User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


FDA Advisory Panel Votes NO on Belantamab for Myeloma
A bid by GlaxoSmithKline (GSK) to bring its multiple myeloma drug belantamab mafodotin (Blenrep) back to the market hit a stumbling block during an FDA panel meeting held on July 17.
The FDA’s Oncologic Drugs Advisory Committee (ODAC) voted 5-3 against belantamab in combination with bortezomib and dexamethasone and 7-1 against belantamab in combination with pomalidamide and dexamethasone on the specific questions of whether the benefits of each treatment regimen at the proposed doses outweigh the risks for patients with relapsed or refractory disease after at least one prior line of therapy.
ODAC members voting no cited concerns about the lack of exploration of optimal dosing, as well as high rates of ocular toxicity and a lack of diversity among trial participants.
“This was a challenging decision because the efficacy data were strong, but the toxicity data were also very strong,” said Neil Vasan, MD, PhD, of New York University Langone Health in New York City.
Regarding optimal dosing, Vasan, who voted no on both questions, cited “a missed opportunity over the course of many years during the development of this drug to explore these different dosages,” but he also noted that “the building blocks are here to explore this question in the future.”
Belantamab, an antibody-drug conjugate targeting B-cell maturation antigen, was granted accelerated approval as a late-line therapy for relapsed or refractory multiple myeloma in August 2020 based on findings from the DREAMM-2 trial. However, GSK voluntarily withdrew the drug from the US market in 2023 after the confirmatory DREAMM-3 trial did not meet its primary endpoint of improved progression-free survival (PFS).
The company continued to explore belantamab in combination with other agents and in earlier lines of therapy. Based on findings from the DREAMM-7 and DREAMM-8 trials, which both showed improved PFS vs standard-of-care triplet therapies, the company submitted a new Biologics License Application in November 2024 seeking approval of the belantamab-based regimens.
Findings from DREAMM-7 and DREAMM-8 were reported at the 2025 American Society of Clinical Oncology conference in Chicago in June.
Both studies met their primary PFS endpoints, but the FDA expressed concerns about adverse events, dosing, and the relevance of the data for US patients and therefore sought input from ODAC members on the proposed dosages of 2.5 mg/kg every 3 weeks for the belantamab plus bortezomib and dexamethasone combination and 2.5 mg/kg in cycle 1, followed by 1.0 mg/kg every 4 weeks for the belantamab plus pomalidamide and dexamethasone combination.
Although GSK and several patients with multiple myeloma touted life-saving benefits of belantamab and argued that ocular toxicity associated with treatment is manageable and transient, most — but not all — ODAC members were unconvinced, at least as to the immediate questions regarding the benefit-risk profile.
“This is probably one of the most difficult votes I’ve done as a member of this committee,” said Grzegorz S. Nowakowski, MD, of the Mayo Clinic, Rochester, Minnesota, who voted yes on belantamab plus bortezomib and dexamethasone.
Nowakowski noted mistakes made from a regulatory perspective, including a lack of appropriate US patient representation in the trials and attention to dose optimization, but ultimately said that, as a practicing hematologist, he couldn’t ignore the drug’s clear activity, including a possible overall survival benefit, and the potential for mitigating toxicity with careful follow-up and dose reductions.
John DeFlice, MD, of Cedars-Sinai Samuel Oschin Cancer Center in Los Angeles — a multiple myeloma survivor and patient representative on the committee — voted yes on both questions, noting that, based on the testimony of patients and the clinical experience of the investigators, belantamab is “an amazing drug for an incurable disease.”
“I think [these] are the wrong issues to be evaluated,” DeFlice said of the specific questions posed by the FDA at the hearing.
The FDA considers the recommendations of its advisory panels in making final approval decisions but is not bound by them.
A version of this article first appeared on Medscape.com.
A bid by GlaxoSmithKline (GSK) to bring its multiple myeloma drug belantamab mafodotin (Blenrep) back to the market hit a stumbling block during an FDA panel meeting held on July 17.
The FDA’s Oncologic Drugs Advisory Committee (ODAC) voted 5-3 against belantamab in combination with bortezomib and dexamethasone and 7-1 against belantamab in combination with pomalidamide and dexamethasone on the specific questions of whether the benefits of each treatment regimen at the proposed doses outweigh the risks for patients with relapsed or refractory disease after at least one prior line of therapy.
ODAC members voting no cited concerns about the lack of exploration of optimal dosing, as well as high rates of ocular toxicity and a lack of diversity among trial participants.
“This was a challenging decision because the efficacy data were strong, but the toxicity data were also very strong,” said Neil Vasan, MD, PhD, of New York University Langone Health in New York City.
Regarding optimal dosing, Vasan, who voted no on both questions, cited “a missed opportunity over the course of many years during the development of this drug to explore these different dosages,” but he also noted that “the building blocks are here to explore this question in the future.”
Belantamab, an antibody-drug conjugate targeting B-cell maturation antigen, was granted accelerated approval as a late-line therapy for relapsed or refractory multiple myeloma in August 2020 based on findings from the DREAMM-2 trial. However, GSK voluntarily withdrew the drug from the US market in 2023 after the confirmatory DREAMM-3 trial did not meet its primary endpoint of improved progression-free survival (PFS).
The company continued to explore belantamab in combination with other agents and in earlier lines of therapy. Based on findings from the DREAMM-7 and DREAMM-8 trials, which both showed improved PFS vs standard-of-care triplet therapies, the company submitted a new Biologics License Application in November 2024 seeking approval of the belantamab-based regimens.
Findings from DREAMM-7 and DREAMM-8 were reported at the 2025 American Society of Clinical Oncology conference in Chicago in June.
Both studies met their primary PFS endpoints, but the FDA expressed concerns about adverse events, dosing, and the relevance of the data for US patients and therefore sought input from ODAC members on the proposed dosages of 2.5 mg/kg every 3 weeks for the belantamab plus bortezomib and dexamethasone combination and 2.5 mg/kg in cycle 1, followed by 1.0 mg/kg every 4 weeks for the belantamab plus pomalidamide and dexamethasone combination.
Although GSK and several patients with multiple myeloma touted life-saving benefits of belantamab and argued that ocular toxicity associated with treatment is manageable and transient, most — but not all — ODAC members were unconvinced, at least as to the immediate questions regarding the benefit-risk profile.
“This is probably one of the most difficult votes I’ve done as a member of this committee,” said Grzegorz S. Nowakowski, MD, of the Mayo Clinic, Rochester, Minnesota, who voted yes on belantamab plus bortezomib and dexamethasone.
Nowakowski noted mistakes made from a regulatory perspective, including a lack of appropriate US patient representation in the trials and attention to dose optimization, but ultimately said that, as a practicing hematologist, he couldn’t ignore the drug’s clear activity, including a possible overall survival benefit, and the potential for mitigating toxicity with careful follow-up and dose reductions.
John DeFlice, MD, of Cedars-Sinai Samuel Oschin Cancer Center in Los Angeles — a multiple myeloma survivor and patient representative on the committee — voted yes on both questions, noting that, based on the testimony of patients and the clinical experience of the investigators, belantamab is “an amazing drug for an incurable disease.”
“I think [these] are the wrong issues to be evaluated,” DeFlice said of the specific questions posed by the FDA at the hearing.
The FDA considers the recommendations of its advisory panels in making final approval decisions but is not bound by them.
A version of this article first appeared on Medscape.com.
A bid by GlaxoSmithKline (GSK) to bring its multiple myeloma drug belantamab mafodotin (Blenrep) back to the market hit a stumbling block during an FDA panel meeting held on July 17.
The FDA’s Oncologic Drugs Advisory Committee (ODAC) voted 5-3 against belantamab in combination with bortezomib and dexamethasone and 7-1 against belantamab in combination with pomalidamide and dexamethasone on the specific questions of whether the benefits of each treatment regimen at the proposed doses outweigh the risks for patients with relapsed or refractory disease after at least one prior line of therapy.
ODAC members voting no cited concerns about the lack of exploration of optimal dosing, as well as high rates of ocular toxicity and a lack of diversity among trial participants.
“This was a challenging decision because the efficacy data were strong, but the toxicity data were also very strong,” said Neil Vasan, MD, PhD, of New York University Langone Health in New York City.
Regarding optimal dosing, Vasan, who voted no on both questions, cited “a missed opportunity over the course of many years during the development of this drug to explore these different dosages,” but he also noted that “the building blocks are here to explore this question in the future.”
Belantamab, an antibody-drug conjugate targeting B-cell maturation antigen, was granted accelerated approval as a late-line therapy for relapsed or refractory multiple myeloma in August 2020 based on findings from the DREAMM-2 trial. However, GSK voluntarily withdrew the drug from the US market in 2023 after the confirmatory DREAMM-3 trial did not meet its primary endpoint of improved progression-free survival (PFS).
The company continued to explore belantamab in combination with other agents and in earlier lines of therapy. Based on findings from the DREAMM-7 and DREAMM-8 trials, which both showed improved PFS vs standard-of-care triplet therapies, the company submitted a new Biologics License Application in November 2024 seeking approval of the belantamab-based regimens.
Findings from DREAMM-7 and DREAMM-8 were reported at the 2025 American Society of Clinical Oncology conference in Chicago in June.
Both studies met their primary PFS endpoints, but the FDA expressed concerns about adverse events, dosing, and the relevance of the data for US patients and therefore sought input from ODAC members on the proposed dosages of 2.5 mg/kg every 3 weeks for the belantamab plus bortezomib and dexamethasone combination and 2.5 mg/kg in cycle 1, followed by 1.0 mg/kg every 4 weeks for the belantamab plus pomalidamide and dexamethasone combination.
Although GSK and several patients with multiple myeloma touted life-saving benefits of belantamab and argued that ocular toxicity associated with treatment is manageable and transient, most — but not all — ODAC members were unconvinced, at least as to the immediate questions regarding the benefit-risk profile.
“This is probably one of the most difficult votes I’ve done as a member of this committee,” said Grzegorz S. Nowakowski, MD, of the Mayo Clinic, Rochester, Minnesota, who voted yes on belantamab plus bortezomib and dexamethasone.
Nowakowski noted mistakes made from a regulatory perspective, including a lack of appropriate US patient representation in the trials and attention to dose optimization, but ultimately said that, as a practicing hematologist, he couldn’t ignore the drug’s clear activity, including a possible overall survival benefit, and the potential for mitigating toxicity with careful follow-up and dose reductions.
John DeFlice, MD, of Cedars-Sinai Samuel Oschin Cancer Center in Los Angeles — a multiple myeloma survivor and patient representative on the committee — voted yes on both questions, noting that, based on the testimony of patients and the clinical experience of the investigators, belantamab is “an amazing drug for an incurable disease.”
“I think [these] are the wrong issues to be evaluated,” DeFlice said of the specific questions posed by the FDA at the hearing.
The FDA considers the recommendations of its advisory panels in making final approval decisions but is not bound by them.
A version of this article first appeared on Medscape.com.
Stay Alert to Sleep Apnea Burden in the Military
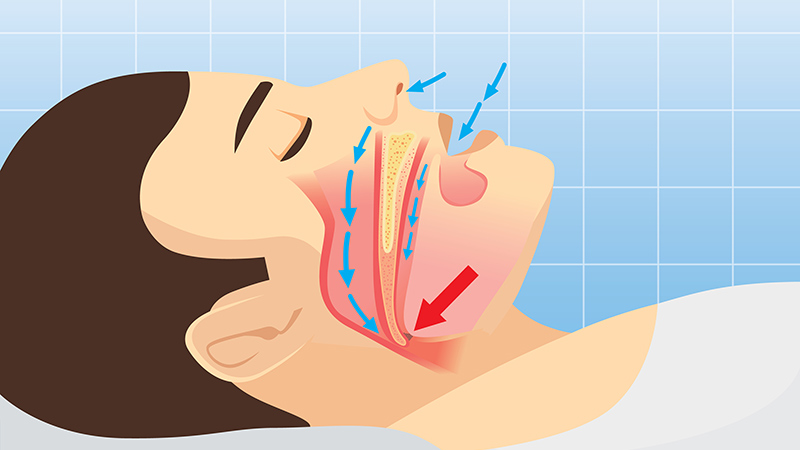
Obstructive sleep apnea (OSA) was associated with a significantly increased risk for adverse health outcomes and health care resource use among military personnel in the US, according to data from about 120,000 active-duty service members.
OSA and other clinical sleep disorders are common among military personnel, driven in part by demanding, nontraditional work schedules that can exacerbate sleep problems, but OSA’s impact in this population has not been well-studied, Emerson M. Wickwire, PhD, of the University of Maryland School of Medicine, Baltimore, and colleagues wrote in a new paper published in Chest.
In the current health economic climate of increasing costs and limited resources, the economic aspects of sleep disorders have never been more important, Wickwire said in an interview. The data in this study are the first to quantify the health and utilization burden of OSA in the US military and can support military decision-makers regarding allocation of scarce resources, he said.
To assess the burden of OSA in the military, they reviewed fully de-identified data from 59,203 active-duty military personnel with diagnoses of OSA and compared them with 59,203 active-duty military personnel without OSA. The participants ranged in age from 18 to 64 years; 7.4% were women and 64.5% were white individuals. Study outcomes included new diagnoses of physical and psychological health conditions, as well as health care resource use in the first year after the index date.
About one third of the participants were in the Army (38.7%), 25.6% were in the Air Force, 23.5% were in the Navy, 5.8% were in the Marines, 5.7% were in the Coast Guard, and 0.7% were in the Public Health Service.
Over the 1-year study period, military personnel with OSA diagnoses were significantly more likely to experience new physical and psychological adverse events than control individuals without OSA, based on proportional hazards models. The physical conditions with the greatest increased risk in the OSA group were traumatic brain injury and cardiovascular disease (which included acute myocardial infarction, atrial fibrillation, ischemic heart disease, and peripheral procedures), with hazard ratios (HRs) 3.27 and 2.32, respectively. The psychological conditions with the greatest increased risk in the OSA group vs control individuals were posttraumatic stress disorder (PTSD) and anxiety (HR, 4.41, and HR, 3.35, respectively).
Individuals with OSA also showed increased use of healthcare resources compared with control individuals without OSA, with an additional 170,511 outpatient visits, 66 inpatient visits, and 1,852 emergency department visits.
Don’t Discount OSA in Military Personnel
“From a clinical perspective, these findings underscore the importance of recognizing OSA as a critical risk factor for a wide array of physical and psychological health outcomes,” the researchers wrote in their discussion.
The results highlight the need for more clinical attention to patient screening, triage, and delivery of care, but efforts are limited by the documented shortage of sleep specialists in the military health system, they noted.
Key limitations of the study include the use of an administrative claims data source, which did not include clinical information such as disease severity or daytime symptoms, and the nonrandomized, observational study design, Wickwire told this news organization.
Looking ahead, the researchers at the University of Maryland School of Medicine and the Uniformed Services University, Bethesda, Maryland, are launching a new trial to assess the clinical effectiveness and cost-effectiveness of telehealth visits for military beneficiaries diagnosed with OSA as a way to manage the shortage of sleep specialists in the military health system, according to a press release from the University of Maryland.
“Although the association between poor sleep and traumatic stress is well-known, present results highlight striking associations between sleep apnea and posttraumatic stress disorder, traumatic brain injury, and musculoskeletal injuries, which are key outcomes from the military perspective,” Wickwire told this news organization.
“Our most important clinical recommendation is for healthcare providers to be on alert for signs and symptoms of OSA, including snoring, daytime sleepiness, and morning dry mouth,” said Wickwire. “Primary care and mental health providers should be especially attuned,” he added.
Results Not Surprising, but Research Gaps Remain
“The sleep health of active-duty military personnel is not only vital for optimal military performance but also impacts the health of Veterans after separation from the military,” said Q. Afifa Shamim-Uzzaman, MD, an associate professor and a sleep medicine specialist at the University of Michigan, Ann Arbor, Michigan, in an interview.
The current study identifies increased utilization of healthcare resources by active-duty personnel with sleep apnea, and outcomes were not surprising, said Shamim-Uzzaman, who is employed by the Veterans’ Health Administration, but was not involved in the current study.
The association between untreated OSA and medical and psychological comorbidities such as cardiovascular disease, diabetes, and mood disorders such as depression and anxiety is well-known, Shamim-Uzzaman said. “Patients with depression who also have sleep disturbances are at higher risk for suicide — the strength of this association is such that it led the Veterans’ Health Administration to mandate suicide screening for Veterans seen in its sleep clinics,” he added.
“We also know that untreated OSA is associated with excessive daytime sleepiness, slowed reaction times, and increased risk of motor vehicle accidents, all of which can contribute to sustaining injuries such as traumatic brain injury,” said Shamim-Uzzaman. “Emerging evidence also suggests that sleep disruption prior to exposure to trauma increases the risk of developing PTSD. Therefore, it is not surprising that patients with sleep apnea would have higher healthcare utilization for non-OSA conditions than those without sleep apnea,” he noted.
In clinical practice, the study underscores the importance of identifying and managing sleep health in military personnel, who frequently work nontraditional schedules with long, sustained shifts in grueling conditions not conducive to healthy sleep, Shamim-Uzzaman told this news organization. “Although the harsh work environments that our active-duty military endure come part and parcel with the job, clinicians caring for these individuals should ask specifically about their sleep and working schedules to optimize sleep as best as possible; this should include, but not be limited to, screening and testing for sleep disordered breathing and insomnia,” he said.
The current study has several limitations, including the inability to control for smoking or alcohol use, which are common in military personnel and associated with increased morbidity, said Shamim-Uzzaman. The study also did not assess the impact of other confounding factors, such as sleep duration and daytime sleepiness, that could impact the results, especially the association of OSA and traumatic brain injury, he noted. “More research is needed to assess the impact of these factors as well as the effect of treatment of OSA on comorbidities and healthcare utilization,” he said.
This study was supported by the Military Health Services Research Program.
Wickwire’s institution had received research funding from the American Academy of Sleep Medicine Foundation, Department of Defense, Merck, National Institutes of Health/National Institute on Aging, ResMed, the ResMed Foundation, and the SRS Foundation. Wickwire disclosed serving as a scientific consultant to Axsome Therapeutics, Dayzz, Eisai, EnsoData, Idorsia, Merck, Nox Health, Primasun, Purdue, and ResMed and is an equity shareholder in Well Tap.
Shamim-Uzzaman is an employee of the Veterans’ Health Administration.
A version of this article first appeared on Medscape.com.
Obstructive sleep apnea (OSA) was associated with a significantly increased risk for adverse health outcomes and health care resource use among military personnel in the US, according to data from about 120,000 active-duty service members.
OSA and other clinical sleep disorders are common among military personnel, driven in part by demanding, nontraditional work schedules that can exacerbate sleep problems, but OSA’s impact in this population has not been well-studied, Emerson M. Wickwire, PhD, of the University of Maryland School of Medicine, Baltimore, and colleagues wrote in a new paper published in Chest.
In the current health economic climate of increasing costs and limited resources, the economic aspects of sleep disorders have never been more important, Wickwire said in an interview. The data in this study are the first to quantify the health and utilization burden of OSA in the US military and can support military decision-makers regarding allocation of scarce resources, he said.
