User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Immunotherapy should not be withheld because of sex, age, or PS
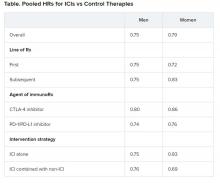
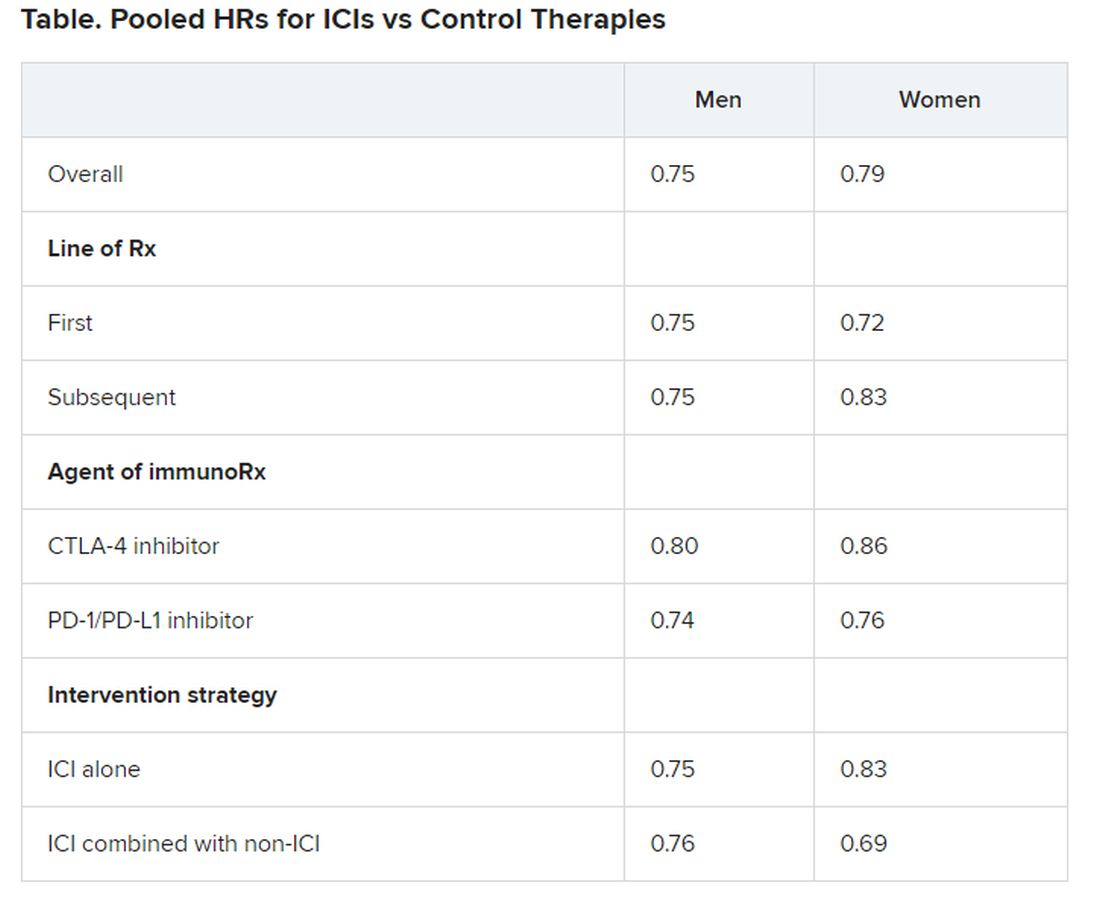
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
Aspirin may accelerate cancer progression in older adults
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
Tailored messaging needed to get cancer screening back on track
In late June, Lisa Richardson, MD, emerged from Atlanta, Georgia’s initial COVID-19 lockdown, and “got back out there” for some overdue doctor’s appointments, including a mammogram.
The mammogram was a particular priority for her, since she is director of the CDC’s Division of Cancer Prevention and Control. But she knows that cancer screening is going to be a much tougher sell for the average person going forward in the pandemic era.
“It really is a challenge trying to get people to feel comfortable coming back in to be screened,” she said. Richardson was speaking recently at the AACR virtual meeting: COVID-19 and Cancer, a virtual symposium on cancer prevention and early detection in the COVID-19 pandemic organized by the American Association for Cancer Research.
While health service shutdowns and stay-at-home orders forced the country’s initial precipitous decline in cancer screening, fear of contracting COVID-19 is a big part of what is preventing patients from returning.
“We’ve known even pre-pandemic that people were hesitant to do cancer screening and in some ways this has really given them an out to say, ‘Well, I’m going to hold off on that colonoscopy,’ ” Amy Leader, MD, from Thomas Jefferson University’s Kimmel Cancer Center in Philadelphia, Pennsylvania, said during the symposium.
Estimating the pandemic’s impact on cancer care
While the impact of the pandemic on cancer can only be estimated at the moment, the prospects are already daunting, said Richardson, speculating that the hard-won 26% drop in cancer mortality over the past two decades “may be put on hold or reversed” by COVID-19.
There could be as many as 10,000 excess deaths in the US from colorectal and breast cancer alone because of COVID-19 delays, predicted Norman E. Sharpless, director of the US National Cancer Institute in Bethesda, Maryland.
But even Sharpless acknowledges that his modeling gives a conservative estimate, “as it does not consider other cancer types, it does not account for the additional nonlethal morbidity from upstaging, and it assumes a moderate disruption in care that completely resolves after 6 months.”
With still no end to the pandemic in sight, the true scope of cancer screening and treatment disruptions will take a long time to assess, but several studies presented during the symposium revealed some early indications.
A national survey launched in mid-May, which involved 534 women either diagnosed with breast cancer or undergoing screening or diagnostic evaluation for it, found that delays in screening were reported by 31.7% of those with breast cancer, and 26.7% of those without. Additionally, 21% of those on active treatment for breast cancer reported treatment delays.
“It’s going to be really important to implement strategies to help patients return to care ... creating a culture and a feeling of safety among patients and communicating through the uncertainty that exists in the pandemic,” said study investigator Erica T. Warner, ScD MPH, from Massachusetts General Hospital, Boston.
Screening for prostate cancer (via prostate-specific antigen testing) also declined, though not as dramatically as that for breast cancer, noted Mara Epstein, ScD, from The Meyers Primary Care Institute, University of Massachusetts Medical School, Worcester. Her study at a large healthcare provider group compared rates of both screening and diagnostic mammographies, and also PSA testing, as well as breast and prostate biopsies in the first five months of 2020 vs the same months in 2019.
While a decrease from 2019 to 2020 was seen in all procedures over the entire study period, the greatest decline was seen in April for screening mammography (down 98%), and tomosynthesis (down 96%), as well as PSA testing (down 83%), she said.
More recent figures are hard to come by, but a recent weekly survey from the Primary Care Collaborative shows 46% of practices are offering preventive and chronic care management visits, but patients are not scheduling them, and 44% report that in-person visit volume is between 30%-50% below normal over the last 4 weeks.
Will COVID-19 exacerbate racial disparities in cancer?
Neither of the studies presented at the symposium analyzed cancer care disruptions by race, but there was concern among some panelists that cancer care disparities that existed before the pandemic will be magnified further.
“Over the next several months and into the next year there’s going to be some catch-up in screening and treatment, and one of my concerns is minority and underserved populations will not partake in that catch-up the way many middle-class Americans will,” said Otis Brawley, MD, from Johns Hopkins University, Baltimore, Maryland.
There is ample evidence that minority populations have been disproportionately hit by COVID-19, job losses, and lost health insurance, said the CDC’s Richardson, and all these factors could widen the cancer gap.
“It’s not a race thing, it’s a ‘what do you do thing,’ and an access to care thing, and what your socioeconomic status is,” Richardson said in an interview. “People who didn’t have sick leave before the pandemic still don’t have sick leave; if they didn’t have time to get their mammogram they still don’t have time.”
But she acknowledges that evidence is still lacking. Could some minority populations actually be less fearful of medical encounters because their work has already prevented them from sheltering in place? “It could go either way,” she said. “They might be less wary of venturing out into the clinic, but they also might reason that they’ve exposed themselves enough already at work and don’t want any additional exposure.”
In that regard, Richardson suggests population-specific messaging will be an important way of communicating with under-served populations to restart screening.
“We’re struggling at CDC with how to develop messages that resonate within different communities, because we’re missing the point of actually speaking to people within their culture and within the places that they live,” she said. “Just saying the same thing and putting a black face on it is not going to make a difference; you actually have to speak the language of the people you’re trying to reach — the same message in different packages.”
To that end, even before the pandemic, the CDC supported the development of Make It Your Own, a website that uses “evidence-based strategies” to assist healthcare organizations in customizing health information “by race, ethnicity, age, gender and location”, and target messages to “specific populations, cultural groups and languages”.
But Mass General’s Warner says she’s not sure she would argue for messages to be tailored by race, “at least not without evidence that values and priorities regarding returning to care differ between racial/ethnic groups.”
“Tailoring in the absence of data requires assumptions that may or may not be correct and ignores within-group heterogeneity,” Warner told Medscape Medical News. “However, I do believe that messaging about return to cancer screening and care should be multifaceted and use diverse imagery. This recognizes that some messages will resonate more or less with individuals based on their own characteristics, of which race may be one.”
Warner does believe in the power of tailored messaging though. “Part of the onus for healthcare institutions and providers is to make some decisions about who it is really important to bring back in soonest,” she said.
“Those are the ones we want to prioritize, as opposed to those who we want to get back into care but we don’t need to get them in right now,” Warner emphasized. “As they are balancing all the needs of their family and their community and their other needs, messaging that adds additional stress, worry, anxiety and shame is not what we want to do. So really we need to distinguish between these populations, identify the priorities, hit the hard message to people who really need it now, and encourage others to come back in as they can.”
Building trust
All the panelists agreed that building trust with the public will be key to getting cancer care back on track.
“I don’t think anyone trusts the healthcare community right now, but we already had this baseline distrust of healthcare among many minority communities, and now with COVID-19, the African American community in particular is seeing people go into the hospital and never come back,” said Richardson.
For Warner, the onus really falls on healthcare institutions. “We have to be proactive and not leave the burden of deciding when and how to return to care up to patients,” she said.
“What we need to focus on as much as possible is to get people to realize it is safe to come see the doctor,” said Johns Hopkins oncologist Brawley. “We have to make it safe for them to come see us, and then we have to convince them it is safe to come see us.”
Venturing out to her mammography appointment in early June, Richardson said she felt safe. “Everything was just the way it was supposed to be, everyone was masked, everyone was washing their hands,” she said.
Yet, by mid-June she had contracted COVID-19. “I don’t know where I got it,” she said. “No matter how careful you are, understand that if you’re in a total red spot, as I am, you can just get it.”
This article first appeared on Medscape.com.
In late June, Lisa Richardson, MD, emerged from Atlanta, Georgia’s initial COVID-19 lockdown, and “got back out there” for some overdue doctor’s appointments, including a mammogram.
The mammogram was a particular priority for her, since she is director of the CDC’s Division of Cancer Prevention and Control. But she knows that cancer screening is going to be a much tougher sell for the average person going forward in the pandemic era.
“It really is a challenge trying to get people to feel comfortable coming back in to be screened,” she said. Richardson was speaking recently at the AACR virtual meeting: COVID-19 and Cancer, a virtual symposium on cancer prevention and early detection in the COVID-19 pandemic organized by the American Association for Cancer Research.
While health service shutdowns and stay-at-home orders forced the country’s initial precipitous decline in cancer screening, fear of contracting COVID-19 is a big part of what is preventing patients from returning.
“We’ve known even pre-pandemic that people were hesitant to do cancer screening and in some ways this has really given them an out to say, ‘Well, I’m going to hold off on that colonoscopy,’ ” Amy Leader, MD, from Thomas Jefferson University’s Kimmel Cancer Center in Philadelphia, Pennsylvania, said during the symposium.
Estimating the pandemic’s impact on cancer care
While the impact of the pandemic on cancer can only be estimated at the moment, the prospects are already daunting, said Richardson, speculating that the hard-won 26% drop in cancer mortality over the past two decades “may be put on hold or reversed” by COVID-19.
There could be as many as 10,000 excess deaths in the US from colorectal and breast cancer alone because of COVID-19 delays, predicted Norman E. Sharpless, director of the US National Cancer Institute in Bethesda, Maryland.
But even Sharpless acknowledges that his modeling gives a conservative estimate, “as it does not consider other cancer types, it does not account for the additional nonlethal morbidity from upstaging, and it assumes a moderate disruption in care that completely resolves after 6 months.”
With still no end to the pandemic in sight, the true scope of cancer screening and treatment disruptions will take a long time to assess, but several studies presented during the symposium revealed some early indications.
A national survey launched in mid-May, which involved 534 women either diagnosed with breast cancer or undergoing screening or diagnostic evaluation for it, found that delays in screening were reported by 31.7% of those with breast cancer, and 26.7% of those without. Additionally, 21% of those on active treatment for breast cancer reported treatment delays.
“It’s going to be really important to implement strategies to help patients return to care ... creating a culture and a feeling of safety among patients and communicating through the uncertainty that exists in the pandemic,” said study investigator Erica T. Warner, ScD MPH, from Massachusetts General Hospital, Boston.
Screening for prostate cancer (via prostate-specific antigen testing) also declined, though not as dramatically as that for breast cancer, noted Mara Epstein, ScD, from The Meyers Primary Care Institute, University of Massachusetts Medical School, Worcester. Her study at a large healthcare provider group compared rates of both screening and diagnostic mammographies, and also PSA testing, as well as breast and prostate biopsies in the first five months of 2020 vs the same months in 2019.
While a decrease from 2019 to 2020 was seen in all procedures over the entire study period, the greatest decline was seen in April for screening mammography (down 98%), and tomosynthesis (down 96%), as well as PSA testing (down 83%), she said.
More recent figures are hard to come by, but a recent weekly survey from the Primary Care Collaborative shows 46% of practices are offering preventive and chronic care management visits, but patients are not scheduling them, and 44% report that in-person visit volume is between 30%-50% below normal over the last 4 weeks.
Will COVID-19 exacerbate racial disparities in cancer?
Neither of the studies presented at the symposium analyzed cancer care disruptions by race, but there was concern among some panelists that cancer care disparities that existed before the pandemic will be magnified further.
“Over the next several months and into the next year there’s going to be some catch-up in screening and treatment, and one of my concerns is minority and underserved populations will not partake in that catch-up the way many middle-class Americans will,” said Otis Brawley, MD, from Johns Hopkins University, Baltimore, Maryland.
There is ample evidence that minority populations have been disproportionately hit by COVID-19, job losses, and lost health insurance, said the CDC’s Richardson, and all these factors could widen the cancer gap.
“It’s not a race thing, it’s a ‘what do you do thing,’ and an access to care thing, and what your socioeconomic status is,” Richardson said in an interview. “People who didn’t have sick leave before the pandemic still don’t have sick leave; if they didn’t have time to get their mammogram they still don’t have time.”
But she acknowledges that evidence is still lacking. Could some minority populations actually be less fearful of medical encounters because their work has already prevented them from sheltering in place? “It could go either way,” she said. “They might be less wary of venturing out into the clinic, but they also might reason that they’ve exposed themselves enough already at work and don’t want any additional exposure.”
In that regard, Richardson suggests population-specific messaging will be an important way of communicating with under-served populations to restart screening.
“We’re struggling at CDC with how to develop messages that resonate within different communities, because we’re missing the point of actually speaking to people within their culture and within the places that they live,” she said. “Just saying the same thing and putting a black face on it is not going to make a difference; you actually have to speak the language of the people you’re trying to reach — the same message in different packages.”
To that end, even before the pandemic, the CDC supported the development of Make It Your Own, a website that uses “evidence-based strategies” to assist healthcare organizations in customizing health information “by race, ethnicity, age, gender and location”, and target messages to “specific populations, cultural groups and languages”.
But Mass General’s Warner says she’s not sure she would argue for messages to be tailored by race, “at least not without evidence that values and priorities regarding returning to care differ between racial/ethnic groups.”
“Tailoring in the absence of data requires assumptions that may or may not be correct and ignores within-group heterogeneity,” Warner told Medscape Medical News. “However, I do believe that messaging about return to cancer screening and care should be multifaceted and use diverse imagery. This recognizes that some messages will resonate more or less with individuals based on their own characteristics, of which race may be one.”
Warner does believe in the power of tailored messaging though. “Part of the onus for healthcare institutions and providers is to make some decisions about who it is really important to bring back in soonest,” she said.
“Those are the ones we want to prioritize, as opposed to those who we want to get back into care but we don’t need to get them in right now,” Warner emphasized. “As they are balancing all the needs of their family and their community and their other needs, messaging that adds additional stress, worry, anxiety and shame is not what we want to do. So really we need to distinguish between these populations, identify the priorities, hit the hard message to people who really need it now, and encourage others to come back in as they can.”
