User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Hypofractionated radiotherapy for prostate cancer stands the test of time
SAN FRANCISCO – an update of the CHHiP trial shows.
The 3,216 men in the phase 3 trial had node-negative T1b-T3a prostate cancer and were evenly assigned to a conventional regimen of 74 Gy delivered in 37 fractions, a hypofractionated regimen of 60 Gy in 20 fractions, or a hypofractionated regimen of 57 Gy in 19 fractions. All regimens were delivered with intensity-modulated techniques.
The trial’s 5-year results, previously reported, showed noninferiority of the 60-Gy regimen, compared with the 74-Gy regimen on risk of biochemical or clinical failure (hazard ratio, 0.84), prompting recommendation of the former as a new standard of care for localized prostate cancer (Lancet Oncol. 2016;17:1047-60). Noninferiority could not be established for the 57-Gy regimen.
The 8-year results were essentially the same, confirming noninferiority of the 60-Gy regimen (HR, 0.85) but not the 57-Gy regimen. Meanwhile, bowel and bladder toxicity continued to be low across regimens.
David P. Dearnaley, MB BCh, MD, of the Royal Marsden NHS Foundation Trust, London, reported the 8-year results at the 2020 Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Study details
At a median follow-up of 9.3 years, the 8-year rate of freedom from biochemical failure (defined by Phoenix consensus guidelines) or clinical failure (cancer recurrence) was 80.6% with 74 Gy, 83.7% with 60 Gy, and 78.5% with 57 Gy, Dr. Dearnaley reported.
Analyses confirmed noninferiority of the 60-Gy regimen (HR, 0.85; 95% confidence interval, 0.72-1.01; P = .11), but not the 57-Gy regimen (HR, 1.17; 95% CI, 1.00-1.36; P = .10), as the upper bound of the confidence interval crossed the predefined 1.21 boundary for noninferiority.
In an unplanned analysis, the pattern among men younger than 75 years was similar to that in the entire trial population. But among men 75 years of age and older, the 57-Gy arm is actually outperforming the 74-Gy arm (HR, 0.77).
The three regimens yielded a similarly high rate of freedom from metastases, at about 95% in each arm. The 60-Gy regimen had an edge in overall survival relative to the 74-Gy regimen (88.6% vs. 85.9%; HR, 0.84) that is hard to explain, according to Dr. Dearnaley.
“Because there is an 8:1 ratio of non–prostate cancer deaths to prostate cancer deaths, you would have to postulate something other than prostate cancer being affected by the radiotherapy fractionation,” he said. “The answers on a postcard, because I can’t think of one.”
On central pathology review, nearly a fifth of evaluated trial patients had high-risk disease. “I know everybody wants to know about high-risk patients, but I’d rather take the trial results as a whole and look to see if there is any heterogeneity between those groups rather than perform a specific high-risk subgroup analysis,” Dr. Dearnaley said, expressing concern about performing too many subgroup analyses.
That said, older patients on the trial tended to have higher risk. “It does seem hypofractionation was particularly useful in those patients,” he noted. “Now, whether that’s anything to do with their pathology or whether it’s due to their age per se, I really don’t know.”
There were no differences between groups on rates of Radiation Therapy Oncology Group toxicity at 5 years, with grade 2 or worse bowel toxicity and bladder toxicity each seen in about 2% of patients.
There were no significant differences in rates of patient-reported “moderate or big” bowel bother (roughly 5%-8%) and urinary bother (roughly 7%-9%). For all regimens, bowel and urinary symptoms remained stable from 2-5 years.
Reassuring for practice
These updated findings “support the continued use of 60 Gy in 20 fractions as the standard of care,” Dr. Dearnaley said.
When the math is run to permit comparison, efficacy findings of the CHHiP trial show “amazing agreement” with those of the similar multinational PROFIT trial, he noted (J Clin Oncol. 2017 Jun 10;35(17):1884-90).
The absolute advantage in the failure-free rate of 3.1% and the overall survival rate of 2.7% for the 60-Gy regimen in CHHiP generated interest among symposium attendees about its possible superiority. “I think the 60 Gy is marginally more effective than the 74 Gy,” Dr. Dearnaley said, but he acknowledged that there are no statistics to prove that.
“This CHHiP update is fantastic,” said session cochair Paul L. Nguyen, MD, of the Dana-Farber Cancer Institute in Boston. “It is very reassuring that the initial results the investigators presented several years ago still hold up in the long term. It’s even more reassuring for the use of hypofractionation, and it’s great to know that we can use it across the age spectrum and it works well.”
This trial is the only noninferiority hypofractionation trial in prostate cancer that includes a sizable share of patients at high risk for poor outcomes, a population for whom efficacy of this strategy is of particular interest, Dr. Nguyen noted.
“That’s always been a question,” he said. “The majority of the data from the noninferiority trials is for the low- and intermediate-risk patients. So it really would be interesting to learn whatever we can about high-risk patients from this trial.”
The trial was funded by Cancer Research UK, Department of Health (UK), and the National Institute for Health Research Cancer Research Network. Dr. Dearnaley and Dr. Nguyen disclosed relationships with a range of pharmaceutical companies.
SOURCE: Dearnaley DP et al. GUCS 2020. Abstract 325.
SAN FRANCISCO – an update of the CHHiP trial shows.
The 3,216 men in the phase 3 trial had node-negative T1b-T3a prostate cancer and were evenly assigned to a conventional regimen of 74 Gy delivered in 37 fractions, a hypofractionated regimen of 60 Gy in 20 fractions, or a hypofractionated regimen of 57 Gy in 19 fractions. All regimens were delivered with intensity-modulated techniques.
The trial’s 5-year results, previously reported, showed noninferiority of the 60-Gy regimen, compared with the 74-Gy regimen on risk of biochemical or clinical failure (hazard ratio, 0.84), prompting recommendation of the former as a new standard of care for localized prostate cancer (Lancet Oncol. 2016;17:1047-60). Noninferiority could not be established for the 57-Gy regimen.
The 8-year results were essentially the same, confirming noninferiority of the 60-Gy regimen (HR, 0.85) but not the 57-Gy regimen. Meanwhile, bowel and bladder toxicity continued to be low across regimens.
David P. Dearnaley, MB BCh, MD, of the Royal Marsden NHS Foundation Trust, London, reported the 8-year results at the 2020 Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Study details
At a median follow-up of 9.3 years, the 8-year rate of freedom from biochemical failure (defined by Phoenix consensus guidelines) or clinical failure (cancer recurrence) was 80.6% with 74 Gy, 83.7% with 60 Gy, and 78.5% with 57 Gy, Dr. Dearnaley reported.
Analyses confirmed noninferiority of the 60-Gy regimen (HR, 0.85; 95% confidence interval, 0.72-1.01; P = .11), but not the 57-Gy regimen (HR, 1.17; 95% CI, 1.00-1.36; P = .10), as the upper bound of the confidence interval crossed the predefined 1.21 boundary for noninferiority.
In an unplanned analysis, the pattern among men younger than 75 years was similar to that in the entire trial population. But among men 75 years of age and older, the 57-Gy arm is actually outperforming the 74-Gy arm (HR, 0.77).
The three regimens yielded a similarly high rate of freedom from metastases, at about 95% in each arm. The 60-Gy regimen had an edge in overall survival relative to the 74-Gy regimen (88.6% vs. 85.9%; HR, 0.84) that is hard to explain, according to Dr. Dearnaley.
“Because there is an 8:1 ratio of non–prostate cancer deaths to prostate cancer deaths, you would have to postulate something other than prostate cancer being affected by the radiotherapy fractionation,” he said. “The answers on a postcard, because I can’t think of one.”
On central pathology review, nearly a fifth of evaluated trial patients had high-risk disease. “I know everybody wants to know about high-risk patients, but I’d rather take the trial results as a whole and look to see if there is any heterogeneity between those groups rather than perform a specific high-risk subgroup analysis,” Dr. Dearnaley said, expressing concern about performing too many subgroup analyses.
That said, older patients on the trial tended to have higher risk. “It does seem hypofractionation was particularly useful in those patients,” he noted. “Now, whether that’s anything to do with their pathology or whether it’s due to their age per se, I really don’t know.”
There were no differences between groups on rates of Radiation Therapy Oncology Group toxicity at 5 years, with grade 2 or worse bowel toxicity and bladder toxicity each seen in about 2% of patients.
There were no significant differences in rates of patient-reported “moderate or big” bowel bother (roughly 5%-8%) and urinary bother (roughly 7%-9%). For all regimens, bowel and urinary symptoms remained stable from 2-5 years.
Reassuring for practice
These updated findings “support the continued use of 60 Gy in 20 fractions as the standard of care,” Dr. Dearnaley said.
When the math is run to permit comparison, efficacy findings of the CHHiP trial show “amazing agreement” with those of the similar multinational PROFIT trial, he noted (J Clin Oncol. 2017 Jun 10;35(17):1884-90).
The absolute advantage in the failure-free rate of 3.1% and the overall survival rate of 2.7% for the 60-Gy regimen in CHHiP generated interest among symposium attendees about its possible superiority. “I think the 60 Gy is marginally more effective than the 74 Gy,” Dr. Dearnaley said, but he acknowledged that there are no statistics to prove that.
“This CHHiP update is fantastic,” said session cochair Paul L. Nguyen, MD, of the Dana-Farber Cancer Institute in Boston. “It is very reassuring that the initial results the investigators presented several years ago still hold up in the long term. It’s even more reassuring for the use of hypofractionation, and it’s great to know that we can use it across the age spectrum and it works well.”
This trial is the only noninferiority hypofractionation trial in prostate cancer that includes a sizable share of patients at high risk for poor outcomes, a population for whom efficacy of this strategy is of particular interest, Dr. Nguyen noted.
“That’s always been a question,” he said. “The majority of the data from the noninferiority trials is for the low- and intermediate-risk patients. So it really would be interesting to learn whatever we can about high-risk patients from this trial.”
The trial was funded by Cancer Research UK, Department of Health (UK), and the National Institute for Health Research Cancer Research Network. Dr. Dearnaley and Dr. Nguyen disclosed relationships with a range of pharmaceutical companies.
SOURCE: Dearnaley DP et al. GUCS 2020. Abstract 325.
SAN FRANCISCO – an update of the CHHiP trial shows.
The 3,216 men in the phase 3 trial had node-negative T1b-T3a prostate cancer and were evenly assigned to a conventional regimen of 74 Gy delivered in 37 fractions, a hypofractionated regimen of 60 Gy in 20 fractions, or a hypofractionated regimen of 57 Gy in 19 fractions. All regimens were delivered with intensity-modulated techniques.
The trial’s 5-year results, previously reported, showed noninferiority of the 60-Gy regimen, compared with the 74-Gy regimen on risk of biochemical or clinical failure (hazard ratio, 0.84), prompting recommendation of the former as a new standard of care for localized prostate cancer (Lancet Oncol. 2016;17:1047-60). Noninferiority could not be established for the 57-Gy regimen.
The 8-year results were essentially the same, confirming noninferiority of the 60-Gy regimen (HR, 0.85) but not the 57-Gy regimen. Meanwhile, bowel and bladder toxicity continued to be low across regimens.
David P. Dearnaley, MB BCh, MD, of the Royal Marsden NHS Foundation Trust, London, reported the 8-year results at the 2020 Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
Study details
At a median follow-up of 9.3 years, the 8-year rate of freedom from biochemical failure (defined by Phoenix consensus guidelines) or clinical failure (cancer recurrence) was 80.6% with 74 Gy, 83.7% with 60 Gy, and 78.5% with 57 Gy, Dr. Dearnaley reported.
Analyses confirmed noninferiority of the 60-Gy regimen (HR, 0.85; 95% confidence interval, 0.72-1.01; P = .11), but not the 57-Gy regimen (HR, 1.17; 95% CI, 1.00-1.36; P = .10), as the upper bound of the confidence interval crossed the predefined 1.21 boundary for noninferiority.
In an unplanned analysis, the pattern among men younger than 75 years was similar to that in the entire trial population. But among men 75 years of age and older, the 57-Gy arm is actually outperforming the 74-Gy arm (HR, 0.77).
The three regimens yielded a similarly high rate of freedom from metastases, at about 95% in each arm. The 60-Gy regimen had an edge in overall survival relative to the 74-Gy regimen (88.6% vs. 85.9%; HR, 0.84) that is hard to explain, according to Dr. Dearnaley.
“Because there is an 8:1 ratio of non–prostate cancer deaths to prostate cancer deaths, you would have to postulate something other than prostate cancer being affected by the radiotherapy fractionation,” he said. “The answers on a postcard, because I can’t think of one.”
On central pathology review, nearly a fifth of evaluated trial patients had high-risk disease. “I know everybody wants to know about high-risk patients, but I’d rather take the trial results as a whole and look to see if there is any heterogeneity between those groups rather than perform a specific high-risk subgroup analysis,” Dr. Dearnaley said, expressing concern about performing too many subgroup analyses.
That said, older patients on the trial tended to have higher risk. “It does seem hypofractionation was particularly useful in those patients,” he noted. “Now, whether that’s anything to do with their pathology or whether it’s due to their age per se, I really don’t know.”
There were no differences between groups on rates of Radiation Therapy Oncology Group toxicity at 5 years, with grade 2 or worse bowel toxicity and bladder toxicity each seen in about 2% of patients.
There were no significant differences in rates of patient-reported “moderate or big” bowel bother (roughly 5%-8%) and urinary bother (roughly 7%-9%). For all regimens, bowel and urinary symptoms remained stable from 2-5 years.
Reassuring for practice
These updated findings “support the continued use of 60 Gy in 20 fractions as the standard of care,” Dr. Dearnaley said.
When the math is run to permit comparison, efficacy findings of the CHHiP trial show “amazing agreement” with those of the similar multinational PROFIT trial, he noted (J Clin Oncol. 2017 Jun 10;35(17):1884-90).
The absolute advantage in the failure-free rate of 3.1% and the overall survival rate of 2.7% for the 60-Gy regimen in CHHiP generated interest among symposium attendees about its possible superiority. “I think the 60 Gy is marginally more effective than the 74 Gy,” Dr. Dearnaley said, but he acknowledged that there are no statistics to prove that.
