User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
FDA approves first drug to treat Alzheimer’s agitation
(AD), making it the first FDA-approved drug for this indication.
“Agitation is one of the most common and challenging aspects of care among patients with dementia due to Alzheimer’s disease,” Tiffany Farchione, MD, director of the division of psychiatry in the FDA’s Center for Drug Evaluation and Research, said in a news release.
Agitation can include symptoms that range from pacing or restlessness to verbal and physical aggression. “These symptoms are leading causes of assisted living or nursing home placement and have been associated with accelerated disease progression,” Dr. Farchione said.
Brexpiprazole was approved by the FDA in 2015 as an adjunctive therapy to antidepressants for adults with major depressive disorder and for adults with schizophrenia.
Approval of the supplemental application for brexpiprazole for agitation associated with AD dementia was based on results of two randomized, double-blind, placebo-controlled studies.
In both studies, patients who received 2 mg or 3 mg of brexpiprazole showed statistically significant and clinically meaningful improvements in agitation symptoms, as shown by total Cohen-Mansfield Agitation Inventory (CMAI) score, compared with patients who received placebo.
The recommended starting dosage for the treatment of agitation associated with AD dementia is 0.5 mg once daily on days 1-7; it was increased to 1 mg once daily on days 8-14 and then to the recommended target dose of 2 mg once daily.
The dosage can be increased to the maximum recommended daily dosage of 3 mg once daily after at least 14 days, depending on clinical response and tolerability.
The most common side effects of brexpiprazole in patients with agitation associated with AD dementia include headache, dizziness, urinary tract infection, nasopharyngitis, and sleep disturbances.
The drug includes a boxed warning for medications in this class that elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death.
The supplemental application for brexpiprazole for agitation had fast-track designation.
A version of this article first appeared on Medscape.com.
(AD), making it the first FDA-approved drug for this indication.
“Agitation is one of the most common and challenging aspects of care among patients with dementia due to Alzheimer’s disease,” Tiffany Farchione, MD, director of the division of psychiatry in the FDA’s Center for Drug Evaluation and Research, said in a news release.
Agitation can include symptoms that range from pacing or restlessness to verbal and physical aggression. “These symptoms are leading causes of assisted living or nursing home placement and have been associated with accelerated disease progression,” Dr. Farchione said.
Brexpiprazole was approved by the FDA in 2015 as an adjunctive therapy to antidepressants for adults with major depressive disorder and for adults with schizophrenia.
Approval of the supplemental application for brexpiprazole for agitation associated with AD dementia was based on results of two randomized, double-blind, placebo-controlled studies.
In both studies, patients who received 2 mg or 3 mg of brexpiprazole showed statistically significant and clinically meaningful improvements in agitation symptoms, as shown by total Cohen-Mansfield Agitation Inventory (CMAI) score, compared with patients who received placebo.
The recommended starting dosage for the treatment of agitation associated with AD dementia is 0.5 mg once daily on days 1-7; it was increased to 1 mg once daily on days 8-14 and then to the recommended target dose of 2 mg once daily.
The dosage can be increased to the maximum recommended daily dosage of 3 mg once daily after at least 14 days, depending on clinical response and tolerability.
The most common side effects of brexpiprazole in patients with agitation associated with AD dementia include headache, dizziness, urinary tract infection, nasopharyngitis, and sleep disturbances.
The drug includes a boxed warning for medications in this class that elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death.
The supplemental application for brexpiprazole for agitation had fast-track designation.
A version of this article first appeared on Medscape.com.
(AD), making it the first FDA-approved drug for this indication.
“Agitation is one of the most common and challenging aspects of care among patients with dementia due to Alzheimer’s disease,” Tiffany Farchione, MD, director of the division of psychiatry in the FDA’s Center for Drug Evaluation and Research, said in a news release.
Agitation can include symptoms that range from pacing or restlessness to verbal and physical aggression. “These symptoms are leading causes of assisted living or nursing home placement and have been associated with accelerated disease progression,” Dr. Farchione said.
Brexpiprazole was approved by the FDA in 2015 as an adjunctive therapy to antidepressants for adults with major depressive disorder and for adults with schizophrenia.
Approval of the supplemental application for brexpiprazole for agitation associated with AD dementia was based on results of two randomized, double-blind, placebo-controlled studies.
In both studies, patients who received 2 mg or 3 mg of brexpiprazole showed statistically significant and clinically meaningful improvements in agitation symptoms, as shown by total Cohen-Mansfield Agitation Inventory (CMAI) score, compared with patients who received placebo.
The recommended starting dosage for the treatment of agitation associated with AD dementia is 0.5 mg once daily on days 1-7; it was increased to 1 mg once daily on days 8-14 and then to the recommended target dose of 2 mg once daily.
The dosage can be increased to the maximum recommended daily dosage of 3 mg once daily after at least 14 days, depending on clinical response and tolerability.
The most common side effects of brexpiprazole in patients with agitation associated with AD dementia include headache, dizziness, urinary tract infection, nasopharyngitis, and sleep disturbances.
The drug includes a boxed warning for medications in this class that elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death.
The supplemental application for brexpiprazole for agitation had fast-track designation.
A version of this article first appeared on Medscape.com.
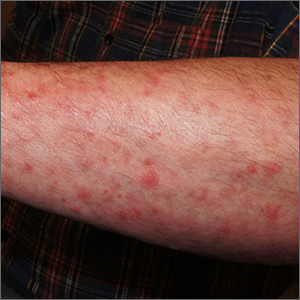
Itchy pustules over hair follicles
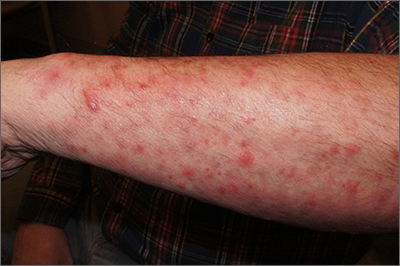
A potassium hydroxide (KOH) preparation of pus and dry superficial skin taken from 1 of the pustules revealed multiple hyphae and confirmed a diagnosis of nodular granulomatous perifolliculitis, also called Majocchi granuloma.
Majocchi granuloma is a reactive process of inflammation caused by infection of the follicular unit(s) by a dermatophyte—most often the same Trichophyton species responsible for more superficial tinea. On exam, there may be a solitary papule, pustule, or nodule. More often, there are multiple papules and pustules grouped within an annular plaque in hair-bearing areas on the head, trunk, or extremities. Majocchi granuloma can occur in patients who are healthy and those who are immunosuppressed.1 It can also occur when a topical steroid is applied to unsuspected tinea, as occurred here. In this case, the patient was accustomed to having multiple skin plaques of psoriasis and assumed this was a stubborn manifestation of that.
Because the infection penetrates deeper than most topical therapies can effectively reach at adequate concentrations, systemic medications are the treatments of choice. Terbinafine, itraconazole, and fluconazole are all effective options but need to be used for several weeks to be effective.
This patient received terbinafine 250 mg/d for 6 weeks and the pustules cleared completely. He continued with his other psoriasis medications throughout his treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. İlkit M, Durdu M, Karakaş M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457. doi: 10.3109/13693786.2012.669503

A potassium hydroxide (KOH) preparation of pus and dry superficial skin taken from 1 of the pustules revealed multiple hyphae and confirmed a diagnosis of nodular granulomatous perifolliculitis, also called Majocchi granuloma.
Majocchi granuloma is a reactive process of inflammation caused by infection of the follicular unit(s) by a dermatophyte—most often the same Trichophyton species responsible for more superficial tinea. On exam, there may be a solitary papule, pustule, or nodule. More often, there are multiple papules and pustules grouped within an annular plaque in hair-bearing areas on the head, trunk, or extremities. Majocchi granuloma can occur in patients who are healthy and those who are immunosuppressed.1 It can also occur when a topical steroid is applied to unsuspected tinea, as occurred here. In this case, the patient was accustomed to having multiple skin plaques of psoriasis and assumed this was a stubborn manifestation of that.
Because the infection penetrates deeper than most topical therapies can effectively reach at adequate concentrations, systemic medications are the treatments of choice. Terbinafine, itraconazole, and fluconazole are all effective options but need to be used for several weeks to be effective.
This patient received terbinafine 250 mg/d for 6 weeks and the pustules cleared completely. He continued with his other psoriasis medications throughout his treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A potassium hydroxide (KOH) preparation of pus and dry superficial skin taken from 1 of the pustules revealed multiple hyphae and confirmed a diagnosis of nodular granulomatous perifolliculitis, also called Majocchi granuloma.
Majocchi granuloma is a reactive process of inflammation caused by infection of the follicular unit(s) by a dermatophyte—most often the same Trichophyton species responsible for more superficial tinea. On exam, there may be a solitary papule, pustule, or nodule. More often, there are multiple papules and pustules grouped within an annular plaque in hair-bearing areas on the head, trunk, or extremities. Majocchi granuloma can occur in patients who are healthy and those who are immunosuppressed.1 It can also occur when a topical steroid is applied to unsuspected tinea, as occurred here. In this case, the patient was accustomed to having multiple skin plaques of psoriasis and assumed this was a stubborn manifestation of that.
Because the infection penetrates deeper than most topical therapies can effectively reach at adequate concentrations, systemic medications are the treatments of choice. Terbinafine, itraconazole, and fluconazole are all effective options but need to be used for several weeks to be effective.
This patient received terbinafine 250 mg/d for 6 weeks and the pustules cleared completely. He continued with his other psoriasis medications throughout his treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. İlkit M, Durdu M, Karakaş M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457. doi: 10.3109/13693786.2012.669503
1. İlkit M, Durdu M, Karakaş M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457. doi: 10.3109/13693786.2012.669503
Part-time physician: Is it a viable career choice?
On average, physicians reported in the Medscape Physician Compensation Report 2023 that they worked 50 hours per week. Five specialties, including critical care, cardiology, and general surgery reported working 55 or more hours weekly.
In 2011, The New England Journal of Medicine reported that part-time physician careers were rising. At the time, part-time doctors made up 21% of the physician workforce, up from 13% in 2005.
In a more recent survey from the California Health Care Foundation, only 12% of California physicians said they devoted 20-29 hours a week to patient care.
Amy Knoup, a senior recruitment adviser with Provider Solutions & Development), has been helping doctors find jobs for over a decade, and she’s noticed a trend.
“Not only are more physicians seeking part-time roles than they were 10 years ago, but more large health care systems are also offering part time or per diem as well,” said Ms. Knoup.
Who’s working part time, and why?
Ten years ago, the fastest growing segment of part-timers were men nearing retirement and early- to mid-career women.
Pediatricians led the part-time pack in 2002, according to an American Academy of Pediatrics study. At the time, 15% of pediatricians reported their hours as part time. However, the numbers may have increased over the years. For example, a 2021 study by the department of pediatrics, Boston Medical Center, and Boston University found that almost 30% of graduating pediatricians sought part-time work at the end of their training.
At PS&D, Ms. Knoup said she has noticed a trend toward part-timers among primary care, behavioral health, and outpatient specialties such as endocrinology. “We’re also seeing it with the inpatient side in roles that are more shift based like hospitalists, radiologists, and critical care and ER doctors.”