To assess the burden of OSA in the military, they reviewed fully de-identified data from 59,203 active-duty military personnel with diagnoses of OSA and compared them with 59,203 active-duty military personnel without OSA. The participants ranged in age from 18 to 64 years; 7.4% were women and 64.5% were white individuals. Study outcomes included new diagnoses of physical and psychological health conditions, as well as health care resource use in the first year after the index date.
About one third of the participants were in the Army (38.7%), 25.6% were in the Air Force, 23.5% were in the Navy, 5.8% were in the Marines, 5.7% were in the Coast Guard, and 0.7% were in the Public Health Service.
Over the 1-year study period, military personnel with OSA diagnoses were significantly more likely to experience new physical and psychological adverse events than control individuals without OSA, based on proportional hazards models. The physical conditions with the greatest increased risk in the OSA group were traumatic brain injury and cardiovascular disease (which included acute myocardial infarction, atrial fibrillation, ischemic heart disease, and peripheral procedures), with hazard ratios (HRs) 3.27 and 2.32, respectively. The psychological conditions with the greatest increased risk in the OSA group vs control individuals were posttraumatic stress disorder (PTSD) and anxiety (HR, 4.41, and HR, 3.35, respectively).
Individuals with OSA also showed increased use of healthcare resources compared with control individuals without OSA, with an additional 170,511 outpatient visits, 66 inpatient visits, and 1,852 emergency department visits.
Don’t Discount OSA in Military Personnel
“From a clinical perspective, these findings underscore the importance of recognizing OSA as a critical risk factor for a wide array of physical and psychological health outcomes,” the researchers wrote in their discussion.
The results highlight the need for more clinical attention to patient screening, triage, and delivery of care, but efforts are limited by the documented shortage of sleep specialists in the military health system, they noted.
Key limitations of the study include the use of an administrative claims data source, which did not include clinical information such as disease severity or daytime symptoms, and the nonrandomized, observational study design, Wickwire told this news organization.
Looking ahead, the researchers at the University of Maryland School of Medicine and the Uniformed Services University, Bethesda, Maryland, are launching a new trial to assess the clinical effectiveness and cost-effectiveness of telehealth visits for military beneficiaries diagnosed with OSA as a way to manage the shortage of sleep specialists in the military health system, according to a press release from the University of Maryland.
“Although the association between poor sleep and traumatic stress is well-known, present results highlight striking associations between sleep apnea and posttraumatic stress disorder, traumatic brain injury, and musculoskeletal injuries, which are key outcomes from the military perspective,” Wickwire told this news organization.
“Our most important clinical recommendation is for healthcare providers to be on alert for signs and symptoms of OSA, including snoring, daytime sleepiness, and morning dry mouth,” said Wickwire. “Primary care and mental health providers should be especially attuned,” he added.
Results Not Surprising, but Research Gaps Remain
“The sleep health of active-duty military personnel is not only vital for optimal military performance but also impacts the health of Veterans after separation from the military,” said Q. Afifa Shamim-Uzzaman, MD, an associate professor and a sleep medicine specialist at the University of Michigan, Ann Arbor, Michigan, in an interview.
The current study identifies increased utilization of healthcare resources by active-duty personnel with sleep apnea, and outcomes were not surprising, said Shamim-Uzzaman, who is employed by the Veterans’ Health Administration, but was not involved in the current study.
The association between untreated OSA and medical and psychological comorbidities such as cardiovascular disease, diabetes, and mood disorders such as depression and anxiety is well-known, Shamim-Uzzaman said. “Patients with depression who also have sleep disturbances are at higher risk for suicide — the strength of this association is such that it led the Veterans’ Health Administration to mandate suicide screening for Veterans seen in its sleep clinics,” he added.
“We also know that untreated OSA is associated with excessive daytime sleepiness, slowed reaction times, and increased risk of motor vehicle accidents, all of which can contribute to sustaining injuries such as traumatic brain injury,” said Shamim-Uzzaman. “Emerging evidence also suggests that sleep disruption prior to exposure to trauma increases the risk of developing PTSD. Therefore, it is not surprising that patients with sleep apnea would have higher healthcare utilization for non-OSA conditions than those without sleep apnea,” he noted.
In clinical practice, the study underscores the importance of identifying and managing sleep health in military personnel, who frequently work nontraditional schedules with long, sustained shifts in grueling conditions not conducive to healthy sleep, Shamim-Uzzaman told this news organization. “Although the harsh work environments that our active-duty military endure come part and parcel with the job, clinicians caring for these individuals should ask specifically about their sleep and working schedules to optimize sleep as best as possible; this should include, but not be limited to, screening and testing for sleep disordered breathing and insomnia,” he said.
The current study has several limitations, including the inability to control for smoking or alcohol use, which are common in military personnel and associated with increased morbidity, said Shamim-Uzzaman. The study also did not assess the impact of other confounding factors, such as sleep duration and daytime sleepiness, that could impact the results, especially the association of OSA and traumatic brain injury, he noted. “More research is needed to assess the impact of these factors as well as the effect of treatment of OSA on comorbidities and healthcare utilization,” he said.
This study was supported by the Military Health Services Research Program.
Wickwire’s institution had received research funding from the American Academy of Sleep Medicine Foundation, Department of Defense, Merck, National Institutes of Health/National Institute on Aging, ResMed, the ResMed Foundation, and the SRS Foundation. Wickwire disclosed serving as a scientific consultant to Axsome Therapeutics, Dayzz, Eisai, EnsoData, Idorsia, Merck, Nox Health, Primasun, Purdue, and ResMed and is an equity shareholder in Well Tap.
Shamim-Uzzaman is an employee of the Veterans’ Health Administration.
A version of this article first appeared on Medscape.com.
Obstructive sleep apnea (OSA) was associated with a significantly increased risk for adverse health outcomes and health care resource use among military personnel in the US, according to data from about 120,000 active-duty service members.
OSA and other clinical sleep disorders are common among military personnel, driven in part by demanding, nontraditional work schedules that can exacerbate sleep problems, but OSA’s impact in this population has not been well-studied, Emerson M. Wickwire, PhD, of the University of Maryland School of Medicine, Baltimore, and colleagues wrote in a new paper published in Chest.
In the current health economic climate of increasing costs and limited resources, the economic aspects of sleep disorders have never been more important, Wickwire said in an interview. The data in this study are the first to quantify the health and utilization burden of OSA in the US military and can support military decision-makers regarding allocation of scarce resources, he said.
To assess the burden of OSA in the military, they reviewed fully de-identified data from 59,203 active-duty military personnel with diagnoses of OSA and compared them with 59,203 active-duty military personnel without OSA. The participants ranged in age from 18 to 64 years; 7.4% were women and 64.5% were white individuals. Study outcomes included new diagnoses of physical and psychological health conditions, as well as health care resource use in the first year after the index date.
About one third of the participants were in the Army (38.7%), 25.6% were in the Air Force, 23.5% were in the Navy, 5.8% were in the Marines, 5.7% were in the Coast Guard, and 0.7% were in the Public Health Service.
Over the 1-year study period, military personnel with OSA diagnoses were significantly more likely to experience new physical and psychological adverse events than control individuals without OSA, based on proportional hazards models. The physical conditions with the greatest increased risk in the OSA group were traumatic brain injury and cardiovascular disease (which included acute myocardial infarction, atrial fibrillation, ischemic heart disease, and peripheral procedures), with hazard ratios (HRs) 3.27 and 2.32, respectively. The psychological conditions with the greatest increased risk in the OSA group vs control individuals were posttraumatic stress disorder (PTSD) and anxiety (HR, 4.41, and HR, 3.35, respectively).
Individuals with OSA also showed increased use of healthcare resources compared with control individuals without OSA, with an additional 170,511 outpatient visits, 66 inpatient visits, and 1,852 emergency department visits.
Don’t Discount OSA in Military Personnel
“From a clinical perspective, these findings underscore the importance of recognizing OSA as a critical risk factor for a wide array of physical and psychological health outcomes,” the researchers wrote in their discussion.
The results highlight the need for more clinical attention to patient screening, triage, and delivery of care, but efforts are limited by the documented shortage of sleep specialists in the military health system, they noted.
Key limitations of the study include the use of an administrative claims data source, which did not include clinical information such as disease severity or daytime symptoms, and the nonrandomized, observational study design, Wickwire told this news organization.
Looking ahead, the researchers at the University of Maryland School of Medicine and the Uniformed Services University, Bethesda, Maryland, are launching a new trial to assess the clinical effectiveness and cost-effectiveness of telehealth visits for military beneficiaries diagnosed with OSA as a way to manage the shortage of sleep specialists in the military health system, according to a press release from the University of Maryland.
“Although the association between poor sleep and traumatic stress is well-known, present results highlight striking associations between sleep apnea and posttraumatic stress disorder, traumatic brain injury, and musculoskeletal injuries, which are key outcomes from the military perspective,” Wickwire told this news organization.
“Our most important clinical recommendation is for healthcare providers to be on alert for signs and symptoms of OSA, including snoring, daytime sleepiness, and morning dry mouth,” said Wickwire. “Primary care and mental health providers should be especially attuned,” he added.
Results Not Surprising, but Research Gaps Remain
“The sleep health of active-duty military personnel is not only vital for optimal military performance but also impacts the health of Veterans after separation from the military,” said Q. Afifa Shamim-Uzzaman, MD, an associate professor and a sleep medicine specialist at the University of Michigan, Ann Arbor, Michigan, in an interview.
The current study identifies increased utilization of healthcare resources by active-duty personnel with sleep apnea, and outcomes were not surprising, said Shamim-Uzzaman, who is employed by the Veterans’ Health Administration, but was not involved in the current study.
The association between untreated OSA and medical and psychological comorbidities such as cardiovascular disease, diabetes, and mood disorders such as depression and anxiety is well-known, Shamim-Uzzaman said. “Patients with depression who also have sleep disturbances are at higher risk for suicide — the strength of this association is such that it led the Veterans’ Health Administration to mandate suicide screening for Veterans seen in its sleep clinics,” he added.
“We also know that untreated OSA is associated with excessive daytime sleepiness, slowed reaction times, and increased risk of motor vehicle accidents, all of which can contribute to sustaining injuries such as traumatic brain injury,” said Shamim-Uzzaman. “Emerging evidence also suggests that sleep disruption prior to exposure to trauma increases the risk of developing PTSD. Therefore, it is not surprising that patients with sleep apnea would have higher healthcare utilization for non-OSA conditions than those without sleep apnea,” he noted.
In clinical practice, the study underscores the importance of identifying and managing sleep health in military personnel, who frequently work nontraditional schedules with long, sustained shifts in grueling conditions not conducive to healthy sleep, Shamim-Uzzaman told this news organization. “Although the harsh work environments that our active-duty military endure come part and parcel with the job, clinicians caring for these individuals should ask specifically about their sleep and working schedules to optimize sleep as best as possible; this should include, but not be limited to, screening and testing for sleep disordered breathing and insomnia,” he said.
The current study has several limitations, including the inability to control for smoking or alcohol use, which are common in military personnel and associated with increased morbidity, said Shamim-Uzzaman. The study also did not assess the impact of other confounding factors, such as sleep duration and daytime sleepiness, that could impact the results, especially the association of OSA and traumatic brain injury, he noted. “More research is needed to assess the impact of these factors as well as the effect of treatment of OSA on comorbidities and healthcare utilization,” he said.
This study was supported by the Military Health Services Research Program.
Wickwire’s institution had received research funding from the American Academy of Sleep Medicine Foundation, Department of Defense, Merck, National Institutes of Health/National Institute on Aging, ResMed, the ResMed Foundation, and the SRS Foundation. Wickwire disclosed serving as a scientific consultant to Axsome Therapeutics, Dayzz, Eisai, EnsoData, Idorsia, Merck, Nox Health, Primasun, Purdue, and ResMed and is an equity shareholder in Well Tap.
Shamim-Uzzaman is an employee of the Veterans’ Health Administration.
A version of this article first appeared on Medscape.com.
Treating Metastatic RCC: From Risk Assessment to Therapy Selection
Treating Metastatic RCC: From Risk Assessment to Therapy Selection
Treatment of metastatic renal cell carcinoma (RCC) is complex and requires careful analysis of risk and treatment options, an oncologist said at the July Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, regarding treating veterans with kidney cancer.
“We’ve come a long way in treating this disease, but individualizing therapy remains critical, especially in complex populations like our veterans,” said Matthew B. Rettig, MD, chief of Hematology-Oncology at the Veterans Affairs Greater Los Angeles Healthcare System and professor of Medicine and Urology at UCLA.
Rettig emphasized 2 critical early questions clinicians should consider when encountering metastatic RCC. First: Can the patient be treated with localized interventions such as metastasectomy, radiation therapy, or nephrectomy? These can be curative, Rettig said.
And second: Does the patient currently need systemic therapy? “[There are] a small subset of patients,” Rettig said, “who go into a durable, complete remission, dare I say ‘cure,’ with immunotherapeutic-based approaches.”
Rettig highlighted the International Metastatic Renal Cell Carcinoma Database Consortium criteria as a guide for clinicians as they determine the best strategy for treatment. The Database Consortium estimates survival in various lines of therapy by incorporating 6 prognostic factors: anemia, hypercalcemia, neutrophilia, thrombocytosis, performance status, and time from diagnosis to treatment.
These criteria classify patients into favorable, intermediate, or poor risk categories that can guide first-line systemic therapy. The criteria also provide estimates of median survival.
Rettig noted a “huge percentage” of veterans mirror the intermediate-risk demographics of clinical trial cohorts but often present with greater comorbidity burdens: “That plays into whether we treat and how we treat,” he said.
Rettig highlighted kidney cancer guidelines from the National Comprehensive Cancer Network and noted that several trials examined first-line use of combinations of vascular endothelial growth factor receptor tyrosine kinase inhibitors (TKIs) and checkpoint inhibitors.
There’s a general theme in the findings, he said: “You have OS (overall survival) and PFS (progression-free survival) benefit in the intermediate/poor risk group, but only PFS benefit in the patients who have favorable-risk disease. And you see higher objective response rates with the combinations.
“If you have a patient who's highly symptomatic or has an organ system threatened by a metastasis, you'd want to use a combination that elicits a higher objective response rate,” Rettig added.
A TKI is going to be the most appropriate second-line therapy for patients who received a prior checkpoint inhibitor, Rettig said.
“Don't change to another checkpoint inhibitor,” he said. “We have enough phase 3 data that indicates checkpoint inhibitors are no longer really adding to benefit once they’ve had a checkpoint inhibitor.”
Rettig said to even consider checkpoint inhibitors for patients who are checkpoint inhibitor-naïve, especially given the potential for durable remissions. As for third-line therapy, he said, “we have both belzutifan and tivozanib, which have been shown to improve PFS. More studies are ongoing.”
There are many adverse events linked to TKIs, Rettig said, including cardiovascular problems, thrombosis, hypertension, heart failure, torsades de pointes, QT prolongation, and gastrointestinal toxicity. TKIs tend to be the major drivers of adverse events in combination therapy.
Rettig emphasized the shorter half-life of the TKI axitinib, which he said allows for easier management of toxicities: “That’s why it’s preferred in the VA RCC clinical pathway.”
Rettig discloses relationships with Ambrx, Amgen, AVEO, Bayer, INmune Bio, Johnson & Johnson Health Care Systems, Lantheus, Merck, Myovant, Novartis, ORIC, and Progenics.
Treatment of metastatic renal cell carcinoma (RCC) is complex and requires careful analysis of risk and treatment options, an oncologist said at the July Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, regarding treating veterans with kidney cancer.
“We’ve come a long way in treating this disease, but individualizing therapy remains critical, especially in complex populations like our veterans,” said Matthew B. Rettig, MD, chief of Hematology-Oncology at the Veterans Affairs Greater Los Angeles Healthcare System and professor of Medicine and Urology at UCLA.
Rettig emphasized 2 critical early questions clinicians should consider when encountering metastatic RCC. First: Can the patient be treated with localized interventions such as metastasectomy, radiation therapy, or nephrectomy? These can be curative, Rettig said.
And second: Does the patient currently need systemic therapy? “[There are] a small subset of patients,” Rettig said, “who go into a durable, complete remission, dare I say ‘cure,’ with immunotherapeutic-based approaches.”
Rettig highlighted the International Metastatic Renal Cell Carcinoma Database Consortium criteria as a guide for clinicians as they determine the best strategy for treatment. The Database Consortium estimates survival in various lines of therapy by incorporating 6 prognostic factors: anemia, hypercalcemia, neutrophilia, thrombocytosis, performance status, and time from diagnosis to treatment.
These criteria classify patients into favorable, intermediate, or poor risk categories that can guide first-line systemic therapy. The criteria also provide estimates of median survival.
Rettig noted a “huge percentage” of veterans mirror the intermediate-risk demographics of clinical trial cohorts but often present with greater comorbidity burdens: “That plays into whether we treat and how we treat,” he said.
Rettig highlighted kidney cancer guidelines from the National Comprehensive Cancer Network and noted that several trials examined first-line use of combinations of vascular endothelial growth factor receptor tyrosine kinase inhibitors (TKIs) and checkpoint inhibitors.
There’s a general theme in the findings, he said: “You have OS (overall survival) and PFS (progression-free survival) benefit in the intermediate/poor risk group, but only PFS benefit in the patients who have favorable-risk disease. And you see higher objective response rates with the combinations.
“If you have a patient who's highly symptomatic or has an organ system threatened by a metastasis, you'd want to use a combination that elicits a higher objective response rate,” Rettig added.
A TKI is going to be the most appropriate second-line therapy for patients who received a prior checkpoint inhibitor, Rettig said.
“Don't change to another checkpoint inhibitor,” he said. “We have enough phase 3 data that indicates checkpoint inhibitors are no longer really adding to benefit once they’ve had a checkpoint inhibitor.”
Rettig said to even consider checkpoint inhibitors for patients who are checkpoint inhibitor-naïve, especially given the potential for durable remissions. As for third-line therapy, he said, “we have both belzutifan and tivozanib, which have been shown to improve PFS. More studies are ongoing.”
There are many adverse events linked to TKIs, Rettig said, including cardiovascular problems, thrombosis, hypertension, heart failure, torsades de pointes, QT prolongation, and gastrointestinal toxicity. TKIs tend to be the major drivers of adverse events in combination therapy.
Rettig emphasized the shorter half-life of the TKI axitinib, which he said allows for easier management of toxicities: “That’s why it’s preferred in the VA RCC clinical pathway.”
Rettig discloses relationships with Ambrx, Amgen, AVEO, Bayer, INmune Bio, Johnson & Johnson Health Care Systems, Lantheus, Merck, Myovant, Novartis, ORIC, and Progenics.
Treatment of metastatic renal cell carcinoma (RCC) is complex and requires careful analysis of risk and treatment options, an oncologist said at the July Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, regarding treating veterans with kidney cancer.
“We’ve come a long way in treating this disease, but individualizing therapy remains critical, especially in complex populations like our veterans,” said Matthew B. Rettig, MD, chief of Hematology-Oncology at the Veterans Affairs Greater Los Angeles Healthcare System and professor of Medicine and Urology at UCLA.
