User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
COVID-19 may discourage pediatric flu vaccination
Parents who did not vaccinate their children against influenza last year were significantly less likely to do so this year than parents whose children were vaccinated last year, based on survey data from more than 2,000 parents with babies and young children.
“Pediatric vaccination will be an important component to mitigating a dual influenza/COVID-19 epidemic,” Rebeccah L. Sokol, PhD, of Wayne State University, Detroit, and Anna H. Grummon, PhD, of Harvard School of Public Health, Boston, reported in Pediatrics.
Although the pandemic has increased acceptance of some healthy behaviors including handwashing and social distancing, the impact on influenza vaccination rates remains unknown, they said.
To assess parents’ current intentions for flu vaccination of young children this season, the researchers conducted an online survey of 2,164 parents or guardians of children aged between 6 months and 5 years in the United States. The 15-minute online survey was conducted in May 2020 and participants received gift cards. The primary outcome was the impact of the COVID-19 pandemic on parental intentions for having their child vaccinated against seasonal flu this year.
“We measured change categorically, with response options ranging from 1 (I became much less likely to get my child the flu shot next year) to 5 (I became much more likely to get my child the flu shot next year),” the researchers said.
Pandemic changes some parents’ plans
Overall, 60% of parents said that the ongoing pandemic had altered their flu vaccination intentions for their children. About 34% percent of parents whose children did not receive flu vaccine last year said they would not seek the vaccine this year because of the pandemic, compared with 25% of parents whose children received last year’s flu vaccine, a statistically significant difference (P < .001).
Approximately 21% of parents whose children received no flu vaccine last year said the pandemic made them more likely to seek vaccination for the 2020-2021 season, compared with 38% of parents whose children received last year’s flu vaccine.
“These results suggest that overall seasonal influenza vaccination rates may not increase simply because of an ongoing infectious disease pandemic. Instead, a significant predictor of future behavior remains past behavior,” Dr. Sokol and Dr. Grummon said.
The study findings were limited by several factors including the use of a convenience sample and the timing of the survey in May 2020, meaning that survey results might not be generalizable this fall as the pandemic persists, they noted. “Additionally, we assessed intentions to vaccinate; future research will clarify the COVID-19 pandemic’s influence on actual vaccination behaviors.”
The challenge of how to increase uptake of the influenza vaccine during the era of COVID-19 remains, and targeted efforts could include social norms messaging through social media, mass media, or health care providers to increase parents’ intentions to vaccinate, as well as vaccination reminders and presumptive announcements from health care providers that present vaccination as the default option, the researchers added.
Potential for ‘twindemic’ is real
The uptake of flu vaccination is especially important this year, Christopher J. Harrison, MD, director of the vaccine and treatment evaluation unit and professor of pediatrics at the University of Missouri–Kansas City, said in an interview.
“This year we are entering a flu season where the certainty of the timing as well as the potential severity of the season are not known. That said, social distancing and wearing masks – to the extent that enough people conform to COVID-19 precautions – could delay or even blunt the usual influenza season,” he noted.
Unfortunately, the Centers for Disease Control and Prevention and the Food and Drug Administration have had their credibility damaged by the challenges of creating a successful response on the fly to a uniquely multifaceted virus to which previous rules do not apply, Dr. Harrison said. In addition, public confidence was eroded when information about testing and reopening policies were released by non-CDC nonscientists and labeled “CDC recommended,” with no opportunity for the scientific community to correct inaccuracies.
“The current study reveals that public trust in influenza vaccine and indirectly in health authorities has been affected by the pandemic,” said Dr. Harrison. “Vaccine hesitancy has increased somewhat even among previous vaccine accepters. One wonders if promises of a quick COVID-19 vaccine increased mistrust of the FDA because of safety concerns, even among the most ardent provaccine population, and whether these concerns are bleeding over into influenza vaccine concerns.
“This only adds to the anxiety that families feel about visiting any medical facility for routine vaccines while the pandemic rages, and we now are in a fall SARS-CoV-2 resurgence,” he added.
Although the current study data are concerning, “there could still be a net gain of pediatric influenza vaccine uptake this season because the 34% less likely to immunize among previously nonimmunizing families would be counterbalanced by 21% of the same group being more likely to immunize their children [theoretical net loss of 13%],” Dr. Harrison explained. “But the pandemic seems to have motivated previously influenza-immunizing families, i.e. while 24% were less likely, 39% are more likely to immunize [theoretical net gain of 15%]. That said, we would still be way short of the number needed to get to herd immunity.”
Dr. Harrison said he found the findings somewhat surprising, but perhaps he should not have. “I had hoped for more acceptance rather than most people staying in their prior vaccine ‘opinion lanes,’ ending up with likely little overall net change in plans to immunize despite increased health awareness caused by a pandemic.”
However, “the U.S. population has been polarized on vaccines and particularly influenza vaccines for more than 50 years, so why would a pandemic make us less polarized, particularly when the pandemic itself has been a polarizing event?” he questioned.
The greatest barriers to flu vaccination for children this year include a lack of motivation among families to visit immunization sites, given the ongoing need for social distancing and masks, Dr. Harrison said.
“Another barrier is the waning public confidence in our medical/scientific national leaders and organizations,” he emphasized. “This makes it crucial that primary care providers step up and be extra strong vaccine advocates, despite the fact that pandemic economics and necessary safety processes have stressed providers and devastated practices. Indeed, in times of medical stress, no one gets more trust from families than their own personal provider.”
Ultimately, avenues for future research include asking diverse groups of families what they feel they need to hear to be more engaged in immunizing children against influenza. But for now, the current study findings identify that “the public is not uniformly responding to the pandemic’s influence on their likelihood of immunizing their children against influenza,” Dr. Harrison said.
“We now know the size of the problem and hopefully governments, public health organizations, pediatric advocates and clinical care givers can find ways to magnify the message that a pandemic year is not a year to avoid seasonal influenza vaccine unless one has a true contraindication,” Dr. Harrison said.
In addition, “one wonders if the poll were taken today – post the president’s COVID-19 illness – would the answers be different?” he noted.
Dr. Sokol’s work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development but otherwise had no financial conflicts to disclose. Dr. Harrison disclosed that his institution receives grant funding from Merck, Pfizer, and GlaxoSmithKline for pediatric noninfluenza vaccine studies on which he is a subinvestigator, and support from the CDC for pediatric respiratory and gastrointestinal virus surveillance studies on which he is an investigator.
SOURCE: Sokol RL, Grummon AH. Pediatrics. 2020 Sep 30. doi: 10.1542/peds.2020-022871.
Parents who did not vaccinate their children against influenza last year were significantly less likely to do so this year than parents whose children were vaccinated last year, based on survey data from more than 2,000 parents with babies and young children.
“Pediatric vaccination will be an important component to mitigating a dual influenza/COVID-19 epidemic,” Rebeccah L. Sokol, PhD, of Wayne State University, Detroit, and Anna H. Grummon, PhD, of Harvard School of Public Health, Boston, reported in Pediatrics.
Although the pandemic has increased acceptance of some healthy behaviors including handwashing and social distancing, the impact on influenza vaccination rates remains unknown, they said.
To assess parents’ current intentions for flu vaccination of young children this season, the researchers conducted an online survey of 2,164 parents or guardians of children aged between 6 months and 5 years in the United States. The 15-minute online survey was conducted in May 2020 and participants received gift cards. The primary outcome was the impact of the COVID-19 pandemic on parental intentions for having their child vaccinated against seasonal flu this year.
“We measured change categorically, with response options ranging from 1 (I became much less likely to get my child the flu shot next year) to 5 (I became much more likely to get my child the flu shot next year),” the researchers said.
Pandemic changes some parents’ plans
Overall, 60% of parents said that the ongoing pandemic had altered their flu vaccination intentions for their children. About 34% percent of parents whose children did not receive flu vaccine last year said they would not seek the vaccine this year because of the pandemic, compared with 25% of parents whose children received last year’s flu vaccine, a statistically significant difference (P < .001).
Approximately 21% of parents whose children received no flu vaccine last year said the pandemic made them more likely to seek vaccination for the 2020-2021 season, compared with 38% of parents whose children received last year’s flu vaccine.
“These results suggest that overall seasonal influenza vaccination rates may not increase simply because of an ongoing infectious disease pandemic. Instead, a significant predictor of future behavior remains past behavior,” Dr. Sokol and Dr. Grummon said.
The study findings were limited by several factors including the use of a convenience sample and the timing of the survey in May 2020, meaning that survey results might not be generalizable this fall as the pandemic persists, they noted. “Additionally, we assessed intentions to vaccinate; future research will clarify the COVID-19 pandemic’s influence on actual vaccination behaviors.”
The challenge of how to increase uptake of the influenza vaccine during the era of COVID-19 remains, and targeted efforts could include social norms messaging through social media, mass media, or health care providers to increase parents’ intentions to vaccinate, as well as vaccination reminders and presumptive announcements from health care providers that present vaccination as the default option, the researchers added.
Potential for ‘twindemic’ is real
The uptake of flu vaccination is especially important this year, Christopher J. Harrison, MD, director of the vaccine and treatment evaluation unit and professor of pediatrics at the University of Missouri–Kansas City, said in an interview.
“This year we are entering a flu season where the certainty of the timing as well as the potential severity of the season are not known. That said, social distancing and wearing masks – to the extent that enough people conform to COVID-19 precautions – could delay or even blunt the usual influenza season,” he noted.
Unfortunately, the Centers for Disease Control and Prevention and the Food and Drug Administration have had their credibility damaged by the challenges of creating a successful response on the fly to a uniquely multifaceted virus to which previous rules do not apply, Dr. Harrison said. In addition, public confidence was eroded when information about testing and reopening policies were released by non-CDC nonscientists and labeled “CDC recommended,” with no opportunity for the scientific community to correct inaccuracies.
“The current study reveals that public trust in influenza vaccine and indirectly in health authorities has been affected by the pandemic,” said Dr. Harrison. “Vaccine hesitancy has increased somewhat even among previous vaccine accepters. One wonders if promises of a quick COVID-19 vaccine increased mistrust of the FDA because of safety concerns, even among the most ardent provaccine population, and whether these concerns are bleeding over into influenza vaccine concerns.
“This only adds to the anxiety that families feel about visiting any medical facility for routine vaccines while the pandemic rages, and we now are in a fall SARS-CoV-2 resurgence,” he added.
Although the current study data are concerning, “there could still be a net gain of pediatric influenza vaccine uptake this season because the 34% less likely to immunize among previously nonimmunizing families would be counterbalanced by 21% of the same group being more likely to immunize their children [theoretical net loss of 13%],” Dr. Harrison explained. “But the pandemic seems to have motivated previously influenza-immunizing families, i.e. while 24% were less likely, 39% are more likely to immunize [theoretical net gain of 15%]. That said, we would still be way short of the number needed to get to herd immunity.”
Dr. Harrison said he found the findings somewhat surprising, but perhaps he should not have. “I had hoped for more acceptance rather than most people staying in their prior vaccine ‘opinion lanes,’ ending up with likely little overall net change in plans to immunize despite increased health awareness caused by a pandemic.”
However, “the U.S. population has been polarized on vaccines and particularly influenza vaccines for more than 50 years, so why would a pandemic make us less polarized, particularly when the pandemic itself has been a polarizing event?” he questioned.
The greatest barriers to flu vaccination for children this year include a lack of motivation among families to visit immunization sites, given the ongoing need for social distancing and masks, Dr. Harrison said.
“Another barrier is the waning public confidence in our medical/scientific national leaders and organizations,” he emphasized. “This makes it crucial that primary care providers step up and be extra strong vaccine advocates, despite the fact that pandemic economics and necessary safety processes have stressed providers and devastated practices. Indeed, in times of medical stress, no one gets more trust from families than their own personal provider.”
Ultimately, avenues for future research include asking diverse groups of families what they feel they need to hear to be more engaged in immunizing children against influenza. But for now, the current study findings identify that “the public is not uniformly responding to the pandemic’s influence on their likelihood of immunizing their children against influenza,” Dr. Harrison said.
“We now know the size of the problem and hopefully governments, public health organizations, pediatric advocates and clinical care givers can find ways to magnify the message that a pandemic year is not a year to avoid seasonal influenza vaccine unless one has a true contraindication,” Dr. Harrison said.
In addition, “one wonders if the poll were taken today – post the president’s COVID-19 illness – would the answers be different?” he noted.
Dr. Sokol’s work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development but otherwise had no financial conflicts to disclose. Dr. Harrison disclosed that his institution receives grant funding from Merck, Pfizer, and GlaxoSmithKline for pediatric noninfluenza vaccine studies on which he is a subinvestigator, and support from the CDC for pediatric respiratory and gastrointestinal virus surveillance studies on which he is an investigator.
SOURCE: Sokol RL, Grummon AH. Pediatrics. 2020 Sep 30. doi: 10.1542/peds.2020-022871.
Parents who did not vaccinate their children against influenza last year were significantly less likely to do so this year than parents whose children were vaccinated last year, based on survey data from more than 2,000 parents with babies and young children.
“Pediatric vaccination will be an important component to mitigating a dual influenza/COVID-19 epidemic,” Rebeccah L. Sokol, PhD, of Wayne State University, Detroit, and Anna H. Grummon, PhD, of Harvard School of Public Health, Boston, reported in Pediatrics.
Although the pandemic has increased acceptance of some healthy behaviors including handwashing and social distancing, the impact on influenza vaccination rates remains unknown, they said.
To assess parents’ current intentions for flu vaccination of young children this season, the researchers conducted an online survey of 2,164 parents or guardians of children aged between 6 months and 5 years in the United States. The 15-minute online survey was conducted in May 2020 and participants received gift cards. The primary outcome was the impact of the COVID-19 pandemic on parental intentions for having their child vaccinated against seasonal flu this year.
“We measured change categorically, with response options ranging from 1 (I became much less likely to get my child the flu shot next year) to 5 (I became much more likely to get my child the flu shot next year),” the researchers said.
Pandemic changes some parents’ plans
Overall, 60% of parents said that the ongoing pandemic had altered their flu vaccination intentions for their children. About 34% percent of parents whose children did not receive flu vaccine last year said they would not seek the vaccine this year because of the pandemic, compared with 25% of parents whose children received last year’s flu vaccine, a statistically significant difference (P < .001).
Approximately 21% of parents whose children received no flu vaccine last year said the pandemic made them more likely to seek vaccination for the 2020-2021 season, compared with 38% of parents whose children received last year’s flu vaccine.
“These results suggest that overall seasonal influenza vaccination rates may not increase simply because of an ongoing infectious disease pandemic. Instead, a significant predictor of future behavior remains past behavior,” Dr. Sokol and Dr. Grummon said.
The study findings were limited by several factors including the use of a convenience sample and the timing of the survey in May 2020, meaning that survey results might not be generalizable this fall as the pandemic persists, they noted. “Additionally, we assessed intentions to vaccinate; future research will clarify the COVID-19 pandemic’s influence on actual vaccination behaviors.”
The challenge of how to increase uptake of the influenza vaccine during the era of COVID-19 remains, and targeted efforts could include social norms messaging through social media, mass media, or health care providers to increase parents’ intentions to vaccinate, as well as vaccination reminders and presumptive announcements from health care providers that present vaccination as the default option, the researchers added.
Potential for ‘twindemic’ is real
The uptake of flu vaccination is especially important this year, Christopher J. Harrison, MD, director of the vaccine and treatment evaluation unit and professor of pediatrics at the University of Missouri–Kansas City, said in an interview.
“This year we are entering a flu season where the certainty of the timing as well as the potential severity of the season are not known. That said, social distancing and wearing masks – to the extent that enough people conform to COVID-19 precautions – could delay or even blunt the usual influenza season,” he noted.
Unfortunately, the Centers for Disease Control and Prevention and the Food and Drug Administration have had their credibility damaged by the challenges of creating a successful response on the fly to a uniquely multifaceted virus to which previous rules do not apply, Dr. Harrison said. In addition, public confidence was eroded when information about testing and reopening policies were released by non-CDC nonscientists and labeled “CDC recommended,” with no opportunity for the scientific community to correct inaccuracies.
“The current study reveals that public trust in influenza vaccine and indirectly in health authorities has been affected by the pandemic,” said Dr. Harrison. “Vaccine hesitancy has increased somewhat even among previous vaccine accepters. One wonders if promises of a quick COVID-19 vaccine increased mistrust of the FDA because of safety concerns, even among the most ardent provaccine population, and whether these concerns are bleeding over into influenza vaccine concerns.
“This only adds to the anxiety that families feel about visiting any medical facility for routine vaccines while the pandemic rages, and we now are in a fall SARS-CoV-2 resurgence,” he added.
Although the current study data are concerning, “there could still be a net gain of pediatric influenza vaccine uptake this season because the 34% less likely to immunize among previously nonimmunizing families would be counterbalanced by 21% of the same group being more likely to immunize their children [theoretical net loss of 13%],” Dr. Harrison explained. “But the pandemic seems to have motivated previously influenza-immunizing families, i.e. while 24% were less likely, 39% are more likely to immunize [theoretical net gain of 15%]. That said, we would still be way short of the number needed to get to herd immunity.”
