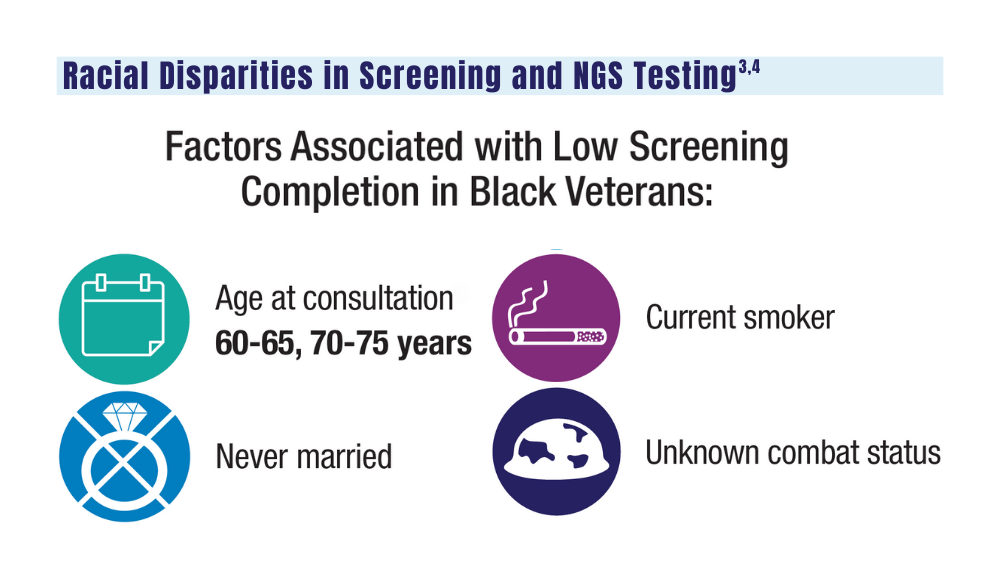
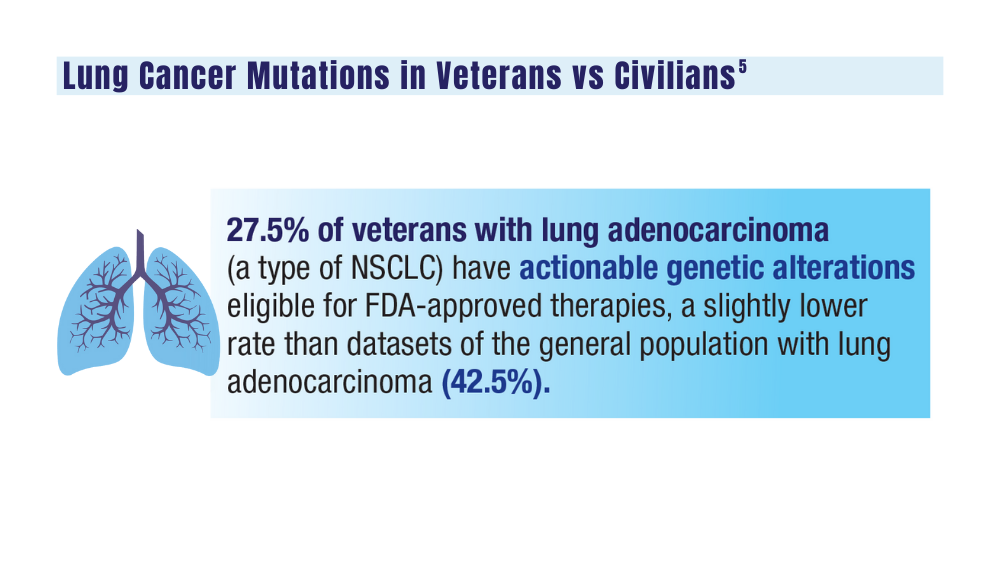
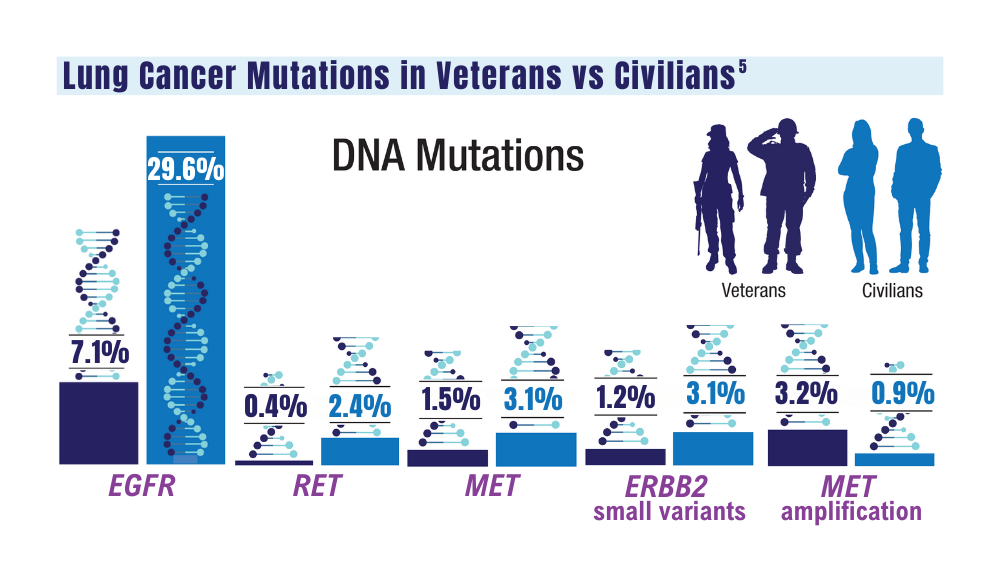
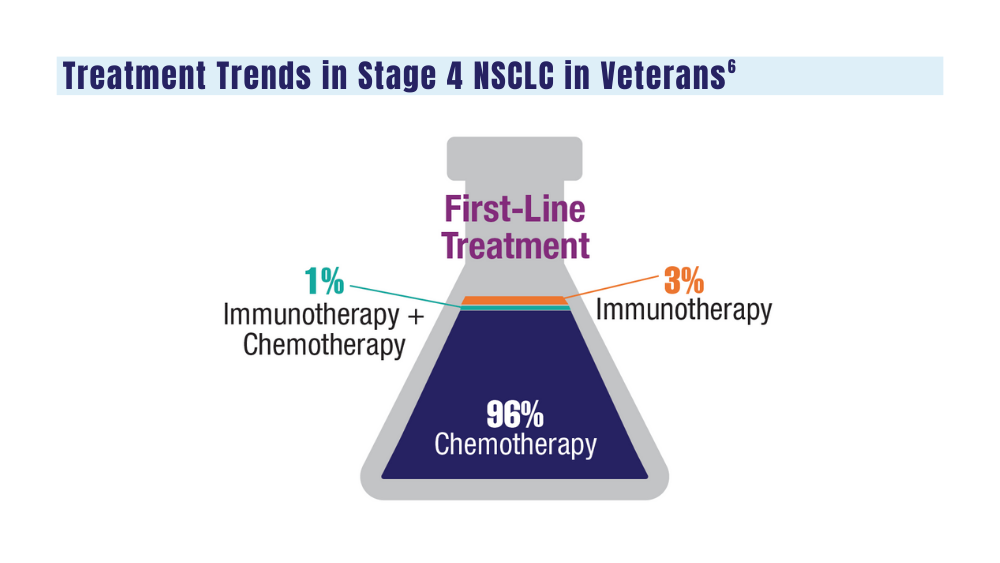
User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


New CRC stool test beats FIT for sensitivity but not specificity
, according to the large prospective BLUE-C study.
The multi-target assay by Exact Sciences Corporation, the makers of Cologuard, includes new biomarkers designed to increase specificity without decreasing sensitivity. It showed a sensitivity for CRC of almost 94%, with more than 43% sensitivity for advanced precancerous lesions and nearly 91% specificity for advanced neoplasia, according to the study results, which were published in The New England Journal of Medicine.
Adherence to CRC screening in the United States is well below the 80% national target, and the quest continues for noninvasive screening assays that might improve screening adherence, noted lead author Thomas F. Imperiale, MD, AGAF, a professor of medicine at Indiana University School of medicine in Indianapolis, and colleagues.
“The test’s manufacturer developed a new version of its existing Cologuard FIT/DNA test because it took to heart the feedback from primary care providers and gastroenterologists about the test’s low specificity,” Dr. Imperiale said in an interview. “The goal of the new test was to improve specificity without losing, and perhaps even gaining, some sensitivity — a goal that is not easily accomplished when you’re trying to improve on a sensitivity for colorectal cancer that was already 92.3% in the current version of Cologuard.”
Compared with the earlier version of Cologuard, he added, the new generation retained sensitivity for CRC and advanced precancerous lesions or polyps while improving specificity by 30% (90.6% vs 86.6%) for advanced neoplasia — a combination of CRC and advanced precancerous lesions, he said. “This with the caveat, however, that the two versions were not compared head-to-head in this new study,” Dr. Imperiale said.
The higher specificity for advanced lesions is expected to translate to a lower false positive rate. Lowering false positive rates is crucial because that reduces the need for costly, invasive, and unnecessary colonoscopies, said Aasma Shaukat, MD, MPH, AGAF, director of outcomes research in NYU Langone Health’s division of gastroenterology and hepatology in New York City.
“Many physicians felt there were too many false positives with the existing version, and that is anxiety-provoking in patients and providers,” said Dr. Shaukat, who was not involved in the study.
In her view, however, the test’s moderate improvements in detecting certain lesions does not make it demonstrably superior to its predecessor, and there is always the possibility of higher cost to consider.
While acknowledging that a higher sensitivity for all advanced precancerous lesions would have been welcome, Dr. Imperiale said the test detected 75% of the most worrisome of such lesions — “the ones containing high-grade dysplastic cells and suggesting near-term conversion to cancer. And its ability to detect other advanced lesions improved as the size of the lesions increased.”
Testing details
Almost 21,000 asymptomatic participants age 40 years and older undergoing screening colonoscopy were evaluated at 186 US sites during the period 2019 to 2023. Of the cohort, 98 had CRC, 2144 had advanced precancerous lesions, 6973 had nonadvanced adenomas, and 10,961 had nonneoplastic findings or negative colonoscopy.
Advanced precancerous lesions included one or more adenomas or sessile serrated lesions measuring at least 1 cm in the longest dimension, lesions with villous histologic features, and high-grade dysplasia. The new DNA test identified 92 of 98 participants with CRC and 76 of 82 participants with screening-relevant cancers. Among the findings for the new assay:
- Sensitivity for any-stage CRC was 93.9% (95% confidence interval [CI], 87.1- 97.7)
- Sensitivity for advanced precancerous lesions was 43.4% (95% CI, 41.3-45.6)
- Sensitivity for high-grade dysplasia was 74.6% (95% CI, 65.6-82.3)
- Specificity for advanced neoplasia was 90.6% (95% CI, 90.1- 91.0).
- Specificity for nonneoplastic findings or negative colonoscopy was 92.7% (95% CI, 92.2-93.1)
- Specificity for negative colonoscopy was 93.3 (95% CI, 92.8-93.9)
- No adverse events occurred.
In the comparator assay, OC-AUTO FIT by Polymedco, sensitivity was 67.3% (95% CI, 57.1-76.5) for CRC, 23.3% (95% CI, 21.5-25.2) for advanced precancerous lesions, and 47.4% (95% CI, 37.9-56.9) for high-grade dysplasia. In the comparator FIT, however, specificity was better across all age groups — at 94.8% (95% CI, 94.4-95.1) for advanced neoplasia, 95.7% (95% CI, 95.3- 96.1) for nonneoplastic findings, and 96.0% (95% CI, 95.5-96.4) for negative colonoscopy.
In another article in the same issue of NEJM, Guardant Health’s cell-free DNA blood-based test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions in an average-risk population.
An age-related decrease in specificity was observed with the new Cologuard test, but that did not concern Dr. Imperiale because the same observation was made with the current version. “In fact, the next-gen version appears to have less of an age-related decrease in specificity than the current version, although, again, the two versions were not tested head-to-head,” he noted.
The effect of age-related background methylation of DNA is well known, he explained. “Clinicians and older patients in the screening age range do need to be aware of this effect on specificity before ordering or agreeing to do the test. I do not see this as a stumbling block to implementation, but it does require discussion between patient and ordering provider.”
The new version of the DNA test is expected to be available in about a year.
According to Dr. Imperiale, further research is needed to ascertain the test’s acceptability and adherence rates and to quantify its yield in population-based screening. Determining its cost-effectiveness and making it easier to use are other goals. “And most importantly, the degree of reduction in the incidence and mortality from colorectal cancer,” he said.
Cost-effectiveness and the selection of the testing interval may play roles in adherence, particularly in populations with lower rates of screening adherence than the general population, John M. Carethers, MD, AGAF, of the University of California, San Diego, noted in a related editorial.
“Adherence to screening varies according to age group, including persons in the 45- to 49-year age group who are now eligible for average-risk screening,” he wrote. “It is hoped that these newer tests will increase use and adherence and elevate the percentage of the population undergoing screening in order to reduce deaths from colorectal cancer.”
This study was sponsored by Exact Sciences Corporation, which conducted the stool testing at its laboratories.
Dr. Imperiale had no competing interests to disclose. Several study co-authors reported employment with Exact Sciences, or stock and intellectual property ownership. Dr. Shaukat disclosed consulting for Freenome. Dr. Carethers reported ties to Avantor Inc. and Geneoscopy.
, according to the large prospective BLUE-C study.
The multi-target assay by Exact Sciences Corporation, the makers of Cologuard, includes new biomarkers designed to increase specificity without decreasing sensitivity. It showed a sensitivity for CRC of almost 94%, with more than 43% sensitivity for advanced precancerous lesions and nearly 91% specificity for advanced neoplasia, according to the study results, which were published in The New England Journal of Medicine.
Adherence to CRC screening in the United States is well below the 80% national target, and the quest continues for noninvasive screening assays that might improve screening adherence, noted lead author Thomas F. Imperiale, MD, AGAF, a professor of medicine at Indiana University School of medicine in Indianapolis, and colleagues.
“The test’s manufacturer developed a new version of its existing Cologuard FIT/DNA test because it took to heart the feedback from primary care providers and gastroenterologists about the test’s low specificity,” Dr. Imperiale said in an interview. “The goal of the new test was to improve specificity without losing, and perhaps even gaining, some sensitivity — a goal that is not easily accomplished when you’re trying to improve on a sensitivity for colorectal cancer that was already 92.3% in the current version of Cologuard.”
Compared with the earlier version of Cologuard, he added, the new generation retained sensitivity for CRC and advanced precancerous lesions or polyps while improving specificity by 30% (90.6% vs 86.6%) for advanced neoplasia — a combination of CRC and advanced precancerous lesions, he said. “This with the caveat, however, that the two versions were not compared head-to-head in this new study,” Dr. Imperiale said.
The higher specificity for advanced lesions is expected to translate to a lower false positive rate. Lowering false positive rates is crucial because that reduces the need for costly, invasive, and unnecessary colonoscopies, said Aasma Shaukat, MD, MPH, AGAF, director of outcomes research in NYU Langone Health’s division of gastroenterology and hepatology in New York City.
“Many physicians felt there were too many false positives with the existing version, and that is anxiety-provoking in patients and providers,” said Dr. Shaukat, who was not involved in the study.
In her view, however, the test’s moderate improvements in detecting certain lesions does not make it demonstrably superior to its predecessor, and there is always the possibility of higher cost to consider.
While acknowledging that a higher sensitivity for all advanced precancerous lesions would have been welcome, Dr. Imperiale said the test detected 75% of the most worrisome of such lesions — “the ones containing high-grade dysplastic cells and suggesting near-term conversion to cancer. And its ability to detect other advanced lesions improved as the size of the lesions increased.”
Testing details
Almost 21,000 asymptomatic participants age 40 years and older undergoing screening colonoscopy were evaluated at 186 US sites during the period 2019 to 2023. Of the cohort, 98 had CRC, 2144 had advanced precancerous lesions, 6973 had nonadvanced adenomas, and 10,961 had nonneoplastic findings or negative colonoscopy.
Advanced precancerous lesions included one or more adenomas or sessile serrated lesions measuring at least 1 cm in the longest dimension, lesions with villous histologic features, and high-grade dysplasia. The new DNA test identified 92 of 98 participants with CRC and 76 of 82 participants with screening-relevant cancers. Among the findings for the new assay:
- Sensitivity for any-stage CRC was 93.9% (95% confidence interval [CI], 87.1- 97.7)
- Sensitivity for advanced precancerous lesions was 43.4% (95% CI, 41.3-45.6)
- Sensitivity for high-grade dysplasia was 74.6% (95% CI, 65.6-82.3)
- Specificity for advanced neoplasia was 90.6% (95% CI, 90.1- 91.0).
- Specificity for nonneoplastic findings or negative colonoscopy was 92.7% (95% CI, 92.2-93.1)
- Specificity for negative colonoscopy was 93.3 (95% CI, 92.8-93.9)
- No adverse events occurred.
In the comparator assay, OC-AUTO FIT by Polymedco, sensitivity was 67.3% (95% CI, 57.1-76.5) for CRC, 23.3% (95% CI, 21.5-25.2) for advanced precancerous lesions, and 47.4% (95% CI, 37.9-56.9) for high-grade dysplasia. In the comparator FIT, however, specificity was better across all age groups — at 94.8% (95% CI, 94.4-95.1) for advanced neoplasia, 95.7% (95% CI, 95.3- 96.1) for nonneoplastic findings, and 96.0% (95% CI, 95.5-96.4) for negative colonoscopy.
In another article in the same issue of NEJM, Guardant Health’s cell-free DNA blood-based test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions in an average-risk population.
An age-related decrease in specificity was observed with the new Cologuard test, but that did not concern Dr. Imperiale because the same observation was made with the current version. “In fact, the next-gen version appears to have less of an age-related decrease in specificity than the current version, although, again, the two versions were not tested head-to-head,” he noted.
The effect of age-related background methylation of DNA is well known, he explained. “Clinicians and older patients in the screening age range do need to be aware of this effect on specificity before ordering or agreeing to do the test. I do not see this as a stumbling block to implementation, but it does require discussion between patient and ordering provider.”
The new version of the DNA test is expected to be available in about a year.
According to Dr. Imperiale, further research is needed to ascertain the test’s acceptability and adherence rates and to quantify its yield in population-based screening. Determining its cost-effectiveness and making it easier to use are other goals. “And most importantly, the degree of reduction in the incidence and mortality from colorectal cancer,” he said.
Cost-effectiveness and the selection of the testing interval may play roles in adherence, particularly in populations with lower rates of screening adherence than the general population, John M. Carethers, MD, AGAF, of the University of California, San Diego, noted in a related editorial.
“Adherence to screening varies according to age group, including persons in the 45- to 49-year age group who are now eligible for average-risk screening,” he wrote. “It is hoped that these newer tests will increase use and adherence and elevate the percentage of the population undergoing screening in order to reduce deaths from colorectal cancer.”
This study was sponsored by Exact Sciences Corporation, which conducted the stool testing at its laboratories.
Dr. Imperiale had no competing interests to disclose. Several study co-authors reported employment with Exact Sciences, or stock and intellectual property ownership. Dr. Shaukat disclosed consulting for Freenome. Dr. Carethers reported ties to Avantor Inc. and Geneoscopy.
, according to the large prospective BLUE-C study.
