User login
-
Are psychiatrists more prepared for COVID-19 than we think?
Helping patients navigate surreal situations is what we do
A meme has been going around the Internet in which a Muppet is dressed as a doctor, and the caption declares: “If you don’t want to be intubated by a psychiatrist, stay home!” This meme is meant as a commentary on health care worker shortages. But it also touches on the concerns of psychiatrists who might be questioning our role in the pandemic, given that we are physicians who do not regularly rely on labs or imaging to guide treatment. And we rarely even touch our patients.
As observed by Henry A. Nasrallah, MD, editor in chief of Current Psychiatry, who referred to anxiety as endemic during a viral pandemic (Current Psychiatry. 2020 April;19[4]:e3-5), our society is experiencing intense psychological repercussions from the pandemic. These repercussions will evolve from anxiety to despair, and for some, to resilience.
All jokes aside about the medical knowledge of psychiatrists, we are on the cutting edge of how to address the pandemic of fear and uncertainty gripping individuals and society across the nation.
Isn’t it our role as psychiatrists to help people face the reality of personal and societal crises? Aren’t we trained to help people find their internal reserves, bolster them with medications and/or psychotherapy, and prepare them to respond to challenges? I propose that our training and particular experience of hearing patients’ stories has indeed prepared us to receive surreal information and package it into a palatable, even therapeutic, form for our patients.
I’d like to present two cases I’ve recently seen during the first stages of the COVID-19 pandemic juxtaposed with patients I saw during “normal” times. These cases show that, as psychiatrists, we are prepared to face the psychological impact of this crisis.
A patient called me about worsened anxiety after she’d been sidelined at home from her job as a waitress and was currently spending 12 hours a day with her overbearing mother. She had always used her work to buffer her anxiety, as the fast pace of the restaurant kept her from ruminating.
The call reminded me of ones I’d receive from female patients during the MeToo movement and particularly during the Brett Kavanaugh confirmation hearings for the Supreme Court, in which a sexual assault victim and alleged perpetrator faced off on television. During therapy and medication management sessions alike, I would talk to women struggling with the number of news stories about victims coming forward after sexual assault. They were reliving their humiliations, and despite the empowering nature of the movement, they felt vulnerable in the shadow of memories of their perpetrators.
The advice I gave then is similar to the guidance I give now, and also is closely related to the Centers for Disease Control and Prevention advice on its website on how to manage the mental health impact of COVID-19. People can be informed without suffering by taking these steps:
- Limit the amount of news and social media consumed, and if possible, try to schedule news consumption into discrete periods that are not close to bedtime or other periods meant for relaxation.
- Reach out to loved ones and friends who remind you of strength and better times.
- Make time to relax and unwind, either through resting or engaging in an activity you enjoy.
- Take care of your body and mind with exercise.
- Try for 8 hours of sleep a night (even if it doesn’t happen).
- Use techniques such as meditating, doing yoga, or breathing to practice focusing your attention somewhere.
All of our lives have been disrupted by COVID-19 and acknowledging this to patients can help them feel less isolated and vulnerable. Our patients with diagnosed psychiatric disorders will be more susceptible to crippling anxiety, exacerbations in panic attacks, obsessive-compulsive disorder symptoms, and resurgence of suicidal ideation in the face of uncertainty and despair. They may also be more likely to experience the socioeconomic fallout of this pandemic. But it’s not just these individuals who will be hit with intense feelings as we wonder what the next day, month, or 6 months hold for us, our families, our friends, our country, and our world.
Recently, I had one of the more surreal experiences of my professional life. I work as a consulation-liaison psychiatrist on the medical wards, and I was consulted to treat a young woman from Central America with schizophrenia who made a serious suicide attempt in mid-February before COVID-19 was part of the lexicon.
After an overdose, she developed aspiration pneumonia and acute respiratory distress syndrome and ended up in the ICU on a respirator for 3 weeks. Her doctors and family were certain she would die, but she miraculously survived. By the time she was extubated and less delirious from her medically induced coma, the hospital had restricted all visitors because of COVID-19.
Because I speak Spanish, we developed as decent a working relationship as we could, considering the patient’s delirium and blunted affect. On top of restarting her antipsychotics, I had to inform her that her family was no longer allowed to come visit her. Outside of this room, I vacillated on how to tell a woman with a history of paranoia that the hospital would not allow her family to visit because we were in the middle of a pandemic. A contagious virus had quickly spread around the world, cases were now spiking in the United States, much of the country was on lockdown, and the hospital was limiting visitors because asymptomatic individuals could bring the virus into the hospital or be infected by asymptomatic staff.
As the words came out of my mouth, she looked at me as I have looked at psychotic individuals as they spin me yarns of impossible explanation for their symptoms when I know they’re simply psychotic and living in an alternate reality. Imagine just waking up from a coma and your doctor coming in to tell you: “The U.S. is on lockdown because a deadly virus is spreading throughout our country.” You’d think you’ve woken up in a zombie film. Yet, the patient simply nodded and asked: “Will I be able to use the phone to call my family?” I sighed with relief and helped her dial her brother’s number.
Haven’t we all listened to insane stories while keeping a straight face and then answered with a politely bland question? Just a few months ago, I treated a homeless woman with schizophrenia who calmly explained to me that her large malignant ovarian tumor (which I could see protruding under her gown) was the unborn heir of Queen Victoria and Prince Albert. If she allowed the doctors to take it out (that is, treat her cancer) she’d be assassinated by the Russian intelligence agency. She refused to let the doctors sentence her to death. Ultimately, we allowed her to refuse treatment. Despite a month of treatment with antipsychotic medication, her psychotic beliefs did not change, and we could not imagine forcing her through surgery and chemotherapy. She died in hospice.
I’ve walked the valleys of bizarro land many times. Working through the dark reality of COVID-19 should be no match for us psychiatrists who have listened to dark stories and responded with words of comfort or empathic silence. As mental health clinicians, I believe we are well equipped to fight on the front lines of the pandemic of fear that has arrested our country. We can make ourselves available to our patients, friends, family, and institutions – medical or otherwise – that are grappling with how to cope with the psychological impact of COVID-19.
Dr. Posada is a consultation-liaison psychiatry fellow with the Inova Fairfax Hospital/George Washington University program in Falls Church, Va., and associate producer of the MDedge Psychcast. She changed key details about the patients discussed to protect their confidentiality. Dr. Posada has no conflicts of interest.
Helping patients navigate surreal situations is what we do
Helping patients navigate surreal situations is what we do
A meme has been going around the Internet in which a Muppet is dressed as a doctor, and the caption declares: “If you don’t want to be intubated by a psychiatrist, stay home!” This meme is meant as a commentary on health care worker shortages. But it also touches on the concerns of psychiatrists who might be questioning our role in the pandemic, given that we are physicians who do not regularly rely on labs or imaging to guide treatment. And we rarely even touch our patients.
As observed by Henry A. Nasrallah, MD, editor in chief of Current Psychiatry, who referred to anxiety as endemic during a viral pandemic (Current Psychiatry. 2020 April;19[4]:e3-5), our society is experiencing intense psychological repercussions from the pandemic. These repercussions will evolve from anxiety to despair, and for some, to resilience.
All jokes aside about the medical knowledge of psychiatrists, we are on the cutting edge of how to address the pandemic of fear and uncertainty gripping individuals and society across the nation.
Isn’t it our role as psychiatrists to help people face the reality of personal and societal crises? Aren’t we trained to help people find their internal reserves, bolster them with medications and/or psychotherapy, and prepare them to respond to challenges? I propose that our training and particular experience of hearing patients’ stories has indeed prepared us to receive surreal information and package it into a palatable, even therapeutic, form for our patients.
I’d like to present two cases I’ve recently seen during the first stages of the COVID-19 pandemic juxtaposed with patients I saw during “normal” times. These cases show that, as psychiatrists, we are prepared to face the psychological impact of this crisis.
A patient called me about worsened anxiety after she’d been sidelined at home from her job as a waitress and was currently spending 12 hours a day with her overbearing mother. She had always used her work to buffer her anxiety, as the fast pace of the restaurant kept her from ruminating.
The call reminded me of ones I’d receive from female patients during the MeToo movement and particularly during the Brett Kavanaugh confirmation hearings for the Supreme Court, in which a sexual assault victim and alleged perpetrator faced off on television. During therapy and medication management sessions alike, I would talk to women struggling with the number of news stories about victims coming forward after sexual assault. They were reliving their humiliations, and despite the empowering nature of the movement, they felt vulnerable in the shadow of memories of their perpetrators.
The advice I gave then is similar to the guidance I give now, and also is closely related to the Centers for Disease Control and Prevention advice on its website on how to manage the mental health impact of COVID-19. People can be informed without suffering by taking these steps:
- Limit the amount of news and social media consumed, and if possible, try to schedule news consumption into discrete periods that are not close to bedtime or other periods meant for relaxation.
- Reach out to loved ones and friends who remind you of strength and better times.
- Make time to relax and unwind, either through resting or engaging in an activity you enjoy.
- Take care of your body and mind with exercise.
- Try for 8 hours of sleep a night (even if it doesn’t happen).
- Use techniques such as meditating, doing yoga, or breathing to practice focusing your attention somewhere.
All of our lives have been disrupted by COVID-19 and acknowledging this to patients can help them feel less isolated and vulnerable. Our patients with diagnosed psychiatric disorders will be more susceptible to crippling anxiety, exacerbations in panic attacks, obsessive-compulsive disorder symptoms, and resurgence of suicidal ideation in the face of uncertainty and despair. They may also be more likely to experience the socioeconomic fallout of this pandemic. But it’s not just these individuals who will be hit with intense feelings as we wonder what the next day, month, or 6 months hold for us, our families, our friends, our country, and our world.
Recently, I had one of the more surreal experiences of my professional life. I work as a consulation-liaison psychiatrist on the medical wards, and I was consulted to treat a young woman from Central America with schizophrenia who made a serious suicide attempt in mid-February before COVID-19 was part of the lexicon.
After an overdose, she developed aspiration pneumonia and acute respiratory distress syndrome and ended up in the ICU on a respirator for 3 weeks. Her doctors and family were certain she would die, but she miraculously survived. By the time she was extubated and less delirious from her medically induced coma, the hospital had restricted all visitors because of COVID-19.
Because I speak Spanish, we developed as decent a working relationship as we could, considering the patient’s delirium and blunted affect. On top of restarting her antipsychotics, I had to inform her that her family was no longer allowed to come visit her. Outside of this room, I vacillated on how to tell a woman with a history of paranoia that the hospital would not allow her family to visit because we were in the middle of a pandemic. A contagious virus had quickly spread around the world, cases were now spiking in the United States, much of the country was on lockdown, and the hospital was limiting visitors because asymptomatic individuals could bring the virus into the hospital or be infected by asymptomatic staff.
As the words came out of my mouth, she looked at me as I have looked at psychotic individuals as they spin me yarns of impossible explanation for their symptoms when I know they’re simply psychotic and living in an alternate reality. Imagine just waking up from a coma and your doctor coming in to tell you: “The U.S. is on lockdown because a deadly virus is spreading throughout our country.” You’d think you’ve woken up in a zombie film. Yet, the patient simply nodded and asked: “Will I be able to use the phone to call my family?” I sighed with relief and helped her dial her brother’s number.
Haven’t we all listened to insane stories while keeping a straight face and then answered with a politely bland question? Just a few months ago, I treated a homeless woman with schizophrenia who calmly explained to me that her large malignant ovarian tumor (which I could see protruding under her gown) was the unborn heir of Queen Victoria and Prince Albert. If she allowed the doctors to take it out (that is, treat her cancer) she’d be assassinated by the Russian intelligence agency. She refused to let the doctors sentence her to death. Ultimately, we allowed her to refuse treatment. Despite a month of treatment with antipsychotic medication, her psychotic beliefs did not change, and we could not imagine forcing her through surgery and chemotherapy. She died in hospice.
I’ve walked the valleys of bizarro land many times. Working through the dark reality of COVID-19 should be no match for us psychiatrists who have listened to dark stories and responded with words of comfort or empathic silence. As mental health clinicians, I believe we are well equipped to fight on the front lines of the pandemic of fear that has arrested our country. We can make ourselves available to our patients, friends, family, and institutions – medical or otherwise – that are grappling with how to cope with the psychological impact of COVID-19.
Dr. Posada is a consultation-liaison psychiatry fellow with the Inova Fairfax Hospital/George Washington University program in Falls Church, Va., and associate producer of the MDedge Psychcast. She changed key details about the patients discussed to protect their confidentiality. Dr. Posada has no conflicts of interest.
A meme has been going around the Internet in which a Muppet is dressed as a doctor, and the caption declares: “If you don’t want to be intubated by a psychiatrist, stay home!” This meme is meant as a commentary on health care worker shortages. But it also touches on the concerns of psychiatrists who might be questioning our role in the pandemic, given that we are physicians who do not regularly rely on labs or imaging to guide treatment. And we rarely even touch our patients.
As observed by Henry A. Nasrallah, MD, editor in chief of Current Psychiatry, who referred to anxiety as endemic during a viral pandemic (Current Psychiatry. 2020 April;19[4]:e3-5), our society is experiencing intense psychological repercussions from the pandemic. These repercussions will evolve from anxiety to despair, and for some, to resilience.
All jokes aside about the medical knowledge of psychiatrists, we are on the cutting edge of how to address the pandemic of fear and uncertainty gripping individuals and society across the nation.
Isn’t it our role as psychiatrists to help people face the reality of personal and societal crises? Aren’t we trained to help people find their internal reserves, bolster them with medications and/or psychotherapy, and prepare them to respond to challenges? I propose that our training and particular experience of hearing patients’ stories has indeed prepared us to receive surreal information and package it into a palatable, even therapeutic, form for our patients.
I’d like to present two cases I’ve recently seen during the first stages of the COVID-19 pandemic juxtaposed with patients I saw during “normal” times. These cases show that, as psychiatrists, we are prepared to face the psychological impact of this crisis.
A patient called me about worsened anxiety after she’d been sidelined at home from her job as a waitress and was currently spending 12 hours a day with her overbearing mother. She had always used her work to buffer her anxiety, as the fast pace of the restaurant kept her from ruminating.
The call reminded me of ones I’d receive from female patients during the MeToo movement and particularly during the Brett Kavanaugh confirmation hearings for the Supreme Court, in which a sexual assault victim and alleged perpetrator faced off on television. During therapy and medication management sessions alike, I would talk to women struggling with the number of news stories about victims coming forward after sexual assault. They were reliving their humiliations, and despite the empowering nature of the movement, they felt vulnerable in the shadow of memories of their perpetrators.
The advice I gave then is similar to the guidance I give now, and also is closely related to the Centers for Disease Control and Prevention advice on its website on how to manage the mental health impact of COVID-19. People can be informed without suffering by taking these steps:
- Limit the amount of news and social media consumed, and if possible, try to schedule news consumption into discrete periods that are not close to bedtime or other periods meant for relaxation.
- Reach out to loved ones and friends who remind you of strength and better times.
- Make time to relax and unwind, either through resting or engaging in an activity you enjoy.
- Take care of your body and mind with exercise.
- Try for 8 hours of sleep a night (even if it doesn’t happen).
- Use techniques such as meditating, doing yoga, or breathing to practice focusing your attention somewhere.