“This CHHiP update is fantastic,” said session cochair Paul L. Nguyen, MD, of the Dana-Farber Cancer Institute in Boston. “It is very reassuring that the initial results the investigators presented several years ago still hold up in the long term. It’s even more reassuring for the use of hypofractionation, and it’s great to know that we can use it across the age spectrum and it works well.”
This trial is the only noninferiority hypofractionation trial in prostate cancer that includes a sizable share of patients at high risk for poor outcomes, a population for whom efficacy of this strategy is of particular interest, Dr. Nguyen noted.
“That’s always been a question,” he said. “The majority of the data from the noninferiority trials is for the low- and intermediate-risk patients. So it really would be interesting to learn whatever we can about high-risk patients from this trial.”
The trial was funded by Cancer Research UK, Department of Health (UK), and the National Institute for Health Research Cancer Research Network. Dr. Dearnaley and Dr. Nguyen disclosed relationships with a range of pharmaceutical companies.
SOURCE: Dearnaley DP et al. GUCS 2020. Abstract 325.
REPORTING FROM GUCS 2020
As novel coronavirus outbreak evolves, critical care providers need to be prepared
ORLANDO – While the impact of the 2019 novel coronavirus outbreak on hospitals outside of China remains to be determined, there are several practical points critical care professionals need to know to be prepared in the face of this dynamic and rapidly evolving outbreak, speakers said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“Priorities for us in our hospitals are early detection, infection prevention, staff safety, and obviously, taking care of sick people,” said Ryan C. Maves, MD, of the Naval Medical Center San Diego in a special session on the 2019 Novel Coronavirus outbreak.*
Approximately 72,000 cases of coronavirus disease 2019 (COVID-19) had been reported as of Feb. 17, 2020, the day of Dr. Maves’ talk, according to statistics from Johns Hopkins Center for Science and Engineering in Baltimore. A total of 1,775 deaths had been recorded, nearly all of which were in Hubei Province, the central point of the outbreak. In the United States, the number of cases stood at 15, with no deaths reported.
While the dynamics of the 2019 novel coronavirus are still being learned, the estimated range of spread for droplet transmission is 2 meters, according to Dr. Maves. The duration of environmental persistence is not yet known, but he said that other coronaviruses persist in low-humidity conditions for up to 4 days.
The number of secondary cases that arise from a primary infection, or R0, is estimated to be between 1.5 and 3, though it can change as exposure evolves; by comparison, the R0 for H1N1 influenza has been reported as 1.5, while measles is 12-18, indicating that it is “very contagious,” said Dr. Maves. Severe acute respiratory syndrome had an initial R0 of about 3.5, which he said declined rapidly to 0.7 as environmental and policy controls were put into place.
Critical care professionals need to know how to identify patients at risk of having COVID-19 and determine whether they need further work-up, according to Dr. Maves, who highlighted recent criteria released by the Centers for Disease Control and Prevention.
The highest-risk category, he said, are individuals exposed to a laboratory-confirmed coronavirus case, which along with fever or signs and symptoms of a lower respiratory illness would be sufficient to classify them as a “person of interest” requiring further evaluation for disease. A history of travel from Hubei Province plus fever and signs/symptoms of lower respiratory illness would also meet criteria for evaluation, according to the CDC, while travel to mainland China would also meet the threshold, if those symptoms required hospitalization.
The CDC also published a step-wise flowchart to evaluate patients who may have been exposed to the 2019 novel coronavirus. According to that flowchart, if an individual has traveled to China or had close contact with someone infected with the 2019 Novel Coronavirus within 14 days of symptoms, and that individual has fever or symptoms of lower respiratory illness such as cough or shortness of breath, then providers should isolate that individual and assess clinical status, in addition to contacting the local health department.
Laura E. Evans, MD, MS, FCCM, of New York University, said she might recommend providers “flip the script” on that CDC algorithm when it comes to identifying patients who may have been exposed.
“I think perhaps what we should be doing at sites of entry is not talking about travel as the first question, but rather fever or symptoms of lower respiratory illnesses as the first question, and use that as the opportunity to implement risk mitigation at that stage,” Dr. Evans said in a presentation on preparing for COVID-19.
Even with “substantial uncertainty” about the potential impact of the 2019 Novel Coronavirus, a significant influx of seriously ill patients would put strain the U.S. health care delivery system, she added.
“None of us have tons of extra capacity in our emergency departments, inpatient units, or ICUs, and I think we need to be prepared for that,” she added. “We need to know what our process is for ‘identify, isolate, and inform,’ and we need to be testing that now.”
Dr. Maves and Dr. Evans both reported that they had no financial conflicts of interest to report. Dr. Maves indicated that the views expressed in his presentation did not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government.
*Correction, 2/19/20: An earlier version of this article misstated the location of the naval center.
ORLANDO – While the impact of the 2019 novel coronavirus outbreak on hospitals outside of China remains to be determined, there are several practical points critical care professionals need to know to be prepared in the face of this dynamic and rapidly evolving outbreak, speakers said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“Priorities for us in our hospitals are early detection, infection prevention, staff safety, and obviously, taking care of sick people,” said Ryan C. Maves, MD, of the Naval Medical Center San Diego in a special session on the 2019 Novel Coronavirus outbreak.*
Approximately 72,000 cases of coronavirus disease 2019 (COVID-19) had been reported as of Feb. 17, 2020, the day of Dr. Maves’ talk, according to statistics from Johns Hopkins Center for Science and Engineering in Baltimore. A total of 1,775 deaths had been recorded, nearly all of which were in Hubei Province, the central point of the outbreak. In the United States, the number of cases stood at 15, with no deaths reported.
While the dynamics of the 2019 novel coronavirus are still being learned, the estimated range of spread for droplet transmission is 2 meters, according to Dr. Maves. The duration of environmental persistence is not yet known, but he said that other coronaviruses persist in low-humidity conditions for up to 4 days.
The number of secondary cases that arise from a primary infection, or R0, is estimated to be between 1.5 and 3, though it can change as exposure evolves; by comparison, the R0 for H1N1 influenza has been reported as 1.5, while measles is 12-18, indicating that it is “very contagious,” said Dr. Maves. Severe acute respiratory syndrome had an initial R0 of about 3.5, which he said declined rapidly to 0.7 as environmental and policy controls were put into place.
Critical care professionals need to know how to identify patients at risk of having COVID-19 and determine whether they need further work-up, according to Dr. Maves, who highlighted recent criteria released by the Centers for Disease Control and Prevention.
The highest-risk category, he said, are individuals exposed to a laboratory-confirmed coronavirus case, which along with fever or signs and symptoms of a lower respiratory illness would be sufficient to classify them as a “person of interest” requiring further evaluation for disease. A history of travel from Hubei Province plus fever and signs/symptoms of lower respiratory illness would also meet criteria for evaluation, according to the CDC, while travel to mainland China would also meet the threshold, if those symptoms required hospitalization.
The CDC also published a step-wise flowchart to evaluate patients who may have been exposed to the 2019 novel coronavirus. According to that flowchart, if an individual has traveled to China or had close contact with someone infected with the 2019 Novel Coronavirus within 14 days of symptoms, and that individual has fever or symptoms of lower respiratory illness such as cough or shortness of breath, then providers should isolate that individual and assess clinical status, in addition to contacting the local health department.
Laura E. Evans, MD, MS, FCCM, of New York University, said she might recommend providers “flip the script” on that CDC algorithm when it comes to identifying patients who may have been exposed.
“I think perhaps what we should be doing at sites of entry is not talking about travel as the first question, but rather fever or symptoms of lower respiratory illnesses as the first question, and use that as the opportunity to implement risk mitigation at that stage,” Dr. Evans said in a presentation on preparing for COVID-19.
Even with “substantial uncertainty” about the potential impact of the 2019 Novel Coronavirus, a significant influx of seriously ill patients would put strain the U.S. health care delivery system, she added.
“None of us have tons of extra capacity in our emergency departments, inpatient units, or ICUs, and I think we need to be prepared for that,” she added. “We need to know what our process is for ‘identify, isolate, and inform,’ and we need to be testing that now.”
Dr. Maves and Dr. Evans both reported that they had no financial conflicts of interest to report. Dr. Maves indicated that the views expressed in his presentation did not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government.
*Correction, 2/19/20: An earlier version of this article misstated the location of the naval center.
ORLANDO – While the impact of the 2019 novel coronavirus outbreak on hospitals outside of China remains to be determined, there are several practical points critical care professionals need to know to be prepared in the face of this dynamic and rapidly evolving outbreak, speakers said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“Priorities for us in our hospitals are early detection, infection prevention, staff safety, and obviously, taking care of sick people,” said Ryan C. Maves, MD, of the Naval Medical Center San Diego in a special session on the 2019 Novel Coronavirus outbreak.*
Approximately 72,000 cases of coronavirus disease 2019 (COVID-19) had been reported as of Feb. 17, 2020, the day of Dr. Maves’ talk, according to statistics from Johns Hopkins Center for Science and Engineering in Baltimore. A total of 1,775 deaths had been recorded, nearly all of which were in Hubei Province, the central point of the outbreak. In the United States, the number of cases stood at 15, with no deaths reported.
While the dynamics of the 2019 novel coronavirus are still being learned, the estimated range of spread for droplet transmission is 2 meters, according to Dr. Maves. The duration of environmental persistence is not yet known, but he said that other coronaviruses persist in low-humidity conditions for up to 4 days.
The number of secondary cases that arise from a primary infection, or R0, is estimated to be between 1.5 and 3, though it can change as exposure evolves; by comparison, the R0 for H1N1 influenza has been reported as 1.5, while measles is 12-18, indicating that it is “very contagious,” said Dr. Maves. Severe acute respiratory syndrome had an initial R0 of about 3.5, which he said declined rapidly to 0.7 as environmental and policy controls were put into place.
Critical care professionals need to know how to identify patients at risk of having COVID-19 and determine whether they need further work-up, according to Dr. Maves, who highlighted recent criteria released by the Centers for Disease Control and Prevention.
The highest-risk category, he said, are individuals exposed to a laboratory-confirmed coronavirus case, which along with fever or signs and symptoms of a lower respiratory illness would be sufficient to classify them as a “person of interest” requiring further evaluation for disease. A history of travel from Hubei Province plus fever and signs/symptoms of lower respiratory illness would also meet criteria for evaluation, according to the CDC, while travel to mainland China would also meet the threshold, if those symptoms required hospitalization.
The CDC also published a step-wise flowchart to evaluate patients who may have been exposed to the 2019 novel coronavirus. According to that flowchart, if an individual has traveled to China or had close contact with someone infected with the 2019 Novel Coronavirus within 14 days of symptoms, and that individual has fever or symptoms of lower respiratory illness such as cough or shortness of breath, then providers should isolate that individual and assess clinical status, in addition to contacting the local health department.
Laura E. Evans, MD, MS, FCCM, of New York University, said she might recommend providers “flip the script” on that CDC algorithm when it comes to identifying patients who may have been exposed.
“I think perhaps what we should be doing at sites of entry is not talking about travel as the first question, but rather fever or symptoms of lower respiratory illnesses as the first question, and use that as the opportunity to implement risk mitigation at that stage,” Dr. Evans said in a presentation on preparing for COVID-19.
Even with “substantial uncertainty” about the potential impact of the 2019 Novel Coronavirus, a significant influx of seriously ill patients would put strain the U.S. health care delivery system, she added.
“None of us have tons of extra capacity in our emergency departments, inpatient units, or ICUs, and I think we need to be prepared for that,” she added. “We need to know what our process is for ‘identify, isolate, and inform,’ and we need to be testing that now.”
Dr. Maves and Dr. Evans both reported that they had no financial conflicts of interest to report. Dr. Maves indicated that the views expressed in his presentation did not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government.
*Correction, 2/19/20: An earlier version of this article misstated the location of the naval center.
EXPERT ANALYSIS FROM CCC49
Some relevant financial conflicts go undisclosed in ACR guidelines
Over one-third of undisclosed industry payments made to physician-authors of American College of Rheumatology clinical practice guidelines were relevant to guideline recommendations, according to a recent review in Arthritis & Rheumatology.
Since 2014, 56 of 89 total physician-authors across five ACR clinical practice guidelines have been paid a total of $9,728,751 from industry sources. Nineteen of 89 authors received $1,961,362 in industry payments that were directly relevant to a guideline’s recommendations, and $699,561 of these payments (35.7%) were undisclosed, according to Cole Wayant, of the Oklahoma State University Center for Health Sciences, Tulsa, and colleagues.
The ACR’s Policy and Procedure Manual for Clinical Practice Guidelines, last updated in January 2015, allows up to 49% of authors in a clinical practice guideline to have financial conflicts of interest, including intellectual conflicts of interest, and requires them to report those relationships. When the ACR creates a call for letters of interest for a guideline, it includes a list of companies and organizations that could be affected by the guideline topic. To be considered conflict free, an author must not have ties to these companies and organizations for 1 year before the deadline on the letter of interest and 1 year after a guideline is published. This policy extends to members of an ACR guideline development group, literature review team, and voting panel. Under these guidelines, an author who has any relationship with a company is considered conflicted, which counts toward this total.
Mr. Wayant and colleagues performed a cross-sectional study of five ACR guidelines published since August 2014 on axial spondyloarthritis (27 authors), glucocorticoid-induced osteoporosis (21 authors), RA (26 authors), perioperative management of antirheumatic medication (31 authors), and polymyalgia rheumatica (46 authors). Using the Open Payments Database, the researchers searched for any general (speaking fees, consulting fees, education, honoraria, travel, food, or beverage payments) research, associated research, and ownership (stocks or dividends) relationships reported by guideline authors in the 12 months before a guideline was published. The guidelines on axial spondyloarthritis, glucocorticoid-induced osteoporosis, and RA contained specific recommendations for classes of medications or branded drugs, and conflicts from authors in those guidelines were assessed to determine relevancy of those payments.
