User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Give bacterial diversity a chance: The antibiotic dichotomy
What’s the opposite of an antibiotic?
Everyone knows that LOTME loves a good dichotomy: yin/yang, good/evil, heads/tails, particle/wave, peanut butter/jelly. They’re all great. We’re also big fans of microbiomes, particularly the gut microbiome. But what if we could combine the two? A healthy and nutritious story about the gut microbiome, with a dash of added dichotomy for flavor. Is such a thing even possible? Let’s find out.
First, we need an antibiotic, a drug designed to fight bacterial infections. If you’ve got strep throat, otitis media, or bubonic plague, there’s a good chance you will receive an antibiotic. That antibiotic will kill the bad bacteria that are making you sick, but it will also kill a lot of the good bacteria that inhabit your gut microbiome, which results in side effects like bloating and diarrhea.
It comes down to diversity, explained Elisa Marroquin, PhD, of Texas Christian University (Go Horned Frogs!): “In a human community, we need people that have different professions because we don’t all know how to do every single job. And so the same happens with bacteria. We need lots of different gut bacteria that know how to do different things.”
She and her colleagues reviewed 29 studies published over the last 7 years and found a way to preserve the diversity of a human gut microbiome that’s dealing with an antibiotic. Their solution? Prescribe a probiotic.
The way to fight the effects of stopping a bacterial infection is to provide food for what are, basically, other bacterial infections. Antibiotic/probiotic is a prescription for dichotomy, and it means we managed to combine gut microbiomes with a dichotomy. And you didn’t think we could do it.
The earphone of hearing aids
It’s estimated that up to 75% of people who need hearing aids don’t wear them. Why? Well, there’s the social stigma about not wanting to appear too old, and then there’s the cost factor.
Is there a cheaper, less stigmatizing option to amplify hearing? The answer, according to otolaryngologist Yen-fu Cheng, MD, of Taipei Veterans General Hospital and associates, is wireless earphones. AirPods, if you want to be brand specific.
Airpods can be on the more expensive side – running about $129 for AirPods 2 and $249 for AirPods Pro – but when compared with premium hearing aids ($10,000), or even basic aids (about $1,500), the Apple products come off inexpensive after all.
The team tested the premium and basic hearing aids against the AirPods 2 and the AirPod Pro using Apple’s Live Listen feature, which helps amplify sound through the company’s wireless earphones and iPhones and was initially designed to assist people with normal hearing in situations such as birdwatching.
The AirPods Pro worked just as well as the basic hearing aid but not quite as well as the premium hearing aid in a quiet setting, while the AirPods 2 performed the worst. When tested in a noisy setting, the AirPods Pro was pretty comparable to the premium hearing aid, as long as the noise came from a lateral direction. Neither of the AirPod models did as well as the hearing aids with head-on noises.
Wireless earbuds may not be the perfect solution from a clinical standpoint, but they’re a good start for people who don’t have access to hearing aids, Dr. Cheng noted.
So who says headphones damage your hearing? They might actually help.
Now I lay me down to sleep, I pray the computer my soul to keep
Radiation is the boring hazard of space travel. No one dies in a space horror movie because they’ve been slowly exposed to too much cosmic radiation. It’s always “thrown out the airlock” this and “eaten by a xenomorph” that.
Radiation, however, is not something that can be ignored, but it turns out that a potential solution is another science fiction staple: artificial hibernation. Generally in sci-fi, hibernation is a plot convenience to get people from point A to point B in a ship that doesn’t break the laws of physics. Here on Earth, though, it is well known that animals naturally entering a state of torpor during hibernation gain significant resistance to radiation.
The problem, of course, is that humans don’t hibernate, and no matter how hard people who work 100-hour weeks for Elon Musk try, sleeping for months on end is simply something we can’t do. However, a new study shows that it’s possible to induce this torpor state in animals that don’t naturally hibernate. By injecting rats with adenosine 5’-monophosphate monohydrate and keeping them in a room held at 16° C, an international team of scientists successfully induced a synthetic torpor state.
That’s not all they did: The scientists also exposed the hibernating rats to a large dose of radiation approximating that found in deep space. Which isn’t something we’d like to explain to our significant other when we got home from work. “So how was your day?” “Oh, I irradiated a bunch of sleeping rats. … Don’t worry they’re fine!” Which they were. Thanks to the hypoxic and hypothermic state, the tissue was spared damage from the high-energy ion radiation.
Obviously, there’s a big difference between a rat and a human and a lot of work to be done, but the study does show that artificial hibernation is possible. Perhaps one day we’ll be able to fall asleep and wake up light-years away under an alien sky, and we won’t be horrifically mutated or riddled with cancer. If, however, you find yourself in hibernation on your way to Jupiter (or Saturn) to investigate a mysterious black monolith, we suggest sleeping with one eye open and gripping your pillow tight.
What’s the opposite of an antibiotic?
Everyone knows that LOTME loves a good dichotomy: yin/yang, good/evil, heads/tails, particle/wave, peanut butter/jelly. They’re all great. We’re also big fans of microbiomes, particularly the gut microbiome. But what if we could combine the two? A healthy and nutritious story about the gut microbiome, with a dash of added dichotomy for flavor. Is such a thing even possible? Let’s find out.
First, we need an antibiotic, a drug designed to fight bacterial infections. If you’ve got strep throat, otitis media, or bubonic plague, there’s a good chance you will receive an antibiotic. That antibiotic will kill the bad bacteria that are making you sick, but it will also kill a lot of the good bacteria that inhabit your gut microbiome, which results in side effects like bloating and diarrhea.
It comes down to diversity, explained Elisa Marroquin, PhD, of Texas Christian University (Go Horned Frogs!): “In a human community, we need people that have different professions because we don’t all know how to do every single job. And so the same happens with bacteria. We need lots of different gut bacteria that know how to do different things.”
She and her colleagues reviewed 29 studies published over the last 7 years and found a way to preserve the diversity of a human gut microbiome that’s dealing with an antibiotic. Their solution? Prescribe a probiotic.
The way to fight the effects of stopping a bacterial infection is to provide food for what are, basically, other bacterial infections. Antibiotic/probiotic is a prescription for dichotomy, and it means we managed to combine gut microbiomes with a dichotomy. And you didn’t think we could do it.
The earphone of hearing aids
It’s estimated that up to 75% of people who need hearing aids don’t wear them. Why? Well, there’s the social stigma about not wanting to appear too old, and then there’s the cost factor.
Is there a cheaper, less stigmatizing option to amplify hearing? The answer, according to otolaryngologist Yen-fu Cheng, MD, of Taipei Veterans General Hospital and associates, is wireless earphones. AirPods, if you want to be brand specific.
Airpods can be on the more expensive side – running about $129 for AirPods 2 and $249 for AirPods Pro – but when compared with premium hearing aids ($10,000), or even basic aids (about $1,500), the Apple products come off inexpensive after all.
The team tested the premium and basic hearing aids against the AirPods 2 and the AirPod Pro using Apple’s Live Listen feature, which helps amplify sound through the company’s wireless earphones and iPhones and was initially designed to assist people with normal hearing in situations such as birdwatching.