Another trend Ms. Knoup has noticed is with early-career doctors. “They have a different mindset,” she said. “Younger generations are acutely aware of burnout. They may have experienced it in residency or during the pandemic. They’ve had a taste of that and don’t want to go down that road again, so they’re seeking part-time roles. It’s an intentional choice.”
Tracey O’Connell, MD, a radiologist, always knew that she wanted to work part time. “I had a baby as a resident, and I was pregnant with my second child as a fellow,” she said. “I was already feeling overwhelmed with medical training and having a family.”
Dr. O’Connell worked in private practice for 16 years on Mondays, Wednesdays, and Fridays, with no nights or weekends.
“I still found it completely overwhelming,” she said. “Even though I had more days not working than working, I felt like the demands of medical life had advanced faster than human beings could adapt, and I still feel that way.”
Today she runs a part-time teleradiology practice from home but spends more time on her second career as a life coach. “Most of my clients are physicians looking for more fulfillment and sustainable ways of practicing medicine while maintaining their own identity as human beings, not just the all-consuming identity of ‘doctor,’ ” she said.
On the other end of the career spectrum is Lois Goodman, MD, an ob.gyn. in her late 70s. After 42 years in a group practice, she started her solo practice at 72, seeing patients 3 days per week. “I’m just happy to be working. That’s a tremendous payoff for me. I need to keep working for my mental health.”
How does part-time work affect physician shortages and care delivery?
Reducing clinical effort is one of the strategies physicians use to scale down overload. Still, it’s not viable as a long-term solution, said Christine Sinsky, MD, AMA’s vice president of professional satisfaction and a nationally regarded researcher on physician burnout.
“If all the physicians in a community went from working 100% FTE clinical to 50% FTE clinical, then the people in that community would have half the access to care that they had,” said Dr. Sinsky. “There’s less capacity in the system to care for patients.”
Some could argue, then, that part-time physician work may contribute to physician shortage predictions. An Association of American Medical Colleges report estimates there will be a shortage of 37,800 to 124,000 physicians by 2034.
But physicians working part-time express a contrasting point of view. “I don’t believe that part-time workers are responsible for the health care shortage but rather, a great solution,” said Dr. O’Connell. “Because in order to continue working for a long time rather than quitting when the demands exceed human capacity, working part time is a great compromise to offer a life of more sustainable well-being and longevity as a physician, and still live a wholehearted life.”
Pros and cons of being a part-time physician
Pros
Less burnout: The American Medical Association has tracked burnout rates for 22 years. By the end of 2021, nearly 63% of physicians reported burnout symptoms, compared with 38% the year before. Going part time appears to reduce burnout, suggests a study published in Mayo Clinic Proceedings.
Better work-life balance: Rachel Miller, MD, an ob.gyn., worked 60-70 hours weekly for 9 years. In 2022, she went to work as an OB hospitalist for a health care system that welcomes part-time clinicians. Since then, she has achieved a better work-life balance, putting in 26-28 hours a week. Dr. Miller now spends more time with her kids and in her additional role as an executive coach to leaders in the medical field.
More focus: “When I’m at work, I’m 100% mentally in and focused,” said Dr. Miller. “My interactions with patients are different because I’m not burned out. My demeanor and my willingness to connect are stronger.”
Better health: Mehmet Cilingiroglu, MD, with CardioSolution, traded full-time work for part time when health issues and a kidney transplant sidelined his 30-year career in 2018. “Despite my significant health issues, I’ve been able to continue working at a pace that suits me rather than having to retire,” he said. “Part-time physicians can still enjoy patient care, research, innovation, education, and training while balancing that with other areas of life.”
Errin Weisman, a DO who gave up full-time work in 2016, said cutting back makes her feel healthier, happier, and more energized. “Part-time work helps me to bring my A game each day I work and deliver the best care.” She’s also a life coach encouraging other physicians to find balance in their professional and personal lives.
Cons
Cut in pay: Obviously, the No. 1 con is you’ll make less working part time, so adjusting to a salary decrease can be a huge issue, especially if you don’t have other sources of income. Physicians paying off student loans, those caring for children or elderly parents, or those in their prime earning years needing to save for retirement may not be able to go part time.
Diminished career: The chance for promotions or being well known in your field can be diminished, as well as a loss of proficiency if you’re only performing surgery or procedures part time. In some specialties, working part time and not keeping up with (or being able to practice) newer technology developments can harm your career or reputation in the long run.
Missing out: While working part time has many benefits, physicians also experience a wide range of drawbacks. Dr. Goodman, for example, said she misses delivering babies and doing surgeries. Dr. Miller said she gave up some aspects of her specialty, like performing hysterectomies, participating in complex cases, and no longer having an office like she did as a full-time ob.gyn.
Loss of fellowship: Dr. O’Connell said she missed the camaraderie and sense of belonging when she scaled back her hours. “I felt like a fish out of water, that my values didn’t align with the group’s values,” she said. This led to self-doubt, frustrated colleagues, and a reduction in benefits.
Lost esteem: Dr. O’Connell also felt she was expected to work overtime without additional pay and was no longer eligible for bonuses. “I was treated as a team player when I was needed, but not when it came to perks and benefits and insider privilege,” she said. There may be a loss of esteem among colleagues and supervisors.
Overcoming stigma: Because part-time physician work is still not prevalent among colleagues, some may resist the idea, have less respect for it, perceive it as not being serious about your career as a physician, or associate it with being lazy or entitled.
Summing it up
Every physician must weigh the value and drawbacks of part-time work, but the more physicians who go this route, the more part-time medicine gains traction and the more physicians can learn about its values versus its drawbacks.
A version of this article first appeared on Medscape.com.
On average, physicians reported in the Medscape Physician Compensation Report 2023 that they worked 50 hours per week. Five specialties, including critical care, cardiology, and general surgery reported working 55 or more hours weekly.
In 2011, The New England Journal of Medicine reported that part-time physician careers were rising. At the time, part-time doctors made up 21% of the physician workforce, up from 13% in 2005.
In a more recent survey from the California Health Care Foundation, only 12% of California physicians said they devoted 20-29 hours a week to patient care.
Amy Knoup, a senior recruitment adviser with Provider Solutions & Development), has been helping doctors find jobs for over a decade, and she’s noticed a trend.
“Not only are more physicians seeking part-time roles than they were 10 years ago, but more large health care systems are also offering part time or per diem as well,” said Ms. Knoup.
Who’s working part time, and why?
Ten years ago, the fastest growing segment of part-timers were men nearing retirement and early- to mid-career women.
Pediatricians led the part-time pack in 2002, according to an American Academy of Pediatrics study. At the time, 15% of pediatricians reported their hours as part time. However, the numbers may have increased over the years. For example, a 2021 study by the department of pediatrics, Boston Medical Center, and Boston University found that almost 30% of graduating pediatricians sought part-time work at the end of their training.
At PS&D, Ms. Knoup said she has noticed a trend toward part-timers among primary care, behavioral health, and outpatient specialties such as endocrinology. “We’re also seeing it with the inpatient side in roles that are more shift based like hospitalists, radiologists, and critical care and ER doctors.”
Another trend Ms. Knoup has noticed is with early-career doctors. “They have a different mindset,” she said. “Younger generations are acutely aware of burnout. They may have experienced it in residency or during the pandemic. They’ve had a taste of that and don’t want to go down that road again, so they’re seeking part-time roles. It’s an intentional choice.”
Tracey O’Connell, MD, a radiologist, always knew that she wanted to work part time. “I had a baby as a resident, and I was pregnant with my second child as a fellow,” she said. “I was already feeling overwhelmed with medical training and having a family.”
Dr. O’Connell worked in private practice for 16 years on Mondays, Wednesdays, and Fridays, with no nights or weekends.
“I still found it completely overwhelming,” she said. “Even though I had more days not working than working, I felt like the demands of medical life had advanced faster than human beings could adapt, and I still feel that way.”
Today she runs a part-time teleradiology practice from home but spends more time on her second career as a life coach. “Most of my clients are physicians looking for more fulfillment and sustainable ways of practicing medicine while maintaining their own identity as human beings, not just the all-consuming identity of ‘doctor,’ ” she said.
On the other end of the career spectrum is Lois Goodman, MD, an ob.gyn. in her late 70s. After 42 years in a group practice, she started her solo practice at 72, seeing patients 3 days per week. “I’m just happy to be working. That’s a tremendous payoff for me. I need to keep working for my mental health.”
How does part-time work affect physician shortages and care delivery?
Reducing clinical effort is one of the strategies physicians use to scale down overload. Still, it’s not viable as a long-term solution, said Christine Sinsky, MD, AMA’s vice president of professional satisfaction and a nationally regarded researcher on physician burnout.
“If all the physicians in a community went from working 100% FTE clinical to 50% FTE clinical, then the people in that community would have half the access to care that they had,” said Dr. Sinsky. “There’s less capacity in the system to care for patients.”
Some could argue, then, that part-time physician work may contribute to physician shortage predictions. An Association of American Medical Colleges report estimates there will be a shortage of 37,800 to 124,000 physicians by 2034.
But physicians working part-time express a contrasting point of view. “I don’t believe that part-time workers are responsible for the health care shortage but rather, a great solution,” said Dr. O’Connell. “Because in order to continue working for a long time rather than quitting when the demands exceed human capacity, working part time is a great compromise to offer a life of more sustainable well-being and longevity as a physician, and still live a wholehearted life.”
Pros and cons of being a part-time physician
Pros
Less burnout: The American Medical Association has tracked burnout rates for 22 years. By the end of 2021, nearly 63% of physicians reported burnout symptoms, compared with 38% the year before. Going part time appears to reduce burnout, suggests a study published in Mayo Clinic Proceedings.
Better work-life balance: Rachel Miller, MD, an ob.gyn., worked 60-70 hours weekly for 9 years. In 2022, she went to work as an OB hospitalist for a health care system that welcomes part-time clinicians. Since then, she has achieved a better work-life balance, putting in 26-28 hours a week. Dr. Miller now spends more time with her kids and in her additional role as an executive coach to leaders in the medical field.
More focus: “When I’m at work, I’m 100% mentally in and focused,” said Dr. Miller. “My interactions with patients are different because I’m not burned out. My demeanor and my willingness to connect are stronger.”
Better health: Mehmet Cilingiroglu, MD, with CardioSolution, traded full-time work for part time when health issues and a kidney transplant sidelined his 30-year career in 2018. “Despite my significant health issues, I’ve been able to continue working at a pace that suits me rather than having to retire,” he said. “Part-time physicians can still enjoy patient care, research, innovation, education, and training while balancing that with other areas of life.”
Errin Weisman, a DO who gave up full-time work in 2016, said cutting back makes her feel healthier, happier, and more energized. “Part-time work helps me to bring my A game each day I work and deliver the best care.” She’s also a life coach encouraging other physicians to find balance in their professional and personal lives.
