User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Smartwatches able to detect very early signs of Parkinson’s
new research shows.
An analysis of wearable motion-tracking data from UK Biobank participants showed a strong correlation between reduced daytime movement over 1 week and a clinical diagnosis of PD up to 7 years later.
“Smartwatch data is easily accessible and low cost. By using this type of data, we would potentially be able to identify individuals in the very early stages of Parkinson’s disease within the general population,” lead researcher Cynthia Sandor, PhD, from Cardiff (Wales) University, said in a statement.
“We have shown here that a single week of data captured can predict events up to 7 years in the future. With these results we could develop a valuable screening tool to aid in the early detection of Parkinson’s,” she added.
“This has implications both for research, in improving recruitment into clinical trials, and in clinical practice, in allowing patients to access treatments at an earlier stage, in future when such treatments become available,” said Dr. Sandor.
The study was published online in Nature Medicine.
Novel biomarker for PD
Using machine learning, the researchers analyzed accelerometry data from 103,712 UK Biobank participants who wore a medical-grade smartwatch for a 7-day period during 2013-2016.
At the time of or within 2 years after accelerometry data collection, 273 participants were diagnosed with PD. An additional 196 individuals received a new PD diagnosis more than 2 years after accelerometry data collection (the prodromal group).
The patients with prodromal symptoms of PD and those who were diagnosed with PD showed a significantly reduced daytime acceleration profile up to 7 years before diagnosis, compared with age- and sex-matched healthy control persons, the researchers found.
The reduction in acceleration both before and following diagnosis was unique to patients with PD, “suggesting this measure to be disease specific with potential for use in early identification of individuals likely to be diagnosed with PD,” they wrote.
Accelerometry data proved more accurate than other risk factors (lifestyle, genetics, blood chemistry) or recognized prodromal symptoms of PD in predicting whether an individual would develop PD.
“Our results suggest that accelerometry collected with wearable devices in the general population could be used to identify those at elevated risk for PD on an unprecedented scale and, importantly, individuals who will likely convert within the next few years can be included in studies for neuroprotective treatments,” the researchers conclude in their article.
High-quality research
In a statement from the U.K.-based nonprofit Science Media Centre, José López Barneo, MD, PhD, with the University of Seville (Spain), said this “good quality” study “fits well with current knowledge.”
Dr. Barneo noted that other investigators have also observed that slowness of movement is a characteristic feature of some people who subsequently develop PD.
But these studies involved preselected cohorts of persons at risk of developing PD, or they were carried out in a hospital that required healthcare staff to conduct the movement analysis. In contrast, the current study was conducted in a very large cohort from the general U.K. population.
Also weighing in, José Luis Lanciego, MD, PhD, with the University of Navarra (Spain), said the “main value of this study is that it has demonstrated that accelerometry measurements obtained using wearable devices (such as a smartwatch or other similar devices) are more useful than the assessment of any other potentially prodromal symptom in identifying which people in the [general] population are at increased risk of developing Parkinson’s disease in the future, as well as being able to estimate how many years it will take to start suffering from this neurodegenerative process.
“In these diseases, early diagnosis is to some extent questionable, as early diagnosis is of little use if neuroprotective treatment is not available,” Dr. Lanciego noted.
“However, it is of great importance for use in clinical trials aimed at evaluating the efficacy of new potentially neuroprotective treatments whose main objective is to slow down – and, ideally, even halt ― the clinical progression that typically characterizes Parkinson’s disease,” Dr. Lanciego added.
The study was funded by the UK Dementia Research Institute, the Welsh government, and Cardiff University. Dr. Sandor, Dr. Barneo, and Dr. Lanciego have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
new research shows.
An analysis of wearable motion-tracking data from UK Biobank participants showed a strong correlation between reduced daytime movement over 1 week and a clinical diagnosis of PD up to 7 years later.
“Smartwatch data is easily accessible and low cost. By using this type of data, we would potentially be able to identify individuals in the very early stages of Parkinson’s disease within the general population,” lead researcher Cynthia Sandor, PhD, from Cardiff (Wales) University, said in a statement.
“We have shown here that a single week of data captured can predict events up to 7 years in the future. With these results we could develop a valuable screening tool to aid in the early detection of Parkinson’s,” she added.
“This has implications both for research, in improving recruitment into clinical trials, and in clinical practice, in allowing patients to access treatments at an earlier stage, in future when such treatments become available,” said Dr. Sandor.
The study was published online in Nature Medicine.
Novel biomarker for PD
Using machine learning, the researchers analyzed accelerometry data from 103,712 UK Biobank participants who wore a medical-grade smartwatch for a 7-day period during 2013-2016.
At the time of or within 2 years after accelerometry data collection, 273 participants were diagnosed with PD. An additional 196 individuals received a new PD diagnosis more than 2 years after accelerometry data collection (the prodromal group).
The patients with prodromal symptoms of PD and those who were diagnosed with PD showed a significantly reduced daytime acceleration profile up to 7 years before diagnosis, compared with age- and sex-matched healthy control persons, the researchers found.
The reduction in acceleration both before and following diagnosis was unique to patients with PD, “suggesting this measure to be disease specific with potential for use in early identification of individuals likely to be diagnosed with PD,” they wrote.
Accelerometry data proved more accurate than other risk factors (lifestyle, genetics, blood chemistry) or recognized prodromal symptoms of PD in predicting whether an individual would develop PD.
“Our results suggest that accelerometry collected with wearable devices in the general population could be used to identify those at elevated risk for PD on an unprecedented scale and, importantly, individuals who will likely convert within the next few years can be included in studies for neuroprotective treatments,” the researchers conclude in their article.
High-quality research
In a statement from the U.K.-based nonprofit Science Media Centre, José López Barneo, MD, PhD, with the University of Seville (Spain), said this “good quality” study “fits well with current knowledge.”
Dr. Barneo noted that other investigators have also observed that slowness of movement is a characteristic feature of some people who subsequently develop PD.
But these studies involved preselected cohorts of persons at risk of developing PD, or they were carried out in a hospital that required healthcare staff to conduct the movement analysis. In contrast, the current study was conducted in a very large cohort from the general U.K. population.
Also weighing in, José Luis Lanciego, MD, PhD, with the University of Navarra (Spain), said the “main value of this study is that it has demonstrated that accelerometry measurements obtained using wearable devices (such as a smartwatch or other similar devices) are more useful than the assessment of any other potentially prodromal symptom in identifying which people in the [general] population are at increased risk of developing Parkinson’s disease in the future, as well as being able to estimate how many years it will take to start suffering from this neurodegenerative process.
“In these diseases, early diagnosis is to some extent questionable, as early diagnosis is of little use if neuroprotective treatment is not available,” Dr. Lanciego noted.
“However, it is of great importance for use in clinical trials aimed at evaluating the efficacy of new potentially neuroprotective treatments whose main objective is to slow down – and, ideally, even halt ― the clinical progression that typically characterizes Parkinson’s disease,” Dr. Lanciego added.
The study was funded by the UK Dementia Research Institute, the Welsh government, and Cardiff University. Dr. Sandor, Dr. Barneo, and Dr. Lanciego have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
new research shows.
An analysis of wearable motion-tracking data from UK Biobank participants showed a strong correlation between reduced daytime movement over 1 week and a clinical diagnosis of PD up to 7 years later.
“Smartwatch data is easily accessible and low cost. By using this type of data, we would potentially be able to identify individuals in the very early stages of Parkinson’s disease within the general population,” lead researcher Cynthia Sandor, PhD, from Cardiff (Wales) University, said in a statement.
“We have shown here that a single week of data captured can predict events up to 7 years in the future. With these results we could develop a valuable screening tool to aid in the early detection of Parkinson’s,” she added.
“This has implications both for research, in improving recruitment into clinical trials, and in clinical practice, in allowing patients to access treatments at an earlier stage, in future when such treatments become available,” said Dr. Sandor.
The study was published online in Nature Medicine.
Novel biomarker for PD
Using machine learning, the researchers analyzed accelerometry data from 103,712 UK Biobank participants who wore a medical-grade smartwatch for a 7-day period during 2013-2016.
At the time of or within 2 years after accelerometry data collection, 273 participants were diagnosed with PD. An additional 196 individuals received a new PD diagnosis more than 2 years after accelerometry data collection (the prodromal group).
The patients with prodromal symptoms of PD and those who were diagnosed with PD showed a significantly reduced daytime acceleration profile up to 7 years before diagnosis, compared with age- and sex-matched healthy control persons, the researchers found.
The reduction in acceleration both before and following diagnosis was unique to patients with PD, “suggesting this measure to be disease specific with potential for use in early identification of individuals likely to be diagnosed with PD,” they wrote.
Accelerometry data proved more accurate than other risk factors (lifestyle, genetics, blood chemistry) or recognized prodromal symptoms of PD in predicting whether an individual would develop PD.
“Our results suggest that accelerometry collected with wearable devices in the general population could be used to identify those at elevated risk for PD on an unprecedented scale and, importantly, individuals who will likely convert within the next few years can be included in studies for neuroprotective treatments,” the researchers conclude in their article.
High-quality research
In a statement from the U.K.-based nonprofit Science Media Centre, José López Barneo, MD, PhD, with the University of Seville (Spain), said this “good quality” study “fits well with current knowledge.”
Dr. Barneo noted that other investigators have also observed that slowness of movement is a characteristic feature of some people who subsequently develop PD.
But these studies involved preselected cohorts of persons at risk of developing PD, or they were carried out in a hospital that required healthcare staff to conduct the movement analysis. In contrast, the current study was conducted in a very large cohort from the general U.K. population.
Also weighing in, José Luis Lanciego, MD, PhD, with the University of Navarra (Spain), said the “main value of this study is that it has demonstrated that accelerometry measurements obtained using wearable devices (such as a smartwatch or other similar devices) are more useful than the assessment of any other potentially prodromal symptom in identifying which people in the [general] population are at increased risk of developing Parkinson’s disease in the future, as well as being able to estimate how many years it will take to start suffering from this neurodegenerative process.
“In these diseases, early diagnosis is to some extent questionable, as early diagnosis is of little use if neuroprotective treatment is not available,” Dr. Lanciego noted.
“However, it is of great importance for use in clinical trials aimed at evaluating the efficacy of new potentially neuroprotective treatments whose main objective is to slow down – and, ideally, even halt ― the clinical progression that typically characterizes Parkinson’s disease,” Dr. Lanciego added.
The study was funded by the UK Dementia Research Institute, the Welsh government, and Cardiff University. Dr. Sandor, Dr. Barneo, and Dr. Lanciego have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
FROM NATURE MEDICINE
Nearly one in five in U.S. still hadn’t gotten COVID by end of 2022
, according to a new estimate.
The findings came from an analysis of blood donations. The Centers for Disease Control and Prevention analyzed donor blood from 143,000 people every 3 months during 2022, looking for the presence of COVID antibodies that meant a person had previously been infected with the virus. The prevalence of antibodies from previous infections steadily rose throughout the year. Antibodies from prior infection were found in 49% of donors as of Feb. 15, 2022, 59% of donors as of May 15, 2022, 70% of donors as of Aug. 15, 2022, and 78% of donors as of Nov. 15, 2022.
Donor blood also was analyzed for the presence of antibodies known to come from COVID vaccination. When the vaccine-induced and infection-induced antibody data were combined, the CDC estimated that 97% of people had antibodies as of the end of the 2022.
In the report, CDC authors explained that while the presence of antibodies is related to protection from infection and to less severe disease, the level of antibodies that a person has can vary. The authors said that no standards have yet been set that show a minimum level of antibodies needed to provide protection.
As of July 3, more than 1.1 million people had died in the United States from COVID-19, according to CDC data. Deaths for the first half of 2023 are down dramatically, compared with the first 3 years of the pandemic, with just 41,538 death certificates this year listing the virus as an underlying or contributing cause. About two in three COVID deaths this year occurred in a hospital or nursing home, and 89% of people who died from the virus this year have been age 65 or older.
A version of this article first appeared on WebMD.com.
, according to a new estimate.
The findings came from an analysis of blood donations. The Centers for Disease Control and Prevention analyzed donor blood from 143,000 people every 3 months during 2022, looking for the presence of COVID antibodies that meant a person had previously been infected with the virus. The prevalence of antibodies from previous infections steadily rose throughout the year. Antibodies from prior infection were found in 49% of donors as of Feb. 15, 2022, 59% of donors as of May 15, 2022, 70% of donors as of Aug. 15, 2022, and 78% of donors as of Nov. 15, 2022.
Donor blood also was analyzed for the presence of antibodies known to come from COVID vaccination. When the vaccine-induced and infection-induced antibody data were combined, the CDC estimated that 97% of people had antibodies as of the end of the 2022.
In the report, CDC authors explained that while the presence of antibodies is related to protection from infection and to less severe disease, the level of antibodies that a person has can vary. The authors said that no standards have yet been set that show a minimum level of antibodies needed to provide protection.
As of July 3, more than 1.1 million people had died in the United States from COVID-19, according to CDC data. Deaths for the first half of 2023 are down dramatically, compared with the first 3 years of the pandemic, with just 41,538 death certificates this year listing the virus as an underlying or contributing cause. About two in three COVID deaths this year occurred in a hospital or nursing home, and 89% of people who died from the virus this year have been age 65 or older.
A version of this article first appeared on WebMD.com.
, according to a new estimate.
The findings came from an analysis of blood donations. The Centers for Disease Control and Prevention analyzed donor blood from 143,000 people every 3 months during 2022, looking for the presence of COVID antibodies that meant a person had previously been infected with the virus. The prevalence of antibodies from previous infections steadily rose throughout the year. Antibodies from prior infection were found in 49% of donors as of Feb. 15, 2022, 59% of donors as of May 15, 2022, 70% of donors as of Aug. 15, 2022, and 78% of donors as of Nov. 15, 2022.
Donor blood also was analyzed for the presence of antibodies known to come from COVID vaccination. When the vaccine-induced and infection-induced antibody data were combined, the CDC estimated that 97% of people had antibodies as of the end of the 2022.
In the report, CDC authors explained that while the presence of antibodies is related to protection from infection and to less severe disease, the level of antibodies that a person has can vary. The authors said that no standards have yet been set that show a minimum level of antibodies needed to provide protection.
As of July 3, more than 1.1 million people had died in the United States from COVID-19, according to CDC data. Deaths for the first half of 2023 are down dramatically, compared with the first 3 years of the pandemic, with just 41,538 death certificates this year listing the virus as an underlying or contributing cause. About two in three COVID deaths this year occurred in a hospital or nursing home, and 89% of people who died from the virus this year have been age 65 or older.
A version of this article first appeared on WebMD.com.
Coffee’s brain-boosting effect goes beyond caffeine
“There is a widespread anticipation that coffee boosts alertness and psychomotor performance. By gaining a deeper understanding of the mechanisms underlying this biological phenomenon, we pave the way for investigating the factors that can influence it and even exploring the potential advantages of those mechanisms,” study investigator Nuno Sousa, MD, PhD, with the University of Minho, Braga, Portugal, said in a statement.
The study was published online in Frontiers in Behavioral Neuroscience.
Caffeine can’t take all the credit
Certain compounds in coffee, including caffeine and chlorogenic acids, have well-documented psychoactive effects, but the psychological impact of coffee/caffeine consumption as a whole remains a matter of debate.
The researchers investigated the neurobiological impact of coffee drinking on brain connectivity using resting-state functional MRI (fMRI).
They recruited 47 generally healthy adults (mean age, 30 years; 31 women) who regularly drank a minimum of one cup of coffee per day. Participants refrained from eating or drinking caffeinated beverages for at least 3 hours prior to undergoing fMRI.