Building trust
All the panelists agreed that building trust with the public will be key to getting cancer care back on track.
“I don’t think anyone trusts the healthcare community right now, but we already had this baseline distrust of healthcare among many minority communities, and now with COVID-19, the African American community in particular is seeing people go into the hospital and never come back,” said Richardson.
For Warner, the onus really falls on healthcare institutions. “We have to be proactive and not leave the burden of deciding when and how to return to care up to patients,” she said.
“What we need to focus on as much as possible is to get people to realize it is safe to come see the doctor,” said Johns Hopkins oncologist Brawley. “We have to make it safe for them to come see us, and then we have to convince them it is safe to come see us.”
Venturing out to her mammography appointment in early June, Richardson said she felt safe. “Everything was just the way it was supposed to be, everyone was masked, everyone was washing their hands,” she said.
Yet, by mid-June she had contracted COVID-19. “I don’t know where I got it,” she said. “No matter how careful you are, understand that if you’re in a total red spot, as I am, you can just get it.”
This article first appeared on Medscape.com.
In late June, Lisa Richardson, MD, emerged from Atlanta, Georgia’s initial COVID-19 lockdown, and “got back out there” for some overdue doctor’s appointments, including a mammogram.
The mammogram was a particular priority for her, since she is director of the CDC’s Division of Cancer Prevention and Control. But she knows that cancer screening is going to be a much tougher sell for the average person going forward in the pandemic era.
“It really is a challenge trying to get people to feel comfortable coming back in to be screened,” she said. Richardson was speaking recently at the AACR virtual meeting: COVID-19 and Cancer, a virtual symposium on cancer prevention and early detection in the COVID-19 pandemic organized by the American Association for Cancer Research.
While health service shutdowns and stay-at-home orders forced the country’s initial precipitous decline in cancer screening, fear of contracting COVID-19 is a big part of what is preventing patients from returning.
“We’ve known even pre-pandemic that people were hesitant to do cancer screening and in some ways this has really given them an out to say, ‘Well, I’m going to hold off on that colonoscopy,’ ” Amy Leader, MD, from Thomas Jefferson University’s Kimmel Cancer Center in Philadelphia, Pennsylvania, said during the symposium.
Estimating the pandemic’s impact on cancer care
While the impact of the pandemic on cancer can only be estimated at the moment, the prospects are already daunting, said Richardson, speculating that the hard-won 26% drop in cancer mortality over the past two decades “may be put on hold or reversed” by COVID-19.
There could be as many as 10,000 excess deaths in the US from colorectal and breast cancer alone because of COVID-19 delays, predicted Norman E. Sharpless, director of the US National Cancer Institute in Bethesda, Maryland.
But even Sharpless acknowledges that his modeling gives a conservative estimate, “as it does not consider other cancer types, it does not account for the additional nonlethal morbidity from upstaging, and it assumes a moderate disruption in care that completely resolves after 6 months.”
With still no end to the pandemic in sight, the true scope of cancer screening and treatment disruptions will take a long time to assess, but several studies presented during the symposium revealed some early indications.
A national survey launched in mid-May, which involved 534 women either diagnosed with breast cancer or undergoing screening or diagnostic evaluation for it, found that delays in screening were reported by 31.7% of those with breast cancer, and 26.7% of those without. Additionally, 21% of those on active treatment for breast cancer reported treatment delays.
“It’s going to be really important to implement strategies to help patients return to care ... creating a culture and a feeling of safety among patients and communicating through the uncertainty that exists in the pandemic,” said study investigator Erica T. Warner, ScD MPH, from Massachusetts General Hospital, Boston.
Screening for prostate cancer (via prostate-specific antigen testing) also declined, though not as dramatically as that for breast cancer, noted Mara Epstein, ScD, from The Meyers Primary Care Institute, University of Massachusetts Medical School, Worcester. Her study at a large healthcare provider group compared rates of both screening and diagnostic mammographies, and also PSA testing, as well as breast and prostate biopsies in the first five months of 2020 vs the same months in 2019.
While a decrease from 2019 to 2020 was seen in all procedures over the entire study period, the greatest decline was seen in April for screening mammography (down 98%), and tomosynthesis (down 96%), as well as PSA testing (down 83%), she said.
More recent figures are hard to come by, but a recent weekly survey from the Primary Care Collaborative shows 46% of practices are offering preventive and chronic care management visits, but patients are not scheduling them, and 44% report that in-person visit volume is between 30%-50% below normal over the last 4 weeks.
Will COVID-19 exacerbate racial disparities in cancer?
Neither of the studies presented at the symposium analyzed cancer care disruptions by race, but there was concern among some panelists that cancer care disparities that existed before the pandemic will be magnified further.
“Over the next several months and into the next year there’s going to be some catch-up in screening and treatment, and one of my concerns is minority and underserved populations will not partake in that catch-up the way many middle-class Americans will,” said Otis Brawley, MD, from Johns Hopkins University, Baltimore, Maryland.
There is ample evidence that minority populations have been disproportionately hit by COVID-19, job losses, and lost health insurance, said the CDC’s Richardson, and all these factors could widen the cancer gap.
“It’s not a race thing, it’s a ‘what do you do thing,’ and an access to care thing, and what your socioeconomic status is,” Richardson said in an interview. “People who didn’t have sick leave before the pandemic still don’t have sick leave; if they didn’t have time to get their mammogram they still don’t have time.”
But she acknowledges that evidence is still lacking. Could some minority populations actually be less fearful of medical encounters because their work has already prevented them from sheltering in place? “It could go either way,” she said. “They might be less wary of venturing out into the clinic, but they also might reason that they’ve exposed themselves enough already at work and don’t want any additional exposure.”
In that regard, Richardson suggests population-specific messaging will be an important way of communicating with under-served populations to restart screening.
“We’re struggling at CDC with how to develop messages that resonate within different communities, because we’re missing the point of actually speaking to people within their culture and within the places that they live,” she said. “Just saying the same thing and putting a black face on it is not going to make a difference; you actually have to speak the language of the people you’re trying to reach — the same message in different packages.”
To that end, even before the pandemic, the CDC supported the development of Make It Your Own, a website that uses “evidence-based strategies” to assist healthcare organizations in customizing health information “by race, ethnicity, age, gender and location”, and target messages to “specific populations, cultural groups and languages”.
But Mass General’s Warner says she’s not sure she would argue for messages to be tailored by race, “at least not without evidence that values and priorities regarding returning to care differ between racial/ethnic groups.”
“Tailoring in the absence of data requires assumptions that may or may not be correct and ignores within-group heterogeneity,” Warner told Medscape Medical News. “However, I do believe that messaging about return to cancer screening and care should be multifaceted and use diverse imagery. This recognizes that some messages will resonate more or less with individuals based on their own characteristics, of which race may be one.”
Warner does believe in the power of tailored messaging though. “Part of the onus for healthcare institutions and providers is to make some decisions about who it is really important to bring back in soonest,” she said.
“Those are the ones we want to prioritize, as opposed to those who we want to get back into care but we don’t need to get them in right now,” Warner emphasized. “As they are balancing all the needs of their family and their community and their other needs, messaging that adds additional stress, worry, anxiety and shame is not what we want to do. So really we need to distinguish between these populations, identify the priorities, hit the hard message to people who really need it now, and encourage others to come back in as they can.”
Building trust
All the panelists agreed that building trust with the public will be key to getting cancer care back on track.
“I don’t think anyone trusts the healthcare community right now, but we already had this baseline distrust of healthcare among many minority communities, and now with COVID-19, the African American community in particular is seeing people go into the hospital and never come back,” said Richardson.
For Warner, the onus really falls on healthcare institutions. “We have to be proactive and not leave the burden of deciding when and how to return to care up to patients,” she said.
“What we need to focus on as much as possible is to get people to realize it is safe to come see the doctor,” said Johns Hopkins oncologist Brawley. “We have to make it safe for them to come see us, and then we have to convince them it is safe to come see us.”
Venturing out to her mammography appointment in early June, Richardson said she felt safe. “Everything was just the way it was supposed to be, everyone was masked, everyone was washing their hands,” she said.
Yet, by mid-June she had contracted COVID-19. “I don’t know where I got it,” she said. “No matter how careful you are, understand that if you’re in a total red spot, as I am, you can just get it.”
This article first appeared on Medscape.com.
Diabetes plus weight loss equals increased risk of pancreatic cancer
A new study has linked recent-onset diabetes and subsequent weight loss to an increased risk of pancreatic cancer, indicating a distinct group of individuals to screen early for this deadly disease.
“The likelihood of a pancreatic cancer diagnosis was even further elevated among individuals with older age, healthy weight before weight loss, and unintentional weight loss,” wrote Chen Yuan, ScD, of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston. The study was published in JAMA Oncology.
To determine whether an association exists between diabetes plus weight change and pancreatic cancer, the researchers analyzed decades of medical history data from the Nurses’ Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS). The study population from the NHS included 112,818 women with a mean age of 59 years; the population from the HPFS included 46,207 men with a mean age of 65 years. Since enrollment – the baseline was 1978 for the NHS and 1988 for the HPFS – participants have provided follow-up information via biennial questionnaires.
Recent diabetes onset, weight loss boost cancer risk
From those combined groups, 1,116 incident cases of pancreatic cancer (0.7%) were identified. Compared with patients with no diabetes, patients with recent-onset diabetes had triple the risk of pancreatic cancer (age-adjusted hazard ratio, 2.97; 95% confidence interval, 2.31-3.82) and patients with longstanding diabetes had more than double the risk (HR, 2.16; 95% CI, 1.78-2.60). Patients with longer disease duration also had more than twice the risk of pancreatic cancer, with HRs of 2.25 for those with diabetes for 4-10 years (95% CI, 1.74-2.92) and 2.07 for more than 10 years (95% CI, 1.61-2.66).
Compared with patients who hadn’t lost any weight, patients who reported a 1- to 4-pound weight loss (HR, 1.25; 95% CI, 1.03-1.52), a 5- to 8-pound weight loss (HR, 1.33; 95% CI, 1.06-1.66), and a more than 8-pound weight loss (HR, 1.92; 95% CI, 1.58-2.32) had higher risks of pancreatic cancer. Patients with recent-onset diabetes and a 1- to 8-pound weight loss (91 incident cases per 100,000 person-years; 95% CI, 55-151) or a weight loss of more than 8 pounds (164 incident cases per 100,000 person years; 95% CI, 114-238) had a much higher incidence of pancreatic cancer, compared with patients with neither (16 incident cases per 100,000 person-years; 95% CI, 14-17).
After stratified analyses of patients with both recent-onset diabetes and weight loss, rates of pancreatic cancer were also notably high in those 70 years or older (234 cases per 100,000 person years), those with a body mass index of less than 25 kg/m2 before weight loss (400 cases per 100,000 person years), and those with a low likelihood of intentional weight loss (334 cases per 100,000 person years).
“I like the study because it reminds us of the importance of not thinking everyone that presents with type 2 diabetes necessarily has garden-variety diabetes,” Paul Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said in an interview. “I have always been concerned when a new-onset diabetic individual presents with no family history of diabetes or prediabetes, especially if they’re neither overweight nor obese. I have sometimes screened those individuals for pancreatic abnormalities.”
A call for screening
“This study highlights the consideration for further screening to those with weight loss at the time of diabetes diagnosis, which is very sensible given how unusual weight loss is as a presenting symptom at the time of diagnosis of typical type 2 diabetes,” Dr. Jellinger added. “The combination of weight loss and no family history of diabetes at the time of diagnosis should be an even stronger signal for pancreatic cancer screening and potential detection at a much earlier stage.”
The authors acknowledged their study’s limitations, including some patients with pancreatic cancer not returning their questionnaires and the timing of the questionnaires meaning that patients could’ve developed diabetes after returning it. In addition, they recognized that the participants were “predominantly White health professionals” and recommended a study of “additional patient populations” in the future.
The authors noted numerous potential conflicts of interest, including receiving grants and personal fees from various initiatives, organizations, and pharmaceutical companies.
SOURCE: Yuan C et al. JAMA Oncol. 2020 Aug 13. doi: 10.1001/jamaoncol.2020.2948.
A new study has linked recent-onset diabetes and subsequent weight loss to an increased risk of pancreatic cancer, indicating a distinct group of individuals to screen early for this deadly disease.
“The likelihood of a pancreatic cancer diagnosis was even further elevated among individuals with older age, healthy weight before weight loss, and unintentional weight loss,” wrote Chen Yuan, ScD, of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston. The study was published in JAMA Oncology.
To determine whether an association exists between diabetes plus weight change and pancreatic cancer, the researchers analyzed decades of medical history data from the Nurses’ Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS). The study population from the NHS included 112,818 women with a mean age of 59 years; the population from the HPFS included 46,207 men with a mean age of 65 years. Since enrollment – the baseline was 1978 for the NHS and 1988 for the HPFS – participants have provided follow-up information via biennial questionnaires.
Recent diabetes onset, weight loss boost cancer risk
From those combined groups, 1,116 incident cases of pancreatic cancer (0.7%) were identified. Compared with patients with no diabetes, patients with recent-onset diabetes had triple the risk of pancreatic cancer (age-adjusted hazard ratio, 2.97; 95% confidence interval, 2.31-3.82) and patients with longstanding diabetes had more than double the risk (HR, 2.16; 95% CI, 1.78-2.60). Patients with longer disease duration also had more than twice the risk of pancreatic cancer, with HRs of 2.25 for those with diabetes for 4-10 years (95% CI, 1.74-2.92) and 2.07 for more than 10 years (95% CI, 1.61-2.66).
Compared with patients who hadn’t lost any weight, patients who reported a 1- to 4-pound weight loss (HR, 1.25; 95% CI, 1.03-1.52), a 5- to 8-pound weight loss (HR, 1.33; 95% CI, 1.06-1.66), and a more than 8-pound weight loss (HR, 1.92; 95% CI, 1.58-2.32) had higher risks of pancreatic cancer. Patients with recent-onset diabetes and a 1- to 8-pound weight loss (91 incident cases per 100,000 person-years; 95% CI, 55-151) or a weight loss of more than 8 pounds (164 incident cases per 100,000 person years; 95% CI, 114-238) had a much higher incidence of pancreatic cancer, compared with patients with neither (16 incident cases per 100,000 person-years; 95% CI, 14-17).
After stratified analyses of patients with both recent-onset diabetes and weight loss, rates of pancreatic cancer were also notably high in those 70 years or older (234 cases per 100,000 person years), those with a body mass index of less than 25 kg/m2 before weight loss (400 cases per 100,000 person years), and those with a low likelihood of intentional weight loss (334 cases per 100,000 person years).
“I like the study because it reminds us of the importance of not thinking everyone that presents with type 2 diabetes necessarily has garden-variety diabetes,” Paul Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said in an interview. “I have always been concerned when a new-onset diabetic individual presents with no family history of diabetes or prediabetes, especially if they’re neither overweight nor obese. I have sometimes screened those individuals for pancreatic abnormalities.”
A call for screening
“This study highlights the consideration for further screening to those with weight loss at the time of diabetes diagnosis, which is very sensible given how unusual weight loss is as a presenting symptom at the time of diagnosis of typical type 2 diabetes,” Dr. Jellinger added. “The combination of weight loss and no family history of diabetes at the time of diagnosis should be an even stronger signal for pancreatic cancer screening and potential detection at a much earlier stage.”
The authors acknowledged their study’s limitations, including some patients with pancreatic cancer not returning their questionnaires and the timing of the questionnaires meaning that patients could’ve developed diabetes after returning it. In addition, they recognized that the participants were “predominantly White health professionals” and recommended a study of “additional patient populations” in the future.
The authors noted numerous potential conflicts of interest, including receiving grants and personal fees from various initiatives, organizations, and pharmaceutical companies.
SOURCE: Yuan C et al. JAMA Oncol. 2020 Aug 13. doi: 10.1001/jamaoncol.2020.2948.
A new study has linked recent-onset diabetes and subsequent weight loss to an increased risk of pancreatic cancer, indicating a distinct group of individuals to screen early for this deadly disease.
“The likelihood of a pancreatic cancer diagnosis was even further elevated among individuals with older age, healthy weight before weight loss, and unintentional weight loss,” wrote Chen Yuan, ScD, of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston. The study was published in JAMA Oncology.
To determine whether an association exists between diabetes plus weight change and pancreatic cancer, the researchers analyzed decades of medical history data from the Nurses’ Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS). The study population from the NHS included 112,818 women with a mean age of 59 years; the population from the HPFS included 46,207 men with a mean age of 65 years. Since enrollment – the baseline was 1978 for the NHS and 1988 for the HPFS – participants have provided follow-up information via biennial questionnaires.
Recent diabetes onset, weight loss boost cancer risk
From those combined groups, 1,116 incident cases of pancreatic cancer (0.7%) were identified. Compared with patients with no diabetes, patients with recent-onset diabetes had triple the risk of pancreatic cancer (age-adjusted hazard ratio, 2.97; 95% confidence interval, 2.31-3.82) and patients with longstanding diabetes had more than double the risk (HR, 2.16; 95% CI, 1.78-2.60). Patients with longer disease duration also had more than twice the risk of pancreatic cancer, with HRs of 2.25 for those with diabetes for 4-10 years (95% CI, 1.74-2.92) and 2.07 for more than 10 years (95% CI, 1.61-2.66).