Of the 56 physician-authors who received at least one payment (62.9%), the median payment was $522. However, 51 authors reported receiving more than $1,000, 42 authors reported more than $10,000, 20 authors reported more than $100,000, and 2 authors reported more than $1 million. Overall, 14 of 56 authors (25.0%) reported having no financial conflicts of interest, but did in fact receive some payment, and $4,189,090 of the $9,728,751 (43.1%) was not reported. The researchers said that the 19 authors with directly relevant payments were members of the voting panel (11 authors), literature review team (6 authors), and core leadership team (3 authors).
Physician-authors of clinical practice guidelines receiving payments from industry is not an issue specific to rheumatology. In an interview, Mr. Wayant said that authors of clinical guidelines across many different medical specialties often work closely with industry and hold “numerous conflicts of interest.”
“If professional societies are meant to be the public face of specialty providers, one would expect the guideline authors to resemble all society members,” Mr. Wayant said. “However, we routinely find that authors of professional society guidelines have large financial conflicts of interest that exceed the national average, indicating that the views and opinions of guideline authors may not reflect the opinion of most providers.”
These financial relationships between industry and physician authors have been shown to affect research results. A Cochrane Review published in 2017 evaluating industry sponsorship and research outcomes found that studies sponsored by industry were more likely to have favorable efficacy results and conclusions, compared with studies not sponsored by industry sources (Cochrane Database Syst Rev. 2017 Feb 16;2:MR000033). As medical societies continue to become more involved with clinical practice guidelines, recommendations from physician-authors with financial ties to industry can present a conflict of interest. Recommendations in clinical practice guidelines often affect reimbursement of a drug from insurance, and an author can vote for a drug recommendation in a guideline that may not match patient values and preferences, noted Mr. Wayant.
“These authors are fundamentally different from the average rheumatologist that stays up to date with the medical literature, in terms of financial ties to industry,” he said. “Removing the influence of for-profit companies from guideline development cannot harm the rigor of the guideline recommendations, since many medical professionals without conflicts are experts in evidence-based medicine and study appraisal.”
Being financially linked to industry does not automatically make one the most qualified candidate for deciding which therapies are best for patients, Mr. Wayant explained, and guidelines should reflect the values of patients and the medical profession, rather than industry.
“Given the importance of guidelines, [we] encourage the ACR and all professional societies to do everything possible to be above reproach and seek out authors who do not have financial conflicts to write the guidelines,” he said.
The authors reported having no funding source for the study. One author reported serving on an advisory board for Janssen involving infliximab and golimumab, for Sanofi Genzyme involving sarilumab, and receiving payment for a survey from Comsort. The other authors reported having no conflicts of interest.
SOURCE: Wayant C et al. Arthritis Rheumatol. 2020 Feb 10. doi: 10.1002/art.41224.
Over one-third of undisclosed industry payments made to physician-authors of American College of Rheumatology clinical practice guidelines were relevant to guideline recommendations, according to a recent review in Arthritis & Rheumatology.
Since 2014, 56 of 89 total physician-authors across five ACR clinical practice guidelines have been paid a total of $9,728,751 from industry sources. Nineteen of 89 authors received $1,961,362 in industry payments that were directly relevant to a guideline’s recommendations, and $699,561 of these payments (35.7%) were undisclosed, according to Cole Wayant, of the Oklahoma State University Center for Health Sciences, Tulsa, and colleagues.
The ACR’s Policy and Procedure Manual for Clinical Practice Guidelines, last updated in January 2015, allows up to 49% of authors in a clinical practice guideline to have financial conflicts of interest, including intellectual conflicts of interest, and requires them to report those relationships. When the ACR creates a call for letters of interest for a guideline, it includes a list of companies and organizations that could be affected by the guideline topic. To be considered conflict free, an author must not have ties to these companies and organizations for 1 year before the deadline on the letter of interest and 1 year after a guideline is published. This policy extends to members of an ACR guideline development group, literature review team, and voting panel. Under these guidelines, an author who has any relationship with a company is considered conflicted, which counts toward this total.
Mr. Wayant and colleagues performed a cross-sectional study of five ACR guidelines published since August 2014 on axial spondyloarthritis (27 authors), glucocorticoid-induced osteoporosis (21 authors), RA (26 authors), perioperative management of antirheumatic medication (31 authors), and polymyalgia rheumatica (46 authors). Using the Open Payments Database, the researchers searched for any general (speaking fees, consulting fees, education, honoraria, travel, food, or beverage payments) research, associated research, and ownership (stocks or dividends) relationships reported by guideline authors in the 12 months before a guideline was published. The guidelines on axial spondyloarthritis, glucocorticoid-induced osteoporosis, and RA contained specific recommendations for classes of medications or branded drugs, and conflicts from authors in those guidelines were assessed to determine relevancy of those payments.
Of the 56 physician-authors who received at least one payment (62.9%), the median payment was $522. However, 51 authors reported receiving more than $1,000, 42 authors reported more than $10,000, 20 authors reported more than $100,000, and 2 authors reported more than $1 million. Overall, 14 of 56 authors (25.0%) reported having no financial conflicts of interest, but did in fact receive some payment, and $4,189,090 of the $9,728,751 (43.1%) was not reported. The researchers said that the 19 authors with directly relevant payments were members of the voting panel (11 authors), literature review team (6 authors), and core leadership team (3 authors).
Physician-authors of clinical practice guidelines receiving payments from industry is not an issue specific to rheumatology. In an interview, Mr. Wayant said that authors of clinical guidelines across many different medical specialties often work closely with industry and hold “numerous conflicts of interest.”
“If professional societies are meant to be the public face of specialty providers, one would expect the guideline authors to resemble all society members,” Mr. Wayant said. “However, we routinely find that authors of professional society guidelines have large financial conflicts of interest that exceed the national average, indicating that the views and opinions of guideline authors may not reflect the opinion of most providers.”
These financial relationships between industry and physician authors have been shown to affect research results. A Cochrane Review published in 2017 evaluating industry sponsorship and research outcomes found that studies sponsored by industry were more likely to have favorable efficacy results and conclusions, compared with studies not sponsored by industry sources (Cochrane Database Syst Rev. 2017 Feb 16;2:MR000033). As medical societies continue to become more involved with clinical practice guidelines, recommendations from physician-authors with financial ties to industry can present a conflict of interest. Recommendations in clinical practice guidelines often affect reimbursement of a drug from insurance, and an author can vote for a drug recommendation in a guideline that may not match patient values and preferences, noted Mr. Wayant.
“These authors are fundamentally different from the average rheumatologist that stays up to date with the medical literature, in terms of financial ties to industry,” he said. “Removing the influence of for-profit companies from guideline development cannot harm the rigor of the guideline recommendations, since many medical professionals without conflicts are experts in evidence-based medicine and study appraisal.”
Being financially linked to industry does not automatically make one the most qualified candidate for deciding which therapies are best for patients, Mr. Wayant explained, and guidelines should reflect the values of patients and the medical profession, rather than industry.
“Given the importance of guidelines, [we] encourage the ACR and all professional societies to do everything possible to be above reproach and seek out authors who do not have financial conflicts to write the guidelines,” he said.
The authors reported having no funding source for the study. One author reported serving on an advisory board for Janssen involving infliximab and golimumab, for Sanofi Genzyme involving sarilumab, and receiving payment for a survey from Comsort. The other authors reported having no conflicts of interest.
SOURCE: Wayant C et al. Arthritis Rheumatol. 2020 Feb 10. doi: 10.1002/art.41224.
Over one-third of undisclosed industry payments made to physician-authors of American College of Rheumatology clinical practice guidelines were relevant to guideline recommendations, according to a recent review in Arthritis & Rheumatology.
Since 2014, 56 of 89 total physician-authors across five ACR clinical practice guidelines have been paid a total of $9,728,751 from industry sources. Nineteen of 89 authors received $1,961,362 in industry payments that were directly relevant to a guideline’s recommendations, and $699,561 of these payments (35.7%) were undisclosed, according to Cole Wayant, of the Oklahoma State University Center for Health Sciences, Tulsa, and colleagues.
The ACR’s Policy and Procedure Manual for Clinical Practice Guidelines, last updated in January 2015, allows up to 49% of authors in a clinical practice guideline to have financial conflicts of interest, including intellectual conflicts of interest, and requires them to report those relationships. When the ACR creates a call for letters of interest for a guideline, it includes a list of companies and organizations that could be affected by the guideline topic. To be considered conflict free, an author must not have ties to these companies and organizations for 1 year before the deadline on the letter of interest and 1 year after a guideline is published. This policy extends to members of an ACR guideline development group, literature review team, and voting panel. Under these guidelines, an author who has any relationship with a company is considered conflicted, which counts toward this total.
Mr. Wayant and colleagues performed a cross-sectional study of five ACR guidelines published since August 2014 on axial spondyloarthritis (27 authors), glucocorticoid-induced osteoporosis (21 authors), RA (26 authors), perioperative management of antirheumatic medication (31 authors), and polymyalgia rheumatica (46 authors). Using the Open Payments Database, the researchers searched for any general (speaking fees, consulting fees, education, honoraria, travel, food, or beverage payments) research, associated research, and ownership (stocks or dividends) relationships reported by guideline authors in the 12 months before a guideline was published. The guidelines on axial spondyloarthritis, glucocorticoid-induced osteoporosis, and RA contained specific recommendations for classes of medications or branded drugs, and conflicts from authors in those guidelines were assessed to determine relevancy of those payments.
Of the 56 physician-authors who received at least one payment (62.9%), the median payment was $522. However, 51 authors reported receiving more than $1,000, 42 authors reported more than $10,000, 20 authors reported more than $100,000, and 2 authors reported more than $1 million. Overall, 14 of 56 authors (25.0%) reported having no financial conflicts of interest, but did in fact receive some payment, and $4,189,090 of the $9,728,751 (43.1%) was not reported. The researchers said that the 19 authors with directly relevant payments were members of the voting panel (11 authors), literature review team (6 authors), and core leadership team (3 authors).
Physician-authors of clinical practice guidelines receiving payments from industry is not an issue specific to rheumatology. In an interview, Mr. Wayant said that authors of clinical guidelines across many different medical specialties often work closely with industry and hold “numerous conflicts of interest.”
“If professional societies are meant to be the public face of specialty providers, one would expect the guideline authors to resemble all society members,” Mr. Wayant said. “However, we routinely find that authors of professional society guidelines have large financial conflicts of interest that exceed the national average, indicating that the views and opinions of guideline authors may not reflect the opinion of most providers.”
These financial relationships between industry and physician authors have been shown to affect research results. A Cochrane Review published in 2017 evaluating industry sponsorship and research outcomes found that studies sponsored by industry were more likely to have favorable efficacy results and conclusions, compared with studies not sponsored by industry sources (Cochrane Database Syst Rev. 2017 Feb 16;2:MR000033). As medical societies continue to become more involved with clinical practice guidelines, recommendations from physician-authors with financial ties to industry can present a conflict of interest. Recommendations in clinical practice guidelines often affect reimbursement of a drug from insurance, and an author can vote for a drug recommendation in a guideline that may not match patient values and preferences, noted Mr. Wayant.
“These authors are fundamentally different from the average rheumatologist that stays up to date with the medical literature, in terms of financial ties to industry,” he said. “Removing the influence of for-profit companies from guideline development cannot harm the rigor of the guideline recommendations, since many medical professionals without conflicts are experts in evidence-based medicine and study appraisal.”
Being financially linked to industry does not automatically make one the most qualified candidate for deciding which therapies are best for patients, Mr. Wayant explained, and guidelines should reflect the values of patients and the medical profession, rather than industry.
“Given the importance of guidelines, [we] encourage the ACR and all professional societies to do everything possible to be above reproach and seek out authors who do not have financial conflicts to write the guidelines,” he said.
The authors reported having no funding source for the study. One author reported serving on an advisory board for Janssen involving infliximab and golimumab, for Sanofi Genzyme involving sarilumab, and receiving payment for a survey from Comsort. The other authors reported having no conflicts of interest.
SOURCE: Wayant C et al. Arthritis Rheumatol. 2020 Feb 10. doi: 10.1002/art.41224.
FROM ARTHRITIS & RHEUMATOLOGY
ACC issues guidance on cardiac implications of coronavirus
The American College of Cardiology on Feb. 13, 2020, released a clinical bulletin that aims to address cardiac implications of the current epidemic of the novel coronavirus, now known as COVID-19.
The bulletin, reviewed and approved by the college’s Science and Quality Oversight Committee, “provides background on the epidemic, which was first reported in late December 2019, and looks at early cardiac implications from case reports,” the ACC noted in a press release. “It also provides information on the potential cardiac implications from analog viral respiratory pandemics and offers early clinical guidance given current COVID-19 uncertainty.”
The document looks at some early cardiac implications of the infection. For example, early case reports suggest patients with underlying conditions are at higher risk of complications or mortality from the virus, with up to 50% of hospitalized patients having a chronic medical illness, the authors wrote.
About 40% of hospitalized patients confirmed to have the virus have cardiovascular or cerebrovascular disease, they noted.
In a recent case report on 138 hospitalized COVID-19 patients, they noted, 19.6% developed acute respiratory distress syndrome, 16.7% developed arrhythmia, 8.7% developed shock, 7.2% developed acute cardiac injury, and 3.6% developed acute kidney injury. “Rates of complication were universally higher for ICU patients,” they wrote.
“The first reported death was a 61-year-old male, with a long history of smoking, who succumbed to acute respiratory distress, heart failure, and cardiac arrest,” the document noted. “Early, unpublished first-hand reports suggest at least some patients develop myocarditis.”
Stressing the current uncertainty about the virus, the bulletin provides the following clinical guidance:
- COVID-19 is spread through droplets and can live for substantial periods outside the body; containment and prevention using standard public health and personal strategies for preventing the spread of communicable disease remains the priority.
- In geographies with active COVID-19 transmission (mainly China), it is reasonable to advise patients with underlying cardiovascular disease of the potential increased risk and to encourage additional, reasonable precautions.