Rettig emphasized 2 critical early questions clinicians should consider when encountering metastatic RCC. First: Can the patient be treated with localized interventions such as metastasectomy, radiation therapy, or nephrectomy? These can be curative, Rettig said.
And second: Does the patient currently need systemic therapy? “[There are] a small subset of patients,” Rettig said, “who go into a durable, complete remission, dare I say ‘cure,’ with immunotherapeutic-based approaches.”
Rettig highlighted the International Metastatic Renal Cell Carcinoma Database Consortium criteria as a guide for clinicians as they determine the best strategy for treatment. The Database Consortium estimates survival in various lines of therapy by incorporating 6 prognostic factors: anemia, hypercalcemia, neutrophilia, thrombocytosis, performance status, and time from diagnosis to treatment.
These criteria classify patients into favorable, intermediate, or poor risk categories that can guide first-line systemic therapy. The criteria also provide estimates of median survival.
Rettig noted a “huge percentage” of veterans mirror the intermediate-risk demographics of clinical trial cohorts but often present with greater comorbidity burdens: “That plays into whether we treat and how we treat,” he said.
Rettig highlighted kidney cancer guidelines from the National Comprehensive Cancer Network and noted that several trials examined first-line use of combinations of vascular endothelial growth factor receptor tyrosine kinase inhibitors (TKIs) and checkpoint inhibitors.
There’s a general theme in the findings, he said: “You have OS (overall survival) and PFS (progression-free survival) benefit in the intermediate/poor risk group, but only PFS benefit in the patients who have favorable-risk disease. And you see higher objective response rates with the combinations.
“If you have a patient who's highly symptomatic or has an organ system threatened by a metastasis, you'd want to use a combination that elicits a higher objective response rate,” Rettig added.
A TKI is going to be the most appropriate second-line therapy for patients who received a prior checkpoint inhibitor, Rettig said.
“Don't change to another checkpoint inhibitor,” he said. “We have enough phase 3 data that indicates checkpoint inhibitors are no longer really adding to benefit once they’ve had a checkpoint inhibitor.”
Rettig said to even consider checkpoint inhibitors for patients who are checkpoint inhibitor-naïve, especially given the potential for durable remissions. As for third-line therapy, he said, “we have both belzutifan and tivozanib, which have been shown to improve PFS. More studies are ongoing.”
There are many adverse events linked to TKIs, Rettig said, including cardiovascular problems, thrombosis, hypertension, heart failure, torsades de pointes, QT prolongation, and gastrointestinal toxicity. TKIs tend to be the major drivers of adverse events in combination therapy.
Rettig emphasized the shorter half-life of the TKI axitinib, which he said allows for easier management of toxicities: “That’s why it’s preferred in the VA RCC clinical pathway.”
Rettig discloses relationships with Ambrx, Amgen, AVEO, Bayer, INmune Bio, Johnson & Johnson Health Care Systems, Lantheus, Merck, Myovant, Novartis, ORIC, and Progenics.
Treating Metastatic RCC: From Risk Assessment to Therapy Selection
Treating Metastatic RCC: From Risk Assessment to Therapy Selection
Renal Cell Carcinoma: What You Need to Know About Hereditary Syndromes
Renal Cell Carcinoma: What You Need to Know About Hereditary Syndromes
The role of hereditary syndromes in renal cell carcinoma (RCC) might be easily missed, a kidney cancer specialist said during a recent Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, though careful clinical evaluation can uncover genetic traits that may affect treatment and familial risk.
“The importance of finding or identifying hereditary forms of kidney cancer really should not be underestimated,” said urologist Brian Shuch, MD, director of the UCLA Kidney Cancer Program, on treating veterans with kidney cancer.
According to Shuch, recent data suggest that about 4.5% of patients with RCC have a hereditary syndrome: “A lot of times, these hide in plain sight. You have to really look deep and try to figure things out and understand that maybe they have a hereditary form of kidney cancer.”
It is important to consider early genetic testing, Shuch said. Red flags for hereditary syndromes include early-onset RCC (age ≤ 45 years), multifocal tumors, bilateral tumors (especially in younger individuals), or a relevant family personal history, he said.
Unusual skin conditions are also potential signs, Shuch said. These can include leiomyomas, fibrofolliculomas, and angiofibromas: “Patients have lots of lumps or bumps.”
“When I look at a patient, I go head to toe and ask if there any issues with your vision, any issues with your hearing, any issues swallowing,” he explained at the meeting. “Do you have any problems with heart issues, adrenal issues? You’ve got to go through each organ, and it can lead you to different things.”
Shuch highlighted Von Hippel-Lindau (VHL) syndrome, which affects 1 in 25,000 people. About 80% to 90% of these patients have a family history, Shuch said.
But the others do not. “Unfortunately, some get diagnosed later in life because they don’t get cascade testing starting at aged 2, which is recommended. These are the patients who might be coming into the ER with a hemangioblastoma or picking up the phone and all of a sudden being deaf in one ear due to an endolymphatic sac tumor.
“We want to limit metastatic spread and preserve the kidneys,” Shuch said. “We don’t want to be doing radical nephrectomies. We want to avoid chronic kidney disease, prevent end-stage renal disease, and maximize quality of life.”
It’s a good idea to avoid surgical removal unless a patient’s tumor grows to be > 3 cm, a line that indicates risk of metastases, he said.
In terms of treatment, Shuch highlighted a 2021 study that showed benefit in VHL from belzutifan (Welireg), an oral HIF-2 α inhibitor approved by the US Food and Drug Administration. The medication significantly reduced the need for surgical intervention.
“Patients go on this drug, and surgeons are putting their scalpels down,” said Shuch, who worked on the 2021 study.
Other hereditary syndromes include the rare hereditary papillary RCC, and Birt-Hogg-Dubé syndrome, believed to affect 1 in 200,000 people but may be more common, he said.
Birt-Hogg-Dubé syndrome is linked to lung cysts, lung collapse, and skin manifestations. The 3 cm surgery rule is appropriate in these cases, Shuch said, and metastases are rare.
Another condition, hereditary leiomyomatosis and RCC, is the most dangerous hereditary form. Originally thought to affect 1 in 200,000 people, hereditary leiomyomatosis and RCC is similar to Birt-Hogg-Dubé syndrome in that it is believed to be more common.
“You will see this,” Shuch predicted.
Shuch advised colleagues to intervene early and take a large margin during surgery.
He also highlighted familial paraganglioma syndrome, which is associated with gastrointestinal stromal tumors, and Cowden syndrome, which is linked to skin manifestations and breast, thyroid, and endometrial cancer.
Shuch reported that he had no disclosures.
The role of hereditary syndromes in renal cell carcinoma (RCC) might be easily missed, a kidney cancer specialist said during a recent Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, though careful clinical evaluation can uncover genetic traits that may affect treatment and familial risk.
“The importance of finding or identifying hereditary forms of kidney cancer really should not be underestimated,” said urologist Brian Shuch, MD, director of the UCLA Kidney Cancer Program, on treating veterans with kidney cancer.
According to Shuch, recent data suggest that about 4.5% of patients with RCC have a hereditary syndrome: “A lot of times, these hide in plain sight. You have to really look deep and try to figure things out and understand that maybe they have a hereditary form of kidney cancer.”
It is important to consider early genetic testing, Shuch said. Red flags for hereditary syndromes include early-onset RCC (age ≤ 45 years), multifocal tumors, bilateral tumors (especially in younger individuals), or a relevant family personal history, he said.
Unusual skin conditions are also potential signs, Shuch said. These can include leiomyomas, fibrofolliculomas, and angiofibromas: “Patients have lots of lumps or bumps.”
“When I look at a patient, I go head to toe and ask if there any issues with your vision, any issues with your hearing, any issues swallowing,” he explained at the meeting. “Do you have any problems with heart issues, adrenal issues? You’ve got to go through each organ, and it can lead you to different things.”
Shuch highlighted Von Hippel-Lindau (VHL) syndrome, which affects 1 in 25,000 people. About 80% to 90% of these patients have a family history, Shuch said.
But the others do not. “Unfortunately, some get diagnosed later in life because they don’t get cascade testing starting at aged 2, which is recommended. These are the patients who might be coming into the ER with a hemangioblastoma or picking up the phone and all of a sudden being deaf in one ear due to an endolymphatic sac tumor.
“We want to limit metastatic spread and preserve the kidneys,” Shuch said. “We don’t want to be doing radical nephrectomies. We want to avoid chronic kidney disease, prevent end-stage renal disease, and maximize quality of life.”
It’s a good idea to avoid surgical removal unless a patient’s tumor grows to be > 3 cm, a line that indicates risk of metastases, he said.
In terms of treatment, Shuch highlighted a 2021 study that showed benefit in VHL from belzutifan (Welireg), an oral HIF-2 α inhibitor approved by the US Food and Drug Administration. The medication significantly reduced the need for surgical intervention.
“Patients go on this drug, and surgeons are putting their scalpels down,” said Shuch, who worked on the 2021 study.
Other hereditary syndromes include the rare hereditary papillary RCC, and Birt-Hogg-Dubé syndrome, believed to affect 1 in 200,000 people but may be more common, he said.
Birt-Hogg-Dubé syndrome is linked to lung cysts, lung collapse, and skin manifestations. The 3 cm surgery rule is appropriate in these cases, Shuch said, and metastases are rare.
Another condition, hereditary leiomyomatosis and RCC, is the most dangerous hereditary form. Originally thought to affect 1 in 200,000 people, hereditary leiomyomatosis and RCC is similar to Birt-Hogg-Dubé syndrome in that it is believed to be more common.
“You will see this,” Shuch predicted.
Shuch advised colleagues to intervene early and take a large margin during surgery.
He also highlighted familial paraganglioma syndrome, which is associated with gastrointestinal stromal tumors, and Cowden syndrome, which is linked to skin manifestations and breast, thyroid, and endometrial cancer.
Shuch reported that he had no disclosures.
The role of hereditary syndromes in renal cell carcinoma (RCC) might be easily missed, a kidney cancer specialist said during a recent Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, though careful clinical evaluation can uncover genetic traits that may affect treatment and familial risk.
“The importance of finding or identifying hereditary forms of kidney cancer really should not be underestimated,” said urologist Brian Shuch, MD, director of the UCLA Kidney Cancer Program, on treating veterans with kidney cancer.
According to Shuch, recent data suggest that about 4.5% of patients with RCC have a hereditary syndrome: “A lot of times, these hide in plain sight. You have to really look deep and try to figure things out and understand that maybe they have a hereditary form of kidney cancer.”
It is important to consider early genetic testing, Shuch said. Red flags for hereditary syndromes include early-onset RCC (age ≤ 45 years), multifocal tumors, bilateral tumors (especially in younger individuals), or a relevant family personal history, he said.
Unusual skin conditions are also potential signs, Shuch said. These can include leiomyomas, fibrofolliculomas, and angiofibromas: “Patients have lots of lumps or bumps.”
“When I look at a patient, I go head to toe and ask if there any issues with your vision, any issues with your hearing, any issues swallowing,” he explained at the meeting. “Do you have any problems with heart issues, adrenal issues? You’ve got to go through each organ, and it can lead you to different things.”
Shuch highlighted Von Hippel-Lindau (VHL) syndrome, which affects 1 in 25,000 people. About 80% to 90% of these patients have a family history, Shuch said.
But the others do not. “Unfortunately, some get diagnosed later in life because they don’t get cascade testing starting at aged 2, which is recommended. These are the patients who might be coming into the ER with a hemangioblastoma or picking up the phone and all of a sudden being deaf in one ear due to an endolymphatic sac tumor.
“We want to limit metastatic spread and preserve the kidneys,” Shuch said. “We don’t want to be doing radical nephrectomies. We want to avoid chronic kidney disease, prevent end-stage renal disease, and maximize quality of life.”
It’s a good idea to avoid surgical removal unless a patient’s tumor grows to be > 3 cm, a line that indicates risk of metastases, he said.
In terms of treatment, Shuch highlighted a 2021 study that showed benefit in VHL from belzutifan (Welireg), an oral HIF-2 α inhibitor approved by the US Food and Drug Administration. The medication significantly reduced the need for surgical intervention.
“Patients go on this drug, and surgeons are putting their scalpels down,” said Shuch, who worked on the 2021 study.
Other hereditary syndromes include the rare hereditary papillary RCC, and Birt-Hogg-Dubé syndrome, believed to affect 1 in 200,000 people but may be more common, he said.
Birt-Hogg-Dubé syndrome is linked to lung cysts, lung collapse, and skin manifestations. The 3 cm surgery rule is appropriate in these cases, Shuch said, and metastases are rare.
Another condition, hereditary leiomyomatosis and RCC, is the most dangerous hereditary form. Originally thought to affect 1 in 200,000 people, hereditary leiomyomatosis and RCC is similar to Birt-Hogg-Dubé syndrome in that it is believed to be more common.
“You will see this,” Shuch predicted.
Shuch advised colleagues to intervene early and take a large margin during surgery.
He also highlighted familial paraganglioma syndrome, which is associated with gastrointestinal stromal tumors, and Cowden syndrome, which is linked to skin manifestations and breast, thyroid, and endometrial cancer.
Shuch reported that he had no disclosures.
Renal Cell Carcinoma: What You Need to Know About Hereditary Syndromes
Renal Cell Carcinoma: What You Need to Know About Hereditary Syndromes
At-Home Alzheimer’s Testing Is Here: Are Physicians Ready?
Given the opportunity, 90% of Americans say they would take a blood biomarker test for Alzheimer’s disease (AD) — even in the absence of symptoms. Notably, 80% say they wouldn’t wait for a physician to order a test, they’d request one themselves.
The findings, from a recent nationwide survey by the Alzheimer’s Association, suggest a growing desire to predict the risk for or show evidence of AD and related dementias with a simple blood test. For consumers with the inclination and the money, that desire can now become reality.
Once limited to research settings or only available via a physician’s order, blood-based diagnostics for specific biomarkers — primarily pTau-217 and beta-amyloid 42/20 — are now offered by at least four companies in the US. Several others sell blood-based “dementia” panels without those biomarkers and screens for apolipoprotein (APOE) genes, including APOE4, a variant that confers a higher risk for AD.
The companies promote testing to all comers, not just those with a family history or concerns about cognitive symptoms. Test prices range from hundreds to thousands of dollars, depending on whether they are included in a company membership, often designed to encourage repeat testing. Blood draws are conducted at home or at certified labs. Buyers don’t need a prescription or to consult with a physician after receiving results.
Knowing results of such tests could be empowering and may encourage people to prepare for their illness, Jessica Mozersky, PhD, assistant professor of medicine at the Bioethics Research Center at Washington University in St. Louis, told this news organization. A direct-to-consumer (DTC) test also eliminates potential physician-created barriers to testing, she added.
But there are also potential harms.
Based on results, individuals may interpret everyday forgetfulness — like misplacing keys — as a sign that dementia is inevitable. This can lead them to change life plans, rethink the way they spend their time, or begin viewing their future negatively. “It creates unnecessary worry and anxiety,” Mozersky said.
The growing availability of DTC tests — heralded by some experts and discouraged by others — comes as AD and dementia specialists continue to debate whether AD diagnostic and staging criteria should be based only on biomarkers or on criteria that includes both pathology and symptomology.
For many, it raises a fundamental concern: If experts haven’t reached a consensus on blood-based AD biomarker testing, how can consumers be expected to interpret at-home test results?
Growing Demand
In 2024, the number of people living with AD passed 7 million. A recent report from the Alzheimer’s Association estimates that number will nearly double by 2060.
The demand for testing also appears to be rising. Similar to the findings in the Alzheimer’s Association’s survey, a small observational study published last year showed that 90% of patients who received a cerebrospinal fluid AD biomarker test ordered by a physician said the decision to get the test was “easy.” For 82%, getting results was positive because it allowed them to plan ahead and to adopt or continue healthy behaviors such as exercise and cognitive activities.
Until now, blood biomarker tests for AD have primarily been available only through a doctor. The tests measure beta-amyloid 42/20 and pTau-217, both of which are strong biomarkers of AD. Some other blood-based biomarkers under investigation include neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP).
As reported by Medscape Medical News, the FDA approved the first blood-based AD diagnostic test in May. The Lumipulse G pTau 217/Beta-Amyloid 1-42 is for the early detection of amyloid plaques associated with AD in adults aged 55 years or older who show signs and symptoms of the disease. But it is only available by prescription.
Quest Diagnostics tested the DTC market in 2023, promoting a consumer-initiated test for beta-amyloid 42/40 that had previously been available only through physicians. It was not well-received by clinicians and ethicists. The company withdrew it later that year but continues to sell beta-amyloid 42/20 and pTau-217 tests through physicians, as does its competitor Labcorp.
Today, at least a handful of companies in the US market AD biomarkers directly to the public: Apollo Health, BetterBrain, Function Health, Neurogen Biomarking, and True Health Labs. None of the companies have disclosed ties to pharmaceutical or device companies or test developers.
What Can Consumers Get?
Some companies direct customers to a lab for blood sample collection, whereas others send a technician to customers’ homes. The extent of biomarker testing and posttest consultation also vary by company.
Apollo Health customers can order a “BrainScan” for $799, which includes screens for pTau-217, GFAP, and NfL. Buyers get a detailed report that explains each test, the result (in nanograms per liter) and optimal range (ng/L) and potential next steps. A pTau-217 result in the normal range, for instance, would come with a recommendation for repeat testing every 2 years. If someone receives an abnormal result, they are contacted by a health coach who can make a physician referral.
At Function Health, members pay $499 a year to have access to hundreds of tests and a written summary of results by a clinician. All of its “Brain Health” tests, including “Beta-Amyloid 42/40 Ratio,” pTau-217, APOE, MTHFR, DNA, and NfL, are available for an additional undisclosed charge.
BetterBrain has a $399 membership that covers an initial 75-minute consultation with cognitive tests, a “personalized brain health plan,” and a blood test that is a basic panel without AD biomarkers. A $499 membership includes all of that plus an APOE test. A pTau-217 test is available for an additional undisclosed fee.
At Neurogen Biomarking, which started in January, a consumer orders an at-home test kit, and a phlebotomist comes to their home for a blood draw. The consumer then fills out an online cognitive assessment. Test results are reviewed by a board-certified neurologist and discussed with the consumer via a virtual visit. If the person is at low-risk, they are given some educational material. Those at higher risk are referred to Neurogen’s “team of specialty-trained neurologists” for continuing care. Testing costs were not provided by the company.
Consumers can order “Beta-Amyloid 42/40” for $749 and pTau-217 for $229 directly through True Health Labs. No consultations or services are offered.
DTC Testing Raises Alarms
It’s unclear where DTC tests fall in terms of regulation. The FDA does not usually review at-home tests for low-risk medical purposes but will generally do so for diagnostics that are for higher-risk conditions “to determine the validity of test claims,” according to the agency’s website.
Consumers, however, don’t usually have easy access to information on biomarker tests’ sensitivity, specificity, or other characteristics that would be used by clinicians or regulatory authorities to assess a test’s validity.
The lack of regulation of consumer-initiated AD testing is one issue cited by critics of at-home tests, including the Alzheimer’s Association.