All of our lives have been disrupted by COVID-19 and acknowledging this to patients can help them feel less isolated and vulnerable. Our patients with diagnosed psychiatric disorders will be more susceptible to crippling anxiety, exacerbations in panic attacks, obsessive-compulsive disorder symptoms, and resurgence of suicidal ideation in the face of uncertainty and despair. They may also be more likely to experience the socioeconomic fallout of this pandemic. But it’s not just these individuals who will be hit with intense feelings as we wonder what the next day, month, or 6 months hold for us, our families, our friends, our country, and our world.
Recently, I had one of the more surreal experiences of my professional life. I work as a consulation-liaison psychiatrist on the medical wards, and I was consulted to treat a young woman from Central America with schizophrenia who made a serious suicide attempt in mid-February before COVID-19 was part of the lexicon.
After an overdose, she developed aspiration pneumonia and acute respiratory distress syndrome and ended up in the ICU on a respirator for 3 weeks. Her doctors and family were certain she would die, but she miraculously survived. By the time she was extubated and less delirious from her medically induced coma, the hospital had restricted all visitors because of COVID-19.
Because I speak Spanish, we developed as decent a working relationship as we could, considering the patient’s delirium and blunted affect. On top of restarting her antipsychotics, I had to inform her that her family was no longer allowed to come visit her. Outside of this room, I vacillated on how to tell a woman with a history of paranoia that the hospital would not allow her family to visit because we were in the middle of a pandemic. A contagious virus had quickly spread around the world, cases were now spiking in the United States, much of the country was on lockdown, and the hospital was limiting visitors because asymptomatic individuals could bring the virus into the hospital or be infected by asymptomatic staff.
As the words came out of my mouth, she looked at me as I have looked at psychotic individuals as they spin me yarns of impossible explanation for their symptoms when I know they’re simply psychotic and living in an alternate reality. Imagine just waking up from a coma and your doctor coming in to tell you: “The U.S. is on lockdown because a deadly virus is spreading throughout our country.” You’d think you’ve woken up in a zombie film. Yet, the patient simply nodded and asked: “Will I be able to use the phone to call my family?” I sighed with relief and helped her dial her brother’s number.
Haven’t we all listened to insane stories while keeping a straight face and then answered with a politely bland question? Just a few months ago, I treated a homeless woman with schizophrenia who calmly explained to me that her large malignant ovarian tumor (which I could see protruding under her gown) was the unborn heir of Queen Victoria and Prince Albert. If she allowed the doctors to take it out (that is, treat her cancer) she’d be assassinated by the Russian intelligence agency. She refused to let the doctors sentence her to death. Ultimately, we allowed her to refuse treatment. Despite a month of treatment with antipsychotic medication, her psychotic beliefs did not change, and we could not imagine forcing her through surgery and chemotherapy. She died in hospice.
I’ve walked the valleys of bizarro land many times. Working through the dark reality of COVID-19 should be no match for us psychiatrists who have listened to dark stories and responded with words of comfort or empathic silence. As mental health clinicians, I believe we are well equipped to fight on the front lines of the pandemic of fear that has arrested our country. We can make ourselves available to our patients, friends, family, and institutions – medical or otherwise – that are grappling with how to cope with the psychological impact of COVID-19.
Dr. Posada is a consultation-liaison psychiatry fellow with the Inova Fairfax Hospital/George Washington University program in Falls Church, Va., and associate producer of the MDedge Psychcast. She changed key details about the patients discussed to protect their confidentiality. Dr. Posada has no conflicts of interest.
In the Phoenix area, we are in a lull before the coronavirus storm
“There is no sound save the throb of the blowers and the vibration of the hard-driven engines. There is little motion as the gun crews man their guns and the fire-control details stand with heads bent and their hands clapped over their headphones. Somewhere out there are the enemy planes.”
That’s from one of my favorite WW2 histories, “Torpedo Junction,” by Robert J. Casey. He was a reporter stationed on board the cruiser USS Salt Lake City. The entry is from a day in February 1942 when the ship was part of a force that bombarded the Japanese encampment on Wake Island. The excerpt describes the scene later that afternoon, as they awaited a counterattack from Japanese planes.
For some reason that paragraph kept going through my mind this past Sunday afternoon, in the comparatively mundane situation of sitting in the hospital library signing off on my dictations and reviewing test results. I certainly was in no danger of being bombed or strafed, yet ...
Around me, the hospital was preparing for battle. As I rounded, most of the beds were empty and many of the floors above me were shut down and darkened. Waiting rooms were empty. If you hadn’t read the news you’d think there was a sudden lull in the health care world.
But the real truth is that it’s the calm before an anticipated storm. The elective procedures have all been canceled. Nonurgent outpatient tests are on hold. Only the sickest are being admitted, and they’re being sent out as soon as possible. Every bed possible is being kept open for the feared onslaught of coronavirus patients in the coming weeks. Protective equipment, already in short supply, is being stockpiled as it becomes available. Plans have been made to erect triage tents in the parking lots.
I sit in the library and think of this. It’s quiet except for the soft hum of the air conditioning blowers as Phoenix starts to warm up for another summer. The muted purr of the computer’s hard drive as I click away on the keys. On the floors above me the nurses and respiratory techs and doctors go about their daily business of patient care, wondering when the real battle will begin (probably 2-3 weeks from the time of this writing, if not sooner).
These are scary times. I’d be lying if I said I wasn’t frightened about what might happen to me, my family, my friends, my coworkers, my patients.
The people working in the hospital above me are in the same boat, all nervous about what’s going to happen. None of them is any more immune to coronavirus than the people they’ll be treating.
But, like the crew of the USS Salt Lake City, they’re ready to do their jobs. Because it’s part of what drove each of us into our own part of this field. Because we care and want to help. And health care doesn’t work unless the whole team does.
I respect them all for it. I always have and always will, and now more than ever.
Good luck.
Dr. Block has a solo neurology practice in Scottsdale, Ariz. He has no relevant disclosures.
“There is no sound save the throb of the blowers and the vibration of the hard-driven engines. There is little motion as the gun crews man their guns and the fire-control details stand with heads bent and their hands clapped over their headphones. Somewhere out there are the enemy planes.”
That’s from one of my favorite WW2 histories, “Torpedo Junction,” by Robert J. Casey. He was a reporter stationed on board the cruiser USS Salt Lake City. The entry is from a day in February 1942 when the ship was part of a force that bombarded the Japanese encampment on Wake Island. The excerpt describes the scene later that afternoon, as they awaited a counterattack from Japanese planes.
For some reason that paragraph kept going through my mind this past Sunday afternoon, in the comparatively mundane situation of sitting in the hospital library signing off on my dictations and reviewing test results. I certainly was in no danger of being bombed or strafed, yet ...
Around me, the hospital was preparing for battle. As I rounded, most of the beds were empty and many of the floors above me were shut down and darkened. Waiting rooms were empty. If you hadn’t read the news you’d think there was a sudden lull in the health care world.
But the real truth is that it’s the calm before an anticipated storm. The elective procedures have all been canceled. Nonurgent outpatient tests are on hold. Only the sickest are being admitted, and they’re being sent out as soon as possible. Every bed possible is being kept open for the feared onslaught of coronavirus patients in the coming weeks. Protective equipment, already in short supply, is being stockpiled as it becomes available. Plans have been made to erect triage tents in the parking lots.
I sit in the library and think of this. It’s quiet except for the soft hum of the air conditioning blowers as Phoenix starts to warm up for another summer. The muted purr of the computer’s hard drive as I click away on the keys. On the floors above me the nurses and respiratory techs and doctors go about their daily business of patient care, wondering when the real battle will begin (probably 2-3 weeks from the time of this writing, if not sooner).
These are scary times. I’d be lying if I said I wasn’t frightened about what might happen to me, my family, my friends, my coworkers, my patients.
The people working in the hospital above me are in the same boat, all nervous about what’s going to happen. None of them is any more immune to coronavirus than the people they’ll be treating.
But, like the crew of the USS Salt Lake City, they’re ready to do their jobs. Because it’s part of what drove each of us into our own part of this field. Because we care and want to help. And health care doesn’t work unless the whole team does.
I respect them all for it. I always have and always will, and now more than ever.
Good luck.
Dr. Block has a solo neurology practice in Scottsdale, Ariz. He has no relevant disclosures.
“There is no sound save the throb of the blowers and the vibration of the hard-driven engines. There is little motion as the gun crews man their guns and the fire-control details stand with heads bent and their hands clapped over their headphones. Somewhere out there are the enemy planes.”
That’s from one of my favorite WW2 histories, “Torpedo Junction,” by Robert J. Casey. He was a reporter stationed on board the cruiser USS Salt Lake City. The entry is from a day in February 1942 when the ship was part of a force that bombarded the Japanese encampment on Wake Island. The excerpt describes the scene later that afternoon, as they awaited a counterattack from Japanese planes.
For some reason that paragraph kept going through my mind this past Sunday afternoon, in the comparatively mundane situation of sitting in the hospital library signing off on my dictations and reviewing test results. I certainly was in no danger of being bombed or strafed, yet ...
Around me, the hospital was preparing for battle. As I rounded, most of the beds were empty and many of the floors above me were shut down and darkened. Waiting rooms were empty. If you hadn’t read the news you’d think there was a sudden lull in the health care world.
But the real truth is that it’s the calm before an anticipated storm. The elective procedures have all been canceled. Nonurgent outpatient tests are on hold. Only the sickest are being admitted, and they’re being sent out as soon as possible. Every bed possible is being kept open for the feared onslaught of coronavirus patients in the coming weeks. Protective equipment, already in short supply, is being stockpiled as it becomes available. Plans have been made to erect triage tents in the parking lots.
I sit in the library and think of this. It’s quiet except for the soft hum of the air conditioning blowers as Phoenix starts to warm up for another summer. The muted purr of the computer’s hard drive as I click away on the keys. On the floors above me the nurses and respiratory techs and doctors go about their daily business of patient care, wondering when the real battle will begin (probably 2-3 weeks from the time of this writing, if not sooner).
These are scary times. I’d be lying if I said I wasn’t frightened about what might happen to me, my family, my friends, my coworkers, my patients.
The people working in the hospital above me are in the same boat, all nervous about what’s going to happen. None of them is any more immune to coronavirus than the people they’ll be treating.
But, like the crew of the USS Salt Lake City, they’re ready to do their jobs. Because it’s part of what drove each of us into our own part of this field. Because we care and want to help. And health care doesn’t work unless the whole team does.
I respect them all for it. I always have and always will, and now more than ever.
Good luck.
Dr. Block has a solo neurology practice in Scottsdale, Ariz. He has no relevant disclosures.
Physician couples draft wills, face tough questions amid COVID-19
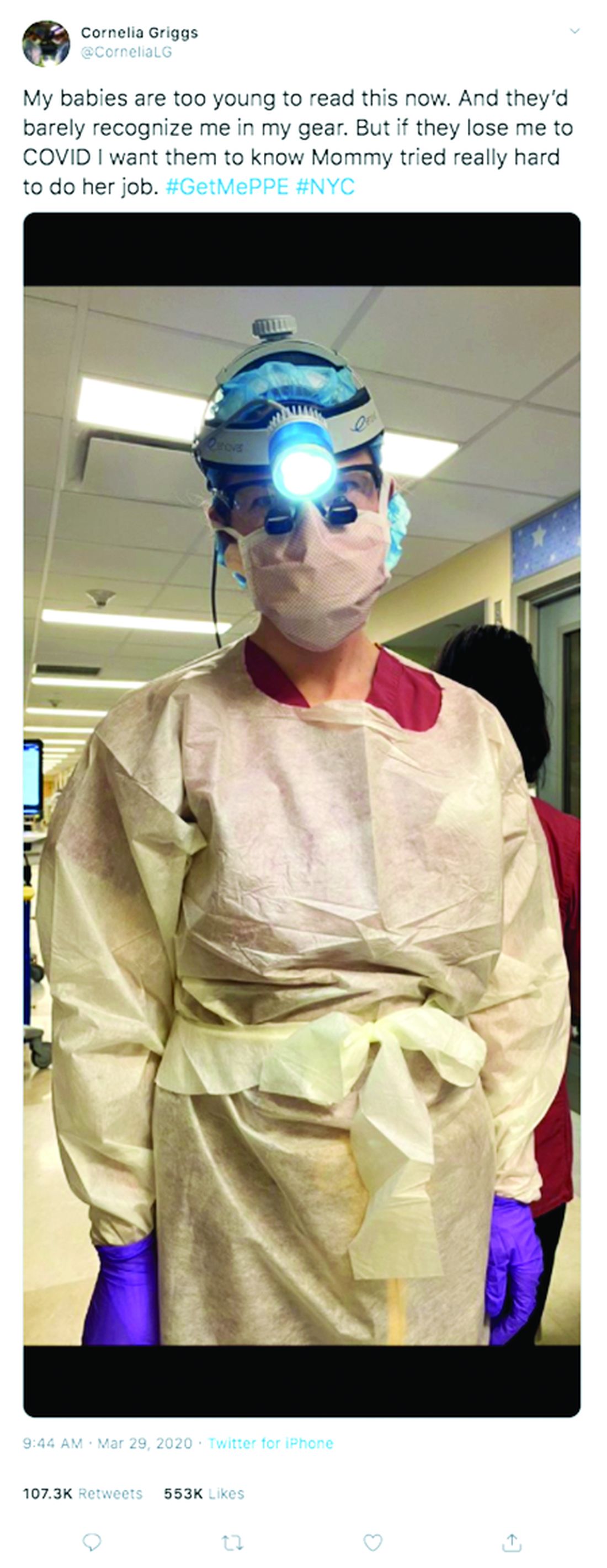
Not long ago, weekends for Cornelia Griggs, MD, meant making trips to the grocery store, chasing after two active toddlers, and eating brunch with her husband after a busy work week. But life has changed dramatically for the family since the spread of COVID-19. On a recent weekend, Dr. Griggs and her husband, Robert Goldstone, MD, spent their days off drafting a will.
“We’re both doctors, and we know that health care workers have an increased risk of contracting COVID,” said Dr. Griggs, a pediatric surgery fellow at Columbia University Irving Medical Center in New York. “It felt like the responsible thing to do: Have a will in place to make sure our wishes are clear about who would manage our property and assets, and who would take care of our kids – God forbid.”
Outlining their final wishes is among many difficult decisions the doctors, both 36, have been forced to make in recent weeks. Dr. Goldstone, a general surgeon at Massachusetts General Hospital in Boston, is no longer returning to New York during his time off, said Dr. Griggs, who has had known COVID-19 exposures. The couple’s children, aged 4 and almost 2, are temporarily living with their grandparents in Connecticut to decrease their exposure risk.
“I felt like it was safer for all of them to be there while I was going back and forth from the hospital,” Dr. Griggs said. “My husband is in Boston. The kids are in Connecticut and I’m in New York. That inherently is hard because our whole family is split up. I don’t know when it will be safe for me to see them again.”
Health professional couples across the country are facing similar challenges as they navigate the risk of contracting COVID-19 at work, while trying to protect their families at home. From childcare dilemmas to quarantine quandaries to end-of-life considerations, partners who work in health care are confronting tough questions as the pandemic continues.
The biggest challenge is the uncertainty, says Angela Weyand, MD, an Ann Arbor, Mich.–based pediatric hematologist/oncologist who shares two young daughters with husband Ted Claflin, MD, a physical medicine and rehabilitation physician. Dr. Weyand said she and her husband are primarily working remotely now, but she knows that one or both could be deployed to the hospital to help care for patients, if the need arises. Nearby Detroit has been labeled a coronavirus “hot spot” by the U.S. Surgeon General.