Compared with patients who hadn’t lost any weight, patients who reported a 1- to 4-pound weight loss (HR, 1.25; 95% CI, 1.03-1.52), a 5- to 8-pound weight loss (HR, 1.33; 95% CI, 1.06-1.66), and a more than 8-pound weight loss (HR, 1.92; 95% CI, 1.58-2.32) had higher risks of pancreatic cancer. Patients with recent-onset diabetes and a 1- to 8-pound weight loss (91 incident cases per 100,000 person-years; 95% CI, 55-151) or a weight loss of more than 8 pounds (164 incident cases per 100,000 person years; 95% CI, 114-238) had a much higher incidence of pancreatic cancer, compared with patients with neither (16 incident cases per 100,000 person-years; 95% CI, 14-17).
After stratified analyses of patients with both recent-onset diabetes and weight loss, rates of pancreatic cancer were also notably high in those 70 years or older (234 cases per 100,000 person years), those with a body mass index of less than 25 kg/m2 before weight loss (400 cases per 100,000 person years), and those with a low likelihood of intentional weight loss (334 cases per 100,000 person years).
“I like the study because it reminds us of the importance of not thinking everyone that presents with type 2 diabetes necessarily has garden-variety diabetes,” Paul Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said in an interview. “I have always been concerned when a new-onset diabetic individual presents with no family history of diabetes or prediabetes, especially if they’re neither overweight nor obese. I have sometimes screened those individuals for pancreatic abnormalities.”
A call for screening
“This study highlights the consideration for further screening to those with weight loss at the time of diabetes diagnosis, which is very sensible given how unusual weight loss is as a presenting symptom at the time of diagnosis of typical type 2 diabetes,” Dr. Jellinger added. “The combination of weight loss and no family history of diabetes at the time of diagnosis should be an even stronger signal for pancreatic cancer screening and potential detection at a much earlier stage.”
The authors acknowledged their study’s limitations, including some patients with pancreatic cancer not returning their questionnaires and the timing of the questionnaires meaning that patients could’ve developed diabetes after returning it. In addition, they recognized that the participants were “predominantly White health professionals” and recommended a study of “additional patient populations” in the future.
The authors noted numerous potential conflicts of interest, including receiving grants and personal fees from various initiatives, organizations, and pharmaceutical companies.
SOURCE: Yuan C et al. JAMA Oncol. 2020 Aug 13. doi: 10.1001/jamaoncol.2020.2948.
FROM JAMA ONCOLOGY
Incidence, prognosis of second lung cancers support long-term surveillance
Second lung cancers occurring up to a decade after the first are on the rise, but their prognosis is similar – especially when detected early – which supports long-term surveillance in survivors, finds a large population-based study.
Although guidelines recommend continued annual low-dose CT scan surveillance extending beyond 4 years for this population based on expert consensus, long-term evidence of benefit is lacking.
Investigators led by John M. Varlotto, MD, a radiation oncologist at the University of Massachusetts Medical Center, Worcester, analyzed Surveillance, Epidemiology & End Results (SEER) data for more than 58,000 patients with first and sometimes second non–small cell lung cancers initially treated by surgical resection.
Study results reported in Lung Cancer showed that the age-adjusted incidence of second lung cancers occurring 4-10 years after the first lung cancer rose sharply during the 1985-2014 study period, driven by a large uptick in women patients.
Among all patients, second lung cancers had similar overall survival as first lung cancers, but poorer lung cancer–specific survival. However, among the subset of patients having early-stage resectable disease (tumors measuring less than 4 cm with negative nodes), both outcomes were statistically indistinguishable.
“Because our investigation noted that the overall survival of patients undergoing a second lung cancer operation was similar to those patients undergoing a first operation, and because there is a rising rate of second lung cancer in lung cancer survivors, we feel that continued surveillance beyond the 4-year interval as recommended by the American Association for Thoracic Surgery as well as the [National Comprehensive Cancer Network] guidelines would be beneficial to long-term survivors of early-stage lung cancer,” Dr. Varlotto and coinvestigators wrote.
“The recent results from recent lung cancer screening studies demonstrate that females may benefit preferentially from screening … and our study suggests that these preferential benefits of increased CT scan surveillance may extend to females who are long-term survivors of lung cancer as well,” they added.
Findings in context
“As this is an observational study, it is challenging to understand what is driving the rise in prevalence of second lung cancers,” Mara Antonoff, MD, of The University of Texas MD Anderson Cancer Center in Houston commented in an interview.
“Overall, the findings are very important, as they suggest that we should continue to perform surveillance imaging for patients beyond recommended guidelines, which may allow us to achieve better survival outcomes for those individuals who develop a second lung cancer years after the first lung cancer,” she agreed.
“Just as lung cancer screening is important to identifying lung cancers at an earlier stage when they are more easily treatable and more likely to be cured, surveillance after an initial treatment for lung cancer would allow a diagnosis of second lung cancers at an earlier stage, so the patients can again achieve durable cure,” Dr. Antonoff concluded.
Study details
For the study, Dr. Varlotto and coinvestigators used data from SEER-13 and SEER-18 to identify patients with a lung cancer diagnosis during 1998-2013, and data from SEER-9, covering the years 1985-2014, to calculate rates of second cancers occurring 4-10 years after a first lung cancer.
Analyses were based on 58,758 patients with a surgically resected first primary lung cancer (55.9% with early-stage disease) and 384 patients with a surgically resected second primary lung cancer (77.6% with early-stage disease). Median follow-up was 76 months for the former and 46 months for the latter.
Results showed that in the 4-10 years after a first lung cancer diagnosis, the age-adjusted incidence of second lung cancers rose by study year but remained less than that of all other second cancers combined until the mid-2000s. Among women, incidence started rising sharply in 2001 and significantly exceeded that of all other second cancers starting in 2005.
In the entire population of study patients, propensity-adjusted analyses showed that second lung cancers were similar to first lung cancers on overall survival (P = .1726) but had worse lung cancer–specific survival (P = .0143). However, in the subset of patients with early-stage resectable disease, second and first lung cancers were similar on both overall survival (P = .3872) and lung cancer–specific survival (P = .1276).
Dr. Varlotto disclosed that he had no conflicts of interest. The study was funded by the Department of Radiation Oncology, University of Massachusetts. Dr. Antonoff disclosed that she had no relevant conflicts of interest.
SOURCE: Varlotto JM et al. Lung Cancer. 2020;147:115-122.
Second lung cancers occurring up to a decade after the first are on the rise, but their prognosis is similar – especially when detected early – which supports long-term surveillance in survivors, finds a large population-based study.
Although guidelines recommend continued annual low-dose CT scan surveillance extending beyond 4 years for this population based on expert consensus, long-term evidence of benefit is lacking.
Investigators led by John M. Varlotto, MD, a radiation oncologist at the University of Massachusetts Medical Center, Worcester, analyzed Surveillance, Epidemiology & End Results (SEER) data for more than 58,000 patients with first and sometimes second non–small cell lung cancers initially treated by surgical resection.
Study results reported in Lung Cancer showed that the age-adjusted incidence of second lung cancers occurring 4-10 years after the first lung cancer rose sharply during the 1985-2014 study period, driven by a large uptick in women patients.
Among all patients, second lung cancers had similar overall survival as first lung cancers, but poorer lung cancer–specific survival. However, among the subset of patients having early-stage resectable disease (tumors measuring less than 4 cm with negative nodes), both outcomes were statistically indistinguishable.
“Because our investigation noted that the overall survival of patients undergoing a second lung cancer operation was similar to those patients undergoing a first operation, and because there is a rising rate of second lung cancer in lung cancer survivors, we feel that continued surveillance beyond the 4-year interval as recommended by the American Association for Thoracic Surgery as well as the [National Comprehensive Cancer Network] guidelines would be beneficial to long-term survivors of early-stage lung cancer,” Dr. Varlotto and coinvestigators wrote.
“The recent results from recent lung cancer screening studies demonstrate that females may benefit preferentially from screening … and our study suggests that these preferential benefits of increased CT scan surveillance may extend to females who are long-term survivors of lung cancer as well,” they added.
Findings in context
“As this is an observational study, it is challenging to understand what is driving the rise in prevalence of second lung cancers,” Mara Antonoff, MD, of The University of Texas MD Anderson Cancer Center in Houston commented in an interview.
“Overall, the findings are very important, as they suggest that we should continue to perform surveillance imaging for patients beyond recommended guidelines, which may allow us to achieve better survival outcomes for those individuals who develop a second lung cancer years after the first lung cancer,” she agreed.
“Just as lung cancer screening is important to identifying lung cancers at an earlier stage when they are more easily treatable and more likely to be cured, surveillance after an initial treatment for lung cancer would allow a diagnosis of second lung cancers at an earlier stage, so the patients can again achieve durable cure,” Dr. Antonoff concluded.
Study details
For the study, Dr. Varlotto and coinvestigators used data from SEER-13 and SEER-18 to identify patients with a lung cancer diagnosis during 1998-2013, and data from SEER-9, covering the years 1985-2014, to calculate rates of second cancers occurring 4-10 years after a first lung cancer.
Analyses were based on 58,758 patients with a surgically resected first primary lung cancer (55.9% with early-stage disease) and 384 patients with a surgically resected second primary lung cancer (77.6% with early-stage disease). Median follow-up was 76 months for the former and 46 months for the latter.
Results showed that in the 4-10 years after a first lung cancer diagnosis, the age-adjusted incidence of second lung cancers rose by study year but remained less than that of all other second cancers combined until the mid-2000s. Among women, incidence started rising sharply in 2001 and significantly exceeded that of all other second cancers starting in 2005.
In the entire population of study patients, propensity-adjusted analyses showed that second lung cancers were similar to first lung cancers on overall survival (P = .1726) but had worse lung cancer–specific survival (P = .0143). However, in the subset of patients with early-stage resectable disease, second and first lung cancers were similar on both overall survival (P = .3872) and lung cancer–specific survival (P = .1276).
Dr. Varlotto disclosed that he had no conflicts of interest. The study was funded by the Department of Radiation Oncology, University of Massachusetts. Dr. Antonoff disclosed that she had no relevant conflicts of interest.
SOURCE: Varlotto JM et al. Lung Cancer. 2020;147:115-122.
Second lung cancers occurring up to a decade after the first are on the rise, but their prognosis is similar – especially when detected early – which supports long-term surveillance in survivors, finds a large population-based study.
Although guidelines recommend continued annual low-dose CT scan surveillance extending beyond 4 years for this population based on expert consensus, long-term evidence of benefit is lacking.
Investigators led by John M. Varlotto, MD, a radiation oncologist at the University of Massachusetts Medical Center, Worcester, analyzed Surveillance, Epidemiology & End Results (SEER) data for more than 58,000 patients with first and sometimes second non–small cell lung cancers initially treated by surgical resection.
Study results reported in Lung Cancer showed that the age-adjusted incidence of second lung cancers occurring 4-10 years after the first lung cancer rose sharply during the 1985-2014 study period, driven by a large uptick in women patients.
Among all patients, second lung cancers had similar overall survival as first lung cancers, but poorer lung cancer–specific survival. However, among the subset of patients having early-stage resectable disease (tumors measuring less than 4 cm with negative nodes), both outcomes were statistically indistinguishable.
“Because our investigation noted that the overall survival of patients undergoing a second lung cancer operation was similar to those patients undergoing a first operation, and because there is a rising rate of second lung cancer in lung cancer survivors, we feel that continued surveillance beyond the 4-year interval as recommended by the American Association for Thoracic Surgery as well as the [National Comprehensive Cancer Network] guidelines would be beneficial to long-term survivors of early-stage lung cancer,” Dr. Varlotto and coinvestigators wrote.
“The recent results from recent lung cancer screening studies demonstrate that females may benefit preferentially from screening … and our study suggests that these preferential benefits of increased CT scan surveillance may extend to females who are long-term survivors of lung cancer as well,” they added.
Findings in context
“As this is an observational study, it is challenging to understand what is driving the rise in prevalence of second lung cancers,” Mara Antonoff, MD, of The University of Texas MD Anderson Cancer Center in Houston commented in an interview.
“Overall, the findings are very important, as they suggest that we should continue to perform surveillance imaging for patients beyond recommended guidelines, which may allow us to achieve better survival outcomes for those individuals who develop a second lung cancer years after the first lung cancer,” she agreed.
“Just as lung cancer screening is important to identifying lung cancers at an earlier stage when they are more easily treatable and more likely to be cured, surveillance after an initial treatment for lung cancer would allow a diagnosis of second lung cancers at an earlier stage, so the patients can again achieve durable cure,” Dr. Antonoff concluded.
Study details
For the study, Dr. Varlotto and coinvestigators used data from SEER-13 and SEER-18 to identify patients with a lung cancer diagnosis during 1998-2013, and data from SEER-9, covering the years 1985-2014, to calculate rates of second cancers occurring 4-10 years after a first lung cancer.
Analyses were based on 58,758 patients with a surgically resected first primary lung cancer (55.9% with early-stage disease) and 384 patients with a surgically resected second primary lung cancer (77.6% with early-stage disease). Median follow-up was 76 months for the former and 46 months for the latter.
Results showed that in the 4-10 years after a first lung cancer diagnosis, the age-adjusted incidence of second lung cancers rose by study year but remained less than that of all other second cancers combined until the mid-2000s. Among women, incidence started rising sharply in 2001 and significantly exceeded that of all other second cancers starting in 2005.
In the entire population of study patients, propensity-adjusted analyses showed that second lung cancers were similar to first lung cancers on overall survival (P = .1726) but had worse lung cancer–specific survival (P = .0143). However, in the subset of patients with early-stage resectable disease, second and first lung cancers were similar on both overall survival (P = .3872) and lung cancer–specific survival (P = .1276).
Dr. Varlotto disclosed that he had no conflicts of interest. The study was funded by the Department of Radiation Oncology, University of Massachusetts. Dr. Antonoff disclosed that she had no relevant conflicts of interest.
SOURCE: Varlotto JM et al. Lung Cancer. 2020;147:115-122.
FROM LUNG CANCER
Mammography starting at 40 cuts risk of breast cancer death
New data will add fuel to the ongoing debate over the age at which mammography screening for breast cancer should begin. Many guidelines recommend starting at age 50.
But yearly mammography between the ages of 40 and 49 years was associated with a “substantial and significant” 25% reduction in breast cancer mortality during the first 10 years of follow-up, according to new data from the UK Age Trial.
The researchers calculated that 1,150 women needed to undergo screening in the age group of 40-49 years to prevent 1 breast cancer death, or about 1 breast cancer death prevented per 1,000 screened.
However, they also noted that, in the years since the trial first began, there have been improvements in the treatment of breast cancer, so “there might be less scope for screening to reduce mortality in our current era.”
The study was published online August 12 in Lancet Oncology.
“Our results do indicate that screening before age 50 does indeed prevent deaths from breast cancer, with a minimal additional burden of overdiagnosis,” said lead author Stephen W. Duffy, MSc, director of the policy research unit in cancer awareness, screening and early diagnosis, at Queen Mary University, London.
That said, Dr. Duffy explained they do not expect policy makers to extend the age range on the basis of these results alone. “For one thing, they will want to consider costs, both human and financial.” “For another, at this time, the services are concentrating on recovering from the hiatus caused by the COVID-19 crisis, and, at this time, it would be impractical to try to expand the eligibility for screening.”
“I would say our results indicate that lowering the age range, although not necessarily to 40 but to some age below 50, will be at least worth considering when the current crisis is over,” he added.
Guideline recommendations differ
Breast cancer screening guidelines have generated debate, much of which has focused on the age at which to begin screening.
The U.S. Preventive Services Task Force and American College of Physicians recommend screening every other year, on average, for women between the ages of 50 and 74 years.
However, other organizations disagree. The American College of Radiology and Society of Breast Imaging both recommend annual mammograms starting at age 40, and continuing “as long as they are in good health.”
In the UK, where the study was conducted, a national breast cancer screening program offers mammography to women aged 50-70 years every 3 years.
Given the uncertainty that continues to exist over the optimal age for average-risk women to begin screening, the UK Age Trial set out to assess if screening should begin at a younger age and if that might lead to overdiagnosis of breast cancer.
Results from the study’s 17-year follow-up, published in 2015, showed a reduction in breast cancer mortality with annual screening, beginning at age 40 years, which was significant in the first 10 years after participants were randomized (Lancet Oncol. 2015;16:1123-32).
In the current study, Dr. Duffy and colleagues report on breast cancer incidence and mortality results in the UK Age trial after 23 years of follow-up.
The cohort included 160,921 women enrolled between Oct. 14, 1990, and Sept. 24, 1997, who were randomized to screening (n = 53,883) or the control group (n = 106,953).