Cons
Cut in pay: Obviously, the No. 1 con is you’ll make less working part time, so adjusting to a salary decrease can be a huge issue, especially if you don’t have other sources of income. Physicians paying off student loans, those caring for children or elderly parents, or those in their prime earning years needing to save for retirement may not be able to go part time.
Diminished career: The chance for promotions or being well known in your field can be diminished, as well as a loss of proficiency if you’re only performing surgery or procedures part time. In some specialties, working part time and not keeping up with (or being able to practice) newer technology developments can harm your career or reputation in the long run.
Missing out: While working part time has many benefits, physicians also experience a wide range of drawbacks. Dr. Goodman, for example, said she misses delivering babies and doing surgeries. Dr. Miller said she gave up some aspects of her specialty, like performing hysterectomies, participating in complex cases, and no longer having an office like she did as a full-time ob.gyn.
Loss of fellowship: Dr. O’Connell said she missed the camaraderie and sense of belonging when she scaled back her hours. “I felt like a fish out of water, that my values didn’t align with the group’s values,” she said. This led to self-doubt, frustrated colleagues, and a reduction in benefits.
Lost esteem: Dr. O’Connell also felt she was expected to work overtime without additional pay and was no longer eligible for bonuses. “I was treated as a team player when I was needed, but not when it came to perks and benefits and insider privilege,” she said. There may be a loss of esteem among colleagues and supervisors.
Overcoming stigma: Because part-time physician work is still not prevalent among colleagues, some may resist the idea, have less respect for it, perceive it as not being serious about your career as a physician, or associate it with being lazy or entitled.
Summing it up
Every physician must weigh the value and drawbacks of part-time work, but the more physicians who go this route, the more part-time medicine gains traction and the more physicians can learn about its values versus its drawbacks.
A version of this article first appeared on Medscape.com.
On average, physicians reported in the Medscape Physician Compensation Report 2023 that they worked 50 hours per week. Five specialties, including critical care, cardiology, and general surgery reported working 55 or more hours weekly.
In 2011, The New England Journal of Medicine reported that part-time physician careers were rising. At the time, part-time doctors made up 21% of the physician workforce, up from 13% in 2005.
In a more recent survey from the California Health Care Foundation, only 12% of California physicians said they devoted 20-29 hours a week to patient care.
Amy Knoup, a senior recruitment adviser with Provider Solutions & Development), has been helping doctors find jobs for over a decade, and she’s noticed a trend.
“Not only are more physicians seeking part-time roles than they were 10 years ago, but more large health care systems are also offering part time or per diem as well,” said Ms. Knoup.
Who’s working part time, and why?
Ten years ago, the fastest growing segment of part-timers were men nearing retirement and early- to mid-career women.
Pediatricians led the part-time pack in 2002, according to an American Academy of Pediatrics study. At the time, 15% of pediatricians reported their hours as part time. However, the numbers may have increased over the years. For example, a 2021 study by the department of pediatrics, Boston Medical Center, and Boston University found that almost 30% of graduating pediatricians sought part-time work at the end of their training.
At PS&D, Ms. Knoup said she has noticed a trend toward part-timers among primary care, behavioral health, and outpatient specialties such as endocrinology. “We’re also seeing it with the inpatient side in roles that are more shift based like hospitalists, radiologists, and critical care and ER doctors.”
Another trend Ms. Knoup has noticed is with early-career doctors. “They have a different mindset,” she said. “Younger generations are acutely aware of burnout. They may have experienced it in residency or during the pandemic. They’ve had a taste of that and don’t want to go down that road again, so they’re seeking part-time roles. It’s an intentional choice.”
Tracey O’Connell, MD, a radiologist, always knew that she wanted to work part time. “I had a baby as a resident, and I was pregnant with my second child as a fellow,” she said. “I was already feeling overwhelmed with medical training and having a family.”
Dr. O’Connell worked in private practice for 16 years on Mondays, Wednesdays, and Fridays, with no nights or weekends.
“I still found it completely overwhelming,” she said. “Even though I had more days not working than working, I felt like the demands of medical life had advanced faster than human beings could adapt, and I still feel that way.”
Today she runs a part-time teleradiology practice from home but spends more time on her second career as a life coach. “Most of my clients are physicians looking for more fulfillment and sustainable ways of practicing medicine while maintaining their own identity as human beings, not just the all-consuming identity of ‘doctor,’ ” she said.
On the other end of the career spectrum is Lois Goodman, MD, an ob.gyn. in her late 70s. After 42 years in a group practice, she started her solo practice at 72, seeing patients 3 days per week. “I’m just happy to be working. That’s a tremendous payoff for me. I need to keep working for my mental health.”
How does part-time work affect physician shortages and care delivery?
Reducing clinical effort is one of the strategies physicians use to scale down overload. Still, it’s not viable as a long-term solution, said Christine Sinsky, MD, AMA’s vice president of professional satisfaction and a nationally regarded researcher on physician burnout.
“If all the physicians in a community went from working 100% FTE clinical to 50% FTE clinical, then the people in that community would have half the access to care that they had,” said Dr. Sinsky. “There’s less capacity in the system to care for patients.”
Some could argue, then, that part-time physician work may contribute to physician shortage predictions. An Association of American Medical Colleges report estimates there will be a shortage of 37,800 to 124,000 physicians by 2034.
But physicians working part-time express a contrasting point of view. “I don’t believe that part-time workers are responsible for the health care shortage but rather, a great solution,” said Dr. O’Connell. “Because in order to continue working for a long time rather than quitting when the demands exceed human capacity, working part time is a great compromise to offer a life of more sustainable well-being and longevity as a physician, and still live a wholehearted life.”
Pros and cons of being a part-time physician
Pros
Less burnout: The American Medical Association has tracked burnout rates for 22 years. By the end of 2021, nearly 63% of physicians reported burnout symptoms, compared with 38% the year before. Going part time appears to reduce burnout, suggests a study published in Mayo Clinic Proceedings.
Better work-life balance: Rachel Miller, MD, an ob.gyn., worked 60-70 hours weekly for 9 years. In 2022, she went to work as an OB hospitalist for a health care system that welcomes part-time clinicians. Since then, she has achieved a better work-life balance, putting in 26-28 hours a week. Dr. Miller now spends more time with her kids and in her additional role as an executive coach to leaders in the medical field.
More focus: “When I’m at work, I’m 100% mentally in and focused,” said Dr. Miller. “My interactions with patients are different because I’m not burned out. My demeanor and my willingness to connect are stronger.”
Better health: Mehmet Cilingiroglu, MD, with CardioSolution, traded full-time work for part time when health issues and a kidney transplant sidelined his 30-year career in 2018. “Despite my significant health issues, I’ve been able to continue working at a pace that suits me rather than having to retire,” he said. “Part-time physicians can still enjoy patient care, research, innovation, education, and training while balancing that with other areas of life.”
Errin Weisman, a DO who gave up full-time work in 2016, said cutting back makes her feel healthier, happier, and more energized. “Part-time work helps me to bring my A game each day I work and deliver the best care.” She’s also a life coach encouraging other physicians to find balance in their professional and personal lives.
Cons
Cut in pay: Obviously, the No. 1 con is you’ll make less working part time, so adjusting to a salary decrease can be a huge issue, especially if you don’t have other sources of income. Physicians paying off student loans, those caring for children or elderly parents, or those in their prime earning years needing to save for retirement may not be able to go part time.
Diminished career: The chance for promotions or being well known in your field can be diminished, as well as a loss of proficiency if you’re only performing surgery or procedures part time. In some specialties, working part time and not keeping up with (or being able to practice) newer technology developments can harm your career or reputation in the long run.
Missing out: While working part time has many benefits, physicians also experience a wide range of drawbacks. Dr. Goodman, for example, said she misses delivering babies and doing surgeries. Dr. Miller said she gave up some aspects of her specialty, like performing hysterectomies, participating in complex cases, and no longer having an office like she did as a full-time ob.gyn.
Loss of fellowship: Dr. O’Connell said she missed the camaraderie and sense of belonging when she scaled back her hours. “I felt like a fish out of water, that my values didn’t align with the group’s values,” she said. This led to self-doubt, frustrated colleagues, and a reduction in benefits.
Lost esteem: Dr. O’Connell also felt she was expected to work overtime without additional pay and was no longer eligible for bonuses. “I was treated as a team player when I was needed, but not when it came to perks and benefits and insider privilege,” she said. There may be a loss of esteem among colleagues and supervisors.
Overcoming stigma: Because part-time physician work is still not prevalent among colleagues, some may resist the idea, have less respect for it, perceive it as not being serious about your career as a physician, or associate it with being lazy or entitled.
Summing it up
Every physician must weigh the value and drawbacks of part-time work, but the more physicians who go this route, the more part-time medicine gains traction and the more physicians can learn about its values versus its drawbacks.
A version of this article first appeared on Medscape.com.
Clinical trials: Top priority for long COVID
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
Study of hospitalizations in Canada quantifies benefit of COVID-19 vaccine to reduce death, ICU admissions
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
Medications that scare me
An 85-year-old woman is brought to the emergency department after a syncopal episode. Her caregivers report a similar episode 2 weeks ago, but she recovered so quickly they did not seek evaluation for her.
Medications: Omeprazole 20 mg, pravastatin 40 mg, citalopram 10 mg, albuterol, donepezil 10 mg, isosorbide mononitrate 60 mg, and calcium. On exam, blood pressure is 100/60 mm Hg, pulse 55. ECG indicates bradycardia with normal intervals. What drug most likely caused her syncope?
A. Citalopram
B. Pravastatin
C. Donepezil
D. Isosorbide
E. Calcium
This woman’s syncope is likely caused by donepezil. Citalopram can lengthen the QT interval, especially in elderly patients, but the normal intervals on ECG eliminate this possibility. Donepezil can cause bradycardia, which can contribute to syncope.
Hernandez and colleagues evaluated a cohort of veterans with dementia over an 8-year period.1 They found that there was a 1.4-fold increased risk of bradycardia in patients with dementia treated with an acetylcholine inhibitor (compared with that in patients who were not taking these medications) and that there was a dose-dependent increase in risk for patients on donepezil.
Park-Wyllie et al. found in a study of 1.4 million older adults a greater than twofold risk of hospitalization for bradycardia in patients treated with a cholinesterase inhibitor.2 Gill and colleagues performed a population-based cohort study of 19,803 elderly patients with dementia who were prescribed cholinesterase inhibitors, and compared them to age-matched controls.3 They found increased hospital visits for syncope in people receiving cholinesterase inhibitors (hazard ratio, 1.76; 95% confidence interval, 1.57-1.98). Other syncope-related events were also more common in people receiving cholinesterase inhibitors, compared with controls: hospital visits for bradycardia (HR, 1.69; 95% CI, 1.32-2.15), permanent pacemaker insertion (HR, 1.49; 95% CI, 1.12-2.00), and hip fracture (HR, 1.18; (95% CI, 1.04-1.34).
