britain, Conn.
Dr. Griffith explained that he prints out the NCCN guidance with “every patient newly diagnosed” and that the preferred designation is a “huge help” in reassuring them about not treating low-risk disease initially.
In a Twitter thread, Benjamin Davies, MD, of the University of Pittsburgh, facetiously wondered if a time warp was at play: “To suggest for a millisecond that active surveillance isn’t the preferred method for low-risk men is bizarre thinking ... Is this 1980?”
“I’m baffled,” said Brian Chapin, MD, of MD Anderson Cancer Center, Houston, in another Twitter thread.
“This is ludicrous,” said Andrew Vickers, PhD, of Memorial Sloan Kettering Cancer Center in New York City in a tweet.
Alexander Kutikov, MD, of Fox Chase Cancer Center in Philadelphia, commented on Twitter that the change “seems off the rails…a bit stunned by this.”
Matthew Cooperberg, MD, of the University of California San Francisco, and Minhaj Siddiqui, MD, of the University of Maryland in Baltimore both called the move a “step backward.”
Many others also expressed disappointment in the NCCN, whose guidelines are hugely influential because of the role they play clinically as well as with payors and the legal system.
“A huge setback & frankly a disgrace for @NCCN and its processes,” commented Fox Chase’s Dr. Kutikov.
Stacy Loeb, MD, of NYU Langone Health in New York City, suggested the new guidance may stunt use of active surveillance in the United States. She tweeted: “The updated NCCN guideline certainly won’t help the lagging and heterogenous uptake of active surveillance in the U.S. We should be carefully expanding the pool for active surveillance, not narrowing it.”
The purpose of active surveillance is to avoid adverse events from treatment, which can be life-changing as they include incontinence and erectile dysfunction.
The rationale is that many men with low-risk prostate cancer may not need treatment for their disease, as the disease may be slow-growing and may never threaten their life. With active surveillance, men are instead monitored with blood tests, scans, and biopsies to watch for worsening disease, and treated only when there are signs of disease progression.
This active surveillance approach has grown in acceptance among American patients since 2010.
The concern now is that the change in guidance from the NCCN will lead to a reduction in active surveillance, and an increase in initial treatment with surgery and radiotherapy for low-risk disease, which is considered by many to be “overtreatment’ of this disease and may not be medically necessary.
For example, UCSF’s Dr. Cooperberg said he feared that the changed guidance “will be used by urologists and radiation oncologists to justify overtreatment of low-risk disease.”
Dr. Kutikov agreed but described that possibility differently, citing the risk of lawsuits. He observed that without the NCCN’s “medico-legal buffer” of active surveillance as the preferred initial treatment, there are “further incentives” for overtreatment.
The new NCCN guidance also conflicts with the American Urological Association’s guidelines and dissolves what was once a mostly united front from the two major organizations on active surveillance and low-risk disease.
The AUA Guideline reads: “Clinicians should recommend active surveillance as the preferable care option for most low-risk localized prostate cancer patients (Moderate Recommendation; Evidence Level: Grade B)
Patients protest change in wording
Not surprisingly, the revised NCCN guidance was criticized by multiple patient advocacy groups, including Active Surveillance Patients International (ASPI), which wrote a letter to the NCCN protesting the change.
In that letter, the ASPI writes that active surveillance is now chosen as the initial approach for low-risk prostate cancer in about 90% of cases in some European nations, and in about 50% of cases in the United States. It also warns that eliminating the word “preferred” from the NCCN guidelines represents a retreat, and “will have repercussions far beyond what we may first conceive.”
“Active surveillance should be the preferred choice to preserve quality of life for men with low-risk cancer,” the advocacy group states. “The PIVOT trials indicate for low-risk disease there is basically no advantage to intervention. Why would one risk the side effects if they knew that?”
Why now?
The NCCN’s move to alter its low-risk prostate cancer guidance is especially striking because, 11 years ago, the NCCN broke new ground in recommending active surveillance as the sole initial treatment option for low-risk men. (It was also the first guidelines group to recommend the same for very low-risk men.)
So why the change now? This news organization requested, but did not receive, comment from the NCCN and its chair of the prostate cancer panel, Edward Schaeffer, MD, of Northwestern University in Chicago.
However, on Twitter, Dr. Schaeffer hinted at what had turned the tables for the NCCN panel – the risk that, over time, some men with low-risk disease who are on active surveillance are reclassified on biopsy as having a higher risk.
He highlighted a 2020 study on that very subject from the University of California, San Francisco, published in the Journal of Urology. Those authors concluded that: “Given the heterogeneity of the disease, some tumors characterized as low risk may merit early treatment while others may be followed much less intensely over some time interval.”
Dr. Schaeffer tweeted: “I think this nicely sums up the low-risk space ...”
Experts reacting to Dr. Schaeffer’s tweet were not swayed.
Looking at additional measures such as genomic scores and PSA density, as advocated by Dr. Schaeffer via the posted 2020 study, is good for assessing individual risk, “but still, active surveillance is the preferred option for low risk,” said MD Anderson’s Dr. Chapin.
UCSF’s Dr. Cooperberg, who was a co-author on that 2020 Journal of Urology paper, commented that the university’s urology department had “spent the past quarter century arguing active surveillance is ‘preferred’ for almost all low risk [disease]!”
“Many on active surveillance need treatment someday, but that does not justify immediate overtreatment,” he concluded.
A version of this article first appeared on Medscape.com.
For over a decade, the influential National Comprehensive Cancer Network (NCCN) has been recommending that men with low-risk prostate cancer be offered active surveillance as the lone “preferred” initial treatment option.
But the NCCN has now reversed this long-standing recommendation in the latest revision of its prostate cancer guideline.
The organization now recommends that low-risk disease be managed with either active surveillance or radiation therapy or surgery, with equal weight given to all three of these initial options.
The complaints were voiced in unusually blunt and strong language for physicians.
“This is a terrible step back that impacts every urologist,” commented John Griffith, MD, of Hartford Healthcare, who practices in New Britain, Conn.
Dr. Griffith explained that he prints out the NCCN guidance with “every patient newly diagnosed” and that the preferred designation is a “huge help” in reassuring them about not treating low-risk disease initially.
In a Twitter thread, Benjamin Davies, MD, of the University of Pittsburgh, facetiously wondered if a time warp was at play: “To suggest for a millisecond that active surveillance isn’t the preferred method for low-risk men is bizarre thinking ... Is this 1980?”
“I’m baffled,” said Brian Chapin, MD, of MD Anderson Cancer Center, Houston, in another Twitter thread.
“This is ludicrous,” said Andrew Vickers, PhD, of Memorial Sloan Kettering Cancer Center in New York City in a tweet.
Alexander Kutikov, MD, of Fox Chase Cancer Center in Philadelphia, commented on Twitter that the change “seems off the rails…a bit stunned by this.”
Matthew Cooperberg, MD, of the University of California San Francisco, and Minhaj Siddiqui, MD, of the University of Maryland in Baltimore both called the move a “step backward.”
Many others also expressed disappointment in the NCCN, whose guidelines are hugely influential because of the role they play clinically as well as with payors and the legal system.
“A huge setback & frankly a disgrace for @NCCN and its processes,” commented Fox Chase’s Dr. Kutikov.
Stacy Loeb, MD, of NYU Langone Health in New York City, suggested the new guidance may stunt use of active surveillance in the United States. She tweeted: “The updated NCCN guideline certainly won’t help the lagging and heterogenous uptake of active surveillance in the U.S. We should be carefully expanding the pool for active surveillance, not narrowing it.”
The purpose of active surveillance is to avoid adverse events from treatment, which can be life-changing as they include incontinence and erectile dysfunction.
The rationale is that many men with low-risk prostate cancer may not need treatment for their disease, as the disease may be slow-growing and may never threaten their life. With active surveillance, men are instead monitored with blood tests, scans, and biopsies to watch for worsening disease, and treated only when there are signs of disease progression.
This active surveillance approach has grown in acceptance among American patients since 2010.
The concern now is that the change in guidance from the NCCN will lead to a reduction in active surveillance, and an increase in initial treatment with surgery and radiotherapy for low-risk disease, which is considered by many to be “overtreatment’ of this disease and may not be medically necessary.
For example, UCSF’s Dr. Cooperberg said he feared that the changed guidance “will be used by urologists and radiation oncologists to justify overtreatment of low-risk disease.”
Dr. Kutikov agreed but described that possibility differently, citing the risk of lawsuits. He observed that without the NCCN’s “medico-legal buffer” of active surveillance as the preferred initial treatment, there are “further incentives” for overtreatment.
The new NCCN guidance also conflicts with the American Urological Association’s guidelines and dissolves what was once a mostly united front from the two major organizations on active surveillance and low-risk disease.
The AUA Guideline reads: “Clinicians should recommend active surveillance as the preferable care option for most low-risk localized prostate cancer patients (Moderate Recommendation; Evidence Level: Grade B)
Patients protest change in wording
Not surprisingly, the revised NCCN guidance was criticized by multiple patient advocacy groups, including Active Surveillance Patients International (ASPI), which wrote a letter to the NCCN protesting the change.
In that letter, the ASPI writes that active surveillance is now chosen as the initial approach for low-risk prostate cancer in about 90% of cases in some European nations, and in about 50% of cases in the United States. It also warns that eliminating the word “preferred” from the NCCN guidelines represents a retreat, and “will have repercussions far beyond what we may first conceive.”
“Active surveillance should be the preferred choice to preserve quality of life for men with low-risk cancer,” the advocacy group states. “The PIVOT trials indicate for low-risk disease there is basically no advantage to intervention. Why would one risk the side effects if they knew that?”
Why now?
The NCCN’s move to alter its low-risk prostate cancer guidance is especially striking because, 11 years ago, the NCCN broke new ground in recommending active surveillance as the sole initial treatment option for low-risk men. (It was also the first guidelines group to recommend the same for very low-risk men.)
So why the change now? This news organization requested, but did not receive, comment from the NCCN and its chair of the prostate cancer panel, Edward Schaeffer, MD, of Northwestern University in Chicago.
However, on Twitter, Dr. Schaeffer hinted at what had turned the tables for the NCCN panel – the risk that, over time, some men with low-risk disease who are on active surveillance are reclassified on biopsy as having a higher risk.
He highlighted a 2020 study on that very subject from the University of California, San Francisco, published in the Journal of Urology. Those authors concluded that: “Given the heterogeneity of the disease, some tumors characterized as low risk may merit early treatment while others may be followed much less intensely over some time interval.”
Dr. Schaeffer tweeted: “I think this nicely sums up the low-risk space ...”
Experts reacting to Dr. Schaeffer’s tweet were not swayed.
Looking at additional measures such as genomic scores and PSA density, as advocated by Dr. Schaeffer via the posted 2020 study, is good for assessing individual risk, “but still, active surveillance is the preferred option for low risk,” said MD Anderson’s Dr. Chapin.
UCSF’s Dr. Cooperberg, who was a co-author on that 2020 Journal of Urology paper, commented that the university’s urology department had “spent the past quarter century arguing active surveillance is ‘preferred’ for almost all low risk [disease]!”
“Many on active surveillance need treatment someday, but that does not justify immediate overtreatment,” he concluded.
A version of this article first appeared on Medscape.com.
For over a decade, the influential National Comprehensive Cancer Network (NCCN) has been recommending that men with low-risk prostate cancer be offered active surveillance as the lone “preferred” initial treatment option.
But the NCCN has now reversed this long-standing recommendation in the latest revision of its prostate cancer guideline.
The organization now recommends that low-risk disease be managed with either active surveillance or radiation therapy or surgery, with equal weight given to all three of these initial options.
The complaints were voiced in unusually blunt and strong language for physicians.
“This is a terrible step back that impacts every urologist,” commented John Griffith, MD, of Hartford Healthcare, who practices in New Britain, Conn.
Dr. Griffith explained that he prints out the NCCN guidance with “every patient newly diagnosed” and that the preferred designation is a “huge help” in reassuring them about not treating low-risk disease initially.
In a Twitter thread, Benjamin Davies, MD, of the University of Pittsburgh, facetiously wondered if a time warp was at play: “To suggest for a millisecond that active surveillance isn’t the preferred method for low-risk men is bizarre thinking ... Is this 1980?”
“I’m baffled,” said Brian Chapin, MD, of MD Anderson Cancer Center, Houston, in another Twitter thread.
“This is ludicrous,” said Andrew Vickers, PhD, of Memorial Sloan Kettering Cancer Center in New York City in a tweet.
Alexander Kutikov, MD, of Fox Chase Cancer Center in Philadelphia, commented on Twitter that the change “seems off the rails…a bit stunned by this.”
Matthew Cooperberg, MD, of the University of California San Francisco, and Minhaj Siddiqui, MD, of the University of Maryland in Baltimore both called the move a “step backward.”
Many others also expressed disappointment in the NCCN, whose guidelines are hugely influential because of the role they play clinically as well as with payors and the legal system.
“A huge setback & frankly a disgrace for @NCCN and its processes,” commented Fox Chase’s Dr. Kutikov.
Stacy Loeb, MD, of NYU Langone Health in New York City, suggested the new guidance may stunt use of active surveillance in the United States. She tweeted: “The updated NCCN guideline certainly won’t help the lagging and heterogenous uptake of active surveillance in the U.S. We should be carefully expanding the pool for active surveillance, not narrowing it.”
The purpose of active surveillance is to avoid adverse events from treatment, which can be life-changing as they include incontinence and erectile dysfunction.
The rationale is that many men with low-risk prostate cancer may not need treatment for their disease, as the disease may be slow-growing and may never threaten their life. With active surveillance, men are instead monitored with blood tests, scans, and biopsies to watch for worsening disease, and treated only when there are signs of disease progression.
This active surveillance approach has grown in acceptance among American patients since 2010.
The concern now is that the change in guidance from the NCCN will lead to a reduction in active surveillance, and an increase in initial treatment with surgery and radiotherapy for low-risk disease, which is considered by many to be “overtreatment’ of this disease and may not be medically necessary.
For example, UCSF’s Dr. Cooperberg said he feared that the changed guidance “will be used by urologists and radiation oncologists to justify overtreatment of low-risk disease.”
Dr. Kutikov agreed but described that possibility differently, citing the risk of lawsuits. He observed that without the NCCN’s “medico-legal buffer” of active surveillance as the preferred initial treatment, there are “further incentives” for overtreatment.
The new NCCN guidance also conflicts with the American Urological Association’s guidelines and dissolves what was once a mostly united front from the two major organizations on active surveillance and low-risk disease.
The AUA Guideline reads: “Clinicians should recommend active surveillance as the preferable care option for most low-risk localized prostate cancer patients (Moderate Recommendation; Evidence Level: Grade B)
Patients protest change in wording
Not surprisingly, the revised NCCN guidance was criticized by multiple patient advocacy groups, including Active Surveillance Patients International (ASPI), which wrote a letter to the NCCN protesting the change.
In that letter, the ASPI writes that active surveillance is now chosen as the initial approach for low-risk prostate cancer in about 90% of cases in some European nations, and in about 50% of cases in the United States. It also warns that eliminating the word “preferred” from the NCCN guidelines represents a retreat, and “will have repercussions far beyond what we may first conceive.”
“Active surveillance should be the preferred choice to preserve quality of life for men with low-risk cancer,” the advocacy group states. “The PIVOT trials indicate for low-risk disease there is basically no advantage to intervention. Why would one risk the side effects if they knew that?”
Why now?
The NCCN’s move to alter its low-risk prostate cancer guidance is especially striking because, 11 years ago, the NCCN broke new ground in recommending active surveillance as the sole initial treatment option for low-risk men. (It was also the first guidelines group to recommend the same for very low-risk men.)
So why the change now? This news organization requested, but did not receive, comment from the NCCN and its chair of the prostate cancer panel, Edward Schaeffer, MD, of Northwestern University in Chicago.
However, on Twitter, Dr. Schaeffer hinted at what had turned the tables for the NCCN panel – the risk that, over time, some men with low-risk disease who are on active surveillance are reclassified on biopsy as having a higher risk.
He highlighted a 2020 study on that very subject from the University of California, San Francisco, published in the Journal of Urology. Those authors concluded that: “Given the heterogeneity of the disease, some tumors characterized as low risk may merit early treatment while others may be followed much less intensely over some time interval.”
Dr. Schaeffer tweeted: “I think this nicely sums up the low-risk space ...”
Experts reacting to Dr. Schaeffer’s tweet were not swayed.
Looking at additional measures such as genomic scores and PSA density, as advocated by Dr. Schaeffer via the posted 2020 study, is good for assessing individual risk, “but still, active surveillance is the preferred option for low risk,” said MD Anderson’s Dr. Chapin.
UCSF’s Dr. Cooperberg, who was a co-author on that 2020 Journal of Urology paper, commented that the university’s urology department had “spent the past quarter century arguing active surveillance is ‘preferred’ for almost all low risk [disease]!”
“Many on active surveillance need treatment someday, but that does not justify immediate overtreatment,” he concluded.
A version of this article first appeared on Medscape.com.
Early SAVR tops watchful waiting in severe, asymptomatic aortic stenosis: AVATAR
Better to intervene early with a new valve in patients with severe aortic stenosis (AS) who are asymptomatic, even during exercise, than to wait for the disease to progress and symptoms to emerge before operating, suggests a small, randomized trial that challenges the guidelines.
Of the trial’s 157 patients, all with negative results on stress tests and normal left ventricular (LV) function despite severe AS, those assigned to early surgical aortic valve replacement (SAVR), compared with standard watchful waiting, showed a better-than-50% drop in risk for death or major adverse cardiac events (MACE) over 2-3 years. The benefit appeared driven by fewer hospitalizations for heart failure (HF) and deaths in the early-surgery group.
The findings “advocate for early surgery once aortic stenosis becomes significant and regardless of symptom status,” Marko Banovic, MD, PhD, said during his presentation at the American Heart Association scientific sessions.
Dr. Banovic, from the University of Belgrade Medical School in Serbia, is coprincipal investigator on the trial, called AVATAR (Aortic Valve Replacement vs. Conservative Treatment in Asymptomatic Severe Aortic Stenosis). He is also lead author on the study’s publication in Circulation, timed to coincide with his AHA presentation.
“The AVATAR findings provide additional evidence to help clinicians in guiding their decision when seeing a patient with significant aortic stenosis, normal left ventricular function, overall low surgical risk, and without significant comorbidities,” Dr. Banovic told this news organization.
