User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Updated heart failure measures add newer meds
Safety measures for lab monitoring of mineralocorticoid receptor agonist therapy, performance measures for sacubitril/valsartan, cardiac resynchronization therapy and titration of medications, and quality measures based on patient-reported outcomes are among the updates the joint task force of the American College of Cardiology and the American Heart Association have made to performance and quality measures for managing adults with heart failure.
The revisions, published online Nov. 2 in the Journal of the American College of Cardiology, update the 2011 ACC/AHA heart failure measure set, writing committee vice chair Gregg C. Fonarow, MD, said in an interview. The 2011 measure set predates the 2015 approval of the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan for heart failure in adults.
Measures stress dosages, strength of evidence
“For the first time the heart failure performance measure sets also focus on not just the use of guideline-recommended medication at any dose, but on utilizing the doses that are evidence-based and guideline recommended so long as they are well tolerated,” said Dr. Fonarow, interim chief of cardiology at the University of California, Los Angeles. “The measure set now includes assessment of patients being treated with doses of medications at 50% or greater of target dose in the absence of contraindications or documented intolerance.”
The update includes seven new performance measures, two quality measures, and one structural measure. The performance measures come from the strongest recommendations – that is, a class of recommendation of 1 (strong) or 3 (no benefit or harmful, process to be avoided) – in the 2017 ACC/AHA/Heart Failure Society of American heart failure guideline update published in Circulation.
In addition to the 2017 update, the writing committee also reviewed existing performance measures. “Those management strategies, diagnostic testing, medications, and devices with the strongest evidence and highest level of guideline recommendations were further considered for inclusion in the performance measure set,” Dr. Fonarow said. “The measures went through extensive review by peer reviewers and approval from the organizations represented.”
Specifically, the update includes measures for monitoring serum potassium after starting mineralocorticoid receptor antagonists therapy, and cardiac resynchronization therapy for patients with heart failure with reduced ejection fraction already on guideline-directed therapy. “This therapy can significantly improve functional capacity and outcomes in appropriately selected patients,” Dr. Fonarow said.
New and retired measures
The update adds two performance measures for titration of medications based on dose, either reaching 50% of the recommended dose for a variety of medications, including ARNI, or documenting that the dose wasn’t tolerated for other reason for not using the dose.
The new structural measure calls for facility participation in a heart failure registry. The revised measure set now consists of 18 measures in all.
The update retired one measure from the 2011 set: left ventricular ejection fraction assessment for inpatients. The committee cited its use above 97% as the reason, but LVEF in outpatients remains a measure.
The following tree measures have been revised:
- Patient self-care education has moved from performance measure to quality measure because of concerns about the accuracy of self-care education documentation and limited evidence of improved outcomes with better documentation.
- ACE inhibitor or angiotensin receptor blocker therapy for left ventricular systolic dysfunction adds ARNI therapy to align with the 2017 ACC/AHA/HFSA update.
- Postdischarge appointments shifts from performance to quality measure and include a 7-day limit.
Measures future research should focus on, noted Dr. Fonarow, include the use of sodium glucose cotransporter 2 (SGLT2) inhibitors for heart failure, including in patients without diabetes. “Since the ACC/AHA heart failure guidelines had not yet been updated to recommend these therapies they could not be included in this performance measure set,” he said.
He also said “an urgent need” exists for further research into treatments for heart failure with preserved ejection fraction along with optimal implementation strategies.
“If these ACC/AHA heart failure performance measures were applied in all settings in which patients with heart failure in the United States are being cared for, and optimal and equitable conformity with each of these measures were achieved, over 100,000 lives a year of patients with heart failure could be saved,” he said. “There’s in an urgent need to measure and improve heart failure care quality.”
Dr. Fonarow reported financial relationships with Abbott, Amgen, AstraZeneca, CHF Solutions, Janssen, Medtronic, Merck, and Novartis.
SOURCE: American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2020 Nov 2;76:2527-64.
Safety measures for lab monitoring of mineralocorticoid receptor agonist therapy, performance measures for sacubitril/valsartan, cardiac resynchronization therapy and titration of medications, and quality measures based on patient-reported outcomes are among the updates the joint task force of the American College of Cardiology and the American Heart Association have made to performance and quality measures for managing adults with heart failure.
The revisions, published online Nov. 2 in the Journal of the American College of Cardiology, update the 2011 ACC/AHA heart failure measure set, writing committee vice chair Gregg C. Fonarow, MD, said in an interview. The 2011 measure set predates the 2015 approval of the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan for heart failure in adults.
Measures stress dosages, strength of evidence
“For the first time the heart failure performance measure sets also focus on not just the use of guideline-recommended medication at any dose, but on utilizing the doses that are evidence-based and guideline recommended so long as they are well tolerated,” said Dr. Fonarow, interim chief of cardiology at the University of California, Los Angeles. “The measure set now includes assessment of patients being treated with doses of medications at 50% or greater of target dose in the absence of contraindications or documented intolerance.”
The update includes seven new performance measures, two quality measures, and one structural measure. The performance measures come from the strongest recommendations – that is, a class of recommendation of 1 (strong) or 3 (no benefit or harmful, process to be avoided) – in the 2017 ACC/AHA/Heart Failure Society of American heart failure guideline update published in Circulation.
In addition to the 2017 update, the writing committee also reviewed existing performance measures. “Those management strategies, diagnostic testing, medications, and devices with the strongest evidence and highest level of guideline recommendations were further considered for inclusion in the performance measure set,” Dr. Fonarow said. “The measures went through extensive review by peer reviewers and approval from the organizations represented.”
Specifically, the update includes measures for monitoring serum potassium after starting mineralocorticoid receptor antagonists therapy, and cardiac resynchronization therapy for patients with heart failure with reduced ejection fraction already on guideline-directed therapy. “This therapy can significantly improve functional capacity and outcomes in appropriately selected patients,” Dr. Fonarow said.
New and retired measures
The update adds two performance measures for titration of medications based on dose, either reaching 50% of the recommended dose for a variety of medications, including ARNI, or documenting that the dose wasn’t tolerated for other reason for not using the dose.
The new structural measure calls for facility participation in a heart failure registry. The revised measure set now consists of 18 measures in all.
The update retired one measure from the 2011 set: left ventricular ejection fraction assessment for inpatients. The committee cited its use above 97% as the reason, but LVEF in outpatients remains a measure.
The following tree measures have been revised:
- Patient self-care education has moved from performance measure to quality measure because of concerns about the accuracy of self-care education documentation and limited evidence of improved outcomes with better documentation.
- ACE inhibitor or angiotensin receptor blocker therapy for left ventricular systolic dysfunction adds ARNI therapy to align with the 2017 ACC/AHA/HFSA update.
- Postdischarge appointments shifts from performance to quality measure and include a 7-day limit.
Measures future research should focus on, noted Dr. Fonarow, include the use of sodium glucose cotransporter 2 (SGLT2) inhibitors for heart failure, including in patients without diabetes. “Since the ACC/AHA heart failure guidelines had not yet been updated to recommend these therapies they could not be included in this performance measure set,” he said.
He also said “an urgent need” exists for further research into treatments for heart failure with preserved ejection fraction along with optimal implementation strategies.
“If these ACC/AHA heart failure performance measures were applied in all settings in which patients with heart failure in the United States are being cared for, and optimal and equitable conformity with each of these measures were achieved, over 100,000 lives a year of patients with heart failure could be saved,” he said. “There’s in an urgent need to measure and improve heart failure care quality.”
Dr. Fonarow reported financial relationships with Abbott, Amgen, AstraZeneca, CHF Solutions, Janssen, Medtronic, Merck, and Novartis.
SOURCE: American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2020 Nov 2;76:2527-64.
Safety measures for lab monitoring of mineralocorticoid receptor agonist therapy, performance measures for sacubitril/valsartan, cardiac resynchronization therapy and titration of medications, and quality measures based on patient-reported outcomes are among the updates the joint task force of the American College of Cardiology and the American Heart Association have made to performance and quality measures for managing adults with heart failure.
The revisions, published online Nov. 2 in the Journal of the American College of Cardiology, update the 2011 ACC/AHA heart failure measure set, writing committee vice chair Gregg C. Fonarow, MD, said in an interview. The 2011 measure set predates the 2015 approval of the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan for heart failure in adults.
Measures stress dosages, strength of evidence
“For the first time the heart failure performance measure sets also focus on not just the use of guideline-recommended medication at any dose, but on utilizing the doses that are evidence-based and guideline recommended so long as they are well tolerated,” said Dr. Fonarow, interim chief of cardiology at the University of California, Los Angeles. “The measure set now includes assessment of patients being treated with doses of medications at 50% or greater of target dose in the absence of contraindications or documented intolerance.”
The update includes seven new performance measures, two quality measures, and one structural measure. The performance measures come from the strongest recommendations – that is, a class of recommendation of 1 (strong) or 3 (no benefit or harmful, process to be avoided) – in the 2017 ACC/AHA/Heart Failure Society of American heart failure guideline update published in Circulation.
In addition to the 2017 update, the writing committee also reviewed existing performance measures. “Those management strategies, diagnostic testing, medications, and devices with the strongest evidence and highest level of guideline recommendations were further considered for inclusion in the performance measure set,” Dr. Fonarow said. “The measures went through extensive review by peer reviewers and approval from the organizations represented.”
Specifically, the update includes measures for monitoring serum potassium after starting mineralocorticoid receptor antagonists therapy, and cardiac resynchronization therapy for patients with heart failure with reduced ejection fraction already on guideline-directed therapy. “This therapy can significantly improve functional capacity and outcomes in appropriately selected patients,” Dr. Fonarow said.
New and retired measures
The update adds two performance measures for titration of medications based on dose, either reaching 50% of the recommended dose for a variety of medications, including ARNI, or documenting that the dose wasn’t tolerated for other reason for not using the dose.
The new structural measure calls for facility participation in a heart failure registry. The revised measure set now consists of 18 measures in all.
The update retired one measure from the 2011 set: left ventricular ejection fraction assessment for inpatients. The committee cited its use above 97% as the reason, but LVEF in outpatients remains a measure.
The following tree measures have been revised:
- Patient self-care education has moved from performance measure to quality measure because of concerns about the accuracy of self-care education documentation and limited evidence of improved outcomes with better documentation.
- ACE inhibitor or angiotensin receptor blocker therapy for left ventricular systolic dysfunction adds ARNI therapy to align with the 2017 ACC/AHA/HFSA update.
- Postdischarge appointments shifts from performance to quality measure and include a 7-day limit.
Measures future research should focus on, noted Dr. Fonarow, include the use of sodium glucose cotransporter 2 (SGLT2) inhibitors for heart failure, including in patients without diabetes. “Since the ACC/AHA heart failure guidelines had not yet been updated to recommend these therapies they could not be included in this performance measure set,” he said.
He also said “an urgent need” exists for further research into treatments for heart failure with preserved ejection fraction along with optimal implementation strategies.
“If these ACC/AHA heart failure performance measures were applied in all settings in which patients with heart failure in the United States are being cared for, and optimal and equitable conformity with each of these measures were achieved, over 100,000 lives a year of patients with heart failure could be saved,” he said. “There’s in an urgent need to measure and improve heart failure care quality.”
Dr. Fonarow reported financial relationships with Abbott, Amgen, AstraZeneca, CHF Solutions, Janssen, Medtronic, Merck, and Novartis.
SOURCE: American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2020 Nov 2;76:2527-64.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Health sector has spent $464 million on lobbying in 2020
, according to the Center for Responsive Politics.
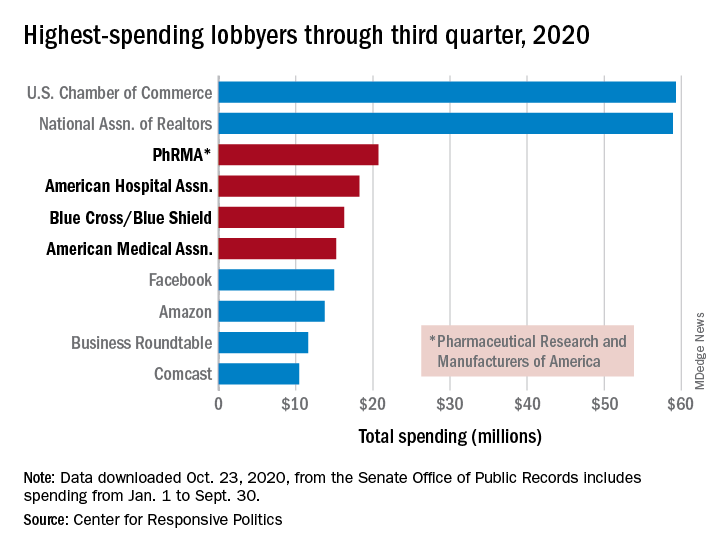
PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
Physician burnout costly to organizations and U.S. health system
Background: Occupational burnout is more prevalent among physicians than among the general population, and physician burnout is associated with several negative clinical outcomes. However, little is known about the economic cost of this widespread issue.
Study design: Cost-consequence analysis using a novel mathematical model.
Setting: Simulated population of U.S. physicians.
Synopsis: Researchers conducted a cost-consequence analysis using a mathematical model designed to determine the financial impact of burnout – or the difference in observed cost and the theoretical cost if physicians did not experience burnout. The model used a hypothetical physician population based on a 2013 profile of U.S. physicians, a 2014 survey of physicians that assessed burnout, and preexisting literature on burnout to generate the input data for their model. The investigators focused on two outcomes: turnover and reduction in clinical hours. They found that approximately $4.6 billion per year is lost in direct cost secondary to physician burnout, with the greatest proportion coming from physician turnover. The figure ranged from $2.6 billion to $6.3 billion in multivariate sensitivity analysis. For an organization, the cost of burnout is about $7,600 per physician per year, with a range of $4,100 to $10,200. Though statistical modeling can be imprecise, and the input data were imperfect, the study was the first to examine the systemwide cost of physician burnout in the United States.
Bottom line: Along with the negative effects on physician and patient well-being, physician burnout is financially costly to the U.S. health care system and to individual organizations. Programs to reduce burnout could be both ethically and economically advantageous.
Citation: Han S et al. Estimating the attributable cost of physician burnout in the United States. Ann Intern Med. 2019;170(11):784-90.
Dr. Suojanen is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: Occupational burnout is more prevalent among physicians than among the general population, and physician burnout is associated with several negative clinical outcomes. However, little is known about the economic cost of this widespread issue.
Study design: Cost-consequence analysis using a novel mathematical model.
Setting: Simulated population of U.S. physicians.
Synopsis: Researchers conducted a cost-consequence analysis using a mathematical model designed to determine the financial impact of burnout – or the difference in observed cost and the theoretical cost if physicians did not experience burnout. The model used a hypothetical physician population based on a 2013 profile of U.S. physicians, a 2014 survey of physicians that assessed burnout, and preexisting literature on burnout to generate the input data for their model. The investigators focused on two outcomes: turnover and reduction in clinical hours. They found that approximately $4.6 billion per year is lost in direct cost secondary to physician burnout, with the greatest proportion coming from physician turnover. The figure ranged from $2.6 billion to $6.3 billion in multivariate sensitivity analysis. For an organization, the cost of burnout is about $7,600 per physician per year, with a range of $4,100 to $10,200. Though statistical modeling can be imprecise, and the input data were imperfect, the study was the first to examine the systemwide cost of physician burnout in the United States.
Bottom line: Along with the negative effects on physician and patient well-being, physician burnout is financially costly to the U.S. health care system and to individual organizations. Programs to reduce burnout could be both ethically and economically advantageous.
Citation: Han S et al. Estimating the attributable cost of physician burnout in the United States. Ann Intern Med. 2019;170(11):784-90.
Dr. Suojanen is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: Occupational burnout is more prevalent among physicians than among the general population, and physician burnout is associated with several negative clinical outcomes. However, little is known about the economic cost of this widespread issue.
Study design: Cost-consequence analysis using a novel mathematical model.
Setting: Simulated population of U.S. physicians.
Synopsis: Researchers conducted a cost-consequence analysis using a mathematical model designed to determine the financial impact of burnout – or the difference in observed cost and the theoretical cost if physicians did not experience burnout. The model used a hypothetical physician population based on a 2013 profile of U.S. physicians, a 2014 survey of physicians that assessed burnout, and preexisting literature on burnout to generate the input data for their model. The investigators focused on two outcomes: turnover and reduction in clinical hours. They found that approximately $4.6 billion per year is lost in direct cost secondary to physician burnout, with the greatest proportion coming from physician turnover. The figure ranged from $2.6 billion to $6.3 billion in multivariate sensitivity analysis. For an organization, the cost of burnout is about $7,600 per physician per year, with a range of $4,100 to $10,200. Though statistical modeling can be imprecise, and the input data were imperfect, the study was the first to examine the systemwide cost of physician burnout in the United States.
Bottom line: Along with the negative effects on physician and patient well-being, physician burnout is financially costly to the U.S. health care system and to individual organizations. Programs to reduce burnout could be both ethically and economically advantageous.
Citation: Han S et al. Estimating the attributable cost of physician burnout in the United States. Ann Intern Med. 2019;170(11):784-90.
Dr. Suojanen is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Med student’s cardiac crisis a COVID-era medical mystery
Within minutes of her arrival at Community North Hospital in Indianapolis, Ramya Yeleti’s vital signs plummeted; her pulse was at 45 beats per minute and her ejection fraction was hovering near 10%. “I definitely thought there was a chance I would close my eyes and never open them again, but I only had a few seconds to process that,” she recalled. Then everything went black. Ramya fell unconscious as shock pads were positioned and a swarm of clinicians prepared to insert an Impella heart pump through a catheter into her aorta.
