User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Cardiorespiratory fitness linked to cancer risk, mortality?
TOPLINE:
a large Swedish cohort study suggests.
METHODOLOGY:
- A prospective cohort study included 177,709 Swedish men (mean age, 42; mean body mass index, 26 kg/m2) who completed an occupational health profile assessment and were followed for a mean of 9.6 years.
- CRF was assessed by determining maximal oxygen consumption during an aerobic fitness test, known as a submaximal Åstrand cycle ergometer test.
- Participants reported physical activity habits, lifestyle, and perceived health.
- Data on prostate, colon, and lung cancer incidence and mortality were derived from national registers.
- Outcomes from three higher CRF groups (low, > 25-35; moderate, > 35-45; high, > 45 mL/min per kg) were compared with those from the very low CRF group (25 mL/min per kg or less). Models were adjusted for various factors, including age, BMI, education, dietary habits, comorbidity, and smoking.
TAKEAWAY:
- During follow-up, investigators identified 1,918 prostate, 499 colon, and 283 lung cancer cases as well as 141 prostate, 207 lung, and 152 colon cancer deaths.
- In the fully adjusted model, higher CRF levels were associated with a significantly lower risk for colon cancer (hazard ratio, 0.72 for moderate; HR, 0.63 for high).
- In this model, higher CRF was also associated with a lower risk of death from prostate cancer (HR, 0.67 for low; HR, 0.57 for moderate; HR, 0.29 for high).
- For lung cancer mortality, only high CRF was associated with a significantly lower risk of death (HR, 0.41).
- An association between CRF and lung cancer incidence (HR, 0.99) and death (HR, 0.99) was only evident among adults aged 60 and older.
IN PRACTICE:
“The clinical implications of these findings further emphasize the importance of CRF for possibly reducing cancer incidence and mortality,” the authors concluded. “It is important for the general public to understand that higher-intensity [physical activity] has greater effects on CRF and is likely to be more protective against the risk of developing and dying from certain cancers.”
SOURCE:
The study was led by Elin Ekblom-Bak, PhD, from the Swedish School of Sport and Health Sciences, Stockholm. It was published online in JAMA Network Open.
LIMITATIONS:
The study was limited by voluntary participation, inclusion of only employed individuals, and estimations of CRF via submaximal tests. Data on smoking status were not optimal and there was a small number of cancer cases and deaths.
DISCLOSURES:
Funding was provided by the Swedish Cancer Society. The authors have reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
a large Swedish cohort study suggests.
METHODOLOGY:
- A prospective cohort study included 177,709 Swedish men (mean age, 42; mean body mass index, 26 kg/m2) who completed an occupational health profile assessment and were followed for a mean of 9.6 years.
- CRF was assessed by determining maximal oxygen consumption during an aerobic fitness test, known as a submaximal Åstrand cycle ergometer test.
- Participants reported physical activity habits, lifestyle, and perceived health.
- Data on prostate, colon, and lung cancer incidence and mortality were derived from national registers.
- Outcomes from three higher CRF groups (low, > 25-35; moderate, > 35-45; high, > 45 mL/min per kg) were compared with those from the very low CRF group (25 mL/min per kg or less). Models were adjusted for various factors, including age, BMI, education, dietary habits, comorbidity, and smoking.
TAKEAWAY:
- During follow-up, investigators identified 1,918 prostate, 499 colon, and 283 lung cancer cases as well as 141 prostate, 207 lung, and 152 colon cancer deaths.
- In the fully adjusted model, higher CRF levels were associated with a significantly lower risk for colon cancer (hazard ratio, 0.72 for moderate; HR, 0.63 for high).
- In this model, higher CRF was also associated with a lower risk of death from prostate cancer (HR, 0.67 for low; HR, 0.57 for moderate; HR, 0.29 for high).
- For lung cancer mortality, only high CRF was associated with a significantly lower risk of death (HR, 0.41).
- An association between CRF and lung cancer incidence (HR, 0.99) and death (HR, 0.99) was only evident among adults aged 60 and older.
IN PRACTICE:
“The clinical implications of these findings further emphasize the importance of CRF for possibly reducing cancer incidence and mortality,” the authors concluded. “It is important for the general public to understand that higher-intensity [physical activity] has greater effects on CRF and is likely to be more protective against the risk of developing and dying from certain cancers.”
SOURCE:
The study was led by Elin Ekblom-Bak, PhD, from the Swedish School of Sport and Health Sciences, Stockholm. It was published online in JAMA Network Open.
LIMITATIONS:
The study was limited by voluntary participation, inclusion of only employed individuals, and estimations of CRF via submaximal tests. Data on smoking status were not optimal and there was a small number of cancer cases and deaths.
DISCLOSURES:
Funding was provided by the Swedish Cancer Society. The authors have reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
a large Swedish cohort study suggests.
METHODOLOGY:
- A prospective cohort study included 177,709 Swedish men (mean age, 42; mean body mass index, 26 kg/m2) who completed an occupational health profile assessment and were followed for a mean of 9.6 years.
- CRF was assessed by determining maximal oxygen consumption during an aerobic fitness test, known as a submaximal Åstrand cycle ergometer test.
- Participants reported physical activity habits, lifestyle, and perceived health.
- Data on prostate, colon, and lung cancer incidence and mortality were derived from national registers.
- Outcomes from three higher CRF groups (low, > 25-35; moderate, > 35-45; high, > 45 mL/min per kg) were compared with those from the very low CRF group (25 mL/min per kg or less). Models were adjusted for various factors, including age, BMI, education, dietary habits, comorbidity, and smoking.
TAKEAWAY:
- During follow-up, investigators identified 1,918 prostate, 499 colon, and 283 lung cancer cases as well as 141 prostate, 207 lung, and 152 colon cancer deaths.
- In the fully adjusted model, higher CRF levels were associated with a significantly lower risk for colon cancer (hazard ratio, 0.72 for moderate; HR, 0.63 for high).
- In this model, higher CRF was also associated with a lower risk of death from prostate cancer (HR, 0.67 for low; HR, 0.57 for moderate; HR, 0.29 for high).
- For lung cancer mortality, only high CRF was associated with a significantly lower risk of death (HR, 0.41).
- An association between CRF and lung cancer incidence (HR, 0.99) and death (HR, 0.99) was only evident among adults aged 60 and older.
IN PRACTICE:
“The clinical implications of these findings further emphasize the importance of CRF for possibly reducing cancer incidence and mortality,” the authors concluded. “It is important for the general public to understand that higher-intensity [physical activity] has greater effects on CRF and is likely to be more protective against the risk of developing and dying from certain cancers.”
SOURCE:
The study was led by Elin Ekblom-Bak, PhD, from the Swedish School of Sport and Health Sciences, Stockholm. It was published online in JAMA Network Open.
LIMITATIONS:
The study was limited by voluntary participation, inclusion of only employed individuals, and estimations of CRF via submaximal tests. Data on smoking status were not optimal and there was a small number of cancer cases and deaths.
DISCLOSURES:
Funding was provided by the Swedish Cancer Society. The authors have reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Diabetes may short circuit pembrolizumab benefits in NSCLC
TOPLINE:
METHODOLOGY:
- Investigators reviewed the medical records of 203 consecutive patients with metastatic NSCLC who received first-line pembrolizumab either alone or in combination with chemotherapy at a single tertiary center in Israel.
- Overall, 1 in 4 patients (n = 51) had diabetes mellitus; most (n = 42) were being treated with oral hypoglycemic agents, frequently metformin, and 7 were taking insulin.
- Rates of tumors with PD‐L1 expression above 50% were not significantly different among patients with diabetes and those without.
TAKEAWAY:
- Overall, among patients with diabetes, median progression-free survival (PFS) was significantly shorter than among patients without diabetes (5.9 vs. 7.1 months), as was overall survival (12 vs. 21 months).
- Shorter overall survival was more pronounced among those with diabetes who received pembrolizumab alone (12 vs. 27 months) in comparison with patients who received pembrolizumab plus chemotherapy (14.3 vs. 19.4 months).
- After adjusting for potential confounders, multivariate analysis confirmed that diabetes was an independent risk factor for shorter PFS (hazard ratio, 1.67) and shorter overall survival (HR, 1.73) for patients with NSCLC.
- In a validation cohort of 452 patients with metastatic NSCLC, only 19.6% of those with diabetes continued to take pembrolizumab at 12 months versus 31.7% of those without diabetes.
IN PRACTICE:
“As NSCLC patients with [diabetes] constitute a significant subgroup, there is an urgent need to validate our findings and explore whether outcomes in these patients can be improved by better glycemic control,” the authors said, adding that “chemotherapy may offset some of the deleterious effects” of diabetes.
SOURCE:
The study was led by Yasmin Leshem, MD, PhD, of the Tel Aviv Sourasky Medical Center, and was published in Cancer.
LIMITATIONS:
- Without access to blood test results outside the hospital, the researchers could not determine whether better glycemic control might have improved outcomes.
- The incidence of type 1 or 2 diabetes was not well documented.
