User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
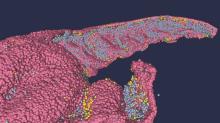
Likely cause of mysterious hepatitis outbreak in children identified
Coinfection with AAV2 and a human adenovirus (HAdV), in particular, appears to leave some children more vulnerable to this acute hepatitis of unknown origin, researchers reported in three studies published online in Nature. Coinfection with Epstein-Barr virus (EBV), herpes, and enterovirus also were found. Adeno-associated viruses are not considered pathogenic on their own and require a “helper” virus for productive infection.
“I am quite confident that we have identified the key viruses involved because we used a comprehensive metagenomic sequencing approach to look for potential infections from any virus or non-viral pathogen,” Charles Chiu, MD, PhD, senior author and professor of laboratory medicine and medicine/infectious diseases at the University of California, San Francisco, said in an interview.
Dr. Chiu and colleagues propose that lockdowns and social isolation during the COVID-19 pandemic left more children susceptible. A major aspect of immunity in childhood is the adaptive immune response – both cell-mediated and humoral – shaped in part by exposure to viruses and other pathogens early in life, Dr. Chiu said.
“Due to COVID-19, a large population of children did not experience this, so it is possible once restrictions were lifted, they were suddenly exposed over a short period of time to multiple viruses that, in a poorly trained immune system, would have increased their risk of developing severe disease,” he said.
This theory has been popular, especially because cases of unexplained acute hepatitis peaked during the height of the COVID-19 pandemic when isolation was common, William F. Balistreri, MD, who was not affiliated with the study, told this news organization. Dr. Balistreri is professor of pediatrics and director emeritus of the Pediatric Liver Care Center at Cincinnati Children’s Hospital Medical Center.
Identifying the culprits
Determining what factors might be involved was the main aim of the etiology study by Dr. Chiu and colleagues published online in Nature.
The journal simultaneously published a genomic study confirming the presence of AAV2 and other suspected viruses and a genomic and laboratory study further corroborating the results.
More than 1,000 children worldwide had been diagnosed with unexplained acute pediatric hepatitis as of August 2022. In the United States, there have been 358 cases, including 22 in which the child required a liver transplant and 13 in which the child died.
This new form of hepatitis, first detected in October 2021, does not fit into existing classifications of types A through E, so some researchers refer to the condition as acute non–A-E hepatitis of unknown etiology.
The investigators started with an important clue based on previous research: the role adenovirus might play. Dr. Chiu and colleagues assessed 27 blood, stool, and other samples from 16 affected children who each previously tested positive for adenoviruses. The researchers included cases of the condition identified up until May 22, 2022. The median age was 3 years, and approximately half were boys.
They compared viruses present in these children with those in 113 controls without the mysterious hepatitis. The control group consisted of 15 children who were hospitalized with a nonhepatitis inflammatory condition, 27 with a noninflammatory condition, 30 with acute hepatitis of known origin, 12 with acute gastroenteritis and an HAdV-positive stool sample, and 11 with acute gastroenteritis and an HAdV-negative stool sample, as well as 18 blood donors. The median age was 7 years.
The researchers assessed samples using multiple technologies, including metagenomic sequencing, tiling multiplex polymerase chain reaction (PCR) amplicon sequencing, metagenomic sequencing with probe capture viral enrichment, and virus-specific PCR. Many of these advanced techniques were not even available 5-10 years ago, Dr. Chiu said.
Key findings
Blood samples were available for 14 of the 16 children with acute hepatitis of unknown origin. Among this study group, AAV2 was found in 13 (93%). No other adeno-associated viruses were found. HAdV was detected in all 14 children: HAdV-41 in 11 children and HAdV-40, HAdV-2, and an untypeable strain in one child each. This finding was not intuitive because HAdVs are not commonly associated with hepatitis, according to the study.
AAV2 was much less common in the control group. For example, it was found in none of the children with hepatitis of known origin and in only four children (3.5%) with acute gastroenteritis and HAdV-positive stool. Of note, neither AAV2 nor HAdV-41 was detected among the 30 pediatric controls with acute hepatitis of defined etiology nor 42 of the hospitalized children without hepatitis, the researchers wrote.
In the search for other viruses in the study group, metagenomic sequencing detected EBV, also known as human herpesvirus (HHV)–4, in two children, cytomegalovirus (CMV) in one child, and HAdV type C in one child.
Analysis of whole blood revealed enterovirus A71 in one patient. HAdV type C also was detected in one child on the basis of a nasopharyngeal swab, and picobirnavirus was found in a stool sample from another patient.
Researchers conducted virus-specific PCR tests on both patient groups to identify additional viruses that may be associated with the unexplained acute hepatitis. EBV/HHV-4 was detected in 11 children (79%) in the study group vs. in 1 child (0.88%) in the control group. HHV-6 was detected in seven children (50%) in the study group, compared with one case in the control group. CMV was not detected in any of the children in the study group versus vs. two children (1.8%) in the control group.
“Although we found significant differences in the relative proportions of EBV and HHV-6 in cases compared to controls, we do not believe that these viruses are the primary cause of acute severe hepatitis,” the researchers wrote. The viral load of the two herpes viruses were very low, so the positive results could represent integrated proviral DNA rather than bona fide low-level herpesvirus. In addition, herpesvirus can be reactivated by an inflammatory condition.
“Nevertheless, it is striking that among the 16 cases (in the study group), dual, triple, or quadruple infections with AAV2, adenovirus, and one or both herpesviruses were detected in whole blood from at least 12 cases (75%),” the researchers wrote.
Management of suspected hepatitis
The study’s key messages for parents and health care providers “are awareness and reassurance,” Dr. Balistreri said in an interview.
Vigilance also is warranted if a child develops prodromal symptoms including respiratory and/or gastrointestinal signs such as nausea, vomiting, diarrhea, and abdomen pain, he said. If jaundice or scleral icterus is noted, then hepatitis should be suspected.
Some patients need hospitalization and quickly recover. In very rare instances, the inflammation may progress to liver failure and transplantation, Dr. Balistreri said.
“Reassurance is based on the good news that most children with acute hepatitis get better. If a case arises, it is good practice to keep the child well hydrated, offer a normal diet, and avoid medications that may be cleared by the liver,” Dr. Balistreri added.
“Of course, COVID-19 vaccination is strongly suggested,” he said.
Some existing treatments could help against unexplained acute hepatitis, Dr. Chiu said. “The findings suggest that antiviral therapy might be effective in these cases.”
Cidofovir can be effective against adenovirus, according to a report in The Lancet . Similarly, ganciclovir or valganciclovir may have activity against EBV/HHV-4 or HHV-6, Dr. Chiu said. “However, antiviral therapy is not available for AAV2.”
The three studies published in Nature “offer compelling evidence, from disparate centers, of a linkage of outbreak cases to infection by AAV2,” Dr. Balistreri said. The studies also suggest that liver injury was related to abnormal immune responses. This is an important clinical distinction, indicating a potential therapeutic approach to future cases – immunosuppression rather than anti-adenoviral agents, he said.
“We await further studies of this important concept,” Dr. Balistreri said.
Many unanswered questions remain about the condition’s etiology, he added. Is there a synergy or shared susceptibility related to SARS-CoV-2? Is the COVID-19 virus helping to trigger these infections, or does it increase the risk once infected? Also, are other epigenetic factors or viruses involved?
Moving forward
The next steps in the research could go beyond identifying presence of these different viruses and determining which one(s) are contributing the most to the acute pediatric hepatitis, Dr. Chiu said.
The researchers also would like to test early results from the United Kingdom that identified a potential association of acute severe hepatitis with the presence of human leukocyte antigen genotype DRB1*04:01, he added.
They also might investigate other unintended potential clinical consequences of the COVID-19 pandemic, including long COVID and resurgence of infections from other viruses, such as respiratory syncytial virus, influenza, and enterovirus D68.
The study was supported by the Centers for Disease Control and Prevention, the National Institutes of Health, the Department of Homeland Security, and other grants. Dr. Chiu is a founder of Delve Bio and on the scientific advisory board for Delve Bio, Mammoth Biosciences, BiomeSense, and Poppy Health. Dr. Balistreri had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Coinfection with AAV2 and a human adenovirus (HAdV), in particular, appears to leave some children more vulnerable to this acute hepatitis of unknown origin, researchers reported in three studies published online in Nature. Coinfection with Epstein-Barr virus (EBV), herpes, and enterovirus also were found. Adeno-associated viruses are not considered pathogenic on their own and require a “helper” virus for productive infection.
“I am quite confident that we have identified the key viruses involved because we used a comprehensive metagenomic sequencing approach to look for potential infections from any virus or non-viral pathogen,” Charles Chiu, MD, PhD, senior author and professor of laboratory medicine and medicine/infectious diseases at the University of California, San Francisco, said in an interview.
Dr. Chiu and colleagues propose that lockdowns and social isolation during the COVID-19 pandemic left more children susceptible. A major aspect of immunity in childhood is the adaptive immune response – both cell-mediated and humoral – shaped in part by exposure to viruses and other pathogens early in life, Dr. Chiu said.
“Due to COVID-19, a large population of children did not experience this, so it is possible once restrictions were lifted, they were suddenly exposed over a short period of time to multiple viruses that, in a poorly trained immune system, would have increased their risk of developing severe disease,” he said.
This theory has been popular, especially because cases of unexplained acute hepatitis peaked during the height of the COVID-19 pandemic when isolation was common, William F. Balistreri, MD, who was not affiliated with the study, told this news organization. Dr. Balistreri is professor of pediatrics and director emeritus of the Pediatric Liver Care Center at Cincinnati Children’s Hospital Medical Center.
Identifying the culprits
Determining what factors might be involved was the main aim of the etiology study by Dr. Chiu and colleagues published online in Nature.
The journal simultaneously published a genomic study confirming the presence of AAV2 and other suspected viruses and a genomic and laboratory study further corroborating the results.
More than 1,000 children worldwide had been diagnosed with unexplained acute pediatric hepatitis as of August 2022. In the United States, there have been 358 cases, including 22 in which the child required a liver transplant and 13 in which the child died.
This new form of hepatitis, first detected in October 2021, does not fit into existing classifications of types A through E, so some researchers refer to the condition as acute non–A-E hepatitis of unknown etiology.
The investigators started with an important clue based on previous research: the role adenovirus might play. Dr. Chiu and colleagues assessed 27 blood, stool, and other samples from 16 affected children who each previously tested positive for adenoviruses. The researchers included cases of the condition identified up until May 22, 2022. The median age was 3 years, and approximately half were boys.