The multi-target assay by Exact Sciences Corporation, the makers of Cologuard, includes new biomarkers designed to increase specificity without decreasing sensitivity. It showed a sensitivity for CRC of almost 94%, with more than 43% sensitivity for advanced precancerous lesions and nearly 91% specificity for advanced neoplasia, according to the study results, which were published in The New England Journal of Medicine.
Adherence to CRC screening in the United States is well below the 80% national target, and the quest continues for noninvasive screening assays that might improve screening adherence, noted lead author Thomas F. Imperiale, MD, AGAF, a professor of medicine at Indiana University School of medicine in Indianapolis, and colleagues.
“The test’s manufacturer developed a new version of its existing Cologuard FIT/DNA test because it took to heart the feedback from primary care providers and gastroenterologists about the test’s low specificity,” Dr. Imperiale said in an interview. “The goal of the new test was to improve specificity without losing, and perhaps even gaining, some sensitivity — a goal that is not easily accomplished when you’re trying to improve on a sensitivity for colorectal cancer that was already 92.3% in the current version of Cologuard.”
Compared with the earlier version of Cologuard, he added, the new generation retained sensitivity for CRC and advanced precancerous lesions or polyps while improving specificity by 30% (90.6% vs 86.6%) for advanced neoplasia — a combination of CRC and advanced precancerous lesions, he said. “This with the caveat, however, that the two versions were not compared head-to-head in this new study,” Dr. Imperiale said.
The higher specificity for advanced lesions is expected to translate to a lower false positive rate. Lowering false positive rates is crucial because that reduces the need for costly, invasive, and unnecessary colonoscopies, said Aasma Shaukat, MD, MPH, AGAF, director of outcomes research in NYU Langone Health’s division of gastroenterology and hepatology in New York City.
“Many physicians felt there were too many false positives with the existing version, and that is anxiety-provoking in patients and providers,” said Dr. Shaukat, who was not involved in the study.
In her view, however, the test’s moderate improvements in detecting certain lesions does not make it demonstrably superior to its predecessor, and there is always the possibility of higher cost to consider.
While acknowledging that a higher sensitivity for all advanced precancerous lesions would have been welcome, Dr. Imperiale said the test detected 75% of the most worrisome of such lesions — “the ones containing high-grade dysplastic cells and suggesting near-term conversion to cancer. And its ability to detect other advanced lesions improved as the size of the lesions increased.”
Testing details
Almost 21,000 asymptomatic participants age 40 years and older undergoing screening colonoscopy were evaluated at 186 US sites during the period 2019 to 2023. Of the cohort, 98 had CRC, 2144 had advanced precancerous lesions, 6973 had nonadvanced adenomas, and 10,961 had nonneoplastic findings or negative colonoscopy.
Advanced precancerous lesions included one or more adenomas or sessile serrated lesions measuring at least 1 cm in the longest dimension, lesions with villous histologic features, and high-grade dysplasia. The new DNA test identified 92 of 98 participants with CRC and 76 of 82 participants with screening-relevant cancers. Among the findings for the new assay:
- Sensitivity for any-stage CRC was 93.9% (95% confidence interval [CI], 87.1- 97.7)
- Sensitivity for advanced precancerous lesions was 43.4% (95% CI, 41.3-45.6)
- Sensitivity for high-grade dysplasia was 74.6% (95% CI, 65.6-82.3)
- Specificity for advanced neoplasia was 90.6% (95% CI, 90.1- 91.0).
- Specificity for nonneoplastic findings or negative colonoscopy was 92.7% (95% CI, 92.2-93.1)
- Specificity for negative colonoscopy was 93.3 (95% CI, 92.8-93.9)
- No adverse events occurred.
In the comparator assay, OC-AUTO FIT by Polymedco, sensitivity was 67.3% (95% CI, 57.1-76.5) for CRC, 23.3% (95% CI, 21.5-25.2) for advanced precancerous lesions, and 47.4% (95% CI, 37.9-56.9) for high-grade dysplasia. In the comparator FIT, however, specificity was better across all age groups — at 94.8% (95% CI, 94.4-95.1) for advanced neoplasia, 95.7% (95% CI, 95.3- 96.1) for nonneoplastic findings, and 96.0% (95% CI, 95.5-96.4) for negative colonoscopy.
In another article in the same issue of NEJM, Guardant Health’s cell-free DNA blood-based test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions in an average-risk population.
An age-related decrease in specificity was observed with the new Cologuard test, but that did not concern Dr. Imperiale because the same observation was made with the current version. “In fact, the next-gen version appears to have less of an age-related decrease in specificity than the current version, although, again, the two versions were not tested head-to-head,” he noted.
The effect of age-related background methylation of DNA is well known, he explained. “Clinicians and older patients in the screening age range do need to be aware of this effect on specificity before ordering or agreeing to do the test. I do not see this as a stumbling block to implementation, but it does require discussion between patient and ordering provider.”
The new version of the DNA test is expected to be available in about a year.
According to Dr. Imperiale, further research is needed to ascertain the test’s acceptability and adherence rates and to quantify its yield in population-based screening. Determining its cost-effectiveness and making it easier to use are other goals. “And most importantly, the degree of reduction in the incidence and mortality from colorectal cancer,” he said.
Cost-effectiveness and the selection of the testing interval may play roles in adherence, particularly in populations with lower rates of screening adherence than the general population, John M. Carethers, MD, AGAF, of the University of California, San Diego, noted in a related editorial.
“Adherence to screening varies according to age group, including persons in the 45- to 49-year age group who are now eligible for average-risk screening,” he wrote. “It is hoped that these newer tests will increase use and adherence and elevate the percentage of the population undergoing screening in order to reduce deaths from colorectal cancer.”
This study was sponsored by Exact Sciences Corporation, which conducted the stool testing at its laboratories.
Dr. Imperiale had no competing interests to disclose. Several study co-authors reported employment with Exact Sciences, or stock and intellectual property ownership. Dr. Shaukat disclosed consulting for Freenome. Dr. Carethers reported ties to Avantor Inc. and Geneoscopy.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Extraordinary Patients Inspired Father of Cancer Immunotherapy
His pioneering research established interleukin-2 (IL-2) as the first U.S. Food and Drug Administration–approved cancer immunotherapy in 1992.
To recognize his trailblazing work and other achievements, the American Association for Cancer Research (AACR) will award Dr. Rosenberg with the 2024 AACR Award for Lifetime Achievement in Cancer Research at its annual meeting in April.
Dr. Rosenberg, a senior investigator for the Center for Cancer Research at the National Cancer Institute (NCI), and chief of the NCI Surgery Branch, shared the history behind his novel research and the patient stories that inspired his discoveries, during an interview.
Tell us a little about yourself and where you grew up.
Dr. Rosenberg: I grew up in the Bronx. My parents both immigrated to the United States from Poland as teenagers.
As a young boy, did you always want to become a doctor?
Dr. Rosenberg: I think some defining moments on why I decided to go into medicine occurred when I was 6 or 7 years old. The second world war was over, and many of the horrors of the Holocaust became apparent to me. I was brought up as an Orthodox Jew. My parents were quite religious, and I remember postcards coming in one after another about relatives that had died in the death camps. That had a profound influence on me.
How did that experience impact your aspirations?
Dr. Rosenberg: It was an example to me of how evil certain people and groups can be toward one another. I decided at that point, that I wanted to do something good for people, and medicine seemed the most likely way to do that. But also, I was developing a broad scientific interest. I ended up at the Bronx High School of Science and knew that I not only wanted to practice the medicine of today, but I wanted to play a role in helping develop the medicine.
What led to your interest in cancer treatment?
Dr. Rosenberg: Well, as a medical student and resident, it became clear that the field of cancer needed major improvement. We had three major ways to treat cancer: surgery, radiation therapy, and chemotherapy. That could cure about half of the people [who] had cancer. But despite the best application of those three specialties, there were over 600,000 deaths from cancer each year in the United States alone. It was clear to me that new approaches were needed, and I became very interested in taking advantage of the body’s immune system as a source of information to try to make progress.
Were there patients who inspired your research?
Dr. Rosenberg: There were two patients that I saw early in my career that impressed me a great deal. One was a patient that I saw when working in the emergency ward as a resident. A patient came in with right upper quadrant pain that looked like a gallbladder attack. That’s what it was. But when I went through his chart, I saw that he had been at that hospital 12 years earlier with a metastatic gastric cancer. The surgeons had operated. They saw tumor had spread to the liver and could not be removed. They closed the belly, not expecting him to survive. Yet he kept showing up for follow-up visits.
Here he was 12 years later. When I helped operate to take out his gallbladder, there was no evidence of any cancer. The cancer had disappeared in the absence of any external treatment. One of the rarest events in medicine, the spontaneous regression of a cancer. Somehow his body had learned how to destroy the tumor.
Was the second patient’s case as impressive?
Dr. Rosenberg: This patient had received a kidney transplant from a gentleman who died in an auto accident. [The donor’s] kidney contained a cancer deposit, a kidney cancer, unbeknownst to the transplant surgeons. [When the kidney was transplanted], the recipient developed widespread metastatic kidney cancer.
[The recipient] was on immunosuppressive drugs, and so the drugs had to be stopped. [When the immunosuppressive drugs were stopped], the patient’s body rejected the kidney and his cancer disappeared.
That showed me that, in fact, if you could stimulate a strong enough immune reaction, in this case, an [allogeneic] reaction, against foreign tissues from a different individual, that you could make large vascularized, invasive cancers disappear based on immune reactivities. Those were clues that led me toward studying the immune system’s impact on cancer.
From there, how did your work evolve?
Dr. Rosenberg: As chief of the surgery branch at NIH, I began doing research. It was very difficult to manipulate immune cells in the laboratory. They wouldn’t stay alive. But I tried to study immune reactions in patients with cancer to see if there was such a thing as an immune reaction against the cancer. There was no such thing known at the time. There were no cancer antigens and no known immune reactions against the disease in the human.
Around this time, investigators were publishing studies about interleukin-2 (IL-2), or white blood cells known as leukocytes. How did interleukin-2 further your research?
Dr. Rosenberg: The advent of interleukin-2 enabled scientists to grow lymphocytes outside the body. [This] enabled us to grow t-lymphocytes, which are some of the major warriors of the immune system against foreign tissue. After [studying] 66 patients in which we studied interleukin-2 and cells that would develop from it, we finally saw a disappearance of melanoma in a patient that received interleukin-2. And we went on to treat hundreds of patients with that hormone, interleukin-2. In fact, interleukin-2 became the first immunotherapy ever approved by the Food and Drug Administration for the treatment of cancer in humans.
How did this finding impact your future discoveries?
Dr. Rosenberg: [It] led to studies of the mechanism of action of interleukin-2 and to do that, we identified a kind of cell called a tumor infiltrating lymphocyte. What better place, intuitively to look for cells doing battle against the cancer than within the cancer itself?
In 1988, we demonstrated for the first time that transfer of lymphocytes with antitumor activity could cause the regression of melanoma. This was a living drug obtained from melanoma deposits that could be grown outside the body and then readministered to the patient under suitable conditions. Interestingly, [in February the FDA approved that drug as treatment for patients with melanoma]. A company developed it to the point where in multi-institutional studies, they reproduced our results.
And we’ve now emphasized the value of using T cell therapy, t cell transfer, for the treatment of patients with the common solid cancers, the cancers that start anywhere from the colon up through the intestine, the stomach, the pancreas, and the esophagus. Solid tumors such as ovarian cancer, uterine cancer and so on, are also potentially susceptible to this T cell therapy.
We’ve published several papers showing in isolated patients that you could cause major regressions, if not complete regressions, of these solid cancers in the liver, in the breast, the cervix, the colon. That’s a major aspect of what we’re doing now.
I think immunotherapy has come to be recognized as a major fourth arm that can be used to attack cancers, adding to surgery, radiation, and chemotherapy.
What guidance would you have for other physician-investigators or young doctors who want to follow in your path?
Dr. Rosenberg: You have to have a broad base of knowledge. You have to be willing to immerse yourself in a problem so that your mind is working on it when you’re doing things where you can only think. [When] you’re taking a shower, [or] waiting at a red light, your mind is working on this problem because you’re immersed in trying to understand it.
You need to have a laser focus on the goals that you have and not get sidetracked by issues that may be interesting but not directly related to the goals that you’re attempting to achieve.
His pioneering research established interleukin-2 (IL-2) as the first U.S. Food and Drug Administration–approved cancer immunotherapy in 1992.
To recognize his trailblazing work and other achievements, the American Association for Cancer Research (AACR) will award Dr. Rosenberg with the 2024 AACR Award for Lifetime Achievement in Cancer Research at its annual meeting in April.
Dr. Rosenberg, a senior investigator for the Center for Cancer Research at the National Cancer Institute (NCI), and chief of the NCI Surgery Branch, shared the history behind his novel research and the patient stories that inspired his discoveries, during an interview.
Tell us a little about yourself and where you grew up.
Dr. Rosenberg: I grew up in the Bronx. My parents both immigrated to the United States from Poland as teenagers.
As a young boy, did you always want to become a doctor?
Dr. Rosenberg: I think some defining moments on why I decided to go into medicine occurred when I was 6 or 7 years old. The second world war was over, and many of the horrors of the Holocaust became apparent to me. I was brought up as an Orthodox Jew. My parents were quite religious, and I remember postcards coming in one after another about relatives that had died in the death camps. That had a profound influence on me.
How did that experience impact your aspirations?
Dr. Rosenberg: It was an example to me of how evil certain people and groups can be toward one another. I decided at that point, that I wanted to do something good for people, and medicine seemed the most likely way to do that. But also, I was developing a broad scientific interest. I ended up at the Bronx High School of Science and knew that I not only wanted to practice the medicine of today, but I wanted to play a role in helping develop the medicine.
What led to your interest in cancer treatment?
Dr. Rosenberg: Well, as a medical student and resident, it became clear that the field of cancer needed major improvement. We had three major ways to treat cancer: surgery, radiation therapy, and chemotherapy. That could cure about half of the people [who] had cancer. But despite the best application of those three specialties, there were over 600,000 deaths from cancer each year in the United States alone. It was clear to me that new approaches were needed, and I became very interested in taking advantage of the body’s immune system as a source of information to try to make progress.
Were there patients who inspired your research?
Dr. Rosenberg: There were two patients that I saw early in my career that impressed me a great deal. One was a patient that I saw when working in the emergency ward as a resident. A patient came in with right upper quadrant pain that looked like a gallbladder attack. That’s what it was. But when I went through his chart, I saw that he had been at that hospital 12 years earlier with a metastatic gastric cancer. The surgeons had operated. They saw tumor had spread to the liver and could not be removed. They closed the belly, not expecting him to survive. Yet he kept showing up for follow-up visits.
Here he was 12 years later. When I helped operate to take out his gallbladder, there was no evidence of any cancer. The cancer had disappeared in the absence of any external treatment. One of the rarest events in medicine, the spontaneous regression of a cancer. Somehow his body had learned how to destroy the tumor.
Was the second patient’s case as impressive?
Dr. Rosenberg: This patient had received a kidney transplant from a gentleman who died in an auto accident. [The donor’s] kidney contained a cancer deposit, a kidney cancer, unbeknownst to the transplant surgeons. [When the kidney was transplanted], the recipient developed widespread metastatic kidney cancer.
[The recipient] was on immunosuppressive drugs, and so the drugs had to be stopped. [When the immunosuppressive drugs were stopped], the patient’s body rejected the kidney and his cancer disappeared.
That showed me that, in fact, if you could stimulate a strong enough immune reaction, in this case, an [allogeneic] reaction, against foreign tissues from a different individual, that you could make large vascularized, invasive cancers disappear based on immune reactivities. Those were clues that led me toward studying the immune system’s impact on cancer.
From there, how did your work evolve?
Dr. Rosenberg: As chief of the surgery branch at NIH, I began doing research. It was very difficult to manipulate immune cells in the laboratory. They wouldn’t stay alive. But I tried to study immune reactions in patients with cancer to see if there was such a thing as an immune reaction against the cancer. There was no such thing known at the time. There were no cancer antigens and no known immune reactions against the disease in the human.
Around this time, investigators were publishing studies about interleukin-2 (IL-2), or white blood cells known as leukocytes. How did interleukin-2 further your research?
Dr. Rosenberg: The advent of interleukin-2 enabled scientists to grow lymphocytes outside the body. [This] enabled us to grow t-lymphocytes, which are some of the major warriors of the immune system against foreign tissue. After [studying] 66 patients in which we studied interleukin-2 and cells that would develop from it, we finally saw a disappearance of melanoma in a patient that received interleukin-2. And we went on to treat hundreds of patients with that hormone, interleukin-2. In fact, interleukin-2 became the first immunotherapy ever approved by the Food and Drug Administration for the treatment of cancer in humans.
How did this finding impact your future discoveries?
Dr. Rosenberg: [It] led to studies of the mechanism of action of interleukin-2 and to do that, we identified a kind of cell called a tumor infiltrating lymphocyte. What better place, intuitively to look for cells doing battle against the cancer than within the cancer itself?
In 1988, we demonstrated for the first time that transfer of lymphocytes with antitumor activity could cause the regression of melanoma. This was a living drug obtained from melanoma deposits that could be grown outside the body and then readministered to the patient under suitable conditions. Interestingly, [in February the FDA approved that drug as treatment for patients with melanoma]. A company developed it to the point where in multi-institutional studies, they reproduced our results.
And we’ve now emphasized the value of using T cell therapy, t cell transfer, for the treatment of patients with the common solid cancers, the cancers that start anywhere from the colon up through the intestine, the stomach, the pancreas, and the esophagus. Solid tumors such as ovarian cancer, uterine cancer and so on, are also potentially susceptible to this T cell therapy.
We’ve published several papers showing in isolated patients that you could cause major regressions, if not complete regressions, of these solid cancers in the liver, in the breast, the cervix, the colon. That’s a major aspect of what we’re doing now.
I think immunotherapy has come to be recognized as a major fourth arm that can be used to attack cancers, adding to surgery, radiation, and chemotherapy.
What guidance would you have for other physician-investigators or young doctors who want to follow in your path?
Dr. Rosenberg: You have to have a broad base of knowledge. You have to be willing to immerse yourself in a problem so that your mind is working on it when you’re doing things where you can only think. [When] you’re taking a shower, [or] waiting at a red light, your mind is working on this problem because you’re immersed in trying to understand it.
You need to have a laser focus on the goals that you have and not get sidetracked by issues that may be interesting but not directly related to the goals that you’re attempting to achieve.
His pioneering research established interleukin-2 (IL-2) as the first U.S. Food and Drug Administration–approved cancer immunotherapy in 1992.
To recognize his trailblazing work and other achievements, the American Association for Cancer Research (AACR) will award Dr. Rosenberg with the 2024 AACR Award for Lifetime Achievement in Cancer Research at its annual meeting in April.
Dr. Rosenberg, a senior investigator for the Center for Cancer Research at the National Cancer Institute (NCI), and chief of the NCI Surgery Branch, shared the history behind his novel research and the patient stories that inspired his discoveries, during an interview.
Tell us a little about yourself and where you grew up.
Dr. Rosenberg: I grew up in the Bronx. My parents both immigrated to the United States from Poland as teenagers.
As a young boy, did you always want to become a doctor?
Dr. Rosenberg: I think some defining moments on why I decided to go into medicine occurred when I was 6 or 7 years old. The second world war was over, and many of the horrors of the Holocaust became apparent to me. I was brought up as an Orthodox Jew. My parents were quite religious, and I remember postcards coming in one after another about relatives that had died in the death camps. That had a profound influence on me.