DISCLOSURES:
- No funding source was reported.
- Two investigators reported receiving consulting and/or other fees from Bristol-Myers Squibb, Roche, Merck, Novartis, and Merck Sharp and Dohme.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Investigators reviewed the medical records of 203 consecutive patients with metastatic NSCLC who received first-line pembrolizumab either alone or in combination with chemotherapy at a single tertiary center in Israel.
- Overall, 1 in 4 patients (n = 51) had diabetes mellitus; most (n = 42) were being treated with oral hypoglycemic agents, frequently metformin, and 7 were taking insulin.
- Rates of tumors with PD‐L1 expression above 50% were not significantly different among patients with diabetes and those without.
TAKEAWAY:
- Overall, among patients with diabetes, median progression-free survival (PFS) was significantly shorter than among patients without diabetes (5.9 vs. 7.1 months), as was overall survival (12 vs. 21 months).
- Shorter overall survival was more pronounced among those with diabetes who received pembrolizumab alone (12 vs. 27 months) in comparison with patients who received pembrolizumab plus chemotherapy (14.3 vs. 19.4 months).
- After adjusting for potential confounders, multivariate analysis confirmed that diabetes was an independent risk factor for shorter PFS (hazard ratio, 1.67) and shorter overall survival (HR, 1.73) for patients with NSCLC.
- In a validation cohort of 452 patients with metastatic NSCLC, only 19.6% of those with diabetes continued to take pembrolizumab at 12 months versus 31.7% of those without diabetes.
IN PRACTICE:
“As NSCLC patients with [diabetes] constitute a significant subgroup, there is an urgent need to validate our findings and explore whether outcomes in these patients can be improved by better glycemic control,” the authors said, adding that “chemotherapy may offset some of the deleterious effects” of diabetes.
SOURCE:
The study was led by Yasmin Leshem, MD, PhD, of the Tel Aviv Sourasky Medical Center, and was published in Cancer.
LIMITATIONS:
- Without access to blood test results outside the hospital, the researchers could not determine whether better glycemic control might have improved outcomes.
- The incidence of type 1 or 2 diabetes was not well documented.
DISCLOSURES:
- No funding source was reported.
- Two investigators reported receiving consulting and/or other fees from Bristol-Myers Squibb, Roche, Merck, Novartis, and Merck Sharp and Dohme.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Investigators reviewed the medical records of 203 consecutive patients with metastatic NSCLC who received first-line pembrolizumab either alone or in combination with chemotherapy at a single tertiary center in Israel.
- Overall, 1 in 4 patients (n = 51) had diabetes mellitus; most (n = 42) were being treated with oral hypoglycemic agents, frequently metformin, and 7 were taking insulin.
- Rates of tumors with PD‐L1 expression above 50% were not significantly different among patients with diabetes and those without.
TAKEAWAY:
- Overall, among patients with diabetes, median progression-free survival (PFS) was significantly shorter than among patients without diabetes (5.9 vs. 7.1 months), as was overall survival (12 vs. 21 months).
- Shorter overall survival was more pronounced among those with diabetes who received pembrolizumab alone (12 vs. 27 months) in comparison with patients who received pembrolizumab plus chemotherapy (14.3 vs. 19.4 months).
- After adjusting for potential confounders, multivariate analysis confirmed that diabetes was an independent risk factor for shorter PFS (hazard ratio, 1.67) and shorter overall survival (HR, 1.73) for patients with NSCLC.
- In a validation cohort of 452 patients with metastatic NSCLC, only 19.6% of those with diabetes continued to take pembrolizumab at 12 months versus 31.7% of those without diabetes.
IN PRACTICE:
“As NSCLC patients with [diabetes] constitute a significant subgroup, there is an urgent need to validate our findings and explore whether outcomes in these patients can be improved by better glycemic control,” the authors said, adding that “chemotherapy may offset some of the deleterious effects” of diabetes.
SOURCE:
The study was led by Yasmin Leshem, MD, PhD, of the Tel Aviv Sourasky Medical Center, and was published in Cancer.
LIMITATIONS:
- Without access to blood test results outside the hospital, the researchers could not determine whether better glycemic control might have improved outcomes.
- The incidence of type 1 or 2 diabetes was not well documented.
DISCLOSURES:
- No funding source was reported.
- Two investigators reported receiving consulting and/or other fees from Bristol-Myers Squibb, Roche, Merck, Novartis, and Merck Sharp and Dohme.
A version of this article first appeared on Medscape.com.
Cannabis for cancer symptoms: Perceived or real benefit?
TOPLINE:
METHODOLOGY:
- Participants included 267 adults (mean age, 58 years; 70% women; 88% White) undergoing treatment for cancer, most commonly breast (47%) and ovarian (29%).
- Participants completed online surveys to characterize cannabis use, reasons for using it, perceived benefits and harms, and physical/psychological symptoms.
- Participants who had used cannabis for more than 1 day during the previous 30 days were compared with those who had not.
TAKEAWAY:
- Overall, 26% of respondents reported cannabis use in the past 30 days, most often edibles (65%) or smoked cannabis (51%).
- Cannabis users were more likely to be younger, male, Black, to have lower income, worse physical/psychological symptoms, and to be disabled or unable to work in comparison with nonusers.
- Cannabis was used to treat pain, cancer, sleep problems, anxiety, nausea, and poor appetite; perceived benefits were greatest with respect to sleep, nausea, pain, muscle spasms, and anxiety.
- Despite perceived benefits, cannabis users reported worse overall distress, anxiety, sleep disturbances, appetite, nausea, fatigue, and pain.
IN PRACTICE:
“The study findings indicate that patients with cancer perceived benefits to using cannabis for many symptoms” but also revealed that “those who used cannabis in the past 30 days had significantly worse symptom profiles overall than those who did not use cannabis,” the authors wrote.
SOURCE:
The study, led by Desiree R. Azizoddin, PsyD, University of Oklahoma Health Science Center, Oklahoma City, was published online in Cancer.
LIMITATIONS:
It’s not known whether adults who used cannabis had significantly worse symptoms at the outset, which may have prompted cannabis use, or whether cannabis use may have exacerbated their symptoms.
DISCLOSURES:
Funding for the study was provided by grants from the National Cancer Institute and the Oklahoma Tobacco Settlement Endowment Trust. Nine of the 10 authors have disclosed no relevant conflicts of interest. One author has relationships with various pharmaceutical companies involved in oncology.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Participants included 267 adults (mean age, 58 years; 70% women; 88% White) undergoing treatment for cancer, most commonly breast (47%) and ovarian (29%).
- Participants completed online surveys to characterize cannabis use, reasons for using it, perceived benefits and harms, and physical/psychological symptoms.
- Participants who had used cannabis for more than 1 day during the previous 30 days were compared with those who had not.
TAKEAWAY:
- Overall, 26% of respondents reported cannabis use in the past 30 days, most often edibles (65%) or smoked cannabis (51%).
- Cannabis users were more likely to be younger, male, Black, to have lower income, worse physical/psychological symptoms, and to be disabled or unable to work in comparison with nonusers.
- Cannabis was used to treat pain, cancer, sleep problems, anxiety, nausea, and poor appetite; perceived benefits were greatest with respect to sleep, nausea, pain, muscle spasms, and anxiety.
- Despite perceived benefits, cannabis users reported worse overall distress, anxiety, sleep disturbances, appetite, nausea, fatigue, and pain.
IN PRACTICE:
“The study findings indicate that patients with cancer perceived benefits to using cannabis for many symptoms” but also revealed that “those who used cannabis in the past 30 days had significantly worse symptom profiles overall than those who did not use cannabis,” the authors wrote.
SOURCE:
The study, led by Desiree R. Azizoddin, PsyD, University of Oklahoma Health Science Center, Oklahoma City, was published online in Cancer.
LIMITATIONS:
It’s not known whether adults who used cannabis had significantly worse symptoms at the outset, which may have prompted cannabis use, or whether cannabis use may have exacerbated their symptoms.
DISCLOSURES:
Funding for the study was provided by grants from the National Cancer Institute and the Oklahoma Tobacco Settlement Endowment Trust. Nine of the 10 authors have disclosed no relevant conflicts of interest. One author has relationships with various pharmaceutical companies involved in oncology.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Participants included 267 adults (mean age, 58 years; 70% women; 88% White) undergoing treatment for cancer, most commonly breast (47%) and ovarian (29%).
- Participants completed online surveys to characterize cannabis use, reasons for using it, perceived benefits and harms, and physical/psychological symptoms.
- Participants who had used cannabis for more than 1 day during the previous 30 days were compared with those who had not.
TAKEAWAY:
- Overall, 26% of respondents reported cannabis use in the past 30 days, most often edibles (65%) or smoked cannabis (51%).
- Cannabis users were more likely to be younger, male, Black, to have lower income, worse physical/psychological symptoms, and to be disabled or unable to work in comparison with nonusers.
- Cannabis was used to treat pain, cancer, sleep problems, anxiety, nausea, and poor appetite; perceived benefits were greatest with respect to sleep, nausea, pain, muscle spasms, and anxiety.
