User login
-
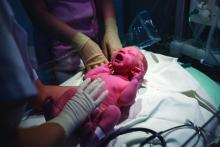
During a pandemic, infusion center nursing team pitches in to keep patients on track
How do you run a chemotherapy infusion center during a pandemic?
Quick action, innovative staffing solutions, and nimble leadership are allowing one cancer center to continue providing care for the most vulnerable patients, while keeping patients and staff safe.
When nursing leaders at Atrium Health’s Levine Cancer Institute in Charlotte, N.C., realized that business was not going to continue as usual for American health care during the COVID-19 pandemic, they knew they had to act quickly to keep the institute’s 82-chair infusion center up and running.
North Carolina had already imposed restrictions on mass gatherings and closed educational facilities and some businesses by mid-March. Stay-at-home orders were being issued in surrounding states (North Carolina came under a statewide order on March 30). Physical distancing and a healthy, resilient team were prerequisites to an effective COVID-19 solution for the infusion clinic, said Angela Hosking, MBA, MSN, RN, director of nursing for Levine Cancer Institute. In an interview, she said that, at meetings on Monday, March 23, “we divided the team exactly in half.”
Infusion center staff members were broken into an “A” and a “B” rotation, with each team either on site or remotely for a 14-day stretch, and then switching at the 2-week mark. The 14-day rotation, she said, was chosen so that each cohort would have a full 2 weeks away after having been in clinic to ensure they were symptom free before returning. The cohorting scheme also serves to minimize between-staff exposure and risk of transmission.
These changes were implemented immediately, said Ms. Hosking, and included all but the most senior leadership – Ms. Hosking alternates days on site with another senior colleague to help with continuity.
Infusion center patients were triaged to determine “who absolutely needed to be seen,” and clinic staff started making phone calls and reshuffling the schedule so the clinic could continue at half-strength staffing.
The clinic was rearranged to ensure each infusion chair had appropriate space but the nursing work flow was still safe with reduced staff, said Jessica Stewart, MSN, RN, Levine Cancer Institute’s hematology–sickle cell nurse manager.
Patients were receptive, said Ms. Stewart. The team that was working remotely made sure all patients were called the day before their appointments, so they could understand what to expect when they arrived. Any needed updates to the medical history and patient teaching can also be done over the phone the day before the visit, she said, noting that patients are also queried about any concerning symptoms such as fever or cough.
In the spirit of providing information and managing expectations, patients are also informed that they will not be able to bring a visitor along and are advised to expect additional screening when they arrive. In addition to a repeat of symptom screening, patients are checked for fever with a temporal thermometer.
Any patient who arrives reporting symptoms or who has a fever is then subject to additional screening. Physician phone consultation is available, if needed, and patients may be routed to a drive-through screening and testing setup, or to the ED if there are concerns the patient may be seriously ill.
Several weeks into the new operations, Ms. Stewart said, “we’ve fine-tuned the processes we currently have in place. There’s new practices with virtual visits to make reaching our patients easier. Our senior leadership is communicating in a weekly video sent to all [Levine Cancer Institute] teammates for updates; it’s very transparent and the team is appreciative of being kept in the loop.”
Thus far, said Ms. Hosking, “it’s gone well – we’ve successfully operationalized this plan. … I think it shows that people that care about each other and their mission can collaborate with each other” to make change happen in a hurry.
Though it’s too soon to know exactly what the future holds once the pandemic has passed, some aspects of the new way of doing things may carry forward, said Ms. Stewart. “Communication has been massively streamlined,” and staff has found the previsit phone calls an efficient and effective way to gather and impart information.
A staff nurse at the infusion center, Whitney Hollifield, RN, added that patients have seen – and appreciate – the added precautions taken by all. “I feel that we have done well with protecting our patients from unneeded exposure and patients have expressed this to me,” said Ms. Hollifield. “They have said: ‘Thank you for doing this because I am scared to come in right now so I appreciate that your office is thinking of protecting us.’ ”
Ms. Hollifield added that “patients have been very responsive to our strategy for their care because we are truly concerned for them and I think that this shows. I believe that we are doing everything we can to keep them safe during a tumultuous time, and they feel genuine care for them during a frightening time is reassuring.”
On the practical side of things, Ms. Stewart noted, patients and families have provided infusion center staff with a seemingly endless supply of food: “We have never been more well fed!”
Rhonda Davis, RN, is a nurse at the Levine Cancer Institute. Speaking of the changes that have been made in recent weeks, she said, “Some of the changes that I think have been meaningful these last 3 weeks are making sure that the patients are the No. 1 priority. We are doing this by allowing patients options such as phone and virtual visits. This helps patients have some control over their health during this scary time for all.”
Ms. Davis acknowledged her own feelings about the uncertain times ahead. “As an individual with good health, I am scared, so to imagine the fear that these patients are facing must be overwhelming to them. Along that line, one of the most meaningful things that has happened for me is calling patients and having them concerned about my health and telling me to be safe.”
Despite her trepidation, she said, it’s meaningful for her to hear from patients who are in the clinic that they appreciate her presence. She found it heartening “that they are also considering our safety as well as their own.”
The two-cohort scheme has been well received by nursing staff, both administrators and clinic staff agreed. “I think that allowing staff to work 2 weeks on and 2 weeks at home helps keep patients and teammates safe,” Ms. Davis said.
Another infusion nurse, Ursel Wallace, RN, said that she appreciated the speed and efficiency with which the pandemic adaptations were made, including the nuts and bolts of reshuffling a complicated infusion schedule. “I know there were many different moving parts and it took a village” to move with such alacrity without dropping balls, she said.
The infusion nursing team’s spirit was summed up by Patricia Ashworth, RN: “Together, we will prevail!”
How do you run a chemotherapy infusion center during a pandemic?
Quick action, innovative staffing solutions, and nimble leadership are allowing one cancer center to continue providing care for the most vulnerable patients, while keeping patients and staff safe.
When nursing leaders at Atrium Health’s Levine Cancer Institute in Charlotte, N.C., realized that business was not going to continue as usual for American health care during the COVID-19 pandemic, they knew they had to act quickly to keep the institute’s 82-chair infusion center up and running.
North Carolina had already imposed restrictions on mass gatherings and closed educational facilities and some businesses by mid-March. Stay-at-home orders were being issued in surrounding states (North Carolina came under a statewide order on March 30). Physical distancing and a healthy, resilient team were prerequisites to an effective COVID-19 solution for the infusion clinic, said Angela Hosking, MBA, MSN, RN, director of nursing for Levine Cancer Institute. In an interview, she said that, at meetings on Monday, March 23, “we divided the team exactly in half.”
Infusion center staff members were broken into an “A” and a “B” rotation, with each team either on site or remotely for a 14-day stretch, and then switching at the 2-week mark. The 14-day rotation, she said, was chosen so that each cohort would have a full 2 weeks away after having been in clinic to ensure they were symptom free before returning. The cohorting scheme also serves to minimize between-staff exposure and risk of transmission.
These changes were implemented immediately, said Ms. Hosking, and included all but the most senior leadership – Ms. Hosking alternates days on site with another senior colleague to help with continuity.
Infusion center patients were triaged to determine “who absolutely needed to be seen,” and clinic staff started making phone calls and reshuffling the schedule so the clinic could continue at half-strength staffing.
The clinic was rearranged to ensure each infusion chair had appropriate space but the nursing work flow was still safe with reduced staff, said Jessica Stewart, MSN, RN, Levine Cancer Institute’s hematology–sickle cell nurse manager.
Patients were receptive, said Ms. Stewart. The team that was working remotely made sure all patients were called the day before their appointments, so they could understand what to expect when they arrived. Any needed updates to the medical history and patient teaching can also be done over the phone the day before the visit, she said, noting that patients are also queried about any concerning symptoms such as fever or cough.
In the spirit of providing information and managing expectations, patients are also informed that they will not be able to bring a visitor along and are advised to expect additional screening when they arrive. In addition to a repeat of symptom screening, patients are checked for fever with a temporal thermometer.
Any patient who arrives reporting symptoms or who has a fever is then subject to additional screening. Physician phone consultation is available, if needed, and patients may be routed to a drive-through screening and testing setup, or to the ED if there are concerns the patient may be seriously ill.
Several weeks into the new operations, Ms. Stewart said, “we’ve fine-tuned the processes we currently have in place. There’s new practices with virtual visits to make reaching our patients easier. Our senior leadership is communicating in a weekly video sent to all [Levine Cancer Institute] teammates for updates; it’s very transparent and the team is appreciative of being kept in the loop.”
Thus far, said Ms. Hosking, “it’s gone well – we’ve successfully operationalized this plan. … I think it shows that people that care about each other and their mission can collaborate with each other” to make change happen in a hurry.
Though it’s too soon to know exactly what the future holds once the pandemic has passed, some aspects of the new way of doing things may carry forward, said Ms. Stewart. “Communication has been massively streamlined,” and staff has found the previsit phone calls an efficient and effective way to gather and impart information.
A staff nurse at the infusion center, Whitney Hollifield, RN, added that patients have seen – and appreciate – the added precautions taken by all. “I feel that we have done well with protecting our patients from unneeded exposure and patients have expressed this to me,” said Ms. Hollifield. “They have said: ‘Thank you for doing this because I am scared to come in right now so I appreciate that your office is thinking of protecting us.’ ”
Ms. Hollifield added that “patients have been very responsive to our strategy for their care because we are truly concerned for them and I think that this shows. I believe that we are doing everything we can to keep them safe during a tumultuous time, and they feel genuine care for them during a frightening time is reassuring.”
On the practical side of things, Ms. Stewart noted, patients and families have provided infusion center staff with a seemingly endless supply of food: “We have never been more well fed!”
Rhonda Davis, RN, is a nurse at the Levine Cancer Institute. Speaking of the changes that have been made in recent weeks, she said, “Some of the changes that I think have been meaningful these last 3 weeks are making sure that the patients are the No. 1 priority. We are doing this by allowing patients options such as phone and virtual visits. This helps patients have some control over their health during this scary time for all.”
Ms. Davis acknowledged her own feelings about the uncertain times ahead. “As an individual with good health, I am scared, so to imagine the fear that these patients are facing must be overwhelming to them. Along that line, one of the most meaningful things that has happened for me is calling patients and having them concerned about my health and telling me to be safe.”
Despite her trepidation, she said, it’s meaningful for her to hear from patients who are in the clinic that they appreciate her presence. She found it heartening “that they are also considering our safety as well as their own.”
The two-cohort scheme has been well received by nursing staff, both administrators and clinic staff agreed. “I think that allowing staff to work 2 weeks on and 2 weeks at home helps keep patients and teammates safe,” Ms. Davis said.
Another infusion nurse, Ursel Wallace, RN, said that she appreciated the speed and efficiency with which the pandemic adaptations were made, including the nuts and bolts of reshuffling a complicated infusion schedule. “I know there were many different moving parts and it took a village” to move with such alacrity without dropping balls, she said.
The infusion nursing team’s spirit was summed up by Patricia Ashworth, RN: “Together, we will prevail!”
How do you run a chemotherapy infusion center during a pandemic?
Quick action, innovative staffing solutions, and nimble leadership are allowing one cancer center to continue providing care for the most vulnerable patients, while keeping patients and staff safe.
When nursing leaders at Atrium Health’s Levine Cancer Institute in Charlotte, N.C., realized that business was not going to continue as usual for American health care during the COVID-19 pandemic, they knew they had to act quickly to keep the institute’s 82-chair infusion center up and running.
North Carolina had already imposed restrictions on mass gatherings and closed educational facilities and some businesses by mid-March. Stay-at-home orders were being issued in surrounding states (North Carolina came under a statewide order on March 30). Physical distancing and a healthy, resilient team were prerequisites to an effective COVID-19 solution for the infusion clinic, said Angela Hosking, MBA, MSN, RN, director of nursing for Levine Cancer Institute. In an interview, she said that, at meetings on Monday, March 23, “we divided the team exactly in half.”
Infusion center staff members were broken into an “A” and a “B” rotation, with each team either on site or remotely for a 14-day stretch, and then switching at the 2-week mark. The 14-day rotation, she said, was chosen so that each cohort would have a full 2 weeks away after having been in clinic to ensure they were symptom free before returning. The cohorting scheme also serves to minimize between-staff exposure and risk of transmission.
These changes were implemented immediately, said Ms. Hosking, and included all but the most senior leadership – Ms. Hosking alternates days on site with another senior colleague to help with continuity.
Infusion center patients were triaged to determine “who absolutely needed to be seen,” and clinic staff started making phone calls and reshuffling the schedule so the clinic could continue at half-strength staffing.
The clinic was rearranged to ensure each infusion chair had appropriate space but the nursing work flow was still safe with reduced staff, said Jessica Stewart, MSN, RN, Levine Cancer Institute’s hematology–sickle cell nurse manager.
Patients were receptive, said Ms. Stewart. The team that was working remotely made sure all patients were called the day before their appointments, so they could understand what to expect when they arrived. Any needed updates to the medical history and patient teaching can also be done over the phone the day before the visit, she said, noting that patients are also queried about any concerning symptoms such as fever or cough.
In the spirit of providing information and managing expectations, patients are also informed that they will not be able to bring a visitor along and are advised to expect additional screening when they arrive. In addition to a repeat of symptom screening, patients are checked for fever with a temporal thermometer.
Any patient who arrives reporting symptoms or who has a fever is then subject to additional screening. Physician phone consultation is available, if needed, and patients may be routed to a drive-through screening and testing setup, or to the ED if there are concerns the patient may be seriously ill.
Several weeks into the new operations, Ms. Stewart said, “we’ve fine-tuned the processes we currently have in place. There’s new practices with virtual visits to make reaching our patients easier. Our senior leadership is communicating in a weekly video sent to all [Levine Cancer Institute] teammates for updates; it’s very transparent and the team is appreciative of being kept in the loop.”
Thus far, said Ms. Hosking, “it’s gone well – we’ve successfully operationalized this plan. … I think it shows that people that care about each other and their mission can collaborate with each other” to make change happen in a hurry.
Though it’s too soon to know exactly what the future holds once the pandemic has passed, some aspects of the new way of doing things may carry forward, said Ms. Stewart. “Communication has been massively streamlined,” and staff has found the previsit phone calls an efficient and effective way to gather and impart information.
A staff nurse at the infusion center, Whitney Hollifield, RN, added that patients have seen – and appreciate – the added precautions taken by all. “I feel that we have done well with protecting our patients from unneeded exposure and patients have expressed this to me,” said Ms. Hollifield. “They have said: ‘Thank you for doing this because I am scared to come in right now so I appreciate that your office is thinking of protecting us.’ ”
Ms. Hollifield added that “patients have been very responsive to our strategy for their care because we are truly concerned for them and I think that this shows. I believe that we are doing everything we can to keep them safe during a tumultuous time, and they feel genuine care for them during a frightening time is reassuring.”
On the practical side of things, Ms. Stewart noted, patients and families have provided infusion center staff with a seemingly endless supply of food: “We have never been more well fed!”
Rhonda Davis, RN, is a nurse at the Levine Cancer Institute. Speaking of the changes that have been made in recent weeks, she said, “Some of the changes that I think have been meaningful these last 3 weeks are making sure that the patients are the No. 1 priority. We are doing this by allowing patients options such as phone and virtual visits. This helps patients have some control over their health during this scary time for all.”
Ms. Davis acknowledged her own feelings about the uncertain times ahead. “As an individual with good health, I am scared, so to imagine the fear that these patients are facing must be overwhelming to them. Along that line, one of the most meaningful things that has happened for me is calling patients and having them concerned about my health and telling me to be safe.”
Despite her trepidation, she said, it’s meaningful for her to hear from patients who are in the clinic that they appreciate her presence. She found it heartening “that they are also considering our safety as well as their own.”
The two-cohort scheme has been well received by nursing staff, both administrators and clinic staff agreed. “I think that allowing staff to work 2 weeks on and 2 weeks at home helps keep patients and teammates safe,” Ms. Davis said.
Another infusion nurse, Ursel Wallace, RN, said that she appreciated the speed and efficiency with which the pandemic adaptations were made, including the nuts and bolts of reshuffling a complicated infusion schedule. “I know there were many different moving parts and it took a village” to move with such alacrity without dropping balls, she said.
The infusion nursing team’s spirit was summed up by Patricia Ashworth, RN: “Together, we will prevail!”
Latest data on COVID-19 patients with rheumatic diseases revealed in registry
An international registry of adult and pediatric rheumatology patients is beginning to identify trends in the types of patients with COVID-19 and who is recovering.
The COVID-19 Global Rheumatology Alliance (GRA) has created pediatric and adult registries for health care providers to enter information on their rheumatology patients with COVID-19. The adult registry is hosted by the University of California, San Francisco, Research Electronic Data Capture system, while the Childhood Arthritis and Rheumatology Research Alliance is supporting the pediatric registry. A separate path for data entry of both adult and pediatric cases has been established through the European League Against Rheumatism for European countries and countries with EULAR member organizations.