- Older adults are less likely to present with fever, thus close assessment for other symptoms such as cough or shortness of breath is warranted.
- Some experts have suggested that the rigorous use of guideline-directed, plaque-stabilizing agents could offer additional protection to cardiovascular disease (CVD) patients during a widespread outbreak (statins, beta-blockers, ACE inhibitors, acetylsalicylic acid); however, such therapies should be tailored to individual patients.
- It is important for patients with CVD to remain current with vaccinations, including the pneumococcal vaccine, given the increased risk of secondary bacterial infection; it would also be prudent to receive vaccination to prevent another source of fever which could be initially confused with coronavirus infection.
- It may be reasonable to triage COVID-19 patients according to the presence of underlying cardiovascular, respiratory, renal, and other chronic diseases for prioritized treatment.
- Providers are cautioned that classic symptoms and presentation of acute MI may be overshadowed in the context of coronavirus, resulting in underdiagnosis.
- For CVD patients in geographies without widespread COVID-19, emphasis should remain on the threat from influenza, the importance of vaccination and frequent handwashing, and continued adherence to all guideline-directed therapy for underlying chronic conditions.
- COVID-19 is a fast-moving epidemic with an uncertain clinical profile; providers should be prepared for guidance to shift as more information becomes available.
The full clinical update is available here.
This article first appeared on Medscape.com.
The American College of Cardiology on Feb. 13, 2020, released a clinical bulletin that aims to address cardiac implications of the current epidemic of the novel coronavirus, now known as COVID-19.
The bulletin, reviewed and approved by the college’s Science and Quality Oversight Committee, “provides background on the epidemic, which was first reported in late December 2019, and looks at early cardiac implications from case reports,” the ACC noted in a press release. “It also provides information on the potential cardiac implications from analog viral respiratory pandemics and offers early clinical guidance given current COVID-19 uncertainty.”
The document looks at some early cardiac implications of the infection. For example, early case reports suggest patients with underlying conditions are at higher risk of complications or mortality from the virus, with up to 50% of hospitalized patients having a chronic medical illness, the authors wrote.
About 40% of hospitalized patients confirmed to have the virus have cardiovascular or cerebrovascular disease, they noted.
In a recent case report on 138 hospitalized COVID-19 patients, they noted, 19.6% developed acute respiratory distress syndrome, 16.7% developed arrhythmia, 8.7% developed shock, 7.2% developed acute cardiac injury, and 3.6% developed acute kidney injury. “Rates of complication were universally higher for ICU patients,” they wrote.
“The first reported death was a 61-year-old male, with a long history of smoking, who succumbed to acute respiratory distress, heart failure, and cardiac arrest,” the document noted. “Early, unpublished first-hand reports suggest at least some patients develop myocarditis.”
Stressing the current uncertainty about the virus, the bulletin provides the following clinical guidance:
- COVID-19 is spread through droplets and can live for substantial periods outside the body; containment and prevention using standard public health and personal strategies for preventing the spread of communicable disease remains the priority.
- In geographies with active COVID-19 transmission (mainly China), it is reasonable to advise patients with underlying cardiovascular disease of the potential increased risk and to encourage additional, reasonable precautions.
- Older adults are less likely to present with fever, thus close assessment for other symptoms such as cough or shortness of breath is warranted.
- Some experts have suggested that the rigorous use of guideline-directed, plaque-stabilizing agents could offer additional protection to cardiovascular disease (CVD) patients during a widespread outbreak (statins, beta-blockers, ACE inhibitors, acetylsalicylic acid); however, such therapies should be tailored to individual patients.
- It is important for patients with CVD to remain current with vaccinations, including the pneumococcal vaccine, given the increased risk of secondary bacterial infection; it would also be prudent to receive vaccination to prevent another source of fever which could be initially confused with coronavirus infection.
- It may be reasonable to triage COVID-19 patients according to the presence of underlying cardiovascular, respiratory, renal, and other chronic diseases for prioritized treatment.
- Providers are cautioned that classic symptoms and presentation of acute MI may be overshadowed in the context of coronavirus, resulting in underdiagnosis.
- For CVD patients in geographies without widespread COVID-19, emphasis should remain on the threat from influenza, the importance of vaccination and frequent handwashing, and continued adherence to all guideline-directed therapy for underlying chronic conditions.
- COVID-19 is a fast-moving epidemic with an uncertain clinical profile; providers should be prepared for guidance to shift as more information becomes available.
The full clinical update is available here.
This article first appeared on Medscape.com.
The American College of Cardiology on Feb. 13, 2020, released a clinical bulletin that aims to address cardiac implications of the current epidemic of the novel coronavirus, now known as COVID-19.
The bulletin, reviewed and approved by the college’s Science and Quality Oversight Committee, “provides background on the epidemic, which was first reported in late December 2019, and looks at early cardiac implications from case reports,” the ACC noted in a press release. “It also provides information on the potential cardiac implications from analog viral respiratory pandemics and offers early clinical guidance given current COVID-19 uncertainty.”
The document looks at some early cardiac implications of the infection. For example, early case reports suggest patients with underlying conditions are at higher risk of complications or mortality from the virus, with up to 50% of hospitalized patients having a chronic medical illness, the authors wrote.
About 40% of hospitalized patients confirmed to have the virus have cardiovascular or cerebrovascular disease, they noted.
In a recent case report on 138 hospitalized COVID-19 patients, they noted, 19.6% developed acute respiratory distress syndrome, 16.7% developed arrhythmia, 8.7% developed shock, 7.2% developed acute cardiac injury, and 3.6% developed acute kidney injury. “Rates of complication were universally higher for ICU patients,” they wrote.
“The first reported death was a 61-year-old male, with a long history of smoking, who succumbed to acute respiratory distress, heart failure, and cardiac arrest,” the document noted. “Early, unpublished first-hand reports suggest at least some patients develop myocarditis.”
Stressing the current uncertainty about the virus, the bulletin provides the following clinical guidance:
- COVID-19 is spread through droplets and can live for substantial periods outside the body; containment and prevention using standard public health and personal strategies for preventing the spread of communicable disease remains the priority.
- In geographies with active COVID-19 transmission (mainly China), it is reasonable to advise patients with underlying cardiovascular disease of the potential increased risk and to encourage additional, reasonable precautions.
- Older adults are less likely to present with fever, thus close assessment for other symptoms such as cough or shortness of breath is warranted.
- Some experts have suggested that the rigorous use of guideline-directed, plaque-stabilizing agents could offer additional protection to cardiovascular disease (CVD) patients during a widespread outbreak (statins, beta-blockers, ACE inhibitors, acetylsalicylic acid); however, such therapies should be tailored to individual patients.
- It is important for patients with CVD to remain current with vaccinations, including the pneumococcal vaccine, given the increased risk of secondary bacterial infection; it would also be prudent to receive vaccination to prevent another source of fever which could be initially confused with coronavirus infection.
- It may be reasonable to triage COVID-19 patients according to the presence of underlying cardiovascular, respiratory, renal, and other chronic diseases for prioritized treatment.
- Providers are cautioned that classic symptoms and presentation of acute MI may be overshadowed in the context of coronavirus, resulting in underdiagnosis.
- For CVD patients in geographies without widespread COVID-19, emphasis should remain on the threat from influenza, the importance of vaccination and frequent handwashing, and continued adherence to all guideline-directed therapy for underlying chronic conditions.
- COVID-19 is a fast-moving epidemic with an uncertain clinical profile; providers should be prepared for guidance to shift as more information becomes available.
The full clinical update is available here.
This article first appeared on Medscape.com.
Oncologists are average in terms of happiness, survey suggests
When it comes to physician happiness both in and outside the workplace, oncologists are about average, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.
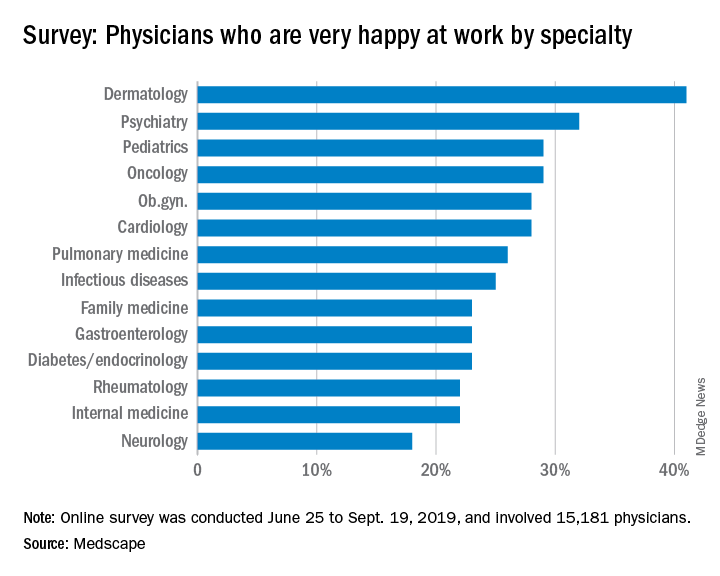
Oncologists landed in the middle of the pack among all physicians surveyed for happiness. Rheumatologists were most likely to report being very or extremely happy outside of work (60%) and neurologists were least likely to do so (44%), but about half of oncologists (51%) reported being very/extremely happy outside of work. For happiness at work, dermatologists topped the list (41%), neurologists came in last (18%), and oncologists remained in the middle (29%).
Oncologists were average when it came to burnout as well, matching the rate of overall physicians. Specifically, 32% of oncologists were burned out, 4% were depressed, and 9% were both burned out and depressed.
The most commonly reported factors contributing to burnout among oncologists were an overabundance of bureaucratic tasks (74%), spending too many hours at work (42%), and a lack of respect from colleagues in the workplace (36%).
Exercise was the most commonly reported way oncologists dealt with burnout (51%), followed by talking with family and friends (49%), and isolating themselves from others (38%). In addition, 57% of oncologists took 3-4 weeks’ vacation, compared with 44% of physicians overall; 29% of oncologists took less than 3 weeks’ vacation.
About 18% of oncologists said they had contemplated suicide, and 1% said they’d attempted it; 72% said they’d never had thoughts of suicide. Just under one-quarter of oncologists said they were currently seeking professional help or were planning to seek help for symptoms of depression and/or burnout.
“The survey results are concerning on several levels,” Maurie Markman, MD, of Cancer Treatment Centers of America, Philadelphia, said in an interview.
“First, the data suggest a considerable number of oncologists are simply burned out from the day-to-day bureaucracy (paperwork, etc.) of medical practice, which has absolutely nothing to do with the actual care delivered. This likely impacts the willingness to continue in this role. Second, one must be concerned for the future recruitment of physicians to become clinical oncologists. And finally, one must wonder about the impact of these concerning figures on the quality of care being provided to cancer patients.”
This survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians. Oncologists made up 1% of the survey pool.
When it comes to physician happiness both in and outside the workplace, oncologists are about average, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.

Oncologists landed in the middle of the pack among all physicians surveyed for happiness. Rheumatologists were most likely to report being very or extremely happy outside of work (60%) and neurologists were least likely to do so (44%), but about half of oncologists (51%) reported being very/extremely happy outside of work. For happiness at work, dermatologists topped the list (41%), neurologists came in last (18%), and oncologists remained in the middle (29%).
Oncologists were average when it came to burnout as well, matching the rate of overall physicians. Specifically, 32% of oncologists were burned out, 4% were depressed, and 9% were both burned out and depressed.
The most commonly reported factors contributing to burnout among oncologists were an overabundance of bureaucratic tasks (74%), spending too many hours at work (42%), and a lack of respect from colleagues in the workplace (36%).
Exercise was the most commonly reported way oncologists dealt with burnout (51%), followed by talking with family and friends (49%), and isolating themselves from others (38%). In addition, 57% of oncologists took 3-4 weeks’ vacation, compared with 44% of physicians overall; 29% of oncologists took less than 3 weeks’ vacation.
About 18% of oncologists said they had contemplated suicide, and 1% said they’d attempted it; 72% said they’d never had thoughts of suicide. Just under one-quarter of oncologists said they were currently seeking professional help or were planning to seek help for symptoms of depression and/or burnout.
“The survey results are concerning on several levels,” Maurie Markman, MD, of Cancer Treatment Centers of America, Philadelphia, said in an interview.
“First, the data suggest a considerable number of oncologists are simply burned out from the day-to-day bureaucracy (paperwork, etc.) of medical practice, which has absolutely nothing to do with the actual care delivered. This likely impacts the willingness to continue in this role. Second, one must be concerned for the future recruitment of physicians to become clinical oncologists. And finally, one must wonder about the impact of these concerning figures on the quality of care being provided to cancer patients.”
This survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians. Oncologists made up 1% of the survey pool.
When it comes to physician happiness both in and outside the workplace, oncologists are about average, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.

Oncologists landed in the middle of the pack among all physicians surveyed for happiness. Rheumatologists were most likely to report being very or extremely happy outside of work (60%) and neurologists were least likely to do so (44%), but about half of oncologists (51%) reported being very/extremely happy outside of work. For happiness at work, dermatologists topped the list (41%), neurologists came in last (18%), and oncologists remained in the middle (29%).
Oncologists were average when it came to burnout as well, matching the rate of overall physicians. Specifically, 32% of oncologists were burned out, 4% were depressed, and 9% were both burned out and depressed.
The most commonly reported factors contributing to burnout among oncologists were an overabundance of bureaucratic tasks (74%), spending too many hours at work (42%), and a lack of respect from colleagues in the workplace (36%).
Exercise was the most commonly reported way oncologists dealt with burnout (51%), followed by talking with family and friends (49%), and isolating themselves from others (38%). In addition, 57% of oncologists took 3-4 weeks’ vacation, compared with 44% of physicians overall; 29% of oncologists took less than 3 weeks’ vacation.
About 18% of oncologists said they had contemplated suicide, and 1% said they’d attempted it; 72% said they’d never had thoughts of suicide. Just under one-quarter of oncologists said they were currently seeking professional help or were planning to seek help for symptoms of depression and/or burnout.