They compared viruses present in these children with those in 113 controls without the mysterious hepatitis. The control group consisted of 15 children who were hospitalized with a nonhepatitis inflammatory condition, 27 with a noninflammatory condition, 30 with acute hepatitis of known origin, 12 with acute gastroenteritis and an HAdV-positive stool sample, and 11 with acute gastroenteritis and an HAdV-negative stool sample, as well as 18 blood donors. The median age was 7 years.
The researchers assessed samples using multiple technologies, including metagenomic sequencing, tiling multiplex polymerase chain reaction (PCR) amplicon sequencing, metagenomic sequencing with probe capture viral enrichment, and virus-specific PCR. Many of these advanced techniques were not even available 5-10 years ago, Dr. Chiu said.
Key findings
Blood samples were available for 14 of the 16 children with acute hepatitis of unknown origin. Among this study group, AAV2 was found in 13 (93%). No other adeno-associated viruses were found. HAdV was detected in all 14 children: HAdV-41 in 11 children and HAdV-40, HAdV-2, and an untypeable strain in one child each. This finding was not intuitive because HAdVs are not commonly associated with hepatitis, according to the study.
AAV2 was much less common in the control group. For example, it was found in none of the children with hepatitis of known origin and in only four children (3.5%) with acute gastroenteritis and HAdV-positive stool. Of note, neither AAV2 nor HAdV-41 was detected among the 30 pediatric controls with acute hepatitis of defined etiology nor 42 of the hospitalized children without hepatitis, the researchers wrote.
In the search for other viruses in the study group, metagenomic sequencing detected EBV, also known as human herpesvirus (HHV)–4, in two children, cytomegalovirus (CMV) in one child, and HAdV type C in one child.
Analysis of whole blood revealed enterovirus A71 in one patient. HAdV type C also was detected in one child on the basis of a nasopharyngeal swab, and picobirnavirus was found in a stool sample from another patient.
Researchers conducted virus-specific PCR tests on both patient groups to identify additional viruses that may be associated with the unexplained acute hepatitis. EBV/HHV-4 was detected in 11 children (79%) in the study group vs. in 1 child (0.88%) in the control group. HHV-6 was detected in seven children (50%) in the study group, compared with one case in the control group. CMV was not detected in any of the children in the study group versus vs. two children (1.8%) in the control group.
“Although we found significant differences in the relative proportions of EBV and HHV-6 in cases compared to controls, we do not believe that these viruses are the primary cause of acute severe hepatitis,” the researchers wrote. The viral load of the two herpes viruses were very low, so the positive results could represent integrated proviral DNA rather than bona fide low-level herpesvirus. In addition, herpesvirus can be reactivated by an inflammatory condition.
“Nevertheless, it is striking that among the 16 cases (in the study group), dual, triple, or quadruple infections with AAV2, adenovirus, and one or both herpesviruses were detected in whole blood from at least 12 cases (75%),” the researchers wrote.
Management of suspected hepatitis
The study’s key messages for parents and health care providers “are awareness and reassurance,” Dr. Balistreri said in an interview.
Vigilance also is warranted if a child develops prodromal symptoms including respiratory and/or gastrointestinal signs such as nausea, vomiting, diarrhea, and abdomen pain, he said. If jaundice or scleral icterus is noted, then hepatitis should be suspected.
Some patients need hospitalization and quickly recover. In very rare instances, the inflammation may progress to liver failure and transplantation, Dr. Balistreri said.
“Reassurance is based on the good news that most children with acute hepatitis get better. If a case arises, it is good practice to keep the child well hydrated, offer a normal diet, and avoid medications that may be cleared by the liver,” Dr. Balistreri added.
“Of course, COVID-19 vaccination is strongly suggested,” he said.
Some existing treatments could help against unexplained acute hepatitis, Dr. Chiu said. “The findings suggest that antiviral therapy might be effective in these cases.”
Cidofovir can be effective against adenovirus, according to a report in The Lancet . Similarly, ganciclovir or valganciclovir may have activity against EBV/HHV-4 or HHV-6, Dr. Chiu said. “However, antiviral therapy is not available for AAV2.”
The three studies published in Nature “offer compelling evidence, from disparate centers, of a linkage of outbreak cases to infection by AAV2,” Dr. Balistreri said. The studies also suggest that liver injury was related to abnormal immune responses. This is an important clinical distinction, indicating a potential therapeutic approach to future cases – immunosuppression rather than anti-adenoviral agents, he said.
“We await further studies of this important concept,” Dr. Balistreri said.
Many unanswered questions remain about the condition’s etiology, he added. Is there a synergy or shared susceptibility related to SARS-CoV-2? Is the COVID-19 virus helping to trigger these infections, or does it increase the risk once infected? Also, are other epigenetic factors or viruses involved?
Moving forward
The next steps in the research could go beyond identifying presence of these different viruses and determining which one(s) are contributing the most to the acute pediatric hepatitis, Dr. Chiu said.
The researchers also would like to test early results from the United Kingdom that identified a potential association of acute severe hepatitis with the presence of human leukocyte antigen genotype DRB1*04:01, he added.
They also might investigate other unintended potential clinical consequences of the COVID-19 pandemic, including long COVID and resurgence of infections from other viruses, such as respiratory syncytial virus, influenza, and enterovirus D68.
The study was supported by the Centers for Disease Control and Prevention, the National Institutes of Health, the Department of Homeland Security, and other grants. Dr. Chiu is a founder of Delve Bio and on the scientific advisory board for Delve Bio, Mammoth Biosciences, BiomeSense, and Poppy Health. Dr. Balistreri had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Coinfection with AAV2 and a human adenovirus (HAdV), in particular, appears to leave some children more vulnerable to this acute hepatitis of unknown origin, researchers reported in three studies published online in Nature. Coinfection with Epstein-Barr virus (EBV), herpes, and enterovirus also were found. Adeno-associated viruses are not considered pathogenic on their own and require a “helper” virus for productive infection.
“I am quite confident that we have identified the key viruses involved because we used a comprehensive metagenomic sequencing approach to look for potential infections from any virus or non-viral pathogen,” Charles Chiu, MD, PhD, senior author and professor of laboratory medicine and medicine/infectious diseases at the University of California, San Francisco, said in an interview.
Dr. Chiu and colleagues propose that lockdowns and social isolation during the COVID-19 pandemic left more children susceptible. A major aspect of immunity in childhood is the adaptive immune response – both cell-mediated and humoral – shaped in part by exposure to viruses and other pathogens early in life, Dr. Chiu said.
“Due to COVID-19, a large population of children did not experience this, so it is possible once restrictions were lifted, they were suddenly exposed over a short period of time to multiple viruses that, in a poorly trained immune system, would have increased their risk of developing severe disease,” he said.
This theory has been popular, especially because cases of unexplained acute hepatitis peaked during the height of the COVID-19 pandemic when isolation was common, William F. Balistreri, MD, who was not affiliated with the study, told this news organization. Dr. Balistreri is professor of pediatrics and director emeritus of the Pediatric Liver Care Center at Cincinnati Children’s Hospital Medical Center.
Identifying the culprits
Determining what factors might be involved was the main aim of the etiology study by Dr. Chiu and colleagues published online in Nature.
The journal simultaneously published a genomic study confirming the presence of AAV2 and other suspected viruses and a genomic and laboratory study further corroborating the results.
More than 1,000 children worldwide had been diagnosed with unexplained acute pediatric hepatitis as of August 2022. In the United States, there have been 358 cases, including 22 in which the child required a liver transplant and 13 in which the child died.
This new form of hepatitis, first detected in October 2021, does not fit into existing classifications of types A through E, so some researchers refer to the condition as acute non–A-E hepatitis of unknown etiology.
The investigators started with an important clue based on previous research: the role adenovirus might play. Dr. Chiu and colleagues assessed 27 blood, stool, and other samples from 16 affected children who each previously tested positive for adenoviruses. The researchers included cases of the condition identified up until May 22, 2022. The median age was 3 years, and approximately half were boys.
They compared viruses present in these children with those in 113 controls without the mysterious hepatitis. The control group consisted of 15 children who were hospitalized with a nonhepatitis inflammatory condition, 27 with a noninflammatory condition, 30 with acute hepatitis of known origin, 12 with acute gastroenteritis and an HAdV-positive stool sample, and 11 with acute gastroenteritis and an HAdV-negative stool sample, as well as 18 blood donors. The median age was 7 years.
The researchers assessed samples using multiple technologies, including metagenomic sequencing, tiling multiplex polymerase chain reaction (PCR) amplicon sequencing, metagenomic sequencing with probe capture viral enrichment, and virus-specific PCR. Many of these advanced techniques were not even available 5-10 years ago, Dr. Chiu said.
Key findings
Blood samples were available for 14 of the 16 children with acute hepatitis of unknown origin. Among this study group, AAV2 was found in 13 (93%). No other adeno-associated viruses were found. HAdV was detected in all 14 children: HAdV-41 in 11 children and HAdV-40, HAdV-2, and an untypeable strain in one child each. This finding was not intuitive because HAdVs are not commonly associated with hepatitis, according to the study.
AAV2 was much less common in the control group. For example, it was found in none of the children with hepatitis of known origin and in only four children (3.5%) with acute gastroenteritis and HAdV-positive stool. Of note, neither AAV2 nor HAdV-41 was detected among the 30 pediatric controls with acute hepatitis of defined etiology nor 42 of the hospitalized children without hepatitis, the researchers wrote.
In the search for other viruses in the study group, metagenomic sequencing detected EBV, also known as human herpesvirus (HHV)–4, in two children, cytomegalovirus (CMV) in one child, and HAdV type C in one child.
Analysis of whole blood revealed enterovirus A71 in one patient. HAdV type C also was detected in one child on the basis of a nasopharyngeal swab, and picobirnavirus was found in a stool sample from another patient.
Researchers conducted virus-specific PCR tests on both patient groups to identify additional viruses that may be associated with the unexplained acute hepatitis. EBV/HHV-4 was detected in 11 children (79%) in the study group vs. in 1 child (0.88%) in the control group. HHV-6 was detected in seven children (50%) in the study group, compared with one case in the control group. CMV was not detected in any of the children in the study group versus vs. two children (1.8%) in the control group.
“Although we found significant differences in the relative proportions of EBV and HHV-6 in cases compared to controls, we do not believe that these viruses are the primary cause of acute severe hepatitis,” the researchers wrote. The viral load of the two herpes viruses were very low, so the positive results could represent integrated proviral DNA rather than bona fide low-level herpesvirus. In addition, herpesvirus can be reactivated by an inflammatory condition.