The third-year medical student and aspiring psychiatrist had been doing in-person neurology rotations in July when she began to experience fever and uncontrolled vomiting. Her initial thought was that she must have caught the flu from a patient.
After all, Ramya, along with her father Ram Yeleti, MD, mother Indira, and twin sister Divya, had all weathered COVID-19 in previous months and later tested positive for SARS-CoV-2 antibodies. The only family member who had been spared was her younger brother Rohith.
Indira suffered a severe case, requiring ICU care for 2 days but no ventilator; the others experienced mostly mild symptoms. Ramya — who was studying for her third-year board exams after classes at Marian University College of Osteopathic Medicine in Indianapolis went virtual in March — was left with lingering fatigue; however, her cough and muscle aches abated and her sense of taste and smell returned. When she started rotations, she thought her life was getting back to normal.
Ramya’s flu symptoms did not improve. A university-mandated rapid COVID test came back negative, but 2 more days of vomiting started to worry both her and her father, who is a cardiologist and chief physician executive at Community Health Network in Indianapolis. After Ramya felt some chest pain, she asked her father to listen to her heart. All sounded normal, and Ram prescribed ondansetron for her nausea.
But the antiemetic didn’t work, and by the next morning both father and daughter were convinced that they needed to head to the emergency department.
“I wanted to double-check if I was missing something about her being dehydrated,” Ram told Medscape Medical News. “Several things can cause protracted nausea, like hepatitis, appendicitis, or another infection. I feel terribly guilty I didn’t realize she had a heart condition.”
A surprising turn for the worst
Ramya’s subtle symptoms quickly gave way to the dramatic cardiac crisis that unfolded just after her arrival at Community North. “Her EKG looked absolutely horrendous, like a 75-year-old having a heart attack,” Ram said.
As a cardiologist, he knew his daughter’s situation was growing dire when he heard physicians shouting that the Impella wasn’t working and she needed extracorporeal membrane oxygenation (ECMO).
“At that point, I didn’t think she’d survive,” her father recalled. “We had 10 physicians in the room who worked on her for 5 hours to get her stabilized.”
“It was especially traumatic because, obviously, I knew exactly what was happening,” he added. “You can’t sugarcoat anything.”
After being connected to the heart–lung equipment, Ramya was transferred to IU Health Methodist Hospital, also in Indianapolis, where she was tested again for COVID-19. Unlike the rapid test administered just days earlier, the PCR assay came back positive.
“I knew she had acute myocarditis, but coronavirus never crossed my mind,” said Ram.
“As we were dealing with her heart, we were also dealing with this challenge: she was coming back positive for COVID-19 again,” said Roopa Rao, MD, the heart failure transplant cardiologist at IU Health who treated Ramya.
“We weren’t sure whether we were dealing with an active infection or dead virus” from her previous infection, Rao said, “so we started treating her like she had active COVID-19 and gave her remdesivir, convalescent plasma, and steroids, which was the protocol in our hospital.”
A biopsy of Ramya’s heart tissue, along with blood tests, indicated a past parvovirus infection. It’s possible that Ramya’s previous coronavirus infection made her susceptible to heart damage from a newer parvovirus infection, said Rao. Either virus, or both together, could have been responsible for the calamity.
Although it was unheard of during Ramya’s cardiac crisis in early August, evolving evidence now raises the possibility that she is one of a handful of people in the world to be reinfected with SARS-CoV-2. Also emerging are cases of COVID-related myocarditis and other extreme heart complications, particularly in young people.
“At the time, it wasn’t really clear if people could have another infection so quickly,” Rao told Medscape Medical News. “It is possible she is one of these rare individuals to have COVID-19 twice. I’m hoping at some point we will have some clarity.”
“I would favor a coinfection as probably the triggering factor for her sickness,” she said. “It may take some time, but like any other disease — and it doesn’t look like COVID will go away magically — I hope we’ll have some answers down the road.”
Another wrinkle
The next 48 hours brought astonishing news: Ramya’s heart function had rebounded to nearly normal, and her ejection fraction increased to about 45%. Heart transplantation wouldn’t be necessary, although Rao stood poised to follow through if ECMO only sustained, rather than improved, Ramya’s prognosis.
“Ramya was so sick that if she didn’t recover, the only option would be a heart transplant,” said Rao. “But we wanted to do everything to keep that heart.”
After steroid and COVID treatment, Ramya’s heart started to come back. “It didn’t make sense to me,” said Rao. “I don’t know what helped. If we hadn’t done ECMO, her heart probably wouldn’t have recovered, so I would say we have to support these patients and give them time for the heart to recover, even to the point of ECMO.”
Despite the good news, Ramya’s survival still hung in the balance. When she was disconnected from ECMO, clinicians discovered that the Impella device had caused a rare complication, damaging her mitral valve. The valve could be repaired surgically, but both Rao and Ram felt great trepidation at the prospect of cardiopulmonary bypass during the open-heart procedure.
“They would need to stop her heart and restart it, and I was concerned it would not restart,” Ram explained. “I didn’t like the idea of open-heart surgery, but my biggest fear was she was not going to survive it because of a really fresh, sick heart.”
The cardiologists’ fears did, in fact, come to pass: it took an hour to coax Ramya’s heart back at the end of surgery. But, just as the surgeon was preparing to reconnect Ramya to ECMO in desperation, “her heart recovered again,” Rao reported.
“Some things you never forget in life,” she said. “I can’t describe how everyone in the OR felt, all taking care of her. I told Ramya, ‘you are a fighter’.”
New strength
Six days would pass before Ramya woke up and learned of the astounding series of events that saved her. She knew “something was really wrong” because of the incision at the center of her chest, but learning she’d been on ECMO and the heart transplant list drove home how close to death she’d actually come.
“Most people don’t get off ECMO; they die on it,” she said. “And the chances of dying on the heart transplant list are very high. It was very strange to me that this was my story all of a sudden, when a week and a half earlier I was on rotation.”
Ongoing physical therapy over the past 3 months has transformed Ramya from a state of profound physical weakness to a place of relative strength. The now-fourth-year med student is turning 26 in November and is hungry to restart in-person rotations. Her downtime has been filled in part with researching myocarditis and collaborating with Rao on her own case study for journal publication.
But the mental trauma from her experience has girded her in ways she knows will make her stronger personally and professionally in the years ahead.
“It’s still very hard. I’m still recovering,” she acknowledged. “I described it to my therapist as an invisible wound on my brain.”
“When I came out of the hospital, I still had ECMO wounds, deep gashes on my legs that affected how fast and how long I could walk,” she said. “I felt like the same thing was going on my brain — a huge cut no one could see.”
Her intention to specialize in psychiatry has become more pressing now that Ramya has realized the impact of trauma on mental health.
“My body failing me was awful, but I could handle it,” she said. “Losing any part of my mind would have been way worse. I want to take care of that in my patients.”
This article first appeared on Medscape.com.
Within minutes of her arrival at Community North Hospital in Indianapolis, Ramya Yeleti’s vital signs plummeted; her pulse was at 45 beats per minute and her ejection fraction was hovering near 10%. “I definitely thought there was a chance I would close my eyes and never open them again, but I only had a few seconds to process that,” she recalled. Then everything went black. Ramya fell unconscious as shock pads were positioned and a swarm of clinicians prepared to insert an Impella heart pump through a catheter into her aorta.
The third-year medical student and aspiring psychiatrist had been doing in-person neurology rotations in July when she began to experience fever and uncontrolled vomiting. Her initial thought was that she must have caught the flu from a patient.
After all, Ramya, along with her father Ram Yeleti, MD, mother Indira, and twin sister Divya, had all weathered COVID-19 in previous months and later tested positive for SARS-CoV-2 antibodies. The only family member who had been spared was her younger brother Rohith.
Indira suffered a severe case, requiring ICU care for 2 days but no ventilator; the others experienced mostly mild symptoms. Ramya — who was studying for her third-year board exams after classes at Marian University College of Osteopathic Medicine in Indianapolis went virtual in March — was left with lingering fatigue; however, her cough and muscle aches abated and her sense of taste and smell returned. When she started rotations, she thought her life was getting back to normal.
Ramya’s flu symptoms did not improve. A university-mandated rapid COVID test came back negative, but 2 more days of vomiting started to worry both her and her father, who is a cardiologist and chief physician executive at Community Health Network in Indianapolis. After Ramya felt some chest pain, she asked her father to listen to her heart. All sounded normal, and Ram prescribed ondansetron for her nausea.
But the antiemetic didn’t work, and by the next morning both father and daughter were convinced that they needed to head to the emergency department.
“I wanted to double-check if I was missing something about her being dehydrated,” Ram told Medscape Medical News. “Several things can cause protracted nausea, like hepatitis, appendicitis, or another infection. I feel terribly guilty I didn’t realize she had a heart condition.”
A surprising turn for the worst
Ramya’s subtle symptoms quickly gave way to the dramatic cardiac crisis that unfolded just after her arrival at Community North. “Her EKG looked absolutely horrendous, like a 75-year-old having a heart attack,” Ram said.
As a cardiologist, he knew his daughter’s situation was growing dire when he heard physicians shouting that the Impella wasn’t working and she needed extracorporeal membrane oxygenation (ECMO).
“At that point, I didn’t think she’d survive,” her father recalled. “We had 10 physicians in the room who worked on her for 5 hours to get her stabilized.”
“It was especially traumatic because, obviously, I knew exactly what was happening,” he added. “You can’t sugarcoat anything.”
After being connected to the heart–lung equipment, Ramya was transferred to IU Health Methodist Hospital, also in Indianapolis, where she was tested again for COVID-19. Unlike the rapid test administered just days earlier, the PCR assay came back positive.
“I knew she had acute myocarditis, but coronavirus never crossed my mind,” said Ram.
“As we were dealing with her heart, we were also dealing with this challenge: she was coming back positive for COVID-19 again,” said Roopa Rao, MD, the heart failure transplant cardiologist at IU Health who treated Ramya.
“We weren’t sure whether we were dealing with an active infection or dead virus” from her previous infection, Rao said, “so we started treating her like she had active COVID-19 and gave her remdesivir, convalescent plasma, and steroids, which was the protocol in our hospital.”
A biopsy of Ramya’s heart tissue, along with blood tests, indicated a past parvovirus infection. It’s possible that Ramya’s previous coronavirus infection made her susceptible to heart damage from a newer parvovirus infection, said Rao. Either virus, or both together, could have been responsible for the calamity.
Although it was unheard of during Ramya’s cardiac crisis in early August, evolving evidence now raises the possibility that she is one of a handful of people in the world to be reinfected with SARS-CoV-2. Also emerging are cases of COVID-related myocarditis and other extreme heart complications, particularly in young people.
“At the time, it wasn’t really clear if people could have another infection so quickly,” Rao told Medscape Medical News. “It is possible she is one of these rare individuals to have COVID-19 twice. I’m hoping at some point we will have some clarity.”
“I would favor a coinfection as probably the triggering factor for her sickness,” she said. “It may take some time, but like any other disease — and it doesn’t look like COVID will go away magically — I hope we’ll have some answers down the road.”
Another wrinkle
The next 48 hours brought astonishing news: Ramya’s heart function had rebounded to nearly normal, and her ejection fraction increased to about 45%. Heart transplantation wouldn’t be necessary, although Rao stood poised to follow through if ECMO only sustained, rather than improved, Ramya’s prognosis.
“Ramya was so sick that if she didn’t recover, the only option would be a heart transplant,” said Rao. “But we wanted to do everything to keep that heart.”
After steroid and COVID treatment, Ramya’s heart started to come back. “It didn’t make sense to me,” said Rao. “I don’t know what helped. If we hadn’t done ECMO, her heart probably wouldn’t have recovered, so I would say we have to support these patients and give them time for the heart to recover, even to the point of ECMO.”
Despite the good news, Ramya’s survival still hung in the balance. When she was disconnected from ECMO, clinicians discovered that the Impella device had caused a rare complication, damaging her mitral valve. The valve could be repaired surgically, but both Rao and Ram felt great trepidation at the prospect of cardiopulmonary bypass during the open-heart procedure.
“They would need to stop her heart and restart it, and I was concerned it would not restart,” Ram explained. “I didn’t like the idea of open-heart surgery, but my biggest fear was she was not going to survive it because of a really fresh, sick heart.”
The cardiologists’ fears did, in fact, come to pass: it took an hour to coax Ramya’s heart back at the end of surgery. But, just as the surgeon was preparing to reconnect Ramya to ECMO in desperation, “her heart recovered again,” Rao reported.
“Some things you never forget in life,” she said. “I can’t describe how everyone in the OR felt, all taking care of her. I told Ramya, ‘you are a fighter’.”
New strength
Six days would pass before Ramya woke up and learned of the astounding series of events that saved her. She knew “something was really wrong” because of the incision at the center of her chest, but learning she’d been on ECMO and the heart transplant list drove home how close to death she’d actually come.
“Most people don’t get off ECMO; they die on it,” she said. “And the chances of dying on the heart transplant list are very high. It was very strange to me that this was my story all of a sudden, when a week and a half earlier I was on rotation.”
Ongoing physical therapy over the past 3 months has transformed Ramya from a state of profound physical weakness to a place of relative strength. The now-fourth-year med student is turning 26 in November and is hungry to restart in-person rotations. Her downtime has been filled in part with researching myocarditis and collaborating with Rao on her own case study for journal publication.
But the mental trauma from her experience has girded her in ways she knows will make her stronger personally and professionally in the years ahead.
“It’s still very hard. I’m still recovering,” she acknowledged. “I described it to my therapist as an invisible wound on my brain.”
“When I came out of the hospital, I still had ECMO wounds, deep gashes on my legs that affected how fast and how long I could walk,” she said. “I felt like the same thing was going on my brain — a huge cut no one could see.”
Her intention to specialize in psychiatry has become more pressing now that Ramya has realized the impact of trauma on mental health.
“My body failing me was awful, but I could handle it,” she said. “Losing any part of my mind would have been way worse. I want to take care of that in my patients.”
This article first appeared on Medscape.com.
Within minutes of her arrival at Community North Hospital in Indianapolis, Ramya Yeleti’s vital signs plummeted; her pulse was at 45 beats per minute and her ejection fraction was hovering near 10%. “I definitely thought there was a chance I would close my eyes and never open them again, but I only had a few seconds to process that,” she recalled. Then everything went black. Ramya fell unconscious as shock pads were positioned and a swarm of clinicians prepared to insert an Impella heart pump through a catheter into her aorta.
The third-year medical student and aspiring psychiatrist had been doing in-person neurology rotations in July when she began to experience fever and uncontrolled vomiting. Her initial thought was that she must have caught the flu from a patient.
After all, Ramya, along with her father Ram Yeleti, MD, mother Indira, and twin sister Divya, had all weathered COVID-19 in previous months and later tested positive for SARS-CoV-2 antibodies. The only family member who had been spared was her younger brother Rohith.
Indira suffered a severe case, requiring ICU care for 2 days but no ventilator; the others experienced mostly mild symptoms. Ramya — who was studying for her third-year board exams after classes at Marian University College of Osteopathic Medicine in Indianapolis went virtual in March — was left with lingering fatigue; however, her cough and muscle aches abated and her sense of taste and smell returned. When she started rotations, she thought her life was getting back to normal.
Ramya’s flu symptoms did not improve. A university-mandated rapid COVID test came back negative, but 2 more days of vomiting started to worry both her and her father, who is a cardiologist and chief physician executive at Community Health Network in Indianapolis. After Ramya felt some chest pain, she asked her father to listen to her heart. All sounded normal, and Ram prescribed ondansetron for her nausea.
But the antiemetic didn’t work, and by the next morning both father and daughter were convinced that they needed to head to the emergency department.
“I wanted to double-check if I was missing something about her being dehydrated,” Ram told Medscape Medical News. “Several things can cause protracted nausea, like hepatitis, appendicitis, or another infection. I feel terribly guilty I didn’t realize she had a heart condition.”
A surprising turn for the worst
Ramya’s subtle symptoms quickly gave way to the dramatic cardiac crisis that unfolded just after her arrival at Community North. “Her EKG looked absolutely horrendous, like a 75-year-old having a heart attack,” Ram said.
As a cardiologist, he knew his daughter’s situation was growing dire when he heard physicians shouting that the Impella wasn’t working and she needed extracorporeal membrane oxygenation (ECMO).
“At that point, I didn’t think she’d survive,” her father recalled. “We had 10 physicians in the room who worked on her for 5 hours to get her stabilized.”
“It was especially traumatic because, obviously, I knew exactly what was happening,” he added. “You can’t sugarcoat anything.”
After being connected to the heart–lung equipment, Ramya was transferred to IU Health Methodist Hospital, also in Indianapolis, where she was tested again for COVID-19. Unlike the rapid test administered just days earlier, the PCR assay came back positive.
“I knew she had acute myocarditis, but coronavirus never crossed my mind,” said Ram.
“As we were dealing with her heart, we were also dealing with this challenge: she was coming back positive for COVID-19 again,” said Roopa Rao, MD, the heart failure transplant cardiologist at IU Health who treated Ramya.
