User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
CDC cuts back hospital data reporting on COVID
When the federal government’s public health emergency (PHE) ended on May 11, the Centers for Disease Control and Prevention scaled back the amount of COVID-related data that it had required hospitals to collect and report during the previous 3 years. The CDC had to do this, an agency spokesman said in an interview, because “CDC’s authorizations to collect certain types of public health data” expired with the PHE.
The CDC insists that it will have enough data to keep up with the virus, which repeatedly defied scientists’ expectations during the course of the pandemic. But some experts have doubts about whether this will turn out to be the case.
While the COVID pandemic is subsiding and transitioning to an endemic phase, many things about the coronavirus are still not understood, noted Marisa Eisenberg, PhD, associate professor of epidemiology at the University of Michigan, Ann Arbor.
“COVID is here to stay, and it ebbs and flows but is staying at fairly consistent levels across the country,” she said in an interview. “Meanwhile, we haven’t established a regular seasonality for COVID that we see for most other respiratory illnesses. We’re still seeing pretty rapidly invading new waves of variants. With flu and other respiratory illnesses, you often see a particular variant in each season. There’s an established pattern. For COVID, that’s still shifting.”
Similarly, Sam Scarpino, PhD, a public health expert at Northeastern University, Boston, told the New York Times: “The CDC is shuffling COVID into the deck of infectious diseases that we’re satisfied living with. One thousand deaths a week is just unacceptable.”
William Schaffner, MD, a professor of preventive medicine and health policy at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview that “how we deal with influenza is something of a template or a model for what the CDC is trying to get to with COVID.” It’s not practical for physicians and hospitals to report every flu case, and the same is now true for COVID. However, “we’re still asking for data on people who are hospitalized with COVID to be reported. That will give us a measure of the major public health impact.”
Dr. Eisenberg doesn’t fully subscribe to this notion. “COVID and influenza are both respiratory illnesses, and our initial pandemic response was based on playbooks that we’d built for potential flu pandemics. But COVID is not the flu. We still have to grapple with the fact that it’s killing a lot more people than the flu does. So maybe it’s a template, but not a perfect one.”
What data is being deleted
The CDC is now requiring hospitals to submit COVID-related data weekly, rather than daily, as it previously had. In addition, the agency has cut the number of data elements that hospitals must report from 62 to 44. Among the data fields that are now optional for hospitals to report are the numbers of hospitalized children with suspected or lab-confirmed COVID; hospitalized and ventilated COVID patients; adults in the ICU with suspected or lab-confirmed COVID; adult and pediatric admissions with suspected COVID; COVID-related emergency department visits; and inpatients with hospital-acquired COVID.
Although widely feared by health care workers and the public, hospital-acquired COVID has never been a major factor in the pandemic, Dr. Schaffner said. “So why ask for something that’s actually not so critical? Let’s keep the emphasis on rapid, accurate reporting of people who are hospitalized because of this disease.”
Akin Demehin, senior director for quality and patient safety policy for the American Hospital Association, agreed that the rate of hospital-acquired COVID cases “has been very low throughout the pandemic.” That was one reason why CDC made this measure optional.
Dr. Eisenberg concurred with this view. “We worried about [hospital-acquired COVID] a lot, and then, because people were very careful, it wasn’t as much of a problem as we feared it would be.” But she added a note of caution: “Masking and other [preventive guidelines] are shifting in hospitals, so it will be interesting to see whether that affects things.”
CDC justifies its new policy
To put the hospital data reporting changes in context, it’s important to know that CDC will no longer directly track community levels of COVID and the percentage of tests that come back positive for COVID, which until now were used to measure transmission rates. (Laboratories no longer have to report these test data, whether they are in hospitals or in the community.) To track death rates, CDC will rely on the National Vital Statistics System, which is accurate but lags other kinds of surveillance by 2-3 weeks, according to the New York Times.
In a recent MMWR report, CDC defended its new COVID surveillance system, saying: “Weekly COVID-19 hospital admission levels and the percentage of all COVID-19–associated deaths will be primary surveillance indicators. Emergency department visits and percentage of positive SARS-CoV-2 laboratory test results will help detect early changes in trends. Genomic surveillance will continue to help identify and monitor SARS-CoV-2 variants.”
Clarifying the latter point, CDC said that national genomic surveillance, along with wastewater surveillance, will continue to be used to estimate COVID variant proportions. Dr. Eisenberg stressed the importance of genomic surveillance at the hundreds of sites that CDC now maintains across the country. But currently, many of these sites are only monitoring the level of COVID.
CDC also observed that COVID-19 hospital admission levels have been shown to be “concordant” with community levels of SARS-CoV-2 infection. Therefore, rates of COVID-associated admissions and the percentages of positive test results, COVID ED visits, and COVID deaths are “suitable and timely indicators of trends in COVID-19 activity and severity.”
Ready to shift to voluntary reporting?
In a news release, AHA praised the “streamlining” of CDC requirements for data reporting but said that it hoped that mandatory reporting would be phased out as soon as possible.
The association noted that this would require action by the Centers for Medicare & Medicaid Services. CMS now enforces the CDC requirements with a “condition of participation” (COP) provision, by which noncompliant hospitals could be excluded from Medicare. CMS has extended this COP to April 30, 2024, although it could choose to ask the Secretary of Health and Human Services to terminate it earlier.
If mandatory reporting were repealed, would most hospitals still report on the key COVID metrics? Mr. Demehin noted that before CMS implemented its COP, hospitals reported COVID data voluntarily, “and the participation rate was well over 90%. So setting up a mechanism similar to that is something we’ve encouraged CMS to consider.”
Dr. Eisenberg is skeptical. While bigger hospitals with more resources might continue reporting voluntarily, she said, safety-net hospitals in underserved areas might not, because they are especially short staffed. “Then you have disparities in which hospitals will report.”
Vaccinations: The sleeping dragon
COVID continues to ravage the nation. According to CDC statistics, there were 1,109 deaths from COVID in the U.S. in the week ending May 6, and total deaths have hit 1.13 million. There were 1,333 new COVID-related hospital admissions, and 7,261 people were in the hospital because of COVID.
Another eye-catching number: Only 16.9% of the U.S. population has received an updated COVID vaccine booster. Dr. Schaffner thinks that this is what we should really keep our eye on. While the combination of vaccinations and widespread SARS-CoV-2 infections has conferred herd immunity on most Americans, he said it’s temporary. “Whether your immunity comes from the virus and recovery from disease or from the vaccines, that immunity will wane over time. Unless we keep our vaccination rate up, we may see more future cases. We’ll have to see how that works out. But I’m nervous about that, because people do appear to be nonchalant.”
A version of this article first appeared on Medscape.com.
When the federal government’s public health emergency (PHE) ended on May 11, the Centers for Disease Control and Prevention scaled back the amount of COVID-related data that it had required hospitals to collect and report during the previous 3 years. The CDC had to do this, an agency spokesman said in an interview, because “CDC’s authorizations to collect certain types of public health data” expired with the PHE.
The CDC insists that it will have enough data to keep up with the virus, which repeatedly defied scientists’ expectations during the course of the pandemic. But some experts have doubts about whether this will turn out to be the case.
While the COVID pandemic is subsiding and transitioning to an endemic phase, many things about the coronavirus are still not understood, noted Marisa Eisenberg, PhD, associate professor of epidemiology at the University of Michigan, Ann Arbor.
“COVID is here to stay, and it ebbs and flows but is staying at fairly consistent levels across the country,” she said in an interview. “Meanwhile, we haven’t established a regular seasonality for COVID that we see for most other respiratory illnesses. We’re still seeing pretty rapidly invading new waves of variants. With flu and other respiratory illnesses, you often see a particular variant in each season. There’s an established pattern. For COVID, that’s still shifting.”
Similarly, Sam Scarpino, PhD, a public health expert at Northeastern University, Boston, told the New York Times: “The CDC is shuffling COVID into the deck of infectious diseases that we’re satisfied living with. One thousand deaths a week is just unacceptable.”
William Schaffner, MD, a professor of preventive medicine and health policy at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview that “how we deal with influenza is something of a template or a model for what the CDC is trying to get to with COVID.” It’s not practical for physicians and hospitals to report every flu case, and the same is now true for COVID. However, “we’re still asking for data on people who are hospitalized with COVID to be reported. That will give us a measure of the major public health impact.”
Dr. Eisenberg doesn’t fully subscribe to this notion. “COVID and influenza are both respiratory illnesses, and our initial pandemic response was based on playbooks that we’d built for potential flu pandemics. But COVID is not the flu. We still have to grapple with the fact that it’s killing a lot more people than the flu does. So maybe it’s a template, but not a perfect one.”
What data is being deleted
The CDC is now requiring hospitals to submit COVID-related data weekly, rather than daily, as it previously had. In addition, the agency has cut the number of data elements that hospitals must report from 62 to 44. Among the data fields that are now optional for hospitals to report are the numbers of hospitalized children with suspected or lab-confirmed COVID; hospitalized and ventilated COVID patients; adults in the ICU with suspected or lab-confirmed COVID; adult and pediatric admissions with suspected COVID; COVID-related emergency department visits; and inpatients with hospital-acquired COVID.
Although widely feared by health care workers and the public, hospital-acquired COVID has never been a major factor in the pandemic, Dr. Schaffner said. “So why ask for something that’s actually not so critical? Let’s keep the emphasis on rapid, accurate reporting of people who are hospitalized because of this disease.”
Akin Demehin, senior director for quality and patient safety policy for the American Hospital Association, agreed that the rate of hospital-acquired COVID cases “has been very low throughout the pandemic.” That was one reason why CDC made this measure optional.
Dr. Eisenberg concurred with this view. “We worried about [hospital-acquired COVID] a lot, and then, because people were very careful, it wasn’t as much of a problem as we feared it would be.” But she added a note of caution: “Masking and other [preventive guidelines] are shifting in hospitals, so it will be interesting to see whether that affects things.”
CDC justifies its new policy
To put the hospital data reporting changes in context, it’s important to know that CDC will no longer directly track community levels of COVID and the percentage of tests that come back positive for COVID, which until now were used to measure transmission rates. (Laboratories no longer have to report these test data, whether they are in hospitals or in the community.) To track death rates, CDC will rely on the National Vital Statistics System, which is accurate but lags other kinds of surveillance by 2-3 weeks, according to the New York Times.
In a recent MMWR report, CDC defended its new COVID surveillance system, saying: “Weekly COVID-19 hospital admission levels and the percentage of all COVID-19–associated deaths will be primary surveillance indicators. Emergency department visits and percentage of positive SARS-CoV-2 laboratory test results will help detect early changes in trends. Genomic surveillance will continue to help identify and monitor SARS-CoV-2 variants.”
Clarifying the latter point, CDC said that national genomic surveillance, along with wastewater surveillance, will continue to be used to estimate COVID variant proportions. Dr. Eisenberg stressed the importance of genomic surveillance at the hundreds of sites that CDC now maintains across the country. But currently, many of these sites are only monitoring the level of COVID.
CDC also observed that COVID-19 hospital admission levels have been shown to be “concordant” with community levels of SARS-CoV-2 infection. Therefore, rates of COVID-associated admissions and the percentages of positive test results, COVID ED visits, and COVID deaths are “suitable and timely indicators of trends in COVID-19 activity and severity.”
Ready to shift to voluntary reporting?
In a news release, AHA praised the “streamlining” of CDC requirements for data reporting but said that it hoped that mandatory reporting would be phased out as soon as possible.
The association noted that this would require action by the Centers for Medicare & Medicaid Services. CMS now enforces the CDC requirements with a “condition of participation” (COP) provision, by which noncompliant hospitals could be excluded from Medicare. CMS has extended this COP to April 30, 2024, although it could choose to ask the Secretary of Health and Human Services to terminate it earlier.
If mandatory reporting were repealed, would most hospitals still report on the key COVID metrics? Mr. Demehin noted that before CMS implemented its COP, hospitals reported COVID data voluntarily, “and the participation rate was well over 90%. So setting up a mechanism similar to that is something we’ve encouraged CMS to consider.”
Dr. Eisenberg is skeptical. While bigger hospitals with more resources might continue reporting voluntarily, she said, safety-net hospitals in underserved areas might not, because they are especially short staffed. “Then you have disparities in which hospitals will report.”
Vaccinations: The sleeping dragon
COVID continues to ravage the nation. According to CDC statistics, there were 1,109 deaths from COVID in the U.S. in the week ending May 6, and total deaths have hit 1.13 million. There were 1,333 new COVID-related hospital admissions, and 7,261 people were in the hospital because of COVID.
Another eye-catching number: Only 16.9% of the U.S. population has received an updated COVID vaccine booster. Dr. Schaffner thinks that this is what we should really keep our eye on. While the combination of vaccinations and widespread SARS-CoV-2 infections has conferred herd immunity on most Americans, he said it’s temporary. “Whether your immunity comes from the virus and recovery from disease or from the vaccines, that immunity will wane over time. Unless we keep our vaccination rate up, we may see more future cases. We’ll have to see how that works out. But I’m nervous about that, because people do appear to be nonchalant.”