To tease out the specific impact of caffeinated coffee intake, 30 habitual coffee drinkers (mean age, 32 years; 27 women) were given hot water containing the same amount of caffeine, but they were not given coffee.
The investigators conducted two fMRI scans – one before, and one 30 minutes after drinking coffee or caffeine-infused water.
Both drinking coffee and drinking plain caffeine in water led to a decrease in functional connectivity of the brain’s default mode network, which is typically active during self-reflection in resting states.
This finding suggests that consuming either coffee or caffeine heightened individuals’ readiness to transition from a state of rest to engaging in task-related activities, the researchers noted.
However, drinking a cup of coffee also boosted connectivity in the higher visual network and the right executive control network, which are linked to working memory, cognitive control, and goal-directed behavior – something that did not occur from drinking caffeinated water.
“Put simply, individuals exhibited a heightened state of preparedness, being more responsive and attentive to external stimuli after drinking coffee,” said first author Maria Picó-Pérez, PhD, with the University of Minho.
Given that some of the effects of coffee also occurred with caffeine alone, it’s “plausible to assume that other caffeinated beverages may share similar effects,” she added.
Still, certain effects were specific to coffee drinking, “likely influenced by factors such as the distinct aroma and taste of coffee or the psychological expectations associated with consuming this particular beverage,” the researcher wrote.
The investigators report that the observations could provide a scientific foundation for the common belief that coffee increases alertness and cognitive functioning. Further research is needed to differentiate the effects of caffeine from the overall experience of drinking coffee.
A limitation of the study is the absence of a nondrinker control sample (to rule out the withdrawal effect) or an alternative group that consumed decaffeinated coffee (to rule out the placebo effect of coffee intake) – something that should be considered in future studies, the researchers noted.
The study was funded by the Institute for the Scientific Information on Coffee. The authors declared no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
“There is a widespread anticipation that coffee boosts alertness and psychomotor performance. By gaining a deeper understanding of the mechanisms underlying this biological phenomenon, we pave the way for investigating the factors that can influence it and even exploring the potential advantages of those mechanisms,” study investigator Nuno Sousa, MD, PhD, with the University of Minho, Braga, Portugal, said in a statement.
The study was published online in Frontiers in Behavioral Neuroscience.
Caffeine can’t take all the credit
Certain compounds in coffee, including caffeine and chlorogenic acids, have well-documented psychoactive effects, but the psychological impact of coffee/caffeine consumption as a whole remains a matter of debate.
The researchers investigated the neurobiological impact of coffee drinking on brain connectivity using resting-state functional MRI (fMRI).
They recruited 47 generally healthy adults (mean age, 30 years; 31 women) who regularly drank a minimum of one cup of coffee per day. Participants refrained from eating or drinking caffeinated beverages for at least 3 hours prior to undergoing fMRI.
To tease out the specific impact of caffeinated coffee intake, 30 habitual coffee drinkers (mean age, 32 years; 27 women) were given hot water containing the same amount of caffeine, but they were not given coffee.
The investigators conducted two fMRI scans – one before, and one 30 minutes after drinking coffee or caffeine-infused water.
Both drinking coffee and drinking plain caffeine in water led to a decrease in functional connectivity of the brain’s default mode network, which is typically active during self-reflection in resting states.
This finding suggests that consuming either coffee or caffeine heightened individuals’ readiness to transition from a state of rest to engaging in task-related activities, the researchers noted.
However, drinking a cup of coffee also boosted connectivity in the higher visual network and the right executive control network, which are linked to working memory, cognitive control, and goal-directed behavior – something that did not occur from drinking caffeinated water.
“Put simply, individuals exhibited a heightened state of preparedness, being more responsive and attentive to external stimuli after drinking coffee,” said first author Maria Picó-Pérez, PhD, with the University of Minho.
Given that some of the effects of coffee also occurred with caffeine alone, it’s “plausible to assume that other caffeinated beverages may share similar effects,” she added.
Still, certain effects were specific to coffee drinking, “likely influenced by factors such as the distinct aroma and taste of coffee or the psychological expectations associated with consuming this particular beverage,” the researcher wrote.
The investigators report that the observations could provide a scientific foundation for the common belief that coffee increases alertness and cognitive functioning. Further research is needed to differentiate the effects of caffeine from the overall experience of drinking coffee.
A limitation of the study is the absence of a nondrinker control sample (to rule out the withdrawal effect) or an alternative group that consumed decaffeinated coffee (to rule out the placebo effect of coffee intake) – something that should be considered in future studies, the researchers noted.
The study was funded by the Institute for the Scientific Information on Coffee. The authors declared no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
“There is a widespread anticipation that coffee boosts alertness and psychomotor performance. By gaining a deeper understanding of the mechanisms underlying this biological phenomenon, we pave the way for investigating the factors that can influence it and even exploring the potential advantages of those mechanisms,” study investigator Nuno Sousa, MD, PhD, with the University of Minho, Braga, Portugal, said in a statement.
The study was published online in Frontiers in Behavioral Neuroscience.
Caffeine can’t take all the credit
Certain compounds in coffee, including caffeine and chlorogenic acids, have well-documented psychoactive effects, but the psychological impact of coffee/caffeine consumption as a whole remains a matter of debate.
The researchers investigated the neurobiological impact of coffee drinking on brain connectivity using resting-state functional MRI (fMRI).
They recruited 47 generally healthy adults (mean age, 30 years; 31 women) who regularly drank a minimum of one cup of coffee per day. Participants refrained from eating or drinking caffeinated beverages for at least 3 hours prior to undergoing fMRI.
To tease out the specific impact of caffeinated coffee intake, 30 habitual coffee drinkers (mean age, 32 years; 27 women) were given hot water containing the same amount of caffeine, but they were not given coffee.
The investigators conducted two fMRI scans – one before, and one 30 minutes after drinking coffee or caffeine-infused water.
Both drinking coffee and drinking plain caffeine in water led to a decrease in functional connectivity of the brain’s default mode network, which is typically active during self-reflection in resting states.
This finding suggests that consuming either coffee or caffeine heightened individuals’ readiness to transition from a state of rest to engaging in task-related activities, the researchers noted.
However, drinking a cup of coffee also boosted connectivity in the higher visual network and the right executive control network, which are linked to working memory, cognitive control, and goal-directed behavior – something that did not occur from drinking caffeinated water.
“Put simply, individuals exhibited a heightened state of preparedness, being more responsive and attentive to external stimuli after drinking coffee,” said first author Maria Picó-Pérez, PhD, with the University of Minho.
Given that some of the effects of coffee also occurred with caffeine alone, it’s “plausible to assume that other caffeinated beverages may share similar effects,” she added.
Still, certain effects were specific to coffee drinking, “likely influenced by factors such as the distinct aroma and taste of coffee or the psychological expectations associated with consuming this particular beverage,” the researcher wrote.
The investigators report that the observations could provide a scientific foundation for the common belief that coffee increases alertness and cognitive functioning. Further research is needed to differentiate the effects of caffeine from the overall experience of drinking coffee.
A limitation of the study is the absence of a nondrinker control sample (to rule out the withdrawal effect) or an alternative group that consumed decaffeinated coffee (to rule out the placebo effect of coffee intake) – something that should be considered in future studies, the researchers noted.
The study was funded by the Institute for the Scientific Information on Coffee. The authors declared no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
FROM FRONTIERS IN BEHAVIORAL NEUROSCIENCE
Long COVID ‘brain fog’ confounds doctors, but new research offers hope
Kate Whitley was petrified of COVID-19 from the beginning of the pandemic because she has Hashimoto disease, an autoimmune disorder that she knew put her at high risk for complications.
She was right to be worried. Two months after contracting the infection in September 2022, the 42-year-old Nashville resident was diagnosed with long COVID. For Ms. Whitley, the resulting brain fog has been the most challenging factor. She is the owner of a successful paper goods store, and she can’t remember basic aspects of her job. She can’t tolerate loud noises and gets so distracted that she has trouble remembering what she was doing.
Ms. Whitley doesn’t like the term “brain fog” because it doesn’t begin to describe the dramatic disruption to her life over the past 7 months.
Brain fog is among the most common symptoms of long COVID, and also one of the most poorly understood. A reported 46% of those diagnosed with long COVID complain of brain fog or a loss of memory. Many clinicians agree that the term is vague and often doesn’t truly represent the condition. That, in turn, makes it harder for doctors to diagnose and treat it. There are no standard tests for it, nor are there guidelines for symptom management or treatment.
“There’s a lot of imprecision in the term because it might mean different things to different patients,” said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University, Nashville, Tenn., and author of a new book, “Clearing the Fog: From Surviving to Thriving With Long COVID – A Practical Guide.”
Dr. Jackson, who began treating Ms. Whitley in February 2023, said that it makes more sense to call brain fog a brain impairment or an acquired brain injury (ABI) because it doesn’t occur gradually. COVID damages the brain and causes injury. For those with long COVID who were previously in the intensive care unit and may have undergone ventilation, hypoxic brain injury may result from the lack of oxygen to the brain.
Even among those with milder cases of acute COVID, there’s some evidence that persistent neuroinflammation in the brain caused by an activated immune system may also cause damage.
In both cases, the results can be debilitating. Ms. Whitley also has dysautonomia – a disorder of the autonomic nervous system that can cause dizziness, sweating, and headaches along with fatigue and heart palpitations.
She said that she’s so forgetful that when she sees people socially, she’s nervous of what she’ll say. “I feel like I’m constantly sticking my foot in my mouth because I can’t remember details of other people’s lives,” she said.
Although brain disorders such as Alzheimer’s disease and other forms of dementia are marked by a slow decline, ABI occurs more suddenly and may include a loss of executive function and attention.
“With a brain injury, you’re doing fine, and then some event happens (in this case COVID), and immediately after that, your cognitive function is different,” said Dr. Jackson.
Additionally, ABI is an actual diagnosis, whereas brain fog is not.
“With a brain injury, there’s a treatment pathway for cognitive rehabilitation,” said Dr. Jackson.
Treatments may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for treatment of the mental and behavioral disorders that may result. Dr. Jackson said that while many patients aren’t functioning cognitively or physically at 100%, they can make enough strides that they don’t have to give up things such as driving and, in some cases, their jobs.
Other experts agree that long COVID may damage the brain. An April 2022 study published in the journal Nature found strong evidence that SARS-CoV-2 infection may cause brain-related abnormalities, for example, a reduction in gray matter in certain parts of the brain, including the prefrontal cortex, hypothalamus, and amygdala.
Additionally, white matter, which is found deeper in the brain and is responsible for the exchange of information between different parts of the brain, may also be at risk of damage as a result of the virus, according to a November 2022 study published in the journal SN Comprehensive Clinical Medicine.
Calling it a “fog” makes it easier for clinicians and the general public to dismiss its severity, said Tyler Reed Bell, PhD, a researcher who specializes in viruses that cause brain injury. He is a fellow in the department of psychiatry at the University of California, San Diego. Brain fog can make driving and returning to work especially dangerous. Because of difficulty focusing, patients are much more likely to make mistakes that cause accidents.
“The COVID virus is very invasive to the brain,” Dr. Bell said.
Others contend this may be a rush to judgment. Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina at Chapel Hill’s COVID Recovery Clinic, agrees that in more serious cases of COVID that cause a lack of oxygen to the brain, it’s reasonable to call it a brain injury. But brain fog can also be associated with other long COVID symptoms, not just damage to the brain.
Chronic fatigue and poor sleep are both commonly reported symptoms of long COVID that negatively affect brain function, she said. Sleep disturbances, cardiac problems, dysautonomia, and emotional distress could also affect the way the brain functions post COVID. Finding the right treatment requires identifying all the factors contributing to cognitive impairment.
Part of the problem in treating long COVID brain fog is that diagnostic technology is not sensitive enough to detect inflammation that could be causing damage.
Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, said her team is working on identifying biomarkers that could detect brain inflammation in a way similar to the manner researchers have identified biomarkers to help diagnose chronic fatigue syndrome. Additionally, a new study published last month in JAMA for the first time clearly defined 12 symptoms of long COVID, and brain fog was listed among them. All of this contributes to the development of clear diagnostic criteria.
“It will make a big difference once we have some consistency among clinicians in diagnosing the condition,” said Dr. McComsey.
Ms. Whitley is thankful for the treatment that she’s received thus far. She’s seeing a cognitive rehabilitation therapist, who assesses her memory, cognition, and attention span and gives her tools to break up simple tasks, such as driving, so that they don’t feel overwhelming. She’s back behind the wheel and back to work.
But perhaps most importantly, Ms. Whitley joined a support group, led by Dr. Jackson, that includes other people experiencing the same symptoms she is. When she was at her darkest, they understood.
“Talking to other survivors has been the only solace in all this,” Ms. Whitley said. “Together, we grieve all that’s been lost.”
A version of this article first appeared on Medscape.com.
Kate Whitley was petrified of COVID-19 from the beginning of the pandemic because she has Hashimoto disease, an autoimmune disorder that she knew put her at high risk for complications.
She was right to be worried. Two months after contracting the infection in September 2022, the 42-year-old Nashville resident was diagnosed with long COVID. For Ms. Whitley, the resulting brain fog has been the most challenging factor. She is the owner of a successful paper goods store, and she can’t remember basic aspects of her job. She can’t tolerate loud noises and gets so distracted that she has trouble remembering what she was doing.
Ms. Whitley doesn’t like the term “brain fog” because it doesn’t begin to describe the dramatic disruption to her life over the past 7 months.
Brain fog is among the most common symptoms of long COVID, and also one of the most poorly understood. A reported 46% of those diagnosed with long COVID complain of brain fog or a loss of memory. Many clinicians agree that the term is vague and often doesn’t truly represent the condition. That, in turn, makes it harder for doctors to diagnose and treat it. There are no standard tests for it, nor are there guidelines for symptom management or treatment.
“There’s a lot of imprecision in the term because it might mean different things to different patients,” said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University, Nashville, Tenn., and author of a new book, “Clearing the Fog: From Surviving to Thriving With Long COVID – A Practical Guide.”
Dr. Jackson, who began treating Ms. Whitley in February 2023, said that it makes more sense to call brain fog a brain impairment or an acquired brain injury (ABI) because it doesn’t occur gradually. COVID damages the brain and causes injury. For those with long COVID who were previously in the intensive care unit and may have undergone ventilation, hypoxic brain injury may result from the lack of oxygen to the brain.
Even among those with milder cases of acute COVID, there’s some evidence that persistent neuroinflammation in the brain caused by an activated immune system may also cause damage.
In both cases, the results can be debilitating. Ms. Whitley also has dysautonomia – a disorder of the autonomic nervous system that can cause dizziness, sweating, and headaches along with fatigue and heart palpitations.
She said that she’s so forgetful that when she sees people socially, she’s nervous of what she’ll say. “I feel like I’m constantly sticking my foot in my mouth because I can’t remember details of other people’s lives,” she said.
Although brain disorders such as Alzheimer’s disease and other forms of dementia are marked by a slow decline, ABI occurs more suddenly and may include a loss of executive function and attention.
“With a brain injury, you’re doing fine, and then some event happens (in this case COVID), and immediately after that, your cognitive function is different,” said Dr. Jackson.
Additionally, ABI is an actual diagnosis, whereas brain fog is not.
“With a brain injury, there’s a treatment pathway for cognitive rehabilitation,” said Dr. Jackson.
