User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
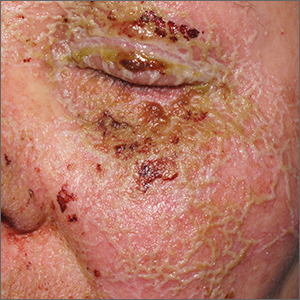
Pustules on face
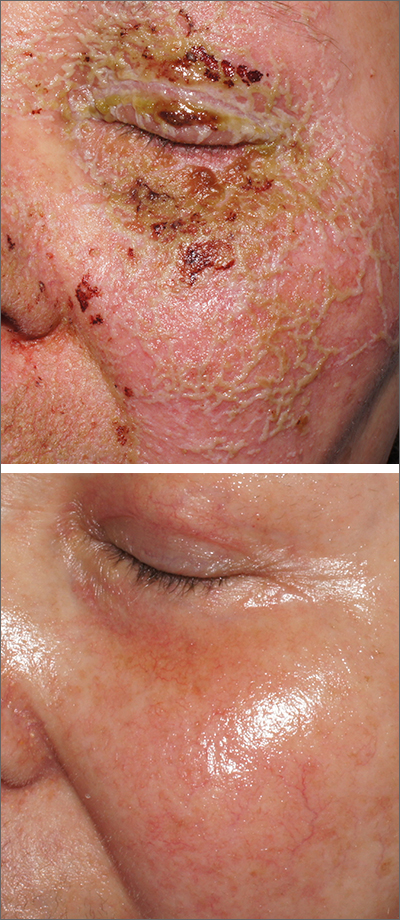
A review of the patient’s chemotherapy medications revealed that 4 weeks earlier, panitumumab had been added to her folinic acid, fluorouracil, and irinotecan (FOLFIRI) regimen. The physician diagnosed this acneiform eruption as an adverse effect of the panitumumab.
Panitumumab is a monoclonal antibody that works to inhibit epidermal growth factor receptor (EGFR) proteins that are overexpressed on some solid tumors and responsible for cancer cell proliferation. EGFR inhibitor–induced acneiform eruptions are common in patients receiving panitumumab.
EGFR proteins have been a target of chemotherapy since the approval of the small molecule erlotinib in 2004. Panitumumab and cetuximab are monoclonal antibodies targeting EGFR and improve long-term survival in patients with metastatic colorectal cancer when added to other standard chemotherapy regimens. EGFR is found throughout the epidermis and all EGFR inhibitors may cause unique skin toxicity not seen with other chemotherapy agents. In 1 study of 229 patients, 59% of patients exhibited skin toxicity at Day 15; the most common examples included widespread acne-like papules and pustules or an eczema-like manifestation.1 Eruptions may be worsened by significant sun exposure while on panitumumab. In this case, the acneiform eruption occurred more intensely along visible facial telangiectasias.
When EGFR inhibitor–induced acneiform eruption occurs, patients commonly develop skin toxicity within the first 2 to 4 weeks of therapy. Pre-therapy doxycycline or minocycline and/or topical steroids may help prevent toxicities from occurring. These same therapies may be used to treat symptoms after they have occurred. More severe cases with systemic symptoms or failure to improve with the above measures may need prednisone or cessation of therapy.
This patient was started on topical hydrocortisone 2.5% ointment twice daily and oral doxycycline 100 mg bid for 6 weeks. She had dramatic improvement within 3 weeks. Doxycycline was subsequently continued at a dose of 100 mg/d and the patient was able to continue with her chemotherapy combination for several more months. Unfortunately, her colon cancer progressed despite therapy and she ultimately died from cancer-related complications.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Bouché O, Ben Abdelghani M, Labourey JL, et al. Management of skin toxicities during panitumumab treatment in metastatic colorectal cancer. World J Gastroenterol. 2019;25:4007-4018. doi: 10.3748/wjg.v25.i29.4007

A review of the patient’s chemotherapy medications revealed that 4 weeks earlier, panitumumab had been added to her folinic acid, fluorouracil, and irinotecan (FOLFIRI) regimen. The physician diagnosed this acneiform eruption as an adverse effect of the panitumumab.
Panitumumab is a monoclonal antibody that works to inhibit epidermal growth factor receptor (EGFR) proteins that are overexpressed on some solid tumors and responsible for cancer cell proliferation. EGFR inhibitor–induced acneiform eruptions are common in patients receiving panitumumab.
EGFR proteins have been a target of chemotherapy since the approval of the small molecule erlotinib in 2004. Panitumumab and cetuximab are monoclonal antibodies targeting EGFR and improve long-term survival in patients with metastatic colorectal cancer when added to other standard chemotherapy regimens. EGFR is found throughout the epidermis and all EGFR inhibitors may cause unique skin toxicity not seen with other chemotherapy agents. In 1 study of 229 patients, 59% of patients exhibited skin toxicity at Day 15; the most common examples included widespread acne-like papules and pustules or an eczema-like manifestation.1 Eruptions may be worsened by significant sun exposure while on panitumumab. In this case, the acneiform eruption occurred more intensely along visible facial telangiectasias.
When EGFR inhibitor–induced acneiform eruption occurs, patients commonly develop skin toxicity within the first 2 to 4 weeks of therapy. Pre-therapy doxycycline or minocycline and/or topical steroids may help prevent toxicities from occurring. These same therapies may be used to treat symptoms after they have occurred. More severe cases with systemic symptoms or failure to improve with the above measures may need prednisone or cessation of therapy.
This patient was started on topical hydrocortisone 2.5% ointment twice daily and oral doxycycline 100 mg bid for 6 weeks. She had dramatic improvement within 3 weeks. Doxycycline was subsequently continued at a dose of 100 mg/d and the patient was able to continue with her chemotherapy combination for several more months. Unfortunately, her colon cancer progressed despite therapy and she ultimately died from cancer-related complications.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A review of the patient’s chemotherapy medications revealed that 4 weeks earlier, panitumumab had been added to her folinic acid, fluorouracil, and irinotecan (FOLFIRI) regimen. The physician diagnosed this acneiform eruption as an adverse effect of the panitumumab.
Panitumumab is a monoclonal antibody that works to inhibit epidermal growth factor receptor (EGFR) proteins that are overexpressed on some solid tumors and responsible for cancer cell proliferation. EGFR inhibitor–induced acneiform eruptions are common in patients receiving panitumumab.
EGFR proteins have been a target of chemotherapy since the approval of the small molecule erlotinib in 2004. Panitumumab and cetuximab are monoclonal antibodies targeting EGFR and improve long-term survival in patients with metastatic colorectal cancer when added to other standard chemotherapy regimens. EGFR is found throughout the epidermis and all EGFR inhibitors may cause unique skin toxicity not seen with other chemotherapy agents. In 1 study of 229 patients, 59% of patients exhibited skin toxicity at Day 15; the most common examples included widespread acne-like papules and pustules or an eczema-like manifestation.1 Eruptions may be worsened by significant sun exposure while on panitumumab. In this case, the acneiform eruption occurred more intensely along visible facial telangiectasias.
When EGFR inhibitor–induced acneiform eruption occurs, patients commonly develop skin toxicity within the first 2 to 4 weeks of therapy. Pre-therapy doxycycline or minocycline and/or topical steroids may help prevent toxicities from occurring. These same therapies may be used to treat symptoms after they have occurred. More severe cases with systemic symptoms or failure to improve with the above measures may need prednisone or cessation of therapy.
This patient was started on topical hydrocortisone 2.5% ointment twice daily and oral doxycycline 100 mg bid for 6 weeks. She had dramatic improvement within 3 weeks. Doxycycline was subsequently continued at a dose of 100 mg/d and the patient was able to continue with her chemotherapy combination for several more months. Unfortunately, her colon cancer progressed despite therapy and she ultimately died from cancer-related complications.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Bouché O, Ben Abdelghani M, Labourey JL, et al. Management of skin toxicities during panitumumab treatment in metastatic colorectal cancer. World J Gastroenterol. 2019;25:4007-4018. doi: 10.3748/wjg.v25.i29.4007
1. Bouché O, Ben Abdelghani M, Labourey JL, et al. Management of skin toxicities during panitumumab treatment in metastatic colorectal cancer. World J Gastroenterol. 2019;25:4007-4018. doi: 10.3748/wjg.v25.i29.4007
Prostate biopsies a laughing (gas) matter?
An old dog – nitrous oxide – can learn new tricks, managing pain in men undergoing transrectal biopsies, researchers reported at the annual meeting of the American Urological Association.
said Heidi Rayala, MD, PhD, assistant professor of surgery at Harvard Medical School, Boston, who helped conduct the study.
Nitrous oxide is best known as a pain medication and anesthetic during dental procedures and childbirth, after trauma, and in end-of-life care.
In the new study, Dr. Rayala and her colleagues at Harvard and Beth Israel-Deaconess Medical Center, Boston, randomly assigned 128 men to self-administered nitrous oxide (SANO) or oxygen as a placebo. Patients in the SANO group had a smaller change in post-biopsy pain score (Visual Analog Scale for pain, 0.43 vs. 1.03; P = .03) and lower odds of experiencing pain during the procedure (odds ratio, 0.45; confidence interval, 0.21-0.97; P = .04).
A comparison of anxiety scores in the two groups failed to find a statistically significant difference between SANO and placebo. However, more men who received nitrous oxide said they tolerated the procedure “better than expected” (61% vs. 41%; P = 0.02), according to the researchers.
Dr. Rayala said that the researchers used the Nitrouseal system (Sedation Systems), in which the patient holds a mask to their face and works with staff to adjust the gas levels to the desired amount. The system is governed to max out at 50% nitrous oxide, ensuring “minimal sedation concentrations, so anesthesia personnel are not required,” she said.
“At levels of less than 50%, patients respond normally to verbal commands and maintain normal airway reflexes,” Dr. Rayala added. “This provides an advantage in that patients do not require the presence of anesthesia personnel.” And because the body eliminates the gas within about 5 minutes, patients do not require an escort home, she said.
This system is also self-scavenging to protect the operating urologist and other personnel from environmental exposure to nitrous oxide.
Dr. Rayala said that three patients (2.3%) found the mask uncomfortable, but in follow-up studies the clinicians have done a better job of preparing patients for the feeling of the mask, making a marked difference. Headaches and nausea are the most commonly reported complaints at concentrations above 50%.
“We did not have patients report headaches or nausea in new study (by the BIDMC group),” she said. This study has been submitted for publication.
Clinicians outside the United States have been quicker to embrace nitrous oxide for prostate procedures.
In a randomized controlled trial, researchers in Australia found no significant improvement in pain scores at 15 minutes from the use of nitrous oxide during transrectal biopsies; however, improvements were seen in patient-reported discomfort, overall experience, and willingness to undergo repeat biopsies.
Stephen McCombie, MD, a consultant at Perth Urology Clinic, Australia, who has been adapting the nitrous oxide protocol for transrectal biopsies to transperineal procedures, said that the Beth Israel study “adds to the evidence to support adjunct use of mild inhalational anesthetics and analgesics during prostate biopsies to improve the patient experience of the procedure.”
He said that the role for these agents may grow with the global trend away from transrectal prostate biopsies and toward transperineal biopsies, largely driven by increasing rates of sepsis after transrectal biopsies.
“While transperineal biopsies can be more painful then transrectal biopsies when performed under local anesthesia, perhaps due to biopsies being taken through the highly sensate perineum as opposed to above the dentate line, optimization of the technique can significantly reduce the discomfort associated with the procedure, which may be further reduced with these agents,” Dr. McCombie said.
“Studies indicate that transperineal biopsies can be more painful than the traditional transrectal biopsies,” Dr. Rayala said. “We do offer transperineal biopsies at BIDMC, and we are gearing up to repeat the SANO study” for those patients.
Dr. Rayala and Dr. McCombie have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An old dog – nitrous oxide – can learn new tricks, managing pain in men undergoing transrectal biopsies, researchers reported at the annual meeting of the American Urological Association.
said Heidi Rayala, MD, PhD, assistant professor of surgery at Harvard Medical School, Boston, who helped conduct the study.
Nitrous oxide is best known as a pain medication and anesthetic during dental procedures and childbirth, after trauma, and in end-of-life care.
In the new study, Dr. Rayala and her colleagues at Harvard and Beth Israel-Deaconess Medical Center, Boston, randomly assigned 128 men to self-administered nitrous oxide (SANO) or oxygen as a placebo. Patients in the SANO group had a smaller change in post-biopsy pain score (Visual Analog Scale for pain, 0.43 vs. 1.03; P = .03) and lower odds of experiencing pain during the procedure (odds ratio, 0.45; confidence interval, 0.21-0.97; P = .04).
A comparison of anxiety scores in the two groups failed to find a statistically significant difference between SANO and placebo. However, more men who received nitrous oxide said they tolerated the procedure “better than expected” (61% vs. 41%; P = 0.02), according to the researchers.
Dr. Rayala said that the researchers used the Nitrouseal system (Sedation Systems), in which the patient holds a mask to their face and works with staff to adjust the gas levels to the desired amount. The system is governed to max out at 50% nitrous oxide, ensuring “minimal sedation concentrations, so anesthesia personnel are not required,” she said.
“At levels of less than 50%, patients respond normally to verbal commands and maintain normal airway reflexes,” Dr. Rayala added. “This provides an advantage in that patients do not require the presence of anesthesia personnel.” And because the body eliminates the gas within about 5 minutes, patients do not require an escort home, she said.
This system is also self-scavenging to protect the operating urologist and other personnel from environmental exposure to nitrous oxide.
Dr. Rayala said that three patients (2.3%) found the mask uncomfortable, but in follow-up studies the clinicians have done a better job of preparing patients for the feeling of the mask, making a marked difference. Headaches and nausea are the most commonly reported complaints at concentrations above 50%.
“We did not have patients report headaches or nausea in new study (by the BIDMC group),” she said. This study has been submitted for publication.
Clinicians outside the United States have been quicker to embrace nitrous oxide for prostate procedures.
In a randomized controlled trial, researchers in Australia found no significant improvement in pain scores at 15 minutes from the use of nitrous oxide during transrectal biopsies; however, improvements were seen in patient-reported discomfort, overall experience, and willingness to undergo repeat biopsies.
Stephen McCombie, MD, a consultant at Perth Urology Clinic, Australia, who has been adapting the nitrous oxide protocol for transrectal biopsies to transperineal procedures, said that the Beth Israel study “adds to the evidence to support adjunct use of mild inhalational anesthetics and analgesics during prostate biopsies to improve the patient experience of the procedure.”
He said that the role for these agents may grow with the global trend away from transrectal prostate biopsies and toward transperineal biopsies, largely driven by increasing rates of sepsis after transrectal biopsies.