European and North American Guidelines favor watchful waiting for asymptomatic patients with severe aortic stenosis, with surgery upon development of symptoms or LV dysfunction, observed Victoria Delgado, MD, PhD, Leiden (the Netherlands) University Medical Center, an invited discussant for the AVATAR presentation.
AVATAR does suggest that “early surgery in truly asymptomatic patients with severe aortic stenosis and preserved ejection fraction seems to provide better outcomes as compared to the conservative treatment,” she said. “But I think that the long-term follow-up for potential events, such as valve durability or endocarditis, is still needed.”
The trial has strengths, compared with the recent RECOVERY trial, which also concluded in favor of early SAVR over watchful waiting in patients described as asymptomatic with severe aortic stenosis. Dr. Delgado and other observers, however, have pointed out limitations of that trial, including questions about whether the patients were truly asymptomatic – stress testing wasn›t routinely performed.
In AVATAR, all patients were negative at stress testing, which required them to reach their estimated maximum heart rate, Dr. Banovic noted. As he and his colleagues write, the trial expands on RECOVERY “by providing evidence of the benefit of early surgery in a setting representative of a dilemma in decision making, in truly asymptomatic patients with severe but not critical aortic stenosis and normal LV function.”
A role for TAVR?
Guidelines in general “can be very conservative and lag behind evidence a bit,” Patricia A. Pellikka, MD, Mayo Clinic, Rochester, Minn., who is not associated with AVATAR, said in an interview.
“I think when we see patients clinically, we can advise them that if they don’t have symptoms and they do have severe aortic stenosis,” she said, “they’re likely going to get symptoms within a reasonably short period of time, according to our retrospective databases, and that doing the intervention early may yield better long-term outcomes.”
The results of AVATAR, in which valve replacement consisted only of SAVR, “probably could be extrapolated” to transcatheter aortic valve replacement (TAVR), Dr. Pellikka observed. “Certainly, TAVR is the procedure that patients come asking for. It’s attractive to avoid a major surgery, and it seems very plausible that TAVR would have yielded similar results if that had been a therapy in this trial.”
In practice, patient age and functional status would figure heavily in deciding whether early valve replacement, and which procedure, is appropriate, Dr. Banovic said in an interview. Importantly, the trial’s patients were at low surgical risk and free of major chronic diseases or other important health concerns.
“Frailty and older age are known risk factors for suboptimal recovery” after SAVR, Dr. Banovic said when interviewed. Therefore, frail patients, who were not many in AVATAR, might be “more suitable for TAVR than SAVR, based on the TAVR-vs.-SAVR results in symptomatic AS patients,” he said.
“One might extrapolate experience from AVATAR trial to TAVR, which may lower the bar for TAVR indications,” but that would require more supporting evidence, Dr. Banovic said.
Confirmed asymptomatic
AVATAR, conducted at nine centers in seven countries in the European Union, randomly assigned 157 adults with severe AS by echocardiography and a LV ejection fraction (LVEF) greater than 50% to early SAVR or conservative management. They averaged 67 years in age, and 43% were women.
The trial excluded anyone with dyspnea, syncope, presyncope, angina, or LV dysfunction and anyone with a history of atrial fibrillation or significant cardiac, renal, or lung disease. The cohort’s average Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 1.7%.
The 78 patients in the early-surgery group “were expected” to have the procedure within 8 weeks of randomization, the published report states; the median time was 55 days. Six of them ultimately did not have the surgery. There was only one periprocedural death, for an operative mortality of 1.4%.
The 79 patients assigned to conservative care were later referred for surgery if they developed symptoms, their LVEF dropped below 50%, or they showed a 0.3-m/sec jump in peak aortic jet velocity at follow-up echocardiography. That occurred with 25 patients a median of 400 days after randomization.
The rate of the primary endpoint – death from any cause, acute myocardial infarction, stroke, or unplanned HF hospitalization – was 16.6% in the early-surgery group and 32.9% for those managed conservatively over a median of 32 months. The hazard ratio by intention-to-treat analysis was 0.46 (95% confidence interval, 0.23-0.90; P = .02). The HR for death from any cause or HF hospitalization was 0.40 (95% CI, 0.19-0.84; P = .013). Any differences in the individual endpoints of death, first HF hospitalizations, thromboembolic complications, or major bleeding were not significant.
If early aortic valve replacement is better for patients like those in AVATAR, some sort of screening for previously unknown severe aortic stenosis may seem attractive for selected populations. “Echocardiography would be the screening test for aortic stenosis, but it’s fairly expensive and therefore has never been advocated as a test to screen everyone,” Dr. Pellikka observed.
“But things are changing,” given innovations such as point-of-care ultrasonography and machine learning, she noted. “Artificial intelligence is progressing in its application to echocardiography, and it’s conceivable that in the future, there might be some abbreviated or screening type of test. But I don’t think we’re quite there yet.”
Dr. Banovic had no conflicts; disclosures for the other authors are in the report. Dr. Delgado disclosed speaker fees from Edwards Lifesciences, Abbott Vascular, Medtronic, Merck, Novartis, and GE Healthcare and unrestricted research grants to her institution from Abbott Vascular, Bayer, Biotronik, Bioventrix, Boston Scientific, Edwards Lifesciences, GE Healthcare, Ionis, and Medtronic. Dr. Pellikka disclosed receiving a research grant from Ultromics and having unspecified modest relationships with GE Healthcare, Lantheus, and OxThera.
A version of this article first appeared on Medscape.com.
Better to intervene early with a new valve in patients with severe aortic stenosis (AS) who are asymptomatic, even during exercise, than to wait for the disease to progress and symptoms to emerge before operating, suggests a small, randomized trial that challenges the guidelines.
Of the trial’s 157 patients, all with negative results on stress tests and normal left ventricular (LV) function despite severe AS, those assigned to early surgical aortic valve replacement (SAVR), compared with standard watchful waiting, showed a better-than-50% drop in risk for death or major adverse cardiac events (MACE) over 2-3 years. The benefit appeared driven by fewer hospitalizations for heart failure (HF) and deaths in the early-surgery group.
The findings “advocate for early surgery once aortic stenosis becomes significant and regardless of symptom status,” Marko Banovic, MD, PhD, said during his presentation at the American Heart Association scientific sessions.
Dr. Banovic, from the University of Belgrade Medical School in Serbia, is coprincipal investigator on the trial, called AVATAR (Aortic Valve Replacement vs. Conservative Treatment in Asymptomatic Severe Aortic Stenosis). He is also lead author on the study’s publication in Circulation, timed to coincide with his AHA presentation.
“The AVATAR findings provide additional evidence to help clinicians in guiding their decision when seeing a patient with significant aortic stenosis, normal left ventricular function, overall low surgical risk, and without significant comorbidities,” Dr. Banovic told this news organization.
European and North American Guidelines favor watchful waiting for asymptomatic patients with severe aortic stenosis, with surgery upon development of symptoms or LV dysfunction, observed Victoria Delgado, MD, PhD, Leiden (the Netherlands) University Medical Center, an invited discussant for the AVATAR presentation.
AVATAR does suggest that “early surgery in truly asymptomatic patients with severe aortic stenosis and preserved ejection fraction seems to provide better outcomes as compared to the conservative treatment,” she said. “But I think that the long-term follow-up for potential events, such as valve durability or endocarditis, is still needed.”
The trial has strengths, compared with the recent RECOVERY trial, which also concluded in favor of early SAVR over watchful waiting in patients described as asymptomatic with severe aortic stenosis. Dr. Delgado and other observers, however, have pointed out limitations of that trial, including questions about whether the patients were truly asymptomatic – stress testing wasn›t routinely performed.
In AVATAR, all patients were negative at stress testing, which required them to reach their estimated maximum heart rate, Dr. Banovic noted. As he and his colleagues write, the trial expands on RECOVERY “by providing evidence of the benefit of early surgery in a setting representative of a dilemma in decision making, in truly asymptomatic patients with severe but not critical aortic stenosis and normal LV function.”
A role for TAVR?
Guidelines in general “can be very conservative and lag behind evidence a bit,” Patricia A. Pellikka, MD, Mayo Clinic, Rochester, Minn., who is not associated with AVATAR, said in an interview.
“I think when we see patients clinically, we can advise them that if they don’t have symptoms and they do have severe aortic stenosis,” she said, “they’re likely going to get symptoms within a reasonably short period of time, according to our retrospective databases, and that doing the intervention early may yield better long-term outcomes.”
The results of AVATAR, in which valve replacement consisted only of SAVR, “probably could be extrapolated” to transcatheter aortic valve replacement (TAVR), Dr. Pellikka observed. “Certainly, TAVR is the procedure that patients come asking for. It’s attractive to avoid a major surgery, and it seems very plausible that TAVR would have yielded similar results if that had been a therapy in this trial.”
In practice, patient age and functional status would figure heavily in deciding whether early valve replacement, and which procedure, is appropriate, Dr. Banovic said in an interview. Importantly, the trial’s patients were at low surgical risk and free of major chronic diseases or other important health concerns.
“Frailty and older age are known risk factors for suboptimal recovery” after SAVR, Dr. Banovic said when interviewed. Therefore, frail patients, who were not many in AVATAR, might be “more suitable for TAVR than SAVR, based on the TAVR-vs.-SAVR results in symptomatic AS patients,” he said.
“One might extrapolate experience from AVATAR trial to TAVR, which may lower the bar for TAVR indications,” but that would require more supporting evidence, Dr. Banovic said.
Confirmed asymptomatic
AVATAR, conducted at nine centers in seven countries in the European Union, randomly assigned 157 adults with severe AS by echocardiography and a LV ejection fraction (LVEF) greater than 50% to early SAVR or conservative management. They averaged 67 years in age, and 43% were women.
The trial excluded anyone with dyspnea, syncope, presyncope, angina, or LV dysfunction and anyone with a history of atrial fibrillation or significant cardiac, renal, or lung disease. The cohort’s average Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 1.7%.
The 78 patients in the early-surgery group “were expected” to have the procedure within 8 weeks of randomization, the published report states; the median time was 55 days. Six of them ultimately did not have the surgery. There was only one periprocedural death, for an operative mortality of 1.4%.
The 79 patients assigned to conservative care were later referred for surgery if they developed symptoms, their LVEF dropped below 50%, or they showed a 0.3-m/sec jump in peak aortic jet velocity at follow-up echocardiography. That occurred with 25 patients a median of 400 days after randomization.
The rate of the primary endpoint – death from any cause, acute myocardial infarction, stroke, or unplanned HF hospitalization – was 16.6% in the early-surgery group and 32.9% for those managed conservatively over a median of 32 months. The hazard ratio by intention-to-treat analysis was 0.46 (95% confidence interval, 0.23-0.90; P = .02). The HR for death from any cause or HF hospitalization was 0.40 (95% CI, 0.19-0.84; P = .013). Any differences in the individual endpoints of death, first HF hospitalizations, thromboembolic complications, or major bleeding were not significant.
If early aortic valve replacement is better for patients like those in AVATAR, some sort of screening for previously unknown severe aortic stenosis may seem attractive for selected populations. “Echocardiography would be the screening test for aortic stenosis, but it’s fairly expensive and therefore has never been advocated as a test to screen everyone,” Dr. Pellikka observed.
“But things are changing,” given innovations such as point-of-care ultrasonography and machine learning, she noted. “Artificial intelligence is progressing in its application to echocardiography, and it’s conceivable that in the future, there might be some abbreviated or screening type of test. But I don’t think we’re quite there yet.”
Dr. Banovic had no conflicts; disclosures for the other authors are in the report. Dr. Delgado disclosed speaker fees from Edwards Lifesciences, Abbott Vascular, Medtronic, Merck, Novartis, and GE Healthcare and unrestricted research grants to her institution from Abbott Vascular, Bayer, Biotronik, Bioventrix, Boston Scientific, Edwards Lifesciences, GE Healthcare, Ionis, and Medtronic. Dr. Pellikka disclosed receiving a research grant from Ultromics and having unspecified modest relationships with GE Healthcare, Lantheus, and OxThera.
A version of this article first appeared on Medscape.com.
Better to intervene early with a new valve in patients with severe aortic stenosis (AS) who are asymptomatic, even during exercise, than to wait for the disease to progress and symptoms to emerge before operating, suggests a small, randomized trial that challenges the guidelines.
Of the trial’s 157 patients, all with negative results on stress tests and normal left ventricular (LV) function despite severe AS, those assigned to early surgical aortic valve replacement (SAVR), compared with standard watchful waiting, showed a better-than-50% drop in risk for death or major adverse cardiac events (MACE) over 2-3 years. The benefit appeared driven by fewer hospitalizations for heart failure (HF) and deaths in the early-surgery group.
The findings “advocate for early surgery once aortic stenosis becomes significant and regardless of symptom status,” Marko Banovic, MD, PhD, said during his presentation at the American Heart Association scientific sessions.
Dr. Banovic, from the University of Belgrade Medical School in Serbia, is coprincipal investigator on the trial, called AVATAR (Aortic Valve Replacement vs. Conservative Treatment in Asymptomatic Severe Aortic Stenosis). He is also lead author on the study’s publication in Circulation, timed to coincide with his AHA presentation.
“The AVATAR findings provide additional evidence to help clinicians in guiding their decision when seeing a patient with significant aortic stenosis, normal left ventricular function, overall low surgical risk, and without significant comorbidities,” Dr. Banovic told this news organization.
European and North American Guidelines favor watchful waiting for asymptomatic patients with severe aortic stenosis, with surgery upon development of symptoms or LV dysfunction, observed Victoria Delgado, MD, PhD, Leiden (the Netherlands) University Medical Center, an invited discussant for the AVATAR presentation.
AVATAR does suggest that “early surgery in truly asymptomatic patients with severe aortic stenosis and preserved ejection fraction seems to provide better outcomes as compared to the conservative treatment,” she said. “But I think that the long-term follow-up for potential events, such as valve durability or endocarditis, is still needed.”
The trial has strengths, compared with the recent RECOVERY trial, which also concluded in favor of early SAVR over watchful waiting in patients described as asymptomatic with severe aortic stenosis. Dr. Delgado and other observers, however, have pointed out limitations of that trial, including questions about whether the patients were truly asymptomatic – stress testing wasn›t routinely performed.
In AVATAR, all patients were negative at stress testing, which required them to reach their estimated maximum heart rate, Dr. Banovic noted. As he and his colleagues write, the trial expands on RECOVERY “by providing evidence of the benefit of early surgery in a setting representative of a dilemma in decision making, in truly asymptomatic patients with severe but not critical aortic stenosis and normal LV function.”
A role for TAVR?
Guidelines in general “can be very conservative and lag behind evidence a bit,” Patricia A. Pellikka, MD, Mayo Clinic, Rochester, Minn., who is not associated with AVATAR, said in an interview.
“I think when we see patients clinically, we can advise them that if they don’t have symptoms and they do have severe aortic stenosis,” she said, “they’re likely going to get symptoms within a reasonably short period of time, according to our retrospective databases, and that doing the intervention early may yield better long-term outcomes.”
The results of AVATAR, in which valve replacement consisted only of SAVR, “probably could be extrapolated” to transcatheter aortic valve replacement (TAVR), Dr. Pellikka observed. “Certainly, TAVR is the procedure that patients come asking for. It’s attractive to avoid a major surgery, and it seems very plausible that TAVR would have yielded similar results if that had been a therapy in this trial.”
In practice, patient age and functional status would figure heavily in deciding whether early valve replacement, and which procedure, is appropriate, Dr. Banovic said in an interview. Importantly, the trial’s patients were at low surgical risk and free of major chronic diseases or other important health concerns.
“Frailty and older age are known risk factors for suboptimal recovery” after SAVR, Dr. Banovic said when interviewed. Therefore, frail patients, who were not many in AVATAR, might be “more suitable for TAVR than SAVR, based on the TAVR-vs.-SAVR results in symptomatic AS patients,” he said.
“One might extrapolate experience from AVATAR trial to TAVR, which may lower the bar for TAVR indications,” but that would require more supporting evidence, Dr. Banovic said.
Confirmed asymptomatic
AVATAR, conducted at nine centers in seven countries in the European Union, randomly assigned 157 adults with severe AS by echocardiography and a LV ejection fraction (LVEF) greater than 50% to early SAVR or conservative management. They averaged 67 years in age, and 43% were women.
The trial excluded anyone with dyspnea, syncope, presyncope, angina, or LV dysfunction and anyone with a history of atrial fibrillation or significant cardiac, renal, or lung disease. The cohort’s average Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 1.7%.
The 78 patients in the early-surgery group “were expected” to have the procedure within 8 weeks of randomization, the published report states; the median time was 55 days. Six of them ultimately did not have the surgery. There was only one periprocedural death, for an operative mortality of 1.4%.
The 79 patients assigned to conservative care were later referred for surgery if they developed symptoms, their LVEF dropped below 50%, or they showed a 0.3-m/sec jump in peak aortic jet velocity at follow-up echocardiography. That occurred with 25 patients a median of 400 days after randomization.
The rate of the primary endpoint – death from any cause, acute myocardial infarction, stroke, or unplanned HF hospitalization – was 16.6% in the early-surgery group and 32.9% for those managed conservatively over a median of 32 months. The hazard ratio by intention-to-treat analysis was 0.46 (95% confidence interval, 0.23-0.90; P = .02). The HR for death from any cause or HF hospitalization was 0.40 (95% CI, 0.19-0.84; P = .013). Any differences in the individual endpoints of death, first HF hospitalizations, thromboembolic complications, or major bleeding were not significant.
If early aortic valve replacement is better for patients like those in AVATAR, some sort of screening for previously unknown severe aortic stenosis may seem attractive for selected populations. “Echocardiography would be the screening test for aortic stenosis, but it’s fairly expensive and therefore has never been advocated as a test to screen everyone,” Dr. Pellikka observed.
“But things are changing,” given innovations such as point-of-care ultrasonography and machine learning, she noted. “Artificial intelligence is progressing in its application to echocardiography, and it’s conceivable that in the future, there might be some abbreviated or screening type of test. But I don’t think we’re quite there yet.”
Dr. Banovic had no conflicts; disclosures for the other authors are in the report. Dr. Delgado disclosed speaker fees from Edwards Lifesciences, Abbott Vascular, Medtronic, Merck, Novartis, and GE Healthcare and unrestricted research grants to her institution from Abbott Vascular, Bayer, Biotronik, Bioventrix, Boston Scientific, Edwards Lifesciences, GE Healthcare, Ionis, and Medtronic. Dr. Pellikka disclosed receiving a research grant from Ultromics and having unspecified modest relationships with GE Healthcare, Lantheus, and OxThera.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
CABG safe 3 days after stopping ticagrelor: RAPID CABG
Patients with acute coronary syndromes who have been taking the antiplatelet medication, ticagrelor, and who need coronary artery bypass surgery (CABG) may be able to safely have the procedure earlier than typically recommended, a new randomized trial suggests.