“Right now, I think our biggest fear is spreading coronavirus to those we love, especially those in higher risk groups,” she said. “At the same time, we are also concerned about our own health and our future ability to be there for our children, a fear that, thankfully, neither one of us has ever had to face before. We are trying to take things one day at a time, acknowledging all that we have to be grateful for, and also learning to accept that many things right now are outside of our control.”
Dr. Weyand, 38, and her husband, 40, finalized their wills in March.
“We have been working on them for quite some time, but before now, there has never been any urgency,” Dr. Weyand said. “Hearing about the high rate of infection in health care workers and the increasing number of deaths in young healthy people made us realize that this should be a priority.”
Dallas internist Bethany Agusala, MD, 36, and her husband, Kartik Agusala, MD, 41, a cardiologist, recently spent time engaged in the same activity. The couple, who work for the University of Texas Southwestern Medical Center, have two children, aged 2 and 4.
“The chances are hopefully small that something bad would happen to either one of us, but it just seemed like a good time to get [a will] in place,” Dr. Bethany Agusala said in an interview. “It’s never an easy thing to think about.
Pediatric surgeon Chethan Sathya, MD, 34, and his wife, 31, a physician assistant, have vastly altered their home routine to prevent the risk of exposure to their 16-month-old daughter. Dr. Sathya works for the Northwell Health System in New York, which has hundreds of hospitalized patients with COVID-19, Dr. Sathya said in an interview. He did not want to disclose his wife's name or institution, but said she works in a COVID-19 unit at a New York hospital.
When his wife returns home, she removes all of her clothes and places them in a bag, showers, and then isolates herself in the bedroom. Dr. Sathya brings his wife meals and then remains in a different room with their baby.
“It’s only been a few days,” he said. “We’re going to decide: Does she just stay in one room at all times or when she doesn’t work for a few days then after 1 day, can she come out? Should she get a hotel room elsewhere? These are the considerations.”
They employ an older nanny whom they also worry about, and with whom they try to limit contact, said Dr. Sathya, who practices at Cohen Children’s Medical Center. In a matter of weeks, Dr. Sathya anticipates he will be called upon to assist in some form with the COVID crisis.
“We haven’t figured that out. I’m not sure what we’ll do,” he said. “There is no perfect solution. You have to adapt. It’s very difficult to do so when you’re living in a condo in New York.”
For Dr. Griggs, life is much quieter at home without her husband and two “laughing, wiggly,” toddlers. Weekends are now defined by resting, video calls with her family, and exercising, when it’s safe, said Dr. Griggs, who recently penned a New York Times opinion piece about the pandemic and is also active on social media regarding personal protective equipment. She calls her husband her “rock” who never fails to put a smile on her face when they chat from across the miles. Her advice for other health care couples is to take it “one day at a time.”
“Don’t try to make plans weeks in advance or let your mind go to a dark place,” she said. “It’s so easy to feel overwhelmed. The only way to get through this is to focus on surviving each day.”
Editor's Note, 3/31/20: Due to incorrect information provided, the hospital where Dr. Sathya's wife works was misidentified. We have removed the name of that hospital. The story does not include his wife's employer, because Dr. Sathya did not have permission to disclose her workplace and she wishes to remain anonymous.
Not long ago, weekends for Cornelia Griggs, MD, meant making trips to the grocery store, chasing after two active toddlers, and eating brunch with her husband after a busy work week. But life has changed dramatically for the family since the spread of COVID-19. On a recent weekend, Dr. Griggs and her husband, Robert Goldstone, MD, spent their days off drafting a will.
“We’re both doctors, and we know that health care workers have an increased risk of contracting COVID,” said Dr. Griggs, a pediatric surgery fellow at Columbia University Irving Medical Center in New York. “It felt like the responsible thing to do: Have a will in place to make sure our wishes are clear about who would manage our property and assets, and who would take care of our kids – God forbid.”
Outlining their final wishes is among many difficult decisions the doctors, both 36, have been forced to make in recent weeks. Dr. Goldstone, a general surgeon at Massachusetts General Hospital in Boston, is no longer returning to New York during his time off, said Dr. Griggs, who has had known COVID-19 exposures. The couple’s children, aged 4 and almost 2, are temporarily living with their grandparents in Connecticut to decrease their exposure risk.
“I felt like it was safer for all of them to be there while I was going back and forth from the hospital,” Dr. Griggs said. “My husband is in Boston. The kids are in Connecticut and I’m in New York. That inherently is hard because our whole family is split up. I don’t know when it will be safe for me to see them again.”
Health professional couples across the country are facing similar challenges as they navigate the risk of contracting COVID-19 at work, while trying to protect their families at home. From childcare dilemmas to quarantine quandaries to end-of-life considerations, partners who work in health care are confronting tough questions as the pandemic continues.
The biggest challenge is the uncertainty, says Angela Weyand, MD, an Ann Arbor, Mich.–based pediatric hematologist/oncologist who shares two young daughters with husband Ted Claflin, MD, a physical medicine and rehabilitation physician. Dr. Weyand said she and her husband are primarily working remotely now, but she knows that one or both could be deployed to the hospital to help care for patients, if the need arises. Nearby Detroit has been labeled a coronavirus “hot spot” by the U.S. Surgeon General.
“Right now, I think our biggest fear is spreading coronavirus to those we love, especially those in higher risk groups,” she said. “At the same time, we are also concerned about our own health and our future ability to be there for our children, a fear that, thankfully, neither one of us has ever had to face before. We are trying to take things one day at a time, acknowledging all that we have to be grateful for, and also learning to accept that many things right now are outside of our control.”
Dr. Weyand, 38, and her husband, 40, finalized their wills in March.
“We have been working on them for quite some time, but before now, there has never been any urgency,” Dr. Weyand said. “Hearing about the high rate of infection in health care workers and the increasing number of deaths in young healthy people made us realize that this should be a priority.”
Dallas internist Bethany Agusala, MD, 36, and her husband, Kartik Agusala, MD, 41, a cardiologist, recently spent time engaged in the same activity. The couple, who work for the University of Texas Southwestern Medical Center, have two children, aged 2 and 4.
“The chances are hopefully small that something bad would happen to either one of us, but it just seemed like a good time to get [a will] in place,” Dr. Bethany Agusala said in an interview. “It’s never an easy thing to think about.
Pediatric surgeon Chethan Sathya, MD, 34, and his wife, 31, a physician assistant, have vastly altered their home routine to prevent the risk of exposure to their 16-month-old daughter. Dr. Sathya works for the Northwell Health System in New York, which has hundreds of hospitalized patients with COVID-19, Dr. Sathya said in an interview. He did not want to disclose his wife's name or institution, but said she works in a COVID-19 unit at a New York hospital.
When his wife returns home, she removes all of her clothes and places them in a bag, showers, and then isolates herself in the bedroom. Dr. Sathya brings his wife meals and then remains in a different room with their baby.
“It’s only been a few days,” he said. “We’re going to decide: Does she just stay in one room at all times or when she doesn’t work for a few days then after 1 day, can she come out? Should she get a hotel room elsewhere? These are the considerations.”
They employ an older nanny whom they also worry about, and with whom they try to limit contact, said Dr. Sathya, who practices at Cohen Children’s Medical Center. In a matter of weeks, Dr. Sathya anticipates he will be called upon to assist in some form with the COVID crisis.
“We haven’t figured that out. I’m not sure what we’ll do,” he said. “There is no perfect solution. You have to adapt. It’s very difficult to do so when you’re living in a condo in New York.”
For Dr. Griggs, life is much quieter at home without her husband and two “laughing, wiggly,” toddlers. Weekends are now defined by resting, video calls with her family, and exercising, when it’s safe, said Dr. Griggs, who recently penned a New York Times opinion piece about the pandemic and is also active on social media regarding personal protective equipment. She calls her husband her “rock” who never fails to put a smile on her face when they chat from across the miles. Her advice for other health care couples is to take it “one day at a time.”

“Don’t try to make plans weeks in advance or let your mind go to a dark place,” she said. “It’s so easy to feel overwhelmed. The only way to get through this is to focus on surviving each day.”
Editor's Note, 3/31/20: Due to incorrect information provided, the hospital where Dr. Sathya's wife works was misidentified. We have removed the name of that hospital. The story does not include his wife's employer, because Dr. Sathya did not have permission to disclose her workplace and she wishes to remain anonymous.
Not long ago, weekends for Cornelia Griggs, MD, meant making trips to the grocery store, chasing after two active toddlers, and eating brunch with her husband after a busy work week. But life has changed dramatically for the family since the spread of COVID-19. On a recent weekend, Dr. Griggs and her husband, Robert Goldstone, MD, spent their days off drafting a will.
“We’re both doctors, and we know that health care workers have an increased risk of contracting COVID,” said Dr. Griggs, a pediatric surgery fellow at Columbia University Irving Medical Center in New York. “It felt like the responsible thing to do: Have a will in place to make sure our wishes are clear about who would manage our property and assets, and who would take care of our kids – God forbid.”
Outlining their final wishes is among many difficult decisions the doctors, both 36, have been forced to make in recent weeks. Dr. Goldstone, a general surgeon at Massachusetts General Hospital in Boston, is no longer returning to New York during his time off, said Dr. Griggs, who has had known COVID-19 exposures. The couple’s children, aged 4 and almost 2, are temporarily living with their grandparents in Connecticut to decrease their exposure risk.
“I felt like it was safer for all of them to be there while I was going back and forth from the hospital,” Dr. Griggs said. “My husband is in Boston. The kids are in Connecticut and I’m in New York. That inherently is hard because our whole family is split up. I don’t know when it will be safe for me to see them again.”
Health professional couples across the country are facing similar challenges as they navigate the risk of contracting COVID-19 at work, while trying to protect their families at home. From childcare dilemmas to quarantine quandaries to end-of-life considerations, partners who work in health care are confronting tough questions as the pandemic continues.
The biggest challenge is the uncertainty, says Angela Weyand, MD, an Ann Arbor, Mich.–based pediatric hematologist/oncologist who shares two young daughters with husband Ted Claflin, MD, a physical medicine and rehabilitation physician. Dr. Weyand said she and her husband are primarily working remotely now, but she knows that one or both could be deployed to the hospital to help care for patients, if the need arises. Nearby Detroit has been labeled a coronavirus “hot spot” by the U.S. Surgeon General.
“Right now, I think our biggest fear is spreading coronavirus to those we love, especially those in higher risk groups,” she said. “At the same time, we are also concerned about our own health and our future ability to be there for our children, a fear that, thankfully, neither one of us has ever had to face before. We are trying to take things one day at a time, acknowledging all that we have to be grateful for, and also learning to accept that many things right now are outside of our control.”
Dr. Weyand, 38, and her husband, 40, finalized their wills in March.
“We have been working on them for quite some time, but before now, there has never been any urgency,” Dr. Weyand said. “Hearing about the high rate of infection in health care workers and the increasing number of deaths in young healthy people made us realize that this should be a priority.”
Dallas internist Bethany Agusala, MD, 36, and her husband, Kartik Agusala, MD, 41, a cardiologist, recently spent time engaged in the same activity. The couple, who work for the University of Texas Southwestern Medical Center, have two children, aged 2 and 4.
“The chances are hopefully small that something bad would happen to either one of us, but it just seemed like a good time to get [a will] in place,” Dr. Bethany Agusala said in an interview. “It’s never an easy thing to think about.
Pediatric surgeon Chethan Sathya, MD, 34, and his wife, 31, a physician assistant, have vastly altered their home routine to prevent the risk of exposure to their 16-month-old daughter. Dr. Sathya works for the Northwell Health System in New York, which has hundreds of hospitalized patients with COVID-19, Dr. Sathya said in an interview. He did not want to disclose his wife's name or institution, but said she works in a COVID-19 unit at a New York hospital.
When his wife returns home, she removes all of her clothes and places them in a bag, showers, and then isolates herself in the bedroom. Dr. Sathya brings his wife meals and then remains in a different room with their baby.
“It’s only been a few days,” he said. “We’re going to decide: Does she just stay in one room at all times or when she doesn’t work for a few days then after 1 day, can she come out? Should she get a hotel room elsewhere? These are the considerations.”
They employ an older nanny whom they also worry about, and with whom they try to limit contact, said Dr. Sathya, who practices at Cohen Children’s Medical Center. In a matter of weeks, Dr. Sathya anticipates he will be called upon to assist in some form with the COVID crisis.
“We haven’t figured that out. I’m not sure what we’ll do,” he said. “There is no perfect solution. You have to adapt. It’s very difficult to do so when you’re living in a condo in New York.”
For Dr. Griggs, life is much quieter at home without her husband and two “laughing, wiggly,” toddlers. Weekends are now defined by resting, video calls with her family, and exercising, when it’s safe, said Dr. Griggs, who recently penned a New York Times opinion piece about the pandemic and is also active on social media regarding personal protective equipment. She calls her husband her “rock” who never fails to put a smile on her face when they chat from across the miles. Her advice for other health care couples is to take it “one day at a time.”

“Don’t try to make plans weeks in advance or let your mind go to a dark place,” she said. “It’s so easy to feel overwhelmed. The only way to get through this is to focus on surviving each day.”
Editor's Note, 3/31/20: Due to incorrect information provided, the hospital where Dr. Sathya's wife works was misidentified. We have removed the name of that hospital. The story does not include his wife's employer, because Dr. Sathya did not have permission to disclose her workplace and she wishes to remain anonymous.
TAILOR-PCI: Clopidogrel genotyping trial narrowly misses endpoint
The largest trial to date investigating the clinical utility of using genetic testing to detect clopidogrel loss-of-function genotype to guide antiplatelet therapy in patients undergoing percutaneous coronary intervention (PCI) missed its primary endpoint of a 50% reduction in cardiovascular events at 1 year.
However, the TAILOR-PCI trial did show a 34% reduction in such events at 1 year, as well as a statistically significant 40% reduction in the total number of events per patient receiving genetically guided treatment compared with patients who received standard treatment.
In addition, a post hoc analysis found a significant 79% reduction in the rate of adverse events in the first 3 months of treatment among patients who received genetically guided therapy compared with those who did not.
The study was presented March 28 during the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology.
“Although these results fell short of the effect size that we predicted, they nevertheless provide a signal that offers support for the benefit of genetically guided therapy, with approximately one-third fewer adverse events in the patients who received genetically guided treatment compared with those who did not,” concluded Naveen L. Pereira, MD, professor of medicine at the Mayo Clinic in Rochester, Minnesota, and co-principal investigator of the study.
Pereira said the post hoc analysis of the first 3 months of treatment was particularly interesting. “This period immediately after PCI is when patients are at the highest risk for adverse events. We now know that antiplatelet drug therapy is critical during the first 3 months after PCI. Our findings suggest that the lion’s share of the benefit of genetically guided therapy may occur during this high-risk period,” he noted.
However, he added, “Because this wasn’t a preplanned analysis, we can’t draw firm conclusions from it, but it merits further study.”
Asked during an ACC virtual press conference how these results may influence clinical practice, Pereira said he hopes it changes practice toward genotyping.
“We set a very high standard in trying to achieve a 50% reduction in events, but we did see a 34% reduction. I think the probability of the results being true is very high,” he said. “I hope people pay attention to that. I’m not sure what the guidelines will do, but I believe if clopidogrel genetic information is made available to the physician, not changing therapy in a patient who has the loss-of-function gene will now be very difficult.”