“While transperineal biopsies can be more painful then transrectal biopsies when performed under local anesthesia, perhaps due to biopsies being taken through the highly sensate perineum as opposed to above the dentate line, optimization of the technique can significantly reduce the discomfort associated with the procedure, which may be further reduced with these agents,” Dr. McCombie said.
“Studies indicate that transperineal biopsies can be more painful than the traditional transrectal biopsies,” Dr. Rayala said. “We do offer transperineal biopsies at BIDMC, and we are gearing up to repeat the SANO study” for those patients.
Dr. Rayala and Dr. McCombie have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An old dog – nitrous oxide – can learn new tricks, managing pain in men undergoing transrectal biopsies, researchers reported at the annual meeting of the American Urological Association.
said Heidi Rayala, MD, PhD, assistant professor of surgery at Harvard Medical School, Boston, who helped conduct the study.
Nitrous oxide is best known as a pain medication and anesthetic during dental procedures and childbirth, after trauma, and in end-of-life care.
In the new study, Dr. Rayala and her colleagues at Harvard and Beth Israel-Deaconess Medical Center, Boston, randomly assigned 128 men to self-administered nitrous oxide (SANO) or oxygen as a placebo. Patients in the SANO group had a smaller change in post-biopsy pain score (Visual Analog Scale for pain, 0.43 vs. 1.03; P = .03) and lower odds of experiencing pain during the procedure (odds ratio, 0.45; confidence interval, 0.21-0.97; P = .04).
A comparison of anxiety scores in the two groups failed to find a statistically significant difference between SANO and placebo. However, more men who received nitrous oxide said they tolerated the procedure “better than expected” (61% vs. 41%; P = 0.02), according to the researchers.
Dr. Rayala said that the researchers used the Nitrouseal system (Sedation Systems), in which the patient holds a mask to their face and works with staff to adjust the gas levels to the desired amount. The system is governed to max out at 50% nitrous oxide, ensuring “minimal sedation concentrations, so anesthesia personnel are not required,” she said.
“At levels of less than 50%, patients respond normally to verbal commands and maintain normal airway reflexes,” Dr. Rayala added. “This provides an advantage in that patients do not require the presence of anesthesia personnel.” And because the body eliminates the gas within about 5 minutes, patients do not require an escort home, she said.
This system is also self-scavenging to protect the operating urologist and other personnel from environmental exposure to nitrous oxide.
Dr. Rayala said that three patients (2.3%) found the mask uncomfortable, but in follow-up studies the clinicians have done a better job of preparing patients for the feeling of the mask, making a marked difference. Headaches and nausea are the most commonly reported complaints at concentrations above 50%.
“We did not have patients report headaches or nausea in new study (by the BIDMC group),” she said. This study has been submitted for publication.
Clinicians outside the United States have been quicker to embrace nitrous oxide for prostate procedures.
In a randomized controlled trial, researchers in Australia found no significant improvement in pain scores at 15 minutes from the use of nitrous oxide during transrectal biopsies; however, improvements were seen in patient-reported discomfort, overall experience, and willingness to undergo repeat biopsies.
Stephen McCombie, MD, a consultant at Perth Urology Clinic, Australia, who has been adapting the nitrous oxide protocol for transrectal biopsies to transperineal procedures, said that the Beth Israel study “adds to the evidence to support adjunct use of mild inhalational anesthetics and analgesics during prostate biopsies to improve the patient experience of the procedure.”
He said that the role for these agents may grow with the global trend away from transrectal prostate biopsies and toward transperineal biopsies, largely driven by increasing rates of sepsis after transrectal biopsies.
“While transperineal biopsies can be more painful then transrectal biopsies when performed under local anesthesia, perhaps due to biopsies being taken through the highly sensate perineum as opposed to above the dentate line, optimization of the technique can significantly reduce the discomfort associated with the procedure, which may be further reduced with these agents,” Dr. McCombie said.
“Studies indicate that transperineal biopsies can be more painful than the traditional transrectal biopsies,” Dr. Rayala said. “We do offer transperineal biopsies at BIDMC, and we are gearing up to repeat the SANO study” for those patients.
Dr. Rayala and Dr. McCombie have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AUA 2023
CPAP not only solution for sleep apnea
Although continuous positive airway pressure (CPAP) machines are the gold standard in the management of sleep apnea, several other treatments should be considered.
“Just because you have a hammer doesn’t mean everything is a nail,” Kimberly Hardin, MD, professor of clinical internal medicine at University of California, Davis, said at the annual meeting of the American College of Physicians.
“Sleep has been underestimated in the health arena for many, many years,” said Dr. Hardin, who likened sound sleep to the “sixth vital sign.” “We know that sleep plays an integral role in our health.”
Surgical options include nasal surgery and maxillomandibular advancement surgery, also known as double-jaw surgery. Such procedures should be considered only for patients who are unwilling or unable to use CPAP or other nonsurgical treatments.
Sleep apnea occurs in 4% of adult men and 2% of adult women aged 30-60. Most commonly, obstructive sleep apnea involves the cessation or significant decrease in airflow while sleeping. The Apnea Hypopnea Index (AHI) is the number of times a patient experiences apnea or hypopnea during one night divided by the hours of sleep. Normal sleep AHI is fewer than five events per hour on average; mild sleep apnea is five to 14 events; moderate, 15-29; and severe, at least 30 events.
To identify sleep apnea, physicians have several tools at their disposal, starting with preliminary questionnaires that query patients as to whether they are having trouble falling asleep, staying asleep, or are tired during the day. Additional assessment tools include sleep lab testing and at-home testing.
At-home testing has come to include more than the common devices that are worn around the chest and nose for a night.
“It’s not very fun looking,” Dr. Hardin said of the weighty, obtrusive monitoring devices. “So lots of folks have come up with some new ways of doing things.”
These new options incorporate headbands, wrist and finger devices, arterial tonometry, and sleep rings.
Studies show that U.S. adults do not get enough sleep, and poor-quality sleep is as inadequate as insufficient sleep. Barely a third of adults get the minimum 7 hours recommended by the Centers for Disease Control and Prevention. Non-Hispanic Black adults are less likely to report sleeping 7-9 hours and are more likely to report sleeping 6 or fewer hours than are non-Hispanic White and Hispanic adults.
Dr. Hardin said doctors can advise patients to keep their bedrooms quiet, dark, and cool with no TVs or electronics, to maintain regular wake and sleep times, and to stop consuming caffeine late in the day.
Insufficient or poor sleep can have wide-ranging implications on medical conditions such as diabetes, heart disease, obesity, immunodeficiency, cognitive function, mental health, and, ultimately, mortality, according to Dr. Hardin.
“Some people say, ‘Oh, never mind, I can sleep when I’m dead,’ “ Dr. Hardin said. But such a mentality can have a bearing on life expectancy.
A version of this article first appeared on Medscape.com.
Although continuous positive airway pressure (CPAP) machines are the gold standard in the management of sleep apnea, several other treatments should be considered.
“Just because you have a hammer doesn’t mean everything is a nail,” Kimberly Hardin, MD, professor of clinical internal medicine at University of California, Davis, said at the annual meeting of the American College of Physicians.
“Sleep has been underestimated in the health arena for many, many years,” said Dr. Hardin, who likened sound sleep to the “sixth vital sign.” “We know that sleep plays an integral role in our health.”
Surgical options include nasal surgery and maxillomandibular advancement surgery, also known as double-jaw surgery. Such procedures should be considered only for patients who are unwilling or unable to use CPAP or other nonsurgical treatments.
Sleep apnea occurs in 4% of adult men and 2% of adult women aged 30-60. Most commonly, obstructive sleep apnea involves the cessation or significant decrease in airflow while sleeping. The Apnea Hypopnea Index (AHI) is the number of times a patient experiences apnea or hypopnea during one night divided by the hours of sleep. Normal sleep AHI is fewer than five events per hour on average; mild sleep apnea is five to 14 events; moderate, 15-29; and severe, at least 30 events.
To identify sleep apnea, physicians have several tools at their disposal, starting with preliminary questionnaires that query patients as to whether they are having trouble falling asleep, staying asleep, or are tired during the day. Additional assessment tools include sleep lab testing and at-home testing.
At-home testing has come to include more than the common devices that are worn around the chest and nose for a night.
“It’s not very fun looking,” Dr. Hardin said of the weighty, obtrusive monitoring devices. “So lots of folks have come up with some new ways of doing things.”
These new options incorporate headbands, wrist and finger devices, arterial tonometry, and sleep rings.
Studies show that U.S. adults do not get enough sleep, and poor-quality sleep is as inadequate as insufficient sleep. Barely a third of adults get the minimum 7 hours recommended by the Centers for Disease Control and Prevention. Non-Hispanic Black adults are less likely to report sleeping 7-9 hours and are more likely to report sleeping 6 or fewer hours than are non-Hispanic White and Hispanic adults.
Dr. Hardin said doctors can advise patients to keep their bedrooms quiet, dark, and cool with no TVs or electronics, to maintain regular wake and sleep times, and to stop consuming caffeine late in the day.
Insufficient or poor sleep can have wide-ranging implications on medical conditions such as diabetes, heart disease, obesity, immunodeficiency, cognitive function, mental health, and, ultimately, mortality, according to Dr. Hardin.
“Some people say, ‘Oh, never mind, I can sleep when I’m dead,’ “ Dr. Hardin said. But such a mentality can have a bearing on life expectancy.
A version of this article first appeared on Medscape.com.
Although continuous positive airway pressure (CPAP) machines are the gold standard in the management of sleep apnea, several other treatments should be considered.
“Just because you have a hammer doesn’t mean everything is a nail,” Kimberly Hardin, MD, professor of clinical internal medicine at University of California, Davis, said at the annual meeting of the American College of Physicians.
“Sleep has been underestimated in the health arena for many, many years,” said Dr. Hardin, who likened sound sleep to the “sixth vital sign.” “We know that sleep plays an integral role in our health.”
Surgical options include nasal surgery and maxillomandibular advancement surgery, also known as double-jaw surgery. Such procedures should be considered only for patients who are unwilling or unable to use CPAP or other nonsurgical treatments.
Sleep apnea occurs in 4% of adult men and 2% of adult women aged 30-60. Most commonly, obstructive sleep apnea involves the cessation or significant decrease in airflow while sleeping. The Apnea Hypopnea Index (AHI) is the number of times a patient experiences apnea or hypopnea during one night divided by the hours of sleep. Normal sleep AHI is fewer than five events per hour on average; mild sleep apnea is five to 14 events; moderate, 15-29; and severe, at least 30 events.
To identify sleep apnea, physicians have several tools at their disposal, starting with preliminary questionnaires that query patients as to whether they are having trouble falling asleep, staying asleep, or are tired during the day. Additional assessment tools include sleep lab testing and at-home testing.
At-home testing has come to include more than the common devices that are worn around the chest and nose for a night.
“It’s not very fun looking,” Dr. Hardin said of the weighty, obtrusive monitoring devices. “So lots of folks have come up with some new ways of doing things.”
These new options incorporate headbands, wrist and finger devices, arterial tonometry, and sleep rings.
Studies show that U.S. adults do not get enough sleep, and poor-quality sleep is as inadequate as insufficient sleep. Barely a third of adults get the minimum 7 hours recommended by the Centers for Disease Control and Prevention. Non-Hispanic Black adults are less likely to report sleeping 7-9 hours and are more likely to report sleeping 6 or fewer hours than are non-Hispanic White and Hispanic adults.
Dr. Hardin said doctors can advise patients to keep their bedrooms quiet, dark, and cool with no TVs or electronics, to maintain regular wake and sleep times, and to stop consuming caffeine late in the day.
Insufficient or poor sleep can have wide-ranging implications on medical conditions such as diabetes, heart disease, obesity, immunodeficiency, cognitive function, mental health, and, ultimately, mortality, according to Dr. Hardin.
“Some people say, ‘Oh, never mind, I can sleep when I’m dead,’ “ Dr. Hardin said. But such a mentality can have a bearing on life expectancy.
A version of this article first appeared on Medscape.com.
FROM INTERNAL MEDICINE 2023
Why is buprenorphine use flatlining?
Opioid overdose deaths are at a record high in the United States, and many of these deaths can be prevented with medications such as buprenorphine, said lead author Kao-Ping Chua, MD, of the University of Michigan, Ann Arbor, in an interview. “However, buprenorphine cannot prevent opioid overdose deaths if patients are never started on the medication or only stay on the medication for a short time. For that reason, rates of buprenorphine initiation and retention are critical metrics for measuring how well the U.S. health care system is responding to the opioid epidemic,” he said.
“At the time we started our study, several other research groups had evaluated U.S. rates of buprenorphine initiation and retention using data through 2020. However, more recent national data were lacking,” Dr. Chua told this news organization. “We felt that this was an important knowledge gap given the many changes in society that have occurred since 2020,” he noted. “For example, it was possible that the relaxation of social distancing measures during 2021 and 2022 might have reduced barriers to health care visits, thereby increasing opportunities to initiate treatment for opioid addiction with buprenorphine,” he said.
Dr. Chua and colleagues used data from the IQVIA Longitudinal Prescription Database, which reports 92% of prescriptions dispensed from retail pharmacies in the United States. “Buprenorphine products included immediate-release and extended-release formulations approved for opioid use disorder but not formulations primarily used to treat pain,” they write.
Monthly buprenorphine initiation was defined as the number of patients initiating therapy per 100,000 individuals. For retention, the researchers used a National Quality Forum-endorsed quality measure that defined retention as continuous use of buprenorphine for at least 180 days.
A total of 3,006,629 patients began buprenorphine therapy during the study period; approximately 43% were female.
During the first years of the study period, from January 2016 through September 2018, the monthly buprenorphine initiation rate increased from 12.5 per 100,000 to 15.9 per 100,000, with a statistically significant monthly percentage change of 0.62% (P < .001).
However, from October 2018 through October 2022, the monthly percentage remained essentially the same (P = .62) with a monthly percentage change of −0.03%.
From March 2020 through December 2020, the median monthly buprenorphine initiation rate was 14.4 per 100,000, only slightly lower than the rates from January 2019 through February 2020 and from January 2021 through October 2022 (15.5 per 100,000 and 15.0 per 100,000, respectively).
Over the entire study period from January 2016 through October 2022, the median monthly retention rate for buprenorphine use was 22.2%. This rate increased minimally, with no significant changes in slope and a monthly percentage change of 0.08% (P = .04).
The study findings were limited by several factors, including a lack of data on race and ethnicity, in-clinic administration of buprenorphine, and buprenorphine dispensing through methadone outpatient programs, the researchers note. Also, data did not indicate whether some patients began buprenorphine to treat pain, they say. The timing of the flattening of buprenorphine use also suggests the influence of factors beyond the COVID-19 pandemic, they write.
