User login
-
COVID-19 effect: Prescription fills mostly down for leading drugs
Prescription fills for hydroxychloroquine/chloroquine spiked right after COVID-19 was declared a national emergency in March, but use of the drugs still remains well above 2019 levels, based on data from more than 58,000 U.S. pharmacies.
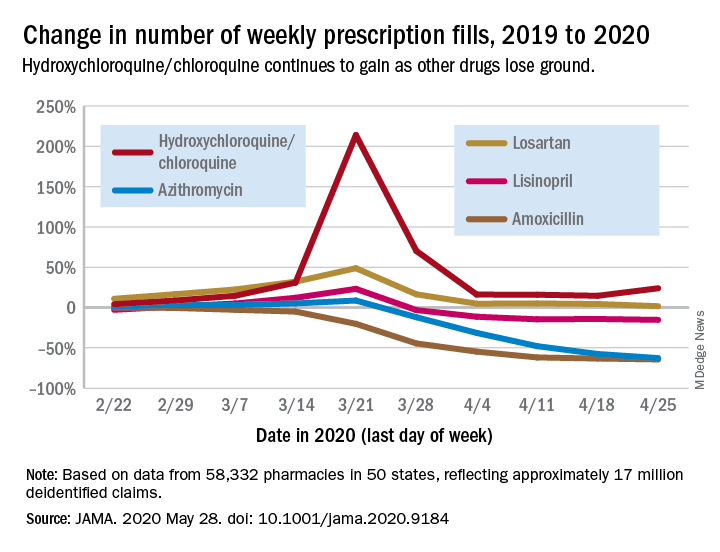
Hydroxychloroquine/chloroquine are also doing better than any of the prescription drugs in the top 10 based on total claims in 2019, Muthiah Vaduganathan, MD, MPH, of Brigham and Women’s Hospital, Boston, and associates reported May 28 in a research letter in JAMA.
Prescription fills for hydroxychloroquine/chloroquine have been above 2019 levels every week since the national emergency was declared on March 13, with the high occurring during the week of March 15-21, when fills were 214% higher than the corresponding week in 2019. The lowest level in that time came during the week of April 12-18, with growth of 14.6% over 2019, the investigators said.
The drugs occupying the top 10 – amlodipine, amoxicillin, atorvastatin, gabapentin, hydrocodone-acetaminophen, levothyroxine, lisinopril, losartan, omeprazole, and sertraline – have not done as well. Losartan, the only one that hasn’t lost ground in any week since March 13, rose by almost 49% during March 15-21, but was down to a 1.7% rise by the end of the study period, they reported.
Meanwhile, the other drug touted as a treatment for COVID-19, azithromycin, has fallen farther than most of the top 10. By April 19-25, the last week of the study period, fills for the antibiotic were down 62.7%, compared with last year, the analysis showed. Only amoxicillin had dropped more (64.4%).
“The modest decline for most common long-term therapies after peak could represent reduced contact with prescribing clinicians, restricted access to pharmacies, pharmacist rationing, loss of insurance from unemployment, or replete supplies from early stockpiling,” Dr. Vaduganathan and associates wrote.
The investigators “used all-payer U.S. pharmacy data from 58,332 chain, independent, and mail-order pharmacies across 14,421 zip codes in 50 states, reflecting approximately 17 million deidentified claims,” to estimate national prescription fills, they explained.
SOURCE: Vaduganathan M et al. JAMA 2020 May 28. doi: 10.1001/jama.2020.9184.
Prescription fills for hydroxychloroquine/chloroquine spiked right after COVID-19 was declared a national emergency in March, but use of the drugs still remains well above 2019 levels, based on data from more than 58,000 U.S. pharmacies.

Hydroxychloroquine/chloroquine are also doing better than any of the prescription drugs in the top 10 based on total claims in 2019, Muthiah Vaduganathan, MD, MPH, of Brigham and Women’s Hospital, Boston, and associates reported May 28 in a research letter in JAMA.
Prescription fills for hydroxychloroquine/chloroquine have been above 2019 levels every week since the national emergency was declared on March 13, with the high occurring during the week of March 15-21, when fills were 214% higher than the corresponding week in 2019. The lowest level in that time came during the week of April 12-18, with growth of 14.6% over 2019, the investigators said.
The drugs occupying the top 10 – amlodipine, amoxicillin, atorvastatin, gabapentin, hydrocodone-acetaminophen, levothyroxine, lisinopril, losartan, omeprazole, and sertraline – have not done as well. Losartan, the only one that hasn’t lost ground in any week since March 13, rose by almost 49% during March 15-21, but was down to a 1.7% rise by the end of the study period, they reported.
Meanwhile, the other drug touted as a treatment for COVID-19, azithromycin, has fallen farther than most of the top 10. By April 19-25, the last week of the study period, fills for the antibiotic were down 62.7%, compared with last year, the analysis showed. Only amoxicillin had dropped more (64.4%).
“The modest decline for most common long-term therapies after peak could represent reduced contact with prescribing clinicians, restricted access to pharmacies, pharmacist rationing, loss of insurance from unemployment, or replete supplies from early stockpiling,” Dr. Vaduganathan and associates wrote.
The investigators “used all-payer U.S. pharmacy data from 58,332 chain, independent, and mail-order pharmacies across 14,421 zip codes in 50 states, reflecting approximately 17 million deidentified claims,” to estimate national prescription fills, they explained.
SOURCE: Vaduganathan M et al. JAMA 2020 May 28. doi: 10.1001/jama.2020.9184.
Prescription fills for hydroxychloroquine/chloroquine spiked right after COVID-19 was declared a national emergency in March, but use of the drugs still remains well above 2019 levels, based on data from more than 58,000 U.S. pharmacies.

Hydroxychloroquine/chloroquine are also doing better than any of the prescription drugs in the top 10 based on total claims in 2019, Muthiah Vaduganathan, MD, MPH, of Brigham and Women’s Hospital, Boston, and associates reported May 28 in a research letter in JAMA.
Prescription fills for hydroxychloroquine/chloroquine have been above 2019 levels every week since the national emergency was declared on March 13, with the high occurring during the week of March 15-21, when fills were 214% higher than the corresponding week in 2019. The lowest level in that time came during the week of April 12-18, with growth of 14.6% over 2019, the investigators said.
The drugs occupying the top 10 – amlodipine, amoxicillin, atorvastatin, gabapentin, hydrocodone-acetaminophen, levothyroxine, lisinopril, losartan, omeprazole, and sertraline – have not done as well. Losartan, the only one that hasn’t lost ground in any week since March 13, rose by almost 49% during March 15-21, but was down to a 1.7% rise by the end of the study period, they reported.
Meanwhile, the other drug touted as a treatment for COVID-19, azithromycin, has fallen farther than most of the top 10. By April 19-25, the last week of the study period, fills for the antibiotic were down 62.7%, compared with last year, the analysis showed. Only amoxicillin had dropped more (64.4%).
“The modest decline for most common long-term therapies after peak could represent reduced contact with prescribing clinicians, restricted access to pharmacies, pharmacist rationing, loss of insurance from unemployment, or replete supplies from early stockpiling,” Dr. Vaduganathan and associates wrote.
The investigators “used all-payer U.S. pharmacy data from 58,332 chain, independent, and mail-order pharmacies across 14,421 zip codes in 50 states, reflecting approximately 17 million deidentified claims,” to estimate national prescription fills, they explained.
SOURCE: Vaduganathan M et al. JAMA 2020 May 28. doi: 10.1001/jama.2020.9184.
FROM JAMA
APA, others lobby to make COVID-19 telehealth waivers permanent
The American Psychiatric Association (APA) is calling on Congress to permanently lift restrictions that have allowed unfettered delivery of telehealth services during the COVID-19 pandemic, which experts say has been a boon to patients and physicians alike.
“We ask Congress to extend the telehealth waiver authority under COVID-19 beyond the emergency and to study its impact while doing so,” said APA President Jeffrey Geller, MD, in a May 27 video briefing with congressional staff and reporters.
The APA is also seeking to make permanent certain waivers granted by the Centers for Medicare & Medicaid Services on April 30, including elimination of geographic restrictions on behavioral health and allowing patients be seen at home, said Dr. Geller.
The APA also is asking for the elimination of the rule that requires clinicians to have an initial face-to-face meeting with patients before they can prescribe controlled substances, Dr. Geller said. The Drug Enforcement Administration waived that requirement, known as the Ryan Haight Act, on March 17 for the duration of the national emergency.
Telemedicine has supporters on both sides of the aisle in Congress, including Rep. Paul Tonko (D-N.Y.) who said at the APA briefing he would fight to make the waivers permanent.
“The expanded use of telehealth has enormous potential during normal times as well, especially in behavioral health,” said Mr. Tonko. “I am pushing fiercely for these current flexibilities to be extended for a reasonable time after the public health emergency so that we can have time to evaluate which should be made permanent,” he said.
Dr. Geller, other clinicians, and advocates in the briefing praised CMS for facilitating telepsychiatry for Medicare. That follows in the footsteps of most private insurers, who have also relaxed requirements into the summer, according to the Medical Group Management Association.
Game changer
The Medicare waivers “have dramatically changed the entire scene for someone like myself as a clinician to allow me to see my patients in a much easier way,” said Peter Yellowlees, MBBS, MD, chief wellness officer, University of California Davis Health. Within 2 weeks in March, the health system converted almost all of its regular outpatient visits to telemedicine, he said.
Dr. Yellowlees added government still needs to address, what he called, outdated HIPAA regulations that ban certain technologies.
“It makes no sense that I can talk to someone on an iPhone, but the moment I talk to them on FaceTime, it’s illegal,” said Dr. Yellowlees, a former president of the American Telemedicine Association.
Dr. Geller said that “psychiatric care provided by telehealth is as effective as in-person psychiatric services,” adding that “some patients prefer telepsychiatry because of its convenience and as a means of reducing stigma associated with seeking help for mental health.”
Shabana Khan, MD, a child psychiatrist and director of telepsychiatry at New York University Langone Health, said audio and video conferencing are helping address a shortage and maldistribution of child and adolescent psychiatrists.
Americans’ mental health is suffering during the pandemic. The U.S. Census Bureau recently released data showing that half of those surveyed reported depressed mood and that one-third are reporting anxiety, depression, or both, as reported by the Washington Post.
“At this very time that anxiety, depression, substance use, and other mental health problems are rising, our nation’s already strained mental health system is really being pushed to the brink,” said Jodi Kwarciany, manager for mental health policy for the National Alliance on Mental Illness, during the briefing.
Telemedicine can help “by connecting people to providers at the time and the place and using the technology that works best for them,” she said, adding that NAMI would press policymakers to address barriers to access.
The clinicians on the briefing said they’ve observed that some patients are more comfortable with video or audio interactions than with in-person visits.
Increased access to care
Telepsychiatry seems to be convincing some to reconsider therapy, since they can do it at home, said Dr. Yellowlees. he said.
For instance, he said, he has been able to consult by phone and video with several patients who receive care through the Indian Health Service who had not be able to get into the physical clinic.
Dr. Yellowlees said video sessions also may encourage patients to be more, not less, talkative. “Video is actually counterintuitively a very intimate experience,” he said, in part because of the perceived distance and people’s tendency to be less inhibited on technology platforms.“It’s less embarrassing,” he said. “If you’ve got really dramatic, difficult, traumatic things to talk about, it’s slightly easier to talk to someone who’s slightly further apart from you on video,” said Dr. Yellowlees.
“Individuals who have a significant amount of anxiety may actually feel more comfortable with the distance that this technology affords,” agreed Dr. Khan. She said telemedicine had made sessions more comfortable for some of her patients with autism spectrum disorder.
Dr. Geller said audio and video have been important to his practice during the pandemic. One of his patients never leaves the house and does not use computers. “He spends his time sequestered at home listening to records on his record player,” said Dr. Geller. But he’s been amenable to phone sessions. “What I’ve found with him, and I’ve found with several other patients, is that they actually talk more easily when they’re not face to face,” he said.
Far fewer no-shows
Another plus for his New England–based practice during the last few months: patients have not been anxious about missing sessions because of the weather. The clinicians all noted that telepsychiatry seemed to reduce missed visits.
Dr. Yellowlees said that no-show rates had decreased by half at UC Davis. “That means no significant loss of income,” during the pandemic, he said.
“The no-show rate is incredibly low, particularly because when you call the patients and they don’t remember they had an appointment, you have the appointment anyway, most of the time,” said Dr. Geller.
For Dr. Khan, being able to conduct audio and video sessions during the pandemic has meant keeping up continuity of care.
As a result of the pandemic, many college students in New York City had to go home – often to another state. The waivers granted by New York’s Medicaid program and other insurers have allowed Dr. Khan to continue care for these patients.
The NYU clinic also operates day programs in rural areas 5 hours from the city. Dr. Khan recently evaluated a 12-year-old girl with significant anxiety and low mood, both of which had worsened.
“She would not have been able to access care otherwise,” said Dr. Khan. And for rural patients who do not have access to broadband or smartphones, audio visits “have been immensely helpful,” she said.
Dr. Khan, Dr. Geller, and Dr. Yellowlees have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The American Psychiatric Association (APA) is calling on Congress to permanently lift restrictions that have allowed unfettered delivery of telehealth services during the COVID-19 pandemic, which experts say has been a boon to patients and physicians alike.
“We ask Congress to extend the telehealth waiver authority under COVID-19 beyond the emergency and to study its impact while doing so,” said APA President Jeffrey Geller, MD, in a May 27 video briefing with congressional staff and reporters.
The APA is also seeking to make permanent certain waivers granted by the Centers for Medicare & Medicaid Services on April 30, including elimination of geographic restrictions on behavioral health and allowing patients be seen at home, said Dr. Geller.
The APA also is asking for the elimination of the rule that requires clinicians to have an initial face-to-face meeting with patients before they can prescribe controlled substances, Dr. Geller said. The Drug Enforcement Administration waived that requirement, known as the Ryan Haight Act, on March 17 for the duration of the national emergency.
Telemedicine has supporters on both sides of the aisle in Congress, including Rep. Paul Tonko (D-N.Y.) who said at the APA briefing he would fight to make the waivers permanent.
“The expanded use of telehealth has enormous potential during normal times as well, especially in behavioral health,” said Mr. Tonko. “I am pushing fiercely for these current flexibilities to be extended for a reasonable time after the public health emergency so that we can have time to evaluate which should be made permanent,” he said.
Dr. Geller, other clinicians, and advocates in the briefing praised CMS for facilitating telepsychiatry for Medicare. That follows in the footsteps of most private insurers, who have also relaxed requirements into the summer, according to the Medical Group Management Association.
Game changer
The Medicare waivers “have dramatically changed the entire scene for someone like myself as a clinician to allow me to see my patients in a much easier way,” said Peter Yellowlees, MBBS, MD, chief wellness officer, University of California Davis Health. Within 2 weeks in March, the health system converted almost all of its regular outpatient visits to telemedicine, he said.
Dr. Yellowlees added government still needs to address, what he called, outdated HIPAA regulations that ban certain technologies.
“It makes no sense that I can talk to someone on an iPhone, but the moment I talk to them on FaceTime, it’s illegal,” said Dr. Yellowlees, a former president of the American Telemedicine Association.
Dr. Geller said that “psychiatric care provided by telehealth is as effective as in-person psychiatric services,” adding that “some patients prefer telepsychiatry because of its convenience and as a means of reducing stigma associated with seeking help for mental health.”
Shabana Khan, MD, a child psychiatrist and director of telepsychiatry at New York University Langone Health, said audio and video conferencing are helping address a shortage and maldistribution of child and adolescent psychiatrists.
Americans’ mental health is suffering during the pandemic. The U.S. Census Bureau recently released data showing that half of those surveyed reported depressed mood and that one-third are reporting anxiety, depression, or both, as reported by the Washington Post.
“At this very time that anxiety, depression, substance use, and other mental health problems are rising, our nation’s already strained mental health system is really being pushed to the brink,” said Jodi Kwarciany, manager for mental health policy for the National Alliance on Mental Illness, during the briefing.
Telemedicine can help “by connecting people to providers at the time and the place and using the technology that works best for them,” she said, adding that NAMI would press policymakers to address barriers to access.
The clinicians on the briefing said they’ve observed that some patients are more comfortable with video or audio interactions than with in-person visits.
Increased access to care
Telepsychiatry seems to be convincing some to reconsider therapy, since they can do it at home, said Dr. Yellowlees. he said.
For instance, he said, he has been able to consult by phone and video with several patients who receive care through the Indian Health Service who had not be able to get into the physical clinic.
Dr. Yellowlees said video sessions also may encourage patients to be more, not less, talkative. “Video is actually counterintuitively a very intimate experience,” he said, in part because of the perceived distance and people’s tendency to be less inhibited on technology platforms.“It’s less embarrassing,” he said. “If you’ve got really dramatic, difficult, traumatic things to talk about, it’s slightly easier to talk to someone who’s slightly further apart from you on video,” said Dr. Yellowlees.
“Individuals who have a significant amount of anxiety may actually feel more comfortable with the distance that this technology affords,” agreed Dr. Khan. She said telemedicine had made sessions more comfortable for some of her patients with autism spectrum disorder.