Prior to the creation of the registries, there were no data available to guide rheumatologists in clinical decision making for their patients, noted Jinoos Yazdany, MD, MPH, COVID-19 GRA steering committee member and chief of the division of rheumatology at Zuckerberg San Francisco General Hospital. “COVID-19 has potential to severely affect those with rheumatologic diseases or those taking immunosuppressive drugs,” she said in an interview. “The GRA registries were designed to answer critical questions that will inform the medical care of this population.”
The GRA began on Twitter, with conversations between Leonard H. Calabrese, DO, of the Cleveland Clinic; Paul Sufka, MD, of HealthPartners in St. Paul, Minn.; Philip Robinson, MBChB, PhD, of the Royal Brisbane (Australia) Hospital; and herself, Dr. Yazdany said. Dr. Robinson started work on the governance of the GRA, Dr. Yazdany designed the data infrastructure, and Dr. Sufka approached his professional networks and social media followings to promote the effort and ask for support. The COVID-19 GRA steering committee representatives include patients, private practice rheumatologists, and international investigators. Listed among official supporters of the alliance are the American College of Rheumatology and EULAR along with more than 290 medical societies, institutions, journals, and other organizations in rheumatology.
The goal of the registries is to examine the health outcomes of patients with rheumatic diseases and COVID-19 based on sociodemographic factors, comorbidities, and clinical presentations of COVID-19 as well as what role taking immunosuppressive drugs prior to a COVID-19 infection play in helping or hindering outcomes. Hydroxychloroquine, used to treat lupus and arthritis, is a potential treatment candidate for COVID-19. Biologics such as tocilizumab (Actemra) and sarilumab (Kevzara), which target interleukin-6, and anakinra (Kineret), which targets IL-1, are treatment candidates for patients who have experienced COVID-related cytokine storm syndrome, which researchers believe may contribute to worsening or fatal cases.
Dr. Yazdany, who is also vice chair of real-world data infrastructure, registry, and institutional review board/ethics for the GRA, said that there are some important high-level trends in the data thus far. “People with lupus and those taking hydroxychloroquine are becoming infected with SARS-CoV-2, which is counter to misinformation on social media. Most people with rheumatic diseases on immunosuppression are recovering, which is great news for our patients.”
One of the major strengths of the registries is that each case is entered by the rheumatologist treating the patient and contains detailed clinical information, Dr. Yazdany said. However, the registry has no control group, it is not a population surveillance study, and it may contain selection bias through rheumatologists omitting milder, undiagnosed cases.
“The Global Alliance case reporting registry represents the collective effort of hundreds of rheumatologists across the world. I have never been more inspired by the strength and collaboration of the rheumatology community,” Dr. Yazdany said.
According to a paper published in the Lancet Rheumatology, which references data on 110 cases from the combined databases up to April 1, about three-fourths of cases presented with fever (79%) and cough (77%), and about half presented with shortness of breath (50%) and myalgia (45%).
Results from the global and UCSF registries
As of April 18, 334 cases were in the global and UCSF registries, with 121 patients (36%) in the database having both COVID-19 and RA, 33 patients (10%) with psoriatic arthritis, 58 patients (17%) with systemic lupus erythematosus, 28 patients (8%) with axial spondyloarthritis, 27 patients (8%) with vasculitis, and 19 patients (6%) with Sjögren’s syndrome. There were less than five cases reported for patients with the following rheumatic diseases: inflammatory myopathy, ocular inflammation, other inflammatory arthritis, polymyalgia rheumatica, sarcoidosis, systemic sclerosis, osteoporosis, psoriasis, isolated pulmonary capillaritis, gout, and autoinflammatory disease. A majority of the patients in the registries are women (74%) aged younger than 65 years (78%) and are white (52%).
The most common comorbid conditions among patients in the registry are hypertension (33%), lung disease (18%), diabetes (11%), cardiovascular disease (10%), chronic renal insufficiency or end-stage renal disease (7%), morbid obesity (7%), and cancer (4%). Before being diagnosed with COVID-19, 219 patients (66%) in the registry were taking conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), which included antimalarials, azathioprine, cyclophosphamide, cyclosporine, leflunomide, methotrexate, mycophenolate mofetil/mycophenolic acid, sulfasalazine, and tacrolimus. A total of 122 patients (37%) were taking biologic DMARDs, 101 patients were taking glucocorticoids (30%), 86 patients (26%) were taking hydroxychloroquine, 41 patients (12%) were taking NSAIDs, and 18 patients (5%) were taking a Janus kinase inhibitor.
The most recent data from the registry show that 128 patients (38%) have been hospitalized for COVID-19, and 19 patients (6%) have died. Although 104 patients (31%) resolved their infections, 177 patients (53%) have a COVID-19 infection status of “unresolved,” and 53 patients (16%) have an unknown infection status.
EULAR registry results
As of April 21, 249 cases were in the EULAR registry, including 110 hospitalizations (44%) and 37 deaths (15%). Overall, 64% of these patients were women, and they had a median age of 60 years.
The top five diagnoses of these patients were RA (39%), psoriatic arthritis (15%), spondyloarthritis (9%), systemic lupus erythematosus (9%), and gout (5%). A total of 27% had no reported comorbidities, while lung disease occurred in 26%, hypertension in 34%, diabetes in 11%, and cardiovascular disease on 11%. The registry also reported use of any DMARD in 80%, including 62% on csDMARDs, 31% on biologics, and 2% on targeted synthetic DMARDs.
Ten authors in the Lancet Rheumatology paper reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speaker’s bureau positions, personal fees, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. The other authors reported no relevant conflicts of interest.
An international registry of adult and pediatric rheumatology patients is beginning to identify trends in the types of patients with COVID-19 and who is recovering.
The COVID-19 Global Rheumatology Alliance (GRA) has created pediatric and adult registries for health care providers to enter information on their rheumatology patients with COVID-19. The adult registry is hosted by the University of California, San Francisco, Research Electronic Data Capture system, while the Childhood Arthritis and Rheumatology Research Alliance is supporting the pediatric registry. A separate path for data entry of both adult and pediatric cases has been established through the European League Against Rheumatism for European countries and countries with EULAR member organizations.
Prior to the creation of the registries, there were no data available to guide rheumatologists in clinical decision making for their patients, noted Jinoos Yazdany, MD, MPH, COVID-19 GRA steering committee member and chief of the division of rheumatology at Zuckerberg San Francisco General Hospital. “COVID-19 has potential to severely affect those with rheumatologic diseases or those taking immunosuppressive drugs,” she said in an interview. “The GRA registries were designed to answer critical questions that will inform the medical care of this population.”
The GRA began on Twitter, with conversations between Leonard H. Calabrese, DO, of the Cleveland Clinic; Paul Sufka, MD, of HealthPartners in St. Paul, Minn.; Philip Robinson, MBChB, PhD, of the Royal Brisbane (Australia) Hospital; and herself, Dr. Yazdany said. Dr. Robinson started work on the governance of the GRA, Dr. Yazdany designed the data infrastructure, and Dr. Sufka approached his professional networks and social media followings to promote the effort and ask for support. The COVID-19 GRA steering committee representatives include patients, private practice rheumatologists, and international investigators. Listed among official supporters of the alliance are the American College of Rheumatology and EULAR along with more than 290 medical societies, institutions, journals, and other organizations in rheumatology.
The goal of the registries is to examine the health outcomes of patients with rheumatic diseases and COVID-19 based on sociodemographic factors, comorbidities, and clinical presentations of COVID-19 as well as what role taking immunosuppressive drugs prior to a COVID-19 infection play in helping or hindering outcomes. Hydroxychloroquine, used to treat lupus and arthritis, is a potential treatment candidate for COVID-19. Biologics such as tocilizumab (Actemra) and sarilumab (Kevzara), which target interleukin-6, and anakinra (Kineret), which targets IL-1, are treatment candidates for patients who have experienced COVID-related cytokine storm syndrome, which researchers believe may contribute to worsening or fatal cases.
Dr. Yazdany, who is also vice chair of real-world data infrastructure, registry, and institutional review board/ethics for the GRA, said that there are some important high-level trends in the data thus far. “People with lupus and those taking hydroxychloroquine are becoming infected with SARS-CoV-2, which is counter to misinformation on social media. Most people with rheumatic diseases on immunosuppression are recovering, which is great news for our patients.”
One of the major strengths of the registries is that each case is entered by the rheumatologist treating the patient and contains detailed clinical information, Dr. Yazdany said. However, the registry has no control group, it is not a population surveillance study, and it may contain selection bias through rheumatologists omitting milder, undiagnosed cases.
“The Global Alliance case reporting registry represents the collective effort of hundreds of rheumatologists across the world. I have never been more inspired by the strength and collaboration of the rheumatology community,” Dr. Yazdany said.
According to a paper published in the Lancet Rheumatology, which references data on 110 cases from the combined databases up to April 1, about three-fourths of cases presented with fever (79%) and cough (77%), and about half presented with shortness of breath (50%) and myalgia (45%).
Results from the global and UCSF registries
As of April 18, 334 cases were in the global and UCSF registries, with 121 patients (36%) in the database having both COVID-19 and RA, 33 patients (10%) with psoriatic arthritis, 58 patients (17%) with systemic lupus erythematosus, 28 patients (8%) with axial spondyloarthritis, 27 patients (8%) with vasculitis, and 19 patients (6%) with Sjögren’s syndrome. There were less than five cases reported for patients with the following rheumatic diseases: inflammatory myopathy, ocular inflammation, other inflammatory arthritis, polymyalgia rheumatica, sarcoidosis, systemic sclerosis, osteoporosis, psoriasis, isolated pulmonary capillaritis, gout, and autoinflammatory disease. A majority of the patients in the registries are women (74%) aged younger than 65 years (78%) and are white (52%).
The most common comorbid conditions among patients in the registry are hypertension (33%), lung disease (18%), diabetes (11%), cardiovascular disease (10%), chronic renal insufficiency or end-stage renal disease (7%), morbid obesity (7%), and cancer (4%). Before being diagnosed with COVID-19, 219 patients (66%) in the registry were taking conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), which included antimalarials, azathioprine, cyclophosphamide, cyclosporine, leflunomide, methotrexate, mycophenolate mofetil/mycophenolic acid, sulfasalazine, and tacrolimus. A total of 122 patients (37%) were taking biologic DMARDs, 101 patients were taking glucocorticoids (30%), 86 patients (26%) were taking hydroxychloroquine, 41 patients (12%) were taking NSAIDs, and 18 patients (5%) were taking a Janus kinase inhibitor.
The most recent data from the registry show that 128 patients (38%) have been hospitalized for COVID-19, and 19 patients (6%) have died. Although 104 patients (31%) resolved their infections, 177 patients (53%) have a COVID-19 infection status of “unresolved,” and 53 patients (16%) have an unknown infection status.
EULAR registry results
As of April 21, 249 cases were in the EULAR registry, including 110 hospitalizations (44%) and 37 deaths (15%). Overall, 64% of these patients were women, and they had a median age of 60 years.
The top five diagnoses of these patients were RA (39%), psoriatic arthritis (15%), spondyloarthritis (9%), systemic lupus erythematosus (9%), and gout (5%). A total of 27% had no reported comorbidities, while lung disease occurred in 26%, hypertension in 34%, diabetes in 11%, and cardiovascular disease on 11%. The registry also reported use of any DMARD in 80%, including 62% on csDMARDs, 31% on biologics, and 2% on targeted synthetic DMARDs.
Ten authors in the Lancet Rheumatology paper reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speaker’s bureau positions, personal fees, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. The other authors reported no relevant conflicts of interest.
An international registry of adult and pediatric rheumatology patients is beginning to identify trends in the types of patients with COVID-19 and who is recovering.
The COVID-19 Global Rheumatology Alliance (GRA) has created pediatric and adult registries for health care providers to enter information on their rheumatology patients with COVID-19. The adult registry is hosted by the University of California, San Francisco, Research Electronic Data Capture system, while the Childhood Arthritis and Rheumatology Research Alliance is supporting the pediatric registry. A separate path for data entry of both adult and pediatric cases has been established through the European League Against Rheumatism for European countries and countries with EULAR member organizations.
Prior to the creation of the registries, there were no data available to guide rheumatologists in clinical decision making for their patients, noted Jinoos Yazdany, MD, MPH, COVID-19 GRA steering committee member and chief of the division of rheumatology at Zuckerberg San Francisco General Hospital. “COVID-19 has potential to severely affect those with rheumatologic diseases or those taking immunosuppressive drugs,” she said in an interview. “The GRA registries were designed to answer critical questions that will inform the medical care of this population.”
The GRA began on Twitter, with conversations between Leonard H. Calabrese, DO, of the Cleveland Clinic; Paul Sufka, MD, of HealthPartners in St. Paul, Minn.; Philip Robinson, MBChB, PhD, of the Royal Brisbane (Australia) Hospital; and herself, Dr. Yazdany said. Dr. Robinson started work on the governance of the GRA, Dr. Yazdany designed the data infrastructure, and Dr. Sufka approached his professional networks and social media followings to promote the effort and ask for support. The COVID-19 GRA steering committee representatives include patients, private practice rheumatologists, and international investigators. Listed among official supporters of the alliance are the American College of Rheumatology and EULAR along with more than 290 medical societies, institutions, journals, and other organizations in rheumatology.
The goal of the registries is to examine the health outcomes of patients with rheumatic diseases and COVID-19 based on sociodemographic factors, comorbidities, and clinical presentations of COVID-19 as well as what role taking immunosuppressive drugs prior to a COVID-19 infection play in helping or hindering outcomes. Hydroxychloroquine, used to treat lupus and arthritis, is a potential treatment candidate for COVID-19. Biologics such as tocilizumab (Actemra) and sarilumab (Kevzara), which target interleukin-6, and anakinra (Kineret), which targets IL-1, are treatment candidates for patients who have experienced COVID-related cytokine storm syndrome, which researchers believe may contribute to worsening or fatal cases.
Dr. Yazdany, who is also vice chair of real-world data infrastructure, registry, and institutional review board/ethics for the GRA, said that there are some important high-level trends in the data thus far. “People with lupus and those taking hydroxychloroquine are becoming infected with SARS-CoV-2, which is counter to misinformation on social media. Most people with rheumatic diseases on immunosuppression are recovering, which is great news for our patients.”
One of the major strengths of the registries is that each case is entered by the rheumatologist treating the patient and contains detailed clinical information, Dr. Yazdany said. However, the registry has no control group, it is not a population surveillance study, and it may contain selection bias through rheumatologists omitting milder, undiagnosed cases.
“The Global Alliance case reporting registry represents the collective effort of hundreds of rheumatologists across the world. I have never been more inspired by the strength and collaboration of the rheumatology community,” Dr. Yazdany said.
According to a paper published in the Lancet Rheumatology, which references data on 110 cases from the combined databases up to April 1, about three-fourths of cases presented with fever (79%) and cough (77%), and about half presented with shortness of breath (50%) and myalgia (45%).
Results from the global and UCSF registries
As of April 18, 334 cases were in the global and UCSF registries, with 121 patients (36%) in the database having both COVID-19 and RA, 33 patients (10%) with psoriatic arthritis, 58 patients (17%) with systemic lupus erythematosus, 28 patients (8%) with axial spondyloarthritis, 27 patients (8%) with vasculitis, and 19 patients (6%) with Sjögren’s syndrome. There were less than five cases reported for patients with the following rheumatic diseases: inflammatory myopathy, ocular inflammation, other inflammatory arthritis, polymyalgia rheumatica, sarcoidosis, systemic sclerosis, osteoporosis, psoriasis, isolated pulmonary capillaritis, gout, and autoinflammatory disease. A majority of the patients in the registries are women (74%) aged younger than 65 years (78%) and are white (52%).
The most common comorbid conditions among patients in the registry are hypertension (33%), lung disease (18%), diabetes (11%), cardiovascular disease (10%), chronic renal insufficiency or end-stage renal disease (7%), morbid obesity (7%), and cancer (4%). Before being diagnosed with COVID-19, 219 patients (66%) in the registry were taking conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), which included antimalarials, azathioprine, cyclophosphamide, cyclosporine, leflunomide, methotrexate, mycophenolate mofetil/mycophenolic acid, sulfasalazine, and tacrolimus. A total of 122 patients (37%) were taking biologic DMARDs, 101 patients were taking glucocorticoids (30%), 86 patients (26%) were taking hydroxychloroquine, 41 patients (12%) were taking NSAIDs, and 18 patients (5%) were taking a Janus kinase inhibitor.
The most recent data from the registry show that 128 patients (38%) have been hospitalized for COVID-19, and 19 patients (6%) have died. Although 104 patients (31%) resolved their infections, 177 patients (53%) have a COVID-19 infection status of “unresolved,” and 53 patients (16%) have an unknown infection status.