Discussant of the trial, Roxana Mehran, MD, Mount Sinai Hospital, New York City, said she thought the results were good enough clinically to justify using genotyping to guide therapy.
“The trial showed an absolute 1.8% reduction and a relative 34% reduction in cardiovascular events, which did not quite meet the P value for significance, and they are supported by a significant reduction in multiple events, and a large difference at 3 months, although these are not primary analyses. So, for me this trial has shown that tailoring antiplatelet therapy by genetic testing is beneficial,” she said.
Another outside commentator, Patrick O’Gara, MD, Brigham and Women’s Hospital, Boston, Massachusetts, described TAILOR-PCI as a “terrific study.”
“Together with the study presented last year showing genotype-guided clopidogrel treatment was noninferior to ticagrelor/prasugrel in STEMI [non-ST-segment elevation myocardial infarction] patients, it chips away at the biologic appropriateness of targeting therapies based on genetic risk,” he said.
“I would hate people to focus on the fact the primary endpoint was missed by one hundredth of a percentage point but hope they would rather consider the bigger picture of making this genotype test more available and accessible to inform clinical decision making,” O’Gara added. “It just makes too much sense to ignore this potential.”
The TAILOR-PCI trial enrolled 5302 patients from 40 centers in the United States, Canada, Mexico, and South Korea who had undergone PCI with stenting. They were randomly assigned to genetic testing for the clopidogrel loss-of-function variant or a group that received standard treatment (clopidogrel) without genetic testing.
In the genetic testing group, 35% of patients were found to have the clopidogrel loss-of-function variant and were therefore prescribed ticagrelor, whereas those without the loss-of-function variant received clopidogrel.
After 1 year, the primary endpoint, a composite of cardiovascular death, MI, stroke, definite or probable stent thrombosis, and severe recurrent ischemia, occurred in 35 patients (4%) of the group that received genetically guided treatment, compared with 54 (5.9%) in the conventionally treated group (adjusted hazard ratio [HR], 0.66; 95% confidence interval [CI], 0.43 - 1.02; P = .56).
A prespecified analysis of total events (rather than just analysis of first event per patient) showed a 40% reduction in the genotyped group (HR, 0.60; 95% CI, 0.41 - 0.89; P = .011).
“Multiple adverse events represent a higher burden on the patient, so it is encouraging to see a significant reduction in cumulative events with genetically guided therapy,” Pereira said.
There was no difference in the safety endpoint of TIMI major bleeding or minor bleeding between the two groups: 1.9% in the genetically guided group vs 1.6% in the conventional treatment group.
The results did not differ between various subgroups in the trial, including race or ethnicity. Although Asian patients have a higher occurrence of the clopidogrel loss-of-function gene, the event risk reductions were similar in Asian and white patients in the study.
Pereira said the study may have been underpowered because of recent improvements in care. When the TAILOR-PCI trial was designed in 2012, around 10% to 12% of patients who received a stent could be expected to have a major adverse event, but during the trial, greater use of drug-coated stents and other treatments significantly reduced the expected rate of adverse events and made it more difficult for the trial to reach its goal of a 50% reduction in adverse events with the number of patients enrolled, he explained.
As part of the discussion, Mehran pointed out that more than 80% of the patients in the trial had acute coronary syndrome (ACS) and yet were being sent home on clopidogrel, which she said she found “daunting.”
“This begs the question of whether they were lower-risk patients and not really the hot unstable ACS patients with large thrombus burden where we see higher event rates,” Mehran commented. She also noted the results must be considered in the new era of platelet monotherapy, where aspirin is being withdrawn, and asked whether clopidogrel monotherapy would be considered safe without aspirin on board.
The researchers are planning a cost-effectiveness analysis of genetically guided therapy based on these data, and they are also continuing to follow patients over the longer term.
The TAILOR-PCI study was funded by the Mayo Clinic in collaboration with the National Heart, Lung, and Blood Institute. Spartan Bioscience Inc supplied the genetic tests used. Pereira reports no relevant disclosures.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology. Abstract 20-LB-20309-ACC. Presented March 28, 2020.
This article first appeared on Medscape.com.
The largest trial to date investigating the clinical utility of using genetic testing to detect clopidogrel loss-of-function genotype to guide antiplatelet therapy in patients undergoing percutaneous coronary intervention (PCI) missed its primary endpoint of a 50% reduction in cardiovascular events at 1 year.
However, the TAILOR-PCI trial did show a 34% reduction in such events at 1 year, as well as a statistically significant 40% reduction in the total number of events per patient receiving genetically guided treatment compared with patients who received standard treatment.
In addition, a post hoc analysis found a significant 79% reduction in the rate of adverse events in the first 3 months of treatment among patients who received genetically guided therapy compared with those who did not.
The study was presented March 28 during the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology.
“Although these results fell short of the effect size that we predicted, they nevertheless provide a signal that offers support for the benefit of genetically guided therapy, with approximately one-third fewer adverse events in the patients who received genetically guided treatment compared with those who did not,” concluded Naveen L. Pereira, MD, professor of medicine at the Mayo Clinic in Rochester, Minnesota, and co-principal investigator of the study.
Pereira said the post hoc analysis of the first 3 months of treatment was particularly interesting. “This period immediately after PCI is when patients are at the highest risk for adverse events. We now know that antiplatelet drug therapy is critical during the first 3 months after PCI. Our findings suggest that the lion’s share of the benefit of genetically guided therapy may occur during this high-risk period,” he noted.
However, he added, “Because this wasn’t a preplanned analysis, we can’t draw firm conclusions from it, but it merits further study.”
Asked during an ACC virtual press conference how these results may influence clinical practice, Pereira said he hopes it changes practice toward genotyping.
“We set a very high standard in trying to achieve a 50% reduction in events, but we did see a 34% reduction. I think the probability of the results being true is very high,” he said. “I hope people pay attention to that. I’m not sure what the guidelines will do, but I believe if clopidogrel genetic information is made available to the physician, not changing therapy in a patient who has the loss-of-function gene will now be very difficult.”
Discussant of the trial, Roxana Mehran, MD, Mount Sinai Hospital, New York City, said she thought the results were good enough clinically to justify using genotyping to guide therapy.
“The trial showed an absolute 1.8% reduction and a relative 34% reduction in cardiovascular events, which did not quite meet the P value for significance, and they are supported by a significant reduction in multiple events, and a large difference at 3 months, although these are not primary analyses. So, for me this trial has shown that tailoring antiplatelet therapy by genetic testing is beneficial,” she said.
Another outside commentator, Patrick O’Gara, MD, Brigham and Women’s Hospital, Boston, Massachusetts, described TAILOR-PCI as a “terrific study.”
“Together with the study presented last year showing genotype-guided clopidogrel treatment was noninferior to ticagrelor/prasugrel in STEMI [non-ST-segment elevation myocardial infarction] patients, it chips away at the biologic appropriateness of targeting therapies based on genetic risk,” he said.
“I would hate people to focus on the fact the primary endpoint was missed by one hundredth of a percentage point but hope they would rather consider the bigger picture of making this genotype test more available and accessible to inform clinical decision making,” O’Gara added. “It just makes too much sense to ignore this potential.”
The TAILOR-PCI trial enrolled 5302 patients from 40 centers in the United States, Canada, Mexico, and South Korea who had undergone PCI with stenting. They were randomly assigned to genetic testing for the clopidogrel loss-of-function variant or a group that received standard treatment (clopidogrel) without genetic testing.
In the genetic testing group, 35% of patients were found to have the clopidogrel loss-of-function variant and were therefore prescribed ticagrelor, whereas those without the loss-of-function variant received clopidogrel.
After 1 year, the primary endpoint, a composite of cardiovascular death, MI, stroke, definite or probable stent thrombosis, and severe recurrent ischemia, occurred in 35 patients (4%) of the group that received genetically guided treatment, compared with 54 (5.9%) in the conventionally treated group (adjusted hazard ratio [HR], 0.66; 95% confidence interval [CI], 0.43 - 1.02; P = .56).
A prespecified analysis of total events (rather than just analysis of first event per patient) showed a 40% reduction in the genotyped group (HR, 0.60; 95% CI, 0.41 - 0.89; P = .011).
“Multiple adverse events represent a higher burden on the patient, so it is encouraging to see a significant reduction in cumulative events with genetically guided therapy,” Pereira said.
There was no difference in the safety endpoint of TIMI major bleeding or minor bleeding between the two groups: 1.9% in the genetically guided group vs 1.6% in the conventional treatment group.
The results did not differ between various subgroups in the trial, including race or ethnicity. Although Asian patients have a higher occurrence of the clopidogrel loss-of-function gene, the event risk reductions were similar in Asian and white patients in the study.
Pereira said the study may have been underpowered because of recent improvements in care. When the TAILOR-PCI trial was designed in 2012, around 10% to 12% of patients who received a stent could be expected to have a major adverse event, but during the trial, greater use of drug-coated stents and other treatments significantly reduced the expected rate of adverse events and made it more difficult for the trial to reach its goal of a 50% reduction in adverse events with the number of patients enrolled, he explained.
As part of the discussion, Mehran pointed out that more than 80% of the patients in the trial had acute coronary syndrome (ACS) and yet were being sent home on clopidogrel, which she said she found “daunting.”
“This begs the question of whether they were lower-risk patients and not really the hot unstable ACS patients with large thrombus burden where we see higher event rates,” Mehran commented. She also noted the results must be considered in the new era of platelet monotherapy, where aspirin is being withdrawn, and asked whether clopidogrel monotherapy would be considered safe without aspirin on board.
The researchers are planning a cost-effectiveness analysis of genetically guided therapy based on these data, and they are also continuing to follow patients over the longer term.
The TAILOR-PCI study was funded by the Mayo Clinic in collaboration with the National Heart, Lung, and Blood Institute. Spartan Bioscience Inc supplied the genetic tests used. Pereira reports no relevant disclosures.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology. Abstract 20-LB-20309-ACC. Presented March 28, 2020.
This article first appeared on Medscape.com.
The largest trial to date investigating the clinical utility of using genetic testing to detect clopidogrel loss-of-function genotype to guide antiplatelet therapy in patients undergoing percutaneous coronary intervention (PCI) missed its primary endpoint of a 50% reduction in cardiovascular events at 1 year.
However, the TAILOR-PCI trial did show a 34% reduction in such events at 1 year, as well as a statistically significant 40% reduction in the total number of events per patient receiving genetically guided treatment compared with patients who received standard treatment.
In addition, a post hoc analysis found a significant 79% reduction in the rate of adverse events in the first 3 months of treatment among patients who received genetically guided therapy compared with those who did not.
The study was presented March 28 during the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology.
“Although these results fell short of the effect size that we predicted, they nevertheless provide a signal that offers support for the benefit of genetically guided therapy, with approximately one-third fewer adverse events in the patients who received genetically guided treatment compared with those who did not,” concluded Naveen L. Pereira, MD, professor of medicine at the Mayo Clinic in Rochester, Minnesota, and co-principal investigator of the study.
Pereira said the post hoc analysis of the first 3 months of treatment was particularly interesting. “This period immediately after PCI is when patients are at the highest risk for adverse events. We now know that antiplatelet drug therapy is critical during the first 3 months after PCI. Our findings suggest that the lion’s share of the benefit of genetically guided therapy may occur during this high-risk period,” he noted.
However, he added, “Because this wasn’t a preplanned analysis, we can’t draw firm conclusions from it, but it merits further study.”
Asked during an ACC virtual press conference how these results may influence clinical practice, Pereira said he hopes it changes practice toward genotyping.
“We set a very high standard in trying to achieve a 50% reduction in events, but we did see a 34% reduction. I think the probability of the results being true is very high,” he said. “I hope people pay attention to that. I’m not sure what the guidelines will do, but I believe if clopidogrel genetic information is made available to the physician, not changing therapy in a patient who has the loss-of-function gene will now be very difficult.”
Discussant of the trial, Roxana Mehran, MD, Mount Sinai Hospital, New York City, said she thought the results were good enough clinically to justify using genotyping to guide therapy.
“The trial showed an absolute 1.8% reduction and a relative 34% reduction in cardiovascular events, which did not quite meet the P value for significance, and they are supported by a significant reduction in multiple events, and a large difference at 3 months, although these are not primary analyses. So, for me this trial has shown that tailoring antiplatelet therapy by genetic testing is beneficial,” she said.
Another outside commentator, Patrick O’Gara, MD, Brigham and Women’s Hospital, Boston, Massachusetts, described TAILOR-PCI as a “terrific study.”
“Together with the study presented last year showing genotype-guided clopidogrel treatment was noninferior to ticagrelor/prasugrel in STEMI [non-ST-segment elevation myocardial infarction] patients, it chips away at the biologic appropriateness of targeting therapies based on genetic risk,” he said.
“I would hate people to focus on the fact the primary endpoint was missed by one hundredth of a percentage point but hope they would rather consider the bigger picture of making this genotype test more available and accessible to inform clinical decision making,” O’Gara added. “It just makes too much sense to ignore this potential.”
The TAILOR-PCI trial enrolled 5302 patients from 40 centers in the United States, Canada, Mexico, and South Korea who had undergone PCI with stenting. They were randomly assigned to genetic testing for the clopidogrel loss-of-function variant or a group that received standard treatment (clopidogrel) without genetic testing.
In the genetic testing group, 35% of patients were found to have the clopidogrel loss-of-function variant and were therefore prescribed ticagrelor, whereas those without the loss-of-function variant received clopidogrel.
After 1 year, the primary endpoint, a composite of cardiovascular death, MI, stroke, definite or probable stent thrombosis, and severe recurrent ischemia, occurred in 35 patients (4%) of the group that received genetically guided treatment, compared with 54 (5.9%) in the conventionally treated group (adjusted hazard ratio [HR], 0.66; 95% confidence interval [CI], 0.43 - 1.02; P = .56).
A prespecified analysis of total events (rather than just analysis of first event per patient) showed a 40% reduction in the genotyped group (HR, 0.60; 95% CI, 0.41 - 0.89; P = .011).
“Multiple adverse events represent a higher burden on the patient, so it is encouraging to see a significant reduction in cumulative events with genetically guided therapy,” Pereira said.
There was no difference in the safety endpoint of TIMI major bleeding or minor bleeding between the two groups: 1.9% in the genetically guided group vs 1.6% in the conventional treatment group.
The results did not differ between various subgroups in the trial, including race or ethnicity. Although Asian patients have a higher occurrence of the clopidogrel loss-of-function gene, the event risk reductions were similar in Asian and white patients in the study.
Pereira said the study may have been underpowered because of recent improvements in care. When the TAILOR-PCI trial was designed in 2012, around 10% to 12% of patients who received a stent could be expected to have a major adverse event, but during the trial, greater use of drug-coated stents and other treatments significantly reduced the expected rate of adverse events and made it more difficult for the trial to reach its goal of a 50% reduction in adverse events with the number of patients enrolled, he explained.
As part of the discussion, Mehran pointed out that more than 80% of the patients in the trial had acute coronary syndrome (ACS) and yet were being sent home on clopidogrel, which she said she found “daunting.”
“This begs the question of whether they were lower-risk patients and not really the hot unstable ACS patients with large thrombus burden where we see higher event rates,” Mehran commented. She also noted the results must be considered in the new era of platelet monotherapy, where aspirin is being withdrawn, and asked whether clopidogrel monotherapy would be considered safe without aspirin on board.