Dr. Geller said audio and video have been important to his practice during the pandemic. One of his patients never leaves the house and does not use computers. “He spends his time sequestered at home listening to records on his record player,” said Dr. Geller. But he’s been amenable to phone sessions. “What I’ve found with him, and I’ve found with several other patients, is that they actually talk more easily when they’re not face to face,” he said.
Far fewer no-shows
Another plus for his New England–based practice during the last few months: patients have not been anxious about missing sessions because of the weather. The clinicians all noted that telepsychiatry seemed to reduce missed visits.
Dr. Yellowlees said that no-show rates had decreased by half at UC Davis. “That means no significant loss of income,” during the pandemic, he said.
“The no-show rate is incredibly low, particularly because when you call the patients and they don’t remember they had an appointment, you have the appointment anyway, most of the time,” said Dr. Geller.
For Dr. Khan, being able to conduct audio and video sessions during the pandemic has meant keeping up continuity of care.
As a result of the pandemic, many college students in New York City had to go home – often to another state. The waivers granted by New York’s Medicaid program and other insurers have allowed Dr. Khan to continue care for these patients.
The NYU clinic also operates day programs in rural areas 5 hours from the city. Dr. Khan recently evaluated a 12-year-old girl with significant anxiety and low mood, both of which had worsened.
“She would not have been able to access care otherwise,” said Dr. Khan. And for rural patients who do not have access to broadband or smartphones, audio visits “have been immensely helpful,” she said.
Dr. Khan, Dr. Geller, and Dr. Yellowlees have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The American Psychiatric Association (APA) is calling on Congress to permanently lift restrictions that have allowed unfettered delivery of telehealth services during the COVID-19 pandemic, which experts say has been a boon to patients and physicians alike.
“We ask Congress to extend the telehealth waiver authority under COVID-19 beyond the emergency and to study its impact while doing so,” said APA President Jeffrey Geller, MD, in a May 27 video briefing with congressional staff and reporters.
The APA is also seeking to make permanent certain waivers granted by the Centers for Medicare & Medicaid Services on April 30, including elimination of geographic restrictions on behavioral health and allowing patients be seen at home, said Dr. Geller.
The APA also is asking for the elimination of the rule that requires clinicians to have an initial face-to-face meeting with patients before they can prescribe controlled substances, Dr. Geller said. The Drug Enforcement Administration waived that requirement, known as the Ryan Haight Act, on March 17 for the duration of the national emergency.
Telemedicine has supporters on both sides of the aisle in Congress, including Rep. Paul Tonko (D-N.Y.) who said at the APA briefing he would fight to make the waivers permanent.
“The expanded use of telehealth has enormous potential during normal times as well, especially in behavioral health,” said Mr. Tonko. “I am pushing fiercely for these current flexibilities to be extended for a reasonable time after the public health emergency so that we can have time to evaluate which should be made permanent,” he said.
Dr. Geller, other clinicians, and advocates in the briefing praised CMS for facilitating telepsychiatry for Medicare. That follows in the footsteps of most private insurers, who have also relaxed requirements into the summer, according to the Medical Group Management Association.
Game changer
The Medicare waivers “have dramatically changed the entire scene for someone like myself as a clinician to allow me to see my patients in a much easier way,” said Peter Yellowlees, MBBS, MD, chief wellness officer, University of California Davis Health. Within 2 weeks in March, the health system converted almost all of its regular outpatient visits to telemedicine, he said.
Dr. Yellowlees added government still needs to address, what he called, outdated HIPAA regulations that ban certain technologies.
“It makes no sense that I can talk to someone on an iPhone, but the moment I talk to them on FaceTime, it’s illegal,” said Dr. Yellowlees, a former president of the American Telemedicine Association.
Dr. Geller said that “psychiatric care provided by telehealth is as effective as in-person psychiatric services,” adding that “some patients prefer telepsychiatry because of its convenience and as a means of reducing stigma associated with seeking help for mental health.”
Shabana Khan, MD, a child psychiatrist and director of telepsychiatry at New York University Langone Health, said audio and video conferencing are helping address a shortage and maldistribution of child and adolescent psychiatrists.
Americans’ mental health is suffering during the pandemic. The U.S. Census Bureau recently released data showing that half of those surveyed reported depressed mood and that one-third are reporting anxiety, depression, or both, as reported by the Washington Post.
“At this very time that anxiety, depression, substance use, and other mental health problems are rising, our nation’s already strained mental health system is really being pushed to the brink,” said Jodi Kwarciany, manager for mental health policy for the National Alliance on Mental Illness, during the briefing.
Telemedicine can help “by connecting people to providers at the time and the place and using the technology that works best for them,” she said, adding that NAMI would press policymakers to address barriers to access.
The clinicians on the briefing said they’ve observed that some patients are more comfortable with video or audio interactions than with in-person visits.
Increased access to care
Telepsychiatry seems to be convincing some to reconsider therapy, since they can do it at home, said Dr. Yellowlees. he said.
For instance, he said, he has been able to consult by phone and video with several patients who receive care through the Indian Health Service who had not be able to get into the physical clinic.
Dr. Yellowlees said video sessions also may encourage patients to be more, not less, talkative. “Video is actually counterintuitively a very intimate experience,” he said, in part because of the perceived distance and people’s tendency to be less inhibited on technology platforms.“It’s less embarrassing,” he said. “If you’ve got really dramatic, difficult, traumatic things to talk about, it’s slightly easier to talk to someone who’s slightly further apart from you on video,” said Dr. Yellowlees.
“Individuals who have a significant amount of anxiety may actually feel more comfortable with the distance that this technology affords,” agreed Dr. Khan. She said telemedicine had made sessions more comfortable for some of her patients with autism spectrum disorder.
Dr. Geller said audio and video have been important to his practice during the pandemic. One of his patients never leaves the house and does not use computers. “He spends his time sequestered at home listening to records on his record player,” said Dr. Geller. But he’s been amenable to phone sessions. “What I’ve found with him, and I’ve found with several other patients, is that they actually talk more easily when they’re not face to face,” he said.
Far fewer no-shows
Another plus for his New England–based practice during the last few months: patients have not been anxious about missing sessions because of the weather. The clinicians all noted that telepsychiatry seemed to reduce missed visits.
Dr. Yellowlees said that no-show rates had decreased by half at UC Davis. “That means no significant loss of income,” during the pandemic, he said.
“The no-show rate is incredibly low, particularly because when you call the patients and they don’t remember they had an appointment, you have the appointment anyway, most of the time,” said Dr. Geller.
For Dr. Khan, being able to conduct audio and video sessions during the pandemic has meant keeping up continuity of care.
As a result of the pandemic, many college students in New York City had to go home – often to another state. The waivers granted by New York’s Medicaid program and other insurers have allowed Dr. Khan to continue care for these patients.
The NYU clinic also operates day programs in rural areas 5 hours from the city. Dr. Khan recently evaluated a 12-year-old girl with significant anxiety and low mood, both of which had worsened.
“She would not have been able to access care otherwise,” said Dr. Khan. And for rural patients who do not have access to broadband or smartphones, audio visits “have been immensely helpful,” she said.
Dr. Khan, Dr. Geller, and Dr. Yellowlees have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 may increase risk of preterm birth and cesarean delivery
Among 57 hospitalized patients with SARS-CoV-2 infection who underwent vaginal or cesarean delivery, 7 had spontaneous preterm or respiratory-indicated preterm delivery, a rate of 12%, according to a study published in Obstetrics & Gynecology. For comparison, 7% of patients had preterm delivery in 2019, researchers reported “We also noted a high cesarean delivery rate in the study population (39% vs. 27% in the same area in 2019), mainly as a result of maternal respiratory-indicated urgent delivery,” wrote Valeria M. Savasi, MD, PhD, of the University of Milan and Luigi Sacco Hospital, also in Milan, and colleagues.
Data do not indicate that pregnant women are more susceptible to severe COVID-19 infection, nor have studies suggested an increased risk of miscarriage, congenital anomalies, or early pregnancy loss in pregnant patients with COVID-19, the authors wrote. Studies have described an increased risk of preterm birth, however.
To study clinical features of maternal SARS-CoV-2 infection and potential factors associated with severe disease and iatrogenic delivery, Dr. Savasi and colleagues conducted a prospective study of 77 women with laboratory-confirmed SARS-CoV-2 infection who were admitted during pregnancy or the immediate postpartum period in 12 maternity hospitals in northern Italy between Feb. 23 and March 28, 2020.
The investigators classified patients as having severe disease if they underwent urgent delivery based on maternal respiratory function or if they were admitted to an ICU or subintensive care department. In all, 14 patients (18%) were classified as having severe disease.
“Three patients were intubated after emergency cesarean delivery performed for maternal deterioration, and one patient underwent extracorporeal membrane oxygenation,” Dr. Savasi and colleagues reported. The results are consistent with epidemiologic data in the nonpregnant population with COVID-19 disease.
Of 11 patients with severe disease who underwent urgent delivery for respiratory compromise, 6 had significant postpartum improvement in clinical conditions. No maternal deaths occurred.
“Increased BMI [body mass index] was a significant risk factor for severe disease,” Dr. Savasi and colleagues wrote. “Fever and dyspnea on admission were symptoms significantly associated with subsequent severe maternal respiratory deterioration.”
Most patients (65%) were admitted during the third trimester, and 20 patients were still pregnant at discharge.
“Nine newborns were admitted to the neonatal intensive care unit,” the authors wrote. “Interestingly, besides prematurity, fetal oxygenation and well-being at delivery were not apparently affected by the maternal acute conditions.” Three newborns with vaginal delivery and one with cesarean delivery tested positive for SARS-CoV-2. The newborns may have been infected after delivery, Dr. Savasi and colleagues added. For all newborns, rooming-in and breastfeeding were performed, and none developed respiratory symptoms.
Criteria for hospital admission and therapeutic protocols may have varied between hospitals, the authors noted. In addition, the study included 12 patients who were asymptomatic and admitted for obstetric indications. These patients were tested for SARS-CoV-2 because of contact with an infected individual. Most patients were symptomatic, however, which explains the high rate of maternal severe outcomes. Hospitals have since adopted a universal SARS-CoV-2 screening policy for hospitalized pregnant patients.
Kristina Adams Waldorf, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, commented in an interview that Savasi et al. describe one of the larger COVID-19 in pregnancy cohorts to date with rates of severe disease and delivery for respiratory compromise, which is remarkably similar to Washington state (severe disease, 18% vs. nearly 15%; delivery for respiratory compromise, 16% vs. 20%). As in Washington state, Italian women with a higher prepregnancy BMI were overrepresented in the severe disease group.
“Data are beginning to emerge that identify women who were overweight or obese prior to pregnancy as a high risk group for developing severe COVID-19. These data are similar to known associations between obesity and critical illness in pregnancy during the 2009 ‘swine flu’ (influenza A virus, H1N1) pandemic,” she said.
“This study and others indicate that the late second and third trimesters may be a time when women are more likely to be symptomatic from COVID-19. It remains unclear if women in the first trimester are protected from severe COVID-19 outcomes or have outcomes similar to nonpregnant women,” concluded Dr. Waldorf.
One study author disclosed receiving funds from Lo Li Pharma and Zambongroup. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Savasi VM et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003979.
Among 57 hospitalized patients with SARS-CoV-2 infection who underwent vaginal or cesarean delivery, 7 had spontaneous preterm or respiratory-indicated preterm delivery, a rate of 12%, according to a study published in Obstetrics & Gynecology. For comparison, 7% of patients had preterm delivery in 2019, researchers reported “We also noted a high cesarean delivery rate in the study population (39% vs. 27% in the same area in 2019), mainly as a result of maternal respiratory-indicated urgent delivery,” wrote Valeria M. Savasi, MD, PhD, of the University of Milan and Luigi Sacco Hospital, also in Milan, and colleagues.
Data do not indicate that pregnant women are more susceptible to severe COVID-19 infection, nor have studies suggested an increased risk of miscarriage, congenital anomalies, or early pregnancy loss in pregnant patients with COVID-19, the authors wrote. Studies have described an increased risk of preterm birth, however.
To study clinical features of maternal SARS-CoV-2 infection and potential factors associated with severe disease and iatrogenic delivery, Dr. Savasi and colleagues conducted a prospective study of 77 women with laboratory-confirmed SARS-CoV-2 infection who were admitted during pregnancy or the immediate postpartum period in 12 maternity hospitals in northern Italy between Feb. 23 and March 28, 2020.
The investigators classified patients as having severe disease if they underwent urgent delivery based on maternal respiratory function or if they were admitted to an ICU or subintensive care department. In all, 14 patients (18%) were classified as having severe disease.
“Three patients were intubated after emergency cesarean delivery performed for maternal deterioration, and one patient underwent extracorporeal membrane oxygenation,” Dr. Savasi and colleagues reported. The results are consistent with epidemiologic data in the nonpregnant population with COVID-19 disease.
Of 11 patients with severe disease who underwent urgent delivery for respiratory compromise, 6 had significant postpartum improvement in clinical conditions. No maternal deaths occurred.
“Increased BMI [body mass index] was a significant risk factor for severe disease,” Dr. Savasi and colleagues wrote. “Fever and dyspnea on admission were symptoms significantly associated with subsequent severe maternal respiratory deterioration.”
Most patients (65%) were admitted during the third trimester, and 20 patients were still pregnant at discharge.
“Nine newborns were admitted to the neonatal intensive care unit,” the authors wrote. “Interestingly, besides prematurity, fetal oxygenation and well-being at delivery were not apparently affected by the maternal acute conditions.” Three newborns with vaginal delivery and one with cesarean delivery tested positive for SARS-CoV-2. The newborns may have been infected after delivery, Dr. Savasi and colleagues added. For all newborns, rooming-in and breastfeeding were performed, and none developed respiratory symptoms.
Criteria for hospital admission and therapeutic protocols may have varied between hospitals, the authors noted. In addition, the study included 12 patients who were asymptomatic and admitted for obstetric indications. These patients were tested for SARS-CoV-2 because of contact with an infected individual. Most patients were symptomatic, however, which explains the high rate of maternal severe outcomes. Hospitals have since adopted a universal SARS-CoV-2 screening policy for hospitalized pregnant patients.
Kristina Adams Waldorf, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, commented in an interview that Savasi et al. describe one of the larger COVID-19 in pregnancy cohorts to date with rates of severe disease and delivery for respiratory compromise, which is remarkably similar to Washington state (severe disease, 18% vs. nearly 15%; delivery for respiratory compromise, 16% vs. 20%). As in Washington state, Italian women with a higher prepregnancy BMI were overrepresented in the severe disease group.
“Data are beginning to emerge that identify women who were overweight or obese prior to pregnancy as a high risk group for developing severe COVID-19. These data are similar to known associations between obesity and critical illness in pregnancy during the 2009 ‘swine flu’ (influenza A virus, H1N1) pandemic,” she said.
“This study and others indicate that the late second and third trimesters may be a time when women are more likely to be symptomatic from COVID-19. It remains unclear if women in the first trimester are protected from severe COVID-19 outcomes or have outcomes similar to nonpregnant women,” concluded Dr. Waldorf.
One study author disclosed receiving funds from Lo Li Pharma and Zambongroup. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Savasi VM et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003979.
Among 57 hospitalized patients with SARS-CoV-2 infection who underwent vaginal or cesarean delivery, 7 had spontaneous preterm or respiratory-indicated preterm delivery, a rate of 12%, according to a study published in Obstetrics & Gynecology. For comparison, 7% of patients had preterm delivery in 2019, researchers reported “We also noted a high cesarean delivery rate in the study population (39% vs. 27% in the same area in 2019), mainly as a result of maternal respiratory-indicated urgent delivery,” wrote Valeria M. Savasi, MD, PhD, of the University of Milan and Luigi Sacco Hospital, also in Milan, and colleagues.
Data do not indicate that pregnant women are more susceptible to severe COVID-19 infection, nor have studies suggested an increased risk of miscarriage, congenital anomalies, or early pregnancy loss in pregnant patients with COVID-19, the authors wrote. Studies have described an increased risk of preterm birth, however.
To study clinical features of maternal SARS-CoV-2 infection and potential factors associated with severe disease and iatrogenic delivery, Dr. Savasi and colleagues conducted a prospective study of 77 women with laboratory-confirmed SARS-CoV-2 infection who were admitted during pregnancy or the immediate postpartum period in 12 maternity hospitals in northern Italy between Feb. 23 and March 28, 2020.
The investigators classified patients as having severe disease if they underwent urgent delivery based on maternal respiratory function or if they were admitted to an ICU or subintensive care department. In all, 14 patients (18%) were classified as having severe disease.
“Three patients were intubated after emergency cesarean delivery performed for maternal deterioration, and one patient underwent extracorporeal membrane oxygenation,” Dr. Savasi and colleagues reported. The results are consistent with epidemiologic data in the nonpregnant population with COVID-19 disease.
Of 11 patients with severe disease who underwent urgent delivery for respiratory compromise, 6 had significant postpartum improvement in clinical conditions. No maternal deaths occurred.