“The survey results are concerning on several levels,” Maurie Markman, MD, of Cancer Treatment Centers of America, Philadelphia, said in an interview.
“First, the data suggest a considerable number of oncologists are simply burned out from the day-to-day bureaucracy (paperwork, etc.) of medical practice, which has absolutely nothing to do with the actual care delivered. This likely impacts the willingness to continue in this role. Second, one must be concerned for the future recruitment of physicians to become clinical oncologists. And finally, one must wonder about the impact of these concerning figures on the quality of care being provided to cancer patients.”
This survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians. Oncologists made up 1% of the survey pool.
Tramadol use for noncancer pain linked with increased hip fracture risk
The risk of hip fracture was higher among patients treated with tramadol for chronic noncancer pain than among those treated with other commonly used NSAIDs in a large population-based cohort in the United Kingdom.
The incidence of hip fracture over a 12-month period among 293,912 propensity score-matched tramadol and codeine recipients in The Health Improvement Network (THIN) database during 2000-2017 was 3.7 vs. 2.9 per 1,000 person-years, respectively (hazard ratio for hip fracture, 1.28), Jie Wei, PhD, of Xiangya Hospital, Central South University, Changsha, China, and colleagues reported in the Journal of Bone and Mineral Research.
Hip fracture incidence per 1,000 person-years was also higher in propensity score–matched cohorts of patients receiving tramadol vs. naproxen (2.9 vs. 1.7; HR, 1.69), ibuprofen (3.4 vs. 2.0; HR, 1.65), celecoxib (3.4 vs. 1.8; HR, 1.85), or etoricoxib (2.9 vs. 1.5; HR, 1.96), the investigators found.
Tramadol is considered a weak opioid and is commonly used for the treatment of pain based on a lower perceived risk of serious cardiovascular and gastrointestinal effects versus NSAIDs, and of addiction and respiratory depression versus traditional opioids, they explained. Several professional organizations also have “strongly or conditionally recommended tramadol” as a first- or second-line treatment for conditions such as osteoarthritis, fibromyalgia, and chronic low back pain.
The potential mechanisms for the association between tramadol and hip fracture require further study, but “[c]onsidering the significant impact of hip fracture on morbidity, mortality, and health care costs, our results point to the need to consider tramadol’s associated risk of fracture in clinical practice and treatment guidelines,” they concluded.
This study was supported by the National Institutes of Health, the National Natural Science Foundation of China, and the Postdoctoral Science Foundation of Central South University. The authors reported having no conflicts of interest.
SOURCE: Wei J et al. J Bone Miner Res. 2019 Feb 5. doi: 10.1002/jbmr.3935.
The risk of hip fracture was higher among patients treated with tramadol for chronic noncancer pain than among those treated with other commonly used NSAIDs in a large population-based cohort in the United Kingdom.
The incidence of hip fracture over a 12-month period among 293,912 propensity score-matched tramadol and codeine recipients in The Health Improvement Network (THIN) database during 2000-2017 was 3.7 vs. 2.9 per 1,000 person-years, respectively (hazard ratio for hip fracture, 1.28), Jie Wei, PhD, of Xiangya Hospital, Central South University, Changsha, China, and colleagues reported in the Journal of Bone and Mineral Research.
Hip fracture incidence per 1,000 person-years was also higher in propensity score–matched cohorts of patients receiving tramadol vs. naproxen (2.9 vs. 1.7; HR, 1.69), ibuprofen (3.4 vs. 2.0; HR, 1.65), celecoxib (3.4 vs. 1.8; HR, 1.85), or etoricoxib (2.9 vs. 1.5; HR, 1.96), the investigators found.
Tramadol is considered a weak opioid and is commonly used for the treatment of pain based on a lower perceived risk of serious cardiovascular and gastrointestinal effects versus NSAIDs, and of addiction and respiratory depression versus traditional opioids, they explained. Several professional organizations also have “strongly or conditionally recommended tramadol” as a first- or second-line treatment for conditions such as osteoarthritis, fibromyalgia, and chronic low back pain.
The potential mechanisms for the association between tramadol and hip fracture require further study, but “[c]onsidering the significant impact of hip fracture on morbidity, mortality, and health care costs, our results point to the need to consider tramadol’s associated risk of fracture in clinical practice and treatment guidelines,” they concluded.
This study was supported by the National Institutes of Health, the National Natural Science Foundation of China, and the Postdoctoral Science Foundation of Central South University. The authors reported having no conflicts of interest.
SOURCE: Wei J et al. J Bone Miner Res. 2019 Feb 5. doi: 10.1002/jbmr.3935.
The risk of hip fracture was higher among patients treated with tramadol for chronic noncancer pain than among those treated with other commonly used NSAIDs in a large population-based cohort in the United Kingdom.
The incidence of hip fracture over a 12-month period among 293,912 propensity score-matched tramadol and codeine recipients in The Health Improvement Network (THIN) database during 2000-2017 was 3.7 vs. 2.9 per 1,000 person-years, respectively (hazard ratio for hip fracture, 1.28), Jie Wei, PhD, of Xiangya Hospital, Central South University, Changsha, China, and colleagues reported in the Journal of Bone and Mineral Research.
Hip fracture incidence per 1,000 person-years was also higher in propensity score–matched cohorts of patients receiving tramadol vs. naproxen (2.9 vs. 1.7; HR, 1.69), ibuprofen (3.4 vs. 2.0; HR, 1.65), celecoxib (3.4 vs. 1.8; HR, 1.85), or etoricoxib (2.9 vs. 1.5; HR, 1.96), the investigators found.
Tramadol is considered a weak opioid and is commonly used for the treatment of pain based on a lower perceived risk of serious cardiovascular and gastrointestinal effects versus NSAIDs, and of addiction and respiratory depression versus traditional opioids, they explained. Several professional organizations also have “strongly or conditionally recommended tramadol” as a first- or second-line treatment for conditions such as osteoarthritis, fibromyalgia, and chronic low back pain.
The potential mechanisms for the association between tramadol and hip fracture require further study, but “[c]onsidering the significant impact of hip fracture on morbidity, mortality, and health care costs, our results point to the need to consider tramadol’s associated risk of fracture in clinical practice and treatment guidelines,” they concluded.
This study was supported by the National Institutes of Health, the National Natural Science Foundation of China, and the Postdoctoral Science Foundation of Central South University. The authors reported having no conflicts of interest.
SOURCE: Wei J et al. J Bone Miner Res. 2019 Feb 5. doi: 10.1002/jbmr.3935.
FROM THE JOURNAL OF BONE AND MINERAL RESEARCH
An epidemic of fear and misinformation
As I write this, the 2019 novel coronavirus* continues to spread, exceeding 59,000 cases and 1,300 deaths worldwide. With it spreads fear. In the modern world of social media, misinformation spreads even faster than disease.
The news about a novel and deadly illness crowds out more substantial worries. Humans are not particularly good at assessing risk or responding rationally and consistently to it. Risk is hard to fully define. If you look up “risk” in Merriam Webster’s online dictionary, you get the simple definition of “possibility of loss or injury; peril.” If you look up risk in Wikipedia, you get 12 pages of explanation and 8 more pages of links and references.
People handle risk differently. Some people are more risk adverse than others. Some get a pleasurable thrill from risk, whether a slot machine or a parachute jump. Most people really don’t comprehend small probabilities, with tens of billions of dollars spent annually on U.S. lotteries.
Because 98% of people who get COVID-19 are recovering, this is not an extinction-level event or the zombie apocalypse. It is a major health hazard, and one where morbidity and mortality might be assuaged by an early and effective public health response, including the population’s adoption of good habits such as hand washing, cough etiquette, and staying home when ill.
Three key factors may help reduce the fear factor.
One key factor is accurate communication of health information to the public. This has been severely harmed in the last few years by the promotion of gossip on social media, such as Facebook, within newsfeeds without any vetting, along with a smaller component of deliberate misinformation from untraceable sources. Compare this situation with the decision in May 1988 when Surgeon General C. Everett Koop chose to snail mail a brochure on AIDS to every household in America. It was unprecedented. One element of this communication is the public’s belief that government and health care officials will responsibly and timely convey the information. There are accusations that the Chinese government initially impeded early warnings about COVID-19. Dr. Koop, to his great credit and lifesaving leadership, overcame queasiness within the Reagan administration about issues of morality and taste in discussing some of the HIV information. Alas, no similar leadership occurred in the decade of the 2010s when deaths from the opioid epidemic in the United States skyrocketed to claim more lives annually than car accidents or suicide.
A second factor is the credibility of the scientists. Antivaxxers, climate change deniers, and mercenary scientists have severely damaged that credibility of science, compared with the trust in scientists 50 years ago during the Apollo moon shot.
A third factor is perspective. Poor journalism and clickbait can focus excessively on the rare events as news. Airline crashes make the front page while fatal car accidents, claiming a hundred times more lives annually, don’t even merit a story in local media. Someone wins the lottery weekly but few pay attention to those suffering from gambling debts.
Influenza is killing many times more people than the 2019 novel coronavirus, but the news is focused on cruise ships. In the United States, influenza annually will strike tens of millions, with about 10 per 1,000 hospitalized and 0.5 per 1,000 dying. The novel coronavirus is more lethal. SARS (a coronavirus epidemic in 2003) had 8,000 cases with a mortality rate of 96 per 1,000 while the novel 2019 strain so far is killing about 20 per 1,000. That value may be an overestimate, because there may be a significant fraction of COVID-19 patients with symptoms mild enough that they do not seek medical care and do not get tested and counted.
For perspective, in 1952 the United States reported 50,000 cases of polio (meningitis or paralytic) annually with 3,000 deaths. As many as 95% of cases of poliovirus infection have no or mild symptoms and would not have been reported, so the case fatality rate estimate is skewed. In the 1950s, the United States averaged about 500,000 cases of measles per year, with about 500 deaths annually for a case fatality rate of about 1 per 1,000 in a population that was well nourished with good medical care. In malnourished children without access to modern health care, the case fatality rate can be as high as 100 per 1,000, which is why globally measles killed 142,000 people in 2018, a substantial improvement from 536,000 deaths globally in 2000, but still a leading killer of children worldwide. Vaccines had reduced the annual death toll of polio and measles in the U.S. to zero.
In comparison, in this country the annual incidences are about 70,000 overdose deaths, 50,000 suicides, and 40,000 traffic deaths.
Reassurance is the most common product sold by pediatricians. We look for low-probability, high-impact bad things. Usually we don’t find them and can reassure parents that the child will be okay. Sometimes we spot a higher-risk situation and intervene. My job is to worry professionally so that parents can worry less.
COVID-19 worries me, but irrational people worry me more. The real enemies are fear, disinformation, discrimination, and economic warfare.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
*This article was updated 2/21/2020.
As I write this, the 2019 novel coronavirus* continues to spread, exceeding 59,000 cases and 1,300 deaths worldwide. With it spreads fear. In the modern world of social media, misinformation spreads even faster than disease.
The news about a novel and deadly illness crowds out more substantial worries. Humans are not particularly good at assessing risk or responding rationally and consistently to it. Risk is hard to fully define. If you look up “risk” in Merriam Webster’s online dictionary, you get the simple definition of “possibility of loss or injury; peril.” If you look up risk in Wikipedia, you get 12 pages of explanation and 8 more pages of links and references.
People handle risk differently. Some people are more risk adverse than others. Some get a pleasurable thrill from risk, whether a slot machine or a parachute jump. Most people really don’t comprehend small probabilities, with tens of billions of dollars spent annually on U.S. lotteries.
Because 98% of people who get COVID-19 are recovering, this is not an extinction-level event or the zombie apocalypse. It is a major health hazard, and one where morbidity and mortality might be assuaged by an early and effective public health response, including the population’s adoption of good habits such as hand washing, cough etiquette, and staying home when ill.
Three key factors may help reduce the fear factor.
One key factor is accurate communication of health information to the public. This has been severely harmed in the last few years by the promotion of gossip on social media, such as Facebook, within newsfeeds without any vetting, along with a smaller component of deliberate misinformation from untraceable sources. Compare this situation with the decision in May 1988 when Surgeon General C. Everett Koop chose to snail mail a brochure on AIDS to every household in America. It was unprecedented. One element of this communication is the public’s belief that government and health care officials will responsibly and timely convey the information. There are accusations that the Chinese government initially impeded early warnings about COVID-19. Dr. Koop, to his great credit and lifesaving leadership, overcame queasiness within the Reagan administration about issues of morality and taste in discussing some of the HIV information. Alas, no similar leadership occurred in the decade of the 2010s when deaths from the opioid epidemic in the United States skyrocketed to claim more lives annually than car accidents or suicide.
A second factor is the credibility of the scientists. Antivaxxers, climate change deniers, and mercenary scientists have severely damaged that credibility of science, compared with the trust in scientists 50 years ago during the Apollo moon shot.
A third factor is perspective. Poor journalism and clickbait can focus excessively on the rare events as news. Airline crashes make the front page while fatal car accidents, claiming a hundred times more lives annually, don’t even merit a story in local media. Someone wins the lottery weekly but few pay attention to those suffering from gambling debts.
Influenza is killing many times more people than the 2019 novel coronavirus, but the news is focused on cruise ships. In the United States, influenza annually will strike tens of millions, with about 10 per 1,000 hospitalized and 0.5 per 1,000 dying. The novel coronavirus is more lethal. SARS (a coronavirus epidemic in 2003) had 8,000 cases with a mortality rate of 96 per 1,000 while the novel 2019 strain so far is killing about 20 per 1,000. That value may be an overestimate, because there may be a significant fraction of COVID-19 patients with symptoms mild enough that they do not seek medical care and do not get tested and counted.
For perspective, in 1952 the United States reported 50,000 cases of polio (meningitis or paralytic) annually with 3,000 deaths. As many as 95% of cases of poliovirus infection have no or mild symptoms and would not have been reported, so the case fatality rate estimate is skewed. In the 1950s, the United States averaged about 500,000 cases of measles per year, with about 500 deaths annually for a case fatality rate of about 1 per 1,000 in a population that was well nourished with good medical care. In malnourished children without access to modern health care, the case fatality rate can be as high as 100 per 1,000, which is why globally measles killed 142,000 people in 2018, a substantial improvement from 536,000 deaths globally in 2000, but still a leading killer of children worldwide. Vaccines had reduced the annual death toll of polio and measles in the U.S. to zero.