How did that experience impact your aspirations?
Dr. Rosenberg: It was an example to me of how evil certain people and groups can be toward one another. I decided at that point, that I wanted to do something good for people, and medicine seemed the most likely way to do that. But also, I was developing a broad scientific interest. I ended up at the Bronx High School of Science and knew that I not only wanted to practice the medicine of today, but I wanted to play a role in helping develop the medicine.
What led to your interest in cancer treatment?
Dr. Rosenberg: Well, as a medical student and resident, it became clear that the field of cancer needed major improvement. We had three major ways to treat cancer: surgery, radiation therapy, and chemotherapy. That could cure about half of the people [who] had cancer. But despite the best application of those three specialties, there were over 600,000 deaths from cancer each year in the United States alone. It was clear to me that new approaches were needed, and I became very interested in taking advantage of the body’s immune system as a source of information to try to make progress.
Were there patients who inspired your research?
Dr. Rosenberg: There were two patients that I saw early in my career that impressed me a great deal. One was a patient that I saw when working in the emergency ward as a resident. A patient came in with right upper quadrant pain that looked like a gallbladder attack. That’s what it was. But when I went through his chart, I saw that he had been at that hospital 12 years earlier with a metastatic gastric cancer. The surgeons had operated. They saw tumor had spread to the liver and could not be removed. They closed the belly, not expecting him to survive. Yet he kept showing up for follow-up visits.
Here he was 12 years later. When I helped operate to take out his gallbladder, there was no evidence of any cancer. The cancer had disappeared in the absence of any external treatment. One of the rarest events in medicine, the spontaneous regression of a cancer. Somehow his body had learned how to destroy the tumor.
Was the second patient’s case as impressive?
Dr. Rosenberg: This patient had received a kidney transplant from a gentleman who died in an auto accident. [The donor’s] kidney contained a cancer deposit, a kidney cancer, unbeknownst to the transplant surgeons. [When the kidney was transplanted], the recipient developed widespread metastatic kidney cancer.
[The recipient] was on immunosuppressive drugs, and so the drugs had to be stopped. [When the immunosuppressive drugs were stopped], the patient’s body rejected the kidney and his cancer disappeared.
That showed me that, in fact, if you could stimulate a strong enough immune reaction, in this case, an [allogeneic] reaction, against foreign tissues from a different individual, that you could make large vascularized, invasive cancers disappear based on immune reactivities. Those were clues that led me toward studying the immune system’s impact on cancer.
From there, how did your work evolve?
Dr. Rosenberg: As chief of the surgery branch at NIH, I began doing research. It was very difficult to manipulate immune cells in the laboratory. They wouldn’t stay alive. But I tried to study immune reactions in patients with cancer to see if there was such a thing as an immune reaction against the cancer. There was no such thing known at the time. There were no cancer antigens and no known immune reactions against the disease in the human.
Around this time, investigators were publishing studies about interleukin-2 (IL-2), or white blood cells known as leukocytes. How did interleukin-2 further your research?
Dr. Rosenberg: The advent of interleukin-2 enabled scientists to grow lymphocytes outside the body. [This] enabled us to grow t-lymphocytes, which are some of the major warriors of the immune system against foreign tissue. After [studying] 66 patients in which we studied interleukin-2 and cells that would develop from it, we finally saw a disappearance of melanoma in a patient that received interleukin-2. And we went on to treat hundreds of patients with that hormone, interleukin-2. In fact, interleukin-2 became the first immunotherapy ever approved by the Food and Drug Administration for the treatment of cancer in humans.
How did this finding impact your future discoveries?
Dr. Rosenberg: [It] led to studies of the mechanism of action of interleukin-2 and to do that, we identified a kind of cell called a tumor infiltrating lymphocyte. What better place, intuitively to look for cells doing battle against the cancer than within the cancer itself?
In 1988, we demonstrated for the first time that transfer of lymphocytes with antitumor activity could cause the regression of melanoma. This was a living drug obtained from melanoma deposits that could be grown outside the body and then readministered to the patient under suitable conditions. Interestingly, [in February the FDA approved that drug as treatment for patients with melanoma]. A company developed it to the point where in multi-institutional studies, they reproduced our results.
And we’ve now emphasized the value of using T cell therapy, t cell transfer, for the treatment of patients with the common solid cancers, the cancers that start anywhere from the colon up through the intestine, the stomach, the pancreas, and the esophagus. Solid tumors such as ovarian cancer, uterine cancer and so on, are also potentially susceptible to this T cell therapy.
We’ve published several papers showing in isolated patients that you could cause major regressions, if not complete regressions, of these solid cancers in the liver, in the breast, the cervix, the colon. That’s a major aspect of what we’re doing now.
I think immunotherapy has come to be recognized as a major fourth arm that can be used to attack cancers, adding to surgery, radiation, and chemotherapy.
What guidance would you have for other physician-investigators or young doctors who want to follow in your path?
Dr. Rosenberg: You have to have a broad base of knowledge. You have to be willing to immerse yourself in a problem so that your mind is working on it when you’re doing things where you can only think. [When] you’re taking a shower, [or] waiting at a red light, your mind is working on this problem because you’re immersed in trying to understand it.
You need to have a laser focus on the goals that you have and not get sidetracked by issues that may be interesting but not directly related to the goals that you’re attempting to achieve.
Why a New Inhalable Lung Cancer Treatment Is So Promising
Cells in the human body chat with each other all the time. One major way they communicate is by releasing tiny spheres called exosomes. These carry fats, proteins, and genetic material that help regulate everything from pregnancy and immune responses to heart health and kidney function.
“Exosomes work like text messages between cells , sending and receiving information,” said lead researcher Ke Cheng, PhD, professor of biomedical engineering at Columbia. “The significance of this study is that exosomes can bring mRNA-based treatment to lung cancer cells locally, unlike systemic chemotherapy that can have side effects throughout the body. And inhalation is totally noninvasive. You don’t need a nurse to use an IV needle to pierce your skin.”
Dr. Cheng expects a human trial could launch within 5 years. For now, his study is attracting attention because it marks an advance in three areas of intense interest by researchers and biotech companies alike: Therapeutic uses of exosomes, inhalable treatments for lung conditions, and the safe delivery of powerful interleukin-12 (IL-12) immunotherapy.
Inside the Study
Dr. Cheng, who has been developing exosome and stem cell therapies for more than 15 years, and his lab team focused on lung cancer because the disease, often detected in later stages, “has a huge mortality rate,” he said. “Therapies have been suboptimal and leave the organ so damaged.”
He wanted to explore new alternatives to systemic treatments. Most are given intravenously, but Dr. Cheng thinks exosomes — also called extracellular vesicles (EVs) — could change that.
“One of the advantages of exosomes is that they are naturally secreted by the body or cultured cells,” he noted. “They have low toxicity and have multiple ways of getting their message into cells.”
The scientists borrowed an approach that captured public attention during the pandemic: Using messenger RNA, which directs cells to make proteins for tasks — including boosting immune response.
IL-12 has shown promise against cancer for decades, but early human trials triggered serious side effects and several deaths. Researchers are now trying new delivery methods that target tumor cells without affecting healthy tissue. Dr. Cheng’s team took a new approach, inserting mRNA for IL-12 into exosomes.
One aim of the study was to compare the effectiveness of inhaled exosomes vs inhaled liposomes, engineered fat droplets also under investigation as drug carriers. The team’s question: Which would work better at introducing IL-12 to the lungs to affect cancer, without triggering side effects?
After lab mice inhaled the particles through the nose, the researchers found that exosomes delivered more mRNA into cancer cells in the lungs and fought lung cancer with few side effects. Three days after treatment, researchers saw an influx of cancer-fighting T cells within tumors — with higher levels for exosome-based treatment. Plus, the exosomes led to more cancer-destroying nature killer cells and more monocytes, a sign of immune-system activation.
Researchers also found the treatment acted as a vaccine, training the immune system to battle newly introduced cancers. Little of the exosome-delivered drug escaped into the bloodstream, and the study found minimal side effects. Inhalation didn’t affect normal breathing, Dr. Cheng added.
The study’s use of inhaled exosomes makes it significant, said Raghu Kalluri, MD, PhD, professor and chair of the Department of Cancer Biology at MD Anderson Cancer Center. “This is an interesting study that explores the inhalable delivery of engineered EVs for the treatment of lung cancer and offers insights into focused delivery of EV-based drugs…with implications for diseases beyond cancer,” he said. Dr. Kalluri is also an exosome researcher.
New Frontiers
Once seen as a “quirky biological phenomenon” or just cellular trash, exosomes are now the subject of intense medical research for their potential as drug carriers, as treatments in their own right for everything from wound healing and pneumonia to heart attacks and bowel disorders, and as measurable biological markers that could lead to new tests for cancer and other conditions. One exosome-based prostate cancer test, the ExoDx Prostate Test, is already on the market.
The explosion in exosome research — the number of published studies has grown from just a handful in the early 1980s to more than 9000 — spotlights a particular focus on cancer. According to a 2021 paper in Annals of Oncology, clinical trials for exosomes in cancer treatments and tests far out-paces those for diabetes, heart disease, or neurologic conditions. Currently, 52 clinical trials using exosomes in cancer diagnosis or treatment have been completed, are underway, or are looking for participants, according to clinicaltrials.gov.
Dr. Cheng’s approach could also be used to deliver other drugs to the lungs and other organs via inhalation. “We’re testing inhalation for a different type of lung disease, acute lung injury,” Dr. Cheng said. Other potential targets include lung disorders like pulmonary hypertension. Inhaled exosomes could potentially reach the brain via the olfactory bulb or the heart as it receives oxygenated blood from the lungs.
Breathing in Medicine
So far, inhalable cancer treatments are not available outside research studies in the United States or Europe , said Remi Rosiere, PhD, a lecturer at the Université libre de Bruxelles in Brussels, Belgium, and chief scientific officer of InhaTarget Therapeutics, a company developing its own inhaled treatments for severe respiratory diseases. “Oncologists are very interested,” he said. “If you concentrate the drug on the tumor site, you can avoid distribution to the body.”
Early research into inhalable chemotherapy began in the 1960s but was unsuccessful because breathing equipment dispersed toxic cancer drugs into the air or delivered only small amounts to the lungs, he said.
New delivery techniques aim to change that. Dr. Rosiere’s company is starting a human trial of a dry powder inhaler with the chemotherapy drug cisplatin for lung cancer. Also in the pipeline is an immunotherapy treatment for lung cancer inserted in lipid nanoparticles, which are tiny fat particles similar to liposomes.
He said Dr. Cheng’s study shows the advantages of sending in exosomes. “The data are very persuasive,” Dr. Rosier said of the study. “Exosomes have a good safety profile and are able to remain in the lung for quite a long time. This prolongs exposure to the drug for greater effectiveness, without causing toxicities.”
Getting from a mouse study to a human trial will take time. “You need to understand this is very early stage,” Dr. Rosiere added. “There will be many challenges to overcome.”
One is purely practical: If the drug approaches human trials, he said, regulators will ask whether the exosomes can be produced in large quantities to meet the huge demand for new lung cancer treatments. “Lung cancer is the number one fatal cancer in the world,” Dr. Rosiere said.
A New Route for ‘Powerful’ Cancer Treatment
Meanwhile, the Columbia University study showed that inhalable exosomes are a unique delivery method for IL-12 — and could help solve a major problem that’s plagued this promising cancer treatment for decades.
Called “one of the most powerful immunotherapy agents ever discovered” in a 2022 literature review, IL-12 showed serious side effects that stalled research in the 1980s , sparking an ongoing search for new delivery methods that continues today. In 2022 and 2023, Big Pharma companies including AstraZenca, Moderna, and Bristol Myers Squib reduced their involvement with IL-12 treatment research, leaving the field open to smaller biotech companies working on a variety of drug-delivery approaches that could make IL-12 safe and effective in humans.
These include injecting it directly into tumors, encasing it in various types of particles, masking the drug so it is activated only in cancer cells, and using IL-12 mRNA, which essentially turns tumor cells into IL-12–producing factories. Another IL-12 mRNA drug, from Pittsburgh-based Krystal Biotech, received a fast-track designation from the US Food and Drug Administration in February 2024 for an inhaled lung cancer treatment that packages mRNA for IL-12 and IL-2 inside an engineered virus.
And of course, there is Dr. Cheng’s inhalable treatment, culminating decades of work across three burgeoning fields.
A version of this article appeared on Medscape.com.
Cells in the human body chat with each other all the time. One major way they communicate is by releasing tiny spheres called exosomes. These carry fats, proteins, and genetic material that help regulate everything from pregnancy and immune responses to heart health and kidney function.
“Exosomes work like text messages between cells , sending and receiving information,” said lead researcher Ke Cheng, PhD, professor of biomedical engineering at Columbia. “The significance of this study is that exosomes can bring mRNA-based treatment to lung cancer cells locally, unlike systemic chemotherapy that can have side effects throughout the body. And inhalation is totally noninvasive. You don’t need a nurse to use an IV needle to pierce your skin.”
Dr. Cheng expects a human trial could launch within 5 years. For now, his study is attracting attention because it marks an advance in three areas of intense interest by researchers and biotech companies alike: Therapeutic uses of exosomes, inhalable treatments for lung conditions, and the safe delivery of powerful interleukin-12 (IL-12) immunotherapy.
Inside the Study
Dr. Cheng, who has been developing exosome and stem cell therapies for more than 15 years, and his lab team focused on lung cancer because the disease, often detected in later stages, “has a huge mortality rate,” he said. “Therapies have been suboptimal and leave the organ so damaged.”
He wanted to explore new alternatives to systemic treatments. Most are given intravenously, but Dr. Cheng thinks exosomes — also called extracellular vesicles (EVs) — could change that.
“One of the advantages of exosomes is that they are naturally secreted by the body or cultured cells,” he noted. “They have low toxicity and have multiple ways of getting their message into cells.”
The scientists borrowed an approach that captured public attention during the pandemic: Using messenger RNA, which directs cells to make proteins for tasks — including boosting immune response.
IL-12 has shown promise against cancer for decades, but early human trials triggered serious side effects and several deaths. Researchers are now trying new delivery methods that target tumor cells without affecting healthy tissue. Dr. Cheng’s team took a new approach, inserting mRNA for IL-12 into exosomes.
One aim of the study was to compare the effectiveness of inhaled exosomes vs inhaled liposomes, engineered fat droplets also under investigation as drug carriers. The team’s question: Which would work better at introducing IL-12 to the lungs to affect cancer, without triggering side effects?
After lab mice inhaled the particles through the nose, the researchers found that exosomes delivered more mRNA into cancer cells in the lungs and fought lung cancer with few side effects. Three days after treatment, researchers saw an influx of cancer-fighting T cells within tumors — with higher levels for exosome-based treatment. Plus, the exosomes led to more cancer-destroying nature killer cells and more monocytes, a sign of immune-system activation.
Researchers also found the treatment acted as a vaccine, training the immune system to battle newly introduced cancers. Little of the exosome-delivered drug escaped into the bloodstream, and the study found minimal side effects. Inhalation didn’t affect normal breathing, Dr. Cheng added.
The study’s use of inhaled exosomes makes it significant, said Raghu Kalluri, MD, PhD, professor and chair of the Department of Cancer Biology at MD Anderson Cancer Center. “This is an interesting study that explores the inhalable delivery of engineered EVs for the treatment of lung cancer and offers insights into focused delivery of EV-based drugs…with implications for diseases beyond cancer,” he said. Dr. Kalluri is also an exosome researcher.
New Frontiers
Once seen as a “quirky biological phenomenon” or just cellular trash, exosomes are now the subject of intense medical research for their potential as drug carriers, as treatments in their own right for everything from wound healing and pneumonia to heart attacks and bowel disorders, and as measurable biological markers that could lead to new tests for cancer and other conditions. One exosome-based prostate cancer test, the ExoDx Prostate Test, is already on the market.
The explosion in exosome research — the number of published studies has grown from just a handful in the early 1980s to more than 9000 — spotlights a particular focus on cancer. According to a 2021 paper in Annals of Oncology, clinical trials for exosomes in cancer treatments and tests far out-paces those for diabetes, heart disease, or neurologic conditions. Currently, 52 clinical trials using exosomes in cancer diagnosis or treatment have been completed, are underway, or are looking for participants, according to clinicaltrials.gov.
Dr. Cheng’s approach could also be used to deliver other drugs to the lungs and other organs via inhalation. “We’re testing inhalation for a different type of lung disease, acute lung injury,” Dr. Cheng said. Other potential targets include lung disorders like pulmonary hypertension. Inhaled exosomes could potentially reach the brain via the olfactory bulb or the heart as it receives oxygenated blood from the lungs.
Breathing in Medicine
So far, inhalable cancer treatments are not available outside research studies in the United States or Europe , said Remi Rosiere, PhD, a lecturer at the Université libre de Bruxelles in Brussels, Belgium, and chief scientific officer of InhaTarget Therapeutics, a company developing its own inhaled treatments for severe respiratory diseases. “Oncologists are very interested,” he said. “If you concentrate the drug on the tumor site, you can avoid distribution to the body.”
Early research into inhalable chemotherapy began in the 1960s but was unsuccessful because breathing equipment dispersed toxic cancer drugs into the air or delivered only small amounts to the lungs, he said.
New delivery techniques aim to change that. Dr. Rosiere’s company is starting a human trial of a dry powder inhaler with the chemotherapy drug cisplatin for lung cancer. Also in the pipeline is an immunotherapy treatment for lung cancer inserted in lipid nanoparticles, which are tiny fat particles similar to liposomes.
He said Dr. Cheng’s study shows the advantages of sending in exosomes. “The data are very persuasive,” Dr. Rosier said of the study. “Exosomes have a good safety profile and are able to remain in the lung for quite a long time. This prolongs exposure to the drug for greater effectiveness, without causing toxicities.”
Getting from a mouse study to a human trial will take time. “You need to understand this is very early stage,” Dr. Rosiere added. “There will be many challenges to overcome.”
One is purely practical: If the drug approaches human trials, he said, regulators will ask whether the exosomes can be produced in large quantities to meet the huge demand for new lung cancer treatments. “Lung cancer is the number one fatal cancer in the world,” Dr. Rosiere said.
A New Route for ‘Powerful’ Cancer Treatment
Meanwhile, the Columbia University study showed that inhalable exosomes are a unique delivery method for IL-12 — and could help solve a major problem that’s plagued this promising cancer treatment for decades.
Called “one of the most powerful immunotherapy agents ever discovered” in a 2022 literature review, IL-12 showed serious side effects that stalled research in the 1980s , sparking an ongoing search for new delivery methods that continues today. In 2022 and 2023, Big Pharma companies including AstraZenca, Moderna, and Bristol Myers Squib reduced their involvement with IL-12 treatment research, leaving the field open to smaller biotech companies working on a variety of drug-delivery approaches that could make IL-12 safe and effective in humans.
These include injecting it directly into tumors, encasing it in various types of particles, masking the drug so it is activated only in cancer cells, and using IL-12 mRNA, which essentially turns tumor cells into IL-12–producing factories. Another IL-12 mRNA drug, from Pittsburgh-based Krystal Biotech, received a fast-track designation from the US Food and Drug Administration in February 2024 for an inhaled lung cancer treatment that packages mRNA for IL-12 and IL-2 inside an engineered virus.