Of those screened during the study period, 7,893 (18.1%) had at least one false-positive result. There were 10,439 deaths, of which 683 (7%) were attributed to breast cancer diagnosed during the study period.
At 10 years of follow-up, death from breast cancer was significantly lower among women in the screening versus control group (83 vs 219 deaths; relative risk, 0.75; P = .029).
However, no significant reduction was observed thereafter, with 126 versus 255 deaths occurring after more than 10 years of follow-up (RR, 0.98; 95% confidence interval, 0.79-1.22; P = .86), the authors note.
“This follow-up indicates that the gain in survival was concentrated in the first 10 years after the women began to be screened,” commented Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England.
“In those first 10 years, out of every 10,000 women invited for screening, on average, about 16 died of breast cancer, while in every 10,000 women in the control group who did not get the screening, on average, 21 died. These numbers indicate that lives were saved,” he said.
“But they also indicate that death from breast cancer was pretty rare in women of that age,” he pointed out.
“Because breast cancer deaths in younger women are not common, the estimates of breast cancer death rates are not very precise, despite the fact that the trial involved 160,000 women,” he said.
“Over the whole follow-up period so far, the difference in numbers of deaths between those who were screened in their 40s and those who were not is 6 deaths for every 10,000 women, but because of the statistical uncertainty, this figure could plausibly be larger, at 13 per 10,000. Or, in fact, the data are also consistent with a very slightly higher death rate [1 death per 10,000 women] in those who had the screening,” Dr. McConway explained.
“But none of those numbers is very large, out of 10,000 women. Allowing for the fact that not every woman invited for screening will actually attend the screening, the researchers estimate that 1,150 women would have to be screened in their 40s to prevent one breast cancer death,” he noted.
U.S. experts support starting screening at 40
“The American Society of Breast Surgeons has continued to recommend screening women at the age of 40,” said Stephanie Bernik, MD, FACS, chief of breast surgery, Mount Sinai West, and associate professor of surgery at the Icahn School of Medicine at Mount Sinai, New York. “There is no question that screening earlier saves more lives, and this study adds to the body of evidence already available.”
She pointed out that the argument against early screening was that there were many false positives, which, in turn, increased cost and anxiety. “Because women in their 40s are in the prime of their lives, often with young children, it seems as though screening would be paramount. Furthermore, it is well known that the sooner you find a cancer, the better, as the treatment needed to cure the cancer is less toxic and less dramatic.”
Catherine Tuite, MD, section chief, breast radiology, Fox Chase Cancer Center, Philadelphia, echoed a similar viewpoint. “There is no real debate on this issue. The USPSTF recommends beginning screening mammography at age 50, and it is no secret that this is a recommendation based on cost, not on saving women’s lives.”
She emphasized that these recommendations were made without the input of expert physicians. “The data, reaffirmed by this publication, have always been clear that the most years of life saved from deaths due to breast cancer are achieved in women who begin screening mammography at age 40. We know that one-sixth of all breast cancers are diagnosed before age 50, and many of these cancers are the most aggressive types of breast cancer.
“The guidelines from every organization representing health care professionals who actually diagnose and care for women with breast cancer recommend that all women of average risk begin annual screening mammography at age 40 and continue as long as the woman is in good health, with life expectancy of 10 years,” she continued.
As for screening intervals, annual mammogram is also recommended for all age groups in the United States. At her institutions, she explained that they are currently enrolling women into the TMIST screening mammogram trial, which is, among other endpoints, evaluating a biannual screening interval for postmenopausal women of lower than average risk, but again, outside of a trial setting, yearly screening for all women is recommended.
Dr. Duffy commented that, in the United Kingdom, the current screening protocol for mammograms is every 3 years, which he said “works well in women over the age of 50 years.” But for younger women, more frequent screening would be need – in this study, screening was done annually.
“The results not only from our study but from others around the world suggest that this [3-year screening interval] would not be very effective in women under 50, due partly to the denser breast tissue of younger women and partly to the faster progression on average of cancers diagnosed in younger women,” he said. “Some counties in Sweden, for example, offer screening to women under 50 at 18-month intervals, which seems more realistic.”
The study was funded by the Health Technology Assessment program of the National Institute for Health Research. Dr. Duffy reported also receiving grants from the NIHR outside this trial. Dr. Bernik, Dr. Tuite, and Dr. Hodgson reported no relevant financial relationships.
This article first appeared on Medscape.com.
New data will add fuel to the ongoing debate over the age at which mammography screening for breast cancer should begin. Many guidelines recommend starting at age 50.
But yearly mammography between the ages of 40 and 49 years was associated with a “substantial and significant” 25% reduction in breast cancer mortality during the first 10 years of follow-up, according to new data from the UK Age Trial.
The researchers calculated that 1,150 women needed to undergo screening in the age group of 40-49 years to prevent 1 breast cancer death, or about 1 breast cancer death prevented per 1,000 screened.
However, they also noted that, in the years since the trial first began, there have been improvements in the treatment of breast cancer, so “there might be less scope for screening to reduce mortality in our current era.”
The study was published online August 12 in Lancet Oncology.
“Our results do indicate that screening before age 50 does indeed prevent deaths from breast cancer, with a minimal additional burden of overdiagnosis,” said lead author Stephen W. Duffy, MSc, director of the policy research unit in cancer awareness, screening and early diagnosis, at Queen Mary University, London.
That said, Dr. Duffy explained they do not expect policy makers to extend the age range on the basis of these results alone. “For one thing, they will want to consider costs, both human and financial.” “For another, at this time, the services are concentrating on recovering from the hiatus caused by the COVID-19 crisis, and, at this time, it would be impractical to try to expand the eligibility for screening.”
“I would say our results indicate that lowering the age range, although not necessarily to 40 but to some age below 50, will be at least worth considering when the current crisis is over,” he added.
Guideline recommendations differ
Breast cancer screening guidelines have generated debate, much of which has focused on the age at which to begin screening.
The U.S. Preventive Services Task Force and American College of Physicians recommend screening every other year, on average, for women between the ages of 50 and 74 years.
However, other organizations disagree. The American College of Radiology and Society of Breast Imaging both recommend annual mammograms starting at age 40, and continuing “as long as they are in good health.”
In the UK, where the study was conducted, a national breast cancer screening program offers mammography to women aged 50-70 years every 3 years.
Given the uncertainty that continues to exist over the optimal age for average-risk women to begin screening, the UK Age Trial set out to assess if screening should begin at a younger age and if that might lead to overdiagnosis of breast cancer.
Results from the study’s 17-year follow-up, published in 2015, showed a reduction in breast cancer mortality with annual screening, beginning at age 40 years, which was significant in the first 10 years after participants were randomized (Lancet Oncol. 2015;16:1123-32).
In the current study, Dr. Duffy and colleagues report on breast cancer incidence and mortality results in the UK Age trial after 23 years of follow-up.
The cohort included 160,921 women enrolled between Oct. 14, 1990, and Sept. 24, 1997, who were randomized to screening (n = 53,883) or the control group (n = 106,953).
Of those screened during the study period, 7,893 (18.1%) had at least one false-positive result. There were 10,439 deaths, of which 683 (7%) were attributed to breast cancer diagnosed during the study period.
At 10 years of follow-up, death from breast cancer was significantly lower among women in the screening versus control group (83 vs 219 deaths; relative risk, 0.75; P = .029).
However, no significant reduction was observed thereafter, with 126 versus 255 deaths occurring after more than 10 years of follow-up (RR, 0.98; 95% confidence interval, 0.79-1.22; P = .86), the authors note.
“This follow-up indicates that the gain in survival was concentrated in the first 10 years after the women began to be screened,” commented Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England.
“In those first 10 years, out of every 10,000 women invited for screening, on average, about 16 died of breast cancer, while in every 10,000 women in the control group who did not get the screening, on average, 21 died. These numbers indicate that lives were saved,” he said.
“But they also indicate that death from breast cancer was pretty rare in women of that age,” he pointed out.
“Because breast cancer deaths in younger women are not common, the estimates of breast cancer death rates are not very precise, despite the fact that the trial involved 160,000 women,” he said.
“Over the whole follow-up period so far, the difference in numbers of deaths between those who were screened in their 40s and those who were not is 6 deaths for every 10,000 women, but because of the statistical uncertainty, this figure could plausibly be larger, at 13 per 10,000. Or, in fact, the data are also consistent with a very slightly higher death rate [1 death per 10,000 women] in those who had the screening,” Dr. McConway explained.
“But none of those numbers is very large, out of 10,000 women. Allowing for the fact that not every woman invited for screening will actually attend the screening, the researchers estimate that 1,150 women would have to be screened in their 40s to prevent one breast cancer death,” he noted.
U.S. experts support starting screening at 40
“The American Society of Breast Surgeons has continued to recommend screening women at the age of 40,” said Stephanie Bernik, MD, FACS, chief of breast surgery, Mount Sinai West, and associate professor of surgery at the Icahn School of Medicine at Mount Sinai, New York. “There is no question that screening earlier saves more lives, and this study adds to the body of evidence already available.”
She pointed out that the argument against early screening was that there were many false positives, which, in turn, increased cost and anxiety. “Because women in their 40s are in the prime of their lives, often with young children, it seems as though screening would be paramount. Furthermore, it is well known that the sooner you find a cancer, the better, as the treatment needed to cure the cancer is less toxic and less dramatic.”
Catherine Tuite, MD, section chief, breast radiology, Fox Chase Cancer Center, Philadelphia, echoed a similar viewpoint. “There is no real debate on this issue. The USPSTF recommends beginning screening mammography at age 50, and it is no secret that this is a recommendation based on cost, not on saving women’s lives.”
She emphasized that these recommendations were made without the input of expert physicians. “The data, reaffirmed by this publication, have always been clear that the most years of life saved from deaths due to breast cancer are achieved in women who begin screening mammography at age 40. We know that one-sixth of all breast cancers are diagnosed before age 50, and many of these cancers are the most aggressive types of breast cancer.
“The guidelines from every organization representing health care professionals who actually diagnose and care for women with breast cancer recommend that all women of average risk begin annual screening mammography at age 40 and continue as long as the woman is in good health, with life expectancy of 10 years,” she continued.
As for screening intervals, annual mammogram is also recommended for all age groups in the United States. At her institutions, she explained that they are currently enrolling women into the TMIST screening mammogram trial, which is, among other endpoints, evaluating a biannual screening interval for postmenopausal women of lower than average risk, but again, outside of a trial setting, yearly screening for all women is recommended.
Dr. Duffy commented that, in the United Kingdom, the current screening protocol for mammograms is every 3 years, which he said “works well in women over the age of 50 years.” But for younger women, more frequent screening would be need – in this study, screening was done annually.
“The results not only from our study but from others around the world suggest that this [3-year screening interval] would not be very effective in women under 50, due partly to the denser breast tissue of younger women and partly to the faster progression on average of cancers diagnosed in younger women,” he said. “Some counties in Sweden, for example, offer screening to women under 50 at 18-month intervals, which seems more realistic.”
The study was funded by the Health Technology Assessment program of the National Institute for Health Research. Dr. Duffy reported also receiving grants from the NIHR outside this trial. Dr. Bernik, Dr. Tuite, and Dr. Hodgson reported no relevant financial relationships.
This article first appeared on Medscape.com.
New data will add fuel to the ongoing debate over the age at which mammography screening for breast cancer should begin. Many guidelines recommend starting at age 50.
But yearly mammography between the ages of 40 and 49 years was associated with a “substantial and significant” 25% reduction in breast cancer mortality during the first 10 years of follow-up, according to new data from the UK Age Trial.
The researchers calculated that 1,150 women needed to undergo screening in the age group of 40-49 years to prevent 1 breast cancer death, or about 1 breast cancer death prevented per 1,000 screened.
However, they also noted that, in the years since the trial first began, there have been improvements in the treatment of breast cancer, so “there might be less scope for screening to reduce mortality in our current era.”
The study was published online August 12 in Lancet Oncology.
“Our results do indicate that screening before age 50 does indeed prevent deaths from breast cancer, with a minimal additional burden of overdiagnosis,” said lead author Stephen W. Duffy, MSc, director of the policy research unit in cancer awareness, screening and early diagnosis, at Queen Mary University, London.
That said, Dr. Duffy explained they do not expect policy makers to extend the age range on the basis of these results alone. “For one thing, they will want to consider costs, both human and financial.” “For another, at this time, the services are concentrating on recovering from the hiatus caused by the COVID-19 crisis, and, at this time, it would be impractical to try to expand the eligibility for screening.”
“I would say our results indicate that lowering the age range, although not necessarily to 40 but to some age below 50, will be at least worth considering when the current crisis is over,” he added.
Guideline recommendations differ
Breast cancer screening guidelines have generated debate, much of which has focused on the age at which to begin screening.
The U.S. Preventive Services Task Force and American College of Physicians recommend screening every other year, on average, for women between the ages of 50 and 74 years.
However, other organizations disagree. The American College of Radiology and Society of Breast Imaging both recommend annual mammograms starting at age 40, and continuing “as long as they are in good health.”
In the UK, where the study was conducted, a national breast cancer screening program offers mammography to women aged 50-70 years every 3 years.
Given the uncertainty that continues to exist over the optimal age for average-risk women to begin screening, the UK Age Trial set out to assess if screening should begin at a younger age and if that might lead to overdiagnosis of breast cancer.
Results from the study’s 17-year follow-up, published in 2015, showed a reduction in breast cancer mortality with annual screening, beginning at age 40 years, which was significant in the first 10 years after participants were randomized (Lancet Oncol. 2015;16:1123-32).
In the current study, Dr. Duffy and colleagues report on breast cancer incidence and mortality results in the UK Age trial after 23 years of follow-up.
The cohort included 160,921 women enrolled between Oct. 14, 1990, and Sept. 24, 1997, who were randomized to screening (n = 53,883) or the control group (n = 106,953).
Of those screened during the study period, 7,893 (18.1%) had at least one false-positive result. There were 10,439 deaths, of which 683 (7%) were attributed to breast cancer diagnosed during the study period.
At 10 years of follow-up, death from breast cancer was significantly lower among women in the screening versus control group (83 vs 219 deaths; relative risk, 0.75; P = .029).
However, no significant reduction was observed thereafter, with 126 versus 255 deaths occurring after more than 10 years of follow-up (RR, 0.98; 95% confidence interval, 0.79-1.22; P = .86), the authors note.
“This follow-up indicates that the gain in survival was concentrated in the first 10 years after the women began to be screened,” commented Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England.
“In those first 10 years, out of every 10,000 women invited for screening, on average, about 16 died of breast cancer, while in every 10,000 women in the control group who did not get the screening, on average, 21 died. These numbers indicate that lives were saved,” he said.
“But they also indicate that death from breast cancer was pretty rare in women of that age,” he pointed out.
“Because breast cancer deaths in younger women are not common, the estimates of breast cancer death rates are not very precise, despite the fact that the trial involved 160,000 women,” he said.
“Over the whole follow-up period so far, the difference in numbers of deaths between those who were screened in their 40s and those who were not is 6 deaths for every 10,000 women, but because of the statistical uncertainty, this figure could plausibly be larger, at 13 per 10,000. Or, in fact, the data are also consistent with a very slightly higher death rate [1 death per 10,000 women] in those who had the screening,” Dr. McConway explained.
“But none of those numbers is very large, out of 10,000 women. Allowing for the fact that not every woman invited for screening will actually attend the screening, the researchers estimate that 1,150 women would have to be screened in their 40s to prevent one breast cancer death,” he noted.
U.S. experts support starting screening at 40
“The American Society of Breast Surgeons has continued to recommend screening women at the age of 40,” said Stephanie Bernik, MD, FACS, chief of breast surgery, Mount Sinai West, and associate professor of surgery at the Icahn School of Medicine at Mount Sinai, New York. “There is no question that screening earlier saves more lives, and this study adds to the body of evidence already available.”
She pointed out that the argument against early screening was that there were many false positives, which, in turn, increased cost and anxiety. “Because women in their 40s are in the prime of their lives, often with young children, it seems as though screening would be paramount. Furthermore, it is well known that the sooner you find a cancer, the better, as the treatment needed to cure the cancer is less toxic and less dramatic.”
Catherine Tuite, MD, section chief, breast radiology, Fox Chase Cancer Center, Philadelphia, echoed a similar viewpoint. “There is no real debate on this issue. The USPSTF recommends beginning screening mammography at age 50, and it is no secret that this is a recommendation based on cost, not on saving women’s lives.”
She emphasized that these recommendations were made without the input of expert physicians. “The data, reaffirmed by this publication, have always been clear that the most years of life saved from deaths due to breast cancer are achieved in women who begin screening mammography at age 40. We know that one-sixth of all breast cancers are diagnosed before age 50, and many of these cancers are the most aggressive types of breast cancer.
“The guidelines from every organization representing health care professionals who actually diagnose and care for women with breast cancer recommend that all women of average risk begin annual screening mammography at age 40 and continue as long as the woman is in good health, with life expectancy of 10 years,” she continued.