Treatments may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for treatment of the mental and behavioral disorders that may result. Dr. Jackson said that while many patients aren’t functioning cognitively or physically at 100%, they can make enough strides that they don’t have to give up things such as driving and, in some cases, their jobs.
Other experts agree that long COVID may damage the brain. An April 2022 study published in the journal Nature found strong evidence that SARS-CoV-2 infection may cause brain-related abnormalities, for example, a reduction in gray matter in certain parts of the brain, including the prefrontal cortex, hypothalamus, and amygdala.
Additionally, white matter, which is found deeper in the brain and is responsible for the exchange of information between different parts of the brain, may also be at risk of damage as a result of the virus, according to a November 2022 study published in the journal SN Comprehensive Clinical Medicine.
Calling it a “fog” makes it easier for clinicians and the general public to dismiss its severity, said Tyler Reed Bell, PhD, a researcher who specializes in viruses that cause brain injury. He is a fellow in the department of psychiatry at the University of California, San Diego. Brain fog can make driving and returning to work especially dangerous. Because of difficulty focusing, patients are much more likely to make mistakes that cause accidents.
“The COVID virus is very invasive to the brain,” Dr. Bell said.
Others contend this may be a rush to judgment. Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina at Chapel Hill’s COVID Recovery Clinic, agrees that in more serious cases of COVID that cause a lack of oxygen to the brain, it’s reasonable to call it a brain injury. But brain fog can also be associated with other long COVID symptoms, not just damage to the brain.
Chronic fatigue and poor sleep are both commonly reported symptoms of long COVID that negatively affect brain function, she said. Sleep disturbances, cardiac problems, dysautonomia, and emotional distress could also affect the way the brain functions post COVID. Finding the right treatment requires identifying all the factors contributing to cognitive impairment.
Part of the problem in treating long COVID brain fog is that diagnostic technology is not sensitive enough to detect inflammation that could be causing damage.
Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, said her team is working on identifying biomarkers that could detect brain inflammation in a way similar to the manner researchers have identified biomarkers to help diagnose chronic fatigue syndrome. Additionally, a new study published last month in JAMA for the first time clearly defined 12 symptoms of long COVID, and brain fog was listed among them. All of this contributes to the development of clear diagnostic criteria.
“It will make a big difference once we have some consistency among clinicians in diagnosing the condition,” said Dr. McComsey.
Ms. Whitley is thankful for the treatment that she’s received thus far. She’s seeing a cognitive rehabilitation therapist, who assesses her memory, cognition, and attention span and gives her tools to break up simple tasks, such as driving, so that they don’t feel overwhelming. She’s back behind the wheel and back to work.
But perhaps most importantly, Ms. Whitley joined a support group, led by Dr. Jackson, that includes other people experiencing the same symptoms she is. When she was at her darkest, they understood.
“Talking to other survivors has been the only solace in all this,” Ms. Whitley said. “Together, we grieve all that’s been lost.”
A version of this article first appeared on Medscape.com.
Kate Whitley was petrified of COVID-19 from the beginning of the pandemic because she has Hashimoto disease, an autoimmune disorder that she knew put her at high risk for complications.
She was right to be worried. Two months after contracting the infection in September 2022, the 42-year-old Nashville resident was diagnosed with long COVID. For Ms. Whitley, the resulting brain fog has been the most challenging factor. She is the owner of a successful paper goods store, and she can’t remember basic aspects of her job. She can’t tolerate loud noises and gets so distracted that she has trouble remembering what she was doing.
Ms. Whitley doesn’t like the term “brain fog” because it doesn’t begin to describe the dramatic disruption to her life over the past 7 months.
Brain fog is among the most common symptoms of long COVID, and also one of the most poorly understood. A reported 46% of those diagnosed with long COVID complain of brain fog or a loss of memory. Many clinicians agree that the term is vague and often doesn’t truly represent the condition. That, in turn, makes it harder for doctors to diagnose and treat it. There are no standard tests for it, nor are there guidelines for symptom management or treatment.
“There’s a lot of imprecision in the term because it might mean different things to different patients,” said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University, Nashville, Tenn., and author of a new book, “Clearing the Fog: From Surviving to Thriving With Long COVID – A Practical Guide.”
Dr. Jackson, who began treating Ms. Whitley in February 2023, said that it makes more sense to call brain fog a brain impairment or an acquired brain injury (ABI) because it doesn’t occur gradually. COVID damages the brain and causes injury. For those with long COVID who were previously in the intensive care unit and may have undergone ventilation, hypoxic brain injury may result from the lack of oxygen to the brain.
Even among those with milder cases of acute COVID, there’s some evidence that persistent neuroinflammation in the brain caused by an activated immune system may also cause damage.
In both cases, the results can be debilitating. Ms. Whitley also has dysautonomia – a disorder of the autonomic nervous system that can cause dizziness, sweating, and headaches along with fatigue and heart palpitations.
She said that she’s so forgetful that when she sees people socially, she’s nervous of what she’ll say. “I feel like I’m constantly sticking my foot in my mouth because I can’t remember details of other people’s lives,” she said.
Although brain disorders such as Alzheimer’s disease and other forms of dementia are marked by a slow decline, ABI occurs more suddenly and may include a loss of executive function and attention.
“With a brain injury, you’re doing fine, and then some event happens (in this case COVID), and immediately after that, your cognitive function is different,” said Dr. Jackson.
Additionally, ABI is an actual diagnosis, whereas brain fog is not.
“With a brain injury, there’s a treatment pathway for cognitive rehabilitation,” said Dr. Jackson.
Treatments may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for treatment of the mental and behavioral disorders that may result. Dr. Jackson said that while many patients aren’t functioning cognitively or physically at 100%, they can make enough strides that they don’t have to give up things such as driving and, in some cases, their jobs.
Other experts agree that long COVID may damage the brain. An April 2022 study published in the journal Nature found strong evidence that SARS-CoV-2 infection may cause brain-related abnormalities, for example, a reduction in gray matter in certain parts of the brain, including the prefrontal cortex, hypothalamus, and amygdala.
Additionally, white matter, which is found deeper in the brain and is responsible for the exchange of information between different parts of the brain, may also be at risk of damage as a result of the virus, according to a November 2022 study published in the journal SN Comprehensive Clinical Medicine.
Calling it a “fog” makes it easier for clinicians and the general public to dismiss its severity, said Tyler Reed Bell, PhD, a researcher who specializes in viruses that cause brain injury. He is a fellow in the department of psychiatry at the University of California, San Diego. Brain fog can make driving and returning to work especially dangerous. Because of difficulty focusing, patients are much more likely to make mistakes that cause accidents.
“The COVID virus is very invasive to the brain,” Dr. Bell said.
Others contend this may be a rush to judgment. Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina at Chapel Hill’s COVID Recovery Clinic, agrees that in more serious cases of COVID that cause a lack of oxygen to the brain, it’s reasonable to call it a brain injury. But brain fog can also be associated with other long COVID symptoms, not just damage to the brain.
Chronic fatigue and poor sleep are both commonly reported symptoms of long COVID that negatively affect brain function, she said. Sleep disturbances, cardiac problems, dysautonomia, and emotional distress could also affect the way the brain functions post COVID. Finding the right treatment requires identifying all the factors contributing to cognitive impairment.
Part of the problem in treating long COVID brain fog is that diagnostic technology is not sensitive enough to detect inflammation that could be causing damage.
Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, said her team is working on identifying biomarkers that could detect brain inflammation in a way similar to the manner researchers have identified biomarkers to help diagnose chronic fatigue syndrome. Additionally, a new study published last month in JAMA for the first time clearly defined 12 symptoms of long COVID, and brain fog was listed among them. All of this contributes to the development of clear diagnostic criteria.
“It will make a big difference once we have some consistency among clinicians in diagnosing the condition,” said Dr. McComsey.
Ms. Whitley is thankful for the treatment that she’s received thus far. She’s seeing a cognitive rehabilitation therapist, who assesses her memory, cognition, and attention span and gives her tools to break up simple tasks, such as driving, so that they don’t feel overwhelming. She’s back behind the wheel and back to work.
But perhaps most importantly, Ms. Whitley joined a support group, led by Dr. Jackson, that includes other people experiencing the same symptoms she is. When she was at her darkest, they understood.
“Talking to other survivors has been the only solace in all this,” Ms. Whitley said. “Together, we grieve all that’s been lost.”
A version of this article first appeared on Medscape.com.
More than 30 experts question validity of serotonin/depression study
The authors of the article, however, stand by their conclusion.
“The methodology doesn’t conform to a conventional umbrella review,” said the commentary’s lead author, Sameer Jauhar, MD, PhD, first author of the commentary criticizing the review, which was published online in Molecular Psychiatry.
In addition, preeminent psychiatrist David J. Nutt, MD, PhD, Edmond J. Safra Professor of Neuropsychopharmacology, Imperial College London, is calling for the review to be retracted. In an interview with The Daily Mail, he said the article is “full of flaws and it should never have been published in the first place. Yet it has been frequently cited and people believe it is true. It’s essentially misinformation. That’s why I’m calling on the journal to retract it.” Dr. Nutt is one of the authors of the published commentary.
‘No convincing evidence’
Led by Joanna Moncrieff, MD, professor of clinical and social psychiatry, University College London, the authors analyzed systematic reviews and meta-analyses to determine whether low serotonin levels are, in fact, associated with depression.
Of 361 potential studies, 17 were selected for the review, including meta-analyses, systematic reviews, and a genetic association study.
The review included examinations of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) in “body fluids,” 5HT1A receptor and serotonin transporter protein (SERT) availability in imaging and postmortem studies, investigations of SERT gene polymorphisms, interactions between SERT and stress in depression, and effects of tryptophan depletion on mood.
The tryptophan hypothesis suggests depression occurs through tryptophan depletion, which lowers available serotonin. According to the review, two crossover studies of patients with depression who were currently receiving or had recently received antidepressant treatment did not show substantial effects of depletion, and data from studies involving volunteers largely showed no effect.
Ultimately, Dr. Moncrieff and colleagues concluded that “there is no convincing evidence that depression is associated with, or caused by, lower serotonin concentrations or activity.”
‘Unconventional, odd’ methodology
However, Dr. Jauhar and the commentary’s coauthors disagree with the study’s conclusion. The researchers claim that “we don’t see depression symptoms in healthy volunteers when given tryptophan depletion; everyone knows that and agrees with that; it’s only in people vulnerable to depression who will have it.”
Furthermore, he said, the study’s conclusion does not consider that experimental medicine studies of tryptophan depletion are difficult to conduct. “You’re not going to have huge sample sizes as you would in a genetic study or big epidemiological studies.
Dr. Jauhar said he found it “unconventional” and “odd” that the review included individual tryptophan depletion studies that were not in the prespecified protocol.
For studies involving molecular imaging, Dr. Jauhar said the review’s inferences were “simplistic” and the review authors are “basically shaping the argument” to fit their desired narrative.
He also noted factual errors in the review. “They make a mistake when they talk about serotonin transporter imaging; they say there are no consistent findings across studies when, in fact, there are.”
With both tryptophan depletion and molecular imaging studies, the review “glosses over findings” from the original studies, said Dr. Jauhar.
For tryptophan depletion, “a more accurate, constructive conclusion would be that acute tryptophan depletion and decreased plasma tryptophan in depression indicate a role for 5-HT in those vulnerable to or suffering from depression, and that molecular imaging suggests the system is perturbed,” the commentators wrote.
“The proven efficacy of SSRIs in a proportion of people with depression lends credibility to this position,” they added.
Dr. Jauhar also took issue with criteria for certainty of finding of these and other studies used in the review. “If you’re setting the criteria yourself, it’s arbitrary.”
No new data
An umbrella review is supposed to be of the highest quality and should entail “taking out the studies and analysing them yourself,” but here, “all they have done is put a synthesis forward of other people’s reviews, so essentially there’s no new data there,” said Dr. Jauhar.
And sometimes the review’s findings differ from the original research. “When you have people who haven’t conducted original research themselves quoting someone else’s work and ignoring what those people say, we’re all in trouble,” said Dr. Jauhar.
In an additional commentary also published in Molecular Psychiatry, Jacob Pade Ramsøe Jacobsen, Evecxia Therapeutics, Durham, N.C., also criticized the review by Dr. Moncrieff and colleagues.
Its authors appear unfamiliar with serotonin biology and pharmacology, Dr. Jacobsen wrote.
“The review contains factual errors, makes conclusions serotonin neurobiology may not support, and quotes the cited literature in a selective manner,” he added.
“Most troubling, they misinterpret some data reviewed and intimate that serotonin reuptake inhibitor antidepressants, e.g., SSRIs, may decrease, rather than increase, serotonin function.”
If accepted by general practitioners and the public, the review’s conclusions “could lead to reduced use of antidepressants among patients in need and increased morbidity related to depression.”
Dr. Moncrieff pushes back
Responding to the torrent of criticism of her study, Dr. Moncrieff told this news organization via email that they stand by the review, adding that Dr. Jauhar and others “don’t want to let the cat out of the bag” that there’s no good evidence to support the hypothesis that low serotonin causes depression because it challenges antidepressant use.
“The idea that antidepressants work by correcting an underlying chemical imbalance or serotonin abnormality has led research up a blind alley and meant scientists have not taken the harmful effects of these drugs seriously enough.”
These critics, she added, “want business as usual – which means people will continue to be misinformed and exposed to harmful effects of drugs that have minimal and uncertain benefits.”
In a letter to the editor of Molecular Psychiatry, Dr. Moncrieff and her fellow authors maintain that they used approved and well-accepted methods for the umbrella review, including preregistering the protocol and using recommended search methods and quality assessments, and that they did not miss certain studies, as has been claimed.
In her blog, Dr. Moncrieff wrote that the “marginal differences between antidepressants and placebo that are apparent in clinical trials are likely to be produced by alternative, more plausible mechanisms like the emotional blunting effects of the drugs or by amplified placebo effects, rather than by targeting underlying biological mechanisms (since these have not been demonstrated).”
It also highlights “how we don’t know what antidepressants do to the brain exactly, which is a cause for concern,” she adds.
Dr. Jauhar has received honoraria for nonpromotional educational talks on antipsychotics from Janssen, Sunovion, and Lundbeck and on causes of schizophrenia for Boehringer-Ingelheim. He has also received honoraria for consulting on antipsychotics for LB Pharmaceuticals. He sits on Council for the British Association for Psychopharmacology and was a recent panel member for the Wellcome Trust.
A version of this article originally appeared on Medscape.com.
The authors of the article, however, stand by their conclusion.
“The methodology doesn’t conform to a conventional umbrella review,” said the commentary’s lead author, Sameer Jauhar, MD, PhD, first author of the commentary criticizing the review, which was published online in Molecular Psychiatry.
In addition, preeminent psychiatrist David J. Nutt, MD, PhD, Edmond J. Safra Professor of Neuropsychopharmacology, Imperial College London, is calling for the review to be retracted. In an interview with The Daily Mail, he said the article is “full of flaws and it should never have been published in the first place. Yet it has been frequently cited and people believe it is true. It’s essentially misinformation. That’s why I’m calling on the journal to retract it.” Dr. Nutt is one of the authors of the published commentary.
‘No convincing evidence’
Led by Joanna Moncrieff, MD, professor of clinical and social psychiatry, University College London, the authors analyzed systematic reviews and meta-analyses to determine whether low serotonin levels are, in fact, associated with depression.
Of 361 potential studies, 17 were selected for the review, including meta-analyses, systematic reviews, and a genetic association study.
The review included examinations of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) in “body fluids,” 5HT1A receptor and serotonin transporter protein (SERT) availability in imaging and postmortem studies, investigations of SERT gene polymorphisms, interactions between SERT and stress in depression, and effects of tryptophan depletion on mood.