The RAPID CABG trial found that early surgery 2-3 days after ticagrelor cessation was noninferior in incurring severe or massive perioperative bleeding, compared with waiting 5-7 days. There was also no significant difference in TIMI CABG or Bleeding Academic Research Consortium (BARC) type 4 or 5 bleeding.
Patients in the delayed group had a numerically higher number of ischemic events requiring earlier surgery and had a longer hospital stay.
The study was presented at the American Heart Association scientific sessions.
“RAPID CABG is the first and only randomized controlled trial evaluating the safety of early surgery in patients taking ticagrelor,” said lead investigator Derek So, MD.
Dr. So, a cardiologist at the University of Ottawa Heart Institute and a professor at the University of Ottawa, explained that ticagrelor is a first-line antiplatelet agent for patients with acute coronary syndromes (ACS), but around 10% of patients presenting with ACS require CABG surgery.
A major concern among patients requiring bypass surgery is perioperative bleeding, and it has been shown that patients undergoing urgent bypass within 24 hours of the last dose of ticagrelor have increased mortality. Accordingly, guidelines suggest a waiting period for patients not requiring urgent bypass surgery, Dr. So noted.
Current North American guidelines suggest a waiting period of at least 5 days after stopping ticagrelor before bypass surgery. In contrast, the updated European and Japanese guidelines suggest a waiting period of 3 days.
Dr. So noted that all of the guidelines are based on cohort studies and pharmacodynamic studies, with no randomized evidence. Pharmacodynamic studies have shown that at 48 hours after the last dose of ticagrelor, the level of platelet inhibition drops to the same levels seen with long-term treatment with clopidogrel, a weaker antiplatelet drug, and after 120 hours (5 days) the effect has completely worn off.
Dr. So concluded that these new results from the RAPID CABG trial “may influence future iterations of North American guidelines with reduced waiting prior to bypass surgery” for patients receiving ticagrelor, and “they could also strengthen the level of evidence in European and Asian guidelines.”
Designated discussant of the RAPID CABG trial, Roxana Mehran, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York, said this was a “very important study,” being the only randomized trial to look at this issue to date.
Dr. Mehran noted that the results showed a similar number of major life-threatening bleeding events in the early and delayed groups and met the noninferiority endpoint, but she pointed out that the trial had a small sample size and a small number of events. “Therefore, larger trials are needed to verify these important and encouraging results.”
However, she concluded that these results should be considered in decisions about the timing of bypass surgery in patients receiving ticagrelor. “I will be changing my practice and sending patients earlier based on this data,” she said.
RAPID CABG
RAPID CABG was a physician-initiated multicenter randomized study evaluating the safety of early surgery at 2-3 days after ticagrelor cessation, compared with a delay of 5-7 days among patients presenting with ACS who required nonemergency CABG surgery.
The study enrolled 143 patients with ACS who were receiving ticagrelor and needed CABG surgery. Patients with stenting for culprit lesions, those requiring urgent surgery (less than 24 hours after presentation), and those requiring valve surgery were excluded.
Three patients declined surgery, and several others underwent surgery outside the assigned time window, so the results were based on the per protocol analysis of patients who actually had CABG in the assigned time window: 65 patients in the early CABG group and 58 in the delayed group.
The mean time from last ticagrelor dose to surgery was 3 days in the early group and 6 days in the delayed group.
Platelet reactivity on the VerifyNow test showed more residual antiplatelet activity in the early group, with P2Y12 reaction unit (PRU) levels of 200 (vs. 251 in the delayed group). This test measures the extent of platelet aggregation in the presence of P2Y12-inhibitor drugs, with lower PRU levels showing stronger antiplatelet effects.
The primary outcome of the study was severe or massive bleeding by Universal Definition of Perioperative Bleeding (UDPB) class 3 or 4. This is defined as a blood transfusions of more than 5 units of red blood cells or plasma within 24 hours of surgical closure, chest tube drainage of over 1,000 mL in the first 12 hours, and reoperation for bleeding.
Results showed that 4.6% of the early-surgery group had a primary outcome bleeding event, compared with 5.2% of the delayed surgery group, meeting the criteria for noninferiority (P = .0253 for noninferiority).
Individual components of the primary endpoint showed three class 3 (severe) bleeding events in both groups and no class 4 (massive) bleeding events in either group.
In terms of other bleeding outcomes, TIMI CABG bleeding occurred in two patients (3.1%) in the early-surgery group vs. no patients in the delayed group; BARC 4 bleeding occurred in two patients (3.1%) in the early group versus none in the delayed group, and there were no BARC 5 bleeding events in either group.
In the intention-to-treat analysis, ischemic events before surgery occurred in six patients (8.7%) in the delayed group (one myocardial infarction, four cases of recurrent ischemia, and one ventricular tachycardia) versus none in the early group.
Cumulative 6-month ischemic events occurred in nine patients (13.0%) in the delayed group vs. four patients (5.6%) in the early group, the difference being driven by nonfatal MI and recurrent ischemia.
There were no cardiovascular deaths in either group and one all-cause death in both groups.
Patients undergoing early surgery also had a shorter hospitalization, with a median length of stay of 9 days versus 12 days in the delayed group.
Larger trial needed
Commenting on the RAPID CABG study at an AHA press conference, Joanna Chikwe, MD, chair of the cardiac surgery department at Cedars-Sinai Medical Center, Los Angeles, said the results were in line with her practice.
“These results confirm what I already think is safe,” she said. “I’m comfortable going within 48 hours. But we individualize our approach, so it was helpful that the study investigators included platelet reactivity data. The interesting thing for me in this study was the number of adverse events in patients who waited longer.”
Dr. Chikwe said her top-line message was that “Surgery looked incredibly safe; there was amazingly low mortality. And if a patient has an indication for surgery, waiting does not serve you well.”
However, she also cautioned that the trial was somewhat underpowered, with a small number of events that drove the primary outcome, leading to some uncertainty on the results.
“The RAPID trial was helpful, and although it confirms my practice, I think physicians may want to see a larger-powered trial to be convincingly compelled that they should change their practice,” Dr. Chikwe noted.
She added that clinical trials in cardiac surgery are driven by inherent challenges. “Cardiac surgery is not very common, and it is hard to recruit patients into these trials, so you are generally tied to a small number of patients, and you therefore have to be extremely thoughtful about the study design. It is almost a given that you will need to use surrogate endpoints, and the choice of the surrogate endpoint can determine which way the trial goes.”
The RAPID CABG study was funded by the Canadian Institutes of Health Research. Dr. So reports research support, consultancy, or speaker’s fees from AggreDyne, Roche Diagnostics, Fujimori Kogyo, and AstraZeneca Canada. Dr. Mehran reports that her institution has received significant trial funding from AstraZeneca (the manufacturer of ticagrelor).
A version of this article first appeared on Medscape.com.
Patients with acute coronary syndromes who have been taking the antiplatelet medication, ticagrelor, and who need coronary artery bypass surgery (CABG) may be able to safely have the procedure earlier than typically recommended, a new randomized trial suggests.
The RAPID CABG trial found that early surgery 2-3 days after ticagrelor cessation was noninferior in incurring severe or massive perioperative bleeding, compared with waiting 5-7 days. There was also no significant difference in TIMI CABG or Bleeding Academic Research Consortium (BARC) type 4 or 5 bleeding.
Patients in the delayed group had a numerically higher number of ischemic events requiring earlier surgery and had a longer hospital stay.
The study was presented at the American Heart Association scientific sessions.
“RAPID CABG is the first and only randomized controlled trial evaluating the safety of early surgery in patients taking ticagrelor,” said lead investigator Derek So, MD.
Dr. So, a cardiologist at the University of Ottawa Heart Institute and a professor at the University of Ottawa, explained that ticagrelor is a first-line antiplatelet agent for patients with acute coronary syndromes (ACS), but around 10% of patients presenting with ACS require CABG surgery.
A major concern among patients requiring bypass surgery is perioperative bleeding, and it has been shown that patients undergoing urgent bypass within 24 hours of the last dose of ticagrelor have increased mortality. Accordingly, guidelines suggest a waiting period for patients not requiring urgent bypass surgery, Dr. So noted.
Current North American guidelines suggest a waiting period of at least 5 days after stopping ticagrelor before bypass surgery. In contrast, the updated European and Japanese guidelines suggest a waiting period of 3 days.
Dr. So noted that all of the guidelines are based on cohort studies and pharmacodynamic studies, with no randomized evidence. Pharmacodynamic studies have shown that at 48 hours after the last dose of ticagrelor, the level of platelet inhibition drops to the same levels seen with long-term treatment with clopidogrel, a weaker antiplatelet drug, and after 120 hours (5 days) the effect has completely worn off.
Dr. So concluded that these new results from the RAPID CABG trial “may influence future iterations of North American guidelines with reduced waiting prior to bypass surgery” for patients receiving ticagrelor, and “they could also strengthen the level of evidence in European and Asian guidelines.”
Designated discussant of the RAPID CABG trial, Roxana Mehran, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York, said this was a “very important study,” being the only randomized trial to look at this issue to date.
Dr. Mehran noted that the results showed a similar number of major life-threatening bleeding events in the early and delayed groups and met the noninferiority endpoint, but she pointed out that the trial had a small sample size and a small number of events. “Therefore, larger trials are needed to verify these important and encouraging results.”
However, she concluded that these results should be considered in decisions about the timing of bypass surgery in patients receiving ticagrelor. “I will be changing my practice and sending patients earlier based on this data,” she said.
RAPID CABG
RAPID CABG was a physician-initiated multicenter randomized study evaluating the safety of early surgery at 2-3 days after ticagrelor cessation, compared with a delay of 5-7 days among patients presenting with ACS who required nonemergency CABG surgery.
The study enrolled 143 patients with ACS who were receiving ticagrelor and needed CABG surgery. Patients with stenting for culprit lesions, those requiring urgent surgery (less than 24 hours after presentation), and those requiring valve surgery were excluded.
Three patients declined surgery, and several others underwent surgery outside the assigned time window, so the results were based on the per protocol analysis of patients who actually had CABG in the assigned time window: 65 patients in the early CABG group and 58 in the delayed group.
The mean time from last ticagrelor dose to surgery was 3 days in the early group and 6 days in the delayed group.
Platelet reactivity on the VerifyNow test showed more residual antiplatelet activity in the early group, with P2Y12 reaction unit (PRU) levels of 200 (vs. 251 in the delayed group). This test measures the extent of platelet aggregation in the presence of P2Y12-inhibitor drugs, with lower PRU levels showing stronger antiplatelet effects.
The primary outcome of the study was severe or massive bleeding by Universal Definition of Perioperative Bleeding (UDPB) class 3 or 4. This is defined as a blood transfusions of more than 5 units of red blood cells or plasma within 24 hours of surgical closure, chest tube drainage of over 1,000 mL in the first 12 hours, and reoperation for bleeding.
Results showed that 4.6% of the early-surgery group had a primary outcome bleeding event, compared with 5.2% of the delayed surgery group, meeting the criteria for noninferiority (P = .0253 for noninferiority).
Individual components of the primary endpoint showed three class 3 (severe) bleeding events in both groups and no class 4 (massive) bleeding events in either group.
In terms of other bleeding outcomes, TIMI CABG bleeding occurred in two patients (3.1%) in the early-surgery group vs. no patients in the delayed group; BARC 4 bleeding occurred in two patients (3.1%) in the early group versus none in the delayed group, and there were no BARC 5 bleeding events in either group.
In the intention-to-treat analysis, ischemic events before surgery occurred in six patients (8.7%) in the delayed group (one myocardial infarction, four cases of recurrent ischemia, and one ventricular tachycardia) versus none in the early group.
Cumulative 6-month ischemic events occurred in nine patients (13.0%) in the delayed group vs. four patients (5.6%) in the early group, the difference being driven by nonfatal MI and recurrent ischemia.
There were no cardiovascular deaths in either group and one all-cause death in both groups.
Patients undergoing early surgery also had a shorter hospitalization, with a median length of stay of 9 days versus 12 days in the delayed group.
Larger trial needed
Commenting on the RAPID CABG study at an AHA press conference, Joanna Chikwe, MD, chair of the cardiac surgery department at Cedars-Sinai Medical Center, Los Angeles, said the results were in line with her practice.
“These results confirm what I already think is safe,” she said. “I’m comfortable going within 48 hours. But we individualize our approach, so it was helpful that the study investigators included platelet reactivity data. The interesting thing for me in this study was the number of adverse events in patients who waited longer.”
Dr. Chikwe said her top-line message was that “Surgery looked incredibly safe; there was amazingly low mortality. And if a patient has an indication for surgery, waiting does not serve you well.”
However, she also cautioned that the trial was somewhat underpowered, with a small number of events that drove the primary outcome, leading to some uncertainty on the results.
“The RAPID trial was helpful, and although it confirms my practice, I think physicians may want to see a larger-powered trial to be convincingly compelled that they should change their practice,” Dr. Chikwe noted.
She added that clinical trials in cardiac surgery are driven by inherent challenges. “Cardiac surgery is not very common, and it is hard to recruit patients into these trials, so you are generally tied to a small number of patients, and you therefore have to be extremely thoughtful about the study design. It is almost a given that you will need to use surrogate endpoints, and the choice of the surrogate endpoint can determine which way the trial goes.”
The RAPID CABG study was funded by the Canadian Institutes of Health Research. Dr. So reports research support, consultancy, or speaker’s fees from AggreDyne, Roche Diagnostics, Fujimori Kogyo, and AstraZeneca Canada. Dr. Mehran reports that her institution has received significant trial funding from AstraZeneca (the manufacturer of ticagrelor).
A version of this article first appeared on Medscape.com.
Patients with acute coronary syndromes who have been taking the antiplatelet medication, ticagrelor, and who need coronary artery bypass surgery (CABG) may be able to safely have the procedure earlier than typically recommended, a new randomized trial suggests.
The RAPID CABG trial found that early surgery 2-3 days after ticagrelor cessation was noninferior in incurring severe or massive perioperative bleeding, compared with waiting 5-7 days. There was also no significant difference in TIMI CABG or Bleeding Academic Research Consortium (BARC) type 4 or 5 bleeding.
Patients in the delayed group had a numerically higher number of ischemic events requiring earlier surgery and had a longer hospital stay.
The study was presented at the American Heart Association scientific sessions.
“RAPID CABG is the first and only randomized controlled trial evaluating the safety of early surgery in patients taking ticagrelor,” said lead investigator Derek So, MD.
Dr. So, a cardiologist at the University of Ottawa Heart Institute and a professor at the University of Ottawa, explained that ticagrelor is a first-line antiplatelet agent for patients with acute coronary syndromes (ACS), but around 10% of patients presenting with ACS require CABG surgery.
A major concern among patients requiring bypass surgery is perioperative bleeding, and it has been shown that patients undergoing urgent bypass within 24 hours of the last dose of ticagrelor have increased mortality. Accordingly, guidelines suggest a waiting period for patients not requiring urgent bypass surgery, Dr. So noted.
Current North American guidelines suggest a waiting period of at least 5 days after stopping ticagrelor before bypass surgery. In contrast, the updated European and Japanese guidelines suggest a waiting period of 3 days.
Dr. So noted that all of the guidelines are based on cohort studies and pharmacodynamic studies, with no randomized evidence. Pharmacodynamic studies have shown that at 48 hours after the last dose of ticagrelor, the level of platelet inhibition drops to the same levels seen with long-term treatment with clopidogrel, a weaker antiplatelet drug, and after 120 hours (5 days) the effect has completely worn off.
Dr. So concluded that these new results from the RAPID CABG trial “may influence future iterations of North American guidelines with reduced waiting prior to bypass surgery” for patients receiving ticagrelor, and “they could also strengthen the level of evidence in European and Asian guidelines.”
Designated discussant of the RAPID CABG trial, Roxana Mehran, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York, said this was a “very important study,” being the only randomized trial to look at this issue to date.
Dr. Mehran noted that the results showed a similar number of major life-threatening bleeding events in the early and delayed groups and met the noninferiority endpoint, but she pointed out that the trial had a small sample size and a small number of events. “Therefore, larger trials are needed to verify these important and encouraging results.”
However, she concluded that these results should be considered in decisions about the timing of bypass surgery in patients receiving ticagrelor. “I will be changing my practice and sending patients earlier based on this data,” she said.
RAPID CABG
RAPID CABG was a physician-initiated multicenter randomized study evaluating the safety of early surgery at 2-3 days after ticagrelor cessation, compared with a delay of 5-7 days among patients presenting with ACS who required nonemergency CABG surgery.
The study enrolled 143 patients with ACS who were receiving ticagrelor and needed CABG surgery. Patients with stenting for culprit lesions, those requiring urgent surgery (less than 24 hours after presentation), and those requiring valve surgery were excluded.
Three patients declined surgery, and several others underwent surgery outside the assigned time window, so the results were based on the per protocol analysis of patients who actually had CABG in the assigned time window: 65 patients in the early CABG group and 58 in the delayed group.
The mean time from last ticagrelor dose to surgery was 3 days in the early group and 6 days in the delayed group.
Platelet reactivity on the VerifyNow test showed more residual antiplatelet activity in the early group, with P2Y12 reaction unit (PRU) levels of 200 (vs. 251 in the delayed group). This test measures the extent of platelet aggregation in the presence of P2Y12-inhibitor drugs, with lower PRU levels showing stronger antiplatelet effects.
The primary outcome of the study was severe or massive bleeding by Universal Definition of Perioperative Bleeding (UDPB) class 3 or 4. This is defined as a blood transfusions of more than 5 units of red blood cells or plasma within 24 hours of surgical closure, chest tube drainage of over 1,000 mL in the first 12 hours, and reoperation for bleeding.
Results showed that 4.6% of the early-surgery group had a primary outcome bleeding event, compared with 5.2% of the delayed surgery group, meeting the criteria for noninferiority (P = .0253 for noninferiority).
Individual components of the primary endpoint showed three class 3 (severe) bleeding events in both groups and no class 4 (massive) bleeding events in either group.
In terms of other bleeding outcomes, TIMI CABG bleeding occurred in two patients (3.1%) in the early-surgery group vs. no patients in the delayed group; BARC 4 bleeding occurred in two patients (3.1%) in the early group versus none in the delayed group, and there were no BARC 5 bleeding events in either group.
