User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Children and COVID: Vaccination a harder sell in the summer
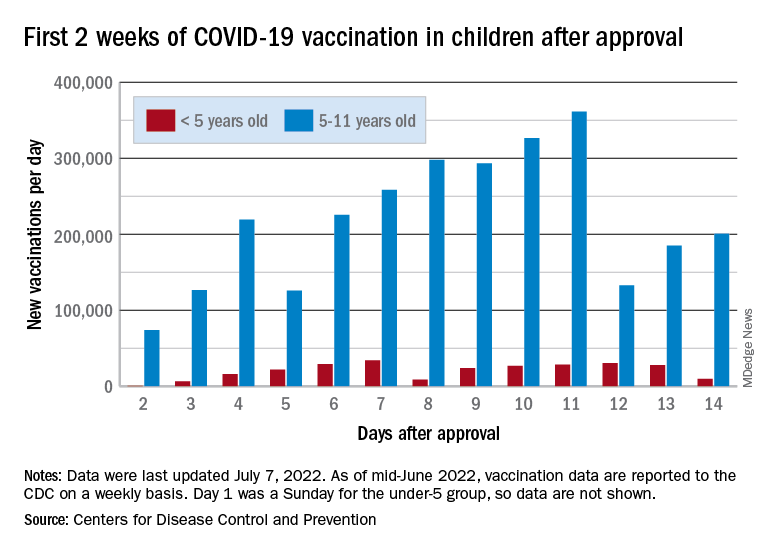
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.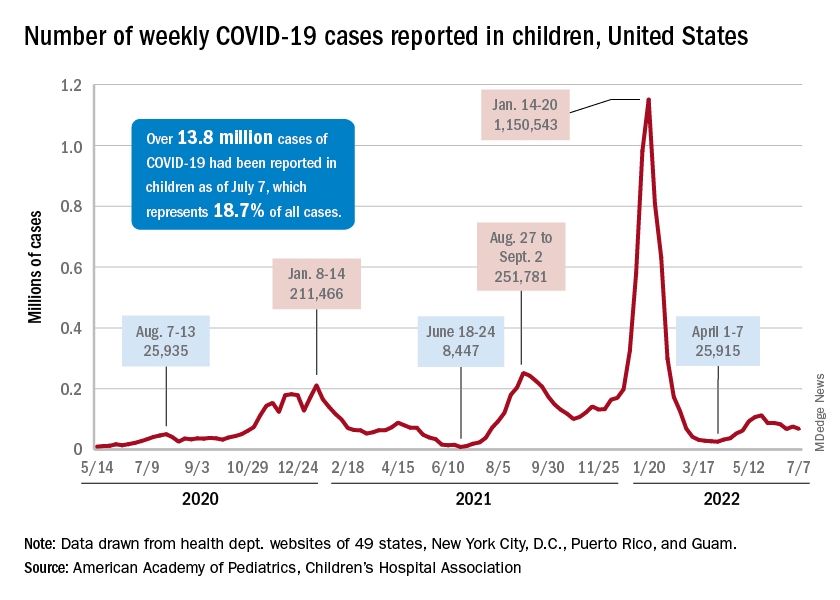
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
Transplanted pig hearts functioned normally in deceased persons on ventilator support
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.
Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).
The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.

Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).

The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.

Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).

The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.
Inflation and health care: The prognosis for doctors
Rampant inflation doesn’t just mean a spike in everyday expenses like gas and groceries. It’s also bound to have a significant impact on the cost of health care – and on your practice. A recent report from McKinsey & Company predicts that the current inflationary spiral will force health care providers to charge higher reimbursement rates, and those costs inevitably will be passed along to both employers and consumers. Bottom line: Your patients will likely have to pay more out of pocket.
How, precisely, will inflation affect your practice, and what’s the best way to minimize the damage?
Step 1: Maintain operational standards
“Based on the conversations we’ve had with our physician clients that own practices, we see the potential for cost inflation to outrun revenue inflation over the next year,” said Michael Ashley Schulman, CFA, partner and chief investment officer at Running Point Capital, El Segundo, Calif. “Staff wages, as well as office equipment and medical supply costs, are increasing faster than insurance and Medicare/Medicaid reimbursement amounts.” Even so, topflight employees are essential to keep your practice running smoothly. Prioritize excellent nursing. Instead of adding a new hire, compensate your best nurse as well as possible. The same goes for an efficient office manager: On that front, too, you should go the extra mile, even if it means trimming expenses elsewhere.
Step 2: Plan ahead for insurance challenges
Many insurers, including Medicare, set health care costs a year in advance, based on projected growth. This means insurance payouts will stay largely the same for the time being. “Almost all physicians employed by large groups won’t see costs due to inflation rise until next year,” said Mark V. Pauly, PhD, Bendheim Professor in the department of health care management at the University of Pennsylvania, Philadelphia. “For self-employed physicians, there will also be a cushion.”
“The big issue with inflation is that more patients will likely be underinsured,” said Tiffany Johnson, MBA, CFP, co-CEO and financial advisor at Piece of Wealth Planning in Atlanta. “With more out-of-pocket costs ... these patients may not seek out medical treatment or go to see a specialist if they do not believe it is necessary.” A new study from Johns Hopkins found that patients under financial pressure often delay or forgo medical treatment because of food insecurity. Compassionate care is the solution: Direct these patients to financial aid and other resources they may qualify for. That way, they can continue to receive the care they need from you, and your need to pass on costs may be lower.
Step 3: Rely on your affiliated health care organization
These are tough times when it comes to expansion. “Since we are in an environment where inflation and interest rates are both high, it will be much harder for physicians to have the capital to invest in new technology to grow or advance their practice,” Ms. Johnson said. With that in mind, keep the lines of communication between you and your affiliated hospital/health care organization more open than ever. Combining practices with another doctor is one way to increase revenue; you might ask if any affiliated doctors are seeking to team up. It’s also vital to attend meetings and pay close attention to budget cuts your organization may be making. And don’t be shy about asking your administrator for profit-boosting recommendations.
Step 4: Revisit vendor relationships
Find out if your vendors will continue to supply you with the goods you need at reasonable rates, and switch now if they won’t. Be proactive. “Test new medical suppliers,” Mr. Schulman advised. “Reread equipment leasing contracts to check if the interest rates have increased. See if buyout, prepay, or refinancing options are more economical. Also, investigate [bringing down] your rental expense by reducing square footage or moving to a lower-cost location.” In light of ongoing supply chain issues, it’s wise to consider alternative products. But stay focused on quality – you don’t want to be stuck with cheap, possibly defective equipment. Spend where it’s essential and cut the fat somewhere else.
Step 5: Don’t waste your assets
Analyze your budget in minute detail. “Now is the time to review your current inventory and overhead costs,” Ms. Johnson said. “Many physicians let their office staff handle the restocking of inventory and office supplies. While this can be efficient for their practice, it also leaves room for unnecessary business expenses.” Take a cold, hard look at your supply closet – what’s in there that you can live without? Don’t reorder it. Then seek out any revenue stream you may be overlooking. “It’s important to review billing to make sure all the services are reimbursable,” Ms. Johnson added. Small mistakes can yield dividends if you find them.
Step 6: Be poised to pivot
Get creative. “To minimize a profit decline, use video consulting – it’s more efficient and less equipment intensive,” Mr. Schulman said. “Look at how remote work and flexible hours can maximize the work your practice accomplishes while cutting office costs.”
Ms. Johnson suggests adding concierge services, noting that “concierge doctors offer personalized care and direct access for an up-front fee.” With this approach, you may see fewer patients, but your payout paperwork will decrease, and that up-front fee can be profitable. Another outside-the-box idea: Start making house calls. A Scripps study found that home health visits requested via app can result in patient care delivered by a doctor and medical assistant in less than 2 hours. House calls can be an effective and profitable solution when it comes to providing nonemergency care and preventive treatment to patients who aren’t mobile, not to mention patients who just appreciate the convenience.
Step 7: Maintain transparency
Any economic changes your practice will implement must be communicated to your staff and patients clearly and directly. Keep everyone in the loop and be ready to answer questions immediately. Show those you work with and care for that, regardless of the economy, it’s they who matter to you most. That simple reassurance will prove invaluable.
A version of this article first appeared on Medscape.com.
Rampant inflation doesn’t just mean a spike in everyday expenses like gas and groceries. It’s also bound to have a significant impact on the cost of health care – and on your practice. A recent report from McKinsey & Company predicts that the current inflationary spiral will force health care providers to charge higher reimbursement rates, and those costs inevitably will be passed along to both employers and consumers. Bottom line: Your patients will likely have to pay more out of pocket.
How, precisely, will inflation affect your practice, and what’s the best way to minimize the damage?
Step 1: Maintain operational standards
“Based on the conversations we’ve had with our physician clients that own practices, we see the potential for cost inflation to outrun revenue inflation over the next year,” said Michael Ashley Schulman, CFA, partner and chief investment officer at Running Point Capital, El Segundo, Calif. “Staff wages, as well as office equipment and medical supply costs, are increasing faster than insurance and Medicare/Medicaid reimbursement amounts.” Even so, topflight employees are essential to keep your practice running smoothly. Prioritize excellent nursing. Instead of adding a new hire, compensate your best nurse as well as possible. The same goes for an efficient office manager: On that front, too, you should go the extra mile, even if it means trimming expenses elsewhere.
Step 2: Plan ahead for insurance challenges
Many insurers, including Medicare, set health care costs a year in advance, based on projected growth. This means insurance payouts will stay largely the same for the time being. “Almost all physicians employed by large groups won’t see costs due to inflation rise until next year,” said Mark V. Pauly, PhD, Bendheim Professor in the department of health care management at the University of Pennsylvania, Philadelphia. “For self-employed physicians, there will also be a cushion.”
“The big issue with inflation is that more patients will likely be underinsured,” said Tiffany Johnson, MBA, CFP, co-CEO and financial advisor at Piece of Wealth Planning in Atlanta. “With more out-of-pocket costs ... these patients may not seek out medical treatment or go to see a specialist if they do not believe it is necessary.” A new study from Johns Hopkins found that patients under financial pressure often delay or forgo medical treatment because of food insecurity. Compassionate care is the solution: Direct these patients to financial aid and other resources they may qualify for. That way, they can continue to receive the care they need from you, and your need to pass on costs may be lower.
Step 3: Rely on your affiliated health care organization
These are tough times when it comes to expansion. “Since we are in an environment where inflation and interest rates are both high, it will be much harder for physicians to have the capital to invest in new technology to grow or advance their practice,” Ms. Johnson said. With that in mind, keep the lines of communication between you and your affiliated hospital/health care organization more open than ever. Combining practices with another doctor is one way to increase revenue; you might ask if any affiliated doctors are seeking to team up. It’s also vital to attend meetings and pay close attention to budget cuts your organization may be making. And don’t be shy about asking your administrator for profit-boosting recommendations.
Step 4: Revisit vendor relationships
Find out if your vendors will continue to supply you with the goods you need at reasonable rates, and switch now if they won’t. Be proactive. “Test new medical suppliers,” Mr. Schulman advised. “Reread equipment leasing contracts to check if the interest rates have increased. See if buyout, prepay, or refinancing options are more economical. Also, investigate [bringing down] your rental expense by reducing square footage or moving to a lower-cost location.” In light of ongoing supply chain issues, it’s wise to consider alternative products. But stay focused on quality – you don’t want to be stuck with cheap, possibly defective equipment. Spend where it’s essential and cut the fat somewhere else.
Step 5: Don’t waste your assets
Analyze your budget in minute detail. “Now is the time to review your current inventory and overhead costs,” Ms. Johnson said. “Many physicians let their office staff handle the restocking of inventory and office supplies. While this can be efficient for their practice, it also leaves room for unnecessary business expenses.” Take a cold, hard look at your supply closet – what’s in there that you can live without? Don’t reorder it. Then seek out any revenue stream you may be overlooking. “It’s important to review billing to make sure all the services are reimbursable,” Ms. Johnson added. Small mistakes can yield dividends if you find them.
Step 6: Be poised to pivot
Get creative. “To minimize a profit decline, use video consulting – it’s more efficient and less equipment intensive,” Mr. Schulman said. “Look at how remote work and flexible hours can maximize the work your practice accomplishes while cutting office costs.”