Nausea, vomiting, and weight loss are much more common than the rarer side effects of bradycardia and syncope. The frequency of gastroenterological side effects is up to 25%. Cholinesterase inhibitors have modest effects on cognitive function with a high number needed to treat (NNT) of 10, and an NNT as high as 100 for global function. The number needed to harm (NNH) is 4, when gastrointestinal symptoms are added in.4 Another important, problematic side effect of cholinesterase inhibitors is urinary incontinence. This often leads to patients receiving medications, to combat this side effect, that may worsen cognitive function.
Another commonly used medication that scares me in certain circumstances is trimethoprim-sulfamethoxazole. My main concern is when it is used in patients who are elderly, have chronic kidney disease, or are taking other medications that can cause hyperkalemia (ACEIs, ARBs, potassium-sparing diuretics including spironolactone). Hyperkalemia is a real concern in these patient populations. Trimethoprim reduces renal potassium excretion through the competitive inhibition of sodium channels in the distal nephron, in a manner similar to the potassium-sparing diuretic amiloride. Hospitalizations for hyperkalemia are more common in patients who take ACEIs and ARBs and are prescribed trimethoprim-sulfamethoxazole, compared with other antibiotics.5
Sudden cardiac death is also more common in patients who are taking ACEIs or ARBs and receive trimethoprim-sulfamethoxazole.6 Trimethoprim-sulfamethoxazole also has a powerful interaction with warfarin, both displacing warfarin from albumin and inhibiting its metabolism. It raises the INR (international normalized ratio) in warfarin-treated patients much greater than do other antibiotics.7
Pearls
- Think carefully about the use of cholinesterase inhibitors because of the unfavorable NNH vs. NNT.
- Use caution prescribing trimethoprim for patients who are elderly, especially if they are on an ACEI, an ARB, or spironolactone, and in patients with chronic kidney disease.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Hernandez RK et al. J Am Geriatr Soc. 2009;57:1997-2003.
2. Park-Wyllie LY et al. PLoS Med. 2009;6:e1000157.
3. Gill SS et al. Arch Intern Med 2009;169:867-73.
4. Peters KR. J Am Geriatr Soc. 2013 Jul;61(7):1170-4.
5. Antoniou TN et al. Arch Intern Med. 2010;170(12):1045-9.
6. Fralick M et al. BMJ. 2014 Oct 30;349:g6196.
7. Glasheen JJ et al. J Gen Intern Med. 2005 Jul;20(7):653-6.
An 85-year-old woman is brought to the emergency department after a syncopal episode. Her caregivers report a similar episode 2 weeks ago, but she recovered so quickly they did not seek evaluation for her.
Medications: Omeprazole 20 mg, pravastatin 40 mg, citalopram 10 mg, albuterol, donepezil 10 mg, isosorbide mononitrate 60 mg, and calcium. On exam, blood pressure is 100/60 mm Hg, pulse 55. ECG indicates bradycardia with normal intervals. What drug most likely caused her syncope?
A. Citalopram
B. Pravastatin
C. Donepezil
D. Isosorbide
E. Calcium
This woman’s syncope is likely caused by donepezil. Citalopram can lengthen the QT interval, especially in elderly patients, but the normal intervals on ECG eliminate this possibility. Donepezil can cause bradycardia, which can contribute to syncope.
Hernandez and colleagues evaluated a cohort of veterans with dementia over an 8-year period.1 They found that there was a 1.4-fold increased risk of bradycardia in patients with dementia treated with an acetylcholine inhibitor (compared with that in patients who were not taking these medications) and that there was a dose-dependent increase in risk for patients on donepezil.
Park-Wyllie et al. found in a study of 1.4 million older adults a greater than twofold risk of hospitalization for bradycardia in patients treated with a cholinesterase inhibitor.2 Gill and colleagues performed a population-based cohort study of 19,803 elderly patients with dementia who were prescribed cholinesterase inhibitors, and compared them to age-matched controls.3 They found increased hospital visits for syncope in people receiving cholinesterase inhibitors (hazard ratio, 1.76; 95% confidence interval, 1.57-1.98). Other syncope-related events were also more common in people receiving cholinesterase inhibitors, compared with controls: hospital visits for bradycardia (HR, 1.69; 95% CI, 1.32-2.15), permanent pacemaker insertion (HR, 1.49; 95% CI, 1.12-2.00), and hip fracture (HR, 1.18; (95% CI, 1.04-1.34).
Nausea, vomiting, and weight loss are much more common than the rarer side effects of bradycardia and syncope. The frequency of gastroenterological side effects is up to 25%. Cholinesterase inhibitors have modest effects on cognitive function with a high number needed to treat (NNT) of 10, and an NNT as high as 100 for global function. The number needed to harm (NNH) is 4, when gastrointestinal symptoms are added in.4 Another important, problematic side effect of cholinesterase inhibitors is urinary incontinence. This often leads to patients receiving medications, to combat this side effect, that may worsen cognitive function.
Another commonly used medication that scares me in certain circumstances is trimethoprim-sulfamethoxazole. My main concern is when it is used in patients who are elderly, have chronic kidney disease, or are taking other medications that can cause hyperkalemia (ACEIs, ARBs, potassium-sparing diuretics including spironolactone). Hyperkalemia is a real concern in these patient populations. Trimethoprim reduces renal potassium excretion through the competitive inhibition of sodium channels in the distal nephron, in a manner similar to the potassium-sparing diuretic amiloride. Hospitalizations for hyperkalemia are more common in patients who take ACEIs and ARBs and are prescribed trimethoprim-sulfamethoxazole, compared with other antibiotics.5
Sudden cardiac death is also more common in patients who are taking ACEIs or ARBs and receive trimethoprim-sulfamethoxazole.6 Trimethoprim-sulfamethoxazole also has a powerful interaction with warfarin, both displacing warfarin from albumin and inhibiting its metabolism. It raises the INR (international normalized ratio) in warfarin-treated patients much greater than do other antibiotics.7
Pearls
- Think carefully about the use of cholinesterase inhibitors because of the unfavorable NNH vs. NNT.
- Use caution prescribing trimethoprim for patients who are elderly, especially if they are on an ACEI, an ARB, or spironolactone, and in patients with chronic kidney disease.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Hernandez RK et al. J Am Geriatr Soc. 2009;57:1997-2003.
2. Park-Wyllie LY et al. PLoS Med. 2009;6:e1000157.
3. Gill SS et al. Arch Intern Med 2009;169:867-73.
4. Peters KR. J Am Geriatr Soc. 2013 Jul;61(7):1170-4.
5. Antoniou TN et al. Arch Intern Med. 2010;170(12):1045-9.
6. Fralick M et al. BMJ. 2014 Oct 30;349:g6196.
7. Glasheen JJ et al. J Gen Intern Med. 2005 Jul;20(7):653-6.
An 85-year-old woman is brought to the emergency department after a syncopal episode. Her caregivers report a similar episode 2 weeks ago, but she recovered so quickly they did not seek evaluation for her.
Medications: Omeprazole 20 mg, pravastatin 40 mg, citalopram 10 mg, albuterol, donepezil 10 mg, isosorbide mononitrate 60 mg, and calcium. On exam, blood pressure is 100/60 mm Hg, pulse 55. ECG indicates bradycardia with normal intervals. What drug most likely caused her syncope?
A. Citalopram
B. Pravastatin
C. Donepezil
D. Isosorbide
E. Calcium
This woman’s syncope is likely caused by donepezil. Citalopram can lengthen the QT interval, especially in elderly patients, but the normal intervals on ECG eliminate this possibility. Donepezil can cause bradycardia, which can contribute to syncope.
Hernandez and colleagues evaluated a cohort of veterans with dementia over an 8-year period.1 They found that there was a 1.4-fold increased risk of bradycardia in patients with dementia treated with an acetylcholine inhibitor (compared with that in patients who were not taking these medications) and that there was a dose-dependent increase in risk for patients on donepezil.
Park-Wyllie et al. found in a study of 1.4 million older adults a greater than twofold risk of hospitalization for bradycardia in patients treated with a cholinesterase inhibitor.2 Gill and colleagues performed a population-based cohort study of 19,803 elderly patients with dementia who were prescribed cholinesterase inhibitors, and compared them to age-matched controls.3 They found increased hospital visits for syncope in people receiving cholinesterase inhibitors (hazard ratio, 1.76; 95% confidence interval, 1.57-1.98). Other syncope-related events were also more common in people receiving cholinesterase inhibitors, compared with controls: hospital visits for bradycardia (HR, 1.69; 95% CI, 1.32-2.15), permanent pacemaker insertion (HR, 1.49; 95% CI, 1.12-2.00), and hip fracture (HR, 1.18; (95% CI, 1.04-1.34).
Nausea, vomiting, and weight loss are much more common than the rarer side effects of bradycardia and syncope. The frequency of gastroenterological side effects is up to 25%. Cholinesterase inhibitors have modest effects on cognitive function with a high number needed to treat (NNT) of 10, and an NNT as high as 100 for global function. The number needed to harm (NNH) is 4, when gastrointestinal symptoms are added in.4 Another important, problematic side effect of cholinesterase inhibitors is urinary incontinence. This often leads to patients receiving medications, to combat this side effect, that may worsen cognitive function.
Another commonly used medication that scares me in certain circumstances is trimethoprim-sulfamethoxazole. My main concern is when it is used in patients who are elderly, have chronic kidney disease, or are taking other medications that can cause hyperkalemia (ACEIs, ARBs, potassium-sparing diuretics including spironolactone). Hyperkalemia is a real concern in these patient populations. Trimethoprim reduces renal potassium excretion through the competitive inhibition of sodium channels in the distal nephron, in a manner similar to the potassium-sparing diuretic amiloride. Hospitalizations for hyperkalemia are more common in patients who take ACEIs and ARBs and are prescribed trimethoprim-sulfamethoxazole, compared with other antibiotics.5
Sudden cardiac death is also more common in patients who are taking ACEIs or ARBs and receive trimethoprim-sulfamethoxazole.6 Trimethoprim-sulfamethoxazole also has a powerful interaction with warfarin, both displacing warfarin from albumin and inhibiting its metabolism. It raises the INR (international normalized ratio) in warfarin-treated patients much greater than do other antibiotics.7
Pearls
- Think carefully about the use of cholinesterase inhibitors because of the unfavorable NNH vs. NNT.
- Use caution prescribing trimethoprim for patients who are elderly, especially if they are on an ACEI, an ARB, or spironolactone, and in patients with chronic kidney disease.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Hernandez RK et al. J Am Geriatr Soc. 2009;57:1997-2003.
2. Park-Wyllie LY et al. PLoS Med. 2009;6:e1000157.
3. Gill SS et al. Arch Intern Med 2009;169:867-73.
4. Peters KR. J Am Geriatr Soc. 2013 Jul;61(7):1170-4.
5. Antoniou TN et al. Arch Intern Med. 2010;170(12):1045-9.
6. Fralick M et al. BMJ. 2014 Oct 30;349:g6196.
7. Glasheen JJ et al. J Gen Intern Med. 2005 Jul;20(7):653-6.
Young men at highest schizophrenia risk from cannabis abuse
A new study confirms the robust link between cannabis use and schizophrenia among men and women but suggests that young men may be especially susceptible to schizophrenia from cannabis abuse.