A version of this article first appeared on Medscape.com.
When the federal government’s public health emergency (PHE) ended on May 11, the Centers for Disease Control and Prevention scaled back the amount of COVID-related data that it had required hospitals to collect and report during the previous 3 years. The CDC had to do this, an agency spokesman said in an interview, because “CDC’s authorizations to collect certain types of public health data” expired with the PHE.
The CDC insists that it will have enough data to keep up with the virus, which repeatedly defied scientists’ expectations during the course of the pandemic. But some experts have doubts about whether this will turn out to be the case.
While the COVID pandemic is subsiding and transitioning to an endemic phase, many things about the coronavirus are still not understood, noted Marisa Eisenberg, PhD, associate professor of epidemiology at the University of Michigan, Ann Arbor.
“COVID is here to stay, and it ebbs and flows but is staying at fairly consistent levels across the country,” she said in an interview. “Meanwhile, we haven’t established a regular seasonality for COVID that we see for most other respiratory illnesses. We’re still seeing pretty rapidly invading new waves of variants. With flu and other respiratory illnesses, you often see a particular variant in each season. There’s an established pattern. For COVID, that’s still shifting.”
Similarly, Sam Scarpino, PhD, a public health expert at Northeastern University, Boston, told the New York Times: “The CDC is shuffling COVID into the deck of infectious diseases that we’re satisfied living with. One thousand deaths a week is just unacceptable.”
William Schaffner, MD, a professor of preventive medicine and health policy at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview that “how we deal with influenza is something of a template or a model for what the CDC is trying to get to with COVID.” It’s not practical for physicians and hospitals to report every flu case, and the same is now true for COVID. However, “we’re still asking for data on people who are hospitalized with COVID to be reported. That will give us a measure of the major public health impact.”
Dr. Eisenberg doesn’t fully subscribe to this notion. “COVID and influenza are both respiratory illnesses, and our initial pandemic response was based on playbooks that we’d built for potential flu pandemics. But COVID is not the flu. We still have to grapple with the fact that it’s killing a lot more people than the flu does. So maybe it’s a template, but not a perfect one.”
What data is being deleted
The CDC is now requiring hospitals to submit COVID-related data weekly, rather than daily, as it previously had. In addition, the agency has cut the number of data elements that hospitals must report from 62 to 44. Among the data fields that are now optional for hospitals to report are the numbers of hospitalized children with suspected or lab-confirmed COVID; hospitalized and ventilated COVID patients; adults in the ICU with suspected or lab-confirmed COVID; adult and pediatric admissions with suspected COVID; COVID-related emergency department visits; and inpatients with hospital-acquired COVID.
Although widely feared by health care workers and the public, hospital-acquired COVID has never been a major factor in the pandemic, Dr. Schaffner said. “So why ask for something that’s actually not so critical? Let’s keep the emphasis on rapid, accurate reporting of people who are hospitalized because of this disease.”
Akin Demehin, senior director for quality and patient safety policy for the American Hospital Association, agreed that the rate of hospital-acquired COVID cases “has been very low throughout the pandemic.” That was one reason why CDC made this measure optional.
Dr. Eisenberg concurred with this view. “We worried about [hospital-acquired COVID] a lot, and then, because people were very careful, it wasn’t as much of a problem as we feared it would be.” But she added a note of caution: “Masking and other [preventive guidelines] are shifting in hospitals, so it will be interesting to see whether that affects things.”
CDC justifies its new policy
To put the hospital data reporting changes in context, it’s important to know that CDC will no longer directly track community levels of COVID and the percentage of tests that come back positive for COVID, which until now were used to measure transmission rates. (Laboratories no longer have to report these test data, whether they are in hospitals or in the community.) To track death rates, CDC will rely on the National Vital Statistics System, which is accurate but lags other kinds of surveillance by 2-3 weeks, according to the New York Times.
In a recent MMWR report, CDC defended its new COVID surveillance system, saying: “Weekly COVID-19 hospital admission levels and the percentage of all COVID-19–associated deaths will be primary surveillance indicators. Emergency department visits and percentage of positive SARS-CoV-2 laboratory test results will help detect early changes in trends. Genomic surveillance will continue to help identify and monitor SARS-CoV-2 variants.”
Clarifying the latter point, CDC said that national genomic surveillance, along with wastewater surveillance, will continue to be used to estimate COVID variant proportions. Dr. Eisenberg stressed the importance of genomic surveillance at the hundreds of sites that CDC now maintains across the country. But currently, many of these sites are only monitoring the level of COVID.
CDC also observed that COVID-19 hospital admission levels have been shown to be “concordant” with community levels of SARS-CoV-2 infection. Therefore, rates of COVID-associated admissions and the percentages of positive test results, COVID ED visits, and COVID deaths are “suitable and timely indicators of trends in COVID-19 activity and severity.”
Ready to shift to voluntary reporting?
In a news release, AHA praised the “streamlining” of CDC requirements for data reporting but said that it hoped that mandatory reporting would be phased out as soon as possible.
The association noted that this would require action by the Centers for Medicare & Medicaid Services. CMS now enforces the CDC requirements with a “condition of participation” (COP) provision, by which noncompliant hospitals could be excluded from Medicare. CMS has extended this COP to April 30, 2024, although it could choose to ask the Secretary of Health and Human Services to terminate it earlier.
If mandatory reporting were repealed, would most hospitals still report on the key COVID metrics? Mr. Demehin noted that before CMS implemented its COP, hospitals reported COVID data voluntarily, “and the participation rate was well over 90%. So setting up a mechanism similar to that is something we’ve encouraged CMS to consider.”
Dr. Eisenberg is skeptical. While bigger hospitals with more resources might continue reporting voluntarily, she said, safety-net hospitals in underserved areas might not, because they are especially short staffed. “Then you have disparities in which hospitals will report.”
Vaccinations: The sleeping dragon
COVID continues to ravage the nation. According to CDC statistics, there were 1,109 deaths from COVID in the U.S. in the week ending May 6, and total deaths have hit 1.13 million. There were 1,333 new COVID-related hospital admissions, and 7,261 people were in the hospital because of COVID.
Another eye-catching number: Only 16.9% of the U.S. population has received an updated COVID vaccine booster. Dr. Schaffner thinks that this is what we should really keep our eye on. While the combination of vaccinations and widespread SARS-CoV-2 infections has conferred herd immunity on most Americans, he said it’s temporary. “Whether your immunity comes from the virus and recovery from disease or from the vaccines, that immunity will wane over time. Unless we keep our vaccination rate up, we may see more future cases. We’ll have to see how that works out. But I’m nervous about that, because people do appear to be nonchalant.”
A version of this article first appeared on Medscape.com.
Will a mindfulness approach to depression boost recovery rates, reduce costs?
Self-help mindfulness-based cognitive therapy (MBCT-SH) produced better outcomes for participants with depression and was more cost-effective than CBT-SH.
Practitioner-supported self-help therapy regimens are growing in popularity as a way to expand access to mental health services and to address the shortage of mental health professionals.
Generally, mindfulness-based cognitive therapy aims to increase awareness of the depression maintenance cycle while fostering a nonjudgmental attitude toward present-moment experiences, the investigators note.
In contrast, CBT aims to challenge negative and unrealistic thought patterns that may perpetuate depression, replacing them with more realistic and objective thoughts.
“Practitioner-supported MBCT-SH should be routinely offered as an intervention for mild to moderate depression alongside practitioner-supported CBT-SH,” the investigators note.
The study was published online in JAMA Psychiatry.
Better recovery rates?
CBT-SH traditionally had been associated with high attrition rates, and alternative forms of self-help therapy are becoming increasingly necessary to fill this treatment gap, the researchers note. To compare the efficacy and cost-effectiveness of both treatment types, the researchers recruited 410 participants with mild to moderate depression at 10 sites in the United Kingdom. Participants were randomly assigned to receive either MBCT-SH or CBT-SH between November 2017 and January 2020. A total of 204 participants received MBCT-SH, and 206 received CBT-SH.
All participants were given specific self-help workbooks, depending on the study group to which they were assigned. Those who received MBCT-SH used “The Mindful Way Workbook: An 8-Week Program to Free Yourself From Depression and Emotional Distress,” while those who received CBT-SH used “Overcoming Depression and Low Mood: A Five Areas Approach, 3rd Edition.”
Investigators asked all participants to guide themselves through six 30- to 45-minute sessions, using the information in the workbooks. Trained psychological well-being practitioners supported participants as they moved through the workbooks during the six sessions.
Participants were assessed at baseline with the Patient Health Questionnaire–9 (PHQ-9) and the Clinical Interview Schedule–Revised at 16 weeks and 24 weeks.
At 16 weeks post randomization, results showed that practitioner-supported MBCT-SH led to significantly greater reductions in depression symptom severity, compared with practitioner-supported CBT-SH (mean [standard deviation] PHQ-9 score, 7.2 [4.8] points vs. 8.6 [5.5] points; between-group difference, –1.5 points; 95% confidence interval, –2.6 to –0.4; P = .009).
Results also showed that on average, the CBT-SH intervention cost $631 more per participant than the MBCT-SH intervention over the 42-week follow-up.
The investigators explain that “a substantial proportion of this additional cost was accounted for by additional face-to-face individual psychological therapy accessed by CBT-SH participants outside of the study intervention.
“In conclusion, this study found that a novel intervention, practitioner-supported MBCT-SH, was clinically superior in targeting depressive symptom severity at postintervention and cost-effective, compared with the criterion standard of practitioner-supported CBT-SH for adults experiencing mild to moderate depression,” the investigators write.
“If study findings are translated into routine practice, this would see many more people recovering from depression while costing health services less money,” they add.
Clinically meaningful?
Commenting on the study for this article, Lauren Bylsma, PhD, professor of psychiatry and psychology at the University of Pittsburgh, cast doubt on the ability of such a short trial to determine meaningful change.
She said that the extra costs incurred by participants in the CBT-SH arm of the study are likely, since it is “difficult to do CBT alone – you need an objective person to guide you as you practice.”
Dr. Bylsma noted that ultimately, more real-world studies of therapy are needed, given the great need for mental health.
The study was funded by the National Institute for Health and Care Research. The original article contains a full list of the authors’ relevant financial relationships.
A version of this article first appeared on Medscape.com.
Self-help mindfulness-based cognitive therapy (MBCT-SH) produced better outcomes for participants with depression and was more cost-effective than CBT-SH.
Practitioner-supported self-help therapy regimens are growing in popularity as a way to expand access to mental health services and to address the shortage of mental health professionals.
Generally, mindfulness-based cognitive therapy aims to increase awareness of the depression maintenance cycle while fostering a nonjudgmental attitude toward present-moment experiences, the investigators note.
In contrast, CBT aims to challenge negative and unrealistic thought patterns that may perpetuate depression, replacing them with more realistic and objective thoughts.
“Practitioner-supported MBCT-SH should be routinely offered as an intervention for mild to moderate depression alongside practitioner-supported CBT-SH,” the investigators note.
The study was published online in JAMA Psychiatry.
Better recovery rates?
CBT-SH traditionally had been associated with high attrition rates, and alternative forms of self-help therapy are becoming increasingly necessary to fill this treatment gap, the researchers note. To compare the efficacy and cost-effectiveness of both treatment types, the researchers recruited 410 participants with mild to moderate depression at 10 sites in the United Kingdom. Participants were randomly assigned to receive either MBCT-SH or CBT-SH between November 2017 and January 2020. A total of 204 participants received MBCT-SH, and 206 received CBT-SH.
All participants were given specific self-help workbooks, depending on the study group to which they were assigned. Those who received MBCT-SH used “The Mindful Way Workbook: An 8-Week Program to Free Yourself From Depression and Emotional Distress,” while those who received CBT-SH used “Overcoming Depression and Low Mood: A Five Areas Approach, 3rd Edition.”
Investigators asked all participants to guide themselves through six 30- to 45-minute sessions, using the information in the workbooks. Trained psychological well-being practitioners supported participants as they moved through the workbooks during the six sessions.
Participants were assessed at baseline with the Patient Health Questionnaire–9 (PHQ-9) and the Clinical Interview Schedule–Revised at 16 weeks and 24 weeks.
At 16 weeks post randomization, results showed that practitioner-supported MBCT-SH led to significantly greater reductions in depression symptom severity, compared with practitioner-supported CBT-SH (mean [standard deviation] PHQ-9 score, 7.2 [4.8] points vs. 8.6 [5.5] points; between-group difference, –1.5 points; 95% confidence interval, –2.6 to –0.4; P = .009).
Results also showed that on average, the CBT-SH intervention cost $631 more per participant than the MBCT-SH intervention over the 42-week follow-up.
The investigators explain that “a substantial proportion of this additional cost was accounted for by additional face-to-face individual psychological therapy accessed by CBT-SH participants outside of the study intervention.
“In conclusion, this study found that a novel intervention, practitioner-supported MBCT-SH, was clinically superior in targeting depressive symptom severity at postintervention and cost-effective, compared with the criterion standard of practitioner-supported CBT-SH for adults experiencing mild to moderate depression,” the investigators write.