Dr. Harrison said he found the findings somewhat surprising, but perhaps he should not have. “I had hoped for more acceptance rather than most people staying in their prior vaccine ‘opinion lanes,’ ending up with likely little overall net change in plans to immunize despite increased health awareness caused by a pandemic.”
However, “the U.S. population has been polarized on vaccines and particularly influenza vaccines for more than 50 years, so why would a pandemic make us less polarized, particularly when the pandemic itself has been a polarizing event?” he questioned.
The greatest barriers to flu vaccination for children this year include a lack of motivation among families to visit immunization sites, given the ongoing need for social distancing and masks, Dr. Harrison said.
“Another barrier is the waning public confidence in our medical/scientific national leaders and organizations,” he emphasized. “This makes it crucial that primary care providers step up and be extra strong vaccine advocates, despite the fact that pandemic economics and necessary safety processes have stressed providers and devastated practices. Indeed, in times of medical stress, no one gets more trust from families than their own personal provider.”
Ultimately, avenues for future research include asking diverse groups of families what they feel they need to hear to be more engaged in immunizing children against influenza. But for now, the current study findings identify that “the public is not uniformly responding to the pandemic’s influence on their likelihood of immunizing their children against influenza,” Dr. Harrison said.
“We now know the size of the problem and hopefully governments, public health organizations, pediatric advocates and clinical care givers can find ways to magnify the message that a pandemic year is not a year to avoid seasonal influenza vaccine unless one has a true contraindication,” Dr. Harrison said.
In addition, “one wonders if the poll were taken today – post the president’s COVID-19 illness – would the answers be different?” he noted.
Dr. Sokol’s work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development but otherwise had no financial conflicts to disclose. Dr. Harrison disclosed that his institution receives grant funding from Merck, Pfizer, and GlaxoSmithKline for pediatric noninfluenza vaccine studies on which he is a subinvestigator, and support from the CDC for pediatric respiratory and gastrointestinal virus surveillance studies on which he is an investigator.
SOURCE: Sokol RL, Grummon AH. Pediatrics. 2020 Sep 30. doi: 10.1542/peds.2020-022871.
FROM PEDIATRICS
Stress tests before knee, hip replacement surgeries down, with no ill effects
Cardiac stress testing before hip and knee replacements has dropped steadily since 2006, according to results from a new study that also showed major cardiac complications to be low in the absence of stress testing – even among people with established risk factors.
Routine stress testing before noncardiac surgeries has come under fire in recent decades as an overuse of resources and a burden on patients. Practice guidelines issued in 2007 and 2014 by the American College of Cardiology and the American Heart Association sought to limit the use of preoperative testing to patients with specific cardiovascular risk factors who might have their management changed by the test results.
For their study, published online in JAMA Cardiology, Daniel S. Rubin, MD, of the University of Chicago and colleagues looked at employee-based insurance data, which included Medicare Advantage claims, for more than 800,000 total hip or knee arthroplasties (28% hip and 72% knee replacements) conducted between 2004 and 2017.
While some 10% of the cohort (mean age 62, 58% women) received a stress test in the 2 months before surgery, the investigators found that the frequency of preoperative stress testing dropped annually starting in late 2006, when it peaked at about 14%, to about 7% in 2017. Older age, male sex and a Revised Cardiac Risk Index score of 1 or greater were all associated with a higher likelihood of being tested.
The overall frequency of myocardial infarction or cardiac arrest was 0.24%, occurring in 1,677 of 686,067 patients. While the rate was higher in patients with at least one RCRI condition, this did not differ significantly between those who received a preoperative stress test and those who did not (0.60%; 221 of 36,554 vs. 0.57%; 694 of 122,466 patients.
The 2007 and 2014 ACC/AHA guidelines make clear that patients with zero RCRI conditions – which comprise a history of ischemic heart disease, heart failure, insulin therapy for diabetes, cerebrovascular disease, or chronic kidney disease – should not receive a stress test before an intermediate-risk surgery such as a hip or knee replacement. But in this study, Dr. Rubin and his colleagues found that almost half of patients who had no RCRI risk factors were stress tested anyway. This means, Dr. Rubin said in an interview, that “there’s still room for improvement” in reducing testing.
“I never want to question how a physician chooses to practice, but I have to applaud physicians for reining in the use of this test. We’re using less of this test and yet the incidence of myocardial infarction and cardiac arrest is also going down, which also calls into question whether we’re getting better at choosing the right patients for the test; or the test doesn’t impact outcomes; or overall health of these patients is improving,” he said.
One surprise finding in the study, Dr. Rubin noted, was a higher rate of complications among people without RCRI conditions who were stress tested, compared with those who were not, with a mean complication rate of 0.27%, compared with 0.14% among those who did not receive a test (P < .001). “The RCRI doesn’t capture certain things,” Dr. Rubin said. “And we know that no risk stratification tool is going to capture everything.”
The RCRI, he noted, is based on a clinical history. “If you haven’t been diagnosed yet, it won’t appear as a risk factor, even if you’re clearly at risk. The question then becomes for a physician, do you do the test or not? On a day-to-day basis it’s hard to make that decision because you want what’s best for the individual patient – and it’s hard to generalize from a study of 800,000 people what’s right for that one patient. That said, it doesn’t appear that stress testing improves outcomes and a decrease in testing appears appropriate.”
Dr. Rubin and his colleagues described as a weakness of their study that it did not capture the full scope of preoperative stress testing among Medicare patients, who are older and therefore more likely to be tested.
That the 2007 and 2014 practice guidelines bore on the drop in testing was not demonstrated by Dr. Rubin and colleagues’ study, which saw declines begin even before the guidelines were published. Nonetheless, the results appear to validate the approach advocated in the guidelines, said guideline coauthor Joshua Beckman, MD, of Vanderbilt University, whose recent research has focused on identifying risk factors for MI after noncardiac surgery.
“I hope that the guidelines have helped in changing the culture for the use of preoperative stress testing as a regular thing,” Dr. Beckman said in an interview. “In fact, the guidelines say you shouldn’t do anything before an operation that you wouldn’t do anyway. So these findings are certainly in agreement with what we’re suggesting and support the idea that unless you have something that is unstable or active, stress testing isn’t likely to help.”
Annemarie Thompson, MD, of Duke University in Durham, N.C., another coauthor on the 2014 guidelines, commented in an interview that Dr. Rubin and colleagues’ findings of a doubled rate of complications among people without RCRI conditions who were stress tested, compared with those who were not might mean something “other than just sheer overuse or overordering of tests inappropriately.”
Rather, she said, physicians might be seeing something in the clinic that cannot be captured by a screening tool reliant on existing diagnoses. “Maybe when they’re sitting in front of you in a clinic, they’re so immobile that you’re left wondering. Or maybe they haven’t been seen by a doctor in a long time,” Dr. Thompson said. “So they don’t have diagnoses if they haven’t been followed. I think what [this finding] shows is that clinicians are detecting something. They may not know what it is. But we have to give a little wiggle room to the clinician who is sitting there looking at a patient who looks like they may not make it through surgery.”
Dr. Thompson said it would be helpful, after a big-data study like this one, to go through the clinical histories of those patients – in this study fewer than 100 – who had no RCRI risk factors and yet were stress tested and ended up having complications. “Until then we’re not going to solve the mystery,” she said. “But it’s a very, very interesting study.”
Dr. Rubin is the president of DRDR Mobile Health, a company that creates mobile applications for health care and from which he has not received compensation. One of his coauthors on the study, Dr. Peter Nagele, reported fee income from Roche Diagnostics. Dr. Beckman disclosed personal fees from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, and other pharmaceutical manufacturers. Dr. Thompson has no disclosures.
SOURCE: Rubin et al. JAMA Cardiol. 2020 Sep 30. doi: 10.1001/jamacardio.2020.4311.
Cardiac stress testing before hip and knee replacements has dropped steadily since 2006, according to results from a new study that also showed major cardiac complications to be low in the absence of stress testing – even among people with established risk factors.
Routine stress testing before noncardiac surgeries has come under fire in recent decades as an overuse of resources and a burden on patients. Practice guidelines issued in 2007 and 2014 by the American College of Cardiology and the American Heart Association sought to limit the use of preoperative testing to patients with specific cardiovascular risk factors who might have their management changed by the test results.
For their study, published online in JAMA Cardiology, Daniel S. Rubin, MD, of the University of Chicago and colleagues looked at employee-based insurance data, which included Medicare Advantage claims, for more than 800,000 total hip or knee arthroplasties (28% hip and 72% knee replacements) conducted between 2004 and 2017.
While some 10% of the cohort (mean age 62, 58% women) received a stress test in the 2 months before surgery, the investigators found that the frequency of preoperative stress testing dropped annually starting in late 2006, when it peaked at about 14%, to about 7% in 2017. Older age, male sex and a Revised Cardiac Risk Index score of 1 or greater were all associated with a higher likelihood of being tested.
The overall frequency of myocardial infarction or cardiac arrest was 0.24%, occurring in 1,677 of 686,067 patients. While the rate was higher in patients with at least one RCRI condition, this did not differ significantly between those who received a preoperative stress test and those who did not (0.60%; 221 of 36,554 vs. 0.57%; 694 of 122,466 patients.
The 2007 and 2014 ACC/AHA guidelines make clear that patients with zero RCRI conditions – which comprise a history of ischemic heart disease, heart failure, insulin therapy for diabetes, cerebrovascular disease, or chronic kidney disease – should not receive a stress test before an intermediate-risk surgery such as a hip or knee replacement. But in this study, Dr. Rubin and his colleagues found that almost half of patients who had no RCRI risk factors were stress tested anyway. This means, Dr. Rubin said in an interview, that “there’s still room for improvement” in reducing testing.
“I never want to question how a physician chooses to practice, but I have to applaud physicians for reining in the use of this test. We’re using less of this test and yet the incidence of myocardial infarction and cardiac arrest is also going down, which also calls into question whether we’re getting better at choosing the right patients for the test; or the test doesn’t impact outcomes; or overall health of these patients is improving,” he said.
One surprise finding in the study, Dr. Rubin noted, was a higher rate of complications among people without RCRI conditions who were stress tested, compared with those who were not, with a mean complication rate of 0.27%, compared with 0.14% among those who did not receive a test (P < .001). “The RCRI doesn’t capture certain things,” Dr. Rubin said. “And we know that no risk stratification tool is going to capture everything.”
The RCRI, he noted, is based on a clinical history. “If you haven’t been diagnosed yet, it won’t appear as a risk factor, even if you’re clearly at risk. The question then becomes for a physician, do you do the test or not? On a day-to-day basis it’s hard to make that decision because you want what’s best for the individual patient – and it’s hard to generalize from a study of 800,000 people what’s right for that one patient. That said, it doesn’t appear that stress testing improves outcomes and a decrease in testing appears appropriate.”
Dr. Rubin and his colleagues described as a weakness of their study that it did not capture the full scope of preoperative stress testing among Medicare patients, who are older and therefore more likely to be tested.
That the 2007 and 2014 practice guidelines bore on the drop in testing was not demonstrated by Dr. Rubin and colleagues’ study, which saw declines begin even before the guidelines were published. Nonetheless, the results appear to validate the approach advocated in the guidelines, said guideline coauthor Joshua Beckman, MD, of Vanderbilt University, whose recent research has focused on identifying risk factors for MI after noncardiac surgery.
“I hope that the guidelines have helped in changing the culture for the use of preoperative stress testing as a regular thing,” Dr. Beckman said in an interview. “In fact, the guidelines say you shouldn’t do anything before an operation that you wouldn’t do anyway. So these findings are certainly in agreement with what we’re suggesting and support the idea that unless you have something that is unstable or active, stress testing isn’t likely to help.”
Annemarie Thompson, MD, of Duke University in Durham, N.C., another coauthor on the 2014 guidelines, commented in an interview that Dr. Rubin and colleagues’ findings of a doubled rate of complications among people without RCRI conditions who were stress tested, compared with those who were not might mean something “other than just sheer overuse or overordering of tests inappropriately.”
Rather, she said, physicians might be seeing something in the clinic that cannot be captured by a screening tool reliant on existing diagnoses. “Maybe when they’re sitting in front of you in a clinic, they’re so immobile that you’re left wondering. Or maybe they haven’t been seen by a doctor in a long time,” Dr. Thompson said. “So they don’t have diagnoses if they haven’t been followed. I think what [this finding] shows is that clinicians are detecting something. They may not know what it is. But we have to give a little wiggle room to the clinician who is sitting there looking at a patient who looks like they may not make it through surgery.”
Dr. Thompson said it would be helpful, after a big-data study like this one, to go through the clinical histories of those patients – in this study fewer than 100 – who had no RCRI risk factors and yet were stress tested and ended up having complications. “Until then we’re not going to solve the mystery,” she said. “But it’s a very, very interesting study.”
Dr. Rubin is the president of DRDR Mobile Health, a company that creates mobile applications for health care and from which he has not received compensation. One of his coauthors on the study, Dr. Peter Nagele, reported fee income from Roche Diagnostics. Dr. Beckman disclosed personal fees from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, and other pharmaceutical manufacturers. Dr. Thompson has no disclosures.
SOURCE: Rubin et al. JAMA Cardiol. 2020 Sep 30. doi: 10.1001/jamacardio.2020.4311.
Cardiac stress testing before hip and knee replacements has dropped steadily since 2006, according to results from a new study that also showed major cardiac complications to be low in the absence of stress testing – even among people with established risk factors.
Routine stress testing before noncardiac surgeries has come under fire in recent decades as an overuse of resources and a burden on patients. Practice guidelines issued in 2007 and 2014 by the American College of Cardiology and the American Heart Association sought to limit the use of preoperative testing to patients with specific cardiovascular risk factors who might have their management changed by the test results.
For their study, published online in JAMA Cardiology, Daniel S. Rubin, MD, of the University of Chicago and colleagues looked at employee-based insurance data, which included Medicare Advantage claims, for more than 800,000 total hip or knee arthroplasties (28% hip and 72% knee replacements) conducted between 2004 and 2017.
While some 10% of the cohort (mean age 62, 58% women) received a stress test in the 2 months before surgery, the investigators found that the frequency of preoperative stress testing dropped annually starting in late 2006, when it peaked at about 14%, to about 7% in 2017. Older age, male sex and a Revised Cardiac Risk Index score of 1 or greater were all associated with a higher likelihood of being tested.
The overall frequency of myocardial infarction or cardiac arrest was 0.24%, occurring in 1,677 of 686,067 patients. While the rate was higher in patients with at least one RCRI condition, this did not differ significantly between those who received a preoperative stress test and those who did not (0.60%; 221 of 36,554 vs. 0.57%; 694 of 122,466 patients.
The 2007 and 2014 ACC/AHA guidelines make clear that patients with zero RCRI conditions – which comprise a history of ischemic heart disease, heart failure, insulin therapy for diabetes, cerebrovascular disease, or chronic kidney disease – should not receive a stress test before an intermediate-risk surgery such as a hip or knee replacement. But in this study, Dr. Rubin and his colleagues found that almost half of patients who had no RCRI risk factors were stress tested anyway. This means, Dr. Rubin said in an interview, that “there’s still room for improvement” in reducing testing.
“I never want to question how a physician chooses to practice, but I have to applaud physicians for reining in the use of this test. We’re using less of this test and yet the incidence of myocardial infarction and cardiac arrest is also going down, which also calls into question whether we’re getting better at choosing the right patients for the test; or the test doesn’t impact outcomes; or overall health of these patients is improving,” he said.
One surprise finding in the study, Dr. Rubin noted, was a higher rate of complications among people without RCRI conditions who were stress tested, compared with those who were not, with a mean complication rate of 0.27%, compared with 0.14% among those who did not receive a test (P < .001). “The RCRI doesn’t capture certain things,” Dr. Rubin said. “And we know that no risk stratification tool is going to capture everything.”
The RCRI, he noted, is based on a clinical history. “If you haven’t been diagnosed yet, it won’t appear as a risk factor, even if you’re clearly at risk. The question then becomes for a physician, do you do the test or not? On a day-to-day basis it’s hard to make that decision because you want what’s best for the individual patient – and it’s hard to generalize from a study of 800,000 people what’s right for that one patient. That said, it doesn’t appear that stress testing improves outcomes and a decrease in testing appears appropriate.”
Dr. Rubin and his colleagues described as a weakness of their study that it did not capture the full scope of preoperative stress testing among Medicare patients, who are older and therefore more likely to be tested.
That the 2007 and 2014 practice guidelines bore on the drop in testing was not demonstrated by Dr. Rubin and colleagues’ study, which saw declines begin even before the guidelines were published. Nonetheless, the results appear to validate the approach advocated in the guidelines, said guideline coauthor Joshua Beckman, MD, of Vanderbilt University, whose recent research has focused on identifying risk factors for MI after noncardiac surgery.
“I hope that the guidelines have helped in changing the culture for the use of preoperative stress testing as a regular thing,” Dr. Beckman said in an interview. “In fact, the guidelines say you shouldn’t do anything before an operation that you wouldn’t do anyway. So these findings are certainly in agreement with what we’re suggesting and support the idea that unless you have something that is unstable or active, stress testing isn’t likely to help.”