“We weren’t sure whether we were dealing with an active infection or dead virus” from her previous infection, Rao said, “so we started treating her like she had active COVID-19 and gave her remdesivir, convalescent plasma, and steroids, which was the protocol in our hospital.”
A biopsy of Ramya’s heart tissue, along with blood tests, indicated a past parvovirus infection. It’s possible that Ramya’s previous coronavirus infection made her susceptible to heart damage from a newer parvovirus infection, said Rao. Either virus, or both together, could have been responsible for the calamity.
Although it was unheard of during Ramya’s cardiac crisis in early August, evolving evidence now raises the possibility that she is one of a handful of people in the world to be reinfected with SARS-CoV-2. Also emerging are cases of COVID-related myocarditis and other extreme heart complications, particularly in young people.
“At the time, it wasn’t really clear if people could have another infection so quickly,” Rao told Medscape Medical News. “It is possible she is one of these rare individuals to have COVID-19 twice. I’m hoping at some point we will have some clarity.”
“I would favor a coinfection as probably the triggering factor for her sickness,” she said. “It may take some time, but like any other disease — and it doesn’t look like COVID will go away magically — I hope we’ll have some answers down the road.”
Another wrinkle
The next 48 hours brought astonishing news: Ramya’s heart function had rebounded to nearly normal, and her ejection fraction increased to about 45%. Heart transplantation wouldn’t be necessary, although Rao stood poised to follow through if ECMO only sustained, rather than improved, Ramya’s prognosis.
“Ramya was so sick that if she didn’t recover, the only option would be a heart transplant,” said Rao. “But we wanted to do everything to keep that heart.”
After steroid and COVID treatment, Ramya’s heart started to come back. “It didn’t make sense to me,” said Rao. “I don’t know what helped. If we hadn’t done ECMO, her heart probably wouldn’t have recovered, so I would say we have to support these patients and give them time for the heart to recover, even to the point of ECMO.”
Despite the good news, Ramya’s survival still hung in the balance. When she was disconnected from ECMO, clinicians discovered that the Impella device had caused a rare complication, damaging her mitral valve. The valve could be repaired surgically, but both Rao and Ram felt great trepidation at the prospect of cardiopulmonary bypass during the open-heart procedure.
“They would need to stop her heart and restart it, and I was concerned it would not restart,” Ram explained. “I didn’t like the idea of open-heart surgery, but my biggest fear was she was not going to survive it because of a really fresh, sick heart.”
The cardiologists’ fears did, in fact, come to pass: it took an hour to coax Ramya’s heart back at the end of surgery. But, just as the surgeon was preparing to reconnect Ramya to ECMO in desperation, “her heart recovered again,” Rao reported.
“Some things you never forget in life,” she said. “I can’t describe how everyone in the OR felt, all taking care of her. I told Ramya, ‘you are a fighter’.”
New strength
Six days would pass before Ramya woke up and learned of the astounding series of events that saved her. She knew “something was really wrong” because of the incision at the center of her chest, but learning she’d been on ECMO and the heart transplant list drove home how close to death she’d actually come.
“Most people don’t get off ECMO; they die on it,” she said. “And the chances of dying on the heart transplant list are very high. It was very strange to me that this was my story all of a sudden, when a week and a half earlier I was on rotation.”
Ongoing physical therapy over the past 3 months has transformed Ramya from a state of profound physical weakness to a place of relative strength. The now-fourth-year med student is turning 26 in November and is hungry to restart in-person rotations. Her downtime has been filled in part with researching myocarditis and collaborating with Rao on her own case study for journal publication.
But the mental trauma from her experience has girded her in ways she knows will make her stronger personally and professionally in the years ahead.
“It’s still very hard. I’m still recovering,” she acknowledged. “I described it to my therapist as an invisible wound on my brain.”
“When I came out of the hospital, I still had ECMO wounds, deep gashes on my legs that affected how fast and how long I could walk,” she said. “I felt like the same thing was going on my brain — a huge cut no one could see.”
Her intention to specialize in psychiatry has become more pressing now that Ramya has realized the impact of trauma on mental health.
“My body failing me was awful, but I could handle it,” she said. “Losing any part of my mind would have been way worse. I want to take care of that in my patients.”
This article first appeared on Medscape.com.
About 17% of COVID-19 survivors retest positive in follow-up study
For reasons unknown, about one in six people who recovered from COVID-19 subsequently retested positive at least 2 weeks later, researchers reported in a study in Italy.
Sore throat and rhinitis were the only symptoms associated with a positive result. “Patients who continued to have respiratory symptoms, especially, were more likely to have a new positive test result,” lead author Francesco Landi, MD, PhD, said in an interview.
“This suggests the persistence of respiratory symptoms should not be underestimated and should be adequately assessed in all patients considered recovered from COVID-19,” he said.
“The study results are interesting,” Akiko Iwasaki, PhD, an immunobiologist at Yale University and the Howard Hughes Medical Institute, both in New Haven, Conn.,, said in an interview. “There are other reports of RNA detection postdischarge, but this study ... found that only two symptoms out of many – sore throat and rhinitis – were higher in those with PCR [polymerase chain reaction]-positive status.”
The study was published online Sept. 18 in the American Journal of Preventive Medicine.
The findings could carry important implications for people who continue to be symptomatic. “It is reasonable to be cautious and avoid close contact with others, wear a face mask, and possibly undergo an additional nasopharyngeal swab,” said Dr. Landi, associate professor of internal medicine at Catholic University of the Sacred Heart in Rome.
“One of most interesting findings is that persistent symptoms do not correlate with PCR positivity, suggesting that symptoms are in many cases not due to ongoing viral replication,” Jonathan Karn, PhD, professor and chair of the department of molecular biology and microbiology at Case Western Reserve University, Cleveland, said in an interview.
“The key technical problem, which they have discussed, is that a viral RNA signal in the PCR assay does not necessarily mean that infectious virus is present,” Dr. Karn said. He added that new comprehensive viral RNA analyses would be needed to answer this question.
Official COVID-19 recovery
To identify risk factors and COVID-19 survivors more likely to retest positive, Dr. Landi and members of the Gemelli Against COVID-19 Post-Acute Care Study Group evaluated 131 people after hospital discharge.
All participants met World Health Organization criteria for release from isolation, including two negative test results at least 24 hours apart, and were studied between April 21 and May 21. Mean age was 56 and 39% were women. Only a slightly higher mean body mass index of 27.6 kg/m2 in the positive group versus 25.9 in the negative group, was significant.
Although 51% of survivors reported fatigue, 44% had dyspnea, and 17% were coughing, the rates did not differ significantly between groups. In contrast, 18% of positive survivors and 4% of negative survivors had a sore throat (P = .04), and 27% versus 12%, respectively, reported rhinitis (P = .05).
People returned for follow-up visits a mean 17 days after the second negative swab test.
Asymptomatic COVID-19 carriers
“These findings indicate that a noteworthy rate of recovered patients with COVID-19 could still be asymptomatic carriers of the virus,” the researchers noted in the paper. “Even in the absence of specific guidelines, the 22 patients who tested positive for COVID-19 again were suggested to quarantine for a second time.”
No family member or close contact of the positive survivors reported SARS-CoV-2 infection. All patients continued to wear masks and observe social distancing recommendations, which makes it “very difficult to affirm whether these patients were really contagious,” the researchers noted.
Next steps
Evaluating all COVID-19 survivors to identify any who retest positive “will be a crucial contribution to a better understanding of both the natural history of COVID-19 as well as the public health implications of viral shedding,” the authors wrote.
One study limitation is that the reverse transcriptase–PCR test reveals genetic sequences specific to COVID-19. “It is important to underline that this is not a viral culture and cannot determine whether the virus is viable and transmissible,” the researchers noted.
“In this respect, we are trying to better understand if the persistence of long-time positive [reverse transcriptase]–PCR test for COVID-19 is really correlated to a potential contagiousness,” they added.
Dr. Landi and colleagues said their findings should be considered preliminary, and larger data samples are warranted to validate the results.
Dr. Landi and Dr. Karn disclosed no relevant financial relationships. Dr. Iwasaki disclosed a research grant from Condair, a 5% or greater equity interest in RIGImmune, and income of $250 or more from PureTec.
A version of this article originally appeared on Medscape.com.
For reasons unknown, about one in six people who recovered from COVID-19 subsequently retested positive at least 2 weeks later, researchers reported in a study in Italy.
Sore throat and rhinitis were the only symptoms associated with a positive result. “Patients who continued to have respiratory symptoms, especially, were more likely to have a new positive test result,” lead author Francesco Landi, MD, PhD, said in an interview.
“This suggests the persistence of respiratory symptoms should not be underestimated and should be adequately assessed in all patients considered recovered from COVID-19,” he said.
“The study results are interesting,” Akiko Iwasaki, PhD, an immunobiologist at Yale University and the Howard Hughes Medical Institute, both in New Haven, Conn.,, said in an interview. “There are other reports of RNA detection postdischarge, but this study ... found that only two symptoms out of many – sore throat and rhinitis – were higher in those with PCR [polymerase chain reaction]-positive status.”
The study was published online Sept. 18 in the American Journal of Preventive Medicine.
The findings could carry important implications for people who continue to be symptomatic. “It is reasonable to be cautious and avoid close contact with others, wear a face mask, and possibly undergo an additional nasopharyngeal swab,” said Dr. Landi, associate professor of internal medicine at Catholic University of the Sacred Heart in Rome.
“One of most interesting findings is that persistent symptoms do not correlate with PCR positivity, suggesting that symptoms are in many cases not due to ongoing viral replication,” Jonathan Karn, PhD, professor and chair of the department of molecular biology and microbiology at Case Western Reserve University, Cleveland, said in an interview.
“The key technical problem, which they have discussed, is that a viral RNA signal in the PCR assay does not necessarily mean that infectious virus is present,” Dr. Karn said. He added that new comprehensive viral RNA analyses would be needed to answer this question.
Official COVID-19 recovery
To identify risk factors and COVID-19 survivors more likely to retest positive, Dr. Landi and members of the Gemelli Against COVID-19 Post-Acute Care Study Group evaluated 131 people after hospital discharge.
All participants met World Health Organization criteria for release from isolation, including two negative test results at least 24 hours apart, and were studied between April 21 and May 21. Mean age was 56 and 39% were women. Only a slightly higher mean body mass index of 27.6 kg/m2 in the positive group versus 25.9 in the negative group, was significant.
Although 51% of survivors reported fatigue, 44% had dyspnea, and 17% were coughing, the rates did not differ significantly between groups. In contrast, 18% of positive survivors and 4% of negative survivors had a sore throat (P = .04), and 27% versus 12%, respectively, reported rhinitis (P = .05).
People returned for follow-up visits a mean 17 days after the second negative swab test.
Asymptomatic COVID-19 carriers
“These findings indicate that a noteworthy rate of recovered patients with COVID-19 could still be asymptomatic carriers of the virus,” the researchers noted in the paper. “Even in the absence of specific guidelines, the 22 patients who tested positive for COVID-19 again were suggested to quarantine for a second time.”
No family member or close contact of the positive survivors reported SARS-CoV-2 infection. All patients continued to wear masks and observe social distancing recommendations, which makes it “very difficult to affirm whether these patients were really contagious,” the researchers noted.
Next steps
Evaluating all COVID-19 survivors to identify any who retest positive “will be a crucial contribution to a better understanding of both the natural history of COVID-19 as well as the public health implications of viral shedding,” the authors wrote.
One study limitation is that the reverse transcriptase–PCR test reveals genetic sequences specific to COVID-19. “It is important to underline that this is not a viral culture and cannot determine whether the virus is viable and transmissible,” the researchers noted.
“In this respect, we are trying to better understand if the persistence of long-time positive [reverse transcriptase]–PCR test for COVID-19 is really correlated to a potential contagiousness,” they added.
Dr. Landi and colleagues said their findings should be considered preliminary, and larger data samples are warranted to validate the results.
Dr. Landi and Dr. Karn disclosed no relevant financial relationships. Dr. Iwasaki disclosed a research grant from Condair, a 5% or greater equity interest in RIGImmune, and income of $250 or more from PureTec.
A version of this article originally appeared on Medscape.com.
For reasons unknown, about one in six people who recovered from COVID-19 subsequently retested positive at least 2 weeks later, researchers reported in a study in Italy.
Sore throat and rhinitis were the only symptoms associated with a positive result. “Patients who continued to have respiratory symptoms, especially, were more likely to have a new positive test result,” lead author Francesco Landi, MD, PhD, said in an interview.
“This suggests the persistence of respiratory symptoms should not be underestimated and should be adequately assessed in all patients considered recovered from COVID-19,” he said.
“The study results are interesting,” Akiko Iwasaki, PhD, an immunobiologist at Yale University and the Howard Hughes Medical Institute, both in New Haven, Conn.,, said in an interview. “There are other reports of RNA detection postdischarge, but this study ... found that only two symptoms out of many – sore throat and rhinitis – were higher in those with PCR [polymerase chain reaction]-positive status.”
The study was published online Sept. 18 in the American Journal of Preventive Medicine.
The findings could carry important implications for people who continue to be symptomatic. “It is reasonable to be cautious and avoid close contact with others, wear a face mask, and possibly undergo an additional nasopharyngeal swab,” said Dr. Landi, associate professor of internal medicine at Catholic University of the Sacred Heart in Rome.
“One of most interesting findings is that persistent symptoms do not correlate with PCR positivity, suggesting that symptoms are in many cases not due to ongoing viral replication,” Jonathan Karn, PhD, professor and chair of the department of molecular biology and microbiology at Case Western Reserve University, Cleveland, said in an interview.
“The key technical problem, which they have discussed, is that a viral RNA signal in the PCR assay does not necessarily mean that infectious virus is present,” Dr. Karn said. He added that new comprehensive viral RNA analyses would be needed to answer this question.
Official COVID-19 recovery
To identify risk factors and COVID-19 survivors more likely to retest positive, Dr. Landi and members of the Gemelli Against COVID-19 Post-Acute Care Study Group evaluated 131 people after hospital discharge.
All participants met World Health Organization criteria for release from isolation, including two negative test results at least 24 hours apart, and were studied between April 21 and May 21. Mean age was 56 and 39% were women. Only a slightly higher mean body mass index of 27.6 kg/m2 in the positive group versus 25.9 in the negative group, was significant.
Although 51% of survivors reported fatigue, 44% had dyspnea, and 17% were coughing, the rates did not differ significantly between groups. In contrast, 18% of positive survivors and 4% of negative survivors had a sore throat (P = .04), and 27% versus 12%, respectively, reported rhinitis (P = .05).
People returned for follow-up visits a mean 17 days after the second negative swab test.
Asymptomatic COVID-19 carriers
“These findings indicate that a noteworthy rate of recovered patients with COVID-19 could still be asymptomatic carriers of the virus,” the researchers noted in the paper. “Even in the absence of specific guidelines, the 22 patients who tested positive for COVID-19 again were suggested to quarantine for a second time.”
No family member or close contact of the positive survivors reported SARS-CoV-2 infection. All patients continued to wear masks and observe social distancing recommendations, which makes it “very difficult to affirm whether these patients were really contagious,” the researchers noted.
Next steps
Evaluating all COVID-19 survivors to identify any who retest positive “will be a crucial contribution to a better understanding of both the natural history of COVID-19 as well as the public health implications of viral shedding,” the authors wrote.
One study limitation is that the reverse transcriptase–PCR test reveals genetic sequences specific to COVID-19. “It is important to underline that this is not a viral culture and cannot determine whether the virus is viable and transmissible,” the researchers noted.
“In this respect, we are trying to better understand if the persistence of long-time positive [reverse transcriptase]–PCR test for COVID-19 is really correlated to a potential contagiousness,” they added.
Dr. Landi and colleagues said their findings should be considered preliminary, and larger data samples are warranted to validate the results.
Dr. Landi and Dr. Karn disclosed no relevant financial relationships. Dr. Iwasaki disclosed a research grant from Condair, a 5% or greater equity interest in RIGImmune, and income of $250 or more from PureTec.
A version of this article originally appeared on Medscape.com.
CDC panel takes on COVID vaccine rollout, risks, and side effects
Federal advisers who will help determine which Americans get the first COVID vaccines took an in-depth look Oct. 30 at the challenges they face in selecting priority groups.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) will face two key decisions once a COVID vaccine wins clearance from the US Food and Drug Administration (FDA).
ACIP will need to decide whether to recommend its use in adults (the age group in which vaccines are currently being tested). The group will also need to offer direction on which groups should get priority in vaccine allocation, inasmuch as early supplies will not be sufficient to vaccinate everyone.
At the Oct. 30 meeting, CDC’s Kathleen Dooling, MD, MPH, suggested that ACIP plan on tackling these issues as two separate questions when it comes time to weigh in on an approved vaccine. Although there was no formal vote among ACIP members at the meeting, Dooling’s proposal for tackling a future recommendation in a two-part fashion drew positive feedback.
ACIP member Katherine A. Poehling, MD, MPH, suggested that the panel and CDC be ready to reexamine the situation frequently regarding COVID vaccination. “Perhaps we could think about reviewing data on a monthly basis and updating the recommendation, so that we can account for the concerns and balance both the benefits and the [potential] harm,” Poehling said.
Dooling agreed. “Both the vaccine recommendation and allocation will be revisited in what is a very dynamic situation,” Dooling replied to Poehling. “So all new evidence will be brought to ACIP, and certainly the allocation as vaccine distribution proceeds will need to be adjusted accordingly.”