Of note,
“The entanglement of substance use disorders and mental illnesses is a major public health issue, requiring urgent action and support for people who need it,” study coauthor Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a news release.
“As access to potent cannabis products continues to expand, it is crucial that we also expand prevention, screening, and treatment for people who may experience mental illnesses associated with cannabis use,” Dr. Volkow added.
The study was published online in Psychological Medicine.
A modifiable risk factor
The researchers analyzed Danish registry data spanning 5 decades and representing more than 6.9 million people in Denmark to estimate the population-level percentage of schizophrenia cases attributable to CUD.
A total of 60,563 participants were diagnosed with CUD. Three-quarters of cases were in men; there were 45,327 incident cases of schizophrenia during the study period.
The overall adjusted hazard ratio for CUD on schizophrenia was slightly higher among males than females (aHR, 2.42 vs. 2.02); however, among those aged 16 to 20 years, the adjusted incidence risk ratio for males was more than twice that for females (aIRR, 3.84 vs. 1.81).
The researchers estimate that, in 2021, about 15% of schizophrenia cases among males aged 16-49 could have been avoided by preventing CUD, compared with 4% among females in this age range.
For young men aged 21-30, the proportion of preventable schizophrenia cases related to CUD may be as high as 30%, the authors reported.
“Alongside the increasing evidence that CUD is a modifiable risk factor for schizophrenia, our findings underscore the importance of evidence-based strategies to regulate cannabis use and to effectively prevent, screen for, and treat CUD as well as schizophrenia,” the researchers wrote.
Legalization sends the wrong message
In a press statement, lead investigator Carsten Hjorthøj, PhD, with the University of Copenhagen, noted that “increases in the legalization of cannabis over the past few decades have made it one of the most frequently used psychoactive substances in the world, while also decreasing the public’s perception of its harm. This study adds to our growing understanding that cannabis use is not harmless, and that risks are not fixed at one point in time.”
In a prior study, Dr. Hjorthøj and colleagues found that the proportion of new schizophrenia cases attributable to CUD has consistently increased over the past 20 years.
“In my view, the association is most likely causative, at least to a large extent,” Dr. Hjorthøj said at the time this research was published.
“It is of course nearly impossible to use epidemiological studies to actually prove causation, but all the numbers behave exactly in the way that would be expected under the theory of causation,” Dr. Hjorthøj added.
The study received no specific funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study confirms the robust link between cannabis use and schizophrenia among men and women but suggests that young men may be especially susceptible to schizophrenia from cannabis abuse.
Of note,
“The entanglement of substance use disorders and mental illnesses is a major public health issue, requiring urgent action and support for people who need it,” study coauthor Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a news release.
“As access to potent cannabis products continues to expand, it is crucial that we also expand prevention, screening, and treatment for people who may experience mental illnesses associated with cannabis use,” Dr. Volkow added.
The study was published online in Psychological Medicine.
A modifiable risk factor
The researchers analyzed Danish registry data spanning 5 decades and representing more than 6.9 million people in Denmark to estimate the population-level percentage of schizophrenia cases attributable to CUD.
A total of 60,563 participants were diagnosed with CUD. Three-quarters of cases were in men; there were 45,327 incident cases of schizophrenia during the study period.
The overall adjusted hazard ratio for CUD on schizophrenia was slightly higher among males than females (aHR, 2.42 vs. 2.02); however, among those aged 16 to 20 years, the adjusted incidence risk ratio for males was more than twice that for females (aIRR, 3.84 vs. 1.81).
The researchers estimate that, in 2021, about 15% of schizophrenia cases among males aged 16-49 could have been avoided by preventing CUD, compared with 4% among females in this age range.
For young men aged 21-30, the proportion of preventable schizophrenia cases related to CUD may be as high as 30%, the authors reported.
“Alongside the increasing evidence that CUD is a modifiable risk factor for schizophrenia, our findings underscore the importance of evidence-based strategies to regulate cannabis use and to effectively prevent, screen for, and treat CUD as well as schizophrenia,” the researchers wrote.
Legalization sends the wrong message
In a press statement, lead investigator Carsten Hjorthøj, PhD, with the University of Copenhagen, noted that “increases in the legalization of cannabis over the past few decades have made it one of the most frequently used psychoactive substances in the world, while also decreasing the public’s perception of its harm. This study adds to our growing understanding that cannabis use is not harmless, and that risks are not fixed at one point in time.”
In a prior study, Dr. Hjorthøj and colleagues found that the proportion of new schizophrenia cases attributable to CUD has consistently increased over the past 20 years.
“In my view, the association is most likely causative, at least to a large extent,” Dr. Hjorthøj said at the time this research was published.
“It is of course nearly impossible to use epidemiological studies to actually prove causation, but all the numbers behave exactly in the way that would be expected under the theory of causation,” Dr. Hjorthøj added.
The study received no specific funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study confirms the robust link between cannabis use and schizophrenia among men and women but suggests that young men may be especially susceptible to schizophrenia from cannabis abuse.
Of note,
“The entanglement of substance use disorders and mental illnesses is a major public health issue, requiring urgent action and support for people who need it,” study coauthor Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a news release.
“As access to potent cannabis products continues to expand, it is crucial that we also expand prevention, screening, and treatment for people who may experience mental illnesses associated with cannabis use,” Dr. Volkow added.
The study was published online in Psychological Medicine.
A modifiable risk factor
The researchers analyzed Danish registry data spanning 5 decades and representing more than 6.9 million people in Denmark to estimate the population-level percentage of schizophrenia cases attributable to CUD.
A total of 60,563 participants were diagnosed with CUD. Three-quarters of cases were in men; there were 45,327 incident cases of schizophrenia during the study period.
The overall adjusted hazard ratio for CUD on schizophrenia was slightly higher among males than females (aHR, 2.42 vs. 2.02); however, among those aged 16 to 20 years, the adjusted incidence risk ratio for males was more than twice that for females (aIRR, 3.84 vs. 1.81).
The researchers estimate that, in 2021, about 15% of schizophrenia cases among males aged 16-49 could have been avoided by preventing CUD, compared with 4% among females in this age range.
For young men aged 21-30, the proportion of preventable schizophrenia cases related to CUD may be as high as 30%, the authors reported.
“Alongside the increasing evidence that CUD is a modifiable risk factor for schizophrenia, our findings underscore the importance of evidence-based strategies to regulate cannabis use and to effectively prevent, screen for, and treat CUD as well as schizophrenia,” the researchers wrote.
Legalization sends the wrong message
In a press statement, lead investigator Carsten Hjorthøj, PhD, with the University of Copenhagen, noted that “increases in the legalization of cannabis over the past few decades have made it one of the most frequently used psychoactive substances in the world, while also decreasing the public’s perception of its harm. This study adds to our growing understanding that cannabis use is not harmless, and that risks are not fixed at one point in time.”
In a prior study, Dr. Hjorthøj and colleagues found that the proportion of new schizophrenia cases attributable to CUD has consistently increased over the past 20 years.
“In my view, the association is most likely causative, at least to a large extent,” Dr. Hjorthøj said at the time this research was published.
“It is of course nearly impossible to use epidemiological studies to actually prove causation, but all the numbers behave exactly in the way that would be expected under the theory of causation,” Dr. Hjorthøj added.
The study received no specific funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PSYCHOLOGICAL MEDICINE
Improving swallowing may mitigate COPD exacerbations
Dysphagia treatment may be a way to reduce risk for chronic obstructive pulmonary disease (COPD) exacerbations, according to Yoshitaka Oku, MD, of Hyogo Medical University, Nishinomiya, Japan.
Gastroesophageal regurgitation disease (GERD) is known to be associated with exacerbations in COPD, but previous studies have shown little impact of standard GERD therapy on COPD exacerbations. However, additional research indicates that delayed swallowing contributes to COPD exacerbations, as reported in a research review.
In an article published recently in Respiratory Physiology & Neurobiology,
Swallowing disorder (dysphagia) is a common comorbidity in patients with COPD and has been reported at a 17%-20% greater prevalence in those with COPD, compared with controls, the researchers said.
Patients with COPD have altered swallowing behavior because of several factors, including decreased maximal laryngeal elevation, Dr. Oku said. Individuals with COPD “are also prone to laryngeal penetration and aspiration when swallowing large volumes of liquid and tend to follow an inspiratory-swallow-expiratory (I-SW-E) pattern when swallowing large volumes,” he explained.
Dr. Oku conducted prospective studies to investigate the impact of breathing-swallowing discoordination on COPD exacerbation. He found that discoordination in swallowing patterns and the inability to produce airway protective mechanism (such as the I-SW-E pattern) may contribute to more frequent aspirations and more frequent exacerbations.
Dr. Oku also examined whether CPAP and bilevel positive airway pressure (BiPAP) might affect breathing-swallowing coordination in healthy controls and patients with COPD. They found a decrease in breathing-swallowing coordination with CPAP, but not BiPAP, in both controls and stable COPD patients. “During BiPAP, a brief negative flow associated with relaxation of the pharyngeal constrictor muscle triggers inspiratory support, which results in the SW-I pattern,” Dr. Oku noted.
Dr. Oku also wrote that interferential current stimulation (IFC) has been used to stimulate muscles. Studies of transcutaneous electrical sensory stimulation using IFC (IFC-TESS) as an intervention to improve swallowing have shown some success, and also may improve airway protection.
“However, its safety and efficacy in patients with COPD remains unknown,” he wrote. Dr. Oku conducted a study of stable COPD patients and found that repeated salivary swallow test (RSST) scores improved significantly after an IFC-TESS intervention.
Breathing-swallowing discoordination may be an early indicator of swallowing disorder in COPD, and interventions can improve these disorders, Dr. Oku added. However, more research is needed to explore whether interventions to improve dysphagia reduce the frequency of exacerbations in COPD patients, he concluded.
The study was supported by a grant from JSPS KAKENHI. Dr. Oku serves as a senior managing director at EuSense Medical Co.
A version of this article originally appeared on Medscape.com.
Dysphagia treatment may be a way to reduce risk for chronic obstructive pulmonary disease (COPD) exacerbations, according to Yoshitaka Oku, MD, of Hyogo Medical University, Nishinomiya, Japan.
Gastroesophageal regurgitation disease (GERD) is known to be associated with exacerbations in COPD, but previous studies have shown little impact of standard GERD therapy on COPD exacerbations. However, additional research indicates that delayed swallowing contributes to COPD exacerbations, as reported in a research review.
In an article published recently in Respiratory Physiology & Neurobiology,
Swallowing disorder (dysphagia) is a common comorbidity in patients with COPD and has been reported at a 17%-20% greater prevalence in those with COPD, compared with controls, the researchers said.
Patients with COPD have altered swallowing behavior because of several factors, including decreased maximal laryngeal elevation, Dr. Oku said. Individuals with COPD “are also prone to laryngeal penetration and aspiration when swallowing large volumes of liquid and tend to follow an inspiratory-swallow-expiratory (I-SW-E) pattern when swallowing large volumes,” he explained.