Annemarie Thompson, MD, of Duke University in Durham, N.C., another coauthor on the 2014 guidelines, commented in an interview that Dr. Rubin and colleagues’ findings of a doubled rate of complications among people without RCRI conditions who were stress tested, compared with those who were not might mean something “other than just sheer overuse or overordering of tests inappropriately.”
Rather, she said, physicians might be seeing something in the clinic that cannot be captured by a screening tool reliant on existing diagnoses. “Maybe when they’re sitting in front of you in a clinic, they’re so immobile that you’re left wondering. Or maybe they haven’t been seen by a doctor in a long time,” Dr. Thompson said. “So they don’t have diagnoses if they haven’t been followed. I think what [this finding] shows is that clinicians are detecting something. They may not know what it is. But we have to give a little wiggle room to the clinician who is sitting there looking at a patient who looks like they may not make it through surgery.”
Dr. Thompson said it would be helpful, after a big-data study like this one, to go through the clinical histories of those patients – in this study fewer than 100 – who had no RCRI risk factors and yet were stress tested and ended up having complications. “Until then we’re not going to solve the mystery,” she said. “But it’s a very, very interesting study.”
Dr. Rubin is the president of DRDR Mobile Health, a company that creates mobile applications for health care and from which he has not received compensation. One of his coauthors on the study, Dr. Peter Nagele, reported fee income from Roche Diagnostics. Dr. Beckman disclosed personal fees from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, and other pharmaceutical manufacturers. Dr. Thompson has no disclosures.
SOURCE: Rubin et al. JAMA Cardiol. 2020 Sep 30. doi: 10.1001/jamacardio.2020.4311.
FROM JAMA CARDIOLOGY
FDA updates info on postmarketing surveillance study of Essure
The Food and Drug Administration has updated its page on Essure information for patients and health care providers to add additional information on adverse events reported by its manufacturer.
Essure was a permanent implantable birth control device approved by the FDA in 2002. FDA ordered Bayer in 2016 to conduct a postmarket surveillance study of Essure following reports of safety concerns, and expanded the study from 3 years to 5 years in 2018. Bayer voluntarily removed Essure from the market at the end of 2018, citing low sales after a “black box” warning was placed on the device. All devices were returned to the company by the end of 2019.
Bayer is required to report variances in Medical Device Reporting (MDR) requirements of Essure related to litigation to the FDA, which includes adverse events such death, serious injury, and “malfunction that would be likely to cause or contribute to a death or serious injury if the malfunction were to recur.” The reports are limited to events Bayer becomes aware of between November 2016 and November 2020. Bayer will continue to provide these reports until April 2021.
The FDA emphasized that the collected data are based on social media reports and already may be reported to the FDA, rather than being a collection of new events. “The limited information provided in the reports prevents the ability to draw any conclusions as to whether the device, or its removal, caused or contributed to any of the events in the reports,” Benjamin Fisher, PhD, director of the Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office in the Center for Devices and Radiological Health, said in an FDA In Brief statement on Aug. 11.
The FDA first uploaded an Essure MDR variance spreadsheet in August 2020, listing 1,453 events, consisting of 53 reports of deaths, 1,376 reports of serious injury, and 24 reports of device malfunction that occurred as of June 2020. In September 2020, FDA uploaded a second variance spreadsheet, which added another 1,934 events that occurred as of July.
Interim analysis of postmarketing surveillance study
An interim analysis of 1,128 patients from 67 centers in the Essure postmarket surveillance study, which compared women who received Essure with those who received laparoscopic tubal sterilization, revealed that 94.6% (265 of 280 patients) in the Essure group had a successful implantation of the device, compared with 99.6% of women who achieved bilateral tubal occlusion from laparoscopic tubal sterilization.
Regarding safety, 9.1% of women in the Essure group and 4.5% in the laparoscopic tubal sterilization group reported chronic lower abdominal and/or pelvic pain, and 16.3% in the Essure group and 10.2% in the laparoscopic tubal sterilization group reported new or worsening abnormal uterine bleeding. In the Essure group, 22.3% of women said they experienced hypersensitivity, an allergic reaction, and new “autoimmune-like reactions” compared with 12.5% of women in the laparoscopic tubal sterilization group.
The interim analysis also showed 19.7% of women in the Essure group and 3.0% in the laparoscopic tubal sterilization group underwent gynecologic surgical procedures, which were “driven primarily by Essure removal and endometrial ablation procedures in Essure patients.” Device removal occurred in 6.8% of women with the Essure device.
Consistent data on Essure
An FDA search of the Manufacturer and User Facility Device Experience (MAUDE) database in January of 2020 revealed 47,856 medical device reports of Essure between November 2002 and December 2019. The most common adverse events observed during this period were:
- Pain or abdominal pain (32,901 cases).
- Heavy or irregular menses (14,573 cases). Headache (8,570 cases).
- Device fragment or foreign body in a patient (8,501 cases).
- Perforation (7,825 cases).
- Fatigue (7,083 cases).
- Gain or loss in weight (5,980 cases).
- Anxiety and/or depression (5,366 cases).
- Rash and/or hypersensitivity (5,077 cases)
- Hair loss (4,999 cases).
Problems with the device itself included reports of:
- Device incompatibility such as an allergy (7,515 cases).
- The device migrating (4,535 cases).
- The device breaking or fracturing (2,297 cases).
- The device dislodging or dislocating (1,797 cases).
- Improper operation including implant failure and pregnancy (1,058 cases).
In 2019, Essure received 15,083 medical device reports, an increase from 6,000 reports in 2018 and 11,854 reports in 2017.
To date, nearly 39,000 women in the United States have made claims to injuries related to the Essure device. In August, Bayer announced it would pay approximately $1.6 billion U.S. dollars to settle 90% of these cases in exchange for claimants to “dismiss their cases or not file.” Bayer also said in a press release that the settlement is not an admission of wrongdoing or liability on the part of the company.
In an interview, Catherine Cansino, MD, MPH, of the department of obstetrics and gynecology at the University of California, Davis, said the latest adverse event reports show “consistent info from [the] MAUDE database when comparing 2019 to previous years, highlighting most common problems related to pain and heavy or irregular bleeding.”
She emphasized ob.gyns with patients who have an Essure device should “consider Essure-related etiology that may necessitate device removal when evaluating patients with gynecological problems, especially with regard to abdominal/pelvic pain and heavy/irregular bleeding.”
Dr. Cansino reported no relevant financial disclosures. She is a member of the Ob.Gyn. News Editorial Advisory Board.
The Food and Drug Administration has updated its page on Essure information for patients and health care providers to add additional information on adverse events reported by its manufacturer.
Essure was a permanent implantable birth control device approved by the FDA in 2002. FDA ordered Bayer in 2016 to conduct a postmarket surveillance study of Essure following reports of safety concerns, and expanded the study from 3 years to 5 years in 2018. Bayer voluntarily removed Essure from the market at the end of 2018, citing low sales after a “black box” warning was placed on the device. All devices were returned to the company by the end of 2019.
Bayer is required to report variances in Medical Device Reporting (MDR) requirements of Essure related to litigation to the FDA, which includes adverse events such death, serious injury, and “malfunction that would be likely to cause or contribute to a death or serious injury if the malfunction were to recur.” The reports are limited to events Bayer becomes aware of between November 2016 and November 2020. Bayer will continue to provide these reports until April 2021.
The FDA emphasized that the collected data are based on social media reports and already may be reported to the FDA, rather than being a collection of new events. “The limited information provided in the reports prevents the ability to draw any conclusions as to whether the device, or its removal, caused or contributed to any of the events in the reports,” Benjamin Fisher, PhD, director of the Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office in the Center for Devices and Radiological Health, said in an FDA In Brief statement on Aug. 11.
The FDA first uploaded an Essure MDR variance spreadsheet in August 2020, listing 1,453 events, consisting of 53 reports of deaths, 1,376 reports of serious injury, and 24 reports of device malfunction that occurred as of June 2020. In September 2020, FDA uploaded a second variance spreadsheet, which added another 1,934 events that occurred as of July.
Interim analysis of postmarketing surveillance study
An interim analysis of 1,128 patients from 67 centers in the Essure postmarket surveillance study, which compared women who received Essure with those who received laparoscopic tubal sterilization, revealed that 94.6% (265 of 280 patients) in the Essure group had a successful implantation of the device, compared with 99.6% of women who achieved bilateral tubal occlusion from laparoscopic tubal sterilization.
Regarding safety, 9.1% of women in the Essure group and 4.5% in the laparoscopic tubal sterilization group reported chronic lower abdominal and/or pelvic pain, and 16.3% in the Essure group and 10.2% in the laparoscopic tubal sterilization group reported new or worsening abnormal uterine bleeding. In the Essure group, 22.3% of women said they experienced hypersensitivity, an allergic reaction, and new “autoimmune-like reactions” compared with 12.5% of women in the laparoscopic tubal sterilization group.
The interim analysis also showed 19.7% of women in the Essure group and 3.0% in the laparoscopic tubal sterilization group underwent gynecologic surgical procedures, which were “driven primarily by Essure removal and endometrial ablation procedures in Essure patients.” Device removal occurred in 6.8% of women with the Essure device.
Consistent data on Essure
An FDA search of the Manufacturer and User Facility Device Experience (MAUDE) database in January of 2020 revealed 47,856 medical device reports of Essure between November 2002 and December 2019. The most common adverse events observed during this period were:
- Pain or abdominal pain (32,901 cases).
- Heavy or irregular menses (14,573 cases). Headache (8,570 cases).
- Device fragment or foreign body in a patient (8,501 cases).
- Perforation (7,825 cases).
- Fatigue (7,083 cases).
- Gain or loss in weight (5,980 cases).
- Anxiety and/or depression (5,366 cases).
- Rash and/or hypersensitivity (5,077 cases)
- Hair loss (4,999 cases).
Problems with the device itself included reports of:
- Device incompatibility such as an allergy (7,515 cases).
- The device migrating (4,535 cases).
- The device breaking or fracturing (2,297 cases).
- The device dislodging or dislocating (1,797 cases).
- Improper operation including implant failure and pregnancy (1,058 cases).
In 2019, Essure received 15,083 medical device reports, an increase from 6,000 reports in 2018 and 11,854 reports in 2017.
To date, nearly 39,000 women in the United States have made claims to injuries related to the Essure device. In August, Bayer announced it would pay approximately $1.6 billion U.S. dollars to settle 90% of these cases in exchange for claimants to “dismiss their cases or not file.” Bayer also said in a press release that the settlement is not an admission of wrongdoing or liability on the part of the company.
In an interview, Catherine Cansino, MD, MPH, of the department of obstetrics and gynecology at the University of California, Davis, said the latest adverse event reports show “consistent info from [the] MAUDE database when comparing 2019 to previous years, highlighting most common problems related to pain and heavy or irregular bleeding.”
She emphasized ob.gyns with patients who have an Essure device should “consider Essure-related etiology that may necessitate device removal when evaluating patients with gynecological problems, especially with regard to abdominal/pelvic pain and heavy/irregular bleeding.”
Dr. Cansino reported no relevant financial disclosures. She is a member of the Ob.Gyn. News Editorial Advisory Board.
The Food and Drug Administration has updated its page on Essure information for patients and health care providers to add additional information on adverse events reported by its manufacturer.
Essure was a permanent implantable birth control device approved by the FDA in 2002. FDA ordered Bayer in 2016 to conduct a postmarket surveillance study of Essure following reports of safety concerns, and expanded the study from 3 years to 5 years in 2018. Bayer voluntarily removed Essure from the market at the end of 2018, citing low sales after a “black box” warning was placed on the device. All devices were returned to the company by the end of 2019.
Bayer is required to report variances in Medical Device Reporting (MDR) requirements of Essure related to litigation to the FDA, which includes adverse events such death, serious injury, and “malfunction that would be likely to cause or contribute to a death or serious injury if the malfunction were to recur.” The reports are limited to events Bayer becomes aware of between November 2016 and November 2020. Bayer will continue to provide these reports until April 2021.
The FDA emphasized that the collected data are based on social media reports and already may be reported to the FDA, rather than being a collection of new events. “The limited information provided in the reports prevents the ability to draw any conclusions as to whether the device, or its removal, caused or contributed to any of the events in the reports,” Benjamin Fisher, PhD, director of the Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office in the Center for Devices and Radiological Health, said in an FDA In Brief statement on Aug. 11.
The FDA first uploaded an Essure MDR variance spreadsheet in August 2020, listing 1,453 events, consisting of 53 reports of deaths, 1,376 reports of serious injury, and 24 reports of device malfunction that occurred as of June 2020. In September 2020, FDA uploaded a second variance spreadsheet, which added another 1,934 events that occurred as of July.
Interim analysis of postmarketing surveillance study
An interim analysis of 1,128 patients from 67 centers in the Essure postmarket surveillance study, which compared women who received Essure with those who received laparoscopic tubal sterilization, revealed that 94.6% (265 of 280 patients) in the Essure group had a successful implantation of the device, compared with 99.6% of women who achieved bilateral tubal occlusion from laparoscopic tubal sterilization.
Regarding safety, 9.1% of women in the Essure group and 4.5% in the laparoscopic tubal sterilization group reported chronic lower abdominal and/or pelvic pain, and 16.3% in the Essure group and 10.2% in the laparoscopic tubal sterilization group reported new or worsening abnormal uterine bleeding. In the Essure group, 22.3% of women said they experienced hypersensitivity, an allergic reaction, and new “autoimmune-like reactions” compared with 12.5% of women in the laparoscopic tubal sterilization group.
The interim analysis also showed 19.7% of women in the Essure group and 3.0% in the laparoscopic tubal sterilization group underwent gynecologic surgical procedures, which were “driven primarily by Essure removal and endometrial ablation procedures in Essure patients.” Device removal occurred in 6.8% of women with the Essure device.
Consistent data on Essure
An FDA search of the Manufacturer and User Facility Device Experience (MAUDE) database in January of 2020 revealed 47,856 medical device reports of Essure between November 2002 and December 2019. The most common adverse events observed during this period were:
- Pain or abdominal pain (32,901 cases).
- Heavy or irregular menses (14,573 cases). Headache (8,570 cases).
- Device fragment or foreign body in a patient (8,501 cases).
- Perforation (7,825 cases).
- Fatigue (7,083 cases).
- Gain or loss in weight (5,980 cases).
- Anxiety and/or depression (5,366 cases).
- Rash and/or hypersensitivity (5,077 cases)
- Hair loss (4,999 cases).
Problems with the device itself included reports of:
- Device incompatibility such as an allergy (7,515 cases).
- The device migrating (4,535 cases).
- The device breaking or fracturing (2,297 cases).
- The device dislodging or dislocating (1,797 cases).
- Improper operation including implant failure and pregnancy (1,058 cases).
In 2019, Essure received 15,083 medical device reports, an increase from 6,000 reports in 2018 and 11,854 reports in 2017.
To date, nearly 39,000 women in the United States have made claims to injuries related to the Essure device. In August, Bayer announced it would pay approximately $1.6 billion U.S. dollars to settle 90% of these cases in exchange for claimants to “dismiss their cases or not file.” Bayer also said in a press release that the settlement is not an admission of wrongdoing or liability on the part of the company.
In an interview, Catherine Cansino, MD, MPH, of the department of obstetrics and gynecology at the University of California, Davis, said the latest adverse event reports show “consistent info from [the] MAUDE database when comparing 2019 to previous years, highlighting most common problems related to pain and heavy or irregular bleeding.”
She emphasized ob.gyns with patients who have an Essure device should “consider Essure-related etiology that may necessitate device removal when evaluating patients with gynecological problems, especially with regard to abdominal/pelvic pain and heavy/irregular bleeding.”
Dr. Cansino reported no relevant financial disclosures. She is a member of the Ob.Gyn. News Editorial Advisory Board.
Inside the flawed White House testing scheme that did not protect Trump
The president has said others are tested before getting close to him, appearing to hold it as an iron shield of safety. He has largely eschewed mask-wearing and social distancing in meetings, travel and public events, while holding rallies for thousands of often maskless supporters.
The Trump administration has increasingly pinned its coronavirus testing strategy for the nation on antigen tests, which do not need a traditional lab for processing and quickly return results to patients. But the results are less accurate than those of the slower PCR tests.
An early antigen test used by the White House was woefully inaccurate. But the new antigen test the White House is using has not been independently evaluated for accuracy and reliability. Moreover, this is the kit the Trump administration is pushing out to thousands of nursing homes to test residents and staff.
Testing “isn’t a ‘get out of jail free card,’” said Dr. Alan Wells, medical director of clinical labs at the University of Pittsburgh Medical Center and creator of its test for the novel coronavirus. In general, antigen tests can miss up to half the cases that are detected by polymerase chain reaction tests, depending on the population of patients tested, he said.
The White House said the president’s diagnosis was confirmed with a PCR test but declined to say which test delivered his initial result. The White House has been using a new antigen test from Abbott Laboratories to screen its staff for COVID-19, according to two administration officials.