Ethics and limited evidence
During the meeting, ACIP members repeatedly expressed discomfort with the prospect of having to weigh in on widespread use of COVID vaccines on the basis of limited evidence.
Within months, FDA may opt for a special clearance, known as an emergency use authorization (EUA), for one or more of the experimental COVID vaccines now in advanced testing. Many of FDA’s past EUA clearances were granted for test kits. For those EUA approvals, the agency considered risks of false results but not longer-term, direct harm to patients from these products.
With a COVID vaccine, there will be strong pressure to distribute doses as quickly as possible with the hope of curbing the pandemic, which has already led to more than 229,000 deaths in the United States alone and has disrupted lives and economies around the world. But questions will persist about the possibility of serious complications from these vaccines, ACIP members noted.
“My personal struggle is the ethical side and how to balance these two,” said ACIP member Robert L. Atmar, MD, of Baylor College of Medicine, Houston, Texas, who noted that he expects his fellow panelists to share this concern.
Currently, four experimental COVID vaccines likely to be used in the United States have advanced to phase 3 testing. Pfizer Inc and BioNtech have enrolled more than 42,000 participants in a test of their candidate, BNT162b2 vaccine, and rival Moderna has enrolled about 30,000 participants in a test of its mRNA-1273 vaccine, CDC staff said.
The other two advanced COVID vaccine candidates have overcome recent hurdles. AstraZeneca Plc on Oct. 23 announced that FDA had removed a hold on the testing of its AZD1222 vaccine candidate; the trial will enroll approximately 30,000 people. Johnson & Johnson’s Janssen unit also announced that day the lifting of a safety pause for its Ad26.COV2.S vaccine; the phase 3 trial for that vaccine will enroll approximately 60,000 volunteers. Federal agencies, states, and territories have developed plans for future distribution of COVID vaccines, CDC staff said in briefing materials for today’s ACIP meeting.
Several ACIP members raised many of the same concerns that members of an FDA advisory committee raised at a meeting earlier in October. ACIP and FDA advisers honed in on the FDA’s decision to set a median follow-up duration of 2 months in phase 3 trials in connection with expected EUA applications for COVID-19 vaccines.
“I struggle with following people for 2 months after their second vaccination as a time point to start making final decisions about safety,” said ACIP member Sharon E. Frey, MD, a professor at St. Louis University School of Medicine, St. Louis, Missouri. “I just want to put that out there.”
Medical front line, then who?
There is consensus that healthcare workers be in the first stage ― Phase 1 ― of distribution. That recommendation was made in a report from the National Academies of Sciences, Engineering, and Medicine (NASEM). Phase 1A would include first responders; Phase 1B might include people of all ages who have two or more comorbidities that put them at significantly higher risk for COVID-19 or death, as well as older adults living in congregate or overcrowded settings, the NASEM report said.
A presentation from the CDC’s Matthew Biggerstaff, ScD, MPH, underscored challenges in distributing what are expected to be limited initial supplies of COVID vaccines.
Biggerstaff showed several scenarios the CDC’s Data, Analytics, and Modeling Task Force had studied. The initial allocation of vaccines would be for healthcare workers, followed by what the CDC called Phase 1B.
Choices for a rollout may include next giving COVID vaccines to people at high risk, such as persons who have one or more chronic medical conditions, including heart disease, diabetes, kidney disease, or obesity. Other options for the rollout could be to vaccinate people aged 65 years and older or essential workers whose employment puts them in contact with the public, thus raising the risk of contracting the virus.
The CDC’s research found that the greatest impact in preventing death was to initially vaccinate adults aged 65 and older in Phase 1B. The agency staff described this approach as likely to result in an about “1 to 11% increase in averted deaths across the scenarios.”
Initially vaccinating essential workers or high-risk adults in Phase 1B would avert the most infections. The agency staff described this approach as yielding about “1 to 5% increase in averted infections across the scenarios,” Biggerstaff said during his presentation.
The following are other findings of the CDC staff:
The earlier the vaccine rollout relative to increasing transmission, the greater the averted percentage and differences between the strategies.
Differences were not substantial in some scenarios.
The need to continue efforts to slow the spread of COVID-19 should be emphasized.
Adverse effects
ACIP members also heard about strategies for tracking potential side effects of future vaccines. A presentation by Tom Shimabukuro, MD, MPH, MBA, from the CDC’s COVID-19 Vaccine Task Force/Vaccine Safety Team, included details about a new smartphone-based active surveillance program for COVID-19 vaccine safety.
Known as v-safe, this system would use Web-based survey monitoring and incorporate text messaging. It would conduct electronic health checks on vaccine recipients, which would occur daily during the first week post vaccination and weekly thereafter for 6 weeks from the time of vaccination.
Clinicians “can play an important role in helping CDC enroll patients in v-safe at the time of vaccination,” Shimabukuro noted in his presentation. This would add another task, though, for clinicians, the CDC staff noted.
Pregnancy and breastfeeding are special concerns
Of special concern with the rollout of a COVID vaccine are recommendations regarding pregnancy and breastfeeding. Women constitute about 75% of the healthcare workforce, CDC staff noted.
At the time the initial ACIP COVID vaccination recommendations are made, there could be approximately 330,000 healthcare personnel who are pregnant or who have recently given birth. Available data indicate potentially increased risks for severe maternal illness and preterm birth associated with SARS-CoV-2 infection, said CDC’s Megan Wallace, DrPH, MPH, in a presentation for the Friday meeting.
In an Oct. 27 letter to ACIP, Chair Jose Romero, the American College of Obstetricians and Gynecologists (ACOG), urged the panel to ensure that pregnant women and new mothers in the healthcare workforce have priority access to a COVID vaccine. Pregnant and lactating women were “noticeably and alarmingly absent from the NASEM vaccine allocation plan for COVID-19,” wrote Christopher M. Zahn, MD, vice president for practice activities at ACOG, in the letter to Romero.
“ACOG urges ACIP to incorporate pregnant and lactating women clearly and explicitly into its COVID-19 vaccine allocation and prioritization framework,” Zahn wrote. “Should an Emergency Use Authorization be executed for one or more COVID-19 vaccines and provide a permissive recommendation for pregnant and lactating women, pregnant health care workers, pregnant first responders, and pregnant individuals with underlying conditions should be prioritized for vaccination alongside their non-pregnant peers.”
This article first appeared on Medscape.com.
Federal advisers who will help determine which Americans get the first COVID vaccines took an in-depth look Oct. 30 at the challenges they face in selecting priority groups.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) will face two key decisions once a COVID vaccine wins clearance from the US Food and Drug Administration (FDA).
ACIP will need to decide whether to recommend its use in adults (the age group in which vaccines are currently being tested). The group will also need to offer direction on which groups should get priority in vaccine allocation, inasmuch as early supplies will not be sufficient to vaccinate everyone.
At the Oct. 30 meeting, CDC’s Kathleen Dooling, MD, MPH, suggested that ACIP plan on tackling these issues as two separate questions when it comes time to weigh in on an approved vaccine. Although there was no formal vote among ACIP members at the meeting, Dooling’s proposal for tackling a future recommendation in a two-part fashion drew positive feedback.
ACIP member Katherine A. Poehling, MD, MPH, suggested that the panel and CDC be ready to reexamine the situation frequently regarding COVID vaccination. “Perhaps we could think about reviewing data on a monthly basis and updating the recommendation, so that we can account for the concerns and balance both the benefits and the [potential] harm,” Poehling said.
Dooling agreed. “Both the vaccine recommendation and allocation will be revisited in what is a very dynamic situation,” Dooling replied to Poehling. “So all new evidence will be brought to ACIP, and certainly the allocation as vaccine distribution proceeds will need to be adjusted accordingly.”
Ethics and limited evidence
During the meeting, ACIP members repeatedly expressed discomfort with the prospect of having to weigh in on widespread use of COVID vaccines on the basis of limited evidence.
Within months, FDA may opt for a special clearance, known as an emergency use authorization (EUA), for one or more of the experimental COVID vaccines now in advanced testing. Many of FDA’s past EUA clearances were granted for test kits. For those EUA approvals, the agency considered risks of false results but not longer-term, direct harm to patients from these products.
With a COVID vaccine, there will be strong pressure to distribute doses as quickly as possible with the hope of curbing the pandemic, which has already led to more than 229,000 deaths in the United States alone and has disrupted lives and economies around the world. But questions will persist about the possibility of serious complications from these vaccines, ACIP members noted.
“My personal struggle is the ethical side and how to balance these two,” said ACIP member Robert L. Atmar, MD, of Baylor College of Medicine, Houston, Texas, who noted that he expects his fellow panelists to share this concern.
Currently, four experimental COVID vaccines likely to be used in the United States have advanced to phase 3 testing. Pfizer Inc and BioNtech have enrolled more than 42,000 participants in a test of their candidate, BNT162b2 vaccine, and rival Moderna has enrolled about 30,000 participants in a test of its mRNA-1273 vaccine, CDC staff said.
The other two advanced COVID vaccine candidates have overcome recent hurdles. AstraZeneca Plc on Oct. 23 announced that FDA had removed a hold on the testing of its AZD1222 vaccine candidate; the trial will enroll approximately 30,000 people. Johnson & Johnson’s Janssen unit also announced that day the lifting of a safety pause for its Ad26.COV2.S vaccine; the phase 3 trial for that vaccine will enroll approximately 60,000 volunteers. Federal agencies, states, and territories have developed plans for future distribution of COVID vaccines, CDC staff said in briefing materials for today’s ACIP meeting.
Several ACIP members raised many of the same concerns that members of an FDA advisory committee raised at a meeting earlier in October. ACIP and FDA advisers honed in on the FDA’s decision to set a median follow-up duration of 2 months in phase 3 trials in connection with expected EUA applications for COVID-19 vaccines.
“I struggle with following people for 2 months after their second vaccination as a time point to start making final decisions about safety,” said ACIP member Sharon E. Frey, MD, a professor at St. Louis University School of Medicine, St. Louis, Missouri. “I just want to put that out there.”
Medical front line, then who?
There is consensus that healthcare workers be in the first stage ― Phase 1 ― of distribution. That recommendation was made in a report from the National Academies of Sciences, Engineering, and Medicine (NASEM). Phase 1A would include first responders; Phase 1B might include people of all ages who have two or more comorbidities that put them at significantly higher risk for COVID-19 or death, as well as older adults living in congregate or overcrowded settings, the NASEM report said.
A presentation from the CDC’s Matthew Biggerstaff, ScD, MPH, underscored challenges in distributing what are expected to be limited initial supplies of COVID vaccines.
Biggerstaff showed several scenarios the CDC’s Data, Analytics, and Modeling Task Force had studied. The initial allocation of vaccines would be for healthcare workers, followed by what the CDC called Phase 1B.
Choices for a rollout may include next giving COVID vaccines to people at high risk, such as persons who have one or more chronic medical conditions, including heart disease, diabetes, kidney disease, or obesity. Other options for the rollout could be to vaccinate people aged 65 years and older or essential workers whose employment puts them in contact with the public, thus raising the risk of contracting the virus.
The CDC’s research found that the greatest impact in preventing death was to initially vaccinate adults aged 65 and older in Phase 1B. The agency staff described this approach as likely to result in an about “1 to 11% increase in averted deaths across the scenarios.”
Initially vaccinating essential workers or high-risk adults in Phase 1B would avert the most infections. The agency staff described this approach as yielding about “1 to 5% increase in averted infections across the scenarios,” Biggerstaff said during his presentation.
The following are other findings of the CDC staff:
The earlier the vaccine rollout relative to increasing transmission, the greater the averted percentage and differences between the strategies.
Differences were not substantial in some scenarios.
The need to continue efforts to slow the spread of COVID-19 should be emphasized.
Adverse effects
ACIP members also heard about strategies for tracking potential side effects of future vaccines. A presentation by Tom Shimabukuro, MD, MPH, MBA, from the CDC’s COVID-19 Vaccine Task Force/Vaccine Safety Team, included details about a new smartphone-based active surveillance program for COVID-19 vaccine safety.
Known as v-safe, this system would use Web-based survey monitoring and incorporate text messaging. It would conduct electronic health checks on vaccine recipients, which would occur daily during the first week post vaccination and weekly thereafter for 6 weeks from the time of vaccination.
Clinicians “can play an important role in helping CDC enroll patients in v-safe at the time of vaccination,” Shimabukuro noted in his presentation. This would add another task, though, for clinicians, the CDC staff noted.
Pregnancy and breastfeeding are special concerns
Of special concern with the rollout of a COVID vaccine are recommendations regarding pregnancy and breastfeeding. Women constitute about 75% of the healthcare workforce, CDC staff noted.
At the time the initial ACIP COVID vaccination recommendations are made, there could be approximately 330,000 healthcare personnel who are pregnant or who have recently given birth. Available data indicate potentially increased risks for severe maternal illness and preterm birth associated with SARS-CoV-2 infection, said CDC’s Megan Wallace, DrPH, MPH, in a presentation for the Friday meeting.
In an Oct. 27 letter to ACIP, Chair Jose Romero, the American College of Obstetricians and Gynecologists (ACOG), urged the panel to ensure that pregnant women and new mothers in the healthcare workforce have priority access to a COVID vaccine. Pregnant and lactating women were “noticeably and alarmingly absent from the NASEM vaccine allocation plan for COVID-19,” wrote Christopher M. Zahn, MD, vice president for practice activities at ACOG, in the letter to Romero.
“ACOG urges ACIP to incorporate pregnant and lactating women clearly and explicitly into its COVID-19 vaccine allocation and prioritization framework,” Zahn wrote. “Should an Emergency Use Authorization be executed for one or more COVID-19 vaccines and provide a permissive recommendation for pregnant and lactating women, pregnant health care workers, pregnant first responders, and pregnant individuals with underlying conditions should be prioritized for vaccination alongside their non-pregnant peers.”
This article first appeared on Medscape.com.
Federal advisers who will help determine which Americans get the first COVID vaccines took an in-depth look Oct. 30 at the challenges they face in selecting priority groups.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) will face two key decisions once a COVID vaccine wins clearance from the US Food and Drug Administration (FDA).
ACIP will need to decide whether to recommend its use in adults (the age group in which vaccines are currently being tested). The group will also need to offer direction on which groups should get priority in vaccine allocation, inasmuch as early supplies will not be sufficient to vaccinate everyone.
At the Oct. 30 meeting, CDC’s Kathleen Dooling, MD, MPH, suggested that ACIP plan on tackling these issues as two separate questions when it comes time to weigh in on an approved vaccine. Although there was no formal vote among ACIP members at the meeting, Dooling’s proposal for tackling a future recommendation in a two-part fashion drew positive feedback.
ACIP member Katherine A. Poehling, MD, MPH, suggested that the panel and CDC be ready to reexamine the situation frequently regarding COVID vaccination. “Perhaps we could think about reviewing data on a monthly basis and updating the recommendation, so that we can account for the concerns and balance both the benefits and the [potential] harm,” Poehling said.
Dooling agreed. “Both the vaccine recommendation and allocation will be revisited in what is a very dynamic situation,” Dooling replied to Poehling. “So all new evidence will be brought to ACIP, and certainly the allocation as vaccine distribution proceeds will need to be adjusted accordingly.”
Ethics and limited evidence
During the meeting, ACIP members repeatedly expressed discomfort with the prospect of having to weigh in on widespread use of COVID vaccines on the basis of limited evidence.
Within months, FDA may opt for a special clearance, known as an emergency use authorization (EUA), for one or more of the experimental COVID vaccines now in advanced testing. Many of FDA’s past EUA clearances were granted for test kits. For those EUA approvals, the agency considered risks of false results but not longer-term, direct harm to patients from these products.
With a COVID vaccine, there will be strong pressure to distribute doses as quickly as possible with the hope of curbing the pandemic, which has already led to more than 229,000 deaths in the United States alone and has disrupted lives and economies around the world. But questions will persist about the possibility of serious complications from these vaccines, ACIP members noted.
“My personal struggle is the ethical side and how to balance these two,” said ACIP member Robert L. Atmar, MD, of Baylor College of Medicine, Houston, Texas, who noted that he expects his fellow panelists to share this concern.
Currently, four experimental COVID vaccines likely to be used in the United States have advanced to phase 3 testing. Pfizer Inc and BioNtech have enrolled more than 42,000 participants in a test of their candidate, BNT162b2 vaccine, and rival Moderna has enrolled about 30,000 participants in a test of its mRNA-1273 vaccine, CDC staff said.
The other two advanced COVID vaccine candidates have overcome recent hurdles. AstraZeneca Plc on Oct. 23 announced that FDA had removed a hold on the testing of its AZD1222 vaccine candidate; the trial will enroll approximately 30,000 people. Johnson & Johnson’s Janssen unit also announced that day the lifting of a safety pause for its Ad26.COV2.S vaccine; the phase 3 trial for that vaccine will enroll approximately 60,000 volunteers. Federal agencies, states, and territories have developed plans for future distribution of COVID vaccines, CDC staff said in briefing materials for today’s ACIP meeting.
Several ACIP members raised many of the same concerns that members of an FDA advisory committee raised at a meeting earlier in October. ACIP and FDA advisers honed in on the FDA’s decision to set a median follow-up duration of 2 months in phase 3 trials in connection with expected EUA applications for COVID-19 vaccines.
“I struggle with following people for 2 months after their second vaccination as a time point to start making final decisions about safety,” said ACIP member Sharon E. Frey, MD, a professor at St. Louis University School of Medicine, St. Louis, Missouri. “I just want to put that out there.”