EULAR registry results
As of April 21, 249 cases were in the EULAR registry, including 110 hospitalizations (44%) and 37 deaths (15%). Overall, 64% of these patients were women, and they had a median age of 60 years.
The top five diagnoses of these patients were RA (39%), psoriatic arthritis (15%), spondyloarthritis (9%), systemic lupus erythematosus (9%), and gout (5%). A total of 27% had no reported comorbidities, while lung disease occurred in 26%, hypertension in 34%, diabetes in 11%, and cardiovascular disease on 11%. The registry also reported use of any DMARD in 80%, including 62% on csDMARDs, 31% on biologics, and 2% on targeted synthetic DMARDs.
Ten authors in the Lancet Rheumatology paper reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speaker’s bureau positions, personal fees, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. The other authors reported no relevant conflicts of interest.
FDA approves ibrutinib-rituximab combo for newly diagnosed CLL, SLL in adults
The Food and Drug Administration has expanded the indication for ibrutinib (Imbruvica) to allow its combination with rituximab for frontline treatment of chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in adults.
The approval, announced April 21, was based on findings from the randomized, controlled, open-label, phase 3 E1912 trial of 529 patients, which demonstrated significantly improved progression-free survival (PFS) among those who received ibrutinib plus rituximab, compared with those who received fludarabine, cyclophosphamide, and rituximab (FCR) (87% vs. 75%; hazard ratio, 0.34). Median PFS was not reached in either arm after a median follow-up of 37 months.
E1912 was the first study to show superiority of a chemotherapy-free regimen over FCR chemoimmunotherapy, considered the gold standard for newly diagnosed CLL and SLL for the past 2 decades.
The recommended dosage for the newly approved combination is a once-daily 420-mg dose of ibrutinib taken with a glass of water, with rituximab initiation in the second cycle at doses of 50 mg/m2 on day 1, 325 mg/m2 on day 2, and 500 mg/m2 on days 1-5 of subsequent cycles for a total of six cycles.
The Food and Drug Administration has expanded the indication for ibrutinib (Imbruvica) to allow its combination with rituximab for frontline treatment of chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in adults.
The approval, announced April 21, was based on findings from the randomized, controlled, open-label, phase 3 E1912 trial of 529 patients, which demonstrated significantly improved progression-free survival (PFS) among those who received ibrutinib plus rituximab, compared with those who received fludarabine, cyclophosphamide, and rituximab (FCR) (87% vs. 75%; hazard ratio, 0.34). Median PFS was not reached in either arm after a median follow-up of 37 months.
E1912 was the first study to show superiority of a chemotherapy-free regimen over FCR chemoimmunotherapy, considered the gold standard for newly diagnosed CLL and SLL for the past 2 decades.
The recommended dosage for the newly approved combination is a once-daily 420-mg dose of ibrutinib taken with a glass of water, with rituximab initiation in the second cycle at doses of 50 mg/m2 on day 1, 325 mg/m2 on day 2, and 500 mg/m2 on days 1-5 of subsequent cycles for a total of six cycles.
The Food and Drug Administration has expanded the indication for ibrutinib (Imbruvica) to allow its combination with rituximab for frontline treatment of chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in adults.
The approval, announced April 21, was based on findings from the randomized, controlled, open-label, phase 3 E1912 trial of 529 patients, which demonstrated significantly improved progression-free survival (PFS) among those who received ibrutinib plus rituximab, compared with those who received fludarabine, cyclophosphamide, and rituximab (FCR) (87% vs. 75%; hazard ratio, 0.34). Median PFS was not reached in either arm after a median follow-up of 37 months.
E1912 was the first study to show superiority of a chemotherapy-free regimen over FCR chemoimmunotherapy, considered the gold standard for newly diagnosed CLL and SLL for the past 2 decades.
The recommended dosage for the newly approved combination is a once-daily 420-mg dose of ibrutinib taken with a glass of water, with rituximab initiation in the second cycle at doses of 50 mg/m2 on day 1, 325 mg/m2 on day 2, and 500 mg/m2 on days 1-5 of subsequent cycles for a total of six cycles.
COVID-19: Helping health care workers on front lines
Psychiatrists are intervening with less conventional strategies
Across the country, psychiatrists are stepping up to provide urgent care to fellow health care workers in need amid the coronavirus pandemic. They’re offering stress management strategies, spearheading unusual partnerships, and discovering that psychotherapy and medication might not be their most helpful tools to help their colleagues at this time.
“This is completely the opposite of the way we practice psychiatry,” said Allison Cotton, MD, of the University of Nevada, Reno. “Our interventions are quite different from a psychotherapeutic standpoint.”
In March, she worked with four colleagues, Suzan Song, MD, MPH, PhD; Ben Cheng, MD; Smita Gautam, MD; and Mona S. Masood, DO, to create the Physician Support Line, a confidential and free hotline that links physicians to volunteer psychiatrists who are available to listen and offer advice on coping. The hotline (888-409-0141) is available every day from 8 a.m. to midnight Eastern time. Calls typically take 15-45 minutes; no appointment is needed, and conversations are not reportable to state medical boards.

“The calls can be very intense,” Dr. Cotton said, and they’re unusual for several reasons. The hotline is not like a suicide or crisis hotline, when “a person calls because they need help, and then they can go get that help – they go to the hospital and get admitted to a psychiatric unit. Our callers don’t have that luxury.”
It’s also impossible to take an extensive history and create a sophisticated, long-term treatment plan as psychiatrists would during normal office visits. At the hotline, Dr. Cotton said, “we’re really focusing on the caller’s strengths and helping them come up with a plan for today to get through whatever they’re facing,” she said.
Stress management is critical
Psychiatrists at the University of Colorado Anschutz Medical Campus are embracing a similar approach to help health care workers cope, said Steven Berkowitz, MD. “We focus on stress management, and the notion that they are generally healthy and understandably struggling with extraordinary circumstances,” he said. “We are conservative in our use of medications and really only prescribe medications, such as trazodone, to help with sleep. We do not use benzodiazepines unless there is a history of more severe psychiatric problems.”
The pressure on health care workers during the pandemic is intense. A survey of 1,257 workers in 34 Chinese hospitals found high levels of symptoms of depression (50%), anxiety (45%), insomnia (35%), and distress (72%). Several groups appeared to be more vulnerable: women, nurses, front-line health care workers, and those in the coronavirus-stricken city of Wuhan (JAMA Netw Open. 2020;3[3]:e203976).
In Colorado, “providers are depleted,” Dr. Berkowitz said. “We are hearing about sleep disturbances and even some traumatic nightmares from ICU staff. During our support sessions, tears come most frequently when they talk about the struggle to care for their families and how they’re putting them at risk.”
Also, he said, “one of the most upsetting issues has been around language and cultural issues. Because of the language barriers, providers cannot explain why families can’t be with their sick members, which has led to acrimony.”
Guilt is a prevailing theme
Guilt also is a common emotion among health care workers, said psychiatrist Tia Konzer, DO, of Charlotte, N.C. “The ones on the front line question whether they were able to do enough to save someone or if they could have done more. Those of us not on the front lines feel guilty that we’re not there with our colleagues, that we don’t face the same fears and are in the safety of our outpatient clinics.”
The focus on social distancing is creating its own strains, she said. “A lot of people are recognizing the power of human touch and how comforting that is,” she said. “The healers aren’t able to comfort the loved ones of the deceased, and we’re not able to comfort each other. And people are having a hard time not being able to hug their kids and their spouses, having to ward off their kids when they come home or avoid them until they’ve showered.”
How can mental health professionals be most helpful to health care workers in need? The simple act of listening is crucial, several such professionals said in interviews.
“Your main job is to bear witness to their experiences and to hear their story, then secondarily to make sure they have a basic self-care plan to recover from what they’re doing each day,” said psychologist Leah Welch, PhD, of the Scripps Health network in San Diego. “Don’t talk too much or try to give advice too quickly before you’ve listened to what the caregiver has shared. They’re accumulating small traumas and need time and space to sort them out, and that takes patience and a listening ear on the part of the provider. Rushing in too quickly with advice deprives them of making sense of their own experience.”
She added that “they should also be thanked for what they’re doing, because it requires skill, empathy, and courage. They are being heroic, and they need to know they’re appreciated by those of us not on the front lines for what they’re putting themselves through.”
Partnerships are forming
At Zuckerberg San Francisco General Hospital and Trauma Center, psychiatry chief Lisa Fortuna, MD, MPH, MDiv, said her team has had success by working closely with the hospital’s chaplains. “A lot of the staff are not saying: ‘We’re stressing out; help us.’ The chaplains had starting rounding, asking how they’re doing, and they’d open up because there was already a relationship. The chaplains are very well trained in dealing with being support for people under situations of death, loss, and immediate stress.”
The chaplains themselves became overwhelmed, and the hospital responded by reaching out to bring in more chaplains. The psychiatry team, meanwhile, worked to partner with the chaplains to provide a continuum of support for staff. “We have an opportunity to build on the trust that they have,” said Dr. Fortuna, who is an ordained Episcopal minister. “They’re the perfect partners.”
What happens now? Dr. Fortuna has seen the long-term aftermath of a crisis. She previously worked in Massachusetts and helped to support health care workers in that state after the Boston Marathon bombing.
She cautioned that health care workers may first run on adrenaline in a crisis, spurred by “heroic high energy.” But then, the full extent of the tragedy begins to set in, and they start to process their feelings. “You have to keep people going through those phases,” she said.
Going forward, she said, “there will be a prolonged tail of stress,” especially if virus outbreaks recur. “We’ll have a long time enduring this.”
Don’t forget the self-care
There was a time during the pandemic when Dr. Cotton had become so overwhelmed by anxiety that she called the Physician Support Line to get some support from fellow psychiatrists.
“I thought, ‘Why not?’” she recalled. “I helped create the hotline. Why wouldn’t I call it?”
The calls took only a few minutes but they made a difference to Dr. Cotton, who had been severely ill with what she believed was an unconfirmed case of the novel coronavirus. “I immediately felt more like I improved my outlook by focusing on what I could control,” she said, “and accepting the things I could not control.”
Many psychiatrists are finding themselves in similar situations. Fortunately, colleagues are highlighting ways for psychiatrists to care for themselves just as they care for patients.
“One of the challenges clinicians are facing is that they are living through a shared experience in this global pandemic with their patients right now,” said psychologist Randi Pochtar, PhD, who is managing support groups for front-line workers at NYU Langone Health in New York City. “Some might find the work to be overwhelming and anxiety-inducing, and others might find their work to be helpful in managing their own anxiety and stress about the pandemic and its impact.”
Dr. Cotton said her breaking points came when she felt panic amid the pandemic. “I had watched too much news, and I’d seen protesters not taking it seriously, and I was scared for my family and myself. I just needed to feel like someone heard me feeling that way.”
The calls to the hotline were helpful, she said, and so was sharing news about her illness with friends. “So many people reached out to me and checked in on me, people I haven’t seen in years, and that was immensely helpful,” she said.
This sort of personal exposure may not come naturally to physicians and nurses, she said. “We don’t seek that kind of attention when we’re ill. Instead, we say: ‘I’m fine; how are you doing?’ That’s what we do every day of our lives at work.”
How can clinicians help themselves and one another? “Clinicians in our practice have been coping and supporting each other through peer supervision, connecting with colleagues in team meetings, and simply checking in on one another,” said Dr. Pochtar. “In addition, we can adopt many of the strategies that we are likely recommending to our patients, such as maintaining routines as much as possible, engaging in regular exercise, eating well and consistently, and connecting with friends and family.”
Managers can play important roles, said Dr. Fortuna. “I’ve been checking in with my faculty, being as supportive as I can be and highlighting the extraordinary things that people are doing, like going from zero to 100 percent in setting up telehealth.”
Dr. Konzer offered another perspective on recognizing the value of the work that psychiatrists are doing. “We’re on the front line of helping heal the front line, and in that responsibility comes an additional stress,” she said. “But there’s an additional gift of being able to contribute where we are most beneficial. We can try to be present now, versus worrying about what may happen or what lies ahead, and appreciate the beauty in the helpers and the small joys of life.”
Dr. Cotton, Dr. Berkowitz, Dr. Konzer, Dr. Welch, Dr. Fortuna, and Dr. Pochtar reported no relevant disclosures.
Psychiatrists are intervening with less conventional strategies
Psychiatrists are intervening with less conventional strategies
Across the country, psychiatrists are stepping up to provide urgent care to fellow health care workers in need amid the coronavirus pandemic. They’re offering stress management strategies, spearheading unusual partnerships, and discovering that psychotherapy and medication might not be their most helpful tools to help their colleagues at this time.
“This is completely the opposite of the way we practice psychiatry,” said Allison Cotton, MD, of the University of Nevada, Reno. “Our interventions are quite different from a psychotherapeutic standpoint.”
In March, she worked with four colleagues, Suzan Song, MD, MPH, PhD; Ben Cheng, MD; Smita Gautam, MD; and Mona S. Masood, DO, to create the Physician Support Line, a confidential and free hotline that links physicians to volunteer psychiatrists who are available to listen and offer advice on coping. The hotline (888-409-0141) is available every day from 8 a.m. to midnight Eastern time. Calls typically take 15-45 minutes; no appointment is needed, and conversations are not reportable to state medical boards.

“The calls can be very intense,” Dr. Cotton said, and they’re unusual for several reasons. The hotline is not like a suicide or crisis hotline, when “a person calls because they need help, and then they can go get that help – they go to the hospital and get admitted to a psychiatric unit. Our callers don’t have that luxury.”
It’s also impossible to take an extensive history and create a sophisticated, long-term treatment plan as psychiatrists would during normal office visits. At the hotline, Dr. Cotton said, “we’re really focusing on the caller’s strengths and helping them come up with a plan for today to get through whatever they’re facing,” she said.
Stress management is critical
Psychiatrists at the University of Colorado Anschutz Medical Campus are embracing a similar approach to help health care workers cope, said Steven Berkowitz, MD. “We focus on stress management, and the notion that they are generally healthy and understandably struggling with extraordinary circumstances,” he said. “We are conservative in our use of medications and really only prescribe medications, such as trazodone, to help with sleep. We do not use benzodiazepines unless there is a history of more severe psychiatric problems.”
The pressure on health care workers during the pandemic is intense. A survey of 1,257 workers in 34 Chinese hospitals found high levels of symptoms of depression (50%), anxiety (45%), insomnia (35%), and distress (72%). Several groups appeared to be more vulnerable: women, nurses, front-line health care workers, and those in the coronavirus-stricken city of Wuhan (JAMA Netw Open. 2020;3[3]:e203976).
In Colorado, “providers are depleted,” Dr. Berkowitz said. “We are hearing about sleep disturbances and even some traumatic nightmares from ICU staff. During our support sessions, tears come most frequently when they talk about the struggle to care for their families and how they’re putting them at risk.”
Also, he said, “one of the most upsetting issues has been around language and cultural issues. Because of the language barriers, providers cannot explain why families can’t be with their sick members, which has led to acrimony.”
Guilt is a prevailing theme
Guilt also is a common emotion among health care workers, said psychiatrist Tia Konzer, DO, of Charlotte, N.C. “The ones on the front line question whether they were able to do enough to save someone or if they could have done more. Those of us not on the front lines feel guilty that we’re not there with our colleagues, that we don’t face the same fears and are in the safety of our outpatient clinics.”
The focus on social distancing is creating its own strains, she said. “A lot of people are recognizing the power of human touch and how comforting that is,” she said. “The healers aren’t able to comfort the loved ones of the deceased, and we’re not able to comfort each other. And people are having a hard time not being able to hug their kids and their spouses, having to ward off their kids when they come home or avoid them until they’ve showered.”
How can mental health professionals be most helpful to health care workers in need? The simple act of listening is crucial, several such professionals said in interviews.
“Your main job is to bear witness to their experiences and to hear their story, then secondarily to make sure they have a basic self-care plan to recover from what they’re doing each day,” said psychologist Leah Welch, PhD, of the Scripps Health network in San Diego. “Don’t talk too much or try to give advice too quickly before you’ve listened to what the caregiver has shared. They’re accumulating small traumas and need time and space to sort them out, and that takes patience and a listening ear on the part of the provider. Rushing in too quickly with advice deprives them of making sense of their own experience.”
She added that “they should also be thanked for what they’re doing, because it requires skill, empathy, and courage. They are being heroic, and they need to know they’re appreciated by those of us not on the front lines for what they’re putting themselves through.”
Partnerships are forming
At Zuckerberg San Francisco General Hospital and Trauma Center, psychiatry chief Lisa Fortuna, MD, MPH, MDiv, said her team has had success by working closely with the hospital’s chaplains. “A lot of the staff are not saying: ‘We’re stressing out; help us.’ The chaplains had starting rounding, asking how they’re doing, and they’d open up because there was already a relationship. The chaplains are very well trained in dealing with being support for people under situations of death, loss, and immediate stress.”