Ms. Johnson suggests adding concierge services, noting that “concierge doctors offer personalized care and direct access for an up-front fee.” With this approach, you may see fewer patients, but your payout paperwork will decrease, and that up-front fee can be profitable. Another outside-the-box idea: Start making house calls. A Scripps study found that home health visits requested via app can result in patient care delivered by a doctor and medical assistant in less than 2 hours. House calls can be an effective and profitable solution when it comes to providing nonemergency care and preventive treatment to patients who aren’t mobile, not to mention patients who just appreciate the convenience.
Step 7: Maintain transparency
Any economic changes your practice will implement must be communicated to your staff and patients clearly and directly. Keep everyone in the loop and be ready to answer questions immediately. Show those you work with and care for that, regardless of the economy, it’s they who matter to you most. That simple reassurance will prove invaluable.
A version of this article first appeared on Medscape.com.
Rampant inflation doesn’t just mean a spike in everyday expenses like gas and groceries. It’s also bound to have a significant impact on the cost of health care – and on your practice. A recent report from McKinsey & Company predicts that the current inflationary spiral will force health care providers to charge higher reimbursement rates, and those costs inevitably will be passed along to both employers and consumers. Bottom line: Your patients will likely have to pay more out of pocket.
How, precisely, will inflation affect your practice, and what’s the best way to minimize the damage?
Step 1: Maintain operational standards
“Based on the conversations we’ve had with our physician clients that own practices, we see the potential for cost inflation to outrun revenue inflation over the next year,” said Michael Ashley Schulman, CFA, partner and chief investment officer at Running Point Capital, El Segundo, Calif. “Staff wages, as well as office equipment and medical supply costs, are increasing faster than insurance and Medicare/Medicaid reimbursement amounts.” Even so, topflight employees are essential to keep your practice running smoothly. Prioritize excellent nursing. Instead of adding a new hire, compensate your best nurse as well as possible. The same goes for an efficient office manager: On that front, too, you should go the extra mile, even if it means trimming expenses elsewhere.
Step 2: Plan ahead for insurance challenges
Many insurers, including Medicare, set health care costs a year in advance, based on projected growth. This means insurance payouts will stay largely the same for the time being. “Almost all physicians employed by large groups won’t see costs due to inflation rise until next year,” said Mark V. Pauly, PhD, Bendheim Professor in the department of health care management at the University of Pennsylvania, Philadelphia. “For self-employed physicians, there will also be a cushion.”
“The big issue with inflation is that more patients will likely be underinsured,” said Tiffany Johnson, MBA, CFP, co-CEO and financial advisor at Piece of Wealth Planning in Atlanta. “With more out-of-pocket costs ... these patients may not seek out medical treatment or go to see a specialist if they do not believe it is necessary.” A new study from Johns Hopkins found that patients under financial pressure often delay or forgo medical treatment because of food insecurity. Compassionate care is the solution: Direct these patients to financial aid and other resources they may qualify for. That way, they can continue to receive the care they need from you, and your need to pass on costs may be lower.
Step 3: Rely on your affiliated health care organization
These are tough times when it comes to expansion. “Since we are in an environment where inflation and interest rates are both high, it will be much harder for physicians to have the capital to invest in new technology to grow or advance their practice,” Ms. Johnson said. With that in mind, keep the lines of communication between you and your affiliated hospital/health care organization more open than ever. Combining practices with another doctor is one way to increase revenue; you might ask if any affiliated doctors are seeking to team up. It’s also vital to attend meetings and pay close attention to budget cuts your organization may be making. And don’t be shy about asking your administrator for profit-boosting recommendations.
Step 4: Revisit vendor relationships
Find out if your vendors will continue to supply you with the goods you need at reasonable rates, and switch now if they won’t. Be proactive. “Test new medical suppliers,” Mr. Schulman advised. “Reread equipment leasing contracts to check if the interest rates have increased. See if buyout, prepay, or refinancing options are more economical. Also, investigate [bringing down] your rental expense by reducing square footage or moving to a lower-cost location.” In light of ongoing supply chain issues, it’s wise to consider alternative products. But stay focused on quality – you don’t want to be stuck with cheap, possibly defective equipment. Spend where it’s essential and cut the fat somewhere else.
Step 5: Don’t waste your assets
Analyze your budget in minute detail. “Now is the time to review your current inventory and overhead costs,” Ms. Johnson said. “Many physicians let their office staff handle the restocking of inventory and office supplies. While this can be efficient for their practice, it also leaves room for unnecessary business expenses.” Take a cold, hard look at your supply closet – what’s in there that you can live without? Don’t reorder it. Then seek out any revenue stream you may be overlooking. “It’s important to review billing to make sure all the services are reimbursable,” Ms. Johnson added. Small mistakes can yield dividends if you find them.
Step 6: Be poised to pivot
Get creative. “To minimize a profit decline, use video consulting – it’s more efficient and less equipment intensive,” Mr. Schulman said. “Look at how remote work and flexible hours can maximize the work your practice accomplishes while cutting office costs.”
Ms. Johnson suggests adding concierge services, noting that “concierge doctors offer personalized care and direct access for an up-front fee.” With this approach, you may see fewer patients, but your payout paperwork will decrease, and that up-front fee can be profitable. Another outside-the-box idea: Start making house calls. A Scripps study found that home health visits requested via app can result in patient care delivered by a doctor and medical assistant in less than 2 hours. House calls can be an effective and profitable solution when it comes to providing nonemergency care and preventive treatment to patients who aren’t mobile, not to mention patients who just appreciate the convenience.
Step 7: Maintain transparency
Any economic changes your practice will implement must be communicated to your staff and patients clearly and directly. Keep everyone in the loop and be ready to answer questions immediately. Show those you work with and care for that, regardless of the economy, it’s they who matter to you most. That simple reassurance will prove invaluable.
A version of this article first appeared on Medscape.com.
BA.4 and BA.5 subvariants are more evasive of antibodies, but not of cellular immunity
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.
High residual liver cancer risk in HCV-cured cirrhosis
A new study confirms the very high risk of hepatocellular carcinoma faced by patients with cirrhosis who have been cured of hepatitis C, a finding the researchers hope will encourage clinicians to communicate risk information to patients and encourage regular HCC screening.
On average, the predicted probability of HCC in cirrhosis patients was 410 times greater than the equivalent probability in the general population, the study team found.
Hamish Innes, PhD, with Public Health Scotland, Glasgow, and colleagues wrote.
“Central to this is ensuring that cured cirrhosis patients understand the risk of HCC and are provided with appropriate surveillance,” they added.
“Most patients with cirrhosis do not adhere to HCC screening guidelines,” Nina Beri, MD, medical oncologist with New York University Perlmutter Cancer Center, who wasn’t involved in the study, said in an interview.
The “important” finding in this study “should be conveyed to patients, as this may help improve screening adherence rates,” Dr. Beri said.
The study was published online in the American Journal of Gastroenterology.
Findings may help promote screening uptake
Dr. Innes and colleagues compared the predicted probability of HCC in 1,803 Scottish adults (mean age, 50 years; 74% male) with cirrhosis and cured hepatitis C to the background risk in the general population of Scotland.
The mean predicted 3-year probability of HCC at the time of sustained viral response (SVR), determined using the aMAP prognostic model, was 3.64% (range, 0.012%-36.12%).
This contrasts with a 3-year HCC probability in the general population ranging from less than 0.0001% to 0.25% depending on demographics.
All patients with cirrhosis – even those at lowest risk – had a higher probability of HCC than the general population, but there was considerable heterogeneity from one patient to the next.
For example, the mean 3-year predicted probability was 18 times higher in the top quintile (9.8%) versus the lowest quintile (0.5%) of risk, the researchers found.
They could not identify a patient subgroup who exhibited a similar HCC risk profile to the general population, as was their hope going into the study.
Dr. Innes and colleagues have developed an online tool that allows clinicians to frame a patient›s 3-year HCC probability against the equivalent probability in the general population.
In the future, they said the scope of the tool could be extended by incorporating general population data from countries beyond Scotland.
“Our hope is that this tool will springboard patient-clinician discussions about HCC risk, and could mitigate low screening uptake,” Dr. Innes and colleagues wrote.
Curing HCV doesn’t eliminate risk
Commenting on the study, Nancy Reau, MD, section chief of hepatology at Rush University Medical Center, Chicago, said curing HCV is “very important and significantly reduces risk for complications, but it doesn’t return you to the normal population.”
Dr. Reau’s advice to cirrhosis patients: “Get screened twice a year.”
Dr. Beri said, in addition to conveying this risk to patients, “it is also important to disseminate this information to the community and to primary care practices, particularly as some patients may not currently follow in a specialized liver disease clinic.”
Also weighing in, Amit Singal, MD, chief of hepatology at the University of Texas Southwestern Medical Center, Dallas, said this study highlights that underlying cirrhosis is “the strongest risk factor for the development of HCC.”
In contrast to other cancers, such as breast and colorectal cancer, in which high risk populations can be identified by readily available information such as age and sex, implementation of HCC screening programs requires identification of patients with cirrhosis, Dr. Singal noted.
“Underuse of HCC screening in clinical practice is often related to providers having difficulty at this step in the process and contributes to the high proportion of HCC detected at late stages,” he told this news organization.
“Availability of accurate noninvasive markers of fibrosis will hopefully help with better identification of patients with cirrhosis moving forward,” Dr. Singal said, “although we as hepatologists need to work closely with our primary care colleagues to ensure these tools are used routinely in at-risk patients, such as those with nonalcoholic fatty liver disease, alcohol-associated liver disease, or history of cured (post-SVR) hepatitis C infection.”
The study was supported by the Medical Research Foundation and Public Health Scotland. Dr. Innes, Dr. Beri, Dr. Reau, and Dr. Singal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study confirms the very high risk of hepatocellular carcinoma faced by patients with cirrhosis who have been cured of hepatitis C, a finding the researchers hope will encourage clinicians to communicate risk information to patients and encourage regular HCC screening.
On average, the predicted probability of HCC in cirrhosis patients was 410 times greater than the equivalent probability in the general population, the study team found.
Hamish Innes, PhD, with Public Health Scotland, Glasgow, and colleagues wrote.
“Central to this is ensuring that cured cirrhosis patients understand the risk of HCC and are provided with appropriate surveillance,” they added.
“Most patients with cirrhosis do not adhere to HCC screening guidelines,” Nina Beri, MD, medical oncologist with New York University Perlmutter Cancer Center, who wasn’t involved in the study, said in an interview.
The “important” finding in this study “should be conveyed to patients, as this may help improve screening adherence rates,” Dr. Beri said.
The study was published online in the American Journal of Gastroenterology.
Findings may help promote screening uptake
Dr. Innes and colleagues compared the predicted probability of HCC in 1,803 Scottish adults (mean age, 50 years; 74% male) with cirrhosis and cured hepatitis C to the background risk in the general population of Scotland.
The mean predicted 3-year probability of HCC at the time of sustained viral response (SVR), determined using the aMAP prognostic model, was 3.64% (range, 0.012%-36.12%).
This contrasts with a 3-year HCC probability in the general population ranging from less than 0.0001% to 0.25% depending on demographics.
All patients with cirrhosis – even those at lowest risk – had a higher probability of HCC than the general population, but there was considerable heterogeneity from one patient to the next.
For example, the mean 3-year predicted probability was 18 times higher in the top quintile (9.8%) versus the lowest quintile (0.5%) of risk, the researchers found.
They could not identify a patient subgroup who exhibited a similar HCC risk profile to the general population, as was their hope going into the study.
Dr. Innes and colleagues have developed an online tool that allows clinicians to frame a patient›s 3-year HCC probability against the equivalent probability in the general population.
In the future, they said the scope of the tool could be extended by incorporating general population data from countries beyond Scotland.
“Our hope is that this tool will springboard patient-clinician discussions about HCC risk, and could mitigate low screening uptake,” Dr. Innes and colleagues wrote.
Curing HCV doesn’t eliminate risk
Commenting on the study, Nancy Reau, MD, section chief of hepatology at Rush University Medical Center, Chicago, said curing HCV is “very important and significantly reduces risk for complications, but it doesn’t return you to the normal population.”
Dr. Reau’s advice to cirrhosis patients: “Get screened twice a year.”
Dr. Beri said, in addition to conveying this risk to patients, “it is also important to disseminate this information to the community and to primary care practices, particularly as some patients may not currently follow in a specialized liver disease clinic.”
Also weighing in, Amit Singal, MD, chief of hepatology at the University of Texas Southwestern Medical Center, Dallas, said this study highlights that underlying cirrhosis is “the strongest risk factor for the development of HCC.”