Dr. Oku conducted prospective studies to investigate the impact of breathing-swallowing discoordination on COPD exacerbation. He found that discoordination in swallowing patterns and the inability to produce airway protective mechanism (such as the I-SW-E pattern) may contribute to more frequent aspirations and more frequent exacerbations.
Dr. Oku also examined whether CPAP and bilevel positive airway pressure (BiPAP) might affect breathing-swallowing coordination in healthy controls and patients with COPD. They found a decrease in breathing-swallowing coordination with CPAP, but not BiPAP, in both controls and stable COPD patients. “During BiPAP, a brief negative flow associated with relaxation of the pharyngeal constrictor muscle triggers inspiratory support, which results in the SW-I pattern,” Dr. Oku noted.
Dr. Oku also wrote that interferential current stimulation (IFC) has been used to stimulate muscles. Studies of transcutaneous electrical sensory stimulation using IFC (IFC-TESS) as an intervention to improve swallowing have shown some success, and also may improve airway protection.
“However, its safety and efficacy in patients with COPD remains unknown,” he wrote. Dr. Oku conducted a study of stable COPD patients and found that repeated salivary swallow test (RSST) scores improved significantly after an IFC-TESS intervention.
Breathing-swallowing discoordination may be an early indicator of swallowing disorder in COPD, and interventions can improve these disorders, Dr. Oku added. However, more research is needed to explore whether interventions to improve dysphagia reduce the frequency of exacerbations in COPD patients, he concluded.
The study was supported by a grant from JSPS KAKENHI. Dr. Oku serves as a senior managing director at EuSense Medical Co.
A version of this article originally appeared on Medscape.com.
Dysphagia treatment may be a way to reduce risk for chronic obstructive pulmonary disease (COPD) exacerbations, according to Yoshitaka Oku, MD, of Hyogo Medical University, Nishinomiya, Japan.
Gastroesophageal regurgitation disease (GERD) is known to be associated with exacerbations in COPD, but previous studies have shown little impact of standard GERD therapy on COPD exacerbations. However, additional research indicates that delayed swallowing contributes to COPD exacerbations, as reported in a research review.
In an article published recently in Respiratory Physiology & Neurobiology,
Swallowing disorder (dysphagia) is a common comorbidity in patients with COPD and has been reported at a 17%-20% greater prevalence in those with COPD, compared with controls, the researchers said.
Patients with COPD have altered swallowing behavior because of several factors, including decreased maximal laryngeal elevation, Dr. Oku said. Individuals with COPD “are also prone to laryngeal penetration and aspiration when swallowing large volumes of liquid and tend to follow an inspiratory-swallow-expiratory (I-SW-E) pattern when swallowing large volumes,” he explained.
Dr. Oku conducted prospective studies to investigate the impact of breathing-swallowing discoordination on COPD exacerbation. He found that discoordination in swallowing patterns and the inability to produce airway protective mechanism (such as the I-SW-E pattern) may contribute to more frequent aspirations and more frequent exacerbations.
Dr. Oku also examined whether CPAP and bilevel positive airway pressure (BiPAP) might affect breathing-swallowing coordination in healthy controls and patients with COPD. They found a decrease in breathing-swallowing coordination with CPAP, but not BiPAP, in both controls and stable COPD patients. “During BiPAP, a brief negative flow associated with relaxation of the pharyngeal constrictor muscle triggers inspiratory support, which results in the SW-I pattern,” Dr. Oku noted.
Dr. Oku also wrote that interferential current stimulation (IFC) has been used to stimulate muscles. Studies of transcutaneous electrical sensory stimulation using IFC (IFC-TESS) as an intervention to improve swallowing have shown some success, and also may improve airway protection.
“However, its safety and efficacy in patients with COPD remains unknown,” he wrote. Dr. Oku conducted a study of stable COPD patients and found that repeated salivary swallow test (RSST) scores improved significantly after an IFC-TESS intervention.
Breathing-swallowing discoordination may be an early indicator of swallowing disorder in COPD, and interventions can improve these disorders, Dr. Oku added. However, more research is needed to explore whether interventions to improve dysphagia reduce the frequency of exacerbations in COPD patients, he concluded.
The study was supported by a grant from JSPS KAKENHI. Dr. Oku serves as a senior managing director at EuSense Medical Co.
A version of this article originally appeared on Medscape.com.
Virtual care not linked with greater ED use during pandemic
Canadian family physicians’ increased use of virtual care during the first years of the pandemic was not associated with increased emergency department use among patients, a new analysis of data from Ontario suggests.
In a cross-sectional study that included almost 14,000 family physicians and almost 13 million patients in Ontario, an adjusted analysis indicated that patients with physicians who provided more than 20% of care virtually had lower rates of ED visits, compared with patients whose physicians provided the least virtual care.
“I was surprised to see that ED visit volumes in fall 2021 were below prepandemic levels,” study author Tara Kiran, MD, who practices family medicine at St. Michael’s Hospital of the University of Toronto, told this news organization.
“At that time, there was a lot in the news about how our EDs were overcrowded and an assumption that this related to higher visit volumes. But our data [suggest] there were other factors at play, including strains in staffing in the ED, hospital inpatient units, and in long-term care.” Dr. Kiran is also the Fidani chair in improvement and innovation and vice-chair of quality and innovation at the department of family and community medicine of the University of Toronto.
The study was published online in JAMA Network Open.
Embrace of telehealth
The investigators analyzed administrative data from Ontario for 13,820 family physicians (mean age, 50 years; 51.5% men) and 12,951,063 patients (mean age, 42.6 years; 51.8% women) under their care.
The family physicians had at least one primary care visit claim between Feb. 1 and Oct. 31, 2021. The researchers categorized the physicians by the percentage of total visits they delivered virtually (via telephone or video) during the study period, as follows: 0% (100% in person), greater than 0%-20%, greater than 20%-40%, greater than 40%-60%, greater than 60%-80%, greater than 80% to less than 100%, or 100%.
The percentage of virtual primary care visits peaked at 82% in the first 2 weeks of the pandemic and decreased to 49% by October 2021. ED visit rates decreased at the start of the pandemic and remained lower than in 2019 throughout the study period.
Most physicians provided between 40% and 80% of care virtually. A greater percentage of those who provided more than 80% of care virtually were aged 65 years or older, were women, and practiced in large cities.
Patient comorbidity and morbidity were similar across all categories of virtual care use. The mean number of ED visits was highest among patients whose physicians provided only in-person care (470.3 per 1,000 patients) and was lowest among those whose physicians provided greater than 0% to less than 100% of care virtually (242 per 1,000 patients).
After adjustment for patient characteristics, patients of physicians who provided more than 20% of care virtually had lower rates of ED visits, compared with patients of physicians who provided the least virtual care (for example, greater than 80% to less than 100% versus 0%-20% virtual visits in big cities; relative rate, 0.77). This pattern was consistent across all rurality of practice categories and after adjustment for 2019 ED visit rates.
The investigators observed a gradient in urban areas. Patients of physicians who provided the highest level of virtual care had the lowest ED visit rates.
Investigating virtual modalities
Some policymakers worried that inappropriate use of virtual care was leading to an increase in ED use. “Findings of this study refute this hypothesis,” the authors write. Increases in ED use seemed to coincide with decreases in COVID-19 cases, not with increases in virtual primary care visits.
Furthermore, at the population level, patients who were cared for by physicians who provided a high percentage of virtual care did not have a higher rate of ED visits, compared with those cared for by physicians who provided the lowest levels of virtual care.
During the pandemic, the switch to virtual care worked well for some of Dr. Kiran’s patients. It was more convenient, because they didn’t have to take time off work, travel to and from the clinic, find and pay for parking, or wait in the clinic before the appointment, she said.
But for others, “virtual care really didn’t work well,” she said. “This was particularly true for people who didn’t have a regular working phone, who didn’t have a private space to take calls, who weren’t fluent in English, and who were hard of hearing or had severe mental illness that resulted in paranoid thoughts.”
Clinicians also may have had different comfort levels and preferences regarding virtual visits, Dr. Kiran hypothesized. Some found it convenient and efficient, whereas others may have found it cumbersome and inefficient. “I personally find it harder to build relationships with patients when I use virtual care,” she said. “I experience more joy in work with in-person visits, but other clinicians may feel differently.”
Dr. Kiran and her colleagues are conducting a public engagement initiative called OurCare to understand public perspectives on the future of primary care. “As part of that work, we want to understand what virtual modalities are most important to the public and how the public thinks these should be integrated into primary care.”
Virtual care can support access, patient-centered care, and equity in primary care, Dr. Kiran added. “Ideally, it should be integrated into an existing relationship with a family physician and be a complement to in-person visits.”
The right dose?
In an accompanying editorial, Jesse M. Pines, MD, chief of clinical innovation at U.S. Acute Care Solutions, Canton, Ohio, writes, “There is no convincing mechanism consistent with the data for the observed outcome of lower ED use at higher telehealth use.”
Additional research is needed, he notes, to answer the “Goldilocks question” – that is, what amount of telehealth optimizes its benefits while minimizing potential problems?
“The right dose of telehealth needs to balance (1) concerns by payers and policymakers that it will increase cost and cause unintended consequences (for example, misdiagnosis or duplicative care) and (2) the desire of its proponents who want to allow clinicians to use it as they see fit, with few restrictions,” writes Dr. Pines.
“Future research would ideally use more robust research design,” he suggested. “For example, randomized trials could test different doses of telehealth, or mixed-methods studies could help elucidate how telehealth may be changing clinical management or care-seeking behavior.”
Equitable reimbursement needed
Priya Nori, MD, associate professor of infectious diseases at Montefiore Health System and associate professor at the Albert Einstein College of Medicine, both in New York, said, “I agree with their conclusions and am reassured about telehealth as a durable form of health care delivery.”
Large, population-level studies such as this one might persuade legislators to require equitable reimbursement for in-person and virtual visits “so providers have comparable incentives to provide both types of care,” she said. “Although only primary care was addressed in the study, I believe that virtual care is here to stay and can be applied to primary care, subspecialty care, and other services, like antimicrobial stewardship, infection prevention, et cetera. We need to embrace it.”
A similar study should be conducted in the United States, along with additional research “to ensure that visits done by telephone have similar outcomes as those done by video, as not all communities have adequate internet access or video conferencing technology,” said Dr. Nori.
The study was supported by ICES and grants from Ontario Health, the Canadian Institutes of Health Research, and the Health Systems Research Program of Ontario MOH. Dr. Kiran, Dr. Pines, and Dr. Nori have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Canadian family physicians’ increased use of virtual care during the first years of the pandemic was not associated with increased emergency department use among patients, a new analysis of data from Ontario suggests.
In a cross-sectional study that included almost 14,000 family physicians and almost 13 million patients in Ontario, an adjusted analysis indicated that patients with physicians who provided more than 20% of care virtually had lower rates of ED visits, compared with patients whose physicians provided the least virtual care.
“I was surprised to see that ED visit volumes in fall 2021 were below prepandemic levels,” study author Tara Kiran, MD, who practices family medicine at St. Michael’s Hospital of the University of Toronto, told this news organization.