The tryptophan hypothesis suggests depression occurs through tryptophan depletion, which lowers available serotonin. According to the review, two crossover studies of patients with depression who were currently receiving or had recently received antidepressant treatment did not show substantial effects of depletion, and data from studies involving volunteers largely showed no effect.
Ultimately, Dr. Moncrieff and colleagues concluded that “there is no convincing evidence that depression is associated with, or caused by, lower serotonin concentrations or activity.”
‘Unconventional, odd’ methodology
However, Dr. Jauhar and the commentary’s coauthors disagree with the study’s conclusion. The researchers claim that “we don’t see depression symptoms in healthy volunteers when given tryptophan depletion; everyone knows that and agrees with that; it’s only in people vulnerable to depression who will have it.”
Furthermore, he said, the study’s conclusion does not consider that experimental medicine studies of tryptophan depletion are difficult to conduct. “You’re not going to have huge sample sizes as you would in a genetic study or big epidemiological studies.
Dr. Jauhar said he found it “unconventional” and “odd” that the review included individual tryptophan depletion studies that were not in the prespecified protocol.
For studies involving molecular imaging, Dr. Jauhar said the review’s inferences were “simplistic” and the review authors are “basically shaping the argument” to fit their desired narrative.
He also noted factual errors in the review. “They make a mistake when they talk about serotonin transporter imaging; they say there are no consistent findings across studies when, in fact, there are.”
With both tryptophan depletion and molecular imaging studies, the review “glosses over findings” from the original studies, said Dr. Jauhar.
For tryptophan depletion, “a more accurate, constructive conclusion would be that acute tryptophan depletion and decreased plasma tryptophan in depression indicate a role for 5-HT in those vulnerable to or suffering from depression, and that molecular imaging suggests the system is perturbed,” the commentators wrote.
“The proven efficacy of SSRIs in a proportion of people with depression lends credibility to this position,” they added.
Dr. Jauhar also took issue with criteria for certainty of finding of these and other studies used in the review. “If you’re setting the criteria yourself, it’s arbitrary.”
No new data
An umbrella review is supposed to be of the highest quality and should entail “taking out the studies and analysing them yourself,” but here, “all they have done is put a synthesis forward of other people’s reviews, so essentially there’s no new data there,” said Dr. Jauhar.
And sometimes the review’s findings differ from the original research. “When you have people who haven’t conducted original research themselves quoting someone else’s work and ignoring what those people say, we’re all in trouble,” said Dr. Jauhar.
In an additional commentary also published in Molecular Psychiatry, Jacob Pade Ramsøe Jacobsen, Evecxia Therapeutics, Durham, N.C., also criticized the review by Dr. Moncrieff and colleagues.
Its authors appear unfamiliar with serotonin biology and pharmacology, Dr. Jacobsen wrote.
“The review contains factual errors, makes conclusions serotonin neurobiology may not support, and quotes the cited literature in a selective manner,” he added.
“Most troubling, they misinterpret some data reviewed and intimate that serotonin reuptake inhibitor antidepressants, e.g., SSRIs, may decrease, rather than increase, serotonin function.”
If accepted by general practitioners and the public, the review’s conclusions “could lead to reduced use of antidepressants among patients in need and increased morbidity related to depression.”
Dr. Moncrieff pushes back
Responding to the torrent of criticism of her study, Dr. Moncrieff told this news organization via email that they stand by the review, adding that Dr. Jauhar and others “don’t want to let the cat out of the bag” that there’s no good evidence to support the hypothesis that low serotonin causes depression because it challenges antidepressant use.
“The idea that antidepressants work by correcting an underlying chemical imbalance or serotonin abnormality has led research up a blind alley and meant scientists have not taken the harmful effects of these drugs seriously enough.”
These critics, she added, “want business as usual – which means people will continue to be misinformed and exposed to harmful effects of drugs that have minimal and uncertain benefits.”
In a letter to the editor of Molecular Psychiatry, Dr. Moncrieff and her fellow authors maintain that they used approved and well-accepted methods for the umbrella review, including preregistering the protocol and using recommended search methods and quality assessments, and that they did not miss certain studies, as has been claimed.
In her blog, Dr. Moncrieff wrote that the “marginal differences between antidepressants and placebo that are apparent in clinical trials are likely to be produced by alternative, more plausible mechanisms like the emotional blunting effects of the drugs or by amplified placebo effects, rather than by targeting underlying biological mechanisms (since these have not been demonstrated).”
It also highlights “how we don’t know what antidepressants do to the brain exactly, which is a cause for concern,” she adds.
Dr. Jauhar has received honoraria for nonpromotional educational talks on antipsychotics from Janssen, Sunovion, and Lundbeck and on causes of schizophrenia for Boehringer-Ingelheim. He has also received honoraria for consulting on antipsychotics for LB Pharmaceuticals. He sits on Council for the British Association for Psychopharmacology and was a recent panel member for the Wellcome Trust.
A version of this article originally appeared on Medscape.com.
The authors of the article, however, stand by their conclusion.
“The methodology doesn’t conform to a conventional umbrella review,” said the commentary’s lead author, Sameer Jauhar, MD, PhD, first author of the commentary criticizing the review, which was published online in Molecular Psychiatry.
In addition, preeminent psychiatrist David J. Nutt, MD, PhD, Edmond J. Safra Professor of Neuropsychopharmacology, Imperial College London, is calling for the review to be retracted. In an interview with The Daily Mail, he said the article is “full of flaws and it should never have been published in the first place. Yet it has been frequently cited and people believe it is true. It’s essentially misinformation. That’s why I’m calling on the journal to retract it.” Dr. Nutt is one of the authors of the published commentary.
‘No convincing evidence’
Led by Joanna Moncrieff, MD, professor of clinical and social psychiatry, University College London, the authors analyzed systematic reviews and meta-analyses to determine whether low serotonin levels are, in fact, associated with depression.
Of 361 potential studies, 17 were selected for the review, including meta-analyses, systematic reviews, and a genetic association study.
The review included examinations of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) in “body fluids,” 5HT1A receptor and serotonin transporter protein (SERT) availability in imaging and postmortem studies, investigations of SERT gene polymorphisms, interactions between SERT and stress in depression, and effects of tryptophan depletion on mood.
The tryptophan hypothesis suggests depression occurs through tryptophan depletion, which lowers available serotonin. According to the review, two crossover studies of patients with depression who were currently receiving or had recently received antidepressant treatment did not show substantial effects of depletion, and data from studies involving volunteers largely showed no effect.
Ultimately, Dr. Moncrieff and colleagues concluded that “there is no convincing evidence that depression is associated with, or caused by, lower serotonin concentrations or activity.”
‘Unconventional, odd’ methodology
However, Dr. Jauhar and the commentary’s coauthors disagree with the study’s conclusion. The researchers claim that “we don’t see depression symptoms in healthy volunteers when given tryptophan depletion; everyone knows that and agrees with that; it’s only in people vulnerable to depression who will have it.”
Furthermore, he said, the study’s conclusion does not consider that experimental medicine studies of tryptophan depletion are difficult to conduct. “You’re not going to have huge sample sizes as you would in a genetic study or big epidemiological studies.
Dr. Jauhar said he found it “unconventional” and “odd” that the review included individual tryptophan depletion studies that were not in the prespecified protocol.
For studies involving molecular imaging, Dr. Jauhar said the review’s inferences were “simplistic” and the review authors are “basically shaping the argument” to fit their desired narrative.
He also noted factual errors in the review. “They make a mistake when they talk about serotonin transporter imaging; they say there are no consistent findings across studies when, in fact, there are.”
With both tryptophan depletion and molecular imaging studies, the review “glosses over findings” from the original studies, said Dr. Jauhar.
For tryptophan depletion, “a more accurate, constructive conclusion would be that acute tryptophan depletion and decreased plasma tryptophan in depression indicate a role for 5-HT in those vulnerable to or suffering from depression, and that molecular imaging suggests the system is perturbed,” the commentators wrote.
“The proven efficacy of SSRIs in a proportion of people with depression lends credibility to this position,” they added.
Dr. Jauhar also took issue with criteria for certainty of finding of these and other studies used in the review. “If you’re setting the criteria yourself, it’s arbitrary.”
No new data
An umbrella review is supposed to be of the highest quality and should entail “taking out the studies and analysing them yourself,” but here, “all they have done is put a synthesis forward of other people’s reviews, so essentially there’s no new data there,” said Dr. Jauhar.
And sometimes the review’s findings differ from the original research. “When you have people who haven’t conducted original research themselves quoting someone else’s work and ignoring what those people say, we’re all in trouble,” said Dr. Jauhar.
In an additional commentary also published in Molecular Psychiatry, Jacob Pade Ramsøe Jacobsen, Evecxia Therapeutics, Durham, N.C., also criticized the review by Dr. Moncrieff and colleagues.
Its authors appear unfamiliar with serotonin biology and pharmacology, Dr. Jacobsen wrote.
“The review contains factual errors, makes conclusions serotonin neurobiology may not support, and quotes the cited literature in a selective manner,” he added.
“Most troubling, they misinterpret some data reviewed and intimate that serotonin reuptake inhibitor antidepressants, e.g., SSRIs, may decrease, rather than increase, serotonin function.”
If accepted by general practitioners and the public, the review’s conclusions “could lead to reduced use of antidepressants among patients in need and increased morbidity related to depression.”
Dr. Moncrieff pushes back
Responding to the torrent of criticism of her study, Dr. Moncrieff told this news organization via email that they stand by the review, adding that Dr. Jauhar and others “don’t want to let the cat out of the bag” that there’s no good evidence to support the hypothesis that low serotonin causes depression because it challenges antidepressant use.
“The idea that antidepressants work by correcting an underlying chemical imbalance or serotonin abnormality has led research up a blind alley and meant scientists have not taken the harmful effects of these drugs seriously enough.”
These critics, she added, “want business as usual – which means people will continue to be misinformed and exposed to harmful effects of drugs that have minimal and uncertain benefits.”
In a letter to the editor of Molecular Psychiatry, Dr. Moncrieff and her fellow authors maintain that they used approved and well-accepted methods for the umbrella review, including preregistering the protocol and using recommended search methods and quality assessments, and that they did not miss certain studies, as has been claimed.
In her blog, Dr. Moncrieff wrote that the “marginal differences between antidepressants and placebo that are apparent in clinical trials are likely to be produced by alternative, more plausible mechanisms like the emotional blunting effects of the drugs or by amplified placebo effects, rather than by targeting underlying biological mechanisms (since these have not been demonstrated).”
It also highlights “how we don’t know what antidepressants do to the brain exactly, which is a cause for concern,” she adds.
Dr. Jauhar has received honoraria for nonpromotional educational talks on antipsychotics from Janssen, Sunovion, and Lundbeck and on causes of schizophrenia for Boehringer-Ingelheim. He has also received honoraria for consulting on antipsychotics for LB Pharmaceuticals. He sits on Council for the British Association for Psychopharmacology and was a recent panel member for the Wellcome Trust.
A version of this article originally appeared on Medscape.com.
Agency issues advisory on mental health symptoms of long COVID
The nine mental health symptoms highlighted in the advisory are fatigue; cognitive impairment, including brain fog; anxiety; depression; obsessive-compulsive disorder; sleep disorders; PTSD; psychotic disorder; and start of a substance use disorder.
The advisory noted that social factors can contribute to the mental health problems for racial and ethnic minorities; people with limited access to health care; people who already have behavioral health conditions and physical disabilities; and people who are lesbian, gay, bisexual, transgender, queer, or intersex.
“Long COVID has a range of burdensome physical symptoms and can take a toll on a person’s mental health. It can be very challenging for a person, whether they are impacted themselves, or they are a caregiver for someone who is affected,” Health and Human Services Secretary Xavier Becerra said in a statement. “This advisory helps to raise awareness, especially among primary care practitioners and clinicians who are often the ones treating patients with long COVID.”
The department says about 10% of people infected with COVID have at least one long COVID symptom. Physical symptoms include dizziness, stomach upset, heart palpitations, issues with sexual desire or capacity, loss of smell or taste, thirst, chronic coughing, chest pain, and abnormal movements.
“We know that people living with long COVID need help today, and providers need help understanding what long COVID is and how to treat it,” Admiral Rachel Levine, MD, assistant secretary for health, said in the statement. “This advisory helps bridge that gap for the behavioral health impacts of long COVID.”
A version of this article first appeared on WebMD.com.
The nine mental health symptoms highlighted in the advisory are fatigue; cognitive impairment, including brain fog; anxiety; depression; obsessive-compulsive disorder; sleep disorders; PTSD; psychotic disorder; and start of a substance use disorder.
The advisory noted that social factors can contribute to the mental health problems for racial and ethnic minorities; people with limited access to health care; people who already have behavioral health conditions and physical disabilities; and people who are lesbian, gay, bisexual, transgender, queer, or intersex.
“Long COVID has a range of burdensome physical symptoms and can take a toll on a person’s mental health. It can be very challenging for a person, whether they are impacted themselves, or they are a caregiver for someone who is affected,” Health and Human Services Secretary Xavier Becerra said in a statement. “This advisory helps to raise awareness, especially among primary care practitioners and clinicians who are often the ones treating patients with long COVID.”
The department says about 10% of people infected with COVID have at least one long COVID symptom. Physical symptoms include dizziness, stomach upset, heart palpitations, issues with sexual desire or capacity, loss of smell or taste, thirst, chronic coughing, chest pain, and abnormal movements.
“We know that people living with long COVID need help today, and providers need help understanding what long COVID is and how to treat it,” Admiral Rachel Levine, MD, assistant secretary for health, said in the statement. “This advisory helps bridge that gap for the behavioral health impacts of long COVID.”
A version of this article first appeared on WebMD.com.
The nine mental health symptoms highlighted in the advisory are fatigue; cognitive impairment, including brain fog; anxiety; depression; obsessive-compulsive disorder; sleep disorders; PTSD; psychotic disorder; and start of a substance use disorder.
The advisory noted that social factors can contribute to the mental health problems for racial and ethnic minorities; people with limited access to health care; people who already have behavioral health conditions and physical disabilities; and people who are lesbian, gay, bisexual, transgender, queer, or intersex.
“Long COVID has a range of burdensome physical symptoms and can take a toll on a person’s mental health. It can be very challenging for a person, whether they are impacted themselves, or they are a caregiver for someone who is affected,” Health and Human Services Secretary Xavier Becerra said in a statement. “This advisory helps to raise awareness, especially among primary care practitioners and clinicians who are often the ones treating patients with long COVID.”
The department says about 10% of people infected with COVID have at least one long COVID symptom. Physical symptoms include dizziness, stomach upset, heart palpitations, issues with sexual desire or capacity, loss of smell or taste, thirst, chronic coughing, chest pain, and abnormal movements.
“We know that people living with long COVID need help today, and providers need help understanding what long COVID is and how to treat it,” Admiral Rachel Levine, MD, assistant secretary for health, said in the statement. “This advisory helps bridge that gap for the behavioral health impacts of long COVID.”
A version of this article first appeared on WebMD.com.