The test, known as BinaxNOW, received an emergency use authorization from the Food and Drug Administration in August. It produces results in 15 minutes. Yet little is independently known about how effective it is. According to the company, the test is 97% accurate in detecting positives and 98.5% accurate in identifying those without disease. Abbott’s stated performance of its antigen test was based on examining people within 7 days of COVID symptoms appearing.
The president and first lady have both had symptoms, according to White House chief of staff Mark Meadows and the first lady’s Twitter account. The president was admitted to Walter Reed National Military Medical Center on Friday evening “out of an abundance of caution,” White House press secretary Kayleigh McEnany said in a statement.
Vice President Mike Pence is also tested daily for the virus and tested negative, spokesperson Devin O’Malley said Friday, but he did not respond to a follow-up question about which test was used.
Trump heavily promoted another Abbott rapid testing device, the ID NOW, earlier this year. But that test relies on different technology than the newer Abbott antigen test.
“I have not seen any independent evaluation of the Binax assay in the literature or in the blogs,” Wells said. “It is an unknown.”
The Department of Health and Human Services announced in August that it had signed a $760 million contract with Abbott for 150 million BinaxNOW antigen tests, which are now being distributed to nursing homes and historically black colleges and universities, as well as to governors to help inform decisions about opening and closing schools. The Big Ten football conference has also pinned playing hopes on the deployment of antigen tests following Trump’s political pressure.
However, even senior federal officials concede that a test alone isn’t likely to stop the spread of a virus that has sickened more than 7 million Americans.
“Testing does not substitute for avoiding crowded indoor spaces, washing hands, or wearing a mask when you can’t physically distance; further, a negative test today does not mean that you won’t be positive tomorrow,” Adm. Brett Giroir, the senior HHS official helming the administration’s testing effort, said in a statement at the time.
Trump could be part of a “super-spreading event,” said Dr. Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Given the timing of Trump’s positive test — which he announced on Twitter early Friday – his infection “likely happened 5 or more days ago,” Osterholm said. “If so, then he was widely infectious as early as Tuesday,” the day of the first presidential debate in Cleveland.
At least seven people who attended a Rose Garden announcement last Saturday, when Trump announced his nomination of Judge Amy Coney Barrett to the Supreme Court, have since tested positive for the coronavirus. They include Trump’s former adviser Kellyanne Conway, Republican Sens. Mike Lee and Thom Tillis, and the president of the University of Notre Dame, the Rev. John Jenkins.
“Having that many infected people there all at one time, we’re still going to see transmission coming off that event for a couple days,” Osterholm said.
Osterholm notes that about 20% of infected people lead to 80% of COVID-19 cases, because “super spreaders” can infect so many people at once.
He notes that participants and audience members at Tuesday’s debate were separated by at least 6 feet. But 6 feet isn’t always enough to prevent infection, he said.
While many COVID-19 infections appear to be spread by respiratory droplets, which usually fall to the ground within 6 feet, people who are singing or speaking loudly can project virus much further. Evidence also suggests that the novel coronavirus can spread through aerosols, floating in the air like a speck of dust.
“I wonder how much virus was floating in that room that night,” Osterholm said.
Other experts say it’s too soon to say whether Trump was infected in a super-spreader event. “The president and his wife have had many exposures to many people in enclosed venues without protection,” so they could have been infected at any number of places, said Dr. William Schaffner, an infectious disease specialist at the Vanderbilt University School of Medicine.
Although Democratic presidential candidate and former Vice President Joe Biden tested negative for the virus with a PCR test Friday, experts note that false-negative results are common in the first few days after infection. Test results over the next several days will yield more useful information.
It can take more than a week for the virus to reproduce enough to be detected, Wells said: “You are probably not detectable for 3, 5, 7, even 10 days after you’re exposed.”
In Minnesota, where Trump held an outdoor campaign rally in Duluth with hundreds of attendees Wednesday, health officials warned that a 14-day quarantine is necessary, regardless of test results.
“Anyone who was a direct contact of President Trump or known COVID-19 cases needs to quarantine and should get tested,” the Minnesota Department of Health said.
Ongoing lapses in test result reporting could hamper efforts to track and isolate sick people. As of Sept. 10, 21 states and the District of Columbia were not reporting all antigen test results, according to a KHN investigation, a lapse in reporting that officials say leaves them blind to disease spread. Since then, public health departments in Arizona, North Carolina and South Dakota all have announced plans to add antigen testing to their case reporting.
Requests for comment to the D.C. Department of Health were referred to Mayor Muriel Bowser’s office, which did not respond. District health officials told KHN in early September that the White House does not report antigen test results to them – a potential violation of federal law under the CARES Act, which says any institution performing tests to diagnose COVID-19 must report all results to local or state public health departments.
Dr. Amesh Adalja, a senior scholar at the Johns Hopkins University Center for Health Security, said it’s not surprising that Trump tested positive, given that so many of his close associates – including his national security adviser and Secret Service officers – have also been infected by the virus.
“When you look at the number of social contacts and travel schedules, it’s not surprising,” Adalja said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
The president has said others are tested before getting close to him, appearing to hold it as an iron shield of safety. He has largely eschewed mask-wearing and social distancing in meetings, travel and public events, while holding rallies for thousands of often maskless supporters.
The Trump administration has increasingly pinned its coronavirus testing strategy for the nation on antigen tests, which do not need a traditional lab for processing and quickly return results to patients. But the results are less accurate than those of the slower PCR tests.
An early antigen test used by the White House was woefully inaccurate. But the new antigen test the White House is using has not been independently evaluated for accuracy and reliability. Moreover, this is the kit the Trump administration is pushing out to thousands of nursing homes to test residents and staff.
Testing “isn’t a ‘get out of jail free card,’” said Dr. Alan Wells, medical director of clinical labs at the University of Pittsburgh Medical Center and creator of its test for the novel coronavirus. In general, antigen tests can miss up to half the cases that are detected by polymerase chain reaction tests, depending on the population of patients tested, he said.
The White House said the president’s diagnosis was confirmed with a PCR test but declined to say which test delivered his initial result. The White House has been using a new antigen test from Abbott Laboratories to screen its staff for COVID-19, according to two administration officials.
The test, known as BinaxNOW, received an emergency use authorization from the Food and Drug Administration in August. It produces results in 15 minutes. Yet little is independently known about how effective it is. According to the company, the test is 97% accurate in detecting positives and 98.5% accurate in identifying those without disease. Abbott’s stated performance of its antigen test was based on examining people within 7 days of COVID symptoms appearing.
The president and first lady have both had symptoms, according to White House chief of staff Mark Meadows and the first lady’s Twitter account. The president was admitted to Walter Reed National Military Medical Center on Friday evening “out of an abundance of caution,” White House press secretary Kayleigh McEnany said in a statement.
Vice President Mike Pence is also tested daily for the virus and tested negative, spokesperson Devin O’Malley said Friday, but he did not respond to a follow-up question about which test was used.
Trump heavily promoted another Abbott rapid testing device, the ID NOW, earlier this year. But that test relies on different technology than the newer Abbott antigen test.
“I have not seen any independent evaluation of the Binax assay in the literature or in the blogs,” Wells said. “It is an unknown.”
The Department of Health and Human Services announced in August that it had signed a $760 million contract with Abbott for 150 million BinaxNOW antigen tests, which are now being distributed to nursing homes and historically black colleges and universities, as well as to governors to help inform decisions about opening and closing schools. The Big Ten football conference has also pinned playing hopes on the deployment of antigen tests following Trump’s political pressure.
However, even senior federal officials concede that a test alone isn’t likely to stop the spread of a virus that has sickened more than 7 million Americans.
“Testing does not substitute for avoiding crowded indoor spaces, washing hands, or wearing a mask when you can’t physically distance; further, a negative test today does not mean that you won’t be positive tomorrow,” Adm. Brett Giroir, the senior HHS official helming the administration’s testing effort, said in a statement at the time.
Trump could be part of a “super-spreading event,” said Dr. Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Given the timing of Trump’s positive test — which he announced on Twitter early Friday – his infection “likely happened 5 or more days ago,” Osterholm said. “If so, then he was widely infectious as early as Tuesday,” the day of the first presidential debate in Cleveland.
At least seven people who attended a Rose Garden announcement last Saturday, when Trump announced his nomination of Judge Amy Coney Barrett to the Supreme Court, have since tested positive for the coronavirus. They include Trump’s former adviser Kellyanne Conway, Republican Sens. Mike Lee and Thom Tillis, and the president of the University of Notre Dame, the Rev. John Jenkins.
“Having that many infected people there all at one time, we’re still going to see transmission coming off that event for a couple days,” Osterholm said.
Osterholm notes that about 20% of infected people lead to 80% of COVID-19 cases, because “super spreaders” can infect so many people at once.
He notes that participants and audience members at Tuesday’s debate were separated by at least 6 feet. But 6 feet isn’t always enough to prevent infection, he said.
While many COVID-19 infections appear to be spread by respiratory droplets, which usually fall to the ground within 6 feet, people who are singing or speaking loudly can project virus much further. Evidence also suggests that the novel coronavirus can spread through aerosols, floating in the air like a speck of dust.
“I wonder how much virus was floating in that room that night,” Osterholm said.
Other experts say it’s too soon to say whether Trump was infected in a super-spreader event. “The president and his wife have had many exposures to many people in enclosed venues without protection,” so they could have been infected at any number of places, said Dr. William Schaffner, an infectious disease specialist at the Vanderbilt University School of Medicine.
Although Democratic presidential candidate and former Vice President Joe Biden tested negative for the virus with a PCR test Friday, experts note that false-negative results are common in the first few days after infection. Test results over the next several days will yield more useful information.
It can take more than a week for the virus to reproduce enough to be detected, Wells said: “You are probably not detectable for 3, 5, 7, even 10 days after you’re exposed.”
In Minnesota, where Trump held an outdoor campaign rally in Duluth with hundreds of attendees Wednesday, health officials warned that a 14-day quarantine is necessary, regardless of test results.
“Anyone who was a direct contact of President Trump or known COVID-19 cases needs to quarantine and should get tested,” the Minnesota Department of Health said.
Ongoing lapses in test result reporting could hamper efforts to track and isolate sick people. As of Sept. 10, 21 states and the District of Columbia were not reporting all antigen test results, according to a KHN investigation, a lapse in reporting that officials say leaves them blind to disease spread. Since then, public health departments in Arizona, North Carolina and South Dakota all have announced plans to add antigen testing to their case reporting.
Requests for comment to the D.C. Department of Health were referred to Mayor Muriel Bowser’s office, which did not respond. District health officials told KHN in early September that the White House does not report antigen test results to them – a potential violation of federal law under the CARES Act, which says any institution performing tests to diagnose COVID-19 must report all results to local or state public health departments.
Dr. Amesh Adalja, a senior scholar at the Johns Hopkins University Center for Health Security, said it’s not surprising that Trump tested positive, given that so many of his close associates – including his national security adviser and Secret Service officers – have also been infected by the virus.
“When you look at the number of social contacts and travel schedules, it’s not surprising,” Adalja said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
The president has said others are tested before getting close to him, appearing to hold it as an iron shield of safety. He has largely eschewed mask-wearing and social distancing in meetings, travel and public events, while holding rallies for thousands of often maskless supporters.
The Trump administration has increasingly pinned its coronavirus testing strategy for the nation on antigen tests, which do not need a traditional lab for processing and quickly return results to patients. But the results are less accurate than those of the slower PCR tests.
An early antigen test used by the White House was woefully inaccurate. But the new antigen test the White House is using has not been independently evaluated for accuracy and reliability. Moreover, this is the kit the Trump administration is pushing out to thousands of nursing homes to test residents and staff.
Testing “isn’t a ‘get out of jail free card,’” said Dr. Alan Wells, medical director of clinical labs at the University of Pittsburgh Medical Center and creator of its test for the novel coronavirus. In general, antigen tests can miss up to half the cases that are detected by polymerase chain reaction tests, depending on the population of patients tested, he said.
The White House said the president’s diagnosis was confirmed with a PCR test but declined to say which test delivered his initial result. The White House has been using a new antigen test from Abbott Laboratories to screen its staff for COVID-19, according to two administration officials.
The test, known as BinaxNOW, received an emergency use authorization from the Food and Drug Administration in August. It produces results in 15 minutes. Yet little is independently known about how effective it is. According to the company, the test is 97% accurate in detecting positives and 98.5% accurate in identifying those without disease. Abbott’s stated performance of its antigen test was based on examining people within 7 days of COVID symptoms appearing.
The president and first lady have both had symptoms, according to White House chief of staff Mark Meadows and the first lady’s Twitter account. The president was admitted to Walter Reed National Military Medical Center on Friday evening “out of an abundance of caution,” White House press secretary Kayleigh McEnany said in a statement.
Vice President Mike Pence is also tested daily for the virus and tested negative, spokesperson Devin O’Malley said Friday, but he did not respond to a follow-up question about which test was used.
Trump heavily promoted another Abbott rapid testing device, the ID NOW, earlier this year. But that test relies on different technology than the newer Abbott antigen test.
“I have not seen any independent evaluation of the Binax assay in the literature or in the blogs,” Wells said. “It is an unknown.”
The Department of Health and Human Services announced in August that it had signed a $760 million contract with Abbott for 150 million BinaxNOW antigen tests, which are now being distributed to nursing homes and historically black colleges and universities, as well as to governors to help inform decisions about opening and closing schools. The Big Ten football conference has also pinned playing hopes on the deployment of antigen tests following Trump’s political pressure.
However, even senior federal officials concede that a test alone isn’t likely to stop the spread of a virus that has sickened more than 7 million Americans.
“Testing does not substitute for avoiding crowded indoor spaces, washing hands, or wearing a mask when you can’t physically distance; further, a negative test today does not mean that you won’t be positive tomorrow,” Adm. Brett Giroir, the senior HHS official helming the administration’s testing effort, said in a statement at the time.
Trump could be part of a “super-spreading event,” said Dr. Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Given the timing of Trump’s positive test — which he announced on Twitter early Friday – his infection “likely happened 5 or more days ago,” Osterholm said. “If so, then he was widely infectious as early as Tuesday,” the day of the first presidential debate in Cleveland.
At least seven people who attended a Rose Garden announcement last Saturday, when Trump announced his nomination of Judge Amy Coney Barrett to the Supreme Court, have since tested positive for the coronavirus. They include Trump’s former adviser Kellyanne Conway, Republican Sens. Mike Lee and Thom Tillis, and the president of the University of Notre Dame, the Rev. John Jenkins.
“Having that many infected people there all at one time, we’re still going to see transmission coming off that event for a couple days,” Osterholm said.
Osterholm notes that about 20% of infected people lead to 80% of COVID-19 cases, because “super spreaders” can infect so many people at once.
He notes that participants and audience members at Tuesday’s debate were separated by at least 6 feet. But 6 feet isn’t always enough to prevent infection, he said.
While many COVID-19 infections appear to be spread by respiratory droplets, which usually fall to the ground within 6 feet, people who are singing or speaking loudly can project virus much further. Evidence also suggests that the novel coronavirus can spread through aerosols, floating in the air like a speck of dust.
“I wonder how much virus was floating in that room that night,” Osterholm said.
Other experts say it’s too soon to say whether Trump was infected in a super-spreader event. “The president and his wife have had many exposures to many people in enclosed venues without protection,” so they could have been infected at any number of places, said Dr. William Schaffner, an infectious disease specialist at the Vanderbilt University School of Medicine.
Although Democratic presidential candidate and former Vice President Joe Biden tested negative for the virus with a PCR test Friday, experts note that false-negative results are common in the first few days after infection. Test results over the next several days will yield more useful information.
It can take more than a week for the virus to reproduce enough to be detected, Wells said: “You are probably not detectable for 3, 5, 7, even 10 days after you’re exposed.”
In Minnesota, where Trump held an outdoor campaign rally in Duluth with hundreds of attendees Wednesday, health officials warned that a 14-day quarantine is necessary, regardless of test results.
“Anyone who was a direct contact of President Trump or known COVID-19 cases needs to quarantine and should get tested,” the Minnesota Department of Health said.
Ongoing lapses in test result reporting could hamper efforts to track and isolate sick people. As of Sept. 10, 21 states and the District of Columbia were not reporting all antigen test results, according to a KHN investigation, a lapse in reporting that officials say leaves them blind to disease spread. Since then, public health departments in Arizona, North Carolina and South Dakota all have announced plans to add antigen testing to their case reporting.
Requests for comment to the D.C. Department of Health were referred to Mayor Muriel Bowser’s office, which did not respond. District health officials told KHN in early September that the White House does not report antigen test results to them – a potential violation of federal law under the CARES Act, which says any institution performing tests to diagnose COVID-19 must report all results to local or state public health departments.
Dr. Amesh Adalja, a senior scholar at the Johns Hopkins University Center for Health Security, said it’s not surprising that Trump tested positive, given that so many of his close associates – including his national security adviser and Secret Service officers – have also been infected by the virus.
“When you look at the number of social contacts and travel schedules, it’s not surprising,” Adalja said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
‘Celebration’ will be ‘short-lived’ if COVID vaccine rushed: Experts
on Wednesday.