The AirPods Pro worked just as well as the basic hearing aid but not quite as well as the premium hearing aid in a quiet setting, while the AirPods 2 performed the worst. When tested in a noisy setting, the AirPods Pro was pretty comparable to the premium hearing aid, as long as the noise came from a lateral direction. Neither of the AirPod models did as well as the hearing aids with head-on noises.
Wireless earbuds may not be the perfect solution from a clinical standpoint, but they’re a good start for people who don’t have access to hearing aids, Dr. Cheng noted.
So who says headphones damage your hearing? They might actually help.
Now I lay me down to sleep, I pray the computer my soul to keep
Radiation is the boring hazard of space travel. No one dies in a space horror movie because they’ve been slowly exposed to too much cosmic radiation. It’s always “thrown out the airlock” this and “eaten by a xenomorph” that.
Radiation, however, is not something that can be ignored, but it turns out that a potential solution is another science fiction staple: artificial hibernation. Generally in sci-fi, hibernation is a plot convenience to get people from point A to point B in a ship that doesn’t break the laws of physics. Here on Earth, though, it is well known that animals naturally entering a state of torpor during hibernation gain significant resistance to radiation.
The problem, of course, is that humans don’t hibernate, and no matter how hard people who work 100-hour weeks for Elon Musk try, sleeping for months on end is simply something we can’t do. However, a new study shows that it’s possible to induce this torpor state in animals that don’t naturally hibernate. By injecting rats with adenosine 5’-monophosphate monohydrate and keeping them in a room held at 16° C, an international team of scientists successfully induced a synthetic torpor state.
That’s not all they did: The scientists also exposed the hibernating rats to a large dose of radiation approximating that found in deep space. Which isn’t something we’d like to explain to our significant other when we got home from work. “So how was your day?” “Oh, I irradiated a bunch of sleeping rats. … Don’t worry they’re fine!” Which they were. Thanks to the hypoxic and hypothermic state, the tissue was spared damage from the high-energy ion radiation.
Obviously, there’s a big difference between a rat and a human and a lot of work to be done, but the study does show that artificial hibernation is possible. Perhaps one day we’ll be able to fall asleep and wake up light-years away under an alien sky, and we won’t be horrifically mutated or riddled with cancer. If, however, you find yourself in hibernation on your way to Jupiter (or Saturn) to investigate a mysterious black monolith, we suggest sleeping with one eye open and gripping your pillow tight.
What’s the opposite of an antibiotic?
Everyone knows that LOTME loves a good dichotomy: yin/yang, good/evil, heads/tails, particle/wave, peanut butter/jelly. They’re all great. We’re also big fans of microbiomes, particularly the gut microbiome. But what if we could combine the two? A healthy and nutritious story about the gut microbiome, with a dash of added dichotomy for flavor. Is such a thing even possible? Let’s find out.
First, we need an antibiotic, a drug designed to fight bacterial infections. If you’ve got strep throat, otitis media, or bubonic plague, there’s a good chance you will receive an antibiotic. That antibiotic will kill the bad bacteria that are making you sick, but it will also kill a lot of the good bacteria that inhabit your gut microbiome, which results in side effects like bloating and diarrhea.
It comes down to diversity, explained Elisa Marroquin, PhD, of Texas Christian University (Go Horned Frogs!): “In a human community, we need people that have different professions because we don’t all know how to do every single job. And so the same happens with bacteria. We need lots of different gut bacteria that know how to do different things.”
She and her colleagues reviewed 29 studies published over the last 7 years and found a way to preserve the diversity of a human gut microbiome that’s dealing with an antibiotic. Their solution? Prescribe a probiotic.
The way to fight the effects of stopping a bacterial infection is to provide food for what are, basically, other bacterial infections. Antibiotic/probiotic is a prescription for dichotomy, and it means we managed to combine gut microbiomes with a dichotomy. And you didn’t think we could do it.
The earphone of hearing aids
It’s estimated that up to 75% of people who need hearing aids don’t wear them. Why? Well, there’s the social stigma about not wanting to appear too old, and then there’s the cost factor.
Is there a cheaper, less stigmatizing option to amplify hearing? The answer, according to otolaryngologist Yen-fu Cheng, MD, of Taipei Veterans General Hospital and associates, is wireless earphones. AirPods, if you want to be brand specific.
Airpods can be on the more expensive side – running about $129 for AirPods 2 and $249 for AirPods Pro – but when compared with premium hearing aids ($10,000), or even basic aids (about $1,500), the Apple products come off inexpensive after all.
The team tested the premium and basic hearing aids against the AirPods 2 and the AirPod Pro using Apple’s Live Listen feature, which helps amplify sound through the company’s wireless earphones and iPhones and was initially designed to assist people with normal hearing in situations such as birdwatching.
The AirPods Pro worked just as well as the basic hearing aid but not quite as well as the premium hearing aid in a quiet setting, while the AirPods 2 performed the worst. When tested in a noisy setting, the AirPods Pro was pretty comparable to the premium hearing aid, as long as the noise came from a lateral direction. Neither of the AirPod models did as well as the hearing aids with head-on noises.
Wireless earbuds may not be the perfect solution from a clinical standpoint, but they’re a good start for people who don’t have access to hearing aids, Dr. Cheng noted.
So who says headphones damage your hearing? They might actually help.
Now I lay me down to sleep, I pray the computer my soul to keep
Radiation is the boring hazard of space travel. No one dies in a space horror movie because they’ve been slowly exposed to too much cosmic radiation. It’s always “thrown out the airlock” this and “eaten by a xenomorph” that.
Radiation, however, is not something that can be ignored, but it turns out that a potential solution is another science fiction staple: artificial hibernation. Generally in sci-fi, hibernation is a plot convenience to get people from point A to point B in a ship that doesn’t break the laws of physics. Here on Earth, though, it is well known that animals naturally entering a state of torpor during hibernation gain significant resistance to radiation.
The problem, of course, is that humans don’t hibernate, and no matter how hard people who work 100-hour weeks for Elon Musk try, sleeping for months on end is simply something we can’t do. However, a new study shows that it’s possible to induce this torpor state in animals that don’t naturally hibernate. By injecting rats with adenosine 5’-monophosphate monohydrate and keeping them in a room held at 16° C, an international team of scientists successfully induced a synthetic torpor state.
That’s not all they did: The scientists also exposed the hibernating rats to a large dose of radiation approximating that found in deep space. Which isn’t something we’d like to explain to our significant other when we got home from work. “So how was your day?” “Oh, I irradiated a bunch of sleeping rats. … Don’t worry they’re fine!” Which they were. Thanks to the hypoxic and hypothermic state, the tissue was spared damage from the high-energy ion radiation.
Obviously, there’s a big difference between a rat and a human and a lot of work to be done, but the study does show that artificial hibernation is possible. Perhaps one day we’ll be able to fall asleep and wake up light-years away under an alien sky, and we won’t be horrifically mutated or riddled with cancer. If, however, you find yourself in hibernation on your way to Jupiter (or Saturn) to investigate a mysterious black monolith, we suggest sleeping with one eye open and gripping your pillow tight.
Don’t let amoxicillin shortage go to waste, antibiotic stewards say
Some experts are encouraging clinicians to see the amoxicillin shortage through pink-colored glasses.
The ongoing shortage, which was first reported in October and was prompted by a surge in demand linked in part to influenza and respiratory syncytial virus (RSV), could be an opportunity for clinicians to refine their prescribing practices and avoid unnecessary and potentially harmful orders for the medication, they say.