And of course, there is Dr. Cheng’s inhalable treatment, culminating decades of work across three burgeoning fields.
A version of this article appeared on Medscape.com.
Cells in the human body chat with each other all the time. One major way they communicate is by releasing tiny spheres called exosomes. These carry fats, proteins, and genetic material that help regulate everything from pregnancy and immune responses to heart health and kidney function.
“Exosomes work like text messages between cells , sending and receiving information,” said lead researcher Ke Cheng, PhD, professor of biomedical engineering at Columbia. “The significance of this study is that exosomes can bring mRNA-based treatment to lung cancer cells locally, unlike systemic chemotherapy that can have side effects throughout the body. And inhalation is totally noninvasive. You don’t need a nurse to use an IV needle to pierce your skin.”
Dr. Cheng expects a human trial could launch within 5 years. For now, his study is attracting attention because it marks an advance in three areas of intense interest by researchers and biotech companies alike: Therapeutic uses of exosomes, inhalable treatments for lung conditions, and the safe delivery of powerful interleukin-12 (IL-12) immunotherapy.
Inside the Study
Dr. Cheng, who has been developing exosome and stem cell therapies for more than 15 years, and his lab team focused on lung cancer because the disease, often detected in later stages, “has a huge mortality rate,” he said. “Therapies have been suboptimal and leave the organ so damaged.”
He wanted to explore new alternatives to systemic treatments. Most are given intravenously, but Dr. Cheng thinks exosomes — also called extracellular vesicles (EVs) — could change that.
“One of the advantages of exosomes is that they are naturally secreted by the body or cultured cells,” he noted. “They have low toxicity and have multiple ways of getting their message into cells.”
The scientists borrowed an approach that captured public attention during the pandemic: Using messenger RNA, which directs cells to make proteins for tasks — including boosting immune response.
IL-12 has shown promise against cancer for decades, but early human trials triggered serious side effects and several deaths. Researchers are now trying new delivery methods that target tumor cells without affecting healthy tissue. Dr. Cheng’s team took a new approach, inserting mRNA for IL-12 into exosomes.
One aim of the study was to compare the effectiveness of inhaled exosomes vs inhaled liposomes, engineered fat droplets also under investigation as drug carriers. The team’s question: Which would work better at introducing IL-12 to the lungs to affect cancer, without triggering side effects?
After lab mice inhaled the particles through the nose, the researchers found that exosomes delivered more mRNA into cancer cells in the lungs and fought lung cancer with few side effects. Three days after treatment, researchers saw an influx of cancer-fighting T cells within tumors — with higher levels for exosome-based treatment. Plus, the exosomes led to more cancer-destroying nature killer cells and more monocytes, a sign of immune-system activation.
Researchers also found the treatment acted as a vaccine, training the immune system to battle newly introduced cancers. Little of the exosome-delivered drug escaped into the bloodstream, and the study found minimal side effects. Inhalation didn’t affect normal breathing, Dr. Cheng added.
The study’s use of inhaled exosomes makes it significant, said Raghu Kalluri, MD, PhD, professor and chair of the Department of Cancer Biology at MD Anderson Cancer Center. “This is an interesting study that explores the inhalable delivery of engineered EVs for the treatment of lung cancer and offers insights into focused delivery of EV-based drugs…with implications for diseases beyond cancer,” he said. Dr. Kalluri is also an exosome researcher.
New Frontiers
Once seen as a “quirky biological phenomenon” or just cellular trash, exosomes are now the subject of intense medical research for their potential as drug carriers, as treatments in their own right for everything from wound healing and pneumonia to heart attacks and bowel disorders, and as measurable biological markers that could lead to new tests for cancer and other conditions. One exosome-based prostate cancer test, the ExoDx Prostate Test, is already on the market.
The explosion in exosome research — the number of published studies has grown from just a handful in the early 1980s to more than 9000 — spotlights a particular focus on cancer. According to a 2021 paper in Annals of Oncology, clinical trials for exosomes in cancer treatments and tests far out-paces those for diabetes, heart disease, or neurologic conditions. Currently, 52 clinical trials using exosomes in cancer diagnosis or treatment have been completed, are underway, or are looking for participants, according to clinicaltrials.gov.
Dr. Cheng’s approach could also be used to deliver other drugs to the lungs and other organs via inhalation. “We’re testing inhalation for a different type of lung disease, acute lung injury,” Dr. Cheng said. Other potential targets include lung disorders like pulmonary hypertension. Inhaled exosomes could potentially reach the brain via the olfactory bulb or the heart as it receives oxygenated blood from the lungs.
Breathing in Medicine
So far, inhalable cancer treatments are not available outside research studies in the United States or Europe , said Remi Rosiere, PhD, a lecturer at the Université libre de Bruxelles in Brussels, Belgium, and chief scientific officer of InhaTarget Therapeutics, a company developing its own inhaled treatments for severe respiratory diseases. “Oncologists are very interested,” he said. “If you concentrate the drug on the tumor site, you can avoid distribution to the body.”
Early research into inhalable chemotherapy began in the 1960s but was unsuccessful because breathing equipment dispersed toxic cancer drugs into the air or delivered only small amounts to the lungs, he said.
New delivery techniques aim to change that. Dr. Rosiere’s company is starting a human trial of a dry powder inhaler with the chemotherapy drug cisplatin for lung cancer. Also in the pipeline is an immunotherapy treatment for lung cancer inserted in lipid nanoparticles, which are tiny fat particles similar to liposomes.
He said Dr. Cheng’s study shows the advantages of sending in exosomes. “The data are very persuasive,” Dr. Rosier said of the study. “Exosomes have a good safety profile and are able to remain in the lung for quite a long time. This prolongs exposure to the drug for greater effectiveness, without causing toxicities.”
Getting from a mouse study to a human trial will take time. “You need to understand this is very early stage,” Dr. Rosiere added. “There will be many challenges to overcome.”
One is purely practical: If the drug approaches human trials, he said, regulators will ask whether the exosomes can be produced in large quantities to meet the huge demand for new lung cancer treatments. “Lung cancer is the number one fatal cancer in the world,” Dr. Rosiere said.
A New Route for ‘Powerful’ Cancer Treatment
Meanwhile, the Columbia University study showed that inhalable exosomes are a unique delivery method for IL-12 — and could help solve a major problem that’s plagued this promising cancer treatment for decades.
Called “one of the most powerful immunotherapy agents ever discovered” in a 2022 literature review, IL-12 showed serious side effects that stalled research in the 1980s , sparking an ongoing search for new delivery methods that continues today. In 2022 and 2023, Big Pharma companies including AstraZenca, Moderna, and Bristol Myers Squib reduced their involvement with IL-12 treatment research, leaving the field open to smaller biotech companies working on a variety of drug-delivery approaches that could make IL-12 safe and effective in humans.
These include injecting it directly into tumors, encasing it in various types of particles, masking the drug so it is activated only in cancer cells, and using IL-12 mRNA, which essentially turns tumor cells into IL-12–producing factories. Another IL-12 mRNA drug, from Pittsburgh-based Krystal Biotech, received a fast-track designation from the US Food and Drug Administration in February 2024 for an inhaled lung cancer treatment that packages mRNA for IL-12 and IL-2 inside an engineered virus.
And of course, there is Dr. Cheng’s inhalable treatment, culminating decades of work across three burgeoning fields.
A version of this article appeared on Medscape.com.
FROM NATURE NANOTECHNOLOGY
RUBY: ‘A Huge Win’ for Patients With Advanced or Recurrent Endometrial Cancer
The benefit of the combination of the programmed death protein 1 (PD-1) inhibitor dostarlimab (Jemperli) and chemotherapy was even more pronounced among patients with DNA mismatch repair deficient/microsatellite instability high (dMMR/MSI-H) tumors.
These results, from the second interim analysis of the phase 3 ENGOT-EN6-NSGO/GOG-3031/RUBY trial, were cheered by audience members when they were reported at the Society of Gynecologic Oncology (SGO)’s Annual Meeting on Women’s Cancer, held in San Diego, California.
“Overall survival benefit to the addition of PD-1 inhibitor to chemotherapy upfront for patients with advanced and recurrent MSI-high endometrial cancer: SOLD!” said invited discussant Gini Fleming, medical director of gynecologic oncology at the University of Chicago.
“I think this is a huge win for our patients. It’s something that none of us have seen before over many years of working with endometrial cancer and should be incorporated into everybody’s practice as of yesterday,” she said.
Continued Improvement
Results from the first interim analysis of the trial showed that dostarlimab and chemotherapy significantly improved progression-free survival (PFS) in the dMMR/MSI-H population, and there was an early trend toward improved overall survival, compared with chemotherapy plus placebo.
As Matthew A. Powell, MD from Washington University School of Medicine in Saint Louis, Missouri reported at SGO 2024, that early trend has become an undeniable survival advantage.
At a median follow-up of 37.2 months, the median overall survival was 44.6 months for patients randomized to the combination, compared with 28.2 months for those assigned to chemotherapy plus placebo.
The respective 3-year overall survival (OS) rates were 54.9% and 42.9%, translating into a hazard ratio (HR) for death with dostarlimab/chemotherapy of 0.69 (P = .002).
Among the subset of patients with dMMR/MSI-H tumors the survival benefit conferred by the combination was even greater, with median OS not reached in the dostarlimab group vs 31.4 months in the chemotherapy-alone arm, with respective 3-year OS rates of 78% and 46%. This difference translated into a HR for death with the combination of 0.32 (P = .0002) for patients with deficient mismatch-repair cancers.
“Dostarlimab plus carboplatin-paclitaxel chemotherapy demonstrated statistically significant and clinically meaningful overall survival improvements in the overall population, a substantial unprecedented overall survival benefit in patients with defective mismatch-repair tumors, and a clinically meaningful; 7-month improvement in the OS difference in patients with proficient mismatch-repair tumors,” Dr. Powell said.
RUBY Details
The trial was conducted in 494 patients with primary advanced stage III or IV or first recurrent endometrial cancer who received first-line treatment with standard chemotherapy with carboplatin (area under the concentration–time curve, 5 mg/mL per minute) and paclitaxel (175 mg/m2 of body surface area), every 3 weeks (six cycles). They were also randomized to receive either dostarlimab (1000 mg) or dostarlimab placebo every 6 weeks for up to 3 years.
Within the cohort, 118 patients (23.9%) had dMMR/MSI-H tumors.
At the time of the first interim analysis the estimated progression-free survival at 24 months in the dMMR–MSI-H subgroup was 61.4% in the dostarlimab group vs 15.7 in the placebo group (HR for progression or death, 0.28; P < .001). For the entire cohort, progression-free survival at 24 months was 36.1% vs 18.1% (HR, 0.64; P < .001).
A prespecified exploratory analysis of progression-free survival in proficient MMR, microsatellite stable (MSS) patients was also done, and a clinically relevant benefit was observed.
Overall survival at that time also favored dostarlimab, although it was only mature for 33% of the population. But at 24 months, OS rates were 71.3% vs 56.0% among placebo recipients; this difference approached but did not reach statistical significance.
The overall response rate in the dMMR–MSI-H population vs the placebo group was 77.6% vs 69%, respectively, and 68.1% and 63.4% in the pMMR/MSS population.
The most common adverse events observed were nausea, alopecia, and fatigue. Grade 3 and higher adverse events at the most recent follow-up were more frequent in the dostarlimab group than in the placebo group (72.2% vs 60.2%).
“Importantly, safety was maintained” at the second interim analysis, Dr. Powell said.
“No new safety signals were noted, no new deaths related to therapy were noted with the subsequent 1-year additional analysis time,” he said.
What’s Next?
Dr. Fleming reviewed potential strategies for further improving care of patients with advanced or recurrent endometrial cancer during her discussion.
“What are the next directions for patients with MSI-high disease? Well, obviously could we use immune checkpoint inhibitors without chemotherapy and not compromise results? There are two ongoing trials or trials that we’re awaiting results of that have compared single-agent immune checkpoint inhibitor to just chemotherapy in mismatch repair-deficient advanced disease, and hopefully we can extrapolate from these trials to determine if this might be a more patient-friendly and equally effective strategy, but we don’t yet know,” she said.
Dr. Fleming also noted that ongoing or planned clinical trials will address questions about potential options for patients with MSI-H tumors whose disease progresses on frontline chemotherapy and immunotherapy. Other trials are assessing whether combining radiotherapy with checkpoint inhibitors will be effective in treating patients with earlier-stage tumors, or whether the addition of a PARP inhibitor might offer additional benefit for these patients.
“Immune checkpoint inhibitor should be given first line to patients with advanced/recurrent microsatellite [instability] endometrial cancer, and they should be considered as front line in patients with microsatellite stable disease. At this point, unfortunately, we have no reasonable predictive factors to know which of those patients with microsatellite stable disease will truly benefit. Multiple other agents are being tested in this setting, and will hopefully prove useful in subgroups,” she said.
The study is funded by GlaxoSmithKline. Dr. Powell reports grants/research support from GSK and honoraria/consultation fees from AstraZeneca, Clovis Oncology, Eisai, GSK, Immunogen, and Merck. Dr. Fleming reports serving as an institutional principal investigator for trials sponsored by multiple companies, not including GSK.
The benefit of the combination of the programmed death protein 1 (PD-1) inhibitor dostarlimab (Jemperli) and chemotherapy was even more pronounced among patients with DNA mismatch repair deficient/microsatellite instability high (dMMR/MSI-H) tumors.
These results, from the second interim analysis of the phase 3 ENGOT-EN6-NSGO/GOG-3031/RUBY trial, were cheered by audience members when they were reported at the Society of Gynecologic Oncology (SGO)’s Annual Meeting on Women’s Cancer, held in San Diego, California.
“Overall survival benefit to the addition of PD-1 inhibitor to chemotherapy upfront for patients with advanced and recurrent MSI-high endometrial cancer: SOLD!” said invited discussant Gini Fleming, medical director of gynecologic oncology at the University of Chicago.
“I think this is a huge win for our patients. It’s something that none of us have seen before over many years of working with endometrial cancer and should be incorporated into everybody’s practice as of yesterday,” she said.
Continued Improvement
Results from the first interim analysis of the trial showed that dostarlimab and chemotherapy significantly improved progression-free survival (PFS) in the dMMR/MSI-H population, and there was an early trend toward improved overall survival, compared with chemotherapy plus placebo.
As Matthew A. Powell, MD from Washington University School of Medicine in Saint Louis, Missouri reported at SGO 2024, that early trend has become an undeniable survival advantage.
At a median follow-up of 37.2 months, the median overall survival was 44.6 months for patients randomized to the combination, compared with 28.2 months for those assigned to chemotherapy plus placebo.
The respective 3-year overall survival (OS) rates were 54.9% and 42.9%, translating into a hazard ratio (HR) for death with dostarlimab/chemotherapy of 0.69 (P = .002).
Among the subset of patients with dMMR/MSI-H tumors the survival benefit conferred by the combination was even greater, with median OS not reached in the dostarlimab group vs 31.4 months in the chemotherapy-alone arm, with respective 3-year OS rates of 78% and 46%. This difference translated into a HR for death with the combination of 0.32 (P = .0002) for patients with deficient mismatch-repair cancers.
“Dostarlimab plus carboplatin-paclitaxel chemotherapy demonstrated statistically significant and clinically meaningful overall survival improvements in the overall population, a substantial unprecedented overall survival benefit in patients with defective mismatch-repair tumors, and a clinically meaningful; 7-month improvement in the OS difference in patients with proficient mismatch-repair tumors,” Dr. Powell said.
RUBY Details
The trial was conducted in 494 patients with primary advanced stage III or IV or first recurrent endometrial cancer who received first-line treatment with standard chemotherapy with carboplatin (area under the concentration–time curve, 5 mg/mL per minute) and paclitaxel (175 mg/m2 of body surface area), every 3 weeks (six cycles). They were also randomized to receive either dostarlimab (1000 mg) or dostarlimab placebo every 6 weeks for up to 3 years.
Within the cohort, 118 patients (23.9%) had dMMR/MSI-H tumors.
At the time of the first interim analysis the estimated progression-free survival at 24 months in the dMMR–MSI-H subgroup was 61.4% in the dostarlimab group vs 15.7 in the placebo group (HR for progression or death, 0.28; P < .001). For the entire cohort, progression-free survival at 24 months was 36.1% vs 18.1% (HR, 0.64; P < .001).
A prespecified exploratory analysis of progression-free survival in proficient MMR, microsatellite stable (MSS) patients was also done, and a clinically relevant benefit was observed.
Overall survival at that time also favored dostarlimab, although it was only mature for 33% of the population. But at 24 months, OS rates were 71.3% vs 56.0% among placebo recipients; this difference approached but did not reach statistical significance.
The overall response rate in the dMMR–MSI-H population vs the placebo group was 77.6% vs 69%, respectively, and 68.1% and 63.4% in the pMMR/MSS population.
The most common adverse events observed were nausea, alopecia, and fatigue. Grade 3 and higher adverse events at the most recent follow-up were more frequent in the dostarlimab group than in the placebo group (72.2% vs 60.2%).
“Importantly, safety was maintained” at the second interim analysis, Dr. Powell said.
“No new safety signals were noted, no new deaths related to therapy were noted with the subsequent 1-year additional analysis time,” he said.
What’s Next?
Dr. Fleming reviewed potential strategies for further improving care of patients with advanced or recurrent endometrial cancer during her discussion.
“What are the next directions for patients with MSI-high disease? Well, obviously could we use immune checkpoint inhibitors without chemotherapy and not compromise results? There are two ongoing trials or trials that we’re awaiting results of that have compared single-agent immune checkpoint inhibitor to just chemotherapy in mismatch repair-deficient advanced disease, and hopefully we can extrapolate from these trials to determine if this might be a more patient-friendly and equally effective strategy, but we don’t yet know,” she said.
Dr. Fleming also noted that ongoing or planned clinical trials will address questions about potential options for patients with MSI-H tumors whose disease progresses on frontline chemotherapy and immunotherapy. Other trials are assessing whether combining radiotherapy with checkpoint inhibitors will be effective in treating patients with earlier-stage tumors, or whether the addition of a PARP inhibitor might offer additional benefit for these patients.
“Immune checkpoint inhibitor should be given first line to patients with advanced/recurrent microsatellite [instability] endometrial cancer, and they should be considered as front line in patients with microsatellite stable disease. At this point, unfortunately, we have no reasonable predictive factors to know which of those patients with microsatellite stable disease will truly benefit. Multiple other agents are being tested in this setting, and will hopefully prove useful in subgroups,” she said.
The study is funded by GlaxoSmithKline. Dr. Powell reports grants/research support from GSK and honoraria/consultation fees from AstraZeneca, Clovis Oncology, Eisai, GSK, Immunogen, and Merck. Dr. Fleming reports serving as an institutional principal investigator for trials sponsored by multiple companies, not including GSK.
The benefit of the combination of the programmed death protein 1 (PD-1) inhibitor dostarlimab (Jemperli) and chemotherapy was even more pronounced among patients with DNA mismatch repair deficient/microsatellite instability high (dMMR/MSI-H) tumors.
These results, from the second interim analysis of the phase 3 ENGOT-EN6-NSGO/GOG-3031/RUBY trial, were cheered by audience members when they were reported at the Society of Gynecologic Oncology (SGO)’s Annual Meeting on Women’s Cancer, held in San Diego, California.
