User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
Is it flu, RSV, or COVID? Experts fear the ‘tripledemic’
Just when we thought this holiday season, finally, would be the back-to-normal one, some infectious disease experts are warning that a so-called “tripledemic” – influenza, COVID-19, and RSV – may be in the forecast.
The warning isn’t without basis.
The flu season has gotten an early start. As of Oct. 21, early increases in seasonal flu activity have been reported in most of the country, the Centers for Disease Control and Prevention said, with the southeast and south-central areas having the highest activity levels.
Children’s hospitals and EDs are seeing a surge in children with RSV.
COVID-19 cases are trending down, according to the CDC, but epidemiologists – scientists who study disease outbreaks – always have their eyes on emerging variants.
said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill. Dr. Lessler is on the coordinating team for the COVID-19 Scenario Modeling Hub, which aims to predict the course COVID-19, and the Flu Scenario Modeling Hub, which does the same for influenza.
For COVID-19, some models are predicting some spikes before Christmas, he said, and others see a new wave in 2023. For the flu, the model is predicting an earlier-than-usual start, as the CDC has reported.
While flu activity is relatively low, the CDC said, the season is off to an early start. For the week ending Oct. 21, 1,674 patients were hospitalized for flu, higher than in the summer months but fewer than the 2,675 hospitalizations for the week of May 15, 2022.
As of Oct. 20, COVID-19 cases have declined 12% over the last 2 weeks, nationwide. But hospitalizations are up 10% in much of the Northeast, The New York Times reports, and the improvement in cases and deaths has been slowing down.
As of Oct. 15, 15% of RSV tests reported nationwide were positive, compared with about 11% at that time in 2021, the CDC said. The surveillance collects information from 75 counties in 12 states.
Experts point out that the viruses – all three are respiratory viruses – are simply playing catchup.
“They spread the same way and along with lots of other viruses, and you tend to see an increase in them during the cold months,” said Timothy Brewer, MD, professor of medicine and epidemiology at UCLA.
The increase in all three viruses “is almost predictable at this point in the pandemic,” said Dean Blumberg, MD, a professor and chief of pediatric infectious diseases at the University of California Davis Health. “All the respiratory viruses are out of whack.”
Last year, RSV cases were up, too, and began to appear very early, he said, in the summer instead of in the cooler months. Flu also appeared early in 2021, as it has in 2022.
That contrasts with the flu season of 2020-2021, when COVID precautions were nearly universal, and cases were down. At UC Davis, “we didn’t have one pediatric admission due to influenza in the 2020-2021 [flu] season,” Dr. Blumberg said.
The number of pediatric flu deaths usually range from 37 to 199 per year, according to CDC records. But in the 2020-2021 season, the CDC recorded one pediatric flu death in the U.S.
Both children and adults have had less contact with others the past two seasons, Dr. Blumberg said, “and they don’t get the immunity they got with those infections [previously]. That’s why we are seeing out-of-season, early season [viruses].”
Eventually, he said, the cases of flu and RSV will return to previous levels. “It could be as soon as next year,” Dr. Blumberg said. And COVID-19, hopefully, will become like influenza, he said.
“RSV has always come around in the fall and winter,” said Elizabeth Murray, DO, a pediatric emergency medicine doctor at the University of Rochester (N.Y.) Medical Center and a spokesperson for the American Academy of Pediatrics. In 2022, children are back in school and for the most part not masking. “It’s a perfect storm for all the germs to spread now. They’ve just been waiting for their opportunity to come back.”
Self-care vs. not
RSV can pose a risk for anyone, but most at risk are children under age 5, especially infants under age 1, and adults over age 65. There is no vaccine for it. Symptoms include a runny nose, decreased appetite, coughing, sneezing, fever, and wheezing. But in young infants, there may only be decreased activity, crankiness, and breathing issues, the CDC said.
Keep an eye on the breathing if RSV is suspected, Dr. Murray tells parents. If your child can’t breathe easily, is unable to lie down comfortably, can’t speak clearly, or is sucking in the chest muscles to breathe, get medical help. Most kids with RSV can stay home and recover, she said, but often will need to be checked by a medical professional.
She advises against getting an oximeter to measure oxygen levels for home use. “They are often not accurate,” she said. If in doubt about how serious your child’s symptoms are, “don’t wait it out,” and don’t hesitate to call 911.
Symptoms of flu, COVID, and RSV can overlap. But each can involve breathing problems, which can be an emergency.
“It’s important to seek medical attention for any concerning symptoms, but especially severe shortness of breath or difficulty breathing, as these could signal the need for supplemental oxygen or other emergency interventions,” said Mandy De Vries, a respiratory therapist and director of education at the American Association for Respiratory Care. Inhalation treatment or mechanical ventilation may be needed for severe respiratory issues.
Precautions
To avoid the tripledemic – or any single infection – Timothy Brewer, MD, a professor of medicine and epidemiology at the University of California, Los Angeles, suggests some familiar measures: “Stay home if you’re feeling sick. Make sure you are up to date on your vaccinations. Wear a mask indoors.”
A version of this article first appeared on Medscape.com.
Just when we thought this holiday season, finally, would be the back-to-normal one, some infectious disease experts are warning that a so-called “tripledemic” – influenza, COVID-19, and RSV – may be in the forecast.
The warning isn’t without basis.
The flu season has gotten an early start. As of Oct. 21, early increases in seasonal flu activity have been reported in most of the country, the Centers for Disease Control and Prevention said, with the southeast and south-central areas having the highest activity levels.
Children’s hospitals and EDs are seeing a surge in children with RSV.
COVID-19 cases are trending down, according to the CDC, but epidemiologists – scientists who study disease outbreaks – always have their eyes on emerging variants.
said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill. Dr. Lessler is on the coordinating team for the COVID-19 Scenario Modeling Hub, which aims to predict the course COVID-19, and the Flu Scenario Modeling Hub, which does the same for influenza.
For COVID-19, some models are predicting some spikes before Christmas, he said, and others see a new wave in 2023. For the flu, the model is predicting an earlier-than-usual start, as the CDC has reported.
While flu activity is relatively low, the CDC said, the season is off to an early start. For the week ending Oct. 21, 1,674 patients were hospitalized for flu, higher than in the summer months but fewer than the 2,675 hospitalizations for the week of May 15, 2022.
As of Oct. 20, COVID-19 cases have declined 12% over the last 2 weeks, nationwide. But hospitalizations are up 10% in much of the Northeast, The New York Times reports, and the improvement in cases and deaths has been slowing down.
As of Oct. 15, 15% of RSV tests reported nationwide were positive, compared with about 11% at that time in 2021, the CDC said. The surveillance collects information from 75 counties in 12 states.
Experts point out that the viruses – all three are respiratory viruses – are simply playing catchup.
“They spread the same way and along with lots of other viruses, and you tend to see an increase in them during the cold months,” said Timothy Brewer, MD, professor of medicine and epidemiology at UCLA.
The increase in all three viruses “is almost predictable at this point in the pandemic,” said Dean Blumberg, MD, a professor and chief of pediatric infectious diseases at the University of California Davis Health. “All the respiratory viruses are out of whack.”
Last year, RSV cases were up, too, and began to appear very early, he said, in the summer instead of in the cooler months. Flu also appeared early in 2021, as it has in 2022.
That contrasts with the flu season of 2020-2021, when COVID precautions were nearly universal, and cases were down. At UC Davis, “we didn’t have one pediatric admission due to influenza in the 2020-2021 [flu] season,” Dr. Blumberg said.
The number of pediatric flu deaths usually range from 37 to 199 per year, according to CDC records. But in the 2020-2021 season, the CDC recorded one pediatric flu death in the U.S.
Both children and adults have had less contact with others the past two seasons, Dr. Blumberg said, “and they don’t get the immunity they got with those infections [previously]. That’s why we are seeing out-of-season, early season [viruses].”
Eventually, he said, the cases of flu and RSV will return to previous levels. “It could be as soon as next year,” Dr. Blumberg said. And COVID-19, hopefully, will become like influenza, he said.
“RSV has always come around in the fall and winter,” said Elizabeth Murray, DO, a pediatric emergency medicine doctor at the University of Rochester (N.Y.) Medical Center and a spokesperson for the American Academy of Pediatrics. In 2022, children are back in school and for the most part not masking. “It’s a perfect storm for all the germs to spread now. They’ve just been waiting for their opportunity to come back.”
Self-care vs. not
RSV can pose a risk for anyone, but most at risk are children under age 5, especially infants under age 1, and adults over age 65. There is no vaccine for it. Symptoms include a runny nose, decreased appetite, coughing, sneezing, fever, and wheezing. But in young infants, there may only be decreased activity, crankiness, and breathing issues, the CDC said.
Keep an eye on the breathing if RSV is suspected, Dr. Murray tells parents. If your child can’t breathe easily, is unable to lie down comfortably, can’t speak clearly, or is sucking in the chest muscles to breathe, get medical help. Most kids with RSV can stay home and recover, she said, but often will need to be checked by a medical professional.
She advises against getting an oximeter to measure oxygen levels for home use. “They are often not accurate,” she said. If in doubt about how serious your child’s symptoms are, “don’t wait it out,” and don’t hesitate to call 911.
Symptoms of flu, COVID, and RSV can overlap. But each can involve breathing problems, which can be an emergency.
“It’s important to seek medical attention for any concerning symptoms, but especially severe shortness of breath or difficulty breathing, as these could signal the need for supplemental oxygen or other emergency interventions,” said Mandy De Vries, a respiratory therapist and director of education at the American Association for Respiratory Care. Inhalation treatment or mechanical ventilation may be needed for severe respiratory issues.
Precautions
To avoid the tripledemic – or any single infection – Timothy Brewer, MD, a professor of medicine and epidemiology at the University of California, Los Angeles, suggests some familiar measures: “Stay home if you’re feeling sick. Make sure you are up to date on your vaccinations. Wear a mask indoors.”
A version of this article first appeared on Medscape.com.
Just when we thought this holiday season, finally, would be the back-to-normal one, some infectious disease experts are warning that a so-called “tripledemic” – influenza, COVID-19, and RSV – may be in the forecast.
The warning isn’t without basis.
The flu season has gotten an early start. As of Oct. 21, early increases in seasonal flu activity have been reported in most of the country, the Centers for Disease Control and Prevention said, with the southeast and south-central areas having the highest activity levels.
Children’s hospitals and EDs are seeing a surge in children with RSV.
COVID-19 cases are trending down, according to the CDC, but epidemiologists – scientists who study disease outbreaks – always have their eyes on emerging variants.
said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill. Dr. Lessler is on the coordinating team for the COVID-19 Scenario Modeling Hub, which aims to predict the course COVID-19, and the Flu Scenario Modeling Hub, which does the same for influenza.
For COVID-19, some models are predicting some spikes before Christmas, he said, and others see a new wave in 2023. For the flu, the model is predicting an earlier-than-usual start, as the CDC has reported.
While flu activity is relatively low, the CDC said, the season is off to an early start. For the week ending Oct. 21, 1,674 patients were hospitalized for flu, higher than in the summer months but fewer than the 2,675 hospitalizations for the week of May 15, 2022.
As of Oct. 20, COVID-19 cases have declined 12% over the last 2 weeks, nationwide. But hospitalizations are up 10% in much of the Northeast, The New York Times reports, and the improvement in cases and deaths has been slowing down.
As of Oct. 15, 15% of RSV tests reported nationwide were positive, compared with about 11% at that time in 2021, the CDC said. The surveillance collects information from 75 counties in 12 states.
Experts point out that the viruses – all three are respiratory viruses – are simply playing catchup.
“They spread the same way and along with lots of other viruses, and you tend to see an increase in them during the cold months,” said Timothy Brewer, MD, professor of medicine and epidemiology at UCLA.
The increase in all three viruses “is almost predictable at this point in the pandemic,” said Dean Blumberg, MD, a professor and chief of pediatric infectious diseases at the University of California Davis Health. “All the respiratory viruses are out of whack.”
Last year, RSV cases were up, too, and began to appear very early, he said, in the summer instead of in the cooler months. Flu also appeared early in 2021, as it has in 2022.
That contrasts with the flu season of 2020-2021, when COVID precautions were nearly universal, and cases were down. At UC Davis, “we didn’t have one pediatric admission due to influenza in the 2020-2021 [flu] season,” Dr. Blumberg said.
The number of pediatric flu deaths usually range from 37 to 199 per year, according to CDC records. But in the 2020-2021 season, the CDC recorded one pediatric flu death in the U.S.
Both children and adults have had less contact with others the past two seasons, Dr. Blumberg said, “and they don’t get the immunity they got with those infections [previously]. That’s why we are seeing out-of-season, early season [viruses].”
Eventually, he said, the cases of flu and RSV will return to previous levels. “It could be as soon as next year,” Dr. Blumberg said. And COVID-19, hopefully, will become like influenza, he said.
“RSV has always come around in the fall and winter,” said Elizabeth Murray, DO, a pediatric emergency medicine doctor at the University of Rochester (N.Y.) Medical Center and a spokesperson for the American Academy of Pediatrics. In 2022, children are back in school and for the most part not masking. “It’s a perfect storm for all the germs to spread now. They’ve just been waiting for their opportunity to come back.”
Self-care vs. not
RSV can pose a risk for anyone, but most at risk are children under age 5, especially infants under age 1, and adults over age 65. There is no vaccine for it. Symptoms include a runny nose, decreased appetite, coughing, sneezing, fever, and wheezing. But in young infants, there may only be decreased activity, crankiness, and breathing issues, the CDC said.
Keep an eye on the breathing if RSV is suspected, Dr. Murray tells parents. If your child can’t breathe easily, is unable to lie down comfortably, can’t speak clearly, or is sucking in the chest muscles to breathe, get medical help. Most kids with RSV can stay home and recover, she said, but often will need to be checked by a medical professional.
She advises against getting an oximeter to measure oxygen levels for home use. “They are often not accurate,” she said. If in doubt about how serious your child’s symptoms are, “don’t wait it out,” and don’t hesitate to call 911.
Symptoms of flu, COVID, and RSV can overlap. But each can involve breathing problems, which can be an emergency.
“It’s important to seek medical attention for any concerning symptoms, but especially severe shortness of breath or difficulty breathing, as these could signal the need for supplemental oxygen or other emergency interventions,” said Mandy De Vries, a respiratory therapist and director of education at the American Association for Respiratory Care. Inhalation treatment or mechanical ventilation may be needed for severe respiratory issues.
Precautions
To avoid the tripledemic – or any single infection – Timothy Brewer, MD, a professor of medicine and epidemiology at the University of California, Los Angeles, suggests some familiar measures: “Stay home if you’re feeling sick. Make sure you are up to date on your vaccinations. Wear a mask indoors.”
A version of this article first appeared on Medscape.com.
HPV-positive women who undergo IVF don’t have worse outcomes
A new study provides more evidence that HPV infection doesn’t raise the risk of poor outcomes in women who undergo fertility treatment via in vitro fertilization with fresh embryos. In fact, HPV-positive women were somewhat more likely than HPV-negative women to become pregnant (relative risk, 1.20; 95% confidence interval, 1.03-1.39) and have live births (RR, 1.39; 95% CI, 1.13-1.70), researchers reported Oct. 24 at the American Society for Reproductive Medicine’s 2022 meeting .