In comparison, in this country the annual incidences are about 70,000 overdose deaths, 50,000 suicides, and 40,000 traffic deaths.
Reassurance is the most common product sold by pediatricians. We look for low-probability, high-impact bad things. Usually we don’t find them and can reassure parents that the child will be okay. Sometimes we spot a higher-risk situation and intervene. My job is to worry professionally so that parents can worry less.
COVID-19 worries me, but irrational people worry me more. The real enemies are fear, disinformation, discrimination, and economic warfare.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
*This article was updated 2/21/2020.
As I write this, the 2019 novel coronavirus* continues to spread, exceeding 59,000 cases and 1,300 deaths worldwide. With it spreads fear. In the modern world of social media, misinformation spreads even faster than disease.
The news about a novel and deadly illness crowds out more substantial worries. Humans are not particularly good at assessing risk or responding rationally and consistently to it. Risk is hard to fully define. If you look up “risk” in Merriam Webster’s online dictionary, you get the simple definition of “possibility of loss or injury; peril.” If you look up risk in Wikipedia, you get 12 pages of explanation and 8 more pages of links and references.
People handle risk differently. Some people are more risk adverse than others. Some get a pleasurable thrill from risk, whether a slot machine or a parachute jump. Most people really don’t comprehend small probabilities, with tens of billions of dollars spent annually on U.S. lotteries.
Because 98% of people who get COVID-19 are recovering, this is not an extinction-level event or the zombie apocalypse. It is a major health hazard, and one where morbidity and mortality might be assuaged by an early and effective public health response, including the population’s adoption of good habits such as hand washing, cough etiquette, and staying home when ill.
Three key factors may help reduce the fear factor.
One key factor is accurate communication of health information to the public. This has been severely harmed in the last few years by the promotion of gossip on social media, such as Facebook, within newsfeeds without any vetting, along with a smaller component of deliberate misinformation from untraceable sources. Compare this situation with the decision in May 1988 when Surgeon General C. Everett Koop chose to snail mail a brochure on AIDS to every household in America. It was unprecedented. One element of this communication is the public’s belief that government and health care officials will responsibly and timely convey the information. There are accusations that the Chinese government initially impeded early warnings about COVID-19. Dr. Koop, to his great credit and lifesaving leadership, overcame queasiness within the Reagan administration about issues of morality and taste in discussing some of the HIV information. Alas, no similar leadership occurred in the decade of the 2010s when deaths from the opioid epidemic in the United States skyrocketed to claim more lives annually than car accidents or suicide.
A second factor is the credibility of the scientists. Antivaxxers, climate change deniers, and mercenary scientists have severely damaged that credibility of science, compared with the trust in scientists 50 years ago during the Apollo moon shot.
A third factor is perspective. Poor journalism and clickbait can focus excessively on the rare events as news. Airline crashes make the front page while fatal car accidents, claiming a hundred times more lives annually, don’t even merit a story in local media. Someone wins the lottery weekly but few pay attention to those suffering from gambling debts.
Influenza is killing many times more people than the 2019 novel coronavirus, but the news is focused on cruise ships. In the United States, influenza annually will strike tens of millions, with about 10 per 1,000 hospitalized and 0.5 per 1,000 dying. The novel coronavirus is more lethal. SARS (a coronavirus epidemic in 2003) had 8,000 cases with a mortality rate of 96 per 1,000 while the novel 2019 strain so far is killing about 20 per 1,000. That value may be an overestimate, because there may be a significant fraction of COVID-19 patients with symptoms mild enough that they do not seek medical care and do not get tested and counted.
For perspective, in 1952 the United States reported 50,000 cases of polio (meningitis or paralytic) annually with 3,000 deaths. As many as 95% of cases of poliovirus infection have no or mild symptoms and would not have been reported, so the case fatality rate estimate is skewed. In the 1950s, the United States averaged about 500,000 cases of measles per year, with about 500 deaths annually for a case fatality rate of about 1 per 1,000 in a population that was well nourished with good medical care. In malnourished children without access to modern health care, the case fatality rate can be as high as 100 per 1,000, which is why globally measles killed 142,000 people in 2018, a substantial improvement from 536,000 deaths globally in 2000, but still a leading killer of children worldwide. Vaccines had reduced the annual death toll of polio and measles in the U.S. to zero.
In comparison, in this country the annual incidences are about 70,000 overdose deaths, 50,000 suicides, and 40,000 traffic deaths.
Reassurance is the most common product sold by pediatricians. We look for low-probability, high-impact bad things. Usually we don’t find them and can reassure parents that the child will be okay. Sometimes we spot a higher-risk situation and intervene. My job is to worry professionally so that parents can worry less.
COVID-19 worries me, but irrational people worry me more. The real enemies are fear, disinformation, discrimination, and economic warfare.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
*This article was updated 2/21/2020.
Lorcaserin withdrawn from U.S. market due to cancer risk
The Food and Drug Administration asked Eisai to voluntary withdraw the weight-loss drug lorcaserin (Belviq and Belviq XR) on Feb. 13 after a post-marketing trial with more than 12,000 subjects revealed an increased occurrence of cancer.
In a Drug Safety Communication, the agency said “health care professionals should stop prescribing and dispensing lorcaserin to patients. Contact patients currently taking lorcaserin, inform them of the increased occurrence of cancer seen in the clinical trial, and ask them to stop taking the medicine. Discuss alternative weight-loss medicines or strategies with your patients.”
Eisai is complying with the withdrawal request.
The decision is based on the agency’s review of the 5-year trial, which was designed to evaluate cardiac risk with the drug and ended in June 2018. In total, 7.7% of patients randomized to 10 mg lorcaserin twice daily were diagnosed with 520 primary cancers, compared with 7.1% of placebo subjects diagnosed with 470 cancers, over a median follow-up of 3 years and 3 months. There was one additional cancer observed for every 470 patients treated for 1 year.
“There was no apparent difference in the incidence of cancer over the initial months of treatment, but the imbalance increased with longer duration on lorcaserin,” FDA said. Pancreatic, colorectal, and lung cancers were among those diagnosed.
In short, “we believe that the risks of lorcaserin outweigh its benefits based on our completed review of” the data, the agency said. The FDA is not recommending special cancer screenings for patients who have taken lorcaserin.
The action follows an FDA alert in January about a possible elevated cancer risk based on its preliminary analysis of the study.
Patients were also advised Feb. 13 to stop taking the drug and talk to their providers about alternative weight-loss medications and weight-management programs.
They were also told to dispose of the pills at a drug take-back location if available, but if not, to mix them with an “unappealing substance” such as dirt, cat litter, or used coffee grounds; seal them in plastic bag; and put them in the trash.
The Food and Drug Administration asked Eisai to voluntary withdraw the weight-loss drug lorcaserin (Belviq and Belviq XR) on Feb. 13 after a post-marketing trial with more than 12,000 subjects revealed an increased occurrence of cancer.
In a Drug Safety Communication, the agency said “health care professionals should stop prescribing and dispensing lorcaserin to patients. Contact patients currently taking lorcaserin, inform them of the increased occurrence of cancer seen in the clinical trial, and ask them to stop taking the medicine. Discuss alternative weight-loss medicines or strategies with your patients.”
Eisai is complying with the withdrawal request.
The decision is based on the agency’s review of the 5-year trial, which was designed to evaluate cardiac risk with the drug and ended in June 2018. In total, 7.7% of patients randomized to 10 mg lorcaserin twice daily were diagnosed with 520 primary cancers, compared with 7.1% of placebo subjects diagnosed with 470 cancers, over a median follow-up of 3 years and 3 months. There was one additional cancer observed for every 470 patients treated for 1 year.
“There was no apparent difference in the incidence of cancer over the initial months of treatment, but the imbalance increased with longer duration on lorcaserin,” FDA said. Pancreatic, colorectal, and lung cancers were among those diagnosed.
In short, “we believe that the risks of lorcaserin outweigh its benefits based on our completed review of” the data, the agency said. The FDA is not recommending special cancer screenings for patients who have taken lorcaserin.
The action follows an FDA alert in January about a possible elevated cancer risk based on its preliminary analysis of the study.
Patients were also advised Feb. 13 to stop taking the drug and talk to their providers about alternative weight-loss medications and weight-management programs.
They were also told to dispose of the pills at a drug take-back location if available, but if not, to mix them with an “unappealing substance” such as dirt, cat litter, or used coffee grounds; seal them in plastic bag; and put them in the trash.
The Food and Drug Administration asked Eisai to voluntary withdraw the weight-loss drug lorcaserin (Belviq and Belviq XR) on Feb. 13 after a post-marketing trial with more than 12,000 subjects revealed an increased occurrence of cancer.
In a Drug Safety Communication, the agency said “health care professionals should stop prescribing and dispensing lorcaserin to patients. Contact patients currently taking lorcaserin, inform them of the increased occurrence of cancer seen in the clinical trial, and ask them to stop taking the medicine. Discuss alternative weight-loss medicines or strategies with your patients.”
Eisai is complying with the withdrawal request.
The decision is based on the agency’s review of the 5-year trial, which was designed to evaluate cardiac risk with the drug and ended in June 2018. In total, 7.7% of patients randomized to 10 mg lorcaserin twice daily were diagnosed with 520 primary cancers, compared with 7.1% of placebo subjects diagnosed with 470 cancers, over a median follow-up of 3 years and 3 months. There was one additional cancer observed for every 470 patients treated for 1 year.
“There was no apparent difference in the incidence of cancer over the initial months of treatment, but the imbalance increased with longer duration on lorcaserin,” FDA said. Pancreatic, colorectal, and lung cancers were among those diagnosed.
In short, “we believe that the risks of lorcaserin outweigh its benefits based on our completed review of” the data, the agency said. The FDA is not recommending special cancer screenings for patients who have taken lorcaserin.
The action follows an FDA alert in January about a possible elevated cancer risk based on its preliminary analysis of the study.
Patients were also advised Feb. 13 to stop taking the drug and talk to their providers about alternative weight-loss medications and weight-management programs.
They were also told to dispose of the pills at a drug take-back location if available, but if not, to mix them with an “unappealing substance” such as dirt, cat litter, or used coffee grounds; seal them in plastic bag; and put them in the trash.
Fast-track surgery for hip fracture does not reduce mortality
An accelerated path to surgery after hip fracture did not improve mortality or major complications, according to a new international randomized trial. However, a fast track to surgery hastened mobilization, weight-bearing, and hospital discharge, and reduced the risk of urinary tract infection and delirium.
The HIP ATTACK (Hip Fracture Accelerated Surgical Treatment and Care Track) study enrolled 2,970 patients (median age, 79 years; 69% women) during March 2014-May 2019. The study excluded patients younger than 45 years, as well as those who were on nonreversible anticoagulation and who had high-energy or more complex hip fractures. In all, 1,487 patients were randomly assigned to the accelerated-surgery group, which received early medical evaluation with a goal of heading to surgery within 6 hours of a hip fracture diagnosis. The goal was achieved, with patients in the intervention arm receiving care at a median 6 hours after diagnosis. Patients in the 69 participating hospitals in 17 countries who were assigned to standard of care received surgery at a median 24 hours after diagnosis (P less than .001).
“Observational data, clinical experience, and biological rationale suggest that the longer a patient is immobile and lying in a bed, the higher the risk of poor outcomes,” wrote principal investigators Philip J. Devereaux, MD, PhD, and Mohit Bhandari, MD, PhD, of McMaster University, Hamilton, Ont., and their colleagues on the HIP ATTACK writing committee.
The study was the first large, randomized trial that directly compared accelerated surgery with standard of care, noted the authors. Previous observational studies had shown worse outcomes for those usual-care patients who waited longer for surgery.
In HIP ATTACK, there was no difference in the primary outcome measures of 90-day mortality and major complications for patients receiving surgery within 6 hours after hip fracture diagnosis, compared with those who received surgery within 24 hours. The coprimary outcome measures included serious complications, such as MI, stroke, venous thromboembolism, sepsis, pneumonia, and life-threatening or major bleeding.
In practice, the researchers found that patients in the accelerated-surgery group received medical clearance in a median time of 2 hours after a diagnosis of hip fracture, whereas the standard of care group was cleared in 4 hours.
At 90 days, 9% of patients in the accelerated-surgery group and 10% of those in the usual-care group had died, a nonsignificant difference between the two groups. In both groups, 22% of patients experienced a major complication. A post hoc analysis that looked for any site-clustering effects did not detect different outcomes, the investigators wrote.
Delirium occurred in 132 patients (9%) of the accelerated-surgery group and in 175 patients (12%) in the usual-care group (odds ratio, 0.72; 95% confidence interval, 0.58-0.92). Infection without sepsis and urinary tract infection were both less common in the accelerated-surgery group (hazard ratio, 0.80 and 0.78, respectively).
The authors noted that the potential benefits of a speedy course to surgery, including reduced immobility and less pain, could be negated if physicians had less time to optimize medical care for older patients with multiple comorbidities and who make up a significant proportion of those who sustain low-energy hip fractures. However, medical complications, such as MI and new-onset atrial fibrillation, were not seen more frequently in the accelerated-surgery group.
In an editorial accompanying the study, Alejandro Lizaur-Utrilla, MD, and Fernando Lopez-Prats, MD, of the Universidad Miguel Hernández, Alicante, Spain, observed that the 6-hour window for hip fracture surgery may be difficult to achieve given clinical practicalities and that, in some cases, the 6-hour window might be unavoidable if severe comorbidities and overall poor health make early surgery inadvisable.
They also expressed concern that, despite the lack of harm shown in the patients who underwent accelerated surgery, the surgery “might negatively affect patients’ outcomes by preventing or limiting the opportunity for optimization of patients’ medical conditions before surgery.” They called for further study to delineate how fitness for surgery affects outcomes in accelerated surgery and to further examine whether the better outcomes are associated with improved cost-effectiveness.