In contrast to other cancers, such as breast and colorectal cancer, in which high risk populations can be identified by readily available information such as age and sex, implementation of HCC screening programs requires identification of patients with cirrhosis, Dr. Singal noted.
“Underuse of HCC screening in clinical practice is often related to providers having difficulty at this step in the process and contributes to the high proportion of HCC detected at late stages,” he told this news organization.
“Availability of accurate noninvasive markers of fibrosis will hopefully help with better identification of patients with cirrhosis moving forward,” Dr. Singal said, “although we as hepatologists need to work closely with our primary care colleagues to ensure these tools are used routinely in at-risk patients, such as those with nonalcoholic fatty liver disease, alcohol-associated liver disease, or history of cured (post-SVR) hepatitis C infection.”
The study was supported by the Medical Research Foundation and Public Health Scotland. Dr. Innes, Dr. Beri, Dr. Reau, and Dr. Singal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study confirms the very high risk of hepatocellular carcinoma faced by patients with cirrhosis who have been cured of hepatitis C, a finding the researchers hope will encourage clinicians to communicate risk information to patients and encourage regular HCC screening.
On average, the predicted probability of HCC in cirrhosis patients was 410 times greater than the equivalent probability in the general population, the study team found.
Hamish Innes, PhD, with Public Health Scotland, Glasgow, and colleagues wrote.
“Central to this is ensuring that cured cirrhosis patients understand the risk of HCC and are provided with appropriate surveillance,” they added.
“Most patients with cirrhosis do not adhere to HCC screening guidelines,” Nina Beri, MD, medical oncologist with New York University Perlmutter Cancer Center, who wasn’t involved in the study, said in an interview.
The “important” finding in this study “should be conveyed to patients, as this may help improve screening adherence rates,” Dr. Beri said.
The study was published online in the American Journal of Gastroenterology.
Findings may help promote screening uptake
Dr. Innes and colleagues compared the predicted probability of HCC in 1,803 Scottish adults (mean age, 50 years; 74% male) with cirrhosis and cured hepatitis C to the background risk in the general population of Scotland.
The mean predicted 3-year probability of HCC at the time of sustained viral response (SVR), determined using the aMAP prognostic model, was 3.64% (range, 0.012%-36.12%).
This contrasts with a 3-year HCC probability in the general population ranging from less than 0.0001% to 0.25% depending on demographics.
All patients with cirrhosis – even those at lowest risk – had a higher probability of HCC than the general population, but there was considerable heterogeneity from one patient to the next.
For example, the mean 3-year predicted probability was 18 times higher in the top quintile (9.8%) versus the lowest quintile (0.5%) of risk, the researchers found.
They could not identify a patient subgroup who exhibited a similar HCC risk profile to the general population, as was their hope going into the study.
Dr. Innes and colleagues have developed an online tool that allows clinicians to frame a patient›s 3-year HCC probability against the equivalent probability in the general population.
In the future, they said the scope of the tool could be extended by incorporating general population data from countries beyond Scotland.
“Our hope is that this tool will springboard patient-clinician discussions about HCC risk, and could mitigate low screening uptake,” Dr. Innes and colleagues wrote.
Curing HCV doesn’t eliminate risk
Commenting on the study, Nancy Reau, MD, section chief of hepatology at Rush University Medical Center, Chicago, said curing HCV is “very important and significantly reduces risk for complications, but it doesn’t return you to the normal population.”
Dr. Reau’s advice to cirrhosis patients: “Get screened twice a year.”
Dr. Beri said, in addition to conveying this risk to patients, “it is also important to disseminate this information to the community and to primary care practices, particularly as some patients may not currently follow in a specialized liver disease clinic.”
Also weighing in, Amit Singal, MD, chief of hepatology at the University of Texas Southwestern Medical Center, Dallas, said this study highlights that underlying cirrhosis is “the strongest risk factor for the development of HCC.”
In contrast to other cancers, such as breast and colorectal cancer, in which high risk populations can be identified by readily available information such as age and sex, implementation of HCC screening programs requires identification of patients with cirrhosis, Dr. Singal noted.
“Underuse of HCC screening in clinical practice is often related to providers having difficulty at this step in the process and contributes to the high proportion of HCC detected at late stages,” he told this news organization.
“Availability of accurate noninvasive markers of fibrosis will hopefully help with better identification of patients with cirrhosis moving forward,” Dr. Singal said, “although we as hepatologists need to work closely with our primary care colleagues to ensure these tools are used routinely in at-risk patients, such as those with nonalcoholic fatty liver disease, alcohol-associated liver disease, or history of cured (post-SVR) hepatitis C infection.”
The study was supported by the Medical Research Foundation and Public Health Scotland. Dr. Innes, Dr. Beri, Dr. Reau, and Dr. Singal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Obesity links to faster fading of COVID vaccine protection
Researchers published the study covered in this summary on medRxiv.org as a preprint that has not yet been peer reviewed.
Key takeaways
- The study results suggest that
- The findings documented evidence of reduced neutralizing antibody capacity 6 months after primary vaccination in people with severe obesity.
- This was a large study involving about more than 3.5 million people who had received at least two doses of COVID-19 vaccine, including more than 650,000 with obesity.
Why this matters
- Obesity is associated with comorbidities that independently increase the risk for severe COVID-19, including type 2 diabetes, chronic kidney disease, and heart failure.
- The authors concluded that additional or more frequent booster doses are likely to be required to maintain protection among people with obesity against COVID-19.
Study design
- Prospective longitudinal study of the incidence and severity of COVID-19 infections and immune responses in a cohort of more than 3.5 million adults from a Scottish healthcare database who received two or three doses of COVID-19 vaccine. The data came from the study, centered at the University of Edinburgh.
- About 16% had obesity with a body mass index of 30-39.9 kg/m2, and an additional 3% had severe obesity with a BMI of 40 or greater.
- Although not specified in this preprint, another said that the vaccines administered in Scotland have been the Pfizer-BioNTech and Oxford-AstraZeneca formulations.
Key results
- Between Sept. 14, 2020, and March 19, 2022, 10,983 people (0.3% of the total cohort; 6.0 events per 1,000 person-years) had severe COVID-19, consisting of 9,733 who were hospitalized and 2,207 who died (957 of those hospitalized also died).
- People with obesity or severe obesity were at higher risk of hospitalization or death from COVID-19 after both a second and third (booster) dose of vaccine.
- Compared with those with normal weight, those with severe obesity (BMI higher than 40) were at significantly increased risk for severe COVID-19 after a second vaccine dose, with an adjusted rate ratio 1.76, whereas those with standard obesity (BMI, 30-40) were at a modestly but significantly increased risk with an adjusted rate ratio of 1.11.
- Breakthrough infections after the second dose for those with severe obesity, obesity, and normal weight occurred on average at 10 weeks, 15 weeks, and 20 weeks, respectively.
- Interaction testing showed that vaccine effectiveness significantly diminished over time across BMI groups, and protection waned more rapidly as BMI increased.
- Results from immunophenotyping studies run in a subgroup of several dozen subjects with severe obesity or normal weight showed significant decrements in the robustness of antibody responses in those with severe obesity 6 months after a second or third vaccine dose.
Limitations
- The authors did not specify any limitations.
Disclosures
- The study received no commercial funding.
- One author received funding from Wellcome.
This is a summary of a preprint research study , “Accelerated waning of the humoral response to SARS-CoV-2 vaccines in obesity,” published by researchers primarily at the University of Cambridge (England), on medRxiv. This study has not yet been peer reviewed. The full text of the study can be found on medRxiv.org.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on medRxiv.org as a preprint that has not yet been peer reviewed.
Key takeaways
- The study results suggest that
- The findings documented evidence of reduced neutralizing antibody capacity 6 months after primary vaccination in people with severe obesity.
- This was a large study involving about more than 3.5 million people who had received at least two doses of COVID-19 vaccine, including more than 650,000 with obesity.
Why this matters
- Obesity is associated with comorbidities that independently increase the risk for severe COVID-19, including type 2 diabetes, chronic kidney disease, and heart failure.
- The authors concluded that additional or more frequent booster doses are likely to be required to maintain protection among people with obesity against COVID-19.
Study design
- Prospective longitudinal study of the incidence and severity of COVID-19 infections and immune responses in a cohort of more than 3.5 million adults from a Scottish healthcare database who received two or three doses of COVID-19 vaccine. The data came from the study, centered at the University of Edinburgh.
- About 16% had obesity with a body mass index of 30-39.9 kg/m2, and an additional 3% had severe obesity with a BMI of 40 or greater.
- Although not specified in this preprint, another said that the vaccines administered in Scotland have been the Pfizer-BioNTech and Oxford-AstraZeneca formulations.
Key results
- Between Sept. 14, 2020, and March 19, 2022, 10,983 people (0.3% of the total cohort; 6.0 events per 1,000 person-years) had severe COVID-19, consisting of 9,733 who were hospitalized and 2,207 who died (957 of those hospitalized also died).
- People with obesity or severe obesity were at higher risk of hospitalization or death from COVID-19 after both a second and third (booster) dose of vaccine.
- Compared with those with normal weight, those with severe obesity (BMI higher than 40) were at significantly increased risk for severe COVID-19 after a second vaccine dose, with an adjusted rate ratio 1.76, whereas those with standard obesity (BMI, 30-40) were at a modestly but significantly increased risk with an adjusted rate ratio of 1.11.
- Breakthrough infections after the second dose for those with severe obesity, obesity, and normal weight occurred on average at 10 weeks, 15 weeks, and 20 weeks, respectively.
- Interaction testing showed that vaccine effectiveness significantly diminished over time across BMI groups, and protection waned more rapidly as BMI increased.
- Results from immunophenotyping studies run in a subgroup of several dozen subjects with severe obesity or normal weight showed significant decrements in the robustness of antibody responses in those with severe obesity 6 months after a second or third vaccine dose.
Limitations
- The authors did not specify any limitations.
Disclosures
- The study received no commercial funding.
- One author received funding from Wellcome.
This is a summary of a preprint research study , “Accelerated waning of the humoral response to SARS-CoV-2 vaccines in obesity,” published by researchers primarily at the University of Cambridge (England), on medRxiv. This study has not yet been peer reviewed. The full text of the study can be found on medRxiv.org.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on medRxiv.org as a preprint that has not yet been peer reviewed.
Key takeaways
- The study results suggest that
- The findings documented evidence of reduced neutralizing antibody capacity 6 months after primary vaccination in people with severe obesity.
- This was a large study involving about more than 3.5 million people who had received at least two doses of COVID-19 vaccine, including more than 650,000 with obesity.
Why this matters
- Obesity is associated with comorbidities that independently increase the risk for severe COVID-19, including type 2 diabetes, chronic kidney disease, and heart failure.
- The authors concluded that additional or more frequent booster doses are likely to be required to maintain protection among people with obesity against COVID-19.
Study design
- Prospective longitudinal study of the incidence and severity of COVID-19 infections and immune responses in a cohort of more than 3.5 million adults from a Scottish healthcare database who received two or three doses of COVID-19 vaccine. The data came from the study, centered at the University of Edinburgh.
- About 16% had obesity with a body mass index of 30-39.9 kg/m2, and an additional 3% had severe obesity with a BMI of 40 or greater.
- Although not specified in this preprint, another said that the vaccines administered in Scotland have been the Pfizer-BioNTech and Oxford-AstraZeneca formulations.
Key results
- Between Sept. 14, 2020, and March 19, 2022, 10,983 people (0.3% of the total cohort; 6.0 events per 1,000 person-years) had severe COVID-19, consisting of 9,733 who were hospitalized and 2,207 who died (957 of those hospitalized also died).
- People with obesity or severe obesity were at higher risk of hospitalization or death from COVID-19 after both a second and third (booster) dose of vaccine.
- Compared with those with normal weight, those with severe obesity (BMI higher than 40) were at significantly increased risk for severe COVID-19 after a second vaccine dose, with an adjusted rate ratio 1.76, whereas those with standard obesity (BMI, 30-40) were at a modestly but significantly increased risk with an adjusted rate ratio of 1.11.
- Breakthrough infections after the second dose for those with severe obesity, obesity, and normal weight occurred on average at 10 weeks, 15 weeks, and 20 weeks, respectively.
- Interaction testing showed that vaccine effectiveness significantly diminished over time across BMI groups, and protection waned more rapidly as BMI increased.
- Results from immunophenotyping studies run in a subgroup of several dozen subjects with severe obesity or normal weight showed significant decrements in the robustness of antibody responses in those with severe obesity 6 months after a second or third vaccine dose.
