User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Radiotherapeutic Care of Patients With Stage IV Lung Cancer with Thoracic Symptoms in the Veterans Health Administration (FULL)
Lung cancer is the leading cause of cancer mortality both in the US and worldwide.1 Many patients diagnosed with lung cancer present with advanced disease with thoracic symptoms such as cough, hemoptysis, dyspnea, and chest pain.2-4 Palliative radiotherapy is routinely used in patients with locally advanced and metastatic lung cancer with the goal of relieving these symptoms and improving quality of life. Guidelines published by the American Society for Radiation Oncology (ASTRO) in 2011, and updated in 2018, provide recommendations on palliation of lung cancer with external beam radiotherapy (EBRT) and clarify the roles of concurrent chemotherapy and endobronchial brachytherapy (EBB) for palliation.5,6
After prostate cancer, lung cancer is the second most frequently diagnosed cancer in the Veterans Health Administration (VHA).7 The VHA consists of 172 medical centers and is the largest integrated health care system in the US. At the time of this study, 40 of these centers had onsite radiation facilities. The VHA Palliative Radiation Taskforce has conducted a series of surveys to evaluate use of palliative radiotherapy in the VHA, determine VHA practice concordance with ASTRO and American College of Radiology (ACR) guidelines, and direct educational efforts towards addressing gaps in knowledge. These efforts are directed at ensuring best practices throughout this large and heterogeneous healthcare system. In 2016 a survey was conducted to evaluate concordance of VHA radiation oncologist (RO) practice with the 2011 ASTRO guidelines on palliative thoracic radiotherapy for non-small cell lung cancer (NSCLC).
Methods
A survey instrument was generated by VHA National Palliative Radiotherapy Taskforce members. It was reviewed and approved for use by the VHA Patient Care Services office. In May of 2016, the online survey was sent to the 88 VHA ROs practicing at the 40 sites with onsite radiation facilities. The survey aimed to determine patterns of practice for palliation of thoracic symptoms secondary to lung cancer.
Demographic information obtained included years in practice, employment status, academic appointment, board certification, and familiarity with ASTRO lung cancer guidelines. Two clinical scenarios were presented to glean opinions on dose/fractionation schemes preferred, use of concurrent chemotherapy, and use of EBB and/or yttrium aluminum garnet (YAG) laser technology. Survey questions also assessed use of EBRT for palliation of hemoptysis, chest wall pain, and/or stridor as well as use of stereotactic body radiotherapy (SBRT) for palliation.
Survey results were assessed for concordance with published ASTRO guidelines. χ2 tests were run to test for associations between demographic factors such as academic appointment, years of practice, full time vs part time employment, and familiarity with ASTRO palliative lung cancer guidelines, with use of EBRT for palliation, dose and fractionation preference, use of concurrent chemotherapy, and strategy for management of endobronchial lesions.
Results
Of the 88 physicians surveyed, 54 responded for a response rate of 61%. Respondents represented 37 of the 40 (93%) VHA radiation oncology departments (Table 1). Among respondents, most were board certified (96%), held academic appointments (91%), and were full-time employees (85%). Forty-four percent of respondents were in practice for > 20 years, 19% for 11 to 20 years, 20% for 6 to 10 years, and 17% for < 6 years. A majority reported familiarity with the ASTRO guidelines (64%), while just 11% reported no familiarity with the guidelines.
When asked about use of SBRT for palliation of hemoptysis, stridor, and/or chest pain, the majority (87%) preferred conventional EBRT. Of the 13% who reported use of SBRT, most (11%) performed it onsite, with 2% of respondents referring offsite to non-VHA centers for the service. When asked about use of EBB for palliation, only 2% reported use of that procedure at their facilities, while 26% reported referral to non-VHA facilities for EBB. The remaining 72% of respondents favor use of conventional EBRT.
Respondents were presented with a case of a male patient aged 70 years who smoked and had widely metastatic NSCLC, a life expectancy of about 3 months, and 10/10 chest wall pain from direct tumor invasion. All respondents recommended palliative radiotherapy. The preferred fractionation was 20 Gray (Gy) in 5 fractions, which was recommended by 69% of respondents. The remainder recommended 30 Gy in 10 fractions (22%) or a single fraction of 10 Gy (9%). No respondent recommended the longer fractionation options of 60 Gy in 30 fractions, 45 Gy in 15 fractions, or 40 Gy in 20 fractions. The majority (98%) did not recommend concurrent chemotherapy.
When the above case was modified for an endobronchial lesion requiring palliation with associated lung collapse, rather than chest wall invasion, 20 respondents (38%) reported they would refer for EBB, and 20 respondents reported they would refer for YAG laser. As > 1 answer could be selected for this question, there were 12 respondents who selected both EBB and YAG laser; 8 selected only EBB, and 8 selected only YAG laser. Many respondents added comments about treating with EBRT, which had not been presented as an answer choice. Nearly half of respondents (49%) were amenable to referral for the use of EBB or YAG laser for lung reexpansion prior to radiotherapy. Three respondents mentioned referral for an endobronchial stent prior to palliative radiotherapy to address this question.
χ2 tests were used to evaluate for significant associations between demographic factors, such as number of years in practice, academic appointment, full-time vs part-time status, and familiarity with ASTRO guidelines with clinical management choices (Table 2). The χ2 analysis revealed that these demographic factors were not significantly associated with familiarity with ASTRO guidelines, offering SBRT for palliation, EBRT fractionation scheme preferred, use of concurrent chemotherapy, or use of EBB or YAG laser.
Discussion
This survey was conducted to evaluate concordance of management of metastatic lung cancer in the VHA with ASTRO guidelines. The relationship between respondents’ familiarity with the guidelines and responses also was evaluated to determine the impact such guidelines have on decision-making. The ASTRO guidelines for palliative thoracic radiation make recommendations regarding 3 issues: (1) radiation doses and fractionations for palliation; (2) the role of EBB; and (3) the use of concurrent chemotherapy.5,6
Radiation Dose and Fractionation for Palliation
A variety of dose/fractionation schemes are considered appropriate in the ASTRO guideline statement, including more prolonged courses such as 30 Gy/10 fractions as well as more hypofractionated regimens (ie, 20 Gy/5 fractions, 17 Gy/2 fractions, and a single fraction of 10 Gy). Higher dose regimens, such as 30 Gy/10 fractions, have been associated with prolonged survival, as well as increased toxicities such as radiation esophagitis.8 Therefore, the guidelines support use of 30 Gy/10 fractions for patients with good performance status while encouraging use of more hypofractionated regimens for patients with poor performance status. In considering more hypofractionated regimens, one must consider the possibility of adverse effects that can be associated with higher dose per fraction. For instance, 17 Gy/2 fractions has been associated with myelopathy; therefore it should be used with caution and careful treatment planning.9
For the survey case example (a male aged 70 years with a 3-month life expectancy who required palliation for chest wall pain), all respondents selected hypofractionated regimens; with no respondent selected the more prolonged fractionations of 60 Gy/30 fractions, 45 Gy/15 fractions, or 40 Gy/20 fractions. These more prolonged fractionations are not endorsed by the guidelines in general, and particularly not for a patient with poor life expectancy. All responses for this case selected by survey respondents are considered appropriate per the consensus guideline statement.
Role of Concurrent Chemotherapy
The ASTRO guidelines do not support use of concurrent chemotherapy for palliation of stage IV NSCLC.5,6 The 2018 updated guidelines established a role for concurrent chemotherapy for patients with stage III NSCLC with good performance status and life expectancy of > 3 months. This updated recommendation is based on data from 2 randomized trials demonstrating improvement in overall survival with the addition of chemotherapy for patients with stage III NSCLC undergoing palliative radiotherapy.10-12
These newer studies are in contrast to an older randomized study by Ball and colleagues that demonstrated greater toxicity from concurrent chemotherapy, with no improvement in outcomes such as palliation of symptoms, overall survival, or progression free survival.13 In contrast to the newer studies that included only patients with stage III NSCLC, about half of the patients in the Ball and colleagues study had known metastatic disease.10-13 Of note, staging for metastatic disease was not carried out routinely, so it is possible that a greater proportion of patients had metastatic disease that would have been seen on imaging. In concordance with the guidelines, 98% of the survey respondents did not recommend concurrent chemotherapy for palliation of intrathoracic symptom; only 1 respondent recommended use of chemotherapy for palliation.
Role of Endobronchial Brachytherapy
EBB involves implantation of radioactive sources for treatment of endobronchial lesions causing obstructive symptoms.14 Given the lack of randomized data that demonstrate a benefit of EBB over EBRT, the ASTRO guidelines do not endorse routine use of EBB for initial palliative management.15,16 The ASTRO guidelines reference a Cochrane Review of 13 trials that concluded that EBRT alone is superior to EBB alone for initial palliation of symptoms from endobronchial NSCLC.17
Of respondents surveyed, only 1 facility offered onsite EBB. The majority of respondents (72%) preferred the use of conventional EBRT techniques, while 26% refer to non-VHA centers for EBB. Lack of incorporation of EBB into routine VHA practice likely is a reflection of the unclear role of this technology based on the available literature and ASTRO guidelines. In the setting of a right lower lung collapse, more respondents (49%) would consider use of EBB or YAG laser technology for lung reexpansion prior to EBRT.
The ASTRO guidelines recommend that initial EBB in conjunction with EBRT be considered based on randomized data demonstrating significant improvement in lung reexpansion and in patient reported dyspnea with addition of EBB to EBRT over EBRT alone.18 However, the guidelines do not mandate the use of EBB in this situation. It is possible that targeted education regarding the role of EBB would improve knowledge of the potential benefit in the setting of lung collapse and increase the percentage of VHA ROs who would recommend this procedure.
Limitations
The study is limited by lack of generalizability of these findings to all ROs in the country. It is also possible that physician responses do not represent practice patterns with complete accuracy. The use of EBB varied among practitioners. Further study of this technology is necessary to clarify its role in the management of endobronchial obstructive symptoms and to determine whether efforts should be made to increase access to EBB within the VHA.
Conclusions
Most of the ROs who responded to our survey were cognizant and compliant with current ASTRO guidelines on management of lung cancer. Furthermore, familiarity with ASTRO guidelines and management choices were not associated with the respondents’ years in practice, academic appointment, full-time vs part-time status, or familiarity with ASTRO guidelines. This study is a nationwide survey of ROs in the VHA system that reflects the radiation-related care received by veterans with metastatic lung cancer. Responses were obtained from 93% of the 40 radiation oncology centers, so it is likely that the survey accurately represents the decision-making process at the majority of centers. It is possible that those who did not respond to the survey do not treat thoracic cases.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 65(2):87-108.
2. Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200.
3. Chute CG, Greenberg ER, Baron J, Korson R, Baker J, Yates J. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107-2111.
4. Hyde L, Hyde Cl. Clinical manifestations of lung cancer. Chest. 1974;65(3):299-306.
5. Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1(2):60-71.
6. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8(4):245-250.
7. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the United States Veterans Affairs (VA) healthcare system. Mil Med. 2012;177(6):693-701.
8. Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001-4011.
9. A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65(6):934-941.
10. Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol. 2010;5(8):1255-1262.
11. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467-1475.
12. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Aasebø U. Poor prognosis patients with inoperable locally advanced NSCLC and large tumors benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol. 2014;9(6):825-833.
13. Ball D, Smith J, Bishop J, et al. A phase III study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75(5):690-697.
14. Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15(1):1-11.
15. Sur R, Ahmed SN, Donde B, Morar R, Mohamed G, Sur M, Pacella JA, Van der Merwe E, Feldman C. Brachytherapy boost vs teletherapy boost in palliation of symptomatic, locally advanced non-small cell lung cancer: preliminary analysis of a randomized prospective study. J Brachytherapy Int. 2001;17(4):309-315.
16. Sur R, Donde B, Mohuiddin M, et al. Randomized prospective study on the role of high dose rate intraluminal brachytherapy (HDRILBT) in palliation of symptoms in advanced non-small cell lung cancer (NSCLC) treated with radiation alone. Int J Radiat Oncol Biol Phys. 2004;60(1):S205.
17. Ung YC, Yu E, Falkson C, et al. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small cell lung cancer: a systematic review. Brachytherapy. 2006;5:189-202.
18. Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol. 2001;58(3):257-268.
Lung cancer is the leading cause of cancer mortality both in the US and worldwide.1 Many patients diagnosed with lung cancer present with advanced disease with thoracic symptoms such as cough, hemoptysis, dyspnea, and chest pain.2-4 Palliative radiotherapy is routinely used in patients with locally advanced and metastatic lung cancer with the goal of relieving these symptoms and improving quality of life. Guidelines published by the American Society for Radiation Oncology (ASTRO) in 2011, and updated in 2018, provide recommendations on palliation of lung cancer with external beam radiotherapy (EBRT) and clarify the roles of concurrent chemotherapy and endobronchial brachytherapy (EBB) for palliation.5,6
After prostate cancer, lung cancer is the second most frequently diagnosed cancer in the Veterans Health Administration (VHA).7 The VHA consists of 172 medical centers and is the largest integrated health care system in the US. At the time of this study, 40 of these centers had onsite radiation facilities. The VHA Palliative Radiation Taskforce has conducted a series of surveys to evaluate use of palliative radiotherapy in the VHA, determine VHA practice concordance with ASTRO and American College of Radiology (ACR) guidelines, and direct educational efforts towards addressing gaps in knowledge. These efforts are directed at ensuring best practices throughout this large and heterogeneous healthcare system. In 2016 a survey was conducted to evaluate concordance of VHA radiation oncologist (RO) practice with the 2011 ASTRO guidelines on palliative thoracic radiotherapy for non-small cell lung cancer (NSCLC).
Methods
A survey instrument was generated by VHA National Palliative Radiotherapy Taskforce members. It was reviewed and approved for use by the VHA Patient Care Services office. In May of 2016, the online survey was sent to the 88 VHA ROs practicing at the 40 sites with onsite radiation facilities. The survey aimed to determine patterns of practice for palliation of thoracic symptoms secondary to lung cancer.
Demographic information obtained included years in practice, employment status, academic appointment, board certification, and familiarity with ASTRO lung cancer guidelines. Two clinical scenarios were presented to glean opinions on dose/fractionation schemes preferred, use of concurrent chemotherapy, and use of EBB and/or yttrium aluminum garnet (YAG) laser technology. Survey questions also assessed use of EBRT for palliation of hemoptysis, chest wall pain, and/or stridor as well as use of stereotactic body radiotherapy (SBRT) for palliation.
Survey results were assessed for concordance with published ASTRO guidelines. χ2 tests were run to test for associations between demographic factors such as academic appointment, years of practice, full time vs part time employment, and familiarity with ASTRO palliative lung cancer guidelines, with use of EBRT for palliation, dose and fractionation preference, use of concurrent chemotherapy, and strategy for management of endobronchial lesions.