Multiple HIP ATTACK coinvestigators reported relationships with pharmaceutical and medical device companies, including companies that manufacture hip prosthesis and orthopedic surgical devices and implants. The study was sponsored by the Canadian Population Health Research Institute, the Ontario Strategy for Patient Oriented Research Support Unit, the Ontario Ministry of Health and Long-Term Care, the Hamilton Health Sciences Foundation, Physicians’ Services Incorporated Foundation, Michael G. DeGroote Institute for Pain Research and Care, Smith & Nephew (to recruit patients in Spain), and Indiegogo Crowdfunding.
SOURCE: Borges F et al. Lancet. 2020 Feb. 9. doi: 10.1016/S0140-6736(20)30058-1.
An accelerated path to surgery after hip fracture did not improve mortality or major complications, according to a new international randomized trial. However, a fast track to surgery hastened mobilization, weight-bearing, and hospital discharge, and reduced the risk of urinary tract infection and delirium.
The HIP ATTACK (Hip Fracture Accelerated Surgical Treatment and Care Track) study enrolled 2,970 patients (median age, 79 years; 69% women) during March 2014-May 2019. The study excluded patients younger than 45 years, as well as those who were on nonreversible anticoagulation and who had high-energy or more complex hip fractures. In all, 1,487 patients were randomly assigned to the accelerated-surgery group, which received early medical evaluation with a goal of heading to surgery within 6 hours of a hip fracture diagnosis. The goal was achieved, with patients in the intervention arm receiving care at a median 6 hours after diagnosis. Patients in the 69 participating hospitals in 17 countries who were assigned to standard of care received surgery at a median 24 hours after diagnosis (P less than .001).
“Observational data, clinical experience, and biological rationale suggest that the longer a patient is immobile and lying in a bed, the higher the risk of poor outcomes,” wrote principal investigators Philip J. Devereaux, MD, PhD, and Mohit Bhandari, MD, PhD, of McMaster University, Hamilton, Ont., and their colleagues on the HIP ATTACK writing committee.
The study was the first large, randomized trial that directly compared accelerated surgery with standard of care, noted the authors. Previous observational studies had shown worse outcomes for those usual-care patients who waited longer for surgery.
In HIP ATTACK, there was no difference in the primary outcome measures of 90-day mortality and major complications for patients receiving surgery within 6 hours after hip fracture diagnosis, compared with those who received surgery within 24 hours. The coprimary outcome measures included serious complications, such as MI, stroke, venous thromboembolism, sepsis, pneumonia, and life-threatening or major bleeding.
In practice, the researchers found that patients in the accelerated-surgery group received medical clearance in a median time of 2 hours after a diagnosis of hip fracture, whereas the standard of care group was cleared in 4 hours.
At 90 days, 9% of patients in the accelerated-surgery group and 10% of those in the usual-care group had died, a nonsignificant difference between the two groups. In both groups, 22% of patients experienced a major complication. A post hoc analysis that looked for any site-clustering effects did not detect different outcomes, the investigators wrote.
Delirium occurred in 132 patients (9%) of the accelerated-surgery group and in 175 patients (12%) in the usual-care group (odds ratio, 0.72; 95% confidence interval, 0.58-0.92). Infection without sepsis and urinary tract infection were both less common in the accelerated-surgery group (hazard ratio, 0.80 and 0.78, respectively).
The authors noted that the potential benefits of a speedy course to surgery, including reduced immobility and less pain, could be negated if physicians had less time to optimize medical care for older patients with multiple comorbidities and who make up a significant proportion of those who sustain low-energy hip fractures. However, medical complications, such as MI and new-onset atrial fibrillation, were not seen more frequently in the accelerated-surgery group.
In an editorial accompanying the study, Alejandro Lizaur-Utrilla, MD, and Fernando Lopez-Prats, MD, of the Universidad Miguel Hernández, Alicante, Spain, observed that the 6-hour window for hip fracture surgery may be difficult to achieve given clinical practicalities and that, in some cases, the 6-hour window might be unavoidable if severe comorbidities and overall poor health make early surgery inadvisable.
They also expressed concern that, despite the lack of harm shown in the patients who underwent accelerated surgery, the surgery “might negatively affect patients’ outcomes by preventing or limiting the opportunity for optimization of patients’ medical conditions before surgery.” They called for further study to delineate how fitness for surgery affects outcomes in accelerated surgery and to further examine whether the better outcomes are associated with improved cost-effectiveness.
Multiple HIP ATTACK coinvestigators reported relationships with pharmaceutical and medical device companies, including companies that manufacture hip prosthesis and orthopedic surgical devices and implants. The study was sponsored by the Canadian Population Health Research Institute, the Ontario Strategy for Patient Oriented Research Support Unit, the Ontario Ministry of Health and Long-Term Care, the Hamilton Health Sciences Foundation, Physicians’ Services Incorporated Foundation, Michael G. DeGroote Institute for Pain Research and Care, Smith & Nephew (to recruit patients in Spain), and Indiegogo Crowdfunding.
SOURCE: Borges F et al. Lancet. 2020 Feb. 9. doi: 10.1016/S0140-6736(20)30058-1.
An accelerated path to surgery after hip fracture did not improve mortality or major complications, according to a new international randomized trial. However, a fast track to surgery hastened mobilization, weight-bearing, and hospital discharge, and reduced the risk of urinary tract infection and delirium.
The HIP ATTACK (Hip Fracture Accelerated Surgical Treatment and Care Track) study enrolled 2,970 patients (median age, 79 years; 69% women) during March 2014-May 2019. The study excluded patients younger than 45 years, as well as those who were on nonreversible anticoagulation and who had high-energy or more complex hip fractures. In all, 1,487 patients were randomly assigned to the accelerated-surgery group, which received early medical evaluation with a goal of heading to surgery within 6 hours of a hip fracture diagnosis. The goal was achieved, with patients in the intervention arm receiving care at a median 6 hours after diagnosis. Patients in the 69 participating hospitals in 17 countries who were assigned to standard of care received surgery at a median 24 hours after diagnosis (P less than .001).
“Observational data, clinical experience, and biological rationale suggest that the longer a patient is immobile and lying in a bed, the higher the risk of poor outcomes,” wrote principal investigators Philip J. Devereaux, MD, PhD, and Mohit Bhandari, MD, PhD, of McMaster University, Hamilton, Ont., and their colleagues on the HIP ATTACK writing committee.
The study was the first large, randomized trial that directly compared accelerated surgery with standard of care, noted the authors. Previous observational studies had shown worse outcomes for those usual-care patients who waited longer for surgery.
In HIP ATTACK, there was no difference in the primary outcome measures of 90-day mortality and major complications for patients receiving surgery within 6 hours after hip fracture diagnosis, compared with those who received surgery within 24 hours. The coprimary outcome measures included serious complications, such as MI, stroke, venous thromboembolism, sepsis, pneumonia, and life-threatening or major bleeding.
In practice, the researchers found that patients in the accelerated-surgery group received medical clearance in a median time of 2 hours after a diagnosis of hip fracture, whereas the standard of care group was cleared in 4 hours.
At 90 days, 9% of patients in the accelerated-surgery group and 10% of those in the usual-care group had died, a nonsignificant difference between the two groups. In both groups, 22% of patients experienced a major complication. A post hoc analysis that looked for any site-clustering effects did not detect different outcomes, the investigators wrote.
Delirium occurred in 132 patients (9%) of the accelerated-surgery group and in 175 patients (12%) in the usual-care group (odds ratio, 0.72; 95% confidence interval, 0.58-0.92). Infection without sepsis and urinary tract infection were both less common in the accelerated-surgery group (hazard ratio, 0.80 and 0.78, respectively).
The authors noted that the potential benefits of a speedy course to surgery, including reduced immobility and less pain, could be negated if physicians had less time to optimize medical care for older patients with multiple comorbidities and who make up a significant proportion of those who sustain low-energy hip fractures. However, medical complications, such as MI and new-onset atrial fibrillation, were not seen more frequently in the accelerated-surgery group.
In an editorial accompanying the study, Alejandro Lizaur-Utrilla, MD, and Fernando Lopez-Prats, MD, of the Universidad Miguel Hernández, Alicante, Spain, observed that the 6-hour window for hip fracture surgery may be difficult to achieve given clinical practicalities and that, in some cases, the 6-hour window might be unavoidable if severe comorbidities and overall poor health make early surgery inadvisable.
They also expressed concern that, despite the lack of harm shown in the patients who underwent accelerated surgery, the surgery “might negatively affect patients’ outcomes by preventing or limiting the opportunity for optimization of patients’ medical conditions before surgery.” They called for further study to delineate how fitness for surgery affects outcomes in accelerated surgery and to further examine whether the better outcomes are associated with improved cost-effectiveness.
Multiple HIP ATTACK coinvestigators reported relationships with pharmaceutical and medical device companies, including companies that manufacture hip prosthesis and orthopedic surgical devices and implants. The study was sponsored by the Canadian Population Health Research Institute, the Ontario Strategy for Patient Oriented Research Support Unit, the Ontario Ministry of Health and Long-Term Care, the Hamilton Health Sciences Foundation, Physicians’ Services Incorporated Foundation, Michael G. DeGroote Institute for Pain Research and Care, Smith & Nephew (to recruit patients in Spain), and Indiegogo Crowdfunding.
SOURCE: Borges F et al. Lancet. 2020 Feb. 9. doi: 10.1016/S0140-6736(20)30058-1.
Antiepileptic drugs may not independently impair cognition
according to research published online ahead of print Feb. 3 in Neurology. Optimizing AED therapy to reduce or prevent seizures is thus unlikely to affect cognition, according to the investigators.
Patients who take AEDs commonly report cognitive problems, but investigations into the cognitive effects of AEDs have yielded inconsistent results. “We were also interested in this association, as we often treat complex patients taking multiple or high-dose AEDs, and our patients often report cognitive dysfunction,” said Emma Foster, MBBS, an epilepsy fellow at Alfred Health and the Royal Melbourne Hospital in Victoria, Australia. “We were particularly interested to examine how much AEDs affect cognition relative to other factors. We commonly see patients in our tertiary epilepsy care unit who have had severe epilepsy for a long time or who have psychiatric disorders, and these factors may also contribute to cognitive dysfunction.”
Researchers analyzed patients admitted for video EEG monitoring
For their study, Dr. Foster and colleagues prospectively enrolled patients admitted to the Royal Melbourne Hospital’s video EEG monitoring unit between January 2009 and December 2016. Patients were included in the study if they were age 18 years or older, had been admitted for diagnostic or surgical evaluation, and had complete data for the relevant variables. Patients were prescribed AED monotherapy or polytherapy.
The researchers based epilepsy diagnoses on the 2014 International League Against Epilepsy criteria. Diagnoses of psychogenic nonepileptic seizures (PNES) were based on a consensus of epileptologists at weekly multidisciplinary clinical meetings, which was supported by evaluation of all available data. Some patients received a diagnosis of comorbid epilepsy and PNES. If data were insufficient to support a diagnosis of epilepsy or PNES, the admission was considered nondiagnostic.
All participants underwent neuropsychologic and neuropsychiatric screening. Researchers assessed patients’ objective, global cognitive function using the Neuropsychiatry Unit Cognitive Assessment Tool (NUCOG), a validated instrument. Patients responded to the Quality of Life in Epilepsy inventory (QOLIE-89) to provide a measure of subjective cognitive function. They also responded to the Hospital Anxiety and Depression Scale (HADS) to screen for mood disorders.
Dr. Foster and colleagues measured seizure frequency through patient self-report. Patients averaged their seizure frequency during the 12-month period before admission to the video EEG unit. They categorized it according to a 12-point system in which 0 denotes patients who are seizure-free and not taking AEDs and 12 denotes patients in status epilepticus. Patients with PNES used the same scale to report event frequency, although the system was not designed for this purpose.
Almost half of patients were prescribed polypharmacy
The researchers included 331 patients in their analysis. The population’s mean age was 39.3 years, and about 62% of patients were female. Approximately 47% of patients had epilepsy, 25.7% had PNES, 6.6% had comorbid epilepsy and PNES, and 20.5% had a nondiagnostic outcome. Among patients with epilepsy, most (54.5%) had temporal lobe epilepsy, followed by extratemporal focal epilepsy (32.1%) and generalized epilepsy (13.5%). The mean number of AEDs prescribed on admission was 1.6, and mean seizure or event frequency score was 7.2, which indicated 1-3 seizures per month. Mean HADS depression score was within the normal range (5.7), and mean HADS anxiety score was in the borderline range (8.2).
Approximately 45% of patients were prescribed AED polypharmacy on admission, 25.1% were prescribed AED monotherapy, and 29.9% were prescribed no AED. Levetiracetam, valproate, and carbamazepine were the most frequently prescribed AEDs. Most patients with epilepsy (73.1%) were on polypharmacy, compared with 17.6% of patients with PNES, 63.6% of patients with epilepsy and PNES, and 8.8% of nondiagnostic patients.
Older age and greater seizure frequency predicted impaired objective cognitive function. Comorbid epilepsy and PNES appeared to predict impaired objective cognitive function as well, but the data were inconclusive. No AED was a significant predictor of objective cognitive function. Higher depression and anxiety scores and greater seizure frequency predicted impaired subjective cognitive function. No AED predicted subjective cognitive function.
Future studies could address particular cognitive domains
Previous studies have suggested that treatment with topiramate predicts objective or subjective cognitive function, but Dr. Foster and colleagues did not observe this result. The current findings suggest that topiramate may have a less significant effect on cognition than the literature suggests, they wrote. In addition, more evidence is needed to fully understand the effects of clobazam, valproate, phenytoin, and gabapentin because the analysis was underpowered for these drugs.
Although NUCOG assesses global cognitive function reliably, its ability to measure particular cognitive subdomains is limited. “We aim to conduct future research investigating the complex associations between different cognitive functions, including processing speed, and specific AEDs in this heterogeneous population,” said Dr. Foster.