“Increased BMI [body mass index] was a significant risk factor for severe disease,” Dr. Savasi and colleagues wrote. “Fever and dyspnea on admission were symptoms significantly associated with subsequent severe maternal respiratory deterioration.”
Most patients (65%) were admitted during the third trimester, and 20 patients were still pregnant at discharge.
“Nine newborns were admitted to the neonatal intensive care unit,” the authors wrote. “Interestingly, besides prematurity, fetal oxygenation and well-being at delivery were not apparently affected by the maternal acute conditions.” Three newborns with vaginal delivery and one with cesarean delivery tested positive for SARS-CoV-2. The newborns may have been infected after delivery, Dr. Savasi and colleagues added. For all newborns, rooming-in and breastfeeding were performed, and none developed respiratory symptoms.
Criteria for hospital admission and therapeutic protocols may have varied between hospitals, the authors noted. In addition, the study included 12 patients who were asymptomatic and admitted for obstetric indications. These patients were tested for SARS-CoV-2 because of contact with an infected individual. Most patients were symptomatic, however, which explains the high rate of maternal severe outcomes. Hospitals have since adopted a universal SARS-CoV-2 screening policy for hospitalized pregnant patients.
Kristina Adams Waldorf, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, commented in an interview that Savasi et al. describe one of the larger COVID-19 in pregnancy cohorts to date with rates of severe disease and delivery for respiratory compromise, which is remarkably similar to Washington state (severe disease, 18% vs. nearly 15%; delivery for respiratory compromise, 16% vs. 20%). As in Washington state, Italian women with a higher prepregnancy BMI were overrepresented in the severe disease group.
“Data are beginning to emerge that identify women who were overweight or obese prior to pregnancy as a high risk group for developing severe COVID-19. These data are similar to known associations between obesity and critical illness in pregnancy during the 2009 ‘swine flu’ (influenza A virus, H1N1) pandemic,” she said.
“This study and others indicate that the late second and third trimesters may be a time when women are more likely to be symptomatic from COVID-19. It remains unclear if women in the first trimester are protected from severe COVID-19 outcomes or have outcomes similar to nonpregnant women,” concluded Dr. Waldorf.
One study author disclosed receiving funds from Lo Li Pharma and Zambongroup. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Savasi VM et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003979.
FROM OBSTETRICS & GYNECOLOGY
Testing the limits of medical technology
On March 9 my team was given a directive by the chief medical officer of our health system. It seemed like an impossible task, involving the mobilization of people, processes, and technology at a scale and speed we had never before achieved. It turned out getting this done was impossible. In spite of our best efforts, we failed to meet the deadline – it actually took us 3 days. Still, by March 12, we had opened the doors on the first community testing site in our area and gained the attention of local and national news outlets for our accomplishment.
Now more than 2 months later, I’m quite proud of what our team was able to achieve for the health system, but I’m still quite frustrated at the state of COVID-19 testing nationwide – there’s simply not enough available, and there is tremendous variability in the reliability of the tests. In this column, we’d like to highlight some of the challenges we’ve faced and reflect on how the shortcomings of modern technology have once again proven that medicine is both a science and an art.
Our dangerous lack of preparation
Prior to the coronavirus pandemic, I had never considered surgical masks, face shields, and nasal swabs to be critical components of medical technology. My opinion quickly changed after opening our drive-through COVID-19 site. I now have a much greater appreciation for the importance of personal protective equipment and basic testing supplies.
I was shocked by how difficult obtaining it has been during the past few months. It seems that no one anticipated the possibility of a pandemic on this grand a scale, so stockpiles of equipment were depleted quickly and couldn’t be replenished. Also, most manufacturing occurs outside the United States, which creates additional barriers to controlling the supply chain. One need not look far to find stories of widespread price-gouging, black market racketeering, and even hijackings that have stood in the way of accessing the necessary supplies. Sadly, the lack of equipment is far from the only challenge we’ve faced. In some cases, it has been a mistrust of results that has prevented widespread testing and mitigation.
The risks of flying blind
When President Trump touted the introduction of a rapid COVID-19 test at the end of March, many people were excited. Promising positive results in as few as 5 minutes, the assay was granted an Emergency Use Authorization (EUA) by the Food and Drug Administration in order to expedite its availability in the market. According to the FDA’s website, an EUA allows “unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions.” This rapid (though untested) approval was all that many health care providers needed to hear – immediately hospitals and physicians scrambled to get their hands on the testing devices. Unfortunately, on May 14th, the FDA issued a press release that raised concerns about that same test because it seemed to be reporting a high number of false-negative results. Just as quickly as the devices had been adopted, health care providers began backing away from them in favor of other assays, and a serious truth about COVID-19 testing was revealed: In many ways, we’re flying blind.
Laboratory manufacturers have been working overtime to create assays for SARS-CoV-2 (the coronavirus that causes COVID-19) and have used different technologies for detection. The most commonly used are polymerase chain reaction (PCR) tests. In these assays, viral RNA is converted to DNA by reverse transcriptase, then amplified through the addition of primers that enable detection. PCR technology has been available for years and is a reliable method for identifying DNA and RNA, but the required heating and cooling process takes time and results can take several hours to return. To address this and expedite testing, other methods of detection have been tried, such as the loop-mediated isothermal amplification (LAMP) technique employed by the rapid assay mentioned above. Regardless of methodology, all laboratory tests have one thing in common: None of them is perfect.
Every assay has a different level of reliability. When screening for a disease such as COVID-19, we are particularly interested in a test’s sensitivity (that is, it’s ability to detect disease); we’d love such a screening test to be 100% sensitive and thereby not miss a single case. In truth, no test’s sensitivity is 100%, and in this particular case even the best assays only score around 98%. This means that out of every 100 patients with COVID-19 who are evaluated, two might test negative for the virus. In a pandemic this can have dire consequences, so health care providers – unable to fully trust their instruments – must employ clinical acumen and years of experience to navigate these cloudy skies. We are hopeful that additional tools will complement our current methods, but with new assays also come new questions.
Is anyone safe?
We receive regular questions from physicians about the value of antibody testing, but it’s not yet clear how best to respond. While the assays seem to be reliable, the utility of the results are still ill defined. Antibodies to SARS-CoV-2 (both IgG and IgM) appear to peak about 2-3 weeks after symptom onset, but we don’t yet know if the presence of those antibodies confers long-term immunity. Therefore, patients should not use the information to change their masking or social-distancing practices, nor should they presume that they are safe from becoming reinfected with COVID-19. While new research looks promising, there are still too many unknowns to be able to confidently reassure providers or patients of the true value of antibody testing. This underscores our final point: Medicine remains an art.
As we are regularly reminded, we’ll never fully anticipate the challenges or barriers to success, and technology will never replace the value of clinical judgment and human experience. While the situation is unsettling in many ways, we are reassured and encouraged by the role we still get to play in keeping our patients healthy in this health care crisis, and we’ll continue to do so through whatever the future holds.
Dr. Notte is a family physician and chief medical officer of Abington Lansdale (Pa.) Hospital - Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
On March 9 my team was given a directive by the chief medical officer of our health system. It seemed like an impossible task, involving the mobilization of people, processes, and technology at a scale and speed we had never before achieved. It turned out getting this done was impossible. In spite of our best efforts, we failed to meet the deadline – it actually took us 3 days. Still, by March 12, we had opened the doors on the first community testing site in our area and gained the attention of local and national news outlets for our accomplishment.
Now more than 2 months later, I’m quite proud of what our team was able to achieve for the health system, but I’m still quite frustrated at the state of COVID-19 testing nationwide – there’s simply not enough available, and there is tremendous variability in the reliability of the tests. In this column, we’d like to highlight some of the challenges we’ve faced and reflect on how the shortcomings of modern technology have once again proven that medicine is both a science and an art.
Our dangerous lack of preparation
Prior to the coronavirus pandemic, I had never considered surgical masks, face shields, and nasal swabs to be critical components of medical technology. My opinion quickly changed after opening our drive-through COVID-19 site. I now have a much greater appreciation for the importance of personal protective equipment and basic testing supplies.
I was shocked by how difficult obtaining it has been during the past few months. It seems that no one anticipated the possibility of a pandemic on this grand a scale, so stockpiles of equipment were depleted quickly and couldn’t be replenished. Also, most manufacturing occurs outside the United States, which creates additional barriers to controlling the supply chain. One need not look far to find stories of widespread price-gouging, black market racketeering, and even hijackings that have stood in the way of accessing the necessary supplies. Sadly, the lack of equipment is far from the only challenge we’ve faced. In some cases, it has been a mistrust of results that has prevented widespread testing and mitigation.
The risks of flying blind
When President Trump touted the introduction of a rapid COVID-19 test at the end of March, many people were excited. Promising positive results in as few as 5 minutes, the assay was granted an Emergency Use Authorization (EUA) by the Food and Drug Administration in order to expedite its availability in the market. According to the FDA’s website, an EUA allows “unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions.” This rapid (though untested) approval was all that many health care providers needed to hear – immediately hospitals and physicians scrambled to get their hands on the testing devices. Unfortunately, on May 14th, the FDA issued a press release that raised concerns about that same test because it seemed to be reporting a high number of false-negative results. Just as quickly as the devices had been adopted, health care providers began backing away from them in favor of other assays, and a serious truth about COVID-19 testing was revealed: In many ways, we’re flying blind.
Laboratory manufacturers have been working overtime to create assays for SARS-CoV-2 (the coronavirus that causes COVID-19) and have used different technologies for detection. The most commonly used are polymerase chain reaction (PCR) tests. In these assays, viral RNA is converted to DNA by reverse transcriptase, then amplified through the addition of primers that enable detection. PCR technology has been available for years and is a reliable method for identifying DNA and RNA, but the required heating and cooling process takes time and results can take several hours to return. To address this and expedite testing, other methods of detection have been tried, such as the loop-mediated isothermal amplification (LAMP) technique employed by the rapid assay mentioned above. Regardless of methodology, all laboratory tests have one thing in common: None of them is perfect.
Every assay has a different level of reliability. When screening for a disease such as COVID-19, we are particularly interested in a test’s sensitivity (that is, it’s ability to detect disease); we’d love such a screening test to be 100% sensitive and thereby not miss a single case. In truth, no test’s sensitivity is 100%, and in this particular case even the best assays only score around 98%. This means that out of every 100 patients with COVID-19 who are evaluated, two might test negative for the virus. In a pandemic this can have dire consequences, so health care providers – unable to fully trust their instruments – must employ clinical acumen and years of experience to navigate these cloudy skies. We are hopeful that additional tools will complement our current methods, but with new assays also come new questions.
Is anyone safe?
We receive regular questions from physicians about the value of antibody testing, but it’s not yet clear how best to respond. While the assays seem to be reliable, the utility of the results are still ill defined. Antibodies to SARS-CoV-2 (both IgG and IgM) appear to peak about 2-3 weeks after symptom onset, but we don’t yet know if the presence of those antibodies confers long-term immunity. Therefore, patients should not use the information to change their masking or social-distancing practices, nor should they presume that they are safe from becoming reinfected with COVID-19. While new research looks promising, there are still too many unknowns to be able to confidently reassure providers or patients of the true value of antibody testing. This underscores our final point: Medicine remains an art.
As we are regularly reminded, we’ll never fully anticipate the challenges or barriers to success, and technology will never replace the value of clinical judgment and human experience. While the situation is unsettling in many ways, we are reassured and encouraged by the role we still get to play in keeping our patients healthy in this health care crisis, and we’ll continue to do so through whatever the future holds.
Dr. Notte is a family physician and chief medical officer of Abington Lansdale (Pa.) Hospital - Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
On March 9 my team was given a directive by the chief medical officer of our health system. It seemed like an impossible task, involving the mobilization of people, processes, and technology at a scale and speed we had never before achieved. It turned out getting this done was impossible. In spite of our best efforts, we failed to meet the deadline – it actually took us 3 days. Still, by March 12, we had opened the doors on the first community testing site in our area and gained the attention of local and national news outlets for our accomplishment.
Now more than 2 months later, I’m quite proud of what our team was able to achieve for the health system, but I’m still quite frustrated at the state of COVID-19 testing nationwide – there’s simply not enough available, and there is tremendous variability in the reliability of the tests. In this column, we’d like to highlight some of the challenges we’ve faced and reflect on how the shortcomings of modern technology have once again proven that medicine is both a science and an art.
Our dangerous lack of preparation
Prior to the coronavirus pandemic, I had never considered surgical masks, face shields, and nasal swabs to be critical components of medical technology. My opinion quickly changed after opening our drive-through COVID-19 site. I now have a much greater appreciation for the importance of personal protective equipment and basic testing supplies.
I was shocked by how difficult obtaining it has been during the past few months. It seems that no one anticipated the possibility of a pandemic on this grand a scale, so stockpiles of equipment were depleted quickly and couldn’t be replenished. Also, most manufacturing occurs outside the United States, which creates additional barriers to controlling the supply chain. One need not look far to find stories of widespread price-gouging, black market racketeering, and even hijackings that have stood in the way of accessing the necessary supplies. Sadly, the lack of equipment is far from the only challenge we’ve faced. In some cases, it has been a mistrust of results that has prevented widespread testing and mitigation.
The risks of flying blind
When President Trump touted the introduction of a rapid COVID-19 test at the end of March, many people were excited. Promising positive results in as few as 5 minutes, the assay was granted an Emergency Use Authorization (EUA) by the Food and Drug Administration in order to expedite its availability in the market. According to the FDA’s website, an EUA allows “unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions.” This rapid (though untested) approval was all that many health care providers needed to hear – immediately hospitals and physicians scrambled to get their hands on the testing devices. Unfortunately, on May 14th, the FDA issued a press release that raised concerns about that same test because it seemed to be reporting a high number of false-negative results. Just as quickly as the devices had been adopted, health care providers began backing away from them in favor of other assays, and a serious truth about COVID-19 testing was revealed: In many ways, we’re flying blind.
Laboratory manufacturers have been working overtime to create assays for SARS-CoV-2 (the coronavirus that causes COVID-19) and have used different technologies for detection. The most commonly used are polymerase chain reaction (PCR) tests. In these assays, viral RNA is converted to DNA by reverse transcriptase, then amplified through the addition of primers that enable detection. PCR technology has been available for years and is a reliable method for identifying DNA and RNA, but the required heating and cooling process takes time and results can take several hours to return. To address this and expedite testing, other methods of detection have been tried, such as the loop-mediated isothermal amplification (LAMP) technique employed by the rapid assay mentioned above. Regardless of methodology, all laboratory tests have one thing in common: None of them is perfect.
Every assay has a different level of reliability. When screening for a disease such as COVID-19, we are particularly interested in a test’s sensitivity (that is, it’s ability to detect disease); we’d love such a screening test to be 100% sensitive and thereby not miss a single case. In truth, no test’s sensitivity is 100%, and in this particular case even the best assays only score around 98%. This means that out of every 100 patients with COVID-19 who are evaluated, two might test negative for the virus. In a pandemic this can have dire consequences, so health care providers – unable to fully trust their instruments – must employ clinical acumen and years of experience to navigate these cloudy skies. We are hopeful that additional tools will complement our current methods, but with new assays also come new questions.
Is anyone safe?
We receive regular questions from physicians about the value of antibody testing, but it’s not yet clear how best to respond. While the assays seem to be reliable, the utility of the results are still ill defined. Antibodies to SARS-CoV-2 (both IgG and IgM) appear to peak about 2-3 weeks after symptom onset, but we don’t yet know if the presence of those antibodies confers long-term immunity. Therefore, patients should not use the information to change their masking or social-distancing practices, nor should they presume that they are safe from becoming reinfected with COVID-19. While new research looks promising, there are still too many unknowns to be able to confidently reassure providers or patients of the true value of antibody testing. This underscores our final point: Medicine remains an art.
As we are regularly reminded, we’ll never fully anticipate the challenges or barriers to success, and technology will never replace the value of clinical judgment and human experience. While the situation is unsettling in many ways, we are reassured and encouraged by the role we still get to play in keeping our patients healthy in this health care crisis, and we’ll continue to do so through whatever the future holds.
Dr. Notte is a family physician and chief medical officer of Abington Lansdale (Pa.) Hospital - Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Scientific doubt tempers COVID-19 vaccine optimism
US government and industry projections that a COVID-19 vaccine will be ready by this fall or even January would take compressing what usually takes at least a decade into months, with little room for error or safety surprises.
“If all the cards fall into the right place and all the stars are aligned, you definitely could get a vaccine by December or January,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said last week.
But Fauci said a more realistic timeline is still 12 to 18 months, and experts interviewed by Medscape Medical News agree. They say that although recent developments are encouraging, history and scientific reason say the day when a COVID-19 vaccine is widely available will not come this year and may not come by the end of 2021.
The encouraging signals come primarily from two recent announcements: the $1.2 billion United States backing last week of one vaccine platform and the announcement on May 18 that the first human trials of another have produced some positive phase 1 results.
Recent developments
On May 21, the US Department of Health and Human Services (HHS) under “Operation Warp Speed” announced that the US will give AstraZeneca $1.2 billion “to make available at least 300 million doses of a coronavirus vaccine called AZD1222, with the first doses delivered as early as October 2020.”