“Nevertheless, it is striking that among the 16 cases (in the study group), dual, triple, or quadruple infections with AAV2, adenovirus, and one or both herpesviruses were detected in whole blood from at least 12 cases (75%),” the researchers wrote.
Management of suspected hepatitis
The study’s key messages for parents and health care providers “are awareness and reassurance,” Dr. Balistreri said in an interview.
Vigilance also is warranted if a child develops prodromal symptoms including respiratory and/or gastrointestinal signs such as nausea, vomiting, diarrhea, and abdomen pain, he said. If jaundice or scleral icterus is noted, then hepatitis should be suspected.
Some patients need hospitalization and quickly recover. In very rare instances, the inflammation may progress to liver failure and transplantation, Dr. Balistreri said.
“Reassurance is based on the good news that most children with acute hepatitis get better. If a case arises, it is good practice to keep the child well hydrated, offer a normal diet, and avoid medications that may be cleared by the liver,” Dr. Balistreri added.
“Of course, COVID-19 vaccination is strongly suggested,” he said.
Some existing treatments could help against unexplained acute hepatitis, Dr. Chiu said. “The findings suggest that antiviral therapy might be effective in these cases.”
Cidofovir can be effective against adenovirus, according to a report in The Lancet . Similarly, ganciclovir or valganciclovir may have activity against EBV/HHV-4 or HHV-6, Dr. Chiu said. “However, antiviral therapy is not available for AAV2.”
The three studies published in Nature “offer compelling evidence, from disparate centers, of a linkage of outbreak cases to infection by AAV2,” Dr. Balistreri said. The studies also suggest that liver injury was related to abnormal immune responses. This is an important clinical distinction, indicating a potential therapeutic approach to future cases – immunosuppression rather than anti-adenoviral agents, he said.
“We await further studies of this important concept,” Dr. Balistreri said.
Many unanswered questions remain about the condition’s etiology, he added. Is there a synergy or shared susceptibility related to SARS-CoV-2? Is the COVID-19 virus helping to trigger these infections, or does it increase the risk once infected? Also, are other epigenetic factors or viruses involved?
Moving forward
The next steps in the research could go beyond identifying presence of these different viruses and determining which one(s) are contributing the most to the acute pediatric hepatitis, Dr. Chiu said.
The researchers also would like to test early results from the United Kingdom that identified a potential association of acute severe hepatitis with the presence of human leukocyte antigen genotype DRB1*04:01, he added.
They also might investigate other unintended potential clinical consequences of the COVID-19 pandemic, including long COVID and resurgence of infections from other viruses, such as respiratory syncytial virus, influenza, and enterovirus D68.
The study was supported by the Centers for Disease Control and Prevention, the National Institutes of Health, the Department of Homeland Security, and other grants. Dr. Chiu is a founder of Delve Bio and on the scientific advisory board for Delve Bio, Mammoth Biosciences, BiomeSense, and Poppy Health. Dr. Balistreri had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE
Deadly bacteria in recalled eye drops can spread person-to-person
according to a new report.
Scientists are concerned that the once-rare treatment-resistant bacteria found in the eyedrops can spread person-to-person, posing a risk of becoming a recurrent problem in the United States, The New York Times reported.
In January, EzriCare and Delsam Pharma artificial tears and ointment products were recalled after being linked to the bacterium P. aeruginosa. The bacteria have caused at least 68 infections, including three deaths and at least eight cases of blindness. The eyedrops were imported to the United States from India, and many of the cases occurred after the bacteria spread person-to-person at a long-term care facility in Connecticut, according to the Times, which cited FDA and Centers for Disease Control and Prevention lead investigator Maroya Walters, PhD.
Dr. Walters said the cases that caused death or blindness were traced to the EzriCare artificial tears product.
“It’s very hard to get rid of,” University of North Carolina at Chapel Hill infectious disease specialist David van Duin, MD, PhD, told the Times, noting that the bacteria cling to sink drains, water faucets, and other moist places.
The FDA said it had halted the import of the recalled products and has since visited the plant in India where they were made, which is owned by Global Pharma Healthcare. In a citation to the company dated March 2, the FDA listed nearly a dozen problems, such as dirty equipment and the absence of safety procedures and tests.
A version of this article originally appeared on WebMD.com.
according to a new report.
Scientists are concerned that the once-rare treatment-resistant bacteria found in the eyedrops can spread person-to-person, posing a risk of becoming a recurrent problem in the United States, The New York Times reported.
In January, EzriCare and Delsam Pharma artificial tears and ointment products were recalled after being linked to the bacterium P. aeruginosa. The bacteria have caused at least 68 infections, including three deaths and at least eight cases of blindness. The eyedrops were imported to the United States from India, and many of the cases occurred after the bacteria spread person-to-person at a long-term care facility in Connecticut, according to the Times, which cited FDA and Centers for Disease Control and Prevention lead investigator Maroya Walters, PhD.
Dr. Walters said the cases that caused death or blindness were traced to the EzriCare artificial tears product.
“It’s very hard to get rid of,” University of North Carolina at Chapel Hill infectious disease specialist David van Duin, MD, PhD, told the Times, noting that the bacteria cling to sink drains, water faucets, and other moist places.
The FDA said it had halted the import of the recalled products and has since visited the plant in India where they were made, which is owned by Global Pharma Healthcare. In a citation to the company dated March 2, the FDA listed nearly a dozen problems, such as dirty equipment and the absence of safety procedures and tests.
A version of this article originally appeared on WebMD.com.
according to a new report.
Scientists are concerned that the once-rare treatment-resistant bacteria found in the eyedrops can spread person-to-person, posing a risk of becoming a recurrent problem in the United States, The New York Times reported.
In January, EzriCare and Delsam Pharma artificial tears and ointment products were recalled after being linked to the bacterium P. aeruginosa. The bacteria have caused at least 68 infections, including three deaths and at least eight cases of blindness. The eyedrops were imported to the United States from India, and many of the cases occurred after the bacteria spread person-to-person at a long-term care facility in Connecticut, according to the Times, which cited FDA and Centers for Disease Control and Prevention lead investigator Maroya Walters, PhD.
Dr. Walters said the cases that caused death or blindness were traced to the EzriCare artificial tears product.
“It’s very hard to get rid of,” University of North Carolina at Chapel Hill infectious disease specialist David van Duin, MD, PhD, told the Times, noting that the bacteria cling to sink drains, water faucets, and other moist places.
The FDA said it had halted the import of the recalled products and has since visited the plant in India where they were made, which is owned by Global Pharma Healthcare. In a citation to the company dated March 2, the FDA listed nearly a dozen problems, such as dirty equipment and the absence of safety procedures and tests.
A version of this article originally appeared on WebMD.com.
Single bivalent COVID booster is enough for now: CDC
“If you have completed your updated booster dose, you are currently up to date. There is not a recommendation to get another updated booster dose,” the CDC website now explains.
In January, the nation’s expert COVID panel recommended that the United States move toward an annual COVID booster shot in the fall, similar to the annual flu shot, that targets the most widely circulating strains of the virus. Recent studies have shown that booster strength wanes after a few months, spurring discussions of whether people at high risk of getting a severe case of COVID may need more than one annual shot.
September was the last time a new booster dose was recommended, when, at the time, the bivalent booster was released, offering new protection against Omicron variants of the virus. Health officials’ focus is now shifting from preventing infections to reducing the likelihood of severe ones, the San Francisco Chronicle reported.
“The bottom line is that there is some waning of protection for those who got boosters more than six months ago and haven’t had an intervening infection,” said Bob Wachter, MD, head of the University of California–San Francisco’s department of medicine, according to the Chronicle. “But the level of protection versus severe infection continues to be fairly high, good enough that people who aren’t at super high risk are probably fine waiting until a new booster comes out in the fall.”
The Wall Street Journal reported recently that many people have been asking their doctors to give them another booster, which is not authorized by the Food and Drug Administration.
About 8 in 10 people in the United States got the initial set of COVID-19 vaccines, which were first approved in August 2021. But just 16.4% of people in the United States have gotten the latest booster that was released in September, CDC data show.
A version of this article originally appeared on WebMD.com.
“If you have completed your updated booster dose, you are currently up to date. There is not a recommendation to get another updated booster dose,” the CDC website now explains.
In January, the nation’s expert COVID panel recommended that the United States move toward an annual COVID booster shot in the fall, similar to the annual flu shot, that targets the most widely circulating strains of the virus. Recent studies have shown that booster strength wanes after a few months, spurring discussions of whether people at high risk of getting a severe case of COVID may need more than one annual shot.
September was the last time a new booster dose was recommended, when, at the time, the bivalent booster was released, offering new protection against Omicron variants of the virus. Health officials’ focus is now shifting from preventing infections to reducing the likelihood of severe ones, the San Francisco Chronicle reported.
“The bottom line is that there is some waning of protection for those who got boosters more than six months ago and haven’t had an intervening infection,” said Bob Wachter, MD, head of the University of California–San Francisco’s department of medicine, according to the Chronicle. “But the level of protection versus severe infection continues to be fairly high, good enough that people who aren’t at super high risk are probably fine waiting until a new booster comes out in the fall.”
The Wall Street Journal reported recently that many people have been asking their doctors to give them another booster, which is not authorized by the Food and Drug Administration.
About 8 in 10 people in the United States got the initial set of COVID-19 vaccines, which were first approved in August 2021. But just 16.4% of people in the United States have gotten the latest booster that was released in September, CDC data show.
A version of this article originally appeared on WebMD.com.
“If you have completed your updated booster dose, you are currently up to date. There is not a recommendation to get another updated booster dose,” the CDC website now explains.
In January, the nation’s expert COVID panel recommended that the United States move toward an annual COVID booster shot in the fall, similar to the annual flu shot, that targets the most widely circulating strains of the virus. Recent studies have shown that booster strength wanes after a few months, spurring discussions of whether people at high risk of getting a severe case of COVID may need more than one annual shot.
September was the last time a new booster dose was recommended, when, at the time, the bivalent booster was released, offering new protection against Omicron variants of the virus. Health officials’ focus is now shifting from preventing infections to reducing the likelihood of severe ones, the San Francisco Chronicle reported.
“The bottom line is that there is some waning of protection for those who got boosters more than six months ago and haven’t had an intervening infection,” said Bob Wachter, MD, head of the University of California–San Francisco’s department of medicine, according to the Chronicle. “But the level of protection versus severe infection continues to be fairly high, good enough that people who aren’t at super high risk are probably fine waiting until a new booster comes out in the fall.”
The Wall Street Journal reported recently that many people have been asking their doctors to give them another booster, which is not authorized by the Food and Drug Administration.