“Overall survival benefit to the addition of PD-1 inhibitor to chemotherapy upfront for patients with advanced and recurrent MSI-high endometrial cancer: SOLD!” said invited discussant Gini Fleming, medical director of gynecologic oncology at the University of Chicago.
“I think this is a huge win for our patients. It’s something that none of us have seen before over many years of working with endometrial cancer and should be incorporated into everybody’s practice as of yesterday,” she said.
Continued Improvement
Results from the first interim analysis of the trial showed that dostarlimab and chemotherapy significantly improved progression-free survival (PFS) in the dMMR/MSI-H population, and there was an early trend toward improved overall survival, compared with chemotherapy plus placebo.
As Matthew A. Powell, MD from Washington University School of Medicine in Saint Louis, Missouri reported at SGO 2024, that early trend has become an undeniable survival advantage.
At a median follow-up of 37.2 months, the median overall survival was 44.6 months for patients randomized to the combination, compared with 28.2 months for those assigned to chemotherapy plus placebo.
The respective 3-year overall survival (OS) rates were 54.9% and 42.9%, translating into a hazard ratio (HR) for death with dostarlimab/chemotherapy of 0.69 (P = .002).
Among the subset of patients with dMMR/MSI-H tumors the survival benefit conferred by the combination was even greater, with median OS not reached in the dostarlimab group vs 31.4 months in the chemotherapy-alone arm, with respective 3-year OS rates of 78% and 46%. This difference translated into a HR for death with the combination of 0.32 (P = .0002) for patients with deficient mismatch-repair cancers.
“Dostarlimab plus carboplatin-paclitaxel chemotherapy demonstrated statistically significant and clinically meaningful overall survival improvements in the overall population, a substantial unprecedented overall survival benefit in patients with defective mismatch-repair tumors, and a clinically meaningful; 7-month improvement in the OS difference in patients with proficient mismatch-repair tumors,” Dr. Powell said.
RUBY Details
The trial was conducted in 494 patients with primary advanced stage III or IV or first recurrent endometrial cancer who received first-line treatment with standard chemotherapy with carboplatin (area under the concentration–time curve, 5 mg/mL per minute) and paclitaxel (175 mg/m2 of body surface area), every 3 weeks (six cycles). They were also randomized to receive either dostarlimab (1000 mg) or dostarlimab placebo every 6 weeks for up to 3 years.
Within the cohort, 118 patients (23.9%) had dMMR/MSI-H tumors.
At the time of the first interim analysis the estimated progression-free survival at 24 months in the dMMR–MSI-H subgroup was 61.4% in the dostarlimab group vs 15.7 in the placebo group (HR for progression or death, 0.28; P < .001). For the entire cohort, progression-free survival at 24 months was 36.1% vs 18.1% (HR, 0.64; P < .001).
A prespecified exploratory analysis of progression-free survival in proficient MMR, microsatellite stable (MSS) patients was also done, and a clinically relevant benefit was observed.
Overall survival at that time also favored dostarlimab, although it was only mature for 33% of the population. But at 24 months, OS rates were 71.3% vs 56.0% among placebo recipients; this difference approached but did not reach statistical significance.
The overall response rate in the dMMR–MSI-H population vs the placebo group was 77.6% vs 69%, respectively, and 68.1% and 63.4% in the pMMR/MSS population.
The most common adverse events observed were nausea, alopecia, and fatigue. Grade 3 and higher adverse events at the most recent follow-up were more frequent in the dostarlimab group than in the placebo group (72.2% vs 60.2%).
“Importantly, safety was maintained” at the second interim analysis, Dr. Powell said.
“No new safety signals were noted, no new deaths related to therapy were noted with the subsequent 1-year additional analysis time,” he said.
What’s Next?
Dr. Fleming reviewed potential strategies for further improving care of patients with advanced or recurrent endometrial cancer during her discussion.
“What are the next directions for patients with MSI-high disease? Well, obviously could we use immune checkpoint inhibitors without chemotherapy and not compromise results? There are two ongoing trials or trials that we’re awaiting results of that have compared single-agent immune checkpoint inhibitor to just chemotherapy in mismatch repair-deficient advanced disease, and hopefully we can extrapolate from these trials to determine if this might be a more patient-friendly and equally effective strategy, but we don’t yet know,” she said.
Dr. Fleming also noted that ongoing or planned clinical trials will address questions about potential options for patients with MSI-H tumors whose disease progresses on frontline chemotherapy and immunotherapy. Other trials are assessing whether combining radiotherapy with checkpoint inhibitors will be effective in treating patients with earlier-stage tumors, or whether the addition of a PARP inhibitor might offer additional benefit for these patients.
“Immune checkpoint inhibitor should be given first line to patients with advanced/recurrent microsatellite [instability] endometrial cancer, and they should be considered as front line in patients with microsatellite stable disease. At this point, unfortunately, we have no reasonable predictive factors to know which of those patients with microsatellite stable disease will truly benefit. Multiple other agents are being tested in this setting, and will hopefully prove useful in subgroups,” she said.
The study is funded by GlaxoSmithKline. Dr. Powell reports grants/research support from GSK and honoraria/consultation fees from AstraZeneca, Clovis Oncology, Eisai, GSK, Immunogen, and Merck. Dr. Fleming reports serving as an institutional principal investigator for trials sponsored by multiple companies, not including GSK.
FROM SGO 2024
Cell-Free DNA Blood Test Has High Accuracy for Detecting Colorectal Cancer
, according to a new study.
The cfDNA blood test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions. Other noninvasive screening methods have sensitivity from 67% to 94% for CRC and 22% to 43% for advanced precancerous lesions.
“The results of the study are a promising step toward developing more convenient tools to detect colorectal cancer early while it is more easily treated,” said senior author William M. Grady, MD, AGAF, medical director of the Gastrointestinal Cancer Prevention Program at the Fred Hutchinson Cancer Center in Seattle.
“The test, which has an accuracy rate for colon cancer detection similar to stool tests used for early detection of cancer, could offer an alternative for patients who may otherwise decline current screening options,” he said.
The study was published online on March 14 in The New England Journal of Medicine.
Analyzing the Blood Test’s Accuracy
Dr. Grady and colleagues conducted a multisite clinical trial called ECLIPSE, which compared the sensitivity and specificity of a cfDNA blood test (Shield, Guardant Health) against that obtained with colonoscopy, the gold standard for CRC screening. Guardant led and funded the study.
Guardant’s Shield test is designed to detect CRC through genomic alterations, aberrant methylation status, and fragmentomic patterns, which show up as an “abnormal signal detected” result. Similar blood tests are being developed as “liquid biopsy” tests for other emerging cancer screenings as well.
The study included 7861 people with average CRC risk who underwent routine screening with colonoscopy at 265 sites in the United States, including primary care and endoscopy centers in academic and community-based institutions. Eligible people were aged 45-84 years (average age, 60 years), and 53.7% were women. The race and ethnicity characteristics of the participants closely mirrored the demographic distribution in the 2020 US Census.
Overall, 54 of 65 (83.1%) participants with colonoscopy-detected CRC had a positive cfDNA blood test. However, 11 participants (16.9%) with CRC had a negative test.
The cfDNA blood test identified 42 of 48 stage I, II, or III CRCs, indicating a sensitivity of 87.5%, including 65% for stage I cancers, 100% for stage II cancers, and 100% for stage III cancers. The test also identified all 10 of the stage IV CRC cases. There were no substantial differences in sensitivity for CRC based on primary tumor location, tumor histologic grade, or demographic characteristics.
Among participants without advanced colorectal neoplasia on colonoscopy, 89.6% had a negative cfDNA blood test, and 10.4% had a positive test.
Among those with a negative colonoscopy — with no CRC, advanced precancerous lesions, or nonadvanced precancerous lesions — specificity was 89.9%.
Among 1116 participants with advanced precancerous lesions identified as the most advanced lesion on colonoscopy, the cfDNA blood test was positive for 147, indicating a sensitivity for advanced precancerous lesions of 13.2%.
Although the blood test has sensitivity similar to stool-based tests for CRC, the accuracy is lower than it is with colonoscopy, which remains the current gold standard for CRC screening, Dr. Grady said.
“Colorectal cancer is common and very preventable with screening, but only about 50% to 60% of people who are eligible for screening actually take those tests,” he said. “Getting people to be screened for cancer works best when we offer them screening options and then let them choose what works best for them.”
Future Research
Colorectal cancer is the second leading cause of cancer-related death among US adults and is now the third most diagnosed cancer for people younger than 50 years, Dr. Grady said. Although overall CRC death rates have declined in recent years, the rates among those younger than 55 years have increased since the mid-2000s.
“When colorectal cancer is found earlier and the cancer has not yet spread throughout the body, patient outcomes are much better, as reflected in 5-year survival being much better. It makes sense that an effective blood-based test could have a potential role, in particular for those not getting screened yet,” said Joshua Melson, MD, AGAF, clinical professor of medicine and director of the High-Risk Clinic for Gastrointestinal Cancers at the University of Arizona Cancer Center in Tucson.
Dr. Melson, who wasn’t involved with this study, noted that blood-based testing shows promise for cancer detection but needs additional support for real-world implementation. For instance, the Shield blood test has difficulty detecting precancerous lesions, and it remains unclear what the optimal intervals for repeat testing would be after a negative test, he said. In addition, screening programs will need to ensure they have capacity to effectively deal with a positive test result.
“For a screening program to actually work, when a noninvasive test (whether blood-based or stool-based) is read as positive, those patients need to have a follow-up colonoscopy,” he said.
Proper communication with patients will be important as well, said Gloria Coronado, PhD, associate director of Population Sciences at the University of Arizona Cancer Center, Tucson. Dr. Coronado, who wasn’t involved with this study, has developed CRC screening messages for specific patient populations and studied patient reactions to CRC blood tests.
In a study by Dr. Coronado and colleagues, among more than 2000 patients who passively declined fecal testing and had an upcoming clinic visit, CRC screening proportions were 17.5 percentage points higher in the group offered the blood test vs those offered usual care. In qualitative interviews, one patient said of the blood-based testing option, “I was screaming hallelujah!”
“Patients believed that a blood test would be more accurate than a stool-based test. However, for the detection of advanced adenomas, the reverse is true,” she said. “It will be important to balance the high acceptance and enthusiasm for the blood test with the lower performance of the blood test compared to other tests already on the market.”
In a statement accompanying the study’s publication, the American Gastroenterological Association welcomed these results as an exciting development, but cautioned that a blood-based test was not interchangeable with colonoscopy.
“The Centers for Medicare and Medicaid Services (CMS) has determined it will cover a blood test for colorectal cancer screening every three years if the test achieves 74% sensitivity for CRC, 90% specificity, and FDA approval,” the statement reads. “However, a blood test that meets only the CMS criteria will be inferior to current recommended tests and should not be recommended to replace current tests. Such a test could be recommended for patients who decline all other recommended tests, since any screening is better than no screening at all.”
Dr. Grady is a paid member of Guardant’s scientific advisory board and advised on the design and procedure of the clinical trial and data analysis. Dr. Melson previously served as consultant for Guardant. Dr. Coronado reported no relevant disclosures.
A version of this article appeared on Medscape.com .
, according to a new study.
The cfDNA blood test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions. Other noninvasive screening methods have sensitivity from 67% to 94% for CRC and 22% to 43% for advanced precancerous lesions.
“The results of the study are a promising step toward developing more convenient tools to detect colorectal cancer early while it is more easily treated,” said senior author William M. Grady, MD, AGAF, medical director of the Gastrointestinal Cancer Prevention Program at the Fred Hutchinson Cancer Center in Seattle.
“The test, which has an accuracy rate for colon cancer detection similar to stool tests used for early detection of cancer, could offer an alternative for patients who may otherwise decline current screening options,” he said.
The study was published online on March 14 in The New England Journal of Medicine.
Analyzing the Blood Test’s Accuracy
Dr. Grady and colleagues conducted a multisite clinical trial called ECLIPSE, which compared the sensitivity and specificity of a cfDNA blood test (Shield, Guardant Health) against that obtained with colonoscopy, the gold standard for CRC screening. Guardant led and funded the study.
Guardant’s Shield test is designed to detect CRC through genomic alterations, aberrant methylation status, and fragmentomic patterns, which show up as an “abnormal signal detected” result. Similar blood tests are being developed as “liquid biopsy” tests for other emerging cancer screenings as well.
The study included 7861 people with average CRC risk who underwent routine screening with colonoscopy at 265 sites in the United States, including primary care and endoscopy centers in academic and community-based institutions. Eligible people were aged 45-84 years (average age, 60 years), and 53.7% were women. The race and ethnicity characteristics of the participants closely mirrored the demographic distribution in the 2020 US Census.
Overall, 54 of 65 (83.1%) participants with colonoscopy-detected CRC had a positive cfDNA blood test. However, 11 participants (16.9%) with CRC had a negative test.
The cfDNA blood test identified 42 of 48 stage I, II, or III CRCs, indicating a sensitivity of 87.5%, including 65% for stage I cancers, 100% for stage II cancers, and 100% for stage III cancers. The test also identified all 10 of the stage IV CRC cases. There were no substantial differences in sensitivity for CRC based on primary tumor location, tumor histologic grade, or demographic characteristics.
Among participants without advanced colorectal neoplasia on colonoscopy, 89.6% had a negative cfDNA blood test, and 10.4% had a positive test.
Among those with a negative colonoscopy — with no CRC, advanced precancerous lesions, or nonadvanced precancerous lesions — specificity was 89.9%.
Among 1116 participants with advanced precancerous lesions identified as the most advanced lesion on colonoscopy, the cfDNA blood test was positive for 147, indicating a sensitivity for advanced precancerous lesions of 13.2%.
Although the blood test has sensitivity similar to stool-based tests for CRC, the accuracy is lower than it is with colonoscopy, which remains the current gold standard for CRC screening, Dr. Grady said.
“Colorectal cancer is common and very preventable with screening, but only about 50% to 60% of people who are eligible for screening actually take those tests,” he said. “Getting people to be screened for cancer works best when we offer them screening options and then let them choose what works best for them.”
Future Research
Colorectal cancer is the second leading cause of cancer-related death among US adults and is now the third most diagnosed cancer for people younger than 50 years, Dr. Grady said. Although overall CRC death rates have declined in recent years, the rates among those younger than 55 years have increased since the mid-2000s.
“When colorectal cancer is found earlier and the cancer has not yet spread throughout the body, patient outcomes are much better, as reflected in 5-year survival being much better. It makes sense that an effective blood-based test could have a potential role, in particular for those not getting screened yet,” said Joshua Melson, MD, AGAF, clinical professor of medicine and director of the High-Risk Clinic for Gastrointestinal Cancers at the University of Arizona Cancer Center in Tucson.
Dr. Melson, who wasn’t involved with this study, noted that blood-based testing shows promise for cancer detection but needs additional support for real-world implementation. For instance, the Shield blood test has difficulty detecting precancerous lesions, and it remains unclear what the optimal intervals for repeat testing would be after a negative test, he said. In addition, screening programs will need to ensure they have capacity to effectively deal with a positive test result.
“For a screening program to actually work, when a noninvasive test (whether blood-based or stool-based) is read as positive, those patients need to have a follow-up colonoscopy,” he said.
Proper communication with patients will be important as well, said Gloria Coronado, PhD, associate director of Population Sciences at the University of Arizona Cancer Center, Tucson. Dr. Coronado, who wasn’t involved with this study, has developed CRC screening messages for specific patient populations and studied patient reactions to CRC blood tests.
In a study by Dr. Coronado and colleagues, among more than 2000 patients who passively declined fecal testing and had an upcoming clinic visit, CRC screening proportions were 17.5 percentage points higher in the group offered the blood test vs those offered usual care. In qualitative interviews, one patient said of the blood-based testing option, “I was screaming hallelujah!”
“Patients believed that a blood test would be more accurate than a stool-based test. However, for the detection of advanced adenomas, the reverse is true,” she said. “It will be important to balance the high acceptance and enthusiasm for the blood test with the lower performance of the blood test compared to other tests already on the market.”
In a statement accompanying the study’s publication, the American Gastroenterological Association welcomed these results as an exciting development, but cautioned that a blood-based test was not interchangeable with colonoscopy.
“The Centers for Medicare and Medicaid Services (CMS) has determined it will cover a blood test for colorectal cancer screening every three years if the test achieves 74% sensitivity for CRC, 90% specificity, and FDA approval,” the statement reads. “However, a blood test that meets only the CMS criteria will be inferior to current recommended tests and should not be recommended to replace current tests. Such a test could be recommended for patients who decline all other recommended tests, since any screening is better than no screening at all.”
Dr. Grady is a paid member of Guardant’s scientific advisory board and advised on the design and procedure of the clinical trial and data analysis. Dr. Melson previously served as consultant for Guardant. Dr. Coronado reported no relevant disclosures.
A version of this article appeared on Medscape.com .
, according to a new study.
The cfDNA blood test had 83% sensitivity for CRC, 90% specificity for advanced neoplasia, and 13% sensitivity for advanced precancerous lesions. Other noninvasive screening methods have sensitivity from 67% to 94% for CRC and 22% to 43% for advanced precancerous lesions.
“The results of the study are a promising step toward developing more convenient tools to detect colorectal cancer early while it is more easily treated,” said senior author William M. Grady, MD, AGAF, medical director of the Gastrointestinal Cancer Prevention Program at the Fred Hutchinson Cancer Center in Seattle.
“The test, which has an accuracy rate for colon cancer detection similar to stool tests used for early detection of cancer, could offer an alternative for patients who may otherwise decline current screening options,” he said.
The study was published online on March 14 in The New England Journal of Medicine.
Analyzing the Blood Test’s Accuracy
Dr. Grady and colleagues conducted a multisite clinical trial called ECLIPSE, which compared the sensitivity and specificity of a cfDNA blood test (Shield, Guardant Health) against that obtained with colonoscopy, the gold standard for CRC screening. Guardant led and funded the study.
Guardant’s Shield test is designed to detect CRC through genomic alterations, aberrant methylation status, and fragmentomic patterns, which show up as an “abnormal signal detected” result. Similar blood tests are being developed as “liquid biopsy” tests for other emerging cancer screenings as well.
The study included 7861 people with average CRC risk who underwent routine screening with colonoscopy at 265 sites in the United States, including primary care and endoscopy centers in academic and community-based institutions. Eligible people were aged 45-84 years (average age, 60 years), and 53.7% were women. The race and ethnicity characteristics of the participants closely mirrored the demographic distribution in the 2020 US Census.
Overall, 54 of 65 (83.1%) participants with colonoscopy-detected CRC had a positive cfDNA blood test. However, 11 participants (16.9%) with CRC had a negative test.
The cfDNA blood test identified 42 of 48 stage I, II, or III CRCs, indicating a sensitivity of 87.5%, including 65% for stage I cancers, 100% for stage II cancers, and 100% for stage III cancers. The test also identified all 10 of the stage IV CRC cases. There were no substantial differences in sensitivity for CRC based on primary tumor location, tumor histologic grade, or demographic characteristics.
Among participants without advanced colorectal neoplasia on colonoscopy, 89.6% had a negative cfDNA blood test, and 10.4% had a positive test.
Among those with a negative colonoscopy — with no CRC, advanced precancerous lesions, or nonadvanced precancerous lesions — specificity was 89.9%.
Among 1116 participants with advanced precancerous lesions identified as the most advanced lesion on colonoscopy, the cfDNA blood test was positive for 147, indicating a sensitivity for advanced precancerous lesions of 13.2%.