Antibiotics are often prescribed to patients who do not need them. In many cases, patients’ symptoms are caused by viral infections, not bacteria, so antibiotics do not help.
Even when symptoms resolve after a patient takes an antibiotic, the drug may have had nothing to do with their improvement.
Time to double-down on assessment; use antibiotics only when needed,” Jason Gallagher, PharmD, of Temple University School of Pharmacy in Philadelphia, posted on Twitter.
When antibiotics are not helping, they still may cause harm. Treatment with antibiotics entails risks for antibiotic resistance, infection with Clostridioides difficile, and side effects, such as rashes and – as Dr. Gallagher noted – diarrhea.
“They say ‘never let a good shortage go to waste,’ ” Michael Cosimini, MD, a pediatrician at Oregon Health & Science University, Portland, tweeted about the lack of amoxicillin in October.
Dr. Cosimini offered his thoughts about “improving our amoxicillin prescribing patterns” in pediatrics and encouraged colleagues to do so.
For example, he highlighted guidelines that state that antimicrobial therapy is not routinely required for preschool-aged children with community-acquired pneumonia (CAP) because most cases are caused by viral pathogens.
And trials show that when antibiotics are used for CAP, a shorter treatment duration, such as 5 days, rather than the standard 7-10 days, can be sufficient.
“As physicians, a shortage like this is an opportunity to do our best in the short term, as well as reflect on our current practice and make changes for the better in the long run,” Dr. Cosimini told this news organization.
Amoxicillin is the most commonly prescribed antibiotic in the outpatient setting and is the first choice among antimicrobial agents for common infections, such as otitis media, strep throat, and pneumonia, he said. “We use it frequently, so even small changes could go a long way to improve our prescribing practice,” Dr. Cosimini said.
Inappropriate antibiotic prescribing may be common
A 2021 statement on antibiotic stewardship from the American Academy of Pediatrics (AAP) declared that while antibiotics have saved countless lives, they can also cause harm and are frequently used inappropriately.
“One in five pediatric ambulatory visits result in an antibiotic prescription, accounting for nearly 50 million antibiotic prescriptions annually in the United States, at least half of which are considered inappropriate. [Acute respiratory tract infections] account for more than two-thirds of antibiotic prescriptions for children, at least one-third of which are unnecessary,” according to the society.
Outpatient antibiotic stewardship efforts could focus on clinical encounters in which the medications could be avoided altogether, the AAP suggested.
“Examples include antibiotic prescribing for nonspecific upper respiratory infection, bronchiolitis, acute bronchitis, asthma exacerbation, or conjunctivitis,” the group said.
Given the epidemiology of bacterial infections seen in ambulatory care settings that warrant antibiotic therapy, researchers conservatively estimate “that antibiotic prescribing could be safely reduced by 30%,” the statement noted.
That said, treatment decisions are not always clear cut.
“Certain infections in children, such as ear infections and lung infections, can be caused by viruses, bacteria, or both at the same time,” Dr. Cosimini said. “As such, it is very difficult to know which children benefit from which antibiotics.”
Watching, waiting, vaccinating
Pediatricians know that many children with ear infections will get better without antibiotics. “Parents should know that their doctor may suggest watching an ear infection without antibiotics, as is the recommendation from the AAP,” Dr. Cosimini said.
Data indicate that doctors are not following this practice as often as they could be, he said.
When antibiotic treatment is needed during the shortage, agents other than amoxicillin suspension can be used.
“Even though amoxicillin suspension is our go-to antibiotic for many infections, there are effective alternative options,” Dr. Cosimini said. “Children’s Hospital of Philadelphia has a good list for doctors looking for alternatives.”
Another approach to reducing the use of antibiotics in the future involves preventing infections through vaccination.
Research shows that routine childhood vaccines may have averted millions of respiratory and ear infections. And because bacterial infections can follow viral infections, the annual flu vaccine and COVID-19 vaccines “are also great tools to reduce antibiotic use,” Dr. Cosimini said.
A turn to more toxic options?
The shortage of amoxicillin oral powder for suspension was reported by the Food and Drug Administration and the American Society of Health-System Pharmacists (ASHP) in October.
On Nov. 4, the Society of Infectious Diseases Pharmacists (SIDP) issued a statement on the amoxicillin shortage, noting that increased demand for the drug coincided with a surge in respiratory viral infections, including RSV and influenza, among children.
“Though supportive care is the mainstay of treatment for viral infections, antibiotics may be indicated for the treatment of superimposed bacterial infections, including pneumonia and acute otitis media,” the SIDP statement said. “While alternative antibiotics may be available depending on the indication, many have a broader spectrum of activity, increased toxicity, and excess cost relative to amoxicillin. Furthermore, it is anticipated alternatives may soon become in short supply as well, given increased usage.”
SIDP “encourages the judicious use of antibiotics” and supports watch-and-wait strategies and the use of the shortest effective duration of therapy when appropriate.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, monitors around 250 drug shortages at any given time.
The amoxicillin shortage, while not “overly worrisome,” stands out because of how widely the drug is used and the fact that the shortage appears to have been sparked by an increase in demand rather than supply chain or manufacturing quality problems that more typically lead to shortages, he said.
Unlike some other shortages, the amoxicillin shortfall largely does not involve disrupting a medication regimen that someone was already receiving, and substitutions should be available.
“That said, it’s very, very disruptive to parents or a caregiver when you have a sick child who needs an antibiotic and it’s not available,” Dr. Ganio said.
Can a poster change practice?
In an unrelated move, the U.S. Agency for Healthcare Research and Quality published new resources and strategies to reduce inappropriate antibiotic use in ambulatory care settings.
One of the tools is a poster that doctors can print and hang in their offices. It states: “We commit to only prescribing antibiotics when they will help you. Taking antibiotics when you do not need them will NOT make you better. You will still feel sick, and the antibiotic may give you a skin rash, diarrhea, or a yeast infection.”
Jeffrey A. Linder, MD, MPH, a general internist and researcher at Northwestern University in Chicago, helped develop some of the approaches to improve prescribing practices in primary care.
Dr. Linder explained on a recent episode of the Freakonomics, M.D. podcast that the poster can be key.
One reason clinicians may prescribe antibiotics inappropriately is because they assume – perhaps erroneously – that patients want and expect them. By addressing the issue up front by displaying the poster, they may be able to “short-circuit” that type of thinking.
A minority of patients do expect antibiotics. “But the vast majority of patients are thinking, ‘I don’t feel well, I want to know what’s going on, and I want to know how to feel better and what’s going to happen.’ ”
For their part, patients can tell their doctors that they want an antibiotic only if they really need it, Dr. Linder said.
A version of this article first appeared on Medscape.com.
Some experts are encouraging clinicians to see the amoxicillin shortage through pink-colored glasses.
The ongoing shortage, which was first reported in October and was prompted by a surge in demand linked in part to influenza and respiratory syncytial virus (RSV), could be an opportunity for clinicians to refine their prescribing practices and avoid unnecessary and potentially harmful orders for the medication, they say.
Antibiotics are often prescribed to patients who do not need them. In many cases, patients’ symptoms are caused by viral infections, not bacteria, so antibiotics do not help.
Even when symptoms resolve after a patient takes an antibiotic, the drug may have had nothing to do with their improvement.