As for screening intervals, annual mammogram is also recommended for all age groups in the United States. At her institutions, she explained that they are currently enrolling women into the TMIST screening mammogram trial, which is, among other endpoints, evaluating a biannual screening interval for postmenopausal women of lower than average risk, but again, outside of a trial setting, yearly screening for all women is recommended.
Dr. Duffy commented that, in the United Kingdom, the current screening protocol for mammograms is every 3 years, which he said “works well in women over the age of 50 years.” But for younger women, more frequent screening would be need – in this study, screening was done annually.
“The results not only from our study but from others around the world suggest that this [3-year screening interval] would not be very effective in women under 50, due partly to the denser breast tissue of younger women and partly to the faster progression on average of cancers diagnosed in younger women,” he said. “Some counties in Sweden, for example, offer screening to women under 50 at 18-month intervals, which seems more realistic.”
The study was funded by the Health Technology Assessment program of the National Institute for Health Research. Dr. Duffy reported also receiving grants from the NIHR outside this trial. Dr. Bernik, Dr. Tuite, and Dr. Hodgson reported no relevant financial relationships.
This article first appeared on Medscape.com.
Swallowable ‘sponge on string’ to diagnose esophageal cancer
An experimental cell-collection device that can be administered without anesthesia in a primary care practice was shown to be better at detecting Barrett esophagus than the standard of care in a community-based clinical trial.
Use of this patient-swallowed device, called Cytosponge-TFF3, could allow clinicians to diagnose esophageal conditions such as dysplasia or cancer at an earlier and potentially curable stage, said the investigators. However, it would also increase the likelihood of unnecessary endoscopies, owing to false-positive results.
“In this multicenter, pragmatic, randomized controlled trial we found that an invitation to have a Cytosponge-TFF3 test led to increased diagnosis of Barrett’s esophagus when compared with usual care by general practitioners,” write Rebecca C. Fitzgerald, MD, from the Hutchison/MRC Research Center in Cambridge, England, and colleagues.
The study was published online on Aug. 1 in The Lancet.
“This is a very important study, a landmark study,” said Stephen J. Meltzer, MD, professor of medicine and oncology at Johns Hopkins University, Baltimore, who was approached for comment.
“What it shows is that if you opt to have this procedure, you’re much more likely to have your Barrett’s diagnosed than if you don’t opt to have it,” he said.
He congratulated Dr. Fitzgerald and colleagues for successful completion of a large, primary practice–based clinical utility study.
“Those studies are very difficult to do. This is looking at the actual impact of an intervention, which is the sponge,” he said in an interview.
Soaking up cells
Dr. Meltzer was senior author of a case-control study published in 2019 in Clinical Cancer Research that described use of a similar device. As previously reported, that device, called EsophaCap, uses a “methylation on bead” technique to collect DNA on a swallowed sponge. The DNA is then extracted from the sponge and analyzed with a methylation biomarker panel.
Like the EsophaCap device, the Cytosponge-TFF3 device consists of a compressed, gelatin-coated collection sponge attached to a thread. The patient swallows the device. After the gelatin dissolves and the sponge expands, it is gently withdrawn through the esophagus, picking up cells as it passes through.
The collected cells are then analyzed with an in vitro test for biomarker trefoil factor 3 (TFF3), a sign of intestinal metaplasia that is a histopathologic hallmark of Barrett esophagus, the authors explained.
Cytosponge-TFF3 study
The study by Dr. Fitzgerald and colleagues was conducted in patients taking medications for gastroesophageal reflux. The patients were undergoing treatment at 109 general practice clinics in England.
Eligible patients included adults aged 50 years and older who had been taking acid-suppressing medication for gastroesophageal reflux for more than 6 months and had not undergone endoscopy within the previous 5 years.
The study was randomized at both the clinic level (cluster randomization) and the individual patient level. Patients were assigned to either standard management of gastroesophageal reflux, with endoscopies performed only if recommended by the practitioner, or to the intervention group, where individuals received usual care and were offered the Cytosponge-TFF3 procedure. Those patients whose samples yielded TFF3-positive cells subsequently underwent endoscopy.
Among 6,834 patients assigned to the intervention group, 2,679 (39%) expressed willingness to undergo the Cytosponge-TFF3 procedure. Of this group, 1,750 patients met all of the eligibility criteria on telephone screening and underwent the procedure.
The large majority of patients (95%) who agreed to undergo the procedure were able to swallow the capsule and the attached thread.
Patients in the intervention group who declined the Cytosponge-TFF3 and all patients assigned to the usual-care arm underwent endoscopy only at the recommendation of their primary practitioner.
During a mean follow-up of 12 months, 140 of the 6,834 patients in the intervention group (2%) were diagnosed with Barrett esophagus, compared with 13 of 6,388 patients in the usual-care group (0.2%). The absolute difference per 1000 person-years, the trial’s primary endpoint, was 18.3. The rate ratio adjusted for cluster randomization was 10.6 (P < .001).
A total of four patients in the intervention group were diagnosed with dysplastic Barrett esophagus, and five were diagnosed with stage I esophagogastric cancer. No patients in the usual-care group were diagnosed with either condition.
Of the 1,654 patients in the intervention group who opted for the Cytosponge device and swallowed it successfully, 221 underwent endoscopy after testing positive for TFF3. Of these patients, 131 (59%) were diagnosed with either Barrett esophagus or cancer.
The most common adverse event with the Cytosponge procedure was sore throat, reported by 4% of those who opted for it. In one patient, the thread became detach from the Cytopsonge, necessitating endoscopy to remove the device.
Promising, but refinements needed
In an editorial accompanying the study, Yuri Hanada, MD, and Kenneth K. Wang, MD, from the department of gastroenterology at the Mayo Clinic in Rochester, Minn., said that the Cytosponge-TFF3 procedure “is a promising nonendoscopic screening tool and will represent a component in the screening for Barrett’s esophagus and esophagogastric cancer.”
They noted, however, that it is unlikely to be the sole screening tool for Barrett esophagus and that its use in primary practice may be problematic during the COVID-19 pandemic, because of the release of aerosolized particles as the sponge is withdrawn from the esophagus.
“It might also be necessary to enrich disease prevalence in the screened population by limiting this population to males and people with other risk factors, in order to make this test more cost-effective than previously shown,” they wrote.
Acceptance rate low?
Dr. Meltzer noted that, despite being less invasive than endoscopy, only 39% of the group who could try it agreed to do so.
“It was kind of surprising, because in my experience, when I offer it to my patients, the acceptance is much higher, but that’s not in a controlled clinical trial situation, so I don’t really know what the true percentage is,” he said.
He pointed out that the patients he sees in his clinic are more likely to be symptomatic and highly motivated to accept a test, in contrast to the general patient population in the study.
He also noted that the endoscopy-confirmed prevalence rate of Barrett esophagus or cancer in 221 patients in the intervention group was 59%, suggesting that 41% underwent an unnecessary endoscopy after the Cytosponge screening.
Dr. Fitzgerald and colleagues acknowledged the potential for overdiagnosis with screening. They noted a debate as to whether 1 cm or short segments of Barrett esophagus are a cause for clinical concern.
They also note that the TFF3 test (used in the CytoSponge device) is sensitive and detects some short segments of Barrett esophagus and that, “since this was a pragmatic trial that relied on a coded diagnosis of Barrett’s esophagus, we also identified patients in the usual care group who had short segments of Barrett’s esophagus (1 cm or less in length) and were diagnosed as having the condition, reflecting the variable practice in U.K. hospitals.
“We expect that these patients can be reassured and probably do not require surveillance,” they continued. “This expectation is consistent with the clinical guidelines, which suggest that patients with over 1 cm of salmon-colored epithelium containing intestinal metaplasia should be monitored.”
The study was funded by Cancer Research UK, the U.K. National Institute for Health Research, the U.K. National Health Service, Medtronic, and the Medical Research Council. Dr. Fitzgerald is named on patents related to the Cytosponge-TFF3 test. Dr. Meltzer has cofounded a company, Capsulomics, to commercialize the methylation biomarker panel used in EsophaCap studies. Dr. Wang has received research funding from eNose for research on a device used in a screening study of Barrett esophagus.
This article first appeared on Medscape.com.
An experimental cell-collection device that can be administered without anesthesia in a primary care practice was shown to be better at detecting Barrett esophagus than the standard of care in a community-based clinical trial.
Use of this patient-swallowed device, called Cytosponge-TFF3, could allow clinicians to diagnose esophageal conditions such as dysplasia or cancer at an earlier and potentially curable stage, said the investigators. However, it would also increase the likelihood of unnecessary endoscopies, owing to false-positive results.
“In this multicenter, pragmatic, randomized controlled trial we found that an invitation to have a Cytosponge-TFF3 test led to increased diagnosis of Barrett’s esophagus when compared with usual care by general practitioners,” write Rebecca C. Fitzgerald, MD, from the Hutchison/MRC Research Center in Cambridge, England, and colleagues.
The study was published online on Aug. 1 in The Lancet.
“This is a very important study, a landmark study,” said Stephen J. Meltzer, MD, professor of medicine and oncology at Johns Hopkins University, Baltimore, who was approached for comment.
“What it shows is that if you opt to have this procedure, you’re much more likely to have your Barrett’s diagnosed than if you don’t opt to have it,” he said.
He congratulated Dr. Fitzgerald and colleagues for successful completion of a large, primary practice–based clinical utility study.
“Those studies are very difficult to do. This is looking at the actual impact of an intervention, which is the sponge,” he said in an interview.
Soaking up cells
Dr. Meltzer was senior author of a case-control study published in 2019 in Clinical Cancer Research that described use of a similar device. As previously reported, that device, called EsophaCap, uses a “methylation on bead” technique to collect DNA on a swallowed sponge. The DNA is then extracted from the sponge and analyzed with a methylation biomarker panel.
Like the EsophaCap device, the Cytosponge-TFF3 device consists of a compressed, gelatin-coated collection sponge attached to a thread. The patient swallows the device. After the gelatin dissolves and the sponge expands, it is gently withdrawn through the esophagus, picking up cells as it passes through.
The collected cells are then analyzed with an in vitro test for biomarker trefoil factor 3 (TFF3), a sign of intestinal metaplasia that is a histopathologic hallmark of Barrett esophagus, the authors explained.
Cytosponge-TFF3 study
The study by Dr. Fitzgerald and colleagues was conducted in patients taking medications for gastroesophageal reflux. The patients were undergoing treatment at 109 general practice clinics in England.
Eligible patients included adults aged 50 years and older who had been taking acid-suppressing medication for gastroesophageal reflux for more than 6 months and had not undergone endoscopy within the previous 5 years.
The study was randomized at both the clinic level (cluster randomization) and the individual patient level. Patients were assigned to either standard management of gastroesophageal reflux, with endoscopies performed only if recommended by the practitioner, or to the intervention group, where individuals received usual care and were offered the Cytosponge-TFF3 procedure. Those patients whose samples yielded TFF3-positive cells subsequently underwent endoscopy.
Among 6,834 patients assigned to the intervention group, 2,679 (39%) expressed willingness to undergo the Cytosponge-TFF3 procedure. Of this group, 1,750 patients met all of the eligibility criteria on telephone screening and underwent the procedure.
The large majority of patients (95%) who agreed to undergo the procedure were able to swallow the capsule and the attached thread.
Patients in the intervention group who declined the Cytosponge-TFF3 and all patients assigned to the usual-care arm underwent endoscopy only at the recommendation of their primary practitioner.
During a mean follow-up of 12 months, 140 of the 6,834 patients in the intervention group (2%) were diagnosed with Barrett esophagus, compared with 13 of 6,388 patients in the usual-care group (0.2%). The absolute difference per 1000 person-years, the trial’s primary endpoint, was 18.3. The rate ratio adjusted for cluster randomization was 10.6 (P < .001).
A total of four patients in the intervention group were diagnosed with dysplastic Barrett esophagus, and five were diagnosed with stage I esophagogastric cancer. No patients in the usual-care group were diagnosed with either condition.
Of the 1,654 patients in the intervention group who opted for the Cytosponge device and swallowed it successfully, 221 underwent endoscopy after testing positive for TFF3. Of these patients, 131 (59%) were diagnosed with either Barrett esophagus or cancer.
The most common adverse event with the Cytosponge procedure was sore throat, reported by 4% of those who opted for it. In one patient, the thread became detach from the Cytopsonge, necessitating endoscopy to remove the device.
Promising, but refinements needed
In an editorial accompanying the study, Yuri Hanada, MD, and Kenneth K. Wang, MD, from the department of gastroenterology at the Mayo Clinic in Rochester, Minn., said that the Cytosponge-TFF3 procedure “is a promising nonendoscopic screening tool and will represent a component in the screening for Barrett’s esophagus and esophagogastric cancer.”
They noted, however, that it is unlikely to be the sole screening tool for Barrett esophagus and that its use in primary practice may be problematic during the COVID-19 pandemic, because of the release of aerosolized particles as the sponge is withdrawn from the esophagus.
“It might also be necessary to enrich disease prevalence in the screened population by limiting this population to males and people with other risk factors, in order to make this test more cost-effective than previously shown,” they wrote.
Acceptance rate low?
Dr. Meltzer noted that, despite being less invasive than endoscopy, only 39% of the group who could try it agreed to do so.
“It was kind of surprising, because in my experience, when I offer it to my patients, the acceptance is much higher, but that’s not in a controlled clinical trial situation, so I don’t really know what the true percentage is,” he said.
He pointed out that the patients he sees in his clinic are more likely to be symptomatic and highly motivated to accept a test, in contrast to the general patient population in the study.
He also noted that the endoscopy-confirmed prevalence rate of Barrett esophagus or cancer in 221 patients in the intervention group was 59%, suggesting that 41% underwent an unnecessary endoscopy after the Cytosponge screening.
Dr. Fitzgerald and colleagues acknowledged the potential for overdiagnosis with screening. They noted a debate as to whether 1 cm or short segments of Barrett esophagus are a cause for clinical concern.
They also note that the TFF3 test (used in the CytoSponge device) is sensitive and detects some short segments of Barrett esophagus and that, “since this was a pragmatic trial that relied on a coded diagnosis of Barrett’s esophagus, we also identified patients in the usual care group who had short segments of Barrett’s esophagus (1 cm or less in length) and were diagnosed as having the condition, reflecting the variable practice in U.K. hospitals.
“We expect that these patients can be reassured and probably do not require surveillance,” they continued. “This expectation is consistent with the clinical guidelines, which suggest that patients with over 1 cm of salmon-colored epithelium containing intestinal metaplasia should be monitored.”
The study was funded by Cancer Research UK, the U.K. National Institute for Health Research, the U.K. National Health Service, Medtronic, and the Medical Research Council. Dr. Fitzgerald is named on patents related to the Cytosponge-TFF3 test. Dr. Meltzer has cofounded a company, Capsulomics, to commercialize the methylation biomarker panel used in EsophaCap studies. Dr. Wang has received research funding from eNose for research on a device used in a screening study of Barrett esophagus.
This article first appeared on Medscape.com.
An experimental cell-collection device that can be administered without anesthesia in a primary care practice was shown to be better at detecting Barrett esophagus than the standard of care in a community-based clinical trial.
Use of this patient-swallowed device, called Cytosponge-TFF3, could allow clinicians to diagnose esophageal conditions such as dysplasia or cancer at an earlier and potentially curable stage, said the investigators. However, it would also increase the likelihood of unnecessary endoscopies, owing to false-positive results.
“In this multicenter, pragmatic, randomized controlled trial we found that an invitation to have a Cytosponge-TFF3 test led to increased diagnosis of Barrett’s esophagus when compared with usual care by general practitioners,” write Rebecca C. Fitzgerald, MD, from the Hutchison/MRC Research Center in Cambridge, England, and colleagues.
The study was published online on Aug. 1 in The Lancet.
“This is a very important study, a landmark study,” said Stephen J. Meltzer, MD, professor of medicine and oncology at Johns Hopkins University, Baltimore, who was approached for comment.
“What it shows is that if you opt to have this procedure, you’re much more likely to have your Barrett’s diagnosed than if you don’t opt to have it,” he said.
He congratulated Dr. Fitzgerald and colleagues for successful completion of a large, primary practice–based clinical utility study.
“Those studies are very difficult to do. This is looking at the actual impact of an intervention, which is the sponge,” he said in an interview.
Soaking up cells
Dr. Meltzer was senior author of a case-control study published in 2019 in Clinical Cancer Research that described use of a similar device. As previously reported, that device, called EsophaCap, uses a “methylation on bead” technique to collect DNA on a swallowed sponge. The DNA is then extracted from the sponge and analyzed with a methylation biomarker panel.
Like the EsophaCap device, the Cytosponge-TFF3 device consists of a compressed, gelatin-coated collection sponge attached to a thread. The patient swallows the device. After the gelatin dissolves and the sponge expands, it is gently withdrawn through the esophagus, picking up cells as it passes through.