The researchers are planning a cost-effectiveness analysis of genetically guided therapy based on these data, and they are also continuing to follow patients over the longer term.
The TAILOR-PCI study was funded by the Mayo Clinic in collaboration with the National Heart, Lung, and Blood Institute. Spartan Bioscience Inc supplied the genetic tests used. Pereira reports no relevant disclosures.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology. Abstract 20-LB-20309-ACC. Presented March 28, 2020.
This article first appeared on Medscape.com.
Rivaroxaban plus aspirin safely benefits PAD patients after limb revascularization
A combined antithrombotic regimen of rivaroxaban plus aspirin was safe and effective for reducing ischemic events in patients with symptomatic peripheral artery disease who had just undergone peripheral artery revascularization in VOYAGER PAD, a multicenter randomized trial with nearly 6,600 patients.
The study and its results were a groundbreaking advance for this patient population, who until now have had no evidence-based treatment available, Mark P. Bonaca, MD, said on March 28 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
The study design excluded a small percentage of patients (about 2%) because of their very high bleeding-risk history. Among the treated patients, in those who received a combination of 2.5 mg rivaroxaban twice daily plus 100 mg of aspirin daily, bleeding events were more common, compared with control patients who received aspirin alone. But the patients who received both drugs showed no excess of fatal bleeds or intracranial hemorrhages, and the rate of ischemic events prevented by rivaroxaban plus aspirin exceeded the excess rate of bleeds by three- to sixfold, depending on how bleeding episodes were defined, noted Dr. Bonaca, executive director of CPC Clinical Research and CPC Community Health, an academic research organization affiliated with the University of Colorado at Denver in Aurora.
“This was a much anticipated and important trial. Those of us who treat patients with lower-limb peripheral artery disease have not had much evidence on how to treat these patients, particularly those who have just undergone revascularization. This trial gives us the evidence,” commented Mark A. Creager, MD, professor of medicine and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center in Lebanon, N.H. “The bleeding risk [from adding rivaroxaban treatment] was substantially less than the benefit from preventing major adverse limb events and major adverse cardiovascular events,” producing a “favorable balance” of benefit, compared with risk, Dr. Creager said in an interview. “In the right patients, the benefit greatly outweighed the risk.”
“This was an incredible trial that will advance care,” commented Joshua A. Beckman, MD, professor of medicine and director of Vascular Medicine at Vanderbilt University in Nashville, Tenn. “The treatment was beneficial for patients across a range of symptom severity, from claudication to critical limb ischemia,” and the results expand the range of patients proven to benefit from the rivaroxaban plus aspirin combination from the types of patients with peripheral artery disease (PAD) enrolled in the COMPASS trial. That pivotal trial showed similar benefit from the dual-antithrombotic regimen, but in patients who had both coronary artery disease as well as atherosclerotic disease in at least one additional vascular bed, such as lower-limb arteries (N Engl J Med. 2017 Oct 5;377[14]:1319-30). In addition to “bringing acute limb ischemia to the cardiovascular community,” the results also identified a very useful time point in the clinical presentation of these patients for starting a combined rivaroxaban plus aspirin regimen: when patients are hospitalized for their revascularization procedure, said Dr. Beckman, a designated discussant for the report.
Among the 6,564 patients randomized in the study, about two-thirds underwent endovascular revascularization within 10 days before starting their study treatment, and the remaining third had undergone surgical revascularization. The study focused on patients “with symptomatic PAD but without known coronary artery disease,” noted Dr. Bonaca.
VOYAGER PAD trial
The VOYAGER PAD (Vascular Outcomes Study of Acetylsalicylic Acid Along With Rivaroxaban in Endovascular Or Surgical Limb Revascularization for Peripheral Artery Disease) trial enrolled patients during 2015-2018 at 534 sites in 34 countries. The study’s primary endpoint was a composite of acute limb ischemia, major amputation for vascular causes, myocardial infarction, ischemic stroke, or death from cardiovascular causes, and was reduced during a median follow-up of 28 months from 19.9% with aspirin alone to 17.3% on the combined regimen, a 2.6% absolute difference and a 15% relative risk reduction that was statistically significant, an endpoint primarily driven by a reduction in acute limb ischemia. The primary safety endpoint was the rate of TIMI (Thrombolysis in Myocardial Infarction) major bleeds, which was 0.8% higher in the patients who received the anticoagulant, a 43% relative increase that just missed statistical significance. But that result demonstrated the small but important increased risk for bleeding events that the dual regimen produced in these patients, Dr. Bonaca said. Simultaneously with his report the findings also appeared in an article published online (N Engl J Med. 2020 Mar 28. doi: 10.1056/NEJMoa2000052).
Dr. Bonaca cautioned that one limitation of his report on the primary outcome of VOYAGER PAD is that the results of an important subgroup analysis won’t be known until a second report during the ACC online sessions on March 29, which will examine the impact that treatment with the antiplatelet drug clopidogrel had on both the efficacy and safety outcomes. Half of the enrolled patients received clopidogrel at the discretion of their treating physicians; addition or exclusion of concurrent clopidogrel treatment was outside of the study’s design. “Is efficacy the same with or without clopidogrel, and what is the bleeding cost,” especially in patients who receive three antithrombotic drugs? “It will be very important to understand,” Dr. Bonaca said.
“Until now, we had no idea of what was the best antithrombotic strategy for patients after a successful peripheral vascular intervention.” VOYAGER PAD was “an unprecedented vascular study that addressed an unmet patient need,” commented Roxana Mehran, MD, a designated discussant for the study and professor of medicine and director of Interventional Cardiovascular Research at Mount Sinai Medical Center in New York.
VOYAGER PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). The institution that Dr. Bonaca directs has received research funding from Bayer and Janssen, and also from Amgen, Aralez, AstraZeneca, Merck, Novo Nordisk, Pfizer, and Sanofi. Dr. Creager had no disclosures. Dr. Beckman has served as a data safety monitor for Bayer and for Novartis, and has been a consultant to Amgen, AstraZeneca, JanOne and Sanofi. Dr. Mehran has received research funding from Bayer and has been a consultant to Janssen, and she has also received research funding or been a consultant to several other companies.
SOURCE: Bonaca MP et al. ACC 20, Abstract 402-10.
A combined antithrombotic regimen of rivaroxaban plus aspirin was safe and effective for reducing ischemic events in patients with symptomatic peripheral artery disease who had just undergone peripheral artery revascularization in VOYAGER PAD, a multicenter randomized trial with nearly 6,600 patients.
The study and its results were a groundbreaking advance for this patient population, who until now have had no evidence-based treatment available, Mark P. Bonaca, MD, said on March 28 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
The study design excluded a small percentage of patients (about 2%) because of their very high bleeding-risk history. Among the treated patients, in those who received a combination of 2.5 mg rivaroxaban twice daily plus 100 mg of aspirin daily, bleeding events were more common, compared with control patients who received aspirin alone. But the patients who received both drugs showed no excess of fatal bleeds or intracranial hemorrhages, and the rate of ischemic events prevented by rivaroxaban plus aspirin exceeded the excess rate of bleeds by three- to sixfold, depending on how bleeding episodes were defined, noted Dr. Bonaca, executive director of CPC Clinical Research and CPC Community Health, an academic research organization affiliated with the University of Colorado at Denver in Aurora.
“This was a much anticipated and important trial. Those of us who treat patients with lower-limb peripheral artery disease have not had much evidence on how to treat these patients, particularly those who have just undergone revascularization. This trial gives us the evidence,” commented Mark A. Creager, MD, professor of medicine and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center in Lebanon, N.H. “The bleeding risk [from adding rivaroxaban treatment] was substantially less than the benefit from preventing major adverse limb events and major adverse cardiovascular events,” producing a “favorable balance” of benefit, compared with risk, Dr. Creager said in an interview. “In the right patients, the benefit greatly outweighed the risk.”
“This was an incredible trial that will advance care,” commented Joshua A. Beckman, MD, professor of medicine and director of Vascular Medicine at Vanderbilt University in Nashville, Tenn. “The treatment was beneficial for patients across a range of symptom severity, from claudication to critical limb ischemia,” and the results expand the range of patients proven to benefit from the rivaroxaban plus aspirin combination from the types of patients with peripheral artery disease (PAD) enrolled in the COMPASS trial. That pivotal trial showed similar benefit from the dual-antithrombotic regimen, but in patients who had both coronary artery disease as well as atherosclerotic disease in at least one additional vascular bed, such as lower-limb arteries (N Engl J Med. 2017 Oct 5;377[14]:1319-30). In addition to “bringing acute limb ischemia to the cardiovascular community,” the results also identified a very useful time point in the clinical presentation of these patients for starting a combined rivaroxaban plus aspirin regimen: when patients are hospitalized for their revascularization procedure, said Dr. Beckman, a designated discussant for the report.
Among the 6,564 patients randomized in the study, about two-thirds underwent endovascular revascularization within 10 days before starting their study treatment, and the remaining third had undergone surgical revascularization. The study focused on patients “with symptomatic PAD but without known coronary artery disease,” noted Dr. Bonaca.
VOYAGER PAD trial
The VOYAGER PAD (Vascular Outcomes Study of Acetylsalicylic Acid Along With Rivaroxaban in Endovascular Or Surgical Limb Revascularization for Peripheral Artery Disease) trial enrolled patients during 2015-2018 at 534 sites in 34 countries. The study’s primary endpoint was a composite of acute limb ischemia, major amputation for vascular causes, myocardial infarction, ischemic stroke, or death from cardiovascular causes, and was reduced during a median follow-up of 28 months from 19.9% with aspirin alone to 17.3% on the combined regimen, a 2.6% absolute difference and a 15% relative risk reduction that was statistically significant, an endpoint primarily driven by a reduction in acute limb ischemia. The primary safety endpoint was the rate of TIMI (Thrombolysis in Myocardial Infarction) major bleeds, which was 0.8% higher in the patients who received the anticoagulant, a 43% relative increase that just missed statistical significance. But that result demonstrated the small but important increased risk for bleeding events that the dual regimen produced in these patients, Dr. Bonaca said. Simultaneously with his report the findings also appeared in an article published online (N Engl J Med. 2020 Mar 28. doi: 10.1056/NEJMoa2000052).
Dr. Bonaca cautioned that one limitation of his report on the primary outcome of VOYAGER PAD is that the results of an important subgroup analysis won’t be known until a second report during the ACC online sessions on March 29, which will examine the impact that treatment with the antiplatelet drug clopidogrel had on both the efficacy and safety outcomes. Half of the enrolled patients received clopidogrel at the discretion of their treating physicians; addition or exclusion of concurrent clopidogrel treatment was outside of the study’s design. “Is efficacy the same with or without clopidogrel, and what is the bleeding cost,” especially in patients who receive three antithrombotic drugs? “It will be very important to understand,” Dr. Bonaca said.
“Until now, we had no idea of what was the best antithrombotic strategy for patients after a successful peripheral vascular intervention.” VOYAGER PAD was “an unprecedented vascular study that addressed an unmet patient need,” commented Roxana Mehran, MD, a designated discussant for the study and professor of medicine and director of Interventional Cardiovascular Research at Mount Sinai Medical Center in New York.
VOYAGER PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). The institution that Dr. Bonaca directs has received research funding from Bayer and Janssen, and also from Amgen, Aralez, AstraZeneca, Merck, Novo Nordisk, Pfizer, and Sanofi. Dr. Creager had no disclosures. Dr. Beckman has served as a data safety monitor for Bayer and for Novartis, and has been a consultant to Amgen, AstraZeneca, JanOne and Sanofi. Dr. Mehran has received research funding from Bayer and has been a consultant to Janssen, and she has also received research funding or been a consultant to several other companies.
SOURCE: Bonaca MP et al. ACC 20, Abstract 402-10.
A combined antithrombotic regimen of rivaroxaban plus aspirin was safe and effective for reducing ischemic events in patients with symptomatic peripheral artery disease who had just undergone peripheral artery revascularization in VOYAGER PAD, a multicenter randomized trial with nearly 6,600 patients.
The study and its results were a groundbreaking advance for this patient population, who until now have had no evidence-based treatment available, Mark P. Bonaca, MD, said on March 28 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
The study design excluded a small percentage of patients (about 2%) because of their very high bleeding-risk history. Among the treated patients, in those who received a combination of 2.5 mg rivaroxaban twice daily plus 100 mg of aspirin daily, bleeding events were more common, compared with control patients who received aspirin alone. But the patients who received both drugs showed no excess of fatal bleeds or intracranial hemorrhages, and the rate of ischemic events prevented by rivaroxaban plus aspirin exceeded the excess rate of bleeds by three- to sixfold, depending on how bleeding episodes were defined, noted Dr. Bonaca, executive director of CPC Clinical Research and CPC Community Health, an academic research organization affiliated with the University of Colorado at Denver in Aurora.
“This was a much anticipated and important trial. Those of us who treat patients with lower-limb peripheral artery disease have not had much evidence on how to treat these patients, particularly those who have just undergone revascularization. This trial gives us the evidence,” commented Mark A. Creager, MD, professor of medicine and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center in Lebanon, N.H. “The bleeding risk [from adding rivaroxaban treatment] was substantially less than the benefit from preventing major adverse limb events and major adverse cardiovascular events,” producing a “favorable balance” of benefit, compared with risk, Dr. Creager said in an interview. “In the right patients, the benefit greatly outweighed the risk.”
“This was an incredible trial that will advance care,” commented Joshua A. Beckman, MD, professor of medicine and director of Vascular Medicine at Vanderbilt University in Nashville, Tenn. “The treatment was beneficial for patients across a range of symptom severity, from claudication to critical limb ischemia,” and the results expand the range of patients proven to benefit from the rivaroxaban plus aspirin combination from the types of patients with peripheral artery disease (PAD) enrolled in the COMPASS trial. That pivotal trial showed similar benefit from the dual-antithrombotic regimen, but in patients who had both coronary artery disease as well as atherosclerotic disease in at least one additional vascular bed, such as lower-limb arteries (N Engl J Med. 2017 Oct 5;377[14]:1319-30). In addition to “bringing acute limb ischemia to the cardiovascular community,” the results also identified a very useful time point in the clinical presentation of these patients for starting a combined rivaroxaban plus aspirin regimen: when patients are hospitalized for their revascularization procedure, said Dr. Beckman, a designated discussant for the report.
Among the 6,564 patients randomized in the study, about two-thirds underwent endovascular revascularization within 10 days before starting their study treatment, and the remaining third had undergone surgical revascularization. The study focused on patients “with symptomatic PAD but without known coronary artery disease,” noted Dr. Bonaca.
VOYAGER PAD trial
The VOYAGER PAD (Vascular Outcomes Study of Acetylsalicylic Acid Along With Rivaroxaban in Endovascular Or Surgical Limb Revascularization for Peripheral Artery Disease) trial enrolled patients during 2015-2018 at 534 sites in 34 countries. The study’s primary endpoint was a composite of acute limb ischemia, major amputation for vascular causes, myocardial infarction, ischemic stroke, or death from cardiovascular causes, and was reduced during a median follow-up of 28 months from 19.9% with aspirin alone to 17.3% on the combined regimen, a 2.6% absolute difference and a 15% relative risk reduction that was statistically significant, an endpoint primarily driven by a reduction in acute limb ischemia. The primary safety endpoint was the rate of TIMI (Thrombolysis in Myocardial Infarction) major bleeds, which was 0.8% higher in the patients who received the anticoagulant, a 43% relative increase that just missed statistical significance. But that result demonstrated the small but important increased risk for bleeding events that the dual regimen produced in these patients, Dr. Bonaca said. Simultaneously with his report the findings also appeared in an article published online (N Engl J Med. 2020 Mar 28. doi: 10.1056/NEJMoa2000052).