The chaplains themselves became overwhelmed, and the hospital responded by reaching out to bring in more chaplains. The psychiatry team, meanwhile, worked to partner with the chaplains to provide a continuum of support for staff. “We have an opportunity to build on the trust that they have,” said Dr. Fortuna, who is an ordained Episcopal minister. “They’re the perfect partners.”
What happens now? Dr. Fortuna has seen the long-term aftermath of a crisis. She previously worked in Massachusetts and helped to support health care workers in that state after the Boston Marathon bombing.
She cautioned that health care workers may first run on adrenaline in a crisis, spurred by “heroic high energy.” But then, the full extent of the tragedy begins to set in, and they start to process their feelings. “You have to keep people going through those phases,” she said.
Going forward, she said, “there will be a prolonged tail of stress,” especially if virus outbreaks recur. “We’ll have a long time enduring this.”
Don’t forget the self-care
There was a time during the pandemic when Dr. Cotton had become so overwhelmed by anxiety that she called the Physician Support Line to get some support from fellow psychiatrists.
“I thought, ‘Why not?’” she recalled. “I helped create the hotline. Why wouldn’t I call it?”
The calls took only a few minutes but they made a difference to Dr. Cotton, who had been severely ill with what she believed was an unconfirmed case of the novel coronavirus. “I immediately felt more like I improved my outlook by focusing on what I could control,” she said, “and accepting the things I could not control.”
Many psychiatrists are finding themselves in similar situations. Fortunately, colleagues are highlighting ways for psychiatrists to care for themselves just as they care for patients.
“One of the challenges clinicians are facing is that they are living through a shared experience in this global pandemic with their patients right now,” said psychologist Randi Pochtar, PhD, who is managing support groups for front-line workers at NYU Langone Health in New York City. “Some might find the work to be overwhelming and anxiety-inducing, and others might find their work to be helpful in managing their own anxiety and stress about the pandemic and its impact.”
Dr. Cotton said her breaking points came when she felt panic amid the pandemic. “I had watched too much news, and I’d seen protesters not taking it seriously, and I was scared for my family and myself. I just needed to feel like someone heard me feeling that way.”
The calls to the hotline were helpful, she said, and so was sharing news about her illness with friends. “So many people reached out to me and checked in on me, people I haven’t seen in years, and that was immensely helpful,” she said.
This sort of personal exposure may not come naturally to physicians and nurses, she said. “We don’t seek that kind of attention when we’re ill. Instead, we say: ‘I’m fine; how are you doing?’ That’s what we do every day of our lives at work.”
How can clinicians help themselves and one another? “Clinicians in our practice have been coping and supporting each other through peer supervision, connecting with colleagues in team meetings, and simply checking in on one another,” said Dr. Pochtar. “In addition, we can adopt many of the strategies that we are likely recommending to our patients, such as maintaining routines as much as possible, engaging in regular exercise, eating well and consistently, and connecting with friends and family.”
Managers can play important roles, said Dr. Fortuna. “I’ve been checking in with my faculty, being as supportive as I can be and highlighting the extraordinary things that people are doing, like going from zero to 100 percent in setting up telehealth.”
Dr. Konzer offered another perspective on recognizing the value of the work that psychiatrists are doing. “We’re on the front line of helping heal the front line, and in that responsibility comes an additional stress,” she said. “But there’s an additional gift of being able to contribute where we are most beneficial. We can try to be present now, versus worrying about what may happen or what lies ahead, and appreciate the beauty in the helpers and the small joys of life.”
Dr. Cotton, Dr. Berkowitz, Dr. Konzer, Dr. Welch, Dr. Fortuna, and Dr. Pochtar reported no relevant disclosures.
Across the country, psychiatrists are stepping up to provide urgent care to fellow health care workers in need amid the coronavirus pandemic. They’re offering stress management strategies, spearheading unusual partnerships, and discovering that psychotherapy and medication might not be their most helpful tools to help their colleagues at this time.
“This is completely the opposite of the way we practice psychiatry,” said Allison Cotton, MD, of the University of Nevada, Reno. “Our interventions are quite different from a psychotherapeutic standpoint.”
In March, she worked with four colleagues, Suzan Song, MD, MPH, PhD; Ben Cheng, MD; Smita Gautam, MD; and Mona S. Masood, DO, to create the Physician Support Line, a confidential and free hotline that links physicians to volunteer psychiatrists who are available to listen and offer advice on coping. The hotline (888-409-0141) is available every day from 8 a.m. to midnight Eastern time. Calls typically take 15-45 minutes; no appointment is needed, and conversations are not reportable to state medical boards.

“The calls can be very intense,” Dr. Cotton said, and they’re unusual for several reasons. The hotline is not like a suicide or crisis hotline, when “a person calls because they need help, and then they can go get that help – they go to the hospital and get admitted to a psychiatric unit. Our callers don’t have that luxury.”
It’s also impossible to take an extensive history and create a sophisticated, long-term treatment plan as psychiatrists would during normal office visits. At the hotline, Dr. Cotton said, “we’re really focusing on the caller’s strengths and helping them come up with a plan for today to get through whatever they’re facing,” she said.
Stress management is critical
Psychiatrists at the University of Colorado Anschutz Medical Campus are embracing a similar approach to help health care workers cope, said Steven Berkowitz, MD. “We focus on stress management, and the notion that they are generally healthy and understandably struggling with extraordinary circumstances,” he said. “We are conservative in our use of medications and really only prescribe medications, such as trazodone, to help with sleep. We do not use benzodiazepines unless there is a history of more severe psychiatric problems.”
The pressure on health care workers during the pandemic is intense. A survey of 1,257 workers in 34 Chinese hospitals found high levels of symptoms of depression (50%), anxiety (45%), insomnia (35%), and distress (72%). Several groups appeared to be more vulnerable: women, nurses, front-line health care workers, and those in the coronavirus-stricken city of Wuhan (JAMA Netw Open. 2020;3[3]:e203976).
In Colorado, “providers are depleted,” Dr. Berkowitz said. “We are hearing about sleep disturbances and even some traumatic nightmares from ICU staff. During our support sessions, tears come most frequently when they talk about the struggle to care for their families and how they’re putting them at risk.”
Also, he said, “one of the most upsetting issues has been around language and cultural issues. Because of the language barriers, providers cannot explain why families can’t be with their sick members, which has led to acrimony.”
Guilt is a prevailing theme
Guilt also is a common emotion among health care workers, said psychiatrist Tia Konzer, DO, of Charlotte, N.C. “The ones on the front line question whether they were able to do enough to save someone or if they could have done more. Those of us not on the front lines feel guilty that we’re not there with our colleagues, that we don’t face the same fears and are in the safety of our outpatient clinics.”
The focus on social distancing is creating its own strains, she said. “A lot of people are recognizing the power of human touch and how comforting that is,” she said. “The healers aren’t able to comfort the loved ones of the deceased, and we’re not able to comfort each other. And people are having a hard time not being able to hug their kids and their spouses, having to ward off their kids when they come home or avoid them until they’ve showered.”
How can mental health professionals be most helpful to health care workers in need? The simple act of listening is crucial, several such professionals said in interviews.
“Your main job is to bear witness to their experiences and to hear their story, then secondarily to make sure they have a basic self-care plan to recover from what they’re doing each day,” said psychologist Leah Welch, PhD, of the Scripps Health network in San Diego. “Don’t talk too much or try to give advice too quickly before you’ve listened to what the caregiver has shared. They’re accumulating small traumas and need time and space to sort them out, and that takes patience and a listening ear on the part of the provider. Rushing in too quickly with advice deprives them of making sense of their own experience.”
She added that “they should also be thanked for what they’re doing, because it requires skill, empathy, and courage. They are being heroic, and they need to know they’re appreciated by those of us not on the front lines for what they’re putting themselves through.”
Partnerships are forming
At Zuckerberg San Francisco General Hospital and Trauma Center, psychiatry chief Lisa Fortuna, MD, MPH, MDiv, said her team has had success by working closely with the hospital’s chaplains. “A lot of the staff are not saying: ‘We’re stressing out; help us.’ The chaplains had starting rounding, asking how they’re doing, and they’d open up because there was already a relationship. The chaplains are very well trained in dealing with being support for people under situations of death, loss, and immediate stress.”
The chaplains themselves became overwhelmed, and the hospital responded by reaching out to bring in more chaplains. The psychiatry team, meanwhile, worked to partner with the chaplains to provide a continuum of support for staff. “We have an opportunity to build on the trust that they have,” said Dr. Fortuna, who is an ordained Episcopal minister. “They’re the perfect partners.”
What happens now? Dr. Fortuna has seen the long-term aftermath of a crisis. She previously worked in Massachusetts and helped to support health care workers in that state after the Boston Marathon bombing.
She cautioned that health care workers may first run on adrenaline in a crisis, spurred by “heroic high energy.” But then, the full extent of the tragedy begins to set in, and they start to process their feelings. “You have to keep people going through those phases,” she said.
Going forward, she said, “there will be a prolonged tail of stress,” especially if virus outbreaks recur. “We’ll have a long time enduring this.”
Don’t forget the self-care
There was a time during the pandemic when Dr. Cotton had become so overwhelmed by anxiety that she called the Physician Support Line to get some support from fellow psychiatrists.
“I thought, ‘Why not?’” she recalled. “I helped create the hotline. Why wouldn’t I call it?”
The calls took only a few minutes but they made a difference to Dr. Cotton, who had been severely ill with what she believed was an unconfirmed case of the novel coronavirus. “I immediately felt more like I improved my outlook by focusing on what I could control,” she said, “and accepting the things I could not control.”
Many psychiatrists are finding themselves in similar situations. Fortunately, colleagues are highlighting ways for psychiatrists to care for themselves just as they care for patients.
“One of the challenges clinicians are facing is that they are living through a shared experience in this global pandemic with their patients right now,” said psychologist Randi Pochtar, PhD, who is managing support groups for front-line workers at NYU Langone Health in New York City. “Some might find the work to be overwhelming and anxiety-inducing, and others might find their work to be helpful in managing their own anxiety and stress about the pandemic and its impact.”
Dr. Cotton said her breaking points came when she felt panic amid the pandemic. “I had watched too much news, and I’d seen protesters not taking it seriously, and I was scared for my family and myself. I just needed to feel like someone heard me feeling that way.”
The calls to the hotline were helpful, she said, and so was sharing news about her illness with friends. “So many people reached out to me and checked in on me, people I haven’t seen in years, and that was immensely helpful,” she said.
This sort of personal exposure may not come naturally to physicians and nurses, she said. “We don’t seek that kind of attention when we’re ill. Instead, we say: ‘I’m fine; how are you doing?’ That’s what we do every day of our lives at work.”
How can clinicians help themselves and one another? “Clinicians in our practice have been coping and supporting each other through peer supervision, connecting with colleagues in team meetings, and simply checking in on one another,” said Dr. Pochtar. “In addition, we can adopt many of the strategies that we are likely recommending to our patients, such as maintaining routines as much as possible, engaging in regular exercise, eating well and consistently, and connecting with friends and family.”
Managers can play important roles, said Dr. Fortuna. “I’ve been checking in with my faculty, being as supportive as I can be and highlighting the extraordinary things that people are doing, like going from zero to 100 percent in setting up telehealth.”
Dr. Konzer offered another perspective on recognizing the value of the work that psychiatrists are doing. “We’re on the front line of helping heal the front line, and in that responsibility comes an additional stress,” she said. “But there’s an additional gift of being able to contribute where we are most beneficial. We can try to be present now, versus worrying about what may happen or what lies ahead, and appreciate the beauty in the helpers and the small joys of life.”
Dr. Cotton, Dr. Berkowitz, Dr. Konzer, Dr. Welch, Dr. Fortuna, and Dr. Pochtar reported no relevant disclosures.
REACH2: Ruxolitinib outperformed control treatment for refractory acute GVHD
Ruxolitinib produced significantly better efficacy outcomes in patients with glucocorticoid-refractory acute graft-versus-host disease (GVHD), compared with investigator’s choice of control therapy, in the phase 3 REACH2 trial.
However, there was a higher incidence of thrombocytopenia with ruxolitinib than with control treatment, according to a report by Robert Zeiser, MD, of University of Freiburg (Germany) and colleagues on behalf of the REACH2 research group. The report was published in the New England Journal of Medicine.
The REACH2 trial (NCT02913261) is a randomized, open-label, phase 3 trial comparing the efficacy and safety of oral ruxolitinib (10 mg twice daily) with investigator’s choice of therapy for control treatment using a list of nine commonly used options.
Patients were 12 years of age or older with glucocorticoid-refractory acute GVHD after allogeneic stem cell transplant. A total of 154 patients were assigned to the ruxolitinib group, and 155 patients were in the control group.
Most patients – 152 in the ruxolitinib group and 150 in the control group – received at least one dose of trial treatment.
Treatment discontinuation occurred in 72% (111/154) of patients in the ruxolitinib group and in 85% (132/155) of those in the control group. The most common reason for discontinuation was lack of efficacy (in 21% and 44%, respectively).
Outcomes
The overall response at day 28 (the primary endpoint) was significantly higher in the ruxolitinib group than in the control group (62% vs. 39%; odds ratio, 2.64; P < .001). The durable overall response at day 56 was also significantly higher in the ruxolitinib group than in the control group (40% vs. 22%; OR, 2.38; P < .001).
The estimated cumulative incidence of loss of response at 6 months was 10% in the ruxolitinib group compared with 39% in the control group.
The median failure-free survival was considerably longer with ruxolitinib than with control treatment (5.0 months vs. 1.0 month; hazard ratio for relapse or progression of hematologic disease, non–relapse-related death, or the use of new systemic therapy for acute GVHD, 0.46).
The median overall survival was 11.1 months in the ruxolitinib group and 6.5 months in the control group (HR, 0.83).
Overall, 72 patients (47%) in the ruxolitinib group and 77 (51%) in the control group died by the data cutoff date. Most deaths were attributed to acute GVHD (22% in the ruxolitinib group and 25% in the control group).
The most common adverse events at day 28 (in the ruxolitinib and control arms, respectively) were thrombocytopenia (33% and 18%), anemia (30% and 28%), and cytomegalovirus infection (26% and 21%).
Praise for ‘successful’ randomized trial in GVHD
“The authors are to be congratulated for completing this successful randomized trial, which showed convincingly that ruxolitinib was more effective than the investigator’s choice of therapy ... in patients in whom glucocorticoid therapy had failed,” wrote Nelson Chao, MD, of Duke University in Durham, N.C., in his invited editorial.
He went on to speculate on the possible mechanism for ruxolitinib in these patients, discussing the possible role of the STAT3 and STAT1 signaling pathways.
Dr. Chao also found it “interesting that the incidence of infectious complications or relapse was apparently not greater with ruxolitinib than with control therapy,” but he noted that the total follow-up time was short.
“As with all good research, these observations raise important questions and set the stage for further work in this area,” he concluded.
The REACH2 trial was funded by Novartis. The study authors disclosed relationships with a variety of pharmaceutical companies, including Novartis. Dr. Chao reported having no relevant disclosures.
SOURCE: Zeiser R et al. N Engl J Med. 2020. doi: 10.1056/NEJMoa1917635.
Ruxolitinib produced significantly better efficacy outcomes in patients with glucocorticoid-refractory acute graft-versus-host disease (GVHD), compared with investigator’s choice of control therapy, in the phase 3 REACH2 trial.
However, there was a higher incidence of thrombocytopenia with ruxolitinib than with control treatment, according to a report by Robert Zeiser, MD, of University of Freiburg (Germany) and colleagues on behalf of the REACH2 research group. The report was published in the New England Journal of Medicine.
The REACH2 trial (NCT02913261) is a randomized, open-label, phase 3 trial comparing the efficacy and safety of oral ruxolitinib (10 mg twice daily) with investigator’s choice of therapy for control treatment using a list of nine commonly used options.
Patients were 12 years of age or older with glucocorticoid-refractory acute GVHD after allogeneic stem cell transplant. A total of 154 patients were assigned to the ruxolitinib group, and 155 patients were in the control group.
Most patients – 152 in the ruxolitinib group and 150 in the control group – received at least one dose of trial treatment.
Treatment discontinuation occurred in 72% (111/154) of patients in the ruxolitinib group and in 85% (132/155) of those in the control group. The most common reason for discontinuation was lack of efficacy (in 21% and 44%, respectively).
Outcomes
The overall response at day 28 (the primary endpoint) was significantly higher in the ruxolitinib group than in the control group (62% vs. 39%; odds ratio, 2.64; P < .001). The durable overall response at day 56 was also significantly higher in the ruxolitinib group than in the control group (40% vs. 22%; OR, 2.38; P < .001).