Results
Of the 88 physicians surveyed, 54 responded for a response rate of 61%. Respondents represented 37 of the 40 (93%) VHA radiation oncology departments (Table 1). Among respondents, most were board certified (96%), held academic appointments (91%), and were full-time employees (85%). Forty-four percent of respondents were in practice for > 20 years, 19% for 11 to 20 years, 20% for 6 to 10 years, and 17% for < 6 years. A majority reported familiarity with the ASTRO guidelines (64%), while just 11% reported no familiarity with the guidelines.
When asked about use of SBRT for palliation of hemoptysis, stridor, and/or chest pain, the majority (87%) preferred conventional EBRT. Of the 13% who reported use of SBRT, most (11%) performed it onsite, with 2% of respondents referring offsite to non-VHA centers for the service. When asked about use of EBB for palliation, only 2% reported use of that procedure at their facilities, while 26% reported referral to non-VHA facilities for EBB. The remaining 72% of respondents favor use of conventional EBRT.
Respondents were presented with a case of a male patient aged 70 years who smoked and had widely metastatic NSCLC, a life expectancy of about 3 months, and 10/10 chest wall pain from direct tumor invasion. All respondents recommended palliative radiotherapy. The preferred fractionation was 20 Gray (Gy) in 5 fractions, which was recommended by 69% of respondents. The remainder recommended 30 Gy in 10 fractions (22%) or a single fraction of 10 Gy (9%). No respondent recommended the longer fractionation options of 60 Gy in 30 fractions, 45 Gy in 15 fractions, or 40 Gy in 20 fractions. The majority (98%) did not recommend concurrent chemotherapy.
When the above case was modified for an endobronchial lesion requiring palliation with associated lung collapse, rather than chest wall invasion, 20 respondents (38%) reported they would refer for EBB, and 20 respondents reported they would refer for YAG laser. As > 1 answer could be selected for this question, there were 12 respondents who selected both EBB and YAG laser; 8 selected only EBB, and 8 selected only YAG laser. Many respondents added comments about treating with EBRT, which had not been presented as an answer choice. Nearly half of respondents (49%) were amenable to referral for the use of EBB or YAG laser for lung reexpansion prior to radiotherapy. Three respondents mentioned referral for an endobronchial stent prior to palliative radiotherapy to address this question.
χ2 tests were used to evaluate for significant associations between demographic factors, such as number of years in practice, academic appointment, full-time vs part-time status, and familiarity with ASTRO guidelines with clinical management choices (Table 2). The χ2 analysis revealed that these demographic factors were not significantly associated with familiarity with ASTRO guidelines, offering SBRT for palliation, EBRT fractionation scheme preferred, use of concurrent chemotherapy, or use of EBB or YAG laser.
Discussion
This survey was conducted to evaluate concordance of management of metastatic lung cancer in the VHA with ASTRO guidelines. The relationship between respondents’ familiarity with the guidelines and responses also was evaluated to determine the impact such guidelines have on decision-making. The ASTRO guidelines for palliative thoracic radiation make recommendations regarding 3 issues: (1) radiation doses and fractionations for palliation; (2) the role of EBB; and (3) the use of concurrent chemotherapy.5,6
Radiation Dose and Fractionation for Palliation
A variety of dose/fractionation schemes are considered appropriate in the ASTRO guideline statement, including more prolonged courses such as 30 Gy/10 fractions as well as more hypofractionated regimens (ie, 20 Gy/5 fractions, 17 Gy/2 fractions, and a single fraction of 10 Gy). Higher dose regimens, such as 30 Gy/10 fractions, have been associated with prolonged survival, as well as increased toxicities such as radiation esophagitis.8 Therefore, the guidelines support use of 30 Gy/10 fractions for patients with good performance status while encouraging use of more hypofractionated regimens for patients with poor performance status. In considering more hypofractionated regimens, one must consider the possibility of adverse effects that can be associated with higher dose per fraction. For instance, 17 Gy/2 fractions has been associated with myelopathy; therefore it should be used with caution and careful treatment planning.9
For the survey case example (a male aged 70 years with a 3-month life expectancy who required palliation for chest wall pain), all respondents selected hypofractionated regimens; with no respondent selected the more prolonged fractionations of 60 Gy/30 fractions, 45 Gy/15 fractions, or 40 Gy/20 fractions. These more prolonged fractionations are not endorsed by the guidelines in general, and particularly not for a patient with poor life expectancy. All responses for this case selected by survey respondents are considered appropriate per the consensus guideline statement.
Role of Concurrent Chemotherapy
The ASTRO guidelines do not support use of concurrent chemotherapy for palliation of stage IV NSCLC.5,6 The 2018 updated guidelines established a role for concurrent chemotherapy for patients with stage III NSCLC with good performance status and life expectancy of > 3 months. This updated recommendation is based on data from 2 randomized trials demonstrating improvement in overall survival with the addition of chemotherapy for patients with stage III NSCLC undergoing palliative radiotherapy.10-12
These newer studies are in contrast to an older randomized study by Ball and colleagues that demonstrated greater toxicity from concurrent chemotherapy, with no improvement in outcomes such as palliation of symptoms, overall survival, or progression free survival.13 In contrast to the newer studies that included only patients with stage III NSCLC, about half of the patients in the Ball and colleagues study had known metastatic disease.10-13 Of note, staging for metastatic disease was not carried out routinely, so it is possible that a greater proportion of patients had metastatic disease that would have been seen on imaging. In concordance with the guidelines, 98% of the survey respondents did not recommend concurrent chemotherapy for palliation of intrathoracic symptom; only 1 respondent recommended use of chemotherapy for palliation.
Role of Endobronchial Brachytherapy
EBB involves implantation of radioactive sources for treatment of endobronchial lesions causing obstructive symptoms.14 Given the lack of randomized data that demonstrate a benefit of EBB over EBRT, the ASTRO guidelines do not endorse routine use of EBB for initial palliative management.15,16 The ASTRO guidelines reference a Cochrane Review of 13 trials that concluded that EBRT alone is superior to EBB alone for initial palliation of symptoms from endobronchial NSCLC.17
Of respondents surveyed, only 1 facility offered onsite EBB. The majority of respondents (72%) preferred the use of conventional EBRT techniques, while 26% refer to non-VHA centers for EBB. Lack of incorporation of EBB into routine VHA practice likely is a reflection of the unclear role of this technology based on the available literature and ASTRO guidelines. In the setting of a right lower lung collapse, more respondents (49%) would consider use of EBB or YAG laser technology for lung reexpansion prior to EBRT.
The ASTRO guidelines recommend that initial EBB in conjunction with EBRT be considered based on randomized data demonstrating significant improvement in lung reexpansion and in patient reported dyspnea with addition of EBB to EBRT over EBRT alone.18 However, the guidelines do not mandate the use of EBB in this situation. It is possible that targeted education regarding the role of EBB would improve knowledge of the potential benefit in the setting of lung collapse and increase the percentage of VHA ROs who would recommend this procedure.
Limitations
The study is limited by lack of generalizability of these findings to all ROs in the country. It is also possible that physician responses do not represent practice patterns with complete accuracy. The use of EBB varied among practitioners. Further study of this technology is necessary to clarify its role in the management of endobronchial obstructive symptoms and to determine whether efforts should be made to increase access to EBB within the VHA.
Conclusions
Most of the ROs who responded to our survey were cognizant and compliant with current ASTRO guidelines on management of lung cancer. Furthermore, familiarity with ASTRO guidelines and management choices were not associated with the respondents’ years in practice, academic appointment, full-time vs part-time status, or familiarity with ASTRO guidelines. This study is a nationwide survey of ROs in the VHA system that reflects the radiation-related care received by veterans with metastatic lung cancer. Responses were obtained from 93% of the 40 radiation oncology centers, so it is likely that the survey accurately represents the decision-making process at the majority of centers. It is possible that those who did not respond to the survey do not treat thoracic cases.
Lung cancer is the leading cause of cancer mortality both in the US and worldwide.1 Many patients diagnosed with lung cancer present with advanced disease with thoracic symptoms such as cough, hemoptysis, dyspnea, and chest pain.2-4 Palliative radiotherapy is routinely used in patients with locally advanced and metastatic lung cancer with the goal of relieving these symptoms and improving quality of life. Guidelines published by the American Society for Radiation Oncology (ASTRO) in 2011, and updated in 2018, provide recommendations on palliation of lung cancer with external beam radiotherapy (EBRT) and clarify the roles of concurrent chemotherapy and endobronchial brachytherapy (EBB) for palliation.5,6
After prostate cancer, lung cancer is the second most frequently diagnosed cancer in the Veterans Health Administration (VHA).7 The VHA consists of 172 medical centers and is the largest integrated health care system in the US. At the time of this study, 40 of these centers had onsite radiation facilities. The VHA Palliative Radiation Taskforce has conducted a series of surveys to evaluate use of palliative radiotherapy in the VHA, determine VHA practice concordance with ASTRO and American College of Radiology (ACR) guidelines, and direct educational efforts towards addressing gaps in knowledge. These efforts are directed at ensuring best practices throughout this large and heterogeneous healthcare system. In 2016 a survey was conducted to evaluate concordance of VHA radiation oncologist (RO) practice with the 2011 ASTRO guidelines on palliative thoracic radiotherapy for non-small cell lung cancer (NSCLC).
Methods
A survey instrument was generated by VHA National Palliative Radiotherapy Taskforce members. It was reviewed and approved for use by the VHA Patient Care Services office. In May of 2016, the online survey was sent to the 88 VHA ROs practicing at the 40 sites with onsite radiation facilities. The survey aimed to determine patterns of practice for palliation of thoracic symptoms secondary to lung cancer.
Demographic information obtained included years in practice, employment status, academic appointment, board certification, and familiarity with ASTRO lung cancer guidelines. Two clinical scenarios were presented to glean opinions on dose/fractionation schemes preferred, use of concurrent chemotherapy, and use of EBB and/or yttrium aluminum garnet (YAG) laser technology. Survey questions also assessed use of EBRT for palliation of hemoptysis, chest wall pain, and/or stridor as well as use of stereotactic body radiotherapy (SBRT) for palliation.
Survey results were assessed for concordance with published ASTRO guidelines. χ2 tests were run to test for associations between demographic factors such as academic appointment, years of practice, full time vs part time employment, and familiarity with ASTRO palliative lung cancer guidelines, with use of EBRT for palliation, dose and fractionation preference, use of concurrent chemotherapy, and strategy for management of endobronchial lesions.
Results
Of the 88 physicians surveyed, 54 responded for a response rate of 61%. Respondents represented 37 of the 40 (93%) VHA radiation oncology departments (Table 1). Among respondents, most were board certified (96%), held academic appointments (91%), and were full-time employees (85%). Forty-four percent of respondents were in practice for > 20 years, 19% for 11 to 20 years, 20% for 6 to 10 years, and 17% for < 6 years. A majority reported familiarity with the ASTRO guidelines (64%), while just 11% reported no familiarity with the guidelines.
When asked about use of SBRT for palliation of hemoptysis, stridor, and/or chest pain, the majority (87%) preferred conventional EBRT. Of the 13% who reported use of SBRT, most (11%) performed it onsite, with 2% of respondents referring offsite to non-VHA centers for the service. When asked about use of EBB for palliation, only 2% reported use of that procedure at their facilities, while 26% reported referral to non-VHA facilities for EBB. The remaining 72% of respondents favor use of conventional EBRT.
Respondents were presented with a case of a male patient aged 70 years who smoked and had widely metastatic NSCLC, a life expectancy of about 3 months, and 10/10 chest wall pain from direct tumor invasion. All respondents recommended palliative radiotherapy. The preferred fractionation was 20 Gray (Gy) in 5 fractions, which was recommended by 69% of respondents. The remainder recommended 30 Gy in 10 fractions (22%) or a single fraction of 10 Gy (9%). No respondent recommended the longer fractionation options of 60 Gy in 30 fractions, 45 Gy in 15 fractions, or 40 Gy in 20 fractions. The majority (98%) did not recommend concurrent chemotherapy.
When the above case was modified for an endobronchial lesion requiring palliation with associated lung collapse, rather than chest wall invasion, 20 respondents (38%) reported they would refer for EBB, and 20 respondents reported they would refer for YAG laser. As > 1 answer could be selected for this question, there were 12 respondents who selected both EBB and YAG laser; 8 selected only EBB, and 8 selected only YAG laser. Many respondents added comments about treating with EBRT, which had not been presented as an answer choice. Nearly half of respondents (49%) were amenable to referral for the use of EBB or YAG laser for lung reexpansion prior to radiotherapy. Three respondents mentioned referral for an endobronchial stent prior to palliative radiotherapy to address this question.
χ2 tests were used to evaluate for significant associations between demographic factors, such as number of years in practice, academic appointment, full-time vs part-time status, and familiarity with ASTRO guidelines with clinical management choices (Table 2). The χ2 analysis revealed that these demographic factors were not significantly associated with familiarity with ASTRO guidelines, offering SBRT for palliation, EBRT fractionation scheme preferred, use of concurrent chemotherapy, or use of EBB or YAG laser.
Discussion
This survey was conducted to evaluate concordance of management of metastatic lung cancer in the VHA with ASTRO guidelines. The relationship between respondents’ familiarity with the guidelines and responses also was evaluated to determine the impact such guidelines have on decision-making. The ASTRO guidelines for palliative thoracic radiation make recommendations regarding 3 issues: (1) radiation doses and fractionations for palliation; (2) the role of EBB; and (3) the use of concurrent chemotherapy.5,6
Radiation Dose and Fractionation for Palliation
A variety of dose/fractionation schemes are considered appropriate in the ASTRO guideline statement, including more prolonged courses such as 30 Gy/10 fractions as well as more hypofractionated regimens (ie, 20 Gy/5 fractions, 17 Gy/2 fractions, and a single fraction of 10 Gy). Higher dose regimens, such as 30 Gy/10 fractions, have been associated with prolonged survival, as well as increased toxicities such as radiation esophagitis.8 Therefore, the guidelines support use of 30 Gy/10 fractions for patients with good performance status while encouraging use of more hypofractionated regimens for patients with poor performance status. In considering more hypofractionated regimens, one must consider the possibility of adverse effects that can be associated with higher dose per fraction. For instance, 17 Gy/2 fractions has been associated with myelopathy; therefore it should be used with caution and careful treatment planning.9
For the survey case example (a male aged 70 years with a 3-month life expectancy who required palliation for chest wall pain), all respondents selected hypofractionated regimens; with no respondent selected the more prolonged fractionations of 60 Gy/30 fractions, 45 Gy/15 fractions, or 40 Gy/20 fractions. These more prolonged fractionations are not endorsed by the guidelines in general, and particularly not for a patient with poor life expectancy. All responses for this case selected by survey respondents are considered appropriate per the consensus guideline statement.