- Despite perceived benefits, cannabis users reported worse overall distress, anxiety, sleep disturbances, appetite, nausea, fatigue, and pain.
IN PRACTICE:
“The study findings indicate that patients with cancer perceived benefits to using cannabis for many symptoms” but also revealed that “those who used cannabis in the past 30 days had significantly worse symptom profiles overall than those who did not use cannabis,” the authors wrote.
SOURCE:
The study, led by Desiree R. Azizoddin, PsyD, University of Oklahoma Health Science Center, Oklahoma City, was published online in Cancer.
LIMITATIONS:
It’s not known whether adults who used cannabis had significantly worse symptoms at the outset, which may have prompted cannabis use, or whether cannabis use may have exacerbated their symptoms.
DISCLOSURES:
Funding for the study was provided by grants from the National Cancer Institute and the Oklahoma Tobacco Settlement Endowment Trust. Nine of the 10 authors have disclosed no relevant conflicts of interest. One author has relationships with various pharmaceutical companies involved in oncology.
A version of this article first appeared on Medscape.com.
Cancer Data Trends 2023
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
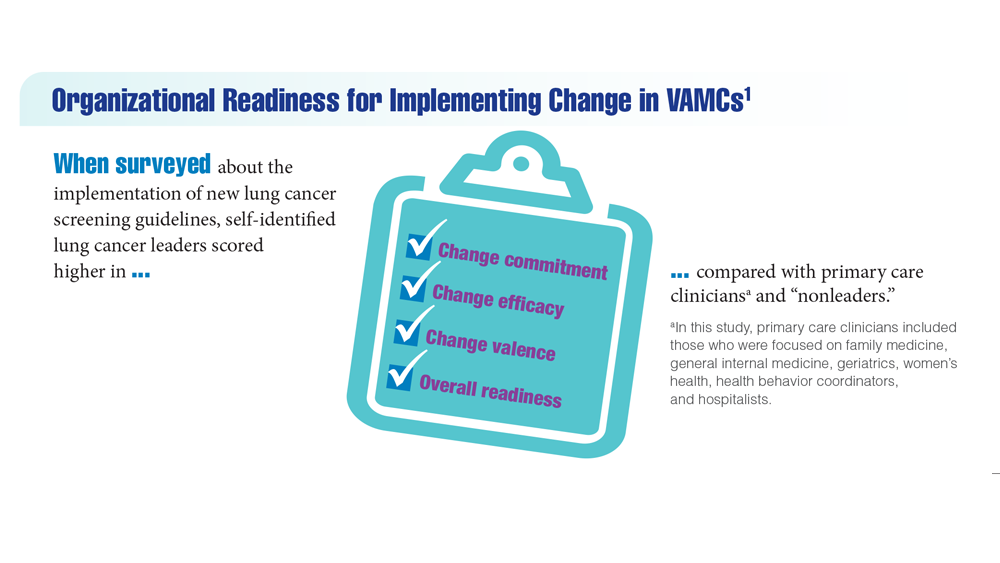
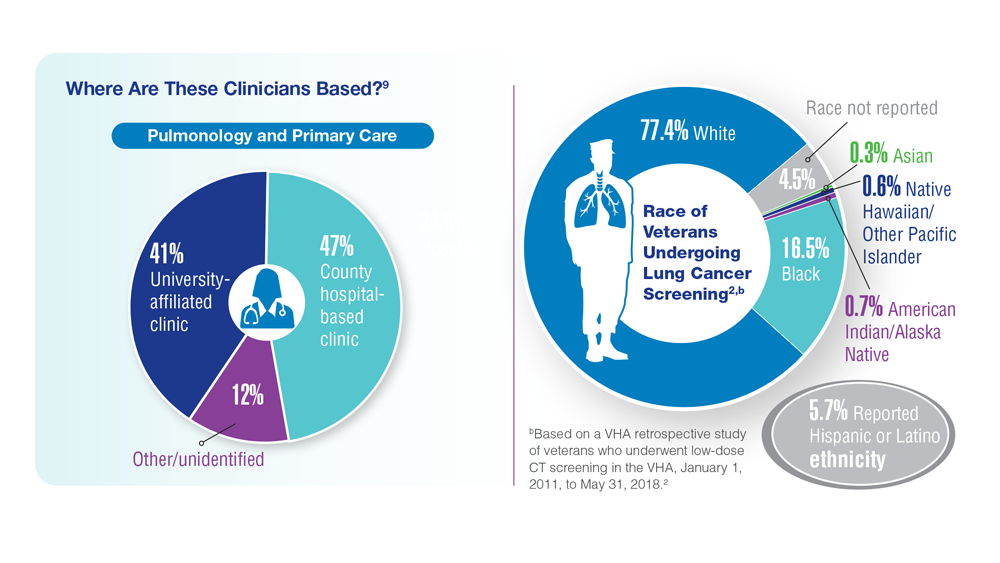
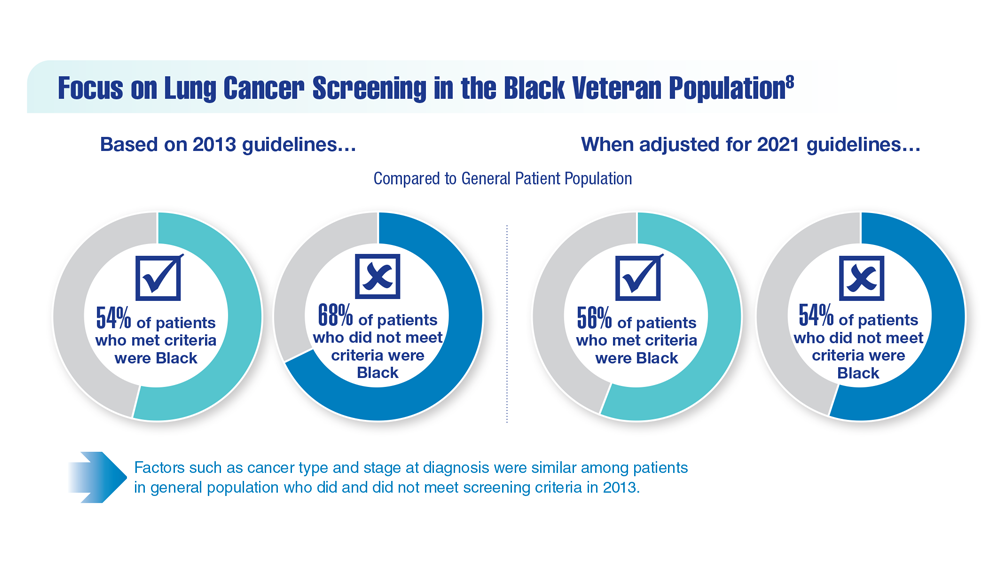
Lung Cancer Screening in Veterans
- Spalluto LB et al. J Am Coll Radiol. 2021;18(6):809-819. doi:10.1016/j.jacr.2020.12.010
- Lewis JA et al. JNCI Cancer Spectr. 2020;4(5):pkaa053. doi:10.1093/jncics/pkaa053
- Wallace C. Largest-ever lung cancer screening study reveals ways to increase screening outreach. Medical University of South Carolina. November 22, 2022. Accessed January 4, 202 https://hollingscancercenter.musc.edu/news/archive/2022/11/22/largest-ever-lung-cancer-screening-study-reveals-ways-to-increase-screening-outreach
- Screening facts & figures. Go2 For Lung Cancer. 2022. Accessed January 4, 2023. https://go2.org/risk-early-detection/screening-facts-figures/
- Dyer O. BMJ. 2021;372:n698. doi:10.1136/bmj.n698
- Boudreau JH et al. Chest. 2021;160(1):358-367. doi:10.1016/j.chest.2021.02.016
- Maurice NM, Tanner NT. Semin Oncol. 2022;S0093-7754(22)00041-0. doi:10.1053/j.seminoncol.2022.06.001
- Rusher TN et al. Fed Pract. 2022;39(suppl 2):S48-S51. doi:10.12788/fp.0269
- Núñez ER et al. JAMA Netw Open. 2021;4(7):e2116233. doi:10.1001/jamanetworkopen.2021.16233
- Lake M et al. BMC Cancer. 2020;20(1):561. doi:1186/s12885-020-06923-0
- Spalluto LB et al. J Am Coll Radiol. 2021;18(6):809-819. doi:10.1016/j.jacr.2020.12.010
- Lewis JA et al. JNCI Cancer Spectr. 2020;4(5):pkaa053. doi:10.1093/jncics/pkaa053
- Wallace C. Largest-ever lung cancer screening study reveals ways to increase screening outreach. Medical University of South Carolina. November 22, 2022. Accessed January 4, 202 https://hollingscancercenter.musc.edu/news/archive/2022/11/22/largest-ever-lung-cancer-screening-study-reveals-ways-to-increase-screening-outreach
- Screening facts & figures. Go2 For Lung Cancer. 2022. Accessed January 4, 2023. https://go2.org/risk-early-detection/screening-facts-figures/
- Dyer O. BMJ. 2021;372:n698. doi:10.1136/bmj.n698
- Boudreau JH et al. Chest. 2021;160(1):358-367. doi:10.1016/j.chest.2021.02.016
- Maurice NM, Tanner NT. Semin Oncol. 2022;S0093-7754(22)00041-0. doi:10.1053/j.seminoncol.2022.06.001
- Rusher TN et al. Fed Pract. 2022;39(suppl 2):S48-S51. doi:10.12788/fp.0269
- Núñez ER et al. JAMA Netw Open. 2021;4(7):e2116233. doi:10.1001/jamanetworkopen.2021.16233
- Lake M et al. BMC Cancer. 2020;20(1):561. doi:1186/s12885-020-06923-0
- Spalluto LB et al. J Am Coll Radiol. 2021;18(6):809-819. doi:10.1016/j.jacr.2020.12.010
- Lewis JA et al. JNCI Cancer Spectr. 2020;4(5):pkaa053. doi:10.1093/jncics/pkaa053
- Wallace C. Largest-ever lung cancer screening study reveals ways to increase screening outreach. Medical University of South Carolina. November 22, 2022. Accessed January 4, 202 https://hollingscancercenter.musc.edu/news/archive/2022/11/22/largest-ever-lung-cancer-screening-study-reveals-ways-to-increase-screening-outreach
- Screening facts & figures. Go2 For Lung Cancer. 2022. Accessed January 4, 2023. https://go2.org/risk-early-detection/screening-facts-figures/
- Dyer O. BMJ. 2021;372:n698. doi:10.1136/bmj.n698
- Boudreau JH et al. Chest. 2021;160(1):358-367. doi:10.1016/j.chest.2021.02.016
- Maurice NM, Tanner NT. Semin Oncol. 2022;S0093-7754(22)00041-0. doi:10.1053/j.seminoncol.2022.06.001
- Rusher TN et al. Fed Pract. 2022;39(suppl 2):S48-S51. doi:10.12788/fp.0269
- Núñez ER et al. JAMA Netw Open. 2021;4(7):e2116233. doi:10.1001/jamanetworkopen.2021.16233
- Lake M et al. BMC Cancer. 2020;20(1):561. doi:1186/s12885-020-06923-0
CLL combo treatment: Phase-3 study inconclusive
The difference in PFS between the IVO arm, 85%, versus 87% in the IO arm was statistically insignificant.