New DEA CME mandate affects 2 million prescribers
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.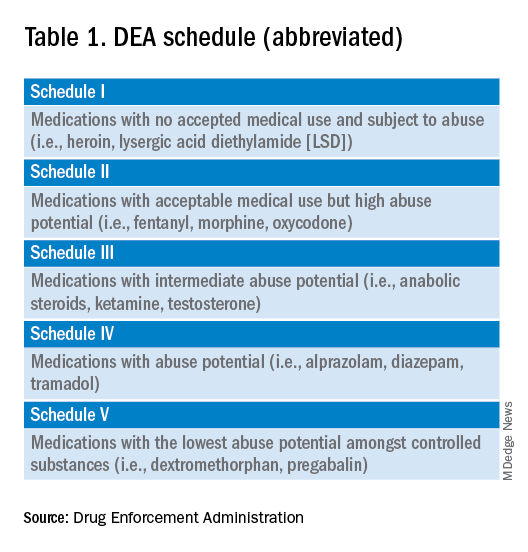
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 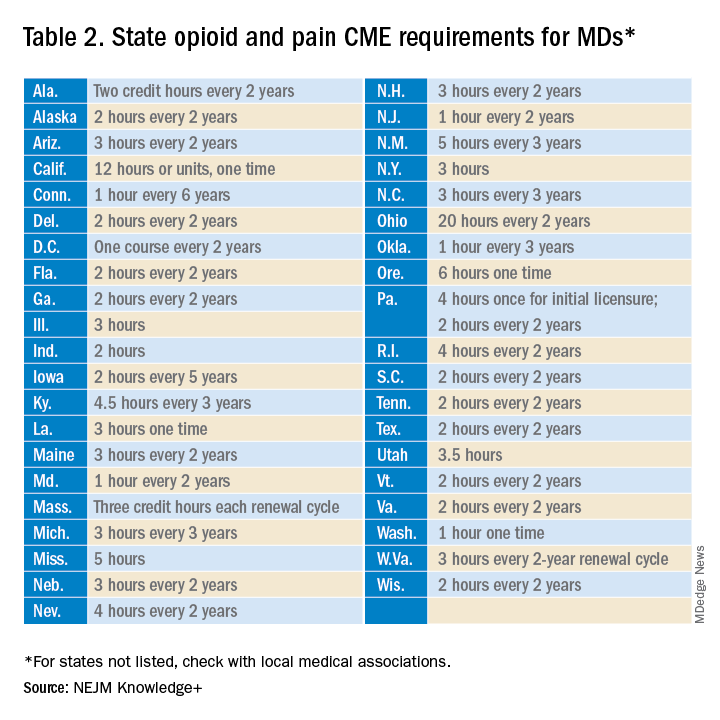
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).
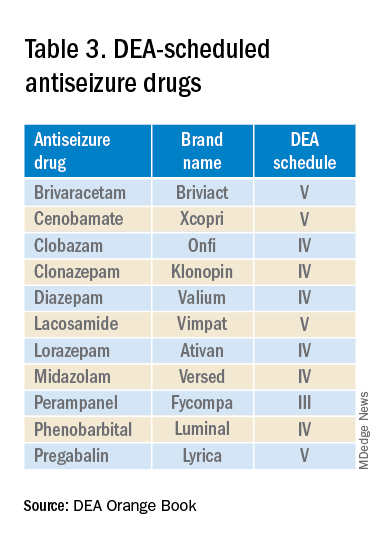
Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).

Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).

Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
HPV rates skyrocket despite safe, effective vaccine
An epidemic of sexually transmitted HPV is now swirling around the United States and the United Kingdom, with some serious cases leading to oropharyngeal cancer, which can affect the back of the throat, tonsils, and tongue.
HPV is the leading cause (70%) of this oropharyngeal cancer, according to the CDC. It is the most common sexually transmitted disease in the nation, and around 3.6% of women and 10% of men report oral HPV specifically. But over the past decade, oropharyngeal cases have been steadily falling a little under 4% and 2%, respectively, according to the National Cancer Institute.
HPV is often undetectable and can clear up within a few months. But unfortunately for some, serious disease, such as throat cancer, can develop.
Studies show the HPV vaccine to be extremely effective in lowering sexually transmitted HPV cases. Yet, only 54.5% of young people aged 13-15 have taken the recommended two to three doses, according to the National Cancer Institute.
Why aren’t more young people taking the vaccine?
Low public awareness of the dangers of HPV may be behind young people’s poor vaccination rates, according to Teresa Lee, MD, of the Fox Chase Cancer Center in Philadelphia. “For example, while the link with head and neck cancers has been well-studied, the FDA labeling was not changed to reflect this as an indication until 2020,” she said.
Other reasons can include one’s socioeconomic background, poor health literacy, cultural or religious stigmas around vaccines, and lack of quality, low-cost health care, says Emmanuel Aguh, MD, a board-certified family medicine physician. “Some individuals and families are still resistant to vaccines and the noted lack of uptake.”
Doctors and other health care professionals should also be sure to tell patients of all ages about the risks of HPV infection and how well the vaccine works, Dr. Lee said. “Not everyone who is now eligible may have been offered the vaccine as a child, and the first time young adults may receive counseling on this subject may not be until they are entering a very busy period of their lives with many responsibilities – when it may be hard to fit in things like health maintenance.”
How safe is the HPV vaccine?
The Food and Drug Administration and Centers for Disease Control and Prevention have studied the HPV vaccine for years to find out how safe it is and how well it works, Dr. Aguh said. No major side effects have been reported, and the most common side effect is soreness where you get the shot (which is normal after most vaccines). Some dizziness and fainting in adolescents can also occur, so young people are usually asked to sit or lie down during the shot and for 15 minutes afterward, he said.
“Serious adverse events have not been reported at higher rates than expected following HPV vaccination, meaning there is no clear evidence they are related to the vaccine,” Dr. Lee said. “The vaccine is highly effective in decreasing rates of detectable infection with the high-risk HPV strains responsible for HPV-associated cancers.”
The HPV vaccine is largely recommended for people aged 9-26, and sometimes up to age 45, depending on the individual, Dr. Aguh said. If you are over 26, talk to your doctor about whether you should consider getting the vaccine.
“It is usually given in two doses for complete protection if taken before the 15th birthday,” Dr. Aguh said. “If taken afterward, or in those with a weak immune system, they might require three doses to be fully protected.”
The vaccine produces antibodies that can stop HPV from infecting cells and lowers your chances of catching an HPV-related cancer, such as throat cancer or cancer of the cervix, he said.
While the vaccine is not guaranteed to protect you from the more than 100 strains of HPV, it can protect you from HPV 16 and HPV 18 – two high-risk strains that cause around 70% of cervical cancers.
What is fueling the rise of HPV cases?
A misconception that oral sex is somehow a “safe and risk-free” alternative to anal or vaginal sex could be one reason, Dr. Aguh said.
“It is important to know that, with oral sex, you are exposed to many of the risks associated with vaginal intercourse, especially if you do not take any measures to protect yourself and/or your partner,” Dr. Aguh said. “[With oral sex] it is possible to end up contracting an infection like chlamydia, gonorrhea, and even HPV, leading to an increased risk of HPV-associated oropharyngeal cancers.”
A lack of public awareness of what can cause throat cancer could also explain this phenomenon. The number of people you have oral sex with, along with the age you begin sexual activity, can greatly determine your risk of the disease, according to Dr. Lee. She echoes a report by Hisham Mehanna, PhD, in The Conversation.
“For oropharyngeal cancer, the main risk factor is the number of lifetime sexual partners, especially oral sex,” wrote Dr. Mehanna, a professor at the Institute of Cancer and Genomic Sciences at the University of Birmingham (England). “Those with six or more lifetime oral-sex partners are 8.5 times more likely to develop oropharyngeal cancer than those who do not practice oral sex.”
What are symptoms of oropharyngeal cancer?
Labored breathing or swallowing, a cough that won’t go away, and crackling or hoarseness of your voice could all be signs of throat cancer. Other symptoms include earaches, swelling of the head or neck, and enlarged lymph nodes, among others, Dr. Aguh said.
“The signs and symptoms of HPV-related throat cancers can be difficult to identify and recognize, as they can be vague and are also associated with other medical conditions. Sometimes, there are no signs at all, or they are not easily noticeable due to the location,” he said.
You should go see your doctor if you have any of these ailments for an extended period.
How to reduce your risk
In addition to having six or more oral-sex partners, smoking and drinking heavily could also raise your risk of throat cancer, said Dr. Lee. Proper dental health – like seeing your dentist regularly and practicing proper oral hygiene – can also shave your risk.
“[Good dental health] can help not just with head and neck cancer risk, but with many other inflammation-related diseases,” Dr. Lee said.
Using dental dams and condoms can also be a good method of protection, Dr. Aguh said. A dental dam is a stretchy sheet of latex, or polyurethane plastic, in the shape of a square that is made for blocking body fluid to lower your risk of contracting an STD via oral sex.
Keep in mind: Even with these protections, make sure you and your partner discuss each other’s sexual history, any prior or current STDs and their preferred protection from STDs, said Dr. Aguh.
If you or your partner is being treated for an STD, consider opting out of oral sex and consulting a doctor.
The HPV vaccine is another common method of protection. The shot is “approved for prevention of nine of the most high-risk strains of HPV,” or those that are most commonly linked to cancer, according to Dr. Lee. The vaccine “reduces the frequency of infection” with these viruses, which can ultimately lower the risk of cancers linked to HPV, including cervical, anal, and vulvar and vaginal cancers, she said.
“The best time to receive treatment for prevention of disease is prior to onset of sexual intercourse,” said Dr. Lee.
To get your HPV vaccine, head to your family doctor, school- or community-based health center, or state health department, suggests the CDC.
A version of this article originally appeared on WebMD.com.
An epidemic of sexually transmitted HPV is now swirling around the United States and the United Kingdom, with some serious cases leading to oropharyngeal cancer, which can affect the back of the throat, tonsils, and tongue.
HPV is the leading cause (70%) of this oropharyngeal cancer, according to the CDC. It is the most common sexually transmitted disease in the nation, and around 3.6% of women and 10% of men report oral HPV specifically. But over the past decade, oropharyngeal cases have been steadily falling a little under 4% and 2%, respectively, according to the National Cancer Institute.
HPV is often undetectable and can clear up within a few months. But unfortunately for some, serious disease, such as throat cancer, can develop.
Studies show the HPV vaccine to be extremely effective in lowering sexually transmitted HPV cases. Yet, only 54.5% of young people aged 13-15 have taken the recommended two to three doses, according to the National Cancer Institute.
Why aren’t more young people taking the vaccine?
Low public awareness of the dangers of HPV may be behind young people’s poor vaccination rates, according to Teresa Lee, MD, of the Fox Chase Cancer Center in Philadelphia. “For example, while the link with head and neck cancers has been well-studied, the FDA labeling was not changed to reflect this as an indication until 2020,” she said.
Other reasons can include one’s socioeconomic background, poor health literacy, cultural or religious stigmas around vaccines, and lack of quality, low-cost health care, says Emmanuel Aguh, MD, a board-certified family medicine physician. “Some individuals and families are still resistant to vaccines and the noted lack of uptake.”
Doctors and other health care professionals should also be sure to tell patients of all ages about the risks of HPV infection and how well the vaccine works, Dr. Lee said. “Not everyone who is now eligible may have been offered the vaccine as a child, and the first time young adults may receive counseling on this subject may not be until they are entering a very busy period of their lives with many responsibilities – when it may be hard to fit in things like health maintenance.”
How safe is the HPV vaccine?
The Food and Drug Administration and Centers for Disease Control and Prevention have studied the HPV vaccine for years to find out how safe it is and how well it works, Dr. Aguh said. No major side effects have been reported, and the most common side effect is soreness where you get the shot (which is normal after most vaccines). Some dizziness and fainting in adolescents can also occur, so young people are usually asked to sit or lie down during the shot and for 15 minutes afterward, he said.
“Serious adverse events have not been reported at higher rates than expected following HPV vaccination, meaning there is no clear evidence they are related to the vaccine,” Dr. Lee said. “The vaccine is highly effective in decreasing rates of detectable infection with the high-risk HPV strains responsible for HPV-associated cancers.”
The HPV vaccine is largely recommended for people aged 9-26, and sometimes up to age 45, depending on the individual, Dr. Aguh said. If you are over 26, talk to your doctor about whether you should consider getting the vaccine.
“It is usually given in two doses for complete protection if taken before the 15th birthday,” Dr. Aguh said. “If taken afterward, or in those with a weak immune system, they might require three doses to be fully protected.”
The vaccine produces antibodies that can stop HPV from infecting cells and lowers your chances of catching an HPV-related cancer, such as throat cancer or cancer of the cervix, he said.
While the vaccine is not guaranteed to protect you from the more than 100 strains of HPV, it can protect you from HPV 16 and HPV 18 – two high-risk strains that cause around 70% of cervical cancers.
What is fueling the rise of HPV cases?
A misconception that oral sex is somehow a “safe and risk-free” alternative to anal or vaginal sex could be one reason, Dr. Aguh said.
“It is important to know that, with oral sex, you are exposed to many of the risks associated with vaginal intercourse, especially if you do not take any measures to protect yourself and/or your partner,” Dr. Aguh said. “[With oral sex] it is possible to end up contracting an infection like chlamydia, gonorrhea, and even HPV, leading to an increased risk of HPV-associated oropharyngeal cancers.”
A lack of public awareness of what can cause throat cancer could also explain this phenomenon. The number of people you have oral sex with, along with the age you begin sexual activity, can greatly determine your risk of the disease, according to Dr. Lee. She echoes a report by Hisham Mehanna, PhD, in The Conversation.
“For oropharyngeal cancer, the main risk factor is the number of lifetime sexual partners, especially oral sex,” wrote Dr. Mehanna, a professor at the Institute of Cancer and Genomic Sciences at the University of Birmingham (England). “Those with six or more lifetime oral-sex partners are 8.5 times more likely to develop oropharyngeal cancer than those who do not practice oral sex.”
What are symptoms of oropharyngeal cancer?
Labored breathing or swallowing, a cough that won’t go away, and crackling or hoarseness of your voice could all be signs of throat cancer. Other symptoms include earaches, swelling of the head or neck, and enlarged lymph nodes, among others, Dr. Aguh said.
“The signs and symptoms of HPV-related throat cancers can be difficult to identify and recognize, as they can be vague and are also associated with other medical conditions. Sometimes, there are no signs at all, or they are not easily noticeable due to the location,” he said.
You should go see your doctor if you have any of these ailments for an extended period.
How to reduce your risk
In addition to having six or more oral-sex partners, smoking and drinking heavily could also raise your risk of throat cancer, said Dr. Lee. Proper dental health – like seeing your dentist regularly and practicing proper oral hygiene – can also shave your risk.
“[Good dental health] can help not just with head and neck cancer risk, but with many other inflammation-related diseases,” Dr. Lee said.
Using dental dams and condoms can also be a good method of protection, Dr. Aguh said. A dental dam is a stretchy sheet of latex, or polyurethane plastic, in the shape of a square that is made for blocking body fluid to lower your risk of contracting an STD via oral sex.
Keep in mind: Even with these protections, make sure you and your partner discuss each other’s sexual history, any prior or current STDs and their preferred protection from STDs, said Dr. Aguh.
If you or your partner is being treated for an STD, consider opting out of oral sex and consulting a doctor.
The HPV vaccine is another common method of protection. The shot is “approved for prevention of nine of the most high-risk strains of HPV,” or those that are most commonly linked to cancer, according to Dr. Lee. The vaccine “reduces the frequency of infection” with these viruses, which can ultimately lower the risk of cancers linked to HPV, including cervical, anal, and vulvar and vaginal cancers, she said.
“The best time to receive treatment for prevention of disease is prior to onset of sexual intercourse,” said Dr. Lee.
To get your HPV vaccine, head to your family doctor, school- or community-based health center, or state health department, suggests the CDC.