Although the blood test has sensitivity similar to stool-based tests for CRC, the accuracy is lower than it is with colonoscopy, which remains the current gold standard for CRC screening, Dr. Grady said.
“Colorectal cancer is common and very preventable with screening, but only about 50% to 60% of people who are eligible for screening actually take those tests,” he said. “Getting people to be screened for cancer works best when we offer them screening options and then let them choose what works best for them.”
Future Research
Colorectal cancer is the second leading cause of cancer-related death among US adults and is now the third most diagnosed cancer for people younger than 50 years, Dr. Grady said. Although overall CRC death rates have declined in recent years, the rates among those younger than 55 years have increased since the mid-2000s.
“When colorectal cancer is found earlier and the cancer has not yet spread throughout the body, patient outcomes are much better, as reflected in 5-year survival being much better. It makes sense that an effective blood-based test could have a potential role, in particular for those not getting screened yet,” said Joshua Melson, MD, AGAF, clinical professor of medicine and director of the High-Risk Clinic for Gastrointestinal Cancers at the University of Arizona Cancer Center in Tucson.
Dr. Melson, who wasn’t involved with this study, noted that blood-based testing shows promise for cancer detection but needs additional support for real-world implementation. For instance, the Shield blood test has difficulty detecting precancerous lesions, and it remains unclear what the optimal intervals for repeat testing would be after a negative test, he said. In addition, screening programs will need to ensure they have capacity to effectively deal with a positive test result.
“For a screening program to actually work, when a noninvasive test (whether blood-based or stool-based) is read as positive, those patients need to have a follow-up colonoscopy,” he said.
Proper communication with patients will be important as well, said Gloria Coronado, PhD, associate director of Population Sciences at the University of Arizona Cancer Center, Tucson. Dr. Coronado, who wasn’t involved with this study, has developed CRC screening messages for specific patient populations and studied patient reactions to CRC blood tests.
In a study by Dr. Coronado and colleagues, among more than 2000 patients who passively declined fecal testing and had an upcoming clinic visit, CRC screening proportions were 17.5 percentage points higher in the group offered the blood test vs those offered usual care. In qualitative interviews, one patient said of the blood-based testing option, “I was screaming hallelujah!”
“Patients believed that a blood test would be more accurate than a stool-based test. However, for the detection of advanced adenomas, the reverse is true,” she said. “It will be important to balance the high acceptance and enthusiasm for the blood test with the lower performance of the blood test compared to other tests already on the market.”
In a statement accompanying the study’s publication, the American Gastroenterological Association welcomed these results as an exciting development, but cautioned that a blood-based test was not interchangeable with colonoscopy.
“The Centers for Medicare and Medicaid Services (CMS) has determined it will cover a blood test for colorectal cancer screening every three years if the test achieves 74% sensitivity for CRC, 90% specificity, and FDA approval,” the statement reads. “However, a blood test that meets only the CMS criteria will be inferior to current recommended tests and should not be recommended to replace current tests. Such a test could be recommended for patients who decline all other recommended tests, since any screening is better than no screening at all.”
Dr. Grady is a paid member of Guardant’s scientific advisory board and advised on the design and procedure of the clinical trial and data analysis. Dr. Melson previously served as consultant for Guardant. Dr. Coronado reported no relevant disclosures.
A version of this article appeared on Medscape.com .
FROM NEJM
Combining Targeted Drugs and Radiation in Breast Cancer: What’s Safe?
One reason is studies of new drugs typically exclude concurrent radiotherapy, said Kathy Miller, MD, a contributor to this news organization and professor of oncology and medicine at the Indiana University School of Medicine, Indianapolis, Indiana.
If trials evaluating new targeted therapies included concurrent radiotherapy, it would be challenging to identify whether toxicities came from the drug itself, the radiation, or the combination, Dr. Miller explained.
Given the limited evidence, “we tend to be cautious and conservative” and not combine therapies that “we don’t know are safe or appropriate for patients,” said Chirag Shah, MD, director of breast radiology at the Cleveland Clinic, Cleveland, Ohio.
Below is a guide to what we do and don’t know about combining radiotherapy and systemic treatments in breast cancer.
1. Immunotherapy plus radiotherapy likely safe but evidence is limited
Safety data on combining immune checkpoint inhibitors and radiotherapy in breast cancer are limited because concurrent radiotherapy has typically been excluded in pivotal trials.
The 2020 KEYNOTE-522 trial did provide a rare look at concurrent radiotherapy and immunotherapy in early triple-negative breast cancer. The analysis found “no safety concerns” with concurrent radiotherapy and pembrolizumab, lead investigator Peter Schmid, MD, of Queen Mary University of London, England, told this news organization.
Research on other solid tumor types also suggests that radiotherapy “can be considered safe” alongside immunotherapy, the authors of a recent ESTRO consensus said.
Despite evidence indicating radiotherapy alongside immunotherapy can be safe in patients with breast cancer, “certain aspects, such as patient selection, total dose, and dose per fraction, remain open for debate to achieve the best therapeutic outcomes,” the ESTRO experts cautioned.
2. CDK4/6 inhibitors may be offered with radiotherapy in some settings, not others
CDK4/6 inhibitors are now standard of care for first- or second-line treatment in patients with advanced or metastatic hormone receptor–positive, human epidermal growth factor receptor 2 (HER2)–negative breast cancer.
“Unfortunately, we found no information regarding concurrent radiotherapy in the adjuvant setting” in pivotal trials for palbociclib, abemaciclib, and ribociclib, the ESTRO authors said. In the pivotal trials for palbociclib and abemaciclib, patients had to discontinue immunotherapy before initiating radiotherapy, and in the trial for ribociclib, palliative radiotherapy was allowed for relieving bone pain only.
However, in 2023, a team of experts from 12 countries attempted to piece together the available evidence, publishing a meta-analysis of 11 retrospective studies on the safety of CDK4/6 inhibitors given concurrently with radiotherapy in patients with metastatic disease.
Although most of these studies had small patient populations, the analysis revealed that CDK4/6 inhibitors given concurrently with radiotherapy in patients with metastatic breast cancer led to a similar side-effect profile to that observed in trials of the inhibitors given sequentially with adjuvant radiotherapy.
“These findings suggest that the simultaneous administration of CDK4/6 inhibitors and radiotherapy is generally well tolerated,” the ESTRO authors concluded but added that CDK4/6 inhibitors and concomitant radiotherapy should be investigated more in the adjuvant locoregional, whole brain, and intracranial stereotactic radiotherapy settings.
The expert panel did note, however, that CDK4/6 inhibitors and concomitant radiotherapy “could be offered” during palliative and ablative extracranial radiotherapy.
3. Only offer poly (ADP-ribose) polymerase (PARP) inhibitors plus radiotherapy in clinical trial setting
PARP inhibitors olaparib (Lynparza) and talazoprib (Talzenna) are standard of care in patients with metastatic breast cancer who have BRCA1/2 gene mutations. Olaparib is also indicated for high-risk early breast cancer following neoadjuvant or adjuvant chemotherapy.
But data on combining PARP inhibitors with radiotherapy in breast cancer also remain limited.
One ongoing phase 2 trial, comparing olaparib plus radiotherapy to radiotherapy alone in 300 people with inflammatory breast cancer, is aiming to tease out the safety of the combination and whether it improves local control in patients with aggressive disease.
“The desire is to explore the exciting possibility that low doses of PARP inhibition may radiosensitize tumor cells more than normal tissues,” Reshma Jagsi, MD, chair of the Department of Radiation Oncology at Emory University School of Medicine in Atlanta, Georgia, who is leading the study.
Because of potential good or bad interactions between new systemic therapies and radiotherapy, “intentional trial design” is important, Dr. Jagsi said, so we “know the best way to combine treatments in practice to optimize outcomes.”
But given the evidence to date, the ESTRO experts advised waiting until “further research provides more comprehensive safety and efficacy data” in the primary, adjuvant, and metastatic settings. The experts also advised not offering PARP inhibitors and concomitant radiotherapy to treat advanced breast cancer outside of clinical trials.
4. Phosphoinositide 3-kinase inhibitors (PI3K) inhibitors, mammalian target of rapamycin (mTOR) inhibitors, and newer targeted agents should not be offered concurrently with radiotherapy
Clinical trial data on the safety of combining PI3K and mTOR inhibitors with radiation are thin, especially in advanced breast cancer. Typically, radiotherapy within 4 weeks before randomization, or 2 weeks for palliative radiation, was excluded in pivotal trials.
For this reason, the ESTRO team recommended that concurrent radiation with either PI3K inhibitors or mTOR inhibitors “should not be offered.”
ESTRO also cautioned against providing radiation concurrently with newer anti-HER2 tyrosine-kinase drugs, such as neratinib or tucatinib, or newer antibody-drug conjugates such as trastuzumab deruxtecan, until more data emerge on the safety of these combinations.
5. Combining older HER2-targeted drugs and radiotherapy generally safe
The ESTRO authors agreed that older anti-HER2 drugs trastuzumab (Herceptin), pertuzumab (Perjeta), and lapatinib (Tykerb) can be safely used concurrently with locoregional radiotherapy as well.
One of the biggest concerns in the field is how to combine radiation with systemic therapies in the setting of brain metastases, and the data on these older anti-HER2 drugs are relatively clear that it’s safe, Dr. Miller said.
For instance, in a 2019 study of 84 patients with 487 brain metastases, stereotactic radiosurgery given alongside lapatinib led to significantly higher rates of complete responses than stereotactic radiosurgery alone (35% vs 11%) with no increased risk for radiation necrosis.
The ESTRO team agreed, noting that the latest evidence supports the use of trastuzumab, pertuzumab, or lapatinib alongside radiotherapy for whole brain and ablative intracranial stereotactic radiotherapy.
As for older antibody-drug conjugates, trastuzumab emtansine (T-DM1) plus radiotherapy “might be considered” during adjuvant locoregional radiotherapy for breast cancer but should not be offered for whole brain and ablative intracranial stereotactic radiotherapy, the ESTRO team said.
Dr. Jagsi declared the following conflicts in a recent 2024 publication: Stock options for advisory board role in Equity Quotient; grants or contracts from Genentech; and expert witness for Kleinbard, LLC, and Hawks Quindel Law. In the Keynote-522 trial publication Dr. Schmid declared relationships with AstraZeneca, Bayer, Boehringer Ingelheim, Celgene, Eisai, Hoffmann-La Roche, Genetech, Merck, Novartis, and Pfizer. Dr. Shah reported consulting for Impedimed, Videra Surgical, and PreludeDX.
A version of this article appeared on Medscape.com.
One reason is studies of new drugs typically exclude concurrent radiotherapy, said Kathy Miller, MD, a contributor to this news organization and professor of oncology and medicine at the Indiana University School of Medicine, Indianapolis, Indiana.
If trials evaluating new targeted therapies included concurrent radiotherapy, it would be challenging to identify whether toxicities came from the drug itself, the radiation, or the combination, Dr. Miller explained.
Given the limited evidence, “we tend to be cautious and conservative” and not combine therapies that “we don’t know are safe or appropriate for patients,” said Chirag Shah, MD, director of breast radiology at the Cleveland Clinic, Cleveland, Ohio.
Below is a guide to what we do and don’t know about combining radiotherapy and systemic treatments in breast cancer.
1. Immunotherapy plus radiotherapy likely safe but evidence is limited
Safety data on combining immune checkpoint inhibitors and radiotherapy in breast cancer are limited because concurrent radiotherapy has typically been excluded in pivotal trials.
The 2020 KEYNOTE-522 trial did provide a rare look at concurrent radiotherapy and immunotherapy in early triple-negative breast cancer. The analysis found “no safety concerns” with concurrent radiotherapy and pembrolizumab, lead investigator Peter Schmid, MD, of Queen Mary University of London, England, told this news organization.
Research on other solid tumor types also suggests that radiotherapy “can be considered safe” alongside immunotherapy, the authors of a recent ESTRO consensus said.
Despite evidence indicating radiotherapy alongside immunotherapy can be safe in patients with breast cancer, “certain aspects, such as patient selection, total dose, and dose per fraction, remain open for debate to achieve the best therapeutic outcomes,” the ESTRO experts cautioned.
2. CDK4/6 inhibitors may be offered with radiotherapy in some settings, not others
CDK4/6 inhibitors are now standard of care for first- or second-line treatment in patients with advanced or metastatic hormone receptor–positive, human epidermal growth factor receptor 2 (HER2)–negative breast cancer.
“Unfortunately, we found no information regarding concurrent radiotherapy in the adjuvant setting” in pivotal trials for palbociclib, abemaciclib, and ribociclib, the ESTRO authors said. In the pivotal trials for palbociclib and abemaciclib, patients had to discontinue immunotherapy before initiating radiotherapy, and in the trial for ribociclib, palliative radiotherapy was allowed for relieving bone pain only.
However, in 2023, a team of experts from 12 countries attempted to piece together the available evidence, publishing a meta-analysis of 11 retrospective studies on the safety of CDK4/6 inhibitors given concurrently with radiotherapy in patients with metastatic disease.
Although most of these studies had small patient populations, the analysis revealed that CDK4/6 inhibitors given concurrently with radiotherapy in patients with metastatic breast cancer led to a similar side-effect profile to that observed in trials of the inhibitors given sequentially with adjuvant radiotherapy.
“These findings suggest that the simultaneous administration of CDK4/6 inhibitors and radiotherapy is generally well tolerated,” the ESTRO authors concluded but added that CDK4/6 inhibitors and concomitant radiotherapy should be investigated more in the adjuvant locoregional, whole brain, and intracranial stereotactic radiotherapy settings.
The expert panel did note, however, that CDK4/6 inhibitors and concomitant radiotherapy “could be offered” during palliative and ablative extracranial radiotherapy.
3. Only offer poly (ADP-ribose) polymerase (PARP) inhibitors plus radiotherapy in clinical trial setting
PARP inhibitors olaparib (Lynparza) and talazoprib (Talzenna) are standard of care in patients with metastatic breast cancer who have BRCA1/2 gene mutations. Olaparib is also indicated for high-risk early breast cancer following neoadjuvant or adjuvant chemotherapy.
But data on combining PARP inhibitors with radiotherapy in breast cancer also remain limited.
One ongoing phase 2 trial, comparing olaparib plus radiotherapy to radiotherapy alone in 300 people with inflammatory breast cancer, is aiming to tease out the safety of the combination and whether it improves local control in patients with aggressive disease.
“The desire is to explore the exciting possibility that low doses of PARP inhibition may radiosensitize tumor cells more than normal tissues,” Reshma Jagsi, MD, chair of the Department of Radiation Oncology at Emory University School of Medicine in Atlanta, Georgia, who is leading the study.
Because of potential good or bad interactions between new systemic therapies and radiotherapy, “intentional trial design” is important, Dr. Jagsi said, so we “know the best way to combine treatments in practice to optimize outcomes.”
But given the evidence to date, the ESTRO experts advised waiting until “further research provides more comprehensive safety and efficacy data” in the primary, adjuvant, and metastatic settings. The experts also advised not offering PARP inhibitors and concomitant radiotherapy to treat advanced breast cancer outside of clinical trials.
4. Phosphoinositide 3-kinase inhibitors (PI3K) inhibitors, mammalian target of rapamycin (mTOR) inhibitors, and newer targeted agents should not be offered concurrently with radiotherapy
Clinical trial data on the safety of combining PI3K and mTOR inhibitors with radiation are thin, especially in advanced breast cancer. Typically, radiotherapy within 4 weeks before randomization, or 2 weeks for palliative radiation, was excluded in pivotal trials.
For this reason, the ESTRO team recommended that concurrent radiation with either PI3K inhibitors or mTOR inhibitors “should not be offered.”
ESTRO also cautioned against providing radiation concurrently with newer anti-HER2 tyrosine-kinase drugs, such as neratinib or tucatinib, or newer antibody-drug conjugates such as trastuzumab deruxtecan, until more data emerge on the safety of these combinations.
5. Combining older HER2-targeted drugs and radiotherapy generally safe
The ESTRO authors agreed that older anti-HER2 drugs trastuzumab (Herceptin), pertuzumab (Perjeta), and lapatinib (Tykerb) can be safely used concurrently with locoregional radiotherapy as well.
One of the biggest concerns in the field is how to combine radiation with systemic therapies in the setting of brain metastases, and the data on these older anti-HER2 drugs are relatively clear that it’s safe, Dr. Miller said.
For instance, in a 2019 study of 84 patients with 487 brain metastases, stereotactic radiosurgery given alongside lapatinib led to significantly higher rates of complete responses than stereotactic radiosurgery alone (35% vs 11%) with no increased risk for radiation necrosis.
The ESTRO team agreed, noting that the latest evidence supports the use of trastuzumab, pertuzumab, or lapatinib alongside radiotherapy for whole brain and ablative intracranial stereotactic radiotherapy.
As for older antibody-drug conjugates, trastuzumab emtansine (T-DM1) plus radiotherapy “might be considered” during adjuvant locoregional radiotherapy for breast cancer but should not be offered for whole brain and ablative intracranial stereotactic radiotherapy, the ESTRO team said.
Dr. Jagsi declared the following conflicts in a recent 2024 publication: Stock options for advisory board role in Equity Quotient; grants or contracts from Genentech; and expert witness for Kleinbard, LLC, and Hawks Quindel Law. In the Keynote-522 trial publication Dr. Schmid declared relationships with AstraZeneca, Bayer, Boehringer Ingelheim, Celgene, Eisai, Hoffmann-La Roche, Genetech, Merck, Novartis, and Pfizer. Dr. Shah reported consulting for Impedimed, Videra Surgical, and PreludeDX.
A version of this article appeared on Medscape.com.
One reason is studies of new drugs typically exclude concurrent radiotherapy, said Kathy Miller, MD, a contributor to this news organization and professor of oncology and medicine at the Indiana University School of Medicine, Indianapolis, Indiana.
If trials evaluating new targeted therapies included concurrent radiotherapy, it would be challenging to identify whether toxicities came from the drug itself, the radiation, or the combination, Dr. Miller explained.
Given the limited evidence, “we tend to be cautious and conservative” and not combine therapies that “we don’t know are safe or appropriate for patients,” said Chirag Shah, MD, director of breast radiology at the Cleveland Clinic, Cleveland, Ohio.
Below is a guide to what we do and don’t know about combining radiotherapy and systemic treatments in breast cancer.
1. Immunotherapy plus radiotherapy likely safe but evidence is limited
Safety data on combining immune checkpoint inhibitors and radiotherapy in breast cancer are limited because concurrent radiotherapy has typically been excluded in pivotal trials.
The 2020 KEYNOTE-522 trial did provide a rare look at concurrent radiotherapy and immunotherapy in early triple-negative breast cancer. The analysis found “no safety concerns” with concurrent radiotherapy and pembrolizumab, lead investigator Peter Schmid, MD, of Queen Mary University of London, England, told this news organization.
Research on other solid tumor types also suggests that radiotherapy “can be considered safe” alongside immunotherapy, the authors of a recent ESTRO consensus said.
Despite evidence indicating radiotherapy alongside immunotherapy can be safe in patients with breast cancer, “certain aspects, such as patient selection, total dose, and dose per fraction, remain open for debate to achieve the best therapeutic outcomes,” the ESTRO experts cautioned.
2. CDK4/6 inhibitors may be offered with radiotherapy in some settings, not others
CDK4/6 inhibitors are now standard of care for first- or second-line treatment in patients with advanced or metastatic hormone receptor–positive, human epidermal growth factor receptor 2 (HER2)–negative breast cancer.