The estimated cumulative incidence of loss of response at 6 months was 10% in the ruxolitinib group compared with 39% in the control group.
The median failure-free survival was considerably longer with ruxolitinib than with control treatment (5.0 months vs. 1.0 month; hazard ratio for relapse or progression of hematologic disease, non–relapse-related death, or the use of new systemic therapy for acute GVHD, 0.46).
The median overall survival was 11.1 months in the ruxolitinib group and 6.5 months in the control group (HR, 0.83).
Overall, 72 patients (47%) in the ruxolitinib group and 77 (51%) in the control group died by the data cutoff date. Most deaths were attributed to acute GVHD (22% in the ruxolitinib group and 25% in the control group).
The most common adverse events at day 28 (in the ruxolitinib and control arms, respectively) were thrombocytopenia (33% and 18%), anemia (30% and 28%), and cytomegalovirus infection (26% and 21%).
Praise for ‘successful’ randomized trial in GVHD
“The authors are to be congratulated for completing this successful randomized trial, which showed convincingly that ruxolitinib was more effective than the investigator’s choice of therapy ... in patients in whom glucocorticoid therapy had failed,” wrote Nelson Chao, MD, of Duke University in Durham, N.C., in his invited editorial.
He went on to speculate on the possible mechanism for ruxolitinib in these patients, discussing the possible role of the STAT3 and STAT1 signaling pathways.
Dr. Chao also found it “interesting that the incidence of infectious complications or relapse was apparently not greater with ruxolitinib than with control therapy,” but he noted that the total follow-up time was short.
“As with all good research, these observations raise important questions and set the stage for further work in this area,” he concluded.
The REACH2 trial was funded by Novartis. The study authors disclosed relationships with a variety of pharmaceutical companies, including Novartis. Dr. Chao reported having no relevant disclosures.
SOURCE: Zeiser R et al. N Engl J Med. 2020. doi: 10.1056/NEJMoa1917635.
Ruxolitinib produced significantly better efficacy outcomes in patients with glucocorticoid-refractory acute graft-versus-host disease (GVHD), compared with investigator’s choice of control therapy, in the phase 3 REACH2 trial.
However, there was a higher incidence of thrombocytopenia with ruxolitinib than with control treatment, according to a report by Robert Zeiser, MD, of University of Freiburg (Germany) and colleagues on behalf of the REACH2 research group. The report was published in the New England Journal of Medicine.
The REACH2 trial (NCT02913261) is a randomized, open-label, phase 3 trial comparing the efficacy and safety of oral ruxolitinib (10 mg twice daily) with investigator’s choice of therapy for control treatment using a list of nine commonly used options.
Patients were 12 years of age or older with glucocorticoid-refractory acute GVHD after allogeneic stem cell transplant. A total of 154 patients were assigned to the ruxolitinib group, and 155 patients were in the control group.
Most patients – 152 in the ruxolitinib group and 150 in the control group – received at least one dose of trial treatment.
Treatment discontinuation occurred in 72% (111/154) of patients in the ruxolitinib group and in 85% (132/155) of those in the control group. The most common reason for discontinuation was lack of efficacy (in 21% and 44%, respectively).
Outcomes
The overall response at day 28 (the primary endpoint) was significantly higher in the ruxolitinib group than in the control group (62% vs. 39%; odds ratio, 2.64; P < .001). The durable overall response at day 56 was also significantly higher in the ruxolitinib group than in the control group (40% vs. 22%; OR, 2.38; P < .001).
The estimated cumulative incidence of loss of response at 6 months was 10% in the ruxolitinib group compared with 39% in the control group.
The median failure-free survival was considerably longer with ruxolitinib than with control treatment (5.0 months vs. 1.0 month; hazard ratio for relapse or progression of hematologic disease, non–relapse-related death, or the use of new systemic therapy for acute GVHD, 0.46).
The median overall survival was 11.1 months in the ruxolitinib group and 6.5 months in the control group (HR, 0.83).
Overall, 72 patients (47%) in the ruxolitinib group and 77 (51%) in the control group died by the data cutoff date. Most deaths were attributed to acute GVHD (22% in the ruxolitinib group and 25% in the control group).
The most common adverse events at day 28 (in the ruxolitinib and control arms, respectively) were thrombocytopenia (33% and 18%), anemia (30% and 28%), and cytomegalovirus infection (26% and 21%).
Praise for ‘successful’ randomized trial in GVHD
“The authors are to be congratulated for completing this successful randomized trial, which showed convincingly that ruxolitinib was more effective than the investigator’s choice of therapy ... in patients in whom glucocorticoid therapy had failed,” wrote Nelson Chao, MD, of Duke University in Durham, N.C., in his invited editorial.
He went on to speculate on the possible mechanism for ruxolitinib in these patients, discussing the possible role of the STAT3 and STAT1 signaling pathways.
Dr. Chao also found it “interesting that the incidence of infectious complications or relapse was apparently not greater with ruxolitinib than with control therapy,” but he noted that the total follow-up time was short.
“As with all good research, these observations raise important questions and set the stage for further work in this area,” he concluded.
The REACH2 trial was funded by Novartis. The study authors disclosed relationships with a variety of pharmaceutical companies, including Novartis. Dr. Chao reported having no relevant disclosures.
SOURCE: Zeiser R et al. N Engl J Med. 2020. doi: 10.1056/NEJMoa1917635.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Ruxolitinib was significantly more effective against acute graft-versus-host disease than was control treatment.
Major finding: The overall response at day 28 was significantly higher in the ruxolitinib group than in the control group (62% vs. 39%; P < .001).
Study details: Phase 3 trial of 154 patients randomized to ruxolitinib and 155 patients randomized to investigator’s choice of control therapy.
Disclosures: The trial was funded by Novartis. Authors disclosed relationships with a variety of pharmaceutical companies, including Novartis.
Source: Zeiser R et al. N Engl J Med. 2020. doi: 10.1056/NEJMoa1917635.
Signature STEMI sign may be less diagnostic in the COVID-19 age
The signature electrocardiographic sign indicating ST-segment-elevation MI may be a less-consistent indicator of actual STEMI at a time when patients with COVID-19 have come to overwhelm many hospital ICUs.
Many of the 18 such patients identified at six New York City hospitals who showed ST-segment elevation on their 12-lead ECG in the city’s first month of fighting the pandemic turned out to be free of either obstructive coronary artery disease by angiography or of regional wall-motion abnormalities (RWMA) by ECG, according to a letter published in the New England Journal of Medicine.
Those 10 patients in the 18-case series were said to have noncoronary myocardial injury, perhaps from myocarditis – a prevalent feature of severe COVID-19 – and the remaining 8 patients with obstructive coronary artery disease, RWMA, or both were diagnosed with STEMI. Of the latter patients, six went to the cath lab and five of those underwent percutaneous coronary intervention, Sripal Bangalore, MD, MHA, of New York University, and colleagues reported.
In an interview, Dr. Bangalore framed the case-series report as a caution against substituting fibrinolytic therapy for primary percutaneous coronary intervention in patients with STE while hospitals are unusually burdened by the COVID-19 pandemic and invasive procedures intensify the threat of SARS-CoV-2 exposure to clinicians.
The strategy was recently advanced as an option for highly selected patients in a statement from the American College of Cardiology and Society for Cardiovascular Angiography and Interventions (SCAI).
“During the COVID-19 pandemic, one of the main reasons fibrinolytic therapy has been pushed is to reduce the exposure to the cath-lab staff,” Dr. Bangalore observed. “But if you pursue that route, it’s problematic because more than half may not have obstructive disease and fibrinolytic therapy may not help. And if you give them fibrinolytics, you’re potentially increasing their risk of bleeding complications.
“The take-home from these 18 patients is that it’s very difficult to guess who is going to have obstructive disease and who is going to have nonobstructive disease,” Dr. Bangalore said. “Maybe we should assess these patients with not just an ECG but with a quick echo, then make a decision. Our practice so far has been to take these patients to the cath lab.”
The ACC/SCAI statement proposed that “fibrinolysis can be considered an option for the relatively stable STEMI patient with active COVID-19” after careful consideration of possible patient benefit versus the risks of cath-lab personnel exposure to the virus.
Only six patients in the current series, including five in the STEMI group, are reported to have had chest pain at about the time of STE, observed Michael J. Blaha, MD, MPH, of Johns Hopkins Hospital, Baltimore.
So, he said in an interview, “one of their points is that you have to take ST elevations with a grain of salt in this [COVID-19] era, because there are a lot of people presenting with ST elevations in the absence of chest pain.”
That, and the high prevalence of nonobstructive disease in the series, indeed argues against the use of fibrinolytic therapy in such patients, Dr. Blaha said.
Normally, when there is STE, “the pretest probability of STEMI is so high, and if you can’t make it to the cath lab for some reason, sure, it makes sense to give lytics.” However, he said, “COVID-19 is changing the clinical landscape. Now, with a variety of virus-mediated myocardial injury presentations, including myocarditis, the pretest probability of MI is lower.”
The current report “confirms that, in the COVID era, ST elevations are not diagnostic for MI and must be considered within the totality of clinical evidence, and a conservative approach to going to the cath lab is probably warranted,” Dr. Blaha said in an interview.
However, with the reduced pretest probability of STE for STEMI, he agreed, “I almost don’t see any scenario where I’d be comfortable, based on ECG changes alone, giving lytics at this time.”
Dr. Bangalore pointed out that all of the 18 patients in the series had elevated levels of the fibrin degradation product D-dimer, a biomarker that reflects ongoing hemostatic activation. Levels were higher in the 8 patients who ultimately received a STEMI diagnosis than in the remaining 10 patients.
But COVID-19 patients in general may have elevated D-dimer and “a lot of microthrombi,” he said. “So the question is, are those microthrombi also causal for any of the ECG changes we are also seeing?”
Aside from microthrombi, global hypoxia and myocarditis could be other potential causes of STE in COVID-19 patients in the absence of STEMI, Dr. Bangalore proposed. “At this point we just generally don’t know.”
Dr. Bangalore reported no conflicts; disclosures for the other authors are available at nejm.org. Dr. Blaha disclosed receiving grants from Amgen and serving on advisory boards for Amgen and other pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
The signature electrocardiographic sign indicating ST-segment-elevation MI may be a less-consistent indicator of actual STEMI at a time when patients with COVID-19 have come to overwhelm many hospital ICUs.
Many of the 18 such patients identified at six New York City hospitals who showed ST-segment elevation on their 12-lead ECG in the city’s first month of fighting the pandemic turned out to be free of either obstructive coronary artery disease by angiography or of regional wall-motion abnormalities (RWMA) by ECG, according to a letter published in the New England Journal of Medicine.
Those 10 patients in the 18-case series were said to have noncoronary myocardial injury, perhaps from myocarditis – a prevalent feature of severe COVID-19 – and the remaining 8 patients with obstructive coronary artery disease, RWMA, or both were diagnosed with STEMI. Of the latter patients, six went to the cath lab and five of those underwent percutaneous coronary intervention, Sripal Bangalore, MD, MHA, of New York University, and colleagues reported.
In an interview, Dr. Bangalore framed the case-series report as a caution against substituting fibrinolytic therapy for primary percutaneous coronary intervention in patients with STE while hospitals are unusually burdened by the COVID-19 pandemic and invasive procedures intensify the threat of SARS-CoV-2 exposure to clinicians.
The strategy was recently advanced as an option for highly selected patients in a statement from the American College of Cardiology and Society for Cardiovascular Angiography and Interventions (SCAI).
“During the COVID-19 pandemic, one of the main reasons fibrinolytic therapy has been pushed is to reduce the exposure to the cath-lab staff,” Dr. Bangalore observed. “But if you pursue that route, it’s problematic because more than half may not have obstructive disease and fibrinolytic therapy may not help. And if you give them fibrinolytics, you’re potentially increasing their risk of bleeding complications.
“The take-home from these 18 patients is that it’s very difficult to guess who is going to have obstructive disease and who is going to have nonobstructive disease,” Dr. Bangalore said. “Maybe we should assess these patients with not just an ECG but with a quick echo, then make a decision. Our practice so far has been to take these patients to the cath lab.”
The ACC/SCAI statement proposed that “fibrinolysis can be considered an option for the relatively stable STEMI patient with active COVID-19” after careful consideration of possible patient benefit versus the risks of cath-lab personnel exposure to the virus.
Only six patients in the current series, including five in the STEMI group, are reported to have had chest pain at about the time of STE, observed Michael J. Blaha, MD, MPH, of Johns Hopkins Hospital, Baltimore.
So, he said in an interview, “one of their points is that you have to take ST elevations with a grain of salt in this [COVID-19] era, because there are a lot of people presenting with ST elevations in the absence of chest pain.”
That, and the high prevalence of nonobstructive disease in the series, indeed argues against the use of fibrinolytic therapy in such patients, Dr. Blaha said.
Normally, when there is STE, “the pretest probability of STEMI is so high, and if you can’t make it to the cath lab for some reason, sure, it makes sense to give lytics.” However, he said, “COVID-19 is changing the clinical landscape. Now, with a variety of virus-mediated myocardial injury presentations, including myocarditis, the pretest probability of MI is lower.”
The current report “confirms that, in the COVID era, ST elevations are not diagnostic for MI and must be considered within the totality of clinical evidence, and a conservative approach to going to the cath lab is probably warranted,” Dr. Blaha said in an interview.
However, with the reduced pretest probability of STE for STEMI, he agreed, “I almost don’t see any scenario where I’d be comfortable, based on ECG changes alone, giving lytics at this time.”
Dr. Bangalore pointed out that all of the 18 patients in the series had elevated levels of the fibrin degradation product D-dimer, a biomarker that reflects ongoing hemostatic activation. Levels were higher in the 8 patients who ultimately received a STEMI diagnosis than in the remaining 10 patients.
But COVID-19 patients in general may have elevated D-dimer and “a lot of microthrombi,” he said. “So the question is, are those microthrombi also causal for any of the ECG changes we are also seeing?”
Aside from microthrombi, global hypoxia and myocarditis could be other potential causes of STE in COVID-19 patients in the absence of STEMI, Dr. Bangalore proposed. “At this point we just generally don’t know.”
Dr. Bangalore reported no conflicts; disclosures for the other authors are available at nejm.org. Dr. Blaha disclosed receiving grants from Amgen and serving on advisory boards for Amgen and other pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
The signature electrocardiographic sign indicating ST-segment-elevation MI may be a less-consistent indicator of actual STEMI at a time when patients with COVID-19 have come to overwhelm many hospital ICUs.
Many of the 18 such patients identified at six New York City hospitals who showed ST-segment elevation on their 12-lead ECG in the city’s first month of fighting the pandemic turned out to be free of either obstructive coronary artery disease by angiography or of regional wall-motion abnormalities (RWMA) by ECG, according to a letter published in the New England Journal of Medicine.
Those 10 patients in the 18-case series were said to have noncoronary myocardial injury, perhaps from myocarditis – a prevalent feature of severe COVID-19 – and the remaining 8 patients with obstructive coronary artery disease, RWMA, or both were diagnosed with STEMI. Of the latter patients, six went to the cath lab and five of those underwent percutaneous coronary intervention, Sripal Bangalore, MD, MHA, of New York University, and colleagues reported.
In an interview, Dr. Bangalore framed the case-series report as a caution against substituting fibrinolytic therapy for primary percutaneous coronary intervention in patients with STE while hospitals are unusually burdened by the COVID-19 pandemic and invasive procedures intensify the threat of SARS-CoV-2 exposure to clinicians.
The strategy was recently advanced as an option for highly selected patients in a statement from the American College of Cardiology and Society for Cardiovascular Angiography and Interventions (SCAI).
“During the COVID-19 pandemic, one of the main reasons fibrinolytic therapy has been pushed is to reduce the exposure to the cath-lab staff,” Dr. Bangalore observed. “But if you pursue that route, it’s problematic because more than half may not have obstructive disease and fibrinolytic therapy may not help. And if you give them fibrinolytics, you’re potentially increasing their risk of bleeding complications.
“The take-home from these 18 patients is that it’s very difficult to guess who is going to have obstructive disease and who is going to have nonobstructive disease,” Dr. Bangalore said. “Maybe we should assess these patients with not just an ECG but with a quick echo, then make a decision. Our practice so far has been to take these patients to the cath lab.”
The ACC/SCAI statement proposed that “fibrinolysis can be considered an option for the relatively stable STEMI patient with active COVID-19” after careful consideration of possible patient benefit versus the risks of cath-lab personnel exposure to the virus.
Only six patients in the current series, including five in the STEMI group, are reported to have had chest pain at about the time of STE, observed Michael J. Blaha, MD, MPH, of Johns Hopkins Hospital, Baltimore.