Time to double-down on assessment; use antibiotics only when needed,” Jason Gallagher, PharmD, of Temple University School of Pharmacy in Philadelphia, posted on Twitter.
When antibiotics are not helping, they still may cause harm. Treatment with antibiotics entails risks for antibiotic resistance, infection with Clostridioides difficile, and side effects, such as rashes and – as Dr. Gallagher noted – diarrhea.
“They say ‘never let a good shortage go to waste,’ ” Michael Cosimini, MD, a pediatrician at Oregon Health & Science University, Portland, tweeted about the lack of amoxicillin in October.
Dr. Cosimini offered his thoughts about “improving our amoxicillin prescribing patterns” in pediatrics and encouraged colleagues to do so.
For example, he highlighted guidelines that state that antimicrobial therapy is not routinely required for preschool-aged children with community-acquired pneumonia (CAP) because most cases are caused by viral pathogens.
And trials show that when antibiotics are used for CAP, a shorter treatment duration, such as 5 days, rather than the standard 7-10 days, can be sufficient.
“As physicians, a shortage like this is an opportunity to do our best in the short term, as well as reflect on our current practice and make changes for the better in the long run,” Dr. Cosimini told this news organization.
Amoxicillin is the most commonly prescribed antibiotic in the outpatient setting and is the first choice among antimicrobial agents for common infections, such as otitis media, strep throat, and pneumonia, he said. “We use it frequently, so even small changes could go a long way to improve our prescribing practice,” Dr. Cosimini said.
Inappropriate antibiotic prescribing may be common
A 2021 statement on antibiotic stewardship from the American Academy of Pediatrics (AAP) declared that while antibiotics have saved countless lives, they can also cause harm and are frequently used inappropriately.
“One in five pediatric ambulatory visits result in an antibiotic prescription, accounting for nearly 50 million antibiotic prescriptions annually in the United States, at least half of which are considered inappropriate. [Acute respiratory tract infections] account for more than two-thirds of antibiotic prescriptions for children, at least one-third of which are unnecessary,” according to the society.
Outpatient antibiotic stewardship efforts could focus on clinical encounters in which the medications could be avoided altogether, the AAP suggested.
“Examples include antibiotic prescribing for nonspecific upper respiratory infection, bronchiolitis, acute bronchitis, asthma exacerbation, or conjunctivitis,” the group said.
Given the epidemiology of bacterial infections seen in ambulatory care settings that warrant antibiotic therapy, researchers conservatively estimate “that antibiotic prescribing could be safely reduced by 30%,” the statement noted.
That said, treatment decisions are not always clear cut.
“Certain infections in children, such as ear infections and lung infections, can be caused by viruses, bacteria, or both at the same time,” Dr. Cosimini said. “As such, it is very difficult to know which children benefit from which antibiotics.”
Watching, waiting, vaccinating
Pediatricians know that many children with ear infections will get better without antibiotics. “Parents should know that their doctor may suggest watching an ear infection without antibiotics, as is the recommendation from the AAP,” Dr. Cosimini said.
Data indicate that doctors are not following this practice as often as they could be, he said.
When antibiotic treatment is needed during the shortage, agents other than amoxicillin suspension can be used.
“Even though amoxicillin suspension is our go-to antibiotic for many infections, there are effective alternative options,” Dr. Cosimini said. “Children’s Hospital of Philadelphia has a good list for doctors looking for alternatives.”
Another approach to reducing the use of antibiotics in the future involves preventing infections through vaccination.
Research shows that routine childhood vaccines may have averted millions of respiratory and ear infections. And because bacterial infections can follow viral infections, the annual flu vaccine and COVID-19 vaccines “are also great tools to reduce antibiotic use,” Dr. Cosimini said.
A turn to more toxic options?
The shortage of amoxicillin oral powder for suspension was reported by the Food and Drug Administration and the American Society of Health-System Pharmacists (ASHP) in October.
On Nov. 4, the Society of Infectious Diseases Pharmacists (SIDP) issued a statement on the amoxicillin shortage, noting that increased demand for the drug coincided with a surge in respiratory viral infections, including RSV and influenza, among children.
“Though supportive care is the mainstay of treatment for viral infections, antibiotics may be indicated for the treatment of superimposed bacterial infections, including pneumonia and acute otitis media,” the SIDP statement said. “While alternative antibiotics may be available depending on the indication, many have a broader spectrum of activity, increased toxicity, and excess cost relative to amoxicillin. Furthermore, it is anticipated alternatives may soon become in short supply as well, given increased usage.”
SIDP “encourages the judicious use of antibiotics” and supports watch-and-wait strategies and the use of the shortest effective duration of therapy when appropriate.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, monitors around 250 drug shortages at any given time.
The amoxicillin shortage, while not “overly worrisome,” stands out because of how widely the drug is used and the fact that the shortage appears to have been sparked by an increase in demand rather than supply chain or manufacturing quality problems that more typically lead to shortages, he said.
Unlike some other shortages, the amoxicillin shortfall largely does not involve disrupting a medication regimen that someone was already receiving, and substitutions should be available.
“That said, it’s very, very disruptive to parents or a caregiver when you have a sick child who needs an antibiotic and it’s not available,” Dr. Ganio said.
Can a poster change practice?
In an unrelated move, the U.S. Agency for Healthcare Research and Quality published new resources and strategies to reduce inappropriate antibiotic use in ambulatory care settings.
One of the tools is a poster that doctors can print and hang in their offices. It states: “We commit to only prescribing antibiotics when they will help you. Taking antibiotics when you do not need them will NOT make you better. You will still feel sick, and the antibiotic may give you a skin rash, diarrhea, or a yeast infection.”
Jeffrey A. Linder, MD, MPH, a general internist and researcher at Northwestern University in Chicago, helped develop some of the approaches to improve prescribing practices in primary care.
Dr. Linder explained on a recent episode of the Freakonomics, M.D. podcast that the poster can be key.
One reason clinicians may prescribe antibiotics inappropriately is because they assume – perhaps erroneously – that patients want and expect them. By addressing the issue up front by displaying the poster, they may be able to “short-circuit” that type of thinking.
A minority of patients do expect antibiotics. “But the vast majority of patients are thinking, ‘I don’t feel well, I want to know what’s going on, and I want to know how to feel better and what’s going to happen.’ ”
For their part, patients can tell their doctors that they want an antibiotic only if they really need it, Dr. Linder said.
A version of this article first appeared on Medscape.com.
Some experts are encouraging clinicians to see the amoxicillin shortage through pink-colored glasses.
The ongoing shortage, which was first reported in October and was prompted by a surge in demand linked in part to influenza and respiratory syncytial virus (RSV), could be an opportunity for clinicians to refine their prescribing practices and avoid unnecessary and potentially harmful orders for the medication, they say.
Antibiotics are often prescribed to patients who do not need them. In many cases, patients’ symptoms are caused by viral infections, not bacteria, so antibiotics do not help.
Even when symptoms resolve after a patient takes an antibiotic, the drug may have had nothing to do with their improvement.
Time to double-down on assessment; use antibiotics only when needed,” Jason Gallagher, PharmD, of Temple University School of Pharmacy in Philadelphia, posted on Twitter.