“Unfortunately, we found no information regarding concurrent radiotherapy in the adjuvant setting” in pivotal trials for palbociclib, abemaciclib, and ribociclib, the ESTRO authors said. In the pivotal trials for palbociclib and abemaciclib, patients had to discontinue immunotherapy before initiating radiotherapy, and in the trial for ribociclib, palliative radiotherapy was allowed for relieving bone pain only.
However, in 2023, a team of experts from 12 countries attempted to piece together the available evidence, publishing a meta-analysis of 11 retrospective studies on the safety of CDK4/6 inhibitors given concurrently with radiotherapy in patients with metastatic disease.
Although most of these studies had small patient populations, the analysis revealed that CDK4/6 inhibitors given concurrently with radiotherapy in patients with metastatic breast cancer led to a similar side-effect profile to that observed in trials of the inhibitors given sequentially with adjuvant radiotherapy.
“These findings suggest that the simultaneous administration of CDK4/6 inhibitors and radiotherapy is generally well tolerated,” the ESTRO authors concluded but added that CDK4/6 inhibitors and concomitant radiotherapy should be investigated more in the adjuvant locoregional, whole brain, and intracranial stereotactic radiotherapy settings.
The expert panel did note, however, that CDK4/6 inhibitors and concomitant radiotherapy “could be offered” during palliative and ablative extracranial radiotherapy.
3. Only offer poly (ADP-ribose) polymerase (PARP) inhibitors plus radiotherapy in clinical trial setting
PARP inhibitors olaparib (Lynparza) and talazoprib (Talzenna) are standard of care in patients with metastatic breast cancer who have BRCA1/2 gene mutations. Olaparib is also indicated for high-risk early breast cancer following neoadjuvant or adjuvant chemotherapy.
But data on combining PARP inhibitors with radiotherapy in breast cancer also remain limited.
One ongoing phase 2 trial, comparing olaparib plus radiotherapy to radiotherapy alone in 300 people with inflammatory breast cancer, is aiming to tease out the safety of the combination and whether it improves local control in patients with aggressive disease.
“The desire is to explore the exciting possibility that low doses of PARP inhibition may radiosensitize tumor cells more than normal tissues,” Reshma Jagsi, MD, chair of the Department of Radiation Oncology at Emory University School of Medicine in Atlanta, Georgia, who is leading the study.
Because of potential good or bad interactions between new systemic therapies and radiotherapy, “intentional trial design” is important, Dr. Jagsi said, so we “know the best way to combine treatments in practice to optimize outcomes.”
But given the evidence to date, the ESTRO experts advised waiting until “further research provides more comprehensive safety and efficacy data” in the primary, adjuvant, and metastatic settings. The experts also advised not offering PARP inhibitors and concomitant radiotherapy to treat advanced breast cancer outside of clinical trials.
4. Phosphoinositide 3-kinase inhibitors (PI3K) inhibitors, mammalian target of rapamycin (mTOR) inhibitors, and newer targeted agents should not be offered concurrently with radiotherapy
Clinical trial data on the safety of combining PI3K and mTOR inhibitors with radiation are thin, especially in advanced breast cancer. Typically, radiotherapy within 4 weeks before randomization, or 2 weeks for palliative radiation, was excluded in pivotal trials.
For this reason, the ESTRO team recommended that concurrent radiation with either PI3K inhibitors or mTOR inhibitors “should not be offered.”
ESTRO also cautioned against providing radiation concurrently with newer anti-HER2 tyrosine-kinase drugs, such as neratinib or tucatinib, or newer antibody-drug conjugates such as trastuzumab deruxtecan, until more data emerge on the safety of these combinations.
5. Combining older HER2-targeted drugs and radiotherapy generally safe
The ESTRO authors agreed that older anti-HER2 drugs trastuzumab (Herceptin), pertuzumab (Perjeta), and lapatinib (Tykerb) can be safely used concurrently with locoregional radiotherapy as well.
One of the biggest concerns in the field is how to combine radiation with systemic therapies in the setting of brain metastases, and the data on these older anti-HER2 drugs are relatively clear that it’s safe, Dr. Miller said.
For instance, in a 2019 study of 84 patients with 487 brain metastases, stereotactic radiosurgery given alongside lapatinib led to significantly higher rates of complete responses than stereotactic radiosurgery alone (35% vs 11%) with no increased risk for radiation necrosis.
The ESTRO team agreed, noting that the latest evidence supports the use of trastuzumab, pertuzumab, or lapatinib alongside radiotherapy for whole brain and ablative intracranial stereotactic radiotherapy.
As for older antibody-drug conjugates, trastuzumab emtansine (T-DM1) plus radiotherapy “might be considered” during adjuvant locoregional radiotherapy for breast cancer but should not be offered for whole brain and ablative intracranial stereotactic radiotherapy, the ESTRO team said.
Dr. Jagsi declared the following conflicts in a recent 2024 publication: Stock options for advisory board role in Equity Quotient; grants or contracts from Genentech; and expert witness for Kleinbard, LLC, and Hawks Quindel Law. In the Keynote-522 trial publication Dr. Schmid declared relationships with AstraZeneca, Bayer, Boehringer Ingelheim, Celgene, Eisai, Hoffmann-La Roche, Genetech, Merck, Novartis, and Pfizer. Dr. Shah reported consulting for Impedimed, Videra Surgical, and PreludeDX.
A version of this article appeared on Medscape.com.
FDA Approves New Esophageal Cancer Drug
The US Food and Drug Administration (FDA) has approved tislelizumab-jsgr (Tevimbra, BeiGene Ltd.) as second-line monotherapy for certain adult patients with unresectable or metastatic esophageal squamous cell carcinoma (ESCC).
Specifically, the novel checkpoint inhibitor is approved for patients with ESCC after prior systemic chemotherapy that did not include a programmed death–ligand 1 (PD-L1) inhibitor.
Approval was based on findings from the open-label, phase 3 RATIONALE 302 trial showing a statistically significant and clinically meaningful overall survival benefit with tislelizumab vs investigator’s choice of chemotherapy.
Study participants included 512 adults enrolled at 123 research sites in 11 countries in Europe, Asia, and North America. Patients were randomly assigned to receive intravenous tislelizumab, a humanized immunoglobulin G4 anti-programmed cell death protein 1 monoclonal antibody, at a dose of 200 mg every 3 weeks or investigator’s choice of standard chemotherapy with paclitaxel, docetaxel, or irinotecan until disease progression, unacceptable toxicity, or study withdrawal.
Median overall survival in the intention-to-treat population, the primary study endpoint, was 8.6 months vs 6.3 months in the chemotherapy arms (hazard ratio [HR], 0.70). The survival benefit was observed across predefined subgroups, including baseline PD-L1 status and region. The new agent was also associated with improved overall response rate (20.4% vs 9.8%) and more durable response (median duration of response of 7.1 vs 4.0 months; HR, 0.42) compared with chemotherapy.
The most common adverse reactions for tislelizumab, each occurring in at least 20% of treated patients, included increased glucose and decreased hemoglobin, lymphocytes, sodium, and albumin as well as increased alkaline phosphatase, anemia, fatigue, increased aspartate aminotransferase, musculoskeletal pain, decreased weight, increased alanine aminotransferase, and cough.
Fewer patients in the tislelizumab arm experienced grade 3 or greater treatment-emergent adverse events compared with the chemotherapy arm (46% vs 68%, respectively), and fewer patients discontinued tislelizumab vs chemotherapy due to such an event (7% vs 14%).
“Patients diagnosed with advanced or metastasized ESCC, the most common histologic subtype of esophageal cancer, often progress following initial therapy and are in need of new options,” Syma Iqbal, MD, of the Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, stated in the BeiGene release. “The RATIONALE 302 trial showed that patients with previously treated ESCC who received Tevimbra saw a clinically meaningful survival benefit, highlighting its potential as an important treatment option for these patients.”
The approval, which was deferred in 2022 due to COVID-19-related restrictions, marks the first for the agent in the United States. Tislelizumab should be available in the United States in the second half of 2024, BeiGene noted.
The FDA is also reviewing a Biologics License Application for the agent as a first-line treatment for patients with unresectable, locally advanced, or metastatic ESCC and for those with locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma, BeiGene announced in a press release.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved tislelizumab-jsgr (Tevimbra, BeiGene Ltd.) as second-line monotherapy for certain adult patients with unresectable or metastatic esophageal squamous cell carcinoma (ESCC).
Specifically, the novel checkpoint inhibitor is approved for patients with ESCC after prior systemic chemotherapy that did not include a programmed death–ligand 1 (PD-L1) inhibitor.
Approval was based on findings from the open-label, phase 3 RATIONALE 302 trial showing a statistically significant and clinically meaningful overall survival benefit with tislelizumab vs investigator’s choice of chemotherapy.
Study participants included 512 adults enrolled at 123 research sites in 11 countries in Europe, Asia, and North America. Patients were randomly assigned to receive intravenous tislelizumab, a humanized immunoglobulin G4 anti-programmed cell death protein 1 monoclonal antibody, at a dose of 200 mg every 3 weeks or investigator’s choice of standard chemotherapy with paclitaxel, docetaxel, or irinotecan until disease progression, unacceptable toxicity, or study withdrawal.
Median overall survival in the intention-to-treat population, the primary study endpoint, was 8.6 months vs 6.3 months in the chemotherapy arms (hazard ratio [HR], 0.70). The survival benefit was observed across predefined subgroups, including baseline PD-L1 status and region. The new agent was also associated with improved overall response rate (20.4% vs 9.8%) and more durable response (median duration of response of 7.1 vs 4.0 months; HR, 0.42) compared with chemotherapy.
The most common adverse reactions for tislelizumab, each occurring in at least 20% of treated patients, included increased glucose and decreased hemoglobin, lymphocytes, sodium, and albumin as well as increased alkaline phosphatase, anemia, fatigue, increased aspartate aminotransferase, musculoskeletal pain, decreased weight, increased alanine aminotransferase, and cough.
Fewer patients in the tislelizumab arm experienced grade 3 or greater treatment-emergent adverse events compared with the chemotherapy arm (46% vs 68%, respectively), and fewer patients discontinued tislelizumab vs chemotherapy due to such an event (7% vs 14%).
“Patients diagnosed with advanced or metastasized ESCC, the most common histologic subtype of esophageal cancer, often progress following initial therapy and are in need of new options,” Syma Iqbal, MD, of the Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, stated in the BeiGene release. “The RATIONALE 302 trial showed that patients with previously treated ESCC who received Tevimbra saw a clinically meaningful survival benefit, highlighting its potential as an important treatment option for these patients.”
The approval, which was deferred in 2022 due to COVID-19-related restrictions, marks the first for the agent in the United States. Tislelizumab should be available in the United States in the second half of 2024, BeiGene noted.
The FDA is also reviewing a Biologics License Application for the agent as a first-line treatment for patients with unresectable, locally advanced, or metastatic ESCC and for those with locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma, BeiGene announced in a press release.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved tislelizumab-jsgr (Tevimbra, BeiGene Ltd.) as second-line monotherapy for certain adult patients with unresectable or metastatic esophageal squamous cell carcinoma (ESCC).
Specifically, the novel checkpoint inhibitor is approved for patients with ESCC after prior systemic chemotherapy that did not include a programmed death–ligand 1 (PD-L1) inhibitor.
Approval was based on findings from the open-label, phase 3 RATIONALE 302 trial showing a statistically significant and clinically meaningful overall survival benefit with tislelizumab vs investigator’s choice of chemotherapy.
Study participants included 512 adults enrolled at 123 research sites in 11 countries in Europe, Asia, and North America. Patients were randomly assigned to receive intravenous tislelizumab, a humanized immunoglobulin G4 anti-programmed cell death protein 1 monoclonal antibody, at a dose of 200 mg every 3 weeks or investigator’s choice of standard chemotherapy with paclitaxel, docetaxel, or irinotecan until disease progression, unacceptable toxicity, or study withdrawal.
Median overall survival in the intention-to-treat population, the primary study endpoint, was 8.6 months vs 6.3 months in the chemotherapy arms (hazard ratio [HR], 0.70). The survival benefit was observed across predefined subgroups, including baseline PD-L1 status and region. The new agent was also associated with improved overall response rate (20.4% vs 9.8%) and more durable response (median duration of response of 7.1 vs 4.0 months; HR, 0.42) compared with chemotherapy.
The most common adverse reactions for tislelizumab, each occurring in at least 20% of treated patients, included increased glucose and decreased hemoglobin, lymphocytes, sodium, and albumin as well as increased alkaline phosphatase, anemia, fatigue, increased aspartate aminotransferase, musculoskeletal pain, decreased weight, increased alanine aminotransferase, and cough.
Fewer patients in the tislelizumab arm experienced grade 3 or greater treatment-emergent adverse events compared with the chemotherapy arm (46% vs 68%, respectively), and fewer patients discontinued tislelizumab vs chemotherapy due to such an event (7% vs 14%).
“Patients diagnosed with advanced or metastasized ESCC, the most common histologic subtype of esophageal cancer, often progress following initial therapy and are in need of new options,” Syma Iqbal, MD, of the Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, stated in the BeiGene release. “The RATIONALE 302 trial showed that patients with previously treated ESCC who received Tevimbra saw a clinically meaningful survival benefit, highlighting its potential as an important treatment option for these patients.”
The approval, which was deferred in 2022 due to COVID-19-related restrictions, marks the first for the agent in the United States. Tislelizumab should be available in the United States in the second half of 2024, BeiGene noted.
The FDA is also reviewing a Biologics License Application for the agent as a first-line treatment for patients with unresectable, locally advanced, or metastatic ESCC and for those with locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma, BeiGene announced in a press release.
A version of this article appeared on Medscape.com.
Cancer Data Trends 2024: Lung Cancer
1. Wolf AMD, Oeffinger KC, Shih TYC, et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J Clin. 2023;10.3322/caac.21811. doi:10.3322/caac.21811
2. US Department of Veterans Affairs. VA promotes high-quality, patient-centered lung cancer screening for veterans. Published June 15, 2023. Accessed December 18, 2023. http://www.hsrd.research.va.gov/impacts/lcs.cfm
3. Navuluri N, Morrison S, Green CL, et al. Racial disparities in lung cancer screening among veterans, 2013 to 2021. JAMA Netw Open. 2023;6(6):e2318795. doi:10.1001/jamanetworkopen.2023.18795
4. Bruno DS, Hess LM, Li X, Su EW, Patel M. Disparities in biomarker testing and clinical trial enrollment among patients with lung, breast, or colorectal cancers in the United States. JCO Precis Oncol. 2022;6:e2100427. doi:10.1200/PO.21.00427
5. Jalal SI, Guo A, Ahmed S, Kelley MJ. Analysis of actionable genetic alterations in lung carcinoma from the VA National Precision Oncology Program. Semin Oncol. 2022;S0093-7754(22)00054-9. doi:10.1053/j.seminoncol.2022.06.014
6. Williams CD, Allo MA, Gu L, Vashistha V, Press A, Kelley M. Health outcomes and healthcare resource utilization among veterans with stage IV non-small cell lung cancer treated with second-line chemotherapy versus immunotherapy. PLoS One. 2023;18(2):e0282020. doi:10.1371/journal.pone.0282020
7. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. Updated December 15, 2023. Accessed December 18, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications
8. Paz-Ares L, Chen Y, Reinmuth N, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open. 2022;7(2):100408. doi:10.1016/j.esmoop.2022.100408
1. Wolf AMD, Oeffinger KC, Shih TYC, et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J Clin. 2023;10.3322/caac.21811. doi:10.3322/caac.21811
2. US Department of Veterans Affairs. VA promotes high-quality, patient-centered lung cancer screening for veterans. Published June 15, 2023. Accessed December 18, 2023. http://www.hsrd.research.va.gov/impacts/lcs.cfm
3. Navuluri N, Morrison S, Green CL, et al. Racial disparities in lung cancer screening among veterans, 2013 to 2021. JAMA Netw Open. 2023;6(6):e2318795. doi:10.1001/jamanetworkopen.2023.18795
4. Bruno DS, Hess LM, Li X, Su EW, Patel M. Disparities in biomarker testing and clinical trial enrollment among patients with lung, breast, or colorectal cancers in the United States. JCO Precis Oncol. 2022;6:e2100427. doi:10.1200/PO.21.00427
5. Jalal SI, Guo A, Ahmed S, Kelley MJ. Analysis of actionable genetic alterations in lung carcinoma from the VA National Precision Oncology Program. Semin Oncol. 2022;S0093-7754(22)00054-9. doi:10.1053/j.seminoncol.2022.06.014
6. Williams CD, Allo MA, Gu L, Vashistha V, Press A, Kelley M. Health outcomes and healthcare resource utilization among veterans with stage IV non-small cell lung cancer treated with second-line chemotherapy versus immunotherapy. PLoS One. 2023;18(2):e0282020. doi:10.1371/journal.pone.0282020
7. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. Updated December 15, 2023. Accessed December 18, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications
8. Paz-Ares L, Chen Y, Reinmuth N, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open. 2022;7(2):100408. doi:10.1016/j.esmoop.2022.100408
1. Wolf AMD, Oeffinger KC, Shih TYC, et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J Clin. 2023;10.3322/caac.21811. doi:10.3322/caac.21811
2. US Department of Veterans Affairs. VA promotes high-quality, patient-centered lung cancer screening for veterans. Published June 15, 2023. Accessed December 18, 2023. http://www.hsrd.research.va.gov/impacts/lcs.cfm
3. Navuluri N, Morrison S, Green CL, et al. Racial disparities in lung cancer screening among veterans, 2013 to 2021. JAMA Netw Open. 2023;6(6):e2318795. doi:10.1001/jamanetworkopen.2023.18795
4. Bruno DS, Hess LM, Li X, Su EW, Patel M. Disparities in biomarker testing and clinical trial enrollment among patients with lung, breast, or colorectal cancers in the United States. JCO Precis Oncol. 2022;6:e2100427. doi:10.1200/PO.21.00427
5. Jalal SI, Guo A, Ahmed S, Kelley MJ. Analysis of actionable genetic alterations in lung carcinoma from the VA National Precision Oncology Program. Semin Oncol. 2022;S0093-7754(22)00054-9. doi:10.1053/j.seminoncol.2022.06.014
6. Williams CD, Allo MA, Gu L, Vashistha V, Press A, Kelley M. Health outcomes and healthcare resource utilization among veterans with stage IV non-small cell lung cancer treated with second-line chemotherapy versus immunotherapy. PLoS One. 2023;18(2):e0282020. doi:10.1371/journal.pone.0282020
7. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. Updated December 15, 2023. Accessed December 18, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications
8. Paz-Ares L, Chen Y, Reinmuth N, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open. 2022;7(2):100408. doi:10.1016/j.esmoop.2022.100408
Cancer Data Trends 2024: Multiple Myeloma
1. Mahmood S, Gupta P, Ma H. Impact of time period of diagnosis, race, and military exposures on the survival of US military veterans with multiple myeloma and/or plasmacytoma. J Clin Oncol. 2023;41(16 suppl). Abstract e20061. https://doi.org/10.1200/jco.2023.41.16_suppl.e20061
2. National Cancer Institute. Cancer stat facts: myeloma. Accessed January 2, 2024. https://seer.cancer.gov/statfacts/html/mulmy.html
3. Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up [published correction appears in Ann Oncol. 2022;33(1):117]. Ann Oncol. 2021;32(3):309-322. doi: 10.1016/j.annonc.2020.11.014
4. Su CT, Chen JC, Sussman JB. Virtual care for multiple myeloma in the COVID-19 era: interrupted time series analysis of Veterans Health Administration data. Leuk Lymphoma. 2023;64(5):1035-1039. doi: 10.1080/10428194.2023.2189989
5. O’Donnell EK, Shapiro YN, Yee AJ, et al. Quality of life, psychological distress, and prognostic perceptions in patients with multiple myeloma. Cancer. 2022;128(10):1996-2004. doi: 10.1002/cncr.34134
6. O’Donnell EK, Shapiro YN, Yee AJ, et al. Quality of life, psychological distress, and prognostic perceptions in caregivers of patients with multiple myeloma. Blood Adv. 2022;6(17):4967-4974. doi: 10.1182/bloodadvances.2022007127
7. Ahmed N, Shahzad M, Shippey E, et al. Socioeconomic and racial disparity in chimeric antigen receptor T cell therapy access. Transplant Cell Ther. 2022;28(7):358-364. doi: 10.1016/j.jtct.2022.04.008
1. Mahmood S, Gupta P, Ma H. Impact of time period of diagnosis, race, and military exposures on the survival of US military veterans with multiple myeloma and/or plasmacytoma. J Clin Oncol. 2023;41(16 suppl). Abstract e20061. https://doi.org/10.1200/jco.2023.41.16_suppl.e20061
2. National Cancer Institute. Cancer stat facts: myeloma. Accessed January 2, 2024. https://seer.cancer.gov/statfacts/html/mulmy.html
3. Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up [published correction appears in Ann Oncol. 2022;33(1):117]. Ann Oncol. 2021;32(3):309-322. doi: 10.1016/j.annonc.2020.11.014
4. Su CT, Chen JC, Sussman JB. Virtual care for multiple myeloma in the COVID-19 era: interrupted time series analysis of Veterans Health Administration data. Leuk Lymphoma. 2023;64(5):1035-1039. doi: 10.1080/10428194.2023.2189989
5. O’Donnell EK, Shapiro YN, Yee AJ, et al. Quality of life, psychological distress, and prognostic perceptions in patients with multiple myeloma. Cancer. 2022;128(10):1996-2004. doi: 10.1002/cncr.34134
6. O’Donnell EK, Shapiro YN, Yee AJ, et al. Quality of life, psychological distress, and prognostic perceptions in caregivers of patients with multiple myeloma. Blood Adv. 2022;6(17):4967-4974. doi: 10.1182/bloodadvances.2022007127
7. Ahmed N, Shahzad M, Shippey E, et al. Socioeconomic and racial disparity in chimeric antigen receptor T cell therapy access. Transplant Cell Ther. 2022;28(7):358-364. doi: 10.1016/j.jtct.2022.04.008
1. Mahmood S, Gupta P, Ma H. Impact of time period of diagnosis, race, and military exposures on the survival of US military veterans with multiple myeloma and/or plasmacytoma. J Clin Oncol. 2023;41(16 suppl). Abstract e20061. https://doi.org/10.1200/jco.2023.41.16_suppl.e20061
2. National Cancer Institute. Cancer stat facts: myeloma. Accessed January 2, 2024. https://seer.cancer.gov/statfacts/html/mulmy.html
3. Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up [published correction appears in Ann Oncol. 2022;33(1):117]. Ann Oncol. 2021;32(3):309-322. doi: 10.1016/j.annonc.2020.11.014
4. Su CT, Chen JC, Sussman JB. Virtual care for multiple myeloma in the COVID-19 era: interrupted time series analysis of Veterans Health Administration data. Leuk Lymphoma. 2023;64(5):1035-1039. doi: 10.1080/10428194.2023.2189989
5. O’Donnell EK, Shapiro YN, Yee AJ, et al. Quality of life, psychological distress, and prognostic perceptions in patients with multiple myeloma. Cancer. 2022;128(10):1996-2004. doi: 10.1002/cncr.34134
6. O’Donnell EK, Shapiro YN, Yee AJ, et al. Quality of life, psychological distress, and prognostic perceptions in caregivers of patients with multiple myeloma. Blood Adv. 2022;6(17):4967-4974. doi: 10.1182/bloodadvances.2022007127
7. Ahmed N, Shahzad M, Shippey E, et al. Socioeconomic and racial disparity in chimeric antigen receptor T cell therapy access. Transplant Cell Ther. 2022;28(7):358-364. doi: 10.1016/j.jtct.2022.04.008
Survival Advantage of Adjuvant IO ‘Big News’ in Renal Cancer
This transcript has been edited for clarity.