On May 18, the Massachusetts-based biotechnology company Moderna announced that phase 1 clinical results showed that its vaccine candidate, which uses a new messenger RNA (mRNA) technology, appeared safe. Eight participants in the human trials were able to produce neutralizing antibodies that researchers believe are important in developing protection from the virus.
Moderna Chief Medical Officer Tal Zaks, MD, PhD told CNN that if the vaccine candidate does well in phase 2, “it could be ready by January 2021.”
The two candidates are among 10 in clinical trials for the SARS-CoV-2 virus, according to the World Health Organization (WHO). The AstraZeneca/ AZD1222 candidate (also called ChAdOx1 nCoV-19, in collaboration with the University of Oxford) has entered phase 2/3.
Moderna’s candidate and another being developed in Beijing, China, are in phase 2, WHO reports. As of yesterday, 115 other candidates are in preclinical evaluation.
Maria Elena Bottazzi, PhD, associate dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, Texas, told Medscape Medical News it’s important to realize that, in the case of the $1.2 billion US investment, “what they’re talking about is manufacturing.”
The idea, she said, is to pay AstraZeneca up front so that manufacturing can start before it is known whether the vaccine candidate is safe or effective, the reverse of how the clinical trial process usually works.
That way, if the candidate is deemed safe and effective, time is not lost by then deciding how to make it and distribute it.
By the end of this year, she said, “Maybe we will have many vaccines made and stored in a refrigerator somewhere. But between now and December, there’s absolutely no way you can show efficacy of the vaccine at the same time you confirm that it’s safe.”
“Take these things with a grain of salt”
Animal testing for the AstraZeneca candidate, made in partnership with the University of Oxford in the United Kingdom, has yielded lackluster results, according to results on the preprint server BioRxiv, which have not been peer-reviewed.
“The results were not bad, but they were not gangbusters,” Bottazzi said. The results show the vaccine offered only partial protection.
“Partial protection is better than no protection,” she noted. “You have to take these things with a grain of salt. We don’t know what’s going to happen in humans.”
As for the Moderna candidate, Bottazzi said, “the good news is they found an appropriate safety profile. But from an eight-person group to make the extrapolation that they have efficacy — it’s unrealistic.”
Nicole Lurie, MD, MSPH, is senior adviser to the CEO for the Coalition for Epidemic Preparedness Innovation (CEPI), a nongovernmental organization funded by the Wellcome Trust, the Bill and Melinda Gates Foundation, the European Commission, and eight countries (Australia, Belgium, Canada, Ethiopia, Germany, Japan, Norway, and the United Kingdom) charged with supporting development of vaccines for pathogens on WHO’s priority list.
She and her colleagues write in a paper published online in the New England Journal of Medicine on March 30 that “it typically takes multiple candidates and many years to produce a licensed vaccine.”
The fastest time for developing a vaccine to date is 4 years, for the mumps vaccine, licensed in 1967.
As to whether she would expect a rollout of any vaccine by the end of the year, Lurie told Medscape Medical News, “If everything goes according to plan in every way, shape or form, well then maybe you can get there. But I wouldn’t hold my breath.”
Lurie and her colleagues write that “it’s far from certain that these new platforms will be scalable or that existing capacity can provide sufficient quantities of vaccine fast enough.”
On a call with reporters today, leaders of some of the words largest pharmaceutical companies said that one of the key bottlenecks is the sheer number of vials needed in order to distribute billions of doses of a successful vaccine.
Pfizer CEO Albert Bourla, DVM, PhD, said, “Typically we are producing vaccines in single-dose vials. We are exploring with governments right now if it would be more convenient if there were 5-dose vials or 10-dose vials. I think we can resolve a significant part of the bottleneck.”
Despite the challenges, experts interviewed for this article agree that it will be possible to make a vaccine for COVID-19. They don’t expect attempts to meet the same complications that HIV researchers have seen over decades as the virus continues to confound with mutations.
Fred Ledley, MD, director of the Center for Integration of Science and Industry at Bentley University in Waltham, Massachusetts, told Medscape Medical News, “There doesn’t appear to be anything terribly diabolical about this virus. The mutation rate doesn’t appear to be anything like HIV. It appears to have some big, ugly proteins on the surface, which is good for vaccines — proteins with a lot of physical features look distinguishable from healthy cells. Signs all point to that it should be possible to make a vaccine.”
History raises safety concerns
However, Ledley said, “The idea of doing it in 6 months is largely unrealistic.”
He says 18 months is more realistic, primarily because of the sheer number of people that would have to be enrolled in a phase 3 study to truly test whether the endpoints are being met.
Vaccines are given to healthy volunteers. If safety signals arise, they may not be apparent until massive numbers of people are tested in phase 3.
“You’re never going to see the rates cut to 0%, but to see the difference between 10 people getting sick and seven people getting sick, takes very, very large numbers,” Ledley said. “There’s no way that can be done in 6 months. You’re talking about tens of thousands of people enrolled.”
He notes at this point it’s unclear what the endpoints will be and what the safety thresholds will be after consideration of risks and benefit.
Another big question for Ledley: “We don’t know what type of immunity we need to protect us against the virus. Do you just need the antibodies in your blood or do you need cells that are primed to attack the virus? Is it more of a chemical clearance or do the cells need to physically go in and digest the virus?”
History also points to the need for rigorous safety precautions that scientists fear could be compromised as trial phases overlap and processes are run in parallel instead of one step at a time.
An early batch of the Salk vaccine for polio in 1955, for example, turned out to be contaminated and caused paralysis in some children and 10 deaths, he points out.
CEPI’s Lurie adds that early candidates for another coronavirus, severe acute respiratory syndrome (SARS), “caused a reaction in the lungs that was very dangerous” before development was halted.
She also pointed to previous findings that a vaccine for dengue fever could worsen the disease in some people through a phenomenon called antibody-dependent enhancement.
Lurie and colleagues write in their paper that “it’s critical that vaccines also be developed using the tried-and-true methods, even if they may take longer to enter clinical trials or to result in large numbers of doses.”
Live attenuated vaccine
Raul Andino, PhD, a virologist at the University of California San Francisco, is among the scientists working with a tried-and-true method — a live attenuated vaccine — and he told Medscape Medical News he’s predicting it will take 2 years to develop.
He said it is cheaper to produce because scientists just have to learn how to grow the virus. Because the technology is already proven, a live attenuated vaccine could be rapidly produced on a worldwide scale.
The hope is also that a live attenuated vaccine would be given once in a lifetime and therefore be more affordable, especially in poorer countries.
“While a Moderna vaccine might be good for Europe and the United States,” he said, “It’s not going to be good for Africa, India, Brazil.”
Andino said, “I would bet money” that the front-runner vaccines so far will not be one-time vaccines.
He points out that most of the vaccine candidates are trying to protect people from disease. While there’s nothing wrong with that, he said, “In my opinion that is the lower-hanging fruit.”
“In my mind we need something that interrupts the chain of transmission and induces protection,” Andino said, important for developing herd immunity.
The reason this type of approach takes longer is because you are introducing a weakened form of the virus to the body and you have to make sure it doesn’t cause disease, not just in a small test population, but in populations who may be more susceptible to the disease, Andino said.
A call for unified strategies
Universities, countries, international consortiums, and public-private partnerships are all racing to find several safe and effective vaccines as no one entity will likely be able to provide the global solution.
Some of the efforts involve overlap of entities but with different focuses.
Along with “Operation Warp Speed” and CEPI, other collaborations include Gavi the Vaccine Alliance, whose core partners include WHO, UNICEF, the World Bank, and the Gates Foundation; and “Accelerating Therapeutic Interventions and Vaccines (ACTIV) partnership,” led by the National Institutes of Health.
Industry partners in ACTIV (18 biopharmaceutical companies), according to a May 18 article published online in the Journal of the American Medical Association, have said they will contribute their respective clinical trial capacities, regardless of which agent is studied.
Some, however, have called for more streamlining of efforts.
“Ideally we’d be working together,” Lurie told Medscape Medical News.
“I’m hopeful we will find ways to collaborate scientifically,” she said. “The US government’s responsibility is to make doses for the US. CEPI’s responsibility is to make doses for the world. A big focus of CEPI is to make sure we have manufacturing capacity outside of the US so those doses can be available to the world and they don’t get seized by wealthy countries.”
Bottazzi, Ledley, Lurie, and Andino report no relevant financial relationships.
This article first appeared on Medscape.com.
US government and industry projections that a COVID-19 vaccine will be ready by this fall or even January would take compressing what usually takes at least a decade into months, with little room for error or safety surprises.
“If all the cards fall into the right place and all the stars are aligned, you definitely could get a vaccine by December or January,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said last week.
But Fauci said a more realistic timeline is still 12 to 18 months, and experts interviewed by Medscape Medical News agree. They say that although recent developments are encouraging, history and scientific reason say the day when a COVID-19 vaccine is widely available will not come this year and may not come by the end of 2021.
The encouraging signals come primarily from two recent announcements: the $1.2 billion United States backing last week of one vaccine platform and the announcement on May 18 that the first human trials of another have produced some positive phase 1 results.
Recent developments
On May 21, the US Department of Health and Human Services (HHS) under “Operation Warp Speed” announced that the US will give AstraZeneca $1.2 billion “to make available at least 300 million doses of a coronavirus vaccine called AZD1222, with the first doses delivered as early as October 2020.”
On May 18, the Massachusetts-based biotechnology company Moderna announced that phase 1 clinical results showed that its vaccine candidate, which uses a new messenger RNA (mRNA) technology, appeared safe. Eight participants in the human trials were able to produce neutralizing antibodies that researchers believe are important in developing protection from the virus.
Moderna Chief Medical Officer Tal Zaks, MD, PhD told CNN that if the vaccine candidate does well in phase 2, “it could be ready by January 2021.”
The two candidates are among 10 in clinical trials for the SARS-CoV-2 virus, according to the World Health Organization (WHO). The AstraZeneca/ AZD1222 candidate (also called ChAdOx1 nCoV-19, in collaboration with the University of Oxford) has entered phase 2/3.
Moderna’s candidate and another being developed in Beijing, China, are in phase 2, WHO reports. As of yesterday, 115 other candidates are in preclinical evaluation.
Maria Elena Bottazzi, PhD, associate dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, Texas, told Medscape Medical News it’s important to realize that, in the case of the $1.2 billion US investment, “what they’re talking about is manufacturing.”
The idea, she said, is to pay AstraZeneca up front so that manufacturing can start before it is known whether the vaccine candidate is safe or effective, the reverse of how the clinical trial process usually works.
That way, if the candidate is deemed safe and effective, time is not lost by then deciding how to make it and distribute it.
By the end of this year, she said, “Maybe we will have many vaccines made and stored in a refrigerator somewhere. But between now and December, there’s absolutely no way you can show efficacy of the vaccine at the same time you confirm that it’s safe.”
“Take these things with a grain of salt”
Animal testing for the AstraZeneca candidate, made in partnership with the University of Oxford in the United Kingdom, has yielded lackluster results, according to results on the preprint server BioRxiv, which have not been peer-reviewed.
“The results were not bad, but they were not gangbusters,” Bottazzi said. The results show the vaccine offered only partial protection.
“Partial protection is better than no protection,” she noted. “You have to take these things with a grain of salt. We don’t know what’s going to happen in humans.”
As for the Moderna candidate, Bottazzi said, “the good news is they found an appropriate safety profile. But from an eight-person group to make the extrapolation that they have efficacy — it’s unrealistic.”
Nicole Lurie, MD, MSPH, is senior adviser to the CEO for the Coalition for Epidemic Preparedness Innovation (CEPI), a nongovernmental organization funded by the Wellcome Trust, the Bill and Melinda Gates Foundation, the European Commission, and eight countries (Australia, Belgium, Canada, Ethiopia, Germany, Japan, Norway, and the United Kingdom) charged with supporting development of vaccines for pathogens on WHO’s priority list.
She and her colleagues write in a paper published online in the New England Journal of Medicine on March 30 that “it typically takes multiple candidates and many years to produce a licensed vaccine.”
The fastest time for developing a vaccine to date is 4 years, for the mumps vaccine, licensed in 1967.
As to whether she would expect a rollout of any vaccine by the end of the year, Lurie told Medscape Medical News, “If everything goes according to plan in every way, shape or form, well then maybe you can get there. But I wouldn’t hold my breath.”
Lurie and her colleagues write that “it’s far from certain that these new platforms will be scalable or that existing capacity can provide sufficient quantities of vaccine fast enough.”
On a call with reporters today, leaders of some of the words largest pharmaceutical companies said that one of the key bottlenecks is the sheer number of vials needed in order to distribute billions of doses of a successful vaccine.
Pfizer CEO Albert Bourla, DVM, PhD, said, “Typically we are producing vaccines in single-dose vials. We are exploring with governments right now if it would be more convenient if there were 5-dose vials or 10-dose vials. I think we can resolve a significant part of the bottleneck.”
Despite the challenges, experts interviewed for this article agree that it will be possible to make a vaccine for COVID-19. They don’t expect attempts to meet the same complications that HIV researchers have seen over decades as the virus continues to confound with mutations.
Fred Ledley, MD, director of the Center for Integration of Science and Industry at Bentley University in Waltham, Massachusetts, told Medscape Medical News, “There doesn’t appear to be anything terribly diabolical about this virus. The mutation rate doesn’t appear to be anything like HIV. It appears to have some big, ugly proteins on the surface, which is good for vaccines — proteins with a lot of physical features look distinguishable from healthy cells. Signs all point to that it should be possible to make a vaccine.”
History raises safety concerns
However, Ledley said, “The idea of doing it in 6 months is largely unrealistic.”
He says 18 months is more realistic, primarily because of the sheer number of people that would have to be enrolled in a phase 3 study to truly test whether the endpoints are being met.
Vaccines are given to healthy volunteers. If safety signals arise, they may not be apparent until massive numbers of people are tested in phase 3.
“You’re never going to see the rates cut to 0%, but to see the difference between 10 people getting sick and seven people getting sick, takes very, very large numbers,” Ledley said. “There’s no way that can be done in 6 months. You’re talking about tens of thousands of people enrolled.”
He notes at this point it’s unclear what the endpoints will be and what the safety thresholds will be after consideration of risks and benefit.
Another big question for Ledley: “We don’t know what type of immunity we need to protect us against the virus. Do you just need the antibodies in your blood or do you need cells that are primed to attack the virus? Is it more of a chemical clearance or do the cells need to physically go in and digest the virus?”
History also points to the need for rigorous safety precautions that scientists fear could be compromised as trial phases overlap and processes are run in parallel instead of one step at a time.
An early batch of the Salk vaccine for polio in 1955, for example, turned out to be contaminated and caused paralysis in some children and 10 deaths, he points out.
CEPI’s Lurie adds that early candidates for another coronavirus, severe acute respiratory syndrome (SARS), “caused a reaction in the lungs that was very dangerous” before development was halted.
She also pointed to previous findings that a vaccine for dengue fever could worsen the disease in some people through a phenomenon called antibody-dependent enhancement.
Lurie and colleagues write in their paper that “it’s critical that vaccines also be developed using the tried-and-true methods, even if they may take longer to enter clinical trials or to result in large numbers of doses.”
Live attenuated vaccine
Raul Andino, PhD, a virologist at the University of California San Francisco, is among the scientists working with a tried-and-true method — a live attenuated vaccine — and he told Medscape Medical News he’s predicting it will take 2 years to develop.
He said it is cheaper to produce because scientists just have to learn how to grow the virus. Because the technology is already proven, a live attenuated vaccine could be rapidly produced on a worldwide scale.
The hope is also that a live attenuated vaccine would be given once in a lifetime and therefore be more affordable, especially in poorer countries.
“While a Moderna vaccine might be good for Europe and the United States,” he said, “It’s not going to be good for Africa, India, Brazil.”
Andino said, “I would bet money” that the front-runner vaccines so far will not be one-time vaccines.
He points out that most of the vaccine candidates are trying to protect people from disease. While there’s nothing wrong with that, he said, “In my opinion that is the lower-hanging fruit.”
“In my mind we need something that interrupts the chain of transmission and induces protection,” Andino said, important for developing herd immunity.
The reason this type of approach takes longer is because you are introducing a weakened form of the virus to the body and you have to make sure it doesn’t cause disease, not just in a small test population, but in populations who may be more susceptible to the disease, Andino said.
A call for unified strategies
Universities, countries, international consortiums, and public-private partnerships are all racing to find several safe and effective vaccines as no one entity will likely be able to provide the global solution.
Some of the efforts involve overlap of entities but with different focuses.
Along with “Operation Warp Speed” and CEPI, other collaborations include Gavi the Vaccine Alliance, whose core partners include WHO, UNICEF, the World Bank, and the Gates Foundation; and “Accelerating Therapeutic Interventions and Vaccines (ACTIV) partnership,” led by the National Institutes of Health.
Industry partners in ACTIV (18 biopharmaceutical companies), according to a May 18 article published online in the Journal of the American Medical Association, have said they will contribute their respective clinical trial capacities, regardless of which agent is studied.
Some, however, have called for more streamlining of efforts.
“Ideally we’d be working together,” Lurie told Medscape Medical News.