The career staff of the Food and Drug Administration can be counted on to appropriately weigh whether a vaccine should be cleared for use in preventing COVID-19, witnesses, including Paul A. Offit, MD, of Children’s Hospital of Philadelphia, told the House Energy and Commerce Committee’s oversight and investigations panel.
FDA staffers would object to attempts by the Trump administration to rush a vaccine to the public without proper vetting, as would veteran federal researchers, including National Institutes of Health Director Francis S. Collins, MD, PhD, and Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, Offit said.
“If COVID-19 vaccines are released before they’re ready to be released, you will hear from these people, and you will also hear from people like Dr. Francis Collins and Tony Fauci, both of whom are trusted by the American public, as well as many other academicians and researchers who wouldn’t stand for this,” he said.
“The public is already nervous about these vaccines,” said Offit, who serves on key FDA and Centers for Disease Control and Prevention committees overseeing vaccine policy. “If trusted health officials stand up and decry a premature release, the celebration by the administration will be short-lived.”
Overly optimistic estimates about a potential approval can only serve to erode the public’s trust in these crucial vaccines, said another witness, Ashish K. Jha, MD, MPH, the dean of Brown University’s School of Public Health, in Providence, Rhode Island.
“All political leaders need to stop talking about things like time lines,” Jha told the lawmakers.
President Donald Trump has several times suggested that a COVID vaccine might be approved ahead of the November 3 election, where he faces a significant challenge from his Democratic rival, former Vice President Joe Biden.
In a Tuesday night debate with Biden, Trump again raised the idea of a quick approval. “Now we’re weeks away from a vaccine,” Trump said during the debate.
Trump’s estimates, though, are not in line with those offered by most firms involved with making vaccines. The most optimistic projections have come from Pfizer Inc. The drugmaker’s chief executive, Albert Bourla, has spoken about his company possibly having data to present to the FDA as early as late October about the safety and effectiveness of a vaccine.
In a September 8 interview with the Today show, Bourla said there was a 60% chance his company would meet that goal. In response to a question, he made it clear his comments applied to a potential Pfizer application, not an approval or release of a vaccine by that time.
In response to concerns about political pressures, the FDA in June issued guidance outlining what its staff would require for approval of a COVID-19 vaccine.
Pushback on politics
Another witness at the Wednesday hearing, Mark McClellan, MD, PhD, a former FDA commissioner (2002 – 2004), pushed back on objections to a potential release of further guidance from the agency.
“Some recent statements from the White House have implied that FDA’s plan to release additional written guidance on its expectations for emergency use authorization of a vaccine is unnecessarily raising the bar on regulatory standards for authorization,” said McClellan in his testimony for the House panel. “That is not the case.”
Instead, further FDA guidance would be a welcome form of feedback for the firms trying to develop COVID-19 vaccines, according to McClellan, who also serves on the board of directors for Johnson & Johnson. Johnson & Johnson is among the firms that have advanced a COVID-19 vaccine candidate to phase 3 testing. In his role as a director, he serves on the board’s regulatory compliance committee.
Along with politics, recent stumbles at FDA with emergency use authorizations (EUAs) of treatments for COVID-19 have eroded the public’s confidence in the agency, Jha told the House panel. The FDA approved hydroxychloroquine, a medicine promoted by Trump for use in COVID, under an EUA in March and then revoked this clearance in June.
Jha said the FDA’s most serious misstep was its handling of convalescent plasma, which was approved through an EUA on August 23 “in a highly advertised and widely televised announcement including the president.
“The announcement solidified in the public conversation the impression that, increasingly with this administration, politics are taking over trusted, nonpartisan scientific institutions,” he said in his testimony.
Approving a COVID-19 vaccine on the limited evidence through an EUA would mark a serious departure from FDA policy, according to Jha.
“While we sometimes accept a certain level of potential harm in experimental treatments for those who are severely ill, vaccines are given to healthy people and therefore need to have a substantially higher measure of safety and effectiveness,” he explained.
Jha said the FDA has only once before used this EUA approach for a vaccine. That was for a vaccine against inhaled anthrax and was mostly distributed to high-risk soldiers and civilians in war zones.
COVID-19, in contrast, is an infection that has changed lives around the world. The virus has contributed to more than 1 million deaths, including more than 200,000 in the United States, according to the World Health Organization.
Scientists are hoping vaccines will help curb this infection, although much of the future success of vaccines depends on how widely they are used, witnesses told the House panel.
Debate on approaches for vaccine effectiveness
In his testimony, Jha also noted concerns about COVID-19 vaccine trials. He included a reference to a Sept. 22 opinion article titled, “These Coronavirus Trials Don›t Answer the One Question We Need to Know,” which was written by Peter Doshi, PhD, of the University of Maryland School of Pharmacy, in Baltimore, and Eric Topol, MD, a professor of molecular medicine at Scripps Research in La Jolla, Calif. Topol is also editor in chief of Medscape.
Topol and Doshi questioned why the firms Moderna, Pfizer, and AstraZeneca structured their competing trials such that “a vaccine could meet the companies’ benchmark for success if it lowered the risk of mild Covid-19, but was never shown to reduce moderate or severe forms of the disease, or the risk of hospitalization, admissions to the intensive care unit or death.”
“To say a vaccine works should mean that most people no longer run the risk of getting seriously sick,” Topol and Doshi wrote. “That’s not what these trials will determine.”
There was disagreement about this point at the hearing. U.S. Representative Morgan Griffith (R-Va.) read the section of the Doshi-Topol article quoted above and asked one witness, Offit, to weigh in.
“Do you agree with those concerns? And either way, tell me why,” Griffith asked.
“I don’t agree,” Offit responded.
“I think it’s actually much harder to prevent asymptomatic infection or mildly symptomatic infection,” he said. “If you can prevent that, you are much more likely to prevent moderate to severe disease. So I think they have it backwards.”
But other researchers also question the approaches used with the current crop of COVID-19 vaccines.
“With the current protocols, it is conceivable that a vaccine might be considered effective – and eventually approved – based primarily on its ability to prevent mild cases alone,” wrote William Haseltine, PhD, president of the nonprofit ACCESS Health International, in a September 22 opinion article in the Washington Post titled: “Beware of COVID-19 Vaccine Trials Designed to Succeed From the Start.”
In an interview with Medscape Medical News on Wednesday, Haseltine said he maintains these concerns about the tests. Earlier in his career, he was a leader in HIV research through his lab at Harvard University in Cambridge, Massachusetts, and he subsequently led a biotech company, Human Genome Sciences.
He fears consumers will not get what they might expect from the vaccines being tested.
“What people care about is if this is going to keep them out of the hospital and will it keep them alive. And that’s not even part of this protocol,” Haseltine said.
This article first appeared on Medscape.com.
on Wednesday.
The career staff of the Food and Drug Administration can be counted on to appropriately weigh whether a vaccine should be cleared for use in preventing COVID-19, witnesses, including Paul A. Offit, MD, of Children’s Hospital of Philadelphia, told the House Energy and Commerce Committee’s oversight and investigations panel.
FDA staffers would object to attempts by the Trump administration to rush a vaccine to the public without proper vetting, as would veteran federal researchers, including National Institutes of Health Director Francis S. Collins, MD, PhD, and Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, Offit said.
“If COVID-19 vaccines are released before they’re ready to be released, you will hear from these people, and you will also hear from people like Dr. Francis Collins and Tony Fauci, both of whom are trusted by the American public, as well as many other academicians and researchers who wouldn’t stand for this,” he said.
“The public is already nervous about these vaccines,” said Offit, who serves on key FDA and Centers for Disease Control and Prevention committees overseeing vaccine policy. “If trusted health officials stand up and decry a premature release, the celebration by the administration will be short-lived.”
Overly optimistic estimates about a potential approval can only serve to erode the public’s trust in these crucial vaccines, said another witness, Ashish K. Jha, MD, MPH, the dean of Brown University’s School of Public Health, in Providence, Rhode Island.
“All political leaders need to stop talking about things like time lines,” Jha told the lawmakers.
President Donald Trump has several times suggested that a COVID vaccine might be approved ahead of the November 3 election, where he faces a significant challenge from his Democratic rival, former Vice President Joe Biden.
In a Tuesday night debate with Biden, Trump again raised the idea of a quick approval. “Now we’re weeks away from a vaccine,” Trump said during the debate.
Trump’s estimates, though, are not in line with those offered by most firms involved with making vaccines. The most optimistic projections have come from Pfizer Inc. The drugmaker’s chief executive, Albert Bourla, has spoken about his company possibly having data to present to the FDA as early as late October about the safety and effectiveness of a vaccine.
In a September 8 interview with the Today show, Bourla said there was a 60% chance his company would meet that goal. In response to a question, he made it clear his comments applied to a potential Pfizer application, not an approval or release of a vaccine by that time.
In response to concerns about political pressures, the FDA in June issued guidance outlining what its staff would require for approval of a COVID-19 vaccine.
Pushback on politics
Another witness at the Wednesday hearing, Mark McClellan, MD, PhD, a former FDA commissioner (2002 – 2004), pushed back on objections to a potential release of further guidance from the agency.
“Some recent statements from the White House have implied that FDA’s plan to release additional written guidance on its expectations for emergency use authorization of a vaccine is unnecessarily raising the bar on regulatory standards for authorization,” said McClellan in his testimony for the House panel. “That is not the case.”
Instead, further FDA guidance would be a welcome form of feedback for the firms trying to develop COVID-19 vaccines, according to McClellan, who also serves on the board of directors for Johnson & Johnson. Johnson & Johnson is among the firms that have advanced a COVID-19 vaccine candidate to phase 3 testing. In his role as a director, he serves on the board’s regulatory compliance committee.
Along with politics, recent stumbles at FDA with emergency use authorizations (EUAs) of treatments for COVID-19 have eroded the public’s confidence in the agency, Jha told the House panel. The FDA approved hydroxychloroquine, a medicine promoted by Trump for use in COVID, under an EUA in March and then revoked this clearance in June.
Jha said the FDA’s most serious misstep was its handling of convalescent plasma, which was approved through an EUA on August 23 “in a highly advertised and widely televised announcement including the president.
“The announcement solidified in the public conversation the impression that, increasingly with this administration, politics are taking over trusted, nonpartisan scientific institutions,” he said in his testimony.
Approving a COVID-19 vaccine on the limited evidence through an EUA would mark a serious departure from FDA policy, according to Jha.
“While we sometimes accept a certain level of potential harm in experimental treatments for those who are severely ill, vaccines are given to healthy people and therefore need to have a substantially higher measure of safety and effectiveness,” he explained.
Jha said the FDA has only once before used this EUA approach for a vaccine. That was for a vaccine against inhaled anthrax and was mostly distributed to high-risk soldiers and civilians in war zones.
COVID-19, in contrast, is an infection that has changed lives around the world. The virus has contributed to more than 1 million deaths, including more than 200,000 in the United States, according to the World Health Organization.
Scientists are hoping vaccines will help curb this infection, although much of the future success of vaccines depends on how widely they are used, witnesses told the House panel.
Debate on approaches for vaccine effectiveness
In his testimony, Jha also noted concerns about COVID-19 vaccine trials. He included a reference to a Sept. 22 opinion article titled, “These Coronavirus Trials Don›t Answer the One Question We Need to Know,” which was written by Peter Doshi, PhD, of the University of Maryland School of Pharmacy, in Baltimore, and Eric Topol, MD, a professor of molecular medicine at Scripps Research in La Jolla, Calif. Topol is also editor in chief of Medscape.
Topol and Doshi questioned why the firms Moderna, Pfizer, and AstraZeneca structured their competing trials such that “a vaccine could meet the companies’ benchmark for success if it lowered the risk of mild Covid-19, but was never shown to reduce moderate or severe forms of the disease, or the risk of hospitalization, admissions to the intensive care unit or death.”
“To say a vaccine works should mean that most people no longer run the risk of getting seriously sick,” Topol and Doshi wrote. “That’s not what these trials will determine.”
There was disagreement about this point at the hearing. U.S. Representative Morgan Griffith (R-Va.) read the section of the Doshi-Topol article quoted above and asked one witness, Offit, to weigh in.
“Do you agree with those concerns? And either way, tell me why,” Griffith asked.
“I don’t agree,” Offit responded.
“I think it’s actually much harder to prevent asymptomatic infection or mildly symptomatic infection,” he said. “If you can prevent that, you are much more likely to prevent moderate to severe disease. So I think they have it backwards.”
But other researchers also question the approaches used with the current crop of COVID-19 vaccines.
“With the current protocols, it is conceivable that a vaccine might be considered effective – and eventually approved – based primarily on its ability to prevent mild cases alone,” wrote William Haseltine, PhD, president of the nonprofit ACCESS Health International, in a September 22 opinion article in the Washington Post titled: “Beware of COVID-19 Vaccine Trials Designed to Succeed From the Start.”
In an interview with Medscape Medical News on Wednesday, Haseltine said he maintains these concerns about the tests. Earlier in his career, he was a leader in HIV research through his lab at Harvard University in Cambridge, Massachusetts, and he subsequently led a biotech company, Human Genome Sciences.
He fears consumers will not get what they might expect from the vaccines being tested.
“What people care about is if this is going to keep them out of the hospital and will it keep them alive. And that’s not even part of this protocol,” Haseltine said.
This article first appeared on Medscape.com.
on Wednesday.
The career staff of the Food and Drug Administration can be counted on to appropriately weigh whether a vaccine should be cleared for use in preventing COVID-19, witnesses, including Paul A. Offit, MD, of Children’s Hospital of Philadelphia, told the House Energy and Commerce Committee’s oversight and investigations panel.
FDA staffers would object to attempts by the Trump administration to rush a vaccine to the public without proper vetting, as would veteran federal researchers, including National Institutes of Health Director Francis S. Collins, MD, PhD, and Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, Offit said.
“If COVID-19 vaccines are released before they’re ready to be released, you will hear from these people, and you will also hear from people like Dr. Francis Collins and Tony Fauci, both of whom are trusted by the American public, as well as many other academicians and researchers who wouldn’t stand for this,” he said.
“The public is already nervous about these vaccines,” said Offit, who serves on key FDA and Centers for Disease Control and Prevention committees overseeing vaccine policy. “If trusted health officials stand up and decry a premature release, the celebration by the administration will be short-lived.”
Overly optimistic estimates about a potential approval can only serve to erode the public’s trust in these crucial vaccines, said another witness, Ashish K. Jha, MD, MPH, the dean of Brown University’s School of Public Health, in Providence, Rhode Island.
“All political leaders need to stop talking about things like time lines,” Jha told the lawmakers.
President Donald Trump has several times suggested that a COVID vaccine might be approved ahead of the November 3 election, where he faces a significant challenge from his Democratic rival, former Vice President Joe Biden.
In a Tuesday night debate with Biden, Trump again raised the idea of a quick approval. “Now we’re weeks away from a vaccine,” Trump said during the debate.
Trump’s estimates, though, are not in line with those offered by most firms involved with making vaccines. The most optimistic projections have come from Pfizer Inc. The drugmaker’s chief executive, Albert Bourla, has spoken about his company possibly having data to present to the FDA as early as late October about the safety and effectiveness of a vaccine.
In a September 8 interview with the Today show, Bourla said there was a 60% chance his company would meet that goal. In response to a question, he made it clear his comments applied to a potential Pfizer application, not an approval or release of a vaccine by that time.
In response to concerns about political pressures, the FDA in June issued guidance outlining what its staff would require for approval of a COVID-19 vaccine.
Pushback on politics
Another witness at the Wednesday hearing, Mark McClellan, MD, PhD, a former FDA commissioner (2002 – 2004), pushed back on objections to a potential release of further guidance from the agency.
“Some recent statements from the White House have implied that FDA’s plan to release additional written guidance on its expectations for emergency use authorization of a vaccine is unnecessarily raising the bar on regulatory standards for authorization,” said McClellan in his testimony for the House panel. “That is not the case.”
Instead, further FDA guidance would be a welcome form of feedback for the firms trying to develop COVID-19 vaccines, according to McClellan, who also serves on the board of directors for Johnson & Johnson. Johnson & Johnson is among the firms that have advanced a COVID-19 vaccine candidate to phase 3 testing. In his role as a director, he serves on the board’s regulatory compliance committee.
Along with politics, recent stumbles at FDA with emergency use authorizations (EUAs) of treatments for COVID-19 have eroded the public’s confidence in the agency, Jha told the House panel. The FDA approved hydroxychloroquine, a medicine promoted by Trump for use in COVID, under an EUA in March and then revoked this clearance in June.
Jha said the FDA’s most serious misstep was its handling of convalescent plasma, which was approved through an EUA on August 23 “in a highly advertised and widely televised announcement including the president.
“The announcement solidified in the public conversation the impression that, increasingly with this administration, politics are taking over trusted, nonpartisan scientific institutions,” he said in his testimony.
Approving a COVID-19 vaccine on the limited evidence through an EUA would mark a serious departure from FDA policy, according to Jha.
“While we sometimes accept a certain level of potential harm in experimental treatments for those who are severely ill, vaccines are given to healthy people and therefore need to have a substantially higher measure of safety and effectiveness,” he explained.