However, the results were strengthened by the large sample size and suggest that efforts to date to increase buprenorphine use have been unsuccessful, the researchers write. “A comprehensive approach is needed to eliminate barriers to buprenorphine initiation and retention, such as stigma and uneven access to prescribers,” they conclude.
Study highlights underuse of buprenorphine option
“Our study shows that buprenorphine initiation rates have been flat since the end of 2018 and that rates of 180-day retention in buprenorphine therapy have remained low throughout 2016-2022,” Dr. Chua told this news organization. “Neither of these findings are particularly surprising, but they are disappointing,” he said. “There were a lot of policy and clinical efforts to maintain and expand access to buprenorphine during the COVID-19 pandemic, such as allowing buprenorphine to be prescribed via telehealth without an in-person visit and eliminating training requirements for the waiver that previously was required to prescribe buprenorphine.
“The fact that buprenorphine initiation and retention did not rise after these efforts were implemented suggests that they were insufficient to meet the rising need for this medication,” he said.
The current study “adds to a growing body of research suggesting that clinicians are not maximizing opportunities to initiate buprenorphine treatment among patients with opioid addiction,” Dr. Chua said. He cited another of his recent studies in which 1 in 12 patients were prescribed buprenorphine within 30 days of an emergency department visit for opioid overdose from August 2019 to April 2021, but half of patients with emergency department visits with anaphylaxis were prescribed anepinephrine auto-injector.
“My hope is that our new study will further underscore to clinicians how much the health care system is underusing a critical tool to prevent opioid overdose deaths,” he said.
The federal government’s recent elimination of the waiver needed to prescribe buprenorphine may move the needle, but to what degree remains to be seen, Dr. Chua added. “It is possible this intervention will be insufficient to overcome the many other barriers to buprenorphine initiation and retention, such as stigma about the drug among clinicians, patients, and pharmacists,” he said.
Lack of education remains a barrier to buprenorphine use
The current study is important to determine whether attempts to increase buprenorphine initiation and treatment retention are working, said Reuben J. Strayer, MD, director of addiction medicine in the emergency medicine department at Maimonides Medical Center, New York, in an interview.
Dr. Strayer was not involved in the current study, but said he was surprised that initiation of buprenorphine didn’t decrease more dramatically during the pandemic, given the significant barriers to accessing care during that time.
However, “efforts to increase buprenorphine initiation and retention have not been sufficiently effective,” Dr. Strayer said. “The rise of fentanyl as a primary street opioid, replacing heroin, has dissuaded both patients and providers from initiating buprenorphine for fear of precipitated withdrawal.”
The elimination of the DATA 2000 (X) waiver was the removal of a potential barrier to increased buprenorphine use, said Dr. Strayer. “Now that the DATA 2000 (X) waiver has been eliminated, the focus of buprenorphine access is educating primary care and inpatient providers on its use, so that patients with OUD [opioid use disorder] can be treated, regardless of the venue at which they seek care,” he said.
Looking ahead, “The priority in buprenorphine research is determining the most effective way to initiate buprenorphine without the risk of precipitated withdrawal,” Dr. Strayer added.
The study was supported in part by the Benter Foundation, the Michigan Department of Health and Human Services, and the Susan B. Meister Child Health Evaluation and Research Center in the department of pediatrics at the University of Michigan. Dr. Chua was supported by the National Institute on Drug Abuse. Dr. Strayer has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths are at a record high in the United States, and many of these deaths can be prevented with medications such as buprenorphine, said lead author Kao-Ping Chua, MD, of the University of Michigan, Ann Arbor, in an interview. “However, buprenorphine cannot prevent opioid overdose deaths if patients are never started on the medication or only stay on the medication for a short time. For that reason, rates of buprenorphine initiation and retention are critical metrics for measuring how well the U.S. health care system is responding to the opioid epidemic,” he said.
“At the time we started our study, several other research groups had evaluated U.S. rates of buprenorphine initiation and retention using data through 2020. However, more recent national data were lacking,” Dr. Chua told this news organization. “We felt that this was an important knowledge gap given the many changes in society that have occurred since 2020,” he noted. “For example, it was possible that the relaxation of social distancing measures during 2021 and 2022 might have reduced barriers to health care visits, thereby increasing opportunities to initiate treatment for opioid addiction with buprenorphine,” he said.
Dr. Chua and colleagues used data from the IQVIA Longitudinal Prescription Database, which reports 92% of prescriptions dispensed from retail pharmacies in the United States. “Buprenorphine products included immediate-release and extended-release formulations approved for opioid use disorder but not formulations primarily used to treat pain,” they write.
Monthly buprenorphine initiation was defined as the number of patients initiating therapy per 100,000 individuals. For retention, the researchers used a National Quality Forum-endorsed quality measure that defined retention as continuous use of buprenorphine for at least 180 days.
A total of 3,006,629 patients began buprenorphine therapy during the study period; approximately 43% were female.
During the first years of the study period, from January 2016 through September 2018, the monthly buprenorphine initiation rate increased from 12.5 per 100,000 to 15.9 per 100,000, with a statistically significant monthly percentage change of 0.62% (P < .001).
However, from October 2018 through October 2022, the monthly percentage remained essentially the same (P = .62) with a monthly percentage change of −0.03%.
From March 2020 through December 2020, the median monthly buprenorphine initiation rate was 14.4 per 100,000, only slightly lower than the rates from January 2019 through February 2020 and from January 2021 through October 2022 (15.5 per 100,000 and 15.0 per 100,000, respectively).
Over the entire study period from January 2016 through October 2022, the median monthly retention rate for buprenorphine use was 22.2%. This rate increased minimally, with no significant changes in slope and a monthly percentage change of 0.08% (P = .04).
The study findings were limited by several factors, including a lack of data on race and ethnicity, in-clinic administration of buprenorphine, and buprenorphine dispensing through methadone outpatient programs, the researchers note. Also, data did not indicate whether some patients began buprenorphine to treat pain, they say. The timing of the flattening of buprenorphine use also suggests the influence of factors beyond the COVID-19 pandemic, they write.
However, the results were strengthened by the large sample size and suggest that efforts to date to increase buprenorphine use have been unsuccessful, the researchers write. “A comprehensive approach is needed to eliminate barriers to buprenorphine initiation and retention, such as stigma and uneven access to prescribers,” they conclude.
Study highlights underuse of buprenorphine option
“Our study shows that buprenorphine initiation rates have been flat since the end of 2018 and that rates of 180-day retention in buprenorphine therapy have remained low throughout 2016-2022,” Dr. Chua told this news organization. “Neither of these findings are particularly surprising, but they are disappointing,” he said. “There were a lot of policy and clinical efforts to maintain and expand access to buprenorphine during the COVID-19 pandemic, such as allowing buprenorphine to be prescribed via telehealth without an in-person visit and eliminating training requirements for the waiver that previously was required to prescribe buprenorphine.
“The fact that buprenorphine initiation and retention did not rise after these efforts were implemented suggests that they were insufficient to meet the rising need for this medication,” he said.
The current study “adds to a growing body of research suggesting that clinicians are not maximizing opportunities to initiate buprenorphine treatment among patients with opioid addiction,” Dr. Chua said. He cited another of his recent studies in which 1 in 12 patients were prescribed buprenorphine within 30 days of an emergency department visit for opioid overdose from August 2019 to April 2021, but half of patients with emergency department visits with anaphylaxis were prescribed anepinephrine auto-injector.
“My hope is that our new study will further underscore to clinicians how much the health care system is underusing a critical tool to prevent opioid overdose deaths,” he said.
The federal government’s recent elimination of the waiver needed to prescribe buprenorphine may move the needle, but to what degree remains to be seen, Dr. Chua added. “It is possible this intervention will be insufficient to overcome the many other barriers to buprenorphine initiation and retention, such as stigma about the drug among clinicians, patients, and pharmacists,” he said.
Lack of education remains a barrier to buprenorphine use
The current study is important to determine whether attempts to increase buprenorphine initiation and treatment retention are working, said Reuben J. Strayer, MD, director of addiction medicine in the emergency medicine department at Maimonides Medical Center, New York, in an interview.
Dr. Strayer was not involved in the current study, but said he was surprised that initiation of buprenorphine didn’t decrease more dramatically during the pandemic, given the significant barriers to accessing care during that time.
However, “efforts to increase buprenorphine initiation and retention have not been sufficiently effective,” Dr. Strayer said. “The rise of fentanyl as a primary street opioid, replacing heroin, has dissuaded both patients and providers from initiating buprenorphine for fear of precipitated withdrawal.”
The elimination of the DATA 2000 (X) waiver was the removal of a potential barrier to increased buprenorphine use, said Dr. Strayer. “Now that the DATA 2000 (X) waiver has been eliminated, the focus of buprenorphine access is educating primary care and inpatient providers on its use, so that patients with OUD [opioid use disorder] can be treated, regardless of the venue at which they seek care,” he said.
Looking ahead, “The priority in buprenorphine research is determining the most effective way to initiate buprenorphine without the risk of precipitated withdrawal,” Dr. Strayer added.
The study was supported in part by the Benter Foundation, the Michigan Department of Health and Human Services, and the Susan B. Meister Child Health Evaluation and Research Center in the department of pediatrics at the University of Michigan. Dr. Chua was supported by the National Institute on Drug Abuse. Dr. Strayer has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths are at a record high in the United States, and many of these deaths can be prevented with medications such as buprenorphine, said lead author Kao-Ping Chua, MD, of the University of Michigan, Ann Arbor, in an interview. “However, buprenorphine cannot prevent opioid overdose deaths if patients are never started on the medication or only stay on the medication for a short time. For that reason, rates of buprenorphine initiation and retention are critical metrics for measuring how well the U.S. health care system is responding to the opioid epidemic,” he said.
“At the time we started our study, several other research groups had evaluated U.S. rates of buprenorphine initiation and retention using data through 2020. However, more recent national data were lacking,” Dr. Chua told this news organization. “We felt that this was an important knowledge gap given the many changes in society that have occurred since 2020,” he noted. “For example, it was possible that the relaxation of social distancing measures during 2021 and 2022 might have reduced barriers to health care visits, thereby increasing opportunities to initiate treatment for opioid addiction with buprenorphine,” he said.
Dr. Chua and colleagues used data from the IQVIA Longitudinal Prescription Database, which reports 92% of prescriptions dispensed from retail pharmacies in the United States. “Buprenorphine products included immediate-release and extended-release formulations approved for opioid use disorder but not formulations primarily used to treat pain,” they write.
Monthly buprenorphine initiation was defined as the number of patients initiating therapy per 100,000 individuals. For retention, the researchers used a National Quality Forum-endorsed quality measure that defined retention as continuous use of buprenorphine for at least 180 days.
A total of 3,006,629 patients began buprenorphine therapy during the study period; approximately 43% were female.
During the first years of the study period, from January 2016 through September 2018, the monthly buprenorphine initiation rate increased from 12.5 per 100,000 to 15.9 per 100,000, with a statistically significant monthly percentage change of 0.62% (P < .001).
However, from October 2018 through October 2022, the monthly percentage remained essentially the same (P = .62) with a monthly percentage change of −0.03%.
From March 2020 through December 2020, the median monthly buprenorphine initiation rate was 14.4 per 100,000, only slightly lower than the rates from January 2019 through February 2020 and from January 2021 through October 2022 (15.5 per 100,000 and 15.0 per 100,000, respectively).
Over the entire study period from January 2016 through October 2022, the median monthly retention rate for buprenorphine use was 22.2%. This rate increased minimally, with no significant changes in slope and a monthly percentage change of 0.08% (P = .04).
The study findings were limited by several factors, including a lack of data on race and ethnicity, in-clinic administration of buprenorphine, and buprenorphine dispensing through methadone outpatient programs, the researchers note. Also, data did not indicate whether some patients began buprenorphine to treat pain, they say. The timing of the flattening of buprenorphine use also suggests the influence of factors beyond the COVID-19 pandemic, they write.
However, the results were strengthened by the large sample size and suggest that efforts to date to increase buprenorphine use have been unsuccessful, the researchers write. “A comprehensive approach is needed to eliminate barriers to buprenorphine initiation and retention, such as stigma and uneven access to prescribers,” they conclude.
Study highlights underuse of buprenorphine option
“Our study shows that buprenorphine initiation rates have been flat since the end of 2018 and that rates of 180-day retention in buprenorphine therapy have remained low throughout 2016-2022,” Dr. Chua told this news organization. “Neither of these findings are particularly surprising, but they are disappointing,” he said. “There were a lot of policy and clinical efforts to maintain and expand access to buprenorphine during the COVID-19 pandemic, such as allowing buprenorphine to be prescribed via telehealth without an in-person visit and eliminating training requirements for the waiver that previously was required to prescribe buprenorphine.
“The fact that buprenorphine initiation and retention did not rise after these efforts were implemented suggests that they were insufficient to meet the rising need for this medication,” he said.
The current study “adds to a growing body of research suggesting that clinicians are not maximizing opportunities to initiate buprenorphine treatment among patients with opioid addiction,” Dr. Chua said. He cited another of his recent studies in which 1 in 12 patients were prescribed buprenorphine within 30 days of an emergency department visit for opioid overdose from August 2019 to April 2021, but half of patients with emergency department visits with anaphylaxis were prescribed anepinephrine auto-injector.
“My hope is that our new study will further underscore to clinicians how much the health care system is underusing a critical tool to prevent opioid overdose deaths,” he said.
The federal government’s recent elimination of the waiver needed to prescribe buprenorphine may move the needle, but to what degree remains to be seen, Dr. Chua added. “It is possible this intervention will be insufficient to overcome the many other barriers to buprenorphine initiation and retention, such as stigma about the drug among clinicians, patients, and pharmacists,” he said.
Lack of education remains a barrier to buprenorphine use
The current study is important to determine whether attempts to increase buprenorphine initiation and treatment retention are working, said Reuben J. Strayer, MD, director of addiction medicine in the emergency medicine department at Maimonides Medical Center, New York, in an interview.
Dr. Strayer was not involved in the current study, but said he was surprised that initiation of buprenorphine didn’t decrease more dramatically during the pandemic, given the significant barriers to accessing care during that time.
However, “efforts to increase buprenorphine initiation and retention have not been sufficiently effective,” Dr. Strayer said. “The rise of fentanyl as a primary street opioid, replacing heroin, has dissuaded both patients and providers from initiating buprenorphine for fear of precipitated withdrawal.”
The elimination of the DATA 2000 (X) waiver was the removal of a potential barrier to increased buprenorphine use, said Dr. Strayer. “Now that the DATA 2000 (X) waiver has been eliminated, the focus of buprenorphine access is educating primary care and inpatient providers on its use, so that patients with OUD [opioid use disorder] can be treated, regardless of the venue at which they seek care,” he said.