“This evidence should reassure women that being HPV positive will not affect live birth rates after a fresh embryo transfer cycle,” said study coauthor and ob.gyn. Nina Vyas, MD, a clinical fellow at Weill Cornell Medicine, New York, in an interview.
According to Dr. Vyas, previous studies have offered conflicting results about whether HPV affects pregnancy outcomes. In 2006, for example, her group performed a pilot study (Fertil Steril. Jun 16. doi: 10.1016/j.fertnstert.2006.01.051) that linked lower pregnancy rates to HPV-positive tests on the day of egg retrieval.
“We sought to reevaluate this finding in a retrospective manner,” Dr. Vyas said. “You’re taking eggs out of their home, injecting with sperm, and putting them back. There’s so much that we don’t know, and we want to make sure there’s no extra risk.”
Also, she added, “prior studies had a relatively low sample size. We sought to use our patient volume to address this question on a larger scale. Our current study benefits from a large sample size and using the clinically meaningful endpoint of live birth as our primary outcome.”
For the new study, researchers retrospectively analyzed 1,333 patients (of 2,209 screened) who received first fresh embryo transfers from 2017 to 2019. All had cytology or HPV status documented per cervical cancer screening guidelines within 6 months before embryos were transferred.
The researchers looked at only fresh embryo transfers “so we could account for pregnancy outcomes closest to the documented HPV status at the time of egg retrieval,” Dr. Vyas said.
Ten percent (133) of patients were HPV positive. Of those, 60.1% became pregnant, and 43.6% of them had live births. Of the HPV-negative women (90% of subjects, n = 1,200), 52.2% became pregnant and 33.5% had live births. The researchers didn’t calculate P values, but Dr. Vyas said an analysis determined that the differences between HPV-positive and HPV-negative women were statistically significant.
The study size doesn’t allow researchers to determine whether HPV actually has a protective effect on pregnancy/live birth rates in IVF, Dr. Vyas said. Even if it did, the virus is dangerous.
What else could explain the discrepancy? “Some elements driving this could the smaller sample size of the HPV-positive group, differences in HPV prevalence between the general population and our population,” she said, “or other confounding factors we were not able to appreciate due to the limitations of the retrospective study.”
Researchers also reported that they found “no significant difference in biochemical or spontaneous abortion rates” between HPV-positive and HPV-negative women.
What is the message of the study? “Women with HPV can rest assured that they won’t have worse outcomes than their non-HPV [infected] counterparts after a fresh embryo transfer cycle,” Dr. Vyas said.
In an interview, McGill University, Montreal, epidemiologist Helen Trottier, PhD, MSc, noted that she recently coauthored a study that linked persistent HPV infection in pregnancy to premature births. The findings appear convincing, she said: “I think we can say that HPV is associated with preterm birth.”
She praised the new study but noted “the relative risks that are reported need to be adjusted for race and possibly other factors.”
Dr. Vyas said that kind of adjustment will occur in a future study that’s in progress. “We are now prospectively enrolling patients and collecting cytology data to understand whether there might be a difference for women with higher malignancy potential/different types of HPV genotypes.”
The study authors have no disclosures. Disclosure information for Dr. Trottier was unavailable.
A new study provides more evidence that HPV infection doesn’t raise the risk of poor outcomes in women who undergo fertility treatment via in vitro fertilization with fresh embryos. In fact, HPV-positive women were somewhat more likely than HPV-negative women to become pregnant (relative risk, 1.20; 95% confidence interval, 1.03-1.39) and have live births (RR, 1.39; 95% CI, 1.13-1.70), researchers reported Oct. 24 at the American Society for Reproductive Medicine’s 2022 meeting .
“This evidence should reassure women that being HPV positive will not affect live birth rates after a fresh embryo transfer cycle,” said study coauthor and ob.gyn. Nina Vyas, MD, a clinical fellow at Weill Cornell Medicine, New York, in an interview.
According to Dr. Vyas, previous studies have offered conflicting results about whether HPV affects pregnancy outcomes. In 2006, for example, her group performed a pilot study (Fertil Steril. Jun 16. doi: 10.1016/j.fertnstert.2006.01.051) that linked lower pregnancy rates to HPV-positive tests on the day of egg retrieval.
“We sought to reevaluate this finding in a retrospective manner,” Dr. Vyas said. “You’re taking eggs out of their home, injecting with sperm, and putting them back. There’s so much that we don’t know, and we want to make sure there’s no extra risk.”
Also, she added, “prior studies had a relatively low sample size. We sought to use our patient volume to address this question on a larger scale. Our current study benefits from a large sample size and using the clinically meaningful endpoint of live birth as our primary outcome.”
For the new study, researchers retrospectively analyzed 1,333 patients (of 2,209 screened) who received first fresh embryo transfers from 2017 to 2019. All had cytology or HPV status documented per cervical cancer screening guidelines within 6 months before embryos were transferred.
The researchers looked at only fresh embryo transfers “so we could account for pregnancy outcomes closest to the documented HPV status at the time of egg retrieval,” Dr. Vyas said.
Ten percent (133) of patients were HPV positive. Of those, 60.1% became pregnant, and 43.6% of them had live births. Of the HPV-negative women (90% of subjects, n = 1,200), 52.2% became pregnant and 33.5% had live births. The researchers didn’t calculate P values, but Dr. Vyas said an analysis determined that the differences between HPV-positive and HPV-negative women were statistically significant.
The study size doesn’t allow researchers to determine whether HPV actually has a protective effect on pregnancy/live birth rates in IVF, Dr. Vyas said. Even if it did, the virus is dangerous.
What else could explain the discrepancy? “Some elements driving this could the smaller sample size of the HPV-positive group, differences in HPV prevalence between the general population and our population,” she said, “or other confounding factors we were not able to appreciate due to the limitations of the retrospective study.”
Researchers also reported that they found “no significant difference in biochemical or spontaneous abortion rates” between HPV-positive and HPV-negative women.
What is the message of the study? “Women with HPV can rest assured that they won’t have worse outcomes than their non-HPV [infected] counterparts after a fresh embryo transfer cycle,” Dr. Vyas said.
In an interview, McGill University, Montreal, epidemiologist Helen Trottier, PhD, MSc, noted that she recently coauthored a study that linked persistent HPV infection in pregnancy to premature births. The findings appear convincing, she said: “I think we can say that HPV is associated with preterm birth.”
She praised the new study but noted “the relative risks that are reported need to be adjusted for race and possibly other factors.”
Dr. Vyas said that kind of adjustment will occur in a future study that’s in progress. “We are now prospectively enrolling patients and collecting cytology data to understand whether there might be a difference for women with higher malignancy potential/different types of HPV genotypes.”
The study authors have no disclosures. Disclosure information for Dr. Trottier was unavailable.
A new study provides more evidence that HPV infection doesn’t raise the risk of poor outcomes in women who undergo fertility treatment via in vitro fertilization with fresh embryos. In fact, HPV-positive women were somewhat more likely than HPV-negative women to become pregnant (relative risk, 1.20; 95% confidence interval, 1.03-1.39) and have live births (RR, 1.39; 95% CI, 1.13-1.70), researchers reported Oct. 24 at the American Society for Reproductive Medicine’s 2022 meeting .
“This evidence should reassure women that being HPV positive will not affect live birth rates after a fresh embryo transfer cycle,” said study coauthor and ob.gyn. Nina Vyas, MD, a clinical fellow at Weill Cornell Medicine, New York, in an interview.
According to Dr. Vyas, previous studies have offered conflicting results about whether HPV affects pregnancy outcomes. In 2006, for example, her group performed a pilot study (Fertil Steril. Jun 16. doi: 10.1016/j.fertnstert.2006.01.051) that linked lower pregnancy rates to HPV-positive tests on the day of egg retrieval.
“We sought to reevaluate this finding in a retrospective manner,” Dr. Vyas said. “You’re taking eggs out of their home, injecting with sperm, and putting them back. There’s so much that we don’t know, and we want to make sure there’s no extra risk.”
Also, she added, “prior studies had a relatively low sample size. We sought to use our patient volume to address this question on a larger scale. Our current study benefits from a large sample size and using the clinically meaningful endpoint of live birth as our primary outcome.”
For the new study, researchers retrospectively analyzed 1,333 patients (of 2,209 screened) who received first fresh embryo transfers from 2017 to 2019. All had cytology or HPV status documented per cervical cancer screening guidelines within 6 months before embryos were transferred.
The researchers looked at only fresh embryo transfers “so we could account for pregnancy outcomes closest to the documented HPV status at the time of egg retrieval,” Dr. Vyas said.
Ten percent (133) of patients were HPV positive. Of those, 60.1% became pregnant, and 43.6% of them had live births. Of the HPV-negative women (90% of subjects, n = 1,200), 52.2% became pregnant and 33.5% had live births. The researchers didn’t calculate P values, but Dr. Vyas said an analysis determined that the differences between HPV-positive and HPV-negative women were statistically significant.
The study size doesn’t allow researchers to determine whether HPV actually has a protective effect on pregnancy/live birth rates in IVF, Dr. Vyas said. Even if it did, the virus is dangerous.
What else could explain the discrepancy? “Some elements driving this could the smaller sample size of the HPV-positive group, differences in HPV prevalence between the general population and our population,” she said, “or other confounding factors we were not able to appreciate due to the limitations of the retrospective study.”
Researchers also reported that they found “no significant difference in biochemical or spontaneous abortion rates” between HPV-positive and HPV-negative women.
What is the message of the study? “Women with HPV can rest assured that they won’t have worse outcomes than their non-HPV [infected] counterparts after a fresh embryo transfer cycle,” Dr. Vyas said.
In an interview, McGill University, Montreal, epidemiologist Helen Trottier, PhD, MSc, noted that she recently coauthored a study that linked persistent HPV infection in pregnancy to premature births. The findings appear convincing, she said: “I think we can say that HPV is associated with preterm birth.”
She praised the new study but noted “the relative risks that are reported need to be adjusted for race and possibly other factors.”
Dr. Vyas said that kind of adjustment will occur in a future study that’s in progress. “We are now prospectively enrolling patients and collecting cytology data to understand whether there might be a difference for women with higher malignancy potential/different types of HPV genotypes.”
The study authors have no disclosures. Disclosure information for Dr. Trottier was unavailable.
FROM ASRM 2022
Many specialists are on the wrong side of the patient-jargon relationship
Doctor, doctor, gimme the news. I got a bad case of misidentifying you
There are a lot of medical specialties out there. A lot. Everything from allergists to urologists, with something like 150 subspecialties grouped in among the larger specialties. Can you name every one? Do you know what they do?
The point is, telling a patient or anyone in the general public that you’re an ophthalmologist may not be as helpful as you might think, if a recent study is to be believed. In a survey of 204 adults, conducted at the Minnesota State Fair of all places, researchers asked volunteers to define 14 different specialties, as well as five medical seniority titles.
The results were less than stellar. While more than 90% of people correctly defined what cardiologists and dermatologists do, 6 of the other 12 specialists were correctly identified by less than half of those surveyed. Nephrology was at the bottom, correctly identified by just 20% of the fair-attending public, followed by internists (21%), intensivists (29%), hospitalists (31%), pulmonologists (43%), and neonatologists at 48%. The hospitalists are particularly concerning. They’re doctors, but in hospitals. How hard is that? (Yes, it’s obviously more complicated than that, but still.)
The general public didn’t fare much better when it came to correctly lining up the order of progression from medical student to attending. Just 12% managed to place all five in the correct order of med student, intern, senior resident, fellow, then attending, with senior resident proving especially troublesome. More than 40% put senior resident at the end, compared with 27% for attending. Which does make a certain amount of sense, since it has senior in the name.
While the results speak for themselves – maybe elaborate on what the heck your fancy title actually means – it’s too bad the researchers didn’t throw in something really tricky. If two-thirds of the population can’t identify a hospitalist, just imagine how many people would misidentify an otolaryngologist.
Beach-to-table sand could fight obesity
People are always looking for the new weight loss solution. Whether it’s to just look good in a new pair of jeans or reduce the risk of cardiovascular disease, there are millions of diets and exercise routines out here. We’re here to tell you that the next new therapy to reduce fat comes from a very unsuspecting place: Sand.
Like sand from the beach and desert, sand? Well, yes and no.
The research involved engineered porous silica particles made from sand that are designed to have a high surface area. Investigators used a two-step GI model in which gastric digestion was modeled for 30 minutes, followed by a 60-minute intestinal phase, to show that the porous silica particles helped prevent fat and sugar adsorption within the GI tract.
By mimicking the gastrointestinal environment during digestion of a high-fat, high-carb meal, the researchers found that the porous silica created an “anti-obesity effect” by restricting the adsorption of those fats and carbohydrates.
Okay, but how is that on the tummy? Much gentler on the stomach than a drug such as orlistat, said senior researcher Paul Joyce, PhD, of the University of South Australia, Adelaide, who noted the lack of effective therapies without side effects, such as bloating, diarrhea, and abdominal pain, that deter people from treatment.
Obesity affects over 1.9 billion people worldwide, so the researchers think this could be a breakthrough. Reducing obesity may be one of the most preventable ways to reduce the risk of type 2 diabetes, heart disease, and other weight-related chronic conditions. A treatment solution this simple could be the answer to this global health crisis.
Who would have thought the solution would be as simple as sand? But how would the sand get in our stomachs? Do we sprinkle it on our food? Mix it in during cooking? Or will the sand come in pill form? We sure hope it’s that third one.
I am Reliebo. I am here to help you
Halloween is almost here, and the LOTME staff has been trying to make the office look as scary as possible: Headless vampires, ghost clowns, Ted Cruz, gray tombstones, pink hearts, green clovers, red balloons. Wait a second, those last three are Lucky Charms marshmallows, aren’t they? We’ll use those some other time.
What are we not using to decorate? Well, besides marshmallows from cereal, we’re not using Reliebo. That’s what we’re not using. Reliebo is a cute little fuzzy robot, and is not at all scary. Reliebo was designed to be the opposite of scary. Reliebo “may reduce fear as well as alleviate the perception of pain during medical treatments, including vaccinations,” senior author Fumihide Tanaka, PhD, of the University of Tsukuba (Japan) said in a written statement.
The soft, fur-covered robot contains small airbags that can inflate in response to hand movements. When study participants were subjected to a moderate heat stimulus on one arm, those who held the robot with the other arm experienced less pain than those who did not have a Reliebo.
The results also were encouraging when Dr. Tanaka and associates measured the levels of oxytocin and cortisol (biomarkers for stress) from the subjects’ saliva samples and evaluated their fear of injections and their psychological state before and after the experiments.
After looking at that photo of Reliebo for a while, though, we have to admit that we’re having a bit of a rethink about its cuteness. Is it cute, or weird-looking? An office full of fuzzy little inflating robots just could be seriously creepy. Please don’t tell the rest of the staff about this. We want to surprise them on Monday.