Dr. Bonaca cautioned that one limitation of his report on the primary outcome of VOYAGER PAD is that the results of an important subgroup analysis won’t be known until a second report during the ACC online sessions on March 29, which will examine the impact that treatment with the antiplatelet drug clopidogrel had on both the efficacy and safety outcomes. Half of the enrolled patients received clopidogrel at the discretion of their treating physicians; addition or exclusion of concurrent clopidogrel treatment was outside of the study’s design. “Is efficacy the same with or without clopidogrel, and what is the bleeding cost,” especially in patients who receive three antithrombotic drugs? “It will be very important to understand,” Dr. Bonaca said.
“Until now, we had no idea of what was the best antithrombotic strategy for patients after a successful peripheral vascular intervention.” VOYAGER PAD was “an unprecedented vascular study that addressed an unmet patient need,” commented Roxana Mehran, MD, a designated discussant for the study and professor of medicine and director of Interventional Cardiovascular Research at Mount Sinai Medical Center in New York.
VOYAGER PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). The institution that Dr. Bonaca directs has received research funding from Bayer and Janssen, and also from Amgen, Aralez, AstraZeneca, Merck, Novo Nordisk, Pfizer, and Sanofi. Dr. Creager had no disclosures. Dr. Beckman has served as a data safety monitor for Bayer and for Novartis, and has been a consultant to Amgen, AstraZeneca, JanOne and Sanofi. Dr. Mehran has received research funding from Bayer and has been a consultant to Janssen, and she has also received research funding or been a consultant to several other companies.
SOURCE: Bonaca MP et al. ACC 20, Abstract 402-10.
REPORTING FROM ACC 20
Key clinical point: Combined treatment with rivaroxaban plus aspirin safely reduced a composite measure of adverse ischemic events in PAD patients following lower-limb revascularization.
Major finding: The primary event outcome occurred in 17.3% of patients on rivaroxaban plus aspirin, and in 19.9% on aspirin alone.
Study details: VOYAGER PAD, a multicenter, international randomized trial with 6,564 patients.
Disclosures: VOYAGER PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). The institution that Dr. Bonaca directs has received research funding from Bayer and Janssen, and also from Amgen, Aralez, AstraZeneca, Merck, Novo Nordisk, Pfizer, and Sanofi. Dr. Creager had no disclosures. Dr. Beckman has served as a data safety monitor for Bayer and for Novartis, and has been a consultant to Amgen, AstraZeneca, JanOne and Sanofi. Dr. Mehran has received research funding from Bayer and has been a consultant to Janssen, and she has also received research funding or been a consultant to several other companies.
Source: Bonaca MP. ACC 20, Abstract 402-10.
Stage I mycosis fungoides is the general dermatologist’s bailiwick
LAHAINA, HAWAII – without bringing in a medical oncologist, Trilokraj Tejasvi, MBBS, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
This approach is in the best interest of patients with stage I mycosis fungoides, the skin-limited, patch/plaque form of the disease that generally responds well to skin-directed therapies without needing to resort to the medical oncologist’s arsenal of toxic treatments.
“For many medical oncologists, a lymphoma is a lymphoma. The first thing they give is CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), and all the variants of CHOP,” cautioned Dr. Tejasvi, a dermatologist who is director of the cutaneous lymphoma program at the University of Michigan, Ann Arbor, and chief of the dermatology service at the Ann Arbor Veteran Affairs Hospital.
Stage IA mycosis fungoides is defined under the TNMB (tumor, node, metastasis, blood) classification as patches and/or plaques covering less than 10% of body surface area along with negative nodes, no metastases, and no or low burden of disease in the blood. Stage IB differs only in that it features 10% or greater body surface area involvement. The extent of body surface area involvement can be estimated by hands-on measurement in which the area of one of the patient’s hands – palm plus fingers – is considered equivalent to 1% of that individual’s total body surface area.
The first question patients newly diagnosed with a cutaneous T-cell lymphoma ask concerns their prognosis. For those with stage IA or IB mycosis fungoides, the news is very good, as highlighted in a retrospective study of nearly 1,400 patients with mycosis fungoides, 71% of whom presented with patch/plaque stage disease (J Clin Oncol. 2010 Nov 1;28[31]:4730-9).
The median overall survival was 35.5 years in patients with stage IA disease and 21.5 years in those with stage IB disease.
“I tell patients with stage IA disease that whether we treat it or not will not change the course of their life,” Dr. Tejasvri said.
His message to patients with stage IB disease is that, because of their 38% risk of disease progression, he wants to see them in follow-up annually for the rest of their life.
Stage IIA disease – that is, patches and/or plaques with lymph node involvement with no effacement – is a tipping point at which serious consideration should be given to possible referral to a specialized multidisciplinary lymphoma center, in his view. That’s because the 10-year overall survival rate is only 52%.
Topical therapies
Topical corticosteroids remain the time-honored first-line skin-directed treatment. The mechanism of benefit involves induction of apoptosis and inhibition of lymphocyte binding. In one prospective study, clobetasol propionate achieved a 94% overall response rate in patients with stage IA or B disease, with minimal toxicity.
Alternatives include topical 5% imiquimod (Aldara), with an overall response rate of 80% and complete response rate of 45% in a 20-patient study. A newer formulation of mechlorethamine gel (Valchlor), is reported to have a 59% overall response rate and a sustained response in 86% of initial responders. For refractory skin lesions, 1% bexarotene gel (Targretin) is an option, with overall response rates of 44%-63% reported in prospective trials.
“I like it if the patient’s insurance covers it. Otherwise, it’s like buying a Prius: it’s $30,000 for a 45-g tube, which is insane,” Dr. Tejasvi commented.
Narrow-band UVB phototherapy is an effective modality for thin plaques and patches, as is PUVA for thicker ones. Dr. Tejasvi typically treats with topical steroids and/or phototherapy for at least 3 months before tapering.
When to suspect mycosis fungoides
“Mycosis fungoides is a great masquerader,” the dermatologist observed. For that reason, it deserves to be included in the differential diagnosis of an atypical psoriasiform or eczematoid rash, any new-onset rash in an elderly patient, or a rash with fever, night sweats, and unintended weight loss in a patient of any age. Generalized erythema with severe itching is another red flag.
“This pruritus is so severe that the only other condition which in my clinical practice would match it is Norwegian scabies,” according to Dr. Tejasvi.
Polychromatic patches or plaques in skin of color warrant further investigation as possible mycosis fungoides, he added.
Dr. Tejasvi reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – without bringing in a medical oncologist, Trilokraj Tejasvi, MBBS, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
This approach is in the best interest of patients with stage I mycosis fungoides, the skin-limited, patch/plaque form of the disease that generally responds well to skin-directed therapies without needing to resort to the medical oncologist’s arsenal of toxic treatments.
“For many medical oncologists, a lymphoma is a lymphoma. The first thing they give is CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), and all the variants of CHOP,” cautioned Dr. Tejasvi, a dermatologist who is director of the cutaneous lymphoma program at the University of Michigan, Ann Arbor, and chief of the dermatology service at the Ann Arbor Veteran Affairs Hospital.
Stage IA mycosis fungoides is defined under the TNMB (tumor, node, metastasis, blood) classification as patches and/or plaques covering less than 10% of body surface area along with negative nodes, no metastases, and no or low burden of disease in the blood. Stage IB differs only in that it features 10% or greater body surface area involvement. The extent of body surface area involvement can be estimated by hands-on measurement in which the area of one of the patient’s hands – palm plus fingers – is considered equivalent to 1% of that individual’s total body surface area.
The first question patients newly diagnosed with a cutaneous T-cell lymphoma ask concerns their prognosis. For those with stage IA or IB mycosis fungoides, the news is very good, as highlighted in a retrospective study of nearly 1,400 patients with mycosis fungoides, 71% of whom presented with patch/plaque stage disease (J Clin Oncol. 2010 Nov 1;28[31]:4730-9).
The median overall survival was 35.5 years in patients with stage IA disease and 21.5 years in those with stage IB disease.
“I tell patients with stage IA disease that whether we treat it or not will not change the course of their life,” Dr. Tejasvri said.
His message to patients with stage IB disease is that, because of their 38% risk of disease progression, he wants to see them in follow-up annually for the rest of their life.
Stage IIA disease – that is, patches and/or plaques with lymph node involvement with no effacement – is a tipping point at which serious consideration should be given to possible referral to a specialized multidisciplinary lymphoma center, in his view. That’s because the 10-year overall survival rate is only 52%.
Topical therapies
Topical corticosteroids remain the time-honored first-line skin-directed treatment. The mechanism of benefit involves induction of apoptosis and inhibition of lymphocyte binding. In one prospective study, clobetasol propionate achieved a 94% overall response rate in patients with stage IA or B disease, with minimal toxicity.
Alternatives include topical 5% imiquimod (Aldara), with an overall response rate of 80% and complete response rate of 45% in a 20-patient study. A newer formulation of mechlorethamine gel (Valchlor), is reported to have a 59% overall response rate and a sustained response in 86% of initial responders. For refractory skin lesions, 1% bexarotene gel (Targretin) is an option, with overall response rates of 44%-63% reported in prospective trials.
“I like it if the patient’s insurance covers it. Otherwise, it’s like buying a Prius: it’s $30,000 for a 45-g tube, which is insane,” Dr. Tejasvi commented.
Narrow-band UVB phototherapy is an effective modality for thin plaques and patches, as is PUVA for thicker ones. Dr. Tejasvi typically treats with topical steroids and/or phototherapy for at least 3 months before tapering.
When to suspect mycosis fungoides
“Mycosis fungoides is a great masquerader,” the dermatologist observed. For that reason, it deserves to be included in the differential diagnosis of an atypical psoriasiform or eczematoid rash, any new-onset rash in an elderly patient, or a rash with fever, night sweats, and unintended weight loss in a patient of any age. Generalized erythema with severe itching is another red flag.
“This pruritus is so severe that the only other condition which in my clinical practice would match it is Norwegian scabies,” according to Dr. Tejasvi.
Polychromatic patches or plaques in skin of color warrant further investigation as possible mycosis fungoides, he added.
Dr. Tejasvi reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – without bringing in a medical oncologist, Trilokraj Tejasvi, MBBS, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
This approach is in the best interest of patients with stage I mycosis fungoides, the skin-limited, patch/plaque form of the disease that generally responds well to skin-directed therapies without needing to resort to the medical oncologist’s arsenal of toxic treatments.
“For many medical oncologists, a lymphoma is a lymphoma. The first thing they give is CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), and all the variants of CHOP,” cautioned Dr. Tejasvi, a dermatologist who is director of the cutaneous lymphoma program at the University of Michigan, Ann Arbor, and chief of the dermatology service at the Ann Arbor Veteran Affairs Hospital.
Stage IA mycosis fungoides is defined under the TNMB (tumor, node, metastasis, blood) classification as patches and/or plaques covering less than 10% of body surface area along with negative nodes, no metastases, and no or low burden of disease in the blood. Stage IB differs only in that it features 10% or greater body surface area involvement. The extent of body surface area involvement can be estimated by hands-on measurement in which the area of one of the patient’s hands – palm plus fingers – is considered equivalent to 1% of that individual’s total body surface area.
The first question patients newly diagnosed with a cutaneous T-cell lymphoma ask concerns their prognosis. For those with stage IA or IB mycosis fungoides, the news is very good, as highlighted in a retrospective study of nearly 1,400 patients with mycosis fungoides, 71% of whom presented with patch/plaque stage disease (J Clin Oncol. 2010 Nov 1;28[31]:4730-9).
The median overall survival was 35.5 years in patients with stage IA disease and 21.5 years in those with stage IB disease.
“I tell patients with stage IA disease that whether we treat it or not will not change the course of their life,” Dr. Tejasvri said.
His message to patients with stage IB disease is that, because of their 38% risk of disease progression, he wants to see them in follow-up annually for the rest of their life.
Stage IIA disease – that is, patches and/or plaques with lymph node involvement with no effacement – is a tipping point at which serious consideration should be given to possible referral to a specialized multidisciplinary lymphoma center, in his view. That’s because the 10-year overall survival rate is only 52%.
Topical therapies
Topical corticosteroids remain the time-honored first-line skin-directed treatment. The mechanism of benefit involves induction of apoptosis and inhibition of lymphocyte binding. In one prospective study, clobetasol propionate achieved a 94% overall response rate in patients with stage IA or B disease, with minimal toxicity.
Alternatives include topical 5% imiquimod (Aldara), with an overall response rate of 80% and complete response rate of 45% in a 20-patient study. A newer formulation of mechlorethamine gel (Valchlor), is reported to have a 59% overall response rate and a sustained response in 86% of initial responders. For refractory skin lesions, 1% bexarotene gel (Targretin) is an option, with overall response rates of 44%-63% reported in prospective trials.
“I like it if the patient’s insurance covers it. Otherwise, it’s like buying a Prius: it’s $30,000 for a 45-g tube, which is insane,” Dr. Tejasvi commented.
Narrow-band UVB phototherapy is an effective modality for thin plaques and patches, as is PUVA for thicker ones. Dr. Tejasvi typically treats with topical steroids and/or phototherapy for at least 3 months before tapering.
When to suspect mycosis fungoides
“Mycosis fungoides is a great masquerader,” the dermatologist observed. For that reason, it deserves to be included in the differential diagnosis of an atypical psoriasiform or eczematoid rash, any new-onset rash in an elderly patient, or a rash with fever, night sweats, and unintended weight loss in a patient of any age. Generalized erythema with severe itching is another red flag.
“This pruritus is so severe that the only other condition which in my clinical practice would match it is Norwegian scabies,” according to Dr. Tejasvi.
Polychromatic patches or plaques in skin of color warrant further investigation as possible mycosis fungoides, he added.
Dr. Tejasvi reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
FDA okays emergency use of convalescent plasma for seriously ill COVID-19 patients
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to [email protected].
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to [email protected].
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to [email protected].
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”
Reports suggest possible in utero transmission of novel coronavirus 2019
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
FROM JAMA
Doctors sound off about future of medical meetings
As most 2020 medical conferences have, one by one, been canceled or rescheduled as virtual meetings in the time of a pandemic, some physicians and other healthcare professionals are wondering if this is the year that will change the scene forever.
Amid the choruses of resignation (“Unfortunately, it’s the right thing to do.”) and optimism (“See you next year!”), there have been plenty of voices describing another broad sentiment – that all was not well with medical meetings even before the coronavirus.
One dominant criticism is that there are too many meetings.
Indeed, there are many, many meetings. During 2005–2015, there were 30,000-plus medical meetings in the United States, according to a report from the Healthcare Convention and Exhibitors Association.
Most of those are of little value, tweeted Dhruv Khullar, MD, an internist at Weill Cornell Medicine, New York City (@DhruvKhullar): “One possible consequence of cancelling so many meetings due to #COVID19 is that we realize we probably don’t need most of them.”
The tweet was liked 1.9K times, which is high for a medical post. Comments were mostly in agreement, with some skepticism.