“If study findings are translated into routine practice, this would see many more people recovering from depression while costing health services less money,” they add.
Clinically meaningful?
Commenting on the study for this article, Lauren Bylsma, PhD, professor of psychiatry and psychology at the University of Pittsburgh, cast doubt on the ability of such a short trial to determine meaningful change.
She said that the extra costs incurred by participants in the CBT-SH arm of the study are likely, since it is “difficult to do CBT alone – you need an objective person to guide you as you practice.”
Dr. Bylsma noted that ultimately, more real-world studies of therapy are needed, given the great need for mental health.
The study was funded by the National Institute for Health and Care Research. The original article contains a full list of the authors’ relevant financial relationships.
A version of this article first appeared on Medscape.com.
Self-help mindfulness-based cognitive therapy (MBCT-SH) produced better outcomes for participants with depression and was more cost-effective than CBT-SH.
Practitioner-supported self-help therapy regimens are growing in popularity as a way to expand access to mental health services and to address the shortage of mental health professionals.
Generally, mindfulness-based cognitive therapy aims to increase awareness of the depression maintenance cycle while fostering a nonjudgmental attitude toward present-moment experiences, the investigators note.
In contrast, CBT aims to challenge negative and unrealistic thought patterns that may perpetuate depression, replacing them with more realistic and objective thoughts.
“Practitioner-supported MBCT-SH should be routinely offered as an intervention for mild to moderate depression alongside practitioner-supported CBT-SH,” the investigators note.
The study was published online in JAMA Psychiatry.
Better recovery rates?
CBT-SH traditionally had been associated with high attrition rates, and alternative forms of self-help therapy are becoming increasingly necessary to fill this treatment gap, the researchers note. To compare the efficacy and cost-effectiveness of both treatment types, the researchers recruited 410 participants with mild to moderate depression at 10 sites in the United Kingdom. Participants were randomly assigned to receive either MBCT-SH or CBT-SH between November 2017 and January 2020. A total of 204 participants received MBCT-SH, and 206 received CBT-SH.
All participants were given specific self-help workbooks, depending on the study group to which they were assigned. Those who received MBCT-SH used “The Mindful Way Workbook: An 8-Week Program to Free Yourself From Depression and Emotional Distress,” while those who received CBT-SH used “Overcoming Depression and Low Mood: A Five Areas Approach, 3rd Edition.”
Investigators asked all participants to guide themselves through six 30- to 45-minute sessions, using the information in the workbooks. Trained psychological well-being practitioners supported participants as they moved through the workbooks during the six sessions.
Participants were assessed at baseline with the Patient Health Questionnaire–9 (PHQ-9) and the Clinical Interview Schedule–Revised at 16 weeks and 24 weeks.
At 16 weeks post randomization, results showed that practitioner-supported MBCT-SH led to significantly greater reductions in depression symptom severity, compared with practitioner-supported CBT-SH (mean [standard deviation] PHQ-9 score, 7.2 [4.8] points vs. 8.6 [5.5] points; between-group difference, –1.5 points; 95% confidence interval, –2.6 to –0.4; P = .009).
Results also showed that on average, the CBT-SH intervention cost $631 more per participant than the MBCT-SH intervention over the 42-week follow-up.
The investigators explain that “a substantial proportion of this additional cost was accounted for by additional face-to-face individual psychological therapy accessed by CBT-SH participants outside of the study intervention.
“In conclusion, this study found that a novel intervention, practitioner-supported MBCT-SH, was clinically superior in targeting depressive symptom severity at postintervention and cost-effective, compared with the criterion standard of practitioner-supported CBT-SH for adults experiencing mild to moderate depression,” the investigators write.
“If study findings are translated into routine practice, this would see many more people recovering from depression while costing health services less money,” they add.
Clinically meaningful?
Commenting on the study for this article, Lauren Bylsma, PhD, professor of psychiatry and psychology at the University of Pittsburgh, cast doubt on the ability of such a short trial to determine meaningful change.
She said that the extra costs incurred by participants in the CBT-SH arm of the study are likely, since it is “difficult to do CBT alone – you need an objective person to guide you as you practice.”
Dr. Bylsma noted that ultimately, more real-world studies of therapy are needed, given the great need for mental health.
The study was funded by the National Institute for Health and Care Research. The original article contains a full list of the authors’ relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
U.S. adults report depression at record rates: Survey
In a survey, 29% of adults said they had been diagnosed with depression during their lifetime, and 18% said they currently have depression or are being treated for it. Those rates are up from the baseline 2015 rates of 20% of people ever having depression and 11% of people with a current diagnosis.
Depression had been steadily rising before the pandemic, and the Gallup analysts wrote that “social isolation, loneliness, fear of infection, psychological exhaustion (particularly among frontline responders such as health care workers), elevated substance abuse, and disruptions in mental health services have all likely played a role” in the increase.
“The fact that Americans are more depressed and struggling after this time of incredible stress and isolation is perhaps not surprising,” American Psychiatric Association president Rebecca Brendel, MD, told CNN. “There are lingering effects on our health, especially our mental health, from the past 3 years that disrupted everything we knew.”
The new estimates are based on online survey responses collected in February from 5,167 adults in the United States who answered the questions:
- Has a doctor or nurse ever told you that you have depression?
- Do you currently have or are you currently being treated for depression?
Depression, which is also called major depressive disorder, is a treatable illness that negatively affects how someone feels, thinks, and acts. The symptoms can be both emotional (such as sadness or loss of interest in activities) and physical (such as fatigue or slowed movements or speech).
The latest study found that depression rates increased the most among women, young adults, Black people, and Hispanic people. For the first time, more Black and Hispanic people than White people reported ever being diagnosed with depression. The lifetime depression rate among Black people was 34%, compared with 31% for Hispanic people and 29% for White people.
The rate of lifetime depression among women jumped 10 percentage points in the past 5 years, to 37%, in February, the survey results showed. About 1 in 4 women said they currently had depression or were being treated for it, up 6 percentage points compared with 5 years ago.
When responses were analyzed by age, those 18-44 years old were the most likely to report ever being diagnosed with depression or currently having the illness. About one-third of younger adults have ever been diagnosed, and more than 1 in 5 said they currently have depression.
Dr. Brendel said awareness and reduced stigma could be adding to the rising rates of depression.
“We’re making it easier to talk about mental health and looking at it as part of our overall wellness, just like physical health,” she said. “People are aware of depression, and people are seeking help for it.”
If you or someone you know needs help, dial 988 for support from the national Suicide & Crisis Lifeline. It’s free, confidential, and available 24 hours a day, 7 days a week. You can also visit 988lifeline.org and choose the chat feature.
A version of this article first appeared on Medscape.com.
In a survey, 29% of adults said they had been diagnosed with depression during their lifetime, and 18% said they currently have depression or are being treated for it. Those rates are up from the baseline 2015 rates of 20% of people ever having depression and 11% of people with a current diagnosis.
Depression had been steadily rising before the pandemic, and the Gallup analysts wrote that “social isolation, loneliness, fear of infection, psychological exhaustion (particularly among frontline responders such as health care workers), elevated substance abuse, and disruptions in mental health services have all likely played a role” in the increase.
“The fact that Americans are more depressed and struggling after this time of incredible stress and isolation is perhaps not surprising,” American Psychiatric Association president Rebecca Brendel, MD, told CNN. “There are lingering effects on our health, especially our mental health, from the past 3 years that disrupted everything we knew.”
The new estimates are based on online survey responses collected in February from 5,167 adults in the United States who answered the questions:
- Has a doctor or nurse ever told you that you have depression?
- Do you currently have or are you currently being treated for depression?
Depression, which is also called major depressive disorder, is a treatable illness that negatively affects how someone feels, thinks, and acts. The symptoms can be both emotional (such as sadness or loss of interest in activities) and physical (such as fatigue or slowed movements or speech).
The latest study found that depression rates increased the most among women, young adults, Black people, and Hispanic people. For the first time, more Black and Hispanic people than White people reported ever being diagnosed with depression. The lifetime depression rate among Black people was 34%, compared with 31% for Hispanic people and 29% for White people.
The rate of lifetime depression among women jumped 10 percentage points in the past 5 years, to 37%, in February, the survey results showed. About 1 in 4 women said they currently had depression or were being treated for it, up 6 percentage points compared with 5 years ago.
When responses were analyzed by age, those 18-44 years old were the most likely to report ever being diagnosed with depression or currently having the illness. About one-third of younger adults have ever been diagnosed, and more than 1 in 5 said they currently have depression.
Dr. Brendel said awareness and reduced stigma could be adding to the rising rates of depression.
“We’re making it easier to talk about mental health and looking at it as part of our overall wellness, just like physical health,” she said. “People are aware of depression, and people are seeking help for it.”
If you or someone you know needs help, dial 988 for support from the national Suicide & Crisis Lifeline. It’s free, confidential, and available 24 hours a day, 7 days a week. You can also visit 988lifeline.org and choose the chat feature.
A version of this article first appeared on Medscape.com.
In a survey, 29% of adults said they had been diagnosed with depression during their lifetime, and 18% said they currently have depression or are being treated for it. Those rates are up from the baseline 2015 rates of 20% of people ever having depression and 11% of people with a current diagnosis.
Depression had been steadily rising before the pandemic, and the Gallup analysts wrote that “social isolation, loneliness, fear of infection, psychological exhaustion (particularly among frontline responders such as health care workers), elevated substance abuse, and disruptions in mental health services have all likely played a role” in the increase.
“The fact that Americans are more depressed and struggling after this time of incredible stress and isolation is perhaps not surprising,” American Psychiatric Association president Rebecca Brendel, MD, told CNN. “There are lingering effects on our health, especially our mental health, from the past 3 years that disrupted everything we knew.”
The new estimates are based on online survey responses collected in February from 5,167 adults in the United States who answered the questions:
- Has a doctor or nurse ever told you that you have depression?
- Do you currently have or are you currently being treated for depression?
Depression, which is also called major depressive disorder, is a treatable illness that negatively affects how someone feels, thinks, and acts. The symptoms can be both emotional (such as sadness or loss of interest in activities) and physical (such as fatigue or slowed movements or speech).
The latest study found that depression rates increased the most among women, young adults, Black people, and Hispanic people. For the first time, more Black and Hispanic people than White people reported ever being diagnosed with depression. The lifetime depression rate among Black people was 34%, compared with 31% for Hispanic people and 29% for White people.
The rate of lifetime depression among women jumped 10 percentage points in the past 5 years, to 37%, in February, the survey results showed. About 1 in 4 women said they currently had depression or were being treated for it, up 6 percentage points compared with 5 years ago.
When responses were analyzed by age, those 18-44 years old were the most likely to report ever being diagnosed with depression or currently having the illness. About one-third of younger adults have ever been diagnosed, and more than 1 in 5 said they currently have depression.
Dr. Brendel said awareness and reduced stigma could be adding to the rising rates of depression.
“We’re making it easier to talk about mental health and looking at it as part of our overall wellness, just like physical health,” she said. “People are aware of depression, and people are seeking help for it.”
If you or someone you know needs help, dial 988 for support from the national Suicide & Crisis Lifeline. It’s free, confidential, and available 24 hours a day, 7 days a week. You can also visit 988lifeline.org and choose the chat feature.
A version of this article first appeared on Medscape.com.
Analysis: 40% of information about cirrhosis on TikTok is incorrect
CHICAGO –
TikTok’s short video format is increasingly becoming a major source for news and information, especially for adolescents and young adults. In 2022 the technology news website The Verge reported that about 10% of all U.S. adults regularly get news from TikTok, including an estimated 26% of adults under 30.
“Our study highlights the alarming prevalence of misinformation related to liver disease and cirrhosis on the TikTok platform. This misinformation can potentially lead to harm and poor health outcomes for individuals seeking accurate information about their conditions,” wrote the study authors in their abstract.
The study, which was led by Macklin Loveland, MD, an internal medicine resident at the University of Arizona, Tucson, found that, among 2,223 TikTok posts related to liver disease or cirrhosis as of November 2022, 60.3% were found to have accurate medical information, but the remaining 39.7% contained misinformation.
Some of the misinformation offered highly dubious and potentially dangerous advice, such as “Reishi mushrooms can reverse liver damage,” when in fact there is evidence to suggest that reishi mushrooms, especially in powder form, may be toxic to the liver and may even cause fatal fulminant hepatitis.
In an interview, Dr. Loveland said that much of the good information about liver disease on TikTok seems to come from patients with cirrhosis or other liver diseases who post videos chronicling their experiences, whereas bad information seems to come from people who are trying to pitch a product such as “natural” or “alternative” medicine.
“People who have real-life, firsthand experiences with cirrhosis have a really nice platform to talk about their disease process and share information with other people with similar disease processes, and the majority of them are accurate,” he said. “Where the inaccuracies come in are when people are trying to profit off a product or sell a dietary supplement, and then things go by the wayside.”
It’s important for TikTok users to understand the intentions of other users, he said, and expressed the hope that content mediators within TikTok would help to keep misinformation at bay.