Doctor, doctor, gimme the news. I got a bad case of misidentifying you
There are a lot of medical specialties out there. A lot. Everything from allergists to urologists, with something like 150 subspecialties grouped in among the larger specialties. Can you name every one? Do you know what they do?
The point is, telling a patient or anyone in the general public that you’re an ophthalmologist may not be as helpful as you might think, if a recent study is to be believed. In a survey of 204 adults, conducted at the Minnesota State Fair of all places, researchers asked volunteers to define 14 different specialties, as well as five medical seniority titles.
The results were less than stellar. While more than 90% of people correctly defined what cardiologists and dermatologists do, 6 of the other 12 specialists were correctly identified by less than half of those surveyed. Nephrology was at the bottom, correctly identified by just 20% of the fair-attending public, followed by internists (21%), intensivists (29%), hospitalists (31%), pulmonologists (43%), and neonatologists at 48%. The hospitalists are particularly concerning. They’re doctors, but in hospitals. How hard is that? (Yes, it’s obviously more complicated than that, but still.)
The general public didn’t fare much better when it came to correctly lining up the order of progression from medical student to attending. Just 12% managed to place all five in the correct order of med student, intern, senior resident, fellow, then attending, with senior resident proving especially troublesome. More than 40% put senior resident at the end, compared with 27% for attending. Which does make a certain amount of sense, since it has senior in the name.
While the results speak for themselves – maybe elaborate on what the heck your fancy title actually means – it’s too bad the researchers didn’t throw in something really tricky. If two-thirds of the population can’t identify a hospitalist, just imagine how many people would misidentify an otolaryngologist.
Beach-to-table sand could fight obesity
People are always looking for the new weight loss solution. Whether it’s to just look good in a new pair of jeans or reduce the risk of cardiovascular disease, there are millions of diets and exercise routines out here. We’re here to tell you that the next new therapy to reduce fat comes from a very unsuspecting place: Sand.
Like sand from the beach and desert, sand? Well, yes and no.
The research involved engineered porous silica particles made from sand that are designed to have a high surface area. Investigators used a two-step GI model in which gastric digestion was modeled for 30 minutes, followed by a 60-minute intestinal phase, to show that the porous silica particles helped prevent fat and sugar adsorption within the GI tract.
By mimicking the gastrointestinal environment during digestion of a high-fat, high-carb meal, the researchers found that the porous silica created an “anti-obesity effect” by restricting the adsorption of those fats and carbohydrates.
Okay, but how is that on the tummy? Much gentler on the stomach than a drug such as orlistat, said senior researcher Paul Joyce, PhD, of the University of South Australia, Adelaide, who noted the lack of effective therapies without side effects, such as bloating, diarrhea, and abdominal pain, that deter people from treatment.
Obesity affects over 1.9 billion people worldwide, so the researchers think this could be a breakthrough. Reducing obesity may be one of the most preventable ways to reduce the risk of type 2 diabetes, heart disease, and other weight-related chronic conditions. A treatment solution this simple could be the answer to this global health crisis.
Who would have thought the solution would be as simple as sand? But how would the sand get in our stomachs? Do we sprinkle it on our food? Mix it in during cooking? Or will the sand come in pill form? We sure hope it’s that third one.
I am Reliebo. I am here to help you
Halloween is almost here, and the LOTME staff has been trying to make the office look as scary as possible: Headless vampires, ghost clowns, Ted Cruz, gray tombstones, pink hearts, green clovers, red balloons. Wait a second, those last three are Lucky Charms marshmallows, aren’t they? We’ll use those some other time.
What are we not using to decorate? Well, besides marshmallows from cereal, we’re not using Reliebo. That’s what we’re not using. Reliebo is a cute little fuzzy robot, and is not at all scary. Reliebo was designed to be the opposite of scary. Reliebo “may reduce fear as well as alleviate the perception of pain during medical treatments, including vaccinations,” senior author Fumihide Tanaka, PhD, of the University of Tsukuba (Japan) said in a written statement.
The soft, fur-covered robot contains small airbags that can inflate in response to hand movements. When study participants were subjected to a moderate heat stimulus on one arm, those who held the robot with the other arm experienced less pain than those who did not have a Reliebo.
The results also were encouraging when Dr. Tanaka and associates measured the levels of oxytocin and cortisol (biomarkers for stress) from the subjects’ saliva samples and evaluated their fear of injections and their psychological state before and after the experiments.
After looking at that photo of Reliebo for a while, though, we have to admit that we’re having a bit of a rethink about its cuteness. Is it cute, or weird-looking? An office full of fuzzy little inflating robots just could be seriously creepy. Please don’t tell the rest of the staff about this. We want to surprise them on Monday.
Doctor, doctor, gimme the news. I got a bad case of misidentifying you
There are a lot of medical specialties out there. A lot. Everything from allergists to urologists, with something like 150 subspecialties grouped in among the larger specialties. Can you name every one? Do you know what they do?
The point is, telling a patient or anyone in the general public that you’re an ophthalmologist may not be as helpful as you might think, if a recent study is to be believed. In a survey of 204 adults, conducted at the Minnesota State Fair of all places, researchers asked volunteers to define 14 different specialties, as well as five medical seniority titles.
The results were less than stellar. While more than 90% of people correctly defined what cardiologists and dermatologists do, 6 of the other 12 specialists were correctly identified by less than half of those surveyed. Nephrology was at the bottom, correctly identified by just 20% of the fair-attending public, followed by internists (21%), intensivists (29%), hospitalists (31%), pulmonologists (43%), and neonatologists at 48%. The hospitalists are particularly concerning. They’re doctors, but in hospitals. How hard is that? (Yes, it’s obviously more complicated than that, but still.)
The general public didn’t fare much better when it came to correctly lining up the order of progression from medical student to attending. Just 12% managed to place all five in the correct order of med student, intern, senior resident, fellow, then attending, with senior resident proving especially troublesome. More than 40% put senior resident at the end, compared with 27% for attending. Which does make a certain amount of sense, since it has senior in the name.
While the results speak for themselves – maybe elaborate on what the heck your fancy title actually means – it’s too bad the researchers didn’t throw in something really tricky. If two-thirds of the population can’t identify a hospitalist, just imagine how many people would misidentify an otolaryngologist.
Beach-to-table sand could fight obesity
People are always looking for the new weight loss solution. Whether it’s to just look good in a new pair of jeans or reduce the risk of cardiovascular disease, there are millions of diets and exercise routines out here. We’re here to tell you that the next new therapy to reduce fat comes from a very unsuspecting place: Sand.
Like sand from the beach and desert, sand? Well, yes and no.
The research involved engineered porous silica particles made from sand that are designed to have a high surface area. Investigators used a two-step GI model in which gastric digestion was modeled for 30 minutes, followed by a 60-minute intestinal phase, to show that the porous silica particles helped prevent fat and sugar adsorption within the GI tract.
By mimicking the gastrointestinal environment during digestion of a high-fat, high-carb meal, the researchers found that the porous silica created an “anti-obesity effect” by restricting the adsorption of those fats and carbohydrates.
Okay, but how is that on the tummy? Much gentler on the stomach than a drug such as orlistat, said senior researcher Paul Joyce, PhD, of the University of South Australia, Adelaide, who noted the lack of effective therapies without side effects, such as bloating, diarrhea, and abdominal pain, that deter people from treatment.
Obesity affects over 1.9 billion people worldwide, so the researchers think this could be a breakthrough. Reducing obesity may be one of the most preventable ways to reduce the risk of type 2 diabetes, heart disease, and other weight-related chronic conditions. A treatment solution this simple could be the answer to this global health crisis.
Who would have thought the solution would be as simple as sand? But how would the sand get in our stomachs? Do we sprinkle it on our food? Mix it in during cooking? Or will the sand come in pill form? We sure hope it’s that third one.
I am Reliebo. I am here to help you
Halloween is almost here, and the LOTME staff has been trying to make the office look as scary as possible: Headless vampires, ghost clowns, Ted Cruz, gray tombstones, pink hearts, green clovers, red balloons. Wait a second, those last three are Lucky Charms marshmallows, aren’t they? We’ll use those some other time.
What are we not using to decorate? Well, besides marshmallows from cereal, we’re not using Reliebo. That’s what we’re not using. Reliebo is a cute little fuzzy robot, and is not at all scary. Reliebo was designed to be the opposite of scary. Reliebo “may reduce fear as well as alleviate the perception of pain during medical treatments, including vaccinations,” senior author Fumihide Tanaka, PhD, of the University of Tsukuba (Japan) said in a written statement.
The soft, fur-covered robot contains small airbags that can inflate in response to hand movements. When study participants were subjected to a moderate heat stimulus on one arm, those who held the robot with the other arm experienced less pain than those who did not have a Reliebo.
The results also were encouraging when Dr. Tanaka and associates measured the levels of oxytocin and cortisol (biomarkers for stress) from the subjects’ saliva samples and evaluated their fear of injections and their psychological state before and after the experiments.
After looking at that photo of Reliebo for a while, though, we have to admit that we’re having a bit of a rethink about its cuteness. Is it cute, or weird-looking? An office full of fuzzy little inflating robots just could be seriously creepy. Please don’t tell the rest of the staff about this. We want to surprise them on Monday.
Ivermectin for COVID-19: Final nail in the coffin
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
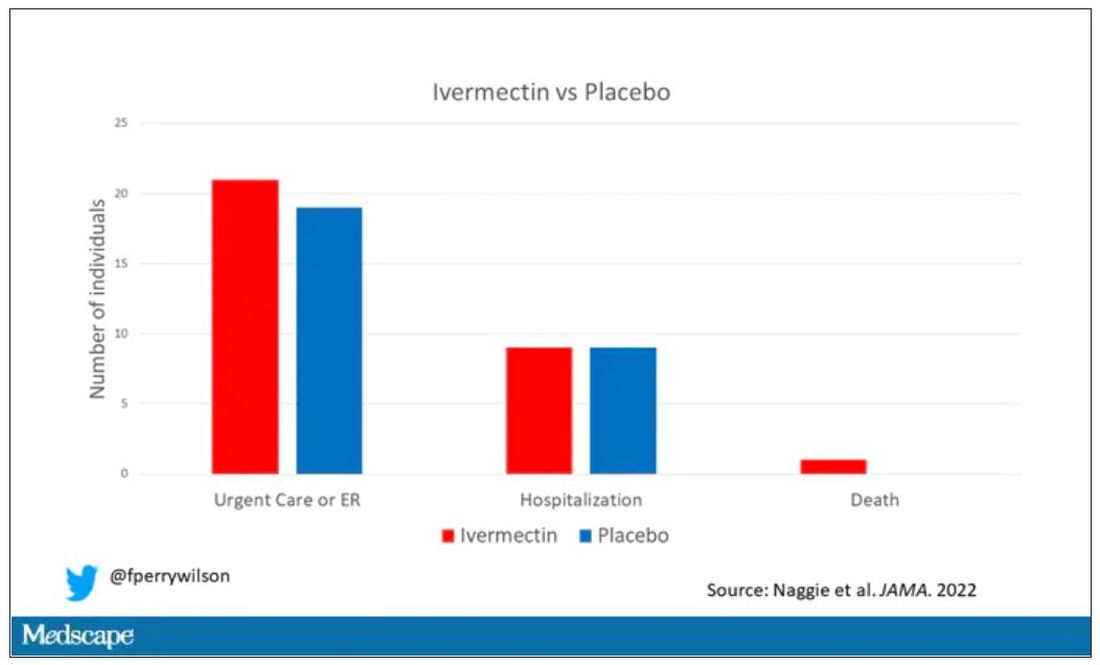
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.
But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.
But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.
But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.
Children and COVID: Weekly cases fall to lowest level in over a year
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.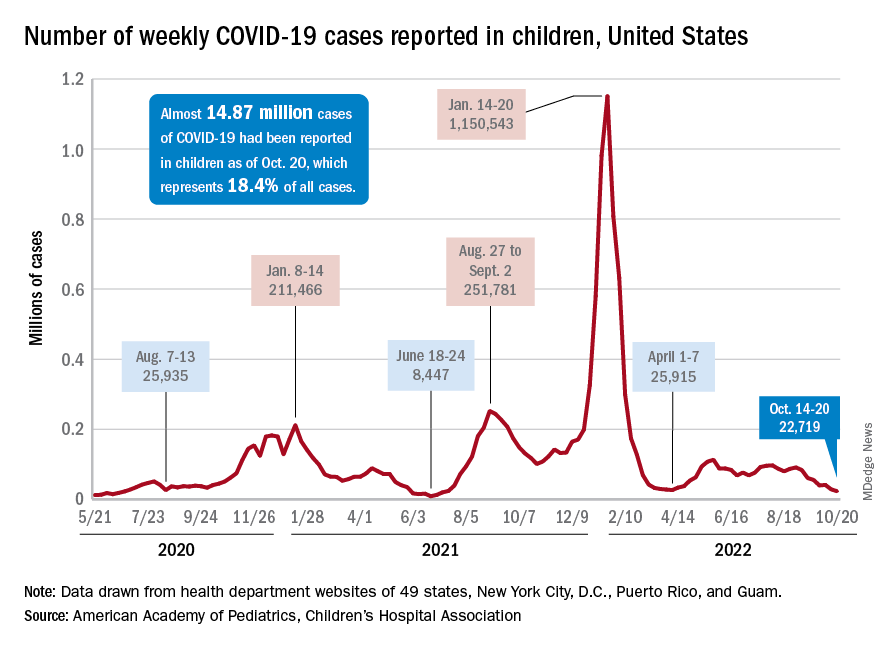
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.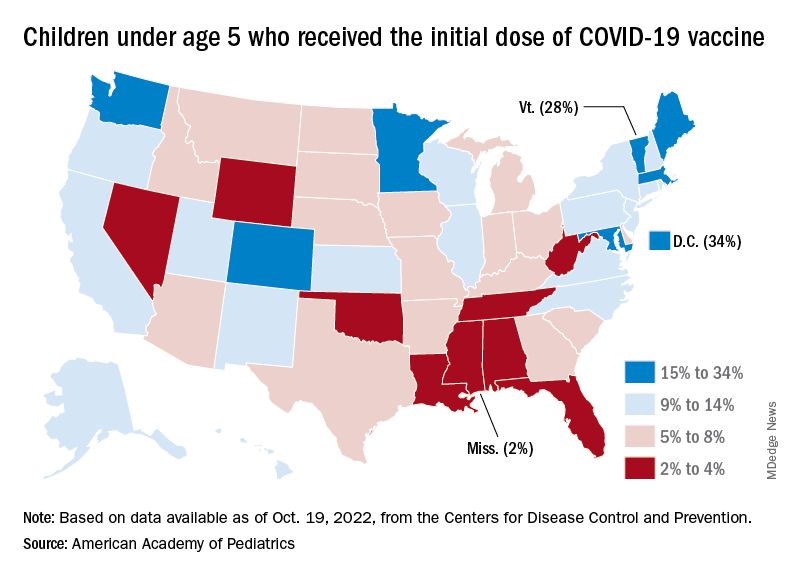
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
Time to ditch clarithromycin for H. pylori?