In the intention-to-treat analysis, ischemic events before surgery occurred in six patients (8.7%) in the delayed group (one myocardial infarction, four cases of recurrent ischemia, and one ventricular tachycardia) versus none in the early group.
Cumulative 6-month ischemic events occurred in nine patients (13.0%) in the delayed group vs. four patients (5.6%) in the early group, the difference being driven by nonfatal MI and recurrent ischemia.
There were no cardiovascular deaths in either group and one all-cause death in both groups.
Patients undergoing early surgery also had a shorter hospitalization, with a median length of stay of 9 days versus 12 days in the delayed group.
Larger trial needed
Commenting on the RAPID CABG study at an AHA press conference, Joanna Chikwe, MD, chair of the cardiac surgery department at Cedars-Sinai Medical Center, Los Angeles, said the results were in line with her practice.
“These results confirm what I already think is safe,” she said. “I’m comfortable going within 48 hours. But we individualize our approach, so it was helpful that the study investigators included platelet reactivity data. The interesting thing for me in this study was the number of adverse events in patients who waited longer.”
Dr. Chikwe said her top-line message was that “Surgery looked incredibly safe; there was amazingly low mortality. And if a patient has an indication for surgery, waiting does not serve you well.”
However, she also cautioned that the trial was somewhat underpowered, with a small number of events that drove the primary outcome, leading to some uncertainty on the results.
“The RAPID trial was helpful, and although it confirms my practice, I think physicians may want to see a larger-powered trial to be convincingly compelled that they should change their practice,” Dr. Chikwe noted.
She added that clinical trials in cardiac surgery are driven by inherent challenges. “Cardiac surgery is not very common, and it is hard to recruit patients into these trials, so you are generally tied to a small number of patients, and you therefore have to be extremely thoughtful about the study design. It is almost a given that you will need to use surrogate endpoints, and the choice of the surrogate endpoint can determine which way the trial goes.”
The RAPID CABG study was funded by the Canadian Institutes of Health Research. Dr. So reports research support, consultancy, or speaker’s fees from AggreDyne, Roche Diagnostics, Fujimori Kogyo, and AstraZeneca Canada. Dr. Mehran reports that her institution has received significant trial funding from AstraZeneca (the manufacturer of ticagrelor).
A version of this article first appeared on Medscape.com.
FROM AHA 2021
Concomitant tricuspid-mitral surgery beneficial but with a trade-off
Tricuspid valve repair at the time of mitral valve surgery reduces tricuspid regurgitation progression, but at the cost of more than a fivefold increase in permanent pacemakers, results of a new Cardiothoracic Surgical Trials Network study show.
The results were presented during the opening late-breaking science session at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
Tricuspid regurgitation (TR) is common among patients undergoing mitral valve surgery, and there’s broad agreement to intervene when a patient has severe TR. There’s uncertainty, however, about the management of moderate or less TR during mitral valve surgery, which is reflected in current guidelines on the basis of observational data, explained coprimary investigator James Gammie, MD, codirector and surgical director of the Johns Hopkins Heart and Vascular Institute, Baltimore. As a result, rates of concomitant tricuspid-mitral surgery range from 5% to 75% at various centers.
To help fill the gap, Dr. Gammie and colleagues screened 5,208 patients at 29 centers in the United States, Canada, and Germany undergoing surgery for degenerative mitral regurgitation, and randomly assigned 401 patients (75% male) to mitral valve surgery alone or with tricuspid annuloplasty.
Patients had either moderate TR (37%) or less than moderate TR with a dilated tricuspid annulus of at least 40 mm or at least 21 mm/m2 indexed for body surface area. Importantly, there was a uniform surgical approach using undersized (26-30 mm) rigid nonplanar annuloplasty rings to repair the tricuspid valve, he said.
The study’s primary outcome of treatment failure at 2 years was defined as the composite of death, reoperation for TR, or progression of TR from baseline by 2 grades or severe TR.
The primary endpoint occurred in 10.2% of patients who underwent mitral valve surgery alone and 3.9% who underwent concomitant tricuspid annuloplasty (relative risk, 0.37; 95% confidence interval, 0.16-0.86; P = .02).
The endpoint was driven exclusively by less TR progression in the annuloplasty group, with no TR reoperations in either group, observed Dr. Gammie. At 2 years, just 0.6% of the annuloplasty group had severe TR, compared with 5.6% of the surgery-alone group.
The rate of permanent pacemaker implantations, however, jumped from 2.5% with surgery alone to 14.1% with concomitant tricuspid annuloplasty (rate ratio, 5.75; 95% CI, 2.27-14.60). More than half of pacemakers were placed during the first 2 days after surgery.
There was no between-group difference in 2-year rates of all-cause mortality, major adverse cardiac and cerebrovascular events, readmission, quality of life, or functional status.
Less than moderate TR
In a post hoc analysis stratified by baseline TR severity, treatment failure was significantly less common with surgery plus tricuspid annuloplasty among patients with moderate TR (4.5% vs. 18.1%) but not among those with less than moderate TR and tricuspid annular dilation (3.4% vs. 6.1%).
Although the trial was not powered for the subgroup analysis, “these results call into question the idea that less than moderate TR with annular dilation should be an indication for tricuspid valve repair,” Dr. Gammie told this news organization.
“I did not repair the tricuspid valve in the setting of less than moderate TR before the trial, and my practice won’t change; but it will be based on much better evidence,” he added. “Of course, long-term data from our trial will be of great interest.”
Discussant Joseph Woo, MD, chair of surgery at Stanford (Calif.) University, congratulated the authors on a “landmark trial” that addresses a highly relevant problem without a clear-cut indication.
In the 2020 AHA/American College of Cardiology heart valve disease guideline, tricuspid valve surgery is a class I recommendation when there’s severe TR (stages C and D) and left-sided valve surgery but a class IIa recommendation in patients with progressive TR (stage B) with an annular dilation of at least 40 mm.
“The interesting findings in this study include that moderate TR was only 37% of the enrolled patients, and only 97% of the patients with degenerative MR received a mitral valve repair,” Dr. Woo said. “This level of mitral valve repair is perhaps lower than what we might expect at these centers and lower, certainly, than what the AHA/ACC guidelines recommend for surgery on asymptomatic severe mitral regurgitation.”
Panelist Roxanna Mehran, MD, of Icahn School of Medicine at Mount Sinai in New York said, “What I was struck by is that we, as clinicians, believe that if you fix the mitral valve, maybe the tricuspid regurgitation will improve. And it seems like that is not what’s happening, and I think that’s a big takeaway.”
Session comoderator Joanna Chikwe, MD, head of cardiac surgery at Cedars-Sinai Medical Center, Los Angeles, said, “I think we can all agree that severe tricuspid regurgitation is a disaster for patients, and I think the fact the trial is designed for an additional 5 years’ follow-up will hopefully give us some insights into the clinical impact of severe tricuspid regurgitation.”
For now, “a back of the envelope calculation suggests that, for every 20 patients with moderate tricuspid regurgitation who we repair the tricuspid valve in, we would prevent severe tricuspid valve regurgitation in 1 at the price of pacemakers in 2,” she said.
Dr. Chikwe said in an interview that “transcatheter tricuspid repair is increasingly helping these patients, but if you could avoid it with a technique that doesn’t cause incremental harm beyond, perhaps, the need for pacemakers, then this is helpful data that supports that approach.”
The pacemaker burden is not negligible, she said, but also not surprising to surgeons. “If you look at national practice of mitral-tricuspid surgery, it’s about 15% after that, and it’s simply because the conduction tissue is so close to the tricuspid annulus.”
Pacemaker implantation rates, like those for concomitant tricuspid-mitral surgery, are also highly variable, and in some single-center series only around 2%, Dr. Chikwe said. “So that suggests there are technical approaches that can minimize the pacemaker rate [such as] being extremely careful to avoid suture placement around the area of the conduction tissues.”
For some the trade-off between reduced TR progression and the risk of a permanent pacemaker is worth it. “But the fact that the trial didn’t show a difference in survival, a difference in symptoms or quality of life, might suggest that patients you anticipated were high risk for surgery or didn’t have a longer projected survival aren’t going to benefit from what is quite an aggressive surgical approach,” Dr. Chikwe said.
In an accompanying editorial, Dr. Chikwe and Mario Gaudino, MD, of Weill Cornell Medicine, New York, also point out that the “very dynamic nature of tricuspid regurgitation and wide variability in assessing tricuspid annular dilatation are additional compelling reasons to leave lesser regurgitation alone.”
Julia Grapsa, MD, PhD, Kings College and tricuspid service lead at Guys and St. Thomas NHS, London, also pointed to the need for longer-term follow-up but said increased use of imaging markers is also needed to help pinpoint TR progression in these patients. “For the moment, the results should remind imagers and clinicians to refer patients earlier.”
“As a valvular heart physician, I see more and more patients coming in with significant severe tricuspid regurgitation post–mitral valve surgery and because of the time that’s passed, there’s dysfunction of the right heart, the left heart, and it’s very hard to suggest an operation because they’re at high risk,” she said. “So we’re discussing with these patients whether to do an intervention or medical management.”
“Now, with this study, and the pending longer follow-up by the authors, I’m optimistic that the class II recommendation will be class I in order to help our patients treat tricuspid regurgitation earlier than late,” said Dr. Grapsa, who is also editor-in-chief of JACC: Case Reports.
The study was funded by the National Heart, Lung, and Blood Institute and the German Center for Cardiovascular Research. Dr. Gammie reports a consultant/stockholder relationship with Edwards Lifesciences. Dr. Grapsa reports no conflicts of interest. Dr. Chikwe reports that as coprincipal investigator/study director of NCT 05051033 (an NHLBI-sponsored Cardiothoracic Surgical Trials Network trial), she collaborates with several of the study authors.
A version of this article first appeared on Medscape.com.
Tricuspid valve repair at the time of mitral valve surgery reduces tricuspid regurgitation progression, but at the cost of more than a fivefold increase in permanent pacemakers, results of a new Cardiothoracic Surgical Trials Network study show.
The results were presented during the opening late-breaking science session at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
Tricuspid regurgitation (TR) is common among patients undergoing mitral valve surgery, and there’s broad agreement to intervene when a patient has severe TR. There’s uncertainty, however, about the management of moderate or less TR during mitral valve surgery, which is reflected in current guidelines on the basis of observational data, explained coprimary investigator James Gammie, MD, codirector and surgical director of the Johns Hopkins Heart and Vascular Institute, Baltimore. As a result, rates of concomitant tricuspid-mitral surgery range from 5% to 75% at various centers.
To help fill the gap, Dr. Gammie and colleagues screened 5,208 patients at 29 centers in the United States, Canada, and Germany undergoing surgery for degenerative mitral regurgitation, and randomly assigned 401 patients (75% male) to mitral valve surgery alone or with tricuspid annuloplasty.
Patients had either moderate TR (37%) or less than moderate TR with a dilated tricuspid annulus of at least 40 mm or at least 21 mm/m2 indexed for body surface area. Importantly, there was a uniform surgical approach using undersized (26-30 mm) rigid nonplanar annuloplasty rings to repair the tricuspid valve, he said.
The study’s primary outcome of treatment failure at 2 years was defined as the composite of death, reoperation for TR, or progression of TR from baseline by 2 grades or severe TR.
The primary endpoint occurred in 10.2% of patients who underwent mitral valve surgery alone and 3.9% who underwent concomitant tricuspid annuloplasty (relative risk, 0.37; 95% confidence interval, 0.16-0.86; P = .02).
The endpoint was driven exclusively by less TR progression in the annuloplasty group, with no TR reoperations in either group, observed Dr. Gammie. At 2 years, just 0.6% of the annuloplasty group had severe TR, compared with 5.6% of the surgery-alone group.
The rate of permanent pacemaker implantations, however, jumped from 2.5% with surgery alone to 14.1% with concomitant tricuspid annuloplasty (rate ratio, 5.75; 95% CI, 2.27-14.60). More than half of pacemakers were placed during the first 2 days after surgery.
There was no between-group difference in 2-year rates of all-cause mortality, major adverse cardiac and cerebrovascular events, readmission, quality of life, or functional status.
Less than moderate TR
In a post hoc analysis stratified by baseline TR severity, treatment failure was significantly less common with surgery plus tricuspid annuloplasty among patients with moderate TR (4.5% vs. 18.1%) but not among those with less than moderate TR and tricuspid annular dilation (3.4% vs. 6.1%).
Although the trial was not powered for the subgroup analysis, “these results call into question the idea that less than moderate TR with annular dilation should be an indication for tricuspid valve repair,” Dr. Gammie told this news organization.
“I did not repair the tricuspid valve in the setting of less than moderate TR before the trial, and my practice won’t change; but it will be based on much better evidence,” he added. “Of course, long-term data from our trial will be of great interest.”
Discussant Joseph Woo, MD, chair of surgery at Stanford (Calif.) University, congratulated the authors on a “landmark trial” that addresses a highly relevant problem without a clear-cut indication.
In the 2020 AHA/American College of Cardiology heart valve disease guideline, tricuspid valve surgery is a class I recommendation when there’s severe TR (stages C and D) and left-sided valve surgery but a class IIa recommendation in patients with progressive TR (stage B) with an annular dilation of at least 40 mm.
“The interesting findings in this study include that moderate TR was only 37% of the enrolled patients, and only 97% of the patients with degenerative MR received a mitral valve repair,” Dr. Woo said. “This level of mitral valve repair is perhaps lower than what we might expect at these centers and lower, certainly, than what the AHA/ACC guidelines recommend for surgery on asymptomatic severe mitral regurgitation.”
Panelist Roxanna Mehran, MD, of Icahn School of Medicine at Mount Sinai in New York said, “What I was struck by is that we, as clinicians, believe that if you fix the mitral valve, maybe the tricuspid regurgitation will improve. And it seems like that is not what’s happening, and I think that’s a big takeaway.”
Session comoderator Joanna Chikwe, MD, head of cardiac surgery at Cedars-Sinai Medical Center, Los Angeles, said, “I think we can all agree that severe tricuspid regurgitation is a disaster for patients, and I think the fact the trial is designed for an additional 5 years’ follow-up will hopefully give us some insights into the clinical impact of severe tricuspid regurgitation.”
For now, “a back of the envelope calculation suggests that, for every 20 patients with moderate tricuspid regurgitation who we repair the tricuspid valve in, we would prevent severe tricuspid valve regurgitation in 1 at the price of pacemakers in 2,” she said.
Dr. Chikwe said in an interview that “transcatheter tricuspid repair is increasingly helping these patients, but if you could avoid it with a technique that doesn’t cause incremental harm beyond, perhaps, the need for pacemakers, then this is helpful data that supports that approach.”
The pacemaker burden is not negligible, she said, but also not surprising to surgeons. “If you look at national practice of mitral-tricuspid surgery, it’s about 15% after that, and it’s simply because the conduction tissue is so close to the tricuspid annulus.”
Pacemaker implantation rates, like those for concomitant tricuspid-mitral surgery, are also highly variable, and in some single-center series only around 2%, Dr. Chikwe said. “So that suggests there are technical approaches that can minimize the pacemaker rate [such as] being extremely careful to avoid suture placement around the area of the conduction tissues.”
For some the trade-off between reduced TR progression and the risk of a permanent pacemaker is worth it. “But the fact that the trial didn’t show a difference in survival, a difference in symptoms or quality of life, might suggest that patients you anticipated were high risk for surgery or didn’t have a longer projected survival aren’t going to benefit from what is quite an aggressive surgical approach,” Dr. Chikwe said.
In an accompanying editorial, Dr. Chikwe and Mario Gaudino, MD, of Weill Cornell Medicine, New York, also point out that the “very dynamic nature of tricuspid regurgitation and wide variability in assessing tricuspid annular dilatation are additional compelling reasons to leave lesser regurgitation alone.”
Julia Grapsa, MD, PhD, Kings College and tricuspid service lead at Guys and St. Thomas NHS, London, also pointed to the need for longer-term follow-up but said increased use of imaging markers is also needed to help pinpoint TR progression in these patients. “For the moment, the results should remind imagers and clinicians to refer patients earlier.”
“As a valvular heart physician, I see more and more patients coming in with significant severe tricuspid regurgitation post–mitral valve surgery and because of the time that’s passed, there’s dysfunction of the right heart, the left heart, and it’s very hard to suggest an operation because they’re at high risk,” she said. “So we’re discussing with these patients whether to do an intervention or medical management.”
“Now, with this study, and the pending longer follow-up by the authors, I’m optimistic that the class II recommendation will be class I in order to help our patients treat tricuspid regurgitation earlier than late,” said Dr. Grapsa, who is also editor-in-chief of JACC: Case Reports.
The study was funded by the National Heart, Lung, and Blood Institute and the German Center for Cardiovascular Research. Dr. Gammie reports a consultant/stockholder relationship with Edwards Lifesciences. Dr. Grapsa reports no conflicts of interest. Dr. Chikwe reports that as coprincipal investigator/study director of NCT 05051033 (an NHLBI-sponsored Cardiothoracic Surgical Trials Network trial), she collaborates with several of the study authors.
A version of this article first appeared on Medscape.com.
Tricuspid valve repair at the time of mitral valve surgery reduces tricuspid regurgitation progression, but at the cost of more than a fivefold increase in permanent pacemakers, results of a new Cardiothoracic Surgical Trials Network study show.
The results were presented during the opening late-breaking science session at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
Tricuspid regurgitation (TR) is common among patients undergoing mitral valve surgery, and there’s broad agreement to intervene when a patient has severe TR. There’s uncertainty, however, about the management of moderate or less TR during mitral valve surgery, which is reflected in current guidelines on the basis of observational data, explained coprimary investigator James Gammie, MD, codirector and surgical director of the Johns Hopkins Heart and Vascular Institute, Baltimore. As a result, rates of concomitant tricuspid-mitral surgery range from 5% to 75% at various centers.
To help fill the gap, Dr. Gammie and colleagues screened 5,208 patients at 29 centers in the United States, Canada, and Germany undergoing surgery for degenerative mitral regurgitation, and randomly assigned 401 patients (75% male) to mitral valve surgery alone or with tricuspid annuloplasty.
Patients had either moderate TR (37%) or less than moderate TR with a dilated tricuspid annulus of at least 40 mm or at least 21 mm/m2 indexed for body surface area. Importantly, there was a uniform surgical approach using undersized (26-30 mm) rigid nonplanar annuloplasty rings to repair the tricuspid valve, he said.