Skyler B. Johnson, MD, assistant professor of radiation oncology at the University of Utah Huntsman Cancer Institute, Salt Lake City, has studied misinformation about cancer on social media, and as he and colleagues reported in the Journal of the National Cancer Institute, of 200 articles on cancer posted on social media sites, 32.5% contained misinformation and 30.5% contained harmful information.
In an interview, Dr. Johnson, who was not involved in the study by Dr. Loveland and colleagues, offered advice for colleagues about countering misinformation.
“What we’re trying to do, as part of our research group, is encourage physicians to take an active role in addressing misinformation with their patients and also in their social networking interactions,” he said. “When I see a new patient with a new diagnosis of cancer, I warn them that they’re going to encounter things online that may or may not be true.”
He noted that, often when physicians instruct patients not to go online to research their disease, the first thing the vast majority of patients do after leaving the office is to jump online.
Instead, Dr. Johnson recommended inoculating patients against misinformation – not to discourage them from going online, but to inform them about the myriad good and bad information sources, and steer them toward trustworthy sites such as government agencies (the National Institutes of Health, Centers for Disease Control and Prevention, etc), academic medical center sites, or select nonprofit organizations, foundations, and medical associations.
“But at the end of the day, even those you have to be somewhat vigilant about, because there are some unscrupulous providers who exploit even those sites,” he added.
Study details
Dr. Loveland and colleagues used the TikTok search engine to look for posts containing the terms “cirrhosis” and “liver disease,” and watched all such videos that turned up in the search from beginning to end. They classified each post as either educational in intent or whether it depicted a firsthand experience with liver disease.
They determined whether each post was accurate or erroneous according to guidelines from the American Association for the Study of Liver Disease, American College of Gastroenterology, and American Gastroenterological Association. They also recorded the number of likes, comments, and the number of times that each video was shared.
Accurate posts were viewed and liked significantly more often than inaccurate ones, and accurate posts had significantly more comments that faulty posts.
However, both accurate and inaccurate posts were shared a similar number of times, suggesting that bad-quality information can metastasize just as easily as good information can be disseminated.
Dr. Loveland and colleagues echoed the recommendations of Dr. Johnson, stating that “health care provides should be aware of the potential for misinformation on social media and should strive to provide their patients with accurate and evidence-based information to inform their health care decisions.”
The study was internally funded. Dr. Loveland and Dr. Johnson reported having no conflicts of interest to disclose.
DDW is sponsored by the American Association for the Study of Liver Diseases, the AGA, the American Society for Gastrointestinal Endoscopy, and The Society for Surgery of the Alimentary Tract.
CHICAGO –
TikTok’s short video format is increasingly becoming a major source for news and information, especially for adolescents and young adults. In 2022 the technology news website The Verge reported that about 10% of all U.S. adults regularly get news from TikTok, including an estimated 26% of adults under 30.
“Our study highlights the alarming prevalence of misinformation related to liver disease and cirrhosis on the TikTok platform. This misinformation can potentially lead to harm and poor health outcomes for individuals seeking accurate information about their conditions,” wrote the study authors in their abstract.
The study, which was led by Macklin Loveland, MD, an internal medicine resident at the University of Arizona, Tucson, found that, among 2,223 TikTok posts related to liver disease or cirrhosis as of November 2022, 60.3% were found to have accurate medical information, but the remaining 39.7% contained misinformation.
Some of the misinformation offered highly dubious and potentially dangerous advice, such as “Reishi mushrooms can reverse liver damage,” when in fact there is evidence to suggest that reishi mushrooms, especially in powder form, may be toxic to the liver and may even cause fatal fulminant hepatitis.
In an interview, Dr. Loveland said that much of the good information about liver disease on TikTok seems to come from patients with cirrhosis or other liver diseases who post videos chronicling their experiences, whereas bad information seems to come from people who are trying to pitch a product such as “natural” or “alternative” medicine.
“People who have real-life, firsthand experiences with cirrhosis have a really nice platform to talk about their disease process and share information with other people with similar disease processes, and the majority of them are accurate,” he said. “Where the inaccuracies come in are when people are trying to profit off a product or sell a dietary supplement, and then things go by the wayside.”
It’s important for TikTok users to understand the intentions of other users, he said, and expressed the hope that content mediators within TikTok would help to keep misinformation at bay.
Skyler B. Johnson, MD, assistant professor of radiation oncology at the University of Utah Huntsman Cancer Institute, Salt Lake City, has studied misinformation about cancer on social media, and as he and colleagues reported in the Journal of the National Cancer Institute, of 200 articles on cancer posted on social media sites, 32.5% contained misinformation and 30.5% contained harmful information.
In an interview, Dr. Johnson, who was not involved in the study by Dr. Loveland and colleagues, offered advice for colleagues about countering misinformation.
“What we’re trying to do, as part of our research group, is encourage physicians to take an active role in addressing misinformation with their patients and also in their social networking interactions,” he said. “When I see a new patient with a new diagnosis of cancer, I warn them that they’re going to encounter things online that may or may not be true.”
He noted that, often when physicians instruct patients not to go online to research their disease, the first thing the vast majority of patients do after leaving the office is to jump online.
Instead, Dr. Johnson recommended inoculating patients against misinformation – not to discourage them from going online, but to inform them about the myriad good and bad information sources, and steer them toward trustworthy sites such as government agencies (the National Institutes of Health, Centers for Disease Control and Prevention, etc), academic medical center sites, or select nonprofit organizations, foundations, and medical associations.
“But at the end of the day, even those you have to be somewhat vigilant about, because there are some unscrupulous providers who exploit even those sites,” he added.
Study details
Dr. Loveland and colleagues used the TikTok search engine to look for posts containing the terms “cirrhosis” and “liver disease,” and watched all such videos that turned up in the search from beginning to end. They classified each post as either educational in intent or whether it depicted a firsthand experience with liver disease.
They determined whether each post was accurate or erroneous according to guidelines from the American Association for the Study of Liver Disease, American College of Gastroenterology, and American Gastroenterological Association. They also recorded the number of likes, comments, and the number of times that each video was shared.
Accurate posts were viewed and liked significantly more often than inaccurate ones, and accurate posts had significantly more comments that faulty posts.
However, both accurate and inaccurate posts were shared a similar number of times, suggesting that bad-quality information can metastasize just as easily as good information can be disseminated.
Dr. Loveland and colleagues echoed the recommendations of Dr. Johnson, stating that “health care provides should be aware of the potential for misinformation on social media and should strive to provide their patients with accurate and evidence-based information to inform their health care decisions.”
The study was internally funded. Dr. Loveland and Dr. Johnson reported having no conflicts of interest to disclose.
DDW is sponsored by the American Association for the Study of Liver Diseases, the AGA, the American Society for Gastrointestinal Endoscopy, and The Society for Surgery of the Alimentary Tract.
CHICAGO –
TikTok’s short video format is increasingly becoming a major source for news and information, especially for adolescents and young adults. In 2022 the technology news website The Verge reported that about 10% of all U.S. adults regularly get news from TikTok, including an estimated 26% of adults under 30.
“Our study highlights the alarming prevalence of misinformation related to liver disease and cirrhosis on the TikTok platform. This misinformation can potentially lead to harm and poor health outcomes for individuals seeking accurate information about their conditions,” wrote the study authors in their abstract.
The study, which was led by Macklin Loveland, MD, an internal medicine resident at the University of Arizona, Tucson, found that, among 2,223 TikTok posts related to liver disease or cirrhosis as of November 2022, 60.3% were found to have accurate medical information, but the remaining 39.7% contained misinformation.
Some of the misinformation offered highly dubious and potentially dangerous advice, such as “Reishi mushrooms can reverse liver damage,” when in fact there is evidence to suggest that reishi mushrooms, especially in powder form, may be toxic to the liver and may even cause fatal fulminant hepatitis.
In an interview, Dr. Loveland said that much of the good information about liver disease on TikTok seems to come from patients with cirrhosis or other liver diseases who post videos chronicling their experiences, whereas bad information seems to come from people who are trying to pitch a product such as “natural” or “alternative” medicine.
“People who have real-life, firsthand experiences with cirrhosis have a really nice platform to talk about their disease process and share information with other people with similar disease processes, and the majority of them are accurate,” he said. “Where the inaccuracies come in are when people are trying to profit off a product or sell a dietary supplement, and then things go by the wayside.”
It’s important for TikTok users to understand the intentions of other users, he said, and expressed the hope that content mediators within TikTok would help to keep misinformation at bay.
Skyler B. Johnson, MD, assistant professor of radiation oncology at the University of Utah Huntsman Cancer Institute, Salt Lake City, has studied misinformation about cancer on social media, and as he and colleagues reported in the Journal of the National Cancer Institute, of 200 articles on cancer posted on social media sites, 32.5% contained misinformation and 30.5% contained harmful information.
In an interview, Dr. Johnson, who was not involved in the study by Dr. Loveland and colleagues, offered advice for colleagues about countering misinformation.
“What we’re trying to do, as part of our research group, is encourage physicians to take an active role in addressing misinformation with their patients and also in their social networking interactions,” he said. “When I see a new patient with a new diagnosis of cancer, I warn them that they’re going to encounter things online that may or may not be true.”
He noted that, often when physicians instruct patients not to go online to research their disease, the first thing the vast majority of patients do after leaving the office is to jump online.
Instead, Dr. Johnson recommended inoculating patients against misinformation – not to discourage them from going online, but to inform them about the myriad good and bad information sources, and steer them toward trustworthy sites such as government agencies (the National Institutes of Health, Centers for Disease Control and Prevention, etc), academic medical center sites, or select nonprofit organizations, foundations, and medical associations.
“But at the end of the day, even those you have to be somewhat vigilant about, because there are some unscrupulous providers who exploit even those sites,” he added.
Study details
Dr. Loveland and colleagues used the TikTok search engine to look for posts containing the terms “cirrhosis” and “liver disease,” and watched all such videos that turned up in the search from beginning to end. They classified each post as either educational in intent or whether it depicted a firsthand experience with liver disease.
They determined whether each post was accurate or erroneous according to guidelines from the American Association for the Study of Liver Disease, American College of Gastroenterology, and American Gastroenterological Association. They also recorded the number of likes, comments, and the number of times that each video was shared.
Accurate posts were viewed and liked significantly more often than inaccurate ones, and accurate posts had significantly more comments that faulty posts.
However, both accurate and inaccurate posts were shared a similar number of times, suggesting that bad-quality information can metastasize just as easily as good information can be disseminated.
Dr. Loveland and colleagues echoed the recommendations of Dr. Johnson, stating that “health care provides should be aware of the potential for misinformation on social media and should strive to provide their patients with accurate and evidence-based information to inform their health care decisions.”
The study was internally funded. Dr. Loveland and Dr. Johnson reported having no conflicts of interest to disclose.
DDW is sponsored by the American Association for the Study of Liver Diseases, the AGA, the American Society for Gastrointestinal Endoscopy, and The Society for Surgery of the Alimentary Tract.
AT DDW 2023
FDA OKs spinal cord stimulation devices for chronic back pain
The Food and Drug Administration has expanded the indication for Abbott Laboratories’ spinal cord stimulation (SCS) devices to include treatment of chronic back pain in patients who have not had, or are not eligible for, back surgery, the company has announced.
The new indication spans all of Abbott’s SCS devices in the United States, which include the recharge-free Proclaim SCS family and the rechargeable Eterna SCS platform.
The devices feature the company’s proprietary, low-energy BurstDR stimulation waveform, a form of stimulation therapy that uses bursts of mild electrical energy without causing an abnormal tingling sensation to help disrupt pain signals before they can reach the brain, the company explained.
The expanded indication was supported by results from the DISTINCT study, which enrolled 270 adults suffering from severe, disabling chronic back pain for an average of more than 12 years and who were not eligible for surgery.
The study showed that significantly more patients who were treated with SCS achieved significant improvements in back pain, function, quality of life, and psychological status than peers treated with conservative medical management.
“To date, we have struggled with how to treat people who weren’t considered a good surgical candidate because we didn’t have clear, data-driven treatment options for non-surgical back pain,” Timothy Deer, MD, president and CEO of the Spine and Nerve Centers of the Virginias in Charleston, W.Va., said in a news release.
“This new indication for Abbott’s SCS devices, together with BurstDR stimulation, allows physicians the ability to identify and treat a new group of people, providing them with relief from chronic back pain,” Dr. Deer said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has expanded the indication for Abbott Laboratories’ spinal cord stimulation (SCS) devices to include treatment of chronic back pain in patients who have not had, or are not eligible for, back surgery, the company has announced.
The new indication spans all of Abbott’s SCS devices in the United States, which include the recharge-free Proclaim SCS family and the rechargeable Eterna SCS platform.
The devices feature the company’s proprietary, low-energy BurstDR stimulation waveform, a form of stimulation therapy that uses bursts of mild electrical energy without causing an abnormal tingling sensation to help disrupt pain signals before they can reach the brain, the company explained.
The expanded indication was supported by results from the DISTINCT study, which enrolled 270 adults suffering from severe, disabling chronic back pain for an average of more than 12 years and who were not eligible for surgery.