Looking ahead, “The priority in buprenorphine research is determining the most effective way to initiate buprenorphine without the risk of precipitated withdrawal,” Dr. Strayer added.
The study was supported in part by the Benter Foundation, the Michigan Department of Health and Human Services, and the Susan B. Meister Child Health Evaluation and Research Center in the department of pediatrics at the University of Michigan. Dr. Chua was supported by the National Institute on Drug Abuse. Dr. Strayer has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Lose weight, gain huge debt: N.Y. provider has sued more than 300 patients who had bariatric surgery
Seven months after Lahavah Wallace’s weight-loss operation, a New York bariatric surgery practice sued her, accusing her of “intentionally” failing to pay nearly $18,000 of her bill.
Long Island Minimally Invasive Surgery, which does business as the New York Bariatric Group, went on to accuse Ms. Wallace of “embezzlement,” alleging she kept insurance payments that should have been turned over to the practice.
Ms. Wallace denies the allegations, which the bariatric practice has leveled against patients in hundreds of debt-collection lawsuits filed over the past 4 years, court records in New York state show.
In about 60 cases, the lawsuits demanded $100,000 or more from patients. Some patients were found liable for tens of thousands of dollars in interest charges or wound up shackled with debt that could take a decade or more to shake. Others are facing the likely prospect of six-figure financial penalties, court records show.
Backed by a major private equity firm, the bariatric practice spends millions each year on advertisements featuring patients who have dropped 100 pounds or more after bariatric procedures, sometimes having had a portion of their stomachs removed. The ads have run on TV, online, and on New York City subway posters.
The online ads, often showcasing the slogan “Stop obesity for life,” appealed to Ms. Wallace, who lives in Brooklyn and works as a legal assistant for the state of New York. She said she turned over checks from her insurer to the bariatric group and was stunned when the medical practice hauled her into court citing an “out-of-network payment agreement” she had signed before her surgery.
“I really didn’t know what I was signing,” Ms. Wallace told KFF Health News. “I didn’t pay enough attention.”
Shawn Garber, MD, a bariatric surgeon who founded the practice in 2000 on Long Island and serves as its CEO, said that “prior to rendering services” his office staff advises patients of the costs and their responsibility to pay the bill.
The bariatric group has cited these out-of-network payment agreements in at least 300 lawsuits filed against patients from January 2019 to 2022 demanding nearly $19 million to cover medical bills, interest charges, and attorney’s fees, a KFF Health News review of New York state court records found.
Danny De Voe, a partner at Sahn Ward Braff Koblenz law firm in Uniondale, N.Y., who filed many of those suits, declined to comment, citing attorney-client privilege.
In most cases, the medical practice had agreed to accept an insurance company’s out-of-network rate as full payment for its services – with caveats, according to court filings.
In the agreements they signed, patients promised to pay any coinsurance, meeting any deductible, and pass on to the medical practice any reimbursement checks they received from their health plans within 7 days.
KFF Health News found – while legal fees and other costs can layer on thousands more.
Elisabeth Benjamin, a lawyer with the Community Service Society of New York, said conflicts can arise when insurers send checks to pay for out-of-network medical services to patients rather than reimbursing a medical provider directly.
“We would prefer to see regulators step in and stop that practice,” she said, adding it “causes tension between providers and patients.”
That’s certainly true for Ms. Wallace. The surgery practice sued her in August 2022demanding $17,981 in fees it said remained unpaid after her January 2022 laparoscopic sleeve gastrectomy, an operation in which much of the stomach is removed to assist weight loss.
The lawsuit also tacked on a demand for $5,993 in attorney’s fees, court records show.
The suit alleges Ms. Wallace signed the contract even though she “had no intention” of paying her bills. The complaint goes on to accuse her of “committing embezzlement” by “willfully, intentionally, deliberately and maliciously” depositing checks from her health plan into her personal account.
The suit doesn’t include details to substantiate these claims, and Ms. Wallace said in her court response they are not true. Ms. Wallace said she turned over checks for the charges.
“They billed the insurance for everything they possibly could,” Ms. Wallace said.
In September, Ms. Wallace filed for bankruptcy, hoping to discharge the bariatric care debt along with about $4,700 in unrelated credit card charges.
The medical practice fired back in November by filing an “adversary complaint” in her Brooklyn bankruptcy court proceeding that argues her medical debt should not be forgiven because Ms. Wallace committed fraud.
The adversary complaint, which is pending in the bankruptcy case, accuses Ms. Wallace of “fraudulently” inducing the surgery center to perform “elective medical procedures” without requiring payment up front.
Both the harsh wording and claims of wrongdoing have infuriated Ms. Wallace and her attorney, Jacob Silver, of Brooklyn.
Mr. Silver wants the medical practice to turn over records of the payments received from Ms. Wallace. “There is no fraud here,” he said. “This is frivolous. We are taking a no-settlement position.”
Gaining debt
Few patients sued by the bariatric practice mount a defense in court and those who do fight often lose, court records show.
The medical practice won default judgments totaling nearly $6 million in about 90 of the 300 cases in the sample reviewed by KFF Health News. Default judgments are entered when the defendant fails to respond.
Many cases either are pending, or it is not clear from court filings how they were resolved.
Some patients tried to argue that the fees were too high or that they didn’t understand going in how much they could owe. One woman, trying to push back against a demand for more than $100,000, said in a legal filing that she “was given numerous papers to sign without anyone of the staff members explaining to me what it actually meant.” Another patient, who was sued for more than $40,000, wrote: “I don’t have the means to pay this bill.”
Among the cases described in court records:
- A Westchester County, N.Y., woman was sued for $102,556 and settled for $72,000 in May 2021. She agreed to pay $7,500 upon signing the settlement and $500 a month from September 2021 to May 2032.
- A Peekskill, N.Y., woman in a December 2019 judgment was held liable for $384,092, which included $94,047 in interest.
- A Newburgh, N.Y., man was sued in 2021 for $252,309 in medical bills, 12% interest, and $84,103 in attorneys’ fees. The case is pending.
Robert Cohen, a longtime attorney for the bariatric practice, testified in a November 2021 hearing that the lawyers take “a contingency fee of one-third of our recovery” in these cases. In that case, Mr. Cohen had requested $13,578 based on his contingency fee arrangement. He testified that he spent 7.3 hours on the case and that his customary billing rate was $475 per hour, which came to $3,467.50. The judge awarded the lower amount, according to a transcript of the hearing.
Teresa LaMasters, MD, president of the American Society for Metabolic and Bariatric Surgery, said suing patients for large sums “is not a common practice” among bariatric surgeons.
“This is not what the vast majority in the field would espouse,” she said.
But Dr. Garber, the NYBG’s chief executive, suggested patients deserve blame.
“These lawsuits stem from these patients stealing the insurance money rather than forwarding it onto NYBG as they are morally and contractually obligated to do,” Dr. Garber wrote in an email to KFF Health News.
Dr. Garber added: “The issue is not with what we bill, but rather with the fact that the insurance companies refuse to send payment directly to us.”
‘A kooky system’
Defense attorneys argue that many patients don’t fully comprehend the perils of failing to pay on time – for whatever reason.
In a few cases, patients admitted pocketing checks they were obligated to turn over to the medical practice. But for the most part, court records don’t specify how many such checks were issued and for what amounts – or whether the patient improperly cashed them.
“It’s a kooky system,” said Paul Brite, an attorney who has faced off against the bariatric practice in court.
“You sign these documents that could cost you tons of money. It shouldn’t be that way,” he said. “This can ruin their financial life.”
New York lawmakers have acted to limit the damage from medical debt, including “surprise bills.”
In November, Democratic Gov. Kathy Hochul signed legislation that prohibits health care providers from slapping liens on a primary residence or garnishing wages.
But contracts with onerous repayment terms represent an “evolving area of law” and an alarming “new twist” on concerns over medical debt, said Ms. Benjamin, the community service society lawyer.
She said contract “accelerator clauses” that trigger severe penalties if patients miss payments should not be permitted for medical debt.
“If you default, the full amount is due,” she said. “This is really a bummer.”
‘Fair market value’
The debt collection lawsuits argue that weight-loss patients had agreed to pay “fair market value” for services – and the doctors are only trying to secure money they are due.
But some prices far exceed typical insurance payments for obesity treatments across the country, according to a medical billing data registry. Surgeons performed about 200,000 bariatric operations in 2020, according to the bariatric surgery society.
Ms. Wallace, the Brooklyn legal assistant, was billed $60,500 for her lap sleeve gastrectomy, though how much her insurance actually paid remains to be hashed out in court.
Michael Arrigo, a California medical billing expert at No World Borders, called the prices “outrageous” and “unreasonable and, in fact, likely unconscionable.”
“I disagree that these are fair market charges,” he said.
Dr. LaMasters called the gastrectomy price billed to Ms. Wallace “really expensive” and “a severe outlier.” While charges vary by region, she quoted a typical price of around $22,000.
Dr. Garber said NYBG “bills at usual and customary rates” determined by Fair Health, a New York City-based repository of insurance claims data. Fair Health “sets these rates based upon the acceptable price for our geographic location,” he said.
But Rachel Kent, Fair Health’s senior director of marketing, told KFF Health News that the group “does not set rates, nor determine or take any position on what constitutes ‘usual and customary rates.’ ” Instead, it reports the prices providers are charging in a given area.
Overall, Fair Health data shows huge price variations even in adjacent ZIP codes in the metro area. In Long Island’s Roslyn Heights neighborhood, where NYBG is based, Fair Health lists the out-of-network price charged by providers in the area as $60,500, the figure Ms. Wallace was billed.
But in several other New York City–area ZIP codes the price charged for the gastrectomy procedure hovers around $20,000, according to the data bank. The price in Manhattan is $17,500, for instance, according to Fair Health.
Nationwide, the average cost in 2021 for bariatric surgery done in a hospital was $32,868, according to a KFF analysis of health insurance claims.
Private equity arrives
Dr. Garber said in a court affidavit in May 2022 that he founded the bariatric practice “with a singular focus: providing safe, effective care to patients suffering from obesity and its resulting complications.”
Under his leadership, the practice has “developed into New York’s elite institution for obesity treatment,” Dr. Garber said. He said the group’s surgeons are “highly sought after to train other bariatric surgeons throughout the country and are active in the development of new, cutting-edge bariatric surgery techniques.”
In 2017, Dr. Garber and partners agreed on a business plan to help spur growth and “attract private equity investment,” according to the affidavit.
They formed a separate company to handle the bariatric practice’s business side. Known as management services organizations, such companies provide a way for private equity investors to circumvent laws in some states that prohibit nonphysicians from owning a stake in a medical practice.
In August 2019, the private equity firm Sentinel Capital Partners bought 65% of the MSO for $156.5 million, according to Dr. Garber’s affidavit. The management company is now known as New You Bariatric Group. The private equity firm did not respond to requests for comment.
Dr. Garber, in a September 2021 American Society for Metabolic and Bariatric Surgery webinar viewable online, said the weight-loss practice spends $6 million a year on media and marketing directly to patients – and is on a roll. Nationally, bariatric surgery is growing 6% annually, he said. NYBG boasts two dozen offices in the tri-state area of New York, New Jersey, and Connecticut and is poised to expand into more states.
“Since private equity, we’ve been growing at 30%-40% year over year,” Dr. Garber said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Seven months after Lahavah Wallace’s weight-loss operation, a New York bariatric surgery practice sued her, accusing her of “intentionally” failing to pay nearly $18,000 of her bill.
Long Island Minimally Invasive Surgery, which does business as the New York Bariatric Group, went on to accuse Ms. Wallace of “embezzlement,” alleging she kept insurance payments that should have been turned over to the practice.
Ms. Wallace denies the allegations, which the bariatric practice has leveled against patients in hundreds of debt-collection lawsuits filed over the past 4 years, court records in New York state show.
In about 60 cases, the lawsuits demanded $100,000 or more from patients. Some patients were found liable for tens of thousands of dollars in interest charges or wound up shackled with debt that could take a decade or more to shake. Others are facing the likely prospect of six-figure financial penalties, court records show.
Backed by a major private equity firm, the bariatric practice spends millions each year on advertisements featuring patients who have dropped 100 pounds or more after bariatric procedures, sometimes having had a portion of their stomachs removed. The ads have run on TV, online, and on New York City subway posters.
The online ads, often showcasing the slogan “Stop obesity for life,” appealed to Ms. Wallace, who lives in Brooklyn and works as a legal assistant for the state of New York. She said she turned over checks from her insurer to the bariatric group and was stunned when the medical practice hauled her into court citing an “out-of-network payment agreement” she had signed before her surgery.
“I really didn’t know what I was signing,” Ms. Wallace told KFF Health News. “I didn’t pay enough attention.”
Shawn Garber, MD, a bariatric surgeon who founded the practice in 2000 on Long Island and serves as its CEO, said that “prior to rendering services” his office staff advises patients of the costs and their responsibility to pay the bill.
The bariatric group has cited these out-of-network payment agreements in at least 300 lawsuits filed against patients from January 2019 to 2022 demanding nearly $19 million to cover medical bills, interest charges, and attorney’s fees, a KFF Health News review of New York state court records found.
Danny De Voe, a partner at Sahn Ward Braff Koblenz law firm in Uniondale, N.Y., who filed many of those suits, declined to comment, citing attorney-client privilege.
In most cases, the medical practice had agreed to accept an insurance company’s out-of-network rate as full payment for its services – with caveats, according to court filings.
In the agreements they signed, patients promised to pay any coinsurance, meeting any deductible, and pass on to the medical practice any reimbursement checks they received from their health plans within 7 days.
KFF Health News found – while legal fees and other costs can layer on thousands more.
Elisabeth Benjamin, a lawyer with the Community Service Society of New York, said conflicts can arise when insurers send checks to pay for out-of-network medical services to patients rather than reimbursing a medical provider directly.
“We would prefer to see regulators step in and stop that practice,” she said, adding it “causes tension between providers and patients.”
That’s certainly true for Ms. Wallace. The surgery practice sued her in August 2022demanding $17,981 in fees it said remained unpaid after her January 2022 laparoscopic sleeve gastrectomy, an operation in which much of the stomach is removed to assist weight loss.
The lawsuit also tacked on a demand for $5,993 in attorney’s fees, court records show.
The suit alleges Ms. Wallace signed the contract even though she “had no intention” of paying her bills. The complaint goes on to accuse her of “committing embezzlement” by “willfully, intentionally, deliberately and maliciously” depositing checks from her health plan into her personal account.
The suit doesn’t include details to substantiate these claims, and Ms. Wallace said in her court response they are not true. Ms. Wallace said she turned over checks for the charges.
“They billed the insurance for everything they possibly could,” Ms. Wallace said.
In September, Ms. Wallace filed for bankruptcy, hoping to discharge the bariatric care debt along with about $4,700 in unrelated credit card charges.