Rates of resistance to clarithromycin among Helicobacter pylori isolates in the United States and Europe are high enough to warrant discontinuation of empiric use of proton pump inhibitor (PPI)–based triple therapy that includes the antibiotic in these regions, a new study has found.
Overall, 22.2% of participants were resistant to clarithromycin – a rate that is above the currently recommended threshold of 15% or higher for avoidance of PPI-based triple therapy that includes clarithromycin.
, study investigator William Chey, MD, professor and chief, Division of Gastroenterology and Hepatology, Michigan Medicine, Ann Arbor, said in an interview.
Judith Kim, MD, a gastroenterologist at NYU Langone Health and clinical instructor of medicine at NYU Grossman School of Medicine, who wasn’t involved in the study, agrees.
“The use of PPI-based triple therapy is still common practice despite recent recommendations to avoid clarithromycin in areas with high resistance rates,” Dr. Kim told this news organization.
“This study shows that multiple parts of the United States and Europe have high resistance rates,” rendering clarithromycin-based regimens “more likely to ineffectively eradicate H pylori,” Dr. Kim said.
The study was published online in The American Journal of Gastroenterology.
Better options now available
Guidelines advise against the use of PPI-based triple regimens with clarithromycin for H. pylori infection in areas where resistance is 15% or higher or for patients who have previously received macrolides. However, up-to-date information on H. pylori antimicrobial resistance patterns is limited, especially in the United States.
Dr. Chey and colleagues assessed resistance rates to antibiotics commonly used to treat H. pylori in isolates from 907 adults with the infection in the United States and Europe. They included four U.S. subregions and five participating European countries.
In all U.S. subregions and European countries, clarithromycin resistance rates were above 15% except possibly in the United Kingdom, where the sample size was too small to provide a reliable estimate.
Three-quarters of the clarithromycin-resistant isolates were also resistant to metronidazole.
The study also found that, overall, 1.2% of patients had isolates that were resistant to amoxicillin, and 69.2% had isolates resistant to metronidazole. Resistance patterns were similar in the United States and Europe; metronidazole resistance was the most common (50%-79% of isolates), and amoxicillin was the least common (≤ 5%).
“Overall, these data provide robust evidence to support a shift away from the default empiric prescription of triple combinations containing a PPI and clarithromycin for H. pylori infection in the United States and Europe,” the study team writes.
The high prevalence of resistance, including dual resistance, highlights the need for antibiotic stewardship and resistance surveillance, as well as novel treatment strategies for H. pylori infection, they add.
Last spring, as previously reported, the United States Food and Drug Administration approved two vonoprazan-based treatments for H. pylori: Voquezna Triple Pak (vonoprazan, amoxicillin, clarithromycin) and Voquezna Dual Pak (vonoprazan, amoxicillin), both from Phathom Pharmaceuticals.
“Vonoprazan-based treatment may be superior to standard PPI triple therapy for clarithromycin-resistant infections based on prior studies and is a potential good option,” Dr. Kim said.
Still, she added, she “would most likely first recommend regimens that do not have clarithromycin, such as bismuth quadruple therapy.”
Study’s importance
Because the study drew upon the largest dataset to date on U.S. resistance rates, it should be used to more precisely guide first-line therapy decisions, said Richard Peek, Jr., MD, professor of medicine and director of gastroenterology at Vanderbilt University Medical Center, Nashville, Tenn.
“To date, there has been a dearth of information in the United States regarding H. pylori resistance rates, which has often led to the use of ineffective empiric therapies and inappropriate exposure to antibiotics,” Dr. Peek, who wasn’t involved in the study, told this news organization.
“These data are particularly exciting when viewed within the context of new genomic sequencing tests that can determine H. pylori resistance patterns using DNA isolated from the stomach or the stool,” he said.
Dr. Peek agreed that the recent approval of vonoprazan-based therapies “adds another regimen to the therapeutic armamentarium available for eradicating H. pylori, and its value seems to be particularly beneficial for eradication failures.”
The research was funded by Phathom Pharmaceuticals. Dr. Chey is a board member of the American College of Gastroenterology, GI on Demand, the International Foundation of Functional GI Disorders, and the Rome Foundation. He has received compensation as a consultant from AbbVie, Alfasigma, Allakos, Alnylam, Bayer, BioAmerica, Cosmo, Intrinsic Medicine, Ironwood Pharmaceuticals, QOL Medical, Nestle, Phathom Pharmaceuticals, RedHill Biopharma, Salix/Valeant, Takeda, Urovant, and Vibrant; grant/research support from BioAmerica, Commonwealth Diagnostics International, QOL Medical, Salix, and Vibrant; owns stock/stock options in GI on Demand and Modify Health; and owns patents relating to methods and kits for identifying food sensitivities and intolerances, digital manometry, and a rectal expulsion device. Dr. Peek and Dr. Kim report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of resistance to clarithromycin among Helicobacter pylori isolates in the United States and Europe are high enough to warrant discontinuation of empiric use of proton pump inhibitor (PPI)–based triple therapy that includes the antibiotic in these regions, a new study has found.
Overall, 22.2% of participants were resistant to clarithromycin – a rate that is above the currently recommended threshold of 15% or higher for avoidance of PPI-based triple therapy that includes clarithromycin.
, study investigator William Chey, MD, professor and chief, Division of Gastroenterology and Hepatology, Michigan Medicine, Ann Arbor, said in an interview.
Judith Kim, MD, a gastroenterologist at NYU Langone Health and clinical instructor of medicine at NYU Grossman School of Medicine, who wasn’t involved in the study, agrees.
“The use of PPI-based triple therapy is still common practice despite recent recommendations to avoid clarithromycin in areas with high resistance rates,” Dr. Kim told this news organization.
“This study shows that multiple parts of the United States and Europe have high resistance rates,” rendering clarithromycin-based regimens “more likely to ineffectively eradicate H pylori,” Dr. Kim said.
The study was published online in The American Journal of Gastroenterology.
Better options now available
Guidelines advise against the use of PPI-based triple regimens with clarithromycin for H. pylori infection in areas where resistance is 15% or higher or for patients who have previously received macrolides. However, up-to-date information on H. pylori antimicrobial resistance patterns is limited, especially in the United States.
Dr. Chey and colleagues assessed resistance rates to antibiotics commonly used to treat H. pylori in isolates from 907 adults with the infection in the United States and Europe. They included four U.S. subregions and five participating European countries.
In all U.S. subregions and European countries, clarithromycin resistance rates were above 15% except possibly in the United Kingdom, where the sample size was too small to provide a reliable estimate.
Three-quarters of the clarithromycin-resistant isolates were also resistant to metronidazole.
The study also found that, overall, 1.2% of patients had isolates that were resistant to amoxicillin, and 69.2% had isolates resistant to metronidazole. Resistance patterns were similar in the United States and Europe; metronidazole resistance was the most common (50%-79% of isolates), and amoxicillin was the least common (≤ 5%).
“Overall, these data provide robust evidence to support a shift away from the default empiric prescription of triple combinations containing a PPI and clarithromycin for H. pylori infection in the United States and Europe,” the study team writes.
The high prevalence of resistance, including dual resistance, highlights the need for antibiotic stewardship and resistance surveillance, as well as novel treatment strategies for H. pylori infection, they add.
Last spring, as previously reported, the United States Food and Drug Administration approved two vonoprazan-based treatments for H. pylori: Voquezna Triple Pak (vonoprazan, amoxicillin, clarithromycin) and Voquezna Dual Pak (vonoprazan, amoxicillin), both from Phathom Pharmaceuticals.
“Vonoprazan-based treatment may be superior to standard PPI triple therapy for clarithromycin-resistant infections based on prior studies and is a potential good option,” Dr. Kim said.
Still, she added, she “would most likely first recommend regimens that do not have clarithromycin, such as bismuth quadruple therapy.”
Study’s importance
Because the study drew upon the largest dataset to date on U.S. resistance rates, it should be used to more precisely guide first-line therapy decisions, said Richard Peek, Jr., MD, professor of medicine and director of gastroenterology at Vanderbilt University Medical Center, Nashville, Tenn.
“To date, there has been a dearth of information in the United States regarding H. pylori resistance rates, which has often led to the use of ineffective empiric therapies and inappropriate exposure to antibiotics,” Dr. Peek, who wasn’t involved in the study, told this news organization.
“These data are particularly exciting when viewed within the context of new genomic sequencing tests that can determine H. pylori resistance patterns using DNA isolated from the stomach or the stool,” he said.
Dr. Peek agreed that the recent approval of vonoprazan-based therapies “adds another regimen to the therapeutic armamentarium available for eradicating H. pylori, and its value seems to be particularly beneficial for eradication failures.”
The research was funded by Phathom Pharmaceuticals. Dr. Chey is a board member of the American College of Gastroenterology, GI on Demand, the International Foundation of Functional GI Disorders, and the Rome Foundation. He has received compensation as a consultant from AbbVie, Alfasigma, Allakos, Alnylam, Bayer, BioAmerica, Cosmo, Intrinsic Medicine, Ironwood Pharmaceuticals, QOL Medical, Nestle, Phathom Pharmaceuticals, RedHill Biopharma, Salix/Valeant, Takeda, Urovant, and Vibrant; grant/research support from BioAmerica, Commonwealth Diagnostics International, QOL Medical, Salix, and Vibrant; owns stock/stock options in GI on Demand and Modify Health; and owns patents relating to methods and kits for identifying food sensitivities and intolerances, digital manometry, and a rectal expulsion device. Dr. Peek and Dr. Kim report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of resistance to clarithromycin among Helicobacter pylori isolates in the United States and Europe are high enough to warrant discontinuation of empiric use of proton pump inhibitor (PPI)–based triple therapy that includes the antibiotic in these regions, a new study has found.
Overall, 22.2% of participants were resistant to clarithromycin – a rate that is above the currently recommended threshold of 15% or higher for avoidance of PPI-based triple therapy that includes clarithromycin.
, study investigator William Chey, MD, professor and chief, Division of Gastroenterology and Hepatology, Michigan Medicine, Ann Arbor, said in an interview.
Judith Kim, MD, a gastroenterologist at NYU Langone Health and clinical instructor of medicine at NYU Grossman School of Medicine, who wasn’t involved in the study, agrees.
“The use of PPI-based triple therapy is still common practice despite recent recommendations to avoid clarithromycin in areas with high resistance rates,” Dr. Kim told this news organization.
“This study shows that multiple parts of the United States and Europe have high resistance rates,” rendering clarithromycin-based regimens “more likely to ineffectively eradicate H pylori,” Dr. Kim said.
The study was published online in The American Journal of Gastroenterology.
Better options now available
Guidelines advise against the use of PPI-based triple regimens with clarithromycin for H. pylori infection in areas where resistance is 15% or higher or for patients who have previously received macrolides. However, up-to-date information on H. pylori antimicrobial resistance patterns is limited, especially in the United States.
Dr. Chey and colleagues assessed resistance rates to antibiotics commonly used to treat H. pylori in isolates from 907 adults with the infection in the United States and Europe. They included four U.S. subregions and five participating European countries.
In all U.S. subregions and European countries, clarithromycin resistance rates were above 15% except possibly in the United Kingdom, where the sample size was too small to provide a reliable estimate.
Three-quarters of the clarithromycin-resistant isolates were also resistant to metronidazole.
The study also found that, overall, 1.2% of patients had isolates that were resistant to amoxicillin, and 69.2% had isolates resistant to metronidazole. Resistance patterns were similar in the United States and Europe; metronidazole resistance was the most common (50%-79% of isolates), and amoxicillin was the least common (≤ 5%).
“Overall, these data provide robust evidence to support a shift away from the default empiric prescription of triple combinations containing a PPI and clarithromycin for H. pylori infection in the United States and Europe,” the study team writes.
The high prevalence of resistance, including dual resistance, highlights the need for antibiotic stewardship and resistance surveillance, as well as novel treatment strategies for H. pylori infection, they add.
Last spring, as previously reported, the United States Food and Drug Administration approved two vonoprazan-based treatments for H. pylori: Voquezna Triple Pak (vonoprazan, amoxicillin, clarithromycin) and Voquezna Dual Pak (vonoprazan, amoxicillin), both from Phathom Pharmaceuticals.
“Vonoprazan-based treatment may be superior to standard PPI triple therapy for clarithromycin-resistant infections based on prior studies and is a potential good option,” Dr. Kim said.
Still, she added, she “would most likely first recommend regimens that do not have clarithromycin, such as bismuth quadruple therapy.”
Study’s importance
Because the study drew upon the largest dataset to date on U.S. resistance rates, it should be used to more precisely guide first-line therapy decisions, said Richard Peek, Jr., MD, professor of medicine and director of gastroenterology at Vanderbilt University Medical Center, Nashville, Tenn.
“To date, there has been a dearth of information in the United States regarding H. pylori resistance rates, which has often led to the use of ineffective empiric therapies and inappropriate exposure to antibiotics,” Dr. Peek, who wasn’t involved in the study, told this news organization.
“These data are particularly exciting when viewed within the context of new genomic sequencing tests that can determine H. pylori resistance patterns using DNA isolated from the stomach or the stool,” he said.
Dr. Peek agreed that the recent approval of vonoprazan-based therapies “adds another regimen to the therapeutic armamentarium available for eradicating H. pylori, and its value seems to be particularly beneficial for eradication failures.”
The research was funded by Phathom Pharmaceuticals. Dr. Chey is a board member of the American College of Gastroenterology, GI on Demand, the International Foundation of Functional GI Disorders, and the Rome Foundation. He has received compensation as a consultant from AbbVie, Alfasigma, Allakos, Alnylam, Bayer, BioAmerica, Cosmo, Intrinsic Medicine, Ironwood Pharmaceuticals, QOL Medical, Nestle, Phathom Pharmaceuticals, RedHill Biopharma, Salix/Valeant, Takeda, Urovant, and Vibrant; grant/research support from BioAmerica, Commonwealth Diagnostics International, QOL Medical, Salix, and Vibrant; owns stock/stock options in GI on Demand and Modify Health; and owns patents relating to methods and kits for identifying food sensitivities and intolerances, digital manometry, and a rectal expulsion device. Dr. Peek and Dr. Kim report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Life expectancy 6.3 years shorter for Black MSM with HIV
WASHINGTON –
Lead author Katherine Rich, MPH, a student at Harvard Medical School, Boston, presented the data during an annual scientific meeting on infectious diseases.
“Substantial disparities in care exist between Black and White MSM here in the United States,” she said. “The 2030 goals of the EHE [Ending the HIV Epidemic in the U.S. initiative] won’t be met until HIV-related disparities are reduced.”
Using modeling, the team was able to measure both the gaps and the potential of interventions to address those gaps.
- The team found that improving engagement in care had the largest benefit in narrowing the gap. Improving engagement and retention in care, they write, would result in a gain of 1.4 life-years for Black MSM and 1 year for White MSM.
- Annual testing would add 0.6 life-years for Black MSM and 0.3 life-years for White MSM, compared with standard care.