When antibiotics are not helping, they still may cause harm. Treatment with antibiotics entails risks for antibiotic resistance, infection with Clostridioides difficile, and side effects, such as rashes and – as Dr. Gallagher noted – diarrhea.
“They say ‘never let a good shortage go to waste,’ ” Michael Cosimini, MD, a pediatrician at Oregon Health & Science University, Portland, tweeted about the lack of amoxicillin in October.
Dr. Cosimini offered his thoughts about “improving our amoxicillin prescribing patterns” in pediatrics and encouraged colleagues to do so.
For example, he highlighted guidelines that state that antimicrobial therapy is not routinely required for preschool-aged children with community-acquired pneumonia (CAP) because most cases are caused by viral pathogens.
And trials show that when antibiotics are used for CAP, a shorter treatment duration, such as 5 days, rather than the standard 7-10 days, can be sufficient.
“As physicians, a shortage like this is an opportunity to do our best in the short term, as well as reflect on our current practice and make changes for the better in the long run,” Dr. Cosimini told this news organization.
Amoxicillin is the most commonly prescribed antibiotic in the outpatient setting and is the first choice among antimicrobial agents for common infections, such as otitis media, strep throat, and pneumonia, he said. “We use it frequently, so even small changes could go a long way to improve our prescribing practice,” Dr. Cosimini said.
Inappropriate antibiotic prescribing may be common
A 2021 statement on antibiotic stewardship from the American Academy of Pediatrics (AAP) declared that while antibiotics have saved countless lives, they can also cause harm and are frequently used inappropriately.
“One in five pediatric ambulatory visits result in an antibiotic prescription, accounting for nearly 50 million antibiotic prescriptions annually in the United States, at least half of which are considered inappropriate. [Acute respiratory tract infections] account for more than two-thirds of antibiotic prescriptions for children, at least one-third of which are unnecessary,” according to the society.
Outpatient antibiotic stewardship efforts could focus on clinical encounters in which the medications could be avoided altogether, the AAP suggested.
“Examples include antibiotic prescribing for nonspecific upper respiratory infection, bronchiolitis, acute bronchitis, asthma exacerbation, or conjunctivitis,” the group said.
Given the epidemiology of bacterial infections seen in ambulatory care settings that warrant antibiotic therapy, researchers conservatively estimate “that antibiotic prescribing could be safely reduced by 30%,” the statement noted.
That said, treatment decisions are not always clear cut.
“Certain infections in children, such as ear infections and lung infections, can be caused by viruses, bacteria, or both at the same time,” Dr. Cosimini said. “As such, it is very difficult to know which children benefit from which antibiotics.”
Watching, waiting, vaccinating
Pediatricians know that many children with ear infections will get better without antibiotics. “Parents should know that their doctor may suggest watching an ear infection without antibiotics, as is the recommendation from the AAP,” Dr. Cosimini said.
Data indicate that doctors are not following this practice as often as they could be, he said.
When antibiotic treatment is needed during the shortage, agents other than amoxicillin suspension can be used.
“Even though amoxicillin suspension is our go-to antibiotic for many infections, there are effective alternative options,” Dr. Cosimini said. “Children’s Hospital of Philadelphia has a good list for doctors looking for alternatives.”
Another approach to reducing the use of antibiotics in the future involves preventing infections through vaccination.
Research shows that routine childhood vaccines may have averted millions of respiratory and ear infections. And because bacterial infections can follow viral infections, the annual flu vaccine and COVID-19 vaccines “are also great tools to reduce antibiotic use,” Dr. Cosimini said.
A turn to more toxic options?
The shortage of amoxicillin oral powder for suspension was reported by the Food and Drug Administration and the American Society of Health-System Pharmacists (ASHP) in October.
On Nov. 4, the Society of Infectious Diseases Pharmacists (SIDP) issued a statement on the amoxicillin shortage, noting that increased demand for the drug coincided with a surge in respiratory viral infections, including RSV and influenza, among children.
“Though supportive care is the mainstay of treatment for viral infections, antibiotics may be indicated for the treatment of superimposed bacterial infections, including pneumonia and acute otitis media,” the SIDP statement said. “While alternative antibiotics may be available depending on the indication, many have a broader spectrum of activity, increased toxicity, and excess cost relative to amoxicillin. Furthermore, it is anticipated alternatives may soon become in short supply as well, given increased usage.”
SIDP “encourages the judicious use of antibiotics” and supports watch-and-wait strategies and the use of the shortest effective duration of therapy when appropriate.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, monitors around 250 drug shortages at any given time.
The amoxicillin shortage, while not “overly worrisome,” stands out because of how widely the drug is used and the fact that the shortage appears to have been sparked by an increase in demand rather than supply chain or manufacturing quality problems that more typically lead to shortages, he said.
Unlike some other shortages, the amoxicillin shortfall largely does not involve disrupting a medication regimen that someone was already receiving, and substitutions should be available.
“That said, it’s very, very disruptive to parents or a caregiver when you have a sick child who needs an antibiotic and it’s not available,” Dr. Ganio said.
Can a poster change practice?
In an unrelated move, the U.S. Agency for Healthcare Research and Quality published new resources and strategies to reduce inappropriate antibiotic use in ambulatory care settings.
One of the tools is a poster that doctors can print and hang in their offices. It states: “We commit to only prescribing antibiotics when they will help you. Taking antibiotics when you do not need them will NOT make you better. You will still feel sick, and the antibiotic may give you a skin rash, diarrhea, or a yeast infection.”
Jeffrey A. Linder, MD, MPH, a general internist and researcher at Northwestern University in Chicago, helped develop some of the approaches to improve prescribing practices in primary care.
Dr. Linder explained on a recent episode of the Freakonomics, M.D. podcast that the poster can be key.
One reason clinicians may prescribe antibiotics inappropriately is because they assume – perhaps erroneously – that patients want and expect them. By addressing the issue up front by displaying the poster, they may be able to “short-circuit” that type of thinking.
A minority of patients do expect antibiotics. “But the vast majority of patients are thinking, ‘I don’t feel well, I want to know what’s going on, and I want to know how to feel better and what’s going to happen.’ ”
For their part, patients can tell their doctors that they want an antibiotic only if they really need it, Dr. Linder said.
A version of this article first appeared on Medscape.com.
‘A huge deal’: Millions have long COVID, and more are expected
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
Is there a doctor on the plane? Tips for providing in-flight assistance
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.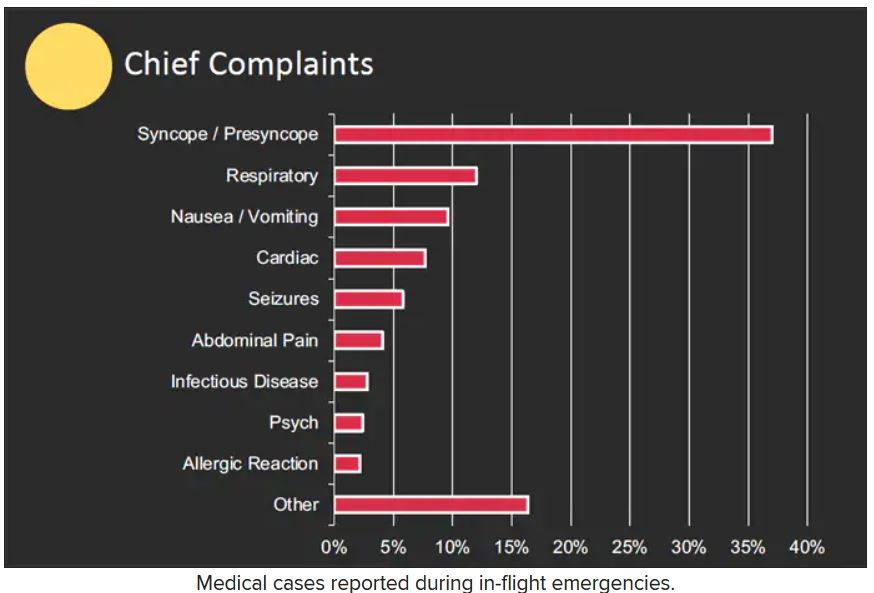
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACEP 2022
RSV causes 1 in 50 deaths in children under age 5: European study
But RSV – formally known as respiratory syncytial virus – is also a problem in high-income nations. In those countries, 1 in 56 otherwise healthy babies are hospitalized with RSV during their first year of life, said the study, which was published in the Lancet Respiratory Medicine.