About 8 in 10 people in the United States got the initial set of COVID-19 vaccines, which were first approved in August 2021. But just 16.4% of people in the United States have gotten the latest booster that was released in September, CDC data show.
A version of this article originally appeared on WebMD.com.
Negative expectations of COVID shots may amplify side effects
It fits the psychosomatic role of “nocebo effects,” the researchers say – when “psychological characteristics including anxiety, depression, and the tendency to amplify benign bodily sensations” cause participants to report more bad effects than others.
In August 2021, researchers in Hamburg, Germany, followed 1,678 adults getting a second shot of Pfizer or Moderna mRNA-based vaccines. Participants reported symptoms in a diary, starting 2 weeks ahead of the vaccinations and going 7 days afterward.
Some participants said they weren’t expecting much benefit. Researchers said these people were more likely to “catastrophize instead of normalize benign bodily sensations.” People who’d had a bad experience with their first shot were more likely to say they felt aches, pains, and other side effects from the second.
The research was published in JAMA Network Open.
“Clinician-patient interactions and public vaccine campaigns may both benefit from these insights by optimizing and contextualizing information provided about COVID-19 vaccines,” the researchers said. “Unfavorable nocebo-related adverse effects could then be prevented, and overall vaccine acceptance could be improved.”
More than half of participants, 52.1%, expected bad effects to happen from the shot. Another 7.6% said they would be hospitalized from those bad effects, and 10.6% said the effects would last in the long term.
The Washington Times reported that “substantial numbers of patients reported adverse effects after vaccination,” but people with positive expectations reported them as minor. “Those who scored higher for anxiety, depression, and other psychosocial factors were more likely to flag these issues as severe.”
A version of this article originally appeared on WebMD.com.
It fits the psychosomatic role of “nocebo effects,” the researchers say – when “psychological characteristics including anxiety, depression, and the tendency to amplify benign bodily sensations” cause participants to report more bad effects than others.
In August 2021, researchers in Hamburg, Germany, followed 1,678 adults getting a second shot of Pfizer or Moderna mRNA-based vaccines. Participants reported symptoms in a diary, starting 2 weeks ahead of the vaccinations and going 7 days afterward.
Some participants said they weren’t expecting much benefit. Researchers said these people were more likely to “catastrophize instead of normalize benign bodily sensations.” People who’d had a bad experience with their first shot were more likely to say they felt aches, pains, and other side effects from the second.
The research was published in JAMA Network Open.
“Clinician-patient interactions and public vaccine campaigns may both benefit from these insights by optimizing and contextualizing information provided about COVID-19 vaccines,” the researchers said. “Unfavorable nocebo-related adverse effects could then be prevented, and overall vaccine acceptance could be improved.”
More than half of participants, 52.1%, expected bad effects to happen from the shot. Another 7.6% said they would be hospitalized from those bad effects, and 10.6% said the effects would last in the long term.
The Washington Times reported that “substantial numbers of patients reported adverse effects after vaccination,” but people with positive expectations reported them as minor. “Those who scored higher for anxiety, depression, and other psychosocial factors were more likely to flag these issues as severe.”
A version of this article originally appeared on WebMD.com.
It fits the psychosomatic role of “nocebo effects,” the researchers say – when “psychological characteristics including anxiety, depression, and the tendency to amplify benign bodily sensations” cause participants to report more bad effects than others.
In August 2021, researchers in Hamburg, Germany, followed 1,678 adults getting a second shot of Pfizer or Moderna mRNA-based vaccines. Participants reported symptoms in a diary, starting 2 weeks ahead of the vaccinations and going 7 days afterward.
Some participants said they weren’t expecting much benefit. Researchers said these people were more likely to “catastrophize instead of normalize benign bodily sensations.” People who’d had a bad experience with their first shot were more likely to say they felt aches, pains, and other side effects from the second.
The research was published in JAMA Network Open.
“Clinician-patient interactions and public vaccine campaigns may both benefit from these insights by optimizing and contextualizing information provided about COVID-19 vaccines,” the researchers said. “Unfavorable nocebo-related adverse effects could then be prevented, and overall vaccine acceptance could be improved.”
More than half of participants, 52.1%, expected bad effects to happen from the shot. Another 7.6% said they would be hospitalized from those bad effects, and 10.6% said the effects would last in the long term.
The Washington Times reported that “substantial numbers of patients reported adverse effects after vaccination,” but people with positive expectations reported them as minor. “Those who scored higher for anxiety, depression, and other psychosocial factors were more likely to flag these issues as severe.”
A version of this article originally appeared on WebMD.com.
FROM JAMA NETWORK OPEN
Nasal COVID treatment shows early promise against multiple variants
if used within 4 hours after infection inside the nose, new research reveals.
Known as TriSb92 (brand name Covidin, from drugmaker Pandemblock Oy in Finland), the viral inhibitor also appears effective against all coronavirus variants of concern, neutralizing even the Omicron variants BA.5, XBB, and BQ.1.1 in laboratory and mice studies.
Unlike a COVID vaccine that boosts a person’s immune system as protection, the antiviral nasal spray works more directly by blocking the virus, acting as a “biological mask in the nasal cavity,” according to the biotechnology company set up to develop the treatment.
The product targets a stable site on the spike protein of the virus that is not known to mutate. This same site is shared among many variants of the COVID virus, so it could be effective against future variants as well, researchers note.
“In animal models, by directly inactivating the virus, TriSb92 offers immediate and robust protection” against coronavirus infection and severe COVID, said Anna R. Mäkelä, PhD, lead author of the study and a senior scientist in the department of virology at the University of Helsinki.
The study was published online in Nature Communications.
A potential first line of defense
Even in cases where the antiviral does not prevent coronavirus infection, the treatment could slow infection. This could happen by limiting how much virus could replicate early in the skin inside the nose and nasopharynx (the upper part of the throat), said Dr. Mäkelä, who is also CEO of Pandemblock Oy, the company set up to develop the product.
“TriSb92 could effectively tip the balance in favor of the [the person] and thereby help to reduce the risk of severe COVID-19 disease,” she said.
The antiviral also could offer an alternative to people who cannot or do not respond to a vaccine.
“Many elderly people as well as individuals who are immunodeficient for various reasons do not respond to vaccines and are in the need of other protective measures,” said Kalle Saksela, MD, PhD, senior author of the study and a virologist at the University of Helsinki.
Multiple doses needed?
TriSb92 is “one of multiple nasal spray approaches but unlikely to be as durable as effective nasal vaccines,” said Eric Topol, MD, a professor of molecular medicine and executive vice president of Scripps Research in La Jolla, Calif. Dr. Topol is also editor-in-chief of Medscape, WebMD’s sister site for medical professionals.
“The sprays generally require multiple doses per day, whereas a single dose of a nasal vaccine may protect for months,” he said.
“Both have the allure of being variant-proof,” Dr. Topol added.
Thinking small
Many laboratories are shifting from treatments using monoclonal antibodies to treatments using smaller antibody fragments called “nanobodies” because they are more cost-effective and are able to last longer in storage, Dr. Mäkelä and colleagues noted.
Several of these nanobodies have shown promise against viruses in cell culture or animal models, including as an intranasal preventive treatment for SARS-CoV-2.
One of these smaller antibodies is being developed from llamas for example; another comes from experiments with yeast to develop synthetic nanobodies; and in a third case, researchers isolated nanobodies from llamas and from mice and showed they could neutralize the SARS-CoV-2 virus.
These nanobodies and TriSb92 target a specific part of the coronavirus spike protein called the receptor-binding domain (RBD). The RBD is where the coronavirus attaches to cells in the body. These agents essentially trick the virus by changing the structure of the outside of cells, so they look like a virus has already fused to them. This way, the virus moves on.
Key findings
The researchers compared mice treated with TriSb92 before and after exposure to SARS-CoV-2. When given in advance, none of the treated mice had SARS-CoV-2 RNA in their lungs, while untreated mice in the comparison group had “abundant” levels.
Other evidence of viral infection showed similar differences between treated and untreated mice in the protective lining of cells called the epithelium inside the nose, nasal mucosa, and airways.
Similarly, when given 2 or 4 hours after SARS-CoV-2 had already infected the epithelium, TriSb92 was linked to a complete lack of the virus’s RNA in the lungs.
It was more effective against the virus, though, when given before infection rather than after, “perhaps due to the initial establishment of the infection,” the researchers note.
The company led by Dr. Mäkelä is now working to secure funding for clinical trials of TriSb92 in humans.
A version of this article first appeared on WebMD.com.
if used within 4 hours after infection inside the nose, new research reveals.
Known as TriSb92 (brand name Covidin, from drugmaker Pandemblock Oy in Finland), the viral inhibitor also appears effective against all coronavirus variants of concern, neutralizing even the Omicron variants BA.5, XBB, and BQ.1.1 in laboratory and mice studies.
Unlike a COVID vaccine that boosts a person’s immune system as protection, the antiviral nasal spray works more directly by blocking the virus, acting as a “biological mask in the nasal cavity,” according to the biotechnology company set up to develop the treatment.
The product targets a stable site on the spike protein of the virus that is not known to mutate. This same site is shared among many variants of the COVID virus, so it could be effective against future variants as well, researchers note.
“In animal models, by directly inactivating the virus, TriSb92 offers immediate and robust protection” against coronavirus infection and severe COVID, said Anna R. Mäkelä, PhD, lead author of the study and a senior scientist in the department of virology at the University of Helsinki.
The study was published online in Nature Communications.
A potential first line of defense
Even in cases where the antiviral does not prevent coronavirus infection, the treatment could slow infection. This could happen by limiting how much virus could replicate early in the skin inside the nose and nasopharynx (the upper part of the throat), said Dr. Mäkelä, who is also CEO of Pandemblock Oy, the company set up to develop the product.
“TriSb92 could effectively tip the balance in favor of the [the person] and thereby help to reduce the risk of severe COVID-19 disease,” she said.
The antiviral also could offer an alternative to people who cannot or do not respond to a vaccine.
“Many elderly people as well as individuals who are immunodeficient for various reasons do not respond to vaccines and are in the need of other protective measures,” said Kalle Saksela, MD, PhD, senior author of the study and a virologist at the University of Helsinki.
Multiple doses needed?
TriSb92 is “one of multiple nasal spray approaches but unlikely to be as durable as effective nasal vaccines,” said Eric Topol, MD, a professor of molecular medicine and executive vice president of Scripps Research in La Jolla, Calif. Dr. Topol is also editor-in-chief of Medscape, WebMD’s sister site for medical professionals.
“The sprays generally require multiple doses per day, whereas a single dose of a nasal vaccine may protect for months,” he said.