The collected cells are then analyzed with an in vitro test for biomarker trefoil factor 3 (TFF3), a sign of intestinal metaplasia that is a histopathologic hallmark of Barrett esophagus, the authors explained.
Cytosponge-TFF3 study
The study by Dr. Fitzgerald and colleagues was conducted in patients taking medications for gastroesophageal reflux. The patients were undergoing treatment at 109 general practice clinics in England.
Eligible patients included adults aged 50 years and older who had been taking acid-suppressing medication for gastroesophageal reflux for more than 6 months and had not undergone endoscopy within the previous 5 years.
The study was randomized at both the clinic level (cluster randomization) and the individual patient level. Patients were assigned to either standard management of gastroesophageal reflux, with endoscopies performed only if recommended by the practitioner, or to the intervention group, where individuals received usual care and were offered the Cytosponge-TFF3 procedure. Those patients whose samples yielded TFF3-positive cells subsequently underwent endoscopy.
Among 6,834 patients assigned to the intervention group, 2,679 (39%) expressed willingness to undergo the Cytosponge-TFF3 procedure. Of this group, 1,750 patients met all of the eligibility criteria on telephone screening and underwent the procedure.
The large majority of patients (95%) who agreed to undergo the procedure were able to swallow the capsule and the attached thread.
Patients in the intervention group who declined the Cytosponge-TFF3 and all patients assigned to the usual-care arm underwent endoscopy only at the recommendation of their primary practitioner.
During a mean follow-up of 12 months, 140 of the 6,834 patients in the intervention group (2%) were diagnosed with Barrett esophagus, compared with 13 of 6,388 patients in the usual-care group (0.2%). The absolute difference per 1000 person-years, the trial’s primary endpoint, was 18.3. The rate ratio adjusted for cluster randomization was 10.6 (P < .001).
A total of four patients in the intervention group were diagnosed with dysplastic Barrett esophagus, and five were diagnosed with stage I esophagogastric cancer. No patients in the usual-care group were diagnosed with either condition.
Of the 1,654 patients in the intervention group who opted for the Cytosponge device and swallowed it successfully, 221 underwent endoscopy after testing positive for TFF3. Of these patients, 131 (59%) were diagnosed with either Barrett esophagus or cancer.
The most common adverse event with the Cytosponge procedure was sore throat, reported by 4% of those who opted for it. In one patient, the thread became detach from the Cytopsonge, necessitating endoscopy to remove the device.
Promising, but refinements needed
In an editorial accompanying the study, Yuri Hanada, MD, and Kenneth K. Wang, MD, from the department of gastroenterology at the Mayo Clinic in Rochester, Minn., said that the Cytosponge-TFF3 procedure “is a promising nonendoscopic screening tool and will represent a component in the screening for Barrett’s esophagus and esophagogastric cancer.”
They noted, however, that it is unlikely to be the sole screening tool for Barrett esophagus and that its use in primary practice may be problematic during the COVID-19 pandemic, because of the release of aerosolized particles as the sponge is withdrawn from the esophagus.
“It might also be necessary to enrich disease prevalence in the screened population by limiting this population to males and people with other risk factors, in order to make this test more cost-effective than previously shown,” they wrote.
Acceptance rate low?
Dr. Meltzer noted that, despite being less invasive than endoscopy, only 39% of the group who could try it agreed to do so.
“It was kind of surprising, because in my experience, when I offer it to my patients, the acceptance is much higher, but that’s not in a controlled clinical trial situation, so I don’t really know what the true percentage is,” he said.
He pointed out that the patients he sees in his clinic are more likely to be symptomatic and highly motivated to accept a test, in contrast to the general patient population in the study.
He also noted that the endoscopy-confirmed prevalence rate of Barrett esophagus or cancer in 221 patients in the intervention group was 59%, suggesting that 41% underwent an unnecessary endoscopy after the Cytosponge screening.
Dr. Fitzgerald and colleagues acknowledged the potential for overdiagnosis with screening. They noted a debate as to whether 1 cm or short segments of Barrett esophagus are a cause for clinical concern.
They also note that the TFF3 test (used in the CytoSponge device) is sensitive and detects some short segments of Barrett esophagus and that, “since this was a pragmatic trial that relied on a coded diagnosis of Barrett’s esophagus, we also identified patients in the usual care group who had short segments of Barrett’s esophagus (1 cm or less in length) and were diagnosed as having the condition, reflecting the variable practice in U.K. hospitals.
“We expect that these patients can be reassured and probably do not require surveillance,” they continued. “This expectation is consistent with the clinical guidelines, which suggest that patients with over 1 cm of salmon-colored epithelium containing intestinal metaplasia should be monitored.”
The study was funded by Cancer Research UK, the U.K. National Institute for Health Research, the U.K. National Health Service, Medtronic, and the Medical Research Council. Dr. Fitzgerald is named on patents related to the Cytosponge-TFF3 test. Dr. Meltzer has cofounded a company, Capsulomics, to commercialize the methylation biomarker panel used in EsophaCap studies. Dr. Wang has received research funding from eNose for research on a device used in a screening study of Barrett esophagus.
This article first appeared on Medscape.com.
AI improves diagnostic accuracy in cervical cancer
This could allow some women to avoid surgery and be treated with chemotherapy instead, suggested researchers.
The model mined tumor information from pelvic sagittal contrast-enhanced T1-weighted MRIs and combined this with clinical MRI lymph node status.
It was 90.62% sensitive and 87.16% specific for predicting lymph node metastases (LNMs) in a validation cohort of women who underwent surgery for cervical cancer.
The area under the curve was 0.933. The approach was significantly associated with disease-free survival (hazard ratio, 4.59; 95% confidence interval, 2.04-10.31; P < .001).
The study was published online on July 24 in JAMA Network Open.
“The findings of this study suggest that deep learning can be used as a preoperative noninvasive tool to diagnose lymph node metastasis in cervical cancer ... This model might be used preoperatively to help gynecologists make decisions,” said investigators led by Qingxia Wu, PhD, of the Northeastern University College of Medicine and Biomedical Information Engineering in Shenyang, China.
“Studies like these suggest that deep learning has the potential to improve the way we care for our patients,” but there’s much to be done “before these types of algorithms will be commonplace,” commented Christiaan Rees, MD, PhD, an internal medicine resident at Brigham and Women’s Hospital, Boston, who has a doctorate in quantitative biomedical sciences.
Next steps include repeated validation across multiple control groups, he said in an interview, as well as “finding ways to effectively integrate these tools into the radiologist’s day-to-day practice. One possibility would be for direct integration of the algorithm into the electronic health record.”
Accurate prediction could lead to skipping surgery
Chemotherapy, rather than surgery, is an option for women with positive lymph nodes (LNs), so accurate prediction can help them avoid an operation and its risks, the authors said.
The problem is that “the traditional methods for assessing LN status in cervical cancer, which rely mainly on assessing the size of LNs on MRI, have limited sensitivity in diagnosing LNM in cervical cancer and might lead to inappropriate treatment decisions,” they wrote.
“Although sentinel LN dissection ... shows good sensitivity and specificity, its application is limited by available facilities and experts,” the team said.
DL is an advanced form of artificial intelligence in which a computer program continuously improves on a given task as it incorporates more data – in Dr. Wu’s study, more than 14 million images. Deep learning has recently shown promise in several imaging tasks, such as diagnosing Alzheimer’s disease and screening for breast cancer.
Once adapted for cervical cancer, DL “does not require precise tumor delineation, making it an easy-to-use method in clinical practice. In many tumor analysis tasks, DL outperforms traditional radiomic features,” the team noted.
The study involved 479 women – 338 during model development, and 141 in the validation cohorts. The mean age of the participants was 49.1 years. They had undergone radical hysterectomy with pelvic lymphadenectomy for stage IB-IIB cervical cancer within 2 weeks of a pelvic MRI. Pathology reports were used to check the accuracy of the model’s predictions.
Specificity, sensitivity, and area under the curve were a little better in the study’s development cohort than its validation group, for whom median disease-free survival was 23 months versus 31 months among the patients in the development cohort. Nodes were positive on lymphadenectomy in a little more than 20% of women in both groups.
Incorporation of both intratumoral and peritumoral regions on contrast-enhanced T1-weighted MRIs versus axial T2-weighted and axial diffusion-weighted imaging, produced the highest sensitivity. Adding MRI-LN status – defined as positive when the short-axis diameter of the largest LN on MRI was ≥1 cm – improved the model’s specificity.
To understand how the model reached its conclusions, the team analyzed how it extracted features from tumor images. “In the shallow convolution layers, the DL model extracted simple tumor edge features ... while in deeper convolution layers, it extracted complex tumor texture information ... In the last convolution layer, the DL model extracted high-level abstract features (the fourteenth layer). Although these high-level features were so intricate that they were hard to interpret by general gross observation, they were associated with LN status,” the investigators said.
The team notes that “both intratumoral and peritumoral regions were necessary for the DL model to make decisions,” which “can probably be explained by the fact that higher lymphatic vessel density in peritumoral regions might lead to higher regional LNM.”
Commenting on the study, Dr. Rees said that “the authors did a [good] job of essentially deconstructing their neural network to see what the algorithm was actually picking up on to make its decision.
“One of the nice features of deep learning is that once the algorithm has been developed and validated, the end user doesn’t need any experience in deep learning in order to use it,” he added.
Even so, “while these resources can be incredibly powerful tools, they should not function in a vacuum without human judgment,” Dr. Rees said.
The work was funded by the National Natural Science Foundation of China, among others. The investigators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
This could allow some women to avoid surgery and be treated with chemotherapy instead, suggested researchers.
The model mined tumor information from pelvic sagittal contrast-enhanced T1-weighted MRIs and combined this with clinical MRI lymph node status.
It was 90.62% sensitive and 87.16% specific for predicting lymph node metastases (LNMs) in a validation cohort of women who underwent surgery for cervical cancer.
The area under the curve was 0.933. The approach was significantly associated with disease-free survival (hazard ratio, 4.59; 95% confidence interval, 2.04-10.31; P < .001).
The study was published online on July 24 in JAMA Network Open.
“The findings of this study suggest that deep learning can be used as a preoperative noninvasive tool to diagnose lymph node metastasis in cervical cancer ... This model might be used preoperatively to help gynecologists make decisions,” said investigators led by Qingxia Wu, PhD, of the Northeastern University College of Medicine and Biomedical Information Engineering in Shenyang, China.
“Studies like these suggest that deep learning has the potential to improve the way we care for our patients,” but there’s much to be done “before these types of algorithms will be commonplace,” commented Christiaan Rees, MD, PhD, an internal medicine resident at Brigham and Women’s Hospital, Boston, who has a doctorate in quantitative biomedical sciences.
Next steps include repeated validation across multiple control groups, he said in an interview, as well as “finding ways to effectively integrate these tools into the radiologist’s day-to-day practice. One possibility would be for direct integration of the algorithm into the electronic health record.”
Accurate prediction could lead to skipping surgery
Chemotherapy, rather than surgery, is an option for women with positive lymph nodes (LNs), so accurate prediction can help them avoid an operation and its risks, the authors said.
The problem is that “the traditional methods for assessing LN status in cervical cancer, which rely mainly on assessing the size of LNs on MRI, have limited sensitivity in diagnosing LNM in cervical cancer and might lead to inappropriate treatment decisions,” they wrote.
“Although sentinel LN dissection ... shows good sensitivity and specificity, its application is limited by available facilities and experts,” the team said.
DL is an advanced form of artificial intelligence in which a computer program continuously improves on a given task as it incorporates more data – in Dr. Wu’s study, more than 14 million images. Deep learning has recently shown promise in several imaging tasks, such as diagnosing Alzheimer’s disease and screening for breast cancer.
Once adapted for cervical cancer, DL “does not require precise tumor delineation, making it an easy-to-use method in clinical practice. In many tumor analysis tasks, DL outperforms traditional radiomic features,” the team noted.
The study involved 479 women – 338 during model development, and 141 in the validation cohorts. The mean age of the participants was 49.1 years. They had undergone radical hysterectomy with pelvic lymphadenectomy for stage IB-IIB cervical cancer within 2 weeks of a pelvic MRI. Pathology reports were used to check the accuracy of the model’s predictions.
Specificity, sensitivity, and area under the curve were a little better in the study’s development cohort than its validation group, for whom median disease-free survival was 23 months versus 31 months among the patients in the development cohort. Nodes were positive on lymphadenectomy in a little more than 20% of women in both groups.
Incorporation of both intratumoral and peritumoral regions on contrast-enhanced T1-weighted MRIs versus axial T2-weighted and axial diffusion-weighted imaging, produced the highest sensitivity. Adding MRI-LN status – defined as positive when the short-axis diameter of the largest LN on MRI was ≥1 cm – improved the model’s specificity.
To understand how the model reached its conclusions, the team analyzed how it extracted features from tumor images. “In the shallow convolution layers, the DL model extracted simple tumor edge features ... while in deeper convolution layers, it extracted complex tumor texture information ... In the last convolution layer, the DL model extracted high-level abstract features (the fourteenth layer). Although these high-level features were so intricate that they were hard to interpret by general gross observation, they were associated with LN status,” the investigators said.
The team notes that “both intratumoral and peritumoral regions were necessary for the DL model to make decisions,” which “can probably be explained by the fact that higher lymphatic vessel density in peritumoral regions might lead to higher regional LNM.”
Commenting on the study, Dr. Rees said that “the authors did a [good] job of essentially deconstructing their neural network to see what the algorithm was actually picking up on to make its decision.
“One of the nice features of deep learning is that once the algorithm has been developed and validated, the end user doesn’t need any experience in deep learning in order to use it,” he added.
Even so, “while these resources can be incredibly powerful tools, they should not function in a vacuum without human judgment,” Dr. Rees said.
The work was funded by the National Natural Science Foundation of China, among others. The investigators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
This could allow some women to avoid surgery and be treated with chemotherapy instead, suggested researchers.
The model mined tumor information from pelvic sagittal contrast-enhanced T1-weighted MRIs and combined this with clinical MRI lymph node status.
It was 90.62% sensitive and 87.16% specific for predicting lymph node metastases (LNMs) in a validation cohort of women who underwent surgery for cervical cancer.
The area under the curve was 0.933. The approach was significantly associated with disease-free survival (hazard ratio, 4.59; 95% confidence interval, 2.04-10.31; P < .001).
The study was published online on July 24 in JAMA Network Open.
“The findings of this study suggest that deep learning can be used as a preoperative noninvasive tool to diagnose lymph node metastasis in cervical cancer ... This model might be used preoperatively to help gynecologists make decisions,” said investigators led by Qingxia Wu, PhD, of the Northeastern University College of Medicine and Biomedical Information Engineering in Shenyang, China.
“Studies like these suggest that deep learning has the potential to improve the way we care for our patients,” but there’s much to be done “before these types of algorithms will be commonplace,” commented Christiaan Rees, MD, PhD, an internal medicine resident at Brigham and Women’s Hospital, Boston, who has a doctorate in quantitative biomedical sciences.
Next steps include repeated validation across multiple control groups, he said in an interview, as well as “finding ways to effectively integrate these tools into the radiologist’s day-to-day practice. One possibility would be for direct integration of the algorithm into the electronic health record.”
Accurate prediction could lead to skipping surgery
Chemotherapy, rather than surgery, is an option for women with positive lymph nodes (LNs), so accurate prediction can help them avoid an operation and its risks, the authors said.
The problem is that “the traditional methods for assessing LN status in cervical cancer, which rely mainly on assessing the size of LNs on MRI, have limited sensitivity in diagnosing LNM in cervical cancer and might lead to inappropriate treatment decisions,” they wrote.
“Although sentinel LN dissection ... shows good sensitivity and specificity, its application is limited by available facilities and experts,” the team said.
DL is an advanced form of artificial intelligence in which a computer program continuously improves on a given task as it incorporates more data – in Dr. Wu’s study, more than 14 million images. Deep learning has recently shown promise in several imaging tasks, such as diagnosing Alzheimer’s disease and screening for breast cancer.
Once adapted for cervical cancer, DL “does not require precise tumor delineation, making it an easy-to-use method in clinical practice. In many tumor analysis tasks, DL outperforms traditional radiomic features,” the team noted.
The study involved 479 women – 338 during model development, and 141 in the validation cohorts. The mean age of the participants was 49.1 years. They had undergone radical hysterectomy with pelvic lymphadenectomy for stage IB-IIB cervical cancer within 2 weeks of a pelvic MRI. Pathology reports were used to check the accuracy of the model’s predictions.
Specificity, sensitivity, and area under the curve were a little better in the study’s development cohort than its validation group, for whom median disease-free survival was 23 months versus 31 months among the patients in the development cohort. Nodes were positive on lymphadenectomy in a little more than 20% of women in both groups.
Incorporation of both intratumoral and peritumoral regions on contrast-enhanced T1-weighted MRIs versus axial T2-weighted and axial diffusion-weighted imaging, produced the highest sensitivity. Adding MRI-LN status – defined as positive when the short-axis diameter of the largest LN on MRI was ≥1 cm – improved the model’s specificity.