Researchers looked at the health records of 9,154 infants born between July 1, 2017, and July 31, 2020, who were treated at health centers across Europe. Previous studies have concentrated on babies with preexisting conditions, but this one looked at otherwise healthy children, researchers said.
“This is the lowest-risk baby who is being hospitalized for this, so really, numbers are really much higher than I think some people would have guessed,” said study coauthor Louis Bont, MD, a professor of pediatric infectious diseases at Wilhelmina Children’s Hospital at University Medical Center Utrecht in the Netherlands, according to CNN. He is also chairman of the ReSViNET foundation, which aims to reduce RSV infection globally.
The study said more than 97% of deaths from RSV occur in low-income and middle-income countries. The study concluded that “maternal vaccination and passive [immunization] could have a profound impact on the RSV burden.”
In developed nations, children who get RSV usually survive because they have access to ventilators and other health care equipment. Still, just being treated for RSV can have long-range negative effects on a child’s health, Kristina Deeter, MD, chair of pediatrics at the University of Nevada, Reno, told CNN.
“Whether that is just traumatic psychosocial, emotional issues after hospitalization or even having more vulnerable lungs – you can develop asthma later on, for instance, if you’ve had a really severe infection at a young age – it can damage your lungs permanently,” she said of the study. “It’s still an important virus in our world and something that we really focus on.”
The Lancet study was published days after the CDC warned public health officials that respiratory viruses, including RSV, are surging among children across the country.
A version of this article first appeared on WebMD.com.
But RSV – formally known as respiratory syncytial virus – is also a problem in high-income nations. In those countries, 1 in 56 otherwise healthy babies are hospitalized with RSV during their first year of life, said the study, which was published in the Lancet Respiratory Medicine.
Researchers looked at the health records of 9,154 infants born between July 1, 2017, and July 31, 2020, who were treated at health centers across Europe. Previous studies have concentrated on babies with preexisting conditions, but this one looked at otherwise healthy children, researchers said.
“This is the lowest-risk baby who is being hospitalized for this, so really, numbers are really much higher than I think some people would have guessed,” said study coauthor Louis Bont, MD, a professor of pediatric infectious diseases at Wilhelmina Children’s Hospital at University Medical Center Utrecht in the Netherlands, according to CNN. He is also chairman of the ReSViNET foundation, which aims to reduce RSV infection globally.
The study said more than 97% of deaths from RSV occur in low-income and middle-income countries. The study concluded that “maternal vaccination and passive [immunization] could have a profound impact on the RSV burden.”
In developed nations, children who get RSV usually survive because they have access to ventilators and other health care equipment. Still, just being treated for RSV can have long-range negative effects on a child’s health, Kristina Deeter, MD, chair of pediatrics at the University of Nevada, Reno, told CNN.
“Whether that is just traumatic psychosocial, emotional issues after hospitalization or even having more vulnerable lungs – you can develop asthma later on, for instance, if you’ve had a really severe infection at a young age – it can damage your lungs permanently,” she said of the study. “It’s still an important virus in our world and something that we really focus on.”
The Lancet study was published days after the CDC warned public health officials that respiratory viruses, including RSV, are surging among children across the country.
A version of this article first appeared on WebMD.com.
But RSV – formally known as respiratory syncytial virus – is also a problem in high-income nations. In those countries, 1 in 56 otherwise healthy babies are hospitalized with RSV during their first year of life, said the study, which was published in the Lancet Respiratory Medicine.
Researchers looked at the health records of 9,154 infants born between July 1, 2017, and July 31, 2020, who were treated at health centers across Europe. Previous studies have concentrated on babies with preexisting conditions, but this one looked at otherwise healthy children, researchers said.
“This is the lowest-risk baby who is being hospitalized for this, so really, numbers are really much higher than I think some people would have guessed,” said study coauthor Louis Bont, MD, a professor of pediatric infectious diseases at Wilhelmina Children’s Hospital at University Medical Center Utrecht in the Netherlands, according to CNN. He is also chairman of the ReSViNET foundation, which aims to reduce RSV infection globally.
The study said more than 97% of deaths from RSV occur in low-income and middle-income countries. The study concluded that “maternal vaccination and passive [immunization] could have a profound impact on the RSV burden.”
In developed nations, children who get RSV usually survive because they have access to ventilators and other health care equipment. Still, just being treated for RSV can have long-range negative effects on a child’s health, Kristina Deeter, MD, chair of pediatrics at the University of Nevada, Reno, told CNN.
“Whether that is just traumatic psychosocial, emotional issues after hospitalization or even having more vulnerable lungs – you can develop asthma later on, for instance, if you’ve had a really severe infection at a young age – it can damage your lungs permanently,” she said of the study. “It’s still an important virus in our world and something that we really focus on.”
The Lancet study was published days after the CDC warned public health officials that respiratory viruses, including RSV, are surging among children across the country.
A version of this article first appeared on WebMD.com.
FROM LANCET RESPIRATORY MEDICINE
Children and COVID: Weekly cases continue to hold fairly steady
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.
The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.

The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.

The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.
Love them or hate them, masks in schools work
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The body of evidence for Paxlovid therapy
Dear Colleagues,
We have a mismatch. The evidence supporting treatment for Paxlovid is compelling for people aged 60 or over, but the older patients in the United States are much less likely to be treated. Not only was there a randomized, placebo-controlled trial of high-risk patients which showed 89% reduction of hospitalizations and deaths (median age, 45), but there have been multiple real-world effectiveness studies subsequently published that have partitioned the benefit for age 65 or older, such as the ones from Israel and Hong Kong (age 60+). Overall, the real-world effectiveness in the first month after treatment is at least as good, if not better, than in the high-risk randomized trial.
We’re doing the current survey to find out, but the most likely reasons include (1) lack of confidence of benefit; (2) medication interactions; and (3) concerns over rebound.
Let me address each of these briefly. The lack of confidence in benefit stems from the fact that the initial high-risk trial was in unvaccinated individuals. That concern can now be put aside because all of the several real-world studies confirming the protective benefit against hospitalizations and deaths are in people who have been vaccinated, and a significant proportion received booster shots.