“Due to the early read-out and the futility boundaries being crossed, long-term follow-up will be critical to understand if there are any long-term benefits to IVO,” said study principal investigator Jennifer A. Woyach MD, professor in the division of hematology at The Ohio State University Comprehensive Care Center (OSUCCC – The James) in Columbus.
The 14-month follow-up data includes results from 465 CLL patients aged 65+ (median age 74 years, 67.5% male) who were treatment naive. The IO and IVO arms had 232 and 233 participants respectively, patients across both arms had Eastern Cooperative Oncology Group scores of 0-1 (97%), occurrence of Del (17p) was 13%, and a Rai stage status of III/IV was 55%, slightly more patients in the IO arm had unmutated IGHV 55% vs. 47% in the IVO arm. Researchers noted that, as expected, patients in the IVO group had a greater occurrence of hematologic adverse events graded at 3 or above, 61% VS 48% in the IO arm, P =.006.
The trial was spurred by the fact that many CLL patients on IO therapy must remain on treatment indefinitely, and an earlier phase II trial suggested that IVO therapy could lead to deep remission and therapy discontinuation.
Looking at the complete response (CR) rates and undetectable minimal residual disease (uMRD) rates across both arms suggested that there may be some hope that IVO could help CLL patients achieve deep remissions and discontinue therapy. Patients in the IVO arm had a CR of 68.5% and uMRD of 86.8% while only 31.3% of those in the IO arm had a CR and 33.3% achieved uMRD status.
“Despite the impressive CR and uMRD results, this study demonstrates that IVO is not superior to IO in terms of progression-free survival. However, because many patients in the IVO arm have discontinued treatment while those in the IO arm remain on ibrutinib, we think that it will be very important to continue to follow these patients long term, to see if there are advantages to this time limited therapy, especially in terms of toxicity, that we cannot appreciate with this follow-up,” said Dr. Woyach.
The Alliance for Clinical Trials in Oncology cooperative group, including OSUCCC James, is currently working to design the next frontline CLL study for older patients that builds on this work.
Dr. Woyach disclosed ties with Abbvie, AstraZeneca, Beigene, Genentech, Janssen, Loxo/Lilly, Merck, Newave, Pharmacyclics, and Schrodinger.
The difference in PFS between the IVO arm, 85%, versus 87% in the IO arm was statistically insignificant.
“Due to the early read-out and the futility boundaries being crossed, long-term follow-up will be critical to understand if there are any long-term benefits to IVO,” said study principal investigator Jennifer A. Woyach MD, professor in the division of hematology at The Ohio State University Comprehensive Care Center (OSUCCC – The James) in Columbus.
The 14-month follow-up data includes results from 465 CLL patients aged 65+ (median age 74 years, 67.5% male) who were treatment naive. The IO and IVO arms had 232 and 233 participants respectively, patients across both arms had Eastern Cooperative Oncology Group scores of 0-1 (97%), occurrence of Del (17p) was 13%, and a Rai stage status of III/IV was 55%, slightly more patients in the IO arm had unmutated IGHV 55% vs. 47% in the IVO arm. Researchers noted that, as expected, patients in the IVO group had a greater occurrence of hematologic adverse events graded at 3 or above, 61% VS 48% in the IO arm, P =.006.
The trial was spurred by the fact that many CLL patients on IO therapy must remain on treatment indefinitely, and an earlier phase II trial suggested that IVO therapy could lead to deep remission and therapy discontinuation.
Looking at the complete response (CR) rates and undetectable minimal residual disease (uMRD) rates across both arms suggested that there may be some hope that IVO could help CLL patients achieve deep remissions and discontinue therapy. Patients in the IVO arm had a CR of 68.5% and uMRD of 86.8% while only 31.3% of those in the IO arm had a CR and 33.3% achieved uMRD status.
“Despite the impressive CR and uMRD results, this study demonstrates that IVO is not superior to IO in terms of progression-free survival. However, because many patients in the IVO arm have discontinued treatment while those in the IO arm remain on ibrutinib, we think that it will be very important to continue to follow these patients long term, to see if there are advantages to this time limited therapy, especially in terms of toxicity, that we cannot appreciate with this follow-up,” said Dr. Woyach.
The Alliance for Clinical Trials in Oncology cooperative group, including OSUCCC James, is currently working to design the next frontline CLL study for older patients that builds on this work.
Dr. Woyach disclosed ties with Abbvie, AstraZeneca, Beigene, Genentech, Janssen, Loxo/Lilly, Merck, Newave, Pharmacyclics, and Schrodinger.
The difference in PFS between the IVO arm, 85%, versus 87% in the IO arm was statistically insignificant.
“Due to the early read-out and the futility boundaries being crossed, long-term follow-up will be critical to understand if there are any long-term benefits to IVO,” said study principal investigator Jennifer A. Woyach MD, professor in the division of hematology at The Ohio State University Comprehensive Care Center (OSUCCC – The James) in Columbus.
The 14-month follow-up data includes results from 465 CLL patients aged 65+ (median age 74 years, 67.5% male) who were treatment naive. The IO and IVO arms had 232 and 233 participants respectively, patients across both arms had Eastern Cooperative Oncology Group scores of 0-1 (97%), occurrence of Del (17p) was 13%, and a Rai stage status of III/IV was 55%, slightly more patients in the IO arm had unmutated IGHV 55% vs. 47% in the IVO arm. Researchers noted that, as expected, patients in the IVO group had a greater occurrence of hematologic adverse events graded at 3 or above, 61% VS 48% in the IO arm, P =.006.
The trial was spurred by the fact that many CLL patients on IO therapy must remain on treatment indefinitely, and an earlier phase II trial suggested that IVO therapy could lead to deep remission and therapy discontinuation.
Looking at the complete response (CR) rates and undetectable minimal residual disease (uMRD) rates across both arms suggested that there may be some hope that IVO could help CLL patients achieve deep remissions and discontinue therapy. Patients in the IVO arm had a CR of 68.5% and uMRD of 86.8% while only 31.3% of those in the IO arm had a CR and 33.3% achieved uMRD status.
“Despite the impressive CR and uMRD results, this study demonstrates that IVO is not superior to IO in terms of progression-free survival. However, because many patients in the IVO arm have discontinued treatment while those in the IO arm remain on ibrutinib, we think that it will be very important to continue to follow these patients long term, to see if there are advantages to this time limited therapy, especially in terms of toxicity, that we cannot appreciate with this follow-up,” said Dr. Woyach.
The Alliance for Clinical Trials in Oncology cooperative group, including OSUCCC James, is currently working to design the next frontline CLL study for older patients that builds on this work.