- In simulating viral suppression, the model-predicted gain would be 1.1 years for Black MSM and 0.3 for White.
Furthermore, a combination of annual testing, 95% engagement in care, and 95% virologic suppression would add 3.4 years for Black MSM (more than double the increase in life-years for any one intervention) and 1.6 years for their White counterparts, the research suggests.
The researchers projected life expectancy from age 15 to be 52.2 years for Black MSM (or 67.2 years old) and 58.5 years from age 15 for White MSM (or 73.5 years old), a difference of 6.3 years.
Kathleen McManus, MD, assistant professor of medicine in infectious diseases and international health at the University of Virginia, Charlottesville, said in an interview that the projected gap in years of life should be a call to action. Dr. McManus was not involved with the study.
Life expectancy gap ‘alarming’
“It is alarming that with current usual HIV care Black MSM with HIV have 6.3 fewer years of life expectancy than White MSM,” she said. “Black MSM having lower retention in care and a lower rate of viral suppression than White MSM demonstrates that there is a problem with our current health care delivery to Black MSM.”
With qualitative, community-engaged research, she said, “we need to ask the Black MSM community what care innovations they need – and then we need clinics and organizations to make the identified changes.”
Researchers used the validated CEPAC (Cost-Effectiveness of Preventing AIDS Complications) microsimulation HIV model to project life expectancy. Using data from the United States Centers for Disease Control and Prevention, they estimated the average age at HIV infection to be 26.8 years for Black MSM and 35 years for White MSM.
They estimated the proportion of time that MSM with diagnosed HIV are retained in care to be 75.2% for Black MSM and 80.6% for White MSM. They calculated the proportion who achieve virologic suppression to be 82% for Black MSM and 91.2% for White MSM.
Senior author Emily P. Hyle, MD, associate professor of medicine at Harvard and infectious diseases physician at Massachusetts General Hospital, both in Boston, said in a press conference before the presentation that strategies to narrow the gap will look different by region.
“Our study highlights that if you can find effective interventions, the effect can be incredibly large. These are very large differences in life-years and life expectancies,” she said.
Ms. Rich gave an example of promising interventions by pointing to work by study coauthor Aima Ahonkhai, MD, MPH, assistant professor of medicine at Vanderbilt University, Nashville, Tenn., who has received federal funding to pursue research on whether preventive care outreach in barbershops can improve prevention for Black men with HIV.
Ms. Rich noted that the modeling has limitations in that it focused on health outcomes and did not simulate transmissions. Results also reflect national data and not local HIV care continuums, which, she acknowledged, differ substantially.
Dr. McManus and the study authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON –
Lead author Katherine Rich, MPH, a student at Harvard Medical School, Boston, presented the data during an annual scientific meeting on infectious diseases.
“Substantial disparities in care exist between Black and White MSM here in the United States,” she said. “The 2030 goals of the EHE [Ending the HIV Epidemic in the U.S. initiative] won’t be met until HIV-related disparities are reduced.”
Using modeling, the team was able to measure both the gaps and the potential of interventions to address those gaps.
- The team found that improving engagement in care had the largest benefit in narrowing the gap. Improving engagement and retention in care, they write, would result in a gain of 1.4 life-years for Black MSM and 1 year for White MSM.
- Annual testing would add 0.6 life-years for Black MSM and 0.3 life-years for White MSM, compared with standard care.
- In simulating viral suppression, the model-predicted gain would be 1.1 years for Black MSM and 0.3 for White.
Furthermore, a combination of annual testing, 95% engagement in care, and 95% virologic suppression would add 3.4 years for Black MSM (more than double the increase in life-years for any one intervention) and 1.6 years for their White counterparts, the research suggests.
The researchers projected life expectancy from age 15 to be 52.2 years for Black MSM (or 67.2 years old) and 58.5 years from age 15 for White MSM (or 73.5 years old), a difference of 6.3 years.
Kathleen McManus, MD, assistant professor of medicine in infectious diseases and international health at the University of Virginia, Charlottesville, said in an interview that the projected gap in years of life should be a call to action. Dr. McManus was not involved with the study.
Life expectancy gap ‘alarming’
“It is alarming that with current usual HIV care Black MSM with HIV have 6.3 fewer years of life expectancy than White MSM,” she said. “Black MSM having lower retention in care and a lower rate of viral suppression than White MSM demonstrates that there is a problem with our current health care delivery to Black MSM.”
With qualitative, community-engaged research, she said, “we need to ask the Black MSM community what care innovations they need – and then we need clinics and organizations to make the identified changes.”
Researchers used the validated CEPAC (Cost-Effectiveness of Preventing AIDS Complications) microsimulation HIV model to project life expectancy. Using data from the United States Centers for Disease Control and Prevention, they estimated the average age at HIV infection to be 26.8 years for Black MSM and 35 years for White MSM.
They estimated the proportion of time that MSM with diagnosed HIV are retained in care to be 75.2% for Black MSM and 80.6% for White MSM. They calculated the proportion who achieve virologic suppression to be 82% for Black MSM and 91.2% for White MSM.
Senior author Emily P. Hyle, MD, associate professor of medicine at Harvard and infectious diseases physician at Massachusetts General Hospital, both in Boston, said in a press conference before the presentation that strategies to narrow the gap will look different by region.
“Our study highlights that if you can find effective interventions, the effect can be incredibly large. These are very large differences in life-years and life expectancies,” she said.
Ms. Rich gave an example of promising interventions by pointing to work by study coauthor Aima Ahonkhai, MD, MPH, assistant professor of medicine at Vanderbilt University, Nashville, Tenn., who has received federal funding to pursue research on whether preventive care outreach in barbershops can improve prevention for Black men with HIV.
Ms. Rich noted that the modeling has limitations in that it focused on health outcomes and did not simulate transmissions. Results also reflect national data and not local HIV care continuums, which, she acknowledged, differ substantially.
Dr. McManus and the study authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON –
Lead author Katherine Rich, MPH, a student at Harvard Medical School, Boston, presented the data during an annual scientific meeting on infectious diseases.
“Substantial disparities in care exist between Black and White MSM here in the United States,” she said. “The 2030 goals of the EHE [Ending the HIV Epidemic in the U.S. initiative] won’t be met until HIV-related disparities are reduced.”
Using modeling, the team was able to measure both the gaps and the potential of interventions to address those gaps.
- The team found that improving engagement in care had the largest benefit in narrowing the gap. Improving engagement and retention in care, they write, would result in a gain of 1.4 life-years for Black MSM and 1 year for White MSM.
- Annual testing would add 0.6 life-years for Black MSM and 0.3 life-years for White MSM, compared with standard care.
- In simulating viral suppression, the model-predicted gain would be 1.1 years for Black MSM and 0.3 for White.
Furthermore, a combination of annual testing, 95% engagement in care, and 95% virologic suppression would add 3.4 years for Black MSM (more than double the increase in life-years for any one intervention) and 1.6 years for their White counterparts, the research suggests.
The researchers projected life expectancy from age 15 to be 52.2 years for Black MSM (or 67.2 years old) and 58.5 years from age 15 for White MSM (or 73.5 years old), a difference of 6.3 years.
Kathleen McManus, MD, assistant professor of medicine in infectious diseases and international health at the University of Virginia, Charlottesville, said in an interview that the projected gap in years of life should be a call to action. Dr. McManus was not involved with the study.
Life expectancy gap ‘alarming’
“It is alarming that with current usual HIV care Black MSM with HIV have 6.3 fewer years of life expectancy than White MSM,” she said. “Black MSM having lower retention in care and a lower rate of viral suppression than White MSM demonstrates that there is a problem with our current health care delivery to Black MSM.”
With qualitative, community-engaged research, she said, “we need to ask the Black MSM community what care innovations they need – and then we need clinics and organizations to make the identified changes.”
Researchers used the validated CEPAC (Cost-Effectiveness of Preventing AIDS Complications) microsimulation HIV model to project life expectancy. Using data from the United States Centers for Disease Control and Prevention, they estimated the average age at HIV infection to be 26.8 years for Black MSM and 35 years for White MSM.
They estimated the proportion of time that MSM with diagnosed HIV are retained in care to be 75.2% for Black MSM and 80.6% for White MSM. They calculated the proportion who achieve virologic suppression to be 82% for Black MSM and 91.2% for White MSM.
Senior author Emily P. Hyle, MD, associate professor of medicine at Harvard and infectious diseases physician at Massachusetts General Hospital, both in Boston, said in a press conference before the presentation that strategies to narrow the gap will look different by region.
“Our study highlights that if you can find effective interventions, the effect can be incredibly large. These are very large differences in life-years and life expectancies,” she said.
Ms. Rich gave an example of promising interventions by pointing to work by study coauthor Aima Ahonkhai, MD, MPH, assistant professor of medicine at Vanderbilt University, Nashville, Tenn., who has received federal funding to pursue research on whether preventive care outreach in barbershops can improve prevention for Black men with HIV.
Ms. Rich noted that the modeling has limitations in that it focused on health outcomes and did not simulate transmissions. Results also reflect national data and not local HIV care continuums, which, she acknowledged, differ substantially.
Dr. McManus and the study authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT IDWEEK 2022
Worse COVID outcomes seen with gout, particularly in women
People with gout, especially women, appear to be at higher risk for poor COVID-19 outcomes, including hospitalization and death, regardless of COVID-19 vaccination status, researchers suggest.
“We found that the risks of SARS-CoV-2 infection, 30-day hospitalization, and 30-day death among individuals with gout were higher than the general population irrespective of the vaccination status,” lead study author Dongxing Xie, MD, PhD, Xiangya Hospital, Central South University, Changsha, China, and his colleagues write in their large population study. “This finding informs individuals with gout, especially women, that additional measures, even after vaccination, should be considered in order to mitigate the risk of SARS-CoV-2 infection and its severe sequelae.”
People with gout, the most common inflammatory arthritis, often have other conditions that are linked to higher risk for SARS-CoV-2 infection and poor outcomes as well, including obesity, cardiovascular disease, and chronic kidney disease, the authors write. And elevated serum urate may contribute to inflammation and possible COVID-19 complications. But unlike in the case of diseases such as lupus and rheumatoid arthritis, little is known about SARS-CoV-2 infection risk among patients with gout.
As reported in Arthritis & Rheumatology, Dr. Xie and his research team used the Health Improvement Network ([THIN], now called IQVIA Medical Research Database) repository of medical conditions, demographics, and other details of around 17 million people in the United Kingdom to estimate the risk for SARS-CoV-2 infection, hospitalization, and death in people with gout. They compared those outcomes with outcomes of people without gout and compared outcomes of vaccinated vs. nonvaccinated participants.
From December 2020 through October 2021, the researchers investigated the risk for SARS-CoV-2 breakthrough infection in vaccinated people between age 18 and 90 years who had gout and were hospitalized within 30 days after the infection diagnosis or who died within 30 days after the diagnosis. They compared these outcomes with the outcomes of people in the general population without gout after COVID-19 vaccination. They also compared the risk for SARS-CoV-2 infection and its severe outcomes between individuals with gout and the general population among unvaccinated people.
They weighted these comparisons on the basis of age, sex, body mass index, socioeconomic deprivation index score, region, and number of previous COVID-19 tests in one model. A more fully adjusted model also weighted the comparisons for lifestyle factors, comorbidities, medications, and healthcare utilization.
The vaccinated cohort consisted of 54,576 people with gout and 1,336,377 without gout from the general population. The unvaccinated cohort included 61,111 individuals with gout and 1,697,168 individuals without gout from the general population.
Women more likely to be hospitalized and die
The risk for breakthrough infection in the vaccinated cohort was significantly higher among people with gout than among those without gout in the general population, particularly for men, who had hazard ratios (HRs) ranging from 1.22 with a fully adjusted exposure score to 1.30 with a partially adjusted score, but this was not seen in women. The overall incidence of breakthrough infection per 1,000 person-months for these groups was 4.68 with gout vs. 3.76 without gout.
The researchers showed a similar pattern of a higher rate of hospitalizations for people with gout vs. without (0.42/1,000 person-months vs. 0.28); in this case, women had higher risks than did men, with HRs for women ranging from 1.55 with a fully adjusted exposure score to 1.91 with a partially adjusted score, compared with 1.22 and 1.43 for men, respectively.
People with gout had significantly higher mortality than did those without (0.06/1,000 person-months vs. 0.04), but the risk for death was only higher for women, with HRs calculated to be 2.23 in fully adjusted exposure scores and 3.01 in partially adjusted scores.
These same comparisons in the unvaccinated cohort all went in the same direction as did those in the vaccinated cohort but showed higher rates for infection (8.69/1,000 person-months vs. 6.89), hospitalization (2.57/1,000 person-months vs. 1.71), and death (0.65/1,000 person-months vs. 0.53). Similar sex-specific links between gout and risks for SARS-CoV-2 infection, hospitalization, and death were seen in the unvaccinated cohort.
Patients with gout and COVID-19 need close monitoring
Four experts who were not involved in the study encourage greater attention to the needs of patients with gout.
Pamela B. Davis, MD, PhD, research professor at Case Western Reserve University, Cleveland, told this news organization, “This study brings to attention yet another potentially vulnerable group for physicians to monitor closely if they are infected with SARS-CoV-2.
“It is not clear why women with gout are more vulnerable, but fewer women than men were in the cohort with gout, and the confidence intervals for the results in women were, in general, larger,” she said.
“The authors suggest that women with gout tend to be older and have more comorbidities than men with gout,” Dr. Davis added. “The excess risk diminishes when the model is fully adjusted for comorbidities, such as obesity, hypertension, or heart disease, suggesting that already-known antecedents of infection severity account for a great deal of the excess risk.”
Kevin D. Deane, MD, PhD, associate professor of medicine and chair in rheumatology research at the University of Colorado at Denver, Aurora, advises physicians to keep in mind other conditions linked with increased risk for severe COVID-19, including advanced age; heart, lung, or kidney problems; and autoimmune diseases.
“I would be very cautious about the finding that there was not a difference in outcomes in individuals with gout based on vaccination status,” he cautioned, urging clinicians to “still strongly recommend vaccines according to guidelines.”
Sarah E. Waldman, MD, associate clinical professor of infectious diseases at UC Davis Health in Sacramento, Calif., called the study interesting but not surprising.
“The reason for increased risk for COVID-19 infection among those with gout may have to do with their underlying inflammatory state. Additional research needs to be done on this topic.
“Retrospective population-based cohort studies can be difficult to interpret due to biases,” she added. Associations identified in this type of study do not determine causation.
“As the researchers noted, those with gout tend to have additional comorbidities as well as advanced age,” she said. “They may also seek medical care more often and be tested for SARS-CoV-2 more frequently.”
Dr. Waldman advises clinicians to counsel patients with gout about their potential increased infection risk and ways they can protect themselves, including COVID-19 vaccinations.
“The strong association between gout and COVID-19 infection could involve coexisting conditions such as diabetes, hypertension, cardiovascular disease, and chronic kidney disease,” Dr. Aung added.