Role of Concurrent Chemotherapy
The ASTRO guidelines do not support use of concurrent chemotherapy for palliation of stage IV NSCLC.5,6 The 2018 updated guidelines established a role for concurrent chemotherapy for patients with stage III NSCLC with good performance status and life expectancy of > 3 months. This updated recommendation is based on data from 2 randomized trials demonstrating improvement in overall survival with the addition of chemotherapy for patients with stage III NSCLC undergoing palliative radiotherapy.10-12
These newer studies are in contrast to an older randomized study by Ball and colleagues that demonstrated greater toxicity from concurrent chemotherapy, with no improvement in outcomes such as palliation of symptoms, overall survival, or progression free survival.13 In contrast to the newer studies that included only patients with stage III NSCLC, about half of the patients in the Ball and colleagues study had known metastatic disease.10-13 Of note, staging for metastatic disease was not carried out routinely, so it is possible that a greater proportion of patients had metastatic disease that would have been seen on imaging. In concordance with the guidelines, 98% of the survey respondents did not recommend concurrent chemotherapy for palliation of intrathoracic symptom; only 1 respondent recommended use of chemotherapy for palliation.
Role of Endobronchial Brachytherapy
EBB involves implantation of radioactive sources for treatment of endobronchial lesions causing obstructive symptoms.14 Given the lack of randomized data that demonstrate a benefit of EBB over EBRT, the ASTRO guidelines do not endorse routine use of EBB for initial palliative management.15,16 The ASTRO guidelines reference a Cochrane Review of 13 trials that concluded that EBRT alone is superior to EBB alone for initial palliation of symptoms from endobronchial NSCLC.17
Of respondents surveyed, only 1 facility offered onsite EBB. The majority of respondents (72%) preferred the use of conventional EBRT techniques, while 26% refer to non-VHA centers for EBB. Lack of incorporation of EBB into routine VHA practice likely is a reflection of the unclear role of this technology based on the available literature and ASTRO guidelines. In the setting of a right lower lung collapse, more respondents (49%) would consider use of EBB or YAG laser technology for lung reexpansion prior to EBRT.
The ASTRO guidelines recommend that initial EBB in conjunction with EBRT be considered based on randomized data demonstrating significant improvement in lung reexpansion and in patient reported dyspnea with addition of EBB to EBRT over EBRT alone.18 However, the guidelines do not mandate the use of EBB in this situation. It is possible that targeted education regarding the role of EBB would improve knowledge of the potential benefit in the setting of lung collapse and increase the percentage of VHA ROs who would recommend this procedure.
Limitations
The study is limited by lack of generalizability of these findings to all ROs in the country. It is also possible that physician responses do not represent practice patterns with complete accuracy. The use of EBB varied among practitioners. Further study of this technology is necessary to clarify its role in the management of endobronchial obstructive symptoms and to determine whether efforts should be made to increase access to EBB within the VHA.
Conclusions
Most of the ROs who responded to our survey were cognizant and compliant with current ASTRO guidelines on management of lung cancer. Furthermore, familiarity with ASTRO guidelines and management choices were not associated with the respondents’ years in practice, academic appointment, full-time vs part-time status, or familiarity with ASTRO guidelines. This study is a nationwide survey of ROs in the VHA system that reflects the radiation-related care received by veterans with metastatic lung cancer. Responses were obtained from 93% of the 40 radiation oncology centers, so it is likely that the survey accurately represents the decision-making process at the majority of centers. It is possible that those who did not respond to the survey do not treat thoracic cases.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 65(2):87-108.
2. Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200.
3. Chute CG, Greenberg ER, Baron J, Korson R, Baker J, Yates J. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107-2111.
4. Hyde L, Hyde Cl. Clinical manifestations of lung cancer. Chest. 1974;65(3):299-306.
5. Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1(2):60-71.
6. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8(4):245-250.
7. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the United States Veterans Affairs (VA) healthcare system. Mil Med. 2012;177(6):693-701.
8. Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001-4011.
9. A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65(6):934-941.
10. Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol. 2010;5(8):1255-1262.
11. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467-1475.
12. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Aasebø U. Poor prognosis patients with inoperable locally advanced NSCLC and large tumors benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol. 2014;9(6):825-833.
13. Ball D, Smith J, Bishop J, et al. A phase III study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75(5):690-697.
14. Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15(1):1-11.
15. Sur R, Ahmed SN, Donde B, Morar R, Mohamed G, Sur M, Pacella JA, Van der Merwe E, Feldman C. Brachytherapy boost vs teletherapy boost in palliation of symptomatic, locally advanced non-small cell lung cancer: preliminary analysis of a randomized prospective study. J Brachytherapy Int. 2001;17(4):309-315.
16. Sur R, Donde B, Mohuiddin M, et al. Randomized prospective study on the role of high dose rate intraluminal brachytherapy (HDRILBT) in palliation of symptoms in advanced non-small cell lung cancer (NSCLC) treated with radiation alone. Int J Radiat Oncol Biol Phys. 2004;60(1):S205.
17. Ung YC, Yu E, Falkson C, et al. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small cell lung cancer: a systematic review. Brachytherapy. 2006;5:189-202.
18. Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol. 2001;58(3):257-268.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 65(2):87-108.
2. Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200.
3. Chute CG, Greenberg ER, Baron J, Korson R, Baker J, Yates J. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107-2111.
4. Hyde L, Hyde Cl. Clinical manifestations of lung cancer. Chest. 1974;65(3):299-306.
5. Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1(2):60-71.
6. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8(4):245-250.
7. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the United States Veterans Affairs (VA) healthcare system. Mil Med. 2012;177(6):693-701.
8. Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001-4011.
9. A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65(6):934-941.
10. Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol. 2010;5(8):1255-1262.
11. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467-1475.
12. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Aasebø U. Poor prognosis patients with inoperable locally advanced NSCLC and large tumors benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol. 2014;9(6):825-833.
13. Ball D, Smith J, Bishop J, et al. A phase III study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75(5):690-697.
14. Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15(1):1-11.
15. Sur R, Ahmed SN, Donde B, Morar R, Mohamed G, Sur M, Pacella JA, Van der Merwe E, Feldman C. Brachytherapy boost vs teletherapy boost in palliation of symptomatic, locally advanced non-small cell lung cancer: preliminary analysis of a randomized prospective study. J Brachytherapy Int. 2001;17(4):309-315.
16. Sur R, Donde B, Mohuiddin M, et al. Randomized prospective study on the role of high dose rate intraluminal brachytherapy (HDRILBT) in palliation of symptoms in advanced non-small cell lung cancer (NSCLC) treated with radiation alone. Int J Radiat Oncol Biol Phys. 2004;60(1):S205.
17. Ung YC, Yu E, Falkson C, et al. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small cell lung cancer: a systematic review. Brachytherapy. 2006;5:189-202.
18. Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol. 2001;58(3):257-268.
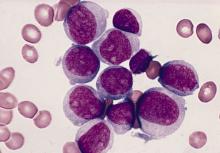
A Pivotal Moment in Cancer Surgery, Captured on Film
Few ever see this side of cancer care. Our cameras go behind the scenes as a surgical oncologist faces a crucial moment in the OR in this first episode of a new video series, The Oncologists.
Medscape Oncology © 2021 WebMD, LLC
Any views expressed above are the author's own and do not necessarily reflect the views of WebMD or Medscape.
Cite this: A Pivotal Moment in Cancer Surgery, Captured on Film - Medscape - Feb 18, 2021.
Few ever see this side of cancer care. Our cameras go behind the scenes as a surgical oncologist faces a crucial moment in the OR in this first episode of a new video series, The Oncologists.
Medscape Oncology © 2021 WebMD, LLC
Any views expressed above are the author's own and do not necessarily reflect the views of WebMD or Medscape.
Cite this: A Pivotal Moment in Cancer Surgery, Captured on Film - Medscape - Feb 18, 2021.
Few ever see this side of cancer care. Our cameras go behind the scenes as a surgical oncologist faces a crucial moment in the OR in this first episode of a new video series, The Oncologists.
Medscape Oncology © 2021 WebMD, LLC
Any views expressed above are the author's own and do not necessarily reflect the views of WebMD or Medscape.
Cite this: A Pivotal Moment in Cancer Surgery, Captured on Film - Medscape - Feb 18, 2021.
Superior survival with sintilimab in squamous NSCLC
Sintilimab improved both overall survival (OS) and progression-free survival (PFS), according to Yuankai Shi, MD, of the Chinese Academy of Medical Sciences & Peking Union Medical College in Beijing.
Dr. Shi presented these findings, from the ORIENT-3 study, at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT041).
ORIENT-3 enrolled and randomized 290 patients with stage IIIB/IIIC or IV sqNSCLC and disease progression during or after first-line platimum-based chemotherapy. They were randomized 1:1 to receive sintilimab at 200 mg or docetaxel at 75 mg/m2intravenously every 3 weeks until disease progression or intolerable toxicity.
The median age was 60 years in the sintilimab arm and 61 years in the docetaxel arm. A majority of patients were men (94% in the sintilimab arm and 90% in the docetaxel arm), most were current or former smokers (90% and 80%, respectively), and more than three-quarters had an ECOG performance status of 1 (76% and 77%, respectively). More than half of patients had a PD-L1 tumor proportion score (TPS) of 1% or greater (57% and 47%, respectively), and 81% of patients in both arms had stage IV disease.
Results: Survival and safety
Patients in the sintilimab arm received a median of 8.0 cycles of therapy (range, 1-45), and those in the docetaxel arm received a median of 2.0 cycles of therapy (range, 1-15).
At a median follow-up of 23.56 months, the median OS was significantly longer in the sintilimab arm than in the docetaxel arm – 11.79 months and 8.25 months, respectively (hazard ratio, 0.74; P = .02489). OS benefits were generally consistent across subgroups.
The secondary endpoints of PFS and objective response rate also favored sintilimab, Dr. Shi reported.
The median PFS was 4.30 months in the sintilimab arm and 2.79 months in the docetaxel arm (HR, 0.52; P < .00001). Confirmed objective response rates were 25.5% and 2.2%, respectively; the median duration of response was 12.45 months and 4.14 months, respectively; and disease control rates were 65.5% and 37.8%, respectively.
“Sintilimab had a favorable safety profile over docetaxel, with a lower frequency of grade 3 or higher treatment-related adverse events, with no new safety signals observed,” Dr. Shi said.
Treatment-related adverse events (TRAEs) occurred in 84.7% of patients receiving sintilimab and 83.1% of those receiving docetaxel. Hypothyroidism was the most common TRAE in the sintilimab arm (18.1%), and alopecia was the most common TRAE in the docetaxel arm (34.6%).
Grade 3 or higher TRAEs were less frequent in the sintilimab arm than in the docetaxel arm (18.1% vs. 36.2%). Rates of discontinuation because of TRAEs were 12.5% and 5.4% in the sintilimab and docetaxel arms, respectively. TRAEs leading to death occurred in five patients in the sintilimab arm and one in the docetaxel arm.
Use in the real world
Noting sintilimab’s significant OS and PFS benefits as well as superior response rate and duration of response, Dr. Shi concluded, “Sintilimab might provide an alternative second-line treatment option for advanced and metastatic sqNSCLC.”
AACR moderator Marina Garassino, MD, of the University of Chicago, commented on the potential utility of sintilimab and tislelizumab, another checkpoint inhibitor that was evaluated in NSCLC in the RATIONALE 303 trial (AACR 2021, Abstract CT039). Dr. Garassino observed that both drugs have demonstrated superiority to docetaxel as second-line therapy in NSCLC.
Although there have been no head-to-head trials, sintilimab and tislelizumab appear to be very similar to the already approved immune checkpoint inhibitors, which are currently being used as first-line treatment.
“That similarity would make them inappropriate for second-line treatment, except in countries where immune checkpoint inhibitors are not yet approved for first-line therapy,” Dr. Garassino noted.
When asked to comment on the higher treatment-related death rate observed with sintilimab, Dr. Garassino said, “We need to remember that these drugs were developed in China with a population that may have a side effect profile differing from that of a Western population. Also, we are very familiar with this class of drugs and know how to treat their side effects. Similar drugs but different populations and different trials, so it’s very hard to judge.”
Dr. Garassino speculated that with the “super expensive” price tags on the new checkpoint inhibitors, having additional agents that could provide choice and drive prices down would be welcome.
ORIENT-3 was funded by Innovent Biologics and Eli Lilly. Dr. Shi disclosed consultancy for Innovent Biologics. Dr. Garassino disclosed relationships with Eli Lilly, AstraZeneca, Novartis, and several other companies, not including Innovent Biologics.
Sintilimab improved both overall survival (OS) and progression-free survival (PFS), according to Yuankai Shi, MD, of the Chinese Academy of Medical Sciences & Peking Union Medical College in Beijing.
Dr. Shi presented these findings, from the ORIENT-3 study, at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT041).
ORIENT-3 enrolled and randomized 290 patients with stage IIIB/IIIC or IV sqNSCLC and disease progression during or after first-line platimum-based chemotherapy. They were randomized 1:1 to receive sintilimab at 200 mg or docetaxel at 75 mg/m2intravenously every 3 weeks until disease progression or intolerable toxicity.
The median age was 60 years in the sintilimab arm and 61 years in the docetaxel arm. A majority of patients were men (94% in the sintilimab arm and 90% in the docetaxel arm), most were current or former smokers (90% and 80%, respectively), and more than three-quarters had an ECOG performance status of 1 (76% and 77%, respectively). More than half of patients had a PD-L1 tumor proportion score (TPS) of 1% or greater (57% and 47%, respectively), and 81% of patients in both arms had stage IV disease.
Results: Survival and safety
Patients in the sintilimab arm received a median of 8.0 cycles of therapy (range, 1-45), and those in the docetaxel arm received a median of 2.0 cycles of therapy (range, 1-15).
At a median follow-up of 23.56 months, the median OS was significantly longer in the sintilimab arm than in the docetaxel arm – 11.79 months and 8.25 months, respectively (hazard ratio, 0.74; P = .02489). OS benefits were generally consistent across subgroups.
The secondary endpoints of PFS and objective response rate also favored sintilimab, Dr. Shi reported.
The median PFS was 4.30 months in the sintilimab arm and 2.79 months in the docetaxel arm (HR, 0.52; P < .00001). Confirmed objective response rates were 25.5% and 2.2%, respectively; the median duration of response was 12.45 months and 4.14 months, respectively; and disease control rates were 65.5% and 37.8%, respectively.