Medical front line, then who?
There is consensus that healthcare workers be in the first stage ― Phase 1 ― of distribution. That recommendation was made in a report from the National Academies of Sciences, Engineering, and Medicine (NASEM). Phase 1A would include first responders; Phase 1B might include people of all ages who have two or more comorbidities that put them at significantly higher risk for COVID-19 or death, as well as older adults living in congregate or overcrowded settings, the NASEM report said.
A presentation from the CDC’s Matthew Biggerstaff, ScD, MPH, underscored challenges in distributing what are expected to be limited initial supplies of COVID vaccines.
Biggerstaff showed several scenarios the CDC’s Data, Analytics, and Modeling Task Force had studied. The initial allocation of vaccines would be for healthcare workers, followed by what the CDC called Phase 1B.
Choices for a rollout may include next giving COVID vaccines to people at high risk, such as persons who have one or more chronic medical conditions, including heart disease, diabetes, kidney disease, or obesity. Other options for the rollout could be to vaccinate people aged 65 years and older or essential workers whose employment puts them in contact with the public, thus raising the risk of contracting the virus.
The CDC’s research found that the greatest impact in preventing death was to initially vaccinate adults aged 65 and older in Phase 1B. The agency staff described this approach as likely to result in an about “1 to 11% increase in averted deaths across the scenarios.”
Initially vaccinating essential workers or high-risk adults in Phase 1B would avert the most infections. The agency staff described this approach as yielding about “1 to 5% increase in averted infections across the scenarios,” Biggerstaff said during his presentation.
The following are other findings of the CDC staff:
The earlier the vaccine rollout relative to increasing transmission, the greater the averted percentage and differences between the strategies.
Differences were not substantial in some scenarios.
The need to continue efforts to slow the spread of COVID-19 should be emphasized.
Adverse effects
ACIP members also heard about strategies for tracking potential side effects of future vaccines. A presentation by Tom Shimabukuro, MD, MPH, MBA, from the CDC’s COVID-19 Vaccine Task Force/Vaccine Safety Team, included details about a new smartphone-based active surveillance program for COVID-19 vaccine safety.
Known as v-safe, this system would use Web-based survey monitoring and incorporate text messaging. It would conduct electronic health checks on vaccine recipients, which would occur daily during the first week post vaccination and weekly thereafter for 6 weeks from the time of vaccination.
Clinicians “can play an important role in helping CDC enroll patients in v-safe at the time of vaccination,” Shimabukuro noted in his presentation. This would add another task, though, for clinicians, the CDC staff noted.
Pregnancy and breastfeeding are special concerns
Of special concern with the rollout of a COVID vaccine are recommendations regarding pregnancy and breastfeeding. Women constitute about 75% of the healthcare workforce, CDC staff noted.
At the time the initial ACIP COVID vaccination recommendations are made, there could be approximately 330,000 healthcare personnel who are pregnant or who have recently given birth. Available data indicate potentially increased risks for severe maternal illness and preterm birth associated with SARS-CoV-2 infection, said CDC’s Megan Wallace, DrPH, MPH, in a presentation for the Friday meeting.
In an Oct. 27 letter to ACIP, Chair Jose Romero, the American College of Obstetricians and Gynecologists (ACOG), urged the panel to ensure that pregnant women and new mothers in the healthcare workforce have priority access to a COVID vaccine. Pregnant and lactating women were “noticeably and alarmingly absent from the NASEM vaccine allocation plan for COVID-19,” wrote Christopher M. Zahn, MD, vice president for practice activities at ACOG, in the letter to Romero.
“ACOG urges ACIP to incorporate pregnant and lactating women clearly and explicitly into its COVID-19 vaccine allocation and prioritization framework,” Zahn wrote. “Should an Emergency Use Authorization be executed for one or more COVID-19 vaccines and provide a permissive recommendation for pregnant and lactating women, pregnant health care workers, pregnant first responders, and pregnant individuals with underlying conditions should be prioritized for vaccination alongside their non-pregnant peers.”
This article first appeared on Medscape.com.
Hospitalists are natural leaders in the COVID-19 battle
Christopher Pribula, MD, a hospitalist at Sanford Broadway Medical Center in Fargo, N.D., didn’t anticipate becoming his hospital’s resident expert on COVID-19. Having just returned from vacation in March, he agreed to cover for a colleague on what would become the special care unit. “When our hospital medicine group decided that it would be the COVID unit, I just ran with it,” he said. Dr. Pribula spent the next 18 days doing 8- to 14-hour shifts and learning as much as he could as the hospital – and the nation – wrestled with the pandemic.
“Because I was the first hospitalist, along with our infectious disease specialist, Dr. Avish Nagpal, to really engage with the virus, people came to me with their questions,” Dr. Pribula said. Working to establish protocols for the care of COVID-19 patients involved a lot of planning, from nursing protocols to discharge planning.
Dr. Pribula was part of the hospital’s incident command structure, thought about how the system could scale up for a potential surge, and worked with the North Dakota Medical Association to reach out to outlying medical centers on safety and infection control. He even drew on his prior work experience as a medical technologist doing negative-pressure containment in a cell-processing facility to help create the hospital’s negative-pressure unit in an old ICU.
“We did a lot of communication from the start. To a certain extent we were making it up as we went along, but we sat down and huddled as a team every day at 9 and 4,” he explained. “We started out with observation and retrospective research, and learned piece by piece. But that’s how science works.”
Hospitalists across the country have played leading roles in their hospitals’ and health systems’ response to the pandemic, and not just because they are on the front lines providing patient care. Their job as doctors who work full-time in the hospital makes them natural leaders in improving clinical quality and hospital administrative protocols as well as studying the latest information and educating their colleagues. Responding to the pandemic has required lots of planning, careful attention to schedules and assignments and staff stress, and working with other departments in the hospital and groups in the community, including public health authorities.
Where is hospital treatment for COVID-19 at today?
As knowledge has grown, Dr. Pribula said, COVID-19 treatment in the hospital has come to incorporate remdesivir, a broad-spectrum antiviral; dexamethasone, a common steroid medication; and convalescent plasma, blood products from people who have recovered from the illness. “We went from no steroids to giving steroids. We went from putting patients on ventilators to avoid acute respiratory distress syndrome (ARDS) initially to now working to avoid intubation at all costs,” he said.
“What we found is that we need to pressure-support these patients. We do proning and CPAP while we let the lungs heal. By the time they arrive at the hospital, more often than not they’re on the backside of the viral load. But now we’re dealing with the body’s inflammatory response.”
Navneet Attri, MD, a hospitalist at Sutter Santa Rosa Regional Hospital in Santa Rosa, Calif., 50 miles north of San Francisco, experienced fears and uncertainties working at a hospital that treated early COVID patients from the Grand Princess cruise ship. Early on, she wrote a post describing her experience for The Hospitalist Leader, the Society of Hospital Medicine’s blog page.
Dr. Attri said she has gone through the gamut of emotions while caring for COVID-19 patients, addressing their fears and trying to support family members who aren’t allowed to enter the hospital to be at their loved one’s side. Sometimes, patient after patient with COVID-19 becomes almost too much. But seeing a lot of them in the intervening 6 months has increased her confidence level.
Understanding of how the disease is spread has continued to evolve, with a recent return to focusing on airborne transmission, she said. Frontline workers need N95 masks and eye shields, even if all of that PPE feels like a burden. Dr. Attri said she hardly notices the PPE anymore. “Putting it on is just a habit.”
She sits on Sonoma County’s COVID-19 surge planning group, which has representatives from the three local hospitals, the public health department, and other community agencies. “I report back to my hospitalist group about the situation in the community. Because our facilities were well prepared, our hospitals have not been overwhelmed,” she said.
The importance of teamwork
Sunil Shah, MD, a hospitalist with Northwell Health’s Southside Hospital in Bay Shore, N.Y., is part of the massive hospital medicine team, including reassigned specialists and volunteers from across the country, deployed at Northwell hospitals in Greater New York City and Long Island during the COVID-19 surge. Northwell probably has cared for more COVID-19 patients than any other health system in the country, and at the height of the surge the intensity of hospital care was like nothing he’s ever seen. But he also expressed gratitude that doctors from other parts of the country were willing to come and help out.
Southside Hospital went almost overnight from a 200-bed acute facility to a full, 350-bed, regional COVID-19–only hospital. “On busy days, our entire hospital was like a floating ICU,” he said. “You’d hear ‘rapid response’ or ‘code blue’ over the intercom every few seconds. Normally we’d have a designated rapid response person for the day, but with COVID, everybody stepped in to help – whoever was closest,” he said.
Majid Sheikh, MD, a hospitalist at Emory University Hospital in Atlanta, also became a go-to COVID-19 expert for his group. “I didn’t specifically volunteer, but my partner and I had the first cases, and the leadership group was happy to have us there,” he explained.
“One interesting thing I learned was the concept of the ‘happy’ hypoxemic patient, who is having a significant drop in oxygen saturation without developing any obvious signs of respiratory distress,” he said. “We’d be checking the accuracy of the reading and trying to figure out if it was real.” Emory was also one of the leaders in studying anticoagulant treatments for COVID-19 patients.
“Six months later I would say we’re definitely getting better outcomes on the floor, and our COVID patients aren’t landing in the ICU as easily,” Dr. Sheikh said. “It was scary at first, and doubly scary when doctors sometimes don’t feel they can say, ‘Hey, I’m scared too,’ or ‘By the way, I really don’t know what I’m doing.’ So, we’d be trying to reassure the patients when the information was coming to us in fragments.”
But he also believes that the pandemic has afforded hospitalists the opportunity to be the clinical detectives they were trained to be, sifting through clues. “I had to think more and really pay attention clinically in a much different way. You could say it was exciting and scary at the same time,” he said.
A human fix in the hospital
Dr. Pribula agreed that the pandemic has been both a difficult experience and a rewarding one. “I think of the people I first admitted. If they had shown up even a month later, would they still be with us?” He believes that his group and his field are going to get to a place where they have solid treatment plans for how to provide optimal care and how to protect providers from exposure.
One of the first COVID-19 patients in Fargo had dementia and was very distressed. “She had no idea why nobody was visiting or why we wouldn’t let her out of her room,” Dr. Pribula said. “Instead of reaching for sedatives, one of our nurses went into the room and talked with her, prayed a rosary, and played two hands of cards with her and didn’t have to sedate her. That’s what people need when they’re alone and scared. It wasn’t a medical fix but a human fix.”
A version of this article originally appeared on Medscape.com.
Christopher Pribula, MD, a hospitalist at Sanford Broadway Medical Center in Fargo, N.D., didn’t anticipate becoming his hospital’s resident expert on COVID-19. Having just returned from vacation in March, he agreed to cover for a colleague on what would become the special care unit. “When our hospital medicine group decided that it would be the COVID unit, I just ran with it,” he said. Dr. Pribula spent the next 18 days doing 8- to 14-hour shifts and learning as much as he could as the hospital – and the nation – wrestled with the pandemic.
“Because I was the first hospitalist, along with our infectious disease specialist, Dr. Avish Nagpal, to really engage with the virus, people came to me with their questions,” Dr. Pribula said. Working to establish protocols for the care of COVID-19 patients involved a lot of planning, from nursing protocols to discharge planning.
Dr. Pribula was part of the hospital’s incident command structure, thought about how the system could scale up for a potential surge, and worked with the North Dakota Medical Association to reach out to outlying medical centers on safety and infection control. He even drew on his prior work experience as a medical technologist doing negative-pressure containment in a cell-processing facility to help create the hospital’s negative-pressure unit in an old ICU.
“We did a lot of communication from the start. To a certain extent we were making it up as we went along, but we sat down and huddled as a team every day at 9 and 4,” he explained. “We started out with observation and retrospective research, and learned piece by piece. But that’s how science works.”
Hospitalists across the country have played leading roles in their hospitals’ and health systems’ response to the pandemic, and not just because they are on the front lines providing patient care. Their job as doctors who work full-time in the hospital makes them natural leaders in improving clinical quality and hospital administrative protocols as well as studying the latest information and educating their colleagues. Responding to the pandemic has required lots of planning, careful attention to schedules and assignments and staff stress, and working with other departments in the hospital and groups in the community, including public health authorities.
Where is hospital treatment for COVID-19 at today?
As knowledge has grown, Dr. Pribula said, COVID-19 treatment in the hospital has come to incorporate remdesivir, a broad-spectrum antiviral; dexamethasone, a common steroid medication; and convalescent plasma, blood products from people who have recovered from the illness. “We went from no steroids to giving steroids. We went from putting patients on ventilators to avoid acute respiratory distress syndrome (ARDS) initially to now working to avoid intubation at all costs,” he said.
“What we found is that we need to pressure-support these patients. We do proning and CPAP while we let the lungs heal. By the time they arrive at the hospital, more often than not they’re on the backside of the viral load. But now we’re dealing with the body’s inflammatory response.”
Navneet Attri, MD, a hospitalist at Sutter Santa Rosa Regional Hospital in Santa Rosa, Calif., 50 miles north of San Francisco, experienced fears and uncertainties working at a hospital that treated early COVID patients from the Grand Princess cruise ship. Early on, she wrote a post describing her experience for The Hospitalist Leader, the Society of Hospital Medicine’s blog page.
Dr. Attri said she has gone through the gamut of emotions while caring for COVID-19 patients, addressing their fears and trying to support family members who aren’t allowed to enter the hospital to be at their loved one’s side. Sometimes, patient after patient with COVID-19 becomes almost too much. But seeing a lot of them in the intervening 6 months has increased her confidence level.
Understanding of how the disease is spread has continued to evolve, with a recent return to focusing on airborne transmission, she said. Frontline workers need N95 masks and eye shields, even if all of that PPE feels like a burden. Dr. Attri said she hardly notices the PPE anymore. “Putting it on is just a habit.”
She sits on Sonoma County’s COVID-19 surge planning group, which has representatives from the three local hospitals, the public health department, and other community agencies. “I report back to my hospitalist group about the situation in the community. Because our facilities were well prepared, our hospitals have not been overwhelmed,” she said.
The importance of teamwork
Sunil Shah, MD, a hospitalist with Northwell Health’s Southside Hospital in Bay Shore, N.Y., is part of the massive hospital medicine team, including reassigned specialists and volunteers from across the country, deployed at Northwell hospitals in Greater New York City and Long Island during the COVID-19 surge. Northwell probably has cared for more COVID-19 patients than any other health system in the country, and at the height of the surge the intensity of hospital care was like nothing he’s ever seen. But he also expressed gratitude that doctors from other parts of the country were willing to come and help out.
Southside Hospital went almost overnight from a 200-bed acute facility to a full, 350-bed, regional COVID-19–only hospital. “On busy days, our entire hospital was like a floating ICU,” he said. “You’d hear ‘rapid response’ or ‘code blue’ over the intercom every few seconds. Normally we’d have a designated rapid response person for the day, but with COVID, everybody stepped in to help – whoever was closest,” he said.
Majid Sheikh, MD, a hospitalist at Emory University Hospital in Atlanta, also became a go-to COVID-19 expert for his group. “I didn’t specifically volunteer, but my partner and I had the first cases, and the leadership group was happy to have us there,” he explained.
“One interesting thing I learned was the concept of the ‘happy’ hypoxemic patient, who is having a significant drop in oxygen saturation without developing any obvious signs of respiratory distress,” he said. “We’d be checking the accuracy of the reading and trying to figure out if it was real.” Emory was also one of the leaders in studying anticoagulant treatments for COVID-19 patients.
“Six months later I would say we’re definitely getting better outcomes on the floor, and our COVID patients aren’t landing in the ICU as easily,” Dr. Sheikh said. “It was scary at first, and doubly scary when doctors sometimes don’t feel they can say, ‘Hey, I’m scared too,’ or ‘By the way, I really don’t know what I’m doing.’ So, we’d be trying to reassure the patients when the information was coming to us in fragments.”
But he also believes that the pandemic has afforded hospitalists the opportunity to be the clinical detectives they were trained to be, sifting through clues. “I had to think more and really pay attention clinically in a much different way. You could say it was exciting and scary at the same time,” he said.
A human fix in the hospital
Dr. Pribula agreed that the pandemic has been both a difficult experience and a rewarding one. “I think of the people I first admitted. If they had shown up even a month later, would they still be with us?” He believes that his group and his field are going to get to a place where they have solid treatment plans for how to provide optimal care and how to protect providers from exposure.
One of the first COVID-19 patients in Fargo had dementia and was very distressed. “She had no idea why nobody was visiting or why we wouldn’t let her out of her room,” Dr. Pribula said. “Instead of reaching for sedatives, one of our nurses went into the room and talked with her, prayed a rosary, and played two hands of cards with her and didn’t have to sedate her. That’s what people need when they’re alone and scared. It wasn’t a medical fix but a human fix.”
A version of this article originally appeared on Medscape.com.
Christopher Pribula, MD, a hospitalist at Sanford Broadway Medical Center in Fargo, N.D., didn’t anticipate becoming his hospital’s resident expert on COVID-19. Having just returned from vacation in March, he agreed to cover for a colleague on what would become the special care unit. “When our hospital medicine group decided that it would be the COVID unit, I just ran with it,” he said. Dr. Pribula spent the next 18 days doing 8- to 14-hour shifts and learning as much as he could as the hospital – and the nation – wrestled with the pandemic.