“Both have the allure of being variant-proof,” Dr. Topol added.
Thinking small
Many laboratories are shifting from treatments using monoclonal antibodies to treatments using smaller antibody fragments called “nanobodies” because they are more cost-effective and are able to last longer in storage, Dr. Mäkelä and colleagues noted.
Several of these nanobodies have shown promise against viruses in cell culture or animal models, including as an intranasal preventive treatment for SARS-CoV-2.
One of these smaller antibodies is being developed from llamas for example; another comes from experiments with yeast to develop synthetic nanobodies; and in a third case, researchers isolated nanobodies from llamas and from mice and showed they could neutralize the SARS-CoV-2 virus.
These nanobodies and TriSb92 target a specific part of the coronavirus spike protein called the receptor-binding domain (RBD). The RBD is where the coronavirus attaches to cells in the body. These agents essentially trick the virus by changing the structure of the outside of cells, so they look like a virus has already fused to them. This way, the virus moves on.
Key findings
The researchers compared mice treated with TriSb92 before and after exposure to SARS-CoV-2. When given in advance, none of the treated mice had SARS-CoV-2 RNA in their lungs, while untreated mice in the comparison group had “abundant” levels.
Other evidence of viral infection showed similar differences between treated and untreated mice in the protective lining of cells called the epithelium inside the nose, nasal mucosa, and airways.
Similarly, when given 2 or 4 hours after SARS-CoV-2 had already infected the epithelium, TriSb92 was linked to a complete lack of the virus’s RNA in the lungs.
It was more effective against the virus, though, when given before infection rather than after, “perhaps due to the initial establishment of the infection,” the researchers note.
The company led by Dr. Mäkelä is now working to secure funding for clinical trials of TriSb92 in humans.
A version of this article first appeared on WebMD.com.
if used within 4 hours after infection inside the nose, new research reveals.
Known as TriSb92 (brand name Covidin, from drugmaker Pandemblock Oy in Finland), the viral inhibitor also appears effective against all coronavirus variants of concern, neutralizing even the Omicron variants BA.5, XBB, and BQ.1.1 in laboratory and mice studies.
Unlike a COVID vaccine that boosts a person’s immune system as protection, the antiviral nasal spray works more directly by blocking the virus, acting as a “biological mask in the nasal cavity,” according to the biotechnology company set up to develop the treatment.
The product targets a stable site on the spike protein of the virus that is not known to mutate. This same site is shared among many variants of the COVID virus, so it could be effective against future variants as well, researchers note.
“In animal models, by directly inactivating the virus, TriSb92 offers immediate and robust protection” against coronavirus infection and severe COVID, said Anna R. Mäkelä, PhD, lead author of the study and a senior scientist in the department of virology at the University of Helsinki.
The study was published online in Nature Communications.
A potential first line of defense
Even in cases where the antiviral does not prevent coronavirus infection, the treatment could slow infection. This could happen by limiting how much virus could replicate early in the skin inside the nose and nasopharynx (the upper part of the throat), said Dr. Mäkelä, who is also CEO of Pandemblock Oy, the company set up to develop the product.
“TriSb92 could effectively tip the balance in favor of the [the person] and thereby help to reduce the risk of severe COVID-19 disease,” she said.
The antiviral also could offer an alternative to people who cannot or do not respond to a vaccine.
“Many elderly people as well as individuals who are immunodeficient for various reasons do not respond to vaccines and are in the need of other protective measures,” said Kalle Saksela, MD, PhD, senior author of the study and a virologist at the University of Helsinki.
Multiple doses needed?
TriSb92 is “one of multiple nasal spray approaches but unlikely to be as durable as effective nasal vaccines,” said Eric Topol, MD, a professor of molecular medicine and executive vice president of Scripps Research in La Jolla, Calif. Dr. Topol is also editor-in-chief of Medscape, WebMD’s sister site for medical professionals.
“The sprays generally require multiple doses per day, whereas a single dose of a nasal vaccine may protect for months,” he said.
“Both have the allure of being variant-proof,” Dr. Topol added.
Thinking small
Many laboratories are shifting from treatments using monoclonal antibodies to treatments using smaller antibody fragments called “nanobodies” because they are more cost-effective and are able to last longer in storage, Dr. Mäkelä and colleagues noted.
Several of these nanobodies have shown promise against viruses in cell culture or animal models, including as an intranasal preventive treatment for SARS-CoV-2.
One of these smaller antibodies is being developed from llamas for example; another comes from experiments with yeast to develop synthetic nanobodies; and in a third case, researchers isolated nanobodies from llamas and from mice and showed they could neutralize the SARS-CoV-2 virus.
These nanobodies and TriSb92 target a specific part of the coronavirus spike protein called the receptor-binding domain (RBD). The RBD is where the coronavirus attaches to cells in the body. These agents essentially trick the virus by changing the structure of the outside of cells, so they look like a virus has already fused to them. This way, the virus moves on.
Key findings
The researchers compared mice treated with TriSb92 before and after exposure to SARS-CoV-2. When given in advance, none of the treated mice had SARS-CoV-2 RNA in their lungs, while untreated mice in the comparison group had “abundant” levels.
Other evidence of viral infection showed similar differences between treated and untreated mice in the protective lining of cells called the epithelium inside the nose, nasal mucosa, and airways.
Similarly, when given 2 or 4 hours after SARS-CoV-2 had already infected the epithelium, TriSb92 was linked to a complete lack of the virus’s RNA in the lungs.
It was more effective against the virus, though, when given before infection rather than after, “perhaps due to the initial establishment of the infection,” the researchers note.
The company led by Dr. Mäkelä is now working to secure funding for clinical trials of TriSb92 in humans.
A version of this article first appeared on WebMD.com.
FROM NATURE COMMUNICATIONS
High-dose prophylactic anticoagulation benefits patients with COVID-19 pneumonia
High-dose prophylactic anticoagulation or therapeutic anticoagulation reduced de novo thrombosis in patients with hypoxemic COVID-19 pneumonia, based on data from 334 adults.
Patients with hypoxemic COVID-19 pneumonia are at increased risk of thrombosis and anticoagulation-related bleeding, therefore data to identify the lowest effective anticoagulant dose are needed, wrote Vincent Labbé, MD, of Sorbonne University, Paris, and colleagues.
Previous studies of different anticoagulation strategies for noncritically ill and critically ill patients with COVID-19 pneumonia have shown contrasting results, but some institutions recommend a high-dose regimen in the wake of data showing macrovascular thrombosis in patients with COVID-19 who were treated with standard anticoagulation, the authors wrote.
However, no previously published studies have compared the effectiveness of the three anticoagulation strategies: high-dose prophylactic anticoagulation (HD-PA), standard dose prophylactic anticoagulation (SD-PA), and therapeutic anticoagulation (TA), they said.
In the open-label Anticoagulation COVID-19 (ANTICOVID) trial, published in JAMA Internal Medicine, the researchers identified consecutively hospitalized adults aged 18 years and older being treated for hypoxemic COVID-19 pneumonia in 23 centers in France between April 2021 and December 2021.
The patients were randomly assigned to SD-PA (116 patients), HD-PA (111 patients), and TA (112 patients) using low-molecular-weight heparin for 14 days, or until either hospital discharge or weaning from supplemental oxygen for 48 consecutive hours, whichever outcome occurred first. The HD-PA patients received two times the SD-PA dose. The mean age of the patients was 58.3 years, and approximately two-thirds were men; race and ethnicity data were not collected. Participants had no macrovascular thrombosis at the start of the study.
The primary outcomes were all-cause mortality and time to clinical improvement (defined as the time from randomization to a 2-point improvement on a 7-category respiratory function scale).
The secondary outcome was a combination of safety and efficacy at day 28 that included a composite of thrombosis (ischemic stroke, noncerebrovascular arterial thrombosis, deep venous thrombosis, pulmonary artery thrombosis, and central venous catheter–related deep venous thrombosis), major bleeding, or all-cause death.
For the primary outcome, results were similar among the groups; HD-PA had no significant benefit over SD-PA or TA. All-cause death rates for SD-PA, HD-PA, and TA patients were 14%, 12%, and 13%, respectively. The time to clinical improvement for the three groups was approximately 8 days, 9 days, and 8 days, respectively. Results for the primary outcome were consistent across all prespecified subgroups.
However, HD-PA was associated with a significant fourfold reduced risk of de novo thrombosis compared with SD-PA (5.5% vs. 20.2%) with no observed increase in major bleeding. TA was not associated with any significant improvement in primary or secondary outcomes compared with HD-PA or SD-PA.
The current study findings of no improvement in survival or disease resolution in patients with a higher anticoagulant dose reflects data from previous studies, the researchers wrote in their discussion. “Our study results together with those of previous RCTs support the premise that the role of microvascular thrombosis in worsening organ dysfunction may be narrower than estimated,” they said.
The findings were limited by several factors including the open-label design and the relatively small sample size, the lack of data on microvascular (vs. macrovascular) thrombosis at baseline, and the predominance of the Delta variant of COVID-19 among the study participants, which may have contributed to a lower mortality rate, the researchers noted.
However, given the significant reduction in de novo thrombosis, the results support the routine use of HD-PA in patients with severe hypoxemic COVID-19 pneumonia, they concluded.
Results inform current clinical practice
Over the course of the COVID-19 pandemic, “Patients hospitalized with COVID-19 manifested the highest risk for thromboembolic complications, especially patients in the intensive care setting,” and early reports suggested that standard prophylactic doses of anticoagulant therapy might be insufficient to prevent thrombotic events, Richard C. Becker, MD, of the University of Cincinnati, and Thomas L. Ortel, MD, of Duke University, Durham, N.C., wrote in an accompanying editorial.
“Although there have been several studies that have investigated the role of anticoagulant therapy in hospitalized patients with COVID-19, this is the first study that specifically compared a standard, prophylactic dose of low-molecular-weight heparin to a ‘high-dose’ prophylactic regimen and to a full therapeutic dose regimen,” Dr. Ortel said in an interview.
“Given the concerns about an increased thrombotic risk with prophylactic dose anticoagulation, and the potential bleeding risk associated with a full therapeutic dose of anticoagulation, this approach enabled the investigators to explore the efficacy and safety of an intermediate dose between these two extremes,” he said.
In the current study, , a finding that was not observed in other studies investigating anticoagulant therapy in hospitalized patients with severe COVID-19,” Dr. Ortel told this news organization. “Much initial concern about progression of disease in patients hospitalized with severe COVID-19 focused on the role of microvascular thrombosis, which appears to be less important in this process, or, alternatively, less responsive to anticoagulant therapy.”