Michaela West, MD, PhD, a surgeon at North Memorial Health, Minneapolis, Minnesota, responded (@MichaelaWst): “Agree. COVID-19 may forever change our perspective regarding medical professional meetings.”
Nwando Olayiwola, MD, chair of family medicine, Ohio State University, Columbus, strongly agreed (@DrNwando): “This is the tweet I wish I tweeted.”
However, Kelly Swords, MD, MPH, urologist, University of California, San Diego, in a dissenting opinion, stated the obvious (@k_dagger): “Except there is no substitute for human interaction.”
Worth the Effort?
The cancellation of medical meetings has given those who regularly attend an opportunity to reassess their value and to question the worth of the effort involved in attending in person.
David Steensma, MD, hematologist-oncologist, Harvard Medical School, Boston, (@DavidSteensma) tweeted that he would like to scale back: “The present crisis is an opportunity to reassess what is actually necessary and rebalance [in terms of meetings].”
Travel to meetings is often unpleasant, said others.
Chris Palatucci, life sciences executive recruiter, Coulter Partners, Boston, tweeted (@LifeSciRcruitr): “I will die a happy man if I never get on another plane. Glorified bus travel.” He also believes that once the coronavirus crisis is over, its “silver lining” will be the realization that “40% of all meetings are unnecessary.”
Many professionals have welcomed the announcements that major conferences have been canceled and will be conducted virtually.
The latest change is from the American Society of Clinical Oncology (ASCO), whose annual meeting was to be held in Chicago at the end of May but will now be held online.
Virtual ASCO will be more manageable – and comfy, said Fumiko Ladd Chino, MD, radiation oncologist, Memorial Sloan Kettering Cancer Center, New York City.
She (@fumikochino) explained why in a recent tweet: “1) I will be finally able to see ALL OF THE PRESENTATIONS I wanted to see instead of wandering around feeling overwhelmed. 2) I will be able to FOCUS on the presentations and not searching for a power outlet. 3) PAJAMAS.”
Virtual meetings already beat real meetings, added Adriana Scheliga, MD, hematologist-oncologist, Brazilian National Cancer Institute (@linfopedia): “I’ve been saying this for a while. For me the best ASCO Meetings, for example, are the “virtual meetings!”
However, meetings in place are also very much about professional community and mutual support, reminds Susan E. Sedory, MA, executive director, Society of Interventional Radiology, which canceled its meeting March 6 in a multifaceted process described by Medscape Medical News.
Is This the Time to Evaluate Meetings?
Coming up soon is the first major conference to go virtual after being canceled – the American College of Cardiology (ACC), which has been one of the top 20 largest meetings in the United States by attendance.
This meeting, which was to have taken place in Chicago on March 28–30, will now occur online on those days. The ACC says it will stream all “live” sessions on demand and provide access to additional videos, abstracts, and slides for at least 90 days after the meeting. And it will be free to anyone with an Internet connection.
Medical meetings in distant locales may bounce back, as they have grown into a very big business. ASCO is illustrative.
The group’s first scientific annual meeting was held in 1965 in Philadelphia, with about 70 members and invited guests in attendance. Fast forward 50-plus years to 2019: there were 42,500 attendees, a 4.4% increase from 2018. Notably, the top countries in attendance in 2019 were the United States and China.
Not everyone is happy that canceled meetings are being held online in the middle of a pandemic.
“In a COVID-19 world, the brain cannot focus on nonviral topics,” said commentator John Mandrola, MD, Baptist Health, Louisville, Kentucky, in his regular column for Medscape Cardiology/theheart.org.
The virtual ACC meeting should be canceled or delayed – to mirror what is happening in the world, he argues. “In hospitals, we have postponed the elective to make room for the coming surge. Shouldn’t ACC do the same? After the crisis passes, we can have a virtual meeting with a proper discussion of the science,” he writes.
But #MedTwitter, with its collective constructive criticism of medical meetings, is perhaps proof that the brain can function – and arrive at clarity – when under pandemic duress.
“Am I the only one experiencing a certain relief at the cancellation of multiple trips and meetings, and vowing to let this revelation affect my decision making in the future,” tweeted Steven Joffe, MD, MPH, University of Pennsylvania, Philadelphia (@Steve Joffe).
Louise Perkins King, MD, a bioethicist at Harvard Medical School, responded to Joffe. Hoping not to “belittle” the suffering from the COVID-19 pandemic, she (@louise_p_king) addressed her healthcare colleagues: “...there is potential for us all to learn what is essential travel and burden and what is not from this. I hope it leads to lasting change.”
This article first appeared on Medscape.com.
As most 2020 medical conferences have, one by one, been canceled or rescheduled as virtual meetings in the time of a pandemic, some physicians and other healthcare professionals are wondering if this is the year that will change the scene forever.
Amid the choruses of resignation (“Unfortunately, it’s the right thing to do.”) and optimism (“See you next year!”), there have been plenty of voices describing another broad sentiment – that all was not well with medical meetings even before the coronavirus.
One dominant criticism is that there are too many meetings.
Indeed, there are many, many meetings. During 2005–2015, there were 30,000-plus medical meetings in the United States, according to a report from the Healthcare Convention and Exhibitors Association.
Most of those are of little value, tweeted Dhruv Khullar, MD, an internist at Weill Cornell Medicine, New York City (@DhruvKhullar): “One possible consequence of cancelling so many meetings due to #COVID19 is that we realize we probably don’t need most of them.”
The tweet was liked 1.9K times, which is high for a medical post. Comments were mostly in agreement, with some skepticism.
Michaela West, MD, PhD, a surgeon at North Memorial Health, Minneapolis, Minnesota, responded (@MichaelaWst): “Agree. COVID-19 may forever change our perspective regarding medical professional meetings.”
Nwando Olayiwola, MD, chair of family medicine, Ohio State University, Columbus, strongly agreed (@DrNwando): “This is the tweet I wish I tweeted.”
However, Kelly Swords, MD, MPH, urologist, University of California, San Diego, in a dissenting opinion, stated the obvious (@k_dagger): “Except there is no substitute for human interaction.”
Worth the Effort?
The cancellation of medical meetings has given those who regularly attend an opportunity to reassess their value and to question the worth of the effort involved in attending in person.
David Steensma, MD, hematologist-oncologist, Harvard Medical School, Boston, (@DavidSteensma) tweeted that he would like to scale back: “The present crisis is an opportunity to reassess what is actually necessary and rebalance [in terms of meetings].”
Travel to meetings is often unpleasant, said others.
Chris Palatucci, life sciences executive recruiter, Coulter Partners, Boston, tweeted (@LifeSciRcruitr): “I will die a happy man if I never get on another plane. Glorified bus travel.” He also believes that once the coronavirus crisis is over, its “silver lining” will be the realization that “40% of all meetings are unnecessary.”
Many professionals have welcomed the announcements that major conferences have been canceled and will be conducted virtually.
The latest change is from the American Society of Clinical Oncology (ASCO), whose annual meeting was to be held in Chicago at the end of May but will now be held online.
Virtual ASCO will be more manageable – and comfy, said Fumiko Ladd Chino, MD, radiation oncologist, Memorial Sloan Kettering Cancer Center, New York City.
She (@fumikochino) explained why in a recent tweet: “1) I will be finally able to see ALL OF THE PRESENTATIONS I wanted to see instead of wandering around feeling overwhelmed. 2) I will be able to FOCUS on the presentations and not searching for a power outlet. 3) PAJAMAS.”
Virtual meetings already beat real meetings, added Adriana Scheliga, MD, hematologist-oncologist, Brazilian National Cancer Institute (@linfopedia): “I’ve been saying this for a while. For me the best ASCO Meetings, for example, are the “virtual meetings!”
However, meetings in place are also very much about professional community and mutual support, reminds Susan E. Sedory, MA, executive director, Society of Interventional Radiology, which canceled its meeting March 6 in a multifaceted process described by Medscape Medical News.
Is This the Time to Evaluate Meetings?
Coming up soon is the first major conference to go virtual after being canceled – the American College of Cardiology (ACC), which has been one of the top 20 largest meetings in the United States by attendance.
This meeting, which was to have taken place in Chicago on March 28–30, will now occur online on those days. The ACC says it will stream all “live” sessions on demand and provide access to additional videos, abstracts, and slides for at least 90 days after the meeting. And it will be free to anyone with an Internet connection.
Medical meetings in distant locales may bounce back, as they have grown into a very big business. ASCO is illustrative.
The group’s first scientific annual meeting was held in 1965 in Philadelphia, with about 70 members and invited guests in attendance. Fast forward 50-plus years to 2019: there were 42,500 attendees, a 4.4% increase from 2018. Notably, the top countries in attendance in 2019 were the United States and China.
Not everyone is happy that canceled meetings are being held online in the middle of a pandemic.
“In a COVID-19 world, the brain cannot focus on nonviral topics,” said commentator John Mandrola, MD, Baptist Health, Louisville, Kentucky, in his regular column for Medscape Cardiology/theheart.org.
The virtual ACC meeting should be canceled or delayed – to mirror what is happening in the world, he argues. “In hospitals, we have postponed the elective to make room for the coming surge. Shouldn’t ACC do the same? After the crisis passes, we can have a virtual meeting with a proper discussion of the science,” he writes.
But #MedTwitter, with its collective constructive criticism of medical meetings, is perhaps proof that the brain can function – and arrive at clarity – when under pandemic duress.
“Am I the only one experiencing a certain relief at the cancellation of multiple trips and meetings, and vowing to let this revelation affect my decision making in the future,” tweeted Steven Joffe, MD, MPH, University of Pennsylvania, Philadelphia (@Steve Joffe).
Louise Perkins King, MD, a bioethicist at Harvard Medical School, responded to Joffe. Hoping not to “belittle” the suffering from the COVID-19 pandemic, she (@louise_p_king) addressed her healthcare colleagues: “...there is potential for us all to learn what is essential travel and burden and what is not from this. I hope it leads to lasting change.”
This article first appeared on Medscape.com.
As most 2020 medical conferences have, one by one, been canceled or rescheduled as virtual meetings in the time of a pandemic, some physicians and other healthcare professionals are wondering if this is the year that will change the scene forever.
Amid the choruses of resignation (“Unfortunately, it’s the right thing to do.”) and optimism (“See you next year!”), there have been plenty of voices describing another broad sentiment – that all was not well with medical meetings even before the coronavirus.
One dominant criticism is that there are too many meetings.
Indeed, there are many, many meetings. During 2005–2015, there were 30,000-plus medical meetings in the United States, according to a report from the Healthcare Convention and Exhibitors Association.
Most of those are of little value, tweeted Dhruv Khullar, MD, an internist at Weill Cornell Medicine, New York City (@DhruvKhullar): “One possible consequence of cancelling so many meetings due to #COVID19 is that we realize we probably don’t need most of them.”
The tweet was liked 1.9K times, which is high for a medical post. Comments were mostly in agreement, with some skepticism.
Michaela West, MD, PhD, a surgeon at North Memorial Health, Minneapolis, Minnesota, responded (@MichaelaWst): “Agree. COVID-19 may forever change our perspective regarding medical professional meetings.”
Nwando Olayiwola, MD, chair of family medicine, Ohio State University, Columbus, strongly agreed (@DrNwando): “This is the tweet I wish I tweeted.”
However, Kelly Swords, MD, MPH, urologist, University of California, San Diego, in a dissenting opinion, stated the obvious (@k_dagger): “Except there is no substitute for human interaction.”
Worth the Effort?
The cancellation of medical meetings has given those who regularly attend an opportunity to reassess their value and to question the worth of the effort involved in attending in person.
David Steensma, MD, hematologist-oncologist, Harvard Medical School, Boston, (@DavidSteensma) tweeted that he would like to scale back: “The present crisis is an opportunity to reassess what is actually necessary and rebalance [in terms of meetings].”
Travel to meetings is often unpleasant, said others.
Chris Palatucci, life sciences executive recruiter, Coulter Partners, Boston, tweeted (@LifeSciRcruitr): “I will die a happy man if I never get on another plane. Glorified bus travel.” He also believes that once the coronavirus crisis is over, its “silver lining” will be the realization that “40% of all meetings are unnecessary.”
Many professionals have welcomed the announcements that major conferences have been canceled and will be conducted virtually.
The latest change is from the American Society of Clinical Oncology (ASCO), whose annual meeting was to be held in Chicago at the end of May but will now be held online.
Virtual ASCO will be more manageable – and comfy, said Fumiko Ladd Chino, MD, radiation oncologist, Memorial Sloan Kettering Cancer Center, New York City.
She (@fumikochino) explained why in a recent tweet: “1) I will be finally able to see ALL OF THE PRESENTATIONS I wanted to see instead of wandering around feeling overwhelmed. 2) I will be able to FOCUS on the presentations and not searching for a power outlet. 3) PAJAMAS.”
Virtual meetings already beat real meetings, added Adriana Scheliga, MD, hematologist-oncologist, Brazilian National Cancer Institute (@linfopedia): “I’ve been saying this for a while. For me the best ASCO Meetings, for example, are the “virtual meetings!”
However, meetings in place are also very much about professional community and mutual support, reminds Susan E. Sedory, MA, executive director, Society of Interventional Radiology, which canceled its meeting March 6 in a multifaceted process described by Medscape Medical News.
Is This the Time to Evaluate Meetings?
Coming up soon is the first major conference to go virtual after being canceled – the American College of Cardiology (ACC), which has been one of the top 20 largest meetings in the United States by attendance.
This meeting, which was to have taken place in Chicago on March 28–30, will now occur online on those days. The ACC says it will stream all “live” sessions on demand and provide access to additional videos, abstracts, and slides for at least 90 days after the meeting. And it will be free to anyone with an Internet connection.
Medical meetings in distant locales may bounce back, as they have grown into a very big business. ASCO is illustrative.
The group’s first scientific annual meeting was held in 1965 in Philadelphia, with about 70 members and invited guests in attendance. Fast forward 50-plus years to 2019: there were 42,500 attendees, a 4.4% increase from 2018. Notably, the top countries in attendance in 2019 were the United States and China.
Not everyone is happy that canceled meetings are being held online in the middle of a pandemic.
“In a COVID-19 world, the brain cannot focus on nonviral topics,” said commentator John Mandrola, MD, Baptist Health, Louisville, Kentucky, in his regular column for Medscape Cardiology/theheart.org.
The virtual ACC meeting should be canceled or delayed – to mirror what is happening in the world, he argues. “In hospitals, we have postponed the elective to make room for the coming surge. Shouldn’t ACC do the same? After the crisis passes, we can have a virtual meeting with a proper discussion of the science,” he writes.
But #MedTwitter, with its collective constructive criticism of medical meetings, is perhaps proof that the brain can function – and arrive at clarity – when under pandemic duress.
“Am I the only one experiencing a certain relief at the cancellation of multiple trips and meetings, and vowing to let this revelation affect my decision making in the future,” tweeted Steven Joffe, MD, MPH, University of Pennsylvania, Philadelphia (@Steve Joffe).
Louise Perkins King, MD, a bioethicist at Harvard Medical School, responded to Joffe. Hoping not to “belittle” the suffering from the COVID-19 pandemic, she (@louise_p_king) addressed her healthcare colleagues: “...there is potential for us all to learn what is essential travel and burden and what is not from this. I hope it leads to lasting change.”
This article first appeared on Medscape.com.
The power and promise of person-generated health data (Part II)
In Part I of our discussion we introduced the concept of person-generated health data (PGHD), defined as wellness and/or health-related data created, recorded, or gathered by individuals.