Earlier studies show links between gout and severe COVID-19 outcomes
Lead author Kanon Jatuworapruk, MD, PhD, of Thammasat University in Pathumthani, Thailand, and his colleagues investigated characteristics and outcomes of people with gout who were hospitalized for COVID-19 between March 2020 and October 2021, using data from the COVID-19 Global Rheumatology Alliance registry.
“This cohort of people with gout and COVID-19 who were hospitalized had high frequencies of ventilatory support and death,” the authors write in ACR Open Rheumatology . “This suggests that patients with gout who were hospitalized for COVID-19 may be at risk of poor outcomes, perhaps related to known risk factors for poor outcomes, such as age and presence of comorbidity.”
In their study, the average age of the 163 patients was 63 years, and 85% were men. Most lived in the Western Pacific Region and North America, and 46% had two or more comorbidities, most commonly hypertension, cardiovascular disease, diabetes, chronic kidney disease, and obesity. The researchers found that:
- Sixty-eight percent of the cohort required supplemental oxygen or ventilatory support during hospitalization.
- Sixteen percent of deaths were related to COVID-19, with 73% of deaths occurring in people with two or more comorbidities.
Ruth K. Topless, assistant research fellow in the department of biochemistry at the University of Otago in Dunedin, New Zealand, is the lead author on a study she and her colleagues are conducting using the UK Biobank databases of 459,837 participants in the United Kingdom, including 15,871 people with gout, through April 6, 2021, to investigate whether gout is a risk factor for diagnosis of COVID-19 and COVID-19–related death.
“Gout is a risk factor for COVID-19-related death in the UK Biobank cohort, with an increased risk in women with gout, which was driven by risk factors independent of the metabolic comorbidities of gout,” the researchers conclude in The Lancet Rheumatology.
In their study, gout was linked with COVID-19 diagnosis (odds ratio, 1.20; 95% confidence interval, 1.11-1.29) but not with risk for COVID-19–related death in the group of patients with COVID-19 (OR, 1.20; 95% CI, 0.96-1.51). In the entire cohort, gout was linked with COVID-19–related death (OR, 1.29; 95% CI, 1.06-1.56); women with gout were at increased risk for COVID-19–related death (OR, 1.98; 95% CI, 1.34-2.94), but men with gout were not (OR, 1.16; 95% CI, 0.93-1.45). The risk for COVID-19 diagnosis was significant in the nonvaccinated group (OR, 1.21; 95% CI, 1.11-1.30) but not in the vaccinated group (OR, 1.09; 95% CI, 0.65-1.85).
Editorial authors join in recommending further related research
In a commentary in The Lancet Rheumatology about the UK Biobank and other related research, Christoffer B. Nissen, MD, of University Hospital of Southern Denmark in Sonderborg, and his co-authors call the Topless and colleagues study “an elegantly conducted analysis of data from the UK Biobank supporting the hypothesis that gout needs attention in patients with COVID-19.”
Further studies are needed to investigate to what degree a diagnosis of gout is a risk factor for COVID-19 and whether treatment modifies the risk of a severe disease course,” they write. “However, in the interim, the results of this study could be considered when risk stratifying patients with gout in view of vaccination recommendations and early treatment interventions.”
Each of the three studies received grant funding. Several of the authors of the studies report financial involvements with pharmaceutical companies. All outside experts commented by email and report no relevant financial involvements.
A version of this article first appeared on Medscape.com.
People with gout, especially women, appear to be at higher risk for poor COVID-19 outcomes, including hospitalization and death, regardless of COVID-19 vaccination status, researchers suggest.
“We found that the risks of SARS-CoV-2 infection, 30-day hospitalization, and 30-day death among individuals with gout were higher than the general population irrespective of the vaccination status,” lead study author Dongxing Xie, MD, PhD, Xiangya Hospital, Central South University, Changsha, China, and his colleagues write in their large population study. “This finding informs individuals with gout, especially women, that additional measures, even after vaccination, should be considered in order to mitigate the risk of SARS-CoV-2 infection and its severe sequelae.”
People with gout, the most common inflammatory arthritis, often have other conditions that are linked to higher risk for SARS-CoV-2 infection and poor outcomes as well, including obesity, cardiovascular disease, and chronic kidney disease, the authors write. And elevated serum urate may contribute to inflammation and possible COVID-19 complications. But unlike in the case of diseases such as lupus and rheumatoid arthritis, little is known about SARS-CoV-2 infection risk among patients with gout.
As reported in Arthritis & Rheumatology, Dr. Xie and his research team used the Health Improvement Network ([THIN], now called IQVIA Medical Research Database) repository of medical conditions, demographics, and other details of around 17 million people in the United Kingdom to estimate the risk for SARS-CoV-2 infection, hospitalization, and death in people with gout. They compared those outcomes with outcomes of people without gout and compared outcomes of vaccinated vs. nonvaccinated participants.
From December 2020 through October 2021, the researchers investigated the risk for SARS-CoV-2 breakthrough infection in vaccinated people between age 18 and 90 years who had gout and were hospitalized within 30 days after the infection diagnosis or who died within 30 days after the diagnosis. They compared these outcomes with the outcomes of people in the general population without gout after COVID-19 vaccination. They also compared the risk for SARS-CoV-2 infection and its severe outcomes between individuals with gout and the general population among unvaccinated people.
They weighted these comparisons on the basis of age, sex, body mass index, socioeconomic deprivation index score, region, and number of previous COVID-19 tests in one model. A more fully adjusted model also weighted the comparisons for lifestyle factors, comorbidities, medications, and healthcare utilization.
The vaccinated cohort consisted of 54,576 people with gout and 1,336,377 without gout from the general population. The unvaccinated cohort included 61,111 individuals with gout and 1,697,168 individuals without gout from the general population.
Women more likely to be hospitalized and die
The risk for breakthrough infection in the vaccinated cohort was significantly higher among people with gout than among those without gout in the general population, particularly for men, who had hazard ratios (HRs) ranging from 1.22 with a fully adjusted exposure score to 1.30 with a partially adjusted score, but this was not seen in women. The overall incidence of breakthrough infection per 1,000 person-months for these groups was 4.68 with gout vs. 3.76 without gout.
The researchers showed a similar pattern of a higher rate of hospitalizations for people with gout vs. without (0.42/1,000 person-months vs. 0.28); in this case, women had higher risks than did men, with HRs for women ranging from 1.55 with a fully adjusted exposure score to 1.91 with a partially adjusted score, compared with 1.22 and 1.43 for men, respectively.
People with gout had significantly higher mortality than did those without (0.06/1,000 person-months vs. 0.04), but the risk for death was only higher for women, with HRs calculated to be 2.23 in fully adjusted exposure scores and 3.01 in partially adjusted scores.
These same comparisons in the unvaccinated cohort all went in the same direction as did those in the vaccinated cohort but showed higher rates for infection (8.69/1,000 person-months vs. 6.89), hospitalization (2.57/1,000 person-months vs. 1.71), and death (0.65/1,000 person-months vs. 0.53). Similar sex-specific links between gout and risks for SARS-CoV-2 infection, hospitalization, and death were seen in the unvaccinated cohort.
Patients with gout and COVID-19 need close monitoring
Four experts who were not involved in the study encourage greater attention to the needs of patients with gout.
Pamela B. Davis, MD, PhD, research professor at Case Western Reserve University, Cleveland, told this news organization, “This study brings to attention yet another potentially vulnerable group for physicians to monitor closely if they are infected with SARS-CoV-2.
“It is not clear why women with gout are more vulnerable, but fewer women than men were in the cohort with gout, and the confidence intervals for the results in women were, in general, larger,” she said.
“The authors suggest that women with gout tend to be older and have more comorbidities than men with gout,” Dr. Davis added. “The excess risk diminishes when the model is fully adjusted for comorbidities, such as obesity, hypertension, or heart disease, suggesting that already-known antecedents of infection severity account for a great deal of the excess risk.”
Kevin D. Deane, MD, PhD, associate professor of medicine and chair in rheumatology research at the University of Colorado at Denver, Aurora, advises physicians to keep in mind other conditions linked with increased risk for severe COVID-19, including advanced age; heart, lung, or kidney problems; and autoimmune diseases.
“I would be very cautious about the finding that there was not a difference in outcomes in individuals with gout based on vaccination status,” he cautioned, urging clinicians to “still strongly recommend vaccines according to guidelines.”
Sarah E. Waldman, MD, associate clinical professor of infectious diseases at UC Davis Health in Sacramento, Calif., called the study interesting but not surprising.
“The reason for increased risk for COVID-19 infection among those with gout may have to do with their underlying inflammatory state. Additional research needs to be done on this topic.
“Retrospective population-based cohort studies can be difficult to interpret due to biases,” she added. Associations identified in this type of study do not determine causation.
“As the researchers noted, those with gout tend to have additional comorbidities as well as advanced age,” she said. “They may also seek medical care more often and be tested for SARS-CoV-2 more frequently.”
Dr. Waldman advises clinicians to counsel patients with gout about their potential increased infection risk and ways they can protect themselves, including COVID-19 vaccinations.
“The strong association between gout and COVID-19 infection could involve coexisting conditions such as diabetes, hypertension, cardiovascular disease, and chronic kidney disease,” Dr. Aung added.
Earlier studies show links between gout and severe COVID-19 outcomes
Lead author Kanon Jatuworapruk, MD, PhD, of Thammasat University in Pathumthani, Thailand, and his colleagues investigated characteristics and outcomes of people with gout who were hospitalized for COVID-19 between March 2020 and October 2021, using data from the COVID-19 Global Rheumatology Alliance registry.
“This cohort of people with gout and COVID-19 who were hospitalized had high frequencies of ventilatory support and death,” the authors write in ACR Open Rheumatology . “This suggests that patients with gout who were hospitalized for COVID-19 may be at risk of poor outcomes, perhaps related to known risk factors for poor outcomes, such as age and presence of comorbidity.”
In their study, the average age of the 163 patients was 63 years, and 85% were men. Most lived in the Western Pacific Region and North America, and 46% had two or more comorbidities, most commonly hypertension, cardiovascular disease, diabetes, chronic kidney disease, and obesity. The researchers found that:
- Sixty-eight percent of the cohort required supplemental oxygen or ventilatory support during hospitalization.
- Sixteen percent of deaths were related to COVID-19, with 73% of deaths occurring in people with two or more comorbidities.
Ruth K. Topless, assistant research fellow in the department of biochemistry at the University of Otago in Dunedin, New Zealand, is the lead author on a study she and her colleagues are conducting using the UK Biobank databases of 459,837 participants in the United Kingdom, including 15,871 people with gout, through April 6, 2021, to investigate whether gout is a risk factor for diagnosis of COVID-19 and COVID-19–related death.
“Gout is a risk factor for COVID-19-related death in the UK Biobank cohort, with an increased risk in women with gout, which was driven by risk factors independent of the metabolic comorbidities of gout,” the researchers conclude in The Lancet Rheumatology.
In their study, gout was linked with COVID-19 diagnosis (odds ratio, 1.20; 95% confidence interval, 1.11-1.29) but not with risk for COVID-19–related death in the group of patients with COVID-19 (OR, 1.20; 95% CI, 0.96-1.51). In the entire cohort, gout was linked with COVID-19–related death (OR, 1.29; 95% CI, 1.06-1.56); women with gout were at increased risk for COVID-19–related death (OR, 1.98; 95% CI, 1.34-2.94), but men with gout were not (OR, 1.16; 95% CI, 0.93-1.45). The risk for COVID-19 diagnosis was significant in the nonvaccinated group (OR, 1.21; 95% CI, 1.11-1.30) but not in the vaccinated group (OR, 1.09; 95% CI, 0.65-1.85).
Editorial authors join in recommending further related research
In a commentary in The Lancet Rheumatology about the UK Biobank and other related research, Christoffer B. Nissen, MD, of University Hospital of Southern Denmark in Sonderborg, and his co-authors call the Topless and colleagues study “an elegantly conducted analysis of data from the UK Biobank supporting the hypothesis that gout needs attention in patients with COVID-19.”
Further studies are needed to investigate to what degree a diagnosis of gout is a risk factor for COVID-19 and whether treatment modifies the risk of a severe disease course,” they write. “However, in the interim, the results of this study could be considered when risk stratifying patients with gout in view of vaccination recommendations and early treatment interventions.”
Each of the three studies received grant funding. Several of the authors of the studies report financial involvements with pharmaceutical companies. All outside experts commented by email and report no relevant financial involvements.
A version of this article first appeared on Medscape.com.
People with gout, especially women, appear to be at higher risk for poor COVID-19 outcomes, including hospitalization and death, regardless of COVID-19 vaccination status, researchers suggest.
“We found that the risks of SARS-CoV-2 infection, 30-day hospitalization, and 30-day death among individuals with gout were higher than the general population irrespective of the vaccination status,” lead study author Dongxing Xie, MD, PhD, Xiangya Hospital, Central South University, Changsha, China, and his colleagues write in their large population study. “This finding informs individuals with gout, especially women, that additional measures, even after vaccination, should be considered in order to mitigate the risk of SARS-CoV-2 infection and its severe sequelae.”
People with gout, the most common inflammatory arthritis, often have other conditions that are linked to higher risk for SARS-CoV-2 infection and poor outcomes as well, including obesity, cardiovascular disease, and chronic kidney disease, the authors write. And elevated serum urate may contribute to inflammation and possible COVID-19 complications. But unlike in the case of diseases such as lupus and rheumatoid arthritis, little is known about SARS-CoV-2 infection risk among patients with gout.
As reported in Arthritis & Rheumatology, Dr. Xie and his research team used the Health Improvement Network ([THIN], now called IQVIA Medical Research Database) repository of medical conditions, demographics, and other details of around 17 million people in the United Kingdom to estimate the risk for SARS-CoV-2 infection, hospitalization, and death in people with gout. They compared those outcomes with outcomes of people without gout and compared outcomes of vaccinated vs. nonvaccinated participants.
From December 2020 through October 2021, the researchers investigated the risk for SARS-CoV-2 breakthrough infection in vaccinated people between age 18 and 90 years who had gout and were hospitalized within 30 days after the infection diagnosis or who died within 30 days after the diagnosis. They compared these outcomes with the outcomes of people in the general population without gout after COVID-19 vaccination. They also compared the risk for SARS-CoV-2 infection and its severe outcomes between individuals with gout and the general population among unvaccinated people.
They weighted these comparisons on the basis of age, sex, body mass index, socioeconomic deprivation index score, region, and number of previous COVID-19 tests in one model. A more fully adjusted model also weighted the comparisons for lifestyle factors, comorbidities, medications, and healthcare utilization.
The vaccinated cohort consisted of 54,576 people with gout and 1,336,377 without gout from the general population. The unvaccinated cohort included 61,111 individuals with gout and 1,697,168 individuals without gout from the general population.
Women more likely to be hospitalized and die
The risk for breakthrough infection in the vaccinated cohort was significantly higher among people with gout than among those without gout in the general population, particularly for men, who had hazard ratios (HRs) ranging from 1.22 with a fully adjusted exposure score to 1.30 with a partially adjusted score, but this was not seen in women. The overall incidence of breakthrough infection per 1,000 person-months for these groups was 4.68 with gout vs. 3.76 without gout.