“Sintilimab had a favorable safety profile over docetaxel, with a lower frequency of grade 3 or higher treatment-related adverse events, with no new safety signals observed,” Dr. Shi said.
Treatment-related adverse events (TRAEs) occurred in 84.7% of patients receiving sintilimab and 83.1% of those receiving docetaxel. Hypothyroidism was the most common TRAE in the sintilimab arm (18.1%), and alopecia was the most common TRAE in the docetaxel arm (34.6%).
Grade 3 or higher TRAEs were less frequent in the sintilimab arm than in the docetaxel arm (18.1% vs. 36.2%). Rates of discontinuation because of TRAEs were 12.5% and 5.4% in the sintilimab and docetaxel arms, respectively. TRAEs leading to death occurred in five patients in the sintilimab arm and one in the docetaxel arm.
Use in the real world
Noting sintilimab’s significant OS and PFS benefits as well as superior response rate and duration of response, Dr. Shi concluded, “Sintilimab might provide an alternative second-line treatment option for advanced and metastatic sqNSCLC.”
AACR moderator Marina Garassino, MD, of the University of Chicago, commented on the potential utility of sintilimab and tislelizumab, another checkpoint inhibitor that was evaluated in NSCLC in the RATIONALE 303 trial (AACR 2021, Abstract CT039). Dr. Garassino observed that both drugs have demonstrated superiority to docetaxel as second-line therapy in NSCLC.
Although there have been no head-to-head trials, sintilimab and tislelizumab appear to be very similar to the already approved immune checkpoint inhibitors, which are currently being used as first-line treatment.
“That similarity would make them inappropriate for second-line treatment, except in countries where immune checkpoint inhibitors are not yet approved for first-line therapy,” Dr. Garassino noted.
When asked to comment on the higher treatment-related death rate observed with sintilimab, Dr. Garassino said, “We need to remember that these drugs were developed in China with a population that may have a side effect profile differing from that of a Western population. Also, we are very familiar with this class of drugs and know how to treat their side effects. Similar drugs but different populations and different trials, so it’s very hard to judge.”
Dr. Garassino speculated that with the “super expensive” price tags on the new checkpoint inhibitors, having additional agents that could provide choice and drive prices down would be welcome.
ORIENT-3 was funded by Innovent Biologics and Eli Lilly. Dr. Shi disclosed consultancy for Innovent Biologics. Dr. Garassino disclosed relationships with Eli Lilly, AstraZeneca, Novartis, and several other companies, not including Innovent Biologics.
Sintilimab improved both overall survival (OS) and progression-free survival (PFS), according to Yuankai Shi, MD, of the Chinese Academy of Medical Sciences & Peking Union Medical College in Beijing.
Dr. Shi presented these findings, from the ORIENT-3 study, at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT041).
ORIENT-3 enrolled and randomized 290 patients with stage IIIB/IIIC or IV sqNSCLC and disease progression during or after first-line platimum-based chemotherapy. They were randomized 1:1 to receive sintilimab at 200 mg or docetaxel at 75 mg/m2intravenously every 3 weeks until disease progression or intolerable toxicity.
The median age was 60 years in the sintilimab arm and 61 years in the docetaxel arm. A majority of patients were men (94% in the sintilimab arm and 90% in the docetaxel arm), most were current or former smokers (90% and 80%, respectively), and more than three-quarters had an ECOG performance status of 1 (76% and 77%, respectively). More than half of patients had a PD-L1 tumor proportion score (TPS) of 1% or greater (57% and 47%, respectively), and 81% of patients in both arms had stage IV disease.
Results: Survival and safety
Patients in the sintilimab arm received a median of 8.0 cycles of therapy (range, 1-45), and those in the docetaxel arm received a median of 2.0 cycles of therapy (range, 1-15).
At a median follow-up of 23.56 months, the median OS was significantly longer in the sintilimab arm than in the docetaxel arm – 11.79 months and 8.25 months, respectively (hazard ratio, 0.74; P = .02489). OS benefits were generally consistent across subgroups.
The secondary endpoints of PFS and objective response rate also favored sintilimab, Dr. Shi reported.
The median PFS was 4.30 months in the sintilimab arm and 2.79 months in the docetaxel arm (HR, 0.52; P < .00001). Confirmed objective response rates were 25.5% and 2.2%, respectively; the median duration of response was 12.45 months and 4.14 months, respectively; and disease control rates were 65.5% and 37.8%, respectively.
“Sintilimab had a favorable safety profile over docetaxel, with a lower frequency of grade 3 or higher treatment-related adverse events, with no new safety signals observed,” Dr. Shi said.
Treatment-related adverse events (TRAEs) occurred in 84.7% of patients receiving sintilimab and 83.1% of those receiving docetaxel. Hypothyroidism was the most common TRAE in the sintilimab arm (18.1%), and alopecia was the most common TRAE in the docetaxel arm (34.6%).
Grade 3 or higher TRAEs were less frequent in the sintilimab arm than in the docetaxel arm (18.1% vs. 36.2%). Rates of discontinuation because of TRAEs were 12.5% and 5.4% in the sintilimab and docetaxel arms, respectively. TRAEs leading to death occurred in five patients in the sintilimab arm and one in the docetaxel arm.
Use in the real world
Noting sintilimab’s significant OS and PFS benefits as well as superior response rate and duration of response, Dr. Shi concluded, “Sintilimab might provide an alternative second-line treatment option for advanced and metastatic sqNSCLC.”
AACR moderator Marina Garassino, MD, of the University of Chicago, commented on the potential utility of sintilimab and tislelizumab, another checkpoint inhibitor that was evaluated in NSCLC in the RATIONALE 303 trial (AACR 2021, Abstract CT039). Dr. Garassino observed that both drugs have demonstrated superiority to docetaxel as second-line therapy in NSCLC.
Although there have been no head-to-head trials, sintilimab and tislelizumab appear to be very similar to the already approved immune checkpoint inhibitors, which are currently being used as first-line treatment.
“That similarity would make them inappropriate for second-line treatment, except in countries where immune checkpoint inhibitors are not yet approved for first-line therapy,” Dr. Garassino noted.
When asked to comment on the higher treatment-related death rate observed with sintilimab, Dr. Garassino said, “We need to remember that these drugs were developed in China with a population that may have a side effect profile differing from that of a Western population. Also, we are very familiar with this class of drugs and know how to treat their side effects. Similar drugs but different populations and different trials, so it’s very hard to judge.”
Dr. Garassino speculated that with the “super expensive” price tags on the new checkpoint inhibitors, having additional agents that could provide choice and drive prices down would be welcome.
ORIENT-3 was funded by Innovent Biologics and Eli Lilly. Dr. Shi disclosed consultancy for Innovent Biologics. Dr. Garassino disclosed relationships with Eli Lilly, AstraZeneca, Novartis, and several other companies, not including Innovent Biologics.
FROM AACR 2021
To stay: Two more cancer indications with ‘dangling approvals’
Two more cancer indications that had been granted accelerated approval by the Food and Drug Administration are going to stay in place, at least for now. This was the verdict after the second day of a historic 3-day meeting (April 27-29) and follows a similar verdict from day 1.
Federal advisers so far have supported the idea of maintaining conditional approvals of some cancer indications for a number of immune checkpoint inhibitors, despite poor results in studies that were meant to confirm the benefit of these medicines for certain patients.
On the second day (April 28) of the FDA meeting, the Oncologic Drugs Advisory Committee (ODAC) supported the views of pharmaceutical companies in two more cases of what top agency staff call “dangling accelerated approvals.”
ODAC voted 10-1 in favor of maintaining the indication for atezolizumab (Tecentriq) for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial.
ODAC also voted 5-3 that day in favor of maintaining accelerated approval for pembrolizumab (Keytruda) for first-line cisplatin- and carboplatin-ineligible patients with advanced/metastatic urothelial carcinoma.
The FDA often follows the advice of its panels, but it is not bound to do so. If the FDA were to decide to strip the indications in question from these PD-1 medicines, such decisions would not remove these drugs from the market. The three drugs have already been approved for a number of other cancer indications.
Off-label prescribing is not uncommon in oncology, but a loss of an approved indication would affect reimbursement for these medicines, Scot Ebbinghaus, MD, vice president of oncology clinical research at Merck (the manufacturer of pembrolizumab), told ODAC members during a discussion of the possible consequences of removing the indications in question.
“Access to those treatments may end up being substantially limited, and really the best way to ensure that there’s access is to maintain FDA approval,” Dr. Ebbinghaus said.
Another participant at the meeting asked the panel and the FDA to consider the burden on patients in paying for medicines that have not yet proved to be beneficial.
Diana Zuckerman, PhD, of the nonprofit National Center for Health Research, noted that the ODAC panel included physicians who see cancer patients.
“You’re used to trying different types of treatments in hopes that something will work,” she said. “Shouldn’t cancer patients be eligible for free treatment in clinical trials instead of paying for treatment that isn’t proven to work?”
Rapid development of PD-1 drugs
Top officials at the FDA framed the challenges with accelerated approvals for immunotherapy drugs in an April 21 article in The New England Journal of Medicine. Over the course of about 6 years, the FDA approved six of these PD-1 or PD-L1 drugs for more than 75 indications in oncology, wrote Richard Pazdur, MD, and Julia A. Beaver, MD, of the FDA.
“Development of drugs in this class occurred more rapidly than that in any other therapeutic area in history,” they wrote.
In 10 cases, the required follow-up trials did not confirm the expected benefit, and yet marketing authorization for these drugs continued, leading Dr. Pazdur and Dr. Beaver to dub these “dangling” accelerated approvals. Four of these indications were voluntarily withdrawn. For the other six indications, the FDA sought feedback from ODAC during the 3-day meeting. Over the first 2 days of the meeting, ODAC recommended that three of these cancer indications remain. Three more will be considered on the last day of the meeting.
A version of this article first appeared on Medscape.com.
Two more cancer indications that had been granted accelerated approval by the Food and Drug Administration are going to stay in place, at least for now. This was the verdict after the second day of a historic 3-day meeting (April 27-29) and follows a similar verdict from day 1.
Federal advisers so far have supported the idea of maintaining conditional approvals of some cancer indications for a number of immune checkpoint inhibitors, despite poor results in studies that were meant to confirm the benefit of these medicines for certain patients.
On the second day (April 28) of the FDA meeting, the Oncologic Drugs Advisory Committee (ODAC) supported the views of pharmaceutical companies in two more cases of what top agency staff call “dangling accelerated approvals.”
ODAC voted 10-1 in favor of maintaining the indication for atezolizumab (Tecentriq) for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial.
ODAC also voted 5-3 that day in favor of maintaining accelerated approval for pembrolizumab (Keytruda) for first-line cisplatin- and carboplatin-ineligible patients with advanced/metastatic urothelial carcinoma.
The FDA often follows the advice of its panels, but it is not bound to do so. If the FDA were to decide to strip the indications in question from these PD-1 medicines, such decisions would not remove these drugs from the market. The three drugs have already been approved for a number of other cancer indications.
Off-label prescribing is not uncommon in oncology, but a loss of an approved indication would affect reimbursement for these medicines, Scot Ebbinghaus, MD, vice president of oncology clinical research at Merck (the manufacturer of pembrolizumab), told ODAC members during a discussion of the possible consequences of removing the indications in question.
“Access to those treatments may end up being substantially limited, and really the best way to ensure that there’s access is to maintain FDA approval,” Dr. Ebbinghaus said.
Another participant at the meeting asked the panel and the FDA to consider the burden on patients in paying for medicines that have not yet proved to be beneficial.
Diana Zuckerman, PhD, of the nonprofit National Center for Health Research, noted that the ODAC panel included physicians who see cancer patients.
“You’re used to trying different types of treatments in hopes that something will work,” she said. “Shouldn’t cancer patients be eligible for free treatment in clinical trials instead of paying for treatment that isn’t proven to work?”
Rapid development of PD-1 drugs
Top officials at the FDA framed the challenges with accelerated approvals for immunotherapy drugs in an April 21 article in The New England Journal of Medicine. Over the course of about 6 years, the FDA approved six of these PD-1 or PD-L1 drugs for more than 75 indications in oncology, wrote Richard Pazdur, MD, and Julia A. Beaver, MD, of the FDA.
“Development of drugs in this class occurred more rapidly than that in any other therapeutic area in history,” they wrote.
In 10 cases, the required follow-up trials did not confirm the expected benefit, and yet marketing authorization for these drugs continued, leading Dr. Pazdur and Dr. Beaver to dub these “dangling” accelerated approvals. Four of these indications were voluntarily withdrawn. For the other six indications, the FDA sought feedback from ODAC during the 3-day meeting. Over the first 2 days of the meeting, ODAC recommended that three of these cancer indications remain. Three more will be considered on the last day of the meeting.
A version of this article first appeared on Medscape.com.
Two more cancer indications that had been granted accelerated approval by the Food and Drug Administration are going to stay in place, at least for now. This was the verdict after the second day of a historic 3-day meeting (April 27-29) and follows a similar verdict from day 1.
Federal advisers so far have supported the idea of maintaining conditional approvals of some cancer indications for a number of immune checkpoint inhibitors, despite poor results in studies that were meant to confirm the benefit of these medicines for certain patients.
On the second day (April 28) of the FDA meeting, the Oncologic Drugs Advisory Committee (ODAC) supported the views of pharmaceutical companies in two more cases of what top agency staff call “dangling accelerated approvals.”
ODAC voted 10-1 in favor of maintaining the indication for atezolizumab (Tecentriq) for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial.
ODAC also voted 5-3 that day in favor of maintaining accelerated approval for pembrolizumab (Keytruda) for first-line cisplatin- and carboplatin-ineligible patients with advanced/metastatic urothelial carcinoma.
The FDA often follows the advice of its panels, but it is not bound to do so. If the FDA were to decide to strip the indications in question from these PD-1 medicines, such decisions would not remove these drugs from the market. The three drugs have already been approved for a number of other cancer indications.
Off-label prescribing is not uncommon in oncology, but a loss of an approved indication would affect reimbursement for these medicines, Scot Ebbinghaus, MD, vice president of oncology clinical research at Merck (the manufacturer of pembrolizumab), told ODAC members during a discussion of the possible consequences of removing the indications in question.
“Access to those treatments may end up being substantially limited, and really the best way to ensure that there’s access is to maintain FDA approval,” Dr. Ebbinghaus said.
Another participant at the meeting asked the panel and the FDA to consider the burden on patients in paying for medicines that have not yet proved to be beneficial.
Diana Zuckerman, PhD, of the nonprofit National Center for Health Research, noted that the ODAC panel included physicians who see cancer patients.
“You’re used to trying different types of treatments in hopes that something will work,” she said. “Shouldn’t cancer patients be eligible for free treatment in clinical trials instead of paying for treatment that isn’t proven to work?”