So, he said in an interview, “one of their points is that you have to take ST elevations with a grain of salt in this [COVID-19] era, because there are a lot of people presenting with ST elevations in the absence of chest pain.”
That, and the high prevalence of nonobstructive disease in the series, indeed argues against the use of fibrinolytic therapy in such patients, Dr. Blaha said.
Normally, when there is STE, “the pretest probability of STEMI is so high, and if you can’t make it to the cath lab for some reason, sure, it makes sense to give lytics.” However, he said, “COVID-19 is changing the clinical landscape. Now, with a variety of virus-mediated myocardial injury presentations, including myocarditis, the pretest probability of MI is lower.”
The current report “confirms that, in the COVID era, ST elevations are not diagnostic for MI and must be considered within the totality of clinical evidence, and a conservative approach to going to the cath lab is probably warranted,” Dr. Blaha said in an interview.
However, with the reduced pretest probability of STE for STEMI, he agreed, “I almost don’t see any scenario where I’d be comfortable, based on ECG changes alone, giving lytics at this time.”
Dr. Bangalore pointed out that all of the 18 patients in the series had elevated levels of the fibrin degradation product D-dimer, a biomarker that reflects ongoing hemostatic activation. Levels were higher in the 8 patients who ultimately received a STEMI diagnosis than in the remaining 10 patients.
But COVID-19 patients in general may have elevated D-dimer and “a lot of microthrombi,” he said. “So the question is, are those microthrombi also causal for any of the ECG changes we are also seeing?”
Aside from microthrombi, global hypoxia and myocarditis could be other potential causes of STE in COVID-19 patients in the absence of STEMI, Dr. Bangalore proposed. “At this point we just generally don’t know.”
Dr. Bangalore reported no conflicts; disclosures for the other authors are available at nejm.org. Dr. Blaha disclosed receiving grants from Amgen and serving on advisory boards for Amgen and other pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
Management of infants born to mothers with COVID-19
Initial guidance for pediatric hospitalists
Clinical question: How should we care for newborns born to mothers with COVID-19?
Background: Around the United States, the SARS-CoV-2 virus is infecting pregnant mothers and causing COVID-19. Current limited data demonstrates that children under the age of 1 year are at risk for severe disease. Clinicians are caring for infants born to mothers with COVID-19 during the pandemic with minimal guidance.
Study design: Clinical practice guidelines.
Synopsis: The American Academy of Pediatrics’ Committee on Fetus and Newborn, Section on Neonatal and Perinatal Medicine and Committee of Infectious Diseases developed guidelines of care for infants born to COVID-19 mothers to help clinicians care for newborns using limited data published before March 30, 2020.
- Neonates should be considered persons under investigation (PUIs) if they are born to mothers with diagnosed COVID-19 or with COVID-19 tests pending at the time of delivery.
- Neonatal clinicians should attend deliveries based on their center’s policies. If clinicians are required to perform stabilization they should use airborne, droplet, and contact personal protective equipment (PPE). This includes, gown, gloves, eye protection (goggles or face shield), and N95 respirator mask or an air-purifying respirator.
- Mother and newborn should be separated to minimize the infant’s risk of postnatal infection.
- Well newborns born at or near term may be admitted to areas physically separated from newborns unaffected by maternal COVID-19. Alternatively, a mother may room-in with her infant with 6 feet of separation between mother and infant. Newborn PUIs should be bathed as soon as possible.
- Newborns requiring intensive care should be admitted to a single negative-pressure room. Alternatively, COVID-19–exposed infants should be grouped with a minimum of 6 feet of separation, or placed in air temperature-controlled isolettes.
- Until the newborn PUI’s virologic status is known, clinical staff caring for the infant should use droplet and contact PPE. This includes gown, gloves, eye protection (goggles or face shield), and a standard surgical mask. Airborne, droplet, and contact precautions should be used for infants requiring CPAP or any form of mechanical ventilation.
- COVID-19–positive mothers who want to breastfeed may feed expressed breast milk using proper breast and hand hygiene or directly breastfeed their infants wearing a mask while practicing proper breast and hand hygiene.
- If testing is available, newborns should be tested for SARS-CoV-2 using molecular arrays. If testing is unavailable, clinicians may monitor newborns clinically. Infants should be tested if they require prolonged intensive care.
- Optimal timing and extent of testing is unknown. Tests should be performed around 24 hours of life and 48 hours of life. If discharge is planned for a well appearing infant before 48 hours of life, the clinician may choose not to do the 48-hour test. A single swab should be taken from the throat followed by the nasopharynx to perform the test.
- Newborns should receive all newborn care, including circumcision if requested.
- Infants who are asymptomatic with positive or pending SARS-CoV-2 tests may be discharged home with plans for frequent outpatient follow-up through 14 days after birth. Infants with negative SARS-CoV-2 testing should be discharged to the care of a noninfected caregiver. If the mother lives in the same household, she must keep a distance of 6 feet as often as possible. When not possible, the mother should wear a mask and practice hand hygiene. The mother may resume caring for her infant normally when she has been afebrile for more than 72 hours (without antipyretics) and has been asymptomatic for 7 days. Alternatively, the mother may resume care if she has two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
- Visitation to infants requiring intensive care should be limited for mothers with COVID-19 until her fever has resolved for more than 72 hours and has improvement of respiratory symptoms and has had two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
Bottom line: Clinicians should protect themselves with contact and droplet PPE at all times until the infant’s viral status is known. Clinicians should use airborne, contact, and droplet PPE when resuscitating the infant and/or when using CPAP/mechanical ventilation. Mothers should be encouraged to feed their infants expressed breast milk while practicing proper hygiene or directly breastfeed while wearing a mask and practicing proper hygiene. Viral testing of every infant born to a mother with COVID-19 should be performed after the infant is 24 hours old. Mothers should resume caring for their infants normally after they have met criteria suggesting they are no longer actively infected.
Article citation: Puopolo KM, Hudak ML, Kimberlin DW, Cummings J. Initial Guidance: Management of Infants born to Mothers with COVID-19. 2020 Apr 2. https://downloads.aap.org/AAP/PDF/COVID%2019%20Initial%20Newborn%20Guidance.pdf. Accessed Apr 2, 2020.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the Pediatrics Editor for The Hospitalist.
Initial guidance for pediatric hospitalists
Initial guidance for pediatric hospitalists
Clinical question: How should we care for newborns born to mothers with COVID-19?
Background: Around the United States, the SARS-CoV-2 virus is infecting pregnant mothers and causing COVID-19. Current limited data demonstrates that children under the age of 1 year are at risk for severe disease. Clinicians are caring for infants born to mothers with COVID-19 during the pandemic with minimal guidance.
Study design: Clinical practice guidelines.
Synopsis: The American Academy of Pediatrics’ Committee on Fetus and Newborn, Section on Neonatal and Perinatal Medicine and Committee of Infectious Diseases developed guidelines of care for infants born to COVID-19 mothers to help clinicians care for newborns using limited data published before March 30, 2020.
- Neonates should be considered persons under investigation (PUIs) if they are born to mothers with diagnosed COVID-19 or with COVID-19 tests pending at the time of delivery.
- Neonatal clinicians should attend deliveries based on their center’s policies. If clinicians are required to perform stabilization they should use airborne, droplet, and contact personal protective equipment (PPE). This includes, gown, gloves, eye protection (goggles or face shield), and N95 respirator mask or an air-purifying respirator.
- Mother and newborn should be separated to minimize the infant’s risk of postnatal infection.
- Well newborns born at or near term may be admitted to areas physically separated from newborns unaffected by maternal COVID-19. Alternatively, a mother may room-in with her infant with 6 feet of separation between mother and infant. Newborn PUIs should be bathed as soon as possible.
- Newborns requiring intensive care should be admitted to a single negative-pressure room. Alternatively, COVID-19–exposed infants should be grouped with a minimum of 6 feet of separation, or placed in air temperature-controlled isolettes.
- Until the newborn PUI’s virologic status is known, clinical staff caring for the infant should use droplet and contact PPE. This includes gown, gloves, eye protection (goggles or face shield), and a standard surgical mask. Airborne, droplet, and contact precautions should be used for infants requiring CPAP or any form of mechanical ventilation.
- COVID-19–positive mothers who want to breastfeed may feed expressed breast milk using proper breast and hand hygiene or directly breastfeed their infants wearing a mask while practicing proper breast and hand hygiene.
- If testing is available, newborns should be tested for SARS-CoV-2 using molecular arrays. If testing is unavailable, clinicians may monitor newborns clinically. Infants should be tested if they require prolonged intensive care.
- Optimal timing and extent of testing is unknown. Tests should be performed around 24 hours of life and 48 hours of life. If discharge is planned for a well appearing infant before 48 hours of life, the clinician may choose not to do the 48-hour test. A single swab should be taken from the throat followed by the nasopharynx to perform the test.
- Newborns should receive all newborn care, including circumcision if requested.
- Infants who are asymptomatic with positive or pending SARS-CoV-2 tests may be discharged home with plans for frequent outpatient follow-up through 14 days after birth. Infants with negative SARS-CoV-2 testing should be discharged to the care of a noninfected caregiver. If the mother lives in the same household, she must keep a distance of 6 feet as often as possible. When not possible, the mother should wear a mask and practice hand hygiene. The mother may resume caring for her infant normally when she has been afebrile for more than 72 hours (without antipyretics) and has been asymptomatic for 7 days. Alternatively, the mother may resume care if she has two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
- Visitation to infants requiring intensive care should be limited for mothers with COVID-19 until her fever has resolved for more than 72 hours and has improvement of respiratory symptoms and has had two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
Bottom line: Clinicians should protect themselves with contact and droplet PPE at all times until the infant’s viral status is known. Clinicians should use airborne, contact, and droplet PPE when resuscitating the infant and/or when using CPAP/mechanical ventilation. Mothers should be encouraged to feed their infants expressed breast milk while practicing proper hygiene or directly breastfeed while wearing a mask and practicing proper hygiene. Viral testing of every infant born to a mother with COVID-19 should be performed after the infant is 24 hours old. Mothers should resume caring for their infants normally after they have met criteria suggesting they are no longer actively infected.
Article citation: Puopolo KM, Hudak ML, Kimberlin DW, Cummings J. Initial Guidance: Management of Infants born to Mothers with COVID-19. 2020 Apr 2. https://downloads.aap.org/AAP/PDF/COVID%2019%20Initial%20Newborn%20Guidance.pdf. Accessed Apr 2, 2020.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the Pediatrics Editor for The Hospitalist.
Clinical question: How should we care for newborns born to mothers with COVID-19?
Background: Around the United States, the SARS-CoV-2 virus is infecting pregnant mothers and causing COVID-19. Current limited data demonstrates that children under the age of 1 year are at risk for severe disease. Clinicians are caring for infants born to mothers with COVID-19 during the pandemic with minimal guidance.
Study design: Clinical practice guidelines.
Synopsis: The American Academy of Pediatrics’ Committee on Fetus and Newborn, Section on Neonatal and Perinatal Medicine and Committee of Infectious Diseases developed guidelines of care for infants born to COVID-19 mothers to help clinicians care for newborns using limited data published before March 30, 2020.
- Neonates should be considered persons under investigation (PUIs) if they are born to mothers with diagnosed COVID-19 or with COVID-19 tests pending at the time of delivery.
- Neonatal clinicians should attend deliveries based on their center’s policies. If clinicians are required to perform stabilization they should use airborne, droplet, and contact personal protective equipment (PPE). This includes, gown, gloves, eye protection (goggles or face shield), and N95 respirator mask or an air-purifying respirator.
- Mother and newborn should be separated to minimize the infant’s risk of postnatal infection.
- Well newborns born at or near term may be admitted to areas physically separated from newborns unaffected by maternal COVID-19. Alternatively, a mother may room-in with her infant with 6 feet of separation between mother and infant. Newborn PUIs should be bathed as soon as possible.
- Newborns requiring intensive care should be admitted to a single negative-pressure room. Alternatively, COVID-19–exposed infants should be grouped with a minimum of 6 feet of separation, or placed in air temperature-controlled isolettes.
- Until the newborn PUI’s virologic status is known, clinical staff caring for the infant should use droplet and contact PPE. This includes gown, gloves, eye protection (goggles or face shield), and a standard surgical mask. Airborne, droplet, and contact precautions should be used for infants requiring CPAP or any form of mechanical ventilation.
- COVID-19–positive mothers who want to breastfeed may feed expressed breast milk using proper breast and hand hygiene or directly breastfeed their infants wearing a mask while practicing proper breast and hand hygiene.
- If testing is available, newborns should be tested for SARS-CoV-2 using molecular arrays. If testing is unavailable, clinicians may monitor newborns clinically. Infants should be tested if they require prolonged intensive care.
- Optimal timing and extent of testing is unknown. Tests should be performed around 24 hours of life and 48 hours of life. If discharge is planned for a well appearing infant before 48 hours of life, the clinician may choose not to do the 48-hour test. A single swab should be taken from the throat followed by the nasopharynx to perform the test.
- Newborns should receive all newborn care, including circumcision if requested.
- Infants who are asymptomatic with positive or pending SARS-CoV-2 tests may be discharged home with plans for frequent outpatient follow-up through 14 days after birth. Infants with negative SARS-CoV-2 testing should be discharged to the care of a noninfected caregiver. If the mother lives in the same household, she must keep a distance of 6 feet as often as possible. When not possible, the mother should wear a mask and practice hand hygiene. The mother may resume caring for her infant normally when she has been afebrile for more than 72 hours (without antipyretics) and has been asymptomatic for 7 days. Alternatively, the mother may resume care if she has two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
- Visitation to infants requiring intensive care should be limited for mothers with COVID-19 until her fever has resolved for more than 72 hours and has improvement of respiratory symptoms and has had two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
Bottom line: Clinicians should protect themselves with contact and droplet PPE at all times until the infant’s viral status is known. Clinicians should use airborne, contact, and droplet PPE when resuscitating the infant and/or when using CPAP/mechanical ventilation. Mothers should be encouraged to feed their infants expressed breast milk while practicing proper hygiene or directly breastfeed while wearing a mask and practicing proper hygiene. Viral testing of every infant born to a mother with COVID-19 should be performed after the infant is 24 hours old. Mothers should resume caring for their infants normally after they have met criteria suggesting they are no longer actively infected.
Article citation: Puopolo KM, Hudak ML, Kimberlin DW, Cummings J. Initial Guidance: Management of Infants born to Mothers with COVID-19. 2020 Apr 2. https://downloads.aap.org/AAP/PDF/COVID%2019%20Initial%20Newborn%20Guidance.pdf. Accessed Apr 2, 2020.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the Pediatrics Editor for The Hospitalist.
Get triage plans in place before COVID-19 surge hits, critical care experts say
, according to authors of recent reports that offer advice on how to prepare for surges in demand.
Even modest numbers of critically ill COVID-19 patients have already rapidly overwhelmed existing hospital capacity in hard-hit areas including Italy, Spain, and New York City, said authors of an expert panel report released in CHEST.
“The ethical burden this places on hospitals, health systems, and society is enormous,” said Ryan C. Maves, MD, FCCP, of the Naval Medical Center in San Diego, lead author of the expert panel report from the Task Force for Mass Critical Care and the American College of Chest Physicians (CHEST).
Triage decisions could be especially daunting for resource-intensive therapies such as extracorporeal membrane oxygenation (ECMO), as physicians may be forced to decide when and if to offer such support after demand outstrips a hospital’s ability to provide it.
“ECMO requires a lot of specialized capability to initiate on a patient, and then, it requires a lot of specialized capability to maintain and do safely,” said Steven P. Keller, MD, of the division of emergency critical care medicine in the department of emergency medicine at Brigham and Women’s Hospital and Harvard Medical School in Boston.
Those resource requirements can present a challenge to health care systems already overtaxed by COVID-19, according to Dr. Keller, coauthor of a guidance document in Annals of the American Thoracic Society. The guidance suggests a pandemic approach to ECMO response that’s tiered depending on the intensity of the surge over usual hospital volumes.
Mild surges call for a focus on increasing ECMO capacity, while a moderate surge may indicate a need to focus on allocating scarce resources, and a major surge may signal the need to limit or defer use of scarce resources, according to the guidance.
“If your health care system is stretched from a resource standpoint, at what point do you say, ‘we don’t even have the capability to even safely do ECMO, and so, perhaps we should not even be offering the support’?” Dr. Keller said in an interview. “That’s what we tried to get at in the paper – helping institutions think about how to prepare for that pandemic, and then when to make decisions on when it should and should not be offered.”
Critical care guidance for COVID-19
The guidance from the Task Force for Mass Critical Care and CHEST offers nine specific actions that authors suggest as part of a framework for communities to establish the infrastructure needed to triage critical care resources and “equitably” meet the needs of the largest number of COVID-19 patients.
“It is the goal of the task force to minimize the need for allocation of scarce resources as much as possible,” the authors stated.