“At that time, there was a lot in the news about how our EDs were overcrowded and an assumption that this related to higher visit volumes. But our data [suggest] there were other factors at play, including strains in staffing in the ED, hospital inpatient units, and in long-term care.” Dr. Kiran is also the Fidani chair in improvement and innovation and vice-chair of quality and innovation at the department of family and community medicine of the University of Toronto.
The study was published online in JAMA Network Open.
Embrace of telehealth
The investigators analyzed administrative data from Ontario for 13,820 family physicians (mean age, 50 years; 51.5% men) and 12,951,063 patients (mean age, 42.6 years; 51.8% women) under their care.
The family physicians had at least one primary care visit claim between Feb. 1 and Oct. 31, 2021. The researchers categorized the physicians by the percentage of total visits they delivered virtually (via telephone or video) during the study period, as follows: 0% (100% in person), greater than 0%-20%, greater than 20%-40%, greater than 40%-60%, greater than 60%-80%, greater than 80% to less than 100%, or 100%.
The percentage of virtual primary care visits peaked at 82% in the first 2 weeks of the pandemic and decreased to 49% by October 2021. ED visit rates decreased at the start of the pandemic and remained lower than in 2019 throughout the study period.
Most physicians provided between 40% and 80% of care virtually. A greater percentage of those who provided more than 80% of care virtually were aged 65 years or older, were women, and practiced in large cities.
Patient comorbidity and morbidity were similar across all categories of virtual care use. The mean number of ED visits was highest among patients whose physicians provided only in-person care (470.3 per 1,000 patients) and was lowest among those whose physicians provided greater than 0% to less than 100% of care virtually (242 per 1,000 patients).
After adjustment for patient characteristics, patients of physicians who provided more than 20% of care virtually had lower rates of ED visits, compared with patients of physicians who provided the least virtual care (for example, greater than 80% to less than 100% versus 0%-20% virtual visits in big cities; relative rate, 0.77). This pattern was consistent across all rurality of practice categories and after adjustment for 2019 ED visit rates.
The investigators observed a gradient in urban areas. Patients of physicians who provided the highest level of virtual care had the lowest ED visit rates.
Investigating virtual modalities
Some policymakers worried that inappropriate use of virtual care was leading to an increase in ED use. “Findings of this study refute this hypothesis,” the authors write. Increases in ED use seemed to coincide with decreases in COVID-19 cases, not with increases in virtual primary care visits.
Furthermore, at the population level, patients who were cared for by physicians who provided a high percentage of virtual care did not have a higher rate of ED visits, compared with those cared for by physicians who provided the lowest levels of virtual care.
During the pandemic, the switch to virtual care worked well for some of Dr. Kiran’s patients. It was more convenient, because they didn’t have to take time off work, travel to and from the clinic, find and pay for parking, or wait in the clinic before the appointment, she said.
But for others, “virtual care really didn’t work well,” she said. “This was particularly true for people who didn’t have a regular working phone, who didn’t have a private space to take calls, who weren’t fluent in English, and who were hard of hearing or had severe mental illness that resulted in paranoid thoughts.”
Clinicians also may have had different comfort levels and preferences regarding virtual visits, Dr. Kiran hypothesized. Some found it convenient and efficient, whereas others may have found it cumbersome and inefficient. “I personally find it harder to build relationships with patients when I use virtual care,” she said. “I experience more joy in work with in-person visits, but other clinicians may feel differently.”
Dr. Kiran and her colleagues are conducting a public engagement initiative called OurCare to understand public perspectives on the future of primary care. “As part of that work, we want to understand what virtual modalities are most important to the public and how the public thinks these should be integrated into primary care.”
Virtual care can support access, patient-centered care, and equity in primary care, Dr. Kiran added. “Ideally, it should be integrated into an existing relationship with a family physician and be a complement to in-person visits.”
The right dose?
In an accompanying editorial, Jesse M. Pines, MD, chief of clinical innovation at U.S. Acute Care Solutions, Canton, Ohio, writes, “There is no convincing mechanism consistent with the data for the observed outcome of lower ED use at higher telehealth use.”
Additional research is needed, he notes, to answer the “Goldilocks question” – that is, what amount of telehealth optimizes its benefits while minimizing potential problems?
“The right dose of telehealth needs to balance (1) concerns by payers and policymakers that it will increase cost and cause unintended consequences (for example, misdiagnosis or duplicative care) and (2) the desire of its proponents who want to allow clinicians to use it as they see fit, with few restrictions,” writes Dr. Pines.
“Future research would ideally use more robust research design,” he suggested. “For example, randomized trials could test different doses of telehealth, or mixed-methods studies could help elucidate how telehealth may be changing clinical management or care-seeking behavior.”
Equitable reimbursement needed
Priya Nori, MD, associate professor of infectious diseases at Montefiore Health System and associate professor at the Albert Einstein College of Medicine, both in New York, said, “I agree with their conclusions and am reassured about telehealth as a durable form of health care delivery.”
Large, population-level studies such as this one might persuade legislators to require equitable reimbursement for in-person and virtual visits “so providers have comparable incentives to provide both types of care,” she said. “Although only primary care was addressed in the study, I believe that virtual care is here to stay and can be applied to primary care, subspecialty care, and other services, like antimicrobial stewardship, infection prevention, et cetera. We need to embrace it.”
A similar study should be conducted in the United States, along with additional research “to ensure that visits done by telephone have similar outcomes as those done by video, as not all communities have adequate internet access or video conferencing technology,” said Dr. Nori.
The study was supported by ICES and grants from Ontario Health, the Canadian Institutes of Health Research, and the Health Systems Research Program of Ontario MOH. Dr. Kiran, Dr. Pines, and Dr. Nori have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Canadian family physicians’ increased use of virtual care during the first years of the pandemic was not associated with increased emergency department use among patients, a new analysis of data from Ontario suggests.
In a cross-sectional study that included almost 14,000 family physicians and almost 13 million patients in Ontario, an adjusted analysis indicated that patients with physicians who provided more than 20% of care virtually had lower rates of ED visits, compared with patients whose physicians provided the least virtual care.
“I was surprised to see that ED visit volumes in fall 2021 were below prepandemic levels,” study author Tara Kiran, MD, who practices family medicine at St. Michael’s Hospital of the University of Toronto, told this news organization.
“At that time, there was a lot in the news about how our EDs were overcrowded and an assumption that this related to higher visit volumes. But our data [suggest] there were other factors at play, including strains in staffing in the ED, hospital inpatient units, and in long-term care.” Dr. Kiran is also the Fidani chair in improvement and innovation and vice-chair of quality and innovation at the department of family and community medicine of the University of Toronto.
The study was published online in JAMA Network Open.
Embrace of telehealth
The investigators analyzed administrative data from Ontario for 13,820 family physicians (mean age, 50 years; 51.5% men) and 12,951,063 patients (mean age, 42.6 years; 51.8% women) under their care.
The family physicians had at least one primary care visit claim between Feb. 1 and Oct. 31, 2021. The researchers categorized the physicians by the percentage of total visits they delivered virtually (via telephone or video) during the study period, as follows: 0% (100% in person), greater than 0%-20%, greater than 20%-40%, greater than 40%-60%, greater than 60%-80%, greater than 80% to less than 100%, or 100%.
The percentage of virtual primary care visits peaked at 82% in the first 2 weeks of the pandemic and decreased to 49% by October 2021. ED visit rates decreased at the start of the pandemic and remained lower than in 2019 throughout the study period.
Most physicians provided between 40% and 80% of care virtually. A greater percentage of those who provided more than 80% of care virtually were aged 65 years or older, were women, and practiced in large cities.
Patient comorbidity and morbidity were similar across all categories of virtual care use. The mean number of ED visits was highest among patients whose physicians provided only in-person care (470.3 per 1,000 patients) and was lowest among those whose physicians provided greater than 0% to less than 100% of care virtually (242 per 1,000 patients).
After adjustment for patient characteristics, patients of physicians who provided more than 20% of care virtually had lower rates of ED visits, compared with patients of physicians who provided the least virtual care (for example, greater than 80% to less than 100% versus 0%-20% virtual visits in big cities; relative rate, 0.77). This pattern was consistent across all rurality of practice categories and after adjustment for 2019 ED visit rates.
The investigators observed a gradient in urban areas. Patients of physicians who provided the highest level of virtual care had the lowest ED visit rates.
Investigating virtual modalities
Some policymakers worried that inappropriate use of virtual care was leading to an increase in ED use. “Findings of this study refute this hypothesis,” the authors write. Increases in ED use seemed to coincide with decreases in COVID-19 cases, not with increases in virtual primary care visits.
Furthermore, at the population level, patients who were cared for by physicians who provided a high percentage of virtual care did not have a higher rate of ED visits, compared with those cared for by physicians who provided the lowest levels of virtual care.
During the pandemic, the switch to virtual care worked well for some of Dr. Kiran’s patients. It was more convenient, because they didn’t have to take time off work, travel to and from the clinic, find and pay for parking, or wait in the clinic before the appointment, she said.
But for others, “virtual care really didn’t work well,” she said. “This was particularly true for people who didn’t have a regular working phone, who didn’t have a private space to take calls, who weren’t fluent in English, and who were hard of hearing or had severe mental illness that resulted in paranoid thoughts.”
Clinicians also may have had different comfort levels and preferences regarding virtual visits, Dr. Kiran hypothesized. Some found it convenient and efficient, whereas others may have found it cumbersome and inefficient. “I personally find it harder to build relationships with patients when I use virtual care,” she said. “I experience more joy in work with in-person visits, but other clinicians may feel differently.”
Dr. Kiran and her colleagues are conducting a public engagement initiative called OurCare to understand public perspectives on the future of primary care. “As part of that work, we want to understand what virtual modalities are most important to the public and how the public thinks these should be integrated into primary care.”
Virtual care can support access, patient-centered care, and equity in primary care, Dr. Kiran added. “Ideally, it should be integrated into an existing relationship with a family physician and be a complement to in-person visits.”
The right dose?
In an accompanying editorial, Jesse M. Pines, MD, chief of clinical innovation at U.S. Acute Care Solutions, Canton, Ohio, writes, “There is no convincing mechanism consistent with the data for the observed outcome of lower ED use at higher telehealth use.”
Additional research is needed, he notes, to answer the “Goldilocks question” – that is, what amount of telehealth optimizes its benefits while minimizing potential problems?
“The right dose of telehealth needs to balance (1) concerns by payers and policymakers that it will increase cost and cause unintended consequences (for example, misdiagnosis or duplicative care) and (2) the desire of its proponents who want to allow clinicians to use it as they see fit, with few restrictions,” writes Dr. Pines.
“Future research would ideally use more robust research design,” he suggested. “For example, randomized trials could test different doses of telehealth, or mixed-methods studies could help elucidate how telehealth may be changing clinical management or care-seeking behavior.”
Equitable reimbursement needed
Priya Nori, MD, associate professor of infectious diseases at Montefiore Health System and associate professor at the Albert Einstein College of Medicine, both in New York, said, “I agree with their conclusions and am reassured about telehealth as a durable form of health care delivery.”