Rapid development of PD-1 drugs
Top officials at the FDA framed the challenges with accelerated approvals for immunotherapy drugs in an April 21 article in The New England Journal of Medicine. Over the course of about 6 years, the FDA approved six of these PD-1 or PD-L1 drugs for more than 75 indications in oncology, wrote Richard Pazdur, MD, and Julia A. Beaver, MD, of the FDA.
“Development of drugs in this class occurred more rapidly than that in any other therapeutic area in history,” they wrote.
In 10 cases, the required follow-up trials did not confirm the expected benefit, and yet marketing authorization for these drugs continued, leading Dr. Pazdur and Dr. Beaver to dub these “dangling” accelerated approvals. Four of these indications were voluntarily withdrawn. For the other six indications, the FDA sought feedback from ODAC during the 3-day meeting. Over the first 2 days of the meeting, ODAC recommended that three of these cancer indications remain. Three more will be considered on the last day of the meeting.
A version of this article first appeared on Medscape.com.
Is it time for universal genetic testing in colorectal cancer?
A prospective study of universal genetic testing suggested that one in six colorectal cancer patients have an inherited genetic predisposition to the cancer. More than half of the patients with genetic mutations identified in this study would have been missed if the patients had undergone genetic testing based on current practice. In 11% of patients, the genetic findings led to a change in treatment, including the type of surgery or targeted cancer therapy.
These results were presented at the American College of Medical Genetics and Genomics annual meeting and published in Clinical Gastroenterology and Hepatology.
“This study shows the limitations of relying on current clinical practice guidelines for genetic evaluation, which prioritize age of cancer diagnosis and family cancer history,” said investigator Niloy Jewel Samadder, MD, of the Mayo Clinic in Phoenix.
“We were surprised that more than 50% of the patients with a genetic mutation would not have been identified had we relied on national practice guidelines or only used a small colon cancer-specific gene panel, as is commonly employed in clinical practice. The fact that more than 10% of patients actually had changes in the type of surgery or chemo/immunotherapy they received gives the strongest indicator of how genetics can revolutionize and individualize cancer care,” Dr. Samadder said.
He added that identifying a germline predisposition has multiple values, including understanding the reason patients and their family members develop specific cancers, preventing the development of new cancers in patients and family members by providing targeted prevention and screening for those at genetic risk, and improving survival in patients with a genetic driver of cancer via targeted therapy.
Study details
The researchers used a next-generation sequencing platform with more than 80 genes in colorectal cancer patients (not selected for age or family history) receiving care at Mayo Clinic Cancer Centers between April 1, 2018, and March 31, 2020.
The study included 361 patients with a median age of 57 years. Pathogenic germline variants were identified in 56 patients (15.5%), including 44 patients with moderate and high penetrance cancer susceptibility genes.
Younger age (under 50 years old) was associated with having a germline mutation, but “more importantly, gender, family cancer history, and stage of cancer were not,” Dr. Samadder said.
“The current clinical guidelines rely heavily on these characteristics to determine who should and should not be referred for genetic testing,” he continued. “Our study suggests that even older patients with colorectal cancer have a high rate of having pathogenic germline mutations [12%], so restricting genetic testing to only those under age 50 will miss a substantial portion of patients who might benefit from this test.”
Genetic testing for all
“Our findings support the broad use of genetic testing in all colorectal cancer patients, regardless of age, gender, ethnicity, family cancer history, or stage of cancer,” Dr. Samadder said.
“This study adds to a growing body of literature that provides evidence that cancer susceptibility due to variants in single genes is more common than previously appreciated,” said Marc S. Williams, MD, president of the American College of Medical Genetics and Genomics. “Current testing guidelines seem to be relatively insensitive and miss opportunities for testing of patients.”
Dr. Williams noted that 11% of patients in this study had a change in disease management related to a genetic testing result, which is “a relatively modest impact.”
“Another potential advantage of [universal] testing was the opportunity to test at-risk relatives,” Dr. Williams added. “However, only 16% of eligible individuals pursued testing. This is consistent with other studies and represents an opportunity for testing new ways to improve uptake of cascade screening.”
Dr. Williams said rigorous prospective studies enrolling large numbers of patients from diverse backgrounds are needed to inform the development and updating of genetic testing guidelines.
He disclosed no conflicts of interest. Dr. Samadder disclosed relationships with Janssen Research and Development, Recursion Pharmaceuticals, and Cancer Prevention Pharmaceuticals. The research was funded by the Mayo Clinic, Desert Mountain Members’ CARE Foundation, David and Twila Woods Foundation, and the Gerstner Foundation.
A prospective study of universal genetic testing suggested that one in six colorectal cancer patients have an inherited genetic predisposition to the cancer. More than half of the patients with genetic mutations identified in this study would have been missed if the patients had undergone genetic testing based on current practice. In 11% of patients, the genetic findings led to a change in treatment, including the type of surgery or targeted cancer therapy.
These results were presented at the American College of Medical Genetics and Genomics annual meeting and published in Clinical Gastroenterology and Hepatology.
“This study shows the limitations of relying on current clinical practice guidelines for genetic evaluation, which prioritize age of cancer diagnosis and family cancer history,” said investigator Niloy Jewel Samadder, MD, of the Mayo Clinic in Phoenix.
“We were surprised that more than 50% of the patients with a genetic mutation would not have been identified had we relied on national practice guidelines or only used a small colon cancer-specific gene panel, as is commonly employed in clinical practice. The fact that more than 10% of patients actually had changes in the type of surgery or chemo/immunotherapy they received gives the strongest indicator of how genetics can revolutionize and individualize cancer care,” Dr. Samadder said.
He added that identifying a germline predisposition has multiple values, including understanding the reason patients and their family members develop specific cancers, preventing the development of new cancers in patients and family members by providing targeted prevention and screening for those at genetic risk, and improving survival in patients with a genetic driver of cancer via targeted therapy.
Study details
The researchers used a next-generation sequencing platform with more than 80 genes in colorectal cancer patients (not selected for age or family history) receiving care at Mayo Clinic Cancer Centers between April 1, 2018, and March 31, 2020.
The study included 361 patients with a median age of 57 years. Pathogenic germline variants were identified in 56 patients (15.5%), including 44 patients with moderate and high penetrance cancer susceptibility genes.
Younger age (under 50 years old) was associated with having a germline mutation, but “more importantly, gender, family cancer history, and stage of cancer were not,” Dr. Samadder said.
“The current clinical guidelines rely heavily on these characteristics to determine who should and should not be referred for genetic testing,” he continued. “Our study suggests that even older patients with colorectal cancer have a high rate of having pathogenic germline mutations [12%], so restricting genetic testing to only those under age 50 will miss a substantial portion of patients who might benefit from this test.”
Genetic testing for all
“Our findings support the broad use of genetic testing in all colorectal cancer patients, regardless of age, gender, ethnicity, family cancer history, or stage of cancer,” Dr. Samadder said.
“This study adds to a growing body of literature that provides evidence that cancer susceptibility due to variants in single genes is more common than previously appreciated,” said Marc S. Williams, MD, president of the American College of Medical Genetics and Genomics. “Current testing guidelines seem to be relatively insensitive and miss opportunities for testing of patients.”
Dr. Williams noted that 11% of patients in this study had a change in disease management related to a genetic testing result, which is “a relatively modest impact.”
“Another potential advantage of [universal] testing was the opportunity to test at-risk relatives,” Dr. Williams added. “However, only 16% of eligible individuals pursued testing. This is consistent with other studies and represents an opportunity for testing new ways to improve uptake of cascade screening.”
Dr. Williams said rigorous prospective studies enrolling large numbers of patients from diverse backgrounds are needed to inform the development and updating of genetic testing guidelines.
He disclosed no conflicts of interest. Dr. Samadder disclosed relationships with Janssen Research and Development, Recursion Pharmaceuticals, and Cancer Prevention Pharmaceuticals. The research was funded by the Mayo Clinic, Desert Mountain Members’ CARE Foundation, David and Twila Woods Foundation, and the Gerstner Foundation.
A prospective study of universal genetic testing suggested that one in six colorectal cancer patients have an inherited genetic predisposition to the cancer. More than half of the patients with genetic mutations identified in this study would have been missed if the patients had undergone genetic testing based on current practice. In 11% of patients, the genetic findings led to a change in treatment, including the type of surgery or targeted cancer therapy.
These results were presented at the American College of Medical Genetics and Genomics annual meeting and published in Clinical Gastroenterology and Hepatology.
“This study shows the limitations of relying on current clinical practice guidelines for genetic evaluation, which prioritize age of cancer diagnosis and family cancer history,” said investigator Niloy Jewel Samadder, MD, of the Mayo Clinic in Phoenix.
“We were surprised that more than 50% of the patients with a genetic mutation would not have been identified had we relied on national practice guidelines or only used a small colon cancer-specific gene panel, as is commonly employed in clinical practice. The fact that more than 10% of patients actually had changes in the type of surgery or chemo/immunotherapy they received gives the strongest indicator of how genetics can revolutionize and individualize cancer care,” Dr. Samadder said.
He added that identifying a germline predisposition has multiple values, including understanding the reason patients and their family members develop specific cancers, preventing the development of new cancers in patients and family members by providing targeted prevention and screening for those at genetic risk, and improving survival in patients with a genetic driver of cancer via targeted therapy.
Study details
The researchers used a next-generation sequencing platform with more than 80 genes in colorectal cancer patients (not selected for age or family history) receiving care at Mayo Clinic Cancer Centers between April 1, 2018, and March 31, 2020.
The study included 361 patients with a median age of 57 years. Pathogenic germline variants were identified in 56 patients (15.5%), including 44 patients with moderate and high penetrance cancer susceptibility genes.
Younger age (under 50 years old) was associated with having a germline mutation, but “more importantly, gender, family cancer history, and stage of cancer were not,” Dr. Samadder said.
“The current clinical guidelines rely heavily on these characteristics to determine who should and should not be referred for genetic testing,” he continued. “Our study suggests that even older patients with colorectal cancer have a high rate of having pathogenic germline mutations [12%], so restricting genetic testing to only those under age 50 will miss a substantial portion of patients who might benefit from this test.”
Genetic testing for all
“Our findings support the broad use of genetic testing in all colorectal cancer patients, regardless of age, gender, ethnicity, family cancer history, or stage of cancer,” Dr. Samadder said.
“This study adds to a growing body of literature that provides evidence that cancer susceptibility due to variants in single genes is more common than previously appreciated,” said Marc S. Williams, MD, president of the American College of Medical Genetics and Genomics. “Current testing guidelines seem to be relatively insensitive and miss opportunities for testing of patients.”
Dr. Williams noted that 11% of patients in this study had a change in disease management related to a genetic testing result, which is “a relatively modest impact.”
“Another potential advantage of [universal] testing was the opportunity to test at-risk relatives,” Dr. Williams added. “However, only 16% of eligible individuals pursued testing. This is consistent with other studies and represents an opportunity for testing new ways to improve uptake of cascade screening.”
Dr. Williams said rigorous prospective studies enrolling large numbers of patients from diverse backgrounds are needed to inform the development and updating of genetic testing guidelines.
He disclosed no conflicts of interest. Dr. Samadder disclosed relationships with Janssen Research and Development, Recursion Pharmaceuticals, and Cancer Prevention Pharmaceuticals. The research was funded by the Mayo Clinic, Desert Mountain Members’ CARE Foundation, David and Twila Woods Foundation, and the Gerstner Foundation.
FROM ACMG 2021
Survival benefit with nivolumab extends to 5 years in NSCLC
Across the two studies – CheckMate 017 and 057 – 854 patients were randomized 1:1 following progression on platinum therapy to either nivolumab at 3 mg/kg once every 2 weeks or docetaxel at 75 mg/m2 once every 3 weeks until further progression or unacceptable toxicity. Previously reported overall survival (OS) outcomes favored nivolumab.
At a minimum follow-up of 5.4 years, 50 nivolumab-treated patients and 9 docetaxel-treated patients were still alive.
The 5-year OS rates were 13.4% in the nivolumab arm and 2.6% in the docetaxel arm. The 5-year progression-free survival (PFS) rates were 8% and 0%, respectively.
There were no new safety signals with nivolumab, and there was no evidence of select late-onset grade 3-4 adverse events.
According to the study authors, this analysis is the longest phase 3 follow-up to date of a PD-1 inhibitor in previously treated, advanced NSCLC, and it suggests that “long-term survival beyond 5 years may ... be possible in NSCLC.”
“The results indicate that some patients with NSCLC can have long-lasting benefit from checkpoint inhibitors. We have seen similar results in terms of long-term OS with pembrolizumab,” said investigator Hossein Borghaei, DO, chief of thoracic medical oncology at the Fox Chase Cancer Center in Philadelphia.
Dr. Borghaei said the question now is “how to identify the population that really benefits from these treatments. We think PD-L1–high [patients] have a better chance, [as do patients with] tumors that have a higher percentage of tumor-infiltrating lymphocytes, but there’s nothing concrete beyond that.”
No baseline clinical or tumor factors emerged to distinguish between long-and short-term survivors, but the 5-year OS rate was 18.3% among nivolumab-treated patients with PD-L1 expression at or above 1% versus 8% among patients with expression below 1%.
The optimal duration of nivolumab treatment beyond 1 year is also uncertain.
The median duration of therapy was 36.9 months in the 5-year survivors treated with nivolumab, and 36% of patients (18/50) were still on nivolumab at the 5-year mark.
The median duration of time off treatment was 41.9 months among patients who discontinued nivolumab. Five patients (10%) were off treatment with no subsequent therapy and had not progressed at 5 years, “suggesting benefit even for patients who stopped nivolumab treatment,” the researchers wrote.
They also found that nivolumab-treated patients who remained alive at 3 years appeared to stabilize and plateau thereafter, with early response suggesting better long-term outcomes. The majority of patients without disease progression at 2, 3, and 4 years, for instance, remained progression free at 5 years. Nearly one-third of patients who achieved an objective response with nivolumab – but none of the patients who responded to docetaxel – had ongoing responses at 5 years.
Similarly, nivolumab-treated patients without disease progression at 2 years and 3 years had an 82% and 93% chance of survival, respectively, and a 59.6% and 78.3% chance of remaining progression free at 5 years.
This research was funded by Bristol-Myers Squibb. Dr. Borghaei and coauthors disclosed numerous ties to the company, including employment.
Across the two studies – CheckMate 017 and 057 – 854 patients were randomized 1:1 following progression on platinum therapy to either nivolumab at 3 mg/kg once every 2 weeks or docetaxel at 75 mg/m2 once every 3 weeks until further progression or unacceptable toxicity. Previously reported overall survival (OS) outcomes favored nivolumab.