The clinical takeaway from the study, Dr. Ortel said, is the decreased risk for venous thromboembolism with a high-dose prophylactic anticoagulation strategy compared with a standard-dose prophylactic regimen for patients hospitalized with hypoxemic COVID-19 pneumonia, “leading to an improved net clinical outcome.”
Looking ahead, “Additional research is needed to determine whether a higher dose of prophylactic anticoagulation would be beneficial for patients hospitalized with COVID-19 pneumonia who are not in an intensive care unit setting,” Dr. Ortel said. Studies are needed to determine whether therapeutic interventions are equally beneficial in patients with different coronavirus variants, since most patients in the current study were infected with the Delta variant, he added.
The study was supported by LEO Pharma. Dr. Labbé disclosed grants from LEO Pharma during the study and fees from AOP Health unrelated to the current study.
Dr. Becker disclosed personal fees from Novartis Data Safety Monitoring Board, Ionis Data Safety Monitoring Board, and Basking Biosciences Scientific Advisory Board unrelated to the current study. Dr. Ortel disclosed grants from the National Institutes of Health, Instrumentation Laboratory, Stago, and Siemens; contract fees from the Centers for Disease Control and Prevention; and honoraria from UpToDate unrelated to the current study.
A version of this article originally appeared on Medscape.com.
High-dose prophylactic anticoagulation or therapeutic anticoagulation reduced de novo thrombosis in patients with hypoxemic COVID-19 pneumonia, based on data from 334 adults.
Patients with hypoxemic COVID-19 pneumonia are at increased risk of thrombosis and anticoagulation-related bleeding, therefore data to identify the lowest effective anticoagulant dose are needed, wrote Vincent Labbé, MD, of Sorbonne University, Paris, and colleagues.
Previous studies of different anticoagulation strategies for noncritically ill and critically ill patients with COVID-19 pneumonia have shown contrasting results, but some institutions recommend a high-dose regimen in the wake of data showing macrovascular thrombosis in patients with COVID-19 who were treated with standard anticoagulation, the authors wrote.
However, no previously published studies have compared the effectiveness of the three anticoagulation strategies: high-dose prophylactic anticoagulation (HD-PA), standard dose prophylactic anticoagulation (SD-PA), and therapeutic anticoagulation (TA), they said.
In the open-label Anticoagulation COVID-19 (ANTICOVID) trial, published in JAMA Internal Medicine, the researchers identified consecutively hospitalized adults aged 18 years and older being treated for hypoxemic COVID-19 pneumonia in 23 centers in France between April 2021 and December 2021.
The patients were randomly assigned to SD-PA (116 patients), HD-PA (111 patients), and TA (112 patients) using low-molecular-weight heparin for 14 days, or until either hospital discharge or weaning from supplemental oxygen for 48 consecutive hours, whichever outcome occurred first. The HD-PA patients received two times the SD-PA dose. The mean age of the patients was 58.3 years, and approximately two-thirds were men; race and ethnicity data were not collected. Participants had no macrovascular thrombosis at the start of the study.
The primary outcomes were all-cause mortality and time to clinical improvement (defined as the time from randomization to a 2-point improvement on a 7-category respiratory function scale).
The secondary outcome was a combination of safety and efficacy at day 28 that included a composite of thrombosis (ischemic stroke, noncerebrovascular arterial thrombosis, deep venous thrombosis, pulmonary artery thrombosis, and central venous catheter–related deep venous thrombosis), major bleeding, or all-cause death.
For the primary outcome, results were similar among the groups; HD-PA had no significant benefit over SD-PA or TA. All-cause death rates for SD-PA, HD-PA, and TA patients were 14%, 12%, and 13%, respectively. The time to clinical improvement for the three groups was approximately 8 days, 9 days, and 8 days, respectively. Results for the primary outcome were consistent across all prespecified subgroups.
However, HD-PA was associated with a significant fourfold reduced risk of de novo thrombosis compared with SD-PA (5.5% vs. 20.2%) with no observed increase in major bleeding. TA was not associated with any significant improvement in primary or secondary outcomes compared with HD-PA or SD-PA.
The current study findings of no improvement in survival or disease resolution in patients with a higher anticoagulant dose reflects data from previous studies, the researchers wrote in their discussion. “Our study results together with those of previous RCTs support the premise that the role of microvascular thrombosis in worsening organ dysfunction may be narrower than estimated,” they said.
The findings were limited by several factors including the open-label design and the relatively small sample size, the lack of data on microvascular (vs. macrovascular) thrombosis at baseline, and the predominance of the Delta variant of COVID-19 among the study participants, which may have contributed to a lower mortality rate, the researchers noted.
However, given the significant reduction in de novo thrombosis, the results support the routine use of HD-PA in patients with severe hypoxemic COVID-19 pneumonia, they concluded.
Results inform current clinical practice
Over the course of the COVID-19 pandemic, “Patients hospitalized with COVID-19 manifested the highest risk for thromboembolic complications, especially patients in the intensive care setting,” and early reports suggested that standard prophylactic doses of anticoagulant therapy might be insufficient to prevent thrombotic events, Richard C. Becker, MD, of the University of Cincinnati, and Thomas L. Ortel, MD, of Duke University, Durham, N.C., wrote in an accompanying editorial.
“Although there have been several studies that have investigated the role of anticoagulant therapy in hospitalized patients with COVID-19, this is the first study that specifically compared a standard, prophylactic dose of low-molecular-weight heparin to a ‘high-dose’ prophylactic regimen and to a full therapeutic dose regimen,” Dr. Ortel said in an interview.
“Given the concerns about an increased thrombotic risk with prophylactic dose anticoagulation, and the potential bleeding risk associated with a full therapeutic dose of anticoagulation, this approach enabled the investigators to explore the efficacy and safety of an intermediate dose between these two extremes,” he said.
In the current study, , a finding that was not observed in other studies investigating anticoagulant therapy in hospitalized patients with severe COVID-19,” Dr. Ortel told this news organization. “Much initial concern about progression of disease in patients hospitalized with severe COVID-19 focused on the role of microvascular thrombosis, which appears to be less important in this process, or, alternatively, less responsive to anticoagulant therapy.”
The clinical takeaway from the study, Dr. Ortel said, is the decreased risk for venous thromboembolism with a high-dose prophylactic anticoagulation strategy compared with a standard-dose prophylactic regimen for patients hospitalized with hypoxemic COVID-19 pneumonia, “leading to an improved net clinical outcome.”
Looking ahead, “Additional research is needed to determine whether a higher dose of prophylactic anticoagulation would be beneficial for patients hospitalized with COVID-19 pneumonia who are not in an intensive care unit setting,” Dr. Ortel said. Studies are needed to determine whether therapeutic interventions are equally beneficial in patients with different coronavirus variants, since most patients in the current study were infected with the Delta variant, he added.
The study was supported by LEO Pharma. Dr. Labbé disclosed grants from LEO Pharma during the study and fees from AOP Health unrelated to the current study.
Dr. Becker disclosed personal fees from Novartis Data Safety Monitoring Board, Ionis Data Safety Monitoring Board, and Basking Biosciences Scientific Advisory Board unrelated to the current study. Dr. Ortel disclosed grants from the National Institutes of Health, Instrumentation Laboratory, Stago, and Siemens; contract fees from the Centers for Disease Control and Prevention; and honoraria from UpToDate unrelated to the current study.
A version of this article originally appeared on Medscape.com.
High-dose prophylactic anticoagulation or therapeutic anticoagulation reduced de novo thrombosis in patients with hypoxemic COVID-19 pneumonia, based on data from 334 adults.
Patients with hypoxemic COVID-19 pneumonia are at increased risk of thrombosis and anticoagulation-related bleeding, therefore data to identify the lowest effective anticoagulant dose are needed, wrote Vincent Labbé, MD, of Sorbonne University, Paris, and colleagues.
Previous studies of different anticoagulation strategies for noncritically ill and critically ill patients with COVID-19 pneumonia have shown contrasting results, but some institutions recommend a high-dose regimen in the wake of data showing macrovascular thrombosis in patients with COVID-19 who were treated with standard anticoagulation, the authors wrote.
However, no previously published studies have compared the effectiveness of the three anticoagulation strategies: high-dose prophylactic anticoagulation (HD-PA), standard dose prophylactic anticoagulation (SD-PA), and therapeutic anticoagulation (TA), they said.
In the open-label Anticoagulation COVID-19 (ANTICOVID) trial, published in JAMA Internal Medicine, the researchers identified consecutively hospitalized adults aged 18 years and older being treated for hypoxemic COVID-19 pneumonia in 23 centers in France between April 2021 and December 2021.
The patients were randomly assigned to SD-PA (116 patients), HD-PA (111 patients), and TA (112 patients) using low-molecular-weight heparin for 14 days, or until either hospital discharge or weaning from supplemental oxygen for 48 consecutive hours, whichever outcome occurred first. The HD-PA patients received two times the SD-PA dose. The mean age of the patients was 58.3 years, and approximately two-thirds were men; race and ethnicity data were not collected. Participants had no macrovascular thrombosis at the start of the study.
The primary outcomes were all-cause mortality and time to clinical improvement (defined as the time from randomization to a 2-point improvement on a 7-category respiratory function scale).
The secondary outcome was a combination of safety and efficacy at day 28 that included a composite of thrombosis (ischemic stroke, noncerebrovascular arterial thrombosis, deep venous thrombosis, pulmonary artery thrombosis, and central venous catheter–related deep venous thrombosis), major bleeding, or all-cause death.
For the primary outcome, results were similar among the groups; HD-PA had no significant benefit over SD-PA or TA. All-cause death rates for SD-PA, HD-PA, and TA patients were 14%, 12%, and 13%, respectively. The time to clinical improvement for the three groups was approximately 8 days, 9 days, and 8 days, respectively. Results for the primary outcome were consistent across all prespecified subgroups.
However, HD-PA was associated with a significant fourfold reduced risk of de novo thrombosis compared with SD-PA (5.5% vs. 20.2%) with no observed increase in major bleeding. TA was not associated with any significant improvement in primary or secondary outcomes compared with HD-PA or SD-PA.
The current study findings of no improvement in survival or disease resolution in patients with a higher anticoagulant dose reflects data from previous studies, the researchers wrote in their discussion. “Our study results together with those of previous RCTs support the premise that the role of microvascular thrombosis in worsening organ dysfunction may be narrower than estimated,” they said.
The findings were limited by several factors including the open-label design and the relatively small sample size, the lack of data on microvascular (vs. macrovascular) thrombosis at baseline, and the predominance of the Delta variant of COVID-19 among the study participants, which may have contributed to a lower mortality rate, the researchers noted.