To understand how the model reached its conclusions, the team analyzed how it extracted features from tumor images. “In the shallow convolution layers, the DL model extracted simple tumor edge features ... while in deeper convolution layers, it extracted complex tumor texture information ... In the last convolution layer, the DL model extracted high-level abstract features (the fourteenth layer). Although these high-level features were so intricate that they were hard to interpret by general gross observation, they were associated with LN status,” the investigators said.
The team notes that “both intratumoral and peritumoral regions were necessary for the DL model to make decisions,” which “can probably be explained by the fact that higher lymphatic vessel density in peritumoral regions might lead to higher regional LNM.”
Commenting on the study, Dr. Rees said that “the authors did a [good] job of essentially deconstructing their neural network to see what the algorithm was actually picking up on to make its decision.
“One of the nice features of deep learning is that once the algorithm has been developed and validated, the end user doesn’t need any experience in deep learning in order to use it,” he added.
Even so, “while these resources can be incredibly powerful tools, they should not function in a vacuum without human judgment,” Dr. Rees said.
The work was funded by the National Natural Science Foundation of China, among others. The investigators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
NSCLC success story: Mortality down, survival improved
“This analysis shows for the first time that nationwide mortality rates for the most common category of lung cancer, NSCLC, are declining faster than its incidence, an advance that correlates with the [FDA] approval of several targeted therapies for this cancer in recent years,” coauthor Douglas Lowy, MD, deputy director, National Cancer Institute, Bethesda, Md., said in a statement.
“Major improvements have been made in NSCLC treatment with the advent of targeted therapies and immunotherapies,” lead author Nadia Howlander, PhD, National Cancer Institute, and colleagues observed.
“The survival benefit for patients with NSCLC treated with targeted therapy has been shown in clinical trials, but our study highlights their possible effect at the population level,” they added.
In contrast, mortality from SCLC has dropped only in tandem with a decline in the incidence of SCLC, and survival has remained largely unchanged, the same analysis showed.
NSCLC is by far the most common type of lung cancer, accounting for more than 75% of all lung cancer cases in the United States. SCLC accounts for about 13%.
The study was published online Aug. 12 in the New England Journal of Medicine.
“Although overall mortality from lung cancer has been declining in the United States, little is known about mortality trends according to cancer subtype at the population level because death certificates do not record subtype information,” the authors commented.
“To address this data limitation, the U.S. Surveillance, Epidemiology and End Results (SEER) program has linked mortality records to incidence cancer cases,” the authors explained. This allowed them to calculate incidence-based mortality among men and women in the United States.
The incidence-based mortality method that the researchers used was applied to the SEER data to describe population-level mortality trends in the United States that were attributable to each subtype of lung cancer as well as gender from 2001 to 2016.
Among men, the incidence of NSCLC decreased gradually by 1.9% a year from 2001 to 2008, then more dramatically by 3.1% a year from 2008 to 2016.
Corresponding incidence-based mortality rates among men dropped by 3.2% a year from 2006 to 2013, then again more dramatically by 6.3% a year from 2013 to 2016.
“The 2-year relative survival among patients with lung cancer improved substantially from 26% among men with NSCLC diagnosed in 2001 to 35% among those with NSCLC diagnosed in 2014,” the researchers added.
Among women, the incidence of NSCLC remained unchanged between 2001 and 2006, after which it began to drop by 1.5% a year from 2006 to 2016.
“In contrast, incidence-based mortality decreased slowly [among women] by 2.3% annually ... from 2006 through 2014 and then at a faster rate of 5.9% annually ... from 2014 through 2016,” the authors noted.
The 2-year relative survival rate for patients with NSCLC was higher among women than among men, improving from 35% in 2001 to 44% in 2014.
Improvements in survival were also observed for all races and ethnicities, despite concerns that new cancer treatments might increase treatment disparities between races, because they are all so expensive, the authors commented.
Mortality from SCLC declined by 4.3% a year among men, but that decline was entirely due to a similar decrease in the incidence of SCLC. Survival at 2 years for patients with this subtype of lung cancer remained largely unchanged over the same interval.
Genetic testing
The accelerating decline in NSCLC mortality starting in 2013 corresponds to the period in which clinicians began to routinely test for molecular alterations in epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK), the authors pointed out.
In 2012, the National Comprehensive Cancer Network recommended that all patients with nonsquamous NSCLC undergo genetic testing for EGFR mutations and ALK rearrangements.
At about the same time, the FDA approved a number of targeted therapies for tumors that are sensitive to targeted tyrosine kinase inhibition.
More recently, immunotherapies that act as programmed cell death inhibitors have substantially improved NSCLC outcomes, the authors noted.
The first of these was approved for NSCLC in 2015 (pembrolizumab). It was followed by a number of similar agents. It is unlikely that their approval contributed to the observed decline in NSCLC mortality, which started to accelerate in 2013 in the United States, the authors commented.
Nevertheless, the effect that the immune checkpoint inhibitors has had on the survival of patients with NSCLC can be expected to continue and to extend improvement in survival beyond the current study endpoint in 2016, they suggest.
Another contributing factor is the decline in smoking that has occurred in the United States since the 1960s. This has led to the decrease in the incidence of lung cancer. The faster decrease in the incidence of SCLC, compared with NSCLC can be explained by the higher relative risk of smoking with regard to SCLC compared to NSCLC, they commented.
Similarly, the faster decrease in lung cancer incidence in men compared to women can be explained by the relative difference in the prevalence of smoking between men and women, they added.
The authors disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
“This analysis shows for the first time that nationwide mortality rates for the most common category of lung cancer, NSCLC, are declining faster than its incidence, an advance that correlates with the [FDA] approval of several targeted therapies for this cancer in recent years,” coauthor Douglas Lowy, MD, deputy director, National Cancer Institute, Bethesda, Md., said in a statement.
“Major improvements have been made in NSCLC treatment with the advent of targeted therapies and immunotherapies,” lead author Nadia Howlander, PhD, National Cancer Institute, and colleagues observed.
“The survival benefit for patients with NSCLC treated with targeted therapy has been shown in clinical trials, but our study highlights their possible effect at the population level,” they added.
In contrast, mortality from SCLC has dropped only in tandem with a decline in the incidence of SCLC, and survival has remained largely unchanged, the same analysis showed.
NSCLC is by far the most common type of lung cancer, accounting for more than 75% of all lung cancer cases in the United States. SCLC accounts for about 13%.
The study was published online Aug. 12 in the New England Journal of Medicine.
“Although overall mortality from lung cancer has been declining in the United States, little is known about mortality trends according to cancer subtype at the population level because death certificates do not record subtype information,” the authors commented.
“To address this data limitation, the U.S. Surveillance, Epidemiology and End Results (SEER) program has linked mortality records to incidence cancer cases,” the authors explained. This allowed them to calculate incidence-based mortality among men and women in the United States.
The incidence-based mortality method that the researchers used was applied to the SEER data to describe population-level mortality trends in the United States that were attributable to each subtype of lung cancer as well as gender from 2001 to 2016.
Among men, the incidence of NSCLC decreased gradually by 1.9% a year from 2001 to 2008, then more dramatically by 3.1% a year from 2008 to 2016.
Corresponding incidence-based mortality rates among men dropped by 3.2% a year from 2006 to 2013, then again more dramatically by 6.3% a year from 2013 to 2016.
“The 2-year relative survival among patients with lung cancer improved substantially from 26% among men with NSCLC diagnosed in 2001 to 35% among those with NSCLC diagnosed in 2014,” the researchers added.
Among women, the incidence of NSCLC remained unchanged between 2001 and 2006, after which it began to drop by 1.5% a year from 2006 to 2016.
“In contrast, incidence-based mortality decreased slowly [among women] by 2.3% annually ... from 2006 through 2014 and then at a faster rate of 5.9% annually ... from 2014 through 2016,” the authors noted.
The 2-year relative survival rate for patients with NSCLC was higher among women than among men, improving from 35% in 2001 to 44% in 2014.
Improvements in survival were also observed for all races and ethnicities, despite concerns that new cancer treatments might increase treatment disparities between races, because they are all so expensive, the authors commented.
Mortality from SCLC declined by 4.3% a year among men, but that decline was entirely due to a similar decrease in the incidence of SCLC. Survival at 2 years for patients with this subtype of lung cancer remained largely unchanged over the same interval.
Genetic testing
The accelerating decline in NSCLC mortality starting in 2013 corresponds to the period in which clinicians began to routinely test for molecular alterations in epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK), the authors pointed out.
In 2012, the National Comprehensive Cancer Network recommended that all patients with nonsquamous NSCLC undergo genetic testing for EGFR mutations and ALK rearrangements.
At about the same time, the FDA approved a number of targeted therapies for tumors that are sensitive to targeted tyrosine kinase inhibition.
More recently, immunotherapies that act as programmed cell death inhibitors have substantially improved NSCLC outcomes, the authors noted.
The first of these was approved for NSCLC in 2015 (pembrolizumab). It was followed by a number of similar agents. It is unlikely that their approval contributed to the observed decline in NSCLC mortality, which started to accelerate in 2013 in the United States, the authors commented.
Nevertheless, the effect that the immune checkpoint inhibitors has had on the survival of patients with NSCLC can be expected to continue and to extend improvement in survival beyond the current study endpoint in 2016, they suggest.
Another contributing factor is the decline in smoking that has occurred in the United States since the 1960s. This has led to the decrease in the incidence of lung cancer. The faster decrease in the incidence of SCLC, compared with NSCLC can be explained by the higher relative risk of smoking with regard to SCLC compared to NSCLC, they commented.
Similarly, the faster decrease in lung cancer incidence in men compared to women can be explained by the relative difference in the prevalence of smoking between men and women, they added.
The authors disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
“This analysis shows for the first time that nationwide mortality rates for the most common category of lung cancer, NSCLC, are declining faster than its incidence, an advance that correlates with the [FDA] approval of several targeted therapies for this cancer in recent years,” coauthor Douglas Lowy, MD, deputy director, National Cancer Institute, Bethesda, Md., said in a statement.
“Major improvements have been made in NSCLC treatment with the advent of targeted therapies and immunotherapies,” lead author Nadia Howlander, PhD, National Cancer Institute, and colleagues observed.
“The survival benefit for patients with NSCLC treated with targeted therapy has been shown in clinical trials, but our study highlights their possible effect at the population level,” they added.
In contrast, mortality from SCLC has dropped only in tandem with a decline in the incidence of SCLC, and survival has remained largely unchanged, the same analysis showed.
NSCLC is by far the most common type of lung cancer, accounting for more than 75% of all lung cancer cases in the United States. SCLC accounts for about 13%.
The study was published online Aug. 12 in the New England Journal of Medicine.
“Although overall mortality from lung cancer has been declining in the United States, little is known about mortality trends according to cancer subtype at the population level because death certificates do not record subtype information,” the authors commented.
“To address this data limitation, the U.S. Surveillance, Epidemiology and End Results (SEER) program has linked mortality records to incidence cancer cases,” the authors explained. This allowed them to calculate incidence-based mortality among men and women in the United States.
The incidence-based mortality method that the researchers used was applied to the SEER data to describe population-level mortality trends in the United States that were attributable to each subtype of lung cancer as well as gender from 2001 to 2016.
Among men, the incidence of NSCLC decreased gradually by 1.9% a year from 2001 to 2008, then more dramatically by 3.1% a year from 2008 to 2016.
Corresponding incidence-based mortality rates among men dropped by 3.2% a year from 2006 to 2013, then again more dramatically by 6.3% a year from 2013 to 2016.
“The 2-year relative survival among patients with lung cancer improved substantially from 26% among men with NSCLC diagnosed in 2001 to 35% among those with NSCLC diagnosed in 2014,” the researchers added.
Among women, the incidence of NSCLC remained unchanged between 2001 and 2006, after which it began to drop by 1.5% a year from 2006 to 2016.
“In contrast, incidence-based mortality decreased slowly [among women] by 2.3% annually ... from 2006 through 2014 and then at a faster rate of 5.9% annually ... from 2014 through 2016,” the authors noted.
The 2-year relative survival rate for patients with NSCLC was higher among women than among men, improving from 35% in 2001 to 44% in 2014.
Improvements in survival were also observed for all races and ethnicities, despite concerns that new cancer treatments might increase treatment disparities between races, because they are all so expensive, the authors commented.
Mortality from SCLC declined by 4.3% a year among men, but that decline was entirely due to a similar decrease in the incidence of SCLC. Survival at 2 years for patients with this subtype of lung cancer remained largely unchanged over the same interval.
Genetic testing
The accelerating decline in NSCLC mortality starting in 2013 corresponds to the period in which clinicians began to routinely test for molecular alterations in epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK), the authors pointed out.
In 2012, the National Comprehensive Cancer Network recommended that all patients with nonsquamous NSCLC undergo genetic testing for EGFR mutations and ALK rearrangements.
At about the same time, the FDA approved a number of targeted therapies for tumors that are sensitive to targeted tyrosine kinase inhibition.
More recently, immunotherapies that act as programmed cell death inhibitors have substantially improved NSCLC outcomes, the authors noted.
The first of these was approved for NSCLC in 2015 (pembrolizumab). It was followed by a number of similar agents. It is unlikely that their approval contributed to the observed decline in NSCLC mortality, which started to accelerate in 2013 in the United States, the authors commented.
Nevertheless, the effect that the immune checkpoint inhibitors has had on the survival of patients with NSCLC can be expected to continue and to extend improvement in survival beyond the current study endpoint in 2016, they suggest.
Another contributing factor is the decline in smoking that has occurred in the United States since the 1960s. This has led to the decrease in the incidence of lung cancer. The faster decrease in the incidence of SCLC, compared with NSCLC can be explained by the higher relative risk of smoking with regard to SCLC compared to NSCLC, they commented.
Similarly, the faster decrease in lung cancer incidence in men compared to women can be explained by the relative difference in the prevalence of smoking between men and women, they added.
The authors disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Sintilimab scintillates in first-line nonsquamous NSCLC
The investigational anti-PD-1 antibody sintilimab (Tyvyt, Innovent Biologics and Eli Lilly) has shown that it improves the efficacy of platinum-based chemotherapy in the first-line treatment of patients with advanced nonsquamous non–small cell lung cancer (NSCLC) in a phase 3 trial dubbed ORIENT-11.
The study was presented at the World Congress on Lung Cancer 2020 Virtual Presidential Symposium, held virtually due to the COVID-19 pandemic, on August 8. It was also published simultaneously in the Journal of Thoracic Oncology.
Sintilimab is a fully human IgG4 monoclonal antibody that blocks the binding of programmed death (PD)-1 to PD-ligand 1 (PD-L1) or PD-L2 with high affinity, and has received market authorization in China for the treatment of Hodgkin lymphoma.
For ORIENT-11, almost 400 patients with advanced nonsquamous NSCLC were randomly assigned to sintilimab or placebo plus pemetrexed and platinum-based chemotherapy in a 2:1 ratio.
“The addition of sintilimab to pemetrexed and platinum significantly improved PFS [progression-free survival], compared to placebo,” reducing progression rates by 52%, noted lead investigator Li Zhang, MD, professor of medical oncology, Sun Yat-Sen University Cancer Center, Guangzhou, China.
Crucially, this benefit “was seen across key clinical subgroups,” he added.
He noted that the overall response rate “was also improved, with a durable response,” while the results, which are not yet mature, suggest the experimental arm was associated with an overall survival (OS) benefit.
Study discussant Misako Nagasaka, MD, a thoracic oncologist and clinical investigator at Karmanos Cancer Institute, Detroit, said that the PFS benefit seen in the study is “certainly encouraging.”
Adding a note of caution, she continued: “But we have seen studies with PFS improvement which did not translate into OS improvement.
“Longer follow-up would allow events to mature and we will ultimately see if there would be a significant OS benefit,” she said.
Dr. Nagasaka also pointed out that the greater benefit with sintilimab seen in patients with high PD-L1 begs the question as to what would be the preferred regimen in those with higher or lower expression.
And, she said, this is not just about what regimen to choose but “more importantly, why?”
“Perhaps you’d like to use something with the best response rate, perhaps you’re sticking to a single agent immunotherapy because the toxicity profile is more favorable, or perhaps you [are] convinced with a certain regimen because of the robust PFS and OS data,” she said.
“Whatever you chose, there was a reason for your choice,” she said, adding that the sintilimab combination would have to “fulfill those reasons for you to consider choosing this regimen.”
Study details
Dr. Zhang began his presentation by noting that previous phase 1b studies have shown that sintilimab plus pemetrexed and platinum-based chemotherapy has a “tolerable safety profile and promising efficacy” in previously untreated non-squamous NSCLC.
They therefore conducted ORIENT-11, a randomized, double-blind, phase 3 study involving 397 patients with untreated stage IIIB/C or IV nonsquamous NSCLC who had neither EGFR nor ALK gene alterations.