“I’m hopeful we will find ways to collaborate scientifically,” she said. “The US government’s responsibility is to make doses for the US. CEPI’s responsibility is to make doses for the world. A big focus of CEPI is to make sure we have manufacturing capacity outside of the US so those doses can be available to the world and they don’t get seized by wealthy countries.”
Bottazzi, Ledley, Lurie, and Andino report no relevant financial relationships.
This article first appeared on Medscape.com.
US government and industry projections that a COVID-19 vaccine will be ready by this fall or even January would take compressing what usually takes at least a decade into months, with little room for error or safety surprises.
“If all the cards fall into the right place and all the stars are aligned, you definitely could get a vaccine by December or January,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said last week.
But Fauci said a more realistic timeline is still 12 to 18 months, and experts interviewed by Medscape Medical News agree. They say that although recent developments are encouraging, history and scientific reason say the day when a COVID-19 vaccine is widely available will not come this year and may not come by the end of 2021.
The encouraging signals come primarily from two recent announcements: the $1.2 billion United States backing last week of one vaccine platform and the announcement on May 18 that the first human trials of another have produced some positive phase 1 results.
Recent developments
On May 21, the US Department of Health and Human Services (HHS) under “Operation Warp Speed” announced that the US will give AstraZeneca $1.2 billion “to make available at least 300 million doses of a coronavirus vaccine called AZD1222, with the first doses delivered as early as October 2020.”
On May 18, the Massachusetts-based biotechnology company Moderna announced that phase 1 clinical results showed that its vaccine candidate, which uses a new messenger RNA (mRNA) technology, appeared safe. Eight participants in the human trials were able to produce neutralizing antibodies that researchers believe are important in developing protection from the virus.
Moderna Chief Medical Officer Tal Zaks, MD, PhD told CNN that if the vaccine candidate does well in phase 2, “it could be ready by January 2021.”
The two candidates are among 10 in clinical trials for the SARS-CoV-2 virus, according to the World Health Organization (WHO). The AstraZeneca/ AZD1222 candidate (also called ChAdOx1 nCoV-19, in collaboration with the University of Oxford) has entered phase 2/3.
Moderna’s candidate and another being developed in Beijing, China, are in phase 2, WHO reports. As of yesterday, 115 other candidates are in preclinical evaluation.
Maria Elena Bottazzi, PhD, associate dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, Texas, told Medscape Medical News it’s important to realize that, in the case of the $1.2 billion US investment, “what they’re talking about is manufacturing.”
The idea, she said, is to pay AstraZeneca up front so that manufacturing can start before it is known whether the vaccine candidate is safe or effective, the reverse of how the clinical trial process usually works.
That way, if the candidate is deemed safe and effective, time is not lost by then deciding how to make it and distribute it.
By the end of this year, she said, “Maybe we will have many vaccines made and stored in a refrigerator somewhere. But between now and December, there’s absolutely no way you can show efficacy of the vaccine at the same time you confirm that it’s safe.”
“Take these things with a grain of salt”
Animal testing for the AstraZeneca candidate, made in partnership with the University of Oxford in the United Kingdom, has yielded lackluster results, according to results on the preprint server BioRxiv, which have not been peer-reviewed.
“The results were not bad, but they were not gangbusters,” Bottazzi said. The results show the vaccine offered only partial protection.
“Partial protection is better than no protection,” she noted. “You have to take these things with a grain of salt. We don’t know what’s going to happen in humans.”
As for the Moderna candidate, Bottazzi said, “the good news is they found an appropriate safety profile. But from an eight-person group to make the extrapolation that they have efficacy — it’s unrealistic.”
Nicole Lurie, MD, MSPH, is senior adviser to the CEO for the Coalition for Epidemic Preparedness Innovation (CEPI), a nongovernmental organization funded by the Wellcome Trust, the Bill and Melinda Gates Foundation, the European Commission, and eight countries (Australia, Belgium, Canada, Ethiopia, Germany, Japan, Norway, and the United Kingdom) charged with supporting development of vaccines for pathogens on WHO’s priority list.
She and her colleagues write in a paper published online in the New England Journal of Medicine on March 30 that “it typically takes multiple candidates and many years to produce a licensed vaccine.”
The fastest time for developing a vaccine to date is 4 years, for the mumps vaccine, licensed in 1967.
As to whether she would expect a rollout of any vaccine by the end of the year, Lurie told Medscape Medical News, “If everything goes according to plan in every way, shape or form, well then maybe you can get there. But I wouldn’t hold my breath.”
Lurie and her colleagues write that “it’s far from certain that these new platforms will be scalable or that existing capacity can provide sufficient quantities of vaccine fast enough.”
On a call with reporters today, leaders of some of the words largest pharmaceutical companies said that one of the key bottlenecks is the sheer number of vials needed in order to distribute billions of doses of a successful vaccine.
Pfizer CEO Albert Bourla, DVM, PhD, said, “Typically we are producing vaccines in single-dose vials. We are exploring with governments right now if it would be more convenient if there were 5-dose vials or 10-dose vials. I think we can resolve a significant part of the bottleneck.”
Despite the challenges, experts interviewed for this article agree that it will be possible to make a vaccine for COVID-19. They don’t expect attempts to meet the same complications that HIV researchers have seen over decades as the virus continues to confound with mutations.
Fred Ledley, MD, director of the Center for Integration of Science and Industry at Bentley University in Waltham, Massachusetts, told Medscape Medical News, “There doesn’t appear to be anything terribly diabolical about this virus. The mutation rate doesn’t appear to be anything like HIV. It appears to have some big, ugly proteins on the surface, which is good for vaccines — proteins with a lot of physical features look distinguishable from healthy cells. Signs all point to that it should be possible to make a vaccine.”
History raises safety concerns
However, Ledley said, “The idea of doing it in 6 months is largely unrealistic.”
He says 18 months is more realistic, primarily because of the sheer number of people that would have to be enrolled in a phase 3 study to truly test whether the endpoints are being met.
Vaccines are given to healthy volunteers. If safety signals arise, they may not be apparent until massive numbers of people are tested in phase 3.
“You’re never going to see the rates cut to 0%, but to see the difference between 10 people getting sick and seven people getting sick, takes very, very large numbers,” Ledley said. “There’s no way that can be done in 6 months. You’re talking about tens of thousands of people enrolled.”
He notes at this point it’s unclear what the endpoints will be and what the safety thresholds will be after consideration of risks and benefit.
Another big question for Ledley: “We don’t know what type of immunity we need to protect us against the virus. Do you just need the antibodies in your blood or do you need cells that are primed to attack the virus? Is it more of a chemical clearance or do the cells need to physically go in and digest the virus?”
History also points to the need for rigorous safety precautions that scientists fear could be compromised as trial phases overlap and processes are run in parallel instead of one step at a time.
An early batch of the Salk vaccine for polio in 1955, for example, turned out to be contaminated and caused paralysis in some children and 10 deaths, he points out.
CEPI’s Lurie adds that early candidates for another coronavirus, severe acute respiratory syndrome (SARS), “caused a reaction in the lungs that was very dangerous” before development was halted.
She also pointed to previous findings that a vaccine for dengue fever could worsen the disease in some people through a phenomenon called antibody-dependent enhancement.
Lurie and colleagues write in their paper that “it’s critical that vaccines also be developed using the tried-and-true methods, even if they may take longer to enter clinical trials or to result in large numbers of doses.”
Live attenuated vaccine
Raul Andino, PhD, a virologist at the University of California San Francisco, is among the scientists working with a tried-and-true method — a live attenuated vaccine — and he told Medscape Medical News he’s predicting it will take 2 years to develop.
He said it is cheaper to produce because scientists just have to learn how to grow the virus. Because the technology is already proven, a live attenuated vaccine could be rapidly produced on a worldwide scale.
The hope is also that a live attenuated vaccine would be given once in a lifetime and therefore be more affordable, especially in poorer countries.
“While a Moderna vaccine might be good for Europe and the United States,” he said, “It’s not going to be good for Africa, India, Brazil.”
Andino said, “I would bet money” that the front-runner vaccines so far will not be one-time vaccines.
He points out that most of the vaccine candidates are trying to protect people from disease. While there’s nothing wrong with that, he said, “In my opinion that is the lower-hanging fruit.”
“In my mind we need something that interrupts the chain of transmission and induces protection,” Andino said, important for developing herd immunity.
The reason this type of approach takes longer is because you are introducing a weakened form of the virus to the body and you have to make sure it doesn’t cause disease, not just in a small test population, but in populations who may be more susceptible to the disease, Andino said.
A call for unified strategies
Universities, countries, international consortiums, and public-private partnerships are all racing to find several safe and effective vaccines as no one entity will likely be able to provide the global solution.
Some of the efforts involve overlap of entities but with different focuses.
Along with “Operation Warp Speed” and CEPI, other collaborations include Gavi the Vaccine Alliance, whose core partners include WHO, UNICEF, the World Bank, and the Gates Foundation; and “Accelerating Therapeutic Interventions and Vaccines (ACTIV) partnership,” led by the National Institutes of Health.
Industry partners in ACTIV (18 biopharmaceutical companies), according to a May 18 article published online in the Journal of the American Medical Association, have said they will contribute their respective clinical trial capacities, regardless of which agent is studied.
Some, however, have called for more streamlining of efforts.
“Ideally we’d be working together,” Lurie told Medscape Medical News.
“I’m hopeful we will find ways to collaborate scientifically,” she said. “The US government’s responsibility is to make doses for the US. CEPI’s responsibility is to make doses for the world. A big focus of CEPI is to make sure we have manufacturing capacity outside of the US so those doses can be available to the world and they don’t get seized by wealthy countries.”
Bottazzi, Ledley, Lurie, and Andino report no relevant financial relationships.
This article first appeared on Medscape.com.
COVID-19: Putting distance between projection and reality
When it comes to COVID-19, studies show that social distancing flattened the curve.
Cumulative hospitalizations in four states with stay-at-home orders were well short of the projected exponential growth curves, Soumya Sen, PhD, of the University of Minnesota, Minneapolis, and associates reported May 27 in a research letter in JAMA. All states were observed through April 28.
The deviations between observed cases and worst-case projections in the four states – Colorado, Minnesota, Ohio, and Virginia – all began within 8-10 days of the stay-at-home orders. In Minnesota, 17 days after the order, there were 361 cumulative hospitalizations, compared with a projection of 988 had no such action been taken. In Virginia, the corresponding numbers were 1,048 observed and 2,335 projected, they reported.
“Observed hospitalizations consistently fell outside of the 95% prediction bands of the projected exponential growth curve,” Dr. Sen and associates noted.
In a separate Canadian study measuring COVID-19 patients occupying ICU beds in Ontario and deaths among those cases, hospitals “would have rapidly exceeded ICU capacity and observed substantially higher mortality” without any physical distancing intervention, Ashleigh R. Tuite, PhD, MPH, of the University of Toronto and associates wrote May 27 in a letter in Annals of Internal Medicine.
Their model, based on a 70% reduction in physical contacts for March 19–May 3, projected 2.0 cases per 100,000 population with physical distancing and 37.4 per 100,000 without. Deaths among those ICU patients were projected at 2.5 per 100,000 with distancing and 12.7 per 100,000 without intervention, they reported.
“Our modeling also shows the challenges associated with relaxation of physical distancing measures without a concomitant increase in other public health measures. Specifically, when the number of contacts between persons returns to more than 50% of normal, we expect disease activity to resurge rapidly and ICUs to quickly reach capacity,” they wrote.
The study published in JAMA used publicly available data from the University of Minnesota COVID-19 Hospitalization Project, which is partially funded by the University of Minnesota Office of Academic Clinical Affairs and United Health Foundation.
SOURCES: Sen S et al. JAMA. 2020 May 27. doi: 10.1001/jama.2020.9176; Tuite AR et al. Ann Intern Med. 2020 May 27. doi: 10.7326/M20-2945.
When it comes to COVID-19, studies show that social distancing flattened the curve.

Cumulative hospitalizations in four states with stay-at-home orders were well short of the projected exponential growth curves, Soumya Sen, PhD, of the University of Minnesota, Minneapolis, and associates reported May 27 in a research letter in JAMA. All states were observed through April 28.
The deviations between observed cases and worst-case projections in the four states – Colorado, Minnesota, Ohio, and Virginia – all began within 8-10 days of the stay-at-home orders. In Minnesota, 17 days after the order, there were 361 cumulative hospitalizations, compared with a projection of 988 had no such action been taken. In Virginia, the corresponding numbers were 1,048 observed and 2,335 projected, they reported.
“Observed hospitalizations consistently fell outside of the 95% prediction bands of the projected exponential growth curve,” Dr. Sen and associates noted.
In a separate Canadian study measuring COVID-19 patients occupying ICU beds in Ontario and deaths among those cases, hospitals “would have rapidly exceeded ICU capacity and observed substantially higher mortality” without any physical distancing intervention, Ashleigh R. Tuite, PhD, MPH, of the University of Toronto and associates wrote May 27 in a letter in Annals of Internal Medicine.
Their model, based on a 70% reduction in physical contacts for March 19–May 3, projected 2.0 cases per 100,000 population with physical distancing and 37.4 per 100,000 without. Deaths among those ICU patients were projected at 2.5 per 100,000 with distancing and 12.7 per 100,000 without intervention, they reported.
“Our modeling also shows the challenges associated with relaxation of physical distancing measures without a concomitant increase in other public health measures. Specifically, when the number of contacts between persons returns to more than 50% of normal, we expect disease activity to resurge rapidly and ICUs to quickly reach capacity,” they wrote.
The study published in JAMA used publicly available data from the University of Minnesota COVID-19 Hospitalization Project, which is partially funded by the University of Minnesota Office of Academic Clinical Affairs and United Health Foundation.
SOURCES: Sen S et al. JAMA. 2020 May 27. doi: 10.1001/jama.2020.9176; Tuite AR et al. Ann Intern Med. 2020 May 27. doi: 10.7326/M20-2945.
When it comes to COVID-19, studies show that social distancing flattened the curve.

Cumulative hospitalizations in four states with stay-at-home orders were well short of the projected exponential growth curves, Soumya Sen, PhD, of the University of Minnesota, Minneapolis, and associates reported May 27 in a research letter in JAMA. All states were observed through April 28.
The deviations between observed cases and worst-case projections in the four states – Colorado, Minnesota, Ohio, and Virginia – all began within 8-10 days of the stay-at-home orders. In Minnesota, 17 days after the order, there were 361 cumulative hospitalizations, compared with a projection of 988 had no such action been taken. In Virginia, the corresponding numbers were 1,048 observed and 2,335 projected, they reported.
“Observed hospitalizations consistently fell outside of the 95% prediction bands of the projected exponential growth curve,” Dr. Sen and associates noted.
In a separate Canadian study measuring COVID-19 patients occupying ICU beds in Ontario and deaths among those cases, hospitals “would have rapidly exceeded ICU capacity and observed substantially higher mortality” without any physical distancing intervention, Ashleigh R. Tuite, PhD, MPH, of the University of Toronto and associates wrote May 27 in a letter in Annals of Internal Medicine.
Their model, based on a 70% reduction in physical contacts for March 19–May 3, projected 2.0 cases per 100,000 population with physical distancing and 37.4 per 100,000 without. Deaths among those ICU patients were projected at 2.5 per 100,000 with distancing and 12.7 per 100,000 without intervention, they reported.
“Our modeling also shows the challenges associated with relaxation of physical distancing measures without a concomitant increase in other public health measures. Specifically, when the number of contacts between persons returns to more than 50% of normal, we expect disease activity to resurge rapidly and ICUs to quickly reach capacity,” they wrote.
The study published in JAMA used publicly available data from the University of Minnesota COVID-19 Hospitalization Project, which is partially funded by the University of Minnesota Office of Academic Clinical Affairs and United Health Foundation.
SOURCES: Sen S et al. JAMA. 2020 May 27. doi: 10.1001/jama.2020.9176; Tuite AR et al. Ann Intern Med. 2020 May 27. doi: 10.7326/M20-2945.
Active cancer increases death risk in patients with COVID-19
Patients with COVID-19 and progressing cancer had a fivefold increase in the risk of 30-day mortality, compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer, according to data from the COVID-19 and Cancer Consortium (CCC19) registry.
Other independent risk factors for death in patients with COVID-19 and cancer were older age, male sex, former smoking, number of comorbidities, Eastern Cooperative Oncology Group (ECOG) performance status of 2 or greater, and treatment with hydroxychloroquine plus azithromycin.
In fact, patients who received hydroxychloroquine and azithromycin had a nearly threefold higher risk of death than did patients who had not received the combination. However, this finding was of “uncertain validity due to a high risk of residual confounding; for example, patients receiving this combination were more likely to have severe disease or more likely to be hospitalized,” said Jeremy L. Warner, MD, of Vanderbilt University Medical Center in Nashville, Tennessee.
Dr. Warner presented these findings in an online press briefing. Additional findings from the CCC19 registry are set to be presented as part of the American Society of Clinical Oncology (ASCO) virtual scientific program. The findings were also published in The Lancet.