However, given the significant reduction in de novo thrombosis, the results support the routine use of HD-PA in patients with severe hypoxemic COVID-19 pneumonia, they concluded.
Results inform current clinical practice
Over the course of the COVID-19 pandemic, “Patients hospitalized with COVID-19 manifested the highest risk for thromboembolic complications, especially patients in the intensive care setting,” and early reports suggested that standard prophylactic doses of anticoagulant therapy might be insufficient to prevent thrombotic events, Richard C. Becker, MD, of the University of Cincinnati, and Thomas L. Ortel, MD, of Duke University, Durham, N.C., wrote in an accompanying editorial.
“Although there have been several studies that have investigated the role of anticoagulant therapy in hospitalized patients with COVID-19, this is the first study that specifically compared a standard, prophylactic dose of low-molecular-weight heparin to a ‘high-dose’ prophylactic regimen and to a full therapeutic dose regimen,” Dr. Ortel said in an interview.
“Given the concerns about an increased thrombotic risk with prophylactic dose anticoagulation, and the potential bleeding risk associated with a full therapeutic dose of anticoagulation, this approach enabled the investigators to explore the efficacy and safety of an intermediate dose between these two extremes,” he said.
In the current study, , a finding that was not observed in other studies investigating anticoagulant therapy in hospitalized patients with severe COVID-19,” Dr. Ortel told this news organization. “Much initial concern about progression of disease in patients hospitalized with severe COVID-19 focused on the role of microvascular thrombosis, which appears to be less important in this process, or, alternatively, less responsive to anticoagulant therapy.”
The clinical takeaway from the study, Dr. Ortel said, is the decreased risk for venous thromboembolism with a high-dose prophylactic anticoagulation strategy compared with a standard-dose prophylactic regimen for patients hospitalized with hypoxemic COVID-19 pneumonia, “leading to an improved net clinical outcome.”
Looking ahead, “Additional research is needed to determine whether a higher dose of prophylactic anticoagulation would be beneficial for patients hospitalized with COVID-19 pneumonia who are not in an intensive care unit setting,” Dr. Ortel said. Studies are needed to determine whether therapeutic interventions are equally beneficial in patients with different coronavirus variants, since most patients in the current study were infected with the Delta variant, he added.
The study was supported by LEO Pharma. Dr. Labbé disclosed grants from LEO Pharma during the study and fees from AOP Health unrelated to the current study.
Dr. Becker disclosed personal fees from Novartis Data Safety Monitoring Board, Ionis Data Safety Monitoring Board, and Basking Biosciences Scientific Advisory Board unrelated to the current study. Dr. Ortel disclosed grants from the National Institutes of Health, Instrumentation Laboratory, Stago, and Siemens; contract fees from the Centers for Disease Control and Prevention; and honoraria from UpToDate unrelated to the current study.
A version of this article originally appeared on Medscape.com.
‘Excess’ deaths surging, but why?
This transcript has been edited for clarity.
“Excess deaths.” You’ve heard the phrase countless times by now. It is one of the myriad of previously esoteric epidemiology terms that the pandemic brought squarely into the zeitgeist.
As a sort of standard candle of the performance of a state or a region or a country in terms of health care, it has a lot of utility – if for nothing more than Monday-morning quarterbacking. But this week, I want to dig in on the concept a bit because, according to a new study, the excess death gap between the United States and Western Europe has never been higher.
You might imagine that the best way to figure this out is for some group of intelligent people to review each death and decide, somehow, whether it was expected or not. But aside from being impractical, this would end up being somewhat subjective. That older person who died from pneumonia – was that an expected death? Could it have been avoided?
Rather, the calculation of excess mortality relies on large numbers and statistical inference to compare an expected number of deaths with those that are observed.
The difference is excess mortality, even if you can never be sure whether any particular death was expected or not.
As always, however, the devil is in the details. What data do you use to define the expected number of deaths?
There are options here. Probably the most straightforward analysis uses past data from the country of interest. You look at annual deaths over some historical period of time and compare those numbers with the rates today. Two issues need to be accounted for here: population growth – a larger population will have more deaths, so you need to adjust the historical population with current levels, and demographic shifts – an older or more male population will have more deaths, so you need to adjust for that as well.
But provided you take care of those factors, you can estimate fairly well how many deaths you can expect to see in any given period of time.
Still, you should see right away that excess mortality is a relative concept. If you think that, just perhaps, the United States has some systematic failure to deliver care that has been stable and persistent over time, you wouldn’t capture that failing in an excess mortality calculation that uses U.S. historical data as the baseline.
The best way to get around that is to use data from other countries, and that’s just what this article – a rare single-author piece by Patrick Heuveline – does, calculating excess deaths in the United States by standardizing our mortality rates to the five largest Western European countries: the United Kingdom, France, Germany, Italy, and Spain.
Controlling for the differences in the demographics of that European population, here is the expected number of deaths in the United States over the past 5 years.
Note that there is a small uptick in expected deaths in 2020, reflecting the pandemic, which returns to baseline levels by 2021. This is because that’s what happened in Europe; by 2021, the excess mortality due to COVID-19 was quite low.
Here are the actual deaths in the US during that time.
Highlighted here in green, then, is the excess mortality over time in the United States.
There are some fascinating and concerning findings here.
First of all, you can see that even before the pandemic, the United States has an excess mortality problem. This is not entirely a surprise; we’ve known that so-called “deaths of despair,” those due to alcohol abuse, drug overdoses, and suicide, are at an all-time high and tend to affect a “prime of life” population that would not otherwise be expected to die. In fact, fully 50% of the excess deaths in the United States occur in those between ages 15 and 64.
Excess deaths are also a concerning percentage of total deaths. In 2017, 17% of total deaths in the United States could be considered “excess.” In 2021, that number had doubled to 35%. Nearly 900,000 individuals in the United States died in 2021 who perhaps didn’t need to.
The obvious culprit to blame here is COVID, but COVID-associated excess deaths only explain about 50% of the excess we see in 2021. The rest reflect something even more concerning: a worsening of the failures of the past, perhaps exacerbated by the pandemic but not due to the virus itself.
Of course, we started this discussion acknowledging that the calculation of excess mortality is exquisitely dependent on how you model the expected number of deaths, and I’m sure some will take issue with the use of European numbers when applied to Americans. After all, Europe has, by and large, a robust public health service, socialized medicine, and healthcare that does not run the risk of bankrupting its citizens. How can we compare our outcomes to a place like that?
How indeed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven,Conn. He reported no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
“Excess deaths.” You’ve heard the phrase countless times by now. It is one of the myriad of previously esoteric epidemiology terms that the pandemic brought squarely into the zeitgeist.
As a sort of standard candle of the performance of a state or a region or a country in terms of health care, it has a lot of utility – if for nothing more than Monday-morning quarterbacking. But this week, I want to dig in on the concept a bit because, according to a new study, the excess death gap between the United States and Western Europe has never been higher.
You might imagine that the best way to figure this out is for some group of intelligent people to review each death and decide, somehow, whether it was expected or not. But aside from being impractical, this would end up being somewhat subjective. That older person who died from pneumonia – was that an expected death? Could it have been avoided?
Rather, the calculation of excess mortality relies on large numbers and statistical inference to compare an expected number of deaths with those that are observed.
The difference is excess mortality, even if you can never be sure whether any particular death was expected or not.
As always, however, the devil is in the details. What data do you use to define the expected number of deaths?
There are options here. Probably the most straightforward analysis uses past data from the country of interest. You look at annual deaths over some historical period of time and compare those numbers with the rates today. Two issues need to be accounted for here: population growth – a larger population will have more deaths, so you need to adjust the historical population with current levels, and demographic shifts – an older or more male population will have more deaths, so you need to adjust for that as well.
But provided you take care of those factors, you can estimate fairly well how many deaths you can expect to see in any given period of time.
Still, you should see right away that excess mortality is a relative concept. If you think that, just perhaps, the United States has some systematic failure to deliver care that has been stable and persistent over time, you wouldn’t capture that failing in an excess mortality calculation that uses U.S. historical data as the baseline.
The best way to get around that is to use data from other countries, and that’s just what this article – a rare single-author piece by Patrick Heuveline – does, calculating excess deaths in the United States by standardizing our mortality rates to the five largest Western European countries: the United Kingdom, France, Germany, Italy, and Spain.
Controlling for the differences in the demographics of that European population, here is the expected number of deaths in the United States over the past 5 years.
Note that there is a small uptick in expected deaths in 2020, reflecting the pandemic, which returns to baseline levels by 2021. This is because that’s what happened in Europe; by 2021, the excess mortality due to COVID-19 was quite low.
Here are the actual deaths in the US during that time.
Highlighted here in green, then, is the excess mortality over time in the United States.
There are some fascinating and concerning findings here.
First of all, you can see that even before the pandemic, the United States has an excess mortality problem. This is not entirely a surprise; we’ve known that so-called “deaths of despair,” those due to alcohol abuse, drug overdoses, and suicide, are at an all-time high and tend to affect a “prime of life” population that would not otherwise be expected to die. In fact, fully 50% of the excess deaths in the United States occur in those between ages 15 and 64.
Excess deaths are also a concerning percentage of total deaths. In 2017, 17% of total deaths in the United States could be considered “excess.” In 2021, that number had doubled to 35%. Nearly 900,000 individuals in the United States died in 2021 who perhaps didn’t need to.
The obvious culprit to blame here is COVID, but COVID-associated excess deaths only explain about 50% of the excess we see in 2021. The rest reflect something even more concerning: a worsening of the failures of the past, perhaps exacerbated by the pandemic but not due to the virus itself.
Of course, we started this discussion acknowledging that the calculation of excess mortality is exquisitely dependent on how you model the expected number of deaths, and I’m sure some will take issue with the use of European numbers when applied to Americans. After all, Europe has, by and large, a robust public health service, socialized medicine, and healthcare that does not run the risk of bankrupting its citizens. How can we compare our outcomes to a place like that?
How indeed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven,Conn. He reported no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
“Excess deaths.” You’ve heard the phrase countless times by now. It is one of the myriad of previously esoteric epidemiology terms that the pandemic brought squarely into the zeitgeist.
As a sort of standard candle of the performance of a state or a region or a country in terms of health care, it has a lot of utility – if for nothing more than Monday-morning quarterbacking. But this week, I want to dig in on the concept a bit because, according to a new study, the excess death gap between the United States and Western Europe has never been higher.