Jha said the FDA has only once before used this EUA approach for a vaccine. That was for a vaccine against inhaled anthrax and was mostly distributed to high-risk soldiers and civilians in war zones.
COVID-19, in contrast, is an infection that has changed lives around the world. The virus has contributed to more than 1 million deaths, including more than 200,000 in the United States, according to the World Health Organization.
Scientists are hoping vaccines will help curb this infection, although much of the future success of vaccines depends on how widely they are used, witnesses told the House panel.
Debate on approaches for vaccine effectiveness
In his testimony, Jha also noted concerns about COVID-19 vaccine trials. He included a reference to a Sept. 22 opinion article titled, “These Coronavirus Trials Don›t Answer the One Question We Need to Know,” which was written by Peter Doshi, PhD, of the University of Maryland School of Pharmacy, in Baltimore, and Eric Topol, MD, a professor of molecular medicine at Scripps Research in La Jolla, Calif. Topol is also editor in chief of Medscape.
Topol and Doshi questioned why the firms Moderna, Pfizer, and AstraZeneca structured their competing trials such that “a vaccine could meet the companies’ benchmark for success if it lowered the risk of mild Covid-19, but was never shown to reduce moderate or severe forms of the disease, or the risk of hospitalization, admissions to the intensive care unit or death.”
“To say a vaccine works should mean that most people no longer run the risk of getting seriously sick,” Topol and Doshi wrote. “That’s not what these trials will determine.”
There was disagreement about this point at the hearing. U.S. Representative Morgan Griffith (R-Va.) read the section of the Doshi-Topol article quoted above and asked one witness, Offit, to weigh in.
“Do you agree with those concerns? And either way, tell me why,” Griffith asked.
“I don’t agree,” Offit responded.
“I think it’s actually much harder to prevent asymptomatic infection or mildly symptomatic infection,” he said. “If you can prevent that, you are much more likely to prevent moderate to severe disease. So I think they have it backwards.”
But other researchers also question the approaches used with the current crop of COVID-19 vaccines.
“With the current protocols, it is conceivable that a vaccine might be considered effective – and eventually approved – based primarily on its ability to prevent mild cases alone,” wrote William Haseltine, PhD, president of the nonprofit ACCESS Health International, in a September 22 opinion article in the Washington Post titled: “Beware of COVID-19 Vaccine Trials Designed to Succeed From the Start.”
In an interview with Medscape Medical News on Wednesday, Haseltine said he maintains these concerns about the tests. Earlier in his career, he was a leader in HIV research through his lab at Harvard University in Cambridge, Massachusetts, and he subsequently led a biotech company, Human Genome Sciences.
He fears consumers will not get what they might expect from the vaccines being tested.
“What people care about is if this is going to keep them out of the hospital and will it keep them alive. And that’s not even part of this protocol,” Haseltine said.
This article first appeared on Medscape.com.
Screening algorithm safely selects patients for OSA treatment before bariatric surgery
A novel algorithm for selecting patients who require treatment for obstructive sleep apnea (OSA) before undergoing bariatric surgery proved safe in a prospective cohort study of 1,103 patients.
Screening for OSA is recommended before bariatric surgery. OSA has been associated in several meta-analyses with increased risk for postoperative complications – not limited to bariatric surgery – and some studies have suggested that this increased risk may be limited to severe OSA, said Frédéric Series, MD, of Université Laval, Quebec City, at the virtual annual meeting of the Associated Sleep Societies.
The preoperative screening algorithm, which utilizes the results of nocturnal home oximetry and morning capillary gas measurements, effectively stratified patients for the risk of postoperative adverse events and “safely selected patients who don’t need [continuous positive airway pressure] before bariatric surgery,” he said. “The risk of postoperative adverse events following bariatric surgery was not increased in untreated OSA patients with low or moderate risk of severe OSA and hypoventilation.”
The study also demonstrated, he said, that patients with severe OSA with or without hypoventilation, even when correctly treated, remain at higher risk for complications.
The algorithm utilizes an oxygen desaturation index (ODI) corresponding to 3% drops in SaO2 and the percent of the total recording time with an SaO2 below 90%, as well as capillary gas measurements (PCO2). Treatment was initiated for those with severe OSA (ODI ≥ 25/hr, < 10% of recording time with a SaO2 below 90%) or OSA with hypoventilation (PCO2 ≥ 45).
“When the ODI was less than 25 per hour, and when the total recording time spent below 90% SaO2 was less than 10%, with PCO2 < 45 mmHg, we expected no need for CPAP treatment,” Dr. Series said. For analysis, the investigators considered part of the untreated group – those with an ODI < 10/hr (no or mild OSA) – as a control group.
Treated patients underwent CPAP/BiPAP for a mean duration of 1.5 months. Good treatment compliance was mandatory for surgery, and treatment was continued immediately after extubation, in the recovery room, in nearly all patients, Dr. Series reported.
The analysis covered 1,103 patients: 447 controls (40.8%), 358 untreated (32.7%), 289 treated for OSA (26.4%) and 9 (0.8%) treated for OSA + hypoventilation. Patients with OSA, particularly those with severe OSA and those with hypoventilation, were older and heavier and significantly more likely to have hypertension and diabetes than controls.
There were no differences between the four groups in 10-day reoperation or 30-day readmission occurrence, and postoperative complications were “particularly infrequent in the control and OSA-untreated groups, with no differences between these two groups,” Dr. Series said.
Cardiac arrhythmia (mainly atrial fibrillation) occurred more frequently in the OSA-treated group (2.4%) and the OSA/hypoventilation patients (11%) than in the other groups (0.5%-0.6%).
Respiratory failure occurred in about one-third of patients with hypoventilation, and admission to the ICU was “dramatically higher” in patients with hypoventilation (67%), because of respiratory failure, arrhythmia, or other unstable medical conditions, Dr. Series said.
There were no differences between the groups in the duration of surgery or the amount of anesthetic used, but the length of stay in the recovery room was significantly longer in the OSA-treated and hypoventilation groups. The length of hospital stay was also longer in these groups. Sleeve gastrectomy was the most frequent bariatric surgical procedure across all groups, including 100% of patients with hypoventilation, he noted.
Asked to comment on the study, Octavian C. Ioachimescu, MD, PhD, of Emory University in Atlanta and the Atlanta Veterans Affairs Medical Center in Decatur, said the algorithm “clearly deserves further validation in other clinical-based cohorts and longer-term outcome assessment.”
Dr. Series reported that he has no relevant disclosures. Dr. Ioachimescu also said he has no relevant disclosures.
A novel algorithm for selecting patients who require treatment for obstructive sleep apnea (OSA) before undergoing bariatric surgery proved safe in a prospective cohort study of 1,103 patients.
Screening for OSA is recommended before bariatric surgery. OSA has been associated in several meta-analyses with increased risk for postoperative complications – not limited to bariatric surgery – and some studies have suggested that this increased risk may be limited to severe OSA, said Frédéric Series, MD, of Université Laval, Quebec City, at the virtual annual meeting of the Associated Sleep Societies.
The preoperative screening algorithm, which utilizes the results of nocturnal home oximetry and morning capillary gas measurements, effectively stratified patients for the risk of postoperative adverse events and “safely selected patients who don’t need [continuous positive airway pressure] before bariatric surgery,” he said. “The risk of postoperative adverse events following bariatric surgery was not increased in untreated OSA patients with low or moderate risk of severe OSA and hypoventilation.”
The study also demonstrated, he said, that patients with severe OSA with or without hypoventilation, even when correctly treated, remain at higher risk for complications.
The algorithm utilizes an oxygen desaturation index (ODI) corresponding to 3% drops in SaO2 and the percent of the total recording time with an SaO2 below 90%, as well as capillary gas measurements (PCO2). Treatment was initiated for those with severe OSA (ODI ≥ 25/hr, < 10% of recording time with a SaO2 below 90%) or OSA with hypoventilation (PCO2 ≥ 45).
“When the ODI was less than 25 per hour, and when the total recording time spent below 90% SaO2 was less than 10%, with PCO2 < 45 mmHg, we expected no need for CPAP treatment,” Dr. Series said. For analysis, the investigators considered part of the untreated group – those with an ODI < 10/hr (no or mild OSA) – as a control group.
Treated patients underwent CPAP/BiPAP for a mean duration of 1.5 months. Good treatment compliance was mandatory for surgery, and treatment was continued immediately after extubation, in the recovery room, in nearly all patients, Dr. Series reported.
The analysis covered 1,103 patients: 447 controls (40.8%), 358 untreated (32.7%), 289 treated for OSA (26.4%) and 9 (0.8%) treated for OSA + hypoventilation. Patients with OSA, particularly those with severe OSA and those with hypoventilation, were older and heavier and significantly more likely to have hypertension and diabetes than controls.
There were no differences between the four groups in 10-day reoperation or 30-day readmission occurrence, and postoperative complications were “particularly infrequent in the control and OSA-untreated groups, with no differences between these two groups,” Dr. Series said.
Cardiac arrhythmia (mainly atrial fibrillation) occurred more frequently in the OSA-treated group (2.4%) and the OSA/hypoventilation patients (11%) than in the other groups (0.5%-0.6%).
Respiratory failure occurred in about one-third of patients with hypoventilation, and admission to the ICU was “dramatically higher” in patients with hypoventilation (67%), because of respiratory failure, arrhythmia, or other unstable medical conditions, Dr. Series said.
There were no differences between the groups in the duration of surgery or the amount of anesthetic used, but the length of stay in the recovery room was significantly longer in the OSA-treated and hypoventilation groups. The length of hospital stay was also longer in these groups. Sleeve gastrectomy was the most frequent bariatric surgical procedure across all groups, including 100% of patients with hypoventilation, he noted.
Asked to comment on the study, Octavian C. Ioachimescu, MD, PhD, of Emory University in Atlanta and the Atlanta Veterans Affairs Medical Center in Decatur, said the algorithm “clearly deserves further validation in other clinical-based cohorts and longer-term outcome assessment.”
Dr. Series reported that he has no relevant disclosures. Dr. Ioachimescu also said he has no relevant disclosures.
A novel algorithm for selecting patients who require treatment for obstructive sleep apnea (OSA) before undergoing bariatric surgery proved safe in a prospective cohort study of 1,103 patients.
Screening for OSA is recommended before bariatric surgery. OSA has been associated in several meta-analyses with increased risk for postoperative complications – not limited to bariatric surgery – and some studies have suggested that this increased risk may be limited to severe OSA, said Frédéric Series, MD, of Université Laval, Quebec City, at the virtual annual meeting of the Associated Sleep Societies.
The preoperative screening algorithm, which utilizes the results of nocturnal home oximetry and morning capillary gas measurements, effectively stratified patients for the risk of postoperative adverse events and “safely selected patients who don’t need [continuous positive airway pressure] before bariatric surgery,” he said. “The risk of postoperative adverse events following bariatric surgery was not increased in untreated OSA patients with low or moderate risk of severe OSA and hypoventilation.”
The study also demonstrated, he said, that patients with severe OSA with or without hypoventilation, even when correctly treated, remain at higher risk for complications.
The algorithm utilizes an oxygen desaturation index (ODI) corresponding to 3% drops in SaO2 and the percent of the total recording time with an SaO2 below 90%, as well as capillary gas measurements (PCO2). Treatment was initiated for those with severe OSA (ODI ≥ 25/hr, < 10% of recording time with a SaO2 below 90%) or OSA with hypoventilation (PCO2 ≥ 45).
“When the ODI was less than 25 per hour, and when the total recording time spent below 90% SaO2 was less than 10%, with PCO2 < 45 mmHg, we expected no need for CPAP treatment,” Dr. Series said. For analysis, the investigators considered part of the untreated group – those with an ODI < 10/hr (no or mild OSA) – as a control group.
Treated patients underwent CPAP/BiPAP for a mean duration of 1.5 months. Good treatment compliance was mandatory for surgery, and treatment was continued immediately after extubation, in the recovery room, in nearly all patients, Dr. Series reported.
The analysis covered 1,103 patients: 447 controls (40.8%), 358 untreated (32.7%), 289 treated for OSA (26.4%) and 9 (0.8%) treated for OSA + hypoventilation. Patients with OSA, particularly those with severe OSA and those with hypoventilation, were older and heavier and significantly more likely to have hypertension and diabetes than controls.
There were no differences between the four groups in 10-day reoperation or 30-day readmission occurrence, and postoperative complications were “particularly infrequent in the control and OSA-untreated groups, with no differences between these two groups,” Dr. Series said.
Cardiac arrhythmia (mainly atrial fibrillation) occurred more frequently in the OSA-treated group (2.4%) and the OSA/hypoventilation patients (11%) than in the other groups (0.5%-0.6%).
Respiratory failure occurred in about one-third of patients with hypoventilation, and admission to the ICU was “dramatically higher” in patients with hypoventilation (67%), because of respiratory failure, arrhythmia, or other unstable medical conditions, Dr. Series said.
There were no differences between the groups in the duration of surgery or the amount of anesthetic used, but the length of stay in the recovery room was significantly longer in the OSA-treated and hypoventilation groups. The length of hospital stay was also longer in these groups. Sleeve gastrectomy was the most frequent bariatric surgical procedure across all groups, including 100% of patients with hypoventilation, he noted.
Asked to comment on the study, Octavian C. Ioachimescu, MD, PhD, of Emory University in Atlanta and the Atlanta Veterans Affairs Medical Center in Decatur, said the algorithm “clearly deserves further validation in other clinical-based cohorts and longer-term outcome assessment.”
Dr. Series reported that he has no relevant disclosures. Dr. Ioachimescu also said he has no relevant disclosures.
REPORTING FROM SLEEP 2020
Nerve damage linked to prone positioning in COVID-19
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.
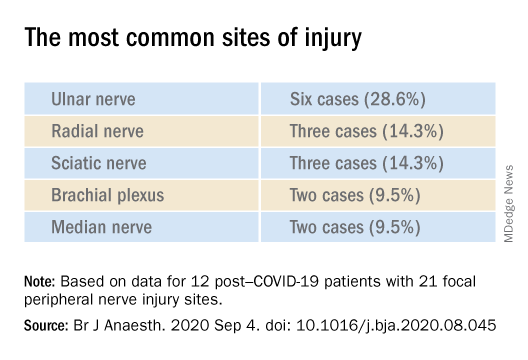
“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.

“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.

“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF ANAESTHESIOLOGY
Trump signs Medicare loan relief bill delaying repayments
President Trump on Oct. 1 signed a bill to keep the federal government running through December 11. This “continuing resolution” (CR), which was approved by the Senate Wednesday on an 84-10 vote, according to The New York Times, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under the Medicare Accelerated and Advance Payments (AAP) program, the Centers for Medicare & Medicaid Services advanced money to physicians who were financially impacted by the pandemic. The program, created in March, was suspended in late April.
Physicians who received the Medicare loans were supposed to start paying them back 120 days after they were made. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with interest of 10.25%.
For the practices that received these advances, that meant their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with interest of 4%.
More than 80% of the $100 billion that CMS loaned to healthcare providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
This article first appeared on Medscape.com.
President Trump on Oct. 1 signed a bill to keep the federal government running through December 11. This “continuing resolution” (CR), which was approved by the Senate Wednesday on an 84-10 vote, according to The New York Times, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under the Medicare Accelerated and Advance Payments (AAP) program, the Centers for Medicare & Medicaid Services advanced money to physicians who were financially impacted by the pandemic. The program, created in March, was suspended in late April.
Physicians who received the Medicare loans were supposed to start paying them back 120 days after they were made. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with interest of 10.25%.
For the practices that received these advances, that meant their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with interest of 4%.
More than 80% of the $100 billion that CMS loaned to healthcare providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
This article first appeared on Medscape.com.
President Trump on Oct. 1 signed a bill to keep the federal government running through December 11. This “continuing resolution” (CR), which was approved by the Senate Wednesday on an 84-10 vote, according to The New York Times, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under the Medicare Accelerated and Advance Payments (AAP) program, the Centers for Medicare & Medicaid Services advanced money to physicians who were financially impacted by the pandemic. The program, created in March, was suspended in late April.
Physicians who received the Medicare loans were supposed to start paying them back 120 days after they were made. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with interest of 10.25%.
For the practices that received these advances, that meant their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with interest of 4%.
More than 80% of the $100 billion that CMS loaned to healthcare providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
This article first appeared on Medscape.com.
Pandemic poses new challenges for rural doctors
These include struggling with seeing patients virtually and treating patients who have politicized the virus. Additionally, the pandemic has exposed rural practices to greater financial difficulties.
Before the pandemic some rurally based primary care physicians were already working through big challenges, such as having few local medical colleagues to consult and working in small practices with lean budgets. In fact, data gathered by the National Rural Health Association showed that there are only 40 primary care physicians per 100,000 patients in rural regions, compared with 53 in urban areas – and the number of physicians overall is 13 per 10,000 in rural areas, compared with 31 in cities.
In the prepandemic world, for some doctors, the challenges were balanced by the benefits of practicing in these sparsely populated communities with scenic, low-traffic roads. Some perks of practicing in rural areas touted by doctors included having a fast commute, being able to swim in a lake near the office before work, having a low cost of living, and feeling like they are making a difference in their communities as they treat generations of the families they see around town.