The study’s primary outcome of treatment failure at 2 years was defined as the composite of death, reoperation for TR, or progression of TR from baseline by 2 grades or severe TR.
The primary endpoint occurred in 10.2% of patients who underwent mitral valve surgery alone and 3.9% who underwent concomitant tricuspid annuloplasty (relative risk, 0.37; 95% confidence interval, 0.16-0.86; P = .02).
The endpoint was driven exclusively by less TR progression in the annuloplasty group, with no TR reoperations in either group, observed Dr. Gammie. At 2 years, just 0.6% of the annuloplasty group had severe TR, compared with 5.6% of the surgery-alone group.
The rate of permanent pacemaker implantations, however, jumped from 2.5% with surgery alone to 14.1% with concomitant tricuspid annuloplasty (rate ratio, 5.75; 95% CI, 2.27-14.60). More than half of pacemakers were placed during the first 2 days after surgery.
There was no between-group difference in 2-year rates of all-cause mortality, major adverse cardiac and cerebrovascular events, readmission, quality of life, or functional status.
Less than moderate TR
In a post hoc analysis stratified by baseline TR severity, treatment failure was significantly less common with surgery plus tricuspid annuloplasty among patients with moderate TR (4.5% vs. 18.1%) but not among those with less than moderate TR and tricuspid annular dilation (3.4% vs. 6.1%).
Although the trial was not powered for the subgroup analysis, “these results call into question the idea that less than moderate TR with annular dilation should be an indication for tricuspid valve repair,” Dr. Gammie told this news organization.
“I did not repair the tricuspid valve in the setting of less than moderate TR before the trial, and my practice won’t change; but it will be based on much better evidence,” he added. “Of course, long-term data from our trial will be of great interest.”
Discussant Joseph Woo, MD, chair of surgery at Stanford (Calif.) University, congratulated the authors on a “landmark trial” that addresses a highly relevant problem without a clear-cut indication.
In the 2020 AHA/American College of Cardiology heart valve disease guideline, tricuspid valve surgery is a class I recommendation when there’s severe TR (stages C and D) and left-sided valve surgery but a class IIa recommendation in patients with progressive TR (stage B) with an annular dilation of at least 40 mm.
“The interesting findings in this study include that moderate TR was only 37% of the enrolled patients, and only 97% of the patients with degenerative MR received a mitral valve repair,” Dr. Woo said. “This level of mitral valve repair is perhaps lower than what we might expect at these centers and lower, certainly, than what the AHA/ACC guidelines recommend for surgery on asymptomatic severe mitral regurgitation.”
Panelist Roxanna Mehran, MD, of Icahn School of Medicine at Mount Sinai in New York said, “What I was struck by is that we, as clinicians, believe that if you fix the mitral valve, maybe the tricuspid regurgitation will improve. And it seems like that is not what’s happening, and I think that’s a big takeaway.”
Session comoderator Joanna Chikwe, MD, head of cardiac surgery at Cedars-Sinai Medical Center, Los Angeles, said, “I think we can all agree that severe tricuspid regurgitation is a disaster for patients, and I think the fact the trial is designed for an additional 5 years’ follow-up will hopefully give us some insights into the clinical impact of severe tricuspid regurgitation.”
For now, “a back of the envelope calculation suggests that, for every 20 patients with moderate tricuspid regurgitation who we repair the tricuspid valve in, we would prevent severe tricuspid valve regurgitation in 1 at the price of pacemakers in 2,” she said.
Dr. Chikwe said in an interview that “transcatheter tricuspid repair is increasingly helping these patients, but if you could avoid it with a technique that doesn’t cause incremental harm beyond, perhaps, the need for pacemakers, then this is helpful data that supports that approach.”
The pacemaker burden is not negligible, she said, but also not surprising to surgeons. “If you look at national practice of mitral-tricuspid surgery, it’s about 15% after that, and it’s simply because the conduction tissue is so close to the tricuspid annulus.”
Pacemaker implantation rates, like those for concomitant tricuspid-mitral surgery, are also highly variable, and in some single-center series only around 2%, Dr. Chikwe said. “So that suggests there are technical approaches that can minimize the pacemaker rate [such as] being extremely careful to avoid suture placement around the area of the conduction tissues.”
For some the trade-off between reduced TR progression and the risk of a permanent pacemaker is worth it. “But the fact that the trial didn’t show a difference in survival, a difference in symptoms or quality of life, might suggest that patients you anticipated were high risk for surgery or didn’t have a longer projected survival aren’t going to benefit from what is quite an aggressive surgical approach,” Dr. Chikwe said.
In an accompanying editorial, Dr. Chikwe and Mario Gaudino, MD, of Weill Cornell Medicine, New York, also point out that the “very dynamic nature of tricuspid regurgitation and wide variability in assessing tricuspid annular dilatation are additional compelling reasons to leave lesser regurgitation alone.”
Julia Grapsa, MD, PhD, Kings College and tricuspid service lead at Guys and St. Thomas NHS, London, also pointed to the need for longer-term follow-up but said increased use of imaging markers is also needed to help pinpoint TR progression in these patients. “For the moment, the results should remind imagers and clinicians to refer patients earlier.”
“As a valvular heart physician, I see more and more patients coming in with significant severe tricuspid regurgitation post–mitral valve surgery and because of the time that’s passed, there’s dysfunction of the right heart, the left heart, and it’s very hard to suggest an operation because they’re at high risk,” she said. “So we’re discussing with these patients whether to do an intervention or medical management.”
“Now, with this study, and the pending longer follow-up by the authors, I’m optimistic that the class II recommendation will be class I in order to help our patients treat tricuspid regurgitation earlier than late,” said Dr. Grapsa, who is also editor-in-chief of JACC: Case Reports.
The study was funded by the National Heart, Lung, and Blood Institute and the German Center for Cardiovascular Research. Dr. Gammie reports a consultant/stockholder relationship with Edwards Lifesciences. Dr. Grapsa reports no conflicts of interest. Dr. Chikwe reports that as coprincipal investigator/study director of NCT 05051033 (an NHLBI-sponsored Cardiothoracic Surgical Trials Network trial), she collaborates with several of the study authors.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
VEST: External sheath for CABG vein grafts shows promise
A novel, stent-shaped device that provides external buttressing to saphenous vein grafts placed during coronary artery bypass surgery was safe, but failed to improve 12-month patency of vein grafts, in a prospective study with 224 patients.
Despite the neutral result, “we are cautiously optimistic” about the prospects for the device to reduce the risk for failure of coronary vein grafts caused by intimal hyperplasia of the internal lining of the vein graft that leads to graft occlusion, said John D. Puskas, MD, lead investigator of the study, who reported the results at the American Heart Association scientific sessions.
In the trial, called VEST, each buttressed vein graft was compared with a similar, unbuttressed graft in the same patient. Perhaps the biggest issue faced by the study was the unexpectedly high 42% rate of vein-graft occlusion or diffuse disease seen in the studied grafts 12 months after placement. This rate included both the vein grafts placed within the external buttressing device and control vein grafts that underwent the same postharvest preparation but weren’t placed within an external sheath, which is formed from woven cobalt chromium wire.
Dr. Puskas attributed this high failure rate to the need to remove all adventitia tissue and fat from the harvested saphenous vein segments before grafting, a step required to allow the vein conduit to fit inside the wire sheath. The potential exists to further optimize this step, he said in an interview.
“I was very surprised by the low 12-month patency rates” in both treatment arms of the study, commented Joanna Chikwe, MD, chair of cardiac surgery at Cedars-Sinai Medical Center in Los Angeles.
External scaffold to counter blood pressure
The concept behind the external buttressing sheath is that the walls of saphenous vein grafts are not structured to accommodate arterial blood pressure, and over time this pressure produces accelerated atherosclerotic changes and premature occlusion and graft failure. The external support is supposed to impede vein wall dilatation, reduce irregularities of the inner lumen surface, and improve hemodynamics and shear stress.
The VEST trial ran at 14 U.S. and 3 Canadian centers and enrolled 224 patients scheduled for coronary artery bypass grafting with planned use of at least two saphenous vein grafts, along with an internal mammary artery graft for the left anterior descending coronary artery. The patients averaged 66 years of age, 21% were women, and 51% had diabetes.
All patients successfully underwent their surgery, with 203 returning after 12 months for their primary follow-up examination by intravascular ultrasound. However, because of the high rate of vein occlusion or development of diffuse intragraft disease, successful intravascular ultrasound (IVUS) examination of both vein grafts occurred in only 113 patients.
The IVUS examinations showed that the study’s primary endpoint, the intimal hyperplasia area in all 224 patients who received vein grafts, averaged 5.11 mm2 in the grafts placed within the wire sleeve and 5.79 mm2 for control grafts not placed in the wire sheath, a difference that fell short of significance (P = .072). However, in a sensitivity analysis that focused on only the 113 patients who had both vein grafts successfully assayed by IVUS, the average area of intimal hyperplasia was 4.58 mm2 in the grafts within a wire sheath and 5.12 mm2 in the control grafts, a significant difference (P = .043).
The combined rate of major adverse cardiovascular events after 12 months was 7%, including a 2% mortality rate, a 3% stroke rate, and 3% rate of Mis, outcomes that suggested “no safety signals,” said Dr. Puskas, chair of cardiovascular surgery at Mount Sinai St. Luke’s in New York.
Although a large body of evidence has shown the superiority of arterial grafts for long-term graft patency, vein grafts have many advantages that have maintained them as the most widely used conduits worldwide for coronary artery bypass surgery, Dr. Puskas said.
Saphenous vein segments are readily available from patients and easy to harvest; they nicely conform to the coronary arteries that require bypass, rarely leak, are easy to work with, and can successfully hold stitches. Surgeons performing coronary artery bypass are unlikely to abandon vein grafts anytime soon, which makes improving the performance of vein grafts a priority, Dr. Puskas said.
The study was sponsored by Vascular Graft Solutions, the company developing the venous graft external support. Dr. Puskas and Dr. Chikwe had no disclosures related to the study.
A novel, stent-shaped device that provides external buttressing to saphenous vein grafts placed during coronary artery bypass surgery was safe, but failed to improve 12-month patency of vein grafts, in a prospective study with 224 patients.
Despite the neutral result, “we are cautiously optimistic” about the prospects for the device to reduce the risk for failure of coronary vein grafts caused by intimal hyperplasia of the internal lining of the vein graft that leads to graft occlusion, said John D. Puskas, MD, lead investigator of the study, who reported the results at the American Heart Association scientific sessions.
In the trial, called VEST, each buttressed vein graft was compared with a similar, unbuttressed graft in the same patient. Perhaps the biggest issue faced by the study was the unexpectedly high 42% rate of vein-graft occlusion or diffuse disease seen in the studied grafts 12 months after placement. This rate included both the vein grafts placed within the external buttressing device and control vein grafts that underwent the same postharvest preparation but weren’t placed within an external sheath, which is formed from woven cobalt chromium wire.
Dr. Puskas attributed this high failure rate to the need to remove all adventitia tissue and fat from the harvested saphenous vein segments before grafting, a step required to allow the vein conduit to fit inside the wire sheath. The potential exists to further optimize this step, he said in an interview.
“I was very surprised by the low 12-month patency rates” in both treatment arms of the study, commented Joanna Chikwe, MD, chair of cardiac surgery at Cedars-Sinai Medical Center in Los Angeles.
External scaffold to counter blood pressure
The concept behind the external buttressing sheath is that the walls of saphenous vein grafts are not structured to accommodate arterial blood pressure, and over time this pressure produces accelerated atherosclerotic changes and premature occlusion and graft failure. The external support is supposed to impede vein wall dilatation, reduce irregularities of the inner lumen surface, and improve hemodynamics and shear stress.
The VEST trial ran at 14 U.S. and 3 Canadian centers and enrolled 224 patients scheduled for coronary artery bypass grafting with planned use of at least two saphenous vein grafts, along with an internal mammary artery graft for the left anterior descending coronary artery. The patients averaged 66 years of age, 21% were women, and 51% had diabetes.
All patients successfully underwent their surgery, with 203 returning after 12 months for their primary follow-up examination by intravascular ultrasound. However, because of the high rate of vein occlusion or development of diffuse intragraft disease, successful intravascular ultrasound (IVUS) examination of both vein grafts occurred in only 113 patients.
The IVUS examinations showed that the study’s primary endpoint, the intimal hyperplasia area in all 224 patients who received vein grafts, averaged 5.11 mm2 in the grafts placed within the wire sleeve and 5.79 mm2 for control grafts not placed in the wire sheath, a difference that fell short of significance (P = .072). However, in a sensitivity analysis that focused on only the 113 patients who had both vein grafts successfully assayed by IVUS, the average area of intimal hyperplasia was 4.58 mm2 in the grafts within a wire sheath and 5.12 mm2 in the control grafts, a significant difference (P = .043).
The combined rate of major adverse cardiovascular events after 12 months was 7%, including a 2% mortality rate, a 3% stroke rate, and 3% rate of Mis, outcomes that suggested “no safety signals,” said Dr. Puskas, chair of cardiovascular surgery at Mount Sinai St. Luke’s in New York.
Although a large body of evidence has shown the superiority of arterial grafts for long-term graft patency, vein grafts have many advantages that have maintained them as the most widely used conduits worldwide for coronary artery bypass surgery, Dr. Puskas said.
Saphenous vein segments are readily available from patients and easy to harvest; they nicely conform to the coronary arteries that require bypass, rarely leak, are easy to work with, and can successfully hold stitches. Surgeons performing coronary artery bypass are unlikely to abandon vein grafts anytime soon, which makes improving the performance of vein grafts a priority, Dr. Puskas said.
The study was sponsored by Vascular Graft Solutions, the company developing the venous graft external support. Dr. Puskas and Dr. Chikwe had no disclosures related to the study.
A novel, stent-shaped device that provides external buttressing to saphenous vein grafts placed during coronary artery bypass surgery was safe, but failed to improve 12-month patency of vein grafts, in a prospective study with 224 patients.
Despite the neutral result, “we are cautiously optimistic” about the prospects for the device to reduce the risk for failure of coronary vein grafts caused by intimal hyperplasia of the internal lining of the vein graft that leads to graft occlusion, said John D. Puskas, MD, lead investigator of the study, who reported the results at the American Heart Association scientific sessions.
In the trial, called VEST, each buttressed vein graft was compared with a similar, unbuttressed graft in the same patient. Perhaps the biggest issue faced by the study was the unexpectedly high 42% rate of vein-graft occlusion or diffuse disease seen in the studied grafts 12 months after placement. This rate included both the vein grafts placed within the external buttressing device and control vein grafts that underwent the same postharvest preparation but weren’t placed within an external sheath, which is formed from woven cobalt chromium wire.
Dr. Puskas attributed this high failure rate to the need to remove all adventitia tissue and fat from the harvested saphenous vein segments before grafting, a step required to allow the vein conduit to fit inside the wire sheath. The potential exists to further optimize this step, he said in an interview.
“I was very surprised by the low 12-month patency rates” in both treatment arms of the study, commented Joanna Chikwe, MD, chair of cardiac surgery at Cedars-Sinai Medical Center in Los Angeles.
External scaffold to counter blood pressure
The concept behind the external buttressing sheath is that the walls of saphenous vein grafts are not structured to accommodate arterial blood pressure, and over time this pressure produces accelerated atherosclerotic changes and premature occlusion and graft failure. The external support is supposed to impede vein wall dilatation, reduce irregularities of the inner lumen surface, and improve hemodynamics and shear stress.
The VEST trial ran at 14 U.S. and 3 Canadian centers and enrolled 224 patients scheduled for coronary artery bypass grafting with planned use of at least two saphenous vein grafts, along with an internal mammary artery graft for the left anterior descending coronary artery. The patients averaged 66 years of age, 21% were women, and 51% had diabetes.
All patients successfully underwent their surgery, with 203 returning after 12 months for their primary follow-up examination by intravascular ultrasound. However, because of the high rate of vein occlusion or development of diffuse intragraft disease, successful intravascular ultrasound (IVUS) examination of both vein grafts occurred in only 113 patients.
The IVUS examinations showed that the study’s primary endpoint, the intimal hyperplasia area in all 224 patients who received vein grafts, averaged 5.11 mm2 in the grafts placed within the wire sleeve and 5.79 mm2 for control grafts not placed in the wire sheath, a difference that fell short of significance (P = .072). However, in a sensitivity analysis that focused on only the 113 patients who had both vein grafts successfully assayed by IVUS, the average area of intimal hyperplasia was 4.58 mm2 in the grafts within a wire sheath and 5.12 mm2 in the control grafts, a significant difference (P = .043).
The combined rate of major adverse cardiovascular events after 12 months was 7%, including a 2% mortality rate, a 3% stroke rate, and 3% rate of Mis, outcomes that suggested “no safety signals,” said Dr. Puskas, chair of cardiovascular surgery at Mount Sinai St. Luke’s in New York.
Although a large body of evidence has shown the superiority of arterial grafts for long-term graft patency, vein grafts have many advantages that have maintained them as the most widely used conduits worldwide for coronary artery bypass surgery, Dr. Puskas said.
Saphenous vein segments are readily available from patients and easy to harvest; they nicely conform to the coronary arteries that require bypass, rarely leak, are easy to work with, and can successfully hold stitches. Surgeons performing coronary artery bypass are unlikely to abandon vein grafts anytime soon, which makes improving the performance of vein grafts a priority, Dr. Puskas said.
The study was sponsored by Vascular Graft Solutions, the company developing the venous graft external support. Dr. Puskas and Dr. Chikwe had no disclosures related to the study.
FROM AHA 2021
Pandemic stresses harder on physician moms than physician dads: Study
COVID-19 has been difficult for parents trying to balance careers, home life, and keeping their loved ones safe. A new study indicates that, not only are physicians not immune to these stressors, but the long-term effects could be devastating for health care overall.
In a study published Nov. 11, 2021, in JAMA Network Open , researchers found that stresses to work/life balance and family life caused by the pandemic have differed among men and women physicians.
Physicians and other health care workers have been at the front lines of the COVID-19 pandemic, and their work lives have been the focus of a lot of attention in the media and by researchers. Their family lives, not so much. But physicians have families, and the pandemic has upended almost everything about their lives, particularly where work life and home life intersect. School and day care closures, working from home, working extra hours, or working less – all of these changes have consequences on family life and the mental health of parents who are also physicians.
Findings from a Medscape survey published in early 2021 indicate that more female physicians than male physicians were either “conflicted” or “very conflicted” as parents because of work demands (42% vs. 23%) nearly 6 months into the pandemic.