You might imagine that the best way to figure this out is for some group of intelligent people to review each death and decide, somehow, whether it was expected or not. But aside from being impractical, this would end up being somewhat subjective. That older person who died from pneumonia – was that an expected death? Could it have been avoided?
Rather, the calculation of excess mortality relies on large numbers and statistical inference to compare an expected number of deaths with those that are observed.
The difference is excess mortality, even if you can never be sure whether any particular death was expected or not.
As always, however, the devil is in the details. What data do you use to define the expected number of deaths?
There are options here. Probably the most straightforward analysis uses past data from the country of interest. You look at annual deaths over some historical period of time and compare those numbers with the rates today. Two issues need to be accounted for here: population growth – a larger population will have more deaths, so you need to adjust the historical population with current levels, and demographic shifts – an older or more male population will have more deaths, so you need to adjust for that as well.
But provided you take care of those factors, you can estimate fairly well how many deaths you can expect to see in any given period of time.
Still, you should see right away that excess mortality is a relative concept. If you think that, just perhaps, the United States has some systematic failure to deliver care that has been stable and persistent over time, you wouldn’t capture that failing in an excess mortality calculation that uses U.S. historical data as the baseline.
The best way to get around that is to use data from other countries, and that’s just what this article – a rare single-author piece by Patrick Heuveline – does, calculating excess deaths in the United States by standardizing our mortality rates to the five largest Western European countries: the United Kingdom, France, Germany, Italy, and Spain.
Controlling for the differences in the demographics of that European population, here is the expected number of deaths in the United States over the past 5 years.
Note that there is a small uptick in expected deaths in 2020, reflecting the pandemic, which returns to baseline levels by 2021. This is because that’s what happened in Europe; by 2021, the excess mortality due to COVID-19 was quite low.
Here are the actual deaths in the US during that time.
Highlighted here in green, then, is the excess mortality over time in the United States.
There are some fascinating and concerning findings here.
First of all, you can see that even before the pandemic, the United States has an excess mortality problem. This is not entirely a surprise; we’ve known that so-called “deaths of despair,” those due to alcohol abuse, drug overdoses, and suicide, are at an all-time high and tend to affect a “prime of life” population that would not otherwise be expected to die. In fact, fully 50% of the excess deaths in the United States occur in those between ages 15 and 64.
Excess deaths are also a concerning percentage of total deaths. In 2017, 17% of total deaths in the United States could be considered “excess.” In 2021, that number had doubled to 35%. Nearly 900,000 individuals in the United States died in 2021 who perhaps didn’t need to.
The obvious culprit to blame here is COVID, but COVID-associated excess deaths only explain about 50% of the excess we see in 2021. The rest reflect something even more concerning: a worsening of the failures of the past, perhaps exacerbated by the pandemic but not due to the virus itself.
Of course, we started this discussion acknowledging that the calculation of excess mortality is exquisitely dependent on how you model the expected number of deaths, and I’m sure some will take issue with the use of European numbers when applied to Americans. After all, Europe has, by and large, a robust public health service, socialized medicine, and healthcare that does not run the risk of bankrupting its citizens. How can we compare our outcomes to a place like that?
How indeed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven,Conn. He reported no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
Spotting STIs: Vaginal swabs work best
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
Sweaty treatment for social anxiety could pass the sniff test
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
Your hippocampus is showing
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
Your hippocampus is showing
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
Your hippocampus is showing
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
COVID-19 potentially induced adult-onset IgA vasculitis
Plasma exchange successfully improved symptoms of immunoglobulin A vasculitis in an adult female patient who developed the condition after infection with COVID-19, according to a case report published in Cureus.
Immunoglobulin A (IgA) vasculitis can affect all ages, but is relatively rare in adults, and the etiology remains unclear, wrote Hassan Alwafi, MD, of Umm Al-Qura University, Makkah, Saudi Arabia, and colleagues.
COVID-19 has been associated with pulmonary and extrapulmonary complications, but , the authors wrote.
The authors described a case of a 41-year-old otherwise healthy Saudi Arabian woman who presented with an ascending rash on both lower extremities, along with arthralgia. Blood tests showed high blood urea nitrogen, creatinine, and inflammatory markers, and a negative immune panel. The patient had been infected with COVID-19 approximately 2 weeks before the onset of symptoms, but she was treated with supportive care and required no antiviral therapy of dexamethasone.
In addition, the patient’s urinalysis showed proteinuria and hematuria. After a kidney biopsy revealed additional abnormalities, the patient was started on intravenous methylprednisolone pulse therapy.
A few days after the initiation of therapy, the patient experienced nosebleeds and coughing up blood. After a chest x-ray showed bilateral pleural effusion, the patient was transferred to the ICU. The patient was started on intravenous piperacillin-tazobactam, and received two doses of intravenous immunoglobulin and plasma exchange after consultation with a nephrologist. Ultimately, the initial rash and other clinical symptoms improved, and the patient was discharged with a tapering schedule of oral prednisolone.
In this case, COVID-19 may have played a role in the development of IgA vasculitis, the authors said.
The authors also listed 21 cases of IgA vasculitis following COVID-19 infection, including 14 children and 7 adults. Of these, three cases had combined kidney and lung involvement, the two pediatric cases died from respiratory failure, while the adult case was successfully treated with steroid monotherapy.
“As COVID-19 is a novel disease and its pathogenic mechanism of causing IgA vasculitis is not well understood, every patient who is infected with or recently recovered from COVID-19 and presents with a skin rash or arthralgia should have baseline blood and urine tests done and should be treated promptly to avoid the emergence of irreversible consequences,” the authors wrote in their discussion.
Although case reports cannot prove a cause-and-effect link, the data from the cases in the current review suggest that COVID-19 infection may be an indirect trigger for IgA vasculitis, including cases associated with pulmonary renal syndrome, they said. However, more research is needed, especially on the efficacy of treatments in adults, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Plasma exchange successfully improved symptoms of immunoglobulin A vasculitis in an adult female patient who developed the condition after infection with COVID-19, according to a case report published in Cureus.
Immunoglobulin A (IgA) vasculitis can affect all ages, but is relatively rare in adults, and the etiology remains unclear, wrote Hassan Alwafi, MD, of Umm Al-Qura University, Makkah, Saudi Arabia, and colleagues.
COVID-19 has been associated with pulmonary and extrapulmonary complications, but , the authors wrote.
The authors described a case of a 41-year-old otherwise healthy Saudi Arabian woman who presented with an ascending rash on both lower extremities, along with arthralgia. Blood tests showed high blood urea nitrogen, creatinine, and inflammatory markers, and a negative immune panel. The patient had been infected with COVID-19 approximately 2 weeks before the onset of symptoms, but she was treated with supportive care and required no antiviral therapy of dexamethasone.
In addition, the patient’s urinalysis showed proteinuria and hematuria. After a kidney biopsy revealed additional abnormalities, the patient was started on intravenous methylprednisolone pulse therapy.
A few days after the initiation of therapy, the patient experienced nosebleeds and coughing up blood. After a chest x-ray showed bilateral pleural effusion, the patient was transferred to the ICU. The patient was started on intravenous piperacillin-tazobactam, and received two doses of intravenous immunoglobulin and plasma exchange after consultation with a nephrologist. Ultimately, the initial rash and other clinical symptoms improved, and the patient was discharged with a tapering schedule of oral prednisolone.
In this case, COVID-19 may have played a role in the development of IgA vasculitis, the authors said.
The authors also listed 21 cases of IgA vasculitis following COVID-19 infection, including 14 children and 7 adults. Of these, three cases had combined kidney and lung involvement, the two pediatric cases died from respiratory failure, while the adult case was successfully treated with steroid monotherapy.
“As COVID-19 is a novel disease and its pathogenic mechanism of causing IgA vasculitis is not well understood, every patient who is infected with or recently recovered from COVID-19 and presents with a skin rash or arthralgia should have baseline blood and urine tests done and should be treated promptly to avoid the emergence of irreversible consequences,” the authors wrote in their discussion.
Although case reports cannot prove a cause-and-effect link, the data from the cases in the current review suggest that COVID-19 infection may be an indirect trigger for IgA vasculitis, including cases associated with pulmonary renal syndrome, they said. However, more research is needed, especially on the efficacy of treatments in adults, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Plasma exchange successfully improved symptoms of immunoglobulin A vasculitis in an adult female patient who developed the condition after infection with COVID-19, according to a case report published in Cureus.
Immunoglobulin A (IgA) vasculitis can affect all ages, but is relatively rare in adults, and the etiology remains unclear, wrote Hassan Alwafi, MD, of Umm Al-Qura University, Makkah, Saudi Arabia, and colleagues.
COVID-19 has been associated with pulmonary and extrapulmonary complications, but , the authors wrote.
The authors described a case of a 41-year-old otherwise healthy Saudi Arabian woman who presented with an ascending rash on both lower extremities, along with arthralgia. Blood tests showed high blood urea nitrogen, creatinine, and inflammatory markers, and a negative immune panel. The patient had been infected with COVID-19 approximately 2 weeks before the onset of symptoms, but she was treated with supportive care and required no antiviral therapy of dexamethasone.
In addition, the patient’s urinalysis showed proteinuria and hematuria. After a kidney biopsy revealed additional abnormalities, the patient was started on intravenous methylprednisolone pulse therapy.
A few days after the initiation of therapy, the patient experienced nosebleeds and coughing up blood. After a chest x-ray showed bilateral pleural effusion, the patient was transferred to the ICU. The patient was started on intravenous piperacillin-tazobactam, and received two doses of intravenous immunoglobulin and plasma exchange after consultation with a nephrologist. Ultimately, the initial rash and other clinical symptoms improved, and the patient was discharged with a tapering schedule of oral prednisolone.
In this case, COVID-19 may have played a role in the development of IgA vasculitis, the authors said.
The authors also listed 21 cases of IgA vasculitis following COVID-19 infection, including 14 children and 7 adults. Of these, three cases had combined kidney and lung involvement, the two pediatric cases died from respiratory failure, while the adult case was successfully treated with steroid monotherapy.
“As COVID-19 is a novel disease and its pathogenic mechanism of causing IgA vasculitis is not well understood, every patient who is infected with or recently recovered from COVID-19 and presents with a skin rash or arthralgia should have baseline blood and urine tests done and should be treated promptly to avoid the emergence of irreversible consequences,” the authors wrote in their discussion.
Although case reports cannot prove a cause-and-effect link, the data from the cases in the current review suggest that COVID-19 infection may be an indirect trigger for IgA vasculitis, including cases associated with pulmonary renal syndrome, they said. However, more research is needed, especially on the efficacy of treatments in adults, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM CUREUS