The study showed that significantly more patients who were treated with SCS achieved significant improvements in back pain, function, quality of life, and psychological status than peers treated with conservative medical management.
“To date, we have struggled with how to treat people who weren’t considered a good surgical candidate because we didn’t have clear, data-driven treatment options for non-surgical back pain,” Timothy Deer, MD, president and CEO of the Spine and Nerve Centers of the Virginias in Charleston, W.Va., said in a news release.
“This new indication for Abbott’s SCS devices, together with BurstDR stimulation, allows physicians the ability to identify and treat a new group of people, providing them with relief from chronic back pain,” Dr. Deer said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has expanded the indication for Abbott Laboratories’ spinal cord stimulation (SCS) devices to include treatment of chronic back pain in patients who have not had, or are not eligible for, back surgery, the company has announced.
The new indication spans all of Abbott’s SCS devices in the United States, which include the recharge-free Proclaim SCS family and the rechargeable Eterna SCS platform.
The devices feature the company’s proprietary, low-energy BurstDR stimulation waveform, a form of stimulation therapy that uses bursts of mild electrical energy without causing an abnormal tingling sensation to help disrupt pain signals before they can reach the brain, the company explained.
The expanded indication was supported by results from the DISTINCT study, which enrolled 270 adults suffering from severe, disabling chronic back pain for an average of more than 12 years and who were not eligible for surgery.
The study showed that significantly more patients who were treated with SCS achieved significant improvements in back pain, function, quality of life, and psychological status than peers treated with conservative medical management.
“To date, we have struggled with how to treat people who weren’t considered a good surgical candidate because we didn’t have clear, data-driven treatment options for non-surgical back pain,” Timothy Deer, MD, president and CEO of the Spine and Nerve Centers of the Virginias in Charleston, W.Va., said in a news release.
“This new indication for Abbott’s SCS devices, together with BurstDR stimulation, allows physicians the ability to identify and treat a new group of people, providing them with relief from chronic back pain,” Dr. Deer said.
A version of this article first appeared on Medscape.com.
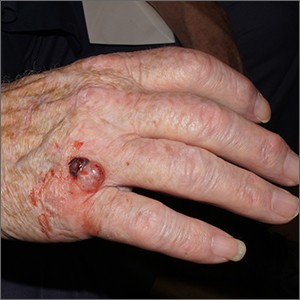
Nodule on farmer’s hand
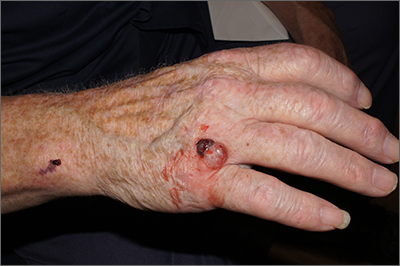
A broad shave biopsy was performed at the base of the lesion and the results were consistent with a thick nodular melanoma with a Breslow depth of 5.5 mm.
Melanoma is the deadliest skin cancer in the United States with mortality risk corresponding with the depth of the tumor.1 Nodular melanomas grow faster than all other types of melanoma. For this reason, a concerning raised lesion with a risk of melanoma should not be observed for change over time; it should be biopsied promptly. In this case, a depth of 5.5 mm was cause for quick action. Patients with tumors > 1 mm in depth (and some tumors > 0.8 mm) should be offered sentinel lymph node biopsy (SLNB) along with wide local excision to evaluate for lymphatic spread. Patients with thinner tumors may undergo wide local excision without SLNB.
In this case, National Comprehensive Cancer Network guidelines would dictate a 2-cm margin for a wide local incision; the patient underwent a modified version of this with Surgical Oncology to accommodate maintenance of hand function. This patient’s SLNB was negative, so the melanoma was classified as Stage IIC.
In the recent past, there were no additional treatments for patients with late Stage II disease (thick tumors without evidence of metastasis). However, in December 2021, the US Food and Drug Administration approved the use of immunotherapy with pembrolizumab in patients with node-negative late Stage II melanoma after demonstration of improved recurrence-free survival in the KEYNOTE trial.2 Evidence of improved long-term survival is mixed with adjuvant therapy, and studies evaluating the best role of adjuvant therapy are ongoing.
This patient was started on a regimen of pembrolizumab 200 mg IV every 3 weeks, which he will continue for as long as 1 year. He has tolerated this regimen without difficulty and has no evidence of disease.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Epstein DS, Lange JR, Gruber SB, et al. Is physician detection associated with thinner melanomas? JAMA. 1999;281:640-643. doi: 10.1001/jama.281.7.640
2. Luke JJ, Rutkowski P, Queirolo P, et al; KEYNOTE-716 Investigators. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet. 2022;399:1718-1729. doi: 10.1016/S0140-6736(22)00562-1

A broad shave biopsy was performed at the base of the lesion and the results were consistent with a thick nodular melanoma with a Breslow depth of 5.5 mm.
Melanoma is the deadliest skin cancer in the United States with mortality risk corresponding with the depth of the tumor.1 Nodular melanomas grow faster than all other types of melanoma. For this reason, a concerning raised lesion with a risk of melanoma should not be observed for change over time; it should be biopsied promptly. In this case, a depth of 5.5 mm was cause for quick action. Patients with tumors > 1 mm in depth (and some tumors > 0.8 mm) should be offered sentinel lymph node biopsy (SLNB) along with wide local excision to evaluate for lymphatic spread. Patients with thinner tumors may undergo wide local excision without SLNB.
In this case, National Comprehensive Cancer Network guidelines would dictate a 2-cm margin for a wide local incision; the patient underwent a modified version of this with Surgical Oncology to accommodate maintenance of hand function. This patient’s SLNB was negative, so the melanoma was classified as Stage IIC.
In the recent past, there were no additional treatments for patients with late Stage II disease (thick tumors without evidence of metastasis). However, in December 2021, the US Food and Drug Administration approved the use of immunotherapy with pembrolizumab in patients with node-negative late Stage II melanoma after demonstration of improved recurrence-free survival in the KEYNOTE trial.2 Evidence of improved long-term survival is mixed with adjuvant therapy, and studies evaluating the best role of adjuvant therapy are ongoing.
This patient was started on a regimen of pembrolizumab 200 mg IV every 3 weeks, which he will continue for as long as 1 year. He has tolerated this regimen without difficulty and has no evidence of disease.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A broad shave biopsy was performed at the base of the lesion and the results were consistent with a thick nodular melanoma with a Breslow depth of 5.5 mm.
Melanoma is the deadliest skin cancer in the United States with mortality risk corresponding with the depth of the tumor.1 Nodular melanomas grow faster than all other types of melanoma. For this reason, a concerning raised lesion with a risk of melanoma should not be observed for change over time; it should be biopsied promptly. In this case, a depth of 5.5 mm was cause for quick action. Patients with tumors > 1 mm in depth (and some tumors > 0.8 mm) should be offered sentinel lymph node biopsy (SLNB) along with wide local excision to evaluate for lymphatic spread. Patients with thinner tumors may undergo wide local excision without SLNB.
In this case, National Comprehensive Cancer Network guidelines would dictate a 2-cm margin for a wide local incision; the patient underwent a modified version of this with Surgical Oncology to accommodate maintenance of hand function. This patient’s SLNB was negative, so the melanoma was classified as Stage IIC.
In the recent past, there were no additional treatments for patients with late Stage II disease (thick tumors without evidence of metastasis). However, in December 2021, the US Food and Drug Administration approved the use of immunotherapy with pembrolizumab in patients with node-negative late Stage II melanoma after demonstration of improved recurrence-free survival in the KEYNOTE trial.2 Evidence of improved long-term survival is mixed with adjuvant therapy, and studies evaluating the best role of adjuvant therapy are ongoing.
This patient was started on a regimen of pembrolizumab 200 mg IV every 3 weeks, which he will continue for as long as 1 year. He has tolerated this regimen without difficulty and has no evidence of disease.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Epstein DS, Lange JR, Gruber SB, et al. Is physician detection associated with thinner melanomas? JAMA. 1999;281:640-643. doi: 10.1001/jama.281.7.640
2. Luke JJ, Rutkowski P, Queirolo P, et al; KEYNOTE-716 Investigators. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet. 2022;399:1718-1729. doi: 10.1016/S0140-6736(22)00562-1
1. Epstein DS, Lange JR, Gruber SB, et al. Is physician detection associated with thinner melanomas? JAMA. 1999;281:640-643. doi: 10.1001/jama.281.7.640
2. Luke JJ, Rutkowski P, Queirolo P, et al; KEYNOTE-716 Investigators. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet. 2022;399:1718-1729. doi: 10.1016/S0140-6736(22)00562-1
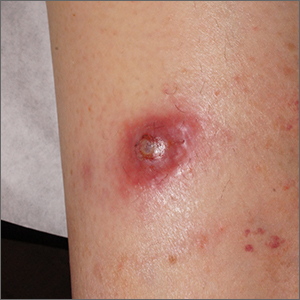
Firm nodule following tick bite
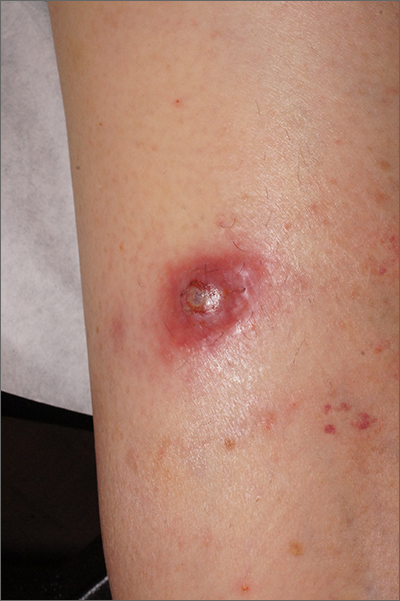
The biopsy revealed abundant lymphocytes, neutrophils, and eosinophils consistent with a diagnosis of cutaneous B cell pseudolymphoma. The pseudolymphoma was caused by an exaggerated response to a tick bite.
As the name implies, B cell pseudolymphomas clinically and histologically mimic the various patterns of cutaneous lymphoma, often appearing as a firm, pink to dark violet nodule. A biopsy is mandatory to distinguish between pseudolymphoma and true lymphoma. There is an association between pseudolymphomas and Borrelia burgdorferi, the organism responsible for Lyme disease. Thus, testing for Lyme disease is recommended for patients with pseudolymphomas who live in endemic areas. Patients who test positive should be treated with doxycycline 100 mg bid for 10 days.
In the absence of Lyme disease, a pseudolymphoma may resolve spontaneously over weeks or months. Resolution can be hastened with topical superpotent steroids, intralesional steroids, or systemic steroids. Treatment can begin with topical clobetasol 0.05% bid. If the lesion does not resolve, the next step would be intralesional triamcinolone 10 mg/mL injected directly into the nodule until it blanches slightly. Injections should be repeated every 3 to 4 weeks for a total of 2 to 3 injections.
The patient in this case had negative Lyme serology and was treated with 2 injections of triamcinolone 10 mg/mL administered 3 weeks apart. She experienced complete resolution.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi: 10.1016/j.path.2017.01.002

The biopsy revealed abundant lymphocytes, neutrophils, and eosinophils consistent with a diagnosis of cutaneous B cell pseudolymphoma. The pseudolymphoma was caused by an exaggerated response to a tick bite.
As the name implies, B cell pseudolymphomas clinically and histologically mimic the various patterns of cutaneous lymphoma, often appearing as a firm, pink to dark violet nodule. A biopsy is mandatory to distinguish between pseudolymphoma and true lymphoma. There is an association between pseudolymphomas and Borrelia burgdorferi, the organism responsible for Lyme disease. Thus, testing for Lyme disease is recommended for patients with pseudolymphomas who live in endemic areas. Patients who test positive should be treated with doxycycline 100 mg bid for 10 days.
In the absence of Lyme disease, a pseudolymphoma may resolve spontaneously over weeks or months. Resolution can be hastened with topical superpotent steroids, intralesional steroids, or systemic steroids. Treatment can begin with topical clobetasol 0.05% bid. If the lesion does not resolve, the next step would be intralesional triamcinolone 10 mg/mL injected directly into the nodule until it blanches slightly. Injections should be repeated every 3 to 4 weeks for a total of 2 to 3 injections.
The patient in this case had negative Lyme serology and was treated with 2 injections of triamcinolone 10 mg/mL administered 3 weeks apart. She experienced complete resolution.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

The biopsy revealed abundant lymphocytes, neutrophils, and eosinophils consistent with a diagnosis of cutaneous B cell pseudolymphoma. The pseudolymphoma was caused by an exaggerated response to a tick bite.
As the name implies, B cell pseudolymphomas clinically and histologically mimic the various patterns of cutaneous lymphoma, often appearing as a firm, pink to dark violet nodule. A biopsy is mandatory to distinguish between pseudolymphoma and true lymphoma. There is an association between pseudolymphomas and Borrelia burgdorferi, the organism responsible for Lyme disease. Thus, testing for Lyme disease is recommended for patients with pseudolymphomas who live in endemic areas. Patients who test positive should be treated with doxycycline 100 mg bid for 10 days.