Limitations
- The authors did not specify any limitations.
Disclosures
- The study received no commercial funding.
- One author received funding from Wellcome.
This is a summary of a preprint research study , “Accelerated waning of the humoral response to SARS-CoV-2 vaccines in obesity,” published by researchers primarily at the University of Cambridge (England), on medRxiv. This study has not yet been peer reviewed. The full text of the study can be found on medRxiv.org.
A version of this article first appeared on Medscape.com.
Liver disease and death rates fall after hepatitis C treatment barriers are dismantled
As obstacles to hepatitis C treatment uptake were removed, rates of hepatitis-related liver disease in marginalized groups plummeted, according to a new study in Baltimore, published in Annals of Internal Medicine.
A community-based cohort study that follows current and former people who inject drugs (PWID) with hepatitis C documented drastic reductions in liver disease and death as effective oral antivirals became more readily accessible there between 2015 and 2019.
The researchers concluded that hepatitis C elimination targets are achievable. But, they warned, uptake is uneven, and more needs to be done to facilitate treatment.
“[The study] gives us a real-world perspective on what’s happening on the ground, in terms of people getting treated,” said first author Javier Cepeda, PhD, MPH, an assistant professor at the Johns Hopkins Bloomberg School of Public Health, Baltimore, in an interview. “Changing policy, reducing barriers, [and] getting them access to treatment really does have this really important public health benefit.”
” said Maria Corcorran, MD, MPH. Dr. Corcorran, an acting assistant professor for the department of medicine at the University of Washington, was not involved with the study. “It’s just further evidence that we need to really be linking people to care and getting people treated and cured.”
The World Health Organization has called for the disease’s global elimination by 2030. Cure rates top 95%. But, there are so many new cases and so many barriers to detection and treatment that how to develop and roll out a public health response is the most important question in the field, wrote study co-author David L. Thomas, MD, MPH, in a review article in The New England Journal of Medicine.
“Folks who inject drugs ... do well on hep C treatment and have similar rates of sustained virologic response or cure,” said Dr. Corcorran, who runs a low-barrier clinic for people experiencing homelessness.
But, she added, “there are barriers that are still put up to treatment in terms of who can treat and what insurance is going to cover.”
A look at a vulnerable population
The authors studied adults enrolled in ALIVE (AIDS Linked to the Intravenous Experience), a cohort study that has recruited current and former PWID in the Baltimore area since 1988.
Participants visit the clinic twice a year for health-related interviews and blood testing, including HIV serology, hepatitis C virus (HCV) antibody and RNA testing, and liver function tests. They are counseled about HCV testing and treatment but do not receive treatment through the study.
Beginning in 2006, researchers added liver stiffness measures (LSMs), a noninvasive measure conducted with transient elastography.
From 2006 to 2019, the authors followed 1,323 ALIVE participants with chronic HCV infection. The primary outcome was LSMs.
Less liver disease, fewer deaths
At baseline, participants’ median age was 49 years; 82% of participants were Black individuals, 71% percent were male, and two-thirds were HIV-negative.
Three percent reported receiving hepatitis C treatment in 2014, which increased to 39% in 2019.
Among 10,350 LSMs, 15% showed cirrhosis at baseline in 2006. In 2015, that rose to 19%, but by 2019, it had fallen to 8%.
By definition, 100% had detectable HCV RNA at baseline. In 2015, 91% still did. By 2019, that rate had fallen to 48%.
Undetectable HCV RNA correlated with lower log LSM in adjusted models (P < .001). It also correlated strongly with lower odds of liver cirrhosis, with an adjusted odds ratio of 0.28 (95% confidence interval, 0.17-0.45; P < .001). In addition, it correlated with lower risk for all-cause mortality, with an adjusted hazard ratio of 0.54 (95% CI, 0.38-0.77; P < .001).
Limitations include the fact that, although transient elastography is considered the most valid way to detect cirrhosis in people with hepatitis C, liver stiffness has not been validated as a measure of fibrosis among people with a sustained virologic response.
In addition, ALIVE participants are older and more likely to be African American individuals, compared with the general population of PWID in Baltimore, wrote co-author Shruti H. Mehta, PhD, a professor of epidemiology at Johns Hopkins University, Baltimore, in an email exchange with this news organization. That could affect generalizability.
Treatment is crucial
The first direct-acting antiviral (DAA) for hepatitis C was approved in 2011, and an oral fixed-dose combination antiviral was approved in 2014, ushering in treatments with cure rates far exceeding those with interferon-based therapy.
But until recently, Medicaid patients in Maryland seeking DAA therapy for hepatitis C required prior authorization, with initial restrictions related to disease stage, substance use, and provider type, according to Dr. Mehta.
Gradually, those restrictions were lifted, Dr. Mehta added, and all were eliminated by 2019.
Dr. Cepeda urges clinicians to treat patients infected with hepatitis C immediately.
“There are really important implications on both reducing liver disease progression and all-cause mortality,” he said.
“Hep C is just one part of a whole constellation of health care delivery [and] of treating all of the other potential problems that might need to be addressed – especially with people who inject drugs,” Dr. Cepeda added. “Getting them into care is really, really important.”
The study was funded by the National Institutes of Health. Dr. Cepeda and Dr. Corcorran report no relevant financial relationships. Dr. Mehta reports receiving payments or honoraria and travel support from Gilead Sciences, the makers of the oral hepatitis C medication ledipasvir/sofosbuvir (Harvoni), as well as equipment, materials, drugs, medical writing, gifts, or other services from Abbott, which sells hepatitis C diagnostics. Dr. Thomas reports ties to Excision Bio and to Merck DSMB.
A version of this article first appeared on Medscape.com.
As obstacles to hepatitis C treatment uptake were removed, rates of hepatitis-related liver disease in marginalized groups plummeted, according to a new study in Baltimore, published in Annals of Internal Medicine.
A community-based cohort study that follows current and former people who inject drugs (PWID) with hepatitis C documented drastic reductions in liver disease and death as effective oral antivirals became more readily accessible there between 2015 and 2019.
The researchers concluded that hepatitis C elimination targets are achievable. But, they warned, uptake is uneven, and more needs to be done to facilitate treatment.
“[The study] gives us a real-world perspective on what’s happening on the ground, in terms of people getting treated,” said first author Javier Cepeda, PhD, MPH, an assistant professor at the Johns Hopkins Bloomberg School of Public Health, Baltimore, in an interview. “Changing policy, reducing barriers, [and] getting them access to treatment really does have this really important public health benefit.”
” said Maria Corcorran, MD, MPH. Dr. Corcorran, an acting assistant professor for the department of medicine at the University of Washington, was not involved with the study. “It’s just further evidence that we need to really be linking people to care and getting people treated and cured.”
The World Health Organization has called for the disease’s global elimination by 2030. Cure rates top 95%. But, there are so many new cases and so many barriers to detection and treatment that how to develop and roll out a public health response is the most important question in the field, wrote study co-author David L. Thomas, MD, MPH, in a review article in The New England Journal of Medicine.
“Folks who inject drugs ... do well on hep C treatment and have similar rates of sustained virologic response or cure,” said Dr. Corcorran, who runs a low-barrier clinic for people experiencing homelessness.
But, she added, “there are barriers that are still put up to treatment in terms of who can treat and what insurance is going to cover.”
A look at a vulnerable population
The authors studied adults enrolled in ALIVE (AIDS Linked to the Intravenous Experience), a cohort study that has recruited current and former PWID in the Baltimore area since 1988.
Participants visit the clinic twice a year for health-related interviews and blood testing, including HIV serology, hepatitis C virus (HCV) antibody and RNA testing, and liver function tests. They are counseled about HCV testing and treatment but do not receive treatment through the study.
Beginning in 2006, researchers added liver stiffness measures (LSMs), a noninvasive measure conducted with transient elastography.
From 2006 to 2019, the authors followed 1,323 ALIVE participants with chronic HCV infection. The primary outcome was LSMs.
Less liver disease, fewer deaths
At baseline, participants’ median age was 49 years; 82% of participants were Black individuals, 71% percent were male, and two-thirds were HIV-negative.
Three percent reported receiving hepatitis C treatment in 2014, which increased to 39% in 2019.
Among 10,350 LSMs, 15% showed cirrhosis at baseline in 2006. In 2015, that rose to 19%, but by 2019, it had fallen to 8%.
By definition, 100% had detectable HCV RNA at baseline. In 2015, 91% still did. By 2019, that rate had fallen to 48%.
Undetectable HCV RNA correlated with lower log LSM in adjusted models (P < .001). It also correlated strongly with lower odds of liver cirrhosis, with an adjusted odds ratio of 0.28 (95% confidence interval, 0.17-0.45; P < .001). In addition, it correlated with lower risk for all-cause mortality, with an adjusted hazard ratio of 0.54 (95% CI, 0.38-0.77; P < .001).
Limitations include the fact that, although transient elastography is considered the most valid way to detect cirrhosis in people with hepatitis C, liver stiffness has not been validated as a measure of fibrosis among people with a sustained virologic response.
In addition, ALIVE participants are older and more likely to be African American individuals, compared with the general population of PWID in Baltimore, wrote co-author Shruti H. Mehta, PhD, a professor of epidemiology at Johns Hopkins University, Baltimore, in an email exchange with this news organization. That could affect generalizability.
Treatment is crucial
The first direct-acting antiviral (DAA) for hepatitis C was approved in 2011, and an oral fixed-dose combination antiviral was approved in 2014, ushering in treatments with cure rates far exceeding those with interferon-based therapy.
But until recently, Medicaid patients in Maryland seeking DAA therapy for hepatitis C required prior authorization, with initial restrictions related to disease stage, substance use, and provider type, according to Dr. Mehta.
Gradually, those restrictions were lifted, Dr. Mehta added, and all were eliminated by 2019.
Dr. Cepeda urges clinicians to treat patients infected with hepatitis C immediately.
“There are really important implications on both reducing liver disease progression and all-cause mortality,” he said.
“Hep C is just one part of a whole constellation of health care delivery [and] of treating all of the other potential problems that might need to be addressed – especially with people who inject drugs,” Dr. Cepeda added. “Getting them into care is really, really important.”
The study was funded by the National Institutes of Health. Dr. Cepeda and Dr. Corcorran report no relevant financial relationships. Dr. Mehta reports receiving payments or honoraria and travel support from Gilead Sciences, the makers of the oral hepatitis C medication ledipasvir/sofosbuvir (Harvoni), as well as equipment, materials, drugs, medical writing, gifts, or other services from Abbott, which sells hepatitis C diagnostics. Dr. Thomas reports ties to Excision Bio and to Merck DSMB.
A version of this article first appeared on Medscape.com.
As obstacles to hepatitis C treatment uptake were removed, rates of hepatitis-related liver disease in marginalized groups plummeted, according to a new study in Baltimore, published in Annals of Internal Medicine.
A community-based cohort study that follows current and former people who inject drugs (PWID) with hepatitis C documented drastic reductions in liver disease and death as effective oral antivirals became more readily accessible there between 2015 and 2019.
The researchers concluded that hepatitis C elimination targets are achievable. But, they warned, uptake is uneven, and more needs to be done to facilitate treatment.
“[The study] gives us a real-world perspective on what’s happening on the ground, in terms of people getting treated,” said first author Javier Cepeda, PhD, MPH, an assistant professor at the Johns Hopkins Bloomberg School of Public Health, Baltimore, in an interview. “Changing policy, reducing barriers, [and] getting them access to treatment really does have this really important public health benefit.”
” said Maria Corcorran, MD, MPH. Dr. Corcorran, an acting assistant professor for the department of medicine at the University of Washington, was not involved with the study. “It’s just further evidence that we need to really be linking people to care and getting people treated and cured.”
The World Health Organization has called for the disease’s global elimination by 2030. Cure rates top 95%. But, there are so many new cases and so many barriers to detection and treatment that how to develop and roll out a public health response is the most important question in the field, wrote study co-author David L. Thomas, MD, MPH, in a review article in The New England Journal of Medicine.
“Folks who inject drugs ... do well on hep C treatment and have similar rates of sustained virologic response or cure,” said Dr. Corcorran, who runs a low-barrier clinic for people experiencing homelessness.
But, she added, “there are barriers that are still put up to treatment in terms of who can treat and what insurance is going to cover.”
A look at a vulnerable population
The authors studied adults enrolled in ALIVE (AIDS Linked to the Intravenous Experience), a cohort study that has recruited current and former PWID in the Baltimore area since 1988.