“None of these tests have been scientifically proven to be accurate,” the association noted in a statement, adding that “the tests can have false positive results, meaning that individuals can have results saying they have dementia when in fact they do not.”
“For these and other reasons, the Alzheimer’s Association believes that home screening tests cannot and should not be used as a substitute for a thorough examination by a skilled physician. The whole process of assessment and diagnosis should be carried out within the context of an ongoing relationship with a responsible and qualified healthcare professional,” the statement said.
The association also said that biomarker tests should not be ordered — even by physicians — for asymptomatic individuals.
The American Academy of Neurology (AAN) does not have a position on DTC tests for AD biomarkers, a spokesperson told this news organization. In a 2021 paper on ethical considerations for diagnosis and care, an AAN committee said that biomarker testing could be clinically useful for some symptomatic patients, but testing asymptomatic individuals is “recommended solely in a research setting” because of potential harms “and the absence of interventions capable of favorably altering the natural history of the disease.”
Eric Topol, MD, chair of the Department of Translational Medicine at Scripps Research in La Jolla, California, is bullish on the potential for blood-based biomarker tests. In a blog post, he called the pTau-217 biomarker “one of the most exciting advances in neurology for decades, giving us a new opportunity to accurately predict and potentially prevent (or at least substantially delay) mild cognitive impairment and Alzheimer’s.”
But, wrote Topol, who is the former editor in chief of MedscapeMedical News, “I don’t think these biomarkers are going to be useful in people at low risk.” He wrote that testing should not be used by people who are “cognitively intact” or to tell someone they have pre-AD. “More work needs to be done to determine whether lowering one’s pTau-217 will alter the brain plaque progression and be seen as a disease-modifier,” wrote Topol.
The Risks of Knowing
Some people don’t want to know their biomarker status. In a study in May in JAMA Network Open, Mozersky and colleagues reported that while 81% of a group of cognitively normal participants in a longitudinal study of dementia said they wanted to see results, only 60% ultimately opted to get results after testing. Participants said they did not want to know because they didn’t want to become a burden on their family or that they felt fine; others had concerns about whether the tests were accurate.
That low number “surprised us,” said Mozersky. “Our study certainly suggests that when you’re really faced with knowing, that your answer is more likely to possibly be no,” she added.
DTC companies tell buyers that results could motivate them to change their lifestyle to reduce their future risk for AD and dementia. But some participants in Mozersky’s study said they didn’t want to know their status because there were no preventive treatments. Test results weren’t seen as “actionable,” she said.
Some studies have shown a degree of fatalism in individuals after receiving a test result, whether it’s positive or negative.
A group of Israeli researchers studied responses of people given PET scans to detect beta-amyloid. Before testing, all participants said they were motivated to adopt lifestyle changes to fight dementia. However, after testing, both those who had elevated beta-amyloid and those who did not reported a much lower desire to change their lifestyle. Those with normal scans probably felt relieved, wrote the researchers. The group with abnormal scans was too small to fully understand their reaction, they wrote.
Concerns about insurance coverage might also deter potential test-takers. Overall, 44% of those responding to the Alzheimer’s Association survey said they were worried that insurers might not cover healthcare costs in the future if they had received a positive test earlier. Respondents also worried about test accuracy, the cost of testing, and whether a positive test might lead to a prohibition on some activities, like driving.
What About the Doctors?
The DTC companies promise buyers that results will be private and won’t be shared with insurers — or with clinicians. And that raises another issue for many who are concerned about the lack of a physician intermediary with at-home testing.
“You remove the opportunity for clinicians to both review the result and figure out how to interpret it before it’s communicated to the patient,” Jalayne J. Arias, JD, a bioethicist and associate professor of Health Policy and Behavioral Sciences at Georgia State University, Atlanta, told this news organization.
Many in the field have been “thinking really carefully about how do we provide guidance to clinicians about biomarker testing,” she said. “Those issues are just heightened when we put it into a direct-to-consumer model,” Arias said.
Arias — who with colleagues published an analysis of potential insurance issues with biomarker tests in JAMA Neurology — said that prohibitions against discrimination based on preexisting conditions means that most likely, health insurers could not use testing data to deny coverage or increase premiums.
But, she said, “there are some question marks around the discrimination risks.” This is especially true for people seeking long-term care, disability or life insurance, she added.
If a test result is not documented in a medical record, it’s not clear whether the individual has an obligation to disclose the result to an insurer, said Arias.
Given all the unanswered questions about how results should be interpreted, to whom the results should be disclosed, and when and how to have discussions with patients, “it’s hard for me to imagine that we’re quite ready for a direct-to-consumer” test, Arias said.
Mozersky noted that Washington University has a financial stake in C2N Diagnostics, which makes the PrecivityAD — biomarker tests for AD. Arias reported having no conflicts of interest.
A version of this article first appeared on Medscape.com.
Given the opportunity, 90% of Americans say they would take a blood biomarker test for Alzheimer’s disease (AD) — even in the absence of symptoms. Notably, 80% say they wouldn’t wait for a physician to order a test, they’d request one themselves.
The findings, from a recent nationwide survey by the Alzheimer’s Association, suggest a growing desire to predict the risk for or show evidence of AD and related dementias with a simple blood test. For consumers with the inclination and the money, that desire can now become reality.
Once limited to research settings or only available via a physician’s order, blood-based diagnostics for specific biomarkers — primarily pTau-217 and beta-amyloid 42/20 — are now offered by at least four companies in the US. Several others sell blood-based “dementia” panels without those biomarkers and screens for apolipoprotein (APOE) genes, including APOE4, a variant that confers a higher risk for AD.
The companies promote testing to all comers, not just those with a family history or concerns about cognitive symptoms. Test prices range from hundreds to thousands of dollars, depending on whether they are included in a company membership, often designed to encourage repeat testing. Blood draws are conducted at home or at certified labs. Buyers don’t need a prescription or to consult with a physician after receiving results.
Knowing results of such tests could be empowering and may encourage people to prepare for their illness, Jessica Mozersky, PhD, assistant professor of medicine at the Bioethics Research Center at Washington University in St. Louis, told this news organization. A direct-to-consumer (DTC) test also eliminates potential physician-created barriers to testing, she added.
But there are also potential harms.
Based on results, individuals may interpret everyday forgetfulness — like misplacing keys — as a sign that dementia is inevitable. This can lead them to change life plans, rethink the way they spend their time, or begin viewing their future negatively. “It creates unnecessary worry and anxiety,” Mozersky said.
The growing availability of DTC tests — heralded by some experts and discouraged by others — comes as AD and dementia specialists continue to debate whether AD diagnostic and staging criteria should be based only on biomarkers or on criteria that includes both pathology and symptomology.
For many, it raises a fundamental concern: If experts haven’t reached a consensus on blood-based AD biomarker testing, how can consumers be expected to interpret at-home test results?
Growing Demand
In 2024, the number of people living with AD passed 7 million. A recent report from the Alzheimer’s Association estimates that number will nearly double by 2060.
The demand for testing also appears to be rising. Similar to the findings in the Alzheimer’s Association’s survey, a small observational study published last year showed that 90% of patients who received a cerebrospinal fluid AD biomarker test ordered by a physician said the decision to get the test was “easy.” For 82%, getting results was positive because it allowed them to plan ahead and to adopt or continue healthy behaviors such as exercise and cognitive activities.
Until now, blood biomarker tests for AD have primarily been available only through a doctor. The tests measure beta-amyloid 42/20 and pTau-217, both of which are strong biomarkers of AD. Some other blood-based biomarkers under investigation include neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP).
As reported by Medscape Medical News, the FDA approved the first blood-based AD diagnostic test in May. The Lumipulse G pTau 217/Beta-Amyloid 1-42 is for the early detection of amyloid plaques associated with AD in adults aged 55 years or older who show signs and symptoms of the disease. But it is only available by prescription.
Quest Diagnostics tested the DTC market in 2023, promoting a consumer-initiated test for beta-amyloid 42/40 that had previously been available only through physicians. It was not well-received by clinicians and ethicists. The company withdrew it later that year but continues to sell beta-amyloid 42/20 and pTau-217 tests through physicians, as does its competitor Labcorp.
Today, at least a handful of companies in the US market AD biomarkers directly to the public: Apollo Health, BetterBrain, Function Health, Neurogen Biomarking, and True Health Labs. None of the companies have disclosed ties to pharmaceutical or device companies or test developers.
What Can Consumers Get?
Some companies direct customers to a lab for blood sample collection, whereas others send a technician to customers’ homes. The extent of biomarker testing and posttest consultation also vary by company.
Apollo Health customers can order a “BrainScan” for $799, which includes screens for pTau-217, GFAP, and NfL. Buyers get a detailed report that explains each test, the result (in nanograms per liter) and optimal range (ng/L) and potential next steps. A pTau-217 result in the normal range, for instance, would come with a recommendation for repeat testing every 2 years. If someone receives an abnormal result, they are contacted by a health coach who can make a physician referral.
At Function Health, members pay $499 a year to have access to hundreds of tests and a written summary of results by a clinician. All of its “Brain Health” tests, including “Beta-Amyloid 42/40 Ratio,” pTau-217, APOE, MTHFR, DNA, and NfL, are available for an additional undisclosed charge.
BetterBrain has a $399 membership that covers an initial 75-minute consultation with cognitive tests, a “personalized brain health plan,” and a blood test that is a basic panel without AD biomarkers. A $499 membership includes all of that plus an APOE test. A pTau-217 test is available for an additional undisclosed fee.
At Neurogen Biomarking, which started in January, a consumer orders an at-home test kit, and a phlebotomist comes to their home for a blood draw. The consumer then fills out an online cognitive assessment. Test results are reviewed by a board-certified neurologist and discussed with the consumer via a virtual visit. If the person is at low-risk, they are given some educational material. Those at higher risk are referred to Neurogen’s “team of specialty-trained neurologists” for continuing care. Testing costs were not provided by the company.
Consumers can order “Beta-Amyloid 42/40” for $749 and pTau-217 for $229 directly through True Health Labs. No consultations or services are offered.
DTC Testing Raises Alarms
It’s unclear where DTC tests fall in terms of regulation. The FDA does not usually review at-home tests for low-risk medical purposes but will generally do so for diagnostics that are for higher-risk conditions “to determine the validity of test claims,” according to the agency’s website.
Consumers, however, don’t usually have easy access to information on biomarker tests’ sensitivity, specificity, or other characteristics that would be used by clinicians or regulatory authorities to assess a test’s validity.
The lack of regulation of consumer-initiated AD testing is one issue cited by critics of at-home tests, including the Alzheimer’s Association.
“None of these tests have been scientifically proven to be accurate,” the association noted in a statement, adding that “the tests can have false positive results, meaning that individuals can have results saying they have dementia when in fact they do not.”
“For these and other reasons, the Alzheimer’s Association believes that home screening tests cannot and should not be used as a substitute for a thorough examination by a skilled physician. The whole process of assessment and diagnosis should be carried out within the context of an ongoing relationship with a responsible and qualified healthcare professional,” the statement said.
The association also said that biomarker tests should not be ordered — even by physicians — for asymptomatic individuals.
The American Academy of Neurology (AAN) does not have a position on DTC tests for AD biomarkers, a spokesperson told this news organization. In a 2021 paper on ethical considerations for diagnosis and care, an AAN committee said that biomarker testing could be clinically useful for some symptomatic patients, but testing asymptomatic individuals is “recommended solely in a research setting” because of potential harms “and the absence of interventions capable of favorably altering the natural history of the disease.”
Eric Topol, MD, chair of the Department of Translational Medicine at Scripps Research in La Jolla, California, is bullish on the potential for blood-based biomarker tests. In a blog post, he called the pTau-217 biomarker “one of the most exciting advances in neurology for decades, giving us a new opportunity to accurately predict and potentially prevent (or at least substantially delay) mild cognitive impairment and Alzheimer’s.”
But, wrote Topol, who is the former editor in chief of MedscapeMedical News, “I don’t think these biomarkers are going to be useful in people at low risk.” He wrote that testing should not be used by people who are “cognitively intact” or to tell someone they have pre-AD. “More work needs to be done to determine whether lowering one’s pTau-217 will alter the brain plaque progression and be seen as a disease-modifier,” wrote Topol.
The Risks of Knowing
Some people don’t want to know their biomarker status. In a study in May in JAMA Network Open, Mozersky and colleagues reported that while 81% of a group of cognitively normal participants in a longitudinal study of dementia said they wanted to see results, only 60% ultimately opted to get results after testing. Participants said they did not want to know because they didn’t want to become a burden on their family or that they felt fine; others had concerns about whether the tests were accurate.
That low number “surprised us,” said Mozersky. “Our study certainly suggests that when you’re really faced with knowing, that your answer is more likely to possibly be no,” she added.
DTC companies tell buyers that results could motivate them to change their lifestyle to reduce their future risk for AD and dementia. But some participants in Mozersky’s study said they didn’t want to know their status because there were no preventive treatments. Test results weren’t seen as “actionable,” she said.
Some studies have shown a degree of fatalism in individuals after receiving a test result, whether it’s positive or negative.
A group of Israeli researchers studied responses of people given PET scans to detect beta-amyloid. Before testing, all participants said they were motivated to adopt lifestyle changes to fight dementia. However, after testing, both those who had elevated beta-amyloid and those who did not reported a much lower desire to change their lifestyle. Those with normal scans probably felt relieved, wrote the researchers. The group with abnormal scans was too small to fully understand their reaction, they wrote.
Concerns about insurance coverage might also deter potential test-takers. Overall, 44% of those responding to the Alzheimer’s Association survey said they were worried that insurers might not cover healthcare costs in the future if they had received a positive test earlier. Respondents also worried about test accuracy, the cost of testing, and whether a positive test might lead to a prohibition on some activities, like driving.
What About the Doctors?
The DTC companies promise buyers that results will be private and won’t be shared with insurers — or with clinicians. And that raises another issue for many who are concerned about the lack of a physician intermediary with at-home testing.
“You remove the opportunity for clinicians to both review the result and figure out how to interpret it before it’s communicated to the patient,” Jalayne J. Arias, JD, a bioethicist and associate professor of Health Policy and Behavioral Sciences at Georgia State University, Atlanta, told this news organization.
Many in the field have been “thinking really carefully about how do we provide guidance to clinicians about biomarker testing,” she said. “Those issues are just heightened when we put it into a direct-to-consumer model,” Arias said.
Arias — who with colleagues published an analysis of potential insurance issues with biomarker tests in JAMA Neurology — said that prohibitions against discrimination based on preexisting conditions means that most likely, health insurers could not use testing data to deny coverage or increase premiums.
But, she said, “there are some question marks around the discrimination risks.” This is especially true for people seeking long-term care, disability or life insurance, she added.
If a test result is not documented in a medical record, it’s not clear whether the individual has an obligation to disclose the result to an insurer, said Arias.
Given all the unanswered questions about how results should be interpreted, to whom the results should be disclosed, and when and how to have discussions with patients, “it’s hard for me to imagine that we’re quite ready for a direct-to-consumer” test, Arias said.
Mozersky noted that Washington University has a financial stake in C2N Diagnostics, which makes the PrecivityAD — biomarker tests for AD. Arias reported having no conflicts of interest.
A version of this article first appeared on Medscape.com.
Given the opportunity, 90% of Americans say they would take a blood biomarker test for Alzheimer’s disease (AD) — even in the absence of symptoms. Notably, 80% say they wouldn’t wait for a physician to order a test, they’d request one themselves.
The findings, from a recent nationwide survey by the Alzheimer’s Association, suggest a growing desire to predict the risk for or show evidence of AD and related dementias with a simple blood test. For consumers with the inclination and the money, that desire can now become reality.
Once limited to research settings or only available via a physician’s order, blood-based diagnostics for specific biomarkers — primarily pTau-217 and beta-amyloid 42/20 — are now offered by at least four companies in the US. Several others sell blood-based “dementia” panels without those biomarkers and screens for apolipoprotein (APOE) genes, including APOE4, a variant that confers a higher risk for AD.
The companies promote testing to all comers, not just those with a family history or concerns about cognitive symptoms. Test prices range from hundreds to thousands of dollars, depending on whether they are included in a company membership, often designed to encourage repeat testing. Blood draws are conducted at home or at certified labs. Buyers don’t need a prescription or to consult with a physician after receiving results.
Knowing results of such tests could be empowering and may encourage people to prepare for their illness, Jessica Mozersky, PhD, assistant professor of medicine at the Bioethics Research Center at Washington University in St. Louis, told this news organization. A direct-to-consumer (DTC) test also eliminates potential physician-created barriers to testing, she added.
But there are also potential harms.
Based on results, individuals may interpret everyday forgetfulness — like misplacing keys — as a sign that dementia is inevitable. This can lead them to change life plans, rethink the way they spend their time, or begin viewing their future negatively. “It creates unnecessary worry and anxiety,” Mozersky said.
The growing availability of DTC tests — heralded by some experts and discouraged by others — comes as AD and dementia specialists continue to debate whether AD diagnostic and staging criteria should be based only on biomarkers or on criteria that includes both pathology and symptomology.
For many, it raises a fundamental concern: If experts haven’t reached a consensus on blood-based AD biomarker testing, how can consumers be expected to interpret at-home test results?
Growing Demand
In 2024, the number of people living with AD passed 7 million. A recent report from the Alzheimer’s Association estimates that number will nearly double by 2060.
The demand for testing also appears to be rising. Similar to the findings in the Alzheimer’s Association’s survey, a small observational study published last year showed that 90% of patients who received a cerebrospinal fluid AD biomarker test ordered by a physician said the decision to get the test was “easy.” For 82%, getting results was positive because it allowed them to plan ahead and to adopt or continue healthy behaviors such as exercise and cognitive activities.
Until now, blood biomarker tests for AD have primarily been available only through a doctor. The tests measure beta-amyloid 42/20 and pTau-217, both of which are strong biomarkers of AD. Some other blood-based biomarkers under investigation include neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP).
As reported by Medscape Medical News, the FDA approved the first blood-based AD diagnostic test in May. The Lumipulse G pTau 217/Beta-Amyloid 1-42 is for the early detection of amyloid plaques associated with AD in adults aged 55 years or older who show signs and symptoms of the disease. But it is only available by prescription.
Quest Diagnostics tested the DTC market in 2023, promoting a consumer-initiated test for beta-amyloid 42/40 that had previously been available only through physicians. It was not well-received by clinicians and ethicists. The company withdrew it later that year but continues to sell beta-amyloid 42/20 and pTau-217 tests through physicians, as does its competitor Labcorp.
Today, at least a handful of companies in the US market AD biomarkers directly to the public: Apollo Health, BetterBrain, Function Health, Neurogen Biomarking, and True Health Labs. None of the companies have disclosed ties to pharmaceutical or device companies or test developers.
What Can Consumers Get?
Some companies direct customers to a lab for blood sample collection, whereas others send a technician to customers’ homes. The extent of biomarker testing and posttest consultation also vary by company.
Apollo Health customers can order a “BrainScan” for $799, which includes screens for pTau-217, GFAP, and NfL. Buyers get a detailed report that explains each test, the result (in nanograms per liter) and optimal range (ng/L) and potential next steps. A pTau-217 result in the normal range, for instance, would come with a recommendation for repeat testing every 2 years. If someone receives an abnormal result, they are contacted by a health coach who can make a physician referral.