A version of this article originally appeared on WebMD.com.
An epidemic of sexually transmitted HPV is now swirling around the United States and the United Kingdom, with some serious cases leading to oropharyngeal cancer, which can affect the back of the throat, tonsils, and tongue.
HPV is the leading cause (70%) of this oropharyngeal cancer, according to the CDC. It is the most common sexually transmitted disease in the nation, and around 3.6% of women and 10% of men report oral HPV specifically. But over the past decade, oropharyngeal cases have been steadily falling a little under 4% and 2%, respectively, according to the National Cancer Institute.
HPV is often undetectable and can clear up within a few months. But unfortunately for some, serious disease, such as throat cancer, can develop.
Studies show the HPV vaccine to be extremely effective in lowering sexually transmitted HPV cases. Yet, only 54.5% of young people aged 13-15 have taken the recommended two to three doses, according to the National Cancer Institute.
Why aren’t more young people taking the vaccine?
Low public awareness of the dangers of HPV may be behind young people’s poor vaccination rates, according to Teresa Lee, MD, of the Fox Chase Cancer Center in Philadelphia. “For example, while the link with head and neck cancers has been well-studied, the FDA labeling was not changed to reflect this as an indication until 2020,” she said.
Other reasons can include one’s socioeconomic background, poor health literacy, cultural or religious stigmas around vaccines, and lack of quality, low-cost health care, says Emmanuel Aguh, MD, a board-certified family medicine physician. “Some individuals and families are still resistant to vaccines and the noted lack of uptake.”
Doctors and other health care professionals should also be sure to tell patients of all ages about the risks of HPV infection and how well the vaccine works, Dr. Lee said. “Not everyone who is now eligible may have been offered the vaccine as a child, and the first time young adults may receive counseling on this subject may not be until they are entering a very busy period of their lives with many responsibilities – when it may be hard to fit in things like health maintenance.”
How safe is the HPV vaccine?
The Food and Drug Administration and Centers for Disease Control and Prevention have studied the HPV vaccine for years to find out how safe it is and how well it works, Dr. Aguh said. No major side effects have been reported, and the most common side effect is soreness where you get the shot (which is normal after most vaccines). Some dizziness and fainting in adolescents can also occur, so young people are usually asked to sit or lie down during the shot and for 15 minutes afterward, he said.
“Serious adverse events have not been reported at higher rates than expected following HPV vaccination, meaning there is no clear evidence they are related to the vaccine,” Dr. Lee said. “The vaccine is highly effective in decreasing rates of detectable infection with the high-risk HPV strains responsible for HPV-associated cancers.”
The HPV vaccine is largely recommended for people aged 9-26, and sometimes up to age 45, depending on the individual, Dr. Aguh said. If you are over 26, talk to your doctor about whether you should consider getting the vaccine.
“It is usually given in two doses for complete protection if taken before the 15th birthday,” Dr. Aguh said. “If taken afterward, or in those with a weak immune system, they might require three doses to be fully protected.”
The vaccine produces antibodies that can stop HPV from infecting cells and lowers your chances of catching an HPV-related cancer, such as throat cancer or cancer of the cervix, he said.
While the vaccine is not guaranteed to protect you from the more than 100 strains of HPV, it can protect you from HPV 16 and HPV 18 – two high-risk strains that cause around 70% of cervical cancers.
What is fueling the rise of HPV cases?
A misconception that oral sex is somehow a “safe and risk-free” alternative to anal or vaginal sex could be one reason, Dr. Aguh said.
“It is important to know that, with oral sex, you are exposed to many of the risks associated with vaginal intercourse, especially if you do not take any measures to protect yourself and/or your partner,” Dr. Aguh said. “[With oral sex] it is possible to end up contracting an infection like chlamydia, gonorrhea, and even HPV, leading to an increased risk of HPV-associated oropharyngeal cancers.”
A lack of public awareness of what can cause throat cancer could also explain this phenomenon. The number of people you have oral sex with, along with the age you begin sexual activity, can greatly determine your risk of the disease, according to Dr. Lee. She echoes a report by Hisham Mehanna, PhD, in The Conversation.
“For oropharyngeal cancer, the main risk factor is the number of lifetime sexual partners, especially oral sex,” wrote Dr. Mehanna, a professor at the Institute of Cancer and Genomic Sciences at the University of Birmingham (England). “Those with six or more lifetime oral-sex partners are 8.5 times more likely to develop oropharyngeal cancer than those who do not practice oral sex.”
What are symptoms of oropharyngeal cancer?
Labored breathing or swallowing, a cough that won’t go away, and crackling or hoarseness of your voice could all be signs of throat cancer. Other symptoms include earaches, swelling of the head or neck, and enlarged lymph nodes, among others, Dr. Aguh said.
“The signs and symptoms of HPV-related throat cancers can be difficult to identify and recognize, as they can be vague and are also associated with other medical conditions. Sometimes, there are no signs at all, or they are not easily noticeable due to the location,” he said.
You should go see your doctor if you have any of these ailments for an extended period.
How to reduce your risk
In addition to having six or more oral-sex partners, smoking and drinking heavily could also raise your risk of throat cancer, said Dr. Lee. Proper dental health – like seeing your dentist regularly and practicing proper oral hygiene – can also shave your risk.
“[Good dental health] can help not just with head and neck cancer risk, but with many other inflammation-related diseases,” Dr. Lee said.
Using dental dams and condoms can also be a good method of protection, Dr. Aguh said. A dental dam is a stretchy sheet of latex, or polyurethane plastic, in the shape of a square that is made for blocking body fluid to lower your risk of contracting an STD via oral sex.
Keep in mind: Even with these protections, make sure you and your partner discuss each other’s sexual history, any prior or current STDs and their preferred protection from STDs, said Dr. Aguh.
If you or your partner is being treated for an STD, consider opting out of oral sex and consulting a doctor.
The HPV vaccine is another common method of protection. The shot is “approved for prevention of nine of the most high-risk strains of HPV,” or those that are most commonly linked to cancer, according to Dr. Lee. The vaccine “reduces the frequency of infection” with these viruses, which can ultimately lower the risk of cancers linked to HPV, including cervical, anal, and vulvar and vaginal cancers, she said.
“The best time to receive treatment for prevention of disease is prior to onset of sexual intercourse,” said Dr. Lee.
To get your HPV vaccine, head to your family doctor, school- or community-based health center, or state health department, suggests the CDC.
A version of this article originally appeared on WebMD.com.
Triple-agonist retatrutide hits new weight loss highs
SAN DIEGO – New designer molecules that target weight loss via multiple mechanisms continue to raise the bar of how many pounds people with overweight or obesity can lose.
Retatrutide (Eli Lilly), an investigational agent that combines agonism to three key hormones that influence eating and metabolism into a single molecule, safely produced weight loss at levels never seen before in a pair of phase 2 studies that together randomized more than 600 people with overweight or obesity, with or without type 2 diabetes.
Among 338 randomized people with overweight or obesity and no type 2 diabetes,
Among 281 randomized people with overweight or obesity and type 2 diabetes, the same dose of retatrutide produced a nearly 17% cut in weight from baseline after 36 weeks of treatment.
Never before seen weight loss
“I have never seen weight loss at this level” after nearly 1 year of treatment, Ania M. Jastreboff, MD, PhD, who led the obesity study, said during a press briefing at the annual scientific sessions of the American Diabetes Association.
The average weight loss by study participants taking high-dose retatrutide in the two studies “is really impressive, way beyond my wildest dreams,” said Carel le Roux, MBChB, PhD, an obesity and diabetes researcher at University College Dublin, Ireland, who was not involved with the retatrutide studies.
And Robert A. Gabbay, MD, chief scientific and medical officer of the ADA, called the results “stunning,” and added, “we are now witnessing the first triple-hormone combination being highly effective for not only weight loss but liver disease and diabetes.”
A prespecified subgroup analysis of the obesity study showed that at both 8-mg and 12-mg weekly doses, 24 weeks of retatrutide produced complete resolution of excess liver fat (hepatic steatosis) in about 80% of the people eligible for the analysis (those with at least 10% of their liver volume as fat at study entry); that figure increased to about 90% of people on these doses after 48 weeks, Lee M. Kaplan, MD, reported during a separate presentation at the meeting.
Adding glucagon agonism ups liver-fat clearance
“When you add glucagon activity,” one of the three agonist actions of retatrutide, “liver-fat clearance goes up tremendously,” said Dr. Kaplan, director of the Obesity, Metabolism and Nutrition Institute at Massachusetts General Hospital in Boston.
“To my knowledge, no mono-agonist of the glucagon-like peptide-1 (GLP-1) receptor [such as semaglutide or liraglutide] produces more than 50% clearance of liver fat,” added Dr. Kaplan.
The separate, randomized study of people with type 2 diabetes showed that in addition to producing an unprecedented average level of weight loss at the highest retatrutide dose, the agent also produced an average reduction from baseline levels of A1c of about 2 percentage points, an efficacy roughly comparable to maximum doses of the most potent GLP-1 mono-agonist semaglutide (Ozempic, Novo Nordisk), as well as by tirzepatide (Mounjaro, Eli Lilly), a dual agonist for the GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptors.
“No other medication has shown an average 17% reduction from baseline bodyweight after 36 weeks in people with type 2 diabetes,” said Julio Rosenstock, MD, director of the Dallas Diabetes Research Center at Medical City, Texas, who presented the results from the type 2 diabetes study of retatrutide.
For the obesity study, people with a body mass index of 27-50 kg/m2 and no diabetes were randomized to placebo or any of four retatrutide target dosages using specified dose-escalation protocols. Participants were an average of 48 years old, and by design, 52% were men. (The study sought to enroll roughly equal numbers of men and women.) Average BMI at study entry was 37 kg/m2.
Weight loss levels after 24 and 48 weeks of retatrutide treatment followed a clear dose-related pattern. (Weight loss averaged about 2% among the 70 controls who received placebo.)
Twenty-six percent without diabetes lost at least 30% of body weight
Every person who escalated to receive the 8-mg or 12-mg weekly dose of retatrutide lost at least 5% of their bodyweight after 48 weeks, 83% of those taking the 12-mg dose lost at least 15%, 63% of those on the 12-mg dose lost at least 20%, and 26% of those on the highest dose lost at least 30% of their starting bodyweight, reported Dr. Jastreboff, director of the Yale Obesity Research Center of Yale University in New Haven, Conn.
The highest dose was also associated with an average 40% relative reduction in triglyceride levels from baseline and an average 22% relative drop in LDL cholesterol levels.
The results were simultaneously published online in the New England Journal of Medicine.
The incidence of serious adverse events with retatrutide was low, similar to the rate in those who received placebo, and showed no dose relationship.
The most common adverse events were gastrointestinal, in as many as 16% of those on the highest dose; these were mild to moderate in severity and usually occurred during dose escalation. In general, adverse events were comparable to what is seen with a GLP-1 agonist or the dual agonist tirzepatide, Dr. Jastreboff said.
A1c normalization in 26% at the highest dose
A similar safety pattern occurred in the study of people with type 2 diabetes, which randomized people with an average A1c of 8.3% and an average BMI of 35.0 kg/m2. After 36 weeks of treatment, the 12-mg weekly dose of retatrutide led to normalization of A1c < 5.7% in 27% of people and A1c ≤ 6.5% in 77%.
“The number of people we were able to revert to a normal A1c was impressive,” said Dr. Rosenstock. These results were simultaneously published online in The Lancet.
The additional findings on liver-fat mobilization in people without diabetes enrolled in the obesity study are notable because no agent currently has labeling from the Food and Drug Administration for the indication of reducing excess liver fat, said Dr. Kaplan.
The researchers measured liver fat at baseline and then during treatment using MRI.
“With the level of fat clearance from the liver that we see with retatrutide it is highly likely that we’ll also see improvements in liver fibrosis” in retatrutide-treated patients, Dr. Kaplan predicted.
Next up for retatrutide is testing in pivotal trials, including the TRIUMPH-3 trial that will enroll about 1,800 people with severe obesity and cardiovascular disease, with findings expected toward the end of 2025.
The retatrutide studies are sponsored by Eli Lilly. Dr. Jastreboff, Dr. Rosenstock, Dr. Kaplan, and Dr. Le Roux have reported financial relationships with Eli Lilly as well as other companies.
A version of this article first appeared on Medscape.com.
SAN DIEGO – New designer molecules that target weight loss via multiple mechanisms continue to raise the bar of how many pounds people with overweight or obesity can lose.
Retatrutide (Eli Lilly), an investigational agent that combines agonism to three key hormones that influence eating and metabolism into a single molecule, safely produced weight loss at levels never seen before in a pair of phase 2 studies that together randomized more than 600 people with overweight or obesity, with or without type 2 diabetes.
Among 338 randomized people with overweight or obesity and no type 2 diabetes,
Among 281 randomized people with overweight or obesity and type 2 diabetes, the same dose of retatrutide produced a nearly 17% cut in weight from baseline after 36 weeks of treatment.
Never before seen weight loss
“I have never seen weight loss at this level” after nearly 1 year of treatment, Ania M. Jastreboff, MD, PhD, who led the obesity study, said during a press briefing at the annual scientific sessions of the American Diabetes Association.
The average weight loss by study participants taking high-dose retatrutide in the two studies “is really impressive, way beyond my wildest dreams,” said Carel le Roux, MBChB, PhD, an obesity and diabetes researcher at University College Dublin, Ireland, who was not involved with the retatrutide studies.
And Robert A. Gabbay, MD, chief scientific and medical officer of the ADA, called the results “stunning,” and added, “we are now witnessing the first triple-hormone combination being highly effective for not only weight loss but liver disease and diabetes.”
A prespecified subgroup analysis of the obesity study showed that at both 8-mg and 12-mg weekly doses, 24 weeks of retatrutide produced complete resolution of excess liver fat (hepatic steatosis) in about 80% of the people eligible for the analysis (those with at least 10% of their liver volume as fat at study entry); that figure increased to about 90% of people on these doses after 48 weeks, Lee M. Kaplan, MD, reported during a separate presentation at the meeting.
Adding glucagon agonism ups liver-fat clearance
“When you add glucagon activity,” one of the three agonist actions of retatrutide, “liver-fat clearance goes up tremendously,” said Dr. Kaplan, director of the Obesity, Metabolism and Nutrition Institute at Massachusetts General Hospital in Boston.
“To my knowledge, no mono-agonist of the glucagon-like peptide-1 (GLP-1) receptor [such as semaglutide or liraglutide] produces more than 50% clearance of liver fat,” added Dr. Kaplan.
The separate, randomized study of people with type 2 diabetes showed that in addition to producing an unprecedented average level of weight loss at the highest retatrutide dose, the agent also produced an average reduction from baseline levels of A1c of about 2 percentage points, an efficacy roughly comparable to maximum doses of the most potent GLP-1 mono-agonist semaglutide (Ozempic, Novo Nordisk), as well as by tirzepatide (Mounjaro, Eli Lilly), a dual agonist for the GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptors.
“No other medication has shown an average 17% reduction from baseline bodyweight after 36 weeks in people with type 2 diabetes,” said Julio Rosenstock, MD, director of the Dallas Diabetes Research Center at Medical City, Texas, who presented the results from the type 2 diabetes study of retatrutide.
For the obesity study, people with a body mass index of 27-50 kg/m2 and no diabetes were randomized to placebo or any of four retatrutide target dosages using specified dose-escalation protocols. Participants were an average of 48 years old, and by design, 52% were men. (The study sought to enroll roughly equal numbers of men and women.) Average BMI at study entry was 37 kg/m2.