The medical practice fired back in November by filing an “adversary complaint” in her Brooklyn bankruptcy court proceeding that argues her medical debt should not be forgiven because Ms. Wallace committed fraud.
The adversary complaint, which is pending in the bankruptcy case, accuses Ms. Wallace of “fraudulently” inducing the surgery center to perform “elective medical procedures” without requiring payment up front.
Both the harsh wording and claims of wrongdoing have infuriated Ms. Wallace and her attorney, Jacob Silver, of Brooklyn.
Mr. Silver wants the medical practice to turn over records of the payments received from Ms. Wallace. “There is no fraud here,” he said. “This is frivolous. We are taking a no-settlement position.”
Gaining debt
Few patients sued by the bariatric practice mount a defense in court and those who do fight often lose, court records show.
The medical practice won default judgments totaling nearly $6 million in about 90 of the 300 cases in the sample reviewed by KFF Health News. Default judgments are entered when the defendant fails to respond.
Many cases either are pending, or it is not clear from court filings how they were resolved.
Some patients tried to argue that the fees were too high or that they didn’t understand going in how much they could owe. One woman, trying to push back against a demand for more than $100,000, said in a legal filing that she “was given numerous papers to sign without anyone of the staff members explaining to me what it actually meant.” Another patient, who was sued for more than $40,000, wrote: “I don’t have the means to pay this bill.”
Among the cases described in court records:
- A Westchester County, N.Y., woman was sued for $102,556 and settled for $72,000 in May 2021. She agreed to pay $7,500 upon signing the settlement and $500 a month from September 2021 to May 2032.
- A Peekskill, N.Y., woman in a December 2019 judgment was held liable for $384,092, which included $94,047 in interest.
- A Newburgh, N.Y., man was sued in 2021 for $252,309 in medical bills, 12% interest, and $84,103 in attorneys’ fees. The case is pending.
Robert Cohen, a longtime attorney for the bariatric practice, testified in a November 2021 hearing that the lawyers take “a contingency fee of one-third of our recovery” in these cases. In that case, Mr. Cohen had requested $13,578 based on his contingency fee arrangement. He testified that he spent 7.3 hours on the case and that his customary billing rate was $475 per hour, which came to $3,467.50. The judge awarded the lower amount, according to a transcript of the hearing.
Teresa LaMasters, MD, president of the American Society for Metabolic and Bariatric Surgery, said suing patients for large sums “is not a common practice” among bariatric surgeons.
“This is not what the vast majority in the field would espouse,” she said.
But Dr. Garber, the NYBG’s chief executive, suggested patients deserve blame.
“These lawsuits stem from these patients stealing the insurance money rather than forwarding it onto NYBG as they are morally and contractually obligated to do,” Dr. Garber wrote in an email to KFF Health News.
Dr. Garber added: “The issue is not with what we bill, but rather with the fact that the insurance companies refuse to send payment directly to us.”
‘A kooky system’
Defense attorneys argue that many patients don’t fully comprehend the perils of failing to pay on time – for whatever reason.
In a few cases, patients admitted pocketing checks they were obligated to turn over to the medical practice. But for the most part, court records don’t specify how many such checks were issued and for what amounts – or whether the patient improperly cashed them.
“It’s a kooky system,” said Paul Brite, an attorney who has faced off against the bariatric practice in court.
“You sign these documents that could cost you tons of money. It shouldn’t be that way,” he said. “This can ruin their financial life.”
New York lawmakers have acted to limit the damage from medical debt, including “surprise bills.”
In November, Democratic Gov. Kathy Hochul signed legislation that prohibits health care providers from slapping liens on a primary residence or garnishing wages.
But contracts with onerous repayment terms represent an “evolving area of law” and an alarming “new twist” on concerns over medical debt, said Ms. Benjamin, the community service society lawyer.
She said contract “accelerator clauses” that trigger severe penalties if patients miss payments should not be permitted for medical debt.
“If you default, the full amount is due,” she said. “This is really a bummer.”
‘Fair market value’
The debt collection lawsuits argue that weight-loss patients had agreed to pay “fair market value” for services – and the doctors are only trying to secure money they are due.
But some prices far exceed typical insurance payments for obesity treatments across the country, according to a medical billing data registry. Surgeons performed about 200,000 bariatric operations in 2020, according to the bariatric surgery society.
Ms. Wallace, the Brooklyn legal assistant, was billed $60,500 for her lap sleeve gastrectomy, though how much her insurance actually paid remains to be hashed out in court.
Michael Arrigo, a California medical billing expert at No World Borders, called the prices “outrageous” and “unreasonable and, in fact, likely unconscionable.”
“I disagree that these are fair market charges,” he said.
Dr. LaMasters called the gastrectomy price billed to Ms. Wallace “really expensive” and “a severe outlier.” While charges vary by region, she quoted a typical price of around $22,000.
Dr. Garber said NYBG “bills at usual and customary rates” determined by Fair Health, a New York City-based repository of insurance claims data. Fair Health “sets these rates based upon the acceptable price for our geographic location,” he said.
But Rachel Kent, Fair Health’s senior director of marketing, told KFF Health News that the group “does not set rates, nor determine or take any position on what constitutes ‘usual and customary rates.’ ” Instead, it reports the prices providers are charging in a given area.
Overall, Fair Health data shows huge price variations even in adjacent ZIP codes in the metro area. In Long Island’s Roslyn Heights neighborhood, where NYBG is based, Fair Health lists the out-of-network price charged by providers in the area as $60,500, the figure Ms. Wallace was billed.
But in several other New York City–area ZIP codes the price charged for the gastrectomy procedure hovers around $20,000, according to the data bank. The price in Manhattan is $17,500, for instance, according to Fair Health.
Nationwide, the average cost in 2021 for bariatric surgery done in a hospital was $32,868, according to a KFF analysis of health insurance claims.
Private equity arrives
Dr. Garber said in a court affidavit in May 2022 that he founded the bariatric practice “with a singular focus: providing safe, effective care to patients suffering from obesity and its resulting complications.”
Under his leadership, the practice has “developed into New York’s elite institution for obesity treatment,” Dr. Garber said. He said the group’s surgeons are “highly sought after to train other bariatric surgeons throughout the country and are active in the development of new, cutting-edge bariatric surgery techniques.”
In 2017, Dr. Garber and partners agreed on a business plan to help spur growth and “attract private equity investment,” according to the affidavit.
They formed a separate company to handle the bariatric practice’s business side. Known as management services organizations, such companies provide a way for private equity investors to circumvent laws in some states that prohibit nonphysicians from owning a stake in a medical practice.
In August 2019, the private equity firm Sentinel Capital Partners bought 65% of the MSO for $156.5 million, according to Dr. Garber’s affidavit. The management company is now known as New You Bariatric Group. The private equity firm did not respond to requests for comment.
Dr. Garber, in a September 2021 American Society for Metabolic and Bariatric Surgery webinar viewable online, said the weight-loss practice spends $6 million a year on media and marketing directly to patients – and is on a roll. Nationally, bariatric surgery is growing 6% annually, he said. NYBG boasts two dozen offices in the tri-state area of New York, New Jersey, and Connecticut and is poised to expand into more states.
“Since private equity, we’ve been growing at 30%-40% year over year,” Dr. Garber said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Seven months after Lahavah Wallace’s weight-loss operation, a New York bariatric surgery practice sued her, accusing her of “intentionally” failing to pay nearly $18,000 of her bill.
Long Island Minimally Invasive Surgery, which does business as the New York Bariatric Group, went on to accuse Ms. Wallace of “embezzlement,” alleging she kept insurance payments that should have been turned over to the practice.
Ms. Wallace denies the allegations, which the bariatric practice has leveled against patients in hundreds of debt-collection lawsuits filed over the past 4 years, court records in New York state show.
In about 60 cases, the lawsuits demanded $100,000 or more from patients. Some patients were found liable for tens of thousands of dollars in interest charges or wound up shackled with debt that could take a decade or more to shake. Others are facing the likely prospect of six-figure financial penalties, court records show.
Backed by a major private equity firm, the bariatric practice spends millions each year on advertisements featuring patients who have dropped 100 pounds or more after bariatric procedures, sometimes having had a portion of their stomachs removed. The ads have run on TV, online, and on New York City subway posters.
The online ads, often showcasing the slogan “Stop obesity for life,” appealed to Ms. Wallace, who lives in Brooklyn and works as a legal assistant for the state of New York. She said she turned over checks from her insurer to the bariatric group and was stunned when the medical practice hauled her into court citing an “out-of-network payment agreement” she had signed before her surgery.
“I really didn’t know what I was signing,” Ms. Wallace told KFF Health News. “I didn’t pay enough attention.”
Shawn Garber, MD, a bariatric surgeon who founded the practice in 2000 on Long Island and serves as its CEO, said that “prior to rendering services” his office staff advises patients of the costs and their responsibility to pay the bill.
The bariatric group has cited these out-of-network payment agreements in at least 300 lawsuits filed against patients from January 2019 to 2022 demanding nearly $19 million to cover medical bills, interest charges, and attorney’s fees, a KFF Health News review of New York state court records found.
Danny De Voe, a partner at Sahn Ward Braff Koblenz law firm in Uniondale, N.Y., who filed many of those suits, declined to comment, citing attorney-client privilege.
In most cases, the medical practice had agreed to accept an insurance company’s out-of-network rate as full payment for its services – with caveats, according to court filings.
In the agreements they signed, patients promised to pay any coinsurance, meeting any deductible, and pass on to the medical practice any reimbursement checks they received from their health plans within 7 days.
KFF Health News found – while legal fees and other costs can layer on thousands more.
Elisabeth Benjamin, a lawyer with the Community Service Society of New York, said conflicts can arise when insurers send checks to pay for out-of-network medical services to patients rather than reimbursing a medical provider directly.
“We would prefer to see regulators step in and stop that practice,” she said, adding it “causes tension between providers and patients.”
That’s certainly true for Ms. Wallace. The surgery practice sued her in August 2022demanding $17,981 in fees it said remained unpaid after her January 2022 laparoscopic sleeve gastrectomy, an operation in which much of the stomach is removed to assist weight loss.
The lawsuit also tacked on a demand for $5,993 in attorney’s fees, court records show.
The suit alleges Ms. Wallace signed the contract even though she “had no intention” of paying her bills. The complaint goes on to accuse her of “committing embezzlement” by “willfully, intentionally, deliberately and maliciously” depositing checks from her health plan into her personal account.
The suit doesn’t include details to substantiate these claims, and Ms. Wallace said in her court response they are not true. Ms. Wallace said she turned over checks for the charges.
“They billed the insurance for everything they possibly could,” Ms. Wallace said.
In September, Ms. Wallace filed for bankruptcy, hoping to discharge the bariatric care debt along with about $4,700 in unrelated credit card charges.
The medical practice fired back in November by filing an “adversary complaint” in her Brooklyn bankruptcy court proceeding that argues her medical debt should not be forgiven because Ms. Wallace committed fraud.
The adversary complaint, which is pending in the bankruptcy case, accuses Ms. Wallace of “fraudulently” inducing the surgery center to perform “elective medical procedures” without requiring payment up front.
Both the harsh wording and claims of wrongdoing have infuriated Ms. Wallace and her attorney, Jacob Silver, of Brooklyn.
Mr. Silver wants the medical practice to turn over records of the payments received from Ms. Wallace. “There is no fraud here,” he said. “This is frivolous. We are taking a no-settlement position.”
Gaining debt
Few patients sued by the bariatric practice mount a defense in court and those who do fight often lose, court records show.
The medical practice won default judgments totaling nearly $6 million in about 90 of the 300 cases in the sample reviewed by KFF Health News. Default judgments are entered when the defendant fails to respond.
Many cases either are pending, or it is not clear from court filings how they were resolved.
Some patients tried to argue that the fees were too high or that they didn’t understand going in how much they could owe. One woman, trying to push back against a demand for more than $100,000, said in a legal filing that she “was given numerous papers to sign without anyone of the staff members explaining to me what it actually meant.” Another patient, who was sued for more than $40,000, wrote: “I don’t have the means to pay this bill.”
Among the cases described in court records:
- A Westchester County, N.Y., woman was sued for $102,556 and settled for $72,000 in May 2021. She agreed to pay $7,500 upon signing the settlement and $500 a month from September 2021 to May 2032.
- A Peekskill, N.Y., woman in a December 2019 judgment was held liable for $384,092, which included $94,047 in interest.
- A Newburgh, N.Y., man was sued in 2021 for $252,309 in medical bills, 12% interest, and $84,103 in attorneys’ fees. The case is pending.
Robert Cohen, a longtime attorney for the bariatric practice, testified in a November 2021 hearing that the lawyers take “a contingency fee of one-third of our recovery” in these cases. In that case, Mr. Cohen had requested $13,578 based on his contingency fee arrangement. He testified that he spent 7.3 hours on the case and that his customary billing rate was $475 per hour, which came to $3,467.50. The judge awarded the lower amount, according to a transcript of the hearing.
Teresa LaMasters, MD, president of the American Society for Metabolic and Bariatric Surgery, said suing patients for large sums “is not a common practice” among bariatric surgeons.
“This is not what the vast majority in the field would espouse,” she said.
But Dr. Garber, the NYBG’s chief executive, suggested patients deserve blame.
“These lawsuits stem from these patients stealing the insurance money rather than forwarding it onto NYBG as they are morally and contractually obligated to do,” Dr. Garber wrote in an email to KFF Health News.
Dr. Garber added: “The issue is not with what we bill, but rather with the fact that the insurance companies refuse to send payment directly to us.”
‘A kooky system’
Defense attorneys argue that many patients don’t fully comprehend the perils of failing to pay on time – for whatever reason.
In a few cases, patients admitted pocketing checks they were obligated to turn over to the medical practice. But for the most part, court records don’t specify how many such checks were issued and for what amounts – or whether the patient improperly cashed them.
“It’s a kooky system,” said Paul Brite, an attorney who has faced off against the bariatric practice in court.
“You sign these documents that could cost you tons of money. It shouldn’t be that way,” he said. “This can ruin their financial life.”
New York lawmakers have acted to limit the damage from medical debt, including “surprise bills.”
In November, Democratic Gov. Kathy Hochul signed legislation that prohibits health care providers from slapping liens on a primary residence or garnishing wages.
But contracts with onerous repayment terms represent an “evolving area of law” and an alarming “new twist” on concerns over medical debt, said Ms. Benjamin, the community service society lawyer.
She said contract “accelerator clauses” that trigger severe penalties if patients miss payments should not be permitted for medical debt.
“If you default, the full amount is due,” she said. “This is really a bummer.”
‘Fair market value’
The debt collection lawsuits argue that weight-loss patients had agreed to pay “fair market value” for services – and the doctors are only trying to secure money they are due.