At Function Health, members pay $499 a year to have access to hundreds of tests and a written summary of results by a clinician. All of its “Brain Health” tests, including “Beta-Amyloid 42/40 Ratio,” pTau-217, APOE, MTHFR, DNA, and NfL, are available for an additional undisclosed charge.
BetterBrain has a $399 membership that covers an initial 75-minute consultation with cognitive tests, a “personalized brain health plan,” and a blood test that is a basic panel without AD biomarkers. A $499 membership includes all of that plus an APOE test. A pTau-217 test is available for an additional undisclosed fee.
At Neurogen Biomarking, which started in January, a consumer orders an at-home test kit, and a phlebotomist comes to their home for a blood draw. The consumer then fills out an online cognitive assessment. Test results are reviewed by a board-certified neurologist and discussed with the consumer via a virtual visit. If the person is at low-risk, they are given some educational material. Those at higher risk are referred to Neurogen’s “team of specialty-trained neurologists” for continuing care. Testing costs were not provided by the company.
Consumers can order “Beta-Amyloid 42/40” for $749 and pTau-217 for $229 directly through True Health Labs. No consultations or services are offered.
DTC Testing Raises Alarms
It’s unclear where DTC tests fall in terms of regulation. The FDA does not usually review at-home tests for low-risk medical purposes but will generally do so for diagnostics that are for higher-risk conditions “to determine the validity of test claims,” according to the agency’s website.
Consumers, however, don’t usually have easy access to information on biomarker tests’ sensitivity, specificity, or other characteristics that would be used by clinicians or regulatory authorities to assess a test’s validity.
The lack of regulation of consumer-initiated AD testing is one issue cited by critics of at-home tests, including the Alzheimer’s Association.
“None of these tests have been scientifically proven to be accurate,” the association noted in a statement, adding that “the tests can have false positive results, meaning that individuals can have results saying they have dementia when in fact they do not.”
“For these and other reasons, the Alzheimer’s Association believes that home screening tests cannot and should not be used as a substitute for a thorough examination by a skilled physician. The whole process of assessment and diagnosis should be carried out within the context of an ongoing relationship with a responsible and qualified healthcare professional,” the statement said.
The association also said that biomarker tests should not be ordered — even by physicians — for asymptomatic individuals.
The American Academy of Neurology (AAN) does not have a position on DTC tests for AD biomarkers, a spokesperson told this news organization. In a 2021 paper on ethical considerations for diagnosis and care, an AAN committee said that biomarker testing could be clinically useful for some symptomatic patients, but testing asymptomatic individuals is “recommended solely in a research setting” because of potential harms “and the absence of interventions capable of favorably altering the natural history of the disease.”
Eric Topol, MD, chair of the Department of Translational Medicine at Scripps Research in La Jolla, California, is bullish on the potential for blood-based biomarker tests. In a blog post, he called the pTau-217 biomarker “one of the most exciting advances in neurology for decades, giving us a new opportunity to accurately predict and potentially prevent (or at least substantially delay) mild cognitive impairment and Alzheimer’s.”
But, wrote Topol, who is the former editor in chief of MedscapeMedical News, “I don’t think these biomarkers are going to be useful in people at low risk.” He wrote that testing should not be used by people who are “cognitively intact” or to tell someone they have pre-AD. “More work needs to be done to determine whether lowering one’s pTau-217 will alter the brain plaque progression and be seen as a disease-modifier,” wrote Topol.
The Risks of Knowing
Some people don’t want to know their biomarker status. In a study in May in JAMA Network Open, Mozersky and colleagues reported that while 81% of a group of cognitively normal participants in a longitudinal study of dementia said they wanted to see results, only 60% ultimately opted to get results after testing. Participants said they did not want to know because they didn’t want to become a burden on their family or that they felt fine; others had concerns about whether the tests were accurate.
That low number “surprised us,” said Mozersky. “Our study certainly suggests that when you’re really faced with knowing, that your answer is more likely to possibly be no,” she added.
DTC companies tell buyers that results could motivate them to change their lifestyle to reduce their future risk for AD and dementia. But some participants in Mozersky’s study said they didn’t want to know their status because there were no preventive treatments. Test results weren’t seen as “actionable,” she said.
Some studies have shown a degree of fatalism in individuals after receiving a test result, whether it’s positive or negative.
A group of Israeli researchers studied responses of people given PET scans to detect beta-amyloid. Before testing, all participants said they were motivated to adopt lifestyle changes to fight dementia. However, after testing, both those who had elevated beta-amyloid and those who did not reported a much lower desire to change their lifestyle. Those with normal scans probably felt relieved, wrote the researchers. The group with abnormal scans was too small to fully understand their reaction, they wrote.
Concerns about insurance coverage might also deter potential test-takers. Overall, 44% of those responding to the Alzheimer’s Association survey said they were worried that insurers might not cover healthcare costs in the future if they had received a positive test earlier. Respondents also worried about test accuracy, the cost of testing, and whether a positive test might lead to a prohibition on some activities, like driving.
What About the Doctors?
The DTC companies promise buyers that results will be private and won’t be shared with insurers — or with clinicians. And that raises another issue for many who are concerned about the lack of a physician intermediary with at-home testing.
“You remove the opportunity for clinicians to both review the result and figure out how to interpret it before it’s communicated to the patient,” Jalayne J. Arias, JD, a bioethicist and associate professor of Health Policy and Behavioral Sciences at Georgia State University, Atlanta, told this news organization.
Many in the field have been “thinking really carefully about how do we provide guidance to clinicians about biomarker testing,” she said. “Those issues are just heightened when we put it into a direct-to-consumer model,” Arias said.
Arias — who with colleagues published an analysis of potential insurance issues with biomarker tests in JAMA Neurology — said that prohibitions against discrimination based on preexisting conditions means that most likely, health insurers could not use testing data to deny coverage or increase premiums.
But, she said, “there are some question marks around the discrimination risks.” This is especially true for people seeking long-term care, disability or life insurance, she added.
If a test result is not documented in a medical record, it’s not clear whether the individual has an obligation to disclose the result to an insurer, said Arias.
Given all the unanswered questions about how results should be interpreted, to whom the results should be disclosed, and when and how to have discussions with patients, “it’s hard for me to imagine that we’re quite ready for a direct-to-consumer” test, Arias said.
Mozersky noted that Washington University has a financial stake in C2N Diagnostics, which makes the PrecivityAD — biomarker tests for AD. Arias reported having no conflicts of interest.
A version of this article first appeared on Medscape.com.
First New PTSD Drug in Two Decades On the Horizon?
The Psychopharmacologic Drugs Advisory Committee of the FDA is set to meet on July 18 to consider a supplemental new drug application for brexpiprazole (Rexulti, Otsuka Pharmaceutical Co., Ltd.), in combination with sertraline, for the treatment of adults with posttraumatic stress disorder (PTSD).
If approved, it would be the first new treatment for PTSD in more than 20 years.
“It is my hope that the FDA does approve this treatment for two related reasons — the data look positive and compelling, and there’s a tremendous unmet need in PTSD,” Roger McIntyre, MD, professor of psychiatry and pharmacology and head of the Mood Disorders Psychopharmacology Unit, University of Toronto, Toronto, Ontario, Canada, told this news organization.
What’s in the Treatment Toolbox Now?
PTSD is a “common, severe, and nonremitting condition,” McIntyre noted. According to the National Center for PTSD, the condition affects roughly 13 million adults in the US in any given year. This represents about 5% of the adult population.
PTSD can develop following exposure to traumatic events such as combat, assault, disasters, or severe accidents. Core symptoms of PTSD include intrusive memories and flashbacks, avoidance behaviors, negative alterations in mood and cognition, and hyperarousal.
Currently, the selective serotonin reuptake inhibitors (SSRI), sertraline and paroxetine, are the only FDA-approved medications for PTSD, and while these medications can be effective, many patients fail to achieve remission or discontinue treatment due to adverse effects or lack of response.
Other medications used off-label to treat PTSD — including prazosin, mirtazapine, atypical antipsychotics, and mood stabilizers — have shown variable efficacy.
There has not been a new FDA-approved drug for PTSD in over two decades, underscoring the need for better therapeutic options, particularly for patients who do not fully respond to SSRI alone.
Why Brexpiprazole Plus Sertraline?
Brexpiprazole is an atypical antipsychotic currently approved as adjunctive treatment of major depressive disorder (MDD) in adults; treatment of schizophrenia in adults and adolescents aged 13 years or older; and treatment of agitation associated with Alzheimer’s dementia.
The combination of brexpiprazole and sertraline could address the limitations of SSRI alone by working synergistically to treat PTSD.
Sertraline increases serotonin levels in the brain to improve mood and reduce anxiety. Brexpiprazole has a complex mechanism of action involving multiple neurotransmitter systems, including but not limited to serotonin and dopamine.
Together, they may target different aspects of PTSD, potentially leading to a more comprehensive reduction in symptoms.
What Do the Phase 3 Data Show?
In a pivotal, double-blind, randomized controlled, phase 3 trial, brexpiprazole plus sertraline provided significantly greater relief of PTSD symptoms than sertraline plus placebo.
The results were published late last year in JAMA Psychiatry and reported by this news organization at that time.
The trial enrolled 416 adults (mean age, 37 years; 75% women) aged 18-65 years with a Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) diagnosis of PTSD and symptoms for at least 6 months prior to screening.
At baseline, participants had a mean Clinician Administered PTSD Scale (CAPS-5) for DSM-5 total score of 38.4, indicating moderate to high severity PTSD. The average time from the index traumatic event was 4 years, and three fourths had no prior exposure to PTSD prescription medications.
Participants underwent a 1-week placebo run-in period followed by randomization to daily oral brexpiprazole 2-3 mg plus sertraline 150 mg or daily sertraline 150 mg plus placebo for 11 weeks.
At week 10, brexpiprazole plus sertraline demonstrated statistically significant greater improvement in the CAPS-5 total score (primary outcome) than sertraline plus placebo (mean change, -19.2 points vs -13.6 points; P < .001).
Brexpiprazole plus sertraline also led to statistically significant greater improvement on all key secondary and other efficacy endpoints, both clinician-reported and patient-reported, including measures of anxiety, depression, intrusive symptoms, hyperarousal, and overall functioning.
Combining an atypical antipsychotic with an antidepressant for PTSD “builds on what we’ve been doing in depression,” Elspeth Ritchie, MD, chair of Psychiatry, MedStar Washington Hospital Center, Washington, DC, noted in an interview with this news organization.
“We have found that a combination of a low-dose antipsychotic and an antidepressant is helpful for depression, so it makes sense that it will be helpful for PTSD. However, this has been mostly based on clinical decisions, without a heavy research background. Good science is always helpful to support those clinical decisions,” Ritchie told this news organization.
What About Safety?
In the phase 3 trial, brexpiprazole plus sertraline had a safety profile consistent with that of brexpiprazole in approved indications. The rate of discontinuation due to adverse events was low (3.9% for brexpiprazole plus sertraline vs 10.2% for sertraline plus placebo), indicating that most participants tolerated the brexpiprazole and sertraline combination treatment, the study team said.
In both treatment groups, the only treatment-emergent adverse event (TEAE) with incidence greater than 10% was nausea, a known adverse effect of sertraline treatment.
Weight gain was greater in participants receiving the combination. At the last visit, a weight gain of 7% or greater from week 1 was experienced by 8% of participants taking brexpiprazole with the sertraline group and 5% of those taking the sertraline plus placebo. Previous analyses in schizophrenia and MDD show that brexpiprazole is associated with moderate weight gain (+3 to 4 kg over 1 year).
The incidence of sedating TEAEs (a concern with some antipsychotics) was generally low, although fatigue (7% vs 4%) and somnolence (5% vs 3%) were more common with brexpiprazole plus sertraline than with sertraline alone.
There were no clinically meaningful between-group differences in changes in laboratory test parameters, vital signs, or ECG and participant-reported TEAEs related to suicidality.
Potential Concerns
As with any new drug application, several questions and issues are likely to be raised by the advisory committee. They could include whether the clinical benefit is substantial enough to warrant approval and how the observed effect sizes compare to existing approved therapies and evidence-based psychotherapies.
McIntyre told this news organization what’s particularly noteworthy is that the magnitude of the improvement in PTSD symptoms with brexpiprazole plus sertraline is greater than with sertraline alone. “That’s a very important statement. And this high level of efficacy was consistent on the secondary outcome measures, and the overall tolerability and safety seemed very acceptable,” he said.
What’s equally important, said McIntyre, is that most people with PTSD have depression and anxiety, and the brexpiprazole plus sertraline combination was more helpful than sertraline alone on the measures of anxiety and depression. “This is really important, especially in light of the fact that this medication [brexpiprazole] is already approved for adults living with major depressive disorder and inadequate response to antidepressants,” McIntyre said.
McIntyre added he suspects some questions the committee may have could relate to the extent to which it’s the case that brexpiprazole is effective in PTSD regardless of the antidepressant that is prescribed with it.
“There also will be the inevitable questions about the absence of long-term data which I think will need to be addressed given how chronic and relapse prone this condition is,” McIntyre said.
The committee may ask how trauma and PTSD will be screened in primary care and how outcomes related to this therapy will be evaluated in everyday clinical practice, McIntyre said.
Overall, McIntyre said brexpiprazole plus sertraline in PTSD is a “very positive” development for the field.
“PTSD is a terrible condition. It’s so darn common, and we just don’t have enough treatments for it. The data look good for my perspective. My fingers are crossed for the patients with PTSD and their families,” said McIntyre.
Ritchie reported having no relevant disclosures. McIntyre received speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and atai Life Sciences. McIntyre is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
The Psychopharmacologic Drugs Advisory Committee of the FDA is set to meet on July 18 to consider a supplemental new drug application for brexpiprazole (Rexulti, Otsuka Pharmaceutical Co., Ltd.), in combination with sertraline, for the treatment of adults with posttraumatic stress disorder (PTSD).
If approved, it would be the first new treatment for PTSD in more than 20 years.
“It is my hope that the FDA does approve this treatment for two related reasons — the data look positive and compelling, and there’s a tremendous unmet need in PTSD,” Roger McIntyre, MD, professor of psychiatry and pharmacology and head of the Mood Disorders Psychopharmacology Unit, University of Toronto, Toronto, Ontario, Canada, told this news organization.
What’s in the Treatment Toolbox Now?
PTSD is a “common, severe, and nonremitting condition,” McIntyre noted. According to the National Center for PTSD, the condition affects roughly 13 million adults in the US in any given year. This represents about 5% of the adult population.
PTSD can develop following exposure to traumatic events such as combat, assault, disasters, or severe accidents. Core symptoms of PTSD include intrusive memories and flashbacks, avoidance behaviors, negative alterations in mood and cognition, and hyperarousal.
Currently, the selective serotonin reuptake inhibitors (SSRI), sertraline and paroxetine, are the only FDA-approved medications for PTSD, and while these medications can be effective, many patients fail to achieve remission or discontinue treatment due to adverse effects or lack of response.
Other medications used off-label to treat PTSD — including prazosin, mirtazapine, atypical antipsychotics, and mood stabilizers — have shown variable efficacy.
There has not been a new FDA-approved drug for PTSD in over two decades, underscoring the need for better therapeutic options, particularly for patients who do not fully respond to SSRI alone.
Why Brexpiprazole Plus Sertraline?
Brexpiprazole is an atypical antipsychotic currently approved as adjunctive treatment of major depressive disorder (MDD) in adults; treatment of schizophrenia in adults and adolescents aged 13 years or older; and treatment of agitation associated with Alzheimer’s dementia.
The combination of brexpiprazole and sertraline could address the limitations of SSRI alone by working synergistically to treat PTSD.
Sertraline increases serotonin levels in the brain to improve mood and reduce anxiety. Brexpiprazole has a complex mechanism of action involving multiple neurotransmitter systems, including but not limited to serotonin and dopamine.
Together, they may target different aspects of PTSD, potentially leading to a more comprehensive reduction in symptoms.
What Do the Phase 3 Data Show?
In a pivotal, double-blind, randomized controlled, phase 3 trial, brexpiprazole plus sertraline provided significantly greater relief of PTSD symptoms than sertraline plus placebo.
The results were published late last year in JAMA Psychiatry and reported by this news organization at that time.
The trial enrolled 416 adults (mean age, 37 years; 75% women) aged 18-65 years with a Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) diagnosis of PTSD and symptoms for at least 6 months prior to screening.
At baseline, participants had a mean Clinician Administered PTSD Scale (CAPS-5) for DSM-5 total score of 38.4, indicating moderate to high severity PTSD. The average time from the index traumatic event was 4 years, and three fourths had no prior exposure to PTSD prescription medications.
Participants underwent a 1-week placebo run-in period followed by randomization to daily oral brexpiprazole 2-3 mg plus sertraline 150 mg or daily sertraline 150 mg plus placebo for 11 weeks.
At week 10, brexpiprazole plus sertraline demonstrated statistically significant greater improvement in the CAPS-5 total score (primary outcome) than sertraline plus placebo (mean change, -19.2 points vs -13.6 points; P < .001).
Brexpiprazole plus sertraline also led to statistically significant greater improvement on all key secondary and other efficacy endpoints, both clinician-reported and patient-reported, including measures of anxiety, depression, intrusive symptoms, hyperarousal, and overall functioning.
Combining an atypical antipsychotic with an antidepressant for PTSD “builds on what we’ve been doing in depression,” Elspeth Ritchie, MD, chair of Psychiatry, MedStar Washington Hospital Center, Washington, DC, noted in an interview with this news organization.
“We have found that a combination of a low-dose antipsychotic and an antidepressant is helpful for depression, so it makes sense that it will be helpful for PTSD. However, this has been mostly based on clinical decisions, without a heavy research background. Good science is always helpful to support those clinical decisions,” Ritchie told this news organization.
What About Safety?
In the phase 3 trial, brexpiprazole plus sertraline had a safety profile consistent with that of brexpiprazole in approved indications. The rate of discontinuation due to adverse events was low (3.9% for brexpiprazole plus sertraline vs 10.2% for sertraline plus placebo), indicating that most participants tolerated the brexpiprazole and sertraline combination treatment, the study team said.
In both treatment groups, the only treatment-emergent adverse event (TEAE) with incidence greater than 10% was nausea, a known adverse effect of sertraline treatment.
Weight gain was greater in participants receiving the combination. At the last visit, a weight gain of 7% or greater from week 1 was experienced by 8% of participants taking brexpiprazole with the sertraline group and 5% of those taking the sertraline plus placebo. Previous analyses in schizophrenia and MDD show that brexpiprazole is associated with moderate weight gain (+3 to 4 kg over 1 year).
The incidence of sedating TEAEs (a concern with some antipsychotics) was generally low, although fatigue (7% vs 4%) and somnolence (5% vs 3%) were more common with brexpiprazole plus sertraline than with sertraline alone.
There were no clinically meaningful between-group differences in changes in laboratory test parameters, vital signs, or ECG and participant-reported TEAEs related to suicidality.