Hi. I’m Brian Rini. I’m an Ingram Professor of Medicine at Vanderbilt-Ingram Cancer Center in Nashville, Tennessee. I think there’s three main areas: adjuvant therapy in kidney cancer, frontline therapy in advanced disease, and the refractory space.
To open with adjuvant therapy, the biggest news in kidney cancer, and probably all of GU cancer at ASCO GU this year, was the adjuvant pembrolizumab overall survival data. This KEYNOTE study had previously shown disease-free survival advantages over placebo in a population with high-risk resected kidney cancer. There was a trend toward overall survival, but it was not significant in those early analyses.
Now with nearly 5 years of follow-up, we see an overall survival advantage, with a hazard ratio in the 0.6 range — so, about a 40% reduction in the risk for death among these patients receiving adjuvant pembrolizumab (pembro). This was really important for the field. It’s been difficult to show a survival advantage, even in diseases like melanoma, which is considered at least as much, if not more immune responsive, and I think puts into perspective whether to offer this drug to high-risk resected patients. And it certainly needs to be considered for this population.
I think the balance on that — and this came out in some of the questions after the session — was around how many of the placebo recipients got salvage immune therapy, which would be a standard of care. But in the countries where this was done, it’s not really clear how many actually got therapy. We know most patients got some salvage therapy, be it local or systemic, and about half the patients got immune therapy. But some more granular detail would be necessary.
The other thing I would mention is that this was paired with the previous presentation, which was adjuvant nivolumab. It was a very similar study, a similar drug in a similar setting, but it did not show any advantages of either disease-free or overall survival. This comes on the heels of other negative studies and a negative ipilimumab/nivolumab (ipi/nivo) study in this setting, part of the same study.
The reasons for these discrepancies are not entirely clear. There’s differences in populations and duration of therapy and mechanism, and all sorts of things. I don’t think anybody’s really been able to come up with one reason why we have some negative immune trials in kidney cancer and one shiningly positive one. But be that as it may, I think the take-home was that adjuvant pembro is certainly a standard of care in high-risk disease, and a benefit/risk discussion needs to be had with each individual patient. And I think pembro will be the building block for future studies, some of which are ongoing.
The second major area of update was in frontline kidney cancer. There weren’t a lot of new data, but there were updates to the existing trials. As you may know, frontline immune-based doublet is a standard of care in this disease: either ipi/nivo or one of the immuno-oncology/tyrosine kinase inhibitor (IO/TKI) regimens. We had two updates. One was an 8-year update on ipi/nivo. It’s a really long follow-up for these patients now, and what was observed was that these results remain remarkably consistent.
The hazard ratios for benefit in terms of survival and durability of response are really consistent over the past several years — again, a hallmark of immune therapy. Over half the responders are still responding now, many years later. I think that only strengthens the position of ipi/nivo as a choice for advanced clear cell kidney cancer patients. Again, there are good long-term toxicity data, and some patients can remain off treatment in what’s called treatment-free survival. So, an important update. We look forward to future, probably 10-year, data.
The CheckMate 9ER cabozantinib/nivolumab (cabo/nivo) study was updated now with many years of follow-up, as some of the other IO/TKI regimens have as well. And I think there is a similar theme, although a few years behind in maturity from the ipi/nivo data. It shows persistence of benefit. With IO/TKI regimens, a lot of the benefit is up front. It’s high response rates. It’s progression-free survival (PFS). But we’re starting to see some of that durability.
Where it’ll land, if there will be a tail of the curve and where it will be, is unclear, but these updates are important in terms of counseling patients. Patients want to know not just what’s going to happen at their first scan but also years from now. And they’re planning to be around years from now. So, I think these data are important.
The last thing I’ll mention is a health-related quality-of-life update from what was called the 005 trial of belzutifan, an oral HIF inhibitor, compared with everolimus. We heard data at the European Society for Medical Oncology (ESMO) Congress 2023 on a PFS and response-rate advantage. The drug was approved by the US Food and Drug Administration (FDA) in late December, and now we see some quality-of-life data.
Quality-of-life questionnaires and scales have a lot of imperfections. I don’t think they necessarily capture everything we want. But in this case, it was fairly clean in that belzutifan is known to be a well-tolerated agent. The toxicity profile is clean. It’s been used for years in patients with Von Hippel-Lindau syndrome, certainly in the trials for years, and has shown good tolerance over time. So, I view these data as complementary to what we already knew about the drug, but they’re nice to see.
It’s nice to see datasets come together and show the same thing: Not only is the drug active in a refractory renal cell carcinoma (RCC) setting, but also it’s really well tolerated and does not adversely impact patients› quality of life. I use this drug a lot in refractory kidney cancer, and because it’s so well tolerated. That means it’s also combinable. And there are some very large studies in the front-end second-line space combining it, in a space where people believe that it has more activity. But there are some complementary data as we wait for the overall survival signal, hopefully, from this regimen.
So, there have been some exciting updates, mostly in the adjuvant space but also in some other spaces in kidney cancer and building upon some of the clinical advances that we had seen from previous meetings. I’m Brian Rini, and I appreciate you attending.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Brian Rini. I’m an Ingram Professor of Medicine at Vanderbilt-Ingram Cancer Center in Nashville, Tennessee. I think there’s three main areas: adjuvant therapy in kidney cancer, frontline therapy in advanced disease, and the refractory space.
To open with adjuvant therapy, the biggest news in kidney cancer, and probably all of GU cancer at ASCO GU this year, was the adjuvant pembrolizumab overall survival data. This KEYNOTE study had previously shown disease-free survival advantages over placebo in a population with high-risk resected kidney cancer. There was a trend toward overall survival, but it was not significant in those early analyses.
Now with nearly 5 years of follow-up, we see an overall survival advantage, with a hazard ratio in the 0.6 range — so, about a 40% reduction in the risk for death among these patients receiving adjuvant pembrolizumab (pembro). This was really important for the field. It’s been difficult to show a survival advantage, even in diseases like melanoma, which is considered at least as much, if not more immune responsive, and I think puts into perspective whether to offer this drug to high-risk resected patients. And it certainly needs to be considered for this population.
I think the balance on that — and this came out in some of the questions after the session — was around how many of the placebo recipients got salvage immune therapy, which would be a standard of care. But in the countries where this was done, it’s not really clear how many actually got therapy. We know most patients got some salvage therapy, be it local or systemic, and about half the patients got immune therapy. But some more granular detail would be necessary.
The other thing I would mention is that this was paired with the previous presentation, which was adjuvant nivolumab. It was a very similar study, a similar drug in a similar setting, but it did not show any advantages of either disease-free or overall survival. This comes on the heels of other negative studies and a negative ipilimumab/nivolumab (ipi/nivo) study in this setting, part of the same study.
The reasons for these discrepancies are not entirely clear. There’s differences in populations and duration of therapy and mechanism, and all sorts of things. I don’t think anybody’s really been able to come up with one reason why we have some negative immune trials in kidney cancer and one shiningly positive one. But be that as it may, I think the take-home was that adjuvant pembro is certainly a standard of care in high-risk disease, and a benefit/risk discussion needs to be had with each individual patient. And I think pembro will be the building block for future studies, some of which are ongoing.
The second major area of update was in frontline kidney cancer. There weren’t a lot of new data, but there were updates to the existing trials. As you may know, frontline immune-based doublet is a standard of care in this disease: either ipi/nivo or one of the immuno-oncology/tyrosine kinase inhibitor (IO/TKI) regimens. We had two updates. One was an 8-year update on ipi/nivo. It’s a really long follow-up for these patients now, and what was observed was that these results remain remarkably consistent.
The hazard ratios for benefit in terms of survival and durability of response are really consistent over the past several years — again, a hallmark of immune therapy. Over half the responders are still responding now, many years later. I think that only strengthens the position of ipi/nivo as a choice for advanced clear cell kidney cancer patients. Again, there are good long-term toxicity data, and some patients can remain off treatment in what’s called treatment-free survival. So, an important update. We look forward to future, probably 10-year, data.
The CheckMate 9ER cabozantinib/nivolumab (cabo/nivo) study was updated now with many years of follow-up, as some of the other IO/TKI regimens have as well. And I think there is a similar theme, although a few years behind in maturity from the ipi/nivo data. It shows persistence of benefit. With IO/TKI regimens, a lot of the benefit is up front. It’s high response rates. It’s progression-free survival (PFS). But we’re starting to see some of that durability.
Where it’ll land, if there will be a tail of the curve and where it will be, is unclear, but these updates are important in terms of counseling patients. Patients want to know not just what’s going to happen at their first scan but also years from now. And they’re planning to be around years from now. So, I think these data are important.
The last thing I’ll mention is a health-related quality-of-life update from what was called the 005 trial of belzutifan, an oral HIF inhibitor, compared with everolimus. We heard data at the European Society for Medical Oncology (ESMO) Congress 2023 on a PFS and response-rate advantage. The drug was approved by the US Food and Drug Administration (FDA) in late December, and now we see some quality-of-life data.
Quality-of-life questionnaires and scales have a lot of imperfections. I don’t think they necessarily capture everything we want. But in this case, it was fairly clean in that belzutifan is known to be a well-tolerated agent. The toxicity profile is clean. It’s been used for years in patients with Von Hippel-Lindau syndrome, certainly in the trials for years, and has shown good tolerance over time. So, I view these data as complementary to what we already knew about the drug, but they’re nice to see.
It’s nice to see datasets come together and show the same thing: Not only is the drug active in a refractory renal cell carcinoma (RCC) setting, but also it’s really well tolerated and does not adversely impact patients› quality of life. I use this drug a lot in refractory kidney cancer, and because it’s so well tolerated. That means it’s also combinable. And there are some very large studies in the front-end second-line space combining it, in a space where people believe that it has more activity. But there are some complementary data as we wait for the overall survival signal, hopefully, from this regimen.
So, there have been some exciting updates, mostly in the adjuvant space but also in some other spaces in kidney cancer and building upon some of the clinical advances that we had seen from previous meetings. I’m Brian Rini, and I appreciate you attending.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Brian Rini. I’m an Ingram Professor of Medicine at Vanderbilt-Ingram Cancer Center in Nashville, Tennessee. I think there’s three main areas: adjuvant therapy in kidney cancer, frontline therapy in advanced disease, and the refractory space.
To open with adjuvant therapy, the biggest news in kidney cancer, and probably all of GU cancer at ASCO GU this year, was the adjuvant pembrolizumab overall survival data. This KEYNOTE study had previously shown disease-free survival advantages over placebo in a population with high-risk resected kidney cancer. There was a trend toward overall survival, but it was not significant in those early analyses.
Now with nearly 5 years of follow-up, we see an overall survival advantage, with a hazard ratio in the 0.6 range — so, about a 40% reduction in the risk for death among these patients receiving adjuvant pembrolizumab (pembro). This was really important for the field. It’s been difficult to show a survival advantage, even in diseases like melanoma, which is considered at least as much, if not more immune responsive, and I think puts into perspective whether to offer this drug to high-risk resected patients. And it certainly needs to be considered for this population.
I think the balance on that — and this came out in some of the questions after the session — was around how many of the placebo recipients got salvage immune therapy, which would be a standard of care. But in the countries where this was done, it’s not really clear how many actually got therapy. We know most patients got some salvage therapy, be it local or systemic, and about half the patients got immune therapy. But some more granular detail would be necessary.
The other thing I would mention is that this was paired with the previous presentation, which was adjuvant nivolumab. It was a very similar study, a similar drug in a similar setting, but it did not show any advantages of either disease-free or overall survival. This comes on the heels of other negative studies and a negative ipilimumab/nivolumab (ipi/nivo) study in this setting, part of the same study.
The reasons for these discrepancies are not entirely clear. There’s differences in populations and duration of therapy and mechanism, and all sorts of things. I don’t think anybody’s really been able to come up with one reason why we have some negative immune trials in kidney cancer and one shiningly positive one. But be that as it may, I think the take-home was that adjuvant pembro is certainly a standard of care in high-risk disease, and a benefit/risk discussion needs to be had with each individual patient. And I think pembro will be the building block for future studies, some of which are ongoing.
The second major area of update was in frontline kidney cancer. There weren’t a lot of new data, but there were updates to the existing trials. As you may know, frontline immune-based doublet is a standard of care in this disease: either ipi/nivo or one of the immuno-oncology/tyrosine kinase inhibitor (IO/TKI) regimens. We had two updates. One was an 8-year update on ipi/nivo. It’s a really long follow-up for these patients now, and what was observed was that these results remain remarkably consistent.
The hazard ratios for benefit in terms of survival and durability of response are really consistent over the past several years — again, a hallmark of immune therapy. Over half the responders are still responding now, many years later. I think that only strengthens the position of ipi/nivo as a choice for advanced clear cell kidney cancer patients. Again, there are good long-term toxicity data, and some patients can remain off treatment in what’s called treatment-free survival. So, an important update. We look forward to future, probably 10-year, data.
The CheckMate 9ER cabozantinib/nivolumab (cabo/nivo) study was updated now with many years of follow-up, as some of the other IO/TKI regimens have as well. And I think there is a similar theme, although a few years behind in maturity from the ipi/nivo data. It shows persistence of benefit. With IO/TKI regimens, a lot of the benefit is up front. It’s high response rates. It’s progression-free survival (PFS). But we’re starting to see some of that durability.
Where it’ll land, if there will be a tail of the curve and where it will be, is unclear, but these updates are important in terms of counseling patients. Patients want to know not just what’s going to happen at their first scan but also years from now. And they’re planning to be around years from now. So, I think these data are important.
The last thing I’ll mention is a health-related quality-of-life update from what was called the 005 trial of belzutifan, an oral HIF inhibitor, compared with everolimus. We heard data at the European Society for Medical Oncology (ESMO) Congress 2023 on a PFS and response-rate advantage. The drug was approved by the US Food and Drug Administration (FDA) in late December, and now we see some quality-of-life data.
Quality-of-life questionnaires and scales have a lot of imperfections. I don’t think they necessarily capture everything we want. But in this case, it was fairly clean in that belzutifan is known to be a well-tolerated agent. The toxicity profile is clean. It’s been used for years in patients with Von Hippel-Lindau syndrome, certainly in the trials for years, and has shown good tolerance over time. So, I view these data as complementary to what we already knew about the drug, but they’re nice to see.
It’s nice to see datasets come together and show the same thing: Not only is the drug active in a refractory renal cell carcinoma (RCC) setting, but also it’s really well tolerated and does not adversely impact patients› quality of life. I use this drug a lot in refractory kidney cancer, and because it’s so well tolerated. That means it’s also combinable. And there are some very large studies in the front-end second-line space combining it, in a space where people believe that it has more activity. But there are some complementary data as we wait for the overall survival signal, hopefully, from this regimen.
So, there have been some exciting updates, mostly in the adjuvant space but also in some other spaces in kidney cancer and building upon some of the clinical advances that we had seen from previous meetings. I’m Brian Rini, and I appreciate you attending.
A version of this article first appeared on Medscape.com.