The researchers showed a similar pattern of a higher rate of hospitalizations for people with gout vs. without (0.42/1,000 person-months vs. 0.28); in this case, women had higher risks than did men, with HRs for women ranging from 1.55 with a fully adjusted exposure score to 1.91 with a partially adjusted score, compared with 1.22 and 1.43 for men, respectively.
People with gout had significantly higher mortality than did those without (0.06/1,000 person-months vs. 0.04), but the risk for death was only higher for women, with HRs calculated to be 2.23 in fully adjusted exposure scores and 3.01 in partially adjusted scores.
These same comparisons in the unvaccinated cohort all went in the same direction as did those in the vaccinated cohort but showed higher rates for infection (8.69/1,000 person-months vs. 6.89), hospitalization (2.57/1,000 person-months vs. 1.71), and death (0.65/1,000 person-months vs. 0.53). Similar sex-specific links between gout and risks for SARS-CoV-2 infection, hospitalization, and death were seen in the unvaccinated cohort.
Patients with gout and COVID-19 need close monitoring
Four experts who were not involved in the study encourage greater attention to the needs of patients with gout.
Pamela B. Davis, MD, PhD, research professor at Case Western Reserve University, Cleveland, told this news organization, “This study brings to attention yet another potentially vulnerable group for physicians to monitor closely if they are infected with SARS-CoV-2.
“It is not clear why women with gout are more vulnerable, but fewer women than men were in the cohort with gout, and the confidence intervals for the results in women were, in general, larger,” she said.
“The authors suggest that women with gout tend to be older and have more comorbidities than men with gout,” Dr. Davis added. “The excess risk diminishes when the model is fully adjusted for comorbidities, such as obesity, hypertension, or heart disease, suggesting that already-known antecedents of infection severity account for a great deal of the excess risk.”
Kevin D. Deane, MD, PhD, associate professor of medicine and chair in rheumatology research at the University of Colorado at Denver, Aurora, advises physicians to keep in mind other conditions linked with increased risk for severe COVID-19, including advanced age; heart, lung, or kidney problems; and autoimmune diseases.
“I would be very cautious about the finding that there was not a difference in outcomes in individuals with gout based on vaccination status,” he cautioned, urging clinicians to “still strongly recommend vaccines according to guidelines.”
Sarah E. Waldman, MD, associate clinical professor of infectious diseases at UC Davis Health in Sacramento, Calif., called the study interesting but not surprising.
“The reason for increased risk for COVID-19 infection among those with gout may have to do with their underlying inflammatory state. Additional research needs to be done on this topic.
“Retrospective population-based cohort studies can be difficult to interpret due to biases,” she added. Associations identified in this type of study do not determine causation.
“As the researchers noted, those with gout tend to have additional comorbidities as well as advanced age,” she said. “They may also seek medical care more often and be tested for SARS-CoV-2 more frequently.”
Dr. Waldman advises clinicians to counsel patients with gout about their potential increased infection risk and ways they can protect themselves, including COVID-19 vaccinations.
“The strong association between gout and COVID-19 infection could involve coexisting conditions such as diabetes, hypertension, cardiovascular disease, and chronic kidney disease,” Dr. Aung added.
Earlier studies show links between gout and severe COVID-19 outcomes
Lead author Kanon Jatuworapruk, MD, PhD, of Thammasat University in Pathumthani, Thailand, and his colleagues investigated characteristics and outcomes of people with gout who were hospitalized for COVID-19 between March 2020 and October 2021, using data from the COVID-19 Global Rheumatology Alliance registry.
“This cohort of people with gout and COVID-19 who were hospitalized had high frequencies of ventilatory support and death,” the authors write in ACR Open Rheumatology . “This suggests that patients with gout who were hospitalized for COVID-19 may be at risk of poor outcomes, perhaps related to known risk factors for poor outcomes, such as age and presence of comorbidity.”
In their study, the average age of the 163 patients was 63 years, and 85% were men. Most lived in the Western Pacific Region and North America, and 46% had two or more comorbidities, most commonly hypertension, cardiovascular disease, diabetes, chronic kidney disease, and obesity. The researchers found that:
- Sixty-eight percent of the cohort required supplemental oxygen or ventilatory support during hospitalization.
- Sixteen percent of deaths were related to COVID-19, with 73% of deaths occurring in people with two or more comorbidities.
Ruth K. Topless, assistant research fellow in the department of biochemistry at the University of Otago in Dunedin, New Zealand, is the lead author on a study she and her colleagues are conducting using the UK Biobank databases of 459,837 participants in the United Kingdom, including 15,871 people with gout, through April 6, 2021, to investigate whether gout is a risk factor for diagnosis of COVID-19 and COVID-19–related death.
“Gout is a risk factor for COVID-19-related death in the UK Biobank cohort, with an increased risk in women with gout, which was driven by risk factors independent of the metabolic comorbidities of gout,” the researchers conclude in The Lancet Rheumatology.
In their study, gout was linked with COVID-19 diagnosis (odds ratio, 1.20; 95% confidence interval, 1.11-1.29) but not with risk for COVID-19–related death in the group of patients with COVID-19 (OR, 1.20; 95% CI, 0.96-1.51). In the entire cohort, gout was linked with COVID-19–related death (OR, 1.29; 95% CI, 1.06-1.56); women with gout were at increased risk for COVID-19–related death (OR, 1.98; 95% CI, 1.34-2.94), but men with gout were not (OR, 1.16; 95% CI, 0.93-1.45). The risk for COVID-19 diagnosis was significant in the nonvaccinated group (OR, 1.21; 95% CI, 1.11-1.30) but not in the vaccinated group (OR, 1.09; 95% CI, 0.65-1.85).
Editorial authors join in recommending further related research
In a commentary in The Lancet Rheumatology about the UK Biobank and other related research, Christoffer B. Nissen, MD, of University Hospital of Southern Denmark in Sonderborg, and his co-authors call the Topless and colleagues study “an elegantly conducted analysis of data from the UK Biobank supporting the hypothesis that gout needs attention in patients with COVID-19.”
Further studies are needed to investigate to what degree a diagnosis of gout is a risk factor for COVID-19 and whether treatment modifies the risk of a severe disease course,” they write. “However, in the interim, the results of this study could be considered when risk stratifying patients with gout in view of vaccination recommendations and early treatment interventions.”
Each of the three studies received grant funding. Several of the authors of the studies report financial involvements with pharmaceutical companies. All outside experts commented by email and report no relevant financial involvements.
A version of this article first appeared on Medscape.com.
Updated Moderna booster shows greater activity against COVID in adults
WASHINGTON –
The bivalent booster was superior regardless of age and whether a person had previously been infected with SARS-CoV-2.
Additionally, no new safety concerns emerged.
Spyros Chalkias, MD, senior medical director of clinical development at Moderna, presented the data during an annual scientific meeting on infectious diseases.
In the phase 2/3 trial, participants received either 50 mcg of the bivalent vaccine mRNA-1273.214 (25 mcg each of the original Wuhan-Hu-1 and Omicron BA.1 spike mRNAs) or 50 mcg of the standard authorized mRNA-1273. The doses were given as second boosters in adults who had previously received a two-dose primary series and a first booster at least 3 months before.
The model-based geometric mean titers (GMTs) ratio of the enhanced booster compared with the standard booster was 1.74 (1.49-2.04), meeting the prespecified bar for superiority against Omicron BA.1.
In participants without prior SARS-CoV-2 infection who received updated booster doses and those who received standard boosters, the neutralizing antibody GMTs against Omicron BA.1 were 2372.4 and 1473.5, respectively.
Additionally, the updated booster elicited higher GMTs (727.4) than the standard booster (492.1) against Omicron subvariants BA.4/BA.5. Safety and reactogenicity were similar for both vaccine groups.
“By the end of this year, we expect to also have clinical trial data from our BA.4/BA.5 bivalent booster,” Dr. Chalkias said.
In the interim, the U.S. Food and Drug Administration recently granted emergency use authorization for Moderna’s BA.4/BA.5 Omicron-targeting bivalent COVID-19 booster vaccine in children and adolescents aged 6-17 years.
Pfizer/BioNTech also has recently issued an announcement that their COVID-19 booster, adapted for the BA.4 and the BA.5 Omicron subvariants, generated a strong immune response and was well tolerated in human tests.
Pfizer/BioNTech said data from roughly 80 adult patients showed that the booster led to a substantial increase in neutralizing antibody levels against the BA.4/BA.5 variants after 1 week.
Separate study of causes of severe breakthrough infections in early vaccine formulations
Though COVID vaccines reduce the incidence of severe outcomes, there are reports of breakthrough infections in persons who received the original vaccines, and some of these have been serious.
In a separate study, also presented at the meeting, researchers led by first author Austin D. Vo, BS, with the VA Boston Healthcare System, used data collected from Dec. 15, 2020, through Feb. 28, 2022, in a U.S. veteran population to assess those at highest risk for severe disease despite vaccination.
Results of the large, nationwide retrospective study were simultaneously published in JAMA Network Open.
The primary outcome was development of severe COVID, defined as a hospitalization within 14 days of a confirmed positive SARS-CoV-2 test, receipt of supplemental oxygen, mechanical ventilation, or death within 28 days.
Among 110,760 participants with severe disease after primary vaccination, 13% (14,690) were hospitalized with severe COVID-19 or died.
The strongest risk factor for severe disease despite vaccination was age, the researchers found.
Presenting author Westyn Branch-Elliman, MD, associate professor of medicine with VA Boston Healthcare System, said, “We found that age greater than 50 was associated with an adjusted odds ratio of 1.42 for every 5-year increase.”
To put that in perspective, she said, “compared to patients who are 45 to 50, those over 80 had an adjusted odds ratio of 16 for hospitalization or death following breakthrough infection.”
Priya Nori, MD, an infectious disease specialist at Montefiore Medical Center in New York, said in an interview that the evidence that age is a strong risk factor for severe disease – even after vaccination – confirms that attention should be focused on those in the highest age groups, particularly those 80 years and older.
Other top risk factors included having immunocompromising conditions; having received cytotoxic chemotherapy within 6 months (adjusted odds ratio, 2.69; 95% confidence interval, 2.25-3.21); having leukemias/lymphomas (aOR, 1.84; 95% CI, 1.59-2.14); and having chronic conditions associated with end-organ disease.
“We also found that receipt of an additional booster dose of vaccine was associated with a 50% reduction in adjusted odds of severe disease,” noted Dr. Branch-Elliman.
Dr. Nori emphasized that, given these data, emphatic messaging is needed to encourage uptake of the updated Omicron-targeted vaccines for these high-risk age groups.
The study by Dr. Chalkias and colleagues was funded by Moderna. Dr. Chalkias and several coauthors are employed by Moderna. One coauthor has relationships with DLA Piper/Medtronic, and Gilead Pharmaceuticals, and one has relationships with Celgene/Bristol-Myers Squibb, ChemoCentryx, Gilead, and Kiniksa. Dr. Nori has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON –
The bivalent booster was superior regardless of age and whether a person had previously been infected with SARS-CoV-2.
Additionally, no new safety concerns emerged.
Spyros Chalkias, MD, senior medical director of clinical development at Moderna, presented the data during an annual scientific meeting on infectious diseases.
In the phase 2/3 trial, participants received either 50 mcg of the bivalent vaccine mRNA-1273.214 (25 mcg each of the original Wuhan-Hu-1 and Omicron BA.1 spike mRNAs) or 50 mcg of the standard authorized mRNA-1273. The doses were given as second boosters in adults who had previously received a two-dose primary series and a first booster at least 3 months before.
The model-based geometric mean titers (GMTs) ratio of the enhanced booster compared with the standard booster was 1.74 (1.49-2.04), meeting the prespecified bar for superiority against Omicron BA.1.
In participants without prior SARS-CoV-2 infection who received updated booster doses and those who received standard boosters, the neutralizing antibody GMTs against Omicron BA.1 were 2372.4 and 1473.5, respectively.
Additionally, the updated booster elicited higher GMTs (727.4) than the standard booster (492.1) against Omicron subvariants BA.4/BA.5. Safety and reactogenicity were similar for both vaccine groups.
“By the end of this year, we expect to also have clinical trial data from our BA.4/BA.5 bivalent booster,” Dr. Chalkias said.
In the interim, the U.S. Food and Drug Administration recently granted emergency use authorization for Moderna’s BA.4/BA.5 Omicron-targeting bivalent COVID-19 booster vaccine in children and adolescents aged 6-17 years.
Pfizer/BioNTech also has recently issued an announcement that their COVID-19 booster, adapted for the BA.4 and the BA.5 Omicron subvariants, generated a strong immune response and was well tolerated in human tests.
Pfizer/BioNTech said data from roughly 80 adult patients showed that the booster led to a substantial increase in neutralizing antibody levels against the BA.4/BA.5 variants after 1 week.
Separate study of causes of severe breakthrough infections in early vaccine formulations
Though COVID vaccines reduce the incidence of severe outcomes, there are reports of breakthrough infections in persons who received the original vaccines, and some of these have been serious.
In a separate study, also presented at the meeting, researchers led by first author Austin D. Vo, BS, with the VA Boston Healthcare System, used data collected from Dec. 15, 2020, through Feb. 28, 2022, in a U.S. veteran population to assess those at highest risk for severe disease despite vaccination.
Results of the large, nationwide retrospective study were simultaneously published in JAMA Network Open.
The primary outcome was development of severe COVID, defined as a hospitalization within 14 days of a confirmed positive SARS-CoV-2 test, receipt of supplemental oxygen, mechanical ventilation, or death within 28 days.
Among 110,760 participants with severe disease after primary vaccination, 13% (14,690) were hospitalized with severe COVID-19 or died.
The strongest risk factor for severe disease despite vaccination was age, the researchers found.
Presenting author Westyn Branch-Elliman, MD, associate professor of medicine with VA Boston Healthcare System, said, “We found that age greater than 50 was associated with an adjusted odds ratio of 1.42 for every 5-year increase.”
To put that in perspective, she said, “compared to patients who are 45 to 50, those over 80 had an adjusted odds ratio of 16 for hospitalization or death following breakthrough infection.”
Priya Nori, MD, an infectious disease specialist at Montefiore Medical Center in New York, said in an interview that the evidence that age is a strong risk factor for severe disease – even after vaccination – confirms that attention should be focused on those in the highest age groups, particularly those 80 years and older.
Other top risk factors included having immunocompromising conditions; having received cytotoxic chemotherapy within 6 months (adjusted odds ratio, 2.69; 95% confidence interval, 2.25-3.21); having leukemias/lymphomas (aOR, 1.84; 95% CI, 1.59-2.14); and having chronic conditions associated with end-organ disease.
“We also found that receipt of an additional booster dose of vaccine was associated with a 50% reduction in adjusted odds of severe disease,” noted Dr. Branch-Elliman.
Dr. Nori emphasized that, given these data, emphatic messaging is needed to encourage uptake of the updated Omicron-targeted vaccines for these high-risk age groups.