The potential medication interactions due to the ritonavir component of the Paxlovid drug combination, attributable to its cytochrome P450 3A4 inhibition, have been unduly emphasized. There are many drug-interaction checkers for Paxlovid, but this one from the University of Liverpool is user friendly, color- and icon-coded, and shows that the vast majority of interactions can be sidestepped by discontinuing the medication of concern for the length of the Paxlovid treatment, 5 days. The simple chart is provided in my recent substack newsletter.
As far as rebound, this problem has unfortunately been exaggerated because of lack of prospective systematic studies and appreciation that a positive test of clinical symptom rebound can occur without Paxlovid. There are soon to be multiple reports that the incidence of Paxlovid rebound is fairly low, in the range of 10%. That concern should not be a reason to withhold treatment.
Now the plot thickens. A new preprint report from the Veterans Health Administration, the largest health care system in the United States, looks at 90-day outcomes of about 9,000 Paxlovid-treated patients and approximately 47,000 controls. Not only was there a 26% reduction in long COVID, but of the breakdown of 12 organs/systems and symptoms, 10 of 12 were significantly reduced with Paxlovid, including pulmonary embolism, deep vein thrombosis, and neurocognitive impairment. There was also a 48% reduction in death and a 30% reduction in hospitalizations after the first 30 days. I have reviewed all of these data and put them in context in a recent newsletter. A key point is that the magnitude of benefit was unaffected by vaccination or booster status, or prior COVID infections, or unvaccinated status. Also, it was the same for men and women, as well as for age > 70 and age < 60. These findings all emphasize a new reason to be using Paxlovid therapy, and if replicated, Paxlovid may even be indicated for younger patients (who are at low risk for hospitalizations and deaths but at increased risk for long COVID).
In summary, for older patients, we should be thinking of why we should be using Paxlovid rather than the reason not to treat. We’ll be interested in the survey results to understand the mismatch better, and we look forward to your ideas and feedback to make better use of this treatment for the people who need it the most.
Sincerely yours, Eric J. Topol, MD
Dr. Topol reports no conflicts of interest with Pfizer; he receives no honoraria or speaker fees, does not serve in an advisory role, and has no financial association with the company.
A version of this article first appeared on Medscape.com.
Dear Colleagues,
We have a mismatch. The evidence supporting treatment for Paxlovid is compelling for people aged 60 or over, but the older patients in the United States are much less likely to be treated. Not only was there a randomized, placebo-controlled trial of high-risk patients which showed 89% reduction of hospitalizations and deaths (median age, 45), but there have been multiple real-world effectiveness studies subsequently published that have partitioned the benefit for age 65 or older, such as the ones from Israel and Hong Kong (age 60+). Overall, the real-world effectiveness in the first month after treatment is at least as good, if not better, than in the high-risk randomized trial.
We’re doing the current survey to find out, but the most likely reasons include (1) lack of confidence of benefit; (2) medication interactions; and (3) concerns over rebound.
Let me address each of these briefly. The lack of confidence in benefit stems from the fact that the initial high-risk trial was in unvaccinated individuals. That concern can now be put aside because all of the several real-world studies confirming the protective benefit against hospitalizations and deaths are in people who have been vaccinated, and a significant proportion received booster shots.
The potential medication interactions due to the ritonavir component of the Paxlovid drug combination, attributable to its cytochrome P450 3A4 inhibition, have been unduly emphasized. There are many drug-interaction checkers for Paxlovid, but this one from the University of Liverpool is user friendly, color- and icon-coded, and shows that the vast majority of interactions can be sidestepped by discontinuing the medication of concern for the length of the Paxlovid treatment, 5 days. The simple chart is provided in my recent substack newsletter.
As far as rebound, this problem has unfortunately been exaggerated because of lack of prospective systematic studies and appreciation that a positive test of clinical symptom rebound can occur without Paxlovid. There are soon to be multiple reports that the incidence of Paxlovid rebound is fairly low, in the range of 10%. That concern should not be a reason to withhold treatment.
Now the plot thickens. A new preprint report from the Veterans Health Administration, the largest health care system in the United States, looks at 90-day outcomes of about 9,000 Paxlovid-treated patients and approximately 47,000 controls. Not only was there a 26% reduction in long COVID, but of the breakdown of 12 organs/systems and symptoms, 10 of 12 were significantly reduced with Paxlovid, including pulmonary embolism, deep vein thrombosis, and neurocognitive impairment. There was also a 48% reduction in death and a 30% reduction in hospitalizations after the first 30 days. I have reviewed all of these data and put them in context in a recent newsletter. A key point is that the magnitude of benefit was unaffected by vaccination or booster status, or prior COVID infections, or unvaccinated status. Also, it was the same for men and women, as well as for age > 70 and age < 60. These findings all emphasize a new reason to be using Paxlovid therapy, and if replicated, Paxlovid may even be indicated for younger patients (who are at low risk for hospitalizations and deaths but at increased risk for long COVID).
In summary, for older patients, we should be thinking of why we should be using Paxlovid rather than the reason not to treat. We’ll be interested in the survey results to understand the mismatch better, and we look forward to your ideas and feedback to make better use of this treatment for the people who need it the most.
Sincerely yours, Eric J. Topol, MD
Dr. Topol reports no conflicts of interest with Pfizer; he receives no honoraria or speaker fees, does not serve in an advisory role, and has no financial association with the company.
A version of this article first appeared on Medscape.com.
Dear Colleagues,
We have a mismatch. The evidence supporting treatment for Paxlovid is compelling for people aged 60 or over, but the older patients in the United States are much less likely to be treated. Not only was there a randomized, placebo-controlled trial of high-risk patients which showed 89% reduction of hospitalizations and deaths (median age, 45), but there have been multiple real-world effectiveness studies subsequently published that have partitioned the benefit for age 65 or older, such as the ones from Israel and Hong Kong (age 60+). Overall, the real-world effectiveness in the first month after treatment is at least as good, if not better, than in the high-risk randomized trial.
We’re doing the current survey to find out, but the most likely reasons include (1) lack of confidence of benefit; (2) medication interactions; and (3) concerns over rebound.
Let me address each of these briefly. The lack of confidence in benefit stems from the fact that the initial high-risk trial was in unvaccinated individuals. That concern can now be put aside because all of the several real-world studies confirming the protective benefit against hospitalizations and deaths are in people who have been vaccinated, and a significant proportion received booster shots.
The potential medication interactions due to the ritonavir component of the Paxlovid drug combination, attributable to its cytochrome P450 3A4 inhibition, have been unduly emphasized. There are many drug-interaction checkers for Paxlovid, but this one from the University of Liverpool is user friendly, color- and icon-coded, and shows that the vast majority of interactions can be sidestepped by discontinuing the medication of concern for the length of the Paxlovid treatment, 5 days. The simple chart is provided in my recent substack newsletter.
As far as rebound, this problem has unfortunately been exaggerated because of lack of prospective systematic studies and appreciation that a positive test of clinical symptom rebound can occur without Paxlovid. There are soon to be multiple reports that the incidence of Paxlovid rebound is fairly low, in the range of 10%. That concern should not be a reason to withhold treatment.