‘Severe impact’ in cancer patients
“For people with cancer, the impact of COVID-19 is especially severe, whether they have been exposed to the virus or not. Patients with cancer are typically older adults, often with other underlying conditions, and their immune systems may be suppressed by the cancer, or due to chemotherapy, radiation, or other treatment,” commented ASCO President Howard A. Burris III, MD, who moderated the press briefing but was not involved in the study of CCC19 registry data.
“ASCO members tell us that they have had to delay or modify treatment plans to reduce patients’ risk of infection, and we’re unclear what the impact of these changes will be. Delays in cancer screening and diagnosis are also a major concern,” Dr. Burris continued.
“This does confirm reports that have come out from other centers, including other parts of the world, where they have found that people who have cancer and COVID-19 have a worse outcome,” said Andrew T. Chan, MD, MPH, of Massachusetts General Hospital in Boston, who was not involved in the research.
Dr. Chan’s group has developed a COVID-19 symptom study app with the aim of defining whether people living with cancer are at increased risk for infections, in addition to whether cancer is an independent risk factor for COVID-19 severity or mortality.
“Using data from our app, we were able to show that people who reported living with cancer did have a higher risk of developing COVID and were more likely to be hospitalized related to COVID,” Dr. Chan said in an interview.
Study details
The CCC19 registry collects information from 104 participating institutions in the United States and Canada, as well as anonymous data from individuals in the United States, Argentina, Canada, the European Union, and the United Kingdom.
The sample of 928 patients Dr. Warner presented was evenly balanced by sex. The median age was 66 years, and 30% of patients were aged 75 years or older.
In all, 39% of patients were on active anticancer therapy, and 43% had measurable disease. Breast cancer was the most common diagnosis, followed by prostate cancer, gastrointestinal cancers, lymphomas, and thoracic cancers.
Two-thirds of the patients (68%) had an ECOG performance status of 0 or 1, 8% had a performance status of 2, and 5% a status of 3 or 4. The remaining patients had unknown performance status.
Slightly more than half of patients (52%) were never smokers, 37% were former smokers, and 5% were current smokers. The remaining 6% of patients had unknown smoking status.
At a median follow-up of 21 days, 121 patients (13%) had died. All deaths occurred within 30 days of COVID-19 diagnosis. Among patients who died, 78 were male, 64 were former smokers, 70 were aged 75 years or older, 41 had active stable or responding cancer, 25 had progressing cancer, and 42 had an ECOG performance status of 2 or higher.
In all, 466 patients were hospitalized, and 106 in this group (23%) died. Among the 132 patients admitted to an ICU, 50 (38%) died, including 27 patients aged 75 years or older, and 15 with an ECOG performance status of 2 or greater. Of the 116 patients who required intubation, 50 (43%) died, including 26 who were 75 years or older, and 11 who had a performance status of 2 or greater.
It’s early days yet, and a larger sample size with longer follow-up will be needed to get a more complete picture of how COVID-19 affects specific patient subsets over time, Dr. Warner said.
ASCO has established its own COVID-19 registry to collect both near-term and longitudinal data during the pandemic.
“We’ll be able to learn about both how the pandemic has impacted delivery of cancer care, as well as the longer-term effects of COVID-19 on cancer patients and understand what care approaches are working best,” said Richard L. Schilsky, MD, chief medical officer and executive vice president of ASCO, during the briefing.
The study of CCC19 registry data was supported in part by the National Institutes of Health and the American Cancer Society. Dr. Warner disclosed stock/ownership in HemOnc.org, consulting for IBM and Westat, and travel expenses from IBM. Dr. Burris, Dr. Schilsky, and Dr. Chan reported no disclosures relevant to the study.
SOURCE: Warner J L et al. ASCO 2020, Abstract LBA110.
Patients with COVID-19 and progressing cancer had a fivefold increase in the risk of 30-day mortality, compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer, according to data from the COVID-19 and Cancer Consortium (CCC19) registry.
Other independent risk factors for death in patients with COVID-19 and cancer were older age, male sex, former smoking, number of comorbidities, Eastern Cooperative Oncology Group (ECOG) performance status of 2 or greater, and treatment with hydroxychloroquine plus azithromycin.
In fact, patients who received hydroxychloroquine and azithromycin had a nearly threefold higher risk of death than did patients who had not received the combination. However, this finding was of “uncertain validity due to a high risk of residual confounding; for example, patients receiving this combination were more likely to have severe disease or more likely to be hospitalized,” said Jeremy L. Warner, MD, of Vanderbilt University Medical Center in Nashville, Tennessee.
Dr. Warner presented these findings in an online press briefing. Additional findings from the CCC19 registry are set to be presented as part of the American Society of Clinical Oncology (ASCO) virtual scientific program. The findings were also published in The Lancet.
‘Severe impact’ in cancer patients
“For people with cancer, the impact of COVID-19 is especially severe, whether they have been exposed to the virus or not. Patients with cancer are typically older adults, often with other underlying conditions, and their immune systems may be suppressed by the cancer, or due to chemotherapy, radiation, or other treatment,” commented ASCO President Howard A. Burris III, MD, who moderated the press briefing but was not involved in the study of CCC19 registry data.
“ASCO members tell us that they have had to delay or modify treatment plans to reduce patients’ risk of infection, and we’re unclear what the impact of these changes will be. Delays in cancer screening and diagnosis are also a major concern,” Dr. Burris continued.
“This does confirm reports that have come out from other centers, including other parts of the world, where they have found that people who have cancer and COVID-19 have a worse outcome,” said Andrew T. Chan, MD, MPH, of Massachusetts General Hospital in Boston, who was not involved in the research.
Dr. Chan’s group has developed a COVID-19 symptom study app with the aim of defining whether people living with cancer are at increased risk for infections, in addition to whether cancer is an independent risk factor for COVID-19 severity or mortality.
“Using data from our app, we were able to show that people who reported living with cancer did have a higher risk of developing COVID and were more likely to be hospitalized related to COVID,” Dr. Chan said in an interview.
Study details
The CCC19 registry collects information from 104 participating institutions in the United States and Canada, as well as anonymous data from individuals in the United States, Argentina, Canada, the European Union, and the United Kingdom.
The sample of 928 patients Dr. Warner presented was evenly balanced by sex. The median age was 66 years, and 30% of patients were aged 75 years or older.
In all, 39% of patients were on active anticancer therapy, and 43% had measurable disease. Breast cancer was the most common diagnosis, followed by prostate cancer, gastrointestinal cancers, lymphomas, and thoracic cancers.
Two-thirds of the patients (68%) had an ECOG performance status of 0 or 1, 8% had a performance status of 2, and 5% a status of 3 or 4. The remaining patients had unknown performance status.
Slightly more than half of patients (52%) were never smokers, 37% were former smokers, and 5% were current smokers. The remaining 6% of patients had unknown smoking status.
At a median follow-up of 21 days, 121 patients (13%) had died. All deaths occurred within 30 days of COVID-19 diagnosis. Among patients who died, 78 were male, 64 were former smokers, 70 were aged 75 years or older, 41 had active stable or responding cancer, 25 had progressing cancer, and 42 had an ECOG performance status of 2 or higher.
In all, 466 patients were hospitalized, and 106 in this group (23%) died. Among the 132 patients admitted to an ICU, 50 (38%) died, including 27 patients aged 75 years or older, and 15 with an ECOG performance status of 2 or greater. Of the 116 patients who required intubation, 50 (43%) died, including 26 who were 75 years or older, and 11 who had a performance status of 2 or greater.
It’s early days yet, and a larger sample size with longer follow-up will be needed to get a more complete picture of how COVID-19 affects specific patient subsets over time, Dr. Warner said.
ASCO has established its own COVID-19 registry to collect both near-term and longitudinal data during the pandemic.
“We’ll be able to learn about both how the pandemic has impacted delivery of cancer care, as well as the longer-term effects of COVID-19 on cancer patients and understand what care approaches are working best,” said Richard L. Schilsky, MD, chief medical officer and executive vice president of ASCO, during the briefing.
The study of CCC19 registry data was supported in part by the National Institutes of Health and the American Cancer Society. Dr. Warner disclosed stock/ownership in HemOnc.org, consulting for IBM and Westat, and travel expenses from IBM. Dr. Burris, Dr. Schilsky, and Dr. Chan reported no disclosures relevant to the study.
SOURCE: Warner J L et al. ASCO 2020, Abstract LBA110.
Patients with COVID-19 and progressing cancer had a fivefold increase in the risk of 30-day mortality, compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer, according to data from the COVID-19 and Cancer Consortium (CCC19) registry.
Other independent risk factors for death in patients with COVID-19 and cancer were older age, male sex, former smoking, number of comorbidities, Eastern Cooperative Oncology Group (ECOG) performance status of 2 or greater, and treatment with hydroxychloroquine plus azithromycin.
In fact, patients who received hydroxychloroquine and azithromycin had a nearly threefold higher risk of death than did patients who had not received the combination. However, this finding was of “uncertain validity due to a high risk of residual confounding; for example, patients receiving this combination were more likely to have severe disease or more likely to be hospitalized,” said Jeremy L. Warner, MD, of Vanderbilt University Medical Center in Nashville, Tennessee.
Dr. Warner presented these findings in an online press briefing. Additional findings from the CCC19 registry are set to be presented as part of the American Society of Clinical Oncology (ASCO) virtual scientific program. The findings were also published in The Lancet.
‘Severe impact’ in cancer patients
“For people with cancer, the impact of COVID-19 is especially severe, whether they have been exposed to the virus or not. Patients with cancer are typically older adults, often with other underlying conditions, and their immune systems may be suppressed by the cancer, or due to chemotherapy, radiation, or other treatment,” commented ASCO President Howard A. Burris III, MD, who moderated the press briefing but was not involved in the study of CCC19 registry data.
“ASCO members tell us that they have had to delay or modify treatment plans to reduce patients’ risk of infection, and we’re unclear what the impact of these changes will be. Delays in cancer screening and diagnosis are also a major concern,” Dr. Burris continued.
“This does confirm reports that have come out from other centers, including other parts of the world, where they have found that people who have cancer and COVID-19 have a worse outcome,” said Andrew T. Chan, MD, MPH, of Massachusetts General Hospital in Boston, who was not involved in the research.
Dr. Chan’s group has developed a COVID-19 symptom study app with the aim of defining whether people living with cancer are at increased risk for infections, in addition to whether cancer is an independent risk factor for COVID-19 severity or mortality.
“Using data from our app, we were able to show that people who reported living with cancer did have a higher risk of developing COVID and were more likely to be hospitalized related to COVID,” Dr. Chan said in an interview.
Study details
The CCC19 registry collects information from 104 participating institutions in the United States and Canada, as well as anonymous data from individuals in the United States, Argentina, Canada, the European Union, and the United Kingdom.
The sample of 928 patients Dr. Warner presented was evenly balanced by sex. The median age was 66 years, and 30% of patients were aged 75 years or older.
In all, 39% of patients were on active anticancer therapy, and 43% had measurable disease. Breast cancer was the most common diagnosis, followed by prostate cancer, gastrointestinal cancers, lymphomas, and thoracic cancers.
Two-thirds of the patients (68%) had an ECOG performance status of 0 or 1, 8% had a performance status of 2, and 5% a status of 3 or 4. The remaining patients had unknown performance status.
Slightly more than half of patients (52%) were never smokers, 37% were former smokers, and 5% were current smokers. The remaining 6% of patients had unknown smoking status.
At a median follow-up of 21 days, 121 patients (13%) had died. All deaths occurred within 30 days of COVID-19 diagnosis. Among patients who died, 78 were male, 64 were former smokers, 70 were aged 75 years or older, 41 had active stable or responding cancer, 25 had progressing cancer, and 42 had an ECOG performance status of 2 or higher.
In all, 466 patients were hospitalized, and 106 in this group (23%) died. Among the 132 patients admitted to an ICU, 50 (38%) died, including 27 patients aged 75 years or older, and 15 with an ECOG performance status of 2 or greater. Of the 116 patients who required intubation, 50 (43%) died, including 26 who were 75 years or older, and 11 who had a performance status of 2 or greater.
It’s early days yet, and a larger sample size with longer follow-up will be needed to get a more complete picture of how COVID-19 affects specific patient subsets over time, Dr. Warner said.
ASCO has established its own COVID-19 registry to collect both near-term and longitudinal data during the pandemic.
“We’ll be able to learn about both how the pandemic has impacted delivery of cancer care, as well as the longer-term effects of COVID-19 on cancer patients and understand what care approaches are working best,” said Richard L. Schilsky, MD, chief medical officer and executive vice president of ASCO, during the briefing.
The study of CCC19 registry data was supported in part by the National Institutes of Health and the American Cancer Society. Dr. Warner disclosed stock/ownership in HemOnc.org, consulting for IBM and Westat, and travel expenses from IBM. Dr. Burris, Dr. Schilsky, and Dr. Chan reported no disclosures relevant to the study.
SOURCE: Warner J L et al. ASCO 2020, Abstract LBA110.
FROM ASCO 2020
Key clinical point: Patients with progressing cancer and COVID-19 are at an especially high risk of 30-day mortality.
Major finding: Patients with COVID-19 whose cancers were progressing had a fivefold increase in the risk of 30-day mortality, compared with COVID-19–positive cancer patients in remission or with no evidence of cancer.
Study details: Analysis of data on 928 patients enrolled in the COVID-19 and Cancer Consortium (CCC19) registry.
Disclosures: The research was supported, in part, by the National Institutes of Health and the American Cancer Society. Dr. Warner disclosed relationships with HemOnc.org, IBM, and Westat.
Source: Warner J L et al. ASCO 2020, Abstract LBA110.
Today’s top news highlights: Coping with addiction during COVID, lung rehab part of recovery
Here are the stories our MDedge editors across specialties think you need to know about today:
Long road to recovery includes lung rehab
For seriously ill COVID-19 patients, there may a long recovery period even after leaving the intensive care unit. Eladio (“Lad”) Braganza, age 77, is one of those patients. For 28 days, he was on a ventilator in a Seattle ICU. Now – after a 46-day hospitalization for SARS-CoV-2 infection – he’s making progress in inpatient rehab. “The vast majority of COVID patients in the ICU have lung disease that is quite severe, much more severe than I have seen in my 20 years of doing this,” said critical care specialist Anna Nolan, MD, of the department of medicine at New York University. READ MORE.
Detox unit keeps running during COVID-19
Substance use disorder doesn’t take a break for a pandemic. In fact, the stressors from the current COVID-19 situation have increased substance use. In a commentary published on MDedge, Keji Fagbemi, MD, a hospitalist at the BronxCare Health System, shared how his hospital kept its inpatient detoxification unit running, despite the challenges presented by COVID-19. “At a time when many inpatient detoxification units within the city were temporarily closed due to fear of inpatient spread of the virus or to provide extra COVID beds in anticipation for the peak surge, we have been able to provide a needed service,” he wrote. “In fact, several other inpatient detoxification programs within the city have been able to refer their patients to our facility.” READ MORE.
Air pollution linked to MS risk
Air pollution may be another environmental risk factor for developing multiple sclerosis, suggests new research released as part of the Congress of the European Academy of Neurology (EAN) 2020. The findings, which are based on a large cohort study of nearly 550,000 individuals in Italy, appear to confirm the relationship between exposure to air pollutants and risk for MS that has been shown in prior studies. “Countermeasures that cut air pollution can be important for public health, not only to reduce deaths related to cardiac and pulmonary diseases but also the risk of chronic autoimmune diseases such as MS,” said Roberto Bergamaschi, MD, PhD, director of the Multiple Sclerosis Center, IRCCS Mondino Foundation, Pavia, Italy. READ MORE.
Trials produce conflicting results in Alzheimer’s disease
High-dose aducanumab, a human monoclonal antibody in development for the treatment of Alzheimer’s disease, significantly reduced clinical decline in people with early disease in one randomized, placebo-controlled phase 3 study. But there was no statistically significant change in outcomes in an identical study. “We believe that the difference between the results was largely due to patients’ greater exposure to the high dose of aducanumab,” said Samantha Budd Haeberlein, PhD, one of the study investigators and senior vice president and head of the neurodegeneration development unit at Biogen, which is developing the drug. READ MORE.
Pregnant patients have asymptomatic SARS-CoV-2 infection
The rate of asymptomatic SARS-CoV-2 infection was 16% among women with a planned delivery in a New York City health system during the first half of April, according to recent study results. “If universal testing of pregnant patients in a high prevalence area is not performed, health care workers will be inadvertently exposed to COVID-19, unless universal precautions with personal protective equipment are taken,” researchers wrote in Obstetrics & Gynecology. READ MORE.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Long road to recovery includes lung rehab
For seriously ill COVID-19 patients, there may a long recovery period even after leaving the intensive care unit. Eladio (“Lad”) Braganza, age 77, is one of those patients. For 28 days, he was on a ventilator in a Seattle ICU. Now – after a 46-day hospitalization for SARS-CoV-2 infection – he’s making progress in inpatient rehab. “The vast majority of COVID patients in the ICU have lung disease that is quite severe, much more severe than I have seen in my 20 years of doing this,” said critical care specialist Anna Nolan, MD, of the department of medicine at New York University. READ MORE.