The study by Dr. Chalkias and colleagues was funded by Moderna. Dr. Chalkias and several coauthors are employed by Moderna. One coauthor has relationships with DLA Piper/Medtronic, and Gilead Pharmaceuticals, and one has relationships with Celgene/Bristol-Myers Squibb, ChemoCentryx, Gilead, and Kiniksa. Dr. Nori has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON –
The bivalent booster was superior regardless of age and whether a person had previously been infected with SARS-CoV-2.
Additionally, no new safety concerns emerged.
Spyros Chalkias, MD, senior medical director of clinical development at Moderna, presented the data during an annual scientific meeting on infectious diseases.
In the phase 2/3 trial, participants received either 50 mcg of the bivalent vaccine mRNA-1273.214 (25 mcg each of the original Wuhan-Hu-1 and Omicron BA.1 spike mRNAs) or 50 mcg of the standard authorized mRNA-1273. The doses were given as second boosters in adults who had previously received a two-dose primary series and a first booster at least 3 months before.
The model-based geometric mean titers (GMTs) ratio of the enhanced booster compared with the standard booster was 1.74 (1.49-2.04), meeting the prespecified bar for superiority against Omicron BA.1.
In participants without prior SARS-CoV-2 infection who received updated booster doses and those who received standard boosters, the neutralizing antibody GMTs against Omicron BA.1 were 2372.4 and 1473.5, respectively.
Additionally, the updated booster elicited higher GMTs (727.4) than the standard booster (492.1) against Omicron subvariants BA.4/BA.5. Safety and reactogenicity were similar for both vaccine groups.
“By the end of this year, we expect to also have clinical trial data from our BA.4/BA.5 bivalent booster,” Dr. Chalkias said.
In the interim, the U.S. Food and Drug Administration recently granted emergency use authorization for Moderna’s BA.4/BA.5 Omicron-targeting bivalent COVID-19 booster vaccine in children and adolescents aged 6-17 years.
Pfizer/BioNTech also has recently issued an announcement that their COVID-19 booster, adapted for the BA.4 and the BA.5 Omicron subvariants, generated a strong immune response and was well tolerated in human tests.
Pfizer/BioNTech said data from roughly 80 adult patients showed that the booster led to a substantial increase in neutralizing antibody levels against the BA.4/BA.5 variants after 1 week.
Separate study of causes of severe breakthrough infections in early vaccine formulations
Though COVID vaccines reduce the incidence of severe outcomes, there are reports of breakthrough infections in persons who received the original vaccines, and some of these have been serious.
In a separate study, also presented at the meeting, researchers led by first author Austin D. Vo, BS, with the VA Boston Healthcare System, used data collected from Dec. 15, 2020, through Feb. 28, 2022, in a U.S. veteran population to assess those at highest risk for severe disease despite vaccination.
Results of the large, nationwide retrospective study were simultaneously published in JAMA Network Open.
The primary outcome was development of severe COVID, defined as a hospitalization within 14 days of a confirmed positive SARS-CoV-2 test, receipt of supplemental oxygen, mechanical ventilation, or death within 28 days.
Among 110,760 participants with severe disease after primary vaccination, 13% (14,690) were hospitalized with severe COVID-19 or died.
The strongest risk factor for severe disease despite vaccination was age, the researchers found.
Presenting author Westyn Branch-Elliman, MD, associate professor of medicine with VA Boston Healthcare System, said, “We found that age greater than 50 was associated with an adjusted odds ratio of 1.42 for every 5-year increase.”
To put that in perspective, she said, “compared to patients who are 45 to 50, those over 80 had an adjusted odds ratio of 16 for hospitalization or death following breakthrough infection.”
Priya Nori, MD, an infectious disease specialist at Montefiore Medical Center in New York, said in an interview that the evidence that age is a strong risk factor for severe disease – even after vaccination – confirms that attention should be focused on those in the highest age groups, particularly those 80 years and older.
Other top risk factors included having immunocompromising conditions; having received cytotoxic chemotherapy within 6 months (adjusted odds ratio, 2.69; 95% confidence interval, 2.25-3.21); having leukemias/lymphomas (aOR, 1.84; 95% CI, 1.59-2.14); and having chronic conditions associated with end-organ disease.
“We also found that receipt of an additional booster dose of vaccine was associated with a 50% reduction in adjusted odds of severe disease,” noted Dr. Branch-Elliman.
Dr. Nori emphasized that, given these data, emphatic messaging is needed to encourage uptake of the updated Omicron-targeted vaccines for these high-risk age groups.
The study by Dr. Chalkias and colleagues was funded by Moderna. Dr. Chalkias and several coauthors are employed by Moderna. One coauthor has relationships with DLA Piper/Medtronic, and Gilead Pharmaceuticals, and one has relationships with Celgene/Bristol-Myers Squibb, ChemoCentryx, Gilead, and Kiniksa. Dr. Nori has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT IDWEEK 2022
25 years of chickenpox vaccine: 91 million cases prevented
WASHINGTON – In the 25 years since the United States first launched its universal vaccinations program to protect children against chickenpox (varicella), the program has seen dramatic results, a data analysis indicates.
Results from 1995 – when universal vaccinations began – through 2019 were presented an annual scientific meeting on infectious diseases by Mona Marin, MD, a medical epidemiologist at the Centers for Disease Control and Prevention. Researchers analyzed published data and surveillance data reported to the CDC.
Deaths in under-20 group all but eliminated
Immunocompromised people or pregnant women and infants too young to be vaccinated also benefited from the children’s immunizations.
Each year, about 3.8 million cases, 10,500 hospitalizations, and 100 deaths from chickenpox are prevented in the United States thanks to the vaccination program, Dr. Marin said.
Over 25 years, 91 million cases, 238,000 hospitalizations, and 1,933 – 2,446 deaths have been prevented.
However, chickenpox is still widespread in most of the world.
U.S. first with universal program
The disease was thought to be of little consequence, Dr. Marin said, until the mid-1950s after the first cases of fatal varicella in immunocompromised children revealed the virus’ lethal potential.
The United States was the first country to introduce a universal vaccination program, Dr. Marin said. At the time, it was a one-dose vaccine. Within the first 10 years of the one-dose program, declines in chickenpox cases, hospitalization, and death rates went from 71% to 90% in comparison with previous years. But health care leaders wanted to close the remaining gap and target transmission in schools.
“It was a burden the United States considered unacceptable,” Dr. Marin said.
The leaders had seen the control of measles and polio and wanted the same for chickenpox.
Two-dose vaccines started in 2007
In 2007, the current two-dose policy was introduced. Administration of the first dose is recommended at age 12–15 months, and the second at age 4–6 years. Vaccination is required before the children enter kindergarten.
Coverage was high – at least 90% – the study authors reported; the two-dose program further reduced the number, size, and duration of outbreaks. Over the 25 years, the proportion of outbreaks with fewer than 10 cases increased from 28% to 73%.
By 2019, incidence had dropped by 97%, hospitalizations were down by 94%, and deaths had dropped by 97%.
The biggest decline was seen in those younger than 20, who were born during the vaccination program. That group saw declines of 97% to 99% in cases, hospitalizations, and incidence compared with rates before vaccinations.
Dr. Marin says one dose of the vaccine is moderately effective in preventing all varicella (82%) and is highly effective in preventing severe varicella (more than 97%).
“The second dose adds 10% or more improved protection against all varicella,” she said.
But there have been gains beyond medical advances.
Researchers calculated the economic benefit and found a net savings of $23 billion in medical costs (which also factored in lost wages from parents staying home to care for sick children).
Jaw-dropping results
Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, said in an interview that “as someone who is not a vaccinologist, the declines in deaths, let alone hospitalizations, were jaw-dropping. I hadn’t really seen a synthesis of the impact of one and two doses.”
She said the declines in zoster among young people were interesting. The big question, she said, is what impact this may have for shingles infections in middle-aged adults over time, since chickenpox and shingles are caused by the same virus.
Dr. Marrazzo also noted the economic savings calculations.
“It’s such a cheap intervention. It’s one of the best examples of how a simple vaccine can affect a cascade of events that are a result of chronic viral infection,” she said.
There are also messages for the current debates over COVID-19 vaccinations.
“For me, it is further evidence of the profound population-level effect safe vaccines can have,” Dr. Marrazzo said.
The authors and Dr. Marrazzo report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON – In the 25 years since the United States first launched its universal vaccinations program to protect children against chickenpox (varicella), the program has seen dramatic results, a data analysis indicates.
Results from 1995 – when universal vaccinations began – through 2019 were presented an annual scientific meeting on infectious diseases by Mona Marin, MD, a medical epidemiologist at the Centers for Disease Control and Prevention. Researchers analyzed published data and surveillance data reported to the CDC.
Deaths in under-20 group all but eliminated
Immunocompromised people or pregnant women and infants too young to be vaccinated also benefited from the children’s immunizations.
Each year, about 3.8 million cases, 10,500 hospitalizations, and 100 deaths from chickenpox are prevented in the United States thanks to the vaccination program, Dr. Marin said.
Over 25 years, 91 million cases, 238,000 hospitalizations, and 1,933 – 2,446 deaths have been prevented.
However, chickenpox is still widespread in most of the world.
U.S. first with universal program
The disease was thought to be of little consequence, Dr. Marin said, until the mid-1950s after the first cases of fatal varicella in immunocompromised children revealed the virus’ lethal potential.
The United States was the first country to introduce a universal vaccination program, Dr. Marin said. At the time, it was a one-dose vaccine. Within the first 10 years of the one-dose program, declines in chickenpox cases, hospitalization, and death rates went from 71% to 90% in comparison with previous years. But health care leaders wanted to close the remaining gap and target transmission in schools.
“It was a burden the United States considered unacceptable,” Dr. Marin said.
The leaders had seen the control of measles and polio and wanted the same for chickenpox.
Two-dose vaccines started in 2007
In 2007, the current two-dose policy was introduced. Administration of the first dose is recommended at age 12–15 months, and the second at age 4–6 years. Vaccination is required before the children enter kindergarten.
Coverage was high – at least 90% – the study authors reported; the two-dose program further reduced the number, size, and duration of outbreaks. Over the 25 years, the proportion of outbreaks with fewer than 10 cases increased from 28% to 73%.
By 2019, incidence had dropped by 97%, hospitalizations were down by 94%, and deaths had dropped by 97%.
The biggest decline was seen in those younger than 20, who were born during the vaccination program. That group saw declines of 97% to 99% in cases, hospitalizations, and incidence compared with rates before vaccinations.
Dr. Marin says one dose of the vaccine is moderately effective in preventing all varicella (82%) and is highly effective in preventing severe varicella (more than 97%).
“The second dose adds 10% or more improved protection against all varicella,” she said.
But there have been gains beyond medical advances.
Researchers calculated the economic benefit and found a net savings of $23 billion in medical costs (which also factored in lost wages from parents staying home to care for sick children).
Jaw-dropping results
Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, said in an interview that “as someone who is not a vaccinologist, the declines in deaths, let alone hospitalizations, were jaw-dropping. I hadn’t really seen a synthesis of the impact of one and two doses.”
She said the declines in zoster among young people were interesting. The big question, she said, is what impact this may have for shingles infections in middle-aged adults over time, since chickenpox and shingles are caused by the same virus.
Dr. Marrazzo also noted the economic savings calculations.
“It’s such a cheap intervention. It’s one of the best examples of how a simple vaccine can affect a cascade of events that are a result of chronic viral infection,” she said.
There are also messages for the current debates over COVID-19 vaccinations.
“For me, it is further evidence of the profound population-level effect safe vaccines can have,” Dr. Marrazzo said.
The authors and Dr. Marrazzo report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON – In the 25 years since the United States first launched its universal vaccinations program to protect children against chickenpox (varicella), the program has seen dramatic results, a data analysis indicates.
Results from 1995 – when universal vaccinations began – through 2019 were presented an annual scientific meeting on infectious diseases by Mona Marin, MD, a medical epidemiologist at the Centers for Disease Control and Prevention. Researchers analyzed published data and surveillance data reported to the CDC.
Deaths in under-20 group all but eliminated
Immunocompromised people or pregnant women and infants too young to be vaccinated also benefited from the children’s immunizations.
Each year, about 3.8 million cases, 10,500 hospitalizations, and 100 deaths from chickenpox are prevented in the United States thanks to the vaccination program, Dr. Marin said.
Over 25 years, 91 million cases, 238,000 hospitalizations, and 1,933 – 2,446 deaths have been prevented.
However, chickenpox is still widespread in most of the world.
U.S. first with universal program
The disease was thought to be of little consequence, Dr. Marin said, until the mid-1950s after the first cases of fatal varicella in immunocompromised children revealed the virus’ lethal potential.
The United States was the first country to introduce a universal vaccination program, Dr. Marin said. At the time, it was a one-dose vaccine. Within the first 10 years of the one-dose program, declines in chickenpox cases, hospitalization, and death rates went from 71% to 90% in comparison with previous years. But health care leaders wanted to close the remaining gap and target transmission in schools.
“It was a burden the United States considered unacceptable,” Dr. Marin said.
The leaders had seen the control of measles and polio and wanted the same for chickenpox.
Two-dose vaccines started in 2007
In 2007, the current two-dose policy was introduced. Administration of the first dose is recommended at age 12–15 months, and the second at age 4–6 years. Vaccination is required before the children enter kindergarten.
Coverage was high – at least 90% – the study authors reported; the two-dose program further reduced the number, size, and duration of outbreaks. Over the 25 years, the proportion of outbreaks with fewer than 10 cases increased from 28% to 73%.
By 2019, incidence had dropped by 97%, hospitalizations were down by 94%, and deaths had dropped by 97%.
The biggest decline was seen in those younger than 20, who were born during the vaccination program. That group saw declines of 97% to 99% in cases, hospitalizations, and incidence compared with rates before vaccinations.
Dr. Marin says one dose of the vaccine is moderately effective in preventing all varicella (82%) and is highly effective in preventing severe varicella (more than 97%).
“The second dose adds 10% or more improved protection against all varicella,” she said.
But there have been gains beyond medical advances.
Researchers calculated the economic benefit and found a net savings of $23 billion in medical costs (which also factored in lost wages from parents staying home to care for sick children).
Jaw-dropping results
Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, said in an interview that “as someone who is not a vaccinologist, the declines in deaths, let alone hospitalizations, were jaw-dropping. I hadn’t really seen a synthesis of the impact of one and two doses.”
She said the declines in zoster among young people were interesting. The big question, she said, is what impact this may have for shingles infections in middle-aged adults over time, since chickenpox and shingles are caused by the same virus.
Dr. Marrazzo also noted the economic savings calculations.
“It’s such a cheap intervention. It’s one of the best examples of how a simple vaccine can affect a cascade of events that are a result of chronic viral infection,” she said.
There are also messages for the current debates over COVID-19 vaccinations.
“For me, it is further evidence of the profound population-level effect safe vaccines can have,” Dr. Marrazzo said.
The authors and Dr. Marrazzo report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT IDWEEK 2022