Potential Concerns
As with any new drug application, several questions and issues are likely to be raised by the advisory committee. They could include whether the clinical benefit is substantial enough to warrant approval and how the observed effect sizes compare to existing approved therapies and evidence-based psychotherapies.
McIntyre told this news organization what’s particularly noteworthy is that the magnitude of the improvement in PTSD symptoms with brexpiprazole plus sertraline is greater than with sertraline alone. “That’s a very important statement. And this high level of efficacy was consistent on the secondary outcome measures, and the overall tolerability and safety seemed very acceptable,” he said.
What’s equally important, said McIntyre, is that most people with PTSD have depression and anxiety, and the brexpiprazole plus sertraline combination was more helpful than sertraline alone on the measures of anxiety and depression. “This is really important, especially in light of the fact that this medication [brexpiprazole] is already approved for adults living with major depressive disorder and inadequate response to antidepressants,” McIntyre said.
McIntyre added he suspects some questions the committee may have could relate to the extent to which it’s the case that brexpiprazole is effective in PTSD regardless of the antidepressant that is prescribed with it.
“There also will be the inevitable questions about the absence of long-term data which I think will need to be addressed given how chronic and relapse prone this condition is,” McIntyre said.
The committee may ask how trauma and PTSD will be screened in primary care and how outcomes related to this therapy will be evaluated in everyday clinical practice, McIntyre said.
Overall, McIntyre said brexpiprazole plus sertraline in PTSD is a “very positive” development for the field.
“PTSD is a terrible condition. It’s so darn common, and we just don’t have enough treatments for it. The data look good for my perspective. My fingers are crossed for the patients with PTSD and their families,” said McIntyre.
Ritchie reported having no relevant disclosures. McIntyre received speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and atai Life Sciences. McIntyre is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
The Psychopharmacologic Drugs Advisory Committee of the FDA is set to meet on July 18 to consider a supplemental new drug application for brexpiprazole (Rexulti, Otsuka Pharmaceutical Co., Ltd.), in combination with sertraline, for the treatment of adults with posttraumatic stress disorder (PTSD).
If approved, it would be the first new treatment for PTSD in more than 20 years.
“It is my hope that the FDA does approve this treatment for two related reasons — the data look positive and compelling, and there’s a tremendous unmet need in PTSD,” Roger McIntyre, MD, professor of psychiatry and pharmacology and head of the Mood Disorders Psychopharmacology Unit, University of Toronto, Toronto, Ontario, Canada, told this news organization.
What’s in the Treatment Toolbox Now?
PTSD is a “common, severe, and nonremitting condition,” McIntyre noted. According to the National Center for PTSD, the condition affects roughly 13 million adults in the US in any given year. This represents about 5% of the adult population.
PTSD can develop following exposure to traumatic events such as combat, assault, disasters, or severe accidents. Core symptoms of PTSD include intrusive memories and flashbacks, avoidance behaviors, negative alterations in mood and cognition, and hyperarousal.
Currently, the selective serotonin reuptake inhibitors (SSRI), sertraline and paroxetine, are the only FDA-approved medications for PTSD, and while these medications can be effective, many patients fail to achieve remission or discontinue treatment due to adverse effects or lack of response.
Other medications used off-label to treat PTSD — including prazosin, mirtazapine, atypical antipsychotics, and mood stabilizers — have shown variable efficacy.
There has not been a new FDA-approved drug for PTSD in over two decades, underscoring the need for better therapeutic options, particularly for patients who do not fully respond to SSRI alone.
Why Brexpiprazole Plus Sertraline?
Brexpiprazole is an atypical antipsychotic currently approved as adjunctive treatment of major depressive disorder (MDD) in adults; treatment of schizophrenia in adults and adolescents aged 13 years or older; and treatment of agitation associated with Alzheimer’s dementia.
The combination of brexpiprazole and sertraline could address the limitations of SSRI alone by working synergistically to treat PTSD.
Sertraline increases serotonin levels in the brain to improve mood and reduce anxiety. Brexpiprazole has a complex mechanism of action involving multiple neurotransmitter systems, including but not limited to serotonin and dopamine.
Together, they may target different aspects of PTSD, potentially leading to a more comprehensive reduction in symptoms.
What Do the Phase 3 Data Show?
In a pivotal, double-blind, randomized controlled, phase 3 trial, brexpiprazole plus sertraline provided significantly greater relief of PTSD symptoms than sertraline plus placebo.
The results were published late last year in JAMA Psychiatry and reported by this news organization at that time.
The trial enrolled 416 adults (mean age, 37 years; 75% women) aged 18-65 years with a Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) diagnosis of PTSD and symptoms for at least 6 months prior to screening.
At baseline, participants had a mean Clinician Administered PTSD Scale (CAPS-5) for DSM-5 total score of 38.4, indicating moderate to high severity PTSD. The average time from the index traumatic event was 4 years, and three fourths had no prior exposure to PTSD prescription medications.
Participants underwent a 1-week placebo run-in period followed by randomization to daily oral brexpiprazole 2-3 mg plus sertraline 150 mg or daily sertraline 150 mg plus placebo for 11 weeks.
At week 10, brexpiprazole plus sertraline demonstrated statistically significant greater improvement in the CAPS-5 total score (primary outcome) than sertraline plus placebo (mean change, -19.2 points vs -13.6 points; P < .001).
Brexpiprazole plus sertraline also led to statistically significant greater improvement on all key secondary and other efficacy endpoints, both clinician-reported and patient-reported, including measures of anxiety, depression, intrusive symptoms, hyperarousal, and overall functioning.
Combining an atypical antipsychotic with an antidepressant for PTSD “builds on what we’ve been doing in depression,” Elspeth Ritchie, MD, chair of Psychiatry, MedStar Washington Hospital Center, Washington, DC, noted in an interview with this news organization.
“We have found that a combination of a low-dose antipsychotic and an antidepressant is helpful for depression, so it makes sense that it will be helpful for PTSD. However, this has been mostly based on clinical decisions, without a heavy research background. Good science is always helpful to support those clinical decisions,” Ritchie told this news organization.
What About Safety?
In the phase 3 trial, brexpiprazole plus sertraline had a safety profile consistent with that of brexpiprazole in approved indications. The rate of discontinuation due to adverse events was low (3.9% for brexpiprazole plus sertraline vs 10.2% for sertraline plus placebo), indicating that most participants tolerated the brexpiprazole and sertraline combination treatment, the study team said.
In both treatment groups, the only treatment-emergent adverse event (TEAE) with incidence greater than 10% was nausea, a known adverse effect of sertraline treatment.
Weight gain was greater in participants receiving the combination. At the last visit, a weight gain of 7% or greater from week 1 was experienced by 8% of participants taking brexpiprazole with the sertraline group and 5% of those taking the sertraline plus placebo. Previous analyses in schizophrenia and MDD show that brexpiprazole is associated with moderate weight gain (+3 to 4 kg over 1 year).
The incidence of sedating TEAEs (a concern with some antipsychotics) was generally low, although fatigue (7% vs 4%) and somnolence (5% vs 3%) were more common with brexpiprazole plus sertraline than with sertraline alone.
There were no clinically meaningful between-group differences in changes in laboratory test parameters, vital signs, or ECG and participant-reported TEAEs related to suicidality.
Potential Concerns
As with any new drug application, several questions and issues are likely to be raised by the advisory committee. They could include whether the clinical benefit is substantial enough to warrant approval and how the observed effect sizes compare to existing approved therapies and evidence-based psychotherapies.
McIntyre told this news organization what’s particularly noteworthy is that the magnitude of the improvement in PTSD symptoms with brexpiprazole plus sertraline is greater than with sertraline alone. “That’s a very important statement. And this high level of efficacy was consistent on the secondary outcome measures, and the overall tolerability and safety seemed very acceptable,” he said.
What’s equally important, said McIntyre, is that most people with PTSD have depression and anxiety, and the brexpiprazole plus sertraline combination was more helpful than sertraline alone on the measures of anxiety and depression. “This is really important, especially in light of the fact that this medication [brexpiprazole] is already approved for adults living with major depressive disorder and inadequate response to antidepressants,” McIntyre said.
McIntyre added he suspects some questions the committee may have could relate to the extent to which it’s the case that brexpiprazole is effective in PTSD regardless of the antidepressant that is prescribed with it.
“There also will be the inevitable questions about the absence of long-term data which I think will need to be addressed given how chronic and relapse prone this condition is,” McIntyre said.
The committee may ask how trauma and PTSD will be screened in primary care and how outcomes related to this therapy will be evaluated in everyday clinical practice, McIntyre said.
Overall, McIntyre said brexpiprazole plus sertraline in PTSD is a “very positive” development for the field.
“PTSD is a terrible condition. It’s so darn common, and we just don’t have enough treatments for it. The data look good for my perspective. My fingers are crossed for the patients with PTSD and their families,” said McIntyre.
Ritchie reported having no relevant disclosures. McIntyre received speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and atai Life Sciences. McIntyre is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
Metastases-Directed Therapy for Pancreatic Cancer: More Questions Than Answers
This transcript has been edited for clarity.
Hello. I’m Dr Maurie Markman, from City of Hope. I’d like to discuss what I consider to be an absolutely fascinating paper, and one that I will say has very interesting results but raises many more questions than it answers. I think that was the intent of the authors.
The paper is entitled, “Addition of metastasis-directed therapy to systemic therapy for oligometastatic pancreatic ductal adenocarcinoma (EXTEND): a multicenter, randomized phase 2 trial,” published in the Journal of Clinical Oncology.
You might ask what metastasis-directed therapy in pancreatic cancer means. Have we really made much of an impact on pancreatic cancer? In fact, in my earlier years of training, if somebody came up with the idea, or suggested as part of a trial or treatment of an individual patient, that they would focus on metastases in pancreas cancer, you might say they’re crazy, or you might say: “Yeah, but they probably don’t know anything about the disease and its natural history.”
Now, fast forward several decades. Even with the recognized, modest advances in systemic therapy, what we see are tremendous, really remarkable advances in innovations in radiation therapy. Of course, this includes not only the use of radiation itself but also the imaging technology that is used to direct the radiation therapy. These advances have permitted asking the questions that are addressed in the current study.
Again, this study is fascinating. They randomized a very small number. Again, it’s a randomized phase 2 study. It’s really more of a proof of principle here. They randomized 41 patients with five or fewer metastatic lesions — with oligometastatic disease, they could have numerous lesions — to undergo what they’ve described as comprehensive metastases-directed therapy.
Most of this was external beam radiation therapy and stereotactic radiation therapy, but there were some localized radiation implants as well, plus chemotherapy. This was comprehensive metastases-directed therapy to each of these sites plus chemotherapy vs chemotherapy alone.
What was shown in this trial? The progression-free survival (PFS) in the metastases-directed therapy group was 10.3 months vs 2.5 months in the group of patients who received chemotherapy only, with a hazard ratio of 0.43 and statistical significance.
Remember, this was a very small study, but we see more than a tripling in the PFS. There was no difference in overall survival, which is not at all surprising because it was a very small sample size.
Very importantly — and essential to doing this trial ethically — a crossover was permitted at the time of progression, meaning that if a patient received chemotherapy only and progressed, they could potentially get stereotactic radiation to sites of metastatic disease. They might have also benefited from that kind of strategy to the metastasis-[therapy] so that overall survival in the small population may not be different. Again, there was a tripling of the time to disease progression.
Clearly, a larger study will be required to be more definitive. We would need more centers involved and maybe some modification in the study design in this trial because of any issues that the investigators may have identified. Of course, overall survival would be a fair endpoint to look at, but again, crossover would be essential, and that might influence an ultimate outcome. PFS is a very valid endpoint.
The only other point to mention is, with these results — and as I mentioned, advances in radiation and imaging — is it reasonable to potentially consider this type of approach for individual patients as a component of aggressive standard of care? Of course, this would be with very adequate informed consent from patients, because we don’t know what the impact will be.
With the limited morbidity associated with the radiation, for an individual patient with pancreatic cancer who has an adequate performance status and limited metastases, if we give them chemotherapy and also directed radiation, is it reasonable to consider that as an appropriate treatment option outside the setting of a clinical trial?
I think this is a very valid question that needs to be addressed. In my opinion, the answer in some settings should be yes, but that needs to be discussed much more widely than simply in this randomized phase 2 trial.
Thank you for your attention.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. I’m Dr Maurie Markman, from City of Hope. I’d like to discuss what I consider to be an absolutely fascinating paper, and one that I will say has very interesting results but raises many more questions than it answers. I think that was the intent of the authors.
The paper is entitled, “Addition of metastasis-directed therapy to systemic therapy for oligometastatic pancreatic ductal adenocarcinoma (EXTEND): a multicenter, randomized phase 2 trial,” published in the Journal of Clinical Oncology.
You might ask what metastasis-directed therapy in pancreatic cancer means. Have we really made much of an impact on pancreatic cancer? In fact, in my earlier years of training, if somebody came up with the idea, or suggested as part of a trial or treatment of an individual patient, that they would focus on metastases in pancreas cancer, you might say they’re crazy, or you might say: “Yeah, but they probably don’t know anything about the disease and its natural history.”
Now, fast forward several decades. Even with the recognized, modest advances in systemic therapy, what we see are tremendous, really remarkable advances in innovations in radiation therapy. Of course, this includes not only the use of radiation itself but also the imaging technology that is used to direct the radiation therapy. These advances have permitted asking the questions that are addressed in the current study.
Again, this study is fascinating. They randomized a very small number. Again, it’s a randomized phase 2 study. It’s really more of a proof of principle here. They randomized 41 patients with five or fewer metastatic lesions — with oligometastatic disease, they could have numerous lesions — to undergo what they’ve described as comprehensive metastases-directed therapy.
Most of this was external beam radiation therapy and stereotactic radiation therapy, but there were some localized radiation implants as well, plus chemotherapy. This was comprehensive metastases-directed therapy to each of these sites plus chemotherapy vs chemotherapy alone.
What was shown in this trial? The progression-free survival (PFS) in the metastases-directed therapy group was 10.3 months vs 2.5 months in the group of patients who received chemotherapy only, with a hazard ratio of 0.43 and statistical significance.
Remember, this was a very small study, but we see more than a tripling in the PFS. There was no difference in overall survival, which is not at all surprising because it was a very small sample size.
Very importantly — and essential to doing this trial ethically — a crossover was permitted at the time of progression, meaning that if a patient received chemotherapy only and progressed, they could potentially get stereotactic radiation to sites of metastatic disease. They might have also benefited from that kind of strategy to the metastasis-[therapy] so that overall survival in the small population may not be different. Again, there was a tripling of the time to disease progression.
Clearly, a larger study will be required to be more definitive. We would need more centers involved and maybe some modification in the study design in this trial because of any issues that the investigators may have identified. Of course, overall survival would be a fair endpoint to look at, but again, crossover would be essential, and that might influence an ultimate outcome. PFS is a very valid endpoint.
The only other point to mention is, with these results — and as I mentioned, advances in radiation and imaging — is it reasonable to potentially consider this type of approach for individual patients as a component of aggressive standard of care? Of course, this would be with very adequate informed consent from patients, because we don’t know what the impact will be.
With the limited morbidity associated with the radiation, for an individual patient with pancreatic cancer who has an adequate performance status and limited metastases, if we give them chemotherapy and also directed radiation, is it reasonable to consider that as an appropriate treatment option outside the setting of a clinical trial?
I think this is a very valid question that needs to be addressed. In my opinion, the answer in some settings should be yes, but that needs to be discussed much more widely than simply in this randomized phase 2 trial.
Thank you for your attention.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. I’m Dr Maurie Markman, from City of Hope. I’d like to discuss what I consider to be an absolutely fascinating paper, and one that I will say has very interesting results but raises many more questions than it answers. I think that was the intent of the authors.
The paper is entitled, “Addition of metastasis-directed therapy to systemic therapy for oligometastatic pancreatic ductal adenocarcinoma (EXTEND): a multicenter, randomized phase 2 trial,” published in the Journal of Clinical Oncology.
You might ask what metastasis-directed therapy in pancreatic cancer means. Have we really made much of an impact on pancreatic cancer? In fact, in my earlier years of training, if somebody came up with the idea, or suggested as part of a trial or treatment of an individual patient, that they would focus on metastases in pancreas cancer, you might say they’re crazy, or you might say: “Yeah, but they probably don’t know anything about the disease and its natural history.”
Now, fast forward several decades. Even with the recognized, modest advances in systemic therapy, what we see are tremendous, really remarkable advances in innovations in radiation therapy. Of course, this includes not only the use of radiation itself but also the imaging technology that is used to direct the radiation therapy. These advances have permitted asking the questions that are addressed in the current study.
Again, this study is fascinating. They randomized a very small number. Again, it’s a randomized phase 2 study. It’s really more of a proof of principle here. They randomized 41 patients with five or fewer metastatic lesions — with oligometastatic disease, they could have numerous lesions — to undergo what they’ve described as comprehensive metastases-directed therapy.
Most of this was external beam radiation therapy and stereotactic radiation therapy, but there were some localized radiation implants as well, plus chemotherapy. This was comprehensive metastases-directed therapy to each of these sites plus chemotherapy vs chemotherapy alone.
What was shown in this trial? The progression-free survival (PFS) in the metastases-directed therapy group was 10.3 months vs 2.5 months in the group of patients who received chemotherapy only, with a hazard ratio of 0.43 and statistical significance.
Remember, this was a very small study, but we see more than a tripling in the PFS. There was no difference in overall survival, which is not at all surprising because it was a very small sample size.
Very importantly — and essential to doing this trial ethically — a crossover was permitted at the time of progression, meaning that if a patient received chemotherapy only and progressed, they could potentially get stereotactic radiation to sites of metastatic disease. They might have also benefited from that kind of strategy to the metastasis-[therapy] so that overall survival in the small population may not be different. Again, there was a tripling of the time to disease progression.
Clearly, a larger study will be required to be more definitive. We would need more centers involved and maybe some modification in the study design in this trial because of any issues that the investigators may have identified. Of course, overall survival would be a fair endpoint to look at, but again, crossover would be essential, and that might influence an ultimate outcome. PFS is a very valid endpoint.
The only other point to mention is, with these results — and as I mentioned, advances in radiation and imaging — is it reasonable to potentially consider this type of approach for individual patients as a component of aggressive standard of care? Of course, this would be with very adequate informed consent from patients, because we don’t know what the impact will be.
With the limited morbidity associated with the radiation, for an individual patient with pancreatic cancer who has an adequate performance status and limited metastases, if we give them chemotherapy and also directed radiation, is it reasonable to consider that as an appropriate treatment option outside the setting of a clinical trial?
I think this is a very valid question that needs to be addressed. In my opinion, the answer in some settings should be yes, but that needs to be discussed much more widely than simply in this randomized phase 2 trial.
Thank you for your attention.
A version of this article first appeared on Medscape.com.
State-Mandated ‘Gold Card’ Programs to Ease Prior Authorization Burdens Offer Little Relief, Experts Say
“Gold card” programs were supposed to make it easier for frustrated physicians to deal with insurers’ burdensome prior authorization demands.
The idea: Insurers would reward doctors whose past prior authorization requests were typically approved by exempting them from red tape in the future.