“Because I was the first hospitalist, along with our infectious disease specialist, Dr. Avish Nagpal, to really engage with the virus, people came to me with their questions,” Dr. Pribula said. Working to establish protocols for the care of COVID-19 patients involved a lot of planning, from nursing protocols to discharge planning.
Dr. Pribula was part of the hospital’s incident command structure, thought about how the system could scale up for a potential surge, and worked with the North Dakota Medical Association to reach out to outlying medical centers on safety and infection control. He even drew on his prior work experience as a medical technologist doing negative-pressure containment in a cell-processing facility to help create the hospital’s negative-pressure unit in an old ICU.
“We did a lot of communication from the start. To a certain extent we were making it up as we went along, but we sat down and huddled as a team every day at 9 and 4,” he explained. “We started out with observation and retrospective research, and learned piece by piece. But that’s how science works.”
Hospitalists across the country have played leading roles in their hospitals’ and health systems’ response to the pandemic, and not just because they are on the front lines providing patient care. Their job as doctors who work full-time in the hospital makes them natural leaders in improving clinical quality and hospital administrative protocols as well as studying the latest information and educating their colleagues. Responding to the pandemic has required lots of planning, careful attention to schedules and assignments and staff stress, and working with other departments in the hospital and groups in the community, including public health authorities.
Where is hospital treatment for COVID-19 at today?
As knowledge has grown, Dr. Pribula said, COVID-19 treatment in the hospital has come to incorporate remdesivir, a broad-spectrum antiviral; dexamethasone, a common steroid medication; and convalescent plasma, blood products from people who have recovered from the illness. “We went from no steroids to giving steroids. We went from putting patients on ventilators to avoid acute respiratory distress syndrome (ARDS) initially to now working to avoid intubation at all costs,” he said.
“What we found is that we need to pressure-support these patients. We do proning and CPAP while we let the lungs heal. By the time they arrive at the hospital, more often than not they’re on the backside of the viral load. But now we’re dealing with the body’s inflammatory response.”
Navneet Attri, MD, a hospitalist at Sutter Santa Rosa Regional Hospital in Santa Rosa, Calif., 50 miles north of San Francisco, experienced fears and uncertainties working at a hospital that treated early COVID patients from the Grand Princess cruise ship. Early on, she wrote a post describing her experience for The Hospitalist Leader, the Society of Hospital Medicine’s blog page.
Dr. Attri said she has gone through the gamut of emotions while caring for COVID-19 patients, addressing their fears and trying to support family members who aren’t allowed to enter the hospital to be at their loved one’s side. Sometimes, patient after patient with COVID-19 becomes almost too much. But seeing a lot of them in the intervening 6 months has increased her confidence level.
Understanding of how the disease is spread has continued to evolve, with a recent return to focusing on airborne transmission, she said. Frontline workers need N95 masks and eye shields, even if all of that PPE feels like a burden. Dr. Attri said she hardly notices the PPE anymore. “Putting it on is just a habit.”
She sits on Sonoma County’s COVID-19 surge planning group, which has representatives from the three local hospitals, the public health department, and other community agencies. “I report back to my hospitalist group about the situation in the community. Because our facilities were well prepared, our hospitals have not been overwhelmed,” she said.
The importance of teamwork
Sunil Shah, MD, a hospitalist with Northwell Health’s Southside Hospital in Bay Shore, N.Y., is part of the massive hospital medicine team, including reassigned specialists and volunteers from across the country, deployed at Northwell hospitals in Greater New York City and Long Island during the COVID-19 surge. Northwell probably has cared for more COVID-19 patients than any other health system in the country, and at the height of the surge the intensity of hospital care was like nothing he’s ever seen. But he also expressed gratitude that doctors from other parts of the country were willing to come and help out.
Southside Hospital went almost overnight from a 200-bed acute facility to a full, 350-bed, regional COVID-19–only hospital. “On busy days, our entire hospital was like a floating ICU,” he said. “You’d hear ‘rapid response’ or ‘code blue’ over the intercom every few seconds. Normally we’d have a designated rapid response person for the day, but with COVID, everybody stepped in to help – whoever was closest,” he said.
Majid Sheikh, MD, a hospitalist at Emory University Hospital in Atlanta, also became a go-to COVID-19 expert for his group. “I didn’t specifically volunteer, but my partner and I had the first cases, and the leadership group was happy to have us there,” he explained.
“One interesting thing I learned was the concept of the ‘happy’ hypoxemic patient, who is having a significant drop in oxygen saturation without developing any obvious signs of respiratory distress,” he said. “We’d be checking the accuracy of the reading and trying to figure out if it was real.” Emory was also one of the leaders in studying anticoagulant treatments for COVID-19 patients.
“Six months later I would say we’re definitely getting better outcomes on the floor, and our COVID patients aren’t landing in the ICU as easily,” Dr. Sheikh said. “It was scary at first, and doubly scary when doctors sometimes don’t feel they can say, ‘Hey, I’m scared too,’ or ‘By the way, I really don’t know what I’m doing.’ So, we’d be trying to reassure the patients when the information was coming to us in fragments.”
But he also believes that the pandemic has afforded hospitalists the opportunity to be the clinical detectives they were trained to be, sifting through clues. “I had to think more and really pay attention clinically in a much different way. You could say it was exciting and scary at the same time,” he said.
A human fix in the hospital
Dr. Pribula agreed that the pandemic has been both a difficult experience and a rewarding one. “I think of the people I first admitted. If they had shown up even a month later, would they still be with us?” He believes that his group and his field are going to get to a place where they have solid treatment plans for how to provide optimal care and how to protect providers from exposure.
One of the first COVID-19 patients in Fargo had dementia and was very distressed. “She had no idea why nobody was visiting or why we wouldn’t let her out of her room,” Dr. Pribula said. “Instead of reaching for sedatives, one of our nurses went into the room and talked with her, prayed a rosary, and played two hands of cards with her and didn’t have to sedate her. That’s what people need when they’re alone and scared. It wasn’t a medical fix but a human fix.”
A version of this article originally appeared on Medscape.com.
Echocardiography in AMI not associated with improved outcomes
Background: Guidelines recommend that patients with AMI undergo universal echocardiography for the assessment of cardiac structure and ejection fraction, despite modest diagnostic yield.
Study design: Retrospective cohort.
Setting: 397 U.S. hospitals contributing to the Premier Healthcare Informatics inpatient database.
Synopsis: ICD-9 codes were used to identify 98,999 hospitalizations with a discharge diagnosis of AMI. Of these, 70.4% had at least one transthoracic echocardiogram performed. Patients who underwent echocardiogram were more likely than patients without an echocardiogram to have heart failure, pulmonary disease, and intensive care unit stays and require interventions such as noninvasive and invasive ventilation, vasopressors, balloon pumps, and inotropic agents.
Risk-standardized echocardiography rates varied significantly across hospitals, ranging from a median of 54% in the lowest quartile to 83% in the highest quartile. The authors found that use of echocardiography was most strongly associated with the hospital, more so than individual patient factors. In adjusted analyses, no difference was seen in inpatient mortality (odds ratio, 1.02; 95% CI, 0.88-1.99) or 3-month readmission (OR, 1.01; 95% CI, 0.93-1.10), but slightly longer mean length of stay (0.23 days; 95% CI, 0.04-0.41; P = .01) and higher mean costs ($3,164; 95% CI, $1,843-$4,485; P < .001) were found in patients treated at hospitals with the highest quartile of echocardiography use, compared with those in the lowest quartile.
Limitations include lack of information about long-term clinical outcomes, inability to adjust for ejection fraction levels, and reliance on administrative data for AMI and procedure codes.
Bottom line: In a cohort of patients with AMI, higher rates of hospital echocardiography use did not appear to be associated with better clinical outcomes but were associated with longer length of stay and greater hospital costs.
Citation: Pack QR et al. Association between inpatient echocardiography use and outcomes in adult patients with acute myocardial infarction. JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.1051.
Dr. Liu is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: Guidelines recommend that patients with AMI undergo universal echocardiography for the assessment of cardiac structure and ejection fraction, despite modest diagnostic yield.
Study design: Retrospective cohort.
Setting: 397 U.S. hospitals contributing to the Premier Healthcare Informatics inpatient database.
Synopsis: ICD-9 codes were used to identify 98,999 hospitalizations with a discharge diagnosis of AMI. Of these, 70.4% had at least one transthoracic echocardiogram performed. Patients who underwent echocardiogram were more likely than patients without an echocardiogram to have heart failure, pulmonary disease, and intensive care unit stays and require interventions such as noninvasive and invasive ventilation, vasopressors, balloon pumps, and inotropic agents.
Risk-standardized echocardiography rates varied significantly across hospitals, ranging from a median of 54% in the lowest quartile to 83% in the highest quartile. The authors found that use of echocardiography was most strongly associated with the hospital, more so than individual patient factors. In adjusted analyses, no difference was seen in inpatient mortality (odds ratio, 1.02; 95% CI, 0.88-1.99) or 3-month readmission (OR, 1.01; 95% CI, 0.93-1.10), but slightly longer mean length of stay (0.23 days; 95% CI, 0.04-0.41; P = .01) and higher mean costs ($3,164; 95% CI, $1,843-$4,485; P < .001) were found in patients treated at hospitals with the highest quartile of echocardiography use, compared with those in the lowest quartile.
Limitations include lack of information about long-term clinical outcomes, inability to adjust for ejection fraction levels, and reliance on administrative data for AMI and procedure codes.
Bottom line: In a cohort of patients with AMI, higher rates of hospital echocardiography use did not appear to be associated with better clinical outcomes but were associated with longer length of stay and greater hospital costs.
Citation: Pack QR et al. Association between inpatient echocardiography use and outcomes in adult patients with acute myocardial infarction. JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.1051.
Dr. Liu is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: Guidelines recommend that patients with AMI undergo universal echocardiography for the assessment of cardiac structure and ejection fraction, despite modest diagnostic yield.
Study design: Retrospective cohort.
Setting: 397 U.S. hospitals contributing to the Premier Healthcare Informatics inpatient database.
Synopsis: ICD-9 codes were used to identify 98,999 hospitalizations with a discharge diagnosis of AMI. Of these, 70.4% had at least one transthoracic echocardiogram performed. Patients who underwent echocardiogram were more likely than patients without an echocardiogram to have heart failure, pulmonary disease, and intensive care unit stays and require interventions such as noninvasive and invasive ventilation, vasopressors, balloon pumps, and inotropic agents.
Risk-standardized echocardiography rates varied significantly across hospitals, ranging from a median of 54% in the lowest quartile to 83% in the highest quartile. The authors found that use of echocardiography was most strongly associated with the hospital, more so than individual patient factors. In adjusted analyses, no difference was seen in inpatient mortality (odds ratio, 1.02; 95% CI, 0.88-1.99) or 3-month readmission (OR, 1.01; 95% CI, 0.93-1.10), but slightly longer mean length of stay (0.23 days; 95% CI, 0.04-0.41; P = .01) and higher mean costs ($3,164; 95% CI, $1,843-$4,485; P < .001) were found in patients treated at hospitals with the highest quartile of echocardiography use, compared with those in the lowest quartile.
Limitations include lack of information about long-term clinical outcomes, inability to adjust for ejection fraction levels, and reliance on administrative data for AMI and procedure codes.
Bottom line: In a cohort of patients with AMI, higher rates of hospital echocardiography use did not appear to be associated with better clinical outcomes but were associated with longer length of stay and greater hospital costs.
Citation: Pack QR et al. Association between inpatient echocardiography use and outcomes in adult patients with acute myocardial infarction. JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.1051.
Dr. Liu is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
COVID and med ed cost: Are future docs paying more for less?
Like most medical students, Kaitlyn Thomas’s education was abruptly interrupted by the pandemic. Her school, an osteopathic medicine institution in the Midwest, followed guidelines issued by the American Association of Medical Colleges in March, shifting lectures online and suspending activities in which students interacted with patients. But even as Ms. Thomas’s learning opportunities dwindled for the sake of safety, the costs kept piling up.
Instead of going home to live with her family, she stayed in her apartment near school – and kept paying rent – so she could be nearby for the two licensing exams she was scheduled to take 3 months later. Both tests were canceled 9 days before she was scheduled to take them, one without any notification. This meant she had to travel to two different testing sites in two different states. All told, she said, the whole thing cost her around $2,000.
Ms. Thomas’s experience isn’t rare. Across the country, medical students find themselves paying substantial costs for a medical education now greatly altered by the pandemic. Despite restrictions on time spent in hospitals, hands-on learning, social events, and access to libraries, gyms, study spaces, and instructors, the price of tuition hasn’t dropped but has remained the same or has even risen.
In response, students have become vocal about the return on their pricey investment. “Am I just going to end up doing most of my year online, and what does that look like for my future patients?” Ms. Thomas asked. “It really doesn’t feel like a time to be limiting education.”
Medical schools and administrators are scrambling to find creative solutions for safely educating students. No matter what those solutions may be, experts say, the pandemic has drawn fresh attention to enduring questions about how the cost of medical education compares to its value. Although many are frustrated, some see the potential for COVID to open new opportunities for lasting innovation. At the very least, the pandemic has sparked conversations about what matters most in terms of producing qualified physicians.
“While this is a challenging time, we will get through it, and we will continue to educate doctors, and we will get them through to practice,” says Robert Cain, president and CEO of the American Association of Colleges of Osteopathic Medicine. Many in the midst of training still have one lingering question: Is the price future doctors are now paying still worth it?
COVID’s “hidden costs” for students
Tom is a third-year student at an allopathic medicine institution in the Caribbean. He asked not to be fully identified here, owing to concern about possible backlash. In March, Tom was doing clinical rotations in New York City when his training was put on hold. He returned home to Connecticut and resumed working 60-80 hours a week as a paramedic. As much as 75% of that income went to pay for the New York City apartment he was no longer living in – an apartment that cost more than $2,000 a month – and for student loans that suddenly came due when his enrollment status changed.
Tom has been able to take some online courses through his school. But he still doesn’t know whether state licensing boards will accept them, how residency programs will view them, or whether he will eventually have to retake those online classes in person. At the end of September, he was allowed to return to the hospital but was relocated to Chicago and was forced to move on short notice.
Like many students, Tom has worried that the pandemic may prevent him from acquiring crucial elements for his residency applications, things like letters of recommendation or key experiences. That could delay his next stage of training, which would mean lost future income, increasing student loan interest, and lost work experience. “This could also mean the difference between getting a residency and being able to practice medicine and not being able to practice my intended specialty,” he said. “This is the real hidden cost we may have to deal with.”
International medical students hoping to practice in the United States face additional costs. Michelle Warncke earned her bachelor’s degree in America but went to the United Kingdom for her master’s and her medical degree, which she completed in 2019. She then moved to North Carolina with her husband and saved money to take the exams she needed for residency in the states. But her scheduled Step 2 CS exam was canceled because of the pandemic. Now, like hundreds or even thousands of other students, she said she is unable to apply for residency, even as her student loans collect interest. An active Facebook group of international medical graduates includes about 1,500 people with comparable dilemmas.
The path to becoming a physician carries a well-known price tag, one that is already quite high. Now, for many, that price is substantially increasing. “The only way I can actually keep my medical credentials up to date and passable, to be able to ever get a shot at a residency in the following years,” she said, “is to move to another country and work for less pay, pay for a visa, pay for my exams, pay for my language test, and wait and hope that I might be able to as an older graduate then be able to apply for residency.”
Scaling back the price of med school?
Questions about the economics of medical education aren’t new, says David Asch, MD, MBA, an internal medicine physician and executive director of the Center for Health Care Innovation at the University of Pennsylvania, Philadelphia. But the changes forced by COVID could lead to innovations that may finally better balance the financial scales.
Such innovations are necessary, many say, given how medical education costs have skyrocketed over the past half century. In the 1960s, 4 years of medical school cost about $40,000 in today’s dollars, Dr. Asch and colleagues wrote in a 2020 analysis, which they conducted before the pandemic began. By 2018, the price of a medical education in the United States had ballooned to about $300,000. About 75% of students were taking out loans. Upon graduating, the average debt was $200,000.
Medical school is expensive for many tangible reasons, Dr. Asch said. Schools must pay for curriculum, faculty, technology, textbooks, lab materials, facilities, administrators, and more. But policy changes could decrease those costs.
He says one idea would be for medical schools to join forces and give students access to the same basic lectures in the early years, delivered online by top-notch instructors. Students could then participate in on-campus programs that might only require 3 years to complete instead of 4. By demonstrating what can be done via online platforms, he said, the pandemic might pave the way to permanent changes that could reduce costs.
“I’m not trying to pick on biochemistry professors and medical schools, but how many do we need in the country?” Dr. Asch asked. “We’re all watching the same episode of Seinfeld. Why can’t we all watch the same episode of the Krebs cycle?” If all 190 or so medical schools in the United States shared such preclinical courses, he says, each would require a fraction of the current cost to produce. “We could save 99.5% of the cost. So why don’t we do that?”
Pandemic as opportunity
Although the price of medical education has yet to decrease, schools are working to leverage the pandemic to provide increased educational value.
This generation of physicians will not only have to cope with the fallout of this pandemic, they will be the ones responsible for confronting the next pandemic as well, says Donald Brady, MD, senior associate dean for health sciences education at Vanderbilt University, Nashville, Tenn. “They will be the leaders in the future who will better be able to know how to handle it [a pandemic] because they were able to watch it and be part of it safely in the current circumstance.”