Participants visit the clinic twice a year for health-related interviews and blood testing, including HIV serology, hepatitis C virus (HCV) antibody and RNA testing, and liver function tests. They are counseled about HCV testing and treatment but do not receive treatment through the study.
Beginning in 2006, researchers added liver stiffness measures (LSMs), a noninvasive measure conducted with transient elastography.
From 2006 to 2019, the authors followed 1,323 ALIVE participants with chronic HCV infection. The primary outcome was LSMs.
Less liver disease, fewer deaths
At baseline, participants’ median age was 49 years; 82% of participants were Black individuals, 71% percent were male, and two-thirds were HIV-negative.
Three percent reported receiving hepatitis C treatment in 2014, which increased to 39% in 2019.
Among 10,350 LSMs, 15% showed cirrhosis at baseline in 2006. In 2015, that rose to 19%, but by 2019, it had fallen to 8%.
By definition, 100% had detectable HCV RNA at baseline. In 2015, 91% still did. By 2019, that rate had fallen to 48%.
Undetectable HCV RNA correlated with lower log LSM in adjusted models (P < .001). It also correlated strongly with lower odds of liver cirrhosis, with an adjusted odds ratio of 0.28 (95% confidence interval, 0.17-0.45; P < .001). In addition, it correlated with lower risk for all-cause mortality, with an adjusted hazard ratio of 0.54 (95% CI, 0.38-0.77; P < .001).
Limitations include the fact that, although transient elastography is considered the most valid way to detect cirrhosis in people with hepatitis C, liver stiffness has not been validated as a measure of fibrosis among people with a sustained virologic response.
In addition, ALIVE participants are older and more likely to be African American individuals, compared with the general population of PWID in Baltimore, wrote co-author Shruti H. Mehta, PhD, a professor of epidemiology at Johns Hopkins University, Baltimore, in an email exchange with this news organization. That could affect generalizability.
Treatment is crucial
The first direct-acting antiviral (DAA) for hepatitis C was approved in 2011, and an oral fixed-dose combination antiviral was approved in 2014, ushering in treatments with cure rates far exceeding those with interferon-based therapy.
But until recently, Medicaid patients in Maryland seeking DAA therapy for hepatitis C required prior authorization, with initial restrictions related to disease stage, substance use, and provider type, according to Dr. Mehta.
Gradually, those restrictions were lifted, Dr. Mehta added, and all were eliminated by 2019.
Dr. Cepeda urges clinicians to treat patients infected with hepatitis C immediately.
“There are really important implications on both reducing liver disease progression and all-cause mortality,” he said.
“Hep C is just one part of a whole constellation of health care delivery [and] of treating all of the other potential problems that might need to be addressed – especially with people who inject drugs,” Dr. Cepeda added. “Getting them into care is really, really important.”
The study was funded by the National Institutes of Health. Dr. Cepeda and Dr. Corcorran report no relevant financial relationships. Dr. Mehta reports receiving payments or honoraria and travel support from Gilead Sciences, the makers of the oral hepatitis C medication ledipasvir/sofosbuvir (Harvoni), as well as equipment, materials, drugs, medical writing, gifts, or other services from Abbott, which sells hepatitis C diagnostics. Dr. Thomas reports ties to Excision Bio and to Merck DSMB.
A version of this article first appeared on Medscape.com.
Long COVID-19 in children and adolescents: What do we know?
Among scientists, the existence of long COVID-19 in children and adolescents has been the subject of debate.
Published by a Mexican multidisciplinary group in Scientific Reports, the first study is a systematic review and meta-analysis. It identified mood symptoms as the most prevalent clinical manifestations of long COVID-19 in children and adolescents. These symptoms included sadness, tension, anger, depression, and anxiety (16.50%); fatigue (9.66%); and sleep disorders (8.42%).
The second study, LongCOVIDKidsDK, was conducted in Denmark. It compared 11,000 children younger than 14 years who had tested positive for COVID-19 with 33,000 children who had no history of COVID-19. The study was published in The Lancet Child and Adolescent Health.
Definitions are changing
In their meta-analysis, the researchers estimated the prevalence and counted signs and symptoms of long COVID-19, as defined by the United Kingdom’s National Institute for Health and Care Excellence. Long COVID-19 was defined as the presence of one or more symptoms more than 4 weeks after SARS-CoV-2 infection. For search terms, the researchers used “COVID-19,” “COVID,” “SARSCOV-2,” “coronavirus,” “long COVID,” “postCOVID,” “PASC,” “long-haulers,” “prolonged,” “post-acute,” “persistent,” “convalescent,” “sequelae,” and “postviral.”
Of the 8,373 citations returned by the search as of Feb. 10, 2022, 21 prospective studies, 2 of them on preprint servers, met the authors’ selection criteria. Those studies included a total of 80,071 children and adolescents younger than 18 years.
In the meta-analysis, the prevalence of long COVID-19 among children and adolescents was reported to be 25.24% (95% confidence interval, 18.17-33.02; I2, 99.61%), regardless of whether the case had been asymptomatic, mild, moderate, severe, or serious. For patients who had been hospitalized, the prevalence was 29.19% (95% CI, 17.83-41.98; I2, 80.84%).
These numbers, while striking, are not the focus of the study, according to first author Sandra Lopez-Leon, MD, PhD, associate professor of pharmacoepidemiology at Rutgers University, New Brunswick, N.J. “It’s important that we don’t focus on that 25%,” she said in an interview. “It’s a disease that we’re learning about, we’re at a time when the definitions are still changing, and, depending on when it is measured, a different number will be given. The message we want to give is that long COVID-19 exists, it’s happening in children and adolescents, and patients need this recognition. And also to show that it can affect the whole body.”
The study showed that the children and adolescents who presented with SARS-CoV-2 infection were at higher risk of subsequent long dyspnea, anosmia/ageusia, or fever, compared with control persons.
In total, in the studies that were included, more than 40 long-term clinical manifestations associated with COVID-19 in the pediatric population were identified.
The most common symptoms among children aged 0-3 years were mood swings, skin rashes, and stomachaches. In 4- to 11-year-olds, the most common symptoms were mood swings, trouble remembering or concentrating, and skin rashes. In 12- to 14-year-olds, they were fatigue, mood swings, and trouble remembering or concentrating. These data are based on parent responses.
The list of signs and symptoms also includes headache, respiratory symptoms, cognitive symptoms (such as decreased concentration, learning difficulties, confusion, and memory loss), loss of appetite, and smell disorder (hyposmia, anosmia, hyperosmia, parosmia, and phantom smell).
In the studies, the prevalence of the following symptoms was less than 5%: hyperhidrosis, chest pain, dizziness, cough, myalgia/arthralgia, changes in body weight, taste disorder, otalgia (tinnitus, ear pain, vertigo), ophthalmologic symptoms (conjunctivitis, dry eye, blurred vision, photophobia, pain), dermatologic symptoms (dry skin, itchy skin, rashes, hives, hair loss), urinary symptoms, abdominal pain, throat pain, chest tightness, variations in heart rate, palpitations, constipation, dysphonia, fever, diarrhea, vomiting/nausea, menstrual changes, neurological abnormalities, speech disorders, and dysphagia.
The authors made it clear that the frequency and severity of these symptoms can fluctuate from one patient to another.
“The meta-analysis is important because it brings together 21 studies selected from more than 8,000 articles – and in them, a large number of children – to study the most common manifestations of long COVID-19,” Gabriela Ensinck, MD, head of the infectious diseases department at the Víctor J. Vilela Children’s Hospital in Rosario, Argentina, told this news organization. Dr. Ensinck did not participate in the study. “The important thing here is that long COVID-19 exists in pediatrics. And that it is a prolongation of signs or symptoms over time, a time for which there is no single definition.”
“It’s a snapshot of all the symptoms that can remain after COVID-19,” Dr. Lopez-Leon explained. “The meta-analysis seeks to see if there’s an association between having had COVID-19 and having the symptoms, but at no time does it speak of causality.”
The prevalence of symptoms largely depends on the time since the onset of acute COVID-19. Most symptoms improve over time. In the studies that were included in the meta-analysis, the follow-up time varied between 1 and 13 months. It is important to understand what symptoms are associated with each period after the onset of infection, the authors said.
Danish parent survey
The Danish study LongCOVIDKidsDK followed the World Health Organization criteria for long COVID-19 and included children and adolescents aged 0-14 years who received a diagnosis of COVID-19 and who experienced symptoms that lasted at least 2 months.
Between July 20, 2021, and Sept. 15, 2021, a questionnaire was sent to 38,152 case patients and 147,212 control persons. Of this group, 10,997 (28.8%) case patients and 33,016 (22.4%) control persons answered the survey.
Children who had been diagnosed with SARS-CoV-2 infection were more likely to experience long-lasting symptoms than children who had never been diagnosed. Approximately one-third of children with a positive SARS-CoV-2 test experienced symptoms that were not present before infection. Children who experienced long-lasting symptoms included 40% of children diagnosed with COVID-19 and 27% of control persons aged 0-3 years, 38% of case patients and 34% of control persons aged 4-11 years, and 46% of case patients and 41% of control persons aged 12-14 years.
Interestingly, those diagnosed with COVID-19 reported fewer psychological and social problems than those in the control group. Among the oldest (aged 12-14 years), quality of life scores were higher and anxiety scores were lower for those who had tested positive for SARS-CoV-2.
More information needed
Given the diversity of symptoms in the meta-analysis and the LongCOVIDKidsDK study, a multidisciplinary approach is imperative. Dr. Lopez-Leon suggests that there is a need to raise awareness among parents, clinicians, researchers, and the health system about the conditions that can occur after COVID-19. Clinicians must better understand the sequelae to provide targeted care and treatment. The authors of the Danish study recommend establishing clinics for long COVID-19 with multispecialty care.
Maren J. Heilskov Rytter, PhD, associate professor of clinical medicine at the University of Copenhagen, wrote an editorial in The Lancet Child and Adolescent Health about the Danish study. Until it is clarified whether SARS-CoV-2 does indeed cause persistent symptoms, she wrote, “it seems excessive and premature to establish specific multidisciplinary clinics for children with long COVID-19.”
Dr. Rytter highlighted the difficulty of interpreting LongCOVIDKidsDK data, owing to recall bias, the failure to exclude other causes of symptoms in the cases analyzed, and the number of symptoms in the control persons. In addition, the data analyzed in Denmark are of limited clinical relevance, she said, given a greater presence of mild symptoms and, paradoxically, a higher quality of life.
She concluded, “In the majority of children with nonspecific symptoms after COVID-19, the symptoms presented are more likely to have been caused by something other than COVID-19, and if they are related to COVID-19, they are likely to go away over time.”
Dr. Ensinck, who is coauthor of the Argentine Ministry of Health’s guide for long COVID-19 monitoring for children and adolescents and who represented the Infectious Diseases Committee of the Argentine Society of Pediatrics, highlighted another aspect of the problem. “What should be taken into account in these data is to see how much the confinement contributed. Children are the ones who suffered the most in the period in which schools were closed; they could not meet their peers, they had sick relatives, they felt fear. … all this must be taken into account.”
There is as yet no agreement on how to define and diagnose long COVID-19 in adults, a population that has been studied more closely. Part of the problem is that long COVID-19 has been linked to more than 200 symptoms, which can range in severity from inconvenient to debilitating, can last for months or years, and can recur, sometimes months after apparent recovery. Thus, there are still disparate answers to basic questions about the syndrome’s frequency and its effects on vaccination, reinfection, and the latest variant of SARS-CoV-2.
This article has been translated from the Medscape Spanish edition. A version appeared on Medscape.com.
Among scientists, the existence of long COVID-19 in children and adolescents has been the subject of debate.
Published by a Mexican multidisciplinary group in Scientific Reports, the first study is a systematic review and meta-analysis. It identified mood symptoms as the most prevalent clinical manifestations of long COVID-19 in children and adolescents. These symptoms included sadness, tension, anger, depression, and anxiety (16.50%); fatigue (9.66%); and sleep disorders (8.42%).
The second study, LongCOVIDKidsDK, was conducted in Denmark. It compared 11,000 children younger than 14 years who had tested positive for COVID-19 with 33,000 children who had no history of COVID-19. The study was published in The Lancet Child and Adolescent Health.