But some prices far exceed typical insurance payments for obesity treatments across the country, according to a medical billing data registry. Surgeons performed about 200,000 bariatric operations in 2020, according to the bariatric surgery society.
Ms. Wallace, the Brooklyn legal assistant, was billed $60,500 for her lap sleeve gastrectomy, though how much her insurance actually paid remains to be hashed out in court.
Michael Arrigo, a California medical billing expert at No World Borders, called the prices “outrageous” and “unreasonable and, in fact, likely unconscionable.”
“I disagree that these are fair market charges,” he said.
Dr. LaMasters called the gastrectomy price billed to Ms. Wallace “really expensive” and “a severe outlier.” While charges vary by region, she quoted a typical price of around $22,000.
Dr. Garber said NYBG “bills at usual and customary rates” determined by Fair Health, a New York City-based repository of insurance claims data. Fair Health “sets these rates based upon the acceptable price for our geographic location,” he said.
But Rachel Kent, Fair Health’s senior director of marketing, told KFF Health News that the group “does not set rates, nor determine or take any position on what constitutes ‘usual and customary rates.’ ” Instead, it reports the prices providers are charging in a given area.
Overall, Fair Health data shows huge price variations even in adjacent ZIP codes in the metro area. In Long Island’s Roslyn Heights neighborhood, where NYBG is based, Fair Health lists the out-of-network price charged by providers in the area as $60,500, the figure Ms. Wallace was billed.
But in several other New York City–area ZIP codes the price charged for the gastrectomy procedure hovers around $20,000, according to the data bank. The price in Manhattan is $17,500, for instance, according to Fair Health.
Nationwide, the average cost in 2021 for bariatric surgery done in a hospital was $32,868, according to a KFF analysis of health insurance claims.
Private equity arrives
Dr. Garber said in a court affidavit in May 2022 that he founded the bariatric practice “with a singular focus: providing safe, effective care to patients suffering from obesity and its resulting complications.”
Under his leadership, the practice has “developed into New York’s elite institution for obesity treatment,” Dr. Garber said. He said the group’s surgeons are “highly sought after to train other bariatric surgeons throughout the country and are active in the development of new, cutting-edge bariatric surgery techniques.”
In 2017, Dr. Garber and partners agreed on a business plan to help spur growth and “attract private equity investment,” according to the affidavit.
They formed a separate company to handle the bariatric practice’s business side. Known as management services organizations, such companies provide a way for private equity investors to circumvent laws in some states that prohibit nonphysicians from owning a stake in a medical practice.
In August 2019, the private equity firm Sentinel Capital Partners bought 65% of the MSO for $156.5 million, according to Dr. Garber’s affidavit. The management company is now known as New You Bariatric Group. The private equity firm did not respond to requests for comment.
Dr. Garber, in a September 2021 American Society for Metabolic and Bariatric Surgery webinar viewable online, said the weight-loss practice spends $6 million a year on media and marketing directly to patients – and is on a roll. Nationally, bariatric surgery is growing 6% annually, he said. NYBG boasts two dozen offices in the tri-state area of New York, New Jersey, and Connecticut and is poised to expand into more states.
“Since private equity, we’ve been growing at 30%-40% year over year,” Dr. Garber said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Two Canadian provinces lift licensing barriers for U.S. doctors
They’ll no longer have to start with a limited license and take additional exams or be supervised for up to a year to become fully licensed.
Canada is experiencing an acute shortage of licensed physicians that’s expected to intensify over the next decade. The shortfall is estimated to be about 44,000 physicians by 2028, with family doctors accounting for 72% of the deficit.
“Reducing licensing barriers should make Canada a more attractive option for U.S. doctors who may be considering a move north,” said Tom Florence, president of AMN Healthcare’s Physician Solutions division, which recruits American physicians to work in Canada.
“Canada also has a truly expedited work visa process for qualifying physicians who have a job offer and wish to practice there,” said Mr. Florence. It usually takes about 6 months compared with at least 18 months for Canadian physicians who want to work in the United States, he said.
Few U.S.-trained physicians work in Canada, which has a population of nearly 39 million. Just 812 of them practiced in Canada in 2019, the last year data was collected, according to the Canadian Medical Association.
But Canada may attract American physicians who find U.S. medicine to be fraught with ethical dilemmas and restrictions from insurance companies and elected officials, said Theresa Rohr-Kirchgraber, MD, an internist and immediate past president of the American Medical Women’s Association.
“Rather than give up practicing medicine, a move to Canada may be a welcome respite for some U.S. physicians,” she said.
Physician recruiters in Ontario and Nova Scotia welcomed the news. About 13% of the population is without a family doctor, according to news reports.
A number of U.S. physicians have started practices in Nova Scotia in recent years, said Katrina Philopoulos, Nova Scotia Health’s director of physician recruitment. “I think this momentum will help us,” she said.
Other Canadian provinces with physician shortages are also considering making similar changes. Alberta recently announced a 5-year pilot project to waive some licensing requirements for family doctors and general practitioners trained in Australia, Ireland, United Kingdom, and the United States.
What are the pros and cons of working in Canada?
“Some U.S. physicians may be attracted by a single-payer system in which all patients have access to coverage, but there are a range of drawbacks and benefits to consider in both systems,” said Mr. Florence.
U.S. physicians generally earn more than their Canadian counterparts, so income is not likely to be a draw, he said.
That appears to be the case for both family medicine physicians and specialists when comparing average net annual salaries. (To obtain Canadian salaries, 2021 gross income data from the Canadian Institute for Health Information were used; 20% was deducted for operation costs; and Canadian dollars were converted into U.S. dollars based on the current exchange rate.)
A family medicine doctor in Canada will earn an annual average salary of $195,853 USD compared with $236,000 in the United States. A cardiologist in Canada will earn $314,051 USD annually compared with $459,000 in the United States. A dermatologist in Canada will earn $270,018 annually compared with $394,000 in the United States.
Everett Fuller, MD, an emergency medicine physician who moved from Texas to Nova Scotia in 2015 for his Canadian wife, recently wrote about the pros and cons of working there compared with the United States. For him, it was a worthwhile move.
“It’s getting back to making medicine and patient care the priority instead of the business of medicine,” Dr. Fuller wrote.
“I have the comfort of knowing that a patient and their family will not go bankrupt trying to pay medical bills if I make a catastrophic diagnosis. There’s no out-of-pocket cost, other than prescriptions (depending on their drug plan).”
Dr. Fuller also doesn’t have to fight insurers for reimbursement or preapprovals, and he pays much less for medical malpractice premiums in a less litigious environment, he said.
But he mentioned a few negatives. Some treatment is rationed, which can lead to long wait times for patients to get appointments. Also, “hospitals aren’t in it for the profit, so you’re not going to get a CT, MRI, and cath lab in every hospital,” he noted.
Mr. Florence doesn’t think either system “offers a panacea for many of the challenges physicians face today. Even with reduced barriers to licensure, we do not anticipate an exodus to U.S. physicians to the north.”
A version of this article first appeared on Medscape.com.
They’ll no longer have to start with a limited license and take additional exams or be supervised for up to a year to become fully licensed.
Canada is experiencing an acute shortage of licensed physicians that’s expected to intensify over the next decade. The shortfall is estimated to be about 44,000 physicians by 2028, with family doctors accounting for 72% of the deficit.
“Reducing licensing barriers should make Canada a more attractive option for U.S. doctors who may be considering a move north,” said Tom Florence, president of AMN Healthcare’s Physician Solutions division, which recruits American physicians to work in Canada.
“Canada also has a truly expedited work visa process for qualifying physicians who have a job offer and wish to practice there,” said Mr. Florence. It usually takes about 6 months compared with at least 18 months for Canadian physicians who want to work in the United States, he said.
Few U.S.-trained physicians work in Canada, which has a population of nearly 39 million. Just 812 of them practiced in Canada in 2019, the last year data was collected, according to the Canadian Medical Association.
But Canada may attract American physicians who find U.S. medicine to be fraught with ethical dilemmas and restrictions from insurance companies and elected officials, said Theresa Rohr-Kirchgraber, MD, an internist and immediate past president of the American Medical Women’s Association.
“Rather than give up practicing medicine, a move to Canada may be a welcome respite for some U.S. physicians,” she said.
Physician recruiters in Ontario and Nova Scotia welcomed the news. About 13% of the population is without a family doctor, according to news reports.
A number of U.S. physicians have started practices in Nova Scotia in recent years, said Katrina Philopoulos, Nova Scotia Health’s director of physician recruitment. “I think this momentum will help us,” she said.
Other Canadian provinces with physician shortages are also considering making similar changes. Alberta recently announced a 5-year pilot project to waive some licensing requirements for family doctors and general practitioners trained in Australia, Ireland, United Kingdom, and the United States.
What are the pros and cons of working in Canada?
“Some U.S. physicians may be attracted by a single-payer system in which all patients have access to coverage, but there are a range of drawbacks and benefits to consider in both systems,” said Mr. Florence.
U.S. physicians generally earn more than their Canadian counterparts, so income is not likely to be a draw, he said.
That appears to be the case for both family medicine physicians and specialists when comparing average net annual salaries. (To obtain Canadian salaries, 2021 gross income data from the Canadian Institute for Health Information were used; 20% was deducted for operation costs; and Canadian dollars were converted into U.S. dollars based on the current exchange rate.)
A family medicine doctor in Canada will earn an annual average salary of $195,853 USD compared with $236,000 in the United States. A cardiologist in Canada will earn $314,051 USD annually compared with $459,000 in the United States. A dermatologist in Canada will earn $270,018 annually compared with $394,000 in the United States.
Everett Fuller, MD, an emergency medicine physician who moved from Texas to Nova Scotia in 2015 for his Canadian wife, recently wrote about the pros and cons of working there compared with the United States. For him, it was a worthwhile move.
“It’s getting back to making medicine and patient care the priority instead of the business of medicine,” Dr. Fuller wrote.
“I have the comfort of knowing that a patient and their family will not go bankrupt trying to pay medical bills if I make a catastrophic diagnosis. There’s no out-of-pocket cost, other than prescriptions (depending on their drug plan).”
Dr. Fuller also doesn’t have to fight insurers for reimbursement or preapprovals, and he pays much less for medical malpractice premiums in a less litigious environment, he said.
But he mentioned a few negatives. Some treatment is rationed, which can lead to long wait times for patients to get appointments. Also, “hospitals aren’t in it for the profit, so you’re not going to get a CT, MRI, and cath lab in every hospital,” he noted.
Mr. Florence doesn’t think either system “offers a panacea for many of the challenges physicians face today. Even with reduced barriers to licensure, we do not anticipate an exodus to U.S. physicians to the north.”
A version of this article first appeared on Medscape.com.
They’ll no longer have to start with a limited license and take additional exams or be supervised for up to a year to become fully licensed.
Canada is experiencing an acute shortage of licensed physicians that’s expected to intensify over the next decade. The shortfall is estimated to be about 44,000 physicians by 2028, with family doctors accounting for 72% of the deficit.
“Reducing licensing barriers should make Canada a more attractive option for U.S. doctors who may be considering a move north,” said Tom Florence, president of AMN Healthcare’s Physician Solutions division, which recruits American physicians to work in Canada.
“Canada also has a truly expedited work visa process for qualifying physicians who have a job offer and wish to practice there,” said Mr. Florence. It usually takes about 6 months compared with at least 18 months for Canadian physicians who want to work in the United States, he said.
Few U.S.-trained physicians work in Canada, which has a population of nearly 39 million. Just 812 of them practiced in Canada in 2019, the last year data was collected, according to the Canadian Medical Association.
But Canada may attract American physicians who find U.S. medicine to be fraught with ethical dilemmas and restrictions from insurance companies and elected officials, said Theresa Rohr-Kirchgraber, MD, an internist and immediate past president of the American Medical Women’s Association.
“Rather than give up practicing medicine, a move to Canada may be a welcome respite for some U.S. physicians,” she said.
Physician recruiters in Ontario and Nova Scotia welcomed the news. About 13% of the population is without a family doctor, according to news reports.
A number of U.S. physicians have started practices in Nova Scotia in recent years, said Katrina Philopoulos, Nova Scotia Health’s director of physician recruitment. “I think this momentum will help us,” she said.
Other Canadian provinces with physician shortages are also considering making similar changes. Alberta recently announced a 5-year pilot project to waive some licensing requirements for family doctors and general practitioners trained in Australia, Ireland, United Kingdom, and the United States.
What are the pros and cons of working in Canada?
“Some U.S. physicians may be attracted by a single-payer system in which all patients have access to coverage, but there are a range of drawbacks and benefits to consider in both systems,” said Mr. Florence.
U.S. physicians generally earn more than their Canadian counterparts, so income is not likely to be a draw, he said.
That appears to be the case for both family medicine physicians and specialists when comparing average net annual salaries. (To obtain Canadian salaries, 2021 gross income data from the Canadian Institute for Health Information were used; 20% was deducted for operation costs; and Canadian dollars were converted into U.S. dollars based on the current exchange rate.)
A family medicine doctor in Canada will earn an annual average salary of $195,853 USD compared with $236,000 in the United States. A cardiologist in Canada will earn $314,051 USD annually compared with $459,000 in the United States. A dermatologist in Canada will earn $270,018 annually compared with $394,000 in the United States.
Everett Fuller, MD, an emergency medicine physician who moved from Texas to Nova Scotia in 2015 for his Canadian wife, recently wrote about the pros and cons of working there compared with the United States. For him, it was a worthwhile move.
“It’s getting back to making medicine and patient care the priority instead of the business of medicine,” Dr. Fuller wrote.
“I have the comfort of knowing that a patient and their family will not go bankrupt trying to pay medical bills if I make a catastrophic diagnosis. There’s no out-of-pocket cost, other than prescriptions (depending on their drug plan).”
Dr. Fuller also doesn’t have to fight insurers for reimbursement or preapprovals, and he pays much less for medical malpractice premiums in a less litigious environment, he said.
But he mentioned a few negatives. Some treatment is rationed, which can lead to long wait times for patients to get appointments. Also, “hospitals aren’t in it for the profit, so you’re not going to get a CT, MRI, and cath lab in every hospital,” he noted.
Mr. Florence doesn’t think either system “offers a panacea for many of the challenges physicians face today. Even with reduced barriers to licensure, we do not anticipate an exodus to U.S. physicians to the north.”
A version of this article first appeared on Medscape.com.
Researchers seek to understand post-COVID autoimmune disease risk
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Bariatric surgery cuts risk for obesity-related cancers in half: Study
For years evidence has pointed to multiple health benefits associated with bariatric surgery, including improvements in diabetes, sleep apnea, and blood pressure. Now researchers are adding cutting cancer risk by more than half to the list.
compared with 8.9% of their peers who did not undergo such surgery.