At least 10 states have required insurers to establish gold card programs amid mounting concerns nationwide that overuse of prior authorization jeopardizes patient health. Last month, leading insurers joined with the White House in a voluntary pledge to reduce their use of the practice, which they contend is necessary to control costs and minimize unnecessary care.
But Texas’ experience with gold card programs may signal the limits of that approach.
Only 3% of Clinicians Qualified
The Lone Star State was an early adopter, passing a 2021 law enabling health providers with a high prior authorization success rate to earn a “gold card” exemption from insurers.
But statewide, only 3% of providers met that bar, according to a testimony provided by the Texas Department of Insurance earlier this year.
“I think it’s safe to say that the impact of this law on prior authorizations for our physicians is underwhelming,” said Ezequiel “Zeke” Silva III, MD, a San Antonio-based interventional radiologist who chairs the Texas Medical Association’s Council on Legislation. “We would have hoped for a greater percentage of our physicians to have been granted the ‘gold card’ status.”
At least nine other states have enacted gold card laws, according to the National Conference of State Legislatures (NCSL).
Care Delayed and Denied
Physicians maintain that excessive prior authorization paperwork impedes timely patient care, with clinicians and staffers devoting 13 hours weekly to documentation, according to a 2024 American Medical Association survey.
Insurers view the review as a guardrail against unnecessary care driving up costs. Studies show that restricting prior authorization could boost premiums by 5.6%-16.7%, a Texas Association of Health Plans official testified during the legislative session.
In June, Texas Gov. Greg Abbott signed a revised version of the state’s “gold card” law — part of an emerging national attempt to streamline the prior review process. Cigna, Humana, UnitedHealthcare, and other large insurers have voluntarily committed to reducing the scope of claims involved, according to the America’s Health Insurance Plans trade group.
Meanwhile, federal officials have finalized requirements that direct some insurers, including Medicaid and Medicare Advantage programs, to speed up responses to prior authorization requests, among other measures. Some of those requirements begin in 2026.
Gold Card Designs
As in other states, Texas’ “gold card” legislation applies only to state-regulated insurers, which comprise about one fifth of the state’s market. Under HB 3812, which takes effect on September 1, insurers will evaluate health providers based on a year of prior authorization requests rather than 6 months under the 2021 law.
To be evaluated, providers must have submitted at least five requests for a specific health service during that period. To achieve “gold card” status, insurers must approve at least 90% of requests, the same threshold as set by the 2021 law. But the new law stipulates that insurers review a broader pool of requests, including those made directly to the health plan as well as any related affiliates, according to the Texas Department of Insurance.
The new law continues to limit exemptions only to “top-performing physicians” who repeatedly provide cost-effective care, said Blake Hutson, director of public affairs at the Texas Association of Health Plans. “Even with the change to 1 year, and the bill also adds in a broader array of claims that will be looked at, you still have to meet 90%.”
A key addition requires insurers to release an annual report detailing how many exemptions they have granted or denied, making decisions more transparent to the public, Silva said. “Not just what’s being approved and what’s not being approved, but to potentially evaluate for trends that presently we just have no ability to evaluate,” he said.
Gold card laws vary from state to state, and some exclude prescription drugs, according to an NCSL legislative summary. Other states with gold card programs include Arkansas, Colorado, Illinois, Louisiana, Michigan, New Mexico, Vermont, West Virginia, and Wyoming.
In Illinois, legislation passed last year targeted hospital services for Medicaid patients, as denial rates were routinely higher in that population, said Dave Gross, senior vice president of Government Relations and Communications at the Illinois Health and Hospital Association, Naperville, Illinois. “We’re not seeing this problem in the commercial space,” he noted.
Real-World Implications
To some degree, the “gold card” concept makes intuitive sense, recognizing physicians who have a track record of getting their medical care requests approved, said Ravi Gupta, MD, an assistant professor of medicine at Johns Hopkins University School of Medicine, Baltimore, who has studied prior authorization patterns.
But Gupta raised equity concerns. Physicians in large medical groups and hospital systems will have access to staff and other resources to better navigate the prior approval process than those in smaller private practices.
Plus, he added, there’s the potential that physicians who achieve exemptions may become “more indiscriminate” about the services that they recommend.
Insurers’ stated aim is to reduce unnecessary and low-value medical care through prior authorization gatekeeping, Gupta said. But a study he helped conduct, assessing policies across five Medicare Advantage insurers, found a significant lack of consensus on what treatments should be included. Treatments comprising only 12% of Medicare spending would have required prior authorization by all five insurers. Most of that consensus, he wrote, “was devoted to a small number of costly services.”
The administrative burdens affect patients as well. Two thirds of patients with cancer in one 2023 study become personally involved, including calling the insurer or appealing a denial. The patients also reported less trust in insurers and the health system overall, which could have worrisome downstream effects, Fumiko Chino, MD, the study’s lead author and an assistant professor of radiation oncology at Houston’s MD Anderson Cancer Center, said.
“If you don’t trust healthcare,” she said, “why on earth would you get a vaccine or get cancer screening or get your blood pressure checked?”
More Than X Percent?
Gupta views the leading health insurers’ pledge as encouraging in concept — but he notes that they are voluntary commitments without any accountability.
In the interim, gold carding remains no more than a workaround, he said.
“Gold cards aren’t really fixing that [prior authorization] problem,” he said. “They’re just rewarding certain clinicians who can demonstrate that they have been able to get through the prior authorization process successfully for X amount of time before they’re rewarded with a gold card.”
In Illinois, regulators are still hashing out gold card rules, including whether the required 90% approval threshold will be based on a specific hospital service or a broader pool of services, Gross said. The hospital association also will closely watch whether Illinois’ experience begins to mirror that in Texas, he said.
“We have some of the best hospitals in the country here in Chicago,” he said. “If we end up with a 3% approval rating of gold cards, we’re going to have to go back to the legislature.”
A version of this article first appeared on Medscape.com.
“Gold card” programs were supposed to make it easier for frustrated physicians to deal with insurers’ burdensome prior authorization demands.
The idea: Insurers would reward doctors whose past prior authorization requests were typically approved by exempting them from red tape in the future.
At least 10 states have required insurers to establish gold card programs amid mounting concerns nationwide that overuse of prior authorization jeopardizes patient health. Last month, leading insurers joined with the White House in a voluntary pledge to reduce their use of the practice, which they contend is necessary to control costs and minimize unnecessary care.
But Texas’ experience with gold card programs may signal the limits of that approach.
Only 3% of Clinicians Qualified
The Lone Star State was an early adopter, passing a 2021 law enabling health providers with a high prior authorization success rate to earn a “gold card” exemption from insurers.
But statewide, only 3% of providers met that bar, according to a testimony provided by the Texas Department of Insurance earlier this year.
“I think it’s safe to say that the impact of this law on prior authorizations for our physicians is underwhelming,” said Ezequiel “Zeke” Silva III, MD, a San Antonio-based interventional radiologist who chairs the Texas Medical Association’s Council on Legislation. “We would have hoped for a greater percentage of our physicians to have been granted the ‘gold card’ status.”
At least nine other states have enacted gold card laws, according to the National Conference of State Legislatures (NCSL).
Care Delayed and Denied
Physicians maintain that excessive prior authorization paperwork impedes timely patient care, with clinicians and staffers devoting 13 hours weekly to documentation, according to a 2024 American Medical Association survey.
Insurers view the review as a guardrail against unnecessary care driving up costs. Studies show that restricting prior authorization could boost premiums by 5.6%-16.7%, a Texas Association of Health Plans official testified during the legislative session.
In June, Texas Gov. Greg Abbott signed a revised version of the state’s “gold card” law — part of an emerging national attempt to streamline the prior review process. Cigna, Humana, UnitedHealthcare, and other large insurers have voluntarily committed to reducing the scope of claims involved, according to the America’s Health Insurance Plans trade group.
Meanwhile, federal officials have finalized requirements that direct some insurers, including Medicaid and Medicare Advantage programs, to speed up responses to prior authorization requests, among other measures. Some of those requirements begin in 2026.
Gold Card Designs
As in other states, Texas’ “gold card” legislation applies only to state-regulated insurers, which comprise about one fifth of the state’s market. Under HB 3812, which takes effect on September 1, insurers will evaluate health providers based on a year of prior authorization requests rather than 6 months under the 2021 law.
To be evaluated, providers must have submitted at least five requests for a specific health service during that period. To achieve “gold card” status, insurers must approve at least 90% of requests, the same threshold as set by the 2021 law. But the new law stipulates that insurers review a broader pool of requests, including those made directly to the health plan as well as any related affiliates, according to the Texas Department of Insurance.
The new law continues to limit exemptions only to “top-performing physicians” who repeatedly provide cost-effective care, said Blake Hutson, director of public affairs at the Texas Association of Health Plans. “Even with the change to 1 year, and the bill also adds in a broader array of claims that will be looked at, you still have to meet 90%.”
A key addition requires insurers to release an annual report detailing how many exemptions they have granted or denied, making decisions more transparent to the public, Silva said. “Not just what’s being approved and what’s not being approved, but to potentially evaluate for trends that presently we just have no ability to evaluate,” he said.
Gold card laws vary from state to state, and some exclude prescription drugs, according to an NCSL legislative summary. Other states with gold card programs include Arkansas, Colorado, Illinois, Louisiana, Michigan, New Mexico, Vermont, West Virginia, and Wyoming.
In Illinois, legislation passed last year targeted hospital services for Medicaid patients, as denial rates were routinely higher in that population, said Dave Gross, senior vice president of Government Relations and Communications at the Illinois Health and Hospital Association, Naperville, Illinois. “We’re not seeing this problem in the commercial space,” he noted.
Real-World Implications
To some degree, the “gold card” concept makes intuitive sense, recognizing physicians who have a track record of getting their medical care requests approved, said Ravi Gupta, MD, an assistant professor of medicine at Johns Hopkins University School of Medicine, Baltimore, who has studied prior authorization patterns.
But Gupta raised equity concerns. Physicians in large medical groups and hospital systems will have access to staff and other resources to better navigate the prior approval process than those in smaller private practices.
Plus, he added, there’s the potential that physicians who achieve exemptions may become “more indiscriminate” about the services that they recommend.
Insurers’ stated aim is to reduce unnecessary and low-value medical care through prior authorization gatekeeping, Gupta said. But a study he helped conduct, assessing policies across five Medicare Advantage insurers, found a significant lack of consensus on what treatments should be included. Treatments comprising only 12% of Medicare spending would have required prior authorization by all five insurers. Most of that consensus, he wrote, “was devoted to a small number of costly services.”
The administrative burdens affect patients as well. Two thirds of patients with cancer in one 2023 study become personally involved, including calling the insurer or appealing a denial. The patients also reported less trust in insurers and the health system overall, which could have worrisome downstream effects, Fumiko Chino, MD, the study’s lead author and an assistant professor of radiation oncology at Houston’s MD Anderson Cancer Center, said.
“If you don’t trust healthcare,” she said, “why on earth would you get a vaccine or get cancer screening or get your blood pressure checked?”
More Than X Percent?
Gupta views the leading health insurers’ pledge as encouraging in concept — but he notes that they are voluntary commitments without any accountability.
In the interim, gold carding remains no more than a workaround, he said.
“Gold cards aren’t really fixing that [prior authorization] problem,” he said. “They’re just rewarding certain clinicians who can demonstrate that they have been able to get through the prior authorization process successfully for X amount of time before they’re rewarded with a gold card.”
In Illinois, regulators are still hashing out gold card rules, including whether the required 90% approval threshold will be based on a specific hospital service or a broader pool of services, Gross said. The hospital association also will closely watch whether Illinois’ experience begins to mirror that in Texas, he said.
“We have some of the best hospitals in the country here in Chicago,” he said. “If we end up with a 3% approval rating of gold cards, we’re going to have to go back to the legislature.”
A version of this article first appeared on Medscape.com.
“Gold card” programs were supposed to make it easier for frustrated physicians to deal with insurers’ burdensome prior authorization demands.
The idea: Insurers would reward doctors whose past prior authorization requests were typically approved by exempting them from red tape in the future.
At least 10 states have required insurers to establish gold card programs amid mounting concerns nationwide that overuse of prior authorization jeopardizes patient health. Last month, leading insurers joined with the White House in a voluntary pledge to reduce their use of the practice, which they contend is necessary to control costs and minimize unnecessary care.
But Texas’ experience with gold card programs may signal the limits of that approach.
Only 3% of Clinicians Qualified
The Lone Star State was an early adopter, passing a 2021 law enabling health providers with a high prior authorization success rate to earn a “gold card” exemption from insurers.
But statewide, only 3% of providers met that bar, according to a testimony provided by the Texas Department of Insurance earlier this year.
“I think it’s safe to say that the impact of this law on prior authorizations for our physicians is underwhelming,” said Ezequiel “Zeke” Silva III, MD, a San Antonio-based interventional radiologist who chairs the Texas Medical Association’s Council on Legislation. “We would have hoped for a greater percentage of our physicians to have been granted the ‘gold card’ status.”
At least nine other states have enacted gold card laws, according to the National Conference of State Legislatures (NCSL).
Care Delayed and Denied
Physicians maintain that excessive prior authorization paperwork impedes timely patient care, with clinicians and staffers devoting 13 hours weekly to documentation, according to a 2024 American Medical Association survey.
Insurers view the review as a guardrail against unnecessary care driving up costs. Studies show that restricting prior authorization could boost premiums by 5.6%-16.7%, a Texas Association of Health Plans official testified during the legislative session.
In June, Texas Gov. Greg Abbott signed a revised version of the state’s “gold card” law — part of an emerging national attempt to streamline the prior review process. Cigna, Humana, UnitedHealthcare, and other large insurers have voluntarily committed to reducing the scope of claims involved, according to the America’s Health Insurance Plans trade group.
Meanwhile, federal officials have finalized requirements that direct some insurers, including Medicaid and Medicare Advantage programs, to speed up responses to prior authorization requests, among other measures. Some of those requirements begin in 2026.
Gold Card Designs
As in other states, Texas’ “gold card” legislation applies only to state-regulated insurers, which comprise about one fifth of the state’s market. Under HB 3812, which takes effect on September 1, insurers will evaluate health providers based on a year of prior authorization requests rather than 6 months under the 2021 law.
To be evaluated, providers must have submitted at least five requests for a specific health service during that period. To achieve “gold card” status, insurers must approve at least 90% of requests, the same threshold as set by the 2021 law. But the new law stipulates that insurers review a broader pool of requests, including those made directly to the health plan as well as any related affiliates, according to the Texas Department of Insurance.
The new law continues to limit exemptions only to “top-performing physicians” who repeatedly provide cost-effective care, said Blake Hutson, director of public affairs at the Texas Association of Health Plans. “Even with the change to 1 year, and the bill also adds in a broader array of claims that will be looked at, you still have to meet 90%.”
A key addition requires insurers to release an annual report detailing how many exemptions they have granted or denied, making decisions more transparent to the public, Silva said. “Not just what’s being approved and what’s not being approved, but to potentially evaluate for trends that presently we just have no ability to evaluate,” he said.
Gold card laws vary from state to state, and some exclude prescription drugs, according to an NCSL legislative summary. Other states with gold card programs include Arkansas, Colorado, Illinois, Louisiana, Michigan, New Mexico, Vermont, West Virginia, and Wyoming.
In Illinois, legislation passed last year targeted hospital services for Medicaid patients, as denial rates were routinely higher in that population, said Dave Gross, senior vice president of Government Relations and Communications at the Illinois Health and Hospital Association, Naperville, Illinois. “We’re not seeing this problem in the commercial space,” he noted.
Real-World Implications
To some degree, the “gold card” concept makes intuitive sense, recognizing physicians who have a track record of getting their medical care requests approved, said Ravi Gupta, MD, an assistant professor of medicine at Johns Hopkins University School of Medicine, Baltimore, who has studied prior authorization patterns.
But Gupta raised equity concerns. Physicians in large medical groups and hospital systems will have access to staff and other resources to better navigate the prior approval process than those in smaller private practices.
Plus, he added, there’s the potential that physicians who achieve exemptions may become “more indiscriminate” about the services that they recommend.
Insurers’ stated aim is to reduce unnecessary and low-value medical care through prior authorization gatekeeping, Gupta said. But a study he helped conduct, assessing policies across five Medicare Advantage insurers, found a significant lack of consensus on what treatments should be included. Treatments comprising only 12% of Medicare spending would have required prior authorization by all five insurers. Most of that consensus, he wrote, “was devoted to a small number of costly services.”
The administrative burdens affect patients as well. Two thirds of patients with cancer in one 2023 study become personally involved, including calling the insurer or appealing a denial. The patients also reported less trust in insurers and the health system overall, which could have worrisome downstream effects, Fumiko Chino, MD, the study’s lead author and an assistant professor of radiation oncology at Houston’s MD Anderson Cancer Center, said.
“If you don’t trust healthcare,” she said, “why on earth would you get a vaccine or get cancer screening or get your blood pressure checked?”
More Than X Percent?
Gupta views the leading health insurers’ pledge as encouraging in concept — but he notes that they are voluntary commitments without any accountability.
In the interim, gold carding remains no more than a workaround, he said.
“Gold cards aren’t really fixing that [prior authorization] problem,” he said. “They’re just rewarding certain clinicians who can demonstrate that they have been able to get through the prior authorization process successfully for X amount of time before they’re rewarded with a gold card.”
In Illinois, regulators are still hashing out gold card rules, including whether the required 90% approval threshold will be based on a specific hospital service or a broader pool of services, Gross said. The hospital association also will closely watch whether Illinois’ experience begins to mirror that in Texas, he said.
“We have some of the best hospitals in the country here in Chicago,” he said. “If we end up with a 3% approval rating of gold cards, we’re going to have to go back to the legislature.”
A version of this article first appeared on Medscape.com.
Are Breast Cancer Survivors Vulnerable to Alzheimer’s Disease?
Despite concerns about cognitive decline after cancer treatment, most breast cancer survivors show no increased risk of developing Alzheimer’s disease, and some may have a slightly lower risk than their cancer-free peers, according to a large retrospective study from Korea.
However, any apparent protective effect faded with time, the investigators reported online in JAMA Network Open.
Overall, this is “reassuring news for cancer survivors,” Tim Ahles, PhD, a psychologist with Memorial Sloan Kettering Cancer Center, New York City, who wasn’t involved in the study, told this news organization.
“I get this question from patients a lot,” Ahles said. And based on these findings, “it doesn’t look like a history of breast cancer and breast cancer treatment increases your risk for Alzheimer’s disease.”
Breast cancer survivors often report cancer-related cognitive impairment, such as difficulties with concentration and memory, both during and after cancer treatment. But evidence surrounding patients’ risk for Alzheimer’s disease is mixed. One large study based in Sweden, for instance, reported a 35% increased risk for Alzheimer’s disease among patients diagnosed with breast cancer after the age of 65 years, but not among younger patients. A population-based study from Taiwan, however, found no increase in the risk for dementia overall compared with cancer-free individuals but did note a lower dementia risk in patients who had received tamoxifen.
To help clarify the evidence, investigators assessed Alzheimer’s disease risk in a large cohort of patients and explored the association by treatment type, age, and important risk factors.