Definitions are changing
In their meta-analysis, the researchers estimated the prevalence and counted signs and symptoms of long COVID-19, as defined by the United Kingdom’s National Institute for Health and Care Excellence. Long COVID-19 was defined as the presence of one or more symptoms more than 4 weeks after SARS-CoV-2 infection. For search terms, the researchers used “COVID-19,” “COVID,” “SARSCOV-2,” “coronavirus,” “long COVID,” “postCOVID,” “PASC,” “long-haulers,” “prolonged,” “post-acute,” “persistent,” “convalescent,” “sequelae,” and “postviral.”
Of the 8,373 citations returned by the search as of Feb. 10, 2022, 21 prospective studies, 2 of them on preprint servers, met the authors’ selection criteria. Those studies included a total of 80,071 children and adolescents younger than 18 years.
In the meta-analysis, the prevalence of long COVID-19 among children and adolescents was reported to be 25.24% (95% confidence interval, 18.17-33.02; I2, 99.61%), regardless of whether the case had been asymptomatic, mild, moderate, severe, or serious. For patients who had been hospitalized, the prevalence was 29.19% (95% CI, 17.83-41.98; I2, 80.84%).
These numbers, while striking, are not the focus of the study, according to first author Sandra Lopez-Leon, MD, PhD, associate professor of pharmacoepidemiology at Rutgers University, New Brunswick, N.J. “It’s important that we don’t focus on that 25%,” she said in an interview. “It’s a disease that we’re learning about, we’re at a time when the definitions are still changing, and, depending on when it is measured, a different number will be given. The message we want to give is that long COVID-19 exists, it’s happening in children and adolescents, and patients need this recognition. And also to show that it can affect the whole body.”
The study showed that the children and adolescents who presented with SARS-CoV-2 infection were at higher risk of subsequent long dyspnea, anosmia/ageusia, or fever, compared with control persons.
In total, in the studies that were included, more than 40 long-term clinical manifestations associated with COVID-19 in the pediatric population were identified.
The most common symptoms among children aged 0-3 years were mood swings, skin rashes, and stomachaches. In 4- to 11-year-olds, the most common symptoms were mood swings, trouble remembering or concentrating, and skin rashes. In 12- to 14-year-olds, they were fatigue, mood swings, and trouble remembering or concentrating. These data are based on parent responses.
The list of signs and symptoms also includes headache, respiratory symptoms, cognitive symptoms (such as decreased concentration, learning difficulties, confusion, and memory loss), loss of appetite, and smell disorder (hyposmia, anosmia, hyperosmia, parosmia, and phantom smell).
In the studies, the prevalence of the following symptoms was less than 5%: hyperhidrosis, chest pain, dizziness, cough, myalgia/arthralgia, changes in body weight, taste disorder, otalgia (tinnitus, ear pain, vertigo), ophthalmologic symptoms (conjunctivitis, dry eye, blurred vision, photophobia, pain), dermatologic symptoms (dry skin, itchy skin, rashes, hives, hair loss), urinary symptoms, abdominal pain, throat pain, chest tightness, variations in heart rate, palpitations, constipation, dysphonia, fever, diarrhea, vomiting/nausea, menstrual changes, neurological abnormalities, speech disorders, and dysphagia.
The authors made it clear that the frequency and severity of these symptoms can fluctuate from one patient to another.
“The meta-analysis is important because it brings together 21 studies selected from more than 8,000 articles – and in them, a large number of children – to study the most common manifestations of long COVID-19,” Gabriela Ensinck, MD, head of the infectious diseases department at the Víctor J. Vilela Children’s Hospital in Rosario, Argentina, told this news organization. Dr. Ensinck did not participate in the study. “The important thing here is that long COVID-19 exists in pediatrics. And that it is a prolongation of signs or symptoms over time, a time for which there is no single definition.”
“It’s a snapshot of all the symptoms that can remain after COVID-19,” Dr. Lopez-Leon explained. “The meta-analysis seeks to see if there’s an association between having had COVID-19 and having the symptoms, but at no time does it speak of causality.”
The prevalence of symptoms largely depends on the time since the onset of acute COVID-19. Most symptoms improve over time. In the studies that were included in the meta-analysis, the follow-up time varied between 1 and 13 months. It is important to understand what symptoms are associated with each period after the onset of infection, the authors said.
Danish parent survey
The Danish study LongCOVIDKidsDK followed the World Health Organization criteria for long COVID-19 and included children and adolescents aged 0-14 years who received a diagnosis of COVID-19 and who experienced symptoms that lasted at least 2 months.
Between July 20, 2021, and Sept. 15, 2021, a questionnaire was sent to 38,152 case patients and 147,212 control persons. Of this group, 10,997 (28.8%) case patients and 33,016 (22.4%) control persons answered the survey.
Children who had been diagnosed with SARS-CoV-2 infection were more likely to experience long-lasting symptoms than children who had never been diagnosed. Approximately one-third of children with a positive SARS-CoV-2 test experienced symptoms that were not present before infection. Children who experienced long-lasting symptoms included 40% of children diagnosed with COVID-19 and 27% of control persons aged 0-3 years, 38% of case patients and 34% of control persons aged 4-11 years, and 46% of case patients and 41% of control persons aged 12-14 years.
Interestingly, those diagnosed with COVID-19 reported fewer psychological and social problems than those in the control group. Among the oldest (aged 12-14 years), quality of life scores were higher and anxiety scores were lower for those who had tested positive for SARS-CoV-2.
More information needed
Given the diversity of symptoms in the meta-analysis and the LongCOVIDKidsDK study, a multidisciplinary approach is imperative. Dr. Lopez-Leon suggests that there is a need to raise awareness among parents, clinicians, researchers, and the health system about the conditions that can occur after COVID-19. Clinicians must better understand the sequelae to provide targeted care and treatment. The authors of the Danish study recommend establishing clinics for long COVID-19 with multispecialty care.
Maren J. Heilskov Rytter, PhD, associate professor of clinical medicine at the University of Copenhagen, wrote an editorial in The Lancet Child and Adolescent Health about the Danish study. Until it is clarified whether SARS-CoV-2 does indeed cause persistent symptoms, she wrote, “it seems excessive and premature to establish specific multidisciplinary clinics for children with long COVID-19.”
Dr. Rytter highlighted the difficulty of interpreting LongCOVIDKidsDK data, owing to recall bias, the failure to exclude other causes of symptoms in the cases analyzed, and the number of symptoms in the control persons. In addition, the data analyzed in Denmark are of limited clinical relevance, she said, given a greater presence of mild symptoms and, paradoxically, a higher quality of life.
She concluded, “In the majority of children with nonspecific symptoms after COVID-19, the symptoms presented are more likely to have been caused by something other than COVID-19, and if they are related to COVID-19, they are likely to go away over time.”
Dr. Ensinck, who is coauthor of the Argentine Ministry of Health’s guide for long COVID-19 monitoring for children and adolescents and who represented the Infectious Diseases Committee of the Argentine Society of Pediatrics, highlighted another aspect of the problem. “What should be taken into account in these data is to see how much the confinement contributed. Children are the ones who suffered the most in the period in which schools were closed; they could not meet their peers, they had sick relatives, they felt fear. … all this must be taken into account.”
There is as yet no agreement on how to define and diagnose long COVID-19 in adults, a population that has been studied more closely. Part of the problem is that long COVID-19 has been linked to more than 200 symptoms, which can range in severity from inconvenient to debilitating, can last for months or years, and can recur, sometimes months after apparent recovery. Thus, there are still disparate answers to basic questions about the syndrome’s frequency and its effects on vaccination, reinfection, and the latest variant of SARS-CoV-2.
This article has been translated from the Medscape Spanish edition. A version appeared on Medscape.com.
Among scientists, the existence of long COVID-19 in children and adolescents has been the subject of debate.
Published by a Mexican multidisciplinary group in Scientific Reports, the first study is a systematic review and meta-analysis. It identified mood symptoms as the most prevalent clinical manifestations of long COVID-19 in children and adolescents. These symptoms included sadness, tension, anger, depression, and anxiety (16.50%); fatigue (9.66%); and sleep disorders (8.42%).
The second study, LongCOVIDKidsDK, was conducted in Denmark. It compared 11,000 children younger than 14 years who had tested positive for COVID-19 with 33,000 children who had no history of COVID-19. The study was published in The Lancet Child and Adolescent Health.
Definitions are changing
In their meta-analysis, the researchers estimated the prevalence and counted signs and symptoms of long COVID-19, as defined by the United Kingdom’s National Institute for Health and Care Excellence. Long COVID-19 was defined as the presence of one or more symptoms more than 4 weeks after SARS-CoV-2 infection. For search terms, the researchers used “COVID-19,” “COVID,” “SARSCOV-2,” “coronavirus,” “long COVID,” “postCOVID,” “PASC,” “long-haulers,” “prolonged,” “post-acute,” “persistent,” “convalescent,” “sequelae,” and “postviral.”
Of the 8,373 citations returned by the search as of Feb. 10, 2022, 21 prospective studies, 2 of them on preprint servers, met the authors’ selection criteria. Those studies included a total of 80,071 children and adolescents younger than 18 years.
In the meta-analysis, the prevalence of long COVID-19 among children and adolescents was reported to be 25.24% (95% confidence interval, 18.17-33.02; I2, 99.61%), regardless of whether the case had been asymptomatic, mild, moderate, severe, or serious. For patients who had been hospitalized, the prevalence was 29.19% (95% CI, 17.83-41.98; I2, 80.84%).
These numbers, while striking, are not the focus of the study, according to first author Sandra Lopez-Leon, MD, PhD, associate professor of pharmacoepidemiology at Rutgers University, New Brunswick, N.J. “It’s important that we don’t focus on that 25%,” she said in an interview. “It’s a disease that we’re learning about, we’re at a time when the definitions are still changing, and, depending on when it is measured, a different number will be given. The message we want to give is that long COVID-19 exists, it’s happening in children and adolescents, and patients need this recognition. And also to show that it can affect the whole body.”
The study showed that the children and adolescents who presented with SARS-CoV-2 infection were at higher risk of subsequent long dyspnea, anosmia/ageusia, or fever, compared with control persons.
In total, in the studies that were included, more than 40 long-term clinical manifestations associated with COVID-19 in the pediatric population were identified.
The most common symptoms among children aged 0-3 years were mood swings, skin rashes, and stomachaches. In 4- to 11-year-olds, the most common symptoms were mood swings, trouble remembering or concentrating, and skin rashes. In 12- to 14-year-olds, they were fatigue, mood swings, and trouble remembering or concentrating. These data are based on parent responses.
The list of signs and symptoms also includes headache, respiratory symptoms, cognitive symptoms (such as decreased concentration, learning difficulties, confusion, and memory loss), loss of appetite, and smell disorder (hyposmia, anosmia, hyperosmia, parosmia, and phantom smell).
In the studies, the prevalence of the following symptoms was less than 5%: hyperhidrosis, chest pain, dizziness, cough, myalgia/arthralgia, changes in body weight, taste disorder, otalgia (tinnitus, ear pain, vertigo), ophthalmologic symptoms (conjunctivitis, dry eye, blurred vision, photophobia, pain), dermatologic symptoms (dry skin, itchy skin, rashes, hives, hair loss), urinary symptoms, abdominal pain, throat pain, chest tightness, variations in heart rate, palpitations, constipation, dysphonia, fever, diarrhea, vomiting/nausea, menstrual changes, neurological abnormalities, speech disorders, and dysphagia.
The authors made it clear that the frequency and severity of these symptoms can fluctuate from one patient to another.
“The meta-analysis is important because it brings together 21 studies selected from more than 8,000 articles – and in them, a large number of children – to study the most common manifestations of long COVID-19,” Gabriela Ensinck, MD, head of the infectious diseases department at the Víctor J. Vilela Children’s Hospital in Rosario, Argentina, told this news organization. Dr. Ensinck did not participate in the study. “The important thing here is that long COVID-19 exists in pediatrics. And that it is a prolongation of signs or symptoms over time, a time for which there is no single definition.”
“It’s a snapshot of all the symptoms that can remain after COVID-19,” Dr. Lopez-Leon explained. “The meta-analysis seeks to see if there’s an association between having had COVID-19 and having the symptoms, but at no time does it speak of causality.”
The prevalence of symptoms largely depends on the time since the onset of acute COVID-19. Most symptoms improve over time. In the studies that were included in the meta-analysis, the follow-up time varied between 1 and 13 months. It is important to understand what symptoms are associated with each period after the onset of infection, the authors said.