As much as possible, Vanderbilt is using the pandemic as an opportunity. As soon as it became clear that students couldn’t be involved in certain hands-on training, instructors developed a course about pandemics that included lectures on ethics, global health, systemic racism, and other topics. It also included experiential components of pandemic management, such as opportunities to work with patients through telehealth.
Students say they feel that they are getting less for their money and that they are paying for experiences that are no longer available, such as hands-on patient contact and community events. However, Dr. Brady said, schools have had to account for new expenses, including various now-required technologies and transitioning to courses online.
Some challenges can’t be solved with money alone. Medical schools across the country are working together to ensure that they are still adequately preparing students. Vanderbilt participates in an AAMC group that meets regularly and is also one of 37 institutions involved in an American Medical Association Consortium (AACOM). These groups discuss challenges, strategies, and opportunities for optimizing medical education during the pandemic.
Some institutions have come up with creative solutions. Ohio University’s Heritage College of Osteopathic Medicine, in Athens, Ohio, in collaboration with the Ohio Department of Health, launched a 4-week rotation for third-year students that focuses on public health. Harvard Medical School, Boston, was one of several schools that allowed students to graduate early in the spring. “We’re constantly talking to our colleagues and friends,” Dr. Brady said. “We learn from each other. There’s a lot of sharing going on.”
Other organizations are also working to make sure students ultimately get what they are paying for: a high-quality education. As soon as the pandemic began, the AACOM organized four working groups to address how schools could better use technology to deliver curricula and how students could participate in public health efforts, among other topics. “For the students, the part they don’t see and can’t really be aware of is all the things that happen behind the scenes,” Mr. Cain said. “People were working really hard to make sure that their education was still delivered, and delivered in a way that was going to assure a good product at the end.”
Ultimately, that product will be held to a rigid standard, said Geoffrey Young, the AAMC’s senior director for student affairs and programs. Medical schools must still meet standards of competency set by the liaison committee on medical education. Mr. Young says that even now those standards remain rigorous enough to ensure that medical students are learning what they need to know. “The core elements for competency may be slightly altered to address the realities that we’re experiencing because of COVID, but the core tenants of competencies will not change,” he said.
Even as conversations continue about what a medical education is worth, the pandemic is drawing new attention to the profession. No signs suggest that the value of tuition or a shift to more virtual offerings are scaring students away. Applications for medical schools were up 17% for the fall of 2021.
Brady expects the surge in interest to continue. “The increased focus and emphasis on public health, the increased focus and emphasis on health equity, the increased focus on the need for a more diverse physician workforce, the interest in basic science research around viruses, the interest in COVID itself – there are a lot of different elements that are setting us up for a potential boom in applications to medical school,” he said.
Beyond increasing interest, the pandemic may also finally force a reckoning on the disconnection between how schools think about costs and how students think about value, Dr. Asch said. “When students say: ‘I’m not getting as much from this,’ they’re saying, ‘you should price this according to its lower value.’ And when the medical schools are saying: ‘Oh, but it’s costing us so much more,’ they’re talking about pricing according to the cost. It’s like one group is speaking Latin and the other group is speaking Greek.” Perhaps, he said, COVID-related changes will finally get them speaking the same language.
This article first appeared on Medscape.com.
Like most medical students, Kaitlyn Thomas’s education was abruptly interrupted by the pandemic. Her school, an osteopathic medicine institution in the Midwest, followed guidelines issued by the American Association of Medical Colleges in March, shifting lectures online and suspending activities in which students interacted with patients. But even as Ms. Thomas’s learning opportunities dwindled for the sake of safety, the costs kept piling up.
Instead of going home to live with her family, she stayed in her apartment near school – and kept paying rent – so she could be nearby for the two licensing exams she was scheduled to take 3 months later. Both tests were canceled 9 days before she was scheduled to take them, one without any notification. This meant she had to travel to two different testing sites in two different states. All told, she said, the whole thing cost her around $2,000.
Ms. Thomas’s experience isn’t rare. Across the country, medical students find themselves paying substantial costs for a medical education now greatly altered by the pandemic. Despite restrictions on time spent in hospitals, hands-on learning, social events, and access to libraries, gyms, study spaces, and instructors, the price of tuition hasn’t dropped but has remained the same or has even risen.
In response, students have become vocal about the return on their pricey investment. “Am I just going to end up doing most of my year online, and what does that look like for my future patients?” Ms. Thomas asked. “It really doesn’t feel like a time to be limiting education.”
Medical schools and administrators are scrambling to find creative solutions for safely educating students. No matter what those solutions may be, experts say, the pandemic has drawn fresh attention to enduring questions about how the cost of medical education compares to its value. Although many are frustrated, some see the potential for COVID to open new opportunities for lasting innovation. At the very least, the pandemic has sparked conversations about what matters most in terms of producing qualified physicians.
“While this is a challenging time, we will get through it, and we will continue to educate doctors, and we will get them through to practice,” says Robert Cain, president and CEO of the American Association of Colleges of Osteopathic Medicine. Many in the midst of training still have one lingering question: Is the price future doctors are now paying still worth it?
COVID’s “hidden costs” for students
Tom is a third-year student at an allopathic medicine institution in the Caribbean. He asked not to be fully identified here, owing to concern about possible backlash. In March, Tom was doing clinical rotations in New York City when his training was put on hold. He returned home to Connecticut and resumed working 60-80 hours a week as a paramedic. As much as 75% of that income went to pay for the New York City apartment he was no longer living in – an apartment that cost more than $2,000 a month – and for student loans that suddenly came due when his enrollment status changed.
Tom has been able to take some online courses through his school. But he still doesn’t know whether state licensing boards will accept them, how residency programs will view them, or whether he will eventually have to retake those online classes in person. At the end of September, he was allowed to return to the hospital but was relocated to Chicago and was forced to move on short notice.
Like many students, Tom has worried that the pandemic may prevent him from acquiring crucial elements for his residency applications, things like letters of recommendation or key experiences. That could delay his next stage of training, which would mean lost future income, increasing student loan interest, and lost work experience. “This could also mean the difference between getting a residency and being able to practice medicine and not being able to practice my intended specialty,” he said. “This is the real hidden cost we may have to deal with.”
International medical students hoping to practice in the United States face additional costs. Michelle Warncke earned her bachelor’s degree in America but went to the United Kingdom for her master’s and her medical degree, which she completed in 2019. She then moved to North Carolina with her husband and saved money to take the exams she needed for residency in the states. But her scheduled Step 2 CS exam was canceled because of the pandemic. Now, like hundreds or even thousands of other students, she said she is unable to apply for residency, even as her student loans collect interest. An active Facebook group of international medical graduates includes about 1,500 people with comparable dilemmas.
The path to becoming a physician carries a well-known price tag, one that is already quite high. Now, for many, that price is substantially increasing. “The only way I can actually keep my medical credentials up to date and passable, to be able to ever get a shot at a residency in the following years,” she said, “is to move to another country and work for less pay, pay for a visa, pay for my exams, pay for my language test, and wait and hope that I might be able to as an older graduate then be able to apply for residency.”
Scaling back the price of med school?
Questions about the economics of medical education aren’t new, says David Asch, MD, MBA, an internal medicine physician and executive director of the Center for Health Care Innovation at the University of Pennsylvania, Philadelphia. But the changes forced by COVID could lead to innovations that may finally better balance the financial scales.
Such innovations are necessary, many say, given how medical education costs have skyrocketed over the past half century. In the 1960s, 4 years of medical school cost about $40,000 in today’s dollars, Dr. Asch and colleagues wrote in a 2020 analysis, which they conducted before the pandemic began. By 2018, the price of a medical education in the United States had ballooned to about $300,000. About 75% of students were taking out loans. Upon graduating, the average debt was $200,000.
Medical school is expensive for many tangible reasons, Dr. Asch said. Schools must pay for curriculum, faculty, technology, textbooks, lab materials, facilities, administrators, and more. But policy changes could decrease those costs.
He says one idea would be for medical schools to join forces and give students access to the same basic lectures in the early years, delivered online by top-notch instructors. Students could then participate in on-campus programs that might only require 3 years to complete instead of 4. By demonstrating what can be done via online platforms, he said, the pandemic might pave the way to permanent changes that could reduce costs.
“I’m not trying to pick on biochemistry professors and medical schools, but how many do we need in the country?” Dr. Asch asked. “We’re all watching the same episode of Seinfeld. Why can’t we all watch the same episode of the Krebs cycle?” If all 190 or so medical schools in the United States shared such preclinical courses, he says, each would require a fraction of the current cost to produce. “We could save 99.5% of the cost. So why don’t we do that?”
Pandemic as opportunity
Although the price of medical education has yet to decrease, schools are working to leverage the pandemic to provide increased educational value.
This generation of physicians will not only have to cope with the fallout of this pandemic, they will be the ones responsible for confronting the next pandemic as well, says Donald Brady, MD, senior associate dean for health sciences education at Vanderbilt University, Nashville, Tenn. “They will be the leaders in the future who will better be able to know how to handle it [a pandemic] because they were able to watch it and be part of it safely in the current circumstance.”
As much as possible, Vanderbilt is using the pandemic as an opportunity. As soon as it became clear that students couldn’t be involved in certain hands-on training, instructors developed a course about pandemics that included lectures on ethics, global health, systemic racism, and other topics. It also included experiential components of pandemic management, such as opportunities to work with patients through telehealth.
Students say they feel that they are getting less for their money and that they are paying for experiences that are no longer available, such as hands-on patient contact and community events. However, Dr. Brady said, schools have had to account for new expenses, including various now-required technologies and transitioning to courses online.
Some challenges can’t be solved with money alone. Medical schools across the country are working together to ensure that they are still adequately preparing students. Vanderbilt participates in an AAMC group that meets regularly and is also one of 37 institutions involved in an American Medical Association Consortium (AACOM). These groups discuss challenges, strategies, and opportunities for optimizing medical education during the pandemic.
Some institutions have come up with creative solutions. Ohio University’s Heritage College of Osteopathic Medicine, in Athens, Ohio, in collaboration with the Ohio Department of Health, launched a 4-week rotation for third-year students that focuses on public health. Harvard Medical School, Boston, was one of several schools that allowed students to graduate early in the spring. “We’re constantly talking to our colleagues and friends,” Dr. Brady said. “We learn from each other. There’s a lot of sharing going on.”
Other organizations are also working to make sure students ultimately get what they are paying for: a high-quality education. As soon as the pandemic began, the AACOM organized four working groups to address how schools could better use technology to deliver curricula and how students could participate in public health efforts, among other topics. “For the students, the part they don’t see and can’t really be aware of is all the things that happen behind the scenes,” Mr. Cain said. “People were working really hard to make sure that their education was still delivered, and delivered in a way that was going to assure a good product at the end.”
Ultimately, that product will be held to a rigid standard, said Geoffrey Young, the AAMC’s senior director for student affairs and programs. Medical schools must still meet standards of competency set by the liaison committee on medical education. Mr. Young says that even now those standards remain rigorous enough to ensure that medical students are learning what they need to know. “The core elements for competency may be slightly altered to address the realities that we’re experiencing because of COVID, but the core tenants of competencies will not change,” he said.
Even as conversations continue about what a medical education is worth, the pandemic is drawing new attention to the profession. No signs suggest that the value of tuition or a shift to more virtual offerings are scaring students away. Applications for medical schools were up 17% for the fall of 2021.
Brady expects the surge in interest to continue. “The increased focus and emphasis on public health, the increased focus and emphasis on health equity, the increased focus on the need for a more diverse physician workforce, the interest in basic science research around viruses, the interest in COVID itself – there are a lot of different elements that are setting us up for a potential boom in applications to medical school,” he said.
Beyond increasing interest, the pandemic may also finally force a reckoning on the disconnection between how schools think about costs and how students think about value, Dr. Asch said. “When students say: ‘I’m not getting as much from this,’ they’re saying, ‘you should price this according to its lower value.’ And when the medical schools are saying: ‘Oh, but it’s costing us so much more,’ they’re talking about pricing according to the cost. It’s like one group is speaking Latin and the other group is speaking Greek.” Perhaps, he said, COVID-related changes will finally get them speaking the same language.
This article first appeared on Medscape.com.
Like most medical students, Kaitlyn Thomas’s education was abruptly interrupted by the pandemic. Her school, an osteopathic medicine institution in the Midwest, followed guidelines issued by the American Association of Medical Colleges in March, shifting lectures online and suspending activities in which students interacted with patients. But even as Ms. Thomas’s learning opportunities dwindled for the sake of safety, the costs kept piling up.
Instead of going home to live with her family, she stayed in her apartment near school – and kept paying rent – so she could be nearby for the two licensing exams she was scheduled to take 3 months later. Both tests were canceled 9 days before she was scheduled to take them, one without any notification. This meant she had to travel to two different testing sites in two different states. All told, she said, the whole thing cost her around $2,000.
Ms. Thomas’s experience isn’t rare. Across the country, medical students find themselves paying substantial costs for a medical education now greatly altered by the pandemic. Despite restrictions on time spent in hospitals, hands-on learning, social events, and access to libraries, gyms, study spaces, and instructors, the price of tuition hasn’t dropped but has remained the same or has even risen.
In response, students have become vocal about the return on their pricey investment. “Am I just going to end up doing most of my year online, and what does that look like for my future patients?” Ms. Thomas asked. “It really doesn’t feel like a time to be limiting education.”
Medical schools and administrators are scrambling to find creative solutions for safely educating students. No matter what those solutions may be, experts say, the pandemic has drawn fresh attention to enduring questions about how the cost of medical education compares to its value. Although many are frustrated, some see the potential for COVID to open new opportunities for lasting innovation. At the very least, the pandemic has sparked conversations about what matters most in terms of producing qualified physicians.
“While this is a challenging time, we will get through it, and we will continue to educate doctors, and we will get them through to practice,” says Robert Cain, president and CEO of the American Association of Colleges of Osteopathic Medicine. Many in the midst of training still have one lingering question: Is the price future doctors are now paying still worth it?
COVID’s “hidden costs” for students
Tom is a third-year student at an allopathic medicine institution in the Caribbean. He asked not to be fully identified here, owing to concern about possible backlash. In March, Tom was doing clinical rotations in New York City when his training was put on hold. He returned home to Connecticut and resumed working 60-80 hours a week as a paramedic. As much as 75% of that income went to pay for the New York City apartment he was no longer living in – an apartment that cost more than $2,000 a month – and for student loans that suddenly came due when his enrollment status changed.
Tom has been able to take some online courses through his school. But he still doesn’t know whether state licensing boards will accept them, how residency programs will view them, or whether he will eventually have to retake those online classes in person. At the end of September, he was allowed to return to the hospital but was relocated to Chicago and was forced to move on short notice.
Like many students, Tom has worried that the pandemic may prevent him from acquiring crucial elements for his residency applications, things like letters of recommendation or key experiences. That could delay his next stage of training, which would mean lost future income, increasing student loan interest, and lost work experience. “This could also mean the difference between getting a residency and being able to practice medicine and not being able to practice my intended specialty,” he said. “This is the real hidden cost we may have to deal with.”
International medical students hoping to practice in the United States face additional costs. Michelle Warncke earned her bachelor’s degree in America but went to the United Kingdom for her master’s and her medical degree, which she completed in 2019. She then moved to North Carolina with her husband and saved money to take the exams she needed for residency in the states. But her scheduled Step 2 CS exam was canceled because of the pandemic. Now, like hundreds or even thousands of other students, she said she is unable to apply for residency, even as her student loans collect interest. An active Facebook group of international medical graduates includes about 1,500 people with comparable dilemmas.
The path to becoming a physician carries a well-known price tag, one that is already quite high. Now, for many, that price is substantially increasing. “The only way I can actually keep my medical credentials up to date and passable, to be able to ever get a shot at a residency in the following years,” she said, “is to move to another country and work for less pay, pay for a visa, pay for my exams, pay for my language test, and wait and hope that I might be able to as an older graduate then be able to apply for residency.”
Scaling back the price of med school?
Questions about the economics of medical education aren’t new, says David Asch, MD, MBA, an internal medicine physician and executive director of the Center for Health Care Innovation at the University of Pennsylvania, Philadelphia. But the changes forced by COVID could lead to innovations that may finally better balance the financial scales.
Such innovations are necessary, many say, given how medical education costs have skyrocketed over the past half century. In the 1960s, 4 years of medical school cost about $40,000 in today’s dollars, Dr. Asch and colleagues wrote in a 2020 analysis, which they conducted before the pandemic began. By 2018, the price of a medical education in the United States had ballooned to about $300,000. About 75% of students were taking out loans. Upon graduating, the average debt was $200,000.
Medical school is expensive for many tangible reasons, Dr. Asch said. Schools must pay for curriculum, faculty, technology, textbooks, lab materials, facilities, administrators, and more. But policy changes could decrease those costs.
He says one idea would be for medical schools to join forces and give students access to the same basic lectures in the early years, delivered online by top-notch instructors. Students could then participate in on-campus programs that might only require 3 years to complete instead of 4. By demonstrating what can be done via online platforms, he said, the pandemic might pave the way to permanent changes that could reduce costs.
“I’m not trying to pick on biochemistry professors and medical schools, but how many do we need in the country?” Dr. Asch asked. “We’re all watching the same episode of Seinfeld. Why can’t we all watch the same episode of the Krebs cycle?” If all 190 or so medical schools in the United States shared such preclinical courses, he says, each would require a fraction of the current cost to produce. “We could save 99.5% of the cost. So why don’t we do that?”