In the current study, researchers from the University of Michigan, Harvard University, and the Medical University of South Carolina teamed up to investigate gender differences in how work/family factors affected the mental health of early-career physician parents in the United States during the first year of the COVID-19 pandemic. The results suggest that the pandemic has increased gender disparity and added disproportionately to the burden of female physicians.
Managing the household falls mostly on moms
Participants were physicians enrolled in the Intern Health Study, a longitudinal study that regularly surveys medical interns in the United States to assess stress and mood. When researchers compared survey results from before the onset of the pandemic (2018) with later results (2020), they found a striking gender difference in how the pandemic has changed family and work duties for physicians.
The authors of the study pointed out that previous research had found that female physicians take on a greater share of household and childcare duties than male physicians. The current study found that their share had increased with the pandemic. Physician moms are now 30 times more likely to be in charge of these tasks than physician dads.
In families in which both parents were physicians, none of the men said they took the primary role in managing the extra demands caused by the pandemic. In addition, women were twice as likely as men to work primarily from home and to work reduced hours.
The extra stress seems to be taking a toll on women physicians. In the 2020 survey, physician mothers had higher scores for anxiety and depression symptoms, compared with men. Notably, the 2018 survey did not show a significant difference in depression scores between men and women. Nor were there significant differences in depression and anxiety scores between women and men who were not parents or in reports of work/family conflict before and after the pandemic.
In general, the results indicate that the pandemic has only widened the gender gap between women and men physicians when it comes to managing family life and dealing with the stresses of maintaining a suitable work-life balance.
‘Long-term repercussions’ for gender equity in medicine
Although these are serious problems for women physicians and their families, the effects go beyond the home and beyond individuals. Even before the pandemic, women in medicine struggled for parity in career advancement and opportunities as well as in pay, and this new setback could make those challenges even greater.
“Even short-term adjustments can have serious long-term repercussions as they may lead to lower earnings and negatively impact opportunities for promotion, further exacerbating gender inequalities in compensation and advancement,” the study’s authors wrote.
The potential damage extends to the entire profession and the health care system itself. The profession is already struggling to retain young female physicians, and this situation is likely to make that problem worse and have long-term consequences. Citing data showing that female physicians spend more time with patients and that their patients may have better outcomes, the authors wrote that the consequences of losing more early-career female physicians “could be devastating to the U.S. health care system, particularly in the context of a global pandemic and an impending physician shortage.”
The sample size was small (276 U.S. physicians), and the study relied on self-reported data. The findings suggest that more research on this topic is needed, especially research that includes other demographic factors, such as sexual orientation and ethnicity. The authors recommend that institutional and public policymakers take into account the effects of the pandemic on physician mothers to ensure that recent gains in gender equity for women physicians do not fall victim to COVID-19.
A version of this article first appeared on Medscape.com.
COVID-19 has been difficult for parents trying to balance careers, home life, and keeping their loved ones safe. A new study indicates that, not only are physicians not immune to these stressors, but the long-term effects could be devastating for health care overall.
In a study published Nov. 11, 2021, in JAMA Network Open , researchers found that stresses to work/life balance and family life caused by the pandemic have differed among men and women physicians.
Physicians and other health care workers have been at the front lines of the COVID-19 pandemic, and their work lives have been the focus of a lot of attention in the media and by researchers. Their family lives, not so much. But physicians have families, and the pandemic has upended almost everything about their lives, particularly where work life and home life intersect. School and day care closures, working from home, working extra hours, or working less – all of these changes have consequences on family life and the mental health of parents who are also physicians.
Findings from a Medscape survey published in early 2021 indicate that more female physicians than male physicians were either “conflicted” or “very conflicted” as parents because of work demands (42% vs. 23%) nearly 6 months into the pandemic.
In the current study, researchers from the University of Michigan, Harvard University, and the Medical University of South Carolina teamed up to investigate gender differences in how work/family factors affected the mental health of early-career physician parents in the United States during the first year of the COVID-19 pandemic. The results suggest that the pandemic has increased gender disparity and added disproportionately to the burden of female physicians.
Managing the household falls mostly on moms
Participants were physicians enrolled in the Intern Health Study, a longitudinal study that regularly surveys medical interns in the United States to assess stress and mood. When researchers compared survey results from before the onset of the pandemic (2018) with later results (2020), they found a striking gender difference in how the pandemic has changed family and work duties for physicians.
The authors of the study pointed out that previous research had found that female physicians take on a greater share of household and childcare duties than male physicians. The current study found that their share had increased with the pandemic. Physician moms are now 30 times more likely to be in charge of these tasks than physician dads.
In families in which both parents were physicians, none of the men said they took the primary role in managing the extra demands caused by the pandemic. In addition, women were twice as likely as men to work primarily from home and to work reduced hours.
The extra stress seems to be taking a toll on women physicians. In the 2020 survey, physician mothers had higher scores for anxiety and depression symptoms, compared with men. Notably, the 2018 survey did not show a significant difference in depression scores between men and women. Nor were there significant differences in depression and anxiety scores between women and men who were not parents or in reports of work/family conflict before and after the pandemic.
In general, the results indicate that the pandemic has only widened the gender gap between women and men physicians when it comes to managing family life and dealing with the stresses of maintaining a suitable work-life balance.
‘Long-term repercussions’ for gender equity in medicine
Although these are serious problems for women physicians and their families, the effects go beyond the home and beyond individuals. Even before the pandemic, women in medicine struggled for parity in career advancement and opportunities as well as in pay, and this new setback could make those challenges even greater.
“Even short-term adjustments can have serious long-term repercussions as they may lead to lower earnings and negatively impact opportunities for promotion, further exacerbating gender inequalities in compensation and advancement,” the study’s authors wrote.
The potential damage extends to the entire profession and the health care system itself. The profession is already struggling to retain young female physicians, and this situation is likely to make that problem worse and have long-term consequences. Citing data showing that female physicians spend more time with patients and that their patients may have better outcomes, the authors wrote that the consequences of losing more early-career female physicians “could be devastating to the U.S. health care system, particularly in the context of a global pandemic and an impending physician shortage.”
The sample size was small (276 U.S. physicians), and the study relied on self-reported data. The findings suggest that more research on this topic is needed, especially research that includes other demographic factors, such as sexual orientation and ethnicity. The authors recommend that institutional and public policymakers take into account the effects of the pandemic on physician mothers to ensure that recent gains in gender equity for women physicians do not fall victim to COVID-19.
A version of this article first appeared on Medscape.com.
COVID-19 has been difficult for parents trying to balance careers, home life, and keeping their loved ones safe. A new study indicates that, not only are physicians not immune to these stressors, but the long-term effects could be devastating for health care overall.
In a study published Nov. 11, 2021, in JAMA Network Open , researchers found that stresses to work/life balance and family life caused by the pandemic have differed among men and women physicians.
Physicians and other health care workers have been at the front lines of the COVID-19 pandemic, and their work lives have been the focus of a lot of attention in the media and by researchers. Their family lives, not so much. But physicians have families, and the pandemic has upended almost everything about their lives, particularly where work life and home life intersect. School and day care closures, working from home, working extra hours, or working less – all of these changes have consequences on family life and the mental health of parents who are also physicians.
Findings from a Medscape survey published in early 2021 indicate that more female physicians than male physicians were either “conflicted” or “very conflicted” as parents because of work demands (42% vs. 23%) nearly 6 months into the pandemic.
In the current study, researchers from the University of Michigan, Harvard University, and the Medical University of South Carolina teamed up to investigate gender differences in how work/family factors affected the mental health of early-career physician parents in the United States during the first year of the COVID-19 pandemic. The results suggest that the pandemic has increased gender disparity and added disproportionately to the burden of female physicians.
Managing the household falls mostly on moms
Participants were physicians enrolled in the Intern Health Study, a longitudinal study that regularly surveys medical interns in the United States to assess stress and mood. When researchers compared survey results from before the onset of the pandemic (2018) with later results (2020), they found a striking gender difference in how the pandemic has changed family and work duties for physicians.
The authors of the study pointed out that previous research had found that female physicians take on a greater share of household and childcare duties than male physicians. The current study found that their share had increased with the pandemic. Physician moms are now 30 times more likely to be in charge of these tasks than physician dads.
In families in which both parents were physicians, none of the men said they took the primary role in managing the extra demands caused by the pandemic. In addition, women were twice as likely as men to work primarily from home and to work reduced hours.
The extra stress seems to be taking a toll on women physicians. In the 2020 survey, physician mothers had higher scores for anxiety and depression symptoms, compared with men. Notably, the 2018 survey did not show a significant difference in depression scores between men and women. Nor were there significant differences in depression and anxiety scores between women and men who were not parents or in reports of work/family conflict before and after the pandemic.
In general, the results indicate that the pandemic has only widened the gender gap between women and men physicians when it comes to managing family life and dealing with the stresses of maintaining a suitable work-life balance.
‘Long-term repercussions’ for gender equity in medicine
Although these are serious problems for women physicians and their families, the effects go beyond the home and beyond individuals. Even before the pandemic, women in medicine struggled for parity in career advancement and opportunities as well as in pay, and this new setback could make those challenges even greater.
“Even short-term adjustments can have serious long-term repercussions as they may lead to lower earnings and negatively impact opportunities for promotion, further exacerbating gender inequalities in compensation and advancement,” the study’s authors wrote.
The potential damage extends to the entire profession and the health care system itself. The profession is already struggling to retain young female physicians, and this situation is likely to make that problem worse and have long-term consequences. Citing data showing that female physicians spend more time with patients and that their patients may have better outcomes, the authors wrote that the consequences of losing more early-career female physicians “could be devastating to the U.S. health care system, particularly in the context of a global pandemic and an impending physician shortage.”
The sample size was small (276 U.S. physicians), and the study relied on self-reported data. The findings suggest that more research on this topic is needed, especially research that includes other demographic factors, such as sexual orientation and ethnicity. The authors recommend that institutional and public policymakers take into account the effects of the pandemic on physician mothers to ensure that recent gains in gender equity for women physicians do not fall victim to COVID-19.
A version of this article first appeared on Medscape.com.
Fully endovascular mitral valve replacement a limited success in feasibility study
It remains early days for transcatheter mitral-valve replacement (TMVR) as a minimally invasive way to treat severe, mitral regurgitation (MR), but it’s even earlier days for TMVR as an endovascular procedure. Most of the technique’s limited experience with a dedicated mitral prosthesis has involved transapical delivery.
But now a 15-patient study of transfemoral, transeptal TMVR – with a prosthesis designed for the mitral position and previously tested only transapically – has shown good 30-day results in that MR was essentially abolished with virtually no paravalvular leakage.
Nor were there adverse clinical events such as death, stroke, reintervention, or new need for a pacemaker in any of the high-surgical-risk patients with MR in this feasibility study of the transfemoral Intrepid TMVR System (Medtronic). Implantation failed, however, in one patient who then received a surgical valve via sternotomy.
The current cohort is part of a larger ongoing trial that will track whether patients implanted transfemorally with the Intrepid also show reverse remodeling and good clinical outcomes over at least a year. That study, called APOLLO, is one of several exploring dedicated TMVR valves from different companies, with names like SUMMIT, MISCEND, and TIARA-2.
Currently, TMVR is approved in the United States only using one device designed for the aortic position and only for treating failed surgical mitral bioprostheses in high-risk patients.
If the Intrepid transfemoral system has an Achilles’ heel, at least in the current iteration, it might be its 35 F catheter delivery system that requires surgical access to the femoral vein. Seven of the patients in the small series experienced major bleeding events, including six at the femoral access site, listed as major vascular complications.
Overall, the study’s patients “were extremely sick with a lot of comorbidity. A lot of them had atrial fibrillation, a lot of them were on anticoagulation to start with,” observed Firas Zahr, MD, Oregon Health & Science University, Portland, as part of his presentation of the study at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held virtually as well as onsite in Orlando, Florida.
All had moderate-to-severe, usually primary MR; two thirds of the cohort had been in NYHA class III or IV at baseline, and 40% had been hospitalized for heart failure within the past year. Eight had a history of cardiovascular surgery, and eight had diabetes. Their mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 4.7, Dr. Zahr reported.
“At 30 days, there was a significant improvement in their heart failure classification; the vast majority of the patients were [NYHA] class I and class II,” said Dr. Zahr, who is also lead author on the study’s Nov. 6 publication in JACC: Cardiovascular Interventions.
Observers of the study at TCT 2021 seemed enthusiastic about the study’s results but recognized that TMVR in its current form still has formidable limitations.
“This is clearly an exciting look into the future and very reassuring to a degree, aside from the complications, which are somewhat expected as we go with 30-plus French devices,” Rajiv Tayal, MD, MPH, said at a press conference on the Intrepid study held before Dr. Zahr’s formal presentation. Dr. Tayal is an interventional cardiologist with Valley Health System, Ridgewood, New Jersey, and New York Medical College, Valhalla.
“I think we’ve all learned that transapical [access] is just not a viable procedure for a lot of these patients, and so we’ve got to get to transfemoral,” Susheel K. Kodali, MD, interventional cardiologist at New York-Presbyterian/Columbia University Irving Medical Center, said at the same forum.
A 35 F device “is going to be too big,” he said. However, “it is the first step to iterate to a smaller device.” Dr. Kodali said his center contributed a patient to the study, and he is listed as a coauthor on the publication.
The delivery system’s large profile is only part of the vascular complication issue. Not only did the procedure require surgical cutdown for venous access, but “we were fairly aggressive in anticoagulating these patients with the fear of thrombus formation,” Dr. Zahr said in the discussion following his presentation.
“A postprocedure anticoagulation regimen is recommended within the protocol, but ultimate therapy was left to the discretion of the treating site physician,” the published report states, noting that all 14 patients with successful TMVR were discharged on warfarin. They included 12 who were also put on a single antiplatelet and one given dual antiplatelet therapy on top of the oral anticoagulant.
“One thing that we learned is that we probably should standardize our approach to perioperative anticoagulation,” Dr. Zahr observed. Also, a 29 F sheath for the system is in the works, “and we’re hoping that with smaller sheath size, and hopefully going even to percutaneous, might have an impact on lowering the vascular complications.”
Explanations for the “higher-than-expected vascular complication rate” remains somewhat unclear, agreed an editorial accompanying the study’s publication, “but may include a learning curve with the system, the large introducer sheath, the need for surgical cutdown, and postprocedural anticoagulation.”
For trans-septal TMVR to become a default approach, “venous access will need to be achieved percutaneously and vascular complications need to be infrequent,” contends the editorial, with lead author Mohamad Alkhouli, MD, Mayo Clinic, Rochester, Minn.
“These data provide a glimpse into the future of TMVR. The excellent short-term safety and effectiveness of this still very early-stage procedure represent a major step forward in the field,” they write.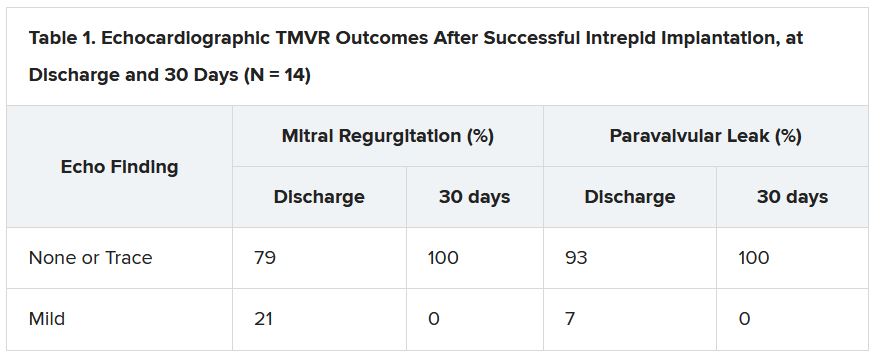
“The main question that the Intrepid early feasibility data raise is whether transfemoral, trans-septal TMVR will evolve to become the preferred strategy over transapical TMVR,” as occurred with transcatheter aortic-valve replacement (TAVR), the editorial states. “The answer is likely yes, but a few matters specific to trans-septal route will need be addressed first.”
Among those matters: The 35 F catheter leaves behind a considerable atrial septal defect (ASD). At operator discretion in this series, 11 patients received an ASD closure device.
None of the remaining four patients “developed significant heart failure or right ventricular dysfunction,” Dr. Zahr observed. “So, it seems like those patients who had their ASD left open tolerated it fairly well, at least until 30 days.”
But “we still need to learn what to do with those ASDs,” he said. “What is an acceptable residual shunt and what is an acceptable ASD size is to be determined.”
In general, the editorial notes, “the TMVR population has a high prevalence of cardiomyopathy, and a large residual iatrogenic ASD may lead to worsening volume overload and heart failure decompensation in some patients.”
Insertion of a closure device has its own issues, it continues. “Closure of the ASD might impede future access to the left atrium, which could impact life-long management of this high-risk population. A large septal occluder may hinder potentially needed procedures such as paravalvular leak closure, left atrial appendage closure, or pulmonary vein isolation.”
Patients like those in the current series, Dr. Kodali observed, will face “a lifetime of management challenges, and you want to make sure you don’t take away other options.”
The study was funded by Medtronic. Dr. Zahr reported institutional grant support from Edwards Lifesciences and Medtronic. Dr. Kodali disclosed consultant fees from Admedus and Dura Biotech; equity in Dura Biotech, Microinterventional Devices, Thubrika Aortic Valve, Supira, Admedus, TriFlo, and Anona; and institutional grant support from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve. The editorial writers have disclosed no relevant financial relationships. Dr. Tayal disclosed consultant fees or honoraria from or serving on a speakers bureau for Abiomed, Edwards Lifesciences, Abbott Vascular, and Shockwave Medical.
A version of this article first appeared on Medscape.com.
It remains early days for transcatheter mitral-valve replacement (TMVR) as a minimally invasive way to treat severe, mitral regurgitation (MR), but it’s even earlier days for TMVR as an endovascular procedure. Most of the technique’s limited experience with a dedicated mitral prosthesis has involved transapical delivery.
But now a 15-patient study of transfemoral, transeptal TMVR – with a prosthesis designed for the mitral position and previously tested only transapically – has shown good 30-day results in that MR was essentially abolished with virtually no paravalvular leakage.
Nor were there adverse clinical events such as death, stroke, reintervention, or new need for a pacemaker in any of the high-surgical-risk patients with MR in this feasibility study of the transfemoral Intrepid TMVR System (Medtronic). Implantation failed, however, in one patient who then received a surgical valve via sternotomy.