Danish parent survey
The Danish study LongCOVIDKidsDK followed the World Health Organization criteria for long COVID-19 and included children and adolescents aged 0-14 years who received a diagnosis of COVID-19 and who experienced symptoms that lasted at least 2 months.
Between July 20, 2021, and Sept. 15, 2021, a questionnaire was sent to 38,152 case patients and 147,212 control persons. Of this group, 10,997 (28.8%) case patients and 33,016 (22.4%) control persons answered the survey.
Children who had been diagnosed with SARS-CoV-2 infection were more likely to experience long-lasting symptoms than children who had never been diagnosed. Approximately one-third of children with a positive SARS-CoV-2 test experienced symptoms that were not present before infection. Children who experienced long-lasting symptoms included 40% of children diagnosed with COVID-19 and 27% of control persons aged 0-3 years, 38% of case patients and 34% of control persons aged 4-11 years, and 46% of case patients and 41% of control persons aged 12-14 years.
Interestingly, those diagnosed with COVID-19 reported fewer psychological and social problems than those in the control group. Among the oldest (aged 12-14 years), quality of life scores were higher and anxiety scores were lower for those who had tested positive for SARS-CoV-2.
More information needed
Given the diversity of symptoms in the meta-analysis and the LongCOVIDKidsDK study, a multidisciplinary approach is imperative. Dr. Lopez-Leon suggests that there is a need to raise awareness among parents, clinicians, researchers, and the health system about the conditions that can occur after COVID-19. Clinicians must better understand the sequelae to provide targeted care and treatment. The authors of the Danish study recommend establishing clinics for long COVID-19 with multispecialty care.
Maren J. Heilskov Rytter, PhD, associate professor of clinical medicine at the University of Copenhagen, wrote an editorial in The Lancet Child and Adolescent Health about the Danish study. Until it is clarified whether SARS-CoV-2 does indeed cause persistent symptoms, she wrote, “it seems excessive and premature to establish specific multidisciplinary clinics for children with long COVID-19.”
Dr. Rytter highlighted the difficulty of interpreting LongCOVIDKidsDK data, owing to recall bias, the failure to exclude other causes of symptoms in the cases analyzed, and the number of symptoms in the control persons. In addition, the data analyzed in Denmark are of limited clinical relevance, she said, given a greater presence of mild symptoms and, paradoxically, a higher quality of life.
She concluded, “In the majority of children with nonspecific symptoms after COVID-19, the symptoms presented are more likely to have been caused by something other than COVID-19, and if they are related to COVID-19, they are likely to go away over time.”
Dr. Ensinck, who is coauthor of the Argentine Ministry of Health’s guide for long COVID-19 monitoring for children and adolescents and who represented the Infectious Diseases Committee of the Argentine Society of Pediatrics, highlighted another aspect of the problem. “What should be taken into account in these data is to see how much the confinement contributed. Children are the ones who suffered the most in the period in which schools were closed; they could not meet their peers, they had sick relatives, they felt fear. … all this must be taken into account.”
There is as yet no agreement on how to define and diagnose long COVID-19 in adults, a population that has been studied more closely. Part of the problem is that long COVID-19 has been linked to more than 200 symptoms, which can range in severity from inconvenient to debilitating, can last for months or years, and can recur, sometimes months after apparent recovery. Thus, there are still disparate answers to basic questions about the syndrome’s frequency and its effects on vaccination, reinfection, and the latest variant of SARS-CoV-2.
This article has been translated from the Medscape Spanish edition. A version appeared on Medscape.com.
FROM SCIENTIFIC REPORTS AND THE LANCET CHILD AND ADOLESCENT HEALTH
Zoster vaccination does not appear to increase flare risk in patients with immune-mediated inflammatory disease
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
Docs reveal perils of giving medical advice to friends and family
Stephen Pribut, DPM, a sports medicine podiatrist based in Washington, has had many friends or family members ask him for medical advice. It’s a scenario every doctor will face at one point or another in their careers, and it’s never an easy one.
Dr. Pribut received a call from a friend about a sore shoulder from swimming, saying that his doctor had dismissed the potential for a rotator cuff injury. “Months later, images revealed it was a rotator cuff tear and he wanted my advice,” says Dr. Pribut.
Not being a shoulder specialist, Dr. Pribut limited his input. “I told him to consider a good physical therapist or a shoulder specialist and gave him some alternative strokes for swimming that hopefully wouldn’t aggravate the injury,” he explains.
But he admits some situations are challenging. “I had a relative asking about a third party with an ankle injury. I advised he hold off on using a balance board until things healed, and to make sure he went to see a specialist. Unfortunately, he went to his general practitioner who likely knows nothing about ankle anatomy,” says Dr. Pribut.
“I finally saw a photo, which revealed swelling higher up on the ankle and no evidence of a hematoma – much lower than we would see in an ankle ligament injury. I would like him to see a sports podiatrist or foot and ankle orthopedist, but now I have to stay calm when the advice isn’t followed,” he says.
When asked, “Do you give medical advice to your friends?” 96% of respondents answered yes.
Yazan Abou-Ismail, MD, assistant professor of medicine in the division of hematology at the University of Utah, Salt Lake City, has often faced questions from friends and family, particularly throughout the COVID-19 pandemic. “How you respond is something all physicians need to analyze carefully,” he says. “I get questions on a regular basis, but this greatly increased with COVID.”
“Sharing general information is okay, and it’s even a requirement that we educate on such topics,” says Dr. Abou-Ismail. “But if someone knows they have COVID, for instance, and wants advice on how to proceed, it’s important to send them to their primary care physician for an evaluation rather than give them instructions on care.”
Dr. Abou-Ismail says that most “curbside consulting” equates to lack of an ethical follow-up. “If you gave medical advice without having assessed them, you’re lacking the medical history, a physical exam, and you should not be giving advice,” he says. “This applies to follow-ups, too.”
Throughout the pandemic, Dr. Abou-Ismail’s requests for advice on COVID even extended to online inquiries, often from strangers. “This is not a place to do a formal assessment,” he reminds. “But there are certain types of advice you can offer appropriately.”
Dr. Abou-Ismail considers safe advice to be simple public health messages that stay far out of specifics. Things like “don’t smoke,” or “eat a healthy diet,” and “get enough sleep,” fall into this safety zone. Even, “What is XYZ disease?” or “How do COVID vaccines work?” are topics he says he answers comfortably.
“But telling someone you need a specific treatment for a condition is inappropriate,” he explains. “This is a general way of practicing medicine – your advice should never venture into the potential of doing harm.”
This approach is exactly in line with legal advice, according to Jeff Caesar Chukwuma, founder and senior partner at Chukwuma Law Group, Miami. “It doesn’t mean that doctors should never give medical advice to friends or family, but if they do, they should make sure to take several precautions to protect both themselves and their family and friends,” he says.
When the request for medical advice from an acquaintance migrates into areas in which a physician is not a specialist, sharing recommendations gets even trickier – and more ethically questionable.
Says Mr. Chukwuma, “Doctors should avoid giving advice in areas outside their area of expertise to lower the possibility of providing erroneous or harmful information,” he says.
How to stay safe when asked for advice
The American Medical Association has weighed in on the topic. In the Code of Medical Ethics Opinion 1.2.1, the AMA states that, “Treating oneself or a member of one’s own family poses several challenges for physicians, including concerns about professional objectivity, patient autonomy, and informed consent.”
What about friends or acquaintances, however?
Even so, some respondents voiced their concerns with the scenario. Responses like, “Due to ethics, I would prefer they go and get first, second, and third opinions,” and “Usually the medical advice is very basic first aid (often mental health first aid), and if it’s anything remotely more complicated, I direct them to the appropriate provider.”
The AMA places advising friends in the same basket as advising and treating family members or oneself. In an article appearing in the AMA Journal of Ethics, Horacio Hojman, MD, of Tufts University School of Medicine, Boston, weighed in: “First and foremost, patients deserve objectivity from their doctors. When a physician is emotionally involved with a patient, that physician’s objectivity can be called into question.”
Why is medical advice so thorny when dealing with friends or relatives?
In some cases, a physician might not ask a friend relevant personal questions about his or her medical history, for instance. Or the friend might not want to share details with the doctor. In either case, the lack of information exchange can lead to improper advice.
The issue of giving medical advice to friends, family, and acquaintances can also wade into legal territory. “Personally or professionally, trust is the decisive factor that puts us at ease with the people we surround ourselves with,” says Mr. Chukwuma. “Nowhere is this truer than in medicine, where we approach doctors with some of the most sensitive matters in our lives and entrust our care to them, especially when the physician in question is a close friend or family member.”
Mr. Chukwuma points out that, while there are few strict legal prohibitions against doctors providing care or advice to family and friends, the AMA’s code of ethics states that such action should be reserved for rare situations, such as emergency settings or isolated settings where there is no other qualified physician available, or for minor, not long-term problems.
This was part of the equation for Dr. Pribut when helping his mother navigate her treatment for breast cancer. “With close relatives, offering advice and help can be very hard,” he says.
“This is to protect both patients and doctors,” says Mr. Chukwuma. “Although seeking advice from a family member or friend who is a doctor may be more convenient for a patient, they run the risk of receiving inadequate care by not going in for a formal medical visit complete with tests, medical examination, and follow-up care.”
Mr. Chukwuma offers guidance on how to share medical advice ethically and legally with family, friends, and acquaintances. “First, as much as possible, speak to general medical facts and knowledge rather than comment directly on the patient’s particular situation,” he says. “In the absence of thorough examination and tests, the doctor’s knowledge of a patient’s condition is limited, therefore, you should take care not to provide seemingly definitive answers on that patient’s unique condition in situations where they can’t rely on data to back up their advice and recommendations.”
The AMA’s Journal of Ethics article shares these tips for staying on the right side of the ethical line when dealing with friends and family members:
- Politely decline.
- Offer other forms of assistance – this might help a friend find the right qualified physician – as Dr. Pribut tends to do. Maybe help in navigating the sometimes-confusing health care system.
- Don’t hesitate in an emergency – the old “is there a doctor on board,” scenario on a plane when someone is in distress is a perfectly acceptable, and recommended, time to step in, even if it is a friend or family member.
Dr. Pribut, a long-time veteran of the tricky medical waters involving friends and family, has this to offer: “Be cautious and always stay in the realm of what you know,” he says. “Always encourage people to seek an opinion from a qualified doctor. Help them find a reputable doctor if that’s useful.”
Mr. Chukwuma adds also that doctors should stand firm when pushed by a friend or family member, especially when offering advice, even if it’s in the form of general education. “The doctor should make it clear to the family member or friend that their advice in no way takes the place of actual treatment or examination by a medical professional and that, if need be, the patient should seek formal medical help from another doctor, ideally one not related to or friends with the patient,” he says.
A version of this article first appeared on Medscape.com.
Stephen Pribut, DPM, a sports medicine podiatrist based in Washington, has had many friends or family members ask him for medical advice. It’s a scenario every doctor will face at one point or another in their careers, and it’s never an easy one.
Dr. Pribut received a call from a friend about a sore shoulder from swimming, saying that his doctor had dismissed the potential for a rotator cuff injury. “Months later, images revealed it was a rotator cuff tear and he wanted my advice,” says Dr. Pribut.
Not being a shoulder specialist, Dr. Pribut limited his input. “I told him to consider a good physical therapist or a shoulder specialist and gave him some alternative strokes for swimming that hopefully wouldn’t aggravate the injury,” he explains.
But he admits some situations are challenging. “I had a relative asking about a third party with an ankle injury. I advised he hold off on using a balance board until things healed, and to make sure he went to see a specialist. Unfortunately, he went to his general practitioner who likely knows nothing about ankle anatomy,” says Dr. Pribut.
“I finally saw a photo, which revealed swelling higher up on the ankle and no evidence of a hematoma – much lower than we would see in an ankle ligament injury. I would like him to see a sports podiatrist or foot and ankle orthopedist, but now I have to stay calm when the advice isn’t followed,” he says.
When asked, “Do you give medical advice to your friends?” 96% of respondents answered yes.
Yazan Abou-Ismail, MD, assistant professor of medicine in the division of hematology at the University of Utah, Salt Lake City, has often faced questions from friends and family, particularly throughout the COVID-19 pandemic. “How you respond is something all physicians need to analyze carefully,” he says. “I get questions on a regular basis, but this greatly increased with COVID.”
“Sharing general information is okay, and it’s even a requirement that we educate on such topics,” says Dr. Abou-Ismail. “But if someone knows they have COVID, for instance, and wants advice on how to proceed, it’s important to send them to their primary care physician for an evaluation rather than give them instructions on care.”