But today, new hurdles to practicing medicine in rural America created by the COVID-19 pandemic have caused the hardships to feel heavier than the joys at times for some physicians interviewed by MDedge.
Many independent rural practices in need of assistance were not able to get much from the federal Provider Relief Funds, said John M. Westfall, MD, who is director of the Robert Graham Center for Policy Studies in Family Medicine and Primary Care, in an interview.
“Rural primary care doctors function independently or in smaller critical access hospitals and community health centers,” said Dr. Westfall, who previously practiced family medicine in a small town in Colorado. “Many of these have much less financial reserves so are at risk of cutbacks and closure.”
Jacqueline W. Fincher, MD, an internist based in a tiny Georgia community along the highway between Atlanta and Augusta, said her small practice works on really thin margins and doesn’t have much cushion. At the beginning of the pandemic, all visits were down, and her practice operated at a loss. To help, Dr. Fincher and her colleagues applied for funding from the Small Business Administration’s Paycheck Protection Program (PPP) through the CARES Act.
“COVID-19 has had a tremendous impact especially on primary care practices. We live and die by volume. … Our volume in mid-March to mid-May really dropped dramatically,” explained Dr. Fincher, who is also president of the American College of Physicians. “The PPP sustained us for 2 months, enabling us to pay our staff and to remain open and get us up and running on telehealth.”
Starting up telemedicine
Experiencing spotty or no access to broadband Internet is nothing new to rural physicians, but having this problem interfere with their ability to provide care to patients is.
As much of the American health system rapidly embraced telehealth during the pandemic, obtaining access to high-speed Internet has been a major challenge for rural patients, noted Dr. Westfall.
“Some practices were able to quickly adopt some telehealth capacity with phone and video. Changes in payment for telehealth helped. But in some rural communities there was not adequate Internet bandwidth for quality video connections. And some patients did not have the means for high-speed video connections,” Dr. Westfall said.
Indeed, according to a 2019 Pew Research Center survey, 63% of rural Americans say they can access the Internet through a broadband connection at home, compared with 75% and 79% in suburban and urban areas, respectively.
In the Appalachian town of Zanesville, Ohio, for example, family physician Shelly L. Dunmyer, MD, and her colleagues discovered that many patients don’t have Internet access at home. Dr. Fincher has to go to the office to conduct telehealth visits because her own Internet access at home is unpredictable. As for patients, it may take 15 minutes for them to work out technical glitches and find good Internet reception, said Dr. Fincher. For internist Y. Ki Shin, MD, who practices in the coastal town of Montesano in Washington state, about 25% of his practice’s telehealth visits must be conducted by phone because of limitations on video, such as lack of high-speed access.
But telephone visits are often insufficient replacements for appointments via video, according to several rural physicians interviewed for this piece.
“Telehealth can be frustrating at times due to connectivity issues which can be difficult at times in the rural areas,” said Dr. Fincher. “In order for telehealth to be reasonably helpful to patients and physicians to care for people with chronic problems, the patients must have things like blood pressure monitors, glucometers, and scales to address problems like hypertension, diabetes myelitis, and congestive heart failure.”
“If you have the audio and video and the data from these devices, you’re good. If you don’t have these data, and/or don’t have the video you just can’t provide good care,” she explained.
Dr. Dunmyer and her colleagues at Medical Home Primary Care Center in Zanesville, Ohio, found a way to get around the problem of patients not being able to access Internet to participate in video visits from their homes. This involved having her patients drive into her practice’s parking lot to participate in modified telehealth visits. Staffers gave iPads to patients in their cars, and Dr. Dunmyer conducted visits from her office, about 50 yards away.
“We were even doing Medicare wellness visits: Instead of asking them to get up and move around the room, we would sit at the window and wave at them, ask them to get out, walk around the car. We were able to check mobility and all kinds of things that we’d normally do in the office,” Dr. Dunmyer explained in an interview.
The family physician noted that her practice is now conducting fewer parking lot visits since her office is allowing in-person appointments, but that they’re still an option for her patients.
Treating political adversaries
Some rural physicians have experienced strained relationships with patients for reasons other than technology – stark differences in opinion over the pandemic itself. Certain patients are following President Trump’s lead and questioning everything from the pandemic death toll to preventive measures recommended by scientists and medical experts, physicians interviewed by MDedge said.
Patients everywhere share these viewpoints, of course, but research and election results confirm that rural areas are more receptive to conservative viewpoints. In 2018, a Pew Research Center survey reported that rural and urban areas are “becoming more polarized politically,” and “rural areas tend to have a higher concentration of Republicans and Republican-leaning independents.” For example, 40% of rural respondents reported “very warm” or “somewhat warm” feelings toward Donald Trump, compared with just 19% in urban areas.
Dr. Shin has struggled to cope with patients who want to argue about pandemic safety precautions like wearing masks and seem to question whether systemic racism exists.
“We are seeing a lot more people who feel that this pandemic is not real, that it’s a political and not-true infection,” he said in an interview. “We’ve had patients who were angry at us because we made them wear masks, and some were demanding hydroxychloroquine and wanted to have an argument because we’re not going to prescribe it for them.”
In one situation, which he found especially disturbing, Dr. Shin had to leave the exam room because a patient wouldn’t stop challenging him regarding the pandemic. Things have gotten so bad that Dr. Shin has even questioned whether he wants to continue his long career in his small town because of local political attitudes such as opposition to mask-wearing and social distancing.
“Mr. Trump’s misinformation on this pandemic made my job much more difficult. As a minority, I feel less safe in my community than ever,” said Dr. Shin, who described himself as Asian American.
Despite these new stressors, Dr. Shin has experienced some joyful moments while practicing medicine in the pandemic.
He said a recent home visit to a patient who had been hospitalized for over 3 months and nearly died helped him put political disputes with his patients into perspective.
“He was discharged home but is bedbound. He had gangrene on his toes, and I could not fully examine him using video,” Dr. Shin recalled. “It was tricky to find the house, but a very large Trump sign was very helpful in locating it. It was a good visit: He was happy to see me, and I was happy to see that he was doing okay at home.”
“I need to remind myself that supporting Mr. Trump does not always mean that my patient supports Mr. Trump’s view on the pandemic and the race issues in our country,” Dr. Shin added.
The Washington-based internist said he also tells himself that, even if his patients refuse to follow his strong advice regarding pandemic precautions, it does not mean he has failed as a doctor.
“I need to continue to educate patients about the dangers of COVID infection but cannot be angry if they don’t choose to follow my recommendations,” he noted.
Dr. Fincher says her close connection with patients has allowed her to smooth over politically charged claims about the pandemic in the town of Thomson, Georgia, with a population 6,800.
“I have a sense that, even though we may differ in our understanding of some basic facts, they appreciate what I say since we have a long-term relationship built on trust,” she said. This kind of trust, Dr. Fincher suggested, may be more common than in urban areas where there’s a larger supply of physicians, and patients don’t see the same doctors for long periods of time.
“It’s more meaningful when it comes from me, rather than doctors who are [new to patients] every year when their employer changes their insurance,” she noted.
These include struggling with seeing patients virtually and treating patients who have politicized the virus. Additionally, the pandemic has exposed rural practices to greater financial difficulties.
Before the pandemic some rurally based primary care physicians were already working through big challenges, such as having few local medical colleagues to consult and working in small practices with lean budgets. In fact, data gathered by the National Rural Health Association showed that there are only 40 primary care physicians per 100,000 patients in rural regions, compared with 53 in urban areas – and the number of physicians overall is 13 per 10,000 in rural areas, compared with 31 in cities.
In the prepandemic world, for some doctors, the challenges were balanced by the benefits of practicing in these sparsely populated communities with scenic, low-traffic roads. Some perks of practicing in rural areas touted by doctors included having a fast commute, being able to swim in a lake near the office before work, having a low cost of living, and feeling like they are making a difference in their communities as they treat generations of the families they see around town.
But today, new hurdles to practicing medicine in rural America created by the COVID-19 pandemic have caused the hardships to feel heavier than the joys at times for some physicians interviewed by MDedge.
Many independent rural practices in need of assistance were not able to get much from the federal Provider Relief Funds, said John M. Westfall, MD, who is director of the Robert Graham Center for Policy Studies in Family Medicine and Primary Care, in an interview.
“Rural primary care doctors function independently or in smaller critical access hospitals and community health centers,” said Dr. Westfall, who previously practiced family medicine in a small town in Colorado. “Many of these have much less financial reserves so are at risk of cutbacks and closure.”
Jacqueline W. Fincher, MD, an internist based in a tiny Georgia community along the highway between Atlanta and Augusta, said her small practice works on really thin margins and doesn’t have much cushion. At the beginning of the pandemic, all visits were down, and her practice operated at a loss. To help, Dr. Fincher and her colleagues applied for funding from the Small Business Administration’s Paycheck Protection Program (PPP) through the CARES Act.
“COVID-19 has had a tremendous impact especially on primary care practices. We live and die by volume. … Our volume in mid-March to mid-May really dropped dramatically,” explained Dr. Fincher, who is also president of the American College of Physicians. “The PPP sustained us for 2 months, enabling us to pay our staff and to remain open and get us up and running on telehealth.”
Starting up telemedicine
Experiencing spotty or no access to broadband Internet is nothing new to rural physicians, but having this problem interfere with their ability to provide care to patients is.
As much of the American health system rapidly embraced telehealth during the pandemic, obtaining access to high-speed Internet has been a major challenge for rural patients, noted Dr. Westfall.
“Some practices were able to quickly adopt some telehealth capacity with phone and video. Changes in payment for telehealth helped. But in some rural communities there was not adequate Internet bandwidth for quality video connections. And some patients did not have the means for high-speed video connections,” Dr. Westfall said.
Indeed, according to a 2019 Pew Research Center survey, 63% of rural Americans say they can access the Internet through a broadband connection at home, compared with 75% and 79% in suburban and urban areas, respectively.
In the Appalachian town of Zanesville, Ohio, for example, family physician Shelly L. Dunmyer, MD, and her colleagues discovered that many patients don’t have Internet access at home. Dr. Fincher has to go to the office to conduct telehealth visits because her own Internet access at home is unpredictable. As for patients, it may take 15 minutes for them to work out technical glitches and find good Internet reception, said Dr. Fincher. For internist Y. Ki Shin, MD, who practices in the coastal town of Montesano in Washington state, about 25% of his practice’s telehealth visits must be conducted by phone because of limitations on video, such as lack of high-speed access.
But telephone visits are often insufficient replacements for appointments via video, according to several rural physicians interviewed for this piece.
“Telehealth can be frustrating at times due to connectivity issues which can be difficult at times in the rural areas,” said Dr. Fincher. “In order for telehealth to be reasonably helpful to patients and physicians to care for people with chronic problems, the patients must have things like blood pressure monitors, glucometers, and scales to address problems like hypertension, diabetes myelitis, and congestive heart failure.”
“If you have the audio and video and the data from these devices, you’re good. If you don’t have these data, and/or don’t have the video you just can’t provide good care,” she explained.
Dr. Dunmyer and her colleagues at Medical Home Primary Care Center in Zanesville, Ohio, found a way to get around the problem of patients not being able to access Internet to participate in video visits from their homes. This involved having her patients drive into her practice’s parking lot to participate in modified telehealth visits. Staffers gave iPads to patients in their cars, and Dr. Dunmyer conducted visits from her office, about 50 yards away.
“We were even doing Medicare wellness visits: Instead of asking them to get up and move around the room, we would sit at the window and wave at them, ask them to get out, walk around the car. We were able to check mobility and all kinds of things that we’d normally do in the office,” Dr. Dunmyer explained in an interview.
The family physician noted that her practice is now conducting fewer parking lot visits since her office is allowing in-person appointments, but that they’re still an option for her patients.
Treating political adversaries
Some rural physicians have experienced strained relationships with patients for reasons other than technology – stark differences in opinion over the pandemic itself. Certain patients are following President Trump’s lead and questioning everything from the pandemic death toll to preventive measures recommended by scientists and medical experts, physicians interviewed by MDedge said.
Patients everywhere share these viewpoints, of course, but research and election results confirm that rural areas are more receptive to conservative viewpoints. In 2018, a Pew Research Center survey reported that rural and urban areas are “becoming more polarized politically,” and “rural areas tend to have a higher concentration of Republicans and Republican-leaning independents.” For example, 40% of rural respondents reported “very warm” or “somewhat warm” feelings toward Donald Trump, compared with just 19% in urban areas.
Dr. Shin has struggled to cope with patients who want to argue about pandemic safety precautions like wearing masks and seem to question whether systemic racism exists.
“We are seeing a lot more people who feel that this pandemic is not real, that it’s a political and not-true infection,” he said in an interview. “We’ve had patients who were angry at us because we made them wear masks, and some were demanding hydroxychloroquine and wanted to have an argument because we’re not going to prescribe it for them.”
In one situation, which he found especially disturbing, Dr. Shin had to leave the exam room because a patient wouldn’t stop challenging him regarding the pandemic. Things have gotten so bad that Dr. Shin has even questioned whether he wants to continue his long career in his small town because of local political attitudes such as opposition to mask-wearing and social distancing.
“Mr. Trump’s misinformation on this pandemic made my job much more difficult. As a minority, I feel less safe in my community than ever,” said Dr. Shin, who described himself as Asian American.
Despite these new stressors, Dr. Shin has experienced some joyful moments while practicing medicine in the pandemic.
He said a recent home visit to a patient who had been hospitalized for over 3 months and nearly died helped him put political disputes with his patients into perspective.
“He was discharged home but is bedbound. He had gangrene on his toes, and I could not fully examine him using video,” Dr. Shin recalled. “It was tricky to find the house, but a very large Trump sign was very helpful in locating it. It was a good visit: He was happy to see me, and I was happy to see that he was doing okay at home.”
“I need to remind myself that supporting Mr. Trump does not always mean that my patient supports Mr. Trump’s view on the pandemic and the race issues in our country,” Dr. Shin added.
The Washington-based internist said he also tells himself that, even if his patients refuse to follow his strong advice regarding pandemic precautions, it does not mean he has failed as a doctor.
“I need to continue to educate patients about the dangers of COVID infection but cannot be angry if they don’t choose to follow my recommendations,” he noted.
Dr. Fincher says her close connection with patients has allowed her to smooth over politically charged claims about the pandemic in the town of Thomson, Georgia, with a population 6,800.
“I have a sense that, even though we may differ in our understanding of some basic facts, they appreciate what I say since we have a long-term relationship built on trust,” she said. This kind of trust, Dr. Fincher suggested, may be more common than in urban areas where there’s a larger supply of physicians, and patients don’t see the same doctors for long periods of time.
“It’s more meaningful when it comes from me, rather than doctors who are [new to patients] every year when their employer changes their insurance,” she noted.
These include struggling with seeing patients virtually and treating patients who have politicized the virus. Additionally, the pandemic has exposed rural practices to greater financial difficulties.
Before the pandemic some rurally based primary care physicians were already working through big challenges, such as having few local medical colleagues to consult and working in small practices with lean budgets. In fact, data gathered by the National Rural Health Association showed that there are only 40 primary care physicians per 100,000 patients in rural regions, compared with 53 in urban areas – and the number of physicians overall is 13 per 10,000 in rural areas, compared with 31 in cities.
In the prepandemic world, for some doctors, the challenges were balanced by the benefits of practicing in these sparsely populated communities with scenic, low-traffic roads. Some perks of practicing in rural areas touted by doctors included having a fast commute, being able to swim in a lake near the office before work, having a low cost of living, and feeling like they are making a difference in their communities as they treat generations of the families they see around town.
But today, new hurdles to practicing medicine in rural America created by the COVID-19 pandemic have caused the hardships to feel heavier than the joys at times for some physicians interviewed by MDedge.
Many independent rural practices in need of assistance were not able to get much from the federal Provider Relief Funds, said John M. Westfall, MD, who is director of the Robert Graham Center for Policy Studies in Family Medicine and Primary Care, in an interview.
“Rural primary care doctors function independently or in smaller critical access hospitals and community health centers,” said Dr. Westfall, who previously practiced family medicine in a small town in Colorado. “Many of these have much less financial reserves so are at risk of cutbacks and closure.”
Jacqueline W. Fincher, MD, an internist based in a tiny Georgia community along the highway between Atlanta and Augusta, said her small practice works on really thin margins and doesn’t have much cushion. At the beginning of the pandemic, all visits were down, and her practice operated at a loss. To help, Dr. Fincher and her colleagues applied for funding from the Small Business Administration’s Paycheck Protection Program (PPP) through the CARES Act.
“COVID-19 has had a tremendous impact especially on primary care practices. We live and die by volume. … Our volume in mid-March to mid-May really dropped dramatically,” explained Dr. Fincher, who is also president of the American College of Physicians. “The PPP sustained us for 2 months, enabling us to pay our staff and to remain open and get us up and running on telehealth.”
Starting up telemedicine
Experiencing spotty or no access to broadband Internet is nothing new to rural physicians, but having this problem interfere with their ability to provide care to patients is.