At a minimum follow-up of 5.4 years, 50 nivolumab-treated patients and 9 docetaxel-treated patients were still alive.
The 5-year OS rates were 13.4% in the nivolumab arm and 2.6% in the docetaxel arm. The 5-year progression-free survival (PFS) rates were 8% and 0%, respectively.
There were no new safety signals with nivolumab, and there was no evidence of select late-onset grade 3-4 adverse events.
According to the study authors, this analysis is the longest phase 3 follow-up to date of a PD-1 inhibitor in previously treated, advanced NSCLC, and it suggests that “long-term survival beyond 5 years may ... be possible in NSCLC.”
“The results indicate that some patients with NSCLC can have long-lasting benefit from checkpoint inhibitors. We have seen similar results in terms of long-term OS with pembrolizumab,” said investigator Hossein Borghaei, DO, chief of thoracic medical oncology at the Fox Chase Cancer Center in Philadelphia.
Dr. Borghaei said the question now is “how to identify the population that really benefits from these treatments. We think PD-L1–high [patients] have a better chance, [as do patients with] tumors that have a higher percentage of tumor-infiltrating lymphocytes, but there’s nothing concrete beyond that.”
No baseline clinical or tumor factors emerged to distinguish between long-and short-term survivors, but the 5-year OS rate was 18.3% among nivolumab-treated patients with PD-L1 expression at or above 1% versus 8% among patients with expression below 1%.
The optimal duration of nivolumab treatment beyond 1 year is also uncertain.
The median duration of therapy was 36.9 months in the 5-year survivors treated with nivolumab, and 36% of patients (18/50) were still on nivolumab at the 5-year mark.
The median duration of time off treatment was 41.9 months among patients who discontinued nivolumab. Five patients (10%) were off treatment with no subsequent therapy and had not progressed at 5 years, “suggesting benefit even for patients who stopped nivolumab treatment,” the researchers wrote.
They also found that nivolumab-treated patients who remained alive at 3 years appeared to stabilize and plateau thereafter, with early response suggesting better long-term outcomes. The majority of patients without disease progression at 2, 3, and 4 years, for instance, remained progression free at 5 years. Nearly one-third of patients who achieved an objective response with nivolumab – but none of the patients who responded to docetaxel – had ongoing responses at 5 years.
Similarly, nivolumab-treated patients without disease progression at 2 years and 3 years had an 82% and 93% chance of survival, respectively, and a 59.6% and 78.3% chance of remaining progression free at 5 years.
This research was funded by Bristol-Myers Squibb. Dr. Borghaei and coauthors disclosed numerous ties to the company, including employment.
Across the two studies – CheckMate 017 and 057 – 854 patients were randomized 1:1 following progression on platinum therapy to either nivolumab at 3 mg/kg once every 2 weeks or docetaxel at 75 mg/m2 once every 3 weeks until further progression or unacceptable toxicity. Previously reported overall survival (OS) outcomes favored nivolumab.
At a minimum follow-up of 5.4 years, 50 nivolumab-treated patients and 9 docetaxel-treated patients were still alive.
The 5-year OS rates were 13.4% in the nivolumab arm and 2.6% in the docetaxel arm. The 5-year progression-free survival (PFS) rates were 8% and 0%, respectively.
There were no new safety signals with nivolumab, and there was no evidence of select late-onset grade 3-4 adverse events.
According to the study authors, this analysis is the longest phase 3 follow-up to date of a PD-1 inhibitor in previously treated, advanced NSCLC, and it suggests that “long-term survival beyond 5 years may ... be possible in NSCLC.”
“The results indicate that some patients with NSCLC can have long-lasting benefit from checkpoint inhibitors. We have seen similar results in terms of long-term OS with pembrolizumab,” said investigator Hossein Borghaei, DO, chief of thoracic medical oncology at the Fox Chase Cancer Center in Philadelphia.
Dr. Borghaei said the question now is “how to identify the population that really benefits from these treatments. We think PD-L1–high [patients] have a better chance, [as do patients with] tumors that have a higher percentage of tumor-infiltrating lymphocytes, but there’s nothing concrete beyond that.”
No baseline clinical or tumor factors emerged to distinguish between long-and short-term survivors, but the 5-year OS rate was 18.3% among nivolumab-treated patients with PD-L1 expression at or above 1% versus 8% among patients with expression below 1%.
The optimal duration of nivolumab treatment beyond 1 year is also uncertain.
The median duration of therapy was 36.9 months in the 5-year survivors treated with nivolumab, and 36% of patients (18/50) were still on nivolumab at the 5-year mark.
The median duration of time off treatment was 41.9 months among patients who discontinued nivolumab. Five patients (10%) were off treatment with no subsequent therapy and had not progressed at 5 years, “suggesting benefit even for patients who stopped nivolumab treatment,” the researchers wrote.
They also found that nivolumab-treated patients who remained alive at 3 years appeared to stabilize and plateau thereafter, with early response suggesting better long-term outcomes. The majority of patients without disease progression at 2, 3, and 4 years, for instance, remained progression free at 5 years. Nearly one-third of patients who achieved an objective response with nivolumab – but none of the patients who responded to docetaxel – had ongoing responses at 5 years.
Similarly, nivolumab-treated patients without disease progression at 2 years and 3 years had an 82% and 93% chance of survival, respectively, and a 59.6% and 78.3% chance of remaining progression free at 5 years.
This research was funded by Bristol-Myers Squibb. Dr. Borghaei and coauthors disclosed numerous ties to the company, including employment.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Predicting outcomes in therapy-related AML
Therapy-related acute myeloid leukemia (t-AML) occurs as a complication of chemotherapy and/or radiotherapy for previous cancer or for nonmalignant disorders, with an estimated prevalence of 10%-15% of all AML cases, according to Ram Vasudevan Nampoothiri, MD, and colleagues at the Princess Margaret Cancer Center, University of Toronto.
Dr. Nampoothiri and colleagues performed a retrospective study of 68 patients with t-AML who underwent hematopoietic stem cell transplantation (HSCT) at their institution. They found significant predictors of reduced overall survival, including chromosomal rearrangements, induction regimens, donor type, patient performance status, and the type of graft-versus-host disease (GVHD) prophylaxis the patients received, as reported in Hematology/Oncology and Stem Cell Therapy.
Some populations benefit
Among the 68 patients studied, a total of 59.9% were women; and the median age was 56.5 years. All patients were analyzed for prior malignancy, therapy, time to diagnosis of t-AML, transplant details, relapse-free survival, overall survival, and predictors of outcomes.
At 2 years, the cumulative incidence of relapse, nonrelapse mortality, relapse-free survival, and overall survival were 17.9%, 34.5%, 47.6%, and 49.3%, respectively. Overall, acute and chronic GVHD occurred in 39 (57.4%) and 23 (33.8%) patients, respectively, according to the researchers.
The significant predictors of reduced overall survival were the presence of the 11q23 chromosomal rearrangement (hazard ratio, 3.24), use of induction regimens other than fludarabine, cytarabine, idarubicin, and granulocyte colony-stimulating factor or 7 + 3 (HR, 3.65), use of haploidentical donors (HR, 3.48), an Eastern Cooperative Oncology Group performance status of 2 or higher (HR, 5.83), and use of cyclosporine A–methotrexate as GVHD prophylaxis (HR, 2.41).
The researchers also found that a significant decrease in survival was seen with an increasing number of any of these prognostic factors.
A growing need
The incidence of t-AML is increasing because of longer life expectancy of the general population and also because of the improved survival of patients treated with chemotherapy and/or radiation for prior malignancies, according to the researchers.
They concluded that, even with this increasing prevalence and normally poor prognosis, “patients of t-AML having good-risk karyotypes, good performance status, and having HLA-matched donors have favorable outcomes after allo-HSCT.”
The authors reported that they had no competing financial interests.
Therapy-related acute myeloid leukemia (t-AML) occurs as a complication of chemotherapy and/or radiotherapy for previous cancer or for nonmalignant disorders, with an estimated prevalence of 10%-15% of all AML cases, according to Ram Vasudevan Nampoothiri, MD, and colleagues at the Princess Margaret Cancer Center, University of Toronto.
Dr. Nampoothiri and colleagues performed a retrospective study of 68 patients with t-AML who underwent hematopoietic stem cell transplantation (HSCT) at their institution. They found significant predictors of reduced overall survival, including chromosomal rearrangements, induction regimens, donor type, patient performance status, and the type of graft-versus-host disease (GVHD) prophylaxis the patients received, as reported in Hematology/Oncology and Stem Cell Therapy.
Some populations benefit
Among the 68 patients studied, a total of 59.9% were women; and the median age was 56.5 years. All patients were analyzed for prior malignancy, therapy, time to diagnosis of t-AML, transplant details, relapse-free survival, overall survival, and predictors of outcomes.
At 2 years, the cumulative incidence of relapse, nonrelapse mortality, relapse-free survival, and overall survival were 17.9%, 34.5%, 47.6%, and 49.3%, respectively. Overall, acute and chronic GVHD occurred in 39 (57.4%) and 23 (33.8%) patients, respectively, according to the researchers.
The significant predictors of reduced overall survival were the presence of the 11q23 chromosomal rearrangement (hazard ratio, 3.24), use of induction regimens other than fludarabine, cytarabine, idarubicin, and granulocyte colony-stimulating factor or 7 + 3 (HR, 3.65), use of haploidentical donors (HR, 3.48), an Eastern Cooperative Oncology Group performance status of 2 or higher (HR, 5.83), and use of cyclosporine A–methotrexate as GVHD prophylaxis (HR, 2.41).
The researchers also found that a significant decrease in survival was seen with an increasing number of any of these prognostic factors.
A growing need
The incidence of t-AML is increasing because of longer life expectancy of the general population and also because of the improved survival of patients treated with chemotherapy and/or radiation for prior malignancies, according to the researchers.
They concluded that, even with this increasing prevalence and normally poor prognosis, “patients of t-AML having good-risk karyotypes, good performance status, and having HLA-matched donors have favorable outcomes after allo-HSCT.”
The authors reported that they had no competing financial interests.
Therapy-related acute myeloid leukemia (t-AML) occurs as a complication of chemotherapy and/or radiotherapy for previous cancer or for nonmalignant disorders, with an estimated prevalence of 10%-15% of all AML cases, according to Ram Vasudevan Nampoothiri, MD, and colleagues at the Princess Margaret Cancer Center, University of Toronto.
Dr. Nampoothiri and colleagues performed a retrospective study of 68 patients with t-AML who underwent hematopoietic stem cell transplantation (HSCT) at their institution. They found significant predictors of reduced overall survival, including chromosomal rearrangements, induction regimens, donor type, patient performance status, and the type of graft-versus-host disease (GVHD) prophylaxis the patients received, as reported in Hematology/Oncology and Stem Cell Therapy.
Some populations benefit
Among the 68 patients studied, a total of 59.9% were women; and the median age was 56.5 years. All patients were analyzed for prior malignancy, therapy, time to diagnosis of t-AML, transplant details, relapse-free survival, overall survival, and predictors of outcomes.
At 2 years, the cumulative incidence of relapse, nonrelapse mortality, relapse-free survival, and overall survival were 17.9%, 34.5%, 47.6%, and 49.3%, respectively. Overall, acute and chronic GVHD occurred in 39 (57.4%) and 23 (33.8%) patients, respectively, according to the researchers.
The significant predictors of reduced overall survival were the presence of the 11q23 chromosomal rearrangement (hazard ratio, 3.24), use of induction regimens other than fludarabine, cytarabine, idarubicin, and granulocyte colony-stimulating factor or 7 + 3 (HR, 3.65), use of haploidentical donors (HR, 3.48), an Eastern Cooperative Oncology Group performance status of 2 or higher (HR, 5.83), and use of cyclosporine A–methotrexate as GVHD prophylaxis (HR, 2.41).
The researchers also found that a significant decrease in survival was seen with an increasing number of any of these prognostic factors.
A growing need
The incidence of t-AML is increasing because of longer life expectancy of the general population and also because of the improved survival of patients treated with chemotherapy and/or radiation for prior malignancies, according to the researchers.
They concluded that, even with this increasing prevalence and normally poor prognosis, “patients of t-AML having good-risk karyotypes, good performance status, and having HLA-matched donors have favorable outcomes after allo-HSCT.”
The authors reported that they had no competing financial interests.
FROM HEMATOLOGY/ONCOLOGY AND STEM CELL THERAPY
Cell-free DNA improves response prediction in breast cancer
When the two techniques were in agreement, the accuracy of response prediction was 92.6% in the study, with a predictive value for complete response of 87.5% and a predictive value for absence of complete response of 94.7%, which was substantially better than either method alone.
“Our work identifies a new parameter that is easily combinable with MRI for a more accurate prediction of response following neoadjuvant treatment, with possible implications for current protocols for the evaluation of nodal residual disease,” researcher Francesco Ravera, MD, PhD, of the University of Genoa (Italy), said in a press release.
Dr. Ravera and colleagues presented their research in a poster at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract LB063).
Accurate response prediction is important because it guides subsequent surgical management, Dr. Ravera and colleagues noted. Pathological complete responders – generally about 25% of patients after neoadjuvant therapy – typically undergo a sentinel lymph node biopsy to ensure cancer hasn’t spread, while incomplete responders often have a complete axillary lymph node dissection.
Response is currently assessed by MRI, but accuracy is suboptimal, the researchers noted. A more accurate method might “allow the omission of sentinel lymph node biopsy in complete responders, which could be replaced by longitudinal radiologic monitoring. This would represent substantial progress in the pursuit of an effective, minimally invasive treatment,” Dr. Ravera said.
He and his colleagues turned to plasma cfDNA because it has shown potential for providing useful diagnostic, recurrence, and treatment response information in neoplastic patients.
When healthy cells die, they release similarly sized DNA fragments into the blood, but cancer cells release fragments of varying sizes. The heart of the research was using electrophoresis to assess the degree of fragmentation – called cfDNA integrity – in plasma samples from 38 patients after anthracycline/taxane-based regimens.
The researchers compared how well cfDNA, preoperative MRI, and the combination of the two methods predicted response according to surgical histology.
A total of 11 patients had pathological complete responses to neoadjuvant therapy.
The ratio of large 321-1,000 base pair sized fragments to smaller 150-220 base pair sized fragments, which the team dubbed the “cfDNA integrity index,” best predicted response. At a cutoff above 2.71, the index was 81.6% accurate in predicting pathological complete response, with a sensitivity of 81.8% and specificity of 81.5%.
The predictive power wasn’t much better than MRI, which was 77.1% accurate, with a sensitivity of 72.7% and a specificity of 81.5%.
The two techniques were concordant in their prediction in over two-thirds of patients. When the techniques agreed, accuracy was over 90%.