Dr. Woyach disclosed ties with Abbvie, AstraZeneca, Beigene, Genentech, Janssen, Loxo/Lilly, Merck, Newave, Pharmacyclics, and Schrodinger.
FDA approves first gene therapy for hemophilia A
Valoctocogene roxaparvovec, a one-time, single-dose IV infusion, is the first gene therapy approved in the United States for severe hemophilia A and will cost around $2.9 million. BioMarin has said the price tag reflects “the possibility of freedom from years” of infusions, which come to about $800,000 each year.
However, last December, the Institute for Clinical and Economic Review (ICER) set the upper bounds for the gene therapy at about $1.96 million. The extent to which the gene therapy will provide freedom from infusions, for how long, and in which patients are not completely understood.
Hemophilia A is caused by a mutation in the gene that produces a protein called coagulation factor VIII, which is essential for blood clotting. Valoctocogene roxaparvovec delivers a functional gene to liver cells via an adeno-associated virus serotype 5; the gene instructs the cells to make the missing clotting factor.
“Adults with severe hemophilia A face a lifelong burden, with frequent infusions and a high risk of health complications, including uncontrolled bleeding and irreversible joint damage,” Steven Pipe, MD, professor of pediatrics and pathology at the University of Michigan, Ann Arbor, and an investigator for the phase 3 study that led to the approval, said in a statement. The approval of valoctocogene roxaparvovec “has the potential to transform the way we treat adults based on years of bleed control following a single, one-time infusion.”
About 6,500 U.S. adults live with severe hemophilia A, and BioMarin said it anticipates approximately 2,500 will be eligible to receive the gene therapy following the approval. The U.S. indication is limited to patients without a history of factor VIII inhibitors and without detectable antibodies to the adeno-associated virus serotype 5.
Last August, the European Medicines Agency authorized the gene therapy for use in Europe, but according to Forbes and PharmaPhorum, uptake in Europe has been delayed because of reimbursement issues, given the cost of treatment and clinical uncertainties.
Data to date, however, are promising for most patients. Approval was based on BioMarin’s open-label, single-arm GENEr8-1 study in 134 men with severe congenital hemophilia A. Patients received a single infusion of 6 x 1013 vector genomes per kilogram.
Among the 132 patients available for 2-year evaluation, median factor VIII activity was in the range for mild hemophilia (6%-39% of normal) with an 84.5% reduction in bleeding events from baseline.
More than 80% of participants had no bleeding events requiring treatment, and there was a 98% reduction from baseline in mean use of exogenous factor VIII.
Overall, at 2 years, only 4.5% of patients had factor VIII activity consistent with severe hemophilia A; 9.1% had activity consistent with moderate disease; 59.8% had activity consistent with mild disease, and 26.5% had activity in the normal range above 40 IU/dL.
Trial investigators estimated that the typical half-life of the transgene-derived factor VIII production system is 123 weeks.
Among the six men who resumed prophylaxis, most had fewer bleeding events than when they were on prophylaxis before the infusion. All patients developed antibodies to the virus delivery vector, precluding retreatment.
Elevated alanine aminotransferase levels were the most common adverse event, occurring in 88.8% of patients, who were treated with immunosuppressants for a median of 33 weeks. Elevations persisted at 2 years in 29% of patients.
The other most common adverse events were headache (38.1%), nausea (37.3%), and increases in aspartate aminotransferase (35.1%).
A version of this article first appeared on Medscape.com.
Valoctocogene roxaparvovec, a one-time, single-dose IV infusion, is the first gene therapy approved in the United States for severe hemophilia A and will cost around $2.9 million. BioMarin has said the price tag reflects “the possibility of freedom from years” of infusions, which come to about $800,000 each year.
However, last December, the Institute for Clinical and Economic Review (ICER) set the upper bounds for the gene therapy at about $1.96 million. The extent to which the gene therapy will provide freedom from infusions, for how long, and in which patients are not completely understood.
Hemophilia A is caused by a mutation in the gene that produces a protein called coagulation factor VIII, which is essential for blood clotting. Valoctocogene roxaparvovec delivers a functional gene to liver cells via an adeno-associated virus serotype 5; the gene instructs the cells to make the missing clotting factor.
“Adults with severe hemophilia A face a lifelong burden, with frequent infusions and a high risk of health complications, including uncontrolled bleeding and irreversible joint damage,” Steven Pipe, MD, professor of pediatrics and pathology at the University of Michigan, Ann Arbor, and an investigator for the phase 3 study that led to the approval, said in a statement. The approval of valoctocogene roxaparvovec “has the potential to transform the way we treat adults based on years of bleed control following a single, one-time infusion.”
About 6,500 U.S. adults live with severe hemophilia A, and BioMarin said it anticipates approximately 2,500 will be eligible to receive the gene therapy following the approval. The U.S. indication is limited to patients without a history of factor VIII inhibitors and without detectable antibodies to the adeno-associated virus serotype 5.
Last August, the European Medicines Agency authorized the gene therapy for use in Europe, but according to Forbes and PharmaPhorum, uptake in Europe has been delayed because of reimbursement issues, given the cost of treatment and clinical uncertainties.
Data to date, however, are promising for most patients. Approval was based on BioMarin’s open-label, single-arm GENEr8-1 study in 134 men with severe congenital hemophilia A. Patients received a single infusion of 6 x 1013 vector genomes per kilogram.
Among the 132 patients available for 2-year evaluation, median factor VIII activity was in the range for mild hemophilia (6%-39% of normal) with an 84.5% reduction in bleeding events from baseline.
More than 80% of participants had no bleeding events requiring treatment, and there was a 98% reduction from baseline in mean use of exogenous factor VIII.
Overall, at 2 years, only 4.5% of patients had factor VIII activity consistent with severe hemophilia A; 9.1% had activity consistent with moderate disease; 59.8% had activity consistent with mild disease, and 26.5% had activity in the normal range above 40 IU/dL.
Trial investigators estimated that the typical half-life of the transgene-derived factor VIII production system is 123 weeks.
Among the six men who resumed prophylaxis, most had fewer bleeding events than when they were on prophylaxis before the infusion. All patients developed antibodies to the virus delivery vector, precluding retreatment.
Elevated alanine aminotransferase levels were the most common adverse event, occurring in 88.8% of patients, who were treated with immunosuppressants for a median of 33 weeks. Elevations persisted at 2 years in 29% of patients.
The other most common adverse events were headache (38.1%), nausea (37.3%), and increases in aspartate aminotransferase (35.1%).
A version of this article first appeared on Medscape.com.
Valoctocogene roxaparvovec, a one-time, single-dose IV infusion, is the first gene therapy approved in the United States for severe hemophilia A and will cost around $2.9 million. BioMarin has said the price tag reflects “the possibility of freedom from years” of infusions, which come to about $800,000 each year.
However, last December, the Institute for Clinical and Economic Review (ICER) set the upper bounds for the gene therapy at about $1.96 million. The extent to which the gene therapy will provide freedom from infusions, for how long, and in which patients are not completely understood.
Hemophilia A is caused by a mutation in the gene that produces a protein called coagulation factor VIII, which is essential for blood clotting. Valoctocogene roxaparvovec delivers a functional gene to liver cells via an adeno-associated virus serotype 5; the gene instructs the cells to make the missing clotting factor.
“Adults with severe hemophilia A face a lifelong burden, with frequent infusions and a high risk of health complications, including uncontrolled bleeding and irreversible joint damage,” Steven Pipe, MD, professor of pediatrics and pathology at the University of Michigan, Ann Arbor, and an investigator for the phase 3 study that led to the approval, said in a statement. The approval of valoctocogene roxaparvovec “has the potential to transform the way we treat adults based on years of bleed control following a single, one-time infusion.”
About 6,500 U.S. adults live with severe hemophilia A, and BioMarin said it anticipates approximately 2,500 will be eligible to receive the gene therapy following the approval. The U.S. indication is limited to patients without a history of factor VIII inhibitors and without detectable antibodies to the adeno-associated virus serotype 5.
Last August, the European Medicines Agency authorized the gene therapy for use in Europe, but according to Forbes and PharmaPhorum, uptake in Europe has been delayed because of reimbursement issues, given the cost of treatment and clinical uncertainties.
Data to date, however, are promising for most patients. Approval was based on BioMarin’s open-label, single-arm GENEr8-1 study in 134 men with severe congenital hemophilia A. Patients received a single infusion of 6 x 1013 vector genomes per kilogram.
Among the 132 patients available for 2-year evaluation, median factor VIII activity was in the range for mild hemophilia (6%-39% of normal) with an 84.5% reduction in bleeding events from baseline.
More than 80% of participants had no bleeding events requiring treatment, and there was a 98% reduction from baseline in mean use of exogenous factor VIII.
Overall, at 2 years, only 4.5% of patients had factor VIII activity consistent with severe hemophilia A; 9.1% had activity consistent with moderate disease; 59.8% had activity consistent with mild disease, and 26.5% had activity in the normal range above 40 IU/dL.
Trial investigators estimated that the typical half-life of the transgene-derived factor VIII production system is 123 weeks.