“We did see a difference in breast cancer, colon cancer, liver cancer, and ovarian cancer incidence. ... with patients in the bariatric surgery group having lower incidence of these four types of cancers when compared to the nonsurgical control group,” said Vibhu Chittajallu, MD, lead author and a gastroenterology fellow at Case Western Reserve University and University Hospitals in Cleveland.
The obesity epidemic is “one of the most serious health challenges in the United States today,” Dr. Chittajallu added at an April 27 media briefing during which select research was previewed for the annual Digestive Disease Week®. Obesity has been associated with multiple serious illnesses, including type 2 diabetes, heart disease, and cancer.
Obesity is also common. The Centers for Disease Control and Prevention reports that nearly 42% of American adults have obesity, and rates continue to rise.
Dr. Chittajallu and colleagues used billing codes in a national database to identify 55,789 patients with obesity who underwent bariatric surgery (sleeve gastrectomy, gastric bypass, or gastric band procedures) and a control group of the same size who did not have surgery.
Investigators controlled for risk factors that contribute to cancer development, including smoking history, alcohol use, heart disease, and hormone therapies.
Key findings
In 10 years of follow-up, 2,206 patients who underwent bariatric surgery developed an obesity-associated cancer, compared with 4,960 patients who did not have bariatric surgery.
The bariatric surgery group had lower numbers of new cases for six types of cancers (Table 1).
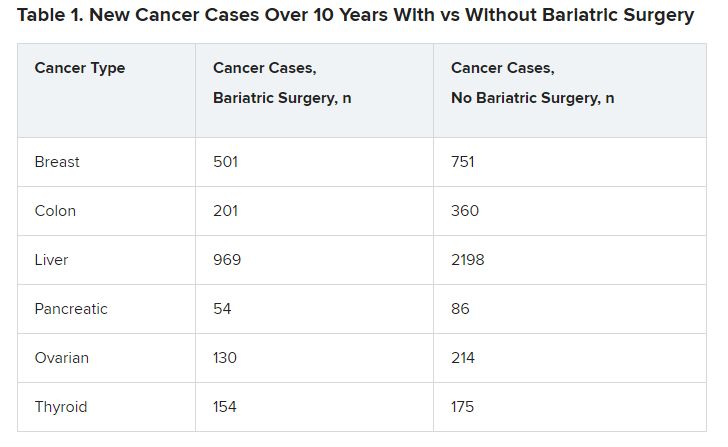
The differences were significant in four cancer types associated with obesity: breast cancer (P = .001), colon cancer (P < .01), liver cancer (P < .01), and ovarian cancer (P = .002).
The incidence of several other cancers, including renal carcinoma, and rectal and endometrial cancers, was not significantly different between the groups.
The mechanisms underlying excess cancer cases in patients with obesity are not completely understood, Dr. Chittajallu said. Bariatric surgery has been shown to decrease excess inflammation, elevate insulin, and moderate hormone levels.
‘Fascinating’ study but questions remain
The study is “fascinating,” said Loren Laine, MD, moderator of the media briefing. “Obesity is clearly associated with a number of different cancers, and that’s very important. So, it makes logical sense that if you lose weight, you will reduce that risk.”
Although investigators controlled for several known cancer risk factors, there are some they couldn’t control for because they were not included in the database, and there could be unknowns that also affected the results, noted Dr. Laine, who is professor of medicine (digestive diseases) and chief of digestive health at Yale University in New Haven, Conn.
“You have to be circumspect when you look at retrospective observational studies,” he added.
It would be helpful to know when most cancers developed over the 10 years, Dr. Laine said. Dr. Chittajallu responded that the research team did not include cancers that developed in the first year after bariatric surgery to minimize incidental findings, but he did not provide a timeline for the cancers that developed.
Another unanswered question, Dr. Laine said, is whether a dose-response relationship exists. If future research shows that the more weight a person loses, the more likely they are to have a reduction in cancer risk, “that would be fascinating,” he said. Also, it would be interesting to know if endoscopic interventions and weight-loss medications decrease cancer risks in people with obesity.
More research is needed to understand how bariatric surgery affects cancer risk, Dr. Chittajallu said. “But the significant findings from this study suggest it’s an exciting avenue for further study.”
DDW 2023 will be held May 6-9 in Chicago and virtually.
The study was independently supported. Dr. Chittajallu and Dr. Laine have reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
For years evidence has pointed to multiple health benefits associated with bariatric surgery, including improvements in diabetes, sleep apnea, and blood pressure. Now researchers are adding cutting cancer risk by more than half to the list.
compared with 8.9% of their peers who did not undergo such surgery.
“We did see a difference in breast cancer, colon cancer, liver cancer, and ovarian cancer incidence. ... with patients in the bariatric surgery group having lower incidence of these four types of cancers when compared to the nonsurgical control group,” said Vibhu Chittajallu, MD, lead author and a gastroenterology fellow at Case Western Reserve University and University Hospitals in Cleveland.
The obesity epidemic is “one of the most serious health challenges in the United States today,” Dr. Chittajallu added at an April 27 media briefing during which select research was previewed for the annual Digestive Disease Week®. Obesity has been associated with multiple serious illnesses, including type 2 diabetes, heart disease, and cancer.
Obesity is also common. The Centers for Disease Control and Prevention reports that nearly 42% of American adults have obesity, and rates continue to rise.
Dr. Chittajallu and colleagues used billing codes in a national database to identify 55,789 patients with obesity who underwent bariatric surgery (sleeve gastrectomy, gastric bypass, or gastric band procedures) and a control group of the same size who did not have surgery.
Investigators controlled for risk factors that contribute to cancer development, including smoking history, alcohol use, heart disease, and hormone therapies.
Key findings
In 10 years of follow-up, 2,206 patients who underwent bariatric surgery developed an obesity-associated cancer, compared with 4,960 patients who did not have bariatric surgery.
The bariatric surgery group had lower numbers of new cases for six types of cancers (Table 1).

The differences were significant in four cancer types associated with obesity: breast cancer (P = .001), colon cancer (P < .01), liver cancer (P < .01), and ovarian cancer (P = .002).
The incidence of several other cancers, including renal carcinoma, and rectal and endometrial cancers, was not significantly different between the groups.
The mechanisms underlying excess cancer cases in patients with obesity are not completely understood, Dr. Chittajallu said. Bariatric surgery has been shown to decrease excess inflammation, elevate insulin, and moderate hormone levels.
‘Fascinating’ study but questions remain
The study is “fascinating,” said Loren Laine, MD, moderator of the media briefing. “Obesity is clearly associated with a number of different cancers, and that’s very important. So, it makes logical sense that if you lose weight, you will reduce that risk.”
Although investigators controlled for several known cancer risk factors, there are some they couldn’t control for because they were not included in the database, and there could be unknowns that also affected the results, noted Dr. Laine, who is professor of medicine (digestive diseases) and chief of digestive health at Yale University in New Haven, Conn.
“You have to be circumspect when you look at retrospective observational studies,” he added.
It would be helpful to know when most cancers developed over the 10 years, Dr. Laine said. Dr. Chittajallu responded that the research team did not include cancers that developed in the first year after bariatric surgery to minimize incidental findings, but he did not provide a timeline for the cancers that developed.
Another unanswered question, Dr. Laine said, is whether a dose-response relationship exists. If future research shows that the more weight a person loses, the more likely they are to have a reduction in cancer risk, “that would be fascinating,” he said. Also, it would be interesting to know if endoscopic interventions and weight-loss medications decrease cancer risks in people with obesity.
More research is needed to understand how bariatric surgery affects cancer risk, Dr. Chittajallu said. “But the significant findings from this study suggest it’s an exciting avenue for further study.”
DDW 2023 will be held May 6-9 in Chicago and virtually.
The study was independently supported. Dr. Chittajallu and Dr. Laine have reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
For years evidence has pointed to multiple health benefits associated with bariatric surgery, including improvements in diabetes, sleep apnea, and blood pressure. Now researchers are adding cutting cancer risk by more than half to the list.
compared with 8.9% of their peers who did not undergo such surgery.
“We did see a difference in breast cancer, colon cancer, liver cancer, and ovarian cancer incidence. ... with patients in the bariatric surgery group having lower incidence of these four types of cancers when compared to the nonsurgical control group,” said Vibhu Chittajallu, MD, lead author and a gastroenterology fellow at Case Western Reserve University and University Hospitals in Cleveland.
The obesity epidemic is “one of the most serious health challenges in the United States today,” Dr. Chittajallu added at an April 27 media briefing during which select research was previewed for the annual Digestive Disease Week®. Obesity has been associated with multiple serious illnesses, including type 2 diabetes, heart disease, and cancer.
Obesity is also common. The Centers for Disease Control and Prevention reports that nearly 42% of American adults have obesity, and rates continue to rise.
Dr. Chittajallu and colleagues used billing codes in a national database to identify 55,789 patients with obesity who underwent bariatric surgery (sleeve gastrectomy, gastric bypass, or gastric band procedures) and a control group of the same size who did not have surgery.
Investigators controlled for risk factors that contribute to cancer development, including smoking history, alcohol use, heart disease, and hormone therapies.
Key findings
In 10 years of follow-up, 2,206 patients who underwent bariatric surgery developed an obesity-associated cancer, compared with 4,960 patients who did not have bariatric surgery.
The bariatric surgery group had lower numbers of new cases for six types of cancers (Table 1).

The differences were significant in four cancer types associated with obesity: breast cancer (P = .001), colon cancer (P < .01), liver cancer (P < .01), and ovarian cancer (P = .002).
The incidence of several other cancers, including renal carcinoma, and rectal and endometrial cancers, was not significantly different between the groups.
The mechanisms underlying excess cancer cases in patients with obesity are not completely understood, Dr. Chittajallu said. Bariatric surgery has been shown to decrease excess inflammation, elevate insulin, and moderate hormone levels.
‘Fascinating’ study but questions remain
The study is “fascinating,” said Loren Laine, MD, moderator of the media briefing. “Obesity is clearly associated with a number of different cancers, and that’s very important. So, it makes logical sense that if you lose weight, you will reduce that risk.”
Although investigators controlled for several known cancer risk factors, there are some they couldn’t control for because they were not included in the database, and there could be unknowns that also affected the results, noted Dr. Laine, who is professor of medicine (digestive diseases) and chief of digestive health at Yale University in New Haven, Conn.
“You have to be circumspect when you look at retrospective observational studies,” he added.
It would be helpful to know when most cancers developed over the 10 years, Dr. Laine said. Dr. Chittajallu responded that the research team did not include cancers that developed in the first year after bariatric surgery to minimize incidental findings, but he did not provide a timeline for the cancers that developed.
Another unanswered question, Dr. Laine said, is whether a dose-response relationship exists. If future research shows that the more weight a person loses, the more likely they are to have a reduction in cancer risk, “that would be fascinating,” he said. Also, it would be interesting to know if endoscopic interventions and weight-loss medications decrease cancer risks in people with obesity.
More research is needed to understand how bariatric surgery affects cancer risk, Dr. Chittajallu said. “But the significant findings from this study suggest it’s an exciting avenue for further study.”
DDW 2023 will be held May 6-9 in Chicago and virtually.
The study was independently supported. Dr. Chittajallu and Dr. Laine have reported no relevant financial relationships.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
AT DDW 2023
FMT in a pill: FDA approves second product to prevent C. diff recurrence
The recent approval of the first oral fecal-derived microbiota therapy to prevent the recurrence of Clostridioides difficile (C. diff) infection in patients was welcome news for physicians who’ve struggled under the weight of having too few treatment options for the prevention of C. diff recurrence.
The product, developed by Massachusetts-based Seres Therepeutics and marketed as Vowst, was approved by the U.S. Food and Drug Administration on April 26. It is approved for use in adults who have already been treated with antibiotics for a recurrent infection with C. diff bacteria.
and is designed to be delivered in four capsules taken daily for 3 days.
Gastroenterologist Phillip I. Tarr, MD, division chief of gastroenterology at Washington University, St. Louis, and chair of the American Gastroenterological Association Center for Gut Microbiome Research and Education, said that prevention of recurrent C. diff infection “remains challenging,” and that Vowst “provides the first FDA-approved, orally administered microbiome therapeutic with which to achieve this goal. This advance also makes us optimistic we might soon be able to prevent other disorders by managing gut microbial communities.”
Vowst is the second therapy derived from human stool to be approved for the indication in less than 6 months. In December, the FDA approved Rebyota (Ferring), a rectally delivered treatment that also uses microbes from donor feces. Both products were given priority review, orphan drug, and breakthrough therapy designations by the agency.
C. diff infection can be aggravated by an alteration of normal gut flora associated with antibiotics treatment, leading to cycles of repeated infections. Infection can produce diarrhea, abdominal pain, fever, and severe morbidity. In the United States, an estimated 15,000 to 30,000 deaths per year are linked to C. diff. Risk factors for recurrent infection include being 65 or older, hospitalization, being in a nursing home, a weakened immune system, and previous infection with C. diff.
Therapies transplanting fecal microbiota from donors have been used since the 1950s as treatments for recurrent C. diff infection, and in the past decade, as stool banks recruiting screened donors have made fecal microbiota transplants, or FMT, standard of care. However, only in recent years have fecal-derived therapies become subject to standardized safety and efficacy testing.
Both the current FDA-approved products, Rebyota and Vowst, were shown in randomized controlled trials to reduce recurrence of C. diff infection, compared with placebo. In a phase 3 clinical trial of Rebyota (n = 262) in antibiotic-treated patients, one rectally administered dose reduced recurrence of C. diff infection by 70.6% at 8 weeks, compared with 57.5% for placebo. A phase 3 study of Vowst (n = 281) showed recurrence in treated subjects to be 12.4% at 8 weeks, compared with nearly 40% of those receiving placebo (relative risk, 0.32; 95% confidence interval, 0.18-0.58; P less than .001).
Despite screening protocols that have become increasingly homogenized and rigorous, FMT is associated with the risk of introducing pathogens. Vowst is manufactured with purified bacterial spores derived from donor feces, not whole stool. Nonetheless, FDA noted in its statement that Vowst could still potentially introduce infectious agents or allergens.
Antibiotics are still first-line treatment
In an interview, Jessica Allegretti, MD, MPH, AGAF, medical director of the Crohn’s and Colitis Center at Brigham & Women’s Hospital, Boston, said that having two FDA-approved therapies with different means of administration “is great for the field and great for patients. These are both meant to be used after a course of antibiotics, so antibiotics are still the mainstay of treatment for C. diff and recurrent C. diff, but we now have more options to prevent recurrence.”
The convenience of an oral therapy that can be taken at home is “very attractive,” Dr. Allegretti added, noting that there will also be patients “who either don’t want to or can’t take capsules, for whom a rectal administration [in a health care setting] may be preferred.”