The patients were randomly assigned in a 2:1 fashion to sintilimab plus pemetrexed and platinum-based chemotherapy (n = 266) or placebo plus pemetrexed and chemo (n = 131) for four cycles, followed by sintilimab or placebo plus pemetrexed for up to 24 months.
Thirty-five patients in the placebo arm crossed over to sintilimab monotherapy, representing 31.3% of the intention-to-treat population.
At the data cutoff of Nov. 15, 2019, 198 events had occurred, at a median follow-up of 8.9 months.
The team found that median PFS was significantly higher with sintilimab than placebo combination therapy, at 8.9 months vs. 5.0 months, or a hazard ratio of 0.482 (P < .00001).
Dr. Zhang noted that the benefit with sintilimab plus pemetrexed and platinum-based chemotherapy was seen across all subgroups.
However, it was notable that the impact of adding sintilimab on PFS was greater in patients with a tumor proportion score (TPS) ≥50%.
The HR for progression vs. the placebo treatment arm was 0.310, with median PFS not reached, which decreased to 0.503 in patients with a TPS of 1%-49% and 0.664 among those with a TPS <1%.
The results also showed that there was a “nominally significant improvement” in overall survival with sintilimab versus placebo, at a HR of 0.609 (P = 0.01921).
The ORR was markedly different between the sintilimab and placebo groups, at 51.9% vs. 29.8%, with the duration of response not reached in the sintilimab arm compared with 5.5 months in the placebo arm.
The sintilimab arm included three (1.1%) complete responses, which was not observed with pemetrexed and platinum-based chemotherapy alone.
Finally, Dr. Zhang observed that the safety profiles of the sintilimab and placebo arms were similar, with comparable rates of any, grade 3-5, and serious adverse events largely driven by high rates of chemotherapy-related events.
While there were fewer adverse events that led to death with sintilimab, at 2.3% vs. 6.9% with placebo, there were, as expected, more immune-related adverse events, at 43.2% vs. 36.6%, respectively.
Comparison with pembrolizumab
In her discussion, Dr. Nagasaka said that the first question that came to mind when she saw the results was: “How does the ORIENT-11 data compare with KEYNOTE-189?”
For that study, pembrolizumab (Keytruda, Merck) was added to pemetrexed plus carboplatin chemotherapy and compared with standard of care alone in patients with untreated metastatic nonsquamous NSCLC.
As reported by Medscape Medical News, pembrolizumab was associated with a 48% reduced risk of disease progression, as well as improved overall survival.
Dr. Nagasaka said that ORIENT-11 “had patients that tended to be younger, there were more males, more with performance status 1, and those who had never smoked” than those in KEYNOTE-189.
“But most importantly, KEYNOTE-189 had a very small number of patients from East Asia, only 1% in the pembro arm and 2.9% in the placebo arm.”
In contrast, all the patients included in ORIENT-11 were from East Asia, making the study of “high importance.”
She added that, “while across-trial comparisons must be taken with caution, the medium PFS of ORIENT-11 ... appears comparable to those of KEYNOTE-189,” while the HR “appears identical.”
This is despite median follow-up time in ORIENT-11 of “only” 8.9 months vs. a median of 23.1 months in the updated KEYNOTE-189 data.
There are plans to register the sintilimab combination therapy in China for the treatment of nonsquamous NSCLC, where it will go up against pembrolizumab as well as, potentially, tislelizumab (BeiGene).
The study was sponsored by Innovent Biologics and Eli Lilly. Dr. Zhang disclosed research grants from Eli Lilly and Pfizer. Dr. Nagasaka disclosed serving on the advisory boards of AstraZeneca, Daiichi Sankyo, Takeda, Novartis, and EMD Serono; as a consultant for Caris Life Sciences; and receiving travel support from An Hearts Therapeutics.
This article first appeared on Medscape.com.
The investigational anti-PD-1 antibody sintilimab (Tyvyt, Innovent Biologics and Eli Lilly) has shown that it improves the efficacy of platinum-based chemotherapy in the first-line treatment of patients with advanced nonsquamous non–small cell lung cancer (NSCLC) in a phase 3 trial dubbed ORIENT-11.
The study was presented at the World Congress on Lung Cancer 2020 Virtual Presidential Symposium, held virtually due to the COVID-19 pandemic, on August 8. It was also published simultaneously in the Journal of Thoracic Oncology.
Sintilimab is a fully human IgG4 monoclonal antibody that blocks the binding of programmed death (PD)-1 to PD-ligand 1 (PD-L1) or PD-L2 with high affinity, and has received market authorization in China for the treatment of Hodgkin lymphoma.
For ORIENT-11, almost 400 patients with advanced nonsquamous NSCLC were randomly assigned to sintilimab or placebo plus pemetrexed and platinum-based chemotherapy in a 2:1 ratio.
“The addition of sintilimab to pemetrexed and platinum significantly improved PFS [progression-free survival], compared to placebo,” reducing progression rates by 52%, noted lead investigator Li Zhang, MD, professor of medical oncology, Sun Yat-Sen University Cancer Center, Guangzhou, China.
Crucially, this benefit “was seen across key clinical subgroups,” he added.
He noted that the overall response rate “was also improved, with a durable response,” while the results, which are not yet mature, suggest the experimental arm was associated with an overall survival (OS) benefit.
Study discussant Misako Nagasaka, MD, a thoracic oncologist and clinical investigator at Karmanos Cancer Institute, Detroit, said that the PFS benefit seen in the study is “certainly encouraging.”
Adding a note of caution, she continued: “But we have seen studies with PFS improvement which did not translate into OS improvement.
“Longer follow-up would allow events to mature and we will ultimately see if there would be a significant OS benefit,” she said.
Dr. Nagasaka also pointed out that the greater benefit with sintilimab seen in patients with high PD-L1 begs the question as to what would be the preferred regimen in those with higher or lower expression.
And, she said, this is not just about what regimen to choose but “more importantly, why?”
“Perhaps you’d like to use something with the best response rate, perhaps you’re sticking to a single agent immunotherapy because the toxicity profile is more favorable, or perhaps you [are] convinced with a certain regimen because of the robust PFS and OS data,” she said.
“Whatever you chose, there was a reason for your choice,” she said, adding that the sintilimab combination would have to “fulfill those reasons for you to consider choosing this regimen.”
Study details
Dr. Zhang began his presentation by noting that previous phase 1b studies have shown that sintilimab plus pemetrexed and platinum-based chemotherapy has a “tolerable safety profile and promising efficacy” in previously untreated non-squamous NSCLC.
They therefore conducted ORIENT-11, a randomized, double-blind, phase 3 study involving 397 patients with untreated stage IIIB/C or IV nonsquamous NSCLC who had neither EGFR nor ALK gene alterations.
The patients were randomly assigned in a 2:1 fashion to sintilimab plus pemetrexed and platinum-based chemotherapy (n = 266) or placebo plus pemetrexed and chemo (n = 131) for four cycles, followed by sintilimab or placebo plus pemetrexed for up to 24 months.
Thirty-five patients in the placebo arm crossed over to sintilimab monotherapy, representing 31.3% of the intention-to-treat population.
At the data cutoff of Nov. 15, 2019, 198 events had occurred, at a median follow-up of 8.9 months.
The team found that median PFS was significantly higher with sintilimab than placebo combination therapy, at 8.9 months vs. 5.0 months, or a hazard ratio of 0.482 (P < .00001).
Dr. Zhang noted that the benefit with sintilimab plus pemetrexed and platinum-based chemotherapy was seen across all subgroups.
However, it was notable that the impact of adding sintilimab on PFS was greater in patients with a tumor proportion score (TPS) ≥50%.
The HR for progression vs. the placebo treatment arm was 0.310, with median PFS not reached, which decreased to 0.503 in patients with a TPS of 1%-49% and 0.664 among those with a TPS <1%.
The results also showed that there was a “nominally significant improvement” in overall survival with sintilimab versus placebo, at a HR of 0.609 (P = 0.01921).
The ORR was markedly different between the sintilimab and placebo groups, at 51.9% vs. 29.8%, with the duration of response not reached in the sintilimab arm compared with 5.5 months in the placebo arm.
The sintilimab arm included three (1.1%) complete responses, which was not observed with pemetrexed and platinum-based chemotherapy alone.
Finally, Dr. Zhang observed that the safety profiles of the sintilimab and placebo arms were similar, with comparable rates of any, grade 3-5, and serious adverse events largely driven by high rates of chemotherapy-related events.
While there were fewer adverse events that led to death with sintilimab, at 2.3% vs. 6.9% with placebo, there were, as expected, more immune-related adverse events, at 43.2% vs. 36.6%, respectively.
Comparison with pembrolizumab
In her discussion, Dr. Nagasaka said that the first question that came to mind when she saw the results was: “How does the ORIENT-11 data compare with KEYNOTE-189?”
For that study, pembrolizumab (Keytruda, Merck) was added to pemetrexed plus carboplatin chemotherapy and compared with standard of care alone in patients with untreated metastatic nonsquamous NSCLC.
As reported by Medscape Medical News, pembrolizumab was associated with a 48% reduced risk of disease progression, as well as improved overall survival.
Dr. Nagasaka said that ORIENT-11 “had patients that tended to be younger, there were more males, more with performance status 1, and those who had never smoked” than those in KEYNOTE-189.
“But most importantly, KEYNOTE-189 had a very small number of patients from East Asia, only 1% in the pembro arm and 2.9% in the placebo arm.”
In contrast, all the patients included in ORIENT-11 were from East Asia, making the study of “high importance.”
She added that, “while across-trial comparisons must be taken with caution, the medium PFS of ORIENT-11 ... appears comparable to those of KEYNOTE-189,” while the HR “appears identical.”
This is despite median follow-up time in ORIENT-11 of “only” 8.9 months vs. a median of 23.1 months in the updated KEYNOTE-189 data.
There are plans to register the sintilimab combination therapy in China for the treatment of nonsquamous NSCLC, where it will go up against pembrolizumab as well as, potentially, tislelizumab (BeiGene).
The study was sponsored by Innovent Biologics and Eli Lilly. Dr. Zhang disclosed research grants from Eli Lilly and Pfizer. Dr. Nagasaka disclosed serving on the advisory boards of AstraZeneca, Daiichi Sankyo, Takeda, Novartis, and EMD Serono; as a consultant for Caris Life Sciences; and receiving travel support from An Hearts Therapeutics.
This article first appeared on Medscape.com.
The investigational anti-PD-1 antibody sintilimab (Tyvyt, Innovent Biologics and Eli Lilly) has shown that it improves the efficacy of platinum-based chemotherapy in the first-line treatment of patients with advanced nonsquamous non–small cell lung cancer (NSCLC) in a phase 3 trial dubbed ORIENT-11.
The study was presented at the World Congress on Lung Cancer 2020 Virtual Presidential Symposium, held virtually due to the COVID-19 pandemic, on August 8. It was also published simultaneously in the Journal of Thoracic Oncology.
Sintilimab is a fully human IgG4 monoclonal antibody that blocks the binding of programmed death (PD)-1 to PD-ligand 1 (PD-L1) or PD-L2 with high affinity, and has received market authorization in China for the treatment of Hodgkin lymphoma.
For ORIENT-11, almost 400 patients with advanced nonsquamous NSCLC were randomly assigned to sintilimab or placebo plus pemetrexed and platinum-based chemotherapy in a 2:1 ratio.
“The addition of sintilimab to pemetrexed and platinum significantly improved PFS [progression-free survival], compared to placebo,” reducing progression rates by 52%, noted lead investigator Li Zhang, MD, professor of medical oncology, Sun Yat-Sen University Cancer Center, Guangzhou, China.
Crucially, this benefit “was seen across key clinical subgroups,” he added.
He noted that the overall response rate “was also improved, with a durable response,” while the results, which are not yet mature, suggest the experimental arm was associated with an overall survival (OS) benefit.
Study discussant Misako Nagasaka, MD, a thoracic oncologist and clinical investigator at Karmanos Cancer Institute, Detroit, said that the PFS benefit seen in the study is “certainly encouraging.”
Adding a note of caution, she continued: “But we have seen studies with PFS improvement which did not translate into OS improvement.
“Longer follow-up would allow events to mature and we will ultimately see if there would be a significant OS benefit,” she said.
Dr. Nagasaka also pointed out that the greater benefit with sintilimab seen in patients with high PD-L1 begs the question as to what would be the preferred regimen in those with higher or lower expression.
And, she said, this is not just about what regimen to choose but “more importantly, why?”
“Perhaps you’d like to use something with the best response rate, perhaps you’re sticking to a single agent immunotherapy because the toxicity profile is more favorable, or perhaps you [are] convinced with a certain regimen because of the robust PFS and OS data,” she said.
“Whatever you chose, there was a reason for your choice,” she said, adding that the sintilimab combination would have to “fulfill those reasons for you to consider choosing this regimen.”
Study details
Dr. Zhang began his presentation by noting that previous phase 1b studies have shown that sintilimab plus pemetrexed and platinum-based chemotherapy has a “tolerable safety profile and promising efficacy” in previously untreated non-squamous NSCLC.
They therefore conducted ORIENT-11, a randomized, double-blind, phase 3 study involving 397 patients with untreated stage IIIB/C or IV nonsquamous NSCLC who had neither EGFR nor ALK gene alterations.
The patients were randomly assigned in a 2:1 fashion to sintilimab plus pemetrexed and platinum-based chemotherapy (n = 266) or placebo plus pemetrexed and chemo (n = 131) for four cycles, followed by sintilimab or placebo plus pemetrexed for up to 24 months.
Thirty-five patients in the placebo arm crossed over to sintilimab monotherapy, representing 31.3% of the intention-to-treat population.
At the data cutoff of Nov. 15, 2019, 198 events had occurred, at a median follow-up of 8.9 months.
The team found that median PFS was significantly higher with sintilimab than placebo combination therapy, at 8.9 months vs. 5.0 months, or a hazard ratio of 0.482 (P < .00001).
Dr. Zhang noted that the benefit with sintilimab plus pemetrexed and platinum-based chemotherapy was seen across all subgroups.
However, it was notable that the impact of adding sintilimab on PFS was greater in patients with a tumor proportion score (TPS) ≥50%.
The HR for progression vs. the placebo treatment arm was 0.310, with median PFS not reached, which decreased to 0.503 in patients with a TPS of 1%-49% and 0.664 among those with a TPS <1%.
The results also showed that there was a “nominally significant improvement” in overall survival with sintilimab versus placebo, at a HR of 0.609 (P = 0.01921).
The ORR was markedly different between the sintilimab and placebo groups, at 51.9% vs. 29.8%, with the duration of response not reached in the sintilimab arm compared with 5.5 months in the placebo arm.
The sintilimab arm included three (1.1%) complete responses, which was not observed with pemetrexed and platinum-based chemotherapy alone.
Finally, Dr. Zhang observed that the safety profiles of the sintilimab and placebo arms were similar, with comparable rates of any, grade 3-5, and serious adverse events largely driven by high rates of chemotherapy-related events.
While there were fewer adverse events that led to death with sintilimab, at 2.3% vs. 6.9% with placebo, there were, as expected, more immune-related adverse events, at 43.2% vs. 36.6%, respectively.
Comparison with pembrolizumab
In her discussion, Dr. Nagasaka said that the first question that came to mind when she saw the results was: “How does the ORIENT-11 data compare with KEYNOTE-189?”
For that study, pembrolizumab (Keytruda, Merck) was added to pemetrexed plus carboplatin chemotherapy and compared with standard of care alone in patients with untreated metastatic nonsquamous NSCLC.
As reported by Medscape Medical News, pembrolizumab was associated with a 48% reduced risk of disease progression, as well as improved overall survival.
Dr. Nagasaka said that ORIENT-11 “had patients that tended to be younger, there were more males, more with performance status 1, and those who had never smoked” than those in KEYNOTE-189.
“But most importantly, KEYNOTE-189 had a very small number of patients from East Asia, only 1% in the pembro arm and 2.9% in the placebo arm.”
In contrast, all the patients included in ORIENT-11 were from East Asia, making the study of “high importance.”
She added that, “while across-trial comparisons must be taken with caution, the medium PFS of ORIENT-11 ... appears comparable to those of KEYNOTE-189,” while the HR “appears identical.”
This is despite median follow-up time in ORIENT-11 of “only” 8.9 months vs. a median of 23.1 months in the updated KEYNOTE-189 data.
There are plans to register the sintilimab combination therapy in China for the treatment of nonsquamous NSCLC, where it will go up against pembrolizumab as well as, potentially, tislelizumab (BeiGene).
The study was sponsored by Innovent Biologics and Eli Lilly. Dr. Zhang disclosed research grants from Eli Lilly and Pfizer. Dr. Nagasaka disclosed serving on the advisory boards of AstraZeneca, Daiichi Sankyo, Takeda, Novartis, and EMD Serono; as a consultant for Caris Life Sciences; and receiving travel support from An Hearts Therapeutics.
This article first appeared on Medscape.com.