Among the six men who resumed prophylaxis, most had fewer bleeding events than when they were on prophylaxis before the infusion. All patients developed antibodies to the virus delivery vector, precluding retreatment.
Elevated alanine aminotransferase levels were the most common adverse event, occurring in 88.8% of patients, who were treated with immunosuppressants for a median of 33 weeks. Elevations persisted at 2 years in 29% of patients.
The other most common adverse events were headache (38.1%), nausea (37.3%), and increases in aspartate aminotransferase (35.1%).
A version of this article first appeared on Medscape.com.
AHA statement addresses equity in cardio-oncology care
A new scientific statement from the American Heart Association focuses on equity in cardio-oncology care and research.
A “growing body of evidence” suggests that women and people from underrepresented patient groups experience disproportionately higher cardiovascular effects from new and emerging anticancer therapies, the writing group, led by Daniel Addison, MD, with the Ohio State University, Columbus, pointed out.
For example, women appear to be at higher risk of immune checkpoint inhibitor–related toxicities, whereas Black patients with cancer face up to a threefold higher risk of cardiotoxicity with anticancer therapies.
With reduced screening and delayed preventive measures, Hispanic patients have more complex heart disease, cancer is diagnosed at later stages, and they receive more cardiotoxic regimens because of a lack of eligibility for novel treatments. Ultimately, this contributes to a higher incidence of treatment complications, cardiac dysfunction, and adverse patient outcomes for this patient group, they write.
Although no studies have specifically addressed cardio-oncology disparities in the LGBTQIA+ population, such disparities can be inferred from known cardiovascular disease and oncology disparities, the writing group noted.
These disparities are supported by “disparately high” risk of death after a cancer diagnosis among women and individuals from underrepresented groups, even after accounting for socioeconomic and behavioral patterns, they pointed out.
The scientific statement was published online in Circulation.
Evidence gaps and the path forward
“Despite advances in strategies to limit the risks of cardiovascular events among cancer survivors, relatively limited guidance is available to address the rapidly growing problem of disparate cardiotoxic risks among women and underrepresented patient populations,” the writing group said.
Decentralized and sporadic evaluations have led to a lack of consensus on the definitions, investigations, and potential optimal strategies to address disparate cardiotoxicity with contemporary cancer immunotherapy, as well as biologic and cytotoxic therapies, they noted.
They said caution is needed when interpreting clinical trial data about cardiotoxicity and in generalizing the results because people from diverse racial and ethnic groups have not been well represented in many trials.
The writing group outlined key evidence gaps and future research directions for addressing cardio-oncology disparities, as well as strategies to improve equity in cardio-oncology care and research.
These include the following:
- Identifying specific predictive factors of long-term cardiotoxic risk with targeted and immune-based cancer therapies in women and underrepresented populations.
- Investigating biological mechanisms that may underlie differences in cardiotoxicities between different patient groups.
- Developing personalized cardioprotection strategies that integrate biological, genetic, and social determinant markers.
- Intentionally diversifying clinical trials and identifying optimal strategies to improve representation in cancer clinical trials.
- Determining the role of technology, such as artificial intelligence, in improving cardiotoxicity disparities.
“Conscientiously leveraging technology and designing trials with outcomes related to these issues in practice (considering feasibility and cost) will critically accelerate the field of cardio-oncology in the 21st century. With tangible goals, we can improve health inequities in cardio-oncology,” the writing group said.
The research had no commercial funding. No conflicts of interest were reported.
A version of this article originally appeared on Medscape.com.
A new scientific statement from the American Heart Association focuses on equity in cardio-oncology care and research.
A “growing body of evidence” suggests that women and people from underrepresented patient groups experience disproportionately higher cardiovascular effects from new and emerging anticancer therapies, the writing group, led by Daniel Addison, MD, with the Ohio State University, Columbus, pointed out.
For example, women appear to be at higher risk of immune checkpoint inhibitor–related toxicities, whereas Black patients with cancer face up to a threefold higher risk of cardiotoxicity with anticancer therapies.
With reduced screening and delayed preventive measures, Hispanic patients have more complex heart disease, cancer is diagnosed at later stages, and they receive more cardiotoxic regimens because of a lack of eligibility for novel treatments. Ultimately, this contributes to a higher incidence of treatment complications, cardiac dysfunction, and adverse patient outcomes for this patient group, they write.
Although no studies have specifically addressed cardio-oncology disparities in the LGBTQIA+ population, such disparities can be inferred from known cardiovascular disease and oncology disparities, the writing group noted.
These disparities are supported by “disparately high” risk of death after a cancer diagnosis among women and individuals from underrepresented groups, even after accounting for socioeconomic and behavioral patterns, they pointed out.
The scientific statement was published online in Circulation.
Evidence gaps and the path forward
“Despite advances in strategies to limit the risks of cardiovascular events among cancer survivors, relatively limited guidance is available to address the rapidly growing problem of disparate cardiotoxic risks among women and underrepresented patient populations,” the writing group said.
Decentralized and sporadic evaluations have led to a lack of consensus on the definitions, investigations, and potential optimal strategies to address disparate cardiotoxicity with contemporary cancer immunotherapy, as well as biologic and cytotoxic therapies, they noted.
They said caution is needed when interpreting clinical trial data about cardiotoxicity and in generalizing the results because people from diverse racial and ethnic groups have not been well represented in many trials.
The writing group outlined key evidence gaps and future research directions for addressing cardio-oncology disparities, as well as strategies to improve equity in cardio-oncology care and research.
These include the following:
- Identifying specific predictive factors of long-term cardiotoxic risk with targeted and immune-based cancer therapies in women and underrepresented populations.
- Investigating biological mechanisms that may underlie differences in cardiotoxicities between different patient groups.
- Developing personalized cardioprotection strategies that integrate biological, genetic, and social determinant markers.
- Intentionally diversifying clinical trials and identifying optimal strategies to improve representation in cancer clinical trials.
- Determining the role of technology, such as artificial intelligence, in improving cardiotoxicity disparities.
“Conscientiously leveraging technology and designing trials with outcomes related to these issues in practice (considering feasibility and cost) will critically accelerate the field of cardio-oncology in the 21st century. With tangible goals, we can improve health inequities in cardio-oncology,” the writing group said.
The research had no commercial funding. No conflicts of interest were reported.
A version of this article originally appeared on Medscape.com.
A new scientific statement from the American Heart Association focuses on equity in cardio-oncology care and research.
A “growing body of evidence” suggests that women and people from underrepresented patient groups experience disproportionately higher cardiovascular effects from new and emerging anticancer therapies, the writing group, led by Daniel Addison, MD, with the Ohio State University, Columbus, pointed out.
For example, women appear to be at higher risk of immune checkpoint inhibitor–related toxicities, whereas Black patients with cancer face up to a threefold higher risk of cardiotoxicity with anticancer therapies.
With reduced screening and delayed preventive measures, Hispanic patients have more complex heart disease, cancer is diagnosed at later stages, and they receive more cardiotoxic regimens because of a lack of eligibility for novel treatments. Ultimately, this contributes to a higher incidence of treatment complications, cardiac dysfunction, and adverse patient outcomes for this patient group, they write.
Although no studies have specifically addressed cardio-oncology disparities in the LGBTQIA+ population, such disparities can be inferred from known cardiovascular disease and oncology disparities, the writing group noted.
These disparities are supported by “disparately high” risk of death after a cancer diagnosis among women and individuals from underrepresented groups, even after accounting for socioeconomic and behavioral patterns, they pointed out.
The scientific statement was published online in Circulation.
Evidence gaps and the path forward
“Despite advances in strategies to limit the risks of cardiovascular events among cancer survivors, relatively limited guidance is available to address the rapidly growing problem of disparate cardiotoxic risks among women and underrepresented patient populations,” the writing group said.
Decentralized and sporadic evaluations have led to a lack of consensus on the definitions, investigations, and potential optimal strategies to address disparate cardiotoxicity with contemporary cancer immunotherapy, as well as biologic and cytotoxic therapies, they noted.
They said caution is needed when interpreting clinical trial data about cardiotoxicity and in generalizing the results because people from diverse racial and ethnic groups have not been well represented in many trials.
The writing group outlined key evidence gaps and future research directions for addressing cardio-oncology disparities, as well as strategies to improve equity in cardio-oncology care and research.
These include the following:
- Identifying specific predictive factors of long-term cardiotoxic risk with targeted and immune-based cancer therapies in women and underrepresented populations.
- Investigating biological mechanisms that may underlie differences in cardiotoxicities between different patient groups.
- Developing personalized cardioprotection strategies that integrate biological, genetic, and social determinant markers.
- Intentionally diversifying clinical trials and identifying optimal strategies to improve representation in cancer clinical trials.
- Determining the role of technology, such as artificial intelligence, in improving cardiotoxicity disparities.
“Conscientiously leveraging technology and designing trials with outcomes related to these issues in practice (considering feasibility and cost) will critically accelerate the field of cardio-oncology in the 21st century. With tangible goals, we can improve health inequities in cardio-oncology,” the writing group said.