In the absence of Lyme disease, a pseudolymphoma may resolve spontaneously over weeks or months. Resolution can be hastened with topical superpotent steroids, intralesional steroids, or systemic steroids. Treatment can begin with topical clobetasol 0.05% bid. If the lesion does not resolve, the next step would be intralesional triamcinolone 10 mg/mL injected directly into the nodule until it blanches slightly. Injections should be repeated every 3 to 4 weeks for a total of 2 to 3 injections.
The patient in this case had negative Lyme serology and was treated with 2 injections of triamcinolone 10 mg/mL administered 3 weeks apart. She experienced complete resolution.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi: 10.1016/j.path.2017.01.002
1. Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi: 10.1016/j.path.2017.01.002
Could vitamin D supplementation help in long COVID?
, in a retrospective, case-matched study.
The lower levels of vitamin D in patients with long COVID were most notable in those with brain fog.
These findings, by Luigi di Filippo, MD, and colleagues, were recently presented at the European Congress of Endocrinology and published in the Journal of Clinical Endocrinology & Metabolism.
“Our data suggest that vitamin D levels should be evaluated in COVID-19 patients after hospital discharge,” wrote the researchers, from San Raffaele Hospital, Milan.
“The role of vitamin D supplementation as a preventive strategy of COVID-19 sequelae should be tested in randomized controlled trials,” they urged.
The researchers also stressed that this was a controlled study in a homogeneous population, it included multiple signs and symptoms of long COVID, and it had a longer follow-up than most previous studies (6 vs. 3 months).
“The highly controlled nature of our study helps us better understand the role of vitamin D deficiency in long COVID and establish that there is likely a link between vitamin D deficiency and long COVID,” senior author Andrea Giustina, MD, said in a press release from the ECE.
“Our study shows that COVID-19 patients with low vitamin D levels are more likely to develop long COVID, but it is not yet known whether vitamin D supplements could improve the symptoms or reduce this risk altogether,” he cautioned.
“If confirmed in large, interventional, randomized controlled trials, [our data suggest] that vitamin D supplementation could represent a possible preventive strategy in reducing the burden of COVID-19 sequelae,” Dr. Giustina and colleagues wrote.
Reasonable to test vitamin D levels, consider supplementation
Invited to comment, Amiel Dror, MD, PhD, who led a related study that showed that people with a vitamin D deficiency were more likely to have severe COVID-19, agreed.
“The novelty and significance of this [new] study lie in the fact that it expands on our current understanding of the interplay between vitamin D and COVID-19, taking it beyond the acute phase of the disease,” said Dr. Dror, from Bar-Ilan University, Safed, Israel.
“It’s striking to see how vitamin D levels continue to influence patients’ health even after recovery from the initial infection,” he noted.
“The findings certainly add weight to the argument for conducting a randomized control trial [RCT],” he continued, which “would enable us to conclusively determine whether vitamin D supplementation can effectively reduce the risk or severity of long COVID.”
“In the interim,” Dr. Dror said, “given the safety profile of vitamin D and its broad health benefits, it could be reasonable to test for vitamin D levels in patients admitted with COVID-19. If levels are found to be low, supplementation could be considered.”
“However, it’s important to note that this should be done under medical supervision,” he cautioned, “and further studies are needed to establish the optimal timing and dosage of supplementation.”
“I anticipate that we’ll see more RCTs [of this] in the future,” he speculated.
Low vitamin D and risk of long COVID
Long COVID is an emerging syndrome that affects 50%-70% of COVID-19 survivors.
Low levels of vitamin D have been associated with increased likelihood of needing mechanical ventilation and worse survival in patients hospitalized with COVID-19, but the risk of long COVID associated with vitamin D has not been known.
Researchers analyzed data from adults aged 18 and older hospitalized at San Raffaele Hospital with a confirmed diagnosis of COVID-19 and discharged during the first pandemic wave, from March to May 2020, and then seen 6-months later for follow-up.
Patients were excluded if they had been admitted to the intensive care unit during hospitalization or had missing medical data or blood samples available to determine (OH) vitamin D levels, at admission and the 6-month follow-up.
Long COVID-19 was defined based on the U.K. National Institute for Health and Care Excellence guidelines as the concomitant presence of at least two or more of 17 signs and symptoms that were absent prior to the COVID-19 infection and could only be attributed to that acute disease.
Researchers identified 50 patients with long COVID at the 6-month follow-up and matched them with 50 patients without long COVID at that time point, based on age, sex, concomitant comorbidities, need for noninvasive mechanical ventilation, and week of evaluation.
Patients were a mean age of 61 years (range, 51-73) and 56% were men; 28% had been on a ventilator during hospitalization for COVID-19.
The most frequent signs and symptoms at 6 months in the patients with long COVID were asthenia (weakness, 38% of patients), dysgeusia (bad taste in the mouth, 34%), dyspnea (shortness of breath, 34%), and anosmia (loss of sense of smell, 24%).
Most symptoms were related to the cardiorespiratory system (42%), the feeling of well-being (42%), or the senses (36%), and fewer patients had symptoms related to neurocognitive impairment (headache or brain fog, 14%), or ear, nose, and throat (12%), or gastrointestinal system (4%).
Patients with long COVID had lower mean 25(OH) vitamin D levels than patients without long COVID (20.1 vs 23.2 ng/mL; P = .03). However, actual vitamin D deficiency levels were similar in both groups.
Two-thirds of patients with low vitamin D levels at hospital admission still presented with low levels at the 6-month follow-up.
Vitamin D levels were significantly lower in patients with neurocognitive symptoms at follow-up (n = 7) than in those without such symptoms (n = 93) (14.6 vs. 20.6 ng/mL; P = .042).
In patients with vitamin D deficiency (< 20 ng/mL) at admission and at follow-up (n = 42), those with long COVID (n = 22) had lower vitamin D levels at follow-up than those without long COVID (n = 20) (12.7 vs. 15.2 ng/mL; P = .041).
And in multiple regression analyses, a lower 25(OH) vitamin D level at follow-up was the only variable that was significantly associated with long COVID (odds ratio, 1.09; 95% confidence interval, 1.01-1.16; P = .008).
The findings “strongly reinforce the clinical usefulness of 25(OH) vitamin D evaluation as a possible modifiable pathophysiological factor underlying this emerging worldwide critical health issue,” the researchers concluded.
The study was supported by Abiogen Pharma. One study author is an employee at Abiogen. Dr. Giustina has reported being a consultant for Abiogen and Takeda and receiving a research grant to his institution from Takeda. Dr. Di Filippo and the other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, in a retrospective, case-matched study.
The lower levels of vitamin D in patients with long COVID were most notable in those with brain fog.
These findings, by Luigi di Filippo, MD, and colleagues, were recently presented at the European Congress of Endocrinology and published in the Journal of Clinical Endocrinology & Metabolism.
“Our data suggest that vitamin D levels should be evaluated in COVID-19 patients after hospital discharge,” wrote the researchers, from San Raffaele Hospital, Milan.
“The role of vitamin D supplementation as a preventive strategy of COVID-19 sequelae should be tested in randomized controlled trials,” they urged.
The researchers also stressed that this was a controlled study in a homogeneous population, it included multiple signs and symptoms of long COVID, and it had a longer follow-up than most previous studies (6 vs. 3 months).
“The highly controlled nature of our study helps us better understand the role of vitamin D deficiency in long COVID and establish that there is likely a link between vitamin D deficiency and long COVID,” senior author Andrea Giustina, MD, said in a press release from the ECE.
“Our study shows that COVID-19 patients with low vitamin D levels are more likely to develop long COVID, but it is not yet known whether vitamin D supplements could improve the symptoms or reduce this risk altogether,” he cautioned.
“If confirmed in large, interventional, randomized controlled trials, [our data suggest] that vitamin D supplementation could represent a possible preventive strategy in reducing the burden of COVID-19 sequelae,” Dr. Giustina and colleagues wrote.
Reasonable to test vitamin D levels, consider supplementation
Invited to comment, Amiel Dror, MD, PhD, who led a related study that showed that people with a vitamin D deficiency were more likely to have severe COVID-19, agreed.
“The novelty and significance of this [new] study lie in the fact that it expands on our current understanding of the interplay between vitamin D and COVID-19, taking it beyond the acute phase of the disease,” said Dr. Dror, from Bar-Ilan University, Safed, Israel.
“It’s striking to see how vitamin D levels continue to influence patients’ health even after recovery from the initial infection,” he noted.
“The findings certainly add weight to the argument for conducting a randomized control trial [RCT],” he continued, which “would enable us to conclusively determine whether vitamin D supplementation can effectively reduce the risk or severity of long COVID.”
“In the interim,” Dr. Dror said, “given the safety profile of vitamin D and its broad health benefits, it could be reasonable to test for vitamin D levels in patients admitted with COVID-19. If levels are found to be low, supplementation could be considered.”
“However, it’s important to note that this should be done under medical supervision,” he cautioned, “and further studies are needed to establish the optimal timing and dosage of supplementation.”
“I anticipate that we’ll see more RCTs [of this] in the future,” he speculated.
Low vitamin D and risk of long COVID
Long COVID is an emerging syndrome that affects 50%-70% of COVID-19 survivors.
Low levels of vitamin D have been associated with increased likelihood of needing mechanical ventilation and worse survival in patients hospitalized with COVID-19, but the risk of long COVID associated with vitamin D has not been known.
Researchers analyzed data from adults aged 18 and older hospitalized at San Raffaele Hospital with a confirmed diagnosis of COVID-19 and discharged during the first pandemic wave, from March to May 2020, and then seen 6-months later for follow-up.
Patients were excluded if they had been admitted to the intensive care unit during hospitalization or had missing medical data or blood samples available to determine (OH) vitamin D levels, at admission and the 6-month follow-up.
Long COVID-19 was defined based on the U.K. National Institute for Health and Care Excellence guidelines as the concomitant presence of at least two or more of 17 signs and symptoms that were absent prior to the COVID-19 infection and could only be attributed to that acute disease.
Researchers identified 50 patients with long COVID at the 6-month follow-up and matched them with 50 patients without long COVID at that time point, based on age, sex, concomitant comorbidities, need for noninvasive mechanical ventilation, and week of evaluation.
Patients were a mean age of 61 years (range, 51-73) and 56% were men; 28% had been on a ventilator during hospitalization for COVID-19.
The most frequent signs and symptoms at 6 months in the patients with long COVID were asthenia (weakness, 38% of patients), dysgeusia (bad taste in the mouth, 34%), dyspnea (shortness of breath, 34%), and anosmia (loss of sense of smell, 24%).
Most symptoms were related to the cardiorespiratory system (42%), the feeling of well-being (42%), or the senses (36%), and fewer patients had symptoms related to neurocognitive impairment (headache or brain fog, 14%), or ear, nose, and throat (12%), or gastrointestinal system (4%).
Patients with long COVID had lower mean 25(OH) vitamin D levels than patients without long COVID (20.1 vs 23.2 ng/mL; P = .03). However, actual vitamin D deficiency levels were similar in both groups.
Two-thirds of patients with low vitamin D levels at hospital admission still presented with low levels at the 6-month follow-up.
Vitamin D levels were significantly lower in patients with neurocognitive symptoms at follow-up (n = 7) than in those without such symptoms (n = 93) (14.6 vs. 20.6 ng/mL; P = .042).
In patients with vitamin D deficiency (< 20 ng/mL) at admission and at follow-up (n = 42), those with long COVID (n = 22) had lower vitamin D levels at follow-up than those without long COVID (n = 20) (12.7 vs. 15.2 ng/mL; P = .041).
And in multiple regression analyses, a lower 25(OH) vitamin D level at follow-up was the only variable that was significantly associated with long COVID (odds ratio, 1.09; 95% confidence interval, 1.01-1.16; P = .008).
The findings “strongly reinforce the clinical usefulness of 25(OH) vitamin D evaluation as a possible modifiable pathophysiological factor underlying this emerging worldwide critical health issue,” the researchers concluded.
The study was supported by Abiogen Pharma. One study author is an employee at Abiogen. Dr. Giustina has reported being a consultant for Abiogen and Takeda and receiving a research grant to his institution from Takeda. Dr. Di Filippo and the other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, in a retrospective, case-matched study.
The lower levels of vitamin D in patients with long COVID were most notable in those with brain fog.
These findings, by Luigi di Filippo, MD, and colleagues, were recently presented at the European Congress of Endocrinology and published in the Journal of Clinical Endocrinology & Metabolism.
“Our data suggest that vitamin D levels should be evaluated in COVID-19 patients after hospital discharge,” wrote the researchers, from San Raffaele Hospital, Milan.
“The role of vitamin D supplementation as a preventive strategy of COVID-19 sequelae should be tested in randomized controlled trials,” they urged.
The researchers also stressed that this was a controlled study in a homogeneous population, it included multiple signs and symptoms of long COVID, and it had a longer follow-up than most previous studies (6 vs. 3 months).