Pandemic as opportunity
Although the price of medical education has yet to decrease, schools are working to leverage the pandemic to provide increased educational value.
This generation of physicians will not only have to cope with the fallout of this pandemic, they will be the ones responsible for confronting the next pandemic as well, says Donald Brady, MD, senior associate dean for health sciences education at Vanderbilt University, Nashville, Tenn. “They will be the leaders in the future who will better be able to know how to handle it [a pandemic] because they were able to watch it and be part of it safely in the current circumstance.”
As much as possible, Vanderbilt is using the pandemic as an opportunity. As soon as it became clear that students couldn’t be involved in certain hands-on training, instructors developed a course about pandemics that included lectures on ethics, global health, systemic racism, and other topics. It also included experiential components of pandemic management, such as opportunities to work with patients through telehealth.
Students say they feel that they are getting less for their money and that they are paying for experiences that are no longer available, such as hands-on patient contact and community events. However, Dr. Brady said, schools have had to account for new expenses, including various now-required technologies and transitioning to courses online.
Some challenges can’t be solved with money alone. Medical schools across the country are working together to ensure that they are still adequately preparing students. Vanderbilt participates in an AAMC group that meets regularly and is also one of 37 institutions involved in an American Medical Association Consortium (AACOM). These groups discuss challenges, strategies, and opportunities for optimizing medical education during the pandemic.
Some institutions have come up with creative solutions. Ohio University’s Heritage College of Osteopathic Medicine, in Athens, Ohio, in collaboration with the Ohio Department of Health, launched a 4-week rotation for third-year students that focuses on public health. Harvard Medical School, Boston, was one of several schools that allowed students to graduate early in the spring. “We’re constantly talking to our colleagues and friends,” Dr. Brady said. “We learn from each other. There’s a lot of sharing going on.”
Other organizations are also working to make sure students ultimately get what they are paying for: a high-quality education. As soon as the pandemic began, the AACOM organized four working groups to address how schools could better use technology to deliver curricula and how students could participate in public health efforts, among other topics. “For the students, the part they don’t see and can’t really be aware of is all the things that happen behind the scenes,” Mr. Cain said. “People were working really hard to make sure that their education was still delivered, and delivered in a way that was going to assure a good product at the end.”
Ultimately, that product will be held to a rigid standard, said Geoffrey Young, the AAMC’s senior director for student affairs and programs. Medical schools must still meet standards of competency set by the liaison committee on medical education. Mr. Young says that even now those standards remain rigorous enough to ensure that medical students are learning what they need to know. “The core elements for competency may be slightly altered to address the realities that we’re experiencing because of COVID, but the core tenants of competencies will not change,” he said.
Even as conversations continue about what a medical education is worth, the pandemic is drawing new attention to the profession. No signs suggest that the value of tuition or a shift to more virtual offerings are scaring students away. Applications for medical schools were up 17% for the fall of 2021.
Brady expects the surge in interest to continue. “The increased focus and emphasis on public health, the increased focus and emphasis on health equity, the increased focus on the need for a more diverse physician workforce, the interest in basic science research around viruses, the interest in COVID itself – there are a lot of different elements that are setting us up for a potential boom in applications to medical school,” he said.
Beyond increasing interest, the pandemic may also finally force a reckoning on the disconnection between how schools think about costs and how students think about value, Dr. Asch said. “When students say: ‘I’m not getting as much from this,’ they’re saying, ‘you should price this according to its lower value.’ And when the medical schools are saying: ‘Oh, but it’s costing us so much more,’ they’re talking about pricing according to the cost. It’s like one group is speaking Latin and the other group is speaking Greek.” Perhaps, he said, COVID-related changes will finally get them speaking the same language.
This article first appeared on Medscape.com.
Two COVID-19 outpatient antibody drugs show encouraging results
Two COVID-19 antibody treatments, one developed by Regeneron and the other by Eli Lilly, show promise in the outpatient setting in results released on Oct. 28.
Regeneron, in a randomized, double-blind trial, is assessing the effect of adding its investigational antibody cocktail REGN-COV2 to usual standard of care in comparison with adding placebo to standard of care. A descriptive analysis from the first 275 patients was previously reported. The data described on Oct. 28, which involve an additional 524 patients, show that the trial met all of the first nine endpoints.
Regeneron announced prospective results from its phase 2/3 trial showing REGN-COV2 significantly reduced viral load and patient medical visits, which included hospitalizations, visits to an emergency department, visits for urgent care, and/or physician office/telemedicine visits.
Interest in the cocktail spiked after President Donald Trump extolled its benefits after it was used in his own COVID-19 treatment earlier in October.
Trump received the highest dose of the drug, 8 g, but, according to a Regeneron news release announcing the latest findings, “results showed no significant difference in virologic or clinical efficacy between the REGN-COV2 high dose (8 grams) and low dose (2.4 grams).”
The company described further results of the industry-funded study in the release: “On the primary endpoint, the average daily change in viral load through day 7 (mean time-weighted average change from baseline) in patients with high viral load (defined as greater than107 copies/mL) was a 0.68 log10 copies/mL greater reduction with REGN-COV2 compared to placebo (combined dose groups; P < .0001). There was a 1.08 log greater reduction with REGN-COV2 treatment by day 5, which corresponds to REGN-COV2 patients having, on average, a greater than 10-fold reduction in viral load, compared to placebo.”
The treatment appears to be most effective in patients most at risk, whether because of high viral load, ineffective baseline antibody immune response, or preexisting conditions, according to the researchers.
According to the press release, these results have not been peer reviewed but have been submitted to the US Food and Drug Administration, which is reviewing a potential emergency use authorization for the treatment in high-risk adults with mild to moderate COVID-19.
Operation Warp Speed, the Trump administration’s treatment and vaccine program, contracted in July with Regeneron for up to 300,000 doses of its antibody cocktail.
Lilly treatment shows drop in hospitalizations, symptoms
Another treatment, also given in the outpatient setting, shows promise as well.
Patients recently diagnosed with mild to moderate COVID-19 who received Eli Lilly’s antibody treatment LY-CoV555 had fewer hospitalizations and symptoms compared with a group that received placebo, an interim analysis of a phase 2 trial indicates.
Peter Chen, MD, with the Department of Medicine, Women’s Guild Lung Institute at Cedars-Sinai Medical Center, Los Angeles, California, and colleagues found that the most profound effects were in the high-risk groups.
The interim findings of the BLAZE-1 study, which was funded by Eli Lilly, were published online October 28 in The New England Journal of Medicine.
Researchers randomly assigned 452 patients to receive an intravenous infusion of LY-CoV555 in one of three doses (700 mg, 2800 mg, or 7000 mg) or placebo.
In the interim analysis, the researchers found that for the entire population, more than 99.97% of viral RNA was eliminated.
For patients who received the 2800-mg dose, the difference from placebo in the decrease from baseline was −0.53 (95% CI, −0.98 to −0.08; P = .02), for a log viral load that was lower by a factor of 3.4. Benefit over placebo was not significant with the other doses.
At day 29, according to the investigators, the percentage of patients hospitalized with COVID-19 was 1.6% (5 of 309 patients) in the treatment group compared with 6.3% (9 of 143 patients) in the placebo group.
Data indicate that the safety profile was similar whether patients received the active treatment or placebo.
“If these results are confirmed in additional analyses in this trial, LY-CoV555 could become a useful treatment for emergency use in patients with recently diagnosed Covid-19,” the authors write.
Deborah Fuller, PhD, professor in the Department of Microbiology at the University of Washington School of Medicine in Seattle, told Medscape Medical News the findings are «exciting» but only part of the treatment solution.
“What’s remarkable about these two studies and others I’ve seen,” she said, “is how consistent they are in terms of the window of time they will be effective, and that’s because they are just targeting the virus itself. They do not have an effect on the inflammation unless they stop the replication early enough.”
The treatments are effective when they are given near the time of diagnosis, she pointed out.
“Once the virus has started that inflammatory cascade in your body, then that train has left the station and you have to deal with the inflammation,” Fuller said.
She says future treatments will likely have to include both the antiviral and anti-inflammatory properties, and physicians will have to assess what’s best, given the stage of the the patient’s disease.
The trial of REGN-COV2 is funded by Regeneron. The BLAZE-1 study is funded by Eli Lilly. Many of the authors have financial ties to Eli Lilly. Fuller has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Two COVID-19 antibody treatments, one developed by Regeneron and the other by Eli Lilly, show promise in the outpatient setting in results released on Oct. 28.
Regeneron, in a randomized, double-blind trial, is assessing the effect of adding its investigational antibody cocktail REGN-COV2 to usual standard of care in comparison with adding placebo to standard of care. A descriptive analysis from the first 275 patients was previously reported. The data described on Oct. 28, which involve an additional 524 patients, show that the trial met all of the first nine endpoints.
Regeneron announced prospective results from its phase 2/3 trial showing REGN-COV2 significantly reduced viral load and patient medical visits, which included hospitalizations, visits to an emergency department, visits for urgent care, and/or physician office/telemedicine visits.
Interest in the cocktail spiked after President Donald Trump extolled its benefits after it was used in his own COVID-19 treatment earlier in October.
Trump received the highest dose of the drug, 8 g, but, according to a Regeneron news release announcing the latest findings, “results showed no significant difference in virologic or clinical efficacy between the REGN-COV2 high dose (8 grams) and low dose (2.4 grams).”
The company described further results of the industry-funded study in the release: “On the primary endpoint, the average daily change in viral load through day 7 (mean time-weighted average change from baseline) in patients with high viral load (defined as greater than107 copies/mL) was a 0.68 log10 copies/mL greater reduction with REGN-COV2 compared to placebo (combined dose groups; P < .0001). There was a 1.08 log greater reduction with REGN-COV2 treatment by day 5, which corresponds to REGN-COV2 patients having, on average, a greater than 10-fold reduction in viral load, compared to placebo.”
The treatment appears to be most effective in patients most at risk, whether because of high viral load, ineffective baseline antibody immune response, or preexisting conditions, according to the researchers.
According to the press release, these results have not been peer reviewed but have been submitted to the US Food and Drug Administration, which is reviewing a potential emergency use authorization for the treatment in high-risk adults with mild to moderate COVID-19.
Operation Warp Speed, the Trump administration’s treatment and vaccine program, contracted in July with Regeneron for up to 300,000 doses of its antibody cocktail.
Lilly treatment shows drop in hospitalizations, symptoms
Another treatment, also given in the outpatient setting, shows promise as well.
Patients recently diagnosed with mild to moderate COVID-19 who received Eli Lilly’s antibody treatment LY-CoV555 had fewer hospitalizations and symptoms compared with a group that received placebo, an interim analysis of a phase 2 trial indicates.
Peter Chen, MD, with the Department of Medicine, Women’s Guild Lung Institute at Cedars-Sinai Medical Center, Los Angeles, California, and colleagues found that the most profound effects were in the high-risk groups.
The interim findings of the BLAZE-1 study, which was funded by Eli Lilly, were published online October 28 in The New England Journal of Medicine.
Researchers randomly assigned 452 patients to receive an intravenous infusion of LY-CoV555 in one of three doses (700 mg, 2800 mg, or 7000 mg) or placebo.
In the interim analysis, the researchers found that for the entire population, more than 99.97% of viral RNA was eliminated.
For patients who received the 2800-mg dose, the difference from placebo in the decrease from baseline was −0.53 (95% CI, −0.98 to −0.08; P = .02), for a log viral load that was lower by a factor of 3.4. Benefit over placebo was not significant with the other doses.
At day 29, according to the investigators, the percentage of patients hospitalized with COVID-19 was 1.6% (5 of 309 patients) in the treatment group compared with 6.3% (9 of 143 patients) in the placebo group.
Data indicate that the safety profile was similar whether patients received the active treatment or placebo.
“If these results are confirmed in additional analyses in this trial, LY-CoV555 could become a useful treatment for emergency use in patients with recently diagnosed Covid-19,” the authors write.
Deborah Fuller, PhD, professor in the Department of Microbiology at the University of Washington School of Medicine in Seattle, told Medscape Medical News the findings are «exciting» but only part of the treatment solution.
“What’s remarkable about these two studies and others I’ve seen,” she said, “is how consistent they are in terms of the window of time they will be effective, and that’s because they are just targeting the virus itself. They do not have an effect on the inflammation unless they stop the replication early enough.”
The treatments are effective when they are given near the time of diagnosis, she pointed out.
“Once the virus has started that inflammatory cascade in your body, then that train has left the station and you have to deal with the inflammation,” Fuller said.
She says future treatments will likely have to include both the antiviral and anti-inflammatory properties, and physicians will have to assess what’s best, given the stage of the the patient’s disease.
The trial of REGN-COV2 is funded by Regeneron. The BLAZE-1 study is funded by Eli Lilly. Many of the authors have financial ties to Eli Lilly. Fuller has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Two COVID-19 antibody treatments, one developed by Regeneron and the other by Eli Lilly, show promise in the outpatient setting in results released on Oct. 28.
Regeneron, in a randomized, double-blind trial, is assessing the effect of adding its investigational antibody cocktail REGN-COV2 to usual standard of care in comparison with adding placebo to standard of care. A descriptive analysis from the first 275 patients was previously reported. The data described on Oct. 28, which involve an additional 524 patients, show that the trial met all of the first nine endpoints.
Regeneron announced prospective results from its phase 2/3 trial showing REGN-COV2 significantly reduced viral load and patient medical visits, which included hospitalizations, visits to an emergency department, visits for urgent care, and/or physician office/telemedicine visits.
Interest in the cocktail spiked after President Donald Trump extolled its benefits after it was used in his own COVID-19 treatment earlier in October.
Trump received the highest dose of the drug, 8 g, but, according to a Regeneron news release announcing the latest findings, “results showed no significant difference in virologic or clinical efficacy between the REGN-COV2 high dose (8 grams) and low dose (2.4 grams).”
The company described further results of the industry-funded study in the release: “On the primary endpoint, the average daily change in viral load through day 7 (mean time-weighted average change from baseline) in patients with high viral load (defined as greater than107 copies/mL) was a 0.68 log10 copies/mL greater reduction with REGN-COV2 compared to placebo (combined dose groups; P < .0001). There was a 1.08 log greater reduction with REGN-COV2 treatment by day 5, which corresponds to REGN-COV2 patients having, on average, a greater than 10-fold reduction in viral load, compared to placebo.”
The treatment appears to be most effective in patients most at risk, whether because of high viral load, ineffective baseline antibody immune response, or preexisting conditions, according to the researchers.
According to the press release, these results have not been peer reviewed but have been submitted to the US Food and Drug Administration, which is reviewing a potential emergency use authorization for the treatment in high-risk adults with mild to moderate COVID-19.
Operation Warp Speed, the Trump administration’s treatment and vaccine program, contracted in July with Regeneron for up to 300,000 doses of its antibody cocktail.
Lilly treatment shows drop in hospitalizations, symptoms
Another treatment, also given in the outpatient setting, shows promise as well.
Patients recently diagnosed with mild to moderate COVID-19 who received Eli Lilly’s antibody treatment LY-CoV555 had fewer hospitalizations and symptoms compared with a group that received placebo, an interim analysis of a phase 2 trial indicates.
Peter Chen, MD, with the Department of Medicine, Women’s Guild Lung Institute at Cedars-Sinai Medical Center, Los Angeles, California, and colleagues found that the most profound effects were in the high-risk groups.
The interim findings of the BLAZE-1 study, which was funded by Eli Lilly, were published online October 28 in The New England Journal of Medicine.
Researchers randomly assigned 452 patients to receive an intravenous infusion of LY-CoV555 in one of three doses (700 mg, 2800 mg, or 7000 mg) or placebo.
In the interim analysis, the researchers found that for the entire population, more than 99.97% of viral RNA was eliminated.
For patients who received the 2800-mg dose, the difference from placebo in the decrease from baseline was −0.53 (95% CI, −0.98 to −0.08; P = .02), for a log viral load that was lower by a factor of 3.4. Benefit over placebo was not significant with the other doses.
At day 29, according to the investigators, the percentage of patients hospitalized with COVID-19 was 1.6% (5 of 309 patients) in the treatment group compared with 6.3% (9 of 143 patients) in the placebo group.
Data indicate that the safety profile was similar whether patients received the active treatment or placebo.
“If these results are confirmed in additional analyses in this trial, LY-CoV555 could become a useful treatment for emergency use in patients with recently diagnosed Covid-19,” the authors write.
Deborah Fuller, PhD, professor in the Department of Microbiology at the University of Washington School of Medicine in Seattle, told Medscape Medical News the findings are «exciting» but only part of the treatment solution.
“What’s remarkable about these two studies and others I’ve seen,” she said, “is how consistent they are in terms of the window of time they will be effective, and that’s because they are just targeting the virus itself. They do not have an effect on the inflammation unless they stop the replication early enough.”
The treatments are effective when they are given near the time of diagnosis, she pointed out.
“Once the virus has started that inflammatory cascade in your body, then that train has left the station and you have to deal with the inflammation,” Fuller said.
She says future treatments will likely have to include both the antiviral and anti-inflammatory properties, and physicians will have to assess what’s best, given the stage of the the patient’s disease.
The trial of REGN-COV2 is funded by Regeneron. The BLAZE-1 study is funded by Eli Lilly. Many of the authors have financial ties to Eli Lilly. Fuller has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.