The current cohort is part of a larger ongoing trial that will track whether patients implanted transfemorally with the Intrepid also show reverse remodeling and good clinical outcomes over at least a year. That study, called APOLLO, is one of several exploring dedicated TMVR valves from different companies, with names like SUMMIT, MISCEND, and TIARA-2.
Currently, TMVR is approved in the United States only using one device designed for the aortic position and only for treating failed surgical mitral bioprostheses in high-risk patients.
If the Intrepid transfemoral system has an Achilles’ heel, at least in the current iteration, it might be its 35 F catheter delivery system that requires surgical access to the femoral vein. Seven of the patients in the small series experienced major bleeding events, including six at the femoral access site, listed as major vascular complications.
Overall, the study’s patients “were extremely sick with a lot of comorbidity. A lot of them had atrial fibrillation, a lot of them were on anticoagulation to start with,” observed Firas Zahr, MD, Oregon Health & Science University, Portland, as part of his presentation of the study at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held virtually as well as onsite in Orlando, Florida.
All had moderate-to-severe, usually primary MR; two thirds of the cohort had been in NYHA class III or IV at baseline, and 40% had been hospitalized for heart failure within the past year. Eight had a history of cardiovascular surgery, and eight had diabetes. Their mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 4.7, Dr. Zahr reported.
“At 30 days, there was a significant improvement in their heart failure classification; the vast majority of the patients were [NYHA] class I and class II,” said Dr. Zahr, who is also lead author on the study’s Nov. 6 publication in JACC: Cardiovascular Interventions.
Observers of the study at TCT 2021 seemed enthusiastic about the study’s results but recognized that TMVR in its current form still has formidable limitations.
“This is clearly an exciting look into the future and very reassuring to a degree, aside from the complications, which are somewhat expected as we go with 30-plus French devices,” Rajiv Tayal, MD, MPH, said at a press conference on the Intrepid study held before Dr. Zahr’s formal presentation. Dr. Tayal is an interventional cardiologist with Valley Health System, Ridgewood, New Jersey, and New York Medical College, Valhalla.
“I think we’ve all learned that transapical [access] is just not a viable procedure for a lot of these patients, and so we’ve got to get to transfemoral,” Susheel K. Kodali, MD, interventional cardiologist at New York-Presbyterian/Columbia University Irving Medical Center, said at the same forum.
A 35 F device “is going to be too big,” he said. However, “it is the first step to iterate to a smaller device.” Dr. Kodali said his center contributed a patient to the study, and he is listed as a coauthor on the publication.
The delivery system’s large profile is only part of the vascular complication issue. Not only did the procedure require surgical cutdown for venous access, but “we were fairly aggressive in anticoagulating these patients with the fear of thrombus formation,” Dr. Zahr said in the discussion following his presentation.
“A postprocedure anticoagulation regimen is recommended within the protocol, but ultimate therapy was left to the discretion of the treating site physician,” the published report states, noting that all 14 patients with successful TMVR were discharged on warfarin. They included 12 who were also put on a single antiplatelet and one given dual antiplatelet therapy on top of the oral anticoagulant.
“One thing that we learned is that we probably should standardize our approach to perioperative anticoagulation,” Dr. Zahr observed. Also, a 29 F sheath for the system is in the works, “and we’re hoping that with smaller sheath size, and hopefully going even to percutaneous, might have an impact on lowering the vascular complications.”
Explanations for the “higher-than-expected vascular complication rate” remains somewhat unclear, agreed an editorial accompanying the study’s publication, “but may include a learning curve with the system, the large introducer sheath, the need for surgical cutdown, and postprocedural anticoagulation.”
For trans-septal TMVR to become a default approach, “venous access will need to be achieved percutaneously and vascular complications need to be infrequent,” contends the editorial, with lead author Mohamad Alkhouli, MD, Mayo Clinic, Rochester, Minn.
“These data provide a glimpse into the future of TMVR. The excellent short-term safety and effectiveness of this still very early-stage procedure represent a major step forward in the field,” they write.
“The main question that the Intrepid early feasibility data raise is whether transfemoral, trans-septal TMVR will evolve to become the preferred strategy over transapical TMVR,” as occurred with transcatheter aortic-valve replacement (TAVR), the editorial states. “The answer is likely yes, but a few matters specific to trans-septal route will need be addressed first.”
Among those matters: The 35 F catheter leaves behind a considerable atrial septal defect (ASD). At operator discretion in this series, 11 patients received an ASD closure device.
None of the remaining four patients “developed significant heart failure or right ventricular dysfunction,” Dr. Zahr observed. “So, it seems like those patients who had their ASD left open tolerated it fairly well, at least until 30 days.”
But “we still need to learn what to do with those ASDs,” he said. “What is an acceptable residual shunt and what is an acceptable ASD size is to be determined.”
In general, the editorial notes, “the TMVR population has a high prevalence of cardiomyopathy, and a large residual iatrogenic ASD may lead to worsening volume overload and heart failure decompensation in some patients.”
Insertion of a closure device has its own issues, it continues. “Closure of the ASD might impede future access to the left atrium, which could impact life-long management of this high-risk population. A large septal occluder may hinder potentially needed procedures such as paravalvular leak closure, left atrial appendage closure, or pulmonary vein isolation.”
Patients like those in the current series, Dr. Kodali observed, will face “a lifetime of management challenges, and you want to make sure you don’t take away other options.”
The study was funded by Medtronic. Dr. Zahr reported institutional grant support from Edwards Lifesciences and Medtronic. Dr. Kodali disclosed consultant fees from Admedus and Dura Biotech; equity in Dura Biotech, Microinterventional Devices, Thubrika Aortic Valve, Supira, Admedus, TriFlo, and Anona; and institutional grant support from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve. The editorial writers have disclosed no relevant financial relationships. Dr. Tayal disclosed consultant fees or honoraria from or serving on a speakers bureau for Abiomed, Edwards Lifesciences, Abbott Vascular, and Shockwave Medical.
A version of this article first appeared on Medscape.com.
It remains early days for transcatheter mitral-valve replacement (TMVR) as a minimally invasive way to treat severe, mitral regurgitation (MR), but it’s even earlier days for TMVR as an endovascular procedure. Most of the technique’s limited experience with a dedicated mitral prosthesis has involved transapical delivery.
But now a 15-patient study of transfemoral, transeptal TMVR – with a prosthesis designed for the mitral position and previously tested only transapically – has shown good 30-day results in that MR was essentially abolished with virtually no paravalvular leakage.
Nor were there adverse clinical events such as death, stroke, reintervention, or new need for a pacemaker in any of the high-surgical-risk patients with MR in this feasibility study of the transfemoral Intrepid TMVR System (Medtronic). Implantation failed, however, in one patient who then received a surgical valve via sternotomy.
The current cohort is part of a larger ongoing trial that will track whether patients implanted transfemorally with the Intrepid also show reverse remodeling and good clinical outcomes over at least a year. That study, called APOLLO, is one of several exploring dedicated TMVR valves from different companies, with names like SUMMIT, MISCEND, and TIARA-2.
Currently, TMVR is approved in the United States only using one device designed for the aortic position and only for treating failed surgical mitral bioprostheses in high-risk patients.
If the Intrepid transfemoral system has an Achilles’ heel, at least in the current iteration, it might be its 35 F catheter delivery system that requires surgical access to the femoral vein. Seven of the patients in the small series experienced major bleeding events, including six at the femoral access site, listed as major vascular complications.
Overall, the study’s patients “were extremely sick with a lot of comorbidity. A lot of them had atrial fibrillation, a lot of them were on anticoagulation to start with,” observed Firas Zahr, MD, Oregon Health & Science University, Portland, as part of his presentation of the study at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held virtually as well as onsite in Orlando, Florida.
All had moderate-to-severe, usually primary MR; two thirds of the cohort had been in NYHA class III or IV at baseline, and 40% had been hospitalized for heart failure within the past year. Eight had a history of cardiovascular surgery, and eight had diabetes. Their mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 4.7, Dr. Zahr reported.
“At 30 days, there was a significant improvement in their heart failure classification; the vast majority of the patients were [NYHA] class I and class II,” said Dr. Zahr, who is also lead author on the study’s Nov. 6 publication in JACC: Cardiovascular Interventions.
Observers of the study at TCT 2021 seemed enthusiastic about the study’s results but recognized that TMVR in its current form still has formidable limitations.
“This is clearly an exciting look into the future and very reassuring to a degree, aside from the complications, which are somewhat expected as we go with 30-plus French devices,” Rajiv Tayal, MD, MPH, said at a press conference on the Intrepid study held before Dr. Zahr’s formal presentation. Dr. Tayal is an interventional cardiologist with Valley Health System, Ridgewood, New Jersey, and New York Medical College, Valhalla.
“I think we’ve all learned that transapical [access] is just not a viable procedure for a lot of these patients, and so we’ve got to get to transfemoral,” Susheel K. Kodali, MD, interventional cardiologist at New York-Presbyterian/Columbia University Irving Medical Center, said at the same forum.
A 35 F device “is going to be too big,” he said. However, “it is the first step to iterate to a smaller device.” Dr. Kodali said his center contributed a patient to the study, and he is listed as a coauthor on the publication.
The delivery system’s large profile is only part of the vascular complication issue. Not only did the procedure require surgical cutdown for venous access, but “we were fairly aggressive in anticoagulating these patients with the fear of thrombus formation,” Dr. Zahr said in the discussion following his presentation.
“A postprocedure anticoagulation regimen is recommended within the protocol, but ultimate therapy was left to the discretion of the treating site physician,” the published report states, noting that all 14 patients with successful TMVR were discharged on warfarin. They included 12 who were also put on a single antiplatelet and one given dual antiplatelet therapy on top of the oral anticoagulant.
“One thing that we learned is that we probably should standardize our approach to perioperative anticoagulation,” Dr. Zahr observed. Also, a 29 F sheath for the system is in the works, “and we’re hoping that with smaller sheath size, and hopefully going even to percutaneous, might have an impact on lowering the vascular complications.”
Explanations for the “higher-than-expected vascular complication rate” remains somewhat unclear, agreed an editorial accompanying the study’s publication, “but may include a learning curve with the system, the large introducer sheath, the need for surgical cutdown, and postprocedural anticoagulation.”
For trans-septal TMVR to become a default approach, “venous access will need to be achieved percutaneously and vascular complications need to be infrequent,” contends the editorial, with lead author Mohamad Alkhouli, MD, Mayo Clinic, Rochester, Minn.
“These data provide a glimpse into the future of TMVR. The excellent short-term safety and effectiveness of this still very early-stage procedure represent a major step forward in the field,” they write.
“The main question that the Intrepid early feasibility data raise is whether transfemoral, trans-septal TMVR will evolve to become the preferred strategy over transapical TMVR,” as occurred with transcatheter aortic-valve replacement (TAVR), the editorial states. “The answer is likely yes, but a few matters specific to trans-septal route will need be addressed first.”
Among those matters: The 35 F catheter leaves behind a considerable atrial septal defect (ASD). At operator discretion in this series, 11 patients received an ASD closure device.
None of the remaining four patients “developed significant heart failure or right ventricular dysfunction,” Dr. Zahr observed. “So, it seems like those patients who had their ASD left open tolerated it fairly well, at least until 30 days.”
But “we still need to learn what to do with those ASDs,” he said. “What is an acceptable residual shunt and what is an acceptable ASD size is to be determined.”
In general, the editorial notes, “the TMVR population has a high prevalence of cardiomyopathy, and a large residual iatrogenic ASD may lead to worsening volume overload and heart failure decompensation in some patients.”
Insertion of a closure device has its own issues, it continues. “Closure of the ASD might impede future access to the left atrium, which could impact life-long management of this high-risk population. A large septal occluder may hinder potentially needed procedures such as paravalvular leak closure, left atrial appendage closure, or pulmonary vein isolation.”
Patients like those in the current series, Dr. Kodali observed, will face “a lifetime of management challenges, and you want to make sure you don’t take away other options.”
The study was funded by Medtronic. Dr. Zahr reported institutional grant support from Edwards Lifesciences and Medtronic. Dr. Kodali disclosed consultant fees from Admedus and Dura Biotech; equity in Dura Biotech, Microinterventional Devices, Thubrika Aortic Valve, Supira, Admedus, TriFlo, and Anona; and institutional grant support from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve. The editorial writers have disclosed no relevant financial relationships. Dr. Tayal disclosed consultant fees or honoraria from or serving on a speakers bureau for Abiomed, Edwards Lifesciences, Abbott Vascular, and Shockwave Medical.
A version of this article first appeared on Medscape.com.
FDA panel slams Endologix response to stent-graft safety issues
The Food and Drug Administration has long kept a watchful eye over successive iterations of endovascular stent graphs in the Endologix AFX line, designed for repair of abdominal aortic aneurysms (AAA). For years, the devices, first approved in 2011, have drawn safety alerts and recalls , stemming from what the agency says was a “higher than expected” risk for potentially injurious or fatal type III endoleaks.
As part of the latest review process, Endologix recently showed regulators data from a rare randomized trial of the AAA endovascular aneurysm repair (EVAR) technique. The company said the recent postmarket study LEOPARD suggested the type III endoleaks – blood seeping around or through the device into the aneurysm – are no more common with the current AFX2 system than with other available AAA stent-grafts.
Technical upgrades to its AFX line of EVAR devices in recent years have largely resolved the safety issues identified in previous models, the company argued.
But the company’s case was unconvincing for a majority of the FDA Circulatory System Devices Advisory Panel that assembled virtually on Nov. 2. A number of panelists questioned the earnestness with which Endologix worked to rectify the safety alert and recall issues. Many also decried the real-world relevance of the randomized trial presented as evidence, with its follow-up time of only a few years.
The panel that included more than a dozen clinicians – mostly surgeons or interventional cardiologists or radiologists – were not instructed to formally vote on the issues. But it ultimately advised the FDA that more exacting studies with longer follow-ups appear needed to show that the device’s benefits in routine use outweigh its risks, especially for type III endoleaks.
“There isn’t a tremendous amount of confidence” that Endologix had enacted sufficient risk-mitigation measures in the wake of the safety alerts and recalls, chair Richard A. Lange, MD, MBA, Foster School of Medicine and Texas Tech University Health Sciences Center, El Paso, said when summarizing the panel’s take on the day’s proceedings.
Although the stent-graft’s safety seemed improved with recent design changes, the panel wasn’t convinced the upgrades could take the credit, or even that they were aimed specifically at preventing endoleaks, Dr. Lange said. “Nobody feels assurance that the problem has been solved.”
“I believe that the type-three endoleaks pose a challenge to patients, and I have not seen enough data to assure me with a degree of certainty that that problem no longer persists,” said panelist Joaquin E. Cigarroa, MD, a cardiologist at Oregon Health & Science University, Portland. His take on the LEOPARD trial, moreover, is that it “does not refute that there is an issue, given the duration of follow-up.”
On the other hand, a majority of the panel agreed that, currently, the AFX2’s benefits would likely outweigh risks for patients in narrowly defined high-risk anatomic or clinical scenarios and those with no other endovascular or surgical option.
“I do believe that there are patient subsets where the Endologix graft can play an important and vital role,” surgeon Keith B. Allen, MD, St. Luke’s Mid America Heart & Vascular Institute, Kansas City, Missouri, offered from the panel.
“In patients that don’t have aneurysmal disease but have distal bifurcation proximal iliac disease, it can be a very nice graft to use and solves a problem,” he said. “To remove that graft completely from the market, I believe, would deny a subset of patients.”
But for aortic aneurysms in routine practice, Dr. Allen said, “I think there are some red flags with it.”
Joining the day’s proceedings as a public commenter, surgeon Mark Conrad, MD, St. Elizabeth’s Hospital, Boston, agreed that “there’s not one commercial device out there that is able to handle every anatomy.”
Having options for patients is important, he said, because “the biggest problems we run into are when somebody only uses one graft, and they try to make that fit everything.”
Another public commenter offered a similar take. “I think we haven’t done a great job in the vascular surgery community really honing in on the detailed nuances that separate one device from another,” said Naiem Nassiri, MD, Yale New Haven Hospital Heart & Vascular Center, Connecticut.
The Endologix device, he said, “serves a very specific role under certain anatomic configurations and limitations, and really, truly fills a gap” left by other available grafts. It suits a very specific niche, “and I think it needs to be explored further for that.”
Endologix representatives who advise clinicians could play a better role in familiarizing operators with the EVAR system’s strengths and limitations, proposed several panelists, including Minhaj S. Khaja, MD, MBA, interventional radiologist at UVA Health and the University of Virginia, Charlottesville.
“There definitely needs to be more education of the clinical reps as well as the physicians implanting these devices,” he said, regarding the type III leaks, patient selection issues, appropriate imaging follow-up, “and the potential for increased reintervention.”
All public commenters, Dr. Lange observed, had been invited to disclose potential conflicts of interest, but it was not mandatory and none did so during the public forum. Disclosures of potential conflicts for the panelists are available on the FDA site.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has long kept a watchful eye over successive iterations of endovascular stent graphs in the Endologix AFX line, designed for repair of abdominal aortic aneurysms (AAA). For years, the devices, first approved in 2011, have drawn safety alerts and recalls , stemming from what the agency says was a “higher than expected” risk for potentially injurious or fatal type III endoleaks.
As part of the latest review process, Endologix recently showed regulators data from a rare randomized trial of the AAA endovascular aneurysm repair (EVAR) technique. The company said the recent postmarket study LEOPARD suggested the type III endoleaks – blood seeping around or through the device into the aneurysm – are no more common with the current AFX2 system than with other available AAA stent-grafts.
Technical upgrades to its AFX line of EVAR devices in recent years have largely resolved the safety issues identified in previous models, the company argued.
But the company’s case was unconvincing for a majority of the FDA Circulatory System Devices Advisory Panel that assembled virtually on Nov. 2. A number of panelists questioned the earnestness with which Endologix worked to rectify the safety alert and recall issues. Many also decried the real-world relevance of the randomized trial presented as evidence, with its follow-up time of only a few years.
The panel that included more than a dozen clinicians – mostly surgeons or interventional cardiologists or radiologists – were not instructed to formally vote on the issues. But it ultimately advised the FDA that more exacting studies with longer follow-ups appear needed to show that the device’s benefits in routine use outweigh its risks, especially for type III endoleaks.