Dr. Abou-Ismail says that most “curbside consulting” equates to lack of an ethical follow-up. “If you gave medical advice without having assessed them, you’re lacking the medical history, a physical exam, and you should not be giving advice,” he says. “This applies to follow-ups, too.”
Throughout the pandemic, Dr. Abou-Ismail’s requests for advice on COVID even extended to online inquiries, often from strangers. “This is not a place to do a formal assessment,” he reminds. “But there are certain types of advice you can offer appropriately.”
Dr. Abou-Ismail considers safe advice to be simple public health messages that stay far out of specifics. Things like “don’t smoke,” or “eat a healthy diet,” and “get enough sleep,” fall into this safety zone. Even, “What is XYZ disease?” or “How do COVID vaccines work?” are topics he says he answers comfortably.
“But telling someone you need a specific treatment for a condition is inappropriate,” he explains. “This is a general way of practicing medicine – your advice should never venture into the potential of doing harm.”
This approach is exactly in line with legal advice, according to Jeff Caesar Chukwuma, founder and senior partner at Chukwuma Law Group, Miami. “It doesn’t mean that doctors should never give medical advice to friends or family, but if they do, they should make sure to take several precautions to protect both themselves and their family and friends,” he says.
When the request for medical advice from an acquaintance migrates into areas in which a physician is not a specialist, sharing recommendations gets even trickier – and more ethically questionable.
Says Mr. Chukwuma, “Doctors should avoid giving advice in areas outside their area of expertise to lower the possibility of providing erroneous or harmful information,” he says.
How to stay safe when asked for advice
The American Medical Association has weighed in on the topic. In the Code of Medical Ethics Opinion 1.2.1, the AMA states that, “Treating oneself or a member of one’s own family poses several challenges for physicians, including concerns about professional objectivity, patient autonomy, and informed consent.”
What about friends or acquaintances, however?
Even so, some respondents voiced their concerns with the scenario. Responses like, “Due to ethics, I would prefer they go and get first, second, and third opinions,” and “Usually the medical advice is very basic first aid (often mental health first aid), and if it’s anything remotely more complicated, I direct them to the appropriate provider.”
The AMA places advising friends in the same basket as advising and treating family members or oneself. In an article appearing in the AMA Journal of Ethics, Horacio Hojman, MD, of Tufts University School of Medicine, Boston, weighed in: “First and foremost, patients deserve objectivity from their doctors. When a physician is emotionally involved with a patient, that physician’s objectivity can be called into question.”
Why is medical advice so thorny when dealing with friends or relatives?
In some cases, a physician might not ask a friend relevant personal questions about his or her medical history, for instance. Or the friend might not want to share details with the doctor. In either case, the lack of information exchange can lead to improper advice.
The issue of giving medical advice to friends, family, and acquaintances can also wade into legal territory. “Personally or professionally, trust is the decisive factor that puts us at ease with the people we surround ourselves with,” says Mr. Chukwuma. “Nowhere is this truer than in medicine, where we approach doctors with some of the most sensitive matters in our lives and entrust our care to them, especially when the physician in question is a close friend or family member.”
Mr. Chukwuma points out that, while there are few strict legal prohibitions against doctors providing care or advice to family and friends, the AMA’s code of ethics states that such action should be reserved for rare situations, such as emergency settings or isolated settings where there is no other qualified physician available, or for minor, not long-term problems.
This was part of the equation for Dr. Pribut when helping his mother navigate her treatment for breast cancer. “With close relatives, offering advice and help can be very hard,” he says.
“This is to protect both patients and doctors,” says Mr. Chukwuma. “Although seeking advice from a family member or friend who is a doctor may be more convenient for a patient, they run the risk of receiving inadequate care by not going in for a formal medical visit complete with tests, medical examination, and follow-up care.”
Mr. Chukwuma offers guidance on how to share medical advice ethically and legally with family, friends, and acquaintances. “First, as much as possible, speak to general medical facts and knowledge rather than comment directly on the patient’s particular situation,” he says. “In the absence of thorough examination and tests, the doctor’s knowledge of a patient’s condition is limited, therefore, you should take care not to provide seemingly definitive answers on that patient’s unique condition in situations where they can’t rely on data to back up their advice and recommendations.”
The AMA’s Journal of Ethics article shares these tips for staying on the right side of the ethical line when dealing with friends and family members:
- Politely decline.
- Offer other forms of assistance – this might help a friend find the right qualified physician – as Dr. Pribut tends to do. Maybe help in navigating the sometimes-confusing health care system.
- Don’t hesitate in an emergency – the old “is there a doctor on board,” scenario on a plane when someone is in distress is a perfectly acceptable, and recommended, time to step in, even if it is a friend or family member.
Dr. Pribut, a long-time veteran of the tricky medical waters involving friends and family, has this to offer: “Be cautious and always stay in the realm of what you know,” he says. “Always encourage people to seek an opinion from a qualified doctor. Help them find a reputable doctor if that’s useful.”
Mr. Chukwuma adds also that doctors should stand firm when pushed by a friend or family member, especially when offering advice, even if it’s in the form of general education. “The doctor should make it clear to the family member or friend that their advice in no way takes the place of actual treatment or examination by a medical professional and that, if need be, the patient should seek formal medical help from another doctor, ideally one not related to or friends with the patient,” he says.
A version of this article first appeared on Medscape.com.
Stephen Pribut, DPM, a sports medicine podiatrist based in Washington, has had many friends or family members ask him for medical advice. It’s a scenario every doctor will face at one point or another in their careers, and it’s never an easy one.
Dr. Pribut received a call from a friend about a sore shoulder from swimming, saying that his doctor had dismissed the potential for a rotator cuff injury. “Months later, images revealed it was a rotator cuff tear and he wanted my advice,” says Dr. Pribut.
Not being a shoulder specialist, Dr. Pribut limited his input. “I told him to consider a good physical therapist or a shoulder specialist and gave him some alternative strokes for swimming that hopefully wouldn’t aggravate the injury,” he explains.
But he admits some situations are challenging. “I had a relative asking about a third party with an ankle injury. I advised he hold off on using a balance board until things healed, and to make sure he went to see a specialist. Unfortunately, he went to his general practitioner who likely knows nothing about ankle anatomy,” says Dr. Pribut.
“I finally saw a photo, which revealed swelling higher up on the ankle and no evidence of a hematoma – much lower than we would see in an ankle ligament injury. I would like him to see a sports podiatrist or foot and ankle orthopedist, but now I have to stay calm when the advice isn’t followed,” he says.
When asked, “Do you give medical advice to your friends?” 96% of respondents answered yes.
Yazan Abou-Ismail, MD, assistant professor of medicine in the division of hematology at the University of Utah, Salt Lake City, has often faced questions from friends and family, particularly throughout the COVID-19 pandemic. “How you respond is something all physicians need to analyze carefully,” he says. “I get questions on a regular basis, but this greatly increased with COVID.”
“Sharing general information is okay, and it’s even a requirement that we educate on such topics,” says Dr. Abou-Ismail. “But if someone knows they have COVID, for instance, and wants advice on how to proceed, it’s important to send them to their primary care physician for an evaluation rather than give them instructions on care.”
Dr. Abou-Ismail says that most “curbside consulting” equates to lack of an ethical follow-up. “If you gave medical advice without having assessed them, you’re lacking the medical history, a physical exam, and you should not be giving advice,” he says. “This applies to follow-ups, too.”
Throughout the pandemic, Dr. Abou-Ismail’s requests for advice on COVID even extended to online inquiries, often from strangers. “This is not a place to do a formal assessment,” he reminds. “But there are certain types of advice you can offer appropriately.”
Dr. Abou-Ismail considers safe advice to be simple public health messages that stay far out of specifics. Things like “don’t smoke,” or “eat a healthy diet,” and “get enough sleep,” fall into this safety zone. Even, “What is XYZ disease?” or “How do COVID vaccines work?” are topics he says he answers comfortably.
“But telling someone you need a specific treatment for a condition is inappropriate,” he explains. “This is a general way of practicing medicine – your advice should never venture into the potential of doing harm.”
This approach is exactly in line with legal advice, according to Jeff Caesar Chukwuma, founder and senior partner at Chukwuma Law Group, Miami. “It doesn’t mean that doctors should never give medical advice to friends or family, but if they do, they should make sure to take several precautions to protect both themselves and their family and friends,” he says.
When the request for medical advice from an acquaintance migrates into areas in which a physician is not a specialist, sharing recommendations gets even trickier – and more ethically questionable.
Says Mr. Chukwuma, “Doctors should avoid giving advice in areas outside their area of expertise to lower the possibility of providing erroneous or harmful information,” he says.
How to stay safe when asked for advice
The American Medical Association has weighed in on the topic. In the Code of Medical Ethics Opinion 1.2.1, the AMA states that, “Treating oneself or a member of one’s own family poses several challenges for physicians, including concerns about professional objectivity, patient autonomy, and informed consent.”
What about friends or acquaintances, however?
Even so, some respondents voiced their concerns with the scenario. Responses like, “Due to ethics, I would prefer they go and get first, second, and third opinions,” and “Usually the medical advice is very basic first aid (often mental health first aid), and if it’s anything remotely more complicated, I direct them to the appropriate provider.”
The AMA places advising friends in the same basket as advising and treating family members or oneself. In an article appearing in the AMA Journal of Ethics, Horacio Hojman, MD, of Tufts University School of Medicine, Boston, weighed in: “First and foremost, patients deserve objectivity from their doctors. When a physician is emotionally involved with a patient, that physician’s objectivity can be called into question.”
Why is medical advice so thorny when dealing with friends or relatives?
In some cases, a physician might not ask a friend relevant personal questions about his or her medical history, for instance. Or the friend might not want to share details with the doctor. In either case, the lack of information exchange can lead to improper advice.
The issue of giving medical advice to friends, family, and acquaintances can also wade into legal territory. “Personally or professionally, trust is the decisive factor that puts us at ease with the people we surround ourselves with,” says Mr. Chukwuma. “Nowhere is this truer than in medicine, where we approach doctors with some of the most sensitive matters in our lives and entrust our care to them, especially when the physician in question is a close friend or family member.”
Mr. Chukwuma points out that, while there are few strict legal prohibitions against doctors providing care or advice to family and friends, the AMA’s code of ethics states that such action should be reserved for rare situations, such as emergency settings or isolated settings where there is no other qualified physician available, or for minor, not long-term problems.
This was part of the equation for Dr. Pribut when helping his mother navigate her treatment for breast cancer. “With close relatives, offering advice and help can be very hard,” he says.
“This is to protect both patients and doctors,” says Mr. Chukwuma. “Although seeking advice from a family member or friend who is a doctor may be more convenient for a patient, they run the risk of receiving inadequate care by not going in for a formal medical visit complete with tests, medical examination, and follow-up care.”
Mr. Chukwuma offers guidance on how to share medical advice ethically and legally with family, friends, and acquaintances. “First, as much as possible, speak to general medical facts and knowledge rather than comment directly on the patient’s particular situation,” he says. “In the absence of thorough examination and tests, the doctor’s knowledge of a patient’s condition is limited, therefore, you should take care not to provide seemingly definitive answers on that patient’s unique condition in situations where they can’t rely on data to back up their advice and recommendations.”
The AMA’s Journal of Ethics article shares these tips for staying on the right side of the ethical line when dealing with friends and family members:
- Politely decline.
- Offer other forms of assistance – this might help a friend find the right qualified physician – as Dr. Pribut tends to do. Maybe help in navigating the sometimes-confusing health care system.
- Don’t hesitate in an emergency – the old “is there a doctor on board,” scenario on a plane when someone is in distress is a perfectly acceptable, and recommended, time to step in, even if it is a friend or family member.
Dr. Pribut, a long-time veteran of the tricky medical waters involving friends and family, has this to offer: “Be cautious and always stay in the realm of what you know,” he says. “Always encourage people to seek an opinion from a qualified doctor. Help them find a reputable doctor if that’s useful.”
Mr. Chukwuma adds also that doctors should stand firm when pushed by a friend or family member, especially when offering advice, even if it’s in the form of general education. “The doctor should make it clear to the family member or friend that their advice in no way takes the place of actual treatment or examination by a medical professional and that, if need be, the patient should seek formal medical help from another doctor, ideally one not related to or friends with the patient,” he says.
A version of this article first appeared on Medscape.com.