Prospective studies are needed to evaluate the cfDNA integrity index in combination with MRI, the researchers concluded.
The study was sponsored by the University of Genoa and others. Dr. Ravera disclosed no conflicts of interest.
When the two techniques were in agreement, the accuracy of response prediction was 92.6% in the study, with a predictive value for complete response of 87.5% and a predictive value for absence of complete response of 94.7%, which was substantially better than either method alone.
“Our work identifies a new parameter that is easily combinable with MRI for a more accurate prediction of response following neoadjuvant treatment, with possible implications for current protocols for the evaluation of nodal residual disease,” researcher Francesco Ravera, MD, PhD, of the University of Genoa (Italy), said in a press release.
Dr. Ravera and colleagues presented their research in a poster at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract LB063).
Accurate response prediction is important because it guides subsequent surgical management, Dr. Ravera and colleagues noted. Pathological complete responders – generally about 25% of patients after neoadjuvant therapy – typically undergo a sentinel lymph node biopsy to ensure cancer hasn’t spread, while incomplete responders often have a complete axillary lymph node dissection.
Response is currently assessed by MRI, but accuracy is suboptimal, the researchers noted. A more accurate method might “allow the omission of sentinel lymph node biopsy in complete responders, which could be replaced by longitudinal radiologic monitoring. This would represent substantial progress in the pursuit of an effective, minimally invasive treatment,” Dr. Ravera said.
He and his colleagues turned to plasma cfDNA because it has shown potential for providing useful diagnostic, recurrence, and treatment response information in neoplastic patients.
When healthy cells die, they release similarly sized DNA fragments into the blood, but cancer cells release fragments of varying sizes. The heart of the research was using electrophoresis to assess the degree of fragmentation – called cfDNA integrity – in plasma samples from 38 patients after anthracycline/taxane-based regimens.
The researchers compared how well cfDNA, preoperative MRI, and the combination of the two methods predicted response according to surgical histology.
A total of 11 patients had pathological complete responses to neoadjuvant therapy.
The ratio of large 321-1,000 base pair sized fragments to smaller 150-220 base pair sized fragments, which the team dubbed the “cfDNA integrity index,” best predicted response. At a cutoff above 2.71, the index was 81.6% accurate in predicting pathological complete response, with a sensitivity of 81.8% and specificity of 81.5%.
The predictive power wasn’t much better than MRI, which was 77.1% accurate, with a sensitivity of 72.7% and a specificity of 81.5%.
The two techniques were concordant in their prediction in over two-thirds of patients. When the techniques agreed, accuracy was over 90%.
Prospective studies are needed to evaluate the cfDNA integrity index in combination with MRI, the researchers concluded.
The study was sponsored by the University of Genoa and others. Dr. Ravera disclosed no conflicts of interest.
When the two techniques were in agreement, the accuracy of response prediction was 92.6% in the study, with a predictive value for complete response of 87.5% and a predictive value for absence of complete response of 94.7%, which was substantially better than either method alone.
“Our work identifies a new parameter that is easily combinable with MRI for a more accurate prediction of response following neoadjuvant treatment, with possible implications for current protocols for the evaluation of nodal residual disease,” researcher Francesco Ravera, MD, PhD, of the University of Genoa (Italy), said in a press release.
Dr. Ravera and colleagues presented their research in a poster at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract LB063).
Accurate response prediction is important because it guides subsequent surgical management, Dr. Ravera and colleagues noted. Pathological complete responders – generally about 25% of patients after neoadjuvant therapy – typically undergo a sentinel lymph node biopsy to ensure cancer hasn’t spread, while incomplete responders often have a complete axillary lymph node dissection.
Response is currently assessed by MRI, but accuracy is suboptimal, the researchers noted. A more accurate method might “allow the omission of sentinel lymph node biopsy in complete responders, which could be replaced by longitudinal radiologic monitoring. This would represent substantial progress in the pursuit of an effective, minimally invasive treatment,” Dr. Ravera said.
He and his colleagues turned to plasma cfDNA because it has shown potential for providing useful diagnostic, recurrence, and treatment response information in neoplastic patients.
When healthy cells die, they release similarly sized DNA fragments into the blood, but cancer cells release fragments of varying sizes. The heart of the research was using electrophoresis to assess the degree of fragmentation – called cfDNA integrity – in plasma samples from 38 patients after anthracycline/taxane-based regimens.
The researchers compared how well cfDNA, preoperative MRI, and the combination of the two methods predicted response according to surgical histology.
A total of 11 patients had pathological complete responses to neoadjuvant therapy.
The ratio of large 321-1,000 base pair sized fragments to smaller 150-220 base pair sized fragments, which the team dubbed the “cfDNA integrity index,” best predicted response. At a cutoff above 2.71, the index was 81.6% accurate in predicting pathological complete response, with a sensitivity of 81.8% and specificity of 81.5%.
The predictive power wasn’t much better than MRI, which was 77.1% accurate, with a sensitivity of 72.7% and a specificity of 81.5%.
The two techniques were concordant in their prediction in over two-thirds of patients. When the techniques agreed, accuracy was over 90%.
Prospective studies are needed to evaluate the cfDNA integrity index in combination with MRI, the researchers concluded.
The study was sponsored by the University of Genoa and others. Dr. Ravera disclosed no conflicts of interest.
FROM AACR 2021
GENUINE improvements: Ublituximab plus ibrutinib for CLL
Chronic lymphocytic leukemia (CLL) is a clinically heterogeneous disease associated with several known genetic abnormalities, including 17p deletion (del[17p]), 11q deletion (del[11q]), and TP53 gene mutations, which are adverse prognostic markers among patients treated with chemoimmunotherapy.
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib is approved for patients with untreated, relapsed, or refractory disease, including those with del(17p). Clinicians will soon have the chance to pair it with ublituximab, a next-generation, glycoengineered, type I, anti-CD20 monoclonal antibody that binds to a unique epitope on CD20, differentiating it from rituximab, ofatumumab, and obinutuzumab. Results from the phase 3 GENUINE trial, which were recently published in The Lancet Haematology, showed that ublituximab plus ibrutinib was superior to ibrutinib alone for patients with relapsed or refractory high-risk CLL.
This news organization spoke with Jennifer R. Brown, MD, PhD, director of the CLL Center and institute physician at the Dana-Farber Cancer Institute and professor of medicine at Harvard Medical School, both in Boston, about the GENUINE trial and its potential impact on treatment choices going forward.
What type of patients were treated in the GENUINE trial?
Dr. Brown: This is a trial among relapsed/refractory CLL patients with 17p or 11q deletion or TP53 mutation. Patients aged 18 years or older with CLL who warranted treatment, as defined by International Workshop on CLL criteria, were eligible if they had previously received at least two cycles of at least one standard treatment regimen, had an Eastern Cooperative Oncology Group performance status of 2 or lower, and had high-risk cytogenetics, defined as the presence of at least one of del(17p), del(11q), or TP53 mutation confirmed by a central laboratory with fluorescence in situ hybridization and/or next-generation sequencing.
What were the main outcomes of the trial?
Originally, the GENUINE trial had coprimary endpoints of progression-free survival (PFS) and overall response rate. Because of slow accrual, it was amended to have one primary endpoint of independent review committee (IRC)–assessed ORR.
IRC-assessed ORR was improved from 65% to 83% with the addition of ublituximab. PFS also improved significantly in the ublituximab group, with an even greater improvement when the analysis was limited to those with del(17p) or TP53 aberrancy, but this outcome was limited by the reduced sample size of the study as well as the relatively short PFS of the ibrutinib arm.
After a median follow-up of 41.6 months, the median IRC-assessed PFS in all treated patients was not reached in the ublituximab plus ibrutinib group after 15 PFS events but was 35.9 months in the ibrutinib group after 25 PFS events (hazard ratio, 0.46; 95% confidence interval, 0.24-0.87; P = .016).
Undetectable minimal residual disease was also seen in 42% of the combination arm, compared with 6% of the ibrutinib arm.
What types of adverse events were found in the trial?
The researchers found mostly mild and known side effects of ibrutinib. More atrial fibrillation and neutropenia were seen in the antibody group, but this was not marked.
Most adverse events were of grade 1 or 2. The most common grade 3 and 4 adverse events were neutropenia (11 [19%] patients in the ublituximab plus ibrutinib group and 7 [12%] in the ibrutinib group), anemia (5 [8%] and 5 [9%], respectively), and diarrhea (6 [10%] and 3 [5%], respectively).
What about serious adverse events?
Hospitalization from infection was seen, as expected. There were two cardiac arrests and an unexplained death, across both arms, which was concerning, given the known association of ibrutinib with ventricular arrhythmia and sudden death. There were also several hemorrhages, including one fatal one, which was again consistent with the known side effects of ibrutinib.
Are there treatments comparable with ublituximab plus ibrutinib that clinicians should perhaps first consider using?
In terms of other anti-CD20 antibodies, we have two randomized trials that have failed to show a benefit from adding rituximab to ibrutinib.
Obinutuzumab, like ublituximab, is also a next-generation glycoengineered antibody, and it is reasonably likely that it might lead to similar results. However, the only data we have on ibrutinib with obinutuzumab are from a single arm in a more heterogeneous, lower-risk patient population, and it is unlikely that a randomized comparison will ever be done.
On the basis of these trial results, how would you use the combination of ublituximab and ibrutinib for your patients?
I would consider the addition of ublituximab to a BTK inhibitor in high-risk patients (once ublituximab is approved). I already usually use a next-generation BTK inhibitor rather than ibrutinib.
Are there any other implications of the GENUINE trial?
I think this trial underscores the importance of studying genetic subgroups of patients separately. In this case, that was done in high-risk patients, but this observation likely also applies to low-risk patients.
Most trials to date have enrolled unselected patient populations, often without stratification, and their results therefore tend to obscure the outcomes in both the very high risk (as studied here) and in the low risk (patients with immunoglobulin heavy chain variable region gene mutations).
Dr. Brown has served as a consultant for AbbVie, Acerta/AstraZeneca, Beigene, Bristol-Myers Squibb/Juno/Celgene, Catapult, Genentech/Roche, Janssen, MEI Pharma, Morphosys, and Novartis, and has received research funding from Gilead, Loxo/Lilly, TG Therapeutics, Verastem/SecuraBio.
A version of this article first appeared on Medscape.com.
Chronic lymphocytic leukemia (CLL) is a clinically heterogeneous disease associated with several known genetic abnormalities, including 17p deletion (del[17p]), 11q deletion (del[11q]), and TP53 gene mutations, which are adverse prognostic markers among patients treated with chemoimmunotherapy.
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib is approved for patients with untreated, relapsed, or refractory disease, including those with del(17p). Clinicians will soon have the chance to pair it with ublituximab, a next-generation, glycoengineered, type I, anti-CD20 monoclonal antibody that binds to a unique epitope on CD20, differentiating it from rituximab, ofatumumab, and obinutuzumab. Results from the phase 3 GENUINE trial, which were recently published in The Lancet Haematology, showed that ublituximab plus ibrutinib was superior to ibrutinib alone for patients with relapsed or refractory high-risk CLL.
This news organization spoke with Jennifer R. Brown, MD, PhD, director of the CLL Center and institute physician at the Dana-Farber Cancer Institute and professor of medicine at Harvard Medical School, both in Boston, about the GENUINE trial and its potential impact on treatment choices going forward.
What type of patients were treated in the GENUINE trial?
Dr. Brown: This is a trial among relapsed/refractory CLL patients with 17p or 11q deletion or TP53 mutation. Patients aged 18 years or older with CLL who warranted treatment, as defined by International Workshop on CLL criteria, were eligible if they had previously received at least two cycles of at least one standard treatment regimen, had an Eastern Cooperative Oncology Group performance status of 2 or lower, and had high-risk cytogenetics, defined as the presence of at least one of del(17p), del(11q), or TP53 mutation confirmed by a central laboratory with fluorescence in situ hybridization and/or next-generation sequencing.
What were the main outcomes of the trial?
Originally, the GENUINE trial had coprimary endpoints of progression-free survival (PFS) and overall response rate. Because of slow accrual, it was amended to have one primary endpoint of independent review committee (IRC)–assessed ORR.
IRC-assessed ORR was improved from 65% to 83% with the addition of ublituximab. PFS also improved significantly in the ublituximab group, with an even greater improvement when the analysis was limited to those with del(17p) or TP53 aberrancy, but this outcome was limited by the reduced sample size of the study as well as the relatively short PFS of the ibrutinib arm.
After a median follow-up of 41.6 months, the median IRC-assessed PFS in all treated patients was not reached in the ublituximab plus ibrutinib group after 15 PFS events but was 35.9 months in the ibrutinib group after 25 PFS events (hazard ratio, 0.46; 95% confidence interval, 0.24-0.87; P = .016).
Undetectable minimal residual disease was also seen in 42% of the combination arm, compared with 6% of the ibrutinib arm.
What types of adverse events were found in the trial?
The researchers found mostly mild and known side effects of ibrutinib. More atrial fibrillation and neutropenia were seen in the antibody group, but this was not marked.
Most adverse events were of grade 1 or 2. The most common grade 3 and 4 adverse events were neutropenia (11 [19%] patients in the ublituximab plus ibrutinib group and 7 [12%] in the ibrutinib group), anemia (5 [8%] and 5 [9%], respectively), and diarrhea (6 [10%] and 3 [5%], respectively).
What about serious adverse events?
Hospitalization from infection was seen, as expected. There were two cardiac arrests and an unexplained death, across both arms, which was concerning, given the known association of ibrutinib with ventricular arrhythmia and sudden death. There were also several hemorrhages, including one fatal one, which was again consistent with the known side effects of ibrutinib.
Are there treatments comparable with ublituximab plus ibrutinib that clinicians should perhaps first consider using?
In terms of other anti-CD20 antibodies, we have two randomized trials that have failed to show a benefit from adding rituximab to ibrutinib.
Obinutuzumab, like ublituximab, is also a next-generation glycoengineered antibody, and it is reasonably likely that it might lead to similar results. However, the only data we have on ibrutinib with obinutuzumab are from a single arm in a more heterogeneous, lower-risk patient population, and it is unlikely that a randomized comparison will ever be done.
On the basis of these trial results, how would you use the combination of ublituximab and ibrutinib for your patients?
I would consider the addition of ublituximab to a BTK inhibitor in high-risk patients (once ublituximab is approved). I already usually use a next-generation BTK inhibitor rather than ibrutinib.
Are there any other implications of the GENUINE trial?
I think this trial underscores the importance of studying genetic subgroups of patients separately. In this case, that was done in high-risk patients, but this observation likely also applies to low-risk patients.