The framework starts with surge planning that includes an inventory of intensive care unit resources such as ventilators, beds, supplies, and staff that could be marshaled to meet a surge in demand, followed by establishing “identification triggers” for triage initiation by a regional authority, should clinical demand reach a crisis stage.
The next step is preparing the triage system, which includes creating a committee at the regional level, identifying members of tertiary triage teams and the support structures they will need, and preparing and distributing training materials.
Agreeing on a triage protocol is important to ensure equitable targeting of resources, and how to allocate limited life-sustaining measures needs to be considered, according to the panel of experts. They also recommend adaptations to the standards of care such as modification of end-of-life care policies, support for health care workers, family, and the public, and consideration of pediatric issues including transport, concentration of care at specific centers, and potential increases in age thresholds to accommodate surges.
Barriers to triage?
When asked about potential barriers to rolling out a triage plan, Dr. Maves said the first is acknowledging the possible need for such a plan: “It is a difficult concept for most in critical care to accept – the idea that we may not be able to provide an individual patient with interventions that we consider routine,” he said.
Beyond acknowledging need, other potential barriers to successful implementation include the limited evidence base to support development of these protocols, as well as the need to address public trust.
“If a triage system is perceived as unjust or biased, or if people think that triage favors or excludes certain groups unfairly, it will undermine any system,” Dr. Maves said. “Making sure the public both understands and has input into system development is critical if we are going to be able to make this work.”
Dr. Maves and coauthors reported that some of the authors of their guidance are United States government employees or military service members, and that their opinions and assertions do not reflect the official views or position of those institutions. Dr. Keller reported no disclosures related to the ECMO guidance.
SOURCES: Maves RC et al. Chest. 2020 Apr 11. pii: S0012-3692(20)30691-7. doi: 10.1016/j.chest.2020.03.063; Seethara R and Keller SP. Ann Am Thorac Soc. 2020 Apr 15. doi: 10.1513/AnnalsATS.202003-233PS.
, according to authors of recent reports that offer advice on how to prepare for surges in demand.
Even modest numbers of critically ill COVID-19 patients have already rapidly overwhelmed existing hospital capacity in hard-hit areas including Italy, Spain, and New York City, said authors of an expert panel report released in CHEST.
“The ethical burden this places on hospitals, health systems, and society is enormous,” said Ryan C. Maves, MD, FCCP, of the Naval Medical Center in San Diego, lead author of the expert panel report from the Task Force for Mass Critical Care and the American College of Chest Physicians (CHEST).
Triage decisions could be especially daunting for resource-intensive therapies such as extracorporeal membrane oxygenation (ECMO), as physicians may be forced to decide when and if to offer such support after demand outstrips a hospital’s ability to provide it.
“ECMO requires a lot of specialized capability to initiate on a patient, and then, it requires a lot of specialized capability to maintain and do safely,” said Steven P. Keller, MD, of the division of emergency critical care medicine in the department of emergency medicine at Brigham and Women’s Hospital and Harvard Medical School in Boston.
Those resource requirements can present a challenge to health care systems already overtaxed by COVID-19, according to Dr. Keller, coauthor of a guidance document in Annals of the American Thoracic Society. The guidance suggests a pandemic approach to ECMO response that’s tiered depending on the intensity of the surge over usual hospital volumes.
Mild surges call for a focus on increasing ECMO capacity, while a moderate surge may indicate a need to focus on allocating scarce resources, and a major surge may signal the need to limit or defer use of scarce resources, according to the guidance.
“If your health care system is stretched from a resource standpoint, at what point do you say, ‘we don’t even have the capability to even safely do ECMO, and so, perhaps we should not even be offering the support’?” Dr. Keller said in an interview. “That’s what we tried to get at in the paper – helping institutions think about how to prepare for that pandemic, and then when to make decisions on when it should and should not be offered.”
Critical care guidance for COVID-19
The guidance from the Task Force for Mass Critical Care and CHEST offers nine specific actions that authors suggest as part of a framework for communities to establish the infrastructure needed to triage critical care resources and “equitably” meet the needs of the largest number of COVID-19 patients.
“It is the goal of the task force to minimize the need for allocation of scarce resources as much as possible,” the authors stated.
The framework starts with surge planning that includes an inventory of intensive care unit resources such as ventilators, beds, supplies, and staff that could be marshaled to meet a surge in demand, followed by establishing “identification triggers” for triage initiation by a regional authority, should clinical demand reach a crisis stage.
The next step is preparing the triage system, which includes creating a committee at the regional level, identifying members of tertiary triage teams and the support structures they will need, and preparing and distributing training materials.
Agreeing on a triage protocol is important to ensure equitable targeting of resources, and how to allocate limited life-sustaining measures needs to be considered, according to the panel of experts. They also recommend adaptations to the standards of care such as modification of end-of-life care policies, support for health care workers, family, and the public, and consideration of pediatric issues including transport, concentration of care at specific centers, and potential increases in age thresholds to accommodate surges.
Barriers to triage?
When asked about potential barriers to rolling out a triage plan, Dr. Maves said the first is acknowledging the possible need for such a plan: “It is a difficult concept for most in critical care to accept – the idea that we may not be able to provide an individual patient with interventions that we consider routine,” he said.
Beyond acknowledging need, other potential barriers to successful implementation include the limited evidence base to support development of these protocols, as well as the need to address public trust.
“If a triage system is perceived as unjust or biased, or if people think that triage favors or excludes certain groups unfairly, it will undermine any system,” Dr. Maves said. “Making sure the public both understands and has input into system development is critical if we are going to be able to make this work.”
Dr. Maves and coauthors reported that some of the authors of their guidance are United States government employees or military service members, and that their opinions and assertions do not reflect the official views or position of those institutions. Dr. Keller reported no disclosures related to the ECMO guidance.
SOURCES: Maves RC et al. Chest. 2020 Apr 11. pii: S0012-3692(20)30691-7. doi: 10.1016/j.chest.2020.03.063; Seethara R and Keller SP. Ann Am Thorac Soc. 2020 Apr 15. doi: 10.1513/AnnalsATS.202003-233PS.
, according to authors of recent reports that offer advice on how to prepare for surges in demand.
Even modest numbers of critically ill COVID-19 patients have already rapidly overwhelmed existing hospital capacity in hard-hit areas including Italy, Spain, and New York City, said authors of an expert panel report released in CHEST.
“The ethical burden this places on hospitals, health systems, and society is enormous,” said Ryan C. Maves, MD, FCCP, of the Naval Medical Center in San Diego, lead author of the expert panel report from the Task Force for Mass Critical Care and the American College of Chest Physicians (CHEST).
Triage decisions could be especially daunting for resource-intensive therapies such as extracorporeal membrane oxygenation (ECMO), as physicians may be forced to decide when and if to offer such support after demand outstrips a hospital’s ability to provide it.
“ECMO requires a lot of specialized capability to initiate on a patient, and then, it requires a lot of specialized capability to maintain and do safely,” said Steven P. Keller, MD, of the division of emergency critical care medicine in the department of emergency medicine at Brigham and Women’s Hospital and Harvard Medical School in Boston.
Those resource requirements can present a challenge to health care systems already overtaxed by COVID-19, according to Dr. Keller, coauthor of a guidance document in Annals of the American Thoracic Society. The guidance suggests a pandemic approach to ECMO response that’s tiered depending on the intensity of the surge over usual hospital volumes.
Mild surges call for a focus on increasing ECMO capacity, while a moderate surge may indicate a need to focus on allocating scarce resources, and a major surge may signal the need to limit or defer use of scarce resources, according to the guidance.
“If your health care system is stretched from a resource standpoint, at what point do you say, ‘we don’t even have the capability to even safely do ECMO, and so, perhaps we should not even be offering the support’?” Dr. Keller said in an interview. “That’s what we tried to get at in the paper – helping institutions think about how to prepare for that pandemic, and then when to make decisions on when it should and should not be offered.”
Critical care guidance for COVID-19
The guidance from the Task Force for Mass Critical Care and CHEST offers nine specific actions that authors suggest as part of a framework for communities to establish the infrastructure needed to triage critical care resources and “equitably” meet the needs of the largest number of COVID-19 patients.
“It is the goal of the task force to minimize the need for allocation of scarce resources as much as possible,” the authors stated.
The framework starts with surge planning that includes an inventory of intensive care unit resources such as ventilators, beds, supplies, and staff that could be marshaled to meet a surge in demand, followed by establishing “identification triggers” for triage initiation by a regional authority, should clinical demand reach a crisis stage.
The next step is preparing the triage system, which includes creating a committee at the regional level, identifying members of tertiary triage teams and the support structures they will need, and preparing and distributing training materials.
Agreeing on a triage protocol is important to ensure equitable targeting of resources, and how to allocate limited life-sustaining measures needs to be considered, according to the panel of experts. They also recommend adaptations to the standards of care such as modification of end-of-life care policies, support for health care workers, family, and the public, and consideration of pediatric issues including transport, concentration of care at specific centers, and potential increases in age thresholds to accommodate surges.
Barriers to triage?
When asked about potential barriers to rolling out a triage plan, Dr. Maves said the first is acknowledging the possible need for such a plan: “It is a difficult concept for most in critical care to accept – the idea that we may not be able to provide an individual patient with interventions that we consider routine,” he said.
Beyond acknowledging need, other potential barriers to successful implementation include the limited evidence base to support development of these protocols, as well as the need to address public trust.
“If a triage system is perceived as unjust or biased, or if people think that triage favors or excludes certain groups unfairly, it will undermine any system,” Dr. Maves said. “Making sure the public both understands and has input into system development is critical if we are going to be able to make this work.”
Dr. Maves and coauthors reported that some of the authors of their guidance are United States government employees or military service members, and that their opinions and assertions do not reflect the official views or position of those institutions. Dr. Keller reported no disclosures related to the ECMO guidance.
SOURCES: Maves RC et al. Chest. 2020 Apr 11. pii: S0012-3692(20)30691-7. doi: 10.1016/j.chest.2020.03.063; Seethara R and Keller SP. Ann Am Thorac Soc. 2020 Apr 15. doi: 10.1513/AnnalsATS.202003-233PS.
FROM CHEST AND ANNALS OF THE AMERICAN THORACIC SOCIETY
Five prognostic indexes come up short for planning early CLL treatment
Prognostic indexes have been developed recently to assess time to first treatment in early-stage chronic lymphocytic leukemia (CLL) patients. However, none of five indexes evaluated in a study showed more than a moderate prognostic value or were precise enough to permit clinical decisions to be made, according to a report by Spanish researchers.
Their study, published in Clinical Lymphoma, Myeloma and Leukemia, examined the comparative prognostic value of five prognostic indexes – the CLL-IPI, the Barcelona-Brno, the IPS-A, the CLL-01, and the Tailored approach – on evaluating 428 Binet A CLL patients from a multicenter Spanish database which contained the relevant necessary clinical and biological information. The predictive value of the scores was assessed with Harrell´s C index and receiver operating characteristic curve (area under the curve, AUC).
The researchers found a significant association between time to first treatment and risk subgroups for all the indexes used. The most accurate index was the IPS-A (Harrell´s C, 0.72; AUC, 0.76), followed by the CLL-01 (Harrell´s C, 0.69; AUC, 0.70), the CLL-IPI (Harrell´s C, .69; AUC, 0.69), the Barcelona-Brno (Harrell´s C: 0.67, AUC, 0.69) and the Tailored approach (Harrell´s C, 0.61 and 0.58, AUCs, 0.58 and 0.54).
However, the concordance between four of the five indexes (the Tailored approach was not included for technical reasons) compared was low (44%): 146 cases were classified as low risk with all four indexes tested, 36 as intermediate risk, and 4 as high risk. In the remaining 242 patients (56%) at least one discrepancy was detected in the allocation among prognostic subgroups between the indexes. However, only 12 patients (3%) were allocated as low and high risk at the same time with different indexes, showing the extremes of the discordance.
These data suggest that, although all of these indexes “significantly improve clinical staging and help physicians in routine clinical practice, it is necessary to harmonize larger cohorts of patients in order to define the best index for treatment decision making in the real world,” the authors stated.
“All the models had a moderate prognostic value to predict time to first therapy. ... None of them was precise enough to allow clinical decisions based exclusively on it,” the authors concluded.
The study was supported by grants from the Spanish government and a variety of nonprofit institutions. The authors reported no commercial disclosures.
SOURCE: Gascon y Marín IG et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 13. doi: 10.1016/j.clml.2020.03.003.
Prognostic indexes have been developed recently to assess time to first treatment in early-stage chronic lymphocytic leukemia (CLL) patients. However, none of five indexes evaluated in a study showed more than a moderate prognostic value or were precise enough to permit clinical decisions to be made, according to a report by Spanish researchers.
Their study, published in Clinical Lymphoma, Myeloma and Leukemia, examined the comparative prognostic value of five prognostic indexes – the CLL-IPI, the Barcelona-Brno, the IPS-A, the CLL-01, and the Tailored approach – on evaluating 428 Binet A CLL patients from a multicenter Spanish database which contained the relevant necessary clinical and biological information. The predictive value of the scores was assessed with Harrell´s C index and receiver operating characteristic curve (area under the curve, AUC).
The researchers found a significant association between time to first treatment and risk subgroups for all the indexes used. The most accurate index was the IPS-A (Harrell´s C, 0.72; AUC, 0.76), followed by the CLL-01 (Harrell´s C, 0.69; AUC, 0.70), the CLL-IPI (Harrell´s C, .69; AUC, 0.69), the Barcelona-Brno (Harrell´s C: 0.67, AUC, 0.69) and the Tailored approach (Harrell´s C, 0.61 and 0.58, AUCs, 0.58 and 0.54).
However, the concordance between four of the five indexes (the Tailored approach was not included for technical reasons) compared was low (44%): 146 cases were classified as low risk with all four indexes tested, 36 as intermediate risk, and 4 as high risk. In the remaining 242 patients (56%) at least one discrepancy was detected in the allocation among prognostic subgroups between the indexes. However, only 12 patients (3%) were allocated as low and high risk at the same time with different indexes, showing the extremes of the discordance.
These data suggest that, although all of these indexes “significantly improve clinical staging and help physicians in routine clinical practice, it is necessary to harmonize larger cohorts of patients in order to define the best index for treatment decision making in the real world,” the authors stated.
“All the models had a moderate prognostic value to predict time to first therapy. ... None of them was precise enough to allow clinical decisions based exclusively on it,” the authors concluded.
The study was supported by grants from the Spanish government and a variety of nonprofit institutions. The authors reported no commercial disclosures.
SOURCE: Gascon y Marín IG et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 13. doi: 10.1016/j.clml.2020.03.003.
Prognostic indexes have been developed recently to assess time to first treatment in early-stage chronic lymphocytic leukemia (CLL) patients. However, none of five indexes evaluated in a study showed more than a moderate prognostic value or were precise enough to permit clinical decisions to be made, according to a report by Spanish researchers.
Their study, published in Clinical Lymphoma, Myeloma and Leukemia, examined the comparative prognostic value of five prognostic indexes – the CLL-IPI, the Barcelona-Brno, the IPS-A, the CLL-01, and the Tailored approach – on evaluating 428 Binet A CLL patients from a multicenter Spanish database which contained the relevant necessary clinical and biological information. The predictive value of the scores was assessed with Harrell´s C index and receiver operating characteristic curve (area under the curve, AUC).
The researchers found a significant association between time to first treatment and risk subgroups for all the indexes used. The most accurate index was the IPS-A (Harrell´s C, 0.72; AUC, 0.76), followed by the CLL-01 (Harrell´s C, 0.69; AUC, 0.70), the CLL-IPI (Harrell´s C, .69; AUC, 0.69), the Barcelona-Brno (Harrell´s C: 0.67, AUC, 0.69) and the Tailored approach (Harrell´s C, 0.61 and 0.58, AUCs, 0.58 and 0.54).
However, the concordance between four of the five indexes (the Tailored approach was not included for technical reasons) compared was low (44%): 146 cases were classified as low risk with all four indexes tested, 36 as intermediate risk, and 4 as high risk. In the remaining 242 patients (56%) at least one discrepancy was detected in the allocation among prognostic subgroups between the indexes. However, only 12 patients (3%) were allocated as low and high risk at the same time with different indexes, showing the extremes of the discordance.
These data suggest that, although all of these indexes “significantly improve clinical staging and help physicians in routine clinical practice, it is necessary to harmonize larger cohorts of patients in order to define the best index for treatment decision making in the real world,” the authors stated.
“All the models had a moderate prognostic value to predict time to first therapy. ... None of them was precise enough to allow clinical decisions based exclusively on it,” the authors concluded.
The study was supported by grants from the Spanish government and a variety of nonprofit institutions. The authors reported no commercial disclosures.
SOURCE: Gascon y Marín IG et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 13. doi: 10.1016/j.clml.2020.03.003.