Weight loss levels after 24 and 48 weeks of retatrutide treatment followed a clear dose-related pattern. (Weight loss averaged about 2% among the 70 controls who received placebo.)
Twenty-six percent without diabetes lost at least 30% of body weight
Every person who escalated to receive the 8-mg or 12-mg weekly dose of retatrutide lost at least 5% of their bodyweight after 48 weeks, 83% of those taking the 12-mg dose lost at least 15%, 63% of those on the 12-mg dose lost at least 20%, and 26% of those on the highest dose lost at least 30% of their starting bodyweight, reported Dr. Jastreboff, director of the Yale Obesity Research Center of Yale University in New Haven, Conn.
The highest dose was also associated with an average 40% relative reduction in triglyceride levels from baseline and an average 22% relative drop in LDL cholesterol levels.
The results were simultaneously published online in the New England Journal of Medicine.
The incidence of serious adverse events with retatrutide was low, similar to the rate in those who received placebo, and showed no dose relationship.
The most common adverse events were gastrointestinal, in as many as 16% of those on the highest dose; these were mild to moderate in severity and usually occurred during dose escalation. In general, adverse events were comparable to what is seen with a GLP-1 agonist or the dual agonist tirzepatide, Dr. Jastreboff said.
A1c normalization in 26% at the highest dose
A similar safety pattern occurred in the study of people with type 2 diabetes, which randomized people with an average A1c of 8.3% and an average BMI of 35.0 kg/m2. After 36 weeks of treatment, the 12-mg weekly dose of retatrutide led to normalization of A1c < 5.7% in 27% of people and A1c ≤ 6.5% in 77%.
“The number of people we were able to revert to a normal A1c was impressive,” said Dr. Rosenstock. These results were simultaneously published online in The Lancet.
The additional findings on liver-fat mobilization in people without diabetes enrolled in the obesity study are notable because no agent currently has labeling from the Food and Drug Administration for the indication of reducing excess liver fat, said Dr. Kaplan.
The researchers measured liver fat at baseline and then during treatment using MRI.
“With the level of fat clearance from the liver that we see with retatrutide it is highly likely that we’ll also see improvements in liver fibrosis” in retatrutide-treated patients, Dr. Kaplan predicted.
Next up for retatrutide is testing in pivotal trials, including the TRIUMPH-3 trial that will enroll about 1,800 people with severe obesity and cardiovascular disease, with findings expected toward the end of 2025.
The retatrutide studies are sponsored by Eli Lilly. Dr. Jastreboff, Dr. Rosenstock, Dr. Kaplan, and Dr. Le Roux have reported financial relationships with Eli Lilly as well as other companies.
A version of this article first appeared on Medscape.com.
SAN DIEGO – New designer molecules that target weight loss via multiple mechanisms continue to raise the bar of how many pounds people with overweight or obesity can lose.
Retatrutide (Eli Lilly), an investigational agent that combines agonism to three key hormones that influence eating and metabolism into a single molecule, safely produced weight loss at levels never seen before in a pair of phase 2 studies that together randomized more than 600 people with overweight or obesity, with or without type 2 diabetes.
Among 338 randomized people with overweight or obesity and no type 2 diabetes,
Among 281 randomized people with overweight or obesity and type 2 diabetes, the same dose of retatrutide produced a nearly 17% cut in weight from baseline after 36 weeks of treatment.
Never before seen weight loss
“I have never seen weight loss at this level” after nearly 1 year of treatment, Ania M. Jastreboff, MD, PhD, who led the obesity study, said during a press briefing at the annual scientific sessions of the American Diabetes Association.
The average weight loss by study participants taking high-dose retatrutide in the two studies “is really impressive, way beyond my wildest dreams,” said Carel le Roux, MBChB, PhD, an obesity and diabetes researcher at University College Dublin, Ireland, who was not involved with the retatrutide studies.
And Robert A. Gabbay, MD, chief scientific and medical officer of the ADA, called the results “stunning,” and added, “we are now witnessing the first triple-hormone combination being highly effective for not only weight loss but liver disease and diabetes.”
A prespecified subgroup analysis of the obesity study showed that at both 8-mg and 12-mg weekly doses, 24 weeks of retatrutide produced complete resolution of excess liver fat (hepatic steatosis) in about 80% of the people eligible for the analysis (those with at least 10% of their liver volume as fat at study entry); that figure increased to about 90% of people on these doses after 48 weeks, Lee M. Kaplan, MD, reported during a separate presentation at the meeting.
Adding glucagon agonism ups liver-fat clearance
“When you add glucagon activity,” one of the three agonist actions of retatrutide, “liver-fat clearance goes up tremendously,” said Dr. Kaplan, director of the Obesity, Metabolism and Nutrition Institute at Massachusetts General Hospital in Boston.
“To my knowledge, no mono-agonist of the glucagon-like peptide-1 (GLP-1) receptor [such as semaglutide or liraglutide] produces more than 50% clearance of liver fat,” added Dr. Kaplan.
The separate, randomized study of people with type 2 diabetes showed that in addition to producing an unprecedented average level of weight loss at the highest retatrutide dose, the agent also produced an average reduction from baseline levels of A1c of about 2 percentage points, an efficacy roughly comparable to maximum doses of the most potent GLP-1 mono-agonist semaglutide (Ozempic, Novo Nordisk), as well as by tirzepatide (Mounjaro, Eli Lilly), a dual agonist for the GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptors.
“No other medication has shown an average 17% reduction from baseline bodyweight after 36 weeks in people with type 2 diabetes,” said Julio Rosenstock, MD, director of the Dallas Diabetes Research Center at Medical City, Texas, who presented the results from the type 2 diabetes study of retatrutide.
For the obesity study, people with a body mass index of 27-50 kg/m2 and no diabetes were randomized to placebo or any of four retatrutide target dosages using specified dose-escalation protocols. Participants were an average of 48 years old, and by design, 52% were men. (The study sought to enroll roughly equal numbers of men and women.) Average BMI at study entry was 37 kg/m2.
Weight loss levels after 24 and 48 weeks of retatrutide treatment followed a clear dose-related pattern. (Weight loss averaged about 2% among the 70 controls who received placebo.)
Twenty-six percent without diabetes lost at least 30% of body weight
Every person who escalated to receive the 8-mg or 12-mg weekly dose of retatrutide lost at least 5% of their bodyweight after 48 weeks, 83% of those taking the 12-mg dose lost at least 15%, 63% of those on the 12-mg dose lost at least 20%, and 26% of those on the highest dose lost at least 30% of their starting bodyweight, reported Dr. Jastreboff, director of the Yale Obesity Research Center of Yale University in New Haven, Conn.
The highest dose was also associated with an average 40% relative reduction in triglyceride levels from baseline and an average 22% relative drop in LDL cholesterol levels.
The results were simultaneously published online in the New England Journal of Medicine.
The incidence of serious adverse events with retatrutide was low, similar to the rate in those who received placebo, and showed no dose relationship.
The most common adverse events were gastrointestinal, in as many as 16% of those on the highest dose; these were mild to moderate in severity and usually occurred during dose escalation. In general, adverse events were comparable to what is seen with a GLP-1 agonist or the dual agonist tirzepatide, Dr. Jastreboff said.
A1c normalization in 26% at the highest dose
A similar safety pattern occurred in the study of people with type 2 diabetes, which randomized people with an average A1c of 8.3% and an average BMI of 35.0 kg/m2. After 36 weeks of treatment, the 12-mg weekly dose of retatrutide led to normalization of A1c < 5.7% in 27% of people and A1c ≤ 6.5% in 77%.
“The number of people we were able to revert to a normal A1c was impressive,” said Dr. Rosenstock. These results were simultaneously published online in The Lancet.
The additional findings on liver-fat mobilization in people without diabetes enrolled in the obesity study are notable because no agent currently has labeling from the Food and Drug Administration for the indication of reducing excess liver fat, said Dr. Kaplan.
The researchers measured liver fat at baseline and then during treatment using MRI.
“With the level of fat clearance from the liver that we see with retatrutide it is highly likely that we’ll also see improvements in liver fibrosis” in retatrutide-treated patients, Dr. Kaplan predicted.
Next up for retatrutide is testing in pivotal trials, including the TRIUMPH-3 trial that will enroll about 1,800 people with severe obesity and cardiovascular disease, with findings expected toward the end of 2025.
The retatrutide studies are sponsored by Eli Lilly. Dr. Jastreboff, Dr. Rosenstock, Dr. Kaplan, and Dr. Le Roux have reported financial relationships with Eli Lilly as well as other companies.
A version of this article first appeared on Medscape.com.
AT ADA 2023
Malpractice lawsuits over denied abortion care may be on the horizon
Some experts predict those providers could soon face a new legal threat: medical malpractice lawsuits alleging they harmed patients by failing to provide timely, necessary abortion care.
“We will absolutely see medical malpractice cases emerge,” said Diana Nordlund, an emergency physician in Grand Rapids, Mich., and former malpractice defense attorney, who chairs the Medical-Legal Committee of the American College of Emergency Physicians. When physicians decide not to provide treatments widely accepted as the standard of care because of these new laws, “that’s perceived as substandard care and there is increased civil liability.”
To some physicians and malpractice attorneys, the question is when – not if – a pregnant patient will die from lack of care and set the stage for a big-dollar wrongful death claim. Abortion rights supporters said such a case could pressure doctors and hospitals to provide appropriate abortion care, counterbalancing their fears of running afoul of state abortion bans, many of which call for criminal prosecution and revocation of medical licenses as punishment for violations.
“If we want to encourage proper care, there has to be some sort of counter-risk to physicians and hospitals for refusing to provide care that should be legal,” said Greer Donley, an associate professor at the University of Pittsburgh school of law who studies the impact of abortion bans. “But most rational people would be more afraid of going to jail.”
Some supporters of abortion bans said they would welcome malpractice lawsuits. Providers are refusing to use the exceptions in some state laws that allow them to perform abortions to save a patient’s life or health, they said.
“It could help achieve our goal if it clarifies that the law did not contradict standard medical practice,” said John Seago, president of Texas Right to Life, referring to the state’s abortion ban.
A new KFF poll found that 59% of ob.gyns. practicing in states with gestational limits on abortion, and 61% of those in states with bans, are somewhat or very concerned about their legal risk when making decisions about the necessity of an abortion.
Some attorneys are exploring lawsuits on behalf of women who they said have been harmed by a state abortion ban. An attorney for Mylissa Farmer, a Missouri woman who was refused an abortion at two hospitals in August after her water broke about 18 weeks into her pregnancy, said she may sue for malpractice. Missouri’s abortion ban, which took effect last year, makes an exception for medical emergencies.
The federal government recently found that the two hospitals violated a federal emergency care law in denying Ms. Farmer an abortion, which experts said could strengthen a malpractice claim. One of the hospitals, Freeman Health System in Joplin, Mo., did not respond to a request for comment. The other, the University of Kansas Health System in Kansas City, said the care provided “was reviewed by the hospital and found to be in accordance with hospital policy,” according to a spokesperson, Jill Chadwick.
Ms. Farmer “experienced permanent physical and emotional damage,” said Michelle Banker, one of her lawyers at the National Women’s Law Center, who added that Ms. Farmer and her attorneys are “considering all our legal options.”
News reports and medical studies show that some women with pregnancy complications have suffered serious health consequences when doctors and hospitals did not provide once-routine abortion care.
Last month, researchers released a study identifying dozens of cases in 14 states in which physicians said deficiencies in care due to abortion restrictions led to preventable complications and hospitalizations, with some patients nearly dying.
“The patients were sent home and told to come back when they had signs of infection,” said Daniel Grossman, an ob.gyn. at the University of California, San Francisco, who led the study. “Many developed serious infections. And it’s clear many of these cases were very emotionally traumatic.”
He said though the researchers did not track patient outcomes, the lack of timely abortion care in such cases could result in severe health harms including loss of fertility, stroke, or heart attack.
“It’s just a matter of time before there will be a death that comes to light,” Dr. Grossman said.
Still, considering the conflict for doctors between medical ethics and personal risk, some stakeholders said patients may be reluctant to sue doctors and juries may balk at finding them liable.
“It’s a terrible position that providers are being put into, and I don’t think juries will blame the doctor unless it’s a super clear case,” said Morgan Murphy, a malpractice plaintiff’s attorney in Missouri.
She said her firm will not pursue malpractice cases based on abortion denials except in “pretty extreme” situations, such as when a patient dies. “Unless a mother is on her deathbed, it’s pretty hard to fault a provider who thinks if they provide treatment they’re going to be criminally liable or will lose their medical license.”
Another hurdle for malpractice cases is that state abortion bans could undermine the argument that abortion is the legal “standard of care,” meaning that it is a widely accepted and prescribed treatment for pregnancy complications such as miscarriage and for fatal fetal abnormalities.
“I absolutely see a breach of the standard of care in these cases,” said Maria A. Phillis, an ob.gyn. and former lawyer in Cleveland. “But if someone goes to trial in a malpractice case, it will come down to a battle of medical experts about whether it’s no longer the standard of care, and the jury would have to decide.”
An additional justification for physicians not to provide abortions is that medical liability insurers generally do not cover damages from criminal acts, which “puts the finger on the scales even more to not do anything,” Dr. Phillis said.
Stuart Grossman, a prominent malpractice plaintiff’s attorney in Florida, said he would be eager to take an abortion-denial case in which the woman suffered serious health or emotional injuries.
Unlike other states with abortion bans, Florida does not cap damage amounts for pain and suffering in malpractice cases, making it more financially viable to sue there.
Mr. Grossman cited the case of Deborah Dorbert, a Florida woman who reportedly was denied an abortion despite being told by her physicians at 24 weeks of pregnancy that her fetus, with no kidneys and underdeveloped lungs, had a fatal condition called Potter syndrome.
Her doctors and the hospital refused to end the pregnancy even though the state’s abortion ban has an exception for fatal fetal abnormalities. Months later, her baby died in his parents’ arms shortly after birth.
“You can see how she’s been devastated mentally,” Mr. Grossman said. “She has a wrongful death case that I’d take in a minute.” He said the couple could file a malpractice suit for Ms. Dorbert’s physical and emotional damages and a separate malpractice and wrongful death suit for the couple’s suffering over the infant’s death.
Failing to counsel patients about their options and connect them with providers willing to terminate a pregnancy is also possible grounds for a malpractice suit, attorneys said. Katie Watson, an associate professor at Northwestern University, Chicago’s school of medicine who has studied state abortion bans, said counseling and referral are not prohibited under these laws and that physicians have an ethical obligation to offer those services.
“I think breaching the obligation for counseling would make a strong malpractice lawsuit,” she said.
Nancy Davis said she received no counseling or referral assistance last July after her doctors at Woman’s Hospital in Baton Rouge, La., told her 10 weeks into her pregnancy that her fetus would not survive because it was missing the top of its skull, a fatal condition called acrania. She said they recommended that she terminate the pregnancy and she agreed.
Ms. Davis said her doctors then told her a hospital executive had denied permission for the procedure because of Louisiana’s abortion ban, even though the law has an exception for fatal fetal abnormalities. A hospital spokesperson declined to comment.
Ms. Davis, who has three children, contacted Planned Parenthood of Greater New York, which arranged for child care and a flight to New York. She had an abortion performed there in September.
“The whole situation has been mentally and physically draining, and my family and I are receiving counseling,” Ms. Davis said. “I’m still very angry at the hospital and the doctors. I feel like I’m owed compensation for the trauma and the heartbreak.”
She sought the counsel of Benjamin Crump, a prominent attorney known for pursuing high-profile cases like wrongful death lawsuits on behalf of the families of Trayvon Martin and George Floyd.