Most trials to date have enrolled unselected patient populations, often without stratification, and their results therefore tend to obscure the outcomes in both the very high risk (as studied here) and in the low risk (patients with immunoglobulin heavy chain variable region gene mutations).
Dr. Brown has served as a consultant for AbbVie, Acerta/AstraZeneca, Beigene, Bristol-Myers Squibb/Juno/Celgene, Catapult, Genentech/Roche, Janssen, MEI Pharma, Morphosys, and Novartis, and has received research funding from Gilead, Loxo/Lilly, TG Therapeutics, Verastem/SecuraBio.
A version of this article first appeared on Medscape.com.
Chronic lymphocytic leukemia (CLL) is a clinically heterogeneous disease associated with several known genetic abnormalities, including 17p deletion (del[17p]), 11q deletion (del[11q]), and TP53 gene mutations, which are adverse prognostic markers among patients treated with chemoimmunotherapy.
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib is approved for patients with untreated, relapsed, or refractory disease, including those with del(17p). Clinicians will soon have the chance to pair it with ublituximab, a next-generation, glycoengineered, type I, anti-CD20 monoclonal antibody that binds to a unique epitope on CD20, differentiating it from rituximab, ofatumumab, and obinutuzumab. Results from the phase 3 GENUINE trial, which were recently published in The Lancet Haematology, showed that ublituximab plus ibrutinib was superior to ibrutinib alone for patients with relapsed or refractory high-risk CLL.
This news organization spoke with Jennifer R. Brown, MD, PhD, director of the CLL Center and institute physician at the Dana-Farber Cancer Institute and professor of medicine at Harvard Medical School, both in Boston, about the GENUINE trial and its potential impact on treatment choices going forward.
What type of patients were treated in the GENUINE trial?
Dr. Brown: This is a trial among relapsed/refractory CLL patients with 17p or 11q deletion or TP53 mutation. Patients aged 18 years or older with CLL who warranted treatment, as defined by International Workshop on CLL criteria, were eligible if they had previously received at least two cycles of at least one standard treatment regimen, had an Eastern Cooperative Oncology Group performance status of 2 or lower, and had high-risk cytogenetics, defined as the presence of at least one of del(17p), del(11q), or TP53 mutation confirmed by a central laboratory with fluorescence in situ hybridization and/or next-generation sequencing.
What were the main outcomes of the trial?
Originally, the GENUINE trial had coprimary endpoints of progression-free survival (PFS) and overall response rate. Because of slow accrual, it was amended to have one primary endpoint of independent review committee (IRC)–assessed ORR.
IRC-assessed ORR was improved from 65% to 83% with the addition of ublituximab. PFS also improved significantly in the ublituximab group, with an even greater improvement when the analysis was limited to those with del(17p) or TP53 aberrancy, but this outcome was limited by the reduced sample size of the study as well as the relatively short PFS of the ibrutinib arm.
After a median follow-up of 41.6 months, the median IRC-assessed PFS in all treated patients was not reached in the ublituximab plus ibrutinib group after 15 PFS events but was 35.9 months in the ibrutinib group after 25 PFS events (hazard ratio, 0.46; 95% confidence interval, 0.24-0.87; P = .016).
Undetectable minimal residual disease was also seen in 42% of the combination arm, compared with 6% of the ibrutinib arm.
What types of adverse events were found in the trial?
The researchers found mostly mild and known side effects of ibrutinib. More atrial fibrillation and neutropenia were seen in the antibody group, but this was not marked.
Most adverse events were of grade 1 or 2. The most common grade 3 and 4 adverse events were neutropenia (11 [19%] patients in the ublituximab plus ibrutinib group and 7 [12%] in the ibrutinib group), anemia (5 [8%] and 5 [9%], respectively), and diarrhea (6 [10%] and 3 [5%], respectively).
What about serious adverse events?
Hospitalization from infection was seen, as expected. There were two cardiac arrests and an unexplained death, across both arms, which was concerning, given the known association of ibrutinib with ventricular arrhythmia and sudden death. There were also several hemorrhages, including one fatal one, which was again consistent with the known side effects of ibrutinib.
Are there treatments comparable with ublituximab plus ibrutinib that clinicians should perhaps first consider using?
In terms of other anti-CD20 antibodies, we have two randomized trials that have failed to show a benefit from adding rituximab to ibrutinib.
Obinutuzumab, like ublituximab, is also a next-generation glycoengineered antibody, and it is reasonably likely that it might lead to similar results. However, the only data we have on ibrutinib with obinutuzumab are from a single arm in a more heterogeneous, lower-risk patient population, and it is unlikely that a randomized comparison will ever be done.
On the basis of these trial results, how would you use the combination of ublituximab and ibrutinib for your patients?
I would consider the addition of ublituximab to a BTK inhibitor in high-risk patients (once ublituximab is approved). I already usually use a next-generation BTK inhibitor rather than ibrutinib.
Are there any other implications of the GENUINE trial?
I think this trial underscores the importance of studying genetic subgroups of patients separately. In this case, that was done in high-risk patients, but this observation likely also applies to low-risk patients.
Most trials to date have enrolled unselected patient populations, often without stratification, and their results therefore tend to obscure the outcomes in both the very high risk (as studied here) and in the low risk (patients with immunoglobulin heavy chain variable region gene mutations).
Dr. Brown has served as a consultant for AbbVie, Acerta/AstraZeneca, Beigene, Bristol-Myers Squibb/Juno/Celgene, Catapult, Genentech/Roche, Janssen, MEI Pharma, Morphosys, and Novartis, and has received research funding from Gilead, Loxo/Lilly, TG Therapeutics, Verastem/SecuraBio.
A version of this article first appeared on Medscape.com.
Hypothesis: Milk and beef ‘causally linked’ to colorectal cancer
Bovine meat and milk factors (BMMF), the recently discovered pathogenic agents found in milk products and beef, could play a role in the development of colorectal cancer, say German scientists.
The team was headed by Harald zur Hausen, MD, PhD, division of episomal-persistent DNA in cancer and chronic diseases, German Cancer Research Center, Heidelberg, who received the Nobel Prize in Physiology or Medicine in 2008 for his discovery of the role of human papillomaviruses in the development of cervical cancer.
The team has been researching the link between BMMF and colorectal cancer for some years, and the latest study was published in the Proceedings of the National Academy of Sciences.
“The results support our hypothesis that the consumption of milk and beef is causally linked to the development of colon cancer,” Dr. Hausen said in a related press statement.
Pathogenic agents, found in vicinity of tumors
BMMF are infectious agents that occur as circular single strands of DNA and have a notable similarity to the sequences of some bacterial plasmids.
A few years ago, Dr. Hausen and colleagues found these pathogenic agents in colon cancer patients, in the immediate vicinity of tumors.
They therefore put forward the hypothesis that BMMF could trigger chronic local inflammation, which in turn could cause genetic mutations via oxidative stress and lead to the development of cancer over the long term.
The German researchers were also able to show in in vitro models that BMMF multiply in human cells where the H1MSB.1 Rep protein, which is required for their replication, is synthesized.
In their latest study, the scientists analyzed tissue samples from colorectal cancer patients and from individuals without the disease.
The team found that BMMF could trigger chronic inflammation in the intestinal tissue of cancer patients, as demonstrated via the presence of proinflammatory macrophages.
The researchers also used anti-Rep antibodies to show that the Rep protein was present around and inside the macrophages. In cancer patients, 7.3% of all the intestinal cells in the tumor environment were positive for Rep versus just 1.7% of those in the control group.
In addition, the researchers reported increased levels of reactive oxygen species close to Rep-positive cells.
“These oxygen radicals promote the development of genetic changes,” Dr. Hausen said.
The inflammation was particularly localized to the immediate vicinity of the intestinal crypts, where the intestinal stem cells, which are responsible for the constant regeneration of the intestinal mucosa, are found.
“We therefore think of the BMMF as indirect carcinogens, some of which will probably have an impact on dividing cells in the intestinal mucosa for decades,” Dr. Hausen explained.
He assumes that infection with BMMF typically occurs early in life. This “opens up the possibility of early intervention,” he suggested.
The early detection of BMMF could allow the identification of individuals particularly at risk, and for these patients to be offered timely colon cancer screening.
The researchers stressed, however, that further study will be required to confirm the results.
They nevertheless believed that BMMF could help explain the link between the consumption of red meat and dairy products and other cancers and diseases, in particular breast, prostate, and lung cancers.
Finally, the pathogenic agents could partially explain the preventive effect of anti-inflammatory dugs such as aspirin and ibuprofen on the incidence of colon cancer and other cancers via the reduction of chronic inflammation.
This work was supported by an unrestricted grant from ORYX Alpha. One coauthor was supported by the EOS Foundation, the SFBTR-179 and 209, and a European Research Council consolidator grant. The other coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Bovine meat and milk factors (BMMF), the recently discovered pathogenic agents found in milk products and beef, could play a role in the development of colorectal cancer, say German scientists.
The team was headed by Harald zur Hausen, MD, PhD, division of episomal-persistent DNA in cancer and chronic diseases, German Cancer Research Center, Heidelberg, who received the Nobel Prize in Physiology or Medicine in 2008 for his discovery of the role of human papillomaviruses in the development of cervical cancer.
The team has been researching the link between BMMF and colorectal cancer for some years, and the latest study was published in the Proceedings of the National Academy of Sciences.
“The results support our hypothesis that the consumption of milk and beef is causally linked to the development of colon cancer,” Dr. Hausen said in a related press statement.
Pathogenic agents, found in vicinity of tumors
BMMF are infectious agents that occur as circular single strands of DNA and have a notable similarity to the sequences of some bacterial plasmids.
A few years ago, Dr. Hausen and colleagues found these pathogenic agents in colon cancer patients, in the immediate vicinity of tumors.
They therefore put forward the hypothesis that BMMF could trigger chronic local inflammation, which in turn could cause genetic mutations via oxidative stress and lead to the development of cancer over the long term.
The German researchers were also able to show in in vitro models that BMMF multiply in human cells where the H1MSB.1 Rep protein, which is required for their replication, is synthesized.
In their latest study, the scientists analyzed tissue samples from colorectal cancer patients and from individuals without the disease.
The team found that BMMF could trigger chronic inflammation in the intestinal tissue of cancer patients, as demonstrated via the presence of proinflammatory macrophages.
The researchers also used anti-Rep antibodies to show that the Rep protein was present around and inside the macrophages. In cancer patients, 7.3% of all the intestinal cells in the tumor environment were positive for Rep versus just 1.7% of those in the control group.
In addition, the researchers reported increased levels of reactive oxygen species close to Rep-positive cells.
“These oxygen radicals promote the development of genetic changes,” Dr. Hausen said.
The inflammation was particularly localized to the immediate vicinity of the intestinal crypts, where the intestinal stem cells, which are responsible for the constant regeneration of the intestinal mucosa, are found.
“We therefore think of the BMMF as indirect carcinogens, some of which will probably have an impact on dividing cells in the intestinal mucosa for decades,” Dr. Hausen explained.
He assumes that infection with BMMF typically occurs early in life. This “opens up the possibility of early intervention,” he suggested.
The early detection of BMMF could allow the identification of individuals particularly at risk, and for these patients to be offered timely colon cancer screening.
The researchers stressed, however, that further study will be required to confirm the results.
They nevertheless believed that BMMF could help explain the link between the consumption of red meat and dairy products and other cancers and diseases, in particular breast, prostate, and lung cancers.
Finally, the pathogenic agents could partially explain the preventive effect of anti-inflammatory dugs such as aspirin and ibuprofen on the incidence of colon cancer and other cancers via the reduction of chronic inflammation.
This work was supported by an unrestricted grant from ORYX Alpha. One coauthor was supported by the EOS Foundation, the SFBTR-179 and 209, and a European Research Council consolidator grant. The other coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Bovine meat and milk factors (BMMF), the recently discovered pathogenic agents found in milk products and beef, could play a role in the development of colorectal cancer, say German scientists.
The team was headed by Harald zur Hausen, MD, PhD, division of episomal-persistent DNA in cancer and chronic diseases, German Cancer Research Center, Heidelberg, who received the Nobel Prize in Physiology or Medicine in 2008 for his discovery of the role of human papillomaviruses in the development of cervical cancer.
The team has been researching the link between BMMF and colorectal cancer for some years, and the latest study was published in the Proceedings of the National Academy of Sciences.
“The results support our hypothesis that the consumption of milk and beef is causally linked to the development of colon cancer,” Dr. Hausen said in a related press statement.
Pathogenic agents, found in vicinity of tumors
BMMF are infectious agents that occur as circular single strands of DNA and have a notable similarity to the sequences of some bacterial plasmids.
A few years ago, Dr. Hausen and colleagues found these pathogenic agents in colon cancer patients, in the immediate vicinity of tumors.
They therefore put forward the hypothesis that BMMF could trigger chronic local inflammation, which in turn could cause genetic mutations via oxidative stress and lead to the development of cancer over the long term.
The German researchers were also able to show in in vitro models that BMMF multiply in human cells where the H1MSB.1 Rep protein, which is required for their replication, is synthesized.
In their latest study, the scientists analyzed tissue samples from colorectal cancer patients and from individuals without the disease.
The team found that BMMF could trigger chronic inflammation in the intestinal tissue of cancer patients, as demonstrated via the presence of proinflammatory macrophages.
The researchers also used anti-Rep antibodies to show that the Rep protein was present around and inside the macrophages. In cancer patients, 7.3% of all the intestinal cells in the tumor environment were positive for Rep versus just 1.7% of those in the control group.
In addition, the researchers reported increased levels of reactive oxygen species close to Rep-positive cells.
“These oxygen radicals promote the development of genetic changes,” Dr. Hausen said.
The inflammation was particularly localized to the immediate vicinity of the intestinal crypts, where the intestinal stem cells, which are responsible for the constant regeneration of the intestinal mucosa, are found.
“We therefore think of the BMMF as indirect carcinogens, some of which will probably have an impact on dividing cells in the intestinal mucosa for decades,” Dr. Hausen explained.
He assumes that infection with BMMF typically occurs early in life. This “opens up the possibility of early intervention,” he suggested.
The early detection of BMMF could allow the identification of individuals particularly at risk, and for these patients to be offered timely colon cancer screening.
The researchers stressed, however, that further study will be required to confirm the results.
They nevertheless believed that BMMF could help explain the link between the consumption of red meat and dairy products and other cancers and diseases, in particular breast, prostate, and lung cancers.
Finally, the pathogenic agents could partially explain the preventive effect of anti-inflammatory dugs such as aspirin and ibuprofen on the incidence of colon cancer and other cancers via the reduction of chronic inflammation.
This work was supported by an unrestricted grant from ORYX Alpha. One coauthor was supported by the EOS Foundation, the SFBTR-179 and 209, and a European Research Council consolidator grant. The other coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.