The research had no commercial funding. No conflicts of interest were reported.
A version of this article originally appeared on Medscape.com.
FROM CIRCULATION
In head and neck cancer, better outcomes seen in patients with overweight
The findings, published in JAMA Network Open, are the latest to parse the complex relationship between body mass index (BMI) and treatment in cancers that is sometimes called the “obesity paradox.” The researchers compared outcomes among patients with normal weight, overweight, and obesity.
While higher BMI is an established risk factor for many types of cancer and for cancer-specific mortality overall, studies in some cancers have shown that patients with higher BMI do better, possibly because excess BMI acts as a nutrient reserve against treatment-associated weight loss.
Methods and results
For their research, Sung Jun Ma, MD, of Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and colleagues looked at records for 445 patients (84% men, median age 61) at Dr. Ma’s institution with nonmetastatic head and neck cancer who underwent chemoradiotherapy between 2005 and 2021. Patients were followed up for a median 48 months, and those with underweight at treatment initiation were excluded.
The researchers found that overweight BMI (25-29.9 kg/m2) was associated with improved overall survival at 5 years (71% vs. 58% of patients with normal weight), as well as 5-year progression-free survival (68% vs. 51%). No overall or progression-free survival benefit link was seen in patients with a BMI of 30 or higher, in contrast to some previous studies of patients with head and neck cancers. BMI was not associated with improved survival outcomes among human papillomavirus–positive patients.
Both overweight and obesity were associated with complete response on follow-up PET-CT, with nearly 92% of patients with overweight and 91% of patients with obesity (defined as having a BMI of 30 or higher) seeing a complete metabolic response, compared with 74% of patients with normal weight.
Having an overweight BMI was also associated with improvements in tumor recurrence, with fewer of patients with this type of BMI experiencing 5-year locoregional failure than patients with normal weight (7% vs 26%). Having an obese BMI was not associated with improvements in recurrence. All the reported differences reached statistical significance.
The study authors surmised that the discrepancies between outcomes for patients with overweight and obesity “may be due to a nonlinear association between BMI and survival, with the highest survival seen in the overweight BMI range.”
It was important to note that this study saw no differences in treatment interruptions between the BMI groups that could account for differences in outcomes. Only three patients in the cohort saw their radiotherapy treatment interrupted, Dr. Ma said in an interview.
“If we felt that the obesity paradox happens because people with normal BMI lose too much weight during the treatment course, treatment gets interrupted, and they get worse outcomes from suboptimal treatments, then we would have seen more treatment interruptions among those with normal BMI. However, that was not the case in our study,” he said. Rather, the results point to “a complex interaction among cancer, [a person’s build], and nutritional status.”
Clinicians should be aware, Dr. Ma added, “that the same head and neck cancer may behave more aggressively among patients with normal BMI, compared to others with overweight BMI. Patients with normal BMI may need to be monitored more closely and carefully for potentially worse outcomes.”
The investigators acknowledged several weaknesses of their study, including its retrospective design, the measure of BMI using cutoffs rather than a continuum, and the collection of BMI information at a single time point. While 84% of patients in the study received cisplatin, the study did not contain information on cumulative cisplatin dose.
Importance of nutritional support during treatment highlighted
In an interview, Ari Rosenberg, MD, of the University of Chicago Medicine, commented that the findings highlighted the importance of expert nutritional supportive care during treatment and monitoring for patients with advanced head and neck cancers undergoing chemoradiation.
“Nutritional status is very important both at baseline and during treatment,” Dr. Rosenberg said. “Even small changes in weight or BMI can be a key indicator of supportive care during chemoradiation and represent a biomarker to guide supportive management. ... The take home message is that patients should be treated at centers that have a high volume of advanced head and neck cancer patients, which have all the supportive components and expertise to optimize treatment delivery and maximize survival.”
Dr. Ma and colleagues’ study was funded by the National Cancer Institute Cancer Center. None of its authors declared financial conflicts of interest. Dr. Rosenberg disclosed receiving consulting fees from EMD Serono related to head and neck cancer treatment.
The findings, published in JAMA Network Open, are the latest to parse the complex relationship between body mass index (BMI) and treatment in cancers that is sometimes called the “obesity paradox.” The researchers compared outcomes among patients with normal weight, overweight, and obesity.
While higher BMI is an established risk factor for many types of cancer and for cancer-specific mortality overall, studies in some cancers have shown that patients with higher BMI do better, possibly because excess BMI acts as a nutrient reserve against treatment-associated weight loss.
Methods and results
For their research, Sung Jun Ma, MD, of Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and colleagues looked at records for 445 patients (84% men, median age 61) at Dr. Ma’s institution with nonmetastatic head and neck cancer who underwent chemoradiotherapy between 2005 and 2021. Patients were followed up for a median 48 months, and those with underweight at treatment initiation were excluded.
The researchers found that overweight BMI (25-29.9 kg/m2) was associated with improved overall survival at 5 years (71% vs. 58% of patients with normal weight), as well as 5-year progression-free survival (68% vs. 51%). No overall or progression-free survival benefit link was seen in patients with a BMI of 30 or higher, in contrast to some previous studies of patients with head and neck cancers. BMI was not associated with improved survival outcomes among human papillomavirus–positive patients.
Both overweight and obesity were associated with complete response on follow-up PET-CT, with nearly 92% of patients with overweight and 91% of patients with obesity (defined as having a BMI of 30 or higher) seeing a complete metabolic response, compared with 74% of patients with normal weight.
Having an overweight BMI was also associated with improvements in tumor recurrence, with fewer of patients with this type of BMI experiencing 5-year locoregional failure than patients with normal weight (7% vs 26%). Having an obese BMI was not associated with improvements in recurrence. All the reported differences reached statistical significance.
The study authors surmised that the discrepancies between outcomes for patients with overweight and obesity “may be due to a nonlinear association between BMI and survival, with the highest survival seen in the overweight BMI range.”
It was important to note that this study saw no differences in treatment interruptions between the BMI groups that could account for differences in outcomes. Only three patients in the cohort saw their radiotherapy treatment interrupted, Dr. Ma said in an interview.
“If we felt that the obesity paradox happens because people with normal BMI lose too much weight during the treatment course, treatment gets interrupted, and they get worse outcomes from suboptimal treatments, then we would have seen more treatment interruptions among those with normal BMI. However, that was not the case in our study,” he said. Rather, the results point to “a complex interaction among cancer, [a person’s build], and nutritional status.”
Clinicians should be aware, Dr. Ma added, “that the same head and neck cancer may behave more aggressively among patients with normal BMI, compared to others with overweight BMI. Patients with normal BMI may need to be monitored more closely and carefully for potentially worse outcomes.”
The investigators acknowledged several weaknesses of their study, including its retrospective design, the measure of BMI using cutoffs rather than a continuum, and the collection of BMI information at a single time point. While 84% of patients in the study received cisplatin, the study did not contain information on cumulative cisplatin dose.
Importance of nutritional support during treatment highlighted
In an interview, Ari Rosenberg, MD, of the University of Chicago Medicine, commented that the findings highlighted the importance of expert nutritional supportive care during treatment and monitoring for patients with advanced head and neck cancers undergoing chemoradiation.
“Nutritional status is very important both at baseline and during treatment,” Dr. Rosenberg said. “Even small changes in weight or BMI can be a key indicator of supportive care during chemoradiation and represent a biomarker to guide supportive management. ... The take home message is that patients should be treated at centers that have a high volume of advanced head and neck cancer patients, which have all the supportive components and expertise to optimize treatment delivery and maximize survival.”
Dr. Ma and colleagues’ study was funded by the National Cancer Institute Cancer Center. None of its authors declared financial conflicts of interest. Dr. Rosenberg disclosed receiving consulting fees from EMD Serono related to head and neck cancer treatment.
The findings, published in JAMA Network Open, are the latest to parse the complex relationship between body mass index (BMI) and treatment in cancers that is sometimes called the “obesity paradox.” The researchers compared outcomes among patients with normal weight, overweight, and obesity.
While higher BMI is an established risk factor for many types of cancer and for cancer-specific mortality overall, studies in some cancers have shown that patients with higher BMI do better, possibly because excess BMI acts as a nutrient reserve against treatment-associated weight loss.
Methods and results
For their research, Sung Jun Ma, MD, of Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and colleagues looked at records for 445 patients (84% men, median age 61) at Dr. Ma’s institution with nonmetastatic head and neck cancer who underwent chemoradiotherapy between 2005 and 2021. Patients were followed up for a median 48 months, and those with underweight at treatment initiation were excluded.
The researchers found that overweight BMI (25-29.9 kg/m2) was associated with improved overall survival at 5 years (71% vs. 58% of patients with normal weight), as well as 5-year progression-free survival (68% vs. 51%). No overall or progression-free survival benefit link was seen in patients with a BMI of 30 or higher, in contrast to some previous studies of patients with head and neck cancers. BMI was not associated with improved survival outcomes among human papillomavirus–positive patients.