Detox unit keeps running during COVID-19
Substance use disorder doesn’t take a break for a pandemic. In fact, the stressors from the current COVID-19 situation have increased substance use. In a commentary published on MDedge, Keji Fagbemi, MD, a hospitalist at the BronxCare Health System, shared how his hospital kept its inpatient detoxification unit running, despite the challenges presented by COVID-19. “At a time when many inpatient detoxification units within the city were temporarily closed due to fear of inpatient spread of the virus or to provide extra COVID beds in anticipation for the peak surge, we have been able to provide a needed service,” he wrote. “In fact, several other inpatient detoxification programs within the city have been able to refer their patients to our facility.” READ MORE.
Air pollution linked to MS risk
Air pollution may be another environmental risk factor for developing multiple sclerosis, suggests new research released as part of the Congress of the European Academy of Neurology (EAN) 2020. The findings, which are based on a large cohort study of nearly 550,000 individuals in Italy, appear to confirm the relationship between exposure to air pollutants and risk for MS that has been shown in prior studies. “Countermeasures that cut air pollution can be important for public health, not only to reduce deaths related to cardiac and pulmonary diseases but also the risk of chronic autoimmune diseases such as MS,” said Roberto Bergamaschi, MD, PhD, director of the Multiple Sclerosis Center, IRCCS Mondino Foundation, Pavia, Italy. READ MORE.
Trials produce conflicting results in Alzheimer’s disease
High-dose aducanumab, a human monoclonal antibody in development for the treatment of Alzheimer’s disease, significantly reduced clinical decline in people with early disease in one randomized, placebo-controlled phase 3 study. But there was no statistically significant change in outcomes in an identical study. “We believe that the difference between the results was largely due to patients’ greater exposure to the high dose of aducanumab,” said Samantha Budd Haeberlein, PhD, one of the study investigators and senior vice president and head of the neurodegeneration development unit at Biogen, which is developing the drug. READ MORE.
Pregnant patients have asymptomatic SARS-CoV-2 infection
The rate of asymptomatic SARS-CoV-2 infection was 16% among women with a planned delivery in a New York City health system during the first half of April, according to recent study results. “If universal testing of pregnant patients in a high prevalence area is not performed, health care workers will be inadvertently exposed to COVID-19, unless universal precautions with personal protective equipment are taken,” researchers wrote in Obstetrics & Gynecology. READ MORE.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Long road to recovery includes lung rehab
For seriously ill COVID-19 patients, there may a long recovery period even after leaving the intensive care unit. Eladio (“Lad”) Braganza, age 77, is one of those patients. For 28 days, he was on a ventilator in a Seattle ICU. Now – after a 46-day hospitalization for SARS-CoV-2 infection – he’s making progress in inpatient rehab. “The vast majority of COVID patients in the ICU have lung disease that is quite severe, much more severe than I have seen in my 20 years of doing this,” said critical care specialist Anna Nolan, MD, of the department of medicine at New York University. READ MORE.
Detox unit keeps running during COVID-19
Substance use disorder doesn’t take a break for a pandemic. In fact, the stressors from the current COVID-19 situation have increased substance use. In a commentary published on MDedge, Keji Fagbemi, MD, a hospitalist at the BronxCare Health System, shared how his hospital kept its inpatient detoxification unit running, despite the challenges presented by COVID-19. “At a time when many inpatient detoxification units within the city were temporarily closed due to fear of inpatient spread of the virus or to provide extra COVID beds in anticipation for the peak surge, we have been able to provide a needed service,” he wrote. “In fact, several other inpatient detoxification programs within the city have been able to refer their patients to our facility.” READ MORE.
Air pollution linked to MS risk
Air pollution may be another environmental risk factor for developing multiple sclerosis, suggests new research released as part of the Congress of the European Academy of Neurology (EAN) 2020. The findings, which are based on a large cohort study of nearly 550,000 individuals in Italy, appear to confirm the relationship between exposure to air pollutants and risk for MS that has been shown in prior studies. “Countermeasures that cut air pollution can be important for public health, not only to reduce deaths related to cardiac and pulmonary diseases but also the risk of chronic autoimmune diseases such as MS,” said Roberto Bergamaschi, MD, PhD, director of the Multiple Sclerosis Center, IRCCS Mondino Foundation, Pavia, Italy. READ MORE.
Trials produce conflicting results in Alzheimer’s disease
High-dose aducanumab, a human monoclonal antibody in development for the treatment of Alzheimer’s disease, significantly reduced clinical decline in people with early disease in one randomized, placebo-controlled phase 3 study. But there was no statistically significant change in outcomes in an identical study. “We believe that the difference between the results was largely due to patients’ greater exposure to the high dose of aducanumab,” said Samantha Budd Haeberlein, PhD, one of the study investigators and senior vice president and head of the neurodegeneration development unit at Biogen, which is developing the drug. READ MORE.
Pregnant patients have asymptomatic SARS-CoV-2 infection
The rate of asymptomatic SARS-CoV-2 infection was 16% among women with a planned delivery in a New York City health system during the first half of April, according to recent study results. “If universal testing of pregnant patients in a high prevalence area is not performed, health care workers will be inadvertently exposed to COVID-19, unless universal precautions with personal protective equipment are taken,” researchers wrote in Obstetrics & Gynecology. READ MORE.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
New York City inpatient detox unit keeps running: Here’s how
Substance use disorder and its daily consequences take no breaks even during a pandemic. The stressors created by COVID-19, including deaths of loved ones and the disruptions to normal life from policies aimed at flattening the curve, seem to have increased substance use.
I practice as a hospitalist with an internal medicine background and specialty in addiction medicine at BronxCare Health System’s inpatient detoxification unit, a 24/7, 20-bed medically-supervised unit in South Bronx in New York City. It is one of the comprehensive services provided by the BronxCare’s life recovery center and addiction services, which also includes an outpatient clinic, opioid treatment program, inpatient rehab, and a half-way house. Inpatient detoxification units like ours are designed to treat serious addictions and chemical dependency and prevent and treat life-threatening withdrawal symptoms and signs or complications. Our patients come from all over the city and its adjoining suburbs, including from emergency room referrals, referral clinics, courts and the justice system, walk-ins, and self-referrals.
At a time when many inpatient detoxification units within the city were temporarily closed due to fear of inpatient spread of the virus or to provide extra COVID beds in anticipation for the peak surge, we have been able to provide a needed service. In fact, several other inpatient detoxification programs within the city have been able to refer their patients to our facility.
Individuals with substance use disorder have historically been a vulnerable and underserved population and possess high risk for multiple health problems as well as preexisting conditions. Many have limited life options financially, educationally, and with housing, and encounter barriers to accessing primary health care services, including preventive services. The introduction of the COVID-19 pandemic into these patients’ precarious health situations only made things worse as many of the limited resources for patients with substance use disorder were diverted to battling the pandemic. Numerous inpatient and outpatient addiction services, for example, were temporarily shut down. This has led to an increase in domestic violence, and psychiatric decompensation, including psychosis, suicidal attempts, and worsening of medical comorbidities in these patients.
Our wake-up call came when the first case of COVID-19 was confirmed in New York in early March. Within a short period of time the state became the epicenter for COVID-19. With the projection of millions of cases being positive and the number of new cases doubling every third day at the onset in New York City, we knew we had a battle brewing and needed to radically transform our mode of operation fast.
Our first task was to ensure the safety of our patients and the dedicated health workers attending to them. We streamlined the patient point of entry through one screening site, while also brushing up on our history-taking to intently screen for COVID-19. This included not just focusing on travels from China, but from Europe and other parts of the world.
Yes, we did ask patients about cough, fever, shortness of breath or difficulty breathing, feeling fatigued, severe body ache, and possible contact with someone who is sick or has traveled overseas. But we were also attuned to the increased rate of community spread and the presentation of other symptoms, such as loss of taste and smell, early in the process. Hence we were able to triage patients with suspected cases to the appropriate sections of the hospital for further screening, testing, and evaluation, instead of having those patients admitted to the detox unit.
Early in the process a huddle team was instituted with daily briefing of staff lasting 30 minutes or less. This team consists of physicians, nurses, a physician assistant, a social worker, and a counselor. In addition to discussing treatment plans for the patient, they deliberate on the public health information from the hospital’s COVID-19 command center, New York State Department of Health, the Office of Mental Health, and the Centers for Disease Control and Prevention concerning the latest evidence-based information. These discussions have helped us modify our policies and practices.
We instituted a no visiting rule during a short hospital stay of 5-7 days, and this was initiated weeks in advance of many institutions, including nursing homes with vulnerable populations. Our admitting criteria was reviewed to allow for admission of only those patients who absolutely needed inpatient substance use disorder treatment, including patients with severe withdrawal symptoms and signs, comorbidities, or neuropsychiatric manifestations that made them unsafe for outpatient or home detoxification. Others were triaged to the outpatient services which was amply supported with telemedicine. Rooms and designated areas of the building were earmarked as places for isolation/quarantine if suspected COVID-19 cases were identified pending testing. To assess patients’ risk of COVID-19, we do point-of-care nasopharyngeal swab testing with polymerase chain reaction.
Regarding face masks, patients and staff were fitted with ones early in the process. Additionally, staff were trained on the importance of face mask use and how to ensure you have a tight seal around the mouth and nose and were provided with other appropriate personal protective equipment. Concerning social distancing, we reduced the patient population capacity for the unit down to 50% and offered only single room admissions. Social distancing was encouraged in the unit, including in the television and recreation room and dining room, and during small treatment groups of less than six individuals. Daily temperature checks with noncontact handheld thermometers were enforced for staff and anyone coming into the life recovery center.
Patients are continuously being educated on the presentations of COVID-19 and encouraged to report any symptoms. Any staff feeling sick or having symptoms are encouraged to stay home. Rigorous and continuous cleaning of surfaces, especially of areas subjected to common use, is done frequently by the hospital housekeeping and environmental crew and is the order of the day.
Dr. Fagbemi is a hospitalist at BronxCare Health System, a not-for-profit health and teaching hospital system serving South and Central Bronx in New York. He has no conflicts of interest to disclose.
Substance use disorder and its daily consequences take no breaks even during a pandemic. The stressors created by COVID-19, including deaths of loved ones and the disruptions to normal life from policies aimed at flattening the curve, seem to have increased substance use.
I practice as a hospitalist with an internal medicine background and specialty in addiction medicine at BronxCare Health System’s inpatient detoxification unit, a 24/7, 20-bed medically-supervised unit in South Bronx in New York City. It is one of the comprehensive services provided by the BronxCare’s life recovery center and addiction services, which also includes an outpatient clinic, opioid treatment program, inpatient rehab, and a half-way house. Inpatient detoxification units like ours are designed to treat serious addictions and chemical dependency and prevent and treat life-threatening withdrawal symptoms and signs or complications. Our patients come from all over the city and its adjoining suburbs, including from emergency room referrals, referral clinics, courts and the justice system, walk-ins, and self-referrals.
At a time when many inpatient detoxification units within the city were temporarily closed due to fear of inpatient spread of the virus or to provide extra COVID beds in anticipation for the peak surge, we have been able to provide a needed service. In fact, several other inpatient detoxification programs within the city have been able to refer their patients to our facility.
Individuals with substance use disorder have historically been a vulnerable and underserved population and possess high risk for multiple health problems as well as preexisting conditions. Many have limited life options financially, educationally, and with housing, and encounter barriers to accessing primary health care services, including preventive services. The introduction of the COVID-19 pandemic into these patients’ precarious health situations only made things worse as many of the limited resources for patients with substance use disorder were diverted to battling the pandemic. Numerous inpatient and outpatient addiction services, for example, were temporarily shut down. This has led to an increase in domestic violence, and psychiatric decompensation, including psychosis, suicidal attempts, and worsening of medical comorbidities in these patients.
Our wake-up call came when the first case of COVID-19 was confirmed in New York in early March. Within a short period of time the state became the epicenter for COVID-19. With the projection of millions of cases being positive and the number of new cases doubling every third day at the onset in New York City, we knew we had a battle brewing and needed to radically transform our mode of operation fast.
Our first task was to ensure the safety of our patients and the dedicated health workers attending to them. We streamlined the patient point of entry through one screening site, while also brushing up on our history-taking to intently screen for COVID-19. This included not just focusing on travels from China, but from Europe and other parts of the world.
Yes, we did ask patients about cough, fever, shortness of breath or difficulty breathing, feeling fatigued, severe body ache, and possible contact with someone who is sick or has traveled overseas. But we were also attuned to the increased rate of community spread and the presentation of other symptoms, such as loss of taste and smell, early in the process. Hence we were able to triage patients with suspected cases to the appropriate sections of the hospital for further screening, testing, and evaluation, instead of having those patients admitted to the detox unit.
Early in the process a huddle team was instituted with daily briefing of staff lasting 30 minutes or less. This team consists of physicians, nurses, a physician assistant, a social worker, and a counselor. In addition to discussing treatment plans for the patient, they deliberate on the public health information from the hospital’s COVID-19 command center, New York State Department of Health, the Office of Mental Health, and the Centers for Disease Control and Prevention concerning the latest evidence-based information. These discussions have helped us modify our policies and practices.
We instituted a no visiting rule during a short hospital stay of 5-7 days, and this was initiated weeks in advance of many institutions, including nursing homes with vulnerable populations. Our admitting criteria was reviewed to allow for admission of only those patients who absolutely needed inpatient substance use disorder treatment, including patients with severe withdrawal symptoms and signs, comorbidities, or neuropsychiatric manifestations that made them unsafe for outpatient or home detoxification. Others were triaged to the outpatient services which was amply supported with telemedicine. Rooms and designated areas of the building were earmarked as places for isolation/quarantine if suspected COVID-19 cases were identified pending testing. To assess patients’ risk of COVID-19, we do point-of-care nasopharyngeal swab testing with polymerase chain reaction.
Regarding face masks, patients and staff were fitted with ones early in the process. Additionally, staff were trained on the importance of face mask use and how to ensure you have a tight seal around the mouth and nose and were provided with other appropriate personal protective equipment. Concerning social distancing, we reduced the patient population capacity for the unit down to 50% and offered only single room admissions. Social distancing was encouraged in the unit, including in the television and recreation room and dining room, and during small treatment groups of less than six individuals. Daily temperature checks with noncontact handheld thermometers were enforced for staff and anyone coming into the life recovery center.
Patients are continuously being educated on the presentations of COVID-19 and encouraged to report any symptoms. Any staff feeling sick or having symptoms are encouraged to stay home. Rigorous and continuous cleaning of surfaces, especially of areas subjected to common use, is done frequently by the hospital housekeeping and environmental crew and is the order of the day.
Dr. Fagbemi is a hospitalist at BronxCare Health System, a not-for-profit health and teaching hospital system serving South and Central Bronx in New York. He has no conflicts of interest to disclose.
Substance use disorder and its daily consequences take no breaks even during a pandemic. The stressors created by COVID-19, including deaths of loved ones and the disruptions to normal life from policies aimed at flattening the curve, seem to have increased substance use.
I practice as a hospitalist with an internal medicine background and specialty in addiction medicine at BronxCare Health System’s inpatient detoxification unit, a 24/7, 20-bed medically-supervised unit in South Bronx in New York City. It is one of the comprehensive services provided by the BronxCare’s life recovery center and addiction services, which also includes an outpatient clinic, opioid treatment program, inpatient rehab, and a half-way house. Inpatient detoxification units like ours are designed to treat serious addictions and chemical dependency and prevent and treat life-threatening withdrawal symptoms and signs or complications. Our patients come from all over the city and its adjoining suburbs, including from emergency room referrals, referral clinics, courts and the justice system, walk-ins, and self-referrals.
At a time when many inpatient detoxification units within the city were temporarily closed due to fear of inpatient spread of the virus or to provide extra COVID beds in anticipation for the peak surge, we have been able to provide a needed service. In fact, several other inpatient detoxification programs within the city have been able to refer their patients to our facility.
Individuals with substance use disorder have historically been a vulnerable and underserved population and possess high risk for multiple health problems as well as preexisting conditions. Many have limited life options financially, educationally, and with housing, and encounter barriers to accessing primary health care services, including preventive services. The introduction of the COVID-19 pandemic into these patients’ precarious health situations only made things worse as many of the limited resources for patients with substance use disorder were diverted to battling the pandemic. Numerous inpatient and outpatient addiction services, for example, were temporarily shut down. This has led to an increase in domestic violence, and psychiatric decompensation, including psychosis, suicidal attempts, and worsening of medical comorbidities in these patients.
Our wake-up call came when the first case of COVID-19 was confirmed in New York in early March. Within a short period of time the state became the epicenter for COVID-19. With the projection of millions of cases being positive and the number of new cases doubling every third day at the onset in New York City, we knew we had a battle brewing and needed to radically transform our mode of operation fast.
Our first task was to ensure the safety of our patients and the dedicated health workers attending to them. We streamlined the patient point of entry through one screening site, while also brushing up on our history-taking to intently screen for COVID-19. This included not just focusing on travels from China, but from Europe and other parts of the world.