Now the plot thickens. A new preprint report from the Veterans Health Administration, the largest health care system in the United States, looks at 90-day outcomes of about 9,000 Paxlovid-treated patients and approximately 47,000 controls. Not only was there a 26% reduction in long COVID, but of the breakdown of 12 organs/systems and symptoms, 10 of 12 were significantly reduced with Paxlovid, including pulmonary embolism, deep vein thrombosis, and neurocognitive impairment. There was also a 48% reduction in death and a 30% reduction in hospitalizations after the first 30 days. I have reviewed all of these data and put them in context in a recent newsletter. A key point is that the magnitude of benefit was unaffected by vaccination or booster status, or prior COVID infections, or unvaccinated status. Also, it was the same for men and women, as well as for age > 70 and age < 60. These findings all emphasize a new reason to be using Paxlovid therapy, and if replicated, Paxlovid may even be indicated for younger patients (who are at low risk for hospitalizations and deaths but at increased risk for long COVID).
In summary, for older patients, we should be thinking of why we should be using Paxlovid rather than the reason not to treat. We’ll be interested in the survey results to understand the mismatch better, and we look forward to your ideas and feedback to make better use of this treatment for the people who need it the most.
Sincerely yours, Eric J. Topol, MD
Dr. Topol reports no conflicts of interest with Pfizer; he receives no honoraria or speaker fees, does not serve in an advisory role, and has no financial association with the company.
A version of this article first appeared on Medscape.com.
Repeat COVID infection doubles mortality risk
Getting COVID-19 a second time doubles a person’s chance of dying and triples the likelihood of being hospitalized in the next 6 months, a new study found.
Vaccination and booster status did not improve survival or hospitalization rates among people who were infected more than once.
“Reinfection with COVID-19 increases the risk of both acute outcomes and long COVID,” study author Ziyad Al-Aly, MD, told Reuters. “This was evident in unvaccinated, vaccinated and boosted people.”
The study was published in the journal Nature Medicine.
Researchers analyzed U.S. Department of Veterans Affairs data, including 443,588 people with a first infection of SARS-CoV-2, 40,947 people who were infected two or more times, and 5.3 million people who had not been infected with coronavirus, whose data served as the control group.
“During the past few months, there’s been an air of invincibility among people who have had COVID-19 or their vaccinations and boosters, and especially among people who have had an infection and also received vaccines; some people started to [refer] to these individuals as having a sort of superimmunity to the virus,” Dr. Al-Aly said in a press release from Washington University in St. Louis. “Without ambiguity, our research showed that getting an infection a second, third or fourth time contributes to additional health risks in the acute phase, meaning the first 30 days after infection, and in the months beyond, meaning the long COVID phase.”
Being infected with COVID-19 more than once also dramatically increased the risk of developing lung problems, heart conditions, or brain conditions. The heightened risks persisted for 6 months.
Researchers said a limitation of their study was that data primarily came from White males.
An expert not involved in the study told Reuters that the Veterans Affairs population does not reflect the general population. Patients at VA health facilities are generally older with more than normal health complications, said John Moore, PhD, a professor of microbiology and immunology at Weill Cornell Medicine, New York.
Dr. Al-Aly encouraged people to be vigilant as they plan for the holiday season, Reuters reported.
“We had started seeing a lot of patients coming to the clinic with an air of invincibility,” he told Reuters. “They wondered, ‘Does getting a reinfection really matter?’ The answer is yes, it absolutely does.”
A version of this article first appeared on WebMD.com.
Getting COVID-19 a second time doubles a person’s chance of dying and triples the likelihood of being hospitalized in the next 6 months, a new study found.
Vaccination and booster status did not improve survival or hospitalization rates among people who were infected more than once.
“Reinfection with COVID-19 increases the risk of both acute outcomes and long COVID,” study author Ziyad Al-Aly, MD, told Reuters. “This was evident in unvaccinated, vaccinated and boosted people.”
The study was published in the journal Nature Medicine.
Researchers analyzed U.S. Department of Veterans Affairs data, including 443,588 people with a first infection of SARS-CoV-2, 40,947 people who were infected two or more times, and 5.3 million people who had not been infected with coronavirus, whose data served as the control group.
“During the past few months, there’s been an air of invincibility among people who have had COVID-19 or their vaccinations and boosters, and especially among people who have had an infection and also received vaccines; some people started to [refer] to these individuals as having a sort of superimmunity to the virus,” Dr. Al-Aly said in a press release from Washington University in St. Louis. “Without ambiguity, our research showed that getting an infection a second, third or fourth time contributes to additional health risks in the acute phase, meaning the first 30 days after infection, and in the months beyond, meaning the long COVID phase.”
Being infected with COVID-19 more than once also dramatically increased the risk of developing lung problems, heart conditions, or brain conditions. The heightened risks persisted for 6 months.
Researchers said a limitation of their study was that data primarily came from White males.
An expert not involved in the study told Reuters that the Veterans Affairs population does not reflect the general population. Patients at VA health facilities are generally older with more than normal health complications, said John Moore, PhD, a professor of microbiology and immunology at Weill Cornell Medicine, New York.
Dr. Al-Aly encouraged people to be vigilant as they plan for the holiday season, Reuters reported.
“We had started seeing a lot of patients coming to the clinic with an air of invincibility,” he told Reuters. “They wondered, ‘Does getting a reinfection really matter?’ The answer is yes, it absolutely does.”
A version of this article first appeared on WebMD.com.
Getting COVID-19 a second time doubles a person’s chance of dying and triples the likelihood of being hospitalized in the next 6 months, a new study found.
Vaccination and booster status did not improve survival or hospitalization rates among people who were infected more than once.
“Reinfection with COVID-19 increases the risk of both acute outcomes and long COVID,” study author Ziyad Al-Aly, MD, told Reuters. “This was evident in unvaccinated, vaccinated and boosted people.”
The study was published in the journal Nature Medicine.
Researchers analyzed U.S. Department of Veterans Affairs data, including 443,588 people with a first infection of SARS-CoV-2, 40,947 people who were infected two or more times, and 5.3 million people who had not been infected with coronavirus, whose data served as the control group.
“During the past few months, there’s been an air of invincibility among people who have had COVID-19 or their vaccinations and boosters, and especially among people who have had an infection and also received vaccines; some people started to [refer] to these individuals as having a sort of superimmunity to the virus,” Dr. Al-Aly said in a press release from Washington University in St. Louis. “Without ambiguity, our research showed that getting an infection a second, third or fourth time contributes to additional health risks in the acute phase, meaning the first 30 days after infection, and in the months beyond, meaning the long COVID phase.”
Being infected with COVID-19 more than once also dramatically increased the risk of developing lung problems, heart conditions, or brain conditions. The heightened risks persisted for 6 months.
Researchers said a limitation of their study was that data primarily came from White males.
An expert not involved in the study told Reuters that the Veterans Affairs population does not reflect the general population. Patients at VA health facilities are generally older with more than normal health complications, said John Moore, PhD, a professor of microbiology and immunology at Weill Cornell Medicine, New York.
Dr. Al-Aly encouraged people to be vigilant as they plan for the holiday season, Reuters reported.
“We had started seeing a lot of patients coming to the clinic with an air of invincibility,” he told Reuters. “They wondered, ‘Does getting a reinfection really matter?’ The answer is yes, it absolutely does.”
A version of this article first appeared on WebMD.com.
FROM NATURE MEDICINE
No benefit of rivaroxaban in COVID outpatients: PREVENT-HD
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
FROM AHA 2022