Both overweight and obesity were associated with complete response on follow-up PET-CT, with nearly 92% of patients with overweight and 91% of patients with obesity (defined as having a BMI of 30 or higher) seeing a complete metabolic response, compared with 74% of patients with normal weight.
Having an overweight BMI was also associated with improvements in tumor recurrence, with fewer of patients with this type of BMI experiencing 5-year locoregional failure than patients with normal weight (7% vs 26%). Having an obese BMI was not associated with improvements in recurrence. All the reported differences reached statistical significance.
The study authors surmised that the discrepancies between outcomes for patients with overweight and obesity “may be due to a nonlinear association between BMI and survival, with the highest survival seen in the overweight BMI range.”
It was important to note that this study saw no differences in treatment interruptions between the BMI groups that could account for differences in outcomes. Only three patients in the cohort saw their radiotherapy treatment interrupted, Dr. Ma said in an interview.
“If we felt that the obesity paradox happens because people with normal BMI lose too much weight during the treatment course, treatment gets interrupted, and they get worse outcomes from suboptimal treatments, then we would have seen more treatment interruptions among those with normal BMI. However, that was not the case in our study,” he said. Rather, the results point to “a complex interaction among cancer, [a person’s build], and nutritional status.”
Clinicians should be aware, Dr. Ma added, “that the same head and neck cancer may behave more aggressively among patients with normal BMI, compared to others with overweight BMI. Patients with normal BMI may need to be monitored more closely and carefully for potentially worse outcomes.”
The investigators acknowledged several weaknesses of their study, including its retrospective design, the measure of BMI using cutoffs rather than a continuum, and the collection of BMI information at a single time point. While 84% of patients in the study received cisplatin, the study did not contain information on cumulative cisplatin dose.
Importance of nutritional support during treatment highlighted
In an interview, Ari Rosenberg, MD, of the University of Chicago Medicine, commented that the findings highlighted the importance of expert nutritional supportive care during treatment and monitoring for patients with advanced head and neck cancers undergoing chemoradiation.
“Nutritional status is very important both at baseline and during treatment,” Dr. Rosenberg said. “Even small changes in weight or BMI can be a key indicator of supportive care during chemoradiation and represent a biomarker to guide supportive management. ... The take home message is that patients should be treated at centers that have a high volume of advanced head and neck cancer patients, which have all the supportive components and expertise to optimize treatment delivery and maximize survival.”
Dr. Ma and colleagues’ study was funded by the National Cancer Institute Cancer Center. None of its authors declared financial conflicts of interest. Dr. Rosenberg disclosed receiving consulting fees from EMD Serono related to head and neck cancer treatment.
FROM JAMA NETWORK OPEN
ESMO helps hematologists assess new cancer drugs
It consists of 11 2- to 3-page forms with checklists to grade treatment trials on the extent to which they meet efficacy and safety thresholds. Each of the 11 forms covers a specific trial scenario, such as a randomized controlled trial with curative intent or a trial of a therapy that is not likely to be curative with a primary endpoint of overall survival.
Treatments with curative intent are graded A, B, or C, while treatments in the noncurative setting are graded on a descending scale from 5 to 1. Scores of A and B in the curative setting and 5 and 4 in the noncurative setting represent substantial benefit.
On the form for RCTs with curative intent, for instance, a survival improvement of 5% or more garners an A but an improvement of less than 3% gets a C. Scores are also annotated for serious acute and/or persistent toxicity if present.
The tool, dubbed the ESMO-MCBS:H (European Society for Medical Oncology Magnitude of Clinical Benefit Scale: Hematology), is explained in an article published in Annals of Oncology. The evaluation forms are available online.
The idea behind the work is to help health care professionals and others to more “accurately assess the value of and prioritise therapies for patients with blood cancers. For clinicians, ESMO-MCBS:H will aid in their clinical decision-making and in the development of evidence-based practice and guidelines,” ESMO said in a press release.
To develop ESMO-MCBS:H, the group tailored its tool for evaluating solid tumor therapies, the ESMO-MCBS, to account for the sometimes different endpoints used in hematologic malignancy trials and the very indolent nature of some blood cancers, such as follicular lymphoma, which hampers development of mature data.
Specific changes include adding a new evaluation form to grade single-arm trials with curative intent, such as those used for CAR-T-cell therapies; incorporating molecular surrogate endpoints used in CML trials; and adding a way to grade outcomes for indolent cancers, among others.
The development process included applying the solid tumor tool to 80 blood cancer studies to identify shortcomings and improve its applicability. The final tool was field tested with 51 international experts from EHA and ESMO who largely agreed on the reasonableness of the trial scores.
ESMO said it expects ESMO-MCBS:H will be useful. The solid tumor tool, first published in 2015, is used by the World Health Organization to screen medications for its essential medicines list as well as by ESMO to generate guidelines and oncology centers across Europe to help with resource allocation decisions.
It consists of 11 2- to 3-page forms with checklists to grade treatment trials on the extent to which they meet efficacy and safety thresholds. Each of the 11 forms covers a specific trial scenario, such as a randomized controlled trial with curative intent or a trial of a therapy that is not likely to be curative with a primary endpoint of overall survival.
Treatments with curative intent are graded A, B, or C, while treatments in the noncurative setting are graded on a descending scale from 5 to 1. Scores of A and B in the curative setting and 5 and 4 in the noncurative setting represent substantial benefit.
On the form for RCTs with curative intent, for instance, a survival improvement of 5% or more garners an A but an improvement of less than 3% gets a C. Scores are also annotated for serious acute and/or persistent toxicity if present.
The tool, dubbed the ESMO-MCBS:H (European Society for Medical Oncology Magnitude of Clinical Benefit Scale: Hematology), is explained in an article published in Annals of Oncology. The evaluation forms are available online.
The idea behind the work is to help health care professionals and others to more “accurately assess the value of and prioritise therapies for patients with blood cancers. For clinicians, ESMO-MCBS:H will aid in their clinical decision-making and in the development of evidence-based practice and guidelines,” ESMO said in a press release.
To develop ESMO-MCBS:H, the group tailored its tool for evaluating solid tumor therapies, the ESMO-MCBS, to account for the sometimes different endpoints used in hematologic malignancy trials and the very indolent nature of some blood cancers, such as follicular lymphoma, which hampers development of mature data.
Specific changes include adding a new evaluation form to grade single-arm trials with curative intent, such as those used for CAR-T-cell therapies; incorporating molecular surrogate endpoints used in CML trials; and adding a way to grade outcomes for indolent cancers, among others.
The development process included applying the solid tumor tool to 80 blood cancer studies to identify shortcomings and improve its applicability. The final tool was field tested with 51 international experts from EHA and ESMO who largely agreed on the reasonableness of the trial scores.
ESMO said it expects ESMO-MCBS:H will be useful. The solid tumor tool, first published in 2015, is used by the World Health Organization to screen medications for its essential medicines list as well as by ESMO to generate guidelines and oncology centers across Europe to help with resource allocation decisions.
It consists of 11 2- to 3-page forms with checklists to grade treatment trials on the extent to which they meet efficacy and safety thresholds. Each of the 11 forms covers a specific trial scenario, such as a randomized controlled trial with curative intent or a trial of a therapy that is not likely to be curative with a primary endpoint of overall survival.
Treatments with curative intent are graded A, B, or C, while treatments in the noncurative setting are graded on a descending scale from 5 to 1. Scores of A and B in the curative setting and 5 and 4 in the noncurative setting represent substantial benefit.
On the form for RCTs with curative intent, for instance, a survival improvement of 5% or more garners an A but an improvement of less than 3% gets a C. Scores are also annotated for serious acute and/or persistent toxicity if present.
The tool, dubbed the ESMO-MCBS:H (European Society for Medical Oncology Magnitude of Clinical Benefit Scale: Hematology), is explained in an article published in Annals of Oncology. The evaluation forms are available online.
The idea behind the work is to help health care professionals and others to more “accurately assess the value of and prioritise therapies for patients with blood cancers. For clinicians, ESMO-MCBS:H will aid in their clinical decision-making and in the development of evidence-based practice and guidelines,” ESMO said in a press release.
To develop ESMO-MCBS:H, the group tailored its tool for evaluating solid tumor therapies, the ESMO-MCBS, to account for the sometimes different endpoints used in hematologic malignancy trials and the very indolent nature of some blood cancers, such as follicular lymphoma, which hampers development of mature data.
Specific changes include adding a new evaluation form to grade single-arm trials with curative intent, such as those used for CAR-T-cell therapies; incorporating molecular surrogate endpoints used in CML trials; and adding a way to grade outcomes for indolent cancers, among others.
The development process included applying the solid tumor tool to 80 blood cancer studies to identify shortcomings and improve its applicability. The final tool was field tested with 51 international experts from EHA and ESMO who largely agreed on the reasonableness of the trial scores.
ESMO said it expects ESMO-MCBS:H will be useful. The solid tumor tool, first published in 2015, is used by the World Health Organization to screen medications for its essential medicines list as well as by ESMO to generate guidelines and oncology centers across Europe to help with resource allocation decisions.
FROM ANNALS OF ONCOLOGY