Yes, we did ask patients about cough, fever, shortness of breath or difficulty breathing, feeling fatigued, severe body ache, and possible contact with someone who is sick or has traveled overseas. But we were also attuned to the increased rate of community spread and the presentation of other symptoms, such as loss of taste and smell, early in the process. Hence we were able to triage patients with suspected cases to the appropriate sections of the hospital for further screening, testing, and evaluation, instead of having those patients admitted to the detox unit.
Early in the process a huddle team was instituted with daily briefing of staff lasting 30 minutes or less. This team consists of physicians, nurses, a physician assistant, a social worker, and a counselor. In addition to discussing treatment plans for the patient, they deliberate on the public health information from the hospital’s COVID-19 command center, New York State Department of Health, the Office of Mental Health, and the Centers for Disease Control and Prevention concerning the latest evidence-based information. These discussions have helped us modify our policies and practices.
We instituted a no visiting rule during a short hospital stay of 5-7 days, and this was initiated weeks in advance of many institutions, including nursing homes with vulnerable populations. Our admitting criteria was reviewed to allow for admission of only those patients who absolutely needed inpatient substance use disorder treatment, including patients with severe withdrawal symptoms and signs, comorbidities, or neuropsychiatric manifestations that made them unsafe for outpatient or home detoxification. Others were triaged to the outpatient services which was amply supported with telemedicine. Rooms and designated areas of the building were earmarked as places for isolation/quarantine if suspected COVID-19 cases were identified pending testing. To assess patients’ risk of COVID-19, we do point-of-care nasopharyngeal swab testing with polymerase chain reaction.
Regarding face masks, patients and staff were fitted with ones early in the process. Additionally, staff were trained on the importance of face mask use and how to ensure you have a tight seal around the mouth and nose and were provided with other appropriate personal protective equipment. Concerning social distancing, we reduced the patient population capacity for the unit down to 50% and offered only single room admissions. Social distancing was encouraged in the unit, including in the television and recreation room and dining room, and during small treatment groups of less than six individuals. Daily temperature checks with noncontact handheld thermometers were enforced for staff and anyone coming into the life recovery center.
Patients are continuously being educated on the presentations of COVID-19 and encouraged to report any symptoms. Any staff feeling sick or having symptoms are encouraged to stay home. Rigorous and continuous cleaning of surfaces, especially of areas subjected to common use, is done frequently by the hospital housekeeping and environmental crew and is the order of the day.
Dr. Fagbemi is a hospitalist at BronxCare Health System, a not-for-profit health and teaching hospital system serving South and Central Bronx in New York. He has no conflicts of interest to disclose.
Placental injury reported in women with COVID-19
Neonates appear healthy so far
Maternal vascular malperfusion and intervillous thrombi were more common in the placentas of women infected with SARS-CoV-2, compared with historic controls, report researchers who conducted the first-of-its-kind case series in the English literature. Nevertheless, the neonates in the report appear to be healthy so far and all tested negative for the virus.
Although the series examining placentas from 16 women is small, it carries a larger implication – that increased antenatal surveillance for pregnant women infected with SARS-CoV-2 may be indicated, the researchers noted.
Furthermore, the results could align with other reports of coagulation and vascular abnormalities among people with COVID-19. “I would say that our findings fit into that larger picture of vascular injury. This is developing, and there are some significant ways that these feeder vessels to the placenta are different, but if this is the emerging paradigm, our findings can fit into it,” Jeffrey A. Goldstein, MD, PhD, assistant professor of pathology at Northwestern University, Chicago, said in an interview.
The research was published in the American Journal of Clinical Pathology.
Prior case series reported in Wuhan, China, do not currently suggest that pregnant women are more likely to experience severe COVID-19, in contrast to observations during severe acute respiratory syndrome and Middle East respiratory syndrome outbreaks. “However,” the researchers noted, “adverse perinatal outcomes have been reported, including increased risks of miscarriage, preeclampsia, preterm birth, and stillbirth.”
To learn more, Dr. Goldstein, lead author Elisheva D. Shanes, MD, and colleagues examined the histology of placentas from women with COVID-19 giving birth between March 18 and May 5, 2020. They compared these placentas with over 17,000 historic controls and 215 women who had their placentas evaluated as part of a melanoma history study.
A total of 10 women were diagnosed with COVID-19 upon presentation to labor and delivery, 4 others were diagnosed approximately 1 month before delivery and the remaining 2 within 1 week of delivery. Ten of the patients were symptomatic and two required oxygen. None of the patients received intubation or died. A total of 14 patients delivered at term, 1 delivered at 34 weeks, and the remaining case experienced a 16-week intrauterine fetal demise (IUFD). The IUFD was excluded from subsequent statistical analysis.
The neonates each had a 5-minute Apgar score of 9. Most infants were discharged on the first or second day of life, and there were no neonatal deaths.
Key findings
Of the 15 placentas, 12 featured maternal vascular malperfusion. This rate was significantly higher than historic controls (P = .046) and melanoma study controls (P = .001).
Specific features varied between groups, with decidual arteriopathy, atherosis and fibrinoid necrosis of maternal vessels, and mural hypertrophy of membrane arterioles observed more often in COVID-19 cases than in all historical controls. In addition, peripheral infarctions, decidual arteriopathy, atherosis, and fibrinoid necrosis, and mural hypertrophy being more common in COVID-19 cases than in placentas of women with a history of melanoma.
In contrast, features of fetal vascular malperfusion were observed in 12 of 15 cases, but not at rates significantly different from the control groups. Chorangiosis, villous edema, and intervillous thrombi also were more common in the COVID-19 cohort.
Dr. Goldstein was surprised they did not observe much acute or chronic inflammation. “We see chronic inflammation in the placenta in response to many viruses, such as cytomegalovirus, so you might expect similar findings, but we didn’t see any increase above the controls.”
There are a couple of case reports of histiocytic intervillositis – a particularly severe form of chronic inflammation – associated with COVID-19, “but we didn’t see that in our study,” he added.
Clinical implications
The healthy neonatal outcomes reported in the study occurred despite the placental injury, which may be caused by the redundancy built into placentas for delivering oxygen and nutrients and for removing waste.
The negative COVID-19 test results in all infants also supports existing evidence that vertical transmission of the virus is uncommon. The finding also suggests that any damage to the placenta is likely related to maternal infection.
Only one mother in the COVID-19 cohort was hypertensive, which surprised the researchers because intervillous thrombi have been associated with maternal high blood pressure. “In the context of research suggesting an increase of thrombotic and thromboembolic disorders in COVID-19,” the researchers noted, “these may represent placental formation or deposition of thrombi in response to the virus.”
One of the priorities for the researchers going forward is to monitor the longer-term outcomes of the infants, Dr. Goldstein said. “We know the people in utero during the 1918-1919 flu pandemic had higher rates of heart disease and other long-term problems, so we want to be on the lookout for something similar.”
Valuable insight
“This is a comprehensive case series of this topic, with findings worth noting and sharing in a timely fashion,” Karen Mestan, MD, associate professor of pediatrics within the division of neonatology at Northwestern University, said when asked to comment on the study.
“The information is valuable to neonatologists as the short- and long-term effects of COVID-19 exposure on newborn infants are still largely unknown,” she added. “Details of placental pathology provide emerging insight and may help us understand mother-baby vertical transmission during the current pandemic.”
Dr. Goldstein and Dr. Mestan had no relevant financial disclosures.
SOURCE: Shanes ED et al. Am J Clin Pathol. 2020 May 22. doi: 10.1093/ajcp/aqaa089.
Neonates appear healthy so far
Neonates appear healthy so far
Maternal vascular malperfusion and intervillous thrombi were more common in the placentas of women infected with SARS-CoV-2, compared with historic controls, report researchers who conducted the first-of-its-kind case series in the English literature. Nevertheless, the neonates in the report appear to be healthy so far and all tested negative for the virus.
Although the series examining placentas from 16 women is small, it carries a larger implication – that increased antenatal surveillance for pregnant women infected with SARS-CoV-2 may be indicated, the researchers noted.
Furthermore, the results could align with other reports of coagulation and vascular abnormalities among people with COVID-19. “I would say that our findings fit into that larger picture of vascular injury. This is developing, and there are some significant ways that these feeder vessels to the placenta are different, but if this is the emerging paradigm, our findings can fit into it,” Jeffrey A. Goldstein, MD, PhD, assistant professor of pathology at Northwestern University, Chicago, said in an interview.
The research was published in the American Journal of Clinical Pathology.
Prior case series reported in Wuhan, China, do not currently suggest that pregnant women are more likely to experience severe COVID-19, in contrast to observations during severe acute respiratory syndrome and Middle East respiratory syndrome outbreaks. “However,” the researchers noted, “adverse perinatal outcomes have been reported, including increased risks of miscarriage, preeclampsia, preterm birth, and stillbirth.”
To learn more, Dr. Goldstein, lead author Elisheva D. Shanes, MD, and colleagues examined the histology of placentas from women with COVID-19 giving birth between March 18 and May 5, 2020. They compared these placentas with over 17,000 historic controls and 215 women who had their placentas evaluated as part of a melanoma history study.
A total of 10 women were diagnosed with COVID-19 upon presentation to labor and delivery, 4 others were diagnosed approximately 1 month before delivery and the remaining 2 within 1 week of delivery. Ten of the patients were symptomatic and two required oxygen. None of the patients received intubation or died. A total of 14 patients delivered at term, 1 delivered at 34 weeks, and the remaining case experienced a 16-week intrauterine fetal demise (IUFD). The IUFD was excluded from subsequent statistical analysis.
The neonates each had a 5-minute Apgar score of 9. Most infants were discharged on the first or second day of life, and there were no neonatal deaths.
Key findings
Of the 15 placentas, 12 featured maternal vascular malperfusion. This rate was significantly higher than historic controls (P = .046) and melanoma study controls (P = .001).
Specific features varied between groups, with decidual arteriopathy, atherosis and fibrinoid necrosis of maternal vessels, and mural hypertrophy of membrane arterioles observed more often in COVID-19 cases than in all historical controls. In addition, peripheral infarctions, decidual arteriopathy, atherosis, and fibrinoid necrosis, and mural hypertrophy being more common in COVID-19 cases than in placentas of women with a history of melanoma.
In contrast, features of fetal vascular malperfusion were observed in 12 of 15 cases, but not at rates significantly different from the control groups. Chorangiosis, villous edema, and intervillous thrombi also were more common in the COVID-19 cohort.
Dr. Goldstein was surprised they did not observe much acute or chronic inflammation. “We see chronic inflammation in the placenta in response to many viruses, such as cytomegalovirus, so you might expect similar findings, but we didn’t see any increase above the controls.”
There are a couple of case reports of histiocytic intervillositis – a particularly severe form of chronic inflammation – associated with COVID-19, “but we didn’t see that in our study,” he added.
Clinical implications
The healthy neonatal outcomes reported in the study occurred despite the placental injury, which may be caused by the redundancy built into placentas for delivering oxygen and nutrients and for removing waste.
The negative COVID-19 test results in all infants also supports existing evidence that vertical transmission of the virus is uncommon. The finding also suggests that any damage to the placenta is likely related to maternal infection.
Only one mother in the COVID-19 cohort was hypertensive, which surprised the researchers because intervillous thrombi have been associated with maternal high blood pressure. “In the context of research suggesting an increase of thrombotic and thromboembolic disorders in COVID-19,” the researchers noted, “these may represent placental formation or deposition of thrombi in response to the virus.”
One of the priorities for the researchers going forward is to monitor the longer-term outcomes of the infants, Dr. Goldstein said. “We know the people in utero during the 1918-1919 flu pandemic had higher rates of heart disease and other long-term problems, so we want to be on the lookout for something similar.”
Valuable insight
“This is a comprehensive case series of this topic, with findings worth noting and sharing in a timely fashion,” Karen Mestan, MD, associate professor of pediatrics within the division of neonatology at Northwestern University, said when asked to comment on the study.
“The information is valuable to neonatologists as the short- and long-term effects of COVID-19 exposure on newborn infants are still largely unknown,” she added. “Details of placental pathology provide emerging insight and may help us understand mother-baby vertical transmission during the current pandemic.”
Dr. Goldstein and Dr. Mestan had no relevant financial disclosures.
SOURCE: Shanes ED et al. Am J Clin Pathol. 2020 May 22. doi: 10.1093/ajcp/aqaa089.
Maternal vascular malperfusion and intervillous thrombi were more common in the placentas of women infected with SARS-CoV-2, compared with historic controls, report researchers who conducted the first-of-its-kind case series in the English literature. Nevertheless, the neonates in the report appear to be healthy so far and all tested negative for the virus.
Although the series examining placentas from 16 women is small, it carries a larger implication – that increased antenatal surveillance for pregnant women infected with SARS-CoV-2 may be indicated, the researchers noted.
Furthermore, the results could align with other reports of coagulation and vascular abnormalities among people with COVID-19. “I would say that our findings fit into that larger picture of vascular injury. This is developing, and there are some significant ways that these feeder vessels to the placenta are different, but if this is the emerging paradigm, our findings can fit into it,” Jeffrey A. Goldstein, MD, PhD, assistant professor of pathology at Northwestern University, Chicago, said in an interview.
The research was published in the American Journal of Clinical Pathology.
Prior case series reported in Wuhan, China, do not currently suggest that pregnant women are more likely to experience severe COVID-19, in contrast to observations during severe acute respiratory syndrome and Middle East respiratory syndrome outbreaks. “However,” the researchers noted, “adverse perinatal outcomes have been reported, including increased risks of miscarriage, preeclampsia, preterm birth, and stillbirth.”
To learn more, Dr. Goldstein, lead author Elisheva D. Shanes, MD, and colleagues examined the histology of placentas from women with COVID-19 giving birth between March 18 and May 5, 2020. They compared these placentas with over 17,000 historic controls and 215 women who had their placentas evaluated as part of a melanoma history study.
A total of 10 women were diagnosed with COVID-19 upon presentation to labor and delivery, 4 others were diagnosed approximately 1 month before delivery and the remaining 2 within 1 week of delivery. Ten of the patients were symptomatic and two required oxygen. None of the patients received intubation or died. A total of 14 patients delivered at term, 1 delivered at 34 weeks, and the remaining case experienced a 16-week intrauterine fetal demise (IUFD). The IUFD was excluded from subsequent statistical analysis.
The neonates each had a 5-minute Apgar score of 9. Most infants were discharged on the first or second day of life, and there were no neonatal deaths.
Key findings
Of the 15 placentas, 12 featured maternal vascular malperfusion. This rate was significantly higher than historic controls (P = .046) and melanoma study controls (P = .001).
Specific features varied between groups, with decidual arteriopathy, atherosis and fibrinoid necrosis of maternal vessels, and mural hypertrophy of membrane arterioles observed more often in COVID-19 cases than in all historical controls. In addition, peripheral infarctions, decidual arteriopathy, atherosis, and fibrinoid necrosis, and mural hypertrophy being more common in COVID-19 cases than in placentas of women with a history of melanoma.
In contrast, features of fetal vascular malperfusion were observed in 12 of 15 cases, but not at rates significantly different from the control groups. Chorangiosis, villous edema, and intervillous thrombi also were more common in the COVID-19 cohort.
Dr. Goldstein was surprised they did not observe much acute or chronic inflammation. “We see chronic inflammation in the placenta in response to many viruses, such as cytomegalovirus, so you might expect similar findings, but we didn’t see any increase above the controls.”
There are a couple of case reports of histiocytic intervillositis – a particularly severe form of chronic inflammation – associated with COVID-19, “but we didn’t see that in our study,” he added.
Clinical implications
The healthy neonatal outcomes reported in the study occurred despite the placental injury, which may be caused by the redundancy built into placentas for delivering oxygen and nutrients and for removing waste.
The negative COVID-19 test results in all infants also supports existing evidence that vertical transmission of the virus is uncommon. The finding also suggests that any damage to the placenta is likely related to maternal infection.
Only one mother in the COVID-19 cohort was hypertensive, which surprised the researchers because intervillous thrombi have been associated with maternal high blood pressure. “In the context of research suggesting an increase of thrombotic and thromboembolic disorders in COVID-19,” the researchers noted, “these may represent placental formation or deposition of thrombi in response to the virus.”
One of the priorities for the researchers going forward is to monitor the longer-term outcomes of the infants, Dr. Goldstein said. “We know the people in utero during the 1918-1919 flu pandemic had higher rates of heart disease and other long-term problems, so we want to be on the lookout for something similar.”
Valuable insight
“This is a comprehensive case series of this topic, with findings worth noting and sharing in a timely fashion,” Karen Mestan, MD, associate professor of pediatrics within the division of neonatology at Northwestern University, said when asked to comment on the study.
“The information is valuable to neonatologists as the short- and long-term effects of COVID-19 exposure on newborn infants are still largely unknown,” she added. “Details of placental pathology provide emerging insight and may help us understand mother-baby vertical transmission during the current pandemic.”
Dr. Goldstein and Dr. Mestan had no relevant financial disclosures.
SOURCE: Shanes ED et al. Am J Clin Pathol. 2020 May 22. doi: 10.1093/ajcp/aqaa089.
FROM THE AMERICAN JOURNAL OF CLINICAL PATHOLOGY