Using the Korean National Health Insurance Service database, the researchers matched 70,701 patients who underwent breast cancer surgery between 2010 and 2016 with 180,360 cancer-free control individuals.
The mean age of breast cancer survivors was 53.1 years. Overall, 72% received radiotherapy. Cyclophosphamide (57%) and anthracycline (50%) were the most commonly used chemotherapies, and tamoxifen (47%) and aromatase inhibitors (30%) were the most commonly used endocrine therapies.
The primary outcome of this study was the incidence of newly diagnosed Alzheimer’s disease, which was defined on the basis of at least one prescription for medications to manage dementia associated with Alzheimer’s disease (donepezil, rivastigmine, galantamine, or memantine).
During a median follow-up of about 7 years, 1229 newly diagnosed Alzheimer’s disease cases were detected in breast cancer survivors and 3430 cases in control individuals — incidence rates of 2.45 and 2.63 per 1000 person-years, respectively.
This corresponded to an 8% lower risk for Alzheimer’s disease in breast cancer survivors compared with cancer-free control individuals at 6 months (subdistribution hazard ratio [SHR], 0.92; 95% CI, 0.86-0.98). The association was especially notable in survivors older than 65 years (SHR, 0.92; 95% CI, 0.85-0.99).
Looking at individual treatment modalities, only radiation therapy was associated with significantly lower risk for Alzheimer’s disease among breast cancer survivors (adjusted HR [aHR], 0.77).
Several risk factors were associated with a significantly higher risk for Alzheimer’s disease: current smoker vs never or ex-smokers (aHR, 2.04), diabetes (aHR, 1.58), and chronic kidney disease (aHR, 3.11). Notably, alcohol use, physical activity level, and hypertension were not associated with Alzheimer’s disease risk.
However, any potential protective effect may be short-lived. The reduced risk for Alzheimer’s disease was no longer significant at 1 year (SHR, 0.94; 95% CI, 0.87-1.01), 3 years (SHR, 0.97; 95% CI, 0.90-1.05), or 5 years (SHR, 0.98; 95% CI, 0.89-1.08).
Even so, breast cancer survivors can still feel reassured by the findings.
“Concerns about chemobrain and the long-term adverse effects of breast cancer treatment on cognition are common, but our findings suggest that this treatment does not directly lead to Alzheimer’s disease,” wrote the authors, led by Su-Min Jeong, MD, with Seoul National University College of Medicine, Seoul, South Korea.
Ahles agreed. The general takeaway from this study is that there is “no strong evidence that the cancer treatment is going to increase your risk for developing Alzheimer’s,” Ahles said. When patients ask about the risk for Alzheimer’s disease, “I can say, ‘Here’s yet another new study that supports the idea that there’s no increased risk.’”
He cautioned, however, that the study doesn’t address whether people with a genetic predisposition to Alzheimer’s might develop it sooner due to cancer treatment.
“Does the cancer treatment increase your probability or nudge you along? The study doesn’t answer that question,” Ahles said.
The study reported having no commercial funding. Jeong and Ahles reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
Despite concerns about cognitive decline after cancer treatment, most breast cancer survivors show no increased risk of developing Alzheimer’s disease, and some may have a slightly lower risk than their cancer-free peers, according to a large retrospective study from Korea.
However, any apparent protective effect faded with time, the investigators reported online in JAMA Network Open.
Overall, this is “reassuring news for cancer survivors,” Tim Ahles, PhD, a psychologist with Memorial Sloan Kettering Cancer Center, New York City, who wasn’t involved in the study, told this news organization.
“I get this question from patients a lot,” Ahles said. And based on these findings, “it doesn’t look like a history of breast cancer and breast cancer treatment increases your risk for Alzheimer’s disease.”
Breast cancer survivors often report cancer-related cognitive impairment, such as difficulties with concentration and memory, both during and after cancer treatment. But evidence surrounding patients’ risk for Alzheimer’s disease is mixed. One large study based in Sweden, for instance, reported a 35% increased risk for Alzheimer’s disease among patients diagnosed with breast cancer after the age of 65 years, but not among younger patients. A population-based study from Taiwan, however, found no increase in the risk for dementia overall compared with cancer-free individuals but did note a lower dementia risk in patients who had received tamoxifen.
To help clarify the evidence, investigators assessed Alzheimer’s disease risk in a large cohort of patients and explored the association by treatment type, age, and important risk factors.
Using the Korean National Health Insurance Service database, the researchers matched 70,701 patients who underwent breast cancer surgery between 2010 and 2016 with 180,360 cancer-free control individuals.
The mean age of breast cancer survivors was 53.1 years. Overall, 72% received radiotherapy. Cyclophosphamide (57%) and anthracycline (50%) were the most commonly used chemotherapies, and tamoxifen (47%) and aromatase inhibitors (30%) were the most commonly used endocrine therapies.
The primary outcome of this study was the incidence of newly diagnosed Alzheimer’s disease, which was defined on the basis of at least one prescription for medications to manage dementia associated with Alzheimer’s disease (donepezil, rivastigmine, galantamine, or memantine).
During a median follow-up of about 7 years, 1229 newly diagnosed Alzheimer’s disease cases were detected in breast cancer survivors and 3430 cases in control individuals — incidence rates of 2.45 and 2.63 per 1000 person-years, respectively.
This corresponded to an 8% lower risk for Alzheimer’s disease in breast cancer survivors compared with cancer-free control individuals at 6 months (subdistribution hazard ratio [SHR], 0.92; 95% CI, 0.86-0.98). The association was especially notable in survivors older than 65 years (SHR, 0.92; 95% CI, 0.85-0.99).
Looking at individual treatment modalities, only radiation therapy was associated with significantly lower risk for Alzheimer’s disease among breast cancer survivors (adjusted HR [aHR], 0.77).
Several risk factors were associated with a significantly higher risk for Alzheimer’s disease: current smoker vs never or ex-smokers (aHR, 2.04), diabetes (aHR, 1.58), and chronic kidney disease (aHR, 3.11). Notably, alcohol use, physical activity level, and hypertension were not associated with Alzheimer’s disease risk.
However, any potential protective effect may be short-lived. The reduced risk for Alzheimer’s disease was no longer significant at 1 year (SHR, 0.94; 95% CI, 0.87-1.01), 3 years (SHR, 0.97; 95% CI, 0.90-1.05), or 5 years (SHR, 0.98; 95% CI, 0.89-1.08).
Even so, breast cancer survivors can still feel reassured by the findings.
“Concerns about chemobrain and the long-term adverse effects of breast cancer treatment on cognition are common, but our findings suggest that this treatment does not directly lead to Alzheimer’s disease,” wrote the authors, led by Su-Min Jeong, MD, with Seoul National University College of Medicine, Seoul, South Korea.
Ahles agreed. The general takeaway from this study is that there is “no strong evidence that the cancer treatment is going to increase your risk for developing Alzheimer’s,” Ahles said. When patients ask about the risk for Alzheimer’s disease, “I can say, ‘Here’s yet another new study that supports the idea that there’s no increased risk.’”
He cautioned, however, that the study doesn’t address whether people with a genetic predisposition to Alzheimer’s might develop it sooner due to cancer treatment.
“Does the cancer treatment increase your probability or nudge you along? The study doesn’t answer that question,” Ahles said.
The study reported having no commercial funding. Jeong and Ahles reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
Despite concerns about cognitive decline after cancer treatment, most breast cancer survivors show no increased risk of developing Alzheimer’s disease, and some may have a slightly lower risk than their cancer-free peers, according to a large retrospective study from Korea.
However, any apparent protective effect faded with time, the investigators reported online in JAMA Network Open.
Overall, this is “reassuring news for cancer survivors,” Tim Ahles, PhD, a psychologist with Memorial Sloan Kettering Cancer Center, New York City, who wasn’t involved in the study, told this news organization.
“I get this question from patients a lot,” Ahles said. And based on these findings, “it doesn’t look like a history of breast cancer and breast cancer treatment increases your risk for Alzheimer’s disease.”
Breast cancer survivors often report cancer-related cognitive impairment, such as difficulties with concentration and memory, both during and after cancer treatment. But evidence surrounding patients’ risk for Alzheimer’s disease is mixed. One large study based in Sweden, for instance, reported a 35% increased risk for Alzheimer’s disease among patients diagnosed with breast cancer after the age of 65 years, but not among younger patients. A population-based study from Taiwan, however, found no increase in the risk for dementia overall compared with cancer-free individuals but did note a lower dementia risk in patients who had received tamoxifen.
To help clarify the evidence, investigators assessed Alzheimer’s disease risk in a large cohort of patients and explored the association by treatment type, age, and important risk factors.
Using the Korean National Health Insurance Service database, the researchers matched 70,701 patients who underwent breast cancer surgery between 2010 and 2016 with 180,360 cancer-free control individuals.
The mean age of breast cancer survivors was 53.1 years. Overall, 72% received radiotherapy. Cyclophosphamide (57%) and anthracycline (50%) were the most commonly used chemotherapies, and tamoxifen (47%) and aromatase inhibitors (30%) were the most commonly used endocrine therapies.
The primary outcome of this study was the incidence of newly diagnosed Alzheimer’s disease, which was defined on the basis of at least one prescription for medications to manage dementia associated with Alzheimer’s disease (donepezil, rivastigmine, galantamine, or memantine).
During a median follow-up of about 7 years, 1229 newly diagnosed Alzheimer’s disease cases were detected in breast cancer survivors and 3430 cases in control individuals — incidence rates of 2.45 and 2.63 per 1000 person-years, respectively.
This corresponded to an 8% lower risk for Alzheimer’s disease in breast cancer survivors compared with cancer-free control individuals at 6 months (subdistribution hazard ratio [SHR], 0.92; 95% CI, 0.86-0.98). The association was especially notable in survivors older than 65 years (SHR, 0.92; 95% CI, 0.85-0.99).
Looking at individual treatment modalities, only radiation therapy was associated with significantly lower risk for Alzheimer’s disease among breast cancer survivors (adjusted HR [aHR], 0.77).
Several risk factors were associated with a significantly higher risk for Alzheimer’s disease: current smoker vs never or ex-smokers (aHR, 2.04), diabetes (aHR, 1.58), and chronic kidney disease (aHR, 3.11). Notably, alcohol use, physical activity level, and hypertension were not associated with Alzheimer’s disease risk.
However, any potential protective effect may be short-lived. The reduced risk for Alzheimer’s disease was no longer significant at 1 year (SHR, 0.94; 95% CI, 0.87-1.01), 3 years (SHR, 0.97; 95% CI, 0.90-1.05), or 5 years (SHR, 0.98; 95% CI, 0.89-1.08).
Even so, breast cancer survivors can still feel reassured by the findings.
“Concerns about chemobrain and the long-term adverse effects of breast cancer treatment on cognition are common, but our findings suggest that this treatment does not directly lead to Alzheimer’s disease,” wrote the authors, led by Su-Min Jeong, MD, with Seoul National University College of Medicine, Seoul, South Korea.
Ahles agreed. The general takeaway from this study is that there is “no strong evidence that the cancer treatment is going to increase your risk for developing Alzheimer’s,” Ahles said. When patients ask about the risk for Alzheimer’s disease, “I can say, ‘Here’s yet another new study that supports the idea that there’s no increased risk.’”
He cautioned, however, that the study doesn’t address whether people with a genetic predisposition to Alzheimer’s might develop it sooner due to cancer treatment.
“Does the cancer treatment increase your probability or nudge you along? The study doesn’t answer that question,” Ahles said.
The study reported having no commercial funding. Jeong and Ahles reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
Paclitaxel Matches Cisplatin HIPEC in Ovarian Cancer
TOPLINE:
Patients with advanced ovarian cancer undergoing interval cytoreductive surgery who received paclitaxel-based hyperthermic intraperitoneal chemotherapy (HIPEC) during surgery appeared to have comparable overall survival and disease-free survival rates to those who received cisplatin-based HIPEC.
METHODOLOGY:
- Although the use of HIPEC remains controversial, cisplatin-based HIPEC during cytoreductive surgery may benefit patients with advanced ovarian cancer; however, there is less evidence for paclitaxel-based HIPEC, typically used in patients who are frail or intolerant to platinum agents.
- To compare the two regimens, researchers analyzed data from the National Registry of Peritoneal Carcinomatosis, which included 846 patients (mean age, 59 years) who underwent interval cytoreductive surgery with either cisplatin-based HIPEC (n = 325) or paclitaxel-based HIPEC (n = 521). After propensity score matching, there were 199 patients per group (total = 398).
- HIPEC was administered post-surgery with cisplatin (75-100 mg/m2 for 90 minutes) or paclitaxel (120 mg/m2 for 60 minutes), both at 42-43 °C.
TAKEAWAY:
- Using cisplatin as the reference group, the median overall survival was not significantly different between the two options (hazard ratio [HR], 0.74; P = .16); however, the median overall survival was 82 months in the paclitaxel group vs 58 months in the cisplatin group.
- Disease-free survival was also not significantly different between the 2 groups, with a median of 20 months in the cisplatin group and 21 months in the paclitaxel groups (HR, 0.95; 95% CI, 0.72-1.25; P = .70).
- Overall survival was comparable during the first 20 months of follow-up and disease-free survival was equivalent during the first 15 months of follow-up, based on a predefined equivalence margin of 0.1.
- Paclitaxel-based HIPEC was not associated with increased morbidity (odds ratio, 1.32; P = .06).
IN PRACTICE:
“Our study suggests that cisplatin and paclitaxel are two safe and effective drugs to be used for HIPEC in [interval cytoreductive surgery] for advanced ovarian cancer. As cisplatin is the preferred drug according to strong evidence, paclitaxel could be a valuable alternative for patients with any contraindication to cisplatin, with similar oncological and perioperative outcomes,” the authors wrote.
SOURCE:
This study, led by Salud González Sánchez, MD, Reina Sofía University Hospital in Córdoba, Spain, was published online in JAMA Network Open.
LIMITATIONS:
The retrospective design of this study limited causal inference. The BRCA mutation status was not captured in the national registry. Additionally, the matching procedure resulted in a moderate sample size, which could have led to residual confounding.
DISCLOSURES:
The authors did not declare any funding information and reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with advanced ovarian cancer undergoing interval cytoreductive surgery who received paclitaxel-based hyperthermic intraperitoneal chemotherapy (HIPEC) during surgery appeared to have comparable overall survival and disease-free survival rates to those who received cisplatin-based HIPEC.
METHODOLOGY:
- Although the use of HIPEC remains controversial, cisplatin-based HIPEC during cytoreductive surgery may benefit patients with advanced ovarian cancer; however, there is less evidence for paclitaxel-based HIPEC, typically used in patients who are frail or intolerant to platinum agents.
- To compare the two regimens, researchers analyzed data from the National Registry of Peritoneal Carcinomatosis, which included 846 patients (mean age, 59 years) who underwent interval cytoreductive surgery with either cisplatin-based HIPEC (n = 325) or paclitaxel-based HIPEC (n = 521). After propensity score matching, there were 199 patients per group (total = 398).
- HIPEC was administered post-surgery with cisplatin (75-100 mg/m2 for 90 minutes) or paclitaxel (120 mg/m2 for 60 minutes), both at 42-43 °C.
TAKEAWAY:
- Using cisplatin as the reference group, the median overall survival was not significantly different between the two options (hazard ratio [HR], 0.74; P = .16); however, the median overall survival was 82 months in the paclitaxel group vs 58 months in the cisplatin group.
- Disease-free survival was also not significantly different between the 2 groups, with a median of 20 months in the cisplatin group and 21 months in the paclitaxel groups (HR, 0.95; 95% CI, 0.72-1.25; P = .70).
- Overall survival was comparable during the first 20 months of follow-up and disease-free survival was equivalent during the first 15 months of follow-up, based on a predefined equivalence margin of 0.1.
- Paclitaxel-based HIPEC was not associated with increased morbidity (odds ratio, 1.32; P = .06).
IN PRACTICE:
“Our study suggests that cisplatin and paclitaxel are two safe and effective drugs to be used for HIPEC in [interval cytoreductive surgery] for advanced ovarian cancer. As cisplatin is the preferred drug according to strong evidence, paclitaxel could be a valuable alternative for patients with any contraindication to cisplatin, with similar oncological and perioperative outcomes,” the authors wrote.
SOURCE:
This study, led by Salud González Sánchez, MD, Reina Sofía University Hospital in Córdoba, Spain, was published online in JAMA Network Open.
LIMITATIONS:
The retrospective design of this study limited causal inference. The BRCA mutation status was not captured in the national registry. Additionally, the matching procedure resulted in a moderate sample size, which could have led to residual confounding.
DISCLOSURES:
The authors did not declare any funding information and reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with advanced ovarian cancer undergoing interval cytoreductive surgery who received paclitaxel-based hyperthermic intraperitoneal chemotherapy (HIPEC) during surgery appeared to have comparable overall survival and disease-free survival rates to those who received cisplatin-based HIPEC.
METHODOLOGY:
- Although the use of HIPEC remains controversial, cisplatin-based HIPEC during cytoreductive surgery may benefit patients with advanced ovarian cancer; however, there is less evidence for paclitaxel-based HIPEC, typically used in patients who are frail or intolerant to platinum agents.
- To compare the two regimens, researchers analyzed data from the National Registry of Peritoneal Carcinomatosis, which included 846 patients (mean age, 59 years) who underwent interval cytoreductive surgery with either cisplatin-based HIPEC (n = 325) or paclitaxel-based HIPEC (n = 521). After propensity score matching, there were 199 patients per group (total = 398).
- HIPEC was administered post-surgery with cisplatin (75-100 mg/m2 for 90 minutes) or paclitaxel (120 mg/m2 for 60 minutes), both at 42-43 °C.
TAKEAWAY:
- Using cisplatin as the reference group, the median overall survival was not significantly different between the two options (hazard ratio [HR], 0.74; P = .16); however, the median overall survival was 82 months in the paclitaxel group vs 58 months in the cisplatin group.
- Disease-free survival was also not significantly different between the 2 groups, with a median of 20 months in the cisplatin group and 21 months in the paclitaxel groups (HR, 0.95; 95% CI, 0.72-1.25; P = .70).
- Overall survival was comparable during the first 20 months of follow-up and disease-free survival was equivalent during the first 15 months of follow-up, based on a predefined equivalence margin of 0.1.
- Paclitaxel-based HIPEC was not associated with increased morbidity (odds ratio, 1.32; P = .06).
IN PRACTICE:
“Our study suggests that cisplatin and paclitaxel are two safe and effective drugs to be used for HIPEC in [interval cytoreductive surgery] for advanced ovarian cancer. As cisplatin is the preferred drug according to strong evidence, paclitaxel could be a valuable alternative for patients with any contraindication to cisplatin, with similar oncological and perioperative outcomes,” the authors wrote.
SOURCE:
This study, led by Salud González Sánchez, MD, Reina Sofía University Hospital in Córdoba, Spain, was published online in JAMA Network Open.
LIMITATIONS:
The retrospective design of this study limited causal inference. The BRCA mutation status was not captured in the national registry. Additionally, the matching procedure resulted in a moderate sample size, which could have led to residual confounding.
DISCLOSURES:
The authors did not declare any funding information and reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.