FROM CLINICAL LYMPHOMA, MYELOMA AND LEUKEMIA
ACEI/ARBs linked with survival in hypertensive, Chinese COVID-19 patients
Hospitalized COVID-19 patients with hypertension and on treatment with an renin-angiotensin system inhibiting drug had significantly better survival, compared with similar hypertensive patients not on these drugs, in observational, propensity score–matched analyses that drew from a pool of more than 3,430 patients hospitalized at any of nine Chinese hospitals during December 2019–February 2020.
“Among patients with hypertension hospitalized with COVID-19, inpatient treatment with ACEI [ACE inhibitor]/ARB [angiotensin receptor blocker] was associated with lower risk of all-cause mortality, compared with ACEI/ARB nonusers, during 28 days of follow-up. While study interpretation needs to consider the potential for residual confounders, it is unlikely that inpatient ACEI/ARB would be associated with an increased risk of mortality,” wrote Peng Zhang, MD, a cardiology researcher at Renmin Hospital of Wuhan University, China, and coauthors in Circulations Research, buttressing recent recommendations from several medical societies to maintain COVID-19 patients on these drugs.
“Our findings in this paper provide evidence supporting continuous use of ACEI/ARB for patients with hypertension infected with SARS-COV-2,” wrote the authors, backing up recent recommendations from cardiology societies that called for not stopping ACEI/ARB prescriptions in patients at risk for contracting or already have COVID-19 infection, including a statement from the American College of Cardiology, American Heart Association, and Heart Failure Society of America, and also guidance from the European Society of Cardiology.
The study included 1,128 patients with a history of hypertension, including 188 (17%) who received an ACEI/ARB drug during hospitalization. During 28-day follow-up, 99 died (9%), including 7 deaths among the 188 patients (4%) on an ACEI/ARB drug and 92 deaths among the 940 other hypertensive patients (10%).
The authors ran several analyses to try to adjust for the influence of possible confounders. A mixed-effect Cox model with four adjusted variables showed that treatment with an ACEI/ARB drug was tied to a statistically significant 58% lower death rate, compared with patients not receiving these drugs.
The researchers also ran several propensity score–adjusted analyses. One matched 174 of the patients who received an ACEI/ARB drug with 522 who did not, and comparing these two matched arms showed that ACEI/ARB use was linked with a statistically significant 63% cut in mortality, compared with patients not getting these drugs. A second propensity score–matched analysis first excluded the 383 patients who were hypertensive but received no antihypertensive medication during hospitalization. From the remaining 745 patients who received at least one antihypertensive medication, the authors identified 181 patients who received an ACEI/ARB and propensity-score matched them with 181 hypertensive patients on a different medication class, finding that ACEI/ARB use linked with a statistically significant 71% lower rate of all-cause mortality.
Additional analyses also showed that patients with hypertension had a statistically significant, 41% increased rate of all-cause death, compared with patients without hypertension, and another propensity score–matched analysis showed that among hypertensives treatment with an ACEI/ARB drug was linked with a statistically significant 68% reduced rate of septic shock.
Although this report was received with caution and some skepticism, it was also acknowledged as a step forward in the creation of an evidence base addressing ACEI/ARB treatment during COVID-19 infection.
“These drugs are lifesaving and should not be discontinued” for patients with hypertension, heart failure, and other cardiovascular disease, commented Gian Paolo Rossi, MD, professor and chair of medicine and director of the high blood pressure unit at the University of Padua (Italy). The analysis by Zhang and associates included the largest number of hospitalized COVID-19 patients with hypertension yet reported to assess the impact of treatment with ACEI/ARB drugs, and adds important evidence in favor of continuing these drugs in patients who develop COVID-19 infection, Dr. Rossi said in an interview. He recently coauthored a review that argued against ACEI/ARB discontinuation in COVID-19 patients based on previously reported evidence (Elife. 2020 Apr 6. doi: 10.7554/eLife.57278).
But other researchers take a wary view of the potential impact of ACEI/ARB agents. “If ACEI/ARB therapy increases ACE2 and the virus down-regulates it, and because ACE2 is the viral entry port into cells, why would ACE2-mediated down-regulation of the renin-angiotensin-aldosterone system lead to amelioration of [COVID-19] disease?” asked Laurence W. Busse, MD, a critical care physician at Emory University, Atlanta. “A number of issues could potentially confound the results, including the definition of COVID-19 and imbalance of antiviral therapy,” added Dr. Busse, who recently coauthored an editorial that posited using angiotensin II (Giapreza), an approved vasopressor drug, as an alternative renin-angiotensin system intervention for COVID-19 patients including both those in shock as well as potentially those not in shock (Crit Care. 2020 Apr 7. doi: 10.1186/s13054-020-02862-1). Despite these caveats, the new Chinese findings reported by Dr. Zhang and associates “are hypothesis generating and worth further exploration.”
The authors of an editorial that accompanied the Zhang study in Circulation Research made similar points. “While the investigators used standard techniques to attempt to reduce bias in this observational study via propensity matching, it is not a randomized study and the residual confounding inherent to this approach renders the conclusions hypothesis generating at best,” wrote Ravi V. Shah, MD, and two coauthors in the editorial (Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317174). They also agreed with the several society statements that have supported continued use of ACEI/ARB drugs in COVID-19 patients. “Withdrawal of these medications in the context of those conditions in which they have proven benefit (e.g., heart failure with reduced left ventricular ejection fraction) may actually inflict more harm than good,” they warned. “In the end we must rely on randomized clinical science,” and while this level of evidence is currently lacking, “the study by Zhang and colleagues is a direct step toward that goal.”
Dr. Zhang and coauthors had no commercial disclosures. Dr. Rossi and Dr. Busse had no disclosures. The authors of the Circulation Research editorial reported several disclosures.
SOURCE: Zhang P et al. Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317134.
Hospitalized COVID-19 patients with hypertension and on treatment with an renin-angiotensin system inhibiting drug had significantly better survival, compared with similar hypertensive patients not on these drugs, in observational, propensity score–matched analyses that drew from a pool of more than 3,430 patients hospitalized at any of nine Chinese hospitals during December 2019–February 2020.
“Among patients with hypertension hospitalized with COVID-19, inpatient treatment with ACEI [ACE inhibitor]/ARB [angiotensin receptor blocker] was associated with lower risk of all-cause mortality, compared with ACEI/ARB nonusers, during 28 days of follow-up. While study interpretation needs to consider the potential for residual confounders, it is unlikely that inpatient ACEI/ARB would be associated with an increased risk of mortality,” wrote Peng Zhang, MD, a cardiology researcher at Renmin Hospital of Wuhan University, China, and coauthors in Circulations Research, buttressing recent recommendations from several medical societies to maintain COVID-19 patients on these drugs.
“Our findings in this paper provide evidence supporting continuous use of ACEI/ARB for patients with hypertension infected with SARS-COV-2,” wrote the authors, backing up recent recommendations from cardiology societies that called for not stopping ACEI/ARB prescriptions in patients at risk for contracting or already have COVID-19 infection, including a statement from the American College of Cardiology, American Heart Association, and Heart Failure Society of America, and also guidance from the European Society of Cardiology.
The study included 1,128 patients with a history of hypertension, including 188 (17%) who received an ACEI/ARB drug during hospitalization. During 28-day follow-up, 99 died (9%), including 7 deaths among the 188 patients (4%) on an ACEI/ARB drug and 92 deaths among the 940 other hypertensive patients (10%).
The authors ran several analyses to try to adjust for the influence of possible confounders. A mixed-effect Cox model with four adjusted variables showed that treatment with an ACEI/ARB drug was tied to a statistically significant 58% lower death rate, compared with patients not receiving these drugs.
The researchers also ran several propensity score–adjusted analyses. One matched 174 of the patients who received an ACEI/ARB drug with 522 who did not, and comparing these two matched arms showed that ACEI/ARB use was linked with a statistically significant 63% cut in mortality, compared with patients not getting these drugs. A second propensity score–matched analysis first excluded the 383 patients who were hypertensive but received no antihypertensive medication during hospitalization. From the remaining 745 patients who received at least one antihypertensive medication, the authors identified 181 patients who received an ACEI/ARB and propensity-score matched them with 181 hypertensive patients on a different medication class, finding that ACEI/ARB use linked with a statistically significant 71% lower rate of all-cause mortality.
Additional analyses also showed that patients with hypertension had a statistically significant, 41% increased rate of all-cause death, compared with patients without hypertension, and another propensity score–matched analysis showed that among hypertensives treatment with an ACEI/ARB drug was linked with a statistically significant 68% reduced rate of septic shock.
Although this report was received with caution and some skepticism, it was also acknowledged as a step forward in the creation of an evidence base addressing ACEI/ARB treatment during COVID-19 infection.
“These drugs are lifesaving and should not be discontinued” for patients with hypertension, heart failure, and other cardiovascular disease, commented Gian Paolo Rossi, MD, professor and chair of medicine and director of the high blood pressure unit at the University of Padua (Italy). The analysis by Zhang and associates included the largest number of hospitalized COVID-19 patients with hypertension yet reported to assess the impact of treatment with ACEI/ARB drugs, and adds important evidence in favor of continuing these drugs in patients who develop COVID-19 infection, Dr. Rossi said in an interview. He recently coauthored a review that argued against ACEI/ARB discontinuation in COVID-19 patients based on previously reported evidence (Elife. 2020 Apr 6. doi: 10.7554/eLife.57278).
But other researchers take a wary view of the potential impact of ACEI/ARB agents. “If ACEI/ARB therapy increases ACE2 and the virus down-regulates it, and because ACE2 is the viral entry port into cells, why would ACE2-mediated down-regulation of the renin-angiotensin-aldosterone system lead to amelioration of [COVID-19] disease?” asked Laurence W. Busse, MD, a critical care physician at Emory University, Atlanta. “A number of issues could potentially confound the results, including the definition of COVID-19 and imbalance of antiviral therapy,” added Dr. Busse, who recently coauthored an editorial that posited using angiotensin II (Giapreza), an approved vasopressor drug, as an alternative renin-angiotensin system intervention for COVID-19 patients including both those in shock as well as potentially those not in shock (Crit Care. 2020 Apr 7. doi: 10.1186/s13054-020-02862-1). Despite these caveats, the new Chinese findings reported by Dr. Zhang and associates “are hypothesis generating and worth further exploration.”
The authors of an editorial that accompanied the Zhang study in Circulation Research made similar points. “While the investigators used standard techniques to attempt to reduce bias in this observational study via propensity matching, it is not a randomized study and the residual confounding inherent to this approach renders the conclusions hypothesis generating at best,” wrote Ravi V. Shah, MD, and two coauthors in the editorial (Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317174). They also agreed with the several society statements that have supported continued use of ACEI/ARB drugs in COVID-19 patients. “Withdrawal of these medications in the context of those conditions in which they have proven benefit (e.g., heart failure with reduced left ventricular ejection fraction) may actually inflict more harm than good,” they warned. “In the end we must rely on randomized clinical science,” and while this level of evidence is currently lacking, “the study by Zhang and colleagues is a direct step toward that goal.”
Dr. Zhang and coauthors had no commercial disclosures. Dr. Rossi and Dr. Busse had no disclosures. The authors of the Circulation Research editorial reported several disclosures.
SOURCE: Zhang P et al. Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317134.
Hospitalized COVID-19 patients with hypertension and on treatment with an renin-angiotensin system inhibiting drug had significantly better survival, compared with similar hypertensive patients not on these drugs, in observational, propensity score–matched analyses that drew from a pool of more than 3,430 patients hospitalized at any of nine Chinese hospitals during December 2019–February 2020.
“Among patients with hypertension hospitalized with COVID-19, inpatient treatment with ACEI [ACE inhibitor]/ARB [angiotensin receptor blocker] was associated with lower risk of all-cause mortality, compared with ACEI/ARB nonusers, during 28 days of follow-up. While study interpretation needs to consider the potential for residual confounders, it is unlikely that inpatient ACEI/ARB would be associated with an increased risk of mortality,” wrote Peng Zhang, MD, a cardiology researcher at Renmin Hospital of Wuhan University, China, and coauthors in Circulations Research, buttressing recent recommendations from several medical societies to maintain COVID-19 patients on these drugs.
“Our findings in this paper provide evidence supporting continuous use of ACEI/ARB for patients with hypertension infected with SARS-COV-2,” wrote the authors, backing up recent recommendations from cardiology societies that called for not stopping ACEI/ARB prescriptions in patients at risk for contracting or already have COVID-19 infection, including a statement from the American College of Cardiology, American Heart Association, and Heart Failure Society of America, and also guidance from the European Society of Cardiology.
The study included 1,128 patients with a history of hypertension, including 188 (17%) who received an ACEI/ARB drug during hospitalization. During 28-day follow-up, 99 died (9%), including 7 deaths among the 188 patients (4%) on an ACEI/ARB drug and 92 deaths among the 940 other hypertensive patients (10%).
The authors ran several analyses to try to adjust for the influence of possible confounders. A mixed-effect Cox model with four adjusted variables showed that treatment with an ACEI/ARB drug was tied to a statistically significant 58% lower death rate, compared with patients not receiving these drugs.
The researchers also ran several propensity score–adjusted analyses. One matched 174 of the patients who received an ACEI/ARB drug with 522 who did not, and comparing these two matched arms showed that ACEI/ARB use was linked with a statistically significant 63% cut in mortality, compared with patients not getting these drugs. A second propensity score–matched analysis first excluded the 383 patients who were hypertensive but received no antihypertensive medication during hospitalization. From the remaining 745 patients who received at least one antihypertensive medication, the authors identified 181 patients who received an ACEI/ARB and propensity-score matched them with 181 hypertensive patients on a different medication class, finding that ACEI/ARB use linked with a statistically significant 71% lower rate of all-cause mortality.
Additional analyses also showed that patients with hypertension had a statistically significant, 41% increased rate of all-cause death, compared with patients without hypertension, and another propensity score–matched analysis showed that among hypertensives treatment with an ACEI/ARB drug was linked with a statistically significant 68% reduced rate of septic shock.
Although this report was received with caution and some skepticism, it was also acknowledged as a step forward in the creation of an evidence base addressing ACEI/ARB treatment during COVID-19 infection.
“These drugs are lifesaving and should not be discontinued” for patients with hypertension, heart failure, and other cardiovascular disease, commented Gian Paolo Rossi, MD, professor and chair of medicine and director of the high blood pressure unit at the University of Padua (Italy). The analysis by Zhang and associates included the largest number of hospitalized COVID-19 patients with hypertension yet reported to assess the impact of treatment with ACEI/ARB drugs, and adds important evidence in favor of continuing these drugs in patients who develop COVID-19 infection, Dr. Rossi said in an interview. He recently coauthored a review that argued against ACEI/ARB discontinuation in COVID-19 patients based on previously reported evidence (Elife. 2020 Apr 6. doi: 10.7554/eLife.57278).
But other researchers take a wary view of the potential impact of ACEI/ARB agents. “If ACEI/ARB therapy increases ACE2 and the virus down-regulates it, and because ACE2 is the viral entry port into cells, why would ACE2-mediated down-regulation of the renin-angiotensin-aldosterone system lead to amelioration of [COVID-19] disease?” asked Laurence W. Busse, MD, a critical care physician at Emory University, Atlanta. “A number of issues could potentially confound the results, including the definition of COVID-19 and imbalance of antiviral therapy,” added Dr. Busse, who recently coauthored an editorial that posited using angiotensin II (Giapreza), an approved vasopressor drug, as an alternative renin-angiotensin system intervention for COVID-19 patients including both those in shock as well as potentially those not in shock (Crit Care. 2020 Apr 7. doi: 10.1186/s13054-020-02862-1). Despite these caveats, the new Chinese findings reported by Dr. Zhang and associates “are hypothesis generating and worth further exploration.”
The authors of an editorial that accompanied the Zhang study in Circulation Research made similar points. “While the investigators used standard techniques to attempt to reduce bias in this observational study via propensity matching, it is not a randomized study and the residual confounding inherent to this approach renders the conclusions hypothesis generating at best,” wrote Ravi V. Shah, MD, and two coauthors in the editorial (Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317174). They also agreed with the several society statements that have supported continued use of ACEI/ARB drugs in COVID-19 patients. “Withdrawal of these medications in the context of those conditions in which they have proven benefit (e.g., heart failure with reduced left ventricular ejection fraction) may actually inflict more harm than good,” they warned. “In the end we must rely on randomized clinical science,” and while this level of evidence is currently lacking, “the study by Zhang and colleagues is a direct step toward that goal.”
Dr. Zhang and coauthors had no commercial disclosures. Dr. Rossi and Dr. Busse had no disclosures. The authors of the Circulation Research editorial reported several disclosures.
SOURCE: Zhang P et al. Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317134.
FROM CIRCULATION RESEARCH