“There isn’t a tremendous amount of confidence” that Endologix had enacted sufficient risk-mitigation measures in the wake of the safety alerts and recalls, chair Richard A. Lange, MD, MBA, Foster School of Medicine and Texas Tech University Health Sciences Center, El Paso, said when summarizing the panel’s take on the day’s proceedings.
Although the stent-graft’s safety seemed improved with recent design changes, the panel wasn’t convinced the upgrades could take the credit, or even that they were aimed specifically at preventing endoleaks, Dr. Lange said. “Nobody feels assurance that the problem has been solved.”
“I believe that the type-three endoleaks pose a challenge to patients, and I have not seen enough data to assure me with a degree of certainty that that problem no longer persists,” said panelist Joaquin E. Cigarroa, MD, a cardiologist at Oregon Health & Science University, Portland. His take on the LEOPARD trial, moreover, is that it “does not refute that there is an issue, given the duration of follow-up.”
On the other hand, a majority of the panel agreed that, currently, the AFX2’s benefits would likely outweigh risks for patients in narrowly defined high-risk anatomic or clinical scenarios and those with no other endovascular or surgical option.
“I do believe that there are patient subsets where the Endologix graft can play an important and vital role,” surgeon Keith B. Allen, MD, St. Luke’s Mid America Heart & Vascular Institute, Kansas City, Missouri, offered from the panel.
“In patients that don’t have aneurysmal disease but have distal bifurcation proximal iliac disease, it can be a very nice graft to use and solves a problem,” he said. “To remove that graft completely from the market, I believe, would deny a subset of patients.”
But for aortic aneurysms in routine practice, Dr. Allen said, “I think there are some red flags with it.”
Joining the day’s proceedings as a public commenter, surgeon Mark Conrad, MD, St. Elizabeth’s Hospital, Boston, agreed that “there’s not one commercial device out there that is able to handle every anatomy.”
Having options for patients is important, he said, because “the biggest problems we run into are when somebody only uses one graft, and they try to make that fit everything.”
Another public commenter offered a similar take. “I think we haven’t done a great job in the vascular surgery community really honing in on the detailed nuances that separate one device from another,” said Naiem Nassiri, MD, Yale New Haven Hospital Heart & Vascular Center, Connecticut.
The Endologix device, he said, “serves a very specific role under certain anatomic configurations and limitations, and really, truly fills a gap” left by other available grafts. It suits a very specific niche, “and I think it needs to be explored further for that.”
Endologix representatives who advise clinicians could play a better role in familiarizing operators with the EVAR system’s strengths and limitations, proposed several panelists, including Minhaj S. Khaja, MD, MBA, interventional radiologist at UVA Health and the University of Virginia, Charlottesville.
“There definitely needs to be more education of the clinical reps as well as the physicians implanting these devices,” he said, regarding the type III leaks, patient selection issues, appropriate imaging follow-up, “and the potential for increased reintervention.”
All public commenters, Dr. Lange observed, had been invited to disclose potential conflicts of interest, but it was not mandatory and none did so during the public forum. Disclosures of potential conflicts for the panelists are available on the FDA site.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has long kept a watchful eye over successive iterations of endovascular stent graphs in the Endologix AFX line, designed for repair of abdominal aortic aneurysms (AAA). For years, the devices, first approved in 2011, have drawn safety alerts and recalls , stemming from what the agency says was a “higher than expected” risk for potentially injurious or fatal type III endoleaks.
As part of the latest review process, Endologix recently showed regulators data from a rare randomized trial of the AAA endovascular aneurysm repair (EVAR) technique. The company said the recent postmarket study LEOPARD suggested the type III endoleaks – blood seeping around or through the device into the aneurysm – are no more common with the current AFX2 system than with other available AAA stent-grafts.
Technical upgrades to its AFX line of EVAR devices in recent years have largely resolved the safety issues identified in previous models, the company argued.
But the company’s case was unconvincing for a majority of the FDA Circulatory System Devices Advisory Panel that assembled virtually on Nov. 2. A number of panelists questioned the earnestness with which Endologix worked to rectify the safety alert and recall issues. Many also decried the real-world relevance of the randomized trial presented as evidence, with its follow-up time of only a few years.
The panel that included more than a dozen clinicians – mostly surgeons or interventional cardiologists or radiologists – were not instructed to formally vote on the issues. But it ultimately advised the FDA that more exacting studies with longer follow-ups appear needed to show that the device’s benefits in routine use outweigh its risks, especially for type III endoleaks.
“There isn’t a tremendous amount of confidence” that Endologix had enacted sufficient risk-mitigation measures in the wake of the safety alerts and recalls, chair Richard A. Lange, MD, MBA, Foster School of Medicine and Texas Tech University Health Sciences Center, El Paso, said when summarizing the panel’s take on the day’s proceedings.
Although the stent-graft’s safety seemed improved with recent design changes, the panel wasn’t convinced the upgrades could take the credit, or even that they were aimed specifically at preventing endoleaks, Dr. Lange said. “Nobody feels assurance that the problem has been solved.”
“I believe that the type-three endoleaks pose a challenge to patients, and I have not seen enough data to assure me with a degree of certainty that that problem no longer persists,” said panelist Joaquin E. Cigarroa, MD, a cardiologist at Oregon Health & Science University, Portland. His take on the LEOPARD trial, moreover, is that it “does not refute that there is an issue, given the duration of follow-up.”
On the other hand, a majority of the panel agreed that, currently, the AFX2’s benefits would likely outweigh risks for patients in narrowly defined high-risk anatomic or clinical scenarios and those with no other endovascular or surgical option.
“I do believe that there are patient subsets where the Endologix graft can play an important and vital role,” surgeon Keith B. Allen, MD, St. Luke’s Mid America Heart & Vascular Institute, Kansas City, Missouri, offered from the panel.
“In patients that don’t have aneurysmal disease but have distal bifurcation proximal iliac disease, it can be a very nice graft to use and solves a problem,” he said. “To remove that graft completely from the market, I believe, would deny a subset of patients.”
But for aortic aneurysms in routine practice, Dr. Allen said, “I think there are some red flags with it.”
Joining the day’s proceedings as a public commenter, surgeon Mark Conrad, MD, St. Elizabeth’s Hospital, Boston, agreed that “there’s not one commercial device out there that is able to handle every anatomy.”
Having options for patients is important, he said, because “the biggest problems we run into are when somebody only uses one graft, and they try to make that fit everything.”
Another public commenter offered a similar take. “I think we haven’t done a great job in the vascular surgery community really honing in on the detailed nuances that separate one device from another,” said Naiem Nassiri, MD, Yale New Haven Hospital Heart & Vascular Center, Connecticut.
The Endologix device, he said, “serves a very specific role under certain anatomic configurations and limitations, and really, truly fills a gap” left by other available grafts. It suits a very specific niche, “and I think it needs to be explored further for that.”
Endologix representatives who advise clinicians could play a better role in familiarizing operators with the EVAR system’s strengths and limitations, proposed several panelists, including Minhaj S. Khaja, MD, MBA, interventional radiologist at UVA Health and the University of Virginia, Charlottesville.
“There definitely needs to be more education of the clinical reps as well as the physicians implanting these devices,” he said, regarding the type III leaks, patient selection issues, appropriate imaging follow-up, “and the potential for increased reintervention.”
All public commenters, Dr. Lange observed, had been invited to disclose potential conflicts of interest, but it was not mandatory and none did so during the public forum. Disclosures of potential conflicts for the panelists are available on the FDA site.
A version of this article first appeared on Medscape.com.
Texas interventional cardiologist subject of anticompetitive lawsuit
Doctors Hospital of Laredo, Texas, and the Laredo Physicians Group have filed a lawsuit against interventional cardiologist Ricardo Cigarroa, MD, alleging that he engaged in anticompetitive conduct over the availability of cardiologists in Laredo.
Also named in the lawsuit are Cigarroa Heart and Vascular Institute, Laredo Texas Hospital Company (doing business as Laredo Medical Center) and Laredo Physician Associates (LPA).
According to the complaint, in August 2020, Doctors Hospital and Laredo Physicians Group began actively recruiting cardiologists to the city of Laredo.
The complaint states that, with more than 260,000 residents, the city should have a minimum of 20 cardiologists. However, Laredo currently has only eight cardiologists and only six are interventional cardiologists.
The lawsuit alleges that when Dr. Cigarroa got wind of these recruitment efforts, he entered into a conspiracy with the Cigarroa Institute (a cardiology outpatient clinic) and Laredo Medical Center, the largest acute-care hospital in the city, to engage in “anticompetitive and tortious behavior.
“Their conspiracy had a simple but pernicious goal: deprive Doctors Hospital and Physicians Group of the doctors and employees needed to compete and provide interventional cardiology services to the Laredo market,” the complaint reads.
The alleged conspiracy unfolded in multiple steps, it notes, with Dr. Cigarroa issuing threats to Doctors Hospital, Laredo Physicians Group, and prospective interventional cardiologists being recruited.
Through threats and coercion, multiple qualified interventional cardiologists who were interested in joining Laredo Physicians Group, and to whom the group extended employment offers, decided not to join, the complaint states.
It further claims that Dr. Cigarroa, his son, and his nephew – who represent more than half of the interventional cardiologists in Laredo – informed Doctors Hospital that they would “no longer respond” to emergency calls at Doctors Hospital.
The complaint further alleges that after “scaring off competitors and further cementing their dominant market power and position,” the defendants targeted Arthur Santos, MD, Laredo’s only cardiovascular surgeon who was employed by Laredo Physicians Group.
“Defendants successfully induced Dr. Santos to agree to join Defendant Laredo Physicians Associates (LPA), breaching his enforceable noncompete contractual provision,” the complaint states.
The defendants then allegedly induced the cardiothoracic surgery technicians at Doctors Hospital to join Laredo Medical Center (LMC) and work with Dr. Cigarroa and Dr. Santos, the complaint says.
“The conspiracy to monopolize Laredo’s interventional cardiology market is a win-win-win for Defendants. Dr Cigarroa and the clinic avoid competition for interventional cardiological services, while LMC is left as the only provider of acute cardiology services in Laredo, gaining additional patients and corresponding increased revenue,” the complaint reads.
“Meanwhile, Doctors Hospital’s acute-care cardiology program will be threatened with extinction and, critically, Laredo patients are left with higher health care costs and greater health risks and without competitive market alternatives,” it states.
Dr. Cigarroa responds
According to the Laredo Times, in a statement responding to the anticompetitive conduct lawsuit, Dr. Cigarroa said: “This lawsuit is a dispute between a for-profit corporation and a physician who has demonstrated over 30 years of commitment to his patients and patient care in this community.
“It’s unfortunate that the executives [at] Doctors Hospital have chosen to put profit above the well-being of their patients and employees. Their actions confirm that they care more about their bottom line than they do about our residents and reaffirms how disconnected they are from our community,” Dr. Cigarroa said.
“My top priority continues to be the health of all Laredo residents. I will never stop caring for the patients that I love, and I will continue to help save lives,” Dr. Cigarroa said, according to the Times article.
“I am humbled to have received numerous calls of support from many Doctors Hospital employees. Their words of encouragement are a true testament to the strong relationships I have within the medical community,” Dr. Cigarroa added.
A version of this article first appeared on Medscape.com.
Doctors Hospital of Laredo, Texas, and the Laredo Physicians Group have filed a lawsuit against interventional cardiologist Ricardo Cigarroa, MD, alleging that he engaged in anticompetitive conduct over the availability of cardiologists in Laredo.
Also named in the lawsuit are Cigarroa Heart and Vascular Institute, Laredo Texas Hospital Company (doing business as Laredo Medical Center) and Laredo Physician Associates (LPA).
According to the complaint, in August 2020, Doctors Hospital and Laredo Physicians Group began actively recruiting cardiologists to the city of Laredo.
The complaint states that, with more than 260,000 residents, the city should have a minimum of 20 cardiologists. However, Laredo currently has only eight cardiologists and only six are interventional cardiologists.
The lawsuit alleges that when Dr. Cigarroa got wind of these recruitment efforts, he entered into a conspiracy with the Cigarroa Institute (a cardiology outpatient clinic) and Laredo Medical Center, the largest acute-care hospital in the city, to engage in “anticompetitive and tortious behavior.
“Their conspiracy had a simple but pernicious goal: deprive Doctors Hospital and Physicians Group of the doctors and employees needed to compete and provide interventional cardiology services to the Laredo market,” the complaint reads.
The alleged conspiracy unfolded in multiple steps, it notes, with Dr. Cigarroa issuing threats to Doctors Hospital, Laredo Physicians Group, and prospective interventional cardiologists being recruited.
Through threats and coercion, multiple qualified interventional cardiologists who were interested in joining Laredo Physicians Group, and to whom the group extended employment offers, decided not to join, the complaint states.
It further claims that Dr. Cigarroa, his son, and his nephew – who represent more than half of the interventional cardiologists in Laredo – informed Doctors Hospital that they would “no longer respond” to emergency calls at Doctors Hospital.
The complaint further alleges that after “scaring off competitors and further cementing their dominant market power and position,” the defendants targeted Arthur Santos, MD, Laredo’s only cardiovascular surgeon who was employed by Laredo Physicians Group.
“Defendants successfully induced Dr. Santos to agree to join Defendant Laredo Physicians Associates (LPA), breaching his enforceable noncompete contractual provision,” the complaint states.
The defendants then allegedly induced the cardiothoracic surgery technicians at Doctors Hospital to join Laredo Medical Center (LMC) and work with Dr. Cigarroa and Dr. Santos, the complaint says.
“The conspiracy to monopolize Laredo’s interventional cardiology market is a win-win-win for Defendants. Dr Cigarroa and the clinic avoid competition for interventional cardiological services, while LMC is left as the only provider of acute cardiology services in Laredo, gaining additional patients and corresponding increased revenue,” the complaint reads.
“Meanwhile, Doctors Hospital’s acute-care cardiology program will be threatened with extinction and, critically, Laredo patients are left with higher health care costs and greater health risks and without competitive market alternatives,” it states.
Dr. Cigarroa responds
According to the Laredo Times, in a statement responding to the anticompetitive conduct lawsuit, Dr. Cigarroa said: “This lawsuit is a dispute between a for-profit corporation and a physician who has demonstrated over 30 years of commitment to his patients and patient care in this community.
“It’s unfortunate that the executives [at] Doctors Hospital have chosen to put profit above the well-being of their patients and employees. Their actions confirm that they care more about their bottom line than they do about our residents and reaffirms how disconnected they are from our community,” Dr. Cigarroa said.
“My top priority continues to be the health of all Laredo residents. I will never stop caring for the patients that I love, and I will continue to help save lives,” Dr. Cigarroa said, according to the Times article.
“I am humbled to have received numerous calls of support from many Doctors Hospital employees. Their words of encouragement are a true testament to the strong relationships I have within the medical community,” Dr. Cigarroa added.
A version of this article first appeared on Medscape.com.
Doctors Hospital of Laredo, Texas, and the Laredo Physicians Group have filed a lawsuit against interventional cardiologist Ricardo Cigarroa, MD, alleging that he engaged in anticompetitive conduct over the availability of cardiologists in Laredo.
Also named in the lawsuit are Cigarroa Heart and Vascular Institute, Laredo Texas Hospital Company (doing business as Laredo Medical Center) and Laredo Physician Associates (LPA).
According to the complaint, in August 2020, Doctors Hospital and Laredo Physicians Group began actively recruiting cardiologists to the city of Laredo.
The complaint states that, with more than 260,000 residents, the city should have a minimum of 20 cardiologists. However, Laredo currently has only eight cardiologists and only six are interventional cardiologists.
The lawsuit alleges that when Dr. Cigarroa got wind of these recruitment efforts, he entered into a conspiracy with the Cigarroa Institute (a cardiology outpatient clinic) and Laredo Medical Center, the largest acute-care hospital in the city, to engage in “anticompetitive and tortious behavior.
“Their conspiracy had a simple but pernicious goal: deprive Doctors Hospital and Physicians Group of the doctors and employees needed to compete and provide interventional cardiology services to the Laredo market,” the complaint reads.
The alleged conspiracy unfolded in multiple steps, it notes, with Dr. Cigarroa issuing threats to Doctors Hospital, Laredo Physicians Group, and prospective interventional cardiologists being recruited.
Through threats and coercion, multiple qualified interventional cardiologists who were interested in joining Laredo Physicians Group, and to whom the group extended employment offers, decided not to join, the complaint states.
It further claims that Dr. Cigarroa, his son, and his nephew – who represent more than half of the interventional cardiologists in Laredo – informed Doctors Hospital that they would “no longer respond” to emergency calls at Doctors Hospital.
The complaint further alleges that after “scaring off competitors and further cementing their dominant market power and position,” the defendants targeted Arthur Santos, MD, Laredo’s only cardiovascular surgeon who was employed by Laredo Physicians Group.
“Defendants successfully induced Dr. Santos to agree to join Defendant Laredo Physicians Associates (LPA), breaching his enforceable noncompete contractual provision,” the complaint states.
The defendants then allegedly induced the cardiothoracic surgery technicians at Doctors Hospital to join Laredo Medical Center (LMC) and work with Dr. Cigarroa and Dr. Santos, the complaint says.
“The conspiracy to monopolize Laredo’s interventional cardiology market is a win-win-win for Defendants. Dr Cigarroa and the clinic avoid competition for interventional cardiological services, while LMC is left as the only provider of acute cardiology services in Laredo, gaining additional patients and corresponding increased revenue,” the complaint reads.
“Meanwhile, Doctors Hospital’s acute-care cardiology program will be threatened with extinction and, critically, Laredo patients are left with higher health care costs and greater health risks and without competitive market alternatives,” it states.
Dr. Cigarroa responds
According to the Laredo Times, in a statement responding to the anticompetitive conduct lawsuit, Dr. Cigarroa said: “This lawsuit is a dispute between a for-profit corporation and a physician who has demonstrated over 30 years of commitment to his patients and patient care in this community.
“It’s unfortunate that the executives [at] Doctors Hospital have chosen to put profit above the well-being of their patients and employees. Their actions confirm that they care more about their bottom line than they do about our residents and reaffirms how disconnected they are from our community,” Dr. Cigarroa said.
“My top priority continues to be the health of all Laredo residents. I will never stop caring for the patients that I love, and I will continue to help save lives,” Dr. Cigarroa said, according to the Times article.
“I am humbled to have received numerous calls of support from many Doctors Hospital employees. Their words of encouragement are a true testament to the strong relationships I have within the medical community,” Dr. Cigarroa added.
A version of this article first appeared on Medscape.com.