As much of the American health system rapidly embraced telehealth during the pandemic, obtaining access to high-speed Internet has been a major challenge for rural patients, noted Dr. Westfall.
“Some practices were able to quickly adopt some telehealth capacity with phone and video. Changes in payment for telehealth helped. But in some rural communities there was not adequate Internet bandwidth for quality video connections. And some patients did not have the means for high-speed video connections,” Dr. Westfall said.
Indeed, according to a 2019 Pew Research Center survey, 63% of rural Americans say they can access the Internet through a broadband connection at home, compared with 75% and 79% in suburban and urban areas, respectively.
In the Appalachian town of Zanesville, Ohio, for example, family physician Shelly L. Dunmyer, MD, and her colleagues discovered that many patients don’t have Internet access at home. Dr. Fincher has to go to the office to conduct telehealth visits because her own Internet access at home is unpredictable. As for patients, it may take 15 minutes for them to work out technical glitches and find good Internet reception, said Dr. Fincher. For internist Y. Ki Shin, MD, who practices in the coastal town of Montesano in Washington state, about 25% of his practice’s telehealth visits must be conducted by phone because of limitations on video, such as lack of high-speed access.
But telephone visits are often insufficient replacements for appointments via video, according to several rural physicians interviewed for this piece.
“Telehealth can be frustrating at times due to connectivity issues which can be difficult at times in the rural areas,” said Dr. Fincher. “In order for telehealth to be reasonably helpful to patients and physicians to care for people with chronic problems, the patients must have things like blood pressure monitors, glucometers, and scales to address problems like hypertension, diabetes myelitis, and congestive heart failure.”
“If you have the audio and video and the data from these devices, you’re good. If you don’t have these data, and/or don’t have the video you just can’t provide good care,” she explained.
Dr. Dunmyer and her colleagues at Medical Home Primary Care Center in Zanesville, Ohio, found a way to get around the problem of patients not being able to access Internet to participate in video visits from their homes. This involved having her patients drive into her practice’s parking lot to participate in modified telehealth visits. Staffers gave iPads to patients in their cars, and Dr. Dunmyer conducted visits from her office, about 50 yards away.
“We were even doing Medicare wellness visits: Instead of asking them to get up and move around the room, we would sit at the window and wave at them, ask them to get out, walk around the car. We were able to check mobility and all kinds of things that we’d normally do in the office,” Dr. Dunmyer explained in an interview.
The family physician noted that her practice is now conducting fewer parking lot visits since her office is allowing in-person appointments, but that they’re still an option for her patients.
Treating political adversaries
Some rural physicians have experienced strained relationships with patients for reasons other than technology – stark differences in opinion over the pandemic itself. Certain patients are following President Trump’s lead and questioning everything from the pandemic death toll to preventive measures recommended by scientists and medical experts, physicians interviewed by MDedge said.
Patients everywhere share these viewpoints, of course, but research and election results confirm that rural areas are more receptive to conservative viewpoints. In 2018, a Pew Research Center survey reported that rural and urban areas are “becoming more polarized politically,” and “rural areas tend to have a higher concentration of Republicans and Republican-leaning independents.” For example, 40% of rural respondents reported “very warm” or “somewhat warm” feelings toward Donald Trump, compared with just 19% in urban areas.
Dr. Shin has struggled to cope with patients who want to argue about pandemic safety precautions like wearing masks and seem to question whether systemic racism exists.
“We are seeing a lot more people who feel that this pandemic is not real, that it’s a political and not-true infection,” he said in an interview. “We’ve had patients who were angry at us because we made them wear masks, and some were demanding hydroxychloroquine and wanted to have an argument because we’re not going to prescribe it for them.”
In one situation, which he found especially disturbing, Dr. Shin had to leave the exam room because a patient wouldn’t stop challenging him regarding the pandemic. Things have gotten so bad that Dr. Shin has even questioned whether he wants to continue his long career in his small town because of local political attitudes such as opposition to mask-wearing and social distancing.
“Mr. Trump’s misinformation on this pandemic made my job much more difficult. As a minority, I feel less safe in my community than ever,” said Dr. Shin, who described himself as Asian American.
Despite these new stressors, Dr. Shin has experienced some joyful moments while practicing medicine in the pandemic.
He said a recent home visit to a patient who had been hospitalized for over 3 months and nearly died helped him put political disputes with his patients into perspective.
“He was discharged home but is bedbound. He had gangrene on his toes, and I could not fully examine him using video,” Dr. Shin recalled. “It was tricky to find the house, but a very large Trump sign was very helpful in locating it. It was a good visit: He was happy to see me, and I was happy to see that he was doing okay at home.”
“I need to remind myself that supporting Mr. Trump does not always mean that my patient supports Mr. Trump’s view on the pandemic and the race issues in our country,” Dr. Shin added.
The Washington-based internist said he also tells himself that, even if his patients refuse to follow his strong advice regarding pandemic precautions, it does not mean he has failed as a doctor.
“I need to continue to educate patients about the dangers of COVID infection but cannot be angry if they don’t choose to follow my recommendations,” he noted.
Dr. Fincher says her close connection with patients has allowed her to smooth over politically charged claims about the pandemic in the town of Thomson, Georgia, with a population 6,800.
“I have a sense that, even though we may differ in our understanding of some basic facts, they appreciate what I say since we have a long-term relationship built on trust,” she said. This kind of trust, Dr. Fincher suggested, may be more common than in urban areas where there’s a larger supply of physicians, and patients don’t see the same doctors for long periods of time.
“It’s more meaningful when it comes from me, rather than doctors who are [new to patients] every year when their employer changes their insurance,” she noted.
Predicting complications of cytoreductive nephrectomy in metastatic RCC
according to an analysis of registry data.
Higher intraoperative blood loss was also associated with low-grade postoperative complications. Intraoperative complications were more likely in patients who had concurrent thrombectomy and surgery on adjacent organs.
Eduard Roussel, MD, of University Hospitals Leuven (Belgium) and colleagues reported these findings in European Urology Oncology.
The authors noted that the role of CN in metastatic RCC is controversial. The CARMENA trial, published in the New England Journal of Medicine, “shifted treatment paradigms” away from surgery by suggesting that sunitinib alone is noninferior to sunitinib after CN.
“Nonetheless, there is a general consensus that certain selected subgroups of patients with low-volume, single-site metastases and few adverse IMDC [International Metastatic Renal Cell Carcinoma Database Consortium] criteria would still benefit from the continued use of upfront CN,” the authors wrote.
They advised clinicians to weigh the risks and benefits of CN, particularly because “postoperative morbidity might preclude or delay the use of subsequent systemic therapies.” However, the risk/benefit calculation for CN has been difficult because past investigations have tended to focus only on survival outcomes, so there isn’t much data on morbidity, the authors wrote.
Patient characteristics and complications
To investigate morbidity associated with CN, Dr. Roussel and colleagues reviewed data from the Registry of Metastatic RCC (REMARCC). The team analyzed the clinical records of 736 patients who underwent nephrectomy for metastatic RCC during 1980-2019.
The patients’ median age was 63 years (range, 55-70 years), and about three-quarters were men. The majority had clear cell carcinoma, and the lungs were the most common site of metastases.
More than 97% of procedures were complete nephrectomies, and the rest were partial. The median estimated blood loss was 400 mL, and the median follow-up was 16.5 months.
There were 69 patients who had intraoperative complications, most commonly bleeding (n = 25), spleen laceration (n = 13), and vascular injury (n = 11).
There were 217 patients who had postoperative complications, including 45 patients with high-grade complications (grade 3-5) and 10 who died.
The most common postoperative complications were vascular/lymphatic in nature. These occurred in 67 patients and included 10 lymphoceles.
“[G]iven the relatively high rate of postoperative lymphoceles, which is probably attributable to the performance of lymph node dissections and the lack of proven oncological survival benefit thereof, surgeons might reconsider the performance of lymphadenectomy during CN,” the investigators wrote.
Other common postoperative complications included infectious and cardiopulmonary issues, which occurred in 42 and 39 patients, respectively.
Predictors of complications
Thrombectomy and adjacent organ removal were significant predictors of intraoperative complications on multivariable analysis. The odds ratios were 1.38 (P = .009) for thrombectomy and 2.71 (P = .004) for adjacent organ removal.
Estimated blood loss was a significant predictor of low- and high-grade postoperative complications. The OR for high-grade complications per 200 mL of blood lost was 1.06 (P = .007), and the OR for low-grade complications per 200 mL blood lost was 1.09 (P = .001).
There was a strong inverse correlation with CN case load at each center and high-grade postoperative complications, which highlights “the impact of centralization of care on postoperative outcomes in complex surgical scenarios,” the investigators wrote. The OR per 25 cases was 0.88 (P = .04).
Results were largely the same when the analysis was limited to the 560 subjects treated since 2006, in the targeted therapy era, except that adjacent organ removal as a predictor of intraoperative complications did not quite reach statistical significance (P = .06).
The presurgery risk factors identified in this study should assist with presurgical counseling, said Zachery Reichert, MD, PhD, a genitourinary medical oncologist and assistant professor at the University of Michigan, Ann Arbor, who was not involved in this study.
“This is especially important for patients who require thrombectomy or adjacent organ removal or do not have access to a surgeon with high cytoreductive nephrectomy caseloads,” he said.
Dr. Reichert reported having no disclosures. There was no external funding for this study, and the investigators didn’t have any disclosures.
SOURCE: Roussel E et al. Eur Urol Oncol. 2020 Aug;3(4):523-9.
according to an analysis of registry data.
Higher intraoperative blood loss was also associated with low-grade postoperative complications. Intraoperative complications were more likely in patients who had concurrent thrombectomy and surgery on adjacent organs.
Eduard Roussel, MD, of University Hospitals Leuven (Belgium) and colleagues reported these findings in European Urology Oncology.
The authors noted that the role of CN in metastatic RCC is controversial. The CARMENA trial, published in the New England Journal of Medicine, “shifted treatment paradigms” away from surgery by suggesting that sunitinib alone is noninferior to sunitinib after CN.
“Nonetheless, there is a general consensus that certain selected subgroups of patients with low-volume, single-site metastases and few adverse IMDC [International Metastatic Renal Cell Carcinoma Database Consortium] criteria would still benefit from the continued use of upfront CN,” the authors wrote.
They advised clinicians to weigh the risks and benefits of CN, particularly because “postoperative morbidity might preclude or delay the use of subsequent systemic therapies.” However, the risk/benefit calculation for CN has been difficult because past investigations have tended to focus only on survival outcomes, so there isn’t much data on morbidity, the authors wrote.
Patient characteristics and complications
To investigate morbidity associated with CN, Dr. Roussel and colleagues reviewed data from the Registry of Metastatic RCC (REMARCC). The team analyzed the clinical records of 736 patients who underwent nephrectomy for metastatic RCC during 1980-2019.
The patients’ median age was 63 years (range, 55-70 years), and about three-quarters were men. The majority had clear cell carcinoma, and the lungs were the most common site of metastases.
More than 97% of procedures were complete nephrectomies, and the rest were partial. The median estimated blood loss was 400 mL, and the median follow-up was 16.5 months.
There were 69 patients who had intraoperative complications, most commonly bleeding (n = 25), spleen laceration (n = 13), and vascular injury (n = 11).
There were 217 patients who had postoperative complications, including 45 patients with high-grade complications (grade 3-5) and 10 who died.
The most common postoperative complications were vascular/lymphatic in nature. These occurred in 67 patients and included 10 lymphoceles.
“[G]iven the relatively high rate of postoperative lymphoceles, which is probably attributable to the performance of lymph node dissections and the lack of proven oncological survival benefit thereof, surgeons might reconsider the performance of lymphadenectomy during CN,” the investigators wrote.
Other common postoperative complications included infectious and cardiopulmonary issues, which occurred in 42 and 39 patients, respectively.
Predictors of complications
Thrombectomy and adjacent organ removal were significant predictors of intraoperative complications on multivariable analysis. The odds ratios were 1.38 (P = .009) for thrombectomy and 2.71 (P = .004) for adjacent organ removal.
Estimated blood loss was a significant predictor of low- and high-grade postoperative complications. The OR for high-grade complications per 200 mL of blood lost was 1.06 (P = .007), and the OR for low-grade complications per 200 mL blood lost was 1.09 (P = .001).
There was a strong inverse correlation with CN case load at each center and high-grade postoperative complications, which highlights “the impact of centralization of care on postoperative outcomes in complex surgical scenarios,” the investigators wrote. The OR per 25 cases was 0.88 (P = .04).
Results were largely the same when the analysis was limited to the 560 subjects treated since 2006, in the targeted therapy era, except that adjacent organ removal as a predictor of intraoperative complications did not quite reach statistical significance (P = .06).
The presurgery risk factors identified in this study should assist with presurgical counseling, said Zachery Reichert, MD, PhD, a genitourinary medical oncologist and assistant professor at the University of Michigan, Ann Arbor, who was not involved in this study.
“This is especially important for patients who require thrombectomy or adjacent organ removal or do not have access to a surgeon with high cytoreductive nephrectomy caseloads,” he said.
Dr. Reichert reported having no disclosures. There was no external funding for this study, and the investigators didn’t have any disclosures.
SOURCE: Roussel E et al. Eur Urol Oncol. 2020 Aug;3(4):523-9.
according to an analysis of registry data.
Higher intraoperative blood loss was also associated with low-grade postoperative complications. Intraoperative complications were more likely in patients who had concurrent thrombectomy and surgery on adjacent organs.
Eduard Roussel, MD, of University Hospitals Leuven (Belgium) and colleagues reported these findings in European Urology Oncology.
The authors noted that the role of CN in metastatic RCC is controversial. The CARMENA trial, published in the New England Journal of Medicine, “shifted treatment paradigms” away from surgery by suggesting that sunitinib alone is noninferior to sunitinib after CN.
“Nonetheless, there is a general consensus that certain selected subgroups of patients with low-volume, single-site metastases and few adverse IMDC [International Metastatic Renal Cell Carcinoma Database Consortium] criteria would still benefit from the continued use of upfront CN,” the authors wrote.
They advised clinicians to weigh the risks and benefits of CN, particularly because “postoperative morbidity might preclude or delay the use of subsequent systemic therapies.” However, the risk/benefit calculation for CN has been difficult because past investigations have tended to focus only on survival outcomes, so there isn’t much data on morbidity, the authors wrote.
Patient characteristics and complications
To investigate morbidity associated with CN, Dr. Roussel and colleagues reviewed data from the Registry of Metastatic RCC (REMARCC). The team analyzed the clinical records of 736 patients who underwent nephrectomy for metastatic RCC during 1980-2019.
The patients’ median age was 63 years (range, 55-70 years), and about three-quarters were men. The majority had clear cell carcinoma, and the lungs were the most common site of metastases.
More than 97% of procedures were complete nephrectomies, and the rest were partial. The median estimated blood loss was 400 mL, and the median follow-up was 16.5 months.
There were 69 patients who had intraoperative complications, most commonly bleeding (n = 25), spleen laceration (n = 13), and vascular injury (n = 11).
There were 217 patients who had postoperative complications, including 45 patients with high-grade complications (grade 3-5) and 10 who died.
The most common postoperative complications were vascular/lymphatic in nature. These occurred in 67 patients and included 10 lymphoceles.
“[G]iven the relatively high rate of postoperative lymphoceles, which is probably attributable to the performance of lymph node dissections and the lack of proven oncological survival benefit thereof, surgeons might reconsider the performance of lymphadenectomy during CN,” the investigators wrote.
Other common postoperative complications included infectious and cardiopulmonary issues, which occurred in 42 and 39 patients, respectively.
Predictors of complications
Thrombectomy and adjacent organ removal were significant predictors of intraoperative complications on multivariable analysis. The odds ratios were 1.38 (P = .009) for thrombectomy and 2.71 (P = .004) for adjacent organ removal.
Estimated blood loss was a significant predictor of low- and high-grade postoperative complications. The OR for high-grade complications per 200 mL of blood lost was 1.06 (P = .007), and the OR for low-grade complications per 200 mL blood lost was 1.09 (P = .001).
There was a strong inverse correlation with CN case load at each center and high-grade postoperative complications, which highlights “the impact of centralization of care on postoperative outcomes in complex surgical scenarios,” the investigators wrote. The OR per 25 cases was 0.88 (P = .04).
Results were largely the same when the analysis was limited to the 560 subjects treated since 2006, in the targeted therapy era, except that adjacent organ removal as a predictor of intraoperative complications did not quite reach statistical significance (P = .06).
The presurgery risk factors identified in this study should assist with presurgical counseling, said Zachery Reichert, MD, PhD, a genitourinary medical oncologist and assistant professor at the University of Michigan, Ann Arbor, who was not involved in this study.
“This is especially important for patients who require thrombectomy or adjacent organ removal or do not have access to a surgeon with high cytoreductive nephrectomy caseloads,” he said.
Dr. Reichert reported having no disclosures. There was no external funding for this study, and the investigators didn’t have any disclosures.
SOURCE: Roussel E et al. Eur Urol Oncol. 2020 Aug;3(4):523-9.
FROM EUROPEAN UROLOGY ONCOLOGY