Large, population-level studies such as this one might persuade legislators to require equitable reimbursement for in-person and virtual visits “so providers have comparable incentives to provide both types of care,” she said. “Although only primary care was addressed in the study, I believe that virtual care is here to stay and can be applied to primary care, subspecialty care, and other services, like antimicrobial stewardship, infection prevention, et cetera. We need to embrace it.”
A similar study should be conducted in the United States, along with additional research “to ensure that visits done by telephone have similar outcomes as those done by video, as not all communities have adequate internet access or video conferencing technology,” said Dr. Nori.
The study was supported by ICES and grants from Ontario Health, the Canadian Institutes of Health Research, and the Health Systems Research Program of Ontario MOH. Dr. Kiran, Dr. Pines, and Dr. Nori have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
CRC screening rates are higher in Medicaid expansion states
CHICAGO – presented on May 6 in Chicago at the annual Digestive Disease Week®.
Researchers from the University of California, Los Angeles, reported that states with expanded Medicaid coverage had significantly higher rates of colorectal cancer (CRC) screening than states where officials refused federal support for Medicaid expansion.
Led by Megan R. McLeod, MD, an internal medicine resident at the University of California, Los Angeles, researchers compared CRC screening rates in states that did not adopt Medicaid expansion in 2021 with screening rates in states that invested Medicaid expansion into 1,284 Federally Qualified Health Centers, which are nonprofit health centers or clinics that serve medically underserved areas and populations. In this study, 76% of these centers were in states that accepted Medicaid expansion. The median colorectal cancer screening rate was 42.1% in Medicaid expansion states, compared with 36.5% in nonexpansion states
“The impact of being uninsured on CRC screening participation was profound in nonexpansion states,” said Dr. McLeod, who will be a UCLA gastroenterology fellow this year.
The study adds to a growing body of evidence that shows Medicaid expansion, which increases access to health care services to previously uninsured or underinsured patients, can improve health outcomes and may reduce racial and economic disparities.
For example, a 2019 study based on electronic health record data presented at the annual meeting of the American Society of Clinical Oncology showed that, after Medicaid expansion, racial differences in timely cancer treatment effectively disappeared. Before Medicaid expansion, Black patients were 4.8% less likely than White patients to receive timely cancer treatment, which is defined as treatment starting within 30 days of the diagnosis of an advanced or metastatic solid tumor. After Medicaid expansion, however, the difference between the racial groups dwindled to 0.8% and was no longer statistically significant.
Researchers at Weill Cornell Medical Center in New York reported in 2020 at the virtual annual meeting of the American Association for the Study of Liver Diseases that, 1 year after Medicaid expansion began on Jan. 1, 2014, the rate of liver-related mortality began to decline in 18 states with expanded coverage, whereas the rate of liver-related deaths continued to climb in 14 states that did not expand Medicaid.
The U.S. Health Resources and Services Administration funds Federally Qualified Health Centers (FQHC) that serve nearly 29 million patients throughout the country, including a large proportion whose care is covered by Medicaid. Among patients cared for in these centers, one in three have incomes below the federal poverty line, and one in five are uninsured.
Screening rates compared
Dr. McLeod and colleagues sought to determine whether Medicaid expansion would have an effect on CRC screening rates at these centers. The final analysis included 6,940,879 patients (between 50 and 74 years), of whom 1.7% were unhoused and 17.6% were uninsured.
Medicaid expansion status appeared to have a direct impact on whether screenings were even offered to patients. Centers in rural areas and those with a high proportion of uninsured patients were found to have significantly higher odds for doing fewer CRC screenings. In Medicaid expansion states, CRC screening rates were significantly lower for patients who were male, Black, Hispanic, had low income, were unhoused, or were uninsured.
In a Q&A that followed the presentation, Steven Itzkowitz, MD, director of the GI fellowship program at the Icahn School of Medicine at Mount Sinai, New York, suggested the type of CRC test patients are offered is directly related to Medicaid expansion status.
“In New York, before Cologuard (a colon and rectal cancer screening test) was covered by Medicaid, it wasn’t used very much, but once it got paid for by Medicaid, rates went up,” he said.
The study was internally supported. Dr. McLeod reported no conflicts of interest. Dr. Itzkowitz has been a consultant for Exact Sciences, the maker of Cologuard.
DDW is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
CHICAGO – presented on May 6 in Chicago at the annual Digestive Disease Week®.
Researchers from the University of California, Los Angeles, reported that states with expanded Medicaid coverage had significantly higher rates of colorectal cancer (CRC) screening than states where officials refused federal support for Medicaid expansion.
Led by Megan R. McLeod, MD, an internal medicine resident at the University of California, Los Angeles, researchers compared CRC screening rates in states that did not adopt Medicaid expansion in 2021 with screening rates in states that invested Medicaid expansion into 1,284 Federally Qualified Health Centers, which are nonprofit health centers or clinics that serve medically underserved areas and populations. In this study, 76% of these centers were in states that accepted Medicaid expansion. The median colorectal cancer screening rate was 42.1% in Medicaid expansion states, compared with 36.5% in nonexpansion states
“The impact of being uninsured on CRC screening participation was profound in nonexpansion states,” said Dr. McLeod, who will be a UCLA gastroenterology fellow this year.
The study adds to a growing body of evidence that shows Medicaid expansion, which increases access to health care services to previously uninsured or underinsured patients, can improve health outcomes and may reduce racial and economic disparities.
For example, a 2019 study based on electronic health record data presented at the annual meeting of the American Society of Clinical Oncology showed that, after Medicaid expansion, racial differences in timely cancer treatment effectively disappeared. Before Medicaid expansion, Black patients were 4.8% less likely than White patients to receive timely cancer treatment, which is defined as treatment starting within 30 days of the diagnosis of an advanced or metastatic solid tumor. After Medicaid expansion, however, the difference between the racial groups dwindled to 0.8% and was no longer statistically significant.
Researchers at Weill Cornell Medical Center in New York reported in 2020 at the virtual annual meeting of the American Association for the Study of Liver Diseases that, 1 year after Medicaid expansion began on Jan. 1, 2014, the rate of liver-related mortality began to decline in 18 states with expanded coverage, whereas the rate of liver-related deaths continued to climb in 14 states that did not expand Medicaid.
The U.S. Health Resources and Services Administration funds Federally Qualified Health Centers (FQHC) that serve nearly 29 million patients throughout the country, including a large proportion whose care is covered by Medicaid. Among patients cared for in these centers, one in three have incomes below the federal poverty line, and one in five are uninsured.
Screening rates compared
Dr. McLeod and colleagues sought to determine whether Medicaid expansion would have an effect on CRC screening rates at these centers. The final analysis included 6,940,879 patients (between 50 and 74 years), of whom 1.7% were unhoused and 17.6% were uninsured.
Medicaid expansion status appeared to have a direct impact on whether screenings were even offered to patients. Centers in rural areas and those with a high proportion of uninsured patients were found to have significantly higher odds for doing fewer CRC screenings. In Medicaid expansion states, CRC screening rates were significantly lower for patients who were male, Black, Hispanic, had low income, were unhoused, or were uninsured.
In a Q&A that followed the presentation, Steven Itzkowitz, MD, director of the GI fellowship program at the Icahn School of Medicine at Mount Sinai, New York, suggested the type of CRC test patients are offered is directly related to Medicaid expansion status.
“In New York, before Cologuard (a colon and rectal cancer screening test) was covered by Medicaid, it wasn’t used very much, but once it got paid for by Medicaid, rates went up,” he said.
The study was internally supported. Dr. McLeod reported no conflicts of interest. Dr. Itzkowitz has been a consultant for Exact Sciences, the maker of Cologuard.
DDW is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
CHICAGO – presented on May 6 in Chicago at the annual Digestive Disease Week®.
Researchers from the University of California, Los Angeles, reported that states with expanded Medicaid coverage had significantly higher rates of colorectal cancer (CRC) screening than states where officials refused federal support for Medicaid expansion.
Led by Megan R. McLeod, MD, an internal medicine resident at the University of California, Los Angeles, researchers compared CRC screening rates in states that did not adopt Medicaid expansion in 2021 with screening rates in states that invested Medicaid expansion into 1,284 Federally Qualified Health Centers, which are nonprofit health centers or clinics that serve medically underserved areas and populations. In this study, 76% of these centers were in states that accepted Medicaid expansion. The median colorectal cancer screening rate was 42.1% in Medicaid expansion states, compared with 36.5% in nonexpansion states
“The impact of being uninsured on CRC screening participation was profound in nonexpansion states,” said Dr. McLeod, who will be a UCLA gastroenterology fellow this year.
The study adds to a growing body of evidence that shows Medicaid expansion, which increases access to health care services to previously uninsured or underinsured patients, can improve health outcomes and may reduce racial and economic disparities.
For example, a 2019 study based on electronic health record data presented at the annual meeting of the American Society of Clinical Oncology showed that, after Medicaid expansion, racial differences in timely cancer treatment effectively disappeared. Before Medicaid expansion, Black patients were 4.8% less likely than White patients to receive timely cancer treatment, which is defined as treatment starting within 30 days of the diagnosis of an advanced or metastatic solid tumor. After Medicaid expansion, however, the difference between the racial groups dwindled to 0.8% and was no longer statistically significant.
Researchers at Weill Cornell Medical Center in New York reported in 2020 at the virtual annual meeting of the American Association for the Study of Liver Diseases that, 1 year after Medicaid expansion began on Jan. 1, 2014, the rate of liver-related mortality began to decline in 18 states with expanded coverage, whereas the rate of liver-related deaths continued to climb in 14 states that did not expand Medicaid.
The U.S. Health Resources and Services Administration funds Federally Qualified Health Centers (FQHC) that serve nearly 29 million patients throughout the country, including a large proportion whose care is covered by Medicaid. Among patients cared for in these centers, one in three have incomes below the federal poverty line, and one in five are uninsured.
Screening rates compared
Dr. McLeod and colleagues sought to determine whether Medicaid expansion would have an effect on CRC screening rates at these centers. The final analysis included 6,940,879 patients (between 50 and 74 years), of whom 1.7% were unhoused and 17.6% were uninsured.
Medicaid expansion status appeared to have a direct impact on whether screenings were even offered to patients. Centers in rural areas and those with a high proportion of uninsured patients were found to have significantly higher odds for doing fewer CRC screenings. In Medicaid expansion states, CRC screening rates were significantly lower for patients who were male, Black, Hispanic, had low income, were unhoused, or were uninsured.
In a Q&A that followed the presentation, Steven Itzkowitz, MD, director of the GI fellowship program at the Icahn School of Medicine at Mount Sinai, New York, suggested the type of CRC test patients are offered is directly related to Medicaid expansion status.
“In New York, before Cologuard (a colon and rectal cancer screening test) was covered by Medicaid, it wasn’t used very much, but once it got paid for by Medicaid, rates went up,” he said.
The study was internally supported. Dr. McLeod reported no conflicts of interest. Dr. Itzkowitz has been a consultant for Exact Sciences, the maker of Cologuard.
DDW is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
AT DDW 2023