But Mr. Crump said that after studying Ms. Davis’ legal options, he decided a judge would likely dismiss a malpractice suit and that Ms. Davis could end up paying the defendants’ legal fees and costs.
“The doctor’s lawyers will say, ‘You can’t expect my client to break the law and go to prison for up to 25 years,’ ” Mr. Crump said. “Unless you change the law, there is no option for her to receive compensation.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – an independent source of health policy research, polling, and journalism. Learn more about KFF.
Some experts predict those providers could soon face a new legal threat: medical malpractice lawsuits alleging they harmed patients by failing to provide timely, necessary abortion care.
“We will absolutely see medical malpractice cases emerge,” said Diana Nordlund, an emergency physician in Grand Rapids, Mich., and former malpractice defense attorney, who chairs the Medical-Legal Committee of the American College of Emergency Physicians. When physicians decide not to provide treatments widely accepted as the standard of care because of these new laws, “that’s perceived as substandard care and there is increased civil liability.”
To some physicians and malpractice attorneys, the question is when – not if – a pregnant patient will die from lack of care and set the stage for a big-dollar wrongful death claim. Abortion rights supporters said such a case could pressure doctors and hospitals to provide appropriate abortion care, counterbalancing their fears of running afoul of state abortion bans, many of which call for criminal prosecution and revocation of medical licenses as punishment for violations.
“If we want to encourage proper care, there has to be some sort of counter-risk to physicians and hospitals for refusing to provide care that should be legal,” said Greer Donley, an associate professor at the University of Pittsburgh school of law who studies the impact of abortion bans. “But most rational people would be more afraid of going to jail.”
Some supporters of abortion bans said they would welcome malpractice lawsuits. Providers are refusing to use the exceptions in some state laws that allow them to perform abortions to save a patient’s life or health, they said.
“It could help achieve our goal if it clarifies that the law did not contradict standard medical practice,” said John Seago, president of Texas Right to Life, referring to the state’s abortion ban.
A new KFF poll found that 59% of ob.gyns. practicing in states with gestational limits on abortion, and 61% of those in states with bans, are somewhat or very concerned about their legal risk when making decisions about the necessity of an abortion.
Some attorneys are exploring lawsuits on behalf of women who they said have been harmed by a state abortion ban. An attorney for Mylissa Farmer, a Missouri woman who was refused an abortion at two hospitals in August after her water broke about 18 weeks into her pregnancy, said she may sue for malpractice. Missouri’s abortion ban, which took effect last year, makes an exception for medical emergencies.
The federal government recently found that the two hospitals violated a federal emergency care law in denying Ms. Farmer an abortion, which experts said could strengthen a malpractice claim. One of the hospitals, Freeman Health System in Joplin, Mo., did not respond to a request for comment. The other, the University of Kansas Health System in Kansas City, said the care provided “was reviewed by the hospital and found to be in accordance with hospital policy,” according to a spokesperson, Jill Chadwick.
Ms. Farmer “experienced permanent physical and emotional damage,” said Michelle Banker, one of her lawyers at the National Women’s Law Center, who added that Ms. Farmer and her attorneys are “considering all our legal options.”
News reports and medical studies show that some women with pregnancy complications have suffered serious health consequences when doctors and hospitals did not provide once-routine abortion care.
Last month, researchers released a study identifying dozens of cases in 14 states in which physicians said deficiencies in care due to abortion restrictions led to preventable complications and hospitalizations, with some patients nearly dying.
“The patients were sent home and told to come back when they had signs of infection,” said Daniel Grossman, an ob.gyn. at the University of California, San Francisco, who led the study. “Many developed serious infections. And it’s clear many of these cases were very emotionally traumatic.”
He said though the researchers did not track patient outcomes, the lack of timely abortion care in such cases could result in severe health harms including loss of fertility, stroke, or heart attack.
“It’s just a matter of time before there will be a death that comes to light,” Dr. Grossman said.
Still, considering the conflict for doctors between medical ethics and personal risk, some stakeholders said patients may be reluctant to sue doctors and juries may balk at finding them liable.
“It’s a terrible position that providers are being put into, and I don’t think juries will blame the doctor unless it’s a super clear case,” said Morgan Murphy, a malpractice plaintiff’s attorney in Missouri.
She said her firm will not pursue malpractice cases based on abortion denials except in “pretty extreme” situations, such as when a patient dies. “Unless a mother is on her deathbed, it’s pretty hard to fault a provider who thinks if they provide treatment they’re going to be criminally liable or will lose their medical license.”
Another hurdle for malpractice cases is that state abortion bans could undermine the argument that abortion is the legal “standard of care,” meaning that it is a widely accepted and prescribed treatment for pregnancy complications such as miscarriage and for fatal fetal abnormalities.
“I absolutely see a breach of the standard of care in these cases,” said Maria A. Phillis, an ob.gyn. and former lawyer in Cleveland. “But if someone goes to trial in a malpractice case, it will come down to a battle of medical experts about whether it’s no longer the standard of care, and the jury would have to decide.”
An additional justification for physicians not to provide abortions is that medical liability insurers generally do not cover damages from criminal acts, which “puts the finger on the scales even more to not do anything,” Dr. Phillis said.
Stuart Grossman, a prominent malpractice plaintiff’s attorney in Florida, said he would be eager to take an abortion-denial case in which the woman suffered serious health or emotional injuries.
Unlike other states with abortion bans, Florida does not cap damage amounts for pain and suffering in malpractice cases, making it more financially viable to sue there.
Mr. Grossman cited the case of Deborah Dorbert, a Florida woman who reportedly was denied an abortion despite being told by her physicians at 24 weeks of pregnancy that her fetus, with no kidneys and underdeveloped lungs, had a fatal condition called Potter syndrome.
Her doctors and the hospital refused to end the pregnancy even though the state’s abortion ban has an exception for fatal fetal abnormalities. Months later, her baby died in his parents’ arms shortly after birth.
“You can see how she’s been devastated mentally,” Mr. Grossman said. “She has a wrongful death case that I’d take in a minute.” He said the couple could file a malpractice suit for Ms. Dorbert’s physical and emotional damages and a separate malpractice and wrongful death suit for the couple’s suffering over the infant’s death.
Failing to counsel patients about their options and connect them with providers willing to terminate a pregnancy is also possible grounds for a malpractice suit, attorneys said. Katie Watson, an associate professor at Northwestern University, Chicago’s school of medicine who has studied state abortion bans, said counseling and referral are not prohibited under these laws and that physicians have an ethical obligation to offer those services.
“I think breaching the obligation for counseling would make a strong malpractice lawsuit,” she said.
Nancy Davis said she received no counseling or referral assistance last July after her doctors at Woman’s Hospital in Baton Rouge, La., told her 10 weeks into her pregnancy that her fetus would not survive because it was missing the top of its skull, a fatal condition called acrania. She said they recommended that she terminate the pregnancy and she agreed.
Ms. Davis said her doctors then told her a hospital executive had denied permission for the procedure because of Louisiana’s abortion ban, even though the law has an exception for fatal fetal abnormalities. A hospital spokesperson declined to comment.
Ms. Davis, who has three children, contacted Planned Parenthood of Greater New York, which arranged for child care and a flight to New York. She had an abortion performed there in September.
“The whole situation has been mentally and physically draining, and my family and I are receiving counseling,” Ms. Davis said. “I’m still very angry at the hospital and the doctors. I feel like I’m owed compensation for the trauma and the heartbreak.”
She sought the counsel of Benjamin Crump, a prominent attorney known for pursuing high-profile cases like wrongful death lawsuits on behalf of the families of Trayvon Martin and George Floyd.
But Mr. Crump said that after studying Ms. Davis’ legal options, he decided a judge would likely dismiss a malpractice suit and that Ms. Davis could end up paying the defendants’ legal fees and costs.
“The doctor’s lawyers will say, ‘You can’t expect my client to break the law and go to prison for up to 25 years,’ ” Mr. Crump said. “Unless you change the law, there is no option for her to receive compensation.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – an independent source of health policy research, polling, and journalism. Learn more about KFF.
Some experts predict those providers could soon face a new legal threat: medical malpractice lawsuits alleging they harmed patients by failing to provide timely, necessary abortion care.
“We will absolutely see medical malpractice cases emerge,” said Diana Nordlund, an emergency physician in Grand Rapids, Mich., and former malpractice defense attorney, who chairs the Medical-Legal Committee of the American College of Emergency Physicians. When physicians decide not to provide treatments widely accepted as the standard of care because of these new laws, “that’s perceived as substandard care and there is increased civil liability.”
To some physicians and malpractice attorneys, the question is when – not if – a pregnant patient will die from lack of care and set the stage for a big-dollar wrongful death claim. Abortion rights supporters said such a case could pressure doctors and hospitals to provide appropriate abortion care, counterbalancing their fears of running afoul of state abortion bans, many of which call for criminal prosecution and revocation of medical licenses as punishment for violations.
“If we want to encourage proper care, there has to be some sort of counter-risk to physicians and hospitals for refusing to provide care that should be legal,” said Greer Donley, an associate professor at the University of Pittsburgh school of law who studies the impact of abortion bans. “But most rational people would be more afraid of going to jail.”
Some supporters of abortion bans said they would welcome malpractice lawsuits. Providers are refusing to use the exceptions in some state laws that allow them to perform abortions to save a patient’s life or health, they said.
“It could help achieve our goal if it clarifies that the law did not contradict standard medical practice,” said John Seago, president of Texas Right to Life, referring to the state’s abortion ban.
A new KFF poll found that 59% of ob.gyns. practicing in states with gestational limits on abortion, and 61% of those in states with bans, are somewhat or very concerned about their legal risk when making decisions about the necessity of an abortion.
Some attorneys are exploring lawsuits on behalf of women who they said have been harmed by a state abortion ban. An attorney for Mylissa Farmer, a Missouri woman who was refused an abortion at two hospitals in August after her water broke about 18 weeks into her pregnancy, said she may sue for malpractice. Missouri’s abortion ban, which took effect last year, makes an exception for medical emergencies.
The federal government recently found that the two hospitals violated a federal emergency care law in denying Ms. Farmer an abortion, which experts said could strengthen a malpractice claim. One of the hospitals, Freeman Health System in Joplin, Mo., did not respond to a request for comment. The other, the University of Kansas Health System in Kansas City, said the care provided “was reviewed by the hospital and found to be in accordance with hospital policy,” according to a spokesperson, Jill Chadwick.
Ms. Farmer “experienced permanent physical and emotional damage,” said Michelle Banker, one of her lawyers at the National Women’s Law Center, who added that Ms. Farmer and her attorneys are “considering all our legal options.”
News reports and medical studies show that some women with pregnancy complications have suffered serious health consequences when doctors and hospitals did not provide once-routine abortion care.
Last month, researchers released a study identifying dozens of cases in 14 states in which physicians said deficiencies in care due to abortion restrictions led to preventable complications and hospitalizations, with some patients nearly dying.
“The patients were sent home and told to come back when they had signs of infection,” said Daniel Grossman, an ob.gyn. at the University of California, San Francisco, who led the study. “Many developed serious infections. And it’s clear many of these cases were very emotionally traumatic.”
He said though the researchers did not track patient outcomes, the lack of timely abortion care in such cases could result in severe health harms including loss of fertility, stroke, or heart attack.
“It’s just a matter of time before there will be a death that comes to light,” Dr. Grossman said.
Still, considering the conflict for doctors between medical ethics and personal risk, some stakeholders said patients may be reluctant to sue doctors and juries may balk at finding them liable.
“It’s a terrible position that providers are being put into, and I don’t think juries will blame the doctor unless it’s a super clear case,” said Morgan Murphy, a malpractice plaintiff’s attorney in Missouri.
She said her firm will not pursue malpractice cases based on abortion denials except in “pretty extreme” situations, such as when a patient dies. “Unless a mother is on her deathbed, it’s pretty hard to fault a provider who thinks if they provide treatment they’re going to be criminally liable or will lose their medical license.”
Another hurdle for malpractice cases is that state abortion bans could undermine the argument that abortion is the legal “standard of care,” meaning that it is a widely accepted and prescribed treatment for pregnancy complications such as miscarriage and for fatal fetal abnormalities.
“I absolutely see a breach of the standard of care in these cases,” said Maria A. Phillis, an ob.gyn. and former lawyer in Cleveland. “But if someone goes to trial in a malpractice case, it will come down to a battle of medical experts about whether it’s no longer the standard of care, and the jury would have to decide.”
An additional justification for physicians not to provide abortions is that medical liability insurers generally do not cover damages from criminal acts, which “puts the finger on the scales even more to not do anything,” Dr. Phillis said.
Stuart Grossman, a prominent malpractice plaintiff’s attorney in Florida, said he would be eager to take an abortion-denial case in which the woman suffered serious health or emotional injuries.
Unlike other states with abortion bans, Florida does not cap damage amounts for pain and suffering in malpractice cases, making it more financially viable to sue there.
Mr. Grossman cited the case of Deborah Dorbert, a Florida woman who reportedly was denied an abortion despite being told by her physicians at 24 weeks of pregnancy that her fetus, with no kidneys and underdeveloped lungs, had a fatal condition called Potter syndrome.
Her doctors and the hospital refused to end the pregnancy even though the state’s abortion ban has an exception for fatal fetal abnormalities. Months later, her baby died in his parents’ arms shortly after birth.
“You can see how she’s been devastated mentally,” Mr. Grossman said. “She has a wrongful death case that I’d take in a minute.” He said the couple could file a malpractice suit for Ms. Dorbert’s physical and emotional damages and a separate malpractice and wrongful death suit for the couple’s suffering over the infant’s death.
Failing to counsel patients about their options and connect them with providers willing to terminate a pregnancy is also possible grounds for a malpractice suit, attorneys said. Katie Watson, an associate professor at Northwestern University, Chicago’s school of medicine who has studied state abortion bans, said counseling and referral are not prohibited under these laws and that physicians have an ethical obligation to offer those services.
“I think breaching the obligation for counseling would make a strong malpractice lawsuit,” she said.
Nancy Davis said she received no counseling or referral assistance last July after her doctors at Woman’s Hospital in Baton Rouge, La., told her 10 weeks into her pregnancy that her fetus would not survive because it was missing the top of its skull, a fatal condition called acrania. She said they recommended that she terminate the pregnancy and she agreed.
Ms. Davis said her doctors then told her a hospital executive had denied permission for the procedure because of Louisiana’s abortion ban, even though the law has an exception for fatal fetal abnormalities. A hospital spokesperson declined to comment.
Ms. Davis, who has three children, contacted Planned Parenthood of Greater New York, which arranged for child care and a flight to New York. She had an abortion performed there in September.
“The whole situation has been mentally and physically draining, and my family and I are receiving counseling,” Ms. Davis said. “I’m still very angry at the hospital and the doctors. I feel like I’m owed compensation for the trauma and the heartbreak.”
She sought the counsel of Benjamin Crump, a prominent attorney known for pursuing high-profile cases like wrongful death lawsuits on behalf of the families of Trayvon Martin and George Floyd.
But Mr. Crump said that after studying Ms. Davis’ legal options, he decided a judge would likely dismiss a malpractice suit and that Ms. Davis could end up paying the defendants’ legal fees and costs.
“The doctor’s lawyers will say, ‘You can’t expect my client to break the law and go to prison for up to 25 years,’ ” Mr. Crump said. “Unless you change the law, there is no option for her to receive compensation.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – an independent source of health policy research, polling, and journalism. Learn more about KFF.