Despite the study’s large sample size, the researchers could not explore potential interactions between various predictor variables. “Epilepsy may interact with the aging process or with other medical conditions associated with aging, such as hypertension and diabetes, and this may increase the risk of cognitive decline,” said Dr. Foster. “Older age may also be associated with reduced capacity to metabolize drugs, increased sensitivity to the cognitive and neurological effects of drugs, less cognitive reserve, and increased likelihood of taking multiple medications, which, along with AEDs, may exert a cognitive effect.”
The current findings may reduce concerns about the effects of AEDs on cognitive function and encourage neurologists to pursue the proper dosing for optimal seizure control, wrote the authors. “However, it is possible that some individuals may be more susceptible than others to AED-related cognitive dysfunction,” said Dr. Foster. “We do not have a robust way to predict who these patients will be, and it is still good practice to make patients aware that some people experience adverse cognitive effects from AEDs. However, it needs to be emphasized that it is unlikely to be the sole reason for their cognitive impairment. Other issues, such as poor seizure control or unrecognized or undertreated mood disorders, are even more important factors for impaired cognition.”
Patients who report cognitive problems should be screened for mood disorders, Dr. Foster continued. “It would also be important to consider whether the patients’ cognitive complaints arise from subtle clinical or subclinical seizure activity and subsequent postictal periods. To investigate this [question] further, clinicians may arrange for prolonged EEG monitoring. This [monitoring] could be done in an ambulatory setting or during an inpatient admission.”
The study was conducted without external funding. Dr. Foster and other investigators reported research funding from professional associations and pharmaceutical companies that was unrelated to the study.
SOURCE: Foster E et al. Neurology. 2020 Feb 3. doi: 10.1212/WNL.0000000000009061.
according to research published online ahead of print Feb. 3 in Neurology. Optimizing AED therapy to reduce or prevent seizures is thus unlikely to affect cognition, according to the investigators.
Patients who take AEDs commonly report cognitive problems, but investigations into the cognitive effects of AEDs have yielded inconsistent results. “We were also interested in this association, as we often treat complex patients taking multiple or high-dose AEDs, and our patients often report cognitive dysfunction,” said Emma Foster, MBBS, an epilepsy fellow at Alfred Health and the Royal Melbourne Hospital in Victoria, Australia. “We were particularly interested to examine how much AEDs affect cognition relative to other factors. We commonly see patients in our tertiary epilepsy care unit who have had severe epilepsy for a long time or who have psychiatric disorders, and these factors may also contribute to cognitive dysfunction.”
Researchers analyzed patients admitted for video EEG monitoring
For their study, Dr. Foster and colleagues prospectively enrolled patients admitted to the Royal Melbourne Hospital’s video EEG monitoring unit between January 2009 and December 2016. Patients were included in the study if they were age 18 years or older, had been admitted for diagnostic or surgical evaluation, and had complete data for the relevant variables. Patients were prescribed AED monotherapy or polytherapy.
The researchers based epilepsy diagnoses on the 2014 International League Against Epilepsy criteria. Diagnoses of psychogenic nonepileptic seizures (PNES) were based on a consensus of epileptologists at weekly multidisciplinary clinical meetings, which was supported by evaluation of all available data. Some patients received a diagnosis of comorbid epilepsy and PNES. If data were insufficient to support a diagnosis of epilepsy or PNES, the admission was considered nondiagnostic.
All participants underwent neuropsychologic and neuropsychiatric screening. Researchers assessed patients’ objective, global cognitive function using the Neuropsychiatry Unit Cognitive Assessment Tool (NUCOG), a validated instrument. Patients responded to the Quality of Life in Epilepsy inventory (QOLIE-89) to provide a measure of subjective cognitive function. They also responded to the Hospital Anxiety and Depression Scale (HADS) to screen for mood disorders.
Dr. Foster and colleagues measured seizure frequency through patient self-report. Patients averaged their seizure frequency during the 12-month period before admission to the video EEG unit. They categorized it according to a 12-point system in which 0 denotes patients who are seizure-free and not taking AEDs and 12 denotes patients in status epilepticus. Patients with PNES used the same scale to report event frequency, although the system was not designed for this purpose.
Almost half of patients were prescribed polypharmacy
The researchers included 331 patients in their analysis. The population’s mean age was 39.3 years, and about 62% of patients were female. Approximately 47% of patients had epilepsy, 25.7% had PNES, 6.6% had comorbid epilepsy and PNES, and 20.5% had a nondiagnostic outcome. Among patients with epilepsy, most (54.5%) had temporal lobe epilepsy, followed by extratemporal focal epilepsy (32.1%) and generalized epilepsy (13.5%). The mean number of AEDs prescribed on admission was 1.6, and mean seizure or event frequency score was 7.2, which indicated 1-3 seizures per month. Mean HADS depression score was within the normal range (5.7), and mean HADS anxiety score was in the borderline range (8.2).
Approximately 45% of patients were prescribed AED polypharmacy on admission, 25.1% were prescribed AED monotherapy, and 29.9% were prescribed no AED. Levetiracetam, valproate, and carbamazepine were the most frequently prescribed AEDs. Most patients with epilepsy (73.1%) were on polypharmacy, compared with 17.6% of patients with PNES, 63.6% of patients with epilepsy and PNES, and 8.8% of nondiagnostic patients.
Older age and greater seizure frequency predicted impaired objective cognitive function. Comorbid epilepsy and PNES appeared to predict impaired objective cognitive function as well, but the data were inconclusive. No AED was a significant predictor of objective cognitive function. Higher depression and anxiety scores and greater seizure frequency predicted impaired subjective cognitive function. No AED predicted subjective cognitive function.
Future studies could address particular cognitive domains
Previous studies have suggested that treatment with topiramate predicts objective or subjective cognitive function, but Dr. Foster and colleagues did not observe this result. The current findings suggest that topiramate may have a less significant effect on cognition than the literature suggests, they wrote. In addition, more evidence is needed to fully understand the effects of clobazam, valproate, phenytoin, and gabapentin because the analysis was underpowered for these drugs.
Although NUCOG assesses global cognitive function reliably, its ability to measure particular cognitive subdomains is limited. “We aim to conduct future research investigating the complex associations between different cognitive functions, including processing speed, and specific AEDs in this heterogeneous population,” said Dr. Foster.
Despite the study’s large sample size, the researchers could not explore potential interactions between various predictor variables. “Epilepsy may interact with the aging process or with other medical conditions associated with aging, such as hypertension and diabetes, and this may increase the risk of cognitive decline,” said Dr. Foster. “Older age may also be associated with reduced capacity to metabolize drugs, increased sensitivity to the cognitive and neurological effects of drugs, less cognitive reserve, and increased likelihood of taking multiple medications, which, along with AEDs, may exert a cognitive effect.”
The current findings may reduce concerns about the effects of AEDs on cognitive function and encourage neurologists to pursue the proper dosing for optimal seizure control, wrote the authors. “However, it is possible that some individuals may be more susceptible than others to AED-related cognitive dysfunction,” said Dr. Foster. “We do not have a robust way to predict who these patients will be, and it is still good practice to make patients aware that some people experience adverse cognitive effects from AEDs. However, it needs to be emphasized that it is unlikely to be the sole reason for their cognitive impairment. Other issues, such as poor seizure control or unrecognized or undertreated mood disorders, are even more important factors for impaired cognition.”
Patients who report cognitive problems should be screened for mood disorders, Dr. Foster continued. “It would also be important to consider whether the patients’ cognitive complaints arise from subtle clinical or subclinical seizure activity and subsequent postictal periods. To investigate this [question] further, clinicians may arrange for prolonged EEG monitoring. This [monitoring] could be done in an ambulatory setting or during an inpatient admission.”
The study was conducted without external funding. Dr. Foster and other investigators reported research funding from professional associations and pharmaceutical companies that was unrelated to the study.
SOURCE: Foster E et al. Neurology. 2020 Feb 3. doi: 10.1212/WNL.0000000000009061.
according to research published online ahead of print Feb. 3 in Neurology. Optimizing AED therapy to reduce or prevent seizures is thus unlikely to affect cognition, according to the investigators.
Patients who take AEDs commonly report cognitive problems, but investigations into the cognitive effects of AEDs have yielded inconsistent results. “We were also interested in this association, as we often treat complex patients taking multiple or high-dose AEDs, and our patients often report cognitive dysfunction,” said Emma Foster, MBBS, an epilepsy fellow at Alfred Health and the Royal Melbourne Hospital in Victoria, Australia. “We were particularly interested to examine how much AEDs affect cognition relative to other factors. We commonly see patients in our tertiary epilepsy care unit who have had severe epilepsy for a long time or who have psychiatric disorders, and these factors may also contribute to cognitive dysfunction.”
Researchers analyzed patients admitted for video EEG monitoring
For their study, Dr. Foster and colleagues prospectively enrolled patients admitted to the Royal Melbourne Hospital’s video EEG monitoring unit between January 2009 and December 2016. Patients were included in the study if they were age 18 years or older, had been admitted for diagnostic or surgical evaluation, and had complete data for the relevant variables. Patients were prescribed AED monotherapy or polytherapy.
The researchers based epilepsy diagnoses on the 2014 International League Against Epilepsy criteria. Diagnoses of psychogenic nonepileptic seizures (PNES) were based on a consensus of epileptologists at weekly multidisciplinary clinical meetings, which was supported by evaluation of all available data. Some patients received a diagnosis of comorbid epilepsy and PNES. If data were insufficient to support a diagnosis of epilepsy or PNES, the admission was considered nondiagnostic.
All participants underwent neuropsychologic and neuropsychiatric screening. Researchers assessed patients’ objective, global cognitive function using the Neuropsychiatry Unit Cognitive Assessment Tool (NUCOG), a validated instrument. Patients responded to the Quality of Life in Epilepsy inventory (QOLIE-89) to provide a measure of subjective cognitive function. They also responded to the Hospital Anxiety and Depression Scale (HADS) to screen for mood disorders.
Dr. Foster and colleagues measured seizure frequency through patient self-report. Patients averaged their seizure frequency during the 12-month period before admission to the video EEG unit. They categorized it according to a 12-point system in which 0 denotes patients who are seizure-free and not taking AEDs and 12 denotes patients in status epilepticus. Patients with PNES used the same scale to report event frequency, although the system was not designed for this purpose.
Almost half of patients were prescribed polypharmacy
The researchers included 331 patients in their analysis. The population’s mean age was 39.3 years, and about 62% of patients were female. Approximately 47% of patients had epilepsy, 25.7% had PNES, 6.6% had comorbid epilepsy and PNES, and 20.5% had a nondiagnostic outcome. Among patients with epilepsy, most (54.5%) had temporal lobe epilepsy, followed by extratemporal focal epilepsy (32.1%) and generalized epilepsy (13.5%). The mean number of AEDs prescribed on admission was 1.6, and mean seizure or event frequency score was 7.2, which indicated 1-3 seizures per month. Mean HADS depression score was within the normal range (5.7), and mean HADS anxiety score was in the borderline range (8.2).
Approximately 45% of patients were prescribed AED polypharmacy on admission, 25.1% were prescribed AED monotherapy, and 29.9% were prescribed no AED. Levetiracetam, valproate, and carbamazepine were the most frequently prescribed AEDs. Most patients with epilepsy (73.1%) were on polypharmacy, compared with 17.6% of patients with PNES, 63.6% of patients with epilepsy and PNES, and 8.8% of nondiagnostic patients.
Older age and greater seizure frequency predicted impaired objective cognitive function. Comorbid epilepsy and PNES appeared to predict impaired objective cognitive function as well, but the data were inconclusive. No AED was a significant predictor of objective cognitive function. Higher depression and anxiety scores and greater seizure frequency predicted impaired subjective cognitive function. No AED predicted subjective cognitive function.
Future studies could address particular cognitive domains
Previous studies have suggested that treatment with topiramate predicts objective or subjective cognitive function, but Dr. Foster and colleagues did not observe this result. The current findings suggest that topiramate may have a less significant effect on cognition than the literature suggests, they wrote. In addition, more evidence is needed to fully understand the effects of clobazam, valproate, phenytoin, and gabapentin because the analysis was underpowered for these drugs.
Although NUCOG assesses global cognitive function reliably, its ability to measure particular cognitive subdomains is limited. “We aim to conduct future research investigating the complex associations between different cognitive functions, including processing speed, and specific AEDs in this heterogeneous population,” said Dr. Foster.
Despite the study’s large sample size, the researchers could not explore potential interactions between various predictor variables. “Epilepsy may interact with the aging process or with other medical conditions associated with aging, such as hypertension and diabetes, and this may increase the risk of cognitive decline,” said Dr. Foster. “Older age may also be associated with reduced capacity to metabolize drugs, increased sensitivity to the cognitive and neurological effects of drugs, less cognitive reserve, and increased likelihood of taking multiple medications, which, along with AEDs, may exert a cognitive effect.”
The current findings may reduce concerns about the effects of AEDs on cognitive function and encourage neurologists to pursue the proper dosing for optimal seizure control, wrote the authors. “However, it is possible that some individuals may be more susceptible than others to AED-related cognitive dysfunction,” said Dr. Foster. “We do not have a robust way to predict who these patients will be, and it is still good practice to make patients aware that some people experience adverse cognitive effects from AEDs. However, it needs to be emphasized that it is unlikely to be the sole reason for their cognitive impairment. Other issues, such as poor seizure control or unrecognized or undertreated mood disorders, are even more important factors for impaired cognition.”
Patients who report cognitive problems should be screened for mood disorders, Dr. Foster continued. “It would also be important to consider whether the patients’ cognitive complaints arise from subtle clinical or subclinical seizure activity and subsequent postictal periods. To investigate this [question] further, clinicians may arrange for prolonged EEG monitoring. This [monitoring] could be done in an ambulatory setting or during an inpatient admission.”
The study was conducted without external funding. Dr. Foster and other investigators reported research funding from professional associations and pharmaceutical companies that was unrelated to the study.
SOURCE: Foster E et al. Neurology. 2020 Feb 3. doi: 10.1212/WNL.0000000000009061.
FROM NEUROLOGY