“The highly controlled nature of our study helps us better understand the role of vitamin D deficiency in long COVID and establish that there is likely a link between vitamin D deficiency and long COVID,” senior author Andrea Giustina, MD, said in a press release from the ECE.
“Our study shows that COVID-19 patients with low vitamin D levels are more likely to develop long COVID, but it is not yet known whether vitamin D supplements could improve the symptoms or reduce this risk altogether,” he cautioned.
“If confirmed in large, interventional, randomized controlled trials, [our data suggest] that vitamin D supplementation could represent a possible preventive strategy in reducing the burden of COVID-19 sequelae,” Dr. Giustina and colleagues wrote.
Reasonable to test vitamin D levels, consider supplementation
Invited to comment, Amiel Dror, MD, PhD, who led a related study that showed that people with a vitamin D deficiency were more likely to have severe COVID-19, agreed.
“The novelty and significance of this [new] study lie in the fact that it expands on our current understanding of the interplay between vitamin D and COVID-19, taking it beyond the acute phase of the disease,” said Dr. Dror, from Bar-Ilan University, Safed, Israel.
“It’s striking to see how vitamin D levels continue to influence patients’ health even after recovery from the initial infection,” he noted.
“The findings certainly add weight to the argument for conducting a randomized control trial [RCT],” he continued, which “would enable us to conclusively determine whether vitamin D supplementation can effectively reduce the risk or severity of long COVID.”
“In the interim,” Dr. Dror said, “given the safety profile of vitamin D and its broad health benefits, it could be reasonable to test for vitamin D levels in patients admitted with COVID-19. If levels are found to be low, supplementation could be considered.”
“However, it’s important to note that this should be done under medical supervision,” he cautioned, “and further studies are needed to establish the optimal timing and dosage of supplementation.”
“I anticipate that we’ll see more RCTs [of this] in the future,” he speculated.
Low vitamin D and risk of long COVID
Long COVID is an emerging syndrome that affects 50%-70% of COVID-19 survivors.
Low levels of vitamin D have been associated with increased likelihood of needing mechanical ventilation and worse survival in patients hospitalized with COVID-19, but the risk of long COVID associated with vitamin D has not been known.
Researchers analyzed data from adults aged 18 and older hospitalized at San Raffaele Hospital with a confirmed diagnosis of COVID-19 and discharged during the first pandemic wave, from March to May 2020, and then seen 6-months later for follow-up.
Patients were excluded if they had been admitted to the intensive care unit during hospitalization or had missing medical data or blood samples available to determine (OH) vitamin D levels, at admission and the 6-month follow-up.
Long COVID-19 was defined based on the U.K. National Institute for Health and Care Excellence guidelines as the concomitant presence of at least two or more of 17 signs and symptoms that were absent prior to the COVID-19 infection and could only be attributed to that acute disease.
Researchers identified 50 patients with long COVID at the 6-month follow-up and matched them with 50 patients without long COVID at that time point, based on age, sex, concomitant comorbidities, need for noninvasive mechanical ventilation, and week of evaluation.
Patients were a mean age of 61 years (range, 51-73) and 56% were men; 28% had been on a ventilator during hospitalization for COVID-19.
The most frequent signs and symptoms at 6 months in the patients with long COVID were asthenia (weakness, 38% of patients), dysgeusia (bad taste in the mouth, 34%), dyspnea (shortness of breath, 34%), and anosmia (loss of sense of smell, 24%).
Most symptoms were related to the cardiorespiratory system (42%), the feeling of well-being (42%), or the senses (36%), and fewer patients had symptoms related to neurocognitive impairment (headache or brain fog, 14%), or ear, nose, and throat (12%), or gastrointestinal system (4%).
Patients with long COVID had lower mean 25(OH) vitamin D levels than patients without long COVID (20.1 vs 23.2 ng/mL; P = .03). However, actual vitamin D deficiency levels were similar in both groups.
Two-thirds of patients with low vitamin D levels at hospital admission still presented with low levels at the 6-month follow-up.
Vitamin D levels were significantly lower in patients with neurocognitive symptoms at follow-up (n = 7) than in those without such symptoms (n = 93) (14.6 vs. 20.6 ng/mL; P = .042).
In patients with vitamin D deficiency (< 20 ng/mL) at admission and at follow-up (n = 42), those with long COVID (n = 22) had lower vitamin D levels at follow-up than those without long COVID (n = 20) (12.7 vs. 15.2 ng/mL; P = .041).
And in multiple regression analyses, a lower 25(OH) vitamin D level at follow-up was the only variable that was significantly associated with long COVID (odds ratio, 1.09; 95% confidence interval, 1.01-1.16; P = .008).
The findings “strongly reinforce the clinical usefulness of 25(OH) vitamin D evaluation as a possible modifiable pathophysiological factor underlying this emerging worldwide critical health issue,” the researchers concluded.
The study was supported by Abiogen Pharma. One study author is an employee at Abiogen. Dr. Giustina has reported being a consultant for Abiogen and Takeda and receiving a research grant to his institution from Takeda. Dr. Di Filippo and the other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECE 2023
Deep sleep may mitigate the impact of Alzheimer’s pathology
Investigators found that deep sleep, also known as non-REM (NREM) slow-wave sleep, can protect memory function in cognitively normal adults with a high beta-amyloid burden.
“Think of deep sleep almost like a life raft that keeps memory afloat, rather than memory getting dragged down by the weight of Alzheimer’s disease pathology,” senior investigator Matthew Walker, PhD, professor of neuroscience and psychology, University of California, Berkeley, said in a news release.
The study was published online in BMC Medicine.
Resilience factor
Studying resilience to existing brain pathology is “an exciting new research direction,” lead author Zsófia Zavecz, PhD, with the Center for Human Sleep Science at the University of California, Berkeley, said in an interview.
“That is, what factors explain the individual differences in cognitive function despite the same level of brain pathology, and how do some people with significant pathology have largely preserved memory?” she added.
The study included 62 cognitively normal older adults from the Berkeley Aging Cohort Study.
Sleep EEG recordings were obtained over 2 nights in a sleep lab and PET scans were used to quantify beta-amyloid. Half of the participants had high beta-amyloid burden and half were beta-amyloid negative.
After the sleep studies, all participants completed a memory task involving matching names to faces.
The results suggest that deep NREM slow-wave sleep significantly moderates the effect of beta-amyloid status on memory function.
Specifically, NREM slow-wave activity selectively supported superior memory function in adults with high beta-amyloid burden, who are most in need of cognitive reserve (B = 2.694, P = .019), the researchers report.
In contrast, adults without significant beta-amyloid pathological burden – and thus without the same need for cognitive reserve – did not similarly benefit from NREM slow-wave activity (B = –0.115, P = .876).
The findings remained significant after adjusting for age, sex, body mass index, gray matter atrophy, and previously identified cognitive reserve factors, such as education and physical activity.
Dr. Zavecz said there are several potential reasons why deep sleep may support cognitive reserve.
One is that during deep sleep specifically, memories are replayed in the brain, and this results in a “neural reorganization” that helps stabilize the memory and make it more permanent.
“Other explanations include deep sleep’s role in maintaining homeostasis in the brain’s capacity to form new neural connections and providing an optimal brain state for the clearance of toxins interfering with healthy brain functioning,” she noted.
“The extent to which sleep could offer a protective buffer against severe cognitive impairment remains to be tested. However, this study is the first step in hopefully a series of new research that will investigate sleep as a cognitive reserve factor,” said Dr. Zavecz.
Encouraging data
Reached for comment, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said although the study sample is small, the results are “encouraging because sleep is a modifiable factor and can therefore be targeted.”
“More work is needed in a larger population before we can fully leverage this stage of sleep to reduce the risk of developing cognitive decline,” Dr. Griffin said.
Also weighing in on this research, Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, said the study is “exciting on two fronts – we may have an additional marker for the development of Alzheimer’s disease to predict risk and track disease, but also targets for early intervention with sleep architecture–enhancing therapies, be they drug, device, or digital.”
“For the sake of our brain health, we all must get very familiar with the concept of cognitive or brain reserve,” said Dr. Lakhan, who was not involved in the study.
“Brain reserve refers to our ability to buttress against the threat of dementia and classically it’s been associated with ongoing brain stimulation (i.e., higher education, cognitively demanding job),” he noted.
“This line of research now opens the door that optimal sleep health – especially deep NREM slow wave sleep – correlates with greater brain reserve against Alzheimer’s disease,” Dr. Lakhan said.
The study was supported by the National Institutes of Health and the University of California, Berkeley. Dr. Walker serves as an advisor to and has equity interest in Bryte, Shuni, Oura, and StimScience. Dr. Zavecz and Dr. Lakhan report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators found that deep sleep, also known as non-REM (NREM) slow-wave sleep, can protect memory function in cognitively normal adults with a high beta-amyloid burden.
“Think of deep sleep almost like a life raft that keeps memory afloat, rather than memory getting dragged down by the weight of Alzheimer’s disease pathology,” senior investigator Matthew Walker, PhD, professor of neuroscience and psychology, University of California, Berkeley, said in a news release.
The study was published online in BMC Medicine.
Resilience factor
Studying resilience to existing brain pathology is “an exciting new research direction,” lead author Zsófia Zavecz, PhD, with the Center for Human Sleep Science at the University of California, Berkeley, said in an interview.
“That is, what factors explain the individual differences in cognitive function despite the same level of brain pathology, and how do some people with significant pathology have largely preserved memory?” she added.
The study included 62 cognitively normal older adults from the Berkeley Aging Cohort Study.
Sleep EEG recordings were obtained over 2 nights in a sleep lab and PET scans were used to quantify beta-amyloid. Half of the participants had high beta-amyloid burden and half were beta-amyloid negative.
After the sleep studies, all participants completed a memory task involving matching names to faces.
The results suggest that deep NREM slow-wave sleep significantly moderates the effect of beta-amyloid status on memory function.
Specifically, NREM slow-wave activity selectively supported superior memory function in adults with high beta-amyloid burden, who are most in need of cognitive reserve (B = 2.694, P = .019), the researchers report.
In contrast, adults without significant beta-amyloid pathological burden – and thus without the same need for cognitive reserve – did not similarly benefit from NREM slow-wave activity (B = –0.115, P = .876).
The findings remained significant after adjusting for age, sex, body mass index, gray matter atrophy, and previously identified cognitive reserve factors, such as education and physical activity.
Dr. Zavecz said there are several potential reasons why deep sleep may support cognitive reserve.
One is that during deep sleep specifically, memories are replayed in the brain, and this results in a “neural reorganization” that helps stabilize the memory and make it more permanent.
“Other explanations include deep sleep’s role in maintaining homeostasis in the brain’s capacity to form new neural connections and providing an optimal brain state for the clearance of toxins interfering with healthy brain functioning,” she noted.
“The extent to which sleep could offer a protective buffer against severe cognitive impairment remains to be tested. However, this study is the first step in hopefully a series of new research that will investigate sleep as a cognitive reserve factor,” said Dr. Zavecz.
Encouraging data
Reached for comment, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said although the study sample is small, the results are “encouraging because sleep is a modifiable factor and can therefore be targeted.”
“More work is needed in a larger population before we can fully leverage this stage of sleep to reduce the risk of developing cognitive decline,” Dr. Griffin said.
Also weighing in on this research, Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, said the study is “exciting on two fronts – we may have an additional marker for the development of Alzheimer’s disease to predict risk and track disease, but also targets for early intervention with sleep architecture–enhancing therapies, be they drug, device, or digital.”
“For the sake of our brain health, we all must get very familiar with the concept of cognitive or brain reserve,” said Dr. Lakhan, who was not involved in the study.
“Brain reserve refers to our ability to buttress against the threat of dementia and classically it’s been associated with ongoing brain stimulation (i.e., higher education, cognitively demanding job),” he noted.
“This line of research now opens the door that optimal sleep health – especially deep NREM slow wave sleep – correlates with greater brain reserve against Alzheimer’s disease,” Dr. Lakhan said.
The study was supported by the National Institutes of Health and the University of California, Berkeley. Dr. Walker serves as an advisor to and has equity interest in Bryte, Shuni, Oura, and StimScience. Dr. Zavecz and Dr. Lakhan report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators found that deep sleep, also known as non-REM (NREM) slow-wave sleep, can protect memory function in cognitively normal adults with a high beta-amyloid burden.
“Think of deep sleep almost like a life raft that keeps memory afloat, rather than memory getting dragged down by the weight of Alzheimer’s disease pathology,” senior investigator Matthew Walker, PhD, professor of neuroscience and psychology, University of California, Berkeley, said in a news release.
The study was published online in BMC Medicine.
Resilience factor
Studying resilience to existing brain pathology is “an exciting new research direction,” lead author Zsófia Zavecz, PhD, with the Center for Human Sleep Science at the University of California, Berkeley, said in an interview.
“That is, what factors explain the individual differences in cognitive function despite the same level of brain pathology, and how do some people with significant pathology have largely preserved memory?” she added.
The study included 62 cognitively normal older adults from the Berkeley Aging Cohort Study.