Dr. Allegretti, who has used FMT to treat recurrent C. difficile for more than a decade, said that she expected traditional FMT using screened donor stool to remain available even as the new products are adopted by clinicians. FMT centers like OpenBiome “will continue to provide access for patients who either don’t have the ability to get the FDA-approved products because of insurance coverage, or for financial reasons, or maybe neither of the new products is appropriate for them,” she said. “I do think there will always be a need for the traditional option. The more options that we have available the better.”
TD Cowen analyst Joseph Thome told Reuters that the drug could be priced close to $20,000 per course, expecting peak sales of $750 million in the U.S. in 2033.
Dr. Allegretti disclosed consulting work for Seres Therapeutics, Ferring, and other manufacturers. She is a member of OpenBiome’s clinical advisory board.
The recent approval of the first oral fecal-derived microbiota therapy to prevent the recurrence of Clostridioides difficile (C. diff) infection in patients was welcome news for physicians who’ve struggled under the weight of having too few treatment options for the prevention of C. diff recurrence.
The product, developed by Massachusetts-based Seres Therepeutics and marketed as Vowst, was approved by the U.S. Food and Drug Administration on April 26. It is approved for use in adults who have already been treated with antibiotics for a recurrent infection with C. diff bacteria.
and is designed to be delivered in four capsules taken daily for 3 days.
Gastroenterologist Phillip I. Tarr, MD, division chief of gastroenterology at Washington University, St. Louis, and chair of the American Gastroenterological Association Center for Gut Microbiome Research and Education, said that prevention of recurrent C. diff infection “remains challenging,” and that Vowst “provides the first FDA-approved, orally administered microbiome therapeutic with which to achieve this goal. This advance also makes us optimistic we might soon be able to prevent other disorders by managing gut microbial communities.”
Vowst is the second therapy derived from human stool to be approved for the indication in less than 6 months. In December, the FDA approved Rebyota (Ferring), a rectally delivered treatment that also uses microbes from donor feces. Both products were given priority review, orphan drug, and breakthrough therapy designations by the agency.
C. diff infection can be aggravated by an alteration of normal gut flora associated with antibiotics treatment, leading to cycles of repeated infections. Infection can produce diarrhea, abdominal pain, fever, and severe morbidity. In the United States, an estimated 15,000 to 30,000 deaths per year are linked to C. diff. Risk factors for recurrent infection include being 65 or older, hospitalization, being in a nursing home, a weakened immune system, and previous infection with C. diff.
Therapies transplanting fecal microbiota from donors have been used since the 1950s as treatments for recurrent C. diff infection, and in the past decade, as stool banks recruiting screened donors have made fecal microbiota transplants, or FMT, standard of care. However, only in recent years have fecal-derived therapies become subject to standardized safety and efficacy testing.
Both the current FDA-approved products, Rebyota and Vowst, were shown in randomized controlled trials to reduce recurrence of C. diff infection, compared with placebo. In a phase 3 clinical trial of Rebyota (n = 262) in antibiotic-treated patients, one rectally administered dose reduced recurrence of C. diff infection by 70.6% at 8 weeks, compared with 57.5% for placebo. A phase 3 study of Vowst (n = 281) showed recurrence in treated subjects to be 12.4% at 8 weeks, compared with nearly 40% of those receiving placebo (relative risk, 0.32; 95% confidence interval, 0.18-0.58; P less than .001).
Despite screening protocols that have become increasingly homogenized and rigorous, FMT is associated with the risk of introducing pathogens. Vowst is manufactured with purified bacterial spores derived from donor feces, not whole stool. Nonetheless, FDA noted in its statement that Vowst could still potentially introduce infectious agents or allergens.
Antibiotics are still first-line treatment
In an interview, Jessica Allegretti, MD, MPH, AGAF, medical director of the Crohn’s and Colitis Center at Brigham & Women’s Hospital, Boston, said that having two FDA-approved therapies with different means of administration “is great for the field and great for patients. These are both meant to be used after a course of antibiotics, so antibiotics are still the mainstay of treatment for C. diff and recurrent C. diff, but we now have more options to prevent recurrence.”
The convenience of an oral therapy that can be taken at home is “very attractive,” Dr. Allegretti added, noting that there will also be patients “who either don’t want to or can’t take capsules, for whom a rectal administration [in a health care setting] may be preferred.”
Dr. Allegretti, who has used FMT to treat recurrent C. difficile for more than a decade, said that she expected traditional FMT using screened donor stool to remain available even as the new products are adopted by clinicians. FMT centers like OpenBiome “will continue to provide access for patients who either don’t have the ability to get the FDA-approved products because of insurance coverage, or for financial reasons, or maybe neither of the new products is appropriate for them,” she said. “I do think there will always be a need for the traditional option. The more options that we have available the better.”
TD Cowen analyst Joseph Thome told Reuters that the drug could be priced close to $20,000 per course, expecting peak sales of $750 million in the U.S. in 2033.
Dr. Allegretti disclosed consulting work for Seres Therapeutics, Ferring, and other manufacturers. She is a member of OpenBiome’s clinical advisory board.
The recent approval of the first oral fecal-derived microbiota therapy to prevent the recurrence of Clostridioides difficile (C. diff) infection in patients was welcome news for physicians who’ve struggled under the weight of having too few treatment options for the prevention of C. diff recurrence.
The product, developed by Massachusetts-based Seres Therepeutics and marketed as Vowst, was approved by the U.S. Food and Drug Administration on April 26. It is approved for use in adults who have already been treated with antibiotics for a recurrent infection with C. diff bacteria.
and is designed to be delivered in four capsules taken daily for 3 days.
Gastroenterologist Phillip I. Tarr, MD, division chief of gastroenterology at Washington University, St. Louis, and chair of the American Gastroenterological Association Center for Gut Microbiome Research and Education, said that prevention of recurrent C. diff infection “remains challenging,” and that Vowst “provides the first FDA-approved, orally administered microbiome therapeutic with which to achieve this goal. This advance also makes us optimistic we might soon be able to prevent other disorders by managing gut microbial communities.”
Vowst is the second therapy derived from human stool to be approved for the indication in less than 6 months. In December, the FDA approved Rebyota (Ferring), a rectally delivered treatment that also uses microbes from donor feces. Both products were given priority review, orphan drug, and breakthrough therapy designations by the agency.
C. diff infection can be aggravated by an alteration of normal gut flora associated with antibiotics treatment, leading to cycles of repeated infections. Infection can produce diarrhea, abdominal pain, fever, and severe morbidity. In the United States, an estimated 15,000 to 30,000 deaths per year are linked to C. diff. Risk factors for recurrent infection include being 65 or older, hospitalization, being in a nursing home, a weakened immune system, and previous infection with C. diff.
Therapies transplanting fecal microbiota from donors have been used since the 1950s as treatments for recurrent C. diff infection, and in the past decade, as stool banks recruiting screened donors have made fecal microbiota transplants, or FMT, standard of care. However, only in recent years have fecal-derived therapies become subject to standardized safety and efficacy testing.
Both the current FDA-approved products, Rebyota and Vowst, were shown in randomized controlled trials to reduce recurrence of C. diff infection, compared with placebo. In a phase 3 clinical trial of Rebyota (n = 262) in antibiotic-treated patients, one rectally administered dose reduced recurrence of C. diff infection by 70.6% at 8 weeks, compared with 57.5% for placebo. A phase 3 study of Vowst (n = 281) showed recurrence in treated subjects to be 12.4% at 8 weeks, compared with nearly 40% of those receiving placebo (relative risk, 0.32; 95% confidence interval, 0.18-0.58; P less than .001).
Despite screening protocols that have become increasingly homogenized and rigorous, FMT is associated with the risk of introducing pathogens. Vowst is manufactured with purified bacterial spores derived from donor feces, not whole stool. Nonetheless, FDA noted in its statement that Vowst could still potentially introduce infectious agents or allergens.
Antibiotics are still first-line treatment
In an interview, Jessica Allegretti, MD, MPH, AGAF, medical director of the Crohn’s and Colitis Center at Brigham & Women’s Hospital, Boston, said that having two FDA-approved therapies with different means of administration “is great for the field and great for patients. These are both meant to be used after a course of antibiotics, so antibiotics are still the mainstay of treatment for C. diff and recurrent C. diff, but we now have more options to prevent recurrence.”
The convenience of an oral therapy that can be taken at home is “very attractive,” Dr. Allegretti added, noting that there will also be patients “who either don’t want to or can’t take capsules, for whom a rectal administration [in a health care setting] may be preferred.”
Dr. Allegretti, who has used FMT to treat recurrent C. difficile for more than a decade, said that she expected traditional FMT using screened donor stool to remain available even as the new products are adopted by clinicians. FMT centers like OpenBiome “will continue to provide access for patients who either don’t have the ability to get the FDA-approved products because of insurance coverage, or for financial reasons, or maybe neither of the new products is appropriate for them,” she said. “I do think there will always be a need for the traditional option. The more options that we have available the better.”
TD Cowen analyst Joseph Thome told Reuters that the drug could be priced close to $20,000 per course, expecting peak sales of $750 million in the U.S. in 2033.
Dr. Allegretti disclosed consulting work for Seres Therapeutics, Ferring, and other manufacturers. She is a member of OpenBiome’s clinical advisory board.
Long-COVID patients respond differently to COVID vaccines
A new study shows that people with long COVID respond differently to COVID vaccines and that the condition may be caused by a dysfunction of the immune system – possibly explaining why some people experience symptoms for months while others recover and resume normal lives.
The study compared people who already had long COVID with people who had recovered from the virus. Both groups had not yet been vaccinated prior to the study. When researchers analyzed blood samples after people received an initial vaccine dose, they found that people with long COVID and people who had already recovered from the virus had similar immune responses at first. But
The long-COVID group also showed an extra immune response that tried to fight the virus in a secondary way that researchers didn’t expect. Both groups showed an initial increase in their blood of antibodies that primarily target what’s known as the “spike” protein of the coronavirus, which allows the virus to invade healthy cells. But the long-COVID group also showed a prolonged increased immune response that tried to fight the part of the virus related to how it replicates.
“Theoretically, the production of these antibodies could mean that people are more protected from infection,” said researcher Catherine Le, MD, in a statement. “We also need to investigate if the elevated immune response corresponds with severity or number of long–COVID-19 symptoms.”
Dr. Le is codirector of the COVID-19 Recovery Program at Cedars-Sinai Medical Center in Los Angeles, where the study was conducted.
Study participants agreed in September 2020 to participate in long-term COVID research at Cedars-Sinai. The new analysis was published earlier this year in BMC Infectious Diseases and included 245 people who had long COVID and 86 health care workers who had recovered from COVID but did not have long-term symptoms.
For the study, long COVID was defined as having symptoms that lasted more than 12 weeks. Common long-COVID symptoms are fatigue, shortness of breath, and brain dysfunction such as confusion and forgetfulness.
The authors said it’s unclear why the two groups had different immune responses and also noted that their study was limited by a small sample size. Their research of blood samples is ongoing, with the goals of identifying a way to diagnose long COVID with a laboratory test and of better understanding what causes the condition.
A version of this article first appeared on WebMD.com.
A new study shows that people with long COVID respond differently to COVID vaccines and that the condition may be caused by a dysfunction of the immune system – possibly explaining why some people experience symptoms for months while others recover and resume normal lives.
The study compared people who already had long COVID with people who had recovered from the virus. Both groups had not yet been vaccinated prior to the study. When researchers analyzed blood samples after people received an initial vaccine dose, they found that people with long COVID and people who had already recovered from the virus had similar immune responses at first. But
The long-COVID group also showed an extra immune response that tried to fight the virus in a secondary way that researchers didn’t expect. Both groups showed an initial increase in their blood of antibodies that primarily target what’s known as the “spike” protein of the coronavirus, which allows the virus to invade healthy cells. But the long-COVID group also showed a prolonged increased immune response that tried to fight the part of the virus related to how it replicates.
“Theoretically, the production of these antibodies could mean that people are more protected from infection,” said researcher Catherine Le, MD, in a statement. “We also need to investigate if the elevated immune response corresponds with severity or number of long–COVID-19 symptoms.”
Dr. Le is codirector of the COVID-19 Recovery Program at Cedars-Sinai Medical Center in Los Angeles, where the study was conducted.
Study participants agreed in September 2020 to participate in long-term COVID research at Cedars-Sinai. The new analysis was published earlier this year in BMC Infectious Diseases and included 245 people who had long COVID and 86 health care workers who had recovered from COVID but did not have long-term symptoms.
For the study, long COVID was defined as having symptoms that lasted more than 12 weeks. Common long-COVID symptoms are fatigue, shortness of breath, and brain dysfunction such as confusion and forgetfulness.
The authors said it’s unclear why the two groups had different immune responses and also noted that their study was limited by a small sample size. Their research of blood samples is ongoing, with the goals of identifying a way to diagnose long COVID with a laboratory test and of better understanding what causes the condition.
A version of this article first appeared on WebMD.com.
A new study shows that people with long COVID respond differently to COVID vaccines and that the condition may be caused by a dysfunction of the immune system – possibly explaining why some people experience symptoms for months while others recover and resume normal lives.
The study compared people who already had long COVID with people who had recovered from the virus. Both groups had not yet been vaccinated prior to the study. When researchers analyzed blood samples after people received an initial vaccine dose, they found that people with long COVID and people who had already recovered from the virus had similar immune responses at first. But
The long-COVID group also showed an extra immune response that tried to fight the virus in a secondary way that researchers didn’t expect. Both groups showed an initial increase in their blood of antibodies that primarily target what’s known as the “spike” protein of the coronavirus, which allows the virus to invade healthy cells. But the long-COVID group also showed a prolonged increased immune response that tried to fight the part of the virus related to how it replicates.
“Theoretically, the production of these antibodies could mean that people are more protected from infection,” said researcher Catherine Le, MD, in a statement. “We also need to investigate if the elevated immune response corresponds with severity or number of long–COVID-19 symptoms.”
Dr. Le is codirector of the COVID-19 Recovery Program at Cedars-Sinai Medical Center in Los Angeles, where the study was conducted.
Study participants agreed in September 2020 to participate in long-term COVID research at Cedars-Sinai. The new analysis was published earlier this year in BMC Infectious Diseases and included 245 people who had long COVID and 86 health care workers who had recovered from COVID but did not have long-term symptoms.
For the study, long COVID was defined as having symptoms that lasted more than 12 weeks. Common long-COVID symptoms are fatigue, shortness of breath, and brain dysfunction such as confusion and forgetfulness.
The authors said it’s unclear why the two groups had different immune responses and also noted that their study was limited by a small sample size. Their research of blood samples is ongoing, with the goals of identifying a way to diagnose long COVID with a laboratory test and of better understanding what causes the condition.
A version of this article first appeared on WebMD.com.