Such rich, longitudinal information is now being used in combination with traditional clinical information to predict, diagnose, and formulate treatment plans for diseases, as well as understand the safety and effectiveness of medical interventions.
Identifying a disease early
One novel example of digital technologies being used for early identification of disease was a promising 2019 study by Eli Lilly (in collaboration with Apple and Evidation Health) called the Lilly Exploratory Digital Assessment Study.
In this study, the feasibility of using PGHD for identifying physiological and behavioral signatures of cognitive impairment was examined for the purpose of seeking new methods to detect mild cognitive impairment (MCI) in a timely and cost-effective manner. The study enrolled 31 study participants with cognitive impairment and 82 without cognitive impairment. It used consumer-grade sensor technologies (the iPhone, Apple Watch, iPad, and Beddit sleep monitor) to continuously and unobtrusively collect data. Among the information the researchers collected were interaction with the phone keyboard, accelerometer data from the Apple Watch, volume of messages sent/received, and sleep cycles.1
A total of 16 terabytes of data were collected over the course of 12 weeks. Data were organized into a behaviorgram (See Figure 1) that gives a holistic picture of a day in a patient’s life. A machine learning model was used to distinguish between behaviorgrams of symptomatic versus healthy controls, identifying typing speed, circadian rhythm shifts, and reliance on helper apps, among other things, as differentiating cognitively impaired from healthy controls. These behaviorgrams may someday serve as “fingerprints” of different diseases, with specific diseases displaying predictable patterns. In the near future, digital measures like the ones investigated in this study are likely to be used to help clinicians predict and diagnose disease, as well as to better understand disease progression and treatment response.
Leading to better health outcomes
The potential of PGHD to detect diseases early and lead to better health outcomes is being investigated in the Heartline study, a collaboration between Johnson & Johnson and Apple, which is supported by Evidation.2
This study aims to enroll 150,000 adults age 65 years and over to analyze the impact of Apple Watch–based early detection of irregular heart rhythms consistent with atrial fibrillation (AFib). The researchers’ hypothesis is that jointly detecting atrial fibrillation early and providing cardiovascular health programs to new AFib patients, will lead to patients being treated by a medical provider for AFib that otherwise would not have been detected. This, in turn, would lead to these AFib patients decreasing their risks of stroke and other serious cardiovascular events, including death, the study authors speculated.
Presenting new challenges
While PGHD has the potential to help people, it also presents new challenges. It is highly sensitive and personal – it can be as identifying as DNA.3
The vast amount of data that PGHD can collect from interaction with consumer wearable devices poses serious privacy risks if done improperly. To address those risks, companies like Evidation have built in protections. Evidation has an app, Achievement, that has enlisted a connected population of more than 3.5 million members who earn rewards for performing health-related actions, as tracked by wearables devices and apps. Through the Achievement app (See Figure 2.), members are provided opportunities to join research studies. As part of these studies, data collected from sensors and apps is used by permission of the member so that it is clear how their data are contributing to specific research questions or use cases.
This is a collaborative model of data collection built upon trust and permission and is substantially different than the collection of data from electronic health records (EHRs) – which is typically aggregated, deidentified, and commercialized, often without the patients’ knowledge or consent. Stringent protections, explicit permission, and transparency are absolutely imperative until privacy frameworks for data outside of HIPAA regulation catches up and protects patients from discrimination and unintended uses of their data.
Large connected cohorts can help advance our understanding of public health. In one study run on Achievement during the 2017-2018 flu season, a survey was sent to the Achievement population every week asking about symptoms of influenza-like illness and requesting permission to access historical data from their wearable around the influenza-like illness event.4 With the data, it was possible to analyze patterns of activity, sleep, and resting heart rate change around flu events. Resting heart rate, in particular, is shown to increase during fever and at the population level. In fact, through the use of PGHD, it is possible to use the fraction of people with resting heart rate above their usual baseline as a proxy to quantify the number of infected people in a region.5 This resting heart rate–informed flu surveillance method, if refined to increased accuracy, can work in near real time. This means it may be able detect influenza outbreaks days earlier than current epidemiological methods.
Health data generated by connected populations are in the early stages of development. It is clear that it will yield novel insights into health and disease. Only time will tell if it will be able to help clinicians and patients better predict, diagnose, and formulate treatment plans for disease.
Neil Skolnik, M.D. is a professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, and associate director of the Family Medicine Residency Program at Abington Jefferson Health. Luca Foschini PhD, is co-founder & chief data scientist at Evidation Health. Bray Patrick-Lake, MFS, is a patient thought leader and director of strategic partnerships at Evidation Health.
References
1. Chen R et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. KDD ’19. August 4–8, 2019 Aug 4-8.
2. The Heartline Study. https://www.heartline.com.
3. Foschini L. Privacy of Wearable and Sensors Data (or, the Lack Thereof?). Data Driven Investor, Medium. 2019.
4. Bradshaw B et al. Influenza surveillance using wearable mobile health devices. Online J Public Health Inform. 2019;11(1):e249.
5. Radin JM et al. Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: a population-based study. Lancet Digital Health. 2020. doi: 10.1016/S2589-7500(19)30222-5.
In Part I of our discussion we introduced the concept of person-generated health data (PGHD), defined as wellness and/or health-related data created, recorded, or gathered by individuals.
Such rich, longitudinal information is now being used in combination with traditional clinical information to predict, diagnose, and formulate treatment plans for diseases, as well as understand the safety and effectiveness of medical interventions.
Identifying a disease early
One novel example of digital technologies being used for early identification of disease was a promising 2019 study by Eli Lilly (in collaboration with Apple and Evidation Health) called the Lilly Exploratory Digital Assessment Study.
In this study, the feasibility of using PGHD for identifying physiological and behavioral signatures of cognitive impairment was examined for the purpose of seeking new methods to detect mild cognitive impairment (MCI) in a timely and cost-effective manner. The study enrolled 31 study participants with cognitive impairment and 82 without cognitive impairment. It used consumer-grade sensor technologies (the iPhone, Apple Watch, iPad, and Beddit sleep monitor) to continuously and unobtrusively collect data. Among the information the researchers collected were interaction with the phone keyboard, accelerometer data from the Apple Watch, volume of messages sent/received, and sleep cycles.1

A total of 16 terabytes of data were collected over the course of 12 weeks. Data were organized into a behaviorgram (See Figure 1) that gives a holistic picture of a day in a patient’s life. A machine learning model was used to distinguish between behaviorgrams of symptomatic versus healthy controls, identifying typing speed, circadian rhythm shifts, and reliance on helper apps, among other things, as differentiating cognitively impaired from healthy controls. These behaviorgrams may someday serve as “fingerprints” of different diseases, with specific diseases displaying predictable patterns. In the near future, digital measures like the ones investigated in this study are likely to be used to help clinicians predict and diagnose disease, as well as to better understand disease progression and treatment response.
Leading to better health outcomes
The potential of PGHD to detect diseases early and lead to better health outcomes is being investigated in the Heartline study, a collaboration between Johnson & Johnson and Apple, which is supported by Evidation.2
This study aims to enroll 150,000 adults age 65 years and over to analyze the impact of Apple Watch–based early detection of irregular heart rhythms consistent with atrial fibrillation (AFib). The researchers’ hypothesis is that jointly detecting atrial fibrillation early and providing cardiovascular health programs to new AFib patients, will lead to patients being treated by a medical provider for AFib that otherwise would not have been detected. This, in turn, would lead to these AFib patients decreasing their risks of stroke and other serious cardiovascular events, including death, the study authors speculated.
Presenting new challenges
While PGHD has the potential to help people, it also presents new challenges. It is highly sensitive and personal – it can be as identifying as DNA.3
The vast amount of data that PGHD can collect from interaction with consumer wearable devices poses serious privacy risks if done improperly. To address those risks, companies like Evidation have built in protections. Evidation has an app, Achievement, that has enlisted a connected population of more than 3.5 million members who earn rewards for performing health-related actions, as tracked by wearables devices and apps. Through the Achievement app (See Figure 2.), members are provided opportunities to join research studies. As part of these studies, data collected from sensors and apps is used by permission of the member so that it is clear how their data are contributing to specific research questions or use cases.
This is a collaborative model of data collection built upon trust and permission and is substantially different than the collection of data from electronic health records (EHRs) – which is typically aggregated, deidentified, and commercialized, often without the patients’ knowledge or consent. Stringent protections, explicit permission, and transparency are absolutely imperative until privacy frameworks for data outside of HIPAA regulation catches up and protects patients from discrimination and unintended uses of their data.
Large connected cohorts can help advance our understanding of public health. In one study run on Achievement during the 2017-2018 flu season, a survey was sent to the Achievement population every week asking about symptoms of influenza-like illness and requesting permission to access historical data from their wearable around the influenza-like illness event.4 With the data, it was possible to analyze patterns of activity, sleep, and resting heart rate change around flu events. Resting heart rate, in particular, is shown to increase during fever and at the population level. In fact, through the use of PGHD, it is possible to use the fraction of people with resting heart rate above their usual baseline as a proxy to quantify the number of infected people in a region.5 This resting heart rate–informed flu surveillance method, if refined to increased accuracy, can work in near real time. This means it may be able detect influenza outbreaks days earlier than current epidemiological methods.
Health data generated by connected populations are in the early stages of development. It is clear that it will yield novel insights into health and disease. Only time will tell if it will be able to help clinicians and patients better predict, diagnose, and formulate treatment plans for disease.
Neil Skolnik, M.D. is a professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, and associate director of the Family Medicine Residency Program at Abington Jefferson Health. Luca Foschini PhD, is co-founder & chief data scientist at Evidation Health. Bray Patrick-Lake, MFS, is a patient thought leader and director of strategic partnerships at Evidation Health.
References
1. Chen R et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. KDD ’19. August 4–8, 2019 Aug 4-8.
2. The Heartline Study. https://www.heartline.com.
3. Foschini L. Privacy of Wearable and Sensors Data (or, the Lack Thereof?). Data Driven Investor, Medium. 2019.
4. Bradshaw B et al. Influenza surveillance using wearable mobile health devices. Online J Public Health Inform. 2019;11(1):e249.
5. Radin JM et al. Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: a population-based study. Lancet Digital Health. 2020. doi: 10.1016/S2589-7500(19)30222-5.
In Part I of our discussion we introduced the concept of person-generated health data (PGHD), defined as wellness and/or health-related data created, recorded, or gathered by individuals.
Such rich, longitudinal information is now being used in combination with traditional clinical information to predict, diagnose, and formulate treatment plans for diseases, as well as understand the safety and effectiveness of medical interventions.
Identifying a disease early
One novel example of digital technologies being used for early identification of disease was a promising 2019 study by Eli Lilly (in collaboration with Apple and Evidation Health) called the Lilly Exploratory Digital Assessment Study.
In this study, the feasibility of using PGHD for identifying physiological and behavioral signatures of cognitive impairment was examined for the purpose of seeking new methods to detect mild cognitive impairment (MCI) in a timely and cost-effective manner. The study enrolled 31 study participants with cognitive impairment and 82 without cognitive impairment. It used consumer-grade sensor technologies (the iPhone, Apple Watch, iPad, and Beddit sleep monitor) to continuously and unobtrusively collect data. Among the information the researchers collected were interaction with the phone keyboard, accelerometer data from the Apple Watch, volume of messages sent/received, and sleep cycles.1

A total of 16 terabytes of data were collected over the course of 12 weeks. Data were organized into a behaviorgram (See Figure 1) that gives a holistic picture of a day in a patient’s life. A machine learning model was used to distinguish between behaviorgrams of symptomatic versus healthy controls, identifying typing speed, circadian rhythm shifts, and reliance on helper apps, among other things, as differentiating cognitively impaired from healthy controls. These behaviorgrams may someday serve as “fingerprints” of different diseases, with specific diseases displaying predictable patterns. In the near future, digital measures like the ones investigated in this study are likely to be used to help clinicians predict and diagnose disease, as well as to better understand disease progression and treatment response.
Leading to better health outcomes
The potential of PGHD to detect diseases early and lead to better health outcomes is being investigated in the Heartline study, a collaboration between Johnson & Johnson and Apple, which is supported by Evidation.2
This study aims to enroll 150,000 adults age 65 years and over to analyze the impact of Apple Watch–based early detection of irregular heart rhythms consistent with atrial fibrillation (AFib). The researchers’ hypothesis is that jointly detecting atrial fibrillation early and providing cardiovascular health programs to new AFib patients, will lead to patients being treated by a medical provider for AFib that otherwise would not have been detected. This, in turn, would lead to these AFib patients decreasing their risks of stroke and other serious cardiovascular events, including death, the study authors speculated.
Presenting new challenges
While PGHD has the potential to help people, it also presents new challenges. It is highly sensitive and personal – it can be as identifying as DNA.3
The vast amount of data that PGHD can collect from interaction with consumer wearable devices poses serious privacy risks if done improperly. To address those risks, companies like Evidation have built in protections. Evidation has an app, Achievement, that has enlisted a connected population of more than 3.5 million members who earn rewards for performing health-related actions, as tracked by wearables devices and apps. Through the Achievement app (See Figure 2.), members are provided opportunities to join research studies. As part of these studies, data collected from sensors and apps is used by permission of the member so that it is clear how their data are contributing to specific research questions or use cases.
This is a collaborative model of data collection built upon trust and permission and is substantially different than the collection of data from electronic health records (EHRs) – which is typically aggregated, deidentified, and commercialized, often without the patients’ knowledge or consent. Stringent protections, explicit permission, and transparency are absolutely imperative until privacy frameworks for data outside of HIPAA regulation catches up and protects patients from discrimination and unintended uses of their data.
Large connected cohorts can help advance our understanding of public health. In one study run on Achievement during the 2017-2018 flu season, a survey was sent to the Achievement population every week asking about symptoms of influenza-like illness and requesting permission to access historical data from their wearable around the influenza-like illness event.4 With the data, it was possible to analyze patterns of activity, sleep, and resting heart rate change around flu events. Resting heart rate, in particular, is shown to increase during fever and at the population level. In fact, through the use of PGHD, it is possible to use the fraction of people with resting heart rate above their usual baseline as a proxy to quantify the number of infected people in a region.5 This resting heart rate–informed flu surveillance method, if refined to increased accuracy, can work in near real time. This means it may be able detect influenza outbreaks days earlier than current epidemiological methods.
Health data generated by connected populations are in the early stages of development. It is clear that it will yield novel insights into health and disease. Only time will tell if it will be able to help clinicians and patients better predict, diagnose, and formulate treatment plans for disease.
Neil Skolnik, M.D. is a professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, and associate director of the Family Medicine Residency Program at Abington Jefferson Health. Luca Foschini PhD, is co-founder & chief data scientist at Evidation Health. Bray Patrick-Lake, MFS, is a patient thought leader and director of strategic partnerships at Evidation Health.
References
1. Chen R et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. KDD ’19. August 4–8, 2019 Aug 4-8.
2. The Heartline Study. https://www.heartline.com.
3. Foschini L. Privacy of Wearable and Sensors Data (or, the Lack Thereof?). Data Driven Investor, Medium. 2019.
4. Bradshaw B et al. Influenza surveillance using wearable mobile health devices. Online J Public Health Inform. 2019;11(1):e249.
5. Radin JM et al. Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: a population-based study. Lancet Digital Health. 2020. doi: 10.1016/S2589-7500(19)30222-5.