Sleep EEG recordings were obtained over 2 nights in a sleep lab and PET scans were used to quantify beta-amyloid. Half of the participants had high beta-amyloid burden and half were beta-amyloid negative.
After the sleep studies, all participants completed a memory task involving matching names to faces.
The results suggest that deep NREM slow-wave sleep significantly moderates the effect of beta-amyloid status on memory function.
Specifically, NREM slow-wave activity selectively supported superior memory function in adults with high beta-amyloid burden, who are most in need of cognitive reserve (B = 2.694, P = .019), the researchers report.
In contrast, adults without significant beta-amyloid pathological burden – and thus without the same need for cognitive reserve – did not similarly benefit from NREM slow-wave activity (B = –0.115, P = .876).
The findings remained significant after adjusting for age, sex, body mass index, gray matter atrophy, and previously identified cognitive reserve factors, such as education and physical activity.
Dr. Zavecz said there are several potential reasons why deep sleep may support cognitive reserve.
One is that during deep sleep specifically, memories are replayed in the brain, and this results in a “neural reorganization” that helps stabilize the memory and make it more permanent.
“Other explanations include deep sleep’s role in maintaining homeostasis in the brain’s capacity to form new neural connections and providing an optimal brain state for the clearance of toxins interfering with healthy brain functioning,” she noted.
“The extent to which sleep could offer a protective buffer against severe cognitive impairment remains to be tested. However, this study is the first step in hopefully a series of new research that will investigate sleep as a cognitive reserve factor,” said Dr. Zavecz.
Encouraging data
Reached for comment, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said although the study sample is small, the results are “encouraging because sleep is a modifiable factor and can therefore be targeted.”
“More work is needed in a larger population before we can fully leverage this stage of sleep to reduce the risk of developing cognitive decline,” Dr. Griffin said.
Also weighing in on this research, Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, said the study is “exciting on two fronts – we may have an additional marker for the development of Alzheimer’s disease to predict risk and track disease, but also targets for early intervention with sleep architecture–enhancing therapies, be they drug, device, or digital.”
“For the sake of our brain health, we all must get very familiar with the concept of cognitive or brain reserve,” said Dr. Lakhan, who was not involved in the study.
“Brain reserve refers to our ability to buttress against the threat of dementia and classically it’s been associated with ongoing brain stimulation (i.e., higher education, cognitively demanding job),” he noted.
“This line of research now opens the door that optimal sleep health – especially deep NREM slow wave sleep – correlates with greater brain reserve against Alzheimer’s disease,” Dr. Lakhan said.
The study was supported by the National Institutes of Health and the University of California, Berkeley. Dr. Walker serves as an advisor to and has equity interest in Bryte, Shuni, Oura, and StimScience. Dr. Zavecz and Dr. Lakhan report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM BMC MEDICINE
Foot ulcers red flag for eye disease in diabetes
Sores on the feet can signal problems with the eyes in patients with diabetes.
Prior research and anecdotal experience show that diabetic foot ulcers and diabetic retinopathy frequently co-occur.
David J. Ramsey, MD, PhD, MPH, director of ophthalmic research at Lahey Hospital & Medical Center, Burlington, Mass., said when clinicians detect either condition, they should involve a team that can intervene to help protect a patient’s vision and mobility.
For example, they should ensure patients receive comprehensive eye and foot evaluations and help them optimize diabetes management.
The new study, presented at the annual meeting of the Association for Research in Vision and Ophthalmology, “adds an important dimension” to understanding the association between the conditions, said Dr. Ramsey, who recently reviewed correlations between diabetic foot ulcers and diabetic retinopathy and their underlying causes.
“Patients with diabetic foot ulcers appear to receive less attention to their diabetic retinopathy and may receive fewer treatments with eye injections targeting vascular endothelial growth factor (VEGF), an important driver of progression of diabetic retinopathy,” said Dr. Ramsey, who is also an associate professor of ophthalmology at Tufts University School of Medicine, Boston. He was not involved in the study presented at ARVO 2023.
In the new study, Christopher T. Zhu, a medical student at UT Health San Antonio, and colleagues analyzed data from 426 eyes of 213 patients with type 2 diabetes who had had at least two eye exams between 2012 and 2022; 72 of the patients had diabetic foot ulcers. Patients were followed for about 4 years on average.
Patients with diabetic foot ulcers had a higher percentage of eyes with macular edema on their initial exam (32.6% vs. 28%). By the final exam, the percentage of eyes with macular edema was significantly greater in the group with diabetic foot ulcers (64.6% vs. 37.6%; P < .0001), Mr. Zhu’s group reported.
Eyes with nonproliferative diabetic retinopathy progressed to proliferative diabetic retinopathy, the worst grade, at a higher rate in the group with foot ulcers (50.6% vs. 35.6%; P = .03). In addition, patients with foot ulcers were more likely to experience vitreous hemorrhage (55.6% vs. 38.7%), the researchers found.
Despite patients with foot ulcers tending to have worse disease, they received fewer treatments for retinopathy. Those without ulcers received an average of 6.9 anti-VEGF injections per eye, while those with ulcers averaged 4.3.
Foot ulcers may hinder the ability of patients to get to appointments to receive the injections, Mr. Zhu and colleagues wrote. “For many patients in our part of the country [South Texas], a lack of transportation is a particular barrier to health care access,” Mr. Zhu told this news organization.
Mr. Zhu’s team conducted their study after noticing that patients with diabetes and foot ulcers who presented to their eye clinics “appeared to progress faster to worse grades of retinopathy” than patients with diabetes who did not have ulcers.
“Similar to how foot ulcers develop due to a severe disruption in blood flow [vascular] and a loss of sensation [neurologic], diabetic retinopathy may have a relation to microvascular disease, neurologic degeneration, and inflammation,” he said.
The findings confirm “that poor perfusion of the eye and foot are linked and can cause ischemic retinopathy leading to the development of proliferative diabetic retinopathy and vitreous hemorrhages, both serious, vision-threatening conditions,” Dr. Ramsey said.
To some extent, fewer treatments with anti-VEGF agents may account for why patients with foot ulcers have more eye complications, Dr. Ramsey added. “Additional research needs to be done to further dissect the cause and the effect, but it’s a very important finding that we need to increase awareness about,” he said.
Dr. Ramsey and Mr. Zhu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sores on the feet can signal problems with the eyes in patients with diabetes.
Prior research and anecdotal experience show that diabetic foot ulcers and diabetic retinopathy frequently co-occur.
David J. Ramsey, MD, PhD, MPH, director of ophthalmic research at Lahey Hospital & Medical Center, Burlington, Mass., said when clinicians detect either condition, they should involve a team that can intervene to help protect a patient’s vision and mobility.
For example, they should ensure patients receive comprehensive eye and foot evaluations and help them optimize diabetes management.
The new study, presented at the annual meeting of the Association for Research in Vision and Ophthalmology, “adds an important dimension” to understanding the association between the conditions, said Dr. Ramsey, who recently reviewed correlations between diabetic foot ulcers and diabetic retinopathy and their underlying causes.
“Patients with diabetic foot ulcers appear to receive less attention to their diabetic retinopathy and may receive fewer treatments with eye injections targeting vascular endothelial growth factor (VEGF), an important driver of progression of diabetic retinopathy,” said Dr. Ramsey, who is also an associate professor of ophthalmology at Tufts University School of Medicine, Boston. He was not involved in the study presented at ARVO 2023.
In the new study, Christopher T. Zhu, a medical student at UT Health San Antonio, and colleagues analyzed data from 426 eyes of 213 patients with type 2 diabetes who had had at least two eye exams between 2012 and 2022; 72 of the patients had diabetic foot ulcers. Patients were followed for about 4 years on average.
Patients with diabetic foot ulcers had a higher percentage of eyes with macular edema on their initial exam (32.6% vs. 28%). By the final exam, the percentage of eyes with macular edema was significantly greater in the group with diabetic foot ulcers (64.6% vs. 37.6%; P < .0001), Mr. Zhu’s group reported.
Eyes with nonproliferative diabetic retinopathy progressed to proliferative diabetic retinopathy, the worst grade, at a higher rate in the group with foot ulcers (50.6% vs. 35.6%; P = .03). In addition, patients with foot ulcers were more likely to experience vitreous hemorrhage (55.6% vs. 38.7%), the researchers found.
Despite patients with foot ulcers tending to have worse disease, they received fewer treatments for retinopathy. Those without ulcers received an average of 6.9 anti-VEGF injections per eye, while those with ulcers averaged 4.3.
Foot ulcers may hinder the ability of patients to get to appointments to receive the injections, Mr. Zhu and colleagues wrote. “For many patients in our part of the country [South Texas], a lack of transportation is a particular barrier to health care access,” Mr. Zhu told this news organization.
Mr. Zhu’s team conducted their study after noticing that patients with diabetes and foot ulcers who presented to their eye clinics “appeared to progress faster to worse grades of retinopathy” than patients with diabetes who did not have ulcers.
“Similar to how foot ulcers develop due to a severe disruption in blood flow [vascular] and a loss of sensation [neurologic], diabetic retinopathy may have a relation to microvascular disease, neurologic degeneration, and inflammation,” he said.
The findings confirm “that poor perfusion of the eye and foot are linked and can cause ischemic retinopathy leading to the development of proliferative diabetic retinopathy and vitreous hemorrhages, both serious, vision-threatening conditions,” Dr. Ramsey said.
To some extent, fewer treatments with anti-VEGF agents may account for why patients with foot ulcers have more eye complications, Dr. Ramsey added. “Additional research needs to be done to further dissect the cause and the effect, but it’s a very important finding that we need to increase awareness about,” he said.
Dr. Ramsey and Mr. Zhu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sores on the feet can signal problems with the eyes in patients with diabetes.
Prior research and anecdotal experience show that diabetic foot ulcers and diabetic retinopathy frequently co-occur.
David J. Ramsey, MD, PhD, MPH, director of ophthalmic research at Lahey Hospital & Medical Center, Burlington, Mass., said when clinicians detect either condition, they should involve a team that can intervene to help protect a patient’s vision and mobility.
For example, they should ensure patients receive comprehensive eye and foot evaluations and help them optimize diabetes management.
The new study, presented at the annual meeting of the Association for Research in Vision and Ophthalmology, “adds an important dimension” to understanding the association between the conditions, said Dr. Ramsey, who recently reviewed correlations between diabetic foot ulcers and diabetic retinopathy and their underlying causes.
“Patients with diabetic foot ulcers appear to receive less attention to their diabetic retinopathy and may receive fewer treatments with eye injections targeting vascular endothelial growth factor (VEGF), an important driver of progression of diabetic retinopathy,” said Dr. Ramsey, who is also an associate professor of ophthalmology at Tufts University School of Medicine, Boston. He was not involved in the study presented at ARVO 2023.
In the new study, Christopher T. Zhu, a medical student at UT Health San Antonio, and colleagues analyzed data from 426 eyes of 213 patients with type 2 diabetes who had had at least two eye exams between 2012 and 2022; 72 of the patients had diabetic foot ulcers. Patients were followed for about 4 years on average.
Patients with diabetic foot ulcers had a higher percentage of eyes with macular edema on their initial exam (32.6% vs. 28%). By the final exam, the percentage of eyes with macular edema was significantly greater in the group with diabetic foot ulcers (64.6% vs. 37.6%; P < .0001), Mr. Zhu’s group reported.
Eyes with nonproliferative diabetic retinopathy progressed to proliferative diabetic retinopathy, the worst grade, at a higher rate in the group with foot ulcers (50.6% vs. 35.6%; P = .03). In addition, patients with foot ulcers were more likely to experience vitreous hemorrhage (55.6% vs. 38.7%), the researchers found.
Despite patients with foot ulcers tending to have worse disease, they received fewer treatments for retinopathy. Those without ulcers received an average of 6.9 anti-VEGF injections per eye, while those with ulcers averaged 4.3.
Foot ulcers may hinder the ability of patients to get to appointments to receive the injections, Mr. Zhu and colleagues wrote. “For many patients in our part of the country [South Texas], a lack of transportation is a particular barrier to health care access,” Mr. Zhu told this news organization.
Mr. Zhu’s team conducted their study after noticing that patients with diabetes and foot ulcers who presented to their eye clinics “appeared to progress faster to worse grades of retinopathy” than patients with diabetes who did not have ulcers.
“Similar to how foot ulcers develop due to a severe disruption in blood flow [vascular] and a loss of sensation [neurologic], diabetic retinopathy may have a relation to microvascular disease, neurologic degeneration, and inflammation,” he said.
The findings confirm “that poor perfusion of the eye and foot are linked and can cause ischemic retinopathy leading to the development of proliferative diabetic retinopathy and vitreous hemorrhages, both serious, vision-threatening conditions,” Dr. Ramsey said.
To some extent, fewer treatments with anti-VEGF agents may account for why patients with foot ulcers have more eye complications, Dr. Ramsey added. “Additional research needs to be done to further dissect the cause and the effect, but it’s a very important finding that we need to increase awareness about,” he said.
Dr. Ramsey and Mr. Zhu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARVO 2023